User login
ASH: First-line ibrutinib beats standard chemo for CLL/SLL in older patients
ORLANDO – Monotherapy with ibrutinib (Imbruvica) prolonged survival longer than did standard chemotherapy using chlorambucil (Leukeran) in the front-line treatment of older patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in the phase III RESONATE-2 study.
Co–drug developers Pharmacyclics and Janssen Biotech announced this summer that ibrutinib, an orally bioavailable, small-molecule inhibitor of Bruton’s tyrosine kinase, had achieved its primary and secondary endpoints.
But the first full look at the data at the annual meeting of the American Society of Hematology showed ibrutinib reduced the risk of progression or death by 84% by independent review compared with chlorambucil, which has been a standard first-line therapy in older CLL patients.
The results were simultaneously published in the New England Journal of Medicine (doi: 10.1056/NEJMoa1509388).
With a median follow-up of 18.4 months, median progression-free survival (PFS) had not been reached with ibrutinib vs. 19 months with chlorambucil (hazard ratio, 0.16; P less than .001).
By investigator assessment, ibrutinib reduced the risk of progression by 91%, with an 18-month PFS rate of 94% vs. 45% with chlorambucil (HR, 0.09; P less than .001).
The PFS benefit with ibrutinib was consistent regardless of patient age, Rai stage, ECOG (Eastern Cooperative Oncology Group) status, bulky disease, and importantly, such high-risk markers as chromosome 11q deletion and unmutated immunoglobulin heavy chain variable (IGHV) mutation status, study author Dr. Alessandra Tedeschi, of Hospital Niguarda Cà Granda, Milan, , said at a press briefing highlighting the study (Ab. 485).
In addition, ibrutinib led to an 84% reduction in the risk of death compared with chlorambucil (HR, 0.16; P = .001). The 24-month overall survival rate was 98% with ibrutinib versus 85% with chlorambucil.
Single-agent ibrutinib was approved in 2014 for patients with CLL who had received at least one prior therapy and for all patients with the deleterious 17p deletion on the basis of the phase III RESONATE trial in relapsed or refractory CLL.
Three-year follow-up in the phase II PCYC-1102 study signaled a benefit with ibrutinib in treatment-naive CLL, showing an overall response rate of 84%, 30-month PFS of 96%, and overall survival rate of 97% in a subset of 31 patients at least 65 years old (Blood. 2015 Apr 16;125[16]:2497-506).
“The phase III RESONATE-2 trial confirms the efficacy of ibrutinib in treatment-naive CLL patients, leading to a 91% reduction in risk of progression and 84% reduction in risk of death when compared to chlorambucil,” Dr. Tedeschi said.
In all, 269 patients, median age of 73 years, were evenly randomized to once-daily ibrutinib 420 mg until progression or unacceptable toxicity or chlorambucil 0.5 mg/kg (up to a maximum of 0.8 mg/kg) on days 1 and 15 of a 28-day cycle for up to 12 cycles. Patients with the deleterious 17p deletion were excluded, as single-agent chlorambucil is not effective in this population.
Ibrutinib significantly improved bone marrow function, as reflected by a sustained increase in hemoglobin and platelets.
“This is very important in this category of elderly patients, in whom bone marrow failure is the most common cause of morbidity,” Dr. Tedeschi said.
There were 3 deaths on the ibrutinib arm and 17 on the chlorambucil arm.
The majority of patients (87%) in this older population with frequent comorbidities was able to continue on oral, once-daily ibrutinib with a median of 1.5 years of follow-up, she said.
The most common adverse events on ibrutinib were grade one diarrhea, fatigue, cough, and nausea that did not result in treatment discontinuation. On the chlorambucil arm, fatigue nausea, vomiting, and cytopenias occurred more frequently than with ibrutinib.
Grade 3 maculopapular rash occurred in 3% with ibrutinib and 2% with chlorambucil, she said.
Ibrutinib was associated with higher and not insignificant rates of atrial fibrillation and major hemorrhage compared with chlorambucil, said Dr. Brian T. Hill of the Taussig Cancer Institute at the Cleveland Clinic, who was not involved in the study. In our interview, Dr. Hill also questions the relevance today of chlorambucil monotherapy as the comparator arm in RESONATE-2.
Pharmacyclics, which is jointly developing ibrutinib with Janssen Biotech, sponsored the study. Dr. Tedeschi reported having nothing to disclose. Several coauthors reported relationships with Pharmacyclics and Janssen.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Monotherapy with ibrutinib (Imbruvica) prolonged survival longer than did standard chemotherapy using chlorambucil (Leukeran) in the front-line treatment of older patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in the phase III RESONATE-2 study.
Co–drug developers Pharmacyclics and Janssen Biotech announced this summer that ibrutinib, an orally bioavailable, small-molecule inhibitor of Bruton’s tyrosine kinase, had achieved its primary and secondary endpoints.
But the first full look at the data at the annual meeting of the American Society of Hematology showed ibrutinib reduced the risk of progression or death by 84% by independent review compared with chlorambucil, which has been a standard first-line therapy in older CLL patients.
The results were simultaneously published in the New England Journal of Medicine (doi: 10.1056/NEJMoa1509388).
With a median follow-up of 18.4 months, median progression-free survival (PFS) had not been reached with ibrutinib vs. 19 months with chlorambucil (hazard ratio, 0.16; P less than .001).
By investigator assessment, ibrutinib reduced the risk of progression by 91%, with an 18-month PFS rate of 94% vs. 45% with chlorambucil (HR, 0.09; P less than .001).
The PFS benefit with ibrutinib was consistent regardless of patient age, Rai stage, ECOG (Eastern Cooperative Oncology Group) status, bulky disease, and importantly, such high-risk markers as chromosome 11q deletion and unmutated immunoglobulin heavy chain variable (IGHV) mutation status, study author Dr. Alessandra Tedeschi, of Hospital Niguarda Cà Granda, Milan, , said at a press briefing highlighting the study (Ab. 485).
In addition, ibrutinib led to an 84% reduction in the risk of death compared with chlorambucil (HR, 0.16; P = .001). The 24-month overall survival rate was 98% with ibrutinib versus 85% with chlorambucil.
Single-agent ibrutinib was approved in 2014 for patients with CLL who had received at least one prior therapy and for all patients with the deleterious 17p deletion on the basis of the phase III RESONATE trial in relapsed or refractory CLL.
Three-year follow-up in the phase II PCYC-1102 study signaled a benefit with ibrutinib in treatment-naive CLL, showing an overall response rate of 84%, 30-month PFS of 96%, and overall survival rate of 97% in a subset of 31 patients at least 65 years old (Blood. 2015 Apr 16;125[16]:2497-506).
“The phase III RESONATE-2 trial confirms the efficacy of ibrutinib in treatment-naive CLL patients, leading to a 91% reduction in risk of progression and 84% reduction in risk of death when compared to chlorambucil,” Dr. Tedeschi said.
In all, 269 patients, median age of 73 years, were evenly randomized to once-daily ibrutinib 420 mg until progression or unacceptable toxicity or chlorambucil 0.5 mg/kg (up to a maximum of 0.8 mg/kg) on days 1 and 15 of a 28-day cycle for up to 12 cycles. Patients with the deleterious 17p deletion were excluded, as single-agent chlorambucil is not effective in this population.
Ibrutinib significantly improved bone marrow function, as reflected by a sustained increase in hemoglobin and platelets.
“This is very important in this category of elderly patients, in whom bone marrow failure is the most common cause of morbidity,” Dr. Tedeschi said.
There were 3 deaths on the ibrutinib arm and 17 on the chlorambucil arm.
The majority of patients (87%) in this older population with frequent comorbidities was able to continue on oral, once-daily ibrutinib with a median of 1.5 years of follow-up, she said.
The most common adverse events on ibrutinib were grade one diarrhea, fatigue, cough, and nausea that did not result in treatment discontinuation. On the chlorambucil arm, fatigue nausea, vomiting, and cytopenias occurred more frequently than with ibrutinib.
Grade 3 maculopapular rash occurred in 3% with ibrutinib and 2% with chlorambucil, she said.
Ibrutinib was associated with higher and not insignificant rates of atrial fibrillation and major hemorrhage compared with chlorambucil, said Dr. Brian T. Hill of the Taussig Cancer Institute at the Cleveland Clinic, who was not involved in the study. In our interview, Dr. Hill also questions the relevance today of chlorambucil monotherapy as the comparator arm in RESONATE-2.
Pharmacyclics, which is jointly developing ibrutinib with Janssen Biotech, sponsored the study. Dr. Tedeschi reported having nothing to disclose. Several coauthors reported relationships with Pharmacyclics and Janssen.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Monotherapy with ibrutinib (Imbruvica) prolonged survival longer than did standard chemotherapy using chlorambucil (Leukeran) in the front-line treatment of older patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in the phase III RESONATE-2 study.
Co–drug developers Pharmacyclics and Janssen Biotech announced this summer that ibrutinib, an orally bioavailable, small-molecule inhibitor of Bruton’s tyrosine kinase, had achieved its primary and secondary endpoints.
But the first full look at the data at the annual meeting of the American Society of Hematology showed ibrutinib reduced the risk of progression or death by 84% by independent review compared with chlorambucil, which has been a standard first-line therapy in older CLL patients.
The results were simultaneously published in the New England Journal of Medicine (doi: 10.1056/NEJMoa1509388).
With a median follow-up of 18.4 months, median progression-free survival (PFS) had not been reached with ibrutinib vs. 19 months with chlorambucil (hazard ratio, 0.16; P less than .001).
By investigator assessment, ibrutinib reduced the risk of progression by 91%, with an 18-month PFS rate of 94% vs. 45% with chlorambucil (HR, 0.09; P less than .001).
The PFS benefit with ibrutinib was consistent regardless of patient age, Rai stage, ECOG (Eastern Cooperative Oncology Group) status, bulky disease, and importantly, such high-risk markers as chromosome 11q deletion and unmutated immunoglobulin heavy chain variable (IGHV) mutation status, study author Dr. Alessandra Tedeschi, of Hospital Niguarda Cà Granda, Milan, , said at a press briefing highlighting the study (Ab. 485).
In addition, ibrutinib led to an 84% reduction in the risk of death compared with chlorambucil (HR, 0.16; P = .001). The 24-month overall survival rate was 98% with ibrutinib versus 85% with chlorambucil.
Single-agent ibrutinib was approved in 2014 for patients with CLL who had received at least one prior therapy and for all patients with the deleterious 17p deletion on the basis of the phase III RESONATE trial in relapsed or refractory CLL.
Three-year follow-up in the phase II PCYC-1102 study signaled a benefit with ibrutinib in treatment-naive CLL, showing an overall response rate of 84%, 30-month PFS of 96%, and overall survival rate of 97% in a subset of 31 patients at least 65 years old (Blood. 2015 Apr 16;125[16]:2497-506).
“The phase III RESONATE-2 trial confirms the efficacy of ibrutinib in treatment-naive CLL patients, leading to a 91% reduction in risk of progression and 84% reduction in risk of death when compared to chlorambucil,” Dr. Tedeschi said.
In all, 269 patients, median age of 73 years, were evenly randomized to once-daily ibrutinib 420 mg until progression or unacceptable toxicity or chlorambucil 0.5 mg/kg (up to a maximum of 0.8 mg/kg) on days 1 and 15 of a 28-day cycle for up to 12 cycles. Patients with the deleterious 17p deletion were excluded, as single-agent chlorambucil is not effective in this population.
Ibrutinib significantly improved bone marrow function, as reflected by a sustained increase in hemoglobin and platelets.
“This is very important in this category of elderly patients, in whom bone marrow failure is the most common cause of morbidity,” Dr. Tedeschi said.
There were 3 deaths on the ibrutinib arm and 17 on the chlorambucil arm.
The majority of patients (87%) in this older population with frequent comorbidities was able to continue on oral, once-daily ibrutinib with a median of 1.5 years of follow-up, she said.
The most common adverse events on ibrutinib were grade one diarrhea, fatigue, cough, and nausea that did not result in treatment discontinuation. On the chlorambucil arm, fatigue nausea, vomiting, and cytopenias occurred more frequently than with ibrutinib.
Grade 3 maculopapular rash occurred in 3% with ibrutinib and 2% with chlorambucil, she said.
Ibrutinib was associated with higher and not insignificant rates of atrial fibrillation and major hemorrhage compared with chlorambucil, said Dr. Brian T. Hill of the Taussig Cancer Institute at the Cleveland Clinic, who was not involved in the study. In our interview, Dr. Hill also questions the relevance today of chlorambucil monotherapy as the comparator arm in RESONATE-2.
Pharmacyclics, which is jointly developing ibrutinib with Janssen Biotech, sponsored the study. Dr. Tedeschi reported having nothing to disclose. Several coauthors reported relationships with Pharmacyclics and Janssen.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2015
Key clinical point: First-line ibrutinib significantly extends survival in older patients with untreated chronic lymphocytic leukemia or small lymphocytic lymphoma, compared with chlorambucil chemotherapy.
Major finding: Median progression-free survival was not reached with ibrutinib vs. 19 months with chlorambucil (HR, 0.16; P less than .001).
Data source: Prospective, phase III study of 269 patients 65 years or older with treatment-naive CLL or SLL.
Disclosures: Pharmacyclics, which is jointly developing ibrutinib with Janssen Biotech, sponsored the study. Dr. Tedeschi reported having nothing to disclose. Several coauthors reported relationships with Pharmacyclics and Janssen.
VIDEO: Midostaurin hits mark in FLT3-mutated AML
ORLANDO – The oral multikinase inhibitor midostaurin improved overall survival by 23% when added to standard chemotherapy and given as maintenance therapy for 1 year in newly diagnosed patients with FLT3-mutated acute myeloid leukemia (AML) in the global phase III CALGB 10603/RATIFY trial.
The results struck a chord at the annual meeting of the American Society of Hematology because the benefits of targeted therapy have so far eluded AML patients despite transforming the treatment of other blood cancers. Currently, there are no approved, targeted treatments for AML.
CALGB 10603/RATIFY is the first large, controlled trial to show an overall survival benefit in the roughly 30% of AML patients with a mutation in the FLT3 gene.
“This is exciting because we haven’t had a new treatment in AML for 30 years,” Dr. Robert Hromas of the University of Florida, Gainesville, said while moderating a press conference highlighting the plenary abstract.
The results were a decade in the making after midostaurin failed previously when used in all AML patients rather than the subset with the FLT3 mutation. But the persistence of researchers, the international collaboration, and funding in cancer research paid off, Dr. Hromas said.
Study author Dr. Richard M. Stone, chief of staff at Dana-Farber Cancer Institute in Boston, reviewed the results of CALGB 10603/RATIFY in an interview.
CALGB 10603/RATIFY was sponsored by the Cancer Therapy Evaluation Program. Dr. Stone reported financial relationships with several drug companies including Novartis, which provided the study drug and sponsored the trial outside North America. Dr. Hromas disclosed serving as an uncompensated advisory board member without equity for Cloud Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – The oral multikinase inhibitor midostaurin improved overall survival by 23% when added to standard chemotherapy and given as maintenance therapy for 1 year in newly diagnosed patients with FLT3-mutated acute myeloid leukemia (AML) in the global phase III CALGB 10603/RATIFY trial.
