User login
U.S. flu activity down again, except in New Jersey
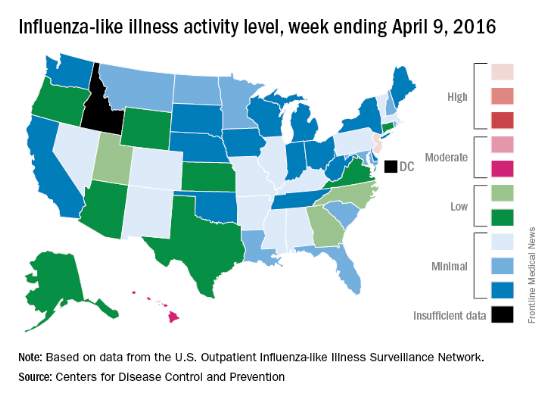
Overall activity of influenza-like illness (ILI) in the United States continued to fall, but New Jersey took a turn for the worse during the week ending April 9, 2016, according to the Centers for Disease Control and Prevention.
New Jersey’s ILI activity level went from 8 the previous week to 10 on the CDC’s 1-10 scale. For the week ending April 9, it was the only U.S. state in the “high” range, with Hawaii the next highest at level 6 – the only state in the “moderate” range, the CDC reported.
Nationwide, the proportion of outpatient visits for ILI was 2.1%, which is at the national baseline of 2.1% and down from 2.5% the week before. That number has now dropped for 4 consecutive weeks since hitting a season high of 3.7% for the week ending March 12. The CDC also reported a cumulative rate of 26.6 influenza-associated hospitalizations per 100,000 population.
Ten flu-related pediatric deaths were reported during the week, of which only one occurred during the week. A total of 50 flu-related pediatric deaths have been reported during the 2015-2016 season, the CDC said. The overall proportion of deaths attributed to pneumonia and influenza was below the system-specific threshold in the National Center for Health Statistics Mortality Surveillance System, but above the system-specific threshold in the 122 Cities Mortality Reporting System.
Overall activity of influenza-like illness (ILI) in the United States continued to fall, but New Jersey took a turn for the worse during the week ending April 9, 2016, according to the Centers for Disease Control and Prevention.
New Jersey’s ILI activity level went from 8 the previous week to 10 on the CDC’s 1-10 scale. For the week ending April 9, it was the only U.S. state in the “high” range, with Hawaii the next highest at level 6 – the only state in the “moderate” range, the CDC reported.

Nationwide, the proportion of outpatient visits for ILI was 2.1%, which is at the national baseline of 2.1% and down from 2.5% the week before. That number has now dropped for 4 consecutive weeks since hitting a season high of 3.7% for the week ending March 12. The CDC also reported a cumulative rate of 26.6 influenza-associated hospitalizations per 100,000 population.
Ten flu-related pediatric deaths were reported during the week, of which only one occurred during the week. A total of 50 flu-related pediatric deaths have been reported during the 2015-2016 season, the CDC said. The overall proportion of deaths attributed to pneumonia and influenza was below the system-specific threshold in the National Center for Health Statistics Mortality Surveillance System, but above the system-specific threshold in the 122 Cities Mortality Reporting System.
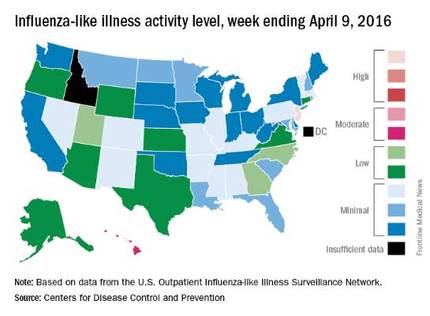
Overall activity of influenza-like illness (ILI) in the United States continued to fall, but New Jersey took a turn for the worse during the week ending April 9, 2016, according to the Centers for Disease Control and Prevention.
New Jersey’s ILI activity level went from 8 the previous week to 10 on the CDC’s 1-10 scale. For the week ending April 9, it was the only U.S. state in the “high” range, with Hawaii the next highest at level 6 – the only state in the “moderate” range, the CDC reported.

Nationwide, the proportion of outpatient visits for ILI was 2.1%, which is at the national baseline of 2.1% and down from 2.5% the week before. That number has now dropped for 4 consecutive weeks since hitting a season high of 3.7% for the week ending March 12. The CDC also reported a cumulative rate of 26.6 influenza-associated hospitalizations per 100,000 population.
Ten flu-related pediatric deaths were reported during the week, of which only one occurred during the week. A total of 50 flu-related pediatric deaths have been reported during the 2015-2016 season, the CDC said. The overall proportion of deaths attributed to pneumonia and influenza was below the system-specific threshold in the National Center for Health Statistics Mortality Surveillance System, but above the system-specific threshold in the 122 Cities Mortality Reporting System.
Pregnancy Considerations for Women With Epilepsy—The WEPOD Study
VANCOUVER—Women with epilepsy seeking pregnancy had comparable likelihood of achieving pregnancy, time to achieve pregnancy, and pregnancy outcomes, compared with a group of healthy peers, according to study findings presented at the 68th Annual Meeting of the American Academy of Neurology. "These findings should reassure women with epilepsy and clinicians when counseling women with epilepsy who are planning pregnancy," said Page B. Pennell, MD, Associate Professor of Neurology at Brigham and Women's Hospital and Harvard Medical School in Boston, and her research colleagues.
Page B. Pennell, MD, Associate Professor of Neurology at Brigham and Women's Hospital and Harvard Medical School in Boston, and her research colleagues.
Previous studies suggested that women with epilepsy have lower fertility compared with healthy controls. Dr. Pennell and colleagues sought to compare time to pregnancy and outcomes (eg, live birth, miscarriage) among women with epilepsy and healthy controls. The Women with Epilepsy: Pregnancy Outcomes and Deliveries (WEPOD) study was a multicenter, prospective, observational study of women with epilepsy and healthy controls.
Dr. Pennell and colleagues enrolled and prospectively followed women with epilepsy and healthy controls, ages 18 to 41, seeking pregnancy within six months of discontinuing contraception. The customized WEPOD electronicdiary captured medication use, seizures, sexual activity, and menstrual bleeding. Pregnancy tests were performed if there was no menses by cycle day 35. Outcomes included proportions of women who achieved pregnancy and time to pregnancy from cessation of birth control. The researchers used a proportional hazard model to evaluate the association between time to pregnancy and certain baseline characteristics.
