User login
Early Tube Feeding Does Not Improve Outcomes in Acute Pancreatitis
Clinical question
Does early nasoenteric feeding decrease the rate of infections or death in patients hospitalized with severe acute pancreatitis?
Bottom line
In patients with severe acute pancreatitis, early nasoenteric feeding initiated within 24 hours of presentation, as compared with oral feeding after 72 hours, does not improve mortality or reduce the rate of major infections. (LOE = 1b-)
Reference
Study design: Randomized controlled trial (nonblinded)
Funding source: Government
Allocation: Concealed
Setting: Inpatient (ward only)
Synopsis
Previous observational studies suggest that early nasoenteric feeding in patients with acute pancreatitis may reduce the rate of major infections by stimulating intestinal motility, reducing bacterial overgrowth, and increasing splanchnic blood flow. Using concealed allocation, these authors randomized patients presenting to the emergency department with severe acute pancreatitis to receive either early nasoenteric tube feeding initiated within 24 hours (n = 102) or oral feeding started at 72 hours (n = 106). If the oral diet was not tolerated, tube feeding was initiated after 96 hours. The 2 groups were similar at baseline: the mean age was 65 years and 60% of the patients had evidence of systemic inflammatory response syndrome (SIRS). Analysis was by intention to treat. One third of patients in the oral group eventually required tube feeding. For the primary composite end point of death or major infection (infected pancreatic necrosis, bacteremia, or pneumonia), there was no significant difference detected between the 2 groups. When the outcomes of major infection and death were examined separately, the 2 groups again had comparable results. Finally, patients in both groups had similar rates of admission to the intensive care unit and similar need for mechanical ventilation. Given fewer-than-expected events in the control group, it is possible that the study was too small to detect a difference in the primary outcome, if such a difference exists.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does early nasoenteric feeding decrease the rate of infections or death in patients hospitalized with severe acute pancreatitis?
Bottom line
In patients with severe acute pancreatitis, early nasoenteric feeding initiated within 24 hours of presentation, as compared with oral feeding after 72 hours, does not improve mortality or reduce the rate of major infections. (LOE = 1b-)
Reference
Study design: Randomized controlled trial (nonblinded)
Funding source: Government
Allocation: Concealed
Setting: Inpatient (ward only)
Synopsis
Previous observational studies suggest that early nasoenteric feeding in patients with acute pancreatitis may reduce the rate of major infections by stimulating intestinal motility, reducing bacterial overgrowth, and increasing splanchnic blood flow. Using concealed allocation, these authors randomized patients presenting to the emergency department with severe acute pancreatitis to receive either early nasoenteric tube feeding initiated within 24 hours (n = 102) or oral feeding started at 72 hours (n = 106). If the oral diet was not tolerated, tube feeding was initiated after 96 hours. The 2 groups were similar at baseline: the mean age was 65 years and 60% of the patients had evidence of systemic inflammatory response syndrome (SIRS). Analysis was by intention to treat. One third of patients in the oral group eventually required tube feeding. For the primary composite end point of death or major infection (infected pancreatic necrosis, bacteremia, or pneumonia), there was no significant difference detected between the 2 groups. When the outcomes of major infection and death were examined separately, the 2 groups again had comparable results. Finally, patients in both groups had similar rates of admission to the intensive care unit and similar need for mechanical ventilation. Given fewer-than-expected events in the control group, it is possible that the study was too small to detect a difference in the primary outcome, if such a difference exists.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does early nasoenteric feeding decrease the rate of infections or death in patients hospitalized with severe acute pancreatitis?
Bottom line
In patients with severe acute pancreatitis, early nasoenteric feeding initiated within 24 hours of presentation, as compared with oral feeding after 72 hours, does not improve mortality or reduce the rate of major infections. (LOE = 1b-)
Reference
Study design: Randomized controlled trial (nonblinded)
Funding source: Government
Allocation: Concealed
Setting: Inpatient (ward only)
Synopsis
Previous observational studies suggest that early nasoenteric feeding in patients with acute pancreatitis may reduce the rate of major infections by stimulating intestinal motility, reducing bacterial overgrowth, and increasing splanchnic blood flow. Using concealed allocation, these authors randomized patients presenting to the emergency department with severe acute pancreatitis to receive either early nasoenteric tube feeding initiated within 24 hours (n = 102) or oral feeding started at 72 hours (n = 106). If the oral diet was not tolerated, tube feeding was initiated after 96 hours. The 2 groups were similar at baseline: the mean age was 65 years and 60% of the patients had evidence of systemic inflammatory response syndrome (SIRS). Analysis was by intention to treat. One third of patients in the oral group eventually required tube feeding. For the primary composite end point of death or major infection (infected pancreatic necrosis, bacteremia, or pneumonia), there was no significant difference detected between the 2 groups. When the outcomes of major infection and death were examined separately, the 2 groups again had comparable results. Finally, patients in both groups had similar rates of admission to the intensive care unit and similar need for mechanical ventilation. Given fewer-than-expected events in the control group, it is possible that the study was too small to detect a difference in the primary outcome, if such a difference exists.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Saying thank you to patients
I say “Thank you” a lot to patients. And I mean it.
I like being a doctor. It’s something I always wanted to do. For all the difficulties that go along with it, I still enjoy the actual job of caring for those who come to me. They’re the reason I’m here, and they keep my practice afloat and let me do what I want in life.
Like any other business, I have competitors. In my area, people have a choice of neurologists, and I appreciate that they picked me. So I always try to thank them when walking up to checkout.
A big part of what makes the job rewarding are those who feel the same way about me. It’s always nice when they thank me for helping, or trying to help, or just listening. I try to be a good doctor, so I’m glad to have someone recognize that. In this field, you can’t make everyone happy, but if I can have a solid majority who understand that I’m doing my best for them, I’ll take it.
I’m not fishing for compliments, or gifts, or a parade. Experience has taught me that patients who are overly flattering are most likely not to mean it. If someone calls me too many wonderful things, I immediately worry about their ulterior motives. Are they looking for narcotics? Disability? A legal action?
But a simple, sincere, “Thank you” from a patient can make it all worthwhile. Even on a bad day, it’s still a bright spot. It’s nice to know I’m making a difference. When I get a small note or appreciative Christmas card from a patient, I save it. They go in a drawer to be taken out and read after a particularly rough time, to remind myself that I must be doing something right.
Being appreciated reminds me why I’m here, and that this was the right choice for me. It lets me know that I’m doing what I set out to do many years ago: to help people.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I say “Thank you” a lot to patients. And I mean it.
I like being a doctor. It’s something I always wanted to do. For all the difficulties that go along with it, I still enjoy the actual job of caring for those who come to me. They’re the reason I’m here, and they keep my practice afloat and let me do what I want in life.
Like any other business, I have competitors. In my area, people have a choice of neurologists, and I appreciate that they picked me. So I always try to thank them when walking up to checkout.
A big part of what makes the job rewarding are those who feel the same way about me. It’s always nice when they thank me for helping, or trying to help, or just listening. I try to be a good doctor, so I’m glad to have someone recognize that. In this field, you can’t make everyone happy, but if I can have a solid majority who understand that I’m doing my best for them, I’ll take it.
I’m not fishing for compliments, or gifts, or a parade. Experience has taught me that patients who are overly flattering are most likely not to mean it. If someone calls me too many wonderful things, I immediately worry about their ulterior motives. Are they looking for narcotics? Disability? A legal action?
But a simple, sincere, “Thank you” from a patient can make it all worthwhile. Even on a bad day, it’s still a bright spot. It’s nice to know I’m making a difference. When I get a small note or appreciative Christmas card from a patient, I save it. They go in a drawer to be taken out and read after a particularly rough time, to remind myself that I must be doing something right.
Being appreciated reminds me why I’m here, and that this was the right choice for me. It lets me know that I’m doing what I set out to do many years ago: to help people.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I say “Thank you” a lot to patients. And I mean it.
I like being a doctor. It’s something I always wanted to do. For all the difficulties that go along with it, I still enjoy the actual job of caring for those who come to me. They’re the reason I’m here, and they keep my practice afloat and let me do what I want in life.
Like any other business, I have competitors. In my area, people have a choice of neurologists, and I appreciate that they picked me. So I always try to thank them when walking up to checkout.
A big part of what makes the job rewarding are those who feel the same way about me. It’s always nice when they thank me for helping, or trying to help, or just listening. I try to be a good doctor, so I’m glad to have someone recognize that. In this field, you can’t make everyone happy, but if I can have a solid majority who understand that I’m doing my best for them, I’ll take it.
I’m not fishing for compliments, or gifts, or a parade. Experience has taught me that patients who are overly flattering are most likely not to mean it. If someone calls me too many wonderful things, I immediately worry about their ulterior motives. Are they looking for narcotics? Disability? A legal action?
But a simple, sincere, “Thank you” from a patient can make it all worthwhile. Even on a bad day, it’s still a bright spot. It’s nice to know I’m making a difference. When I get a small note or appreciative Christmas card from a patient, I save it. They go in a drawer to be taken out and read after a particularly rough time, to remind myself that I must be doing something right.
