User login
Cut to the Quick
A 41‐year‐old woman with dwarfism was referred for evaluation of an isolated elevated alkaline phosphatase (ALP) of 792 U/L (normal value, 3195 U/L) and a gamma‐glutamyl transferase (GGT) of 729 U/L (normal value, 737 U/L), found incidentally on routine laboratory screening. She denied any fevers, chills, weight loss, abdominal pain, nausea, or vomiting.
The presence of an isolated ALP elevation, presumably of hepatobiliary origin given the increase in GGT, in a relatively young woman immediately calls to mind the diagnosis of primary biliary cirrhosis, and I would specifically inquire about pruritus, which occurs commonly in this setting. The absence of abdominal pain argues against the diagnosis of extrahepatic biliary obstruction. Other processes that could result in this asymptomatic presentation include infiltrative diseases such as amyloidosis, sarcoidosis, and other causes of granulomatous hepatitis. The absence of systemic symptoms makes disseminated infection or malignancy with hepatic involvement less likely. I would query whether underlying dwarfism can be associated with metabolic abnormalities that cause infiltrative liver disease, functional or anatomical hepatobiliary abnormalities, or malignancy.
The patient's medical history was notable for chronic constipation, allergic rhinitis, and basal‐cell carcinoma. She had reconstructive surgeries of the left hip and knee 28 years ago without complications. She underwent a right total hip replacement for hip dysplasia 6 months prior, which was complicated by a postoperative joint infection with Enterobacter cloacae. The hardware was retained, and she was treated with incision and drainage and a prolonged fluoroquinolone course. Furthermore, she had a history of immune thrombocytopenic purpura (ITP), which manifested at the age of 20 years. A bone‐marrow biopsy at that time showed no evidence of hematologic malignancy. For her ITP, she had initially received intravenous immunoglobulin (Ig) and cyclosporine without sustained benefit. She underwent a splenectomy at the age of 26 years and was treated intermittently with rituximab over 11 years prior to admission. Her medications included cetirizine. Her parents were nonconsanguineous, of European and Southeast Asian ancestry, and healthy. She was in a long‐term monogamous relationship. The patient had been employed as an educator.
