User login
Statins post PCI: Moderate intensity plus ezetimibe may be preferable
, suggests a “real-world” cohort study that is consistent with trial evidence.
In the observational study with more than 273,000 patients who received percutaneous coronary intervention (PCI) with drug-eluting stents (DES), risk for a broad composite clinical primary endpoint fell by one-fourth (P < .001) among those put on the two-drug regimen with a moderate-intensity statin, compared with those getting a high-intensity statin alone.
The dual-agent approach was also associated with a 15% drop in statin discontinuation and a 20% reduced risk for new-onset diabetes requiring medication (P < .001 for both benefits), reported investigators in the Journal of the American College of Cardiology.
The study’s primary endpoint – a composite of cardiovascular (CV) death, myocardial infarction (MI), coronary revascularization, heart failure (HF) hospitalization, or nonfatal stroke at 3 years – replicated that of the randomized RACING trial conducted by many of the same researchers and published about a year ago in The Lancet.
RACING demonstrated that ezetimibe plus a moderate-intensity statin could be as effective as a high-intensity statin in patients with CV disease, “but have fewer side effects and better compliance,” Myeong-Ki Hong, MD, PhD, Severance Hospital, Yonsei University, Seoul, South Korea, said in an interview.
Dr. Hong is senior author on the current observational study based on the CONNECT-DES registry, which compared rosuvastatin 10 mg/day plus ezetimibe 10 mg/day – used in RACING – with rosuvastatin 20 mg/day in a nationwide cohort of 72,050 patients.
“As we know, populations who are enrolled in randomized studies do not sufficiently represent real patients in practice,” he observed, “so we wanted to evaluate the generalizability of the RACING results in daily clinical practice.”
Deepak L. Bhatt, MD, said he likes studies that look at whether clinical trial results “play out in the real world,” as this one did. “They have largely replicated the results of the RACING trial,” suggesting the approach using a moderate-intensity statin “is the way to go,” Dr. Bhatt of Mount Sinai Health System, New York, who was not affiliated with the current report, said in an interview. “In fact, the moderate-intensity combination regimen was actually better in this study.”
He said the observed reduction in new-onset diabetes with the moderate-intensity statin approach is also important. “There is a link between high-dose statins and diabetes. So, if given the choice, if you can get the benefits from a cardiovascular perspective with a lower risk of diabetes, it makes sense to use the combination therapy.”
Dr. Bhatt said he had been using high-intensity statin monotherapy in his high-risk patients, but RACING made him reconsider the value of moderate-dose statin combination therapy. “Going with lower doses of two drugs instead of high doses of one drug minimizes side effects and, in some cases, can even enhance efficacy – so this is not an unreasonable paradigm.”
In the current cohort study of patients prescribed rosuvastatin after DES implantation, 10,794 received rosuvastatin 10 mg/day plus ezetimibe 10 mg/day, and 61,256 were put on rosuvastatin 20 mg/day.
Hazard ratio risk reductions with the dual-agent lipid-lowering therapy approach, compared with high-intensity statin monotherapy, were more favorable for the primary composite clinical endpoint and important secondary events:
- HR, 0.75; 95% confidence interval, 0.70-0.79; P < .001) for CV death, MI, coronary artery revascularization, HF, or stroke at 3 years.
- HR, 0.85; 95% CI, 0.78-0.94; P = .001) for statin discontinuation.
- HR, 0.80; 95% CI, 0.72-0.88; P < .001) for new-onset diabetes requiring medication.
But HRs for rhabdomyolysis, cholecystectomy, or a new cancer diagnosis did not indicate significant differences between the two groups.
“Now that there is evidence to support the favorable clinical outcomes of combination lipid-lowering therapy with moderate-intensity statin plus ezetimibe” for secondary prevention from both RACING and a study reflecting daily clinical practice, Dr. Hong said, “physicians may feel more comfortable with this approach.”
The registry analysis “is remarkable not only for validating the results of the RACING trial in routine clinical practice in a high-risk secondary prevention population, but also for its innovative methodology,” states an accompanying editorial by Ori Ben-Yehuda, MD, Sulpizio Cardiovascular Center, University of California, San Diego.
Use of such a large single-payer database in their study “affords even greater external validity to the findings, complementing the internal validity of the randomized RACING trial,” Dr. Ben-Yehuda writes.
The rationale for combination therapy is strong, but additional data would be helpful, particularly for informing guidelines, he continues. “A pragmatic trial randomizing a broad racial and ethnic group of patients to low-dose statin,” such as a starting dose of 10 mg/day atorvastatin or 5 mg/day rosuvastatin “plus ezetimibe vs. high-intensity statin alone would provide much needed data to help guide lipid-lowering therapy for millions of patients and hopefully increase persistence on therapy.”
The study was supported by the Cardiovascular Research Center, Seoul, South Korea. Dr. Hong and Dr. Ben-Yehuda have disclosed no relevant financial relationships. Dr. Bhatt has previously disclosed grants and/or personal fees from many companies; personal fees from WebMD and other publications or organizations; and having other relationships with Medscape Cardiology and other publications or organizations.
A version of this article appeared on Medscape.com.
, suggests a “real-world” cohort study that is consistent with trial evidence.
In the observational study with more than 273,000 patients who received percutaneous coronary intervention (PCI) with drug-eluting stents (DES), risk for a broad composite clinical primary endpoint fell by one-fourth (P < .001) among those put on the two-drug regimen with a moderate-intensity statin, compared with those getting a high-intensity statin alone.
The dual-agent approach was also associated with a 15% drop in statin discontinuation and a 20% reduced risk for new-onset diabetes requiring medication (P < .001 for both benefits), reported investigators in the Journal of the American College of Cardiology.
The study’s primary endpoint – a composite of cardiovascular (CV) death, myocardial infarction (MI), coronary revascularization, heart failure (HF) hospitalization, or nonfatal stroke at 3 years – replicated that of the randomized RACING trial conducted by many of the same researchers and published about a year ago in The Lancet.
RACING demonstrated that ezetimibe plus a moderate-intensity statin could be as effective as a high-intensity statin in patients with CV disease, “but have fewer side effects and better compliance,” Myeong-Ki Hong, MD, PhD, Severance Hospital, Yonsei University, Seoul, South Korea, said in an interview.
Dr. Hong is senior author on the current observational study based on the CONNECT-DES registry, which compared rosuvastatin 10 mg/day plus ezetimibe 10 mg/day – used in RACING – with rosuvastatin 20 mg/day in a nationwide cohort of 72,050 patients.
“As we know, populations who are enrolled in randomized studies do not sufficiently represent real patients in practice,” he observed, “so we wanted to evaluate the generalizability of the RACING results in daily clinical practice.”
Deepak L. Bhatt, MD, said he likes studies that look at whether clinical trial results “play out in the real world,” as this one did. “They have largely replicated the results of the RACING trial,” suggesting the approach using a moderate-intensity statin “is the way to go,” Dr. Bhatt of Mount Sinai Health System, New York, who was not affiliated with the current report, said in an interview. “In fact, the moderate-intensity combination regimen was actually better in this study.”
He said the observed reduction in new-onset diabetes with the moderate-intensity statin approach is also important. “There is a link between high-dose statins and diabetes. So, if given the choice, if you can get the benefits from a cardiovascular perspective with a lower risk of diabetes, it makes sense to use the combination therapy.”
Dr. Bhatt said he had been using high-intensity statin monotherapy in his high-risk patients, but RACING made him reconsider the value of moderate-dose statin combination therapy. “Going with lower doses of two drugs instead of high doses of one drug minimizes side effects and, in some cases, can even enhance efficacy – so this is not an unreasonable paradigm.”
In the current cohort study of patients prescribed rosuvastatin after DES implantation, 10,794 received rosuvastatin 10 mg/day plus ezetimibe 10 mg/day, and 61,256 were put on rosuvastatin 20 mg/day.
Hazard ratio risk reductions with the dual-agent lipid-lowering therapy approach, compared with high-intensity statin monotherapy, were more favorable for the primary composite clinical endpoint and important secondary events:
- HR, 0.75; 95% confidence interval, 0.70-0.79; P < .001) for CV death, MI, coronary artery revascularization, HF, or stroke at 3 years.
- HR, 0.85; 95% CI, 0.78-0.94; P = .001) for statin discontinuation.
- HR, 0.80; 95% CI, 0.72-0.88; P < .001) for new-onset diabetes requiring medication.
But HRs for rhabdomyolysis, cholecystectomy, or a new cancer diagnosis did not indicate significant differences between the two groups.
“Now that there is evidence to support the favorable clinical outcomes of combination lipid-lowering therapy with moderate-intensity statin plus ezetimibe” for secondary prevention from both RACING and a study reflecting daily clinical practice, Dr. Hong said, “physicians may feel more comfortable with this approach.”
The registry analysis “is remarkable not only for validating the results of the RACING trial in routine clinical practice in a high-risk secondary prevention population, but also for its innovative methodology,” states an accompanying editorial by Ori Ben-Yehuda, MD, Sulpizio Cardiovascular Center, University of California, San Diego.
Use of such a large single-payer database in their study “affords even greater external validity to the findings, complementing the internal validity of the randomized RACING trial,” Dr. Ben-Yehuda writes.
The rationale for combination therapy is strong, but additional data would be helpful, particularly for informing guidelines, he continues. “A pragmatic trial randomizing a broad racial and ethnic group of patients to low-dose statin,” such as a starting dose of 10 mg/day atorvastatin or 5 mg/day rosuvastatin “plus ezetimibe vs. high-intensity statin alone would provide much needed data to help guide lipid-lowering therapy for millions of patients and hopefully increase persistence on therapy.”
The study was supported by the Cardiovascular Research Center, Seoul, South Korea. Dr. Hong and Dr. Ben-Yehuda have disclosed no relevant financial relationships. Dr. Bhatt has previously disclosed grants and/or personal fees from many companies; personal fees from WebMD and other publications or organizations; and having other relationships with Medscape Cardiology and other publications or organizations.
A version of this article appeared on Medscape.com.
, suggests a “real-world” cohort study that is consistent with trial evidence.
In the observational study with more than 273,000 patients who received percutaneous coronary intervention (PCI) with drug-eluting stents (DES), risk for a broad composite clinical primary endpoint fell by one-fourth (P < .001) among those put on the two-drug regimen with a moderate-intensity statin, compared with those getting a high-intensity statin alone.
The dual-agent approach was also associated with a 15% drop in statin discontinuation and a 20% reduced risk for new-onset diabetes requiring medication (P < .001 for both benefits), reported investigators in the Journal of the American College of Cardiology.
The study’s primary endpoint – a composite of cardiovascular (CV) death, myocardial infarction (MI), coronary revascularization, heart failure (HF) hospitalization, or nonfatal stroke at 3 years – replicated that of the randomized RACING trial conducted by many of the same researchers and published about a year ago in The Lancet.
RACING demonstrated that ezetimibe plus a moderate-intensity statin could be as effective as a high-intensity statin in patients with CV disease, “but have fewer side effects and better compliance,” Myeong-Ki Hong, MD, PhD, Severance Hospital, Yonsei University, Seoul, South Korea, said in an interview.
Dr. Hong is senior author on the current observational study based on the CONNECT-DES registry, which compared rosuvastatin 10 mg/day plus ezetimibe 10 mg/day – used in RACING – with rosuvastatin 20 mg/day in a nationwide cohort of 72,050 patients.
“As we know, populations who are enrolled in randomized studies do not sufficiently represent real patients in practice,” he observed, “so we wanted to evaluate the generalizability of the RACING results in daily clinical practice.”
Deepak L. Bhatt, MD, said he likes studies that look at whether clinical trial results “play out in the real world,” as this one did. “They have largely replicated the results of the RACING trial,” suggesting the approach using a moderate-intensity statin “is the way to go,” Dr. Bhatt of Mount Sinai Health System, New York, who was not affiliated with the current report, said in an interview. “In fact, the moderate-intensity combination regimen was actually better in this study.”
He said the observed reduction in new-onset diabetes with the moderate-intensity statin approach is also important. “There is a link between high-dose statins and diabetes. So, if given the choice, if you can get the benefits from a cardiovascular perspective with a lower risk of diabetes, it makes sense to use the combination therapy.”
Dr. Bhatt said he had been using high-intensity statin monotherapy in his high-risk patients, but RACING made him reconsider the value of moderate-dose statin combination therapy. “Going with lower doses of two drugs instead of high doses of one drug minimizes side effects and, in some cases, can even enhance efficacy – so this is not an unreasonable paradigm.”
In the current cohort study of patients prescribed rosuvastatin after DES implantation, 10,794 received rosuvastatin 10 mg/day plus ezetimibe 10 mg/day, and 61,256 were put on rosuvastatin 20 mg/day.
Hazard ratio risk reductions with the dual-agent lipid-lowering therapy approach, compared with high-intensity statin monotherapy, were more favorable for the primary composite clinical endpoint and important secondary events:
- HR, 0.75; 95% confidence interval, 0.70-0.79; P < .001) for CV death, MI, coronary artery revascularization, HF, or stroke at 3 years.
- HR, 0.85; 95% CI, 0.78-0.94; P = .001) for statin discontinuation.
- HR, 0.80; 95% CI, 0.72-0.88; P < .001) for new-onset diabetes requiring medication.
But HRs for rhabdomyolysis, cholecystectomy, or a new cancer diagnosis did not indicate significant differences between the two groups.
“Now that there is evidence to support the favorable clinical outcomes of combination lipid-lowering therapy with moderate-intensity statin plus ezetimibe” for secondary prevention from both RACING and a study reflecting daily clinical practice, Dr. Hong said, “physicians may feel more comfortable with this approach.”
The registry analysis “is remarkable not only for validating the results of the RACING trial in routine clinical practice in a high-risk secondary prevention population, but also for its innovative methodology,” states an accompanying editorial by Ori Ben-Yehuda, MD, Sulpizio Cardiovascular Center, University of California, San Diego.
Use of such a large single-payer database in their study “affords even greater external validity to the findings, complementing the internal validity of the randomized RACING trial,” Dr. Ben-Yehuda writes.
The rationale for combination therapy is strong, but additional data would be helpful, particularly for informing guidelines, he continues. “A pragmatic trial randomizing a broad racial and ethnic group of patients to low-dose statin,” such as a starting dose of 10 mg/day atorvastatin or 5 mg/day rosuvastatin “plus ezetimibe vs. high-intensity statin alone would provide much needed data to help guide lipid-lowering therapy for millions of patients and hopefully increase persistence on therapy.”
The study was supported by the Cardiovascular Research Center, Seoul, South Korea. Dr. Hong and Dr. Ben-Yehuda have disclosed no relevant financial relationships. Dr. Bhatt has previously disclosed grants and/or personal fees from many companies; personal fees from WebMD and other publications or organizations; and having other relationships with Medscape Cardiology and other publications or organizations.
A version of this article appeared on Medscape.com.
FROM JACC
S-ICD shows virtues, limits in ‘real-world’ postmarket study
In the latest chapter in the U.S. saga of the subcutaneous implantable cardioverter-defibrillator (S-ICD) system (Boston Scientific) – a large postmarket, multicenter registry study – the device performed at least as well as it did in earlier trials, researchers say.
The device met all prespecified safety and efficacy endpoints in a study that enrolled more than 1,600 patients and followed them for about 5 years, they noted in their report on the S-ICD Post-Approval Study (S-ICD-PAS) published online in the Journal of the American College of Cardiology.
the group reported.
The team was “pleasantly surprised” that the device’s safety and efficacy performance “was as good if not better than previous studies,” despite a sicker group of patients, lead author Michael R. Gold, MD, PhD, Medical University of South Carolina, Charleston, said in an interview.
No predictors of initial-shock failure were identified, suggesting the S-ICD should be effective in a broad range of ICD candidates without indications for pacing, Dr. Gold said.
The S-ICD was approved in Europe in 2008 and by the Food and Drug Administration in 2012. Clinical trials have suggested its performance and risk for inappropriate shocks are in line with transvenous-lead systems for most patients with ICD indications who don’t need pacing, while avoiding the sometimes serious risks posed by transvenous leads.
The S-ICD doesn’t have antitachycardia pacing (ATP), an alternative way to stop some arrhythmias and a universal feature of transvenous-lead systems. Its lack of ATP may be partly responsible for the device’s weak uptake in practice, some observers noted.
The S-ICD-PAS study “laudably included centers with variable prior experience with the S-ICD; however, data were not analyzed by center experience,” Jonathan S. Steinberg, MD, and Valentina Kutyifa, MD, PhD, University of Rochester (N.Y.) Medical Center, New York, wrote in an accompanying editorial regarding the report’s potential limitations.
Also of concern, they wrote, is the large proportion of patients who were lost to follow-up; almost 42% left the study before its prospectively defined end.
Still, wrote Dr. Steinberg and Dr. Kutyifa, S-ICD-PAS “provides robust, long-term evidence in favor of S-ICD use in a diverse cohort of younger patients receiving implants for primary or secondary prevention of sudden arrhythmic death.” Further analyses are needed, however, to clarify its performance “in centers with low vs high volume as well as in important clinical subgroups.”
It’s “reassuring to see the phase 4 postapproval study results sort of corroborate what the initial clinical study shows,” Miguel Leal, MD, Emory University, Atlanta, said in an interview.
The study’s significant attrition rate “does not negate the results because the performance curves of the device remained approximately stable over the 5 years,” he said, “suggesting that the patients who were lost [and whose clinical outcomes were not included] may not have made a significant impact when it comes to the final results.”
