User login
Video: Eltrombopag boosted standard therapy in severe, newly-diagnosed aplastic anemia
ORLANDO – Adding eltrombopag to standard immunosuppressive treatment for newly-diagnosed severe aplastic anemia boosted overall response rates from around 65% to over 90%, based on a late-breaker study presented by Dr. Danielle M. Townsley at the annual meeting of the American Society of Hematology.
Dr. Townsley said that starting the combination treatment immediately at diagnosis, rather than waiting to introduce eltrombopag at either 2 weeks or 3 months after initiating standard immunosuppressive therapy, was associated with better outcomes. As a result of the findings, a cohort extension of the trial will continue and is enrolling patients.
In our video interview, Dr. Townsley discusses the top-level results and says that it’s too early to introduce the protocol into practice outside a clinical trial. She urges hematologists to enroll their patients in the ongoing cohort study.
On Twitter @maryjodales
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Adding eltrombopag to standard immunosuppressive treatment for newly-diagnosed severe aplastic anemia boosted overall response rates from around 65% to over 90%, based on a late-breaker study presented by Dr. Danielle M. Townsley at the annual meeting of the American Society of Hematology.
Dr. Townsley said that starting the combination treatment immediately at diagnosis, rather than waiting to introduce eltrombopag at either 2 weeks or 3 months after initiating standard immunosuppressive therapy, was associated with better outcomes. As a result of the findings, a cohort extension of the trial will continue and is enrolling patients.
In our video interview, Dr. Townsley discusses the top-level results and says that it’s too early to introduce the protocol into practice outside a clinical trial. She urges hematologists to enroll their patients in the ongoing cohort study.
On Twitter @maryjodales
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Adding eltrombopag to standard immunosuppressive treatment for newly-diagnosed severe aplastic anemia boosted overall response rates from around 65% to over 90%, based on a late-breaker study presented by Dr. Danielle M. Townsley at the annual meeting of the American Society of Hematology.
Dr. Townsley said that starting the combination treatment immediately at diagnosis, rather than waiting to introduce eltrombopag at either 2 weeks or 3 months after initiating standard immunosuppressive therapy, was associated with better outcomes. As a result of the findings, a cohort extension of the trial will continue and is enrolling patients.
In our video interview, Dr. Townsley discusses the top-level results and says that it’s too early to introduce the protocol into practice outside a clinical trial. She urges hematologists to enroll their patients in the ongoing cohort study.
On Twitter @maryjodales
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2015
VIDEO: High-intensity conditioning stands ground in MDS, AML
ORLANDO – Reduced-intensity conditioning regimens failed to show a significant survival benefit over high-intensity regimens in myelodysplastic syndrome or acute myeloid leukemia in the phase III MAVERICK trial.
Pretransplant reduced intensity conditioning (RIC) also resulted in a significantly higher risk of relapse and inferior relapse-free survival vs. myeloablative conditioning (MAC).
The findings, reported in the late-breaking abstract (LBA-8) session at the annual meeting of the American Society of Hematology, generated a lively debate over the study construct and whether its conclusion that MAC remain “the treatment of choice” in appropriate candidates should be applied to both diseases.
Session comoderator Dr. David P. Steensma, a myelodysplasia physician at the Dana-Farber Cancer Institute in Boston, said in an interview that the data will not change his practice and that physicians should continue to “push the envelope” and provide as intense a conditioning regimen as their patients can tolerate.
“The take-home message is that using a reduced conditioning regimen whatever your choice might be, even though it is gentler on the patient and may be easier for them to go through the transplant, the biggest risk is still the disease, the underlying leukemia or myelodysplasia coming back. And the benefit from reduced intensity is not enough to outweigh that risk.”
To sort out this complex trial, we spoke with study author Dr. Bart L. Scott of the Fred Hutchinson Cancer Research Center at the University of Washington, Seattle.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Reduced-intensity conditioning regimens failed to show a significant survival benefit over high-intensity regimens in myelodysplastic syndrome or acute myeloid leukemia in the phase III MAVERICK trial.
Pretransplant reduced intensity conditioning (RIC) also resulted in a significantly higher risk of relapse and inferior relapse-free survival vs. myeloablative conditioning (MAC).
The findings, reported in the late-breaking abstract (LBA-8) session at the annual meeting of the American Society of Hematology, generated a lively debate over the study construct and whether its conclusion that MAC remain “the treatment of choice” in appropriate candidates should be applied to both diseases.
Session comoderator Dr. David P. Steensma, a myelodysplasia physician at the Dana-Farber Cancer Institute in Boston, said in an interview that the data will not change his practice and that physicians should continue to “push the envelope” and provide as intense a conditioning regimen as their patients can tolerate.
“The take-home message is that using a reduced conditioning regimen whatever your choice might be, even though it is gentler on the patient and may be easier for them to go through the transplant, the biggest risk is still the disease, the underlying leukemia or myelodysplasia coming back. And the benefit from reduced intensity is not enough to outweigh that risk.”
To sort out this complex trial, we spoke with study author Dr. Bart L. Scott of the Fred Hutchinson Cancer Research Center at the University of Washington, Seattle.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Reduced-intensity conditioning regimens failed to show a significant survival benefit over high-intensity regimens in myelodysplastic syndrome or acute myeloid leukemia in the phase III MAVERICK trial.
Pretransplant reduced intensity conditioning (RIC) also resulted in a significantly higher risk of relapse and inferior relapse-free survival vs. myeloablative conditioning (MAC).
The findings, reported in the late-breaking abstract (LBA-8) session at the annual meeting of the American Society of Hematology, generated a lively debate over the study construct and whether its conclusion that MAC remain “the treatment of choice” in appropriate candidates should be applied to both diseases.
Session comoderator Dr. David P. Steensma, a myelodysplasia physician at the Dana-Farber Cancer Institute in Boston, said in an interview that the data will not change his practice and that physicians should continue to “push the envelope” and provide as intense a conditioning regimen as their patients can tolerate.
“The take-home message is that using a reduced conditioning regimen whatever your choice might be, even though it is gentler on the patient and may be easier for them to go through the transplant, the biggest risk is still the disease, the underlying leukemia or myelodysplasia coming back. And the benefit from reduced intensity is not enough to outweigh that risk.”
To sort out this complex trial, we spoke with study author Dr. Bart L. Scott of the Fred Hutchinson Cancer Research Center at the University of Washington, Seattle.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2015
Stent Thrombosis Risk Linked to Bioresorbable Scaffold
NEW YORK - Restenosis rates are similar one year after implantation of an everolimus-eluting bioresorbable vascular scaffold or an everolimus-eluting metallic stent, but the scaffold has a higher risk of device thrombosis within 30 days, a new meta-analysis shows.
The meta-analysis suggests that the two interventions have a "similar requirement of repeat revascularization out to 1-year follow-up, despite inferior angiographic performance," first author Dr. Salvatore Cassese said by email.
This higher risk of stent thrombosis, twice as high with the bioresorbable device compared with the metallic stent after one year, "is somewhat surprising," said Dr. Cassese, of the Technical University of Munich's German Heart Center Munich. "The higher risk of scaffold thrombosis in relatively simple clinical (lesion) settings represents the new important finding showed by this meta-analysis."
A number of recently completed randomized trials showed comparable mid-term outcomes with the two devices, but data from routine clinical practice suggests a "somewhat higher rate of adverse events" with the bioresorbable scaffold, he said.
Most randomized trials comparing the two types of devices were small and not adequately powered to assess clinical endpoints, the authors noted. For their meta-analysis, they identified six trials involving 3,738 patients (mainly men, median age 62.3 years) that met their inclusion criteria (randomized design, an analysis by intention to treat, and a follow-up of at least six months).
The meta-analysis included 2,337 patients who received a bioresorbable scaffold and 1,401 who received a metallic stent. Median follow-up was 12 months.
Both groups had a 3% rate of target lesion revascularization, the primary efficacy outcome, the researchers noted in a report online November 16 in The Lancet.
The risk of the primary safety outcome, definite or probable stent/scaffold thrombosis, was significantly higher for those treated with a bioresorbable scaffold compared with those who received a metallic stent (1.3% versus 0.5%; odds ratio, 1.99), with the highest risk within 30 days after implantation.
In-device late lumen loss was also significantly greater in lesions treated with the bioresorbable device compared with the metallic stent.
Risk of myocardial infarction appeared to be higher in patients with the bioresorbable scaffolds than in those with metallic stents, but the difference was not statistically significant (5.2% versus 3.5%, p=0.06). The groups had similar rates of target lesion failure and risk of death.
The authors noted that their finding of at least similar efficacy of the bioresorbable scaffold versus the existing best-in-class drug-eluting stent at 12 months was achieved in a highly selected population that included mainly stable patients with single de-novo non-complex target lesions and excluded patients who had a higher risk for device failure.
Two large-scale randomized trials are under way that are expected to shed more light on the devices' relative efficacy in higher-risk populations.
Although the study's findings "should heighten concerns about the current generation of bioresorbable vascular scaffold technology, they should by no means be interpreted to mean that bioresorbable scaffolds are not worth pursuing," noted an editorial that accompanied the new meta-analysis. "Just as with first-generation drug-eluting stents, a complete understanding of the limitations of such technology is necessary before further advancements can be made."
"Little information appears available regarding the incidence of symptoms of angina in the comparison groups," Dr. Richard Chazal, who was not involved in the study, said in an email.
"This is important, as the principal utility of stents in stable patients is the relief of such symptoms," noted Dr. Chazal, president-elect of the American College of Cardiology and medical director of the Lee Memorial Health Systems' Heart and Vascular Institute in Fort Myers, Fla.
"Disappearing" bioresorbable scaffolds are viewed as a possible solution to potential problems of leaving metallic stents permanently inside a coronary vessel. These problems include impairing the function of the wall of the artery and limiting future options for treating the artery, especially with a bypass operation, explained Dr. Chazal.
Emergence of their anticipated benefit over metallic stents is expected several years after implantation, when elution of the anti-restenotic drug has stopped and the scaffold has dissolved.
"Longer-term follow-up will be needed to clarify whether these newer devices provide hoped-for advantages over metallic stents, or whether the early issues with thrombosis/clotting and vessel narrowing eventually results in more clinical problems," Dr. Chazal said.
The study had no funding source. Two of the 10 coauthors reported receiving fees from stent manufacturers or holding patents related to drug-eluting stent technologies, "outside the submitted work." A third coauthor is a member of the advisory board of Abbott, which includes a division that makes an everolimus-eluting bioresorbable vascular scaffold.
NEW YORK - Restenosis rates are similar one year after implantation of an everolimus-eluting bioresorbable vascular scaffold or an everolimus-eluting metallic stent, but the scaffold has a higher risk of device thrombosis within 30 days, a new meta-analysis shows.
The meta-analysis suggests that the two interventions have a "similar requirement of repeat revascularization out to 1-year follow-up, despite inferior angiographic performance," first author Dr. Salvatore Cassese said by email.
This higher risk of stent thrombosis, twice as high with the bioresorbable device compared with the metallic stent after one year, "is somewhat surprising," said Dr. Cassese, of the Technical University of Munich's German Heart Center Munich. "The higher risk of scaffold thrombosis in relatively simple clinical (lesion) settings represents the new important finding showed by this meta-analysis."
A number of recently completed randomized trials showed comparable mid-term outcomes with the two devices, but data from routine clinical practice suggests a "somewhat higher rate of adverse events" with the bioresorbable scaffold, he said.
