User login
Families with pulmonary fibrosis share trends in disease evolution
Family members with pulmonary fibrosis showed correlations for predicted forced vital capacity trajectories and computed tomography patterns, based on data from 101 individuals in 45 families.
Patients with familial pulmonary fibrosis (FPF), defined as fibrotic interstitial lung disease among two or more first-degree or second-degree relatives, have worse survival than that of patients with sporadic pulmonary fibrosis, wrote Tinne Goos, MD, of KU Leuven, Belgium, and colleagues. Diagnosis of FPF diagnosis is mainly based on family history, and data on intrafamilial correlations are lacking, they said.
In a study published in the journal Chest, the researchers identified FPF patients treated at a single center. The study population included 101 patients from 45 families; most of these (34) were siblings. Overall, 61.4% of the participants were men, 69.3% were ever-smokers, and 84.2% had idiopathic pulmonary fibrosis.
The analysis included data on computed tomography (CT) scanning and predicted forced vital capacity (FVC%), as well as age at diagnosis, treatment type, gender, smoking history, and date of diagnosis.
Overall, FVC%predicted was significantly correlated within families, with a correlation of 0.75. The annual change in FVC was –158.2 mL, and the annual change in FVC%predicted was –6.3%.
Sixty-five patients received antifibrotic treatment, and 18 received immunosuppressive treatment. Immunosuppressive treatment remained significantly correlated among families in a multivariate analysis, with a correlation of 0.77.
“Age at diagnosis correlated within a generation, while patients from a second generation were diagnosed younger,” the researchers noted. The current study findings and results from other studies suggest a genetic basis for FPF age of onset, and determining an age range for screening unaffected relatives based on the age at diagnosis of affected relatives might be useful, they said.
In addition, 42.2% of families showed concordance of CT scan patterns. Typical usual interstitial pneumonia (UIP) appeared in 35 patients, atypical UIP in 36 patients, UIP with emphysema in 9 patients, and findings incompatible with UIP in 21 patients.
The study findings were limited by several factors including the retrospective design and use of data from a single center, as well as by the changes in clinical practice guidelines between 2004 and 2019, with increases in genetic testing, the researchers noted.
However, the current study is the first known to report on FVC evolution within families in FPF, they said. Future studies of both intra- and interfamilial correlation and variability are needed to identify the genetic and environmental factors that may affect disease manifestation and progression, they concluded.
The study was supported by Research Foundation-Flanders. Dr. Goos had no financial conflicts to disclose.
Family members with pulmonary fibrosis showed correlations for predicted forced vital capacity trajectories and computed tomography patterns, based on data from 101 individuals in 45 families.
Patients with familial pulmonary fibrosis (FPF), defined as fibrotic interstitial lung disease among two or more first-degree or second-degree relatives, have worse survival than that of patients with sporadic pulmonary fibrosis, wrote Tinne Goos, MD, of KU Leuven, Belgium, and colleagues. Diagnosis of FPF diagnosis is mainly based on family history, and data on intrafamilial correlations are lacking, they said.
In a study published in the journal Chest, the researchers identified FPF patients treated at a single center. The study population included 101 patients from 45 families; most of these (34) were siblings. Overall, 61.4% of the participants were men, 69.3% were ever-smokers, and 84.2% had idiopathic pulmonary fibrosis.
The analysis included data on computed tomography (CT) scanning and predicted forced vital capacity (FVC%), as well as age at diagnosis, treatment type, gender, smoking history, and date of diagnosis.
Overall, FVC%predicted was significantly correlated within families, with a correlation of 0.75. The annual change in FVC was –158.2 mL, and the annual change in FVC%predicted was –6.3%.
Sixty-five patients received antifibrotic treatment, and 18 received immunosuppressive treatment. Immunosuppressive treatment remained significantly correlated among families in a multivariate analysis, with a correlation of 0.77.
“Age at diagnosis correlated within a generation, while patients from a second generation were diagnosed younger,” the researchers noted. The current study findings and results from other studies suggest a genetic basis for FPF age of onset, and determining an age range for screening unaffected relatives based on the age at diagnosis of affected relatives might be useful, they said.
In addition, 42.2% of families showed concordance of CT scan patterns. Typical usual interstitial pneumonia (UIP) appeared in 35 patients, atypical UIP in 36 patients, UIP with emphysema in 9 patients, and findings incompatible with UIP in 21 patients.
The study findings were limited by several factors including the retrospective design and use of data from a single center, as well as by the changes in clinical practice guidelines between 2004 and 2019, with increases in genetic testing, the researchers noted.
However, the current study is the first known to report on FVC evolution within families in FPF, they said. Future studies of both intra- and interfamilial correlation and variability are needed to identify the genetic and environmental factors that may affect disease manifestation and progression, they concluded.
The study was supported by Research Foundation-Flanders. Dr. Goos had no financial conflicts to disclose.
Family members with pulmonary fibrosis showed correlations for predicted forced vital capacity trajectories and computed tomography patterns, based on data from 101 individuals in 45 families.
Patients with familial pulmonary fibrosis (FPF), defined as fibrotic interstitial lung disease among two or more first-degree or second-degree relatives, have worse survival than that of patients with sporadic pulmonary fibrosis, wrote Tinne Goos, MD, of KU Leuven, Belgium, and colleagues. Diagnosis of FPF diagnosis is mainly based on family history, and data on intrafamilial correlations are lacking, they said.
In a study published in the journal Chest, the researchers identified FPF patients treated at a single center. The study population included 101 patients from 45 families; most of these (34) were siblings. Overall, 61.4% of the participants were men, 69.3% were ever-smokers, and 84.2% had idiopathic pulmonary fibrosis.
The analysis included data on computed tomography (CT) scanning and predicted forced vital capacity (FVC%), as well as age at diagnosis, treatment type, gender, smoking history, and date of diagnosis.
Overall, FVC%predicted was significantly correlated within families, with a correlation of 0.75. The annual change in FVC was –158.2 mL, and the annual change in FVC%predicted was –6.3%.
Sixty-five patients received antifibrotic treatment, and 18 received immunosuppressive treatment. Immunosuppressive treatment remained significantly correlated among families in a multivariate analysis, with a correlation of 0.77.
“Age at diagnosis correlated within a generation, while patients from a second generation were diagnosed younger,” the researchers noted. The current study findings and results from other studies suggest a genetic basis for FPF age of onset, and determining an age range for screening unaffected relatives based on the age at diagnosis of affected relatives might be useful, they said.
In addition, 42.2% of families showed concordance of CT scan patterns. Typical usual interstitial pneumonia (UIP) appeared in 35 patients, atypical UIP in 36 patients, UIP with emphysema in 9 patients, and findings incompatible with UIP in 21 patients.
The study findings were limited by several factors including the retrospective design and use of data from a single center, as well as by the changes in clinical practice guidelines between 2004 and 2019, with increases in genetic testing, the researchers noted.
However, the current study is the first known to report on FVC evolution within families in FPF, they said. Future studies of both intra- and interfamilial correlation and variability are needed to identify the genetic and environmental factors that may affect disease manifestation and progression, they concluded.
The study was supported by Research Foundation-Flanders. Dr. Goos had no financial conflicts to disclose.
FROM THE JOURNAL CHEST
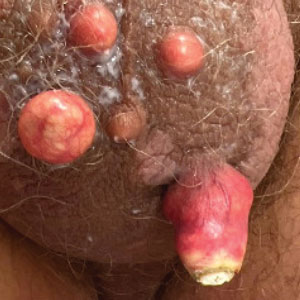
Multiple Nodules on the Scrotum
The Diagnosis: Scrotal Calcinosis
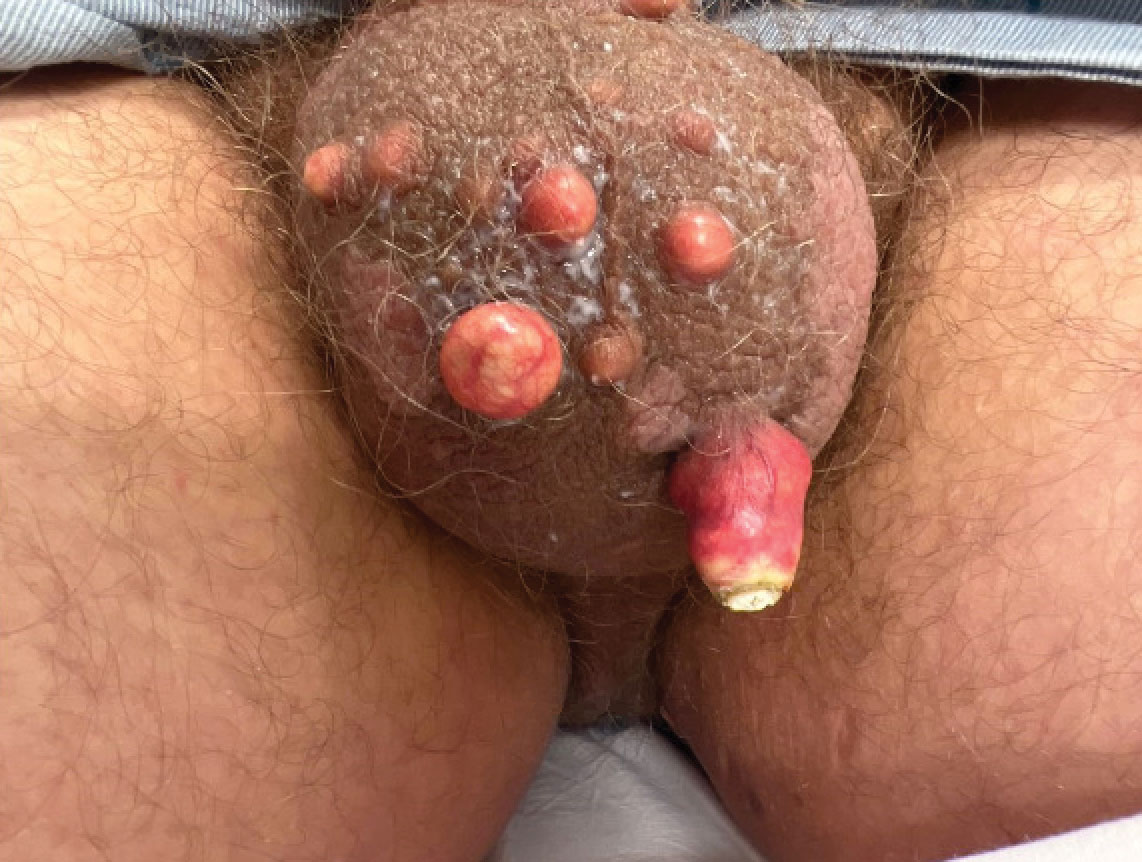
Scrotal calcinosis is a rare benign disease that results from the deposition of calcium, magnesium, phosphate, and carbonate within the dermis and subcutaneous layer of the skin in the absence of underlying systemic disease or serum calcium and phosphorus abnormalities.1,2 Lesions usually are asymptomatic but can be mildly painful or pruritic. They usually present in childhood or early adulthood as yellow-white firm nodules ranging in size from a few millimeters to a few centimeters that increase in size and number over time. Additionally, lesions can ulcerate and discharge a chalklike exudative material. Although benign in nature, the quality-of-life impact in patients with this condition can be substantial, specifically regarding cosmesis, which may cause patients to feel embarrassed and even avoid sexual activity. This condition rarely has been associated with infection.1
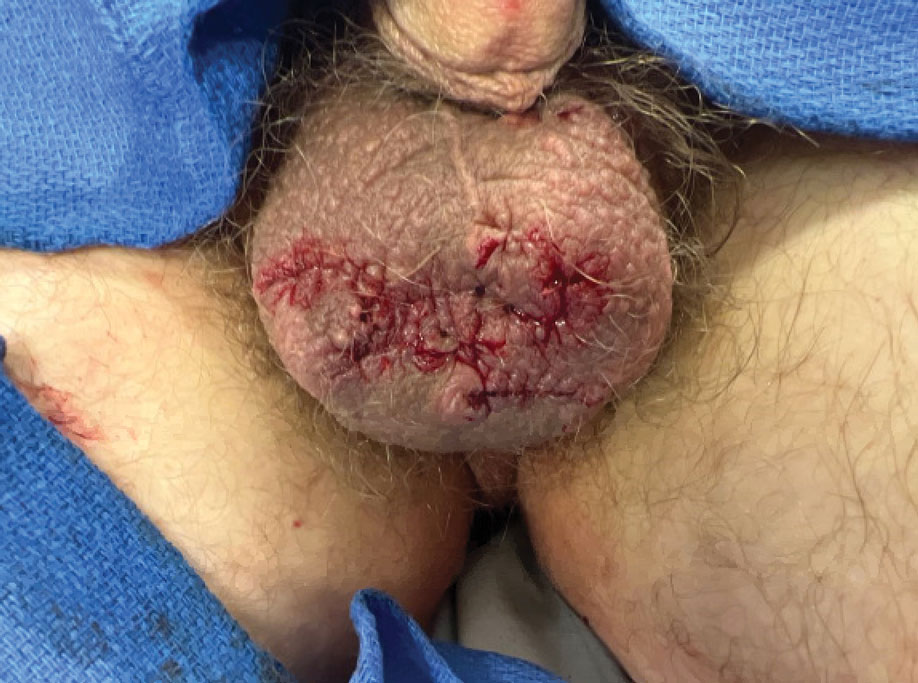
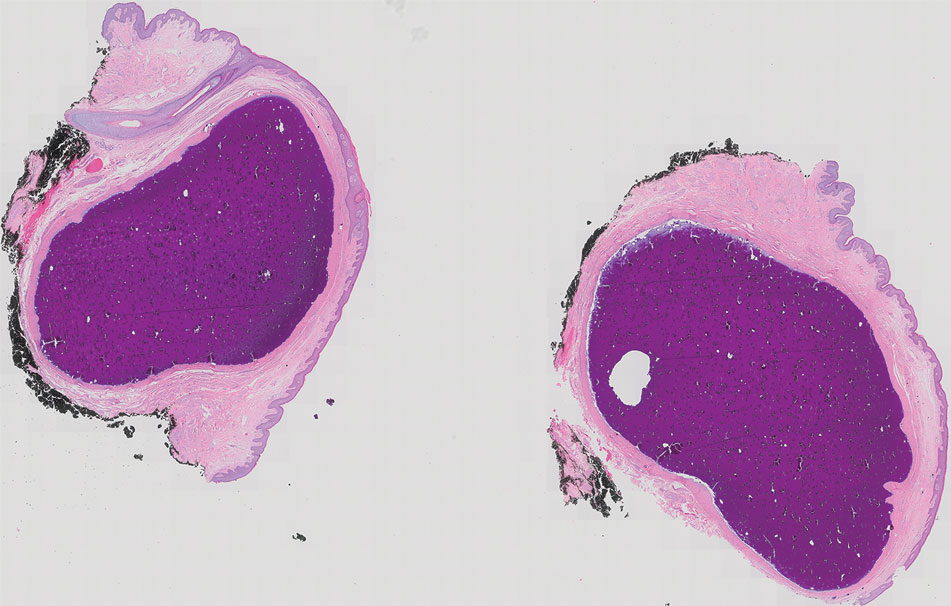
Our patient elected to undergo surgical excision under local anesthesia, and the lesions were sent for histopathologic examination. His postoperative course was unremarkable, and he was pleased with the cosmetic result of the surgery (Figure 1). Histopathology revealed calcified deposits that appeared as intradermal basophilic nodules lacking an epithelial lining (Figure 2), consistent with the diagnosis of scrotal calcinosis.2 No recurrence of the lesions was documented over the course of 18 months.
The pathogenesis of this condition is not clear. Most research supports scrotal calcinosis resulting from dystrophic calcification of epidermal inclusion cysts.3 There have been cases of scrotal calcinosis coinciding with epidermal inclusion cysts of the scrotum in varying stages of inflammation (some intact and some ruptured).2 Some research also suggests dystrophic calcification of eccrine epithelial cysts and degenerated dartos muscle as the origin of scrotal calcinosis.3
The differential diagnosis for this case included calcified steatocystoma multiplex, eruptive xanthomas, nodular scabies, and epidermal inclusion cysts. Steatocystoma multiplex can be inherited in an autosomal-dominant fashion or can develop sporadically with mutations in the KRT17 gene.4 It is characterized by multiple sebum-filled, cystic lesions of the pilosebaceous unit that may become calcified. Calcified lesions appear as yellow, firm, irregularly shaped papules or nodules ranging from a few millimeters to centimeters in size. Cysts can develop anywhere on the body with a predilection for the chest, upper extremities, axillae, trunk, groin, and scrotum.4 Histologically, our patient’s lesions were not associated with the pilosebaceous unit. Additionally, our patient denied a family history of similar skin lesions, which made calcified steatocystoma multiplex an unlikely diagnosis.
Eruptive xanthomas result from localized deposition of lipids within the dermis, typically in the setting of dyslipidemia or poorly controlled diabetes mellitus. They commonly appear on the extremities or buttocks as pruritic crops of yellow-red papules or nodules that are a few millimeters in size. Although our patient has a history of hyperlipidemia, his lesions differed substantially from eruptive xanthomas in clinical presentation.
Nodular scabies is a manifestation of classic scabies that presents with intensely pruritic erythematous papules and nodules that are a few millimeters in size and commonly occur on the axillae, groin, and genitalia. Our patient’s skin lesions were not pruritic and differed in appearance from nodular scabies.
Although research indicates scrotal calcinosis may result from dystrophic calcification of epidermal inclusion cysts,2 the latter present as dome-shaped, flesh-colored nodules with central pores representing the opening of hair follicles. Our patient lacked characteristic findings of epidermal inclusion cysts on histology.