Although the S-ICD seems unlikely to cause complications related to endovascular occlusions or infection, “it can still cause complications related to the implant technique, particularly a device site erosion or device dislodgement,” said Dr. Leal, who chairs the American Heart Association Council on Clinical Cardiology–Electrocardiography & Arrhythmia Committee.
The S-ICD’s biggest contribution has been “the ability to promote efficacious therapy without a need for penetrating the endovascular space,” he observed. “We need to continue to push the envelope towards developing device-based technologies that spare the endovascular space.”
The study enrolled 1,643 patients at 86 U.S. centers; their mean age was 53 and 32% were women. Of the total, 1637 were implanted with the device, 665 completed the study, 288 died, and 686 left the study before completing follow-up. Of the latter, 102 (6.2% of the total) underwent S-ICD explantation, often because of infection.
In addition to the overall shock efficacy rate of 98.4%, induced-arrhythmia shock efficacy was 98.7%, first-shock efficacy for spontaneous arrhythmias was 92.2%, and the rate for either induced or spontaneous arrhythmia was 94.7%. A mean of 1.1 shocks was needed to terminate the arrhythmias; time to shock delivery averaged 17.5 seconds.
The rate of inappropriate shocks was 6.7% at 1 year and 15.8% at 5 years, notably with no significant differences between patients who had and had not undergone defibrillation threshold testing at implantation.
Of 516 inappropriate-shock episodes in 224 patients, almost 86% resulted from inappropriate sensing. Inappropriate shocks became less frequent with longer implantation times and during the course of the study.
The rate of freedom from type 1 complications, the primary safety endpoint, was 93.4%, besting the 85% performance goal. The rate of freedom from electrode-related complications was 99.3%, compared with the performance goal of 92.5%.
The S-ICD was replaced by a transvenous system because of the need for pacing in 1.6% of the cohort.
Sana M. Al-Khatib, MD, MHS, who chaired a 2017 multisociety guideline for managing ventricular arrhythmias and prevention of sudden death, acknowledged the 5-year safety and effectiveness of the S-ICD but also highlighted the “very high dropout rate.”
Moreover, given the cohort’s average age, “these results cannot be generalized to much older patients, in their 70s and 80s [for example]. More data on the S-ICD in older patients are needed, especially because some of these patients will need pacing, which is not provided by the S-ICD,” Dr. Al-Khatib, Duke University, Durham, N.C., said in an interview.
Longer follow-up of patients with the S-ICD is also needed, she added, and “having an S-ICD that is smaller with longer battery life would be great for my patients.”
The study was sponsored by Boston Scientific. Dr. Gold reported receiving consulting fees from Boston Scientific and Medtronic and participating in clinical trials with Boston Scientific, Medtronic, and Abbott. Dr. Steinberg, Dr. Kutyifa, Dr. Al-Khatib, and Dr. Leal reported no relevant relationships.
A version of this article first appeared on Medscape.com.
In the latest chapter in the U.S. saga of the subcutaneous implantable cardioverter-defibrillator (S-ICD) system (Boston Scientific) – a large postmarket, multicenter registry study – the device performed at least as well as it did in earlier trials, researchers say.
The device met all prespecified safety and efficacy endpoints in a study that enrolled more than 1,600 patients and followed them for about 5 years, they noted in their report on the S-ICD Post-Approval Study (S-ICD-PAS) published online in the Journal of the American College of Cardiology.
the group reported.
The team was “pleasantly surprised” that the device’s safety and efficacy performance “was as good if not better than previous studies,” despite a sicker group of patients, lead author Michael R. Gold, MD, PhD, Medical University of South Carolina, Charleston, said in an interview.
No predictors of initial-shock failure were identified, suggesting the S-ICD should be effective in a broad range of ICD candidates without indications for pacing, Dr. Gold said.
The S-ICD was approved in Europe in 2008 and by the Food and Drug Administration in 2012. Clinical trials have suggested its performance and risk for inappropriate shocks are in line with transvenous-lead systems for most patients with ICD indications who don’t need pacing, while avoiding the sometimes serious risks posed by transvenous leads.
The S-ICD doesn’t have antitachycardia pacing (ATP), an alternative way to stop some arrhythmias and a universal feature of transvenous-lead systems. Its lack of ATP may be partly responsible for the device’s weak uptake in practice, some observers noted.
The S-ICD-PAS study “laudably included centers with variable prior experience with the S-ICD; however, data were not analyzed by center experience,” Jonathan S. Steinberg, MD, and Valentina Kutyifa, MD, PhD, University of Rochester (N.Y.) Medical Center, New York, wrote in an accompanying editorial regarding the report’s potential limitations.
Also of concern, they wrote, is the large proportion of patients who were lost to follow-up; almost 42% left the study before its prospectively defined end.
Still, wrote Dr. Steinberg and Dr. Kutyifa, S-ICD-PAS “provides robust, long-term evidence in favor of S-ICD use in a diverse cohort of younger patients receiving implants for primary or secondary prevention of sudden arrhythmic death.” Further analyses are needed, however, to clarify its performance “in centers with low vs high volume as well as in important clinical subgroups.”
It’s “reassuring to see the phase 4 postapproval study results sort of corroborate what the initial clinical study shows,” Miguel Leal, MD, Emory University, Atlanta, said in an interview.
The study’s significant attrition rate “does not negate the results because the performance curves of the device remained approximately stable over the 5 years,” he said, “suggesting that the patients who were lost [and whose clinical outcomes were not included] may not have made a significant impact when it comes to the final results.”
Although the S-ICD seems unlikely to cause complications related to endovascular occlusions or infection, “it can still cause complications related to the implant technique, particularly a device site erosion or device dislodgement,” said Dr. Leal, who chairs the American Heart Association Council on Clinical Cardiology–Electrocardiography & Arrhythmia Committee.
The S-ICD’s biggest contribution has been “the ability to promote efficacious therapy without a need for penetrating the endovascular space,” he observed. “We need to continue to push the envelope towards developing device-based technologies that spare the endovascular space.”
The study enrolled 1,643 patients at 86 U.S. centers; their mean age was 53 and 32% were women. Of the total, 1637 were implanted with the device, 665 completed the study, 288 died, and 686 left the study before completing follow-up. Of the latter, 102 (6.2% of the total) underwent S-ICD explantation, often because of infection.
In addition to the overall shock efficacy rate of 98.4%, induced-arrhythmia shock efficacy was 98.7%, first-shock efficacy for spontaneous arrhythmias was 92.2%, and the rate for either induced or spontaneous arrhythmia was 94.7%. A mean of 1.1 shocks was needed to terminate the arrhythmias; time to shock delivery averaged 17.5 seconds.
The rate of inappropriate shocks was 6.7% at 1 year and 15.8% at 5 years, notably with no significant differences between patients who had and had not undergone defibrillation threshold testing at implantation.
Of 516 inappropriate-shock episodes in 224 patients, almost 86% resulted from inappropriate sensing. Inappropriate shocks became less frequent with longer implantation times and during the course of the study.
The rate of freedom from type 1 complications, the primary safety endpoint, was 93.4%, besting the 85% performance goal. The rate of freedom from electrode-related complications was 99.3%, compared with the performance goal of 92.5%.
The S-ICD was replaced by a transvenous system because of the need for pacing in 1.6% of the cohort.
Sana M. Al-Khatib, MD, MHS, who chaired a 2017 multisociety guideline for managing ventricular arrhythmias and prevention of sudden death, acknowledged the 5-year safety and effectiveness of the S-ICD but also highlighted the “very high dropout rate.”
Moreover, given the cohort’s average age, “these results cannot be generalized to much older patients, in their 70s and 80s [for example]. More data on the S-ICD in older patients are needed, especially because some of these patients will need pacing, which is not provided by the S-ICD,” Dr. Al-Khatib, Duke University, Durham, N.C., said in an interview.
Longer follow-up of patients with the S-ICD is also needed, she added, and “having an S-ICD that is smaller with longer battery life would be great for my patients.”
The study was sponsored by Boston Scientific. Dr. Gold reported receiving consulting fees from Boston Scientific and Medtronic and participating in clinical trials with Boston Scientific, Medtronic, and Abbott. Dr. Steinberg, Dr. Kutyifa, Dr. Al-Khatib, and Dr. Leal reported no relevant relationships.
A version of this article first appeared on Medscape.com.
In the latest chapter in the U.S. saga of the subcutaneous implantable cardioverter-defibrillator (S-ICD) system (Boston Scientific) – a large postmarket, multicenter registry study – the device performed at least as well as it did in earlier trials, researchers say.
The device met all prespecified safety and efficacy endpoints in a study that enrolled more than 1,600 patients and followed them for about 5 years, they noted in their report on the S-ICD Post-Approval Study (S-ICD-PAS) published online in the Journal of the American College of Cardiology.
the group reported.
The team was “pleasantly surprised” that the device’s safety and efficacy performance “was as good if not better than previous studies,” despite a sicker group of patients, lead author Michael R. Gold, MD, PhD, Medical University of South Carolina, Charleston, said in an interview.
No predictors of initial-shock failure were identified, suggesting the S-ICD should be effective in a broad range of ICD candidates without indications for pacing, Dr. Gold said.
The S-ICD was approved in Europe in 2008 and by the Food and Drug Administration in 2012. Clinical trials have suggested its performance and risk for inappropriate shocks are in line with transvenous-lead systems for most patients with ICD indications who don’t need pacing, while avoiding the sometimes serious risks posed by transvenous leads.
The S-ICD doesn’t have antitachycardia pacing (ATP), an alternative way to stop some arrhythmias and a universal feature of transvenous-lead systems. Its lack of ATP may be partly responsible for the device’s weak uptake in practice, some observers noted.
The S-ICD-PAS study “laudably included centers with variable prior experience with the S-ICD; however, data were not analyzed by center experience,” Jonathan S. Steinberg, MD, and Valentina Kutyifa, MD, PhD, University of Rochester (N.Y.) Medical Center, New York, wrote in an accompanying editorial regarding the report’s potential limitations.
Also of concern, they wrote, is the large proportion of patients who were lost to follow-up; almost 42% left the study before its prospectively defined end.
Still, wrote Dr. Steinberg and Dr. Kutyifa, S-ICD-PAS “provides robust, long-term evidence in favor of S-ICD use in a diverse cohort of younger patients receiving implants for primary or secondary prevention of sudden arrhythmic death.” Further analyses are needed, however, to clarify its performance “in centers with low vs high volume as well as in important clinical subgroups.”
It’s “reassuring to see the phase 4 postapproval study results sort of corroborate what the initial clinical study shows,” Miguel Leal, MD, Emory University, Atlanta, said in an interview.
The study’s significant attrition rate “does not negate the results because the performance curves of the device remained approximately stable over the 5 years,” he said, “suggesting that the patients who were lost [and whose clinical outcomes were not included] may not have made a significant impact when it comes to the final results.”
Although the S-ICD seems unlikely to cause complications related to endovascular occlusions or infection, “it can still cause complications related to the implant technique, particularly a device site erosion or device dislodgement,” said Dr. Leal, who chairs the American Heart Association Council on Clinical Cardiology–Electrocardiography & Arrhythmia Committee.
The S-ICD’s biggest contribution has been “the ability to promote efficacious therapy without a need for penetrating the endovascular space,” he observed. “We need to continue to push the envelope towards developing device-based technologies that spare the endovascular space.”
The study enrolled 1,643 patients at 86 U.S. centers; their mean age was 53 and 32% were women. Of the total, 1637 were implanted with the device, 665 completed the study, 288 died, and 686 left the study before completing follow-up. Of the latter, 102 (6.2% of the total) underwent S-ICD explantation, often because of infection.
In addition to the overall shock efficacy rate of 98.4%, induced-arrhythmia shock efficacy was 98.7%, first-shock efficacy for spontaneous arrhythmias was 92.2%, and the rate for either induced or spontaneous arrhythmia was 94.7%. A mean of 1.1 shocks was needed to terminate the arrhythmias; time to shock delivery averaged 17.5 seconds.
The rate of inappropriate shocks was 6.7% at 1 year and 15.8% at 5 years, notably with no significant differences between patients who had and had not undergone defibrillation threshold testing at implantation.
Of 516 inappropriate-shock episodes in 224 patients, almost 86% resulted from inappropriate sensing. Inappropriate shocks became less frequent with longer implantation times and during the course of the study.
The rate of freedom from type 1 complications, the primary safety endpoint, was 93.4%, besting the 85% performance goal. The rate of freedom from electrode-related complications was 99.3%, compared with the performance goal of 92.5%.
The S-ICD was replaced by a transvenous system because of the need for pacing in 1.6% of the cohort.
Sana M. Al-Khatib, MD, MHS, who chaired a 2017 multisociety guideline for managing ventricular arrhythmias and prevention of sudden death, acknowledged the 5-year safety and effectiveness of the S-ICD but also highlighted the “very high dropout rate.”
Moreover, given the cohort’s average age, “these results cannot be generalized to much older patients, in their 70s and 80s [for example]. More data on the S-ICD in older patients are needed, especially because some of these patients will need pacing, which is not provided by the S-ICD,” Dr. Al-Khatib, Duke University, Durham, N.C., said in an interview.
Longer follow-up of patients with the S-ICD is also needed, she added, and “having an S-ICD that is smaller with longer battery life would be great for my patients.”
The study was sponsored by Boston Scientific. Dr. Gold reported receiving consulting fees from Boston Scientific and Medtronic and participating in clinical trials with Boston Scientific, Medtronic, and Abbott. Dr. Steinberg, Dr. Kutyifa, Dr. Al-Khatib, and Dr. Leal reported no relevant relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
New guidelines on diabetes-related laboratory testing
The document, titled, “Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus,” is primarily aimed at both laboratory professionals and clinicians involved in diabetes care.
The guidance is focused “on the practical aspects of care in order to assist with decisions regarding the use or interpretation of laboratory tests while screening, diagnosing, or monitoring patients with diabetes,” wrote David B. Sacks, MBChB, chief of the clinical chemistry service at the National Institutes of Health (NIH), Bethesda, Md., and coauthors. It was published online in both Clinical Chemistry and Diabetes Care, including the guidelines and executive summary.
Coauthor M. Sue Kirkman, MD, of the University of North Carolina, Chapel Hill, said in an interview: “One objective of the guidelines is to increase clinicians’ understanding of the strengths and limitations of tests done in a laboratory and also at the point of care, or in daily life, by people with diabetes.”
The evidence-based recommendations, an update of prior versions published in 2011 and 2002, are meant as a supplement to the ADA Standards of Care in Diabetes and do not address aspects of clinical management, she stressed.
Addition of advice on CGM
A significant addition since 2011 is detailed information regarding the use of real-time continuous glucose monitoring (CGM), with a “strong” recommendation based on a “high” level of evidence for use in teens and adults with type 1 diabetes who meet certain criteria, and lower-grade advice to use real-time or intermittently scanned CGM in other populations, including children with diabetes, pregnant women with type 1 diabetes, and adults with type 2 diabetes taking insulin.
The document also reminds clinicians to consider test limitations, Dr. Kirkman pointed out.
“We do a lot of testing in screening, diagnosis, and monitoring of diabetes and its complications, yet for many clinicians we think that any result we get – or that a patient gets from home testing – is perfect. We often don’t think about the accuracy or precision of some tests, things that might interfere with the result, intra-individual variation of the test, or how one test may compare to a test of higher accuracy,” she said.
One example is a recommendation to collect blood samples for glucose analysis in tubes containing a rapidly effective inhibitor of glycolysis such as a granulated citrate buffer. If unavailable, the sample tube should be placed immediately into an ice water slurry and centrifuged within 15-30 minutes to remove the cells.
Without those measures, “red cells in blood sitting in the test tube continue to break down glucose, so the concentration of glucose will start to fall very soon. ... How the specimen is handled makes a huge difference in the result,” Dr. Kirkman emphasized.
Another is the recommendation of a confirmatory test when diagnosing diabetes, regardless of the initial test used (A1c, fasting glucose, or oral glucose tolerance test). “There is large intra-individual variation of fasting glucose and even larger for 2-hour glucose on the oral glucose tolerance test. ... This means if you do the test one week and then repeat it the next day or a week later, the results will be quite different. This is a reason why confirmation of an abnormal test is important. Yet many times this isn’t done,” she noted.
Other “strong” recommendations based on “high” evidence levels include:
- Fasting glucose should be measured in venous plasma when used to establish the diagnosis of diabetes, with a diagnostic cutoff of > 7.0 mmol/L (> 126 mg/dL) for diabetes.
- Frequent blood glucose monitoring is recommended for all people with diabetes treated with intensive insulin regimens (with multiple daily injections or insulin pump therapy) and who are not using CGM.
- Routine use of blood glucose monitoring is not recommended for people with type 2 diabetes who are treated with diet and/or oral agents alone.
- Treatment goals should be based on ADA recommendations, i.e., A1c < 7% (< 53 mmol/mol) if it can be achieved without significant hypoglycemia or other adverse treatment effects, with higher targets for special populations.
- Annual testing for albuminuria should begin in pubertal or postpubertal individuals 5 years after diagnosis of type 1 diabetes and at time of diagnosis of type 2 diabetes, regardless of treatment.
- Urine albumin should be measured annually in adults with diabetes using morning spot urine albumin-to-creatinine ratio.
Other guidance in the document pertains to use of ketone testing, genetic markers, autoimmune markers, and C-peptide.
According to Dr. Sacks, “It’s important to measure accurately, but it’s also very important to communicate the relevance to clinicians and to listen to them and share information. ... Patient care is a team effort.”
Dr. Sachs has reported receiving funding from the NIH. Dr. Kirkman has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The document, titled, “Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus,” is primarily aimed at both laboratory professionals and clinicians involved in diabetes care.
