User login
Managing Superutilizers
We have known for years that the distribution of healthcare expenditures in the United States is skewed, with a small portion of the population consuming a disproportionately high share of resources. In 2010, 1% of the population accounted for 21.4% of the $1.3 trillion spent on healthcare.[1] Growing evidence documents that most of these high‐cost patients are not receiving coordinated care, preventive care, or care in the most appropriate settings.[2] The term superutilizer describes individuals with complex physical, behavioral, and social needs who have frequent emergency department (ED) visits and multiple costly hospital admissions.[3] Not surprisingly, multiple superutilizer programs and new funding opportunities target this population attempting to reduce their healthcare costs while improving their care, as public and private insurers shift to value‐based care.[4]
Beginning in 2006, the Robert Wood Johnson Foundation supported the Camden Coalition[5] with 3 grants to develop a community‐based approach to identify high‐utilizer patients and provide them with coordinated medical and social services.[6] These programs include community‐based teams that focus on the highest utilizers in a specific geographic area and provide intensive outpatient case management. Building on these efforts, the Center for Medicare and Medicaid Innovation (CMMI) awarded 2 Health Care Innovation Awards totaling $17.2 million to target Medicaid superutilizers.[7] Through its State Innovation Models initiative, CMMI also encourages states to pilot superutilizer programs to increase care coordination and support of persons with certain risk factors such as homelessness or mental illness.[8] Additionally, the National Governors Association developed a 1‐year, multistate policy academy to develop state‐level capacity and state action plans that guide how to improve the delivery and financing of care for superutilizers.[9]
With all these ongoing activities in the setting of a paucity of research identifying the most cost‐efficient practices to manage super‐utilizers, we are glad to see the Journal of Hospital Medicine publish an evaluation of a quality‐improvement project targeting superutilizers.[10] Mercer and colleagues at Duke University Hospital show that developing an individualized care plan and integrating it into their electronic health record (EHR) reduced hospital admissions, but not ED visits. Although we applaud the reportedly individualized patient approach and recognize the effort required to refer patients to a more appropriate care setting, we believe the researchers neglected 3 important components for the intervention: (1) patient engagement in developing individualized care plans, (2) care coordination integrated with community collaboration, and (3) feedback on continuum of care relayed back to providers. The managing strategies mentioned in the article seem to have evolved exclusively from the provider's perspective, a common mistake that the Patient‐Centered Outcomes Research Institute emphasizes must be avoided. We are concerned about the lack of clarity regarding the set of management strategies focused on providing high‐quality care while limiting unnecessary admissions reported by them. We fear this strategy was imposed on patients and not developed collaboratively with them. Effective interventions for superutilizers should do more than just guide providers actions, but also connect services to the patient's needs. There should be coordination and continuous improvement of these efforts, which requires engagement of the patient and their community with feedback to the system.
Possibly most important, an individualized approach to superutilizers needs to be patient‐centeredprioritizing patient goals and preferences, selecting interventions and services guided by the needs of the individual, and emphasizing modifiable outcomes that matter to the patient. Such a patient‐centered approach goes beyond the individual patient to incorporate information about social support and family dynamics, highlighting the role of caregivers. Patients and their caregivers must be engaged or activated to ensure adherence to appropriate care and behaviors in any superutilizer programs. Additionally, individualized patient‐centered care plans should be dynamic and bidirectional to accommodate changes in health priorities that may occur over time. Such lack of patient and community engagement may explain why ED‐visit frequency was unchanged in their study.
The approach of having a Complex Care Plan Committee deserves attention as it appropriately included the right people at the academic medical center. However, why is it voluntary? Should not an important, or even essential, committee such as this be supported by the health system? Moreover, although the care plan developed by members of the committee possesses understandable aspects to be considered in a patient's care, why is this not shown to the patient for their input? Instead of being done to the patient, we recommend including patients in this process, believing such patient engagement would improve care further and likely yield sustained changes. We suggest the researchers remember the maxim nothing about me, without me.
Patients who use the most healthcare services typically have complicated social situations that directly impact their ability to improve their health and stay well.[2, 11] Addressing the social determinants of health is not a new concept; however, creating healthy communities as a core responsibility of the healthcare industry is. Contributing to the dizzying state of change in US healthcare are efforts to shift to value‐based purchasing and population health management.[12] This transformation from a fee‐for‐service hospital‐centric industry into one focused on the continuum of care requires outreach into communities where superutilizers live. Ultimately, all healthcare is local, as this is where patients receive the vast majority of their care. Improving quality and reducing costs requires healthcare providers to work together on a collaborative mission that focuses on the needs of patients and community, not just efforts to reduce utilization. Even hospitalists must forge collaborative relationships with skilled nursing facilities and patient‐centered medical homes.
Given the successes of some superutilizer programs,[3] a key issue is how to scale or disseminate such labor‐intensive highly individualized programs. Each patient has very complex and specific medical, behavioral, and social needs that require creativity and flexibility to adequately address these needs. Without question, patients and/or their caregivers should be members of the care team aiming to optimize their care. Unfortunately, our current healthcare system is not designed to address the complexity and uniqueness of each superutilizer. Nonetheless, summarizing patients history into the EHR and integrating recommendations offers an opportunity to share information as originally hoped by the transition from paper‐based records. It additionally offers an opportunity to learn from use of this information as academic medical centers aim to become learning health systems.[13] Future implementation science research in this area should assess how to scale patient‐centered approaches to care, particularly for those with chronic illness and other vulnerabilities. We must eschew efforts that solely focus on reducing utilization by patients without involving them; after all, they are the focus of healthcare.
Disclosure
Nothing to report.
- , . Differentials in the concentration in the level of health expenditures across population subgroups in the U.S, 2010. Statistical brief #421. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , . Preventing avoidable rehospitalizations by understanding the characteristics of “frequent fliers.” J Nurs Care Qual. 2012;27(1):77–82.
- Robert Wood Johnson Foundation. Super‐utilizer summit: common themes from innovative complex care management programs. Available at: http://www.rwjf.org/en/library/research/2013/10/super‐utilizer‐summit.html. Published October 2013; accessed March 22, 2015.
- . Setting value‐based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899.
- . Medical Report: The hot spotters—can we lower medical costs by giving the neediest patients better care? Available at: http://www.newyorker.com/magazine/2011/01/24/the-hot-spotters. Published January 24, 2011; accessed March 22, 2015.
- Robert Wood Johnson Foundation. A coalition creates a citywide care management system. Available at: http://www.rwjf.org/content/dam/farm/reports/program_results_reports/2014/rwjf69151. Published January 13, 2011; revised June 13, 2014; accessed March 22, 2015.
- Centers for Medicare 10(XX):XXX–XXX.
- , , , . The faces of Medicaid II: recognizing the care needs of people with multiple chronic conditions. Center for Health Care Strategies, Inc. Available at: http://www.chcs.org/resource/the-faces-of-medicaid-ii-recognizing-the-care-needs-of-people-with-multiple-chronic-conditions. Published October 2007; accessed March 22, 2015.
- . Accountable care organizations—the risk of failure and the risks of success. N Engl J Med. 2014;371(18):1750–1751.
- , , . Implementing the learning health system: from concept to action. Ann Intern Med. 2012;157(3):207–210.
We have known for years that the distribution of healthcare expenditures in the United States is skewed, with a small portion of the population consuming a disproportionately high share of resources. In 2010, 1% of the population accounted for 21.4% of the $1.3 trillion spent on healthcare.[1] Growing evidence documents that most of these high‐cost patients are not receiving coordinated care, preventive care, or care in the most appropriate settings.[2] The term superutilizer describes individuals with complex physical, behavioral, and social needs who have frequent emergency department (ED) visits and multiple costly hospital admissions.[3] Not surprisingly, multiple superutilizer programs and new funding opportunities target this population attempting to reduce their healthcare costs while improving their care, as public and private insurers shift to value‐based care.[4]
Beginning in 2006, the Robert Wood Johnson Foundation supported the Camden Coalition[5] with 3 grants to develop a community‐based approach to identify high‐utilizer patients and provide them with coordinated medical and social services.[6] These programs include community‐based teams that focus on the highest utilizers in a specific geographic area and provide intensive outpatient case management. Building on these efforts, the Center for Medicare and Medicaid Innovation (CMMI) awarded 2 Health Care Innovation Awards totaling $17.2 million to target Medicaid superutilizers.[7] Through its State Innovation Models initiative, CMMI also encourages states to pilot superutilizer programs to increase care coordination and support of persons with certain risk factors such as homelessness or mental illness.[8] Additionally, the National Governors Association developed a 1‐year, multistate policy academy to develop state‐level capacity and state action plans that guide how to improve the delivery and financing of care for superutilizers.[9]
With all these ongoing activities in the setting of a paucity of research identifying the most cost‐efficient practices to manage super‐utilizers, we are glad to see the Journal of Hospital Medicine publish an evaluation of a quality‐improvement project targeting superutilizers.[10] Mercer and colleagues at Duke University Hospital show that developing an individualized care plan and integrating it into their electronic health record (EHR) reduced hospital admissions, but not ED visits. Although we applaud the reportedly individualized patient approach and recognize the effort required to refer patients to a more appropriate care setting, we believe the researchers neglected 3 important components for the intervention: (1) patient engagement in developing individualized care plans, (2) care coordination integrated with community collaboration, and (3) feedback on continuum of care relayed back to providers. The managing strategies mentioned in the article seem to have evolved exclusively from the provider's perspective, a common mistake that the Patient‐Centered Outcomes Research Institute emphasizes must be avoided. We are concerned about the lack of clarity regarding the set of management strategies focused on providing high‐quality care while limiting unnecessary admissions reported by them. We fear this strategy was imposed on patients and not developed collaboratively with them. Effective interventions for superutilizers should do more than just guide providers actions, but also connect services to the patient's needs. There should be coordination and continuous improvement of these efforts, which requires engagement of the patient and their community with feedback to the system.
Possibly most important, an individualized approach to superutilizers needs to be patient‐centeredprioritizing patient goals and preferences, selecting interventions and services guided by the needs of the individual, and emphasizing modifiable outcomes that matter to the patient. Such a patient‐centered approach goes beyond the individual patient to incorporate information about social support and family dynamics, highlighting the role of caregivers. Patients and their caregivers must be engaged or activated to ensure adherence to appropriate care and behaviors in any superutilizer programs. Additionally, individualized patient‐centered care plans should be dynamic and bidirectional to accommodate changes in health priorities that may occur over time. Such lack of patient and community engagement may explain why ED‐visit frequency was unchanged in their study.
The approach of having a Complex Care Plan Committee deserves attention as it appropriately included the right people at the academic medical center. However, why is it voluntary? Should not an important, or even essential, committee such as this be supported by the health system? Moreover, although the care plan developed by members of the committee possesses understandable aspects to be considered in a patient's care, why is this not shown to the patient for their input? Instead of being done to the patient, we recommend including patients in this process, believing such patient engagement would improve care further and likely yield sustained changes. We suggest the researchers remember the maxim nothing about me, without me.
Patients who use the most healthcare services typically have complicated social situations that directly impact their ability to improve their health and stay well.[2, 11] Addressing the social determinants of health is not a new concept; however, creating healthy communities as a core responsibility of the healthcare industry is. Contributing to the dizzying state of change in US healthcare are efforts to shift to value‐based purchasing and population health management.[12] This transformation from a fee‐for‐service hospital‐centric industry into one focused on the continuum of care requires outreach into communities where superutilizers live. Ultimately, all healthcare is local, as this is where patients receive the vast majority of their care. Improving quality and reducing costs requires healthcare providers to work together on a collaborative mission that focuses on the needs of patients and community, not just efforts to reduce utilization. Even hospitalists must forge collaborative relationships with skilled nursing facilities and patient‐centered medical homes.
Given the successes of some superutilizer programs,[3] a key issue is how to scale or disseminate such labor‐intensive highly individualized programs. Each patient has very complex and specific medical, behavioral, and social needs that require creativity and flexibility to adequately address these needs. Without question, patients and/or their caregivers should be members of the care team aiming to optimize their care. Unfortunately, our current healthcare system is not designed to address the complexity and uniqueness of each superutilizer. Nonetheless, summarizing patients history into the EHR and integrating recommendations offers an opportunity to share information as originally hoped by the transition from paper‐based records. It additionally offers an opportunity to learn from use of this information as academic medical centers aim to become learning health systems.[13] Future implementation science research in this area should assess how to scale patient‐centered approaches to care, particularly for those with chronic illness and other vulnerabilities. We must eschew efforts that solely focus on reducing utilization by patients without involving them; after all, they are the focus of healthcare.
Disclosure
Nothing to report.
We have known for years that the distribution of healthcare expenditures in the United States is skewed, with a small portion of the population consuming a disproportionately high share of resources. In 2010, 1% of the population accounted for 21.4% of the $1.3 trillion spent on healthcare.[1] Growing evidence documents that most of these high‐cost patients are not receiving coordinated care, preventive care, or care in the most appropriate settings.[2] The term superutilizer describes individuals with complex physical, behavioral, and social needs who have frequent emergency department (ED) visits and multiple costly hospital admissions.[3] Not surprisingly, multiple superutilizer programs and new funding opportunities target this population attempting to reduce their healthcare costs while improving their care, as public and private insurers shift to value‐based care.[4]
Beginning in 2006, the Robert Wood Johnson Foundation supported the Camden Coalition[5] with 3 grants to develop a community‐based approach to identify high‐utilizer patients and provide them with coordinated medical and social services.[6] These programs include community‐based teams that focus on the highest utilizers in a specific geographic area and provide intensive outpatient case management. Building on these efforts, the Center for Medicare and Medicaid Innovation (CMMI) awarded 2 Health Care Innovation Awards totaling $17.2 million to target Medicaid superutilizers.[7] Through its State Innovation Models initiative, CMMI also encourages states to pilot superutilizer programs to increase care coordination and support of persons with certain risk factors such as homelessness or mental illness.[8] Additionally, the National Governors Association developed a 1‐year, multistate policy academy to develop state‐level capacity and state action plans that guide how to improve the delivery and financing of care for superutilizers.[9]
With all these ongoing activities in the setting of a paucity of research identifying the most cost‐efficient practices to manage super‐utilizers, we are glad to see the Journal of Hospital Medicine publish an evaluation of a quality‐improvement project targeting superutilizers.[10] Mercer and colleagues at Duke University Hospital show that developing an individualized care plan and integrating it into their electronic health record (EHR) reduced hospital admissions, but not ED visits. Although we applaud the reportedly individualized patient approach and recognize the effort required to refer patients to a more appropriate care setting, we believe the researchers neglected 3 important components for the intervention: (1) patient engagement in developing individualized care plans, (2) care coordination integrated with community collaboration, and (3) feedback on continuum of care relayed back to providers. The managing strategies mentioned in the article seem to have evolved exclusively from the provider's perspective, a common mistake that the Patient‐Centered Outcomes Research Institute emphasizes must be avoided. We are concerned about the lack of clarity regarding the set of management strategies focused on providing high‐quality care while limiting unnecessary admissions reported by them. We fear this strategy was imposed on patients and not developed collaboratively with them. Effective interventions for superutilizers should do more than just guide providers actions, but also connect services to the patient's needs. There should be coordination and continuous improvement of these efforts, which requires engagement of the patient and their community with feedback to the system.
Possibly most important, an individualized approach to superutilizers needs to be patient‐centeredprioritizing patient goals and preferences, selecting interventions and services guided by the needs of the individual, and emphasizing modifiable outcomes that matter to the patient. Such a patient‐centered approach goes beyond the individual patient to incorporate information about social support and family dynamics, highlighting the role of caregivers. Patients and their caregivers must be engaged or activated to ensure adherence to appropriate care and behaviors in any superutilizer programs. Additionally, individualized patient‐centered care plans should be dynamic and bidirectional to accommodate changes in health priorities that may occur over time. Such lack of patient and community engagement may explain why ED‐visit frequency was unchanged in their study.
The approach of having a Complex Care Plan Committee deserves attention as it appropriately included the right people at the academic medical center. However, why is it voluntary? Should not an important, or even essential, committee such as this be supported by the health system? Moreover, although the care plan developed by members of the committee possesses understandable aspects to be considered in a patient's care, why is this not shown to the patient for their input? Instead of being done to the patient, we recommend including patients in this process, believing such patient engagement would improve care further and likely yield sustained changes. We suggest the researchers remember the maxim nothing about me, without me.
Patients who use the most healthcare services typically have complicated social situations that directly impact their ability to improve their health and stay well.[2, 11] Addressing the social determinants of health is not a new concept; however, creating healthy communities as a core responsibility of the healthcare industry is. Contributing to the dizzying state of change in US healthcare are efforts to shift to value‐based purchasing and population health management.[12] This transformation from a fee‐for‐service hospital‐centric industry into one focused on the continuum of care requires outreach into communities where superutilizers live. Ultimately, all healthcare is local, as this is where patients receive the vast majority of their care. Improving quality and reducing costs requires healthcare providers to work together on a collaborative mission that focuses on the needs of patients and community, not just efforts to reduce utilization. Even hospitalists must forge collaborative relationships with skilled nursing facilities and patient‐centered medical homes.
Given the successes of some superutilizer programs,[3] a key issue is how to scale or disseminate such labor‐intensive highly individualized programs. Each patient has very complex and specific medical, behavioral, and social needs that require creativity and flexibility to adequately address these needs. Without question, patients and/or their caregivers should be members of the care team aiming to optimize their care. Unfortunately, our current healthcare system is not designed to address the complexity and uniqueness of each superutilizer. Nonetheless, summarizing patients history into the EHR and integrating recommendations offers an opportunity to share information as originally hoped by the transition from paper‐based records. It additionally offers an opportunity to learn from use of this information as academic medical centers aim to become learning health systems.[13] Future implementation science research in this area should assess how to scale patient‐centered approaches to care, particularly for those with chronic illness and other vulnerabilities. We must eschew efforts that solely focus on reducing utilization by patients without involving them; after all, they are the focus of healthcare.
Disclosure
Nothing to report.
- , . Differentials in the concentration in the level of health expenditures across population subgroups in the U.S, 2010. Statistical brief #421. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , . Preventing avoidable rehospitalizations by understanding the characteristics of “frequent fliers.” J Nurs Care Qual. 2012;27(1):77–82.
- Robert Wood Johnson Foundation. Super‐utilizer summit: common themes from innovative complex care management programs. Available at: http://www.rwjf.org/en/library/research/2013/10/super‐utilizer‐summit.html. Published October 2013; accessed March 22, 2015.
- . Setting value‐based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899.
- . Medical Report: The hot spotters—can we lower medical costs by giving the neediest patients better care? Available at: http://www.newyorker.com/magazine/2011/01/24/the-hot-spotters. Published January 24, 2011; accessed March 22, 2015.
- Robert Wood Johnson Foundation. A coalition creates a citywide care management system. Available at: http://www.rwjf.org/content/dam/farm/reports/program_results_reports/2014/rwjf69151. Published January 13, 2011; revised June 13, 2014; accessed March 22, 2015.
- Centers for Medicare 10(XX):XXX–XXX.
- , , , . The faces of Medicaid II: recognizing the care needs of people with multiple chronic conditions. Center for Health Care Strategies, Inc. Available at: http://www.chcs.org/resource/the-faces-of-medicaid-ii-recognizing-the-care-needs-of-people-with-multiple-chronic-conditions. Published October 2007; accessed March 22, 2015.
- . Accountable care organizations—the risk of failure and the risks of success. N Engl J Med. 2014;371(18):1750–1751.
- , , . Implementing the learning health system: from concept to action. Ann Intern Med. 2012;157(3):207–210.
- , . Differentials in the concentration in the level of health expenditures across population subgroups in the U.S, 2010. Statistical brief #421. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , . Preventing avoidable rehospitalizations by understanding the characteristics of “frequent fliers.” J Nurs Care Qual. 2012;27(1):77–82.
- Robert Wood Johnson Foundation. Super‐utilizer summit: common themes from innovative complex care management programs. Available at: http://www.rwjf.org/en/library/research/2013/10/super‐utilizer‐summit.html. Published October 2013; accessed March 22, 2015.
- . Setting value‐based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899.
- . Medical Report: The hot spotters—can we lower medical costs by giving the neediest patients better care? Available at: http://www.newyorker.com/magazine/2011/01/24/the-hot-spotters. Published January 24, 2011; accessed March 22, 2015.
- Robert Wood Johnson Foundation. A coalition creates a citywide care management system. Available at: http://www.rwjf.org/content/dam/farm/reports/program_results_reports/2014/rwjf69151. Published January 13, 2011; revised June 13, 2014; accessed March 22, 2015.
- Centers for Medicare 10(XX):XXX–XXX.
- , , , . The faces of Medicaid II: recognizing the care needs of people with multiple chronic conditions. Center for Health Care Strategies, Inc. Available at: http://www.chcs.org/resource/the-faces-of-medicaid-ii-recognizing-the-care-needs-of-people-with-multiple-chronic-conditions. Published October 2007; accessed March 22, 2015.
- . Accountable care organizations—the risk of failure and the risks of success. N Engl J Med. 2014;371(18):1750–1751.
- , , . Implementing the learning health system: from concept to action. Ann Intern Med. 2012;157(3):207–210.
Patient Complexities and Antibiotics
Clinical management of patients with medical and social comorbidities has become increasingly complex.[1, 2, 3, 4] This complexity stems from lack of data for these groups of patients who are often excluded from clinical trials.[5] There are data demonstrating that older patients and patients with multiple comorbidities including diabetes, renal disease, obesity, limited mobility, and poor access to healthcare have worse outcomes for specific conditions compared to otherwise equal counterparts, and that the cost of care for these patients is more expensive.[3, 4, 6] Moreover, traditional risk assessment of disease severity and outcomes are not accurate when applied to medically and socially complex patients.[7, 8]
Treatment decisions regarding antibiotic use add additional complexity. Specifically, physicians antibiotic prescribing decisions can promote the emergence of multidrug resistant pathogens in a hospital or population.[9, 10] Multidrug resistant organisms (MDROs) are particularly problematic, as their prevalence is increasing while the development of new antimicrobial agents is declining.[11] Infections caused by antibiotic‐resistant pathogens are associated with increased morbidity and mortality and healthcare costs.[12, 13, 14] Antibiotic use, although potentially lifesaving, can also result in severe complications such as Clostridium difficile‐associated diarrhea, acute kidney injury, and anaphylaxis, among other adverse events, particularly in older patients with medical comorbidities.[15, 16, 17, 18] Judicious antibiotic use is critical to halt the epidemic of MDROs and to minimize antibiotic‐associated adverse effects.[19, 20, 21]
Evidence‐based guidelines have the potential to assist physicians in choosing the antibiotic that achieves the best clinical outcome for a specific infection or situation.[11] This includes using the narrowest spectrum agent to minimize selection pressure on microorganisms and avoiding unneeded drugs to minimize adverse drug effects.[9, 11] Importantly, guideline adherence regarding antibiotic selection has been shown to be associated with increased clinical success and decreased mortality.[22, 23] Unfortunately, 30% to 50% of antibiotic use in hospitalized patients is inconsistent with national guidelines.[24, 25, 26] Reasons for physicians ordering of tests and treatments inconsistent with guidelines are not fully understood, and potentially include patient and physician factors, and the cultural and social context of the healthcare system.[27]
To optimize the use of antibiotics, it is important to understand how medical complexities (defined as demographic, comorbid, and limited healthcare access characteristics that are associated with suboptimal patient care and outcomes) influence physicians antibiotic prescribing practices.[28] We created 3 clinical vignettes for common diagnoses (dyspnea with initial concern for pneumonia, skin and soft tissue infection, and asymptomatic bacteriuria) among hospitalized patients. We selected these conditions because of their high prevalence, frequent management by hospitalists, generalist physicians, and noninfectious disease specialists, and because well‐documented evidence suggests either no antibiotics or narrower spectrum antibiotics are usually the treatments of choice. Using the Infectious Diseases Society of America (IDSA) guidelines relevant to each clinical vignette,[29, 30, 31] we assessed physicians recommendations for guideline‐appropriate antibiotic management for patients without and with medical complexities using an electronic multiple‐choice survey.
METHODS
Survey Participants
We surveyed internal medicine generalist and subspecialty inpatient physicians from 3 academic medical centers in the metropolitan Los Angeles, California area. Potential participants included attending and housestaff physicians in the departments of internal medicine and family medicine at the 3 medical centers associated with the University of California Los Angeles (UCLA) Clinical and Translational Science Institute: (1) Ronald ReaganUCLA Medical Center, a tertiary care academic medical center; (2) Harbor UCLA Medical Center, a county (public) medical center; and (3) CedarsSinai Medical Center, a tertiary care medical center. Each center was affiliated with a residency training program, although not all attending physicians were associated with the training programs. Physicians were eligible to perform the survey if they attended 2 weeks per year in the inpatient setting. We collected physician‐level information including level of training (resident/fellow vs attending), specialization or not, proportion of time spent working in the hospital, and proportion of time spent providing direct clinical care (compared to activities such as administration and research). All eligible participants were emailed a brief study description with a hyperlink to the electronic survey created in REDCap (Research Electronic Data Capture version 5.6.0, 2013). Administrative staff provided email lists for potential participants at 2 of the hospitals. Per hospital policy, an email list was not provided by the third hospital, and potential participants were emailed the survey link directly by the hospital administrative staff. We incentivized study participation by entering participants who completed the survey into a raffle to win either a $100 gift card or a computer tablet. Physicians had 3 months to complete the survey and were sent up to 5 emails encouraging them to complete it.
