User login
Osteoid Osteomas of the Foot and Ankle: A Study of Patients Over a 20-Year Period
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
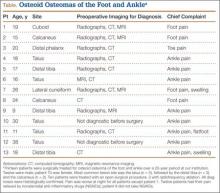
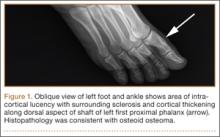
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
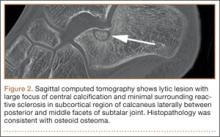
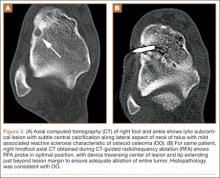
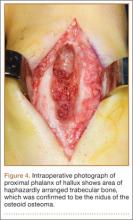
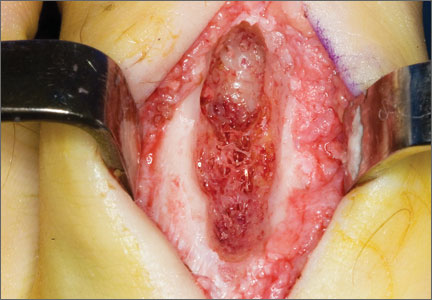
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
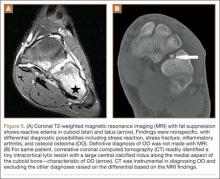
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
VIDEO: Study reignites dental antibiotic prophylaxis controversy
CHICAGO – The first guidelines recommending antibiotic prophylaxis for invasive dental procedures were issued in 1955, and controversy has gone hand in hand with each revision that has called for shorter treatment duration and fewer eligible patients.
A study presented at the American Heart Association scientific sessions adds to that controversy – and has prompted the United Kingdom’s National Institute for Health and Care Excellence to immediately review its 2008 guidelines.
Those guidelines recommend that antibiotics should not be prescribed to prevent infective endocarditis (IE) for people undergoing dental procedures or procedures in the upper and lower gastrointestinal tract, genitourinary tract, and upper and lower respiratory tract.
Five years post NICE, the new study found that antibiotic prophylaxis prescribing fell almost 90% in the United Kingdom, from 10,900 prescriptions per month to 1,307 per month in the last 6 months of the study, reported Dr. Mark Dayer of Taunton and Somerset NHS Trust, Somerset, England. The study was simultaneously published in the Lancet (2014 Nov. 18[doi:10.1016/S0140-6736(14)62007-9]).
In a video interview, study coauthor Dr. Martin Thornhill of the University of Sheffield, England, and AHA President-Elect Dr. Mark Creager, director of the vascular center at Brigham and Women’s Hospital, Boston, talked about the findings, their potential limitations, and whether it’s time for clinicians to change their approach to antibiotic prophylaxis.
The study was funded by the National Institutes of Dental and Cranofacial Research, Heart Research–UK, and Simplyhealth. Dr. Thornhill and Dr. Creager reported no conflicting interests.
CHICAGO – The first guidelines recommending antibiotic prophylaxis for invasive dental procedures were issued in 1955, and controversy has gone hand in hand with each revision that has called for shorter treatment duration and fewer eligible patients.
A study presented at the American Heart Association scientific sessions adds to that controversy – and has prompted the United Kingdom’s National Institute for Health and Care Excellence to immediately review its 2008 guidelines.
Those guidelines recommend that antibiotics should not be prescribed to prevent infective endocarditis (IE) for people undergoing dental procedures or procedures in the upper and lower gastrointestinal tract, genitourinary tract, and upper and lower respiratory tract.
Five years post NICE, the new study found that antibiotic prophylaxis prescribing fell almost 90% in the United Kingdom, from 10,900 prescriptions per month to 1,307 per month in the last 6 months of the study, reported Dr. Mark Dayer of Taunton and Somerset NHS Trust, Somerset, England. The study was simultaneously published in the Lancet (2014 Nov. 18[doi:10.1016/S0140-6736(14)62007-9]).
In a video interview, study coauthor Dr. Martin Thornhill of the University of Sheffield, England, and AHA President-Elect Dr. Mark Creager, director of the vascular center at Brigham and Women’s Hospital, Boston, talked about the findings, their potential limitations, and whether it’s time for clinicians to change their approach to antibiotic prophylaxis.
The study was funded by the National Institutes of Dental and Cranofacial Research, Heart Research–UK, and Simplyhealth. Dr. Thornhill and Dr. Creager reported no conflicting interests.
CHICAGO – The first guidelines recommending antibiotic prophylaxis for invasive dental procedures were issued in 1955, and controversy has gone hand in hand with each revision that has called for shorter treatment duration and fewer eligible patients.
A study presented at the American Heart Association scientific sessions adds to that controversy – and has prompted the United Kingdom’s National Institute for Health and Care Excellence to immediately review its 2008 guidelines.
Those guidelines recommend that antibiotics should not be prescribed to prevent infective endocarditis (IE) for people undergoing dental procedures or procedures in the upper and lower gastrointestinal tract, genitourinary tract, and upper and lower respiratory tract.
Five years post NICE, the new study found that antibiotic prophylaxis prescribing fell almost 90% in the United Kingdom, from 10,900 prescriptions per month to 1,307 per month in the last 6 months of the study, reported Dr. Mark Dayer of Taunton and Somerset NHS Trust, Somerset, England. The study was simultaneously published in the Lancet (2014 Nov. 18[doi:10.1016/S0140-6736(14)62007-9]).
In a video interview, study coauthor Dr. Martin Thornhill of the University of Sheffield, England, and AHA President-Elect Dr. Mark Creager, director of the vascular center at Brigham and Women’s Hospital, Boston, talked about the findings, their potential limitations, and whether it’s time for clinicians to change their approach to antibiotic prophylaxis.
The study was funded by the National Institutes of Dental and Cranofacial Research, Heart Research–UK, and Simplyhealth. Dr. Thornhill and Dr. Creager reported no conflicting interests.
AT THE AHA SCIENTIFIC SESSIONS
Midurethral slings
Minimally invasive synthetic midurethral slings may be considered the standard of care for the surgical treatment of stress urinary incontinence – and a first-line treatment for severe cases of the condition – based on the publication of numerous level 1 randomized trials, high-quality reviews, and recent position statements from professional societies.
The current evidence base shows that midurethral sling operations are as effective as bladder neck slings and colposuspension, with less morbidity. Operating times are shorter, and local anesthesia is possible. Compared with pubovaginal slings, which are fixed at the bladder neck, midurethral slings are associated with less postoperative voiding dysfunction and fewer de novo urgency symptoms.
Midurethral slings (MUS) also have been shown to be more successful – and more cost-effective – than pelvic floor physiotherapy for stress urinary incontinence (SUI) overall, with the possible exception of mild SUI.
Physiotherapy involving pelvic floor muscle therapy has long been advocated as a first-line treatment for SUI, with MUS surgery often recommended when physiotherapy is unsuccessful. In recent years, however, with high success rates for MUS, the role of physiotherapy as a first-line treatment has become more debatable.
A multicenter randomized trial in 660 women published last year in the New England Journal of Medicine substantiated what many of us have seen in our practices and in other published studies: significantly lower rates of improvement and cure with initial physiotherapy than with primary surgery.
Initial MUS surgery resulted in higher rates of subjective improvement, compared with initial physiotherapy (91% vs. 64%), subjective cure (85% v. 53%), and objective cure (77% v. 59%) at 1 year. Moreover, a significant number of women – 49% – chose to abandon conservative therapy and have MUS surgery for their SUI during the study period (N. Engl. J. Med. 2013;369:1124-33).
A joint position statement published in early 2014 by the American Urogynecologic Society (AUGS) and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) calls MUS the most extensively studied anti-incontinence procedure and “probably the most important advancement in the treatment of SUI in the last 50 years.” More than 2,000 publications in the literature have described the procedure for SUI, and multiple randomized controlled trials have compared various types of MUS procedures as well as MUS to other nonmesh SUI procedures, the statement says.
My colleague and I recently modeled the cost-effectiveness of pelvic floor muscle therapy and continence pessaries vs. surgical treatment with MUS for initial treatment of SUI. Initial treatment with MUS was the best strategy, with an incremental cost-effectiveness ratio of $32,132 per quality-adjusted life-year, compared with initial treatment with pelvic floor muscle therapy. Under our model, treatment with a continence pessary would never be the preferred choice due to low subjective cure rates (Am. J. Obstet. Gynecol. 2014;211:565.e1-6).
I now tell patients who present with a history of severe stress incontinence, and who leak on a cough stress test, that a trial of pelvic floor physiotherapy is an option but one with a lower likelihood of success. I recommend an MUS as primary treatment for these patients, and the question then often becomes which sling to use.
Sling selection
There are two broad approaches to MUS surgery – retropubic and transobturator – and within each approach, there are different routes for the delivery of the polypropylene mesh sling.
Retropubic slings. Retropubic slings are passed transvaginally at the midurethral level through the retropubic space. Tension-free vaginal tape (TVT) has been used in millions of women worldwide, with good long-term outcomes, since it was introduced by Dr. Ulf Ulmsten in 1995. The TVT procedure utilizes a bottom-up approach, with curved needles being passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
A second type of retropubic sling – the suprapubic urethral support sling (SPARC, American Medical Systems) – utilizes a downward-pass, or top-down, approach in which a metal trocar is passed through suprapubic incisions and down through the retropubic space to exit a vaginal incision.
The theoretical advantages of this modification to the TVT procedure have included more control over the needle introducer near the rectus fascia, and a lower risk of bowel and vascular injury. However, comparisons during the last decade of the two retropubic approaches have suggested slightly better outcomes – relating both to cure rates and to complication rates – with TVT compared with SPARC.
A Cochrane Review published in 2009, titled “Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women,” provided higher-level evidence in favor of bottom-up slings. A sub-meta-analysis of five randomized controlled trials – part of a broader intervention review – showed that a retropubic bottom-up approach was more effective than a top-down route (risk ratio, 1.10), with higher subjective and objective SUI cure rates (Cochrane Database Syst. Rev. 2009(4): CD006375). There also was significantly less bladder perforation, less mesh erosion, and less voiding dysfunction.
TVT slings, therefore, appear to be somewhat superior, with statistically significant differences in each of the domains of efficacy and morbidity. Still, surgeon experience and skill remain factors in sling selection; the surgeon who feels comfortable and skilled with a top-down approach and has little experience with a bottom-up approach should continue with SPARC. For surgeons who are skilled with both approaches, it might well be preferable to favor TVT.
Transobturator slings. The transobturator approach was developed to minimize the potential for bladder and bowel injuries by avoiding the pelvic organs in the retropubic space. The sling is introduced either through an inside-out technique, with the needle passed from a vaginal incision and out through the obturator foramen, or through an outside-in technique, with the needle passed through the thigh and then out through the vaginal incision.
A meta-analysis of trials of transobturator sling procedures – including four direct-comparison, randomized controlled trials of the inside-out technique vs. the outside-in technique – showed no significant differences between the two approaches in subjective and objective SUI cure rates in the short term. Rates of postoperative voiding difficulties and de novo urgency symptoms were similar (BJU Int. 2010;106:68-76).
Making a choice. Each of the currently available midurethral slings appears to work well, overall, with few clinically significant differences in outcomes. On the other hand, midurethral slings are not all the same. It is important to appreciate the more subtle differences, to be aware of the evidence, and to be appropriately trained. Often, sling selection involves weighing the risks and benefits for the individual.
On a broad scale, the most recent high-level comparison of the retropubic and transobturator slings appears to be a meta-analysis in which retropubic midurethral slings showed better objective and subjective cure rates than transobturator midurethral slings. Women treated with retropubic slings had a 35% higher odds of objective cure and a 24% higher odds of subjective cure. (The weighted average objective cure rates were 87% for retropubic slings vs. 83% for transobturator slings with a weighted average follow-up of approximately 17 months. The weighted average subjective cure rates were 76% and 73%, respectively.)
Operating times were longer with retropubic slings, but lengths of stay were equivalent between the two types of procedures. This was based on 17 studies of about 3,000 women (J. Urology 2014 [doi: 10.1016/j.juro.2014.09.104]).
The types of complications seen with each approach differed. Bladder perforation was significantly more common with retropubic slings (3.2% vs. 0.2%), as was bleeding (3.2% v. 1.1%). Transobturator slings were associated with more cases of neurologic symptoms (9.4% v. 3.5%) and vaginal perforation (3.6% v. 0.9%).
This new review provides updated information to the 2009 Cochrane Review mentioned above, which reported that women were less likely to be continent after operations performed via the obturator route, but also less likely to have encountered complications. More specifically, objective cure rates were slightly higher with retropubic slings than with transobturator slings (88% vs. 84%) in the 2009 review. There was no difference in subjective cure rates. With the obturator route, there was less voiding dysfunction, blood loss, and bladder perforation (0.3% v. 5.5%).
Other pivotal trials since the 2009 Cochrane Review include a multicenter randomized equivalence trial published in the New England Journal of Medicine in 2010. The trial randomized 597 women to transobturator or retropubic sling surgery, and found no significant differences in subjective success (56% vs. 62%) or in objective success (78% vs. 81%) at 12 months (N. Engl. J. Med. 2010;362:2066-76).
There is some level 1 evidence suggesting that for severe incontinence involving intrinsic sphincter deficiency (ISD), a retropubic TVT sling is the more effective procedure. A randomized trial of 164 women with urodynamic SUI and ISD, for instance, found that 21% of those in the TVT group and 45% of those in the transobturator group had urodynamic SUI 6 months postoperatively.