Most randomized trials comparing the two types of devices were small and not adequately powered to assess clinical endpoints, the authors noted. For their meta-analysis, they identified six trials involving 3,738 patients (mainly men, median age 62.3 years) that met their inclusion criteria (randomized design, an analysis by intention to treat, and a follow-up of at least six months).
The meta-analysis included 2,337 patients who received a bioresorbable scaffold and 1,401 who received a metallic stent. Median follow-up was 12 months.
Both groups had a 3% rate of target lesion revascularization, the primary efficacy outcome, the researchers noted in a report online November 16 in The Lancet.
The risk of the primary safety outcome, definite or probable stent/scaffold thrombosis, was significantly higher for those treated with a bioresorbable scaffold compared with those who received a metallic stent (1.3% versus 0.5%; odds ratio, 1.99), with the highest risk within 30 days after implantation.
In-device late lumen loss was also significantly greater in lesions treated with the bioresorbable device compared with the metallic stent.
Risk of myocardial infarction appeared to be higher in patients with the bioresorbable scaffolds than in those with metallic stents, but the difference was not statistically significant (5.2% versus 3.5%, p=0.06). The groups had similar rates of target lesion failure and risk of death.
The authors noted that their finding of at least similar efficacy of the bioresorbable scaffold versus the existing best-in-class drug-eluting stent at 12 months was achieved in a highly selected population that included mainly stable patients with single de-novo non-complex target lesions and excluded patients who had a higher risk for device failure.
Two large-scale randomized trials are under way that are expected to shed more light on the devices' relative efficacy in higher-risk populations.
Although the study's findings "should heighten concerns about the current generation of bioresorbable vascular scaffold technology, they should by no means be interpreted to mean that bioresorbable scaffolds are not worth pursuing," noted an editorial that accompanied the new meta-analysis. "Just as with first-generation drug-eluting stents, a complete understanding of the limitations of such technology is necessary before further advancements can be made."
"Little information appears available regarding the incidence of symptoms of angina in the comparison groups," Dr. Richard Chazal, who was not involved in the study, said in an email.
"This is important, as the principal utility of stents in stable patients is the relief of such symptoms," noted Dr. Chazal, president-elect of the American College of Cardiology and medical director of the Lee Memorial Health Systems' Heart and Vascular Institute in Fort Myers, Fla.
"Disappearing" bioresorbable scaffolds are viewed as a possible solution to potential problems of leaving metallic stents permanently inside a coronary vessel. These problems include impairing the function of the wall of the artery and limiting future options for treating the artery, especially with a bypass operation, explained Dr. Chazal.
Emergence of their anticipated benefit over metallic stents is expected several years after implantation, when elution of the anti-restenotic drug has stopped and the scaffold has dissolved.
"Longer-term follow-up will be needed to clarify whether these newer devices provide hoped-for advantages over metallic stents, or whether the early issues with thrombosis/clotting and vessel narrowing eventually results in more clinical problems," Dr. Chazal said.
The study had no funding source. Two of the 10 coauthors reported receiving fees from stent manufacturers or holding patents related to drug-eluting stent technologies, "outside the submitted work." A third coauthor is a member of the advisory board of Abbott, which includes a division that makes an everolimus-eluting bioresorbable vascular scaffold.
NEW YORK - Restenosis rates are similar one year after implantation of an everolimus-eluting bioresorbable vascular scaffold or an everolimus-eluting metallic stent, but the scaffold has a higher risk of device thrombosis within 30 days, a new meta-analysis shows.
The meta-analysis suggests that the two interventions have a "similar requirement of repeat revascularization out to 1-year follow-up, despite inferior angiographic performance," first author Dr. Salvatore Cassese said by email.
This higher risk of stent thrombosis, twice as high with the bioresorbable device compared with the metallic stent after one year, "is somewhat surprising," said Dr. Cassese, of the Technical University of Munich's German Heart Center Munich. "The higher risk of scaffold thrombosis in relatively simple clinical (lesion) settings represents the new important finding showed by this meta-analysis."
A number of recently completed randomized trials showed comparable mid-term outcomes with the two devices, but data from routine clinical practice suggests a "somewhat higher rate of adverse events" with the bioresorbable scaffold, he said.
Most randomized trials comparing the two types of devices were small and not adequately powered to assess clinical endpoints, the authors noted. For their meta-analysis, they identified six trials involving 3,738 patients (mainly men, median age 62.3 years) that met their inclusion criteria (randomized design, an analysis by intention to treat, and a follow-up of at least six months).
The meta-analysis included 2,337 patients who received a bioresorbable scaffold and 1,401 who received a metallic stent. Median follow-up was 12 months.
Both groups had a 3% rate of target lesion revascularization, the primary efficacy outcome, the researchers noted in a report online November 16 in The Lancet.
The risk of the primary safety outcome, definite or probable stent/scaffold thrombosis, was significantly higher for those treated with a bioresorbable scaffold compared with those who received a metallic stent (1.3% versus 0.5%; odds ratio, 1.99), with the highest risk within 30 days after implantation.
In-device late lumen loss was also significantly greater in lesions treated with the bioresorbable device compared with the metallic stent.
Risk of myocardial infarction appeared to be higher in patients with the bioresorbable scaffolds than in those with metallic stents, but the difference was not statistically significant (5.2% versus 3.5%, p=0.06). The groups had similar rates of target lesion failure and risk of death.
The authors noted that their finding of at least similar efficacy of the bioresorbable scaffold versus the existing best-in-class drug-eluting stent at 12 months was achieved in a highly selected population that included mainly stable patients with single de-novo non-complex target lesions and excluded patients who had a higher risk for device failure.
Two large-scale randomized trials are under way that are expected to shed more light on the devices' relative efficacy in higher-risk populations.
Although the study's findings "should heighten concerns about the current generation of bioresorbable vascular scaffold technology, they should by no means be interpreted to mean that bioresorbable scaffolds are not worth pursuing," noted an editorial that accompanied the new meta-analysis. "Just as with first-generation drug-eluting stents, a complete understanding of the limitations of such technology is necessary before further advancements can be made."
"Little information appears available regarding the incidence of symptoms of angina in the comparison groups," Dr. Richard Chazal, who was not involved in the study, said in an email.
"This is important, as the principal utility of stents in stable patients is the relief of such symptoms," noted Dr. Chazal, president-elect of the American College of Cardiology and medical director of the Lee Memorial Health Systems' Heart and Vascular Institute in Fort Myers, Fla.
"Disappearing" bioresorbable scaffolds are viewed as a possible solution to potential problems of leaving metallic stents permanently inside a coronary vessel. These problems include impairing the function of the wall of the artery and limiting future options for treating the artery, especially with a bypass operation, explained Dr. Chazal.
Emergence of their anticipated benefit over metallic stents is expected several years after implantation, when elution of the anti-restenotic drug has stopped and the scaffold has dissolved.
"Longer-term follow-up will be needed to clarify whether these newer devices provide hoped-for advantages over metallic stents, or whether the early issues with thrombosis/clotting and vessel narrowing eventually results in more clinical problems," Dr. Chazal said.
The study had no funding source. Two of the 10 coauthors reported receiving fees from stent manufacturers or holding patents related to drug-eluting stent technologies, "outside the submitted work." A third coauthor is a member of the advisory board of Abbott, which includes a division that makes an everolimus-eluting bioresorbable vascular scaffold.
Upfront idelalisib carries high risk for acute liver toxicity
ORLANDO – Idelalisib given as first-line therapy for patients with chronic lymphocytic leukemia carries a high risk of early fulminant hepatotoxicity requiring drug interruption and steroids, investigators reported.
Among 24 patients who received idelalisib (Zydelig) monotherapy in a phase II trial of a combination of idelalisib followed by idelalisib concurrent with ofatumumab (Arzerra) as first-line therapy for chronic lymphocytic leukemia (CLL), 12 patients developed acute hepatotoxicity, marked by rapidly soaring levels of transaminase within about 28 days of starting therapy. An additional four patients developed hepatotoxicity at around 130 days, noted Dr. Benjamin L. Lampson, a clinical fellow in medicine at the Dana-Farber Cancer Institute in Boston.
“Multiple lines of evidence suggest that this early hepatotoxicity is immune mediated. The proportion of regulatory T cells in the peripheral blood decrease on idelalisib therapy, providing a possible explanation for the development of early hepatotoxicity,” he said at the annual meeting of the American Society of Hematology.
The toxicities occur more frequently among younger and less heavily pretreated patients, and are likely due to on-target, immune-mediated effects, he noted.
Dr. Lampson presented data on the first 24 patients in an ongoing phase II trial. Patients with previously untreated CLL receive idelalisib 150 mg twice daily for 56 days, in an attempt to mobilize neoplastic B cells from the peripheral lymphoid tissues and into the bloodstream.
Following the monotherapy phase, patients are given ofatumumab in an attempt to clear the disease from peripheral blood.
“This dosing strategy is slightly different than what has been previously been used in trials combining these particular drugs. Specifically, previously reported trials have started these agents simultaneously without a lead-in period of monotherapy,” Dr. Lampson explained.
When the lead-in phase is completed, patients receive idelalisib plus ofatumumab infusions once weekly for 8 weeks, followed by once-monthly infusions for 4 months. Patients then continue on idelalisib indefinitely. The primary endpoint is the overall response rate assessed 2 months after the completion of the combination therapy.
For the first 24 patients treated as of Nov. 9, 2015, the median time on therapy was 7.7 months and median follow-up was 14.7 months.
The median patient age was 67.4 years (range 57.6-84.9). CLL genetics showed that 13 patients had unmutated immunoglobulin heavy chain variable region (IgHV) disease, 4 had the 17p deletion and TP53 mutation, 1 had deletion 11q, and 13 had deletion 13q; some patients had more than one mutation.
“What we began to notice after enrolling just a few subjects on the trial was that severe hepatotoxicity was occurring shortly after initiating idelalisib,” Dr. Lampson said.
He presented one case, a 58-year-old man who was in the idelalisib monotherapy phase of the study. He developed grade 3 hepatotoxicity 28 days after starting the drug, despite having a normal liver function test just 1 day earlier. The drug was stopped, but his liver function tests continued to rise, suggesting a self-perpetuating or self-sustaining process.
On day 32, the patient was admitted to the hospital, and on day 33 he was started on steroids, based on the hypothesis that the hepatotoxicity might have been immune mediated. Two days after initiation of steroids, his liver function tests continued to rise, whereupon he was started on mycophenolate mofetil.
“With these two forms of immunosuppression, the [liver function tests] did eventually normalize, although the steroids and mycophenolate had to be tapered over a period of many weeks. And this patient was not the only patient to experience toxicity; in fact, hepatotoxicity was frequent and often severe,” he said.
At the time of maximum incidence, week 4, the percentage of patients with any hepatotoxicity was 46%, with 13% at grade 4, and 21% at grade 3.
“The median time to initial development of hepatotoxicity is 28 days. This suggests that the mechanism of hepatotoxicity is not immediate, but takes time to develop, consistent with an adaptive immune response. Furthermore, hepatotoxicity is typically occurring before the first dose of ofatumumab is occurring at week 8, suggesting idelalisib alone is the cause of the hepatotoxicity,” Dr. Lampson said.
A comparison of data from the ongoing study and from three previous studies – two with idelalisib in relapsed refractory disease, and one as first-line therapy in patients 65 and older – showed that grade 3 or greater hepatotoxicity was lowest in a phase I trial of idelalisib in which patients had received a median of five prior lines of therapy, occurring in only 1.9% of patients. In contrast, in the current study, 52% of patients experienced grade 3 or 4 transaminitis at some point in the trial.
