User login
Endocarditis in dental patients rises after guidelines discourage prophylaxis
The number of prescriptions for antibiotic prophylaxis before invasive dental procedures has dropped sharply in England since 2008, while the incidence of infective endocarditis has risen significantly in the same time period, researchers found.
A study led by Dr. Martin Thornhill of the University of Sheffield (England) School of Clinical Dentistry, and published online Nov. 18 in the Lancet (doi: 10.1016/S0140-6736(14)62007-9) showed that after the National Institute for Health and Care Excellence issued guidelines against antibiotic prophylaxis, even for patients at high risk of endocarditis, prescriptions fell precipitously from a mean 10,900 per month in 2004-2008 in England to a mean 2,236 a month between April 2008 and April 2013, with only 1,235 issued in the last month of the study period. The NICE guidance, which went further than other published recommendations that have aimed to limit, but not eliminate, the use of antibiotic prophylaxis as a form of endocarditis prevention, cited the absence of a robust evidence base supporting its effectiveness, and also the risk of adverse drug reactions.
Dr. Thornhill and his colleagues reviewed both national prescription records and hospital discharge records for patients with a primary diagnosis of infective endocarditis. Prescriptions of antibiotic prophylaxis for the prevention of infective endocarditis fell significantly after introduction of the NICE guidance.
The incidence of infective endocarditis, in contrast, rose by 0.11 cases per 10 million people per month following the 2008 guidance (95% confidence interval, 0.05-0.16; P < .0001). By March 2013, the researchers found that there were 34.9 more cases per month than would have been expected had the previous trend continued (95% CI, 7.9-61.9). Moreover, the increase was significant for patients determined to be at low or moderate risk as well as for those deemed high risk. The researchers did not find a statistically significant increase in endocarditis-related mortality corresponding to the drop in prescriptions.
Dr. Thornhill and his colleagues cautioned that their results did not establish a causal association between the drop in prescriptions and the rise in cases, and that further investigations were now warranted.
The study was funded by Heart Research UK, Simplyhealth, and the U.S. National Institutes of Health. Two of its authors were involved in guidelines on infective endocarditis issued by the American Heart Association in 2007. One author helped produce European Society of Cardiology endocarditis guidelines in 2009, and also acted as a consultant to NICE during the drafting of the 2008 guidelines on antibiotic prophylaxis in endocarditis.
The number of prescriptions for antibiotic prophylaxis before invasive dental procedures has dropped sharply in England since 2008, while the incidence of infective endocarditis has risen significantly in the same time period, researchers found.
A study led by Dr. Martin Thornhill of the University of Sheffield (England) School of Clinical Dentistry, and published online Nov. 18 in the Lancet (doi: 10.1016/S0140-6736(14)62007-9) showed that after the National Institute for Health and Care Excellence issued guidelines against antibiotic prophylaxis, even for patients at high risk of endocarditis, prescriptions fell precipitously from a mean 10,900 per month in 2004-2008 in England to a mean 2,236 a month between April 2008 and April 2013, with only 1,235 issued in the last month of the study period. The NICE guidance, which went further than other published recommendations that have aimed to limit, but not eliminate, the use of antibiotic prophylaxis as a form of endocarditis prevention, cited the absence of a robust evidence base supporting its effectiveness, and also the risk of adverse drug reactions.
Dr. Thornhill and his colleagues reviewed both national prescription records and hospital discharge records for patients with a primary diagnosis of infective endocarditis. Prescriptions of antibiotic prophylaxis for the prevention of infective endocarditis fell significantly after introduction of the NICE guidance.
The incidence of infective endocarditis, in contrast, rose by 0.11 cases per 10 million people per month following the 2008 guidance (95% confidence interval, 0.05-0.16; P < .0001). By March 2013, the researchers found that there were 34.9 more cases per month than would have been expected had the previous trend continued (95% CI, 7.9-61.9). Moreover, the increase was significant for patients determined to be at low or moderate risk as well as for those deemed high risk. The researchers did not find a statistically significant increase in endocarditis-related mortality corresponding to the drop in prescriptions.
Dr. Thornhill and his colleagues cautioned that their results did not establish a causal association between the drop in prescriptions and the rise in cases, and that further investigations were now warranted.
The study was funded by Heart Research UK, Simplyhealth, and the U.S. National Institutes of Health. Two of its authors were involved in guidelines on infective endocarditis issued by the American Heart Association in 2007. One author helped produce European Society of Cardiology endocarditis guidelines in 2009, and also acted as a consultant to NICE during the drafting of the 2008 guidelines on antibiotic prophylaxis in endocarditis.
The number of prescriptions for antibiotic prophylaxis before invasive dental procedures has dropped sharply in England since 2008, while the incidence of infective endocarditis has risen significantly in the same time period, researchers found.
A study led by Dr. Martin Thornhill of the University of Sheffield (England) School of Clinical Dentistry, and published online Nov. 18 in the Lancet (doi: 10.1016/S0140-6736(14)62007-9) showed that after the National Institute for Health and Care Excellence issued guidelines against antibiotic prophylaxis, even for patients at high risk of endocarditis, prescriptions fell precipitously from a mean 10,900 per month in 2004-2008 in England to a mean 2,236 a month between April 2008 and April 2013, with only 1,235 issued in the last month of the study period. The NICE guidance, which went further than other published recommendations that have aimed to limit, but not eliminate, the use of antibiotic prophylaxis as a form of endocarditis prevention, cited the absence of a robust evidence base supporting its effectiveness, and also the risk of adverse drug reactions.
Dr. Thornhill and his colleagues reviewed both national prescription records and hospital discharge records for patients with a primary diagnosis of infective endocarditis. Prescriptions of antibiotic prophylaxis for the prevention of infective endocarditis fell significantly after introduction of the NICE guidance.
The incidence of infective endocarditis, in contrast, rose by 0.11 cases per 10 million people per month following the 2008 guidance (95% confidence interval, 0.05-0.16; P < .0001). By March 2013, the researchers found that there were 34.9 more cases per month than would have been expected had the previous trend continued (95% CI, 7.9-61.9). Moreover, the increase was significant for patients determined to be at low or moderate risk as well as for those deemed high risk. The researchers did not find a statistically significant increase in endocarditis-related mortality corresponding to the drop in prescriptions.
Dr. Thornhill and his colleagues cautioned that their results did not establish a causal association between the drop in prescriptions and the rise in cases, and that further investigations were now warranted.
The study was funded by Heart Research UK, Simplyhealth, and the U.S. National Institutes of Health. Two of its authors were involved in guidelines on infective endocarditis issued by the American Heart Association in 2007. One author helped produce European Society of Cardiology endocarditis guidelines in 2009, and also acted as a consultant to NICE during the drafting of the 2008 guidelines on antibiotic prophylaxis in endocarditis.
FROM THE LANCET
Key clinical point: Antibiotic prophylaxis before invasive dental procedures dropped sharply while the incidence of infective endocarditis rose significantly.
Major finding: The incidence of infective endocarditis rose by 0.11 cases per 10 million people per month following the 2008 guidance.
Data source: Researchers reviewed both national prescription records and hospital discharge records for patients with a primary diagnosis of infective endocarditis in the United Kingdom.
Disclosures: The study was funded by Heart Research UK, Simplyhealth, and the U.S. National Institutes of Health. Two of its authors were involved in guidelines on infective endocarditis issued by the American Heart Association in 2007. One author helped produce European Society of Cardiology endocarditis guidelines in 2009, and also acted as a consultant to NICE during the drafting of the 2008 guidelines on antibiotic prophylaxis in endocarditis.
CLINICAL GUIDELINES: Primary care bronchiolitis guidelines
Bronchiolitis is the most common cause of hospitalization among infants during the first 12 months of life. Approximately 100,000 bronchiolitis admissions occur annually in children in the United States, at an estimated cost of $1.73 billion. The American Academy of Pediatrics recently published new guidelines for the diagnosis, management, and prevention of bronchiolitis in children younger than 2 years.
Diagnosis
Diagnosis is based on patient history and physical examination. The course and severity of bronchiolitis vary, ranging from mild disease with simple runny nose and cough, to transient apneic events, and on to progressive respiratory distress secondary to airway obstruction. Management of bronchiolitis must be determined in the context of increased risk factors for severe disease, including age less than 12 weeks, history of prematurity, underlying cardiopulmonary disease, and immunodeficiency. Current evidence does not support routine labs or diagnostic imaging as helping with risk assessment. Abnormalities on chest x-ray, which are common in children with bronchiolitis, do reliably predict severity of disease, so chest x-rays are only indicated when another etiology of respiratory distress such as pneumothorax or pneumonia is a concern. Routine virologic testing is not recommended, as it does not appear to aid in guiding the treatment of the child with bronchiolitis.
