User login
Is brain damage an ‘inevitable consequence or an avoidable risk’ of American football?
More research is needed to better understand and prevent traumatic brain injury such as chronic traumatic encephalopathy in American football players, Chad A. Asplund and Dr. Thomas M. Best wrote in an editorial published March 24 in BMJ.
Currently, chronic traumatic encephalopathy (CTE) can be formally diagnosed only at autopsy.
Though the National Football League denies a relationship between football and CTE, all confirmed cases of the disease in American football players to date were in those with a history of repetitive blows to the head. Athletes who began playing football before 12 years of age show greater cognitive impairment in older age than do those who started later, according to the authors.
“Further work into risk mitigation, paralleled with increased research into the pathophysiology of both concussion and CTE, is needed,” the authors wrote. “For now, it seems that the more we learn about CTE, the more questions are left unanswered – it still remains unclear if brain damage is an inevitable consequence or an avoidable risk of American football.”
Read the full article here: BMJ 2015;349:h1381 (doi:10.1136/bmj.h1381).
More research is needed to better understand and prevent traumatic brain injury such as chronic traumatic encephalopathy in American football players, Chad A. Asplund and Dr. Thomas M. Best wrote in an editorial published March 24 in BMJ.
Currently, chronic traumatic encephalopathy (CTE) can be formally diagnosed only at autopsy.
Though the National Football League denies a relationship between football and CTE, all confirmed cases of the disease in American football players to date were in those with a history of repetitive blows to the head. Athletes who began playing football before 12 years of age show greater cognitive impairment in older age than do those who started later, according to the authors.
“Further work into risk mitigation, paralleled with increased research into the pathophysiology of both concussion and CTE, is needed,” the authors wrote. “For now, it seems that the more we learn about CTE, the more questions are left unanswered – it still remains unclear if brain damage is an inevitable consequence or an avoidable risk of American football.”
Read the full article here: BMJ 2015;349:h1381 (doi:10.1136/bmj.h1381).
More research is needed to better understand and prevent traumatic brain injury such as chronic traumatic encephalopathy in American football players, Chad A. Asplund and Dr. Thomas M. Best wrote in an editorial published March 24 in BMJ.
Currently, chronic traumatic encephalopathy (CTE) can be formally diagnosed only at autopsy.
Though the National Football League denies a relationship between football and CTE, all confirmed cases of the disease in American football players to date were in those with a history of repetitive blows to the head. Athletes who began playing football before 12 years of age show greater cognitive impairment in older age than do those who started later, according to the authors.
“Further work into risk mitigation, paralleled with increased research into the pathophysiology of both concussion and CTE, is needed,” the authors wrote. “For now, it seems that the more we learn about CTE, the more questions are left unanswered – it still remains unclear if brain damage is an inevitable consequence or an avoidable risk of American football.”
Read the full article here: BMJ 2015;349:h1381 (doi:10.1136/bmj.h1381).
Dos and don’ts for handling common sling complications
Large-scale randomized trials have not only documented the efficacy of minimally invasive midurethral slings for stress urinary continence, they have also provided more adequate data on the incidence of complications. In practice, meanwhile, we are seeing more complications as the number of midurethral sling placements increases.
Often times, complications can be significantly more impactful than the original urinary incontinence. It is important to take the complications of sling placement seriously. Let patients know that their symptoms matter, and that there are ways to manage complications.
With more long-term data and experience, we have learned more about what to do, and what not to do, to prevent, diagnose, and manage the complications associated with midurethral slings. Here is my approach to the complications most commonly encountered, including bladder perforation, voiding dysfunction, erosion, pain, and recurrent stress urinary incontinence.
I will not address vascular injury in this article, but certainly, this is a surgical emergency that needs to be handled as such. As described in the February 2015 edition of Master Class on midurethral sling technique, accurate visualization toward the ipsilateral shoulder during needle passage is an essential part of preventing vascular injuries during retropubic sling placement.
Bladder perforation
Bladder perforation has consistently been shown to be significantly more common with retropubic slings than with transobturator slings. Reported incidence has ranged from 0.8% to 34% for tension-free vaginal tape (TVT) procedures, with the higher rates seen mainly in teaching institutions. Most commonly, the reported incidence is less than 10%.
Bladder perforation has no effect on the efficacy of the treatment, and no apparent long-term consequences, as long as the injury is identified. Especially with a retropubic sling, cystoscopy should be performed after both needles are placed but prior to advancing the needles all the way through the retropubic space. Simply withdrawing a needle will cause little bladder injury while retracting deployed mesh is significantly more consequential.
I recommend filling the bladder to approximately 300 cc, or to the point where you can see evidence of full distension such as flattened urethral orifices. This confirms that the bladder is under enough distension to preclude any mucosal wrinkles or folds that can hide a trocar injury.
The first step upon recognition of a perforation is to stay calm. In the vast majority of cases, simply withdrawing the needle, replacing it, and verifying correct replacement will prevent any long-term consequences. On the other hand, you must be fully alert to the possibility that the needle wandered away from the pubic bone, and consequently may have entered a space such as the peritoneum. Suspicion for visceral injury should be increased.
Resist the temptation to replace the needle more laterally. This course correction is often an unhelpful instinct, because a more lateral replacement will not move the needle farther from the bladder; it will instead bring it closer to the iliac vessels. Vascular injuries resulting from the surgeon’s attempts at needle replacement are unfortunate, as a minor complication becomes a major one. The key is to be as distal as possible – as close to the pubic bone as possible – and not to replace the needles more laterally.
Postoperative drainage for 1-2 days may be considered, but there is nothing in the literature to require this, and many surgeons do not employ any sort of extra catheterization after surgery where perforation has been observed.
Voiding dysfunction
Some degree of voiding dysfunction is not uncommon in the short term, but when a patient is still unable to void normally or completely after several days, an evaluation is warranted. As with bladder perforation, reported incidence of voiding dysfunction has varied widely, from 2% to 45% with the newer midurethral slings. Generally, the need for surgical revision is about 2%.
There are two reasons for urinary retention: Insufficient contraction force in the bladder or too much resistance. If retention persists beyond a week – in the 7-10 day postop time period – I assess whether the problem is resulting from too much obstruction from the sling, some form of hypotonic bladder, other surgery performed in conjunction with sling placement, medications, or something else.
Difficulty in passing a small urethra catheter in the office may indicate excessive obstruction, for instance, and there may be indications on vaginal examination or through cystoscopy that the sling is too tight. A midurethral “speed bump,” or elevation at the midpoint, with either catheterization or the scope is consistent with over-correction.
Do not dilate or pull down on the sling with any kind of urethra dilator. The sling is more robust than the urethral mucosa, and we now appreciate that this practice is associated with urethral erosion.
If the problem is deemed to be excessive obstruction or over-resistance, and it is fewer than 10 days postop, the patient may be offered a minor revision; the original incision is reopened, the sling material is identified, and the sling arms (lateral to the urethra) are grasped with clamps. Gentle downward traction can loosen the sling.
The sling should be grasped laterally and not at the midpoint; some sling materials will stretch and fracture where the force is applied. A little bit of gentle downward traction (3-5 mm) will often give you the needed amount of space for relieving some of the obstruction.
Beyond 10 days postop, tissue in-growth makes such a sling adjustment difficult, if not impossible. At this point, I recommend transecting the entire sling in the midline.There is differing opinion about whether a portion of the mesh should be resected; I believe that such a resection is usually unnecessary, and that a simple midline release procedure is the best approach.
A study we performed more than a decade ago on surgical release of TVT showed that persistent post-TVT voiding dysfunction can be successfully managed with a simple midline release. Of 1,175 women who underwent TVT placement for stress urinary incontinence and/or intrinsic sphincter deficiency, 23 (1.9%) had persistent voiding dysfunction. All cases of impaired emptying were completely resolved with a release of the tape, and the majority remained cured in terms of their continence or went from “cured” to “improved” over baseline. Three patients (13%) had recurrence of stress incontinence (Obstet. Gynecol. 2002;100:898-902).
We used to wait longer before revising the sling out of fear of losing the entire benefit of the sling. As it turns out, a simple midline release (leaving most, if not all, of the mesh in place) is usually just enough to treat the new complaint while still providing enough lateral support so that the patient retains most or all of the continence achieved with the sling.
Complaints of de novo urge incontinence, or overactive bladder, should be taken seriously. Urge incontinence has even more significant associations with depression and poor quality of life than stress incontinence. In the absence of retention, usual first-line therapies for overactive bladder can be employed, including anticholinergic medications, behavioral therapies, and physical therapy. Failing these interventions, my assessment for this complaint will be similar to that for retention; I’ll look for evidence of too much resistance, such as difficulty in passing a catheter, a “speed bump” cystoscopically, or an elevated pDet on pressure-flow studies, for instance.
If any of these are present, I usually offer sling release first. If, on the other hand, there is no evidence of over resistance in a patient who has de novo urge incontinence or overactive bladder and is refractory to conservative measures, a trial of sacral neuromodulation or botox injections is considered the next step.
Erosion
Erosion remains a difficult complication to understand. Long-term follow-up data show that it occurs after 3%-4% of sling placements, rather than 1% as originally believed. Data are inconsistent, but there probably is a slightly higher incidence of vaginal erosion with a transobturator sling, given more contact between the sling and the anterior vaginal wall.
There are hints in the literature that erosion may be related to technique – perhaps to the depth of dissection during surgery – but this is difficult to quantify. Moreover, many of the reported cases of erosion occur several years, or longer, after surgery. It is hard to blame surgical technique for such delayed erosion.
As we’ve seen with previous generations of mesh, there does not appear to be any window of time after which erosion is no longer a risk. We need to recognize that there is a medium- and long-term risk of erosion and appreciate its presenting symptoms: Recurrent urinary tract infection, pain with voiding, urgency, urinary incontinence, and microscopic hematuria of new onset.
Prevention may well entail preoperative estrogenization. The science looking at the effect of estrogen on sling placement is becoming more robust. While there are uncertainties, I believe that studies likely will show that topical estrogen in the preoperative and perioperative phases plays an important role in preventing erosion from occurring. Personally, I am using it much more than I was 10 years ago.
I like the convenience of the Vagifem tablet (Novo Nordisk Inc., Plainsboro, N.J.), and am reassured by data on systemic absorption with the 10-mcg dose, but any vaginal cream or compounded suppository can be used. I usually advise 4-6 weeks of preoperative preparation, with nightly use for 2 weeks followed by 2-3 nights per week thereafter. Smoking is also a likely risk factor. Data are not entirely consistent, but I believe we should provide counseling and encourage smoking cessation before the implant of mesh.
Management is dependent on when the erosion occurs or is recognized. When erosion occurs within 6 weeks post operatively, primary repair is an option. When erosion is detected after the 6-week window and is causing symptoms, a conservative trim of bristles poking through the vaginal mucosa is worth a try. I do not advise more than one such conservative trim, however, as repeated attempts and series of small resections can make the sling exceedingly difficult to remove if more complete resection is ultimately needed. After one unsuccessful trim, I usually remove the whole sling belly, or most of the vaginal part of the sling.
For slings made of type 1 macroporous mesh, resection of the retropubic or transobturator portions of the mesh usually is not required. In the more rare situation where those pelvic areas of the mesh are associated with pain, I favor a laparoscopic approach to the retropubic space to facilitate minimally invasive removal.
Postop pain, sling failure
Groin pain, or thigh pain, sometimes occurs after placement of a transobturator sling. As I discussed in the previous Master Class on midurethral sling technique, I have seen a significant decrease in groin pain in my patients – without any reduction in benefit – with the use of a shorter transobturator sling that does not leave mesh in the adductor compartment of the thigh and groin.
For persistent groin pain, I favor the use of trigger point injection. Sometimes one injection will impact the inflammatory cycle such that the patient derives long-term benefit. At other times, the trigger point injection will serve as a diagnostic; if pain returns after a period of benefit, I am inclined to resect that part of the mesh.
Pain inside the pelvis, especially on the pelvic sidewall (obturator or puborectalis complex) usually is related to mechanical tension. In my experience, this type of discomfort is slightly more likely to occur with the transobturator slings, which penetrate through the muscular pelvic sidewall and lead to more fibrosis and scar tissue formation.
In most cases of pain and discomfort, attempting to reproduce the patient’s symptoms by putting tension on particular parts of the sling during the office exam helps guide management. If I find that palpating or putting the sling on tension recreates her complaints, and conservative injections have provided temporary or inadequate relief, I usually advocate resecting the vaginal portion of the mesh to relieve that tension.
In cases of recurrent stress urinary incontinence (when the sling has failed), a TVT or repeat TVT is often warranted. The TVT sling has been demonstrated to work after nearly every other previous kind of anti-incontinence procedure, even after a previous retropubic sling. There is little data on mesh removal in such cases. I believe that unless a previously placed but failed sling is causing symptoms, there is no need to resect it. Mesh removal is significantly more traumatic than mesh placement, and in most cases it is not necessary.
Dr. Rardin reported that he has no relevant financial disclosures.
Large-scale randomized trials have not only documented the efficacy of minimally invasive midurethral slings for stress urinary continence, they have also provided more adequate data on the incidence of complications. In practice, meanwhile, we are seeing more complications as the number of midurethral sling placements increases.
Often times, complications can be significantly more impactful than the original urinary incontinence. It is important to take the complications of sling placement seriously. Let patients know that their symptoms matter, and that there are ways to manage complications.
With more long-term data and experience, we have learned more about what to do, and what not to do, to prevent, diagnose, and manage the complications associated with midurethral slings. Here is my approach to the complications most commonly encountered, including bladder perforation, voiding dysfunction, erosion, pain, and recurrent stress urinary incontinence.
I will not address vascular injury in this article, but certainly, this is a surgical emergency that needs to be handled as such. As described in the February 2015 edition of Master Class on midurethral sling technique, accurate visualization toward the ipsilateral shoulder during needle passage is an essential part of preventing vascular injuries during retropubic sling placement.
Bladder perforation
Bladder perforation has consistently been shown to be significantly more common with retropubic slings than with transobturator slings. Reported incidence has ranged from 0.8% to 34% for tension-free vaginal tape (TVT) procedures, with the higher rates seen mainly in teaching institutions. Most commonly, the reported incidence is less than 10%.
Bladder perforation has no effect on the efficacy of the treatment, and no apparent long-term consequences, as long as the injury is identified. Especially with a retropubic sling, cystoscopy should be performed after both needles are placed but prior to advancing the needles all the way through the retropubic space. Simply withdrawing a needle will cause little bladder injury while retracting deployed mesh is significantly more consequential.
I recommend filling the bladder to approximately 300 cc, or to the point where you can see evidence of full distension such as flattened urethral orifices. This confirms that the bladder is under enough distension to preclude any mucosal wrinkles or folds that can hide a trocar injury.
The first step upon recognition of a perforation is to stay calm. In the vast majority of cases, simply withdrawing the needle, replacing it, and verifying correct replacement will prevent any long-term consequences. On the other hand, you must be fully alert to the possibility that the needle wandered away from the pubic bone, and consequently may have entered a space such as the peritoneum. Suspicion for visceral injury should be increased.
Resist the temptation to replace the needle more laterally. This course correction is often an unhelpful instinct, because a more lateral replacement will not move the needle farther from the bladder; it will instead bring it closer to the iliac vessels. Vascular injuries resulting from the surgeon’s attempts at needle replacement are unfortunate, as a minor complication becomes a major one. The key is to be as distal as possible – as close to the pubic bone as possible – and not to replace the needles more laterally.
Postoperative drainage for 1-2 days may be considered, but there is nothing in the literature to require this, and many surgeons do not employ any sort of extra catheterization after surgery where perforation has been observed.
Voiding dysfunction
Some degree of voiding dysfunction is not uncommon in the short term, but when a patient is still unable to void normally or completely after several days, an evaluation is warranted. As with bladder perforation, reported incidence of voiding dysfunction has varied widely, from 2% to 45% with the newer midurethral slings. Generally, the need for surgical revision is about 2%.
There are two reasons for urinary retention: Insufficient contraction force in the bladder or too much resistance. If retention persists beyond a week – in the 7-10 day postop time period – I assess whether the problem is resulting from too much obstruction from the sling, some form of hypotonic bladder, other surgery performed in conjunction with sling placement, medications, or something else.
Difficulty in passing a small urethra catheter in the office may indicate excessive obstruction, for instance, and there may be indications on vaginal examination or through cystoscopy that the sling is too tight. A midurethral “speed bump,” or elevation at the midpoint, with either catheterization or the scope is consistent with over-correction.
Do not dilate or pull down on the sling with any kind of urethra dilator. The sling is more robust than the urethral mucosa, and we now appreciate that this practice is associated with urethral erosion.
If the problem is deemed to be excessive obstruction or over-resistance, and it is fewer than 10 days postop, the patient may be offered a minor revision; the original incision is reopened, the sling material is identified, and the sling arms (lateral to the urethra) are grasped with clamps. Gentle downward traction can loosen the sling.
The sling should be grasped laterally and not at the midpoint; some sling materials will stretch and fracture where the force is applied. A little bit of gentle downward traction (3-5 mm) will often give you the needed amount of space for relieving some of the obstruction.
Beyond 10 days postop, tissue in-growth makes such a sling adjustment difficult, if not impossible. At this point, I recommend transecting the entire sling in the midline.There is differing opinion about whether a portion of the mesh should be resected; I believe that such a resection is usually unnecessary, and that a simple midline release procedure is the best approach.
A study we performed more than a decade ago on surgical release of TVT showed that persistent post-TVT voiding dysfunction can be successfully managed with a simple midline release. Of 1,175 women who underwent TVT placement for stress urinary incontinence and/or intrinsic sphincter deficiency, 23 (1.9%) had persistent voiding dysfunction. All cases of impaired emptying were completely resolved with a release of the tape, and the majority remained cured in terms of their continence or went from “cured” to “improved” over baseline. Three patients (13%) had recurrence of stress incontinence (Obstet. Gynecol. 2002;100:898-902).
We used to wait longer before revising the sling out of fear of losing the entire benefit of the sling. As it turns out, a simple midline release (leaving most, if not all, of the mesh in place) is usually just enough to treat the new complaint while still providing enough lateral support so that the patient retains most or all of the continence achieved with the sling.
Complaints of de novo urge incontinence, or overactive bladder, should be taken seriously. Urge incontinence has even more significant associations with depression and poor quality of life than stress incontinence. In the absence of retention, usual first-line therapies for overactive bladder can be employed, including anticholinergic medications, behavioral therapies, and physical therapy. Failing these interventions, my assessment for this complaint will be similar to that for retention; I’ll look for evidence of too much resistance, such as difficulty in passing a catheter, a “speed bump” cystoscopically, or an elevated pDet on pressure-flow studies, for instance.
If any of these are present, I usually offer sling release first. If, on the other hand, there is no evidence of over resistance in a patient who has de novo urge incontinence or overactive bladder and is refractory to conservative measures, a trial of sacral neuromodulation or botox injections is considered the next step.
Erosion
Erosion remains a difficult complication to understand. Long-term follow-up data show that it occurs after 3%-4% of sling placements, rather than 1% as originally believed. Data are inconsistent, but there probably is a slightly higher incidence of vaginal erosion with a transobturator sling, given more contact between the sling and the anterior vaginal wall.
There are hints in the literature that erosion may be related to technique – perhaps to the depth of dissection during surgery – but this is difficult to quantify. Moreover, many of the reported cases of erosion occur several years, or longer, after surgery. It is hard to blame surgical technique for such delayed erosion.
As we’ve seen with previous generations of mesh, there does not appear to be any window of time after which erosion is no longer a risk. We need to recognize that there is a medium- and long-term risk of erosion and appreciate its presenting symptoms: Recurrent urinary tract infection, pain with voiding, urgency, urinary incontinence, and microscopic hematuria of new onset.