The preferred treatment for scrotal calcinosis is surgical excision, which improves the aesthetic appearance, relieves itch, and removes ulcerative lesions.5 Additionally, surgical excision provides histological diagnostic confirmation. Recurrence with incomplete excision is possible; therefore, all lesions should be completely excised to reduce the risk for recurrence.3
- Pompeo A, Molina WR, Pohlman GD, et al. Idiopathic scrotal calcinosis: a rare entity and a review of the literature. Can Urol Assoc J. 2013;7:E439-E441. doi:10.5489/cuaj.1387
- Swinehart JM, Golitz LE. Scrotal calcinosis: dystrophic calcification of epidermoid cysts. Arch Dermatol. 1982;118:985-988. doi:10.1001 /archderm.1982.01650240029016
- Khallouk A, Yazami OE, Mellas S, et al. Idiopathic scrotal calcinosis: a nonelucidated pathogenesis and its surgical treatment. Rev Urol. 2011;13:95-97.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480. doi:10.1046/j.1365-2133.1998.02413.x
- Solanki A, Narang S, Kathpalia R, et al. Scrotal calcinosis: pathogenetic link with epidermal cyst. BMJ Case Rep. 2015;2015:bcr2015211163. doi:10.1136/bcr-2015-211163
The Diagnosis: Scrotal Calcinosis
Scrotal calcinosis is a rare benign disease that results from the deposition of calcium, magnesium, phosphate, and carbonate within the dermis and subcutaneous layer of the skin in the absence of underlying systemic disease or serum calcium and phosphorus abnormalities.1,2 Lesions usually are asymptomatic but can be mildly painful or pruritic. They usually present in childhood or early adulthood as yellow-white firm nodules ranging in size from a few millimeters to a few centimeters that increase in size and number over time. Additionally, lesions can ulcerate and discharge a chalklike exudative material. Although benign in nature, the quality-of-life impact in patients with this condition can be substantial, specifically regarding cosmesis, which may cause patients to feel embarrassed and even avoid sexual activity. This condition rarely has been associated with infection.1
Our patient elected to undergo surgical excision under local anesthesia, and the lesions were sent for histopathologic examination. His postoperative course was unremarkable, and he was pleased with the cosmetic result of the surgery (Figure 1). Histopathology revealed calcified deposits that appeared as intradermal basophilic nodules lacking an epithelial lining (Figure 2), consistent with the diagnosis of scrotal calcinosis.2 No recurrence of the lesions was documented over the course of 18 months.

The pathogenesis of this condition is not clear. Most research supports scrotal calcinosis resulting from dystrophic calcification of epidermal inclusion cysts.3 There have been cases of scrotal calcinosis coinciding with epidermal inclusion cysts of the scrotum in varying stages of inflammation (some intact and some ruptured).2 Some research also suggests dystrophic calcification of eccrine epithelial cysts and degenerated dartos muscle as the origin of scrotal calcinosis.3

The differential diagnosis for this case included calcified steatocystoma multiplex, eruptive xanthomas, nodular scabies, and epidermal inclusion cysts. Steatocystoma multiplex can be inherited in an autosomal-dominant fashion or can develop sporadically with mutations in the KRT17 gene.4 It is characterized by multiple sebum-filled, cystic lesions of the pilosebaceous unit that may become calcified. Calcified lesions appear as yellow, firm, irregularly shaped papules or nodules ranging from a few millimeters to centimeters in size. Cysts can develop anywhere on the body with a predilection for the chest, upper extremities, axillae, trunk, groin, and scrotum.4 Histologically, our patient’s lesions were not associated with the pilosebaceous unit. Additionally, our patient denied a family history of similar skin lesions, which made calcified steatocystoma multiplex an unlikely diagnosis.
Eruptive xanthomas result from localized deposition of lipids within the dermis, typically in the setting of dyslipidemia or poorly controlled diabetes mellitus. They commonly appear on the extremities or buttocks as pruritic crops of yellow-red papules or nodules that are a few millimeters in size. Although our patient has a history of hyperlipidemia, his lesions differed substantially from eruptive xanthomas in clinical presentation.
Nodular scabies is a manifestation of classic scabies that presents with intensely pruritic erythematous papules and nodules that are a few millimeters in size and commonly occur on the axillae, groin, and genitalia. Our patient’s skin lesions were not pruritic and differed in appearance from nodular scabies.
Although research indicates scrotal calcinosis may result from dystrophic calcification of epidermal inclusion cysts,2 the latter present as dome-shaped, flesh-colored nodules with central pores representing the opening of hair follicles. Our patient lacked characteristic findings of epidermal inclusion cysts on histology.
The preferred treatment for scrotal calcinosis is surgical excision, which improves the aesthetic appearance, relieves itch, and removes ulcerative lesions.5 Additionally, surgical excision provides histological diagnostic confirmation. Recurrence with incomplete excision is possible; therefore, all lesions should be completely excised to reduce the risk for recurrence.3
The Diagnosis: Scrotal Calcinosis
Scrotal calcinosis is a rare benign disease that results from the deposition of calcium, magnesium, phosphate, and carbonate within the dermis and subcutaneous layer of the skin in the absence of underlying systemic disease or serum calcium and phosphorus abnormalities.1,2 Lesions usually are asymptomatic but can be mildly painful or pruritic. They usually present in childhood or early adulthood as yellow-white firm nodules ranging in size from a few millimeters to a few centimeters that increase in size and number over time. Additionally, lesions can ulcerate and discharge a chalklike exudative material. Although benign in nature, the quality-of-life impact in patients with this condition can be substantial, specifically regarding cosmesis, which may cause patients to feel embarrassed and even avoid sexual activity. This condition rarely has been associated with infection.1
Our patient elected to undergo surgical excision under local anesthesia, and the lesions were sent for histopathologic examination. His postoperative course was unremarkable, and he was pleased with the cosmetic result of the surgery (Figure 1). Histopathology revealed calcified deposits that appeared as intradermal basophilic nodules lacking an epithelial lining (Figure 2), consistent with the diagnosis of scrotal calcinosis.2 No recurrence of the lesions was documented over the course of 18 months.

The pathogenesis of this condition is not clear. Most research supports scrotal calcinosis resulting from dystrophic calcification of epidermal inclusion cysts.3 There have been cases of scrotal calcinosis coinciding with epidermal inclusion cysts of the scrotum in varying stages of inflammation (some intact and some ruptured).2 Some research also suggests dystrophic calcification of eccrine epithelial cysts and degenerated dartos muscle as the origin of scrotal calcinosis.3

The differential diagnosis for this case included calcified steatocystoma multiplex, eruptive xanthomas, nodular scabies, and epidermal inclusion cysts. Steatocystoma multiplex can be inherited in an autosomal-dominant fashion or can develop sporadically with mutations in the KRT17 gene.4 It is characterized by multiple sebum-filled, cystic lesions of the pilosebaceous unit that may become calcified. Calcified lesions appear as yellow, firm, irregularly shaped papules or nodules ranging from a few millimeters to centimeters in size. Cysts can develop anywhere on the body with a predilection for the chest, upper extremities, axillae, trunk, groin, and scrotum.4 Histologically, our patient’s lesions were not associated with the pilosebaceous unit. Additionally, our patient denied a family history of similar skin lesions, which made calcified steatocystoma multiplex an unlikely diagnosis.
Eruptive xanthomas result from localized deposition of lipids within the dermis, typically in the setting of dyslipidemia or poorly controlled diabetes mellitus. They commonly appear on the extremities or buttocks as pruritic crops of yellow-red papules or nodules that are a few millimeters in size. Although our patient has a history of hyperlipidemia, his lesions differed substantially from eruptive xanthomas in clinical presentation.
Nodular scabies is a manifestation of classic scabies that presents with intensely pruritic erythematous papules and nodules that are a few millimeters in size and commonly occur on the axillae, groin, and genitalia. Our patient’s skin lesions were not pruritic and differed in appearance from nodular scabies.
Although research indicates scrotal calcinosis may result from dystrophic calcification of epidermal inclusion cysts,2 the latter present as dome-shaped, flesh-colored nodules with central pores representing the opening of hair follicles. Our patient lacked characteristic findings of epidermal inclusion cysts on histology.
The preferred treatment for scrotal calcinosis is surgical excision, which improves the aesthetic appearance, relieves itch, and removes ulcerative lesions.5 Additionally, surgical excision provides histological diagnostic confirmation. Recurrence with incomplete excision is possible; therefore, all lesions should be completely excised to reduce the risk for recurrence.3
- Pompeo A, Molina WR, Pohlman GD, et al. Idiopathic scrotal calcinosis: a rare entity and a review of the literature. Can Urol Assoc J. 2013;7:E439-E441. doi:10.5489/cuaj.1387
- Swinehart JM, Golitz LE. Scrotal calcinosis: dystrophic calcification of epidermoid cysts. Arch Dermatol. 1982;118:985-988. doi:10.1001 /archderm.1982.01650240029016
- Khallouk A, Yazami OE, Mellas S, et al. Idiopathic scrotal calcinosis: a nonelucidated pathogenesis and its surgical treatment. Rev Urol. 2011;13:95-97.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480. doi:10.1046/j.1365-2133.1998.02413.x
- Solanki A, Narang S, Kathpalia R, et al. Scrotal calcinosis: pathogenetic link with epidermal cyst. BMJ Case Rep. 2015;2015:bcr2015211163. doi:10.1136/bcr-2015-211163
- Pompeo A, Molina WR, Pohlman GD, et al. Idiopathic scrotal calcinosis: a rare entity and a review of the literature. Can Urol Assoc J. 2013;7:E439-E441. doi:10.5489/cuaj.1387
- Swinehart JM, Golitz LE. Scrotal calcinosis: dystrophic calcification of epidermoid cysts. Arch Dermatol. 1982;118:985-988. doi:10.1001 /archderm.1982.01650240029016
- Khallouk A, Yazami OE, Mellas S, et al. Idiopathic scrotal calcinosis: a nonelucidated pathogenesis and its surgical treatment. Rev Urol. 2011;13:95-97.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480. doi:10.1046/j.1365-2133.1998.02413.x
- Solanki A, Narang S, Kathpalia R, et al. Scrotal calcinosis: pathogenetic link with epidermal cyst. BMJ Case Rep. 2015;2015:bcr2015211163. doi:10.1136/bcr-2015-211163
A 33-year-old man presented with progressively enlarging bumps on the scrotum that were present since adolescence. He had a history of hyperlipidemia but no history of systemic or autoimmune disease. The lesions were asymptomatic without associated pruritus, pain, or discharge. No treatments had been administered, and he had no known personal or family history of similar skin conditions or skin cancer. He endorsed a monogamous relationship with his wife. Physical examination revealed 15 firm, yellow-white, subcutaneous nodules on the scrotum that varied in size.
New Alzheimer’s drugs: Setting realistic expectations
With the Food and Drug Administration’s full stamp of approval in hand, Leqembi (lecanemab) is poised to catapult us into a new era of treatment for Alzheimer’s disease. And now that the donanemab trial data are out, there’s another antiamyloid drug waiting in the wings.
To finally have true disease-modifying therapies for Alzheimer’s disease is a massive step forward for a field that’s been plagued with disappointment. But these drugs come with serious concerns and unknowns. They will require complex decision-making, putting doctors, patients, and their families in a medical quandary.
Striking the right balance between cautious optimism and realistic expectations will be a formidable challenge.
Managing patient and family expectations
These drugs are no magic bullet. They slow down the dementia’s progression, buying patients more time (on the order of months) before they begin to experience significant worsening. We’ll need a lot more information from research and clinical experience before we can understand how meaningful that treatment effect is. Right now, it is unclear whether eligible patients and their families will even perceive tangible differences.
In the CLARITY-AD trial, participants on lecanemab experienced a 27% slowing in the rate of cognitive decline over 18 months. Donanemab was shown to slow decline in memory and cognition by about 35% over the same time frame in the TRAILBLAZER-ALZ 2 trial. That translates to more time for patients and their families to enjoy independence, maintain normal life, and stave off the most distressing parts of the disease.
But what happens after 18 months of treatment – will the treatment effect magnify or dissipate? How much time are we really buying in the long run? Counseling patients and their families is made all the more difficult when the answers to important questions like these remain to be seen.
Only a sliver of Alzheimer’s patients are current candidates
The fact is that most patients living with Alzheimer’s disease will not qualify for treatment with these drugs. Lecanemab is approved for people with early-stage disease, meaning their dementia is mild or they have mild cognitive impairment, which is a precursor to full-blown Alzheimer’s disease. Of the 6 million people in the United States living with Alzheimer’s, about 1.5 million are estimated to fall into that category. We can expect to see a similar qualifier for donanemab if it receives FDA approval, especially because that trial suggested a more pronounced treatment effect for patients in the earliest stages of the disease.
Even if a patient hits the sweet spot where they have just enough cognitive impairment, but not too much, they aren’t technically therapeutic candidates until prerequisite testing confirms amyloid protein accumulation in the brain via PET scan or cerebrospinal fluid analysis.
Even then, the FDA’s boxed warning for lecanemab recommends that patients undergo genetic testing for the apo E4 mutation to identify those at a particularly high risk for severe adverse effects including brain bleeding and swelling. This recommendation is not unreasonable considering that 15% of the Alzheimer’s population has two copies of the apo E4 mutation and fall into that high-risk group.
Significant risks
Antiamyloid drugs are well-known to cause serious side effects. In the lecanemab trial, 13% of participants receiving Leqembi experienced brain swelling (vs. 2% of participants receiving placebo) and 17% of participants had brain bleeding (vs. 9% of participants on placebo). In the donanemab trial, brain bleeding occurred in 31.4% of participants on the drug (vs. 13.6% on placebo) and swelling occurred in 24% (vs. 2.1% receiving placebo). Thankfully, in both trials, most of these adverse events did not produce significant symptoms, but in rare cases these events caused severe or catastrophic neurologic injury, including death.
How can we best guide patients and their families to weigh the uncertain benefits against potentially serious risks? We can start by considering the patient characteristics most likely to portend increased risk for serious side effects: apo E4 mutations, blood thinner use, and the presence of microhemorrhages on brain imaging. But after that, we’re left with a lot of uncertainty in terms of which patients are most likely to see meaningful clinical improvements from the drug and unknown factors that may increase the risks of treatment.
A costly therapy
Medicare plans to cover 80% of lecanemab’s steep cost of $26,500 per year. Still, that will leave many patients with a hefty copay, potentially over $6,000 per year. But that only scratches the surface. Consider the frequent medical visits, repeated brain scans, laboratory tests, and infusion center appointments. It’s been estimated that all-in, the treatment will actually cost about $90,000 per year.
Yes, Medicare will reimburse a large portion of that cost, but it adds up to an estimated $2 billion per year for about 85,000 patients. This will probably spur increases to Medicare premiums, among other economic consequences for the health care system.
We’ll probably have to wait for an FDA approval decision before we know where donanemab will be priced.
Logistical challenges could be a rate-limiting step
Ask anyone who’s tried to see a neurologist recently, and they’ll tell you that the wait for a new patient appointment is months long. The shortage of neurologists in the United States is already a crisis, and there are even fewer cognitive neurologists. How long will patients be forced to wait for their diagnosis?
Many geriatricians will get comfortable prescribing these drugs, but will our already overburdened primary care providers have the bandwidth to do the same? It’s a tall order.
A new world of Alzheimer’s treatments also means that the infrastructure of our health care systems will need to be ramped up. Lecanemab infusions are administered every 2 weeks and donanemab every 4 weeks. Infusion centers will need to accommodate a lot more patients. And those patients will need frequent brain scans, so neuroimaging centers will need to increase their capacity to perform many more brain MRI and PET scans.
Antiamyloid drugs: An exciting first step
The bottom line is that these drugs aren’t the Alzheimer’s holy grail: An accessible treatment that could stop the disease in its tracks or reverse cognitive impairment. They are, however, a very promising breakthrough.
Yes, there are a ton of kinks to work out here, but this is an exciting start. Alzheimer’s research is entering a renaissance era that will hopefully bring more groundbreaking developments. Better biomarkers to facilitate faster, easier diagnosis. More drugs that go beyond amyloid proteins for their therapeutic targets. Treatments for later-stage disease. Drugs that prevent dementia altogether.
Ultimately, these new antiamyloid beta drugs are an exciting indication that we will eventually have a toolkit of Alzheimer’s drugs to choose from. For now, we’ve taken a solid step forward and there is ample reason to be hopeful for the future.
Dr. Croll is assistant professor of neurology at Temple University, Philadelphia. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
With the Food and Drug Administration’s full stamp of approval in hand, Leqembi (lecanemab) is poised to catapult us into a new era of treatment for Alzheimer’s disease. And now that the donanemab trial data are out, there’s another antiamyloid drug waiting in the wings.
To finally have true disease-modifying therapies for Alzheimer’s disease is a massive step forward for a field that’s been plagued with disappointment. But these drugs come with serious concerns and unknowns. They will require complex decision-making, putting doctors, patients, and their families in a medical quandary.
Striking the right balance between cautious optimism and realistic expectations will be a formidable challenge.
Managing patient and family expectations
These drugs are no magic bullet. They slow down the dementia’s progression, buying patients more time (on the order of months) before they begin to experience significant worsening. We’ll need a lot more information from research and clinical experience before we can understand how meaningful that treatment effect is. Right now, it is unclear whether eligible patients and their families will even perceive tangible differences.
In the CLARITY-AD trial, participants on lecanemab experienced a 27% slowing in the rate of cognitive decline over 18 months. Donanemab was shown to slow decline in memory and cognition by about 35% over the same time frame in the TRAILBLAZER-ALZ 2 trial. That translates to more time for patients and their families to enjoy independence, maintain normal life, and stave off the most distressing parts of the disease.