Enrolled in the study were 88 women with epilepsy and 109 healthy controls with similar demographic characteristics. Among women with epilepsy, 61.4% achieved pregnancy versus 60.6% for healthy controls. Median time to pregnancy was six months in women with epilepsy, compared with nine months for healthy controls. Time to pregnancy was no different across the two groups after controlling for age, BMI, parity, and race. Race and parity were significantly associated with time to pregnancy.
Of the pregnancies that occurred, a similar proportion resulted in miscarriage (12.9% among women with epilepsy and 19.7% among controls), live birth (80.0% among women with epilepsy and 80.3% controls) or other outcome (5.0% versus 0.0%).
The WEPOD study was supported by the Epilepsy Foundation.
VANCOUVER—Women with epilepsy seeking pregnancy had comparable likelihood of achieving pregnancy, time to achieve pregnancy, and pregnancy outcomes, compared with a group of healthy peers, according to study findings presented at the 68th Annual Meeting of the American Academy of Neurology. "These findings should reassure women with epilepsy and clinicians when counseling women with epilepsy who are planning pregnancy," said Page B. Pennell, MD, Associate Professor of Neurology at Brigham and Women's Hospital and Harvard Medical School in Boston, and her research colleagues.
Page B. Pennell, MD, Associate Professor of Neurology at Brigham and Women's Hospital and Harvard Medical School in Boston, and her research colleagues.
Previous studies suggested that women with epilepsy have lower fertility compared with healthy controls. Dr. Pennell and colleagues sought to compare time to pregnancy and outcomes (eg, live birth, miscarriage) among women with epilepsy and healthy controls. The Women with Epilepsy: Pregnancy Outcomes and Deliveries (WEPOD) study was a multicenter, prospective, observational study of women with epilepsy and healthy controls.
Dr. Pennell and colleagues enrolled and prospectively followed women with epilepsy and healthy controls, ages 18 to 41, seeking pregnancy within six months of discontinuing contraception. The customized WEPOD electronicdiary captured medication use, seizures, sexual activity, and menstrual bleeding. Pregnancy tests were performed if there was no menses by cycle day 35. Outcomes included proportions of women who achieved pregnancy and time to pregnancy from cessation of birth control. The researchers used a proportional hazard model to evaluate the association between time to pregnancy and certain baseline characteristics.
Enrolled in the study were 88 women with epilepsy and 109 healthy controls with similar demographic characteristics. Among women with epilepsy, 61.4% achieved pregnancy versus 60.6% for healthy controls. Median time to pregnancy was six months in women with epilepsy, compared with nine months for healthy controls. Time to pregnancy was no different across the two groups after controlling for age, BMI, parity, and race. Race and parity were significantly associated with time to pregnancy.
Of the pregnancies that occurred, a similar proportion resulted in miscarriage (12.9% among women with epilepsy and 19.7% among controls), live birth (80.0% among women with epilepsy and 80.3% controls) or other outcome (5.0% versus 0.0%).
The WEPOD study was supported by the Epilepsy Foundation.
VANCOUVER—Women with epilepsy seeking pregnancy had comparable likelihood of achieving pregnancy, time to achieve pregnancy, and pregnancy outcomes, compared with a group of healthy peers, according to study findings presented at the 68th Annual Meeting of the American Academy of Neurology. "These findings should reassure women with epilepsy and clinicians when counseling women with epilepsy who are planning pregnancy," said Page B. Pennell, MD, Associate Professor of Neurology at Brigham and Women's Hospital and Harvard Medical School in Boston, and her research colleagues.
Page B. Pennell, MD, Associate Professor of Neurology at Brigham and Women's Hospital and Harvard Medical School in Boston, and her research colleagues.
Previous studies suggested that women with epilepsy have lower fertility compared with healthy controls. Dr. Pennell and colleagues sought to compare time to pregnancy and outcomes (eg, live birth, miscarriage) among women with epilepsy and healthy controls. The Women with Epilepsy: Pregnancy Outcomes and Deliveries (WEPOD) study was a multicenter, prospective, observational study of women with epilepsy and healthy controls.
Dr. Pennell and colleagues enrolled and prospectively followed women with epilepsy and healthy controls, ages 18 to 41, seeking pregnancy within six months of discontinuing contraception. The customized WEPOD electronicdiary captured medication use, seizures, sexual activity, and menstrual bleeding. Pregnancy tests were performed if there was no menses by cycle day 35. Outcomes included proportions of women who achieved pregnancy and time to pregnancy from cessation of birth control. The researchers used a proportional hazard model to evaluate the association between time to pregnancy and certain baseline characteristics.
Enrolled in the study were 88 women with epilepsy and 109 healthy controls with similar demographic characteristics. Among women with epilepsy, 61.4% achieved pregnancy versus 60.6% for healthy controls. Median time to pregnancy was six months in women with epilepsy, compared with nine months for healthy controls. Time to pregnancy was no different across the two groups after controlling for age, BMI, parity, and race. Race and parity were significantly associated with time to pregnancy.
Of the pregnancies that occurred, a similar proportion resulted in miscarriage (12.9% among women with epilepsy and 19.7% among controls), live birth (80.0% among women with epilepsy and 80.3% controls) or other outcome (5.0% versus 0.0%).
The WEPOD study was supported by the Epilepsy Foundation.
VRIC is Just Around the Corner
The Vascular Research Initiatives Conference - VRIC - is less than three weeks away. The one-day conference, on May 4 in Nashville, focuses on translational research. This year’s theme is “Outside In: Paradigm Shifts in Vascular Disease.”
VRIC takes place the day before the American Heart Association’s Arteriosclerosis, Thrombosis and Vascular Biology (ATVB) Scientific Sessions, and at the same venue in Nashville. VRIC is considered a key event for meeting and reconnecting with vascular research collaborators.
The Vascular Research Initiatives Conference - VRIC - is less than three weeks away. The one-day conference, on May 4 in Nashville, focuses on translational research. This year’s theme is “Outside In: Paradigm Shifts in Vascular Disease.”