The results struck a chord at the annual meeting of the American Society of Hematology because the benefits of targeted therapy have so far eluded AML patients despite transforming the treatment of other blood cancers. Currently, there are no approved, targeted treatments for AML.
CALGB 10603/RATIFY is the first large, controlled trial to show an overall survival benefit in the roughly 30% of AML patients with a mutation in the FLT3 gene.
“This is exciting because we haven’t had a new treatment in AML for 30 years,” Dr. Robert Hromas of the University of Florida, Gainesville, said while moderating a press conference highlighting the plenary abstract.
The results were a decade in the making after midostaurin failed previously when used in all AML patients rather than the subset with the FLT3 mutation. But the persistence of researchers, the international collaboration, and funding in cancer research paid off, Dr. Hromas said.
Study author Dr. Richard M. Stone, chief of staff at Dana-Farber Cancer Institute in Boston, reviewed the results of CALGB 10603/RATIFY in an interview.
CALGB 10603/RATIFY was sponsored by the Cancer Therapy Evaluation Program. Dr. Stone reported financial relationships with several drug companies including Novartis, which provided the study drug and sponsored the trial outside North America. Dr. Hromas disclosed serving as an uncompensated advisory board member without equity for Cloud Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – The oral multikinase inhibitor midostaurin improved overall survival by 23% when added to standard chemotherapy and given as maintenance therapy for 1 year in newly diagnosed patients with FLT3-mutated acute myeloid leukemia (AML) in the global phase III CALGB 10603/RATIFY trial.
The results struck a chord at the annual meeting of the American Society of Hematology because the benefits of targeted therapy have so far eluded AML patients despite transforming the treatment of other blood cancers. Currently, there are no approved, targeted treatments for AML.
CALGB 10603/RATIFY is the first large, controlled trial to show an overall survival benefit in the roughly 30% of AML patients with a mutation in the FLT3 gene.
“This is exciting because we haven’t had a new treatment in AML for 30 years,” Dr. Robert Hromas of the University of Florida, Gainesville, said while moderating a press conference highlighting the plenary abstract.
The results were a decade in the making after midostaurin failed previously when used in all AML patients rather than the subset with the FLT3 mutation. But the persistence of researchers, the international collaboration, and funding in cancer research paid off, Dr. Hromas said.
Study author Dr. Richard M. Stone, chief of staff at Dana-Farber Cancer Institute in Boston, reviewed the results of CALGB 10603/RATIFY in an interview.
CALGB 10603/RATIFY was sponsored by the Cancer Therapy Evaluation Program. Dr. Stone reported financial relationships with several drug companies including Novartis, which provided the study drug and sponsored the trial outside North America. Dr. Hromas disclosed serving as an uncompensated advisory board member without equity for Cloud Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2015
IVUS Stent Implantation Could Reduce Cardiac Events
NEW YORK - Intravascular ultrasound-guided (IVUS) stent implantation can lead to fewer adverse cardiac events compared with angiography-guided implantation, according to a new trial.
"Among patients requiring long coronary stent implantation, the use of IVUS-guided everolimus-eluting stent implantation, compared with angiography-guided stent implantation, resulted in a significantly lower rate of the composite of major adverse cardiac events at one year," Dr. Myeong-Ki Hong of Severance Cardiovascular Hospital and Yonsei University College of Medicine in Seoul, Korea, and colleagues report.
"These differences were primarily due to lower risk of target lesion revascularization," they note in an article online November 10 in JAMA. They presented their findings simultaneously at the American Heart Association Scientific Sessions in Orlando, Fla.
Dr. Hong and colleagues conducted a trial involving 1,400 patients with long coronary lesions between 2010 and 2014 at 20 centers in Korea. They randomized 700 patients to IVUS-guided stent implantation and 700 to angiography-guided stent implantation. They had one-year follow-up results on 94.5%.
Patient mean age was 64 and 69% were men. The mean stented target length was 39.3 mm.
The composite endpoint of major adverse cardiac events, including cardiac death, target lesion-related myocardial infarction (MI), or ischemia-driven target lesion revascularization, occurred in 19 (2.9%) patients who underwent IVUS implantation and 39 (5.8%) patients who underwent angiography-guided implantation (hazard ratio, 0.48; p=0.007).
Cardiac death and target-related MI were not significantly different between the two groups. However, ischemia-driven target lesion revascularization occurred in 17 (2.5%) IVUS
patients and 33 (5%) angiography patients (HR 0.51, p=0.02).
Clinicians performed post-stent balloon dilation more frequently in IVUS patients than in angiography patients (76% vs. 57%, p<0.001), and the mean final balloon size was larger in IVUS patients.
Patients who met IVUS criteria for stent optimization (363, 54%) had significantly greater mean post-intervention minimal lumen area at the stented segment compared with patients who did not meet IVUS criteria.
"The clinical benefit of IVUS-guided (drug-eluting stent) implantation may be attributed to the larger minimal lumen diameter followed by the more frequent adjunct postdilation with a large-sized balloon in the IVUS-guided group," the researchers write.
"To our knowledge, the current study is the first demonstration of the clinical benefit of IVUS guidance in second generation (drug-eluting stent) implantation in an adequately powered randomized clinical trial," they note.
However, even though recent guidelines recommend IVUS-guided implantation for some patients, evidence for improved outcomes based on adequately powered trials remains inadequate, they caution.
Dr. Hong did not respond to a request for comments. Abbott Vascular funded this research. The authors reported no conflicts of interest.
NEW YORK - Intravascular ultrasound-guided (IVUS) stent implantation can lead to fewer adverse cardiac events compared with angiography-guided implantation, according to a new trial.
"Among patients requiring long coronary stent implantation, the use of IVUS-guided everolimus-eluting stent implantation, compared with angiography-guided stent implantation, resulted in a significantly lower rate of the composite of major adverse cardiac events at one year," Dr. Myeong-Ki Hong of Severance Cardiovascular Hospital and Yonsei University College of Medicine in Seoul, Korea, and colleagues report.
"These differences were primarily due to lower risk of target lesion revascularization," they note in an article online November 10 in JAMA. They presented their findings simultaneously at the American Heart Association Scientific Sessions in Orlando, Fla.
Dr. Hong and colleagues conducted a trial involving 1,400 patients with long coronary lesions between 2010 and 2014 at 20 centers in Korea. They randomized 700 patients to IVUS-guided stent implantation and 700 to angiography-guided stent implantation. They had one-year follow-up results on 94.5%.
Patient mean age was 64 and 69% were men. The mean stented target length was 39.3 mm.
The composite endpoint of major adverse cardiac events, including cardiac death, target lesion-related myocardial infarction (MI), or ischemia-driven target lesion revascularization, occurred in 19 (2.9%) patients who underwent IVUS implantation and 39 (5.8%) patients who underwent angiography-guided implantation (hazard ratio, 0.48; p=0.007).
Cardiac death and target-related MI were not significantly different between the two groups. However, ischemia-driven target lesion revascularization occurred in 17 (2.5%) IVUS
patients and 33 (5%) angiography patients (HR 0.51, p=0.02).
Clinicians performed post-stent balloon dilation more frequently in IVUS patients than in angiography patients (76% vs. 57%, p<0.001), and the mean final balloon size was larger in IVUS patients.
Patients who met IVUS criteria for stent optimization (363, 54%) had significantly greater mean post-intervention minimal lumen area at the stented segment compared with patients who did not meet IVUS criteria.
"The clinical benefit of IVUS-guided (drug-eluting stent) implantation may be attributed to the larger minimal lumen diameter followed by the more frequent adjunct postdilation with a large-sized balloon in the IVUS-guided group," the researchers write.
"To our knowledge, the current study is the first demonstration of the clinical benefit of IVUS guidance in second generation (drug-eluting stent) implantation in an adequately powered randomized clinical trial," they note.
However, even though recent guidelines recommend IVUS-guided implantation for some patients, evidence for improved outcomes based on adequately powered trials remains inadequate, they caution.
Dr. Hong did not respond to a request for comments. Abbott Vascular funded this research. The authors reported no conflicts of interest.
NEW YORK - Intravascular ultrasound-guided (IVUS) stent implantation can lead to fewer adverse cardiac events compared with angiography-guided implantation, according to a new trial.
"Among patients requiring long coronary stent implantation, the use of IVUS-guided everolimus-eluting stent implantation, compared with angiography-guided stent implantation, resulted in a significantly lower rate of the composite of major adverse cardiac events at one year," Dr. Myeong-Ki Hong of Severance Cardiovascular Hospital and Yonsei University College of Medicine in Seoul, Korea, and colleagues report.
"These differences were primarily due to lower risk of target lesion revascularization," they note in an article online November 10 in JAMA. They presented their findings simultaneously at the American Heart Association Scientific Sessions in Orlando, Fla.
Dr. Hong and colleagues conducted a trial involving 1,400 patients with long coronary lesions between 2010 and 2014 at 20 centers in Korea. They randomized 700 patients to IVUS-guided stent implantation and 700 to angiography-guided stent implantation. They had one-year follow-up results on 94.5%.
Patient mean age was 64 and 69% were men. The mean stented target length was 39.3 mm.
The composite endpoint of major adverse cardiac events, including cardiac death, target lesion-related myocardial infarction (MI), or ischemia-driven target lesion revascularization, occurred in 19 (2.9%) patients who underwent IVUS implantation and 39 (5.8%) patients who underwent angiography-guided implantation (hazard ratio, 0.48; p=0.007).
Cardiac death and target-related MI were not significantly different between the two groups. However, ischemia-driven target lesion revascularization occurred in 17 (2.5%) IVUS
patients and 33 (5%) angiography patients (HR 0.51, p=0.02).
Clinicians performed post-stent balloon dilation more frequently in IVUS patients than in angiography patients (76% vs. 57%, p<0.001), and the mean final balloon size was larger in IVUS patients.
Patients who met IVUS criteria for stent optimization (363, 54%) had significantly greater mean post-intervention minimal lumen area at the stented segment compared with patients who did not meet IVUS criteria.
"The clinical benefit of IVUS-guided (drug-eluting stent) implantation may be attributed to the larger minimal lumen diameter followed by the more frequent adjunct postdilation with a large-sized balloon in the IVUS-guided group," the researchers write.
"To our knowledge, the current study is the first demonstration of the clinical benefit of IVUS guidance in second generation (drug-eluting stent) implantation in an adequately powered randomized clinical trial," they note.
However, even though recent guidelines recommend IVUS-guided implantation for some patients, evidence for improved outcomes based on adequately powered trials remains inadequate, they caution.
Dr. Hong did not respond to a request for comments. Abbott Vascular funded this research. The authors reported no conflicts of interest.
‘Moderate’ flu activity seen in two U.S. states
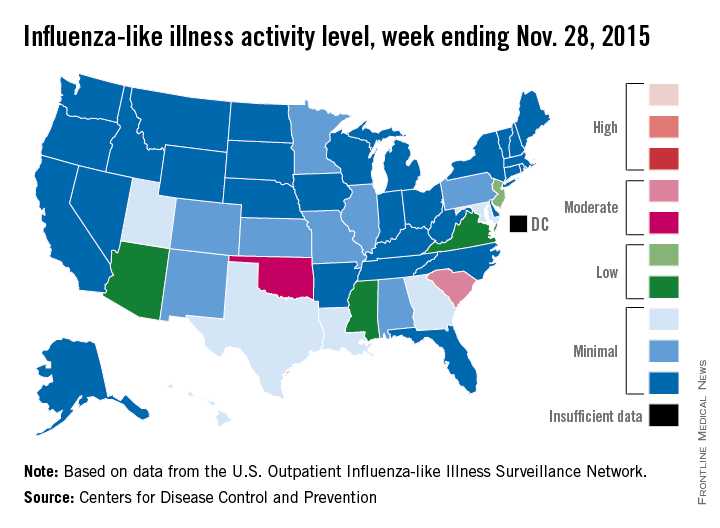
Two U.S. states experienced “moderate” activity of influenza-like illness for the week ending Nov. 28, 2015 – week 7 of the 2015-2016 flu season – the Centers for Disease Control and Prevention reported Dec. 4.
South Carolina had the highest (level 7) activity for the week, with Oklahoma joined by Puerto Rico at level 6. New Jersey was at the highest level (level 5) of “low” activity, while Arizona, Mississippi, and Virginia were a notch lower (level 4) but still in the “low” zone. All told, 20 states had flu activity of level 2 or higher, according to the CDC.
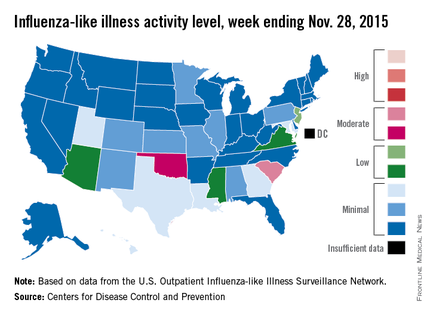
There were no influenza-associated pediatric deaths reported during the week, with two such deaths reported for the 2015-2016 season so far. For week 7 nationwide, 1.9% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network were the result of influenza-like illness – defined as a temperature of 100° F or greater and cough and/or sore throat – which is below the national baseline of 2.1%, the CDC said.
During week 7, 1.5% of the 11,288 specimens tested were positive for influenza, with 60% positive for influenza A and 40% positive for influenza B. For the season overall, 1.2% of the 102,675 specimens tested have been positive, with a 61/39 split for influenza A and B, the CDC noted.
Two U.S. states experienced “moderate” activity of influenza-like illness for the week ending Nov. 28, 2015 – week 7 of the 2015-2016 flu season – the Centers for Disease Control and Prevention reported Dec. 4.
South Carolina had the highest (level 7) activity for the week, with Oklahoma joined by Puerto Rico at level 6. New Jersey was at the highest level (level 5) of “low” activity, while Arizona, Mississippi, and Virginia were a notch lower (level 4) but still in the “low” zone. All told, 20 states had flu activity of level 2 or higher, according to the CDC.

There were no influenza-associated pediatric deaths reported during the week, with two such deaths reported for the 2015-2016 season so far. For week 7 nationwide, 1.9% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network were the result of influenza-like illness – defined as a temperature of 100° F or greater and cough and/or sore throat – which is below the national baseline of 2.1%, the CDC said.
During week 7, 1.5% of the 11,288 specimens tested were positive for influenza, with 60% positive for influenza A and 40% positive for influenza B. For the season overall, 1.2% of the 102,675 specimens tested have been positive, with a 61/39 split for influenza A and B, the CDC noted.
Two U.S. states experienced “moderate” activity of influenza-like illness for the week ending Nov. 28, 2015 – week 7 of the 2015-2016 flu season – the Centers for Disease Control and Prevention reported Dec. 4.
South Carolina had the highest (level 7) activity for the week, with Oklahoma joined by Puerto Rico at level 6. New Jersey was at the highest level (level 5) of “low” activity, while Arizona, Mississippi, and Virginia were a notch lower (level 4) but still in the “low” zone. All told, 20 states had flu activity of level 2 or higher, according to the CDC.

