User login
Can we be too efficient?
“We were all of us cogs in a great machine which sometimes rolled forward, nobody knew where, sometimes backwards, nobody knew why.” – Ernst Toller
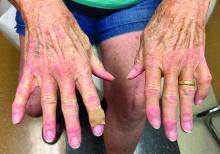
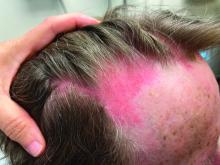
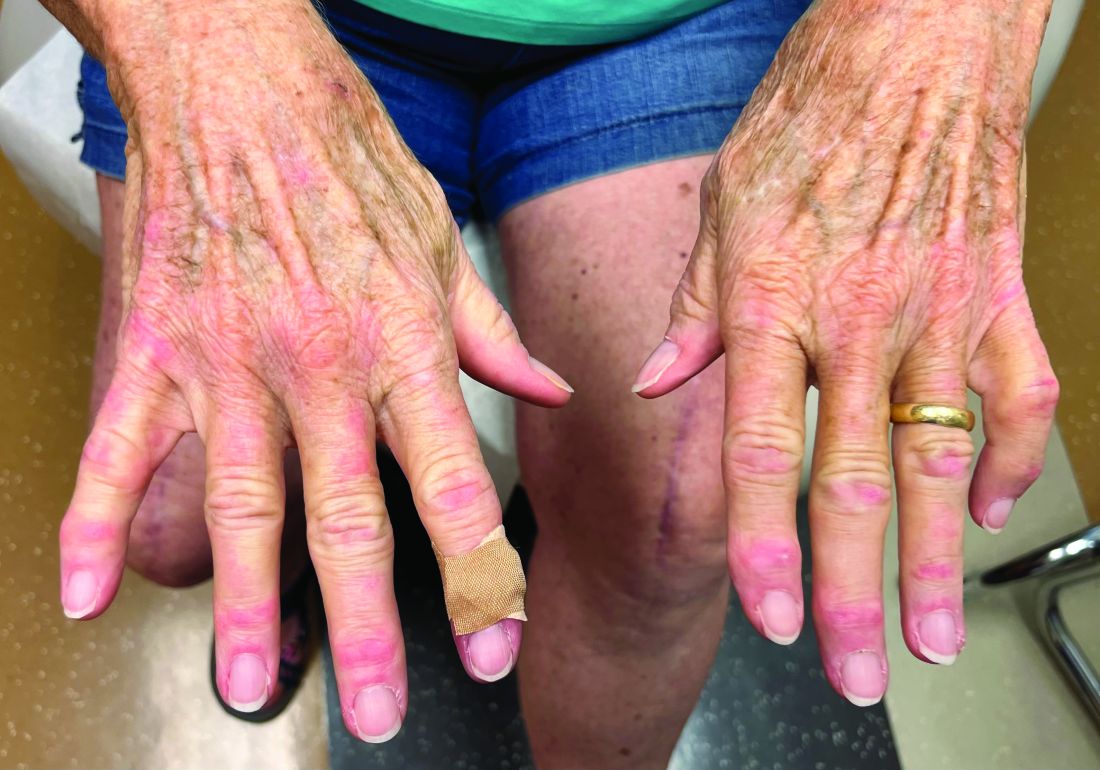
A nice feature of the Apple watch is the stopwatch. With it, I can discreetly click the timer and watch seconds tick away. Tap. There’s one lap. Tap. Two. Tap. That was a quick visit, 6 minutes and 42 seconds. Tap. Under 2 minutes to close the chart. Let’s see if I can beat it. Tap. Tap. What if I moved my Mayo stand over to this side of the room? How about a sign, “All patients must have clothes off if you want a skin exam.” You think ob.gyns. are quick from skin to baby in a stat C-section? You should see how fast I can go from alcohol wipe to Drysol on a biopsy. Seconds. Tick, tick, tap.
Every day I look for ways to go faster. This is not so I can be out the door by 3. Rather, it’s simply to make it through the day without having to log on after we put the kids to bed at night.
Speaking of bedtimes, another nice feature of the Apple watch is the timer. With it, I can set a timer and a lovely chimey alarm will go off. This comes in handy with 3-year-olds. “Sloan, in two minutes we are going to brush your teeth.” Ding. “Sloan, you have one minute to get your pajamas on.” Ding. “Sloanie, I’ll give you 3 more minutes to put the kitties away, then get into bed.” Ding, ding, ding ...
As you can see, using the stopwatch to time a bedtime routine would be demoralizing. If you’ve tried to put a toddler to bed in summer you know. They explore every option to avoid sleeping: one more book (that would make 3), “accidentally” putting their pajamas on backwards, offering to brush their teeth a second time. And once the light is off, “Papa, I have to potty.” No, bedtime routines cannot be standardized. They resist being made efficient.
In contrast, , Frederick Taylor. Taylor, a mechanical engineer, observed inefficiencies on the factory floor. His work was seminal in the development of the second industrial revolution. Before then no one had applied scientific rigor to productivity. His book, “The Principles of Scientific Management,” written in 1909, is considered the most influential management book of the 20th century. He was the first to use stopwatches to perform time studies, noting how long each task took with the belief that there was one best way. The worker was an extension of the machine, tuned by management such that he was as efficient as possible.

Others built on this idea including Frank and Lillian Gilbreth who added video recording, creating time and motion studies to further drive efficiency. This technique is still used in manufacturing and service industries today, including health care. In the 1980s, W. Edwards Deming modernized this effort, empowering workers with techniques taken from Japanese manufacturing. This, too, has been widely adopted in health care and evolved into the Lean and Lean Six Sigma quality movements about a decade ago. The common theme is to reduce waste to make health care as efficient as possible. Lately, this idea seems to have failed us.
The difficulty lies in the belief that efficient is always better. I’m unsure. Efficiency helps to reduce costs. It can also improve access. Yet, it comes at a cost. Eliminating slack concomitantly eliminates resilience. As such, when unexpected and significant changes impact a system, the gears of productivity jam. It’s in part why we are seeing rising wait times and patient dissatisfaction post pandemic. There was no slack and our system was too brittle.
A more insidious downside on the drive to efficiency lies in the nature of what we do. We aren’t factory workers punching out widgets, we’re physicians caring for people and people cannot be standardized. In this way, seeing patients is more like putting a toddler to bed than like assembling an iPhone. There will always be by-the-ways, basal cells hiding behind the ear, traffic jams, and bags of products that they want to review. Not sure how to use your fluorouracil? Let’s go over it again. Need to talk more about why you have granuloma annulare? Let me explain. Despite Taylor’s vision, some work simply cannot be optimized. And shouldn’t.
“Where’s my 11:30 patient who checked in half an hour ago?!” I asked my medical assistant. “Oh, she had to go to the bathroom.” Tap.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“We were all of us cogs in a great machine which sometimes rolled forward, nobody knew where, sometimes backwards, nobody knew why.” – Ernst Toller
A nice feature of the Apple watch is the stopwatch. With it, I can discreetly click the timer and watch seconds tick away. Tap. There’s one lap. Tap. Two. Tap. That was a quick visit, 6 minutes and 42 seconds. Tap. Under 2 minutes to close the chart. Let’s see if I can beat it. Tap. Tap. What if I moved my Mayo stand over to this side of the room? How about a sign, “All patients must have clothes off if you want a skin exam.” You think ob.gyns. are quick from skin to baby in a stat C-section? You should see how fast I can go from alcohol wipe to Drysol on a biopsy. Seconds. Tick, tick, tap.
Every day I look for ways to go faster. This is not so I can be out the door by 3. Rather, it’s simply to make it through the day without having to log on after we put the kids to bed at night.
Speaking of bedtimes, another nice feature of the Apple watch is the timer. With it, I can set a timer and a lovely chimey alarm will go off. This comes in handy with 3-year-olds. “Sloan, in two minutes we are going to brush your teeth.” Ding. “Sloan, you have one minute to get your pajamas on.” Ding. “Sloanie, I’ll give you 3 more minutes to put the kitties away, then get into bed.” Ding, ding, ding ...
As you can see, using the stopwatch to time a bedtime routine would be demoralizing. If you’ve tried to put a toddler to bed in summer you know. They explore every option to avoid sleeping: one more book (that would make 3), “accidentally” putting their pajamas on backwards, offering to brush their teeth a second time. And once the light is off, “Papa, I have to potty.” No, bedtime routines cannot be standardized. They resist being made efficient.
In contrast, , Frederick Taylor. Taylor, a mechanical engineer, observed inefficiencies on the factory floor. His work was seminal in the development of the second industrial revolution. Before then no one had applied scientific rigor to productivity. His book, “The Principles of Scientific Management,” written in 1909, is considered the most influential management book of the 20th century. He was the first to use stopwatches to perform time studies, noting how long each task took with the belief that there was one best way. The worker was an extension of the machine, tuned by management such that he was as efficient as possible.

Others built on this idea including Frank and Lillian Gilbreth who added video recording, creating time and motion studies to further drive efficiency. This technique is still used in manufacturing and service industries today, including health care. In the 1980s, W. Edwards Deming modernized this effort, empowering workers with techniques taken from Japanese manufacturing. This, too, has been widely adopted in health care and evolved into the Lean and Lean Six Sigma quality movements about a decade ago. The common theme is to reduce waste to make health care as efficient as possible. Lately, this idea seems to have failed us.
The difficulty lies in the belief that efficient is always better. I’m unsure. Efficiency helps to reduce costs. It can also improve access. Yet, it comes at a cost. Eliminating slack concomitantly eliminates resilience. As such, when unexpected and significant changes impact a system, the gears of productivity jam. It’s in part why we are seeing rising wait times and patient dissatisfaction post pandemic. There was no slack and our system was too brittle.
A more insidious downside on the drive to efficiency lies in the nature of what we do. We aren’t factory workers punching out widgets, we’re physicians caring for people and people cannot be standardized. In this way, seeing patients is more like putting a toddler to bed than like assembling an iPhone. There will always be by-the-ways, basal cells hiding behind the ear, traffic jams, and bags of products that they want to review. Not sure how to use your fluorouracil? Let’s go over it again. Need to talk more about why you have granuloma annulare? Let me explain. Despite Taylor’s vision, some work simply cannot be optimized. And shouldn’t.
“Where’s my 11:30 patient who checked in half an hour ago?!” I asked my medical assistant. “Oh, she had to go to the bathroom.” Tap.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“We were all of us cogs in a great machine which sometimes rolled forward, nobody knew where, sometimes backwards, nobody knew why.” – Ernst Toller
A nice feature of the Apple watch is the stopwatch. With it, I can discreetly click the timer and watch seconds tick away. Tap. There’s one lap. Tap. Two. Tap. That was a quick visit, 6 minutes and 42 seconds. Tap. Under 2 minutes to close the chart. Let’s see if I can beat it. Tap. Tap. What if I moved my Mayo stand over to this side of the room? How about a sign, “All patients must have clothes off if you want a skin exam.” You think ob.gyns. are quick from skin to baby in a stat C-section? You should see how fast I can go from alcohol wipe to Drysol on a biopsy. Seconds. Tick, tick, tap.
Every day I look for ways to go faster. This is not so I can be out the door by 3. Rather, it’s simply to make it through the day without having to log on after we put the kids to bed at night.
Speaking of bedtimes, another nice feature of the Apple watch is the timer. With it, I can set a timer and a lovely chimey alarm will go off. This comes in handy with 3-year-olds. “Sloan, in two minutes we are going to brush your teeth.” Ding. “Sloan, you have one minute to get your pajamas on.” Ding. “Sloanie, I’ll give you 3 more minutes to put the kitties away, then get into bed.” Ding, ding, ding ...
As you can see, using the stopwatch to time a bedtime routine would be demoralizing. If you’ve tried to put a toddler to bed in summer you know. They explore every option to avoid sleeping: one more book (that would make 3), “accidentally” putting their pajamas on backwards, offering to brush their teeth a second time. And once the light is off, “Papa, I have to potty.” No, bedtime routines cannot be standardized. They resist being made efficient.
In contrast, , Frederick Taylor. Taylor, a mechanical engineer, observed inefficiencies on the factory floor. His work was seminal in the development of the second industrial revolution. Before then no one had applied scientific rigor to productivity. His book, “The Principles of Scientific Management,” written in 1909, is considered the most influential management book of the 20th century. He was the first to use stopwatches to perform time studies, noting how long each task took with the belief that there was one best way. The worker was an extension of the machine, tuned by management such that he was as efficient as possible.

Others built on this idea including Frank and Lillian Gilbreth who added video recording, creating time and motion studies to further drive efficiency. This technique is still used in manufacturing and service industries today, including health care. In the 1980s, W. Edwards Deming modernized this effort, empowering workers with techniques taken from Japanese manufacturing. This, too, has been widely adopted in health care and evolved into the Lean and Lean Six Sigma quality movements about a decade ago. The common theme is to reduce waste to make health care as efficient as possible. Lately, this idea seems to have failed us.
The difficulty lies in the belief that efficient is always better. I’m unsure. Efficiency helps to reduce costs. It can also improve access. Yet, it comes at a cost. Eliminating slack concomitantly eliminates resilience. As such, when unexpected and significant changes impact a system, the gears of productivity jam. It’s in part why we are seeing rising wait times and patient dissatisfaction post pandemic. There was no slack and our system was too brittle.
A more insidious downside on the drive to efficiency lies in the nature of what we do. We aren’t factory workers punching out widgets, we’re physicians caring for people and people cannot be standardized. In this way, seeing patients is more like putting a toddler to bed than like assembling an iPhone. There will always be by-the-ways, basal cells hiding behind the ear, traffic jams, and bags of products that they want to review. Not sure how to use your fluorouracil? Let’s go over it again. Need to talk more about why you have granuloma annulare? Let me explain. Despite Taylor’s vision, some work simply cannot be optimized. And shouldn’t.
“Where’s my 11:30 patient who checked in half an hour ago?!” I asked my medical assistant. “Oh, she had to go to the bathroom.” Tap.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Could colchicine replace aspirin after PCI for ACS?
Dual antiplatelet therapy (DAPT) consisting of aspirin plus a P2Y12 inhibitor has been the standard of care to prevent thrombotic events in patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI).
in these patients, while mitigating the increased bleeding risk associated with aspirin.
Investigators conducted a pilot trial in ACS patients treated with drug-eluting stents (DES) who received low-dose colchicine the day after PCI, together with P2Y12 inhibitor (ticagrelor or prasugrel) maintenance therapy. Aspirin use was discontinued.
At 3 months, only 1% of the patients experienced stent thrombosis, and only 1 patient showed high platelet reactivity. Moreover, at 1 month, high-sensitivity C-reactive protein (hs-CRP) and platelet reactivity both decreased, pointing to reduced inflammation.