But what happens after 18 months of treatment – will the treatment effect magnify or dissipate? How much time are we really buying in the long run? Counseling patients and their families is made all the more difficult when the answers to important questions like these remain to be seen.
Only a sliver of Alzheimer’s patients are current candidates
The fact is that most patients living with Alzheimer’s disease will not qualify for treatment with these drugs. Lecanemab is approved for people with early-stage disease, meaning their dementia is mild or they have mild cognitive impairment, which is a precursor to full-blown Alzheimer’s disease. Of the 6 million people in the United States living with Alzheimer’s, about 1.5 million are estimated to fall into that category. We can expect to see a similar qualifier for donanemab if it receives FDA approval, especially because that trial suggested a more pronounced treatment effect for patients in the earliest stages of the disease.
Even if a patient hits the sweet spot where they have just enough cognitive impairment, but not too much, they aren’t technically therapeutic candidates until prerequisite testing confirms amyloid protein accumulation in the brain via PET scan or cerebrospinal fluid analysis.
Even then, the FDA’s boxed warning for lecanemab recommends that patients undergo genetic testing for the apo E4 mutation to identify those at a particularly high risk for severe adverse effects including brain bleeding and swelling. This recommendation is not unreasonable considering that 15% of the Alzheimer’s population has two copies of the apo E4 mutation and fall into that high-risk group.
Significant risks
Antiamyloid drugs are well-known to cause serious side effects. In the lecanemab trial, 13% of participants receiving Leqembi experienced brain swelling (vs. 2% of participants receiving placebo) and 17% of participants had brain bleeding (vs. 9% of participants on placebo). In the donanemab trial, brain bleeding occurred in 31.4% of participants on the drug (vs. 13.6% on placebo) and swelling occurred in 24% (vs. 2.1% receiving placebo). Thankfully, in both trials, most of these adverse events did not produce significant symptoms, but in rare cases these events caused severe or catastrophic neurologic injury, including death.
How can we best guide patients and their families to weigh the uncertain benefits against potentially serious risks? We can start by considering the patient characteristics most likely to portend increased risk for serious side effects: apo E4 mutations, blood thinner use, and the presence of microhemorrhages on brain imaging. But after that, we’re left with a lot of uncertainty in terms of which patients are most likely to see meaningful clinical improvements from the drug and unknown factors that may increase the risks of treatment.
A costly therapy
Medicare plans to cover 80% of lecanemab’s steep cost of $26,500 per year. Still, that will leave many patients with a hefty copay, potentially over $6,000 per year. But that only scratches the surface. Consider the frequent medical visits, repeated brain scans, laboratory tests, and infusion center appointments. It’s been estimated that all-in, the treatment will actually cost about $90,000 per year.
Yes, Medicare will reimburse a large portion of that cost, but it adds up to an estimated $2 billion per year for about 85,000 patients. This will probably spur increases to Medicare premiums, among other economic consequences for the health care system.
We’ll probably have to wait for an FDA approval decision before we know where donanemab will be priced.
Logistical challenges could be a rate-limiting step
Ask anyone who’s tried to see a neurologist recently, and they’ll tell you that the wait for a new patient appointment is months long. The shortage of neurologists in the United States is already a crisis, and there are even fewer cognitive neurologists. How long will patients be forced to wait for their diagnosis?
Many geriatricians will get comfortable prescribing these drugs, but will our already overburdened primary care providers have the bandwidth to do the same? It’s a tall order.
A new world of Alzheimer’s treatments also means that the infrastructure of our health care systems will need to be ramped up. Lecanemab infusions are administered every 2 weeks and donanemab every 4 weeks. Infusion centers will need to accommodate a lot more patients. And those patients will need frequent brain scans, so neuroimaging centers will need to increase their capacity to perform many more brain MRI and PET scans.
Antiamyloid drugs: An exciting first step
The bottom line is that these drugs aren’t the Alzheimer’s holy grail: An accessible treatment that could stop the disease in its tracks or reverse cognitive impairment. They are, however, a very promising breakthrough.
Yes, there are a ton of kinks to work out here, but this is an exciting start. Alzheimer’s research is entering a renaissance era that will hopefully bring more groundbreaking developments. Better biomarkers to facilitate faster, easier diagnosis. More drugs that go beyond amyloid proteins for their therapeutic targets. Treatments for later-stage disease. Drugs that prevent dementia altogether.
Ultimately, these new antiamyloid beta drugs are an exciting indication that we will eventually have a toolkit of Alzheimer’s drugs to choose from. For now, we’ve taken a solid step forward and there is ample reason to be hopeful for the future.
Dr. Croll is assistant professor of neurology at Temple University, Philadelphia. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
With the Food and Drug Administration’s full stamp of approval in hand, Leqembi (lecanemab) is poised to catapult us into a new era of treatment for Alzheimer’s disease. And now that the donanemab trial data are out, there’s another antiamyloid drug waiting in the wings.
To finally have true disease-modifying therapies for Alzheimer’s disease is a massive step forward for a field that’s been plagued with disappointment. But these drugs come with serious concerns and unknowns. They will require complex decision-making, putting doctors, patients, and their families in a medical quandary.
Striking the right balance between cautious optimism and realistic expectations will be a formidable challenge.
Managing patient and family expectations
These drugs are no magic bullet. They slow down the dementia’s progression, buying patients more time (on the order of months) before they begin to experience significant worsening. We’ll need a lot more information from research and clinical experience before we can understand how meaningful that treatment effect is. Right now, it is unclear whether eligible patients and their families will even perceive tangible differences.
In the CLARITY-AD trial, participants on lecanemab experienced a 27% slowing in the rate of cognitive decline over 18 months. Donanemab was shown to slow decline in memory and cognition by about 35% over the same time frame in the TRAILBLAZER-ALZ 2 trial. That translates to more time for patients and their families to enjoy independence, maintain normal life, and stave off the most distressing parts of the disease.
But what happens after 18 months of treatment – will the treatment effect magnify or dissipate? How much time are we really buying in the long run? Counseling patients and their families is made all the more difficult when the answers to important questions like these remain to be seen.
Only a sliver of Alzheimer’s patients are current candidates
The fact is that most patients living with Alzheimer’s disease will not qualify for treatment with these drugs. Lecanemab is approved for people with early-stage disease, meaning their dementia is mild or they have mild cognitive impairment, which is a precursor to full-blown Alzheimer’s disease. Of the 6 million people in the United States living with Alzheimer’s, about 1.5 million are estimated to fall into that category. We can expect to see a similar qualifier for donanemab if it receives FDA approval, especially because that trial suggested a more pronounced treatment effect for patients in the earliest stages of the disease.
Even if a patient hits the sweet spot where they have just enough cognitive impairment, but not too much, they aren’t technically therapeutic candidates until prerequisite testing confirms amyloid protein accumulation in the brain via PET scan or cerebrospinal fluid analysis.
Even then, the FDA’s boxed warning for lecanemab recommends that patients undergo genetic testing for the apo E4 mutation to identify those at a particularly high risk for severe adverse effects including brain bleeding and swelling. This recommendation is not unreasonable considering that 15% of the Alzheimer’s population has two copies of the apo E4 mutation and fall into that high-risk group.
Significant risks
Antiamyloid drugs are well-known to cause serious side effects. In the lecanemab trial, 13% of participants receiving Leqembi experienced brain swelling (vs. 2% of participants receiving placebo) and 17% of participants had brain bleeding (vs. 9% of participants on placebo). In the donanemab trial, brain bleeding occurred in 31.4% of participants on the drug (vs. 13.6% on placebo) and swelling occurred in 24% (vs. 2.1% receiving placebo). Thankfully, in both trials, most of these adverse events did not produce significant symptoms, but in rare cases these events caused severe or catastrophic neurologic injury, including death.
How can we best guide patients and their families to weigh the uncertain benefits against potentially serious risks? We can start by considering the patient characteristics most likely to portend increased risk for serious side effects: apo E4 mutations, blood thinner use, and the presence of microhemorrhages on brain imaging. But after that, we’re left with a lot of uncertainty in terms of which patients are most likely to see meaningful clinical improvements from the drug and unknown factors that may increase the risks of treatment.
A costly therapy
Medicare plans to cover 80% of lecanemab’s steep cost of $26,500 per year. Still, that will leave many patients with a hefty copay, potentially over $6,000 per year. But that only scratches the surface. Consider the frequent medical visits, repeated brain scans, laboratory tests, and infusion center appointments. It’s been estimated that all-in, the treatment will actually cost about $90,000 per year.
Yes, Medicare will reimburse a large portion of that cost, but it adds up to an estimated $2 billion per year for about 85,000 patients. This will probably spur increases to Medicare premiums, among other economic consequences for the health care system.
We’ll probably have to wait for an FDA approval decision before we know where donanemab will be priced.
Logistical challenges could be a rate-limiting step
Ask anyone who’s tried to see a neurologist recently, and they’ll tell you that the wait for a new patient appointment is months long. The shortage of neurologists in the United States is already a crisis, and there are even fewer cognitive neurologists. How long will patients be forced to wait for their diagnosis?
Many geriatricians will get comfortable prescribing these drugs, but will our already overburdened primary care providers have the bandwidth to do the same? It’s a tall order.
A new world of Alzheimer’s treatments also means that the infrastructure of our health care systems will need to be ramped up. Lecanemab infusions are administered every 2 weeks and donanemab every 4 weeks. Infusion centers will need to accommodate a lot more patients. And those patients will need frequent brain scans, so neuroimaging centers will need to increase their capacity to perform many more brain MRI and PET scans.
Antiamyloid drugs: An exciting first step
The bottom line is that these drugs aren’t the Alzheimer’s holy grail: An accessible treatment that could stop the disease in its tracks or reverse cognitive impairment. They are, however, a very promising breakthrough.
Yes, there are a ton of kinks to work out here, but this is an exciting start. Alzheimer’s research is entering a renaissance era that will hopefully bring more groundbreaking developments. Better biomarkers to facilitate faster, easier diagnosis. More drugs that go beyond amyloid proteins for their therapeutic targets. Treatments for later-stage disease. Drugs that prevent dementia altogether.
Ultimately, these new antiamyloid beta drugs are an exciting indication that we will eventually have a toolkit of Alzheimer’s drugs to choose from. For now, we’ve taken a solid step forward and there is ample reason to be hopeful for the future.
Dr. Croll is assistant professor of neurology at Temple University, Philadelphia. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Methemoglobinemia Induced by Application of an Anesthetic Cream
To the Editor:
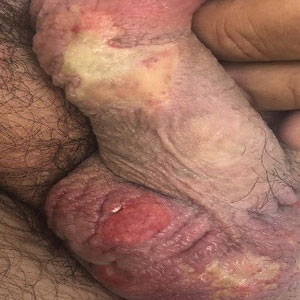
Methemoglobinemia (MetHb) is a condition caused by elevated levels of methemoglobin in the blood, which leads to an overall reduced ability of red blood cells to release oxygen to tissues, causing tissue hypoxia. Methemoglobinemia may be congenital or acquired. Various antibiotics and local anesthetics have been reported to induce acquired MetHb.1 We describe an adult who presented with MetHb resulting from excessive topical application of local anesthetics for painful scrotal ulcers.
A 54-year-old man presented with multiple scrotal and penile shaft ulcers of a few weeks’ duration with no systemic concerns. His medical history included chronic hepatitis C virus (HCV) and lumbar disc disease. Physical examination revealed multiple erosions and ulcers on an erythematous base involving the scrotal skin and distal penile shaft (Figure). Histopathology revealed acute leukocytoclastic vasculitis, and a laboratory workup was positive for mixed cryoglobulinemia that was thought to be HCV related. The patient was started on a systemic corticosteroid treatment in addition to sofosbuvir-velpatasvir for the treatment of HCV-related mixed cryoglobulinemic vasculitis. Concomitantly, the patient self-treated for pain with a local anesthetic cream containing lidocaine 2.5% and prilocaine 2.5%, applying it excessively every few hours daily for 2 weeks. He also intermittently used occlusive dressings.
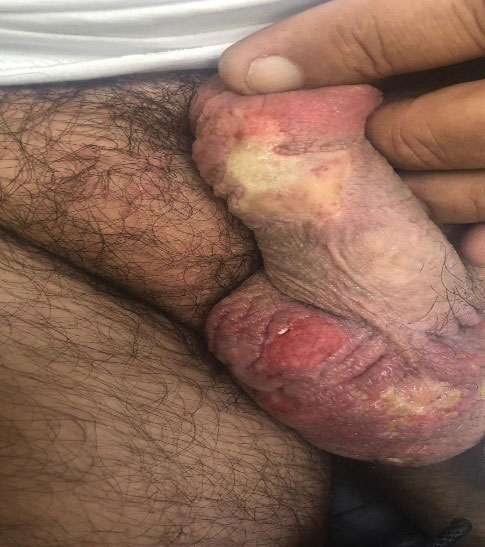
After 2 weeks of application, the patient developed lightheadedness and shortness of breath. He returned and was admitted for further evaluation. He had dyspnea and tachypnea of 22 breaths per minute. He also had mild tachycardia (109 beats per minute). He did not have a fever, and his blood pressure was normal. The oxygen saturation measured in ambient room air by pulse oximetry was 82%. A neurologic examination was normal except for mild drowsiness. The lungs were clear, and heart sounds were normal. A 12-lead electrocardiogram also was normal. A complete blood cell count showed severe macrocytic anemia with a hemoglobin level of 7 g/dL, which was a severe decline from the patient’s baseline level of 14 g/dL (reference range, 13–17 g/dL). A MetHb blood level of 11% was reported on co-oximetry. An arterial blood gas analysis revealed a pH of 7.46; partial pressure of carbon dioxide of 41 mm Hg; and partial pressure of oxygen of 63 mm Hg. The haptoglobin level was low at 2.6 mg/dL (reference range, 30–200 mg/dL). An absolute reticulocyte count was markedly elevated at 0.4×106/mL (reference range, 0.03–0.08×106/mL), lactate dehydrogenase was elevated at 430 U/L (reference range, 125–220 U/L), and indirect billirubin was high at 0.9 mg/dL (reference range, 0–0.5 mg/dL), consistent with hemolytic anemia. Electrolyte serum levels and renal function tests were within reference range. A diagnosis of MetHb induced by the lidocaine-prilocaine cream was rendered, and intravenous methylene blue 72 mg (1 mg/kg) was administered over 10 minutes. Within the next 60 minutes, the patient’s drowsiness and arterial desaturation resolved. A subsequent MetHb measurement taken several hours later was reduced to 4%. The patient remained asymptomatic and was eventually discharged.
Methemoglobinemia is an altered state of hemoglobin where the ferrous (Fe2+) ions of heme are oxidized to the ferric (Fe3+) state. These ferric ions are unable to bind oxygen, resulting in impaired oxygen delivery to tissues.1 Local anesthetics, which are strong oxidizers, have been reported to induce MetHb.2 In our patient, the extensive use of lidocaine 2.5%–prilocaine 2.5% cream resulted in severe life-threatening MetHb. The oxidizing properties of local anesthetics can be attributed to their chemical structure. Benzocaine is metabolized to potent oxidizers such as aniline, phenylhydroxylamine, and nitrobenzene.3 Prilocaine and another potent oxidizer, ortho-toluidine, which is a metabolite of prilocaine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+), leading to MetHb.2,3
Cases of anesthetic-induced MetHb primarily are associated with overuse of the product by applying it to large surface areas or using it for prolonged periods of time. In one case report, the occlusive dressing of the lidocaine-prilocaine cream applied to skin of the legs that was already abraded by laser epilation therapy resulted in MetHb.4 In our patient, applying the topical anesthetic to the eroded high-absorptive mucosal surface of the scrotal skin and the use of occlusive dressings increased the risk for toxicity. Absorption from scrotal skin is 40-times higher than the forearm.5 The face, axillae, and scalp also exhibit increased absorption compared to the forearm—10-, 4-, and 3-times higher, respectively.
In recent years, the use of topical anesthetics has greatly expanded due to the popularity of aesthetic and cosmetic procedures. These procedures often are performed in an outpatient setting.6 Dermatologists should be well aware of MetHb as a serious adverse effect and guide patients accordingly, as patients do not tend to consider a local anesthetic to be a drug. Drug interactions also may affect free lidocaine concentrations by liver cytochrome P450 metabolism; although this was not the case with our patient, special attention should be given to potential interactions that may exacerbate this serious adverse effect. Consideration should be given to patients applying the anesthetic to areas with high absorption capacity.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646-656.
- Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108:837-845.
- Jakobson B, Nilsson A. Methemoglobinemia associated with a prilocaine-lidocaine cream and trimethoprim-sulphamethoxazole. a case report. Acta Anaesthesiol Scand. 1985;29:453-455.
- Hahn I, Hoffman RS, Nelson LS. EMLA®-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26:85-88.
- Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181-183.
- Alster T. Review of lidocaine/tetracaine cream as a topical anesthetic for dermatologic laser procedures. Pain Ther. 2013;2:11-19.
To the Editor:
Methemoglobinemia (MetHb) is a condition caused by elevated levels of methemoglobin in the blood, which leads to an overall reduced ability of red blood cells to release oxygen to tissues, causing tissue hypoxia. Methemoglobinemia may be congenital or acquired. Various antibiotics and local anesthetics have been reported to induce acquired MetHb.1 We describe an adult who presented with MetHb resulting from excessive topical application of local anesthetics for painful scrotal ulcers.