Evidence for the hepatotoxicity being an on-target immune-mediated effect comes from lymphocytic infiltrate on liver biopsy and lymphocytic colitis in idelalisib-treated patients. Additional evidence comes from the fact that the toxicity is both treatable and preventable with steroids, he said.
He cautioned that hepatotoxicity can recur rapidly when the drug is reintroduced.
“In general, our experience has been if idelalisib is resumed while the subject remains on steroids, the drug is more likely to be tolerated and the subject eventually can be tapered off steroids,” he said.
Asked by an audience member whether patients who are receiving idelalisib in the first-line setting should also receive steroids, Dr. Lampson said that they closely monitor patient liver enzymes around 28 days, and if grade 1 transaminitis is detected, patients are automatically started on low-dose steroids.
The study is sponsored by the Dana-Farber Cancer Institute in collaboration with Gilead Sciences and GlaxoSmithKline. Dr. Lampson and colleagues declared no relevant conflicts of interest.
ORLANDO – Idelalisib given as first-line therapy for patients with chronic lymphocytic leukemia carries a high risk of early fulminant hepatotoxicity requiring drug interruption and steroids, investigators reported.
Among 24 patients who received idelalisib (Zydelig) monotherapy in a phase II trial of a combination of idelalisib followed by idelalisib concurrent with ofatumumab (Arzerra) as first-line therapy for chronic lymphocytic leukemia (CLL), 12 patients developed acute hepatotoxicity, marked by rapidly soaring levels of transaminase within about 28 days of starting therapy. An additional four patients developed hepatotoxicity at around 130 days, noted Dr. Benjamin L. Lampson, a clinical fellow in medicine at the Dana-Farber Cancer Institute in Boston.
“Multiple lines of evidence suggest that this early hepatotoxicity is immune mediated. The proportion of regulatory T cells in the peripheral blood decrease on idelalisib therapy, providing a possible explanation for the development of early hepatotoxicity,” he said at the annual meeting of the American Society of Hematology.
The toxicities occur more frequently among younger and less heavily pretreated patients, and are likely due to on-target, immune-mediated effects, he noted.
Dr. Lampson presented data on the first 24 patients in an ongoing phase II trial. Patients with previously untreated CLL receive idelalisib 150 mg twice daily for 56 days, in an attempt to mobilize neoplastic B cells from the peripheral lymphoid tissues and into the bloodstream.
Following the monotherapy phase, patients are given ofatumumab in an attempt to clear the disease from peripheral blood.
“This dosing strategy is slightly different than what has been previously been used in trials combining these particular drugs. Specifically, previously reported trials have started these agents simultaneously without a lead-in period of monotherapy,” Dr. Lampson explained.
When the lead-in phase is completed, patients receive idelalisib plus ofatumumab infusions once weekly for 8 weeks, followed by once-monthly infusions for 4 months. Patients then continue on idelalisib indefinitely. The primary endpoint is the overall response rate assessed 2 months after the completion of the combination therapy.
For the first 24 patients treated as of Nov. 9, 2015, the median time on therapy was 7.7 months and median follow-up was 14.7 months.
The median patient age was 67.4 years (range 57.6-84.9). CLL genetics showed that 13 patients had unmutated immunoglobulin heavy chain variable region (IgHV) disease, 4 had the 17p deletion and TP53 mutation, 1 had deletion 11q, and 13 had deletion 13q; some patients had more than one mutation.
“What we began to notice after enrolling just a few subjects on the trial was that severe hepatotoxicity was occurring shortly after initiating idelalisib,” Dr. Lampson said.
He presented one case, a 58-year-old man who was in the idelalisib monotherapy phase of the study. He developed grade 3 hepatotoxicity 28 days after starting the drug, despite having a normal liver function test just 1 day earlier. The drug was stopped, but his liver function tests continued to rise, suggesting a self-perpetuating or self-sustaining process.
On day 32, the patient was admitted to the hospital, and on day 33 he was started on steroids, based on the hypothesis that the hepatotoxicity might have been immune mediated. Two days after initiation of steroids, his liver function tests continued to rise, whereupon he was started on mycophenolate mofetil.
“With these two forms of immunosuppression, the [liver function tests] did eventually normalize, although the steroids and mycophenolate had to be tapered over a period of many weeks. And this patient was not the only patient to experience toxicity; in fact, hepatotoxicity was frequent and often severe,” he said.
At the time of maximum incidence, week 4, the percentage of patients with any hepatotoxicity was 46%, with 13% at grade 4, and 21% at grade 3.
“The median time to initial development of hepatotoxicity is 28 days. This suggests that the mechanism of hepatotoxicity is not immediate, but takes time to develop, consistent with an adaptive immune response. Furthermore, hepatotoxicity is typically occurring before the first dose of ofatumumab is occurring at week 8, suggesting idelalisib alone is the cause of the hepatotoxicity,” Dr. Lampson said.
A comparison of data from the ongoing study and from three previous studies – two with idelalisib in relapsed refractory disease, and one as first-line therapy in patients 65 and older – showed that grade 3 or greater hepatotoxicity was lowest in a phase I trial of idelalisib in which patients had received a median of five prior lines of therapy, occurring in only 1.9% of patients. In contrast, in the current study, 52% of patients experienced grade 3 or 4 transaminitis at some point in the trial.
Evidence for the hepatotoxicity being an on-target immune-mediated effect comes from lymphocytic infiltrate on liver biopsy and lymphocytic colitis in idelalisib-treated patients. Additional evidence comes from the fact that the toxicity is both treatable and preventable with steroids, he said.
He cautioned that hepatotoxicity can recur rapidly when the drug is reintroduced.
“In general, our experience has been if idelalisib is resumed while the subject remains on steroids, the drug is more likely to be tolerated and the subject eventually can be tapered off steroids,” he said.
Asked by an audience member whether patients who are receiving idelalisib in the first-line setting should also receive steroids, Dr. Lampson said that they closely monitor patient liver enzymes around 28 days, and if grade 1 transaminitis is detected, patients are automatically started on low-dose steroids.
The study is sponsored by the Dana-Farber Cancer Institute in collaboration with Gilead Sciences and GlaxoSmithKline. Dr. Lampson and colleagues declared no relevant conflicts of interest.
ORLANDO – Idelalisib given as first-line therapy for patients with chronic lymphocytic leukemia carries a high risk of early fulminant hepatotoxicity requiring drug interruption and steroids, investigators reported.
Among 24 patients who received idelalisib (Zydelig) monotherapy in a phase II trial of a combination of idelalisib followed by idelalisib concurrent with ofatumumab (Arzerra) as first-line therapy for chronic lymphocytic leukemia (CLL), 12 patients developed acute hepatotoxicity, marked by rapidly soaring levels of transaminase within about 28 days of starting therapy. An additional four patients developed hepatotoxicity at around 130 days, noted Dr. Benjamin L. Lampson, a clinical fellow in medicine at the Dana-Farber Cancer Institute in Boston.
“Multiple lines of evidence suggest that this early hepatotoxicity is immune mediated. The proportion of regulatory T cells in the peripheral blood decrease on idelalisib therapy, providing a possible explanation for the development of early hepatotoxicity,” he said at the annual meeting of the American Society of Hematology.
The toxicities occur more frequently among younger and less heavily pretreated patients, and are likely due to on-target, immune-mediated effects, he noted.
Dr. Lampson presented data on the first 24 patients in an ongoing phase II trial. Patients with previously untreated CLL receive idelalisib 150 mg twice daily for 56 days, in an attempt to mobilize neoplastic B cells from the peripheral lymphoid tissues and into the bloodstream.
Following the monotherapy phase, patients are given ofatumumab in an attempt to clear the disease from peripheral blood.
“This dosing strategy is slightly different than what has been previously been used in trials combining these particular drugs. Specifically, previously reported trials have started these agents simultaneously without a lead-in period of monotherapy,” Dr. Lampson explained.
When the lead-in phase is completed, patients receive idelalisib plus ofatumumab infusions once weekly for 8 weeks, followed by once-monthly infusions for 4 months. Patients then continue on idelalisib indefinitely. The primary endpoint is the overall response rate assessed 2 months after the completion of the combination therapy.
For the first 24 patients treated as of Nov. 9, 2015, the median time on therapy was 7.7 months and median follow-up was 14.7 months.
The median patient age was 67.4 years (range 57.6-84.9). CLL genetics showed that 13 patients had unmutated immunoglobulin heavy chain variable region (IgHV) disease, 4 had the 17p deletion and TP53 mutation, 1 had deletion 11q, and 13 had deletion 13q; some patients had more than one mutation.
“What we began to notice after enrolling just a few subjects on the trial was that severe hepatotoxicity was occurring shortly after initiating idelalisib,” Dr. Lampson said.
He presented one case, a 58-year-old man who was in the idelalisib monotherapy phase of the study. He developed grade 3 hepatotoxicity 28 days after starting the drug, despite having a normal liver function test just 1 day earlier. The drug was stopped, but his liver function tests continued to rise, suggesting a self-perpetuating or self-sustaining process.
On day 32, the patient was admitted to the hospital, and on day 33 he was started on steroids, based on the hypothesis that the hepatotoxicity might have been immune mediated. Two days after initiation of steroids, his liver function tests continued to rise, whereupon he was started on mycophenolate mofetil.
“With these two forms of immunosuppression, the [liver function tests] did eventually normalize, although the steroids and mycophenolate had to be tapered over a period of many weeks. And this patient was not the only patient to experience toxicity; in fact, hepatotoxicity was frequent and often severe,” he said.
At the time of maximum incidence, week 4, the percentage of patients with any hepatotoxicity was 46%, with 13% at grade 4, and 21% at grade 3.
“The median time to initial development of hepatotoxicity is 28 days. This suggests that the mechanism of hepatotoxicity is not immediate, but takes time to develop, consistent with an adaptive immune response. Furthermore, hepatotoxicity is typically occurring before the first dose of ofatumumab is occurring at week 8, suggesting idelalisib alone is the cause of the hepatotoxicity,” Dr. Lampson said.
A comparison of data from the ongoing study and from three previous studies – two with idelalisib in relapsed refractory disease, and one as first-line therapy in patients 65 and older – showed that grade 3 or greater hepatotoxicity was lowest in a phase I trial of idelalisib in which patients had received a median of five prior lines of therapy, occurring in only 1.9% of patients. In contrast, in the current study, 52% of patients experienced grade 3 or 4 transaminitis at some point in the trial.
Evidence for the hepatotoxicity being an on-target immune-mediated effect comes from lymphocytic infiltrate on liver biopsy and lymphocytic colitis in idelalisib-treated patients. Additional evidence comes from the fact that the toxicity is both treatable and preventable with steroids, he said.
He cautioned that hepatotoxicity can recur rapidly when the drug is reintroduced.
“In general, our experience has been if idelalisib is resumed while the subject remains on steroids, the drug is more likely to be tolerated and the subject eventually can be tapered off steroids,” he said.
Asked by an audience member whether patients who are receiving idelalisib in the first-line setting should also receive steroids, Dr. Lampson said that they closely monitor patient liver enzymes around 28 days, and if grade 1 transaminitis is detected, patients are automatically started on low-dose steroids.
The study is sponsored by the Dana-Farber Cancer Institute in collaboration with Gilead Sciences and GlaxoSmithKline. Dr. Lampson and colleagues declared no relevant conflicts of interest.