The guidance is focused “on the practical aspects of care in order to assist with decisions regarding the use or interpretation of laboratory tests while screening, diagnosing, or monitoring patients with diabetes,” wrote David B. Sacks, MBChB, chief of the clinical chemistry service at the National Institutes of Health (NIH), Bethesda, Md., and coauthors. It was published online in both Clinical Chemistry and Diabetes Care, including the guidelines and executive summary.
Coauthor M. Sue Kirkman, MD, of the University of North Carolina, Chapel Hill, said in an interview: “One objective of the guidelines is to increase clinicians’ understanding of the strengths and limitations of tests done in a laboratory and also at the point of care, or in daily life, by people with diabetes.”
The evidence-based recommendations, an update of prior versions published in 2011 and 2002, are meant as a supplement to the ADA Standards of Care in Diabetes and do not address aspects of clinical management, she stressed.
Addition of advice on CGM
A significant addition since 2011 is detailed information regarding the use of real-time continuous glucose monitoring (CGM), with a “strong” recommendation based on a “high” level of evidence for use in teens and adults with type 1 diabetes who meet certain criteria, and lower-grade advice to use real-time or intermittently scanned CGM in other populations, including children with diabetes, pregnant women with type 1 diabetes, and adults with type 2 diabetes taking insulin.
The document also reminds clinicians to consider test limitations, Dr. Kirkman pointed out.
“We do a lot of testing in screening, diagnosis, and monitoring of diabetes and its complications, yet for many clinicians we think that any result we get – or that a patient gets from home testing – is perfect. We often don’t think about the accuracy or precision of some tests, things that might interfere with the result, intra-individual variation of the test, or how one test may compare to a test of higher accuracy,” she said.
One example is a recommendation to collect blood samples for glucose analysis in tubes containing a rapidly effective inhibitor of glycolysis such as a granulated citrate buffer. If unavailable, the sample tube should be placed immediately into an ice water slurry and centrifuged within 15-30 minutes to remove the cells.
Without those measures, “red cells in blood sitting in the test tube continue to break down glucose, so the concentration of glucose will start to fall very soon. ... How the specimen is handled makes a huge difference in the result,” Dr. Kirkman emphasized.
Another is the recommendation of a confirmatory test when diagnosing diabetes, regardless of the initial test used (A1c, fasting glucose, or oral glucose tolerance test). “There is large intra-individual variation of fasting glucose and even larger for 2-hour glucose on the oral glucose tolerance test. ... This means if you do the test one week and then repeat it the next day or a week later, the results will be quite different. This is a reason why confirmation of an abnormal test is important. Yet many times this isn’t done,” she noted.
Other “strong” recommendations based on “high” evidence levels include:
- Fasting glucose should be measured in venous plasma when used to establish the diagnosis of diabetes, with a diagnostic cutoff of > 7.0 mmol/L (> 126 mg/dL) for diabetes.
- Frequent blood glucose monitoring is recommended for all people with diabetes treated with intensive insulin regimens (with multiple daily injections or insulin pump therapy) and who are not using CGM.
- Routine use of blood glucose monitoring is not recommended for people with type 2 diabetes who are treated with diet and/or oral agents alone.
- Treatment goals should be based on ADA recommendations, i.e., A1c < 7% (< 53 mmol/mol) if it can be achieved without significant hypoglycemia or other adverse treatment effects, with higher targets for special populations.
- Annual testing for albuminuria should begin in pubertal or postpubertal individuals 5 years after diagnosis of type 1 diabetes and at time of diagnosis of type 2 diabetes, regardless of treatment.
- Urine albumin should be measured annually in adults with diabetes using morning spot urine albumin-to-creatinine ratio.
Other guidance in the document pertains to use of ketone testing, genetic markers, autoimmune markers, and C-peptide.
According to Dr. Sacks, “It’s important to measure accurately, but it’s also very important to communicate the relevance to clinicians and to listen to them and share information. ... Patient care is a team effort.”
Dr. Sachs has reported receiving funding from the NIH. Dr. Kirkman has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The document, titled, “Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus,” is primarily aimed at both laboratory professionals and clinicians involved in diabetes care.
The guidance is focused “on the practical aspects of care in order to assist with decisions regarding the use or interpretation of laboratory tests while screening, diagnosing, or monitoring patients with diabetes,” wrote David B. Sacks, MBChB, chief of the clinical chemistry service at the National Institutes of Health (NIH), Bethesda, Md., and coauthors. It was published online in both Clinical Chemistry and Diabetes Care, including the guidelines and executive summary.
Coauthor M. Sue Kirkman, MD, of the University of North Carolina, Chapel Hill, said in an interview: “One objective of the guidelines is to increase clinicians’ understanding of the strengths and limitations of tests done in a laboratory and also at the point of care, or in daily life, by people with diabetes.”
The evidence-based recommendations, an update of prior versions published in 2011 and 2002, are meant as a supplement to the ADA Standards of Care in Diabetes and do not address aspects of clinical management, she stressed.
Addition of advice on CGM
A significant addition since 2011 is detailed information regarding the use of real-time continuous glucose monitoring (CGM), with a “strong” recommendation based on a “high” level of evidence for use in teens and adults with type 1 diabetes who meet certain criteria, and lower-grade advice to use real-time or intermittently scanned CGM in other populations, including children with diabetes, pregnant women with type 1 diabetes, and adults with type 2 diabetes taking insulin.
The document also reminds clinicians to consider test limitations, Dr. Kirkman pointed out.
“We do a lot of testing in screening, diagnosis, and monitoring of diabetes and its complications, yet for many clinicians we think that any result we get – or that a patient gets from home testing – is perfect. We often don’t think about the accuracy or precision of some tests, things that might interfere with the result, intra-individual variation of the test, or how one test may compare to a test of higher accuracy,” she said.
One example is a recommendation to collect blood samples for glucose analysis in tubes containing a rapidly effective inhibitor of glycolysis such as a granulated citrate buffer. If unavailable, the sample tube should be placed immediately into an ice water slurry and centrifuged within 15-30 minutes to remove the cells.
Without those measures, “red cells in blood sitting in the test tube continue to break down glucose, so the concentration of glucose will start to fall very soon. ... How the specimen is handled makes a huge difference in the result,” Dr. Kirkman emphasized.
Another is the recommendation of a confirmatory test when diagnosing diabetes, regardless of the initial test used (A1c, fasting glucose, or oral glucose tolerance test). “There is large intra-individual variation of fasting glucose and even larger for 2-hour glucose on the oral glucose tolerance test. ... This means if you do the test one week and then repeat it the next day or a week later, the results will be quite different. This is a reason why confirmation of an abnormal test is important. Yet many times this isn’t done,” she noted.
Other “strong” recommendations based on “high” evidence levels include:
- Fasting glucose should be measured in venous plasma when used to establish the diagnosis of diabetes, with a diagnostic cutoff of > 7.0 mmol/L (> 126 mg/dL) for diabetes.
- Frequent blood glucose monitoring is recommended for all people with diabetes treated with intensive insulin regimens (with multiple daily injections or insulin pump therapy) and who are not using CGM.
- Routine use of blood glucose monitoring is not recommended for people with type 2 diabetes who are treated with diet and/or oral agents alone.
- Treatment goals should be based on ADA recommendations, i.e., A1c < 7% (< 53 mmol/mol) if it can be achieved without significant hypoglycemia or other adverse treatment effects, with higher targets for special populations.
- Annual testing for albuminuria should begin in pubertal or postpubertal individuals 5 years after diagnosis of type 1 diabetes and at time of diagnosis of type 2 diabetes, regardless of treatment.
- Urine albumin should be measured annually in adults with diabetes using morning spot urine albumin-to-creatinine ratio.
Other guidance in the document pertains to use of ketone testing, genetic markers, autoimmune markers, and C-peptide.
According to Dr. Sacks, “It’s important to measure accurately, but it’s also very important to communicate the relevance to clinicians and to listen to them and share information. ... Patient care is a team effort.”
Dr. Sachs has reported receiving funding from the NIH. Dr. Kirkman has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL CHEMISTRY AND DIABETES CARE
Does screening kids with acute sinusitis symptoms for bacterial infection cut unnecessary antibiotic use?
Testing children with acute sinusitis symptoms for specific bacteria may dramatically decrease unnecessary antibiotic use, new research suggests.
The study, published in JAMA, found that children with positive nasopharyngeal tests for one or more of Haemophilus influenzae, Streptococcus pneumoniae, or Moraxella catarrhalis had better resolution of symptoms with antibiotics than those without these bacteria.
If antibiotic use was limited to children with H. influenzae or S. pneumoniae in their nasopharynx at the time of diagnosis, antibiotic use would decrease by 53%, according to the study authors.
Sinusitis is common in children, and symptoms are similar with uncomplicated viral upper respiratory infections.
“We have not had a good way to predict which children will benefit from antibiotics,” said Nader Shaikh, MD, MPH, professor of pediatrics and clinical and translational science at the University of Pittsburgh, and the lead study author. “When a child comes in with a sore throat, we test for strep. If the test is positive, we prescribe antibiotics.”
Dr. Shaikh and his colleagues found that the same approach – swabbing the nose and testing for various bacteria – worked for children with sinusitis.
“Children who tested negative for bacteria did not benefit from antibiotics,” Dr. Shaikh said.
In the double-blind clinical trial, Dr. Shaikh and his colleagues randomized 510 children between ages 2 and 11 with acute sinusitis at six academic primary care offices over a 6-year period. Almost two-thirds of participants were between ages 2 and 5, around half were male, and around half were White. All participants had an initial score of nine or higher on the validated Pediatric Rhinosinusitis Symptom Scale (PRSS).
For 10 days, 254 children received oral amoxicillin (90 mg/kg/day) and clavulanate (6.4mg/kg/day) and 256 received placebo.
In children receiving antibiotics, symptoms resolved over a median of 7 days, compared with 9 days for those given placebo (P = .003).
Children without detected nasopharyngeal pathogens did not benefit from antibiotics as much as those with the pathogens, the researchers found. Among those with pathogens, the mean symptom burden score was 1.95 points lower in the group that received antibiotics, compared with the group that received placebo. For those without pathogens, there was a 0.88-point difference between the antibiotic and placebo groups (P = .02).
The researchers also took nasal swabs at the first and final study visits and tested for S. pneumoniae, H. influenzae, and M. catarrhalis. During that time, parents or caregivers used the PRSS to assess their child’s symptoms, and they recorded the nasal discharge color. Nasal discharge color, Dr. Shaikh and colleagues found, was not linked with antibiotic effect.
Welcome findings
Pediatricians and primary care providers face a significant clinical dilemma when they consider using antibiotics with upper respiratory tract infections (URTIs), according to John H. Greinwald Jr., MD, professor in the department of pediatrics at Cincinnati Children’s Hospital Medical Center.
“These findings certainly make sense because most respiratory infections in children are viral,” Dr. Greinwald said. “The investigators follow the appropriate clinical guidelines for considering antibiotic use in patients with URTIs, which include URTI symptoms lasting longer than 10 days or symptoms initially getting better, then worsening again day 6 through 10.”
Not only is antibiotic resistance a major public health concern, but the drugs can have side effects such as diarrhea, and their long-term effects on the microbiome are unknown.
“Differentiating who has acute sinusitis from who has a viral infection is difficult for primary care providers,” said Eelam A. Adil, MD, MBA, assistant professor of otolaryngology at Harvard Medical School in Boston.
The findings may help clinicians be more selective with antibiotic prescriptions, according to Jacob G. Eide, MD, a head and neck surgeon at Henry Ford Health in Detroit.
“However, we do not want to deny antibiotics when they are beneficial,” Dr. Eide said. “And the difficulty and costs involved in developing the tests need to be considered.”
Dr. Shaikh and his team are studying ways to bring nasal testing into clinical practice, potentially utilizing commercially available molecular testing and rapid antigen tests that work like COVID-19 at-home tests. They are also exploring if other biomarkers in nasal discharge may indicate the presence of bacteria.
All study authors as well as outside experts reported no relevant financial relationships. The study was supported by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
Testing children with acute sinusitis symptoms for specific bacteria may dramatically decrease unnecessary antibiotic use, new research suggests.
The study, published in JAMA, found that children with positive nasopharyngeal tests for one or more of Haemophilus influenzae, Streptococcus pneumoniae, or Moraxella catarrhalis had better resolution of symptoms with antibiotics than those without these bacteria.
If antibiotic use was limited to children with H. influenzae or S. pneumoniae in their nasopharynx at the time of diagnosis, antibiotic use would decrease by 53%, according to the study authors.
Sinusitis is common in children, and symptoms are similar with uncomplicated viral upper respiratory infections.
“We have not had a good way to predict which children will benefit from antibiotics,” said Nader Shaikh, MD, MPH, professor of pediatrics and clinical and translational science at the University of Pittsburgh, and the lead study author. “When a child comes in with a sore throat, we test for strep. If the test is positive, we prescribe antibiotics.”
Dr. Shaikh and his colleagues found that the same approach – swabbing the nose and testing for various bacteria – worked for children with sinusitis.
“Children who tested negative for bacteria did not benefit from antibiotics,” Dr. Shaikh said.
In the double-blind clinical trial, Dr. Shaikh and his colleagues randomized 510 children between ages 2 and 11 with acute sinusitis at six academic primary care offices over a 6-year period. Almost two-thirds of participants were between ages 2 and 5, around half were male, and around half were White. All participants had an initial score of nine or higher on the validated Pediatric Rhinosinusitis Symptom Scale (PRSS).
For 10 days, 254 children received oral amoxicillin (90 mg/kg/day) and clavulanate (6.4mg/kg/day) and 256 received placebo.
In children receiving antibiotics, symptoms resolved over a median of 7 days, compared with 9 days for those given placebo (P = .003).
Children without detected nasopharyngeal pathogens did not benefit from antibiotics as much as those with the pathogens, the researchers found. Among those with pathogens, the mean symptom burden score was 1.95 points lower in the group that received antibiotics, compared with the group that received placebo. For those without pathogens, there was a 0.88-point difference between the antibiotic and placebo groups (P = .02).
The researchers also took nasal swabs at the first and final study visits and tested for S. pneumoniae, H. influenzae, and M. catarrhalis. During that time, parents or caregivers used the PRSS to assess their child’s symptoms, and they recorded the nasal discharge color. Nasal discharge color, Dr. Shaikh and colleagues found, was not linked with antibiotic effect.
Welcome findings
Pediatricians and primary care providers face a significant clinical dilemma when they consider using antibiotics with upper respiratory tract infections (URTIs), according to John H. Greinwald Jr., MD, professor in the department of pediatrics at Cincinnati Children’s Hospital Medical Center.
“These findings certainly make sense because most respiratory infections in children are viral,” Dr. Greinwald said. “The investigators follow the appropriate clinical guidelines for considering antibiotic use in patients with URTIs, which include URTI symptoms lasting longer than 10 days or symptoms initially getting better, then worsening again day 6 through 10.”
Not only is antibiotic resistance a major public health concern, but the drugs can have side effects such as diarrhea, and their long-term effects on the microbiome are unknown.
“Differentiating who has acute sinusitis from who has a viral infection is difficult for primary care providers,” said Eelam A. Adil, MD, MBA, assistant professor of otolaryngology at Harvard Medical School in Boston.
The findings may help clinicians be more selective with antibiotic prescriptions, according to Jacob G. Eide, MD, a head and neck surgeon at Henry Ford Health in Detroit.
“However, we do not want to deny antibiotics when they are beneficial,” Dr. Eide said. “And the difficulty and costs involved in developing the tests need to be considered.”
Dr. Shaikh and his team are studying ways to bring nasal testing into clinical practice, potentially utilizing commercially available molecular testing and rapid antigen tests that work like COVID-19 at-home tests. They are also exploring if other biomarkers in nasal discharge may indicate the presence of bacteria.
All study authors as well as outside experts reported no relevant financial relationships. The study was supported by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
Testing children with acute sinusitis symptoms for specific bacteria may dramatically decrease unnecessary antibiotic use, new research suggests.
The study, published in JAMA, found that children with positive nasopharyngeal tests for one or more of Haemophilus influenzae, Streptococcus pneumoniae, or Moraxella catarrhalis had better resolution of symptoms with antibiotics than those without these bacteria.
If antibiotic use was limited to children with H. influenzae or S. pneumoniae in their nasopharynx at the time of diagnosis, antibiotic use would decrease by 53%, according to the study authors.
Sinusitis is common in children, and symptoms are similar with uncomplicated viral upper respiratory infections.
“We have not had a good way to predict which children will benefit from antibiotics,” said Nader Shaikh, MD, MPH, professor of pediatrics and clinical and translational science at the University of Pittsburgh, and the lead study author. “When a child comes in with a sore throat, we test for strep. If the test is positive, we prescribe antibiotics.”
Dr. Shaikh and his colleagues found that the same approach – swabbing the nose and testing for various bacteria – worked for children with sinusitis.
“Children who tested negative for bacteria did not benefit from antibiotics,” Dr. Shaikh said.
In the double-blind clinical trial, Dr. Shaikh and his colleagues randomized 510 children between ages 2 and 11 with acute sinusitis at six academic primary care offices over a 6-year period. Almost two-thirds of participants were between ages 2 and 5, around half were male, and around half were White. All participants had an initial score of nine or higher on the validated Pediatric Rhinosinusitis Symptom Scale (PRSS).
For 10 days, 254 children received oral amoxicillin (90 mg/kg/day) and clavulanate (6.4mg/kg/day) and 256 received placebo.
In children receiving antibiotics, symptoms resolved over a median of 7 days, compared with 9 days for those given placebo (P = .003).