Survey
The survey consisted of 3 clinical vignettes describing common hospital‐based situations that required decision making about antibiotic use. The 3 clinical vignettes described: (1) a patient with dyspnea and no infiltrate on chest radiograph who is initially treated empirically with antibiotics for pneumonia but is ultimately diagnosed with a congestive heart failure exacerbation, (2) a patient admitted with a skin infection that grows methicillin‐sensitive Staphylococcus aureus, and (3) a patient with a urinary catheter who develops asymptomatic bacteriuria. The first vignette was chosen because congestive heart failure and pneumonia are among the most common reasons for hospitalization in the United States, and their overlapping syndromes can make the diagnosis challenging.[32, 33, 34, 35] The second vignette was chosen because skin infections are some of the most common infectious diseases, with an incidence that is twice that of urinary tract infections and 10 times that of pneumonia, and can lead to serious complications among hospitalized patients.[30, 36] The third vignette was chosen because the prevalence of asymptomatic bacteriuria approaches 100% among catheterized patients, and rates of unnecessary treatment for this condition are as high as 80%.[31, 37]
Each clinical vignette was then modified to include 1 of the 4 studied patient complexities (Table 1). Medical comorbidities were represented by modifying the baseline vignette to describe patients with poorly controlled diabetes, morbid obesity, chronic kidney disease, and/or heavy tobacco use (Table 2). Patients with poor functional status were described in the vignettes as having difficulty with ambulation, requiring a mobility device, or needing assistance with self‐care (Table 2). Clinical vignettes varied the age of the patient from 47 years in the baseline case to 86 years (Table 2). Patients expected to have limited postdischarge follow‐up were described as being uninsured, with the first available follow‐up occurring in a public clinic no sooner than 2 weeks after their hospital discharge (Table 2). These complexities were chosen because they are common among hospitalized patients and have been shown to be associated with worse outcomes in a variety of conditions.[38, 39, 40, 41, 42] The asymptomatic bacteriuria vignette did not have a question about limited postdischarge follow‐up, because the clinical decision making in this question only pertained to the initial diagnosis and management. All physicians were queried about antibiotic use in each of the 3 baseline vignettes and in the subsequent 4 modified vignettes for each baseline scenario, with each physician making antibiotic management decisions for 15 vignettes in total. Physicians responses were recorded using categorical responses describing treatment options.
|
| Dyspnea case (baseline scenario) |
| A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath and a nonproductive cough. In the ED, he had a temp 99.6F, a HR of 120, and an RR 30. Exam was notable for bilateral crackles. CXR on admission was interpreted as having cardiomegaly, bilateral base atelectasis versus infiltrate and prominent pulmonary arteries with cephalization consistent with cardiogenic pulmonary edema. Admission laboratories were notable for an elevated BNP (950 pg/mL) and WBC (11.5 cells 10*9/L) with 75% neutrophils. Troponin and CK‐MB were not elevated. The patient was treated with diuretics, ceftriaxone, and azithromycin, and over the course of the next 48 hours has improved shortness of breath and no fevers. Because of his improvement he is prepared for hospital discharge. A repeat chest x‐ray shows cardiomegaly and bibasilar atelectasis. Blood cultures have been negative. He is insured and can follow with his primary physician within 3 days of discharge. Upon discharge you: |
| A. Discharge on his usual cardiac medications. |
| B. Discharge on his cardiac medications plus azithromycin to complete a 5‐day course of antibiotics. |
| C. Discharge on his cardiac medications plus azithromycin to complete a 7‐day course of antibiotics. |
| D. Discharge on usual cardiac medications plus levofloxacin to complete a 7‐day course of antibiotics. |
| Skin infection case (baseline scenario) |
| A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. Admission temperature was 101.4F. In the ED, she was given vancomycin, and piperacillin/tazobactam and underwent incision and drainage of a thigh abscess, which drained a large amount of pus. The wound was packed. On day 3 she is afebrile, and the pain has improved. Cultures from the wound grow methicillin‐susceptible Staphylococcus aureus. Blood cultures are negative to date. She is ready for discharge. She has no allergies. She is insured and can be followed up with her primary physician within a few days of discharge. You: |
| A. Discharge on vancomycin and piperacillin/tazobactam through a PICC line to complete a 10‐day course. |
| B. Discharge on amoxicillin/clavulanate orally to complete a 10‐day course. |
| C. Discharge on cephalexin to complete a 10‐day course. |
| Asymptomatic bacteriuria case (baseline scenario) |
| A generally healthy 47‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. Labs from that day show a WBC of 8900 and a urinalysis with 12 WBCs/HPF and 2+ bacteria. She has surgical site discomfort and no dysuria or other lower urinary tract symptoms. She has not had a fever during her hospital stay. Two days later her urine culture grows 100,000 CFU/mL of Escherichia coli susceptible to ciprofloxacin but resistant to all other oral antibiotics. That day, the patient is still afebrile and has no dysuria or other lower urinary tract symptoms. Her Foley is changed and a repeat urinalysis shows that the urine has persistent leukocytes and bacteria. You: |
| A. Initiate intravenous ciprofloxacin. |
| B. Initiate oral ciprofloxacin. |
| C. Give no antibiotics. |
| |
| Dyspnea case | |
| Comorbidities | A 47‐year‐old male with a history of stage III congestive heart failure, moderate to heavy tobacco use, poorly controlled type 2 diabetes (last HbA1C=10.9), chronic renal insufficiency (baseline creatinine of 1.3), diabetic retinopathy, and diabetic neuropathy is hospitalized after presenting with shortness of breath. |
| Poor functional status | A 47‐year‐old male with a history of stage III congestive heart failure, and poor functional status with difficulty ambulating and with self‐care is hospitalized after presenting with shortness of breath. |
| Older age | An 86‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath. |
| Limited follow‐up | A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath.Blood cultures have been negative. He is uninsured and will be referred for follow‐up to a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Skin infection case | |
| Comorbidities | A 47‐year‐old morbidly obese female (BMI=32.9) with type 2 diabetes is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Poor functional status | A 47‐year‐old obese female (BMI=32.9) and poor functional status due to her obesity (poor mobility, uses either a walker or an electric scooter at all times to move around) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Older age | An 86‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Limited follow‐up | A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. She is uninsured and will be referred for follow‐up in a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Asymptomatic bacteriuria case | |
| Comorbidities | A 47‐year‐old female with a history type 2 diabetes with diabetic nephropathy and retinopathy is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Poor functional status | A 47‐year‐old female with a history of poor functional status (needs assistance with activities of daily living) due to her obesity and musculoskeletal comorbidities, such as osteoarthritis, is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Older age | A generally healthy 86‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
The institutional review boards at all 3 medical centers approved the study.
Statistical Analysis
We used physician self‐report of recommended antibiotic use stratified by baseline and modified clinical vignettes to calculate the proportion of physicians recommending antibiotic use. We examined how physicians antibiotic use varied according to both patient complexity (medical comorbidity, poor functional status, older age, and limited follow‐up after hospital discharge), and provider characteristics (level of training, degree of specialization, time spent working in the hospital, and time spent providing direct patient care).
Data were analyzed using SAS (version 9.1.3; SAS Institute, Cary, NC). Antibiotic use inconsistent with IDSA guideline recommendations was considered the outcome of interest for the data analysis. Statistical analyses were performed using [2] or Fischer exact test, Student t test, and analysis of variance, as appropriate.
RESULTS
Physician Survey
Of the 874 invited physicians, 255 (29%) responded to the survey. Of these, 8/255 (3.1%) responded that they did not spend 2 or more weeks in the inpatient setting and were thus ineligible for the survey. We analyzed data from the remaining 247 physician respondents. Most respondents (217/233, 93%) reported their primary role was direct clinical care (Table 3). Most (185/241, 77%) reported at least half of their clinical work occurred in the hospital. Approximately three‐quarters (183/241, 76%) of the respondents were residents; the remaining respondents were attending physicians (57/241, 24%). Almost half of attending physicians (46%) were internal medicine subspecialists.
| Physician Characteristic | No. (%) Completing the Survey | % of Physicians Not Adhering to Guidelines in Baseline Scenarios | P Value |
|---|---|---|---|
| |||
| Affiliated medical center, n =241 | |||
| Ronald Reagan UCLA | 47 (20%) | 37% | 0.37 |
| Harbor‐UCLA | 106 (44%) | 41% | |
| Cedars‐Sinai | 86 (35%) | 43% | |
| Primary professional activity, n=233 | |||
| Direct clinical care/teaching | 217 (93%) | 42% | 0.90 |
| Research/administration | 16 (7%) | 27% | |
| Percent of clinical duties in the hospital, n=241 | |||
| 1%25% | 57 (23%) | 41% | 0.71 |
| 51%75% | 93 (39%) | 42% | |
| 76%100% | 92 (38%) | 41% | |
| Level of training and subspecialization, n=241 | |||
| Resident/fellow | 183 (76%) | 43% | 0.05 |
| Attending | 58 (24%) | 34% | |
| Subspecialist | 27 (47%) | 34% | 0.90 |
| Hospitalist | 28 (48%) | 33% | |
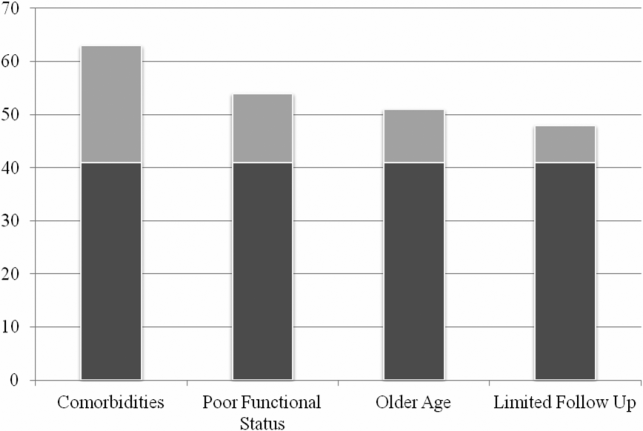
Physician recommendation for the use of antibiotics inconsistent with IDSA guidelines was prevalent in the baseline vignettes: 42% (303/729) overall, and 49% (120/246) for the dyspnea, 28% (68/242) for the skin infection, and 48% (115/241) for the asymptomatic bacteriuria cases. When the vignettes were modified to include patient complexities, the proportion of physicians recommending antibiotics increased significantly compared to the baseline vignette (63% (459/728), 54% (393/728), 51% (371/728), and 48% (232/487) for medical comorbidities, poor functional status, older age, and limited follow‐up respectively, P<0.001 for all comparisons) (Figure 1). The increase in the proportion of physicians recommending antibiotics inconsistent with guidelines for patients with medical complexities was the same when stratified by case (data not shown).

We found no association between provider characteristics (medical center, degree of attending physician specialization, percentage of clinical time spent practicing hospital‐based medicine, or percentage of time providing direct clinical care) and prescribing antibiotics in the baseline vignettes (Table 3). However, resident physicians (n=183) were more likely than attending physicians (n=57) to have recommended antibiotics in the baseline vignettes (43% vs 34%, P<0.05) (Table 3) and in all 4 vignettes with patient complexities (data not shown).
DISCUSSION
In our survey, almost half of the physician respondents recommended antibiotics that were inconsistent with national guidelines in the baseline vignettes. One explanation for this finding is that physicians may underestimate the risk associated with antibiotic use, such as the emergence of antimicrobial resistant pathogens and drug‐associated adverse effects. Although physicians generally agree that antibiotic resistance is an important problem, many believe that it is not a prominent issue in their practice or through their antibiotic prescribing practices.[43, 44] Others have shown that physicians underestimate the risk and severity of antibiotic‐associated complications such as C difficile.[45, 46] An accurate assessment and heightened awareness of the risks associated with antibiotics is important in clinical decision making, and should potentially be included not only in antibiotic stewardship educational efforts, but in national guideline recommendations as well.
Our survey also demonstrated that the tendency of physician respondents to recommend antibiotics was amplified for patients with medical complexities. This suggests that patient characteristics related to medical and social complexities play an important role in physicians clinical decision making about prescribing antibiotics. Previous investigations have shown that when physicians are deciding whether or not to prescribe antibiotics, they tend to deprioritize guideline recommendations and give greater weight to the risk of disease progression and complications that might occur if antibiotics are withheld.[47, 48, 49] Physicians are believed to prescribe antibiotics for complex patients more often, in part, because complex patients are more likely to suffer bad outcomes if undertreated.[50, 51] Axiomatically, patients with medical complexities are also at higher risk for antibiotic‐associated adverse effects including polypharmacy, drug‐drug interactions, and more severe side effects.[52, 53, 54]
An additional factor contributing to the overuse of antibiotics in our survey could be the lack of clear guideline recommendations for antibiotic management, especially among patients with complexities. We reviewed 20 national guidelines that addressed the medical decision making relevant to the survey's 3 clinical vignettes (see Supporting Information, Appendix 1, in the online version of this article). Fifteen of the guidelines provided recommendations for antibiotic management in the baseline vignettes, though most of the recommendations were not explicit about stopping or de‐escalating antibiotics. Furthermore, when antibiotic recommendations were present, they often lacked supporting data for the recommendation. Guidelines were even less complete for patients with medical complexities. For the asymptomatic bacteriuria vignette, 4 of 6 guidelines provided recommendations for patients with medical comorbidities described in our survey's modified vignettes.[31, 55, 56, 57] None of the guidelines related to the dyspnea or skin infection vignette provided specific antibiotic recommendations for complex patients with medical comorbidities, poor functional status, older age, or limited follow‐up (see Supporting Information, Appendix 1, in the online version of this article). Given these findings, along with evidence that medically complex patients are more likely to receive antibiotics compared to their less complex counterparts, there is a need for subsequent guidelines to more explicitly recommend best antibiotic practices for patients with medical and social complexities.
We also found that resident physicians were significantly more likely to recommend antibiotics inconsistent with guidelines compared to attending physicians. Although the underlying explanations for this finding were not explored in this study, possibilities include a lack of familiarity with guideline recommendations, less comfort in discontinuing antibiotics in the setting of clinical uncertainty, and/or a preference to accept the risks of overtreatment over the risks of undertreatment.
There are limitations to our study. First, although previous investigations have shown a high degree of correlation between actual antibiotic prescribing practices and antibiotic prescribing decisions self‐reported in clinical vignettes, physician responses in our survey may not reflect actual practices.[58] Second, we were not able to directly measure how physicians knowledge and interpretation of national guidelines influenced their antibiotic management decisions in the survey. Third, while our study was multisite including physicians from 3 different centers, not all invited physicians responded. Because of the confidentiality procedures surrounding the email distribution lists of potential participants, we were unable to obtain additional details about the non‐responders. Finally, because the large majority of respondents were trainees (76%), the generalizability of our findings to attending physicians may be limited. Nevertheless, because residents soon become staff physicians, resident‐reported data supplemented by that from attending physicians seems relevant to identifying opportunities for improving medical care.
In conclusion, we found that a large proportion of physicians recommended antibiotics that were not indicated based on IDSA guidelines for 3 vignettes depicting common hospital‐based clinical scenarios. This pattern of physicians recommending antibiotics inconsistent with guidelines was accentuated with significantly higher reported use for patients with medical comorbidities, poor functional status, older age, and limited healthcare access. Although good clinical judgment requires increased monitoring of patients with medical complexities, it is important for clinicians not to conflate the need for increased patient monitoring with the need for increased antibiotic use. Additional studies and corresponding guideline recommendations for frail, complex patients could be instrumental in reducing frequent use of antibiotics and the resultant cost, adverse effects, and emergence of antibiotic resistant pathogens. Educational efforts, particularly among trainees, regarding appropriate antibiotic use for clinical indications among patients without and with medical complexities would also likely contribute to these aims. Treatment guidelines should consider explicitly addressing medically complex patients in the context of management of infectious syndromes.
Disclosure
Nothing to report.
- , , , , , . Heart failure and chronic obstructive pulmonary disease multimorbidity at hospital discharge transition: a study of patient and carer experience [published online ahead of print May 16. 2014]. Health Expect. doi: 10.1111/hex.12208.
- , , , , , . Development of clinical practice guidelines for patients with comorbidity and multiple diseases [in Spanish]. Aten Primaria. 2014;46(7):385–392.
- , , , et al. Cardiac complications in patients with community‐acquired pneumonia: a systematic review and meta‐analysis of observational studies. PLoS Med. 2011;8(6):e1001048.
- , , , , , . Cost and incidence of social comorbidities in low‐risk patients with community‐acquired pneumonia admitted to a public hospital. Chest. 2003;124(6):2148–2155.
- , , , et al. Why do GPs exclude patients from participating in research? An exploration of adherence to and divergence from trial criteria. Fam Pract. 2014;31(3):364–370.
- , , , , . The impact of pre‐existing heart failure on pneumonia prognosis: population‐based cohort study. J Gen Intern Med. 2008;23(9):1407–1413.
- , , , et al. Severe community‐acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717–723.
- . Decisions about treating community‐acquired pneumonia. Ann Intern Med. 2005;142(3):215–216.
- , , , et al. The epidemic of antibiotic‐resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–164.
- Will antibiotic misuse now stop? Nat Rev Microbiol. 2003;1(2):85.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- . Economic impact of antimicrobial resistance. Emerg Infect Dis. 2001;7(2):286–292.
- , , , , . Guideline‐concordant therapy and reduced mortality and length of stay in adults with community‐acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169(16):1525–1531.
- , , , et al. Adherence to guidelines' empirical antibiotic recommendations and community‐acquired pneumonia outcome. Eur Respir J. 2008;32(4):892–901.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951–1961.
- , , , . Vancomycin‐induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68(9):1243–1255.
- , . Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol. 2013;45(1):131–142.
- , . Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 2011;72(3):381–393.
- , , , et al. A clinician's guide to the appropriate and accurate use of antibiotics: the Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am J Med. 2005;118(suppl 7A):1S–6S.
- , , , , . Evaluation of rational antibiotic use. Int J Antimicrob Agents. 2000;15(2):131–135.
- , . Rational antibiotic prescribing. Challenges and successes [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(11–12):1418–1426.
- , , , et al. Antibiotic prescription for community‐acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis. 2005;41(12):1709–1716.
- , , . Guideline‐adherent initial intravenous antibiotic therapy for hospital‐acquired/ventilator‐associated pneumonia is clinically superior, saves lives and is cheaper than non guideline adherent therapy. Eur J Med Res. 2011;16(7):315–323.
- , , , , , . Antibiotic misuse: a prospective clinical audit in a French university hospital. Eur J Clin Microbiol Infect Dis. 2007;26(4):277–280.
- , , , , . Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972–978.
- , , , . A systematic review and meta‐analysis of misuse of antibiotic therapies in the community. Int J Antimicrob Agents. 2005;26(2):106–113.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. The complexity of care for patients with rheumatoid arthritis: metrics for better understanding chronic disease care. Med Care. 2007;45(1):55–65.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , , , . Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654.
- , , , et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
- , , , et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29(19):2388–2442.
- , , , . Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med. 2006;47(1):13–18.
- , , , . Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117(5):305–311.
- , , , et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis. In press.
- . Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2012;9(2):85–93.
- , , , et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–1226.
- , , , . Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814.
- , , , , . Frailty in elderly people. Lancet. 2013;381(9868):752–762.
- , , , , , . Risk factors for death in homeless adults in Boston. Arch Intern Med. 1998;158(13):1454–1460.
- . Taking it to the streets: homelessness, health, and health care in the United States. J Gen Intern Med. 2003;18(11):964–965.
- , , , , , . Antibiotic resistance: a survey of physician perceptions. Arch Intern Med. 2002;162(19):2210–2216.
- , , , et al. Primary care clinicians' perceptions of antibiotic resistance: a multi‐country qualitative interview study. J Antimicrob Chemother. 2013;68(1):237–243.
- , , , , , . Underestimation of Clostridium difficile infection among clinicians: an international survey. Eur J Clin Microbiol Infect Dis. 2012;31(9):2439–2444.
- , , , , , . Unnecessary antimicrobial use in patients with current or recent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;34(2):109–116.
- , , , , . Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: a qualitative study from Spain. Fam Pract. 2012;29(3):352–360.
- , , , , . Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41(3):203–212.
- , , , . Guidelines on uncomplicated urinary tract infections are difficult to follow: perceived barriers and suggested interventions. BMC Fam Pract. 2010;11:51.
- , , , . Why don't physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 2009;4:54.
- , , , , . Perceived barriers to guideline adherence: a survey among general practitioners. BMC Fam Pract. 2011;12:98.
- , , , . Clostridium difficile in acute and long‐stay elderly patients. Age Ageing. 1988;17(5):333–336.
- , , , , , . Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin‐resistant Staphylococcus aureus hospital‐acquired pneumonia. Clin Interv Aging. 2013;8:1015–1021.
- , , , et al. Empiric guideline‐recommended weight‐based vancomycin dosing and nephrotoxicity rates in patients with methicillin‐resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Pharmacol Toxicol. 2013;14:12.
- , , , , , , , , . Guidelines on urological infections. Arnhem, The Netherlands: European Association of Urology (EAU); 2011. p. 15–27.
- Scottish Intercollegiate Guidelines Network. Management of suspected bacterial urinary tract infection in adults. Available at: http://www.sign.ac.uk/guidelines/fulltext/88/. Accessed on July 25, 2014.
- , , , et al. [Optimisation of the antibiotic policy in the Netherlands. X. The SWAB guideline for antimicrobial treatment of complicated urinary tract infections]. Ned Tijdschr Geneeskd 2006;150(43):2370–2376.
- , , , et al. Do case vignettes accurately reflect antibiotic prescription? Infect Control Hosp Epidemiol. 2011;32(10):1003–1009.
- The committee for The Japanese Respiratory Society guidelines in management of respiratory infections. Principles for the development of the guidelines. Respirology 2004;9(suppl 1):S1–S2.
Clinical management of patients with medical and social comorbidities has become increasingly complex.[1, 2, 3, 4] This complexity stems from lack of data for these groups of patients who are often excluded from clinical trials.[5] There are data demonstrating that older patients and patients with multiple comorbidities including diabetes, renal disease, obesity, limited mobility, and poor access to healthcare have worse outcomes for specific conditions compared to otherwise equal counterparts, and that the cost of care for these patients is more expensive.[3, 4, 6] Moreover, traditional risk assessment of disease severity and outcomes are not accurate when applied to medically and socially complex patients.[7, 8]
Treatment decisions regarding antibiotic use add additional complexity. Specifically, physicians antibiotic prescribing decisions can promote the emergence of multidrug resistant pathogens in a hospital or population.[9, 10] Multidrug resistant organisms (MDROs) are particularly problematic, as their prevalence is increasing while the development of new antimicrobial agents is declining.[11] Infections caused by antibiotic‐resistant pathogens are associated with increased morbidity and mortality and healthcare costs.[12, 13, 14] Antibiotic use, although potentially lifesaving, can also result in severe complications such as Clostridium difficile‐associated diarrhea, acute kidney injury, and anaphylaxis, among other adverse events, particularly in older patients with medical comorbidities.[15, 16, 17, 18] Judicious antibiotic use is critical to halt the epidemic of MDROs and to minimize antibiotic‐associated adverse effects.[19, 20, 21]
Evidence‐based guidelines have the potential to assist physicians in choosing the antibiotic that achieves the best clinical outcome for a specific infection or situation.[11] This includes using the narrowest spectrum agent to minimize selection pressure on microorganisms and avoiding unneeded drugs to minimize adverse drug effects.[9, 11] Importantly, guideline adherence regarding antibiotic selection has been shown to be associated with increased clinical success and decreased mortality.[22, 23] Unfortunately, 30% to 50% of antibiotic use in hospitalized patients is inconsistent with national guidelines.[24, 25, 26] Reasons for physicians ordering of tests and treatments inconsistent with guidelines are not fully understood, and potentially include patient and physician factors, and the cultural and social context of the healthcare system.[27]
To optimize the use of antibiotics, it is important to understand how medical complexities (defined as demographic, comorbid, and limited healthcare access characteristics that are associated with suboptimal patient care and outcomes) influence physicians antibiotic prescribing practices.[28] We created 3 clinical vignettes for common diagnoses (dyspnea with initial concern for pneumonia, skin and soft tissue infection, and asymptomatic bacteriuria) among hospitalized patients. We selected these conditions because of their high prevalence, frequent management by hospitalists, generalist physicians, and noninfectious disease specialists, and because well‐documented evidence suggests either no antibiotics or narrower spectrum antibiotics are usually the treatments of choice. Using the Infectious Diseases Society of America (IDSA) guidelines relevant to each clinical vignette,[29, 30, 31] we assessed physicians recommendations for guideline‐appropriate antibiotic management for patients without and with medical complexities using an electronic multiple‐choice survey.
METHODS
Survey Participants
We surveyed internal medicine generalist and subspecialty inpatient physicians from 3 academic medical centers in the metropolitan Los Angeles, California area. Potential participants included attending and housestaff physicians in the departments of internal medicine and family medicine at the 3 medical centers associated with the University of California Los Angeles (UCLA) Clinical and Translational Science Institute: (1) Ronald ReaganUCLA Medical Center, a tertiary care academic medical center; (2) Harbor UCLA Medical Center, a county (public) medical center; and (3) CedarsSinai Medical Center, a tertiary care medical center. Each center was affiliated with a residency training program, although not all attending physicians were associated with the training programs. Physicians were eligible to perform the survey if they attended 2 weeks per year in the inpatient setting. We collected physician‐level information including level of training (resident/fellow vs attending), specialization or not, proportion of time spent working in the hospital, and proportion of time spent providing direct clinical care (compared to activities such as administration and research). All eligible participants were emailed a brief study description with a hyperlink to the electronic survey created in REDCap (Research Electronic Data Capture version 5.6.0, 2013). Administrative staff provided email lists for potential participants at 2 of the hospitals. Per hospital policy, an email list was not provided by the third hospital, and potential participants were emailed the survey link directly by the hospital administrative staff. We incentivized study participation by entering participants who completed the survey into a raffle to win either a $100 gift card or a computer tablet. Physicians had 3 months to complete the survey and were sent up to 5 emails encouraging them to complete it.
Survey
The survey consisted of 3 clinical vignettes describing common hospital‐based situations that required decision making about antibiotic use. The 3 clinical vignettes described: (1) a patient with dyspnea and no infiltrate on chest radiograph who is initially treated empirically with antibiotics for pneumonia but is ultimately diagnosed with a congestive heart failure exacerbation, (2) a patient admitted with a skin infection that grows methicillin‐sensitive Staphylococcus aureus, and (3) a patient with a urinary catheter who develops asymptomatic bacteriuria. The first vignette was chosen because congestive heart failure and pneumonia are among the most common reasons for hospitalization in the United States, and their overlapping syndromes can make the diagnosis challenging.[32, 33, 34, 35] The second vignette was chosen because skin infections are some of the most common infectious diseases, with an incidence that is twice that of urinary tract infections and 10 times that of pneumonia, and can lead to serious complications among hospitalized patients.[30, 36] The third vignette was chosen because the prevalence of asymptomatic bacteriuria approaches 100% among catheterized patients, and rates of unnecessary treatment for this condition are as high as 80%.[31, 37]
Each clinical vignette was then modified to include 1 of the 4 studied patient complexities (Table 1). Medical comorbidities were represented by modifying the baseline vignette to describe patients with poorly controlled diabetes, morbid obesity, chronic kidney disease, and/or heavy tobacco use (Table 2). Patients with poor functional status were described in the vignettes as having difficulty with ambulation, requiring a mobility device, or needing assistance with self‐care (Table 2). Clinical vignettes varied the age of the patient from 47 years in the baseline case to 86 years (Table 2). Patients expected to have limited postdischarge follow‐up were described as being uninsured, with the first available follow‐up occurring in a public clinic no sooner than 2 weeks after their hospital discharge (Table 2). These complexities were chosen because they are common among hospitalized patients and have been shown to be associated with worse outcomes in a variety of conditions.[38, 39, 40, 41, 42] The asymptomatic bacteriuria vignette did not have a question about limited postdischarge follow‐up, because the clinical decision making in this question only pertained to the initial diagnosis and management. All physicians were queried about antibiotic use in each of the 3 baseline vignettes and in the subsequent 4 modified vignettes for each baseline scenario, with each physician making antibiotic management decisions for 15 vignettes in total. Physicians responses were recorded using categorical responses describing treatment options.
|
| Dyspnea case (baseline scenario) |
| A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath and a nonproductive cough. In the ED, he had a temp 99.6F, a HR of 120, and an RR 30. Exam was notable for bilateral crackles. CXR on admission was interpreted as having cardiomegaly, bilateral base atelectasis versus infiltrate and prominent pulmonary arteries with cephalization consistent with cardiogenic pulmonary edema. Admission laboratories were notable for an elevated BNP (950 pg/mL) and WBC (11.5 cells 10*9/L) with 75% neutrophils. Troponin and CK‐MB were not elevated. The patient was treated with diuretics, ceftriaxone, and azithromycin, and over the course of the next 48 hours has improved shortness of breath and no fevers. Because of his improvement he is prepared for hospital discharge. A repeat chest x‐ray shows cardiomegaly and bibasilar atelectasis. Blood cultures have been negative. He is insured and can follow with his primary physician within 3 days of discharge. Upon discharge you: |
| A. Discharge on his usual cardiac medications. |
| B. Discharge on his cardiac medications plus azithromycin to complete a 5‐day course of antibiotics. |
| C. Discharge on his cardiac medications plus azithromycin to complete a 7‐day course of antibiotics. |
| D. Discharge on usual cardiac medications plus levofloxacin to complete a 7‐day course of antibiotics. |
| Skin infection case (baseline scenario) |
| A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. Admission temperature was 101.4F. In the ED, she was given vancomycin, and piperacillin/tazobactam and underwent incision and drainage of a thigh abscess, which drained a large amount of pus. The wound was packed. On day 3 she is afebrile, and the pain has improved. Cultures from the wound grow methicillin‐susceptible Staphylococcus aureus. Blood cultures are negative to date. She is ready for discharge. She has no allergies. She is insured and can be followed up with her primary physician within a few days of discharge. You: |
| A. Discharge on vancomycin and piperacillin/tazobactam through a PICC line to complete a 10‐day course. |
| B. Discharge on amoxicillin/clavulanate orally to complete a 10‐day course. |
| C. Discharge on cephalexin to complete a 10‐day course. |
| Asymptomatic bacteriuria case (baseline scenario) |
| A generally healthy 47‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. Labs from that day show a WBC of 8900 and a urinalysis with 12 WBCs/HPF and 2+ bacteria. She has surgical site discomfort and no dysuria or other lower urinary tract symptoms. She has not had a fever during her hospital stay. Two days later her urine culture grows 100,000 CFU/mL of Escherichia coli susceptible to ciprofloxacin but resistant to all other oral antibiotics. That day, the patient is still afebrile and has no dysuria or other lower urinary tract symptoms. Her Foley is changed and a repeat urinalysis shows that the urine has persistent leukocytes and bacteria. You: |
| A. Initiate intravenous ciprofloxacin. |
| B. Initiate oral ciprofloxacin. |
| C. Give no antibiotics. |
| |
| Dyspnea case | |
| Comorbidities | A 47‐year‐old male with a history of stage III congestive heart failure, moderate to heavy tobacco use, poorly controlled type 2 diabetes (last HbA1C=10.9), chronic renal insufficiency (baseline creatinine of 1.3), diabetic retinopathy, and diabetic neuropathy is hospitalized after presenting with shortness of breath. |
| Poor functional status | A 47‐year‐old male with a history of stage III congestive heart failure, and poor functional status with difficulty ambulating and with self‐care is hospitalized after presenting with shortness of breath. |
| Older age | An 86‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath. |
| Limited follow‐up | A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath.Blood cultures have been negative. He is uninsured and will be referred for follow‐up to a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Skin infection case | |
| Comorbidities | A 47‐year‐old morbidly obese female (BMI=32.9) with type 2 diabetes is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Poor functional status | A 47‐year‐old obese female (BMI=32.9) and poor functional status due to her obesity (poor mobility, uses either a walker or an electric scooter at all times to move around) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Older age | An 86‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Limited follow‐up | A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. She is uninsured and will be referred for follow‐up in a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Asymptomatic bacteriuria case | |
| Comorbidities | A 47‐year‐old female with a history type 2 diabetes with diabetic nephropathy and retinopathy is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Poor functional status | A 47‐year‐old female with a history of poor functional status (needs assistance with activities of daily living) due to her obesity and musculoskeletal comorbidities, such as osteoarthritis, is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Older age | A generally healthy 86‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
The institutional review boards at all 3 medical centers approved the study.