The risk ratio of repeat surgery was 2.6 times higher in the transobturator group than in the retropubic TVT group (Obstet. Gynecol. 2008;112:1253-61). TVT was more effective both with and without concurrent pelvic organ prolapse repair.
I tell my patients with severe SUI or ISD, therefore, that retropubic sling procedures appear to be preferable. (Exceptions include the patient who has a history of retropubic surgeries, in whom passing the sling through this route may not be the safest approach, as well as the patient who has had mesh erosion into the bladder.)
In patients whose SUI is less severe, I counsel that a transobturator sling confers satisfaction rates similar to those of a retropubic sling and has a lower risk of complications, such as postoperative voiding dysfunction and bladder perforations, but with the possible trade-off of more thigh discomfort. I also might recommend a transobturator sling to patients with more pronounced initial complaints of urinary urgency and frequency, and to patients who have minor voiding dysfunction or a low level of incomplete bladder emptying.
While often short-lived, the small risk of thigh pain with a transobturator sling makes me less likely to recommend this type of sling for a woman who is a marathon runner or competitive athlete. In her case, an analysis of possible complications includes the consideration that bladder perforation can be addressed relatively quickly in the operating room, while persistent thigh discomfort, though relatively rare, could be a debilitating problem.
Single-incision slings
There appears to be emerging evidence suggesting that some of the fixed and adjustable single-incision slings currently available may have efficacy similar to that of the slings that are now widely used.
A Cochrane Review presented at the 2014 AUGS-IUGA scientific meeting and published this summer concludes that there is not enough evidence on single-incision slings compared with retropubic or transobturator slings to allow reliable comparisons, and that additional, adequately powered, high-quality trials with longer-term follow-up are needed (Cochrane Database Sys. Rev. 2014;6:CD008709). However, research completed since the review offers additional data.
For instance, at the 2014 AUGS-IUGA scientific meeting this summer, an oral paper presentation highlighted findings of a randomized controlled trial that showed similar cure rates after surgery with the MiniArc, a fixed single-incision sling, and the Monarc transobturator sling (both by American Medical Systems) at 24 months. The study randomized 234 women to either sling and found no significant differences in subjective outcomes, objective outcomes, or results on various quality-of-life questionnaires.
As such studies are published and more evidence emerges, we will gain a clearer picture of how the newer single-incision slings compare to the well-tested retropubic and transobturator slings with respect to efficacy and safety.
Single-incision slings require only a small vaginal incision and no exit points. Without abdominal or thigh incisions, these new procedures – intended for less severe SUI (no ISD) – may offer improved perioperative and postoperative patient comfort and a potentially decreased risk of surgical injury to the adductor muscles, as well as a decreased risk of vascular and nerve injury. Candidates for these slings may include those who are very athletic, those who are obese, and those with a history of prior retropubic or pelvic surgery.
Research appears to be progressing, but at this time we do not yet have level 1 evidence to support their routine use.
Dr. Sokol reported that he owns stock in Pelvalon, and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems, and the recipient of research grants from Acell and several other companies.
Minimally invasive synthetic midurethral slings may be considered the standard of care for the surgical treatment of stress urinary incontinence – and a first-line treatment for severe cases of the condition – based on the publication of numerous level 1 randomized trials, high-quality reviews, and recent position statements from professional societies.
The current evidence base shows that midurethral sling operations are as effective as bladder neck slings and colposuspension, with less morbidity. Operating times are shorter, and local anesthesia is possible. Compared with pubovaginal slings, which are fixed at the bladder neck, midurethral slings are associated with less postoperative voiding dysfunction and fewer de novo urgency symptoms.
Midurethral slings (MUS) also have been shown to be more successful – and more cost-effective – than pelvic floor physiotherapy for stress urinary incontinence (SUI) overall, with the possible exception of mild SUI.
Physiotherapy involving pelvic floor muscle therapy has long been advocated as a first-line treatment for SUI, with MUS surgery often recommended when physiotherapy is unsuccessful. In recent years, however, with high success rates for MUS, the role of physiotherapy as a first-line treatment has become more debatable.
A multicenter randomized trial in 660 women published last year in the New England Journal of Medicine substantiated what many of us have seen in our practices and in other published studies: significantly lower rates of improvement and cure with initial physiotherapy than with primary surgery.
Initial MUS surgery resulted in higher rates of subjective improvement, compared with initial physiotherapy (91% vs. 64%), subjective cure (85% v. 53%), and objective cure (77% v. 59%) at 1 year. Moreover, a significant number of women – 49% – chose to abandon conservative therapy and have MUS surgery for their SUI during the study period (N. Engl. J. Med. 2013;369:1124-33).
A joint position statement published in early 2014 by the American Urogynecologic Society (AUGS) and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) calls MUS the most extensively studied anti-incontinence procedure and “probably the most important advancement in the treatment of SUI in the last 50 years.” More than 2,000 publications in the literature have described the procedure for SUI, and multiple randomized controlled trials have compared various types of MUS procedures as well as MUS to other nonmesh SUI procedures, the statement says.
My colleague and I recently modeled the cost-effectiveness of pelvic floor muscle therapy and continence pessaries vs. surgical treatment with MUS for initial treatment of SUI. Initial treatment with MUS was the best strategy, with an incremental cost-effectiveness ratio of $32,132 per quality-adjusted life-year, compared with initial treatment with pelvic floor muscle therapy. Under our model, treatment with a continence pessary would never be the preferred choice due to low subjective cure rates (Am. J. Obstet. Gynecol. 2014;211:565.e1-6).
I now tell patients who present with a history of severe stress incontinence, and who leak on a cough stress test, that a trial of pelvic floor physiotherapy is an option but one with a lower likelihood of success. I recommend an MUS as primary treatment for these patients, and the question then often becomes which sling to use.
Sling selection
There are two broad approaches to MUS surgery – retropubic and transobturator – and within each approach, there are different routes for the delivery of the polypropylene mesh sling.
Retropubic slings. Retropubic slings are passed transvaginally at the midurethral level through the retropubic space. Tension-free vaginal tape (TVT) has been used in millions of women worldwide, with good long-term outcomes, since it was introduced by Dr. Ulf Ulmsten in 1995. The TVT procedure utilizes a bottom-up approach, with curved needles being passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
A second type of retropubic sling – the suprapubic urethral support sling (SPARC, American Medical Systems) – utilizes a downward-pass, or top-down, approach in which a metal trocar is passed through suprapubic incisions and down through the retropubic space to exit a vaginal incision.
The theoretical advantages of this modification to the TVT procedure have included more control over the needle introducer near the rectus fascia, and a lower risk of bowel and vascular injury. However, comparisons during the last decade of the two retropubic approaches have suggested slightly better outcomes – relating both to cure rates and to complication rates – with TVT compared with SPARC.
A Cochrane Review published in 2009, titled “Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women,” provided higher-level evidence in favor of bottom-up slings. A sub-meta-analysis of five randomized controlled trials – part of a broader intervention review – showed that a retropubic bottom-up approach was more effective than a top-down route (risk ratio, 1.10), with higher subjective and objective SUI cure rates (Cochrane Database Syst. Rev. 2009(4): CD006375). There also was significantly less bladder perforation, less mesh erosion, and less voiding dysfunction.
TVT slings, therefore, appear to be somewhat superior, with statistically significant differences in each of the domains of efficacy and morbidity. Still, surgeon experience and skill remain factors in sling selection; the surgeon who feels comfortable and skilled with a top-down approach and has little experience with a bottom-up approach should continue with SPARC. For surgeons who are skilled with both approaches, it might well be preferable to favor TVT.
Transobturator slings. The transobturator approach was developed to minimize the potential for bladder and bowel injuries by avoiding the pelvic organs in the retropubic space. The sling is introduced either through an inside-out technique, with the needle passed from a vaginal incision and out through the obturator foramen, or through an outside-in technique, with the needle passed through the thigh and then out through the vaginal incision.
A meta-analysis of trials of transobturator sling procedures – including four direct-comparison, randomized controlled trials of the inside-out technique vs. the outside-in technique – showed no significant differences between the two approaches in subjective and objective SUI cure rates in the short term. Rates of postoperative voiding difficulties and de novo urgency symptoms were similar (BJU Int. 2010;106:68-76).
Making a choice. Each of the currently available midurethral slings appears to work well, overall, with few clinically significant differences in outcomes. On the other hand, midurethral slings are not all the same. It is important to appreciate the more subtle differences, to be aware of the evidence, and to be appropriately trained. Often, sling selection involves weighing the risks and benefits for the individual.
On a broad scale, the most recent high-level comparison of the retropubic and transobturator slings appears to be a meta-analysis in which retropubic midurethral slings showed better objective and subjective cure rates than transobturator midurethral slings. Women treated with retropubic slings had a 35% higher odds of objective cure and a 24% higher odds of subjective cure. (The weighted average objective cure rates were 87% for retropubic slings vs. 83% for transobturator slings with a weighted average follow-up of approximately 17 months. The weighted average subjective cure rates were 76% and 73%, respectively.)
Operating times were longer with retropubic slings, but lengths of stay were equivalent between the two types of procedures. This was based on 17 studies of about 3,000 women (J. Urology 2014 [doi: 10.1016/j.juro.2014.09.104]).
The types of complications seen with each approach differed. Bladder perforation was significantly more common with retropubic slings (3.2% vs. 0.2%), as was bleeding (3.2% v. 1.1%). Transobturator slings were associated with more cases of neurologic symptoms (9.4% v. 3.5%) and vaginal perforation (3.6% v. 0.9%).
This new review provides updated information to the 2009 Cochrane Review mentioned above, which reported that women were less likely to be continent after operations performed via the obturator route, but also less likely to have encountered complications. More specifically, objective cure rates were slightly higher with retropubic slings than with transobturator slings (88% vs. 84%) in the 2009 review. There was no difference in subjective cure rates. With the obturator route, there was less voiding dysfunction, blood loss, and bladder perforation (0.3% v. 5.5%).
Other pivotal trials since the 2009 Cochrane Review include a multicenter randomized equivalence trial published in the New England Journal of Medicine in 2010. The trial randomized 597 women to transobturator or retropubic sling surgery, and found no significant differences in subjective success (56% vs. 62%) or in objective success (78% vs. 81%) at 12 months (N. Engl. J. Med. 2010;362:2066-76).
There is some level 1 evidence suggesting that for severe incontinence involving intrinsic sphincter deficiency (ISD), a retropubic TVT sling is the more effective procedure. A randomized trial of 164 women with urodynamic SUI and ISD, for instance, found that 21% of those in the TVT group and 45% of those in the transobturator group had urodynamic SUI 6 months postoperatively.
The risk ratio of repeat surgery was 2.6 times higher in the transobturator group than in the retropubic TVT group (Obstet. Gynecol. 2008;112:1253-61). TVT was more effective both with and without concurrent pelvic organ prolapse repair.
I tell my patients with severe SUI or ISD, therefore, that retropubic sling procedures appear to be preferable. (Exceptions include the patient who has a history of retropubic surgeries, in whom passing the sling through this route may not be the safest approach, as well as the patient who has had mesh erosion into the bladder.)
In patients whose SUI is less severe, I counsel that a transobturator sling confers satisfaction rates similar to those of a retropubic sling and has a lower risk of complications, such as postoperative voiding dysfunction and bladder perforations, but with the possible trade-off of more thigh discomfort. I also might recommend a transobturator sling to patients with more pronounced initial complaints of urinary urgency and frequency, and to patients who have minor voiding dysfunction or a low level of incomplete bladder emptying.
While often short-lived, the small risk of thigh pain with a transobturator sling makes me less likely to recommend this type of sling for a woman who is a marathon runner or competitive athlete. In her case, an analysis of possible complications includes the consideration that bladder perforation can be addressed relatively quickly in the operating room, while persistent thigh discomfort, though relatively rare, could be a debilitating problem.
Single-incision slings
There appears to be emerging evidence suggesting that some of the fixed and adjustable single-incision slings currently available may have efficacy similar to that of the slings that are now widely used.
A Cochrane Review presented at the 2014 AUGS-IUGA scientific meeting and published this summer concludes that there is not enough evidence on single-incision slings compared with retropubic or transobturator slings to allow reliable comparisons, and that additional, adequately powered, high-quality trials with longer-term follow-up are needed (Cochrane Database Sys. Rev. 2014;6:CD008709). However, research completed since the review offers additional data.
For instance, at the 2014 AUGS-IUGA scientific meeting this summer, an oral paper presentation highlighted findings of a randomized controlled trial that showed similar cure rates after surgery with the MiniArc, a fixed single-incision sling, and the Monarc transobturator sling (both by American Medical Systems) at 24 months. The study randomized 234 women to either sling and found no significant differences in subjective outcomes, objective outcomes, or results on various quality-of-life questionnaires.
As such studies are published and more evidence emerges, we will gain a clearer picture of how the newer single-incision slings compare to the well-tested retropubic and transobturator slings with respect to efficacy and safety.
Single-incision slings require only a small vaginal incision and no exit points. Without abdominal or thigh incisions, these new procedures – intended for less severe SUI (no ISD) – may offer improved perioperative and postoperative patient comfort and a potentially decreased risk of surgical injury to the adductor muscles, as well as a decreased risk of vascular and nerve injury. Candidates for these slings may include those who are very athletic, those who are obese, and those with a history of prior retropubic or pelvic surgery.
Research appears to be progressing, but at this time we do not yet have level 1 evidence to support their routine use.
Dr. Sokol reported that he owns stock in Pelvalon, and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems, and the recipient of research grants from Acell and several other companies.