Children without detected nasopharyngeal pathogens did not benefit from antibiotics as much as those with the pathogens, the researchers found. Among those with pathogens, the mean symptom burden score was 1.95 points lower in the group that received antibiotics, compared with the group that received placebo. For those without pathogens, there was a 0.88-point difference between the antibiotic and placebo groups (P = .02).
The researchers also took nasal swabs at the first and final study visits and tested for S. pneumoniae, H. influenzae, and M. catarrhalis. During that time, parents or caregivers used the PRSS to assess their child’s symptoms, and they recorded the nasal discharge color. Nasal discharge color, Dr. Shaikh and colleagues found, was not linked with antibiotic effect.
Welcome findings
Pediatricians and primary care providers face a significant clinical dilemma when they consider using antibiotics with upper respiratory tract infections (URTIs), according to John H. Greinwald Jr., MD, professor in the department of pediatrics at Cincinnati Children’s Hospital Medical Center.
“These findings certainly make sense because most respiratory infections in children are viral,” Dr. Greinwald said. “The investigators follow the appropriate clinical guidelines for considering antibiotic use in patients with URTIs, which include URTI symptoms lasting longer than 10 days or symptoms initially getting better, then worsening again day 6 through 10.”
Not only is antibiotic resistance a major public health concern, but the drugs can have side effects such as diarrhea, and their long-term effects on the microbiome are unknown.
“Differentiating who has acute sinusitis from who has a viral infection is difficult for primary care providers,” said Eelam A. Adil, MD, MBA, assistant professor of otolaryngology at Harvard Medical School in Boston.
The findings may help clinicians be more selective with antibiotic prescriptions, according to Jacob G. Eide, MD, a head and neck surgeon at Henry Ford Health in Detroit.
“However, we do not want to deny antibiotics when they are beneficial,” Dr. Eide said. “And the difficulty and costs involved in developing the tests need to be considered.”
Dr. Shaikh and his team are studying ways to bring nasal testing into clinical practice, potentially utilizing commercially available molecular testing and rapid antigen tests that work like COVID-19 at-home tests. They are also exploring if other biomarkers in nasal discharge may indicate the presence of bacteria.
All study authors as well as outside experts reported no relevant financial relationships. The study was supported by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
FROM JAMA
Adjuvant Scalp Rolling for Patients With Refractory Alopecia Areata
To the Editor:
Alopecia areata (AA) is an autoimmune nonscarring hair loss disorder that can present at any age. Patients with AA have a disproportionately high comorbidity burden and low quality of life, often grappling with anxiety, depression, and psychosocial sequelae involving identity, such as reduced self-esteem.1,2 Although conventional therapies aim to reduce hair loss, none are curative.3 Response to treatment is highly unpredictable, with current data suggesting that up to 50% of patients recover within 1 year while 14% to 25% progress to either alopecia totalis (total scalp hair loss) or alopecia universalis (total body hair loss).4 Options for therapeutic intervention remain limited and vary in safety and effectiveness, warranting further research to identify optimal modalities and minimize side effects. Interestingly, scalp rolling has been used as an adjuvant to topical triamcinolone acetonide.3,5 However, the extent of its effect in combination with other therapies remains unclear. We report 3 pediatric patients with confirmed AA refractory to conventional topical treatment who experienced remarkable scalp hair regrowth after adding biweekly scalp rolling as an adjuvant therapy.
A 7-year-old boy with AA presented with 95% scalp hair loss of 7 months’ duration (Figure 1A)(patient 1). Prior treatments included mometasone solution and clobetasol solution 0.05%. After 3 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 1B). No pain, bleeding, or other side effects were reported.

An 11-year-old girl with AA presented with 100% hair loss of 7 months’ duration (Figure 2A)(patient 2). Prior treatments included fluocinonide solution and intralesional Kenalog injections. After 4 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 2B). No pain, bleeding, or other side effects were reported.

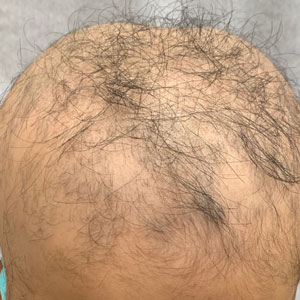
A 16-year-old boy with AA presented with 30% hair loss of 4 years’ duration (Figure 3A)(patient 3). Prior treatments included squaric acid and intralesional Kenalog injections. After 2 years of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth at 17 months (Figure 3B). No pain, bleeding, or other side effects were reported.

Scalp rolling—also known as microneedling—provides a multifactorial approach to hair regrowth in patients with AA. The mechanism of action involves both the hair cycle and wound repair pathways by stimulation of the dermal papillae and stem cells.6 Scalp rolling has been observed to induce the expression of several hair growth pathway mediators, such as WNT3A, β-catenin, vascular endothelial growth factor, and WNT10B.7 Wnt/β-catenin pathway signaling is integral to multiple aspects of the hair regrowth process, including hair morphogenesis, follicle regeneration, and growth of the shaft itself.8,9 Scalp rolling causes microinjuries to the skin, thereby diverting blood supply to the follicles and stimulating wound regeneration, a process suggested to induce follicle regeneration. This effect is due to increased expression of vascular endothelial growth factor after cutaneous injury, a mediator of both hair growth and cycling as well as wound repair.7 Adjuvant scalp rolling creates a synergistic effect by facilitating absorption of topical and intralesional therapies. The physical breakdown of dermal capillary barriers creates microchannels that traverse the stratum corneum, improving the permeability of small-molecule substances and allowing for relatively painless and uniform delivery of combination therapies. A secondary benefit is hypertrophy, which counteracts the atrophy caused by topical steroids via collagen induction.7
Additionally, scalp rolling confers minimal risk to the patient, making it safer than conventional pharmacologic therapies such as corticosteroids or Janus kinase (JAK) inhibitors. Although intralesional steroid injections are first-line treatments for limited disease, they can cause pain and skin atrophy.10 In one cohort of 54 patients, topical steroids were inferior to both oral and intralesional treatment, and oral steroids carried a systemic side-effect profile and worsening of comorbidities including hyperglycemia and hypertension as well as negative effects on bone density.11 Baricitinib, a JAK inhibitor, was the first systemic treatment to gain US Food and Drug Administration approval for severe AA.12 However, this novel therapeutic confers adverse effects including infection, acne, and hypercholesterolemia, as reported in the BRAVE-AA trials.13 More broadly, the US Food and Drug Administration warns of serious long-term risks such as cardiovascular events and malignancy.14 Given the tremendous potential of JAK inhibitors, further research is warranted to understand both the efficacy of topical formulations as well as the possible role of scalp rolling as its adjuvant.
Finally, scalp rolling is easily accessible and affordable to patients. Scalp rolling devices are readily available and affordable online, and they can be used autonomously at home. This pragmatic option allows patients to take control of their own treatment course and offers a financially feasible alternative to navigating insurance coverage as well as the need for extra office visits for medication refills and monitoring.
We report 3 cases of the use of scalp rolling as an adjuvant to conventional therapy for refractory AA in young patients. Although prospective research is required to establish causality and characterize age-related trends in treatment response, consideration of scalp rolling as an adjuvant to conventional therapy may help to optimize treatment regimens. Given its low risk for side effects and potential benefits, we recommend scalp rolling for patients with refractory AA.
1. Senna M, Ko J, Tosti A, et al. Alopecia areata treatment patterns, healthcare resource utilization, and comorbidities in the US population using insurance claims. Adv Ther. 2021;38:4646-4658.
2. Huang CH, Fu Y, Chi CC. Health-related quality of life, depression, and self-esteem in patients with androgenetic alopecia: a systematic review and meta-analysis. JAMA Dermatol. 2021;157:963-970.
3. Deepak SH, Shwetha S. Scalp roller therapy in resistant alopecia areata. J Cutan Aesthet Surg. 2014;7:61-62.
4. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options.Int J Trichology. 2018;10:51-60.
5. Ito T, Yoshimasu T, Furukawa F, et al. Three-microneedle device as an effective option for intralesional corticosteroid administration for the treatment of alopecia areata. J Dermatol. 2017;44:304-305.
6. Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
7. Kim YS, Jeong KH, Kim JE, et al. Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol. 2016;28:586-592.
8. Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166:1035-1042.
9. Myung PS, Takeo M, Ito M, et al. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 2013;133:31-41.
10. Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12.
11. Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276
12.
13. King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699.
14. US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. September 1, 2021.
To the Editor:
Alopecia areata (AA) is an autoimmune nonscarring hair loss disorder that can present at any age. Patients with AA have a disproportionately high comorbidity burden and low quality of life, often grappling with anxiety, depression, and psychosocial sequelae involving identity, such as reduced self-esteem.1,2 Although conventional therapies aim to reduce hair loss, none are curative.3 Response to treatment is highly unpredictable, with current data suggesting that up to 50% of patients recover within 1 year while 14% to 25% progress to either alopecia totalis (total scalp hair loss) or alopecia universalis (total body hair loss).4 Options for therapeutic intervention remain limited and vary in safety and effectiveness, warranting further research to identify optimal modalities and minimize side effects. Interestingly, scalp rolling has been used as an adjuvant to topical triamcinolone acetonide.3,5 However, the extent of its effect in combination with other therapies remains unclear. We report 3 pediatric patients with confirmed AA refractory to conventional topical treatment who experienced remarkable scalp hair regrowth after adding biweekly scalp rolling as an adjuvant therapy.
A 7-year-old boy with AA presented with 95% scalp hair loss of 7 months’ duration (Figure 1A)(patient 1). Prior treatments included mometasone solution and clobetasol solution 0.05%. After 3 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 1B). No pain, bleeding, or other side effects were reported.

An 11-year-old girl with AA presented with 100% hair loss of 7 months’ duration (Figure 2A)(patient 2). Prior treatments included fluocinonide solution and intralesional Kenalog injections. After 4 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 2B). No pain, bleeding, or other side effects were reported.

A 16-year-old boy with AA presented with 30% hair loss of 4 years’ duration (Figure 3A)(patient 3). Prior treatments included squaric acid and intralesional Kenalog injections. After 2 years of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth at 17 months (Figure 3B). No pain, bleeding, or other side effects were reported.

Scalp rolling—also known as microneedling—provides a multifactorial approach to hair regrowth in patients with AA. The mechanism of action involves both the hair cycle and wound repair pathways by stimulation of the dermal papillae and stem cells.6 Scalp rolling has been observed to induce the expression of several hair growth pathway mediators, such as WNT3A, β-catenin, vascular endothelial growth factor, and WNT10B.7 Wnt/β-catenin pathway signaling is integral to multiple aspects of the hair regrowth process, including hair morphogenesis, follicle regeneration, and growth of the shaft itself.8,9 Scalp rolling causes microinjuries to the skin, thereby diverting blood supply to the follicles and stimulating wound regeneration, a process suggested to induce follicle regeneration. This effect is due to increased expression of vascular endothelial growth factor after cutaneous injury, a mediator of both hair growth and cycling as well as wound repair.7 Adjuvant scalp rolling creates a synergistic effect by facilitating absorption of topical and intralesional therapies. The physical breakdown of dermal capillary barriers creates microchannels that traverse the stratum corneum, improving the permeability of small-molecule substances and allowing for relatively painless and uniform delivery of combination therapies. A secondary benefit is hypertrophy, which counteracts the atrophy caused by topical steroids via collagen induction.7
Additionally, scalp rolling confers minimal risk to the patient, making it safer than conventional pharmacologic therapies such as corticosteroids or Janus kinase (JAK) inhibitors. Although intralesional steroid injections are first-line treatments for limited disease, they can cause pain and skin atrophy.10 In one cohort of 54 patients, topical steroids were inferior to both oral and intralesional treatment, and oral steroids carried a systemic side-effect profile and worsening of comorbidities including hyperglycemia and hypertension as well as negative effects on bone density.11 Baricitinib, a JAK inhibitor, was the first systemic treatment to gain US Food and Drug Administration approval for severe AA.12 However, this novel therapeutic confers adverse effects including infection, acne, and hypercholesterolemia, as reported in the BRAVE-AA trials.13 More broadly, the US Food and Drug Administration warns of serious long-term risks such as cardiovascular events and malignancy.14 Given the tremendous potential of JAK inhibitors, further research is warranted to understand both the efficacy of topical formulations as well as the possible role of scalp rolling as its adjuvant.
Finally, scalp rolling is easily accessible and affordable to patients. Scalp rolling devices are readily available and affordable online, and they can be used autonomously at home. This pragmatic option allows patients to take control of their own treatment course and offers a financially feasible alternative to navigating insurance coverage as well as the need for extra office visits for medication refills and monitoring.
We report 3 cases of the use of scalp rolling as an adjuvant to conventional therapy for refractory AA in young patients. Although prospective research is required to establish causality and characterize age-related trends in treatment response, consideration of scalp rolling as an adjuvant to conventional therapy may help to optimize treatment regimens. Given its low risk for side effects and potential benefits, we recommend scalp rolling for patients with refractory AA.
To the Editor:
Alopecia areata (AA) is an autoimmune nonscarring hair loss disorder that can present at any age. Patients with AA have a disproportionately high comorbidity burden and low quality of life, often grappling with anxiety, depression, and psychosocial sequelae involving identity, such as reduced self-esteem.1,2 Although conventional therapies aim to reduce hair loss, none are curative.3 Response to treatment is highly unpredictable, with current data suggesting that up to 50% of patients recover within 1 year while 14% to 25% progress to either alopecia totalis (total scalp hair loss) or alopecia universalis (total body hair loss).4 Options for therapeutic intervention remain limited and vary in safety and effectiveness, warranting further research to identify optimal modalities and minimize side effects. Interestingly, scalp rolling has been used as an adjuvant to topical triamcinolone acetonide.3,5 However, the extent of its effect in combination with other therapies remains unclear. We report 3 pediatric patients with confirmed AA refractory to conventional topical treatment who experienced remarkable scalp hair regrowth after adding biweekly scalp rolling as an adjuvant therapy.
A 7-year-old boy with AA presented with 95% scalp hair loss of 7 months’ duration (Figure 1A)(patient 1). Prior treatments included mometasone solution and clobetasol solution 0.05%. After 3 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 1B). No pain, bleeding, or other side effects were reported.

An 11-year-old girl with AA presented with 100% hair loss of 7 months’ duration (Figure 2A)(patient 2). Prior treatments included fluocinonide solution and intralesional Kenalog injections. After 4 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 2B). No pain, bleeding, or other side effects were reported.

A 16-year-old boy with AA presented with 30% hair loss of 4 years’ duration (Figure 3A)(patient 3). Prior treatments included squaric acid and intralesional Kenalog injections. After 2 years of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth at 17 months (Figure 3B). No pain, bleeding, or other side effects were reported.

Scalp rolling—also known as microneedling—provides a multifactorial approach to hair regrowth in patients with AA. The mechanism of action involves both the hair cycle and wound repair pathways by stimulation of the dermal papillae and stem cells.6 Scalp rolling has been observed to induce the expression of several hair growth pathway mediators, such as WNT3A, β-catenin, vascular endothelial growth factor, and WNT10B.7 Wnt/β-catenin pathway signaling is integral to multiple aspects of the hair regrowth process, including hair morphogenesis, follicle regeneration, and growth of the shaft itself.8,9 Scalp rolling causes microinjuries to the skin, thereby diverting blood supply to the follicles and stimulating wound regeneration, a process suggested to induce follicle regeneration. This effect is due to increased expression of vascular endothelial growth factor after cutaneous injury, a mediator of both hair growth and cycling as well as wound repair.7 Adjuvant scalp rolling creates a synergistic effect by facilitating absorption of topical and intralesional therapies. The physical breakdown of dermal capillary barriers creates microchannels that traverse the stratum corneum, improving the permeability of small-molecule substances and allowing for relatively painless and uniform delivery of combination therapies. A secondary benefit is hypertrophy, which counteracts the atrophy caused by topical steroids via collagen induction.7
Additionally, scalp rolling confers minimal risk to the patient, making it safer than conventional pharmacologic therapies such as corticosteroids or Janus kinase (JAK) inhibitors. Although intralesional steroid injections are first-line treatments for limited disease, they can cause pain and skin atrophy.10 In one cohort of 54 patients, topical steroids were inferior to both oral and intralesional treatment, and oral steroids carried a systemic side-effect profile and worsening of comorbidities including hyperglycemia and hypertension as well as negative effects on bone density.11 Baricitinib, a JAK inhibitor, was the first systemic treatment to gain US Food and Drug Administration approval for severe AA.12 However, this novel therapeutic confers adverse effects including infection, acne, and hypercholesterolemia, as reported in the BRAVE-AA trials.13 More broadly, the US Food and Drug Administration warns of serious long-term risks such as cardiovascular events and malignancy.14 Given the tremendous potential of JAK inhibitors, further research is warranted to understand both the efficacy of topical formulations as well as the possible role of scalp rolling as its adjuvant.
Finally, scalp rolling is easily accessible and affordable to patients. Scalp rolling devices are readily available and affordable online, and they can be used autonomously at home. This pragmatic option allows patients to take control of their own treatment course and offers a financially feasible alternative to navigating insurance coverage as well as the need for extra office visits for medication refills and monitoring.
We report 3 cases of the use of scalp rolling as an adjuvant to conventional therapy for refractory AA in young patients. Although prospective research is required to establish causality and characterize age-related trends in treatment response, consideration of scalp rolling as an adjuvant to conventional therapy may help to optimize treatment regimens. Given its low risk for side effects and potential benefits, we recommend scalp rolling for patients with refractory AA.
