User login
Benefit of lumbar fusion for spinal stenosis found to be small to nonexistent
The benefit of adding lumbar fusion surgery to decompression surgery for spinal stenosis was nonexistent in one large clinical trial and very modest in another, according to separate reports published online April 13 in the New England Journal of Medicine.
Both studies indicated that, given the considerable cost and the potential complications associated with lumbar fusion, it may not be worthwhile to add it to decompression surgery for spinal stenosis. “The goal of surgery in lumbar spinal stenosis is to improve walking distance and to relieve pain by decompression of the nerve roots. The addition of instrumented fusion – ‘just to be sure’ – for the treatment of the most frequent forms of lumbar spinal stenosis does not create any added value for patients and might be regarded as an overcautious and unnecessary treatment,” Dr. Wilco C. Peul and Dr. Wouter A. Moojen said in an editorial accompanying the two reports.
Surgical decompression of spinal stenosis using laminectomy is increasingly being supplemented with lumbar fusion, which is thought to firm up spinal instability and minimize the risk of future deformity. In the United States, approximately half of patients who have surgery for spinal stenosis undergo fusion procedures. Of those who also show degenerative spondylolisthesis on preoperative imaging studies, 96% undergo fusion procedures because many spine surgeons see this as a sign of instability and a mandatory indication for fusion. However, the evidence supporting the use of fusion plus decompression, as opposed to decompression alone, is weak, according to the investigators who conducted the Swedish Spinal Stenosis Study. The other study in the New England Journal of Medicine, the Spinal Laminectomy Versus Instrumented Pedicle Screw (SLIP) trial, was conducted in the United States.
Both of those clinical trials were performed to shed further light on the issue.
In the Swedish Spinal Stenosis Study, the investigators assessed outcomes in 247 patients aged 50-80 years who were treated at seven Swedish hospitals over the course of 6 years. This open-label, superiority trial randomly assigned 124 patients to decompression surgery alone and 123 to decompression plus fusion. The primary outcome measure was score on the Oswestry Disability Index (ODI), which measures disability and quality of life in patients with low-back pain, 2 years after surgery. The ODI scale runs from 0 to 100, with higher scores indicating more severe disability, said Dr. Peter Försth of the department of surgical sciences at Uppsala (Sweden) University and the Stockholm Spine Center and his associates.
At 2 years, there was no significant difference between the two study groups; the decompression-only group had a mean ODI score of 24, and the fusion group had a mean score of 27. The ODI scores in both groups had improved from baseline to a similar degree: by 17 points with decompression alone and by 15 points with fusion. In addition, fusion surgery was not superior to decompression alone regardless of whether patients had any degree of spondylolisthesis and regardless of whether they had severe spondylolisthesis involving a vertebral slip of 7.4 mm or more, the investigators reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1513721).The two study groups also showed no significant differences in secondary outcome measures, including performance on the 6-minute walk test and subjective patient assessment of improvement in walking ability. Moreover, these results persisted in the 144 patients who were assessed at 5-year follow-up.
In contrast, decompression alone was associated with fewer complications than decompression plus fusion. Postoperative wound infection developed in only 4% of the decompression-only group, compared with 10% of the fusion group. Although this study wasn’t adequately powered to draw firm conclusions regarding complications, a previous analysis of registry data reported that adding fusion surgery to decompression surgery doubles the risk of severe adverse events in older patients, Dr. Försth and his associates said.
Decompression alone also was markedly less expensive than fusion surgery. Mean direct costs were $6,800 higher for fusion than for decompression alone, because of the additional operating time needed, the extended hospital stay, and the cost of the implant.
In the SLIP trial, the researchers compared outcomes in 66 patients aged 50-80 years who all had spinal stenosis with grade 1 degenerative spondylolisthesis. The participants were randomly assigned to undergo decompression alone (35 patients) or decompression plus fusion (31 patients) at five U.S. medical centers, said Dr. Zoher Ghogawala of the Alan and Jacqueline B. Stuart Spine Research Center in the department of neurosurgery at Lahey Hospital and Medical Center, Burlington, Mass., and his associates.
The primary outcome measure was the physical-component summary score on the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) 2 years after surgery. This scale also runs from 0 to 100, but higher scores indicate better physical health. Five points was prespecified as the minimal clinically important difference on the SF-36.
At 2 years, patients in the fusion group showed a small but significant advantage of 5.7 points on the SF-36, with a mean score of 15.2, compared with patients in the decompression-only group (mean score, 9.5). However, the ODI scores, a secondary outcome measure in this study, were not significantly different between the two study groups, Dr. Ghogawala and his associates reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1508788).Surgical complications, blood loss, and length of stay all were significantly greater with fusion than with decompression alone.
Dr. Försth’s study was supported by Uppsala University, Uppsala County Council, the Stockholm Spine Center, and Johnson & Johnson. Two of his associates reported ties to Medtronic and Quantify Research. Dr. Ghogawala’s study was supported by the Jean and David Wallace Foundation, the Greenwich Lumbar Stenosis SLIP Study Fund. His associates reported ties to numerous industry sources.
Both of these studies clearly demonstrated that for most patients, stenosis surgery should be limited to decompression when no overt instability is present. Dr. Ghogawala and his colleagues correctly concluded that the modest difference in SF-36 score in favor of fusion doesn’t justify that procedure’s higher cost and complication rate.
Fusion surgery is no longer best practice and should be restricted to patients who have proven spinal instability; vertebral destruction caused by trauma, tumors, infections, or spinal deformities; or possibly neuroforamen stenosis with compressed exiting nerves due to postsurgical disk collapse.
Dr. Wilco C. Peul is at Leiden (the Netherlands) University Medical Center and at Medical Center Haaglanden, the Hague. Dr. Wouter A. Moojen is at Medical Center Haaglanden. Dr. Peul reported receiving grants from ZonMW, Paradigm Spine, Medtronic, Eurospine Foundation, and CVZ. Dr. Moojen reported having no relevant financial disclosures. Dr. Peul and Dr. Moojen made these remarks in an editorial accompanying the reports on the Swedish Spinal Stenosis Study and the Spinal Laminectomy Versus Instrumented Pedicle Screw trial (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMe1600955).
Both of these studies clearly demonstrated that for most patients, stenosis surgery should be limited to decompression when no overt instability is present. Dr. Ghogawala and his colleagues correctly concluded that the modest difference in SF-36 score in favor of fusion doesn’t justify that procedure’s higher cost and complication rate.
Fusion surgery is no longer best practice and should be restricted to patients who have proven spinal instability; vertebral destruction caused by trauma, tumors, infections, or spinal deformities; or possibly neuroforamen stenosis with compressed exiting nerves due to postsurgical disk collapse.
Dr. Wilco C. Peul is at Leiden (the Netherlands) University Medical Center and at Medical Center Haaglanden, the Hague. Dr. Wouter A. Moojen is at Medical Center Haaglanden. Dr. Peul reported receiving grants from ZonMW, Paradigm Spine, Medtronic, Eurospine Foundation, and CVZ. Dr. Moojen reported having no relevant financial disclosures. Dr. Peul and Dr. Moojen made these remarks in an editorial accompanying the reports on the Swedish Spinal Stenosis Study and the Spinal Laminectomy Versus Instrumented Pedicle Screw trial (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMe1600955).
Both of these studies clearly demonstrated that for most patients, stenosis surgery should be limited to decompression when no overt instability is present. Dr. Ghogawala and his colleagues correctly concluded that the modest difference in SF-36 score in favor of fusion doesn’t justify that procedure’s higher cost and complication rate.
Fusion surgery is no longer best practice and should be restricted to patients who have proven spinal instability; vertebral destruction caused by trauma, tumors, infections, or spinal deformities; or possibly neuroforamen stenosis with compressed exiting nerves due to postsurgical disk collapse.
Dr. Wilco C. Peul is at Leiden (the Netherlands) University Medical Center and at Medical Center Haaglanden, the Hague. Dr. Wouter A. Moojen is at Medical Center Haaglanden. Dr. Peul reported receiving grants from ZonMW, Paradigm Spine, Medtronic, Eurospine Foundation, and CVZ. Dr. Moojen reported having no relevant financial disclosures. Dr. Peul and Dr. Moojen made these remarks in an editorial accompanying the reports on the Swedish Spinal Stenosis Study and the Spinal Laminectomy Versus Instrumented Pedicle Screw trial (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMe1600955).
The benefit of adding lumbar fusion surgery to decompression surgery for spinal stenosis was nonexistent in one large clinical trial and very modest in another, according to separate reports published online April 13 in the New England Journal of Medicine.
Both studies indicated that, given the considerable cost and the potential complications associated with lumbar fusion, it may not be worthwhile to add it to decompression surgery for spinal stenosis. “The goal of surgery in lumbar spinal stenosis is to improve walking distance and to relieve pain by decompression of the nerve roots. The addition of instrumented fusion – ‘just to be sure’ – for the treatment of the most frequent forms of lumbar spinal stenosis does not create any added value for patients and might be regarded as an overcautious and unnecessary treatment,” Dr. Wilco C. Peul and Dr. Wouter A. Moojen said in an editorial accompanying the two reports.
Surgical decompression of spinal stenosis using laminectomy is increasingly being supplemented with lumbar fusion, which is thought to firm up spinal instability and minimize the risk of future deformity. In the United States, approximately half of patients who have surgery for spinal stenosis undergo fusion procedures. Of those who also show degenerative spondylolisthesis on preoperative imaging studies, 96% undergo fusion procedures because many spine surgeons see this as a sign of instability and a mandatory indication for fusion. However, the evidence supporting the use of fusion plus decompression, as opposed to decompression alone, is weak, according to the investigators who conducted the Swedish Spinal Stenosis Study. The other study in the New England Journal of Medicine, the Spinal Laminectomy Versus Instrumented Pedicle Screw (SLIP) trial, was conducted in the United States.
Both of those clinical trials were performed to shed further light on the issue.
In the Swedish Spinal Stenosis Study, the investigators assessed outcomes in 247 patients aged 50-80 years who were treated at seven Swedish hospitals over the course of 6 years. This open-label, superiority trial randomly assigned 124 patients to decompression surgery alone and 123 to decompression plus fusion. The primary outcome measure was score on the Oswestry Disability Index (ODI), which measures disability and quality of life in patients with low-back pain, 2 years after surgery. The ODI scale runs from 0 to 100, with higher scores indicating more severe disability, said Dr. Peter Försth of the department of surgical sciences at Uppsala (Sweden) University and the Stockholm Spine Center and his associates.
At 2 years, there was no significant difference between the two study groups; the decompression-only group had a mean ODI score of 24, and the fusion group had a mean score of 27. The ODI scores in both groups had improved from baseline to a similar degree: by 17 points with decompression alone and by 15 points with fusion. In addition, fusion surgery was not superior to decompression alone regardless of whether patients had any degree of spondylolisthesis and regardless of whether they had severe spondylolisthesis involving a vertebral slip of 7.4 mm or more, the investigators reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1513721).The two study groups also showed no significant differences in secondary outcome measures, including performance on the 6-minute walk test and subjective patient assessment of improvement in walking ability. Moreover, these results persisted in the 144 patients who were assessed at 5-year follow-up.
In contrast, decompression alone was associated with fewer complications than decompression plus fusion. Postoperative wound infection developed in only 4% of the decompression-only group, compared with 10% of the fusion group. Although this study wasn’t adequately powered to draw firm conclusions regarding complications, a previous analysis of registry data reported that adding fusion surgery to decompression surgery doubles the risk of severe adverse events in older patients, Dr. Försth and his associates said.
Decompression alone also was markedly less expensive than fusion surgery. Mean direct costs were $6,800 higher for fusion than for decompression alone, because of the additional operating time needed, the extended hospital stay, and the cost of the implant.
In the SLIP trial, the researchers compared outcomes in 66 patients aged 50-80 years who all had spinal stenosis with grade 1 degenerative spondylolisthesis. The participants were randomly assigned to undergo decompression alone (35 patients) or decompression plus fusion (31 patients) at five U.S. medical centers, said Dr. Zoher Ghogawala of the Alan and Jacqueline B. Stuart Spine Research Center in the department of neurosurgery at Lahey Hospital and Medical Center, Burlington, Mass., and his associates.