“In ACS patients undergoing PCI, it is feasible to discontinue aspirin therapy and administer low-dose colchicine on the day after PCI in addition to ticagrelor or prasugrel P2Y12 inhibitors,” write Seung-Yul Lee, MD, CHA Bundang Medical Center, CHA University, Seongnam, South Korea, and colleagues. “This approach is associated with favorable platelet function and inflammatory profiles.”
The study was published online in JACC: Cardiovascular Interventions.
Safety without compromised efficacy
The U.S. Food and Drug Administration recently approved colchicine 0.5-mg tablets (Lodoco, Agepha Pharma) as the first anti-inflammatory drug shown to reduce the risk for myocardial infarction, stroke, coronary revascularization, and cardiovascular death in adult patients with either established atherosclerotic disease or multiple risk factors for cardiovascular disease. It targets residual inflammation as an underlying cause of cardiovascular events.
Patients after PCI are generally treated using DAPT, but given the risk for increased bleeding associated with aspirin – especially when used long-term – there is a “need to identify strategies associated with a more favorable safety profile without compromising efficacy,” the authors write.
Previous research has yielded mixed results in terms of the discontinuation of aspirin therapy after 1-3 months and maintenance on P2Y12 inhibitor monotherapy. But one trial found colchicine to be effective in reducing recurrent ischemia, and its benefits may be more beneficial with early initiation in the hospital.
In this new study, researchers tested a “strategy that substitutes aspirin with colchicine during the acute phase to maximize the treatment effect of reducing recurrent ischemia and bleeding,” they write. The Mono Antiplatelet and Colchicine Therapy (MACT) single-arm, open-label proof-of-concept study was designed to investigate this approach.
The researchers studied 200 patients with non–ST-segment elevation ACS and ST-segment elevation myocardial infarction (STEMI) who underwent PCI with DES (mean [SD] age, 61.4 [10.7] years; 90% male; 100% of Asian ethnicity), who were receiving either ticagrelor or prasugrel plus a loading dose of aspirin.
On the day after PCI, aspirin was discontinued, and low-dose colchicine (0.6 mg once daily) was administered in addition to the P2Y12 inhibitor. In the case of staged PCI, it was performed under the maintenance of colchicine and ticagrelor or prasugrel.
No other antiplatelet or anticoagulant agents were permitted.
Patients underwent platelet function testing using the VerifyNow P2Y12 assay before discharge. Levels of hs-CRP were measured at admission, at 24 and 48 hours after PCI, and at 1-month follow-up. Clinical follow-up was performed at 1 and at 3 months.
The primary outcome was stent thrombosis within 3 months of follow-up. Secondary outcomes included all-cause mortality, MI, revascularization, major bleeding, a composite of cardiac death, target vessel MI, or target lesion revascularization, P2Y12 reaction units (PRUs), and change in hs-CRP levels between 24 hours post-PCI and 1-month follow-up.
The role of inflammation
Of the original 200 patients, 190 completed the full protocol and were available for follow-up.
The primary outcome occurred in only two patients. It turned out that one of the patients had not been adherent with antiplatelet medications.
“Although bleeding occurred in 36 patients, major bleeding occurred in only 1 patient,” the authors report.
The level of platelet reactivity at discharge was 27 ± 42 PRUs. Most patients (91%) met the criteria for low platelet reactivity, while only 0.5% met the criteria for high platelet reactivity. Platelet reactivity was similar, regardless of which P2Y12 inhibitor (ticagrelor or prasugrel) the patients were taking.
In all patients, the level of inflammation was “reduced considerably” over time: After 1 month, the hs-CRP level decreased from 6.1 mg/L (interquartile range [IQR], 2.6-15.9 mg/L) at 24 hours after PCI to 0.6 mg/L (IQR, 0.4-1.2 mg/L; P < .001).
The prevalence of high-inflammation criteria, defined as hs-CRP ≥ 2 mg/L, decreased significantly, from 81.8% at 24 hours after PCI to 11.8% at 1 month (P < .001).
Major bleeding was rare, they report, with a 3-month incidence of 0.5%.
“Inflammation plays a fundamental role in the development and progression of the atherothrombotic process,” the authors explain. A series of factors also trigger “an intense inflammatory response” in the acute phase of MI, which may lead to adverse myocardial remodeling. In the present study, inflammatory levels were rapidly reduced.
They noted several limitations. For example, all enrolled patients were Asian and were at relatively low bleeding and ischemic risk. “Although ticagrelor or prasugrel is effective regardless of ethnicity, clinical data supporting this de-escalation strategy are limited,” they state. Additionally, there was no control group for comparison.
The findings warrant further investigation, they conclude.
Promising but preliminary
Commenting for this news organization, Francesco Costa, MD, PhD, interventional cardiologist and assistant professor, University of Messina, Sicily, Italy, said he thinks it’s “too early for extensive clinical translation of these findings.”
Rather, larger and more extensive randomized trials are “on their way to give more precise estimates regarding the risks and benefits of early aspirin withdrawal in ACS.”
However, added Dr. Costa, who was not involved with the current research, “in this setting, adding colchicine early looks very promising to mitigate potential thrombotic risk without increasing bleeding risk.”
In the meantime, the study “provides novel insights on early aspirin withdrawal and P2Y12 monotherapy in an unselected population, including [those with] STEMI,” said Dr. Costa, also the coauthor of an accompanying editorial. The findings “could be of particular interest for those patients at extremely high bleeding risk or who are truly intolerant to aspirin, a scenario in which options are limited.”
This study was supported by the Cardiovascular Research Center, Seoul, South Korea. Dr. Lee reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Costa has served on an advisory board for AstraZeneca and has received speaker fees from Chiesi Farmaceutici. His coauthor reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
Dual antiplatelet therapy (DAPT) consisting of aspirin plus a P2Y12 inhibitor has been the standard of care to prevent thrombotic events in patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI).
in these patients, while mitigating the increased bleeding risk associated with aspirin.
Investigators conducted a pilot trial in ACS patients treated with drug-eluting stents (DES) who received low-dose colchicine the day after PCI, together with P2Y12 inhibitor (ticagrelor or prasugrel) maintenance therapy. Aspirin use was discontinued.
At 3 months, only 1% of the patients experienced stent thrombosis, and only 1 patient showed high platelet reactivity. Moreover, at 1 month, high-sensitivity C-reactive protein (hs-CRP) and platelet reactivity both decreased, pointing to reduced inflammation.
“In ACS patients undergoing PCI, it is feasible to discontinue aspirin therapy and administer low-dose colchicine on the day after PCI in addition to ticagrelor or prasugrel P2Y12 inhibitors,” write Seung-Yul Lee, MD, CHA Bundang Medical Center, CHA University, Seongnam, South Korea, and colleagues. “This approach is associated with favorable platelet function and inflammatory profiles.”
The study was published online in JACC: Cardiovascular Interventions.
Safety without compromised efficacy
The U.S. Food and Drug Administration recently approved colchicine 0.5-mg tablets (Lodoco, Agepha Pharma) as the first anti-inflammatory drug shown to reduce the risk for myocardial infarction, stroke, coronary revascularization, and cardiovascular death in adult patients with either established atherosclerotic disease or multiple risk factors for cardiovascular disease. It targets residual inflammation as an underlying cause of cardiovascular events.
Patients after PCI are generally treated using DAPT, but given the risk for increased bleeding associated with aspirin – especially when used long-term – there is a “need to identify strategies associated with a more favorable safety profile without compromising efficacy,” the authors write.
Previous research has yielded mixed results in terms of the discontinuation of aspirin therapy after 1-3 months and maintenance on P2Y12 inhibitor monotherapy. But one trial found colchicine to be effective in reducing recurrent ischemia, and its benefits may be more beneficial with early initiation in the hospital.
In this new study, researchers tested a “strategy that substitutes aspirin with colchicine during the acute phase to maximize the treatment effect of reducing recurrent ischemia and bleeding,” they write. The Mono Antiplatelet and Colchicine Therapy (MACT) single-arm, open-label proof-of-concept study was designed to investigate this approach.
The researchers studied 200 patients with non–ST-segment elevation ACS and ST-segment elevation myocardial infarction (STEMI) who underwent PCI with DES (mean [SD] age, 61.4 [10.7] years; 90% male; 100% of Asian ethnicity), who were receiving either ticagrelor or prasugrel plus a loading dose of aspirin.
On the day after PCI, aspirin was discontinued, and low-dose colchicine (0.6 mg once daily) was administered in addition to the P2Y12 inhibitor. In the case of staged PCI, it was performed under the maintenance of colchicine and ticagrelor or prasugrel.
No other antiplatelet or anticoagulant agents were permitted.
Patients underwent platelet function testing using the VerifyNow P2Y12 assay before discharge. Levels of hs-CRP were measured at admission, at 24 and 48 hours after PCI, and at 1-month follow-up. Clinical follow-up was performed at 1 and at 3 months.
The primary outcome was stent thrombosis within 3 months of follow-up. Secondary outcomes included all-cause mortality, MI, revascularization, major bleeding, a composite of cardiac death, target vessel MI, or target lesion revascularization, P2Y12 reaction units (PRUs), and change in hs-CRP levels between 24 hours post-PCI and 1-month follow-up.
The role of inflammation
Of the original 200 patients, 190 completed the full protocol and were available for follow-up.
The primary outcome occurred in only two patients. It turned out that one of the patients had not been adherent with antiplatelet medications.
“Although bleeding occurred in 36 patients, major bleeding occurred in only 1 patient,” the authors report.
The level of platelet reactivity at discharge was 27 ± 42 PRUs. Most patients (91%) met the criteria for low platelet reactivity, while only 0.5% met the criteria for high platelet reactivity. Platelet reactivity was similar, regardless of which P2Y12 inhibitor (ticagrelor or prasugrel) the patients were taking.
In all patients, the level of inflammation was “reduced considerably” over time: After 1 month, the hs-CRP level decreased from 6.1 mg/L (interquartile range [IQR], 2.6-15.9 mg/L) at 24 hours after PCI to 0.6 mg/L (IQR, 0.4-1.2 mg/L; P < .001).
The prevalence of high-inflammation criteria, defined as hs-CRP ≥ 2 mg/L, decreased significantly, from 81.8% at 24 hours after PCI to 11.8% at 1 month (P < .001).
Major bleeding was rare, they report, with a 3-month incidence of 0.5%.
“Inflammation plays a fundamental role in the development and progression of the atherothrombotic process,” the authors explain. A series of factors also trigger “an intense inflammatory response” in the acute phase of MI, which may lead to adverse myocardial remodeling. In the present study, inflammatory levels were rapidly reduced.
They noted several limitations. For example, all enrolled patients were Asian and were at relatively low bleeding and ischemic risk. “Although ticagrelor or prasugrel is effective regardless of ethnicity, clinical data supporting this de-escalation strategy are limited,” they state. Additionally, there was no control group for comparison.
The findings warrant further investigation, they conclude.
Promising but preliminary
Commenting for this news organization, Francesco Costa, MD, PhD, interventional cardiologist and assistant professor, University of Messina, Sicily, Italy, said he thinks it’s “too early for extensive clinical translation of these findings.”
Rather, larger and more extensive randomized trials are “on their way to give more precise estimates regarding the risks and benefits of early aspirin withdrawal in ACS.”
However, added Dr. Costa, who was not involved with the current research, “in this setting, adding colchicine early looks very promising to mitigate potential thrombotic risk without increasing bleeding risk.”
In the meantime, the study “provides novel insights on early aspirin withdrawal and P2Y12 monotherapy in an unselected population, including [those with] STEMI,” said Dr. Costa, also the coauthor of an accompanying editorial. The findings “could be of particular interest for those patients at extremely high bleeding risk or who are truly intolerant to aspirin, a scenario in which options are limited.”
This study was supported by the Cardiovascular Research Center, Seoul, South Korea. Dr. Lee reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Costa has served on an advisory board for AstraZeneca and has received speaker fees from Chiesi Farmaceutici. His coauthor reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
Dual antiplatelet therapy (DAPT) consisting of aspirin plus a P2Y12 inhibitor has been the standard of care to prevent thrombotic events in patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI).
in these patients, while mitigating the increased bleeding risk associated with aspirin.
Investigators conducted a pilot trial in ACS patients treated with drug-eluting stents (DES) who received low-dose colchicine the day after PCI, together with P2Y12 inhibitor (ticagrelor or prasugrel) maintenance therapy. Aspirin use was discontinued.
At 3 months, only 1% of the patients experienced stent thrombosis, and only 1 patient showed high platelet reactivity. Moreover, at 1 month, high-sensitivity C-reactive protein (hs-CRP) and platelet reactivity both decreased, pointing to reduced inflammation.
“In ACS patients undergoing PCI, it is feasible to discontinue aspirin therapy and administer low-dose colchicine on the day after PCI in addition to ticagrelor or prasugrel P2Y12 inhibitors,” write Seung-Yul Lee, MD, CHA Bundang Medical Center, CHA University, Seongnam, South Korea, and colleagues. “This approach is associated with favorable platelet function and inflammatory profiles.”
The study was published online in JACC: Cardiovascular Interventions.
Safety without compromised efficacy
The U.S. Food and Drug Administration recently approved colchicine 0.5-mg tablets (Lodoco, Agepha Pharma) as the first anti-inflammatory drug shown to reduce the risk for myocardial infarction, stroke, coronary revascularization, and cardiovascular death in adult patients with either established atherosclerotic disease or multiple risk factors for cardiovascular disease. It targets residual inflammation as an underlying cause of cardiovascular events.
Patients after PCI are generally treated using DAPT, but given the risk for increased bleeding associated with aspirin – especially when used long-term – there is a “need to identify strategies associated with a more favorable safety profile without compromising efficacy,” the authors write.
Previous research has yielded mixed results in terms of the discontinuation of aspirin therapy after 1-3 months and maintenance on P2Y12 inhibitor monotherapy. But one trial found colchicine to be effective in reducing recurrent ischemia, and its benefits may be more beneficial with early initiation in the hospital.
In this new study, researchers tested a “strategy that substitutes aspirin with colchicine during the acute phase to maximize the treatment effect of reducing recurrent ischemia and bleeding,” they write. The Mono Antiplatelet and Colchicine Therapy (MACT) single-arm, open-label proof-of-concept study was designed to investigate this approach.
The researchers studied 200 patients with non–ST-segment elevation ACS and ST-segment elevation myocardial infarction (STEMI) who underwent PCI with DES (mean [SD] age, 61.4 [10.7] years; 90% male; 100% of Asian ethnicity), who were receiving either ticagrelor or prasugrel plus a loading dose of aspirin.
On the day after PCI, aspirin was discontinued, and low-dose colchicine (0.6 mg once daily) was administered in addition to the P2Y12 inhibitor. In the case of staged PCI, it was performed under the maintenance of colchicine and ticagrelor or prasugrel.
No other antiplatelet or anticoagulant agents were permitted.