There were no influenza-associated pediatric deaths reported during the week, with two such deaths reported for the 2015-2016 season so far. For week 7 nationwide, 1.9% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network were the result of influenza-like illness – defined as a temperature of 100° F or greater and cough and/or sore throat – which is below the national baseline of 2.1%, the CDC said.
During week 7, 1.5% of the 11,288 specimens tested were positive for influenza, with 60% positive for influenza A and 40% positive for influenza B. For the season overall, 1.2% of the 102,675 specimens tested have been positive, with a 61/39 split for influenza A and B, the CDC noted.
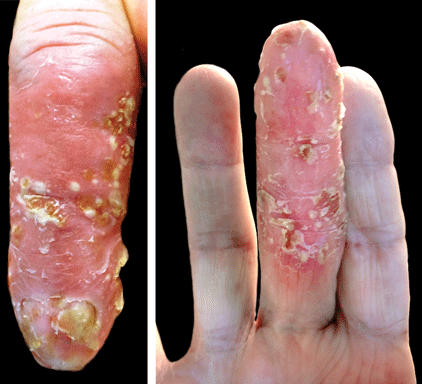
Scaly Plaque With Pustules and Anonychia on the Middle Finger
The Diagnosis: Acrodermatitis Continua of Hallopeau
Acrodermatitis continua of Hallopeau (ACH) is considered to be a form of acropustular psoriasis that presents as a sterile, pustular eruption initially affecting the fingertips and/or toes.1 The slow-growing pustules typically progress locally and can lead to onychodystrophy and/or osteolysis of the underlying bone.2,3 Most commonly affecting adult women, ACH often begins following local trauma to or infection of a single digit.4 As the disease progresses proximally, the small pustules burst, leaving a shiny, erythematous surface on which new pustules can develop. These pustules have a tendency to amalgamate, leading to the characteristic clinical finding of lakes of pus. Pustules frequently appear on the nail matrix and nail bed presenting as severe onychodystrophy and ultimately anonychia.5,6 Rarely, ACH can be associated with generalized pustular psoriasis as well as conjunctivitis, balanitis, and fissuring or annulus migrans of the tongue.2,7
Diagnosis can be established based on clinical findings, biopsy, and bacterial and fungal cultures revealing sterile pustules.8,9 Histologic findings are similar to those seen in pustular psoriasis, demonstrating subcorneal neutrophilic pustules, Munro microabscesses, and dilated blood vessels with lymphocytic infiltrate in the papillary dermis.10
Due to the refractory nature of the disease, there are no recommended guidelines for treatment of ACH. Most successful treatment regimens consist of topical psoriasis medications combined with systemic psoriatic therapies such as cyclosporine, methotrexate, acitretin, or biologic therapy.8,11-16 Our patient achieved satisfactory clinical improvement with clobetasol propionate ointment 0.05% twice daily alternating with calcipotriene cream 0.005% twice daily.
- Suchanek J. Relation of Hallopeau’s acrodermatitis continua to psoriasis. Przegl Dermatol. 1951;1:165-181.
- Adam BA, Loh CL. Acropustulosis (acrodermatitis continua) with resorption of terminal phalanges. Med J Malaysia. 1972;27:30-32.
- Mrowietz U. Pustular eruptions of palms and soles. In: Wolff K, Goldsmith LS, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2007:215-218.
- Yerushalmi J, Grunwald MH, Hallel-Halevy D, et al. Chronic pustular eruption of the thumbs. diagnosis: acrodermatitis continue of Hallopeau (ACH). Arch Dermatol. 2000:136:925-930.
- Granelli U. Impetigo herpetiformis; acrodermatitis continue of Hallopeau and pustular psoriasis; etiology and pathogenesis and differential diagnosis. Minerva Dermatol. 1956;31:120-126.
- Mobini N, Toussaint S, Kamino H. Noninfectious erythematous, papular, and squamous diseases. In: Elder DE, Elenitsas R, Johnson B, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005:174-210.
- Radcliff-Crocker H. Diseases of the Skin: Their Descriptions, Pathology, Diagnosis and Treatment. Philadelphia, PA: P. Blakiston, Son, & Co; 1888.
- Sehgal VN, Verma P, Sharma S, et al. Review: acrodermatitis continua of Hallopeau: evolution of treatment options. Int J Dermatol. 2011;50:1195-1211.
- Post CF, Hopper ME. Dermatitis repens: a report of two cases with bacteriologic studies. AMA Arc Derm Syphilol. 1951;63:220-223.
- Sehgal VN, Sharma S. The significance of Gram’s stain smear, potassium hydroxide mount, culture and microscopic pathology in the diagnosis of acrodermatitis continua of Hallopeau. Skinmed. 2011;9:260-261.
- Mosser G, Pillekamp H, Peter RU. Suppurative acrodermatitis continua of Hallopeau. a differential diagnosis of paronychia. Dtsch Med Wochenschr. 1998;123:386-390.
- Piquero-Casals J, Fonseca de Mello AP, Dal Coleto C, et al. Using oral tetracycline and topical betamethasone valerate to treat acrodermatitis continua of Hallopeau. Cutis. 2002;70:106-108.
- Tsuji T, Nishimura M. Topically administered fluorouracil in acrodermatitis continua of Hallopeau. Arch Dermatol. 1991;127:27-28.
- Van de Kerkhof PCM. In vivo effects of vitamin D3 analogs. J Dermatolog Treat. 1998;(suppl 3):S25-S29.
- Kokelj F, Plozzer C, Trevisan G. Uselessness of topical calcipotriol as monotherapy for acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2001;81:153.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
The Diagnosis: Acrodermatitis Continua of Hallopeau
Acrodermatitis continua of Hallopeau (ACH) is considered to be a form of acropustular psoriasis that presents as a sterile, pustular eruption initially affecting the fingertips and/or toes.1 The slow-growing pustules typically progress locally and can lead to onychodystrophy and/or osteolysis of the underlying bone.2,3 Most commonly affecting adult women, ACH often begins following local trauma to or infection of a single digit.4 As the disease progresses proximally, the small pustules burst, leaving a shiny, erythematous surface on which new pustules can develop. These pustules have a tendency to amalgamate, leading to the characteristic clinical finding of lakes of pus. Pustules frequently appear on the nail matrix and nail bed presenting as severe onychodystrophy and ultimately anonychia.5,6 Rarely, ACH can be associated with generalized pustular psoriasis as well as conjunctivitis, balanitis, and fissuring or annulus migrans of the tongue.2,7
Diagnosis can be established based on clinical findings, biopsy, and bacterial and fungal cultures revealing sterile pustules.8,9 Histologic findings are similar to those seen in pustular psoriasis, demonstrating subcorneal neutrophilic pustules, Munro microabscesses, and dilated blood vessels with lymphocytic infiltrate in the papillary dermis.10
Due to the refractory nature of the disease, there are no recommended guidelines for treatment of ACH. Most successful treatment regimens consist of topical psoriasis medications combined with systemic psoriatic therapies such as cyclosporine, methotrexate, acitretin, or biologic therapy.8,11-16 Our patient achieved satisfactory clinical improvement with clobetasol propionate ointment 0.05% twice daily alternating with calcipotriene cream 0.005% twice daily.
The Diagnosis: Acrodermatitis Continua of Hallopeau
Acrodermatitis continua of Hallopeau (ACH) is considered to be a form of acropustular psoriasis that presents as a sterile, pustular eruption initially affecting the fingertips and/or toes.1 The slow-growing pustules typically progress locally and can lead to onychodystrophy and/or osteolysis of the underlying bone.2,3 Most commonly affecting adult women, ACH often begins following local trauma to or infection of a single digit.4 As the disease progresses proximally, the small pustules burst, leaving a shiny, erythematous surface on which new pustules can develop. These pustules have a tendency to amalgamate, leading to the characteristic clinical finding of lakes of pus. Pustules frequently appear on the nail matrix and nail bed presenting as severe onychodystrophy and ultimately anonychia.5,6 Rarely, ACH can be associated with generalized pustular psoriasis as well as conjunctivitis, balanitis, and fissuring or annulus migrans of the tongue.2,7
Diagnosis can be established based on clinical findings, biopsy, and bacterial and fungal cultures revealing sterile pustules.8,9 Histologic findings are similar to those seen in pustular psoriasis, demonstrating subcorneal neutrophilic pustules, Munro microabscesses, and dilated blood vessels with lymphocytic infiltrate in the papillary dermis.10
Due to the refractory nature of the disease, there are no recommended guidelines for treatment of ACH. Most successful treatment regimens consist of topical psoriasis medications combined with systemic psoriatic therapies such as cyclosporine, methotrexate, acitretin, or biologic therapy.8,11-16 Our patient achieved satisfactory clinical improvement with clobetasol propionate ointment 0.05% twice daily alternating with calcipotriene cream 0.005% twice daily.
- Suchanek J. Relation of Hallopeau’s acrodermatitis continua to psoriasis. Przegl Dermatol. 1951;1:165-181.
- Adam BA, Loh CL. Acropustulosis (acrodermatitis continua) with resorption of terminal phalanges. Med J Malaysia. 1972;27:30-32.
- Mrowietz U. Pustular eruptions of palms and soles. In: Wolff K, Goldsmith LS, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2007:215-218.
- Yerushalmi J, Grunwald MH, Hallel-Halevy D, et al. Chronic pustular eruption of the thumbs. diagnosis: acrodermatitis continue of Hallopeau (ACH). Arch Dermatol. 2000:136:925-930.
- Granelli U. Impetigo herpetiformis; acrodermatitis continue of Hallopeau and pustular psoriasis; etiology and pathogenesis and differential diagnosis. Minerva Dermatol. 1956;31:120-126.
- Mobini N, Toussaint S, Kamino H. Noninfectious erythematous, papular, and squamous diseases. In: Elder DE, Elenitsas R, Johnson B, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005:174-210.
- Radcliff-Crocker H. Diseases of the Skin: Their Descriptions, Pathology, Diagnosis and Treatment. Philadelphia, PA: P. Blakiston, Son, & Co; 1888.
- Sehgal VN, Verma P, Sharma S, et al. Review: acrodermatitis continua of Hallopeau: evolution of treatment options. Int J Dermatol. 2011;50:1195-1211.
- Post CF, Hopper ME. Dermatitis repens: a report of two cases with bacteriologic studies. AMA Arc Derm Syphilol. 1951;63:220-223.
- Sehgal VN, Sharma S. The significance of Gram’s stain smear, potassium hydroxide mount, culture and microscopic pathology in the diagnosis of acrodermatitis continua of Hallopeau. Skinmed. 2011;9:260-261.
- Mosser G, Pillekamp H, Peter RU. Suppurative acrodermatitis continua of Hallopeau. a differential diagnosis of paronychia. Dtsch Med Wochenschr. 1998;123:386-390.
- Piquero-Casals J, Fonseca de Mello AP, Dal Coleto C, et al. Using oral tetracycline and topical betamethasone valerate to treat acrodermatitis continua of Hallopeau. Cutis. 2002;70:106-108.
- Tsuji T, Nishimura M. Topically administered fluorouracil in acrodermatitis continua of Hallopeau. Arch Dermatol. 1991;127:27-28.
- Van de Kerkhof PCM. In vivo effects of vitamin D3 analogs. J Dermatolog Treat. 1998;(suppl 3):S25-S29.
- Kokelj F, Plozzer C, Trevisan G. Uselessness of topical calcipotriol as monotherapy for acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2001;81:153.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Suchanek J. Relation of Hallopeau’s acrodermatitis continua to psoriasis. Przegl Dermatol. 1951;1:165-181.
- Adam BA, Loh CL. Acropustulosis (acrodermatitis continua) with resorption of terminal phalanges. Med J Malaysia. 1972;27:30-32.
- Mrowietz U. Pustular eruptions of palms and soles. In: Wolff K, Goldsmith LS, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2007:215-218.
- Yerushalmi J, Grunwald MH, Hallel-Halevy D, et al. Chronic pustular eruption of the thumbs. diagnosis: acrodermatitis continue of Hallopeau (ACH). Arch Dermatol. 2000:136:925-930.
- Granelli U. Impetigo herpetiformis; acrodermatitis continue of Hallopeau and pustular psoriasis; etiology and pathogenesis and differential diagnosis. Minerva Dermatol. 1956;31:120-126.
- Mobini N, Toussaint S, Kamino H. Noninfectious erythematous, papular, and squamous diseases. In: Elder DE, Elenitsas R, Johnson B, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005:174-210.
- Radcliff-Crocker H. Diseases of the Skin: Their Descriptions, Pathology, Diagnosis and Treatment. Philadelphia, PA: P. Blakiston, Son, & Co; 1888.
- Sehgal VN, Verma P, Sharma S, et al. Review: acrodermatitis continua of Hallopeau: evolution of treatment options. Int J Dermatol. 2011;50:1195-1211.
- Post CF, Hopper ME. Dermatitis repens: a report of two cases with bacteriologic studies. AMA Arc Derm Syphilol. 1951;63:220-223.
- Sehgal VN, Sharma S. The significance of Gram’s stain smear, potassium hydroxide mount, culture and microscopic pathology in the diagnosis of acrodermatitis continua of Hallopeau. Skinmed. 2011;9:260-261.
- Mosser G, Pillekamp H, Peter RU. Suppurative acrodermatitis continua of Hallopeau. a differential diagnosis of paronychia. Dtsch Med Wochenschr. 1998;123:386-390.
- Piquero-Casals J, Fonseca de Mello AP, Dal Coleto C, et al. Using oral tetracycline and topical betamethasone valerate to treat acrodermatitis continua of Hallopeau. Cutis. 2002;70:106-108.
- Tsuji T, Nishimura M. Topically administered fluorouracil in acrodermatitis continua of Hallopeau. Arch Dermatol. 1991;127:27-28.
- Van de Kerkhof PCM. In vivo effects of vitamin D3 analogs. J Dermatolog Treat. 1998;(suppl 3):S25-S29.
- Kokelj F, Plozzer C, Trevisan G. Uselessness of topical calcipotriol as monotherapy for acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2001;81:153.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
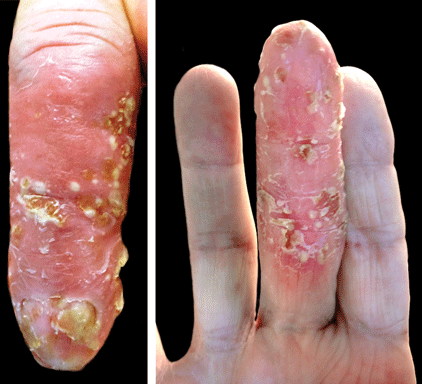
A 69-year-old man presented to our dermatology clinic with a persistent rash on the right middle finger of 5 years’ duration (left). Physical examination revealed a well-demarcated scaly plaque with pustules and anonychia localized to the right middle finger (right). Fungal and bacterial cultures revealed sterile pustules. The patient was successfully treated with an occluded superpotent topical steroid alternating with a topical vitamin D analogue.