Management
Randomized trials have not shown any benefit from alpha- or beta-adrenergic agonist administration. Bronchodilators can lessen symptoms scores, but their use does not speed disease resolution or decrease the length of stay or need for hospitalization. A Cochrane analysis concluded that there was no benefit to giving bronchodilators to infants with bronchiolitis. Adverse effects included tachycardia, tremors, and cost, all of which outweigh potential benefits. While previous versions of the AAP guidelines recommended bronchodilators as an option, the 2014 guidelines state, “Clinicians should not administer albuterol (or salbutamol) to infants and children with a diagnosis of bronchiolitis (Evidence Quality: B; Recommendation Strength: Strong).” It is noted that there may be some children who have reversible airway obstruction, but it is impossible to tell ahead of time who they are; and due to the variability of the disease, it is even hard to tell in whom the medication is effective. It is acknowledged that children with severe disease were usually excluded from the studies of bronchodilators. Epinephrine should also not be used except potentially as a rescue agent in severe disease.
Nebulized hypertonic saline appears to increase mucociliary clearance. Nebulized 3% saline is safe and effective in improving symptoms of mild to moderate bronchiolitis when measured after 24 hours of use, and it possibly decreases the length of hospital stay in studies where the length of stay exceeded 3 days. The guidelines conclude that hypertonic saline may be helpful to infants who are hospitalized with bronchiolitis, but probably is of very little benefit when administered in an emergency department setting.
Although there is strong evidence of benefit of systemic corticosteroids in asthma and croup, there is no evidence that systemic corticosteroids provide benefit in bronchiolitis. In addition, there is some evidence that corticosteroids may prolong viral shedding. For these reasons, the 2014 guidelines state, “Clinicians should not administer systemic corticosteroids to infants with a diagnosis of bronchiolitis in any setting (Evidence Quality: A; Recommendation Strength: Strong Recommendation).”
Physicians may choose not to give supplemental oxygen if oxyhemoglobin saturation is more than 90%, and also not to use continuous pulse oximetry given that it is prone to errors of measurement. Chest physiotherapy is not recommended. Antibiotics use is not recommended unless there is a concomitant bacterial infection or strong suspicion of one.
Prevention
The guidelines advise that palivizumab (Synagis) should not be given to otherwise healthy infants with a gestational age of 29 weeks or greater. Palivizumab should be given in the first year of life to infants with hemodynamically significant heart disease or chronic lung disease of prematurity (defined as infants of less than 32 weeks’ gestation who required more than 21% oxygen for at least the first 28 days of life). Infants who qualify for palivizumab at the start of respiratory syncytial virus season should receive a maximum of five monthly doses (15 mg/kg per dose) of palivizumab or until the end of RSV season, whichever comes first. Because of the low risk of RSV hospitalization in the second year of life, palivizumab prophylaxis is not recommended for children in the second year of life, unless the child meets the criteria for chronic lung disease and continues to require supplemental oxygen or is on chronic corticosteroids or diuretic therapy within 6 months of the onset of the second RSV season.
Reference
Ralston S.L. "Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis." Pediatrics 2014;134:e1474-502).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Rastogi is a third-year resident in the family medicine residency program at Abington Memorial Hospital.
Ralston S.L. “Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis.” Pediatrics 2014;134:e1474-502).
Bronchiolitis is the most common cause of hospitalization among infants during the first 12 months of life. Approximately 100,000 bronchiolitis admissions occur annually in children in the United States, at an estimated cost of $1.73 billion. The American Academy of Pediatrics recently published new guidelines for the diagnosis, management, and prevention of bronchiolitis in children younger than 2 years.
Diagnosis
Diagnosis is based on patient history and physical examination. The course and severity of bronchiolitis vary, ranging from mild disease with simple runny nose and cough, to transient apneic events, and on to progressive respiratory distress secondary to airway obstruction. Management of bronchiolitis must be determined in the context of increased risk factors for severe disease, including age less than 12 weeks, history of prematurity, underlying cardiopulmonary disease, and immunodeficiency. Current evidence does not support routine labs or diagnostic imaging as helping with risk assessment. Abnormalities on chest x-ray, which are common in children with bronchiolitis, do reliably predict severity of disease, so chest x-rays are only indicated when another etiology of respiratory distress such as pneumothorax or pneumonia is a concern. Routine virologic testing is not recommended, as it does not appear to aid in guiding the treatment of the child with bronchiolitis.
Management
Randomized trials have not shown any benefit from alpha- or beta-adrenergic agonist administration. Bronchodilators can lessen symptoms scores, but their use does not speed disease resolution or decrease the length of stay or need for hospitalization. A Cochrane analysis concluded that there was no benefit to giving bronchodilators to infants with bronchiolitis. Adverse effects included tachycardia, tremors, and cost, all of which outweigh potential benefits. While previous versions of the AAP guidelines recommended bronchodilators as an option, the 2014 guidelines state, “Clinicians should not administer albuterol (or salbutamol) to infants and children with a diagnosis of bronchiolitis (Evidence Quality: B; Recommendation Strength: Strong).” It is noted that there may be some children who have reversible airway obstruction, but it is impossible to tell ahead of time who they are; and due to the variability of the disease, it is even hard to tell in whom the medication is effective. It is acknowledged that children with severe disease were usually excluded from the studies of bronchodilators. Epinephrine should also not be used except potentially as a rescue agent in severe disease.
Nebulized hypertonic saline appears to increase mucociliary clearance. Nebulized 3% saline is safe and effective in improving symptoms of mild to moderate bronchiolitis when measured after 24 hours of use, and it possibly decreases the length of hospital stay in studies where the length of stay exceeded 3 days. The guidelines conclude that hypertonic saline may be helpful to infants who are hospitalized with bronchiolitis, but probably is of very little benefit when administered in an emergency department setting.
Although there is strong evidence of benefit of systemic corticosteroids in asthma and croup, there is no evidence that systemic corticosteroids provide benefit in bronchiolitis. In addition, there is some evidence that corticosteroids may prolong viral shedding. For these reasons, the 2014 guidelines state, “Clinicians should not administer systemic corticosteroids to infants with a diagnosis of bronchiolitis in any setting (Evidence Quality: A; Recommendation Strength: Strong Recommendation).”
Physicians may choose not to give supplemental oxygen if oxyhemoglobin saturation is more than 90%, and also not to use continuous pulse oximetry given that it is prone to errors of measurement. Chest physiotherapy is not recommended. Antibiotics use is not recommended unless there is a concomitant bacterial infection or strong suspicion of one.
Prevention
The guidelines advise that palivizumab (Synagis) should not be given to otherwise healthy infants with a gestational age of 29 weeks or greater. Palivizumab should be given in the first year of life to infants with hemodynamically significant heart disease or chronic lung disease of prematurity (defined as infants of less than 32 weeks’ gestation who required more than 21% oxygen for at least the first 28 days of life). Infants who qualify for palivizumab at the start of respiratory syncytial virus season should receive a maximum of five monthly doses (15 mg/kg per dose) of palivizumab or until the end of RSV season, whichever comes first. Because of the low risk of RSV hospitalization in the second year of life, palivizumab prophylaxis is not recommended for children in the second year of life, unless the child meets the criteria for chronic lung disease and continues to require supplemental oxygen or is on chronic corticosteroids or diuretic therapy within 6 months of the onset of the second RSV season.
Reference
Ralston S.L. "Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis." Pediatrics 2014;134:e1474-502).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Rastogi is a third-year resident in the family medicine residency program at Abington Memorial Hospital.
Bronchiolitis is the most common cause of hospitalization among infants during the first 12 months of life. Approximately 100,000 bronchiolitis admissions occur annually in children in the United States, at an estimated cost of $1.73 billion. The American Academy of Pediatrics recently published new guidelines for the diagnosis, management, and prevention of bronchiolitis in children younger than 2 years.
Diagnosis
Diagnosis is based on patient history and physical examination. The course and severity of bronchiolitis vary, ranging from mild disease with simple runny nose and cough, to transient apneic events, and on to progressive respiratory distress secondary to airway obstruction. Management of bronchiolitis must be determined in the context of increased risk factors for severe disease, including age less than 12 weeks, history of prematurity, underlying cardiopulmonary disease, and immunodeficiency. Current evidence does not support routine labs or diagnostic imaging as helping with risk assessment. Abnormalities on chest x-ray, which are common in children with bronchiolitis, do reliably predict severity of disease, so chest x-rays are only indicated when another etiology of respiratory distress such as pneumothorax or pneumonia is a concern. Routine virologic testing is not recommended, as it does not appear to aid in guiding the treatment of the child with bronchiolitis.
Management
Randomized trials have not shown any benefit from alpha- or beta-adrenergic agonist administration. Bronchodilators can lessen symptoms scores, but their use does not speed disease resolution or decrease the length of stay or need for hospitalization. A Cochrane analysis concluded that there was no benefit to giving bronchodilators to infants with bronchiolitis. Adverse effects included tachycardia, tremors, and cost, all of which outweigh potential benefits. While previous versions of the AAP guidelines recommended bronchodilators as an option, the 2014 guidelines state, “Clinicians should not administer albuterol (or salbutamol) to infants and children with a diagnosis of bronchiolitis (Evidence Quality: B; Recommendation Strength: Strong).” It is noted that there may be some children who have reversible airway obstruction, but it is impossible to tell ahead of time who they are; and due to the variability of the disease, it is even hard to tell in whom the medication is effective. It is acknowledged that children with severe disease were usually excluded from the studies of bronchodilators. Epinephrine should also not be used except potentially as a rescue agent in severe disease.