A 54-year-old man presented with multiple scrotal and penile shaft ulcers of a few weeks’ duration with no systemic concerns. His medical history included chronic hepatitis C virus (HCV) and lumbar disc disease. Physical examination revealed multiple erosions and ulcers on an erythematous base involving the scrotal skin and distal penile shaft (Figure). Histopathology revealed acute leukocytoclastic vasculitis, and a laboratory workup was positive for mixed cryoglobulinemia that was thought to be HCV related. The patient was started on a systemic corticosteroid treatment in addition to sofosbuvir-velpatasvir for the treatment of HCV-related mixed cryoglobulinemic vasculitis. Concomitantly, the patient self-treated for pain with a local anesthetic cream containing lidocaine 2.5% and prilocaine 2.5%, applying it excessively every few hours daily for 2 weeks. He also intermittently used occlusive dressings.

After 2 weeks of application, the patient developed lightheadedness and shortness of breath. He returned and was admitted for further evaluation. He had dyspnea and tachypnea of 22 breaths per minute. He also had mild tachycardia (109 beats per minute). He did not have a fever, and his blood pressure was normal. The oxygen saturation measured in ambient room air by pulse oximetry was 82%. A neurologic examination was normal except for mild drowsiness. The lungs were clear, and heart sounds were normal. A 12-lead electrocardiogram also was normal. A complete blood cell count showed severe macrocytic anemia with a hemoglobin level of 7 g/dL, which was a severe decline from the patient’s baseline level of 14 g/dL (reference range, 13–17 g/dL). A MetHb blood level of 11% was reported on co-oximetry. An arterial blood gas analysis revealed a pH of 7.46; partial pressure of carbon dioxide of 41 mm Hg; and partial pressure of oxygen of 63 mm Hg. The haptoglobin level was low at 2.6 mg/dL (reference range, 30–200 mg/dL). An absolute reticulocyte count was markedly elevated at 0.4×106/mL (reference range, 0.03–0.08×106/mL), lactate dehydrogenase was elevated at 430 U/L (reference range, 125–220 U/L), and indirect billirubin was high at 0.9 mg/dL (reference range, 0–0.5 mg/dL), consistent with hemolytic anemia. Electrolyte serum levels and renal function tests were within reference range. A diagnosis of MetHb induced by the lidocaine-prilocaine cream was rendered, and intravenous methylene blue 72 mg (1 mg/kg) was administered over 10 minutes. Within the next 60 minutes, the patient’s drowsiness and arterial desaturation resolved. A subsequent MetHb measurement taken several hours later was reduced to 4%. The patient remained asymptomatic and was eventually discharged.
Methemoglobinemia is an altered state of hemoglobin where the ferrous (Fe2+) ions of heme are oxidized to the ferric (Fe3+) state. These ferric ions are unable to bind oxygen, resulting in impaired oxygen delivery to tissues.1 Local anesthetics, which are strong oxidizers, have been reported to induce MetHb.2 In our patient, the extensive use of lidocaine 2.5%–prilocaine 2.5% cream resulted in severe life-threatening MetHb. The oxidizing properties of local anesthetics can be attributed to their chemical structure. Benzocaine is metabolized to potent oxidizers such as aniline, phenylhydroxylamine, and nitrobenzene.3 Prilocaine and another potent oxidizer, ortho-toluidine, which is a metabolite of prilocaine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+), leading to MetHb.2,3
Cases of anesthetic-induced MetHb primarily are associated with overuse of the product by applying it to large surface areas or using it for prolonged periods of time. In one case report, the occlusive dressing of the lidocaine-prilocaine cream applied to skin of the legs that was already abraded by laser epilation therapy resulted in MetHb.4 In our patient, applying the topical anesthetic to the eroded high-absorptive mucosal surface of the scrotal skin and the use of occlusive dressings increased the risk for toxicity. Absorption from scrotal skin is 40-times higher than the forearm.5 The face, axillae, and scalp also exhibit increased absorption compared to the forearm—10-, 4-, and 3-times higher, respectively.
In recent years, the use of topical anesthetics has greatly expanded due to the popularity of aesthetic and cosmetic procedures. These procedures often are performed in an outpatient setting.6 Dermatologists should be well aware of MetHb as a serious adverse effect and guide patients accordingly, as patients do not tend to consider a local anesthetic to be a drug. Drug interactions also may affect free lidocaine concentrations by liver cytochrome P450 metabolism; although this was not the case with our patient, special attention should be given to potential interactions that may exacerbate this serious adverse effect. Consideration should be given to patients applying the anesthetic to areas with high absorption capacity.
To the Editor:
Methemoglobinemia (MetHb) is a condition caused by elevated levels of methemoglobin in the blood, which leads to an overall reduced ability of red blood cells to release oxygen to tissues, causing tissue hypoxia. Methemoglobinemia may be congenital or acquired. Various antibiotics and local anesthetics have been reported to induce acquired MetHb.1 We describe an adult who presented with MetHb resulting from excessive topical application of local anesthetics for painful scrotal ulcers.
A 54-year-old man presented with multiple scrotal and penile shaft ulcers of a few weeks’ duration with no systemic concerns. His medical history included chronic hepatitis C virus (HCV) and lumbar disc disease. Physical examination revealed multiple erosions and ulcers on an erythematous base involving the scrotal skin and distal penile shaft (Figure). Histopathology revealed acute leukocytoclastic vasculitis, and a laboratory workup was positive for mixed cryoglobulinemia that was thought to be HCV related. The patient was started on a systemic corticosteroid treatment in addition to sofosbuvir-velpatasvir for the treatment of HCV-related mixed cryoglobulinemic vasculitis. Concomitantly, the patient self-treated for pain with a local anesthetic cream containing lidocaine 2.5% and prilocaine 2.5%, applying it excessively every few hours daily for 2 weeks. He also intermittently used occlusive dressings.

After 2 weeks of application, the patient developed lightheadedness and shortness of breath. He returned and was admitted for further evaluation. He had dyspnea and tachypnea of 22 breaths per minute. He also had mild tachycardia (109 beats per minute). He did not have a fever, and his blood pressure was normal. The oxygen saturation measured in ambient room air by pulse oximetry was 82%. A neurologic examination was normal except for mild drowsiness. The lungs were clear, and heart sounds were normal. A 12-lead electrocardiogram also was normal. A complete blood cell count showed severe macrocytic anemia with a hemoglobin level of 7 g/dL, which was a severe decline from the patient’s baseline level of 14 g/dL (reference range, 13–17 g/dL). A MetHb blood level of 11% was reported on co-oximetry. An arterial blood gas analysis revealed a pH of 7.46; partial pressure of carbon dioxide of 41 mm Hg; and partial pressure of oxygen of 63 mm Hg. The haptoglobin level was low at 2.6 mg/dL (reference range, 30–200 mg/dL). An absolute reticulocyte count was markedly elevated at 0.4×106/mL (reference range, 0.03–0.08×106/mL), lactate dehydrogenase was elevated at 430 U/L (reference range, 125–220 U/L), and indirect billirubin was high at 0.9 mg/dL (reference range, 0–0.5 mg/dL), consistent with hemolytic anemia. Electrolyte serum levels and renal function tests were within reference range. A diagnosis of MetHb induced by the lidocaine-prilocaine cream was rendered, and intravenous methylene blue 72 mg (1 mg/kg) was administered over 10 minutes. Within the next 60 minutes, the patient’s drowsiness and arterial desaturation resolved. A subsequent MetHb measurement taken several hours later was reduced to 4%. The patient remained asymptomatic and was eventually discharged.
Methemoglobinemia is an altered state of hemoglobin where the ferrous (Fe2+) ions of heme are oxidized to the ferric (Fe3+) state. These ferric ions are unable to bind oxygen, resulting in impaired oxygen delivery to tissues.1 Local anesthetics, which are strong oxidizers, have been reported to induce MetHb.2 In our patient, the extensive use of lidocaine 2.5%–prilocaine 2.5% cream resulted in severe life-threatening MetHb. The oxidizing properties of local anesthetics can be attributed to their chemical structure. Benzocaine is metabolized to potent oxidizers such as aniline, phenylhydroxylamine, and nitrobenzene.3 Prilocaine and another potent oxidizer, ortho-toluidine, which is a metabolite of prilocaine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+), leading to MetHb.2,3
Cases of anesthetic-induced MetHb primarily are associated with overuse of the product by applying it to large surface areas or using it for prolonged periods of time. In one case report, the occlusive dressing of the lidocaine-prilocaine cream applied to skin of the legs that was already abraded by laser epilation therapy resulted in MetHb.4 In our patient, applying the topical anesthetic to the eroded high-absorptive mucosal surface of the scrotal skin and the use of occlusive dressings increased the risk for toxicity. Absorption from scrotal skin is 40-times higher than the forearm.5 The face, axillae, and scalp also exhibit increased absorption compared to the forearm—10-, 4-, and 3-times higher, respectively.
In recent years, the use of topical anesthetics has greatly expanded due to the popularity of aesthetic and cosmetic procedures. These procedures often are performed in an outpatient setting.6 Dermatologists should be well aware of MetHb as a serious adverse effect and guide patients accordingly, as patients do not tend to consider a local anesthetic to be a drug. Drug interactions also may affect free lidocaine concentrations by liver cytochrome P450 metabolism; although this was not the case with our patient, special attention should be given to potential interactions that may exacerbate this serious adverse effect. Consideration should be given to patients applying the anesthetic to areas with high absorption capacity.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646-656.
- Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108:837-845.
- Jakobson B, Nilsson A. Methemoglobinemia associated with a prilocaine-lidocaine cream and trimethoprim-sulphamethoxazole. a case report. Acta Anaesthesiol Scand. 1985;29:453-455.
- Hahn I, Hoffman RS, Nelson LS. EMLA®-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26:85-88.
- Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181-183.
- Alster T. Review of lidocaine/tetracaine cream as a topical anesthetic for dermatologic laser procedures. Pain Ther. 2013;2:11-19.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646-656.
- Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108:837-845.
- Jakobson B, Nilsson A. Methemoglobinemia associated with a prilocaine-lidocaine cream and trimethoprim-sulphamethoxazole. a case report. Acta Anaesthesiol Scand. 1985;29:453-455.
- Hahn I, Hoffman RS, Nelson LS. EMLA®-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26:85-88.
- Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181-183.
- Alster T. Review of lidocaine/tetracaine cream as a topical anesthetic for dermatologic laser procedures. Pain Ther. 2013;2:11-19.
Practice Points
- Consideration should be given to patients applying anesthetic creams to areas with high absorption capacity.
- Dermatologists should be aware of methemoglobinemia as a serious adverse effect of local anesthetics and guide patients accordingly, as patients do not tend to consider these products to be drugs.
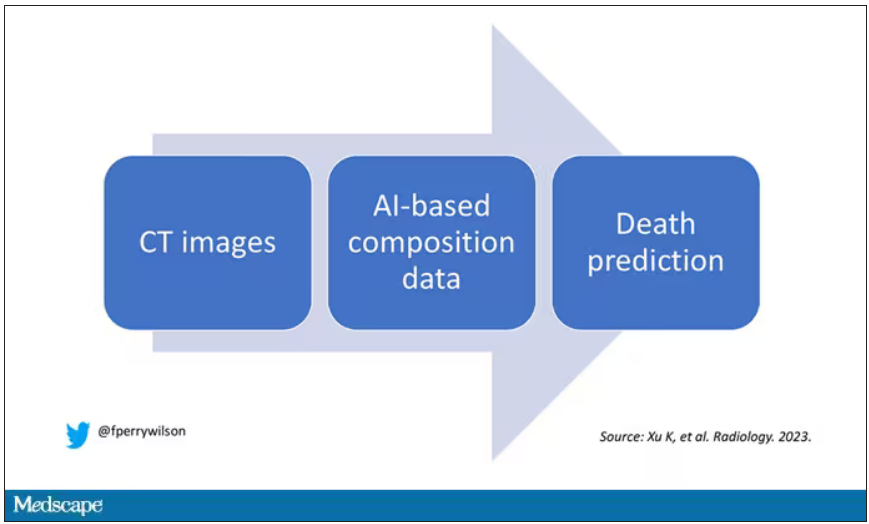
What AI can see in CT scans that humans can’t
This transcript has been edited for clarity.
If a picture is worth a thousand words, then a CT scan of the chest might as well be Atlas Shrugged. When you think of the sheer information content in one of those scans, it becomes immediately clear that our usual method of CT scan interpretation must be leaving a lot on the table. After all, we can go through all that information and come out with simply “normal” and call it a day.
Of course, radiologists can glean a lot from a CT scan, but they are trained to look for abnormalities. They can find pneumonia, emboli, fractures, and pneumothoraces, but the presence or absence of life-threatening abnormalities is still just a fraction of the data contained within a CT scan.
Pulling out more data from those images – data that may not indicate disease per se, but nevertheless tell us something important about patients and their risks – might just fall to those entities that are primed to take a bunch of data and interpret it in new ways: artificial intelligence (AI).
I’m thinking about AI and CT scans this week thanks to this study, appearing in the journal Radiology, from Kaiwen Xu and colleagues at Vanderbilt.
In a previous study, the team had developed an AI algorithm to take chest CT images and convert that data into information about body composition: skeletal muscle mass, fat mass, muscle lipid content – that sort of thing.
While the radiologists are busy looking for cancer or pneumonia, the AI can create a body composition report – two results from one data stream.
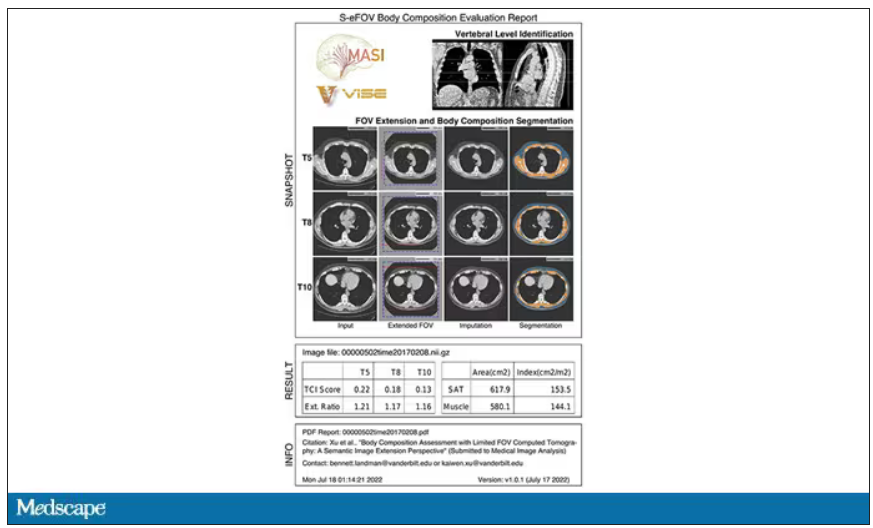
Here’s an example of a report generated from a CT scan from the authors’ GitHub page.
The cool thing here is that this is a clinically collected CT scan of the chest, not a special protocol designed to assess body composition. In fact, this comes from the low-dose lung cancer screening trial dataset.
As you may know, the U.S. Preventive Services Task Force recommends low-dose CT screening of the chest every year for those aged 50-80 with at least a 20 pack-year smoking history. These CT scans form an incredible dataset, actually, as they are all collected with nearly the same parameters. Obviously, the important thing to look for in these CT scans is whether there is early lung cancer. But the new paper asks, as long as we can get information about body composition from these scans, why don’t we? Can it help to risk-stratify these patients?
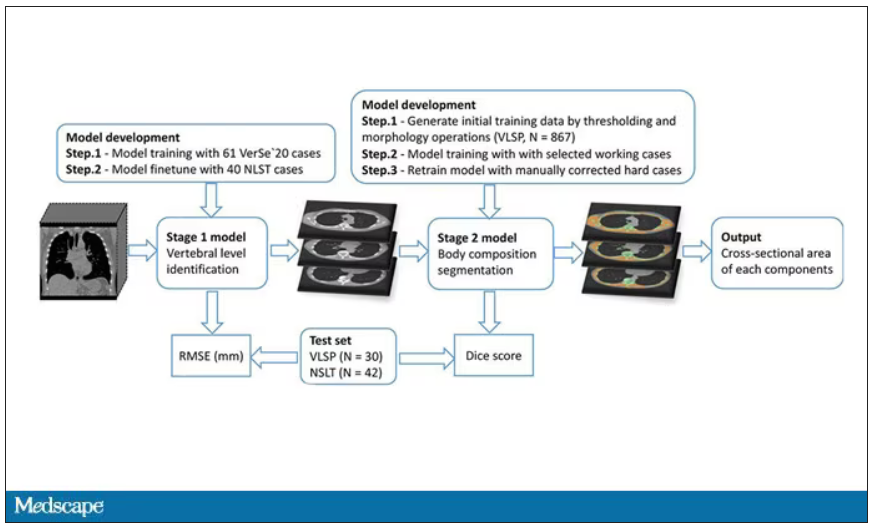
They took 20,768 individuals with CT scans done as part of the low-dose lung cancer screening trial and passed their scans through their automated data pipeline.