Cancer drug prices vary widely from country to country

Photo by Bill Branson
The price of cancer drugs varies widely between European countries, Australia, and New Zealand, according to a study published in The Lancet Oncology.
The study indicates that, overall, the UK and Mediterranean countries such as Greece, Spain, and Portugal pay the lowest average unit manufacturer prices for a group of 31 originator cancer drugs (new drugs under patent).
And Sweden, Switzerland, and Germany pay the highest prices.
The greatest differences in price were noted for gemcitabine, which costs €209 per vial in New Zealand and €43 in Australia, and zoledronic acid, which costs €330 per vial in New Zealand but €128 in Greece.*
“Public payers in Germany are paying 223% more in terms of official prices for interferon alfa 2b for melanoma and leukemia treatment than those in Greece,” noted study author Sabine Vogler, PhD, of the WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies in Vienna, Austria.
“For gefitinib to treat non-small-lung cancer, the price in Germany is 172% higher than in New Zealand.”
To uncover these price differences, Dr Vogler and her colleagues reviewed official drug price data from the Pharma Price Information (PPI) service of the Austrian Public Health Institute for 16 European countries**, and from the pharmaceutical schedules in Australia and New Zealand.
The researchers compared what manufacturers charged for a unit (ie, price per tablet or vial) of 31 originator cancer drugs in June 2013.
None of these drugs had a unit price lower than €10. Four drugs (13%) had an average unit manufacturer price between €250 and €500, and 2 drugs (6%) had an average unit price between €500 and €1000.
Seven drugs (23%) had an average unit price higher than €1000. For example, plerixafor cost over €5000 per injection.
The price differences between the highest- and lowest-priced countries ranged from 28% to 50% for a third of the drugs sampled, between 50% and 100% for half of the drugs, and between 100% and 200% for 3 drugs (10%).
The researchers noted that information on real drug prices is scarce. The cancer drug prices they surveyed did not include confidential discounts such as those agreed upon in managed-entry arrangements that are increasingly used in countries such as Australia, Italy, the UK, and the Netherlands.
“Some high-income countries have managed to barter the manufacturers down to lower prices, but these agreements, including the agreed prices, are confidential,” Dr Vogler explained.
“Although these agreements ensure patient access to new drugs, other countries risk overpaying when setting drug prices through the common practice of external price referencing, or international price comparison, because they can only use the official undiscounted prices as a benchmark. There needs to be far more transparency.”
“We hope that our findings will provide concrete evidence for policymakers to take action to address high prices and ensure more transparency in cancer drug pricing so that costs and access to new drugs does not depend on where a patient lives.” ![]()
*Gemcitabine and zoledronic acid have generic versions in several countries, and originator prices were decreased in some countries following patent expiry but not in others.
**Austria, Belgium, Denmark, Germany, Greece, Finland, France, Italy, Ireland, the Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, and the UK.

Photo by Bill Branson
The price of cancer drugs varies widely between European countries, Australia, and New Zealand, according to a study published in The Lancet Oncology.
The study indicates that, overall, the UK and Mediterranean countries such as Greece, Spain, and Portugal pay the lowest average unit manufacturer prices for a group of 31 originator cancer drugs (new drugs under patent).
And Sweden, Switzerland, and Germany pay the highest prices.
The greatest differences in price were noted for gemcitabine, which costs €209 per vial in New Zealand and €43 in Australia, and zoledronic acid, which costs €330 per vial in New Zealand but €128 in Greece.*
“Public payers in Germany are paying 223% more in terms of official prices for interferon alfa 2b for melanoma and leukemia treatment than those in Greece,” noted study author Sabine Vogler, PhD, of the WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies in Vienna, Austria.
“For gefitinib to treat non-small-lung cancer, the price in Germany is 172% higher than in New Zealand.”
To uncover these price differences, Dr Vogler and her colleagues reviewed official drug price data from the Pharma Price Information (PPI) service of the Austrian Public Health Institute for 16 European countries**, and from the pharmaceutical schedules in Australia and New Zealand.
The researchers compared what manufacturers charged for a unit (ie, price per tablet or vial) of 31 originator cancer drugs in June 2013.
None of these drugs had a unit price lower than €10. Four drugs (13%) had an average unit manufacturer price between €250 and €500, and 2 drugs (6%) had an average unit price between €500 and €1000.
Seven drugs (23%) had an average unit price higher than €1000. For example, plerixafor cost over €5000 per injection.
The price differences between the highest- and lowest-priced countries ranged from 28% to 50% for a third of the drugs sampled, between 50% and 100% for half of the drugs, and between 100% and 200% for 3 drugs (10%).
The researchers noted that information on real drug prices is scarce. The cancer drug prices they surveyed did not include confidential discounts such as those agreed upon in managed-entry arrangements that are increasingly used in countries such as Australia, Italy, the UK, and the Netherlands.
“Some high-income countries have managed to barter the manufacturers down to lower prices, but these agreements, including the agreed prices, are confidential,” Dr Vogler explained.
“Although these agreements ensure patient access to new drugs, other countries risk overpaying when setting drug prices through the common practice of external price referencing, or international price comparison, because they can only use the official undiscounted prices as a benchmark. There needs to be far more transparency.”
“We hope that our findings will provide concrete evidence for policymakers to take action to address high prices and ensure more transparency in cancer drug pricing so that costs and access to new drugs does not depend on where a patient lives.” ![]()
*Gemcitabine and zoledronic acid have generic versions in several countries, and originator prices were decreased in some countries following patent expiry but not in others.
**Austria, Belgium, Denmark, Germany, Greece, Finland, France, Italy, Ireland, the Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, and the UK.

Photo by Bill Branson
The price of cancer drugs varies widely between European countries, Australia, and New Zealand, according to a study published in The Lancet Oncology.
The study indicates that, overall, the UK and Mediterranean countries such as Greece, Spain, and Portugal pay the lowest average unit manufacturer prices for a group of 31 originator cancer drugs (new drugs under patent).
And Sweden, Switzerland, and Germany pay the highest prices.
The greatest differences in price were noted for gemcitabine, which costs €209 per vial in New Zealand and €43 in Australia, and zoledronic acid, which costs €330 per vial in New Zealand but €128 in Greece.*
“Public payers in Germany are paying 223% more in terms of official prices for interferon alfa 2b for melanoma and leukemia treatment than those in Greece,” noted study author Sabine Vogler, PhD, of the WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies in Vienna, Austria.
“For gefitinib to treat non-small-lung cancer, the price in Germany is 172% higher than in New Zealand.”
To uncover these price differences, Dr Vogler and her colleagues reviewed official drug price data from the Pharma Price Information (PPI) service of the Austrian Public Health Institute for 16 European countries**, and from the pharmaceutical schedules in Australia and New Zealand.
The researchers compared what manufacturers charged for a unit (ie, price per tablet or vial) of 31 originator cancer drugs in June 2013.
None of these drugs had a unit price lower than €10. Four drugs (13%) had an average unit manufacturer price between €250 and €500, and 2 drugs (6%) had an average unit price between €500 and €1000.
Seven drugs (23%) had an average unit price higher than €1000. For example, plerixafor cost over €5000 per injection.
The price differences between the highest- and lowest-priced countries ranged from 28% to 50% for a third of the drugs sampled, between 50% and 100% for half of the drugs, and between 100% and 200% for 3 drugs (10%).
The researchers noted that information on real drug prices is scarce. The cancer drug prices they surveyed did not include confidential discounts such as those agreed upon in managed-entry arrangements that are increasingly used in countries such as Australia, Italy, the UK, and the Netherlands.
“Some high-income countries have managed to barter the manufacturers down to lower prices, but these agreements, including the agreed prices, are confidential,” Dr Vogler explained.
“Although these agreements ensure patient access to new drugs, other countries risk overpaying when setting drug prices through the common practice of external price referencing, or international price comparison, because they can only use the official undiscounted prices as a benchmark. There needs to be far more transparency.”
“We hope that our findings will provide concrete evidence for policymakers to take action to address high prices and ensure more transparency in cancer drug pricing so that costs and access to new drugs does not depend on where a patient lives.” ![]()
*Gemcitabine and zoledronic acid have generic versions in several countries, and originator prices were decreased in some countries following patent expiry but not in others.
**Austria, Belgium, Denmark, Germany, Greece, Finland, France, Italy, Ireland, the Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, and the UK.
Fish-related findings may have implications for HSCT

Photo by Richard Ling
Research involving the stonefish—an animal that protects itself using razor-sharp, venom-filled spines—has provided unexpected insight into the human immune response that causes hematopoietic stem cell transplants (HSCTs) to fail.
The insight is now being used to develop immunosuppressants that could potentially improve the success rate of HSCTs.
Researchers explained this surprising connection in PNAS.
Their study indicated that the lethal component of stonefish venom, a protein called stonustoxin, is an ancient relative of the human immune protein perforin.
The body unleashes perforin to destroy virally infected and cancerous cells. Unwanted or excessive perforin activity is responsible for a range of medical problems, including the rejection of HSCTs.
Perforin proteins attach themselves to a cell and assemble to form giant pores on the cell surface. Each pore contains around 20 perforin proteins that stick together in a symmetrical fashion. The pores are big enough to allow toxins to enter the cell, killing it from within.
How these pores form is a mystery, but the current study has revealed a key part of the pore-assembly mechanism.
To make this discovery, the researchers used synchrotron radiation to visualize the atomic structure of stonustoxin. They found the toxin contains 2 perforin-like proteins stuck together.
Seeing how these 2 proteins interact has helped the researchers on their way to understanding how the full assembly of 20 perforin molecules forms a complete pore.
The team is also using their new insight to develop perforin inhibitors.
“Already, the structure of stonustoxin is starting to inform our drug development program, and we now understand the very first stages of perforin pore formation,” said James Whisstock, PhD, of Monash University in Melbourne, Victoria, Australia.
“This type of mechanistic information is extremely useful in developing new strategies to inhibit perforin itself.” ![]()

Photo by Richard Ling
Research involving the stonefish—an animal that protects itself using razor-sharp, venom-filled spines—has provided unexpected insight into the human immune response that causes hematopoietic stem cell transplants (HSCTs) to fail.
The insight is now being used to develop immunosuppressants that could potentially improve the success rate of HSCTs.
Researchers explained this surprising connection in PNAS.
Their study indicated that the lethal component of stonefish venom, a protein called stonustoxin, is an ancient relative of the human immune protein perforin.
The body unleashes perforin to destroy virally infected and cancerous cells. Unwanted or excessive perforin activity is responsible for a range of medical problems, including the rejection of HSCTs.
Perforin proteins attach themselves to a cell and assemble to form giant pores on the cell surface. Each pore contains around 20 perforin proteins that stick together in a symmetrical fashion. The pores are big enough to allow toxins to enter the cell, killing it from within.
How these pores form is a mystery, but the current study has revealed a key part of the pore-assembly mechanism.
To make this discovery, the researchers used synchrotron radiation to visualize the atomic structure of stonustoxin. They found the toxin contains 2 perforin-like proteins stuck together.
Seeing how these 2 proteins interact has helped the researchers on their way to understanding how the full assembly of 20 perforin molecules forms a complete pore.
The team is also using their new insight to develop perforin inhibitors.
“Already, the structure of stonustoxin is starting to inform our drug development program, and we now understand the very first stages of perforin pore formation,” said James Whisstock, PhD, of Monash University in Melbourne, Victoria, Australia.
“This type of mechanistic information is extremely useful in developing new strategies to inhibit perforin itself.” ![]()

Photo by Richard Ling
Research involving the stonefish—an animal that protects itself using razor-sharp, venom-filled spines—has provided unexpected insight into the human immune response that causes hematopoietic stem cell transplants (HSCTs) to fail.
The insight is now being used to develop immunosuppressants that could potentially improve the success rate of HSCTs.
Researchers explained this surprising connection in PNAS.
Their study indicated that the lethal component of stonefish venom, a protein called stonustoxin, is an ancient relative of the human immune protein perforin.
The body unleashes perforin to destroy virally infected and cancerous cells. Unwanted or excessive perforin activity is responsible for a range of medical problems, including the rejection of HSCTs.
Perforin proteins attach themselves to a cell and assemble to form giant pores on the cell surface. Each pore contains around 20 perforin proteins that stick together in a symmetrical fashion. The pores are big enough to allow toxins to enter the cell, killing it from within.
How these pores form is a mystery, but the current study has revealed a key part of the pore-assembly mechanism.
To make this discovery, the researchers used synchrotron radiation to visualize the atomic structure of stonustoxin. They found the toxin contains 2 perforin-like proteins stuck together.
Seeing how these 2 proteins interact has helped the researchers on their way to understanding how the full assembly of 20 perforin molecules forms a complete pore.
The team is also using their new insight to develop perforin inhibitors.
“Already, the structure of stonustoxin is starting to inform our drug development program, and we now understand the very first stages of perforin pore formation,” said James Whisstock, PhD, of Monash University in Melbourne, Victoria, Australia.
“This type of mechanistic information is extremely useful in developing new strategies to inhibit perforin itself.” ![]()
CK‐MB for Chest Pain and Suspected ACS
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 45‐year‐old man with medically controlled hypertension and a 40‐pack‐year smoking history presents to the emergency room complaining of intermittent chest pain for several days. He first noticed a sharp, knifelike sensation in the center of his chest when he reached for a glass in his kitchen a few days ago. The pain lasted for 30 seconds and resolved spontaneously. Since this time, he has had 2 subsequent episodes unrelated to exertion or rest. His physical exam is unremarkable, except for a body mass index of 29. An initial electrocardiogram shows no ischemic changes and no evidence of prior myocardial infarction.
He is currently chest‐painfree and admitted to the inpatient telemetry floor. Is ordering serial sets of creatine kinase (CK), creatine kinase‐myocardial band (CK‐MB), and troponin the most high‐value method to evaluate him for acute coronary syndrome (ACS)?
WHY YOU MIGHT THINK CK‐MB TESTING IS HELPFUL
CK‐MB has been used for 4 decades in the diagnostic evaluation of patients with chest pain and suspected ACS. Despite the advent of a more sensitive and specific test for myocardial injurythe cardiac troponinnearly 3 decades ago, 75% of US clinical pathology laboratories perform both CK‐MB and troponin assays, suggesting that many US physicians continue to order both tests in evaluating patients with chest pain.[1] There are several clinical scenarios in which physicians generally regard CK‐MB testing as useful in addition to troponin. These scenarios include CK‐MB testing (1) for the diagnosis of ACS in special patient populations, like those with acute or chronic renal disease, who are thought to have chronically elevated troponins as a function of their renal disease and not myocardial disease; (2) for additional prognostic information in the setting of a minimally elevated troponin; (3) for the detection of reinfarction, in which troponin is thought to be inferior to CK‐MB; and (4) for estimation of infarct size.
WHY CK‐MB TESTING ADDS NO ADDITIONAL VALUE TO TROPONIN TESTING IN DIAGNOSIS OF ACS
Is CK‐MB More Accurate Than Troponin in the Diagnosis of ACS?