The history of immune‐mediated thrombocytopenia raises the possibility that the present illness may be part of a broader autoimmune diathesis. Other causes of secondary ITP, such as drug‐induced reactions, hematologic malignancies, and viral infections, are unlikely, as her ITP has been persistent for more than 20 years. She has not evolved into a common phenotypic pattern of autoimmune disease such as systemic lupus erythematosus after the appearance of ITP, nor does she endorse a history of thromboembolic complications that would suggest antiphospholipid syndrome.
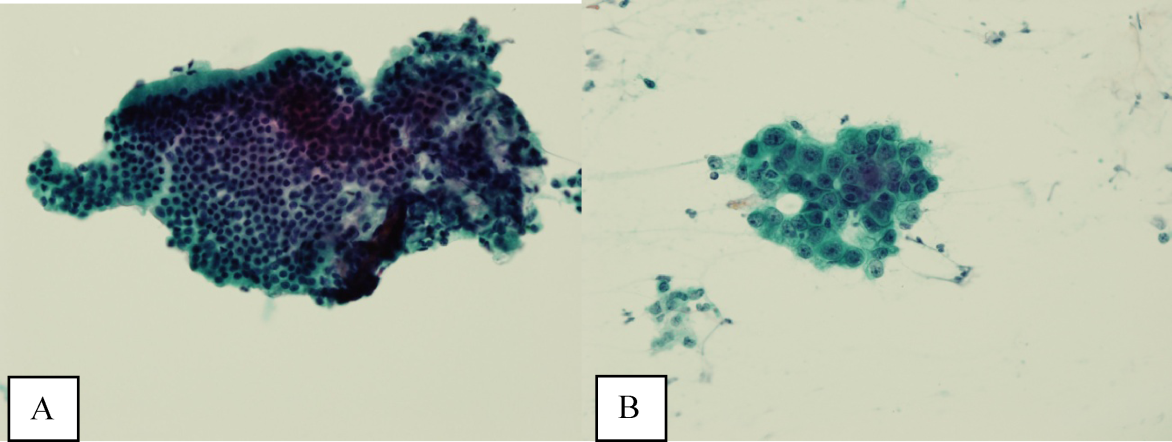
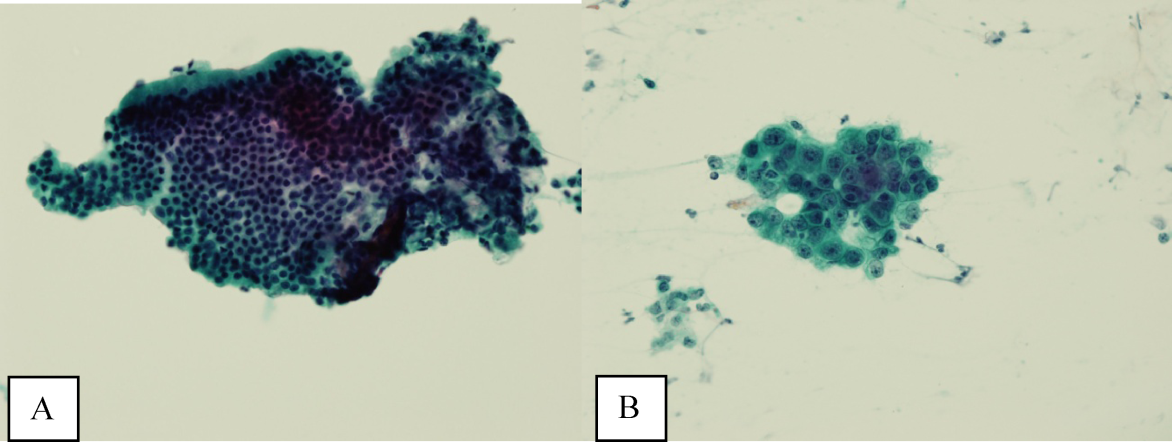
Ultrasound of the abdomen demonstrated narrowing of the extrahepatic biliary duct in the region of the pancreas without evidence of a mass lesion. Computerized tomography (CT) of the abdomen and pelvis similarly showed mild intrahepatic biliary ductal dilatation with narrowing of the extrahepatic duct in the region of the pancreas without apparent pancreatic mass. Endoscopic retrograde cholangiopancreatography (ERCP) confirmed a stricture in the distal common bile duct and dilatation of the common bile duct. Cytology brushings obtained during ERCP showed groups of overlapping, enlarged cells with pleomorphic irregular nuclei, one or more prominent nucleoli, and focal nuclear molding, leading to a diagnosis of adenocarcinoma (Figure 1).
The absence of jaundice and pruritus indicates incomplete biliary obstruction. Commonbile duct strictures are most commonly seen after manipulation of the biliary tree. Neoplasms including pancreatic cancer, adenocarcinoma of the ampulla of Vater, and cholangiocarcinoma may cause compression and obstruction of the common bile duct, as well as stricture formation mediated by a desmoplastic reaction to the tumor. Occasionally, metastatic malignancy or lymphoma may involve the porta hepatis and cause extrinsic compression of the common bile duct. Other etiologies of strictures include sclerosing cholangitis and opportunistic infections such as Cryptosporidium, cytomegalovirus, and microsporidiosis, which are not supported by this patient's history.
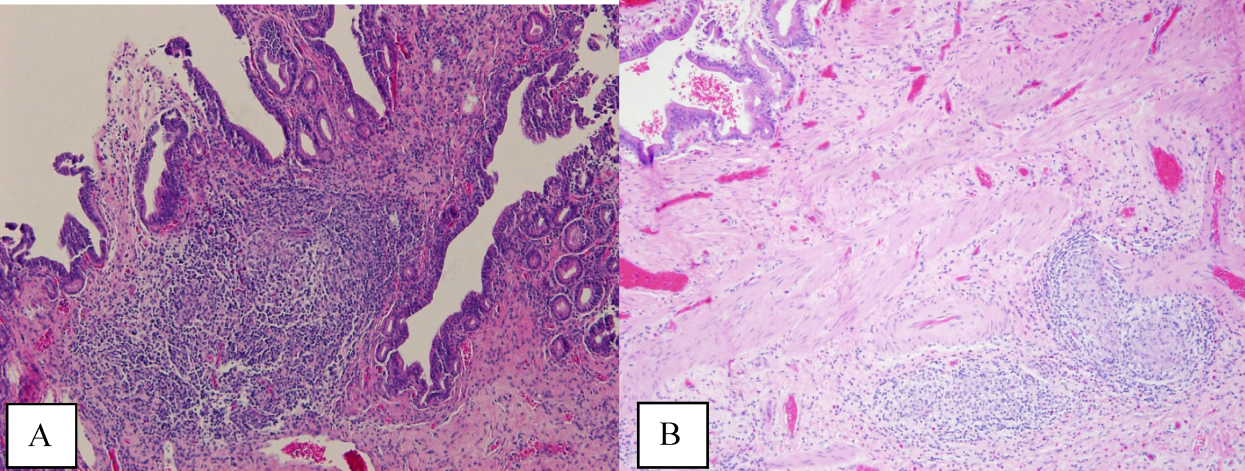
The atypical cells seen on ERCP brushings were interpreted as evidence of cholangiocarcinoma. The patient underwent a pylorus‐sparing Whipple procedure. Examination of the surgical pathology specimens revealed diffuse non‐necrotizing granulomatous inflammation involving the bile duct and gallbladder (Figure 2). There was focal atypia of the bile‐duct epithelial cells, but no evidence of malignancy. There were non‐necrotizing granulomas in numerous lymph nodes, some with significant sclerosis; stains and cultures for acid‐fast bacilli and fungi were negative, and stains for IgG4 and CD1a for Langerhans‐cell histiocytosis were negative.