Patients underwent platelet function testing using the VerifyNow P2Y12 assay before discharge. Levels of hs-CRP were measured at admission, at 24 and 48 hours after PCI, and at 1-month follow-up. Clinical follow-up was performed at 1 and at 3 months.
The primary outcome was stent thrombosis within 3 months of follow-up. Secondary outcomes included all-cause mortality, MI, revascularization, major bleeding, a composite of cardiac death, target vessel MI, or target lesion revascularization, P2Y12 reaction units (PRUs), and change in hs-CRP levels between 24 hours post-PCI and 1-month follow-up.
The role of inflammation
Of the original 200 patients, 190 completed the full protocol and were available for follow-up.
The primary outcome occurred in only two patients. It turned out that one of the patients had not been adherent with antiplatelet medications.
“Although bleeding occurred in 36 patients, major bleeding occurred in only 1 patient,” the authors report.
The level of platelet reactivity at discharge was 27 ± 42 PRUs. Most patients (91%) met the criteria for low platelet reactivity, while only 0.5% met the criteria for high platelet reactivity. Platelet reactivity was similar, regardless of which P2Y12 inhibitor (ticagrelor or prasugrel) the patients were taking.
In all patients, the level of inflammation was “reduced considerably” over time: After 1 month, the hs-CRP level decreased from 6.1 mg/L (interquartile range [IQR], 2.6-15.9 mg/L) at 24 hours after PCI to 0.6 mg/L (IQR, 0.4-1.2 mg/L; P < .001).
The prevalence of high-inflammation criteria, defined as hs-CRP ≥ 2 mg/L, decreased significantly, from 81.8% at 24 hours after PCI to 11.8% at 1 month (P < .001).
Major bleeding was rare, they report, with a 3-month incidence of 0.5%.
“Inflammation plays a fundamental role in the development and progression of the atherothrombotic process,” the authors explain. A series of factors also trigger “an intense inflammatory response” in the acute phase of MI, which may lead to adverse myocardial remodeling. In the present study, inflammatory levels were rapidly reduced.
They noted several limitations. For example, all enrolled patients were Asian and were at relatively low bleeding and ischemic risk. “Although ticagrelor or prasugrel is effective regardless of ethnicity, clinical data supporting this de-escalation strategy are limited,” they state. Additionally, there was no control group for comparison.
The findings warrant further investigation, they conclude.
Promising but preliminary
Commenting for this news organization, Francesco Costa, MD, PhD, interventional cardiologist and assistant professor, University of Messina, Sicily, Italy, said he thinks it’s “too early for extensive clinical translation of these findings.”
Rather, larger and more extensive randomized trials are “on their way to give more precise estimates regarding the risks and benefits of early aspirin withdrawal in ACS.”
However, added Dr. Costa, who was not involved with the current research, “in this setting, adding colchicine early looks very promising to mitigate potential thrombotic risk without increasing bleeding risk.”
In the meantime, the study “provides novel insights on early aspirin withdrawal and P2Y12 monotherapy in an unselected population, including [those with] STEMI,” said Dr. Costa, also the coauthor of an accompanying editorial. The findings “could be of particular interest for those patients at extremely high bleeding risk or who are truly intolerant to aspirin, a scenario in which options are limited.”
This study was supported by the Cardiovascular Research Center, Seoul, South Korea. Dr. Lee reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Costa has served on an advisory board for AstraZeneca and has received speaker fees from Chiesi Farmaceutici. His coauthor reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM JACC: CARIOVASCULAR INTERVENTIONS
FDA warns AstraZeneca over ‘misleading claims’ about COPD drug
Promotional materials for the drug Breztri (budesonide/formoterol fumarate/glycopyrrolate inhaled) suggest that the drug has a positive effect on all-cause mortality for COPD patients, but the referenced clinical trial does not support that claim, the FDA letter states.
The FDA issued the warning letter on Aug. 4 and published the letter online on Aug. 15.
The sales aid highlights a 49% observed relative difference in time to all-cause mortality (ACM) over 1 year between Breztri and long-acting muscarinic antagonist/long-acting beta agonist (LAMA/LABA) inhalers.
Because of “statistical testing hierarchy failure” as well as confounding factors such as the removal of patients from inhaled corticosteroids (ICS) prior to entering the treatment arm of the trial, “no conclusions about the effect of Breztri on ACM can be drawn from the [clinical] trial,” the FDA wrote. “To date, no drug has been shown to improve ACM in COPD.”
The Breztri sales aid also states that there was a 20% reduction of severe exacerbations in patients using Breztri compared with patients using ICS/LABA. However, in the cited clinical trial, “the reduction in severe exacerbations was not statistically significant for patients treated with Breztri relative to comparator groups,” according to the FDA.
AstraZeneca has 15 working days from the receipt of the letter to respond in writing with “any plan for discontinuing use of such communications, or for ceasing distribution of Breztri,” the agency wrote.
A version of this article appeared on Medscape.com.
Promotional materials for the drug Breztri (budesonide/formoterol fumarate/glycopyrrolate inhaled) suggest that the drug has a positive effect on all-cause mortality for COPD patients, but the referenced clinical trial does not support that claim, the FDA letter states.
The FDA issued the warning letter on Aug. 4 and published the letter online on Aug. 15.
The sales aid highlights a 49% observed relative difference in time to all-cause mortality (ACM) over 1 year between Breztri and long-acting muscarinic antagonist/long-acting beta agonist (LAMA/LABA) inhalers.
Because of “statistical testing hierarchy failure” as well as confounding factors such as the removal of patients from inhaled corticosteroids (ICS) prior to entering the treatment arm of the trial, “no conclusions about the effect of Breztri on ACM can be drawn from the [clinical] trial,” the FDA wrote. “To date, no drug has been shown to improve ACM in COPD.”
The Breztri sales aid also states that there was a 20% reduction of severe exacerbations in patients using Breztri compared with patients using ICS/LABA. However, in the cited clinical trial, “the reduction in severe exacerbations was not statistically significant for patients treated with Breztri relative to comparator groups,” according to the FDA.
AstraZeneca has 15 working days from the receipt of the letter to respond in writing with “any plan for discontinuing use of such communications, or for ceasing distribution of Breztri,” the agency wrote.
A version of this article appeared on Medscape.com.
Promotional materials for the drug Breztri (budesonide/formoterol fumarate/glycopyrrolate inhaled) suggest that the drug has a positive effect on all-cause mortality for COPD patients, but the referenced clinical trial does not support that claim, the FDA letter states.
The FDA issued the warning letter on Aug. 4 and published the letter online on Aug. 15.
The sales aid highlights a 49% observed relative difference in time to all-cause mortality (ACM) over 1 year between Breztri and long-acting muscarinic antagonist/long-acting beta agonist (LAMA/LABA) inhalers.
Because of “statistical testing hierarchy failure” as well as confounding factors such as the removal of patients from inhaled corticosteroids (ICS) prior to entering the treatment arm of the trial, “no conclusions about the effect of Breztri on ACM can be drawn from the [clinical] trial,” the FDA wrote. “To date, no drug has been shown to improve ACM in COPD.”
The Breztri sales aid also states that there was a 20% reduction of severe exacerbations in patients using Breztri compared with patients using ICS/LABA. However, in the cited clinical trial, “the reduction in severe exacerbations was not statistically significant for patients treated with Breztri relative to comparator groups,” according to the FDA.
AstraZeneca has 15 working days from the receipt of the letter to respond in writing with “any plan for discontinuing use of such communications, or for ceasing distribution of Breztri,” the agency wrote.
A version of this article appeared on Medscape.com.
FDA okays first-ever new drug for rare bone disorder
Affecting roughly 400 people in the United States and 900 worldwide, FOP is an autosomal dominant condition in which bone develops in soft connective tissue areas of the body where it isn’t normally present (heterotopic ossification), such as the ligaments, tendons, and skeletal muscles. This leads to severe restriction in mobility and function, to the point that people lose the ability to feed or care for themselves. Most are completely disabled by age 30 years and median life expectancy is 56 years, with death often caused by bone formation around the rib cage restricting respiration.
“As a clinician caring for patients with FOP, I personally see the daily challenges and stresses that our patients and their families must contend with ... since the accumulation of heterotopic ossification in FOP is progressive, irreversible, and life altering. This medication is an important treatment option for our FOP community,” said endocrinologist Edward Hsiao, MD, professor of medicine at the University of California, San Francisco, in a statement from Ipsen.
Taken orally, palovarotene selectively targets the gamma subtype of retinoic acid receptors that regulate skeletal development and ectopic bone in the retinoid signaling pathway. The drug mediates interactions between these receptors, growth factors, and proteins within that pathway to reduce new abnormal bone formation.
It is now FDA approved for the treatment of FOP in female patients aged 8 years or older and male patients aged 10 years or older. The recommended dosing is 5 mg daily or weight-based equivalent for pediatric patients under 14 years of age, which can be modified or increased for flare-up symptoms. It is contraindicated during pregnancy.
The FDA approval was based on 18-month data from the phase 3, multicenter, open-label MOVE trial that included 107 adult and pediatric patients, over 10% of the world’s population with FOP. All received oral palovarotene and were compared with untreated individuals from a prior natural history study of the condition. The drug reduced annualized heterotopic ossification volume by 54%.
Side effects were typical of those seen with other systemic retinoid drugs, including mucocutaneous events such as dryness of the skin and mucous membranes, alopecia, drug eruption, rash, and pruritus, and musculoskeletal events, such as arthralgia and premature growth plate closure in growing children.
According to Dr. Hsiao, who was a MOVE investigator, the study “showed that Sohonos can decrease new heterotopic ossification, and that palovarotene can be tolerated by many patients with FOP. Sohonos is not for everyone. As with all medicines there are risks in this case especially for young children who may develop early growth plate closure. In addition, Sohonos has the same side effects as other retinoids.”
The FDA approval of palovarotene follows its rejection for marketing authorization in the European Union in July 2023.
Reached for comment, an Ipsen spokesperson said in an interview: “We reached the end of the regulatory process in the European Union for Sohonos and are disappointed the European Commission decided not to approved palovarotene for people with FOP in Europe.”
The company is developing another drug, fidrisertib, for treating FOP. A pivotal phase 2 trial for that drug is now recruiting patients. Asked where Ipsen might try to market fidrisertib, the spokesperson replied:“At this point, our focus is on the completion of the pivotal trial.”
Meanwhile, in the United States, the FOP community is celebrating the palovarotene approval. In a statement, Michelle Davis, executive director of the International Fibrodysplasia Ossificans Progressiva Association, said: “FOP is life altering to the individuals diagnosed and their families. There’s not a day that goes by where those impacted don’t worry about the debilitating physical pain of muscle that is replaced by bone, another joint locking, or the relentless emotional toll of losing the ability to do an activity they love, or hold a loved one close. ... The first treatment for FOP has been proven to reduce the volume of new abnormal bone growth, which may result in better health outcomes for people living with FOP.”
Ipsen is offering a patient support program to assist with education, coverage, and reimbursement (1-866-435-5677).
A version of this article appeared on Medscape.com.
Affecting roughly 400 people in the United States and 900 worldwide, FOP is an autosomal dominant condition in which bone develops in soft connective tissue areas of the body where it isn’t normally present (heterotopic ossification), such as the ligaments, tendons, and skeletal muscles. This leads to severe restriction in mobility and function, to the point that people lose the ability to feed or care for themselves. Most are completely disabled by age 30 years and median life expectancy is 56 years, with death often caused by bone formation around the rib cage restricting respiration.
“As a clinician caring for patients with FOP, I personally see the daily challenges and stresses that our patients and their families must contend with ... since the accumulation of heterotopic ossification in FOP is progressive, irreversible, and life altering. This medication is an important treatment option for our FOP community,” said endocrinologist Edward Hsiao, MD, professor of medicine at the University of California, San Francisco, in a statement from Ipsen.
Taken orally, palovarotene selectively targets the gamma subtype of retinoic acid receptors that regulate skeletal development and ectopic bone in the retinoid signaling pathway. The drug mediates interactions between these receptors, growth factors, and proteins within that pathway to reduce new abnormal bone formation.
It is now FDA approved for the treatment of FOP in female patients aged 8 years or older and male patients aged 10 years or older. The recommended dosing is 5 mg daily or weight-based equivalent for pediatric patients under 14 years of age, which can be modified or increased for flare-up symptoms. It is contraindicated during pregnancy.
The FDA approval was based on 18-month data from the phase 3, multicenter, open-label MOVE trial that included 107 adult and pediatric patients, over 10% of the world’s population with FOP. All received oral palovarotene and were compared with untreated individuals from a prior natural history study of the condition. The drug reduced annualized heterotopic ossification volume by 54%.
Side effects were typical of those seen with other systemic retinoid drugs, including mucocutaneous events such as dryness of the skin and mucous membranes, alopecia, drug eruption, rash, and pruritus, and musculoskeletal events, such as arthralgia and premature growth plate closure in growing children.
According to Dr. Hsiao, who was a MOVE investigator, the study “showed that Sohonos can decrease new heterotopic ossification, and that palovarotene can be tolerated by many patients with FOP. Sohonos is not for everyone. As with all medicines there are risks in this case especially for young children who may develop early growth plate closure. In addition, Sohonos has the same side effects as other retinoids.”
The FDA approval of palovarotene follows its rejection for marketing authorization in the European Union in July 2023.
Reached for comment, an Ipsen spokesperson said in an interview: “We reached the end of the regulatory process in the European Union for Sohonos and are disappointed the European Commission decided not to approved palovarotene for people with FOP in Europe.”
The company is developing another drug, fidrisertib, for treating FOP. A pivotal phase 2 trial for that drug is now recruiting patients. Asked where Ipsen might try to market fidrisertib, the spokesperson replied:“At this point, our focus is on the completion of the pivotal trial.”
Meanwhile, in the United States, the FOP community is celebrating the palovarotene approval. In a statement, Michelle Davis, executive director of the International Fibrodysplasia Ossificans Progressiva Association, said: “FOP is life altering to the individuals diagnosed and their families. There’s not a day that goes by where those impacted don’t worry about the debilitating physical pain of muscle that is replaced by bone, another joint locking, or the relentless emotional toll of losing the ability to do an activity they love, or hold a loved one close. ... The first treatment for FOP has been proven to reduce the volume of new abnormal bone growth, which may result in better health outcomes for people living with FOP.”
Ipsen is offering a patient support program to assist with education, coverage, and reimbursement (1-866-435-5677).
A version of this article appeared on Medscape.com.
Affecting roughly 400 people in the United States and 900 worldwide, FOP is an autosomal dominant condition in which bone develops in soft connective tissue areas of the body where it isn’t normally present (heterotopic ossification), such as the ligaments, tendons, and skeletal muscles. This leads to severe restriction in mobility and function, to the point that people lose the ability to feed or care for themselves. Most are completely disabled by age 30 years and median life expectancy is 56 years, with death often caused by bone formation around the rib cage restricting respiration.