AT ASH 2015
Key clinical point: Idelalisib in the first-line setting is associated with significant risk of hepatotoxicity, with a peak incidence at about 28 days of therapy.
Major finding: More than half (52%) of patients with newly diagnosed chronic lymphocytic leukemia had grade 3 or 4 hepatotoxicity with idelalisib monotherapy.
Data source: Ongoing phase II clinical trial with data on 24 patients.
Disclosures: The study is sponsored by the Dana-Farber Cancer Institute in collaboration with Gilead Sciences and GlaxoSmithKline. Dr. Lampson and colleagues declared no relevant conflicts of interest.
ASH: Prasugrel does not reduce vaso-occlusive crises in sickle cell anemia
Platelet inhibitor prasugrel has failed to show a significant reduction in the rate of vaso-occlusive crises events in children and adolescents with sickle cell anemia, according to data presented Dec. 8 at the annual meeting of the American Society of Hematology.
The phase III randomized placebo-controlled trial of 341 children and adolescents (aged 2-17 years), known as the Determining Effects of Platelet Inhibition on Vaso-Occlusive Events (DOVE) trial – simultaneously published in the New England Journal of Medicine – showed the rate of vaso-occlusive crises was 2.30 per person-year in the prasugrel group and 2.77 in the placebo group (rate ratio 0.83, 95% confidence interval 0.66-1.05, P = 0.12), with a slightly greater but still nonsignificant reduction among the older patients aged 12-17 years.
Treatment with prasugrel did not achieve any significant reductions in secondary outcomes of hospitalizations for vaso-occlusive crises, red-cell transfusions, pain rate or intensity, analgesic use, or school absences, compared with placebo. Platelet reactivity, however, was significantly lower in the prasugrel group (N Engl J Med. 2015, Dec 8. doi: 10.1056/NEJMoa1512021).
“Sickle cell anemia is a heterogeneous and complex disease in which platelet activation is only one of several mechanisms of vascular injury, which perhaps explains why prasugrel was ineffective,” wrote Dr. Matthew M. Heeney of Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, and his coauthors.
“However, the nonsignificant effect of prasugrel in the oldest age group may suggest that platelet activation is relatively more important in these older patients, a hypothesis that is consistent with the fact that endothelial dysfunction in sickle cell disease is progressive.”
Daiichi Sankyo and Eli Lilly funded the study. Several authors disclosed ties with Eli Lilly or other pharmaceutical companies. Three authors were employees of Eli Lilly, and one was an employee of Daiichi Sankyo.
Platelet inhibitor prasugrel has failed to show a significant reduction in the rate of vaso-occlusive crises events in children and adolescents with sickle cell anemia, according to data presented Dec. 8 at the annual meeting of the American Society of Hematology.
The phase III randomized placebo-controlled trial of 341 children and adolescents (aged 2-17 years), known as the Determining Effects of Platelet Inhibition on Vaso-Occlusive Events (DOVE) trial – simultaneously published in the New England Journal of Medicine – showed the rate of vaso-occlusive crises was 2.30 per person-year in the prasugrel group and 2.77 in the placebo group (rate ratio 0.83, 95% confidence interval 0.66-1.05, P = 0.12), with a slightly greater but still nonsignificant reduction among the older patients aged 12-17 years.
Treatment with prasugrel did not achieve any significant reductions in secondary outcomes of hospitalizations for vaso-occlusive crises, red-cell transfusions, pain rate or intensity, analgesic use, or school absences, compared with placebo. Platelet reactivity, however, was significantly lower in the prasugrel group (N Engl J Med. 2015, Dec 8. doi: 10.1056/NEJMoa1512021).
“Sickle cell anemia is a heterogeneous and complex disease in which platelet activation is only one of several mechanisms of vascular injury, which perhaps explains why prasugrel was ineffective,” wrote Dr. Matthew M. Heeney of Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, and his coauthors.
“However, the nonsignificant effect of prasugrel in the oldest age group may suggest that platelet activation is relatively more important in these older patients, a hypothesis that is consistent with the fact that endothelial dysfunction in sickle cell disease is progressive.”
Daiichi Sankyo and Eli Lilly funded the study. Several authors disclosed ties with Eli Lilly or other pharmaceutical companies. Three authors were employees of Eli Lilly, and one was an employee of Daiichi Sankyo.
Platelet inhibitor prasugrel has failed to show a significant reduction in the rate of vaso-occlusive crises events in children and adolescents with sickle cell anemia, according to data presented Dec. 8 at the annual meeting of the American Society of Hematology.
The phase III randomized placebo-controlled trial of 341 children and adolescents (aged 2-17 years), known as the Determining Effects of Platelet Inhibition on Vaso-Occlusive Events (DOVE) trial – simultaneously published in the New England Journal of Medicine – showed the rate of vaso-occlusive crises was 2.30 per person-year in the prasugrel group and 2.77 in the placebo group (rate ratio 0.83, 95% confidence interval 0.66-1.05, P = 0.12), with a slightly greater but still nonsignificant reduction among the older patients aged 12-17 years.
Treatment with prasugrel did not achieve any significant reductions in secondary outcomes of hospitalizations for vaso-occlusive crises, red-cell transfusions, pain rate or intensity, analgesic use, or school absences, compared with placebo. Platelet reactivity, however, was significantly lower in the prasugrel group (N Engl J Med. 2015, Dec 8. doi: 10.1056/NEJMoa1512021).
“Sickle cell anemia is a heterogeneous and complex disease in which platelet activation is only one of several mechanisms of vascular injury, which perhaps explains why prasugrel was ineffective,” wrote Dr. Matthew M. Heeney of Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, and his coauthors.
“However, the nonsignificant effect of prasugrel in the oldest age group may suggest that platelet activation is relatively more important in these older patients, a hypothesis that is consistent with the fact that endothelial dysfunction in sickle cell disease is progressive.”
Daiichi Sankyo and Eli Lilly funded the study. Several authors disclosed ties with Eli Lilly or other pharmaceutical companies. Three authors were employees of Eli Lilly, and one was an employee of Daiichi Sankyo.
FROM ASH 2015
Key clinical point: Platelet inhibitor prasugrel does not reduce the rate of vaso-occlusive crises events in young patients with sickle cell anemia.
Major finding: Prasugrel did not achieve a significant reduction in vaso-occlusive crises, compared with placebo.
Data source: A phase III randomized placebo-controlled trial of 341 children and adolescents with sickle cell anemia.
Disclosures: Daiichi Sankyo and Eli Lilly funded the study. Several authors disclosed ties with Eli Lilly or other pharmaceutical companies. Three authors were employees of Eli Lilly, and one was an employee of Daiichi Sankyo.
Analysis finds 28.8% prevalence of depression in residents
The estimated prevalence of depression or depressive symptoms was 28.8% among residents and interns worldwide in a meta-analysis of 54 studies of the issue, according to a report published online December 8 in JAMA.
The depression rate ranged from 20.9% to 43.2%, depending on the instrument used to assess symptoms. Eleven studies used the Beck Depression Inventory (BDI), 11 used the Center for Epidemiological Studies Depression Scale (CES-D), 8 used the two-item Primary Care Evaluation of Mental Disorders questionnaire (PRIME-MD), 7 used the nine-item Patient Health Questionnaire (PHQ-9), 4 used the Zung Self-Rating Depression Scale (SDS), 3 used the Harvard Department of Psychiatry/National Depression Screening Day Scale (HANDS), and 11 used other validated methods, said Dr. Douglas A. Mata of the department of pathology, Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
“It is important to note that the vast majority of participants were assessed through self-report inventories that measured depressive symptoms, rather than gold-standard diagnostic clinical interviews for major depressive disorder,” they said.
The meta-analysis included 31 cross-sectional and 23 longitudinal studies published in peer-reviewed journals since 1963 and involving 17,560 residents or interns in North America (35 studies), Asia (9 studies), Europe (5 studies), South America (4 studies), and Africa (1 study). When the results were pooled, the overall prevalence of depression or depressive symptoms was 28.8% (4,969 of 17,560 participants).
In a sensitivity analysis, no individual study affected the overall prevalence estimate by more than 1%. Further analyses showed no significant differences in the prevalence of depression between cross-sectional and longitudinal studies, between U.S. studies and those performed in other countries, between studies of nonsurgical residents only vs. studies of all types of residents, or between studies of interns only vs. studies of upper level residents only. This suggests that the underlying causes of depressive symptoms “are common to the residency experience,” Dr. Mata and his associates said (JAMA. 2015 Dec 8. doi: 10.1001/jama.2015.15845).
The prevalence of depression increased over time. Although this rise was characterized as modest, “it is notable, given efforts by the Accreditation Council for Graduate Medical Education, European Working Time Directive, and others to limit trainee duty hours and improve work conditions. [This] trend may reflect the medical community’s increased awareness of depression or developments external to medical education. Future studies should explore specific factors that may explain this trend,” the investigators said.
The study findings indicate that the long-term health of physicians may be affected, since depression has been linked to a higher risk of future depressive episodes and greater long-term morbidity. Patient care may also be affected, given the established association between physician depression and lower-quality care, they added.

|
Dr. Thomas L. Schwenk |
The meta-analysis by Mata et al. makes it clear that the extent of significant depressive symptomatology, if not overt clinical depression, among physicians-in-training is extraordinarily and unacceptably high. Relieving the burden of depression in these individuals is an issue of professional performance in addition to one of human compassion.
A national conversation about the fundamental structure and function of the graduate medical education system is long overdue, not unlike the discussion that reformed undergraduate medical education after the Flexner Report. The high burden of depressive symptoms among residents and interns has reached a crisis level. It is a marker for deeper and more profound problems in the medical education system, which require equally profound solutions.
Dr. Thomas L. Schwenk is at the University of Nevada, Reno. He reported having no relevant financial disclosures. Dr. Schwenk made these remarks in an editorial accompanying Dr. Mata’s report (JAMA 2015;314:2357-8).

|
Dr. Thomas L. Schwenk |
The meta-analysis by Mata et al. makes it clear that the extent of significant depressive symptomatology, if not overt clinical depression, among physicians-in-training is extraordinarily and unacceptably high. Relieving the burden of depression in these individuals is an issue of professional performance in addition to one of human compassion.
A national conversation about the fundamental structure and function of the graduate medical education system is long overdue, not unlike the discussion that reformed undergraduate medical education after the Flexner Report. The high burden of depressive symptoms among residents and interns has reached a crisis level. It is a marker for deeper and more profound problems in the medical education system, which require equally profound solutions.
Dr. Thomas L. Schwenk is at the University of Nevada, Reno. He reported having no relevant financial disclosures. Dr. Schwenk made these remarks in an editorial accompanying Dr. Mata’s report (JAMA 2015;314:2357-8).

|
Dr. Thomas L. Schwenk |
The meta-analysis by Mata et al. makes it clear that the extent of significant depressive symptomatology, if not overt clinical depression, among physicians-in-training is extraordinarily and unacceptably high. Relieving the burden of depression in these individuals is an issue of professional performance in addition to one of human compassion.
A national conversation about the fundamental structure and function of the graduate medical education system is long overdue, not unlike the discussion that reformed undergraduate medical education after the Flexner Report. The high burden of depressive symptoms among residents and interns has reached a crisis level. It is a marker for deeper and more profound problems in the medical education system, which require equally profound solutions.
Dr. Thomas L. Schwenk is at the University of Nevada, Reno. He reported having no relevant financial disclosures. Dr. Schwenk made these remarks in an editorial accompanying Dr. Mata’s report (JAMA 2015;314:2357-8).