Prevention may well entail preoperative estrogenization. The science looking at the effect of estrogen on sling placement is becoming more robust. While there are uncertainties, I believe that studies likely will show that topical estrogen in the preoperative and perioperative phases plays an important role in preventing erosion from occurring. Personally, I am using it much more than I was 10 years ago.
I like the convenience of the Vagifem tablet (Novo Nordisk Inc., Plainsboro, N.J.), and am reassured by data on systemic absorption with the 10-mcg dose, but any vaginal cream or compounded suppository can be used. I usually advise 4-6 weeks of preoperative preparation, with nightly use for 2 weeks followed by 2-3 nights per week thereafter. Smoking is also a likely risk factor. Data are not entirely consistent, but I believe we should provide counseling and encourage smoking cessation before the implant of mesh.
Management is dependent on when the erosion occurs or is recognized. When erosion occurs within 6 weeks post operatively, primary repair is an option. When erosion is detected after the 6-week window and is causing symptoms, a conservative trim of bristles poking through the vaginal mucosa is worth a try. I do not advise more than one such conservative trim, however, as repeated attempts and series of small resections can make the sling exceedingly difficult to remove if more complete resection is ultimately needed. After one unsuccessful trim, I usually remove the whole sling belly, or most of the vaginal part of the sling.
For slings made of type 1 macroporous mesh, resection of the retropubic or transobturator portions of the mesh usually is not required. In the more rare situation where those pelvic areas of the mesh are associated with pain, I favor a laparoscopic approach to the retropubic space to facilitate minimally invasive removal.
Postop pain, sling failure
Groin pain, or thigh pain, sometimes occurs after placement of a transobturator sling. As I discussed in the previous Master Class on midurethral sling technique, I have seen a significant decrease in groin pain in my patients – without any reduction in benefit – with the use of a shorter transobturator sling that does not leave mesh in the adductor compartment of the thigh and groin.
For persistent groin pain, I favor the use of trigger point injection. Sometimes one injection will impact the inflammatory cycle such that the patient derives long-term benefit. At other times, the trigger point injection will serve as a diagnostic; if pain returns after a period of benefit, I am inclined to resect that part of the mesh.
Pain inside the pelvis, especially on the pelvic sidewall (obturator or puborectalis complex) usually is related to mechanical tension. In my experience, this type of discomfort is slightly more likely to occur with the transobturator slings, which penetrate through the muscular pelvic sidewall and lead to more fibrosis and scar tissue formation.
In most cases of pain and discomfort, attempting to reproduce the patient’s symptoms by putting tension on particular parts of the sling during the office exam helps guide management. If I find that palpating or putting the sling on tension recreates her complaints, and conservative injections have provided temporary or inadequate relief, I usually advocate resecting the vaginal portion of the mesh to relieve that tension.
In cases of recurrent stress urinary incontinence (when the sling has failed), a TVT or repeat TVT is often warranted. The TVT sling has been demonstrated to work after nearly every other previous kind of anti-incontinence procedure, even after a previous retropubic sling. There is little data on mesh removal in such cases. I believe that unless a previously placed but failed sling is causing symptoms, there is no need to resect it. Mesh removal is significantly more traumatic than mesh placement, and in most cases it is not necessary.
Dr. Rardin reported that he has no relevant financial disclosures.
Large-scale randomized trials have not only documented the efficacy of minimally invasive midurethral slings for stress urinary continence, they have also provided more adequate data on the incidence of complications. In practice, meanwhile, we are seeing more complications as the number of midurethral sling placements increases.
Often times, complications can be significantly more impactful than the original urinary incontinence. It is important to take the complications of sling placement seriously. Let patients know that their symptoms matter, and that there are ways to manage complications.
With more long-term data and experience, we have learned more about what to do, and what not to do, to prevent, diagnose, and manage the complications associated with midurethral slings. Here is my approach to the complications most commonly encountered, including bladder perforation, voiding dysfunction, erosion, pain, and recurrent stress urinary incontinence.
I will not address vascular injury in this article, but certainly, this is a surgical emergency that needs to be handled as such. As described in the February 2015 edition of Master Class on midurethral sling technique, accurate visualization toward the ipsilateral shoulder during needle passage is an essential part of preventing vascular injuries during retropubic sling placement.
Bladder perforation
Bladder perforation has consistently been shown to be significantly more common with retropubic slings than with transobturator slings. Reported incidence has ranged from 0.8% to 34% for tension-free vaginal tape (TVT) procedures, with the higher rates seen mainly in teaching institutions. Most commonly, the reported incidence is less than 10%.
Bladder perforation has no effect on the efficacy of the treatment, and no apparent long-term consequences, as long as the injury is identified. Especially with a retropubic sling, cystoscopy should be performed after both needles are placed but prior to advancing the needles all the way through the retropubic space. Simply withdrawing a needle will cause little bladder injury while retracting deployed mesh is significantly more consequential.
I recommend filling the bladder to approximately 300 cc, or to the point where you can see evidence of full distension such as flattened urethral orifices. This confirms that the bladder is under enough distension to preclude any mucosal wrinkles or folds that can hide a trocar injury.
The first step upon recognition of a perforation is to stay calm. In the vast majority of cases, simply withdrawing the needle, replacing it, and verifying correct replacement will prevent any long-term consequences. On the other hand, you must be fully alert to the possibility that the needle wandered away from the pubic bone, and consequently may have entered a space such as the peritoneum. Suspicion for visceral injury should be increased.
Resist the temptation to replace the needle more laterally. This course correction is often an unhelpful instinct, because a more lateral replacement will not move the needle farther from the bladder; it will instead bring it closer to the iliac vessels. Vascular injuries resulting from the surgeon’s attempts at needle replacement are unfortunate, as a minor complication becomes a major one. The key is to be as distal as possible – as close to the pubic bone as possible – and not to replace the needles more laterally.
Postoperative drainage for 1-2 days may be considered, but there is nothing in the literature to require this, and many surgeons do not employ any sort of extra catheterization after surgery where perforation has been observed.
Voiding dysfunction
Some degree of voiding dysfunction is not uncommon in the short term, but when a patient is still unable to void normally or completely after several days, an evaluation is warranted. As with bladder perforation, reported incidence of voiding dysfunction has varied widely, from 2% to 45% with the newer midurethral slings. Generally, the need for surgical revision is about 2%.
There are two reasons for urinary retention: Insufficient contraction force in the bladder or too much resistance. If retention persists beyond a week – in the 7-10 day postop time period – I assess whether the problem is resulting from too much obstruction from the sling, some form of hypotonic bladder, other surgery performed in conjunction with sling placement, medications, or something else.
Difficulty in passing a small urethra catheter in the office may indicate excessive obstruction, for instance, and there may be indications on vaginal examination or through cystoscopy that the sling is too tight. A midurethral “speed bump,” or elevation at the midpoint, with either catheterization or the scope is consistent with over-correction.
Do not dilate or pull down on the sling with any kind of urethra dilator. The sling is more robust than the urethral mucosa, and we now appreciate that this practice is associated with urethral erosion.
If the problem is deemed to be excessive obstruction or over-resistance, and it is fewer than 10 days postop, the patient may be offered a minor revision; the original incision is reopened, the sling material is identified, and the sling arms (lateral to the urethra) are grasped with clamps. Gentle downward traction can loosen the sling.
The sling should be grasped laterally and not at the midpoint; some sling materials will stretch and fracture where the force is applied. A little bit of gentle downward traction (3-5 mm) will often give you the needed amount of space for relieving some of the obstruction.
Beyond 10 days postop, tissue in-growth makes such a sling adjustment difficult, if not impossible. At this point, I recommend transecting the entire sling in the midline.There is differing opinion about whether a portion of the mesh should be resected; I believe that such a resection is usually unnecessary, and that a simple midline release procedure is the best approach.
A study we performed more than a decade ago on surgical release of TVT showed that persistent post-TVT voiding dysfunction can be successfully managed with a simple midline release. Of 1,175 women who underwent TVT placement for stress urinary incontinence and/or intrinsic sphincter deficiency, 23 (1.9%) had persistent voiding dysfunction. All cases of impaired emptying were completely resolved with a release of the tape, and the majority remained cured in terms of their continence or went from “cured” to “improved” over baseline. Three patients (13%) had recurrence of stress incontinence (Obstet. Gynecol. 2002;100:898-902).
We used to wait longer before revising the sling out of fear of losing the entire benefit of the sling. As it turns out, a simple midline release (leaving most, if not all, of the mesh in place) is usually just enough to treat the new complaint while still providing enough lateral support so that the patient retains most or all of the continence achieved with the sling.
Complaints of de novo urge incontinence, or overactive bladder, should be taken seriously. Urge incontinence has even more significant associations with depression and poor quality of life than stress incontinence. In the absence of retention, usual first-line therapies for overactive bladder can be employed, including anticholinergic medications, behavioral therapies, and physical therapy. Failing these interventions, my assessment for this complaint will be similar to that for retention; I’ll look for evidence of too much resistance, such as difficulty in passing a catheter, a “speed bump” cystoscopically, or an elevated pDet on pressure-flow studies, for instance.
If any of these are present, I usually offer sling release first. If, on the other hand, there is no evidence of over resistance in a patient who has de novo urge incontinence or overactive bladder and is refractory to conservative measures, a trial of sacral neuromodulation or botox injections is considered the next step.
Erosion
Erosion remains a difficult complication to understand. Long-term follow-up data show that it occurs after 3%-4% of sling placements, rather than 1% as originally believed. Data are inconsistent, but there probably is a slightly higher incidence of vaginal erosion with a transobturator sling, given more contact between the sling and the anterior vaginal wall.
There are hints in the literature that erosion may be related to technique – perhaps to the depth of dissection during surgery – but this is difficult to quantify. Moreover, many of the reported cases of erosion occur several years, or longer, after surgery. It is hard to blame surgical technique for such delayed erosion.
As we’ve seen with previous generations of mesh, there does not appear to be any window of time after which erosion is no longer a risk. We need to recognize that there is a medium- and long-term risk of erosion and appreciate its presenting symptoms: Recurrent urinary tract infection, pain with voiding, urgency, urinary incontinence, and microscopic hematuria of new onset.
Prevention may well entail preoperative estrogenization. The science looking at the effect of estrogen on sling placement is becoming more robust. While there are uncertainties, I believe that studies likely will show that topical estrogen in the preoperative and perioperative phases plays an important role in preventing erosion from occurring. Personally, I am using it much more than I was 10 years ago.
I like the convenience of the Vagifem tablet (Novo Nordisk Inc., Plainsboro, N.J.), and am reassured by data on systemic absorption with the 10-mcg dose, but any vaginal cream or compounded suppository can be used. I usually advise 4-6 weeks of preoperative preparation, with nightly use for 2 weeks followed by 2-3 nights per week thereafter. Smoking is also a likely risk factor. Data are not entirely consistent, but I believe we should provide counseling and encourage smoking cessation before the implant of mesh.
Management is dependent on when the erosion occurs or is recognized. When erosion occurs within 6 weeks post operatively, primary repair is an option. When erosion is detected after the 6-week window and is causing symptoms, a conservative trim of bristles poking through the vaginal mucosa is worth a try. I do not advise more than one such conservative trim, however, as repeated attempts and series of small resections can make the sling exceedingly difficult to remove if more complete resection is ultimately needed. After one unsuccessful trim, I usually remove the whole sling belly, or most of the vaginal part of the sling.
For slings made of type 1 macroporous mesh, resection of the retropubic or transobturator portions of the mesh usually is not required. In the more rare situation where those pelvic areas of the mesh are associated with pain, I favor a laparoscopic approach to the retropubic space to facilitate minimally invasive removal.
Postop pain, sling failure
Groin pain, or thigh pain, sometimes occurs after placement of a transobturator sling. As I discussed in the previous Master Class on midurethral sling technique, I have seen a significant decrease in groin pain in my patients – without any reduction in benefit – with the use of a shorter transobturator sling that does not leave mesh in the adductor compartment of the thigh and groin.
For persistent groin pain, I favor the use of trigger point injection. Sometimes one injection will impact the inflammatory cycle such that the patient derives long-term benefit. At other times, the trigger point injection will serve as a diagnostic; if pain returns after a period of benefit, I am inclined to resect that part of the mesh.
Pain inside the pelvis, especially on the pelvic sidewall (obturator or puborectalis complex) usually is related to mechanical tension. In my experience, this type of discomfort is slightly more likely to occur with the transobturator slings, which penetrate through the muscular pelvic sidewall and lead to more fibrosis and scar tissue formation.
In most cases of pain and discomfort, attempting to reproduce the patient’s symptoms by putting tension on particular parts of the sling during the office exam helps guide management. If I find that palpating or putting the sling on tension recreates her complaints, and conservative injections have provided temporary or inadequate relief, I usually advocate resecting the vaginal portion of the mesh to relieve that tension.
In cases of recurrent stress urinary incontinence (when the sling has failed), a TVT or repeat TVT is often warranted. The TVT sling has been demonstrated to work after nearly every other previous kind of anti-incontinence procedure, even after a previous retropubic sling. There is little data on mesh removal in such cases. I believe that unless a previously placed but failed sling is causing symptoms, there is no need to resect it. Mesh removal is significantly more traumatic than mesh placement, and in most cases it is not necessary.
Dr. Rardin reported that he has no relevant financial disclosures.
Tackling midurethral sling complications
Over the past 2 decades, midurethral slings, both via a retropubic and a transobturator approach have become the first-line therapy for the surgical correction of female stress urinary incontinence. Not only are cure rates excellent for both techniques, but the incidence of complications are low.
Intraoperatively, major concerns include vascular lesions, nerve injuries, and injuries to the bowel. More minor concerns are related to the bladder.
Perioperative complications include retropubic hematoma, blood loss, urinary tract infection, and spondylitis. Postoperative risks include transient versus permanent urinary retention, vaginal versus urethral erosion, de novo urgency, bladder erosion, and urethral obstruction.
In this edition of Master Class in gynecologic surgery, I am pleased to solicit the help of Dr. Charles Rardin, who will make recommendations regarding the management of some of the most common complications related to midurethral sling procedures.
Dr. Rardin is the director of the Robotic Surgery Program at Women & Infants Hospital of Rhode Island, in Providence; a surgeon in Women & Infants’ division of urogynecology and Reconstructive Pelvic Surgery; and is the director of the hospital’s fellowship urogynecology and reconstructive pelvic surgery.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
Over the past 2 decades, midurethral slings, both via a retropubic and a transobturator approach have become the first-line therapy for the surgical correction of female stress urinary incontinence. Not only are cure rates excellent for both techniques, but the incidence of complications are low.
Intraoperatively, major concerns include vascular lesions, nerve injuries, and injuries to the bowel. More minor concerns are related to the bladder.
Perioperative complications include retropubic hematoma, blood loss, urinary tract infection, and spondylitis. Postoperative risks include transient versus permanent urinary retention, vaginal versus urethral erosion, de novo urgency, bladder erosion, and urethral obstruction.
In this edition of Master Class in gynecologic surgery, I am pleased to solicit the help of Dr. Charles Rardin, who will make recommendations regarding the management of some of the most common complications related to midurethral sling procedures.
Dr. Rardin is the director of the Robotic Surgery Program at Women & Infants Hospital of Rhode Island, in Providence; a surgeon in Women & Infants’ division of urogynecology and Reconstructive Pelvic Surgery; and is the director of the hospital’s fellowship urogynecology and reconstructive pelvic surgery.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
Over the past 2 decades, midurethral slings, both via a retropubic and a transobturator approach have become the first-line therapy for the surgical correction of female stress urinary incontinence. Not only are cure rates excellent for both techniques, but the incidence of complications are low.
Intraoperatively, major concerns include vascular lesions, nerve injuries, and injuries to the bowel. More minor concerns are related to the bladder.
Perioperative complications include retropubic hematoma, blood loss, urinary tract infection, and spondylitis. Postoperative risks include transient versus permanent urinary retention, vaginal versus urethral erosion, de novo urgency, bladder erosion, and urethral obstruction.
In this edition of Master Class in gynecologic surgery, I am pleased to solicit the help of Dr. Charles Rardin, who will make recommendations regarding the management of some of the most common complications related to midurethral sling procedures.
Dr. Rardin is the director of the Robotic Surgery Program at Women & Infants Hospital of Rhode Island, in Providence; a surgeon in Women & Infants’ division of urogynecology and Reconstructive Pelvic Surgery; and is the director of the hospital’s fellowship urogynecology and reconstructive pelvic surgery.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
Hospitalist Continuity Doesn’t Affect Adverse Events among Inpatients
Hospitalist continuity does not appear to be associated with the incidence of adverse events (AEs), according to a new report in the Journal of Hospital Medicine.
Authors used two methods to measure continuity: the Number of Physicians Index (NPI) represented the total number of unique hospitalists caring for a patient, while the Usual Provider of Care (UPC) Index was the proportion of encounters with the most frequently encountered hospitalist.
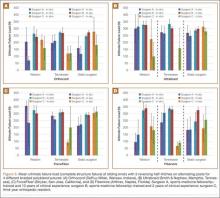
Researchers reported that, in unadjusted models, each one-unit increase in the NPI—meaning less continuity—was significantly associated with the incidence of one or more AEs (odds ratio, 1.75; P<0.001). In addition, UPC was not associated with incidence of AEs. Across all adjusted models, neither index was "significantly associated" with the incidence of AEs.
Lead author Kevin O'Leary, MD, MS, SFHM, of Northwestern University's Feinberg School of Medicine in Chicago, says that the data could be used to help determine how best to structure handoffs.
"Where I think this has a major impact is that a whole lot of groups [are] trying to figure out how long should our rotation length be," Dr. O'Leary says. "All of those programs that are really trying to maximize continuity because they think it's the safest thing and best thing for patient outcomes, they can probably relax a little bit and swing the pendulum a little bit further toward what they think is the right model for the work-life balance of their hospitalist. [They can] worry a little bit less about the impact on the patients because there doesn't seem to be much." TH
Visit our website for more information on transitions of care.
Hospitalist continuity does not appear to be associated with the incidence of adverse events (AEs), according to a new report in the Journal of Hospital Medicine.
Authors used two methods to measure continuity: the Number of Physicians Index (NPI) represented the total number of unique hospitalists caring for a patient, while the Usual Provider of Care (UPC) Index was the proportion of encounters with the most frequently encountered hospitalist.
Researchers reported that, in unadjusted models, each one-unit increase in the NPI—meaning less continuity—was significantly associated with the incidence of one or more AEs (odds ratio, 1.75; P<0.001). In addition, UPC was not associated with incidence of AEs. Across all adjusted models, neither index was "significantly associated" with the incidence of AEs.
Lead author Kevin O'Leary, MD, MS, SFHM, of Northwestern University's Feinberg School of Medicine in Chicago, says that the data could be used to help determine how best to structure handoffs.
"Where I think this has a major impact is that a whole lot of groups [are] trying to figure out how long should our rotation length be," Dr. O'Leary says. "All of those programs that are really trying to maximize continuity because they think it's the safest thing and best thing for patient outcomes, they can probably relax a little bit and swing the pendulum a little bit further toward what they think is the right model for the work-life balance of their hospitalist. [They can] worry a little bit less about the impact on the patients because there doesn't seem to be much." TH
Visit our website for more information on transitions of care.
Hospitalist continuity does not appear to be associated with the incidence of adverse events (AEs), according to a new report in the Journal of Hospital Medicine.
Authors used two methods to measure continuity: the Number of Physicians Index (NPI) represented the total number of unique hospitalists caring for a patient, while the Usual Provider of Care (UPC) Index was the proportion of encounters with the most frequently encountered hospitalist.