“As a clinician caring for patients with FOP, I personally see the daily challenges and stresses that our patients and their families must contend with ... since the accumulation of heterotopic ossification in FOP is progressive, irreversible, and life altering. This medication is an important treatment option for our FOP community,” said endocrinologist Edward Hsiao, MD, professor of medicine at the University of California, San Francisco, in a statement from Ipsen.
Taken orally, palovarotene selectively targets the gamma subtype of retinoic acid receptors that regulate skeletal development and ectopic bone in the retinoid signaling pathway. The drug mediates interactions between these receptors, growth factors, and proteins within that pathway to reduce new abnormal bone formation.
It is now FDA approved for the treatment of FOP in female patients aged 8 years or older and male patients aged 10 years or older. The recommended dosing is 5 mg daily or weight-based equivalent for pediatric patients under 14 years of age, which can be modified or increased for flare-up symptoms. It is contraindicated during pregnancy.
The FDA approval was based on 18-month data from the phase 3, multicenter, open-label MOVE trial that included 107 adult and pediatric patients, over 10% of the world’s population with FOP. All received oral palovarotene and were compared with untreated individuals from a prior natural history study of the condition. The drug reduced annualized heterotopic ossification volume by 54%.
Side effects were typical of those seen with other systemic retinoid drugs, including mucocutaneous events such as dryness of the skin and mucous membranes, alopecia, drug eruption, rash, and pruritus, and musculoskeletal events, such as arthralgia and premature growth plate closure in growing children.
According to Dr. Hsiao, who was a MOVE investigator, the study “showed that Sohonos can decrease new heterotopic ossification, and that palovarotene can be tolerated by many patients with FOP. Sohonos is not for everyone. As with all medicines there are risks in this case especially for young children who may develop early growth plate closure. In addition, Sohonos has the same side effects as other retinoids.”
The FDA approval of palovarotene follows its rejection for marketing authorization in the European Union in July 2023.
Reached for comment, an Ipsen spokesperson said in an interview: “We reached the end of the regulatory process in the European Union for Sohonos and are disappointed the European Commission decided not to approved palovarotene for people with FOP in Europe.”
The company is developing another drug, fidrisertib, for treating FOP. A pivotal phase 2 trial for that drug is now recruiting patients. Asked where Ipsen might try to market fidrisertib, the spokesperson replied:“At this point, our focus is on the completion of the pivotal trial.”
Meanwhile, in the United States, the FOP community is celebrating the palovarotene approval. In a statement, Michelle Davis, executive director of the International Fibrodysplasia Ossificans Progressiva Association, said: “FOP is life altering to the individuals diagnosed and their families. There’s not a day that goes by where those impacted don’t worry about the debilitating physical pain of muscle that is replaced by bone, another joint locking, or the relentless emotional toll of losing the ability to do an activity they love, or hold a loved one close. ... The first treatment for FOP has been proven to reduce the volume of new abnormal bone growth, which may result in better health outcomes for people living with FOP.”
Ipsen is offering a patient support program to assist with education, coverage, and reimbursement (1-866-435-5677).
A version of this article appeared on Medscape.com.
What can you do during a mass shooting? This MD found out
Sunday night. Las Vegas. Jason Aldean had just started playing.
My wife and I were at the 2017 Route 91 Harvest Festival with three other couples; two of them were our close friends. We were sitting in the VIP section, a tented area right next to the stage. We started hearing what I was convinced were fireworks.
I’ve been in the Army for 20 some years. I’ve been deployed and shot at multiple times. But these shots were far away. And you don’t expect people to be shooting at you at a concert.
I was on the edge of the VIP area, so I could see around the corner of the tent. I looked up at the Mandalay Bay and saw the muzzle flash in the hotel window. That’s when I knew.
I screamed: “Somebody’s shooting at us! Everybody get down!”
It took a while for people to realize what was going on. When the first couple volleys sprayed into the crowd, nobody understood. But once enough people had been hit and dropped, everyone knew, and it was just mass exodus.
People screamed and ran everywhere. Some of them tried to jump over the front barrier so they could get underneath the stage. Others were trying to pick up loved ones who’d been shot.
The next 15 minutes are a little foggy. I was helping my wife and the people around us to get down. Funny things come back to you afterward. One of my friends was carrying a 16-ounce beer in his hand. Somebody’s shooting at him and he’s walking around with his beer like he’s afraid to put it down. It was so surreal.
We got everybody underneath the tent, and then we just sat there. There would be shooting and then a pause. You’d think it was over. And then there would be more shooting and another pause. It felt like it never was going to stop.
After a short period of time, somebody came in with an official badge, maybe FBI, who knows. They said: “Okay, everybody up. We’ve got to get you out of here.” So, we all got up and headed across the stage. The gate they were taking us to was in full view of the shooter, so it wasn’t very safe.
As I got up, I looked out at the field. Bodies were scattered everywhere. I’m a trauma surgeon by trade. I couldn’t just leave.
I told my two best friends to take my wife with them. My wife lost her mind at that point. She didn’t want me to run out on the field. But I had to. I saw the injured and they needed help. Another buddy and I jumped over the fence and started taking care of people.
The feeling of being out on the field was one of complete frustration. I was in sandals, shorts, and a t-shirt. We had no stretchers, no medical supplies, no nothing. I didn’t have a belt to use as a tourniquet. I didn’t even have a bandage.
Worse: We were seeing high-velocity gunshot wounds that I’ve seen for 20 years in the Army. I know how to take care of them. I know how to fix them. But there wasn’t a single thing I could do.
We had to get people off the field, so we started gathering up as many as we could. We didn’t know if we were going to get shot at again, so we were trying to hide behind things as we ran. Our main objective was just to get people to a place of safety.
A lot of it is a blur. But a few patients stick out in my mind.
A father and son. The father had been shot through the abdomen, exited out through his back. He was in severe pain and couldn’t walk.
A young girl shot in the arm. Her parents carrying her.
A group of people doing CPR on a young lady. She had a gunshot wound to the head or neck. She was obviously dead. But they were still doing chest compressions in the middle of the field. I had to say to them: “She’s dead. You can’t save her. You need to get off the field.” But they wouldn’t stop. We picked her up and took her out while they continued to do CPR.
Later, I realized I knew that woman. She was part of a group of friends that we would see at the festival. I hadn’t recognized her. I also didn’t know that my friend Marco was there. A month or 2 later, we figured out that he was one of the people doing CPR. And I was the guy who came up and said his friend was dead.
Some people were so badly injured we couldn’t lift them. We started tearing apart the fencing used to separate the crowd and slid sections of the barricades under the wounded to carry them. We also carried off a bunch of people who were dead.
We were moving patients to a covered bar area where we thought they would be safer. What we didn’t know was there was an ambulance rally point at the very far end of the field. Unfortunately, we had no idea it was there.
I saw a lot of other first responders out there, people from the fire department, corpsmen from the Navy, medics. I ran into an anesthesia provider and a series of nurses.
When we got everybody off the field, we started moving them into vehicles. People were bringing their trucks up. One guy even stole a truck so he could drive people to the ED. There wasn’t a lot of triage. We were just stacking whoever we could into the backs of these pickups.
I tried to help a nurse taking care of a lady who had been shot in the neck. She was sitting sort of half upright with the patient lying in her arms. When I reached to help her, she said: “You can’t move her.”
“We need to get her to the hospital,” I replied.
“This is the only position that this lady has an airway,” she said. “You’re going to have to move both of us together. If I move at all, she loses her airway.”
So, a group of us managed to slide something underneath and lift them into the back of a truck.
Loading the wounded went on for a while. And then, just like that, everybody was gone.
I walked back out onto this field which not too long ago held 30,000 people. It was as if aliens had just suddenly beamed everyone out.
There was stuff on the ground everywhere – blankets, clothing, single boots, wallets, purses. I walked past a food stand with food still cooking on the grill. There was a beer tap still running. It was the weirdest feeling I’d ever had in my life.
After that, things got a little crazy again. There had been a report of a second shooter, and no one knew if it was real or not. The police started herding a group of us across the street to the Tropicana. We were still trying to take cover as we walked there. We went past a big lion statue in front of one of the casinos. I have a picture from two years earlier of me sitting on the back of that lion. I remember thinking: Now I’m hunkered down behind the same lion hiding from a shooter. Times change.
They brought about 50 of us into a food court, which was closed. They wouldn’t tell us what was going on. And they wouldn’t let us leave. This went on for hours. Meanwhile, I had dropped my cell phone on the field, so my wife couldn’t get hold of me, and later she told me she assumed I’d been shot. I was just hoping that she was safe.
People were huddled together, crying, holding each other. Most were wearing Western concert–going stuff, which for a lot of them wasn’t very much clothing. The hotel eventually brought some blankets.
I was covered in blood. My shirt, shorts, and sandals were soaked. It was running down my legs. I couldn’t find anything to eat or drink. At one point, I sat down at a slot machine, put a hundred dollars in, and started playing slots. I didn’t know what else to do. It didn’t take me very long to lose it all.
Finally, I started looking for a way to get out. I checked all the exits, but there were security and police there. Then I ran into a guy who said he had found a fire exit. When we opened the fire door, there was a big security guard there, and he said: “You can’t leave.”
We said: “Try to stop us. We’re out of here.”
Another thing I’ll always remember – after I broke out of the Tropicana, I was low crawling through the bushes along the Strip toward my hotel. I got a block away and stood up to cross the street. I pushed the crosswalk button and waited. There were no cars, no people. I’ve just broken all the rules, violated police orders, and now I’m standing there waiting for a blinking light to allow me to cross the street!
I made it back to my hotel room around 3:30 or 4:00 in the morning. My wife was hysterical because I hadn’t been answering my cell phone. I came in, and she gave me a big hug, and I got in the shower. Our plane was leaving in a few hours, so we laid down, but didn’t sleep.
As we were getting ready to leave, my wife’s phone rang, and it was my number. A guy at the same hotel had found my phone on the field and called the “in case of emergency” number. So, I got my phone back.
It wasn’t easy to deal with the aftermath. It really affected everybody’s life. To this day, I’m particular about where we sit at concerts. My wife isn’t comfortable if she can’t see an exit. I now have a med bag in my car with tourniquets, pressure dressings, airway masks for CPR.
I’ll never forget that feeling of absolute frustration. That lady without an airway – I could’ve put a trach in her very quickly and made a difference. Were they able to keep her airway? Did she live?
The father and son – did the father make it? I have no idea what happened to any of them. Later, I went through and looked at the pictures of all the people who had died, but I couldn’t recognize anybody.
The hardest part was being there with my wife. I’ve been in places where people are shooting at you, in vehicles that are getting bombed. I’ve always believed that when it’s your time, it’s your time. If I get shot, well, okay, that happens. But if she got shot or my friends ... that would be really tough.
A year later, I gave a talk about it at a conference. I thought I had worked through everything. But all of those feelings, all of that helplessness, that anger, everything came roaring back to the surface again. They asked me how I deal with it, and I said: “Well ... poorly.” I’m the guy who sticks it in a box in the back of his brain, tucks it in and buries it with a bunch of other boxes, and hopes it never comes out again. But every once in a while, it does.
There were all kinds of people out on that field, some with medical training, some without, all determined to help, trying to get those injured people where they needed to be. In retrospect, it does make you feel good. Somebody was shooting at us, but people were still willing to stand up and risk their lives to help others.
We still talk with our friends about what happened that night. Over the years, it’s become less and less. But there’s still a text sent out every year on that day: “Today is the anniversary. Glad we’re all alive. Thanks for being our friends.”
Dr. Sebesta is a bariatric surgeon with MultiCare Health System in Tacoma, Wash.
A version of this article first appeared on Medscape.com.
Sunday night. Las Vegas. Jason Aldean had just started playing.
My wife and I were at the 2017 Route 91 Harvest Festival with three other couples; two of them were our close friends. We were sitting in the VIP section, a tented area right next to the stage. We started hearing what I was convinced were fireworks.
I’ve been in the Army for 20 some years. I’ve been deployed and shot at multiple times. But these shots were far away. And you don’t expect people to be shooting at you at a concert.
I was on the edge of the VIP area, so I could see around the corner of the tent. I looked up at the Mandalay Bay and saw the muzzle flash in the hotel window. That’s when I knew.
I screamed: “Somebody’s shooting at us! Everybody get down!”
It took a while for people to realize what was going on. When the first couple volleys sprayed into the crowd, nobody understood. But once enough people had been hit and dropped, everyone knew, and it was just mass exodus.
People screamed and ran everywhere. Some of them tried to jump over the front barrier so they could get underneath the stage. Others were trying to pick up loved ones who’d been shot.
The next 15 minutes are a little foggy. I was helping my wife and the people around us to get down. Funny things come back to you afterward. One of my friends was carrying a 16-ounce beer in his hand. Somebody’s shooting at him and he’s walking around with his beer like he’s afraid to put it down. It was so surreal.
We got everybody underneath the tent, and then we just sat there. There would be shooting and then a pause. You’d think it was over. And then there would be more shooting and another pause. It felt like it never was going to stop.
After a short period of time, somebody came in with an official badge, maybe FBI, who knows. They said: “Okay, everybody up. We’ve got to get you out of here.” So, we all got up and headed across the stage. The gate they were taking us to was in full view of the shooter, so it wasn’t very safe.
As I got up, I looked out at the field. Bodies were scattered everywhere. I’m a trauma surgeon by trade. I couldn’t just leave.
I told my two best friends to take my wife with them. My wife lost her mind at that point. She didn’t want me to run out on the field. But I had to. I saw the injured and they needed help. Another buddy and I jumped over the fence and started taking care of people.
The feeling of being out on the field was one of complete frustration. I was in sandals, shorts, and a t-shirt. We had no stretchers, no medical supplies, no nothing. I didn’t have a belt to use as a tourniquet. I didn’t even have a bandage.
Worse: We were seeing high-velocity gunshot wounds that I’ve seen for 20 years in the Army. I know how to take care of them. I know how to fix them. But there wasn’t a single thing I could do.
We had to get people off the field, so we started gathering up as many as we could. We didn’t know if we were going to get shot at again, so we were trying to hide behind things as we ran. Our main objective was just to get people to a place of safety.
A lot of it is a blur. But a few patients stick out in my mind.
A father and son. The father had been shot through the abdomen, exited out through his back. He was in severe pain and couldn’t walk.
A young girl shot in the arm. Her parents carrying her.
A group of people doing CPR on a young lady. She had a gunshot wound to the head or neck. She was obviously dead. But they were still doing chest compressions in the middle of the field. I had to say to them: “She’s dead. You can’t save her. You need to get off the field.” But they wouldn’t stop. We picked her up and took her out while they continued to do CPR.