The estimated prevalence of depression or depressive symptoms was 28.8% among residents and interns worldwide in a meta-analysis of 54 studies of the issue, according to a report published online December 8 in JAMA.
The depression rate ranged from 20.9% to 43.2%, depending on the instrument used to assess symptoms. Eleven studies used the Beck Depression Inventory (BDI), 11 used the Center for Epidemiological Studies Depression Scale (CES-D), 8 used the two-item Primary Care Evaluation of Mental Disorders questionnaire (PRIME-MD), 7 used the nine-item Patient Health Questionnaire (PHQ-9), 4 used the Zung Self-Rating Depression Scale (SDS), 3 used the Harvard Department of Psychiatry/National Depression Screening Day Scale (HANDS), and 11 used other validated methods, said Dr. Douglas A. Mata of the department of pathology, Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
“It is important to note that the vast majority of participants were assessed through self-report inventories that measured depressive symptoms, rather than gold-standard diagnostic clinical interviews for major depressive disorder,” they said.
The meta-analysis included 31 cross-sectional and 23 longitudinal studies published in peer-reviewed journals since 1963 and involving 17,560 residents or interns in North America (35 studies), Asia (9 studies), Europe (5 studies), South America (4 studies), and Africa (1 study). When the results were pooled, the overall prevalence of depression or depressive symptoms was 28.8% (4,969 of 17,560 participants).
In a sensitivity analysis, no individual study affected the overall prevalence estimate by more than 1%. Further analyses showed no significant differences in the prevalence of depression between cross-sectional and longitudinal studies, between U.S. studies and those performed in other countries, between studies of nonsurgical residents only vs. studies of all types of residents, or between studies of interns only vs. studies of upper level residents only. This suggests that the underlying causes of depressive symptoms “are common to the residency experience,” Dr. Mata and his associates said (JAMA. 2015 Dec 8. doi: 10.1001/jama.2015.15845).
The prevalence of depression increased over time. Although this rise was characterized as modest, “it is notable, given efforts by the Accreditation Council for Graduate Medical Education, European Working Time Directive, and others to limit trainee duty hours and improve work conditions. [This] trend may reflect the medical community’s increased awareness of depression or developments external to medical education. Future studies should explore specific factors that may explain this trend,” the investigators said.
The study findings indicate that the long-term health of physicians may be affected, since depression has been linked to a higher risk of future depressive episodes and greater long-term morbidity. Patient care may also be affected, given the established association between physician depression and lower-quality care, they added.
The estimated prevalence of depression or depressive symptoms was 28.8% among residents and interns worldwide in a meta-analysis of 54 studies of the issue, according to a report published online December 8 in JAMA.
The depression rate ranged from 20.9% to 43.2%, depending on the instrument used to assess symptoms. Eleven studies used the Beck Depression Inventory (BDI), 11 used the Center for Epidemiological Studies Depression Scale (CES-D), 8 used the two-item Primary Care Evaluation of Mental Disorders questionnaire (PRIME-MD), 7 used the nine-item Patient Health Questionnaire (PHQ-9), 4 used the Zung Self-Rating Depression Scale (SDS), 3 used the Harvard Department of Psychiatry/National Depression Screening Day Scale (HANDS), and 11 used other validated methods, said Dr. Douglas A. Mata of the department of pathology, Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
“It is important to note that the vast majority of participants were assessed through self-report inventories that measured depressive symptoms, rather than gold-standard diagnostic clinical interviews for major depressive disorder,” they said.
The meta-analysis included 31 cross-sectional and 23 longitudinal studies published in peer-reviewed journals since 1963 and involving 17,560 residents or interns in North America (35 studies), Asia (9 studies), Europe (5 studies), South America (4 studies), and Africa (1 study). When the results were pooled, the overall prevalence of depression or depressive symptoms was 28.8% (4,969 of 17,560 participants).
In a sensitivity analysis, no individual study affected the overall prevalence estimate by more than 1%. Further analyses showed no significant differences in the prevalence of depression between cross-sectional and longitudinal studies, between U.S. studies and those performed in other countries, between studies of nonsurgical residents only vs. studies of all types of residents, or between studies of interns only vs. studies of upper level residents only. This suggests that the underlying causes of depressive symptoms “are common to the residency experience,” Dr. Mata and his associates said (JAMA. 2015 Dec 8. doi: 10.1001/jama.2015.15845).
The prevalence of depression increased over time. Although this rise was characterized as modest, “it is notable, given efforts by the Accreditation Council for Graduate Medical Education, European Working Time Directive, and others to limit trainee duty hours and improve work conditions. [This] trend may reflect the medical community’s increased awareness of depression or developments external to medical education. Future studies should explore specific factors that may explain this trend,” the investigators said.
The study findings indicate that the long-term health of physicians may be affected, since depression has been linked to a higher risk of future depressive episodes and greater long-term morbidity. Patient care may also be affected, given the established association between physician depression and lower-quality care, they added.
FROM JAMA
Key clinical point: The prevalence of depression or depressive symptoms was 28.8% (range, 20.9%-43.2%) among residents in a meta-analysis of 54 studies.
Major finding: The overall prevalence of depression or depressive symptoms was 28.8% (4,969 of 17,560 participants) across all countries, all types of studies, and all types of graduate medical education programs.
Data source: A meta-analysis of 31 cross-sectional and 23 longitudinal studies involving 17,560 residents and interns worldwide.
Disclosures: This study was supported by the U.S. Department of State Fulbright Scholarship program, the National Institutes of Health, and the NIH Medical Scientist Training Program. Dr. Mata and his associates reported having no relevant financial disclosures.
Changing trends in diet pill use, from weight loss agent to recreational drug
The prevalence of obesity and obesity-related conditions in the United States is increasing. Many weight-loss products and dietary supplements are used in an attempt to combat this epidemic, but little evidence exists of their efficacy and safety.
We present a case report of a middle-age woman who developed severe psychotic symptoms while taking phentermine hydrochloride (HCl), a psychostimulant similar to amphetamine that is used as a weight-loss agent and for recreational purposes. Phentermine has been associated with mood and psychotic symptoms and has a tendency to cause psychological dependence and tolerance.
To investigate the risks and potential effects of using this drug, we searched OVID and PubMed databases using the search string “phentermine + psychosis.” We conclude that there is a need for awareness about early detection and treatment of reversible psychotic and mood symptoms caused by what might appear to be harmless weight-loss and energy pills.
Obesity epidemic, wide-ranging weight-loss effortsThere has been a dramatic increase in obesity in the United States in the past 20 years: More than one-third of adults and approximately 17% of children and adolescents are obese. Obesity-related conditions, such as heart disease, stroke, and type 2 diabetes mellitus, are leading causes of preventable death.1 Weight monitoring, a healthy lifestyle, surgical intervention, traditional herbs, and diet-pill supplements are some of the modalities used to address this epidemic.
Most so-called supplements for weight loss are exempt from FDA regulation. They do not undergo rigorous testing for safety. Furthermore, many contain controlled substances; some supplements are anti-seizure medications or other prescription drugs; and some are drugs not approved in the United States.2 Since the 1930s, such drugs as dinitrophenol, ephedrine, amphetamine, fenfluramine, and phentermine have flooded the market with the promise of quick weight loss.3,4
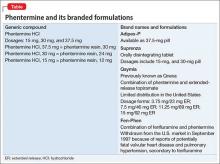
Phentermine, a contraction of “phenyltertiary-butylamine,” and its various types (Table) is a psychostimulant of the phenethylamine class, with a pharmacologic profile similar to that of amphetamine. It is known to yield false-positive immunoassay screening results for amphetamines.
CASE REPORT Acute psychotic break
Ms. B, age 37, with a history of postpartum depression, arrives at the emergency room reporting auditory hallucinations of her son and boyfriend; vivid visual hallucinations; and persecutory ideas toward her boyfriend, whom she believes had kidnapped her son. She also complains of insomnia and intermittent confusion for the past week.
Speech is pressured, fast, and difficult to comprehend at times; affect is labile and irritable. Ms. B denies suicidal ideation and is oriented to time, place, and person.
A urine drug screen is positive for amphetamine.
Pre-admission medications include alprazolam, 1 mg as needed, and zolpidem, 10 mg at bedtime, prescribed by Ms. B’s primary care physician for anxiety and insomnia. She discontinued these medications 3 weeks ago because of increased drowsiness at work. She denies other substance use and is unable to account for the positive urine drug screen.
Her medical history, physical examination, and a CT scan of the head are unremarkable. The components of a comprehensive metabolic panel and complete blood count are within normal limits.
After admission, in-depth assessment reveals that Ms. B has been taking phentermine, 37.5 mg (under the brand name Adipex-P), once daily since age 16 for weight loss. She recently discontinued the drug, abruptly, for 1 month, then resumed taking it at an unspecified higher dosage 1 week before she came to the emergency room, for what she said was recreational use and to meet the demands of her job, which required shift work and long hours.
Over the next few days in the hospital, Ms. B’s symptoms resolve as the drug is eliminated from her body. Speech becomes comprehensible and sleep improves. Affective distress diminishes considerably after admission; slight mood lability persists. She no longer reports perceptual disturbances or distress secondary to intrusive thoughts.
Ms. B is discharged 1 week after admission, with instructions to follow up at a dual-diagnosis outpatient program.
Pharmacologic profilePhentermine acts through sympathomimetic pathways by increasing brain noradrenaline and dopamine. The drug has no effect on serotonin.4,5 Phentermine can lead to elevated blood pressure and heart rate, palpitations, restlessness, and insomnia, and can suppress appetite. Increased sympathomimetic activity has been implicated in the ability of phentermine to induce psychotic symptoms.
The literature. Our PubMed search of “phentermine + psychosis” produced 13 results, including 6 case reports of phentermine use. Five citations were more than 4 decades old5-12; only 1 could be considered recent (2011).13
Patients in these reports developed psychotic or manic features after chronic or acute phentermine use, mainly for weight reduction. The most recent article13 mentioned 4 patients who were abusing diet pills recreationally (including “for lethargy”). As with Ms. B, in all 4 of those patients, phentermine precipitated the primary pathology (mania in bipolar disorder; depression in postpartum depression and substance abuse) or revealed underlying illness.
Changing landscape of use and abuseThere has been a trend observed in the pattern of diet pill use: Initially marketed as an appetite suppressant, these pills are now being abused across ethnic, racial, and socioeconomic groups, by males and females.14 There is also a scarcity of useful guidance for clinicians.
Not only are diet pills used by people with an eating disorder; their recreational use is an emerging problem. If reports12,13 continue to reveal that phentermine is a substance of abuse and has catastrophic effects on the user’s psyche, the need for stronger warnings and guidelines might be warranted to allow consumers to make an informed choice about using the drug.
Call for awarenessThe case we presented here exemplifies the importance of tighter regulation of both over-the-counter and prescription stimulant analogs. There is a need for awareness among practitioners about early detection and treatment of reversible psychotic and mood symptoms secondary to what might be promoted as, or appear to be, “harmless” weight loss and energy pills.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
This unfunded study was presented as a case report poster at the Annual Meeting of the Academy of Psychosomatic Medicine, November 2013, Tucson, Arizona, and at the Colloquium of Scholars of the Philadelphia Psychiatric Society, March 2014, Philadelphia, Pennsylvania.