Nebulized hypertonic saline appears to increase mucociliary clearance. Nebulized 3% saline is safe and effective in improving symptoms of mild to moderate bronchiolitis when measured after 24 hours of use, and it possibly decreases the length of hospital stay in studies where the length of stay exceeded 3 days. The guidelines conclude that hypertonic saline may be helpful to infants who are hospitalized with bronchiolitis, but probably is of very little benefit when administered in an emergency department setting.
Although there is strong evidence of benefit of systemic corticosteroids in asthma and croup, there is no evidence that systemic corticosteroids provide benefit in bronchiolitis. In addition, there is some evidence that corticosteroids may prolong viral shedding. For these reasons, the 2014 guidelines state, “Clinicians should not administer systemic corticosteroids to infants with a diagnosis of bronchiolitis in any setting (Evidence Quality: A; Recommendation Strength: Strong Recommendation).”
Physicians may choose not to give supplemental oxygen if oxyhemoglobin saturation is more than 90%, and also not to use continuous pulse oximetry given that it is prone to errors of measurement. Chest physiotherapy is not recommended. Antibiotics use is not recommended unless there is a concomitant bacterial infection or strong suspicion of one.
Prevention
The guidelines advise that palivizumab (Synagis) should not be given to otherwise healthy infants with a gestational age of 29 weeks or greater. Palivizumab should be given in the first year of life to infants with hemodynamically significant heart disease or chronic lung disease of prematurity (defined as infants of less than 32 weeks’ gestation who required more than 21% oxygen for at least the first 28 days of life). Infants who qualify for palivizumab at the start of respiratory syncytial virus season should receive a maximum of five monthly doses (15 mg/kg per dose) of palivizumab or until the end of RSV season, whichever comes first. Because of the low risk of RSV hospitalization in the second year of life, palivizumab prophylaxis is not recommended for children in the second year of life, unless the child meets the criteria for chronic lung disease and continues to require supplemental oxygen or is on chronic corticosteroids or diuretic therapy within 6 months of the onset of the second RSV season.
Reference
Ralston S.L. "Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis." Pediatrics 2014;134:e1474-502).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Rastogi is a third-year resident in the family medicine residency program at Abington Memorial Hospital.
Ralston S.L. “Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis.” Pediatrics 2014;134:e1474-502).
Ralston S.L. “Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis.” Pediatrics 2014;134:e1474-502).
More to the Story Than a Skull Fracture
ANSWER
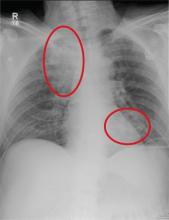
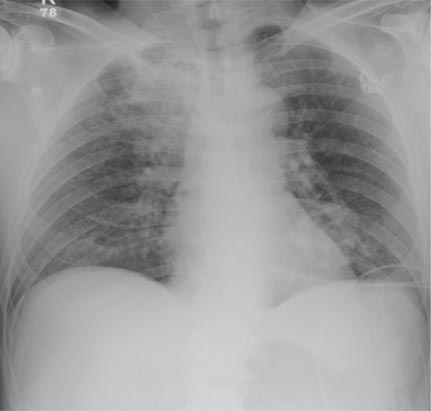
The radiograph shows two areas of concern: Within the apex of the right lung, there is a vague haziness that, in the setting of trauma, is suggestive of a contusion or even aspiration pneumonia. Another possibility is some sort of neoplasm. In addition, the patient has what appears to be a rounded density within the left lung, also suspicious for neoplasm. Additional work-up with contrast-enhanced CT is warranted.
Through further questioning, the patient denies any current symptoms or previous/recent diagnosis of cancer. CT of the chest confirmed the presence of masses in the right upper and left lower lobes. Subsequent biopsy was consistent with a moderate to poorly differentiated squamous cell carcinoma.
ANSWER
The radiograph shows two areas of concern: Within the apex of the right lung, there is a vague haziness that, in the setting of trauma, is suggestive of a contusion or even aspiration pneumonia. Another possibility is some sort of neoplasm. In addition, the patient has what appears to be a rounded density within the left lung, also suspicious for neoplasm. Additional work-up with contrast-enhanced CT is warranted.
Through further questioning, the patient denies any current symptoms or previous/recent diagnosis of cancer. CT of the chest confirmed the presence of masses in the right upper and left lower lobes. Subsequent biopsy was consistent with a moderate to poorly differentiated squamous cell carcinoma.
ANSWER
The radiograph shows two areas of concern: Within the apex of the right lung, there is a vague haziness that, in the setting of trauma, is suggestive of a contusion or even aspiration pneumonia. Another possibility is some sort of neoplasm. In addition, the patient has what appears to be a rounded density within the left lung, also suspicious for neoplasm. Additional work-up with contrast-enhanced CT is warranted.
Through further questioning, the patient denies any current symptoms or previous/recent diagnosis of cancer. CT of the chest confirmed the presence of masses in the right upper and left lower lobes. Subsequent biopsy was consistent with a moderate to poorly differentiated squamous cell carcinoma.
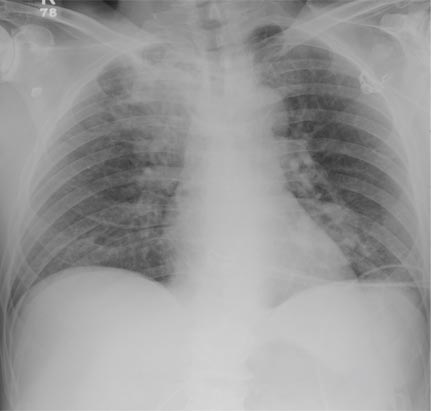
A 63-year-old man is transferred to your facility with a skull fracture secondary to a fall. He thinks he tripped and fell, hitting his head. He does not recall experiencing dizziness or syncope. He states he was momentarily dazed but does not think he lost consciousness. He is complaining of a mild headache and has reported drainage from his left ear. He denies any noteworthy medical history and takes no medications regularly. He admits to smoking one to one-and-a-half packs of cigarettes per day. Initial assessment reveals an older-appearing male who is awake, alert, oriented, and in no obvious distress. His vital signs, including O2 saturation, are normal. His pupils are equal and react briskly. He does have obvious otorrhea from his left ear. He is moving all his extremities well and appears to have no deficits. You review his imaging studies, which include a chest radiograph (shown). What is your impression?
Dr. Robert L. Barbieri’s Editor’s Picks November 2014
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s November 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the November 2014 issue here.
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s November 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the November 2014 issue here.
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s November 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the November 2014 issue here.
VIDEO: End of the road for aspirin in primary prevention?
CHICAGO – Once-daily, low-dose aspirin failed to reduce the combined outcome of cardiovascular death, nonfatal stroke, and nonfatal MI in elderly Japanese patients with atherosclerotic risk factors in the JPPP study.
The cumulative rate of the composite primary outcome was 2.77% with 100 mg/day of aspirin and 2.96% with no aspirin (HR, 0.94; P = .54), Dr. Kazuyuki Shimada reported at the American Heart Association scientific sessions.
However, patients randomized to aspirin did have reductions of 47% and 43% in the individual endpoints of nonfatal MI and transient ischemic attack, respectively.
These benefits had to be weighed against an 85% increase in serious extracranial hemorrhage in those on aspirin, according to results of the Japanese Primary Prevention Project (JPPP), simultaneously published online in JAMA (doi:10.1001/jama.2014.15690).
How generalizable are these results, and is this the end of the road for aspirin in primary prevention? We asked several experts, including invited discussant Dr. Dorairaj Prabhakaran of the Public Health Foundation of India, Dr. Karol Watson of the UCLA Center for Cholesterol and Lipid Management, and Dr. Donald Lloyd-Jonesof Northwestern University in Chicago.
The study was sponsored by the Japanese Ministry of Health, Labor, and Welfare, and the Waksman Foundation of Japan. Bayer Yakuhin provided the aspirin. Dr. Shimada reported honorarium from MSD, Shionogi, Takeda, Daiichi-Sankyo, and Dainippon-Sumitomo, and serving as a consultant/advisory board member for Omron.
Dr. Prabhakaran and Dr. Jones reported no conflicting interests. Dr. Watson reported participating in the clinical trials adjudication committee for Merck.
CHICAGO – Once-daily, low-dose aspirin failed to reduce the combined outcome of cardiovascular death, nonfatal stroke, and nonfatal MI in elderly Japanese patients with atherosclerotic risk factors in the JPPP study.
The cumulative rate of the composite primary outcome was 2.77% with 100 mg/day of aspirin and 2.96% with no aspirin (HR, 0.94; P = .54), Dr. Kazuyuki Shimada reported at the American Heart Association scientific sessions.
However, patients randomized to aspirin did have reductions of 47% and 43% in the individual endpoints of nonfatal MI and transient ischemic attack, respectively.
These benefits had to be weighed against an 85% increase in serious extracranial hemorrhage in those on aspirin, according to results of the Japanese Primary Prevention Project (JPPP), simultaneously published online in JAMA (doi:10.1001/jama.2014.15690).