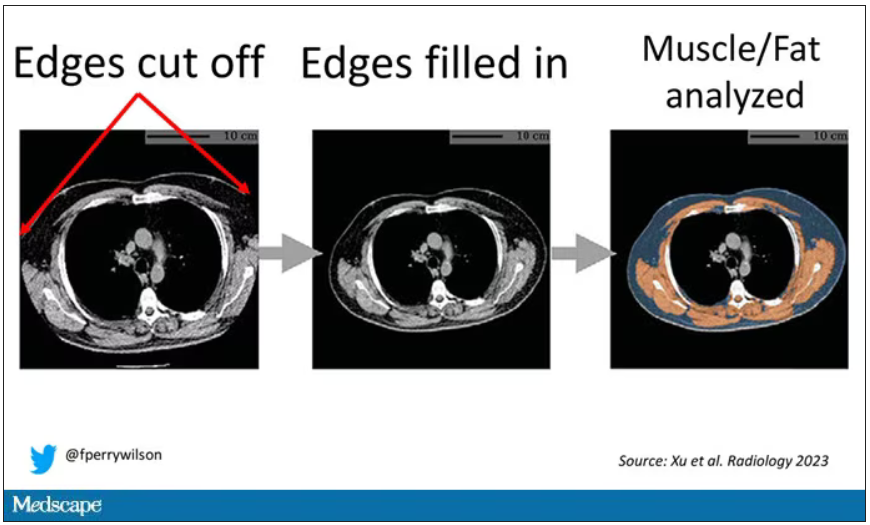
One cool feature here: Depending on body size, sometimes the edges of people in CT scans are not visible. That’s not a big deal for lung-cancer screening as long as you can see both lungs. But it does matter for assessment of muscle and body fat because that stuff lives on the edges of the thoracic cavity. The authors’ data pipeline actually accounts for this, extrapolating what the missing pieces look like from what is able to be seen. It’s quite clever.
On to some results. Would knowledge about the patient’s body composition help predict their ultimate outcome?
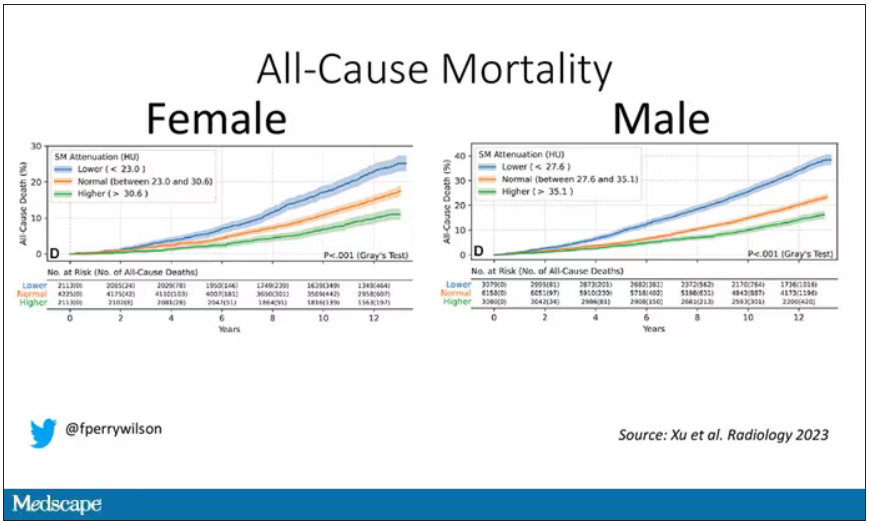
It would. And the best single predictor found was skeletal muscle attenuation – lower levels of skeletal muscle attenuation mean more fat infiltrating the muscle – so lower is worse here. You can see from these all-cause mortality curves that lower levels were associated with substantially worse life expectancy.
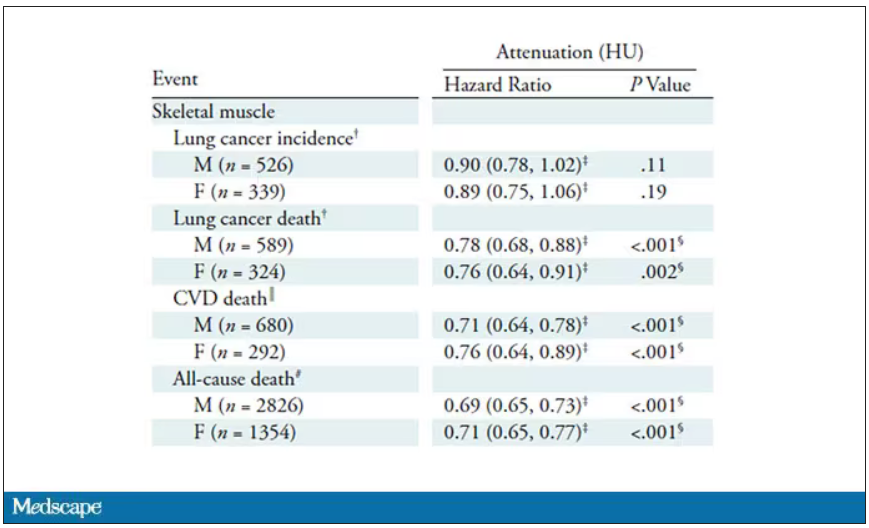
It’s worth noting that these are unadjusted curves. While AI prediction from CT images is very cool, we might be able to make similar predictions knowing, for example, the age of the patient. To account for this, the authors adjusted the findings for age, diabetes, heart disease, stroke, and coronary calcium score (also calculated from those same CT scans). Even after adjustment, skeletal muscle attenuation was significantly associated with all-cause mortality, cardiovascular mortality, and lung-cancer mortality – but not lung cancer incidence.
Those results tell us that there is likely a physiologic significance to skeletal muscle attenuation, and they provide a great proof-of-concept that automated data extraction techniques can be applied broadly to routinely collected radiology images.
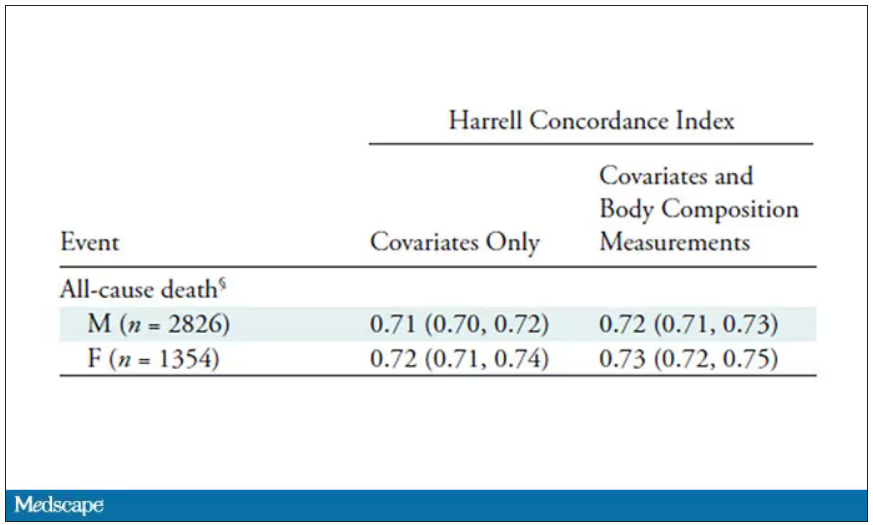
That said, it’s one thing to show that something is physiologically relevant. In terms of actually predicting outcomes, adding this information to a model that contains just those clinical factors like age and diabetes doesn’t actually improve things very much. We measure this with something called the concordance index. This tells us the probability, given two individuals, of how often we can identify the person who has the outcome of interest sooner – if at all. (You can probably guess that the worst possible score is thus 0.5 and the best is 1.) A model without the AI data gives a concordance index for all-cause mortality of 0.71 or 0.72, depending on sex. Adding in the body composition data bumps that up only by a percent or so.
This honestly feels a bit like a missed opportunity to me. The authors pass the imaging data through an AI to get body composition data and then see how that predicts death.
Why not skip the middleman? Train a model using the imaging data to predict death directly, using whatever signal the AI chooses: body composition, lung size, rib thickness – whatever.
I’d be very curious to see how that model might improve our ability to predict these outcomes. In the end, this is a space where AI can make some massive gains – not by trying to do radiologists’ jobs better than radiologists, but by extracting information that radiologists aren’t looking for in the first place.
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
If a picture is worth a thousand words, then a CT scan of the chest might as well be Atlas Shrugged. When you think of the sheer information content in one of those scans, it becomes immediately clear that our usual method of CT scan interpretation must be leaving a lot on the table. After all, we can go through all that information and come out with simply “normal” and call it a day.
Of course, radiologists can glean a lot from a CT scan, but they are trained to look for abnormalities. They can find pneumonia, emboli, fractures, and pneumothoraces, but the presence or absence of life-threatening abnormalities is still just a fraction of the data contained within a CT scan.
Pulling out more data from those images – data that may not indicate disease per se, but nevertheless tell us something important about patients and their risks – might just fall to those entities that are primed to take a bunch of data and interpret it in new ways: artificial intelligence (AI).
I’m thinking about AI and CT scans this week thanks to this study, appearing in the journal Radiology, from Kaiwen Xu and colleagues at Vanderbilt.
In a previous study, the team had developed an AI algorithm to take chest CT images and convert that data into information about body composition: skeletal muscle mass, fat mass, muscle lipid content – that sort of thing.
While the radiologists are busy looking for cancer or pneumonia, the AI can create a body composition report – two results from one data stream.
Here’s an example of a report generated from a CT scan from the authors’ GitHub page.
The cool thing here is that this is a clinically collected CT scan of the chest, not a special protocol designed to assess body composition. In fact, this comes from the low-dose lung cancer screening trial dataset.
As you may know, the U.S. Preventive Services Task Force recommends low-dose CT screening of the chest every year for those aged 50-80 with at least a 20 pack-year smoking history. These CT scans form an incredible dataset, actually, as they are all collected with nearly the same parameters. Obviously, the important thing to look for in these CT scans is whether there is early lung cancer. But the new paper asks, as long as we can get information about body composition from these scans, why don’t we? Can it help to risk-stratify these patients?
They took 20,768 individuals with CT scans done as part of the low-dose lung cancer screening trial and passed their scans through their automated data pipeline.
One cool feature here: Depending on body size, sometimes the edges of people in CT scans are not visible. That’s not a big deal for lung-cancer screening as long as you can see both lungs. But it does matter for assessment of muscle and body fat because that stuff lives on the edges of the thoracic cavity. The authors’ data pipeline actually accounts for this, extrapolating what the missing pieces look like from what is able to be seen. It’s quite clever.
On to some results. Would knowledge about the patient’s body composition help predict their ultimate outcome?
It would. And the best single predictor found was skeletal muscle attenuation – lower levels of skeletal muscle attenuation mean more fat infiltrating the muscle – so lower is worse here. You can see from these all-cause mortality curves that lower levels were associated with substantially worse life expectancy.
It’s worth noting that these are unadjusted curves. While AI prediction from CT images is very cool, we might be able to make similar predictions knowing, for example, the age of the patient. To account for this, the authors adjusted the findings for age, diabetes, heart disease, stroke, and coronary calcium score (also calculated from those same CT scans). Even after adjustment, skeletal muscle attenuation was significantly associated with all-cause mortality, cardiovascular mortality, and lung-cancer mortality – but not lung cancer incidence.
Those results tell us that there is likely a physiologic significance to skeletal muscle attenuation, and they provide a great proof-of-concept that automated data extraction techniques can be applied broadly to routinely collected radiology images.
That said, it’s one thing to show that something is physiologically relevant. In terms of actually predicting outcomes, adding this information to a model that contains just those clinical factors like age and diabetes doesn’t actually improve things very much. We measure this with something called the concordance index. This tells us the probability, given two individuals, of how often we can identify the person who has the outcome of interest sooner – if at all. (You can probably guess that the worst possible score is thus 0.5 and the best is 1.) A model without the AI data gives a concordance index for all-cause mortality of 0.71 or 0.72, depending on sex. Adding in the body composition data bumps that up only by a percent or so.
This honestly feels a bit like a missed opportunity to me. The authors pass the imaging data through an AI to get body composition data and then see how that predicts death.
Why not skip the middleman? Train a model using the imaging data to predict death directly, using whatever signal the AI chooses: body composition, lung size, rib thickness – whatever.
I’d be very curious to see how that model might improve our ability to predict these outcomes. In the end, this is a space where AI can make some massive gains – not by trying to do radiologists’ jobs better than radiologists, but by extracting information that radiologists aren’t looking for in the first place.
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
If a picture is worth a thousand words, then a CT scan of the chest might as well be Atlas Shrugged. When you think of the sheer information content in one of those scans, it becomes immediately clear that our usual method of CT scan interpretation must be leaving a lot on the table. After all, we can go through all that information and come out with simply “normal” and call it a day.
Of course, radiologists can glean a lot from a CT scan, but they are trained to look for abnormalities. They can find pneumonia, emboli, fractures, and pneumothoraces, but the presence or absence of life-threatening abnormalities is still just a fraction of the data contained within a CT scan.
Pulling out more data from those images – data that may not indicate disease per se, but nevertheless tell us something important about patients and their risks – might just fall to those entities that are primed to take a bunch of data and interpret it in new ways: artificial intelligence (AI).
I’m thinking about AI and CT scans this week thanks to this study, appearing in the journal Radiology, from Kaiwen Xu and colleagues at Vanderbilt.
In a previous study, the team had developed an AI algorithm to take chest CT images and convert that data into information about body composition: skeletal muscle mass, fat mass, muscle lipid content – that sort of thing.
While the radiologists are busy looking for cancer or pneumonia, the AI can create a body composition report – two results from one data stream.
Here’s an example of a report generated from a CT scan from the authors’ GitHub page.
The cool thing here is that this is a clinically collected CT scan of the chest, not a special protocol designed to assess body composition. In fact, this comes from the low-dose lung cancer screening trial dataset.
As you may know, the U.S. Preventive Services Task Force recommends low-dose CT screening of the chest every year for those aged 50-80 with at least a 20 pack-year smoking history. These CT scans form an incredible dataset, actually, as they are all collected with nearly the same parameters. Obviously, the important thing to look for in these CT scans is whether there is early lung cancer. But the new paper asks, as long as we can get information about body composition from these scans, why don’t we? Can it help to risk-stratify these patients?
They took 20,768 individuals with CT scans done as part of the low-dose lung cancer screening trial and passed their scans through their automated data pipeline.
One cool feature here: Depending on body size, sometimes the edges of people in CT scans are not visible. That’s not a big deal for lung-cancer screening as long as you can see both lungs. But it does matter for assessment of muscle and body fat because that stuff lives on the edges of the thoracic cavity. The authors’ data pipeline actually accounts for this, extrapolating what the missing pieces look like from what is able to be seen. It’s quite clever.
On to some results. Would knowledge about the patient’s body composition help predict their ultimate outcome?
It would. And the best single predictor found was skeletal muscle attenuation – lower levels of skeletal muscle attenuation mean more fat infiltrating the muscle – so lower is worse here. You can see from these all-cause mortality curves that lower levels were associated with substantially worse life expectancy.
It’s worth noting that these are unadjusted curves. While AI prediction from CT images is very cool, we might be able to make similar predictions knowing, for example, the age of the patient. To account for this, the authors adjusted the findings for age, diabetes, heart disease, stroke, and coronary calcium score (also calculated from those same CT scans). Even after adjustment, skeletal muscle attenuation was significantly associated with all-cause mortality, cardiovascular mortality, and lung-cancer mortality – but not lung cancer incidence.
Those results tell us that there is likely a physiologic significance to skeletal muscle attenuation, and they provide a great proof-of-concept that automated data extraction techniques can be applied broadly to routinely collected radiology images.
That said, it’s one thing to show that something is physiologically relevant. In terms of actually predicting outcomes, adding this information to a model that contains just those clinical factors like age and diabetes doesn’t actually improve things very much. We measure this with something called the concordance index. This tells us the probability, given two individuals, of how often we can identify the person who has the outcome of interest sooner – if at all. (You can probably guess that the worst possible score is thus 0.5 and the best is 1.) A model without the AI data gives a concordance index for all-cause mortality of 0.71 or 0.72, depending on sex. Adding in the body composition data bumps that up only by a percent or so.
This honestly feels a bit like a missed opportunity to me. The authors pass the imaging data through an AI to get body composition data and then see how that predicts death.
Why not skip the middleman? Train a model using the imaging data to predict death directly, using whatever signal the AI chooses: body composition, lung size, rib thickness – whatever.
I’d be very curious to see how that model might improve our ability to predict these outcomes. In the end, this is a space where AI can make some massive gains – not by trying to do radiologists’ jobs better than radiologists, but by extracting information that radiologists aren’t looking for in the first place.
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Commentary: Meningioma, Radiotherapy Interruptions, Therapy Persistence, and Lymphocytes in BC, August 2023
Data are limited regarding the effect of interrupting radiation therapy for patients with BC. A retrospective study by Chow and colleagues looked at 35,845 patients with nonmetastatic triple-negative BC from the National Cancer Database who had received external beam radiation therapy as part of the management of their BC. The analysis showed inferior overall survival in patients with a longer duration of radiation treatment (hazard ratio 1.023; 95% CI 1.015-1.031) The more days of interruption, the higher the likelihood of mortality seen. In reference to 0-1 days of interruption, patients with 2-5 interrupted days (hazard ratio 1.069; 95% CI 1.002-1.140), 6-10 interrupted days (hazard ratio 1.239; 95% CI 1.140-1.348), and 11-15 interrupted days (hazard ratio 1.265; 95% CI 1.126-1.431) did worse. These findings should encourage further studies to explore ways to minimize treatment interruptions among patients with BC.
A lack of adherence to adjuvant endocrine therapy has been associated with increased mortality among women with BC. The retrospective study by Zheng and Thomas included 25,796 older women (> 65 years old) diagnosed with stage I-III hormone receptor–positive BC and looked at associations between adherence to and persistence with adjuvant endocrine therapy and mortality in this cohort. Their findings showed that the risk for all-cause mortality was reduced by 25% in patients with vs without cumulative adherence to endocrine therapy (hazard ratio 0.75; P < .001), although no association was seen with BC-specific mortality. Persistence with endocrine therapy, which was defined as having taken the treatment for ≥ 180 continuous days, was associated with 11% reduction in all-cause mortality and 37% reduction in BC-specific mortality. This study supports prior studies in highlighting the importance of endocrine therapy adherence among women with hormone-positive BC.