Numerous studies have established that CK‐MB is not as specific as troponin for detecting myocardial injury and will result in more false‐positive tests.[2, 3] CK‐MB can be elevated in the setting of acute muscle injury (in 60% of patients), as well as chronic muscle disease (in 80% of patients). In contrast, troponin (I or T), a protein exclusively found in cardiac myocytes, is only elevated due to myocardial injury and is therefore more specific for ACS than CK‐MB.[2] In a study of patients with both skeletal muscle injury and suspected ACS, the respective specificities of troponin and CK‐MB were 94% and 63%, respectively.[3] In special patient populations, like those with chronic renal disease, both troponin and CK‐MB can be elevated in the absence of ACS; the mechanism for cardiac enzyme elevation is unclear. Importantly, there is no evidence to support the incremental value of CK‐MB over troponin alone in this population.[4, 5] Despite chronic troponin and CK‐MB elevations in some patients with chronic renal failure, it is still possible for these patients to have acute changes from baseline that represent myocardial injury. In these patients, cardiac biomarker results must be considered in the context of other clinical features (ie, the patient history, physical exam, and electrocardiogram findings) in making or excluding the diagnosis of ACS.
Does CK‐MB Diagnose ACS More Rapidly Than Troponin?
In patients with myocardial injury, both troponin and CK‐MB typically are detectable in the bloodstream within 2 to 4 hours of symptom onset and peak within 12 to 18 hours; neither has been established as a more rapid biomarker for the detection of myocardial infarction.[6] Furthermore, a systemic review of point‐of‐care cardiac enzyme testing reported that troponin and CK‐MB had similar positive and negative predictive values for diagnosing acute myocardial infarction (AMI) within the first 6 hours of symptom onset.[7]
Does CK‐MB Add Prognostic Information in Addition to Troponin in Patients With ACS?
If CK‐MB adds additional prognostic information in patients with suspected ACS and normal troponin values, then we should continue using it. Based on several large registries of patients with chest pain and/or ACS, approximately 8% to 28% of patients have discordant CK‐MB and troponin values, where 1 value is normal while the other value is abnormal. Several studies have examined whether an abnormal CK‐MB, in the setting of a normal troponin, offers additional prognostic information in comparison with normal values of both biomarkers.
In the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association guidelines) registry, a cohort of 29,357 patients with ACS was retrospectively divided into 4 groups: (1) patients with abnormal CK‐MB (CK‐MB+) and troponin (Tn+) values (ie, double‐positive group); (2) patients with normal CK‐MB (CK‐MB) and troponin (Tn) values (ie, double‐negative group); (3) patients with CK‐MB+/Tn; and (4) patients with CK‐MB/Tn+ values. Among the 4 groups, the rate of in‐hospital mortality was not significantly different between CK‐MB+/Tn (group 3) and patients with double‐negative (ie, normal) values. However, the presence of an abnormal troponin, regardless of CK‐MB status, was associated with an increased risk of in‐hospital death. The authors concluded that in clinical practice, there is little advantage of simultaneous CK‐MB and cTn testing for risk stratification in patients with high‐risk ACS presentations.[8]
In addition to the CRUSADE registry, 2 smaller registries, involving different patient populations, have reported similar results. An analysis of the Global Registry of Acute Coronary Events (GRACE) registry of 10,719 patients with ACS reported no difference between CK‐MB+/Tn patients and double‐negative patients with respect to in‐hospital mortality, as well as 6‐month mortality.[9] In the Internet Tracking Registry of Acute Coronary Syndromes (ITRACS) registry, 8769 patients presenting to emergency rooms with chest pain were analyzed. A minority (18.4%) were ultimately diagnosed with ACS. The authors found that an abnormal troponin, irrespective of CK‐MB status, was associated with an increased in‐hospital mortality rate. In‐hospital death rates were similar between CK‐MB+/Tn and double‐negative patients.[10]
In summary, troponin offers important prognostic information regardless of the CK‐MB result.
Is CK‐MB More Accurate for Diagnosing Reinfarction (Repeat Infarction in Patients With Recent Acute Myocardial Infarction)?
Whereas CK‐MB typically returns to normal within 2 to 3 days, troponin can be elevated for up to 5 to 14 days. Consequently, some have argued that CK‐MB may be more accurate in detecting reinfarction. In the only study to date comparing CK‐MB and troponin patterns in 9 patients with reinfarction, the rise and fall of both biomarkers were similar. Furthermore, those patients with persistently elevated troponin values from baseline (after the initial infarction) experienced a significant rise in troponin with reinfarction.[11]
Is CK‐MB More Accurate for Estimating Infarct Size?
Some have argued that a peak CK‐MB value is more accurate than a peak troponin value for estimating infarct size. However, 2 comparative studies have reported that troponin is as good as and possibly superior to CK‐MB for estimating infarct size. In a study of 65 patients with AMI, a single troponin T measurement obtained 72 hours after coronary care unit admission significantly correlated with peak CK‐MB in estimating infarct size (r=0.76, P<0.001), using single‐photon emission computed tomography imaging as the gold standard.[12] In a similar study of 37 patients with AMI, a single troponin T value had a significantly higher correlation with infarct size than serial and peak CK‐MB. Unlike CK‐MB, the ability of troponin T to predict infarct size was independent of coronary reperfusion.[13]
What do Guidelines and Thought Leaders Say About Using CK‐MB?
The most recent Third Universal Definition of Myocardial Infarction states that troponin is the preferred (cardiac) biomarker‐overall and for each specific category of MI, and that CK‐MB should be considered an alternative if troponin is not available.[14] Several national guidelines endorse troponin as the primary cardiac biomarker for diagnosis of ACS.[15, 16, 17] Finally, several groups have called for the elimination of CK‐MB. In 2008, 2 experts in the field of cardiovascular laboratory medicine argued that CK‐MB test adds little to no incremental information but does add cost andconfusion. Their institution, the Mayo Clinic, removed CK‐MB from their cardiac biomarker panel without any discernible negative effects on clinical care.[6] In a more recent publication, a group of authors from the departments of pathology and laboratory medicine of 7 major US academic medical centers identified CK‐MB as part of a top 10 list of antiquated tests that no longer provide value.[18]
WHAT YOU SHOULD DO INSTEAD: ORDER TROPONIN ALONE
In all cases where a patient presents with chest pain and/or symptoms concerning for ACS, we recommend that troponin be ordered alone. CK‐MB is no longer necessary as an additional test. As healthcare providers, we aim to provide the highest healthcare valuedefined as clinical benefit divided by cost. Routine ordering of CK‐MB offers essentially no benefit but does come at a significant cost. Each CK‐MB costs roughly $40 to $50 a test. If CK‐MB is used in approximately 2 million patients annually diagnosed with ACS and a proportion of the 17 million patients annually evaluated for chest pain, the potential cost, without clear benefit, is substantial.[19]
RECOMMENDATIONS
- In patients suspected of having ACS, troponin should be measured in lieu of CK‐MB and serial CK testing to evaluate for myocardial injury.
- CK‐MB tests should not be ordered routinely for patients suspected of having ACS. Hospitals should remove CK‐MB from pathology lab catalogs or require specific permission to order it.
CONCLUSION
Because CK‐MB, as compared to troponin, is detectable in the bloodstream in a similar timeframe, adds no additional prognostic information, estimates infarct size no differently, and appears to diagnose reinfarction no differently (Table 1), the authors believe that CK‐MB should no longer be ordered for patients with suspected ACS, unless ordering troponin is not an option. Ordering CK‐MB and serial CK for the evaluation of ACS is a Thing We Do for No Reason.
| Test Characteristic | CK‐MB | Troponin |
|---|---|---|
| ||
| Sensitivity | Lower than troponin | Higher than CK‐MB |
| Specificity | 60% to 70% | >94% |
| Diagnostic accuracy in patients with chronic renal failure | Equivalent | Equivalent |
| Rapidity of diagnosis | 24 hours | 2‐4 hours |
| Estimation of infarct size | Equivalent or possibly inferior to troponin | Equivalent or possibly superior to CK‐MB |
| Diagnosis of reinfarction | Equivalent | Equivalent |
Disclosures
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing
- , Creatine kinase‐MB: the journey to obsolescence. Am J Clin Pathol. 2014;141:415–419.
- , , , et al. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991;83:902–912.
- , , , et al. Cardiac troponin I: a marker with high specificity for cardiac injury. Circulation. 1993:88:101–106.
- , Cardiac troponin I in patients with chronic kidney disease stage 3 to 5 in conditions other than acute coronary syndrome. Clin Lab. 2014;60(2):281–290.
- , , , , Unmasking artifactual increases in creatine kinase isoenzymes in patients with renal failure. J Clin Lab Med. 1984;104:193–202.
- , Requiem for a heavyweight: the demise of creatine kinase‐MB. Circulation. 2008;118(21):2200–2206.
- , , , , Int J Cardiol. 2013;168(6):5355–5362.
- , , , et al. Frequency and clinical implications of discordant creatine kinase‐MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol. 2006;47(2):312–318.
- , , , et al.; GRACE Investigators. The diagnostic and prognostic impact of the redefinition of acute myocardial infarction: lessons from the Global Registry of Acute Coronary Events (GRACE). Am Heart J. 2006;151(3):654–660.
- , , , et al.; EMCREG‐i*trACS Investigators. Discordant cardiac biomarkers: frequency and outcomes in emergency department patients with chest pain. Ann Emerg Med. 2006:48(6):660–665.
- , Cardiac troponin and creatine kinase MB monitoring during in‐hospital myocardial reinfarction. Clin Chem. 2005;51:460–463.
- , , , , , Single‐point cardiac troponin T at coronary care unit discharge after myocardial infarction correlates with infarct size and ejection fraction. Clin Chem. 2002;48:1432–1436
- , , , , , Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart. 2002;87:520–524.
- , , , et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035.
- , , , et al. Institute for Clinical Systems Improvement. Diagnosis and Treatment of Chest Pain and Acute Coronary Syndrome (ACS). Available at: http://bit.ly.ACS1112. Updated November 2012.
- , , , et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction. J Am Coll Cardiol. 2013;61(23):e179–e347.
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions, , , , et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction. J Am Coll Cardiol. 2013;61(4):e78–e140.
- , , , , , Antiquated tests within the clinical pathology laboratory. Am J Manag Care. 2010;16(9):e220–e227.
- , , , , Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468–1474.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 45‐year‐old man with medically controlled hypertension and a 40‐pack‐year smoking history presents to the emergency room complaining of intermittent chest pain for several days. He first noticed a sharp, knifelike sensation in the center of his chest when he reached for a glass in his kitchen a few days ago. The pain lasted for 30 seconds and resolved spontaneously. Since this time, he has had 2 subsequent episodes unrelated to exertion or rest. His physical exam is unremarkable, except for a body mass index of 29. An initial electrocardiogram shows no ischemic changes and no evidence of prior myocardial infarction.
He is currently chest‐painfree and admitted to the inpatient telemetry floor. Is ordering serial sets of creatine kinase (CK), creatine kinase‐myocardial band (CK‐MB), and troponin the most high‐value method to evaluate him for acute coronary syndrome (ACS)?
WHY YOU MIGHT THINK CK‐MB TESTING IS HELPFUL
CK‐MB has been used for 4 decades in the diagnostic evaluation of patients with chest pain and suspected ACS. Despite the advent of a more sensitive and specific test for myocardial injurythe cardiac troponinnearly 3 decades ago, 75% of US clinical pathology laboratories perform both CK‐MB and troponin assays, suggesting that many US physicians continue to order both tests in evaluating patients with chest pain.[1] There are several clinical scenarios in which physicians generally regard CK‐MB testing as useful in addition to troponin. These scenarios include CK‐MB testing (1) for the diagnosis of ACS in special patient populations, like those with acute or chronic renal disease, who are thought to have chronically elevated troponins as a function of their renal disease and not myocardial disease; (2) for additional prognostic information in the setting of a minimally elevated troponin; (3) for the detection of reinfarction, in which troponin is thought to be inferior to CK‐MB; and (4) for estimation of infarct size.
WHY CK‐MB TESTING ADDS NO ADDITIONAL VALUE TO TROPONIN TESTING IN DIAGNOSIS OF ACS
Is CK‐MB More Accurate Than Troponin in the Diagnosis of ACS?
Numerous studies have established that CK‐MB is not as specific as troponin for detecting myocardial injury and will result in more false‐positive tests.[2, 3] CK‐MB can be elevated in the setting of acute muscle injury (in 60% of patients), as well as chronic muscle disease (in 80% of patients). In contrast, troponin (I or T), a protein exclusively found in cardiac myocytes, is only elevated due to myocardial injury and is therefore more specific for ACS than CK‐MB.[2] In a study of patients with both skeletal muscle injury and suspected ACS, the respective specificities of troponin and CK‐MB were 94% and 63%, respectively.[3] In special patient populations, like those with chronic renal disease, both troponin and CK‐MB can be elevated in the absence of ACS; the mechanism for cardiac enzyme elevation is unclear. Importantly, there is no evidence to support the incremental value of CK‐MB over troponin alone in this population.[4, 5] Despite chronic troponin and CK‐MB elevations in some patients with chronic renal failure, it is still possible for these patients to have acute changes from baseline that represent myocardial injury. In these patients, cardiac biomarker results must be considered in the context of other clinical features (ie, the patient history, physical exam, and electrocardiogram findings) in making or excluding the diagnosis of ACS.
Does CK‐MB Diagnose ACS More Rapidly Than Troponin?
In patients with myocardial injury, both troponin and CK‐MB typically are detectable in the bloodstream within 2 to 4 hours of symptom onset and peak within 12 to 18 hours; neither has been established as a more rapid biomarker for the detection of myocardial infarction.[6] Furthermore, a systemic review of point‐of‐care cardiac enzyme testing reported that troponin and CK‐MB had similar positive and negative predictive values for diagnosing acute myocardial infarction (AMI) within the first 6 hours of symptom onset.[7]
Does CK‐MB Add Prognostic Information in Addition to Troponin in Patients With ACS?
If CK‐MB adds additional prognostic information in patients with suspected ACS and normal troponin values, then we should continue using it. Based on several large registries of patients with chest pain and/or ACS, approximately 8% to 28% of patients have discordant CK‐MB and troponin values, where 1 value is normal while the other value is abnormal. Several studies have examined whether an abnormal CK‐MB, in the setting of a normal troponin, offers additional prognostic information in comparison with normal values of both biomarkers.