How generalizable are these results, and is this the end of the road for aspirin in primary prevention? We asked several experts, including invited discussant Dr. Dorairaj Prabhakaran of the Public Health Foundation of India, Dr. Karol Watson of the UCLA Center for Cholesterol and Lipid Management, and Dr. Donald Lloyd-Jonesof Northwestern University in Chicago.
The study was sponsored by the Japanese Ministry of Health, Labor, and Welfare, and the Waksman Foundation of Japan. Bayer Yakuhin provided the aspirin. Dr. Shimada reported honorarium from MSD, Shionogi, Takeda, Daiichi-Sankyo, and Dainippon-Sumitomo, and serving as a consultant/advisory board member for Omron.
Dr. Prabhakaran and Dr. Jones reported no conflicting interests. Dr. Watson reported participating in the clinical trials adjudication committee for Merck.
CHICAGO – Once-daily, low-dose aspirin failed to reduce the combined outcome of cardiovascular death, nonfatal stroke, and nonfatal MI in elderly Japanese patients with atherosclerotic risk factors in the JPPP study.
The cumulative rate of the composite primary outcome was 2.77% with 100 mg/day of aspirin and 2.96% with no aspirin (HR, 0.94; P = .54), Dr. Kazuyuki Shimada reported at the American Heart Association scientific sessions.
However, patients randomized to aspirin did have reductions of 47% and 43% in the individual endpoints of nonfatal MI and transient ischemic attack, respectively.
These benefits had to be weighed against an 85% increase in serious extracranial hemorrhage in those on aspirin, according to results of the Japanese Primary Prevention Project (JPPP), simultaneously published online in JAMA (doi:10.1001/jama.2014.15690).
How generalizable are these results, and is this the end of the road for aspirin in primary prevention? We asked several experts, including invited discussant Dr. Dorairaj Prabhakaran of the Public Health Foundation of India, Dr. Karol Watson of the UCLA Center for Cholesterol and Lipid Management, and Dr. Donald Lloyd-Jonesof Northwestern University in Chicago.
The study was sponsored by the Japanese Ministry of Health, Labor, and Welfare, and the Waksman Foundation of Japan. Bayer Yakuhin provided the aspirin. Dr. Shimada reported honorarium from MSD, Shionogi, Takeda, Daiichi-Sankyo, and Dainippon-Sumitomo, and serving as a consultant/advisory board member for Omron.
Dr. Prabhakaran and Dr. Jones reported no conflicting interests. Dr. Watson reported participating in the clinical trials adjudication committee for Merck.
FROM THE AHA SCIENTIFIC SESSIONS
Levofloxacin didn’t prevent BK virus after kidney transplant, increased quinolone resistance
PHILADELPHIA – A 3-month course of levofloxacin after kidney transplant didn’t prevent BK virus from colonizing the urine, setting the stage for viremia in these immunosuppressed patients.
However, the antibiotic was associated with a significant 75% increase in the risk of developing a quinolone-resistant infection, compared with placebo, Dr. Greg A. Knoll and his colleagues reported in the Nov. 15 issue of JAMA (2014 [doi:10.1001/jama.2014.14721]).In a randomized trial of 154 kidney transplant patients, BK virus developed in 29% of those taking levofloxacin and in 33% of those taking placebo, a nonsignificant difference, coauthor Greg Knoll said at a late-breaking poster session during Kidney Week 2014, where the study was simultaneously presented.Levofloxacin, a quinolone antibiotic, has been shown to have some antiviral properties, especially against polyomaviruses – including BK virus, said Dr. Knoll of the University of Ottawa and the Ottawa Hospital.
Almost everyone harbors latent BK virus, Dr. Knoll said in an interview. It sometimes causes mild cold symptoms when first contracted, but often there’s no indication of illness at all. “If you’re otherwise healthy, it goes dormant and stays that way,” he noted.
But it can cause serious problems in immunocompromised patients, especially those with a kidney transplant. “BK virus tends to live in the bladder and urinary tract,” Dr. Knoll said, “So, when it reactivates, that’s the site where it does its damage.”
The virus will first appear in the urine, and then follow a logical progression through the ureters and into the kidney. If it remains unchecked, it causes very severe kidney inflammation. That inflammation “looks a lot like rejection,” Dr. Knoll said. “In fact, for years, we were very confused about this and ended up giving patients with BK viremia more immunosuppressants – when we actually should have been giving them less.”
It’s been tough to find the right balance of treatment to combat BK infections and immunosuppressants to maintain the allograft, he said.
Some retrospective studies suggested that quinolone antibiotics – including levofloxacin – could help fight cytomegalovirus infections and viral pneumonia, and decrease inflammatory markers in the urine of kidney transplant patients. “This was the little bit of evidence we needed to launch this study,” Dr. Knoll said.
The study investigators examined the time to first occurrence of BK viruria within the first year of transplant. Secondary outcomes included BK viremia, peak viral load, rejection, and patient and allograft survival.
Patients’ mean age was 48 years. They had undergone kidney transplant for a variety of reasons, including glomerulonephritis, polycystic kidney disease, diabetes, and hypertension. Comorbidities were common and included diabetes, history of cancer, cardiovascular disease, and hepatitis C and B infections. Most had received a living donor transplant (60%); the rest had kidneys from deceased donors.
Treatment began soon after transplantation. Patients were randomized to a target dose of 500 mg/day levofloxacin for 3 months. The mean length of follow-up was 46 weeks.
The primary outcome of BK viruria occurred in 46 patients – 29% of those in the levofloxacin group and 33.3% of those in the placebo group. That translated to a nonsignificant increased viruria risk of about 4%.
The time to viruria was not significantly different between the groups, with nearly 25% of each group developing it by 25 weeks. Nor was there a between-group difference in the occurrence of sustained viruria.
The secondary endpoint of BK viremia occurred in 7.9% of the levofloxacin group and 11.5% of the placebo group, also a nonsignificant finding. Infections were similar in both group, occurring in 59% of those taking levofloxacin and 45% of those taking placebo. The types of infections were similar: urinary tract/pyelonephritis (37% active vs. 38% placebo); cytomegalovirus (35% vs. 33%); pneumonia (3.5% vs. 1.7%); cellulitis (2.7% vs. 0.8%); and line infections and bacteremias, which were less than 1% in each group. No patient developed a Clostridium difficile infection.
However, patients taking levofloxacin developed significantly more quinolone-resistant infections (46.7% vs. 32.6%). Among isolates that are usually sensitive to quinolones, those patients taking the study drug were 75% more likely than were placebo patients to have a resistant strain (58.3% vs. 33.3%).
Because quinolones are an important part of infection prophylaxis in kidney transplant patients, “This would have significant implications for the management of common infections after transplantation,” Dr. Knoll said. “Our results don’t support the use of levofloxacin for preventing infections in patients with kidney transplants.”
The researchers were disappointed in the outcomes, “but there were people doing this already, and now we have the evidence to tell them to stop,” Dr. Knoll explained. “Of course, we are back to square one, with no proven treatment.”
He added that the quinolones remain critical antibiotics for kidney transplant patients – and that the study in no way suggests that should change.
“We were using these daily for 3 months, and that’s where we got into the resistance trouble,” he said. “That’s not anything like a 7- to 10-day course for a urinary tract infection.”
Dr. Knoll reported receiving investigator-initiated research grants from Astellas Canada and Pfizer Canada. The other coauthors had a number of financial relationships with pharmaceutical companies.
On Twitter @alz_gal
PHILADELPHIA – A 3-month course of levofloxacin after kidney transplant didn’t prevent BK virus from colonizing the urine, setting the stage for viremia in these immunosuppressed patients.
However, the antibiotic was associated with a significant 75% increase in the risk of developing a quinolone-resistant infection, compared with placebo, Dr. Greg A. Knoll and his colleagues reported in the Nov. 15 issue of JAMA (2014 [doi:10.1001/jama.2014.14721]).In a randomized trial of 154 kidney transplant patients, BK virus developed in 29% of those taking levofloxacin and in 33% of those taking placebo, a nonsignificant difference, coauthor Greg Knoll said at a late-breaking poster session during Kidney Week 2014, where the study was simultaneously presented.Levofloxacin, a quinolone antibiotic, has been shown to have some antiviral properties, especially against polyomaviruses – including BK virus, said Dr. Knoll of the University of Ottawa and the Ottawa Hospital.
Almost everyone harbors latent BK virus, Dr. Knoll said in an interview. It sometimes causes mild cold symptoms when first contracted, but often there’s no indication of illness at all. “If you’re otherwise healthy, it goes dormant and stays that way,” he noted.
But it can cause serious problems in immunocompromised patients, especially those with a kidney transplant. “BK virus tends to live in the bladder and urinary tract,” Dr. Knoll said, “So, when it reactivates, that’s the site where it does its damage.”
The virus will first appear in the urine, and then follow a logical progression through the ureters and into the kidney. If it remains unchecked, it causes very severe kidney inflammation. That inflammation “looks a lot like rejection,” Dr. Knoll said. “In fact, for years, we were very confused about this and ended up giving patients with BK viremia more immunosuppressants – when we actually should have been giving them less.”