The primary outcome measure was the physical-component summary score on the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) 2 years after surgery. This scale also runs from 0 to 100, but higher scores indicate better physical health. Five points was prespecified as the minimal clinically important difference on the SF-36.
At 2 years, patients in the fusion group showed a small but significant advantage of 5.7 points on the SF-36, with a mean score of 15.2, compared with patients in the decompression-only group (mean score, 9.5). However, the ODI scores, a secondary outcome measure in this study, were not significantly different between the two study groups, Dr. Ghogawala and his associates reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1508788).Surgical complications, blood loss, and length of stay all were significantly greater with fusion than with decompression alone.
Dr. Försth’s study was supported by Uppsala University, Uppsala County Council, the Stockholm Spine Center, and Johnson & Johnson. Two of his associates reported ties to Medtronic and Quantify Research. Dr. Ghogawala’s study was supported by the Jean and David Wallace Foundation, the Greenwich Lumbar Stenosis SLIP Study Fund. His associates reported ties to numerous industry sources.
The benefit of adding lumbar fusion surgery to decompression surgery for spinal stenosis was nonexistent in one large clinical trial and very modest in another, according to separate reports published online April 13 in the New England Journal of Medicine.
Both studies indicated that, given the considerable cost and the potential complications associated with lumbar fusion, it may not be worthwhile to add it to decompression surgery for spinal stenosis. “The goal of surgery in lumbar spinal stenosis is to improve walking distance and to relieve pain by decompression of the nerve roots. The addition of instrumented fusion – ‘just to be sure’ – for the treatment of the most frequent forms of lumbar spinal stenosis does not create any added value for patients and might be regarded as an overcautious and unnecessary treatment,” Dr. Wilco C. Peul and Dr. Wouter A. Moojen said in an editorial accompanying the two reports.
Surgical decompression of spinal stenosis using laminectomy is increasingly being supplemented with lumbar fusion, which is thought to firm up spinal instability and minimize the risk of future deformity. In the United States, approximately half of patients who have surgery for spinal stenosis undergo fusion procedures. Of those who also show degenerative spondylolisthesis on preoperative imaging studies, 96% undergo fusion procedures because many spine surgeons see this as a sign of instability and a mandatory indication for fusion. However, the evidence supporting the use of fusion plus decompression, as opposed to decompression alone, is weak, according to the investigators who conducted the Swedish Spinal Stenosis Study. The other study in the New England Journal of Medicine, the Spinal Laminectomy Versus Instrumented Pedicle Screw (SLIP) trial, was conducted in the United States.
Both of those clinical trials were performed to shed further light on the issue.
In the Swedish Spinal Stenosis Study, the investigators assessed outcomes in 247 patients aged 50-80 years who were treated at seven Swedish hospitals over the course of 6 years. This open-label, superiority trial randomly assigned 124 patients to decompression surgery alone and 123 to decompression plus fusion. The primary outcome measure was score on the Oswestry Disability Index (ODI), which measures disability and quality of life in patients with low-back pain, 2 years after surgery. The ODI scale runs from 0 to 100, with higher scores indicating more severe disability, said Dr. Peter Försth of the department of surgical sciences at Uppsala (Sweden) University and the Stockholm Spine Center and his associates.
At 2 years, there was no significant difference between the two study groups; the decompression-only group had a mean ODI score of 24, and the fusion group had a mean score of 27. The ODI scores in both groups had improved from baseline to a similar degree: by 17 points with decompression alone and by 15 points with fusion. In addition, fusion surgery was not superior to decompression alone regardless of whether patients had any degree of spondylolisthesis and regardless of whether they had severe spondylolisthesis involving a vertebral slip of 7.4 mm or more, the investigators reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1513721).The two study groups also showed no significant differences in secondary outcome measures, including performance on the 6-minute walk test and subjective patient assessment of improvement in walking ability. Moreover, these results persisted in the 144 patients who were assessed at 5-year follow-up.
In contrast, decompression alone was associated with fewer complications than decompression plus fusion. Postoperative wound infection developed in only 4% of the decompression-only group, compared with 10% of the fusion group. Although this study wasn’t adequately powered to draw firm conclusions regarding complications, a previous analysis of registry data reported that adding fusion surgery to decompression surgery doubles the risk of severe adverse events in older patients, Dr. Försth and his associates said.
Decompression alone also was markedly less expensive than fusion surgery. Mean direct costs were $6,800 higher for fusion than for decompression alone, because of the additional operating time needed, the extended hospital stay, and the cost of the implant.
In the SLIP trial, the researchers compared outcomes in 66 patients aged 50-80 years who all had spinal stenosis with grade 1 degenerative spondylolisthesis. The participants were randomly assigned to undergo decompression alone (35 patients) or decompression plus fusion (31 patients) at five U.S. medical centers, said Dr. Zoher Ghogawala of the Alan and Jacqueline B. Stuart Spine Research Center in the department of neurosurgery at Lahey Hospital and Medical Center, Burlington, Mass., and his associates.
The primary outcome measure was the physical-component summary score on the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) 2 years after surgery. This scale also runs from 0 to 100, but higher scores indicate better physical health. Five points was prespecified as the minimal clinically important difference on the SF-36.
At 2 years, patients in the fusion group showed a small but significant advantage of 5.7 points on the SF-36, with a mean score of 15.2, compared with patients in the decompression-only group (mean score, 9.5). However, the ODI scores, a secondary outcome measure in this study, were not significantly different between the two study groups, Dr. Ghogawala and his associates reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1508788).Surgical complications, blood loss, and length of stay all were significantly greater with fusion than with decompression alone.
Dr. Försth’s study was supported by Uppsala University, Uppsala County Council, the Stockholm Spine Center, and Johnson & Johnson. Two of his associates reported ties to Medtronic and Quantify Research. Dr. Ghogawala’s study was supported by the Jean and David Wallace Foundation, the Greenwich Lumbar Stenosis SLIP Study Fund. His associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: The benefit of adding lumbar fusion surgery to decompression surgery for spinal stenosis was nonexistent in one large trial and very modest in another.
Major finding: At 2 years in the Swedish Spinal Stenosis Study, there was no significant difference between the two study groups; the decompression-only group had a mean Oswestry Disability Index score of 24, and the fusion group had a mean score of 27.
Data source: Two multicenter, randomized trials involving 247 patients and 66 patients, comparing decompression surgery alone with decompression plus fusion.
Disclosures: Dr. Försth’s study was supported by Uppsala University, Uppsala County Council, Stockholm Spine Center, and Johnson & Johnson. Two of his associates reported ties to Medtronic and Quantify Research. Dr. Ghogawala’s study was supported by the Jean and David Wallace Foundation, the Greenwich Lumbar Stenosis SLIP Study Fund. His associates reported ties to numerous industry sources.
Pre-PCI beta-blockers offer no clinical benefit
CHICAGO – Early intravenous administration of the beta-blocker metoprolol before primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction (STEMI) was safe but did not reduce infarct size in the randomized, placebo-controlled Early-BAMI trial.
No difference was seen in infarct size, as measured by magnetic resonance imaging at 30 days, between 336 patients with STEMI who presented within 12 hours of symptom onset and were randomized to receive intravenous metoprolol (2 vials with 5 mg) before undergoing angioplasty, and 347 such patients who received placebo (left ventricular volume, 15.3% and 14.9%, respectively), Dr. Vincent Roolvink of Isala Hospital, Zwolle, the Netherlands, reported at the annual meeting of the American College of Cardiology.
No differences were seen between the groups for the secondary endpoints of blood flow from the left ventricle or levels of cardiac enzymes, Dr. Roolvink noted.
Further, while significantly fewer cases of ventricular arrhythmia occurred in the metoprolol patients (3.6% vs. 6.9%), this difference was not clinically significant, he said.
No significant differences were seen with respect to safety endpoints, including abnormally slow heart rate, low blood pressure, or cardiogenic shock.
The Early-BAMI subjects had a mean age of 62 years, and most (75%) were men. They were enrolled at centers throughout the Netherlands and Spain.
“In this nonrestricted STEMI population, early intravenous metoprolol before primary percutaneous intervention did not reduce infarct size,” Dr Roolvink said, noting that the findings follow conflicting results from prior studies, with some suggesting that beta-blockers could reduce heart attack severity or improve blood flow from the left ventricle when given to STEMI patients prior to angioplasty.
However only one randomized trial took place in the primary percutaneous coronary intervention era, and that trial – METOCARD-CNIC (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction) – involved only patients with STEMIs involving the anterior wall of the left ventricle (J Am Coll Cardiol. 2014;63[22]:2356-62).
Early-BAMI (The Effect of Early Administration of Intravenous Beta Blockers in Patients with ST-elevation Myocardial Infarction Before Primary Percutaneous Coronary Intervention) was the first double blind, placebo-controlled international multicenter study to test this approach.
“Our results do not confirm the effect observed in the METOCARD-CNIC trial,” Dr. Roolvink said.
He noted, however, that the current findings are limited by the fact that study subjects had lower than expected overall heart attack severity.
Additional large randomized trials are needed to clarify whether early beta-blocker treatment is of benefit before angioplasty in STEMI patients. The safety profile, low cost of beta-blocker administration, and the reduction of acute malignant arrhythmias among those receiving beta-blocker treatment in the current trial should encourage the performance of additional larger trials, he said.
The findings were simultaneously published online (J Am Coll Cardiol. 2016 Apr 3. doi:10.1016/j.jacc.2016.03.522)
Early-BAMI was funded by the Dutch Heart Foundation and Medtronic. Dr. Roolvink reported having no disclosures.
CHICAGO – Early intravenous administration of the beta-blocker metoprolol before primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction (STEMI) was safe but did not reduce infarct size in the randomized, placebo-controlled Early-BAMI trial.
No difference was seen in infarct size, as measured by magnetic resonance imaging at 30 days, between 336 patients with STEMI who presented within 12 hours of symptom onset and were randomized to receive intravenous metoprolol (2 vials with 5 mg) before undergoing angioplasty, and 347 such patients who received placebo (left ventricular volume, 15.3% and 14.9%, respectively), Dr. Vincent Roolvink of Isala Hospital, Zwolle, the Netherlands, reported at the annual meeting of the American College of Cardiology.
No differences were seen between the groups for the secondary endpoints of blood flow from the left ventricle or levels of cardiac enzymes, Dr. Roolvink noted.
Further, while significantly fewer cases of ventricular arrhythmia occurred in the metoprolol patients (3.6% vs. 6.9%), this difference was not clinically significant, he said.
No significant differences were seen with respect to safety endpoints, including abnormally slow heart rate, low blood pressure, or cardiogenic shock.
The Early-BAMI subjects had a mean age of 62 years, and most (75%) were men. They were enrolled at centers throughout the Netherlands and Spain.
“In this nonrestricted STEMI population, early intravenous metoprolol before primary percutaneous intervention did not reduce infarct size,” Dr Roolvink said, noting that the findings follow conflicting results from prior studies, with some suggesting that beta-blockers could reduce heart attack severity or improve blood flow from the left ventricle when given to STEMI patients prior to angioplasty.
However only one randomized trial took place in the primary percutaneous coronary intervention era, and that trial – METOCARD-CNIC (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction) – involved only patients with STEMIs involving the anterior wall of the left ventricle (J Am Coll Cardiol. 2014;63[22]:2356-62).
Early-BAMI (The Effect of Early Administration of Intravenous Beta Blockers in Patients with ST-elevation Myocardial Infarction Before Primary Percutaneous Coronary Intervention) was the first double blind, placebo-controlled international multicenter study to test this approach.
“Our results do not confirm the effect observed in the METOCARD-CNIC trial,” Dr. Roolvink said.
He noted, however, that the current findings are limited by the fact that study subjects had lower than expected overall heart attack severity.
Additional large randomized trials are needed to clarify whether early beta-blocker treatment is of benefit before angioplasty in STEMI patients. The safety profile, low cost of beta-blocker administration, and the reduction of acute malignant arrhythmias among those receiving beta-blocker treatment in the current trial should encourage the performance of additional larger trials, he said.
The findings were simultaneously published online (J Am Coll Cardiol. 2016 Apr 3. doi:10.1016/j.jacc.2016.03.522)
Early-BAMI was funded by the Dutch Heart Foundation and Medtronic. Dr. Roolvink reported having no disclosures.