VRIC takes place the day before the American Heart Association’s Arteriosclerosis, Thrombosis and Vascular Biology (ATVB) Scientific Sessions, and at the same venue in Nashville. VRIC is considered a key event for meeting and reconnecting with vascular research collaborators.
The Vascular Research Initiatives Conference - VRIC - is less than three weeks away. The one-day conference, on May 4 in Nashville, focuses on translational research. This year’s theme is “Outside In: Paradigm Shifts in Vascular Disease.”
VRIC takes place the day before the American Heart Association’s Arteriosclerosis, Thrombosis and Vascular Biology (ATVB) Scientific Sessions, and at the same venue in Nashville. VRIC is considered a key event for meeting and reconnecting with vascular research collaborators.
PCORI to Fund Pragmatic Trials and Studies
The Patient-Centered Outcomes Research Institute (PCORI) has announced new funding for pragmatic clinical trials, large simple trials or large-scale observational studies that compare the relative effectiveness of two or more alternatives for improving patient-centered outcomes.
Up to $1 million in direct costs for each study will be available through the initiative, “Pragmatic Clinical Studies to Evaluate Patient-Centered Outcomes.”
PCORI has a list of priority topics, though any study that addresses critical choices faced by patients, caregivers, clinicians and/or delivery systems will be considered.
Deadline for letters of intent is May 4. Application deadline is Aug. 8, and awards will be announced in January 2017.
The Patient-Centered Outcomes Research Institute (PCORI) has announced new funding for pragmatic clinical trials, large simple trials or large-scale observational studies that compare the relative effectiveness of two or more alternatives for improving patient-centered outcomes.
Up to $1 million in direct costs for each study will be available through the initiative, “Pragmatic Clinical Studies to Evaluate Patient-Centered Outcomes.”
PCORI has a list of priority topics, though any study that addresses critical choices faced by patients, caregivers, clinicians and/or delivery systems will be considered.
Deadline for letters of intent is May 4. Application deadline is Aug. 8, and awards will be announced in January 2017.
The Patient-Centered Outcomes Research Institute (PCORI) has announced new funding for pragmatic clinical trials, large simple trials or large-scale observational studies that compare the relative effectiveness of two or more alternatives for improving patient-centered outcomes.
Up to $1 million in direct costs for each study will be available through the initiative, “Pragmatic Clinical Studies to Evaluate Patient-Centered Outcomes.”
PCORI has a list of priority topics, though any study that addresses critical choices faced by patients, caregivers, clinicians and/or delivery systems will be considered.
Deadline for letters of intent is May 4. Application deadline is Aug. 8, and awards will be announced in January 2017.
Register for VAM Hands-On Workshops
SVS members have plenty of chances to learn by doing at hands-on workshops at the Vascular Annual Meeting. Twelve topics will be offered in four separate 90-minute sessions on Wednesday, June 8.
The workshops are $100 each (preregistration is required) and are not included in the VAM registration fee. Simply add the desired workshops when registering. Already registered? Just return to the registration page and add the workshops separately. The workshops are not eligible for CME credit.
Visit the VAM website for more information.
SVS members have plenty of chances to learn by doing at hands-on workshops at the Vascular Annual Meeting. Twelve topics will be offered in four separate 90-minute sessions on Wednesday, June 8.
The workshops are $100 each (preregistration is required) and are not included in the VAM registration fee. Simply add the desired workshops when registering. Already registered? Just return to the registration page and add the workshops separately. The workshops are not eligible for CME credit.
Visit the VAM website for more information.
SVS members have plenty of chances to learn by doing at hands-on workshops at the Vascular Annual Meeting. Twelve topics will be offered in four separate 90-minute sessions on Wednesday, June 8.
The workshops are $100 each (preregistration is required) and are not included in the VAM registration fee. Simply add the desired workshops when registering. Already registered? Just return to the registration page and add the workshops separately. The workshops are not eligible for CME credit.
Visit the VAM website for more information.
Edoxban has Advantages over Warfarin for Patients with Venous Thromboembolism
NEW YORK (Reuters Health) - Edoxaban (Savaysa, Daiichi-Sankyo) shows advantages over warfarin in long-term treatment of patients with venous thromboembolism (VTE), according to a post-hoc analysis of multinational trial data.
As Dr. Gary Raskob told Reuters Health by email, "Our results indicate that once-daily edoxaban provides an effective
and more convenient alternative to warfarin, with lower major bleeding risk, for patients who require extended treatment
beyond three months to prevent recurrent venous thromboembolism."
In a March 22 online paper in the Lancet Haematology, Dr.Raskob, of the University of Oklahoma, Oklahoma City, and colleagues note that guidelines recommend anticoagulant treatment for at least three months. However, "The risk of recurrence is substantial for patients with unprovoked venous thromboembolism or continuing risk factors and many of these
patients need extended anticoagulation therapy beyond three months."
To shed more light on longer term effects, the team examined outcome after three to 12 months in 3,633 patients treated with heparin and edoxaban and 3,594 treated with heparin and warfarin who took part in a randomized, double-blind trial. Median treatment duration was close to 9 months.
At three months, recurrent VTE was seen in 1.1% of the edoxaban group and 1.2% of the warfarin patients. At three to six months, the corresponding proportions were 0.7% and 0.5%. At more than six but less than 12 months, they were 0.2% and 0.8%.
Among other findings was that the cumulative incidence of major bleeding was 0.3% in the edoxaban-treated group and 0.7%
in the warfarin-treated patients. Intention-to-treat analysis gave similar results to these per-protocol findings.
Use of edoxaban, Dr. Raskob concluded, "may enable more patients to stay on extended anticoagulant treatment and help reduce the burden from recurrent venous thromboembolism."
Commenting on the findings by email, Dr. Jerrold H. Levy, coauthor of an accompanying editorial, told Reuters Health, "This post-hoc analysis reports that edoxaban is an alternative to warfarin for extended use in the secondary prevention of venous thromboembolism."
Dr. Levy, of Duke University Hospital, Durham, North Carolina, concluded, "The only other study where a direct oral anticoagulant was compared with warfarin for extended use in this setting was the RE-MEDY trial that compared dabigatran with warfarin in patients for six to 36 months and found dabigatran was similar to warfarin for efficacy with a lower incidence of clinically relevant major bleeding."
