User login
Good Midlife Dietary Habits May Increase Likelihood of Healthy Aging
Study Overview
Objective. To evaluate the contribution of dietary habits in midlife on healthy aging.
Study design. Observational investigation of an ongoing cohort study.
Setting and participants. Participants were gathered from the Nurses’ Health Study, a cohort of 121,700 married female nurses who have completed health-related questionnaires every 2 years since 1976. Data on race was not originally collected, but a subsample analysis revealed that the cohort of nurses was > 98% white [1]. A subset of this cohort (n = 19,415) older than age 70 years from 1995 and 2002 and who received additional cognitive testing was chosen as the population of interest for this study. The investigators excluded participants with missing data (n = 5878) on important covariates and participants who had any of 11 chronic diseases in midlife (n = 2585), obtained from questionnaires in the 1980s. 10,670 participants were included in the final analysis.
Main outcome measures. Participants were dichotomized as “healthy agers” or “usual agers” on the basis of 4 health domains measured in 2000. Persons free of 11 chronic diseases, without cognitive impairment, without physical limitations, and with intact mental health were designated “healthy agers,” with the remainder designated “usual agers.” For each domain, specific criteria were employed to indicate impairment. Cognitive impairment was defined as a score of 31 or greater on the Telephone Interview for Cognitive Status. Investigators used the Medical Outcomes Short-Form 36 health survey (SF-36) to measure physical impairment and mental health. Participants who reported being limited at least “a little” on moderate activities or limited “a lot” on strenuous activities were classified as physically impaired. Intact mental health was defined as a score above the cohort’s median on the mental health subscale of the SF-36.
Dietary habits were ascertained at midlife by an average of the 1984 and 1986 food frequency questionnaire (FFQ) data. Using these data, the authors calculated the Alternative Healthy Eating-2010 (AHEI-2010) and the Alternate Mediterranean Diet (A-MeDi) scores. AHEH-2010 incorporates the latest knowledge on the benefits and harms of foods and nutrients on the risk of chronic disease. It has 11 domains (including whole grain intake, vegetable intake, and lower intake of trans fats, among others) which are each scored 0 (worst) to 10 (best). The A-MeDi score assesses adherence to the traditional Mediterranean diet, which includes intake of vegetables, fruits, nuts, legumes, and moderate alcohol intake, among others. Each of 9 categories is rated 0 or 1, with 1 representing healthy intake.
Covariates included sociodemographic, lifestyle, and health-related measures obtained either in 1984 or 1986. These included age; educational level; household income and home value estimated from census tract data; marital status; family history of diabetes, cancer, and myocardial infarction; physical activity; smoking; multivitamin and aspirin use; BMI; history of high blood pressure; and hypercholesterolemia. BMI was obtained via self-report and averaged from among values obtained in 1984 and 1986; these have previously been shown to have excellent correlation (r = 0.97) to standardized examinations [2].
The authors standardized baseline characteristics for each study participant based upon the age at which they entered the study. They used logistic regression to estimate the odds of being a “healthy ager” in the year 2000 by quintile of AHEI-2010 and A-MeDi scores.
Main results. Of the 10,670 participants, 1171 (11%) were labeled “healthy agers” and 9499 (89%) were labeled “usual agers.” Prevalence in each of the 4 health domains varied widely: 9599 (90%) of the 10670 participants had no cognitive impairment, 7234 (67.8%) had no chronic diseases, 4606 (43.2%) had no mental health limitations, and 2905 (27.2%) had no impairment of physical functioning.
Investigators presented data comparing healthy agers and usual agers at baseline without tests for significance. The mean age of healthy agers and usual agers was comparable (58.6 [SD = 2.5] vs. 59.1 [SD = 2.5]). Healthy agers had lower prevalence of obesity (3% vs. 13%), ever smoking (54% vs. 47%), higher mean physical activity (19.4 MET h/wk [SD = 21.7] vs. 14.1 MET h/wk [SD = 19.8]), lower energy intake (1692 kcal/d [SD = 472] vs. 1743 kcal/d [SD = 477]) and lower prevalence of hypertension (20% vs. 32%) and hypercholesterolemia (12% vs. 17%). Healthy agers also had higher baseline AHEI-2010 (53.2 [SD = 10.3] vs. 50.6 [SD = 10.1]) and A-MeDi scores in midlife (4.5 [SD = 1.6] vs. 4.3 [SD = 1.7]).
Greater scores on the AHEI-2010 and A-MeDi measures in midlife were associated with greater odds of healthy aging in multivariate analysis. After adjusting for all covariates, women in the highest quintile of AHEI-2010 scores at baseline had 34% greater odds (95% CI, 9% to 66%) of being healthy agers compared to women in the lowest quintile. Likewise, adjusted analyses reported women in the highest quintile of A-MeDi scores had 46% greater odds (95% CI, 17% to 83%) of being healthy agers.
Secondary analyses tested each component of healthy aging for associations with AHEI-2010 and A-MeDi scores in midlife. Associations were overall weaker, but no impairment of physical function and no limitation of mental health were both found to be significant after adjustment for covariates. Women in the highest quintile of AHEI-2010 scores at baseline had 23% (95% CI, 11% to 36%) and 13% (95% CI, 5% to 22%) greater odds, respectively, of not having any physical limitations or mental health impairments in late life compared to women in the lowest quintile. Likewise, women in the highest quintile of A-MeDi scores at baseline had 14% (95% CI, 3% to 26%) and 12% (95% CI, 4% to 20%) greater odds, respectively, of not having any physical limitations or mental health impairments in late life compared to women in the lowest quintile.
The authors also tested the effect of individual components of dietary patterns on healthy aging, comparing those in the highest quintile versus those in the lowest quintile for each measure. Persons with the greatest intake of fruits had 46% (95% CI, 15% to 85%) greater odds of being healthy agers compared to those with the lowest intake of fruits. Persons with the highest intake of alcohol had 28% greater odds (95% CI, 4% to 56%) of being healthy agers compared to those with the lowest intake of alcohol. Conversely, those with lower intake of sugar-sweetened beverages (OR, 1.28 [95% CI, 1.03 to 1.58]) and non-omega 3 polyunsaturated fatty acids (OR, 1.38 [CI, 1.10 to 1.73]) had better odds of being healthy agers compared to those with higher intakes.
Conclusion. Women with healthy dietary patterns at midlife had significantly greater odds of being healthy agers in later life after adjusting for potential con-founders. Results were consistent in direction and effect size when using either the AHEI-2010 score or the A-MeDi score. The effects of healthy diet at midlife seemed to have the strongest association with physical impairment scores and mental health scores. Higher intake of fruits and alcohol along with lower intake of sugar-sweetened beverages and polyunsaturated fatty acids seemed to have the most power for predicting healthy aging.
Commentary
These results are consistent with current knowledge, which indicates the health benefits of a balanced, healthy diet high in fruits, vegetables, whole grains, nuts, and legumes and low in red or other processed meats. There is high quality evidence linking each dietary measure to health outcomes. Adherence to the Alternative Healthy Eating Index has been related to lower mortality rates [3], decreased risk of cardiovascular disease [4], and decreased risk of type 2 diabetes and the metabolic syndrome [5]. Likewise, adherence to the Mediterranean diet is associated with reductions in overall mortality, cardiovascular incidence and mortality, cancer incidence and mortality, and neurodegenerative diseases [6]. Both diets endorse moderate alcohol intake, which was associated with lower rates of all-cause and cardiovascular mortality in a meta-analysis [7]. Alcohol is theorized to produce decreased platelet aggregation, increase HDL cholesterol, and increase endothelial vasorelaxation [8]. Polyphenols, most prominent in red wines, may have additional effects which include vaso-relaxation of aortic rings, reduced thrombosis and inflammation, and increased fibrinolysis [9]. Nevertheless, heavy alcohol use may increase cardiovascular mortality, hypertension, and hyperlipidemia [8]. This study concluded that higher alcohol intake was related to being a healthy ager; this may be because there are few heavy alcohol users in this cohort, though this hypothesis was not tested in the study.
Any observational study is subject to debate about the confounders chosen for analysis and potential biases. The authors report that the most powerful confounders in this analysis were BMI, physical activity, and smoking, all of which have been well established as predictors for health in later life [10]. Nonetheless, important potential confounders not used in analysis included the baseline prevalence of mental health problems, cognitive limitations, and physical limitations, all of which were not available.
The greatest concern about of this study is a potential lack of generalizability given the population surveyed. The Nurses’ Health Study consists of a cohort of female, married, predominantly white registered nurses [1]. For instance, African Americans have a greater burden of hypertension than non-Hispanic whites after accounting for dietary differences [11], a higher degree of late-life cognitive dysfunction [12], and greater risk of developing late-life physical disability [13]. Also, race and ethnicity may impact eating patterns, food preferences, and food availability in ways that are difficult to predict. In addition, nurses in the cohort were probably of similar socioeconomic status given their shared occupation, though the authors did not report the variation in median household incomes obtained from census tract analysis in this study [14]. Results might change if the sample was less homogeneous. Nonetheless, the results are consistent with current knowledge, biologically plausible, and clinically meaningful.
Applications for Clinical Practice
Integrating dietary changes in middle-aged women may be an important means of decreasing morbidity in older age and improving physical and mental health functioning later in life. Health care providers should discuss the future benefits of healthy eating on quality of life in order to encourage patients in midlife to alter their diet in meaningful ways. While it may be difficult to generalize these findings to patients of different genders, races, or ethnicities, the biological underpinnings of the data make it hard dispute the conclusions presented in the study.
—Hector Perez, MD, and Melanie Jay, MD, MS
1. Hemenway D, Colditz GA, Willett WC, et al. Fractures and lifestyle: effect of cigarette smoking, alcohol intake, and relative weight on the risk of hip and forearm fractures in middle-aged women. Am J Public Health 1988;78:1554–8.
2. Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–73.
3. Akbaraly TN, Ferrie JE, Berr C, et al. Alternative Healthy Eating Index and mortality over 18 y of follow-up: results from the Whitehall II cohort. Am J Clin Nutr 2011;94:247–53.
4. McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–71.
5. Akbaraly TN, Singh-Manoux A, Tabak AG, et al. Overall diet history and reversibility of the metabolic syndrome over 5 years: the Whitehall II prospective cohort study. Diabetes Care 2010;33:2339–41.
6. Sofi F, Abbate R, Gensini GF, et al. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 2010;92:1189–96.
7. Di Castelnuovo A, Costanzo S, Bagnardi V, et al. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 2006;166:2437–45.
8. Costanzo S, Di Castelnuovo A, Donati MB, et al. Cardiovascular and overall mortality risk in relation to alcohol consumption in patients with cardiovascular disease. Circulation 2010;121:1951–9.
9. Booyse FM, Pan W, Grenett HE, et al. Mechanism by which alcohol and wine polyphenols affect coronary heart disease risk. Ann Epidemiol 2007;17:S24–S31.
10. Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med 2012;55:163–70.
11. Diaz VA, Mainous AG, Koopman RJ, et al. Race and diet in the overweight: association with cardiovascular risk in a nationally representative sample. Nutrition 2005;21:718–25.
12. Sloan FA, Wang J. Disparities among older adults in measures of cognitive function by race or ethnicity. J Gerontol B Psychol Sci Soc Sci 2005;60:P242–50.
13. Dunlop DD, Song J, Manheim LM, et al. Racial/ethnic differences in the development of disability among older adults. Am J Public Health 2007;97:2209–15.
14. Puett RC, Schwartz J, Hart JE, et al. Chronic particulate exposure, mortality, and coronary heart disease in the nurses’ health study. Am J Epidemiol 2008;168:1161–8.
Study Overview
Objective. To evaluate the contribution of dietary habits in midlife on healthy aging.
Study design. Observational investigation of an ongoing cohort study.
Setting and participants. Participants were gathered from the Nurses’ Health Study, a cohort of 121,700 married female nurses who have completed health-related questionnaires every 2 years since 1976. Data on race was not originally collected, but a subsample analysis revealed that the cohort of nurses was > 98% white [1]. A subset of this cohort (n = 19,415) older than age 70 years from 1995 and 2002 and who received additional cognitive testing was chosen as the population of interest for this study. The investigators excluded participants with missing data (n = 5878) on important covariates and participants who had any of 11 chronic diseases in midlife (n = 2585), obtained from questionnaires in the 1980s. 10,670 participants were included in the final analysis.
Main outcome measures. Participants were dichotomized as “healthy agers” or “usual agers” on the basis of 4 health domains measured in 2000. Persons free of 11 chronic diseases, without cognitive impairment, without physical limitations, and with intact mental health were designated “healthy agers,” with the remainder designated “usual agers.” For each domain, specific criteria were employed to indicate impairment. Cognitive impairment was defined as a score of 31 or greater on the Telephone Interview for Cognitive Status. Investigators used the Medical Outcomes Short-Form 36 health survey (SF-36) to measure physical impairment and mental health. Participants who reported being limited at least “a little” on moderate activities or limited “a lot” on strenuous activities were classified as physically impaired. Intact mental health was defined as a score above the cohort’s median on the mental health subscale of the SF-36.
Dietary habits were ascertained at midlife by an average of the 1984 and 1986 food frequency questionnaire (FFQ) data. Using these data, the authors calculated the Alternative Healthy Eating-2010 (AHEI-2010) and the Alternate Mediterranean Diet (A-MeDi) scores. AHEH-2010 incorporates the latest knowledge on the benefits and harms of foods and nutrients on the risk of chronic disease. It has 11 domains (including whole grain intake, vegetable intake, and lower intake of trans fats, among others) which are each scored 0 (worst) to 10 (best). The A-MeDi score assesses adherence to the traditional Mediterranean diet, which includes intake of vegetables, fruits, nuts, legumes, and moderate alcohol intake, among others. Each of 9 categories is rated 0 or 1, with 1 representing healthy intake.
Covariates included sociodemographic, lifestyle, and health-related measures obtained either in 1984 or 1986. These included age; educational level; household income and home value estimated from census tract data; marital status; family history of diabetes, cancer, and myocardial infarction; physical activity; smoking; multivitamin and aspirin use; BMI; history of high blood pressure; and hypercholesterolemia. BMI was obtained via self-report and averaged from among values obtained in 1984 and 1986; these have previously been shown to have excellent correlation (r = 0.97) to standardized examinations [2].
The authors standardized baseline characteristics for each study participant based upon the age at which they entered the study. They used logistic regression to estimate the odds of being a “healthy ager” in the year 2000 by quintile of AHEI-2010 and A-MeDi scores.
Main results. Of the 10,670 participants, 1171 (11%) were labeled “healthy agers” and 9499 (89%) were labeled “usual agers.” Prevalence in each of the 4 health domains varied widely: 9599 (90%) of the 10670 participants had no cognitive impairment, 7234 (67.8%) had no chronic diseases, 4606 (43.2%) had no mental health limitations, and 2905 (27.2%) had no impairment of physical functioning.
Investigators presented data comparing healthy agers and usual agers at baseline without tests for significance. The mean age of healthy agers and usual agers was comparable (58.6 [SD = 2.5] vs. 59.1 [SD = 2.5]). Healthy agers had lower prevalence of obesity (3% vs. 13%), ever smoking (54% vs. 47%), higher mean physical activity (19.4 MET h/wk [SD = 21.7] vs. 14.1 MET h/wk [SD = 19.8]), lower energy intake (1692 kcal/d [SD = 472] vs. 1743 kcal/d [SD = 477]) and lower prevalence of hypertension (20% vs. 32%) and hypercholesterolemia (12% vs. 17%). Healthy agers also had higher baseline AHEI-2010 (53.2 [SD = 10.3] vs. 50.6 [SD = 10.1]) and A-MeDi scores in midlife (4.5 [SD = 1.6] vs. 4.3 [SD = 1.7]).
Greater scores on the AHEI-2010 and A-MeDi measures in midlife were associated with greater odds of healthy aging in multivariate analysis. After adjusting for all covariates, women in the highest quintile of AHEI-2010 scores at baseline had 34% greater odds (95% CI, 9% to 66%) of being healthy agers compared to women in the lowest quintile. Likewise, adjusted analyses reported women in the highest quintile of A-MeDi scores had 46% greater odds (95% CI, 17% to 83%) of being healthy agers.
Secondary analyses tested each component of healthy aging for associations with AHEI-2010 and A-MeDi scores in midlife. Associations were overall weaker, but no impairment of physical function and no limitation of mental health were both found to be significant after adjustment for covariates. Women in the highest quintile of AHEI-2010 scores at baseline had 23% (95% CI, 11% to 36%) and 13% (95% CI, 5% to 22%) greater odds, respectively, of not having any physical limitations or mental health impairments in late life compared to women in the lowest quintile. Likewise, women in the highest quintile of A-MeDi scores at baseline had 14% (95% CI, 3% to 26%) and 12% (95% CI, 4% to 20%) greater odds, respectively, of not having any physical limitations or mental health impairments in late life compared to women in the lowest quintile.
The authors also tested the effect of individual components of dietary patterns on healthy aging, comparing those in the highest quintile versus those in the lowest quintile for each measure. Persons with the greatest intake of fruits had 46% (95% CI, 15% to 85%) greater odds of being healthy agers compared to those with the lowest intake of fruits. Persons with the highest intake of alcohol had 28% greater odds (95% CI, 4% to 56%) of being healthy agers compared to those with the lowest intake of alcohol. Conversely, those with lower intake of sugar-sweetened beverages (OR, 1.28 [95% CI, 1.03 to 1.58]) and non-omega 3 polyunsaturated fatty acids (OR, 1.38 [CI, 1.10 to 1.73]) had better odds of being healthy agers compared to those with higher intakes.
Conclusion. Women with healthy dietary patterns at midlife had significantly greater odds of being healthy agers in later life after adjusting for potential con-founders. Results were consistent in direction and effect size when using either the AHEI-2010 score or the A-MeDi score. The effects of healthy diet at midlife seemed to have the strongest association with physical impairment scores and mental health scores. Higher intake of fruits and alcohol along with lower intake of sugar-sweetened beverages and polyunsaturated fatty acids seemed to have the most power for predicting healthy aging.
Commentary
These results are consistent with current knowledge, which indicates the health benefits of a balanced, healthy diet high in fruits, vegetables, whole grains, nuts, and legumes and low in red or other processed meats. There is high quality evidence linking each dietary measure to health outcomes. Adherence to the Alternative Healthy Eating Index has been related to lower mortality rates [3], decreased risk of cardiovascular disease [4], and decreased risk of type 2 diabetes and the metabolic syndrome [5]. Likewise, adherence to the Mediterranean diet is associated with reductions in overall mortality, cardiovascular incidence and mortality, cancer incidence and mortality, and neurodegenerative diseases [6]. Both diets endorse moderate alcohol intake, which was associated with lower rates of all-cause and cardiovascular mortality in a meta-analysis [7]. Alcohol is theorized to produce decreased platelet aggregation, increase HDL cholesterol, and increase endothelial vasorelaxation [8]. Polyphenols, most prominent in red wines, may have additional effects which include vaso-relaxation of aortic rings, reduced thrombosis and inflammation, and increased fibrinolysis [9]. Nevertheless, heavy alcohol use may increase cardiovascular mortality, hypertension, and hyperlipidemia [8]. This study concluded that higher alcohol intake was related to being a healthy ager; this may be because there are few heavy alcohol users in this cohort, though this hypothesis was not tested in the study.
Any observational study is subject to debate about the confounders chosen for analysis and potential biases. The authors report that the most powerful confounders in this analysis were BMI, physical activity, and smoking, all of which have been well established as predictors for health in later life [10]. Nonetheless, important potential confounders not used in analysis included the baseline prevalence of mental health problems, cognitive limitations, and physical limitations, all of which were not available.
The greatest concern about of this study is a potential lack of generalizability given the population surveyed. The Nurses’ Health Study consists of a cohort of female, married, predominantly white registered nurses [1]. For instance, African Americans have a greater burden of hypertension than non-Hispanic whites after accounting for dietary differences [11], a higher degree of late-life cognitive dysfunction [12], and greater risk of developing late-life physical disability [13]. Also, race and ethnicity may impact eating patterns, food preferences, and food availability in ways that are difficult to predict. In addition, nurses in the cohort were probably of similar socioeconomic status given their shared occupation, though the authors did not report the variation in median household incomes obtained from census tract analysis in this study [14]. Results might change if the sample was less homogeneous. Nonetheless, the results are consistent with current knowledge, biologically plausible, and clinically meaningful.
Applications for Clinical Practice
Integrating dietary changes in middle-aged women may be an important means of decreasing morbidity in older age and improving physical and mental health functioning later in life. Health care providers should discuss the future benefits of healthy eating on quality of life in order to encourage patients in midlife to alter their diet in meaningful ways. While it may be difficult to generalize these findings to patients of different genders, races, or ethnicities, the biological underpinnings of the data make it hard dispute the conclusions presented in the study.
—Hector Perez, MD, and Melanie Jay, MD, MS
Study Overview
Objective. To evaluate the contribution of dietary habits in midlife on healthy aging.
Study design. Observational investigation of an ongoing cohort study.
Setting and participants. Participants were gathered from the Nurses’ Health Study, a cohort of 121,700 married female nurses who have completed health-related questionnaires every 2 years since 1976. Data on race was not originally collected, but a subsample analysis revealed that the cohort of nurses was > 98% white [1]. A subset of this cohort (n = 19,415) older than age 70 years from 1995 and 2002 and who received additional cognitive testing was chosen as the population of interest for this study. The investigators excluded participants with missing data (n = 5878) on important covariates and participants who had any of 11 chronic diseases in midlife (n = 2585), obtained from questionnaires in the 1980s. 10,670 participants were included in the final analysis.
Main outcome measures. Participants were dichotomized as “healthy agers” or “usual agers” on the basis of 4 health domains measured in 2000. Persons free of 11 chronic diseases, without cognitive impairment, without physical limitations, and with intact mental health were designated “healthy agers,” with the remainder designated “usual agers.” For each domain, specific criteria were employed to indicate impairment. Cognitive impairment was defined as a score of 31 or greater on the Telephone Interview for Cognitive Status. Investigators used the Medical Outcomes Short-Form 36 health survey (SF-36) to measure physical impairment and mental health. Participants who reported being limited at least “a little” on moderate activities or limited “a lot” on strenuous activities were classified as physically impaired. Intact mental health was defined as a score above the cohort’s median on the mental health subscale of the SF-36.
Dietary habits were ascertained at midlife by an average of the 1984 and 1986 food frequency questionnaire (FFQ) data. Using these data, the authors calculated the Alternative Healthy Eating-2010 (AHEI-2010) and the Alternate Mediterranean Diet (A-MeDi) scores. AHEH-2010 incorporates the latest knowledge on the benefits and harms of foods and nutrients on the risk of chronic disease. It has 11 domains (including whole grain intake, vegetable intake, and lower intake of trans fats, among others) which are each scored 0 (worst) to 10 (best). The A-MeDi score assesses adherence to the traditional Mediterranean diet, which includes intake of vegetables, fruits, nuts, legumes, and moderate alcohol intake, among others. Each of 9 categories is rated 0 or 1, with 1 representing healthy intake.
Covariates included sociodemographic, lifestyle, and health-related measures obtained either in 1984 or 1986. These included age; educational level; household income and home value estimated from census tract data; marital status; family history of diabetes, cancer, and myocardial infarction; physical activity; smoking; multivitamin and aspirin use; BMI; history of high blood pressure; and hypercholesterolemia. BMI was obtained via self-report and averaged from among values obtained in 1984 and 1986; these have previously been shown to have excellent correlation (r = 0.97) to standardized examinations [2].
The authors standardized baseline characteristics for each study participant based upon the age at which they entered the study. They used logistic regression to estimate the odds of being a “healthy ager” in the year 2000 by quintile of AHEI-2010 and A-MeDi scores.
Main results. Of the 10,670 participants, 1171 (11%) were labeled “healthy agers” and 9499 (89%) were labeled “usual agers.” Prevalence in each of the 4 health domains varied widely: 9599 (90%) of the 10670 participants had no cognitive impairment, 7234 (67.8%) had no chronic diseases, 4606 (43.2%) had no mental health limitations, and 2905 (27.2%) had no impairment of physical functioning.
Investigators presented data comparing healthy agers and usual agers at baseline without tests for significance. The mean age of healthy agers and usual agers was comparable (58.6 [SD = 2.5] vs. 59.1 [SD = 2.5]). Healthy agers had lower prevalence of obesity (3% vs. 13%), ever smoking (54% vs. 47%), higher mean physical activity (19.4 MET h/wk [SD = 21.7] vs. 14.1 MET h/wk [SD = 19.8]), lower energy intake (1692 kcal/d [SD = 472] vs. 1743 kcal/d [SD = 477]) and lower prevalence of hypertension (20% vs. 32%) and hypercholesterolemia (12% vs. 17%). Healthy agers also had higher baseline AHEI-2010 (53.2 [SD = 10.3] vs. 50.6 [SD = 10.1]) and A-MeDi scores in midlife (4.5 [SD = 1.6] vs. 4.3 [SD = 1.7]).
Greater scores on the AHEI-2010 and A-MeDi measures in midlife were associated with greater odds of healthy aging in multivariate analysis. After adjusting for all covariates, women in the highest quintile of AHEI-2010 scores at baseline had 34% greater odds (95% CI, 9% to 66%) of being healthy agers compared to women in the lowest quintile. Likewise, adjusted analyses reported women in the highest quintile of A-MeDi scores had 46% greater odds (95% CI, 17% to 83%) of being healthy agers.
Secondary analyses tested each component of healthy aging for associations with AHEI-2010 and A-MeDi scores in midlife. Associations were overall weaker, but no impairment of physical function and no limitation of mental health were both found to be significant after adjustment for covariates. Women in the highest quintile of AHEI-2010 scores at baseline had 23% (95% CI, 11% to 36%) and 13% (95% CI, 5% to 22%) greater odds, respectively, of not having any physical limitations or mental health impairments in late life compared to women in the lowest quintile. Likewise, women in the highest quintile of A-MeDi scores at baseline had 14% (95% CI, 3% to 26%) and 12% (95% CI, 4% to 20%) greater odds, respectively, of not having any physical limitations or mental health impairments in late life compared to women in the lowest quintile.
The authors also tested the effect of individual components of dietary patterns on healthy aging, comparing those in the highest quintile versus those in the lowest quintile for each measure. Persons with the greatest intake of fruits had 46% (95% CI, 15% to 85%) greater odds of being healthy agers compared to those with the lowest intake of fruits. Persons with the highest intake of alcohol had 28% greater odds (95% CI, 4% to 56%) of being healthy agers compared to those with the lowest intake of alcohol. Conversely, those with lower intake of sugar-sweetened beverages (OR, 1.28 [95% CI, 1.03 to 1.58]) and non-omega 3 polyunsaturated fatty acids (OR, 1.38 [CI, 1.10 to 1.73]) had better odds of being healthy agers compared to those with higher intakes.
Conclusion. Women with healthy dietary patterns at midlife had significantly greater odds of being healthy agers in later life after adjusting for potential con-founders. Results were consistent in direction and effect size when using either the AHEI-2010 score or the A-MeDi score. The effects of healthy diet at midlife seemed to have the strongest association with physical impairment scores and mental health scores. Higher intake of fruits and alcohol along with lower intake of sugar-sweetened beverages and polyunsaturated fatty acids seemed to have the most power for predicting healthy aging.
Commentary
These results are consistent with current knowledge, which indicates the health benefits of a balanced, healthy diet high in fruits, vegetables, whole grains, nuts, and legumes and low in red or other processed meats. There is high quality evidence linking each dietary measure to health outcomes. Adherence to the Alternative Healthy Eating Index has been related to lower mortality rates [3], decreased risk of cardiovascular disease [4], and decreased risk of type 2 diabetes and the metabolic syndrome [5]. Likewise, adherence to the Mediterranean diet is associated with reductions in overall mortality, cardiovascular incidence and mortality, cancer incidence and mortality, and neurodegenerative diseases [6]. Both diets endorse moderate alcohol intake, which was associated with lower rates of all-cause and cardiovascular mortality in a meta-analysis [7]. Alcohol is theorized to produce decreased platelet aggregation, increase HDL cholesterol, and increase endothelial vasorelaxation [8]. Polyphenols, most prominent in red wines, may have additional effects which include vaso-relaxation of aortic rings, reduced thrombosis and inflammation, and increased fibrinolysis [9]. Nevertheless, heavy alcohol use may increase cardiovascular mortality, hypertension, and hyperlipidemia [8]. This study concluded that higher alcohol intake was related to being a healthy ager; this may be because there are few heavy alcohol users in this cohort, though this hypothesis was not tested in the study.
Any observational study is subject to debate about the confounders chosen for analysis and potential biases. The authors report that the most powerful confounders in this analysis were BMI, physical activity, and smoking, all of which have been well established as predictors for health in later life [10]. Nonetheless, important potential confounders not used in analysis included the baseline prevalence of mental health problems, cognitive limitations, and physical limitations, all of which were not available.
The greatest concern about of this study is a potential lack of generalizability given the population surveyed. The Nurses’ Health Study consists of a cohort of female, married, predominantly white registered nurses [1]. For instance, African Americans have a greater burden of hypertension than non-Hispanic whites after accounting for dietary differences [11], a higher degree of late-life cognitive dysfunction [12], and greater risk of developing late-life physical disability [13]. Also, race and ethnicity may impact eating patterns, food preferences, and food availability in ways that are difficult to predict. In addition, nurses in the cohort were probably of similar socioeconomic status given their shared occupation, though the authors did not report the variation in median household incomes obtained from census tract analysis in this study [14]. Results might change if the sample was less homogeneous. Nonetheless, the results are consistent with current knowledge, biologically plausible, and clinically meaningful.
Applications for Clinical Practice
Integrating dietary changes in middle-aged women may be an important means of decreasing morbidity in older age and improving physical and mental health functioning later in life. Health care providers should discuss the future benefits of healthy eating on quality of life in order to encourage patients in midlife to alter their diet in meaningful ways. While it may be difficult to generalize these findings to patients of different genders, races, or ethnicities, the biological underpinnings of the data make it hard dispute the conclusions presented in the study.
—Hector Perez, MD, and Melanie Jay, MD, MS
1. Hemenway D, Colditz GA, Willett WC, et al. Fractures and lifestyle: effect of cigarette smoking, alcohol intake, and relative weight on the risk of hip and forearm fractures in middle-aged women. Am J Public Health 1988;78:1554–8.
2. Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–73.
3. Akbaraly TN, Ferrie JE, Berr C, et al. Alternative Healthy Eating Index and mortality over 18 y of follow-up: results from the Whitehall II cohort. Am J Clin Nutr 2011;94:247–53.
4. McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–71.
5. Akbaraly TN, Singh-Manoux A, Tabak AG, et al. Overall diet history and reversibility of the metabolic syndrome over 5 years: the Whitehall II prospective cohort study. Diabetes Care 2010;33:2339–41.
6. Sofi F, Abbate R, Gensini GF, et al. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 2010;92:1189–96.
7. Di Castelnuovo A, Costanzo S, Bagnardi V, et al. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 2006;166:2437–45.
8. Costanzo S, Di Castelnuovo A, Donati MB, et al. Cardiovascular and overall mortality risk in relation to alcohol consumption in patients with cardiovascular disease. Circulation 2010;121:1951–9.
9. Booyse FM, Pan W, Grenett HE, et al. Mechanism by which alcohol and wine polyphenols affect coronary heart disease risk. Ann Epidemiol 2007;17:S24–S31.
10. Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med 2012;55:163–70.
11. Diaz VA, Mainous AG, Koopman RJ, et al. Race and diet in the overweight: association with cardiovascular risk in a nationally representative sample. Nutrition 2005;21:718–25.
12. Sloan FA, Wang J. Disparities among older adults in measures of cognitive function by race or ethnicity. J Gerontol B Psychol Sci Soc Sci 2005;60:P242–50.
13. Dunlop DD, Song J, Manheim LM, et al. Racial/ethnic differences in the development of disability among older adults. Am J Public Health 2007;97:2209–15.
14. Puett RC, Schwartz J, Hart JE, et al. Chronic particulate exposure, mortality, and coronary heart disease in the nurses’ health study. Am J Epidemiol 2008;168:1161–8.
1. Hemenway D, Colditz GA, Willett WC, et al. Fractures and lifestyle: effect of cigarette smoking, alcohol intake, and relative weight on the risk of hip and forearm fractures in middle-aged women. Am J Public Health 1988;78:1554–8.
2. Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–73.
3. Akbaraly TN, Ferrie JE, Berr C, et al. Alternative Healthy Eating Index and mortality over 18 y of follow-up: results from the Whitehall II cohort. Am J Clin Nutr 2011;94:247–53.
4. McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–71.
5. Akbaraly TN, Singh-Manoux A, Tabak AG, et al. Overall diet history and reversibility of the metabolic syndrome over 5 years: the Whitehall II prospective cohort study. Diabetes Care 2010;33:2339–41.
6. Sofi F, Abbate R, Gensini GF, et al. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 2010;92:1189–96.
7. Di Castelnuovo A, Costanzo S, Bagnardi V, et al. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 2006;166:2437–45.
8. Costanzo S, Di Castelnuovo A, Donati MB, et al. Cardiovascular and overall mortality risk in relation to alcohol consumption in patients with cardiovascular disease. Circulation 2010;121:1951–9.
9. Booyse FM, Pan W, Grenett HE, et al. Mechanism by which alcohol and wine polyphenols affect coronary heart disease risk. Ann Epidemiol 2007;17:S24–S31.
10. Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med 2012;55:163–70.
11. Diaz VA, Mainous AG, Koopman RJ, et al. Race and diet in the overweight: association with cardiovascular risk in a nationally representative sample. Nutrition 2005;21:718–25.
12. Sloan FA, Wang J. Disparities among older adults in measures of cognitive function by race or ethnicity. J Gerontol B Psychol Sci Soc Sci 2005;60:P242–50.
13. Dunlop DD, Song J, Manheim LM, et al. Racial/ethnic differences in the development of disability among older adults. Am J Public Health 2007;97:2209–15.
14. Puett RC, Schwartz J, Hart JE, et al. Chronic particulate exposure, mortality, and coronary heart disease in the nurses’ health study. Am J Epidemiol 2008;168:1161–8.
Technology-Supported Apprenticeship in the Management of Hypertension: A Randomized Controlled Trial
From the Massachusetts Institute of Technology (Dr. Moore, Dr. Moss and Mr. Gilroy) and the Massachusetts General Hospital (Ms. Marshall, Dr. Judge, Dr. Crocker, and Dr. Zusman), Boston, MA.
Abstract
- Objective: To compare technology-supported appren-ticeship in hypertension management with a successful coaching model at Massachusetts General Hospital.
- Methods: A randomized controlled trial was conducted. Adult patients with uncontrolled essential hypertension (average blood pressure (BP) 148/87 mm Hg) were recruited in a staggered fashion for a 12-week study period. Intervention subjects received apprenticeship support from a nurse health coach through the CollaboRhythm application on a tablet computer. Patients self-tracked medication adherence and blood pressure (via wireless device) and the coach helped them to continuously progress through lifestyle change and medication adjustment using integrated messaging. Control subjects received support from the same coach but through traditional channels of office visits, phone calls, and e-mail.
- Results: 42 of 44 subjects completed the study. Intervention subjects achieved a greater decrease in systolic BP at 12 weeks than control subjects (26.3 mm Hg vs. 16.0 mm Hg, P = 0.009). A greater percentage of intervention subjects achieved goal BP ≤ 130/80 mm Hg (75.0% vs. 31.8%, P = 0.003) and 100% of them achieved goal BP ≤ 140/90 mmHg. They also rated the experience higher, although this finding was not statistically significant (8.9 vs. 7.6, P = 0.12). There was a trend toward increased cost for intervention subjects ($67.50 vs. $53.41, P = 0.15), but the projected cost is much less than standard care ($248/patient/year).
- Conclusion: This study provides encouraging evidence that technology-supported apprenticeship can improve the outcomes, cost, and experience of care in managing hypertension.
Hypertension affects approximately 33% of the U.S. adult population [1]. Antihypertensive treatment has been shown to be effective at preventing complications [2,3]. Unfortunately, estimates suggest that the majority of those diagnosed with hypertension do not have their blood pressure controlled [1]. This failure is due to both clinician and patient factors. Mean adherence of clinicians to guidelines is estimated at 53.5% [4]. An electronic monitoring study showed that half of patients who are prescribed medications stop taking them within 1 year [5]. Of those who take their medications, about 10% have adherence issues on any given day and about 50% have significant adherence issues in the course of their treatment [5]. Adherence to diet and exercise self-management is even more dismal, with rates below 20% [6]. Hypertension is an expensive problem with direct medical costs (treatment and complications) greater than $100 billion a year and equally high indirect costs (lost productivity) [7–9].
In the management of chronic diseases, there is a significant trend toward empowering patients with more control and toward providing more longitudinal coaching from clinicians and from peers [10–15]. Technology-supported apprenticeship is a model of chronic disease management that builds on the success of self-management and coaching, but it is more ambitious in that its goal is for patients to lead their care with the support of health coaches and supervising clinicians. It is informed by the field of learning science, particularly in how technology can be used effectively to support learning [16–20]. Apprenticeship refers to the tutelage of a community of novices in a skill or trade by one or more masters through situated learning. Situated learning refers to the process in which novices learn through participation in legitimate tasks in the same physical and social context where they will need to perform them once independent [21,22]. It is opposed to learning through contrived exercises in an artificial environment like a classroom.
In technology-supported apprenticeship, patients are the novice apprentices of master coaches. Technology provides scaffolding and communication tools to allow coaches to support the patient in the management of disease within the context of daily life rather than in the clinician’s office. Patients are supported in gradually developing self-efficacy and independence until they are skilled enough to lead their care and even to help coach others to success. The hypothesis is that embracing the contribution of the patient to this extent will reap unparalleled rewards in the experience, outcomes, and cost of care.
We conducted a randomized controlled trial to assess whether a technology-supported apprenticeship in hyper-tension management would improve blood pressure control compared with a successful coaching model.
Methods
Setting
The health coaching model at the Ambulatory Practice of the Future (APF) at the Massachusetts General Hospital is designed to impact the whole health ofpatients. In the scope of hypertension management, a nurse health coach, under the supervision of a physician, is responsible for improving outcomes through longitudinal support via office visits, phone calls, and e-mails. Diet, exercise, stress management, and medication therapy are all emphasized through health coaching techniques, such as motivational interviewing and appreciative inquiry [23,24]. Patients are referred to dieticians, exercise coaches, mind-body specialists, and others as desired. Prior to the start of this study, the APF was outperforming the national average with ~70% of hypertensive patients in the practice below clinically recommended goal blood pressures.
Recruitment
The study was advertised to all clinical staff of the APF through word of mouth. Since the practice was relatively young, it also had a registry of patients with elevated blood pressure that had not been addressed. Adult patients (> 18 years old) from the registry or from routine visits with essential hypertension (average blood pressure ≥ 140/90 and ≤ 180/120) who were taking 0 or 1 medications and had internet connectivity were eligible for inclusion in the study. Patients with a history of hypotension, syncope, hypertensive urgency, hypertensive emergency, labile hypertension, and proven coronary artery disease were excluded, as were patients with significant visual, auditory, or cognitive impairment and patients who were not proficient in English. Clinical staff notified the nurse health coach for the study on identification of any eligible subjects. The same nurse health coach recruited and cared for all intervention and control subjects.
The nurse health coach contacted each eligible patient to assess interest. Interested patients were scheduled for an appointment to commence the study. A process of written informed consent was carried out with each study subject. Each subject was assigned the next sequential study number, which was pre-randomized to either the intervention or the control group.
The study period for each subject was 12 weeks +/– 2 weeks with staggered recruitment. Twelve weeks represents the minimal time required to progress through the standardized Medication Adjustment Plan that was used, assuming a minimum time of 2 weeks on each medication before a change is considered. A recruitment window from 1 March 2013 until 31 May 2013 was used. The goal was to recruit between 40 and 60 subjects, since statistical analysis revealed that a sample size of 36 was sufficient to detect a 5 mm Hg difference in the decrease in systolic blood pressure between groups given a 4 mm Hg standard deviation.
Intervention
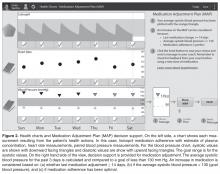
CollaboRhythm is a software platform that was developed at the MIT Media Lab and designed to support the principles of technology-supported apprenticeship. Patient tracking tools document progress, visualizations highlight associations between actions and outcomes, and personalized decision support encourages self-efficacy. Powerful virtual visits and instant messaging allow master clinicians to provide adaptive coaching within the context of daily life rather than in the artificial environment of the office. Applications can be deployed cross-platform to cell phones, tablets, computers, etc.
A simplified system was created for hypertension management. It is optimized for tablet deployment and focuses on instant messaging for communications. The patient uses a wireless blood pressure monitor (D40b model by ForaCare®) to take blood pressure measurements. The device is paired with a tablet application to automatically report measurements. The patient self-reports medication adherence using the tablet application. Patient data is visualized in a daily clock and weekly charts to promote proactive behavior and self-reflection. Decision support for medication adjustment is paired with the charts. The patient’s data are synchronized with the coach’s tablet application via a collaborative health record server implemented using the Indivo X personally controlled health record (Children’s Hospital Boston, indivohealth.org) code base. Instant messaging between the patient and coach is implemented using a real-time messaging server. The applications for the patient and the coach are identical, but the coach’s application is configured to allow switching between patient records in order to manage a number of patients.
The user experience was designed based on our years of exploration at the MIT Media Lab of the psychology of illness and the impact that technology could have on patient empowerment and patient-clinician collaboration. It is important to note that the CollaboRhythm application is different from typical electronic reminder applications. In fact, the application never alarms at patients, even if they forget to perform their health actions. Instead, the application focuses on providing awareness for proactive decision-making, self-reflection, and support from the coach.
Protocol Common to Control and Intervention Subjects
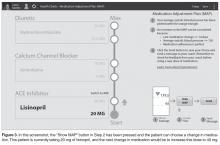
Following informed consent, a baseline blood pressure and heart rate were measured for each subject using the same automated sphygmomanometer. Weight was also measured and a hypertension knowledge assessment (a simple survey about normal blood pressures and the effects of diet, exercise, and medication that was created for the purpose of this study) was completed. The initial medication load was determined using the Medication Adjustment Plan as a reference and each dose step as a unit of 1. For example, if a subject was already on lisinopril 40 mg at the start of the study, the initial medication load was 2.
The nurse health coach conducted an introductory visit with each subject including motivational interviewing to assess their health values and to discuss
hypertension management goals. The subject and coach made shared decisions about diet, exercise, stress management, and medication. The same medication titration algorithm was used for both control and interventional
subjects.
At the conclusion of the study period, subjects in both groups returned for an exit visit. Blood pressure and heart rate were measured using the same automated sphygmomanometer that was used at the initial visit. Weight was measured using the same scale as the initial visit. A hypertension knowledge assessment was completed and an exit interview was conducted. The change in medication load was determined as well. For example, if a subject started the study on lisinopril 20 mg and ended the study on lisinopril 40 mg, the increase in medication load was 1.
Protocol Specifics for Intervention Subjects
The apprenticeship model of chronic disease management was discussed with the nurse health coach and with APF team prior to the study. It was also implicit in the design of the CollaboRhythm tablet application.
During the introduction visit, subjects in the inter-vention group received instructions from the nurse health coach on how to use the CollaboRhythm tablet application to self-track their medication adherence, to review progress using the charts, to propose or agree with changes in hypertension medications, and to communicate with messages.
Subjects in the intervention group received their hyper-tension care through the CollaboRhythm tablet application. All instant messages between the intervention subjects and the nurse health coach were automatically documented electronically. Other forms of communication were documented in a spreadsheet. During the exit visit, the tablet computer and the wireless blood pressure meter were returned.
Protocol Specifics for Control Subjects
Between the introduction and exit visits, subjects in the control group received standard hypertension care from the nurse health coach at the APF. The coach instructed subjects to check their blood pressures 3 to 5 times a week. They had the option of purchasing a cuff, using a monitor at work, or using a monitor at a facility such as a pharmacy or grocery store. Communication took place through
office visits, phone calls, and e-mails. All communications with control subjects were documented for the purposes of this study by the nurse health coach in a spread-
sheet.
Outcomes of Interest
The primary outcomes of interest were the absolute decrease in systolic and diastolic blood pressure and the number of subjects who reached the blood pressure goal of less than or equal to 130/80 mm Hg. This more aggressive blood pressure goal was chosen for a number of reasons: (1) it is not uncommon to set a lower blood pressure goal for home measurements to avoid patients hovering just at the 140/90 goal and not getting the true benefit of reduced blood pressure; and (2) subjects were supported in improving diet, exercise, and stress management in addition to pharmacologic therapy. The 140/90 cut-off (now 150/90 in patients over 60) is focused on pharmacologic therapy [2,3].