CHICAGO – Early intravenous administration of the beta-blocker metoprolol before primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction (STEMI) was safe but did not reduce infarct size in the randomized, placebo-controlled Early-BAMI trial.
No difference was seen in infarct size, as measured by magnetic resonance imaging at 30 days, between 336 patients with STEMI who presented within 12 hours of symptom onset and were randomized to receive intravenous metoprolol (2 vials with 5 mg) before undergoing angioplasty, and 347 such patients who received placebo (left ventricular volume, 15.3% and 14.9%, respectively), Dr. Vincent Roolvink of Isala Hospital, Zwolle, the Netherlands, reported at the annual meeting of the American College of Cardiology.
No differences were seen between the groups for the secondary endpoints of blood flow from the left ventricle or levels of cardiac enzymes, Dr. Roolvink noted.
Further, while significantly fewer cases of ventricular arrhythmia occurred in the metoprolol patients (3.6% vs. 6.9%), this difference was not clinically significant, he said.
No significant differences were seen with respect to safety endpoints, including abnormally slow heart rate, low blood pressure, or cardiogenic shock.
The Early-BAMI subjects had a mean age of 62 years, and most (75%) were men. They were enrolled at centers throughout the Netherlands and Spain.
“In this nonrestricted STEMI population, early intravenous metoprolol before primary percutaneous intervention did not reduce infarct size,” Dr Roolvink said, noting that the findings follow conflicting results from prior studies, with some suggesting that beta-blockers could reduce heart attack severity or improve blood flow from the left ventricle when given to STEMI patients prior to angioplasty.
However only one randomized trial took place in the primary percutaneous coronary intervention era, and that trial – METOCARD-CNIC (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction) – involved only patients with STEMIs involving the anterior wall of the left ventricle (J Am Coll Cardiol. 2014;63[22]:2356-62).
Early-BAMI (The Effect of Early Administration of Intravenous Beta Blockers in Patients with ST-elevation Myocardial Infarction Before Primary Percutaneous Coronary Intervention) was the first double blind, placebo-controlled international multicenter study to test this approach.
“Our results do not confirm the effect observed in the METOCARD-CNIC trial,” Dr. Roolvink said.
He noted, however, that the current findings are limited by the fact that study subjects had lower than expected overall heart attack severity.
Additional large randomized trials are needed to clarify whether early beta-blocker treatment is of benefit before angioplasty in STEMI patients. The safety profile, low cost of beta-blocker administration, and the reduction of acute malignant arrhythmias among those receiving beta-blocker treatment in the current trial should encourage the performance of additional larger trials, he said.
The findings were simultaneously published online (J Am Coll Cardiol. 2016 Apr 3. doi:10.1016/j.jacc.2016.03.522)
Early-BAMI was funded by the Dutch Heart Foundation and Medtronic. Dr. Roolvink reported having no disclosures.
AT ACC 16
Key clinical point: Early intravenous administration of metoprolol before primary percutaneous coronary intervention in patients with STEMI was safe but did not reduce infarct size in the randomized, placebo-controlled Early-BAMI trial.
Major finding: Infarct size on 30-day MRI was 15.3% and 14.9% of left ventricular volume in patients who received metoprolol and placebo, respectively.
Data source: The randomized, placebo-controlled Early-BAMI trial of 683 patients.
Disclosures: Early-BAMI was funded by the Dutch Heart Foundation and Medtronic. Dr. Roolvink reported having no disclosures.
Ticagrelor cuts post-MI events in diabetes patients
CHICAGO – The benefit from dual-antiplatelet therapy in high-risk patients following a myocardial infarction was especially apparent in post-MI patients with diabetes in a prespecified secondary analysis from a multicenter trial of ticagrelor with more than 21,000 patients.
Among post-MI patients with diabetes, treatment with ticagrelor plus aspirin led to an absolute 1.5% reduction in the rate of cardiovascular death, MI, or stroke during a median 33-month follow-up, compared with an absolute 1.1% cut in patients without diabetes, Dr. Deepak L. Bhatt said at the annual meeting of the American College of Cardiology. The relative risk reduction, compared with placebo was 16% in both the diabetes and no diabetes subgroups, statistically significant differences in both subgroups.
“Long-term treatment with ticagrelor reduced the composite of cardiovascular death, MI, or stroke in diabetic patients with a greater absolute risk reduction than in nondiabetic patients,” said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital in Boston. Treatment with ticagrelor plus aspirin in post-MI patients with diabetes also led to an increased number of major bleeding episodes, compared with patients on aspirin alone, but no excess of intracerebral hemorrhages or fatal bleeds, he noted.
This finding of a significant benefit from ticagrelor in post-MI patients with diabetes confirms similar, prior findings with other antiplatelet drugs (including clopidogrel, prasugrel, and vorapaxar) and prior findings with ticagrelor, Dr. Bhatt noted.
The new analysis used data collected in the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. The primary results from PEGASUS-TIMI 54 had shown that adding ticagrelor to aspirin treatment of high-risk post-MI patients, including those who both had or did not have diabetes, significantly cut the composite rate of cardiovascular death, MI, and stroke, compared with aspirin alone (N Engl J Med. 2015 May 7;372[19]:1791-800). The study group included 6,806 patients with diabetes (type 2 diabetes in 99% of these patients), and 14,355 without diabetes. All patients had their MI 1-3 years before entering the study.
Dr. Bhatt and his associates examined the incidence of the various clinical endpoints measured in the study among only the patients with diabetes divided into those who received any dosage of ticagrelor (60 mg b.i.d. or 90 mg b.i.d.) or placebo, and also among the patients without diabetes. In addition to the primary endpoint, the new analysis showed that the rate of cardiovascular death during follow-up was 3.9% in the diabetes patients on dual therapy and 5.0% among the diabetes patients on aspirin only, a 22% relative risk reduction with ticagrelor added that was statistically significant. In contrast, among patients without diabetes the rates of cardiovascular death between those on and not on ticagrelor only differed by 0.2%, a 9% relative risk reduction that was not statistically significant. The same pattern occurred for the endpoint of death from coronary artery disease.
Concurrent with Dr. Bhatt’s report, the results appeared in an article published online (J Am Coll Cardiol. 2016 Apr; doi: 10.1016/S0735-1097[16]30023-7).
A new study, THEMIS, is examining the safety and efficacy of combined ticagrelor and aspirin treatment in a lower-risk group of patients with diabetes, those with coronary artery disease who have not had a prior MI. Those results may be available in 2018.
PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company that markets ticagrelor (Brilinta). Dr. Bhatt has been an advisor to Cardax and Regado Biosciences and has received research support from AstraZeneca and several other companies.
On Twitter @mitchelzoler
CHICAGO – The benefit from dual-antiplatelet therapy in high-risk patients following a myocardial infarction was especially apparent in post-MI patients with diabetes in a prespecified secondary analysis from a multicenter trial of ticagrelor with more than 21,000 patients.
Among post-MI patients with diabetes, treatment with ticagrelor plus aspirin led to an absolute 1.5% reduction in the rate of cardiovascular death, MI, or stroke during a median 33-month follow-up, compared with an absolute 1.1% cut in patients without diabetes, Dr. Deepak L. Bhatt said at the annual meeting of the American College of Cardiology. The relative risk reduction, compared with placebo was 16% in both the diabetes and no diabetes subgroups, statistically significant differences in both subgroups.
“Long-term treatment with ticagrelor reduced the composite of cardiovascular death, MI, or stroke in diabetic patients with a greater absolute risk reduction than in nondiabetic patients,” said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital in Boston. Treatment with ticagrelor plus aspirin in post-MI patients with diabetes also led to an increased number of major bleeding episodes, compared with patients on aspirin alone, but no excess of intracerebral hemorrhages or fatal bleeds, he noted.
This finding of a significant benefit from ticagrelor in post-MI patients with diabetes confirms similar, prior findings with other antiplatelet drugs (including clopidogrel, prasugrel, and vorapaxar) and prior findings with ticagrelor, Dr. Bhatt noted.
The new analysis used data collected in the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. The primary results from PEGASUS-TIMI 54 had shown that adding ticagrelor to aspirin treatment of high-risk post-MI patients, including those who both had or did not have diabetes, significantly cut the composite rate of cardiovascular death, MI, and stroke, compared with aspirin alone (N Engl J Med. 2015 May 7;372[19]:1791-800). The study group included 6,806 patients with diabetes (type 2 diabetes in 99% of these patients), and 14,355 without diabetes. All patients had their MI 1-3 years before entering the study.
Dr. Bhatt and his associates examined the incidence of the various clinical endpoints measured in the study among only the patients with diabetes divided into those who received any dosage of ticagrelor (60 mg b.i.d. or 90 mg b.i.d.) or placebo, and also among the patients without diabetes. In addition to the primary endpoint, the new analysis showed that the rate of cardiovascular death during follow-up was 3.9% in the diabetes patients on dual therapy and 5.0% among the diabetes patients on aspirin only, a 22% relative risk reduction with ticagrelor added that was statistically significant. In contrast, among patients without diabetes the rates of cardiovascular death between those on and not on ticagrelor only differed by 0.2%, a 9% relative risk reduction that was not statistically significant. The same pattern occurred for the endpoint of death from coronary artery disease.
Concurrent with Dr. Bhatt’s report, the results appeared in an article published online (J Am Coll Cardiol. 2016 Apr; doi: 10.1016/S0735-1097[16]30023-7).
A new study, THEMIS, is examining the safety and efficacy of combined ticagrelor and aspirin treatment in a lower-risk group of patients with diabetes, those with coronary artery disease who have not had a prior MI. Those results may be available in 2018.
PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company that markets ticagrelor (Brilinta). Dr. Bhatt has been an advisor to Cardax and Regado Biosciences and has received research support from AstraZeneca and several other companies.
On Twitter @mitchelzoler
CHICAGO – The benefit from dual-antiplatelet therapy in high-risk patients following a myocardial infarction was especially apparent in post-MI patients with diabetes in a prespecified secondary analysis from a multicenter trial of ticagrelor with more than 21,000 patients.
Among post-MI patients with diabetes, treatment with ticagrelor plus aspirin led to an absolute 1.5% reduction in the rate of cardiovascular death, MI, or stroke during a median 33-month follow-up, compared with an absolute 1.1% cut in patients without diabetes, Dr. Deepak L. Bhatt said at the annual meeting of the American College of Cardiology. The relative risk reduction, compared with placebo was 16% in both the diabetes and no diabetes subgroups, statistically significant differences in both subgroups.
“Long-term treatment with ticagrelor reduced the composite of cardiovascular death, MI, or stroke in diabetic patients with a greater absolute risk reduction than in nondiabetic patients,” said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital in Boston. Treatment with ticagrelor plus aspirin in post-MI patients with diabetes also led to an increased number of major bleeding episodes, compared with patients on aspirin alone, but no excess of intracerebral hemorrhages or fatal bleeds, he noted.
This finding of a significant benefit from ticagrelor in post-MI patients with diabetes confirms similar, prior findings with other antiplatelet drugs (including clopidogrel, prasugrel, and vorapaxar) and prior findings with ticagrelor, Dr. Bhatt noted.
The new analysis used data collected in the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. The primary results from PEGASUS-TIMI 54 had shown that adding ticagrelor to aspirin treatment of high-risk post-MI patients, including those who both had or did not have diabetes, significantly cut the composite rate of cardiovascular death, MI, and stroke, compared with aspirin alone (N Engl J Med. 2015 May 7;372[19]:1791-800). The study group included 6,806 patients with diabetes (type 2 diabetes in 99% of these patients), and 14,355 without diabetes. All patients had their MI 1-3 years before entering the study.
Dr. Bhatt and his associates examined the incidence of the various clinical endpoints measured in the study among only the patients with diabetes divided into those who received any dosage of ticagrelor (60 mg b.i.d. or 90 mg b.i.d.) or placebo, and also among the patients without diabetes. In addition to the primary endpoint, the new analysis showed that the rate of cardiovascular death during follow-up was 3.9% in the diabetes patients on dual therapy and 5.0% among the diabetes patients on aspirin only, a 22% relative risk reduction with ticagrelor added that was statistically significant. In contrast, among patients without diabetes the rates of cardiovascular death between those on and not on ticagrelor only differed by 0.2%, a 9% relative risk reduction that was not statistically significant. The same pattern occurred for the endpoint of death from coronary artery disease.