Researchers reported that, in unadjusted models, each one-unit increase in the NPI—meaning less continuity—was significantly associated with the incidence of one or more AEs (odds ratio, 1.75; P<0.001). In addition, UPC was not associated with incidence of AEs. Across all adjusted models, neither index was "significantly associated" with the incidence of AEs.
Lead author Kevin O'Leary, MD, MS, SFHM, of Northwestern University's Feinberg School of Medicine in Chicago, says that the data could be used to help determine how best to structure handoffs.
"Where I think this has a major impact is that a whole lot of groups [are] trying to figure out how long should our rotation length be," Dr. O'Leary says. "All of those programs that are really trying to maximize continuity because they think it's the safest thing and best thing for patient outcomes, they can probably relax a little bit and swing the pendulum a little bit further toward what they think is the right model for the work-life balance of their hospitalist. [They can] worry a little bit less about the impact on the patients because there doesn't seem to be much." TH
Visit our website for more information on transitions of care.
Perioperative Hyperglycemia Increases Risk of Poor Outcomes in Nondiabetics
Clinical question: How does perioperative hyperglycemia affect the risk of adverse events in diabetic patients compared to nondiabetic patients?
Background: Perioperative hyperglycemia is associated with increased rates of infection, myocardial infarction, stroke, and death. Recent studies suggest that nondiabetics are more prone to hyperglycemia-related complications than diabetics. This study sought to analyze the effect and mechanism by which nondiabetics may be at increased risk for such complications.
Study design: Retrospective cohort study.
Setting: Fifty-three hospitals in Washington.
Synopsis: Among 40,836 patients who underwent surgery, diabetics had a higher rate of perioperative adverse events overall compared to nondiabetics (12% versus 9%, P<0.001). Perioperative hyperglycemia, defined as blood glucose 180 or greater, was also associated with an increased rate of adverse events. Ironically, this association was more significant in nondiabetic patients (odds ratio, 1.6; 95% CI, 1.3–2.1) than in diabetic patients (odds ratio, 0.8; 95% CI, 0.6–1.0). Although the exact reason for this is unknown, existing theories include the following:
- Diabetics are more apt to receive insulin for perioperative hyperglycemia than nondiabetics (P<0.001);
- Hyperglycemia in diabetics may be a less-reliable marker of surgical stress than in nondiabetics; and
- Diabetics may be better adapted to hyperglycemia than nondiabetics.
Bottom line: Perioperative hyperglycemia leads to an increased risk of adverse events; this relationship is more pronounced in nondiabetic patients than in diabetic patients.
Citation: Kotagal M, Symons RG, Hirsch IB, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015;261(1):97–103. TH
Visit our website for more physician reviews of hospitalist-focused literature.
Clinical question: How does perioperative hyperglycemia affect the risk of adverse events in diabetic patients compared to nondiabetic patients?
Background: Perioperative hyperglycemia is associated with increased rates of infection, myocardial infarction, stroke, and death. Recent studies suggest that nondiabetics are more prone to hyperglycemia-related complications than diabetics. This study sought to analyze the effect and mechanism by which nondiabetics may be at increased risk for such complications.
Study design: Retrospective cohort study.
Setting: Fifty-three hospitals in Washington.
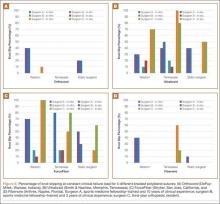
Synopsis: Among 40,836 patients who underwent surgery, diabetics had a higher rate of perioperative adverse events overall compared to nondiabetics (12% versus 9%, P<0.001). Perioperative hyperglycemia, defined as blood glucose 180 or greater, was also associated with an increased rate of adverse events. Ironically, this association was more significant in nondiabetic patients (odds ratio, 1.6; 95% CI, 1.3–2.1) than in diabetic patients (odds ratio, 0.8; 95% CI, 0.6–1.0). Although the exact reason for this is unknown, existing theories include the following:
- Diabetics are more apt to receive insulin for perioperative hyperglycemia than nondiabetics (P<0.001);
- Hyperglycemia in diabetics may be a less-reliable marker of surgical stress than in nondiabetics; and
- Diabetics may be better adapted to hyperglycemia than nondiabetics.
Bottom line: Perioperative hyperglycemia leads to an increased risk of adverse events; this relationship is more pronounced in nondiabetic patients than in diabetic patients.
Citation: Kotagal M, Symons RG, Hirsch IB, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015;261(1):97–103. TH
Visit our website for more physician reviews of hospitalist-focused literature.
Clinical question: How does perioperative hyperglycemia affect the risk of adverse events in diabetic patients compared to nondiabetic patients?
Background: Perioperative hyperglycemia is associated with increased rates of infection, myocardial infarction, stroke, and death. Recent studies suggest that nondiabetics are more prone to hyperglycemia-related complications than diabetics. This study sought to analyze the effect and mechanism by which nondiabetics may be at increased risk for such complications.
Study design: Retrospective cohort study.
Setting: Fifty-three hospitals in Washington.
Synopsis: Among 40,836 patients who underwent surgery, diabetics had a higher rate of perioperative adverse events overall compared to nondiabetics (12% versus 9%, P<0.001). Perioperative hyperglycemia, defined as blood glucose 180 or greater, was also associated with an increased rate of adverse events. Ironically, this association was more significant in nondiabetic patients (odds ratio, 1.6; 95% CI, 1.3–2.1) than in diabetic patients (odds ratio, 0.8; 95% CI, 0.6–1.0). Although the exact reason for this is unknown, existing theories include the following:
- Diabetics are more apt to receive insulin for perioperative hyperglycemia than nondiabetics (P<0.001);
- Hyperglycemia in diabetics may be a less-reliable marker of surgical stress than in nondiabetics; and
- Diabetics may be better adapted to hyperglycemia than nondiabetics.
Bottom line: Perioperative hyperglycemia leads to an increased risk of adverse events; this relationship is more pronounced in nondiabetic patients than in diabetic patients.
Citation: Kotagal M, Symons RG, Hirsch IB, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015;261(1):97–103. TH
Visit our website for more physician reviews of hospitalist-focused literature.
Greater Auricular Nerve Palsy After Arthroscopic Anterior-Inferior and Posterior-Inferior Labral Tear Repair Using Beach-Chair Positioning and a Standard Universal Headrest
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
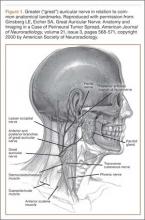
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
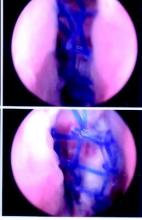
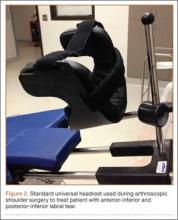
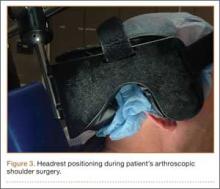
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
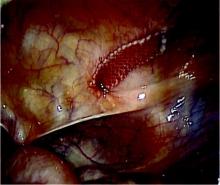
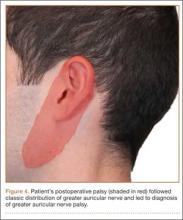
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
Private-academic surgeon salary gap raises concerns Lifestyle choice important Not just the money
LAKE BUENA VISTA, FLA. – Academic surgeons earn an average of 10% or $1.3 million less in gross income across their lifetime than surgeons in private practice, an analysis shows.
Some surgical specialties fare better than others, with academic neurosurgeons having the largest reduction in gross income at $4.2 million (–24.2%), while academic pediatric surgeons earn $238,376 more (1.53%) than their private practice counterparts. They were the only ones to do so.
Several academic surgical specialties did not make the 10% average, including trauma surgeons whose lifetime earnings were down 12% or $2.4 million, vascular surgeons at 13.8% or $1.7 million, and surgical oncologists at 12.2% or $1.3 million.
“The concern that we have is that the academic surgeons are where the education of the future lies,” lead study author Dr. Joseph Martin Lopez said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma (EAST).
Every year a new class of surgeons is faced with the question of academic practice or private practice, but they are also struggling with increasing student loan debt and longer training as more surgical residents elect to enter fellowship rather than general practice.
This growing financial liability coupled with declining physician reimbursement could rapidly shift physician practices and thus threaten the fiscal viability of certain surgical fields or academic surgical careers.
“The more financially irresponsible you make it to become an academic surgeon, the more we put at risk our current mode of training,” Dr. Lopez of Wake Forest University in Winston-Salem, N.C., said.
To account for additional factors outside gross income, the investigators ran the numbers using a second analysis, a net present value calculation, however, and came up with roughly the same salary gap to contend with.
Net present value (NPV) calculations are commonly used in business to calculate the profitability of an investment and also have been used in the medical field to gauge return on investment for various careers. The NPV calculation accounts for positive and negative cash flows over the entire length of a career, using in this case, a 5% discount rate and adjusting for inflation, Dr. Lopez explained.
Both the lifetime gross income and 5% NPV calculation used data from the Medical Group Management Association’s 2012 physician salary report, the 2012 Association of American Medical Colleges physician salary report, and the AAMC database for residency and fellow salary.
The NPV assumed a career length of 37-39 years, based on a retirement age of 65 years for all specialties. Positive cash flows included annual salary less federal income tax. Negative cash flows included the average principal for student loans, according to the AAMC, and interest at 5%, the average for the three largest student loan lenders in 2014, he said. Student loan repayment was calculated for a fixed-rate loan to be paid over 25 years beginning after residency or any required fellowship.
The average reduction in 5% NPV across surgical specialties for an academic surgeon versus a privately employed surgeon was 12.8% or $246,499, Dr. Lopez said.
Once again, academic neurosurgeons had the largest reduction in 5% NPV at 25.5% or a loss of $619,681, followed closely by trauma surgeons (23% or $381,179) and surgical oncologists (16.3% or $256,373). Academic pediatric surgeons had the smallest reduction in 5% NPV at 4.2% or $88,827.
During a discussion of the provocative poster, attendees questioned whether it was fair to say that private surgeons make more money without acknowledging the risk they face, compared with surgeons employed in an academic setting.
Dr. Lopez countered that, increasingly, even private surgeons are no longer truly private surgeons.
“More and more surgical groups are being bought up by hospitals, and even the private surgical groups are being bought up by hospitals, which does stabilize your income to some extent,” he said.
“We all still have [relative value unit] goals to meet and RVU incentives that make it so you can get paid a little more, but it’s something that’s a consideration. It is a risk-reward to be a private surgeon. Depending on how your contract is structured or how your group decides to pay the partners, it may be that if you don’t take very much call or take that many cases, you’ll end up on the short end of the stick.”
Dr. Ben L. Zarzaur, a general surgeon at Indiana University in Indianapolis who comoderated the poster discussion, pointed out that market pressures unaccounted for in the model can dramatically influence a surgeon’s salary over a lifetime.
Dr. Lopez agreed, citing how the increasing number of stent placements by cardiologists, for example, has impacted the bottom line of cardiothoracic surgeons. The NPV calculation was specifically used, however, because it gets at market forces such as inflation and return on investment, not addressed by gross income figures alone.
Finally, Dr. Zarzaur turned and asked the relatively young crowd what they would do if offered $600,000 a year, but had to work 110 hours a week or could get $250,000 and work only 40 hours a week.
Most responded that they’d choose the former to repay their student loans and then switch to the lower-paying position.
Responders made much of job satisfaction, work-life balance, and the ability of surgeons in academic practice to take time away from clinical work to conduct research, their ready access to continuing medical education, and their ability to educate the next generation of surgeons.
“Any time we see this academic-private disparity, you have to think about these secondary gains,” Dr. Zarzaur said.
“This is really interesting work. It gets into why we choose what we do, why we’d take $600,000, work 110 hours a week, and get our rear ends kicked. The flip side is, if I saw this, why would you ever go into academics? But people still choose to do it. I’m in academics so there’s a bias, but we choose to do it anyway up to a point. I don’t know where that point is, but up to a point we do.”
Not just the money

|
| Dr. Laura Drudi |
In the United States, academic vascular surgeons earn 13.8% or $1.7 million less than private vascular surgeons. This financial incentive may influence graduating residents and fellows to enter into private practice. This article indicates that this financial disparity may cost academic institutions the expertise needed to train future physicians. Unfortunately, I believe this analysis falls into one of the many myths between academic and private practice; that is, it’s not only about making the most money possible.
The ongoing debate of academic versus private practice shouldn’t really be a debate at all. It is all about personal choices concerning research, education, work-life balance, and finances to name a few. In the end, anyone can shape the ideal practice they want to have. There are many private practices that are involved in resident education, publish extensively and present at national and international meetings. No job is weaved perfectly, but there will usually be a job that fits an individual’s specific goals and desires.
Dr. Laura Drudi is the resident medical editor for Vascular Specialist.
Lifestyle choice important
The basic finding of the disparity is in fact true leaving aside the flawed methodology of too many assumptions by including all academic ranks, practitioners of different durations in practice, difference in benefits, tuition assistance, and assuming student loans for all surgeons plus a risk free rate that is too high.
Our analysis of both vascular and general surgeon compensation points to a larger disparity at junior academic ranks over the last decade. With our own studies showing a shortage of vascular surgeons, retention of practitioners is paramount for all health systems. Academic centers rely on faculty giving up a percentage of their compensation for the pleasure of teaching, research and intellectual stimulation. The unanswered question is: How much of a disparity will junior academic surgeons tolerate, and how do they value lifestyle against additional compensation? Time will tell.
Dr. Bhagwan Satiani is a professor of vascular surgery at the Wexner Medical Center, Ohio State University.
Not just the money

|
| Dr. Laura Drudi |
In the United States, academic vascular surgeons earn 13.8% or $1.7 million less than private vascular surgeons. This financial incentive may influence graduating residents and fellows to enter into private practice. This article indicates that this financial disparity may cost academic institutions the expertise needed to train future physicians. Unfortunately, I believe this analysis falls into one of the many myths between academic and private practice; that is, it’s not only about making the most money possible.
The ongoing debate of academic versus private practice shouldn’t really be a debate at all. It is all about personal choices concerning research, education, work-life balance, and finances to name a few. In the end, anyone can shape the ideal practice they want to have. There are many private practices that are involved in resident education, publish extensively and present at national and international meetings. No job is weaved perfectly, but there will usually be a job that fits an individual’s specific goals and desires.
Dr. Laura Drudi is the resident medical editor for Vascular Specialist.
Lifestyle choice important
The basic finding of the disparity is in fact true leaving aside the flawed methodology of too many assumptions by including all academic ranks, practitioners of different durations in practice, difference in benefits, tuition assistance, and assuming student loans for all surgeons plus a risk free rate that is too high.
Our analysis of both vascular and general surgeon compensation points to a larger disparity at junior academic ranks over the last decade. With our own studies showing a shortage of vascular surgeons, retention of practitioners is paramount for all health systems. Academic centers rely on faculty giving up a percentage of their compensation for the pleasure of teaching, research and intellectual stimulation. The unanswered question is: How much of a disparity will junior academic surgeons tolerate, and how do they value lifestyle against additional compensation? Time will tell.
Dr. Bhagwan Satiani is a professor of vascular surgery at the Wexner Medical Center, Ohio State University.
Not just the money

|
| Dr. Laura Drudi |
In the United States, academic vascular surgeons earn 13.8% or $1.7 million less than private vascular surgeons. This financial incentive may influence graduating residents and fellows to enter into private practice. This article indicates that this financial disparity may cost academic institutions the expertise needed to train future physicians. Unfortunately, I believe this analysis falls into one of the many myths between academic and private practice; that is, it’s not only about making the most money possible.
The ongoing debate of academic versus private practice shouldn’t really be a debate at all. It is all about personal choices concerning research, education, work-life balance, and finances to name a few. In the end, anyone can shape the ideal practice they want to have. There are many private practices that are involved in resident education, publish extensively and present at national and international meetings. No job is weaved perfectly, but there will usually be a job that fits an individual’s specific goals and desires.
Dr. Laura Drudi is the resident medical editor for Vascular Specialist.
Lifestyle choice important
The basic finding of the disparity is in fact true leaving aside the flawed methodology of too many assumptions by including all academic ranks, practitioners of different durations in practice, difference in benefits, tuition assistance, and assuming student loans for all surgeons plus a risk free rate that is too high.
Our analysis of both vascular and general surgeon compensation points to a larger disparity at junior academic ranks over the last decade. With our own studies showing a shortage of vascular surgeons, retention of practitioners is paramount for all health systems. Academic centers rely on faculty giving up a percentage of their compensation for the pleasure of teaching, research and intellectual stimulation. The unanswered question is: How much of a disparity will junior academic surgeons tolerate, and how do they value lifestyle against additional compensation? Time will tell.
Dr. Bhagwan Satiani is a professor of vascular surgery at the Wexner Medical Center, Ohio State University.
LAKE BUENA VISTA, FLA. – Academic surgeons earn an average of 10% or $1.3 million less in gross income across their lifetime than surgeons in private practice, an analysis shows.
Some surgical specialties fare better than others, with academic neurosurgeons having the largest reduction in gross income at $4.2 million (–24.2%), while academic pediatric surgeons earn $238,376 more (1.53%) than their private practice counterparts. They were the only ones to do so.
Several academic surgical specialties did not make the 10% average, including trauma surgeons whose lifetime earnings were down 12% or $2.4 million, vascular surgeons at 13.8% or $1.7 million, and surgical oncologists at 12.2% or $1.3 million.
“The concern that we have is that the academic surgeons are where the education of the future lies,” lead study author Dr. Joseph Martin Lopez said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma (EAST).
Every year a new class of surgeons is faced with the question of academic practice or private practice, but they are also struggling with increasing student loan debt and longer training as more surgical residents elect to enter fellowship rather than general practice.
This growing financial liability coupled with declining physician reimbursement could rapidly shift physician practices and thus threaten the fiscal viability of certain surgical fields or academic surgical careers.
“The more financially irresponsible you make it to become an academic surgeon, the more we put at risk our current mode of training,” Dr. Lopez of Wake Forest University in Winston-Salem, N.C., said.
To account for additional factors outside gross income, the investigators ran the numbers using a second analysis, a net present value calculation, however, and came up with roughly the same salary gap to contend with.
Net present value (NPV) calculations are commonly used in business to calculate the profitability of an investment and also have been used in the medical field to gauge return on investment for various careers. The NPV calculation accounts for positive and negative cash flows over the entire length of a career, using in this case, a 5% discount rate and adjusting for inflation, Dr. Lopez explained.
Both the lifetime gross income and 5% NPV calculation used data from the Medical Group Management Association’s 2012 physician salary report, the 2012 Association of American Medical Colleges physician salary report, and the AAMC database for residency and fellow salary.
The NPV assumed a career length of 37-39 years, based on a retirement age of 65 years for all specialties. Positive cash flows included annual salary less federal income tax. Negative cash flows included the average principal for student loans, according to the AAMC, and interest at 5%, the average for the three largest student loan lenders in 2014, he said. Student loan repayment was calculated for a fixed-rate loan to be paid over 25 years beginning after residency or any required fellowship.
The average reduction in 5% NPV across surgical specialties for an academic surgeon versus a privately employed surgeon was 12.8% or $246,499, Dr. Lopez said.
Once again, academic neurosurgeons had the largest reduction in 5% NPV at 25.5% or a loss of $619,681, followed closely by trauma surgeons (23% or $381,179) and surgical oncologists (16.3% or $256,373). Academic pediatric surgeons had the smallest reduction in 5% NPV at 4.2% or $88,827.
During a discussion of the provocative poster, attendees questioned whether it was fair to say that private surgeons make more money without acknowledging the risk they face, compared with surgeons employed in an academic setting.
Dr. Lopez countered that, increasingly, even private surgeons are no longer truly private surgeons.
“More and more surgical groups are being bought up by hospitals, and even the private surgical groups are being bought up by hospitals, which does stabilize your income to some extent,” he said.