The secondary outcomes included the number of subjects who reached the blood pressure goal of less than or equal to 140/90 mm Hg (for comparison to other published literature), the number of subjects who achieved greater than a 10 mm Hg decrease in systolic and greater than a 5 mm Hg decrease in diastolic blood pressure, the change in medication load, the absolute decrease in weight, the number of subjects who lost at least 5 lb, a hypertension knowledge score assessed by a pre- and post-study test, a rating of satisfaction in care, and the amount of clinician time required in the care.
Statistical Methods
The study results were analyzed by the intention-to-treat approach. All subjects who returned for the final study visit were included in the analysis regardless of their adherence to the protocol. Subjects who did not return for the study exit visit were not included in the analysis because it would be impossible to determine the change in blood pressure without a final measurement. A 2-tailed Student’s t test for independent samples was used for all comparisons of the mean of continuous variables between the control and intervention groups. A 2-tailed Student’s t test for dependent samples was used for all comparisons between pre- and post-study variables within the control and the intervention group. A chi-square test was used for all comparisons of categorical variables between the groups, except when the expected values in the 2x2 table were below 5. Fisher’s exact test was used in this scenario, which applied to the secondary outcome of the number of subjects who reached the blood pressure goal of less than or equal to 140/90 mm Hg.
Oversight
This study was approved by the institutional review boards at the Massachusetts Institute of Technology and the Massachusetts General Hospital.
Results
There were a total of 44 subjects recruited for the study. One intervention subject and one control subject were lost to follow-up, leaving 42 subjects who completed the study exit visit.
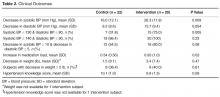
Baseline Statistics
Clinical Outcomes
Cost
 Table 3 presents a summary of nurse coach communications with subjects and the corresponding cost. The coach communicated with intervention subjects primarily through instant messages using the CollaboRhythm tablet application. There were trends toward more e-mails, phone calls, and office visits in control subjects that were not statistically significant. The total nurse coach time spent per patient was calculated using the following assumptions: instant message = 5 minutes, e-mail = 10 minutes, phone call = 15 minutes, office visit = 30 minutes. It is of note that, although virtual visits (video conferences with the ability to co-navigate patient data) were available as a feature, none were conducted during the course of the study. There was a trend toward intervention subjects receiving more support from the nurse coach that was not statistically significant. The cost associated with nurse coach time was calculated for both control and intervention subjects using the assumption of $50/hour (based on $100,000/year average salary plus benefits for nurse with health coaching certification). On average, intervention subjects received 0.28 hours or 16.8 minutes more time at an additional cost of $14.09.
Table 3 presents a summary of nurse coach communications with subjects and the corresponding cost. The coach communicated with intervention subjects primarily through instant messages using the CollaboRhythm tablet application. There were trends toward more e-mails, phone calls, and office visits in control subjects that were not statistically significant. The total nurse coach time spent per patient was calculated using the following assumptions: instant message = 5 minutes, e-mail = 10 minutes, phone call = 15 minutes, office visit = 30 minutes. It is of note that, although virtual visits (video conferences with the ability to co-navigate patient data) were available as a feature, none were conducted during the course of the study. There was a trend toward intervention subjects receiving more support from the nurse coach that was not statistically significant. The cost associated with nurse coach time was calculated for both control and intervention subjects using the assumption of $50/hour (based on $100,000/year average salary plus benefits for nurse with health coaching certification). On average, intervention subjects received 0.28 hours or 16.8 minutes more time at an additional cost of $14.09.
Experience
Thirteen control subjects and 16 intervention subjects provided an experience rating during their exit interview from the study. There was a trend toward greater satisfaction by intervention subjects that was not statistically significant (8.9 vs. 7.6, P = 0.12). The majority of feedback from intervention subjects was obtained through the exit interview. The feedback was overwhelmingly positive. All of the subjects wanted to continue using the CollaboRhythm application after the end of the study. Some who had reached goal only wanted to use it sporadically as a “check-in,” but the majority wanted to use it daily as they had for the study for an indefinite period of time. They felt that the burden of reporting was easily balanced by the value of being able to track progress and get the efficient support of a health coach. They related that awareness of the associations between actions (diet, exercise, stress management, medication adherence) and blood pressure outcomes was integral to their success and their positive experience with the application. Subjects responded very favorably to the concept of leading their care. Some comments from patients include: “It felt good to take responsibility,” “No one has ever asked me to take responsibility for my health,” and “I developed confidence that I never would have had.” The nurse coach commented multiple times how delighted she was with the level of patient engagement and excitement. She also did not want to stop using the application at the end of the study.
The main complaints about the program from both subjects and the health coach were on performance. They wanted the application to load faster and to save data faster.
Discussion
This study provides encouraging evidence that technology-supported apprenticeship can dramatically improve the outcomes, cost, and experience of care in the management of hypertension.
A 26.3 mm Hg average decrease in systolic blood pressure for intervention subjects in 3 months is significantly better than the standard of care and the best of the best in published interventions. [1,11–14]. The same is true for a rate of 100% of patients achieving goal blood pressure < 140/90 mm Hg.
We believe several factors contribute to the success of our program: (1) supporting subjects in the mission of leading their care, (2) rich real-time feedback for self-reflection, (3) emphasis on short-term goals, (4) promise of medication reduction with goal achievement, and (5) social support and accountability that come with having a continuously available coach who had immediate data access.
Other studies have had web communication components, self-titration components, and coaching components. In a progressive study by McManus [11], subjects in the intervention group self-tracked their blood pressure and self-titrated their hypertension medications while control subjects received routine care. Self-managing patients achieved a decrease in systolic blood pressure of 17.6 mm Hg over 12 months while controls only achieved a decrease of 12.2 mm Hg. Self-managing patients were more aggressive in adding new medications and did not have increased incidence of side effects. A study by Green [12] showed that subjects with home blood pressure monitoring, a web training course, and web-based pharmacist coaching achieved a 14.2 mm Hg decrease in systolic blood pressure over 12 months and that 56% reached goal blood pressure (< 140/90 mm Hg). Subjects who received routine care only achieved a 5.3 mm Hg decrease in systolic blood pressure and only 31% reached goal blood pressure. Margolis [13] and Magid [14] conducted studies similar to Green's with pharmacist-led care. In Margolis’ study, 71.8% of inter-vention subjects study achieved goal at 6 months with a mean decrease in systolic blood pressure of 21.5 mm Hg. These results were impressively stable at 18 months [13]. In Magid’s study, 54.1% of intervention subjects achieved goal at 6 months with a mean decrease in systolic blood pressure of 20.7 mm Hg [14].
The technology-supported apprenticeship model was inspired by the exceptional results of these studies, but we believe that it represents a significant step forward in patient engagement. In these studies, the tools were typically more burdensome than empowering, the titration more prescriptive than motivational, and the coaching more timed and paternalistic than continuous and nurturing. Technology-supported apprenticeship relies on more powerful tools for collaboration and aspires for the patient to lead the care rather than the clinician. It has the potential to produce greater self-efficacy, which results in better outcomes at lower cost.
Cost Implications
The 100% rate of attainment of systolic blood pressure < 140 mm Hg in our intervention subjects has the poten-tial to contribute to substantial cost savings through reducing complications. The cost of complications per patient per year is approximately $1275, and attainment of goal blood pressure on average results in approximately 35% decrease in complications [2,7–9] Therefore, it is reasonable to expect at least $446 savings in downstream costs per patient per year with this intervention. This has notable public health implications given that there are approximately 32 million patients in the United States with diagnosed hypertension that is uncontrolled [1]. Cost saving at scale could be more than $14 billion.
Typically, interventions that produce downstream savings in health care require some upstream investment. An exciting aspect of this intervention is that it was significantly less costly, at $67.50 per patient, as compared with the standard of care, at $248 per patient per year [7–9]. Most of the responsibility was embraced by the patients, and the nurse coach needed only to provide occasional social and clinical support. Return office visits were almost completely eliminated and goals were achieved in a fraction of the time. The cost of the technology is effectively negligible given that patients will be able to use their own cell phones and tablets and their own blood pressure cuffs. Based on the prevalence of hypertension, a primary care practice with 2000 patients would be expected to have 600 with hypertension. At $180.50 in savings per patient per year, a profit of $108,300 could be retained if the same reimbursement was provided for this more effective care. At the same time, this more efficient and scalable model of care could allow a practice to care for a larger number of patients without compromising the experience for patients or clinicians.
Limitations and Future Directions
An important limitation of this study is its small sample size. The efficiency and cost analysis suggests that the intervention is highly suitable for scaling but, until this is studied, there is the risk that the small sample size may misrepresent the average effect on a larger population and overestimate the size of a population that a coach can manage. Future work will need to include much larger populations.
Another limitation was the short follow-up period. A key benefit of apprenticeship is that it theoretically results in greater and more sustainable self-efficacy than other models of patient engagement that are not as grounded in learning science and that do not engage the patient as deeply. Follow-up visits for both control and intervention subjects will be conducted at 1 year to assess the sustainability of the blood pressure improvements.
The fact that the population was from a single practice also presents the possibility that the subjects being studied were more interested and/or capable than a larger, more general population. This is likely to be true, since many of the patients at the APF sought it out in the hope of receiving a new health care experience. Future work will need to address patient populations from diverse socioeconomic situations and diverse cultures. Based on pilot studies that we have done with diverse populations with HIV, hypertension, and diabetes, we believe that patients who are more disempowered and disenfranchised at baseline attain greater benefit from becoming apprentices in their care.
Another potential limitation is that hypertension was treated in isolation in this study. In fact, many of the subjects had comorbid conditions and were being treated for them simultaneously at the APF. Future work will need to address how the apprenticeship approach and accompanying technology can support the management of multiple comorbid conditions. At the same time, we believe that setting aggressive goals for one condition at a time and achieving these goals over a short period, such as 3 months, will be an important strategy in effectively engaging patients as active participants in their care and helping them to develop self-efficacy. As they develop competency for one condition, they become more capable of managing the others. If they were to attempt to tackle all of the problems simultaneously, it could be overwhelming and ineffective.
Conclusions
This study provides encouraging evidence that, in the management of hypertension, technology-supported apprenticeship can improve the outcomes, cost, and experience of care. By embracing the potential of patients while at the same time optimizing support from clinicians and leveraging information technology for cost-effective scaling, it has the potential to have a tremendous impact across the spectrum of chronic disease.
Three core tenants of technology-supported apprenticeship have surfaced as the product of this work, and they may serve as a useful guide to other efforts improving care delivery: First, patients are the most underutilized resource in health care. Technology-supported apprenticeship harnesses the contribution of the patient and values the development of self-efficacy to the extent that apprentices can become masters. It supports patients in achieving a pinnacle of patient empowerment.
Second, health takes place in the everyday lives of patients, not in a doctor’s office or hospital. Technology-supported apprenticeship appreciates that situated learning is the key to mastery of chronic disease management and strives to support patients continuously in the context of their lives.
Third, collaboration and information transparency, not just information access, are critical. Technology-supported apprenticeship embraces information technology to maximize collaboration among novices and masters and to provide common ground through complete transparency.
Acknowledgments: This study was completed as part of John O. Moore’s PhD thesis at the MIT Media Lab. Mitchel Resnick, PhD, and Pattie Maes, PhD, were members of the thesis committee along with authors Franklin H. Moss, PhD, and David C. Judge, MD, and their advice was critical in the development of the technology-supported apprenticeship approach.
Corresponding author: John O. Moore, MD, PhD, [email protected]
Funding/support: This work was supported, in part, by the CIMIT Prize for Primary Healthcare from the Center for Integration of Medicine & Innovative Technology, Boston, MA.
Financial disclosures: John O. Moore, Frank Moss, and Scott Gilroy have a health information technology company focused on building software solutions for improving chronic disease management. This company does not yet have a product for sale.
1. Recent trends in the prevalence of high blood pressure and its treatment and control. National Center for Health Statistics. Hyattsville, MD; 2010.
2. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72. Erratum in: JAMA 2003;290:197.
3. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20.
4. Ardery G, Carter BL, Milchak JL, et al. Explicit and implicit evaluation of physician adherence to hypertension guidelines. J Clin Hypertens (Greenwich) 2007;9:113–9.
5. Vrijens B, Vincze G, Kristanto P, et al. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 2008;336:1114–7.
6. Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med 2008;168:308–14.
7. Davis K. Expenditures for hypertension among adults age 18 and older, 2009: estimates for the U.S. civilian noninstitutionalized population. Rockville, MD: Agency for Healthcare Research and Quality; June 2012. Available at http://meps.ahrq.gov/mepsweb/data_files/publications/st371/stat371.shtml.
8. Hodgson TA, Liming C. Medical care expenditures for hypertension, its complications, and its comorbidities. Med Care 2001;39:599–615.
9. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011;123:933–44.
10. Wagner EH, Austin BT, Davis C, et al. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20:64–78.
11. McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomized controlled trial. Lancet 2010;376:163–72.
12. Green BB, Cook AJ, Ralston JD, et al. Effectiveness of home blood pressure monitoring, web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA 2008;299:2857–67.
13. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA 2013;310:46–56.
14. Magid DJ, Olson KL, Billups SJ, et al. A pharmacist-led, American Heart Association Heart360 Web-enabled home blood pressure monitoring program. Circ Cardiovasc Qual Outcomes 2013;6:157–63.
15. Lorig K, Ritter PL, Villa FJ, Armas J. Community-based peer-led diabetes self-management: a randomized trial. Diabetes Educ 2009;35:641–51.
16. Collins A, Brown J, Newman SE. Cognitive apprenticeship: Teaching the crafts of reading, writing, and mathematics. In: Knowing, learning, and instruction: Essays in honor of Robert Glaser. Lawrence Erlbaum Associates; 1989:453–94.
17. Resnick M. Rethinking learning in the digital age. In: Kirkman G, editor. The global information technology report: readiness for a networked world. Oxford University Press; 2002.
18. Koschmann T. Paradigm shifts and instructional technology. In: Koschmann T, editor. CSCL: Theory and practice of an emerging paradigm. Lawrence Erlbaum Associates; 1996:83–124.
19. Brown JS, Adler RP. Minds on fire: open education, the long tail, and learning 2.0. Educause Rev 2008;43:16–32.
20. Collins A, Halverson R. The second educational revolution: rethinking education in the age of technology. J Comp Assist Learn 2010;26:18–27.
21. Dewey J. Experience and education. New York: Collier Books; 1938.
22. Lave J. Situated learning: legitimate peripheral participation (Learning in Doing: Social, Cognitive, and Computational Perspectives). Cambridge University Press; 1991.
23. Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol 2009;64:527–37.
24. Moore SM, Charvat J. Promoting health behavior change using appreciative inquiry: moving from deficit models to affirmation models of care. Fam Community Health 2007;30 Supp 1:S64–74.
From the Massachusetts Institute of Technology (Dr. Moore, Dr. Moss and Mr. Gilroy) and the Massachusetts General Hospital (Ms. Marshall, Dr. Judge, Dr. Crocker, and Dr. Zusman), Boston, MA.
Abstract
- Objective: To compare technology-supported appren-ticeship in hypertension management with a successful coaching model at Massachusetts General Hospital.
- Methods: A randomized controlled trial was conducted. Adult patients with uncontrolled essential hypertension (average blood pressure (BP) 148/87 mm Hg) were recruited in a staggered fashion for a 12-week study period. Intervention subjects received apprenticeship support from a nurse health coach through the CollaboRhythm application on a tablet computer. Patients self-tracked medication adherence and blood pressure (via wireless device) and the coach helped them to continuously progress through lifestyle change and medication adjustment using integrated messaging. Control subjects received support from the same coach but through traditional channels of office visits, phone calls, and e-mail.
- Results: 42 of 44 subjects completed the study. Intervention subjects achieved a greater decrease in systolic BP at 12 weeks than control subjects (26.3 mm Hg vs. 16.0 mm Hg, P = 0.009). A greater percentage of intervention subjects achieved goal BP ≤ 130/80 mm Hg (75.0% vs. 31.8%, P = 0.003) and 100% of them achieved goal BP ≤ 140/90 mmHg. They also rated the experience higher, although this finding was not statistically significant (8.9 vs. 7.6, P = 0.12). There was a trend toward increased cost for intervention subjects ($67.50 vs. $53.41, P = 0.15), but the projected cost is much less than standard care ($248/patient/year).
- Conclusion: This study provides encouraging evidence that technology-supported apprenticeship can improve the outcomes, cost, and experience of care in managing hypertension.
Hypertension affects approximately 33% of the U.S. adult population [1]. Antihypertensive treatment has been shown to be effective at preventing complications [2,3]. Unfortunately, estimates suggest that the majority of those diagnosed with hypertension do not have their blood pressure controlled [1]. This failure is due to both clinician and patient factors. Mean adherence of clinicians to guidelines is estimated at 53.5% [4]. An electronic monitoring study showed that half of patients who are prescribed medications stop taking them within 1 year [5]. Of those who take their medications, about 10% have adherence issues on any given day and about 50% have significant adherence issues in the course of their treatment [5]. Adherence to diet and exercise self-management is even more dismal, with rates below 20% [6]. Hypertension is an expensive problem with direct medical costs (treatment and complications) greater than $100 billion a year and equally high indirect costs (lost productivity) [7–9].
In the management of chronic diseases, there is a significant trend toward empowering patients with more control and toward providing more longitudinal coaching from clinicians and from peers [10–15]. Technology-supported apprenticeship is a model of chronic disease management that builds on the success of self-management and coaching, but it is more ambitious in that its goal is for patients to lead their care with the support of health coaches and supervising clinicians. It is informed by the field of learning science, particularly in how technology can be used effectively to support learning [16–20]. Apprenticeship refers to the tutelage of a community of novices in a skill or trade by one or more masters through situated learning. Situated learning refers to the process in which novices learn through participation in legitimate tasks in the same physical and social context where they will need to perform them once independent [21,22]. It is opposed to learning through contrived exercises in an artificial environment like a classroom.
In technology-supported apprenticeship, patients are the novice apprentices of master coaches. Technology provides scaffolding and communication tools to allow coaches to support the patient in the management of disease within the context of daily life rather than in the clinician’s office. Patients are supported in gradually developing self-efficacy and independence until they are skilled enough to lead their care and even to help coach others to success. The hypothesis is that embracing the contribution of the patient to this extent will reap unparalleled rewards in the experience, outcomes, and cost of care.
We conducted a randomized controlled trial to assess whether a technology-supported apprenticeship in hyper-tension management would improve blood pressure control compared with a successful coaching model.
Methods
Setting
The health coaching model at the Ambulatory Practice of the Future (APF) at the Massachusetts General Hospital is designed to impact the whole health ofpatients. In the scope of hypertension management, a nurse health coach, under the supervision of a physician, is responsible for improving outcomes through longitudinal support via office visits, phone calls, and e-mails. Diet, exercise, stress management, and medication therapy are all emphasized through health coaching techniques, such as motivational interviewing and appreciative inquiry [23,24]. Patients are referred to dieticians, exercise coaches, mind-body specialists, and others as desired. Prior to the start of this study, the APF was outperforming the national average with ~70% of hypertensive patients in the practice below clinically recommended goal blood pressures.
Recruitment
The study was advertised to all clinical staff of the APF through word of mouth. Since the practice was relatively young, it also had a registry of patients with elevated blood pressure that had not been addressed. Adult patients (> 18 years old) from the registry or from routine visits with essential hypertension (average blood pressure ≥ 140/90 and ≤ 180/120) who were taking 0 or 1 medications and had internet connectivity were eligible for inclusion in the study. Patients with a history of hypotension, syncope, hypertensive urgency, hypertensive emergency, labile hypertension, and proven coronary artery disease were excluded, as were patients with significant visual, auditory, or cognitive impairment and patients who were not proficient in English. Clinical staff notified the nurse health coach for the study on identification of any eligible subjects. The same nurse health coach recruited and cared for all intervention and control subjects.
The nurse health coach contacted each eligible patient to assess interest. Interested patients were scheduled for an appointment to commence the study. A process of written informed consent was carried out with each study subject. Each subject was assigned the next sequential study number, which was pre-randomized to either the intervention or the control group.
The study period for each subject was 12 weeks +/– 2 weeks with staggered recruitment. Twelve weeks represents the minimal time required to progress through the standardized Medication Adjustment Plan that was used, assuming a minimum time of 2 weeks on each medication before a change is considered. A recruitment window from 1 March 2013 until 31 May 2013 was used. The goal was to recruit between 40 and 60 subjects, since statistical analysis revealed that a sample size of 36 was sufficient to detect a 5 mm Hg difference in the decrease in systolic blood pressure between groups given a 4 mm Hg standard deviation.
Intervention
CollaboRhythm is a software platform that was developed at the MIT Media Lab and designed to support the principles of technology-supported apprenticeship. Patient tracking tools document progress, visualizations highlight associations between actions and outcomes, and personalized decision support encourages self-efficacy. Powerful virtual visits and instant messaging allow master clinicians to provide adaptive coaching within the context of daily life rather than in the artificial environment of the office. Applications can be deployed cross-platform to cell phones, tablets, computers, etc.
A simplified system was created for hypertension management. It is optimized for tablet deployment and focuses on instant messaging for communications. The patient uses a wireless blood pressure monitor (D40b model by ForaCare®) to take blood pressure measurements. The device is paired with a tablet application to automatically report measurements. The patient self-reports medication adherence using the tablet application. Patient data is visualized in a daily clock and weekly charts to promote proactive behavior and self-reflection. Decision support for medication adjustment is paired with the charts. The patient’s data are synchronized with the coach’s tablet application via a collaborative health record server implemented using the Indivo X personally controlled health record (Children’s Hospital Boston, indivohealth.org) code base. Instant messaging between the patient and coach is implemented using a real-time messaging server. The applications for the patient and the coach are identical, but the coach’s application is configured to allow switching between patient records in order to manage a number of patients.
The user experience was designed based on our years of exploration at the MIT Media Lab of the psychology of illness and the impact that technology could have on patient empowerment and patient-clinician collaboration. It is important to note that the CollaboRhythm application is different from typical electronic reminder applications. In fact, the application never alarms at patients, even if they forget to perform their health actions. Instead, the application focuses on providing awareness for proactive decision-making, self-reflection, and support from the coach.
Protocol Common to Control and Intervention Subjects
Following informed consent, a baseline blood pressure and heart rate were measured for each subject using the same automated sphygmomanometer. Weight was also measured and a hypertension knowledge assessment (a simple survey about normal blood pressures and the effects of diet, exercise, and medication that was created for the purpose of this study) was completed. The initial medication load was determined using the Medication Adjustment Plan as a reference and each dose step as a unit of 1. For example, if a subject was already on lisinopril 40 mg at the start of the study, the initial medication load was 2.
The nurse health coach conducted an introductory visit with each subject including motivational interviewing to assess their health values and to discuss
hypertension management goals. The subject and coach made shared decisions about diet, exercise, stress management, and medication. The same medication titration algorithm was used for both control and interventional
subjects.
At the conclusion of the study period, subjects in both groups returned for an exit visit. Blood pressure and heart rate were measured using the same automated sphygmomanometer that was used at the initial visit. Weight was measured using the same scale as the initial visit. A hypertension knowledge assessment was completed and an exit interview was conducted. The change in medication load was determined as well. For example, if a subject started the study on lisinopril 20 mg and ended the study on lisinopril 40 mg, the increase in medication load was 1.
Protocol Specifics for Intervention Subjects
The apprenticeship model of chronic disease management was discussed with the nurse health coach and with APF team prior to the study. It was also implicit in the design of the CollaboRhythm tablet application.
During the introduction visit, subjects in the inter-vention group received instructions from the nurse health coach on how to use the CollaboRhythm tablet application to self-track their medication adherence, to review progress using the charts, to propose or agree with changes in hypertension medications, and to communicate with messages.
Subjects in the intervention group received their hyper-tension care through the CollaboRhythm tablet application. All instant messages between the intervention subjects and the nurse health coach were automatically documented electronically. Other forms of communication were documented in a spreadsheet. During the exit visit, the tablet computer and the wireless blood pressure meter were returned.
Protocol Specifics for Control Subjects
Between the introduction and exit visits, subjects in the control group received standard hypertension care from the nurse health coach at the APF. The coach instructed subjects to check their blood pressures 3 to 5 times a week. They had the option of purchasing a cuff, using a monitor at work, or using a monitor at a facility such as a pharmacy or grocery store. Communication took place through
office visits, phone calls, and e-mails. All communications with control subjects were documented for the purposes of this study by the nurse health coach in a spread-
sheet.
Outcomes of Interest
The primary outcomes of interest were the absolute decrease in systolic and diastolic blood pressure and the number of subjects who reached the blood pressure goal of less than or equal to 130/80 mm Hg. This more aggressive blood pressure goal was chosen for a number of reasons: (1) it is not uncommon to set a lower blood pressure goal for home measurements to avoid patients hovering just at the 140/90 goal and not getting the true benefit of reduced blood pressure; and (2) subjects were supported in improving diet, exercise, and stress management in addition to pharmacologic therapy. The 140/90 cut-off (now 150/90 in patients over 60) is focused on pharmacologic therapy [2,3].
The secondary outcomes included the number of subjects who reached the blood pressure goal of less than or equal to 140/90 mm Hg (for comparison to other published literature), the number of subjects who achieved greater than a 10 mm Hg decrease in systolic and greater than a 5 mm Hg decrease in diastolic blood pressure, the change in medication load, the absolute decrease in weight, the number of subjects who lost at least 5 lb, a hypertension knowledge score assessed by a pre- and post-study test, a rating of satisfaction in care, and the amount of clinician time required in the care.
Statistical Methods
The study results were analyzed by the intention-to-treat approach. All subjects who returned for the final study visit were included in the analysis regardless of their adherence to the protocol. Subjects who did not return for the study exit visit were not included in the analysis because it would be impossible to determine the change in blood pressure without a final measurement. A 2-tailed Student’s t test for independent samples was used for all comparisons of the mean of continuous variables between the control and intervention groups. A 2-tailed Student’s t test for dependent samples was used for all comparisons between pre- and post-study variables within the control and the intervention group. A chi-square test was used for all comparisons of categorical variables between the groups, except when the expected values in the 2x2 table were below 5. Fisher’s exact test was used in this scenario, which applied to the secondary outcome of the number of subjects who reached the blood pressure goal of less than or equal to 140/90 mm Hg.
Oversight
This study was approved by the institutional review boards at the Massachusetts Institute of Technology and the Massachusetts General Hospital.
Results
There were a total of 44 subjects recruited for the study. One intervention subject and one control subject were lost to follow-up, leaving 42 subjects who completed the study exit visit.
Baseline Statistics
Clinical Outcomes
Cost
 Table 3 presents a summary of nurse coach communications with subjects and the corresponding cost. The coach communicated with intervention subjects primarily through instant messages using the CollaboRhythm tablet application. There were trends toward more e-mails, phone calls, and office visits in control subjects that were not statistically significant. The total nurse coach time spent per patient was calculated using the following assumptions: instant message = 5 minutes, e-mail = 10 minutes, phone call = 15 minutes, office visit = 30 minutes. It is of note that, although virtual visits (video conferences with the ability to co-navigate patient data) were available as a feature, none were conducted during the course of the study. There was a trend toward intervention subjects receiving more support from the nurse coach that was not statistically significant. The cost associated with nurse coach time was calculated for both control and intervention subjects using the assumption of $50/hour (based on $100,000/year average salary plus benefits for nurse with health coaching certification). On average, intervention subjects received 0.28 hours or 16.8 minutes more time at an additional cost of $14.09.
Table 3 presents a summary of nurse coach communications with subjects and the corresponding cost. The coach communicated with intervention subjects primarily through instant messages using the CollaboRhythm tablet application. There were trends toward more e-mails, phone calls, and office visits in control subjects that were not statistically significant. The total nurse coach time spent per patient was calculated using the following assumptions: instant message = 5 minutes, e-mail = 10 minutes, phone call = 15 minutes, office visit = 30 minutes. It is of note that, although virtual visits (video conferences with the ability to co-navigate patient data) were available as a feature, none were conducted during the course of the study. There was a trend toward intervention subjects receiving more support from the nurse coach that was not statistically significant. The cost associated with nurse coach time was calculated for both control and intervention subjects using the assumption of $50/hour (based on $100,000/year average salary plus benefits for nurse with health coaching certification). On average, intervention subjects received 0.28 hours or 16.8 minutes more time at an additional cost of $14.09.
Experience
Thirteen control subjects and 16 intervention subjects provided an experience rating during their exit interview from the study. There was a trend toward greater satisfaction by intervention subjects that was not statistically significant (8.9 vs. 7.6, P = 0.12). The majority of feedback from intervention subjects was obtained through the exit interview. The feedback was overwhelmingly positive. All of the subjects wanted to continue using the CollaboRhythm application after the end of the study. Some who had reached goal only wanted to use it sporadically as a “check-in,” but the majority wanted to use it daily as they had for the study for an indefinite period of time. They felt that the burden of reporting was easily balanced by the value of being able to track progress and get the efficient support of a health coach. They related that awareness of the associations between actions (diet, exercise, stress management, medication adherence) and blood pressure outcomes was integral to their success and their positive experience with the application. Subjects responded very favorably to the concept of leading their care. Some comments from patients include: “It felt good to take responsibility,” “No one has ever asked me to take responsibility for my health,” and “I developed confidence that I never would have had.” The nurse coach commented multiple times how delighted she was with the level of patient engagement and excitement. She also did not want to stop using the application at the end of the study.
The main complaints about the program from both subjects and the health coach were on performance. They wanted the application to load faster and to save data faster.
Discussion
This study provides encouraging evidence that technology-supported apprenticeship can dramatically improve the outcomes, cost, and experience of care in the management of hypertension.
A 26.3 mm Hg average decrease in systolic blood pressure for intervention subjects in 3 months is significantly better than the standard of care and the best of the best in published interventions. [1,11–14]. The same is true for a rate of 100% of patients achieving goal blood pressure < 140/90 mm Hg.
We believe several factors contribute to the success of our program: (1) supporting subjects in the mission of leading their care, (2) rich real-time feedback for self-reflection, (3) emphasis on short-term goals, (4) promise of medication reduction with goal achievement, and (5) social support and accountability that come with having a continuously available coach who had immediate data access.
Other studies have had web communication components, self-titration components, and coaching components. In a progressive study by McManus [11], subjects in the intervention group self-tracked their blood pressure and self-titrated their hypertension medications while control subjects received routine care. Self-managing patients achieved a decrease in systolic blood pressure of 17.6 mm Hg over 12 months while controls only achieved a decrease of 12.2 mm Hg. Self-managing patients were more aggressive in adding new medications and did not have increased incidence of side effects. A study by Green [12] showed that subjects with home blood pressure monitoring, a web training course, and web-based pharmacist coaching achieved a 14.2 mm Hg decrease in systolic blood pressure over 12 months and that 56% reached goal blood pressure (< 140/90 mm Hg). Subjects who received routine care only achieved a 5.3 mm Hg decrease in systolic blood pressure and only 31% reached goal blood pressure. Margolis [13] and Magid [14] conducted studies similar to Green's with pharmacist-led care. In Margolis’ study, 71.8% of inter-vention subjects study achieved goal at 6 months with a mean decrease in systolic blood pressure of 21.5 mm Hg. These results were impressively stable at 18 months [13]. In Magid’s study, 54.1% of intervention subjects achieved goal at 6 months with a mean decrease in systolic blood pressure of 20.7 mm Hg [14].
The technology-supported apprenticeship model was inspired by the exceptional results of these studies, but we believe that it represents a significant step forward in patient engagement. In these studies, the tools were typically more burdensome than empowering, the titration more prescriptive than motivational, and the coaching more timed and paternalistic than continuous and nurturing. Technology-supported apprenticeship relies on more powerful tools for collaboration and aspires for the patient to lead the care rather than the clinician. It has the potential to produce greater self-efficacy, which results in better outcomes at lower cost.
Cost Implications
The 100% rate of attainment of systolic blood pressure < 140 mm Hg in our intervention subjects has the poten-tial to contribute to substantial cost savings through reducing complications. The cost of complications per patient per year is approximately $1275, and attainment of goal blood pressure on average results in approximately 35% decrease in complications [2,7–9] Therefore, it is reasonable to expect at least $446 savings in downstream costs per patient per year with this intervention. This has notable public health implications given that there are approximately 32 million patients in the United States with diagnosed hypertension that is uncontrolled [1]. Cost saving at scale could be more than $14 billion.
Typically, interventions that produce downstream savings in health care require some upstream investment. An exciting aspect of this intervention is that it was significantly less costly, at $67.50 per patient, as compared with the standard of care, at $248 per patient per year [7–9]. Most of the responsibility was embraced by the patients, and the nurse coach needed only to provide occasional social and clinical support. Return office visits were almost completely eliminated and goals were achieved in a fraction of the time. The cost of the technology is effectively negligible given that patients will be able to use their own cell phones and tablets and their own blood pressure cuffs. Based on the prevalence of hypertension, a primary care practice with 2000 patients would be expected to have 600 with hypertension. At $180.50 in savings per patient per year, a profit of $108,300 could be retained if the same reimbursement was provided for this more effective care. At the same time, this more efficient and scalable model of care could allow a practice to care for a larger number of patients without compromising the experience for patients or clinicians.
Limitations and Future Directions
An important limitation of this study is its small sample size. The efficiency and cost analysis suggests that the intervention is highly suitable for scaling but, until this is studied, there is the risk that the small sample size may misrepresent the average effect on a larger population and overestimate the size of a population that a coach can manage. Future work will need to include much larger populations.
Another limitation was the short follow-up period. A key benefit of apprenticeship is that it theoretically results in greater and more sustainable self-efficacy than other models of patient engagement that are not as grounded in learning science and that do not engage the patient as deeply. Follow-up visits for both control and intervention subjects will be conducted at 1 year to assess the sustainability of the blood pressure improvements.
The fact that the population was from a single practice also presents the possibility that the subjects being studied were more interested and/or capable than a larger, more general population. This is likely to be true, since many of the patients at the APF sought it out in the hope of receiving a new health care experience. Future work will need to address patient populations from diverse socioeconomic situations and diverse cultures. Based on pilot studies that we have done with diverse populations with HIV, hypertension, and diabetes, we believe that patients who are more disempowered and disenfranchised at baseline attain greater benefit from becoming apprentices in their care.
Another potential limitation is that hypertension was treated in isolation in this study. In fact, many of the subjects had comorbid conditions and were being treated for them simultaneously at the APF. Future work will need to address how the apprenticeship approach and accompanying technology can support the management of multiple comorbid conditions. At the same time, we believe that setting aggressive goals for one condition at a time and achieving these goals over a short period, such as 3 months, will be an important strategy in effectively engaging patients as active participants in their care and helping them to develop self-efficacy. As they develop competency for one condition, they become more capable of managing the others. If they were to attempt to tackle all of the problems simultaneously, it could be overwhelming and ineffective.
Conclusions
This study provides encouraging evidence that, in the management of hypertension, technology-supported apprenticeship can improve the outcomes, cost, and experience of care. By embracing the potential of patients while at the same time optimizing support from clinicians and leveraging information technology for cost-effective scaling, it has the potential to have a tremendous impact across the spectrum of chronic disease.
Three core tenants of technology-supported apprenticeship have surfaced as the product of this work, and they may serve as a useful guide to other efforts improving care delivery: First, patients are the most underutilized resource in health care. Technology-supported apprenticeship harnesses the contribution of the patient and values the development of self-efficacy to the extent that apprentices can become masters. It supports patients in achieving a pinnacle of patient empowerment.
Second, health takes place in the everyday lives of patients, not in a doctor’s office or hospital. Technology-supported apprenticeship appreciates that situated learning is the key to mastery of chronic disease management and strives to support patients continuously in the context of their lives.
Third, collaboration and information transparency, not just information access, are critical. Technology-supported apprenticeship embraces information technology to maximize collaboration among novices and masters and to provide common ground through complete transparency.
Acknowledgments: This study was completed as part of John O. Moore’s PhD thesis at the MIT Media Lab. Mitchel Resnick, PhD, and Pattie Maes, PhD, were members of the thesis committee along with authors Franklin H. Moss, PhD, and David C. Judge, MD, and their advice was critical in the development of the technology-supported apprenticeship approach.
Corresponding author: John O. Moore, MD, PhD, [email protected]
Funding/support: This work was supported, in part, by the CIMIT Prize for Primary Healthcare from the Center for Integration of Medicine & Innovative Technology, Boston, MA.
Financial disclosures: John O. Moore, Frank Moss, and Scott Gilroy have a health information technology company focused on building software solutions for improving chronic disease management. This company does not yet have a product for sale.
From the Massachusetts Institute of Technology (Dr. Moore, Dr. Moss and Mr. Gilroy) and the Massachusetts General Hospital (Ms. Marshall, Dr. Judge, Dr. Crocker, and Dr. Zusman), Boston, MA.
Abstract
- Objective: To compare technology-supported appren-ticeship in hypertension management with a successful coaching model at Massachusetts General Hospital.
- Methods: A randomized controlled trial was conducted. Adult patients with uncontrolled essential hypertension (average blood pressure (BP) 148/87 mm Hg) were recruited in a staggered fashion for a 12-week study period. Intervention subjects received apprenticeship support from a nurse health coach through the CollaboRhythm application on a tablet computer. Patients self-tracked medication adherence and blood pressure (via wireless device) and the coach helped them to continuously progress through lifestyle change and medication adjustment using integrated messaging. Control subjects received support from the same coach but through traditional channels of office visits, phone calls, and e-mail.
- Results: 42 of 44 subjects completed the study. Intervention subjects achieved a greater decrease in systolic BP at 12 weeks than control subjects (26.3 mm Hg vs. 16.0 mm Hg, P = 0.009). A greater percentage of intervention subjects achieved goal BP ≤ 130/80 mm Hg (75.0% vs. 31.8%, P = 0.003) and 100% of them achieved goal BP ≤ 140/90 mmHg. They also rated the experience higher, although this finding was not statistically significant (8.9 vs. 7.6, P = 0.12). There was a trend toward increased cost for intervention subjects ($67.50 vs. $53.41, P = 0.15), but the projected cost is much less than standard care ($248/patient/year).
- Conclusion: This study provides encouraging evidence that technology-supported apprenticeship can improve the outcomes, cost, and experience of care in managing hypertension.
Hypertension affects approximately 33% of the U.S. adult population [1]. Antihypertensive treatment has been shown to be effective at preventing complications [2,3]. Unfortunately, estimates suggest that the majority of those diagnosed with hypertension do not have their blood pressure controlled [1]. This failure is due to both clinician and patient factors. Mean adherence of clinicians to guidelines is estimated at 53.5% [4]. An electronic monitoring study showed that half of patients who are prescribed medications stop taking them within 1 year [5]. Of those who take their medications, about 10% have adherence issues on any given day and about 50% have significant adherence issues in the course of their treatment [5]. Adherence to diet and exercise self-management is even more dismal, with rates below 20% [6]. Hypertension is an expensive problem with direct medical costs (treatment and complications) greater than $100 billion a year and equally high indirect costs (lost productivity) [7–9].
In the management of chronic diseases, there is a significant trend toward empowering patients with more control and toward providing more longitudinal coaching from clinicians and from peers [10–15]. Technology-supported apprenticeship is a model of chronic disease management that builds on the success of self-management and coaching, but it is more ambitious in that its goal is for patients to lead their care with the support of health coaches and supervising clinicians. It is informed by the field of learning science, particularly in how technology can be used effectively to support learning [16–20]. Apprenticeship refers to the tutelage of a community of novices in a skill or trade by one or more masters through situated learning. Situated learning refers to the process in which novices learn through participation in legitimate tasks in the same physical and social context where they will need to perform them once independent [21,22]. It is opposed to learning through contrived exercises in an artificial environment like a classroom.
In technology-supported apprenticeship, patients are the novice apprentices of master coaches. Technology provides scaffolding and communication tools to allow coaches to support the patient in the management of disease within the context of daily life rather than in the clinician’s office. Patients are supported in gradually developing self-efficacy and independence until they are skilled enough to lead their care and even to help coach others to success. The hypothesis is that embracing the contribution of the patient to this extent will reap unparalleled rewards in the experience, outcomes, and cost of care.
We conducted a randomized controlled trial to assess whether a technology-supported apprenticeship in hyper-tension management would improve blood pressure control compared with a successful coaching model.
Methods
Setting
The health coaching model at the Ambulatory Practice of the Future (APF) at the Massachusetts General Hospital is designed to impact the whole health ofpatients. In the scope of hypertension management, a nurse health coach, under the supervision of a physician, is responsible for improving outcomes through longitudinal support via office visits, phone calls, and e-mails. Diet, exercise, stress management, and medication therapy are all emphasized through health coaching techniques, such as motivational interviewing and appreciative inquiry [23,24]. Patients are referred to dieticians, exercise coaches, mind-body specialists, and others as desired. Prior to the start of this study, the APF was outperforming the national average with ~70% of hypertensive patients in the practice below clinically recommended goal blood pressures.
Recruitment
The study was advertised to all clinical staff of the APF through word of mouth. Since the practice was relatively young, it also had a registry of patients with elevated blood pressure that had not been addressed. Adult patients (> 18 years old) from the registry or from routine visits with essential hypertension (average blood pressure ≥ 140/90 and ≤ 180/120) who were taking 0 or 1 medications and had internet connectivity were eligible for inclusion in the study. Patients with a history of hypotension, syncope, hypertensive urgency, hypertensive emergency, labile hypertension, and proven coronary artery disease were excluded, as were patients with significant visual, auditory, or cognitive impairment and patients who were not proficient in English. Clinical staff notified the nurse health coach for the study on identification of any eligible subjects. The same nurse health coach recruited and cared for all intervention and control subjects.
The nurse health coach contacted each eligible patient to assess interest. Interested patients were scheduled for an appointment to commence the study. A process of written informed consent was carried out with each study subject. Each subject was assigned the next sequential study number, which was pre-randomized to either the intervention or the control group.
The study period for each subject was 12 weeks +/– 2 weeks with staggered recruitment. Twelve weeks represents the minimal time required to progress through the standardized Medication Adjustment Plan that was used, assuming a minimum time of 2 weeks on each medication before a change is considered. A recruitment window from 1 March 2013 until 31 May 2013 was used. The goal was to recruit between 40 and 60 subjects, since statistical analysis revealed that a sample size of 36 was sufficient to detect a 5 mm Hg difference in the decrease in systolic blood pressure between groups given a 4 mm Hg standard deviation.
Intervention
CollaboRhythm is a software platform that was developed at the MIT Media Lab and designed to support the principles of technology-supported apprenticeship. Patient tracking tools document progress, visualizations highlight associations between actions and outcomes, and personalized decision support encourages self-efficacy. Powerful virtual visits and instant messaging allow master clinicians to provide adaptive coaching within the context of daily life rather than in the artificial environment of the office. Applications can be deployed cross-platform to cell phones, tablets, computers, etc.
A simplified system was created for hypertension management. It is optimized for tablet deployment and focuses on instant messaging for communications. The patient uses a wireless blood pressure monitor (D40b model by ForaCare®) to take blood pressure measurements. The device is paired with a tablet application to automatically report measurements. The patient self-reports medication adherence using the tablet application. Patient data is visualized in a daily clock and weekly charts to promote proactive behavior and self-reflection. Decision support for medication adjustment is paired with the charts. The patient’s data are synchronized with the coach’s tablet application via a collaborative health record server implemented using the Indivo X personally controlled health record (Children’s Hospital Boston, indivohealth.org) code base. Instant messaging between the patient and coach is implemented using a real-time messaging server. The applications for the patient and the coach are identical, but the coach’s application is configured to allow switching between patient records in order to manage a number of patients.
The user experience was designed based on our years of exploration at the MIT Media Lab of the psychology of illness and the impact that technology could have on patient empowerment and patient-clinician collaboration. It is important to note that the CollaboRhythm application is different from typical electronic reminder applications. In fact, the application never alarms at patients, even if they forget to perform their health actions. Instead, the application focuses on providing awareness for proactive decision-making, self-reflection, and support from the coach.
Protocol Common to Control and Intervention Subjects
Following informed consent, a baseline blood pressure and heart rate were measured for each subject using the same automated sphygmomanometer. Weight was also measured and a hypertension knowledge assessment (a simple survey about normal blood pressures and the effects of diet, exercise, and medication that was created for the purpose of this study) was completed. The initial medication load was determined using the Medication Adjustment Plan as a reference and each dose step as a unit of 1. For example, if a subject was already on lisinopril 40 mg at the start of the study, the initial medication load was 2.