It’s been tough to find the right balance of treatment to combat BK infections and immunosuppressants to maintain the allograft, he said.
Some retrospective studies suggested that quinolone antibiotics – including levofloxacin – could help fight cytomegalovirus infections and viral pneumonia, and decrease inflammatory markers in the urine of kidney transplant patients. “This was the little bit of evidence we needed to launch this study,” Dr. Knoll said.
The study investigators examined the time to first occurrence of BK viruria within the first year of transplant. Secondary outcomes included BK viremia, peak viral load, rejection, and patient and allograft survival.
Patients’ mean age was 48 years. They had undergone kidney transplant for a variety of reasons, including glomerulonephritis, polycystic kidney disease, diabetes, and hypertension. Comorbidities were common and included diabetes, history of cancer, cardiovascular disease, and hepatitis C and B infections. Most had received a living donor transplant (60%); the rest had kidneys from deceased donors.
Treatment began soon after transplantation. Patients were randomized to a target dose of 500 mg/day levofloxacin for 3 months. The mean length of follow-up was 46 weeks.
The primary outcome of BK viruria occurred in 46 patients – 29% of those in the levofloxacin group and 33.3% of those in the placebo group. That translated to a nonsignificant increased viruria risk of about 4%.
The time to viruria was not significantly different between the groups, with nearly 25% of each group developing it by 25 weeks. Nor was there a between-group difference in the occurrence of sustained viruria.
The secondary endpoint of BK viremia occurred in 7.9% of the levofloxacin group and 11.5% of the placebo group, also a nonsignificant finding. Infections were similar in both group, occurring in 59% of those taking levofloxacin and 45% of those taking placebo. The types of infections were similar: urinary tract/pyelonephritis (37% active vs. 38% placebo); cytomegalovirus (35% vs. 33%); pneumonia (3.5% vs. 1.7%); cellulitis (2.7% vs. 0.8%); and line infections and bacteremias, which were less than 1% in each group. No patient developed a Clostridium difficile infection.
However, patients taking levofloxacin developed significantly more quinolone-resistant infections (46.7% vs. 32.6%). Among isolates that are usually sensitive to quinolones, those patients taking the study drug were 75% more likely than were placebo patients to have a resistant strain (58.3% vs. 33.3%).
Because quinolones are an important part of infection prophylaxis in kidney transplant patients, “This would have significant implications for the management of common infections after transplantation,” Dr. Knoll said. “Our results don’t support the use of levofloxacin for preventing infections in patients with kidney transplants.”
The researchers were disappointed in the outcomes, “but there were people doing this already, and now we have the evidence to tell them to stop,” Dr. Knoll explained. “Of course, we are back to square one, with no proven treatment.”
He added that the quinolones remain critical antibiotics for kidney transplant patients – and that the study in no way suggests that should change.
“We were using these daily for 3 months, and that’s where we got into the resistance trouble,” he said. “That’s not anything like a 7- to 10-day course for a urinary tract infection.”
Dr. Knoll reported receiving investigator-initiated research grants from Astellas Canada and Pfizer Canada. The other coauthors had a number of financial relationships with pharmaceutical companies.
On Twitter @alz_gal
PHILADELPHIA – A 3-month course of levofloxacin after kidney transplant didn’t prevent BK virus from colonizing the urine, setting the stage for viremia in these immunosuppressed patients.
However, the antibiotic was associated with a significant 75% increase in the risk of developing a quinolone-resistant infection, compared with placebo, Dr. Greg A. Knoll and his colleagues reported in the Nov. 15 issue of JAMA (2014 [doi:10.1001/jama.2014.14721]).In a randomized trial of 154 kidney transplant patients, BK virus developed in 29% of those taking levofloxacin and in 33% of those taking placebo, a nonsignificant difference, coauthor Greg Knoll said at a late-breaking poster session during Kidney Week 2014, where the study was simultaneously presented.Levofloxacin, a quinolone antibiotic, has been shown to have some antiviral properties, especially against polyomaviruses – including BK virus, said Dr. Knoll of the University of Ottawa and the Ottawa Hospital.
Almost everyone harbors latent BK virus, Dr. Knoll said in an interview. It sometimes causes mild cold symptoms when first contracted, but often there’s no indication of illness at all. “If you’re otherwise healthy, it goes dormant and stays that way,” he noted.
But it can cause serious problems in immunocompromised patients, especially those with a kidney transplant. “BK virus tends to live in the bladder and urinary tract,” Dr. Knoll said, “So, when it reactivates, that’s the site where it does its damage.”
The virus will first appear in the urine, and then follow a logical progression through the ureters and into the kidney. If it remains unchecked, it causes very severe kidney inflammation. That inflammation “looks a lot like rejection,” Dr. Knoll said. “In fact, for years, we were very confused about this and ended up giving patients with BK viremia more immunosuppressants – when we actually should have been giving them less.”
It’s been tough to find the right balance of treatment to combat BK infections and immunosuppressants to maintain the allograft, he said.
Some retrospective studies suggested that quinolone antibiotics – including levofloxacin – could help fight cytomegalovirus infections and viral pneumonia, and decrease inflammatory markers in the urine of kidney transplant patients. “This was the little bit of evidence we needed to launch this study,” Dr. Knoll said.
The study investigators examined the time to first occurrence of BK viruria within the first year of transplant. Secondary outcomes included BK viremia, peak viral load, rejection, and patient and allograft survival.
Patients’ mean age was 48 years. They had undergone kidney transplant for a variety of reasons, including glomerulonephritis, polycystic kidney disease, diabetes, and hypertension. Comorbidities were common and included diabetes, history of cancer, cardiovascular disease, and hepatitis C and B infections. Most had received a living donor transplant (60%); the rest had kidneys from deceased donors.
Treatment began soon after transplantation. Patients were randomized to a target dose of 500 mg/day levofloxacin for 3 months. The mean length of follow-up was 46 weeks.
The primary outcome of BK viruria occurred in 46 patients – 29% of those in the levofloxacin group and 33.3% of those in the placebo group. That translated to a nonsignificant increased viruria risk of about 4%.
The time to viruria was not significantly different between the groups, with nearly 25% of each group developing it by 25 weeks. Nor was there a between-group difference in the occurrence of sustained viruria.
The secondary endpoint of BK viremia occurred in 7.9% of the levofloxacin group and 11.5% of the placebo group, also a nonsignificant finding. Infections were similar in both group, occurring in 59% of those taking levofloxacin and 45% of those taking placebo. The types of infections were similar: urinary tract/pyelonephritis (37% active vs. 38% placebo); cytomegalovirus (35% vs. 33%); pneumonia (3.5% vs. 1.7%); cellulitis (2.7% vs. 0.8%); and line infections and bacteremias, which were less than 1% in each group. No patient developed a Clostridium difficile infection.
However, patients taking levofloxacin developed significantly more quinolone-resistant infections (46.7% vs. 32.6%). Among isolates that are usually sensitive to quinolones, those patients taking the study drug were 75% more likely than were placebo patients to have a resistant strain (58.3% vs. 33.3%).
Because quinolones are an important part of infection prophylaxis in kidney transplant patients, “This would have significant implications for the management of common infections after transplantation,” Dr. Knoll said. “Our results don’t support the use of levofloxacin for preventing infections in patients with kidney transplants.”
The researchers were disappointed in the outcomes, “but there were people doing this already, and now we have the evidence to tell them to stop,” Dr. Knoll explained. “Of course, we are back to square one, with no proven treatment.”
He added that the quinolones remain critical antibiotics for kidney transplant patients – and that the study in no way suggests that should change.
“We were using these daily for 3 months, and that’s where we got into the resistance trouble,” he said. “That’s not anything like a 7- to 10-day course for a urinary tract infection.”
Dr. Knoll reported receiving investigator-initiated research grants from Astellas Canada and Pfizer Canada. The other coauthors had a number of financial relationships with pharmaceutical companies.
On Twitter @alz_gal
FROM KIDNEY WEEK 2014
Key clinical point: Levofloxacin prophylaxis isn’t recommended after kidney transplant, because it failed to prevent BK viruria and was associated with an increase in quinolone-resistant bacterial isolates.
Major finding: Levofloxacin 500 mg daily for 3 months did not reduce the risk of BK viruria in patients who had a kidney transplant.
Data source: The randomized trial comprised 154 patients.