1. Senna M, Ko J, Tosti A, et al. Alopecia areata treatment patterns, healthcare resource utilization, and comorbidities in the US population using insurance claims. Adv Ther. 2021;38:4646-4658.
2. Huang CH, Fu Y, Chi CC. Health-related quality of life, depression, and self-esteem in patients with androgenetic alopecia: a systematic review and meta-analysis. JAMA Dermatol. 2021;157:963-970.
3. Deepak SH, Shwetha S. Scalp roller therapy in resistant alopecia areata. J Cutan Aesthet Surg. 2014;7:61-62.
4. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options.Int J Trichology. 2018;10:51-60.
5. Ito T, Yoshimasu T, Furukawa F, et al. Three-microneedle device as an effective option for intralesional corticosteroid administration for the treatment of alopecia areata. J Dermatol. 2017;44:304-305.
6. Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
7. Kim YS, Jeong KH, Kim JE, et al. Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol. 2016;28:586-592.
8. Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166:1035-1042.
9. Myung PS, Takeo M, Ito M, et al. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 2013;133:31-41.
10. Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12.
11. Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276
12.
13. King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699.
14. US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. September 1, 2021.
1. Senna M, Ko J, Tosti A, et al. Alopecia areata treatment patterns, healthcare resource utilization, and comorbidities in the US population using insurance claims. Adv Ther. 2021;38:4646-4658.
2. Huang CH, Fu Y, Chi CC. Health-related quality of life, depression, and self-esteem in patients with androgenetic alopecia: a systematic review and meta-analysis. JAMA Dermatol. 2021;157:963-970.
3. Deepak SH, Shwetha S. Scalp roller therapy in resistant alopecia areata. J Cutan Aesthet Surg. 2014;7:61-62.
4. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options.Int J Trichology. 2018;10:51-60.
5. Ito T, Yoshimasu T, Furukawa F, et al. Three-microneedle device as an effective option for intralesional corticosteroid administration for the treatment of alopecia areata. J Dermatol. 2017;44:304-305.
6. Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
7. Kim YS, Jeong KH, Kim JE, et al. Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol. 2016;28:586-592.
8. Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166:1035-1042.
9. Myung PS, Takeo M, Ito M, et al. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 2013;133:31-41.
10. Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12.
11. Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276
12.
13. King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699.
14. US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. September 1, 2021.
Practice Points
- Alopecia areata (AA) is an autoimmune hair loss disorder with few effective treatments and no cure.
- Scalp rolling is a promising new treatment option that may stimulate hair regrowth by both direct collagen induction and indirect synergy with the use of topical medications.
- Dermatologists should be aware of scalp rolling as a safe, affordable, and potentially effective adjuvant to conventional therapy for AA.
Cryptococcus neoformans Panniculitis Unmasked: A Paradoxical Reaction to Therapy
To the Editor:
Cryptococcus neoformans is an opportunistic fungus with a predilection for immunocompromised hosts, including solid organ transplant recipients (SOTRs). However, the rapid emergence of diffuse panniculitis only upon the start of therapy for extracutaneous disease is a rare phenomenon. We report the case of a liver transplant recipient who developed a paradoxical inflammatory reaction after initiating liposomal amphotericin B therapy for disseminated C neoformans, which manifested as progressive indurated plaques histologically consistent with cryptococcal panniculitis.
A 44-year-old man who received an orthotopic liver transplant 12 months prior and was on prednisone (20 mg daily) and tacrolimus (7 mg total daily) was admitted for multifocal pneumonia complicated by septic shock. Blood and respiratory cultures grew C neoformans, and lumbar puncture evaluation of cerebrospinal fluid revealed the presence of Cryptococcus antigen in 1:40 titers. Liposomal amphotericin B 5 mg/kg intravenous daily and fluconazole 400 mg intravenous daily were administered starting on the fourth day of admission; maintenance tacrolimus and steroids were stopped. Within 36 hours of treatment initiation, an erythematous papular rash was noted on the extremities, which initially was deemed an infusion reaction. Over the next 6 days, the rash became progressively confluent and hyperpigmented. A dermatologist was consulted on the fifteenth day of admission.
Physical examination by dermatology revealed diffuse, hyperpigmented to erythematous macules on the torso, back, arms, and legs that coalesced into dusky indurated plaques along the thighs, right side of the flank, and right upper arm (Figure 1). Laboratory analysis revealed thrombocytopenia but was otherwise unremarkable. Histoplasma antigen and Coccidioides IgG and IgM enzyme immunoassays were negative, as were cytomegalovirus, HIV, and rapid plasma reagin test results. Blood culture testing was repeated, and the findings were negative.
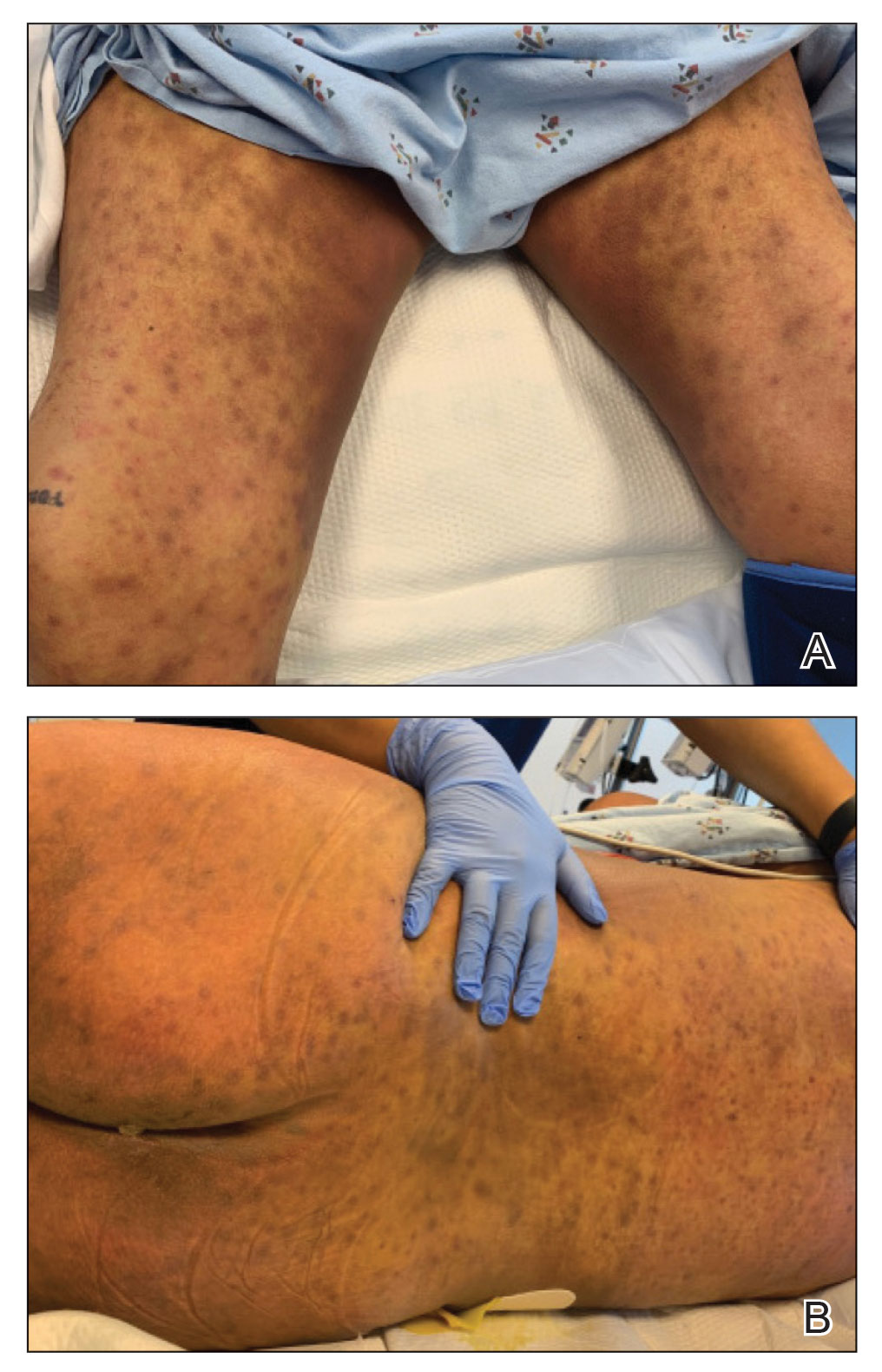
The emergence of the rash after amphotericin initiation prompted concern that the cause was due to a drug reaction rather than cutaneous involvement of cryptococcal infection. Punch biopsies were obtained from the thigh plaque. Hematoxylin and eosin and Grocott-Gomori methenamine-silver stains revealed cryptococcal organisms in the dermis and subcutaneous fat (Figure 2). Bacterial, acid-fast bacillus, and fungal cultures showed no growth.
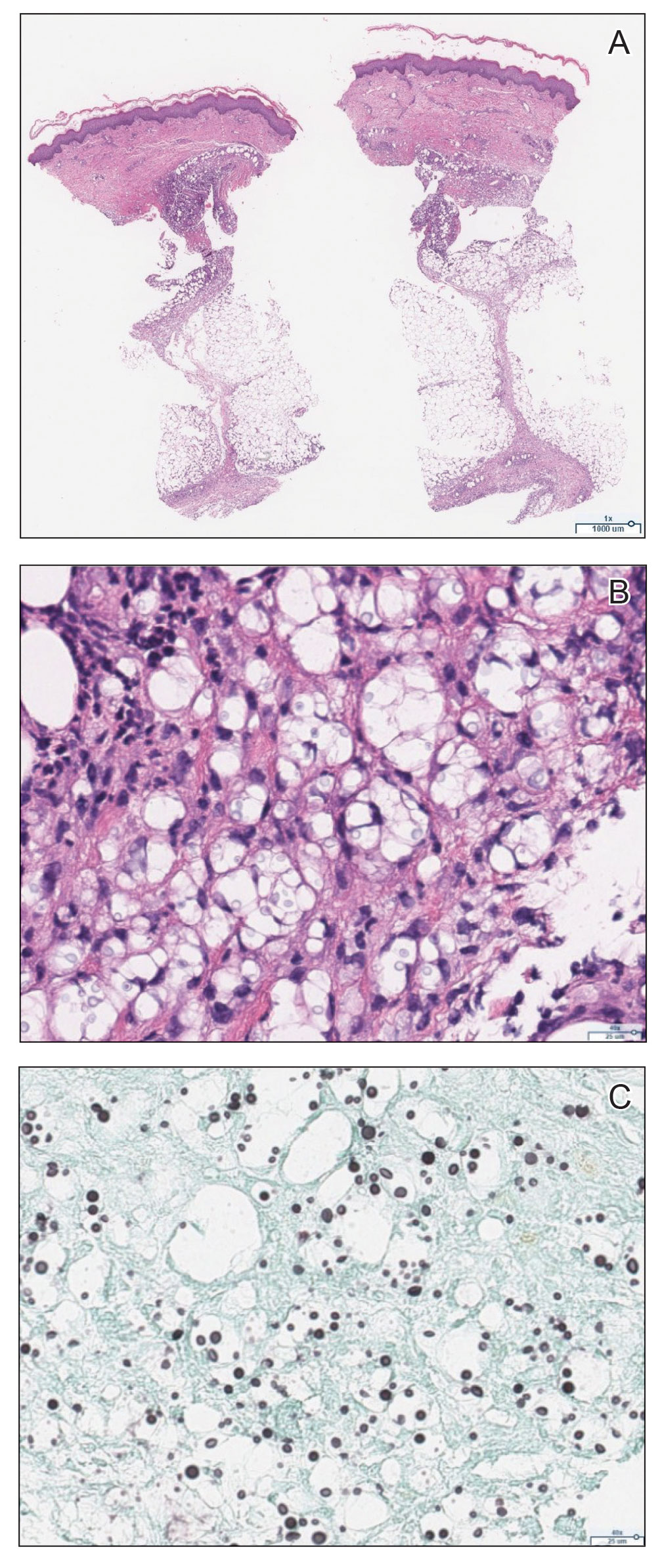
The patient was diagnosed with cryptococcal panniculitis. Induction therapy with liposomal amphotericin B 5 mg/kg daily and flucytosine 25 mg/kg twice daily was pursued. During the treatment, cutaneous involvement evolved into superficial desquamation. The patient ultimately died from shock secondary to persistent cryptococcal fungemia.
Cryptococcus neoformans is an opportunistic fungal infection that represents a notable hazard to SOTR, inflicting 1.5% to 2.8% of this population and carrying a 19% to 42% mortality rate.1,2 This infection occurs at a median of 1.6 to 2.3 years after transplantation,1,3 though liver transplant recipients and those with immune reconstitution inflammatory syndrome (IRIS)–like complications may present sooner (8.8 and 10.5 months, respectively).4 Cutaneous involvement comprises 17% to 21% of cases and is associated with extensive dissemination, including the central nervous system, lung, and bloodstream (61.5%, 23.1%, and 38.5%, respectively).1-3 When Cryptococcus infects the skin, it classically manifests as multiple nodules, umbilicated papules, ulcers, or cellulitis.3 Involvement of subcutaneous adipose tissue is uncommon and primarily is observed at initial presentation alongside disseminated disease.5-8 Our case is unique because cutaneous involvement was absent until treatment initiation.
Similar patterns of worsened or unmasked disease following treatment initiation have been observed in SOTRs with extracutaneous cryptococcus and were attributed to IRIS-like phenomena that generate a hyperactive inflammatory response to infection.4,9 Common immunosuppressive regimens, particularly tacrolimus, depress helper T cell (TH1) cytokine release and promote a TH2-dominant, anti-inflammatory state.10 In cryptococcosis, the fungus itself may stimulate a comparable cytokine milieu to promote immunologic evasion and dissemination. Cryptococcal IRIS-like responses in SOTRs are precipitated by rapid reduction or withdrawal of calcineurin inhibitors and corticosteroids, in combination with the inherent mitogenicity of the C neoformans polysaccharide capsule and antifungal agents.10 In our patient, cryptococcal yeasts may have invaded subcutaneous tissues when he became fungemic but remained subclinical due to minimal inflammatory recruitment. As treatment began and immunosuppressants diminished, fungal recognition and massive cytokine release resulted in frank panniculitis via precipitous immune dysregulation.
First-line therapy of cryptococcosis entails the use of liposomal amphotericin B and flucytosine for induction, followed by fluconazole for consolidation and maintenance. Use of corticosteroids is atypical to the antifungal regimen; however, a role for them has been suggested in severe IRIS involving individuals who are HIV positive, such as those with lesions demonstrating mass effect.11 Rare case reports have described their utility as adjunctive therapies against cryptococcus in SOTRs when treatment with antifungal agents alone failed.12 Given the paucity of prospective trials to support corticosteroid use in SOTRs as well as the worse global outcomes in cases of cryptococcal meningitis,13 therapeutic corticosteroids were not administered in our patient.
Although our case represents a rare event, cutaneous cryptococcosis and IRIS-like phenomena are clinically relevant complications in immunocompromised patients. In particular, they should be promptly considered in SOTRs receiving maintenance immunosuppressants who demonstrate symptom aggravation despite negative microbial culture results and uninterrupted antifungal therapy.
1. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375-381.
2. Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566-1576.
3. Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
4. Singh N, Lortholary O, Alexander BD, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40:1756-1761.
5. Reddy BY, Shaigany S, Schulman L, et al. Resident rounds part III: case report: fatal cryptococcal panniculitis in a lung transplant recipient. J Drugs Dermatol. 2015;14:519-252.
6. Bhowmik D, Dinda AK, Xess I, et al. Fungal panniculitis in renal transplant recipients. Transpl Infect Dis. 2008;10:286-289.
7. Gloster HM, Swerlick RA, Solomon AR. Cryptococcal cellulitis in a diabetic, kidney transplant patient. J Am Acad Dermatol. 1994;30:1025-1026.
8. Carlson KC, Mehlmauer M, Evans S, et al. Cryptococcal cellulitis in renal transplant recipients. J Am Acad Dermatol. 1987;17:469-472.
9. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101-107.
10. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395-401.
11. World Health Organization. Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Published March 1, 2018. Accessed September 6, 2020. https://www.who.int/publications/i/item/9789241550277
12. Lanternier F, Chandesris MO, Poirée S, et al. Cellulitis revealing a cryptococcosis-related immune reconstitution inflammatory syndrome in a renal allograft recipient. Am J Transpl. 2007;7:2826-2828.
13. Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374:542-554.
To the Editor:
Cryptococcus neoformans is an opportunistic fungus with a predilection for immunocompromised hosts, including solid organ transplant recipients (SOTRs). However, the rapid emergence of diffuse panniculitis only upon the start of therapy for extracutaneous disease is a rare phenomenon. We report the case of a liver transplant recipient who developed a paradoxical inflammatory reaction after initiating liposomal amphotericin B therapy for disseminated C neoformans, which manifested as progressive indurated plaques histologically consistent with cryptococcal panniculitis.
A 44-year-old man who received an orthotopic liver transplant 12 months prior and was on prednisone (20 mg daily) and tacrolimus (7 mg total daily) was admitted for multifocal pneumonia complicated by septic shock. Blood and respiratory cultures grew C neoformans, and lumbar puncture evaluation of cerebrospinal fluid revealed the presence of Cryptococcus antigen in 1:40 titers. Liposomal amphotericin B 5 mg/kg intravenous daily and fluconazole 400 mg intravenous daily were administered starting on the fourth day of admission; maintenance tacrolimus and steroids were stopped. Within 36 hours of treatment initiation, an erythematous papular rash was noted on the extremities, which initially was deemed an infusion reaction. Over the next 6 days, the rash became progressively confluent and hyperpigmented. A dermatologist was consulted on the fifteenth day of admission.