In the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association guidelines) registry, a cohort of 29,357 patients with ACS was retrospectively divided into 4 groups: (1) patients with abnormal CK‐MB (CK‐MB+) and troponin (Tn+) values (ie, double‐positive group); (2) patients with normal CK‐MB (CK‐MB) and troponin (Tn) values (ie, double‐negative group); (3) patients with CK‐MB+/Tn; and (4) patients with CK‐MB/Tn+ values. Among the 4 groups, the rate of in‐hospital mortality was not significantly different between CK‐MB+/Tn (group 3) and patients with double‐negative (ie, normal) values. However, the presence of an abnormal troponin, regardless of CK‐MB status, was associated with an increased risk of in‐hospital death. The authors concluded that in clinical practice, there is little advantage of simultaneous CK‐MB and cTn testing for risk stratification in patients with high‐risk ACS presentations.[8]
In addition to the CRUSADE registry, 2 smaller registries, involving different patient populations, have reported similar results. An analysis of the Global Registry of Acute Coronary Events (GRACE) registry of 10,719 patients with ACS reported no difference between CK‐MB+/Tn patients and double‐negative patients with respect to in‐hospital mortality, as well as 6‐month mortality.[9] In the Internet Tracking Registry of Acute Coronary Syndromes (ITRACS) registry, 8769 patients presenting to emergency rooms with chest pain were analyzed. A minority (18.4%) were ultimately diagnosed with ACS. The authors found that an abnormal troponin, irrespective of CK‐MB status, was associated with an increased in‐hospital mortality rate. In‐hospital death rates were similar between CK‐MB+/Tn and double‐negative patients.[10]
In summary, troponin offers important prognostic information regardless of the CK‐MB result.
Is CK‐MB More Accurate for Diagnosing Reinfarction (Repeat Infarction in Patients With Recent Acute Myocardial Infarction)?
Whereas CK‐MB typically returns to normal within 2 to 3 days, troponin can be elevated for up to 5 to 14 days. Consequently, some have argued that CK‐MB may be more accurate in detecting reinfarction. In the only study to date comparing CK‐MB and troponin patterns in 9 patients with reinfarction, the rise and fall of both biomarkers were similar. Furthermore, those patients with persistently elevated troponin values from baseline (after the initial infarction) experienced a significant rise in troponin with reinfarction.[11]
Is CK‐MB More Accurate for Estimating Infarct Size?
Some have argued that a peak CK‐MB value is more accurate than a peak troponin value for estimating infarct size. However, 2 comparative studies have reported that troponin is as good as and possibly superior to CK‐MB for estimating infarct size. In a study of 65 patients with AMI, a single troponin T measurement obtained 72 hours after coronary care unit admission significantly correlated with peak CK‐MB in estimating infarct size (r=0.76, P<0.001), using single‐photon emission computed tomography imaging as the gold standard.[12] In a similar study of 37 patients with AMI, a single troponin T value had a significantly higher correlation with infarct size than serial and peak CK‐MB. Unlike CK‐MB, the ability of troponin T to predict infarct size was independent of coronary reperfusion.[13]
What do Guidelines and Thought Leaders Say About Using CK‐MB?
The most recent Third Universal Definition of Myocardial Infarction states that troponin is the preferred (cardiac) biomarker‐overall and for each specific category of MI, and that CK‐MB should be considered an alternative if troponin is not available.[14] Several national guidelines endorse troponin as the primary cardiac biomarker for diagnosis of ACS.[15, 16, 17] Finally, several groups have called for the elimination of CK‐MB. In 2008, 2 experts in the field of cardiovascular laboratory medicine argued that CK‐MB test adds little to no incremental information but does add cost andconfusion. Their institution, the Mayo Clinic, removed CK‐MB from their cardiac biomarker panel without any discernible negative effects on clinical care.[6] In a more recent publication, a group of authors from the departments of pathology and laboratory medicine of 7 major US academic medical centers identified CK‐MB as part of a top 10 list of antiquated tests that no longer provide value.[18]
WHAT YOU SHOULD DO INSTEAD: ORDER TROPONIN ALONE
In all cases where a patient presents with chest pain and/or symptoms concerning for ACS, we recommend that troponin be ordered alone. CK‐MB is no longer necessary as an additional test. As healthcare providers, we aim to provide the highest healthcare valuedefined as clinical benefit divided by cost. Routine ordering of CK‐MB offers essentially no benefit but does come at a significant cost. Each CK‐MB costs roughly $40 to $50 a test. If CK‐MB is used in approximately 2 million patients annually diagnosed with ACS and a proportion of the 17 million patients annually evaluated for chest pain, the potential cost, without clear benefit, is substantial.[19]
RECOMMENDATIONS
- In patients suspected of having ACS, troponin should be measured in lieu of CK‐MB and serial CK testing to evaluate for myocardial injury.
- CK‐MB tests should not be ordered routinely for patients suspected of having ACS. Hospitals should remove CK‐MB from pathology lab catalogs or require specific permission to order it.
CONCLUSION
Because CK‐MB, as compared to troponin, is detectable in the bloodstream in a similar timeframe, adds no additional prognostic information, estimates infarct size no differently, and appears to diagnose reinfarction no differently (Table 1), the authors believe that CK‐MB should no longer be ordered for patients with suspected ACS, unless ordering troponin is not an option. Ordering CK‐MB and serial CK for the evaluation of ACS is a Thing We Do for No Reason.
| Test Characteristic | CK‐MB | Troponin |
|---|---|---|
| ||
| Sensitivity | Lower than troponin | Higher than CK‐MB |
| Specificity | 60% to 70% | >94% |
| Diagnostic accuracy in patients with chronic renal failure | Equivalent | Equivalent |
| Rapidity of diagnosis | 24 hours | 2‐4 hours |
| Estimation of infarct size | Equivalent or possibly inferior to troponin | Equivalent or possibly superior to CK‐MB |
| Diagnosis of reinfarction | Equivalent | Equivalent |
Disclosures
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 45‐year‐old man with medically controlled hypertension and a 40‐pack‐year smoking history presents to the emergency room complaining of intermittent chest pain for several days. He first noticed a sharp, knifelike sensation in the center of his chest when he reached for a glass in his kitchen a few days ago. The pain lasted for 30 seconds and resolved spontaneously. Since this time, he has had 2 subsequent episodes unrelated to exertion or rest. His physical exam is unremarkable, except for a body mass index of 29. An initial electrocardiogram shows no ischemic changes and no evidence of prior myocardial infarction.
He is currently chest‐painfree and admitted to the inpatient telemetry floor. Is ordering serial sets of creatine kinase (CK), creatine kinase‐myocardial band (CK‐MB), and troponin the most high‐value method to evaluate him for acute coronary syndrome (ACS)?
WHY YOU MIGHT THINK CK‐MB TESTING IS HELPFUL
CK‐MB has been used for 4 decades in the diagnostic evaluation of patients with chest pain and suspected ACS. Despite the advent of a more sensitive and specific test for myocardial injurythe cardiac troponinnearly 3 decades ago, 75% of US clinical pathology laboratories perform both CK‐MB and troponin assays, suggesting that many US physicians continue to order both tests in evaluating patients with chest pain.[1] There are several clinical scenarios in which physicians generally regard CK‐MB testing as useful in addition to troponin. These scenarios include CK‐MB testing (1) for the diagnosis of ACS in special patient populations, like those with acute or chronic renal disease, who are thought to have chronically elevated troponins as a function of their renal disease and not myocardial disease; (2) for additional prognostic information in the setting of a minimally elevated troponin; (3) for the detection of reinfarction, in which troponin is thought to be inferior to CK‐MB; and (4) for estimation of infarct size.
WHY CK‐MB TESTING ADDS NO ADDITIONAL VALUE TO TROPONIN TESTING IN DIAGNOSIS OF ACS
Is CK‐MB More Accurate Than Troponin in the Diagnosis of ACS?
Numerous studies have established that CK‐MB is not as specific as troponin for detecting myocardial injury and will result in more false‐positive tests.[2, 3] CK‐MB can be elevated in the setting of acute muscle injury (in 60% of patients), as well as chronic muscle disease (in 80% of patients). In contrast, troponin (I or T), a protein exclusively found in cardiac myocytes, is only elevated due to myocardial injury and is therefore more specific for ACS than CK‐MB.[2] In a study of patients with both skeletal muscle injury and suspected ACS, the respective specificities of troponin and CK‐MB were 94% and 63%, respectively.[3] In special patient populations, like those with chronic renal disease, both troponin and CK‐MB can be elevated in the absence of ACS; the mechanism for cardiac enzyme elevation is unclear. Importantly, there is no evidence to support the incremental value of CK‐MB over troponin alone in this population.[4, 5] Despite chronic troponin and CK‐MB elevations in some patients with chronic renal failure, it is still possible for these patients to have acute changes from baseline that represent myocardial injury. In these patients, cardiac biomarker results must be considered in the context of other clinical features (ie, the patient history, physical exam, and electrocardiogram findings) in making or excluding the diagnosis of ACS.
Does CK‐MB Diagnose ACS More Rapidly Than Troponin?
In patients with myocardial injury, both troponin and CK‐MB typically are detectable in the bloodstream within 2 to 4 hours of symptom onset and peak within 12 to 18 hours; neither has been established as a more rapid biomarker for the detection of myocardial infarction.[6] Furthermore, a systemic review of point‐of‐care cardiac enzyme testing reported that troponin and CK‐MB had similar positive and negative predictive values for diagnosing acute myocardial infarction (AMI) within the first 6 hours of symptom onset.[7]
Does CK‐MB Add Prognostic Information in Addition to Troponin in Patients With ACS?
If CK‐MB adds additional prognostic information in patients with suspected ACS and normal troponin values, then we should continue using it. Based on several large registries of patients with chest pain and/or ACS, approximately 8% to 28% of patients have discordant CK‐MB and troponin values, where 1 value is normal while the other value is abnormal. Several studies have examined whether an abnormal CK‐MB, in the setting of a normal troponin, offers additional prognostic information in comparison with normal values of both biomarkers.
In the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association guidelines) registry, a cohort of 29,357 patients with ACS was retrospectively divided into 4 groups: (1) patients with abnormal CK‐MB (CK‐MB+) and troponin (Tn+) values (ie, double‐positive group); (2) patients with normal CK‐MB (CK‐MB) and troponin (Tn) values (ie, double‐negative group); (3) patients with CK‐MB+/Tn; and (4) patients with CK‐MB/Tn+ values. Among the 4 groups, the rate of in‐hospital mortality was not significantly different between CK‐MB+/Tn (group 3) and patients with double‐negative (ie, normal) values. However, the presence of an abnormal troponin, regardless of CK‐MB status, was associated with an increased risk of in‐hospital death. The authors concluded that in clinical practice, there is little advantage of simultaneous CK‐MB and cTn testing for risk stratification in patients with high‐risk ACS presentations.[8]
In addition to the CRUSADE registry, 2 smaller registries, involving different patient populations, have reported similar results. An analysis of the Global Registry of Acute Coronary Events (GRACE) registry of 10,719 patients with ACS reported no difference between CK‐MB+/Tn patients and double‐negative patients with respect to in‐hospital mortality, as well as 6‐month mortality.[9] In the Internet Tracking Registry of Acute Coronary Syndromes (ITRACS) registry, 8769 patients presenting to emergency rooms with chest pain were analyzed. A minority (18.4%) were ultimately diagnosed with ACS. The authors found that an abnormal troponin, irrespective of CK‐MB status, was associated with an increased in‐hospital mortality rate. In‐hospital death rates were similar between CK‐MB+/Tn and double‐negative patients.[10]
In summary, troponin offers important prognostic information regardless of the CK‐MB result.
Is CK‐MB More Accurate for Diagnosing Reinfarction (Repeat Infarction in Patients With Recent Acute Myocardial Infarction)?
Whereas CK‐MB typically returns to normal within 2 to 3 days, troponin can be elevated for up to 5 to 14 days. Consequently, some have argued that CK‐MB may be more accurate in detecting reinfarction. In the only study to date comparing CK‐MB and troponin patterns in 9 patients with reinfarction, the rise and fall of both biomarkers were similar. Furthermore, those patients with persistently elevated troponin values from baseline (after the initial infarction) experienced a significant rise in troponin with reinfarction.[11]
Is CK‐MB More Accurate for Estimating Infarct Size?
Some have argued that a peak CK‐MB value is more accurate than a peak troponin value for estimating infarct size. However, 2 comparative studies have reported that troponin is as good as and possibly superior to CK‐MB for estimating infarct size. In a study of 65 patients with AMI, a single troponin T measurement obtained 72 hours after coronary care unit admission significantly correlated with peak CK‐MB in estimating infarct size (r=0.76, P<0.001), using single‐photon emission computed tomography imaging as the gold standard.[12] In a similar study of 37 patients with AMI, a single troponin T value had a significantly higher correlation with infarct size than serial and peak CK‐MB. Unlike CK‐MB, the ability of troponin T to predict infarct size was independent of coronary reperfusion.[13]
What do Guidelines and Thought Leaders Say About Using CK‐MB?
The most recent Third Universal Definition of Myocardial Infarction states that troponin is the preferred (cardiac) biomarker‐overall and for each specific category of MI, and that CK‐MB should be considered an alternative if troponin is not available.[14] Several national guidelines endorse troponin as the primary cardiac biomarker for diagnosis of ACS.[15, 16, 17] Finally, several groups have called for the elimination of CK‐MB. In 2008, 2 experts in the field of cardiovascular laboratory medicine argued that CK‐MB test adds little to no incremental information but does add cost andconfusion. Their institution, the Mayo Clinic, removed CK‐MB from their cardiac biomarker panel without any discernible negative effects on clinical care.[6] In a more recent publication, a group of authors from the departments of pathology and laboratory medicine of 7 major US academic medical centers identified CK‐MB as part of a top 10 list of antiquated tests that no longer provide value.[18]
WHAT YOU SHOULD DO INSTEAD: ORDER TROPONIN ALONE
In all cases where a patient presents with chest pain and/or symptoms concerning for ACS, we recommend that troponin be ordered alone. CK‐MB is no longer necessary as an additional test. As healthcare providers, we aim to provide the highest healthcare valuedefined as clinical benefit divided by cost. Routine ordering of CK‐MB offers essentially no benefit but does come at a significant cost. Each CK‐MB costs roughly $40 to $50 a test. If CK‐MB is used in approximately 2 million patients annually diagnosed with ACS and a proportion of the 17 million patients annually evaluated for chest pain, the potential cost, without clear benefit, is substantial.[19]
RECOMMENDATIONS
- In patients suspected of having ACS, troponin should be measured in lieu of CK‐MB and serial CK testing to evaluate for myocardial injury.
- CK‐MB tests should not be ordered routinely for patients suspected of having ACS. Hospitals should remove CK‐MB from pathology lab catalogs or require specific permission to order it.
CONCLUSION
Because CK‐MB, as compared to troponin, is detectable in the bloodstream in a similar timeframe, adds no additional prognostic information, estimates infarct size no differently, and appears to diagnose reinfarction no differently (Table 1), the authors believe that CK‐MB should no longer be ordered for patients with suspected ACS, unless ordering troponin is not an option. Ordering CK‐MB and serial CK for the evaluation of ACS is a Thing We Do for No Reason.
| Test Characteristic | CK‐MB | Troponin |
|---|---|---|
| ||
| Sensitivity | Lower than troponin | Higher than CK‐MB |
| Specificity | 60% to 70% | >94% |
| Diagnostic accuracy in patients with chronic renal failure | Equivalent | Equivalent |
| Rapidity of diagnosis | 24 hours | 2‐4 hours |
| Estimation of infarct size | Equivalent or possibly inferior to troponin | Equivalent or possibly superior to CK‐MB |
| Diagnosis of reinfarction | Equivalent | Equivalent |
Disclosures
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing
- , Creatine kinase‐MB: the journey to obsolescence. Am J Clin Pathol. 2014;141:415–419.