Statistical Analysis
We used physician self‐report of recommended antibiotic use stratified by baseline and modified clinical vignettes to calculate the proportion of physicians recommending antibiotic use. We examined how physicians antibiotic use varied according to both patient complexity (medical comorbidity, poor functional status, older age, and limited follow‐up after hospital discharge), and provider characteristics (level of training, degree of specialization, time spent working in the hospital, and time spent providing direct patient care).
Data were analyzed using SAS (version 9.1.3; SAS Institute, Cary, NC). Antibiotic use inconsistent with IDSA guideline recommendations was considered the outcome of interest for the data analysis. Statistical analyses were performed using [2] or Fischer exact test, Student t test, and analysis of variance, as appropriate.
RESULTS
Physician Survey
Of the 874 invited physicians, 255 (29%) responded to the survey. Of these, 8/255 (3.1%) responded that they did not spend 2 or more weeks in the inpatient setting and were thus ineligible for the survey. We analyzed data from the remaining 247 physician respondents. Most respondents (217/233, 93%) reported their primary role was direct clinical care (Table 3). Most (185/241, 77%) reported at least half of their clinical work occurred in the hospital. Approximately three‐quarters (183/241, 76%) of the respondents were residents; the remaining respondents were attending physicians (57/241, 24%). Almost half of attending physicians (46%) were internal medicine subspecialists.
| Physician Characteristic | No. (%) Completing the Survey | % of Physicians Not Adhering to Guidelines in Baseline Scenarios | P Value |
|---|---|---|---|
| |||
| Affiliated medical center, n =241 | |||
| Ronald Reagan UCLA | 47 (20%) | 37% | 0.37 |
| Harbor‐UCLA | 106 (44%) | 41% | |
| Cedars‐Sinai | 86 (35%) | 43% | |
| Primary professional activity, n=233 | |||
| Direct clinical care/teaching | 217 (93%) | 42% | 0.90 |
| Research/administration | 16 (7%) | 27% | |
| Percent of clinical duties in the hospital, n=241 | |||
| 1%25% | 57 (23%) | 41% | 0.71 |
| 51%75% | 93 (39%) | 42% | |
| 76%100% | 92 (38%) | 41% | |
| Level of training and subspecialization, n=241 | |||
| Resident/fellow | 183 (76%) | 43% | 0.05 |
| Attending | 58 (24%) | 34% | |
| Subspecialist | 27 (47%) | 34% | 0.90 |
| Hospitalist | 28 (48%) | 33% | |
Physician recommendation for the use of antibiotics inconsistent with IDSA guidelines was prevalent in the baseline vignettes: 42% (303/729) overall, and 49% (120/246) for the dyspnea, 28% (68/242) for the skin infection, and 48% (115/241) for the asymptomatic bacteriuria cases. When the vignettes were modified to include patient complexities, the proportion of physicians recommending antibiotics increased significantly compared to the baseline vignette (63% (459/728), 54% (393/728), 51% (371/728), and 48% (232/487) for medical comorbidities, poor functional status, older age, and limited follow‐up respectively, P<0.001 for all comparisons) (Figure 1). The increase in the proportion of physicians recommending antibiotics inconsistent with guidelines for patients with medical complexities was the same when stratified by case (data not shown).

We found no association between provider characteristics (medical center, degree of attending physician specialization, percentage of clinical time spent practicing hospital‐based medicine, or percentage of time providing direct clinical care) and prescribing antibiotics in the baseline vignettes (Table 3). However, resident physicians (n=183) were more likely than attending physicians (n=57) to have recommended antibiotics in the baseline vignettes (43% vs 34%, P<0.05) (Table 3) and in all 4 vignettes with patient complexities (data not shown).
DISCUSSION
In our survey, almost half of the physician respondents recommended antibiotics that were inconsistent with national guidelines in the baseline vignettes. One explanation for this finding is that physicians may underestimate the risk associated with antibiotic use, such as the emergence of antimicrobial resistant pathogens and drug‐associated adverse effects. Although physicians generally agree that antibiotic resistance is an important problem, many believe that it is not a prominent issue in their practice or through their antibiotic prescribing practices.[43, 44] Others have shown that physicians underestimate the risk and severity of antibiotic‐associated complications such as C difficile.[45, 46] An accurate assessment and heightened awareness of the risks associated with antibiotics is important in clinical decision making, and should potentially be included not only in antibiotic stewardship educational efforts, but in national guideline recommendations as well.
Our survey also demonstrated that the tendency of physician respondents to recommend antibiotics was amplified for patients with medical complexities. This suggests that patient characteristics related to medical and social complexities play an important role in physicians clinical decision making about prescribing antibiotics. Previous investigations have shown that when physicians are deciding whether or not to prescribe antibiotics, they tend to deprioritize guideline recommendations and give greater weight to the risk of disease progression and complications that might occur if antibiotics are withheld.[47, 48, 49] Physicians are believed to prescribe antibiotics for complex patients more often, in part, because complex patients are more likely to suffer bad outcomes if undertreated.[50, 51] Axiomatically, patients with medical complexities are also at higher risk for antibiotic‐associated adverse effects including polypharmacy, drug‐drug interactions, and more severe side effects.[52, 53, 54]
An additional factor contributing to the overuse of antibiotics in our survey could be the lack of clear guideline recommendations for antibiotic management, especially among patients with complexities. We reviewed 20 national guidelines that addressed the medical decision making relevant to the survey's 3 clinical vignettes (see Supporting Information, Appendix 1, in the online version of this article). Fifteen of the guidelines provided recommendations for antibiotic management in the baseline vignettes, though most of the recommendations were not explicit about stopping or de‐escalating antibiotics. Furthermore, when antibiotic recommendations were present, they often lacked supporting data for the recommendation. Guidelines were even less complete for patients with medical complexities. For the asymptomatic bacteriuria vignette, 4 of 6 guidelines provided recommendations for patients with medical comorbidities described in our survey's modified vignettes.[31, 55, 56, 57] None of the guidelines related to the dyspnea or skin infection vignette provided specific antibiotic recommendations for complex patients with medical comorbidities, poor functional status, older age, or limited follow‐up (see Supporting Information, Appendix 1, in the online version of this article). Given these findings, along with evidence that medically complex patients are more likely to receive antibiotics compared to their less complex counterparts, there is a need for subsequent guidelines to more explicitly recommend best antibiotic practices for patients with medical and social complexities.
We also found that resident physicians were significantly more likely to recommend antibiotics inconsistent with guidelines compared to attending physicians. Although the underlying explanations for this finding were not explored in this study, possibilities include a lack of familiarity with guideline recommendations, less comfort in discontinuing antibiotics in the setting of clinical uncertainty, and/or a preference to accept the risks of overtreatment over the risks of undertreatment.
There are limitations to our study. First, although previous investigations have shown a high degree of correlation between actual antibiotic prescribing practices and antibiotic prescribing decisions self‐reported in clinical vignettes, physician responses in our survey may not reflect actual practices.[58] Second, we were not able to directly measure how physicians knowledge and interpretation of national guidelines influenced their antibiotic management decisions in the survey. Third, while our study was multisite including physicians from 3 different centers, not all invited physicians responded. Because of the confidentiality procedures surrounding the email distribution lists of potential participants, we were unable to obtain additional details about the non‐responders. Finally, because the large majority of respondents were trainees (76%), the generalizability of our findings to attending physicians may be limited. Nevertheless, because residents soon become staff physicians, resident‐reported data supplemented by that from attending physicians seems relevant to identifying opportunities for improving medical care.
In conclusion, we found that a large proportion of physicians recommended antibiotics that were not indicated based on IDSA guidelines for 3 vignettes depicting common hospital‐based clinical scenarios. This pattern of physicians recommending antibiotics inconsistent with guidelines was accentuated with significantly higher reported use for patients with medical comorbidities, poor functional status, older age, and limited healthcare access. Although good clinical judgment requires increased monitoring of patients with medical complexities, it is important for clinicians not to conflate the need for increased patient monitoring with the need for increased antibiotic use. Additional studies and corresponding guideline recommendations for frail, complex patients could be instrumental in reducing frequent use of antibiotics and the resultant cost, adverse effects, and emergence of antibiotic resistant pathogens. Educational efforts, particularly among trainees, regarding appropriate antibiotic use for clinical indications among patients without and with medical complexities would also likely contribute to these aims. Treatment guidelines should consider explicitly addressing medically complex patients in the context of management of infectious syndromes.
Disclosure
Nothing to report.
Clinical management of patients with medical and social comorbidities has become increasingly complex.[1, 2, 3, 4] This complexity stems from lack of data for these groups of patients who are often excluded from clinical trials.[5] There are data demonstrating that older patients and patients with multiple comorbidities including diabetes, renal disease, obesity, limited mobility, and poor access to healthcare have worse outcomes for specific conditions compared to otherwise equal counterparts, and that the cost of care for these patients is more expensive.[3, 4, 6] Moreover, traditional risk assessment of disease severity and outcomes are not accurate when applied to medically and socially complex patients.[7, 8]
Treatment decisions regarding antibiotic use add additional complexity. Specifically, physicians antibiotic prescribing decisions can promote the emergence of multidrug resistant pathogens in a hospital or population.[9, 10] Multidrug resistant organisms (MDROs) are particularly problematic, as their prevalence is increasing while the development of new antimicrobial agents is declining.[11] Infections caused by antibiotic‐resistant pathogens are associated with increased morbidity and mortality and healthcare costs.[12, 13, 14] Antibiotic use, although potentially lifesaving, can also result in severe complications such as Clostridium difficile‐associated diarrhea, acute kidney injury, and anaphylaxis, among other adverse events, particularly in older patients with medical comorbidities.[15, 16, 17, 18] Judicious antibiotic use is critical to halt the epidemic of MDROs and to minimize antibiotic‐associated adverse effects.[19, 20, 21]
Evidence‐based guidelines have the potential to assist physicians in choosing the antibiotic that achieves the best clinical outcome for a specific infection or situation.[11] This includes using the narrowest spectrum agent to minimize selection pressure on microorganisms and avoiding unneeded drugs to minimize adverse drug effects.[9, 11] Importantly, guideline adherence regarding antibiotic selection has been shown to be associated with increased clinical success and decreased mortality.[22, 23] Unfortunately, 30% to 50% of antibiotic use in hospitalized patients is inconsistent with national guidelines.[24, 25, 26] Reasons for physicians ordering of tests and treatments inconsistent with guidelines are not fully understood, and potentially include patient and physician factors, and the cultural and social context of the healthcare system.[27]
To optimize the use of antibiotics, it is important to understand how medical complexities (defined as demographic, comorbid, and limited healthcare access characteristics that are associated with suboptimal patient care and outcomes) influence physicians antibiotic prescribing practices.[28] We created 3 clinical vignettes for common diagnoses (dyspnea with initial concern for pneumonia, skin and soft tissue infection, and asymptomatic bacteriuria) among hospitalized patients. We selected these conditions because of their high prevalence, frequent management by hospitalists, generalist physicians, and noninfectious disease specialists, and because well‐documented evidence suggests either no antibiotics or narrower spectrum antibiotics are usually the treatments of choice. Using the Infectious Diseases Society of America (IDSA) guidelines relevant to each clinical vignette,[29, 30, 31] we assessed physicians recommendations for guideline‐appropriate antibiotic management for patients without and with medical complexities using an electronic multiple‐choice survey.
METHODS
Survey Participants
We surveyed internal medicine generalist and subspecialty inpatient physicians from 3 academic medical centers in the metropolitan Los Angeles, California area. Potential participants included attending and housestaff physicians in the departments of internal medicine and family medicine at the 3 medical centers associated with the University of California Los Angeles (UCLA) Clinical and Translational Science Institute: (1) Ronald ReaganUCLA Medical Center, a tertiary care academic medical center; (2) Harbor UCLA Medical Center, a county (public) medical center; and (3) CedarsSinai Medical Center, a tertiary care medical center. Each center was affiliated with a residency training program, although not all attending physicians were associated with the training programs. Physicians were eligible to perform the survey if they attended 2 weeks per year in the inpatient setting. We collected physician‐level information including level of training (resident/fellow vs attending), specialization or not, proportion of time spent working in the hospital, and proportion of time spent providing direct clinical care (compared to activities such as administration and research). All eligible participants were emailed a brief study description with a hyperlink to the electronic survey created in REDCap (Research Electronic Data Capture version 5.6.0, 2013). Administrative staff provided email lists for potential participants at 2 of the hospitals. Per hospital policy, an email list was not provided by the third hospital, and potential participants were emailed the survey link directly by the hospital administrative staff. We incentivized study participation by entering participants who completed the survey into a raffle to win either a $100 gift card or a computer tablet. Physicians had 3 months to complete the survey and were sent up to 5 emails encouraging them to complete it.
Survey
The survey consisted of 3 clinical vignettes describing common hospital‐based situations that required decision making about antibiotic use. The 3 clinical vignettes described: (1) a patient with dyspnea and no infiltrate on chest radiograph who is initially treated empirically with antibiotics for pneumonia but is ultimately diagnosed with a congestive heart failure exacerbation, (2) a patient admitted with a skin infection that grows methicillin‐sensitive Staphylococcus aureus, and (3) a patient with a urinary catheter who develops asymptomatic bacteriuria. The first vignette was chosen because congestive heart failure and pneumonia are among the most common reasons for hospitalization in the United States, and their overlapping syndromes can make the diagnosis challenging.[32, 33, 34, 35] The second vignette was chosen because skin infections are some of the most common infectious diseases, with an incidence that is twice that of urinary tract infections and 10 times that of pneumonia, and can lead to serious complications among hospitalized patients.[30, 36] The third vignette was chosen because the prevalence of asymptomatic bacteriuria approaches 100% among catheterized patients, and rates of unnecessary treatment for this condition are as high as 80%.[31, 37]
Each clinical vignette was then modified to include 1 of the 4 studied patient complexities (Table 1). Medical comorbidities were represented by modifying the baseline vignette to describe patients with poorly controlled diabetes, morbid obesity, chronic kidney disease, and/or heavy tobacco use (Table 2). Patients with poor functional status were described in the vignettes as having difficulty with ambulation, requiring a mobility device, or needing assistance with self‐care (Table 2). Clinical vignettes varied the age of the patient from 47 years in the baseline case to 86 years (Table 2). Patients expected to have limited postdischarge follow‐up were described as being uninsured, with the first available follow‐up occurring in a public clinic no sooner than 2 weeks after their hospital discharge (Table 2). These complexities were chosen because they are common among hospitalized patients and have been shown to be associated with worse outcomes in a variety of conditions.[38, 39, 40, 41, 42] The asymptomatic bacteriuria vignette did not have a question about limited postdischarge follow‐up, because the clinical decision making in this question only pertained to the initial diagnosis and management. All physicians were queried about antibiotic use in each of the 3 baseline vignettes and in the subsequent 4 modified vignettes for each baseline scenario, with each physician making antibiotic management decisions for 15 vignettes in total. Physicians responses were recorded using categorical responses describing treatment options.
|
| Dyspnea case (baseline scenario) |
| A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath and a nonproductive cough. In the ED, he had a temp 99.6F, a HR of 120, and an RR 30. Exam was notable for bilateral crackles. CXR on admission was interpreted as having cardiomegaly, bilateral base atelectasis versus infiltrate and prominent pulmonary arteries with cephalization consistent with cardiogenic pulmonary edema. Admission laboratories were notable for an elevated BNP (950 pg/mL) and WBC (11.5 cells 10*9/L) with 75% neutrophils. Troponin and CK‐MB were not elevated. The patient was treated with diuretics, ceftriaxone, and azithromycin, and over the course of the next 48 hours has improved shortness of breath and no fevers. Because of his improvement he is prepared for hospital discharge. A repeat chest x‐ray shows cardiomegaly and bibasilar atelectasis. Blood cultures have been negative. He is insured and can follow with his primary physician within 3 days of discharge. Upon discharge you: |
| A. Discharge on his usual cardiac medications. |
| B. Discharge on his cardiac medications plus azithromycin to complete a 5‐day course of antibiotics. |
| C. Discharge on his cardiac medications plus azithromycin to complete a 7‐day course of antibiotics. |
| D. Discharge on usual cardiac medications plus levofloxacin to complete a 7‐day course of antibiotics. |
| Skin infection case (baseline scenario) |
| A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. Admission temperature was 101.4F. In the ED, she was given vancomycin, and piperacillin/tazobactam and underwent incision and drainage of a thigh abscess, which drained a large amount of pus. The wound was packed. On day 3 she is afebrile, and the pain has improved. Cultures from the wound grow methicillin‐susceptible Staphylococcus aureus. Blood cultures are negative to date. She is ready for discharge. She has no allergies. She is insured and can be followed up with her primary physician within a few days of discharge. You: |
| A. Discharge on vancomycin and piperacillin/tazobactam through a PICC line to complete a 10‐day course. |
| B. Discharge on amoxicillin/clavulanate orally to complete a 10‐day course. |
| C. Discharge on cephalexin to complete a 10‐day course. |
| Asymptomatic bacteriuria case (baseline scenario) |
| A generally healthy 47‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. Labs from that day show a WBC of 8900 and a urinalysis with 12 WBCs/HPF and 2+ bacteria. She has surgical site discomfort and no dysuria or other lower urinary tract symptoms. She has not had a fever during her hospital stay. Two days later her urine culture grows 100,000 CFU/mL of Escherichia coli susceptible to ciprofloxacin but resistant to all other oral antibiotics. That day, the patient is still afebrile and has no dysuria or other lower urinary tract symptoms. Her Foley is changed and a repeat urinalysis shows that the urine has persistent leukocytes and bacteria. You: |
| A. Initiate intravenous ciprofloxacin. |
| B. Initiate oral ciprofloxacin. |
| C. Give no antibiotics. |
| |
| Dyspnea case | |
| Comorbidities | A 47‐year‐old male with a history of stage III congestive heart failure, moderate to heavy tobacco use, poorly controlled type 2 diabetes (last HbA1C=10.9), chronic renal insufficiency (baseline creatinine of 1.3), diabetic retinopathy, and diabetic neuropathy is hospitalized after presenting with shortness of breath. |
| Poor functional status | A 47‐year‐old male with a history of stage III congestive heart failure, and poor functional status with difficulty ambulating and with self‐care is hospitalized after presenting with shortness of breath. |
| Older age | An 86‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath. |
| Limited follow‐up | A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath.Blood cultures have been negative. He is uninsured and will be referred for follow‐up to a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Skin infection case | |
| Comorbidities | A 47‐year‐old morbidly obese female (BMI=32.9) with type 2 diabetes is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Poor functional status | A 47‐year‐old obese female (BMI=32.9) and poor functional status due to her obesity (poor mobility, uses either a walker or an electric scooter at all times to move around) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Older age | An 86‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Limited follow‐up | A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. She is uninsured and will be referred for follow‐up in a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Asymptomatic bacteriuria case | |
| Comorbidities | A 47‐year‐old female with a history type 2 diabetes with diabetic nephropathy and retinopathy is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Poor functional status | A 47‐year‐old female with a history of poor functional status (needs assistance with activities of daily living) due to her obesity and musculoskeletal comorbidities, such as osteoarthritis, is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Older age | A generally healthy 86‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
The institutional review boards at all 3 medical centers approved the study.
Statistical Analysis
We used physician self‐report of recommended antibiotic use stratified by baseline and modified clinical vignettes to calculate the proportion of physicians recommending antibiotic use. We examined how physicians antibiotic use varied according to both patient complexity (medical comorbidity, poor functional status, older age, and limited follow‐up after hospital discharge), and provider characteristics (level of training, degree of specialization, time spent working in the hospital, and time spent providing direct patient care).
Data were analyzed using SAS (version 9.1.3; SAS Institute, Cary, NC). Antibiotic use inconsistent with IDSA guideline recommendations was considered the outcome of interest for the data analysis. Statistical analyses were performed using [2] or Fischer exact test, Student t test, and analysis of variance, as appropriate.
RESULTS
Physician Survey
Of the 874 invited physicians, 255 (29%) responded to the survey. Of these, 8/255 (3.1%) responded that they did not spend 2 or more weeks in the inpatient setting and were thus ineligible for the survey. We analyzed data from the remaining 247 physician respondents. Most respondents (217/233, 93%) reported their primary role was direct clinical care (Table 3). Most (185/241, 77%) reported at least half of their clinical work occurred in the hospital. Approximately three‐quarters (183/241, 76%) of the respondents were residents; the remaining respondents were attending physicians (57/241, 24%). Almost half of attending physicians (46%) were internal medicine subspecialists.
| Physician Characteristic | No. (%) Completing the Survey | % of Physicians Not Adhering to Guidelines in Baseline Scenarios | P Value |
|---|---|---|---|
| |||
| Affiliated medical center, n =241 | |||
| Ronald Reagan UCLA | 47 (20%) | 37% | 0.37 |
| Harbor‐UCLA | 106 (44%) | 41% | |
| Cedars‐Sinai | 86 (35%) | 43% | |
| Primary professional activity, n=233 | |||
| Direct clinical care/teaching | 217 (93%) | 42% | 0.90 |
| Research/administration | 16 (7%) | 27% | |
| Percent of clinical duties in the hospital, n=241 | |||
| 1%25% | 57 (23%) | 41% | 0.71 |
| 51%75% | 93 (39%) | 42% | |
| 76%100% | 92 (38%) | 41% | |
| Level of training and subspecialization, n=241 | |||
| Resident/fellow | 183 (76%) | 43% | 0.05 |
| Attending | 58 (24%) | 34% | |
| Subspecialist | 27 (47%) | 34% | 0.90 |
| Hospitalist | 28 (48%) | 33% | |
Physician recommendation for the use of antibiotics inconsistent with IDSA guidelines was prevalent in the baseline vignettes: 42% (303/729) overall, and 49% (120/246) for the dyspnea, 28% (68/242) for the skin infection, and 48% (115/241) for the asymptomatic bacteriuria cases. When the vignettes were modified to include patient complexities, the proportion of physicians recommending antibiotics increased significantly compared to the baseline vignette (63% (459/728), 54% (393/728), 51% (371/728), and 48% (232/487) for medical comorbidities, poor functional status, older age, and limited follow‐up respectively, P<0.001 for all comparisons) (Figure 1). The increase in the proportion of physicians recommending antibiotics inconsistent with guidelines for patients with medical complexities was the same when stratified by case (data not shown).

We found no association between provider characteristics (medical center, degree of attending physician specialization, percentage of clinical time spent practicing hospital‐based medicine, or percentage of time providing direct clinical care) and prescribing antibiotics in the baseline vignettes (Table 3). However, resident physicians (n=183) were more likely than attending physicians (n=57) to have recommended antibiotics in the baseline vignettes (43% vs 34%, P<0.05) (Table 3) and in all 4 vignettes with patient complexities (data not shown).
DISCUSSION
In our survey, almost half of the physician respondents recommended antibiotics that were inconsistent with national guidelines in the baseline vignettes. One explanation for this finding is that physicians may underestimate the risk associated with antibiotic use, such as the emergence of antimicrobial resistant pathogens and drug‐associated adverse effects. Although physicians generally agree that antibiotic resistance is an important problem, many believe that it is not a prominent issue in their practice or through their antibiotic prescribing practices.[43, 44] Others have shown that physicians underestimate the risk and severity of antibiotic‐associated complications such as C difficile.[45, 46] An accurate assessment and heightened awareness of the risks associated with antibiotics is important in clinical decision making, and should potentially be included not only in antibiotic stewardship educational efforts, but in national guideline recommendations as well.
Our survey also demonstrated that the tendency of physician respondents to recommend antibiotics was amplified for patients with medical complexities. This suggests that patient characteristics related to medical and social complexities play an important role in physicians clinical decision making about prescribing antibiotics. Previous investigations have shown that when physicians are deciding whether or not to prescribe antibiotics, they tend to deprioritize guideline recommendations and give greater weight to the risk of disease progression and complications that might occur if antibiotics are withheld.[47, 48, 49] Physicians are believed to prescribe antibiotics for complex patients more often, in part, because complex patients are more likely to suffer bad outcomes if undertreated.[50, 51] Axiomatically, patients with medical complexities are also at higher risk for antibiotic‐associated adverse effects including polypharmacy, drug‐drug interactions, and more severe side effects.[52, 53, 54]
An additional factor contributing to the overuse of antibiotics in our survey could be the lack of clear guideline recommendations for antibiotic management, especially among patients with complexities. We reviewed 20 national guidelines that addressed the medical decision making relevant to the survey's 3 clinical vignettes (see Supporting Information, Appendix 1, in the online version of this article). Fifteen of the guidelines provided recommendations for antibiotic management in the baseline vignettes, though most of the recommendations were not explicit about stopping or de‐escalating antibiotics. Furthermore, when antibiotic recommendations were present, they often lacked supporting data for the recommendation. Guidelines were even less complete for patients with medical complexities. For the asymptomatic bacteriuria vignette, 4 of 6 guidelines provided recommendations for patients with medical comorbidities described in our survey's modified vignettes.[31, 55, 56, 57] None of the guidelines related to the dyspnea or skin infection vignette provided specific antibiotic recommendations for complex patients with medical comorbidities, poor functional status, older age, or limited follow‐up (see Supporting Information, Appendix 1, in the online version of this article). Given these findings, along with evidence that medically complex patients are more likely to receive antibiotics compared to their less complex counterparts, there is a need for subsequent guidelines to more explicitly recommend best antibiotic practices for patients with medical and social complexities.
We also found that resident physicians were significantly more likely to recommend antibiotics inconsistent with guidelines compared to attending physicians. Although the underlying explanations for this finding were not explored in this study, possibilities include a lack of familiarity with guideline recommendations, less comfort in discontinuing antibiotics in the setting of clinical uncertainty, and/or a preference to accept the risks of overtreatment over the risks of undertreatment.