“We all still have [relative value unit] goals to meet and RVU incentives that make it so you can get paid a little more, but it’s something that’s a consideration. It is a risk-reward to be a private surgeon. Depending on how your contract is structured or how your group decides to pay the partners, it may be that if you don’t take very much call or take that many cases, you’ll end up on the short end of the stick.”
Dr. Ben L. Zarzaur, a general surgeon at Indiana University in Indianapolis who comoderated the poster discussion, pointed out that market pressures unaccounted for in the model can dramatically influence a surgeon’s salary over a lifetime.
Dr. Lopez agreed, citing how the increasing number of stent placements by cardiologists, for example, has impacted the bottom line of cardiothoracic surgeons. The NPV calculation was specifically used, however, because it gets at market forces such as inflation and return on investment, not addressed by gross income figures alone.
Finally, Dr. Zarzaur turned and asked the relatively young crowd what they would do if offered $600,000 a year, but had to work 110 hours a week or could get $250,000 and work only 40 hours a week.
Most responded that they’d choose the former to repay their student loans and then switch to the lower-paying position.
Responders made much of job satisfaction, work-life balance, and the ability of surgeons in academic practice to take time away from clinical work to conduct research, their ready access to continuing medical education, and their ability to educate the next generation of surgeons.
“Any time we see this academic-private disparity, you have to think about these secondary gains,” Dr. Zarzaur said.
“This is really interesting work. It gets into why we choose what we do, why we’d take $600,000, work 110 hours a week, and get our rear ends kicked. The flip side is, if I saw this, why would you ever go into academics? But people still choose to do it. I’m in academics so there’s a bias, but we choose to do it anyway up to a point. I don’t know where that point is, but up to a point we do.”
LAKE BUENA VISTA, FLA. – Academic surgeons earn an average of 10% or $1.3 million less in gross income across their lifetime than surgeons in private practice, an analysis shows.
Some surgical specialties fare better than others, with academic neurosurgeons having the largest reduction in gross income at $4.2 million (–24.2%), while academic pediatric surgeons earn $238,376 more (1.53%) than their private practice counterparts. They were the only ones to do so.
Several academic surgical specialties did not make the 10% average, including trauma surgeons whose lifetime earnings were down 12% or $2.4 million, vascular surgeons at 13.8% or $1.7 million, and surgical oncologists at 12.2% or $1.3 million.
“The concern that we have is that the academic surgeons are where the education of the future lies,” lead study author Dr. Joseph Martin Lopez said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma (EAST).
Every year a new class of surgeons is faced with the question of academic practice or private practice, but they are also struggling with increasing student loan debt and longer training as more surgical residents elect to enter fellowship rather than general practice.
This growing financial liability coupled with declining physician reimbursement could rapidly shift physician practices and thus threaten the fiscal viability of certain surgical fields or academic surgical careers.
“The more financially irresponsible you make it to become an academic surgeon, the more we put at risk our current mode of training,” Dr. Lopez of Wake Forest University in Winston-Salem, N.C., said.
To account for additional factors outside gross income, the investigators ran the numbers using a second analysis, a net present value calculation, however, and came up with roughly the same salary gap to contend with.
Net present value (NPV) calculations are commonly used in business to calculate the profitability of an investment and also have been used in the medical field to gauge return on investment for various careers. The NPV calculation accounts for positive and negative cash flows over the entire length of a career, using in this case, a 5% discount rate and adjusting for inflation, Dr. Lopez explained.
Both the lifetime gross income and 5% NPV calculation used data from the Medical Group Management Association’s 2012 physician salary report, the 2012 Association of American Medical Colleges physician salary report, and the AAMC database for residency and fellow salary.
The NPV assumed a career length of 37-39 years, based on a retirement age of 65 years for all specialties. Positive cash flows included annual salary less federal income tax. Negative cash flows included the average principal for student loans, according to the AAMC, and interest at 5%, the average for the three largest student loan lenders in 2014, he said. Student loan repayment was calculated for a fixed-rate loan to be paid over 25 years beginning after residency or any required fellowship.
The average reduction in 5% NPV across surgical specialties for an academic surgeon versus a privately employed surgeon was 12.8% or $246,499, Dr. Lopez said.
Once again, academic neurosurgeons had the largest reduction in 5% NPV at 25.5% or a loss of $619,681, followed closely by trauma surgeons (23% or $381,179) and surgical oncologists (16.3% or $256,373). Academic pediatric surgeons had the smallest reduction in 5% NPV at 4.2% or $88,827.
During a discussion of the provocative poster, attendees questioned whether it was fair to say that private surgeons make more money without acknowledging the risk they face, compared with surgeons employed in an academic setting.
Dr. Lopez countered that, increasingly, even private surgeons are no longer truly private surgeons.
“More and more surgical groups are being bought up by hospitals, and even the private surgical groups are being bought up by hospitals, which does stabilize your income to some extent,” he said.
“We all still have [relative value unit] goals to meet and RVU incentives that make it so you can get paid a little more, but it’s something that’s a consideration. It is a risk-reward to be a private surgeon. Depending on how your contract is structured or how your group decides to pay the partners, it may be that if you don’t take very much call or take that many cases, you’ll end up on the short end of the stick.”
Dr. Ben L. Zarzaur, a general surgeon at Indiana University in Indianapolis who comoderated the poster discussion, pointed out that market pressures unaccounted for in the model can dramatically influence a surgeon’s salary over a lifetime.
Dr. Lopez agreed, citing how the increasing number of stent placements by cardiologists, for example, has impacted the bottom line of cardiothoracic surgeons. The NPV calculation was specifically used, however, because it gets at market forces such as inflation and return on investment, not addressed by gross income figures alone.
Finally, Dr. Zarzaur turned and asked the relatively young crowd what they would do if offered $600,000 a year, but had to work 110 hours a week or could get $250,000 and work only 40 hours a week.
Most responded that they’d choose the former to repay their student loans and then switch to the lower-paying position.
Responders made much of job satisfaction, work-life balance, and the ability of surgeons in academic practice to take time away from clinical work to conduct research, their ready access to continuing medical education, and their ability to educate the next generation of surgeons.
“Any time we see this academic-private disparity, you have to think about these secondary gains,” Dr. Zarzaur said.
“This is really interesting work. It gets into why we choose what we do, why we’d take $600,000, work 110 hours a week, and get our rear ends kicked. The flip side is, if I saw this, why would you ever go into academics? But people still choose to do it. I’m in academics so there’s a bias, but we choose to do it anyway up to a point. I don’t know where that point is, but up to a point we do.”
In Vitro and In Situ Characterization of Arthroscopic Loop Security and Knot Security of Braided Polyblend Sutures: A Biomechanical Study
Open-surgery knot tying is easily learned and performed, but knot tying during arthroscopic procedures can be both challenging and frustrating. According to Burkhart and colleagues,1,2 knot security is defined as the effectiveness of the knot in resisting slippage when load is applied, whereas loop security is the effectiveness in maintaining a tight suture loop while a knot is being tied. Arthroscopic knots commonly begin with an initial slipknot locked in place with a series of half-hitches. During arthroscopic surgery, the surgeon usually must tie an arthroscopic knot to obtain secure tissue fixation, an essential component of soft-tissue repair. A secure knot provides optimal tissue apposition for healing, which will ultimately improve functional outcome. For a knot to be effective, it must have both knot security and loop security. Knot security depends on knot configuration, the coefficient of friction, ductility, handling properties, solubility and diameter of suture material, internal interference, slack between throws, and surgeon experience. Tissue fluid and tissue reaction to suture material may affect knot and loop security.
The ideal knot would be easy to tie and reproducible and would not slip or stretch before tissue is healed. The ideal suture material should provide adequate strength to hold soft tissue in an anatomically correct position until healing can occur. It should also be easily and efficiently manipulated by arthroscopic means when tissues are being secured with knots and secure suture loops. Studies have been conducted to evaluate the security of knots tied with arthroscopic techniques, knot configurations, and suture materials, and these investigations have often evaluated knot performance under single load-to-failure (LTF) test scenarios and cyclic loading in vitro (dry environment) in a room-temperature environment.2-10 To our knowledge, few if any attempts have been made to simulate in situ conditions at body temperature when testing knot security. The fluid environment and the temperature could potentially affect the effectiveness of knots, as knot security depends on friction, internal interference, and slack between throws.1
We conducted a study to evaluate biomechanical performance (knot security, loop security) during destructive testing of several different suture materials with various arthroscopic knot configurations. The study was performed under in vitro (dry environment) and in situ (wet environment) conditions by surgeons with different levels of experience.
Materials and Methods
This investigation was conducted at the Orthopaedic Research Institute at Via Christi Health in Wichita, Kansas. The study compared 4 different suture materials tied with 3 different commonly used arthroscopic knots by 3 surgeons with different levels of experience. The 4 types of braided polyblend polyethylene sutures were Fiberwire (Arthrex, Naples, Florida), ForceFiber (Stryker, San Jose, California), Orthocord (DePuy-Mitek, Warsaw, Indiana), and Ultrabraid (Smith & Nephew, Memphis, Tennessee). Each suture material was tied with 3 arthroscopic knots—static surgeon’s knot, Weston knot,11 Tennessee slider12—and a series of 3 reversing half-hitches on alternating posts (RHAPs) (Figure 1). These knots were chosen based on studies showing they have a higher maximum force to failure when combined with 3 RHAPs.1,2,5,9,13-17
We evaluated performer variability with the help of 3 investigator-surgeons who differed in their level of experience tying arthroscopic knots. This experience was defined on the basis of total number of arthroscopies performed—one of the most important factors predicting basic arthroscopic skills. Our surgeon A was a sports medicine fellowship–trained surgeon with 10 years of experience and a significant number of arthroscopies performed annually (350); surgeon B was a sports medicine fellowship–trained surgeon with 3 years of experience and an annual arthroscopy volume of more than 250 procedures; and surgeon C was a third-year orthopedic resident with about 100 arthroscopies performed.
All knots were tied on a standardized post 30 mm in circumference, which provided a consistent starting circumference for each knot and replicated the suture loop created during arthroscopic rotator cuff repair. All knots were tied using standard arthroscopic techniques, with a standard knot pusher and a modified arthroscopic cannula, in a dry environment (Figure 2). Servohydraulic materials testing system instruments (model 810; MTS Systems, Eden Prairie, Minnesota) were used to test the knot security and loop security of each combination of knots and suture types. Two round hooks (diameter, 3.9 mm) were attached to the actuator and the load cell (Figure 3). Loops were preloaded to 6 N to avoid potential errors caused by slack in the loops or by stretching of suture materials and to provide a well-defined starting point for data recording.
LTF testing was performed for both in vitro and in situ conditions using 10 samples of each suture–knot configuration for each mechanical testing. Each type of testing was conducted for a total of 240 suture–knot combinations per investigator. For the in vitro condition, each suture loop was initiated with 5 preconditioning loading cycles, from 6 N to 30 N at 1 Hz. The load was then applied continuously at a crosshead speed of 1 mm/s until “clinical failure” (3 mm crosshead displacement). We used this criterion for clinical failure, as studies have indicated that 3 mm is the point at which tissue apposition is lost.15,18-21 After the crosshead reached the 3-mm displacement, the loads (under load control) were held for 5 minutes at maximum load, and then load was applied continuously at a crosshead speed of 1 mm/s until complete structure failure. Load and displacement data were collected at a frequency of 20 Hz.
For the in situ condition, the same test parameters were used, except that each combination of the suture loop was preloaded to 6 N and soaked in physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing in an effort to simulate the aqueous medium in vivo after surgery. The in situ tests were performed under physiologic solution maintained at 37°C to approximate postoperative physical conditions.
Statistical Analysis
Means and standard deviations of the knot security and loop security achieved by the surgeons (different experience levels) were calculated for each test configuration and each test condition. These values were used to determine the statistical relevance of the difference in arthroscopic loop security and knot security in each configuration. One-way analysis of variance (ANOVA) performed with SPSS Version 19.0 software (SPSS, Chicago, Illinois) with the least significant difference (LSD) multiple comparisons post hoc analysis was used to determine if any observed differences between the types of braided polyblend sutures, the types of sliding knots, the test conditions (in vitro, in situ), and the levels of surgeon experience were significant for each knot configuration. The level of significance of differences was set at P < .001.
Results
Figure 4 shows the mean maximum clinical failure load (3 mm of displacement) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. In the comparison of biomechanical performance (knot and loop security) under in vitro and in situ conditions, no significant difference was detected when Ultrabraid suture material was used, regardless of surgeon experience, for all knot configurations. For surgeon B, there was no significant difference between in vitro and in situ conditions for any knot configurations or suture materials. When Orthocord suture material was used, Weston knots tied by surgeon A, and static surgeon’s knots by surgeons A and C, resulted in a significant difference between the in vitro and in situ conditions. When ForceFiber suture material was used, only Weston knots and Tennessee slider knots by surgeon A had a significant difference between in vitro and in situ conditions. Weston knots by surgeon A exhibited a significant difference between in vitro and in situ conditions, except when Ultrabraid suture material was used.
Surgeon C’s Tennessee slider knots with all polyblend sutures showed significantly lower loads at clinical failure compared with all the other knot configurations and with knots tied by the other 2 surgeons under both in vitro and in situ conditions. Overall, knots tied by surgeon B had higher clinical failure load than knots tied by the other 2 surgeons.
Figure 5 shows the mean ultimate failure load (complete structural failure) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. Knots tied with Orthocord suture material had the overall lower ultimate failure load compared with other suture materials, whereas knots tied with Ultrabraid suture material had the overall highest ultimate failure load. However, the ultimate failure loads for all the knots tied using any suture material, regardless of surgeon experience, were more than 61 N, which is the estimated minimum required ultimate load per suture during a maximum muscle contraction.1
Figure 6 shows the percentage of knot slipping at constant clinical failure load. Orthocord and Fiberwire suture materials had the lowest incidence of knot slippage. Surgeon C had complete knot slippage at constant clinical failure load using ForceFiber with the Weston knot and Ultrabraid with the Tennessee slider knot. When using Ultrabraid or ForceFiber, surgeons A and C had at least 2 knots slip for all knot configurations.
Discussion
Optimization of knot security for any given knot configuration, suture material, and surgeon experience level during arthroscopic knot tying is crucial.1-10 Our study results showed that, under single LTF test scenarios, there was a significant difference between in vitro and in situ conditions with respect to both knot configuration and surgeon experience level, except when Ultrabraid suture material was used. Arthroscopic sliding knots are lockable or nonlockable.7,12 With lockable sliding knots, slippage may be prevented by tensioning the wrapping limb, which distorts the post in the distal part of the knot, resulting in a kink in the post, thereby increasing the internal interference that increases the resistance of the knot from backing off. With nonlockable sliding knots, slippage may be prevented by the tight grip of the wrappings around the initial post.7 The static surgeon’s knot and the Tennessee slider knot are nonlockable, whereas the Weston knot is a distal lockable sliding knot. Compared with nonlockable sliding knots, lockable sliding knots cause less suture loop enlargement. In 1976, Tera and Aberg22 studied the strength of knotted thread for 12 different types of suture knots combined with 11 types of suture material. They conducted their study 1 week after suture material was inserted into the subcutaneous tissue of rabbits. Their results show a greater propensity for certain suture materials to slip when tested in an aqueous environment. In 1998, Babetty and colleagues23 used Wistar rats to compare the in vivo strength, knot efficiency, and knot security of 4 types of sliding knots and to assess tissue reaction as a result of knot configuration, knot volume, and suture size. They found that 4/0 knots lost more strength than 2/0 knots did, and they concluded that the tissue response to all the knots, except 2/0 nylon, was similar. They indicated that the inflammatory sheath volume varied with knot volume, suture size, and knot configuration. Our results agree with observations that exposure to an aqueous environment alters the force to clinical failure of comparable suture and knot configurations.
In addition, our findings indicate that surgeon familiarity with certain knots has a major effect on knot security. The difference in our 3 surgeons’ levels of familiarity with certain knots was somewhat minimized by the knot tying they practiced before submitting knots for testing. The findings contrast with those of Milia and colleagues,24 who conducted a biomechanical study to determine the effect of experience level on knot security. They compared an experienced arthroscopic shoulder surgeon with a junior-level orthopedic resident surgeon and concluded that experience did not affect knot security. However, the knots in their study were tied by hand, not through an arthroscopic cannula with instruments. Our findings suggest that both experienced and less experienced orthopedic residents should be encouraged to practice arthroscopic knot tying in a nonsurgical environment in order to become comfortable tying arthroscopic knots.
Braided nonabsorbable polyester suture traditionally has been found to be stronger than monofilament absorbable polydioxanone (PDS) and to have less slippage potential.8,9,25 Several studies have determined that the braided polyblend sutures now commonly used for arthroscopic knots have better strength profiles over more traditional materials.12,26,27 Orthocord has a dyed absorbable core (PDS, 68%), an undyed nonabsorbable ultrahigh-molecular-weight polyethylene (UHMWPE, 32%) sleeve, and a polyglactin coating.9,10 Both Ultrabraid and ForceFiber are made with braided UHMWPE and have just a few variations in weave patterns. Fiberwire has a multifiber UHMWPE core covered with braided polyester suture material. Several biomechanical studies25,26,28 have evaluated different arthroscopic sliding knot configurations with different suture materials, and all concluded that a surgeon who is choosing an arthroscopic repair technique should know the differences in suture materials and the knot strengths afforded by different knot configurations, as suture material is an important aspect of loop security. Our findings agree with their findings, that suture materials have a major effect on knot security, even with a series of 3 RHAPs, as in theory the RHAPs should minimize suture friction, internal interference, and slack between knot loops—emphasizing the effect of material selection. Furthermore, our findings also indicated that suture materials with a core in their design (Fiberwire, Orthocord) tend to have the lowest incidence of knot slippage. We had suspected that suture surface characteristics and suture construction could be important factors in knot slippage.
Our experimental design had its limitations. First, although we simulated factors such as temperature, plasma environment, and surgeon experience, tying a knot on a standardized post (30 mm in circumference) differed from what is typically done clinically. Second, the metal hooks used in this study were not compressible and did not interpose in the substance of the knot as soft tissue does in the clinical setting. Third, knots were tied with no tension against the sutures, whereas clinically knots are tied under tension as tissues are pulled together in reconstructions. Fourth, it was assumed that soaking in a physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing was sufficient to simulate the aqueous medium in vivo after surgery, but these parameters may not represent conditions in a patient who has just undergone an arthroscopic shoulder repair and adheres to a passive motion protocol. Fifth, there was no blinding of knot type, and there was no randomization of tying order or testing order. Sixth, only a single LTF test was performed, and incremental cyclic loading can be more useful, as it has long been recognized as a leading source of failure in orthopedic repairs.
Conclusion
These study results advance our overall understanding of the biomechanics of the different knot configurations and loop security levels of the different braided polyblend sutures used in arthroscopic procedures through LTF in both in vitro and in situ conditions. Overall, no suture material was superior to any other in a fluid environment, as the combination of aqueous environment and surgeon level of experience with arthroscopic knot tying has a major effect on knot security under single LTF test scenarios. However, our data showed that Ultrabraid suture material had no effect on knot effectiveness over the fluid environment and the temperature. Furthermore, the study showed that the Tennessee slider knot had the steepest learning curve. This study may provide an alternative arthroscopic knots option for soft-tissue repair in which use of certain suture materials is limited.
1. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202-207.
2. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Loop security as a determinant of tissue fixation security. Arthroscopy. 1998;14(7):773-776.
3. Elkousy H, Hammerman SM, Edwards TB, et al. The arthroscopic square knot: a biomechanical comparison with open and arthroscopic knots. Arthroscopy. 2006;22(7):736-741.