Granulomatous inflammation may be caused by a variety of intracellular infections, environmental and occupational exposures, and drug hypersensitivity, or may be associated with malignancy such as lymphoma. In the absence of an alternative explanation, the presence of non‐necrotizing granulomas in multiple organs suggests the diagnosis of sarcoidosis, even if classic intrathoracic involvement is not present. Hepatic involvement with sarcoidosis is common but rarely symptomatic, whereas biliary disease is distinctly uncommon. Interestingly, there is an association between both primary biliary cirrhosis and sclerosing cholangitis with sarcoidosis. The pathologic findings could indicate an autoimmune process that has led to widespread granulomas with this unusual distribution. Disseminated infections such as mycobacterial or fungal diseases seem much less plausible in this woman, who had no prior systemic complaints. The atypical cells seen on the ERCP brushings were almost certainly caused by inflammation and a fibroproliferative response rather than malignancy.
On further questioning, the patient endorsed a history of multiple childhood ear infections that required bilateral myringotomy tubes, and multiple episodes of sinusitis, but both problems improved in adulthood. She had experienced 2 episodes of dermatomal zoster in her lifetime. She also noted frequent vaginal yeast infections. She denied any history of pneumonias or thrush. In her second decade of life, she developed allergic rhinitis and eczema. She denied any chemical or environmental exposures. She had had negative tuberculin skin tests as part of her occupational screening and denied any recent travel.
The additional history of recurrent upper‐respiratory infections early in life and subsequent episodes of dermatomal zoster and candidal infections increases the likelihood that this patient has a primary immunodeficiency. A combined cellular and humoral immunodeficiency would predispose to both bacterial sinopulmonary infections, generally a result of Ig isotype or IgG subclass deficiencies, and recurrent zoster and candidal infection. Any evaluation of her Igs at this time may be confounded by her receipt of anti‐CD20 monoclonal antibody therapy, which may decrease serum Ig levels.
The relatively benign course in terms of infection is consistent with the heterogeneous immunodeficiencies classified as combined immunodeficiency (CID), a less‐penetrant phenotype of severe combined immunodeficiency (SCID), or common variable immunodeficiency (CVID). Autoimmunity is a frequent manifestation of CID and CVID, and affected patients have an increased risk of lymphoma and other malignancies. Granulomatous disease may also be a manifestation of both CID and CVID.
Postoperatively, she developed progressive abdominal distension and pain. A CT of the abdomen and pelvis showed colonic dilatation consistent with Ogilvie pseudo‐obstruction. On postoperative day 9, she developed fevers. On physical examination, her temperature was 38.5C, the blood pressure was 104/56 mm Hg, and the heart rate was 131 beats per minute. Her oxygen saturation was 95% on room air. Her height was 105 cm. She had diffuse alopecia without scarring. She did not have a malar rash or oral ulcerations. Both lungs were clear to auscultation. A cardiac examination showed tachycardia with a regular rhythm, normal heart sounds, and no murmurs. Her musculoskeletal exam was notable for short limbs and phalanges, without synovitis. Bilateral hip exam demonstrated internal and external range of motion without abnormalities. No rashes were present. Her abdominal exam revealed diffuse tenderness with postoperative drains in place. She had nonbloody loose stools.
Although autoimmune diseases such as sarcoidosis can rarely manifest with fevers, evaluation of postoperative fever in this patient should focus first on common processes that also occur in immunocompetent patients. Since she has had a splenectomy and we are now suspicious of an underlying immunodeficiency, appropriate cultures should be obtained and broad‐spectrum intravenous antibiotics should be initiated without delay. The presence of nonscarring alopecia could either represent autoimmune alopecia, if the onset was recent, or it could be part of this patient's underlying skeletal dysplasia syndrome.
Piperacillin/tazobactam and oral metronidazole were started for presumed intra‐abdominal infection. The white cell count was 20,500/mm3 with 96% neutrophils, 1.4% lymphocytes with an absolute lymphocyte count 0.33 109/L (normal value, >1.0 109/L), and 2.6% monocytes. The hematocrit was 27.8% with a mean corpuscular volume of 95 fL. The platelet count was 323,000/mm3. Serum aminotransferase and total bilirubin levels were normal, and ALP was 904 U/L. The serum albumin was 1.2 g/dL (normal value, 3.54.8 g/dL) and prealbumin was 6 mg/dL (normal value, 2037 mg/dL).
Blood cultures returned positive for E. cloacae. Clostridium difficile toxin assay was negative. Piperacillin/tazobactam was switched to meroperem, and metronidazole was discontinued. She continued to have fevers, and on postoperative day 16, repeat blood cultures and urine cultures grew Candida albicans; caspofungin was initiated.
In addition to the neutrophilic leukocytosis in response to gram‐negative bacteremia, there is marked lymphopenia. Although sepsis may cause transient declines in the total lymphocyte count, I do not believe that this entirely accounts for such severe lymphopenia. The albumin is also profoundly low. Her catabolic postsurgical state might explain part of this abnormality, but taken together with her prior gastrointestinal symptoms, these findings could be consistent with intestinal malabsorption or a protein‐losing enteropathy, which can also be associated with primary immunodeficiency.