Concurrent with Dr. Bhatt’s report, the results appeared in an article published online (J Am Coll Cardiol. 2016 Apr; doi: 10.1016/S0735-1097[16]30023-7).
A new study, THEMIS, is examining the safety and efficacy of combined ticagrelor and aspirin treatment in a lower-risk group of patients with diabetes, those with coronary artery disease who have not had a prior MI. Those results may be available in 2018.
PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company that markets ticagrelor (Brilinta). Dr. Bhatt has been an advisor to Cardax and Regado Biosciences and has received research support from AstraZeneca and several other companies.
On Twitter @mitchelzoler
AT ACC 2016
Key clinical point: Among post-MI patients with diabetes, dual-antiplatelet therapy with aspirin and ticagrelor produced a significant drop in the rate of cardiovascular death and other ischemic events.
Major finding: Post-MI patients with diabetes had a 10.1% combined endpoint rate on ticagrelor and a 11.6% rate on placebo.
Data source: Prespecified secondary analysis of data from PEGASUS-TIMI 54, a multicenter randomized trial with 21,162 patients.
Disclosures: PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company that markets ticagrelor (Brilinta). Dr. Bhatt has been an advisor to Cardax and Regado Biosciences and has received research support from AstraZeneca and several other companies.
VIDEO: Stenting to improve quality of life in esophageal cancer
PHILADELPHIA – An esophageal stent can improve quality of life for patients with advanced esophageal cancer, according to Dr. Sushil Ahlawat, director of endoscopy at Rutgers University, New Brunswick, N.J.
“An esophageal stent can be an important modality for palliating patients’ dysphagia, [which] can happen because the tumor is obstructing the esophagus,” says Dr. Ahlawat in this video. He also discusses how this minimally invasive procedure can support those undergoing chemoradiation therapy or surgery for esophageal cancer.
The video was recorded at this year’s meeting, held by Global Academy for Medical Education and Rutgers, the State University of New Jersey. Global Academy for Medical Education and this news organization are owned by the same company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
PHILADELPHIA – An esophageal stent can improve quality of life for patients with advanced esophageal cancer, according to Dr. Sushil Ahlawat, director of endoscopy at Rutgers University, New Brunswick, N.J.
“An esophageal stent can be an important modality for palliating patients’ dysphagia, [which] can happen because the tumor is obstructing the esophagus,” says Dr. Ahlawat in this video. He also discusses how this minimally invasive procedure can support those undergoing chemoradiation therapy or surgery for esophageal cancer.
The video was recorded at this year’s meeting, held by Global Academy for Medical Education and Rutgers, the State University of New Jersey. Global Academy for Medical Education and this news organization are owned by the same company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
PHILADELPHIA – An esophageal stent can improve quality of life for patients with advanced esophageal cancer, according to Dr. Sushil Ahlawat, director of endoscopy at Rutgers University, New Brunswick, N.J.
“An esophageal stent can be an important modality for palliating patients’ dysphagia, [which] can happen because the tumor is obstructing the esophagus,” says Dr. Ahlawat in this video. He also discusses how this minimally invasive procedure can support those undergoing chemoradiation therapy or surgery for esophageal cancer.
The video was recorded at this year’s meeting, held by Global Academy for Medical Education and Rutgers, the State University of New Jersey. Global Academy for Medical Education and this news organization are owned by the same company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM DIGESTIVE DISEASES: NEW ADVANCES
Older patients with soft tissue sarcoma may receive greater benefit from RT
Older patients appeared to receive greater benefit from adjuvant radiotherapy (RT) for soft tissue sarcoma (STS) than younger patients, according an analysis of the Surveillance, Epidemiology, and End Results (SEER) database.
Several STS subtypes were associated with significant RT benefits to overall survival (OS) and disease-specific survival (DSS) in patients older than 65 years, but survival differences were not significant in younger patients. These seven subtypes included leiomyosarcoma (hazard ratio, 0.84; P = .04), sarcoma not otherwise specified (HR, 0.66; P less than or equal to .001), liposarcoma not otherwise specified (HR, 0.72; P = .05), myxoid liposarcoma (HR, 0.50; P = .02), rhabdomyosarcoma (HR, 0.23; P less than or equal to .001), epithelioid (HR, 0.01; P less than .01) and myxoid chondrosarcoma (HR, 0.02; P = .04). One STS subtype, synovial sarcoma, was associated with significant RT benefit only in younger patients (HR, 0.73; P = .04). Malignant fibrous histiocytoma was the only subtype to show significant benefit in the overall cohort as well as both age groups.
“We observed a statistically significant improvement in OS and DSS in all patients receiving RT compared to surgery alone across the majority of histological subgroups. More importantly, there was no significant improvement in younger patients compared to a significant improvement in older patients, suggesting that survival benefits in response to RT are significantly affected by age-related differences,” wrote Dr. Noah K. Yuen of the Department of Surgery, University of California, Davis, and his colleagues (Anticancer Res. 2016 Apr;36(4):1745-50). The findings suggest that older patients may benefit more than previously appreciated, and while implementation of RT among the elderly may present challenges, according to the investigators, “our data suggest that this approach deserves greater attention.”
Previous population-based retrospective studies have demonstrated a similar benefit with surgery and adjuvant RT; however, randomized clinical trials have shown significant improvement in local control but have failed to show significant improvement in OS. The authors acknowledged the potential impact of unmeasured confounding factors on the retrospective study. Selection bias may be present if healthier older patients preferentially received RT; a subpopulation of healthier individuals would be expected to have better survival.
The SEER database analysis included 15,380 patients with non-metastatic STS who underwent surgery during 1990 to 2011. The mean age of the cohort was 56.6 years and one-third were age 65 years or more. As the most common histologic subtype, leiomyosarcoma accounted for 30.1% of all tumors. Most of the patients treated with RT (68.3%) had high-grade tumors.
Older patients appeared to receive greater benefit from adjuvant radiotherapy (RT) for soft tissue sarcoma (STS) than younger patients, according an analysis of the Surveillance, Epidemiology, and End Results (SEER) database.
Several STS subtypes were associated with significant RT benefits to overall survival (OS) and disease-specific survival (DSS) in patients older than 65 years, but survival differences were not significant in younger patients. These seven subtypes included leiomyosarcoma (hazard ratio, 0.84; P = .04), sarcoma not otherwise specified (HR, 0.66; P less than or equal to .001), liposarcoma not otherwise specified (HR, 0.72; P = .05), myxoid liposarcoma (HR, 0.50; P = .02), rhabdomyosarcoma (HR, 0.23; P less than or equal to .001), epithelioid (HR, 0.01; P less than .01) and myxoid chondrosarcoma (HR, 0.02; P = .04). One STS subtype, synovial sarcoma, was associated with significant RT benefit only in younger patients (HR, 0.73; P = .04). Malignant fibrous histiocytoma was the only subtype to show significant benefit in the overall cohort as well as both age groups.
“We observed a statistically significant improvement in OS and DSS in all patients receiving RT compared to surgery alone across the majority of histological subgroups. More importantly, there was no significant improvement in younger patients compared to a significant improvement in older patients, suggesting that survival benefits in response to RT are significantly affected by age-related differences,” wrote Dr. Noah K. Yuen of the Department of Surgery, University of California, Davis, and his colleagues (Anticancer Res. 2016 Apr;36(4):1745-50). The findings suggest that older patients may benefit more than previously appreciated, and while implementation of RT among the elderly may present challenges, according to the investigators, “our data suggest that this approach deserves greater attention.”
Previous population-based retrospective studies have demonstrated a similar benefit with surgery and adjuvant RT; however, randomized clinical trials have shown significant improvement in local control but have failed to show significant improvement in OS. The authors acknowledged the potential impact of unmeasured confounding factors on the retrospective study. Selection bias may be present if healthier older patients preferentially received RT; a subpopulation of healthier individuals would be expected to have better survival.
The SEER database analysis included 15,380 patients with non-metastatic STS who underwent surgery during 1990 to 2011. The mean age of the cohort was 56.6 years and one-third were age 65 years or more. As the most common histologic subtype, leiomyosarcoma accounted for 30.1% of all tumors. Most of the patients treated with RT (68.3%) had high-grade tumors.
Older patients appeared to receive greater benefit from adjuvant radiotherapy (RT) for soft tissue sarcoma (STS) than younger patients, according an analysis of the Surveillance, Epidemiology, and End Results (SEER) database.
Several STS subtypes were associated with significant RT benefits to overall survival (OS) and disease-specific survival (DSS) in patients older than 65 years, but survival differences were not significant in younger patients. These seven subtypes included leiomyosarcoma (hazard ratio, 0.84; P = .04), sarcoma not otherwise specified (HR, 0.66; P less than or equal to .001), liposarcoma not otherwise specified (HR, 0.72; P = .05), myxoid liposarcoma (HR, 0.50; P = .02), rhabdomyosarcoma (HR, 0.23; P less than or equal to .001), epithelioid (HR, 0.01; P less than .01) and myxoid chondrosarcoma (HR, 0.02; P = .04). One STS subtype, synovial sarcoma, was associated with significant RT benefit only in younger patients (HR, 0.73; P = .04). Malignant fibrous histiocytoma was the only subtype to show significant benefit in the overall cohort as well as both age groups.
“We observed a statistically significant improvement in OS and DSS in all patients receiving RT compared to surgery alone across the majority of histological subgroups. More importantly, there was no significant improvement in younger patients compared to a significant improvement in older patients, suggesting that survival benefits in response to RT are significantly affected by age-related differences,” wrote Dr. Noah K. Yuen of the Department of Surgery, University of California, Davis, and his colleagues (Anticancer Res. 2016 Apr;36(4):1745-50). The findings suggest that older patients may benefit more than previously appreciated, and while implementation of RT among the elderly may present challenges, according to the investigators, “our data suggest that this approach deserves greater attention.”
Previous population-based retrospective studies have demonstrated a similar benefit with surgery and adjuvant RT; however, randomized clinical trials have shown significant improvement in local control but have failed to show significant improvement in OS. The authors acknowledged the potential impact of unmeasured confounding factors on the retrospective study. Selection bias may be present if healthier older patients preferentially received RT; a subpopulation of healthier individuals would be expected to have better survival.
The SEER database analysis included 15,380 patients with non-metastatic STS who underwent surgery during 1990 to 2011. The mean age of the cohort was 56.6 years and one-third were age 65 years or more. As the most common histologic subtype, leiomyosarcoma accounted for 30.1% of all tumors. Most of the patients treated with RT (68.3%) had high-grade tumors.
FROM ANTICANCER RESEARCH
Key clinical point: For many subtypes of soft tissue sarcoma (STS), overall survival (OS) was significantly greater in older patients who received adjuvant RT, compared with those who received surgery alone.
Major finding: Among patients age 65 years and older who received RT, most STS subtypes were associated with significantly improved OS; among younger patients, only three subtypes were associated with significantly improved OS.
Data sources: The retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER) database included 15,380 patients with non-metastatic STS who underwent surgery during 1990 to 2011.
Disclosures: Dr. Yuen and his coauthors reported having no disclosures.
Lars Edvinsson, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
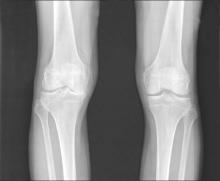
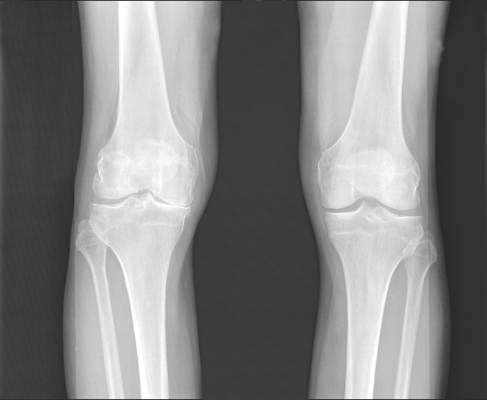
Blood biomarker panel studies aim to predict knee osteoarthritis, progression
AMSTERDAM – Results of separate U.S. and European studies hold promise that predictive blood tests for the diagnosis and progression of knee osteoarthritis could soon be developed.