4. Elkousy HA, Sekiya JK, Stabile KJ, McMahon PJ. A biomechanical comparison of arthroscopic sliding and sliding-locking knots. Arthroscopy. 2005;21(2):204-210.
5. Ilahi OA, Younas SA, Alexander J, Noble PC. Cyclic testing of arthroscopic knot security. Arthroscopy. 2004;20(1):62-68.
6. Loutzenheiser TD, Harryman DT 2nd, Ziegler DW, Yung SW. Optimizing arthroscopic knots using braided or monofilament suture. Arthroscopy. 1998;14(1):57-65.
7. Chan KC, Burkhart SS, Thiagarajan P, Goh JC. Optimization of stacked half-hitch knots for arthroscopic surgery. Arthroscopy. 2001;17(7):752-759.
8. Lee TQ, Matsuura PA, Fogolin RP, Lin AC, Kim D, McMahon PJ. Arthroscopic suture tying: a comparison of knot types and suture materials. Arthroscopy. 2001;17(4):348-352.
9. Mishra DK, Cannon WD Jr, Lucas DJ, Belzer JP. Elongation of arthroscopically tied knots. Am J Sports Med. 1997;25(1):113-117.
10. Kim SH, Ha KI, Kim SH, Kim JS. Significance of the internal locking mechanism for loop security enhancement in the arthroscopic knot. Arthroscopy. 2001;17(8):850-855.
11. Weston PV. A new clinch knot. Obstet Gynecol. 1991;78(1):144-147.
12. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
13. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
14. De Beer JF, van Rooyen K, Boezaart AP. Nicky’s knot—a new slip knot for arthroscopic surgery. Arthroscopy. 1998;14(1):109-110.
15. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
16. Wetzler MJ, Bartolozzi AR, Gillespie MJ, et al. Fatigue properties of suture anchors in anterior shoulder reconstructions: Mitek GII. Arthroscopy. 1996;12(6):687-693.
17. Barber FA, Herbert MA, Beavis RC. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy. 2009;25(2):192-199.
18. Richmond JC. A comparison of ultrasonic suture welding and traditional knot tying. Am J Sports Med. 200;29(3):297-299.
19. James JD, Wu MM, Batra EK, Rodeheaver GT, Edlich RF. Technical considerations in manual and instrument tying techniques. J Emerg Med. 1992;10(4):469-480.
20. Batra EK, Franz DA, Towler MA, et al. Influence of emergency physician’s tying technique on knot security. J Emerg Med. 1992;10(3):309-316.
21. Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop. 2010;39(12):569-576.
22. Tera H, Aberg C. The strength of suture knots after one week in vivo. Acta Chir Scand. 1976;142(4):301-307.
23. Babetty Z, Sümer A, Altintaş S, Ergüney S, Göksel S. Changes in knot-holding capacity of sliding knots in vivo and tissue reaction. Arch Surg. 1998;133(7):727-734.
24. Milia MJ, Peindl RD, Connor PM. Arthroscopic knot tying: the role of instrumentation in achieving knot security. Arthroscopy. 2005;21(1):69-76.
25. Lieurance RK, Pflaster DS, Abbott D, Nottage WM. Failure characteristics of various arthroscopically tied knots. Clin Orthop. 2003;(408):311-318.
26. Abbi G, Espinoza L, Odell T, Mahar A, Pedowitz R. Evaluation of 5 knots and 2 suture materials for arthroscopic rotator cuff repair: very strong sutures can still slip. Arthroscopy. 2006;22(1):38-43.
27. Wüst DM, Meyer DC, Favre P, Gerber C. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22(11):1146-1153.
28. Mahar AT, Moezzi DM, Serra-Hsu F, Pedowitz RA. Comparison and performance characteristics of 3 different knots when tied with 2 suture materials used for shoulder arthroscopy. Arthroscopy. 2006;22(6):614.e1-e2.
Open-surgery knot tying is easily learned and performed, but knot tying during arthroscopic procedures can be both challenging and frustrating. According to Burkhart and colleagues,1,2 knot security is defined as the effectiveness of the knot in resisting slippage when load is applied, whereas loop security is the effectiveness in maintaining a tight suture loop while a knot is being tied. Arthroscopic knots commonly begin with an initial slipknot locked in place with a series of half-hitches. During arthroscopic surgery, the surgeon usually must tie an arthroscopic knot to obtain secure tissue fixation, an essential component of soft-tissue repair. A secure knot provides optimal tissue apposition for healing, which will ultimately improve functional outcome. For a knot to be effective, it must have both knot security and loop security. Knot security depends on knot configuration, the coefficient of friction, ductility, handling properties, solubility and diameter of suture material, internal interference, slack between throws, and surgeon experience. Tissue fluid and tissue reaction to suture material may affect knot and loop security.
The ideal knot would be easy to tie and reproducible and would not slip or stretch before tissue is healed. The ideal suture material should provide adequate strength to hold soft tissue in an anatomically correct position until healing can occur. It should also be easily and efficiently manipulated by arthroscopic means when tissues are being secured with knots and secure suture loops. Studies have been conducted to evaluate the security of knots tied with arthroscopic techniques, knot configurations, and suture materials, and these investigations have often evaluated knot performance under single load-to-failure (LTF) test scenarios and cyclic loading in vitro (dry environment) in a room-temperature environment.2-10 To our knowledge, few if any attempts have been made to simulate in situ conditions at body temperature when testing knot security. The fluid environment and the temperature could potentially affect the effectiveness of knots, as knot security depends on friction, internal interference, and slack between throws.1
We conducted a study to evaluate biomechanical performance (knot security, loop security) during destructive testing of several different suture materials with various arthroscopic knot configurations. The study was performed under in vitro (dry environment) and in situ (wet environment) conditions by surgeons with different levels of experience.
Materials and Methods
This investigation was conducted at the Orthopaedic Research Institute at Via Christi Health in Wichita, Kansas. The study compared 4 different suture materials tied with 3 different commonly used arthroscopic knots by 3 surgeons with different levels of experience. The 4 types of braided polyblend polyethylene sutures were Fiberwire (Arthrex, Naples, Florida), ForceFiber (Stryker, San Jose, California), Orthocord (DePuy-Mitek, Warsaw, Indiana), and Ultrabraid (Smith & Nephew, Memphis, Tennessee). Each suture material was tied with 3 arthroscopic knots—static surgeon’s knot, Weston knot,11 Tennessee slider12—and a series of 3 reversing half-hitches on alternating posts (RHAPs) (Figure 1). These knots were chosen based on studies showing they have a higher maximum force to failure when combined with 3 RHAPs.1,2,5,9,13-17
We evaluated performer variability with the help of 3 investigator-surgeons who differed in their level of experience tying arthroscopic knots. This experience was defined on the basis of total number of arthroscopies performed—one of the most important factors predicting basic arthroscopic skills. Our surgeon A was a sports medicine fellowship–trained surgeon with 10 years of experience and a significant number of arthroscopies performed annually (350); surgeon B was a sports medicine fellowship–trained surgeon with 3 years of experience and an annual arthroscopy volume of more than 250 procedures; and surgeon C was a third-year orthopedic resident with about 100 arthroscopies performed.
All knots were tied on a standardized post 30 mm in circumference, which provided a consistent starting circumference for each knot and replicated the suture loop created during arthroscopic rotator cuff repair. All knots were tied using standard arthroscopic techniques, with a standard knot pusher and a modified arthroscopic cannula, in a dry environment (Figure 2). Servohydraulic materials testing system instruments (model 810; MTS Systems, Eden Prairie, Minnesota) were used to test the knot security and loop security of each combination of knots and suture types. Two round hooks (diameter, 3.9 mm) were attached to the actuator and the load cell (Figure 3). Loops were preloaded to 6 N to avoid potential errors caused by slack in the loops or by stretching of suture materials and to provide a well-defined starting point for data recording.
LTF testing was performed for both in vitro and in situ conditions using 10 samples of each suture–knot configuration for each mechanical testing. Each type of testing was conducted for a total of 240 suture–knot combinations per investigator. For the in vitro condition, each suture loop was initiated with 5 preconditioning loading cycles, from 6 N to 30 N at 1 Hz. The load was then applied continuously at a crosshead speed of 1 mm/s until “clinical failure” (3 mm crosshead displacement). We used this criterion for clinical failure, as studies have indicated that 3 mm is the point at which tissue apposition is lost.15,18-21 After the crosshead reached the 3-mm displacement, the loads (under load control) were held for 5 minutes at maximum load, and then load was applied continuously at a crosshead speed of 1 mm/s until complete structure failure. Load and displacement data were collected at a frequency of 20 Hz.
For the in situ condition, the same test parameters were used, except that each combination of the suture loop was preloaded to 6 N and soaked in physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing in an effort to simulate the aqueous medium in vivo after surgery. The in situ tests were performed under physiologic solution maintained at 37°C to approximate postoperative physical conditions.
Statistical Analysis
Means and standard deviations of the knot security and loop security achieved by the surgeons (different experience levels) were calculated for each test configuration and each test condition. These values were used to determine the statistical relevance of the difference in arthroscopic loop security and knot security in each configuration. One-way analysis of variance (ANOVA) performed with SPSS Version 19.0 software (SPSS, Chicago, Illinois) with the least significant difference (LSD) multiple comparisons post hoc analysis was used to determine if any observed differences between the types of braided polyblend sutures, the types of sliding knots, the test conditions (in vitro, in situ), and the levels of surgeon experience were significant for each knot configuration. The level of significance of differences was set at P < .001.
Results
Figure 4 shows the mean maximum clinical failure load (3 mm of displacement) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. In the comparison of biomechanical performance (knot and loop security) under in vitro and in situ conditions, no significant difference was detected when Ultrabraid suture material was used, regardless of surgeon experience, for all knot configurations. For surgeon B, there was no significant difference between in vitro and in situ conditions for any knot configurations or suture materials. When Orthocord suture material was used, Weston knots tied by surgeon A, and static surgeon’s knots by surgeons A and C, resulted in a significant difference between the in vitro and in situ conditions. When ForceFiber suture material was used, only Weston knots and Tennessee slider knots by surgeon A had a significant difference between in vitro and in situ conditions. Weston knots by surgeon A exhibited a significant difference between in vitro and in situ conditions, except when Ultrabraid suture material was used.
Surgeon C’s Tennessee slider knots with all polyblend sutures showed significantly lower loads at clinical failure compared with all the other knot configurations and with knots tied by the other 2 surgeons under both in vitro and in situ conditions. Overall, knots tied by surgeon B had higher clinical failure load than knots tied by the other 2 surgeons.
Figure 5 shows the mean ultimate failure load (complete structural failure) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. Knots tied with Orthocord suture material had the overall lower ultimate failure load compared with other suture materials, whereas knots tied with Ultrabraid suture material had the overall highest ultimate failure load. However, the ultimate failure loads for all the knots tied using any suture material, regardless of surgeon experience, were more than 61 N, which is the estimated minimum required ultimate load per suture during a maximum muscle contraction.1
Figure 6 shows the percentage of knot slipping at constant clinical failure load. Orthocord and Fiberwire suture materials had the lowest incidence of knot slippage. Surgeon C had complete knot slippage at constant clinical failure load using ForceFiber with the Weston knot and Ultrabraid with the Tennessee slider knot. When using Ultrabraid or ForceFiber, surgeons A and C had at least 2 knots slip for all knot configurations.
Discussion
Optimization of knot security for any given knot configuration, suture material, and surgeon experience level during arthroscopic knot tying is crucial.1-10 Our study results showed that, under single LTF test scenarios, there was a significant difference between in vitro and in situ conditions with respect to both knot configuration and surgeon experience level, except when Ultrabraid suture material was used. Arthroscopic sliding knots are lockable or nonlockable.7,12 With lockable sliding knots, slippage may be prevented by tensioning the wrapping limb, which distorts the post in the distal part of the knot, resulting in a kink in the post, thereby increasing the internal interference that increases the resistance of the knot from backing off. With nonlockable sliding knots, slippage may be prevented by the tight grip of the wrappings around the initial post.7 The static surgeon’s knot and the Tennessee slider knot are nonlockable, whereas the Weston knot is a distal lockable sliding knot. Compared with nonlockable sliding knots, lockable sliding knots cause less suture loop enlargement. In 1976, Tera and Aberg22 studied the strength of knotted thread for 12 different types of suture knots combined with 11 types of suture material. They conducted their study 1 week after suture material was inserted into the subcutaneous tissue of rabbits. Their results show a greater propensity for certain suture materials to slip when tested in an aqueous environment. In 1998, Babetty and colleagues23 used Wistar rats to compare the in vivo strength, knot efficiency, and knot security of 4 types of sliding knots and to assess tissue reaction as a result of knot configuration, knot volume, and suture size. They found that 4/0 knots lost more strength than 2/0 knots did, and they concluded that the tissue response to all the knots, except 2/0 nylon, was similar. They indicated that the inflammatory sheath volume varied with knot volume, suture size, and knot configuration. Our results agree with observations that exposure to an aqueous environment alters the force to clinical failure of comparable suture and knot configurations.
In addition, our findings indicate that surgeon familiarity with certain knots has a major effect on knot security. The difference in our 3 surgeons’ levels of familiarity with certain knots was somewhat minimized by the knot tying they practiced before submitting knots for testing. The findings contrast with those of Milia and colleagues,24 who conducted a biomechanical study to determine the effect of experience level on knot security. They compared an experienced arthroscopic shoulder surgeon with a junior-level orthopedic resident surgeon and concluded that experience did not affect knot security. However, the knots in their study were tied by hand, not through an arthroscopic cannula with instruments. Our findings suggest that both experienced and less experienced orthopedic residents should be encouraged to practice arthroscopic knot tying in a nonsurgical environment in order to become comfortable tying arthroscopic knots.
Braided nonabsorbable polyester suture traditionally has been found to be stronger than monofilament absorbable polydioxanone (PDS) and to have less slippage potential.8,9,25 Several studies have determined that the braided polyblend sutures now commonly used for arthroscopic knots have better strength profiles over more traditional materials.12,26,27 Orthocord has a dyed absorbable core (PDS, 68%), an undyed nonabsorbable ultrahigh-molecular-weight polyethylene (UHMWPE, 32%) sleeve, and a polyglactin coating.9,10 Both Ultrabraid and ForceFiber are made with braided UHMWPE and have just a few variations in weave patterns. Fiberwire has a multifiber UHMWPE core covered with braided polyester suture material. Several biomechanical studies25,26,28 have evaluated different arthroscopic sliding knot configurations with different suture materials, and all concluded that a surgeon who is choosing an arthroscopic repair technique should know the differences in suture materials and the knot strengths afforded by different knot configurations, as suture material is an important aspect of loop security. Our findings agree with their findings, that suture materials have a major effect on knot security, even with a series of 3 RHAPs, as in theory the RHAPs should minimize suture friction, internal interference, and slack between knot loops—emphasizing the effect of material selection. Furthermore, our findings also indicated that suture materials with a core in their design (Fiberwire, Orthocord) tend to have the lowest incidence of knot slippage. We had suspected that suture surface characteristics and suture construction could be important factors in knot slippage.
Our experimental design had its limitations. First, although we simulated factors such as temperature, plasma environment, and surgeon experience, tying a knot on a standardized post (30 mm in circumference) differed from what is typically done clinically. Second, the metal hooks used in this study were not compressible and did not interpose in the substance of the knot as soft tissue does in the clinical setting. Third, knots were tied with no tension against the sutures, whereas clinically knots are tied under tension as tissues are pulled together in reconstructions. Fourth, it was assumed that soaking in a physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing was sufficient to simulate the aqueous medium in vivo after surgery, but these parameters may not represent conditions in a patient who has just undergone an arthroscopic shoulder repair and adheres to a passive motion protocol. Fifth, there was no blinding of knot type, and there was no randomization of tying order or testing order. Sixth, only a single LTF test was performed, and incremental cyclic loading can be more useful, as it has long been recognized as a leading source of failure in orthopedic repairs.
Conclusion
These study results advance our overall understanding of the biomechanics of the different knot configurations and loop security levels of the different braided polyblend sutures used in arthroscopic procedures through LTF in both in vitro and in situ conditions. Overall, no suture material was superior to any other in a fluid environment, as the combination of aqueous environment and surgeon level of experience with arthroscopic knot tying has a major effect on knot security under single LTF test scenarios. However, our data showed that Ultrabraid suture material had no effect on knot effectiveness over the fluid environment and the temperature. Furthermore, the study showed that the Tennessee slider knot had the steepest learning curve. This study may provide an alternative arthroscopic knots option for soft-tissue repair in which use of certain suture materials is limited.
Open-surgery knot tying is easily learned and performed, but knot tying during arthroscopic procedures can be both challenging and frustrating. According to Burkhart and colleagues,1,2 knot security is defined as the effectiveness of the knot in resisting slippage when load is applied, whereas loop security is the effectiveness in maintaining a tight suture loop while a knot is being tied. Arthroscopic knots commonly begin with an initial slipknot locked in place with a series of half-hitches. During arthroscopic surgery, the surgeon usually must tie an arthroscopic knot to obtain secure tissue fixation, an essential component of soft-tissue repair. A secure knot provides optimal tissue apposition for healing, which will ultimately improve functional outcome. For a knot to be effective, it must have both knot security and loop security. Knot security depends on knot configuration, the coefficient of friction, ductility, handling properties, solubility and diameter of suture material, internal interference, slack between throws, and surgeon experience. Tissue fluid and tissue reaction to suture material may affect knot and loop security.
The ideal knot would be easy to tie and reproducible and would not slip or stretch before tissue is healed. The ideal suture material should provide adequate strength to hold soft tissue in an anatomically correct position until healing can occur. It should also be easily and efficiently manipulated by arthroscopic means when tissues are being secured with knots and secure suture loops. Studies have been conducted to evaluate the security of knots tied with arthroscopic techniques, knot configurations, and suture materials, and these investigations have often evaluated knot performance under single load-to-failure (LTF) test scenarios and cyclic loading in vitro (dry environment) in a room-temperature environment.2-10 To our knowledge, few if any attempts have been made to simulate in situ conditions at body temperature when testing knot security. The fluid environment and the temperature could potentially affect the effectiveness of knots, as knot security depends on friction, internal interference, and slack between throws.1
We conducted a study to evaluate biomechanical performance (knot security, loop security) during destructive testing of several different suture materials with various arthroscopic knot configurations. The study was performed under in vitro (dry environment) and in situ (wet environment) conditions by surgeons with different levels of experience.
Materials and Methods
This investigation was conducted at the Orthopaedic Research Institute at Via Christi Health in Wichita, Kansas. The study compared 4 different suture materials tied with 3 different commonly used arthroscopic knots by 3 surgeons with different levels of experience. The 4 types of braided polyblend polyethylene sutures were Fiberwire (Arthrex, Naples, Florida), ForceFiber (Stryker, San Jose, California), Orthocord (DePuy-Mitek, Warsaw, Indiana), and Ultrabraid (Smith & Nephew, Memphis, Tennessee). Each suture material was tied with 3 arthroscopic knots—static surgeon’s knot, Weston knot,11 Tennessee slider12—and a series of 3 reversing half-hitches on alternating posts (RHAPs) (Figure 1). These knots were chosen based on studies showing they have a higher maximum force to failure when combined with 3 RHAPs.1,2,5,9,13-17
We evaluated performer variability with the help of 3 investigator-surgeons who differed in their level of experience tying arthroscopic knots. This experience was defined on the basis of total number of arthroscopies performed—one of the most important factors predicting basic arthroscopic skills. Our surgeon A was a sports medicine fellowship–trained surgeon with 10 years of experience and a significant number of arthroscopies performed annually (350); surgeon B was a sports medicine fellowship–trained surgeon with 3 years of experience and an annual arthroscopy volume of more than 250 procedures; and surgeon C was a third-year orthopedic resident with about 100 arthroscopies performed.