The nurse health coach conducted an introductory visit with each subject including motivational interviewing to assess their health values and to discuss
hypertension management goals. The subject and coach made shared decisions about diet, exercise, stress management, and medication. The same medication titration algorithm was used for both control and interventional
subjects.
At the conclusion of the study period, subjects in both groups returned for an exit visit. Blood pressure and heart rate were measured using the same automated sphygmomanometer that was used at the initial visit. Weight was measured using the same scale as the initial visit. A hypertension knowledge assessment was completed and an exit interview was conducted. The change in medication load was determined as well. For example, if a subject started the study on lisinopril 20 mg and ended the study on lisinopril 40 mg, the increase in medication load was 1.
Protocol Specifics for Intervention Subjects
The apprenticeship model of chronic disease management was discussed with the nurse health coach and with APF team prior to the study. It was also implicit in the design of the CollaboRhythm tablet application.
During the introduction visit, subjects in the inter-vention group received instructions from the nurse health coach on how to use the CollaboRhythm tablet application to self-track their medication adherence, to review progress using the charts, to propose or agree with changes in hypertension medications, and to communicate with messages.
Subjects in the intervention group received their hyper-tension care through the CollaboRhythm tablet application. All instant messages between the intervention subjects and the nurse health coach were automatically documented electronically. Other forms of communication were documented in a spreadsheet. During the exit visit, the tablet computer and the wireless blood pressure meter were returned.
Protocol Specifics for Control Subjects
Between the introduction and exit visits, subjects in the control group received standard hypertension care from the nurse health coach at the APF. The coach instructed subjects to check their blood pressures 3 to 5 times a week. They had the option of purchasing a cuff, using a monitor at work, or using a monitor at a facility such as a pharmacy or grocery store. Communication took place through
office visits, phone calls, and e-mails. All communications with control subjects were documented for the purposes of this study by the nurse health coach in a spread-
sheet.
Outcomes of Interest
The primary outcomes of interest were the absolute decrease in systolic and diastolic blood pressure and the number of subjects who reached the blood pressure goal of less than or equal to 130/80 mm Hg. This more aggressive blood pressure goal was chosen for a number of reasons: (1) it is not uncommon to set a lower blood pressure goal for home measurements to avoid patients hovering just at the 140/90 goal and not getting the true benefit of reduced blood pressure; and (2) subjects were supported in improving diet, exercise, and stress management in addition to pharmacologic therapy. The 140/90 cut-off (now 150/90 in patients over 60) is focused on pharmacologic therapy [2,3].
The secondary outcomes included the number of subjects who reached the blood pressure goal of less than or equal to 140/90 mm Hg (for comparison to other published literature), the number of subjects who achieved greater than a 10 mm Hg decrease in systolic and greater than a 5 mm Hg decrease in diastolic blood pressure, the change in medication load, the absolute decrease in weight, the number of subjects who lost at least 5 lb, a hypertension knowledge score assessed by a pre- and post-study test, a rating of satisfaction in care, and the amount of clinician time required in the care.
Statistical Methods
The study results were analyzed by the intention-to-treat approach. All subjects who returned for the final study visit were included in the analysis regardless of their adherence to the protocol. Subjects who did not return for the study exit visit were not included in the analysis because it would be impossible to determine the change in blood pressure without a final measurement. A 2-tailed Student’s t test for independent samples was used for all comparisons of the mean of continuous variables between the control and intervention groups. A 2-tailed Student’s t test for dependent samples was used for all comparisons between pre- and post-study variables within the control and the intervention group. A chi-square test was used for all comparisons of categorical variables between the groups, except when the expected values in the 2x2 table were below 5. Fisher’s exact test was used in this scenario, which applied to the secondary outcome of the number of subjects who reached the blood pressure goal of less than or equal to 140/90 mm Hg.
Oversight
This study was approved by the institutional review boards at the Massachusetts Institute of Technology and the Massachusetts General Hospital.
Results
There were a total of 44 subjects recruited for the study. One intervention subject and one control subject were lost to follow-up, leaving 42 subjects who completed the study exit visit.
Baseline Statistics
Clinical Outcomes
Cost
 Table 3 presents a summary of nurse coach communications with subjects and the corresponding cost. The coach communicated with intervention subjects primarily through instant messages using the CollaboRhythm tablet application. There were trends toward more e-mails, phone calls, and office visits in control subjects that were not statistically significant. The total nurse coach time spent per patient was calculated using the following assumptions: instant message = 5 minutes, e-mail = 10 minutes, phone call = 15 minutes, office visit = 30 minutes. It is of note that, although virtual visits (video conferences with the ability to co-navigate patient data) were available as a feature, none were conducted during the course of the study. There was a trend toward intervention subjects receiving more support from the nurse coach that was not statistically significant. The cost associated with nurse coach time was calculated for both control and intervention subjects using the assumption of $50/hour (based on $100,000/year average salary plus benefits for nurse with health coaching certification). On average, intervention subjects received 0.28 hours or 16.8 minutes more time at an additional cost of $14.09.
Table 3 presents a summary of nurse coach communications with subjects and the corresponding cost. The coach communicated with intervention subjects primarily through instant messages using the CollaboRhythm tablet application. There were trends toward more e-mails, phone calls, and office visits in control subjects that were not statistically significant. The total nurse coach time spent per patient was calculated using the following assumptions: instant message = 5 minutes, e-mail = 10 minutes, phone call = 15 minutes, office visit = 30 minutes. It is of note that, although virtual visits (video conferences with the ability to co-navigate patient data) were available as a feature, none were conducted during the course of the study. There was a trend toward intervention subjects receiving more support from the nurse coach that was not statistically significant. The cost associated with nurse coach time was calculated for both control and intervention subjects using the assumption of $50/hour (based on $100,000/year average salary plus benefits for nurse with health coaching certification). On average, intervention subjects received 0.28 hours or 16.8 minutes more time at an additional cost of $14.09.
Experience
Thirteen control subjects and 16 intervention subjects provided an experience rating during their exit interview from the study. There was a trend toward greater satisfaction by intervention subjects that was not statistically significant (8.9 vs. 7.6, P = 0.12). The majority of feedback from intervention subjects was obtained through the exit interview. The feedback was overwhelmingly positive. All of the subjects wanted to continue using the CollaboRhythm application after the end of the study. Some who had reached goal only wanted to use it sporadically as a “check-in,” but the majority wanted to use it daily as they had for the study for an indefinite period of time. They felt that the burden of reporting was easily balanced by the value of being able to track progress and get the efficient support of a health coach. They related that awareness of the associations between actions (diet, exercise, stress management, medication adherence) and blood pressure outcomes was integral to their success and their positive experience with the application. Subjects responded very favorably to the concept of leading their care. Some comments from patients include: “It felt good to take responsibility,” “No one has ever asked me to take responsibility for my health,” and “I developed confidence that I never would have had.” The nurse coach commented multiple times how delighted she was with the level of patient engagement and excitement. She also did not want to stop using the application at the end of the study.
The main complaints about the program from both subjects and the health coach were on performance. They wanted the application to load faster and to save data faster.
Discussion
This study provides encouraging evidence that technology-supported apprenticeship can dramatically improve the outcomes, cost, and experience of care in the management of hypertension.
A 26.3 mm Hg average decrease in systolic blood pressure for intervention subjects in 3 months is significantly better than the standard of care and the best of the best in published interventions. [1,11–14]. The same is true for a rate of 100% of patients achieving goal blood pressure < 140/90 mm Hg.
We believe several factors contribute to the success of our program: (1) supporting subjects in the mission of leading their care, (2) rich real-time feedback for self-reflection, (3) emphasis on short-term goals, (4) promise of medication reduction with goal achievement, and (5) social support and accountability that come with having a continuously available coach who had immediate data access.
Other studies have had web communication components, self-titration components, and coaching components. In a progressive study by McManus [11], subjects in the intervention group self-tracked their blood pressure and self-titrated their hypertension medications while control subjects received routine care. Self-managing patients achieved a decrease in systolic blood pressure of 17.6 mm Hg over 12 months while controls only achieved a decrease of 12.2 mm Hg. Self-managing patients were more aggressive in adding new medications and did not have increased incidence of side effects. A study by Green [12] showed that subjects with home blood pressure monitoring, a web training course, and web-based pharmacist coaching achieved a 14.2 mm Hg decrease in systolic blood pressure over 12 months and that 56% reached goal blood pressure (< 140/90 mm Hg). Subjects who received routine care only achieved a 5.3 mm Hg decrease in systolic blood pressure and only 31% reached goal blood pressure. Margolis [13] and Magid [14] conducted studies similar to Green's with pharmacist-led care. In Margolis’ study, 71.8% of inter-vention subjects study achieved goal at 6 months with a mean decrease in systolic blood pressure of 21.5 mm Hg. These results were impressively stable at 18 months [13]. In Magid’s study, 54.1% of intervention subjects achieved goal at 6 months with a mean decrease in systolic blood pressure of 20.7 mm Hg [14].
The technology-supported apprenticeship model was inspired by the exceptional results of these studies, but we believe that it represents a significant step forward in patient engagement. In these studies, the tools were typically more burdensome than empowering, the titration more prescriptive than motivational, and the coaching more timed and paternalistic than continuous and nurturing. Technology-supported apprenticeship relies on more powerful tools for collaboration and aspires for the patient to lead the care rather than the clinician. It has the potential to produce greater self-efficacy, which results in better outcomes at lower cost.
Cost Implications
The 100% rate of attainment of systolic blood pressure < 140 mm Hg in our intervention subjects has the poten-tial to contribute to substantial cost savings through reducing complications. The cost of complications per patient per year is approximately $1275, and attainment of goal blood pressure on average results in approximately 35% decrease in complications [2,7–9] Therefore, it is reasonable to expect at least $446 savings in downstream costs per patient per year with this intervention. This has notable public health implications given that there are approximately 32 million patients in the United States with diagnosed hypertension that is uncontrolled [1]. Cost saving at scale could be more than $14 billion.
Typically, interventions that produce downstream savings in health care require some upstream investment. An exciting aspect of this intervention is that it was significantly less costly, at $67.50 per patient, as compared with the standard of care, at $248 per patient per year [7–9]. Most of the responsibility was embraced by the patients, and the nurse coach needed only to provide occasional social and clinical support. Return office visits were almost completely eliminated and goals were achieved in a fraction of the time. The cost of the technology is effectively negligible given that patients will be able to use their own cell phones and tablets and their own blood pressure cuffs. Based on the prevalence of hypertension, a primary care practice with 2000 patients would be expected to have 600 with hypertension. At $180.50 in savings per patient per year, a profit of $108,300 could be retained if the same reimbursement was provided for this more effective care. At the same time, this more efficient and scalable model of care could allow a practice to care for a larger number of patients without compromising the experience for patients or clinicians.
Limitations and Future Directions
An important limitation of this study is its small sample size. The efficiency and cost analysis suggests that the intervention is highly suitable for scaling but, until this is studied, there is the risk that the small sample size may misrepresent the average effect on a larger population and overestimate the size of a population that a coach can manage. Future work will need to include much larger populations.
Another limitation was the short follow-up period. A key benefit of apprenticeship is that it theoretically results in greater and more sustainable self-efficacy than other models of patient engagement that are not as grounded in learning science and that do not engage the patient as deeply. Follow-up visits for both control and intervention subjects will be conducted at 1 year to assess the sustainability of the blood pressure improvements.
The fact that the population was from a single practice also presents the possibility that the subjects being studied were more interested and/or capable than a larger, more general population. This is likely to be true, since many of the patients at the APF sought it out in the hope of receiving a new health care experience. Future work will need to address patient populations from diverse socioeconomic situations and diverse cultures. Based on pilot studies that we have done with diverse populations with HIV, hypertension, and diabetes, we believe that patients who are more disempowered and disenfranchised at baseline attain greater benefit from becoming apprentices in their care.
Another potential limitation is that hypertension was treated in isolation in this study. In fact, many of the subjects had comorbid conditions and were being treated for them simultaneously at the APF. Future work will need to address how the apprenticeship approach and accompanying technology can support the management of multiple comorbid conditions. At the same time, we believe that setting aggressive goals for one condition at a time and achieving these goals over a short period, such as 3 months, will be an important strategy in effectively engaging patients as active participants in their care and helping them to develop self-efficacy. As they develop competency for one condition, they become more capable of managing the others. If they were to attempt to tackle all of the problems simultaneously, it could be overwhelming and ineffective.
Conclusions
This study provides encouraging evidence that, in the management of hypertension, technology-supported apprenticeship can improve the outcomes, cost, and experience of care. By embracing the potential of patients while at the same time optimizing support from clinicians and leveraging information technology for cost-effective scaling, it has the potential to have a tremendous impact across the spectrum of chronic disease.
Three core tenants of technology-supported apprenticeship have surfaced as the product of this work, and they may serve as a useful guide to other efforts improving care delivery: First, patients are the most underutilized resource in health care. Technology-supported apprenticeship harnesses the contribution of the patient and values the development of self-efficacy to the extent that apprentices can become masters. It supports patients in achieving a pinnacle of patient empowerment.
Second, health takes place in the everyday lives of patients, not in a doctor’s office or hospital. Technology-supported apprenticeship appreciates that situated learning is the key to mastery of chronic disease management and strives to support patients continuously in the context of their lives.
Third, collaboration and information transparency, not just information access, are critical. Technology-supported apprenticeship embraces information technology to maximize collaboration among novices and masters and to provide common ground through complete transparency.
Acknowledgments: This study was completed as part of John O. Moore’s PhD thesis at the MIT Media Lab. Mitchel Resnick, PhD, and Pattie Maes, PhD, were members of the thesis committee along with authors Franklin H. Moss, PhD, and David C. Judge, MD, and their advice was critical in the development of the technology-supported apprenticeship approach.
Corresponding author: John O. Moore, MD, PhD, [email protected]
Funding/support: This work was supported, in part, by the CIMIT Prize for Primary Healthcare from the Center for Integration of Medicine & Innovative Technology, Boston, MA.
Financial disclosures: John O. Moore, Frank Moss, and Scott Gilroy have a health information technology company focused on building software solutions for improving chronic disease management. This company does not yet have a product for sale.
1. Recent trends in the prevalence of high blood pressure and its treatment and control. National Center for Health Statistics. Hyattsville, MD; 2010.
2. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72. Erratum in: JAMA 2003;290:197.
3. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20.
4. Ardery G, Carter BL, Milchak JL, et al. Explicit and implicit evaluation of physician adherence to hypertension guidelines. J Clin Hypertens (Greenwich) 2007;9:113–9.
5. Vrijens B, Vincze G, Kristanto P, et al. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 2008;336:1114–7.
6. Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med 2008;168:308–14.
7. Davis K. Expenditures for hypertension among adults age 18 and older, 2009: estimates for the U.S. civilian noninstitutionalized population. Rockville, MD: Agency for Healthcare Research and Quality; June 2012. Available at http://meps.ahrq.gov/mepsweb/data_files/publications/st371/stat371.shtml.
8. Hodgson TA, Liming C. Medical care expenditures for hypertension, its complications, and its comorbidities. Med Care 2001;39:599–615.
9. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011;123:933–44.
10. Wagner EH, Austin BT, Davis C, et al. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20:64–78.
11. McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomized controlled trial. Lancet 2010;376:163–72.
12. Green BB, Cook AJ, Ralston JD, et al. Effectiveness of home blood pressure monitoring, web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA 2008;299:2857–67.
13. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA 2013;310:46–56.
14. Magid DJ, Olson KL, Billups SJ, et al. A pharmacist-led, American Heart Association Heart360 Web-enabled home blood pressure monitoring program. Circ Cardiovasc Qual Outcomes 2013;6:157–63.
15. Lorig K, Ritter PL, Villa FJ, Armas J. Community-based peer-led diabetes self-management: a randomized trial. Diabetes Educ 2009;35:641–51.
16. Collins A, Brown J, Newman SE. Cognitive apprenticeship: Teaching the crafts of reading, writing, and mathematics. In: Knowing, learning, and instruction: Essays in honor of Robert Glaser. Lawrence Erlbaum Associates; 1989:453–94.
17. Resnick M. Rethinking learning in the digital age. In: Kirkman G, editor. The global information technology report: readiness for a networked world. Oxford University Press; 2002.
18. Koschmann T. Paradigm shifts and instructional technology. In: Koschmann T, editor. CSCL: Theory and practice of an emerging paradigm. Lawrence Erlbaum Associates; 1996:83–124.
19. Brown JS, Adler RP. Minds on fire: open education, the long tail, and learning 2.0. Educause Rev 2008;43:16–32.
20. Collins A, Halverson R. The second educational revolution: rethinking education in the age of technology. J Comp Assist Learn 2010;26:18–27.
21. Dewey J. Experience and education. New York: Collier Books; 1938.
22. Lave J. Situated learning: legitimate peripheral participation (Learning in Doing: Social, Cognitive, and Computational Perspectives). Cambridge University Press; 1991.
23. Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol 2009;64:527–37.
24. Moore SM, Charvat J. Promoting health behavior change using appreciative inquiry: moving from deficit models to affirmation models of care. Fam Community Health 2007;30 Supp 1:S64–74.
1. Recent trends in the prevalence of high blood pressure and its treatment and control. National Center for Health Statistics. Hyattsville, MD; 2010.
2. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72. Erratum in: JAMA 2003;290:197.
3. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20.
4. Ardery G, Carter BL, Milchak JL, et al. Explicit and implicit evaluation of physician adherence to hypertension guidelines. J Clin Hypertens (Greenwich) 2007;9:113–9.
5. Vrijens B, Vincze G, Kristanto P, et al. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 2008;336:1114–7.
6. Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med 2008;168:308–14.
7. Davis K. Expenditures for hypertension among adults age 18 and older, 2009: estimates for the U.S. civilian noninstitutionalized population. Rockville, MD: Agency for Healthcare Research and Quality; June 2012. Available at http://meps.ahrq.gov/mepsweb/data_files/publications/st371/stat371.shtml.
8. Hodgson TA, Liming C. Medical care expenditures for hypertension, its complications, and its comorbidities. Med Care 2001;39:599–615.
9. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011;123:933–44.
10. Wagner EH, Austin BT, Davis C, et al. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20:64–78.
11. McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomized controlled trial. Lancet 2010;376:163–72.
12. Green BB, Cook AJ, Ralston JD, et al. Effectiveness of home blood pressure monitoring, web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA 2008;299:2857–67.
13. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA 2013;310:46–56.
14. Magid DJ, Olson KL, Billups SJ, et al. A pharmacist-led, American Heart Association Heart360 Web-enabled home blood pressure monitoring program. Circ Cardiovasc Qual Outcomes 2013;6:157–63.
15. Lorig K, Ritter PL, Villa FJ, Armas J. Community-based peer-led diabetes self-management: a randomized trial. Diabetes Educ 2009;35:641–51.
16. Collins A, Brown J, Newman SE. Cognitive apprenticeship: Teaching the crafts of reading, writing, and mathematics. In: Knowing, learning, and instruction: Essays in honor of Robert Glaser. Lawrence Erlbaum Associates; 1989:453–94.
17. Resnick M. Rethinking learning in the digital age. In: Kirkman G, editor. The global information technology report: readiness for a networked world. Oxford University Press; 2002.
18. Koschmann T. Paradigm shifts and instructional technology. In: Koschmann T, editor. CSCL: Theory and practice of an emerging paradigm. Lawrence Erlbaum Associates; 1996:83–124.
19. Brown JS, Adler RP. Minds on fire: open education, the long tail, and learning 2.0. Educause Rev 2008;43:16–32.
20. Collins A, Halverson R. The second educational revolution: rethinking education in the age of technology. J Comp Assist Learn 2010;26:18–27.
21. Dewey J. Experience and education. New York: Collier Books; 1938.
22. Lave J. Situated learning: legitimate peripheral participation (Learning in Doing: Social, Cognitive, and Computational Perspectives). Cambridge University Press; 1991.
23. Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol 2009;64:527–37.
24. Moore SM, Charvat J. Promoting health behavior change using appreciative inquiry: moving from deficit models to affirmation models of care. Fam Community Health 2007;30 Supp 1:S64–74.
Acceptance and Commitment Therapy for Chronic Pain
Abstract
- Objective: To describe Acceptance and Commitment Therapy (ACT) and its application in the treatment of chronic pain.
- Methods: Review of the theoretical and clinical literature and presentation of a case example.
- Results: General cognitive behavioral approaches for chronic pain have a consistent and large evidence base supporting their benefits. Even so, these treatments continue to develop with the aim to improve. One example of a relatively new development within the cognitive behavioral approaches is ACT, a treatment that focuses on increasing psychological flexibility. Here we describe ACT and the therapeutic model on which it is based, present its distinguishing features, and summarize the evidence for it as a treatment for chronic pain. We also discuss such issues as dissemination, implementation, and training.
- Conclusion: There are now 7 randomized controlled trials, a number of innovative uncontrolled trials, and at least 1 systematic review that support the clinical efficacy and effectiveness of ACT for chronic pain. Further research and development of this approach is underway.
The introduction of the gate control theory of pain [1] in 1965, among other events, signaled a shift in our understanding of pain, particularly chronic pain. This shift, which continues today, is a shift from a predominantly biomedical model of chronic pain to a biospsychosocial model. This model, as the name suggests, includes psycho-social influences in a key role in relation to the experience of pain and the impact of this experience. During this same period of time, psychosocial models and treatment methods have also shifted and evolved. This evolution has included the operant approach [2], the cognitive behavioral approach [3], and the latest developments, contextual cognitive behavioral approaches [4,5], among which Acceptance and Commitment Therapy (ACT) and mindfulness-based therapies are key examples.
Until about 10 years ago, the mainstream of psycho-logical treatments for chronic pain and other physical health problems was dominated almost exclusively by concepts and methods of what we will refer to as “traditional” cognitive behavioral therapy (CBT). Specific constructs within what is called the “common sense model” [6], such as illness perceptions, beliefs about control over one’s illness, amongst other constructs such as self-efficacy, catastrophising, fear avoidance, and pain-related anxiety, captured a substantial focus of research and treatment development during most of the past 3 decades [7]. The treatment methods that have emerged and persisted from this work have included relaxation, attention-based and cognitive coping strategies, cognitive restructuring, the use of imagery, and certain activity management strategies [8]. However, despite consistent supportive evidence for CBT interventions for chronic pain [9], there remain gaps and areas of relative weakness, both in the conceptual models underlying this work and in the base of evidence. Research clearly shows that not all patients benefit from traditional CBT interventions, and recent reviews of CBT for chronic pain generally show effect sizes that are usually small or mediumat best [9–11].
The Problem with Pain
Pain hurts and is often viewed as harmful, and this leads to fear or anxiety, avoidance, or attempts to control the pain. Seeking to control pain is entirely natural and even seems necessary to reduce the undesirable effects of pain in one’s life.
Dependent on the situation, pain avoidance, sometimes also referred to as “fear avoidance,” in studies of chronic pain can present itself in many forms. Avoidance behavior can include refusal to engage in any activity believed to cause an increase in pain. It may also include “guarding” or bracing around an area of pain, information seeking, treatment seeking, taking medications, overdosing on medications, using aids like heat or ice, withdrawing from social activity, as well as being unwilling to talk about emotional experiences, amongst others [5]. Today, avoidance is recognized as a key foundation element in pain-related suffering and disability [12], and addressing it effectively has become a prime focus in many or most current treatments.
Acceptance and Psychological Flexibility
In recent years, the concept of “acceptance” has gained prominence as a potentially important process for addressing a broad array of psychological problems, including those associated with chronic pain. From this new interest, a fundamentally different treatment emphasis has emerged. This includes a shift away from a predominant focus on changing thoughts and feelings, a focus sometimes adopted within some traditional CBT methods, towards a focus on reducing the influence of thoughts and feelings on our actions instead. This can be a rather confusing distinction. This is because the influence of our thoughts and feelings is often automatic and even invisible to us as it occurs. As such, the influences of our thoughts and feelings appear directly tied to the content of thoughts and feelings, but the matter is not that simple. Clearly there are occasions when our actions contradict our thoughts and feelings, such as when we have perfectly confident beliefs and fail, or significant anxiety and perform successfully. Such instances illustrate what we might call a “2-dimensional” quality of experience; it is the content of experience and the context of experience that determine the influence exerted. Suffice it to say acceptance-based methods are designed to address the difference between experiences that are difficult to control, such as thoughts and feelings, and things that are easier to control: the actions we take in relation to our thoughts and feelings. They do this by taking a focus on creating changes in context and ultimately in behavior. Acceptance includes especially a focus on allowing or opening up to feelings rather than struggling with them or retreating from them. Here, the capacity for openness is a contextual process.
Acceptance methods are not used in isolation. They are usually used in combination with other traditional behavior change strategies, with methods to facilitate values clarification, committed action, and other methods from ACT. Notions of acceptance have even been incorporated into many behavioral and cognitive therapies before, including dialectical behavior therapy [13] and mindfulness-based treatment [14,15], and so this process is not the exclusive domain of ACT. In implementing acceptance-based methods, patients are taught skills, such as to (a) notice feelings specifically in detail, (b) notice that thoughts about pain are products of thinking and not the same as direct experience, (c) notice urges to struggle with thoughts and feelings, (d) to practice refraining from struggling and adopt an observing, allowing, and “making room”–type posture, and (e) take action in line with their goals [4,5].
The wider processes around acceptance in combination are referred to as psychological flexibility [16]. Psychological flexibility relates to one’s ability to directly contact the present moment; to be aware of the thoughts, feelings and potentially unwanted internal experiences it brings; and to follow through with a behavior change or persist with a chosen behavior in the direction of chosen values. Psychological flexibility is the model for psychological health from an ACT perspective [17].
Psychological Flexibility and the 6 Core ACT Processes
Acceptance
Acceptance involves the patients’ willingness to have pain while remaining able to actively choose to continue participating in their life as they want it to be. ACT encour-ages patients to act in ways that are consistent with direct experiences rather than what the mind interprets these events to mean.
Cognitive Defusion
Cognitive defusion is the process of modifying one’s reaction to thoughts by constructing contexts where the influences of these thoughts on behavior are lessened [18]. Unlike traditional cognitive behavior approaches, in ACT it is not the content and actual validity of these thoughts that is challenged but the functions, or influences, of thoughts [19].
Present Moment Awareness
Contact with the present moment reflects the process wherein the person is aware of the situation in “the now” as opposed to focusing on events that happened in the past or might happen in the future [18]. To be “present” requires the individual to flexibly focus attention on experiences as they are happening in the environment, in real time, and to be fully open to what is taking place [20]. It is important the individual is able to notice when he or she is not acting in relation to the present moment and has the ability to shift attention to the present if this shift benefits them.
Self as Context
The sense of self-as-context or self-as-observer is considered the ability to adopt a perspective or point of view that is separate from and not defined by thoughts and feelings or even the physical body. This contrasts a sense of self as made up of personality characteristics, self-evaluations, or a narrative about who we are [5,16]. In ACT, perspective taking can be trained to help people connect with the experience of a distinction between self and psychological experiences. From this, one can choose to follow one’s inner verbal constructions of what defines us, our “stories” of who we are, in certain situations when it works to do so, and not in situations where it leads to unhealthy responses and behavioral restriction.
Values
Values are defined as guiding principles in one’s life. Values are often contrasted with goals, where the difference is that goals can be achieved while values are part of an ongoing process of action and cannot be completed once and for all. In a sense, goals represent set plans of action to be achieved while values are general life directions. If life is like a journey, then goals would be the chosen destination and values would simply be represented by a general direction of travel. Values are helpful when patients struggle with unwanted internal experiences like pain, as they not only serve as a guide for the client to persist in behavior change but also function as a motivating element. Values clarification exercise in therapy encourages the patient to define their values in specific domains of “career, family, intimate relationships, friendships, health, education and spirituality” [4,21] regardless of the primary problem. Personally chosen and clarified values can function as guides when people have difficulty initiating and maintaining behavior change in the presence of unwanted internal experiences.
Committed Action
Committed action is an ongoing process of redirecting behavior in order to create patterns of flexible and effective action in line with a defined value [22]. Patients are encouraged to follow through with their chosen actions that are in line with their values, and to persist or alter their course flexibly. Without the capacity for committed action, behavior change is less likely to persist and integrate into patterns of behavior more generally.
Case Study
Initial Presentation and History
Ms X, a 45-year-old woman, presents with the chief complaint of low back pain, which she has experienced for 3 years. She works part-time due to her pain problem. When she is not at work, she busies herself with seeking both conventional and alternative treatments for her pain condition. In the past, during periods where she experienced pain relief, she attempted to engage in her hobby of photography. However, this often led to a pain flare the next day and required 2 to 3 days of medical leave with increased medication from her PCP before she is able to return to work. As a result, Ms X chose to give up her hobby and focus on treating her pain instead. Ms X in in a constant struggle with her pain condition and believes that she can only return to photography, and live a more normal life, after her pain is cured.
• What are considerations for applying ACT in this scenario?
From an ACT conceptualization this case shows patterns of avoidance that are apparently not helping the person to reach her goals but are causing her distress and restrictions in functioning. An ACT therapist would approach this scenario by first reflecting how normal it is to struggle with pain and stop activities when in pain. From there they might (a) identify what the patient wants from treatment, (b) look at what has been done so far to attain this, (c) examine how well those things have been working, (d) consider the costs of the approach being taken, and (e) if the approach is not working and the cost is high, see if the patient is willing to stop this approach [23].
Therapist’s Initial Approach
Therapist: By what you have told me, your pain has become a big problem for you and it has been going on a long time—3 years. I can see some of the impacts it has had in your life, such as on your work, your photography, and time spent seeking treatment.
Ms X: Yes, it seems like pain has taken over …
Therapist: Exactly, it seems that is a good way to say it. So, understanding that pain has taken over, can I ask you another question?
Ms X: If your question will help me get over this problem, of course.
Therapist: Ok. What is it you want from coming here to participate in this treatment?
Ms X: Well, I want to get rid of this pain, obviously. It’s ruining my life.
Therapist: Ah, that makes sense. You want to eliminate your pain because it has, as you say, ruined your life, and then I guess your life will be better again.
Ms X: Correct.
Therapist: So, can I check in with the things you have been doing so far to reach this goal to eliminate pain?
Ms X: You name it, I’ve tried it: acupuncture, medication, herbs, rest, exercise, magnets, yoga, and more.
Therapist: Ok, you have tried many treatments focused on trying to get rid of the pain. I think that’s a very natural thing to do. In your experience have these methods been successful?
Ms X: Well, some of them seem to work at the time but it all becomes very confusing, because here I am looking for another treatment. It can feel good to get away from the pain for a little while, but soon I will experience a pain flare bringing me back to square one.
Therapist: I see what you are saying. Let me ask my earlier question in a different way. What would your life look like, and what would you be doing, if your pain were not the problem it is today?
Ms X: I would be taking pictures again, be more consistent at work, and spend less time seeking treatments.
Therapist: So, is it your experience that the methods you have been using have helped you to live life this way?
Ms X: … I never thought about it that way ...
• What exercises or techniques are used in ACT?
In practice, ACT is somewhat unique in that it often relies on the use of metaphors and experiential exercises in treatment delivery. Metaphors and stories are used in treatment and communicated in terms that fit with the experience and background of the person seeking treatment. Although therapists can select from among many widely used and often appropriate metaphors and stories, an experienced therapist is likely to create patient specific metaphors “live,” within the context of a particular session. This is consistent with the philosophical underpinning of ACT in its aims for individual tailoring of methods. Unlike other current psychotherapeutic approaches that place a higher value on sticking to a specified protocol, the theory and philosophy behind ACT allow for flexibility and are open to creativity, individual style, and situational sensitivity of the therapist. This is expected to allow the patient to also adopt a similar sensitivity to changing environmental contingencies [19]. In ACT, the techniques typically do not follow a cookbook style of treatment delivery.
Case Continued
Therapist: What if trying to control your thoughts and feelings were not the answer?
Ms X: I have no idea what you mean.
Therapist: Well, you certainly have focused a lot of your effort on trying not to have the thoughts and feelings that seem to block you.
Ms X: What else is there to do, really?
Therapist: If you are willing to experiment with something, try this. Don’t think of a pineapple. (pause for 30 to 60 seconds). Ok, what happens.
Ms X: It didn’t work—I kept thinking about a pineapple.
Therapist: Weird, huh? Notice what is happening here. I wonder if some of your struggles with your experiences are just like this. It’s like by trying to get rid of something, there it is! I wonder if there were another way to do this, do you think you might be willing to test it out?
Ms X: Yes, I can try.
Further ACT Methods
ACT includes numerous experience-based methods and also direct rehearsal of targeted skills. In the previous scen-ario, the therapist might then proceed to instruction and practice of one or another type of acceptance-based skill, something like an “exposure” session or a mindfulness type of exercise that includes having the participant sit with the experience without doing anything else but observe it. The other type of method used includes metaphors that reveal how circumstances and behavior often work in life [4,16].
An Acceptance-Based Metaphor
Therapist: Imagine that you are new to the neighborhood and you invited all your neighbors over to a housewarming party. Everyone in the neighborhood is invited. On that day, the party’s going great, and here comes Joe, who smells and looks like he has not bathed in days. You are embarrassed by the way he looks and smells and try to close the door on him. However, he shows you a flyer that you put up stating that everyone in the neighborhood is invited. So you let him in and quickly shove him to the kitchen so that he will not embarrass you and disrupt your party. However, to stop him from leaving the kitchen, you end up having to stand guard at the doorway. Meanwhile the party is going on and your guests are enjoying themselves, but do you notice what else is happening here?
Ms X: I’ve stopped myself from enjoying my party in order to keep Joe away.
Therapist: What if your pain was like Joe?
Ms X: Huh? … Ah, I think I see what you are saying…
Therapist: It’s like if you allow Joe to simply be another guest, you can do whatever you like at your party. On the other hand, if you say “no” to Joe you also say “no” to the party.
Ms X: Are you saying that it is for me to choose?
• What is the role of therapist in modeling behavior change in ACT?
An important distinction can be made between talking about behavior change and doing behavior change. Within the psychological flexibility model the emphasis is placed on the latter. Here, especially through the use of experiential exercises, clients are put into contact with the experiences that have coordinated unhealthy behavior patterns in the past so that more effective behavior patterns can be acquired. Treatment delivery is guided by the underlying behavioral philosophy and theory. Patients learn to reduce the dominant influence of the literal meaning of language as the only tool for behavior change. Direct experience is moved to the front of awareness and literal meaning, mental and verbal analysis, and so forth, are moved to the back [20]. In treatment, the therapist models for the patient the behavior change processes that are being targeted and also may use examples from his or her life as well as that of the patient’s to develop psychological flexibility [22]. An example might include a therapist’s response to a person who shows an experience of emotional distress and struggling to manage this distress. Here the therapist, in line with ACT, instead of acting in some way to attempt to lessen the distress, would consciously show openness to the experiences and to their own reactions to helplessness around these experiences.
The therapist might say:
“I would feel tired and probably in pain too if I did what you just did. Could we do a little closed-eyes exercise? Shall we put the distressing thought you are having on the table, and focus on it, and we can “observe” what your mind does, and what happens in your body and your emotions when that thought shows up? Are you willing?”
“I’m feeling confused about this issue myself - how about both of us sitting quietly for a moment or two and observe what our minds do in response to this, just slowing things down, and watching?”
“I feel anxious when I believe that my thoughts about pain are true - like I have to do something to make it go away but I don’t know how. What shows up for you when you believe such thoughts about pain?”
• How and when should ACT be used?
Based on current evidence how and when ACT ought to be used, as opposed to other treatment options, will be largely up to the individual professional and their level of competence. ACT is a form of CBT and many of the same guides pertain. In line with the pragmatic approach of ACT, an approach that makes ACT broadly applicable, there is no one particular manualised or scripted treatment protocol that must be adhered to in treatment for one specific condition or another. As mentioned earlier, the ACT approach does not usually follow a cookbook style of delivery, nor is it rigidly guided by strict protocols. There are protocols shared by researchers to support further development but there is no process by which these are deemed “official” or “recognized” or approved by anyone in particular.
A wide range of metaphors and exercises based on a set of behavioral principles that target a particular function has been proposed in ACT and this is part of its uniqueness as a therapeutic model.
Those developing ACT also have not required a standardised certification process to delivering ACT. Instead, they have chosen to create an open community of contributing researchers and clinicians who are “members” by virtue of their commitment to the same approach to clinical development and the same clinical model. Practicing ACT requires that the clinician is aware of their own competencies and delivers treatment accordingly.
• How effective is ACT?
Numerous studies have supported a general role of psychological flexibility in improving the well-being and physical functioning of patients with chronic pain, including patients in specialty care [24,28] and primary care [26]. Many studies support the particular role of acceptance of pain in adjustment to chronic pain [27–30]. Pain acceptance is a better predictor of outcomes than pain severity itself [31,32].
There are now several relatively large-scale studies conducted in actual clinical practice settings that demonstrate the effectiveness of ACT for chronic pain [25,27,33,34]. A more recent study, also conducted in an actual clinical practice setting, provided support for the specific treatment processes proposed within this approach [35]. This study showed that changes in traditionally conceived methods of pain management were unrelated to treatment improvements of pain intensity, physical disability, anxiety and depression for those who participated in treatment, while changes in psychological flexibility were consistently and significantly related to these improvements, with the exception of the results for depression.
Randomised Controlled Trials (RCTs)
To date, there are a total of 7 RCTs related to ACT and chronic pain [36–42], each providing supportive evidence. For example, in one of the early studies, Dahl and colleagues [36] showed that in comparison to treatment as usual, a group of workers who were at risk of long-term absenteeism from work due to pain or stress had a significant reduction in sick leave and healthcare usage after attending four hours of ACT sessions.
Wicksell and colleagues [37,38] conducted 2 separate RCTs with participants who suffered whiplash-associated disorder (WAD) and fibromyalgia, respectively. Post-treatment results of both RCTs showed an improvement in physical functioning, depression and psychological flexibility in the treatment group with gains maintained at follow-up. In addition, participants in the treatment group with WAD showed an improvement in life satisfaction and fear of movement while those in the treatment group with fibromyalgia showed significant improvements in fibromyalgia impact, self-efficacy and anxiety. There was however no change in pain intensity in those who received the ACT-based treatments.
An ACT-based treatment including a self-help manual showed a significant increase in acceptance, satisfaction in life with a higher level of function and decreased pain intensity compared with a wait-list condition and with applied relaxation (AR) [40]. In comparison to the AR condition, participants in the ACT condition also reported a significantly higher level of engagement in meaningful activities and a willingness to experience pain. Follow-up data support the maintenance of these improvements at first follow-up but differences were not significant at the second follow-up. Both depression and anxiety scores improved in both treatment groups.
Wetherell and colleagues [39] compared the effectiveness of ACT and traditional CBT and found that they both produced positive results. Results from the study also showed higher satisfaction in participants who attended ACT treatment than those that attended CBT treatment, suggesting that ACT “is an effective and acceptable” intervention for patients with chronic pain. Overall acceptance of pain was shown to differentiate patients who could function well with chronic pain from those that continued to suffer with it after treatment.
More recently the first internet-based RCT for ACT with chronic pain was conducted [41]. The authors found a reduction in measures of pain-related distress, depressive symptoms, and anxiety, with these gains maintained at 6 months follow-up in the ACT treatment group compared with controls. The most recent RCT was a pilot trial of a group-based treatment of people with chronic pain recruited from general practices in the UK [42]. Participants were randomised to either an ACT-based treatment or treatment as usual. Participants in the ACT-based group underwent 4 sessions each lasting 4 hours with the first 3 sessions completed in 1 week and the last session completed a week later. At 3 months follow-up, participants in the ACT group had lower disability, depression, and higher pain acceptance.
In general, results from the ACT-based RCTs on chronic pain support the efficacy of the treatment and reflect a high degree of versatility, based on the wide variety of modes of delivery tested. However, RCTs for chronic pain are still relatively few with some studies limited to small sample sizes, thus making it difficult to reach definitive conclusions on the general efficacy of ACT in chronic pain treatment. What the studies do seem to show is that ACT is a good alternative treatment option to more traditionally conceived current CBT-based treatments for chronic pain. Larger sample sizes and higher quality studies are needed to strengthen and establish the effectiveness of ACT and to understand the potential impact of wider implementation in clinical practice.
Meta-Analyses
A total of 4 meta-analyses [43–46] have been conducted on acceptance- or ACT-based treatment studies. Although the earlier meta-analyses [43,44] did not separately report the effectiveness of ACT for chronic pain, they reported a moderate effect size for ACT in general, with no evidence that ACT is more effective than established treatments.
Ruiz [46] conducted a review focusing on outcome or mediation/moderation type studies that compared ACT and CBT treatments. His review was not specific to chronic pain, although one study [39] involving a sample of chronic pain patients was included. Moderate effect sizes were found that favored ACT, with ACT showing a greater impact on change processes (g = 0.38) compared to no impact found in CBT (g = 0.05).
Essentially, only one meta-analysis [45] specifically reviewed the efficacy in chronic pain studies. Pain inten-sity and depression were selected as primary outcome measures, with anxiety, physical well-being, and quality of life selected as secondary outcomes. Out of 22 studies that were included in the review, only 2 studies [36,37] were ACT-based RCTs, with the rest of the studies mindfulness-based interventions. The overall effect size of 0.37 was found for pain and 0.32 for depression. In general, results showed significant effect sizes for both primary and secondary outcome measures in favor of the “acceptance-based treatments.” The authors concluded that at present, mindfulness-based stress reduction programs and ACT-based programs may not be superior to CBT but could be good alternatives for people with chronic pain.
The appropriateness of using pain intensity as a primary outcome measure for ACT-based studies is questionable [45]. The focus of ACT is to increase function rather than to reduce pain symptoms; hence possibly including interference of pain in daily life might be a more appropriate outcome measure.
Other Studies
A particularly important question to answer about ACT concerns its cost-effectivness, and we still know relatively little about this. We do know that when people participate in ACT-based treatments they are able to reduce medication use and health care visits and return to work after extended periods away from work [27,28]. It remains to conduct full health economic analyses of this type of approach for chronic pain.
ACT is known to produce significant benefits widely, in other applications apart from chronic pain, such as in workplace stress [47], psychosis [48], obsessive-compulsive disorder [49], and depression [50], among other mental health conditions [51].
• What are implications for policy makers?
Results from studies of ACT in chronic pain and in other areas are disseminating rapidly. This dissemination is aided in part by a professional organization devoted to ACT and psychological flexibility (Association for Contextual Behavioral Science; www.contextualscience.org), which has a new journal, the Journal of Contextual Behavioral Science, started in 2012.
With the development of ACT a focus on implementation, training, and treatment integrity began early. There was an implementation study of ACT was published by Strosahl and colleagues in 1998. Their study showed that training clinicians in ACT produced better outcomes and better treatment completion rates in an outpatient setting in comparison to clinicians not receiving this training.
Processes of training have also appeared during relatively early phases of research into ACT. Lappalainen and colleagues [52] compared the impact of treatment provided by trainee therapists trained in both a traditional CBT model and ACT. Here each trainee therapist treated one patient with traditional CBT and one with ACT. Although the therapists reported higher confidence in delivering traditional CBT, patients treated within an ACT model showed better symptoms improvement. Also, improved acceptance during treatment significantly predicted improvements across both groups of patients. Essentially, therapists with only a limited amount of training in both models demonstrated better clinical results with ACT.
A group-based ACT intervention has also been shown to be effective in reducing stress and improving the professional performance of clinical psychology trainees [53]. Here the trainees found the intervention personally and professionally useful and a majority showed a significant increase in psychological flexibility. This supports the applicability of ACT not only as a model to guide therapy but also as a model to guide training and professional performance [54]. Other results in a pain management setting show that transitioning to ACT as a treatment model can have similar benefits and may increase job satisfaction and staff well-
being [55].
• What are criticisms of ACT?
Many strong supporters of cognitive therapy and more traditional versions of CBT in the field claim that ACT is not new nor better than other current versions of CBT [56]. The proponents of ACT openly acknowledge that many methods used within ACT are adopted or modified from other established therapies [4]. Criticisms are not specific to the application of ACT with chronic pain but are based on others’ perceptions of ACT as a treatment approach and treatment techniques used in ACT in general.
Ost [43] criticised ACT and the third-wave therapies on 2 main grounds. First, he concluded that ACT and the rest of the third-wave therapies were not meeting the criteria of empirically supported treatments. He further concluded that there is no strong evidence to show that ACT is more effective than cognitive therapy. The methods of the Ost review have been challenged [57], yet to a certain degree the points raised are correct. Most of the limitations noted reflect a difference in the maturity of the evidence base for ACT versus traditional CBT-based approaches. Indeed, in comparison to CBT, which is the most empirically established form of psychotherapy and an active area of research for more than 40 years, ACT can be considered to be in its infancy stage of empirically supported treatments, where treatment evidence and availability of high-quality RCTs in general are few at present. Specific research on ACT for chronic pain though supportive is still preliminary to a certain degree. Even so, ACT for pain is regarded as an empirically supported treatment by the body within the American Psychological Association authorized to make this determination [58].