Being appreciated reminds me why I’m here, and that this was the right choice for me. It lets me know that I’m doing what I set out to do many years ago: to help people.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Computer model simulates blood development

Photo courtesy of
University of Cambridge
A new computer model that simulates blood cell development could aid the discovery of novel treatments for hematologic malignancies, according to researchers.
“With this new computer model, we can carry out simulated experiments in seconds that would take many weeks to perform in the laboratory, dramatically speeding up research into blood development and the genetic mutations that cause leukemia,” said Bertie Gottgens, PhD, of the University of Cambridge in the UK.
Dr Gottgens and his colleagues explained this research in Nature Biotechnology.
To start, the researchers measured the activity of 48 genes in 3934 hematopoietic progenitor cells. They used the resulting dataset to construct the computer model of blood cell development, using computational approaches originally developed at Microsoft Research for the synthesis of computer code.
Subsequent lab experiments validated the accuracy of the model.
The researchers noted that the model can be used to simulate the activity of key genes implicated in hematologic malignancies. For example, around 1 in 5 children who develop leukemia have a faulty version of the RUNX1 gene, as do a similar proportion of adults with acute myeloid leukemia.
The computer model shows how RUNX1 interacts with other genes to control blood cell development. The gene produces the RUNX1 protein, which, in healthy patients, activates a network of key genes. In leukemia patients, an altered form of the protein is thought to suppress the network.
If the researchers change the “rules” in the network model, they can simulate the formation of abnormal leukemia cells. By tweaking the leukemia model until the behavior of the network reverts back to normal, the team can identify pathways that, potentially, could be targeted with drugs.
“Because the computer simulations are very fast, we can quickly screen through lots of possibilities to pick the most promising ones as pathways for drug development,” Dr Gottgens said.
“The cost of developing a new drug is enormous, and much of this cost comes from new candidate drugs failing late in the drug development process. Our model could significantly reduce the risk of failure, with the potential to make drug discovery faster and cheaper.” ![]()

Photo courtesy of
University of Cambridge
A new computer model that simulates blood cell development could aid the discovery of novel treatments for hematologic malignancies, according to researchers.
“With this new computer model, we can carry out simulated experiments in seconds that would take many weeks to perform in the laboratory, dramatically speeding up research into blood development and the genetic mutations that cause leukemia,” said Bertie Gottgens, PhD, of the University of Cambridge in the UK.
Dr Gottgens and his colleagues explained this research in Nature Biotechnology.
To start, the researchers measured the activity of 48 genes in 3934 hematopoietic progenitor cells. They used the resulting dataset to construct the computer model of blood cell development, using computational approaches originally developed at Microsoft Research for the synthesis of computer code.
Subsequent lab experiments validated the accuracy of the model.
The researchers noted that the model can be used to simulate the activity of key genes implicated in hematologic malignancies. For example, around 1 in 5 children who develop leukemia have a faulty version of the RUNX1 gene, as do a similar proportion of adults with acute myeloid leukemia.
The computer model shows how RUNX1 interacts with other genes to control blood cell development. The gene produces the RUNX1 protein, which, in healthy patients, activates a network of key genes. In leukemia patients, an altered form of the protein is thought to suppress the network.
If the researchers change the “rules” in the network model, they can simulate the formation of abnormal leukemia cells. By tweaking the leukemia model until the behavior of the network reverts back to normal, the team can identify pathways that, potentially, could be targeted with drugs.
“Because the computer simulations are very fast, we can quickly screen through lots of possibilities to pick the most promising ones as pathways for drug development,” Dr Gottgens said.
“The cost of developing a new drug is enormous, and much of this cost comes from new candidate drugs failing late in the drug development process. Our model could significantly reduce the risk of failure, with the potential to make drug discovery faster and cheaper.” ![]()

Photo courtesy of
University of Cambridge
A new computer model that simulates blood cell development could aid the discovery of novel treatments for hematologic malignancies, according to researchers.
“With this new computer model, we can carry out simulated experiments in seconds that would take many weeks to perform in the laboratory, dramatically speeding up research into blood development and the genetic mutations that cause leukemia,” said Bertie Gottgens, PhD, of the University of Cambridge in the UK.
Dr Gottgens and his colleagues explained this research in Nature Biotechnology.
To start, the researchers measured the activity of 48 genes in 3934 hematopoietic progenitor cells. They used the resulting dataset to construct the computer model of blood cell development, using computational approaches originally developed at Microsoft Research for the synthesis of computer code.
Subsequent lab experiments validated the accuracy of the model.
The researchers noted that the model can be used to simulate the activity of key genes implicated in hematologic malignancies. For example, around 1 in 5 children who develop leukemia have a faulty version of the RUNX1 gene, as do a similar proportion of adults with acute myeloid leukemia.
The computer model shows how RUNX1 interacts with other genes to control blood cell development. The gene produces the RUNX1 protein, which, in healthy patients, activates a network of key genes. In leukemia patients, an altered form of the protein is thought to suppress the network.
If the researchers change the “rules” in the network model, they can simulate the formation of abnormal leukemia cells. By tweaking the leukemia model until the behavior of the network reverts back to normal, the team can identify pathways that, potentially, could be targeted with drugs.
“Because the computer simulations are very fast, we can quickly screen through lots of possibilities to pick the most promising ones as pathways for drug development,” Dr Gottgens said.
“The cost of developing a new drug is enormous, and much of this cost comes from new candidate drugs failing late in the drug development process. Our model could significantly reduce the risk of failure, with the potential to make drug discovery faster and cheaper.” ![]()
Ro-CHOP: Toxicity increases with efficacy
SAN FRANCISCO—Adding romidepsin to CHOP can enhance the regimen’s efficacy against peripheral T-cell lymphoma (PTCL), but the combination can also induce severe toxicity, results of a phase 1b/2 study have shown.
In patients with previously untreated PTCL, romidepsin plus CHOP elicited an overall response rate of about 69%.
But all patients experienced adverse events, a median of 49 per patient. In addition, rates of hematologic toxicities were high, and 3 patients experienced acute cardiac toxicity.
Bertrand Coiffier, MD, PhD, of CHU Lyon Sud in Pierre Benite, France, presented these findings at the 7th Annual T-cell Lymphoma Forum. Dr Coiffier and other researchers involved in this study receive funds from Celgene, the company developing romidepsin.
“CHOP is widely accepted,” Dr Coiffier noted. “It’s the most-used regimen for peripheral T-cell lymphoma, but it’s not the best one, and we certainly have regimens that do produce more [complete responses] and longer responses.”
He said researchers decided to test romidepsin in combination with CHOP because studies have suggested that romidepsin has very good efficacy in relapsed/refractory peripheral T-cell lymphoma, and the toxicities associated with romidepsin and CHOP alone have been managable.
So the researchers tested the combination in 37 patients with untreated PTCL, most of whom were male (n=20). The median age was 57, and 37.8% were older than 60. About 95% of patients had stage III/IV disease, and about 89% had an ECOG performance status less than 2.
Most patients had angioimmunoblastic T-cell lymphoma (n=17), followed by PTCL not otherwise specified (n=13), ALK- anaplastic large-cell lymphoma (n=3), enteropathy-associated T-cell lymphoma (n=1), hepatosplenic T-cell lymphoma (n=1), primary cutaneous CD4+ small/medium T-cell lymphoma (n=1), and “other” (n=1).
Early DLTs
The researchers used a standard “3+3” dose-escalation scheme, starting with a romidepsin dose of 10 mg/m2 given on days 1 and 8.
In the first 2 cycles, there were 3 dose-limiting toxicities (DLTs)—1 case of grade 3 syncope, 1 case of grade 3 general status alteration, and 1 case of grade 3 hematologic toxicity (neutropenia and thrombocytopenia) lasting longer than 7 days.
“So we looked at the definition of the criteria for DLT, and we thought that, this time, they were too severe,” Dr Coiffier said. “After a lot of discussion between all the investigators, we decided to modify the criteria for DLT regarding neutropenia or thrombocytopenia and to allow a little more toxicity before saying it’s a DLT.”
A DLT was initially defined as grade 3/4 non-hematologic toxicity, grade 3 hematologic toxicity lasting more than 7 days, or grade 4 hematologic toxicity lasting more than 3 days. The researchers modified the criteria so that hematologic toxicities would not be considered DLTs if they lasted less than 10 days for grade 3 or less than 7 days for grade 4.
When the team decreased the romidepsin dose to 8 mg/m2, they did not observe any DLTs according to the new criteria. The same was true when they raised the dose back up to 10 mg/m2.
There were, however, DLTs when the dose was increased to 12 mg/m2. In cohort 5, there was a case of grade 3 cardiac failure, and in cohort 6, there were 2 cases of grade 3 nausea.
Nevertheless, 12 mg/m2 became the phase 2 dose. In all, 25 patients received romidepsin at that dose.
Safety data
Twenty-six of 37 patients completed the 8 planned cycles of treatment. Five patients discontinued treatment due to progression and 6 due to toxicity (5 due to thrombocytopenia).
“One hundred percent of patients experienced at least one adverse event, but most of them were grade 1 or 2 [84%] and occurred during the first 2 cycles [38%],” Dr Coiffier said. “There were no deaths related to adverse events.”