All knots were tied on a standardized post 30 mm in circumference, which provided a consistent starting circumference for each knot and replicated the suture loop created during arthroscopic rotator cuff repair. All knots were tied using standard arthroscopic techniques, with a standard knot pusher and a modified arthroscopic cannula, in a dry environment (Figure 2). Servohydraulic materials testing system instruments (model 810; MTS Systems, Eden Prairie, Minnesota) were used to test the knot security and loop security of each combination of knots and suture types. Two round hooks (diameter, 3.9 mm) were attached to the actuator and the load cell (Figure 3). Loops were preloaded to 6 N to avoid potential errors caused by slack in the loops or by stretching of suture materials and to provide a well-defined starting point for data recording.
LTF testing was performed for both in vitro and in situ conditions using 10 samples of each suture–knot configuration for each mechanical testing. Each type of testing was conducted for a total of 240 suture–knot combinations per investigator. For the in vitro condition, each suture loop was initiated with 5 preconditioning loading cycles, from 6 N to 30 N at 1 Hz. The load was then applied continuously at a crosshead speed of 1 mm/s until “clinical failure” (3 mm crosshead displacement). We used this criterion for clinical failure, as studies have indicated that 3 mm is the point at which tissue apposition is lost.15,18-21 After the crosshead reached the 3-mm displacement, the loads (under load control) were held for 5 minutes at maximum load, and then load was applied continuously at a crosshead speed of 1 mm/s until complete structure failure. Load and displacement data were collected at a frequency of 20 Hz.
For the in situ condition, the same test parameters were used, except that each combination of the suture loop was preloaded to 6 N and soaked in physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing in an effort to simulate the aqueous medium in vivo after surgery. The in situ tests were performed under physiologic solution maintained at 37°C to approximate postoperative physical conditions.
Statistical Analysis
Means and standard deviations of the knot security and loop security achieved by the surgeons (different experience levels) were calculated for each test configuration and each test condition. These values were used to determine the statistical relevance of the difference in arthroscopic loop security and knot security in each configuration. One-way analysis of variance (ANOVA) performed with SPSS Version 19.0 software (SPSS, Chicago, Illinois) with the least significant difference (LSD) multiple comparisons post hoc analysis was used to determine if any observed differences between the types of braided polyblend sutures, the types of sliding knots, the test conditions (in vitro, in situ), and the levels of surgeon experience were significant for each knot configuration. The level of significance of differences was set at P < .001.
Results
Figure 4 shows the mean maximum clinical failure load (3 mm of displacement) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. In the comparison of biomechanical performance (knot and loop security) under in vitro and in situ conditions, no significant difference was detected when Ultrabraid suture material was used, regardless of surgeon experience, for all knot configurations. For surgeon B, there was no significant difference between in vitro and in situ conditions for any knot configurations or suture materials. When Orthocord suture material was used, Weston knots tied by surgeon A, and static surgeon’s knots by surgeons A and C, resulted in a significant difference between the in vitro and in situ conditions. When ForceFiber suture material was used, only Weston knots and Tennessee slider knots by surgeon A had a significant difference between in vitro and in situ conditions. Weston knots by surgeon A exhibited a significant difference between in vitro and in situ conditions, except when Ultrabraid suture material was used.
Surgeon C’s Tennessee slider knots with all polyblend sutures showed significantly lower loads at clinical failure compared with all the other knot configurations and with knots tied by the other 2 surgeons under both in vitro and in situ conditions. Overall, knots tied by surgeon B had higher clinical failure load than knots tied by the other 2 surgeons.
Figure 5 shows the mean ultimate failure load (complete structural failure) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. Knots tied with Orthocord suture material had the overall lower ultimate failure load compared with other suture materials, whereas knots tied with Ultrabraid suture material had the overall highest ultimate failure load. However, the ultimate failure loads for all the knots tied using any suture material, regardless of surgeon experience, were more than 61 N, which is the estimated minimum required ultimate load per suture during a maximum muscle contraction.1
Figure 6 shows the percentage of knot slipping at constant clinical failure load. Orthocord and Fiberwire suture materials had the lowest incidence of knot slippage. Surgeon C had complete knot slippage at constant clinical failure load using ForceFiber with the Weston knot and Ultrabraid with the Tennessee slider knot. When using Ultrabraid or ForceFiber, surgeons A and C had at least 2 knots slip for all knot configurations.
Discussion
Optimization of knot security for any given knot configuration, suture material, and surgeon experience level during arthroscopic knot tying is crucial.1-10 Our study results showed that, under single LTF test scenarios, there was a significant difference between in vitro and in situ conditions with respect to both knot configuration and surgeon experience level, except when Ultrabraid suture material was used. Arthroscopic sliding knots are lockable or nonlockable.7,12 With lockable sliding knots, slippage may be prevented by tensioning the wrapping limb, which distorts the post in the distal part of the knot, resulting in a kink in the post, thereby increasing the internal interference that increases the resistance of the knot from backing off. With nonlockable sliding knots, slippage may be prevented by the tight grip of the wrappings around the initial post.7 The static surgeon’s knot and the Tennessee slider knot are nonlockable, whereas the Weston knot is a distal lockable sliding knot. Compared with nonlockable sliding knots, lockable sliding knots cause less suture loop enlargement. In 1976, Tera and Aberg22 studied the strength of knotted thread for 12 different types of suture knots combined with 11 types of suture material. They conducted their study 1 week after suture material was inserted into the subcutaneous tissue of rabbits. Their results show a greater propensity for certain suture materials to slip when tested in an aqueous environment. In 1998, Babetty and colleagues23 used Wistar rats to compare the in vivo strength, knot efficiency, and knot security of 4 types of sliding knots and to assess tissue reaction as a result of knot configuration, knot volume, and suture size. They found that 4/0 knots lost more strength than 2/0 knots did, and they concluded that the tissue response to all the knots, except 2/0 nylon, was similar. They indicated that the inflammatory sheath volume varied with knot volume, suture size, and knot configuration. Our results agree with observations that exposure to an aqueous environment alters the force to clinical failure of comparable suture and knot configurations.
In addition, our findings indicate that surgeon familiarity with certain knots has a major effect on knot security. The difference in our 3 surgeons’ levels of familiarity with certain knots was somewhat minimized by the knot tying they practiced before submitting knots for testing. The findings contrast with those of Milia and colleagues,24 who conducted a biomechanical study to determine the effect of experience level on knot security. They compared an experienced arthroscopic shoulder surgeon with a junior-level orthopedic resident surgeon and concluded that experience did not affect knot security. However, the knots in their study were tied by hand, not through an arthroscopic cannula with instruments. Our findings suggest that both experienced and less experienced orthopedic residents should be encouraged to practice arthroscopic knot tying in a nonsurgical environment in order to become comfortable tying arthroscopic knots.
Braided nonabsorbable polyester suture traditionally has been found to be stronger than monofilament absorbable polydioxanone (PDS) and to have less slippage potential.8,9,25 Several studies have determined that the braided polyblend sutures now commonly used for arthroscopic knots have better strength profiles over more traditional materials.12,26,27 Orthocord has a dyed absorbable core (PDS, 68%), an undyed nonabsorbable ultrahigh-molecular-weight polyethylene (UHMWPE, 32%) sleeve, and a polyglactin coating.9,10 Both Ultrabraid and ForceFiber are made with braided UHMWPE and have just a few variations in weave patterns. Fiberwire has a multifiber UHMWPE core covered with braided polyester suture material. Several biomechanical studies25,26,28 have evaluated different arthroscopic sliding knot configurations with different suture materials, and all concluded that a surgeon who is choosing an arthroscopic repair technique should know the differences in suture materials and the knot strengths afforded by different knot configurations, as suture material is an important aspect of loop security. Our findings agree with their findings, that suture materials have a major effect on knot security, even with a series of 3 RHAPs, as in theory the RHAPs should minimize suture friction, internal interference, and slack between knot loops—emphasizing the effect of material selection. Furthermore, our findings also indicated that suture materials with a core in their design (Fiberwire, Orthocord) tend to have the lowest incidence of knot slippage. We had suspected that suture surface characteristics and suture construction could be important factors in knot slippage.
Our experimental design had its limitations. First, although we simulated factors such as temperature, plasma environment, and surgeon experience, tying a knot on a standardized post (30 mm in circumference) differed from what is typically done clinically. Second, the metal hooks used in this study were not compressible and did not interpose in the substance of the knot as soft tissue does in the clinical setting. Third, knots were tied with no tension against the sutures, whereas clinically knots are tied under tension as tissues are pulled together in reconstructions. Fourth, it was assumed that soaking in a physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing was sufficient to simulate the aqueous medium in vivo after surgery, but these parameters may not represent conditions in a patient who has just undergone an arthroscopic shoulder repair and adheres to a passive motion protocol. Fifth, there was no blinding of knot type, and there was no randomization of tying order or testing order. Sixth, only a single LTF test was performed, and incremental cyclic loading can be more useful, as it has long been recognized as a leading source of failure in orthopedic repairs.
Conclusion
These study results advance our overall understanding of the biomechanics of the different knot configurations and loop security levels of the different braided polyblend sutures used in arthroscopic procedures through LTF in both in vitro and in situ conditions. Overall, no suture material was superior to any other in a fluid environment, as the combination of aqueous environment and surgeon level of experience with arthroscopic knot tying has a major effect on knot security under single LTF test scenarios. However, our data showed that Ultrabraid suture material had no effect on knot effectiveness over the fluid environment and the temperature. Furthermore, the study showed that the Tennessee slider knot had the steepest learning curve. This study may provide an alternative arthroscopic knots option for soft-tissue repair in which use of certain suture materials is limited.
1. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202-207.
2. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Loop security as a determinant of tissue fixation security. Arthroscopy. 1998;14(7):773-776.
3. Elkousy H, Hammerman SM, Edwards TB, et al. The arthroscopic square knot: a biomechanical comparison with open and arthroscopic knots. Arthroscopy. 2006;22(7):736-741.
4. Elkousy HA, Sekiya JK, Stabile KJ, McMahon PJ. A biomechanical comparison of arthroscopic sliding and sliding-locking knots. Arthroscopy. 2005;21(2):204-210.
5. Ilahi OA, Younas SA, Alexander J, Noble PC. Cyclic testing of arthroscopic knot security. Arthroscopy. 2004;20(1):62-68.
6. Loutzenheiser TD, Harryman DT 2nd, Ziegler DW, Yung SW. Optimizing arthroscopic knots using braided or monofilament suture. Arthroscopy. 1998;14(1):57-65.
7. Chan KC, Burkhart SS, Thiagarajan P, Goh JC. Optimization of stacked half-hitch knots for arthroscopic surgery. Arthroscopy. 2001;17(7):752-759.
8. Lee TQ, Matsuura PA, Fogolin RP, Lin AC, Kim D, McMahon PJ. Arthroscopic suture tying: a comparison of knot types and suture materials. Arthroscopy. 2001;17(4):348-352.
9. Mishra DK, Cannon WD Jr, Lucas DJ, Belzer JP. Elongation of arthroscopically tied knots. Am J Sports Med. 1997;25(1):113-117.
10. Kim SH, Ha KI, Kim SH, Kim JS. Significance of the internal locking mechanism for loop security enhancement in the arthroscopic knot. Arthroscopy. 2001;17(8):850-855.
11. Weston PV. A new clinch knot. Obstet Gynecol. 1991;78(1):144-147.
12. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
13. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
14. De Beer JF, van Rooyen K, Boezaart AP. Nicky’s knot—a new slip knot for arthroscopic surgery. Arthroscopy. 1998;14(1):109-110.
15. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
16. Wetzler MJ, Bartolozzi AR, Gillespie MJ, et al. Fatigue properties of suture anchors in anterior shoulder reconstructions: Mitek GII. Arthroscopy. 1996;12(6):687-693.
17. Barber FA, Herbert MA, Beavis RC. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy. 2009;25(2):192-199.
18. Richmond JC. A comparison of ultrasonic suture welding and traditional knot tying. Am J Sports Med. 200;29(3):297-299.
19. James JD, Wu MM, Batra EK, Rodeheaver GT, Edlich RF. Technical considerations in manual and instrument tying techniques. J Emerg Med. 1992;10(4):469-480.
20. Batra EK, Franz DA, Towler MA, et al. Influence of emergency physician’s tying technique on knot security. J Emerg Med. 1992;10(3):309-316.
21. Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop. 2010;39(12):569-576.
22. Tera H, Aberg C. The strength of suture knots after one week in vivo. Acta Chir Scand. 1976;142(4):301-307.
23. Babetty Z, Sümer A, Altintaş S, Ergüney S, Göksel S. Changes in knot-holding capacity of sliding knots in vivo and tissue reaction. Arch Surg. 1998;133(7):727-734.
24. Milia MJ, Peindl RD, Connor PM. Arthroscopic knot tying: the role of instrumentation in achieving knot security. Arthroscopy. 2005;21(1):69-76.
25. Lieurance RK, Pflaster DS, Abbott D, Nottage WM. Failure characteristics of various arthroscopically tied knots. Clin Orthop. 2003;(408):311-318.
26. Abbi G, Espinoza L, Odell T, Mahar A, Pedowitz R. Evaluation of 5 knots and 2 suture materials for arthroscopic rotator cuff repair: very strong sutures can still slip. Arthroscopy. 2006;22(1):38-43.
27. Wüst DM, Meyer DC, Favre P, Gerber C. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22(11):1146-1153.
28. Mahar AT, Moezzi DM, Serra-Hsu F, Pedowitz RA. Comparison and performance characteristics of 3 different knots when tied with 2 suture materials used for shoulder arthroscopy. Arthroscopy. 2006;22(6):614.e1-e2.
1. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202-207.
2. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Loop security as a determinant of tissue fixation security. Arthroscopy. 1998;14(7):773-776.
3. Elkousy H, Hammerman SM, Edwards TB, et al. The arthroscopic square knot: a biomechanical comparison with open and arthroscopic knots. Arthroscopy. 2006;22(7):736-741.
4. Elkousy HA, Sekiya JK, Stabile KJ, McMahon PJ. A biomechanical comparison of arthroscopic sliding and sliding-locking knots. Arthroscopy. 2005;21(2):204-210.
5. Ilahi OA, Younas SA, Alexander J, Noble PC. Cyclic testing of arthroscopic knot security. Arthroscopy. 2004;20(1):62-68.
6. Loutzenheiser TD, Harryman DT 2nd, Ziegler DW, Yung SW. Optimizing arthroscopic knots using braided or monofilament suture. Arthroscopy. 1998;14(1):57-65.
7. Chan KC, Burkhart SS, Thiagarajan P, Goh JC. Optimization of stacked half-hitch knots for arthroscopic surgery. Arthroscopy. 2001;17(7):752-759.
8. Lee TQ, Matsuura PA, Fogolin RP, Lin AC, Kim D, McMahon PJ. Arthroscopic suture tying: a comparison of knot types and suture materials. Arthroscopy. 2001;17(4):348-352.
9. Mishra DK, Cannon WD Jr, Lucas DJ, Belzer JP. Elongation of arthroscopically tied knots. Am J Sports Med. 1997;25(1):113-117.
10. Kim SH, Ha KI, Kim SH, Kim JS. Significance of the internal locking mechanism for loop security enhancement in the arthroscopic knot. Arthroscopy. 2001;17(8):850-855.
11. Weston PV. A new clinch knot. Obstet Gynecol. 1991;78(1):144-147.
12. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
13. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
14. De Beer JF, van Rooyen K, Boezaart AP. Nicky’s knot—a new slip knot for arthroscopic surgery. Arthroscopy. 1998;14(1):109-110.
15. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
16. Wetzler MJ, Bartolozzi AR, Gillespie MJ, et al. Fatigue properties of suture anchors in anterior shoulder reconstructions: Mitek GII. Arthroscopy. 1996;12(6):687-693.
17. Barber FA, Herbert MA, Beavis RC. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy. 2009;25(2):192-199.
18. Richmond JC. A comparison of ultrasonic suture welding and traditional knot tying. Am J Sports Med. 200;29(3):297-299.
19. James JD, Wu MM, Batra EK, Rodeheaver GT, Edlich RF. Technical considerations in manual and instrument tying techniques. J Emerg Med. 1992;10(4):469-480.
20. Batra EK, Franz DA, Towler MA, et al. Influence of emergency physician’s tying technique on knot security. J Emerg Med. 1992;10(3):309-316.
21. Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop. 2010;39(12):569-576.
22. Tera H, Aberg C. The strength of suture knots after one week in vivo. Acta Chir Scand. 1976;142(4):301-307.
23. Babetty Z, Sümer A, Altintaş S, Ergüney S, Göksel S. Changes in knot-holding capacity of sliding knots in vivo and tissue reaction. Arch Surg. 1998;133(7):727-734.
24. Milia MJ, Peindl RD, Connor PM. Arthroscopic knot tying: the role of instrumentation in achieving knot security. Arthroscopy. 2005;21(1):69-76.
25. Lieurance RK, Pflaster DS, Abbott D, Nottage WM. Failure characteristics of various arthroscopically tied knots. Clin Orthop. 2003;(408):311-318.
26. Abbi G, Espinoza L, Odell T, Mahar A, Pedowitz R. Evaluation of 5 knots and 2 suture materials for arthroscopic rotator cuff repair: very strong sutures can still slip. Arthroscopy. 2006;22(1):38-43.
27. Wüst DM, Meyer DC, Favre P, Gerber C. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22(11):1146-1153.
28. Mahar AT, Moezzi DM, Serra-Hsu F, Pedowitz RA. Comparison and performance characteristics of 3 different knots when tied with 2 suture materials used for shoulder arthroscopy. Arthroscopy. 2006;22(6):614.e1-e2.
Arthroscopic Anterior Cruciate Ligament Reconstruction Using a Flexible Guide Pin With a Rigid Reamer
Anterior cruciate ligament (ACL) injuries are common, and arthroscopic ACL reconstruction is a routine procedure. Successful ACL reconstruction requires correct placement of the graft within the anatomical insertion of the native ACL.1-3 Errors in surgical technique—specifically, improper femoral tunnel placement—are the most common cause of graft failure in patients who present with recurrent instability after ACL reconstruction.4 There has been much emphasis on placing the tunnel more centrally in the ACL footprint as well as in a more horizontal position, which is thought to provide better rotational control and anterior-to-posterior translational stability.5-7
Two common techniques for creating the femoral tunnel, transtibial and anteromedial drilling, have their unique limitations. Transtibial drilling can place the tunnel high in the notch, resulting in nonanatomical, vertical graft placement.8,9 This technique can be modified to obtain a more anatomical tunnel, but the risk is the tunnel will be short and close to the joint line.10 To avoid these difficulties, surgeons began using an anteromedial portal.11,12 Although anteromedial drilling places the tunnel in a more anatomical position, it too has drawbacks, including the need to hyperflex the knee, a short tunnel, damage to articular cartilage, proximity to neurovascular structures, and difficulty in visualization during drilling.13-16
Femoral tunnel drilling techniques using flexible guide pins and reamers have been developed to address the limitations of rigid instruments. When we first started using flexible instruments through anteromedial portals, there were multiple incidents of reamer breakage during drilling. We therefore developed a technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position. The patient described in this article provided written informed consent for print and electronic publication of this report.
Technique
We begin with our standard arthroscopic portals, including superolateral outflow, lateral parapatellar, and medial parapatellar portals. The medial parapatellar portal is placed under direct visualization with insertion of an 18-gauge spinal needle, ensuring the trajectory reaches the anatomical location of the native ACL on the lateral femoral condyle (LFC). The ACL stump is débrided with a shaver and a radiofrequency ablator, leaving a remnant of tissue to assist with tunnel placement. We do not routinely perform a notchplasty unless there is a concern about possible graft impingement, or the notch is abnormally small. The anatomical footprint is marked with a small awl (Figure 1), and the arthroscope is moved into the anteromedial portal to confirm anatomical placement of the awl mark (Figure 2).