Serum angiotensin‐converting enzyme was 32 U/L (normal value, 967 U/L). A CT of the chest was performed and did not reveal mediastinal lymphadenopathy, nodules, or consolidations. Antinuclear, antismooth muscle, and antimitochondrial antibodies were negative. Human immunodeficiency virus antibody was negative. Serum quantitative Igs, including IgG, IgM, IgA, and IgE, were undetectable.
Serum lymphocyte subset analysis revealed a CD3 T‐cell count of 101 106/L (normal value, >690 106/L), CD4 T cells 46 106/L (normal value, >410 106/L), CD8 T cells 55 106/L (normal value, >190 106/L), CD19 B cells undetectable at 2 106/L (normal value, >90 106/L), CD16 CD56 NK cells 134 106/L (normal value, >90 106/L). T‐cell lymphocyte proliferation assay showed a completely absent response to candida and tetanus antigens, and a very low response to mitogens.
The immunologic evaluation is confounded by her critical illness and by the prior administration of anti‐CD20 monoclonal antibody. Despite these caveats, the results of these studies are profoundly abnormal and suggest a combined B‐cell and T‐cell immunodeficiency that is more severe from a laboratory standpoint than her history prior to surgery has suggested. Low T lymphocyte numbers, with or without functional abnormalities, are a hallmark of CID and can be also be seen in CVID. The extremely low Ig levels in the presence of severe infections warrant replacement with intravenous Ig.
Combined immunodeficiency and CVID may be associated with a number of mutations; elucidating the genetics and molecular mechanism of immunodeficiency may be important in identifying patients whose immunodeficiency may be cured by stem‐cell transplantation.
Intravenous Ig was administered. Her serum was sent for sequencing of the RMRP gene, mutations of which are found in patients who have cartilage‐hair hypoplasia (CHH), a rare autosomal recessive skeletal dysplasia characterized by short‐limbed dwarfism; fine, sparse hair; and variable degrees of immunodeficiency. She was found to have 2 RMRP mutations, a 126 CT transition and a 218 AC transversion.
The patient developed multiple abdominal abscesses, which were drained and grew vancomycin‐resistant enterococcus (VRE) and C. albicans. Blood cultures also turned positive for VRE. A colonoscopy was performed because of radiographic evidence suggestive of colitis. Biopsies taken from the colonoscopy were negative for cytomegalovirus or other infections, but did reveal rare non‐necrotizing granulomas. The patient developed progressive multiorgan failure requiring mechanical ventilation and continuous venovenous hemofiltration. On postoperative day 36, the patient was transitioned to comfort care, and she expired the next day. A unifying diagnosis of CHH‐related immunodeficiency and disseminated granulomatous disease, complicated by postoperative sepsis, was made. An autopsy was declined.
COMMENTARY
Evaluation of abnormal liver tests is a frequent diagnostic challenge faced by clinicians in both ambulatory and inpatient settings. Identifying the pattern of liver injuryhepatocellular, cholestatic, or infiltrativemay guide the initial workup. This patient's presentation of a normal bilirubin and transaminases with elevations in ALP was consistent with infiltrative hepatic disease. The radiographic finding of extrahepatic biliary strictures, on the other hand, raised concern for an obstructive etiology and prompted an ERCP. Brush cytology has high specificity for malignancy, but interpretation of atypical cells can rarely be inconclusive or be associated with false positives.[1]
The suspicion for infiltrative hepatitis was supported postoperatively by the discovery of diffuse hepatobiliary granulomatous disease, which can be associated with a spectrum of disease states including sarcoidosis, autoimmune disorders, intracellular infections, immunodeficiency, malignancy, environmental or occupational exposures, and drug reactions.[2, 3] During the patient's hospital course and case presentation to the discussant, the possibility of sarcoidosis was raised based on the operative findings. Additional history‐taking was essential to evaluate other etiologies of granulomatous inflammation, and this clinical correlation prevented a second erroneous pathologic diagnosis.