Later, I realized I knew that woman. She was part of a group of friends that we would see at the festival. I hadn’t recognized her. I also didn’t know that my friend Marco was there. A month or 2 later, we figured out that he was one of the people doing CPR. And I was the guy who came up and said his friend was dead.
Some people were so badly injured we couldn’t lift them. We started tearing apart the fencing used to separate the crowd and slid sections of the barricades under the wounded to carry them. We also carried off a bunch of people who were dead.
We were moving patients to a covered bar area where we thought they would be safer. What we didn’t know was there was an ambulance rally point at the very far end of the field. Unfortunately, we had no idea it was there.
I saw a lot of other first responders out there, people from the fire department, corpsmen from the Navy, medics. I ran into an anesthesia provider and a series of nurses.
When we got everybody off the field, we started moving them into vehicles. People were bringing their trucks up. One guy even stole a truck so he could drive people to the ED. There wasn’t a lot of triage. We were just stacking whoever we could into the backs of these pickups.
I tried to help a nurse taking care of a lady who had been shot in the neck. She was sitting sort of half upright with the patient lying in her arms. When I reached to help her, she said: “You can’t move her.”
“We need to get her to the hospital,” I replied.
“This is the only position that this lady has an airway,” she said. “You’re going to have to move both of us together. If I move at all, she loses her airway.”
So, a group of us managed to slide something underneath and lift them into the back of a truck.
Loading the wounded went on for a while. And then, just like that, everybody was gone.
I walked back out onto this field which not too long ago held 30,000 people. It was as if aliens had just suddenly beamed everyone out.
There was stuff on the ground everywhere – blankets, clothing, single boots, wallets, purses. I walked past a food stand with food still cooking on the grill. There was a beer tap still running. It was the weirdest feeling I’d ever had in my life.
After that, things got a little crazy again. There had been a report of a second shooter, and no one knew if it was real or not. The police started herding a group of us across the street to the Tropicana. We were still trying to take cover as we walked there. We went past a big lion statue in front of one of the casinos. I have a picture from two years earlier of me sitting on the back of that lion. I remember thinking: Now I’m hunkered down behind the same lion hiding from a shooter. Times change.
They brought about 50 of us into a food court, which was closed. They wouldn’t tell us what was going on. And they wouldn’t let us leave. This went on for hours. Meanwhile, I had dropped my cell phone on the field, so my wife couldn’t get hold of me, and later she told me she assumed I’d been shot. I was just hoping that she was safe.
People were huddled together, crying, holding each other. Most were wearing Western concert–going stuff, which for a lot of them wasn’t very much clothing. The hotel eventually brought some blankets.
I was covered in blood. My shirt, shorts, and sandals were soaked. It was running down my legs. I couldn’t find anything to eat or drink. At one point, I sat down at a slot machine, put a hundred dollars in, and started playing slots. I didn’t know what else to do. It didn’t take me very long to lose it all.
Finally, I started looking for a way to get out. I checked all the exits, but there were security and police there. Then I ran into a guy who said he had found a fire exit. When we opened the fire door, there was a big security guard there, and he said: “You can’t leave.”
We said: “Try to stop us. We’re out of here.”
Another thing I’ll always remember – after I broke out of the Tropicana, I was low crawling through the bushes along the Strip toward my hotel. I got a block away and stood up to cross the street. I pushed the crosswalk button and waited. There were no cars, no people. I’ve just broken all the rules, violated police orders, and now I’m standing there waiting for a blinking light to allow me to cross the street!
I made it back to my hotel room around 3:30 or 4:00 in the morning. My wife was hysterical because I hadn’t been answering my cell phone. I came in, and she gave me a big hug, and I got in the shower. Our plane was leaving in a few hours, so we laid down, but didn’t sleep.
As we were getting ready to leave, my wife’s phone rang, and it was my number. A guy at the same hotel had found my phone on the field and called the “in case of emergency” number. So, I got my phone back.
It wasn’t easy to deal with the aftermath. It really affected everybody’s life. To this day, I’m particular about where we sit at concerts. My wife isn’t comfortable if she can’t see an exit. I now have a med bag in my car with tourniquets, pressure dressings, airway masks for CPR.
I’ll never forget that feeling of absolute frustration. That lady without an airway – I could’ve put a trach in her very quickly and made a difference. Were they able to keep her airway? Did she live?
The father and son – did the father make it? I have no idea what happened to any of them. Later, I went through and looked at the pictures of all the people who had died, but I couldn’t recognize anybody.
The hardest part was being there with my wife. I’ve been in places where people are shooting at you, in vehicles that are getting bombed. I’ve always believed that when it’s your time, it’s your time. If I get shot, well, okay, that happens. But if she got shot or my friends ... that would be really tough.
A year later, I gave a talk about it at a conference. I thought I had worked through everything. But all of those feelings, all of that helplessness, that anger, everything came roaring back to the surface again. They asked me how I deal with it, and I said: “Well ... poorly.” I’m the guy who sticks it in a box in the back of his brain, tucks it in and buries it with a bunch of other boxes, and hopes it never comes out again. But every once in a while, it does.
There were all kinds of people out on that field, some with medical training, some without, all determined to help, trying to get those injured people where they needed to be. In retrospect, it does make you feel good. Somebody was shooting at us, but people were still willing to stand up and risk their lives to help others.
We still talk with our friends about what happened that night. Over the years, it’s become less and less. But there’s still a text sent out every year on that day: “Today is the anniversary. Glad we’re all alive. Thanks for being our friends.”
Dr. Sebesta is a bariatric surgeon with MultiCare Health System in Tacoma, Wash.
A version of this article first appeared on Medscape.com.
Sunday night. Las Vegas. Jason Aldean had just started playing.
My wife and I were at the 2017 Route 91 Harvest Festival with three other couples; two of them were our close friends. We were sitting in the VIP section, a tented area right next to the stage. We started hearing what I was convinced were fireworks.
I’ve been in the Army for 20 some years. I’ve been deployed and shot at multiple times. But these shots were far away. And you don’t expect people to be shooting at you at a concert.
I was on the edge of the VIP area, so I could see around the corner of the tent. I looked up at the Mandalay Bay and saw the muzzle flash in the hotel window. That’s when I knew.
I screamed: “Somebody’s shooting at us! Everybody get down!”
It took a while for people to realize what was going on. When the first couple volleys sprayed into the crowd, nobody understood. But once enough people had been hit and dropped, everyone knew, and it was just mass exodus.
People screamed and ran everywhere. Some of them tried to jump over the front barrier so they could get underneath the stage. Others were trying to pick up loved ones who’d been shot.
The next 15 minutes are a little foggy. I was helping my wife and the people around us to get down. Funny things come back to you afterward. One of my friends was carrying a 16-ounce beer in his hand. Somebody’s shooting at him and he’s walking around with his beer like he’s afraid to put it down. It was so surreal.
We got everybody underneath the tent, and then we just sat there. There would be shooting and then a pause. You’d think it was over. And then there would be more shooting and another pause. It felt like it never was going to stop.
After a short period of time, somebody came in with an official badge, maybe FBI, who knows. They said: “Okay, everybody up. We’ve got to get you out of here.” So, we all got up and headed across the stage. The gate they were taking us to was in full view of the shooter, so it wasn’t very safe.
As I got up, I looked out at the field. Bodies were scattered everywhere. I’m a trauma surgeon by trade. I couldn’t just leave.
I told my two best friends to take my wife with them. My wife lost her mind at that point. She didn’t want me to run out on the field. But I had to. I saw the injured and they needed help. Another buddy and I jumped over the fence and started taking care of people.
The feeling of being out on the field was one of complete frustration. I was in sandals, shorts, and a t-shirt. We had no stretchers, no medical supplies, no nothing. I didn’t have a belt to use as a tourniquet. I didn’t even have a bandage.
Worse: We were seeing high-velocity gunshot wounds that I’ve seen for 20 years in the Army. I know how to take care of them. I know how to fix them. But there wasn’t a single thing I could do.
We had to get people off the field, so we started gathering up as many as we could. We didn’t know if we were going to get shot at again, so we were trying to hide behind things as we ran. Our main objective was just to get people to a place of safety.
A lot of it is a blur. But a few patients stick out in my mind.
A father and son. The father had been shot through the abdomen, exited out through his back. He was in severe pain and couldn’t walk.
A young girl shot in the arm. Her parents carrying her.
A group of people doing CPR on a young lady. She had a gunshot wound to the head or neck. She was obviously dead. But they were still doing chest compressions in the middle of the field. I had to say to them: “She’s dead. You can’t save her. You need to get off the field.” But they wouldn’t stop. We picked her up and took her out while they continued to do CPR.
Later, I realized I knew that woman. She was part of a group of friends that we would see at the festival. I hadn’t recognized her. I also didn’t know that my friend Marco was there. A month or 2 later, we figured out that he was one of the people doing CPR. And I was the guy who came up and said his friend was dead.
Some people were so badly injured we couldn’t lift them. We started tearing apart the fencing used to separate the crowd and slid sections of the barricades under the wounded to carry them. We also carried off a bunch of people who were dead.
We were moving patients to a covered bar area where we thought they would be safer. What we didn’t know was there was an ambulance rally point at the very far end of the field. Unfortunately, we had no idea it was there.
I saw a lot of other first responders out there, people from the fire department, corpsmen from the Navy, medics. I ran into an anesthesia provider and a series of nurses.
When we got everybody off the field, we started moving them into vehicles. People were bringing their trucks up. One guy even stole a truck so he could drive people to the ED. There wasn’t a lot of triage. We were just stacking whoever we could into the backs of these pickups.
I tried to help a nurse taking care of a lady who had been shot in the neck. She was sitting sort of half upright with the patient lying in her arms. When I reached to help her, she said: “You can’t move her.”
“We need to get her to the hospital,” I replied.
“This is the only position that this lady has an airway,” she said. “You’re going to have to move both of us together. If I move at all, she loses her airway.”
So, a group of us managed to slide something underneath and lift them into the back of a truck.
Loading the wounded went on for a while. And then, just like that, everybody was gone.
I walked back out onto this field which not too long ago held 30,000 people. It was as if aliens had just suddenly beamed everyone out.
There was stuff on the ground everywhere – blankets, clothing, single boots, wallets, purses. I walked past a food stand with food still cooking on the grill. There was a beer tap still running. It was the weirdest feeling I’d ever had in my life.
After that, things got a little crazy again. There had been a report of a second shooter, and no one knew if it was real or not. The police started herding a group of us across the street to the Tropicana. We were still trying to take cover as we walked there. We went past a big lion statue in front of one of the casinos. I have a picture from two years earlier of me sitting on the back of that lion. I remember thinking: Now I’m hunkered down behind the same lion hiding from a shooter. Times change.
They brought about 50 of us into a food court, which was closed. They wouldn’t tell us what was going on. And they wouldn’t let us leave. This went on for hours. Meanwhile, I had dropped my cell phone on the field, so my wife couldn’t get hold of me, and later she told me she assumed I’d been shot. I was just hoping that she was safe.
People were huddled together, crying, holding each other. Most were wearing Western concert–going stuff, which for a lot of them wasn’t very much clothing. The hotel eventually brought some blankets.
I was covered in blood. My shirt, shorts, and sandals were soaked. It was running down my legs. I couldn’t find anything to eat or drink. At one point, I sat down at a slot machine, put a hundred dollars in, and started playing slots. I didn’t know what else to do. It didn’t take me very long to lose it all.
Finally, I started looking for a way to get out. I checked all the exits, but there were security and police there. Then I ran into a guy who said he had found a fire exit. When we opened the fire door, there was a big security guard there, and he said: “You can’t leave.”
We said: “Try to stop us. We’re out of here.”
Another thing I’ll always remember – after I broke out of the Tropicana, I was low crawling through the bushes along the Strip toward my hotel. I got a block away and stood up to cross the street. I pushed the crosswalk button and waited. There were no cars, no people. I’ve just broken all the rules, violated police orders, and now I’m standing there waiting for a blinking light to allow me to cross the street!
I made it back to my hotel room around 3:30 or 4:00 in the morning. My wife was hysterical because I hadn’t been answering my cell phone. I came in, and she gave me a big hug, and I got in the shower. Our plane was leaving in a few hours, so we laid down, but didn’t sleep.
As we were getting ready to leave, my wife’s phone rang, and it was my number. A guy at the same hotel had found my phone on the field and called the “in case of emergency” number. So, I got my phone back.
It wasn’t easy to deal with the aftermath. It really affected everybody’s life. To this day, I’m particular about where we sit at concerts. My wife isn’t comfortable if she can’t see an exit. I now have a med bag in my car with tourniquets, pressure dressings, airway masks for CPR.
I’ll never forget that feeling of absolute frustration. That lady without an airway – I could’ve put a trach in her very quickly and made a difference. Were they able to keep her airway? Did she live?
The father and son – did the father make it? I have no idea what happened to any of them. Later, I went through and looked at the pictures of all the people who had died, but I couldn’t recognize anybody.
The hardest part was being there with my wife. I’ve been in places where people are shooting at you, in vehicles that are getting bombed. I’ve always believed that when it’s your time, it’s your time. If I get shot, well, okay, that happens. But if she got shot or my friends ... that would be really tough.
A year later, I gave a talk about it at a conference. I thought I had worked through everything. But all of those feelings, all of that helplessness, that anger, everything came roaring back to the surface again. They asked me how I deal with it, and I said: “Well ... poorly.” I’m the guy who sticks it in a box in the back of his brain, tucks it in and buries it with a bunch of other boxes, and hopes it never comes out again. But every once in a while, it does.
There were all kinds of people out on that field, some with medical training, some without, all determined to help, trying to get those injured people where they needed to be. In retrospect, it does make you feel good. Somebody was shooting at us, but people were still willing to stand up and risk their lives to help others.
We still talk with our friends about what happened that night. Over the years, it’s become less and less. But there’s still a text sent out every year on that day: “Today is the anniversary. Glad we’re all alive. Thanks for being our friends.”
Dr. Sebesta is a bariatric surgeon with MultiCare Health System in Tacoma, Wash.
A version of this article first appeared on Medscape.com.
Pig kidneys show ‘life-sustaining’ function in human
– marking another important step toward opening up a new supply of much-needed organs for those with end-stage kidney disease.
A team of researchers in Alabama removed a brain-dead person’s kidneys and transplanted two kidneys that had been taken from a genetically modified pig. The researchers monitored the patient’s response to the organs and tracked the kidneys’ function over a 7-day period. The findings were published in JAMA Surgery.
During the first 24 hours after transplantation, the pig kidneys made more than 37 liters of urine. “It was really a remarkable thing to see,” lead investigator Jayme Locke, MD, professor of surgery and the Arnold G. Diethelm Endowed Chair in Transplantation Surgery, University of Alabama at Birmingham, said in a press release.