- , , , et al. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991;83:902–912.
- , , , et al. Cardiac troponin I: a marker with high specificity for cardiac injury. Circulation. 1993:88:101–106.
- , Cardiac troponin I in patients with chronic kidney disease stage 3 to 5 in conditions other than acute coronary syndrome. Clin Lab. 2014;60(2):281–290.
- , , , , Unmasking artifactual increases in creatine kinase isoenzymes in patients with renal failure. J Clin Lab Med. 1984;104:193–202.
- , Requiem for a heavyweight: the demise of creatine kinase‐MB. Circulation. 2008;118(21):2200–2206.
- , , , , Int J Cardiol. 2013;168(6):5355–5362.
- , , , et al. Frequency and clinical implications of discordant creatine kinase‐MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol. 2006;47(2):312–318.
- , , , et al.; GRACE Investigators. The diagnostic and prognostic impact of the redefinition of acute myocardial infarction: lessons from the Global Registry of Acute Coronary Events (GRACE). Am Heart J. 2006;151(3):654–660.
- , , , et al.; EMCREG‐i*trACS Investigators. Discordant cardiac biomarkers: frequency and outcomes in emergency department patients with chest pain. Ann Emerg Med. 2006:48(6):660–665.
- , Cardiac troponin and creatine kinase MB monitoring during in‐hospital myocardial reinfarction. Clin Chem. 2005;51:460–463.
- , , , , , Single‐point cardiac troponin T at coronary care unit discharge after myocardial infarction correlates with infarct size and ejection fraction. Clin Chem. 2002;48:1432–1436
- , , , , , Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart. 2002;87:520–524.
- , , , et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035.
- , , , et al. Institute for Clinical Systems Improvement. Diagnosis and Treatment of Chest Pain and Acute Coronary Syndrome (ACS). Available at: http://bit.ly.ACS1112. Updated November 2012.
- , , , et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction. J Am Coll Cardiol. 2013;61(23):e179–e347.
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions, , , , et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction. J Am Coll Cardiol. 2013;61(4):e78–e140.
- , , , , , Antiquated tests within the clinical pathology laboratory. Am J Manag Care. 2010;16(9):e220–e227.
- , , , , Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468–1474.
- , Creatine kinase‐MB: the journey to obsolescence. Am J Clin Pathol. 2014;141:415–419.
- , , , et al. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991;83:902–912.
- , , , et al. Cardiac troponin I: a marker with high specificity for cardiac injury. Circulation. 1993:88:101–106.
- , Cardiac troponin I in patients with chronic kidney disease stage 3 to 5 in conditions other than acute coronary syndrome. Clin Lab. 2014;60(2):281–290.
- , , , , Unmasking artifactual increases in creatine kinase isoenzymes in patients with renal failure. J Clin Lab Med. 1984;104:193–202.
- , Requiem for a heavyweight: the demise of creatine kinase‐MB. Circulation. 2008;118(21):2200–2206.
- , , , , Int J Cardiol. 2013;168(6):5355–5362.
- , , , et al. Frequency and clinical implications of discordant creatine kinase‐MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol. 2006;47(2):312–318.
- , , , et al.; GRACE Investigators. The diagnostic and prognostic impact of the redefinition of acute myocardial infarction: lessons from the Global Registry of Acute Coronary Events (GRACE). Am Heart J. 2006;151(3):654–660.
- , , , et al.; EMCREG‐i*trACS Investigators. Discordant cardiac biomarkers: frequency and outcomes in emergency department patients with chest pain. Ann Emerg Med. 2006:48(6):660–665.
- , Cardiac troponin and creatine kinase MB monitoring during in‐hospital myocardial reinfarction. Clin Chem. 2005;51:460–463.
- , , , , , Single‐point cardiac troponin T at coronary care unit discharge after myocardial infarction correlates with infarct size and ejection fraction. Clin Chem. 2002;48:1432–1436
- , , , , , Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart. 2002;87:520–524.
- , , , et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035.
- , , , et al. Institute for Clinical Systems Improvement. Diagnosis and Treatment of Chest Pain and Acute Coronary Syndrome (ACS). Available at: http://bit.ly.ACS1112. Updated November 2012.
- , , , et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction. J Am Coll Cardiol. 2013;61(23):e179–e347.
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions, , , , et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction. J Am Coll Cardiol. 2013;61(4):e78–e140.
- , , , , , Antiquated tests within the clinical pathology laboratory. Am J Manag Care. 2010;16(9):e220–e227.
- , , , , Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468–1474.
© 2015 Society of Hospital Medicine
ASH: Novel GBT440 reduces sickle cells, improves hematologic parameters
ORLANDO – An experimental agent that restores plasticity to red blood cells significantly improved hematologic parameters and reduced the deformation of red blood cells from sickle cell disease, suggest preliminary results from a phase I/II randomized clinical trial.
The drug, labeled GBT440, was well tolerated over one month, with no serious drug-related adverse events and no evidence of tissue hypoxia, said Dr. Claire Hemmaway from Queens Hospital in Essex, United Kingdom.
“We have emerging data with 28 days of dosing, and this supports the hypothesis that this drug inhibits polymerization of sickle hemoglobin, improves hemolysis, reduces red blood cell damage even in the micro-circulation, and improves oxygen delivery. Longer-term dosing is clearly required and this will define the optimal hematologic effects and the clinical benefit of this drug,” she said at the American Society of Hematology annual meeting.
Although its mechanism of action is not completely understood, GBT440 is a small-molecule hemoglobin modifier which is known to increase hemoglobin oxygen affinity. This first-in-class agent has been shown in both in vitro and in vivo studies to be a strong and direct anti-sickling agent. The drug has also been shown to inhibit polymerization of hemoglobin S, the central event in sickle cell disease, Dr. Hemmaway said.
“We hypothesized that patients on GBT440 will see a reduction in the red cell damage, see a reduction in hemolysis, and an improvement in the anemia, and with an inhibition of the formation of the sickle cells we’ll also see an improvement in blood flow, and this could potentially modify the course of sickle cell disease in our patients,” she said at a briefing prior to her presentation of the data in an oral abstract session.
To test the safety and efficacy of the drug, the investigators enrolled 64 healthy volunteers and 16 patients with homozygous HbSS sickle-cell disease into a phase I/II randomized, double-placebo controlled, parallel group trial.
The patients all had baseline hemoglobin levels from 6 to 10 g/dL, and had not had a vaso-occlusive crisis or transfusion with 30 days of screening.
The study was divided into parts, with part A testing single ascending doses, and part B testing multiple ascending doses compared with placebo on a 6:2 randomization basis.
As of July 24 2015, 54 healthy volunteers had completed the study, two were discontinued due to mild-to-moderate rash and/or headache, and eight were still on follow-up.
Eight patients with sickle cell disease completed part A, and eight were on follow-up in part B. No patients dropped out of the study, although one had a dose reduction from 700 mg to 400 mg because of abdominal discomfort.
Patients and volunteers generally tolerated the drug well, with generally mild adverse events, no deaths, and just one serious adverse event, an acute painful crisis in a patient on placebo.
The drug was shown to increase hemoglobin in both healthy volunteers and patients, reduce reticulocytosis, and improve biomarkers of hemolysis and inflammation. The hematologic effects correlate with GBT440 blood levels, Dr. Hemmaway said.
“You can see dramatic reductions in the reticulocyte counts, which maximally are reduced by more than 50%, and these are maintained over 28 days. The reduction in reticulocyte counts suggests an improvement in red cell life span,” she said.
One patient who received a 700 mg daily oral dose of GBT440 had evidence of a complete absence of sickled cells 28 days after starting on therapy, she noted.
The investigators have not been able to determine whether the hematologic improvements seen in the study correlate with symptomatic improvements, because they have only 28 days of dosing data and the results are still blinded, Dr. Hemmaway said.
A pediatric hematologist who was not involved in the study said that the early results offer hope for patients.
“I think many of us in the field continue to come back to the painful reality that as of today there is only one FDA-approved medication for sickle cell disease. That fundamentally is something that I think many of us are extremely troubled by, especially at a meeting like this where we see so many other opportunities in other conditions,” commented Dr. Alexis Thompson, a professor of hematology at the Robert H. Lurie Children’s Hospital of Chicago, Illinois.
Although the data are early, they are encouraging enough to justify moving forward with a phase II trial, which is planned to start in early 2016.
“I think the person who is going to be the ultimate beneficiary from many of these advances will be a child who can look forward to not only a longer lifespan but a lifespan that is less hampered by the complications of this disease,” she said.
Dr. Thompson moderated the briefing in which Dr. Hemmaway presented the data.
The study is supported by Global Blood Therapeutics. Dr. Hemmaway had no relevant disclosures. Several co-investigators are employees of the company. Dr, Thompson had no disclosures relevant to the study.
ORLANDO – An experimental agent that restores plasticity to red blood cells significantly improved hematologic parameters and reduced the deformation of red blood cells from sickle cell disease, suggest preliminary results from a phase I/II randomized clinical trial.
The drug, labeled GBT440, was well tolerated over one month, with no serious drug-related adverse events and no evidence of tissue hypoxia, said Dr. Claire Hemmaway from Queens Hospital in Essex, United Kingdom.
“We have emerging data with 28 days of dosing, and this supports the hypothesis that this drug inhibits polymerization of sickle hemoglobin, improves hemolysis, reduces red blood cell damage even in the micro-circulation, and improves oxygen delivery. Longer-term dosing is clearly required and this will define the optimal hematologic effects and the clinical benefit of this drug,” she said at the American Society of Hematology annual meeting.
Although its mechanism of action is not completely understood, GBT440 is a small-molecule hemoglobin modifier which is known to increase hemoglobin oxygen affinity. This first-in-class agent has been shown in both in vitro and in vivo studies to be a strong and direct anti-sickling agent. The drug has also been shown to inhibit polymerization of hemoglobin S, the central event in sickle cell disease, Dr. Hemmaway said.
“We hypothesized that patients on GBT440 will see a reduction in the red cell damage, see a reduction in hemolysis, and an improvement in the anemia, and with an inhibition of the formation of the sickle cells we’ll also see an improvement in blood flow, and this could potentially modify the course of sickle cell disease in our patients,” she said at a briefing prior to her presentation of the data in an oral abstract session.
To test the safety and efficacy of the drug, the investigators enrolled 64 healthy volunteers and 16 patients with homozygous HbSS sickle-cell disease into a phase I/II randomized, double-placebo controlled, parallel group trial.
The patients all had baseline hemoglobin levels from 6 to 10 g/dL, and had not had a vaso-occlusive crisis or transfusion with 30 days of screening.
The study was divided into parts, with part A testing single ascending doses, and part B testing multiple ascending doses compared with placebo on a 6:2 randomization basis.
As of July 24 2015, 54 healthy volunteers had completed the study, two were discontinued due to mild-to-moderate rash and/or headache, and eight were still on follow-up.
Eight patients with sickle cell disease completed part A, and eight were on follow-up in part B. No patients dropped out of the study, although one had a dose reduction from 700 mg to 400 mg because of abdominal discomfort.
Patients and volunteers generally tolerated the drug well, with generally mild adverse events, no deaths, and just one serious adverse event, an acute painful crisis in a patient on placebo.
The drug was shown to increase hemoglobin in both healthy volunteers and patients, reduce reticulocytosis, and improve biomarkers of hemolysis and inflammation. The hematologic effects correlate with GBT440 blood levels, Dr. Hemmaway said.
“You can see dramatic reductions in the reticulocyte counts, which maximally are reduced by more than 50%, and these are maintained over 28 days. The reduction in reticulocyte counts suggests an improvement in red cell life span,” she said.
One patient who received a 700 mg daily oral dose of GBT440 had evidence of a complete absence of sickled cells 28 days after starting on therapy, she noted.
The investigators have not been able to determine whether the hematologic improvements seen in the study correlate with symptomatic improvements, because they have only 28 days of dosing data and the results are still blinded, Dr. Hemmaway said.
A pediatric hematologist who was not involved in the study said that the early results offer hope for patients.
“I think many of us in the field continue to come back to the painful reality that as of today there is only one FDA-approved medication for sickle cell disease. That fundamentally is something that I think many of us are extremely troubled by, especially at a meeting like this where we see so many other opportunities in other conditions,” commented Dr. Alexis Thompson, a professor of hematology at the Robert H. Lurie Children’s Hospital of Chicago, Illinois.
Although the data are early, they are encouraging enough to justify moving forward with a phase II trial, which is planned to start in early 2016.
“I think the person who is going to be the ultimate beneficiary from many of these advances will be a child who can look forward to not only a longer lifespan but a lifespan that is less hampered by the complications of this disease,” she said.
Dr. Thompson moderated the briefing in which Dr. Hemmaway presented the data.
The study is supported by Global Blood Therapeutics. Dr. Hemmaway had no relevant disclosures. Several co-investigators are employees of the company. Dr, Thompson had no disclosures relevant to the study.
ORLANDO – An experimental agent that restores plasticity to red blood cells significantly improved hematologic parameters and reduced the deformation of red blood cells from sickle cell disease, suggest preliminary results from a phase I/II randomized clinical trial.
The drug, labeled GBT440, was well tolerated over one month, with no serious drug-related adverse events and no evidence of tissue hypoxia, said Dr. Claire Hemmaway from Queens Hospital in Essex, United Kingdom.
“We have emerging data with 28 days of dosing, and this supports the hypothesis that this drug inhibits polymerization of sickle hemoglobin, improves hemolysis, reduces red blood cell damage even in the micro-circulation, and improves oxygen delivery. Longer-term dosing is clearly required and this will define the optimal hematologic effects and the clinical benefit of this drug,” she said at the American Society of Hematology annual meeting.
Although its mechanism of action is not completely understood, GBT440 is a small-molecule hemoglobin modifier which is known to increase hemoglobin oxygen affinity. This first-in-class agent has been shown in both in vitro and in vivo studies to be a strong and direct anti-sickling agent. The drug has also been shown to inhibit polymerization of hemoglobin S, the central event in sickle cell disease, Dr. Hemmaway said.
“We hypothesized that patients on GBT440 will see a reduction in the red cell damage, see a reduction in hemolysis, and an improvement in the anemia, and with an inhibition of the formation of the sickle cells we’ll also see an improvement in blood flow, and this could potentially modify the course of sickle cell disease in our patients,” she said at a briefing prior to her presentation of the data in an oral abstract session.
To test the safety and efficacy of the drug, the investigators enrolled 64 healthy volunteers and 16 patients with homozygous HbSS sickle-cell disease into a phase I/II randomized, double-placebo controlled, parallel group trial.
The patients all had baseline hemoglobin levels from 6 to 10 g/dL, and had not had a vaso-occlusive crisis or transfusion with 30 days of screening.
The study was divided into parts, with part A testing single ascending doses, and part B testing multiple ascending doses compared with placebo on a 6:2 randomization basis.