Severe toxicities occurred during the expansion phase. There was a case of severe peripheral sensory neuropathy that led to treatment discontinuation, and there were 3 cases of acute cardiac toxicity. They all occurred after the first cycle, and none were fatal.
The rate of hematologic toxicity was high. Neutropenia occurred in all patients, thrombocytopenia in 94%, and anemia in 89%.
Grade 3/4 adverse events included neutropenia (85%), thrombocytopenia (35%), febrile neutropenia (19%), general status deterioration (13%), nausea/vomiting (10%), anemia (8%), hypophosphatemia (8%), fatigue (5%), mucositis (5%), decreased appetite (5%), hypocalcemia (3%), hyponatremia (3%), hypokalemia (3%), hypomagnesemia (3%), dysgeusia (3%), and peripheral sensory neuropathy (3%).
Response, survival, and next steps
About 51% of patients (18/35) achieved a complete response, and 17% (n=6) had a partial response. Twenty-six percent of patients (n=9) progressed.
The median follow-up was 30 months. The estimated 1-year progression-free survival was 57%, and the estimated 1-year overall survival was 82%.
“The [overall survival] curve is certainly much better than you would expect with just standard CHOP,” Dr Coiffier noted.
He added that this research has progressed to a phase 3 study comparing romidepsin and CHOP in combination to CHOP alone. There are 7 countries participating (France, Belgium, South Korea, Spain, Italy, Germany, and Portugal), and 100 patients have been enrolled thus far. ![]()
SAN FRANCISCO—Adding romidepsin to CHOP can enhance the regimen’s efficacy against peripheral T-cell lymphoma (PTCL), but the combination can also induce severe toxicity, results of a phase 1b/2 study have shown.
In patients with previously untreated PTCL, romidepsin plus CHOP elicited an overall response rate of about 69%.
But all patients experienced adverse events, a median of 49 per patient. In addition, rates of hematologic toxicities were high, and 3 patients experienced acute cardiac toxicity.
Bertrand Coiffier, MD, PhD, of CHU Lyon Sud in Pierre Benite, France, presented these findings at the 7th Annual T-cell Lymphoma Forum. Dr Coiffier and other researchers involved in this study receive funds from Celgene, the company developing romidepsin.
“CHOP is widely accepted,” Dr Coiffier noted. “It’s the most-used regimen for peripheral T-cell lymphoma, but it’s not the best one, and we certainly have regimens that do produce more [complete responses] and longer responses.”
He said researchers decided to test romidepsin in combination with CHOP because studies have suggested that romidepsin has very good efficacy in relapsed/refractory peripheral T-cell lymphoma, and the toxicities associated with romidepsin and CHOP alone have been managable.
So the researchers tested the combination in 37 patients with untreated PTCL, most of whom were male (n=20). The median age was 57, and 37.8% were older than 60. About 95% of patients had stage III/IV disease, and about 89% had an ECOG performance status less than 2.
Most patients had angioimmunoblastic T-cell lymphoma (n=17), followed by PTCL not otherwise specified (n=13), ALK- anaplastic large-cell lymphoma (n=3), enteropathy-associated T-cell lymphoma (n=1), hepatosplenic T-cell lymphoma (n=1), primary cutaneous CD4+ small/medium T-cell lymphoma (n=1), and “other” (n=1).
Early DLTs
The researchers used a standard “3+3” dose-escalation scheme, starting with a romidepsin dose of 10 mg/m2 given on days 1 and 8.
In the first 2 cycles, there were 3 dose-limiting toxicities (DLTs)—1 case of grade 3 syncope, 1 case of grade 3 general status alteration, and 1 case of grade 3 hematologic toxicity (neutropenia and thrombocytopenia) lasting longer than 7 days.
“So we looked at the definition of the criteria for DLT, and we thought that, this time, they were too severe,” Dr Coiffier said. “After a lot of discussion between all the investigators, we decided to modify the criteria for DLT regarding neutropenia or thrombocytopenia and to allow a little more toxicity before saying it’s a DLT.”
A DLT was initially defined as grade 3/4 non-hematologic toxicity, grade 3 hematologic toxicity lasting more than 7 days, or grade 4 hematologic toxicity lasting more than 3 days. The researchers modified the criteria so that hematologic toxicities would not be considered DLTs if they lasted less than 10 days for grade 3 or less than 7 days for grade 4.
When the team decreased the romidepsin dose to 8 mg/m2, they did not observe any DLTs according to the new criteria. The same was true when they raised the dose back up to 10 mg/m2.
There were, however, DLTs when the dose was increased to 12 mg/m2. In cohort 5, there was a case of grade 3 cardiac failure, and in cohort 6, there were 2 cases of grade 3 nausea.
Nevertheless, 12 mg/m2 became the phase 2 dose. In all, 25 patients received romidepsin at that dose.
Safety data
Twenty-six of 37 patients completed the 8 planned cycles of treatment. Five patients discontinued treatment due to progression and 6 due to toxicity (5 due to thrombocytopenia).
“One hundred percent of patients experienced at least one adverse event, but most of them were grade 1 or 2 [84%] and occurred during the first 2 cycles [38%],” Dr Coiffier said. “There were no deaths related to adverse events.”
Severe toxicities occurred during the expansion phase. There was a case of severe peripheral sensory neuropathy that led to treatment discontinuation, and there were 3 cases of acute cardiac toxicity. They all occurred after the first cycle, and none were fatal.
The rate of hematologic toxicity was high. Neutropenia occurred in all patients, thrombocytopenia in 94%, and anemia in 89%.
Grade 3/4 adverse events included neutropenia (85%), thrombocytopenia (35%), febrile neutropenia (19%), general status deterioration (13%), nausea/vomiting (10%), anemia (8%), hypophosphatemia (8%), fatigue (5%), mucositis (5%), decreased appetite (5%), hypocalcemia (3%), hyponatremia (3%), hypokalemia (3%), hypomagnesemia (3%), dysgeusia (3%), and peripheral sensory neuropathy (3%).
Response, survival, and next steps
About 51% of patients (18/35) achieved a complete response, and 17% (n=6) had a partial response. Twenty-six percent of patients (n=9) progressed.
The median follow-up was 30 months. The estimated 1-year progression-free survival was 57%, and the estimated 1-year overall survival was 82%.
“The [overall survival] curve is certainly much better than you would expect with just standard CHOP,” Dr Coiffier noted.
He added that this research has progressed to a phase 3 study comparing romidepsin and CHOP in combination to CHOP alone. There are 7 countries participating (France, Belgium, South Korea, Spain, Italy, Germany, and Portugal), and 100 patients have been enrolled thus far. ![]()
SAN FRANCISCO—Adding romidepsin to CHOP can enhance the regimen’s efficacy against peripheral T-cell lymphoma (PTCL), but the combination can also induce severe toxicity, results of a phase 1b/2 study have shown.
In patients with previously untreated PTCL, romidepsin plus CHOP elicited an overall response rate of about 69%.
But all patients experienced adverse events, a median of 49 per patient. In addition, rates of hematologic toxicities were high, and 3 patients experienced acute cardiac toxicity.
Bertrand Coiffier, MD, PhD, of CHU Lyon Sud in Pierre Benite, France, presented these findings at the 7th Annual T-cell Lymphoma Forum. Dr Coiffier and other researchers involved in this study receive funds from Celgene, the company developing romidepsin.
“CHOP is widely accepted,” Dr Coiffier noted. “It’s the most-used regimen for peripheral T-cell lymphoma, but it’s not the best one, and we certainly have regimens that do produce more [complete responses] and longer responses.”
He said researchers decided to test romidepsin in combination with CHOP because studies have suggested that romidepsin has very good efficacy in relapsed/refractory peripheral T-cell lymphoma, and the toxicities associated with romidepsin and CHOP alone have been managable.
So the researchers tested the combination in 37 patients with untreated PTCL, most of whom were male (n=20). The median age was 57, and 37.8% were older than 60. About 95% of patients had stage III/IV disease, and about 89% had an ECOG performance status less than 2.
Most patients had angioimmunoblastic T-cell lymphoma (n=17), followed by PTCL not otherwise specified (n=13), ALK- anaplastic large-cell lymphoma (n=3), enteropathy-associated T-cell lymphoma (n=1), hepatosplenic T-cell lymphoma (n=1), primary cutaneous CD4+ small/medium T-cell lymphoma (n=1), and “other” (n=1).
Early DLTs
The researchers used a standard “3+3” dose-escalation scheme, starting with a romidepsin dose of 10 mg/m2 given on days 1 and 8.
In the first 2 cycles, there were 3 dose-limiting toxicities (DLTs)—1 case of grade 3 syncope, 1 case of grade 3 general status alteration, and 1 case of grade 3 hematologic toxicity (neutropenia and thrombocytopenia) lasting longer than 7 days.
“So we looked at the definition of the criteria for DLT, and we thought that, this time, they were too severe,” Dr Coiffier said. “After a lot of discussion between all the investigators, we decided to modify the criteria for DLT regarding neutropenia or thrombocytopenia and to allow a little more toxicity before saying it’s a DLT.”