With the knee flexed to 100° to 110°, a flexible 2.7-mm nitonol guide pin (Smith & Nephew, Memphis, Tennessee) is placed freehand through the anteromedial portal into the anatomical footprint of the ACL, marked by the awl, and is passed through the femur before exiting the lateral skin. In most cases, we prefer freehand placement of the awl and pin; however, a femoral drill guide may be used to place the pin into the anatomical footprint of the ACL (Figure 3). The flexible pin allows for knee hyperflexion, clearance of the medial femoral condyle, central placement of the pin between the footprints of the anteromedial and posterolateral bundles for anatomical single-bundle reconstruction, and drilling of a long tunnel (average, 35-40 mm). The pin has a black laser marking that should be placed at the edge of the articular surface of the LFC to ensure appropriate depth of insertion (Figure 4).
A small incision is then made around the guide wire on the lateral thigh, and an outside-in depth gauge is used to obtain an accurate length for the femoral tunnel. The gauge must abut the femoral cortex for accurate assessment of tunnel length. We use an Endobutton (Smith & Nephew) for fixation of the graft in the tunnel. The measured length of the tunnel is used to select an Endobutton of appropriate size and the proper reaming depth for suspension. We routinely use a 10- or 15-mm Endobutton, which provides an average 20 to 25 mm of graft inside the bony tunnel. The knee may then be relaxed to a normal resting flexion angle off the side of the bed, and the arthroscope is inserted into a medial portal or an accessory anteromedial portal to ensure anatomical placement of the pin. Using a flexible guide pin allows the knee to be relatively extended, providing good visualization of overall positioning in relation to the posterior wall of the LFC, whereas keeping the knee in a flexed position (as with a rigid guide pin) can often compromise this visualization.
Using a solid reamer corresponding to the size of the graft, we drill over the guide pin to the appropriate depth, again with the knee hyperflexed (Figure 5), making sure not to breach the lateral femoral cortex, which would compromise fixation with the Endobutton. After drilling with the rigid reamer is completed, placement of the tunnel in an anatomical position is again confirmed with the knee in the normal resting flexion angle (Figure 6). Once the tibial tunnel is drilled at the anatomical footprint, the graft is passed with the proper-length Endobutton and is fixed on the tibial side with a bioabsorbable interference screw 1 to 2 mm larger than the soft-tissue graft and tibial tunnel size. The knee is flexed to 30° while the tibial screw is placed. Graft tension and impingement are then checked (Figure 7). Postoperative anteroposterior and lateral radiographs of the knee may be obtained to confirm anatomical placement of the tunnels as well as proper positioning of the Endobutton (Figures 8A, 8B).
Discussion
Successful ACL reconstruction depends heavily on anatomical tunnel positioning. Failure to place the femoral tunnel in the anatomical footprint of the native ACL results in incomplete restoration of knee kinematics, rotational instability, and graft failure.1-7 Two common techniques for creating this tunnel, transtibial and anteromedial drilling, can reliably place it in an anatomical position. Each technique, however, has limitations. Transtibial drilling can place the tunnel too vertical and high in the notch, or produce a short tibial tunnel close to the joint line.8-10 Anteromedial drilling requires knee hyperflexion, risks damaging the articular cartilage and nearby neurovascular structures, and makes visualization difficult.13-16
One option for addressing some of the difficulties and limitations with anteromedial drilling is to use flexible guide pins and reamers, as first introduced by Cain and Clancy.1 In a cadaveric study, Silver and colleagues17 demonstrated that interosseous tunnels drilled with flexible guide pins were on average more than 6 mm longer than those drilled with rigid pins and consistently were 40 mm or longer. In addition, all tunnels drilled with flexible guide pins were on average 42.3 mm away from the peroneal nerve and 26.1 mm away from the femoral origin of the lateral collateral ligament—safe distances.
Steiner and Smart18 compared flexible and rigid instruments used to drill transtibial and anteromedial (without hyperflexion) anatomical femoral tunnels in ACL reconstruction in cadaveric knees. Although transtibial drilling with flexible pins produced anatomical tunnels, the tunnels were shorter, and the pins exited more posterior in comparison with anteromedial drilling with flexible pins. Transtibial tunnels drilled with rigid pins were nonanatomical and exited more superior and anterior on the femur, resulting in longer tunnels. Anteromedial tunnels drilled with rigid and flexible pins were placed anatomically, but flexible pins produced longer tunnels, did not require hyperflexion (120°), could easily be placed with the knee in 90° of flexion, and did not violate the posterior femoral cortex.
Five times in our early experience with flexible guide pins and reamers, the reamer broke when LFC reaming was initiated. In each case, the broken reamer was retrieved. However, these complications resulted in increased surgical time and cost. In addition, an unretrievable reamer could have caused further injury and suboptimal outcomes. We subsequently developed an anteromedial technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position (Figure 9). The flexible pin provides consistent placement of anatomical tunnels averaging 35 to 40 mm in length. Use of the flexible pin does not require constant hyperflexion of the knee, and it allows for better visualization of the posterior wall of the LFC, ensures anatomical graft placement, and decreases the risk of damaging articular cartilage and causing neurovascular injury. Use of the rigid reamer negates the risks and additional costs associated with reamer breakage. It is unclear why 5 flexible reamers broke during our early use of flexible guide pins and reamers, but it is possible that, because of the patients’ anatomy, placement of the pin in the correct anatomical position in the ACL footprint put a significant amount of abnormal stress on the reamer during tunnel reaming, leading to breakage and failure.
A short femoral tunnel is a common complication of using an anteromedial portal for tunnel drilling.13-16 With the technique we have been using, tunnel lengths average 35 to 40 mm. To address the occasional shorter tunnel, we use Endobutton Direct (Smith & Nephew), which allows for direct fixation of the graft on the button, maximizing the amount of graft in the femoral tunnel and minimizing graft–tunnel length mismatch. In the event there is a lateral wall breach during overdrilling with the reamer, the femoral graft may be secured with screw and post, with interference screw, or with the larger Xtendobuton (Smith & Nephew).
We have successfully used this technique with bone–patellar tendon–bone (BPTB) and hamstring autografts, as well as allografts. Complications, such as graft–tunnel length mismatch, have been uncommon, but, when using BPTB grafts, passing the bone block into the femoral tunnel can be difficult because of the sharp turn required.
Conclusion
Successful ACL reconstruction depends heavily on placement of the graft within the anatomical insertion of the native ACL. With the development of techniques that use flexible guide pins and reamers, it has become possible to place longer anatomical femoral tunnels without the need for hyperflexion. Use of a flexible guide pin with a rigid reamer allows placement of longer anatomical tunnels through an anteromedial portal, reduces time spent with the knee in hyperflexion, provides better viewing, poses less risk of damage to the articular cartilage and neurovascular structures, and at a lower cost with less risk of reamer breakage. In addition, this technique can be used with a variety of graft options, including BPTB grafts, hamstring autografts, and allografts.
1. Cain EL Jr, Clancy WG Jr. Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am. 2002;33(4):717-725.
2. Chhabra A, Starman JS, Ferretti M, Vidal AF, Zantop T, Fu FH. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(suppl 4):2-10.
3. Christel P, Sahasrabudhe A, Basdekis G. Anatomic double-bundle anterior cruciate ligament reconstruction with anatomic aimers. Arthroscopy. 2008;24(10):1146-1151.
4. Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34(1):79-98.
5. Miller CD, Gerdeman AC, Hart JM, et al. A comparison of 2 drilling techniques on the femoral tunnel for anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(3):372-379.
6. Seon JK, Park SJ, Lee KB, Seo HY, Kim MS, Song EK. In vivo stability and clinical comparison of anterior cruciate ligament reconstruction using low or high femoral tunnel positions. Am J Sports Med. 2011;39(1):127-133.
7. Steiner ME, Battaglia TC, Heming JF, Rand JD, Festa A, Baria M. Independent drilling outperforms conventional transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(10):1912-1919.
8. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
9. Tompkins M, Milewski MD, Brockmeier SF, Gaskin CM, Hart JM, Miller MD. Anatomic femoral tunnel drilling in anterior cruciate ligament reconstruction: use of an accessory medial portal versus traditional transtibial drilling. Am J Sports Med. 2012;40(6):1313-1321.
10. Heming JF, Rand J, Steiner ME. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35(10):1708-1715.
11. Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24(1):113-115.
12. Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25(1):95-101.
13. Basdekis G, Abisafi C, Christel P. Influence of knee flexion angle on femoral tunnel characteristics when drilled through the anteromedial portal during anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(4):459-464.
14. Zantop T, Haase AK, Fu FH, Petersen W. Potential risk of cartilage damage in double bundle ACL reconstruction: impact of knee flexion angle and portal location on the femoral PL bundle tunnel. Arch Orthop Trauma Surg. 2008;128(5):509-513.
15. Farrow LD, Parker RD. The relationship of lateral anatomic structures to exiting guide pins during femoral tunnel preparation utilizing an accessory medial portal. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):747-753.
16. Nakamura M, Deie M, Shibuya H, et al. Potential risks of femoral tunnel drilling through the far anteromedial portal: a cadaveric study. Arthroscopy. 2009;25(5):481-487.
17. Silver AG, Kaar SG, Grisell MK, Reagan JM, Farrow LD. Comparison between rigid and flexible systems for drilling the femoral tunnel through an anteromedial portal in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(6):790-795.
18. Steiner ME, Smart LR. Flexible instruments outperform rigid instruments to place anatomic anterior cruciate ligament femoral tunnels without hyperflexion. Arthroscopy. 2012;28(6):835-843.
Anterior cruciate ligament (ACL) injuries are common, and arthroscopic ACL reconstruction is a routine procedure. Successful ACL reconstruction requires correct placement of the graft within the anatomical insertion of the native ACL.1-3 Errors in surgical technique—specifically, improper femoral tunnel placement—are the most common cause of graft failure in patients who present with recurrent instability after ACL reconstruction.4 There has been much emphasis on placing the tunnel more centrally in the ACL footprint as well as in a more horizontal position, which is thought to provide better rotational control and anterior-to-posterior translational stability.5-7
Two common techniques for creating the femoral tunnel, transtibial and anteromedial drilling, have their unique limitations. Transtibial drilling can place the tunnel high in the notch, resulting in nonanatomical, vertical graft placement.8,9 This technique can be modified to obtain a more anatomical tunnel, but the risk is the tunnel will be short and close to the joint line.10 To avoid these difficulties, surgeons began using an anteromedial portal.11,12 Although anteromedial drilling places the tunnel in a more anatomical position, it too has drawbacks, including the need to hyperflex the knee, a short tunnel, damage to articular cartilage, proximity to neurovascular structures, and difficulty in visualization during drilling.13-16
Femoral tunnel drilling techniques using flexible guide pins and reamers have been developed to address the limitations of rigid instruments. When we first started using flexible instruments through anteromedial portals, there were multiple incidents of reamer breakage during drilling. We therefore developed a technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position. The patient described in this article provided written informed consent for print and electronic publication of this report.
Technique
We begin with our standard arthroscopic portals, including superolateral outflow, lateral parapatellar, and medial parapatellar portals. The medial parapatellar portal is placed under direct visualization with insertion of an 18-gauge spinal needle, ensuring the trajectory reaches the anatomical location of the native ACL on the lateral femoral condyle (LFC). The ACL stump is débrided with a shaver and a radiofrequency ablator, leaving a remnant of tissue to assist with tunnel placement. We do not routinely perform a notchplasty unless there is a concern about possible graft impingement, or the notch is abnormally small. The anatomical footprint is marked with a small awl (Figure 1), and the arthroscope is moved into the anteromedial portal to confirm anatomical placement of the awl mark (Figure 2).
With the knee flexed to 100° to 110°, a flexible 2.7-mm nitonol guide pin (Smith & Nephew, Memphis, Tennessee) is placed freehand through the anteromedial portal into the anatomical footprint of the ACL, marked by the awl, and is passed through the femur before exiting the lateral skin. In most cases, we prefer freehand placement of the awl and pin; however, a femoral drill guide may be used to place the pin into the anatomical footprint of the ACL (Figure 3). The flexible pin allows for knee hyperflexion, clearance of the medial femoral condyle, central placement of the pin between the footprints of the anteromedial and posterolateral bundles for anatomical single-bundle reconstruction, and drilling of a long tunnel (average, 35-40 mm). The pin has a black laser marking that should be placed at the edge of the articular surface of the LFC to ensure appropriate depth of insertion (Figure 4).
A small incision is then made around the guide wire on the lateral thigh, and an outside-in depth gauge is used to obtain an accurate length for the femoral tunnel. The gauge must abut the femoral cortex for accurate assessment of tunnel length. We use an Endobutton (Smith & Nephew) for fixation of the graft in the tunnel. The measured length of the tunnel is used to select an Endobutton of appropriate size and the proper reaming depth for suspension. We routinely use a 10- or 15-mm Endobutton, which provides an average 20 to 25 mm of graft inside the bony tunnel. The knee may then be relaxed to a normal resting flexion angle off the side of the bed, and the arthroscope is inserted into a medial portal or an accessory anteromedial portal to ensure anatomical placement of the pin. Using a flexible guide pin allows the knee to be relatively extended, providing good visualization of overall positioning in relation to the posterior wall of the LFC, whereas keeping the knee in a flexed position (as with a rigid guide pin) can often compromise this visualization.
Using a solid reamer corresponding to the size of the graft, we drill over the guide pin to the appropriate depth, again with the knee hyperflexed (Figure 5), making sure not to breach the lateral femoral cortex, which would compromise fixation with the Endobutton. After drilling with the rigid reamer is completed, placement of the tunnel in an anatomical position is again confirmed with the knee in the normal resting flexion angle (Figure 6). Once the tibial tunnel is drilled at the anatomical footprint, the graft is passed with the proper-length Endobutton and is fixed on the tibial side with a bioabsorbable interference screw 1 to 2 mm larger than the soft-tissue graft and tibial tunnel size. The knee is flexed to 30° while the tibial screw is placed. Graft tension and impingement are then checked (Figure 7). Postoperative anteroposterior and lateral radiographs of the knee may be obtained to confirm anatomical placement of the tunnels as well as proper positioning of the Endobutton (Figures 8A, 8B).
Discussion
Successful ACL reconstruction depends heavily on anatomical tunnel positioning. Failure to place the femoral tunnel in the anatomical footprint of the native ACL results in incomplete restoration of knee kinematics, rotational instability, and graft failure.1-7 Two common techniques for creating this tunnel, transtibial and anteromedial drilling, can reliably place it in an anatomical position. Each technique, however, has limitations. Transtibial drilling can place the tunnel too vertical and high in the notch, or produce a short tibial tunnel close to the joint line.8-10 Anteromedial drilling requires knee hyperflexion, risks damaging the articular cartilage and nearby neurovascular structures, and makes visualization difficult.13-16
One option for addressing some of the difficulties and limitations with anteromedial drilling is to use flexible guide pins and reamers, as first introduced by Cain and Clancy.1 In a cadaveric study, Silver and colleagues17 demonstrated that interosseous tunnels drilled with flexible guide pins were on average more than 6 mm longer than those drilled with rigid pins and consistently were 40 mm or longer. In addition, all tunnels drilled with flexible guide pins were on average 42.3 mm away from the peroneal nerve and 26.1 mm away from the femoral origin of the lateral collateral ligament—safe distances.
Steiner and Smart18 compared flexible and rigid instruments used to drill transtibial and anteromedial (without hyperflexion) anatomical femoral tunnels in ACL reconstruction in cadaveric knees. Although transtibial drilling with flexible pins produced anatomical tunnels, the tunnels were shorter, and the pins exited more posterior in comparison with anteromedial drilling with flexible pins. Transtibial tunnels drilled with rigid pins were nonanatomical and exited more superior and anterior on the femur, resulting in longer tunnels. Anteromedial tunnels drilled with rigid and flexible pins were placed anatomically, but flexible pins produced longer tunnels, did not require hyperflexion (120°), could easily be placed with the knee in 90° of flexion, and did not violate the posterior femoral cortex.
Five times in our early experience with flexible guide pins and reamers, the reamer broke when LFC reaming was initiated. In each case, the broken reamer was retrieved. However, these complications resulted in increased surgical time and cost. In addition, an unretrievable reamer could have caused further injury and suboptimal outcomes. We subsequently developed an anteromedial technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position (Figure 9). The flexible pin provides consistent placement of anatomical tunnels averaging 35 to 40 mm in length. Use of the flexible pin does not require constant hyperflexion of the knee, and it allows for better visualization of the posterior wall of the LFC, ensures anatomical graft placement, and decreases the risk of damaging articular cartilage and causing neurovascular injury. Use of the rigid reamer negates the risks and additional costs associated with reamer breakage. It is unclear why 5 flexible reamers broke during our early use of flexible guide pins and reamers, but it is possible that, because of the patients’ anatomy, placement of the pin in the correct anatomical position in the ACL footprint put a significant amount of abnormal stress on the reamer during tunnel reaming, leading to breakage and failure.
A short femoral tunnel is a common complication of using an anteromedial portal for tunnel drilling.13-16 With the technique we have been using, tunnel lengths average 35 to 40 mm. To address the occasional shorter tunnel, we use Endobutton Direct (Smith & Nephew), which allows for direct fixation of the graft on the button, maximizing the amount of graft in the femoral tunnel and minimizing graft–tunnel length mismatch. In the event there is a lateral wall breach during overdrilling with the reamer, the femoral graft may be secured with screw and post, with interference screw, or with the larger Xtendobuton (Smith & Nephew).
We have successfully used this technique with bone–patellar tendon–bone (BPTB) and hamstring autografts, as well as allografts. Complications, such as graft–tunnel length mismatch, have been uncommon, but, when using BPTB grafts, passing the bone block into the femoral tunnel can be difficult because of the sharp turn required.
Conclusion
Successful ACL reconstruction depends heavily on placement of the graft within the anatomical insertion of the native ACL. With the development of techniques that use flexible guide pins and reamers, it has become possible to place longer anatomical femoral tunnels without the need for hyperflexion. Use of a flexible guide pin with a rigid reamer allows placement of longer anatomical tunnels through an anteromedial portal, reduces time spent with the knee in hyperflexion, provides better viewing, poses less risk of damage to the articular cartilage and neurovascular structures, and at a lower cost with less risk of reamer breakage. In addition, this technique can be used with a variety of graft options, including BPTB grafts, hamstring autografts, and allografts.
Anterior cruciate ligament (ACL) injuries are common, and arthroscopic ACL reconstruction is a routine procedure. Successful ACL reconstruction requires correct placement of the graft within the anatomical insertion of the native ACL.1-3 Errors in surgical technique—specifically, improper femoral tunnel placement—are the most common cause of graft failure in patients who present with recurrent instability after ACL reconstruction.4 There has been much emphasis on placing the tunnel more centrally in the ACL footprint as well as in a more horizontal position, which is thought to provide better rotational control and anterior-to-posterior translational stability.5-7
Two common techniques for creating the femoral tunnel, transtibial and anteromedial drilling, have their unique limitations. Transtibial drilling can place the tunnel high in the notch, resulting in nonanatomical, vertical graft placement.8,9 This technique can be modified to obtain a more anatomical tunnel, but the risk is the tunnel will be short and close to the joint line.10 To avoid these difficulties, surgeons began using an anteromedial portal.11,12 Although anteromedial drilling places the tunnel in a more anatomical position, it too has drawbacks, including the need to hyperflex the knee, a short tunnel, damage to articular cartilage, proximity to neurovascular structures, and difficulty in visualization during drilling.13-16
Femoral tunnel drilling techniques using flexible guide pins and reamers have been developed to address the limitations of rigid instruments. When we first started using flexible instruments through anteromedial portals, there were multiple incidents of reamer breakage during drilling. We therefore developed a technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position. The patient described in this article provided written informed consent for print and electronic publication of this report.