Disclosures: Dr. Knoll reported receiving investigator-initiated research grants from Astellas Canada and Pfizer Canada. The other coauthors had a number of financial relationships with pharmaceutical companies
Guideline: Bi-level positive airway pressure (BPAP) devices
Recommendations
- Appropriate for patients with obstructive sleep apnea (OSA) who have failed continuous positive airway pressure (CPAP)/auto-titrating positive airway pressure (APAP) or require supplemental ventilatory support due to a hypoventilation syndrome
BPAP (with back-up rate feature)
- Appropriate for patients with established central sleep apnea (CSA) diagnosed by an in-lab sleep study demonstrating all of the following:
- OSA has been excluded or treated
- Oxygen saturation level is 88% or less for at least five (5) continuous minutes while the patient breathes his/her usual fraction of inspired oxygen (FiO2) OR the patient demonstrates Cheyne-Stokes respiration for five (5) continuous minutes with oxygen saturation falling to 88% or less at least once during that 5 minute interval
- A titration study (split-night or whole-night) has demonstrated significant improvement of sleep-related hypoventilation adjusted to the settings that will be prescribed for home use (while breathing the individual's usual FiO2)
BPAP (with or without back-up rate feature)
- Appropriate in the management of patients with severe chronic obstructive pulmonary disease (COPD) demonstrating either of the following:
- Partial pressure of arterial carbon dioxide (PaCO2) measured by arterial blood gas drawn while the patient is awake and breathing his/her usual FiO2 is 45 mmHg or greater; OR
- Sleep oximetry demonstrates oxygen saturation of 88% or less for at least five continuous minutes while the patient breathes oxygen at 2 L per minute or his/her usual FiO2 (whichever is higher)
BPAP (with or without back-up rate feature)
- Appropriate in the management of patients with certain restrictive thoracic disorders when both a and b below are true:
- The patient has an established diagnosis of a progressive neuromuscular disease (e.g., amyotrophic lateral sclerosis [ALS]) OR a severe thoracic cage abnormality; AND
- One of the following statements is true:
- PaCO2 measured by arterial blood gas drawn while the patient is awake and breathing his/her usual FiO2 is 45 mmHg or greater.
- Sleep oximetry demonstrates oxygen saturation of 88% or less for at least five continuous minutes while the patient breathes his/her usual FiO2
- Maximal inspiratory pressure is less than 60 cm H2O or forced vital capacity is less than 50% of predicted (applies to patients with progressive neuromuscular disease only)
Ongoing Treatment with BPAP
Ongoing treatment is indicated for patients who demonstrate compliance with therapy. Demonstration of compliance is required every 90 days for the first year of treatment and annually thereafter. Compliance is defined as:
- Use of the BPAP device for greater than or equal to four (4) hours per night on 70% of nights during a consecutive thirty (30) day period within the preceding 90 days; OR
- There is clinical evidence submitted by the treating provider that demonstrates continued clinical benefit from use of the positive airway pressure device.
Recommendations
- Appropriate for patients with obstructive sleep apnea (OSA) who have failed continuous positive airway pressure (CPAP)/auto-titrating positive airway pressure (APAP) or require supplemental ventilatory support due to a hypoventilation syndrome
BPAP (with back-up rate feature)
- Appropriate for patients with established central sleep apnea (CSA) diagnosed by an in-lab sleep study demonstrating all of the following:
- OSA has been excluded or treated
- Oxygen saturation level is 88% or less for at least five (5) continuous minutes while the patient breathes his/her usual fraction of inspired oxygen (FiO2) OR the patient demonstrates Cheyne-Stokes respiration for five (5) continuous minutes with oxygen saturation falling to 88% or less at least once during that 5 minute interval
- A titration study (split-night or whole-night) has demonstrated significant improvement of sleep-related hypoventilation adjusted to the settings that will be prescribed for home use (while breathing the individual's usual FiO2)
BPAP (with or without back-up rate feature)
- Appropriate in the management of patients with severe chronic obstructive pulmonary disease (COPD) demonstrating either of the following:
- Partial pressure of arterial carbon dioxide (PaCO2) measured by arterial blood gas drawn while the patient is awake and breathing his/her usual FiO2 is 45 mmHg or greater; OR
- Sleep oximetry demonstrates oxygen saturation of 88% or less for at least five continuous minutes while the patient breathes oxygen at 2 L per minute or his/her usual FiO2 (whichever is higher)
BPAP (with or without back-up rate feature)
- Appropriate in the management of patients with certain restrictive thoracic disorders when both a and b below are true:
- The patient has an established diagnosis of a progressive neuromuscular disease (e.g., amyotrophic lateral sclerosis [ALS]) OR a severe thoracic cage abnormality; AND
- One of the following statements is true:
- PaCO2 measured by arterial blood gas drawn while the patient is awake and breathing his/her usual FiO2 is 45 mmHg or greater.
- Sleep oximetry demonstrates oxygen saturation of 88% or less for at least five continuous minutes while the patient breathes his/her usual FiO2
- Maximal inspiratory pressure is less than 60 cm H2O or forced vital capacity is less than 50% of predicted (applies to patients with progressive neuromuscular disease only)
Ongoing Treatment with BPAP
Ongoing treatment is indicated for patients who demonstrate compliance with therapy. Demonstration of compliance is required every 90 days for the first year of treatment and annually thereafter. Compliance is defined as:
- Use of the BPAP device for greater than or equal to four (4) hours per night on 70% of nights during a consecutive thirty (30) day period within the preceding 90 days; OR
- There is clinical evidence submitted by the treating provider that demonstrates continued clinical benefit from use of the positive airway pressure device.
Recommendations
- Appropriate for patients with obstructive sleep apnea (OSA) who have failed continuous positive airway pressure (CPAP)/auto-titrating positive airway pressure (APAP) or require supplemental ventilatory support due to a hypoventilation syndrome
BPAP (with back-up rate feature)
- Appropriate for patients with established central sleep apnea (CSA) diagnosed by an in-lab sleep study demonstrating all of the following:
- OSA has been excluded or treated
- Oxygen saturation level is 88% or less for at least five (5) continuous minutes while the patient breathes his/her usual fraction of inspired oxygen (FiO2) OR the patient demonstrates Cheyne-Stokes respiration for five (5) continuous minutes with oxygen saturation falling to 88% or less at least once during that 5 minute interval
- A titration study (split-night or whole-night) has demonstrated significant improvement of sleep-related hypoventilation adjusted to the settings that will be prescribed for home use (while breathing the individual's usual FiO2)
BPAP (with or without back-up rate feature)
- Appropriate in the management of patients with severe chronic obstructive pulmonary disease (COPD) demonstrating either of the following:
- Partial pressure of arterial carbon dioxide (PaCO2) measured by arterial blood gas drawn while the patient is awake and breathing his/her usual FiO2 is 45 mmHg or greater; OR
- Sleep oximetry demonstrates oxygen saturation of 88% or less for at least five continuous minutes while the patient breathes oxygen at 2 L per minute or his/her usual FiO2 (whichever is higher)
BPAP (with or without back-up rate feature)
- Appropriate in the management of patients with certain restrictive thoracic disorders when both a and b below are true:
- The patient has an established diagnosis of a progressive neuromuscular disease (e.g., amyotrophic lateral sclerosis [ALS]) OR a severe thoracic cage abnormality; AND
- One of the following statements is true:
- PaCO2 measured by arterial blood gas drawn while the patient is awake and breathing his/her usual FiO2 is 45 mmHg or greater.
- Sleep oximetry demonstrates oxygen saturation of 88% or less for at least five continuous minutes while the patient breathes his/her usual FiO2
- Maximal inspiratory pressure is less than 60 cm H2O or forced vital capacity is less than 50% of predicted (applies to patients with progressive neuromuscular disease only)
Ongoing Treatment with BPAP
Ongoing treatment is indicated for patients who demonstrate compliance with therapy. Demonstration of compliance is required every 90 days for the first year of treatment and annually thereafter. Compliance is defined as:
- Use of the BPAP device for greater than or equal to four (4) hours per night on 70% of nights during a consecutive thirty (30) day period within the preceding 90 days; OR
- There is clinical evidence submitted by the treating provider that demonstrates continued clinical benefit from use of the positive airway pressure device.
Agent can reverse anticoagulant effect of apixaban

Credit: Andre E.X. Brown
CHICAGO—An experimental agent known as andexanet alfa can reverse the anticoagulant effect of apixaban in healthy volunteers, a phase 3 study suggests.
All subjects who received andexanet alfa after treatment with apixaban exhibited at least a 90% reversal of anti‐factor Xa activity, and thrombin generation was restored to baseline levels.
None of the subjects experienced serious adverse events or thrombotic events, and none developed antibodies to factor X or Xa.
Mark Crowther, MD, of McMaster University in Hamilton, Ontario, Canada, presented these results at the American Heart Association 2014 Scientific Sessions. Dr Crowther is a consultant for Portola Pharmaceuticals, the makers of andexanet alfa.
Andexanet alfa is a recombinant, modified factor Xa molecule. It is being developed as an antidote for patients receiving a factor Xa inhibitor who suffer a major bleeding episode or who may require emergency surgery.
In the phase 3 ANNEXA-A study, researchers evaluated the safety and efficacy of andexanet alfa in reversing apixaban-induced anticoagulation in healthy volunteers.
Dr Crowther presented results from the first part of the study, which included 33 subjects ages 50 to 73. The subjects received apixaban at 5 mg twice daily for 4 days. Then, they were randomized in a 3:1 ratio to receive andexanet alfa as a 400 mg intravenous bolus (n=24) or placebo (n=9).
Two to 5 minutes after subjects received andexanet alfa, the anticoagulant activity of apixaban was reversed by approximately 94% compared to placebo (P<0.0001). And the effect lasted 1 to 2 hours.