Conclusion
ACT is essentially a form of CBT, considered broadly. ACT brings with it a different philosophy and approach to science compared with some other forms of CBT—this can lead to some distinctive strategies and methods in treatment for chronic pain. Like traditionally designed CBT, however, ACT similarly aims for behavior change as the end point.
ACT is grounded in specific philosophical assumptions and includes the model of psychological flexibility at its core. Preliminary findings in broad clinical and nonclinical populations support the efficacy, effectiveness, and processes in the psychological flexibility model as mediators of change, in ACT [46,59]. Research has shown that most of the 6 ACT processes, all of those so far investigated, correlate with improved daily functioning and emotional well-being in patients with chronic pain. The evidence base for ACT is still developing. Larger trials, more carefully designed trials, and a continued focus on processes of change will be needed to strengthen this base.
Corresponding author: Su-Yin Yang, Health Psychology Section, Psychology Department, Institute of Psychiatry, King’s College London, 5th Fl, Bermondsey Wing, Guy’s Campus, London SE1 9RT, [email protected].
Financial disclosures: None.
Author contributions: conception and design, SY, LMM; analysis and interpretation of data, SY; drafting of article, SY, LMM; critical revision of the article, LMM; administrative or technical support, LMM; collection and assembly of data, SY.
1. Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971–9.
2. Fordyce WE. Behavioral methods for chronic pain and illness. St Louis: Mosby; 1976.
3. Turk DC, Meichenbaum D, Genest M. Pain and behavioral medicine: A cognitive-behavioral perspective. New York: Guildford Press; 1983.
4. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: An experiential approach to behavior change. New York: Guilford Press; 1999.
5. McCracken LM. Contextual cognitive-behavioral therapy for chronic pain. Seattle: IASP Press; 2005.
6. Leventhal H, Brissette I, Leventhal EA. The common-sense model of self-regulation of health and illness. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behaviour. London: Routledge; 2003:42–65.
7. Gatchel RJ, Peng YB, Peters ML, et al. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133:581–624.
8. Kerns RD, Sellinger J, Goodin BR. Psychological treatment of chronic pain. Ann Rev Clin Psychol 2011;7:411–34.
9. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Sys Rev 2012, Issue 11.
10. Vlaeyen JWS, Morley S. Cognitive-behavioral treatments for chronic pain: what works for whom? Clin J Pain 2005;21:1–8.
11. Eccleston C, Williams AC, Morley S. Psychological therapies for the management of chronic pain (excluding headaches) in adults. Cochrane Database Sys Rev 2009, Issue 2.
12. McCracken LM, Samuel VM. The role of avoidance, pacing, and other activity patterns in chronic pain. Pain 2007;130:119–25.
13. Lynch TR, Chapman AL, Rosenthal MZ, et al. Mechanisms of change in dialectical behavior therapy: theoretical and empirical observations. J Clin Psych 2006;62:459–80.
14. Esmer G, Blum J, Rulf J, et al. Mindfulness-based stress reduction for failed back surgery syndrome: a randomized controlled trial. J Am Osteopath Assoc 2010;10:646–52.
15. Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study. Pain 2008;134:310–9.
16. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: the process and practice of mindful change. 2nd ed. New York: Guilford Press; 2012.
17. Hayes SC, Villatte M, Levin M, Hildebrandt M. Open, aware and active: Contextual approaches as an emerging trend in the behavioural and cognitive therapies. Annu Rev Clin Psychol 2011;7:141–68.
18. Hayes S, Luoma J, Bond F, et al. Acceptance and commitment therapy: model processes and outcomes. Behav Res Ther 2006;44:1–25.
19. Gaudiano BA. A review of acceptance and commitment therapy (ACT) and recommendations for continued scientific advancement. Sci Rev Mental Health Prac 2011;8:5–22.
20. Twohig MP. Introduction: the basics of acceptance and commitment therapy. Cog and Behav Pract 2012;19:499–618.
21. McCracken LM, Yang S. The role of values in a contextual cognitive-behavioral approach to chronic pain. Pain 2006;123:137–45.
22. Luoma JB, Hayes SC, Walser RD. Learning ACT: an acceptance and commitment therapy skills-training manual for therapists. Oakland, CA: New Harbinger Pub; 2007.
23. Strosahl K, Robinson P, Gustavsson T. Brief interventions for radical change: principles and practice of focused acceptance and commitment therapy. Oakland, CA: New Harbinger Pub; 2012.
24. McCracken LM, Vowles KE, Zhao-O’Brien J. Further development of an instrument to assess psychological flexibility in people with chronic pain. J Behav Med 2010;33:346–54.
25. McCracken LM, Gutierrez-Martinez O. Processes of change in psychological flexibility in an interdisciplinary group –based treatment for chronic pain based on acceptance and commitment therapy. Behav Res Ther 2011;49:267–74.
26. McCracken LM, Velleman SC. Psychological flexibility in adults with chronic pain: a study of acceptance, mindfulness, and values-based action in primary care. Pain 2010;148:141–7.
27. McCracken LM, Vowles KE, Eccleston C. Acceptance-based treatment for persons with complex, long standing chronic pain: a preliminary analysis of treatment outcome in comparison to a waiting phase. Behav Res Ther 2005;43:1335–46.
28. McCracken LM, MacKichan F, Eccleston C. Contextual cognitive-behavioural therapy for severely disabled chronic pain sufferers: Effectiveness and clinically significant change. Eur J Pain 2007;11:314–22.
29. Wicksell RK, Melin L, Olsson GL. Exposure and acceptance in the rehabilitation of adolescents with idiopathic chronic pain--a pilot study. Eur J Pain 2007;11:779–87.
30. Wicksell RK, Olsson GL, Hayes SC. Psychological flexibility as a mediator of improvement in acceptance and commitment therapy for patients with chronic pain following whiplash. Eur J Pain 2010;14:1059.e1–1059.e11.
31. McCracken LM, Vowles KE, Eccleston C. Acceptance of chronic pain: component analysis and a revised assessment method. Pain 2004;107:159–66.
32. McCracken LM, Eccleston C. Coping or acceptance: what to do about chronic pain? Pain 2003;105:197–204.
33. Vowles KE, McCracken LM. Acceptance and values-based action in chronic pain: a study treatment effectiveness and process. J Consult Clin Psychol 2008;76:397–407.
34. Vowles KE, McCracken LM, Eccleston C. Processes of behaviour change in interdisciplinary treatment of chronic pain: Contributions of pain intensity, catastrophizing, and acceptance. Eur J Pain 2007;11:779–87.
35. Vowles KE, McCracken LM. Comparing the role of psychological flexibility and traditional pain management coping strategies in chronic pain treatment outcomes. Beh Res Ther 2010;48:141–6.
36. Dahl J, Wilson KG, Nilsson A. Acceptance and commitment therapy and the treatment of persons at risk for long-term disability resulting from stress and pain symptoms: a preliminary randomized trial. Behav Ther 2004;35:785–802.
37. Wicksell RK, Ahlqvist J, Bring A, et al. Can exposure and acceptance strategies improve functioning and life satisfaction in people with chronic pain and whiplash-associated disorders (WAD)? A randomized controlled trial. Cog Behav Ther 2009;38:169–82.
38. Wicksell RK, Kemani M, Jensen K, et al. Acceptance and commitment therapy for fibromyalgia: a randomized controlled trial. Eur J Pain 2013;17:599–611.
39. Wetherell JL, Afari N, Rutledge T, et al. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioural therapy for chronic pain. Pain 2011;152:2098–107.
40. Thorsell J, Finnes A, Dahl J, et al. A comparative study of 2 manual-based self-help interventions, acceptance and commitment therapy and applied relaxation for person with chronic pain. Clin J Pain 2011;27:716–23.
41. Buhrman M, Skoglund A, Husell J, et al. Guided internet-delivered acceptance and commitment therapy for chronic pain patients: a randomized controlled trial. Beh Res Ther 2013;51:307–15.
42. McCracken LM, Sato A, Taylor GJ. A trial of a brief group-based form of acceptance and commitment therapy (ACT) for chronic pain in general practice: pilot outcome and process results. J Pain 2013;14:1398–406.
43. Ost L-G. Efficacy of the third wave of behavioral therapies: A systematic review and meta-analysis. Behav Res Ther 2008;46:296–321.
44. Powers MB, Zum Vorde Sive Vording MB, Emmelkamp PMG. Acceptance and commitment therapy: a meta-analytic review. Psychother Psychosom 2009;78:73–80.
45. Veehof MM, Oskam MJ, Schereurs KM, Bohlmeijer ET. Acceptance-based intervention for the treatment of chronic pain: a systematic review and meta-analysis. Pain 2011;152:533–42.
46. Ruiz FJ. Acceptance and commitment therapy versus traditional cognitive behavioral therapy: a systematic review and meta-analysis of current empirical evidence. Int J Psychol Psycholog Ther 2012;12:333–57.
47. Bond FW, Bunce D. The role of acceptance and job control in mental health, job sarsifaction, and work performance. J App Psych 2003;88:1057–67.
48. Gaudiano BA, Herbert JD. Acute treatment of inpatients with psychotic symptoms using acceptance and commitment therapy: pilot results. Behav Res Ther 2006;44:415–37.
49. Twohig MP, Hayes SC, Masuda A. Increasing willingness to experience obsessions: Acceptance and commitment therapy as a treatment for obsessive-compusive disorder. Behav Ther 2006;37:3–13.
50. Zettle RD, Hayes SC. Dysfunctional control by client verbal behaviour. The context of reason giving. Analys Verbal Behav 1986;4:30–8.
51. Forman EM, Herbert JD, Morita E, et al. A randomised controlled effectiveness trial of acceptance and commitment therapy and cognitive therapy for anxiety and depression. Behav Mod 2007;31:772–99.
52. Lappalainen R, Lehtonen T, Skarp E, et al. The impact of CBT and ACT models using psychology trainee therapists: A preliminary controlled effectiveness trial. Behav Modif 2007;31:488–511.
53. Stafford-Brown J, Pakenham KI. The effectiveness of an ACT informed intervention for managing stress and improving therapist qualities in clinical psychology trainees. J Clin Psychol 2012;68:592–613.
54. Pakenhan KI, Stafford-Brown J. Postgraduate clinical psychology students’ perceptions of an acceptance and commitment therapy stress management intervention and clinical training. Clin Psych 2012;17:56–66.
55. Barker E, McCracken LM. From traditional cognitive behavioral therapy to acceptance and commitment therapy for chronic pain: a mixed method study of staff experiences of change. Brit J Pain published online 19 Jul 2013.
56. Hoffmann SG, Asmundson GJ. Acceptance and mindfulness-based therapy: new wave or old hat? Clin Psych Rev 2008;28:1–16.
57. Gaudiano BA. Ost’s (2008) methodological comparison of clinical trials of acceptance and commitment therapy versus cognitive behavior therapy: matching apples with oranges? Behav Res Ther 2009;47:1066–70.
58. Division 12. APA psychological treatments. Niwot, CO: American Psychological Association. Available at www.div12.org/PsychologicalTreatments/treatments.html.
59. Levin ME, Hildebrandt MJ, Lillis J, Hayes SC. The impact of treatment components suggested by the psychological flexibility model: A meta-analysis of laboratory-based component studies. Behav Ther 2012;43:741–56.
Abstract
- Objective: To describe Acceptance and Commitment Therapy (ACT) and its application in the treatment of chronic pain.
- Methods: Review of the theoretical and clinical literature and presentation of a case example.
- Results: General cognitive behavioral approaches for chronic pain have a consistent and large evidence base supporting their benefits. Even so, these treatments continue to develop with the aim to improve. One example of a relatively new development within the cognitive behavioral approaches is ACT, a treatment that focuses on increasing psychological flexibility. Here we describe ACT and the therapeutic model on which it is based, present its distinguishing features, and summarize the evidence for it as a treatment for chronic pain. We also discuss such issues as dissemination, implementation, and training.
- Conclusion: There are now 7 randomized controlled trials, a number of innovative uncontrolled trials, and at least 1 systematic review that support the clinical efficacy and effectiveness of ACT for chronic pain. Further research and development of this approach is underway.
The introduction of the gate control theory of pain [1] in 1965, among other events, signaled a shift in our understanding of pain, particularly chronic pain. This shift, which continues today, is a shift from a predominantly biomedical model of chronic pain to a biospsychosocial model. This model, as the name suggests, includes psycho-social influences in a key role in relation to the experience of pain and the impact of this experience. During this same period of time, psychosocial models and treatment methods have also shifted and evolved. This evolution has included the operant approach [2], the cognitive behavioral approach [3], and the latest developments, contextual cognitive behavioral approaches [4,5], among which Acceptance and Commitment Therapy (ACT) and mindfulness-based therapies are key examples.
Until about 10 years ago, the mainstream of psycho-logical treatments for chronic pain and other physical health problems was dominated almost exclusively by concepts and methods of what we will refer to as “traditional” cognitive behavioral therapy (CBT). Specific constructs within what is called the “common sense model” [6], such as illness perceptions, beliefs about control over one’s illness, amongst other constructs such as self-efficacy, catastrophising, fear avoidance, and pain-related anxiety, captured a substantial focus of research and treatment development during most of the past 3 decades [7]. The treatment methods that have emerged and persisted from this work have included relaxation, attention-based and cognitive coping strategies, cognitive restructuring, the use of imagery, and certain activity management strategies [8]. However, despite consistent supportive evidence for CBT interventions for chronic pain [9], there remain gaps and areas of relative weakness, both in the conceptual models underlying this work and in the base of evidence. Research clearly shows that not all patients benefit from traditional CBT interventions, and recent reviews of CBT for chronic pain generally show effect sizes that are usually small or mediumat best [9–11].
The Problem with Pain
Pain hurts and is often viewed as harmful, and this leads to fear or anxiety, avoidance, or attempts to control the pain. Seeking to control pain is entirely natural and even seems necessary to reduce the undesirable effects of pain in one’s life.
Dependent on the situation, pain avoidance, sometimes also referred to as “fear avoidance,” in studies of chronic pain can present itself in many forms. Avoidance behavior can include refusal to engage in any activity believed to cause an increase in pain. It may also include “guarding” or bracing around an area of pain, information seeking, treatment seeking, taking medications, overdosing on medications, using aids like heat or ice, withdrawing from social activity, as well as being unwilling to talk about emotional experiences, amongst others [5]. Today, avoidance is recognized as a key foundation element in pain-related suffering and disability [12], and addressing it effectively has become a prime focus in many or most current treatments.
Acceptance and Psychological Flexibility
In recent years, the concept of “acceptance” has gained prominence as a potentially important process for addressing a broad array of psychological problems, including those associated with chronic pain. From this new interest, a fundamentally different treatment emphasis has emerged. This includes a shift away from a predominant focus on changing thoughts and feelings, a focus sometimes adopted within some traditional CBT methods, towards a focus on reducing the influence of thoughts and feelings on our actions instead. This can be a rather confusing distinction. This is because the influence of our thoughts and feelings is often automatic and even invisible to us as it occurs. As such, the influences of our thoughts and feelings appear directly tied to the content of thoughts and feelings, but the matter is not that simple. Clearly there are occasions when our actions contradict our thoughts and feelings, such as when we have perfectly confident beliefs and fail, or significant anxiety and perform successfully. Such instances illustrate what we might call a “2-dimensional” quality of experience; it is the content of experience and the context of experience that determine the influence exerted. Suffice it to say acceptance-based methods are designed to address the difference between experiences that are difficult to control, such as thoughts and feelings, and things that are easier to control: the actions we take in relation to our thoughts and feelings. They do this by taking a focus on creating changes in context and ultimately in behavior. Acceptance includes especially a focus on allowing or opening up to feelings rather than struggling with them or retreating from them. Here, the capacity for openness is a contextual process.
Acceptance methods are not used in isolation. They are usually used in combination with other traditional behavior change strategies, with methods to facilitate values clarification, committed action, and other methods from ACT. Notions of acceptance have even been incorporated into many behavioral and cognitive therapies before, including dialectical behavior therapy [13] and mindfulness-based treatment [14,15], and so this process is not the exclusive domain of ACT. In implementing acceptance-based methods, patients are taught skills, such as to (a) notice feelings specifically in detail, (b) notice that thoughts about pain are products of thinking and not the same as direct experience, (c) notice urges to struggle with thoughts and feelings, (d) to practice refraining from struggling and adopt an observing, allowing, and “making room”–type posture, and (e) take action in line with their goals [4,5].
The wider processes around acceptance in combination are referred to as psychological flexibility [16]. Psychological flexibility relates to one’s ability to directly contact the present moment; to be aware of the thoughts, feelings and potentially unwanted internal experiences it brings; and to follow through with a behavior change or persist with a chosen behavior in the direction of chosen values. Psychological flexibility is the model for psychological health from an ACT perspective [17].
Psychological Flexibility and the 6 Core ACT Processes
Acceptance
Acceptance involves the patients’ willingness to have pain while remaining able to actively choose to continue participating in their life as they want it to be. ACT encour-ages patients to act in ways that are consistent with direct experiences rather than what the mind interprets these events to mean.
Cognitive Defusion
Cognitive defusion is the process of modifying one’s reaction to thoughts by constructing contexts where the influences of these thoughts on behavior are lessened [18]. Unlike traditional cognitive behavior approaches, in ACT it is not the content and actual validity of these thoughts that is challenged but the functions, or influences, of thoughts [19].
Present Moment Awareness
Contact with the present moment reflects the process wherein the person is aware of the situation in “the now” as opposed to focusing on events that happened in the past or might happen in the future [18]. To be “present” requires the individual to flexibly focus attention on experiences as they are happening in the environment, in real time, and to be fully open to what is taking place [20]. It is important the individual is able to notice when he or she is not acting in relation to the present moment and has the ability to shift attention to the present if this shift benefits them.
Self as Context
The sense of self-as-context or self-as-observer is considered the ability to adopt a perspective or point of view that is separate from and not defined by thoughts and feelings or even the physical body. This contrasts a sense of self as made up of personality characteristics, self-evaluations, or a narrative about who we are [5,16]. In ACT, perspective taking can be trained to help people connect with the experience of a distinction between self and psychological experiences. From this, one can choose to follow one’s inner verbal constructions of what defines us, our “stories” of who we are, in certain situations when it works to do so, and not in situations where it leads to unhealthy responses and behavioral restriction.
Values
Values are defined as guiding principles in one’s life. Values are often contrasted with goals, where the difference is that goals can be achieved while values are part of an ongoing process of action and cannot be completed once and for all. In a sense, goals represent set plans of action to be achieved while values are general life directions. If life is like a journey, then goals would be the chosen destination and values would simply be represented by a general direction of travel. Values are helpful when patients struggle with unwanted internal experiences like pain, as they not only serve as a guide for the client to persist in behavior change but also function as a motivating element. Values clarification exercise in therapy encourages the patient to define their values in specific domains of “career, family, intimate relationships, friendships, health, education and spirituality” [4,21] regardless of the primary problem. Personally chosen and clarified values can function as guides when people have difficulty initiating and maintaining behavior change in the presence of unwanted internal experiences.
Committed Action
Committed action is an ongoing process of redirecting behavior in order to create patterns of flexible and effective action in line with a defined value [22]. Patients are encouraged to follow through with their chosen actions that are in line with their values, and to persist or alter their course flexibly. Without the capacity for committed action, behavior change is less likely to persist and integrate into patterns of behavior more generally.
Case Study
Initial Presentation and History
Ms X, a 45-year-old woman, presents with the chief complaint of low back pain, which she has experienced for 3 years. She works part-time due to her pain problem. When she is not at work, she busies herself with seeking both conventional and alternative treatments for her pain condition. In the past, during periods where she experienced pain relief, she attempted to engage in her hobby of photography. However, this often led to a pain flare the next day and required 2 to 3 days of medical leave with increased medication from her PCP before she is able to return to work. As a result, Ms X chose to give up her hobby and focus on treating her pain instead. Ms X in in a constant struggle with her pain condition and believes that she can only return to photography, and live a more normal life, after her pain is cured.
• What are considerations for applying ACT in this scenario?
From an ACT conceptualization this case shows patterns of avoidance that are apparently not helping the person to reach her goals but are causing her distress and restrictions in functioning. An ACT therapist would approach this scenario by first reflecting how normal it is to struggle with pain and stop activities when in pain. From there they might (a) identify what the patient wants from treatment, (b) look at what has been done so far to attain this, (c) examine how well those things have been working, (d) consider the costs of the approach being taken, and (e) if the approach is not working and the cost is high, see if the patient is willing to stop this approach [23].
Therapist’s Initial Approach
Therapist: By what you have told me, your pain has become a big problem for you and it has been going on a long time—3 years. I can see some of the impacts it has had in your life, such as on your work, your photography, and time spent seeking treatment.
Ms X: Yes, it seems like pain has taken over …
Therapist: Exactly, it seems that is a good way to say it. So, understanding that pain has taken over, can I ask you another question?
Ms X: If your question will help me get over this problem, of course.
Therapist: Ok. What is it you want from coming here to participate in this treatment?
Ms X: Well, I want to get rid of this pain, obviously. It’s ruining my life.
Therapist: Ah, that makes sense. You want to eliminate your pain because it has, as you say, ruined your life, and then I guess your life will be better again.
Ms X: Correct.
Therapist: So, can I check in with the things you have been doing so far to reach this goal to eliminate pain?
Ms X: You name it, I’ve tried it: acupuncture, medication, herbs, rest, exercise, magnets, yoga, and more.
Therapist: Ok, you have tried many treatments focused on trying to get rid of the pain. I think that’s a very natural thing to do. In your experience have these methods been successful?
Ms X: Well, some of them seem to work at the time but it all becomes very confusing, because here I am looking for another treatment. It can feel good to get away from the pain for a little while, but soon I will experience a pain flare bringing me back to square one.
Therapist: I see what you are saying. Let me ask my earlier question in a different way. What would your life look like, and what would you be doing, if your pain were not the problem it is today?
Ms X: I would be taking pictures again, be more consistent at work, and spend less time seeking treatments.
Therapist: So, is it your experience that the methods you have been using have helped you to live life this way?
Ms X: … I never thought about it that way ...
• What exercises or techniques are used in ACT?
In practice, ACT is somewhat unique in that it often relies on the use of metaphors and experiential exercises in treatment delivery. Metaphors and stories are used in treatment and communicated in terms that fit with the experience and background of the person seeking treatment. Although therapists can select from among many widely used and often appropriate metaphors and stories, an experienced therapist is likely to create patient specific metaphors “live,” within the context of a particular session. This is consistent with the philosophical underpinning of ACT in its aims for individual tailoring of methods. Unlike other current psychotherapeutic approaches that place a higher value on sticking to a specified protocol, the theory and philosophy behind ACT allow for flexibility and are open to creativity, individual style, and situational sensitivity of the therapist. This is expected to allow the patient to also adopt a similar sensitivity to changing environmental contingencies [19]. In ACT, the techniques typically do not follow a cookbook style of treatment delivery.
Case Continued
Therapist: What if trying to control your thoughts and feelings were not the answer?
Ms X: I have no idea what you mean.
Therapist: Well, you certainly have focused a lot of your effort on trying not to have the thoughts and feelings that seem to block you.
Ms X: What else is there to do, really?
Therapist: If you are willing to experiment with something, try this. Don’t think of a pineapple. (pause for 30 to 60 seconds). Ok, what happens.
Ms X: It didn’t work—I kept thinking about a pineapple.
Therapist: Weird, huh? Notice what is happening here. I wonder if some of your struggles with your experiences are just like this. It’s like by trying to get rid of something, there it is! I wonder if there were another way to do this, do you think you might be willing to test it out?
Ms X: Yes, I can try.
Further ACT Methods
ACT includes numerous experience-based methods and also direct rehearsal of targeted skills. In the previous scen-ario, the therapist might then proceed to instruction and practice of one or another type of acceptance-based skill, something like an “exposure” session or a mindfulness type of exercise that includes having the participant sit with the experience without doing anything else but observe it. The other type of method used includes metaphors that reveal how circumstances and behavior often work in life [4,16].
An Acceptance-Based Metaphor
Therapist: Imagine that you are new to the neighborhood and you invited all your neighbors over to a housewarming party. Everyone in the neighborhood is invited. On that day, the party’s going great, and here comes Joe, who smells and looks like he has not bathed in days. You are embarrassed by the way he looks and smells and try to close the door on him. However, he shows you a flyer that you put up stating that everyone in the neighborhood is invited. So you let him in and quickly shove him to the kitchen so that he will not embarrass you and disrupt your party. However, to stop him from leaving the kitchen, you end up having to stand guard at the doorway. Meanwhile the party is going on and your guests are enjoying themselves, but do you notice what else is happening here?
Ms X: I’ve stopped myself from enjoying my party in order to keep Joe away.
Therapist: What if your pain was like Joe?
Ms X: Huh? … Ah, I think I see what you are saying…
Therapist: It’s like if you allow Joe to simply be another guest, you can do whatever you like at your party. On the other hand, if you say “no” to Joe you also say “no” to the party.
Ms X: Are you saying that it is for me to choose?
• What is the role of therapist in modeling behavior change in ACT?
An important distinction can be made between talking about behavior change and doing behavior change. Within the psychological flexibility model the emphasis is placed on the latter. Here, especially through the use of experiential exercises, clients are put into contact with the experiences that have coordinated unhealthy behavior patterns in the past so that more effective behavior patterns can be acquired. Treatment delivery is guided by the underlying behavioral philosophy and theory. Patients learn to reduce the dominant influence of the literal meaning of language as the only tool for behavior change. Direct experience is moved to the front of awareness and literal meaning, mental and verbal analysis, and so forth, are moved to the back [20]. In treatment, the therapist models for the patient the behavior change processes that are being targeted and also may use examples from his or her life as well as that of the patient’s to develop psychological flexibility [22]. An example might include a therapist’s response to a person who shows an experience of emotional distress and struggling to manage this distress. Here the therapist, in line with ACT, instead of acting in some way to attempt to lessen the distress, would consciously show openness to the experiences and to their own reactions to helplessness around these experiences.
The therapist might say:
“I would feel tired and probably in pain too if I did what you just did. Could we do a little closed-eyes exercise? Shall we put the distressing thought you are having on the table, and focus on it, and we can “observe” what your mind does, and what happens in your body and your emotions when that thought shows up? Are you willing?”
“I’m feeling confused about this issue myself - how about both of us sitting quietly for a moment or two and observe what our minds do in response to this, just slowing things down, and watching?”
“I feel anxious when I believe that my thoughts about pain are true - like I have to do something to make it go away but I don’t know how. What shows up for you when you believe such thoughts about pain?”
• How and when should ACT be used?
Based on current evidence how and when ACT ought to be used, as opposed to other treatment options, will be largely up to the individual professional and their level of competence. ACT is a form of CBT and many of the same guides pertain. In line with the pragmatic approach of ACT, an approach that makes ACT broadly applicable, there is no one particular manualised or scripted treatment protocol that must be adhered to in treatment for one specific condition or another. As mentioned earlier, the ACT approach does not usually follow a cookbook style of delivery, nor is it rigidly guided by strict protocols. There are protocols shared by researchers to support further development but there is no process by which these are deemed “official” or “recognized” or approved by anyone in particular.
A wide range of metaphors and exercises based on a set of behavioral principles that target a particular function has been proposed in ACT and this is part of its uniqueness as a therapeutic model.
Those developing ACT also have not required a standardised certification process to delivering ACT. Instead, they have chosen to create an open community of contributing researchers and clinicians who are “members” by virtue of their commitment to the same approach to clinical development and the same clinical model. Practicing ACT requires that the clinician is aware of their own competencies and delivers treatment accordingly.
• How effective is ACT?
Numerous studies have supported a general role of psychological flexibility in improving the well-being and physical functioning of patients with chronic pain, including patients in specialty care [24,28] and primary care [26]. Many studies support the particular role of acceptance of pain in adjustment to chronic pain [27–30]. Pain acceptance is a better predictor of outcomes than pain severity itself [31,32].
There are now several relatively large-scale studies conducted in actual clinical practice settings that demonstrate the effectiveness of ACT for chronic pain [25,27,33,34]. A more recent study, also conducted in an actual clinical practice setting, provided support for the specific treatment processes proposed within this approach [35]. This study showed that changes in traditionally conceived methods of pain management were unrelated to treatment improvements of pain intensity, physical disability, anxiety and depression for those who participated in treatment, while changes in psychological flexibility were consistently and significantly related to these improvements, with the exception of the results for depression.
Randomised Controlled Trials (RCTs)
To date, there are a total of 7 RCTs related to ACT and chronic pain [36–42], each providing supportive evidence. For example, in one of the early studies, Dahl and colleagues [36] showed that in comparison to treatment as usual, a group of workers who were at risk of long-term absenteeism from work due to pain or stress had a significant reduction in sick leave and healthcare usage after attending four hours of ACT sessions.
Wicksell and colleagues [37,38] conducted 2 separate RCTs with participants who suffered whiplash-associated disorder (WAD) and fibromyalgia, respectively. Post-treatment results of both RCTs showed an improvement in physical functioning, depression and psychological flexibility in the treatment group with gains maintained at follow-up. In addition, participants in the treatment group with WAD showed an improvement in life satisfaction and fear of movement while those in the treatment group with fibromyalgia showed significant improvements in fibromyalgia impact, self-efficacy and anxiety. There was however no change in pain intensity in those who received the ACT-based treatments.
An ACT-based treatment including a self-help manual showed a significant increase in acceptance, satisfaction in life with a higher level of function and decreased pain intensity compared with a wait-list condition and with applied relaxation (AR) [40]. In comparison to the AR condition, participants in the ACT condition also reported a significantly higher level of engagement in meaningful activities and a willingness to experience pain. Follow-up data support the maintenance of these improvements at first follow-up but differences were not significant at the second follow-up. Both depression and anxiety scores improved in both treatment groups.
Wetherell and colleagues [39] compared the effectiveness of ACT and traditional CBT and found that they both produced positive results. Results from the study also showed higher satisfaction in participants who attended ACT treatment than those that attended CBT treatment, suggesting that ACT “is an effective and acceptable” intervention for patients with chronic pain. Overall acceptance of pain was shown to differentiate patients who could function well with chronic pain from those that continued to suffer with it after treatment.
More recently the first internet-based RCT for ACT with chronic pain was conducted [41]. The authors found a reduction in measures of pain-related distress, depressive symptoms, and anxiety, with these gains maintained at 6 months follow-up in the ACT treatment group compared with controls. The most recent RCT was a pilot trial of a group-based treatment of people with chronic pain recruited from general practices in the UK [42]. Participants were randomised to either an ACT-based treatment or treatment as usual. Participants in the ACT-based group underwent 4 sessions each lasting 4 hours with the first 3 sessions completed in 1 week and the last session completed a week later. At 3 months follow-up, participants in the ACT group had lower disability, depression, and higher pain acceptance.
In general, results from the ACT-based RCTs on chronic pain support the efficacy of the treatment and reflect a high degree of versatility, based on the wide variety of modes of delivery tested. However, RCTs for chronic pain are still relatively few with some studies limited to small sample sizes, thus making it difficult to reach definitive conclusions on the general efficacy of ACT in chronic pain treatment. What the studies do seem to show is that ACT is a good alternative treatment option to more traditionally conceived current CBT-based treatments for chronic pain. Larger sample sizes and higher quality studies are needed to strengthen and establish the effectiveness of ACT and to understand the potential impact of wider implementation in clinical practice.
Meta-Analyses
A total of 4 meta-analyses [43–46] have been conducted on acceptance- or ACT-based treatment studies. Although the earlier meta-analyses [43,44] did not separately report the effectiveness of ACT for chronic pain, they reported a moderate effect size for ACT in general, with no evidence that ACT is more effective than established treatments.
Ruiz [46] conducted a review focusing on outcome or mediation/moderation type studies that compared ACT and CBT treatments. His review was not specific to chronic pain, although one study [39] involving a sample of chronic pain patients was included. Moderate effect sizes were found that favored ACT, with ACT showing a greater impact on change processes (g = 0.38) compared to no impact found in CBT (g = 0.05).
Essentially, only one meta-analysis [45] specifically reviewed the efficacy in chronic pain studies. Pain inten-sity and depression were selected as primary outcome measures, with anxiety, physical well-being, and quality of life selected as secondary outcomes. Out of 22 studies that were included in the review, only 2 studies [36,37] were ACT-based RCTs, with the rest of the studies mindfulness-based interventions. The overall effect size of 0.37 was found for pain and 0.32 for depression. In general, results showed significant effect sizes for both primary and secondary outcome measures in favor of the “acceptance-based treatments.” The authors concluded that at present, mindfulness-based stress reduction programs and ACT-based programs may not be superior to CBT but could be good alternatives for people with chronic pain.
The appropriateness of using pain intensity as a primary outcome measure for ACT-based studies is questionable [45]. The focus of ACT is to increase function rather than to reduce pain symptoms; hence possibly including interference of pain in daily life might be a more appropriate outcome measure.
Other Studies
A particularly important question to answer about ACT concerns its cost-effectivness, and we still know relatively little about this. We do know that when people participate in ACT-based treatments they are able to reduce medication use and health care visits and return to work after extended periods away from work [27,28]. It remains to conduct full health economic analyses of this type of approach for chronic pain.
ACT is known to produce significant benefits widely, in other applications apart from chronic pain, such as in workplace stress [47], psychosis [48], obsessive-compulsive disorder [49], and depression [50], among other mental health conditions [51].
• What are implications for policy makers?
Results from studies of ACT in chronic pain and in other areas are disseminating rapidly. This dissemination is aided in part by a professional organization devoted to ACT and psychological flexibility (Association for Contextual Behavioral Science; www.contextualscience.org), which has a new journal, the Journal of Contextual Behavioral Science, started in 2012.
With the development of ACT a focus on implementation, training, and treatment integrity began early. There was an implementation study of ACT was published by Strosahl and colleagues in 1998. Their study showed that training clinicians in ACT produced better outcomes and better treatment completion rates in an outpatient setting in comparison to clinicians not receiving this training.
Processes of training have also appeared during relatively early phases of research into ACT. Lappalainen and colleagues [52] compared the impact of treatment provided by trainee therapists trained in both a traditional CBT model and ACT. Here each trainee therapist treated one patient with traditional CBT and one with ACT. Although the therapists reported higher confidence in delivering traditional CBT, patients treated within an ACT model showed better symptoms improvement. Also, improved acceptance during treatment significantly predicted improvements across both groups of patients. Essentially, therapists with only a limited amount of training in both models demonstrated better clinical results with ACT.
A group-based ACT intervention has also been shown to be effective in reducing stress and improving the professional performance of clinical psychology trainees [53]. Here the trainees found the intervention personally and professionally useful and a majority showed a significant increase in psychological flexibility. This supports the applicability of ACT not only as a model to guide therapy but also as a model to guide training and professional performance [54]. Other results in a pain management setting show that transitioning to ACT as a treatment model can have similar benefits and may increase job satisfaction and staff well-
being [55].
• What are criticisms of ACT?
Many strong supporters of cognitive therapy and more traditional versions of CBT in the field claim that ACT is not new nor better than other current versions of CBT [56]. The proponents of ACT openly acknowledge that many methods used within ACT are adopted or modified from other established therapies [4]. Criticisms are not specific to the application of ACT with chronic pain but are based on others’ perceptions of ACT as a treatment approach and treatment techniques used in ACT in general.
Ost [43] criticised ACT and the third-wave therapies on 2 main grounds. First, he concluded that ACT and the rest of the third-wave therapies were not meeting the criteria of empirically supported treatments. He further concluded that there is no strong evidence to show that ACT is more effective than cognitive therapy. The methods of the Ost review have been challenged [57], yet to a certain degree the points raised are correct. Most of the limitations noted reflect a difference in the maturity of the evidence base for ACT versus traditional CBT-based approaches. Indeed, in comparison to CBT, which is the most empirically established form of psychotherapy and an active area of research for more than 40 years, ACT can be considered to be in its infancy stage of empirically supported treatments, where treatment evidence and availability of high-quality RCTs in general are few at present. Specific research on ACT for chronic pain though supportive is still preliminary to a certain degree. Even so, ACT for pain is regarded as an empirically supported treatment by the body within the American Psychological Association authorized to make this determination [58].
Conclusion
ACT is essentially a form of CBT, considered broadly. ACT brings with it a different philosophy and approach to science compared with some other forms of CBT—this can lead to some distinctive strategies and methods in treatment for chronic pain. Like traditionally designed CBT, however, ACT similarly aims for behavior change as the end point.
ACT is grounded in specific philosophical assumptions and includes the model of psychological flexibility at its core. Preliminary findings in broad clinical and nonclinical populations support the efficacy, effectiveness, and processes in the psychological flexibility model as mediators of change, in ACT [46,59]. Research has shown that most of the 6 ACT processes, all of those so far investigated, correlate with improved daily functioning and emotional well-being in patients with chronic pain. The evidence base for ACT is still developing. Larger trials, more carefully designed trials, and a continued focus on processes of change will be needed to strengthen this base.
Corresponding author: Su-Yin Yang, Health Psychology Section, Psychology Department, Institute of Psychiatry, King’s College London, 5th Fl, Bermondsey Wing, Guy’s Campus, London SE1 9RT, [email protected].
Financial disclosures: None.
Author contributions: conception and design, SY, LMM; analysis and interpretation of data, SY; drafting of article, SY, LMM; critical revision of the article, LMM; administrative or technical support, LMM; collection and assembly of data, SY.
Abstract
- Objective: To describe Acceptance and Commitment Therapy (ACT) and its application in the treatment of chronic pain.
- Methods: Review of the theoretical and clinical literature and presentation of a case example.
- Results: General cognitive behavioral approaches for chronic pain have a consistent and large evidence base supporting their benefits. Even so, these treatments continue to develop with the aim to improve. One example of a relatively new development within the cognitive behavioral approaches is ACT, a treatment that focuses on increasing psychological flexibility. Here we describe ACT and the therapeutic model on which it is based, present its distinguishing features, and summarize the evidence for it as a treatment for chronic pain. We also discuss such issues as dissemination, implementation, and training.
- Conclusion: There are now 7 randomized controlled trials, a number of innovative uncontrolled trials, and at least 1 systematic review that support the clinical efficacy and effectiveness of ACT for chronic pain. Further research and development of this approach is underway.
The introduction of the gate control theory of pain [1] in 1965, among other events, signaled a shift in our understanding of pain, particularly chronic pain. This shift, which continues today, is a shift from a predominantly biomedical model of chronic pain to a biospsychosocial model. This model, as the name suggests, includes psycho-social influences in a key role in relation to the experience of pain and the impact of this experience. During this same period of time, psychosocial models and treatment methods have also shifted and evolved. This evolution has included the operant approach [2], the cognitive behavioral approach [3], and the latest developments, contextual cognitive behavioral approaches [4,5], among which Acceptance and Commitment Therapy (ACT) and mindfulness-based therapies are key examples.
Until about 10 years ago, the mainstream of psycho-logical treatments for chronic pain and other physical health problems was dominated almost exclusively by concepts and methods of what we will refer to as “traditional” cognitive behavioral therapy (CBT). Specific constructs within what is called the “common sense model” [6], such as illness perceptions, beliefs about control over one’s illness, amongst other constructs such as self-efficacy, catastrophising, fear avoidance, and pain-related anxiety, captured a substantial focus of research and treatment development during most of the past 3 decades [7]. The treatment methods that have emerged and persisted from this work have included relaxation, attention-based and cognitive coping strategies, cognitive restructuring, the use of imagery, and certain activity management strategies [8]. However, despite consistent supportive evidence for CBT interventions for chronic pain [9], there remain gaps and areas of relative weakness, both in the conceptual models underlying this work and in the base of evidence. Research clearly shows that not all patients benefit from traditional CBT interventions, and recent reviews of CBT for chronic pain generally show effect sizes that are usually small or mediumat best [9–11].
The Problem with Pain
Pain hurts and is often viewed as harmful, and this leads to fear or anxiety, avoidance, or attempts to control the pain. Seeking to control pain is entirely natural and even seems necessary to reduce the undesirable effects of pain in one’s life.
Dependent on the situation, pain avoidance, sometimes also referred to as “fear avoidance,” in studies of chronic pain can present itself in many forms. Avoidance behavior can include refusal to engage in any activity believed to cause an increase in pain. It may also include “guarding” or bracing around an area of pain, information seeking, treatment seeking, taking medications, overdosing on medications, using aids like heat or ice, withdrawing from social activity, as well as being unwilling to talk about emotional experiences, amongst others [5]. Today, avoidance is recognized as a key foundation element in pain-related suffering and disability [12], and addressing it effectively has become a prime focus in many or most current treatments.
Acceptance and Psychological Flexibility
In recent years, the concept of “acceptance” has gained prominence as a potentially important process for addressing a broad array of psychological problems, including those associated with chronic pain. From this new interest, a fundamentally different treatment emphasis has emerged. This includes a shift away from a predominant focus on changing thoughts and feelings, a focus sometimes adopted within some traditional CBT methods, towards a focus on reducing the influence of thoughts and feelings on our actions instead. This can be a rather confusing distinction. This is because the influence of our thoughts and feelings is often automatic and even invisible to us as it occurs. As such, the influences of our thoughts and feelings appear directly tied to the content of thoughts and feelings, but the matter is not that simple. Clearly there are occasions when our actions contradict our thoughts and feelings, such as when we have perfectly confident beliefs and fail, or significant anxiety and perform successfully. Such instances illustrate what we might call a “2-dimensional” quality of experience; it is the content of experience and the context of experience that determine the influence exerted. Suffice it to say acceptance-based methods are designed to address the difference between experiences that are difficult to control, such as thoughts and feelings, and things that are easier to control: the actions we take in relation to our thoughts and feelings. They do this by taking a focus on creating changes in context and ultimately in behavior. Acceptance includes especially a focus on allowing or opening up to feelings rather than struggling with them or retreating from them. Here, the capacity for openness is a contextual process.
Acceptance methods are not used in isolation. They are usually used in combination with other traditional behavior change strategies, with methods to facilitate values clarification, committed action, and other methods from ACT. Notions of acceptance have even been incorporated into many behavioral and cognitive therapies before, including dialectical behavior therapy [13] and mindfulness-based treatment [14,15], and so this process is not the exclusive domain of ACT. In implementing acceptance-based methods, patients are taught skills, such as to (a) notice feelings specifically in detail, (b) notice that thoughts about pain are products of thinking and not the same as direct experience, (c) notice urges to struggle with thoughts and feelings, (d) to practice refraining from struggling and adopt an observing, allowing, and “making room”–type posture, and (e) take action in line with their goals [4,5].
The wider processes around acceptance in combination are referred to as psychological flexibility [16]. Psychological flexibility relates to one’s ability to directly contact the present moment; to be aware of the thoughts, feelings and potentially unwanted internal experiences it brings; and to follow through with a behavior change or persist with a chosen behavior in the direction of chosen values. Psychological flexibility is the model for psychological health from an ACT perspective [17].
Psychological Flexibility and the 6 Core ACT Processes
Acceptance
Acceptance involves the patients’ willingness to have pain while remaining able to actively choose to continue participating in their life as they want it to be. ACT encour-ages patients to act in ways that are consistent with direct experiences rather than what the mind interprets these events to mean.
Cognitive Defusion
Cognitive defusion is the process of modifying one’s reaction to thoughts by constructing contexts where the influences of these thoughts on behavior are lessened [18]. Unlike traditional cognitive behavior approaches, in ACT it is not the content and actual validity of these thoughts that is challenged but the functions, or influences, of thoughts [19].
Present Moment Awareness
Contact with the present moment reflects the process wherein the person is aware of the situation in “the now” as opposed to focusing on events that happened in the past or might happen in the future [18]. To be “present” requires the individual to flexibly focus attention on experiences as they are happening in the environment, in real time, and to be fully open to what is taking place [20]. It is important the individual is able to notice when he or she is not acting in relation to the present moment and has the ability to shift attention to the present if this shift benefits them.
Self as Context
The sense of self-as-context or self-as-observer is considered the ability to adopt a perspective or point of view that is separate from and not defined by thoughts and feelings or even the physical body. This contrasts a sense of self as made up of personality characteristics, self-evaluations, or a narrative about who we are [5,16]. In ACT, perspective taking can be trained to help people connect with the experience of a distinction between self and psychological experiences. From this, one can choose to follow one’s inner verbal constructions of what defines us, our “stories” of who we are, in certain situations when it works to do so, and not in situations where it leads to unhealthy responses and behavioral restriction.