Minimally invasive synthetic midurethral slings may be considered the standard of care for the surgical treatment of stress urinary incontinence – and a first-line treatment for severe cases of the condition – based on the publication of numerous level 1 randomized trials, high-quality reviews, and recent position statements from professional societies.
The current evidence base shows that midurethral sling operations are as effective as bladder neck slings and colposuspension, with less morbidity. Operating times are shorter, and local anesthesia is possible. Compared with pubovaginal slings, which are fixed at the bladder neck, midurethral slings are associated with less postoperative voiding dysfunction and fewer de novo urgency symptoms.
Midurethral slings (MUS) also have been shown to be more successful – and more cost-effective – than pelvic floor physiotherapy for stress urinary incontinence (SUI) overall, with the possible exception of mild SUI.
Physiotherapy involving pelvic floor muscle therapy has long been advocated as a first-line treatment for SUI, with MUS surgery often recommended when physiotherapy is unsuccessful. In recent years, however, with high success rates for MUS, the role of physiotherapy as a first-line treatment has become more debatable.
A multicenter randomized trial in 660 women published last year in the New England Journal of Medicine substantiated what many of us have seen in our practices and in other published studies: significantly lower rates of improvement and cure with initial physiotherapy than with primary surgery.
Initial MUS surgery resulted in higher rates of subjective improvement, compared with initial physiotherapy (91% vs. 64%), subjective cure (85% v. 53%), and objective cure (77% v. 59%) at 1 year. Moreover, a significant number of women – 49% – chose to abandon conservative therapy and have MUS surgery for their SUI during the study period (N. Engl. J. Med. 2013;369:1124-33).
A joint position statement published in early 2014 by the American Urogynecologic Society (AUGS) and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) calls MUS the most extensively studied anti-incontinence procedure and “probably the most important advancement in the treatment of SUI in the last 50 years.” More than 2,000 publications in the literature have described the procedure for SUI, and multiple randomized controlled trials have compared various types of MUS procedures as well as MUS to other nonmesh SUI procedures, the statement says.
My colleague and I recently modeled the cost-effectiveness of pelvic floor muscle therapy and continence pessaries vs. surgical treatment with MUS for initial treatment of SUI. Initial treatment with MUS was the best strategy, with an incremental cost-effectiveness ratio of $32,132 per quality-adjusted life-year, compared with initial treatment with pelvic floor muscle therapy. Under our model, treatment with a continence pessary would never be the preferred choice due to low subjective cure rates (Am. J. Obstet. Gynecol. 2014;211:565.e1-6).
I now tell patients who present with a history of severe stress incontinence, and who leak on a cough stress test, that a trial of pelvic floor physiotherapy is an option but one with a lower likelihood of success. I recommend an MUS as primary treatment for these patients, and the question then often becomes which sling to use.
Sling selection
There are two broad approaches to MUS surgery – retropubic and transobturator – and within each approach, there are different routes for the delivery of the polypropylene mesh sling.
Retropubic slings. Retropubic slings are passed transvaginally at the midurethral level through the retropubic space. Tension-free vaginal tape (TVT) has been used in millions of women worldwide, with good long-term outcomes, since it was introduced by Dr. Ulf Ulmsten in 1995. The TVT procedure utilizes a bottom-up approach, with curved needles being passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
A second type of retropubic sling – the suprapubic urethral support sling (SPARC, American Medical Systems) – utilizes a downward-pass, or top-down, approach in which a metal trocar is passed through suprapubic incisions and down through the retropubic space to exit a vaginal incision.
The theoretical advantages of this modification to the TVT procedure have included more control over the needle introducer near the rectus fascia, and a lower risk of bowel and vascular injury. However, comparisons during the last decade of the two retropubic approaches have suggested slightly better outcomes – relating both to cure rates and to complication rates – with TVT compared with SPARC.
A Cochrane Review published in 2009, titled “Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women,” provided higher-level evidence in favor of bottom-up slings. A sub-meta-analysis of five randomized controlled trials – part of a broader intervention review – showed that a retropubic bottom-up approach was more effective than a top-down route (risk ratio, 1.10), with higher subjective and objective SUI cure rates (Cochrane Database Syst. Rev. 2009(4): CD006375). There also was significantly less bladder perforation, less mesh erosion, and less voiding dysfunction.
TVT slings, therefore, appear to be somewhat superior, with statistically significant differences in each of the domains of efficacy and morbidity. Still, surgeon experience and skill remain factors in sling selection; the surgeon who feels comfortable and skilled with a top-down approach and has little experience with a bottom-up approach should continue with SPARC. For surgeons who are skilled with both approaches, it might well be preferable to favor TVT.
Transobturator slings. The transobturator approach was developed to minimize the potential for bladder and bowel injuries by avoiding the pelvic organs in the retropubic space. The sling is introduced either through an inside-out technique, with the needle passed from a vaginal incision and out through the obturator foramen, or through an outside-in technique, with the needle passed through the thigh and then out through the vaginal incision.
A meta-analysis of trials of transobturator sling procedures – including four direct-comparison, randomized controlled trials of the inside-out technique vs. the outside-in technique – showed no significant differences between the two approaches in subjective and objective SUI cure rates in the short term. Rates of postoperative voiding difficulties and de novo urgency symptoms were similar (BJU Int. 2010;106:68-76).
Making a choice. Each of the currently available midurethral slings appears to work well, overall, with few clinically significant differences in outcomes. On the other hand, midurethral slings are not all the same. It is important to appreciate the more subtle differences, to be aware of the evidence, and to be appropriately trained. Often, sling selection involves weighing the risks and benefits for the individual.
On a broad scale, the most recent high-level comparison of the retropubic and transobturator slings appears to be a meta-analysis in which retropubic midurethral slings showed better objective and subjective cure rates than transobturator midurethral slings. Women treated with retropubic slings had a 35% higher odds of objective cure and a 24% higher odds of subjective cure. (The weighted average objective cure rates were 87% for retropubic slings vs. 83% for transobturator slings with a weighted average follow-up of approximately 17 months. The weighted average subjective cure rates were 76% and 73%, respectively.)
Operating times were longer with retropubic slings, but lengths of stay were equivalent between the two types of procedures. This was based on 17 studies of about 3,000 women (J. Urology 2014 [doi: 10.1016/j.juro.2014.09.104]).
The types of complications seen with each approach differed. Bladder perforation was significantly more common with retropubic slings (3.2% vs. 0.2%), as was bleeding (3.2% v. 1.1%). Transobturator slings were associated with more cases of neurologic symptoms (9.4% v. 3.5%) and vaginal perforation (3.6% v. 0.9%).
This new review provides updated information to the 2009 Cochrane Review mentioned above, which reported that women were less likely to be continent after operations performed via the obturator route, but also less likely to have encountered complications. More specifically, objective cure rates were slightly higher with retropubic slings than with transobturator slings (88% vs. 84%) in the 2009 review. There was no difference in subjective cure rates. With the obturator route, there was less voiding dysfunction, blood loss, and bladder perforation (0.3% v. 5.5%).
Other pivotal trials since the 2009 Cochrane Review include a multicenter randomized equivalence trial published in the New England Journal of Medicine in 2010. The trial randomized 597 women to transobturator or retropubic sling surgery, and found no significant differences in subjective success (56% vs. 62%) or in objective success (78% vs. 81%) at 12 months (N. Engl. J. Med. 2010;362:2066-76).
There is some level 1 evidence suggesting that for severe incontinence involving intrinsic sphincter deficiency (ISD), a retropubic TVT sling is the more effective procedure. A randomized trial of 164 women with urodynamic SUI and ISD, for instance, found that 21% of those in the TVT group and 45% of those in the transobturator group had urodynamic SUI 6 months postoperatively.
The risk ratio of repeat surgery was 2.6 times higher in the transobturator group than in the retropubic TVT group (Obstet. Gynecol. 2008;112:1253-61). TVT was more effective both with and without concurrent pelvic organ prolapse repair.
I tell my patients with severe SUI or ISD, therefore, that retropubic sling procedures appear to be preferable. (Exceptions include the patient who has a history of retropubic surgeries, in whom passing the sling through this route may not be the safest approach, as well as the patient who has had mesh erosion into the bladder.)
In patients whose SUI is less severe, I counsel that a transobturator sling confers satisfaction rates similar to those of a retropubic sling and has a lower risk of complications, such as postoperative voiding dysfunction and bladder perforations, but with the possible trade-off of more thigh discomfort. I also might recommend a transobturator sling to patients with more pronounced initial complaints of urinary urgency and frequency, and to patients who have minor voiding dysfunction or a low level of incomplete bladder emptying.
While often short-lived, the small risk of thigh pain with a transobturator sling makes me less likely to recommend this type of sling for a woman who is a marathon runner or competitive athlete. In her case, an analysis of possible complications includes the consideration that bladder perforation can be addressed relatively quickly in the operating room, while persistent thigh discomfort, though relatively rare, could be a debilitating problem.
Single-incision slings
There appears to be emerging evidence suggesting that some of the fixed and adjustable single-incision slings currently available may have efficacy similar to that of the slings that are now widely used.
A Cochrane Review presented at the 2014 AUGS-IUGA scientific meeting and published this summer concludes that there is not enough evidence on single-incision slings compared with retropubic or transobturator slings to allow reliable comparisons, and that additional, adequately powered, high-quality trials with longer-term follow-up are needed (Cochrane Database Sys. Rev. 2014;6:CD008709). However, research completed since the review offers additional data.
For instance, at the 2014 AUGS-IUGA scientific meeting this summer, an oral paper presentation highlighted findings of a randomized controlled trial that showed similar cure rates after surgery with the MiniArc, a fixed single-incision sling, and the Monarc transobturator sling (both by American Medical Systems) at 24 months. The study randomized 234 women to either sling and found no significant differences in subjective outcomes, objective outcomes, or results on various quality-of-life questionnaires.
As such studies are published and more evidence emerges, we will gain a clearer picture of how the newer single-incision slings compare to the well-tested retropubic and transobturator slings with respect to efficacy and safety.
Single-incision slings require only a small vaginal incision and no exit points. Without abdominal or thigh incisions, these new procedures – intended for less severe SUI (no ISD) – may offer improved perioperative and postoperative patient comfort and a potentially decreased risk of surgical injury to the adductor muscles, as well as a decreased risk of vascular and nerve injury. Candidates for these slings may include those who are very athletic, those who are obese, and those with a history of prior retropubic or pelvic surgery.
Research appears to be progressing, but at this time we do not yet have level 1 evidence to support their routine use.
Dr. Sokol reported that he owns stock in Pelvalon, and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems, and the recipient of research grants from Acell and several other companies.
Evidence builds for risk-based antihypertension guidelines
CHICAGO – The next time the U.S. hypertension management guideline gets revised, possibly within another couple of years, it may abandon the current approach of focusing primarily on a person’s blood pressure numbers and center instead on assessing a patient’s overall cardiovascular risk and using that status to guide the need for antihypertensive treatment and how aggressively it is applied.
In short, what some preventive cardiologists see coming down the pike is a blood pressure management guideline that follows the same path carved by the cholesterol management guideline issued by the American College of Cardiology and American Heart Association in 2013 (Circulation 2014 [doi: 10.1161/01.cir.0000437738.63853.7a]) that linked use of lipid-lowering treatment with a statin mostly to a patient’s atherosclerotic cardiovascular disease (ASCVD) risk rather than to their level of LDL cholesterol.
One example of the evidence driving this revisionist approach to thinking about hypertension definition came in a report at the American Heart Association Scientific Sessions that showed “a blood pressure treatment strategy focused just on blood pressure leaves substantial CVD risk unaddressed,” said Dr. Kunal N. Karmali, a cardiologist at Northwestern University, Chicago. “Multivariate risk estimation may help identify types of individuals who are likely to benefit from risk-reducing therapy across the spectrum of blood pressure.”
Dr. Karmali and his associates ran their analysis using data from two well-defined U.S. population data bases that included long-term follow-up, the Atherosclerosis Risk in Communities study and the Framingham Offspring Study, which together provided data for 18,898 people. The researchers categorized these people by their baseline systolic blood pressures into six groups, from below 120 mm Hg to 160 mm Hg and above, and calculated each person’s risk for an incident ASCVD event according to their baseline ASCVD risk score using the risk calculator that accompanied release of the 2013 cholesterol management guidelines. By subtracting the baseline risk–derived prediction of the CVD event rate from the actual number of events during follow-up, Dr. Karmali’s group came up with an estimate of the level of “excess” risk for people within each 10 mm Hg blood pressure stratum.
Ten-year follow-up of the two cohorts identified 739 ASCVD events, of which more than 500 were “excess;” the people in the two cohorts had substantially more ASCVD events than would have been predicted by their risk factors alone. These excess events occurred at all levels of blood pressure. The 6,656 people with baseline systolic blood pressures of 120-139 mm Hg, 35% of the people included in the study, had 45% of the excess events, Dr. Karmali reported.
While people with blood pressures of 120-139 mm Hg would generally not be candidates for antihypertensive treatment based on the existing U.S. guideline (JAMA 2014;311[5]:507-20), a sizable majority, 73%, had high baseline ASCVD risk levels, with predicted 10-year CVD rates of 7.5% or greater.
“An implication of this finding is to think beyond just blood pressure,” Dr. Karmali said. “You need to consider multiple risk factors and use overall risk assessment to inform your management decisions. Looking at just the single risk factor of blood pressure doesn’t capture the true benefits of treatment and weigh that against the risks from treatment,” he said in an interview.
He gave the example of an otherwise healthy woman aged 40 years with a systolic blood pressure of 142 mm Hg, who clearly has a different 10-year risk level than a man aged 70 years who has diabetes and smokes and also has a systolic pressure of 142 mm Hg.