There are limitations to our study. First, although previous investigations have shown a high degree of correlation between actual antibiotic prescribing practices and antibiotic prescribing decisions self‐reported in clinical vignettes, physician responses in our survey may not reflect actual practices.[58] Second, we were not able to directly measure how physicians knowledge and interpretation of national guidelines influenced their antibiotic management decisions in the survey. Third, while our study was multisite including physicians from 3 different centers, not all invited physicians responded. Because of the confidentiality procedures surrounding the email distribution lists of potential participants, we were unable to obtain additional details about the non‐responders. Finally, because the large majority of respondents were trainees (76%), the generalizability of our findings to attending physicians may be limited. Nevertheless, because residents soon become staff physicians, resident‐reported data supplemented by that from attending physicians seems relevant to identifying opportunities for improving medical care.
In conclusion, we found that a large proportion of physicians recommended antibiotics that were not indicated based on IDSA guidelines for 3 vignettes depicting common hospital‐based clinical scenarios. This pattern of physicians recommending antibiotics inconsistent with guidelines was accentuated with significantly higher reported use for patients with medical comorbidities, poor functional status, older age, and limited healthcare access. Although good clinical judgment requires increased monitoring of patients with medical complexities, it is important for clinicians not to conflate the need for increased patient monitoring with the need for increased antibiotic use. Additional studies and corresponding guideline recommendations for frail, complex patients could be instrumental in reducing frequent use of antibiotics and the resultant cost, adverse effects, and emergence of antibiotic resistant pathogens. Educational efforts, particularly among trainees, regarding appropriate antibiotic use for clinical indications among patients without and with medical complexities would also likely contribute to these aims. Treatment guidelines should consider explicitly addressing medically complex patients in the context of management of infectious syndromes.
Disclosure
Nothing to report.
- , , , , , . Heart failure and chronic obstructive pulmonary disease multimorbidity at hospital discharge transition: a study of patient and carer experience [published online ahead of print May 16. 2014]. Health Expect. doi: 10.1111/hex.12208.
- , , , , , . Development of clinical practice guidelines for patients with comorbidity and multiple diseases [in Spanish]. Aten Primaria. 2014;46(7):385–392.
- , , , et al. Cardiac complications in patients with community‐acquired pneumonia: a systematic review and meta‐analysis of observational studies. PLoS Med. 2011;8(6):e1001048.
- , , , , , . Cost and incidence of social comorbidities in low‐risk patients with community‐acquired pneumonia admitted to a public hospital. Chest. 2003;124(6):2148–2155.
- , , , et al. Why do GPs exclude patients from participating in research? An exploration of adherence to and divergence from trial criteria. Fam Pract. 2014;31(3):364–370.
- , , , , . The impact of pre‐existing heart failure on pneumonia prognosis: population‐based cohort study. J Gen Intern Med. 2008;23(9):1407–1413.
- , , , et al. Severe community‐acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717–723.
- . Decisions about treating community‐acquired pneumonia. Ann Intern Med. 2005;142(3):215–216.
- , , , et al. The epidemic of antibiotic‐resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–164.
- Will antibiotic misuse now stop? Nat Rev Microbiol. 2003;1(2):85.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- . Economic impact of antimicrobial resistance. Emerg Infect Dis. 2001;7(2):286–292.
- , , , , . Guideline‐concordant therapy and reduced mortality and length of stay in adults with community‐acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169(16):1525–1531.
- , , , et al. Adherence to guidelines' empirical antibiotic recommendations and community‐acquired pneumonia outcome. Eur Respir J. 2008;32(4):892–901.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951–1961.
- , , , . Vancomycin‐induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68(9):1243–1255.
- , . Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol. 2013;45(1):131–142.
- , . Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 2011;72(3):381–393.
- , , , et al. A clinician's guide to the appropriate and accurate use of antibiotics: the Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am J Med. 2005;118(suppl 7A):1S–6S.
- , , , , . Evaluation of rational antibiotic use. Int J Antimicrob Agents. 2000;15(2):131–135.
- , . Rational antibiotic prescribing. Challenges and successes [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(11–12):1418–1426.
- , , , et al. Antibiotic prescription for community‐acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis. 2005;41(12):1709–1716.
- , , . Guideline‐adherent initial intravenous antibiotic therapy for hospital‐acquired/ventilator‐associated pneumonia is clinically superior, saves lives and is cheaper than non guideline adherent therapy. Eur J Med Res. 2011;16(7):315–323.
- , , , , , . Antibiotic misuse: a prospective clinical audit in a French university hospital. Eur J Clin Microbiol Infect Dis. 2007;26(4):277–280.
- , , , , . Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972–978.
- , , , . A systematic review and meta‐analysis of misuse of antibiotic therapies in the community. Int J Antimicrob Agents. 2005;26(2):106–113.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. The complexity of care for patients with rheumatoid arthritis: metrics for better understanding chronic disease care. Med Care. 2007;45(1):55–65.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , , , . Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654.
- , , , et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
- , , , et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29(19):2388–2442.
- , , , . Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med. 2006;47(1):13–18.
- , , , . Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117(5):305–311.
- , , , et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis. In press.
- . Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2012;9(2):85–93.
- , , , et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–1226.
- , , , . Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814.
- , , , , . Frailty in elderly people. Lancet. 2013;381(9868):752–762.
- , , , , , . Risk factors for death in homeless adults in Boston. Arch Intern Med. 1998;158(13):1454–1460.
- . Taking it to the streets: homelessness, health, and health care in the United States. J Gen Intern Med. 2003;18(11):964–965.
- , , , , , . Antibiotic resistance: a survey of physician perceptions. Arch Intern Med. 2002;162(19):2210–2216.
- , , , et al. Primary care clinicians' perceptions of antibiotic resistance: a multi‐country qualitative interview study. J Antimicrob Chemother. 2013;68(1):237–243.
- , , , , , . Underestimation of Clostridium difficile infection among clinicians: an international survey. Eur J Clin Microbiol Infect Dis. 2012;31(9):2439–2444.
- , , , , , . Unnecessary antimicrobial use in patients with current or recent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;34(2):109–116.
- , , , , . Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: a qualitative study from Spain. Fam Pract. 2012;29(3):352–360.
- , , , , . Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41(3):203–212.
- , , , . Guidelines on uncomplicated urinary tract infections are difficult to follow: perceived barriers and suggested interventions. BMC Fam Pract. 2010;11:51.
- , , , . Why don't physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 2009;4:54.
- , , , , . Perceived barriers to guideline adherence: a survey among general practitioners. BMC Fam Pract. 2011;12:98.
- , , , . Clostridium difficile in acute and long‐stay elderly patients. Age Ageing. 1988;17(5):333–336.
- , , , , , . Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin‐resistant Staphylococcus aureus hospital‐acquired pneumonia. Clin Interv Aging. 2013;8:1015–1021.
- , , , et al. Empiric guideline‐recommended weight‐based vancomycin dosing and nephrotoxicity rates in patients with methicillin‐resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Pharmacol Toxicol. 2013;14:12.
- , , , , , , , , . Guidelines on urological infections. Arnhem, The Netherlands: European Association of Urology (EAU); 2011. p. 15–27.
- Scottish Intercollegiate Guidelines Network. Management of suspected bacterial urinary tract infection in adults. Available at: http://www.sign.ac.uk/guidelines/fulltext/88/. Accessed on July 25, 2014.
- , , , et al. [Optimisation of the antibiotic policy in the Netherlands. X. The SWAB guideline for antimicrobial treatment of complicated urinary tract infections]. Ned Tijdschr Geneeskd 2006;150(43):2370–2376.
- , , , et al. Do case vignettes accurately reflect antibiotic prescription? Infect Control Hosp Epidemiol. 2011;32(10):1003–1009.
- The committee for The Japanese Respiratory Society guidelines in management of respiratory infections. Principles for the development of the guidelines. Respirology 2004;9(suppl 1):S1–S2.
- , , , , , . Heart failure and chronic obstructive pulmonary disease multimorbidity at hospital discharge transition: a study of patient and carer experience [published online ahead of print May 16. 2014]. Health Expect. doi: 10.1111/hex.12208.
- , , , , , . Development of clinical practice guidelines for patients with comorbidity and multiple diseases [in Spanish]. Aten Primaria. 2014;46(7):385–392.
- , , , et al. Cardiac complications in patients with community‐acquired pneumonia: a systematic review and meta‐analysis of observational studies. PLoS Med. 2011;8(6):e1001048.
- , , , , , . Cost and incidence of social comorbidities in low‐risk patients with community‐acquired pneumonia admitted to a public hospital. Chest. 2003;124(6):2148–2155.
- , , , et al. Why do GPs exclude patients from participating in research? An exploration of adherence to and divergence from trial criteria. Fam Pract. 2014;31(3):364–370.
- , , , , . The impact of pre‐existing heart failure on pneumonia prognosis: population‐based cohort study. J Gen Intern Med. 2008;23(9):1407–1413.
- , , , et al. Severe community‐acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717–723.
- . Decisions about treating community‐acquired pneumonia. Ann Intern Med. 2005;142(3):215–216.
- , , , et al. The epidemic of antibiotic‐resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–164.
- Will antibiotic misuse now stop? Nat Rev Microbiol. 2003;1(2):85.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- . Economic impact of antimicrobial resistance. Emerg Infect Dis. 2001;7(2):286–292.
- , , , , . Guideline‐concordant therapy and reduced mortality and length of stay in adults with community‐acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169(16):1525–1531.
- , , , et al. Adherence to guidelines' empirical antibiotic recommendations and community‐acquired pneumonia outcome. Eur Respir J. 2008;32(4):892–901.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951–1961.
- , , , . Vancomycin‐induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68(9):1243–1255.
- , . Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol. 2013;45(1):131–142.
- , . Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 2011;72(3):381–393.
- , , , et al. A clinician's guide to the appropriate and accurate use of antibiotics: the Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am J Med. 2005;118(suppl 7A):1S–6S.
- , , , , . Evaluation of rational antibiotic use. Int J Antimicrob Agents. 2000;15(2):131–135.
- , . Rational antibiotic prescribing. Challenges and successes [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(11–12):1418–1426.
- , , , et al. Antibiotic prescription for community‐acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis. 2005;41(12):1709–1716.
- , , . Guideline‐adherent initial intravenous antibiotic therapy for hospital‐acquired/ventilator‐associated pneumonia is clinically superior, saves lives and is cheaper than non guideline adherent therapy. Eur J Med Res. 2011;16(7):315–323.
- , , , , , . Antibiotic misuse: a prospective clinical audit in a French university hospital. Eur J Clin Microbiol Infect Dis. 2007;26(4):277–280.
- , , , , . Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972–978.
- , , , . A systematic review and meta‐analysis of misuse of antibiotic therapies in the community. Int J Antimicrob Agents. 2005;26(2):106–113.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. The complexity of care for patients with rheumatoid arthritis: metrics for better understanding chronic disease care. Med Care. 2007;45(1):55–65.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , , , . Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654.
- , , , et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
- , , , et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29(19):2388–2442.
- , , , . Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med. 2006;47(1):13–18.
- , , , . Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117(5):305–311.
- , , , et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis. In press.
- . Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2012;9(2):85–93.
- , , , et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–1226.
- , , , . Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814.
- , , , , . Frailty in elderly people. Lancet. 2013;381(9868):752–762.
- , , , , , . Risk factors for death in homeless adults in Boston. Arch Intern Med. 1998;158(13):1454–1460.
- . Taking it to the streets: homelessness, health, and health care in the United States. J Gen Intern Med. 2003;18(11):964–965.
- , , , , , . Antibiotic resistance: a survey of physician perceptions. Arch Intern Med. 2002;162(19):2210–2216.
- , , , et al. Primary care clinicians' perceptions of antibiotic resistance: a multi‐country qualitative interview study. J Antimicrob Chemother. 2013;68(1):237–243.
- , , , , , . Underestimation of Clostridium difficile infection among clinicians: an international survey. Eur J Clin Microbiol Infect Dis. 2012;31(9):2439–2444.
- , , , , , . Unnecessary antimicrobial use in patients with current or recent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;34(2):109–116.
- , , , , . Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: a qualitative study from Spain. Fam Pract. 2012;29(3):352–360.
- , , , , . Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41(3):203–212.
- , , , . Guidelines on uncomplicated urinary tract infections are difficult to follow: perceived barriers and suggested interventions. BMC Fam Pract. 2010;11:51.
- , , , . Why don't physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 2009;4:54.
- , , , , . Perceived barriers to guideline adherence: a survey among general practitioners. BMC Fam Pract. 2011;12:98.
- , , , . Clostridium difficile in acute and long‐stay elderly patients. Age Ageing. 1988;17(5):333–336.
- , , , , , . Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin‐resistant Staphylococcus aureus hospital‐acquired pneumonia. Clin Interv Aging. 2013;8:1015–1021.
- , , , et al. Empiric guideline‐recommended weight‐based vancomycin dosing and nephrotoxicity rates in patients with methicillin‐resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Pharmacol Toxicol. 2013;14:12.
- , , , , , , , , . Guidelines on urological infections. Arnhem, The Netherlands: European Association of Urology (EAU); 2011. p. 15–27.
- Scottish Intercollegiate Guidelines Network. Management of suspected bacterial urinary tract infection in adults. Available at: http://www.sign.ac.uk/guidelines/fulltext/88/. Accessed on July 25, 2014.
- , , , et al. [Optimisation of the antibiotic policy in the Netherlands. X. The SWAB guideline for antimicrobial treatment of complicated urinary tract infections]. Ned Tijdschr Geneeskd 2006;150(43):2370–2376.
- , , , et al. Do case vignettes accurately reflect antibiotic prescription? Infect Control Hosp Epidemiol. 2011;32(10):1003–1009.
- The committee for The Japanese Respiratory Society guidelines in management of respiratory infections. Principles for the development of the guidelines. Respirology 2004;9(suppl 1):S1–S2.
© 2015 Society of Hospital Medicine
False Alarms and Patient Safety
Despite 15 years of national and local investment in improving the safety of hospital care, patient safety remains a leading problem in both adult and pediatric hospitals. A 2010 study found that 180,000 Medicare beneficiaries likely die each year due to harm suffered as a result of medical care,[1] a death toll surpassed only by deaths due to cardiovascular disease and cancer. Even though initial efforts in the field have shown great promise for stemming the tide of healthcare‐associated infections,[2] surgical errors,[3] handoff failures,[4] and errors in the care of adults hospitalized for myocardial infarction and congestive heart failure,[5] much work remains to be done.[6] The root causes of many adverse events are poorly understood and unaddressed. Resultant tragedies remain all too common.
In the current issue of the Journal of Hospital Medicine, Bonafide and colleagues report the results of an innovative observational pilot study designed to assess the role of an inadequately addressed root cause of serious errors: alarm fatigue.[7] Alarm fatigue is the phenomenon of desensitization to alarms, particularly in the context of excessive false alarms. In a videotaped observational assessment of nurse response times to 5070 alarms on a pediatric ward and intensive care unit (ICU), the authors found that nurses responded significantly more slowly as the number of nonactionable alarms in the preceding 2 hours increased. Although a substantial majority of these alarms were technically valid (ie, representing true deviations of vital signs outside of the normal range rather than sensor or equipment problems), the vast majority required no action to be takenapproximately 7 out of 8 in the ICU and an astonishing 99 out of 100 on the ward.
As any hospitalist, intensivist, or nurse knows well, alarms are rampant throughout hospitals. It is impossible to walk down any hallway on a busy hospital wardnever mind an ICUwithout seeing a flashing light or 2 above a doorway, and hearing the incessant beeping of oxygen saturation and cardiovascular/respiratory monitors, a thousand bits of technology forever crying wolf. The problem, of course, is that sometimes there really is a wolf, but it is hard to take the risk seriously when the false alarms happen not just twice before a true threat materializes, as in Aesop's fable, but 7 times in the ICU, or worse, 99 times in the setting where most hospitalists practice. Moreover, even when the threat is real, in most cases it is caught in time one way or another, and no lasting harm results.
So why not simply shut off the unremitting noise? In 1987, outside of Baltimore, Amtrak experienced what at the time was the deadliest rail crash in its history after 1 of its passenger trains collided with a Conrail freight train. A major root cause of the crash was that the crew on the freight train had placed duct tape over an annoying automated signal alarm.[8, 9] Tragically, on this particular day, the suppressed alarm was all too relevant. Identifying the real alarm, however, can be nearly impossible when it sounds the same as 100 irritating sounds constantly emanating from the environment. It is the challenge of identifying the needle in the haystack, after you have developed an allergy to the hay.
What then to do? More research like that conducted by Bonafide and colleagues is needed to better understand how healthcare providers respond to the onslaught of alarms they encounter, and to inform refinement of these systems. Understanding how alarm fatigue plays out in the context of different clinical settings, with different workloads, varying levels of distraction, and different rates of true and false‐positive alarms will be critical. Furthermore, understanding how individuals' physiologic fatigue, circadian misalignment, mood, stress, and cognitive state may play into alarm response is likewise essential, if we are to design appropriate alarm systems that function effectively in the busy 24‐hour environment of healthcare. Ongoing work suggests that smart alarms, using algorithms that integrate data from multiple vital sign readings over time, may reduce the frequency of false alarms and better identify clinically significant events.[10] Replacing existing range‐limit monitors with these types of smart alarms has the potential to greatly improve both the sensitivity and specificity of hospital alarms, but further work in this area is needed.
Ultimately, if we can better separate out the signal, we will be better poised to respond to the true emergencies that arise that are currently obscured by the ever‐present noise. Better trust in the alarm systems we have would help all of us focus our energies on the problems that matter most. Doing so, we could better care for our patients, and better identify the system failures that cause them harm in our hospitals.
Disclosures: Dr. Landrigan is supported in part by the Children's Hospital Association for his work as an Executive Council Member of the Pediatric Research in Inpatient Settings network. Dr. Landrigan serves as a consultant to Virgin Pulse regarding sleep, safety, and health. In addition, Dr. Landrigan has received monetary awards, honoraria, and travel reimbursement from multiple academic and professional organizations for delivering lectures on sleep deprivation, physician performance, handoffs, and patient safety, and has served as an expert witness in cases regarding patient safety.
- Office of the Inspector General. Adverse events in hospitals: national incidence among Medicare beneficiaries. OEI‐06‐09‐00090. Available at: https://oig.hhs.gov/oei/reports/oei‐06‐09‐00090.pdf. Published November 2010. Accessed February 27, 2015.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732.
- , , , et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499.
- , , , et al. Changes in medical errors after implementation of a resident handoff program. New Engl J Med. 2014;371:1803–1812.
- , , , et al. National trends in patient safety for four common conditions, 2005–2011. N Engl J Med. 2014;370:341–351.
- , , , et al. Temporal trends in rates of patient harm due to medical care. New Engl J Med. 2010;363:2124–2134.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- . Why are people turning off our alarms? J Acoust Soc Am. 1988;84:1107–1108.
- 1987 Maryland train collision. Wikipedia. Available at: http://en.wikipedia.org/wiki/1987_Maryland_train_collision. Accessed February 27, 2015.
- , , , et al. Collection of annotated data in a clinical validation study for alarm algorithms in intensive care—a methodologic framework. J Crit Care. 2010;25:128–135.
Despite 15 years of national and local investment in improving the safety of hospital care, patient safety remains a leading problem in both adult and pediatric hospitals. A 2010 study found that 180,000 Medicare beneficiaries likely die each year due to harm suffered as a result of medical care,[1] a death toll surpassed only by deaths due to cardiovascular disease and cancer. Even though initial efforts in the field have shown great promise for stemming the tide of healthcare‐associated infections,[2] surgical errors,[3] handoff failures,[4] and errors in the care of adults hospitalized for myocardial infarction and congestive heart failure,[5] much work remains to be done.[6] The root causes of many adverse events are poorly understood and unaddressed. Resultant tragedies remain all too common.
In the current issue of the Journal of Hospital Medicine, Bonafide and colleagues report the results of an innovative observational pilot study designed to assess the role of an inadequately addressed root cause of serious errors: alarm fatigue.[7] Alarm fatigue is the phenomenon of desensitization to alarms, particularly in the context of excessive false alarms. In a videotaped observational assessment of nurse response times to 5070 alarms on a pediatric ward and intensive care unit (ICU), the authors found that nurses responded significantly more slowly as the number of nonactionable alarms in the preceding 2 hours increased. Although a substantial majority of these alarms were technically valid (ie, representing true deviations of vital signs outside of the normal range rather than sensor or equipment problems), the vast majority required no action to be takenapproximately 7 out of 8 in the ICU and an astonishing 99 out of 100 on the ward.
As any hospitalist, intensivist, or nurse knows well, alarms are rampant throughout hospitals. It is impossible to walk down any hallway on a busy hospital wardnever mind an ICUwithout seeing a flashing light or 2 above a doorway, and hearing the incessant beeping of oxygen saturation and cardiovascular/respiratory monitors, a thousand bits of technology forever crying wolf. The problem, of course, is that sometimes there really is a wolf, but it is hard to take the risk seriously when the false alarms happen not just twice before a true threat materializes, as in Aesop's fable, but 7 times in the ICU, or worse, 99 times in the setting where most hospitalists practice. Moreover, even when the threat is real, in most cases it is caught in time one way or another, and no lasting harm results.
So why not simply shut off the unremitting noise? In 1987, outside of Baltimore, Amtrak experienced what at the time was the deadliest rail crash in its history after 1 of its passenger trains collided with a Conrail freight train. A major root cause of the crash was that the crew on the freight train had placed duct tape over an annoying automated signal alarm.[8, 9] Tragically, on this particular day, the suppressed alarm was all too relevant. Identifying the real alarm, however, can be nearly impossible when it sounds the same as 100 irritating sounds constantly emanating from the environment. It is the challenge of identifying the needle in the haystack, after you have developed an allergy to the hay.
What then to do? More research like that conducted by Bonafide and colleagues is needed to better understand how healthcare providers respond to the onslaught of alarms they encounter, and to inform refinement of these systems. Understanding how alarm fatigue plays out in the context of different clinical settings, with different workloads, varying levels of distraction, and different rates of true and false‐positive alarms will be critical. Furthermore, understanding how individuals' physiologic fatigue, circadian misalignment, mood, stress, and cognitive state may play into alarm response is likewise essential, if we are to design appropriate alarm systems that function effectively in the busy 24‐hour environment of healthcare. Ongoing work suggests that smart alarms, using algorithms that integrate data from multiple vital sign readings over time, may reduce the frequency of false alarms and better identify clinically significant events.[10] Replacing existing range‐limit monitors with these types of smart alarms has the potential to greatly improve both the sensitivity and specificity of hospital alarms, but further work in this area is needed.
Ultimately, if we can better separate out the signal, we will be better poised to respond to the true emergencies that arise that are currently obscured by the ever‐present noise. Better trust in the alarm systems we have would help all of us focus our energies on the problems that matter most. Doing so, we could better care for our patients, and better identify the system failures that cause them harm in our hospitals.
Disclosures: Dr. Landrigan is supported in part by the Children's Hospital Association for his work as an Executive Council Member of the Pediatric Research in Inpatient Settings network. Dr. Landrigan serves as a consultant to Virgin Pulse regarding sleep, safety, and health. In addition, Dr. Landrigan has received monetary awards, honoraria, and travel reimbursement from multiple academic and professional organizations for delivering lectures on sleep deprivation, physician performance, handoffs, and patient safety, and has served as an expert witness in cases regarding patient safety.
Despite 15 years of national and local investment in improving the safety of hospital care, patient safety remains a leading problem in both adult and pediatric hospitals. A 2010 study found that 180,000 Medicare beneficiaries likely die each year due to harm suffered as a result of medical care,[1] a death toll surpassed only by deaths due to cardiovascular disease and cancer. Even though initial efforts in the field have shown great promise for stemming the tide of healthcare‐associated infections,[2] surgical errors,[3] handoff failures,[4] and errors in the care of adults hospitalized for myocardial infarction and congestive heart failure,[5] much work remains to be done.[6] The root causes of many adverse events are poorly understood and unaddressed. Resultant tragedies remain all too common.
In the current issue of the Journal of Hospital Medicine, Bonafide and colleagues report the results of an innovative observational pilot study designed to assess the role of an inadequately addressed root cause of serious errors: alarm fatigue.[7] Alarm fatigue is the phenomenon of desensitization to alarms, particularly in the context of excessive false alarms. In a videotaped observational assessment of nurse response times to 5070 alarms on a pediatric ward and intensive care unit (ICU), the authors found that nurses responded significantly more slowly as the number of nonactionable alarms in the preceding 2 hours increased. Although a substantial majority of these alarms were technically valid (ie, representing true deviations of vital signs outside of the normal range rather than sensor or equipment problems), the vast majority required no action to be takenapproximately 7 out of 8 in the ICU and an astonishing 99 out of 100 on the ward.
As any hospitalist, intensivist, or nurse knows well, alarms are rampant throughout hospitals. It is impossible to walk down any hallway on a busy hospital wardnever mind an ICUwithout seeing a flashing light or 2 above a doorway, and hearing the incessant beeping of oxygen saturation and cardiovascular/respiratory monitors, a thousand bits of technology forever crying wolf. The problem, of course, is that sometimes there really is a wolf, but it is hard to take the risk seriously when the false alarms happen not just twice before a true threat materializes, as in Aesop's fable, but 7 times in the ICU, or worse, 99 times in the setting where most hospitalists practice. Moreover, even when the threat is real, in most cases it is caught in time one way or another, and no lasting harm results.
So why not simply shut off the unremitting noise? In 1987, outside of Baltimore, Amtrak experienced what at the time was the deadliest rail crash in its history after 1 of its passenger trains collided with a Conrail freight train. A major root cause of the crash was that the crew on the freight train had placed duct tape over an annoying automated signal alarm.[8, 9] Tragically, on this particular day, the suppressed alarm was all too relevant. Identifying the real alarm, however, can be nearly impossible when it sounds the same as 100 irritating sounds constantly emanating from the environment. It is the challenge of identifying the needle in the haystack, after you have developed an allergy to the hay.
What then to do? More research like that conducted by Bonafide and colleagues is needed to better understand how healthcare providers respond to the onslaught of alarms they encounter, and to inform refinement of these systems. Understanding how alarm fatigue plays out in the context of different clinical settings, with different workloads, varying levels of distraction, and different rates of true and false‐positive alarms will be critical. Furthermore, understanding how individuals' physiologic fatigue, circadian misalignment, mood, stress, and cognitive state may play into alarm response is likewise essential, if we are to design appropriate alarm systems that function effectively in the busy 24‐hour environment of healthcare. Ongoing work suggests that smart alarms, using algorithms that integrate data from multiple vital sign readings over time, may reduce the frequency of false alarms and better identify clinically significant events.[10] Replacing existing range‐limit monitors with these types of smart alarms has the potential to greatly improve both the sensitivity and specificity of hospital alarms, but further work in this area is needed.
Ultimately, if we can better separate out the signal, we will be better poised to respond to the true emergencies that arise that are currently obscured by the ever‐present noise. Better trust in the alarm systems we have would help all of us focus our energies on the problems that matter most. Doing so, we could better care for our patients, and better identify the system failures that cause them harm in our hospitals.
Disclosures: Dr. Landrigan is supported in part by the Children's Hospital Association for his work as an Executive Council Member of the Pediatric Research in Inpatient Settings network. Dr. Landrigan serves as a consultant to Virgin Pulse regarding sleep, safety, and health. In addition, Dr. Landrigan has received monetary awards, honoraria, and travel reimbursement from multiple academic and professional organizations for delivering lectures on sleep deprivation, physician performance, handoffs, and patient safety, and has served as an expert witness in cases regarding patient safety.
- Office of the Inspector General. Adverse events in hospitals: national incidence among Medicare beneficiaries. OEI‐06‐09‐00090. Available at: https://oig.hhs.gov/oei/reports/oei‐06‐09‐00090.pdf. Published November 2010. Accessed February 27, 2015.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732.
- , , , et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499.