Drug Brand Names
Alprazolam • Xanax
Fenfluramine • Pondimin
Phentermine HCl • Adipex-P, Fen-Phen, Qsymia, Suprenza
Topiramate • Topamax, Trokendi XR
Zolpidem • Ambien
1. Center for Disease Control. Division of nutrition, physical activity, and obesity. http://www.cdc.gov/obesity/data/adult. html. Updated September 14, 2015. Accessed October 27, 2015.
2. Retamero C, Rivera T, Murphy K. “Ephedra-free” diet pill-induced psychosis. Psychosomatics. 2011;52(6):579-582.
3. Cohen PA, Goday A, Swann JP. The return of rainbow diet pills. Am J Public Health. 2012;102(9):1676-1686.
4. Wellman PJ. Overview of adrenergic anorectic agents. Am J Clin Nutr. 1992;55(suppl 1):193S-198S.
5. Devan GS. Phentermine and psychosis. Br J Psychiatry. 1990;156:442-443.
6. Hoffman BF. Diet pill psychosis. Can Med Assoc J. 1977;116(4):351-355.
7. Hoffman BF. Diet pill psychosis: follow-up after 6 years. Can Med Assoc J. 1983;129(10):1077-1078.
8. Rubin RT. Acute psychotic reaction following ingestion of phentermine. Am J Psychiatry. 1964;120:1124-1125.
9. Schaffer CB, Pauli MW. Psychotic reaction caused by proprietary oral diet agent. Am J Psychiatry. 1980;137(10):1256-12567.
10. Lee SH, Liu CY, Yang YY. Schizophreniform-like psychotic disorder induced by phentermine: a case report. Zhonghua Yi Xue Za Zhi (Taipei). 1998;61(1):44-47.
11. Zimmer JE, Gregory RJ. Bipolar depression associated with fenfluramine and phentermine. J Clin Psychiatry. 1998;59(7):383-384.
12. Bagri S, Reddy G. Delirium with manic symptoms induced by diet pills. J Clin Psychiatry. 1998;59(2):83.
13. Alexander J, Cheng Y, Choudhary J, et al. Phentermine (Duromine) precipitated psychosis. Aust N Z J Psychiatry. 2011;45(8):684-685.
14. Pomeranz JL, Taylor LM, Austin SB. Over-the-counter and out-of-control: legal strategies to protect youths from abusing products for weight control. Am J Public Health. 2013;103(2):222-2253.
The prevalence of obesity and obesity-related conditions in the United States is increasing. Many weight-loss products and dietary supplements are used in an attempt to combat this epidemic, but little evidence exists of their efficacy and safety.
We present a case report of a middle-age woman who developed severe psychotic symptoms while taking phentermine hydrochloride (HCl), a psychostimulant similar to amphetamine that is used as a weight-loss agent and for recreational purposes. Phentermine has been associated with mood and psychotic symptoms and has a tendency to cause psychological dependence and tolerance.
To investigate the risks and potential effects of using this drug, we searched OVID and PubMed databases using the search string “phentermine + psychosis.” We conclude that there is a need for awareness about early detection and treatment of reversible psychotic and mood symptoms caused by what might appear to be harmless weight-loss and energy pills.
Obesity epidemic, wide-ranging weight-loss effortsThere has been a dramatic increase in obesity in the United States in the past 20 years: More than one-third of adults and approximately 17% of children and adolescents are obese. Obesity-related conditions, such as heart disease, stroke, and type 2 diabetes mellitus, are leading causes of preventable death.1 Weight monitoring, a healthy lifestyle, surgical intervention, traditional herbs, and diet-pill supplements are some of the modalities used to address this epidemic.
Most so-called supplements for weight loss are exempt from FDA regulation. They do not undergo rigorous testing for safety. Furthermore, many contain controlled substances; some supplements are anti-seizure medications or other prescription drugs; and some are drugs not approved in the United States.2 Since the 1930s, such drugs as dinitrophenol, ephedrine, amphetamine, fenfluramine, and phentermine have flooded the market with the promise of quick weight loss.3,4
Phentermine, a contraction of “phenyltertiary-butylamine,” and its various types (Table) is a psychostimulant of the phenethylamine class, with a pharmacologic profile similar to that of amphetamine. It is known to yield false-positive immunoassay screening results for amphetamines.
CASE REPORT Acute psychotic break
Ms. B, age 37, with a history of postpartum depression, arrives at the emergency room reporting auditory hallucinations of her son and boyfriend; vivid visual hallucinations; and persecutory ideas toward her boyfriend, whom she believes had kidnapped her son. She also complains of insomnia and intermittent confusion for the past week.
Speech is pressured, fast, and difficult to comprehend at times; affect is labile and irritable. Ms. B denies suicidal ideation and is oriented to time, place, and person.
A urine drug screen is positive for amphetamine.
Pre-admission medications include alprazolam, 1 mg as needed, and zolpidem, 10 mg at bedtime, prescribed by Ms. B’s primary care physician for anxiety and insomnia. She discontinued these medications 3 weeks ago because of increased drowsiness at work. She denies other substance use and is unable to account for the positive urine drug screen.
Her medical history, physical examination, and a CT scan of the head are unremarkable. The components of a comprehensive metabolic panel and complete blood count are within normal limits.
After admission, in-depth assessment reveals that Ms. B has been taking phentermine, 37.5 mg (under the brand name Adipex-P), once daily since age 16 for weight loss. She recently discontinued the drug, abruptly, for 1 month, then resumed taking it at an unspecified higher dosage 1 week before she came to the emergency room, for what she said was recreational use and to meet the demands of her job, which required shift work and long hours.
Over the next few days in the hospital, Ms. B’s symptoms resolve as the drug is eliminated from her body. Speech becomes comprehensible and sleep improves. Affective distress diminishes considerably after admission; slight mood lability persists. She no longer reports perceptual disturbances or distress secondary to intrusive thoughts.
Ms. B is discharged 1 week after admission, with instructions to follow up at a dual-diagnosis outpatient program.
Pharmacologic profilePhentermine acts through sympathomimetic pathways by increasing brain noradrenaline and dopamine. The drug has no effect on serotonin.4,5 Phentermine can lead to elevated blood pressure and heart rate, palpitations, restlessness, and insomnia, and can suppress appetite. Increased sympathomimetic activity has been implicated in the ability of phentermine to induce psychotic symptoms.
The literature. Our PubMed search of “phentermine + psychosis” produced 13 results, including 6 case reports of phentermine use. Five citations were more than 4 decades old5-12; only 1 could be considered recent (2011).13
Patients in these reports developed psychotic or manic features after chronic or acute phentermine use, mainly for weight reduction. The most recent article13 mentioned 4 patients who were abusing diet pills recreationally (including “for lethargy”). As with Ms. B, in all 4 of those patients, phentermine precipitated the primary pathology (mania in bipolar disorder; depression in postpartum depression and substance abuse) or revealed underlying illness.
Changing landscape of use and abuseThere has been a trend observed in the pattern of diet pill use: Initially marketed as an appetite suppressant, these pills are now being abused across ethnic, racial, and socioeconomic groups, by males and females.14 There is also a scarcity of useful guidance for clinicians.
Not only are diet pills used by people with an eating disorder; their recreational use is an emerging problem. If reports12,13 continue to reveal that phentermine is a substance of abuse and has catastrophic effects on the user’s psyche, the need for stronger warnings and guidelines might be warranted to allow consumers to make an informed choice about using the drug.
Call for awarenessThe case we presented here exemplifies the importance of tighter regulation of both over-the-counter and prescription stimulant analogs. There is a need for awareness among practitioners about early detection and treatment of reversible psychotic and mood symptoms secondary to what might be promoted as, or appear to be, “harmless” weight loss and energy pills.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
This unfunded study was presented as a case report poster at the Annual Meeting of the Academy of Psychosomatic Medicine, November 2013, Tucson, Arizona, and at the Colloquium of Scholars of the Philadelphia Psychiatric Society, March 2014, Philadelphia, Pennsylvania.
Drug Brand Names
Alprazolam • Xanax
Fenfluramine • Pondimin
Phentermine HCl • Adipex-P, Fen-Phen, Qsymia, Suprenza
Topiramate • Topamax, Trokendi XR
Zolpidem • Ambien
The prevalence of obesity and obesity-related conditions in the United States is increasing. Many weight-loss products and dietary supplements are used in an attempt to combat this epidemic, but little evidence exists of their efficacy and safety.
We present a case report of a middle-age woman who developed severe psychotic symptoms while taking phentermine hydrochloride (HCl), a psychostimulant similar to amphetamine that is used as a weight-loss agent and for recreational purposes. Phentermine has been associated with mood and psychotic symptoms and has a tendency to cause psychological dependence and tolerance.
To investigate the risks and potential effects of using this drug, we searched OVID and PubMed databases using the search string “phentermine + psychosis.” We conclude that there is a need for awareness about early detection and treatment of reversible psychotic and mood symptoms caused by what might appear to be harmless weight-loss and energy pills.
Obesity epidemic, wide-ranging weight-loss effortsThere has been a dramatic increase in obesity in the United States in the past 20 years: More than one-third of adults and approximately 17% of children and adolescents are obese. Obesity-related conditions, such as heart disease, stroke, and type 2 diabetes mellitus, are leading causes of preventable death.1 Weight monitoring, a healthy lifestyle, surgical intervention, traditional herbs, and diet-pill supplements are some of the modalities used to address this epidemic.
Most so-called supplements for weight loss are exempt from FDA regulation. They do not undergo rigorous testing for safety. Furthermore, many contain controlled substances; some supplements are anti-seizure medications or other prescription drugs; and some are drugs not approved in the United States.2 Since the 1930s, such drugs as dinitrophenol, ephedrine, amphetamine, fenfluramine, and phentermine have flooded the market with the promise of quick weight loss.3,4
Phentermine, a contraction of “phenyltertiary-butylamine,” and its various types (Table) is a psychostimulant of the phenethylamine class, with a pharmacologic profile similar to that of amphetamine. It is known to yield false-positive immunoassay screening results for amphetamines.
CASE REPORT Acute psychotic break
Ms. B, age 37, with a history of postpartum depression, arrives at the emergency room reporting auditory hallucinations of her son and boyfriend; vivid visual hallucinations; and persecutory ideas toward her boyfriend, whom she believes had kidnapped her son. She also complains of insomnia and intermittent confusion for the past week.
Speech is pressured, fast, and difficult to comprehend at times; affect is labile and irritable. Ms. B denies suicidal ideation and is oriented to time, place, and person.
A urine drug screen is positive for amphetamine.
Pre-admission medications include alprazolam, 1 mg as needed, and zolpidem, 10 mg at bedtime, prescribed by Ms. B’s primary care physician for anxiety and insomnia. She discontinued these medications 3 weeks ago because of increased drowsiness at work. She denies other substance use and is unable to account for the positive urine drug screen.
Her medical history, physical examination, and a CT scan of the head are unremarkable. The components of a comprehensive metabolic panel and complete blood count are within normal limits.
After admission, in-depth assessment reveals that Ms. B has been taking phentermine, 37.5 mg (under the brand name Adipex-P), once daily since age 16 for weight loss. She recently discontinued the drug, abruptly, for 1 month, then resumed taking it at an unspecified higher dosage 1 week before she came to the emergency room, for what she said was recreational use and to meet the demands of her job, which required shift work and long hours.
Over the next few days in the hospital, Ms. B’s symptoms resolve as the drug is eliminated from her body. Speech becomes comprehensible and sleep improves. Affective distress diminishes considerably after admission; slight mood lability persists. She no longer reports perceptual disturbances or distress secondary to intrusive thoughts.