“For cholesterol, we now direct our interventions at people who are at the highest risk, but for blood pressure we still focus on just the single number. Multivariate risk assessment would allow us to direct the interventions at the people who are more likely to benefit,” Dr. Kamali said.
Other recent analyses have also supported this approach, he noted. For example, a metaanalysis published in August used data from nearly 52,000 people collected in 11 studies to show that blood pressure–lowering treatment had a very similar impact on reducing future cardiovascular disease events regardless of a person’s baseline cardiovascular risk level (Lancet 2014;384:591-8).
For middle-aged adults and older people, “I think we would become much more efficient in our selection of people with modestly elevated blood pressure [who need drug treatment] by considering their global risk,” said Dr. Donald M. Lloyd-Jones, professor and chairman of preventive medicine at Northwestern University, and a collaborator on Dr. Karmali’s study.
Another advantage of a risk-based approach to blood pressure management over a number-based approach is that “putting blood pressure into the global context of risk makes me think about global risk management, and that is where clinicians need to move,” Dr. Lloyd-Jones said in an interview. “It’s not just, ‘get a number, treat it, and you’re done.’ You need to also think about statin treatment.”
He acknowledged the need for some caution in this approach, such as recognizing that blood pressure must be carefully reduced in, for example, people with a systolic pressure of 120-129 mm Hg so that blood pressure is not reduced to a dangerously low level (unlike cholesterol, which so far has not shown been shown to cause problems when reduced to very low levels). He also noted that a young adult with a fairly high systolic pressure of, say, 160 mm Hg should receive antihypertensive treatment even if the person has an otherwise low ASCVD risk. But in general a risk-based approach should provide better patient care, he said.
“If this is where new hypertension management guidelines go it would be a significant change,” Dr. Lloyd-Jones acknowledged, “but I think it would help patients. I think this approach merits real consideration” by the panel that will soon create the next revision to the U.S. hypertension management guideline.
On Twitter @mitchelzoler
The concept of a risk-based approach to diagnosing and managing patients with hypertension is ready for more thought among U.S. physicians and policy makers. It is very logical that we could prevent more events by focusing on people who are at higher risk for cardiovascular disease events, and there is more to risk reduction than just treating to a certain blood pressure level.
This approach to managing hypertension has been in place in New Zealand for at least a decade, and was woven into the 2013 hypertension management guidelines issued by the European Society of Cardiology (Eur. Heart J. 2013;34:2159-2219). There was also some attention given to the concept in the JNC VI U.S. hypertension guidelines, but then the guidelines panels backed away from it in the next two revisions.

|
| Mitchel L. Zoler/Frontline Medical News Dr. David C. Goff Jr. |
Several lines of evidence support the risk-based approach. In addition to Dr. Karmali’s new report, there was an analysis published in August that sowed the relative risk reduction from reducing blood pressure was similar across a range of risk levels (Lancet 2014;384:591-8). And a 2011 analysis showed that if you prioritize antihypertensive treatment based on risk level rather than just on systolic pressure you could treat the same number of people but prevent far more cardiovascular disease events (Ann. Int. Med. 2011;154:627-34).
These two prior reports, Dr. Karmali’s new study, and the change in approach introduced by guidelines in New Zealand and in Europe make it the right time to give this approach serious consideration by U.S. policy makers. I think many people agree it makes sense to focus attention on people with the highest cardiovascular risk. But a significant outstanding issue is whether clinicians will be willing to withhold blood pressure lowering treatment from selected people with systolic blood pressures above 140 mm Hg.
Dr. David C. Goff Jr., dean and professor of epidemiology at the Colorado School of Public Health, Aurora, made these comments in an interview. He had no disclosures.
The concept of a risk-based approach to diagnosing and managing patients with hypertension is ready for more thought among U.S. physicians and policy makers. It is very logical that we could prevent more events by focusing on people who are at higher risk for cardiovascular disease events, and there is more to risk reduction than just treating to a certain blood pressure level.
This approach to managing hypertension has been in place in New Zealand for at least a decade, and was woven into the 2013 hypertension management guidelines issued by the European Society of Cardiology (Eur. Heart J. 2013;34:2159-2219). There was also some attention given to the concept in the JNC VI U.S. hypertension guidelines, but then the guidelines panels backed away from it in the next two revisions.

|
| Mitchel L. Zoler/Frontline Medical News Dr. David C. Goff Jr. |
Several lines of evidence support the risk-based approach. In addition to Dr. Karmali’s new report, there was an analysis published in August that sowed the relative risk reduction from reducing blood pressure was similar across a range of risk levels (Lancet 2014;384:591-8). And a 2011 analysis showed that if you prioritize antihypertensive treatment based on risk level rather than just on systolic pressure you could treat the same number of people but prevent far more cardiovascular disease events (Ann. Int. Med. 2011;154:627-34).
These two prior reports, Dr. Karmali’s new study, and the change in approach introduced by guidelines in New Zealand and in Europe make it the right time to give this approach serious consideration by U.S. policy makers. I think many people agree it makes sense to focus attention on people with the highest cardiovascular risk. But a significant outstanding issue is whether clinicians will be willing to withhold blood pressure lowering treatment from selected people with systolic blood pressures above 140 mm Hg.
Dr. David C. Goff Jr., dean and professor of epidemiology at the Colorado School of Public Health, Aurora, made these comments in an interview. He had no disclosures.
The concept of a risk-based approach to diagnosing and managing patients with hypertension is ready for more thought among U.S. physicians and policy makers. It is very logical that we could prevent more events by focusing on people who are at higher risk for cardiovascular disease events, and there is more to risk reduction than just treating to a certain blood pressure level.
This approach to managing hypertension has been in place in New Zealand for at least a decade, and was woven into the 2013 hypertension management guidelines issued by the European Society of Cardiology (Eur. Heart J. 2013;34:2159-2219). There was also some attention given to the concept in the JNC VI U.S. hypertension guidelines, but then the guidelines panels backed away from it in the next two revisions.

|
| Mitchel L. Zoler/Frontline Medical News Dr. David C. Goff Jr. |
Several lines of evidence support the risk-based approach. In addition to Dr. Karmali’s new report, there was an analysis published in August that sowed the relative risk reduction from reducing blood pressure was similar across a range of risk levels (Lancet 2014;384:591-8). And a 2011 analysis showed that if you prioritize antihypertensive treatment based on risk level rather than just on systolic pressure you could treat the same number of people but prevent far more cardiovascular disease events (Ann. Int. Med. 2011;154:627-34).
These two prior reports, Dr. Karmali’s new study, and the change in approach introduced by guidelines in New Zealand and in Europe make it the right time to give this approach serious consideration by U.S. policy makers. I think many people agree it makes sense to focus attention on people with the highest cardiovascular risk. But a significant outstanding issue is whether clinicians will be willing to withhold blood pressure lowering treatment from selected people with systolic blood pressures above 140 mm Hg.
Dr. David C. Goff Jr., dean and professor of epidemiology at the Colorado School of Public Health, Aurora, made these comments in an interview. He had no disclosures.
CHICAGO – The next time the U.S. hypertension management guideline gets revised, possibly within another couple of years, it may abandon the current approach of focusing primarily on a person’s blood pressure numbers and center instead on assessing a patient’s overall cardiovascular risk and using that status to guide the need for antihypertensive treatment and how aggressively it is applied.
In short, what some preventive cardiologists see coming down the pike is a blood pressure management guideline that follows the same path carved by the cholesterol management guideline issued by the American College of Cardiology and American Heart Association in 2013 (Circulation 2014 [doi: 10.1161/01.cir.0000437738.63853.7a]) that linked use of lipid-lowering treatment with a statin mostly to a patient’s atherosclerotic cardiovascular disease (ASCVD) risk rather than to their level of LDL cholesterol.
One example of the evidence driving this revisionist approach to thinking about hypertension definition came in a report at the American Heart Association Scientific Sessions that showed “a blood pressure treatment strategy focused just on blood pressure leaves substantial CVD risk unaddressed,” said Dr. Kunal N. Karmali, a cardiologist at Northwestern University, Chicago. “Multivariate risk estimation may help identify types of individuals who are likely to benefit from risk-reducing therapy across the spectrum of blood pressure.”
Dr. Karmali and his associates ran their analysis using data from two well-defined U.S. population data bases that included long-term follow-up, the Atherosclerosis Risk in Communities study and the Framingham Offspring Study, which together provided data for 18,898 people. The researchers categorized these people by their baseline systolic blood pressures into six groups, from below 120 mm Hg to 160 mm Hg and above, and calculated each person’s risk for an incident ASCVD event according to their baseline ASCVD risk score using the risk calculator that accompanied release of the 2013 cholesterol management guidelines. By subtracting the baseline risk–derived prediction of the CVD event rate from the actual number of events during follow-up, Dr. Karmali’s group came up with an estimate of the level of “excess” risk for people within each 10 mm Hg blood pressure stratum.
Ten-year follow-up of the two cohorts identified 739 ASCVD events, of which more than 500 were “excess;” the people in the two cohorts had substantially more ASCVD events than would have been predicted by their risk factors alone. These excess events occurred at all levels of blood pressure. The 6,656 people with baseline systolic blood pressures of 120-139 mm Hg, 35% of the people included in the study, had 45% of the excess events, Dr. Karmali reported.
While people with blood pressures of 120-139 mm Hg would generally not be candidates for antihypertensive treatment based on the existing U.S. guideline (JAMA 2014;311[5]:507-20), a sizable majority, 73%, had high baseline ASCVD risk levels, with predicted 10-year CVD rates of 7.5% or greater.
“An implication of this finding is to think beyond just blood pressure,” Dr. Karmali said. “You need to consider multiple risk factors and use overall risk assessment to inform your management decisions. Looking at just the single risk factor of blood pressure doesn’t capture the true benefits of treatment and weigh that against the risks from treatment,” he said in an interview.
He gave the example of an otherwise healthy woman aged 40 years with a systolic blood pressure of 142 mm Hg, who clearly has a different 10-year risk level than a man aged 70 years who has diabetes and smokes and also has a systolic pressure of 142 mm Hg.
“For cholesterol, we now direct our interventions at people who are at the highest risk, but for blood pressure we still focus on just the single number. Multivariate risk assessment would allow us to direct the interventions at the people who are more likely to benefit,” Dr. Kamali said.
Other recent analyses have also supported this approach, he noted. For example, a metaanalysis published in August used data from nearly 52,000 people collected in 11 studies to show that blood pressure–lowering treatment had a very similar impact on reducing future cardiovascular disease events regardless of a person’s baseline cardiovascular risk level (Lancet 2014;384:591-8).
For middle-aged adults and older people, “I think we would become much more efficient in our selection of people with modestly elevated blood pressure [who need drug treatment] by considering their global risk,” said Dr. Donald M. Lloyd-Jones, professor and chairman of preventive medicine at Northwestern University, and a collaborator on Dr. Karmali’s study.
Another advantage of a risk-based approach to blood pressure management over a number-based approach is that “putting blood pressure into the global context of risk makes me think about global risk management, and that is where clinicians need to move,” Dr. Lloyd-Jones said in an interview. “It’s not just, ‘get a number, treat it, and you’re done.’ You need to also think about statin treatment.”
He acknowledged the need for some caution in this approach, such as recognizing that blood pressure must be carefully reduced in, for example, people with a systolic pressure of 120-129 mm Hg so that blood pressure is not reduced to a dangerously low level (unlike cholesterol, which so far has not shown been shown to cause problems when reduced to very low levels). He also noted that a young adult with a fairly high systolic pressure of, say, 160 mm Hg should receive antihypertensive treatment even if the person has an otherwise low ASCVD risk. But in general a risk-based approach should provide better patient care, he said.
“If this is where new hypertension management guidelines go it would be a significant change,” Dr. Lloyd-Jones acknowledged, “but I think it would help patients. I think this approach merits real consideration” by the panel that will soon create the next revision to the U.S. hypertension management guideline.
On Twitter @mitchelzoler
CHICAGO – The next time the U.S. hypertension management guideline gets revised, possibly within another couple of years, it may abandon the current approach of focusing primarily on a person’s blood pressure numbers and center instead on assessing a patient’s overall cardiovascular risk and using that status to guide the need for antihypertensive treatment and how aggressively it is applied.
In short, what some preventive cardiologists see coming down the pike is a blood pressure management guideline that follows the same path carved by the cholesterol management guideline issued by the American College of Cardiology and American Heart Association in 2013 (Circulation 2014 [doi: 10.1161/01.cir.0000437738.63853.7a]) that linked use of lipid-lowering treatment with a statin mostly to a patient’s atherosclerotic cardiovascular disease (ASCVD) risk rather than to their level of LDL cholesterol.
One example of the evidence driving this revisionist approach to thinking about hypertension definition came in a report at the American Heart Association Scientific Sessions that showed “a blood pressure treatment strategy focused just on blood pressure leaves substantial CVD risk unaddressed,” said Dr. Kunal N. Karmali, a cardiologist at Northwestern University, Chicago. “Multivariate risk estimation may help identify types of individuals who are likely to benefit from risk-reducing therapy across the spectrum of blood pressure.”