Values
Values are defined as guiding principles in one’s life. Values are often contrasted with goals, where the difference is that goals can be achieved while values are part of an ongoing process of action and cannot be completed once and for all. In a sense, goals represent set plans of action to be achieved while values are general life directions. If life is like a journey, then goals would be the chosen destination and values would simply be represented by a general direction of travel. Values are helpful when patients struggle with unwanted internal experiences like pain, as they not only serve as a guide for the client to persist in behavior change but also function as a motivating element. Values clarification exercise in therapy encourages the patient to define their values in specific domains of “career, family, intimate relationships, friendships, health, education and spirituality” [4,21] regardless of the primary problem. Personally chosen and clarified values can function as guides when people have difficulty initiating and maintaining behavior change in the presence of unwanted internal experiences.
Committed Action
Committed action is an ongoing process of redirecting behavior in order to create patterns of flexible and effective action in line with a defined value [22]. Patients are encouraged to follow through with their chosen actions that are in line with their values, and to persist or alter their course flexibly. Without the capacity for committed action, behavior change is less likely to persist and integrate into patterns of behavior more generally.
Case Study
Initial Presentation and History
Ms X, a 45-year-old woman, presents with the chief complaint of low back pain, which she has experienced for 3 years. She works part-time due to her pain problem. When she is not at work, she busies herself with seeking both conventional and alternative treatments for her pain condition. In the past, during periods where she experienced pain relief, she attempted to engage in her hobby of photography. However, this often led to a pain flare the next day and required 2 to 3 days of medical leave with increased medication from her PCP before she is able to return to work. As a result, Ms X chose to give up her hobby and focus on treating her pain instead. Ms X in in a constant struggle with her pain condition and believes that she can only return to photography, and live a more normal life, after her pain is cured.
• What are considerations for applying ACT in this scenario?
From an ACT conceptualization this case shows patterns of avoidance that are apparently not helping the person to reach her goals but are causing her distress and restrictions in functioning. An ACT therapist would approach this scenario by first reflecting how normal it is to struggle with pain and stop activities when in pain. From there they might (a) identify what the patient wants from treatment, (b) look at what has been done so far to attain this, (c) examine how well those things have been working, (d) consider the costs of the approach being taken, and (e) if the approach is not working and the cost is high, see if the patient is willing to stop this approach [23].
Therapist’s Initial Approach
Therapist: By what you have told me, your pain has become a big problem for you and it has been going on a long time—3 years. I can see some of the impacts it has had in your life, such as on your work, your photography, and time spent seeking treatment.
Ms X: Yes, it seems like pain has taken over …
Therapist: Exactly, it seems that is a good way to say it. So, understanding that pain has taken over, can I ask you another question?
Ms X: If your question will help me get over this problem, of course.
Therapist: Ok. What is it you want from coming here to participate in this treatment?
Ms X: Well, I want to get rid of this pain, obviously. It’s ruining my life.
Therapist: Ah, that makes sense. You want to eliminate your pain because it has, as you say, ruined your life, and then I guess your life will be better again.
Ms X: Correct.
Therapist: So, can I check in with the things you have been doing so far to reach this goal to eliminate pain?
Ms X: You name it, I’ve tried it: acupuncture, medication, herbs, rest, exercise, magnets, yoga, and more.
Therapist: Ok, you have tried many treatments focused on trying to get rid of the pain. I think that’s a very natural thing to do. In your experience have these methods been successful?
Ms X: Well, some of them seem to work at the time but it all becomes very confusing, because here I am looking for another treatment. It can feel good to get away from the pain for a little while, but soon I will experience a pain flare bringing me back to square one.
Therapist: I see what you are saying. Let me ask my earlier question in a different way. What would your life look like, and what would you be doing, if your pain were not the problem it is today?
Ms X: I would be taking pictures again, be more consistent at work, and spend less time seeking treatments.
Therapist: So, is it your experience that the methods you have been using have helped you to live life this way?
Ms X: … I never thought about it that way ...
• What exercises or techniques are used in ACT?
In practice, ACT is somewhat unique in that it often relies on the use of metaphors and experiential exercises in treatment delivery. Metaphors and stories are used in treatment and communicated in terms that fit with the experience and background of the person seeking treatment. Although therapists can select from among many widely used and often appropriate metaphors and stories, an experienced therapist is likely to create patient specific metaphors “live,” within the context of a particular session. This is consistent with the philosophical underpinning of ACT in its aims for individual tailoring of methods. Unlike other current psychotherapeutic approaches that place a higher value on sticking to a specified protocol, the theory and philosophy behind ACT allow for flexibility and are open to creativity, individual style, and situational sensitivity of the therapist. This is expected to allow the patient to also adopt a similar sensitivity to changing environmental contingencies [19]. In ACT, the techniques typically do not follow a cookbook style of treatment delivery.
Case Continued
Therapist: What if trying to control your thoughts and feelings were not the answer?
Ms X: I have no idea what you mean.
Therapist: Well, you certainly have focused a lot of your effort on trying not to have the thoughts and feelings that seem to block you.
Ms X: What else is there to do, really?
Therapist: If you are willing to experiment with something, try this. Don’t think of a pineapple. (pause for 30 to 60 seconds). Ok, what happens.
Ms X: It didn’t work—I kept thinking about a pineapple.
Therapist: Weird, huh? Notice what is happening here. I wonder if some of your struggles with your experiences are just like this. It’s like by trying to get rid of something, there it is! I wonder if there were another way to do this, do you think you might be willing to test it out?
Ms X: Yes, I can try.
Further ACT Methods
ACT includes numerous experience-based methods and also direct rehearsal of targeted skills. In the previous scen-ario, the therapist might then proceed to instruction and practice of one or another type of acceptance-based skill, something like an “exposure” session or a mindfulness type of exercise that includes having the participant sit with the experience without doing anything else but observe it. The other type of method used includes metaphors that reveal how circumstances and behavior often work in life [4,16].
An Acceptance-Based Metaphor
Therapist: Imagine that you are new to the neighborhood and you invited all your neighbors over to a housewarming party. Everyone in the neighborhood is invited. On that day, the party’s going great, and here comes Joe, who smells and looks like he has not bathed in days. You are embarrassed by the way he looks and smells and try to close the door on him. However, he shows you a flyer that you put up stating that everyone in the neighborhood is invited. So you let him in and quickly shove him to the kitchen so that he will not embarrass you and disrupt your party. However, to stop him from leaving the kitchen, you end up having to stand guard at the doorway. Meanwhile the party is going on and your guests are enjoying themselves, but do you notice what else is happening here?
Ms X: I’ve stopped myself from enjoying my party in order to keep Joe away.
Therapist: What if your pain was like Joe?
Ms X: Huh? … Ah, I think I see what you are saying…
Therapist: It’s like if you allow Joe to simply be another guest, you can do whatever you like at your party. On the other hand, if you say “no” to Joe you also say “no” to the party.
Ms X: Are you saying that it is for me to choose?
• What is the role of therapist in modeling behavior change in ACT?
An important distinction can be made between talking about behavior change and doing behavior change. Within the psychological flexibility model the emphasis is placed on the latter. Here, especially through the use of experiential exercises, clients are put into contact with the experiences that have coordinated unhealthy behavior patterns in the past so that more effective behavior patterns can be acquired. Treatment delivery is guided by the underlying behavioral philosophy and theory. Patients learn to reduce the dominant influence of the literal meaning of language as the only tool for behavior change. Direct experience is moved to the front of awareness and literal meaning, mental and verbal analysis, and so forth, are moved to the back [20]. In treatment, the therapist models for the patient the behavior change processes that are being targeted and also may use examples from his or her life as well as that of the patient’s to develop psychological flexibility [22]. An example might include a therapist’s response to a person who shows an experience of emotional distress and struggling to manage this distress. Here the therapist, in line with ACT, instead of acting in some way to attempt to lessen the distress, would consciously show openness to the experiences and to their own reactions to helplessness around these experiences.
The therapist might say:
“I would feel tired and probably in pain too if I did what you just did. Could we do a little closed-eyes exercise? Shall we put the distressing thought you are having on the table, and focus on it, and we can “observe” what your mind does, and what happens in your body and your emotions when that thought shows up? Are you willing?”
“I’m feeling confused about this issue myself - how about both of us sitting quietly for a moment or two and observe what our minds do in response to this, just slowing things down, and watching?”
“I feel anxious when I believe that my thoughts about pain are true - like I have to do something to make it go away but I don’t know how. What shows up for you when you believe such thoughts about pain?”
• How and when should ACT be used?
Based on current evidence how and when ACT ought to be used, as opposed to other treatment options, will be largely up to the individual professional and their level of competence. ACT is a form of CBT and many of the same guides pertain. In line with the pragmatic approach of ACT, an approach that makes ACT broadly applicable, there is no one particular manualised or scripted treatment protocol that must be adhered to in treatment for one specific condition or another. As mentioned earlier, the ACT approach does not usually follow a cookbook style of delivery, nor is it rigidly guided by strict protocols. There are protocols shared by researchers to support further development but there is no process by which these are deemed “official” or “recognized” or approved by anyone in particular.
A wide range of metaphors and exercises based on a set of behavioral principles that target a particular function has been proposed in ACT and this is part of its uniqueness as a therapeutic model.
Those developing ACT also have not required a standardised certification process to delivering ACT. Instead, they have chosen to create an open community of contributing researchers and clinicians who are “members” by virtue of their commitment to the same approach to clinical development and the same clinical model. Practicing ACT requires that the clinician is aware of their own competencies and delivers treatment accordingly.
• How effective is ACT?
Numerous studies have supported a general role of psychological flexibility in improving the well-being and physical functioning of patients with chronic pain, including patients in specialty care [24,28] and primary care [26]. Many studies support the particular role of acceptance of pain in adjustment to chronic pain [27–30]. Pain acceptance is a better predictor of outcomes than pain severity itself [31,32].
There are now several relatively large-scale studies conducted in actual clinical practice settings that demonstrate the effectiveness of ACT for chronic pain [25,27,33,34]. A more recent study, also conducted in an actual clinical practice setting, provided support for the specific treatment processes proposed within this approach [35]. This study showed that changes in traditionally conceived methods of pain management were unrelated to treatment improvements of pain intensity, physical disability, anxiety and depression for those who participated in treatment, while changes in psychological flexibility were consistently and significantly related to these improvements, with the exception of the results for depression.
Randomised Controlled Trials (RCTs)
To date, there are a total of 7 RCTs related to ACT and chronic pain [36–42], each providing supportive evidence. For example, in one of the early studies, Dahl and colleagues [36] showed that in comparison to treatment as usual, a group of workers who were at risk of long-term absenteeism from work due to pain or stress had a significant reduction in sick leave and healthcare usage after attending four hours of ACT sessions.
Wicksell and colleagues [37,38] conducted 2 separate RCTs with participants who suffered whiplash-associated disorder (WAD) and fibromyalgia, respectively. Post-treatment results of both RCTs showed an improvement in physical functioning, depression and psychological flexibility in the treatment group with gains maintained at follow-up. In addition, participants in the treatment group with WAD showed an improvement in life satisfaction and fear of movement while those in the treatment group with fibromyalgia showed significant improvements in fibromyalgia impact, self-efficacy and anxiety. There was however no change in pain intensity in those who received the ACT-based treatments.
An ACT-based treatment including a self-help manual showed a significant increase in acceptance, satisfaction in life with a higher level of function and decreased pain intensity compared with a wait-list condition and with applied relaxation (AR) [40]. In comparison to the AR condition, participants in the ACT condition also reported a significantly higher level of engagement in meaningful activities and a willingness to experience pain. Follow-up data support the maintenance of these improvements at first follow-up but differences were not significant at the second follow-up. Both depression and anxiety scores improved in both treatment groups.
Wetherell and colleagues [39] compared the effectiveness of ACT and traditional CBT and found that they both produced positive results. Results from the study also showed higher satisfaction in participants who attended ACT treatment than those that attended CBT treatment, suggesting that ACT “is an effective and acceptable” intervention for patients with chronic pain. Overall acceptance of pain was shown to differentiate patients who could function well with chronic pain from those that continued to suffer with it after treatment.
More recently the first internet-based RCT for ACT with chronic pain was conducted [41]. The authors found a reduction in measures of pain-related distress, depressive symptoms, and anxiety, with these gains maintained at 6 months follow-up in the ACT treatment group compared with controls. The most recent RCT was a pilot trial of a group-based treatment of people with chronic pain recruited from general practices in the UK [42]. Participants were randomised to either an ACT-based treatment or treatment as usual. Participants in the ACT-based group underwent 4 sessions each lasting 4 hours with the first 3 sessions completed in 1 week and the last session completed a week later. At 3 months follow-up, participants in the ACT group had lower disability, depression, and higher pain acceptance.
In general, results from the ACT-based RCTs on chronic pain support the efficacy of the treatment and reflect a high degree of versatility, based on the wide variety of modes of delivery tested. However, RCTs for chronic pain are still relatively few with some studies limited to small sample sizes, thus making it difficult to reach definitive conclusions on the general efficacy of ACT in chronic pain treatment. What the studies do seem to show is that ACT is a good alternative treatment option to more traditionally conceived current CBT-based treatments for chronic pain. Larger sample sizes and higher quality studies are needed to strengthen and establish the effectiveness of ACT and to understand the potential impact of wider implementation in clinical practice.
Meta-Analyses
A total of 4 meta-analyses [43–46] have been conducted on acceptance- or ACT-based treatment studies. Although the earlier meta-analyses [43,44] did not separately report the effectiveness of ACT for chronic pain, they reported a moderate effect size for ACT in general, with no evidence that ACT is more effective than established treatments.
Ruiz [46] conducted a review focusing on outcome or mediation/moderation type studies that compared ACT and CBT treatments. His review was not specific to chronic pain, although one study [39] involving a sample of chronic pain patients was included. Moderate effect sizes were found that favored ACT, with ACT showing a greater impact on change processes (g = 0.38) compared to no impact found in CBT (g = 0.05).
Essentially, only one meta-analysis [45] specifically reviewed the efficacy in chronic pain studies. Pain inten-sity and depression were selected as primary outcome measures, with anxiety, physical well-being, and quality of life selected as secondary outcomes. Out of 22 studies that were included in the review, only 2 studies [36,37] were ACT-based RCTs, with the rest of the studies mindfulness-based interventions. The overall effect size of 0.37 was found for pain and 0.32 for depression. In general, results showed significant effect sizes for both primary and secondary outcome measures in favor of the “acceptance-based treatments.” The authors concluded that at present, mindfulness-based stress reduction programs and ACT-based programs may not be superior to CBT but could be good alternatives for people with chronic pain.
The appropriateness of using pain intensity as a primary outcome measure for ACT-based studies is questionable [45]. The focus of ACT is to increase function rather than to reduce pain symptoms; hence possibly including interference of pain in daily life might be a more appropriate outcome measure.
Other Studies
A particularly important question to answer about ACT concerns its cost-effectivness, and we still know relatively little about this. We do know that when people participate in ACT-based treatments they are able to reduce medication use and health care visits and return to work after extended periods away from work [27,28]. It remains to conduct full health economic analyses of this type of approach for chronic pain.
ACT is known to produce significant benefits widely, in other applications apart from chronic pain, such as in workplace stress [47], psychosis [48], obsessive-compulsive disorder [49], and depression [50], among other mental health conditions [51].
• What are implications for policy makers?
Results from studies of ACT in chronic pain and in other areas are disseminating rapidly. This dissemination is aided in part by a professional organization devoted to ACT and psychological flexibility (Association for Contextual Behavioral Science; www.contextualscience.org), which has a new journal, the Journal of Contextual Behavioral Science, started in 2012.
With the development of ACT a focus on implementation, training, and treatment integrity began early. There was an implementation study of ACT was published by Strosahl and colleagues in 1998. Their study showed that training clinicians in ACT produced better outcomes and better treatment completion rates in an outpatient setting in comparison to clinicians not receiving this training.
Processes of training have also appeared during relatively early phases of research into ACT. Lappalainen and colleagues [52] compared the impact of treatment provided by trainee therapists trained in both a traditional CBT model and ACT. Here each trainee therapist treated one patient with traditional CBT and one with ACT. Although the therapists reported higher confidence in delivering traditional CBT, patients treated within an ACT model showed better symptoms improvement. Also, improved acceptance during treatment significantly predicted improvements across both groups of patients. Essentially, therapists with only a limited amount of training in both models demonstrated better clinical results with ACT.
A group-based ACT intervention has also been shown to be effective in reducing stress and improving the professional performance of clinical psychology trainees [53]. Here the trainees found the intervention personally and professionally useful and a majority showed a significant increase in psychological flexibility. This supports the applicability of ACT not only as a model to guide therapy but also as a model to guide training and professional performance [54]. Other results in a pain management setting show that transitioning to ACT as a treatment model can have similar benefits and may increase job satisfaction and staff well-
being [55].
• What are criticisms of ACT?
Many strong supporters of cognitive therapy and more traditional versions of CBT in the field claim that ACT is not new nor better than other current versions of CBT [56]. The proponents of ACT openly acknowledge that many methods used within ACT are adopted or modified from other established therapies [4]. Criticisms are not specific to the application of ACT with chronic pain but are based on others’ perceptions of ACT as a treatment approach and treatment techniques used in ACT in general.
Ost [43] criticised ACT and the third-wave therapies on 2 main grounds. First, he concluded that ACT and the rest of the third-wave therapies were not meeting the criteria of empirically supported treatments. He further concluded that there is no strong evidence to show that ACT is more effective than cognitive therapy. The methods of the Ost review have been challenged [57], yet to a certain degree the points raised are correct. Most of the limitations noted reflect a difference in the maturity of the evidence base for ACT versus traditional CBT-based approaches. Indeed, in comparison to CBT, which is the most empirically established form of psychotherapy and an active area of research for more than 40 years, ACT can be considered to be in its infancy stage of empirically supported treatments, where treatment evidence and availability of high-quality RCTs in general are few at present. Specific research on ACT for chronic pain though supportive is still preliminary to a certain degree. Even so, ACT for pain is regarded as an empirically supported treatment by the body within the American Psychological Association authorized to make this determination [58].
Conclusion
ACT is essentially a form of CBT, considered broadly. ACT brings with it a different philosophy and approach to science compared with some other forms of CBT—this can lead to some distinctive strategies and methods in treatment for chronic pain. Like traditionally designed CBT, however, ACT similarly aims for behavior change as the end point.
ACT is grounded in specific philosophical assumptions and includes the model of psychological flexibility at its core. Preliminary findings in broad clinical and nonclinical populations support the efficacy, effectiveness, and processes in the psychological flexibility model as mediators of change, in ACT [46,59]. Research has shown that most of the 6 ACT processes, all of those so far investigated, correlate with improved daily functioning and emotional well-being in patients with chronic pain. The evidence base for ACT is still developing. Larger trials, more carefully designed trials, and a continued focus on processes of change will be needed to strengthen this base.
Corresponding author: Su-Yin Yang, Health Psychology Section, Psychology Department, Institute of Psychiatry, King’s College London, 5th Fl, Bermondsey Wing, Guy’s Campus, London SE1 9RT, [email protected].
Financial disclosures: None.
Author contributions: conception and design, SY, LMM; analysis and interpretation of data, SY; drafting of article, SY, LMM; critical revision of the article, LMM; administrative or technical support, LMM; collection and assembly of data, SY.
1. Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971–9.
2. Fordyce WE. Behavioral methods for chronic pain and illness. St Louis: Mosby; 1976.
3. Turk DC, Meichenbaum D, Genest M. Pain and behavioral medicine: A cognitive-behavioral perspective. New York: Guildford Press; 1983.
4. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: An experiential approach to behavior change. New York: Guilford Press; 1999.
5. McCracken LM. Contextual cognitive-behavioral therapy for chronic pain. Seattle: IASP Press; 2005.
6. Leventhal H, Brissette I, Leventhal EA. The common-sense model of self-regulation of health and illness. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behaviour. London: Routledge; 2003:42–65.
7. Gatchel RJ, Peng YB, Peters ML, et al. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133:581–624.
8. Kerns RD, Sellinger J, Goodin BR. Psychological treatment of chronic pain. Ann Rev Clin Psychol 2011;7:411–34.
9. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Sys Rev 2012, Issue 11.
10. Vlaeyen JWS, Morley S. Cognitive-behavioral treatments for chronic pain: what works for whom? Clin J Pain 2005;21:1–8.
11. Eccleston C, Williams AC, Morley S. Psychological therapies for the management of chronic pain (excluding headaches) in adults. Cochrane Database Sys Rev 2009, Issue 2.
12. McCracken LM, Samuel VM. The role of avoidance, pacing, and other activity patterns in chronic pain. Pain 2007;130:119–25.
13. Lynch TR, Chapman AL, Rosenthal MZ, et al. Mechanisms of change in dialectical behavior therapy: theoretical and empirical observations. J Clin Psych 2006;62:459–80.
14. Esmer G, Blum J, Rulf J, et al. Mindfulness-based stress reduction for failed back surgery syndrome: a randomized controlled trial. J Am Osteopath Assoc 2010;10:646–52.
15. Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study. Pain 2008;134:310–9.
16. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: the process and practice of mindful change. 2nd ed. New York: Guilford Press; 2012.
17. Hayes SC, Villatte M, Levin M, Hildebrandt M. Open, aware and active: Contextual approaches as an emerging trend in the behavioural and cognitive therapies. Annu Rev Clin Psychol 2011;7:141–68.
18. Hayes S, Luoma J, Bond F, et al. Acceptance and commitment therapy: model processes and outcomes. Behav Res Ther 2006;44:1–25.
19. Gaudiano BA. A review of acceptance and commitment therapy (ACT) and recommendations for continued scientific advancement. Sci Rev Mental Health Prac 2011;8:5–22.
20. Twohig MP. Introduction: the basics of acceptance and commitment therapy. Cog and Behav Pract 2012;19:499–618.
21. McCracken LM, Yang S. The role of values in a contextual cognitive-behavioral approach to chronic pain. Pain 2006;123:137–45.
22. Luoma JB, Hayes SC, Walser RD. Learning ACT: an acceptance and commitment therapy skills-training manual for therapists. Oakland, CA: New Harbinger Pub; 2007.
23. Strosahl K, Robinson P, Gustavsson T. Brief interventions for radical change: principles and practice of focused acceptance and commitment therapy. Oakland, CA: New Harbinger Pub; 2012.
24. McCracken LM, Vowles KE, Zhao-O’Brien J. Further development of an instrument to assess psychological flexibility in people with chronic pain. J Behav Med 2010;33:346–54.
25. McCracken LM, Gutierrez-Martinez O. Processes of change in psychological flexibility in an interdisciplinary group –based treatment for chronic pain based on acceptance and commitment therapy. Behav Res Ther 2011;49:267–74.
26. McCracken LM, Velleman SC. Psychological flexibility in adults with chronic pain: a study of acceptance, mindfulness, and values-based action in primary care. Pain 2010;148:141–7.
27. McCracken LM, Vowles KE, Eccleston C. Acceptance-based treatment for persons with complex, long standing chronic pain: a preliminary analysis of treatment outcome in comparison to a waiting phase. Behav Res Ther 2005;43:1335–46.
28. McCracken LM, MacKichan F, Eccleston C. Contextual cognitive-behavioural therapy for severely disabled chronic pain sufferers: Effectiveness and clinically significant change. Eur J Pain 2007;11:314–22.
29. Wicksell RK, Melin L, Olsson GL. Exposure and acceptance in the rehabilitation of adolescents with idiopathic chronic pain--a pilot study. Eur J Pain 2007;11:779–87.
30. Wicksell RK, Olsson GL, Hayes SC. Psychological flexibility as a mediator of improvement in acceptance and commitment therapy for patients with chronic pain following whiplash. Eur J Pain 2010;14:1059.e1–1059.e11.
31. McCracken LM, Vowles KE, Eccleston C. Acceptance of chronic pain: component analysis and a revised assessment method. Pain 2004;107:159–66.
32. McCracken LM, Eccleston C. Coping or acceptance: what to do about chronic pain? Pain 2003;105:197–204.
33. Vowles KE, McCracken LM. Acceptance and values-based action in chronic pain: a study treatment effectiveness and process. J Consult Clin Psychol 2008;76:397–407.
34. Vowles KE, McCracken LM, Eccleston C. Processes of behaviour change in interdisciplinary treatment of chronic pain: Contributions of pain intensity, catastrophizing, and acceptance. Eur J Pain 2007;11:779–87.
35. Vowles KE, McCracken LM. Comparing the role of psychological flexibility and traditional pain management coping strategies in chronic pain treatment outcomes. Beh Res Ther 2010;48:141–6.
36. Dahl J, Wilson KG, Nilsson A. Acceptance and commitment therapy and the treatment of persons at risk for long-term disability resulting from stress and pain symptoms: a preliminary randomized trial. Behav Ther 2004;35:785–802.
37. Wicksell RK, Ahlqvist J, Bring A, et al. Can exposure and acceptance strategies improve functioning and life satisfaction in people with chronic pain and whiplash-associated disorders (WAD)? A randomized controlled trial. Cog Behav Ther 2009;38:169–82.
38. Wicksell RK, Kemani M, Jensen K, et al. Acceptance and commitment therapy for fibromyalgia: a randomized controlled trial. Eur J Pain 2013;17:599–611.
39. Wetherell JL, Afari N, Rutledge T, et al. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioural therapy for chronic pain. Pain 2011;152:2098–107.
40. Thorsell J, Finnes A, Dahl J, et al. A comparative study of 2 manual-based self-help interventions, acceptance and commitment therapy and applied relaxation for person with chronic pain. Clin J Pain 2011;27:716–23.
41. Buhrman M, Skoglund A, Husell J, et al. Guided internet-delivered acceptance and commitment therapy for chronic pain patients: a randomized controlled trial. Beh Res Ther 2013;51:307–15.
42. McCracken LM, Sato A, Taylor GJ. A trial of a brief group-based form of acceptance and commitment therapy (ACT) for chronic pain in general practice: pilot outcome and process results. J Pain 2013;14:1398–406.
43. Ost L-G. Efficacy of the third wave of behavioral therapies: A systematic review and meta-analysis. Behav Res Ther 2008;46:296–321.
44. Powers MB, Zum Vorde Sive Vording MB, Emmelkamp PMG. Acceptance and commitment therapy: a meta-analytic review. Psychother Psychosom 2009;78:73–80.
45. Veehof MM, Oskam MJ, Schereurs KM, Bohlmeijer ET. Acceptance-based intervention for the treatment of chronic pain: a systematic review and meta-analysis. Pain 2011;152:533–42.
46. Ruiz FJ. Acceptance and commitment therapy versus traditional cognitive behavioral therapy: a systematic review and meta-analysis of current empirical evidence. Int J Psychol Psycholog Ther 2012;12:333–57.
47. Bond FW, Bunce D. The role of acceptance and job control in mental health, job sarsifaction, and work performance. J App Psych 2003;88:1057–67.
48. Gaudiano BA, Herbert JD. Acute treatment of inpatients with psychotic symptoms using acceptance and commitment therapy: pilot results. Behav Res Ther 2006;44:415–37.
49. Twohig MP, Hayes SC, Masuda A. Increasing willingness to experience obsessions: Acceptance and commitment therapy as a treatment for obsessive-compusive disorder. Behav Ther 2006;37:3–13.
50. Zettle RD, Hayes SC. Dysfunctional control by client verbal behaviour. The context of reason giving. Analys Verbal Behav 1986;4:30–8.
51. Forman EM, Herbert JD, Morita E, et al. A randomised controlled effectiveness trial of acceptance and commitment therapy and cognitive therapy for anxiety and depression. Behav Mod 2007;31:772–99.
52. Lappalainen R, Lehtonen T, Skarp E, et al. The impact of CBT and ACT models using psychology trainee therapists: A preliminary controlled effectiveness trial. Behav Modif 2007;31:488–511.
53. Stafford-Brown J, Pakenham KI. The effectiveness of an ACT informed intervention for managing stress and improving therapist qualities in clinical psychology trainees. J Clin Psychol 2012;68:592–613.
54. Pakenhan KI, Stafford-Brown J. Postgraduate clinical psychology students’ perceptions of an acceptance and commitment therapy stress management intervention and clinical training. Clin Psych 2012;17:56–66.
55. Barker E, McCracken LM. From traditional cognitive behavioral therapy to acceptance and commitment therapy for chronic pain: a mixed method study of staff experiences of change. Brit J Pain published online 19 Jul 2013.
56. Hoffmann SG, Asmundson GJ. Acceptance and mindfulness-based therapy: new wave or old hat? Clin Psych Rev 2008;28:1–16.
57. Gaudiano BA. Ost’s (2008) methodological comparison of clinical trials of acceptance and commitment therapy versus cognitive behavior therapy: matching apples with oranges? Behav Res Ther 2009;47:1066–70.
58. Division 12. APA psychological treatments. Niwot, CO: American Psychological Association. Available at www.div12.org/PsychologicalTreatments/treatments.html.
59. Levin ME, Hildebrandt MJ, Lillis J, Hayes SC. The impact of treatment components suggested by the psychological flexibility model: A meta-analysis of laboratory-based component studies. Behav Ther 2012;43:741–56.
1. Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971–9.
2. Fordyce WE. Behavioral methods for chronic pain and illness. St Louis: Mosby; 1976.
3. Turk DC, Meichenbaum D, Genest M. Pain and behavioral medicine: A cognitive-behavioral perspective. New York: Guildford Press; 1983.
4. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: An experiential approach to behavior change. New York: Guilford Press; 1999.
5. McCracken LM. Contextual cognitive-behavioral therapy for chronic pain. Seattle: IASP Press; 2005.
6. Leventhal H, Brissette I, Leventhal EA. The common-sense model of self-regulation of health and illness. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behaviour. London: Routledge; 2003:42–65.
7. Gatchel RJ, Peng YB, Peters ML, et al. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133:581–624.
8. Kerns RD, Sellinger J, Goodin BR. Psychological treatment of chronic pain. Ann Rev Clin Psychol 2011;7:411–34.
9. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Sys Rev 2012, Issue 11.
10. Vlaeyen JWS, Morley S. Cognitive-behavioral treatments for chronic pain: what works for whom? Clin J Pain 2005;21:1–8.
11. Eccleston C, Williams AC, Morley S. Psychological therapies for the management of chronic pain (excluding headaches) in adults. Cochrane Database Sys Rev 2009, Issue 2.
12. McCracken LM, Samuel VM. The role of avoidance, pacing, and other activity patterns in chronic pain. Pain 2007;130:119–25.
13. Lynch TR, Chapman AL, Rosenthal MZ, et al. Mechanisms of change in dialectical behavior therapy: theoretical and empirical observations. J Clin Psych 2006;62:459–80.
14. Esmer G, Blum J, Rulf J, et al. Mindfulness-based stress reduction for failed back surgery syndrome: a randomized controlled trial. J Am Osteopath Assoc 2010;10:646–52.
15. Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study. Pain 2008;134:310–9.
16. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: the process and practice of mindful change. 2nd ed. New York: Guilford Press; 2012.
17. Hayes SC, Villatte M, Levin M, Hildebrandt M. Open, aware and active: Contextual approaches as an emerging trend in the behavioural and cognitive therapies. Annu Rev Clin Psychol 2011;7:141–68.
18. Hayes S, Luoma J, Bond F, et al. Acceptance and commitment therapy: model processes and outcomes. Behav Res Ther 2006;44:1–25.
19. Gaudiano BA. A review of acceptance and commitment therapy (ACT) and recommendations for continued scientific advancement. Sci Rev Mental Health Prac 2011;8:5–22.
20. Twohig MP. Introduction: the basics of acceptance and commitment therapy. Cog and Behav Pract 2012;19:499–618.
21. McCracken LM, Yang S. The role of values in a contextual cognitive-behavioral approach to chronic pain. Pain 2006;123:137–45.
22. Luoma JB, Hayes SC, Walser RD. Learning ACT: an acceptance and commitment therapy skills-training manual for therapists. Oakland, CA: New Harbinger Pub; 2007.
23. Strosahl K, Robinson P, Gustavsson T. Brief interventions for radical change: principles and practice of focused acceptance and commitment therapy. Oakland, CA: New Harbinger Pub; 2012.
24. McCracken LM, Vowles KE, Zhao-O’Brien J. Further development of an instrument to assess psychological flexibility in people with chronic pain. J Behav Med 2010;33:346–54.
25. McCracken LM, Gutierrez-Martinez O. Processes of change in psychological flexibility in an interdisciplinary group –based treatment for chronic pain based on acceptance and commitment therapy. Behav Res Ther 2011;49:267–74.
26. McCracken LM, Velleman SC. Psychological flexibility in adults with chronic pain: a study of acceptance, mindfulness, and values-based action in primary care. Pain 2010;148:141–7.
27. McCracken LM, Vowles KE, Eccleston C. Acceptance-based treatment for persons with complex, long standing chronic pain: a preliminary analysis of treatment outcome in comparison to a waiting phase. Behav Res Ther 2005;43:1335–46.
28. McCracken LM, MacKichan F, Eccleston C. Contextual cognitive-behavioural therapy for severely disabled chronic pain sufferers: Effectiveness and clinically significant change. Eur J Pain 2007;11:314–22.
29. Wicksell RK, Melin L, Olsson GL. Exposure and acceptance in the rehabilitation of adolescents with idiopathic chronic pain--a pilot study. Eur J Pain 2007;11:779–87.
30. Wicksell RK, Olsson GL, Hayes SC. Psychological flexibility as a mediator of improvement in acceptance and commitment therapy for patients with chronic pain following whiplash. Eur J Pain 2010;14:1059.e1–1059.e11.
31. McCracken LM, Vowles KE, Eccleston C. Acceptance of chronic pain: component analysis and a revised assessment method. Pain 2004;107:159–66.
32. McCracken LM, Eccleston C. Coping or acceptance: what to do about chronic pain? Pain 2003;105:197–204.
33. Vowles KE, McCracken LM. Acceptance and values-based action in chronic pain: a study treatment effectiveness and process. J Consult Clin Psychol 2008;76:397–407.
34. Vowles KE, McCracken LM, Eccleston C. Processes of behaviour change in interdisciplinary treatment of chronic pain: Contributions of pain intensity, catastrophizing, and acceptance. Eur J Pain 2007;11:779–87.
35. Vowles KE, McCracken LM. Comparing the role of psychological flexibility and traditional pain management coping strategies in chronic pain treatment outcomes. Beh Res Ther 2010;48:141–6.
36. Dahl J, Wilson KG, Nilsson A. Acceptance and commitment therapy and the treatment of persons at risk for long-term disability resulting from stress and pain symptoms: a preliminary randomized trial. Behav Ther 2004;35:785–802.
37. Wicksell RK, Ahlqvist J, Bring A, et al. Can exposure and acceptance strategies improve functioning and life satisfaction in people with chronic pain and whiplash-associated disorders (WAD)? A randomized controlled trial. Cog Behav Ther 2009;38:169–82.
38. Wicksell RK, Kemani M, Jensen K, et al. Acceptance and commitment therapy for fibromyalgia: a randomized controlled trial. Eur J Pain 2013;17:599–611.
39. Wetherell JL, Afari N, Rutledge T, et al. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioural therapy for chronic pain. Pain 2011;152:2098–107.
40. Thorsell J, Finnes A, Dahl J, et al. A comparative study of 2 manual-based self-help interventions, acceptance and commitment therapy and applied relaxation for person with chronic pain. Clin J Pain 2011;27:716–23.
41. Buhrman M, Skoglund A, Husell J, et al. Guided internet-delivered acceptance and commitment therapy for chronic pain patients: a randomized controlled trial. Beh Res Ther 2013;51:307–15.
42. McCracken LM, Sato A, Taylor GJ. A trial of a brief group-based form of acceptance and commitment therapy (ACT) for chronic pain in general practice: pilot outcome and process results. J Pain 2013;14:1398–406.
43. Ost L-G. Efficacy of the third wave of behavioral therapies: A systematic review and meta-analysis. Behav Res Ther 2008;46:296–321.
44. Powers MB, Zum Vorde Sive Vording MB, Emmelkamp PMG. Acceptance and commitment therapy: a meta-analytic review. Psychother Psychosom 2009;78:73–80.
45. Veehof MM, Oskam MJ, Schereurs KM, Bohlmeijer ET. Acceptance-based intervention for the treatment of chronic pain: a systematic review and meta-analysis. Pain 2011;152:533–42.
46. Ruiz FJ. Acceptance and commitment therapy versus traditional cognitive behavioral therapy: a systematic review and meta-analysis of current empirical evidence. Int J Psychol Psycholog Ther 2012;12:333–57.
47. Bond FW, Bunce D. The role of acceptance and job control in mental health, job sarsifaction, and work performance. J App Psych 2003;88:1057–67.
48. Gaudiano BA, Herbert JD. Acute treatment of inpatients with psychotic symptoms using acceptance and commitment therapy: pilot results. Behav Res Ther 2006;44:415–37.
49. Twohig MP, Hayes SC, Masuda A. Increasing willingness to experience obsessions: Acceptance and commitment therapy as a treatment for obsessive-compusive disorder. Behav Ther 2006;37:3–13.
50. Zettle RD, Hayes SC. Dysfunctional control by client verbal behaviour. The context of reason giving. Analys Verbal Behav 1986;4:30–8.
51. Forman EM, Herbert JD, Morita E, et al. A randomised controlled effectiveness trial of acceptance and commitment therapy and cognitive therapy for anxiety and depression. Behav Mod 2007;31:772–99.
52. Lappalainen R, Lehtonen T, Skarp E, et al. The impact of CBT and ACT models using psychology trainee therapists: A preliminary controlled effectiveness trial. Behav Modif 2007;31:488–511.
53. Stafford-Brown J, Pakenham KI. The effectiveness of an ACT informed intervention for managing stress and improving therapist qualities in clinical psychology trainees. J Clin Psychol 2012;68:592–613.
54. Pakenhan KI, Stafford-Brown J. Postgraduate clinical psychology students’ perceptions of an acceptance and commitment therapy stress management intervention and clinical training. Clin Psych 2012;17:56–66.
55. Barker E, McCracken LM. From traditional cognitive behavioral therapy to acceptance and commitment therapy for chronic pain: a mixed method study of staff experiences of change. Brit J Pain published online 19 Jul 2013.
56. Hoffmann SG, Asmundson GJ. Acceptance and mindfulness-based therapy: new wave or old hat? Clin Psych Rev 2008;28:1–16.
57. Gaudiano BA. Ost’s (2008) methodological comparison of clinical trials of acceptance and commitment therapy versus cognitive behavior therapy: matching apples with oranges? Behav Res Ther 2009;47:1066–70.
58. Division 12. APA psychological treatments. Niwot, CO: American Psychological Association. Available at www.div12.org/PsychologicalTreatments/treatments.html.
59. Levin ME, Hildebrandt MJ, Lillis J, Hayes SC. The impact of treatment components suggested by the psychological flexibility model: A meta-analysis of laboratory-based component studies. Behav Ther 2012;43:741–56.
Overcoming Challenges to Obesity Counseling: Suggestions for the Primary Care Provider
From the Kaiser Permanente Center for Health Research Southeast, Atlanta, GA (Dr. Lewis) and the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, MD (Dr. Gudzune).
Abstract
- Objective: To review challenges to obesity counseling in the primary care setting and suggest potential solutions.
- Methods: Review of the literature.
- Results: There are many challenges to obesity counseling in the primary care setting, including lack of primary care provider (PCP) training, provider weight bias, lack of reimbursement, lack of time during outpatient encounters, and limited ability to refer patients to structured weight loss support programs. However, there are potential solutions to overcome these challenges. By seeking continuing medical education on weight management and communication skills, PCPs can address any training gaps and establish rapport with patients when delivering obesity counseling. Recent policy changes including Medicare coverage of obesity counseling visits may reduce PCPs' concern about lack of reimbursement and time, and the rise of new models of care delivery and reimbursement, such as patient-centered medical homes or accountable care organizations, may facilitate referrals to ancillary providers like registered dietitians or multi-component weight loss programs.
- Conclusion: Although providers face several challenges in delivering effective obesity counseling, PCPs may overcome these obstacles by pursuing continuing medical education in this area and taking advantage of new health care benefits coverage and care delivery models.
Over one-third of U.S. adults are now obese [1] and the prevalence of obesity is rising globally (2). In 2003 and 2012, the U.S. Preventive Services Task Force (USPSTF) issued a recommendation that health care providers screen all patients for obesity and offer intensive, multicomponent behavioral interventions to obese patients [3,4]. Screening for obesity typically involves assessment and classification of a patient’s body mass index (BMI). In the primary care setting, weight management may include a range of therapeutic options such as intensive behavioral counseling, prescription anti-obesity medications, and referral to bariatric surgery. Behavioral interventions typically include activities such as goal setting, diet and exercise change, and self-monitoring. A recent systematic review showed that primary care–based behavioral interventions could result in modest weight losses of 3 kg over a 12-month period, and prevent the development of diabetes and hypertension in at-risk patients [5].
PCP Concern: “I never learned about weight management during my training”
One of the most common barriers to providing the recommended counseling reported by health care providers is inadequate training in nutrition, exercise, and weight loss counseling [10–12]. Many providers have knowledge deficiencies in basic weight management [13,14]. In addition, few PCPs who have received obesity-related training rate that training as good quality during medical school (23%) and residency (35%) [15].
Pursuing Additional Training in Weight Management
Providers could address their lack of training in weight management by participating in an obesity curriculum. When surveyed, PCPs have identified that additional training in nutrition counseling (93%) and exercise counseling (92%) would help them improve the care for obese patients, and many (60%) reported receiving good continuing medical education (CME) on this topic [15]. Much research in this area has examined the impact of such training on residents’ provision of obesity counseling. Residents who completed training improved the quality of obesity care that they provided [16], and those who learned appropriate obesity screening and counseling practices were more likely to report discussing lifestyle changes with their patients [17]. The vast majority of surveyed PCPs (86%) also felt that motivational interviewing [15], a technique that can effectively promote weight loss, would help them improve obesity care [18,19]. Patients demonstrated greater confidence in their ability to change their diet when their PCP used motivational interviewing–consistent techniques during counseling [20]; however, few PCPs utilize motivational interviewing techniques [20,21]. Offering CME opportunities for practicing PCPs to obtain skills in nutrition, exercise, and motivational interviewing would likely improve the quality of obesity care and weight loss counseling that are being delivered. PCPs could also consider attending an in-depth weight management and obesity
Applying a Universal Behavior Change Approach to Obesity and Other Behaviors
Another option may be encouraging PCPs to use a universal approach to behavioral counseling across multiple domains [22]. Using a single technique may lend familiarity and efficiency to the health care providers’ counseling [23]. The 5A’s—Assess, Advise, Agree, Assist, Arrange—has been proposed as a possible “universal” strategy that has demonstrated efficacy in both smoking cessation [24] and weight loss [25,26]. Using the 5A’s has been associated with increased motivation to lose weight [25] and increased weight loss [26]. Many physicians are familiar with the 5A’s; however, few physicians use the complete technique. PCPs have been found to most frequently “assess” and “advise” when using the 5A’s technique for weight loss counseling [26,27], although assisting and arranging are the components that have been associated with dietary change and weight loss [26]. PCPs could incorporate these A’s into their counseling routine by ensuring that they “assist” the patient by establishing appropriate lifestyle changes (eg, calorie tracking to achieve a 500 to 1000 calorie reduction per day) or referring to a weight loss program, and “arrange” for follow-up by scheduling an appointment in a few weeks to discuss the patient’s progress [23]. While the 5A’s can effectively promote weight loss, many PCPs would likely require training or retraining in this method to ensure its proper use. For PCPs interested in integrating the 5A’s into their weight management practice, we refer them to the algorithm described by Serdula and colleagues [23].
Cultural Influences on Weight Management
A final weight management training consideration relates to cultural awareness for patients who are from different racial or ethnic backgrounds than the PCP. In the United States, racial and ethnic minority groups are disproportionately burdened by obesity. Nearly 60% of non-Hispanic black women and 41% of Hispanic women are obese, compared with 33% of non-Hispanic whites [28]. Despite this fact, obese non-Hispanic black and Hispanic patients are more likely than white patients to perceive themselves as “slightly overweight” and to rate their health as good to excellent despite their obesity [29,30]. As a result, they may be less likely to seek out weight loss strategies on their own or ask for weight control advice from their providers [31]. Additionally, racial and ethnic disparities in access to healthy foods [32,33], safe areas for engaging in physical activity [34], and lack of social support for healthy behaviors may make it much more difficult for some minority patients to act on their PCP's advice.