Multiple elements of this patient's presentation led to recognition of an underlying primary immunodeficiency. Her prior history of recurrent childhood infections, dermatomal zoster, and vaginal infections suggested a congenital immunodeficiency. The additional features of refractory autoimmune cytopenias (ie, ITP), granulomatous inflammation, undetectable serum Igs, and low T‐cell and B‐cell counts, were consistent with CID or CVID. By definition, CID involves defects in both B and T cells; CVID represents a predominantly B‐cell disorder characterized by abnormalities in Ig production, though concomitant T‐cell dysfunction may also be found.[4] It is worth noting that although this patient had previously received anti‐CD20 monoclonal antibody, which depletes CD20‐positive B lymphocytes, Ig levels are not typically depleted by anti‐CD20 unless there is preexisting antibody deficiency.[5]
We were able to make the unifying diagnosis of CHH to explain her constellation of physical findings, laboratory abnormalities, and histopathology. Also known as McKusick type metaphyseal chondrodysplasia, CHH has a relatively high carrier frequency in the Amish (1:19) and Finnish (1:76) populations.[6, 7] Additional clinical features can include gastrointestinal disorders, poorly pigmented skin and hair, and joint disorders. Dysregulation of immunity is a particular challenge and can be manifested by malignancy, lymphoproliferative disease, cytopenias, or primary immunodeficiencies. Combined immunodeficiency and T cellmediated defects are most common, although there are case reports of CHH associated with severe humoral defects.[8, 9] Primary immunodeficiency, if severe and recognized early, can be treated with bone‐marrow transplantation.[10, 11] Granulomatous inflammation also has been described in CHH.[12]
Although tissue biopsy is often viewed as the gold standard for establishing a definitive diagnosis, this case highlights the significance of applying clinical context to pathologic interpretation and medical decision‐making. Prior to any diagnostic procedure, the patient's history of dwarfism, recurrent infections, and refractory ITP provided clues to an immunodeficiency syndrome, CHH. Knowledge of this immunodeficiency might have better informed the initial pathologic interpretation of atypical cells, which were misread as adenocarcinoma. Furthermore, awareness of the patient's profound immunodeficiency would have given pause to proceeding with invasive surgery without prior Ig and antibiotic support and may have averted a fatal outcome.
KEY TEACHING POINTS
- Infiltrative hepatobiliary diseases may manifest with isolated elevations in ALP.
- Granulomas and autoimmune cytopenias may be features of primary immunodeficiency states.
- A history of recurrent childhood infections should raise suspicion for congenital immunodeficiencies.
- Unique medical complications, including immunodeficiency, can be associated with dwarfism subtypes.
Acknowledgements
The authors thank Jennifer M. Puck, MD, from the University of California San Francisco, Departments of Immunology and Pediatrics, for her invaluable contribution to the discussion on immunodeficiencies.
Disclosure
Nothing to report.
- , , , . Brush cytology of ductal strictures during ERCP. Acta Gastroenterol Belg. 2000;63:254–259.
- , . Granulomatous lung disease: an approach to the differential diagnosis. Arch Pathol Lab Med. 2010;134;667–690.
- James DG, Zumla A, eds. The Granulomatous Disorders. Cambridge, UK: Cambridge University Press; 1999:17–27.
- , , , et al. Unraveling the complexity of T cell abnormalities in common variable immunodeficiency. J Immunol. 2007;178:3932–3943.
- , , , et al. Does rituximab aggravate pre‐existing hypogammaglobulinaemia? J Clin Pathol. 2010;63:275–277.
- . Cartilage‐hair hypoplasia in Finland: epidemiological and genetic aspects of 107 patients. J Med Genet. 1992;29:652–655.
- , , , et al. High‐resolution genetic mapping of the cartilage‐hair hypoplasia (CHH) gene in Amish and Finnish families. Genomics. 1994;20:347–353.
- , , , et al. Combined immunodeficiency and vaccine‐related poliomyelitis in a child with cartilage‐hair hypoplasia. J Pediatr. 1975;86:868–872.
- , , . Deficiency of humoral immunity in cartilage‐hair hypoplasia. J Pediatr. 2000;137:487–492.
- , , , , . Bone marrow transplantation for cartilage‐hair hypoplasia. Bone Marrow Transplant. 2006;38:751–756.
- , , , et al. Clinical and immunologic outcome of patients with cartilage hair hypoplasia after hematopoietic stem cell transplantation [published corrections appear in Blood. 2010;116:2402 and Blood. 2011;117:2077]. Blood. 2010;116:27–35.
- , , , et al. Granulomatous inflammation in cartilage‐hair hypoplasia: risks and benefits of anti‐TNF‐α mAbs. J Allergy Clin Immunol. 2011;128:847–853.
A 41‐year‐old woman with dwarfism was referred for evaluation of an isolated elevated alkaline phosphatase (ALP) of 792 U/L (normal value, 3195 U/L) and a gamma‐glutamyl transferase (GGT) of 729 U/L (normal value, 737 U/L), found incidentally on routine laboratory screening. She denied any fevers, chills, weight loss, abdominal pain, nausea, or vomiting.
The presence of an isolated ALP elevation, presumably of hepatobiliary origin given the increase in GGT, in a relatively young woman immediately calls to mind the diagnosis of primary biliary cirrhosis, and I would specifically inquire about pruritus, which occurs commonly in this setting. The absence of abdominal pain argues against the diagnosis of extrahepatic biliary obstruction. Other processes that could result in this asymptomatic presentation include infiltrative diseases such as amyloidosis, sarcoidosis, and other causes of granulomatous hepatitis. The absence of systemic symptoms makes disseminated infection or malignancy with hepatic involvement less likely. I would query whether underlying dwarfism can be associated with metabolic abnormalities that cause infiltrative liver disease, functional or anatomical hepatobiliary abnormalities, or malignancy.