Dr. Karmali and his associates ran their analysis using data from two well-defined U.S. population data bases that included long-term follow-up, the Atherosclerosis Risk in Communities study and the Framingham Offspring Study, which together provided data for 18,898 people. The researchers categorized these people by their baseline systolic blood pressures into six groups, from below 120 mm Hg to 160 mm Hg and above, and calculated each person’s risk for an incident ASCVD event according to their baseline ASCVD risk score using the risk calculator that accompanied release of the 2013 cholesterol management guidelines. By subtracting the baseline risk–derived prediction of the CVD event rate from the actual number of events during follow-up, Dr. Karmali’s group came up with an estimate of the level of “excess” risk for people within each 10 mm Hg blood pressure stratum.
Ten-year follow-up of the two cohorts identified 739 ASCVD events, of which more than 500 were “excess;” the people in the two cohorts had substantially more ASCVD events than would have been predicted by their risk factors alone. These excess events occurred at all levels of blood pressure. The 6,656 people with baseline systolic blood pressures of 120-139 mm Hg, 35% of the people included in the study, had 45% of the excess events, Dr. Karmali reported.
While people with blood pressures of 120-139 mm Hg would generally not be candidates for antihypertensive treatment based on the existing U.S. guideline (JAMA 2014;311[5]:507-20), a sizable majority, 73%, had high baseline ASCVD risk levels, with predicted 10-year CVD rates of 7.5% or greater.
“An implication of this finding is to think beyond just blood pressure,” Dr. Karmali said. “You need to consider multiple risk factors and use overall risk assessment to inform your management decisions. Looking at just the single risk factor of blood pressure doesn’t capture the true benefits of treatment and weigh that against the risks from treatment,” he said in an interview.
He gave the example of an otherwise healthy woman aged 40 years with a systolic blood pressure of 142 mm Hg, who clearly has a different 10-year risk level than a man aged 70 years who has diabetes and smokes and also has a systolic pressure of 142 mm Hg.
“For cholesterol, we now direct our interventions at people who are at the highest risk, but for blood pressure we still focus on just the single number. Multivariate risk assessment would allow us to direct the interventions at the people who are more likely to benefit,” Dr. Kamali said.
Other recent analyses have also supported this approach, he noted. For example, a metaanalysis published in August used data from nearly 52,000 people collected in 11 studies to show that blood pressure–lowering treatment had a very similar impact on reducing future cardiovascular disease events regardless of a person’s baseline cardiovascular risk level (Lancet 2014;384:591-8).
For middle-aged adults and older people, “I think we would become much more efficient in our selection of people with modestly elevated blood pressure [who need drug treatment] by considering their global risk,” said Dr. Donald M. Lloyd-Jones, professor and chairman of preventive medicine at Northwestern University, and a collaborator on Dr. Karmali’s study.
Another advantage of a risk-based approach to blood pressure management over a number-based approach is that “putting blood pressure into the global context of risk makes me think about global risk management, and that is where clinicians need to move,” Dr. Lloyd-Jones said in an interview. “It’s not just, ‘get a number, treat it, and you’re done.’ You need to also think about statin treatment.”
He acknowledged the need for some caution in this approach, such as recognizing that blood pressure must be carefully reduced in, for example, people with a systolic pressure of 120-129 mm Hg so that blood pressure is not reduced to a dangerously low level (unlike cholesterol, which so far has not shown been shown to cause problems when reduced to very low levels). He also noted that a young adult with a fairly high systolic pressure of, say, 160 mm Hg should receive antihypertensive treatment even if the person has an otherwise low ASCVD risk. But in general a risk-based approach should provide better patient care, he said.
“If this is where new hypertension management guidelines go it would be a significant change,” Dr. Lloyd-Jones acknowledged, “but I think it would help patients. I think this approach merits real consideration” by the panel that will soon create the next revision to the U.S. hypertension management guideline.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Many people with systolic blood pressures of 120-139 mm Hg have significant cardiovascular disease risk that often goes untreated.
Major finding: People with a systolic blood pressure of 120-139 mm Hg generated 45% of excess cardiovascular disease events.
Data source: Analysis of 10-year outcomes of 18,898 American adults followed in either the ARIC study or in the Framingham Offspring Study.
Disclosures: Dr. Karmali and Dr. Lloyd-Jones had no disclosures.
Treatment of stress urinary incontinence
According to a 2004 article by Dr. Eric S. Rovner and Dr. Alan J. Wein, 200 different surgical procedures have been described to treat stress urinary incontinence (Rev. Urol. 2004;6(Suppl 3):S29-47). Two goals exist in such surgical procedures:
1. Urethra repositioning or stabilization of the urethra and bladder neck through creation of retropubic support that is impervious to intraabdominal pressure changes.
2. Augmentation of the ureteral resistance provided by the intrinsic sphincter unit, with or without impacting urethra and bladder neck support (sling vs. periurethral injectables, or a combination of the two).
Sling procedures were initially introduced almost a century ago and have recently become increasingly popular – in part, secondary to a decrease in associated morbidity. Unlike transabdominal or transvaginal urethropexy, a sling not only provides support to the vesicourethral junction, but also may create some aspect of urethral coaptation or compression.
Midurethral slings were introduced nearly 20 years ago. These procedures can be performed with a local anesthetic or with minimal regional anesthesia – thus, in an outpatient setting. In addition, midurethral slings are associated with decreased pain and postoperative convalescence.
I have asked Dr. Eric Russell Sokol to lead this state-of-the-art discussion on midurethral slings. Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals. It is a pleasure and an honor to welcome Dr. Sokol to this edition of Master Class in Gynecologic Surgery, the second installment on urinary incontinence.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
According to a 2004 article by Dr. Eric S. Rovner and Dr. Alan J. Wein, 200 different surgical procedures have been described to treat stress urinary incontinence (Rev. Urol. 2004;6(Suppl 3):S29-47). Two goals exist in such surgical procedures:
1. Urethra repositioning or stabilization of the urethra and bladder neck through creation of retropubic support that is impervious to intraabdominal pressure changes.
2. Augmentation of the ureteral resistance provided by the intrinsic sphincter unit, with or without impacting urethra and bladder neck support (sling vs. periurethral injectables, or a combination of the two).
Sling procedures were initially introduced almost a century ago and have recently become increasingly popular – in part, secondary to a decrease in associated morbidity. Unlike transabdominal or transvaginal urethropexy, a sling not only provides support to the vesicourethral junction, but also may create some aspect of urethral coaptation or compression.
Midurethral slings were introduced nearly 20 years ago. These procedures can be performed with a local anesthetic or with minimal regional anesthesia – thus, in an outpatient setting. In addition, midurethral slings are associated with decreased pain and postoperative convalescence.
I have asked Dr. Eric Russell Sokol to lead this state-of-the-art discussion on midurethral slings. Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals. It is a pleasure and an honor to welcome Dr. Sokol to this edition of Master Class in Gynecologic Surgery, the second installment on urinary incontinence.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
According to a 2004 article by Dr. Eric S. Rovner and Dr. Alan J. Wein, 200 different surgical procedures have been described to treat stress urinary incontinence (Rev. Urol. 2004;6(Suppl 3):S29-47). Two goals exist in such surgical procedures:
1. Urethra repositioning or stabilization of the urethra and bladder neck through creation of retropubic support that is impervious to intraabdominal pressure changes.
2. Augmentation of the ureteral resistance provided by the intrinsic sphincter unit, with or without impacting urethra and bladder neck support (sling vs. periurethral injectables, or a combination of the two).
Sling procedures were initially introduced almost a century ago and have recently become increasingly popular – in part, secondary to a decrease in associated morbidity. Unlike transabdominal or transvaginal urethropexy, a sling not only provides support to the vesicourethral junction, but also may create some aspect of urethral coaptation or compression.
Midurethral slings were introduced nearly 20 years ago. These procedures can be performed with a local anesthetic or with minimal regional anesthesia – thus, in an outpatient setting. In addition, midurethral slings are associated with decreased pain and postoperative convalescence.
I have asked Dr. Eric Russell Sokol to lead this state-of-the-art discussion on midurethral slings. Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals. It is a pleasure and an honor to welcome Dr. Sokol to this edition of Master Class in Gynecologic Surgery, the second installment on urinary incontinence.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
Combo proves active in newly diagnosed MM

Credit: Esther Dyson
Combining the proteasome inhibitor ixazomib with lenalidomide and dexamethasone shows promise for treating patients with newly diagnosed multiple myeloma (MM), according to researchers.
In a phase 1/2 study, the all-oral combination produced a 92% overall response rate and a 27% complete response rate.
Drug-related adverse events occurred in 100% of patients, with events of grade 3 or higher occurring in 63% of patients.
These results appear in The Lancet Oncology. The study was funded by Millennium Pharmaceuticals, the company developing ixazomib.
“Ixazomib is an investigational, oral proteasome inhibitor with promising anti-myeloma effects and low rates of peripheral neuropathy,” said study author Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“While it is well known that a combination of bortezomib, lenalidomide, and dexamethasone is highly effective in treating newly diagnosed multiple myeloma, we wanted to study the safety, tolerability, and activity of ixazomib in combination with lenalidomide and dexamethasone in newly diagnosed multiple myeloma.”
Dr Kumar and colleagues enrolled 65 patients—15 for phase 1 and 50 for phase 2 of the study—who were newly diagnosed with MM and 18 years of age or older. Patients had measurable disease, ECOG performance status of 0 to 2, and no grade 2 or higher peripheral neuropathy.
They received ixazomib (on days 1, 8, and 15) plus lenalidomide at 25 mg (on days 1 to 21) and dexamethasone at 40 mg (on days 1, 8, 15, and 22) for up to twelve 28-day cycles, followed by maintenance therapy with ixazomib alone.
In phase 1, patients received escalating doses of ixazomib, from 1.68 mg/m2 to 3.95 mg/m2, to establish the recommended dose for phase 2. The researchers established 2.97 mg/m2 as the maximum-tolerated dose and recommended the phase 2 dose be 2.23 mg/m2. This was converted to a 4.0 mg fixed dose based on population pharmacokinetic results.
Adverse events
The researchers said the combination was well tolerated, and most toxic effects were managed through dose modifications.
All patients reported at least one treatment-emergent adverse event, and 75% reported at least one treatment-emergent event that was grade 3 or higher.
Fifty-seven percent of patients had adverse events that led to dose reductions, including 53% of patients in the dose-escalation cohort and 58% of patients in the phase 2 cohort.
The most common adverse events resulting in dose reductions included skin and subcutaneous tissue disorders (20%), fatigue (14%), diarrhea (8%), peripheral neuropathy not elsewhere classified (8%), insomnia (6%), and increased body weight (6%). Five patients had adverse events leading to treatment discontinuation.
Two patients in the phase 2 cohort died while on study. One patient died of respiratory syncytial viral pneumonia that was thought to be treatment-related. The other patient died from cardiorespiratory arrest, which was considered not related to treatment.
Response and survival rates
Of the 64 response-evaluable patients, 92% responded to treatment. Fifty-eight percent had a partial response or better, and 27% had a complete response.
Responses deepened with an increasing number of treatment cycles. In the 25 patients continuing with maintenance therapy, 5 (20%) had an improvement in the depth of response during this phase.
The median duration of response has not been reached, but patients maintained responses for up to 2 years.
At last follow-up, 9 patients had progressed or died. The estimated 1-year progression-free survival was 88%, and 2-year progression-free survival was 67%.
The median overall survival has not been reached, but the estimated 1-year overall survival was 94%.
“The all-oral combination of weekly ixazomib plus lenalidomide and dexamethasone was generally well-tolerated and appeared active in patients with newly diagnosed multiple myeloma,” Dr Kumar said. “Our results support the development of a phase 3 trial studying this combination for multiple myeloma.” ![]()

Credit: Esther Dyson
Combining the proteasome inhibitor ixazomib with lenalidomide and dexamethasone shows promise for treating patients with newly diagnosed multiple myeloma (MM), according to researchers.
In a phase 1/2 study, the all-oral combination produced a 92% overall response rate and a 27% complete response rate.
Drug-related adverse events occurred in 100% of patients, with events of grade 3 or higher occurring in 63% of patients.
These results appear in The Lancet Oncology. The study was funded by Millennium Pharmaceuticals, the company developing ixazomib.
“Ixazomib is an investigational, oral proteasome inhibitor with promising anti-myeloma effects and low rates of peripheral neuropathy,” said study author Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“While it is well known that a combination of bortezomib, lenalidomide, and dexamethasone is highly effective in treating newly diagnosed multiple myeloma, we wanted to study the safety, tolerability, and activity of ixazomib in combination with lenalidomide and dexamethasone in newly diagnosed multiple myeloma.”
Dr Kumar and colleagues enrolled 65 patients—15 for phase 1 and 50 for phase 2 of the study—who were newly diagnosed with MM and 18 years of age or older. Patients had measurable disease, ECOG performance status of 0 to 2, and no grade 2 or higher peripheral neuropathy.
They received ixazomib (on days 1, 8, and 15) plus lenalidomide at 25 mg (on days 1 to 21) and dexamethasone at 40 mg (on days 1, 8, 15, and 22) for up to twelve 28-day cycles, followed by maintenance therapy with ixazomib alone.
In phase 1, patients received escalating doses of ixazomib, from 1.68 mg/m2 to 3.95 mg/m2, to establish the recommended dose for phase 2. The researchers established 2.97 mg/m2 as the maximum-tolerated dose and recommended the phase 2 dose be 2.23 mg/m2. This was converted to a 4.0 mg fixed dose based on population pharmacokinetic results.
Adverse events
The researchers said the combination was well tolerated, and most toxic effects were managed through dose modifications.
All patients reported at least one treatment-emergent adverse event, and 75% reported at least one treatment-emergent event that was grade 3 or higher.
Fifty-seven percent of patients had adverse events that led to dose reductions, including 53% of patients in the dose-escalation cohort and 58% of patients in the phase 2 cohort.