Because of different cultures, social influences, and norms, what an individual patient perceives as obese or unhealthy may differ dramatically from what his or her physician views as obese or unhealthy [35–38]. Therefore, it is important that PCPs have a discussion with their patients about their subjective weight and health perceptions before beginning any prescriptive weight management strategies or discussions of “normal BMI” [39,40]. If an obese patient views herself as being at a normal weight for her culture, she is unlikely to respond well to being told by her doctor that she needs to lose 40 pounds to get to a healthy weight. Recent research suggests that alternative goals, such as encouraging weight maintenance for non-Hispanic black women, may be a successful alternative to the traditional pathway of encouraging weight loss [41].
In addition to understanding cultural context during weight status discussions, it is also important to give behavior change advice that is sensitive to the culture, race, and ethnicity of the patient. Dietary recommendations should take into account the patient’s culture. For example, Lindbergh et al have noted that cooking in traditional Hispanic culture does not rely as much on measurements as does cooking for non-Hispanic whites [42]. Therefore, measurement-based dietary advice (the cornerstone of portion control) may be a more problematic concept for these patients to incorporate into their home cooking styles [42]. Physical activity recommendations should also be given in context of cultural acceptability. A recent study by Hall and others concluded that some African-American women may be reluctant to follow exercise advice for fear that sweating will ruin their hairstyles [43]. Although providers need not be experts on the cultural norms of all of their patients, they should be open to discussing them, and to asking about the patient’s goals, ideal body type, comfort with physical activity, diet advice and other issues that will make individualized counseling much more effective.
PCP Concern: “Weight gain reflects the patient’s lack of will power and laziness”
Bias towards obese patients has been documented among health care providers [44,45]. Studies have shown that some providers have less respect for obese patients [46], perceive obese patients as nonadherent to medications [47], and associate obesity with “laziness,” “stupidity,” and “worthlessness” [48]. Furthermore, obese patients identify physicians as a primary source of stigma [49] and many report stigmatizing experiences during interactions with the healthcare system [44,45]. In one study, a considerable proportion of obese patients reported ever experiencing stigma from a doctor (69%) or a nurse (46%) [49]. As a result of these negative experiences, obese patients have reported avoiding or delaying medical services such as gynecological cancer screening [50]. A recent study by Gudzune et al found that obese patients had significantly greater odds of “doctor shopping,” where individuals saw 5 or more primary care providers in a 2-year period [51]. This doctor shopping behavior may also be motivated by dissatisfaction with care, as focus groups of obese women have reported doctor shopping until they find a health care provider who is comfortable, experienced, and skilled in treating obese patients [50].
Assessing Implicit and Explicit Weight Bias
In addition to explicit negative attitudes, health care providers may also hold implicit biases towards obese patients [52]. A recent study found that over half of medical students held an implicit anti-fat bias [53]. These implicit attitudes may manifest more subtly during patient encounters. PCPs engage in less emotional rapport building during visits with overweight and obese patients as compared to normal weight patients [54], which include behaviors such as expressing empathy, concern, reassurance, and partnership. The lack of rapport building could negatively influence the patient-provider relationship and decrease the effectiveness of weight loss counseling. PCPs may need to consider undergoing self-assessment to determine whether or not they hold negative implicit and/or explicit attitudes towards obese patients. PCPs can complete the Weight Implicit Association Test (IAT) for free online at https://implicit.harvard.edu/implicit/demo/. To determine whether they hold negative explicit attitudes, PCPs can download and complete assessments offered by the Yale Rudd Center for Food Policy and Obesity (www.yaleruddcenter.org/resources/bias_toolkit/index.html).
Pursuing Additional Training in Communication Skills
If weight bias is indeed present, PCPs may benefit from additional training in communication skills as well as specific guidance on how to discuss weight loss with overweight and obese patients. For example, an observational study found that patients lost more weight when they had weight loss counseling visits with physicians who used motivational interviewing strategies [20,21]. Additional PCP training in this area would benefit the patient-provider relationship, as research has shown that such patient-centered communication strategies lead to greater patient satisfaction [55,56], improvement in some clinical outcomes [57,58], and less physician burnout [59]. In fact, some medical schools address student weight bias during their obesity curricula [60]. Building communication skills helps improve PCPs’ capacity to show concern and empathy for patients’ struggles, avoid judgment and criticism, and give emotional support and encouragement, which may all improve PCPs’ ability to execute more sensitive weight loss discussions. For providers who are more interested in CME opportunities, the American Academy on Communication in Healthcare offers an online interactive learning program in this area called “Doc Com” (http://doccom.aachonline.org/dnn/Home.aspx).
PCP Concern: “I may not get reimbursed for weight management services”
Traditional metrics for how doctors are reimbursed and how the quality of their care is measured have not promoted weight loss counseling by PCPs. Prior to 2012, physicians could not bill Medicare for obesity-specific counseling visits [61]. Given that many private insurers follow the lead of the Centers for Medicare and Medicaid Services (CMS) for patterns of reimbursement, this issue has been pervasive in U.S. medical practice for a number of years, with considerable variability between plans on which obesity-related services are covered [62]. A recent study of U.S. health plans indicated that most would reject a claim for an office visit where obesity was the only coded diagnosis [62]. Additionally, the quality improvement movement has only recently begun to focus on issues of obesity. In 2009, the National Committee for Quality Assurance’s (NCQA) Healthcare Effectiveness Data and Information Set (HEDIS) added 2 new measures pertaining to the documentation of a patient’s BMI status. Prior to this time, even the simple act of acknowledging obesity was routinely underperformed and quite variable across health plans in the United States [63].
Obesity Screening and Counseling Benefits Coverage
In 2012, CMS made a major coverage change decision when they agreed to reimburse providers for delivering intensive behavioral interventions for obesity [61]. Namely, CMS will now cover a 6-month series of visits for Medicare patients (weekly for month 1, every other week for months 2–6), followed by monthly visits for an additional 6 months in patients who have been able to lose 3 kg. For PCPs and other providers who have long hoped for more opportunity to discuss nutrition, weight, and physical activity with their Medicare patients, these policy changes are exciting. Hopefully, this move by CMS will stimulate similar changes in the private insurance market.
Greater reimbursement of obesity-related care is also more likely given the overall trend of the U.S. health care system—with the focus shifting away from traditional fee-for-service models that have de-emphasized preventive care and counseling and toward a model that rewards well care [64]. Large employer groups, who represent an important voice in any discussion of health insurance and reimbursement, are also increasingly interested in the use of wellness programs and weight loss to decrease their own health care costs. This trend could further stimulate insurers to cover programs that allow providers to engage in weight counseling as a way of attracting or retaining large employer groups as customers [62].
Obesity Screening and Counseling Quality Metrics
A parallel movement in the quality of care realm would serve to bolster any forthcoming changes in reimbursement. For example, an expansion of the HEDIS “wellness and health promotion” measures, or going beyond “BMI assessment” to include a brief assessment of key dietary factors or physical activity level as a routine quality measure, would go a long way toward emphasizing to payers and providers the need for more routine obesity counseling. Professional provider organizations have been increasingly engaged in this area as well. The recent recognition by the American Medical Association of obesity as a disease may also influence organizations such as the NCQA and payers who may be considering how to encourage providers to better address this important issue.
PCP Concern: “I don’t have time to discuss weight loss during outpatient visits”
The average continuity visit for an adult patient in the United States is about 20 minutes in duration, with a mean of 6 to 7 clinical items to be addressed during that time-period [65]. This leaves little time for providers to perform the necessary history and physical portions of the visit, educate patients on various topics, and write out prescriptions or referrals. Not surprisingly, such extreme time pressure leads many PCPs to feel overwhelmed and burned out [66], and the idea of adding another “to-do” to office visits may be resisted. For obese patients, many of whom are likely to have multiple chronic conditions, PCPs are faced with the task of both discussing active issues such as hypertension, diabetes, and sleep apnea, and also potentially discussing the patient’s weight status in a very brief amount of time. Under such time pressures, PCPs often adopt a “putting out fires” mentality and therefore tackle what they see as the most pressing issues—eg, deal with out of control blood pressure by adding a new medication, or lowering hemoglobin A1c by upping the insulin dose, rather than dealing with the 20-lb weight gain that might be leading to the high pressures and hyperglycemia.
Compounding this problem is the fact that well-delivered preventive health advice can be time-consuming, and with so many topics to choose from, it may be difficult for providers to know which issues make the most sense to prioritize [67]. A recent study estimated that PCPs routinely under-counsel patients about nutrition (an advice topic that earns a “B” rating from the USPSTF), while they over-counsel them on exercise and PSA testing (topics that earn an “I” rating from the USPSTF) [68]. Topics of discussion and the time spent on them may reflect patient priorities or PCP comfort with various issues, but it is clear that some improvements could be made to better utilize available time with patients.
In the face of time and resource pressures, many PCPs may not be ideally suited to deliver the kind of intensive behavioral weight loss interventions that are supported by the best scientific evidence [69]. In fact, there is little evidence to support even brief weight counseling sessions by PCPs [70]. However, for busy providers, there are several brief and potentially impactful tasks that could enable them to better support their obese patients.
Brief Counseling Interventions in the Primary Care Setting
First, primary care providers should routinely measure and discuss their patients BMIs as they would any other vital sign. In addition, other brief measures such as “Exercise as a Vital Sign” [71] can be incorporated into the visit, so that behaviors linked to weight can inform the strategy adopted and monitored over time. After a brief discussion is initiated, a referral can be placed for patients who wish to pursue more intense therapy for weight loss—this may be to behavioral health, nutrition, bariatric surgery or a comprehensive weight management clinic. Practices can support their providers by streamlining this referral process and educating providers and patients on available resources. PCPs also may be able to engage their patients in self-monitoring (eg, calorie tracking, exercise tracking, self weighing) so that most of the work and learning takes place outside of the primary care office. For example, PCPs can promote the use of a food diary, a practice that has been shown to improve weight loss success [72]. Review of the diary could take place at a separate visit with the PCP or in follow-up with a weight loss specialist or dietitian.
A major strength of the primary care setting is its longitudinal nature. Even if available time at individual visits is short, advice and support can be given repeatedly over a longer period of time than may often be achieved with a specialist consultant. For patients who are in the maintenance phase of weight loss, having long-term frequent contacts with a provider has been shown to prevent weight regain [73]. The use of group visits and physician extenders (RNs, NPs, PAs) for delivering obesity-related behavioral advice might offer another way to relieve some of the time pressures faced by PCPs in the one-on-one chronic disease management visit [69,74].
PCP Concern: “I don’t know where to refer patients for weight management”
Surveys of obese patients and their doctors indicate that PCPs may not often enough refer patients to structured weight loss programs or registered dietitians [75,76]. Furthermore, PCPs are often isolated from other providers who might be important in a team-based model of obesity care, such as pharmacists, registered dietitians, endocrinologists, and bariatric surgeons. The implementation of the Affordable Care Act, including payment reform and the rise of accountable care organizations, should begin changing the relative isolation of the PCP. If more practices attempt to conform to medical home models, the interconnectedness of PCPs to other health care team members may increase, thus facilitating a more team-based approach to obesity care and easier referrals to specialized team members [77].
Weight Management Resources
Aside from some academic centers and large private health care institutions, many primary care practices lack access to structured obesity care clinics that can help manage the challenges of guiding patients through their weight loss options. For providers who practice in areas that do not afford them easy access to obesity care clinics, it is worth seeking out available resources in the nonmedical community that might provide a structured support system for patients. One low-cost community-based program, Take Off Pounds Sensibly (TOPS; www.tops.org), can achieve and sustain a 6% weight loss for active members [78]. Groups such as Overeaters Anonymous are found in most U.S. cities, and have helpful websites including podcasts that patients can access even in the absence of a local branch (www.oa.org). Organizations like the YMCA, which have good penetration into most areas of the country, offer affordable access to physical activity and health programs including coaching that can promote all around healthier living and improved dietary habits (www.ymca.net). A final consideration could be referral to a commercial weight loss program. A 2005 review of the major U.S. commercial weight loss programs concluded that there was suboptimal evidence for or against these programs’ efficacy [79]. A recent randomized controlled trial showed that patients referred by their PCP to a commercial weight loss program (Weight Watchers) lost significantly more weight (2.3 kg) at 12 months as compared to patients who only received weight loss advice from their PCP [80]. However, it is important to keep in mind that not all commercial programs are the same and some programs can be ineffective or even dangerous for some patients. The PCP may need to take an active role monitoring their patient’s health and safety when using these programs.
A Strategy to Incorporate Weight Management into Current Practice
Summary
Given the obesity epidemic, PCPs will need to begin addressing weight loss as a part of their normal practice; however, providers face several challenges in implementing weight management services. Many PCPs report receiving inadequate training in weight management during their training; however, many CME opportunities exist for providers to reduce their knowledge and skills deficit. Depending upon the prevalence of obesity in their practice and interest in offering weight management services, PCPs may need to consider more intensive weight management training or even pursue certification as an obesity medicine provider through the American Board of Obesity Medicine. For providers with a more general interest in obesity counseling, applying a consistent counseling approach like the 5A’s to several behaviors (eg, obesity, smoking cessation) may facilitate such counseling as a regular part of the outpatient encounter. PCPs should also be aware of different cultural considerations with respect to obesity including different body image perceptions and cooking styles. Obesity bias is pervasive in our society; therefore, PCPs may similarly hold negative explicit or implicit attitudes towards these patients. Providers can engage in online self-assessment about their explicit and implicit biases in order to understand whether they hold any negative attitudes towards obese patients. Additional training in communication skills and empathy may improve these patient-provider relationships and translate into more effective behavioral counseling. PCPs may be concerned about a lack of reimbursement for weight management services or a lack of time to perform counseling during outpatient encounters. With the new obesity counseling benefits coverage by CMS, PCPs should be reimbursed for obesity counseling services and provide additional time through dedicated weight management visits for Medicare patients. The new primary care practice models including the patient-centered medical home may facilitate PCP referrals to other weight management providers such as registered dieticians and health coaches, which could offset the PCP’s time pressures. Finally, PCPs can consider referrals to community resources, such as programs like Overeaters Anonymous, TOPS or the YMCA, to help provide patients group support for behavior change. In summary, PCPs may need to consider additional training to be prepared to deliver high quality obesity care in collaboration with other local partners and weight management specialists.
Corresponding author: Kimberly A. Gudzune, MD, MPH, 2024 E. Monument St, Room 2-611, Baltimore, MD 21287, [email protected].
Funding/support: Dr. Gudzune received support through a career development award from the National Heart, Lung, and Blood Institute (K23HL116601).
Financial disclosures: None
1. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–7.
2. Stevens GA, Singh GM, Lu Y, et al. National, regional, and global rends in adult overweight and obesity prevalences. Popul Health Metr 2012;10:22.
3. McTigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2003;139:933–49.
4. Moyer VA. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:373–8.
5. LeBlanc ES, O’Conner E, Whitlock EP, et al. Effectiveness of primary care – relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155:434–47.
6. Jackson JE, Doescher MP, Saver BG, Hart LG. Trends in professional advice to lose weight among obese adults, 1994 to 2000. J Gen Intern Med 2005;20:814–8.
7. McAlpine DD, Wilson AR. Trends in obesity-related counseling in primary care. Med Care 2007;45:322–9.
8. Bleich SN, Pickett-Blakley O, Cooper LA. Physician practice patterns of obesity diagnosis and weight-related counseling. Patient Educ Couns 2011;82:123–9.
9. Felix H, West DS, Bursac Z. Impact of USPSTF practice guidelines on provider weight loss counseling as reported by obese patients. Prev Med 2008;47:394–7.
10. Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med 1995;24:546–52.
11. Huang J, Yu H, Marin E, et al. Physicians’ weight loss counseling in two public hospital primary care clinics. Acad Med 2004;79:156–61.
12. Alexander SC, Ostbye T, Pollak KI, et al. Physicians’ beliefs about discussing obesity: results from focus groups. Am J Health Promot 2007;21:498–500.
13. Block JP, DeSalvo KB, Fisher WP. Are physicians equipped to address the obesity epidemic? Knowledge and attitudes of internal medicine residents. Prev Med 2003;36:669–75.
14. Jay M, Gillespie C, Ark T, et al. Do internists, pediatricians, and psychiatrists feel competent in obesity care?: using a needs assessment to drive curriculum design. J Gen Intern Med 2008;23:1066–70.
15. Bleich SN, Bennett WL, Gudzune KA, Cooper LA. National survey of US primary care physicians’ perspectives about causes of obesity and solutions to improve care. BMJ Open 2012;2(6).
16. Jay M, Schlair S, Caldwell R, et al. From the patient’s perspective: the impact of training on residnet physician’s obesity counseling. J Gen Intern Med 2010;25:415–22.
17. Forman-Hoffman V, Little A, Wahls T. Barriers to obesity management: a pilot study of primary care clinicians. BMC Fam Pract 2006;7:35.
18. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev 2011;12:709–23.
19. Martins RK, McNeil DW. Review of motivational interviewing in promoting health behaviors. Clin Psychol Rev 2009;29:283–93.
20. Cox ME, Yancy WS Jr, Coffman CJ, et al. Effects of counseling techniques on patients’ weight-related attitudes and behaviors in a primary care clinic. Patient Educ Couns 2011;5:363–8.
21. Pollak KI, Alexander SC, Coffman CJ, et al. Physician communication techniques and weight loss in adults: Project CHAT. Am J Prev Med 2010;39:321–8.
22. Whitlock EP, Orleans CT, Pender N, Allan J. Evaluating primary care behavioral counseling interventions: an evidence-based approach. Am J Prev Med 2002;22:267–84.
23. Serdula MK, Khan LK, Dietz WH. Weight loss counseling revisited. J Amer Med Assoc 2003;289:1747–50.
24. Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update—clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008.
25. Jay M, Gillespie C, Schlair S, et al. Physicians’ use of the 5As in counseling obese patients: is the quality of counseling associated with patients’ motivation and intention to lose weight? BMC Health Serv Res 2010;10:159.
26. Alexander SC, Cox ME, Boling Turner CL, et al Do the five A’s work when physicians counsel about weight loss? Fam Med 2011;43:179–84.
27. Flocke SA, Clark A, Schlessman K, Pomiecko G. Exercise, diet, and weight loss advice in the family medicine outpatient setting. Fam Med 2005;37:415–21.
28. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief 2012;(82):1–8.
29. Burroughs VJ, et al. Self-reported comorbidities among self-described overweight African-American and Hispanic adults in the United States: results of a national survey. Obesity 2008;16:1400–6.
30. Dorsey RR, Eberhardt MS, Ogden CL. Racial/ethnic differences in weight perception. Obesity 2009;17:790–5.
31. Dorsey RR, Eberhardt MS, Ogden CL. Racial and ethnic differences in weight management behavior by weight perception status. Ethnic Dis 2010;20:244–50.
32. Baker EA, Schootman M, Barnidge E, Kelly C. The role of race and poverty in access to foods that enable individuals to adhere to dietary guidelines. Prev Chronic Dis 2006;3(3):A76.
33. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med 2009;36:74–81.
34. Gordon-Larsen P, et al. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics 2006;117:417–24.
35. Kumanyika S, Wilson JF, Guilford-Davenport M. Weight-related attitudes and behaviors of black women. J Am Dietetic Assoc 1993;93:416–22.
36. Chithambo TP, Huey SJ. Black/white differences in perceived weight and attractiveness among overweight women. J Obes 2013;2013:320–6.
37. Paeratakul S, et al. Sex, race/ethnicity, socioeconomic status, and BMI in relation to self-perception of overweight. Obesity Res 2002;10:345–50.
38. Bennett GG, et al. Attitudes regarding overweight, exercise, and health among blacks (United States). Cancer Causes Control 2006;17:95–101.
39. Kumanyika SK, et al. Expanding the obesity research paradigm to reach African American communities. Prev Chronic Disease 2007;4(4).
40. Stuart-Shor EM, Berra KA, Kamau MW, Kumanyika SK. Behavioral strategies for cardiovascular risk reduction in diverse and underserved racial/ethnic groups. Circulation 2012;125:171–84.
41. Bennett GG, Foley P, Levine E, et al. Behavioral treatment for weight gain prevention among black women in primary care practice: a randomized clinical trial. JAMA Intern Med 2013;173:1770–7.
42. Lindberg NM, Stevens VJ, Halperin RO. Weight-loss interventions for Hispanic populations: the role of culture. J Obes 2012:542736.
43. Hall RR, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatology 2013;149:310–4.
44. Puhl RM, Brownell KD. Bias, discrimination, and obesity. Obes Res 2001;9:788–905.
45. Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring) 2009;17:941–64.
46. Huizinga MM, Cooper LA, Bleich SN, et al. Physician respect for patients with obesity. J Gen Intern Med 2009;24:1236–9.
47. Huizinga MM, Bleich SN, Beach MC, et al. Disparity in physician perception of patients’ adherence to medications by obesity status. Obesity (Silver Spring) 2010;18:1932–7.
48. Schwartz MB, Chambliss HO, Brownell KD, et al. Weight bias among health professionals specializing in obesity. Obes Res 2003;11:1033–9.
49. Puhl RM, Brownell KD. Confronting and coping with weight stigma: an investigation of overweight and obese adults. Obesity (Silver Spring) 2006;14:1802–15.
50. Amy NK, Aalborg A, Lyons P, Keranen L. Barriers to routine gynecological cancer screening for White and African-American obese women. Int J Obes (Lond) 2006;30:147–55.
51. Gudzune KA, Bleich SN, Richards TM, et al. Doctor shopping by overweight and obese patients is associated with increased healthcare utilization. Obesity (Silver Spring) 2013;21:1328–34.
52. Teachman BA, Brownell KD. Implicit anti-fat bias among health professionals: is anyone immune? Int J Obes Relat Metab Disord 2001;25:1525–31.
53. Miller DP Jr, Spangler JG, Vitolins MZ, et al. Are medical students aware of their anti-obesity bias? Acad Med 2013;88:978–82.
54. Gudzune KA, Beach MC, Roter DL, Cooper LA. Physicians build less rapport with obese patients. Obesity (Silver Spring) 2013;21:2146–52.
55. Beck RS, Daughtridge R, Sloane PD. Physician-patient communication in the primary care office: a systematic review. J Am Board Fam Pract 2002;15:25–38.
56. Dwamena F, Holmes-Rovner M, Gaulden CM, et al. Interventions for providers to promote patient-center approach in clinical consultations. Cochrane Database Syst Rev 2012;12:CD003267.
57. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ 1995;152:1423–33.
58. Hojat M, Louis DZ, Markham FW, et al. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med 2011;86:359–64.
59. Krasner MS, Epstein RM, Beckman H, et al. Association of an educational program in mindful communication with burnout, empathy, and attitudes among primary care physicians. JAMA 2009;302:1284––93.
60. Vitolins MZ, Crandall S, Miller D, et al. Obesity educational interventions in U.S. medical schools: a systematic review and identified gaps. Teach Learn Med 2012;24:267–72.
61. Centers for Medicare and Medicaid Services, Decision memo for intensive behavioral therapy for obesity. 2012.
62. Simpson LA, Cooper J. Paying for obesity: a changing landscape. Pediatrics 2009;123 Suppl 5:5301–7.
63. Arterburn DE, et al. Body mass index measurement and obesity prevalence in ten U.S. health plans. Clin Med Res 2010;8:126–30.
64. Koh HK, Sebelius KG. Promoting prevention through the Affordable Care Act. N Engl J Med 2010; 363:1296–9.
65. Abbo ED, et al. The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med 2008;23:2058–65.
66. Mechanic D. Physician discontent: challenges and opportunities. JAMA 2003;290:941–6.
67. Yarnall KS, Pollak KI, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health 2003;93:635–41.
68. Pollak KI, Krause KM, Yarnall KS, et al. Estimated time spent on preventive services by primary care physicians. BMC Health Services Research 2008;8:245.
69. Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med 2009;24:1073–9.
70. Carvajal R, Wadden TA, Tsai AG, et al. Managing obesity in primary care practice: a narrative review. Ann N Y Acad Sci 2013;1281:191–06.
71. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc 2012;44:2071–6.
72. Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity 2007;15:3091–6.
73. Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psych Clin North Am 2011;34:841–59.
74. Lavoie JG, Wong ST, Chongo M, et al. Group medical visits can deliver on patient-centered care objectives: results from a qualitative study. BMC Health Serv Res 2013;13:2013.
75. Wadden TA, Anderson DA, Foster GD, et al. Obese women’s perceptions of their physicians’ weight management attitudes and practices. Arch Fam Med 2000;9:854–60.
76. Phelan SP, et al. What do physicians recommend to their overweight and obese patients? J Am Board Fam Med 2009;22:115–22.
77. Grant RMA, Greene DD. The health care home model: primary health care meeting public health goals. Am J Public Health 2012;102:1096–103.
78. Mitchell NS, Dickinson LM, Kempe A, Tsai AG. Determining the effectiveness of Take Off Pounds Sensibly (TOPS), a nationally available nonprofit weight loss program. Obesity (Silver Spring) 2011;19:568–73.
79. Tsai AG, Wadden TA. Systematic review: An evaluation of major commercial weight loss programs in the United States. Ann Intern Med 2005;142:56–66.
80. Jebb SA, Ahern AL, Olson AD, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet 2011;378:1485–92.
81. Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379–87.
82. Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81–95.
83. Scott JG, Cohen D, DiCicco-Bloom B, et al. Speaking of weight: how patients and primary care clinicians initiate weight loss counseling. Prev Med 2004;38:819–27.
84. Brown I, Thompson J, Tod A, Jones G. Primary care support for tacking obesity: a qualitative study of the perceptions of obese patients. Br J Gen Pract 2006;56:666–72.
85. Tailor A, Ogden J. Avoiding the term ‘obesity:’ an experimental study of the impact of doctors’ language on patients’ beliefs. Patient Educ Couns 2009;76:260–4.
86. Pollak KI, Ostbye T, Alexander SC, et al. Empathy goes a long way in weight loss discussions. J Fam Pract 2007;56:1031–6.
From the Kaiser Permanente Center for Health Research Southeast, Atlanta, GA (Dr. Lewis) and the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, MD (Dr. Gudzune).
Abstract
- Objective: To review challenges to obesity counseling in the primary care setting and suggest potential solutions.
- Methods: Review of the literature.
- Results: There are many challenges to obesity counseling in the primary care setting, including lack of primary care provider (PCP) training, provider weight bias, lack of reimbursement, lack of time during outpatient encounters, and limited ability to refer patients to structured weight loss support programs. However, there are potential solutions to overcome these challenges. By seeking continuing medical education on weight management and communication skills, PCPs can address any training gaps and establish rapport with patients when delivering obesity counseling. Recent policy changes including Medicare coverage of obesity counseling visits may reduce PCPs' concern about lack of reimbursement and time, and the rise of new models of care delivery and reimbursement, such as patient-centered medical homes or accountable care organizations, may facilitate referrals to ancillary providers like registered dietitians or multi-component weight loss programs.
- Conclusion: Although providers face several challenges in delivering effective obesity counseling, PCPs may overcome these obstacles by pursuing continuing medical education in this area and taking advantage of new health care benefits coverage and care delivery models.
Over one-third of U.S. adults are now obese [1] and the prevalence of obesity is rising globally (2). In 2003 and 2012, the U.S. Preventive Services Task Force (USPSTF) issued a recommendation that health care providers screen all patients for obesity and offer intensive, multicomponent behavioral interventions to obese patients [3,4]. Screening for obesity typically involves assessment and classification of a patient’s body mass index (BMI). In the primary care setting, weight management may include a range of therapeutic options such as intensive behavioral counseling, prescription anti-obesity medications, and referral to bariatric surgery. Behavioral interventions typically include activities such as goal setting, diet and exercise change, and self-monitoring. A recent systematic review showed that primary care–based behavioral interventions could result in modest weight losses of 3 kg over a 12-month period, and prevent the development of diabetes and hypertension in at-risk patients [5].
PCP Concern: “I never learned about weight management during my training”
One of the most common barriers to providing the recommended counseling reported by health care providers is inadequate training in nutrition, exercise, and weight loss counseling [10–12]. Many providers have knowledge deficiencies in basic weight management [13,14]. In addition, few PCPs who have received obesity-related training rate that training as good quality during medical school (23%) and residency (35%) [15].
Pursuing Additional Training in Weight Management
Providers could address their lack of training in weight management by participating in an obesity curriculum. When surveyed, PCPs have identified that additional training in nutrition counseling (93%) and exercise counseling (92%) would help them improve the care for obese patients, and many (60%) reported receiving good continuing medical education (CME) on this topic [15]. Much research in this area has examined the impact of such training on residents’ provision of obesity counseling. Residents who completed training improved the quality of obesity care that they provided [16], and those who learned appropriate obesity screening and counseling practices were more likely to report discussing lifestyle changes with their patients [17]. The vast majority of surveyed PCPs (86%) also felt that motivational interviewing [15], a technique that can effectively promote weight loss, would help them improve obesity care [18,19]. Patients demonstrated greater confidence in their ability to change their diet when their PCP used motivational interviewing–consistent techniques during counseling [20]; however, few PCPs utilize motivational interviewing techniques [20,21]. Offering CME opportunities for practicing PCPs to obtain skills in nutrition, exercise, and motivational interviewing would likely improve the quality of obesity care and weight loss counseling that are being delivered. PCPs could also consider attending an in-depth weight management and obesity
Applying a Universal Behavior Change Approach to Obesity and Other Behaviors
Another option may be encouraging PCPs to use a universal approach to behavioral counseling across multiple domains [22]. Using a single technique may lend familiarity and efficiency to the health care providers’ counseling [23]. The 5A’s—Assess, Advise, Agree, Assist, Arrange—has been proposed as a possible “universal” strategy that has demonstrated efficacy in both smoking cessation [24] and weight loss [25,26]. Using the 5A’s has been associated with increased motivation to lose weight [25] and increased weight loss [26]. Many physicians are familiar with the 5A’s; however, few physicians use the complete technique. PCPs have been found to most frequently “assess” and “advise” when using the 5A’s technique for weight loss counseling [26,27], although assisting and arranging are the components that have been associated with dietary change and weight loss [26]. PCPs could incorporate these A’s into their counseling routine by ensuring that they “assist” the patient by establishing appropriate lifestyle changes (eg, calorie tracking to achieve a 500 to 1000 calorie reduction per day) or referring to a weight loss program, and “arrange” for follow-up by scheduling an appointment in a few weeks to discuss the patient’s progress [23]. While the 5A’s can effectively promote weight loss, many PCPs would likely require training or retraining in this method to ensure its proper use. For PCPs interested in integrating the 5A’s into their weight management practice, we refer them to the algorithm described by Serdula and colleagues [23].
Cultural Influences on Weight Management
A final weight management training consideration relates to cultural awareness for patients who are from different racial or ethnic backgrounds than the PCP. In the United States, racial and ethnic minority groups are disproportionately burdened by obesity. Nearly 60% of non-Hispanic black women and 41% of Hispanic women are obese, compared with 33% of non-Hispanic whites [28]. Despite this fact, obese non-Hispanic black and Hispanic patients are more likely than white patients to perceive themselves as “slightly overweight” and to rate their health as good to excellent despite their obesity [29,30]. As a result, they may be less likely to seek out weight loss strategies on their own or ask for weight control advice from their providers [31]. Additionally, racial and ethnic disparities in access to healthy foods [32,33], safe areas for engaging in physical activity [34], and lack of social support for healthy behaviors may make it much more difficult for some minority patients to act on their PCP's advice.
Because of different cultures, social influences, and norms, what an individual patient perceives as obese or unhealthy may differ dramatically from what his or her physician views as obese or unhealthy [35–38]. Therefore, it is important that PCPs have a discussion with their patients about their subjective weight and health perceptions before beginning any prescriptive weight management strategies or discussions of “normal BMI” [39,40]. If an obese patient views herself as being at a normal weight for her culture, she is unlikely to respond well to being told by her doctor that she needs to lose 40 pounds to get to a healthy weight. Recent research suggests that alternative goals, such as encouraging weight maintenance for non-Hispanic black women, may be a successful alternative to the traditional pathway of encouraging weight loss [41].
In addition to understanding cultural context during weight status discussions, it is also important to give behavior change advice that is sensitive to the culture, race, and ethnicity of the patient. Dietary recommendations should take into account the patient’s culture. For example, Lindbergh et al have noted that cooking in traditional Hispanic culture does not rely as much on measurements as does cooking for non-Hispanic whites [42]. Therefore, measurement-based dietary advice (the cornerstone of portion control) may be a more problematic concept for these patients to incorporate into their home cooking styles [42]. Physical activity recommendations should also be given in context of cultural acceptability. A recent study by Hall and others concluded that some African-American women may be reluctant to follow exercise advice for fear that sweating will ruin their hairstyles [43]. Although providers need not be experts on the cultural norms of all of their patients, they should be open to discussing them, and to asking about the patient’s goals, ideal body type, comfort with physical activity, diet advice and other issues that will make individualized counseling much more effective.
PCP Concern: “Weight gain reflects the patient’s lack of will power and laziness”
Bias towards obese patients has been documented among health care providers [44,45]. Studies have shown that some providers have less respect for obese patients [46], perceive obese patients as nonadherent to medications [47], and associate obesity with “laziness,” “stupidity,” and “worthlessness” [48]. Furthermore, obese patients identify physicians as a primary source of stigma [49] and many report stigmatizing experiences during interactions with the healthcare system [44,45]. In one study, a considerable proportion of obese patients reported ever experiencing stigma from a doctor (69%) or a nurse (46%) [49]. As a result of these negative experiences, obese patients have reported avoiding or delaying medical services such as gynecological cancer screening [50]. A recent study by Gudzune et al found that obese patients had significantly greater odds of “doctor shopping,” where individuals saw 5 or more primary care providers in a 2-year period [51]. This doctor shopping behavior may also be motivated by dissatisfaction with care, as focus groups of obese women have reported doctor shopping until they find a health care provider who is comfortable, experienced, and skilled in treating obese patients [50].
Assessing Implicit and Explicit Weight Bias
In addition to explicit negative attitudes, health care providers may also hold implicit biases towards obese patients [52]. A recent study found that over half of medical students held an implicit anti-fat bias [53]. These implicit attitudes may manifest more subtly during patient encounters. PCPs engage in less emotional rapport building during visits with overweight and obese patients as compared to normal weight patients [54], which include behaviors such as expressing empathy, concern, reassurance, and partnership. The lack of rapport building could negatively influence the patient-provider relationship and decrease the effectiveness of weight loss counseling. PCPs may need to consider undergoing self-assessment to determine whether or not they hold negative implicit and/or explicit attitudes towards obese patients. PCPs can complete the Weight Implicit Association Test (IAT) for free online at https://implicit.harvard.edu/implicit/demo/. To determine whether they hold negative explicit attitudes, PCPs can download and complete assessments offered by the Yale Rudd Center for Food Policy and Obesity (www.yaleruddcenter.org/resources/bias_toolkit/index.html).
Pursuing Additional Training in Communication Skills
If weight bias is indeed present, PCPs may benefit from additional training in communication skills as well as specific guidance on how to discuss weight loss with overweight and obese patients. For example, an observational study found that patients lost more weight when they had weight loss counseling visits with physicians who used motivational interviewing strategies [20,21]. Additional PCP training in this area would benefit the patient-provider relationship, as research has shown that such patient-centered communication strategies lead to greater patient satisfaction [55,56], improvement in some clinical outcomes [57,58], and less physician burnout [59]. In fact, some medical schools address student weight bias during their obesity curricula [60]. Building communication skills helps improve PCPs’ capacity to show concern and empathy for patients’ struggles, avoid judgment and criticism, and give emotional support and encouragement, which may all improve PCPs’ ability to execute more sensitive weight loss discussions. For providers who are more interested in CME opportunities, the American Academy on Communication in Healthcare offers an online interactive learning program in this area called “Doc Com” (http://doccom.aachonline.org/dnn/Home.aspx).
PCP Concern: “I may not get reimbursed for weight management services”
Traditional metrics for how doctors are reimbursed and how the quality of their care is measured have not promoted weight loss counseling by PCPs. Prior to 2012, physicians could not bill Medicare for obesity-specific counseling visits [61]. Given that many private insurers follow the lead of the Centers for Medicare and Medicaid Services (CMS) for patterns of reimbursement, this issue has been pervasive in U.S. medical practice for a number of years, with considerable variability between plans on which obesity-related services are covered [62]. A recent study of U.S. health plans indicated that most would reject a claim for an office visit where obesity was the only coded diagnosis [62]. Additionally, the quality improvement movement has only recently begun to focus on issues of obesity. In 2009, the National Committee for Quality Assurance’s (NCQA) Healthcare Effectiveness Data and Information Set (HEDIS) added 2 new measures pertaining to the documentation of a patient’s BMI status. Prior to this time, even the simple act of acknowledging obesity was routinely underperformed and quite variable across health plans in the United States [63].
Obesity Screening and Counseling Benefits Coverage
In 2012, CMS made a major coverage change decision when they agreed to reimburse providers for delivering intensive behavioral interventions for obesity [61]. Namely, CMS will now cover a 6-month series of visits for Medicare patients (weekly for month 1, every other week for months 2–6), followed by monthly visits for an additional 6 months in patients who have been able to lose 3 kg. For PCPs and other providers who have long hoped for more opportunity to discuss nutrition, weight, and physical activity with their Medicare patients, these policy changes are exciting. Hopefully, this move by CMS will stimulate similar changes in the private insurance market.
Greater reimbursement of obesity-related care is also more likely given the overall trend of the U.S. health care system—with the focus shifting away from traditional fee-for-service models that have de-emphasized preventive care and counseling and toward a model that rewards well care [64]. Large employer groups, who represent an important voice in any discussion of health insurance and reimbursement, are also increasingly interested in the use of wellness programs and weight loss to decrease their own health care costs. This trend could further stimulate insurers to cover programs that allow providers to engage in weight counseling as a way of attracting or retaining large employer groups as customers [62].
Obesity Screening and Counseling Quality Metrics
A parallel movement in the quality of care realm would serve to bolster any forthcoming changes in reimbursement. For example, an expansion of the HEDIS “wellness and health promotion” measures, or going beyond “BMI assessment” to include a brief assessment of key dietary factors or physical activity level as a routine quality measure, would go a long way toward emphasizing to payers and providers the need for more routine obesity counseling. Professional provider organizations have been increasingly engaged in this area as well. The recent recognition by the American Medical Association of obesity as a disease may also influence organizations such as the NCQA and payers who may be considering how to encourage providers to better address this important issue.
PCP Concern: “I don’t have time to discuss weight loss during outpatient visits”
The average continuity visit for an adult patient in the United States is about 20 minutes in duration, with a mean of 6 to 7 clinical items to be addressed during that time-period [65]. This leaves little time for providers to perform the necessary history and physical portions of the visit, educate patients on various topics, and write out prescriptions or referrals. Not surprisingly, such extreme time pressure leads many PCPs to feel overwhelmed and burned out [66], and the idea of adding another “to-do” to office visits may be resisted. For obese patients, many of whom are likely to have multiple chronic conditions, PCPs are faced with the task of both discussing active issues such as hypertension, diabetes, and sleep apnea, and also potentially discussing the patient’s weight status in a very brief amount of time. Under such time pressures, PCPs often adopt a “putting out fires” mentality and therefore tackle what they see as the most pressing issues—eg, deal with out of control blood pressure by adding a new medication, or lowering hemoglobin A1c by upping the insulin dose, rather than dealing with the 20-lb weight gain that might be leading to the high pressures and hyperglycemia.
Compounding this problem is the fact that well-delivered preventive health advice can be time-consuming, and with so many topics to choose from, it may be difficult for providers to know which issues make the most sense to prioritize [67]. A recent study estimated that PCPs routinely under-counsel patients about nutrition (an advice topic that earns a “B” rating from the USPSTF), while they over-counsel them on exercise and PSA testing (topics that earn an “I” rating from the USPSTF) [68]. Topics of discussion and the time spent on them may reflect patient priorities or PCP comfort with various issues, but it is clear that some improvements could be made to better utilize available time with patients.
In the face of time and resource pressures, many PCPs may not be ideally suited to deliver the kind of intensive behavioral weight loss interventions that are supported by the best scientific evidence [69]. In fact, there is little evidence to support even brief weight counseling sessions by PCPs [70]. However, for busy providers, there are several brief and potentially impactful tasks that could enable them to better support their obese patients.
Brief Counseling Interventions in the Primary Care Setting
First, primary care providers should routinely measure and discuss their patients BMIs as they would any other vital sign. In addition, other brief measures such as “Exercise as a Vital Sign” [71] can be incorporated into the visit, so that behaviors linked to weight can inform the strategy adopted and monitored over time. After a brief discussion is initiated, a referral can be placed for patients who wish to pursue more intense therapy for weight loss—this may be to behavioral health, nutrition, bariatric surgery or a comprehensive weight management clinic. Practices can support their providers by streamlining this referral process and educating providers and patients on available resources. PCPs also may be able to engage their patients in self-monitoring (eg, calorie tracking, exercise tracking, self weighing) so that most of the work and learning takes place outside of the primary care office. For example, PCPs can promote the use of a food diary, a practice that has been shown to improve weight loss success [72]. Review of the diary could take place at a separate visit with the PCP or in follow-up with a weight loss specialist or dietitian.
A major strength of the primary care setting is its longitudinal nature. Even if available time at individual visits is short, advice and support can be given repeatedly over a longer period of time than may often be achieved with a specialist consultant. For patients who are in the maintenance phase of weight loss, having long-term frequent contacts with a provider has been shown to prevent weight regain [73]. The use of group visits and physician extenders (RNs, NPs, PAs) for delivering obesity-related behavioral advice might offer another way to relieve some of the time pressures faced by PCPs in the one-on-one chronic disease management visit [69,74].
PCP Concern: “I don’t know where to refer patients for weight management”
Surveys of obese patients and their doctors indicate that PCPs may not often enough refer patients to structured weight loss programs or registered dietitians [75,76]. Furthermore, PCPs are often isolated from other providers who might be important in a team-based model of obesity care, such as pharmacists, registered dietitians, endocrinologists, and bariatric surgeons. The implementation of the Affordable Care Act, including payment reform and the rise of accountable care organizations, should begin changing the relative isolation of the PCP. If more practices attempt to conform to medical home models, the interconnectedness of PCPs to other health care team members may increase, thus facilitating a more team-based approach to obesity care and easier referrals to specialized team members [77].
Weight Management Resources
Aside from some academic centers and large private health care institutions, many primary care practices lack access to structured obesity care clinics that can help manage the challenges of guiding patients through their weight loss options. For providers who practice in areas that do not afford them easy access to obesity care clinics, it is worth seeking out available resources in the nonmedical community that might provide a structured support system for patients. One low-cost community-based program, Take Off Pounds Sensibly (TOPS; www.tops.org), can achieve and sustain a 6% weight loss for active members [78]. Groups such as Overeaters Anonymous are found in most U.S. cities, and have helpful websites including podcasts that patients can access even in the absence of a local branch (www.oa.org). Organizations like the YMCA, which have good penetration into most areas of the country, offer affordable access to physical activity and health programs including coaching that can promote all around healthier living and improved dietary habits (www.ymca.net). A final consideration could be referral to a commercial weight loss program. A 2005 review of the major U.S. commercial weight loss programs concluded that there was suboptimal evidence for or against these programs’ efficacy [79]. A recent randomized controlled trial showed that patients referred by their PCP to a commercial weight loss program (Weight Watchers) lost significantly more weight (2.3 kg) at 12 months as compared to patients who only received weight loss advice from their PCP [80]. However, it is important to keep in mind that not all commercial programs are the same and some programs can be ineffective or even dangerous for some patients. The PCP may need to take an active role monitoring their patient’s health and safety when using these programs.
A Strategy to Incorporate Weight Management into Current Practice
Summary
Given the obesity epidemic, PCPs will need to begin addressing weight loss as a part of their normal practice; however, providers face several challenges in implementing weight management services. Many PCPs report receiving inadequate training in weight management during their training; however, many CME opportunities exist for providers to reduce their knowledge and skills deficit. Depending upon the prevalence of obesity in their practice and interest in offering weight management services, PCPs may need to consider more intensive weight management training or even pursue certification as an obesity medicine provider through the American Board of Obesity Medicine. For providers with a more general interest in obesity counseling, applying a consistent counseling approach like the 5A’s to several behaviors (eg, obesity, smoking cessation) may facilitate such counseling as a regular part of the outpatient encounter. PCPs should also be aware of different cultural considerations with respect to obesity including different body image perceptions and cooking styles. Obesity bias is pervasive in our society; therefore, PCPs may similarly hold negative explicit or implicit attitudes towards these patients. Providers can engage in online self-assessment about their explicit and implicit biases in order to understand whether they hold any negative attitudes towards obese patients. Additional training in communication skills and empathy may improve these patient-provider relationships and translate into more effective behavioral counseling. PCPs may be concerned about a lack of reimbursement for weight management services or a lack of time to perform counseling during outpatient encounters. With the new obesity counseling benefits coverage by CMS, PCPs should be reimbursed for obesity counseling services and provide additional time through dedicated weight management visits for Medicare patients. The new primary care practice models including the patient-centered medical home may facilitate PCP referrals to other weight management providers such as registered dieticians and health coaches, which could offset the PCP’s time pressures. Finally, PCPs can consider referrals to community resources, such as programs like Overeaters Anonymous, TOPS or the YMCA, to help provide patients group support for behavior change. In summary, PCPs may need to consider additional training to be prepared to deliver high quality obesity care in collaboration with other local partners and weight management specialists.