- , , , et al. Changes in medical errors after implementation of a resident handoff program. New Engl J Med. 2014;371:1803–1812.
- , , , et al. National trends in patient safety for four common conditions, 2005–2011. N Engl J Med. 2014;370:341–351.
- , , , et al. Temporal trends in rates of patient harm due to medical care. New Engl J Med. 2010;363:2124–2134.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- . Why are people turning off our alarms? J Acoust Soc Am. 1988;84:1107–1108.
- 1987 Maryland train collision. Wikipedia. Available at: http://en.wikipedia.org/wiki/1987_Maryland_train_collision. Accessed February 27, 2015.
- , , , et al. Collection of annotated data in a clinical validation study for alarm algorithms in intensive care—a methodologic framework. J Crit Care. 2010;25:128–135.
- Office of the Inspector General. Adverse events in hospitals: national incidence among Medicare beneficiaries. OEI‐06‐09‐00090. Available at: https://oig.hhs.gov/oei/reports/oei‐06‐09‐00090.pdf. Published November 2010. Accessed February 27, 2015.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732.
- , , , et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499.
- , , , et al. Changes in medical errors after implementation of a resident handoff program. New Engl J Med. 2014;371:1803–1812.
- , , , et al. National trends in patient safety for four common conditions, 2005–2011. N Engl J Med. 2014;370:341–351.
- , , , et al. Temporal trends in rates of patient harm due to medical care. New Engl J Med. 2010;363:2124–2134.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- . Why are people turning off our alarms? J Acoust Soc Am. 1988;84:1107–1108.
- 1987 Maryland train collision. Wikipedia. Available at: http://en.wikipedia.org/wiki/1987_Maryland_train_collision. Accessed February 27, 2015.
- , , , et al. Collection of annotated data in a clinical validation study for alarm algorithms in intensive care—a methodologic framework. J Crit Care. 2010;25:128–135.
Monitor Alarms and Response Time
Hospital physiologic monitors can alert clinicians to early signs of physiologic deterioration, and thus have great potential to save lives. However, monitors generate frequent alarms,[1, 2, 3, 4, 5, 6, 7, 8] and most are not relevant to the patient's safety (over 90% of pediatric intensive care unit (PICU)[1, 2] and over 70% of adult intensive care alarms).[5, 6] In psychology experiments, humans rapidly learn to ignore or respond more slowly to alarms when exposed to high false‐alarm rates, exhibiting alarm fatigue.[9, 10] In 2013, The Joint Commission named alarm fatigue the most common contributing factor to alarm‐related sentinel events in hospitals.[11, 12]
Although alarm fatigue has been implicated as a major threat to patient safety, little empirical data support its existence in hospitals. In this study, we aimed to determine if there was an association between nurses' recent exposure to nonactionable physiologic monitor alarms and their response time to future alarms for the same patients. This exploratory work was designed to inform future research in this area, acknowledging that the sample size would be too small for multivariable modeling.
METHODS
Study Definitions
The alarm classification scheme is shown in Figure 1. Note that, for clarity, we have intentionally avoided using the terms true and false alarms because their interpretations vary across studies and can be misleading.
Potentially Critical Alarm
A potentially critical alarm is any alarm for a clinical condition for which a timely response is important to determine if the alarm requires intervention to save the patient's life. This is based on the alarm type alone, including alarms for life‐threatening arrhythmias such as asystole and ventricular tachycardia, as well as alarms for vital signs outside the set limits. Supporting Table 1 in the online version of this article lists the breakdown of alarm types that we defined a priori as potentially and not potentially critical.
| PICU | Ward | |||||||
|---|---|---|---|---|---|---|---|---|
| Alarm type | No. | % of Total | % Valid | % Actionable | No. | % of Total | % Valid | % Actionable |
| ||||||||
| Oxygen saturation | 197 | 19.4 | 82.7 | 38.6 | 590 | 41.2 | 24.4 | 1.9 |
| Heart rate | 194 | 19.1 | 95.4 | 1.0 | 266 | 18.6 | 87.2 | 0.0 |
| Respiratory rate | 229 | 22.6 | 80.8 | 13.5 | 316 | 22.1 | 48.1 | 1.0 |
| Blood pressure | 259 | 25.5 | 83.8 | 5.8 | 11 | 0.8 | 72.7 | 0.0 |
| Critical arrhythmia | 1 | 0.1 | 0.0 | 0.0 | 4 | 0.3 | 0.0 | 0.0 |
| Noncritical arrhythmia | 71 | 7.0 | 2.8 | 0.0 | 244 | 17.1 | 8.6 | 0.0 |
| Central venous pressure | 49 | 4.8 | 0.0 | 0.0 | 0 | 0.0 | N/A | N/A |
| Exhaled carbon dioxide | 14 | 1.4 | 92.9 | 50.0 | 0 | 0.0 | N/A | N/A |
| Total | 1014 | 100.0 | 75.6 | 12.9 | 1,431 | 100.0 | 38.9 | 1.0 |
Valid Alarm
A valid alarm is any alarm that correctly identifies the physiologic status of the patient. Validity was based on waveform quality, lead signal strength indicators, and artifact conditions, referencing each monitor's operator's manual.
Actionable Alarm
An actionable alarm is any valid alarm for a clinical condition that either: (1) leads to a clinical intervention; (2) leads to a consultation with another clinician at the bedside (and thus visible on camera); or (3) is a situation that should have led to intervention or consultation, but the alarm was unwitnessed or misinterpreted by the staff at the bedside.
Nonactionable Alarm
An unactionable alarm is any alarm that does not meet the actionable definition above, including invalid alarms such as those caused by motion artifact, equipment/technical alarms, and alarms that are valid but nonactionable (nuisance alarms).[13]
Response Time
The response time is the time elapsed from when the alarm fired at the bedside to when the nurse entered the room or peered through a window or door, measured in seconds.
Setting and Subjects
We performed this study between August 2012 and July 2013 at a freestanding children's hospital. We evaluated nurses caring for 2 populations: (1) PICU patients with heart and/or lung failure (requiring inotropic support and/or invasive mechanical ventilation), and (2) medical patients on a general inpatient ward. Nurses caring for heart and/or lung failure patients in the PICU typically were assigned 1 to 2 total patients. Nurses on the medical ward typically were assigned 2 to 4 patients. We identified subjects from the population of nurses caring for eligible patients with parents available to provide in‐person consent in each setting. Our primary interest was to evaluate the association between nonactionable alarms and response time, and not to study the epidemiology of alarms in a random sample. Therefore, when alarm data were available prior to screening, we first approached nurses caring for patients in the top 25% of alarm rates for that unit over the preceding 4 hours. We identified preceding alarm rates using BedMasterEx (Excel Medical Electronics, Jupiter, FL).
Human Subjects Protection
This study was approved by the institutional review board of The Children's Hospital of Philadelphia. We obtained written in‐person consent from the patient's parent and the nurse subject. We obtained a Certificate of Confidentiality from the National Institutes of Health to further protect study participants.[14]
Monitoring Equipment
All patients in the PICU were monitored continuously using General Electric (GE) (Fairfield, CT) solar devices. All bed spaces on the wards include GE Dash monitors that are used if ordered. On the ward we studied, 30% to 50% of patients are typically monitored at any given time. In addition to alarming at the bedside, most clinical alarms also generated a text message sent to the nurse's wireless phone listing the room number and the word monitor. Messages did not provide any clinical information about the alarm or patient's status. There were no technicians reviewing alarms centrally.
Physicians used an order set to order monitoring, selecting 1 of 4 available preconfigured profiles: infant <6 months, infant 6 months to 1 year, child, and adult. The parameters for each age group are in Supporting Figure 1, available in the online version of this article. A physician order is required for a nurse to change the parameters. Participating in the study did not affect this workflow.
Primary Outcome
The primary outcome was the nurse's response time to potentially critical monitor alarms that occurred while neither they nor any other clinicians were in the patient's room.
Primary Exposure and Alarm Classification
The primary exposure was the number of nonactionable alarms in the same patient over the preceding 120 minutes (rolling and updated each minute). The alarm classification scheme is shown in Figure 1.
Due to technical limitations with obtaining time‐stamped alarm data from the different ventilators in use during the study period, we were unable to identify the causes of all ventilator alarms. Therefore, we included ventilator alarms that did not lead to clinical interventions as nonactionable alarm exposures, but we did not evaluate the response time to any ventilator alarms.
Data Collection
We combined video recordings with monitor time‐stamp data to evaluate the association between nonactionable alarms and the nurse's response time. Our detailed video recording and annotation methods have been published separately.[15] Briefly, we mounted up to 6 small video cameras in patients' rooms and recorded up to 6 hours per session. The cameras captured the monitor display, a wide view of the room, a close‐up view of the patient, and all windows and doors through which staff could visually assess the patient without entering the room.
Video Processing, Review, and Annotation
The first 5 video sessions were reviewed in a group training setting. Research assistants received instruction on how to determine alarm validity and actionability in accordance with the study definitions. Following the training period, the review workflow was as follows. First, a research assistant entered basic information and a preliminary assessment of the alarm's clinical validity and actionability into a REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN) database.[16] Later, a physician investigator secondarily reviewed all alarms and confirmed the assessments of the research assistants or, when disagreements occurred, discussed and reconciled the database. Alarms that remained unresolved after secondary review were flagged for review with an additional physician or nurse investigator in a team meeting.
Data Analysis
We summarized the patient and nurse subjects, the distributions of alarms, and the response times to potentially critical monitor alarms that occurred while neither the nurse nor any other clinicians were in the patient's room. We explored the data using plots of alarms and response times occurring within individual video sessions as well as with simple linear regression. Hypothesizing that any alarm fatigue effect would be strongest in the highest alarm patients, and having observed that alarms are distributed very unevenly across patients in both the PICU and ward, we made the decision not to use quartiles, but rather to form clinically meaningful categories. We also hypothesized that nurses might not exhibit alarm fatigue unless they were inundated with alarms. We thus divided the nonactionable alarm counts over the preceding 120 minutes into 3 categories: 0 to 29 alarms to represent a low to average alarm rate exhibited by the bottom 50% of the patients, 30 to 79 alarms to represent an elevated alarm rate, and 80+ alarms to represent an extremely high alarm rate exhibited by the top 5%. Because the exposure time was 120 minutes, we conducted the analysis on the alarms occurring after a nurse had been video recorded for at least 120 minutes.
We further evaluated the relationship between nonactionable alarms and nurse response time with Kaplan‐Meier plots by nonactionable alarm count category using the observed response‐time data. The Kaplan‐Meier plots compared response time across the nonactionable alarm exposure group, without any statistical modeling. A log‐rank test stratified by nurse evaluated whether the distributions of response time in the Kaplan‐Meier plots differed across the 3 alarm exposure groups, accounting for within‐nurse clustering.
Accelerated failure‐time regression based on the Weibull distribution then allowed us to compare response time across each alarm exposure group and provided confidence intervals. Accelerated failure‐time models are comparable to Cox models, but emphasize time to event rather than hazards.[17, 18] We determined that the Weibull distribution was suitable by evaluating smoothed hazard and log‐hazard plots, the confidence intervals of the shape parameters in the Weibull models that did not include 1, and by demonstrating that the Weibull model had better fit than an alternative (exponential) model using the likelihood‐ratio test (P<0.0001 for PICU, P=0.02 for ward). Due to the small sample size of nurses and patients, we could not adjust for nurse‐ or patient‐level covariates in the model. When comparing the nonactionable alarm exposure groups in the regression model (029 vs 3079, 3079 vs 80+, and 029 vs 80+), we Bonferroni corrected the critical P value for the 3 comparisons, for a critical P value of 0.05/3=0.0167.
Nurse Questionnaire
At the session's conclusion, nurses completed a questionnaire that included demographics and asked, Did you respond more quickly to monitor alarms during this study because you knew you were being filmed? to measure if nurses would report experiencing a Hawthorne‐like effect.[19, 20, 21]
RESULTS
We performed 40 sessions among 40 patients and 36 nurses over 210 hours. We performed 20 sessions in children with heart and/or lung failure in the PICU and 20 sessions in children on a general ward. Sessions took place on weekdays between 9:00 am and 6:00 pm. There were 3 occasions when we filmed 2 patients cared for by the same nurse at the same time.
Nurses were mostly female (94.4%) and had between 2 months and 28 years of experience (median, 4.8 years). Patients on the ward ranged from 5 days to 5.4 years old (median, 6 months). Patients in the PICU ranged from 5 months to 16 years old (median, 2.5 years). Among the PICU patients, 14 (70%) were receiving mechanical ventilation only, 3 (15%) were receiving vasopressors only, and 3 (15%) were receiving mechanical ventilation and vasopressors.
We observed 5070 alarms during the 40 sessions. We excluded 108 (2.1%) that occurred at the end of video recording sessions with the nurse absent from the room because the nurse's response could not be determined. Alarms per session ranged from 10 to 1430 (median, 75; interquartile range [IQR], 35138). We excluded the outlier PICU patient with 1430 alarms in 5 hours from the analysis to avoid the potential for biasing the results. Figure 2 depicts the data flow.
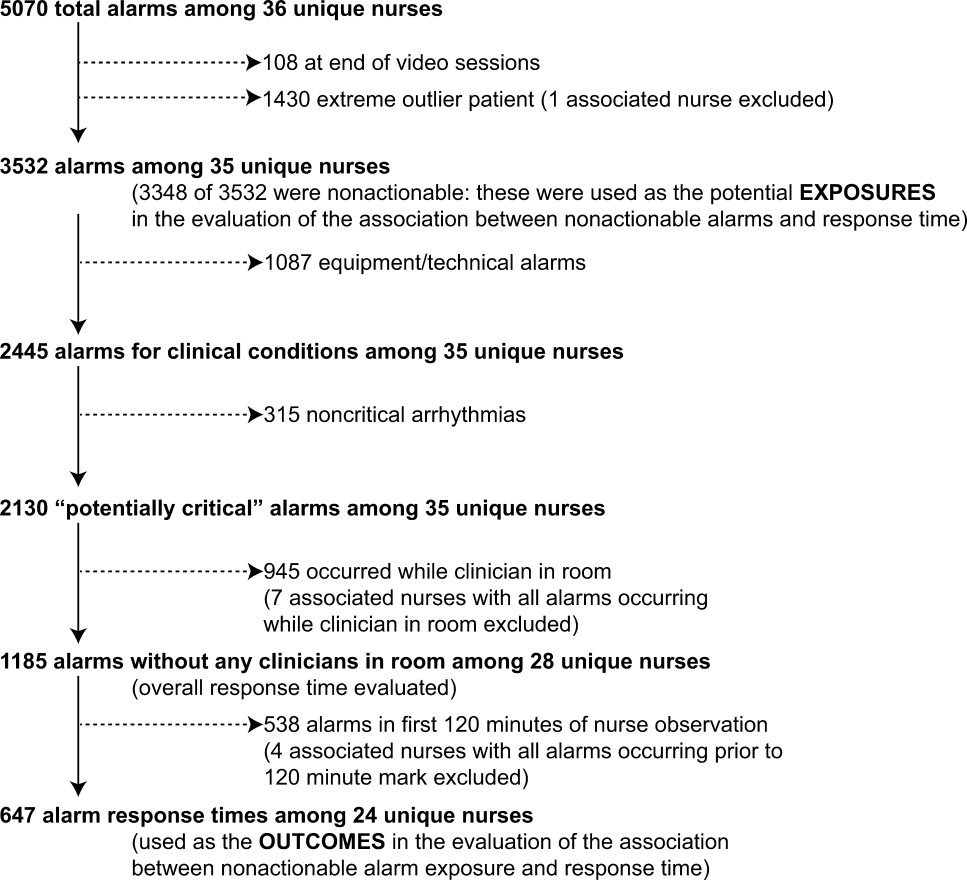
Following the 5 training sessions, research assistants independently reviewed and made preliminary assessments on 4674 alarms; these alarms were all secondarily reviewed by a physician. Using the physician reviewer as the gold standard, the research assistant's sensitivity (assess alarm as actionable when physician also assesses as actionable) was 96.8% and specificity (assess alarm as nonactionable when physician also assesses as nonactionable) was 96.9%. We had to review 54 of 4674 alarms (1.2%) with an additional physician or nurse investigator to achieve consensus.
Characteristics of the 2445 alarms for clinical conditions are shown in Table 1. Only 12.9% of alarms in heart‐ and/or lung‐failure patients in the PICU were actionable, and only 1.0% of alarms in medical patients on a general inpatient ward were actionable.
Overall Response Times for Out‐of‐Room Alarms
We first evaluated response times without excluding alarms occurring prior to the 120‐minute mark. Of the 2445 clinical condition alarms, we excluded the 315 noncritical arrhythmia types from analysis of response time because they did not meet our definition of potentially critical alarms. Of the 2130 potentially critical alarms, 1185 (55.6%) occurred while neither the nurse nor any other clinician was in the patient's room. We proceeded to analyze the response time to these 1185 alarms (307 in the PICU and 878 on the ward). In the PICU, median response time was 3.3 minutes (IQR, 0.814.4). On the ward, median response time was 9.8 minutes (IQR, 3.222.4).
Response‐Time Association With Nonactionable Alarm Exposure
Next, we analyzed the association between response time to potentially critical alarms that occurred when the nurse was not in the patient's room and the number of nonactionable alarms occurring over the preceding 120‐minute window. This required excluding the alarms that occurred in the first 120 minutes of each session, leaving 647 alarms with eligible response times to evaluate the exposure between prior nonactionable alarm exposure and response time: 219 in the PICU and 428 on the ward. Kaplan‐Meier plots and tabulated response times demonstrated the incremental relationships between each nonactionable alarm exposure category in the observed data, with the effects most prominent as the Kaplan‐Meier plots diverged beyond the median (Figure 3 and Table 2). Excluding the extreme outlier patient had no effect on the results, because 1378 of the 1430 alarms occurred with the nurse present at the bedside, and only 2 of the remaining alarms were potentially critical.
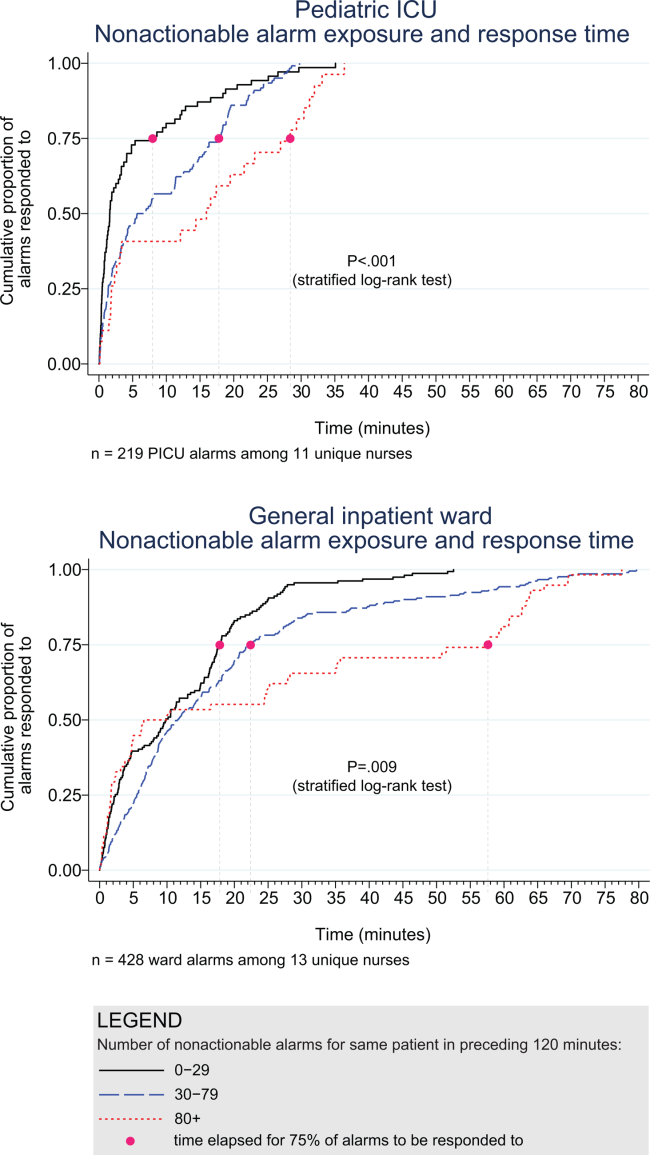
| Observed Data | Accelerated Failure‐Time Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of Potentially Critical Alarms | Minutes Elapsed Until This Percentage of Alarms Was Responded to | Modeled Response Time, min | 95% CI, min | P Value* | ||||
| 50% (Median) | 75% | 90% | 95% | |||||
| ||||||||
| PICU | ||||||||
| 029 nonactionable alarms | 70 | 1.6 | 8.0 | 18.6 | 25.1 | 2.8 | 1.9‐3.8 | Reference |
| 3079 nonactionable alarms | 122 | 6.3 | 17.8 | 22.5 | 26.0 | 5.3 | 4.06.7 | 0.001 (vs 029) |
| 80+ nonactionable alarms | 27 | 16.0 | 28.4 | 32.0 | 33.1 | 8.5 | 4.312.7 | 0.009 (vs 029), 0.15 (vs 3079) |
| Ward | ||||||||
| 029 nonactionable alarms | 159 | 9.8 | 17.8 | 25.0 | 28.9 | 7.7 | 6.39.1 | Reference |
| 3079 nonactionable alarms | 211 | 11.6 | 22.4 | 44.6 | 63.2 | 11.5 | 9.613.3 | 0.001 (vs 029) |
| 80+ nonactionable alarms | 58 | 8.3 | 57.6 | 63.8 | 69.5 | 15.6 | 11.020.1 | 0.001 (vs 029), 0.09 (vs 3079) |
Accelerated failure‐time regressions revealed significant incremental increases in the modeled response time as the number of preceding nonactionable alarms increased in both the PICU and ward settings (Table 2).
Hawthorne‐like Effects
Four of the 36 nurses reported that they responded more quickly to monitor alarms because they knew they were being filmed.
DISCUSSION
Alarm fatigue has recently generated interest among nurses,[22] physicians,[23] regulatory bodies,[24] patient safety organizations,[25] and even attorneys,[26] despite a lack of prior evidence linking nonactionable alarm exposure to response time or other adverse patient‐relevant outcomes. This study's main findings were that (1) the vast majority of alarms were nonactionable, (2) response time to alarms occurring while the nurse was out of the room increased as the number of nonactionable alarms over the preceding 120 minutes increased. These findings may be explained by alarm fatigue.
Our results build upon the findings of other related studies. The nonactionable alarm proportions we found were similar to other pediatric studies, reporting greater than 90% nonactionable alarms.[1, 2] One other study has reported a relationship between alarm exposure and response time. In that study, Voepel‐Lewis and colleagues evaluated nurse responses to pulse oximetry desaturation alarms in adult orthopedic surgery patients using time‐stamp data from their monitor notification system.[27] They found that alarm response time was significantly longer for patients in the highest quartile of alarms compared to those in lower quartiles. Our study provides new data suggesting a relationship between nonactionable alarm exposure and nurse response time.
Our study has several limitations. First, as a preliminary study to investigate feasibility and possible association, the sample of patients and nurses was necessarily limited and did not permit adjustment for nurse‐ or patient‐level covariates. A multivariable analysis with a larger sample might provide insight into alternate explanations for these findings other than alarm fatigue, including measures of nurse workload and patient factors (such as age and illness severity). Additional factors that are not as easily measured can also contribute to the complex decision of when and how to respond to alarms.[28, 29] Second, nurses were aware that they were being video recorded as part of a study of nonactionable alarms, although they did not know the specific details of measurement. Although this lack of blinding might lead to a Hawthorne‐like effect, our positive results suggest that this effect, if present, did not fully obscure the association. Third, all sessions took place on weekdays during daytime hours, but effects of nonactionable alarms might vary by time and day. Finally, we suspect that when nurses experience critical alarms that require them to intervene and rescue a patient, their response times to that patient's alarms that occur later in their shift will be quicker due to a heightened concern for the alarm being actionable. We were unable to explore that relationship in this analysis because the number of critical alarms requiring intervention was very small. This is a topic of future study.
CONCLUSIONS
We identified an association between a nurse's prior exposure to nonactionable alarms and response time to future alarms. This finding is consistent with alarm fatigue, but requires further study to more clearly delineate other factors that might confound or modify that relationship.
Disclosures
This project was funded by the Health Research Formula Fund Grant 4100050891 from the Pennsylvania Department of Public Health Commonwealth Universal Research Enhancement Program (awarded to Drs. Keren and Bonafide). Dr. Bonafide is also supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , . Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997;25(4):614–619.
- , , , , . Clinical evaluation of alarm efficiency in intensive care [in French]. Ann Fr Anesth Reanim. 2000;19:459–466.
- , , , . Reducing false alarms of intensive care online‐monitoring systems: an evaluation of two signal extraction algorithms. Comput Math Methods Med. 2011;2011:143480.
- , , , , , . Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med. 1999;25:1360–1366.
- , , . Improving alarm performance in the medical intensive care unit using delays and clinical context. Anesth Analg. 2009;108:1546–1552.
- , . Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19:28–34.
- , , , , , . Intensive care unit alarms—how many do we need? Crit Care Med. 2010;38:451–456.
- , , , . System operator response to warnings of danger: a laboratory investigation of the effects of the predictive value of a warning on human response time. J Exp Psychol Appl. 1995;1:19–33.
- , , . Human probability matching behaviour in response to alarms of varying reliability. Ergonomics. 1995;38:2300–2312.
- The Joint Commission. Sentinel event alert: medical device alarm safety in hospitals. 2013. Available at: http://www.jointcommission.org/sea_issue_50/. Accessed October 9, 2014.
- . Joint commission warns of alarm fatigue: multitude of alarms from monitoring devices problematic. JAMA. 2013;309(22):2315–2316.
- . Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–277.
- NIH Certificates of Confidentiality Kiosk. Available at: http://grants.nih.gov/grants/policy/coc/. Accessed April 21, 2014.
- , , , et al. Video methods for evaluating physiologic monitor alarms and alarm responses. Biomed Instrum Technol. 2014;48(3):220–230.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381.
- . Accelerated failure time and other parametric models. In: Modelling Survival Data in Medical Research. 2nd ed. Boca Raton, FL: Chapman 2003:197–229.
- , , , . Parametric models. In: An Introduction to Survival Analysis Using Stata, 3rd ed. College Station, TX: Stata Press; 2010:229–244.
- , . Management and the Worker. Cambridge, MA: Harvard University Press; 1939.
- . What happened at Hawthorne? Science. 1974;183(4128):922–932.
- , , , , . Validation of the Work Observation Method By Activity Timing (WOMBAT) method of conducting time‐motion observations in critical care settings: an observational study. BMC Med Inf Decis Mak. 2011;11:32.
- , . Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378–386.
- , . Redesigning hospital alarms for patient safety: alarmed and potentially dangerous. JAMA. 2014;311(12):1199–1200.
- The Joint Commission. The Joint Commission announces 2014 National Patient Safety Goal. Jt Comm Perspect. 2013;33:1–4.
- Top 10 health technology hazards for 2014. Health Devices. 2013;42(11):354–380.
- My Philly Lawyer. Medical malpractice: alarm fatigue threatens patient safety. 2014. Available at: http://www.myphillylawyer.com/Resources/Legal-Articles/Medical-Malpractice-Alarm-Fatigue-Threatens-Patient-Safety.shtml. Accessed April 4, 2014.
- , , , et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud. 2013;50(10):1351–1358.
- , , , , . A description of nurses' decision‐making in managing electrocardiographic monitor alarms [published online ahead of print May 10, 2014]. J Clin Nurs. doi:10.1111/jocn.12625.
- . Nurses' response to frequency and types of electrocardiography alarms in a non‐critical care setting: a descriptive study. Int J Nurs Stud. 2014;51(2):190–197.