The most common adverse events resulting in dose reductions included skin and subcutaneous tissue disorders (20%), fatigue (14%), diarrhea (8%), peripheral neuropathy not elsewhere classified (8%), insomnia (6%), and increased body weight (6%). Five patients had adverse events leading to treatment discontinuation.
Two patients in the phase 2 cohort died while on study. One patient died of respiratory syncytial viral pneumonia that was thought to be treatment-related. The other patient died from cardiorespiratory arrest, which was considered not related to treatment.
Response and survival rates
Of the 64 response-evaluable patients, 92% responded to treatment. Fifty-eight percent had a partial response or better, and 27% had a complete response.
Responses deepened with an increasing number of treatment cycles. In the 25 patients continuing with maintenance therapy, 5 (20%) had an improvement in the depth of response during this phase.
The median duration of response has not been reached, but patients maintained responses for up to 2 years.
At last follow-up, 9 patients had progressed or died. The estimated 1-year progression-free survival was 88%, and 2-year progression-free survival was 67%.
The median overall survival has not been reached, but the estimated 1-year overall survival was 94%.
“The all-oral combination of weekly ixazomib plus lenalidomide and dexamethasone was generally well-tolerated and appeared active in patients with newly diagnosed multiple myeloma,” Dr Kumar said. “Our results support the development of a phase 3 trial studying this combination for multiple myeloma.” ![]()

Credit: Esther Dyson
Combining the proteasome inhibitor ixazomib with lenalidomide and dexamethasone shows promise for treating patients with newly diagnosed multiple myeloma (MM), according to researchers.
In a phase 1/2 study, the all-oral combination produced a 92% overall response rate and a 27% complete response rate.
Drug-related adverse events occurred in 100% of patients, with events of grade 3 or higher occurring in 63% of patients.
These results appear in The Lancet Oncology. The study was funded by Millennium Pharmaceuticals, the company developing ixazomib.
“Ixazomib is an investigational, oral proteasome inhibitor with promising anti-myeloma effects and low rates of peripheral neuropathy,” said study author Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“While it is well known that a combination of bortezomib, lenalidomide, and dexamethasone is highly effective in treating newly diagnosed multiple myeloma, we wanted to study the safety, tolerability, and activity of ixazomib in combination with lenalidomide and dexamethasone in newly diagnosed multiple myeloma.”
Dr Kumar and colleagues enrolled 65 patients—15 for phase 1 and 50 for phase 2 of the study—who were newly diagnosed with MM and 18 years of age or older. Patients had measurable disease, ECOG performance status of 0 to 2, and no grade 2 or higher peripheral neuropathy.
They received ixazomib (on days 1, 8, and 15) plus lenalidomide at 25 mg (on days 1 to 21) and dexamethasone at 40 mg (on days 1, 8, 15, and 22) for up to twelve 28-day cycles, followed by maintenance therapy with ixazomib alone.
In phase 1, patients received escalating doses of ixazomib, from 1.68 mg/m2 to 3.95 mg/m2, to establish the recommended dose for phase 2. The researchers established 2.97 mg/m2 as the maximum-tolerated dose and recommended the phase 2 dose be 2.23 mg/m2. This was converted to a 4.0 mg fixed dose based on population pharmacokinetic results.
Adverse events
The researchers said the combination was well tolerated, and most toxic effects were managed through dose modifications.
All patients reported at least one treatment-emergent adverse event, and 75% reported at least one treatment-emergent event that was grade 3 or higher.
Fifty-seven percent of patients had adverse events that led to dose reductions, including 53% of patients in the dose-escalation cohort and 58% of patients in the phase 2 cohort.
The most common adverse events resulting in dose reductions included skin and subcutaneous tissue disorders (20%), fatigue (14%), diarrhea (8%), peripheral neuropathy not elsewhere classified (8%), insomnia (6%), and increased body weight (6%). Five patients had adverse events leading to treatment discontinuation.
Two patients in the phase 2 cohort died while on study. One patient died of respiratory syncytial viral pneumonia that was thought to be treatment-related. The other patient died from cardiorespiratory arrest, which was considered not related to treatment.
Response and survival rates
Of the 64 response-evaluable patients, 92% responded to treatment. Fifty-eight percent had a partial response or better, and 27% had a complete response.
Responses deepened with an increasing number of treatment cycles. In the 25 patients continuing with maintenance therapy, 5 (20%) had an improvement in the depth of response during this phase.
The median duration of response has not been reached, but patients maintained responses for up to 2 years.
At last follow-up, 9 patients had progressed or died. The estimated 1-year progression-free survival was 88%, and 2-year progression-free survival was 67%.
The median overall survival has not been reached, but the estimated 1-year overall survival was 94%.
“The all-oral combination of weekly ixazomib plus lenalidomide and dexamethasone was generally well-tolerated and appeared active in patients with newly diagnosed multiple myeloma,” Dr Kumar said. “Our results support the development of a phase 3 trial studying this combination for multiple myeloma.” ![]()
Recovering From Military Sexual Trauma
Military sexual trauma (MST) refers to experiences of sexual assault or repeated, threatening sexual harassment experienced while on federal active duty or active duty for training. About 1 in 4 women and 1 in 100 men have reported MST to their VA doctors. However, these numbers do not account for those who have not sought health care for their MST experience or who have sought care for MST outside the VA.
Military sexual trauma is:
Military sexual trauma is the term VA uses to refer to sexual assault or sexual harassment that occurred while the veteran was in the military. A victim of MST may have been:
- Involved in sexual activity against his or her will, by physical force or nonphysical pressure. Nonphysical pressure includes threats of negative consequences for refusing to be sexually cooperative, or indirect promises of faster promotions or better treatment in exchange for sex.
- Unable to consent to sexual activities. This includes intoxication by alcohol or other substances.
Other experiences include sexual touching or grabbing, threatening or offensive remarks about a person’s body or sexual activities, as well as threatening and unwelcome sexual advances.
How do I know if I’m at risk?
Military sexual trauma can occur on or off base and while a service member—man or woman—is on or off duty. Those who commit sexual assault or sexual harassment can be men or women, military personnel or civilians, commanding officers or subordinates, strangers, friends, spouses, or intimate partners.
When do I need medical attention?
The VA reports sexual assault is more likely to result in symptoms of PTSD (posttraumatic stress disorder) than are most other types of trauma, including combat. You should seek medical attention from your primary care doctor, a mental health professional, or your VA facility’s MST Coordinator following a MST experience, especially if you experience any of the following symptoms:
- Depression
- Suicidal thoughts
- Feeling angry or irritable most of the time
- Strong emotional reactions
- Feeling emotionally numb
- Trouble falling or staying asleep
- Nightmares
- Trouble focusing
- Difficulty remembering things
- Substance abuse
- Trouble feeling safe or trusting others
- Feeling isolated or disconnected from others
- Headaches
- Gastrointestinal difficulties
- Sexual dysfunction
- Chronic pain
- Chronic fatigue
- Weight or eating problems
Survivors who are not formally diagnosed may still struggle in certain situations with emotional reactions, memories related to their experiences of MST, or other interpersonal issues.
How is MST treated?
Because MST is an experience, not a diagnosis, treatment needs may vary from patient to patient. However, VA provides free, confidential counseling and treatment to male and female veterans for mental and physical health conditions related to experiences of MST. It is important to note that treatment is available even if the MST incident was not reported at the time it happened.
Your doctor may recommend individual therapy, group therapy, or medication, depending on your symptoms. Therapies that may be used to treat MST include:
- Cognitive behavioral therapy. The main goal of this therapy is to help you change your thought patterns, which will help you change the emotions and behavior connected with the MST experience. A counselor might encourage you to reimagine your trauma repeatedly under controlled conditions—an approach called exposure therapy—as a way of learning to cope.
- Stress inoculation (in-ock-you-lay-shun) training. This therapy involves combining stress management strategies with stress-relieving techniques, such as muscle relaxation, breathing retraining, self-dialogue, and thought stopping.
- Group therapy. This therapy enables you to discuss your trauma with others who have had similar experiences.
- Inpatient therapy. Nationwide, there are programs that offer specialized sexual trauma treatment for veterans who need more intense treatment and support, including that for severe depression or substance abuse.
- Medication. If your MST experience resulted in PTSD, your doctor may prescribe medication to help control symptoms of anxiety or to help you sleep. Your doctor will monitor you closely for any possible adverse effects of these medications. It is also possible that an STD (sexually transmitted disease) was passed during the trauma, so your doctor may choose to screen you for an STD. If the test comes back positive, your doctor will prescribe the appropriate drug for treatment.
Services are designed to help veterans at all stages of their recovery, whether that is focusing on strategies for coping with emotions and memories or, for veterans who are ready, talking about their MST experiences in depth.
What can I do to cope?
When trauma survivors take direct action to cope with their stress reactions, they put themselves in a position of power. This is called active coping, which involves accepting the impact the trauma had on your life and taking direct action to make improvements.
It is important to remember that recovery is a process and takes time. Healing from trauma, including MST, does not happen all at once, and healing does not mean that you must forget the experience. Instead, it means you have less pain and fewer bad feelings when you think about the experience, and any associated symptoms will over time bother you less.
Discussing your trauma with other survivors in a controlled setting can help you learn that you are not alone or weak. In addition, surrounding yourself with people you can talk to about your MST experience may help you feel more understood. You may even choose to distract yourself with positive recreational or work activities.
When you experience unwanted or distressing memories, remind yourself that they are just memories, and talk about them with someone you trust. If you feel that the trauma is happening again, which is known as a flashback, keep your eyes open and remind yourself where you presently are and that the trauma happened in the past.
Although VA and DoD are working to put an end to MST, it is an ongoing problem that affects a large percentage of women and men who proudly serve in the armed forces. For information on how to access free VA services and to determine your eligibility in MST benefits, visit http://www.mentalhealth.va.gov/msthome.asp.
Military sexual trauma (MST) refers to experiences of sexual assault or repeated, threatening sexual harassment experienced while on federal active duty or active duty for training. About 1 in 4 women and 1 in 100 men have reported MST to their VA doctors. However, these numbers do not account for those who have not sought health care for their MST experience or who have sought care for MST outside the VA.
Military sexual trauma is:
Military sexual trauma is the term VA uses to refer to sexual assault or sexual harassment that occurred while the veteran was in the military. A victim of MST may have been:
- Involved in sexual activity against his or her will, by physical force or nonphysical pressure. Nonphysical pressure includes threats of negative consequences for refusing to be sexually cooperative, or indirect promises of faster promotions or better treatment in exchange for sex.
- Unable to consent to sexual activities. This includes intoxication by alcohol or other substances.
Other experiences include sexual touching or grabbing, threatening or offensive remarks about a person’s body or sexual activities, as well as threatening and unwelcome sexual advances.
How do I know if I’m at risk?
Military sexual trauma can occur on or off base and while a service member—man or woman—is on or off duty. Those who commit sexual assault or sexual harassment can be men or women, military personnel or civilians, commanding officers or subordinates, strangers, friends, spouses, or intimate partners.
When do I need medical attention?
The VA reports sexual assault is more likely to result in symptoms of PTSD (posttraumatic stress disorder) than are most other types of trauma, including combat. You should seek medical attention from your primary care doctor, a mental health professional, or your VA facility’s MST Coordinator following a MST experience, especially if you experience any of the following symptoms:
- Depression
- Suicidal thoughts
- Feeling angry or irritable most of the time
- Strong emotional reactions
- Feeling emotionally numb
- Trouble falling or staying asleep
- Nightmares
- Trouble focusing
- Difficulty remembering things
- Substance abuse
- Trouble feeling safe or trusting others
- Feeling isolated or disconnected from others
- Headaches
- Gastrointestinal difficulties
- Sexual dysfunction
- Chronic pain
- Chronic fatigue
- Weight or eating problems
Survivors who are not formally diagnosed may still struggle in certain situations with emotional reactions, memories related to their experiences of MST, or other interpersonal issues.
How is MST treated?
Because MST is an experience, not a diagnosis, treatment needs may vary from patient to patient. However, VA provides free, confidential counseling and treatment to male and female veterans for mental and physical health conditions related to experiences of MST. It is important to note that treatment is available even if the MST incident was not reported at the time it happened.
Your doctor may recommend individual therapy, group therapy, or medication, depending on your symptoms. Therapies that may be used to treat MST include:
- Cognitive behavioral therapy. The main goal of this therapy is to help you change your thought patterns, which will help you change the emotions and behavior connected with the MST experience. A counselor might encourage you to reimagine your trauma repeatedly under controlled conditions—an approach called exposure therapy—as a way of learning to cope.
- Stress inoculation (in-ock-you-lay-shun) training. This therapy involves combining stress management strategies with stress-relieving techniques, such as muscle relaxation, breathing retraining, self-dialogue, and thought stopping.
- Group therapy. This therapy enables you to discuss your trauma with others who have had similar experiences.
- Inpatient therapy. Nationwide, there are programs that offer specialized sexual trauma treatment for veterans who need more intense treatment and support, including that for severe depression or substance abuse.
- Medication. If your MST experience resulted in PTSD, your doctor may prescribe medication to help control symptoms of anxiety or to help you sleep. Your doctor will monitor you closely for any possible adverse effects of these medications. It is also possible that an STD (sexually transmitted disease) was passed during the trauma, so your doctor may choose to screen you for an STD. If the test comes back positive, your doctor will prescribe the appropriate drug for treatment.
Services are designed to help veterans at all stages of their recovery, whether that is focusing on strategies for coping with emotions and memories or, for veterans who are ready, talking about their MST experiences in depth.