Every subject treated with andexanet alfa had between 90% and 96% reversal of the anticoagulant activity of apixaban. The reversal of anti-factor Xa activity was correlated with a significant reduction in the level of free, unbound apixaban in the plasma (P<0.0001).
In addition, andexanet alfa restored thrombin generation to baseline levels. And there was no rebound effect on thrombin generation after andexanet and/or apixaban were cleared.
Three subjects experienced mild infusion reactions after receiving andexanet alfa. But there were no serious adverse events, thrombotic events, or antibodies to factor X or Xa reported.
Now, researchers are going ahead with the second part of the ANNEXA-A study, hoping to demonstrate
that prolonged reversal can be sustained with a continuous infusion of andexanet alfa after the bolus dose.
Thirty-two healthy volunteers will receive apixaban at 5 mg twice daily for 4 days and then be randomized to placebo or andexanet alfa administered as a 400 mg intravenous bolus followed by a continuous infusion of 4 mg/min for 120 minutes. Data from this part of the study are expected to be available in early 2015. ![]()

Credit: Andre E.X. Brown
CHICAGO—An experimental agent known as andexanet alfa can reverse the anticoagulant effect of apixaban in healthy volunteers, a phase 3 study suggests.
All subjects who received andexanet alfa after treatment with apixaban exhibited at least a 90% reversal of anti‐factor Xa activity, and thrombin generation was restored to baseline levels.
None of the subjects experienced serious adverse events or thrombotic events, and none developed antibodies to factor X or Xa.
Mark Crowther, MD, of McMaster University in Hamilton, Ontario, Canada, presented these results at the American Heart Association 2014 Scientific Sessions. Dr Crowther is a consultant for Portola Pharmaceuticals, the makers of andexanet alfa.
Andexanet alfa is a recombinant, modified factor Xa molecule. It is being developed as an antidote for patients receiving a factor Xa inhibitor who suffer a major bleeding episode or who may require emergency surgery.
In the phase 3 ANNEXA-A study, researchers evaluated the safety and efficacy of andexanet alfa in reversing apixaban-induced anticoagulation in healthy volunteers.
Dr Crowther presented results from the first part of the study, which included 33 subjects ages 50 to 73. The subjects received apixaban at 5 mg twice daily for 4 days. Then, they were randomized in a 3:1 ratio to receive andexanet alfa as a 400 mg intravenous bolus (n=24) or placebo (n=9).
Two to 5 minutes after subjects received andexanet alfa, the anticoagulant activity of apixaban was reversed by approximately 94% compared to placebo (P<0.0001). And the effect lasted 1 to 2 hours.
Every subject treated with andexanet alfa had between 90% and 96% reversal of the anticoagulant activity of apixaban. The reversal of anti-factor Xa activity was correlated with a significant reduction in the level of free, unbound apixaban in the plasma (P<0.0001).
In addition, andexanet alfa restored thrombin generation to baseline levels. And there was no rebound effect on thrombin generation after andexanet and/or apixaban were cleared.
Three subjects experienced mild infusion reactions after receiving andexanet alfa. But there were no serious adverse events, thrombotic events, or antibodies to factor X or Xa reported.
Now, researchers are going ahead with the second part of the ANNEXA-A study, hoping to demonstrate
that prolonged reversal can be sustained with a continuous infusion of andexanet alfa after the bolus dose.
Thirty-two healthy volunteers will receive apixaban at 5 mg twice daily for 4 days and then be randomized to placebo or andexanet alfa administered as a 400 mg intravenous bolus followed by a continuous infusion of 4 mg/min for 120 minutes. Data from this part of the study are expected to be available in early 2015. ![]()

Credit: Andre E.X. Brown
CHICAGO—An experimental agent known as andexanet alfa can reverse the anticoagulant effect of apixaban in healthy volunteers, a phase 3 study suggests.
All subjects who received andexanet alfa after treatment with apixaban exhibited at least a 90% reversal of anti‐factor Xa activity, and thrombin generation was restored to baseline levels.
None of the subjects experienced serious adverse events or thrombotic events, and none developed antibodies to factor X or Xa.
Mark Crowther, MD, of McMaster University in Hamilton, Ontario, Canada, presented these results at the American Heart Association 2014 Scientific Sessions. Dr Crowther is a consultant for Portola Pharmaceuticals, the makers of andexanet alfa.
Andexanet alfa is a recombinant, modified factor Xa molecule. It is being developed as an antidote for patients receiving a factor Xa inhibitor who suffer a major bleeding episode or who may require emergency surgery.
In the phase 3 ANNEXA-A study, researchers evaluated the safety and efficacy of andexanet alfa in reversing apixaban-induced anticoagulation in healthy volunteers.
Dr Crowther presented results from the first part of the study, which included 33 subjects ages 50 to 73. The subjects received apixaban at 5 mg twice daily for 4 days. Then, they were randomized in a 3:1 ratio to receive andexanet alfa as a 400 mg intravenous bolus (n=24) or placebo (n=9).
Two to 5 minutes after subjects received andexanet alfa, the anticoagulant activity of apixaban was reversed by approximately 94% compared to placebo (P<0.0001). And the effect lasted 1 to 2 hours.
Every subject treated with andexanet alfa had between 90% and 96% reversal of the anticoagulant activity of apixaban. The reversal of anti-factor Xa activity was correlated with a significant reduction in the level of free, unbound apixaban in the plasma (P<0.0001).
In addition, andexanet alfa restored thrombin generation to baseline levels. And there was no rebound effect on thrombin generation after andexanet and/or apixaban were cleared.
Three subjects experienced mild infusion reactions after receiving andexanet alfa. But there were no serious adverse events, thrombotic events, or antibodies to factor X or Xa reported.
Now, researchers are going ahead with the second part of the ANNEXA-A study, hoping to demonstrate
that prolonged reversal can be sustained with a continuous infusion of andexanet alfa after the bolus dose.
Thirty-two healthy volunteers will receive apixaban at 5 mg twice daily for 4 days and then be randomized to placebo or andexanet alfa administered as a 400 mg intravenous bolus followed by a continuous infusion of 4 mg/min for 120 minutes. Data from this part of the study are expected to be available in early 2015. ![]()
Group silences microRNA to treat DLBCL

Scientists believe they’ve devised a way to use antimiRs as anticancer drugs by showing that a specific antimiR could treat diffuse large B-cell lymphoma (DLBCL) in mice.
In a letter to Nature, the group explained that microRNAs known as oncomiRs can play a causal role in the onset and maintenance of cancer when they are overexpressed.
So inhibiting oncomiRs using antisense oligomers, or antimiRs, has seemed a promising therapeutic strategy. But physiological and cellular barriers have prevented targeted delivery.
Frank Slack, PhD, of Beth Israel Deaconess Medical Center in Boston, Massachusetts, and his colleagues have devised a new antimiR delivery platform and shown that it can inhibit DLBCL growth in vivo.
The team created a mouse model to study miR-155, an oncomiR that, when overexpressed, leads to DLBCL.
“We hypothesized that we could inhibit the function of miR-155 by way of an antisense molecule that would bind to miR-155,” Dr Slack said. “[However,] there are a number of significant obstacles to reaching the tumor cell target. Some roadblocks are clearance through the kidneys and accumulation in the liver, which absorbs any systemically injected agent.”
“Furthermore, even if you are able to reach your targeted cells, the molecules must cross cell membranes and escape degradation from a process known as endocytosis. If you can picture our antisense molecule being a warhead, we had to find the right ‘rocket’ to actually transport it to its target.”
The “rocket” turned out to be a peptide with a low-pH induced transmembrane structure (pHLIP), meaning it inserts into cell membranes only when cells are low in pH. And tumor cells provided the ideal environment.
“When we attached our antisense warhead to the pHLIP peptide, not only did it successfully insert itself into the tumor cell, but it also dragged the antisense molecule itself into the cell,” Dr Slack said. “Now the ‘warhead’ could deploy and actually inhibit microRNA function and control cancer growth.”
In the miR-155/DLBCL mouse models, pHLIP-anti155 slowed tumor growth, suppressed the metastatic spread of neoplastic lymphocytes to other organs, reduced the onset of splenomegaly, and delayed the development of conspicuous lymphadenopathy, when compared to control mice.
Responses with pHLIP-anti155 were similar to those observed in mice that received doxorubicin or CHOP, but pHLIP-anti155 proved less toxic than the other treatments.
“With this delivery platform, we should also be able to transform other RNAs into druggable targets,” Dr Slack said, adding that low pH is also an issue in kidney disease, myocardial infarction, stroke, and infection, among other conditions. So this type of therapy could have wide applications. ![]()

Scientists believe they’ve devised a way to use antimiRs as anticancer drugs by showing that a specific antimiR could treat diffuse large B-cell lymphoma (DLBCL) in mice.
In a letter to Nature, the group explained that microRNAs known as oncomiRs can play a causal role in the onset and maintenance of cancer when they are overexpressed.
So inhibiting oncomiRs using antisense oligomers, or antimiRs, has seemed a promising therapeutic strategy. But physiological and cellular barriers have prevented targeted delivery.