Corresponding author: Kimberly A. Gudzune, MD, MPH, 2024 E. Monument St, Room 2-611, Baltimore, MD 21287, [email protected].
Funding/support: Dr. Gudzune received support through a career development award from the National Heart, Lung, and Blood Institute (K23HL116601).
Financial disclosures: None
From the Kaiser Permanente Center for Health Research Southeast, Atlanta, GA (Dr. Lewis) and the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, MD (Dr. Gudzune).
Abstract
- Objective: To review challenges to obesity counseling in the primary care setting and suggest potential solutions.
- Methods: Review of the literature.
- Results: There are many challenges to obesity counseling in the primary care setting, including lack of primary care provider (PCP) training, provider weight bias, lack of reimbursement, lack of time during outpatient encounters, and limited ability to refer patients to structured weight loss support programs. However, there are potential solutions to overcome these challenges. By seeking continuing medical education on weight management and communication skills, PCPs can address any training gaps and establish rapport with patients when delivering obesity counseling. Recent policy changes including Medicare coverage of obesity counseling visits may reduce PCPs' concern about lack of reimbursement and time, and the rise of new models of care delivery and reimbursement, such as patient-centered medical homes or accountable care organizations, may facilitate referrals to ancillary providers like registered dietitians or multi-component weight loss programs.
- Conclusion: Although providers face several challenges in delivering effective obesity counseling, PCPs may overcome these obstacles by pursuing continuing medical education in this area and taking advantage of new health care benefits coverage and care delivery models.
Over one-third of U.S. adults are now obese [1] and the prevalence of obesity is rising globally (2). In 2003 and 2012, the U.S. Preventive Services Task Force (USPSTF) issued a recommendation that health care providers screen all patients for obesity and offer intensive, multicomponent behavioral interventions to obese patients [3,4]. Screening for obesity typically involves assessment and classification of a patient’s body mass index (BMI). In the primary care setting, weight management may include a range of therapeutic options such as intensive behavioral counseling, prescription anti-obesity medications, and referral to bariatric surgery. Behavioral interventions typically include activities such as goal setting, diet and exercise change, and self-monitoring. A recent systematic review showed that primary care–based behavioral interventions could result in modest weight losses of 3 kg over a 12-month period, and prevent the development of diabetes and hypertension in at-risk patients [5].
PCP Concern: “I never learned about weight management during my training”
One of the most common barriers to providing the recommended counseling reported by health care providers is inadequate training in nutrition, exercise, and weight loss counseling [10–12]. Many providers have knowledge deficiencies in basic weight management [13,14]. In addition, few PCPs who have received obesity-related training rate that training as good quality during medical school (23%) and residency (35%) [15].
Pursuing Additional Training in Weight Management
Providers could address their lack of training in weight management by participating in an obesity curriculum. When surveyed, PCPs have identified that additional training in nutrition counseling (93%) and exercise counseling (92%) would help them improve the care for obese patients, and many (60%) reported receiving good continuing medical education (CME) on this topic [15]. Much research in this area has examined the impact of such training on residents’ provision of obesity counseling. Residents who completed training improved the quality of obesity care that they provided [16], and those who learned appropriate obesity screening and counseling practices were more likely to report discussing lifestyle changes with their patients [17]. The vast majority of surveyed PCPs (86%) also felt that motivational interviewing [15], a technique that can effectively promote weight loss, would help them improve obesity care [18,19]. Patients demonstrated greater confidence in their ability to change their diet when their PCP used motivational interviewing–consistent techniques during counseling [20]; however, few PCPs utilize motivational interviewing techniques [20,21]. Offering CME opportunities for practicing PCPs to obtain skills in nutrition, exercise, and motivational interviewing would likely improve the quality of obesity care and weight loss counseling that are being delivered. PCPs could also consider attending an in-depth weight management and obesity
Applying a Universal Behavior Change Approach to Obesity and Other Behaviors
Another option may be encouraging PCPs to use a universal approach to behavioral counseling across multiple domains [22]. Using a single technique may lend familiarity and efficiency to the health care providers’ counseling [23]. The 5A’s—Assess, Advise, Agree, Assist, Arrange—has been proposed as a possible “universal” strategy that has demonstrated efficacy in both smoking cessation [24] and weight loss [25,26]. Using the 5A’s has been associated with increased motivation to lose weight [25] and increased weight loss [26]. Many physicians are familiar with the 5A’s; however, few physicians use the complete technique. PCPs have been found to most frequently “assess” and “advise” when using the 5A’s technique for weight loss counseling [26,27], although assisting and arranging are the components that have been associated with dietary change and weight loss [26]. PCPs could incorporate these A’s into their counseling routine by ensuring that they “assist” the patient by establishing appropriate lifestyle changes (eg, calorie tracking to achieve a 500 to 1000 calorie reduction per day) or referring to a weight loss program, and “arrange” for follow-up by scheduling an appointment in a few weeks to discuss the patient’s progress [23]. While the 5A’s can effectively promote weight loss, many PCPs would likely require training or retraining in this method to ensure its proper use. For PCPs interested in integrating the 5A’s into their weight management practice, we refer them to the algorithm described by Serdula and colleagues [23].
Cultural Influences on Weight Management
A final weight management training consideration relates to cultural awareness for patients who are from different racial or ethnic backgrounds than the PCP. In the United States, racial and ethnic minority groups are disproportionately burdened by obesity. Nearly 60% of non-Hispanic black women and 41% of Hispanic women are obese, compared with 33% of non-Hispanic whites [28]. Despite this fact, obese non-Hispanic black and Hispanic patients are more likely than white patients to perceive themselves as “slightly overweight” and to rate their health as good to excellent despite their obesity [29,30]. As a result, they may be less likely to seek out weight loss strategies on their own or ask for weight control advice from their providers [31]. Additionally, racial and ethnic disparities in access to healthy foods [32,33], safe areas for engaging in physical activity [34], and lack of social support for healthy behaviors may make it much more difficult for some minority patients to act on their PCP's advice.
Because of different cultures, social influences, and norms, what an individual patient perceives as obese or unhealthy may differ dramatically from what his or her physician views as obese or unhealthy [35–38]. Therefore, it is important that PCPs have a discussion with their patients about their subjective weight and health perceptions before beginning any prescriptive weight management strategies or discussions of “normal BMI” [39,40]. If an obese patient views herself as being at a normal weight for her culture, she is unlikely to respond well to being told by her doctor that she needs to lose 40 pounds to get to a healthy weight. Recent research suggests that alternative goals, such as encouraging weight maintenance for non-Hispanic black women, may be a successful alternative to the traditional pathway of encouraging weight loss [41].
In addition to understanding cultural context during weight status discussions, it is also important to give behavior change advice that is sensitive to the culture, race, and ethnicity of the patient. Dietary recommendations should take into account the patient’s culture. For example, Lindbergh et al have noted that cooking in traditional Hispanic culture does not rely as much on measurements as does cooking for non-Hispanic whites [42]. Therefore, measurement-based dietary advice (the cornerstone of portion control) may be a more problematic concept for these patients to incorporate into their home cooking styles [42]. Physical activity recommendations should also be given in context of cultural acceptability. A recent study by Hall and others concluded that some African-American women may be reluctant to follow exercise advice for fear that sweating will ruin their hairstyles [43]. Although providers need not be experts on the cultural norms of all of their patients, they should be open to discussing them, and to asking about the patient’s goals, ideal body type, comfort with physical activity, diet advice and other issues that will make individualized counseling much more effective.
PCP Concern: “Weight gain reflects the patient’s lack of will power and laziness”
Bias towards obese patients has been documented among health care providers [44,45]. Studies have shown that some providers have less respect for obese patients [46], perceive obese patients as nonadherent to medications [47], and associate obesity with “laziness,” “stupidity,” and “worthlessness” [48]. Furthermore, obese patients identify physicians as a primary source of stigma [49] and many report stigmatizing experiences during interactions with the healthcare system [44,45]. In one study, a considerable proportion of obese patients reported ever experiencing stigma from a doctor (69%) or a nurse (46%) [49]. As a result of these negative experiences, obese patients have reported avoiding or delaying medical services such as gynecological cancer screening [50]. A recent study by Gudzune et al found that obese patients had significantly greater odds of “doctor shopping,” where individuals saw 5 or more primary care providers in a 2-year period [51]. This doctor shopping behavior may also be motivated by dissatisfaction with care, as focus groups of obese women have reported doctor shopping until they find a health care provider who is comfortable, experienced, and skilled in treating obese patients [50].
Assessing Implicit and Explicit Weight Bias
In addition to explicit negative attitudes, health care providers may also hold implicit biases towards obese patients [52]. A recent study found that over half of medical students held an implicit anti-fat bias [53]. These implicit attitudes may manifest more subtly during patient encounters. PCPs engage in less emotional rapport building during visits with overweight and obese patients as compared to normal weight patients [54], which include behaviors such as expressing empathy, concern, reassurance, and partnership. The lack of rapport building could negatively influence the patient-provider relationship and decrease the effectiveness of weight loss counseling. PCPs may need to consider undergoing self-assessment to determine whether or not they hold negative implicit and/or explicit attitudes towards obese patients. PCPs can complete the Weight Implicit Association Test (IAT) for free online at https://implicit.harvard.edu/implicit/demo/. To determine whether they hold negative explicit attitudes, PCPs can download and complete assessments offered by the Yale Rudd Center for Food Policy and Obesity (www.yaleruddcenter.org/resources/bias_toolkit/index.html).
Pursuing Additional Training in Communication Skills
If weight bias is indeed present, PCPs may benefit from additional training in communication skills as well as specific guidance on how to discuss weight loss with overweight and obese patients. For example, an observational study found that patients lost more weight when they had weight loss counseling visits with physicians who used motivational interviewing strategies [20,21]. Additional PCP training in this area would benefit the patient-provider relationship, as research has shown that such patient-centered communication strategies lead to greater patient satisfaction [55,56], improvement in some clinical outcomes [57,58], and less physician burnout [59]. In fact, some medical schools address student weight bias during their obesity curricula [60]. Building communication skills helps improve PCPs’ capacity to show concern and empathy for patients’ struggles, avoid judgment and criticism, and give emotional support and encouragement, which may all improve PCPs’ ability to execute more sensitive weight loss discussions. For providers who are more interested in CME opportunities, the American Academy on Communication in Healthcare offers an online interactive learning program in this area called “Doc Com” (http://doccom.aachonline.org/dnn/Home.aspx).
PCP Concern: “I may not get reimbursed for weight management services”
Traditional metrics for how doctors are reimbursed and how the quality of their care is measured have not promoted weight loss counseling by PCPs. Prior to 2012, physicians could not bill Medicare for obesity-specific counseling visits [61]. Given that many private insurers follow the lead of the Centers for Medicare and Medicaid Services (CMS) for patterns of reimbursement, this issue has been pervasive in U.S. medical practice for a number of years, with considerable variability between plans on which obesity-related services are covered [62]. A recent study of U.S. health plans indicated that most would reject a claim for an office visit where obesity was the only coded diagnosis [62]. Additionally, the quality improvement movement has only recently begun to focus on issues of obesity. In 2009, the National Committee for Quality Assurance’s (NCQA) Healthcare Effectiveness Data and Information Set (HEDIS) added 2 new measures pertaining to the documentation of a patient’s BMI status. Prior to this time, even the simple act of acknowledging obesity was routinely underperformed and quite variable across health plans in the United States [63].
Obesity Screening and Counseling Benefits Coverage
In 2012, CMS made a major coverage change decision when they agreed to reimburse providers for delivering intensive behavioral interventions for obesity [61]. Namely, CMS will now cover a 6-month series of visits for Medicare patients (weekly for month 1, every other week for months 2–6), followed by monthly visits for an additional 6 months in patients who have been able to lose 3 kg. For PCPs and other providers who have long hoped for more opportunity to discuss nutrition, weight, and physical activity with their Medicare patients, these policy changes are exciting. Hopefully, this move by CMS will stimulate similar changes in the private insurance market.
Greater reimbursement of obesity-related care is also more likely given the overall trend of the U.S. health care system—with the focus shifting away from traditional fee-for-service models that have de-emphasized preventive care and counseling and toward a model that rewards well care [64]. Large employer groups, who represent an important voice in any discussion of health insurance and reimbursement, are also increasingly interested in the use of wellness programs and weight loss to decrease their own health care costs. This trend could further stimulate insurers to cover programs that allow providers to engage in weight counseling as a way of attracting or retaining large employer groups as customers [62].
Obesity Screening and Counseling Quality Metrics
A parallel movement in the quality of care realm would serve to bolster any forthcoming changes in reimbursement. For example, an expansion of the HEDIS “wellness and health promotion” measures, or going beyond “BMI assessment” to include a brief assessment of key dietary factors or physical activity level as a routine quality measure, would go a long way toward emphasizing to payers and providers the need for more routine obesity counseling. Professional provider organizations have been increasingly engaged in this area as well. The recent recognition by the American Medical Association of obesity as a disease may also influence organizations such as the NCQA and payers who may be considering how to encourage providers to better address this important issue.
PCP Concern: “I don’t have time to discuss weight loss during outpatient visits”
The average continuity visit for an adult patient in the United States is about 20 minutes in duration, with a mean of 6 to 7 clinical items to be addressed during that time-period [65]. This leaves little time for providers to perform the necessary history and physical portions of the visit, educate patients on various topics, and write out prescriptions or referrals. Not surprisingly, such extreme time pressure leads many PCPs to feel overwhelmed and burned out [66], and the idea of adding another “to-do” to office visits may be resisted. For obese patients, many of whom are likely to have multiple chronic conditions, PCPs are faced with the task of both discussing active issues such as hypertension, diabetes, and sleep apnea, and also potentially discussing the patient’s weight status in a very brief amount of time. Under such time pressures, PCPs often adopt a “putting out fires” mentality and therefore tackle what they see as the most pressing issues—eg, deal with out of control blood pressure by adding a new medication, or lowering hemoglobin A1c by upping the insulin dose, rather than dealing with the 20-lb weight gain that might be leading to the high pressures and hyperglycemia.
Compounding this problem is the fact that well-delivered preventive health advice can be time-consuming, and with so many topics to choose from, it may be difficult for providers to know which issues make the most sense to prioritize [67]. A recent study estimated that PCPs routinely under-counsel patients about nutrition (an advice topic that earns a “B” rating from the USPSTF), while they over-counsel them on exercise and PSA testing (topics that earn an “I” rating from the USPSTF) [68]. Topics of discussion and the time spent on them may reflect patient priorities or PCP comfort with various issues, but it is clear that some improvements could be made to better utilize available time with patients.
In the face of time and resource pressures, many PCPs may not be ideally suited to deliver the kind of intensive behavioral weight loss interventions that are supported by the best scientific evidence [69]. In fact, there is little evidence to support even brief weight counseling sessions by PCPs [70]. However, for busy providers, there are several brief and potentially impactful tasks that could enable them to better support their obese patients.
Brief Counseling Interventions in the Primary Care Setting
First, primary care providers should routinely measure and discuss their patients BMIs as they would any other vital sign. In addition, other brief measures such as “Exercise as a Vital Sign” [71] can be incorporated into the visit, so that behaviors linked to weight can inform the strategy adopted and monitored over time. After a brief discussion is initiated, a referral can be placed for patients who wish to pursue more intense therapy for weight loss—this may be to behavioral health, nutrition, bariatric surgery or a comprehensive weight management clinic. Practices can support their providers by streamlining this referral process and educating providers and patients on available resources. PCPs also may be able to engage their patients in self-monitoring (eg, calorie tracking, exercise tracking, self weighing) so that most of the work and learning takes place outside of the primary care office. For example, PCPs can promote the use of a food diary, a practice that has been shown to improve weight loss success [72]. Review of the diary could take place at a separate visit with the PCP or in follow-up with a weight loss specialist or dietitian.
A major strength of the primary care setting is its longitudinal nature. Even if available time at individual visits is short, advice and support can be given repeatedly over a longer period of time than may often be achieved with a specialist consultant. For patients who are in the maintenance phase of weight loss, having long-term frequent contacts with a provider has been shown to prevent weight regain [73]. The use of group visits and physician extenders (RNs, NPs, PAs) for delivering obesity-related behavioral advice might offer another way to relieve some of the time pressures faced by PCPs in the one-on-one chronic disease management visit [69,74].
PCP Concern: “I don’t know where to refer patients for weight management”
Surveys of obese patients and their doctors indicate that PCPs may not often enough refer patients to structured weight loss programs or registered dietitians [75,76]. Furthermore, PCPs are often isolated from other providers who might be important in a team-based model of obesity care, such as pharmacists, registered dietitians, endocrinologists, and bariatric surgeons. The implementation of the Affordable Care Act, including payment reform and the rise of accountable care organizations, should begin changing the relative isolation of the PCP. If more practices attempt to conform to medical home models, the interconnectedness of PCPs to other health care team members may increase, thus facilitating a more team-based approach to obesity care and easier referrals to specialized team members [77].
Weight Management Resources
Aside from some academic centers and large private health care institutions, many primary care practices lack access to structured obesity care clinics that can help manage the challenges of guiding patients through their weight loss options. For providers who practice in areas that do not afford them easy access to obesity care clinics, it is worth seeking out available resources in the nonmedical community that might provide a structured support system for patients. One low-cost community-based program, Take Off Pounds Sensibly (TOPS; www.tops.org), can achieve and sustain a 6% weight loss for active members [78]. Groups such as Overeaters Anonymous are found in most U.S. cities, and have helpful websites including podcasts that patients can access even in the absence of a local branch (www.oa.org). Organizations like the YMCA, which have good penetration into most areas of the country, offer affordable access to physical activity and health programs including coaching that can promote all around healthier living and improved dietary habits (www.ymca.net). A final consideration could be referral to a commercial weight loss program. A 2005 review of the major U.S. commercial weight loss programs concluded that there was suboptimal evidence for or against these programs’ efficacy [79]. A recent randomized controlled trial showed that patients referred by their PCP to a commercial weight loss program (Weight Watchers) lost significantly more weight (2.3 kg) at 12 months as compared to patients who only received weight loss advice from their PCP [80]. However, it is important to keep in mind that not all commercial programs are the same and some programs can be ineffective or even dangerous for some patients. The PCP may need to take an active role monitoring their patient’s health and safety when using these programs.
A Strategy to Incorporate Weight Management into Current Practice
Summary
Given the obesity epidemic, PCPs will need to begin addressing weight loss as a part of their normal practice; however, providers face several challenges in implementing weight management services. Many PCPs report receiving inadequate training in weight management during their training; however, many CME opportunities exist for providers to reduce their knowledge and skills deficit. Depending upon the prevalence of obesity in their practice and interest in offering weight management services, PCPs may need to consider more intensive weight management training or even pursue certification as an obesity medicine provider through the American Board of Obesity Medicine. For providers with a more general interest in obesity counseling, applying a consistent counseling approach like the 5A’s to several behaviors (eg, obesity, smoking cessation) may facilitate such counseling as a regular part of the outpatient encounter. PCPs should also be aware of different cultural considerations with respect to obesity including different body image perceptions and cooking styles. Obesity bias is pervasive in our society; therefore, PCPs may similarly hold negative explicit or implicit attitudes towards these patients. Providers can engage in online self-assessment about their explicit and implicit biases in order to understand whether they hold any negative attitudes towards obese patients. Additional training in communication skills and empathy may improve these patient-provider relationships and translate into more effective behavioral counseling. PCPs may be concerned about a lack of reimbursement for weight management services or a lack of time to perform counseling during outpatient encounters. With the new obesity counseling benefits coverage by CMS, PCPs should be reimbursed for obesity counseling services and provide additional time through dedicated weight management visits for Medicare patients. The new primary care practice models including the patient-centered medical home may facilitate PCP referrals to other weight management providers such as registered dieticians and health coaches, which could offset the PCP’s time pressures. Finally, PCPs can consider referrals to community resources, such as programs like Overeaters Anonymous, TOPS or the YMCA, to help provide patients group support for behavior change. In summary, PCPs may need to consider additional training to be prepared to deliver high quality obesity care in collaboration with other local partners and weight management specialists.
Corresponding author: Kimberly A. Gudzune, MD, MPH, 2024 E. Monument St, Room 2-611, Baltimore, MD 21287, [email protected].
Funding/support: Dr. Gudzune received support through a career development award from the National Heart, Lung, and Blood Institute (K23HL116601).
Financial disclosures: None
1. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–7.
2. Stevens GA, Singh GM, Lu Y, et al. National, regional, and global rends in adult overweight and obesity prevalences. Popul Health Metr 2012;10:22.
3. McTigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2003;139:933–49.
4. Moyer VA. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:373–8.
5. LeBlanc ES, O’Conner E, Whitlock EP, et al. Effectiveness of primary care – relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155:434–47.
6. Jackson JE, Doescher MP, Saver BG, Hart LG. Trends in professional advice to lose weight among obese adults, 1994 to 2000. J Gen Intern Med 2005;20:814–8.
7. McAlpine DD, Wilson AR. Trends in obesity-related counseling in primary care. Med Care 2007;45:322–9.
8. Bleich SN, Pickett-Blakley O, Cooper LA. Physician practice patterns of obesity diagnosis and weight-related counseling. Patient Educ Couns 2011;82:123–9.
9. Felix H, West DS, Bursac Z. Impact of USPSTF practice guidelines on provider weight loss counseling as reported by obese patients. Prev Med 2008;47:394–7.
10. Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med 1995;24:546–52.
11. Huang J, Yu H, Marin E, et al. Physicians’ weight loss counseling in two public hospital primary care clinics. Acad Med 2004;79:156–61.
12. Alexander SC, Ostbye T, Pollak KI, et al. Physicians’ beliefs about discussing obesity: results from focus groups. Am J Health Promot 2007;21:498–500.
13. Block JP, DeSalvo KB, Fisher WP. Are physicians equipped to address the obesity epidemic? Knowledge and attitudes of internal medicine residents. Prev Med 2003;36:669–75.
14. Jay M, Gillespie C, Ark T, et al. Do internists, pediatricians, and psychiatrists feel competent in obesity care?: using a needs assessment to drive curriculum design. J Gen Intern Med 2008;23:1066–70.
15. Bleich SN, Bennett WL, Gudzune KA, Cooper LA. National survey of US primary care physicians’ perspectives about causes of obesity and solutions to improve care. BMJ Open 2012;2(6).
16. Jay M, Schlair S, Caldwell R, et al. From the patient’s perspective: the impact of training on residnet physician’s obesity counseling. J Gen Intern Med 2010;25:415–22.
17. Forman-Hoffman V, Little A, Wahls T. Barriers to obesity management: a pilot study of primary care clinicians. BMC Fam Pract 2006;7:35.
18. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev 2011;12:709–23.
19. Martins RK, McNeil DW. Review of motivational interviewing in promoting health behaviors. Clin Psychol Rev 2009;29:283–93.
20. Cox ME, Yancy WS Jr, Coffman CJ, et al. Effects of counseling techniques on patients’ weight-related attitudes and behaviors in a primary care clinic. Patient Educ Couns 2011;5:363–8.
21. Pollak KI, Alexander SC, Coffman CJ, et al. Physician communication techniques and weight loss in adults: Project CHAT. Am J Prev Med 2010;39:321–8.
22. Whitlock EP, Orleans CT, Pender N, Allan J. Evaluating primary care behavioral counseling interventions: an evidence-based approach. Am J Prev Med 2002;22:267–84.
23. Serdula MK, Khan LK, Dietz WH. Weight loss counseling revisited. J Amer Med Assoc 2003;289:1747–50.
24. Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update—clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008.
25. Jay M, Gillespie C, Schlair S, et al. Physicians’ use of the 5As in counseling obese patients: is the quality of counseling associated with patients’ motivation and intention to lose weight? BMC Health Serv Res 2010;10:159.
26. Alexander SC, Cox ME, Boling Turner CL, et al Do the five A’s work when physicians counsel about weight loss? Fam Med 2011;43:179–84.
27. Flocke SA, Clark A, Schlessman K, Pomiecko G. Exercise, diet, and weight loss advice in the family medicine outpatient setting. Fam Med 2005;37:415–21.
28. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief 2012;(82):1–8.
29. Burroughs VJ, et al. Self-reported comorbidities among self-described overweight African-American and Hispanic adults in the United States: results of a national survey. Obesity 2008;16:1400–6.
30. Dorsey RR, Eberhardt MS, Ogden CL. Racial/ethnic differences in weight perception. Obesity 2009;17:790–5.
31. Dorsey RR, Eberhardt MS, Ogden CL. Racial and ethnic differences in weight management behavior by weight perception status. Ethnic Dis 2010;20:244–50.
32. Baker EA, Schootman M, Barnidge E, Kelly C. The role of race and poverty in access to foods that enable individuals to adhere to dietary guidelines. Prev Chronic Dis 2006;3(3):A76.
33. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med 2009;36:74–81.
34. Gordon-Larsen P, et al. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics 2006;117:417–24.
35. Kumanyika S, Wilson JF, Guilford-Davenport M. Weight-related attitudes and behaviors of black women. J Am Dietetic Assoc 1993;93:416–22.
36. Chithambo TP, Huey SJ. Black/white differences in perceived weight and attractiveness among overweight women. J Obes 2013;2013:320–6.
37. Paeratakul S, et al. Sex, race/ethnicity, socioeconomic status, and BMI in relation to self-perception of overweight. Obesity Res 2002;10:345–50.
38. Bennett GG, et al. Attitudes regarding overweight, exercise, and health among blacks (United States). Cancer Causes Control 2006;17:95–101.
39. Kumanyika SK, et al. Expanding the obesity research paradigm to reach African American communities. Prev Chronic Disease 2007;4(4).
40. Stuart-Shor EM, Berra KA, Kamau MW, Kumanyika SK. Behavioral strategies for cardiovascular risk reduction in diverse and underserved racial/ethnic groups. Circulation 2012;125:171–84.
41. Bennett GG, Foley P, Levine E, et al. Behavioral treatment for weight gain prevention among black women in primary care practice: a randomized clinical trial. JAMA Intern Med 2013;173:1770–7.
42. Lindberg NM, Stevens VJ, Halperin RO. Weight-loss interventions for Hispanic populations: the role of culture. J Obes 2012:542736.
43. Hall RR, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatology 2013;149:310–4.
44. Puhl RM, Brownell KD. Bias, discrimination, and obesity. Obes Res 2001;9:788–905.
45. Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring) 2009;17:941–64.
46. Huizinga MM, Cooper LA, Bleich SN, et al. Physician respect for patients with obesity. J Gen Intern Med 2009;24:1236–9.
47. Huizinga MM, Bleich SN, Beach MC, et al. Disparity in physician perception of patients’ adherence to medications by obesity status. Obesity (Silver Spring) 2010;18:1932–7.
48. Schwartz MB, Chambliss HO, Brownell KD, et al. Weight bias among health professionals specializing in obesity. Obes Res 2003;11:1033–9.
49. Puhl RM, Brownell KD. Confronting and coping with weight stigma: an investigation of overweight and obese adults. Obesity (Silver Spring) 2006;14:1802–15.
50. Amy NK, Aalborg A, Lyons P, Keranen L. Barriers to routine gynecological cancer screening for White and African-American obese women. Int J Obes (Lond) 2006;30:147–55.
51. Gudzune KA, Bleich SN, Richards TM, et al. Doctor shopping by overweight and obese patients is associated with increased healthcare utilization. Obesity (Silver Spring) 2013;21:1328–34.
52. Teachman BA, Brownell KD. Implicit anti-fat bias among health professionals: is anyone immune? Int J Obes Relat Metab Disord 2001;25:1525–31.
53. Miller DP Jr, Spangler JG, Vitolins MZ, et al. Are medical students aware of their anti-obesity bias? Acad Med 2013;88:978–82.
54. Gudzune KA, Beach MC, Roter DL, Cooper LA. Physicians build less rapport with obese patients. Obesity (Silver Spring) 2013;21:2146–52.
55. Beck RS, Daughtridge R, Sloane PD. Physician-patient communication in the primary care office: a systematic review. J Am Board Fam Pract 2002;15:25–38.
56. Dwamena F, Holmes-Rovner M, Gaulden CM, et al. Interventions for providers to promote patient-center approach in clinical consultations. Cochrane Database Syst Rev 2012;12:CD003267.
57. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ 1995;152:1423–33.
58. Hojat M, Louis DZ, Markham FW, et al. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med 2011;86:359–64.
59. Krasner MS, Epstein RM, Beckman H, et al. Association of an educational program in mindful communication with burnout, empathy, and attitudes among primary care physicians. JAMA 2009;302:1284––93.
60. Vitolins MZ, Crandall S, Miller D, et al. Obesity educational interventions in U.S. medical schools: a systematic review and identified gaps. Teach Learn Med 2012;24:267–72.
61. Centers for Medicare and Medicaid Services, Decision memo for intensive behavioral therapy for obesity. 2012.
62. Simpson LA, Cooper J. Paying for obesity: a changing landscape. Pediatrics 2009;123 Suppl 5:5301–7.
63. Arterburn DE, et al. Body mass index measurement and obesity prevalence in ten U.S. health plans. Clin Med Res 2010;8:126–30.
64. Koh HK, Sebelius KG. Promoting prevention through the Affordable Care Act. N Engl J Med 2010; 363:1296–9.
65. Abbo ED, et al. The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med 2008;23:2058–65.
66. Mechanic D. Physician discontent: challenges and opportunities. JAMA 2003;290:941–6.
67. Yarnall KS, Pollak KI, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health 2003;93:635–41.
68. Pollak KI, Krause KM, Yarnall KS, et al. Estimated time spent on preventive services by primary care physicians. BMC Health Services Research 2008;8:245.
69. Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med 2009;24:1073–9.
70. Carvajal R, Wadden TA, Tsai AG, et al. Managing obesity in primary care practice: a narrative review. Ann N Y Acad Sci 2013;1281:191–06.
71. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc 2012;44:2071–6.
72. Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity 2007;15:3091–6.
73. Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psych Clin North Am 2011;34:841–59.
74. Lavoie JG, Wong ST, Chongo M, et al. Group medical visits can deliver on patient-centered care objectives: results from a qualitative study. BMC Health Serv Res 2013;13:2013.
75. Wadden TA, Anderson DA, Foster GD, et al. Obese women’s perceptions of their physicians’ weight management attitudes and practices. Arch Fam Med 2000;9:854–60.
76. Phelan SP, et al. What do physicians recommend to their overweight and obese patients? J Am Board Fam Med 2009;22:115–22.
77. Grant RMA, Greene DD. The health care home model: primary health care meeting public health goals. Am J Public Health 2012;102:1096–103.
78. Mitchell NS, Dickinson LM, Kempe A, Tsai AG. Determining the effectiveness of Take Off Pounds Sensibly (TOPS), a nationally available nonprofit weight loss program. Obesity (Silver Spring) 2011;19:568–73.
79. Tsai AG, Wadden TA. Systematic review: An evaluation of major commercial weight loss programs in the United States. Ann Intern Med 2005;142:56–66.
80. Jebb SA, Ahern AL, Olson AD, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet 2011;378:1485–92.
81. Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379–87.
82. Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81–95.
83. Scott JG, Cohen D, DiCicco-Bloom B, et al. Speaking of weight: how patients and primary care clinicians initiate weight loss counseling. Prev Med 2004;38:819–27.
84. Brown I, Thompson J, Tod A, Jones G. Primary care support for tacking obesity: a qualitative study of the perceptions of obese patients. Br J Gen Pract 2006;56:666–72.
85. Tailor A, Ogden J. Avoiding the term ‘obesity:’ an experimental study of the impact of doctors’ language on patients’ beliefs. Patient Educ Couns 2009;76:260–4.
86. Pollak KI, Ostbye T, Alexander SC, et al. Empathy goes a long way in weight loss discussions. J Fam Pract 2007;56:1031–6.
1. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–7.
2. Stevens GA, Singh GM, Lu Y, et al. National, regional, and global rends in adult overweight and obesity prevalences. Popul Health Metr 2012;10:22.
3. McTigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2003;139:933–49.
4. Moyer VA. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:373–8.
5. LeBlanc ES, O’Conner E, Whitlock EP, et al. Effectiveness of primary care – relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155:434–47.
6. Jackson JE, Doescher MP, Saver BG, Hart LG. Trends in professional advice to lose weight among obese adults, 1994 to 2000. J Gen Intern Med 2005;20:814–8.
7. McAlpine DD, Wilson AR. Trends in obesity-related counseling in primary care. Med Care 2007;45:322–9.
8. Bleich SN, Pickett-Blakley O, Cooper LA. Physician practice patterns of obesity diagnosis and weight-related counseling. Patient Educ Couns 2011;82:123–9.
9. Felix H, West DS, Bursac Z. Impact of USPSTF practice guidelines on provider weight loss counseling as reported by obese patients. Prev Med 2008;47:394–7.
10. Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med 1995;24:546–52.
11. Huang J, Yu H, Marin E, et al. Physicians’ weight loss counseling in two public hospital primary care clinics. Acad Med 2004;79:156–61.
12. Alexander SC, Ostbye T, Pollak KI, et al. Physicians’ beliefs about discussing obesity: results from focus groups. Am J Health Promot 2007;21:498–500.
13. Block JP, DeSalvo KB, Fisher WP. Are physicians equipped to address the obesity epidemic? Knowledge and attitudes of internal medicine residents. Prev Med 2003;36:669–75.
14. Jay M, Gillespie C, Ark T, et al. Do internists, pediatricians, and psychiatrists feel competent in obesity care?: using a needs assessment to drive curriculum design. J Gen Intern Med 2008;23:1066–70.
15. Bleich SN, Bennett WL, Gudzune KA, Cooper LA. National survey of US primary care physicians’ perspectives about causes of obesity and solutions to improve care. BMJ Open 2012;2(6).
16. Jay M, Schlair S, Caldwell R, et al. From the patient’s perspective: the impact of training on residnet physician’s obesity counseling. J Gen Intern Med 2010;25:415–22.
17. Forman-Hoffman V, Little A, Wahls T. Barriers to obesity management: a pilot study of primary care clinicians. BMC Fam Pract 2006;7:35.
18. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev 2011;12:709–23.
19. Martins RK, McNeil DW. Review of motivational interviewing in promoting health behaviors. Clin Psychol Rev 2009;29:283–93.
20. Cox ME, Yancy WS Jr, Coffman CJ, et al. Effects of counseling techniques on patients’ weight-related attitudes and behaviors in a primary care clinic. Patient Educ Couns 2011;5:363–8.
21. Pollak KI, Alexander SC, Coffman CJ, et al. Physician communication techniques and weight loss in adults: Project CHAT. Am J Prev Med 2010;39:321–8.
22. Whitlock EP, Orleans CT, Pender N, Allan J. Evaluating primary care behavioral counseling interventions: an evidence-based approach. Am J Prev Med 2002;22:267–84.
23. Serdula MK, Khan LK, Dietz WH. Weight loss counseling revisited. J Amer Med Assoc 2003;289:1747–50.
24. Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update—clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008.
25. Jay M, Gillespie C, Schlair S, et al. Physicians’ use of the 5As in counseling obese patients: is the quality of counseling associated with patients’ motivation and intention to lose weight? BMC Health Serv Res 2010;10:159.
26. Alexander SC, Cox ME, Boling Turner CL, et al Do the five A’s work when physicians counsel about weight loss? Fam Med 2011;43:179–84.
27. Flocke SA, Clark A, Schlessman K, Pomiecko G. Exercise, diet, and weight loss advice in the family medicine outpatient setting. Fam Med 2005;37:415–21.
28. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief 2012;(82):1–8.
29. Burroughs VJ, et al. Self-reported comorbidities among self-described overweight African-American and Hispanic adults in the United States: results of a national survey. Obesity 2008;16:1400–6.
30. Dorsey RR, Eberhardt MS, Ogden CL. Racial/ethnic differences in weight perception. Obesity 2009;17:790–5.
31. Dorsey RR, Eberhardt MS, Ogden CL. Racial and ethnic differences in weight management behavior by weight perception status. Ethnic Dis 2010;20:244–50.
32. Baker EA, Schootman M, Barnidge E, Kelly C. The role of race and poverty in access to foods that enable individuals to adhere to dietary guidelines. Prev Chronic Dis 2006;3(3):A76.
33. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med 2009;36:74–81.
34. Gordon-Larsen P, et al. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics 2006;117:417–24.
35. Kumanyika S, Wilson JF, Guilford-Davenport M. Weight-related attitudes and behaviors of black women. J Am Dietetic Assoc 1993;93:416–22.
36. Chithambo TP, Huey SJ. Black/white differences in perceived weight and attractiveness among overweight women. J Obes 2013;2013:320–6.
37. Paeratakul S, et al. Sex, race/ethnicity, socioeconomic status, and BMI in relation to self-perception of overweight. Obesity Res 2002;10:345–50.
38. Bennett GG, et al. Attitudes regarding overweight, exercise, and health among blacks (United States). Cancer Causes Control 2006;17:95–101.
39. Kumanyika SK, et al. Expanding the obesity research paradigm to reach African American communities. Prev Chronic Disease 2007;4(4).
40. Stuart-Shor EM, Berra KA, Kamau MW, Kumanyika SK. Behavioral strategies for cardiovascular risk reduction in diverse and underserved racial/ethnic groups. Circulation 2012;125:171–84.
41. Bennett GG, Foley P, Levine E, et al. Behavioral treatment for weight gain prevention among black women in primary care practice: a randomized clinical trial. JAMA Intern Med 2013;173:1770–7.
42. Lindberg NM, Stevens VJ, Halperin RO. Weight-loss interventions for Hispanic populations: the role of culture. J Obes 2012:542736.
43. Hall RR, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatology 2013;149:310–4.
44. Puhl RM, Brownell KD. Bias, discrimination, and obesity. Obes Res 2001;9:788–905.
45. Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring) 2009;17:941–64.
46. Huizinga MM, Cooper LA, Bleich SN, et al. Physician respect for patients with obesity. J Gen Intern Med 2009;24:1236–9.
47. Huizinga MM, Bleich SN, Beach MC, et al. Disparity in physician perception of patients’ adherence to medications by obesity status. Obesity (Silver Spring) 2010;18:1932–7.
48. Schwartz MB, Chambliss HO, Brownell KD, et al. Weight bias among health professionals specializing in obesity. Obes Res 2003;11:1033–9.
49. Puhl RM, Brownell KD. Confronting and coping with weight stigma: an investigation of overweight and obese adults. Obesity (Silver Spring) 2006;14:1802–15.
50. Amy NK, Aalborg A, Lyons P, Keranen L. Barriers to routine gynecological cancer screening for White and African-American obese women. Int J Obes (Lond) 2006;30:147–55.
51. Gudzune KA, Bleich SN, Richards TM, et al. Doctor shopping by overweight and obese patients is associated with increased healthcare utilization. Obesity (Silver Spring) 2013;21:1328–34.
52. Teachman BA, Brownell KD. Implicit anti-fat bias among health professionals: is anyone immune? Int J Obes Relat Metab Disord 2001;25:1525–31.
53. Miller DP Jr, Spangler JG, Vitolins MZ, et al. Are medical students aware of their anti-obesity bias? Acad Med 2013;88:978–82.
54. Gudzune KA, Beach MC, Roter DL, Cooper LA. Physicians build less rapport with obese patients. Obesity (Silver Spring) 2013;21:2146–52.
55. Beck RS, Daughtridge R, Sloane PD. Physician-patient communication in the primary care office: a systematic review. J Am Board Fam Pract 2002;15:25–38.
56. Dwamena F, Holmes-Rovner M, Gaulden CM, et al. Interventions for providers to promote patient-center approach in clinical consultations. Cochrane Database Syst Rev 2012;12:CD003267.
57. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ 1995;152:1423–33.
58. Hojat M, Louis DZ, Markham FW, et al. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med 2011;86:359–64.
59. Krasner MS, Epstein RM, Beckman H, et al. Association of an educational program in mindful communication with burnout, empathy, and attitudes among primary care physicians. JAMA 2009;302:1284––93.
60. Vitolins MZ, Crandall S, Miller D, et al. Obesity educational interventions in U.S. medical schools: a systematic review and identified gaps. Teach Learn Med 2012;24:267–72.
61. Centers for Medicare and Medicaid Services, Decision memo for intensive behavioral therapy for obesity. 2012.
62. Simpson LA, Cooper J. Paying for obesity: a changing landscape. Pediatrics 2009;123 Suppl 5:5301–7.
63. Arterburn DE, et al. Body mass index measurement and obesity prevalence in ten U.S. health plans. Clin Med Res 2010;8:126–30.
64. Koh HK, Sebelius KG. Promoting prevention through the Affordable Care Act. N Engl J Med 2010; 363:1296–9.
65. Abbo ED, et al. The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med 2008;23:2058–65.
66. Mechanic D. Physician discontent: challenges and opportunities. JAMA 2003;290:941–6.
67. Yarnall KS, Pollak KI, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health 2003;93:635–41.
68. Pollak KI, Krause KM, Yarnall KS, et al. Estimated time spent on preventive services by primary care physicians. BMC Health Services Research 2008;8:245.
69. Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med 2009;24:1073–9.
70. Carvajal R, Wadden TA, Tsai AG, et al. Managing obesity in primary care practice: a narrative review. Ann N Y Acad Sci 2013;1281:191–06.
71. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc 2012;44:2071–6.
72. Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity 2007;15:3091–6.
73. Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psych Clin North Am 2011;34:841–59.
74. Lavoie JG, Wong ST, Chongo M, et al. Group medical visits can deliver on patient-centered care objectives: results from a qualitative study. BMC Health Serv Res 2013;13:2013.
75. Wadden TA, Anderson DA, Foster GD, et al. Obese women’s perceptions of their physicians’ weight management attitudes and practices. Arch Fam Med 2000;9:854–60.
76. Phelan SP, et al. What do physicians recommend to their overweight and obese patients? J Am Board Fam Med 2009;22:115–22.
77. Grant RMA, Greene DD. The health care home model: primary health care meeting public health goals. Am J Public Health 2012;102:1096–103.
78. Mitchell NS, Dickinson LM, Kempe A, Tsai AG. Determining the effectiveness of Take Off Pounds Sensibly (TOPS), a nationally available nonprofit weight loss program. Obesity (Silver Spring) 2011;19:568–73.
79. Tsai AG, Wadden TA. Systematic review: An evaluation of major commercial weight loss programs in the United States. Ann Intern Med 2005;142:56–66.
80. Jebb SA, Ahern AL, Olson AD, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet 2011;378:1485–92.
81. Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379–87.
82. Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81–95.
83. Scott JG, Cohen D, DiCicco-Bloom B, et al. Speaking of weight: how patients and primary care clinicians initiate weight loss counseling. Prev Med 2004;38:819–27.
84. Brown I, Thompson J, Tod A, Jones G. Primary care support for tacking obesity: a qualitative study of the perceptions of obese patients. Br J Gen Pract 2006;56:666–72.
85. Tailor A, Ogden J. Avoiding the term ‘obesity:’ an experimental study of the impact of doctors’ language on patients’ beliefs. Patient Educ Couns 2009;76:260–4.
86. Pollak KI, Ostbye T, Alexander SC, et al. Empathy goes a long way in weight loss discussions. J Fam Pract 2007;56:1031–6.
Investigation reveals ‘inappropriate data handling’ but no misconduct
generated from STAP cells
Credit: Haruko Obokata
The Japanese research institute RIKEN has released early results of its investigation into allegations of misconduct leveled against the creators of STAP cells (stimulus-triggered acquisition of pluripotency cells).
RIKEN has confirmed 2 cases of “inappropriate data handling” but said the circumstances did not constitute misconduct.
The investigation is ongoing, with 4 issues—including charges of plagiarism and doctored figures—still to be resolved.
Research prompts questions, criticism
The investigation began shortly after a group of RIKEN scientists and colleagues from a few other institutions announced their creation of STAP cells.
The researchers said they could induce pluripotency in somatic cells by introducing the cells to a low-pH environment, and they reported this discovery in an article and a letter to Nature.
Not long after the papers were published, members of the scientific community began questioning the validity of the research, citing issues with images, possible plagiarism, and an inability to replicate the experiments described.