This study was funded by Daiichi-Sankyo. Dr. Raskob received fees from the company during the study. Other coauthors
also have ties to the company and a number are employees of Daiichi-Sankyo.
NEW YORK (Reuters Health) - Edoxaban (Savaysa, Daiichi-Sankyo) shows advantages over warfarin in long-term treatment of patients with venous thromboembolism (VTE), according to a post-hoc analysis of multinational trial data.
As Dr. Gary Raskob told Reuters Health by email, "Our results indicate that once-daily edoxaban provides an effective
and more convenient alternative to warfarin, with lower major bleeding risk, for patients who require extended treatment
beyond three months to prevent recurrent venous thromboembolism."
In a March 22 online paper in the Lancet Haematology, Dr.Raskob, of the University of Oklahoma, Oklahoma City, and colleagues note that guidelines recommend anticoagulant treatment for at least three months. However, "The risk of recurrence is substantial for patients with unprovoked venous thromboembolism or continuing risk factors and many of these
patients need extended anticoagulation therapy beyond three months."
To shed more light on longer term effects, the team examined outcome after three to 12 months in 3,633 patients treated with heparin and edoxaban and 3,594 treated with heparin and warfarin who took part in a randomized, double-blind trial. Median treatment duration was close to 9 months.
At three months, recurrent VTE was seen in 1.1% of the edoxaban group and 1.2% of the warfarin patients. At three to six months, the corresponding proportions were 0.7% and 0.5%. At more than six but less than 12 months, they were 0.2% and 0.8%.
Among other findings was that the cumulative incidence of major bleeding was 0.3% in the edoxaban-treated group and 0.7%
in the warfarin-treated patients. Intention-to-treat analysis gave similar results to these per-protocol findings.
Use of edoxaban, Dr. Raskob concluded, "may enable more patients to stay on extended anticoagulant treatment and help reduce the burden from recurrent venous thromboembolism."
Commenting on the findings by email, Dr. Jerrold H. Levy, coauthor of an accompanying editorial, told Reuters Health, "This post-hoc analysis reports that edoxaban is an alternative to warfarin for extended use in the secondary prevention of venous thromboembolism."
Dr. Levy, of Duke University Hospital, Durham, North Carolina, concluded, "The only other study where a direct oral anticoagulant was compared with warfarin for extended use in this setting was the RE-MEDY trial that compared dabigatran with warfarin in patients for six to 36 months and found dabigatran was similar to warfarin for efficacy with a lower incidence of clinically relevant major bleeding."
This study was funded by Daiichi-Sankyo. Dr. Raskob received fees from the company during the study. Other coauthors
also have ties to the company and a number are employees of Daiichi-Sankyo.
NEW YORK (Reuters Health) - Edoxaban (Savaysa, Daiichi-Sankyo) shows advantages over warfarin in long-term treatment of patients with venous thromboembolism (VTE), according to a post-hoc analysis of multinational trial data.
As Dr. Gary Raskob told Reuters Health by email, "Our results indicate that once-daily edoxaban provides an effective
and more convenient alternative to warfarin, with lower major bleeding risk, for patients who require extended treatment
beyond three months to prevent recurrent venous thromboembolism."
In a March 22 online paper in the Lancet Haematology, Dr.Raskob, of the University of Oklahoma, Oklahoma City, and colleagues note that guidelines recommend anticoagulant treatment for at least three months. However, "The risk of recurrence is substantial for patients with unprovoked venous thromboembolism or continuing risk factors and many of these
patients need extended anticoagulation therapy beyond three months."
To shed more light on longer term effects, the team examined outcome after three to 12 months in 3,633 patients treated with heparin and edoxaban and 3,594 treated with heparin and warfarin who took part in a randomized, double-blind trial. Median treatment duration was close to 9 months.
At three months, recurrent VTE was seen in 1.1% of the edoxaban group and 1.2% of the warfarin patients. At three to six months, the corresponding proportions were 0.7% and 0.5%. At more than six but less than 12 months, they were 0.2% and 0.8%.
Among other findings was that the cumulative incidence of major bleeding was 0.3% in the edoxaban-treated group and 0.7%
in the warfarin-treated patients. Intention-to-treat analysis gave similar results to these per-protocol findings.
Use of edoxaban, Dr. Raskob concluded, "may enable more patients to stay on extended anticoagulant treatment and help reduce the burden from recurrent venous thromboembolism."
Commenting on the findings by email, Dr. Jerrold H. Levy, coauthor of an accompanying editorial, told Reuters Health, "This post-hoc analysis reports that edoxaban is an alternative to warfarin for extended use in the secondary prevention of venous thromboembolism."
Dr. Levy, of Duke University Hospital, Durham, North Carolina, concluded, "The only other study where a direct oral anticoagulant was compared with warfarin for extended use in this setting was the RE-MEDY trial that compared dabigatran with warfarin in patients for six to 36 months and found dabigatran was similar to warfarin for efficacy with a lower incidence of clinically relevant major bleeding."
This study was funded by Daiichi-Sankyo. Dr. Raskob received fees from the company during the study. Other coauthors
also have ties to the company and a number are employees of Daiichi-Sankyo.
Hybrid drug could treat resistant malaria

Photo by James Gathany
A newly developed hybrid drug can treat malaria that is resistant to other therapies, according to preclinical research.
With previous work, researchers found that chemoreversal agents can re-sensitize resistant malaria parasites to the antimalarial agent chloroquine.
For the current study, the team created hybrid compounds that combine chloroquine and a chemoreversal agent.
One of these compounds, 35, proved particularly active, killing malaria parasites that were resistant to chloroquine and/or artemisinin.
Compound 35 was significantly more effective than chloroquine at killing these resistant strains, which included Hb3 (P<0.001), Dd2 (P<0.001), ARS-233 (P<0.001), ARS-272 (P<0.01), NHP-04559 (P<0.01), NHP04773 (P<0.001), and 7G8 (P<0.01).
In addition, the researchers said compound 35 has a “good therapeutic window” and “favorable drug-like properties,” but they are continuing to refine the compound to make it more effective.