Tumor-infiltrating lymphocytes (TIL) are considered significant prognostic markers in patients with BC, although the prognostic effect of TIL in human epidermal growth factor reception 2 (HER2)–low BC has not been identified. A large-cohort, single-institution retrospective analysis by Sun and colleagues investigated the prognostic role of TIL in HER2-low early-stage BC. The analysis included 1763 patients with early-stage BC who underwent surgery, of whom 429 patients were HER2+, 739 were HER2-low, and 595 were HER2-0. No differences in disease-free survival (DFS) were seen between the three cohorts. However, in patients with HER2-low BC, high (>10%) vs low (≤10%) TIL levels were associated with a 53% improvement in DFS overall (hazard ratio 0.47; P = .035), and a 58% improvement in DFS was seen for the hormone receptor–positive/HER2-low cohort (hazard ratio 0.42; P = .032).
Data are limited regarding the effect of interrupting radiation therapy for patients with BC. A retrospective study by Chow and colleagues looked at 35,845 patients with nonmetastatic triple-negative BC from the National Cancer Database who had received external beam radiation therapy as part of the management of their BC. The analysis showed inferior overall survival in patients with a longer duration of radiation treatment (hazard ratio 1.023; 95% CI 1.015-1.031) The more days of interruption, the higher the likelihood of mortality seen. In reference to 0-1 days of interruption, patients with 2-5 interrupted days (hazard ratio 1.069; 95% CI 1.002-1.140), 6-10 interrupted days (hazard ratio 1.239; 95% CI 1.140-1.348), and 11-15 interrupted days (hazard ratio 1.265; 95% CI 1.126-1.431) did worse. These findings should encourage further studies to explore ways to minimize treatment interruptions among patients with BC.
A lack of adherence to adjuvant endocrine therapy has been associated with increased mortality among women with BC. The retrospective study by Zheng and Thomas included 25,796 older women (> 65 years old) diagnosed with stage I-III hormone receptor–positive BC and looked at associations between adherence to and persistence with adjuvant endocrine therapy and mortality in this cohort. Their findings showed that the risk for all-cause mortality was reduced by 25% in patients with vs without cumulative adherence to endocrine therapy (hazard ratio 0.75; P < .001), although no association was seen with BC-specific mortality. Persistence with endocrine therapy, which was defined as having taken the treatment for ≥ 180 continuous days, was associated with 11% reduction in all-cause mortality and 37% reduction in BC-specific mortality. This study supports prior studies in highlighting the importance of endocrine therapy adherence among women with hormone-positive BC.
Tumor-infiltrating lymphocytes (TIL) are considered significant prognostic markers in patients with BC, although the prognostic effect of TIL in human epidermal growth factor reception 2 (HER2)–low BC has not been identified. A large-cohort, single-institution retrospective analysis by Sun and colleagues investigated the prognostic role of TIL in HER2-low early-stage BC. The analysis included 1763 patients with early-stage BC who underwent surgery, of whom 429 patients were HER2+, 739 were HER2-low, and 595 were HER2-0. No differences in disease-free survival (DFS) were seen between the three cohorts. However, in patients with HER2-low BC, high (>10%) vs low (≤10%) TIL levels were associated with a 53% improvement in DFS overall (hazard ratio 0.47; P = .035), and a 58% improvement in DFS was seen for the hormone receptor–positive/HER2-low cohort (hazard ratio 0.42; P = .032).
Data are limited regarding the effect of interrupting radiation therapy for patients with BC. A retrospective study by Chow and colleagues looked at 35,845 patients with nonmetastatic triple-negative BC from the National Cancer Database who had received external beam radiation therapy as part of the management of their BC. The analysis showed inferior overall survival in patients with a longer duration of radiation treatment (hazard ratio 1.023; 95% CI 1.015-1.031) The more days of interruption, the higher the likelihood of mortality seen. In reference to 0-1 days of interruption, patients with 2-5 interrupted days (hazard ratio 1.069; 95% CI 1.002-1.140), 6-10 interrupted days (hazard ratio 1.239; 95% CI 1.140-1.348), and 11-15 interrupted days (hazard ratio 1.265; 95% CI 1.126-1.431) did worse. These findings should encourage further studies to explore ways to minimize treatment interruptions among patients with BC.
A lack of adherence to adjuvant endocrine therapy has been associated with increased mortality among women with BC. The retrospective study by Zheng and Thomas included 25,796 older women (> 65 years old) diagnosed with stage I-III hormone receptor–positive BC and looked at associations between adherence to and persistence with adjuvant endocrine therapy and mortality in this cohort. Their findings showed that the risk for all-cause mortality was reduced by 25% in patients with vs without cumulative adherence to endocrine therapy (hazard ratio 0.75; P < .001), although no association was seen with BC-specific mortality. Persistence with endocrine therapy, which was defined as having taken the treatment for ≥ 180 continuous days, was associated with 11% reduction in all-cause mortality and 37% reduction in BC-specific mortality. This study supports prior studies in highlighting the importance of endocrine therapy adherence among women with hormone-positive BC.
Tumor-infiltrating lymphocytes (TIL) are considered significant prognostic markers in patients with BC, although the prognostic effect of TIL in human epidermal growth factor reception 2 (HER2)–low BC has not been identified. A large-cohort, single-institution retrospective analysis by Sun and colleagues investigated the prognostic role of TIL in HER2-low early-stage BC. The analysis included 1763 patients with early-stage BC who underwent surgery, of whom 429 patients were HER2+, 739 were HER2-low, and 595 were HER2-0. No differences in disease-free survival (DFS) were seen between the three cohorts. However, in patients with HER2-low BC, high (>10%) vs low (≤10%) TIL levels were associated with a 53% improvement in DFS overall (hazard ratio 0.47; P = .035), and a 58% improvement in DFS was seen for the hormone receptor–positive/HER2-low cohort (hazard ratio 0.42; P = .032).
Physician not held liable for child’s necrotizing pancreatitis, jury finds
, according to a report posted on the website of Courtroom View Network.
In 2018, the parents of the then 9-year-old child brought him to Wellstar Paulding Hospital in Hiram, Ga., because of his severe abdominal pain and distention, among other symptoms. Following their examination, medical personnel at the hospital suspected the child’s symptoms were the result of severe constipation.
That evening, he was transferred to Children’s Healthcare of Atlanta, where a pediatric gastroenterologist oversaw his care. (Neither the Atlanta hospital nor Wellstar Paulding were defendants in the subsequent lawsuit.)
Late the following day, the child went into hypovolemic shock, a condition that interrupted the blood supply to his body. Admitted to the pediatric ICU, he was diagnosed with a dangerous complication of acute pancreatitis, necrotizing pancreatitis.
Further complications of his original disease led to a 4-month hospital stay, multiple surgeries, and other interventions. To this point, his medical expenses totaled more than $2.5 million.
His parents then sued the pediatric gastroenterologist who had overseen their child’s care. At issue during the 4-day trial was whether the doctor had properly monitored and treated his patient before his hypovolemic shock set in.
Their attorney sketched the “timeline” of the child’s decline, including his rapid heart rate and repeated vomiting. Given these symptoms, he argued, the standard of care required that steps be taken – including the proper tests and other interventions – to prevent the child’s acute pancreatitis from progressing even further.
“We are not asking you to say, ‘Should [the doctor] have immediately diagnosed pancreatitis,’ “ the attorney told the jury. “But the totality here requires you to think, ‘This might be more than just a backed-up kid.’ ”
The defense pushed back strenuously, however. It argued that the pediatric gastroenterologist had acted appropriately given the prevailing consensus, namely that the child was suffering from extreme constipation. Doctors at Wellstar Paulding, the first hospital where he was seen, suspected this diagnosis – and so, based on his exam and the child’s “non-specific” symptoms, did their client, the pediatric gastroenterologist, who saw him subsequently. “The only clinicians who actually laid hands on [the child] all thought constipation,” the attorney said during his closing argument.
The jury agreed, finding that the pediatric gastroenterologist had acted appropriately, based on the available evidence. Following the jury verdict, the defense attorney noted: Absent the “classic” symptoms of pancreatitis, the jury saw that his client “was working with a reasonable diagnosis until [the child’s] clinical picture deteriorated.”
ED doctors can reduce system errors, study says
Emergency physicians are often blamed for system errors beyond their control, asserts a study in the June issue of Emergency Medicine News.
The study – conducted by Tom Belanger, MD, an emergency physician in Texas and chair-elect of the American College of Emergency Physicians Workforce Section – sought to understand to what extent doctors themselves were aware of systemic problems affecting their job. Dr. Belanger surveyed 99 doctors who were asked to comment on a series of ED–related adverse outcomes.
To mitigate response bias, he randomly manipulated the degree to which system error was a perceived factor in each of the adverse cases. In other words, in some cases, the system was represented as a major factor leading to error, while, in other cases, its role was diminished.
Dr. Belanger also divided his doctor/respondents into two groups: The first was asked about his or her personal experience with systemic issues before being presented with the adverse cases; the second group was queried about this experience after being presented with the cases.
The result confirmed Dr. Belanger’s suspicions: Physicians in the first group – that is, those asked about “system factors” before reading about the cases – “were 1.7 times more likely ... to attribute the adverse outcomes in the cases to system factors. (Other significant variables – including whether their shift was busy – also contributed to doctors’ perceptions of adverse outcomes.)
Concluded Dr. Belanger: Since doctors “can identify factors that increase their chances of making mistakes,” system designers should take heed and make efforts to reduce “the probability of error.” If they drag their heels or continue to point to individual doctor error, “they should be held medically and legally liable.”
A version of this article first appeared on Medscape.com.
, according to a report posted on the website of Courtroom View Network.
In 2018, the parents of the then 9-year-old child brought him to Wellstar Paulding Hospital in Hiram, Ga., because of his severe abdominal pain and distention, among other symptoms. Following their examination, medical personnel at the hospital suspected the child’s symptoms were the result of severe constipation.
That evening, he was transferred to Children’s Healthcare of Atlanta, where a pediatric gastroenterologist oversaw his care. (Neither the Atlanta hospital nor Wellstar Paulding were defendants in the subsequent lawsuit.)
Late the following day, the child went into hypovolemic shock, a condition that interrupted the blood supply to his body. Admitted to the pediatric ICU, he was diagnosed with a dangerous complication of acute pancreatitis, necrotizing pancreatitis.
Further complications of his original disease led to a 4-month hospital stay, multiple surgeries, and other interventions. To this point, his medical expenses totaled more than $2.5 million.
His parents then sued the pediatric gastroenterologist who had overseen their child’s care. At issue during the 4-day trial was whether the doctor had properly monitored and treated his patient before his hypovolemic shock set in.
Their attorney sketched the “timeline” of the child’s decline, including his rapid heart rate and repeated vomiting. Given these symptoms, he argued, the standard of care required that steps be taken – including the proper tests and other interventions – to prevent the child’s acute pancreatitis from progressing even further.
“We are not asking you to say, ‘Should [the doctor] have immediately diagnosed pancreatitis,’ “ the attorney told the jury. “But the totality here requires you to think, ‘This might be more than just a backed-up kid.’ ”
The defense pushed back strenuously, however. It argued that the pediatric gastroenterologist had acted appropriately given the prevailing consensus, namely that the child was suffering from extreme constipation. Doctors at Wellstar Paulding, the first hospital where he was seen, suspected this diagnosis – and so, based on his exam and the child’s “non-specific” symptoms, did their client, the pediatric gastroenterologist, who saw him subsequently. “The only clinicians who actually laid hands on [the child] all thought constipation,” the attorney said during his closing argument.
The jury agreed, finding that the pediatric gastroenterologist had acted appropriately, based on the available evidence. Following the jury verdict, the defense attorney noted: Absent the “classic” symptoms of pancreatitis, the jury saw that his client “was working with a reasonable diagnosis until [the child’s] clinical picture deteriorated.”
ED doctors can reduce system errors, study says
Emergency physicians are often blamed for system errors beyond their control, asserts a study in the June issue of Emergency Medicine News.
The study – conducted by Tom Belanger, MD, an emergency physician in Texas and chair-elect of the American College of Emergency Physicians Workforce Section – sought to understand to what extent doctors themselves were aware of systemic problems affecting their job. Dr. Belanger surveyed 99 doctors who were asked to comment on a series of ED–related adverse outcomes.
To mitigate response bias, he randomly manipulated the degree to which system error was a perceived factor in each of the adverse cases. In other words, in some cases, the system was represented as a major factor leading to error, while, in other cases, its role was diminished.
Dr. Belanger also divided his doctor/respondents into two groups: The first was asked about his or her personal experience with systemic issues before being presented with the adverse cases; the second group was queried about this experience after being presented with the cases.
The result confirmed Dr. Belanger’s suspicions: Physicians in the first group – that is, those asked about “system factors” before reading about the cases – “were 1.7 times more likely ... to attribute the adverse outcomes in the cases to system factors. (Other significant variables – including whether their shift was busy – also contributed to doctors’ perceptions of adverse outcomes.)
Concluded Dr. Belanger: Since doctors “can identify factors that increase their chances of making mistakes,” system designers should take heed and make efforts to reduce “the probability of error.” If they drag their heels or continue to point to individual doctor error, “they should be held medically and legally liable.”
A version of this article first appeared on Medscape.com.
, according to a report posted on the website of Courtroom View Network.
In 2018, the parents of the then 9-year-old child brought him to Wellstar Paulding Hospital in Hiram, Ga., because of his severe abdominal pain and distention, among other symptoms. Following their examination, medical personnel at the hospital suspected the child’s symptoms were the result of severe constipation.
That evening, he was transferred to Children’s Healthcare of Atlanta, where a pediatric gastroenterologist oversaw his care. (Neither the Atlanta hospital nor Wellstar Paulding were defendants in the subsequent lawsuit.)
Late the following day, the child went into hypovolemic shock, a condition that interrupted the blood supply to his body. Admitted to the pediatric ICU, he was diagnosed with a dangerous complication of acute pancreatitis, necrotizing pancreatitis.
Further complications of his original disease led to a 4-month hospital stay, multiple surgeries, and other interventions. To this point, his medical expenses totaled more than $2.5 million.
His parents then sued the pediatric gastroenterologist who had overseen their child’s care. At issue during the 4-day trial was whether the doctor had properly monitored and treated his patient before his hypovolemic shock set in.
Their attorney sketched the “timeline” of the child’s decline, including his rapid heart rate and repeated vomiting. Given these symptoms, he argued, the standard of care required that steps be taken – including the proper tests and other interventions – to prevent the child’s acute pancreatitis from progressing even further.
“We are not asking you to say, ‘Should [the doctor] have immediately diagnosed pancreatitis,’ “ the attorney told the jury. “But the totality here requires you to think, ‘This might be more than just a backed-up kid.’ ”
The defense pushed back strenuously, however. It argued that the pediatric gastroenterologist had acted appropriately given the prevailing consensus, namely that the child was suffering from extreme constipation. Doctors at Wellstar Paulding, the first hospital where he was seen, suspected this diagnosis – and so, based on his exam and the child’s “non-specific” symptoms, did their client, the pediatric gastroenterologist, who saw him subsequently. “The only clinicians who actually laid hands on [the child] all thought constipation,” the attorney said during his closing argument.
The jury agreed, finding that the pediatric gastroenterologist had acted appropriately, based on the available evidence. Following the jury verdict, the defense attorney noted: Absent the “classic” symptoms of pancreatitis, the jury saw that his client “was working with a reasonable diagnosis until [the child’s] clinical picture deteriorated.”
ED doctors can reduce system errors, study says
Emergency physicians are often blamed for system errors beyond their control, asserts a study in the June issue of Emergency Medicine News.
The study – conducted by Tom Belanger, MD, an emergency physician in Texas and chair-elect of the American College of Emergency Physicians Workforce Section – sought to understand to what extent doctors themselves were aware of systemic problems affecting their job. Dr. Belanger surveyed 99 doctors who were asked to comment on a series of ED–related adverse outcomes.
To mitigate response bias, he randomly manipulated the degree to which system error was a perceived factor in each of the adverse cases. In other words, in some cases, the system was represented as a major factor leading to error, while, in other cases, its role was diminished.
Dr. Belanger also divided his doctor/respondents into two groups: The first was asked about his or her personal experience with systemic issues before being presented with the adverse cases; the second group was queried about this experience after being presented with the cases.
The result confirmed Dr. Belanger’s suspicions: Physicians in the first group – that is, those asked about “system factors” before reading about the cases – “were 1.7 times more likely ... to attribute the adverse outcomes in the cases to system factors. (Other significant variables – including whether their shift was busy – also contributed to doctors’ perceptions of adverse outcomes.)
Concluded Dr. Belanger: Since doctors “can identify factors that increase their chances of making mistakes,” system designers should take heed and make efforts to reduce “the probability of error.” If they drag their heels or continue to point to individual doctor error, “they should be held medically and legally liable.”
A version of this article first appeared on Medscape.com.
Pediatric dermatologists encouraged to counter misinformation on TikTok, other social media sites
ASHEVILLE, N.C. – , warned an expert at the annual meeting of the Society for Pediatric Dermatology.
“If we don’t get involved, we are basically letting misinformation win. We need to be there,” said Angelo Landriscina, MD, director of dermatology at a Mount Sinai Doctors Clinic in New York.
Most of the content currently available on medical topics, including dermatology and pediatric dermatology, is not created by health care professionals, Dr. Landriscina noted. Not surprisingly, given that much of the content is based on personal opinion from individuals who have no expertise in medical care, he described the information as being of “low quality” when not fully erroneous.
Dr. Landriscina has been active on social media, including TikTok, for several years. Most of his posts involve responses to misinformation. When he sets the record straight on the basis of existing evidence, he often supports his counterargument with references.