What can I do to cope?
When trauma survivors take direct action to cope with their stress reactions, they put themselves in a position of power. This is called active coping, which involves accepting the impact the trauma had on your life and taking direct action to make improvements.
It is important to remember that recovery is a process and takes time. Healing from trauma, including MST, does not happen all at once, and healing does not mean that you must forget the experience. Instead, it means you have less pain and fewer bad feelings when you think about the experience, and any associated symptoms will over time bother you less.
Discussing your trauma with other survivors in a controlled setting can help you learn that you are not alone or weak. In addition, surrounding yourself with people you can talk to about your MST experience may help you feel more understood. You may even choose to distract yourself with positive recreational or work activities.
When you experience unwanted or distressing memories, remind yourself that they are just memories, and talk about them with someone you trust. If you feel that the trauma is happening again, which is known as a flashback, keep your eyes open and remind yourself where you presently are and that the trauma happened in the past.
Although VA and DoD are working to put an end to MST, it is an ongoing problem that affects a large percentage of women and men who proudly serve in the armed forces. For information on how to access free VA services and to determine your eligibility in MST benefits, visit http://www.mentalhealth.va.gov/msthome.asp.
Military sexual trauma (MST) refers to experiences of sexual assault or repeated, threatening sexual harassment experienced while on federal active duty or active duty for training. About 1 in 4 women and 1 in 100 men have reported MST to their VA doctors. However, these numbers do not account for those who have not sought health care for their MST experience or who have sought care for MST outside the VA.
Military sexual trauma is:
Military sexual trauma is the term VA uses to refer to sexual assault or sexual harassment that occurred while the veteran was in the military. A victim of MST may have been:
- Involved in sexual activity against his or her will, by physical force or nonphysical pressure. Nonphysical pressure includes threats of negative consequences for refusing to be sexually cooperative, or indirect promises of faster promotions or better treatment in exchange for sex.
- Unable to consent to sexual activities. This includes intoxication by alcohol or other substances.
Other experiences include sexual touching or grabbing, threatening or offensive remarks about a person’s body or sexual activities, as well as threatening and unwelcome sexual advances.
How do I know if I’m at risk?
Military sexual trauma can occur on or off base and while a service member—man or woman—is on or off duty. Those who commit sexual assault or sexual harassment can be men or women, military personnel or civilians, commanding officers or subordinates, strangers, friends, spouses, or intimate partners.
When do I need medical attention?
The VA reports sexual assault is more likely to result in symptoms of PTSD (posttraumatic stress disorder) than are most other types of trauma, including combat. You should seek medical attention from your primary care doctor, a mental health professional, or your VA facility’s MST Coordinator following a MST experience, especially if you experience any of the following symptoms:
- Depression
- Suicidal thoughts
- Feeling angry or irritable most of the time
- Strong emotional reactions
- Feeling emotionally numb
- Trouble falling or staying asleep
- Nightmares
- Trouble focusing
- Difficulty remembering things
- Substance abuse
- Trouble feeling safe or trusting others
- Feeling isolated or disconnected from others
- Headaches
- Gastrointestinal difficulties
- Sexual dysfunction
- Chronic pain
- Chronic fatigue
- Weight or eating problems
Survivors who are not formally diagnosed may still struggle in certain situations with emotional reactions, memories related to their experiences of MST, or other interpersonal issues.
How is MST treated?
Because MST is an experience, not a diagnosis, treatment needs may vary from patient to patient. However, VA provides free, confidential counseling and treatment to male and female veterans for mental and physical health conditions related to experiences of MST. It is important to note that treatment is available even if the MST incident was not reported at the time it happened.
Your doctor may recommend individual therapy, group therapy, or medication, depending on your symptoms. Therapies that may be used to treat MST include:
- Cognitive behavioral therapy. The main goal of this therapy is to help you change your thought patterns, which will help you change the emotions and behavior connected with the MST experience. A counselor might encourage you to reimagine your trauma repeatedly under controlled conditions—an approach called exposure therapy—as a way of learning to cope.
- Stress inoculation (in-ock-you-lay-shun) training. This therapy involves combining stress management strategies with stress-relieving techniques, such as muscle relaxation, breathing retraining, self-dialogue, and thought stopping.
- Group therapy. This therapy enables you to discuss your trauma with others who have had similar experiences.
- Inpatient therapy. Nationwide, there are programs that offer specialized sexual trauma treatment for veterans who need more intense treatment and support, including that for severe depression or substance abuse.
- Medication. If your MST experience resulted in PTSD, your doctor may prescribe medication to help control symptoms of anxiety or to help you sleep. Your doctor will monitor you closely for any possible adverse effects of these medications. It is also possible that an STD (sexually transmitted disease) was passed during the trauma, so your doctor may choose to screen you for an STD. If the test comes back positive, your doctor will prescribe the appropriate drug for treatment.
Services are designed to help veterans at all stages of their recovery, whether that is focusing on strategies for coping with emotions and memories or, for veterans who are ready, talking about their MST experiences in depth.
What can I do to cope?
When trauma survivors take direct action to cope with their stress reactions, they put themselves in a position of power. This is called active coping, which involves accepting the impact the trauma had on your life and taking direct action to make improvements.
It is important to remember that recovery is a process and takes time. Healing from trauma, including MST, does not happen all at once, and healing does not mean that you must forget the experience. Instead, it means you have less pain and fewer bad feelings when you think about the experience, and any associated symptoms will over time bother you less.
Discussing your trauma with other survivors in a controlled setting can help you learn that you are not alone or weak. In addition, surrounding yourself with people you can talk to about your MST experience may help you feel more understood. You may even choose to distract yourself with positive recreational or work activities.
When you experience unwanted or distressing memories, remind yourself that they are just memories, and talk about them with someone you trust. If you feel that the trauma is happening again, which is known as a flashback, keep your eyes open and remind yourself where you presently are and that the trauma happened in the past.
Although VA and DoD are working to put an end to MST, it is an ongoing problem that affects a large percentage of women and men who proudly serve in the armed forces. For information on how to access free VA services and to determine your eligibility in MST benefits, visit http://www.mentalhealth.va.gov/msthome.asp.
Potential treatment method induces severe side effects

Previous studies have shown that inhibiting the activity of the Malt1 protein can kill lymphoma cells.
Now, research published in Cell Reports has revealed that it also causes the immune system to malfunction.
Malt1 carries out a variety of tasks in lymphocytes, including acting as a protease that breaks down messenger substances and controls their quantity.
Until now, researchers were unsure about the role the protease function plays in immune cell development.
Several years ago, Jürgen Ruland, PhD, of Technische Universität München in Germany, and his colleagues turned their attention to this question.
Via cell culture experiments, the researchers found that blocking the protease function of Malt1 kills lymphoma cells. The team decided to test this strategy in an animal model to shed light on the exact function of Malt1 protease.
“It’s only possible to study complex interactions in the immune system, which comprises a finely orchestrated interplay of various cell types, in an intact organism, not in cell cultures,” Dr Ruland noted. “The processes are too complex to recreate in cells outside the body.”
The mice the researchers used were genetically modified so their Malt1 protein could no longer act as a protease but was still able to carry out all of its other functions.
The team was surprised to find that these mice developed severe signs of inflammation. Moreover, the immune system attacked and destroyed key neurons that coordinate movements.
The researchers were able to explain how this serious malfunction occurred and, in doing so, discovered an unexpected function of Malt1.
They found that, in the absence of the protease function, the mice were unable to produce regulatory T cells, and this caused their immune responses to spin
out of control.
The team also found that normal lymphocytes can be activated without the protease function of Malt1, but they release messenger substances uncontrollably, which causes inflammation.
“Our study showed that Malt1 protease is surprisingly important for the development of regulatory T cells and for damping the immune response in general,” Dr Ruland said. “Since the blockade of the protease function in the organism produces undesirable effects, new alternatives should urgently be sought for the treatment of lymphoma.” ![]()

Previous studies have shown that inhibiting the activity of the Malt1 protein can kill lymphoma cells.
Now, research published in Cell Reports has revealed that it also causes the immune system to malfunction.
Malt1 carries out a variety of tasks in lymphocytes, including acting as a protease that breaks down messenger substances and controls their quantity.
Until now, researchers were unsure about the role the protease function plays in immune cell development.
Several years ago, Jürgen Ruland, PhD, of Technische Universität München in Germany, and his colleagues turned their attention to this question.
Via cell culture experiments, the researchers found that blocking the protease function of Malt1 kills lymphoma cells. The team decided to test this strategy in an animal model to shed light on the exact function of Malt1 protease.
“It’s only possible to study complex interactions in the immune system, which comprises a finely orchestrated interplay of various cell types, in an intact organism, not in cell cultures,” Dr Ruland noted. “The processes are too complex to recreate in cells outside the body.”
The mice the researchers used were genetically modified so their Malt1 protein could no longer act as a protease but was still able to carry out all of its other functions.
The team was surprised to find that these mice developed severe signs of inflammation. Moreover, the immune system attacked and destroyed key neurons that coordinate movements.
The researchers were able to explain how this serious malfunction occurred and, in doing so, discovered an unexpected function of Malt1.
They found that, in the absence of the protease function, the mice were unable to produce regulatory T cells, and this caused their immune responses to spin
out of control.
The team also found that normal lymphocytes can be activated without the protease function of Malt1, but they release messenger substances uncontrollably, which causes inflammation.
“Our study showed that Malt1 protease is surprisingly important for the development of regulatory T cells and for damping the immune response in general,” Dr Ruland said. “Since the blockade of the protease function in the organism produces undesirable effects, new alternatives should urgently be sought for the treatment of lymphoma.” ![]()

Previous studies have shown that inhibiting the activity of the Malt1 protein can kill lymphoma cells.
Now, research published in Cell Reports has revealed that it also causes the immune system to malfunction.
Malt1 carries out a variety of tasks in lymphocytes, including acting as a protease that breaks down messenger substances and controls their quantity.
Until now, researchers were unsure about the role the protease function plays in immune cell development.
Several years ago, Jürgen Ruland, PhD, of Technische Universität München in Germany, and his colleagues turned their attention to this question.
Via cell culture experiments, the researchers found that blocking the protease function of Malt1 kills lymphoma cells. The team decided to test this strategy in an animal model to shed light on the exact function of Malt1 protease.
“It’s only possible to study complex interactions in the immune system, which comprises a finely orchestrated interplay of various cell types, in an intact organism, not in cell cultures,” Dr Ruland noted. “The processes are too complex to recreate in cells outside the body.”
The mice the researchers used were genetically modified so their Malt1 protein could no longer act as a protease but was still able to carry out all of its other functions.
The team was surprised to find that these mice developed severe signs of inflammation. Moreover, the immune system attacked and destroyed key neurons that coordinate movements.
The researchers were able to explain how this serious malfunction occurred and, in doing so, discovered an unexpected function of Malt1.
They found that, in the absence of the protease function, the mice were unable to produce regulatory T cells, and this caused their immune responses to spin
out of control.
The team also found that normal lymphocytes can be activated without the protease function of Malt1, but they release messenger substances uncontrollably, which causes inflammation.
“Our study showed that Malt1 protease is surprisingly important for the development of regulatory T cells and for damping the immune response in general,” Dr Ruland said. “Since the blockade of the protease function in the organism produces undesirable effects, new alternatives should urgently be sought for the treatment of lymphoma.” ![]()
Reintroducing heparin in patients with a history of HIT
Two tests used to measure antibodies in patients with a history of heparin-induced thrombocytopenia (HIT) can produce radically different results, new research shows.
The anti-PF4/heparin IgG-specific enzyme-immunoassay can show that HIT antibody levels are high, while the functional platelet serotonin-release assay indicates that levels are low.
And researchers said the functional assay’s results may be the better indicator of a patient’s readiness for re-exposure to heparin.
Theodore Warkentin, MD, of McMaster University in Hamilton, Ontario, Canada, and his colleagues expressed this viewpoint and detailed the research supporting it in Blood.
When patients with a history of HIT require urgent heart surgery, physicians must test for the presence of HIT antibodies to determine whether the patient can be re-exposed to heparin during the procedure.
Hematologists use two types of tests to measure HIT antibodies—a functional platelet serotonin-release assay and an anti-PF4/heparin IgG-specific enzyme-immunoassay. If the more widely used immunoassay indicates the presence of HIT antibodies in a patient, surgery is usually delayed or plasma exchange is performed to lower the antibodies.
Practitioners have historically understood the two assays to provide similar conclusions, but a case report suggested otherwise.
A 76-year-old female with kidney cancer and previous HIT required urgent cardiac surgery to remove a tumor that had spread to her heart. After both her initial functional and immunoassays indicated the presence of HIT antibodies, her doctors deemed her ineligible for surgery.
But after repeated plasma exchange, the researchers performed both the functional and immunoassays on the patient again, and, this time, they observed strikingly different results.
“We were surprised to see that levels of HIT antibodies in this patient fell very quickly according to the functional assay, yet the antibodies detected by the immunoassay remained high,” Dr Warkentin said.
“This suggested to us that, while physicians in many situations may be waiting for the immunoassay to indicate lower antibody levels, patients in urgent need of heart surgery may be ready much earlier than the results suggest.”
To better understand the dissociation between the results of the two tests, Dr Warkentin’s team developed a model comparing functional and immunoassay results among 15 HIT blood samples. The samples were sequentially diluted and tested with both assays to mimic the effects of repeated plasma exchange.
The researchers observed that HIT antibody levels as measured by the functional assay decreased rather quickly, while the immunoassay continued to indicate high levels.