Researchers at Duke University in Durham, N.C., and at the Hospital Universitario de A Coruña, Spain, reported their studies’ findings at the World Congress on Osteoarthritis sponsored by the Osteoarthritis Research Society International.
Clinical trials are often statistically underpowered to measure progression of knee osteoarthritis, noted Dr. Virginia B. Kraus of Duke University, because of inefficient methods for identifying patients who would progress.
“This is because, with the exception of generalized OA, traditional risk factors are very poor predictors, and that includes age, gender, BMI [body mass index], knee pain, and baseline joint space width,” she explained. Radiographic changes and changes in Kellgren-Lawrence grade are often slow to appear and may only occur in a few subjects at a time. Imaging biomarkers have been identified but are a “rather expensive way of predicting progression,” Dr. Kraus said. There have been some associations between OA progression and biomarkers in the urine and serum, she said, but still not at a level that could be put to clinical use.
“We hypothesized that we could improve the utility of OA biomarkers by using a nonbiased, nontargeted [mass spectrometry] approach,” Dr. Kraus said, explaining that this allowed them to create a Multiple Reaction Monitoring (MRM) panel of 146 serum peptides that were based on analyses of serum, synovial fluid, and urine samples from knee OA radiographic progressors and nonprogressors. The MRM panel was used first to identify candidate biomarkers and then confirm their differential expression patterns, followed by a process of verifying and validating the selected serum biomarkers.
The main aim of the study was to look for serum biomarkers in data from the Prediction of Osteoarthritis Progression (POP) and Genetics of Generalized Osteoarthritis (GOGO) patient cohorts that would be highly predictive for knee OA progression, with a secondary aim to look at potential early diagnostic biomarkers. The investigators used data from 83 patients with symptomatic knee progression to search for prognostic biomarkers and 126 with symptoms but without knee progression to find diagnostic biomarkers. The mean age of patients was 64 years, and 82% were female. The mean BMI was 28 kg/m2.
As expected, clinical predictors alone – age, gender, and BMI – had low predictive power for OA progression, with an area under the curve (AUC) of about 0.7. However, these clinical parameters in conjunction with a panel of 10 markers identified via MRM improved the AUC to 0.9 in the verification set and 0.8 in the validation set. For diagnosis, the AUC for clinical covariates alone was 0.8, but combined with a set of 19 MRM-identified markers, the AUC was 1.0 for the verification set and 0.9 for the validation set.
Dr. Kraus said that the serum peptide biomarkers identified from the MRM panel predicted that OA progression pathways would involve aggregation of cells, complement activation, cell movement of leukocytes, and cellular infiltration of leukocytes. The pathways predicted to be involved in a diagnosis of OA included skeletal function, cell movement, formation of filaments, cellular protrusions, and hemostasis.
“Results from this work in progress suggest that a select set of biomarkers from nontargeted analyses could significantly improve prediction of knee OA progression,” Dr. Kraus concluded. They also suggest a set of biomarkers could be used for diagnosis because of their distinction from those for progression, with one exception. Future work will look at whether the diagnostic panel could be used in patients with nonradiographic OA to potentially diagnose and perhaps help treat OA early before structural damage is apparent, she said.
The proteomics group at the Hospital Universitario de A Coruña collaborated with researchers at the KTH-Royal Institute of Technology in Stockholm and the University of Oxford (England) to also identify a serum protein biomarker panel that might prove useful for the diagnosis of OA.
Their study compared serum samples taken from 461 patients with knee OA with those taken from 412 patients with rheumatoid arthritis and 159 nonarthritic individuals. Serum samples from each of these groups were divided into a screening (n = 593) and a verification set (n = 439). The data were adjusted for variations in participant’s age, BMI, and gender.
In the screening phase, the investigators used an antibody suspension bead array composed of 174 antibodies to examine 78 protein targets to try to identify the best serum protein biomarker candidates. They replicated the findings in an adapted array of 79 antibodies and 34 protein targets and then used this array in the verification set of serum samples.
There were three proteins that could potentially differentiate patients with OA from controls and perhaps be used to diagnose OA. These were: complement 3, which is involved in inflammation; inter-alpha-trypsin inhibitor heavy chain I, which is involved in the stability of the extracellular matrix; and S100 calcium-binding protein A6, which has a role in chondrocyte differentiation and thus bone remodeling.
“Compared to rheumatoid arthritis patients, we observed that the level of complement 3 and inter-alpha-trypsin inhibitor heavy chain I are significantly increased in osteoarthritis patients,” said the presenting study author Lucía Maria Lourido Salas, PhD. “This suggests the potential of these two proteins as specific proteins for osteoarthritis,” she proposed. A further 28 proteins were found that might also help to differentiate OA from RA; 16 of these were increased and 12 decreased in OA patients. Together these 30 proteins could potentially help identify a specific serum protein signature of OA, Dr. Salas said.
Dr. Kraus and Dr. Salas did not report having any financial disclosures. Dr. Kraus’ research is funded by the Duke Biomarker Factory.
AMSTERDAM – Results of separate U.S. and European studies hold promise that predictive blood tests for the diagnosis and progression of knee osteoarthritis could soon be developed.
Researchers at Duke University in Durham, N.C., and at the Hospital Universitario de A Coruña, Spain, reported their studies’ findings at the World Congress on Osteoarthritis sponsored by the Osteoarthritis Research Society International.
Clinical trials are often statistically underpowered to measure progression of knee osteoarthritis, noted Dr. Virginia B. Kraus of Duke University, because of inefficient methods for identifying patients who would progress.
“This is because, with the exception of generalized OA, traditional risk factors are very poor predictors, and that includes age, gender, BMI [body mass index], knee pain, and baseline joint space width,” she explained. Radiographic changes and changes in Kellgren-Lawrence grade are often slow to appear and may only occur in a few subjects at a time. Imaging biomarkers have been identified but are a “rather expensive way of predicting progression,” Dr. Kraus said. There have been some associations between OA progression and biomarkers in the urine and serum, she said, but still not at a level that could be put to clinical use.
“We hypothesized that we could improve the utility of OA biomarkers by using a nonbiased, nontargeted [mass spectrometry] approach,” Dr. Kraus said, explaining that this allowed them to create a Multiple Reaction Monitoring (MRM) panel of 146 serum peptides that were based on analyses of serum, synovial fluid, and urine samples from knee OA radiographic progressors and nonprogressors. The MRM panel was used first to identify candidate biomarkers and then confirm their differential expression patterns, followed by a process of verifying and validating the selected serum biomarkers.
The main aim of the study was to look for serum biomarkers in data from the Prediction of Osteoarthritis Progression (POP) and Genetics of Generalized Osteoarthritis (GOGO) patient cohorts that would be highly predictive for knee OA progression, with a secondary aim to look at potential early diagnostic biomarkers. The investigators used data from 83 patients with symptomatic knee progression to search for prognostic biomarkers and 126 with symptoms but without knee progression to find diagnostic biomarkers. The mean age of patients was 64 years, and 82% were female. The mean BMI was 28 kg/m2.
As expected, clinical predictors alone – age, gender, and BMI – had low predictive power for OA progression, with an area under the curve (AUC) of about 0.7. However, these clinical parameters in conjunction with a panel of 10 markers identified via MRM improved the AUC to 0.9 in the verification set and 0.8 in the validation set. For diagnosis, the AUC for clinical covariates alone was 0.8, but combined with a set of 19 MRM-identified markers, the AUC was 1.0 for the verification set and 0.9 for the validation set.
Dr. Kraus said that the serum peptide biomarkers identified from the MRM panel predicted that OA progression pathways would involve aggregation of cells, complement activation, cell movement of leukocytes, and cellular infiltration of leukocytes. The pathways predicted to be involved in a diagnosis of OA included skeletal function, cell movement, formation of filaments, cellular protrusions, and hemostasis.
“Results from this work in progress suggest that a select set of biomarkers from nontargeted analyses could significantly improve prediction of knee OA progression,” Dr. Kraus concluded. They also suggest a set of biomarkers could be used for diagnosis because of their distinction from those for progression, with one exception. Future work will look at whether the diagnostic panel could be used in patients with nonradiographic OA to potentially diagnose and perhaps help treat OA early before structural damage is apparent, she said.
The proteomics group at the Hospital Universitario de A Coruña collaborated with researchers at the KTH-Royal Institute of Technology in Stockholm and the University of Oxford (England) to also identify a serum protein biomarker panel that might prove useful for the diagnosis of OA.
Their study compared serum samples taken from 461 patients with knee OA with those taken from 412 patients with rheumatoid arthritis and 159 nonarthritic individuals. Serum samples from each of these groups were divided into a screening (n = 593) and a verification set (n = 439). The data were adjusted for variations in participant’s age, BMI, and gender.
In the screening phase, the investigators used an antibody suspension bead array composed of 174 antibodies to examine 78 protein targets to try to identify the best serum protein biomarker candidates. They replicated the findings in an adapted array of 79 antibodies and 34 protein targets and then used this array in the verification set of serum samples.
There were three proteins that could potentially differentiate patients with OA from controls and perhaps be used to diagnose OA. These were: complement 3, which is involved in inflammation; inter-alpha-trypsin inhibitor heavy chain I, which is involved in the stability of the extracellular matrix; and S100 calcium-binding protein A6, which has a role in chondrocyte differentiation and thus bone remodeling.
“Compared to rheumatoid arthritis patients, we observed that the level of complement 3 and inter-alpha-trypsin inhibitor heavy chain I are significantly increased in osteoarthritis patients,” said the presenting study author Lucía Maria Lourido Salas, PhD. “This suggests the potential of these two proteins as specific proteins for osteoarthritis,” she proposed. A further 28 proteins were found that might also help to differentiate OA from RA; 16 of these were increased and 12 decreased in OA patients. Together these 30 proteins could potentially help identify a specific serum protein signature of OA, Dr. Salas said.
Dr. Kraus and Dr. Salas did not report having any financial disclosures. Dr. Kraus’ research is funded by the Duke Biomarker Factory.
AMSTERDAM – Results of separate U.S. and European studies hold promise that predictive blood tests for the diagnosis and progression of knee osteoarthritis could soon be developed.
Researchers at Duke University in Durham, N.C., and at the Hospital Universitario de A Coruña, Spain, reported their studies’ findings at the World Congress on Osteoarthritis sponsored by the Osteoarthritis Research Society International.
Clinical trials are often statistically underpowered to measure progression of knee osteoarthritis, noted Dr. Virginia B. Kraus of Duke University, because of inefficient methods for identifying patients who would progress.
“This is because, with the exception of generalized OA, traditional risk factors are very poor predictors, and that includes age, gender, BMI [body mass index], knee pain, and baseline joint space width,” she explained. Radiographic changes and changes in Kellgren-Lawrence grade are often slow to appear and may only occur in a few subjects at a time. Imaging biomarkers have been identified but are a “rather expensive way of predicting progression,” Dr. Kraus said. There have been some associations between OA progression and biomarkers in the urine and serum, she said, but still not at a level that could be put to clinical use.
“We hypothesized that we could improve the utility of OA biomarkers by using a nonbiased, nontargeted [mass spectrometry] approach,” Dr. Kraus said, explaining that this allowed them to create a Multiple Reaction Monitoring (MRM) panel of 146 serum peptides that were based on analyses of serum, synovial fluid, and urine samples from knee OA radiographic progressors and nonprogressors. The MRM panel was used first to identify candidate biomarkers and then confirm their differential expression patterns, followed by a process of verifying and validating the selected serum biomarkers.
The main aim of the study was to look for serum biomarkers in data from the Prediction of Osteoarthritis Progression (POP) and Genetics of Generalized Osteoarthritis (GOGO) patient cohorts that would be highly predictive for knee OA progression, with a secondary aim to look at potential early diagnostic biomarkers. The investigators used data from 83 patients with symptomatic knee progression to search for prognostic biomarkers and 126 with symptoms but without knee progression to find diagnostic biomarkers. The mean age of patients was 64 years, and 82% were female. The mean BMI was 28 kg/m2.