As of July 24 2015, 54 healthy volunteers had completed the study, two were discontinued due to mild-to-moderate rash and/or headache, and eight were still on follow-up.
Eight patients with sickle cell disease completed part A, and eight were on follow-up in part B. No patients dropped out of the study, although one had a dose reduction from 700 mg to 400 mg because of abdominal discomfort.
Patients and volunteers generally tolerated the drug well, with generally mild adverse events, no deaths, and just one serious adverse event, an acute painful crisis in a patient on placebo.
The drug was shown to increase hemoglobin in both healthy volunteers and patients, reduce reticulocytosis, and improve biomarkers of hemolysis and inflammation. The hematologic effects correlate with GBT440 blood levels, Dr. Hemmaway said.
“You can see dramatic reductions in the reticulocyte counts, which maximally are reduced by more than 50%, and these are maintained over 28 days. The reduction in reticulocyte counts suggests an improvement in red cell life span,” she said.
One patient who received a 700 mg daily oral dose of GBT440 had evidence of a complete absence of sickled cells 28 days after starting on therapy, she noted.
The investigators have not been able to determine whether the hematologic improvements seen in the study correlate with symptomatic improvements, because they have only 28 days of dosing data and the results are still blinded, Dr. Hemmaway said.
A pediatric hematologist who was not involved in the study said that the early results offer hope for patients.
“I think many of us in the field continue to come back to the painful reality that as of today there is only one FDA-approved medication for sickle cell disease. That fundamentally is something that I think many of us are extremely troubled by, especially at a meeting like this where we see so many other opportunities in other conditions,” commented Dr. Alexis Thompson, a professor of hematology at the Robert H. Lurie Children’s Hospital of Chicago, Illinois.
Although the data are early, they are encouraging enough to justify moving forward with a phase II trial, which is planned to start in early 2016.
“I think the person who is going to be the ultimate beneficiary from many of these advances will be a child who can look forward to not only a longer lifespan but a lifespan that is less hampered by the complications of this disease,” she said.
Dr. Thompson moderated the briefing in which Dr. Hemmaway presented the data.
The study is supported by Global Blood Therapeutics. Dr. Hemmaway had no relevant disclosures. Several co-investigators are employees of the company. Dr, Thompson had no disclosures relevant to the study.
AT ASH 2015
Key clinical point: A first-in class small molecule drug improved hematologic parameters and reduced sickling of red blood cells.
Major finding: GBT440 inhibits polymerization of sickle hemoglobin, improves hemolysis, reduces red blood cell damage, and improves oxygen delivery.
Data source: Randomized, double blind, placebo-controlled phase I/II trial.
Disclosures: The study is supported by Global Blood Therapeutics. Dr. Hemmaway had no relevant disclosures. Several co-investigators are employees of the company. Dr, Thompson had no disclosures relevant to the study.
ASH: HLA-identical sibling transplants “excellent” in eligble SCD patients
ORLANDO – For patients with severe sickle cell disease, stem cell transplants from an HLA identical sibling are associated with “excellent” 3-year overall and event-free survival, results of a retrospective analysis show.
Data on 1,000 hematopoietic stem cell transplants (HSCT) in children and adults with sickle-cell disease performed in 88 centers in 23 countries showed a 3-year event-free-survival (EFS) rate of 90%, and 3-year overall survival (OS) rate of 94%, reported Dr. Barbara Cappelli from the Eurocord International Registry in Paris, France.
“Early referral to transplant for patients with severe sickle-cell disease is warranted, as age is an independent predictor for both event-free survival and overall survival,” she said about the study at a briefing at the American Society of Hematology annual meeting.
Patients who received donor cord blood had the best 3-year OS, at 99%, compared with 94% for patients who received bone marrow grafts, and 80% for those who received peripheral blood stem cells (PBSC).
“Transplants from peripheral blood are not recommended, because the result that there is higher mortality with transplants with peripheral blood,” she said.
The findings provide further evidence that for patients with severe sickle-disease who have an HLA-identical sibling available as a donor, HSCT can be curative with good safety, commented Dr. Alexis Thompson, a professor of hematology at the Robert H. Lurie Children’s Hospital of Chicago, Illinois.
Dr. Thompson was not involved in the study, but moderated a briefing where the data were presented.
International Registries
HSCT is the only therapy currently known to be curative for sickle-cell disease, but due to the risk for early and late complications it is generally reserved for patients with the most severe anemia or most disabling symptoms.
To evaluate the efficacy and safety of HSCT for sickle-cell disease, the investigators took a retrospective look at data from bone marrow transplant registries covering Europe, Brazil, the United States, and other reporting centers.
They searched for data on all patients with sickle-cell disease, children and adults, who received a transplant from an HLA identical sibling from 1986 through 2013.
The majority of patients (85%) were younger than 16, with the median age at HSCT of 9 years (range 1 to 54 years). Stroke was the most common indication for HSCT.
In all, 87% of patients received a myeloablative conditioning regimen based on busulfan combined with either cyclophosphamide or fludarabine. The remaining 13% of patients underwent a reduced-intensity conditioning regimen, primarily with fludarabine and cyclophosphamide.
In vivo T-cell depletion was performed in 70% of patients, either with anti-thymocyte globulin (630 patients) or with alemtumumab (76).
Prophylaxis for graft vs. host disease (GVHD) was predominantly cyclosporine as monotherapy or in combination with methotrexate.
A large majority of patients (84%) received bone marrow grafts, 7% got PBSCs, and 9% received umbilical cord blood.
By 60 days, the cumulative incidence of engraftment was 98%, with a median cumulative incidence of platelet engraftment of 25 days.
The cumulative incidence of acute GVHD (within 100 days of transplant) 14.3%, and the cumulative incidence of chronic GVHD (out to 3 years) was 13.3%
In all, 71 patients (7%) required autologous reconstitution, 45 because of late graft failure, 31 patients (3%) underwent a second transplant and 67 patients (7%) died. Of the patients who died, 6% had received bone marrow, 1% had received cord blood, and 21% had received PBSC.
As noted before, respective EFS and OS 3 years after transplant were 90% and 94%.
In multivariate analysis controlled for transplant and demographic characteristics, the authors found that younger age at transplant and use of bone marrow or cord blood were independently associated with better EFS and OS. In addition, 3-year overall survival was significantly better among patients who received transplants from the year 2000 on.
The risk for acute GVHD was significantly associated with increasing age, but none of the variables tested were associated with chronic GVHD.
Dr. Cappelli said that ways to improve outcomes include performing pre-or post-natal diagnosis in at-risk families, performing HLA typing of all family members at the time of diagnosis, and going to transplant as soon as the criteria for severe sickle-cell disease are met.
The study was supported by Eurocord-Monacord, EBMT Paediatric Disease Working Party and CIBMTR. Dr. Cappelli and Dr. Johnson reported having no conflicts of interest.
ORLANDO – For patients with severe sickle cell disease, stem cell transplants from an HLA identical sibling are associated with “excellent” 3-year overall and event-free survival, results of a retrospective analysis show.
Data on 1,000 hematopoietic stem cell transplants (HSCT) in children and adults with sickle-cell disease performed in 88 centers in 23 countries showed a 3-year event-free-survival (EFS) rate of 90%, and 3-year overall survival (OS) rate of 94%, reported Dr. Barbara Cappelli from the Eurocord International Registry in Paris, France.
“Early referral to transplant for patients with severe sickle-cell disease is warranted, as age is an independent predictor for both event-free survival and overall survival,” she said about the study at a briefing at the American Society of Hematology annual meeting.
Patients who received donor cord blood had the best 3-year OS, at 99%, compared with 94% for patients who received bone marrow grafts, and 80% for those who received peripheral blood stem cells (PBSC).
“Transplants from peripheral blood are not recommended, because the result that there is higher mortality with transplants with peripheral blood,” she said.
The findings provide further evidence that for patients with severe sickle-disease who have an HLA-identical sibling available as a donor, HSCT can be curative with good safety, commented Dr. Alexis Thompson, a professor of hematology at the Robert H. Lurie Children’s Hospital of Chicago, Illinois.
Dr. Thompson was not involved in the study, but moderated a briefing where the data were presented.
International Registries
HSCT is the only therapy currently known to be curative for sickle-cell disease, but due to the risk for early and late complications it is generally reserved for patients with the most severe anemia or most disabling symptoms.
To evaluate the efficacy and safety of HSCT for sickle-cell disease, the investigators took a retrospective look at data from bone marrow transplant registries covering Europe, Brazil, the United States, and other reporting centers.
They searched for data on all patients with sickle-cell disease, children and adults, who received a transplant from an HLA identical sibling from 1986 through 2013.
The majority of patients (85%) were younger than 16, with the median age at HSCT of 9 years (range 1 to 54 years). Stroke was the most common indication for HSCT.
In all, 87% of patients received a myeloablative conditioning regimen based on busulfan combined with either cyclophosphamide or fludarabine. The remaining 13% of patients underwent a reduced-intensity conditioning regimen, primarily with fludarabine and cyclophosphamide.
In vivo T-cell depletion was performed in 70% of patients, either with anti-thymocyte globulin (630 patients) or with alemtumumab (76).
Prophylaxis for graft vs. host disease (GVHD) was predominantly cyclosporine as monotherapy or in combination with methotrexate.
A large majority of patients (84%) received bone marrow grafts, 7% got PBSCs, and 9% received umbilical cord blood.
By 60 days, the cumulative incidence of engraftment was 98%, with a median cumulative incidence of platelet engraftment of 25 days.
The cumulative incidence of acute GVHD (within 100 days of transplant) 14.3%, and the cumulative incidence of chronic GVHD (out to 3 years) was 13.3%
In all, 71 patients (7%) required autologous reconstitution, 45 because of late graft failure, 31 patients (3%) underwent a second transplant and 67 patients (7%) died. Of the patients who died, 6% had received bone marrow, 1% had received cord blood, and 21% had received PBSC.
As noted before, respective EFS and OS 3 years after transplant were 90% and 94%.
In multivariate analysis controlled for transplant and demographic characteristics, the authors found that younger age at transplant and use of bone marrow or cord blood were independently associated with better EFS and OS. In addition, 3-year overall survival was significantly better among patients who received transplants from the year 2000 on.
The risk for acute GVHD was significantly associated with increasing age, but none of the variables tested were associated with chronic GVHD.
Dr. Cappelli said that ways to improve outcomes include performing pre-or post-natal diagnosis in at-risk families, performing HLA typing of all family members at the time of diagnosis, and going to transplant as soon as the criteria for severe sickle-cell disease are met.
The study was supported by Eurocord-Monacord, EBMT Paediatric Disease Working Party and CIBMTR. Dr. Cappelli and Dr. Johnson reported having no conflicts of interest.
ORLANDO – For patients with severe sickle cell disease, stem cell transplants from an HLA identical sibling are associated with “excellent” 3-year overall and event-free survival, results of a retrospective analysis show.
Data on 1,000 hematopoietic stem cell transplants (HSCT) in children and adults with sickle-cell disease performed in 88 centers in 23 countries showed a 3-year event-free-survival (EFS) rate of 90%, and 3-year overall survival (OS) rate of 94%, reported Dr. Barbara Cappelli from the Eurocord International Registry in Paris, France.
“Early referral to transplant for patients with severe sickle-cell disease is warranted, as age is an independent predictor for both event-free survival and overall survival,” she said about the study at a briefing at the American Society of Hematology annual meeting.
Patients who received donor cord blood had the best 3-year OS, at 99%, compared with 94% for patients who received bone marrow grafts, and 80% for those who received peripheral blood stem cells (PBSC).
“Transplants from peripheral blood are not recommended, because the result that there is higher mortality with transplants with peripheral blood,” she said.
The findings provide further evidence that for patients with severe sickle-disease who have an HLA-identical sibling available as a donor, HSCT can be curative with good safety, commented Dr. Alexis Thompson, a professor of hematology at the Robert H. Lurie Children’s Hospital of Chicago, Illinois.
Dr. Thompson was not involved in the study, but moderated a briefing where the data were presented.
International Registries
HSCT is the only therapy currently known to be curative for sickle-cell disease, but due to the risk for early and late complications it is generally reserved for patients with the most severe anemia or most disabling symptoms.
To evaluate the efficacy and safety of HSCT for sickle-cell disease, the investigators took a retrospective look at data from bone marrow transplant registries covering Europe, Brazil, the United States, and other reporting centers.
They searched for data on all patients with sickle-cell disease, children and adults, who received a transplant from an HLA identical sibling from 1986 through 2013.
The majority of patients (85%) were younger than 16, with the median age at HSCT of 9 years (range 1 to 54 years). Stroke was the most common indication for HSCT.
In all, 87% of patients received a myeloablative conditioning regimen based on busulfan combined with either cyclophosphamide or fludarabine. The remaining 13% of patients underwent a reduced-intensity conditioning regimen, primarily with fludarabine and cyclophosphamide.
In vivo T-cell depletion was performed in 70% of patients, either with anti-thymocyte globulin (630 patients) or with alemtumumab (76).
Prophylaxis for graft vs. host disease (GVHD) was predominantly cyclosporine as monotherapy or in combination with methotrexate.
A large majority of patients (84%) received bone marrow grafts, 7% got PBSCs, and 9% received umbilical cord blood.
By 60 days, the cumulative incidence of engraftment was 98%, with a median cumulative incidence of platelet engraftment of 25 days.
The cumulative incidence of acute GVHD (within 100 days of transplant) 14.3%, and the cumulative incidence of chronic GVHD (out to 3 years) was 13.3%
In all, 71 patients (7%) required autologous reconstitution, 45 because of late graft failure, 31 patients (3%) underwent a second transplant and 67 patients (7%) died. Of the patients who died, 6% had received bone marrow, 1% had received cord blood, and 21% had received PBSC.
As noted before, respective EFS and OS 3 years after transplant were 90% and 94%.
In multivariate analysis controlled for transplant and demographic characteristics, the authors found that younger age at transplant and use of bone marrow or cord blood were independently associated with better EFS and OS. In addition, 3-year overall survival was significantly better among patients who received transplants from the year 2000 on.
The risk for acute GVHD was significantly associated with increasing age, but none of the variables tested were associated with chronic GVHD.
Dr. Cappelli said that ways to improve outcomes include performing pre-or post-natal diagnosis in at-risk families, performing HLA typing of all family members at the time of diagnosis, and going to transplant as soon as the criteria for severe sickle-cell disease are met.
The study was supported by Eurocord-Monacord, EBMT Paediatric Disease Working Party and CIBMTR. Dr. Cappelli and Dr. Johnson reported having no conflicts of interest.
AT ASH 2015
Key clinical point: Bone marrow transplants from HLA-identical siblings are frequently curative of sickle-cell disease.
Major finding: Three-year event-free survival among 1000 patients was 90%, and 3-year overall survival was 94%.
Data source: Retrospective review of registry data on 1000 patients who underwent hematopoietic stem cell transplants with HLA-identical sibling donors.
Disclosures: The study was supported by Eurocord-Monacord, EBMT Paediatric Disease Working Party and CIBMTR. Dr. Cappelli and Dr. Johnson reported having no conflicts of interest.