A DLT was initially defined as grade 3/4 non-hematologic toxicity, grade 3 hematologic toxicity lasting more than 7 days, or grade 4 hematologic toxicity lasting more than 3 days. The researchers modified the criteria so that hematologic toxicities would not be considered DLTs if they lasted less than 10 days for grade 3 or less than 7 days for grade 4.
When the team decreased the romidepsin dose to 8 mg/m2, they did not observe any DLTs according to the new criteria. The same was true when they raised the dose back up to 10 mg/m2.
There were, however, DLTs when the dose was increased to 12 mg/m2. In cohort 5, there was a case of grade 3 cardiac failure, and in cohort 6, there were 2 cases of grade 3 nausea.
Nevertheless, 12 mg/m2 became the phase 2 dose. In all, 25 patients received romidepsin at that dose.
Safety data
Twenty-six of 37 patients completed the 8 planned cycles of treatment. Five patients discontinued treatment due to progression and 6 due to toxicity (5 due to thrombocytopenia).
“One hundred percent of patients experienced at least one adverse event, but most of them were grade 1 or 2 [84%] and occurred during the first 2 cycles [38%],” Dr Coiffier said. “There were no deaths related to adverse events.”
Severe toxicities occurred during the expansion phase. There was a case of severe peripheral sensory neuropathy that led to treatment discontinuation, and there were 3 cases of acute cardiac toxicity. They all occurred after the first cycle, and none were fatal.
The rate of hematologic toxicity was high. Neutropenia occurred in all patients, thrombocytopenia in 94%, and anemia in 89%.
Grade 3/4 adverse events included neutropenia (85%), thrombocytopenia (35%), febrile neutropenia (19%), general status deterioration (13%), nausea/vomiting (10%), anemia (8%), hypophosphatemia (8%), fatigue (5%), mucositis (5%), decreased appetite (5%), hypocalcemia (3%), hyponatremia (3%), hypokalemia (3%), hypomagnesemia (3%), dysgeusia (3%), and peripheral sensory neuropathy (3%).
Response, survival, and next steps
About 51% of patients (18/35) achieved a complete response, and 17% (n=6) had a partial response. Twenty-six percent of patients (n=9) progressed.
The median follow-up was 30 months. The estimated 1-year progression-free survival was 57%, and the estimated 1-year overall survival was 82%.
“The [overall survival] curve is certainly much better than you would expect with just standard CHOP,” Dr Coiffier noted.
He added that this research has progressed to a phase 3 study comparing romidepsin and CHOP in combination to CHOP alone. There are 7 countries participating (France, Belgium, South Korea, Spain, Italy, Germany, and Portugal), and 100 patients have been enrolled thus far. ![]()
Anticoagulant now available in US pharmacies

Photo by Rhoda Baer
The oral factor Xa inhibitor edoxaban (Savaysa) is now available in US pharmacies, according to Daiichi Sankyo Company, Limited, the company developing the drug.
Savaysa is approved by the US Food and Drug Administration to reduce the risk of stroke and systemic embolism (SE) in patients with non-valvular atrial fibrillation (NVAF), as well as for the treatment of deep vein thrombosis and pulmonary embolism following 5 to 10 days of initial therapy with a parenteral anticoagulant.
According to Savaysa’s label, it should not be used in NVAF patients with creatinine clearance levels greater than 95 mL/min because, in that population, there is an increased risk of ischemic stroke with the drug compared to warfarin.
Daiichi Sankyo has developed resources for physicians and patients to help ensure patients can begin and/or remain on Savaysa per physician instructions.
The Savaysa Savings Plus program will include a reimbursement hotline to assist patients and prescribers who request help understanding a patient’s available coverage. Eligible patients who are prescribed Savaysa can enroll in a copay savings program and pay $4 per month through the Savaysa Savings Card.
Vouchers will also be available to provide patients and doctors with a way to try Savaysa at no cost to see if it is right for the patient.
In addition, the Savaysa Patient Assistance Program will offer assistance to qualified individuals, providing free product to eligible patients who are prescribed Savaysa, are uninsured, and are unable to identify alternative payment sources.
The approval of Savaysa in the US is based on data from the ENGAGE AF-TIMI 48 trial and the Hokusai-VTE trial. ![]()

Photo by Rhoda Baer
The oral factor Xa inhibitor edoxaban (Savaysa) is now available in US pharmacies, according to Daiichi Sankyo Company, Limited, the company developing the drug.
Savaysa is approved by the US Food and Drug Administration to reduce the risk of stroke and systemic embolism (SE) in patients with non-valvular atrial fibrillation (NVAF), as well as for the treatment of deep vein thrombosis and pulmonary embolism following 5 to 10 days of initial therapy with a parenteral anticoagulant.
According to Savaysa’s label, it should not be used in NVAF patients with creatinine clearance levels greater than 95 mL/min because, in that population, there is an increased risk of ischemic stroke with the drug compared to warfarin.
Daiichi Sankyo has developed resources for physicians and patients to help ensure patients can begin and/or remain on Savaysa per physician instructions.
The Savaysa Savings Plus program will include a reimbursement hotline to assist patients and prescribers who request help understanding a patient’s available coverage. Eligible patients who are prescribed Savaysa can enroll in a copay savings program and pay $4 per month through the Savaysa Savings Card.
Vouchers will also be available to provide patients and doctors with a way to try Savaysa at no cost to see if it is right for the patient.
In addition, the Savaysa Patient Assistance Program will offer assistance to qualified individuals, providing free product to eligible patients who are prescribed Savaysa, are uninsured, and are unable to identify alternative payment sources.
The approval of Savaysa in the US is based on data from the ENGAGE AF-TIMI 48 trial and the Hokusai-VTE trial. ![]()

Photo by Rhoda Baer
The oral factor Xa inhibitor edoxaban (Savaysa) is now available in US pharmacies, according to Daiichi Sankyo Company, Limited, the company developing the drug.
Savaysa is approved by the US Food and Drug Administration to reduce the risk of stroke and systemic embolism (SE) in patients with non-valvular atrial fibrillation (NVAF), as well as for the treatment of deep vein thrombosis and pulmonary embolism following 5 to 10 days of initial therapy with a parenteral anticoagulant.
According to Savaysa’s label, it should not be used in NVAF patients with creatinine clearance levels greater than 95 mL/min because, in that population, there is an increased risk of ischemic stroke with the drug compared to warfarin.
Daiichi Sankyo has developed resources for physicians and patients to help ensure patients can begin and/or remain on Savaysa per physician instructions.
The Savaysa Savings Plus program will include a reimbursement hotline to assist patients and prescribers who request help understanding a patient’s available coverage. Eligible patients who are prescribed Savaysa can enroll in a copay savings program and pay $4 per month through the Savaysa Savings Card.
Vouchers will also be available to provide patients and doctors with a way to try Savaysa at no cost to see if it is right for the patient.
In addition, the Savaysa Patient Assistance Program will offer assistance to qualified individuals, providing free product to eligible patients who are prescribed Savaysa, are uninsured, and are unable to identify alternative payment sources.
The approval of Savaysa in the US is based on data from the ENGAGE AF-TIMI 48 trial and the Hokusai-VTE trial. ![]()
FDA clears new blood-draw device
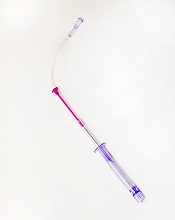
Photo courtesy of
Velano Vascular
The US Food and Drug Administration (FDA) has cleared for marketing a device that reduces the need for venipunctures for in-hospital blood draws.
Velano Vascular’s blood-draw device resembles a common syringe.
It allows peripheral intravenous catheters to be repurposed to draw blood from patients, thereby reducing the need for additional needle sticks among patients receiving medications and hydration via intravenous delivery.
The single-use device will soon be used for clinical evaluation in select hospitals, including the University of Pennsylvania in Philadelphia and University Hospitals Case Medical Center of Cleveland in Ohio.
“A fundamental benefit of this technology is reducing the ‘pin cushion effect,’ in which hospitalized patients are ‘stuck’ several times daily to obtain blood tests,” said Eric M. Stone, co-founder and chief executive officer of Velano Vascular, the company developing the blood-draw device.
“Oftentimes, the draw procedure is plagued by multiple failed attempts. The FDA’s clearance of this novel technology validates the existing clinical need and will allow us to expedite our efforts to bring this innovation to patients, healthcare providers, and hospitals around the world.”
According to research conducted by Velano Vascular, 1 of every 3 hospital patients is stuck 2 or more times daily for blood draws, with a significant subset of these patients receiving 3 or more blood draws, along with numerous needle sticks.
Twenty-eight percent of adult venipunctures and 44% of pediatric venipunctures require more than one stick to successfully draw blood.
“Traditional blood draws are one of the most common and most problematic healthcare procedures,” said Karen Daley, PhD, RN, past president of the American Nurses Association and a healthcare worker safety advocate.
“It is an antiquated technology that creates pain and anxiety for many patients, a significant safety risk for healthcare professionals, and a real inefficiency in our healthcare system. Velano Vascular has developed a common-sense solution to this pervasive, long-standing problem.” ![]()

Photo courtesy of
Velano Vascular
The US Food and Drug Administration (FDA) has cleared for marketing a device that reduces the need for venipunctures for in-hospital blood draws.