Physical examination by dermatology revealed diffuse, hyperpigmented to erythematous macules on the torso, back, arms, and legs that coalesced into dusky indurated plaques along the thighs, right side of the flank, and right upper arm (Figure 1). Laboratory analysis revealed thrombocytopenia but was otherwise unremarkable. Histoplasma antigen and Coccidioides IgG and IgM enzyme immunoassays were negative, as were cytomegalovirus, HIV, and rapid plasma reagin test results. Blood culture testing was repeated, and the findings were negative.

The emergence of the rash after amphotericin initiation prompted concern that the cause was due to a drug reaction rather than cutaneous involvement of cryptococcal infection. Punch biopsies were obtained from the thigh plaque. Hematoxylin and eosin and Grocott-Gomori methenamine-silver stains revealed cryptococcal organisms in the dermis and subcutaneous fat (Figure 2). Bacterial, acid-fast bacillus, and fungal cultures showed no growth.

The patient was diagnosed with cryptococcal panniculitis. Induction therapy with liposomal amphotericin B 5 mg/kg daily and flucytosine 25 mg/kg twice daily was pursued. During the treatment, cutaneous involvement evolved into superficial desquamation. The patient ultimately died from shock secondary to persistent cryptococcal fungemia.
Cryptococcus neoformans is an opportunistic fungal infection that represents a notable hazard to SOTR, inflicting 1.5% to 2.8% of this population and carrying a 19% to 42% mortality rate.1,2 This infection occurs at a median of 1.6 to 2.3 years after transplantation,1,3 though liver transplant recipients and those with immune reconstitution inflammatory syndrome (IRIS)–like complications may present sooner (8.8 and 10.5 months, respectively).4 Cutaneous involvement comprises 17% to 21% of cases and is associated with extensive dissemination, including the central nervous system, lung, and bloodstream (61.5%, 23.1%, and 38.5%, respectively).1-3 When Cryptococcus infects the skin, it classically manifests as multiple nodules, umbilicated papules, ulcers, or cellulitis.3 Involvement of subcutaneous adipose tissue is uncommon and primarily is observed at initial presentation alongside disseminated disease.5-8 Our case is unique because cutaneous involvement was absent until treatment initiation.
Similar patterns of worsened or unmasked disease following treatment initiation have been observed in SOTRs with extracutaneous cryptococcus and were attributed to IRIS-like phenomena that generate a hyperactive inflammatory response to infection.4,9 Common immunosuppressive regimens, particularly tacrolimus, depress helper T cell (TH1) cytokine release and promote a TH2-dominant, anti-inflammatory state.10 In cryptococcosis, the fungus itself may stimulate a comparable cytokine milieu to promote immunologic evasion and dissemination. Cryptococcal IRIS-like responses in SOTRs are precipitated by rapid reduction or withdrawal of calcineurin inhibitors and corticosteroids, in combination with the inherent mitogenicity of the C neoformans polysaccharide capsule and antifungal agents.10 In our patient, cryptococcal yeasts may have invaded subcutaneous tissues when he became fungemic but remained subclinical due to minimal inflammatory recruitment. As treatment began and immunosuppressants diminished, fungal recognition and massive cytokine release resulted in frank panniculitis via precipitous immune dysregulation.
First-line therapy of cryptococcosis entails the use of liposomal amphotericin B and flucytosine for induction, followed by fluconazole for consolidation and maintenance. Use of corticosteroids is atypical to the antifungal regimen; however, a role for them has been suggested in severe IRIS involving individuals who are HIV positive, such as those with lesions demonstrating mass effect.11 Rare case reports have described their utility as adjunctive therapies against cryptococcus in SOTRs when treatment with antifungal agents alone failed.12 Given the paucity of prospective trials to support corticosteroid use in SOTRs as well as the worse global outcomes in cases of cryptococcal meningitis,13 therapeutic corticosteroids were not administered in our patient.
Although our case represents a rare event, cutaneous cryptococcosis and IRIS-like phenomena are clinically relevant complications in immunocompromised patients. In particular, they should be promptly considered in SOTRs receiving maintenance immunosuppressants who demonstrate symptom aggravation despite negative microbial culture results and uninterrupted antifungal therapy.
To the Editor:
Cryptococcus neoformans is an opportunistic fungus with a predilection for immunocompromised hosts, including solid organ transplant recipients (SOTRs). However, the rapid emergence of diffuse panniculitis only upon the start of therapy for extracutaneous disease is a rare phenomenon. We report the case of a liver transplant recipient who developed a paradoxical inflammatory reaction after initiating liposomal amphotericin B therapy for disseminated C neoformans, which manifested as progressive indurated plaques histologically consistent with cryptococcal panniculitis.
A 44-year-old man who received an orthotopic liver transplant 12 months prior and was on prednisone (20 mg daily) and tacrolimus (7 mg total daily) was admitted for multifocal pneumonia complicated by septic shock. Blood and respiratory cultures grew C neoformans, and lumbar puncture evaluation of cerebrospinal fluid revealed the presence of Cryptococcus antigen in 1:40 titers. Liposomal amphotericin B 5 mg/kg intravenous daily and fluconazole 400 mg intravenous daily were administered starting on the fourth day of admission; maintenance tacrolimus and steroids were stopped. Within 36 hours of treatment initiation, an erythematous papular rash was noted on the extremities, which initially was deemed an infusion reaction. Over the next 6 days, the rash became progressively confluent and hyperpigmented. A dermatologist was consulted on the fifteenth day of admission.
Physical examination by dermatology revealed diffuse, hyperpigmented to erythematous macules on the torso, back, arms, and legs that coalesced into dusky indurated plaques along the thighs, right side of the flank, and right upper arm (Figure 1). Laboratory analysis revealed thrombocytopenia but was otherwise unremarkable. Histoplasma antigen and Coccidioides IgG and IgM enzyme immunoassays were negative, as were cytomegalovirus, HIV, and rapid plasma reagin test results. Blood culture testing was repeated, and the findings were negative.

The emergence of the rash after amphotericin initiation prompted concern that the cause was due to a drug reaction rather than cutaneous involvement of cryptococcal infection. Punch biopsies were obtained from the thigh plaque. Hematoxylin and eosin and Grocott-Gomori methenamine-silver stains revealed cryptococcal organisms in the dermis and subcutaneous fat (Figure 2). Bacterial, acid-fast bacillus, and fungal cultures showed no growth.

The patient was diagnosed with cryptococcal panniculitis. Induction therapy with liposomal amphotericin B 5 mg/kg daily and flucytosine 25 mg/kg twice daily was pursued. During the treatment, cutaneous involvement evolved into superficial desquamation. The patient ultimately died from shock secondary to persistent cryptococcal fungemia.
Cryptococcus neoformans is an opportunistic fungal infection that represents a notable hazard to SOTR, inflicting 1.5% to 2.8% of this population and carrying a 19% to 42% mortality rate.1,2 This infection occurs at a median of 1.6 to 2.3 years after transplantation,1,3 though liver transplant recipients and those with immune reconstitution inflammatory syndrome (IRIS)–like complications may present sooner (8.8 and 10.5 months, respectively).4 Cutaneous involvement comprises 17% to 21% of cases and is associated with extensive dissemination, including the central nervous system, lung, and bloodstream (61.5%, 23.1%, and 38.5%, respectively).1-3 When Cryptococcus infects the skin, it classically manifests as multiple nodules, umbilicated papules, ulcers, or cellulitis.3 Involvement of subcutaneous adipose tissue is uncommon and primarily is observed at initial presentation alongside disseminated disease.5-8 Our case is unique because cutaneous involvement was absent until treatment initiation.
Similar patterns of worsened or unmasked disease following treatment initiation have been observed in SOTRs with extracutaneous cryptococcus and were attributed to IRIS-like phenomena that generate a hyperactive inflammatory response to infection.4,9 Common immunosuppressive regimens, particularly tacrolimus, depress helper T cell (TH1) cytokine release and promote a TH2-dominant, anti-inflammatory state.10 In cryptococcosis, the fungus itself may stimulate a comparable cytokine milieu to promote immunologic evasion and dissemination. Cryptococcal IRIS-like responses in SOTRs are precipitated by rapid reduction or withdrawal of calcineurin inhibitors and corticosteroids, in combination with the inherent mitogenicity of the C neoformans polysaccharide capsule and antifungal agents.10 In our patient, cryptococcal yeasts may have invaded subcutaneous tissues when he became fungemic but remained subclinical due to minimal inflammatory recruitment. As treatment began and immunosuppressants diminished, fungal recognition and massive cytokine release resulted in frank panniculitis via precipitous immune dysregulation.
First-line therapy of cryptococcosis entails the use of liposomal amphotericin B and flucytosine for induction, followed by fluconazole for consolidation and maintenance. Use of corticosteroids is atypical to the antifungal regimen; however, a role for them has been suggested in severe IRIS involving individuals who are HIV positive, such as those with lesions demonstrating mass effect.11 Rare case reports have described their utility as adjunctive therapies against cryptococcus in SOTRs when treatment with antifungal agents alone failed.12 Given the paucity of prospective trials to support corticosteroid use in SOTRs as well as the worse global outcomes in cases of cryptococcal meningitis,13 therapeutic corticosteroids were not administered in our patient.
Although our case represents a rare event, cutaneous cryptococcosis and IRIS-like phenomena are clinically relevant complications in immunocompromised patients. In particular, they should be promptly considered in SOTRs receiving maintenance immunosuppressants who demonstrate symptom aggravation despite negative microbial culture results and uninterrupted antifungal therapy.
1. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375-381.
2. Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566-1576.
3. Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
4. Singh N, Lortholary O, Alexander BD, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40:1756-1761.
5. Reddy BY, Shaigany S, Schulman L, et al. Resident rounds part III: case report: fatal cryptococcal panniculitis in a lung transplant recipient. J Drugs Dermatol. 2015;14:519-252.
6. Bhowmik D, Dinda AK, Xess I, et al. Fungal panniculitis in renal transplant recipients. Transpl Infect Dis. 2008;10:286-289.
7. Gloster HM, Swerlick RA, Solomon AR. Cryptococcal cellulitis in a diabetic, kidney transplant patient. J Am Acad Dermatol. 1994;30:1025-1026.
8. Carlson KC, Mehlmauer M, Evans S, et al. Cryptococcal cellulitis in renal transplant recipients. J Am Acad Dermatol. 1987;17:469-472.
9. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101-107.
10. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395-401.
11. World Health Organization. Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Published March 1, 2018. Accessed September 6, 2020. https://www.who.int/publications/i/item/9789241550277
12. Lanternier F, Chandesris MO, Poirée S, et al. Cellulitis revealing a cryptococcosis-related immune reconstitution inflammatory syndrome in a renal allograft recipient. Am J Transpl. 2007;7:2826-2828.
13. Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374:542-554.
1. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375-381.
2. Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566-1576.
3. Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
4. Singh N, Lortholary O, Alexander BD, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40:1756-1761.
5. Reddy BY, Shaigany S, Schulman L, et al. Resident rounds part III: case report: fatal cryptococcal panniculitis in a lung transplant recipient. J Drugs Dermatol. 2015;14:519-252.
6. Bhowmik D, Dinda AK, Xess I, et al. Fungal panniculitis in renal transplant recipients. Transpl Infect Dis. 2008;10:286-289.
7. Gloster HM, Swerlick RA, Solomon AR. Cryptococcal cellulitis in a diabetic, kidney transplant patient. J Am Acad Dermatol. 1994;30:1025-1026.
8. Carlson KC, Mehlmauer M, Evans S, et al. Cryptococcal cellulitis in renal transplant recipients. J Am Acad Dermatol. 1987;17:469-472.
9. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101-107.
10. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395-401.
11. World Health Organization. Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Published March 1, 2018. Accessed September 6, 2020. https://www.who.int/publications/i/item/9789241550277
12. Lanternier F, Chandesris MO, Poirée S, et al. Cellulitis revealing a cryptococcosis-related immune reconstitution inflammatory syndrome in a renal allograft recipient. Am J Transpl. 2007;7:2826-2828.
13. Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374:542-554.
Practice Points
- Panniculitis caused by Cryptococcus neoformans is a rare complication in solid organ transplant recipients.
- Subclinical panniculitis from C neoformans may be unmasked during paradoxical inflammatory reactions as early as days following immunosuppressant withdrawal and treatment initiation.
Vitamin D deficiency linked to psoriasis severity
, suggesting that some people who increase their intake of the vitamin could better control this skin condition that affects up to 8 million people in the United States alone.
Brown University researchers studied almost 500 psoriasis cases taken from the National Health and Nutrition Examination Survey (NHANES), the scientists told attendees at the conference of the American Society for Nutrition. They compared the peoples’ reports on how much of their body surface was affected by psoriasis to vitamin D levels collected in blood samples.
“After adjusting for lifestyle factors such as smoking, the analysis showed that lower vitamin D levels and vitamin D deficiency were significantly associated with greater psoriasis severity,” the ASN said in a news release. “The researchers also found that patients with the least amount of body surface affected by psoriasis had the highest average vitamin D levels while those with the greatest affected area had the lowest average levels of vitamin D.”
The researchers said that people with psoriasis might improve their condition by getting more vitamin D in their diet and through supplements.
“Topical synthetic vitamin D creams are emerging as new therapies for psoriasis, but these usually require a doctor’s prescription,” said researcher Rachel K. Lim, an MD candidate at Brown University, Providence, R.I. “Our results suggest that a vitamin D–rich diet or oral vitamin D supplementation may also provide some benefit to psoriasis patients.”
The researchers said that vitamin D toxicity is rare but that people should consult with their medical caregivers before they start taking supplements.
A version of this article first appeared on WebMD.com.
, suggesting that some people who increase their intake of the vitamin could better control this skin condition that affects up to 8 million people in the United States alone.
Brown University researchers studied almost 500 psoriasis cases taken from the National Health and Nutrition Examination Survey (NHANES), the scientists told attendees at the conference of the American Society for Nutrition. They compared the peoples’ reports on how much of their body surface was affected by psoriasis to vitamin D levels collected in blood samples.
“After adjusting for lifestyle factors such as smoking, the analysis showed that lower vitamin D levels and vitamin D deficiency were significantly associated with greater psoriasis severity,” the ASN said in a news release. “The researchers also found that patients with the least amount of body surface affected by psoriasis had the highest average vitamin D levels while those with the greatest affected area had the lowest average levels of vitamin D.”
The researchers said that people with psoriasis might improve their condition by getting more vitamin D in their diet and through supplements.
“Topical synthetic vitamin D creams are emerging as new therapies for psoriasis, but these usually require a doctor’s prescription,” said researcher Rachel K. Lim, an MD candidate at Brown University, Providence, R.I. “Our results suggest that a vitamin D–rich diet or oral vitamin D supplementation may also provide some benefit to psoriasis patients.”
The researchers said that vitamin D toxicity is rare but that people should consult with their medical caregivers before they start taking supplements.
A version of this article first appeared on WebMD.com.
, suggesting that some people who increase their intake of the vitamin could better control this skin condition that affects up to 8 million people in the United States alone.
Brown University researchers studied almost 500 psoriasis cases taken from the National Health and Nutrition Examination Survey (NHANES), the scientists told attendees at the conference of the American Society for Nutrition. They compared the peoples’ reports on how much of their body surface was affected by psoriasis to vitamin D levels collected in blood samples.
“After adjusting for lifestyle factors such as smoking, the analysis showed that lower vitamin D levels and vitamin D deficiency were significantly associated with greater psoriasis severity,” the ASN said in a news release. “The researchers also found that patients with the least amount of body surface affected by psoriasis had the highest average vitamin D levels while those with the greatest affected area had the lowest average levels of vitamin D.”
The researchers said that people with psoriasis might improve their condition by getting more vitamin D in their diet and through supplements.
“Topical synthetic vitamin D creams are emerging as new therapies for psoriasis, but these usually require a doctor’s prescription,” said researcher Rachel K. Lim, an MD candidate at Brown University, Providence, R.I. “Our results suggest that a vitamin D–rich diet or oral vitamin D supplementation may also provide some benefit to psoriasis patients.”
The researchers said that vitamin D toxicity is rare but that people should consult with their medical caregivers before they start taking supplements.
A version of this article first appeared on WebMD.com.
FROM NUTRITION 2023
Lawsuit against insurer claims retaliation against docs for out-of-network referrals
The case, which has bounced around courts in the Golden State since 2012, pits the nearly 50,000-member California Medical Association (CMA) against Aetna, one of the nation’s largest health insurers. The physician group alleges that Aetna illegally retaliated against physicians who sent patients to certain out-of-network clinics.
Out-of-network providers and clinics were involved in just 4.7% of professional medical claims in 2020, according to a federal report released July 6, 2023. Such claims are more likely than others to be denied, and they result in unexpected medical bills, which have led to the passage of state and federal laws that target “surprise billing.”
In a July 17 ruling, the California Supreme Court unanimously resurrected the CMA v. Aetna case after a judge and a state appeals court killed it on the grounds that the CMA - which is affiliated with the American Medical Association (AMA) - had no standing to sue Aetna. The high state court declared that the CMA could sue on its own behalf, but the justices noted that their ruling says nothing about the merits of the case.
The ruling appears to mean that CMA’s lawsuit will head back to Superior Court in Los Angeles County. The outcome of the case won’t have a direct national effect, since the case is in state court, not federal court. However, state rulings can influence the thinking of judges elsewhere.
The case, filed in 2012, alleges that Aetna harmed patient care by harassing and sacking contract physicians who referred patients to out-of-network ambulatory surgery centers.