Ms. B is discharged 1 week after admission, with instructions to follow up at a dual-diagnosis outpatient program.
Pharmacologic profilePhentermine acts through sympathomimetic pathways by increasing brain noradrenaline and dopamine. The drug has no effect on serotonin.4,5 Phentermine can lead to elevated blood pressure and heart rate, palpitations, restlessness, and insomnia, and can suppress appetite. Increased sympathomimetic activity has been implicated in the ability of phentermine to induce psychotic symptoms.
The literature. Our PubMed search of “phentermine + psychosis” produced 13 results, including 6 case reports of phentermine use. Five citations were more than 4 decades old5-12; only 1 could be considered recent (2011).13
Patients in these reports developed psychotic or manic features after chronic or acute phentermine use, mainly for weight reduction. The most recent article13 mentioned 4 patients who were abusing diet pills recreationally (including “for lethargy”). As with Ms. B, in all 4 of those patients, phentermine precipitated the primary pathology (mania in bipolar disorder; depression in postpartum depression and substance abuse) or revealed underlying illness.
Changing landscape of use and abuseThere has been a trend observed in the pattern of diet pill use: Initially marketed as an appetite suppressant, these pills are now being abused across ethnic, racial, and socioeconomic groups, by males and females.14 There is also a scarcity of useful guidance for clinicians.
Not only are diet pills used by people with an eating disorder; their recreational use is an emerging problem. If reports12,13 continue to reveal that phentermine is a substance of abuse and has catastrophic effects on the user’s psyche, the need for stronger warnings and guidelines might be warranted to allow consumers to make an informed choice about using the drug.
Call for awarenessThe case we presented here exemplifies the importance of tighter regulation of both over-the-counter and prescription stimulant analogs. There is a need for awareness among practitioners about early detection and treatment of reversible psychotic and mood symptoms secondary to what might be promoted as, or appear to be, “harmless” weight loss and energy pills.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
This unfunded study was presented as a case report poster at the Annual Meeting of the Academy of Psychosomatic Medicine, November 2013, Tucson, Arizona, and at the Colloquium of Scholars of the Philadelphia Psychiatric Society, March 2014, Philadelphia, Pennsylvania.
Drug Brand Names
Alprazolam • Xanax
Fenfluramine • Pondimin
Phentermine HCl • Adipex-P, Fen-Phen, Qsymia, Suprenza
Topiramate • Topamax, Trokendi XR
Zolpidem • Ambien
1. Center for Disease Control. Division of nutrition, physical activity, and obesity. http://www.cdc.gov/obesity/data/adult. html. Updated September 14, 2015. Accessed October 27, 2015.
2. Retamero C, Rivera T, Murphy K. “Ephedra-free” diet pill-induced psychosis. Psychosomatics. 2011;52(6):579-582.
3. Cohen PA, Goday A, Swann JP. The return of rainbow diet pills. Am J Public Health. 2012;102(9):1676-1686.
4. Wellman PJ. Overview of adrenergic anorectic agents. Am J Clin Nutr. 1992;55(suppl 1):193S-198S.
5. Devan GS. Phentermine and psychosis. Br J Psychiatry. 1990;156:442-443.
6. Hoffman BF. Diet pill psychosis. Can Med Assoc J. 1977;116(4):351-355.
7. Hoffman BF. Diet pill psychosis: follow-up after 6 years. Can Med Assoc J. 1983;129(10):1077-1078.
8. Rubin RT. Acute psychotic reaction following ingestion of phentermine. Am J Psychiatry. 1964;120:1124-1125.
9. Schaffer CB, Pauli MW. Psychotic reaction caused by proprietary oral diet agent. Am J Psychiatry. 1980;137(10):1256-12567.
10. Lee SH, Liu CY, Yang YY. Schizophreniform-like psychotic disorder induced by phentermine: a case report. Zhonghua Yi Xue Za Zhi (Taipei). 1998;61(1):44-47.
11. Zimmer JE, Gregory RJ. Bipolar depression associated with fenfluramine and phentermine. J Clin Psychiatry. 1998;59(7):383-384.
12. Bagri S, Reddy G. Delirium with manic symptoms induced by diet pills. J Clin Psychiatry. 1998;59(2):83.
13. Alexander J, Cheng Y, Choudhary J, et al. Phentermine (Duromine) precipitated psychosis. Aust N Z J Psychiatry. 2011;45(8):684-685.
14. Pomeranz JL, Taylor LM, Austin SB. Over-the-counter and out-of-control: legal strategies to protect youths from abusing products for weight control. Am J Public Health. 2013;103(2):222-2253.
1. Center for Disease Control. Division of nutrition, physical activity, and obesity. http://www.cdc.gov/obesity/data/adult. html. Updated September 14, 2015. Accessed October 27, 2015.
2. Retamero C, Rivera T, Murphy K. “Ephedra-free” diet pill-induced psychosis. Psychosomatics. 2011;52(6):579-582.
3. Cohen PA, Goday A, Swann JP. The return of rainbow diet pills. Am J Public Health. 2012;102(9):1676-1686.
4. Wellman PJ. Overview of adrenergic anorectic agents. Am J Clin Nutr. 1992;55(suppl 1):193S-198S.
5. Devan GS. Phentermine and psychosis. Br J Psychiatry. 1990;156:442-443.
6. Hoffman BF. Diet pill psychosis. Can Med Assoc J. 1977;116(4):351-355.
7. Hoffman BF. Diet pill psychosis: follow-up after 6 years. Can Med Assoc J. 1983;129(10):1077-1078.
8. Rubin RT. Acute psychotic reaction following ingestion of phentermine. Am J Psychiatry. 1964;120:1124-1125.
9. Schaffer CB, Pauli MW. Psychotic reaction caused by proprietary oral diet agent. Am J Psychiatry. 1980;137(10):1256-12567.
10. Lee SH, Liu CY, Yang YY. Schizophreniform-like psychotic disorder induced by phentermine: a case report. Zhonghua Yi Xue Za Zhi (Taipei). 1998;61(1):44-47.
11. Zimmer JE, Gregory RJ. Bipolar depression associated with fenfluramine and phentermine. J Clin Psychiatry. 1998;59(7):383-384.
12. Bagri S, Reddy G. Delirium with manic symptoms induced by diet pills. J Clin Psychiatry. 1998;59(2):83.
13. Alexander J, Cheng Y, Choudhary J, et al. Phentermine (Duromine) precipitated psychosis. Aust N Z J Psychiatry. 2011;45(8):684-685.
14. Pomeranz JL, Taylor LM, Austin SB. Over-the-counter and out-of-control: legal strategies to protect youths from abusing products for weight control. Am J Public Health. 2013;103(2):222-2253.
VIDEO: ASH highlights five Choosing Wisely initiatives
ORLANDO – Five “Choosing Wisely” initiatives selected from other specialties were featured at the annual meeting of the American Society of Hematology.
Dr. Lisa Hicks of St. Michael’s Hospital in Toronto led ASH’s Choosing Wisely list and moderated their presentation and the discussion of this year’s recommendations at the meeting. In a video interview, Dr. Hicks discussed the five recommendations and how hematologists can influence better patient care through cross-specialty collaborations. The complete Choosing Wisely list is available at www.hematology.org/choosingwisely
The 2015 Choosing Wisely recommendations, selected from recommendations made previously by other organizations, are:
• Don’t image for suspected pulmonary embolism (PE) without moderate or high pre-test probability of PE. (American College of Radiology)
• Don’t perform repetitive CBC and chemistry testing in the face of clinical and lab stability. (Society of Hospital Medicine)
• Don’t routinely order thrombophilia testing on patients undergoing a routine infertility evaluation. (American Society of Reproductive Medicine)
• Don’t transfuse red blood cells for iron deficiency without hemodynamic instability. (American Association of Blood Banks)
• Avoid using PET or PET-CT scanning as part of routine follow-up care to monitor for a cancer recurrence in asymptomatic patients who have finished initial treatment to eliminate the cancer unless there is high-level evidence that such imaging will change the outcome. (American Society of Clinical Oncology)
Choosing Wisely is an initiative of the ABIM Foundation. Dr. Hicks had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Five “Choosing Wisely” initiatives selected from other specialties were featured at the annual meeting of the American Society of Hematology.
Dr. Lisa Hicks of St. Michael’s Hospital in Toronto led ASH’s Choosing Wisely list and moderated their presentation and the discussion of this year’s recommendations at the meeting. In a video interview, Dr. Hicks discussed the five recommendations and how hematologists can influence better patient care through cross-specialty collaborations. The complete Choosing Wisely list is available at www.hematology.org/choosingwisely
The 2015 Choosing Wisely recommendations, selected from recommendations made previously by other organizations, are:
• Don’t image for suspected pulmonary embolism (PE) without moderate or high pre-test probability of PE. (American College of Radiology)
• Don’t perform repetitive CBC and chemistry testing in the face of clinical and lab stability. (Society of Hospital Medicine)
• Don’t routinely order thrombophilia testing on patients undergoing a routine infertility evaluation. (American Society of Reproductive Medicine)
• Don’t transfuse red blood cells for iron deficiency without hemodynamic instability. (American Association of Blood Banks)
• Avoid using PET or PET-CT scanning as part of routine follow-up care to monitor for a cancer recurrence in asymptomatic patients who have finished initial treatment to eliminate the cancer unless there is high-level evidence that such imaging will change the outcome. (American Society of Clinical Oncology)
Choosing Wisely is an initiative of the ABIM Foundation. Dr. Hicks had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Five “Choosing Wisely” initiatives selected from other specialties were featured at the annual meeting of the American Society of Hematology.
Dr. Lisa Hicks of St. Michael’s Hospital in Toronto led ASH’s Choosing Wisely list and moderated their presentation and the discussion of this year’s recommendations at the meeting. In a video interview, Dr. Hicks discussed the five recommendations and how hematologists can influence better patient care through cross-specialty collaborations. The complete Choosing Wisely list is available at www.hematology.org/choosingwisely
The 2015 Choosing Wisely recommendations, selected from recommendations made previously by other organizations, are:
• Don’t image for suspected pulmonary embolism (PE) without moderate or high pre-test probability of PE. (American College of Radiology)
• Don’t perform repetitive CBC and chemistry testing in the face of clinical and lab stability. (Society of Hospital Medicine)
• Don’t routinely order thrombophilia testing on patients undergoing a routine infertility evaluation. (American Society of Reproductive Medicine)
• Don’t transfuse red blood cells for iron deficiency without hemodynamic instability. (American Association of Blood Banks)
• Avoid using PET or PET-CT scanning as part of routine follow-up care to monitor for a cancer recurrence in asymptomatic patients who have finished initial treatment to eliminate the cancer unless there is high-level evidence that such imaging will change the outcome. (American Society of Clinical Oncology)
Choosing Wisely is an initiative of the ABIM Foundation. Dr. Hicks had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2015
AHA: COPD doubles sudden cardiac death risk in hypertensives
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Two large studies link chronic obstructive pulmonary disease with increased risk of sudden cardiac death.
Major finding: Patients with COPD and hypertension had nearly a twofold increased risk of sudden cardiac death, compared with hypertensives without the pulmonary disease.
Data source: This was a secondary analysis comparing sudden cardiac death rates in 385 hypertensive patients with and nearly 12,000 without COPD, all participants in the LIFE trial.
Disclosures: The presenter reported serving as a consultant to Novartis.