He acknowledged that when he became involved in social media he faced criticism from colleagues about participating on an entertainment platform that many considered unworthy of providing objective information. If that was ever true, he argued, it is no longer the case.
“TikTok has adopted a new strategy. The goal is to unseat Google as a search tool, and it’s working,” he said. He explained that many people now use TikTok and other social media sites as their primary source of information on essentially every topic, from where to eat to whether to be screened for cancer.
The particular problem with TikTok – one of the most popular social media outlets – is that there is no mechanism for vetting the source of information. YouTube, by contrast, now requires some sort of validation for anyone who claims to have a medical degree or any other verifiable qualification, according to Dr. Landriscina. TikTok, like many other platforms, has no such requirement.
“Anyone can buy a pair of scrubs [implying expertise] and then post a video,” Dr. Landriscina said.
Even if information from one content provider is more valid than information from others, the TikTok algorithm is specifically designed to emphasize content that has the potential for going viral, which means it favors videos that are provocative over those that are not.
“The algorithm favors any content that is more controversial, more surprising, and keeps viewers engaged,” Dr. Landriscina pointed out.
This does not mean that objective and factual information is ignored, but the algorithm is indifferent to the validity of information, meaning that it allows videos to be posted without regard to whether the content is true, untrue, purposefully misleading, or utter nonsense. For that reason, it is often easier to attract attention by responding to a post that has already gone viral. Information that is clear and digestible can attract viewers and therefore is distributed more widely with the TikTok algorithm.
Parents are on Tiktok too
There is a misperception that the TikTok audience is younger, according to Dr. Landriscina. While peak use in the United States fell among people between the ages of 25 and 34 years in 2022, he said the number of users falls off relatively slowly with subsequent 10-year increments in age. In 2022, there were nearly 20 million users in the peak 10-year age range, but 7.5 million users were 55 years of age or older.
“Pediatric dermatologists should recognize that it is not just kids who are looking for information about their skin diseases, but also their parents,” Dr. Landriscina said.
The top three dermatology topics searched on TikTok in a recent period were acne, alopecia, and cysts. But top searches are very fluid and are extremely hard to quantify, because the basis of the algorithm, which is a proprietary secret, is not only unknown but produces different results for every user.
“The second you touch the app, it changes,” Dr. Landriscina said. He explained that an inquiry about any subject, including those that are medically related, yields content that is different, or at least ordered differently, “depending on how you behaved on the app in the past.”
The phenomenon that drives social media predates this technology. Dr. Landriscina cited a study in 1956 that described the “parasocial interaction theory.” The theory was based on the observation that those who consume media, such as television, which was relatively new in 1956, believed that they had a personal relationship with media figures.
“The users begin to trust influencers as a source, like a friend providing them advice,” Dr. Landriscina said. As an example, he suggested that a fan of the television show Friends who follows actor Jennifer Aniston on social media platforms may begin to think of her as a trusted source of information on any topic, including those for which she may not have expertise.
The reason that he urges medical professionals to become active on TikTok and other social media platforms is that they have a potentially critical role in responding to information that is not just wrong but harmful.
On TikTok and other social media platforms, “there is a lot of interest in content about dermatologic conditions in children. There is a real need for accurate information,” he said,
In the question-and-answer session following his presentation, Dr. Landriscina’s message was not uniformly embraced. One risk, according to an audience member, is that medical professionals will begin to express their own personal opinions rather than rely on evidence, with the result that they will “just add to the sea of misinformation.”
However, this opinion appeared to be the minority view. Most of those who commented took a “that-ship-has-sailed” stance, recognizing the irreversible ascendancy of social media.
“Whether you like it or not, social media is here to stay. We cannot fight it. Rather, we need to embrace it in a responsible way,” said Dakara R. Wright, MD, a dermatologist at the Mid-Atlantic Kaiser Permanente Group, Halethorpe, Md. She, like others, reported that she has come to recognize that social media is a major source of medical information for her patients.
“We need to be a presence on these platforms for the benefit of our patients and their parents,” she said. She acknowledged that she has not been active in posting on social media in the past but said that she has been speaking with administrators in her organization about how to become involved in a responsible way that can be useful to patients.
Candrice R. Heath, MD, assistant professor of dermatology at Temple University, Philadelphia, has been active on social media for several years, posting content on her own account, which is not related to her academic affiliation. She posts for many reasons, not least of which is drawing attention to her expertise.
Like Dr. Landriscina, she recognizes that users of these platforms are guided by the content to make decisions about health care. She also agreed that physicians should not ignore this phenomenon.
Tips on providing content
Given the fact that the algorithm is intended to produce posts that go viral, Dr. Landriscina urged clinicians to make their content easy to watch. He said it is not necessary to overthink content beyond providing accurate information, but he advised that videos be made with attention to adequate lighting and other simple factors to promote visual quality. He said that accurate information is not necessarily dull.
“Some facts can actually be surprising to patients,” he said. He noted that a calm, coherent video can be particularly effective in attracting an audience when it is in reaction to information that has gone viral but is misleading or patently incorrect.
Dr. Landriscina has been an influencer associated with multiple social media platforms, including TikTok. He has in the past been paid for consulting work for TikTok. Dr. Wright and Dr. Heath reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , warned an expert at the annual meeting of the Society for Pediatric Dermatology.
“If we don’t get involved, we are basically letting misinformation win. We need to be there,” said Angelo Landriscina, MD, director of dermatology at a Mount Sinai Doctors Clinic in New York.
Most of the content currently available on medical topics, including dermatology and pediatric dermatology, is not created by health care professionals, Dr. Landriscina noted. Not surprisingly, given that much of the content is based on personal opinion from individuals who have no expertise in medical care, he described the information as being of “low quality” when not fully erroneous.
Dr. Landriscina has been active on social media, including TikTok, for several years. Most of his posts involve responses to misinformation. When he sets the record straight on the basis of existing evidence, he often supports his counterargument with references.
He acknowledged that when he became involved in social media he faced criticism from colleagues about participating on an entertainment platform that many considered unworthy of providing objective information. If that was ever true, he argued, it is no longer the case.
“TikTok has adopted a new strategy. The goal is to unseat Google as a search tool, and it’s working,” he said. He explained that many people now use TikTok and other social media sites as their primary source of information on essentially every topic, from where to eat to whether to be screened for cancer.
The particular problem with TikTok – one of the most popular social media outlets – is that there is no mechanism for vetting the source of information. YouTube, by contrast, now requires some sort of validation for anyone who claims to have a medical degree or any other verifiable qualification, according to Dr. Landriscina. TikTok, like many other platforms, has no such requirement.
“Anyone can buy a pair of scrubs [implying expertise] and then post a video,” Dr. Landriscina said.
Even if information from one content provider is more valid than information from others, the TikTok algorithm is specifically designed to emphasize content that has the potential for going viral, which means it favors videos that are provocative over those that are not.
“The algorithm favors any content that is more controversial, more surprising, and keeps viewers engaged,” Dr. Landriscina pointed out.
This does not mean that objective and factual information is ignored, but the algorithm is indifferent to the validity of information, meaning that it allows videos to be posted without regard to whether the content is true, untrue, purposefully misleading, or utter nonsense. For that reason, it is often easier to attract attention by responding to a post that has already gone viral. Information that is clear and digestible can attract viewers and therefore is distributed more widely with the TikTok algorithm.
Parents are on Tiktok too
There is a misperception that the TikTok audience is younger, according to Dr. Landriscina. While peak use in the United States fell among people between the ages of 25 and 34 years in 2022, he said the number of users falls off relatively slowly with subsequent 10-year increments in age. In 2022, there were nearly 20 million users in the peak 10-year age range, but 7.5 million users were 55 years of age or older.
“Pediatric dermatologists should recognize that it is not just kids who are looking for information about their skin diseases, but also their parents,” Dr. Landriscina said.
The top three dermatology topics searched on TikTok in a recent period were acne, alopecia, and cysts. But top searches are very fluid and are extremely hard to quantify, because the basis of the algorithm, which is a proprietary secret, is not only unknown but produces different results for every user.
“The second you touch the app, it changes,” Dr. Landriscina said. He explained that an inquiry about any subject, including those that are medically related, yields content that is different, or at least ordered differently, “depending on how you behaved on the app in the past.”
The phenomenon that drives social media predates this technology. Dr. Landriscina cited a study in 1956 that described the “parasocial interaction theory.” The theory was based on the observation that those who consume media, such as television, which was relatively new in 1956, believed that they had a personal relationship with media figures.
“The users begin to trust influencers as a source, like a friend providing them advice,” Dr. Landriscina said. As an example, he suggested that a fan of the television show Friends who follows actor Jennifer Aniston on social media platforms may begin to think of her as a trusted source of information on any topic, including those for which she may not have expertise.
The reason that he urges medical professionals to become active on TikTok and other social media platforms is that they have a potentially critical role in responding to information that is not just wrong but harmful.
On TikTok and other social media platforms, “there is a lot of interest in content about dermatologic conditions in children. There is a real need for accurate information,” he said,
In the question-and-answer session following his presentation, Dr. Landriscina’s message was not uniformly embraced. One risk, according to an audience member, is that medical professionals will begin to express their own personal opinions rather than rely on evidence, with the result that they will “just add to the sea of misinformation.”
However, this opinion appeared to be the minority view. Most of those who commented took a “that-ship-has-sailed” stance, recognizing the irreversible ascendancy of social media.
“Whether you like it or not, social media is here to stay. We cannot fight it. Rather, we need to embrace it in a responsible way,” said Dakara R. Wright, MD, a dermatologist at the Mid-Atlantic Kaiser Permanente Group, Halethorpe, Md. She, like others, reported that she has come to recognize that social media is a major source of medical information for her patients.
“We need to be a presence on these platforms for the benefit of our patients and their parents,” she said. She acknowledged that she has not been active in posting on social media in the past but said that she has been speaking with administrators in her organization about how to become involved in a responsible way that can be useful to patients.
Candrice R. Heath, MD, assistant professor of dermatology at Temple University, Philadelphia, has been active on social media for several years, posting content on her own account, which is not related to her academic affiliation. She posts for many reasons, not least of which is drawing attention to her expertise.
Like Dr. Landriscina, she recognizes that users of these platforms are guided by the content to make decisions about health care. She also agreed that physicians should not ignore this phenomenon.
Tips on providing content
Given the fact that the algorithm is intended to produce posts that go viral, Dr. Landriscina urged clinicians to make their content easy to watch. He said it is not necessary to overthink content beyond providing accurate information, but he advised that videos be made with attention to adequate lighting and other simple factors to promote visual quality. He said that accurate information is not necessarily dull.
“Some facts can actually be surprising to patients,” he said. He noted that a calm, coherent video can be particularly effective in attracting an audience when it is in reaction to information that has gone viral but is misleading or patently incorrect.
Dr. Landriscina has been an influencer associated with multiple social media platforms, including TikTok. He has in the past been paid for consulting work for TikTok. Dr. Wright and Dr. Heath reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , warned an expert at the annual meeting of the Society for Pediatric Dermatology.
“If we don’t get involved, we are basically letting misinformation win. We need to be there,” said Angelo Landriscina, MD, director of dermatology at a Mount Sinai Doctors Clinic in New York.
Most of the content currently available on medical topics, including dermatology and pediatric dermatology, is not created by health care professionals, Dr. Landriscina noted. Not surprisingly, given that much of the content is based on personal opinion from individuals who have no expertise in medical care, he described the information as being of “low quality” when not fully erroneous.
Dr. Landriscina has been active on social media, including TikTok, for several years. Most of his posts involve responses to misinformation. When he sets the record straight on the basis of existing evidence, he often supports his counterargument with references.
He acknowledged that when he became involved in social media he faced criticism from colleagues about participating on an entertainment platform that many considered unworthy of providing objective information. If that was ever true, he argued, it is no longer the case.
“TikTok has adopted a new strategy. The goal is to unseat Google as a search tool, and it’s working,” he said. He explained that many people now use TikTok and other social media sites as their primary source of information on essentially every topic, from where to eat to whether to be screened for cancer.
The particular problem with TikTok – one of the most popular social media outlets – is that there is no mechanism for vetting the source of information. YouTube, by contrast, now requires some sort of validation for anyone who claims to have a medical degree or any other verifiable qualification, according to Dr. Landriscina. TikTok, like many other platforms, has no such requirement.
“Anyone can buy a pair of scrubs [implying expertise] and then post a video,” Dr. Landriscina said.
Even if information from one content provider is more valid than information from others, the TikTok algorithm is specifically designed to emphasize content that has the potential for going viral, which means it favors videos that are provocative over those that are not.
“The algorithm favors any content that is more controversial, more surprising, and keeps viewers engaged,” Dr. Landriscina pointed out.
This does not mean that objective and factual information is ignored, but the algorithm is indifferent to the validity of information, meaning that it allows videos to be posted without regard to whether the content is true, untrue, purposefully misleading, or utter nonsense. For that reason, it is often easier to attract attention by responding to a post that has already gone viral. Information that is clear and digestible can attract viewers and therefore is distributed more widely with the TikTok algorithm.
Parents are on Tiktok too
There is a misperception that the TikTok audience is younger, according to Dr. Landriscina. While peak use in the United States fell among people between the ages of 25 and 34 years in 2022, he said the number of users falls off relatively slowly with subsequent 10-year increments in age. In 2022, there were nearly 20 million users in the peak 10-year age range, but 7.5 million users were 55 years of age or older.
“Pediatric dermatologists should recognize that it is not just kids who are looking for information about their skin diseases, but also their parents,” Dr. Landriscina said.
The top three dermatology topics searched on TikTok in a recent period were acne, alopecia, and cysts. But top searches are very fluid and are extremely hard to quantify, because the basis of the algorithm, which is a proprietary secret, is not only unknown but produces different results for every user.
“The second you touch the app, it changes,” Dr. Landriscina said. He explained that an inquiry about any subject, including those that are medically related, yields content that is different, or at least ordered differently, “depending on how you behaved on the app in the past.”
The phenomenon that drives social media predates this technology. Dr. Landriscina cited a study in 1956 that described the “parasocial interaction theory.” The theory was based on the observation that those who consume media, such as television, which was relatively new in 1956, believed that they had a personal relationship with media figures.
“The users begin to trust influencers as a source, like a friend providing them advice,” Dr. Landriscina said. As an example, he suggested that a fan of the television show Friends who follows actor Jennifer Aniston on social media platforms may begin to think of her as a trusted source of information on any topic, including those for which she may not have expertise.
The reason that he urges medical professionals to become active on TikTok and other social media platforms is that they have a potentially critical role in responding to information that is not just wrong but harmful.
On TikTok and other social media platforms, “there is a lot of interest in content about dermatologic conditions in children. There is a real need for accurate information,” he said,
In the question-and-answer session following his presentation, Dr. Landriscina’s message was not uniformly embraced. One risk, according to an audience member, is that medical professionals will begin to express their own personal opinions rather than rely on evidence, with the result that they will “just add to the sea of misinformation.”
However, this opinion appeared to be the minority view. Most of those who commented took a “that-ship-has-sailed” stance, recognizing the irreversible ascendancy of social media.
“Whether you like it or not, social media is here to stay. We cannot fight it. Rather, we need to embrace it in a responsible way,” said Dakara R. Wright, MD, a dermatologist at the Mid-Atlantic Kaiser Permanente Group, Halethorpe, Md. She, like others, reported that she has come to recognize that social media is a major source of medical information for her patients.
“We need to be a presence on these platforms for the benefit of our patients and their parents,” she said. She acknowledged that she has not been active in posting on social media in the past but said that she has been speaking with administrators in her organization about how to become involved in a responsible way that can be useful to patients.
Candrice R. Heath, MD, assistant professor of dermatology at Temple University, Philadelphia, has been active on social media for several years, posting content on her own account, which is not related to her academic affiliation. She posts for many reasons, not least of which is drawing attention to her expertise.
Like Dr. Landriscina, she recognizes that users of these platforms are guided by the content to make decisions about health care. She also agreed that physicians should not ignore this phenomenon.
Tips on providing content
Given the fact that the algorithm is intended to produce posts that go viral, Dr. Landriscina urged clinicians to make their content easy to watch. He said it is not necessary to overthink content beyond providing accurate information, but he advised that videos be made with attention to adequate lighting and other simple factors to promote visual quality. He said that accurate information is not necessarily dull.
“Some facts can actually be surprising to patients,” he said. He noted that a calm, coherent video can be particularly effective in attracting an audience when it is in reaction to information that has gone viral but is misleading or patently incorrect.
Dr. Landriscina has been an influencer associated with multiple social media platforms, including TikTok. He has in the past been paid for consulting work for TikTok. Dr. Wright and Dr. Heath reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
AT SPD 2023
Commentary: Node irradiation, HER2+ treatment, and diet in BC, August 2023
The addition of pertuzumab to trastuzumab plus chemotherapy has demonstrated improvement in pathologic complete response (pCR) rates compared with trastuzumab plus chemotherapy in early-stage HER2-positive breast cancer.3 The framework of oncology is built on clinical trials through their rigorous design, enrollment, and synthesis of data; however, real-world studies are an integral component of cancer research because they provide a more representative sample of the general population treated in routine clinical practice. Neopearl was a retrospective, observational, real-world study that evaluated the efficacy and safety of trastuzumab plus chemotherapy with or without pertuzumab among 271 patients with stage II-III HER2-positive breast cancer (Fabbri et al). The addition of pertuzumab led to an increase in pCR rate (49% vs 62%; odds ratio 1.74; P = .032) and improvement in 5-year event-free survival (81% vs 93%; hazard ratio 2.22; P = .041), and the benefit on univariate analysis was restricted to patients with positive axillary nodes. Furthermore, there were no significant differences in adverse events, including cardiac, between the two groups. These results serve to strengthen the available data regarding the clinical efficacy and favorable safety profile of dual HER2-targeted therapy combined with neoadjuvant chemotherapy.