Technique
We begin with our standard arthroscopic portals, including superolateral outflow, lateral parapatellar, and medial parapatellar portals. The medial parapatellar portal is placed under direct visualization with insertion of an 18-gauge spinal needle, ensuring the trajectory reaches the anatomical location of the native ACL on the lateral femoral condyle (LFC). The ACL stump is débrided with a shaver and a radiofrequency ablator, leaving a remnant of tissue to assist with tunnel placement. We do not routinely perform a notchplasty unless there is a concern about possible graft impingement, or the notch is abnormally small. The anatomical footprint is marked with a small awl (Figure 1), and the arthroscope is moved into the anteromedial portal to confirm anatomical placement of the awl mark (Figure 2).
With the knee flexed to 100° to 110°, a flexible 2.7-mm nitonol guide pin (Smith & Nephew, Memphis, Tennessee) is placed freehand through the anteromedial portal into the anatomical footprint of the ACL, marked by the awl, and is passed through the femur before exiting the lateral skin. In most cases, we prefer freehand placement of the awl and pin; however, a femoral drill guide may be used to place the pin into the anatomical footprint of the ACL (Figure 3). The flexible pin allows for knee hyperflexion, clearance of the medial femoral condyle, central placement of the pin between the footprints of the anteromedial and posterolateral bundles for anatomical single-bundle reconstruction, and drilling of a long tunnel (average, 35-40 mm). The pin has a black laser marking that should be placed at the edge of the articular surface of the LFC to ensure appropriate depth of insertion (Figure 4).
A small incision is then made around the guide wire on the lateral thigh, and an outside-in depth gauge is used to obtain an accurate length for the femoral tunnel. The gauge must abut the femoral cortex for accurate assessment of tunnel length. We use an Endobutton (Smith & Nephew) for fixation of the graft in the tunnel. The measured length of the tunnel is used to select an Endobutton of appropriate size and the proper reaming depth for suspension. We routinely use a 10- or 15-mm Endobutton, which provides an average 20 to 25 mm of graft inside the bony tunnel. The knee may then be relaxed to a normal resting flexion angle off the side of the bed, and the arthroscope is inserted into a medial portal or an accessory anteromedial portal to ensure anatomical placement of the pin. Using a flexible guide pin allows the knee to be relatively extended, providing good visualization of overall positioning in relation to the posterior wall of the LFC, whereas keeping the knee in a flexed position (as with a rigid guide pin) can often compromise this visualization.
Using a solid reamer corresponding to the size of the graft, we drill over the guide pin to the appropriate depth, again with the knee hyperflexed (Figure 5), making sure not to breach the lateral femoral cortex, which would compromise fixation with the Endobutton. After drilling with the rigid reamer is completed, placement of the tunnel in an anatomical position is again confirmed with the knee in the normal resting flexion angle (Figure 6). Once the tibial tunnel is drilled at the anatomical footprint, the graft is passed with the proper-length Endobutton and is fixed on the tibial side with a bioabsorbable interference screw 1 to 2 mm larger than the soft-tissue graft and tibial tunnel size. The knee is flexed to 30° while the tibial screw is placed. Graft tension and impingement are then checked (Figure 7). Postoperative anteroposterior and lateral radiographs of the knee may be obtained to confirm anatomical placement of the tunnels as well as proper positioning of the Endobutton (Figures 8A, 8B).
Discussion
Successful ACL reconstruction depends heavily on anatomical tunnel positioning. Failure to place the femoral tunnel in the anatomical footprint of the native ACL results in incomplete restoration of knee kinematics, rotational instability, and graft failure.1-7 Two common techniques for creating this tunnel, transtibial and anteromedial drilling, can reliably place it in an anatomical position. Each technique, however, has limitations. Transtibial drilling can place the tunnel too vertical and high in the notch, or produce a short tibial tunnel close to the joint line.8-10 Anteromedial drilling requires knee hyperflexion, risks damaging the articular cartilage and nearby neurovascular structures, and makes visualization difficult.13-16
One option for addressing some of the difficulties and limitations with anteromedial drilling is to use flexible guide pins and reamers, as first introduced by Cain and Clancy.1 In a cadaveric study, Silver and colleagues17 demonstrated that interosseous tunnels drilled with flexible guide pins were on average more than 6 mm longer than those drilled with rigid pins and consistently were 40 mm or longer. In addition, all tunnels drilled with flexible guide pins were on average 42.3 mm away from the peroneal nerve and 26.1 mm away from the femoral origin of the lateral collateral ligament—safe distances.
Steiner and Smart18 compared flexible and rigid instruments used to drill transtibial and anteromedial (without hyperflexion) anatomical femoral tunnels in ACL reconstruction in cadaveric knees. Although transtibial drilling with flexible pins produced anatomical tunnels, the tunnels were shorter, and the pins exited more posterior in comparison with anteromedial drilling with flexible pins. Transtibial tunnels drilled with rigid pins were nonanatomical and exited more superior and anterior on the femur, resulting in longer tunnels. Anteromedial tunnels drilled with rigid and flexible pins were placed anatomically, but flexible pins produced longer tunnels, did not require hyperflexion (120°), could easily be placed with the knee in 90° of flexion, and did not violate the posterior femoral cortex.
Five times in our early experience with flexible guide pins and reamers, the reamer broke when LFC reaming was initiated. In each case, the broken reamer was retrieved. However, these complications resulted in increased surgical time and cost. In addition, an unretrievable reamer could have caused further injury and suboptimal outcomes. We subsequently developed an anteromedial technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position (Figure 9). The flexible pin provides consistent placement of anatomical tunnels averaging 35 to 40 mm in length. Use of the flexible pin does not require constant hyperflexion of the knee, and it allows for better visualization of the posterior wall of the LFC, ensures anatomical graft placement, and decreases the risk of damaging articular cartilage and causing neurovascular injury. Use of the rigid reamer negates the risks and additional costs associated with reamer breakage. It is unclear why 5 flexible reamers broke during our early use of flexible guide pins and reamers, but it is possible that, because of the patients’ anatomy, placement of the pin in the correct anatomical position in the ACL footprint put a significant amount of abnormal stress on the reamer during tunnel reaming, leading to breakage and failure.
A short femoral tunnel is a common complication of using an anteromedial portal for tunnel drilling.13-16 With the technique we have been using, tunnel lengths average 35 to 40 mm. To address the occasional shorter tunnel, we use Endobutton Direct (Smith & Nephew), which allows for direct fixation of the graft on the button, maximizing the amount of graft in the femoral tunnel and minimizing graft–tunnel length mismatch. In the event there is a lateral wall breach during overdrilling with the reamer, the femoral graft may be secured with screw and post, with interference screw, or with the larger Xtendobuton (Smith & Nephew).
We have successfully used this technique with bone–patellar tendon–bone (BPTB) and hamstring autografts, as well as allografts. Complications, such as graft–tunnel length mismatch, have been uncommon, but, when using BPTB grafts, passing the bone block into the femoral tunnel can be difficult because of the sharp turn required.
Conclusion
Successful ACL reconstruction depends heavily on placement of the graft within the anatomical insertion of the native ACL. With the development of techniques that use flexible guide pins and reamers, it has become possible to place longer anatomical femoral tunnels without the need for hyperflexion. Use of a flexible guide pin with a rigid reamer allows placement of longer anatomical tunnels through an anteromedial portal, reduces time spent with the knee in hyperflexion, provides better viewing, poses less risk of damage to the articular cartilage and neurovascular structures, and at a lower cost with less risk of reamer breakage. In addition, this technique can be used with a variety of graft options, including BPTB grafts, hamstring autografts, and allografts.
1. Cain EL Jr, Clancy WG Jr. Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am. 2002;33(4):717-725.
2. Chhabra A, Starman JS, Ferretti M, Vidal AF, Zantop T, Fu FH. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(suppl 4):2-10.
3. Christel P, Sahasrabudhe A, Basdekis G. Anatomic double-bundle anterior cruciate ligament reconstruction with anatomic aimers. Arthroscopy. 2008;24(10):1146-1151.
4. Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34(1):79-98.
5. Miller CD, Gerdeman AC, Hart JM, et al. A comparison of 2 drilling techniques on the femoral tunnel for anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(3):372-379.
6. Seon JK, Park SJ, Lee KB, Seo HY, Kim MS, Song EK. In vivo stability and clinical comparison of anterior cruciate ligament reconstruction using low or high femoral tunnel positions. Am J Sports Med. 2011;39(1):127-133.
7. Steiner ME, Battaglia TC, Heming JF, Rand JD, Festa A, Baria M. Independent drilling outperforms conventional transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(10):1912-1919.
8. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
9. Tompkins M, Milewski MD, Brockmeier SF, Gaskin CM, Hart JM, Miller MD. Anatomic femoral tunnel drilling in anterior cruciate ligament reconstruction: use of an accessory medial portal versus traditional transtibial drilling. Am J Sports Med. 2012;40(6):1313-1321.
10. Heming JF, Rand J, Steiner ME. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35(10):1708-1715.
11. Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24(1):113-115.
12. Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25(1):95-101.
13. Basdekis G, Abisafi C, Christel P. Influence of knee flexion angle on femoral tunnel characteristics when drilled through the anteromedial portal during anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(4):459-464.
14. Zantop T, Haase AK, Fu FH, Petersen W. Potential risk of cartilage damage in double bundle ACL reconstruction: impact of knee flexion angle and portal location on the femoral PL bundle tunnel. Arch Orthop Trauma Surg. 2008;128(5):509-513.
15. Farrow LD, Parker RD. The relationship of lateral anatomic structures to exiting guide pins during femoral tunnel preparation utilizing an accessory medial portal. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):747-753.
16. Nakamura M, Deie M, Shibuya H, et al. Potential risks of femoral tunnel drilling through the far anteromedial portal: a cadaveric study. Arthroscopy. 2009;25(5):481-487.
17. Silver AG, Kaar SG, Grisell MK, Reagan JM, Farrow LD. Comparison between rigid and flexible systems for drilling the femoral tunnel through an anteromedial portal in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(6):790-795.
18. Steiner ME, Smart LR. Flexible instruments outperform rigid instruments to place anatomic anterior cruciate ligament femoral tunnels without hyperflexion. Arthroscopy. 2012;28(6):835-843.
1. Cain EL Jr, Clancy WG Jr. Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am. 2002;33(4):717-725.
2. Chhabra A, Starman JS, Ferretti M, Vidal AF, Zantop T, Fu FH. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(suppl 4):2-10.
3. Christel P, Sahasrabudhe A, Basdekis G. Anatomic double-bundle anterior cruciate ligament reconstruction with anatomic aimers. Arthroscopy. 2008;24(10):1146-1151.
4. Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34(1):79-98.
5. Miller CD, Gerdeman AC, Hart JM, et al. A comparison of 2 drilling techniques on the femoral tunnel for anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(3):372-379.
6. Seon JK, Park SJ, Lee KB, Seo HY, Kim MS, Song EK. In vivo stability and clinical comparison of anterior cruciate ligament reconstruction using low or high femoral tunnel positions. Am J Sports Med. 2011;39(1):127-133.
7. Steiner ME, Battaglia TC, Heming JF, Rand JD, Festa A, Baria M. Independent drilling outperforms conventional transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(10):1912-1919.
8. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
9. Tompkins M, Milewski MD, Brockmeier SF, Gaskin CM, Hart JM, Miller MD. Anatomic femoral tunnel drilling in anterior cruciate ligament reconstruction: use of an accessory medial portal versus traditional transtibial drilling. Am J Sports Med. 2012;40(6):1313-1321.
10. Heming JF, Rand J, Steiner ME. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35(10):1708-1715.
11. Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24(1):113-115.
12. Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25(1):95-101.
13. Basdekis G, Abisafi C, Christel P. Influence of knee flexion angle on femoral tunnel characteristics when drilled through the anteromedial portal during anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(4):459-464.
14. Zantop T, Haase AK, Fu FH, Petersen W. Potential risk of cartilage damage in double bundle ACL reconstruction: impact of knee flexion angle and portal location on the femoral PL bundle tunnel. Arch Orthop Trauma Surg. 2008;128(5):509-513.
15. Farrow LD, Parker RD. The relationship of lateral anatomic structures to exiting guide pins during femoral tunnel preparation utilizing an accessory medial portal. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):747-753.
16. Nakamura M, Deie M, Shibuya H, et al. Potential risks of femoral tunnel drilling through the far anteromedial portal: a cadaveric study. Arthroscopy. 2009;25(5):481-487.
17. Silver AG, Kaar SG, Grisell MK, Reagan JM, Farrow LD. Comparison between rigid and flexible systems for drilling the femoral tunnel through an anteromedial portal in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(6):790-795.
18. Steiner ME, Smart LR. Flexible instruments outperform rigid instruments to place anatomic anterior cruciate ligament femoral tunnels without hyperflexion. Arthroscopy. 2012;28(6):835-843.
VIDEO: Data support switching to transradial PCI access
SAN DIEGO – Cardiologists should switch from transfemoral to transradial access in acute coronary syndrome patients undergoing percutaneous coronary intervention, given the reduced mortality rates associated with the transradial approach in the MATRIX study and other studies, Dr. Cindy L. Grines said at the annual meeting of the American College of Cardiology.
Because U.S. interventionalists are “under the clock” when treating patients with ST-elevation myocardial infarction, “many physicians have been unwilling to risk having a difficult transradial case that would take too much time,” explained Dr. Grines, an interventional cardiologist at the Detroit Medical Center.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
For that and other reasons, American interventionalists have been “slow adopters” of the transradial approach, currently using it for about 20% of PCIs, compared with a worldwide rate of about 70%.
It may take instituting incentives to get U.S. cardiologists to change their practice, Dr. Grines suggested in an interview. That could involve increased reimbursement for PCIs done transradially, an increased allowance on acceptable door-to-balloon times for STEMI patients treated transradially, or imposition of new standards for quality assurance that mandate use of transradial in a certain percentage of PCI cases, she said.
The MATRIX study included a second, independent, prespecified analysis that compared outcomes in patients randomized to treatment with two different antithrombin drugs, either bivalirudin (Angiomax) or unfractionated heparin.
That part of the study showed that while treatment with either of the two drugs resulted in no statistically significant difference in the study’s two primary endpoints, treatment with bivalirudin led to statistically significant reductions in all-cause death and cardiovascular death, as well as in major bleeding events, compared with patients treated with unfractionated heparin (Lancet 2015 [doi:10.1016/S0140-6736(15)60292-6]).
Although bivalirudin has generally been the more commonly used antithrombin drug in this clinical setting by U.S. interventionalists in recent years, results reported last year from the HEAT-PCI trial (Lancet 2014;384:1849-58) that showed better outcomes with unfractionated heparin have led to reduced use of bivalirudin, Dr. Grines said.
The new results from MATRIX coupled with results from other trials that compared those drugs can make clinicians “more confident about the benefit of bivalirudin,” she said.
Dr. Grines has been a consultant to and received honoraria from the Medicines Company, which markets Angiomax, and from Abbott Vascular, Merck, and the Volcano Group.
On Twitter @mitchelzoler
SAN DIEGO – Cardiologists should switch from transfemoral to transradial access in acute coronary syndrome patients undergoing percutaneous coronary intervention, given the reduced mortality rates associated with the transradial approach in the MATRIX study and other studies, Dr. Cindy L. Grines said at the annual meeting of the American College of Cardiology.
Because U.S. interventionalists are “under the clock” when treating patients with ST-elevation myocardial infarction, “many physicians have been unwilling to risk having a difficult transradial case that would take too much time,” explained Dr. Grines, an interventional cardiologist at the Detroit Medical Center.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
For that and other reasons, American interventionalists have been “slow adopters” of the transradial approach, currently using it for about 20% of PCIs, compared with a worldwide rate of about 70%.
It may take instituting incentives to get U.S. cardiologists to change their practice, Dr. Grines suggested in an interview. That could involve increased reimbursement for PCIs done transradially, an increased allowance on acceptable door-to-balloon times for STEMI patients treated transradially, or imposition of new standards for quality assurance that mandate use of transradial in a certain percentage of PCI cases, she said.
The MATRIX study included a second, independent, prespecified analysis that compared outcomes in patients randomized to treatment with two different antithrombin drugs, either bivalirudin (Angiomax) or unfractionated heparin.
That part of the study showed that while treatment with either of the two drugs resulted in no statistically significant difference in the study’s two primary endpoints, treatment with bivalirudin led to statistically significant reductions in all-cause death and cardiovascular death, as well as in major bleeding events, compared with patients treated with unfractionated heparin (Lancet 2015 [doi:10.1016/S0140-6736(15)60292-6]).
Although bivalirudin has generally been the more commonly used antithrombin drug in this clinical setting by U.S. interventionalists in recent years, results reported last year from the HEAT-PCI trial (Lancet 2014;384:1849-58) that showed better outcomes with unfractionated heparin have led to reduced use of bivalirudin, Dr. Grines said.
The new results from MATRIX coupled with results from other trials that compared those drugs can make clinicians “more confident about the benefit of bivalirudin,” she said.
Dr. Grines has been a consultant to and received honoraria from the Medicines Company, which markets Angiomax, and from Abbott Vascular, Merck, and the Volcano Group.
On Twitter @mitchelzoler
SAN DIEGO – Cardiologists should switch from transfemoral to transradial access in acute coronary syndrome patients undergoing percutaneous coronary intervention, given the reduced mortality rates associated with the transradial approach in the MATRIX study and other studies, Dr. Cindy L. Grines said at the annual meeting of the American College of Cardiology.
Because U.S. interventionalists are “under the clock” when treating patients with ST-elevation myocardial infarction, “many physicians have been unwilling to risk having a difficult transradial case that would take too much time,” explained Dr. Grines, an interventional cardiologist at the Detroit Medical Center.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
For that and other reasons, American interventionalists have been “slow adopters” of the transradial approach, currently using it for about 20% of PCIs, compared with a worldwide rate of about 70%.
It may take instituting incentives to get U.S. cardiologists to change their practice, Dr. Grines suggested in an interview. That could involve increased reimbursement for PCIs done transradially, an increased allowance on acceptable door-to-balloon times for STEMI patients treated transradially, or imposition of new standards for quality assurance that mandate use of transradial in a certain percentage of PCI cases, she said.
The MATRIX study included a second, independent, prespecified analysis that compared outcomes in patients randomized to treatment with two different antithrombin drugs, either bivalirudin (Angiomax) or unfractionated heparin.
That part of the study showed that while treatment with either of the two drugs resulted in no statistically significant difference in the study’s two primary endpoints, treatment with bivalirudin led to statistically significant reductions in all-cause death and cardiovascular death, as well as in major bleeding events, compared with patients treated with unfractionated heparin (Lancet 2015 [doi:10.1016/S0140-6736(15)60292-6]).
Although bivalirudin has generally been the more commonly used antithrombin drug in this clinical setting by U.S. interventionalists in recent years, results reported last year from the HEAT-PCI trial (Lancet 2014;384:1849-58) that showed better outcomes with unfractionated heparin have led to reduced use of bivalirudin, Dr. Grines said.
The new results from MATRIX coupled with results from other trials that compared those drugs can make clinicians “more confident about the benefit of bivalirudin,” she said.
Dr. Grines has been a consultant to and received honoraria from the Medicines Company, which markets Angiomax, and from Abbott Vascular, Merck, and the Volcano Group.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM ACC 15