Hospital physiologic monitors can alert clinicians to early signs of physiologic deterioration, and thus have great potential to save lives. However, monitors generate frequent alarms,[1, 2, 3, 4, 5, 6, 7, 8] and most are not relevant to the patient's safety (over 90% of pediatric intensive care unit (PICU)[1, 2] and over 70% of adult intensive care alarms).[5, 6] In psychology experiments, humans rapidly learn to ignore or respond more slowly to alarms when exposed to high false‐alarm rates, exhibiting alarm fatigue.[9, 10] In 2013, The Joint Commission named alarm fatigue the most common contributing factor to alarm‐related sentinel events in hospitals.[11, 12]
Although alarm fatigue has been implicated as a major threat to patient safety, little empirical data support its existence in hospitals. In this study, we aimed to determine if there was an association between nurses' recent exposure to nonactionable physiologic monitor alarms and their response time to future alarms for the same patients. This exploratory work was designed to inform future research in this area, acknowledging that the sample size would be too small for multivariable modeling.
METHODS
Study Definitions
The alarm classification scheme is shown in Figure 1. Note that, for clarity, we have intentionally avoided using the terms true and false alarms because their interpretations vary across studies and can be misleading.

Potentially Critical Alarm
A potentially critical alarm is any alarm for a clinical condition for which a timely response is important to determine if the alarm requires intervention to save the patient's life. This is based on the alarm type alone, including alarms for life‐threatening arrhythmias such as asystole and ventricular tachycardia, as well as alarms for vital signs outside the set limits. Supporting Table 1 in the online version of this article lists the breakdown of alarm types that we defined a priori as potentially and not potentially critical.
| PICU | Ward | |||||||
|---|---|---|---|---|---|---|---|---|
| Alarm type | No. | % of Total | % Valid | % Actionable | No. | % of Total | % Valid | % Actionable |
| ||||||||
| Oxygen saturation | 197 | 19.4 | 82.7 | 38.6 | 590 | 41.2 | 24.4 | 1.9 |
| Heart rate | 194 | 19.1 | 95.4 | 1.0 | 266 | 18.6 | 87.2 | 0.0 |
| Respiratory rate | 229 | 22.6 | 80.8 | 13.5 | 316 | 22.1 | 48.1 | 1.0 |
| Blood pressure | 259 | 25.5 | 83.8 | 5.8 | 11 | 0.8 | 72.7 | 0.0 |
| Critical arrhythmia | 1 | 0.1 | 0.0 | 0.0 | 4 | 0.3 | 0.0 | 0.0 |
| Noncritical arrhythmia | 71 | 7.0 | 2.8 | 0.0 | 244 | 17.1 | 8.6 | 0.0 |
| Central venous pressure | 49 | 4.8 | 0.0 | 0.0 | 0 | 0.0 | N/A | N/A |
| Exhaled carbon dioxide | 14 | 1.4 | 92.9 | 50.0 | 0 | 0.0 | N/A | N/A |
| Total | 1014 | 100.0 | 75.6 | 12.9 | 1,431 | 100.0 | 38.9 | 1.0 |
Valid Alarm
A valid alarm is any alarm that correctly identifies the physiologic status of the patient. Validity was based on waveform quality, lead signal strength indicators, and artifact conditions, referencing each monitor's operator's manual.
Actionable Alarm
An actionable alarm is any valid alarm for a clinical condition that either: (1) leads to a clinical intervention; (2) leads to a consultation with another clinician at the bedside (and thus visible on camera); or (3) is a situation that should have led to intervention or consultation, but the alarm was unwitnessed or misinterpreted by the staff at the bedside.
Nonactionable Alarm
An unactionable alarm is any alarm that does not meet the actionable definition above, including invalid alarms such as those caused by motion artifact, equipment/technical alarms, and alarms that are valid but nonactionable (nuisance alarms).[13]
Response Time
The response time is the time elapsed from when the alarm fired at the bedside to when the nurse entered the room or peered through a window or door, measured in seconds.
Setting and Subjects
We performed this study between August 2012 and July 2013 at a freestanding children's hospital. We evaluated nurses caring for 2 populations: (1) PICU patients with heart and/or lung failure (requiring inotropic support and/or invasive mechanical ventilation), and (2) medical patients on a general inpatient ward. Nurses caring for heart and/or lung failure patients in the PICU typically were assigned 1 to 2 total patients. Nurses on the medical ward typically were assigned 2 to 4 patients. We identified subjects from the population of nurses caring for eligible patients with parents available to provide in‐person consent in each setting. Our primary interest was to evaluate the association between nonactionable alarms and response time, and not to study the epidemiology of alarms in a random sample. Therefore, when alarm data were available prior to screening, we first approached nurses caring for patients in the top 25% of alarm rates for that unit over the preceding 4 hours. We identified preceding alarm rates using BedMasterEx (Excel Medical Electronics, Jupiter, FL).
Human Subjects Protection
This study was approved by the institutional review board of The Children's Hospital of Philadelphia. We obtained written in‐person consent from the patient's parent and the nurse subject. We obtained a Certificate of Confidentiality from the National Institutes of Health to further protect study participants.[14]
Monitoring Equipment
All patients in the PICU were monitored continuously using General Electric (GE) (Fairfield, CT) solar devices. All bed spaces on the wards include GE Dash monitors that are used if ordered. On the ward we studied, 30% to 50% of patients are typically monitored at any given time. In addition to alarming at the bedside, most clinical alarms also generated a text message sent to the nurse's wireless phone listing the room number and the word monitor. Messages did not provide any clinical information about the alarm or patient's status. There were no technicians reviewing alarms centrally.
Physicians used an order set to order monitoring, selecting 1 of 4 available preconfigured profiles: infant <6 months, infant 6 months to 1 year, child, and adult. The parameters for each age group are in Supporting Figure 1, available in the online version of this article. A physician order is required for a nurse to change the parameters. Participating in the study did not affect this workflow.
Primary Outcome
The primary outcome was the nurse's response time to potentially critical monitor alarms that occurred while neither they nor any other clinicians were in the patient's room.
Primary Exposure and Alarm Classification
The primary exposure was the number of nonactionable alarms in the same patient over the preceding 120 minutes (rolling and updated each minute). The alarm classification scheme is shown in Figure 1.
Due to technical limitations with obtaining time‐stamped alarm data from the different ventilators in use during the study period, we were unable to identify the causes of all ventilator alarms. Therefore, we included ventilator alarms that did not lead to clinical interventions as nonactionable alarm exposures, but we did not evaluate the response time to any ventilator alarms.
Data Collection
We combined video recordings with monitor time‐stamp data to evaluate the association between nonactionable alarms and the nurse's response time. Our detailed video recording and annotation methods have been published separately.[15] Briefly, we mounted up to 6 small video cameras in patients' rooms and recorded up to 6 hours per session. The cameras captured the monitor display, a wide view of the room, a close‐up view of the patient, and all windows and doors through which staff could visually assess the patient without entering the room.
Video Processing, Review, and Annotation
The first 5 video sessions were reviewed in a group training setting. Research assistants received instruction on how to determine alarm validity and actionability in accordance with the study definitions. Following the training period, the review workflow was as follows. First, a research assistant entered basic information and a preliminary assessment of the alarm's clinical validity and actionability into a REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN) database.[16] Later, a physician investigator secondarily reviewed all alarms and confirmed the assessments of the research assistants or, when disagreements occurred, discussed and reconciled the database. Alarms that remained unresolved after secondary review were flagged for review with an additional physician or nurse investigator in a team meeting.
Data Analysis
We summarized the patient and nurse subjects, the distributions of alarms, and the response times to potentially critical monitor alarms that occurred while neither the nurse nor any other clinicians were in the patient's room. We explored the data using plots of alarms and response times occurring within individual video sessions as well as with simple linear regression. Hypothesizing that any alarm fatigue effect would be strongest in the highest alarm patients, and having observed that alarms are distributed very unevenly across patients in both the PICU and ward, we made the decision not to use quartiles, but rather to form clinically meaningful categories. We also hypothesized that nurses might not exhibit alarm fatigue unless they were inundated with alarms. We thus divided the nonactionable alarm counts over the preceding 120 minutes into 3 categories: 0 to 29 alarms to represent a low to average alarm rate exhibited by the bottom 50% of the patients, 30 to 79 alarms to represent an elevated alarm rate, and 80+ alarms to represent an extremely high alarm rate exhibited by the top 5%. Because the exposure time was 120 minutes, we conducted the analysis on the alarms occurring after a nurse had been video recorded for at least 120 minutes.
We further evaluated the relationship between nonactionable alarms and nurse response time with Kaplan‐Meier plots by nonactionable alarm count category using the observed response‐time data. The Kaplan‐Meier plots compared response time across the nonactionable alarm exposure group, without any statistical modeling. A log‐rank test stratified by nurse evaluated whether the distributions of response time in the Kaplan‐Meier plots differed across the 3 alarm exposure groups, accounting for within‐nurse clustering.
Accelerated failure‐time regression based on the Weibull distribution then allowed us to compare response time across each alarm exposure group and provided confidence intervals. Accelerated failure‐time models are comparable to Cox models, but emphasize time to event rather than hazards.[17, 18] We determined that the Weibull distribution was suitable by evaluating smoothed hazard and log‐hazard plots, the confidence intervals of the shape parameters in the Weibull models that did not include 1, and by demonstrating that the Weibull model had better fit than an alternative (exponential) model using the likelihood‐ratio test (P<0.0001 for PICU, P=0.02 for ward). Due to the small sample size of nurses and patients, we could not adjust for nurse‐ or patient‐level covariates in the model. When comparing the nonactionable alarm exposure groups in the regression model (029 vs 3079, 3079 vs 80+, and 029 vs 80+), we Bonferroni corrected the critical P value for the 3 comparisons, for a critical P value of 0.05/3=0.0167.
Nurse Questionnaire
At the session's conclusion, nurses completed a questionnaire that included demographics and asked, Did you respond more quickly to monitor alarms during this study because you knew you were being filmed? to measure if nurses would report experiencing a Hawthorne‐like effect.[19, 20, 21]
RESULTS
We performed 40 sessions among 40 patients and 36 nurses over 210 hours. We performed 20 sessions in children with heart and/or lung failure in the PICU and 20 sessions in children on a general ward. Sessions took place on weekdays between 9:00 am and 6:00 pm. There were 3 occasions when we filmed 2 patients cared for by the same nurse at the same time.
Nurses were mostly female (94.4%) and had between 2 months and 28 years of experience (median, 4.8 years). Patients on the ward ranged from 5 days to 5.4 years old (median, 6 months). Patients in the PICU ranged from 5 months to 16 years old (median, 2.5 years). Among the PICU patients, 14 (70%) were receiving mechanical ventilation only, 3 (15%) were receiving vasopressors only, and 3 (15%) were receiving mechanical ventilation and vasopressors.
We observed 5070 alarms during the 40 sessions. We excluded 108 (2.1%) that occurred at the end of video recording sessions with the nurse absent from the room because the nurse's response could not be determined. Alarms per session ranged from 10 to 1430 (median, 75; interquartile range [IQR], 35138). We excluded the outlier PICU patient with 1430 alarms in 5 hours from the analysis to avoid the potential for biasing the results. Figure 2 depicts the data flow.

Following the 5 training sessions, research assistants independently reviewed and made preliminary assessments on 4674 alarms; these alarms were all secondarily reviewed by a physician. Using the physician reviewer as the gold standard, the research assistant's sensitivity (assess alarm as actionable when physician also assesses as actionable) was 96.8% and specificity (assess alarm as nonactionable when physician also assesses as nonactionable) was 96.9%. We had to review 54 of 4674 alarms (1.2%) with an additional physician or nurse investigator to achieve consensus.
Characteristics of the 2445 alarms for clinical conditions are shown in Table 1. Only 12.9% of alarms in heart‐ and/or lung‐failure patients in the PICU were actionable, and only 1.0% of alarms in medical patients on a general inpatient ward were actionable.
Overall Response Times for Out‐of‐Room Alarms
We first evaluated response times without excluding alarms occurring prior to the 120‐minute mark. Of the 2445 clinical condition alarms, we excluded the 315 noncritical arrhythmia types from analysis of response time because they did not meet our definition of potentially critical alarms. Of the 2130 potentially critical alarms, 1185 (55.6%) occurred while neither the nurse nor any other clinician was in the patient's room. We proceeded to analyze the response time to these 1185 alarms (307 in the PICU and 878 on the ward). In the PICU, median response time was 3.3 minutes (IQR, 0.814.4). On the ward, median response time was 9.8 minutes (IQR, 3.222.4).
Response‐Time Association With Nonactionable Alarm Exposure
Next, we analyzed the association between response time to potentially critical alarms that occurred when the nurse was not in the patient's room and the number of nonactionable alarms occurring over the preceding 120‐minute window. This required excluding the alarms that occurred in the first 120 minutes of each session, leaving 647 alarms with eligible response times to evaluate the exposure between prior nonactionable alarm exposure and response time: 219 in the PICU and 428 on the ward. Kaplan‐Meier plots and tabulated response times demonstrated the incremental relationships between each nonactionable alarm exposure category in the observed data, with the effects most prominent as the Kaplan‐Meier plots diverged beyond the median (Figure 3 and Table 2). Excluding the extreme outlier patient had no effect on the results, because 1378 of the 1430 alarms occurred with the nurse present at the bedside, and only 2 of the remaining alarms were potentially critical.

| Observed Data | Accelerated Failure‐Time Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of Potentially Critical Alarms | Minutes Elapsed Until This Percentage of Alarms Was Responded to | Modeled Response Time, min | 95% CI, min | P Value* | ||||
| 50% (Median) | 75% | 90% | 95% | |||||
| ||||||||
| PICU | ||||||||
| 029 nonactionable alarms | 70 | 1.6 | 8.0 | 18.6 | 25.1 | 2.8 | 1.9‐3.8 | Reference |
| 3079 nonactionable alarms | 122 | 6.3 | 17.8 | 22.5 | 26.0 | 5.3 | 4.06.7 | 0.001 (vs 029) |
| 80+ nonactionable alarms | 27 | 16.0 | 28.4 | 32.0 | 33.1 | 8.5 | 4.312.7 | 0.009 (vs 029), 0.15 (vs 3079) |
| Ward | ||||||||
| 029 nonactionable alarms | 159 | 9.8 | 17.8 | 25.0 | 28.9 | 7.7 | 6.39.1 | Reference |
| 3079 nonactionable alarms | 211 | 11.6 | 22.4 | 44.6 | 63.2 | 11.5 | 9.613.3 | 0.001 (vs 029) |
| 80+ nonactionable alarms | 58 | 8.3 | 57.6 | 63.8 | 69.5 | 15.6 | 11.020.1 | 0.001 (vs 029), 0.09 (vs 3079) |
Accelerated failure‐time regressions revealed significant incremental increases in the modeled response time as the number of preceding nonactionable alarms increased in both the PICU and ward settings (Table 2).
Hawthorne‐like Effects
Four of the 36 nurses reported that they responded more quickly to monitor alarms because they knew they were being filmed.
DISCUSSION
Alarm fatigue has recently generated interest among nurses,[22] physicians,[23] regulatory bodies,[24] patient safety organizations,[25] and even attorneys,[26] despite a lack of prior evidence linking nonactionable alarm exposure to response time or other adverse patient‐relevant outcomes. This study's main findings were that (1) the vast majority of alarms were nonactionable, (2) response time to alarms occurring while the nurse was out of the room increased as the number of nonactionable alarms over the preceding 120 minutes increased. These findings may be explained by alarm fatigue.
Our results build upon the findings of other related studies. The nonactionable alarm proportions we found were similar to other pediatric studies, reporting greater than 90% nonactionable alarms.[1, 2] One other study has reported a relationship between alarm exposure and response time. In that study, Voepel‐Lewis and colleagues evaluated nurse responses to pulse oximetry desaturation alarms in adult orthopedic surgery patients using time‐stamp data from their monitor notification system.[27] They found that alarm response time was significantly longer for patients in the highest quartile of alarms compared to those in lower quartiles. Our study provides new data suggesting a relationship between nonactionable alarm exposure and nurse response time.
Our study has several limitations. First, as a preliminary study to investigate feasibility and possible association, the sample of patients and nurses was necessarily limited and did not permit adjustment for nurse‐ or patient‐level covariates. A multivariable analysis with a larger sample might provide insight into alternate explanations for these findings other than alarm fatigue, including measures of nurse workload and patient factors (such as age and illness severity). Additional factors that are not as easily measured can also contribute to the complex decision of when and how to respond to alarms.[28, 29] Second, nurses were aware that they were being video recorded as part of a study of nonactionable alarms, although they did not know the specific details of measurement. Although this lack of blinding might lead to a Hawthorne‐like effect, our positive results suggest that this effect, if present, did not fully obscure the association. Third, all sessions took place on weekdays during daytime hours, but effects of nonactionable alarms might vary by time and day. Finally, we suspect that when nurses experience critical alarms that require them to intervene and rescue a patient, their response times to that patient's alarms that occur later in their shift will be quicker due to a heightened concern for the alarm being actionable. We were unable to explore that relationship in this analysis because the number of critical alarms requiring intervention was very small. This is a topic of future study.
CONCLUSIONS
We identified an association between a nurse's prior exposure to nonactionable alarms and response time to future alarms. This finding is consistent with alarm fatigue, but requires further study to more clearly delineate other factors that might confound or modify that relationship.
Disclosures
This project was funded by the Health Research Formula Fund Grant 4100050891 from the Pennsylvania Department of Public Health Commonwealth Universal Research Enhancement Program (awarded to Drs. Keren and Bonafide). Dr. Bonafide is also supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Hospital physiologic monitors can alert clinicians to early signs of physiologic deterioration, and thus have great potential to save lives. However, monitors generate frequent alarms,[1, 2, 3, 4, 5, 6, 7, 8] and most are not relevant to the patient's safety (over 90% of pediatric intensive care unit (PICU)[1, 2] and over 70% of adult intensive care alarms).[5, 6] In psychology experiments, humans rapidly learn to ignore or respond more slowly to alarms when exposed to high false‐alarm rates, exhibiting alarm fatigue.[9, 10] In 2013, The Joint Commission named alarm fatigue the most common contributing factor to alarm‐related sentinel events in hospitals.[11, 12]
Although alarm fatigue has been implicated as a major threat to patient safety, little empirical data support its existence in hospitals. In this study, we aimed to determine if there was an association between nurses' recent exposure to nonactionable physiologic monitor alarms and their response time to future alarms for the same patients. This exploratory work was designed to inform future research in this area, acknowledging that the sample size would be too small for multivariable modeling.
METHODS
Study Definitions
The alarm classification scheme is shown in Figure 1. Note that, for clarity, we have intentionally avoided using the terms true and false alarms because their interpretations vary across studies and can be misleading.

Potentially Critical Alarm
A potentially critical alarm is any alarm for a clinical condition for which a timely response is important to determine if the alarm requires intervention to save the patient's life. This is based on the alarm type alone, including alarms for life‐threatening arrhythmias such as asystole and ventricular tachycardia, as well as alarms for vital signs outside the set limits. Supporting Table 1 in the online version of this article lists the breakdown of alarm types that we defined a priori as potentially and not potentially critical.
| PICU | Ward | |||||||
|---|---|---|---|---|---|---|---|---|
| Alarm type | No. | % of Total | % Valid | % Actionable | No. | % of Total | % Valid | % Actionable |
| ||||||||
| Oxygen saturation | 197 | 19.4 | 82.7 | 38.6 | 590 | 41.2 | 24.4 | 1.9 |
| Heart rate | 194 | 19.1 | 95.4 | 1.0 | 266 | 18.6 | 87.2 | 0.0 |
| Respiratory rate | 229 | 22.6 | 80.8 | 13.5 | 316 | 22.1 | 48.1 | 1.0 |
| Blood pressure | 259 | 25.5 | 83.8 | 5.8 | 11 | 0.8 | 72.7 | 0.0 |
| Critical arrhythmia | 1 | 0.1 | 0.0 | 0.0 | 4 | 0.3 | 0.0 | 0.0 |
| Noncritical arrhythmia | 71 | 7.0 | 2.8 | 0.0 | 244 | 17.1 | 8.6 | 0.0 |
| Central venous pressure | 49 | 4.8 | 0.0 | 0.0 | 0 | 0.0 | N/A | N/A |
| Exhaled carbon dioxide | 14 | 1.4 | 92.9 | 50.0 | 0 | 0.0 | N/A | N/A |
| Total | 1014 | 100.0 | 75.6 | 12.9 | 1,431 | 100.0 | 38.9 | 1.0 |
Valid Alarm
A valid alarm is any alarm that correctly identifies the physiologic status of the patient. Validity was based on waveform quality, lead signal strength indicators, and artifact conditions, referencing each monitor's operator's manual.
Actionable Alarm
An actionable alarm is any valid alarm for a clinical condition that either: (1) leads to a clinical intervention; (2) leads to a consultation with another clinician at the bedside (and thus visible on camera); or (3) is a situation that should have led to intervention or consultation, but the alarm was unwitnessed or misinterpreted by the staff at the bedside.
Nonactionable Alarm
An unactionable alarm is any alarm that does not meet the actionable definition above, including invalid alarms such as those caused by motion artifact, equipment/technical alarms, and alarms that are valid but nonactionable (nuisance alarms).[13]
Response Time
The response time is the time elapsed from when the alarm fired at the bedside to when the nurse entered the room or peered through a window or door, measured in seconds.
Setting and Subjects
We performed this study between August 2012 and July 2013 at a freestanding children's hospital. We evaluated nurses caring for 2 populations: (1) PICU patients with heart and/or lung failure (requiring inotropic support and/or invasive mechanical ventilation), and (2) medical patients on a general inpatient ward. Nurses caring for heart and/or lung failure patients in the PICU typically were assigned 1 to 2 total patients. Nurses on the medical ward typically were assigned 2 to 4 patients. We identified subjects from the population of nurses caring for eligible patients with parents available to provide in‐person consent in each setting. Our primary interest was to evaluate the association between nonactionable alarms and response time, and not to study the epidemiology of alarms in a random sample. Therefore, when alarm data were available prior to screening, we first approached nurses caring for patients in the top 25% of alarm rates for that unit over the preceding 4 hours. We identified preceding alarm rates using BedMasterEx (Excel Medical Electronics, Jupiter, FL).
Human Subjects Protection
This study was approved by the institutional review board of The Children's Hospital of Philadelphia. We obtained written in‐person consent from the patient's parent and the nurse subject. We obtained a Certificate of Confidentiality from the National Institutes of Health to further protect study participants.[14]
Monitoring Equipment
All patients in the PICU were monitored continuously using General Electric (GE) (Fairfield, CT) solar devices. All bed spaces on the wards include GE Dash monitors that are used if ordered. On the ward we studied, 30% to 50% of patients are typically monitored at any given time. In addition to alarming at the bedside, most clinical alarms also generated a text message sent to the nurse's wireless phone listing the room number and the word monitor. Messages did not provide any clinical information about the alarm or patient's status. There were no technicians reviewing alarms centrally.
Physicians used an order set to order monitoring, selecting 1 of 4 available preconfigured profiles: infant <6 months, infant 6 months to 1 year, child, and adult. The parameters for each age group are in Supporting Figure 1, available in the online version of this article. A physician order is required for a nurse to change the parameters. Participating in the study did not affect this workflow.
Primary Outcome
The primary outcome was the nurse's response time to potentially critical monitor alarms that occurred while neither they nor any other clinicians were in the patient's room.
Primary Exposure and Alarm Classification
The primary exposure was the number of nonactionable alarms in the same patient over the preceding 120 minutes (rolling and updated each minute). The alarm classification scheme is shown in Figure 1.
Due to technical limitations with obtaining time‐stamped alarm data from the different ventilators in use during the study period, we were unable to identify the causes of all ventilator alarms. Therefore, we included ventilator alarms that did not lead to clinical interventions as nonactionable alarm exposures, but we did not evaluate the response time to any ventilator alarms.
Data Collection
We combined video recordings with monitor time‐stamp data to evaluate the association between nonactionable alarms and the nurse's response time. Our detailed video recording and annotation methods have been published separately.[15] Briefly, we mounted up to 6 small video cameras in patients' rooms and recorded up to 6 hours per session. The cameras captured the monitor display, a wide view of the room, a close‐up view of the patient, and all windows and doors through which staff could visually assess the patient without entering the room.
Video Processing, Review, and Annotation
The first 5 video sessions were reviewed in a group training setting. Research assistants received instruction on how to determine alarm validity and actionability in accordance with the study definitions. Following the training period, the review workflow was as follows. First, a research assistant entered basic information and a preliminary assessment of the alarm's clinical validity and actionability into a REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN) database.[16] Later, a physician investigator secondarily reviewed all alarms and confirmed the assessments of the research assistants or, when disagreements occurred, discussed and reconciled the database. Alarms that remained unresolved after secondary review were flagged for review with an additional physician or nurse investigator in a team meeting.
Data Analysis
We summarized the patient and nurse subjects, the distributions of alarms, and the response times to potentially critical monitor alarms that occurred while neither the nurse nor any other clinicians were in the patient's room. We explored the data using plots of alarms and response times occurring within individual video sessions as well as with simple linear regression. Hypothesizing that any alarm fatigue effect would be strongest in the highest alarm patients, and having observed that alarms are distributed very unevenly across patients in both the PICU and ward, we made the decision not to use quartiles, but rather to form clinically meaningful categories. We also hypothesized that nurses might not exhibit alarm fatigue unless they were inundated with alarms. We thus divided the nonactionable alarm counts over the preceding 120 minutes into 3 categories: 0 to 29 alarms to represent a low to average alarm rate exhibited by the bottom 50% of the patients, 30 to 79 alarms to represent an elevated alarm rate, and 80+ alarms to represent an extremely high alarm rate exhibited by the top 5%. Because the exposure time was 120 minutes, we conducted the analysis on the alarms occurring after a nurse had been video recorded for at least 120 minutes.
We further evaluated the relationship between nonactionable alarms and nurse response time with Kaplan‐Meier plots by nonactionable alarm count category using the observed response‐time data. The Kaplan‐Meier plots compared response time across the nonactionable alarm exposure group, without any statistical modeling. A log‐rank test stratified by nurse evaluated whether the distributions of response time in the Kaplan‐Meier plots differed across the 3 alarm exposure groups, accounting for within‐nurse clustering.
Accelerated failure‐time regression based on the Weibull distribution then allowed us to compare response time across each alarm exposure group and provided confidence intervals. Accelerated failure‐time models are comparable to Cox models, but emphasize time to event rather than hazards.[17, 18] We determined that the Weibull distribution was suitable by evaluating smoothed hazard and log‐hazard plots, the confidence intervals of the shape parameters in the Weibull models that did not include 1, and by demonstrating that the Weibull model had better fit than an alternative (exponential) model using the likelihood‐ratio test (P<0.0001 for PICU, P=0.02 for ward). Due to the small sample size of nurses and patients, we could not adjust for nurse‐ or patient‐level covariates in the model. When comparing the nonactionable alarm exposure groups in the regression model (029 vs 3079, 3079 vs 80+, and 029 vs 80+), we Bonferroni corrected the critical P value for the 3 comparisons, for a critical P value of 0.05/3=0.0167.
Nurse Questionnaire
At the session's conclusion, nurses completed a questionnaire that included demographics and asked, Did you respond more quickly to monitor alarms during this study because you knew you were being filmed? to measure if nurses would report experiencing a Hawthorne‐like effect.[19, 20, 21]
RESULTS
We performed 40 sessions among 40 patients and 36 nurses over 210 hours. We performed 20 sessions in children with heart and/or lung failure in the PICU and 20 sessions in children on a general ward. Sessions took place on weekdays between 9:00 am and 6:00 pm. There were 3 occasions when we filmed 2 patients cared for by the same nurse at the same time.
Nurses were mostly female (94.4%) and had between 2 months and 28 years of experience (median, 4.8 years). Patients on the ward ranged from 5 days to 5.4 years old (median, 6 months). Patients in the PICU ranged from 5 months to 16 years old (median, 2.5 years). Among the PICU patients, 14 (70%) were receiving mechanical ventilation only, 3 (15%) were receiving vasopressors only, and 3 (15%) were receiving mechanical ventilation and vasopressors.