As expected, clinical predictors alone – age, gender, and BMI – had low predictive power for OA progression, with an area under the curve (AUC) of about 0.7. However, these clinical parameters in conjunction with a panel of 10 markers identified via MRM improved the AUC to 0.9 in the verification set and 0.8 in the validation set. For diagnosis, the AUC for clinical covariates alone was 0.8, but combined with a set of 19 MRM-identified markers, the AUC was 1.0 for the verification set and 0.9 for the validation set.
Dr. Kraus said that the serum peptide biomarkers identified from the MRM panel predicted that OA progression pathways would involve aggregation of cells, complement activation, cell movement of leukocytes, and cellular infiltration of leukocytes. The pathways predicted to be involved in a diagnosis of OA included skeletal function, cell movement, formation of filaments, cellular protrusions, and hemostasis.
“Results from this work in progress suggest that a select set of biomarkers from nontargeted analyses could significantly improve prediction of knee OA progression,” Dr. Kraus concluded. They also suggest a set of biomarkers could be used for diagnosis because of their distinction from those for progression, with one exception. Future work will look at whether the diagnostic panel could be used in patients with nonradiographic OA to potentially diagnose and perhaps help treat OA early before structural damage is apparent, she said.
The proteomics group at the Hospital Universitario de A Coruña collaborated with researchers at the KTH-Royal Institute of Technology in Stockholm and the University of Oxford (England) to also identify a serum protein biomarker panel that might prove useful for the diagnosis of OA.
Their study compared serum samples taken from 461 patients with knee OA with those taken from 412 patients with rheumatoid arthritis and 159 nonarthritic individuals. Serum samples from each of these groups were divided into a screening (n = 593) and a verification set (n = 439). The data were adjusted for variations in participant’s age, BMI, and gender.
In the screening phase, the investigators used an antibody suspension bead array composed of 174 antibodies to examine 78 protein targets to try to identify the best serum protein biomarker candidates. They replicated the findings in an adapted array of 79 antibodies and 34 protein targets and then used this array in the verification set of serum samples.
There were three proteins that could potentially differentiate patients with OA from controls and perhaps be used to diagnose OA. These were: complement 3, which is involved in inflammation; inter-alpha-trypsin inhibitor heavy chain I, which is involved in the stability of the extracellular matrix; and S100 calcium-binding protein A6, which has a role in chondrocyte differentiation and thus bone remodeling.
“Compared to rheumatoid arthritis patients, we observed that the level of complement 3 and inter-alpha-trypsin inhibitor heavy chain I are significantly increased in osteoarthritis patients,” said the presenting study author Lucía Maria Lourido Salas, PhD. “This suggests the potential of these two proteins as specific proteins for osteoarthritis,” she proposed. A further 28 proteins were found that might also help to differentiate OA from RA; 16 of these were increased and 12 decreased in OA patients. Together these 30 proteins could potentially help identify a specific serum protein signature of OA, Dr. Salas said.
Dr. Kraus and Dr. Salas did not report having any financial disclosures. Dr. Kraus’ research is funded by the Duke Biomarker Factory.
AT OARSI 2016
Key clinical point: Distinct serum biomarker panels have been identified for OA progression and diagnosis.
Major finding: The predictive power of clinical variables was improved by using serum biomarker panels in one U.S. study, with area under the curve of 0.7 for progression and 0.8 for diagnosis increasing to 0.9 and 1.0.
Data source: U.S. and European studies investigating serum biomarkers for OA diagnosis and progression.
Disclosures: Dr. Kraus and Dr. Salas did not report having any financial disclosures.
Study: Few HCV-infected heroin users linked to outpatient care
The results of a hepatitis C virus screening program in a suburban New Jersey acute opioid detoxification program reveal that, despite the ease of access to HCV screening and the availability of curative therapies, linkage to care after detection of infection in persons who inject drugs continues to present a challenge.
Eda Akyar, a clinical research coordinator at ID Care, New Jersey’s largest network of infectious disease specialists, and her colleagues examined the results of an HIV, HCV, and HBV infection screening program instituted between Oct. 1, 2014, and June 9, 2015, for patients admitted to an acute opioid detoxification program at Princeton House in suburban New Jersey. Study goals included assessing the prevalence of HIV, HCV, and HBV infections for these patients, the HCV genotype (GT) carried, and the subsequent linkage to care after discharge from the program. The report was published online April 13 in Emerging Infectious Diseases.
During the screening period, patients representing 10 of New Jersey’s 21 counties were tested for the presence of HCV antibodies. Approximately two-thirds of all study participants (66.6%) were between 17 and 35 years of age. In this important age demographic, 237 patients (41.4%) screened positive for HCV antibodies, and 187 were available for further study.
HCV viral load data were available from 172 patients (92.0%), with approximately one-fifth (18.6%) screening undetectable. The HCV GTs obtained from 102 patients revealed that most (62.7%) were GT1a, followed by GT3 (25.5%). In a very surprising result, all HCV antibody positive patients were also HIV antibody negative, the researchers noted.
Regarding linkage to care, 16 of the patients (8.6%) in the 17- to 35-year-old age group attended outpatient follow-up appointments, with three (1.6%) starting on an oral, direct-acting antiviral treatment regimen. Two of these three patients were nonadherent to their treatment regimens. Two additional patients expressed willingness to accept treatment, but were denied their prescriptions by their insurance providers. None of the other participants returned for continued care.
The authors said that their results indicated a high prevalence of HCV among young suburban heroin users attending an acute detoxification program serving a wide geographic area, suggesting that New Jersey is part of the second wave of HCV infection following that seen in those born from 1946 to 1964. They also highlighted their findings of a GT3 prevalence more than twice the national average, a complete lack of HIV infection in a population known to be susceptible to it, and disappointing linkage to care outcomes.
The authors said that easy-to-use curative therapy should underscore a need for improved linkage to care and treatment of HCV-infected persons who inject drugs as part of an important public health effort to prevent its continued spread.
This work was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. Conflict of interest information was not disclosed.
The results of a hepatitis C virus screening program in a suburban New Jersey acute opioid detoxification program reveal that, despite the ease of access to HCV screening and the availability of curative therapies, linkage to care after detection of infection in persons who inject drugs continues to present a challenge.
Eda Akyar, a clinical research coordinator at ID Care, New Jersey’s largest network of infectious disease specialists, and her colleagues examined the results of an HIV, HCV, and HBV infection screening program instituted between Oct. 1, 2014, and June 9, 2015, for patients admitted to an acute opioid detoxification program at Princeton House in suburban New Jersey. Study goals included assessing the prevalence of HIV, HCV, and HBV infections for these patients, the HCV genotype (GT) carried, and the subsequent linkage to care after discharge from the program. The report was published online April 13 in Emerging Infectious Diseases.
During the screening period, patients representing 10 of New Jersey’s 21 counties were tested for the presence of HCV antibodies. Approximately two-thirds of all study participants (66.6%) were between 17 and 35 years of age. In this important age demographic, 237 patients (41.4%) screened positive for HCV antibodies, and 187 were available for further study.
HCV viral load data were available from 172 patients (92.0%), with approximately one-fifth (18.6%) screening undetectable. The HCV GTs obtained from 102 patients revealed that most (62.7%) were GT1a, followed by GT3 (25.5%). In a very surprising result, all HCV antibody positive patients were also HIV antibody negative, the researchers noted.
Regarding linkage to care, 16 of the patients (8.6%) in the 17- to 35-year-old age group attended outpatient follow-up appointments, with three (1.6%) starting on an oral, direct-acting antiviral treatment regimen. Two of these three patients were nonadherent to their treatment regimens. Two additional patients expressed willingness to accept treatment, but were denied their prescriptions by their insurance providers. None of the other participants returned for continued care.
The authors said that their results indicated a high prevalence of HCV among young suburban heroin users attending an acute detoxification program serving a wide geographic area, suggesting that New Jersey is part of the second wave of HCV infection following that seen in those born from 1946 to 1964. They also highlighted their findings of a GT3 prevalence more than twice the national average, a complete lack of HIV infection in a population known to be susceptible to it, and disappointing linkage to care outcomes.
The authors said that easy-to-use curative therapy should underscore a need for improved linkage to care and treatment of HCV-infected persons who inject drugs as part of an important public health effort to prevent its continued spread.
This work was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. Conflict of interest information was not disclosed.
The results of a hepatitis C virus screening program in a suburban New Jersey acute opioid detoxification program reveal that, despite the ease of access to HCV screening and the availability of curative therapies, linkage to care after detection of infection in persons who inject drugs continues to present a challenge.
Eda Akyar, a clinical research coordinator at ID Care, New Jersey’s largest network of infectious disease specialists, and her colleagues examined the results of an HIV, HCV, and HBV infection screening program instituted between Oct. 1, 2014, and June 9, 2015, for patients admitted to an acute opioid detoxification program at Princeton House in suburban New Jersey. Study goals included assessing the prevalence of HIV, HCV, and HBV infections for these patients, the HCV genotype (GT) carried, and the subsequent linkage to care after discharge from the program. The report was published online April 13 in Emerging Infectious Diseases.
During the screening period, patients representing 10 of New Jersey’s 21 counties were tested for the presence of HCV antibodies. Approximately two-thirds of all study participants (66.6%) were between 17 and 35 years of age. In this important age demographic, 237 patients (41.4%) screened positive for HCV antibodies, and 187 were available for further study.
HCV viral load data were available from 172 patients (92.0%), with approximately one-fifth (18.6%) screening undetectable. The HCV GTs obtained from 102 patients revealed that most (62.7%) were GT1a, followed by GT3 (25.5%). In a very surprising result, all HCV antibody positive patients were also HIV antibody negative, the researchers noted.
Regarding linkage to care, 16 of the patients (8.6%) in the 17- to 35-year-old age group attended outpatient follow-up appointments, with three (1.6%) starting on an oral, direct-acting antiviral treatment regimen. Two of these three patients were nonadherent to their treatment regimens. Two additional patients expressed willingness to accept treatment, but were denied their prescriptions by their insurance providers. None of the other participants returned for continued care.
The authors said that their results indicated a high prevalence of HCV among young suburban heroin users attending an acute detoxification program serving a wide geographic area, suggesting that New Jersey is part of the second wave of HCV infection following that seen in those born from 1946 to 1964. They also highlighted their findings of a GT3 prevalence more than twice the national average, a complete lack of HIV infection in a population known to be susceptible to it, and disappointing linkage to care outcomes.
The authors said that easy-to-use curative therapy should underscore a need for improved linkage to care and treatment of HCV-infected persons who inject drugs as part of an important public health effort to prevent its continued spread.
This work was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. Conflict of interest information was not disclosed.
FROM EMERGING INFECTIOUS DISEASES
Key clinical point: Despite the ease of access to hepatitis C virus screening and the availability of curative therapies, linkage to care after detection of infection in persons who inject drugs continues to present a challenge, with larger implications for public health efforts to prevent its spread.
Major finding: Hepatitis C virus genotype 3 infection was highly prevalent in individuals who inject drugs who were participating in an acute heroin detoxification program serving a wide geographic area in suburban New Jersey.
Data sources: Patients admitted to Princeton House, a psychiatric facility in suburban New Jersey with an active opioid detoxification program, screened for hepatitis C virus between Oct. 1, 2014, and June 9, 2015.
Disclosures: This work was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. Conflict of interest information was not disclosed.
VIDEO: Serial lung fluid measurement improved heart failure outcomes
CHICAGO – Regular assessment of a heart failure patient’s lung fluid volume using a device that measures electrical conduction through the chest – lung impedance – helped guide clinicians to make timely adjustments in a patient’s medications and thereby significantly reduce mortality and hospitalizations during an average 4 years of follow-up in a randomized, controlled study with 256 patients.
Monthly measurement of lung impedance and medication adjustments based on the information led to a 58% reduction in hospitalizations for acute heart failure during the first year of the study, compared with control patients, and a 56% reduction in heart failure hospitalizations, compared with controls, during the entire course of the study, the study’s two primary endpoints, Dr. Michael K. Shochat reported at the annual meeting of the American College of Cardiology.