The recipient was given standard maintenance immunosuppression - tacrolimus, mycophenolate mofetil, and prednisone. The target tacrolimus level (8-10 ng/dL) was reached by postoperative day 2 and was maintained through study completion.
At the end of the study, the serum creatinine level was 0.9 mg/dL, and creatinine clearance was 200 mL/min. Creatinine levels are an indicator of kidney function and demonstrate the organ’s ability to filter waste from blood, according to Roger Lord, PhD, senior lecturer (medical sciences) in the School of Behavioural and Health Sciences, Australian Catholic University, who was not involved in the research.
This is the first time that it has been demonstrated that a standard immunosuppression regimen may be sufficient to support xenotransplantation with pig kidneys and in which creatinine clearance was achieved.
The finding comes less than 2 years after the same team published results from a similar experiment. In that transplant, the investigators didn’t observe significant creatinine excretion into the urine.
In the team’s previous attempts, kidney function was delayed because the brain-dead recipients had deteriorated physiologically. This time, the subject was stable, and the team was able to observe urine production within 4 minutes of restoration of blood flow to the transplanted pig organs.
“This new work firmly establishes that the xenografts not only make urine but provide life-sustaining kidney function by clearing serum creatinine,” Locke said in an interview. “This is the first time in history this has been shown.”
The investigators are hoping animal-sourced organs could become an alternative for human transplantations, potentially solving the serious shortage of human organs available for patients on transplant waiting lists.
Organ transplantation can treat patients with advanced kidney disease and kidney failure, but there are not enough human organs available to meet the need. More than 92,000 people in the United States are waiting for a kidney, according to the American Kidney Fund.
Organ rejection is a risk with xenotransplants – animal-to-human organ transplants. Investigators in this study used kidneys from pigs with 10 gene modifications. The modifications were intended to decrease the likelihood of the organs being rejected by a human host.
The kidneys were still viable at the end of the 7-day period. In addition, there was no microscopic blood clot formation, another indicator of normal kidney function, according to Dr. Lord, who provided comments to the UK Science Media Centre.
The long-term outcomes of animal-to-human organ transplantation remain unclear. Dr. Lord describes the operation as a “first step” to demonstrate that genetically modified, transplanted pig kidneys can function normally so as to remove creatinine over a 7-day period.
Dr. Locke and colleagues said: “Future research in living human recipients is necessary to determine long-term xenograft kidney function and whether xenografts could serve as a bridge or destination therapy for end-stage kidney disease.
“Because our study represents a single case, generalizability of the findings is limited. This study showcases xenotransplant as a viable potential solution to an organ shortage crisis responsible for thousands of preventable deaths annually,” they concluded.
A version of this article first appeared on Medscape.com .
– marking another important step toward opening up a new supply of much-needed organs for those with end-stage kidney disease.
A team of researchers in Alabama removed a brain-dead person’s kidneys and transplanted two kidneys that had been taken from a genetically modified pig. The researchers monitored the patient’s response to the organs and tracked the kidneys’ function over a 7-day period. The findings were published in JAMA Surgery.
During the first 24 hours after transplantation, the pig kidneys made more than 37 liters of urine. “It was really a remarkable thing to see,” lead investigator Jayme Locke, MD, professor of surgery and the Arnold G. Diethelm Endowed Chair in Transplantation Surgery, University of Alabama at Birmingham, said in a press release.
The recipient was given standard maintenance immunosuppression - tacrolimus, mycophenolate mofetil, and prednisone. The target tacrolimus level (8-10 ng/dL) was reached by postoperative day 2 and was maintained through study completion.
At the end of the study, the serum creatinine level was 0.9 mg/dL, and creatinine clearance was 200 mL/min. Creatinine levels are an indicator of kidney function and demonstrate the organ’s ability to filter waste from blood, according to Roger Lord, PhD, senior lecturer (medical sciences) in the School of Behavioural and Health Sciences, Australian Catholic University, who was not involved in the research.
This is the first time that it has been demonstrated that a standard immunosuppression regimen may be sufficient to support xenotransplantation with pig kidneys and in which creatinine clearance was achieved.
The finding comes less than 2 years after the same team published results from a similar experiment. In that transplant, the investigators didn’t observe significant creatinine excretion into the urine.
In the team’s previous attempts, kidney function was delayed because the brain-dead recipients had deteriorated physiologically. This time, the subject was stable, and the team was able to observe urine production within 4 minutes of restoration of blood flow to the transplanted pig organs.
“This new work firmly establishes that the xenografts not only make urine but provide life-sustaining kidney function by clearing serum creatinine,” Locke said in an interview. “This is the first time in history this has been shown.”
The investigators are hoping animal-sourced organs could become an alternative for human transplantations, potentially solving the serious shortage of human organs available for patients on transplant waiting lists.
Organ transplantation can treat patients with advanced kidney disease and kidney failure, but there are not enough human organs available to meet the need. More than 92,000 people in the United States are waiting for a kidney, according to the American Kidney Fund.
Organ rejection is a risk with xenotransplants – animal-to-human organ transplants. Investigators in this study used kidneys from pigs with 10 gene modifications. The modifications were intended to decrease the likelihood of the organs being rejected by a human host.
The kidneys were still viable at the end of the 7-day period. In addition, there was no microscopic blood clot formation, another indicator of normal kidney function, according to Dr. Lord, who provided comments to the UK Science Media Centre.
The long-term outcomes of animal-to-human organ transplantation remain unclear. Dr. Lord describes the operation as a “first step” to demonstrate that genetically modified, transplanted pig kidneys can function normally so as to remove creatinine over a 7-day period.
Dr. Locke and colleagues said: “Future research in living human recipients is necessary to determine long-term xenograft kidney function and whether xenografts could serve as a bridge or destination therapy for end-stage kidney disease.
“Because our study represents a single case, generalizability of the findings is limited. This study showcases xenotransplant as a viable potential solution to an organ shortage crisis responsible for thousands of preventable deaths annually,” they concluded.
A version of this article first appeared on Medscape.com .
– marking another important step toward opening up a new supply of much-needed organs for those with end-stage kidney disease.
A team of researchers in Alabama removed a brain-dead person’s kidneys and transplanted two kidneys that had been taken from a genetically modified pig. The researchers monitored the patient’s response to the organs and tracked the kidneys’ function over a 7-day period. The findings were published in JAMA Surgery.
During the first 24 hours after transplantation, the pig kidneys made more than 37 liters of urine. “It was really a remarkable thing to see,” lead investigator Jayme Locke, MD, professor of surgery and the Arnold G. Diethelm Endowed Chair in Transplantation Surgery, University of Alabama at Birmingham, said in a press release.
The recipient was given standard maintenance immunosuppression - tacrolimus, mycophenolate mofetil, and prednisone. The target tacrolimus level (8-10 ng/dL) was reached by postoperative day 2 and was maintained through study completion.
At the end of the study, the serum creatinine level was 0.9 mg/dL, and creatinine clearance was 200 mL/min. Creatinine levels are an indicator of kidney function and demonstrate the organ’s ability to filter waste from blood, according to Roger Lord, PhD, senior lecturer (medical sciences) in the School of Behavioural and Health Sciences, Australian Catholic University, who was not involved in the research.
This is the first time that it has been demonstrated that a standard immunosuppression regimen may be sufficient to support xenotransplantation with pig kidneys and in which creatinine clearance was achieved.
The finding comes less than 2 years after the same team published results from a similar experiment. In that transplant, the investigators didn’t observe significant creatinine excretion into the urine.
In the team’s previous attempts, kidney function was delayed because the brain-dead recipients had deteriorated physiologically. This time, the subject was stable, and the team was able to observe urine production within 4 minutes of restoration of blood flow to the transplanted pig organs.
“This new work firmly establishes that the xenografts not only make urine but provide life-sustaining kidney function by clearing serum creatinine,” Locke said in an interview. “This is the first time in history this has been shown.”
The investigators are hoping animal-sourced organs could become an alternative for human transplantations, potentially solving the serious shortage of human organs available for patients on transplant waiting lists.
Organ transplantation can treat patients with advanced kidney disease and kidney failure, but there are not enough human organs available to meet the need. More than 92,000 people in the United States are waiting for a kidney, according to the American Kidney Fund.
Organ rejection is a risk with xenotransplants – animal-to-human organ transplants. Investigators in this study used kidneys from pigs with 10 gene modifications. The modifications were intended to decrease the likelihood of the organs being rejected by a human host.
The kidneys were still viable at the end of the 7-day period. In addition, there was no microscopic blood clot formation, another indicator of normal kidney function, according to Dr. Lord, who provided comments to the UK Science Media Centre.
The long-term outcomes of animal-to-human organ transplantation remain unclear. Dr. Lord describes the operation as a “first step” to demonstrate that genetically modified, transplanted pig kidneys can function normally so as to remove creatinine over a 7-day period.
Dr. Locke and colleagues said: “Future research in living human recipients is necessary to determine long-term xenograft kidney function and whether xenografts could serve as a bridge or destination therapy for end-stage kidney disease.
“Because our study represents a single case, generalizability of the findings is limited. This study showcases xenotransplant as a viable potential solution to an organ shortage crisis responsible for thousands of preventable deaths annually,” they concluded.
A version of this article first appeared on Medscape.com .
FROM JAMA SURGERY
Pain 1 year after MI tied to all-cause mortality
, new research suggests.
In the analysis of post-MI health data for more than 18,300 Swedish adults, those with moderate pain were 35% more likely to die from any cause during follow-up, compared with those with no pain, and those with extreme pain were more than twice as likely to die.
Furthermore, pain was a stronger predictor of mortality than smoking.
“For a long time, pain has been regarded as merely a symptom of disease rather than a disease” in its own right, Linda Vixner, PT, PhD, of Dalarna University in Falun, Sweden, said in an interview.
Updated definitions of chronic pain in the ICD-11, as well as a recent study using data from the UK Biobank showing that chronic pain is associated with an increased risk of cardiovascular disease, prompted the current study, which looks at the effect of pain on long-term survival after an MI.
“We did not expect that pain would have such a strong impact on the risk of death, and it also surprised us that the risk was more pronounced than that of smoking,” Dr. Vixner said. “Clinicians should consider pain an important cardiovascular risk factor.”
The study was published online in the Journal of the American Heart Association.
‘Experienced pain’ prognostic
The investigators analyzed data from the SWEDEHEART registry of 18,376 patients who had an MI in 2004-2013. The mean age of patients was 62 years and 75% were men. Follow-up time was 8.5 years (median, 3.37).
Self-reported levels of experienced pain according to the EuroQol five-dimension instrument were recorded 12 months after hospital discharge.
Moderate pain was reported by 38.2% of patients and extreme pain by 4.5%.
In the extreme pain category, women were overrepresented (7.5% vs. 3.6% of men), as were current smokers, and patients with diabetes, previous MI, previous stroke, previous percutaneous coronary intervention, non-ST-segment–elevation MI, and any kind of chest pain. Patients classified as physically inactive also were overrepresented in this category.
In addition, those with extreme pain had a higher body mass index and waist circumference 12 months after hospital discharge.
Most (73%) of the 7,889 patients who reported no pain at the 2-month follow-up after MI were also pain-free at the 12-month follow-up, and 65% of those experiencing pain at 2 months were also experiencing pain at 12 months.
There were 1,067 deaths. The adjusted hazard ratio was 1.35 for moderate pain and 2.06 for extreme pain.
As noted, pain was a stronger mortality predictor than smoking: C-statistics for pain were 0.60, and for smoking, 0.55.
“Clinicians managing patients after MI should recognize the need to consider experienced pain as a prognostic factor comparable to persistent smoking and to address this when designing individually adjusted [cardiac rehabilitation] and secondary prevention treatments,” the authors write.
Pain should be assessed at follow-up after MI, they add, and, as Dr. Vixner suggested, it should be “acknowledged as an important risk factor.”
Managing risks
“These findings parallel prior studies and my own clinical experience,” American Heart Association volunteer expert Gregg C. Fonarow, MD, interim chief of the division of cardiology at the University of California, Los Angeles, and director, Ahmanson-UCLA Cardiomyopathy Center, told this news organization.
“There are many potential causes for patient-reported pain in the year after a heart attack,” he said, including a greater cardiovascular risk burden, more comorbid conditions, less physical activity, and chronic use of nonsteroidal anti-inflammatory medications or opioids for pain control – all of which can contribute to the increased risk of mortality.
Factors beyond those evaluated and adjusted for in the observational study may contribute to the observed associations, he added. “Socioeconomic factors were not accounted for [and] there was no information on the types, doses, and frequency of pain medication use.”
“Clinicians managing patients with prior MI should carefully assess experienced pain and utilize this information to optimize risk factor control recommendations, inform treatment decisions, and consider in terms of prognosis,” he advised.
Further studies should evaluate whether the associations hold true for other patient populations, Dr. Fonarow said. “In addition, intervention trials could evaluate if enhanced management strategies in these higher-risk patients with self-reported pain can successfully lower the mortality risk.”
Dr. Vixner sees a role for physical activity in lowering the mortality risk.
“One of the core treatments for chronic pain is physical activity,” she said. “It positively influences quality of life, activities of daily living, pain intensity, and overall physical function, and reduces the risk of social isolation” and cardiovascular diseases.
Her team recently developed the “eVISualisation of physical activity and pain” (eVIS) intervention, which aims to promote healthy physical activity levels in persons living with chronic pain. The intervention is currently being evaluated in an ongoing registry-based, randomized controlled trial.
The study was supported by Svenska Försäkringsföreningen, Dalarna University, Region Dalarna. Dr. Vixner and coauthors have reported no relevant financial relationships. Dr. Fonarow has disclosed consulting for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer.
A version of this article first appeared on Medscape.com.
, new research suggests.
In the analysis of post-MI health data for more than 18,300 Swedish adults, those with moderate pain were 35% more likely to die from any cause during follow-up, compared with those with no pain, and those with extreme pain were more than twice as likely to die.
Furthermore, pain was a stronger predictor of mortality than smoking.
“For a long time, pain has been regarded as merely a symptom of disease rather than a disease” in its own right, Linda Vixner, PT, PhD, of Dalarna University in Falun, Sweden, said in an interview.
Updated definitions of chronic pain in the ICD-11, as well as a recent study using data from the UK Biobank showing that chronic pain is associated with an increased risk of cardiovascular disease, prompted the current study, which looks at the effect of pain on long-term survival after an MI.
“We did not expect that pain would have such a strong impact on the risk of death, and it also surprised us that the risk was more pronounced than that of smoking,” Dr. Vixner said. “Clinicians should consider pain an important cardiovascular risk factor.”
The study was published online in the Journal of the American Heart Association.
‘Experienced pain’ prognostic
The investigators analyzed data from the SWEDEHEART registry of 18,376 patients who had an MI in 2004-2013. The mean age of patients was 62 years and 75% were men. Follow-up time was 8.5 years (median, 3.37).