According to the new ruling, Aetna responded by saying that “its policy, rather than interfering in medical judgments, was designed simply to encourage participating physicians, consistent with their judgment, to use in-network care providers, such as ambulatory surgery centers, and was adopted in part in response to physicians referring patients to facilities in which they had financial interests.”
In a 2012 letter to CMA, as reported by the Los Angeles Times, an Aetna attorney went further and claimed that “physicians and their business partners secure outsized and improper windfalls at the expense of Aetna’s plan members and employer plan sponsors.”
The CMA received support for its lawsuit via friend-of-the-court legal briefs from the California attorney general, city attorneys for several major California cities, the AMA, several major labor unions, the AIDS Healthcare Foundation, and the advocacy organization Consumer Watchdog. The U.S. Chamber of Commerce, the California Association of Health Plans, and the Association of California Life and Health Insurance Companies filed briefs supporting Aetna.
Aetna, now part of CVS Health, declined to comment about the new ruling.
The CMA released a statement from its president, internist/hospitalist Donaldo M. Hernandez, MD: “The practice of threatening physicians who refer patients to out-of-network providers is unlawful, and we are pleased that the court agrees that CMA has the right to challenge these practices in court.”
In an interview, research professor emeritus Jack Hoadley, PhD, of the Health Policy Institute at Georgetown University’s McCourt School of Public Policy, noted that many health plans don’t cover out-of-network care. Those that do – including PPOs and hybrid plans – often require that patients pay a larger share of the total cost or pay a separate or higher deductible, he said.
So why would an insurer punish doctors who refer patients to health care providers who are outside the insurer’s approved network? In some cases, patients may blame insurers when they’re forced to pay higher rates for out-of-network care, Dr. Hoadley said. Insurers may also be miffed when physicians send patients out of network, he said, because insurers contract with physicians to send a certain number of patients within the insurer’s network.
The federal No Surprises Act, passed by Congress in 2020, hasn’t decreased tension between providers and insurers over out-of-network fees, Dr. Hoadley said. As the effects of the law are hammered out in court, he said, there’s still an adversarial relationship.
In California, the out-of-network landscape changed 3 years before the No Surprises Act. In 2017, the state passed its own no-surprise-billing law, which “protects consumers from surprise medical bills when they get non-emergency services, go to an in-network health facility and receive care from an out-of-network provider without their consent.” In these cases, the law says patients need to pay only in accordance with in-network cost sharing.
In 2019, a USC-Brookings Schaeffer Initiative for Health Policy report found signs that out-of-network care was fading in California other than in the emergency setting, possibly as a result of the law.
A version of this article first appeared on Medscape.com.
The case, which has bounced around courts in the Golden State since 2012, pits the nearly 50,000-member California Medical Association (CMA) against Aetna, one of the nation’s largest health insurers. The physician group alleges that Aetna illegally retaliated against physicians who sent patients to certain out-of-network clinics.
Out-of-network providers and clinics were involved in just 4.7% of professional medical claims in 2020, according to a federal report released July 6, 2023. Such claims are more likely than others to be denied, and they result in unexpected medical bills, which have led to the passage of state and federal laws that target “surprise billing.”
In a July 17 ruling, the California Supreme Court unanimously resurrected the CMA v. Aetna case after a judge and a state appeals court killed it on the grounds that the CMA - which is affiliated with the American Medical Association (AMA) - had no standing to sue Aetna. The high state court declared that the CMA could sue on its own behalf, but the justices noted that their ruling says nothing about the merits of the case.
The ruling appears to mean that CMA’s lawsuit will head back to Superior Court in Los Angeles County. The outcome of the case won’t have a direct national effect, since the case is in state court, not federal court. However, state rulings can influence the thinking of judges elsewhere.
The case, filed in 2012, alleges that Aetna harmed patient care by harassing and sacking contract physicians who referred patients to out-of-network ambulatory surgery centers.
According to the new ruling, Aetna responded by saying that “its policy, rather than interfering in medical judgments, was designed simply to encourage participating physicians, consistent with their judgment, to use in-network care providers, such as ambulatory surgery centers, and was adopted in part in response to physicians referring patients to facilities in which they had financial interests.”
In a 2012 letter to CMA, as reported by the Los Angeles Times, an Aetna attorney went further and claimed that “physicians and their business partners secure outsized and improper windfalls at the expense of Aetna’s plan members and employer plan sponsors.”
The CMA received support for its lawsuit via friend-of-the-court legal briefs from the California attorney general, city attorneys for several major California cities, the AMA, several major labor unions, the AIDS Healthcare Foundation, and the advocacy organization Consumer Watchdog. The U.S. Chamber of Commerce, the California Association of Health Plans, and the Association of California Life and Health Insurance Companies filed briefs supporting Aetna.
Aetna, now part of CVS Health, declined to comment about the new ruling.
The CMA released a statement from its president, internist/hospitalist Donaldo M. Hernandez, MD: “The practice of threatening physicians who refer patients to out-of-network providers is unlawful, and we are pleased that the court agrees that CMA has the right to challenge these practices in court.”
In an interview, research professor emeritus Jack Hoadley, PhD, of the Health Policy Institute at Georgetown University’s McCourt School of Public Policy, noted that many health plans don’t cover out-of-network care. Those that do – including PPOs and hybrid plans – often require that patients pay a larger share of the total cost or pay a separate or higher deductible, he said.
So why would an insurer punish doctors who refer patients to health care providers who are outside the insurer’s approved network? In some cases, patients may blame insurers when they’re forced to pay higher rates for out-of-network care, Dr. Hoadley said. Insurers may also be miffed when physicians send patients out of network, he said, because insurers contract with physicians to send a certain number of patients within the insurer’s network.
The federal No Surprises Act, passed by Congress in 2020, hasn’t decreased tension between providers and insurers over out-of-network fees, Dr. Hoadley said. As the effects of the law are hammered out in court, he said, there’s still an adversarial relationship.
In California, the out-of-network landscape changed 3 years before the No Surprises Act. In 2017, the state passed its own no-surprise-billing law, which “protects consumers from surprise medical bills when they get non-emergency services, go to an in-network health facility and receive care from an out-of-network provider without their consent.” In these cases, the law says patients need to pay only in accordance with in-network cost sharing.
In 2019, a USC-Brookings Schaeffer Initiative for Health Policy report found signs that out-of-network care was fading in California other than in the emergency setting, possibly as a result of the law.
A version of this article first appeared on Medscape.com.
The case, which has bounced around courts in the Golden State since 2012, pits the nearly 50,000-member California Medical Association (CMA) against Aetna, one of the nation’s largest health insurers. The physician group alleges that Aetna illegally retaliated against physicians who sent patients to certain out-of-network clinics.
Out-of-network providers and clinics were involved in just 4.7% of professional medical claims in 2020, according to a federal report released July 6, 2023. Such claims are more likely than others to be denied, and they result in unexpected medical bills, which have led to the passage of state and federal laws that target “surprise billing.”
In a July 17 ruling, the California Supreme Court unanimously resurrected the CMA v. Aetna case after a judge and a state appeals court killed it on the grounds that the CMA - which is affiliated with the American Medical Association (AMA) - had no standing to sue Aetna. The high state court declared that the CMA could sue on its own behalf, but the justices noted that their ruling says nothing about the merits of the case.
The ruling appears to mean that CMA’s lawsuit will head back to Superior Court in Los Angeles County. The outcome of the case won’t have a direct national effect, since the case is in state court, not federal court. However, state rulings can influence the thinking of judges elsewhere.
The case, filed in 2012, alleges that Aetna harmed patient care by harassing and sacking contract physicians who referred patients to out-of-network ambulatory surgery centers.
According to the new ruling, Aetna responded by saying that “its policy, rather than interfering in medical judgments, was designed simply to encourage participating physicians, consistent with their judgment, to use in-network care providers, such as ambulatory surgery centers, and was adopted in part in response to physicians referring patients to facilities in which they had financial interests.”
In a 2012 letter to CMA, as reported by the Los Angeles Times, an Aetna attorney went further and claimed that “physicians and their business partners secure outsized and improper windfalls at the expense of Aetna’s plan members and employer plan sponsors.”
The CMA received support for its lawsuit via friend-of-the-court legal briefs from the California attorney general, city attorneys for several major California cities, the AMA, several major labor unions, the AIDS Healthcare Foundation, and the advocacy organization Consumer Watchdog. The U.S. Chamber of Commerce, the California Association of Health Plans, and the Association of California Life and Health Insurance Companies filed briefs supporting Aetna.
Aetna, now part of CVS Health, declined to comment about the new ruling.
The CMA released a statement from its president, internist/hospitalist Donaldo M. Hernandez, MD: “The practice of threatening physicians who refer patients to out-of-network providers is unlawful, and we are pleased that the court agrees that CMA has the right to challenge these practices in court.”
In an interview, research professor emeritus Jack Hoadley, PhD, of the Health Policy Institute at Georgetown University’s McCourt School of Public Policy, noted that many health plans don’t cover out-of-network care. Those that do – including PPOs and hybrid plans – often require that patients pay a larger share of the total cost or pay a separate or higher deductible, he said.
So why would an insurer punish doctors who refer patients to health care providers who are outside the insurer’s approved network? In some cases, patients may blame insurers when they’re forced to pay higher rates for out-of-network care, Dr. Hoadley said. Insurers may also be miffed when physicians send patients out of network, he said, because insurers contract with physicians to send a certain number of patients within the insurer’s network.
The federal No Surprises Act, passed by Congress in 2020, hasn’t decreased tension between providers and insurers over out-of-network fees, Dr. Hoadley said. As the effects of the law are hammered out in court, he said, there’s still an adversarial relationship.
In California, the out-of-network landscape changed 3 years before the No Surprises Act. In 2017, the state passed its own no-surprise-billing law, which “protects consumers from surprise medical bills when they get non-emergency services, go to an in-network health facility and receive care from an out-of-network provider without their consent.” In these cases, the law says patients need to pay only in accordance with in-network cost sharing.
In 2019, a USC-Brookings Schaeffer Initiative for Health Policy report found signs that out-of-network care was fading in California other than in the emergency setting, possibly as a result of the law.
A version of this article first appeared on Medscape.com.
Social isolation linked to lower brain volume
Further, the association between social isolation and reduced brain volume appears to be at least partly mediated by depressive symptoms.
“We believe that efforts should be made to reduce social isolation among the elderly as much as possible,” investigator Toshiharu Ninomiya, MD, PhD, professor of epidemiology and public health at Kyushu University in Fukuoka, Japan, said in an interview.
The study was published online in Neurology.
A dementia prevention strategy
Dr. Ninomiya noted there have been several studies suggesting that social interaction is beneficial in preventing cognitive decline and the onset of dementia.
In addition, recent epidemiological studies have shown social isolation is associated with a risk for cognitive decline and dementia.
Although the investigators note that very little is known about the link between the two, some studies have shown that social isolation is linked with depressive symptoms in older adults, and late-life depression has been associated with brain atrophy.
To explore the potential link between social isolation and brain atrophy, as well as the role of depression as a potential mediator, the investigators studied nearly 9,000 citizens aged 65 and older as part of the Japan Prospective Studies Collaboration for Aging and Dementia (JPSC-AD), an ongoing, community-based nationwide cohort study of dementia in Japan.
Participants were recruited from eight research sites across Japan, and each had a baseline MRI scan between 2016 and 2018. The investigators excluded those with a dementia diagnosis at baseline. Self-reported frequency of social contact was categorized as every day, several times a week, several times a month, or seldom.
Participants also answered questions about medical history and treatment, antihypertensive or antidiabetic medications, exercise, current alcohol intake, and smoking habits. Depressive symptoms were assessed with the Geriatric Depression Scale. Of the participants, 57% were women, and the mean age was 73 years.
Lower brain volume
Total brain volume was lower in those with the lowest frequency of social contact vs. those with the highest frequency (67.3% vs. 67.8%). Less social contact was also linked to smaller temporal lobe, occipital lobe, cingulum, hippocampus, and amygdala volumes.
White matter lesion volume increased with fewer social interactions, from 0.26% in the most social group to 0.30% in the least.
Cognitive function was higher in participants who had daily social contact, compared with those who had the least contact (28 vs. 27 on the Mini-Mental State Examination; P < .001). Scores between 25 and 30 are considered normal.
Depressive symptoms were lower in the daily contact group, compared with the seldom-contact group (P < .001).
The team also found that lower frequency of social contact was significantly associated with the smaller superior, middle, or inferior temporal gyrus; and a smaller fusiform gyrus, transverse temporal gyrus, temporal pole, and entorhinal cortex, among other subregions.
Mediation analyses indicated that depressive symptoms accounted for only 15%-29% of the associations of lower frequency of social contact with each regional volume.
Worse physical health
The results also showed that socially isolated participants were more likely to have diabetes, to have hypertension, to smoke, and to be physically inactive.
“Cardiovascular risk factors have been reported to cause endothelial dysfunction in the brain, which could in turn lead to problems in maintaining microcirculation and blood-brain barrier function,” the investigators write.
Some epidemiological studies have associated cardiovascular risk factors with brain atrophy, they noted, which could have been one of the underlying mechanisms.
Another possibility is that reduced cognitive stimulation due to social isolation may cause brain atrophy, they add.
“Ultimately,” Dr. Ninomiya said, “the detailed mechanism of the relationship between social isolation and brain volume is not yet clear.”
He also said more research is needed to know whether the findings would apply to people in other countries.
In an accompanying editorial, Alexa Walter, PhD, and Danielle Sandsmark, MD, PhD, from the University of Pennsylvania, Philadelphia, note that isolation has been associated with many adverse health outcomes, including increased risk of heart disease, stroke, and premature death.
“Given these findings, future work considering social health factors in the context of neurological disease is an important area of research to consider. Additionally, leveraging other existing longitudinal studies could provide us with an opportunity to better understand these relationships within populations and inform public policy to address these issues,” Dr. Walter and Dr. Sandsmark write.
The study was funded by the Japan Agency for Medical Research and Development and Suntory Holdings Limited. Dr. Ninomiya reports receiving grants from Suntory Holdings Limited.
A version of this article first appeared on Medscape.com.
Further, the association between social isolation and reduced brain volume appears to be at least partly mediated by depressive symptoms.
“We believe that efforts should be made to reduce social isolation among the elderly as much as possible,” investigator Toshiharu Ninomiya, MD, PhD, professor of epidemiology and public health at Kyushu University in Fukuoka, Japan, said in an interview.
The study was published online in Neurology.
A dementia prevention strategy
Dr. Ninomiya noted there have been several studies suggesting that social interaction is beneficial in preventing cognitive decline and the onset of dementia.
In addition, recent epidemiological studies have shown social isolation is associated with a risk for cognitive decline and dementia.
Although the investigators note that very little is known about the link between the two, some studies have shown that social isolation is linked with depressive symptoms in older adults, and late-life depression has been associated with brain atrophy.
To explore the potential link between social isolation and brain atrophy, as well as the role of depression as a potential mediator, the investigators studied nearly 9,000 citizens aged 65 and older as part of the Japan Prospective Studies Collaboration for Aging and Dementia (JPSC-AD), an ongoing, community-based nationwide cohort study of dementia in Japan.
Participants were recruited from eight research sites across Japan, and each had a baseline MRI scan between 2016 and 2018. The investigators excluded those with a dementia diagnosis at baseline. Self-reported frequency of social contact was categorized as every day, several times a week, several times a month, or seldom.
Participants also answered questions about medical history and treatment, antihypertensive or antidiabetic medications, exercise, current alcohol intake, and smoking habits. Depressive symptoms were assessed with the Geriatric Depression Scale. Of the participants, 57% were women, and the mean age was 73 years.
Lower brain volume
Total brain volume was lower in those with the lowest frequency of social contact vs. those with the highest frequency (67.3% vs. 67.8%). Less social contact was also linked to smaller temporal lobe, occipital lobe, cingulum, hippocampus, and amygdala volumes.
White matter lesion volume increased with fewer social interactions, from 0.26% in the most social group to 0.30% in the least.
Cognitive function was higher in participants who had daily social contact, compared with those who had the least contact (28 vs. 27 on the Mini-Mental State Examination; P < .001). Scores between 25 and 30 are considered normal.
Depressive symptoms were lower in the daily contact group, compared with the seldom-contact group (P < .001).
The team also found that lower frequency of social contact was significantly associated with the smaller superior, middle, or inferior temporal gyrus; and a smaller fusiform gyrus, transverse temporal gyrus, temporal pole, and entorhinal cortex, among other subregions.
Mediation analyses indicated that depressive symptoms accounted for only 15%-29% of the associations of lower frequency of social contact with each regional volume.
Worse physical health
The results also showed that socially isolated participants were more likely to have diabetes, to have hypertension, to smoke, and to be physically inactive.
“Cardiovascular risk factors have been reported to cause endothelial dysfunction in the brain, which could in turn lead to problems in maintaining microcirculation and blood-brain barrier function,” the investigators write.
Some epidemiological studies have associated cardiovascular risk factors with brain atrophy, they noted, which could have been one of the underlying mechanisms.
Another possibility is that reduced cognitive stimulation due to social isolation may cause brain atrophy, they add.
“Ultimately,” Dr. Ninomiya said, “the detailed mechanism of the relationship between social isolation and brain volume is not yet clear.”
He also said more research is needed to know whether the findings would apply to people in other countries.
In an accompanying editorial, Alexa Walter, PhD, and Danielle Sandsmark, MD, PhD, from the University of Pennsylvania, Philadelphia, note that isolation has been associated with many adverse health outcomes, including increased risk of heart disease, stroke, and premature death.