Velano Vascular’s blood-draw device resembles a common syringe.
It allows peripheral intravenous catheters to be repurposed to draw blood from patients, thereby reducing the need for additional needle sticks among patients receiving medications and hydration via intravenous delivery.
The single-use device will soon be used for clinical evaluation in select hospitals, including the University of Pennsylvania in Philadelphia and University Hospitals Case Medical Center of Cleveland in Ohio.
“A fundamental benefit of this technology is reducing the ‘pin cushion effect,’ in which hospitalized patients are ‘stuck’ several times daily to obtain blood tests,” said Eric M. Stone, co-founder and chief executive officer of Velano Vascular, the company developing the blood-draw device.
“Oftentimes, the draw procedure is plagued by multiple failed attempts. The FDA’s clearance of this novel technology validates the existing clinical need and will allow us to expedite our efforts to bring this innovation to patients, healthcare providers, and hospitals around the world.”
According to research conducted by Velano Vascular, 1 of every 3 hospital patients is stuck 2 or more times daily for blood draws, with a significant subset of these patients receiving 3 or more blood draws, along with numerous needle sticks.
Twenty-eight percent of adult venipunctures and 44% of pediatric venipunctures require more than one stick to successfully draw blood.
“Traditional blood draws are one of the most common and most problematic healthcare procedures,” said Karen Daley, PhD, RN, past president of the American Nurses Association and a healthcare worker safety advocate.
“It is an antiquated technology that creates pain and anxiety for many patients, a significant safety risk for healthcare professionals, and a real inefficiency in our healthcare system. Velano Vascular has developed a common-sense solution to this pervasive, long-standing problem.” ![]()

Photo courtesy of
Velano Vascular
The US Food and Drug Administration (FDA) has cleared for marketing a device that reduces the need for venipunctures for in-hospital blood draws.
Velano Vascular’s blood-draw device resembles a common syringe.
It allows peripheral intravenous catheters to be repurposed to draw blood from patients, thereby reducing the need for additional needle sticks among patients receiving medications and hydration via intravenous delivery.
The single-use device will soon be used for clinical evaluation in select hospitals, including the University of Pennsylvania in Philadelphia and University Hospitals Case Medical Center of Cleveland in Ohio.
“A fundamental benefit of this technology is reducing the ‘pin cushion effect,’ in which hospitalized patients are ‘stuck’ several times daily to obtain blood tests,” said Eric M. Stone, co-founder and chief executive officer of Velano Vascular, the company developing the blood-draw device.
“Oftentimes, the draw procedure is plagued by multiple failed attempts. The FDA’s clearance of this novel technology validates the existing clinical need and will allow us to expedite our efforts to bring this innovation to patients, healthcare providers, and hospitals around the world.”
According to research conducted by Velano Vascular, 1 of every 3 hospital patients is stuck 2 or more times daily for blood draws, with a significant subset of these patients receiving 3 or more blood draws, along with numerous needle sticks.
Twenty-eight percent of adult venipunctures and 44% of pediatric venipunctures require more than one stick to successfully draw blood.
“Traditional blood draws are one of the most common and most problematic healthcare procedures,” said Karen Daley, PhD, RN, past president of the American Nurses Association and a healthcare worker safety advocate.
“It is an antiquated technology that creates pain and anxiety for many patients, a significant safety risk for healthcare professionals, and a real inefficiency in our healthcare system. Velano Vascular has developed a common-sense solution to this pervasive, long-standing problem.” ![]()
More donated hearts rejected, even as wait list grows
Only about one in three available hearts was accepted for transplant in the United States in 2010, down from 44% 2 decades ago, researchers reported online Feb. 9 in the American Journal of Transplantation.
The decline stems in part from transplant centers rejecting “marginal” donor hearts, belying a growing need for heart transplants, longer waiting times, and multiple campaigns to expand the use of organs donated for transplantation, said Dr. Kiran Khush of Stanford (Calif.) University and her associates.
The researchers analyzed data on 82,053 potential donor hearts from the Organ Procurement and Transplantation Network. In 1995, 44% of available hearts were accepted for transplant, compared with only 29% in 2006 and 32% in 2010, they found. Meanwhile, rejection rates for donor hearts rose from 37% in 1995 to 52% in 2010, they reported (Am. J. Transplant. 2015 Feb. 10 [doi:10.1111/ajt.13055]).
Several factors might explain the trends, the investigators said. Potential heart donors tended to be older and more often had hypertension and diabetes by the final years of the study period, and transplant centers were less likely to accept hearts from such individuals. Also, mechanical circulatory devices were more commonly used, and centers might hesitate to transplant “marginal” hearts into “stable” recipients of such devices, Dr. Khush and her associates said. Furthermore, government scrutiny of post-transplant outcomes might make centers more conservative when evaluating potential donors, they added.
The study also uncovered regional variations in acceptance rates for donor hearts, with the lowest – about 25%-28% – found primarily in the southeastern United States. “Unfortunately, there are no standard guidelines for donor heart evaluation and acceptance, resulting in considerable inconsistencies in the types of donor hearts that are accepted by different transplant centers, and likely resulting in nonrecovery of potentially useful organs,” the investigators said. The findings “lend support to research and policy efforts aimed at establishing evidence-based criteria for donor heart evaluation and acceptance,” they added.
The work was supported by the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; and the Health Resources and Services Administration. The authors reported having no conflicts of interest.
Only about one in three available hearts was accepted for transplant in the United States in 2010, down from 44% 2 decades ago, researchers reported online Feb. 9 in the American Journal of Transplantation.
The decline stems in part from transplant centers rejecting “marginal” donor hearts, belying a growing need for heart transplants, longer waiting times, and multiple campaigns to expand the use of organs donated for transplantation, said Dr. Kiran Khush of Stanford (Calif.) University and her associates.
The researchers analyzed data on 82,053 potential donor hearts from the Organ Procurement and Transplantation Network. In 1995, 44% of available hearts were accepted for transplant, compared with only 29% in 2006 and 32% in 2010, they found. Meanwhile, rejection rates for donor hearts rose from 37% in 1995 to 52% in 2010, they reported (Am. J. Transplant. 2015 Feb. 10 [doi:10.1111/ajt.13055]).
Several factors might explain the trends, the investigators said. Potential heart donors tended to be older and more often had hypertension and diabetes by the final years of the study period, and transplant centers were less likely to accept hearts from such individuals. Also, mechanical circulatory devices were more commonly used, and centers might hesitate to transplant “marginal” hearts into “stable” recipients of such devices, Dr. Khush and her associates said. Furthermore, government scrutiny of post-transplant outcomes might make centers more conservative when evaluating potential donors, they added.
The study also uncovered regional variations in acceptance rates for donor hearts, with the lowest – about 25%-28% – found primarily in the southeastern United States. “Unfortunately, there are no standard guidelines for donor heart evaluation and acceptance, resulting in considerable inconsistencies in the types of donor hearts that are accepted by different transplant centers, and likely resulting in nonrecovery of potentially useful organs,” the investigators said. The findings “lend support to research and policy efforts aimed at establishing evidence-based criteria for donor heart evaluation and acceptance,” they added.
The work was supported by the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; and the Health Resources and Services Administration. The authors reported having no conflicts of interest.
Only about one in three available hearts was accepted for transplant in the United States in 2010, down from 44% 2 decades ago, researchers reported online Feb. 9 in the American Journal of Transplantation.
The decline stems in part from transplant centers rejecting “marginal” donor hearts, belying a growing need for heart transplants, longer waiting times, and multiple campaigns to expand the use of organs donated for transplantation, said Dr. Kiran Khush of Stanford (Calif.) University and her associates.
The researchers analyzed data on 82,053 potential donor hearts from the Organ Procurement and Transplantation Network. In 1995, 44% of available hearts were accepted for transplant, compared with only 29% in 2006 and 32% in 2010, they found. Meanwhile, rejection rates for donor hearts rose from 37% in 1995 to 52% in 2010, they reported (Am. J. Transplant. 2015 Feb. 10 [doi:10.1111/ajt.13055]).
Several factors might explain the trends, the investigators said. Potential heart donors tended to be older and more often had hypertension and diabetes by the final years of the study period, and transplant centers were less likely to accept hearts from such individuals. Also, mechanical circulatory devices were more commonly used, and centers might hesitate to transplant “marginal” hearts into “stable” recipients of such devices, Dr. Khush and her associates said. Furthermore, government scrutiny of post-transplant outcomes might make centers more conservative when evaluating potential donors, they added.
The study also uncovered regional variations in acceptance rates for donor hearts, with the lowest – about 25%-28% – found primarily in the southeastern United States. “Unfortunately, there are no standard guidelines for donor heart evaluation and acceptance, resulting in considerable inconsistencies in the types of donor hearts that are accepted by different transplant centers, and likely resulting in nonrecovery of potentially useful organs,” the investigators said. The findings “lend support to research and policy efforts aimed at establishing evidence-based criteria for donor heart evaluation and acceptance,” they added.
The work was supported by the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; and the Health Resources and Services Administration. The authors reported having no conflicts of interest.