The patient's medical history was notable for chronic constipation, allergic rhinitis, and basal‐cell carcinoma. She had reconstructive surgeries of the left hip and knee 28 years ago without complications. She underwent a right total hip replacement for hip dysplasia 6 months prior, which was complicated by a postoperative joint infection with Enterobacter cloacae. The hardware was retained, and she was treated with incision and drainage and a prolonged fluoroquinolone course. Furthermore, she had a history of immune thrombocytopenic purpura (ITP), which manifested at the age of 20 years. A bone‐marrow biopsy at that time showed no evidence of hematologic malignancy. For her ITP, she had initially received intravenous immunoglobulin (Ig) and cyclosporine without sustained benefit. She underwent a splenectomy at the age of 26 years and was treated intermittently with rituximab over 11 years prior to admission. Her medications included cetirizine. Her parents were nonconsanguineous, of European and Southeast Asian ancestry, and healthy. She was in a long‐term monogamous relationship. The patient had been employed as an educator.
The history of immune‐mediated thrombocytopenia raises the possibility that the present illness may be part of a broader autoimmune diathesis. Other causes of secondary ITP, such as drug‐induced reactions, hematologic malignancies, and viral infections, are unlikely, as her ITP has been persistent for more than 20 years. She has not evolved into a common phenotypic pattern of autoimmune disease such as systemic lupus erythematosus after the appearance of ITP, nor does she endorse a history of thromboembolic complications that would suggest antiphospholipid syndrome.
Ultrasound of the abdomen demonstrated narrowing of the extrahepatic biliary duct in the region of the pancreas without evidence of a mass lesion. Computerized tomography (CT) of the abdomen and pelvis similarly showed mild intrahepatic biliary ductal dilatation with narrowing of the extrahepatic duct in the region of the pancreas without apparent pancreatic mass. Endoscopic retrograde cholangiopancreatography (ERCP) confirmed a stricture in the distal common bile duct and dilatation of the common bile duct. Cytology brushings obtained during ERCP showed groups of overlapping, enlarged cells with pleomorphic irregular nuclei, one or more prominent nucleoli, and focal nuclear molding, leading to a diagnosis of adenocarcinoma (Figure 1).

The absence of jaundice and pruritus indicates incomplete biliary obstruction. Commonbile duct strictures are most commonly seen after manipulation of the biliary tree. Neoplasms including pancreatic cancer, adenocarcinoma of the ampulla of Vater, and cholangiocarcinoma may cause compression and obstruction of the common bile duct, as well as stricture formation mediated by a desmoplastic reaction to the tumor. Occasionally, metastatic malignancy or lymphoma may involve the porta hepatis and cause extrinsic compression of the common bile duct. Other etiologies of strictures include sclerosing cholangitis and opportunistic infections such as Cryptosporidium, cytomegalovirus, and microsporidiosis, which are not supported by this patient's history.
The atypical cells seen on ERCP brushings were interpreted as evidence of cholangiocarcinoma. The patient underwent a pylorus‐sparing Whipple procedure. Examination of the surgical pathology specimens revealed diffuse non‐necrotizing granulomatous inflammation involving the bile duct and gallbladder (Figure 2). There was focal atypia of the bile‐duct epithelial cells, but no evidence of malignancy. There were non‐necrotizing granulomas in numerous lymph nodes, some with significant sclerosis; stains and cultures for acid‐fast bacilli and fungi were negative, and stains for IgG4 and CD1a for Langerhans‐cell histiocytosis were negative.

Granulomatous inflammation may be caused by a variety of intracellular infections, environmental and occupational exposures, and drug hypersensitivity, or may be associated with malignancy such as lymphoma. In the absence of an alternative explanation, the presence of non‐necrotizing granulomas in multiple organs suggests the diagnosis of sarcoidosis, even if classic intrathoracic involvement is not present. Hepatic involvement with sarcoidosis is common but rarely symptomatic, whereas biliary disease is distinctly uncommon. Interestingly, there is an association between both primary biliary cirrhosis and sclerosing cholangitis with sarcoidosis. The pathologic findings could indicate an autoimmune process that has led to widespread granulomas with this unusual distribution. Disseminated infections such as mycobacterial or fungal diseases seem much less plausible in this woman, who had no prior systemic complaints. The atypical cells seen on the ERCP brushings were almost certainly caused by inflammation and a fibroproliferative response rather than malignancy.
On further questioning, the patient endorsed a history of multiple childhood ear infections that required bilateral myringotomy tubes, and multiple episodes of sinusitis, but both problems improved in adulthood. She had experienced 2 episodes of dermatomal zoster in her lifetime. She also noted frequent vaginal yeast infections. She denied any history of pneumonias or thrush. In her second decade of life, she developed allergic rhinitis and eczema. She denied any chemical or environmental exposures. She had had negative tuberculin skin tests as part of her occupational screening and denied any recent travel.
The additional history of recurrent upper‐respiratory infections early in life and subsequent episodes of dermatomal zoster and candidal infections increases the likelihood that this patient has a primary immunodeficiency. A combined cellular and humoral immunodeficiency would predispose to both bacterial sinopulmonary infections, generally a result of Ig isotype or IgG subclass deficiencies, and recurrent zoster and candidal infection. Any evaluation of her Igs at this time may be confounded by her receipt of anti‐CD20 monoclonal antibody therapy, which may decrease serum Ig levels.