The results also showed that performing regular lung impedance measurements and using the results to guide treatment led to a 43% reduction in all-cause mortality and a 62% drop in heart failure mortality during the average 4-year course of the study, said Dr. Shochat, a cardiologist at the Heart Institute of Hillel Yaffe Medical Center in Hadera, Israel. Concurrent with Dr. Shochat’s report at the meeting the results also appeared in an article published online (J Card Failure. 2016;doi:10.1016/j.cardfail.2016.03.015).
A key aspect of the study was that the clinicians who treated the enrolled patients who underwent lung impedance monitoring used this information to adjust medications the patients received. Overall, patients who underwent monitoring had more than twice the number of medication dose adjustments, compared with the control patients. These adjustments particularly focused on diuretic dosages, which changed three times as often in the monitored patients, compared with controls, Dr. Shochat reported. Changes in the dosages of beta-blockers and ACE inhibitors also showed marked increases in the monitored patients, compared with the controls.
The Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients (IMPEDANCE-HF) trial enrolled 256 patients at two centers in Israel during 2005-2014. Patients had New York Heart Association class II-IV heart failure and a left ventricular ejection fraction of 35% or less. The enrolled patients averaged 67 years of age, and 80% were men.
Clinicians measured lung impedance using a proprietary device that places external electrodes on opposite sides of the patient’s chest. Calculation of impedance used a formula that eliminated the noise from chest wall impedance and focused exclusively on lung impedance. Once the electrodes are placed collection of the impedance data takes about 1 minute, Dr. Shochat said. The study protocol called for impedance data to be collected monthly, and in practice it occurred about 11 times a year during the study.
The investigators calculated for each patient in the active arm of the study a “basal” lung impedance level that reflected their level of lung conductivity when their lungs were clear of excess fluid. Participating clinicians were instructed to intervene by altering medications when the impedance level dropped more than 18% below the basal level. Their goal was to prevent impedance from dropping to more than 24% below the basal level, which correlated with when heart failure patients usually required hospitalization for acute decompensation. The specifics of how to adjust medications to manage patients who showed these signs of fluid overload were left to the discretion of each attending physician.
MPEDANCE-HF was sponsored by the RSMM Company, which is developing the lung impedance measurement device used in the study. Dr. Shochat is a cofounder of RSMM and is a member of the company’s board of directors.
On Twitter @mitchelzoler
The very exciting results reported by Dr. Shochat came from a small, positive trial that showed impedance monitoring was an effective way to detect an increased amount of fluid in a heart failure patient’s lungs. This resulted in improved outcomes, compared with patients managed using usual care, including fewer hospitalizations and reduced mortality.
These results suggest that when physicians had lung impedance information, they identified episodes of acute heart failure decompensation sooner and that they used this alert to change treatment and prevent patient worsening. Heart failure exacerbations and decompensation events are a recurring problem for heart failure patients, and the earlier they are identified and addressed with altered treatment, the better it is for the patient’s well being. The next step is to see if these positive results can be confirmed by other research groups and in larger numbers of patients.
These results contrast with the findings from a German study reported in 2015 that used lung impedance information collected by implantable cardioverter defibrillators in heart failure patients to identify episodes of fluid buildup and decompensation. That study failed to show a statistically significant impact on patient outcomes. The researchers speculated that this may have been because patients often did not go online to allow their information to get transmitted to their physician, and physicians often did not act on the information because the patients reported no coincident change in symptoms.
This problem with the German study highlights that collecting lung impedance information will only improve outcomes if physicians then act on the information and modify a patient’s treatment. In the new study reported by Dr. Shochat, patients consistently underwent evaluation for their lung impedance status every month, and when the results suggested a growing problem of fluid overload the physicians consistently acted on the information by adjusting medication dosages.
Use of lung impedance measurement is similar to another approach for monitoring patients with heart failure that recently entered routine U.S. practice, an implanted device to monitor pulmonary artery pressure and identify episodes of fluid overload and acute decompensation. In the future, it will be interesting to compare the efficacy and ease of use of managing heart failure patients with pulmonary artery pressure monitoring with an implanted device and monitoring fluid build up in the lungs with lung impedance.
Dr. John A. Jarcho is a cardiologist at Brigham and Women’s Hospital, Boston. He had no disclosures. He made these comments as a discussant of Dr. Shochat’s report and in an interview.
The very exciting results reported by Dr. Shochat came from a small, positive trial that showed impedance monitoring was an effective way to detect an increased amount of fluid in a heart failure patient’s lungs. This resulted in improved outcomes, compared with patients managed using usual care, including fewer hospitalizations and reduced mortality.
These results suggest that when physicians had lung impedance information, they identified episodes of acute heart failure decompensation sooner and that they used this alert to change treatment and prevent patient worsening. Heart failure exacerbations and decompensation events are a recurring problem for heart failure patients, and the earlier they are identified and addressed with altered treatment, the better it is for the patient’s well being. The next step is to see if these positive results can be confirmed by other research groups and in larger numbers of patients.
These results contrast with the findings from a German study reported in 2015 that used lung impedance information collected by implantable cardioverter defibrillators in heart failure patients to identify episodes of fluid buildup and decompensation. That study failed to show a statistically significant impact on patient outcomes. The researchers speculated that this may have been because patients often did not go online to allow their information to get transmitted to their physician, and physicians often did not act on the information because the patients reported no coincident change in symptoms.
This problem with the German study highlights that collecting lung impedance information will only improve outcomes if physicians then act on the information and modify a patient’s treatment. In the new study reported by Dr. Shochat, patients consistently underwent evaluation for their lung impedance status every month, and when the results suggested a growing problem of fluid overload the physicians consistently acted on the information by adjusting medication dosages.
Use of lung impedance measurement is similar to another approach for monitoring patients with heart failure that recently entered routine U.S. practice, an implanted device to monitor pulmonary artery pressure and identify episodes of fluid overload and acute decompensation. In the future, it will be interesting to compare the efficacy and ease of use of managing heart failure patients with pulmonary artery pressure monitoring with an implanted device and monitoring fluid build up in the lungs with lung impedance.
Dr. John A. Jarcho is a cardiologist at Brigham and Women’s Hospital, Boston. He had no disclosures. He made these comments as a discussant of Dr. Shochat’s report and in an interview.
The very exciting results reported by Dr. Shochat came from a small, positive trial that showed impedance monitoring was an effective way to detect an increased amount of fluid in a heart failure patient’s lungs. This resulted in improved outcomes, compared with patients managed using usual care, including fewer hospitalizations and reduced mortality.
These results suggest that when physicians had lung impedance information, they identified episodes of acute heart failure decompensation sooner and that they used this alert to change treatment and prevent patient worsening. Heart failure exacerbations and decompensation events are a recurring problem for heart failure patients, and the earlier they are identified and addressed with altered treatment, the better it is for the patient’s well being. The next step is to see if these positive results can be confirmed by other research groups and in larger numbers of patients.
These results contrast with the findings from a German study reported in 2015 that used lung impedance information collected by implantable cardioverter defibrillators in heart failure patients to identify episodes of fluid buildup and decompensation. That study failed to show a statistically significant impact on patient outcomes. The researchers speculated that this may have been because patients often did not go online to allow their information to get transmitted to their physician, and physicians often did not act on the information because the patients reported no coincident change in symptoms.
This problem with the German study highlights that collecting lung impedance information will only improve outcomes if physicians then act on the information and modify a patient’s treatment. In the new study reported by Dr. Shochat, patients consistently underwent evaluation for their lung impedance status every month, and when the results suggested a growing problem of fluid overload the physicians consistently acted on the information by adjusting medication dosages.
Use of lung impedance measurement is similar to another approach for monitoring patients with heart failure that recently entered routine U.S. practice, an implanted device to monitor pulmonary artery pressure and identify episodes of fluid overload and acute decompensation. In the future, it will be interesting to compare the efficacy and ease of use of managing heart failure patients with pulmonary artery pressure monitoring with an implanted device and monitoring fluid build up in the lungs with lung impedance.
Dr. John A. Jarcho is a cardiologist at Brigham and Women’s Hospital, Boston. He had no disclosures. He made these comments as a discussant of Dr. Shochat’s report and in an interview.
CHICAGO – Regular assessment of a heart failure patient’s lung fluid volume using a device that measures electrical conduction through the chest – lung impedance – helped guide clinicians to make timely adjustments in a patient’s medications and thereby significantly reduce mortality and hospitalizations during an average 4 years of follow-up in a randomized, controlled study with 256 patients.
Monthly measurement of lung impedance and medication adjustments based on the information led to a 58% reduction in hospitalizations for acute heart failure during the first year of the study, compared with control patients, and a 56% reduction in heart failure hospitalizations, compared with controls, during the entire course of the study, the study’s two primary endpoints, Dr. Michael K. Shochat reported at the annual meeting of the American College of Cardiology.
The results also showed that performing regular lung impedance measurements and using the results to guide treatment led to a 43% reduction in all-cause mortality and a 62% drop in heart failure mortality during the average 4-year course of the study, said Dr. Shochat, a cardiologist at the Heart Institute of Hillel Yaffe Medical Center in Hadera, Israel. Concurrent with Dr. Shochat’s report at the meeting the results also appeared in an article published online (J Card Failure. 2016;doi:10.1016/j.cardfail.2016.03.015).
A key aspect of the study was that the clinicians who treated the enrolled patients who underwent lung impedance monitoring used this information to adjust medications the patients received. Overall, patients who underwent monitoring had more than twice the number of medication dose adjustments, compared with the control patients. These adjustments particularly focused on diuretic dosages, which changed three times as often in the monitored patients, compared with controls, Dr. Shochat reported. Changes in the dosages of beta-blockers and ACE inhibitors also showed marked increases in the monitored patients, compared with the controls.
The Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients (IMPEDANCE-HF) trial enrolled 256 patients at two centers in Israel during 2005-2014. Patients had New York Heart Association class II-IV heart failure and a left ventricular ejection fraction of 35% or less. The enrolled patients averaged 67 years of age, and 80% were men.
Clinicians measured lung impedance using a proprietary device that places external electrodes on opposite sides of the patient’s chest. Calculation of impedance used a formula that eliminated the noise from chest wall impedance and focused exclusively on lung impedance. Once the electrodes are placed collection of the impedance data takes about 1 minute, Dr. Shochat said. The study protocol called for impedance data to be collected monthly, and in practice it occurred about 11 times a year during the study.
The investigators calculated for each patient in the active arm of the study a “basal” lung impedance level that reflected their level of lung conductivity when their lungs were clear of excess fluid. Participating clinicians were instructed to intervene by altering medications when the impedance level dropped more than 18% below the basal level. Their goal was to prevent impedance from dropping to more than 24% below the basal level, which correlated with when heart failure patients usually required hospitalization for acute decompensation. The specifics of how to adjust medications to manage patients who showed these signs of fluid overload were left to the discretion of each attending physician.
MPEDANCE-HF was sponsored by the RSMM Company, which is developing the lung impedance measurement device used in the study. Dr. Shochat is a cofounder of RSMM and is a member of the company’s board of directors.
On Twitter @mitchelzoler
CHICAGO – Regular assessment of a heart failure patient’s lung fluid volume using a device that measures electrical conduction through the chest – lung impedance – helped guide clinicians to make timely adjustments in a patient’s medications and thereby significantly reduce mortality and hospitalizations during an average 4 years of follow-up in a randomized, controlled study with 256 patients.
Monthly measurement of lung impedance and medication adjustments based on the information led to a 58% reduction in hospitalizations for acute heart failure during the first year of the study, compared with control patients, and a 56% reduction in heart failure hospitalizations, compared with controls, during the entire course of the study, the study’s two primary endpoints, Dr. Michael K. Shochat reported at the annual meeting of the American College of Cardiology.
The results also showed that performing regular lung impedance measurements and using the results to guide treatment led to a 43% reduction in all-cause mortality and a 62% drop in heart failure mortality during the average 4-year course of the study, said Dr. Shochat, a cardiologist at the Heart Institute of Hillel Yaffe Medical Center in Hadera, Israel. Concurrent with Dr. Shochat’s report at the meeting the results also appeared in an article published online (J Card Failure. 2016;doi:10.1016/j.cardfail.2016.03.015).