FROM THE AMERICAN JOURNAL OF TRANSPLANTATION
Key clinical point: Acceptance rates of hearts donated for transplantation have declined substantially in the United States.
Major finding: Only 32% of donated hearts were accepted for transplant in 2010, compared with 44% in 1995.
Data source: Analysis of 82,053 potential donor hearts from the Organ Procurement and Transplantation Network.
Disclosures: The study was supported by the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; and the Health Resources and Services Administration. The authors reported having no conflicts of interest.
Overall survival plateaus at 3 years for ipilimumab-treated melanoma patients
Among patients with advanced melanoma who were treated with ipilimumab, about 20%-26% survived to 3 years, and these patients are likely to have a good long-term outcome, according to a pooled analysis of survival data published online Feb. 9 in the Journal of Clinical Oncology.
Investigators pooled data from ten prospective (including two phase III trials) and two retrospective studies with a total of 1,257 previously treated and 604 treatment-naive patients. At least 3 years after receiving ipilimumab, 254 patients were still alive, with a median follow up for this subset of 69 months. Around year 3, the Kaplan-Meier overall survival (OS) curve began to plateau and extended to 9.9 years for the longest survival follow-up.
“These results suggest that the majority of patients who reached this milestone time point had a low risk of death thereafter,” wrote Dr. Dirk Schadendorf and his associates (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.56.2736]).
Compared with patients who were previously treated, treatment-naive patients had a higher median overall survival (13.5 months [95% confidence interval, 11.9-15.4] vs. 10.7 months [9.6-11.4]) and higher 3-year-survival rates (26% [21%-30%] vs. 20% [18%-23%]). No definitive conclusion could be drawn from this observation, however, since nonrandomized subsets were used for this analysis. Subset analysis by dose showed similar median OS and 3-year survival rates for ipilimumab 3 mg/kg, 10 mg/kg, and other dosing regimens.
The researchers expanded the study to include overall survival (OS) data from 2,985 patients enrolled in a U.S. multicenter, open-label, expanded-access treatment protocol (EAP). This group included patients with poorer prognostic factors, some of whom were ineligible for clinical trials. The expanded group showed a lower median OS of 9.5 months and 3 year–survival rate of 21%, with the familiar OS curve plateau around 3 years that extended up to 10 years in some patients.
While this analysis only examined overall survival rates, individual ipilimumab studies that tracked patient responses to the drug have shown that some proportion of long-term survivors did not achieve a response. Identifying the specific disease characteristics of the long-term survivors will require further study.
“Considering the historic median OS of approximately 8-10 months and a 5-year survival rate of approximately 10% in advanced melanoma, the results presented herein are encouraging for patients diagnosed with this aggressive disease,” the authors wrote.
Dr. Schadendorf and his associates demonstrate a plateau in the survival curve of ipilimumab-treated patients beginning at about 3 years and representing about 21% of the treatment group. The curve suggests that those who survive to 3 years are highly likely to have a good long-term outcome, which provides a strong motivating factor in the decision to consider ipilimumab treatment. While pooled data adds information far beyond individual trials, a major drawback lies in the loss of control data necessary to isolate the added benefit of the study drug.
An indirect comparison using historic control series, in this case a large cohort documented in the American Joint Committee on Cancer (AJCC) Melanoma Staging Database, can substitute for missing control data in the pooled analysis. Reviewing data for stage IIIc and IV patients, the overall survival Kaplan-Meier curves in this population also show a plateau, but much later than that reported for ipilimumab, at beyond 8 years.
The AJCC melanoma classification gives survival rates at 3, 5, and 10 years of 19%, 13%, and 9%, respectively. Comparison with ipilimumab data suggests that survival at 3 years is similar, but thereafter improves with ipilimumab by 10% over other treatments that were available at the time. This difference is similar to the percentage of patients who achieved objective responses with ipilimumab. Although assessing response rate and progression-free survival in patients treated with ipilimumab presents challenges, the long-term benefits of ipilimumab could be better ascertained if information on the number of patients in the 21% plateau who were disease free or stably maintaining response had been collected.
Evaluation of long-term benefits of ipilimumab should consider toxicities and costs, as it is one of the most costly systemic therapies used for cancer treatment. The phase III trial using the drug at 3 mg/kg demonstrated that the large majority of patients had no serious adverse effects. If older patients and those with advanced disease are candidates, then the 10%-15% of grade 3 or 4 adverse events may translate to hospitalization and added expense, putting health regulatory systems in the position to deny widespread use of the agent despite proven benefit.
As the first agent to benefit overall survival of patients with advanced melanoma, ipilimumab may pave the way to broader improvements in a larger proportion of patients by combining with targeted therapies, such as BRAF and MEK inhibitors, and other new immunotherapies, such as anti-PD-1 antibodies.
Dr. Antoni Ribas is an oncologist with the Jonsson Comprehensive Cancer Center, Los Angles, and Dr. Keith T. Flaherty is an oncologist with Massachusetts General Hospital Cancer Center, Boston. These remarks were part of an editorial accompanying the report (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.56.2736]). Dr. Ribas has an advisory role with Merck, Amgen, Novartis, GlaxoSmithKline, and Genentech/Roche. Dr. Flaherty has an advisory role with GlaxoSmithKline, Genentech/Roche, Novartis, and Merck.
Dr. Schadendorf and his associates demonstrate a plateau in the survival curve of ipilimumab-treated patients beginning at about 3 years and representing about 21% of the treatment group. The curve suggests that those who survive to 3 years are highly likely to have a good long-term outcome, which provides a strong motivating factor in the decision to consider ipilimumab treatment. While pooled data adds information far beyond individual trials, a major drawback lies in the loss of control data necessary to isolate the added benefit of the study drug.
An indirect comparison using historic control series, in this case a large cohort documented in the American Joint Committee on Cancer (AJCC) Melanoma Staging Database, can substitute for missing control data in the pooled analysis. Reviewing data for stage IIIc and IV patients, the overall survival Kaplan-Meier curves in this population also show a plateau, but much later than that reported for ipilimumab, at beyond 8 years.
The AJCC melanoma classification gives survival rates at 3, 5, and 10 years of 19%, 13%, and 9%, respectively. Comparison with ipilimumab data suggests that survival at 3 years is similar, but thereafter improves with ipilimumab by 10% over other treatments that were available at the time. This difference is similar to the percentage of patients who achieved objective responses with ipilimumab. Although assessing response rate and progression-free survival in patients treated with ipilimumab presents challenges, the long-term benefits of ipilimumab could be better ascertained if information on the number of patients in the 21% plateau who were disease free or stably maintaining response had been collected.
Evaluation of long-term benefits of ipilimumab should consider toxicities and costs, as it is one of the most costly systemic therapies used for cancer treatment. The phase III trial using the drug at 3 mg/kg demonstrated that the large majority of patients had no serious adverse effects. If older patients and those with advanced disease are candidates, then the 10%-15% of grade 3 or 4 adverse events may translate to hospitalization and added expense, putting health regulatory systems in the position to deny widespread use of the agent despite proven benefit.
As the first agent to benefit overall survival of patients with advanced melanoma, ipilimumab may pave the way to broader improvements in a larger proportion of patients by combining with targeted therapies, such as BRAF and MEK inhibitors, and other new immunotherapies, such as anti-PD-1 antibodies.
Dr. Antoni Ribas is an oncologist with the Jonsson Comprehensive Cancer Center, Los Angles, and Dr. Keith T. Flaherty is an oncologist with Massachusetts General Hospital Cancer Center, Boston. These remarks were part of an editorial accompanying the report (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.56.2736]). Dr. Ribas has an advisory role with Merck, Amgen, Novartis, GlaxoSmithKline, and Genentech/Roche. Dr. Flaherty has an advisory role with GlaxoSmithKline, Genentech/Roche, Novartis, and Merck.
Dr. Schadendorf and his associates demonstrate a plateau in the survival curve of ipilimumab-treated patients beginning at about 3 years and representing about 21% of the treatment group. The curve suggests that those who survive to 3 years are highly likely to have a good long-term outcome, which provides a strong motivating factor in the decision to consider ipilimumab treatment. While pooled data adds information far beyond individual trials, a major drawback lies in the loss of control data necessary to isolate the added benefit of the study drug.
An indirect comparison using historic control series, in this case a large cohort documented in the American Joint Committee on Cancer (AJCC) Melanoma Staging Database, can substitute for missing control data in the pooled analysis. Reviewing data for stage IIIc and IV patients, the overall survival Kaplan-Meier curves in this population also show a plateau, but much later than that reported for ipilimumab, at beyond 8 years.
The AJCC melanoma classification gives survival rates at 3, 5, and 10 years of 19%, 13%, and 9%, respectively. Comparison with ipilimumab data suggests that survival at 3 years is similar, but thereafter improves with ipilimumab by 10% over other treatments that were available at the time. This difference is similar to the percentage of patients who achieved objective responses with ipilimumab. Although assessing response rate and progression-free survival in patients treated with ipilimumab presents challenges, the long-term benefits of ipilimumab could be better ascertained if information on the number of patients in the 21% plateau who were disease free or stably maintaining response had been collected.