In light of these issues, one of the study authors recently called for the research to be retracted.
Teruhiko Wakayama, PhD, formerly of RIKEN but now a professor at the University of Yamanashi, said there are “too many uncertainties” surrounding the research at this point. After a retraction, the researchers could collect new data and images to ensure their accuracy and resubmit the research for publication.
On the other hand, fellow study author Charles Vacanti, MD, of Brigham and Women’s Hospital in Boston, has said a retraction is unnecessary.
“I firmly believe that the questions and concerns raised about our STAP cell paper published in Nature do not affect our findings or conclusions,” Dr Vacanti said.
Investigation launched
In response to the questions and allegations, RIKEN formed a committee to investigate the possibility of misconduct.
The investigation is focusing on 4 of the researchers involved: Haruko Obokata, PhD; Yoshiki Sasai, MD, PhD; Hitoshi Niwa, MD, PhD; and Dr Wakayama.
The committee is also looking into 6 issues with the research, 2 of which have been resolved.
Resolved issues
(1) Critics have questioned the “unnatural appearance of colored cell parts shown by arrows in d2 and d3 images of Figure 1f” in the article.
RIKEN concluded that the process of preparing these images did not constitute fabrication within the context of research misconduct.
(2) Questions have been raised about a “strong resemblance between the rightmost panel in Figure 1b and the lower panel in Figure 2g, both showing a fluorescence image of mice placenta” in the letter.
There is no reference to the figures in the figure legends or the main body of text, and RIKEN does define this sort of discrepancy as fabrication. However, the researchers claimed they had intended to delete one of the figures prior to publication but forgot, and there is no evidence to contradict that explanation. So RIKEN concluded that no malice was intended, and this should not be considered misconduct.
Issues under investigation
(1) In Figure 1i of the article, lane 3 appears to have been inserted.
(2) A part of the article’s “Methods” section on karyotyping analysis appears to have been copied from another paper.
(3) Some of the description of karyotyping in the “Methods” section of the article is different from the procedure the researchers followed.
(4) In the article, the image of differentiated cells for Figures 2d and 2e and the image of chimera mouse immunostaining data are incorrect, and investigation revealed that these images closely resemble images Dr Obokata used in her doctoral dissertation.
Next steps
RIKEN said it will continue with the investigation and issue a full report upon its completion. The institute also aims to determine whether the STAP cell experiments can be reproduced.
“The reproducibility and credibility of the STAP phenomenon must be rigorously validated, not only by RIKEN scientists, but also by others,” said RIKEN President Ryoji Noyori, PhD.
“I have instructed our people to cooperate fully with researchers at outside institutions in their efforts to replicate the STAP cell results.”
Dr Noyori added that RIKEN is prepared to withdraw the Nature papers and take “strict disciplinary action” against the researchers involved if the investigation reveals deliberate misconduct. ![]()

generated from STAP cells
Credit: Haruko Obokata
The Japanese research institute RIKEN has released early results of its investigation into allegations of misconduct leveled against the creators of STAP cells (stimulus-triggered acquisition of pluripotency cells).
RIKEN has confirmed 2 cases of “inappropriate data handling” but said the circumstances did not constitute misconduct.
The investigation is ongoing, with 4 issues—including charges of plagiarism and doctored figures—still to be resolved.
Research prompts questions, criticism
The investigation began shortly after a group of RIKEN scientists and colleagues from a few other institutions announced their creation of STAP cells.
The researchers said they could induce pluripotency in somatic cells by introducing the cells to a low-pH environment, and they reported this discovery in an article and a letter to Nature.
Not long after the papers were published, members of the scientific community began questioning the validity of the research, citing issues with images, possible plagiarism, and an inability to replicate the experiments described.
In light of these issues, one of the study authors recently called for the research to be retracted.
Teruhiko Wakayama, PhD, formerly of RIKEN but now a professor at the University of Yamanashi, said there are “too many uncertainties” surrounding the research at this point. After a retraction, the researchers could collect new data and images to ensure their accuracy and resubmit the research for publication.
On the other hand, fellow study author Charles Vacanti, MD, of Brigham and Women’s Hospital in Boston, has said a retraction is unnecessary.
“I firmly believe that the questions and concerns raised about our STAP cell paper published in Nature do not affect our findings or conclusions,” Dr Vacanti said.
Investigation launched
In response to the questions and allegations, RIKEN formed a committee to investigate the possibility of misconduct.
The investigation is focusing on 4 of the researchers involved: Haruko Obokata, PhD; Yoshiki Sasai, MD, PhD; Hitoshi Niwa, MD, PhD; and Dr Wakayama.
The committee is also looking into 6 issues with the research, 2 of which have been resolved.
Resolved issues
(1) Critics have questioned the “unnatural appearance of colored cell parts shown by arrows in d2 and d3 images of Figure 1f” in the article.
RIKEN concluded that the process of preparing these images did not constitute fabrication within the context of research misconduct.
(2) Questions have been raised about a “strong resemblance between the rightmost panel in Figure 1b and the lower panel in Figure 2g, both showing a fluorescence image of mice placenta” in the letter.
There is no reference to the figures in the figure legends or the main body of text, and RIKEN does define this sort of discrepancy as fabrication. However, the researchers claimed they had intended to delete one of the figures prior to publication but forgot, and there is no evidence to contradict that explanation. So RIKEN concluded that no malice was intended, and this should not be considered misconduct.
Issues under investigation
(1) In Figure 1i of the article, lane 3 appears to have been inserted.
(2) A part of the article’s “Methods” section on karyotyping analysis appears to have been copied from another paper.
(3) Some of the description of karyotyping in the “Methods” section of the article is different from the procedure the researchers followed.
(4) In the article, the image of differentiated cells for Figures 2d and 2e and the image of chimera mouse immunostaining data are incorrect, and investigation revealed that these images closely resemble images Dr Obokata used in her doctoral dissertation.
Next steps
RIKEN said it will continue with the investigation and issue a full report upon its completion. The institute also aims to determine whether the STAP cell experiments can be reproduced.
“The reproducibility and credibility of the STAP phenomenon must be rigorously validated, not only by RIKEN scientists, but also by others,” said RIKEN President Ryoji Noyori, PhD.
“I have instructed our people to cooperate fully with researchers at outside institutions in their efforts to replicate the STAP cell results.”
Dr Noyori added that RIKEN is prepared to withdraw the Nature papers and take “strict disciplinary action” against the researchers involved if the investigation reveals deliberate misconduct. ![]()

generated from STAP cells
Credit: Haruko Obokata
The Japanese research institute RIKEN has released early results of its investigation into allegations of misconduct leveled against the creators of STAP cells (stimulus-triggered acquisition of pluripotency cells).
RIKEN has confirmed 2 cases of “inappropriate data handling” but said the circumstances did not constitute misconduct.
The investigation is ongoing, with 4 issues—including charges of plagiarism and doctored figures—still to be resolved.
Research prompts questions, criticism
The investigation began shortly after a group of RIKEN scientists and colleagues from a few other institutions announced their creation of STAP cells.
The researchers said they could induce pluripotency in somatic cells by introducing the cells to a low-pH environment, and they reported this discovery in an article and a letter to Nature.
Not long after the papers were published, members of the scientific community began questioning the validity of the research, citing issues with images, possible plagiarism, and an inability to replicate the experiments described.
In light of these issues, one of the study authors recently called for the research to be retracted.
Teruhiko Wakayama, PhD, formerly of RIKEN but now a professor at the University of Yamanashi, said there are “too many uncertainties” surrounding the research at this point. After a retraction, the researchers could collect new data and images to ensure their accuracy and resubmit the research for publication.
On the other hand, fellow study author Charles Vacanti, MD, of Brigham and Women’s Hospital in Boston, has said a retraction is unnecessary.
“I firmly believe that the questions and concerns raised about our STAP cell paper published in Nature do not affect our findings or conclusions,” Dr Vacanti said.
Investigation launched
In response to the questions and allegations, RIKEN formed a committee to investigate the possibility of misconduct.
The investigation is focusing on 4 of the researchers involved: Haruko Obokata, PhD; Yoshiki Sasai, MD, PhD; Hitoshi Niwa, MD, PhD; and Dr Wakayama.
The committee is also looking into 6 issues with the research, 2 of which have been resolved.
Resolved issues
(1) Critics have questioned the “unnatural appearance of colored cell parts shown by arrows in d2 and d3 images of Figure 1f” in the article.
RIKEN concluded that the process of preparing these images did not constitute fabrication within the context of research misconduct.
(2) Questions have been raised about a “strong resemblance between the rightmost panel in Figure 1b and the lower panel in Figure 2g, both showing a fluorescence image of mice placenta” in the letter.
There is no reference to the figures in the figure legends or the main body of text, and RIKEN does define this sort of discrepancy as fabrication. However, the researchers claimed they had intended to delete one of the figures prior to publication but forgot, and there is no evidence to contradict that explanation. So RIKEN concluded that no malice was intended, and this should not be considered misconduct.
Issues under investigation
(1) In Figure 1i of the article, lane 3 appears to have been inserted.
(2) A part of the article’s “Methods” section on karyotyping analysis appears to have been copied from another paper.
(3) Some of the description of karyotyping in the “Methods” section of the article is different from the procedure the researchers followed.
(4) In the article, the image of differentiated cells for Figures 2d and 2e and the image of chimera mouse immunostaining data are incorrect, and investigation revealed that these images closely resemble images Dr Obokata used in her doctoral dissertation.
Next steps
RIKEN said it will continue with the investigation and issue a full report upon its completion. The institute also aims to determine whether the STAP cell experiments can be reproduced.
“The reproducibility and credibility of the STAP phenomenon must be rigorously validated, not only by RIKEN scientists, but also by others,” said RIKEN President Ryoji Noyori, PhD.
“I have instructed our people to cooperate fully with researchers at outside institutions in their efforts to replicate the STAP cell results.”
Dr Noyori added that RIKEN is prepared to withdraw the Nature papers and take “strict disciplinary action” against the researchers involved if the investigation reveals deliberate misconduct. ![]()
VIDEO: Generational dermatology teaches patients to think long term
CHAMPIONSGATE, FLA. – "Aging doesn’t happen overnight," according to Dr. Wendy Roberts, medical director of Desert Dermatology in Rancho Mirage, Calif.
In a video interview at the Orlando Dermatology Aesthetic and Clinical Conference, Dr. Roberts explained the concept of "generational dermatology" and how dermatologists are uniquely qualified to educate patients about taking a long-term, preventative approach to skin care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHAMPIONSGATE, FLA. – "Aging doesn’t happen overnight," according to Dr. Wendy Roberts, medical director of Desert Dermatology in Rancho Mirage, Calif.
In a video interview at the Orlando Dermatology Aesthetic and Clinical Conference, Dr. Roberts explained the concept of "generational dermatology" and how dermatologists are uniquely qualified to educate patients about taking a long-term, preventative approach to skin care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHAMPIONSGATE, FLA. – "Aging doesn’t happen overnight," according to Dr. Wendy Roberts, medical director of Desert Dermatology in Rancho Mirage, Calif.
In a video interview at the Orlando Dermatology Aesthetic and Clinical Conference, Dr. Roberts explained the concept of "generational dermatology" and how dermatologists are uniquely qualified to educate patients about taking a long-term, preventative approach to skin care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE ODAC CONFERENCE
VIDEO: Try ‘restaurant menu’ approach to laser treatment of scars
CHAMPIONSGATE, FLA. – Patients seeking treatment for scars can benefit from a "restaurant menu" approach that involves using a series of techniques in a single visit, according to Dr. Jill Waibel, medical director of the Miami Dermatology and Laser Institute. At the Orlando Dermatology Aesthetic and Clinical Conference, Dr. Waibel spoke to us about one of her favorite strategies for scar treatment: a multiprocedure, multilaser protocol in a single visit that includes an "appetizer," such as a pulsed dye laser; followed by the "main course" of scar treatment, a fractional ablative device; and then "dessert" of adjunctive and topical therapies.
CHAMPIONSGATE, FLA. – Patients seeking treatment for scars can benefit from a "restaurant menu" approach that involves using a series of techniques in a single visit, according to Dr. Jill Waibel, medical director of the Miami Dermatology and Laser Institute. At the Orlando Dermatology Aesthetic and Clinical Conference, Dr. Waibel spoke to us about one of her favorite strategies for scar treatment: a multiprocedure, multilaser protocol in a single visit that includes an "appetizer," such as a pulsed dye laser; followed by the "main course" of scar treatment, a fractional ablative device; and then "dessert" of adjunctive and topical therapies.
CHAMPIONSGATE, FLA. – Patients seeking treatment for scars can benefit from a "restaurant menu" approach that involves using a series of techniques in a single visit, according to Dr. Jill Waibel, medical director of the Miami Dermatology and Laser Institute. At the Orlando Dermatology Aesthetic and Clinical Conference, Dr. Waibel spoke to us about one of her favorite strategies for scar treatment: a multiprocedure, multilaser protocol in a single visit that includes an "appetizer," such as a pulsed dye laser; followed by the "main course" of scar treatment, a fractional ablative device; and then "dessert" of adjunctive and topical therapies.
EXPERT ANALYSIS FROM THE ODAC CONFERENCE
Cell adherence linked to treatment resistance in CML

Credit: UC San Diego
Preclinical research in chronic myeloid leukemia (CML) has pointed to a relationship between cell adherence and treatment resistance.
Investigators found that a population of plastic-adherent K562 cells with increased expression of BCR-ABL exhibited greater resistance to the tyrosine kinase inhibitor imatinib than nonadherent K562 cells.
“Previous studies have linked high levels of the BCR-ABL mutation with drug resistance,” said Richard Byers, PhD, of The University of Manchester in the UK.
“We wanted to see how expression of BCR-ABL differed across groups of CML cells and, in particular, whether there were differences between adherent and nonadherent populations.”
Dr Byers and his colleagues described this investigation in Experimental Hematology.
The researchers evaluated the heterogeneity of BCR-ABL expression at DNA, messenger RNA, and protein levels, using the CML-derived K562 cell line.
They grew cells in suspension and found that some cells adhered to the plastic dish. The investigators then separated the plastic-adherent and nonadherent cell populations and studied them as single cells and in bulk.
The first discovery was that adherent and nonadherent cells had similar BCR-ABL fusion gene copy numbers.
In bulk-cell analysis, the mean relative normalized ratio for genomic ABL DNA copy number was 47.73 for adherent cells and 53.40 for nonadherent cells (P=0.11). In single-cell analysis, the mean copy numbers were 13.83 and 14.22, respectively (P=0.63).
On the other hand, there was a significant difference in BCR-ABL messenger RNA expression between adherent and nonadherent cells.
In bulk cells, the level of BCR-ABL messenger RNA transcripts was 11-fold higher in adherent cells than in nondherent cells (P=0.022). And single-cell analysis revealed the mean BCR-ABL copy number was 53.11 for adherent cells and 14.06 for nonadherent cells (P=0.0013).
Adherent cells also exhibited significantly upregulated phosphorylation of BCR protein compared to nonadherent cells.
Flow cytometry showed that a mean of 61.9% of adherent cells were positive for phosphor-BCR, compared to 14.5% of nonadherent cells (P=0.0074). And single-cell analysis revealed a mean signal number per cell of 8.23 among adherent cells and 3.02 among nonadherent cells (P<0.0001).
In addition, whole-genome microRNA profiling showed that adherent and nonadherent cell populations expressed significantly different microRNA species.
Finally, the researchers found that treatment with imatinib reduced cell viability more rapidly in nonadherent cells than in adherent cells (P<0.005). The adherent cells showed a decrease in cell viability at 24 hours, compared to 4 hours for nonadherent cells.
The investigators said this research suggests that CML patients may have a similar adherent cell population that mediates resistance to imatinib. And the study highlights the importance of single-cell analysis.
“The small number of cells that show high levels of BCR-ABL may not be detectable through bulk analysis of large samples,” Dr Byers said. “It looks like it is important to look at protein levels in single cells.”
“In future, it may be possible to measure BCR-ABL levels in individual cells in the clinic. This will help us identify the resistant, high-BCR-ABL cells and better understand how patients develop resistance to imatinib treatment, with the aim of combating this resistance to make response more durable and the treatment more effective.” ![]()

Credit: UC San Diego
Preclinical research in chronic myeloid leukemia (CML) has pointed to a relationship between cell adherence and treatment resistance.
Investigators found that a population of plastic-adherent K562 cells with increased expression of BCR-ABL exhibited greater resistance to the tyrosine kinase inhibitor imatinib than nonadherent K562 cells.
“Previous studies have linked high levels of the BCR-ABL mutation with drug resistance,” said Richard Byers, PhD, of The University of Manchester in the UK.
“We wanted to see how expression of BCR-ABL differed across groups of CML cells and, in particular, whether there were differences between adherent and nonadherent populations.”
Dr Byers and his colleagues described this investigation in Experimental Hematology.
The researchers evaluated the heterogeneity of BCR-ABL expression at DNA, messenger RNA, and protein levels, using the CML-derived K562 cell line.
They grew cells in suspension and found that some cells adhered to the plastic dish. The investigators then separated the plastic-adherent and nonadherent cell populations and studied them as single cells and in bulk.
The first discovery was that adherent and nonadherent cells had similar BCR-ABL fusion gene copy numbers.
In bulk-cell analysis, the mean relative normalized ratio for genomic ABL DNA copy number was 47.73 for adherent cells and 53.40 for nonadherent cells (P=0.11). In single-cell analysis, the mean copy numbers were 13.83 and 14.22, respectively (P=0.63).
On the other hand, there was a significant difference in BCR-ABL messenger RNA expression between adherent and nonadherent cells.
In bulk cells, the level of BCR-ABL messenger RNA transcripts was 11-fold higher in adherent cells than in nondherent cells (P=0.022). And single-cell analysis revealed the mean BCR-ABL copy number was 53.11 for adherent cells and 14.06 for nonadherent cells (P=0.0013).
Adherent cells also exhibited significantly upregulated phosphorylation of BCR protein compared to nonadherent cells.
Flow cytometry showed that a mean of 61.9% of adherent cells were positive for phosphor-BCR, compared to 14.5% of nonadherent cells (P=0.0074). And single-cell analysis revealed a mean signal number per cell of 8.23 among adherent cells and 3.02 among nonadherent cells (P<0.0001).
In addition, whole-genome microRNA profiling showed that adherent and nonadherent cell populations expressed significantly different microRNA species.
Finally, the researchers found that treatment with imatinib reduced cell viability more rapidly in nonadherent cells than in adherent cells (P<0.005). The adherent cells showed a decrease in cell viability at 24 hours, compared to 4 hours for nonadherent cells.
The investigators said this research suggests that CML patients may have a similar adherent cell population that mediates resistance to imatinib. And the study highlights the importance of single-cell analysis.
“The small number of cells that show high levels of BCR-ABL may not be detectable through bulk analysis of large samples,” Dr Byers said. “It looks like it is important to look at protein levels in single cells.”
“In future, it may be possible to measure BCR-ABL levels in individual cells in the clinic. This will help us identify the resistant, high-BCR-ABL cells and better understand how patients develop resistance to imatinib treatment, with the aim of combating this resistance to make response more durable and the treatment more effective.” ![]()

Credit: UC San Diego
Preclinical research in chronic myeloid leukemia (CML) has pointed to a relationship between cell adherence and treatment resistance.
Investigators found that a population of plastic-adherent K562 cells with increased expression of BCR-ABL exhibited greater resistance to the tyrosine kinase inhibitor imatinib than nonadherent K562 cells.
“Previous studies have linked high levels of the BCR-ABL mutation with drug resistance,” said Richard Byers, PhD, of The University of Manchester in the UK.
“We wanted to see how expression of BCR-ABL differed across groups of CML cells and, in particular, whether there were differences between adherent and nonadherent populations.”
Dr Byers and his colleagues described this investigation in Experimental Hematology.
The researchers evaluated the heterogeneity of BCR-ABL expression at DNA, messenger RNA, and protein levels, using the CML-derived K562 cell line.
They grew cells in suspension and found that some cells adhered to the plastic dish. The investigators then separated the plastic-adherent and nonadherent cell populations and studied them as single cells and in bulk.
The first discovery was that adherent and nonadherent cells had similar BCR-ABL fusion gene copy numbers.
In bulk-cell analysis, the mean relative normalized ratio for genomic ABL DNA copy number was 47.73 for adherent cells and 53.40 for nonadherent cells (P=0.11). In single-cell analysis, the mean copy numbers were 13.83 and 14.22, respectively (P=0.63).
On the other hand, there was a significant difference in BCR-ABL messenger RNA expression between adherent and nonadherent cells.
In bulk cells, the level of BCR-ABL messenger RNA transcripts was 11-fold higher in adherent cells than in nondherent cells (P=0.022). And single-cell analysis revealed the mean BCR-ABL copy number was 53.11 for adherent cells and 14.06 for nonadherent cells (P=0.0013).
Adherent cells also exhibited significantly upregulated phosphorylation of BCR protein compared to nonadherent cells.
Flow cytometry showed that a mean of 61.9% of adherent cells were positive for phosphor-BCR, compared to 14.5% of nonadherent cells (P=0.0074). And single-cell analysis revealed a mean signal number per cell of 8.23 among adherent cells and 3.02 among nonadherent cells (P<0.0001).
In addition, whole-genome microRNA profiling showed that adherent and nonadherent cell populations expressed significantly different microRNA species.
Finally, the researchers found that treatment with imatinib reduced cell viability more rapidly in nonadherent cells than in adherent cells (P<0.005). The adherent cells showed a decrease in cell viability at 24 hours, compared to 4 hours for nonadherent cells.
The investigators said this research suggests that CML patients may have a similar adherent cell population that mediates resistance to imatinib. And the study highlights the importance of single-cell analysis.
“The small number of cells that show high levels of BCR-ABL may not be detectable through bulk analysis of large samples,” Dr Byers said. “It looks like it is important to look at protein levels in single cells.”
“In future, it may be possible to measure BCR-ABL levels in individual cells in the clinic. This will help us identify the resistant, high-BCR-ABL cells and better understand how patients develop resistance to imatinib treatment, with the aim of combating this resistance to make response more durable and the treatment more effective.” ![]()
Team finds hidden reservoir of HCMV

Credit: Chad McNeeley
Researchers have found evidence suggesting that perivascular mesenchymal stromal cells (MSCs) are a reservoir of human cytomegalovirus (HCMV).
This opens up the possibility of therapeutically targeting these cells, which surround blood vessels in the organs and can be found in the bone marrow.
If effective, such a treatment method could prove life-saving for individuals who experience HCMV reactivation, such as transplant recipients and patients receiving chemotherapy.
“There are antiviral medications designed to prevent HCMV from re-activating, but HCMV infection remains one of the major complications after both organ and bone marrow transplants,” said study author Graca Almeida-Porada, MD, PhD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
“The question scientists have been asking for years is, ‘Where does the virus hide when it is latent?’ Maybe if we knew, we could target it.”
Previous research showed that hematopoietic stem cells can harbor HCMV. Dr Almeida-Porada and her colleagues hypothesized that other cell populations may also harbor the virus, and they suspected that perivascular MSCs were a likely culprit.
The team’s suspicions were confirmed when testing revealed that perivascular MSCs are susceptible to HCMV infection and that the virus can grow within these cells.
The researchers also compared the susceptibility of perivascular MSCs from the liver, brain, lung, and bone marrow. And they found the highest rate of HCMV infection in cells from the lung.
“This may explain why pneumonia is the primary manifestation of the HCMV infection in bone marrow transplant recipients,” Dr Almeida-Porada said.
To expand upon these findings, she and her colleagues analyzed bone marrow samples from 19 healthy individuals who had tested positive for HCMV. Quantitative PCR revealed HCMV DNA in perivascular MSCs from 7 of the subjects.
This suggests bone marrow-derived perivascular MSCs may be a natural HCMV reservoir, according to the researchers.
“We have found another source of cells that can harbor HCMV virus,” Dr Almeida-Porada concluded. “Knowing the identity of the cells opens the possibility of targeting treatments to stop its re-activation.”
Dr Almeida-Porada and her colleagues recounted their discoveries in the American Journal of Transplantation. ![]()

Credit: Chad McNeeley
Researchers have found evidence suggesting that perivascular mesenchymal stromal cells (MSCs) are a reservoir of human cytomegalovirus (HCMV).
This opens up the possibility of therapeutically targeting these cells, which surround blood vessels in the organs and can be found in the bone marrow.
If effective, such a treatment method could prove life-saving for individuals who experience HCMV reactivation, such as transplant recipients and patients receiving chemotherapy.
“There are antiviral medications designed to prevent HCMV from re-activating, but HCMV infection remains one of the major complications after both organ and bone marrow transplants,” said study author Graca Almeida-Porada, MD, PhD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
“The question scientists have been asking for years is, ‘Where does the virus hide when it is latent?’ Maybe if we knew, we could target it.”
Previous research showed that hematopoietic stem cells can harbor HCMV. Dr Almeida-Porada and her colleagues hypothesized that other cell populations may also harbor the virus, and they suspected that perivascular MSCs were a likely culprit.
The team’s suspicions were confirmed when testing revealed that perivascular MSCs are susceptible to HCMV infection and that the virus can grow within these cells.
The researchers also compared the susceptibility of perivascular MSCs from the liver, brain, lung, and bone marrow. And they found the highest rate of HCMV infection in cells from the lung.
“This may explain why pneumonia is the primary manifestation of the HCMV infection in bone marrow transplant recipients,” Dr Almeida-Porada said.
To expand upon these findings, she and her colleagues analyzed bone marrow samples from 19 healthy individuals who had tested positive for HCMV. Quantitative PCR revealed HCMV DNA in perivascular MSCs from 7 of the subjects.
This suggests bone marrow-derived perivascular MSCs may be a natural HCMV reservoir, according to the researchers.
“We have found another source of cells that can harbor HCMV virus,” Dr Almeida-Porada concluded. “Knowing the identity of the cells opens the possibility of targeting treatments to stop its re-activation.”
Dr Almeida-Porada and her colleagues recounted their discoveries in the American Journal of Transplantation. ![]()

Credit: Chad McNeeley
Researchers have found evidence suggesting that perivascular mesenchymal stromal cells (MSCs) are a reservoir of human cytomegalovirus (HCMV).
This opens up the possibility of therapeutically targeting these cells, which surround blood vessels in the organs and can be found in the bone marrow.
If effective, such a treatment method could prove life-saving for individuals who experience HCMV reactivation, such as transplant recipients and patients receiving chemotherapy.
“There are antiviral medications designed to prevent HCMV from re-activating, but HCMV infection remains one of the major complications after both organ and bone marrow transplants,” said study author Graca Almeida-Porada, MD, PhD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
“The question scientists have been asking for years is, ‘Where does the virus hide when it is latent?’ Maybe if we knew, we could target it.”
Previous research showed that hematopoietic stem cells can harbor HCMV. Dr Almeida-Porada and her colleagues hypothesized that other cell populations may also harbor the virus, and they suspected that perivascular MSCs were a likely culprit.
The team’s suspicions were confirmed when testing revealed that perivascular MSCs are susceptible to HCMV infection and that the virus can grow within these cells.
The researchers also compared the susceptibility of perivascular MSCs from the liver, brain, lung, and bone marrow. And they found the highest rate of HCMV infection in cells from the lung.
“This may explain why pneumonia is the primary manifestation of the HCMV infection in bone marrow transplant recipients,” Dr Almeida-Porada said.
To expand upon these findings, she and her colleagues analyzed bone marrow samples from 19 healthy individuals who had tested positive for HCMV. Quantitative PCR revealed HCMV DNA in perivascular MSCs from 7 of the subjects.
This suggests bone marrow-derived perivascular MSCs may be a natural HCMV reservoir, according to the researchers.
“We have found another source of cells that can harbor HCMV virus,” Dr Almeida-Porada concluded. “Knowing the identity of the cells opens the possibility of targeting treatments to stop its re-activation.”
Dr Almeida-Porada and her colleagues recounted their discoveries in the American Journal of Transplantation. ![]()
Analysis details effects of HLA mismatch
GRAPEVINE, TEXAS—An analysis of more than 8000 patients provides insights regarding HLA disparity that may help optimize outcomes in individuals undergoing unrelated-donor hematopoietic stem cell transplant.
The study showed that a single allele- or antigen-level HLA mismatch (7/8) increased the risk for acute and chronic graft-vs-host disease (GVHD) and worsened survival rates.
However, there were no locus-specific effects on survival, and there was no impact of allele- vs antigen-level mismatch on survival.
In addition, patients with an 8/8 matched graft had an increased risk of acute GVHD if they had a DQB1 mismatch or a DPB1 mismatch. DPB1 mismatch also decreased the risk of relapse, and nonpermissive DPB1 mismatch was associated with worse survival.
“Thus, we believe that consideration of DPB1 in donor selection may permit skewing toward donors with permissive DPB1 mismatch and may improve outcomes,” said study investigator Joseph Pidala, MD, of the H. Lee Moffitt Cancer Center in Tampa, Florida.
He presented these findings at the 2014 BMT Tandem Meetings as abstract 5, which was designated one of the meeting’s “Best Abstracts.”
Patient characteristics
Dr Pidala and his colleagues evaluated data from 8003 adult and pediatric patients who had undergone their first myeloablative, unrelated transplant between 1999 and 2011. The patients had been diagnosed with acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, or myelodysplastic syndrome.
Patients and their donors had high-resolution typing for HLA-A, -B, -C, -DRB1, -DQB1, and -DPB1. Most cases were 8/8 matched (n=5449), followed by 7/8 (n=2071) and 6/8 (n=483).
“Those with a greater extent of HLA mismatch were younger, less likely to be Caucasian, they differed according to disease indication for transplant, and had a greater proportion of advanced disease stage,” Dr Pidala noted.
“Additionally, those with a greater extent of HLA mismatch were more likely to receive bone marrow grafts in comparison to peripheral blood, total body irradiation, and also to receive in vivo T-cell depletion.”
Furthermore, there was a declining proportion of 6/8 match over time. It decreased from 49% (1999-2002) to 37% (2003-2006) to 14% (2007-2011).
Effects of HLA mismatch
In all analyses, the researchers considered findings significant if the P value was less than 0.01.
Compared to an 8/8 matched graft, receiving a 7/8 graft was significantly associated with an increase in acute grade 2-4, acute grade 3-4, and chronic GVHD; higher transplant-related mortality (TRM); lower disease-free survival (DFS) among patients with early stage or intermediate (but not advanced) disease; and lower overall survival (OS) regardless of disease stage.
Receiving a 6/8 graft was significantly associated with an increase in acute grade 2-4 and 3-4 GVHD, increased TRM, decreased DFS (early stage or intermediate disease), and decreased OS (early stage or intermediate disease).
“In no cases did we find [that mismatch had] an impact on the incidence of primary disease relapse,” Dr Pidala said. “Comparing 7/8 to 6/8 cases, we found that those with 7/8 had improved transplant-related mortality and disease-free and overall survival [only] in the context of early stage disease.”
The team also confirmed these findings in an analysis of 5846 unique cases. In this cohort, 2528 patients were 8/8 matched, 882 were 7/8, and 157 were 6/8.
Locus-specific effects
Mismatch at HLA-A (n=743) was significantly associated with an increase in acute grade 2-4/3-4 and chronic GVHD, increased TRM, decreased DFS, and decreased OS.
Mismatch at HLA-B (n=345) was significantly associated with an increase in grade 2-4/3-4 acute GHVD, chronic GVHD, and TRM. And mismatch at HLA-C (n=766) was significantly associated with an increase in acute grade 3-4 GVHD and TRM and a decrease in DFS and OS.
There were no significant differences in the case of DRB1 mismatch. However, Dr Pidala noted that this group had the smallest number of patients, at 217.
“I’d also like to point out that in direct, pair-wise comparisons across the mismatched loci, we did not observe any significant differences in overall survival,” Dr Pidala said. “Similarly, we found no impact of allele- vs antigen-level mismatch on overall survival.”
DP and DQ mismatch
Among 8/8 matched cases, DPB1 mismatch led to a significantly increased risk for acute grade 2-4 and 3-4 GHVD, as well as a significant reduction in the risk of relapse. This was the case whether patients had a single- or double-allele mismatch.
The addition of DQB1 mismatch significantly increased the risk of grade 2-4 acute GVHD among 8/8 matched cases only if it was a single-antigen mismatch.
Neither DPB1 nor DQB1 mismatch had a significant effect on OS, DFS, or TRM in 8/8 matched cases. And neither DPB1 nor DQB1 mismatch had a significant effect on outcomes of patients who received 7/8 matched grafts.
Among 8/8 matched cases, those with permissive DPB1 mismatch had a significantly lower risk of acute grade 2-4 and 3-4 GVHD and a significantly higher risk of relapse than patients with nonpermissive DPB1 mismatch. Patients with nonpermissive mismatch also had significantly higher TRM and significantly lower DFS and OS.
However, there were no significant differences between permissive and nonpermissive mismatches for patients with 7/8 matched grafts.
Treatment implications
Based on these results and those of previous studies, Dr Pidala said we can conclude that 8/8 matched grafts confer better survival than mismatched grafts. And a permissive DPB1 mismatch can further improve survival in 8/8 cases.
Among patients receiving a 7/8 matched graft, it is preferable to identify those with HLA-C 03:03/03:04 mismatches, as these patients appear to have mortality rates comparable to 8/8 matched cases.
Among 7/8 matched cases, we should avoid nonpermissive DPB1 mismatch, having 3 or more low-expression loci mismatches (DP, DQ, DRB3/4/5), and nonpermissive allele combinations and amino acid substitutions. ![]()
GRAPEVINE, TEXAS—An analysis of more than 8000 patients provides insights regarding HLA disparity that may help optimize outcomes in individuals undergoing unrelated-donor hematopoietic stem cell transplant.
The study showed that a single allele- or antigen-level HLA mismatch (7/8) increased the risk for acute and chronic graft-vs-host disease (GVHD) and worsened survival rates.
However, there were no locus-specific effects on survival, and there was no impact of allele- vs antigen-level mismatch on survival.
In addition, patients with an 8/8 matched graft had an increased risk of acute GVHD if they had a DQB1 mismatch or a DPB1 mismatch. DPB1 mismatch also decreased the risk of relapse, and nonpermissive DPB1 mismatch was associated with worse survival.
“Thus, we believe that consideration of DPB1 in donor selection may permit skewing toward donors with permissive DPB1 mismatch and may improve outcomes,” said study investigator Joseph Pidala, MD, of the H. Lee Moffitt Cancer Center in Tampa, Florida.
He presented these findings at the 2014 BMT Tandem Meetings as abstract 5, which was designated one of the meeting’s “Best Abstracts.”
Patient characteristics
Dr Pidala and his colleagues evaluated data from 8003 adult and pediatric patients who had undergone their first myeloablative, unrelated transplant between 1999 and 2011. The patients had been diagnosed with acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, or myelodysplastic syndrome.
Patients and their donors had high-resolution typing for HLA-A, -B, -C, -DRB1, -DQB1, and -DPB1. Most cases were 8/8 matched (n=5449), followed by 7/8 (n=2071) and 6/8 (n=483).
“Those with a greater extent of HLA mismatch were younger, less likely to be Caucasian, they differed according to disease indication for transplant, and had a greater proportion of advanced disease stage,” Dr Pidala noted.
“Additionally, those with a greater extent of HLA mismatch were more likely to receive bone marrow grafts in comparison to peripheral blood, total body irradiation, and also to receive in vivo T-cell depletion.”
Furthermore, there was a declining proportion of 6/8 match over time. It decreased from 49% (1999-2002) to 37% (2003-2006) to 14% (2007-2011).
Effects of HLA mismatch
In all analyses, the researchers considered findings significant if the P value was less than 0.01.
Compared to an 8/8 matched graft, receiving a 7/8 graft was significantly associated with an increase in acute grade 2-4, acute grade 3-4, and chronic GVHD; higher transplant-related mortality (TRM); lower disease-free survival (DFS) among patients with early stage or intermediate (but not advanced) disease; and lower overall survival (OS) regardless of disease stage.
Receiving a 6/8 graft was significantly associated with an increase in acute grade 2-4 and 3-4 GVHD, increased TRM, decreased DFS (early stage or intermediate disease), and decreased OS (early stage or intermediate disease).
“In no cases did we find [that mismatch had] an impact on the incidence of primary disease relapse,” Dr Pidala said. “Comparing 7/8 to 6/8 cases, we found that those with 7/8 had improved transplant-related mortality and disease-free and overall survival [only] in the context of early stage disease.”
The team also confirmed these findings in an analysis of 5846 unique cases. In this cohort, 2528 patients were 8/8 matched, 882 were 7/8, and 157 were 6/8.
Locus-specific effects
Mismatch at HLA-A (n=743) was significantly associated with an increase in acute grade 2-4/3-4 and chronic GVHD, increased TRM, decreased DFS, and decreased OS.
Mismatch at HLA-B (n=345) was significantly associated with an increase in grade 2-4/3-4 acute GHVD, chronic GVHD, and TRM. And mismatch at HLA-C (n=766) was significantly associated with an increase in acute grade 3-4 GVHD and TRM and a decrease in DFS and OS.
There were no significant differences in the case of DRB1 mismatch. However, Dr Pidala noted that this group had the smallest number of patients, at 217.
“I’d also like to point out that in direct, pair-wise comparisons across the mismatched loci, we did not observe any significant differences in overall survival,” Dr Pidala said. “Similarly, we found no impact of allele- vs antigen-level mismatch on overall survival.”
DP and DQ mismatch
Among 8/8 matched cases, DPB1 mismatch led to a significantly increased risk for acute grade 2-4 and 3-4 GHVD, as well as a significant reduction in the risk of relapse. This was the case whether patients had a single- or double-allele mismatch.
The addition of DQB1 mismatch significantly increased the risk of grade 2-4 acute GVHD among 8/8 matched cases only if it was a single-antigen mismatch.
Neither DPB1 nor DQB1 mismatch had a significant effect on OS, DFS, or TRM in 8/8 matched cases. And neither DPB1 nor DQB1 mismatch had a significant effect on outcomes of patients who received 7/8 matched grafts.
Among 8/8 matched cases, those with permissive DPB1 mismatch had a significantly lower risk of acute grade 2-4 and 3-4 GVHD and a significantly higher risk of relapse than patients with nonpermissive DPB1 mismatch. Patients with nonpermissive mismatch also had significantly higher TRM and significantly lower DFS and OS.
However, there were no significant differences between permissive and nonpermissive mismatches for patients with 7/8 matched grafts.
Treatment implications
Based on these results and those of previous studies, Dr Pidala said we can conclude that 8/8 matched grafts confer better survival than mismatched grafts. And a permissive DPB1 mismatch can further improve survival in 8/8 cases.
Among patients receiving a 7/8 matched graft, it is preferable to identify those with HLA-C 03:03/03:04 mismatches, as these patients appear to have mortality rates comparable to 8/8 matched cases.
Among 7/8 matched cases, we should avoid nonpermissive DPB1 mismatch, having 3 or more low-expression loci mismatches (DP, DQ, DRB3/4/5), and nonpermissive allele combinations and amino acid substitutions. ![]()
GRAPEVINE, TEXAS—An analysis of more than 8000 patients provides insights regarding HLA disparity that may help optimize outcomes in individuals undergoing unrelated-donor hematopoietic stem cell transplant.
The study showed that a single allele- or antigen-level HLA mismatch (7/8) increased the risk for acute and chronic graft-vs-host disease (GVHD) and worsened survival rates.
However, there were no locus-specific effects on survival, and there was no impact of allele- vs antigen-level mismatch on survival.
In addition, patients with an 8/8 matched graft had an increased risk of acute GVHD if they had a DQB1 mismatch or a DPB1 mismatch. DPB1 mismatch also decreased the risk of relapse, and nonpermissive DPB1 mismatch was associated with worse survival.
“Thus, we believe that consideration of DPB1 in donor selection may permit skewing toward donors with permissive DPB1 mismatch and may improve outcomes,” said study investigator Joseph Pidala, MD, of the H. Lee Moffitt Cancer Center in Tampa, Florida.
He presented these findings at the 2014 BMT Tandem Meetings as abstract 5, which was designated one of the meeting’s “Best Abstracts.”
Patient characteristics
Dr Pidala and his colleagues evaluated data from 8003 adult and pediatric patients who had undergone their first myeloablative, unrelated transplant between 1999 and 2011. The patients had been diagnosed with acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, or myelodysplastic syndrome.
Patients and their donors had high-resolution typing for HLA-A, -B, -C, -DRB1, -DQB1, and -DPB1. Most cases were 8/8 matched (n=5449), followed by 7/8 (n=2071) and 6/8 (n=483).
“Those with a greater extent of HLA mismatch were younger, less likely to be Caucasian, they differed according to disease indication for transplant, and had a greater proportion of advanced disease stage,” Dr Pidala noted.
“Additionally, those with a greater extent of HLA mismatch were more likely to receive bone marrow grafts in comparison to peripheral blood, total body irradiation, and also to receive in vivo T-cell depletion.”
Furthermore, there was a declining proportion of 6/8 match over time. It decreased from 49% (1999-2002) to 37% (2003-2006) to 14% (2007-2011).
Effects of HLA mismatch
In all analyses, the researchers considered findings significant if the P value was less than 0.01.
Compared to an 8/8 matched graft, receiving a 7/8 graft was significantly associated with an increase in acute grade 2-4, acute grade 3-4, and chronic GVHD; higher transplant-related mortality (TRM); lower disease-free survival (DFS) among patients with early stage or intermediate (but not advanced) disease; and lower overall survival (OS) regardless of disease stage.
Receiving a 6/8 graft was significantly associated with an increase in acute grade 2-4 and 3-4 GVHD, increased TRM, decreased DFS (early stage or intermediate disease), and decreased OS (early stage or intermediate disease).
“In no cases did we find [that mismatch had] an impact on the incidence of primary disease relapse,” Dr Pidala said. “Comparing 7/8 to 6/8 cases, we found that those with 7/8 had improved transplant-related mortality and disease-free and overall survival [only] in the context of early stage disease.”
The team also confirmed these findings in an analysis of 5846 unique cases. In this cohort, 2528 patients were 8/8 matched, 882 were 7/8, and 157 were 6/8.
Locus-specific effects
Mismatch at HLA-A (n=743) was significantly associated with an increase in acute grade 2-4/3-4 and chronic GVHD, increased TRM, decreased DFS, and decreased OS.
Mismatch at HLA-B (n=345) was significantly associated with an increase in grade 2-4/3-4 acute GHVD, chronic GVHD, and TRM. And mismatch at HLA-C (n=766) was significantly associated with an increase in acute grade 3-4 GVHD and TRM and a decrease in DFS and OS.
There were no significant differences in the case of DRB1 mismatch. However, Dr Pidala noted that this group had the smallest number of patients, at 217.
“I’d also like to point out that in direct, pair-wise comparisons across the mismatched loci, we did not observe any significant differences in overall survival,” Dr Pidala said. “Similarly, we found no impact of allele- vs antigen-level mismatch on overall survival.”
DP and DQ mismatch
Among 8/8 matched cases, DPB1 mismatch led to a significantly increased risk for acute grade 2-4 and 3-4 GHVD, as well as a significant reduction in the risk of relapse. This was the case whether patients had a single- or double-allele mismatch.
The addition of DQB1 mismatch significantly increased the risk of grade 2-4 acute GVHD among 8/8 matched cases only if it was a single-antigen mismatch.
Neither DPB1 nor DQB1 mismatch had a significant effect on OS, DFS, or TRM in 8/8 matched cases. And neither DPB1 nor DQB1 mismatch had a significant effect on outcomes of patients who received 7/8 matched grafts.
Among 8/8 matched cases, those with permissive DPB1 mismatch had a significantly lower risk of acute grade 2-4 and 3-4 GVHD and a significantly higher risk of relapse than patients with nonpermissive DPB1 mismatch. Patients with nonpermissive mismatch also had significantly higher TRM and significantly lower DFS and OS.
However, there were no significant differences between permissive and nonpermissive mismatches for patients with 7/8 matched grafts.
Treatment implications
Based on these results and those of previous studies, Dr Pidala said we can conclude that 8/8 matched grafts confer better survival than mismatched grafts. And a permissive DPB1 mismatch can further improve survival in 8/8 cases.
Among patients receiving a 7/8 matched graft, it is preferable to identify those with HLA-C 03:03/03:04 mismatches, as these patients appear to have mortality rates comparable to 8/8 matched cases.
Among 7/8 matched cases, we should avoid nonpermissive DPB1 mismatch, having 3 or more low-expression loci mismatches (DP, DQ, DRB3/4/5), and nonpermissive allele combinations and amino acid substitutions. ![]()