RBC storage duration doesn’t affect outcomes in kids

Photo by Elise Amendola
ORLANDO, FL—The storage duration of red blood cells (RBCs) doesn’t affect transfusion outcomes in children with lactic acidosis due to severe anemia, according to a new study.
Investigators found no significant differences in lactate levels, 30-day recovery, survival, or adverse events between children who received RBCs stored for 25 to 35 days and children who received RBCs stored for 1 to 10 days.
These results were published in JAMA and presented at the 2015 ASH Annual Meeting as abstract 769.
Christine Cserti-Gazdewich, MD, of University Health Network in Toronto, Canada, provided insights on the data during a press conference at ASH.
A concern of the investigators, according to Dr Cserti-Gazdewich, was that previous studies on blood storage were conducted in “high-income countries in high-technology care settings with blood inventory wealth, and findings may not be generalizable to the other half of the world.”
She pointed out that, in less developed countries, the shortfall in blood availability compared to the need is up to 3-fold.
So the investigators examined whether longer-stored red blood cells actually deliver oxygen in a manner not worse than shorter-stored or fresh blood and examined it at the extremes of storage duration in the context of a very high-dose need.
The team evaluated 290 children (aged 6 months to 60 months) with elevated blood lactate levels due to severe anemia who presented at a university-affiliated national referral hospital in Kampala, Uganda.
The children were randomized to receive RBC units stored for 25 to 35 days (longer-storage group, n=145) or RBCs stored for 1 to 10 days (shorter-storage group, n=145).
All units were leukoreduced prior to storage. All patients received 10 mL/kg of RBCs during hours 0 through 2 and, if indicated per protocol, an additional 10 mL/kg during hours 4 through 6.
In the entire population, the mean presenting hemoglobin level was 3.7 g/dL, and the mean lactate level was 9.3 mmol/L. The median RBC unit storage duration was 8 days (range, 7-9) for the shorter-storage group and 32 days (range, 30-34) for the longer-storage group.
Results
The investigators found that RBC units maintained under standard storage conditions for 25 to 35 days were not inferior to RBC units stored for up to 10 days.
The study’s primary endpoint was the proportion of patients with a lactate level of 3 mmol/L or lower at 8 hours, using a margin of noninferiority equal to an absolute difference of 25%.
The proportion of patients meeting this endpoint was 0.61 in the longer-storage group and 0.58 in the shorter-storage group (P<0.001 for noninferiority).
Average lactate levels were not statistically different between the 2 groups at 0, 2, 4, 6, 8, or 24 hours. And there was no statistical difference in the median time to achieve a blood lactate of 3 mmol/L or lower at 4 hours (hazard ratio=0.99, P=0.92).
Cerebral tissue oxygen saturation rose significantly during transfusion, but there was no significant difference between the 2 storage groups. The median area under the curve of cerebral tissue oxygen saturation during transfusion was 679 (range, 334-1156) for the longer-storage group and 521 (range, 303-835) for the shorter-storage group (P=0.25).
There was no significant difference between the longer-storage group and the shorter-storage group in the persistence of stupor or coma 8 hours after transfusion (12.6% and 19.6%, respectively, P=0.11) or the persistence of respiratory distress at 8 hours (28.7% and 30%, respectively, P=0.79).
The median length of hospital stay was 4 days (range, 2-6) in the longer-storage group and 4 days (range, 3-7) in the shorter-storage group.
There were 8 deaths, 3 in the longer-storage group and 5 in the shorter-storage group, during the 24 hours from the start of transfusion. Four additional patients, 2 in each group, died in the hospital after the initial 24-hour observation period.
The proportion of patients who had returned to good health by 30 days was 86% of the longer-storage group and 93% of the shorter-storage group (P=0.13).
“By every single measure we explored, and we explored many, we found that long-stored blood was not inferior . . . ,” Dr Csert-Gazdewich said. “We truly found no justification to shorten the current storage duration of red cells as judged by the fundamental role to deliver oxygen.” ![]()

Photo by Elise Amendola
ORLANDO, FL—The storage duration of red blood cells (RBCs) doesn’t affect transfusion outcomes in children with lactic acidosis due to severe anemia, according to a new study.
Investigators found no significant differences in lactate levels, 30-day recovery, survival, or adverse events between children who received RBCs stored for 25 to 35 days and children who received RBCs stored for 1 to 10 days.
These results were published in JAMA and presented at the 2015 ASH Annual Meeting as abstract 769.
Christine Cserti-Gazdewich, MD, of University Health Network in Toronto, Canada, provided insights on the data during a press conference at ASH.
A concern of the investigators, according to Dr Cserti-Gazdewich, was that previous studies on blood storage were conducted in “high-income countries in high-technology care settings with blood inventory wealth, and findings may not be generalizable to the other half of the world.”
She pointed out that, in less developed countries, the shortfall in blood availability compared to the need is up to 3-fold.
So the investigators examined whether longer-stored red blood cells actually deliver oxygen in a manner not worse than shorter-stored or fresh blood and examined it at the extremes of storage duration in the context of a very high-dose need.
The team evaluated 290 children (aged 6 months to 60 months) with elevated blood lactate levels due to severe anemia who presented at a university-affiliated national referral hospital in Kampala, Uganda.
The children were randomized to receive RBC units stored for 25 to 35 days (longer-storage group, n=145) or RBCs stored for 1 to 10 days (shorter-storage group, n=145).
All units were leukoreduced prior to storage. All patients received 10 mL/kg of RBCs during hours 0 through 2 and, if indicated per protocol, an additional 10 mL/kg during hours 4 through 6.
In the entire population, the mean presenting hemoglobin level was 3.7 g/dL, and the mean lactate level was 9.3 mmol/L. The median RBC unit storage duration was 8 days (range, 7-9) for the shorter-storage group and 32 days (range, 30-34) for the longer-storage group.
Results
The investigators found that RBC units maintained under standard storage conditions for 25 to 35 days were not inferior to RBC units stored for up to 10 days.
The study’s primary endpoint was the proportion of patients with a lactate level of 3 mmol/L or lower at 8 hours, using a margin of noninferiority equal to an absolute difference of 25%.
The proportion of patients meeting this endpoint was 0.61 in the longer-storage group and 0.58 in the shorter-storage group (P<0.001 for noninferiority).
Average lactate levels were not statistically different between the 2 groups at 0, 2, 4, 6, 8, or 24 hours. And there was no statistical difference in the median time to achieve a blood lactate of 3 mmol/L or lower at 4 hours (hazard ratio=0.99, P=0.92).
Cerebral tissue oxygen saturation rose significantly during transfusion, but there was no significant difference between the 2 storage groups. The median area under the curve of cerebral tissue oxygen saturation during transfusion was 679 (range, 334-1156) for the longer-storage group and 521 (range, 303-835) for the shorter-storage group (P=0.25).
There was no significant difference between the longer-storage group and the shorter-storage group in the persistence of stupor or coma 8 hours after transfusion (12.6% and 19.6%, respectively, P=0.11) or the persistence of respiratory distress at 8 hours (28.7% and 30%, respectively, P=0.79).
The median length of hospital stay was 4 days (range, 2-6) in the longer-storage group and 4 days (range, 3-7) in the shorter-storage group.
There were 8 deaths, 3 in the longer-storage group and 5 in the shorter-storage group, during the 24 hours from the start of transfusion. Four additional patients, 2 in each group, died in the hospital after the initial 24-hour observation period.
The proportion of patients who had returned to good health by 30 days was 86% of the longer-storage group and 93% of the shorter-storage group (P=0.13).
“By every single measure we explored, and we explored many, we found that long-stored blood was not inferior . . . ,” Dr Csert-Gazdewich said. “We truly found no justification to shorten the current storage duration of red cells as judged by the fundamental role to deliver oxygen.” ![]()

Photo by Elise Amendola
ORLANDO, FL—The storage duration of red blood cells (RBCs) doesn’t affect transfusion outcomes in children with lactic acidosis due to severe anemia, according to a new study.
Investigators found no significant differences in lactate levels, 30-day recovery, survival, or adverse events between children who received RBCs stored for 25 to 35 days and children who received RBCs stored for 1 to 10 days.
These results were published in JAMA and presented at the 2015 ASH Annual Meeting as abstract 769.
Christine Cserti-Gazdewich, MD, of University Health Network in Toronto, Canada, provided insights on the data during a press conference at ASH.
A concern of the investigators, according to Dr Cserti-Gazdewich, was that previous studies on blood storage were conducted in “high-income countries in high-technology care settings with blood inventory wealth, and findings may not be generalizable to the other half of the world.”
She pointed out that, in less developed countries, the shortfall in blood availability compared to the need is up to 3-fold.
So the investigators examined whether longer-stored red blood cells actually deliver oxygen in a manner not worse than shorter-stored or fresh blood and examined it at the extremes of storage duration in the context of a very high-dose need.
The team evaluated 290 children (aged 6 months to 60 months) with elevated blood lactate levels due to severe anemia who presented at a university-affiliated national referral hospital in Kampala, Uganda.
The children were randomized to receive RBC units stored for 25 to 35 days (longer-storage group, n=145) or RBCs stored for 1 to 10 days (shorter-storage group, n=145).
All units were leukoreduced prior to storage. All patients received 10 mL/kg of RBCs during hours 0 through 2 and, if indicated per protocol, an additional 10 mL/kg during hours 4 through 6.
In the entire population, the mean presenting hemoglobin level was 3.7 g/dL, and the mean lactate level was 9.3 mmol/L. The median RBC unit storage duration was 8 days (range, 7-9) for the shorter-storage group and 32 days (range, 30-34) for the longer-storage group.
Results
The investigators found that RBC units maintained under standard storage conditions for 25 to 35 days were not inferior to RBC units stored for up to 10 days.
The study’s primary endpoint was the proportion of patients with a lactate level of 3 mmol/L or lower at 8 hours, using a margin of noninferiority equal to an absolute difference of 25%.
The proportion of patients meeting this endpoint was 0.61 in the longer-storage group and 0.58 in the shorter-storage group (P<0.001 for noninferiority).
Average lactate levels were not statistically different between the 2 groups at 0, 2, 4, 6, 8, or 24 hours. And there was no statistical difference in the median time to achieve a blood lactate of 3 mmol/L or lower at 4 hours (hazard ratio=0.99, P=0.92).
Cerebral tissue oxygen saturation rose significantly during transfusion, but there was no significant difference between the 2 storage groups. The median area under the curve of cerebral tissue oxygen saturation during transfusion was 679 (range, 334-1156) for the longer-storage group and 521 (range, 303-835) for the shorter-storage group (P=0.25).
There was no significant difference between the longer-storage group and the shorter-storage group in the persistence of stupor or coma 8 hours after transfusion (12.6% and 19.6%, respectively, P=0.11) or the persistence of respiratory distress at 8 hours (28.7% and 30%, respectively, P=0.79).
The median length of hospital stay was 4 days (range, 2-6) in the longer-storage group and 4 days (range, 3-7) in the shorter-storage group.
There were 8 deaths, 3 in the longer-storage group and 5 in the shorter-storage group, during the 24 hours from the start of transfusion. Four additional patients, 2 in each group, died in the hospital after the initial 24-hour observation period.
The proportion of patients who had returned to good health by 30 days was 86% of the longer-storage group and 93% of the shorter-storage group (P=0.13).
“By every single measure we explored, and we explored many, we found that long-stored blood was not inferior . . . ,” Dr Csert-Gazdewich said. “We truly found no justification to shorten the current storage duration of red cells as judged by the fundamental role to deliver oxygen.” ![]()