Evaluation of long-term benefits of ipilimumab should consider toxicities and costs, as it is one of the most costly systemic therapies used for cancer treatment. The phase III trial using the drug at 3 mg/kg demonstrated that the large majority of patients had no serious adverse effects. If older patients and those with advanced disease are candidates, then the 10%-15% of grade 3 or 4 adverse events may translate to hospitalization and added expense, putting health regulatory systems in the position to deny widespread use of the agent despite proven benefit.
As the first agent to benefit overall survival of patients with advanced melanoma, ipilimumab may pave the way to broader improvements in a larger proportion of patients by combining with targeted therapies, such as BRAF and MEK inhibitors, and other new immunotherapies, such as anti-PD-1 antibodies.
Dr. Antoni Ribas is an oncologist with the Jonsson Comprehensive Cancer Center, Los Angles, and Dr. Keith T. Flaherty is an oncologist with Massachusetts General Hospital Cancer Center, Boston. These remarks were part of an editorial accompanying the report (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.56.2736]). Dr. Ribas has an advisory role with Merck, Amgen, Novartis, GlaxoSmithKline, and Genentech/Roche. Dr. Flaherty has an advisory role with GlaxoSmithKline, Genentech/Roche, Novartis, and Merck.
Among patients with advanced melanoma who were treated with ipilimumab, about 20%-26% survived to 3 years, and these patients are likely to have a good long-term outcome, according to a pooled analysis of survival data published online Feb. 9 in the Journal of Clinical Oncology.
Investigators pooled data from ten prospective (including two phase III trials) and two retrospective studies with a total of 1,257 previously treated and 604 treatment-naive patients. At least 3 years after receiving ipilimumab, 254 patients were still alive, with a median follow up for this subset of 69 months. Around year 3, the Kaplan-Meier overall survival (OS) curve began to plateau and extended to 9.9 years for the longest survival follow-up.
“These results suggest that the majority of patients who reached this milestone time point had a low risk of death thereafter,” wrote Dr. Dirk Schadendorf and his associates (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.56.2736]).
Compared with patients who were previously treated, treatment-naive patients had a higher median overall survival (13.5 months [95% confidence interval, 11.9-15.4] vs. 10.7 months [9.6-11.4]) and higher 3-year-survival rates (26% [21%-30%] vs. 20% [18%-23%]). No definitive conclusion could be drawn from this observation, however, since nonrandomized subsets were used for this analysis. Subset analysis by dose showed similar median OS and 3-year survival rates for ipilimumab 3 mg/kg, 10 mg/kg, and other dosing regimens.
The researchers expanded the study to include overall survival (OS) data from 2,985 patients enrolled in a U.S. multicenter, open-label, expanded-access treatment protocol (EAP). This group included patients with poorer prognostic factors, some of whom were ineligible for clinical trials. The expanded group showed a lower median OS of 9.5 months and 3 year–survival rate of 21%, with the familiar OS curve plateau around 3 years that extended up to 10 years in some patients.
While this analysis only examined overall survival rates, individual ipilimumab studies that tracked patient responses to the drug have shown that some proportion of long-term survivors did not achieve a response. Identifying the specific disease characteristics of the long-term survivors will require further study.
“Considering the historic median OS of approximately 8-10 months and a 5-year survival rate of approximately 10% in advanced melanoma, the results presented herein are encouraging for patients diagnosed with this aggressive disease,” the authors wrote.
Among patients with advanced melanoma who were treated with ipilimumab, about 20%-26% survived to 3 years, and these patients are likely to have a good long-term outcome, according to a pooled analysis of survival data published online Feb. 9 in the Journal of Clinical Oncology.
Investigators pooled data from ten prospective (including two phase III trials) and two retrospective studies with a total of 1,257 previously treated and 604 treatment-naive patients. At least 3 years after receiving ipilimumab, 254 patients were still alive, with a median follow up for this subset of 69 months. Around year 3, the Kaplan-Meier overall survival (OS) curve began to plateau and extended to 9.9 years for the longest survival follow-up.
“These results suggest that the majority of patients who reached this milestone time point had a low risk of death thereafter,” wrote Dr. Dirk Schadendorf and his associates (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.56.2736]).
Compared with patients who were previously treated, treatment-naive patients had a higher median overall survival (13.5 months [95% confidence interval, 11.9-15.4] vs. 10.7 months [9.6-11.4]) and higher 3-year-survival rates (26% [21%-30%] vs. 20% [18%-23%]). No definitive conclusion could be drawn from this observation, however, since nonrandomized subsets were used for this analysis. Subset analysis by dose showed similar median OS and 3-year survival rates for ipilimumab 3 mg/kg, 10 mg/kg, and other dosing regimens.
The researchers expanded the study to include overall survival (OS) data from 2,985 patients enrolled in a U.S. multicenter, open-label, expanded-access treatment protocol (EAP). This group included patients with poorer prognostic factors, some of whom were ineligible for clinical trials. The expanded group showed a lower median OS of 9.5 months and 3 year–survival rate of 21%, with the familiar OS curve plateau around 3 years that extended up to 10 years in some patients.
While this analysis only examined overall survival rates, individual ipilimumab studies that tracked patient responses to the drug have shown that some proportion of long-term survivors did not achieve a response. Identifying the specific disease characteristics of the long-term survivors will require further study.
“Considering the historic median OS of approximately 8-10 months and a 5-year survival rate of approximately 10% in advanced melanoma, the results presented herein are encouraging for patients diagnosed with this aggressive disease,” the authors wrote.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Ipilimumab-treated advanced melanoma patients alive at 3 years tend to have good long-term outcomes.
Major finding: Around year 3, the Kaplan-Meier OS curve began to plateau and extended to 9.9 years for the longest survival follow-up.
Data source: Pooled overall survival data from 12 studies including 1,861 ipilimumab-treated patients with advanced melanoma.
Disclosures: Dr. Schadendorf disclosed that he is a consultant for Bristol-Myers Squibb. Bristol-Myers Squibb sponsored this study.
ASCO endorses ACS guidelines for prostate cancer survivor care
The American Society of Clinical Oncology has endorsed the American Cancer Society Prostate Cancer Survivorship Care Guidelines, a 39-point list with recommendations on continuing care for prostate care survivors, but with a number of qualifying statements and modifications.
The guidelines, developed by a workgroup of 16 multidisciplinary experts specializing in the care of prostate cancer patients and the long-term effects of their treatments, are intended as points of reference for primary care providers, medical oncologists, urologists, and other health care providers.
Areas covered in the guidelines include health promotion, surveillance for recurrence, screening and early detection of second primary cancers, assessment and management of physical and psychosocial long-term and late effects, and care coordination and practice implications.Read the full list of recommendations here: (doi: 10.1200/JCO.2014.60.2557).
The American Society of Clinical Oncology has endorsed the American Cancer Society Prostate Cancer Survivorship Care Guidelines, a 39-point list with recommendations on continuing care for prostate care survivors, but with a number of qualifying statements and modifications.
The guidelines, developed by a workgroup of 16 multidisciplinary experts specializing in the care of prostate cancer patients and the long-term effects of their treatments, are intended as points of reference for primary care providers, medical oncologists, urologists, and other health care providers.
Areas covered in the guidelines include health promotion, surveillance for recurrence, screening and early detection of second primary cancers, assessment and management of physical and psychosocial long-term and late effects, and care coordination and practice implications.Read the full list of recommendations here: (doi: 10.1200/JCO.2014.60.2557).
The American Society of Clinical Oncology has endorsed the American Cancer Society Prostate Cancer Survivorship Care Guidelines, a 39-point list with recommendations on continuing care for prostate care survivors, but with a number of qualifying statements and modifications.
The guidelines, developed by a workgroup of 16 multidisciplinary experts specializing in the care of prostate cancer patients and the long-term effects of their treatments, are intended as points of reference for primary care providers, medical oncologists, urologists, and other health care providers.
Areas covered in the guidelines include health promotion, surveillance for recurrence, screening and early detection of second primary cancers, assessment and management of physical and psychosocial long-term and late effects, and care coordination and practice implications.Read the full list of recommendations here: (doi: 10.1200/JCO.2014.60.2557).
Psychopharmacology in primary care faces challenges
NEW YORK – Incorporating psychiatric assessment and treatment into a busy primary care practice is not easy, but it is doable.
“Every time I start a patient on a [psychiatric] medication I have a moment of trepidation, even though I have now done this for about 4 years,” Dr. Diane E. Bloomfield said at a psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry. “It still does not come easily to me,” said Dr. Bloomfield, a general-practice pediatrician at the family care center of Montefiore Medical Center in New York.
Inclusion of mental health as part of routine pediatric practice is a new concept. “Until recently, we pediatricians did not think of mental health as part of daily practice,” she said.
Dr. Bloomfield cited three factors that pose the greatest challenges to integrating psychiatry into her practice: time constraints, reimbursement, and knowledge gaps.
Reimbursement limitations contribute to the time issue. Most of Dr. Bloomfield’s patients are covered by Medicaid, which allows for a 15-minute session with each patient and family. That’s barely enough time to assess a child’s social and emotional development, in addition to all the other bases she must cover during an appointment, but she tries to carve out time for more challenging cases by scheduling them near the end of her day.