The relatively benign course in terms of infection is consistent with the heterogeneous immunodeficiencies classified as combined immunodeficiency (CID), a less‐penetrant phenotype of severe combined immunodeficiency (SCID), or common variable immunodeficiency (CVID). Autoimmunity is a frequent manifestation of CID and CVID, and affected patients have an increased risk of lymphoma and other malignancies. Granulomatous disease may also be a manifestation of both CID and CVID.
Postoperatively, she developed progressive abdominal distension and pain. A CT of the abdomen and pelvis showed colonic dilatation consistent with Ogilvie pseudo‐obstruction. On postoperative day 9, she developed fevers. On physical examination, her temperature was 38.5C, the blood pressure was 104/56 mm Hg, and the heart rate was 131 beats per minute. Her oxygen saturation was 95% on room air. Her height was 105 cm. She had diffuse alopecia without scarring. She did not have a malar rash or oral ulcerations. Both lungs were clear to auscultation. A cardiac examination showed tachycardia with a regular rhythm, normal heart sounds, and no murmurs. Her musculoskeletal exam was notable for short limbs and phalanges, without synovitis. Bilateral hip exam demonstrated internal and external range of motion without abnormalities. No rashes were present. Her abdominal exam revealed diffuse tenderness with postoperative drains in place. She had nonbloody loose stools.
Although autoimmune diseases such as sarcoidosis can rarely manifest with fevers, evaluation of postoperative fever in this patient should focus first on common processes that also occur in immunocompetent patients. Since she has had a splenectomy and we are now suspicious of an underlying immunodeficiency, appropriate cultures should be obtained and broad‐spectrum intravenous antibiotics should be initiated without delay. The presence of nonscarring alopecia could either represent autoimmune alopecia, if the onset was recent, or it could be part of this patient's underlying skeletal dysplasia syndrome.
Piperacillin/tazobactam and oral metronidazole were started for presumed intra‐abdominal infection. The white cell count was 20,500/mm3 with 96% neutrophils, 1.4% lymphocytes with an absolute lymphocyte count 0.33 109/L (normal value, >1.0 109/L), and 2.6% monocytes. The hematocrit was 27.8% with a mean corpuscular volume of 95 fL. The platelet count was 323,000/mm3. Serum aminotransferase and total bilirubin levels were normal, and ALP was 904 U/L. The serum albumin was 1.2 g/dL (normal value, 3.54.8 g/dL) and prealbumin was 6 mg/dL (normal value, 2037 mg/dL).
Blood cultures returned positive for E. cloacae. Clostridium difficile toxin assay was negative. Piperacillin/tazobactam was switched to meroperem, and metronidazole was discontinued. She continued to have fevers, and on postoperative day 16, repeat blood cultures and urine cultures grew Candida albicans; caspofungin was initiated.
In addition to the neutrophilic leukocytosis in response to gram‐negative bacteremia, there is marked lymphopenia. Although sepsis may cause transient declines in the total lymphocyte count, I do not believe that this entirely accounts for such severe lymphopenia. The albumin is also profoundly low. Her catabolic postsurgical state might explain part of this abnormality, but taken together with her prior gastrointestinal symptoms, these findings could be consistent with intestinal malabsorption or a protein‐losing enteropathy, which can also be associated with primary immunodeficiency.
Serum angiotensin‐converting enzyme was 32 U/L (normal value, 967 U/L). A CT of the chest was performed and did not reveal mediastinal lymphadenopathy, nodules, or consolidations. Antinuclear, antismooth muscle, and antimitochondrial antibodies were negative. Human immunodeficiency virus antibody was negative. Serum quantitative Igs, including IgG, IgM, IgA, and IgE, were undetectable.
Serum lymphocyte subset analysis revealed a CD3 T‐cell count of 101 106/L (normal value, >690 106/L), CD4 T cells 46 106/L (normal value, >410 106/L), CD8 T cells 55 106/L (normal value, >190 106/L), CD19 B cells undetectable at 2 106/L (normal value, >90 106/L), CD16 CD56 NK cells 134 106/L (normal value, >90 106/L). T‐cell lymphocyte proliferation assay showed a completely absent response to candida and tetanus antigens, and a very low response to mitogens.
The immunologic evaluation is confounded by her critical illness and by the prior administration of anti‐CD20 monoclonal antibody. Despite these caveats, the results of these studies are profoundly abnormal and suggest a combined B‐cell and T‐cell immunodeficiency that is more severe from a laboratory standpoint than her history prior to surgery has suggested. Low T lymphocyte numbers, with or without functional abnormalities, are a hallmark of CID and can be also be seen in CVID. The extremely low Ig levels in the presence of severe infections warrant replacement with intravenous Ig.
Combined immunodeficiency and CVID may be associated with a number of mutations; elucidating the genetics and molecular mechanism of immunodeficiency may be important in identifying patients whose immunodeficiency may be cured by stem‐cell transplantation.