A key aspect of the study was that the clinicians who treated the enrolled patients who underwent lung impedance monitoring used this information to adjust medications the patients received. Overall, patients who underwent monitoring had more than twice the number of medication dose adjustments, compared with the control patients. These adjustments particularly focused on diuretic dosages, which changed three times as often in the monitored patients, compared with controls, Dr. Shochat reported. Changes in the dosages of beta-blockers and ACE inhibitors also showed marked increases in the monitored patients, compared with the controls.
The Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients (IMPEDANCE-HF) trial enrolled 256 patients at two centers in Israel during 2005-2014. Patients had New York Heart Association class II-IV heart failure and a left ventricular ejection fraction of 35% or less. The enrolled patients averaged 67 years of age, and 80% were men.
Clinicians measured lung impedance using a proprietary device that places external electrodes on opposite sides of the patient’s chest. Calculation of impedance used a formula that eliminated the noise from chest wall impedance and focused exclusively on lung impedance. Once the electrodes are placed collection of the impedance data takes about 1 minute, Dr. Shochat said. The study protocol called for impedance data to be collected monthly, and in practice it occurred about 11 times a year during the study.
The investigators calculated for each patient in the active arm of the study a “basal” lung impedance level that reflected their level of lung conductivity when their lungs were clear of excess fluid. Participating clinicians were instructed to intervene by altering medications when the impedance level dropped more than 18% below the basal level. Their goal was to prevent impedance from dropping to more than 24% below the basal level, which correlated with when heart failure patients usually required hospitalization for acute decompensation. The specifics of how to adjust medications to manage patients who showed these signs of fluid overload were left to the discretion of each attending physician.
MPEDANCE-HF was sponsored by the RSMM Company, which is developing the lung impedance measurement device used in the study. Dr. Shochat is a cofounder of RSMM and is a member of the company’s board of directors.
On Twitter @mitchelzoler
AT ACC 2016
Key clinical point: Monthly, noninvasive measurement of lung fluid levels using lung impedance produced better fluid control in heart failure patients and significantly fewer deaths and heart failure hospitalizations.
Major finding: Lung impedance–based management produced a 56% cut in heart failure hospitalizations, compared with standard care.
Data source: IMPEDANCE-HF, a randomized study with 256 heart failure patients at two Israeli centers.
Disclosures: IMPEDANCE-HF was sponsored by the RSMM Company, which is developing the lung impedance measurement device used in the study. Dr. Shochat is a cofounder of RSMM and is a member of the company’s board of directors.
It’s imperative to protect adolescents with HPV vaccine
Low vaccination rates in the United States are in large part due to parental and religious objections to this vaccination that can be overcome by better education and support by pediatricians. It’s been demonstrated that physicians encourage the HPV vaccine less strongly than the other adolescent vaccinations such as Tdap and the meningococcal conjugate vaccine1. In addition, there is a lack of legislation promoting this vaccine. There are only two states and Washington, D.C., that currently have opt-out state mandates for HPV immunization, compared with 46 states plus Washington, D.C., with similar policies for Tdap2. The HPV vaccine is safe, efficacious, and has been demonstrated to reduce mortality and morbidity in our population. It is imperative that we, as pediatricians, strongly encourage and provide these vaccinations to our adolescent patients to help protect our community.
The human papillomavirus (HPV) vaccine was approved for girls in 2008 and for boys in 20113. However, the adoption rate for HPV immunization in the United States has been dismal. Data from 2014 show only 60% of adolescent girls have received at least one HPV vaccine dose, and only 40% have received three doses4. The rates for boys are far worse, with 42% of adolescent boys receiving at least one dose, and only 22% receiving three doses4.
HPV is currently the most common sexually transmitted infection in the United States, with an estimated 14 million new infections per year3. Of these, approximately 11,000 will progress to cervical cancers and 9,000 to male anogenital cancers each year. Annually there are about 4,000 cervical cancer deaths in the United States3.
Three vaccines are approved by the Food and Drug Administration to prevent HPV infection: Gardasil, Gardasil 9, and Cervarix. All three vaccines prevent infections with HPV types 16 and 18, two high-risk HPVs that cause about 70% of cervical cancers (as well as oropharyngeal and other anogenital cancers). Gardasil also prevents infection with HPV types 6 and 11, associated with approximately 90% of anogenital warts. Gardasil 9 prevents infection with the same four HPV types plus five other high-risk HPV types (31, 33, 45, 52, and 58); it is called a nonavalent, or 9-valent, vaccine.
The HPV vaccines have the ability to reduce mortality and morbidity in our patients. A study published in the New England Journal of Medicine in 2011 demonstrated that vaccination with the full HPV series in males 16-26 years prior to HPV exposure led to a 90% efficacy in preventing HPV-related disease5, compared with a 60% efficacy if given after HPV exposure or if the individual received an incomplete dose series.
A recent Pediatrics study reported data since initiation of the vaccination program in American adolescent and young adult women6. The data showed a reduction in HPV-related disease of 64% in the 14- to 19-year age group and a reduction of 34% in the older age group (aged 20-24 years). This is impressive given our currently low vaccination rates.
A Lancet meta-analysis shows evidence of herd immunity and cross-protective effects when vaccination rates are greater than 50% in the population7. This allows groups that aren’t currently approved for vaccination to have a reduction in disease and allows for some protection against HPV types that aren’t currently covered by the vaccines.
So we pediatricians have a job to do in protecting our patients by encouraging HPV immunization.
References
1. Pediatrics. 2016 Feb;137(2):1-9.
3. www.cdc.gov/hpv/hcp/clinician-factsheet.html
5. N Engl J Med. 2011;364(5):401-11.
6. Pediatrics. 2016;137(3):1-9.
7. Lancet Infect Dis. 2015;15(5):565-80.
Dr. Denby is a second-year internal medicine/pediatrics resident at Vanderbilt University Medical Center in Nashville, Tenn.
Low vaccination rates in the United States are in large part due to parental and religious objections to this vaccination that can be overcome by better education and support by pediatricians. It’s been demonstrated that physicians encourage the HPV vaccine less strongly than the other adolescent vaccinations such as Tdap and the meningococcal conjugate vaccine1. In addition, there is a lack of legislation promoting this vaccine. There are only two states and Washington, D.C., that currently have opt-out state mandates for HPV immunization, compared with 46 states plus Washington, D.C., with similar policies for Tdap2. The HPV vaccine is safe, efficacious, and has been demonstrated to reduce mortality and morbidity in our population. It is imperative that we, as pediatricians, strongly encourage and provide these vaccinations to our adolescent patients to help protect our community.
The human papillomavirus (HPV) vaccine was approved for girls in 2008 and for boys in 20113. However, the adoption rate for HPV immunization in the United States has been dismal. Data from 2014 show only 60% of adolescent girls have received at least one HPV vaccine dose, and only 40% have received three doses4. The rates for boys are far worse, with 42% of adolescent boys receiving at least one dose, and only 22% receiving three doses4.
HPV is currently the most common sexually transmitted infection in the United States, with an estimated 14 million new infections per year3. Of these, approximately 11,000 will progress to cervical cancers and 9,000 to male anogenital cancers each year. Annually there are about 4,000 cervical cancer deaths in the United States3.
Three vaccines are approved by the Food and Drug Administration to prevent HPV infection: Gardasil, Gardasil 9, and Cervarix. All three vaccines prevent infections with HPV types 16 and 18, two high-risk HPVs that cause about 70% of cervical cancers (as well as oropharyngeal and other anogenital cancers). Gardasil also prevents infection with HPV types 6 and 11, associated with approximately 90% of anogenital warts. Gardasil 9 prevents infection with the same four HPV types plus five other high-risk HPV types (31, 33, 45, 52, and 58); it is called a nonavalent, or 9-valent, vaccine.
The HPV vaccines have the ability to reduce mortality and morbidity in our patients. A study published in the New England Journal of Medicine in 2011 demonstrated that vaccination with the full HPV series in males 16-26 years prior to HPV exposure led to a 90% efficacy in preventing HPV-related disease5, compared with a 60% efficacy if given after HPV exposure or if the individual received an incomplete dose series.
A recent Pediatrics study reported data since initiation of the vaccination program in American adolescent and young adult women6. The data showed a reduction in HPV-related disease of 64% in the 14- to 19-year age group and a reduction of 34% in the older age group (aged 20-24 years). This is impressive given our currently low vaccination rates.
A Lancet meta-analysis shows evidence of herd immunity and cross-protective effects when vaccination rates are greater than 50% in the population7. This allows groups that aren’t currently approved for vaccination to have a reduction in disease and allows for some protection against HPV types that aren’t currently covered by the vaccines.
So we pediatricians have a job to do in protecting our patients by encouraging HPV immunization.
References
1. Pediatrics. 2016 Feb;137(2):1-9.
3. www.cdc.gov/hpv/hcp/clinician-factsheet.html
5. N Engl J Med. 2011;364(5):401-11.
6. Pediatrics. 2016;137(3):1-9.
7. Lancet Infect Dis. 2015;15(5):565-80.
Dr. Denby is a second-year internal medicine/pediatrics resident at Vanderbilt University Medical Center in Nashville, Tenn.
Low vaccination rates in the United States are in large part due to parental and religious objections to this vaccination that can be overcome by better education and support by pediatricians. It’s been demonstrated that physicians encourage the HPV vaccine less strongly than the other adolescent vaccinations such as Tdap and the meningococcal conjugate vaccine1. In addition, there is a lack of legislation promoting this vaccine. There are only two states and Washington, D.C., that currently have opt-out state mandates for HPV immunization, compared with 46 states plus Washington, D.C., with similar policies for Tdap2. The HPV vaccine is safe, efficacious, and has been demonstrated to reduce mortality and morbidity in our population. It is imperative that we, as pediatricians, strongly encourage and provide these vaccinations to our adolescent patients to help protect our community.
The human papillomavirus (HPV) vaccine was approved for girls in 2008 and for boys in 20113. However, the adoption rate for HPV immunization in the United States has been dismal. Data from 2014 show only 60% of adolescent girls have received at least one HPV vaccine dose, and only 40% have received three doses4. The rates for boys are far worse, with 42% of adolescent boys receiving at least one dose, and only 22% receiving three doses4.
HPV is currently the most common sexually transmitted infection in the United States, with an estimated 14 million new infections per year3. Of these, approximately 11,000 will progress to cervical cancers and 9,000 to male anogenital cancers each year. Annually there are about 4,000 cervical cancer deaths in the United States3.
Three vaccines are approved by the Food and Drug Administration to prevent HPV infection: Gardasil, Gardasil 9, and Cervarix. All three vaccines prevent infections with HPV types 16 and 18, two high-risk HPVs that cause about 70% of cervical cancers (as well as oropharyngeal and other anogenital cancers). Gardasil also prevents infection with HPV types 6 and 11, associated with approximately 90% of anogenital warts. Gardasil 9 prevents infection with the same four HPV types plus five other high-risk HPV types (31, 33, 45, 52, and 58); it is called a nonavalent, or 9-valent, vaccine.
The HPV vaccines have the ability to reduce mortality and morbidity in our patients. A study published in the New England Journal of Medicine in 2011 demonstrated that vaccination with the full HPV series in males 16-26 years prior to HPV exposure led to a 90% efficacy in preventing HPV-related disease5, compared with a 60% efficacy if given after HPV exposure or if the individual received an incomplete dose series.
A recent Pediatrics study reported data since initiation of the vaccination program in American adolescent and young adult women6. The data showed a reduction in HPV-related disease of 64% in the 14- to 19-year age group and a reduction of 34% in the older age group (aged 20-24 years). This is impressive given our currently low vaccination rates.
A Lancet meta-analysis shows evidence of herd immunity and cross-protective effects when vaccination rates are greater than 50% in the population7. This allows groups that aren’t currently approved for vaccination to have a reduction in disease and allows for some protection against HPV types that aren’t currently covered by the vaccines.
So we pediatricians have a job to do in protecting our patients by encouraging HPV immunization.
References
1. Pediatrics. 2016 Feb;137(2):1-9.
3. www.cdc.gov/hpv/hcp/clinician-factsheet.html
5. N Engl J Med. 2011;364(5):401-11.
6. Pediatrics. 2016;137(3):1-9.
7. Lancet Infect Dis. 2015;15(5):565-80.
Dr. Denby is a second-year internal medicine/pediatrics resident at Vanderbilt University Medical Center in Nashville, Tenn.