The team noted that malaria drugs and chemoreversal agents have been used to treat drug-resistant malaria before. But this is the first time a hybrid of chloroquine and a newly discovered chemoreversal factor has been used in a single novel molecule for this purpose.
The researchers said a single therapy has several advantages over combination therapy. Besides being more convenient to take, it has less risk of drug-drug interactions, may be better absorbed and distributed in the body, and could result in slower development of new resistant strains of malaria.
Kevin S. W. Tan, PhD, of the National University of Singapore, and his colleagues described this research in Antimicrobial Agents and Chemotherapy. ![]()

Photo by James Gathany
A newly developed hybrid drug can treat malaria that is resistant to other therapies, according to preclinical research.
With previous work, researchers found that chemoreversal agents can re-sensitize resistant malaria parasites to the antimalarial agent chloroquine.
For the current study, the team created hybrid compounds that combine chloroquine and a chemoreversal agent.
One of these compounds, 35, proved particularly active, killing malaria parasites that were resistant to chloroquine and/or artemisinin.
Compound 35 was significantly more effective than chloroquine at killing these resistant strains, which included Hb3 (P<0.001), Dd2 (P<0.001), ARS-233 (P<0.001), ARS-272 (P<0.01), NHP-04559 (P<0.01), NHP04773 (P<0.001), and 7G8 (P<0.01).
In addition, the researchers said compound 35 has a “good therapeutic window” and “favorable drug-like properties,” but they are continuing to refine the compound to make it more effective.
The team noted that malaria drugs and chemoreversal agents have been used to treat drug-resistant malaria before. But this is the first time a hybrid of chloroquine and a newly discovered chemoreversal factor has been used in a single novel molecule for this purpose.
The researchers said a single therapy has several advantages over combination therapy. Besides being more convenient to take, it has less risk of drug-drug interactions, may be better absorbed and distributed in the body, and could result in slower development of new resistant strains of malaria.
Kevin S. W. Tan, PhD, of the National University of Singapore, and his colleagues described this research in Antimicrobial Agents and Chemotherapy. ![]()

Photo by James Gathany
A newly developed hybrid drug can treat malaria that is resistant to other therapies, according to preclinical research.
With previous work, researchers found that chemoreversal agents can re-sensitize resistant malaria parasites to the antimalarial agent chloroquine.
For the current study, the team created hybrid compounds that combine chloroquine and a chemoreversal agent.
One of these compounds, 35, proved particularly active, killing malaria parasites that were resistant to chloroquine and/or artemisinin.
Compound 35 was significantly more effective than chloroquine at killing these resistant strains, which included Hb3 (P<0.001), Dd2 (P<0.001), ARS-233 (P<0.001), ARS-272 (P<0.01), NHP-04559 (P<0.01), NHP04773 (P<0.001), and 7G8 (P<0.01).
In addition, the researchers said compound 35 has a “good therapeutic window” and “favorable drug-like properties,” but they are continuing to refine the compound to make it more effective.
The team noted that malaria drugs and chemoreversal agents have been used to treat drug-resistant malaria before. But this is the first time a hybrid of chloroquine and a newly discovered chemoreversal factor has been used in a single novel molecule for this purpose.
The researchers said a single therapy has several advantages over combination therapy. Besides being more convenient to take, it has less risk of drug-drug interactions, may be better absorbed and distributed in the body, and could result in slower development of new resistant strains of malaria.
Kevin S. W. Tan, PhD, of the National University of Singapore, and his colleagues described this research in Antimicrobial Agents and Chemotherapy. ![]()
Patients may be uninformed about risks of warfarin

ATHENS—A study of patients taking warfarin suggests many do not fully understand the risks associated with the drug.
Researchers asked patients to complete a questionnaire on warfarin use and found that, on average, patients answered 64% of the questions correctly.
The patients tended to be the least informed about food and drug interactions and which side effects necessitate a call or visit to the doctor.
Kjersti Oterhals, RN, PhD, of Haukeland University Hospital in Bergen, Norway, and her colleagues presented these findings at EuroHeartCare 2016 (abstract 36).
“The stroke and bleeding complications from warfarin can be fatal,” Dr Oterhals noted. “Worldwide, warfarin causes the most deaths from drug-related side effects. Patients need to know what foods and drugs have an impact on how warfarin works and what to do if they have symptoms of an overdose or underdose.”
Dr Oterhals and her colleagues evaluated warfarin knowledge in 404 patients with aortic stenosis. The patients’ mean age was 68, and 70% were male.
Nearly two-thirds of the patients (63%) were taking warfarin because they had a mechanical valve, and 24% were taking the drug because they had atrial fibrillation. The remaining patients were taking the drug for unknown reasons (6%) or “other” reasons (7%).
The patients received a postal questionnaire with 28 multiple-choice questions about warfarin. On average, patients answered 18 of the 28 questions correctly. However, 22% of patients answered less than half of the questions correctly.
The questions that were most often answered incorrectly were those concerning food and drug interactions and when to call or see a doctor.
For example, patients were asked which of the following foods would interfere with warfarin: celery, carrots, coleslaw, or green beans. Only 25% correctly said coleslaw. Most patients answered green beans.
“Patients often think green vegetables have the most vitamin K, but that’s not true,” Dr Oterhals said. “Brassica vegetables such as cabbage, broccoli, and cauliflower are rich sources.”
“Patients do not have to avoid these foods, but they should eat an equal amount every week because the vitamin K will decrease their INR and put them at increased risk of thrombosis or embolism. Patients who like to eat a lot of vitamin K-containing foods can take a higher warfarin dosage, but they need to be consistent.”
Dr Oterhals and her colleagues also found that 80% of patients knew they should go directly to the emergency room if they had nose bleeding that would not stop. However, only 45% of patients correctly said diarrhea for more than one day necessitates a visit to the doctor.
The study also showed that increased age was associated with a decrease in correct answers.
“We can only speculate why,” Dr Oterhals said. “Younger people tend to seek out information about how to manage their disease, while the older generation wants the doctor to tell them what to do.”