Dr. Bloomfield said that she routinely administers the Pediatric Symptom Checklist to all her patients who are 4-18 years old. She recommended that pediatricians take advantage of all the screening tools that the American Academy of Pediatrics (AAP) includes with its practice guidelines, along with the other mental health resources on the AAP website. Using improved coding on her billings also allowed her to arrange reimbursement for more of the time she spends on mental health conditions.
Reducing the knowledge gap can be more complicated. Many pediatricians, Dr. Bloomfield included, did not prescribe methylphenidate or selective serotonin reuptake inhibitors (SSRIs) during training. The boxed warning that the Food and Drug Administration put on antidepressants starting in 2004 has been another factor dampening drug psychotherapy by pediatricians, dissuading them from treating depression, she said.
Some of these dilemmas decreased when the AAP released in 2010 two algorithms that provided a framework for identifying and managing mental health and substance abuse concerns in primary care (Pediatrics 2010;125:S109-25). Neither algorithm, however, dealt with psychopharmacology.
Survey results have shown that many pediatricians become more willing to prescribe SSRIs if they can consult with a psychiatrist about the diagnosis and treatment. Pediatricians are generally more comfortable prescribing stimulants for attention-deficit/hyperactivity disorder (ADHD). “We see a lot of kids with ADHD, so we think we need to do something for them. In addition, medications for ADHD either work or don’t work, but they don’t cause suicidality,” Dr. Bloomfield said.
An AAP working group that included Dr. Bloomfield recently introduced a pilot program for a revised residency curriculum that includes a mental health module as well as a second module that focuses on anxiety diagnosis and management. In addition, certain states, including Massachusetts and New York, have introduced postresidency education programs that deal with child and adolescent psychiatry, including drug treatment.
Dr. Bloomfield said that she had taken training courses in the New York program. “It gave me the tools for evaluating patients and it taught me how to start medications in a safe way.” The midcareer training she received through New York’s Child and Adolescent Psychiatry for Primary Care program “made me much more confident that I could address my patients’ psychosocial needs.” Today, Dr. Bloomfield said she tries to manage children and adolescents with mild depression herself and not refer them to a specialist.
“Pediatricians are quite willing” to include psychiatric interventions in their practice, but we need support from psychiatrists to receive the necessary education and adequate reimbursement,” Dr. Bloomfield said.
On Twitter @mitchelzoler
NEW YORK – Incorporating psychiatric assessment and treatment into a busy primary care practice is not easy, but it is doable.
“Every time I start a patient on a [psychiatric] medication I have a moment of trepidation, even though I have now done this for about 4 years,” Dr. Diane E. Bloomfield said at a psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry. “It still does not come easily to me,” said Dr. Bloomfield, a general-practice pediatrician at the family care center of Montefiore Medical Center in New York.
Inclusion of mental health as part of routine pediatric practice is a new concept. “Until recently, we pediatricians did not think of mental health as part of daily practice,” she said.
Dr. Bloomfield cited three factors that pose the greatest challenges to integrating psychiatry into her practice: time constraints, reimbursement, and knowledge gaps.
Reimbursement limitations contribute to the time issue. Most of Dr. Bloomfield’s patients are covered by Medicaid, which allows for a 15-minute session with each patient and family. That’s barely enough time to assess a child’s social and emotional development, in addition to all the other bases she must cover during an appointment, but she tries to carve out time for more challenging cases by scheduling them near the end of her day.
Dr. Bloomfield said that she routinely administers the Pediatric Symptom Checklist to all her patients who are 4-18 years old. She recommended that pediatricians take advantage of all the screening tools that the American Academy of Pediatrics (AAP) includes with its practice guidelines, along with the other mental health resources on the AAP website. Using improved coding on her billings also allowed her to arrange reimbursement for more of the time she spends on mental health conditions.
Reducing the knowledge gap can be more complicated. Many pediatricians, Dr. Bloomfield included, did not prescribe methylphenidate or selective serotonin reuptake inhibitors (SSRIs) during training. The boxed warning that the Food and Drug Administration put on antidepressants starting in 2004 has been another factor dampening drug psychotherapy by pediatricians, dissuading them from treating depression, she said.
Some of these dilemmas decreased when the AAP released in 2010 two algorithms that provided a framework for identifying and managing mental health and substance abuse concerns in primary care (Pediatrics 2010;125:S109-25). Neither algorithm, however, dealt with psychopharmacology.
Survey results have shown that many pediatricians become more willing to prescribe SSRIs if they can consult with a psychiatrist about the diagnosis and treatment. Pediatricians are generally more comfortable prescribing stimulants for attention-deficit/hyperactivity disorder (ADHD). “We see a lot of kids with ADHD, so we think we need to do something for them. In addition, medications for ADHD either work or don’t work, but they don’t cause suicidality,” Dr. Bloomfield said.
An AAP working group that included Dr. Bloomfield recently introduced a pilot program for a revised residency curriculum that includes a mental health module as well as a second module that focuses on anxiety diagnosis and management. In addition, certain states, including Massachusetts and New York, have introduced postresidency education programs that deal with child and adolescent psychiatry, including drug treatment.
Dr. Bloomfield said that she had taken training courses in the New York program. “It gave me the tools for evaluating patients and it taught me how to start medications in a safe way.” The midcareer training she received through New York’s Child and Adolescent Psychiatry for Primary Care program “made me much more confident that I could address my patients’ psychosocial needs.” Today, Dr. Bloomfield said she tries to manage children and adolescents with mild depression herself and not refer them to a specialist.
“Pediatricians are quite willing” to include psychiatric interventions in their practice, but we need support from psychiatrists to receive the necessary education and adequate reimbursement,” Dr. Bloomfield said.
On Twitter @mitchelzoler
NEW YORK – Incorporating psychiatric assessment and treatment into a busy primary care practice is not easy, but it is doable.
“Every time I start a patient on a [psychiatric] medication I have a moment of trepidation, even though I have now done this for about 4 years,” Dr. Diane E. Bloomfield said at a psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry. “It still does not come easily to me,” said Dr. Bloomfield, a general-practice pediatrician at the family care center of Montefiore Medical Center in New York.
Inclusion of mental health as part of routine pediatric practice is a new concept. “Until recently, we pediatricians did not think of mental health as part of daily practice,” she said.
Dr. Bloomfield cited three factors that pose the greatest challenges to integrating psychiatry into her practice: time constraints, reimbursement, and knowledge gaps.
Reimbursement limitations contribute to the time issue. Most of Dr. Bloomfield’s patients are covered by Medicaid, which allows for a 15-minute session with each patient and family. That’s barely enough time to assess a child’s social and emotional development, in addition to all the other bases she must cover during an appointment, but she tries to carve out time for more challenging cases by scheduling them near the end of her day.
Dr. Bloomfield said that she routinely administers the Pediatric Symptom Checklist to all her patients who are 4-18 years old. She recommended that pediatricians take advantage of all the screening tools that the American Academy of Pediatrics (AAP) includes with its practice guidelines, along with the other mental health resources on the AAP website. Using improved coding on her billings also allowed her to arrange reimbursement for more of the time she spends on mental health conditions.
Reducing the knowledge gap can be more complicated. Many pediatricians, Dr. Bloomfield included, did not prescribe methylphenidate or selective serotonin reuptake inhibitors (SSRIs) during training. The boxed warning that the Food and Drug Administration put on antidepressants starting in 2004 has been another factor dampening drug psychotherapy by pediatricians, dissuading them from treating depression, she said.
Some of these dilemmas decreased when the AAP released in 2010 two algorithms that provided a framework for identifying and managing mental health and substance abuse concerns in primary care (Pediatrics 2010;125:S109-25). Neither algorithm, however, dealt with psychopharmacology.
Survey results have shown that many pediatricians become more willing to prescribe SSRIs if they can consult with a psychiatrist about the diagnosis and treatment. Pediatricians are generally more comfortable prescribing stimulants for attention-deficit/hyperactivity disorder (ADHD). “We see a lot of kids with ADHD, so we think we need to do something for them. In addition, medications for ADHD either work or don’t work, but they don’t cause suicidality,” Dr. Bloomfield said.
An AAP working group that included Dr. Bloomfield recently introduced a pilot program for a revised residency curriculum that includes a mental health module as well as a second module that focuses on anxiety diagnosis and management. In addition, certain states, including Massachusetts and New York, have introduced postresidency education programs that deal with child and adolescent psychiatry, including drug treatment.
Dr. Bloomfield said that she had taken training courses in the New York program. “It gave me the tools for evaluating patients and it taught me how to start medications in a safe way.” The midcareer training she received through New York’s Child and Adolescent Psychiatry for Primary Care program “made me much more confident that I could address my patients’ psychosocial needs.” Today, Dr. Bloomfield said she tries to manage children and adolescents with mild depression herself and not refer them to a specialist.
“Pediatricians are quite willing” to include psychiatric interventions in their practice, but we need support from psychiatrists to receive the necessary education and adequate reimbursement,” Dr. Bloomfield said.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE PSYCHOPHARMACOLOGY UPDATE INSTITUTE