Frank Slack, PhD, of Beth Israel Deaconess Medical Center in Boston, Massachusetts, and his colleagues have devised a new antimiR delivery platform and shown that it can inhibit DLBCL growth in vivo.
The team created a mouse model to study miR-155, an oncomiR that, when overexpressed, leads to DLBCL.
“We hypothesized that we could inhibit the function of miR-155 by way of an antisense molecule that would bind to miR-155,” Dr Slack said. “[However,] there are a number of significant obstacles to reaching the tumor cell target. Some roadblocks are clearance through the kidneys and accumulation in the liver, which absorbs any systemically injected agent.”
“Furthermore, even if you are able to reach your targeted cells, the molecules must cross cell membranes and escape degradation from a process known as endocytosis. If you can picture our antisense molecule being a warhead, we had to find the right ‘rocket’ to actually transport it to its target.”
The “rocket” turned out to be a peptide with a low-pH induced transmembrane structure (pHLIP), meaning it inserts into cell membranes only when cells are low in pH. And tumor cells provided the ideal environment.
“When we attached our antisense warhead to the pHLIP peptide, not only did it successfully insert itself into the tumor cell, but it also dragged the antisense molecule itself into the cell,” Dr Slack said. “Now the ‘warhead’ could deploy and actually inhibit microRNA function and control cancer growth.”
In the miR-155/DLBCL mouse models, pHLIP-anti155 slowed tumor growth, suppressed the metastatic spread of neoplastic lymphocytes to other organs, reduced the onset of splenomegaly, and delayed the development of conspicuous lymphadenopathy, when compared to control mice.
Responses with pHLIP-anti155 were similar to those observed in mice that received doxorubicin or CHOP, but pHLIP-anti155 proved less toxic than the other treatments.
“With this delivery platform, we should also be able to transform other RNAs into druggable targets,” Dr Slack said, adding that low pH is also an issue in kidney disease, myocardial infarction, stroke, and infection, among other conditions. So this type of therapy could have wide applications. ![]()

Scientists believe they’ve devised a way to use antimiRs as anticancer drugs by showing that a specific antimiR could treat diffuse large B-cell lymphoma (DLBCL) in mice.
In a letter to Nature, the group explained that microRNAs known as oncomiRs can play a causal role in the onset and maintenance of cancer when they are overexpressed.
So inhibiting oncomiRs using antisense oligomers, or antimiRs, has seemed a promising therapeutic strategy. But physiological and cellular barriers have prevented targeted delivery.
Frank Slack, PhD, of Beth Israel Deaconess Medical Center in Boston, Massachusetts, and his colleagues have devised a new antimiR delivery platform and shown that it can inhibit DLBCL growth in vivo.
The team created a mouse model to study miR-155, an oncomiR that, when overexpressed, leads to DLBCL.
“We hypothesized that we could inhibit the function of miR-155 by way of an antisense molecule that would bind to miR-155,” Dr Slack said. “[However,] there are a number of significant obstacles to reaching the tumor cell target. Some roadblocks are clearance through the kidneys and accumulation in the liver, which absorbs any systemically injected agent.”
“Furthermore, even if you are able to reach your targeted cells, the molecules must cross cell membranes and escape degradation from a process known as endocytosis. If you can picture our antisense molecule being a warhead, we had to find the right ‘rocket’ to actually transport it to its target.”
The “rocket” turned out to be a peptide with a low-pH induced transmembrane structure (pHLIP), meaning it inserts into cell membranes only when cells are low in pH. And tumor cells provided the ideal environment.
“When we attached our antisense warhead to the pHLIP peptide, not only did it successfully insert itself into the tumor cell, but it also dragged the antisense molecule itself into the cell,” Dr Slack said. “Now the ‘warhead’ could deploy and actually inhibit microRNA function and control cancer growth.”
In the miR-155/DLBCL mouse models, pHLIP-anti155 slowed tumor growth, suppressed the metastatic spread of neoplastic lymphocytes to other organs, reduced the onset of splenomegaly, and delayed the development of conspicuous lymphadenopathy, when compared to control mice.
Responses with pHLIP-anti155 were similar to those observed in mice that received doxorubicin or CHOP, but pHLIP-anti155 proved less toxic than the other treatments.
“With this delivery platform, we should also be able to transform other RNAs into druggable targets,” Dr Slack said, adding that low pH is also an issue in kidney disease, myocardial infarction, stroke, and infection, among other conditions. So this type of therapy could have wide applications. ![]()
Device could streamline preparation of frozen RBCs

Engineers have devised a method that could allow for faster preparation of frozen red blood cells (RBCs), according to research published in Biomicrofluidics.
It’s already possible to cryopreserve RBCs in the presence of 40% glycerol, but the post-thaw washing process can take an hour or more.
Initial experiments and computer modeling suggest this process can be streamlined to take as little as 3 minutes, using a membrane-based microfluidic device.
This could make it more feasible to use frozen blood in emergency or time-constrained medical situations.
“Only a small fraction of our blood supply is now frozen because it’s often impractical to wait so long when a transfusion is needed immediately,” said study author Adam Higgins, PhD, of Oregon State University in Corvallis.
“Because of that, our entire system depends on constantly balancing the use and supply of blood products that can only last 6 weeks or less with refrigeration. This is difficult and can lead to loss of outdated blood, periodic shortages, and other inefficiencies that could be solved with the use of frozen blood.”
With this in mind, the researchers explored the potential for rapid glycerol extraction using a membrane-based microfluidic device.
They theorized that extremely tiny microchannel plates and membranes could be used to precisely control the removal of glycerol at a time scale of seconds, without causing excessive osmotic damage.
The team developed a mass transfer model that allowed them to predict glycerol removal, as well as the resulting cell volume changes.
Results of the researchers’ experiments were in line with the predictions and suggest it is possible to reduce the glycerol concentration by more than 50% without excessive hemolysis.
Now, the team hopes to create working prototypes of the needed technology for further development and testing of this concept. They believe an optimized process may be even faster than the 3 minutes now being predicted. ![]()

Engineers have devised a method that could allow for faster preparation of frozen red blood cells (RBCs), according to research published in Biomicrofluidics.
It’s already possible to cryopreserve RBCs in the presence of 40% glycerol, but the post-thaw washing process can take an hour or more.
Initial experiments and computer modeling suggest this process can be streamlined to take as little as 3 minutes, using a membrane-based microfluidic device.
This could make it more feasible to use frozen blood in emergency or time-constrained medical situations.
“Only a small fraction of our blood supply is now frozen because it’s often impractical to wait so long when a transfusion is needed immediately,” said study author Adam Higgins, PhD, of Oregon State University in Corvallis.
“Because of that, our entire system depends on constantly balancing the use and supply of blood products that can only last 6 weeks or less with refrigeration. This is difficult and can lead to loss of outdated blood, periodic shortages, and other inefficiencies that could be solved with the use of frozen blood.”
With this in mind, the researchers explored the potential for rapid glycerol extraction using a membrane-based microfluidic device.
They theorized that extremely tiny microchannel plates and membranes could be used to precisely control the removal of glycerol at a time scale of seconds, without causing excessive osmotic damage.
The team developed a mass transfer model that allowed them to predict glycerol removal, as well as the resulting cell volume changes.
Results of the researchers’ experiments were in line with the predictions and suggest it is possible to reduce the glycerol concentration by more than 50% without excessive hemolysis.
Now, the team hopes to create working prototypes of the needed technology for further development and testing of this concept. They believe an optimized process may be even faster than the 3 minutes now being predicted. ![]()

Engineers have devised a method that could allow for faster preparation of frozen red blood cells (RBCs), according to research published in Biomicrofluidics.
It’s already possible to cryopreserve RBCs in the presence of 40% glycerol, but the post-thaw washing process can take an hour or more.
Initial experiments and computer modeling suggest this process can be streamlined to take as little as 3 minutes, using a membrane-based microfluidic device.
This could make it more feasible to use frozen blood in emergency or time-constrained medical situations.
“Only a small fraction of our blood supply is now frozen because it’s often impractical to wait so long when a transfusion is needed immediately,” said study author Adam Higgins, PhD, of Oregon State University in Corvallis.
“Because of that, our entire system depends on constantly balancing the use and supply of blood products that can only last 6 weeks or less with refrigeration. This is difficult and can lead to loss of outdated blood, periodic shortages, and other inefficiencies that could be solved with the use of frozen blood.”
With this in mind, the researchers explored the potential for rapid glycerol extraction using a membrane-based microfluidic device.
They theorized that extremely tiny microchannel plates and membranes could be used to precisely control the removal of glycerol at a time scale of seconds, without causing excessive osmotic damage.
The team developed a mass transfer model that allowed them to predict glycerol removal, as well as the resulting cell volume changes.
Results of the researchers’ experiments were in line with the predictions and suggest it is possible to reduce the glycerol concentration by more than 50% without excessive hemolysis.
Now, the team hopes to create working prototypes of the needed technology for further development and testing of this concept. They believe an optimized process may be even faster than the 3 minutes now being predicted. ![]()