Lifestyle factors, including physical activity and diet, are becoming increasingly recognized as important determinants of various cancer-specific outcomes and overall health. Furthermore, because these are modifiable, there is often motivation on behalf of an individual to change behaviors that can affect their outcome. Adherence to the Mediterranean diet (MD) has been associated with reduced risk for breast cancer development and lower mortality among women with breast cancer.4,5 Data from a prospective multicenter European cohort including 13,270 breast cancer survivors demonstrated that low compared with medium adherence to a MD before a breast cancer diagnosis was associated with a 13% higher risk for all-cause mortality (hazard ratio 1.13; 95% CI 1.01-1.26). A three-unit increase in the adapted relative MD score was associated with an 8% reduced risk for overall mortality (hazard ratio3-unit 0.92; 95% CI 0.87-0.97); this result was sustained in the postmenopausal population and strengthened in metastatic disease (Castro-Espin et al). The connection between diet and cancer outcomes is complex, and future research evaluating specific dietary interventions and the underlying biologic pathways by which nutrition exerts its effects will be important to inform our counseling for patients with breast cancer in the survivorship setting.
Additional References
- Whelan TJ, Olivotto IA, Parulekar WR, et al, for the MA.20 Study Investigators. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307-16. doi:10.1056/NEJMoa1415340
- ClinicalTrials.gov. Regional radiotherapy in biomarker low-risk node positive and T3N0 breast cancer (TAILOR RT). National Library of Medicine. Last updated November 23, 2022. https://www.clinicaltrials.gov/study/NCT03488693
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25-32. doi:10.1016/S1470-2045(11)70336-9
- Buckland G, Travier N, Cottet V, et al. Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int J Cancer. 2013;132:2918-27. doi:10.1002/ijc.27958
- Haslam DE, John EM, Knight JA, et al. Diet quality and all-cause mortality in women with breast cancer from the Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2023;32:678-686. doi:10.1158/1055-9965.EPI-22-1198
The addition of pertuzumab to trastuzumab plus chemotherapy has demonstrated improvement in pathologic complete response (pCR) rates compared with trastuzumab plus chemotherapy in early-stage HER2-positive breast cancer.3 The framework of oncology is built on clinical trials through their rigorous design, enrollment, and synthesis of data; however, real-world studies are an integral component of cancer research because they provide a more representative sample of the general population treated in routine clinical practice. Neopearl was a retrospective, observational, real-world study that evaluated the efficacy and safety of trastuzumab plus chemotherapy with or without pertuzumab among 271 patients with stage II-III HER2-positive breast cancer (Fabbri et al). The addition of pertuzumab led to an increase in pCR rate (49% vs 62%; odds ratio 1.74; P = .032) and improvement in 5-year event-free survival (81% vs 93%; hazard ratio 2.22; P = .041), and the benefit on univariate analysis was restricted to patients with positive axillary nodes. Furthermore, there were no significant differences in adverse events, including cardiac, between the two groups. These results serve to strengthen the available data regarding the clinical efficacy and favorable safety profile of dual HER2-targeted therapy combined with neoadjuvant chemotherapy.
Lifestyle factors, including physical activity and diet, are becoming increasingly recognized as important determinants of various cancer-specific outcomes and overall health. Furthermore, because these are modifiable, there is often motivation on behalf of an individual to change behaviors that can affect their outcome. Adherence to the Mediterranean diet (MD) has been associated with reduced risk for breast cancer development and lower mortality among women with breast cancer.4,5 Data from a prospective multicenter European cohort including 13,270 breast cancer survivors demonstrated that low compared with medium adherence to a MD before a breast cancer diagnosis was associated with a 13% higher risk for all-cause mortality (hazard ratio 1.13; 95% CI 1.01-1.26). A three-unit increase in the adapted relative MD score was associated with an 8% reduced risk for overall mortality (hazard ratio3-unit 0.92; 95% CI 0.87-0.97); this result was sustained in the postmenopausal population and strengthened in metastatic disease (Castro-Espin et al). The connection between diet and cancer outcomes is complex, and future research evaluating specific dietary interventions and the underlying biologic pathways by which nutrition exerts its effects will be important to inform our counseling for patients with breast cancer in the survivorship setting.
Additional References
- Whelan TJ, Olivotto IA, Parulekar WR, et al, for the MA.20 Study Investigators. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307-16. doi:10.1056/NEJMoa1415340
- ClinicalTrials.gov. Regional radiotherapy in biomarker low-risk node positive and T3N0 breast cancer (TAILOR RT). National Library of Medicine. Last updated November 23, 2022. https://www.clinicaltrials.gov/study/NCT03488693
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25-32. doi:10.1016/S1470-2045(11)70336-9
- Buckland G, Travier N, Cottet V, et al. Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int J Cancer. 2013;132:2918-27. doi:10.1002/ijc.27958
- Haslam DE, John EM, Knight JA, et al. Diet quality and all-cause mortality in women with breast cancer from the Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2023;32:678-686. doi:10.1158/1055-9965.EPI-22-1198
The addition of pertuzumab to trastuzumab plus chemotherapy has demonstrated improvement in pathologic complete response (pCR) rates compared with trastuzumab plus chemotherapy in early-stage HER2-positive breast cancer.3 The framework of oncology is built on clinical trials through their rigorous design, enrollment, and synthesis of data; however, real-world studies are an integral component of cancer research because they provide a more representative sample of the general population treated in routine clinical practice. Neopearl was a retrospective, observational, real-world study that evaluated the efficacy and safety of trastuzumab plus chemotherapy with or without pertuzumab among 271 patients with stage II-III HER2-positive breast cancer (Fabbri et al). The addition of pertuzumab led to an increase in pCR rate (49% vs 62%; odds ratio 1.74; P = .032) and improvement in 5-year event-free survival (81% vs 93%; hazard ratio 2.22; P = .041), and the benefit on univariate analysis was restricted to patients with positive axillary nodes. Furthermore, there were no significant differences in adverse events, including cardiac, between the two groups. These results serve to strengthen the available data regarding the clinical efficacy and favorable safety profile of dual HER2-targeted therapy combined with neoadjuvant chemotherapy.
Lifestyle factors, including physical activity and diet, are becoming increasingly recognized as important determinants of various cancer-specific outcomes and overall health. Furthermore, because these are modifiable, there is often motivation on behalf of an individual to change behaviors that can affect their outcome. Adherence to the Mediterranean diet (MD) has been associated with reduced risk for breast cancer development and lower mortality among women with breast cancer.4,5 Data from a prospective multicenter European cohort including 13,270 breast cancer survivors demonstrated that low compared with medium adherence to a MD before a breast cancer diagnosis was associated with a 13% higher risk for all-cause mortality (hazard ratio 1.13; 95% CI 1.01-1.26). A three-unit increase in the adapted relative MD score was associated with an 8% reduced risk for overall mortality (hazard ratio3-unit 0.92; 95% CI 0.87-0.97); this result was sustained in the postmenopausal population and strengthened in metastatic disease (Castro-Espin et al). The connection between diet and cancer outcomes is complex, and future research evaluating specific dietary interventions and the underlying biologic pathways by which nutrition exerts its effects will be important to inform our counseling for patients with breast cancer in the survivorship setting.
Additional References
- Whelan TJ, Olivotto IA, Parulekar WR, et al, for the MA.20 Study Investigators. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307-16. doi:10.1056/NEJMoa1415340
- ClinicalTrials.gov. Regional radiotherapy in biomarker low-risk node positive and T3N0 breast cancer (TAILOR RT). National Library of Medicine. Last updated November 23, 2022. https://www.clinicaltrials.gov/study/NCT03488693
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25-32. doi:10.1016/S1470-2045(11)70336-9
- Buckland G, Travier N, Cottet V, et al. Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int J Cancer. 2013;132:2918-27. doi:10.1002/ijc.27958
- Haslam DE, John EM, Knight JA, et al. Diet quality and all-cause mortality in women with breast cancer from the Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2023;32:678-686. doi:10.1158/1055-9965.EPI-22-1198
No cognitive benefit from meditation, learning a language?
The findings are similar to results from another study published last year but are contrary to previous findings showing cognitive benefits for practicing meditation and learning a new language later in life.
“Based on existing literature, which has provided support for the efficacy of meditation and foreign language training in promoting cognition among older adults, perhaps the most surprising outcome of our study was the lack of evidence indicating cognitive benefits after 18 months of either intervention,” lead author Harriet Demnitz-King, MSc, a doctoral candidate at University College London, said in an interview. The findings were published online in JAMA Network Open.
Contradictory findings
For the study, 135 French-speaking, cognitively healthy people were randomized to English-language training, meditation, or a control group. All participants were aged 65 years or older, had been retired for at least 1 year, and had completed at least 7 years of education.
The meditation and English-language training interventions were both 18 months long and included a 2-hour weekly group session, daily home practice of at least 20 minutes, and 1-day intensive 5-hour practice.
Researchers found no significant changes in global cognition, episodic memory, executive function, or attention with either intervention, compared with the control group or to each other.
The findings contradict the researchers’ earlier work that found mindfulness meditation boosted cognitive function in older adults with subjective cognitive decline.
“We are still trying to reconcile these findings,” senior author Natalie Marchant, PhD, associate professor in the division of psychiatry at University College London, said. “It may be that mindfulness meditation may not improve cognition beyond normally functioning levels but may help to preserve cognition in the face of cognitive decline.”
This study was the longest randomized controlled trial in older adults to investigate the effects of non-native language learning on cognition, Dr. Marchant said.
“It may be that language-learning may buffer against age-related cognitive decline but does not boost cognition in high-functioning individuals,” Dr. Marchant said. “While language learning may not improve cognition, we do not want to discard the other possibility without first examining it.”
Dr. Marchant plans to follow participants for years to come to study that very question.
More to learn
The results harken to those of a study last year with a similar participant group and similar results. In that work, mindfulness meditation and exercise also failed to boost cognition in healthy adults. But that may not be the whole story, according to Eric Lenze, MD, professor and chair of psychiatry at Washington University School of Medicine, St. Louis.
Dr. Lenze was a lead author on that earlier research, known as the MEDEX trial, but was not involved with this study. He commented on the new findings for this news organization.
“People may read these results, and ours that were published in JAMA in December, as suggesting that lifestyle and cognitive interventions don’t work in older adults, but that’s not what this shows, in my opinion,” Dr. Lenze said. “It shows that we don’t understand the science of the aging brain as much as we would like to.”
Participants in most of these studies were mostly White, highly educated, and in good cognitive health, all characteristics that could have skewed these findings, he added.
“It may be that interventions to improve cognitive function in older adults would be more likely to help people who have more room to benefit,” Dr. Lenze said. “If you’re already highly educated, healthy, and cognitively normal, why should we expect that you could do even better than that?”
The Age-Well study was funded by European Union in Horizon 2020 program and Inserm, Région Normandie, Fondation d’entreprise MMA des Entrepreneurs du Futur. Dr. Marchant reports grants from Alzheimer’s Society and the U.K. Medical Research Council. Dr. Lenze reports funding from Takeda pharmaceuticals and has been a consultant for Pritikin Intensive Cardiac Rehabilitation.
A version of this article first appeared on Medscape.com.
The findings are similar to results from another study published last year but are contrary to previous findings showing cognitive benefits for practicing meditation and learning a new language later in life.
“Based on existing literature, which has provided support for the efficacy of meditation and foreign language training in promoting cognition among older adults, perhaps the most surprising outcome of our study was the lack of evidence indicating cognitive benefits after 18 months of either intervention,” lead author Harriet Demnitz-King, MSc, a doctoral candidate at University College London, said in an interview. The findings were published online in JAMA Network Open.
Contradictory findings
For the study, 135 French-speaking, cognitively healthy people were randomized to English-language training, meditation, or a control group. All participants were aged 65 years or older, had been retired for at least 1 year, and had completed at least 7 years of education.
The meditation and English-language training interventions were both 18 months long and included a 2-hour weekly group session, daily home practice of at least 20 minutes, and 1-day intensive 5-hour practice.
Researchers found no significant changes in global cognition, episodic memory, executive function, or attention with either intervention, compared with the control group or to each other.
The findings contradict the researchers’ earlier work that found mindfulness meditation boosted cognitive function in older adults with subjective cognitive decline.
“We are still trying to reconcile these findings,” senior author Natalie Marchant, PhD, associate professor in the division of psychiatry at University College London, said. “It may be that mindfulness meditation may not improve cognition beyond normally functioning levels but may help to preserve cognition in the face of cognitive decline.”
This study was the longest randomized controlled trial in older adults to investigate the effects of non-native language learning on cognition, Dr. Marchant said.
“It may be that language-learning may buffer against age-related cognitive decline but does not boost cognition in high-functioning individuals,” Dr. Marchant said. “While language learning may not improve cognition, we do not want to discard the other possibility without first examining it.”
Dr. Marchant plans to follow participants for years to come to study that very question.
More to learn
The results harken to those of a study last year with a similar participant group and similar results. In that work, mindfulness meditation and exercise also failed to boost cognition in healthy adults. But that may not be the whole story, according to Eric Lenze, MD, professor and chair of psychiatry at Washington University School of Medicine, St. Louis.
Dr. Lenze was a lead author on that earlier research, known as the MEDEX trial, but was not involved with this study. He commented on the new findings for this news organization.
“People may read these results, and ours that were published in JAMA in December, as suggesting that lifestyle and cognitive interventions don’t work in older adults, but that’s not what this shows, in my opinion,” Dr. Lenze said. “It shows that we don’t understand the science of the aging brain as much as we would like to.”
Participants in most of these studies were mostly White, highly educated, and in good cognitive health, all characteristics that could have skewed these findings, he added.
“It may be that interventions to improve cognitive function in older adults would be more likely to help people who have more room to benefit,” Dr. Lenze said. “If you’re already highly educated, healthy, and cognitively normal, why should we expect that you could do even better than that?”
The Age-Well study was funded by European Union in Horizon 2020 program and Inserm, Région Normandie, Fondation d’entreprise MMA des Entrepreneurs du Futur. Dr. Marchant reports grants from Alzheimer’s Society and the U.K. Medical Research Council. Dr. Lenze reports funding from Takeda pharmaceuticals and has been a consultant for Pritikin Intensive Cardiac Rehabilitation.
A version of this article first appeared on Medscape.com.
The findings are similar to results from another study published last year but are contrary to previous findings showing cognitive benefits for practicing meditation and learning a new language later in life.
“Based on existing literature, which has provided support for the efficacy of meditation and foreign language training in promoting cognition among older adults, perhaps the most surprising outcome of our study was the lack of evidence indicating cognitive benefits after 18 months of either intervention,” lead author Harriet Demnitz-King, MSc, a doctoral candidate at University College London, said in an interview. The findings were published online in JAMA Network Open.
Contradictory findings
For the study, 135 French-speaking, cognitively healthy people were randomized to English-language training, meditation, or a control group. All participants were aged 65 years or older, had been retired for at least 1 year, and had completed at least 7 years of education.
The meditation and English-language training interventions were both 18 months long and included a 2-hour weekly group session, daily home practice of at least 20 minutes, and 1-day intensive 5-hour practice.
Researchers found no significant changes in global cognition, episodic memory, executive function, or attention with either intervention, compared with the control group or to each other.
The findings contradict the researchers’ earlier work that found mindfulness meditation boosted cognitive function in older adults with subjective cognitive decline.
“We are still trying to reconcile these findings,” senior author Natalie Marchant, PhD, associate professor in the division of psychiatry at University College London, said. “It may be that mindfulness meditation may not improve cognition beyond normally functioning levels but may help to preserve cognition in the face of cognitive decline.”
This study was the longest randomized controlled trial in older adults to investigate the effects of non-native language learning on cognition, Dr. Marchant said.
“It may be that language-learning may buffer against age-related cognitive decline but does not boost cognition in high-functioning individuals,” Dr. Marchant said. “While language learning may not improve cognition, we do not want to discard the other possibility without first examining it.”
Dr. Marchant plans to follow participants for years to come to study that very question.
More to learn
The results harken to those of a study last year with a similar participant group and similar results. In that work, mindfulness meditation and exercise also failed to boost cognition in healthy adults. But that may not be the whole story, according to Eric Lenze, MD, professor and chair of psychiatry at Washington University School of Medicine, St. Louis.
Dr. Lenze was a lead author on that earlier research, known as the MEDEX trial, but was not involved with this study. He commented on the new findings for this news organization.
“People may read these results, and ours that were published in JAMA in December, as suggesting that lifestyle and cognitive interventions don’t work in older adults, but that’s not what this shows, in my opinion,” Dr. Lenze said. “It shows that we don’t understand the science of the aging brain as much as we would like to.”
Participants in most of these studies were mostly White, highly educated, and in good cognitive health, all characteristics that could have skewed these findings, he added.
“It may be that interventions to improve cognitive function in older adults would be more likely to help people who have more room to benefit,” Dr. Lenze said. “If you’re already highly educated, healthy, and cognitively normal, why should we expect that you could do even better than that?”
The Age-Well study was funded by European Union in Horizon 2020 program and Inserm, Région Normandie, Fondation d’entreprise MMA des Entrepreneurs du Futur. Dr. Marchant reports grants from Alzheimer’s Society and the U.K. Medical Research Council. Dr. Lenze reports funding from Takeda pharmaceuticals and has been a consultant for Pritikin Intensive Cardiac Rehabilitation.
A version of this article first appeared on Medscape.com.
FROM JAMA Network Open