This suggests the sensitivity of the immunoassay may provide an overly conservative estimate of HIT antibody levels and their clinical relevance. The analysis illustrates how quickly platelet-activating properties can decline in a patient, either naturally or by using plasma exchange.
The observations also support the use of repeated plasma exchange as a therapeutic strategy prior to planned heparin re-exposure among patients with a recent HIT episode who require urgent cardiac surgery.
“Based on these findings, physicians should consider utilizing both of these tests when preparing a patient with a history of HIT for urgent heart surgery, considering the functional assay result as the stronger indicator of a patient’s readiness,” Dr Warkentin said.
“For these patients, [plasma exchange] can be a useful option to help rapidly reduce their remaining HIT antibody levels, minimize their risk of developing clots, and get them into the operating room sooner.” ![]()

Two tests used to measure antibodies in patients with a history of heparin-induced thrombocytopenia (HIT) can produce radically different results, new research shows.
The anti-PF4/heparin IgG-specific enzyme-immunoassay can show that HIT antibody levels are high, while the functional platelet serotonin-release assay indicates that levels are low.
And researchers said the functional assay’s results may be the better indicator of a patient’s readiness for re-exposure to heparin.
Theodore Warkentin, MD, of McMaster University in Hamilton, Ontario, Canada, and his colleagues expressed this viewpoint and detailed the research supporting it in Blood.
When patients with a history of HIT require urgent heart surgery, physicians must test for the presence of HIT antibodies to determine whether the patient can be re-exposed to heparin during the procedure.
Hematologists use two types of tests to measure HIT antibodies—a functional platelet serotonin-release assay and an anti-PF4/heparin IgG-specific enzyme-immunoassay. If the more widely used immunoassay indicates the presence of HIT antibodies in a patient, surgery is usually delayed or plasma exchange is performed to lower the antibodies.
Practitioners have historically understood the two assays to provide similar conclusions, but a case report suggested otherwise.
A 76-year-old female with kidney cancer and previous HIT required urgent cardiac surgery to remove a tumor that had spread to her heart. After both her initial functional and immunoassays indicated the presence of HIT antibodies, her doctors deemed her ineligible for surgery.
But after repeated plasma exchange, the researchers performed both the functional and immunoassays on the patient again, and, this time, they observed strikingly different results.
“We were surprised to see that levels of HIT antibodies in this patient fell very quickly according to the functional assay, yet the antibodies detected by the immunoassay remained high,” Dr Warkentin said.
“This suggested to us that, while physicians in many situations may be waiting for the immunoassay to indicate lower antibody levels, patients in urgent need of heart surgery may be ready much earlier than the results suggest.”
To better understand the dissociation between the results of the two tests, Dr Warkentin’s team developed a model comparing functional and immunoassay results among 15 HIT blood samples. The samples were sequentially diluted and tested with both assays to mimic the effects of repeated plasma exchange.
The researchers observed that HIT antibody levels as measured by the functional assay decreased rather quickly, while the immunoassay continued to indicate high levels.
This suggests the sensitivity of the immunoassay may provide an overly conservative estimate of HIT antibody levels and their clinical relevance. The analysis illustrates how quickly platelet-activating properties can decline in a patient, either naturally or by using plasma exchange.
The observations also support the use of repeated plasma exchange as a therapeutic strategy prior to planned heparin re-exposure among patients with a recent HIT episode who require urgent cardiac surgery.
“Based on these findings, physicians should consider utilizing both of these tests when preparing a patient with a history of HIT for urgent heart surgery, considering the functional assay result as the stronger indicator of a patient’s readiness,” Dr Warkentin said.
“For these patients, [plasma exchange] can be a useful option to help rapidly reduce their remaining HIT antibody levels, minimize their risk of developing clots, and get them into the operating room sooner.” ![]()

Two tests used to measure antibodies in patients with a history of heparin-induced thrombocytopenia (HIT) can produce radically different results, new research shows.
The anti-PF4/heparin IgG-specific enzyme-immunoassay can show that HIT antibody levels are high, while the functional platelet serotonin-release assay indicates that levels are low.
And researchers said the functional assay’s results may be the better indicator of a patient’s readiness for re-exposure to heparin.
Theodore Warkentin, MD, of McMaster University in Hamilton, Ontario, Canada, and his colleagues expressed this viewpoint and detailed the research supporting it in Blood.
When patients with a history of HIT require urgent heart surgery, physicians must test for the presence of HIT antibodies to determine whether the patient can be re-exposed to heparin during the procedure.
Hematologists use two types of tests to measure HIT antibodies—a functional platelet serotonin-release assay and an anti-PF4/heparin IgG-specific enzyme-immunoassay. If the more widely used immunoassay indicates the presence of HIT antibodies in a patient, surgery is usually delayed or plasma exchange is performed to lower the antibodies.
Practitioners have historically understood the two assays to provide similar conclusions, but a case report suggested otherwise.
A 76-year-old female with kidney cancer and previous HIT required urgent cardiac surgery to remove a tumor that had spread to her heart. After both her initial functional and immunoassays indicated the presence of HIT antibodies, her doctors deemed her ineligible for surgery.
But after repeated plasma exchange, the researchers performed both the functional and immunoassays on the patient again, and, this time, they observed strikingly different results.
“We were surprised to see that levels of HIT antibodies in this patient fell very quickly according to the functional assay, yet the antibodies detected by the immunoassay remained high,” Dr Warkentin said.
“This suggested to us that, while physicians in many situations may be waiting for the immunoassay to indicate lower antibody levels, patients in urgent need of heart surgery may be ready much earlier than the results suggest.”
To better understand the dissociation between the results of the two tests, Dr Warkentin’s team developed a model comparing functional and immunoassay results among 15 HIT blood samples. The samples were sequentially diluted and tested with both assays to mimic the effects of repeated plasma exchange.
The researchers observed that HIT antibody levels as measured by the functional assay decreased rather quickly, while the immunoassay continued to indicate high levels.
This suggests the sensitivity of the immunoassay may provide an overly conservative estimate of HIT antibody levels and their clinical relevance. The analysis illustrates how quickly platelet-activating properties can decline in a patient, either naturally or by using plasma exchange.
The observations also support the use of repeated plasma exchange as a therapeutic strategy prior to planned heparin re-exposure among patients with a recent HIT episode who require urgent cardiac surgery.
“Based on these findings, physicians should consider utilizing both of these tests when preparing a patient with a history of HIT for urgent heart surgery, considering the functional assay result as the stronger indicator of a patient’s readiness,” Dr Warkentin said.
“For these patients, [plasma exchange] can be a useful option to help rapidly reduce their remaining HIT antibody levels, minimize their risk of developing clots, and get them into the operating room sooner.” ![]()
FDA grants CAR T-cell therapy orphan designation

The US Food and Drug Administration (FDA) has granted orphan drug designation for the chimeric antigen receptor (CAR) T-cell therapy JCAR015 to treat acute lymphoblastic leukemia (ALL).
The designation will provide the product’s developer, Juno Therapeutics, with multiple benefits, including the availability of grant money, certain tax credits, and 7 years of market exclusivity, as well as the possibility of an expedited regulatory process.
JCAR015 consists of autologous T cells expressing a CD19-specific, CD28/CD3z CAR. The treatment has shown promise in an ongoing phase 1 trial of patients with B-cell ALL.
Initial results from this study were published in Science Translational Medicine last year and in February. Updated results were presented at the AACR Annual Meeting in April.
At that point, the researchers had enrolled 22 adult patients with relapsed or refractory B-ALL who were minimal residual disease-positive or were in first complete remission at enrollment. Patients in complete remission were monitored and only received JCAR015 if they relapsed.
The remaining patients received re-induction chemotherapy (physician’s choice), followed by an infusion of JCAR015. After treatment, patients could receive allogeneic transplant, a different salvage therapy, or monitoring.
Eighty-two percent of patients achieved a complete response to JCAR015. The average time to complete response was about 24.5 days.
Twelve of the responders were eligible for transplant. Of the 8 patients who ultimately underwent transplant and survived, 1 relapsed, but the rest remained in remission.
Ten patients had died at the time of the AACR presentation. Six deaths were a result of disease relapse or progression, and 2 patients died of complications from stem cell transplant.
The 2 remaining deaths prompted a temporary suspension of enrollment in this trial.
Those deaths were related to complications from cytokine release syndrome. One patient died of cardiovascular disease, and the other died following “persistent seizure activity.”
So researchers at the Memorial Sloan-Kettering Cancer Center, where the trial is being conducted, reviewed these cases.
The results prompted them to amend trial enrollment criteria and dosing recommendations. Now, patients with cardiac disease are ineligible to receive JCAR015.
And the T-cell dose a patient receives will depend on the extent of his or her disease. The hope is that this will reduce the risk of cytokine release syndrome and any resulting seizures.
The researchers also noted that the monoclonal antibody tocilizumab has proven effective in treating cytokine release syndrome.
In addition to this trial, JCAR015 is under investigation in another phase 1 trial of patients with relapsed and refractory non-Hodgkin lymphoma. ![]()

The US Food and Drug Administration (FDA) has granted orphan drug designation for the chimeric antigen receptor (CAR) T-cell therapy JCAR015 to treat acute lymphoblastic leukemia (ALL).
The designation will provide the product’s developer, Juno Therapeutics, with multiple benefits, including the availability of grant money, certain tax credits, and 7 years of market exclusivity, as well as the possibility of an expedited regulatory process.
JCAR015 consists of autologous T cells expressing a CD19-specific, CD28/CD3z CAR. The treatment has shown promise in an ongoing phase 1 trial of patients with B-cell ALL.
Initial results from this study were published in Science Translational Medicine last year and in February. Updated results were presented at the AACR Annual Meeting in April.
At that point, the researchers had enrolled 22 adult patients with relapsed or refractory B-ALL who were minimal residual disease-positive or were in first complete remission at enrollment. Patients in complete remission were monitored and only received JCAR015 if they relapsed.
The remaining patients received re-induction chemotherapy (physician’s choice), followed by an infusion of JCAR015. After treatment, patients could receive allogeneic transplant, a different salvage therapy, or monitoring.
Eighty-two percent of patients achieved a complete response to JCAR015. The average time to complete response was about 24.5 days.
Twelve of the responders were eligible for transplant. Of the 8 patients who ultimately underwent transplant and survived, 1 relapsed, but the rest remained in remission.
Ten patients had died at the time of the AACR presentation. Six deaths were a result of disease relapse or progression, and 2 patients died of complications from stem cell transplant.
The 2 remaining deaths prompted a temporary suspension of enrollment in this trial.
Those deaths were related to complications from cytokine release syndrome. One patient died of cardiovascular disease, and the other died following “persistent seizure activity.”
So researchers at the Memorial Sloan-Kettering Cancer Center, where the trial is being conducted, reviewed these cases.
The results prompted them to amend trial enrollment criteria and dosing recommendations. Now, patients with cardiac disease are ineligible to receive JCAR015.
And the T-cell dose a patient receives will depend on the extent of his or her disease. The hope is that this will reduce the risk of cytokine release syndrome and any resulting seizures.
The researchers also noted that the monoclonal antibody tocilizumab has proven effective in treating cytokine release syndrome.
In addition to this trial, JCAR015 is under investigation in another phase 1 trial of patients with relapsed and refractory non-Hodgkin lymphoma. ![]()

The US Food and Drug Administration (FDA) has granted orphan drug designation for the chimeric antigen receptor (CAR) T-cell therapy JCAR015 to treat acute lymphoblastic leukemia (ALL).
The designation will provide the product’s developer, Juno Therapeutics, with multiple benefits, including the availability of grant money, certain tax credits, and 7 years of market exclusivity, as well as the possibility of an expedited regulatory process.
JCAR015 consists of autologous T cells expressing a CD19-specific, CD28/CD3z CAR. The treatment has shown promise in an ongoing phase 1 trial of patients with B-cell ALL.
Initial results from this study were published in Science Translational Medicine last year and in February. Updated results were presented at the AACR Annual Meeting in April.
At that point, the researchers had enrolled 22 adult patients with relapsed or refractory B-ALL who were minimal residual disease-positive or were in first complete remission at enrollment. Patients in complete remission were monitored and only received JCAR015 if they relapsed.
The remaining patients received re-induction chemotherapy (physician’s choice), followed by an infusion of JCAR015. After treatment, patients could receive allogeneic transplant, a different salvage therapy, or monitoring.
Eighty-two percent of patients achieved a complete response to JCAR015. The average time to complete response was about 24.5 days.
Twelve of the responders were eligible for transplant. Of the 8 patients who ultimately underwent transplant and survived, 1 relapsed, but the rest remained in remission.
Ten patients had died at the time of the AACR presentation. Six deaths were a result of disease relapse or progression, and 2 patients died of complications from stem cell transplant.
The 2 remaining deaths prompted a temporary suspension of enrollment in this trial.
Those deaths were related to complications from cytokine release syndrome. One patient died of cardiovascular disease, and the other died following “persistent seizure activity.”
So researchers at the Memorial Sloan-Kettering Cancer Center, where the trial is being conducted, reviewed these cases.
The results prompted them to amend trial enrollment criteria and dosing recommendations. Now, patients with cardiac disease are ineligible to receive JCAR015.
And the T-cell dose a patient receives will depend on the extent of his or her disease. The hope is that this will reduce the risk of cytokine release syndrome and any resulting seizures.
The researchers also noted that the monoclonal antibody tocilizumab has proven effective in treating cytokine release syndrome.
In addition to this trial, JCAR015 is under investigation in another phase 1 trial of patients with relapsed and refractory non-Hodgkin lymphoma. ![]()