“Motivated patients should be offered an INR testing kit so that they can monitor their levels and adjust the warfarin dose themselves, just as patients with diabetes who use insulin do. It enables patients to travel and try new foods without having to find a clinic to get tested. Patients tell me that hot weather increases their INR, while another found out while in Asia that nori decreased his INR.”
“Warfarin is a life-saving drug but can be deadly if not used carefully. Health professionals have a responsibility to educate patients, but, unfortunately, even cardiac nurses do not know enough. There is an urgent need to improve health professionals’ warfarin knowledge so they can educate patients.” ![]()

ATHENS—A study of patients taking warfarin suggests many do not fully understand the risks associated with the drug.
Researchers asked patients to complete a questionnaire on warfarin use and found that, on average, patients answered 64% of the questions correctly.
The patients tended to be the least informed about food and drug interactions and which side effects necessitate a call or visit to the doctor.
Kjersti Oterhals, RN, PhD, of Haukeland University Hospital in Bergen, Norway, and her colleagues presented these findings at EuroHeartCare 2016 (abstract 36).
“The stroke and bleeding complications from warfarin can be fatal,” Dr Oterhals noted. “Worldwide, warfarin causes the most deaths from drug-related side effects. Patients need to know what foods and drugs have an impact on how warfarin works and what to do if they have symptoms of an overdose or underdose.”
Dr Oterhals and her colleagues evaluated warfarin knowledge in 404 patients with aortic stenosis. The patients’ mean age was 68, and 70% were male.
Nearly two-thirds of the patients (63%) were taking warfarin because they had a mechanical valve, and 24% were taking the drug because they had atrial fibrillation. The remaining patients were taking the drug for unknown reasons (6%) or “other” reasons (7%).
The patients received a postal questionnaire with 28 multiple-choice questions about warfarin. On average, patients answered 18 of the 28 questions correctly. However, 22% of patients answered less than half of the questions correctly.
The questions that were most often answered incorrectly were those concerning food and drug interactions and when to call or see a doctor.
For example, patients were asked which of the following foods would interfere with warfarin: celery, carrots, coleslaw, or green beans. Only 25% correctly said coleslaw. Most patients answered green beans.
“Patients often think green vegetables have the most vitamin K, but that’s not true,” Dr Oterhals said. “Brassica vegetables such as cabbage, broccoli, and cauliflower are rich sources.”
“Patients do not have to avoid these foods, but they should eat an equal amount every week because the vitamin K will decrease their INR and put them at increased risk of thrombosis or embolism. Patients who like to eat a lot of vitamin K-containing foods can take a higher warfarin dosage, but they need to be consistent.”
Dr Oterhals and her colleagues also found that 80% of patients knew they should go directly to the emergency room if they had nose bleeding that would not stop. However, only 45% of patients correctly said diarrhea for more than one day necessitates a visit to the doctor.
The study also showed that increased age was associated with a decrease in correct answers.
“We can only speculate why,” Dr Oterhals said. “Younger people tend to seek out information about how to manage their disease, while the older generation wants the doctor to tell them what to do.”
“Motivated patients should be offered an INR testing kit so that they can monitor their levels and adjust the warfarin dose themselves, just as patients with diabetes who use insulin do. It enables patients to travel and try new foods without having to find a clinic to get tested. Patients tell me that hot weather increases their INR, while another found out while in Asia that nori decreased his INR.”
“Warfarin is a life-saving drug but can be deadly if not used carefully. Health professionals have a responsibility to educate patients, but, unfortunately, even cardiac nurses do not know enough. There is an urgent need to improve health professionals’ warfarin knowledge so they can educate patients.” ![]()

ATHENS—A study of patients taking warfarin suggests many do not fully understand the risks associated with the drug.
Researchers asked patients to complete a questionnaire on warfarin use and found that, on average, patients answered 64% of the questions correctly.
The patients tended to be the least informed about food and drug interactions and which side effects necessitate a call or visit to the doctor.
Kjersti Oterhals, RN, PhD, of Haukeland University Hospital in Bergen, Norway, and her colleagues presented these findings at EuroHeartCare 2016 (abstract 36).
“The stroke and bleeding complications from warfarin can be fatal,” Dr Oterhals noted. “Worldwide, warfarin causes the most deaths from drug-related side effects. Patients need to know what foods and drugs have an impact on how warfarin works and what to do if they have symptoms of an overdose or underdose.”
Dr Oterhals and her colleagues evaluated warfarin knowledge in 404 patients with aortic stenosis. The patients’ mean age was 68, and 70% were male.
Nearly two-thirds of the patients (63%) were taking warfarin because they had a mechanical valve, and 24% were taking the drug because they had atrial fibrillation. The remaining patients were taking the drug for unknown reasons (6%) or “other” reasons (7%).
The patients received a postal questionnaire with 28 multiple-choice questions about warfarin. On average, patients answered 18 of the 28 questions correctly. However, 22% of patients answered less than half of the questions correctly.
The questions that were most often answered incorrectly were those concerning food and drug interactions and when to call or see a doctor.
For example, patients were asked which of the following foods would interfere with warfarin: celery, carrots, coleslaw, or green beans. Only 25% correctly said coleslaw. Most patients answered green beans.
“Patients often think green vegetables have the most vitamin K, but that’s not true,” Dr Oterhals said. “Brassica vegetables such as cabbage, broccoli, and cauliflower are rich sources.”
“Patients do not have to avoid these foods, but they should eat an equal amount every week because the vitamin K will decrease their INR and put them at increased risk of thrombosis or embolism. Patients who like to eat a lot of vitamin K-containing foods can take a higher warfarin dosage, but they need to be consistent.”
Dr Oterhals and her colleagues also found that 80% of patients knew they should go directly to the emergency room if they had nose bleeding that would not stop. However, only 45% of patients correctly said diarrhea for more than one day necessitates a visit to the doctor.
The study also showed that increased age was associated with a decrease in correct answers.
“We can only speculate why,” Dr Oterhals said. “Younger people tend to seek out information about how to manage their disease, while the older generation wants the doctor to tell them what to do.”
“Motivated patients should be offered an INR testing kit so that they can monitor their levels and adjust the warfarin dose themselves, just as patients with diabetes who use insulin do. It enables patients to travel and try new foods without having to find a clinic to get tested. Patients tell me that hot weather increases their INR, while another found out while in Asia that nori decreased his INR.”