Self-reported levels of experienced pain according to the EuroQol five-dimension instrument were recorded 12 months after hospital discharge.
Moderate pain was reported by 38.2% of patients and extreme pain by 4.5%.
In the extreme pain category, women were overrepresented (7.5% vs. 3.6% of men), as were current smokers, and patients with diabetes, previous MI, previous stroke, previous percutaneous coronary intervention, non-ST-segment–elevation MI, and any kind of chest pain. Patients classified as physically inactive also were overrepresented in this category.
In addition, those with extreme pain had a higher body mass index and waist circumference 12 months after hospital discharge.
Most (73%) of the 7,889 patients who reported no pain at the 2-month follow-up after MI were also pain-free at the 12-month follow-up, and 65% of those experiencing pain at 2 months were also experiencing pain at 12 months.
There were 1,067 deaths. The adjusted hazard ratio was 1.35 for moderate pain and 2.06 for extreme pain.
As noted, pain was a stronger mortality predictor than smoking: C-statistics for pain were 0.60, and for smoking, 0.55.
“Clinicians managing patients after MI should recognize the need to consider experienced pain as a prognostic factor comparable to persistent smoking and to address this when designing individually adjusted [cardiac rehabilitation] and secondary prevention treatments,” the authors write.
Pain should be assessed at follow-up after MI, they add, and, as Dr. Vixner suggested, it should be “acknowledged as an important risk factor.”
Managing risks
“These findings parallel prior studies and my own clinical experience,” American Heart Association volunteer expert Gregg C. Fonarow, MD, interim chief of the division of cardiology at the University of California, Los Angeles, and director, Ahmanson-UCLA Cardiomyopathy Center, told this news organization.
“There are many potential causes for patient-reported pain in the year after a heart attack,” he said, including a greater cardiovascular risk burden, more comorbid conditions, less physical activity, and chronic use of nonsteroidal anti-inflammatory medications or opioids for pain control – all of which can contribute to the increased risk of mortality.
Factors beyond those evaluated and adjusted for in the observational study may contribute to the observed associations, he added. “Socioeconomic factors were not accounted for [and] there was no information on the types, doses, and frequency of pain medication use.”
“Clinicians managing patients with prior MI should carefully assess experienced pain and utilize this information to optimize risk factor control recommendations, inform treatment decisions, and consider in terms of prognosis,” he advised.
Further studies should evaluate whether the associations hold true for other patient populations, Dr. Fonarow said. “In addition, intervention trials could evaluate if enhanced management strategies in these higher-risk patients with self-reported pain can successfully lower the mortality risk.”
Dr. Vixner sees a role for physical activity in lowering the mortality risk.
“One of the core treatments for chronic pain is physical activity,” she said. “It positively influences quality of life, activities of daily living, pain intensity, and overall physical function, and reduces the risk of social isolation” and cardiovascular diseases.
Her team recently developed the “eVISualisation of physical activity and pain” (eVIS) intervention, which aims to promote healthy physical activity levels in persons living with chronic pain. The intervention is currently being evaluated in an ongoing registry-based, randomized controlled trial.
The study was supported by Svenska Försäkringsföreningen, Dalarna University, Region Dalarna. Dr. Vixner and coauthors have reported no relevant financial relationships. Dr. Fonarow has disclosed consulting for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer.
A version of this article first appeared on Medscape.com.
, new research suggests.
In the analysis of post-MI health data for more than 18,300 Swedish adults, those with moderate pain were 35% more likely to die from any cause during follow-up, compared with those with no pain, and those with extreme pain were more than twice as likely to die.
Furthermore, pain was a stronger predictor of mortality than smoking.
“For a long time, pain has been regarded as merely a symptom of disease rather than a disease” in its own right, Linda Vixner, PT, PhD, of Dalarna University in Falun, Sweden, said in an interview.
Updated definitions of chronic pain in the ICD-11, as well as a recent study using data from the UK Biobank showing that chronic pain is associated with an increased risk of cardiovascular disease, prompted the current study, which looks at the effect of pain on long-term survival after an MI.
“We did not expect that pain would have such a strong impact on the risk of death, and it also surprised us that the risk was more pronounced than that of smoking,” Dr. Vixner said. “Clinicians should consider pain an important cardiovascular risk factor.”
The study was published online in the Journal of the American Heart Association.
‘Experienced pain’ prognostic
The investigators analyzed data from the SWEDEHEART registry of 18,376 patients who had an MI in 2004-2013. The mean age of patients was 62 years and 75% were men. Follow-up time was 8.5 years (median, 3.37).
Self-reported levels of experienced pain according to the EuroQol five-dimension instrument were recorded 12 months after hospital discharge.
Moderate pain was reported by 38.2% of patients and extreme pain by 4.5%.
In the extreme pain category, women were overrepresented (7.5% vs. 3.6% of men), as were current smokers, and patients with diabetes, previous MI, previous stroke, previous percutaneous coronary intervention, non-ST-segment–elevation MI, and any kind of chest pain. Patients classified as physically inactive also were overrepresented in this category.
In addition, those with extreme pain had a higher body mass index and waist circumference 12 months after hospital discharge.
Most (73%) of the 7,889 patients who reported no pain at the 2-month follow-up after MI were also pain-free at the 12-month follow-up, and 65% of those experiencing pain at 2 months were also experiencing pain at 12 months.
There were 1,067 deaths. The adjusted hazard ratio was 1.35 for moderate pain and 2.06 for extreme pain.
As noted, pain was a stronger mortality predictor than smoking: C-statistics for pain were 0.60, and for smoking, 0.55.
“Clinicians managing patients after MI should recognize the need to consider experienced pain as a prognostic factor comparable to persistent smoking and to address this when designing individually adjusted [cardiac rehabilitation] and secondary prevention treatments,” the authors write.
Pain should be assessed at follow-up after MI, they add, and, as Dr. Vixner suggested, it should be “acknowledged as an important risk factor.”
Managing risks
“These findings parallel prior studies and my own clinical experience,” American Heart Association volunteer expert Gregg C. Fonarow, MD, interim chief of the division of cardiology at the University of California, Los Angeles, and director, Ahmanson-UCLA Cardiomyopathy Center, told this news organization.
“There are many potential causes for patient-reported pain in the year after a heart attack,” he said, including a greater cardiovascular risk burden, more comorbid conditions, less physical activity, and chronic use of nonsteroidal anti-inflammatory medications or opioids for pain control – all of which can contribute to the increased risk of mortality.
Factors beyond those evaluated and adjusted for in the observational study may contribute to the observed associations, he added. “Socioeconomic factors were not accounted for [and] there was no information on the types, doses, and frequency of pain medication use.”
“Clinicians managing patients with prior MI should carefully assess experienced pain and utilize this information to optimize risk factor control recommendations, inform treatment decisions, and consider in terms of prognosis,” he advised.
Further studies should evaluate whether the associations hold true for other patient populations, Dr. Fonarow said. “In addition, intervention trials could evaluate if enhanced management strategies in these higher-risk patients with self-reported pain can successfully lower the mortality risk.”
Dr. Vixner sees a role for physical activity in lowering the mortality risk.
“One of the core treatments for chronic pain is physical activity,” she said. “It positively influences quality of life, activities of daily living, pain intensity, and overall physical function, and reduces the risk of social isolation” and cardiovascular diseases.
Her team recently developed the “eVISualisation of physical activity and pain” (eVIS) intervention, which aims to promote healthy physical activity levels in persons living with chronic pain. The intervention is currently being evaluated in an ongoing registry-based, randomized controlled trial.
The study was supported by Svenska Försäkringsföreningen, Dalarna University, Region Dalarna. Dr. Vixner and coauthors have reported no relevant financial relationships. Dr. Fonarow has disclosed consulting for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Two historical events that changed the field of gastroenterology
The first event took place in 1822 at Fort Mackinac, which today is known as Mackinac Island on northern Lake Huron in Michigan. Alexis St. Martin, a French-Canadian fur trapper, was standing outside of the general store when a shotgun blast accidentally struck him in the stomach. Ordinarily, this would have been a fatal wound, but St. Martin miraculously survived--but with a gastric fistula that permanently exposed the interior of his stomach.
William Beaumont, the post surgeon at Fort Mackinac, engaged in a series of experiments – purportedly 238 – to study human digestion. In one experiment, Dr. Beaumont would pull food in and out of the stomach to study digestion. In another, he would withdraw fluid from the stomach to observe digestion outside of the body. The experiments caused St. Martin considerable discomfort. He eventually returned to Canada, but returned later when the U.S. Army agreed to compensate him for some of his expenses. Today, the experiments would be called into question as having crossed ethical boundaries. Dr. Beaumont published the results from his experiments in a book that established the fundamental basics of our current beliefs about digestion. The experiments arguably mark the first example of gastrointestinal research in the United States.
The second historical event – the invention of the fiber-optic endoscope – also occurred in Michigan. At the University of Michigan, Basil Hirschowitz, MD, invented a flexible, fiber-optic instrument that could be used to look into the stomach, and perhaps even the duodenum. He first tried the invention on himself, and in 1957, he demonstrated it at the national meeting of the American Gastroscopic Society by reading a telephone directory through the new device.
The instrument was soon adopted for clinical use by physicians. Whether the fiber-optic machine was superior for visualizing the stomach was hotly debated, but what was very clear was that the fiber-optic tool was more comfortable for patients. By the mid-1960s, the fiber-optic invention had become the instrument of choice for gastrointestinal endoscopy. Many advances have since been made to the original instrument.
Dr. Howell is the Elizabeth Farrand Professor and a professor of internal medicine, history, and health management and policy at the University of Michigan, Ann Arbor. He has no financial disclosures.
The first event took place in 1822 at Fort Mackinac, which today is known as Mackinac Island on northern Lake Huron in Michigan. Alexis St. Martin, a French-Canadian fur trapper, was standing outside of the general store when a shotgun blast accidentally struck him in the stomach. Ordinarily, this would have been a fatal wound, but St. Martin miraculously survived--but with a gastric fistula that permanently exposed the interior of his stomach.
William Beaumont, the post surgeon at Fort Mackinac, engaged in a series of experiments – purportedly 238 – to study human digestion. In one experiment, Dr. Beaumont would pull food in and out of the stomach to study digestion. In another, he would withdraw fluid from the stomach to observe digestion outside of the body. The experiments caused St. Martin considerable discomfort. He eventually returned to Canada, but returned later when the U.S. Army agreed to compensate him for some of his expenses. Today, the experiments would be called into question as having crossed ethical boundaries. Dr. Beaumont published the results from his experiments in a book that established the fundamental basics of our current beliefs about digestion. The experiments arguably mark the first example of gastrointestinal research in the United States.
The second historical event – the invention of the fiber-optic endoscope – also occurred in Michigan. At the University of Michigan, Basil Hirschowitz, MD, invented a flexible, fiber-optic instrument that could be used to look into the stomach, and perhaps even the duodenum. He first tried the invention on himself, and in 1957, he demonstrated it at the national meeting of the American Gastroscopic Society by reading a telephone directory through the new device.
The instrument was soon adopted for clinical use by physicians. Whether the fiber-optic machine was superior for visualizing the stomach was hotly debated, but what was very clear was that the fiber-optic tool was more comfortable for patients. By the mid-1960s, the fiber-optic invention had become the instrument of choice for gastrointestinal endoscopy. Many advances have since been made to the original instrument.
Dr. Howell is the Elizabeth Farrand Professor and a professor of internal medicine, history, and health management and policy at the University of Michigan, Ann Arbor. He has no financial disclosures.
The first event took place in 1822 at Fort Mackinac, which today is known as Mackinac Island on northern Lake Huron in Michigan. Alexis St. Martin, a French-Canadian fur trapper, was standing outside of the general store when a shotgun blast accidentally struck him in the stomach. Ordinarily, this would have been a fatal wound, but St. Martin miraculously survived--but with a gastric fistula that permanently exposed the interior of his stomach.
William Beaumont, the post surgeon at Fort Mackinac, engaged in a series of experiments – purportedly 238 – to study human digestion. In one experiment, Dr. Beaumont would pull food in and out of the stomach to study digestion. In another, he would withdraw fluid from the stomach to observe digestion outside of the body. The experiments caused St. Martin considerable discomfort. He eventually returned to Canada, but returned later when the U.S. Army agreed to compensate him for some of his expenses. Today, the experiments would be called into question as having crossed ethical boundaries. Dr. Beaumont published the results from his experiments in a book that established the fundamental basics of our current beliefs about digestion. The experiments arguably mark the first example of gastrointestinal research in the United States.
The second historical event – the invention of the fiber-optic endoscope – also occurred in Michigan. At the University of Michigan, Basil Hirschowitz, MD, invented a flexible, fiber-optic instrument that could be used to look into the stomach, and perhaps even the duodenum. He first tried the invention on himself, and in 1957, he demonstrated it at the national meeting of the American Gastroscopic Society by reading a telephone directory through the new device.
The instrument was soon adopted for clinical use by physicians. Whether the fiber-optic machine was superior for visualizing the stomach was hotly debated, but what was very clear was that the fiber-optic tool was more comfortable for patients. By the mid-1960s, the fiber-optic invention had become the instrument of choice for gastrointestinal endoscopy. Many advances have since been made to the original instrument.
Dr. Howell is the Elizabeth Farrand Professor and a professor of internal medicine, history, and health management and policy at the University of Michigan, Ann Arbor. He has no financial disclosures.
A 75-year-old White woman presented with diffuse erythema, scale, and pruritus on her scalp
The classical presentation includes symmetric proximal muscle weakness and underlying malignancy and is very common in adult patients. The etiology is unknown, however.
Some studies suggest people with certain HLA subtypes are at higher risk, and various infectious and pharmacological triggers are suspected to play a role in the pathogenesis of dermatomyositis. Infectious causes include Coxsackie B, enterovirus, and parvovirus. Drugs such as antineoplastic agents, antibiotics, and NSAIDs have been found to be triggers.
The pathogenesis of dermatomyositis involves immune-mediated damage to muscle capillaries and the endothelium of arterioles. In the typical humoral immune response, complement activation occurs. One mechanism of damage in dermatomyositis occurs when the membrane attack complex formed at the end of the complement process deposits in blood vessels, causing inflammation. B cells, autoantibodies, and interferon overexpression may also play a role in damaging the vasculature and muscle fibers. Hypoxia leads to muscular atrophy, resulting in degeneration and death of the fibers. On muscle biopsy, a perivascular and perimysial inflammatory infiltrate, perifascicular atrophy, and microangiopathy may be present. Skin histology reveals vacuolar changes in the basal layer, a lymphocytic infiltrate, and increased mucin production in the dermis.
On clinical examination, patients will have proximal muscle weakness and a skin rash that may include Gottron’s papules, heliotrope erythema, V-sign, shawl sign, holster sign, scalp erythema, midfacial erythema, and photosensitivity. Scalp erythema in dermatomyositis is highly linked to pruritus, alopecia, and telogen effluvium. Patients may experience small fiber neuropathy in dermatomyositis.