We observed 5070 alarms during the 40 sessions. We excluded 108 (2.1%) that occurred at the end of video recording sessions with the nurse absent from the room because the nurse's response could not be determined. Alarms per session ranged from 10 to 1430 (median, 75; interquartile range [IQR], 35138). We excluded the outlier PICU patient with 1430 alarms in 5 hours from the analysis to avoid the potential for biasing the results. Figure 2 depicts the data flow.

Following the 5 training sessions, research assistants independently reviewed and made preliminary assessments on 4674 alarms; these alarms were all secondarily reviewed by a physician. Using the physician reviewer as the gold standard, the research assistant's sensitivity (assess alarm as actionable when physician also assesses as actionable) was 96.8% and specificity (assess alarm as nonactionable when physician also assesses as nonactionable) was 96.9%. We had to review 54 of 4674 alarms (1.2%) with an additional physician or nurse investigator to achieve consensus.
Characteristics of the 2445 alarms for clinical conditions are shown in Table 1. Only 12.9% of alarms in heart‐ and/or lung‐failure patients in the PICU were actionable, and only 1.0% of alarms in medical patients on a general inpatient ward were actionable.
Overall Response Times for Out‐of‐Room Alarms
We first evaluated response times without excluding alarms occurring prior to the 120‐minute mark. Of the 2445 clinical condition alarms, we excluded the 315 noncritical arrhythmia types from analysis of response time because they did not meet our definition of potentially critical alarms. Of the 2130 potentially critical alarms, 1185 (55.6%) occurred while neither the nurse nor any other clinician was in the patient's room. We proceeded to analyze the response time to these 1185 alarms (307 in the PICU and 878 on the ward). In the PICU, median response time was 3.3 minutes (IQR, 0.814.4). On the ward, median response time was 9.8 minutes (IQR, 3.222.4).
Response‐Time Association With Nonactionable Alarm Exposure
Next, we analyzed the association between response time to potentially critical alarms that occurred when the nurse was not in the patient's room and the number of nonactionable alarms occurring over the preceding 120‐minute window. This required excluding the alarms that occurred in the first 120 minutes of each session, leaving 647 alarms with eligible response times to evaluate the exposure between prior nonactionable alarm exposure and response time: 219 in the PICU and 428 on the ward. Kaplan‐Meier plots and tabulated response times demonstrated the incremental relationships between each nonactionable alarm exposure category in the observed data, with the effects most prominent as the Kaplan‐Meier plots diverged beyond the median (Figure 3 and Table 2). Excluding the extreme outlier patient had no effect on the results, because 1378 of the 1430 alarms occurred with the nurse present at the bedside, and only 2 of the remaining alarms were potentially critical.

| Observed Data | Accelerated Failure‐Time Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of Potentially Critical Alarms | Minutes Elapsed Until This Percentage of Alarms Was Responded to | Modeled Response Time, min | 95% CI, min | P Value* | ||||
| 50% (Median) | 75% | 90% | 95% | |||||
| ||||||||
| PICU | ||||||||
| 029 nonactionable alarms | 70 | 1.6 | 8.0 | 18.6 | 25.1 | 2.8 | 1.9‐3.8 | Reference |
| 3079 nonactionable alarms | 122 | 6.3 | 17.8 | 22.5 | 26.0 | 5.3 | 4.06.7 | 0.001 (vs 029) |
| 80+ nonactionable alarms | 27 | 16.0 | 28.4 | 32.0 | 33.1 | 8.5 | 4.312.7 | 0.009 (vs 029), 0.15 (vs 3079) |
| Ward | ||||||||
| 029 nonactionable alarms | 159 | 9.8 | 17.8 | 25.0 | 28.9 | 7.7 | 6.39.1 | Reference |
| 3079 nonactionable alarms | 211 | 11.6 | 22.4 | 44.6 | 63.2 | 11.5 | 9.613.3 | 0.001 (vs 029) |
| 80+ nonactionable alarms | 58 | 8.3 | 57.6 | 63.8 | 69.5 | 15.6 | 11.020.1 | 0.001 (vs 029), 0.09 (vs 3079) |
Accelerated failure‐time regressions revealed significant incremental increases in the modeled response time as the number of preceding nonactionable alarms increased in both the PICU and ward settings (Table 2).
Hawthorne‐like Effects
Four of the 36 nurses reported that they responded more quickly to monitor alarms because they knew they were being filmed.
DISCUSSION
Alarm fatigue has recently generated interest among nurses,[22] physicians,[23] regulatory bodies,[24] patient safety organizations,[25] and even attorneys,[26] despite a lack of prior evidence linking nonactionable alarm exposure to response time or other adverse patient‐relevant outcomes. This study's main findings were that (1) the vast majority of alarms were nonactionable, (2) response time to alarms occurring while the nurse was out of the room increased as the number of nonactionable alarms over the preceding 120 minutes increased. These findings may be explained by alarm fatigue.
Our results build upon the findings of other related studies. The nonactionable alarm proportions we found were similar to other pediatric studies, reporting greater than 90% nonactionable alarms.[1, 2] One other study has reported a relationship between alarm exposure and response time. In that study, Voepel‐Lewis and colleagues evaluated nurse responses to pulse oximetry desaturation alarms in adult orthopedic surgery patients using time‐stamp data from their monitor notification system.[27] They found that alarm response time was significantly longer for patients in the highest quartile of alarms compared to those in lower quartiles. Our study provides new data suggesting a relationship between nonactionable alarm exposure and nurse response time.
Our study has several limitations. First, as a preliminary study to investigate feasibility and possible association, the sample of patients and nurses was necessarily limited and did not permit adjustment for nurse‐ or patient‐level covariates. A multivariable analysis with a larger sample might provide insight into alternate explanations for these findings other than alarm fatigue, including measures of nurse workload and patient factors (such as age and illness severity). Additional factors that are not as easily measured can also contribute to the complex decision of when and how to respond to alarms.[28, 29] Second, nurses were aware that they were being video recorded as part of a study of nonactionable alarms, although they did not know the specific details of measurement. Although this lack of blinding might lead to a Hawthorne‐like effect, our positive results suggest that this effect, if present, did not fully obscure the association. Third, all sessions took place on weekdays during daytime hours, but effects of nonactionable alarms might vary by time and day. Finally, we suspect that when nurses experience critical alarms that require them to intervene and rescue a patient, their response times to that patient's alarms that occur later in their shift will be quicker due to a heightened concern for the alarm being actionable. We were unable to explore that relationship in this analysis because the number of critical alarms requiring intervention was very small. This is a topic of future study.
CONCLUSIONS
We identified an association between a nurse's prior exposure to nonactionable alarms and response time to future alarms. This finding is consistent with alarm fatigue, but requires further study to more clearly delineate other factors that might confound or modify that relationship.
Disclosures
This project was funded by the Health Research Formula Fund Grant 4100050891 from the Pennsylvania Department of Public Health Commonwealth Universal Research Enhancement Program (awarded to Drs. Keren and Bonafide). Dr. Bonafide is also supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , . Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997;25(4):614–619.
- , , , , . Clinical evaluation of alarm efficiency in intensive care [in French]. Ann Fr Anesth Reanim. 2000;19:459–466.
- , , , . Reducing false alarms of intensive care online‐monitoring systems: an evaluation of two signal extraction algorithms. Comput Math Methods Med. 2011;2011:143480.
- , , , , , . Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med. 1999;25:1360–1366.
- , , . Improving alarm performance in the medical intensive care unit using delays and clinical context. Anesth Analg. 2009;108:1546–1552.
- , . Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19:28–34.
- , , , , , . Intensive care unit alarms—how many do we need? Crit Care Med. 2010;38:451–456.
- , , , . System operator response to warnings of danger: a laboratory investigation of the effects of the predictive value of a warning on human response time. J Exp Psychol Appl. 1995;1:19–33.
- , , . Human probability matching behaviour in response to alarms of varying reliability. Ergonomics. 1995;38:2300–2312.
- The Joint Commission. Sentinel event alert: medical device alarm safety in hospitals. 2013. Available at: http://www.jointcommission.org/sea_issue_50/. Accessed October 9, 2014.
- . Joint commission warns of alarm fatigue: multitude of alarms from monitoring devices problematic. JAMA. 2013;309(22):2315–2316.
- . Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–277.
- NIH Certificates of Confidentiality Kiosk. Available at: http://grants.nih.gov/grants/policy/coc/. Accessed April 21, 2014.
- , , , et al. Video methods for evaluating physiologic monitor alarms and alarm responses. Biomed Instrum Technol. 2014;48(3):220–230.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381.
- . Accelerated failure time and other parametric models. In: Modelling Survival Data in Medical Research. 2nd ed. Boca Raton, FL: Chapman 2003:197–229.
- , , , . Parametric models. In: An Introduction to Survival Analysis Using Stata, 3rd ed. College Station, TX: Stata Press; 2010:229–244.
- , . Management and the Worker. Cambridge, MA: Harvard University Press; 1939.
- . What happened at Hawthorne? Science. 1974;183(4128):922–932.
- , , , , . Validation of the Work Observation Method By Activity Timing (WOMBAT) method of conducting time‐motion observations in critical care settings: an observational study. BMC Med Inf Decis Mak. 2011;11:32.
- , . Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378–386.
- , . Redesigning hospital alarms for patient safety: alarmed and potentially dangerous. JAMA. 2014;311(12):1199–1200.
- The Joint Commission. The Joint Commission announces 2014 National Patient Safety Goal. Jt Comm Perspect. 2013;33:1–4.
- Top 10 health technology hazards for 2014. Health Devices. 2013;42(11):354–380.
- My Philly Lawyer. Medical malpractice: alarm fatigue threatens patient safety. 2014. Available at: http://www.myphillylawyer.com/Resources/Legal-Articles/Medical-Malpractice-Alarm-Fatigue-Threatens-Patient-Safety.shtml. Accessed April 4, 2014.
- , , , et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud. 2013;50(10):1351–1358.
- , , , , . A description of nurses' decision‐making in managing electrocardiographic monitor alarms [published online ahead of print May 10, 2014]. J Clin Nurs. doi:10.1111/jocn.12625.
- . Nurses' response to frequency and types of electrocardiography alarms in a non‐critical care setting: a descriptive study. Int J Nurs Stud. 2014;51(2):190–197.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , . Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997;25(4):614–619.
- , , , , . Clinical evaluation of alarm efficiency in intensive care [in French]. Ann Fr Anesth Reanim. 2000;19:459–466.
- , , , . Reducing false alarms of intensive care online‐monitoring systems: an evaluation of two signal extraction algorithms. Comput Math Methods Med. 2011;2011:143480.
- , , , , , . Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med. 1999;25:1360–1366.
- , , . Improving alarm performance in the medical intensive care unit using delays and clinical context. Anesth Analg. 2009;108:1546–1552.
- , . Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19:28–34.
- , , , , , . Intensive care unit alarms—how many do we need? Crit Care Med. 2010;38:451–456.
- , , , . System operator response to warnings of danger: a laboratory investigation of the effects of the predictive value of a warning on human response time. J Exp Psychol Appl. 1995;1:19–33.
- , , . Human probability matching behaviour in response to alarms of varying reliability. Ergonomics. 1995;38:2300–2312.
- The Joint Commission. Sentinel event alert: medical device alarm safety in hospitals. 2013. Available at: http://www.jointcommission.org/sea_issue_50/. Accessed October 9, 2014.
- . Joint commission warns of alarm fatigue: multitude of alarms from monitoring devices problematic. JAMA. 2013;309(22):2315–2316.
- . Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–277.
- NIH Certificates of Confidentiality Kiosk. Available at: http://grants.nih.gov/grants/policy/coc/. Accessed April 21, 2014.
- , , , et al. Video methods for evaluating physiologic monitor alarms and alarm responses. Biomed Instrum Technol. 2014;48(3):220–230.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381.
- . Accelerated failure time and other parametric models. In: Modelling Survival Data in Medical Research. 2nd ed. Boca Raton, FL: Chapman 2003:197–229.
- , , , . Parametric models. In: An Introduction to Survival Analysis Using Stata, 3rd ed. College Station, TX: Stata Press; 2010:229–244.
- , . Management and the Worker. Cambridge, MA: Harvard University Press; 1939.
- . What happened at Hawthorne? Science. 1974;183(4128):922–932.
- , , , , . Validation of the Work Observation Method By Activity Timing (WOMBAT) method of conducting time‐motion observations in critical care settings: an observational study. BMC Med Inf Decis Mak. 2011;11:32.
- , . Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378–386.
- , . Redesigning hospital alarms for patient safety: alarmed and potentially dangerous. JAMA. 2014;311(12):1199–1200.
- The Joint Commission. The Joint Commission announces 2014 National Patient Safety Goal. Jt Comm Perspect. 2013;33:1–4.
- Top 10 health technology hazards for 2014. Health Devices. 2013;42(11):354–380.
- My Philly Lawyer. Medical malpractice: alarm fatigue threatens patient safety. 2014. Available at: http://www.myphillylawyer.com/Resources/Legal-Articles/Medical-Malpractice-Alarm-Fatigue-Threatens-Patient-Safety.shtml. Accessed April 4, 2014.
- , , , et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud. 2013;50(10):1351–1358.
- , , , , . A description of nurses' decision‐making in managing electrocardiographic monitor alarms [published online ahead of print May 10, 2014]. J Clin Nurs. doi:10.1111/jocn.12625.
- . Nurses' response to frequency and types of electrocardiography alarms in a non‐critical care setting: a descriptive study. Int J Nurs Stud. 2014;51(2):190–197.
© 2015 Society of Hospital Medicine
Code Status Documentation
In the hospital, cardiopulmonary resuscitation (CPR) is the default treatment for a patient who suffers a cardiac arrest. Clinician assessment of patient preferences regarding resuscitation, with appropriate documentation in the medical record, is therefore essential for patients who do not wish to be resuscitated.[1] In addition, given frequent patient handoffs between physicians, consistent documentation of patient preferences is critical.[2] Unfortunately, multiple deficiencies in the quality of code status documentation have been identified in prior work.[3, 4] In this issue of the Journal of Hospital Medicine, Weinerman and colleagues[5] build on this literature by not only evaluating the completeness of code status documentation in multiple documentation sites, but also its consistency.
In this Canadian multihospital study, the authors found that only 38 of the 187 patients (20%) admitted to 1 of 4 medicine services had complete and consistent documentation of code status. Even more worrisome is that two‐thirds of the patients had inconsistent code status documentation. Although most of these inconsistencies involved missing information in 1 of the 5 sites of documentation (progress note, physician order, electronic resident sign‐out lists, nursing‐care plan, and do‐not‐resuscitate [DNR] face sheet), 31% were deemed clinically significant (eg, DNR in 1 source and full code in another). Such inconsistent documentation represents a serious threat to patient safety, and highlights the need for interventions aimed at improving the quality and reliability of code status documentation.
The authors identified 71 cases where code status documentation in progress notes was missing or inconsistent with documentation in other sites. Sixty of these notes lacked mention of a preference for full code status, 10 lacked documentation of DNR status, and 1 note incorrectly documented full code rather than DNR status. Interpretation of these findings requires consensus on whether the progress note is an appropriate location for code status documentation. With the evolution of the electronic medical record, the role of the progress note has changed, and unfortunately, these notes have become a lengthy chronicle of a patient's hospital course that includes all clinical data, medical problems, and an array of bottom‐of‐the‐list items such as code status. Information is easily added, but rarely removed, and what remains often goes unedited even for high‐stakes issues such as code status. Given the potential for copying and pasting of progress notes day after day, it is critical that clinicians periodically review the code status documented in the patient's notes and update this information as those preferences change. One solution that may minimize the potential for inaccurate documentation in progress notes is for institutions to utilize a separate note for code status documentation that the clinician fills out following any code status discussion. Having this note clearly labeled (eg, Code Status Note) and in a universal place within the electronic record may provide a reliable and efficient way for both physicians and nurses to identify a patient's preferences, while minimizing the inclusion of repetitive information in daily notes. Furthermore, if entered into a discrete field within the electronic record, this information could then autopopulate other sites (eg, sign‐out, nursing forms), thereby maintaining consistency. Use of note templates can provide a way to then help standardize the quality of information that is included in this type of code status note.
An alternate solution that may minimize the potential for inaccurate implementation of code status preferences is to focus on the fact that they are orders. As this study highlights, there is a need to improve both the completeness and consistency of code status documentation and, to this end, orders such as the Medical Orders for Life‐Sustaining Treatment (MOLST) or Physician Orders for Life‐Sustaining Treatment (POLST) may help.[6] Not only do these orders expand upon resuscitation preferences to include broader preferences for treatment in the context of serious illness, but they are also meant to serve as a standard way to document patient care preferences across healthcare settings. Although the MOLST and POLST primarily aim to translate patient preferences into medical orders to be followed outside of the hospital, their implementation into the electronic medical record may provide a more consistent way to document patient preferences in the hospital as well.
Although many studies have identified the need to improve the quality of code status discussions,[7, 8, 9, 10] the work by Weinerman and colleagues reminds us that attention to documentation is also critical. Ensuring that the electronic medical record contains documentation of the patient's resuscitation preferences and overall goals of care, and that this information can be found easily and reliably by physicians and nurses, should drive future quality improvement and research in this area.
Disclosure
Nothing to report.
- , , , et al. Physician understanding of patient resuscitation preferences: insights and clinical implications. J Am Geriatr Soc. 2000;48(5 suppl):S44–S51.
- , , , et al. The patient handoff: a comprehensive curricular blueprint for resident education to improve continuity of care. Acad Med. 2012;87(4):411–418.
- , , , . Documentation quality of inpatient code status discussions. J Pain Symptom Manage. 2014;48(4):632–638.
- , , , et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437–445.
- , , , , , . Frequency and clinical relevance of inconsistent code status documentation. J Hosp Med. 2015;10(8):491–496.
- , , . Use of the Physician Orders for Life‐Sustaining Treatment Program in the clinical setting: a systematic review of the literature. J Am Geriatr Soc. 2015;63(2):341–350.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. JAMA. 1995;274(20):1591–1598.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Code status discussions: agreement between internal medicine residents and hospitalized patients. Teach Learn Med. 2010;22(4):251–256.
In the hospital, cardiopulmonary resuscitation (CPR) is the default treatment for a patient who suffers a cardiac arrest. Clinician assessment of patient preferences regarding resuscitation, with appropriate documentation in the medical record, is therefore essential for patients who do not wish to be resuscitated.[1] In addition, given frequent patient handoffs between physicians, consistent documentation of patient preferences is critical.[2] Unfortunately, multiple deficiencies in the quality of code status documentation have been identified in prior work.[3, 4] In this issue of the Journal of Hospital Medicine, Weinerman and colleagues[5] build on this literature by not only evaluating the completeness of code status documentation in multiple documentation sites, but also its consistency.
In this Canadian multihospital study, the authors found that only 38 of the 187 patients (20%) admitted to 1 of 4 medicine services had complete and consistent documentation of code status. Even more worrisome is that two‐thirds of the patients had inconsistent code status documentation. Although most of these inconsistencies involved missing information in 1 of the 5 sites of documentation (progress note, physician order, electronic resident sign‐out lists, nursing‐care plan, and do‐not‐resuscitate [DNR] face sheet), 31% were deemed clinically significant (eg, DNR in 1 source and full code in another). Such inconsistent documentation represents a serious threat to patient safety, and highlights the need for interventions aimed at improving the quality and reliability of code status documentation.
The authors identified 71 cases where code status documentation in progress notes was missing or inconsistent with documentation in other sites. Sixty of these notes lacked mention of a preference for full code status, 10 lacked documentation of DNR status, and 1 note incorrectly documented full code rather than DNR status. Interpretation of these findings requires consensus on whether the progress note is an appropriate location for code status documentation. With the evolution of the electronic medical record, the role of the progress note has changed, and unfortunately, these notes have become a lengthy chronicle of a patient's hospital course that includes all clinical data, medical problems, and an array of bottom‐of‐the‐list items such as code status. Information is easily added, but rarely removed, and what remains often goes unedited even for high‐stakes issues such as code status. Given the potential for copying and pasting of progress notes day after day, it is critical that clinicians periodically review the code status documented in the patient's notes and update this information as those preferences change. One solution that may minimize the potential for inaccurate documentation in progress notes is for institutions to utilize a separate note for code status documentation that the clinician fills out following any code status discussion. Having this note clearly labeled (eg, Code Status Note) and in a universal place within the electronic record may provide a reliable and efficient way for both physicians and nurses to identify a patient's preferences, while minimizing the inclusion of repetitive information in daily notes. Furthermore, if entered into a discrete field within the electronic record, this information could then autopopulate other sites (eg, sign‐out, nursing forms), thereby maintaining consistency. Use of note templates can provide a way to then help standardize the quality of information that is included in this type of code status note.
An alternate solution that may minimize the potential for inaccurate implementation of code status preferences is to focus on the fact that they are orders. As this study highlights, there is a need to improve both the completeness and consistency of code status documentation and, to this end, orders such as the Medical Orders for Life‐Sustaining Treatment (MOLST) or Physician Orders for Life‐Sustaining Treatment (POLST) may help.[6] Not only do these orders expand upon resuscitation preferences to include broader preferences for treatment in the context of serious illness, but they are also meant to serve as a standard way to document patient care preferences across healthcare settings. Although the MOLST and POLST primarily aim to translate patient preferences into medical orders to be followed outside of the hospital, their implementation into the electronic medical record may provide a more consistent way to document patient preferences in the hospital as well.
Although many studies have identified the need to improve the quality of code status discussions,[7, 8, 9, 10] the work by Weinerman and colleagues reminds us that attention to documentation is also critical. Ensuring that the electronic medical record contains documentation of the patient's resuscitation preferences and overall goals of care, and that this information can be found easily and reliably by physicians and nurses, should drive future quality improvement and research in this area.
Disclosure
Nothing to report.
In the hospital, cardiopulmonary resuscitation (CPR) is the default treatment for a patient who suffers a cardiac arrest. Clinician assessment of patient preferences regarding resuscitation, with appropriate documentation in the medical record, is therefore essential for patients who do not wish to be resuscitated.[1] In addition, given frequent patient handoffs between physicians, consistent documentation of patient preferences is critical.[2] Unfortunately, multiple deficiencies in the quality of code status documentation have been identified in prior work.[3, 4] In this issue of the Journal of Hospital Medicine, Weinerman and colleagues[5] build on this literature by not only evaluating the completeness of code status documentation in multiple documentation sites, but also its consistency.
In this Canadian multihospital study, the authors found that only 38 of the 187 patients (20%) admitted to 1 of 4 medicine services had complete and consistent documentation of code status. Even more worrisome is that two‐thirds of the patients had inconsistent code status documentation. Although most of these inconsistencies involved missing information in 1 of the 5 sites of documentation (progress note, physician order, electronic resident sign‐out lists, nursing‐care plan, and do‐not‐resuscitate [DNR] face sheet), 31% were deemed clinically significant (eg, DNR in 1 source and full code in another). Such inconsistent documentation represents a serious threat to patient safety, and highlights the need for interventions aimed at improving the quality and reliability of code status documentation.
The authors identified 71 cases where code status documentation in progress notes was missing or inconsistent with documentation in other sites. Sixty of these notes lacked mention of a preference for full code status, 10 lacked documentation of DNR status, and 1 note incorrectly documented full code rather than DNR status. Interpretation of these findings requires consensus on whether the progress note is an appropriate location for code status documentation. With the evolution of the electronic medical record, the role of the progress note has changed, and unfortunately, these notes have become a lengthy chronicle of a patient's hospital course that includes all clinical data, medical problems, and an array of bottom‐of‐the‐list items such as code status. Information is easily added, but rarely removed, and what remains often goes unedited even for high‐stakes issues such as code status. Given the potential for copying and pasting of progress notes day after day, it is critical that clinicians periodically review the code status documented in the patient's notes and update this information as those preferences change. One solution that may minimize the potential for inaccurate documentation in progress notes is for institutions to utilize a separate note for code status documentation that the clinician fills out following any code status discussion. Having this note clearly labeled (eg, Code Status Note) and in a universal place within the electronic record may provide a reliable and efficient way for both physicians and nurses to identify a patient's preferences, while minimizing the inclusion of repetitive information in daily notes. Furthermore, if entered into a discrete field within the electronic record, this information could then autopopulate other sites (eg, sign‐out, nursing forms), thereby maintaining consistency. Use of note templates can provide a way to then help standardize the quality of information that is included in this type of code status note.
An alternate solution that may minimize the potential for inaccurate implementation of code status preferences is to focus on the fact that they are orders. As this study highlights, there is a need to improve both the completeness and consistency of code status documentation and, to this end, orders such as the Medical Orders for Life‐Sustaining Treatment (MOLST) or Physician Orders for Life‐Sustaining Treatment (POLST) may help.[6] Not only do these orders expand upon resuscitation preferences to include broader preferences for treatment in the context of serious illness, but they are also meant to serve as a standard way to document patient care preferences across healthcare settings. Although the MOLST and POLST primarily aim to translate patient preferences into medical orders to be followed outside of the hospital, their implementation into the electronic medical record may provide a more consistent way to document patient preferences in the hospital as well.
Although many studies have identified the need to improve the quality of code status discussions,[7, 8, 9, 10] the work by Weinerman and colleagues reminds us that attention to documentation is also critical. Ensuring that the electronic medical record contains documentation of the patient's resuscitation preferences and overall goals of care, and that this information can be found easily and reliably by physicians and nurses, should drive future quality improvement and research in this area.
Disclosure
Nothing to report.
- , , , et al. Physician understanding of patient resuscitation preferences: insights and clinical implications. J Am Geriatr Soc. 2000;48(5 suppl):S44–S51.
- , , , et al. The patient handoff: a comprehensive curricular blueprint for resident education to improve continuity of care. Acad Med. 2012;87(4):411–418.
- , , , . Documentation quality of inpatient code status discussions. J Pain Symptom Manage. 2014;48(4):632–638.
- , , , et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437–445.
- , , , , , . Frequency and clinical relevance of inconsistent code status documentation. J Hosp Med. 2015;10(8):491–496.
- , , . Use of the Physician Orders for Life‐Sustaining Treatment Program in the clinical setting: a systematic review of the literature. J Am Geriatr Soc. 2015;63(2):341–350.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. JAMA. 1995;274(20):1591–1598.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Code status discussions: agreement between internal medicine residents and hospitalized patients. Teach Learn Med. 2010;22(4):251–256.
- , , , et al. Physician understanding of patient resuscitation preferences: insights and clinical implications. J Am Geriatr Soc. 2000;48(5 suppl):S44–S51.
- , , , et al. The patient handoff: a comprehensive curricular blueprint for resident education to improve continuity of care. Acad Med. 2012;87(4):411–418.
- , , , . Documentation quality of inpatient code status discussions. J Pain Symptom Manage. 2014;48(4):632–638.
- , , , et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437–445.
- , , , , , . Frequency and clinical relevance of inconsistent code status documentation. J Hosp Med. 2015;10(8):491–496.
- , , . Use of the Physician Orders for Life‐Sustaining Treatment Program in the clinical setting: a systematic review of the literature. J Am Geriatr Soc. 2015;63(2):341–350.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. JAMA. 1995;274(20):1591–1598.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Code status discussions: agreement between internal medicine residents and hospitalized patients. Teach Learn Med. 2010;22(4):251–256.
HHS secretary tells how to combat drug abuse
More people are dying of drug overdoses in the United States than in car crashes, Nicholas Garlow writes in a blog entry for the U.S. Department of Health & Human Services.
Sylvia Mathews Burwell, secretary of the HHS, announced ways to combat opioid abuse during a speech at the 4th annual National Rx Drug Abuse Summit in Atlanta.
The strategies Ms. Burwell cited are:
• Provide the training, tools, and educational resources that health care professionals need to make more informed prescribing decisions.