“Given these findings, future work considering social health factors in the context of neurological disease is an important area of research to consider. Additionally, leveraging other existing longitudinal studies could provide us with an opportunity to better understand these relationships within populations and inform public policy to address these issues,” Dr. Walter and Dr. Sandsmark write.
The study was funded by the Japan Agency for Medical Research and Development and Suntory Holdings Limited. Dr. Ninomiya reports receiving grants from Suntory Holdings Limited.
A version of this article first appeared on Medscape.com.
Further, the association between social isolation and reduced brain volume appears to be at least partly mediated by depressive symptoms.
“We believe that efforts should be made to reduce social isolation among the elderly as much as possible,” investigator Toshiharu Ninomiya, MD, PhD, professor of epidemiology and public health at Kyushu University in Fukuoka, Japan, said in an interview.
The study was published online in Neurology.
A dementia prevention strategy
Dr. Ninomiya noted there have been several studies suggesting that social interaction is beneficial in preventing cognitive decline and the onset of dementia.
In addition, recent epidemiological studies have shown social isolation is associated with a risk for cognitive decline and dementia.
Although the investigators note that very little is known about the link between the two, some studies have shown that social isolation is linked with depressive symptoms in older adults, and late-life depression has been associated with brain atrophy.
To explore the potential link between social isolation and brain atrophy, as well as the role of depression as a potential mediator, the investigators studied nearly 9,000 citizens aged 65 and older as part of the Japan Prospective Studies Collaboration for Aging and Dementia (JPSC-AD), an ongoing, community-based nationwide cohort study of dementia in Japan.
Participants were recruited from eight research sites across Japan, and each had a baseline MRI scan between 2016 and 2018. The investigators excluded those with a dementia diagnosis at baseline. Self-reported frequency of social contact was categorized as every day, several times a week, several times a month, or seldom.
Participants also answered questions about medical history and treatment, antihypertensive or antidiabetic medications, exercise, current alcohol intake, and smoking habits. Depressive symptoms were assessed with the Geriatric Depression Scale. Of the participants, 57% were women, and the mean age was 73 years.
Lower brain volume
Total brain volume was lower in those with the lowest frequency of social contact vs. those with the highest frequency (67.3% vs. 67.8%). Less social contact was also linked to smaller temporal lobe, occipital lobe, cingulum, hippocampus, and amygdala volumes.
White matter lesion volume increased with fewer social interactions, from 0.26% in the most social group to 0.30% in the least.
Cognitive function was higher in participants who had daily social contact, compared with those who had the least contact (28 vs. 27 on the Mini-Mental State Examination; P < .001). Scores between 25 and 30 are considered normal.
Depressive symptoms were lower in the daily contact group, compared with the seldom-contact group (P < .001).
The team also found that lower frequency of social contact was significantly associated with the smaller superior, middle, or inferior temporal gyrus; and a smaller fusiform gyrus, transverse temporal gyrus, temporal pole, and entorhinal cortex, among other subregions.
Mediation analyses indicated that depressive symptoms accounted for only 15%-29% of the associations of lower frequency of social contact with each regional volume.
Worse physical health
The results also showed that socially isolated participants were more likely to have diabetes, to have hypertension, to smoke, and to be physically inactive.
“Cardiovascular risk factors have been reported to cause endothelial dysfunction in the brain, which could in turn lead to problems in maintaining microcirculation and blood-brain barrier function,” the investigators write.
Some epidemiological studies have associated cardiovascular risk factors with brain atrophy, they noted, which could have been one of the underlying mechanisms.
Another possibility is that reduced cognitive stimulation due to social isolation may cause brain atrophy, they add.
“Ultimately,” Dr. Ninomiya said, “the detailed mechanism of the relationship between social isolation and brain volume is not yet clear.”
He also said more research is needed to know whether the findings would apply to people in other countries.
In an accompanying editorial, Alexa Walter, PhD, and Danielle Sandsmark, MD, PhD, from the University of Pennsylvania, Philadelphia, note that isolation has been associated with many adverse health outcomes, including increased risk of heart disease, stroke, and premature death.
“Given these findings, future work considering social health factors in the context of neurological disease is an important area of research to consider. Additionally, leveraging other existing longitudinal studies could provide us with an opportunity to better understand these relationships within populations and inform public policy to address these issues,” Dr. Walter and Dr. Sandsmark write.
The study was funded by the Japan Agency for Medical Research and Development and Suntory Holdings Limited. Dr. Ninomiya reports receiving grants from Suntory Holdings Limited.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Stiff arteries may cause metabolic syndrome
New research published in the American Journal of Physiology found that arterial stiffness occurred before the presence of metabolic syndrome. A progressive rise in stiffness was associated with a cumulative increase in risk for the condition among the 3,862 people studied over a 7-year period starting in late adolescence.
Results revealed a notable sex difference: Arterial stiffness increased the risk for metabolic syndrome by 9% for males but only by 1% for females. Males were also five times more likely than females to have metabolic syndrome.
“It seems metabolic syndrome has a new risk factor we haven’t thought about,” said author Andrew O. Agbaje, MD, clinical epidemiologist and researcher, University of Eastern Finland, Kuopio.
Arterial stiffness previously was associated with metabolic syndrome in numerous studies. But the new work is the first to find evidence for causality, Dr. Agbaje said in an interview.
“Interventions have focused on addressing the components of metabolic syndrome such as obesity, dyslipidemia, hyperglycemia, and hypertension,” Dr. Agbaje said. “But arterial stiffness may independently cause metabolic syndrome in 1 out of 10 male teens. I encourage clinicians to think about its role in preventing and managing metabolic syndrome, not just as a consequence but as a cause.”
The results have important implications for physicians, according to Sissi Cossio, MD, pediatric endocrinologist, Pediatrix Medical Group, Fort Lauderdale, Fla.
“The fact that arterial stiffness progression preceded metabolic syndrome is important because it could be used as an earlier detection marker of disease,” Dr. Cossio said.
To conduct the study, Dr. Agbaje and his research team used data collected by the Avon Longitudinal Study of Parents and Children at the University of Bristol in England. Arterial stiffness was measured using carotid-femoral pulse wave velocity, the speed of blood flow from the upper to the lower aorta. They assessed for metabolic syndrome by the presence of three or more risk factors, including high cholesterol, high triglycerides, and high trunk fat mass.
Participants were studied starting in gestation in the early 1990s, and were measured for arterial stiffness and metabolic syndrome starting at age 17 through age 24.
The overall risk for metabolic syndrome doubled within the 7-year study period of follow-up between 2009 and 2017, indicating that early intervention during adolescence is essential.
Dr. Agbaje recommended that physicians start treating arterial stiffness and other markers of metabolic syndrome as early as possible, noting that, “potentially irreversible cardiovascular health damage might occur after age 17.”
Arterial stiffness can be negated through physical activity and dietary changes that lower inflammation. Physicians should refer at-risk teens to a preventative clinic where they can be monitored and receive repeated measurements of arterial stiffness, lipid levels, blood pressure, glucose levels, and obesity every 3 months, Dr. Agbaje said.
“The health progress made after a year would be an indicator for physicians whether a more aggressive therapeutic approach is needed since it takes about 7 years for the risk of metabolic syndrome attributed to arterial stiffness to worsen remarkably in the young population,” he said.
Dr. Agbaje pointed to a few potential pathways through which arterial stiffness might create a disease cascade. Stiffer arteries disrupt blood flow to the liver and pancreas, which could adversely affect their functioning, he said. Damage to these organs may increase insulin and LDL cholesterol blood levels, increasing the risk for metabolic syndrome.
Arterial stiffness also can lead to higher blood pressure and insulin resistance, potentially inducing musculogenesis and vasculogenesis. The resulting excessive muscle mass may also increase the risk for the condition, he said.
Dr. Cossio acknowledged that treatments for metabolic syndrome become less effective with age, but emphasized that reversal is possible in adults with lifestyle changes and medications.
“Early detection will give patients the best chance at reversing the disease, and [primary care physicians] are a key factor in this process,” she said.
Dr. Cossio said that at-risk teens should receive treatment in a weight loss or endocrinology clinic. Treatment may include behavioral, surgical, and pharmacotherapeutic interventions.
“Teens with signs of insulin resistance and impaired fasting glucose, acanthosis, or prediabetes, should start metformin as the first line of therapy,” Dr. Cossio said.
For weight management, she recommends antiobesity medications such as liraglutide, semaglutide, and the combination of phentermine/topiramate in children aged 12 years or older. In teenagers 16 years or older, phentermine alone is another option.
The research group that conducted the study reported received funding from the Jenny and Antti Wihuri Foundation, the North Savo Regional Fund and Central Finnish Cultural Foundation, the Aarne Koskelo Foundation, the Foundation for Pediatric Research, and the Finnish Foundation for Cardiovascular Research, among others. The authors declared no conflicts of interest, financial or otherwise.
A version of this article appeared on Medscape.com.
New research published in the American Journal of Physiology found that arterial stiffness occurred before the presence of metabolic syndrome. A progressive rise in stiffness was associated with a cumulative increase in risk for the condition among the 3,862 people studied over a 7-year period starting in late adolescence.
Results revealed a notable sex difference: Arterial stiffness increased the risk for metabolic syndrome by 9% for males but only by 1% for females. Males were also five times more likely than females to have metabolic syndrome.
“It seems metabolic syndrome has a new risk factor we haven’t thought about,” said author Andrew O. Agbaje, MD, clinical epidemiologist and researcher, University of Eastern Finland, Kuopio.
Arterial stiffness previously was associated with metabolic syndrome in numerous studies. But the new work is the first to find evidence for causality, Dr. Agbaje said in an interview.
“Interventions have focused on addressing the components of metabolic syndrome such as obesity, dyslipidemia, hyperglycemia, and hypertension,” Dr. Agbaje said. “But arterial stiffness may independently cause metabolic syndrome in 1 out of 10 male teens. I encourage clinicians to think about its role in preventing and managing metabolic syndrome, not just as a consequence but as a cause.”
The results have important implications for physicians, according to Sissi Cossio, MD, pediatric endocrinologist, Pediatrix Medical Group, Fort Lauderdale, Fla.
“The fact that arterial stiffness progression preceded metabolic syndrome is important because it could be used as an earlier detection marker of disease,” Dr. Cossio said.
To conduct the study, Dr. Agbaje and his research team used data collected by the Avon Longitudinal Study of Parents and Children at the University of Bristol in England. Arterial stiffness was measured using carotid-femoral pulse wave velocity, the speed of blood flow from the upper to the lower aorta. They assessed for metabolic syndrome by the presence of three or more risk factors, including high cholesterol, high triglycerides, and high trunk fat mass.
Participants were studied starting in gestation in the early 1990s, and were measured for arterial stiffness and metabolic syndrome starting at age 17 through age 24.
The overall risk for metabolic syndrome doubled within the 7-year study period of follow-up between 2009 and 2017, indicating that early intervention during adolescence is essential.
Dr. Agbaje recommended that physicians start treating arterial stiffness and other markers of metabolic syndrome as early as possible, noting that, “potentially irreversible cardiovascular health damage might occur after age 17.”
Arterial stiffness can be negated through physical activity and dietary changes that lower inflammation. Physicians should refer at-risk teens to a preventative clinic where they can be monitored and receive repeated measurements of arterial stiffness, lipid levels, blood pressure, glucose levels, and obesity every 3 months, Dr. Agbaje said.
“The health progress made after a year would be an indicator for physicians whether a more aggressive therapeutic approach is needed since it takes about 7 years for the risk of metabolic syndrome attributed to arterial stiffness to worsen remarkably in the young population,” he said.
Dr. Agbaje pointed to a few potential pathways through which arterial stiffness might create a disease cascade. Stiffer arteries disrupt blood flow to the liver and pancreas, which could adversely affect their functioning, he said. Damage to these organs may increase insulin and LDL cholesterol blood levels, increasing the risk for metabolic syndrome.
Arterial stiffness also can lead to higher blood pressure and insulin resistance, potentially inducing musculogenesis and vasculogenesis. The resulting excessive muscle mass may also increase the risk for the condition, he said.
Dr. Cossio acknowledged that treatments for metabolic syndrome become less effective with age, but emphasized that reversal is possible in adults with lifestyle changes and medications.
“Early detection will give patients the best chance at reversing the disease, and [primary care physicians] are a key factor in this process,” she said.
Dr. Cossio said that at-risk teens should receive treatment in a weight loss or endocrinology clinic. Treatment may include behavioral, surgical, and pharmacotherapeutic interventions.
“Teens with signs of insulin resistance and impaired fasting glucose, acanthosis, or prediabetes, should start metformin as the first line of therapy,” Dr. Cossio said.
For weight management, she recommends antiobesity medications such as liraglutide, semaglutide, and the combination of phentermine/topiramate in children aged 12 years or older. In teenagers 16 years or older, phentermine alone is another option.
The research group that conducted the study reported received funding from the Jenny and Antti Wihuri Foundation, the North Savo Regional Fund and Central Finnish Cultural Foundation, the Aarne Koskelo Foundation, the Foundation for Pediatric Research, and the Finnish Foundation for Cardiovascular Research, among others. The authors declared no conflicts of interest, financial or otherwise.
A version of this article appeared on Medscape.com.
New research published in the American Journal of Physiology found that arterial stiffness occurred before the presence of metabolic syndrome. A progressive rise in stiffness was associated with a cumulative increase in risk for the condition among the 3,862 people studied over a 7-year period starting in late adolescence.
Results revealed a notable sex difference: Arterial stiffness increased the risk for metabolic syndrome by 9% for males but only by 1% for females. Males were also five times more likely than females to have metabolic syndrome.
“It seems metabolic syndrome has a new risk factor we haven’t thought about,” said author Andrew O. Agbaje, MD, clinical epidemiologist and researcher, University of Eastern Finland, Kuopio.
Arterial stiffness previously was associated with metabolic syndrome in numerous studies. But the new work is the first to find evidence for causality, Dr. Agbaje said in an interview.
“Interventions have focused on addressing the components of metabolic syndrome such as obesity, dyslipidemia, hyperglycemia, and hypertension,” Dr. Agbaje said. “But arterial stiffness may independently cause metabolic syndrome in 1 out of 10 male teens. I encourage clinicians to think about its role in preventing and managing metabolic syndrome, not just as a consequence but as a cause.”
The results have important implications for physicians, according to Sissi Cossio, MD, pediatric endocrinologist, Pediatrix Medical Group, Fort Lauderdale, Fla.
“The fact that arterial stiffness progression preceded metabolic syndrome is important because it could be used as an earlier detection marker of disease,” Dr. Cossio said.
To conduct the study, Dr. Agbaje and his research team used data collected by the Avon Longitudinal Study of Parents and Children at the University of Bristol in England. Arterial stiffness was measured using carotid-femoral pulse wave velocity, the speed of blood flow from the upper to the lower aorta. They assessed for metabolic syndrome by the presence of three or more risk factors, including high cholesterol, high triglycerides, and high trunk fat mass.
Participants were studied starting in gestation in the early 1990s, and were measured for arterial stiffness and metabolic syndrome starting at age 17 through age 24.
The overall risk for metabolic syndrome doubled within the 7-year study period of follow-up between 2009 and 2017, indicating that early intervention during adolescence is essential.
Dr. Agbaje recommended that physicians start treating arterial stiffness and other markers of metabolic syndrome as early as possible, noting that, “potentially irreversible cardiovascular health damage might occur after age 17.”
Arterial stiffness can be negated through physical activity and dietary changes that lower inflammation. Physicians should refer at-risk teens to a preventative clinic where they can be monitored and receive repeated measurements of arterial stiffness, lipid levels, blood pressure, glucose levels, and obesity every 3 months, Dr. Agbaje said.
“The health progress made after a year would be an indicator for physicians whether a more aggressive therapeutic approach is needed since it takes about 7 years for the risk of metabolic syndrome attributed to arterial stiffness to worsen remarkably in the young population,” he said.
Dr. Agbaje pointed to a few potential pathways through which arterial stiffness might create a disease cascade. Stiffer arteries disrupt blood flow to the liver and pancreas, which could adversely affect their functioning, he said. Damage to these organs may increase insulin and LDL cholesterol blood levels, increasing the risk for metabolic syndrome.
Arterial stiffness also can lead to higher blood pressure and insulin resistance, potentially inducing musculogenesis and vasculogenesis. The resulting excessive muscle mass may also increase the risk for the condition, he said.
Dr. Cossio acknowledged that treatments for metabolic syndrome become less effective with age, but emphasized that reversal is possible in adults with lifestyle changes and medications.
“Early detection will give patients the best chance at reversing the disease, and [primary care physicians] are a key factor in this process,” she said.
Dr. Cossio said that at-risk teens should receive treatment in a weight loss or endocrinology clinic. Treatment may include behavioral, surgical, and pharmacotherapeutic interventions.
“Teens with signs of insulin resistance and impaired fasting glucose, acanthosis, or prediabetes, should start metformin as the first line of therapy,” Dr. Cossio said.
For weight management, she recommends antiobesity medications such as liraglutide, semaglutide, and the combination of phentermine/topiramate in children aged 12 years or older. In teenagers 16 years or older, phentermine alone is another option.
The research group that conducted the study reported received funding from the Jenny and Antti Wihuri Foundation, the North Savo Regional Fund and Central Finnish Cultural Foundation, the Aarne Koskelo Foundation, the Foundation for Pediatric Research, and the Finnish Foundation for Cardiovascular Research, among others. The authors declared no conflicts of interest, financial or otherwise.
A version of this article appeared on Medscape.com.
FROM AMERICAN JOURNAL OF PHYSIOLOGY