“Warfarin is a life-saving drug but can be deadly if not used carefully. Health professionals have a responsibility to educate patients, but, unfortunately, even cardiac nurses do not know enough. There is an urgent need to improve health professionals’ warfarin knowledge so they can educate patients.” ![]()
Most atopic lesions colonized with Staph
Patients with atopic dermatitis are at an increased risk of Staphylococcus aureus colonization of both their lesional and nonlesional skin, as well as their nose, compared with healthy controls, according to a report in the British Journal of Dermatology.
Dr. J.E.E. Totté of the department of dermatology at the Erasmus MC University Medical Centre, Rotterdam, and associates conducted a systematic literature review and meta-analysis to derive pooled estimates of the prevalence and odds of colonization with S. aureus in patients with atopic dermatitis. They focused on original, human experimental, and observational studies including patients of any age with a confirmed diagnosis of atopic dermatitis (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14566).
Dr. Totté and colleagues identified a total of 4,909 articles, of which 95 were found to meet the study inclusion criteria. All of the included studies were observational, with 30 comparing atopic dermatitis patients with healthy controls.
Almost three-quarters (70%) of patients had S. aureus colonization of lesional skin, while 39% had colonization of nonlesional skin, based on 81 studies including 5,231 patients and 30 studies including 1,496 patients, respectively. Nasal colonization was found in 62% of patients, based on analysis of 43 studies including 2,476 patients.
S. aureus colonization is an important factor in the pathogenesis of atopic dermatitis and should lead to evaluations of targeted antistaphylococcal therapy for the skin and nose, the investigators advised.
The authors reported that the department of dermatology of the Erasmus MC University Medical Centre Rotterdam received an unrestricted grant from Micreos Human Health. Two coauthors disclosed ties to industry sources.
Patients with atopic dermatitis are at an increased risk of Staphylococcus aureus colonization of both their lesional and nonlesional skin, as well as their nose, compared with healthy controls, according to a report in the British Journal of Dermatology.
Dr. J.E.E. Totté of the department of dermatology at the Erasmus MC University Medical Centre, Rotterdam, and associates conducted a systematic literature review and meta-analysis to derive pooled estimates of the prevalence and odds of colonization with S. aureus in patients with atopic dermatitis. They focused on original, human experimental, and observational studies including patients of any age with a confirmed diagnosis of atopic dermatitis (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14566).
Dr. Totté and colleagues identified a total of 4,909 articles, of which 95 were found to meet the study inclusion criteria. All of the included studies were observational, with 30 comparing atopic dermatitis patients with healthy controls.
Almost three-quarters (70%) of patients had S. aureus colonization of lesional skin, while 39% had colonization of nonlesional skin, based on 81 studies including 5,231 patients and 30 studies including 1,496 patients, respectively. Nasal colonization was found in 62% of patients, based on analysis of 43 studies including 2,476 patients.
S. aureus colonization is an important factor in the pathogenesis of atopic dermatitis and should lead to evaluations of targeted antistaphylococcal therapy for the skin and nose, the investigators advised.
The authors reported that the department of dermatology of the Erasmus MC University Medical Centre Rotterdam received an unrestricted grant from Micreos Human Health. Two coauthors disclosed ties to industry sources.
Patients with atopic dermatitis are at an increased risk of Staphylococcus aureus colonization of both their lesional and nonlesional skin, as well as their nose, compared with healthy controls, according to a report in the British Journal of Dermatology.
Dr. J.E.E. Totté of the department of dermatology at the Erasmus MC University Medical Centre, Rotterdam, and associates conducted a systematic literature review and meta-analysis to derive pooled estimates of the prevalence and odds of colonization with S. aureus in patients with atopic dermatitis. They focused on original, human experimental, and observational studies including patients of any age with a confirmed diagnosis of atopic dermatitis (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14566).
Dr. Totté and colleagues identified a total of 4,909 articles, of which 95 were found to meet the study inclusion criteria. All of the included studies were observational, with 30 comparing atopic dermatitis patients with healthy controls.
Almost three-quarters (70%) of patients had S. aureus colonization of lesional skin, while 39% had colonization of nonlesional skin, based on 81 studies including 5,231 patients and 30 studies including 1,496 patients, respectively. Nasal colonization was found in 62% of patients, based on analysis of 43 studies including 2,476 patients.
S. aureus colonization is an important factor in the pathogenesis of atopic dermatitis and should lead to evaluations of targeted antistaphylococcal therapy for the skin and nose, the investigators advised.
The authors reported that the department of dermatology of the Erasmus MC University Medical Centre Rotterdam received an unrestricted grant from Micreos Human Health. Two coauthors disclosed ties to industry sources.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Consider addressing S. aureus colonization in atopic dermatitis patients.
Major finding: Most patients (70%) were colonized with S. aureus on lesional skin, while 39% were colonized on nonlesional skin.
Data source: Literature review and meta-analysis involving 95 studies, 30 with healthy controls.
Disclosures: The study was funded by an unrestricted grant from Micreos Human Health to Erasmus MC University Medical Centre. Two coauthors disclosed ties to industry sources.
Conflicts in the physician-patient relationship
The basic premise of the physician-patient relationship is simple: Patients are ill and we want to make them better. But the true nature of the relationship is not so monochromatic. Patients and physicians can differ, either on what ails the patient or on what will make the patient better, and it can be challenging to navigate that divide.
One common source of conflict for me is test ordering. I tend to be somewhat conservative when it comes to ordering tests, but patients will often feel shortchanged if they are not subjected to needlesticks and radiation. One of the most common requests I get, being in New England, is for Lyme testing. Patients have gotten so sophisticated now that they expect a Western blot. “My primary care doc just refuses to order a Western blot for me,” one lady complained.
Settling on a mutually acceptable diagnosis can be tricky as well. Of course, “mutually acceptable” is not the issue: a patient either has a diagnosis or does not. But in order for the patient to accept your recommended therapy, they have to believe that you have the right diagnosis. And some diagnoses are hard to accept and even harder to prove than others. A few patients refuse to believe that they have rheumatoid arthritis, particularly if they test negative. And how many of your patients refuse to believe that they have fibromyalgia? How many insist that they have that catch-all, “autoimmune disease,” despite evidence to the contrary?