• Increase the use of naloxone, a drug that can reverse opioid overdose*.
• Use medication-assisted treatment to help lift people from opioid addiction.
Drug overdose is the leading cause of injury death in the country. In fact, the number of drug overdoses resulting in deaths has increased fivefold since 1980, Mr. Garlow writes.
*Correction, 4/16/2015: An earlier version of this article misstated naloxone's indication.
More people are dying of drug overdoses in the United States than in car crashes, Nicholas Garlow writes in a blog entry for the U.S. Department of Health & Human Services.
Sylvia Mathews Burwell, secretary of the HHS, announced ways to combat opioid abuse during a speech at the 4th annual National Rx Drug Abuse Summit in Atlanta.
The strategies Ms. Burwell cited are:
• Provide the training, tools, and educational resources that health care professionals need to make more informed prescribing decisions.
• Increase the use of naloxone, a drug that can reverse opioid overdose*.
• Use medication-assisted treatment to help lift people from opioid addiction.
Drug overdose is the leading cause of injury death in the country. In fact, the number of drug overdoses resulting in deaths has increased fivefold since 1980, Mr. Garlow writes.
*Correction, 4/16/2015: An earlier version of this article misstated naloxone's indication.
More people are dying of drug overdoses in the United States than in car crashes, Nicholas Garlow writes in a blog entry for the U.S. Department of Health & Human Services.
Sylvia Mathews Burwell, secretary of the HHS, announced ways to combat opioid abuse during a speech at the 4th annual National Rx Drug Abuse Summit in Atlanta.
The strategies Ms. Burwell cited are:
• Provide the training, tools, and educational resources that health care professionals need to make more informed prescribing decisions.
• Increase the use of naloxone, a drug that can reverse opioid overdose*.
• Use medication-assisted treatment to help lift people from opioid addiction.
Drug overdose is the leading cause of injury death in the country. In fact, the number of drug overdoses resulting in deaths has increased fivefold since 1980, Mr. Garlow writes.
*Correction, 4/16/2015: An earlier version of this article misstated naloxone's indication.
Ablation during mitral valve surgery offers up mixed results
SAN DIEGO – Surgical ablation of atrial fibrillation at the time of mitral valve surgery provides significantly greater rhythm control than mitral valve surgery alone, a study showed.
Freedom from atrial fibrillation (AF) at both 6 months and 1 year was 63% in patients undergoing mitral valve surgery (MVS) plus ablation and 29% in those undergoing MVS alone, a statistically significant difference.
However, patients who had ablation plus MVS were 2.5 times more likely to have a permanent pacemaker implanted than were those who had MVS alone, at 21.5% and 8.1%, respectively, also a significant difference.
Ablation did not increase mortality or major adverse cardiac or cerebrovascular events, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Preoperative AF is present in up to 50% of patients undergoing mitral valve operations and is associated with an increased risk of death and stroke.
The study enrolled 260 relatively elderly patients (mean age 69 years) with AF that was persistent (non–self-terminating for at least 7 days) or long-standing persistent (continuous for at least a year), in addition to mitral valve disease. A total of 133 patients were randomly assigned to MVS plus ablation and 127 to MVS alone. The ablation group was further randomized to pulmonary vein isolation or a biatrial maze procedure; all underwent closure of the left atrial appendage.
There was no significant difference in freedom from AF at 6 months and 1 year between patients who had pulmonary vein isolation or a biatrial maze procedure, at 61% and 66%, respectively, said Dr. Gillinov, a cardiac surgeon at Cleveland Clinic.
One-year mortality was similar among all patients undergoing MVS plus ablation vs. MVS alone, at 6.8% and 8.7%.
The two groups also had similar Short Form-12 questionnaire scores for physical function and mental function, although AF occurring at least once daily was significantly less common with ablation, at 19.8%, compared with 45.2% in the MVS-alone patients, he said.
The heart rhythm endpoint was “stringent,” with 3-day Holter monitors obtained at both 6 and 12 months and repeat ablation procedures and death considered treatment failures, Dr. Gillinov said.
He acknowledged that 20% of patients did not have data for the primary endpoint and that the endpoint was not a clinical one, but said a trial with mortality or stroke as the endpoint would require more than 1,000 patients and many years follow-up.
Regarding whether ablation should now be performed routinely, “the glass is half full or half empty,” remarked discussant Dr. Bernard Gersh of Mayo Clinic in Rochester, Minn. “On one hand, you have shown less atrial fibrillation [with ablation], but no effect on quality of life, and the price to be paid was a higher rate of pacemaker implantation,” he said.
The pacemaker implantation rate was higher than expected – 17% in-hospital – and does represent a potential cost, but he would routinely do a maze procedure, Dr. Gillinov said.
Discussant Dr. Alice Jacobs of the Cardiovascular Center at Boston Medical Center, said she expected Dr. Gillinov to say the procedure should not be used in everyone given the lack of benefit in stroke, probably because they tied off the left atrium appendage, and the increase in pacemaker implantations.
About half of the pacemaker implantations were due to atrioventricular block, possibly a consequence of the valve surgery, and one-third to sinus-node dysfunction, which is common in elderly patients, Dr. Gillinov explained.
The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
SAN DIEGO – Surgical ablation of atrial fibrillation at the time of mitral valve surgery provides significantly greater rhythm control than mitral valve surgery alone, a study showed.
Freedom from atrial fibrillation (AF) at both 6 months and 1 year was 63% in patients undergoing mitral valve surgery (MVS) plus ablation and 29% in those undergoing MVS alone, a statistically significant difference.
However, patients who had ablation plus MVS were 2.5 times more likely to have a permanent pacemaker implanted than were those who had MVS alone, at 21.5% and 8.1%, respectively, also a significant difference.
Ablation did not increase mortality or major adverse cardiac or cerebrovascular events, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Preoperative AF is present in up to 50% of patients undergoing mitral valve operations and is associated with an increased risk of death and stroke.
The study enrolled 260 relatively elderly patients (mean age 69 years) with AF that was persistent (non–self-terminating for at least 7 days) or long-standing persistent (continuous for at least a year), in addition to mitral valve disease. A total of 133 patients were randomly assigned to MVS plus ablation and 127 to MVS alone. The ablation group was further randomized to pulmonary vein isolation or a biatrial maze procedure; all underwent closure of the left atrial appendage.
There was no significant difference in freedom from AF at 6 months and 1 year between patients who had pulmonary vein isolation or a biatrial maze procedure, at 61% and 66%, respectively, said Dr. Gillinov, a cardiac surgeon at Cleveland Clinic.
One-year mortality was similar among all patients undergoing MVS plus ablation vs. MVS alone, at 6.8% and 8.7%.
The two groups also had similar Short Form-12 questionnaire scores for physical function and mental function, although AF occurring at least once daily was significantly less common with ablation, at 19.8%, compared with 45.2% in the MVS-alone patients, he said.
The heart rhythm endpoint was “stringent,” with 3-day Holter monitors obtained at both 6 and 12 months and repeat ablation procedures and death considered treatment failures, Dr. Gillinov said.
He acknowledged that 20% of patients did not have data for the primary endpoint and that the endpoint was not a clinical one, but said a trial with mortality or stroke as the endpoint would require more than 1,000 patients and many years follow-up.
Regarding whether ablation should now be performed routinely, “the glass is half full or half empty,” remarked discussant Dr. Bernard Gersh of Mayo Clinic in Rochester, Minn. “On one hand, you have shown less atrial fibrillation [with ablation], but no effect on quality of life, and the price to be paid was a higher rate of pacemaker implantation,” he said.
The pacemaker implantation rate was higher than expected – 17% in-hospital – and does represent a potential cost, but he would routinely do a maze procedure, Dr. Gillinov said.
Discussant Dr. Alice Jacobs of the Cardiovascular Center at Boston Medical Center, said she expected Dr. Gillinov to say the procedure should not be used in everyone given the lack of benefit in stroke, probably because they tied off the left atrium appendage, and the increase in pacemaker implantations.
About half of the pacemaker implantations were due to atrioventricular block, possibly a consequence of the valve surgery, and one-third to sinus-node dysfunction, which is common in elderly patients, Dr. Gillinov explained.
The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
SAN DIEGO – Surgical ablation of atrial fibrillation at the time of mitral valve surgery provides significantly greater rhythm control than mitral valve surgery alone, a study showed.
Freedom from atrial fibrillation (AF) at both 6 months and 1 year was 63% in patients undergoing mitral valve surgery (MVS) plus ablation and 29% in those undergoing MVS alone, a statistically significant difference.
However, patients who had ablation plus MVS were 2.5 times more likely to have a permanent pacemaker implanted than were those who had MVS alone, at 21.5% and 8.1%, respectively, also a significant difference.
Ablation did not increase mortality or major adverse cardiac or cerebrovascular events, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Preoperative AF is present in up to 50% of patients undergoing mitral valve operations and is associated with an increased risk of death and stroke.
The study enrolled 260 relatively elderly patients (mean age 69 years) with AF that was persistent (non–self-terminating for at least 7 days) or long-standing persistent (continuous for at least a year), in addition to mitral valve disease. A total of 133 patients were randomly assigned to MVS plus ablation and 127 to MVS alone. The ablation group was further randomized to pulmonary vein isolation or a biatrial maze procedure; all underwent closure of the left atrial appendage.
There was no significant difference in freedom from AF at 6 months and 1 year between patients who had pulmonary vein isolation or a biatrial maze procedure, at 61% and 66%, respectively, said Dr. Gillinov, a cardiac surgeon at Cleveland Clinic.
One-year mortality was similar among all patients undergoing MVS plus ablation vs. MVS alone, at 6.8% and 8.7%.
The two groups also had similar Short Form-12 questionnaire scores for physical function and mental function, although AF occurring at least once daily was significantly less common with ablation, at 19.8%, compared with 45.2% in the MVS-alone patients, he said.
The heart rhythm endpoint was “stringent,” with 3-day Holter monitors obtained at both 6 and 12 months and repeat ablation procedures and death considered treatment failures, Dr. Gillinov said.
He acknowledged that 20% of patients did not have data for the primary endpoint and that the endpoint was not a clinical one, but said a trial with mortality or stroke as the endpoint would require more than 1,000 patients and many years follow-up.
Regarding whether ablation should now be performed routinely, “the glass is half full or half empty,” remarked discussant Dr. Bernard Gersh of Mayo Clinic in Rochester, Minn. “On one hand, you have shown less atrial fibrillation [with ablation], but no effect on quality of life, and the price to be paid was a higher rate of pacemaker implantation,” he said.
The pacemaker implantation rate was higher than expected – 17% in-hospital – and does represent a potential cost, but he would routinely do a maze procedure, Dr. Gillinov said.
Discussant Dr. Alice Jacobs of the Cardiovascular Center at Boston Medical Center, said she expected Dr. Gillinov to say the procedure should not be used in everyone given the lack of benefit in stroke, probably because they tied off the left atrium appendage, and the increase in pacemaker implantations.
About half of the pacemaker implantations were due to atrioventricular block, possibly a consequence of the valve surgery, and one-third to sinus-node dysfunction, which is common in elderly patients, Dr. Gillinov explained.
The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
AT ACC 15
Key clinical point: Surgical ablation of atrial fibrillation during mitral valve surgery decreases AF at 6 months and 1 year, but increases pacemaker implantations.
Major finding: Freedom from AF at both 6 months and 1 year was 63% with mitral valve surgery plus ablation and 29% for MVS alone.
Data source: Prospective, randomized study in 260 patients with persistent or longstanding persistent AF who required mitral valve surgery.
Disclosures: The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
Three factors boost dabigatran adherence
Patient adherence to dabigatran therapy varied enormously in a nationwide study of pharmacy data, and three factors were identified that markedly enhanced adherence, according to a report published online April 14 in JAMA.
After research suggested that adherence to dabigatran was suboptimal among patients taking the drug for nonvalvular atrial fibrillation, investigators explored variations in adherence across thousands of sites using a Veterans Health Administration pharmacy database. They found that among 4,863 patients filling dabigatran prescriptions at 67 pharmacies during a 2-year period, adherence ranged from a low of 42% to a high of 93%, said Dr. Supriya Shore, a cardiology fellow at Emory University, Atlanta, and her associates.
The single most important factor that influenced dabigatran adherence was appropriate patient selection before dispensing the drug. This was defined as the pharmacist assessing the indication for treatment and ruling out contraindications after the physician ordered the prescription, as well as checking the patient’s adherence to other medications. Pharmacist monitoring of dabigatran use and adverse events, either alone or in collaboration with the treating clinician, also boosted adherence.
In addition, pharmacists working with clinicians to identify and address nonadherence also enhanced patient adherence. Prescribing physicians may not be able to routinely monitor adherence because of their large workload and limited time during clinic visits. Having a pharmacist do so mitigated patients’ tendency to stop taking the drug when minor adverse effects developed, the investigators reported (JAMA 2015 April 14 [doi:101001/jama.2015.2761]).
“These findings suggest that such site-level practices provide modifiable targets to improve patient adherence to dabigatran, as opposed to patient characteristics that frequently cannot be modified,” Dr. Shore and her associates wrote.
This study was funded in part by VA Health Services Research & Development, an American Heart Association National Scientist Development Grant, and a Gilead Sciences Cardiovascular Research Scholars Program award. Dr. Shore reported having no financial disclosures; one of her associates reported serving as a consultant to Precision Health Economics, Medtronic, and St. Jude Medical.
Patient adherence to dabigatran therapy varied enormously in a nationwide study of pharmacy data, and three factors were identified that markedly enhanced adherence, according to a report published online April 14 in JAMA.
After research suggested that adherence to dabigatran was suboptimal among patients taking the drug for nonvalvular atrial fibrillation, investigators explored variations in adherence across thousands of sites using a Veterans Health Administration pharmacy database. They found that among 4,863 patients filling dabigatran prescriptions at 67 pharmacies during a 2-year period, adherence ranged from a low of 42% to a high of 93%, said Dr. Supriya Shore, a cardiology fellow at Emory University, Atlanta, and her associates.
The single most important factor that influenced dabigatran adherence was appropriate patient selection before dispensing the drug. This was defined as the pharmacist assessing the indication for treatment and ruling out contraindications after the physician ordered the prescription, as well as checking the patient’s adherence to other medications. Pharmacist monitoring of dabigatran use and adverse events, either alone or in collaboration with the treating clinician, also boosted adherence.
In addition, pharmacists working with clinicians to identify and address nonadherence also enhanced patient adherence. Prescribing physicians may not be able to routinely monitor adherence because of their large workload and limited time during clinic visits. Having a pharmacist do so mitigated patients’ tendency to stop taking the drug when minor adverse effects developed, the investigators reported (JAMA 2015 April 14 [doi:101001/jama.2015.2761]).
“These findings suggest that such site-level practices provide modifiable targets to improve patient adherence to dabigatran, as opposed to patient characteristics that frequently cannot be modified,” Dr. Shore and her associates wrote.
This study was funded in part by VA Health Services Research & Development, an American Heart Association National Scientist Development Grant, and a Gilead Sciences Cardiovascular Research Scholars Program award. Dr. Shore reported having no financial disclosures; one of her associates reported serving as a consultant to Precision Health Economics, Medtronic, and St. Jude Medical.
Patient adherence to dabigatran therapy varied enormously in a nationwide study of pharmacy data, and three factors were identified that markedly enhanced adherence, according to a report published online April 14 in JAMA.
After research suggested that adherence to dabigatran was suboptimal among patients taking the drug for nonvalvular atrial fibrillation, investigators explored variations in adherence across thousands of sites using a Veterans Health Administration pharmacy database. They found that among 4,863 patients filling dabigatran prescriptions at 67 pharmacies during a 2-year period, adherence ranged from a low of 42% to a high of 93%, said Dr. Supriya Shore, a cardiology fellow at Emory University, Atlanta, and her associates.
The single most important factor that influenced dabigatran adherence was appropriate patient selection before dispensing the drug. This was defined as the pharmacist assessing the indication for treatment and ruling out contraindications after the physician ordered the prescription, as well as checking the patient’s adherence to other medications. Pharmacist monitoring of dabigatran use and adverse events, either alone or in collaboration with the treating clinician, also boosted adherence.
In addition, pharmacists working with clinicians to identify and address nonadherence also enhanced patient adherence. Prescribing physicians may not be able to routinely monitor adherence because of their large workload and limited time during clinic visits. Having a pharmacist do so mitigated patients’ tendency to stop taking the drug when minor adverse effects developed, the investigators reported (JAMA 2015 April 14 [doi:101001/jama.2015.2761]).
“These findings suggest that such site-level practices provide modifiable targets to improve patient adherence to dabigatran, as opposed to patient characteristics that frequently cannot be modified,” Dr. Shore and her associates wrote.
This study was funded in part by VA Health Services Research & Development, an American Heart Association National Scientist Development Grant, and a Gilead Sciences Cardiovascular Research Scholars Program award. Dr. Shore reported having no financial disclosures; one of her associates reported serving as a consultant to Precision Health Economics, Medtronic, and St. Jude Medical.
FROM JAMA
Key clinical point: Three modifiable factors improved AF patients’ adherence to dabigatran therapy.
Major finding: Among 4,863 patients filling dabigatran prescriptions at 67 pharmacies across the country during a 2-year period, adherence ranged from 42% to 93%.
Data source: A retrospective quantitative analysis and a cross-sectional qualitative analysis of data concerning 4,863 patients who filled dabigatran prescriptions at 67 sites.
Disclosures: This study was funded in part by VA Health Services Research & Development, an American Heart Association National Scientist Development Grant, and a Gilead Sciences Cardiovascular Research Scholars Program award. Dr. Shore reported having no financial disclosures; one of her associates reported serving as a consultant to Precision Health Economics, Medtronic, and St. Jude Medical.
Make the Diagnosis - April 2015
Diagnosis: Lichen Planus
Lichen planus is a common inflammatory condition involving the skin, nails, mucous membranes, and hair follicles. It has no racial predilection, and often affects men and women aged 20-60 years. It is less common in children, who account for only 4% of cases. The lesions are often atypical.
Clinically, patients often present with erythematous to violaceous small, flat-topped, polygonal papules that may coalesce into plaques. Lesions are generally pruritic, and may be tender or painful. Older lesions may be hyperpigmented. White streaks known as Wickham striae can cross the surface of lesions. These striae also can be present orally, such as in the patient described here. Oral lesions also may be atrophic or erosive.
Common body locations with involvement are the inner wrists, legs, torso, or genitals (glans penis). The face is rarely involved. Nail changes, such as longitudinal ridging and splitting, onycholysis, red lunula, yellow nail syndrome, and pterygium formation, can occur.
Lichen planus often spontaneously resolves on its own, with 2/3 of patients resolving in a year. Mucous membrane disease tends to be more chronic. The etiology of lichen planus is unknown. It may have an autoimmune mechanism in which T cells induce keratinocytes to undergo apoptosis. Between 4% and 60% of lichen planus patients also have hepatitis C infections. The differential diagnosis for cutaneous lesions include lichenoid drug eruption, guttate psoriasis, syphilis, and pityriasis lichenoides et varioliformis acuta. Oral lesions may resemble candidiasis, leukoplakia, malignancies, and bullous disease.
Topical and intralesional steroids are often effective for localized disease. Systemic corticosteroids can be useful when lesions are widespread. Phototherapy, isotretinoin, acitretin, hydroxychloroquine, and oral immunosuppressive agents (such as cyclosporine and mycophenolate mofetil) all have been described in the treatment of lichen planus.
Diagnosis: Lichen Planus
Lichen planus is a common inflammatory condition involving the skin, nails, mucous membranes, and hair follicles. It has no racial predilection, and often affects men and women aged 20-60 years. It is less common in children, who account for only 4% of cases. The lesions are often atypical.
Clinically, patients often present with erythematous to violaceous small, flat-topped, polygonal papules that may coalesce into plaques. Lesions are generally pruritic, and may be tender or painful. Older lesions may be hyperpigmented. White streaks known as Wickham striae can cross the surface of lesions. These striae also can be present orally, such as in the patient described here. Oral lesions also may be atrophic or erosive.
Common body locations with involvement are the inner wrists, legs, torso, or genitals (glans penis). The face is rarely involved. Nail changes, such as longitudinal ridging and splitting, onycholysis, red lunula, yellow nail syndrome, and pterygium formation, can occur.
Lichen planus often spontaneously resolves on its own, with 2/3 of patients resolving in a year. Mucous membrane disease tends to be more chronic. The etiology of lichen planus is unknown. It may have an autoimmune mechanism in which T cells induce keratinocytes to undergo apoptosis. Between 4% and 60% of lichen planus patients also have hepatitis C infections. The differential diagnosis for cutaneous lesions include lichenoid drug eruption, guttate psoriasis, syphilis, and pityriasis lichenoides et varioliformis acuta. Oral lesions may resemble candidiasis, leukoplakia, malignancies, and bullous disease.
Topical and intralesional steroids are often effective for localized disease. Systemic corticosteroids can be useful when lesions are widespread. Phototherapy, isotretinoin, acitretin, hydroxychloroquine, and oral immunosuppressive agents (such as cyclosporine and mycophenolate mofetil) all have been described in the treatment of lichen planus.
Diagnosis: Lichen Planus
Lichen planus is a common inflammatory condition involving the skin, nails, mucous membranes, and hair follicles. It has no racial predilection, and often affects men and women aged 20-60 years. It is less common in children, who account for only 4% of cases. The lesions are often atypical.
Clinically, patients often present with erythematous to violaceous small, flat-topped, polygonal papules that may coalesce into plaques. Lesions are generally pruritic, and may be tender or painful. Older lesions may be hyperpigmented. White streaks known as Wickham striae can cross the surface of lesions. These striae also can be present orally, such as in the patient described here. Oral lesions also may be atrophic or erosive.
Common body locations with involvement are the inner wrists, legs, torso, or genitals (glans penis). The face is rarely involved. Nail changes, such as longitudinal ridging and splitting, onycholysis, red lunula, yellow nail syndrome, and pterygium formation, can occur.
Lichen planus often spontaneously resolves on its own, with 2/3 of patients resolving in a year. Mucous membrane disease tends to be more chronic. The etiology of lichen planus is unknown. It may have an autoimmune mechanism in which T cells induce keratinocytes to undergo apoptosis. Between 4% and 60% of lichen planus patients also have hepatitis C infections. The differential diagnosis for cutaneous lesions include lichenoid drug eruption, guttate psoriasis, syphilis, and pityriasis lichenoides et varioliformis acuta. Oral lesions may resemble candidiasis, leukoplakia, malignancies, and bullous disease.
Topical and intralesional steroids are often effective for localized disease. Systemic corticosteroids can be useful when lesions are widespread. Phototherapy, isotretinoin, acitretin, hydroxychloroquine, and oral immunosuppressive agents (such as cyclosporine and mycophenolate mofetil) all have been described in the treatment of lichen planus.
This case and photo were submitted by Dr. Damon McClain, a dermatologist in Camp Lejeune, N.C. A 34-year-old male presented with a 1-month history of an itchy rash on his penis and feet. Upon physical examination, these lesions were seen orally. Blood work, including hepatitis serologies, was negative. His skin lesions improved with topical steroids.
Gene appears key to HSC regulation

in the bone marrow
The gene Ash1l plays a key role in regulating the maintenance and self-renewal of hematopoietic stem cells (HSCs), according to a study published in The Journal of Clinical Investigation.
The research provides new insight into how the body creates and maintains a healthy blood supply and immune system. It also opens new lines of inquiry about Ash1l’s potential role in cancers—like leukemia—that involve other members of the same gene family.
“If we find that Ash1l plays a role [in leukemia], that would open up avenues to try to block or slow down its activity pharmacologically,” said study author Ivan Maillard, MD, of the University of Michigan Medical School in Ann Arbor.
The Ash1l gene regulates the expression of multiple downstream homeotic genes, which help ensure the correct anatomical structure of a developing organism. And Ash1l is part of a family of genes that includes MLL1.
The researchers found that both Ash1l and MLL1 contribute to blood renewal. They observed mild defects in mice missing one gene or the other, but lacking both genes led to catastrophic deficiencies.
“We now have clear evidence that these genes cooperate to develop a healthy blood system,” Dr Maillard said.
He and his colleagues also found that Ash1l-deficient mice had normal numbers of HSCs during early development but a lack of HSCs in maturity—an indication the cells were not able to properly maintain themselves in the bone marrow.
Ash1l-deficient HSCs were unable to establish normal blood renewal after an HSC transplant. Moreover, Ash1l-deficient stem cells competed poorly with normal HSCs in the bone marrow and could easily be dislodged.
“By continuing to investigate the basic, underlying mechanisms [of blood renewal], we are helping to untangle the complex machinery . . . that may lay the foundation for new human treatments 5, 10, or 20 years from now,” Dr Maillard said. ![]()

in the bone marrow
The gene Ash1l plays a key role in regulating the maintenance and self-renewal of hematopoietic stem cells (HSCs), according to a study published in The Journal of Clinical Investigation.
The research provides new insight into how the body creates and maintains a healthy blood supply and immune system. It also opens new lines of inquiry about Ash1l’s potential role in cancers—like leukemia—that involve other members of the same gene family.
“If we find that Ash1l plays a role [in leukemia], that would open up avenues to try to block or slow down its activity pharmacologically,” said study author Ivan Maillard, MD, of the University of Michigan Medical School in Ann Arbor.
The Ash1l gene regulates the expression of multiple downstream homeotic genes, which help ensure the correct anatomical structure of a developing organism. And Ash1l is part of a family of genes that includes MLL1.
The researchers found that both Ash1l and MLL1 contribute to blood renewal. They observed mild defects in mice missing one gene or the other, but lacking both genes led to catastrophic deficiencies.
“We now have clear evidence that these genes cooperate to develop a healthy blood system,” Dr Maillard said.
He and his colleagues also found that Ash1l-deficient mice had normal numbers of HSCs during early development but a lack of HSCs in maturity—an indication the cells were not able to properly maintain themselves in the bone marrow.
Ash1l-deficient HSCs were unable to establish normal blood renewal after an HSC transplant. Moreover, Ash1l-deficient stem cells competed poorly with normal HSCs in the bone marrow and could easily be dislodged.
“By continuing to investigate the basic, underlying mechanisms [of blood renewal], we are helping to untangle the complex machinery . . . that may lay the foundation for new human treatments 5, 10, or 20 years from now,” Dr Maillard said. ![]()

in the bone marrow
The gene Ash1l plays a key role in regulating the maintenance and self-renewal of hematopoietic stem cells (HSCs), according to a study published in The Journal of Clinical Investigation.
The research provides new insight into how the body creates and maintains a healthy blood supply and immune system. It also opens new lines of inquiry about Ash1l’s potential role in cancers—like leukemia—that involve other members of the same gene family.
“If we find that Ash1l plays a role [in leukemia], that would open up avenues to try to block or slow down its activity pharmacologically,” said study author Ivan Maillard, MD, of the University of Michigan Medical School in Ann Arbor.
The Ash1l gene regulates the expression of multiple downstream homeotic genes, which help ensure the correct anatomical structure of a developing organism. And Ash1l is part of a family of genes that includes MLL1.
The researchers found that both Ash1l and MLL1 contribute to blood renewal. They observed mild defects in mice missing one gene or the other, but lacking both genes led to catastrophic deficiencies.
“We now have clear evidence that these genes cooperate to develop a healthy blood system,” Dr Maillard said.
He and his colleagues also found that Ash1l-deficient mice had normal numbers of HSCs during early development but a lack of HSCs in maturity—an indication the cells were not able to properly maintain themselves in the bone marrow.
Ash1l-deficient HSCs were unable to establish normal blood renewal after an HSC transplant. Moreover, Ash1l-deficient stem cells competed poorly with normal HSCs in the bone marrow and could easily be dislodged.
“By continuing to investigate the basic, underlying mechanisms [of blood renewal], we are helping to untangle the complex machinery . . . that may lay the foundation for new human treatments 5, 10, or 20 years from now,” Dr Maillard said. ![]()