Serologies for this patient, who had previously been diagnosed and treated for dermatomyositis, were significant for a positive ANA 1:2560. Anti-Jo-1 antibody was negative. Her liver function tests, aldolase, creatinine kinase, sedimentation rate, C-reactive protein, and serum protein electrophoresis were normal. Imaging revealed mild chronic interstitial lung disease. A malignancy workup was negative.
Treatment of dermatomyositis involves lifestyle changes and pharmacologic therapy. Because of the intense photosensitivity, patients should be diligent with their sun protection. Methotrexate, azathioprine, and mycophenolate mofetil are considered first-line therapies for dermatomyositis. Therapies such as cyclophosphamide, rituximab, IVIg, and plasmapheresis may also be indicated in severe or refractory cases. Additionally, patients with pulmonary involvement should be given systemic steroids. The side effects of these drugs must be considered in the context of the patient’s demographics, comorbidities and lifestyle.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Natalie Y. Nasser, MD, of Kaiser Permanente Riverside Medical Center, Riverside, Calif. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Qudsiya Z and Waseem M. Dermatomyositis, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2023 Jan.
2. Kamperman RG et al. Int J Mol Sci. 2022 Apr 13;23(8):4301.
3. Kassamali B et al. Int J WomensDermatol. 2021 Sep 24;7(5Part A):576-82.
4. Vázquez-Herrera NE et al. Skin Appendage Disord. 2018 Aug;4(3):187-99.
The classical presentation includes symmetric proximal muscle weakness and underlying malignancy and is very common in adult patients. The etiology is unknown, however.
Some studies suggest people with certain HLA subtypes are at higher risk, and various infectious and pharmacological triggers are suspected to play a role in the pathogenesis of dermatomyositis. Infectious causes include Coxsackie B, enterovirus, and parvovirus. Drugs such as antineoplastic agents, antibiotics, and NSAIDs have been found to be triggers.
The pathogenesis of dermatomyositis involves immune-mediated damage to muscle capillaries and the endothelium of arterioles. In the typical humoral immune response, complement activation occurs. One mechanism of damage in dermatomyositis occurs when the membrane attack complex formed at the end of the complement process deposits in blood vessels, causing inflammation. B cells, autoantibodies, and interferon overexpression may also play a role in damaging the vasculature and muscle fibers. Hypoxia leads to muscular atrophy, resulting in degeneration and death of the fibers. On muscle biopsy, a perivascular and perimysial inflammatory infiltrate, perifascicular atrophy, and microangiopathy may be present. Skin histology reveals vacuolar changes in the basal layer, a lymphocytic infiltrate, and increased mucin production in the dermis.
On clinical examination, patients will have proximal muscle weakness and a skin rash that may include Gottron’s papules, heliotrope erythema, V-sign, shawl sign, holster sign, scalp erythema, midfacial erythema, and photosensitivity. Scalp erythema in dermatomyositis is highly linked to pruritus, alopecia, and telogen effluvium. Patients may experience small fiber neuropathy in dermatomyositis.
Serologies for this patient, who had previously been diagnosed and treated for dermatomyositis, were significant for a positive ANA 1:2560. Anti-Jo-1 antibody was negative. Her liver function tests, aldolase, creatinine kinase, sedimentation rate, C-reactive protein, and serum protein electrophoresis were normal. Imaging revealed mild chronic interstitial lung disease. A malignancy workup was negative.
Treatment of dermatomyositis involves lifestyle changes and pharmacologic therapy. Because of the intense photosensitivity, patients should be diligent with their sun protection. Methotrexate, azathioprine, and mycophenolate mofetil are considered first-line therapies for dermatomyositis. Therapies such as cyclophosphamide, rituximab, IVIg, and plasmapheresis may also be indicated in severe or refractory cases. Additionally, patients with pulmonary involvement should be given systemic steroids. The side effects of these drugs must be considered in the context of the patient’s demographics, comorbidities and lifestyle.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Natalie Y. Nasser, MD, of Kaiser Permanente Riverside Medical Center, Riverside, Calif. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Qudsiya Z and Waseem M. Dermatomyositis, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2023 Jan.
2. Kamperman RG et al. Int J Mol Sci. 2022 Apr 13;23(8):4301.
3. Kassamali B et al. Int J WomensDermatol. 2021 Sep 24;7(5Part A):576-82.
4. Vázquez-Herrera NE et al. Skin Appendage Disord. 2018 Aug;4(3):187-99.
The classical presentation includes symmetric proximal muscle weakness and underlying malignancy and is very common in adult patients. The etiology is unknown, however.
Some studies suggest people with certain HLA subtypes are at higher risk, and various infectious and pharmacological triggers are suspected to play a role in the pathogenesis of dermatomyositis. Infectious causes include Coxsackie B, enterovirus, and parvovirus. Drugs such as antineoplastic agents, antibiotics, and NSAIDs have been found to be triggers.
The pathogenesis of dermatomyositis involves immune-mediated damage to muscle capillaries and the endothelium of arterioles. In the typical humoral immune response, complement activation occurs. One mechanism of damage in dermatomyositis occurs when the membrane attack complex formed at the end of the complement process deposits in blood vessels, causing inflammation. B cells, autoantibodies, and interferon overexpression may also play a role in damaging the vasculature and muscle fibers. Hypoxia leads to muscular atrophy, resulting in degeneration and death of the fibers. On muscle biopsy, a perivascular and perimysial inflammatory infiltrate, perifascicular atrophy, and microangiopathy may be present. Skin histology reveals vacuolar changes in the basal layer, a lymphocytic infiltrate, and increased mucin production in the dermis.
On clinical examination, patients will have proximal muscle weakness and a skin rash that may include Gottron’s papules, heliotrope erythema, V-sign, shawl sign, holster sign, scalp erythema, midfacial erythema, and photosensitivity. Scalp erythema in dermatomyositis is highly linked to pruritus, alopecia, and telogen effluvium. Patients may experience small fiber neuropathy in dermatomyositis.
Serologies for this patient, who had previously been diagnosed and treated for dermatomyositis, were significant for a positive ANA 1:2560. Anti-Jo-1 antibody was negative. Her liver function tests, aldolase, creatinine kinase, sedimentation rate, C-reactive protein, and serum protein electrophoresis were normal. Imaging revealed mild chronic interstitial lung disease. A malignancy workup was negative.
Treatment of dermatomyositis involves lifestyle changes and pharmacologic therapy. Because of the intense photosensitivity, patients should be diligent with their sun protection. Methotrexate, azathioprine, and mycophenolate mofetil are considered first-line therapies for dermatomyositis. Therapies such as cyclophosphamide, rituximab, IVIg, and plasmapheresis may also be indicated in severe or refractory cases. Additionally, patients with pulmonary involvement should be given systemic steroids. The side effects of these drugs must be considered in the context of the patient’s demographics, comorbidities and lifestyle.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Natalie Y. Nasser, MD, of Kaiser Permanente Riverside Medical Center, Riverside, Calif. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Qudsiya Z and Waseem M. Dermatomyositis, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2023 Jan.
2. Kamperman RG et al. Int J Mol Sci. 2022 Apr 13;23(8):4301.
3. Kassamali B et al. Int J WomensDermatol. 2021 Sep 24;7(5Part A):576-82.
4. Vázquez-Herrera NE et al. Skin Appendage Disord. 2018 Aug;4(3):187-99.
CDC alerts clinicians to signs of alpha-gal syndrome
AGS causes patients to become allergic to meat, and in some cases the reaction can be life-threatening. Symptoms typically start 2-6 hours after eating the meat.
The American Gastroenterological Association published a Clinical Practice Update in February notifying gastroenterologists that a subset of AGS patients are presenting with abdominal pain, nausea, diarrhea or vomiting, without skin changes or anaphylaxis. If alpha-gal is suspected, serum tests for immunoglobulin E (IgE) antibodies should be performed.
“It is important for gastroenterologists to be aware of this condition and to be capable of diagnosing and treating it in a timely manner,” wrote authors of the clinical practice update in Clinical Gastroenterology and Hepatology.
A Morbidity and Mortality Weekly Report demonstrates that health care provider knowledge is low surrounding AGS. Almost half of the 1,500 health care providers surveyed (42%) had never heard of the syndrome and another 35% were not confident in diagnosing or managing affected patients.
The low knowledge is concerning because the range of the lone star tick, which is the species primarily associated with this syndrome, is expanding. The knowledge gaps may lead to delayed or overlooked diagnoses.
“Improved health care provider education might facilitate a rapid diagnosis of AGS, improve patient care, and support public health understanding of this emerging condition,” write the report authors, led by Ann Carpenter, DVM, with the CDC.
Another Morbidity and Mortality Weekly Report, with lead author Johanna S. Salzer, DVM, PhD, of the CDC, also issued on July 28, notes that specific symptoms and severity of AGS vary and no cure or treatment is currently available. From 2010 to 2018, there were more than 34,000 suspected cases of AGS in the United States, but current knowledge of where the cases have occurred is limited, the study authors write.
According to the report, the suspected AGS cases were concentrated in areas where the lone star tick is known to be found, particularly throughout Arkansas, Kentucky, Missouri, and Suffolk County, N.Y.
The report also notes that, “during 2017-2021, there was an annual increase in positive test results for AGS in the United States. More than 90,000 suspected AGS cases were identified during the study period, and the number of new suspected cases increased by approximately 15,000 each year during the study.”
An AGS diagnosis “can be made with GI distress and increased serum alpha-gal IgE antibodies whose symptoms are relieved adequately on an alpha-gal avoidance diet that eliminates pork, beef, and mammalian-derived products,” the practice update says.
Patients whose symptoms also include facial swelling, urticaria, and trouble breathing should be referred to allergists, the AGA update states.
Patients should also be counseled to avoid further tick bites because additional bites can worsen the allergy.
The authors declare no relevant financial relationships.
AGS causes patients to become allergic to meat, and in some cases the reaction can be life-threatening. Symptoms typically start 2-6 hours after eating the meat.
The American Gastroenterological Association published a Clinical Practice Update in February notifying gastroenterologists that a subset of AGS patients are presenting with abdominal pain, nausea, diarrhea or vomiting, without skin changes or anaphylaxis. If alpha-gal is suspected, serum tests for immunoglobulin E (IgE) antibodies should be performed.
“It is important for gastroenterologists to be aware of this condition and to be capable of diagnosing and treating it in a timely manner,” wrote authors of the clinical practice update in Clinical Gastroenterology and Hepatology.
A Morbidity and Mortality Weekly Report demonstrates that health care provider knowledge is low surrounding AGS. Almost half of the 1,500 health care providers surveyed (42%) had never heard of the syndrome and another 35% were not confident in diagnosing or managing affected patients.
The low knowledge is concerning because the range of the lone star tick, which is the species primarily associated with this syndrome, is expanding. The knowledge gaps may lead to delayed or overlooked diagnoses.
“Improved health care provider education might facilitate a rapid diagnosis of AGS, improve patient care, and support public health understanding of this emerging condition,” write the report authors, led by Ann Carpenter, DVM, with the CDC.
Another Morbidity and Mortality Weekly Report, with lead author Johanna S. Salzer, DVM, PhD, of the CDC, also issued on July 28, notes that specific symptoms and severity of AGS vary and no cure or treatment is currently available. From 2010 to 2018, there were more than 34,000 suspected cases of AGS in the United States, but current knowledge of where the cases have occurred is limited, the study authors write.
According to the report, the suspected AGS cases were concentrated in areas where the lone star tick is known to be found, particularly throughout Arkansas, Kentucky, Missouri, and Suffolk County, N.Y.
The report also notes that, “during 2017-2021, there was an annual increase in positive test results for AGS in the United States. More than 90,000 suspected AGS cases were identified during the study period, and the number of new suspected cases increased by approximately 15,000 each year during the study.”
An AGS diagnosis “can be made with GI distress and increased serum alpha-gal IgE antibodies whose symptoms are relieved adequately on an alpha-gal avoidance diet that eliminates pork, beef, and mammalian-derived products,” the practice update says.
Patients whose symptoms also include facial swelling, urticaria, and trouble breathing should be referred to allergists, the AGA update states.
Patients should also be counseled to avoid further tick bites because additional bites can worsen the allergy.
The authors declare no relevant financial relationships.
AGS causes patients to become allergic to meat, and in some cases the reaction can be life-threatening. Symptoms typically start 2-6 hours after eating the meat.
The American Gastroenterological Association published a Clinical Practice Update in February notifying gastroenterologists that a subset of AGS patients are presenting with abdominal pain, nausea, diarrhea or vomiting, without skin changes or anaphylaxis. If alpha-gal is suspected, serum tests for immunoglobulin E (IgE) antibodies should be performed.
“It is important for gastroenterologists to be aware of this condition and to be capable of diagnosing and treating it in a timely manner,” wrote authors of the clinical practice update in Clinical Gastroenterology and Hepatology.
A Morbidity and Mortality Weekly Report demonstrates that health care provider knowledge is low surrounding AGS. Almost half of the 1,500 health care providers surveyed (42%) had never heard of the syndrome and another 35% were not confident in diagnosing or managing affected patients.
The low knowledge is concerning because the range of the lone star tick, which is the species primarily associated with this syndrome, is expanding. The knowledge gaps may lead to delayed or overlooked diagnoses.
“Improved health care provider education might facilitate a rapid diagnosis of AGS, improve patient care, and support public health understanding of this emerging condition,” write the report authors, led by Ann Carpenter, DVM, with the CDC.
Another Morbidity and Mortality Weekly Report, with lead author Johanna S. Salzer, DVM, PhD, of the CDC, also issued on July 28, notes that specific symptoms and severity of AGS vary and no cure or treatment is currently available. From 2010 to 2018, there were more than 34,000 suspected cases of AGS in the United States, but current knowledge of where the cases have occurred is limited, the study authors write.
According to the report, the suspected AGS cases were concentrated in areas where the lone star tick is known to be found, particularly throughout Arkansas, Kentucky, Missouri, and Suffolk County, N.Y.
The report also notes that, “during 2017-2021, there was an annual increase in positive test results for AGS in the United States. More than 90,000 suspected AGS cases were identified during the study period, and the number of new suspected cases increased by approximately 15,000 each year during the study.”
An AGS diagnosis “can be made with GI distress and increased serum alpha-gal IgE antibodies whose symptoms are relieved adequately on an alpha-gal avoidance diet that eliminates pork, beef, and mammalian-derived products,” the practice update says.
Patients whose symptoms also include facial swelling, urticaria, and trouble breathing should be referred to allergists, the AGA update states.
Patients should also be counseled to avoid further tick bites because additional bites can worsen the allergy.
The authors declare no relevant financial relationships.