On the matter of treatment, there are disagreements, too. The most obvious example, and one of the biggest challenges, is narcotic prescription. Patients with chronic pain often rely on narcotics to feel better, but narcotic use is not recommended in such patients. Physicians and patients can expect to be in a perennial tension over who prevails.
Mental health issues are the most challenging for me. For example, I have a young patient who has a polysubstance use disorder and gets admitted repeatedly for alcohol-induced pancreatitis, yet refuses to get mental health therapy for it despite multiple inpatient psychiatric consultations exhorting her to do so. “It doesn’t do anything for me,” she says. She lies about everything, from medication compliance to where she gets medications to how much she drinks, yet I do not feel equipped to handle these problems.
If any of the above scenarios were board exam questions, choosing the proverbial next best step would be simple. But when does life really operate so neatly? The old paradigm of doctoring was that the physician rendered an opinion informed by his or her education. Today, our exam-room interactions often take the shape of a democracy, one in which an overworked, bandwidth-depleted physician might butt heads with a strong-willed patient, armed with all the wisdom of anecdotes and the Internet. If I were fresh out of med school, I might have had more energy to explain to you why you don’t need that Lyme test or that narcotic prescription. These days though, I find myself waving the white flag far more than I should.
Dr. Chan practices rheumatology in Pawtucket, R.I.
The basic premise of the physician-patient relationship is simple: Patients are ill and we want to make them better. But the true nature of the relationship is not so monochromatic. Patients and physicians can differ, either on what ails the patient or on what will make the patient better, and it can be challenging to navigate that divide.
One common source of conflict for me is test ordering. I tend to be somewhat conservative when it comes to ordering tests, but patients will often feel shortchanged if they are not subjected to needlesticks and radiation. One of the most common requests I get, being in New England, is for Lyme testing. Patients have gotten so sophisticated now that they expect a Western blot. “My primary care doc just refuses to order a Western blot for me,” one lady complained.
Settling on a mutually acceptable diagnosis can be tricky as well. Of course, “mutually acceptable” is not the issue: a patient either has a diagnosis or does not. But in order for the patient to accept your recommended therapy, they have to believe that you have the right diagnosis. And some diagnoses are hard to accept and even harder to prove than others. A few patients refuse to believe that they have rheumatoid arthritis, particularly if they test negative. And how many of your patients refuse to believe that they have fibromyalgia? How many insist that they have that catch-all, “autoimmune disease,” despite evidence to the contrary?
On the matter of treatment, there are disagreements, too. The most obvious example, and one of the biggest challenges, is narcotic prescription. Patients with chronic pain often rely on narcotics to feel better, but narcotic use is not recommended in such patients. Physicians and patients can expect to be in a perennial tension over who prevails.
Mental health issues are the most challenging for me. For example, I have a young patient who has a polysubstance use disorder and gets admitted repeatedly for alcohol-induced pancreatitis, yet refuses to get mental health therapy for it despite multiple inpatient psychiatric consultations exhorting her to do so. “It doesn’t do anything for me,” she says. She lies about everything, from medication compliance to where she gets medications to how much she drinks, yet I do not feel equipped to handle these problems.
If any of the above scenarios were board exam questions, choosing the proverbial next best step would be simple. But when does life really operate so neatly? The old paradigm of doctoring was that the physician rendered an opinion informed by his or her education. Today, our exam-room interactions often take the shape of a democracy, one in which an overworked, bandwidth-depleted physician might butt heads with a strong-willed patient, armed with all the wisdom of anecdotes and the Internet. If I were fresh out of med school, I might have had more energy to explain to you why you don’t need that Lyme test or that narcotic prescription. These days though, I find myself waving the white flag far more than I should.
Dr. Chan practices rheumatology in Pawtucket, R.I.
The basic premise of the physician-patient relationship is simple: Patients are ill and we want to make them better. But the true nature of the relationship is not so monochromatic. Patients and physicians can differ, either on what ails the patient or on what will make the patient better, and it can be challenging to navigate that divide.
One common source of conflict for me is test ordering. I tend to be somewhat conservative when it comes to ordering tests, but patients will often feel shortchanged if they are not subjected to needlesticks and radiation. One of the most common requests I get, being in New England, is for Lyme testing. Patients have gotten so sophisticated now that they expect a Western blot. “My primary care doc just refuses to order a Western blot for me,” one lady complained.
Settling on a mutually acceptable diagnosis can be tricky as well. Of course, “mutually acceptable” is not the issue: a patient either has a diagnosis or does not. But in order for the patient to accept your recommended therapy, they have to believe that you have the right diagnosis. And some diagnoses are hard to accept and even harder to prove than others. A few patients refuse to believe that they have rheumatoid arthritis, particularly if they test negative. And how many of your patients refuse to believe that they have fibromyalgia? How many insist that they have that catch-all, “autoimmune disease,” despite evidence to the contrary?
On the matter of treatment, there are disagreements, too. The most obvious example, and one of the biggest challenges, is narcotic prescription. Patients with chronic pain often rely on narcotics to feel better, but narcotic use is not recommended in such patients. Physicians and patients can expect to be in a perennial tension over who prevails.
Mental health issues are the most challenging for me. For example, I have a young patient who has a polysubstance use disorder and gets admitted repeatedly for alcohol-induced pancreatitis, yet refuses to get mental health therapy for it despite multiple inpatient psychiatric consultations exhorting her to do so. “It doesn’t do anything for me,” she says. She lies about everything, from medication compliance to where she gets medications to how much she drinks, yet I do not feel equipped to handle these problems.
If any of the above scenarios were board exam questions, choosing the proverbial next best step would be simple. But when does life really operate so neatly? The old paradigm of doctoring was that the physician rendered an opinion informed by his or her education. Today, our exam-room interactions often take the shape of a democracy, one in which an overworked, bandwidth-depleted physician might butt heads with a strong-willed patient, armed with all the wisdom of anecdotes and the Internet. If I were fresh out of med school, I might have had more energy to explain to you why you don’t need that Lyme test or that narcotic prescription. These days though, I find myself waving the white flag far more than I should.
Dr. Chan practices rheumatology in Pawtucket, R.I.