Intravenous Ig was administered. Her serum was sent for sequencing of the RMRP gene, mutations of which are found in patients who have cartilage‐hair hypoplasia (CHH), a rare autosomal recessive skeletal dysplasia characterized by short‐limbed dwarfism; fine, sparse hair; and variable degrees of immunodeficiency. She was found to have 2 RMRP mutations, a 126 CT transition and a 218 AC transversion.
The patient developed multiple abdominal abscesses, which were drained and grew vancomycin‐resistant enterococcus (VRE) and C. albicans. Blood cultures also turned positive for VRE. A colonoscopy was performed because of radiographic evidence suggestive of colitis. Biopsies taken from the colonoscopy were negative for cytomegalovirus or other infections, but did reveal rare non‐necrotizing granulomas. The patient developed progressive multiorgan failure requiring mechanical ventilation and continuous venovenous hemofiltration. On postoperative day 36, the patient was transitioned to comfort care, and she expired the next day. A unifying diagnosis of CHH‐related immunodeficiency and disseminated granulomatous disease, complicated by postoperative sepsis, was made. An autopsy was declined.
COMMENTARY
Evaluation of abnormal liver tests is a frequent diagnostic challenge faced by clinicians in both ambulatory and inpatient settings. Identifying the pattern of liver injuryhepatocellular, cholestatic, or infiltrativemay guide the initial workup. This patient's presentation of a normal bilirubin and transaminases with elevations in ALP was consistent with infiltrative hepatic disease. The radiographic finding of extrahepatic biliary strictures, on the other hand, raised concern for an obstructive etiology and prompted an ERCP. Brush cytology has high specificity for malignancy, but interpretation of atypical cells can rarely be inconclusive or be associated with false positives.[1]
The suspicion for infiltrative hepatitis was supported postoperatively by the discovery of diffuse hepatobiliary granulomatous disease, which can be associated with a spectrum of disease states including sarcoidosis, autoimmune disorders, intracellular infections, immunodeficiency, malignancy, environmental or occupational exposures, and drug reactions.[2, 3] During the patient's hospital course and case presentation to the discussant, the possibility of sarcoidosis was raised based on the operative findings. Additional history‐taking was essential to evaluate other etiologies of granulomatous inflammation, and this clinical correlation prevented a second erroneous pathologic diagnosis.
Multiple elements of this patient's presentation led to recognition of an underlying primary immunodeficiency. Her prior history of recurrent childhood infections, dermatomal zoster, and vaginal infections suggested a congenital immunodeficiency. The additional features of refractory autoimmune cytopenias (ie, ITP), granulomatous inflammation, undetectable serum Igs, and low T‐cell and B‐cell counts, were consistent with CID or CVID. By definition, CID involves defects in both B and T cells; CVID represents a predominantly B‐cell disorder characterized by abnormalities in Ig production, though concomitant T‐cell dysfunction may also be found.[4] It is worth noting that although this patient had previously received anti‐CD20 monoclonal antibody, which depletes CD20‐positive B lymphocytes, Ig levels are not typically depleted by anti‐CD20 unless there is preexisting antibody deficiency.[5]
We were able to make the unifying diagnosis of CHH to explain her constellation of physical findings, laboratory abnormalities, and histopathology. Also known as McKusick type metaphyseal chondrodysplasia, CHH has a relatively high carrier frequency in the Amish (1:19) and Finnish (1:76) populations.[6, 7] Additional clinical features can include gastrointestinal disorders, poorly pigmented skin and hair, and joint disorders. Dysregulation of immunity is a particular challenge and can be manifested by malignancy, lymphoproliferative disease, cytopenias, or primary immunodeficiencies. Combined immunodeficiency and T cellmediated defects are most common, although there are case reports of CHH associated with severe humoral defects.[8, 9] Primary immunodeficiency, if severe and recognized early, can be treated with bone‐marrow transplantation.[10, 11] Granulomatous inflammation also has been described in CHH.[12]
Although tissue biopsy is often viewed as the gold standard for establishing a definitive diagnosis, this case highlights the significance of applying clinical context to pathologic interpretation and medical decision‐making. Prior to any diagnostic procedure, the patient's history of dwarfism, recurrent infections, and refractory ITP provided clues to an immunodeficiency syndrome, CHH. Knowledge of this immunodeficiency might have better informed the initial pathologic interpretation of atypical cells, which were misread as adenocarcinoma. Furthermore, awareness of the patient's profound immunodeficiency would have given pause to proceeding with invasive surgery without prior Ig and antibiotic support and may have averted a fatal outcome.
KEY TEACHING POINTS
- Infiltrative hepatobiliary diseases may manifest with isolated elevations in ALP.
- Granulomas and autoimmune cytopenias may be features of primary immunodeficiency states.
- A history of recurrent childhood infections should raise suspicion for congenital immunodeficiencies.
- Unique medical complications, including immunodeficiency, can be associated with dwarfism subtypes.
Acknowledgements
The authors thank Jennifer M. Puck, MD, from the University of California San Francisco, Departments of Immunology and Pediatrics, for her invaluable contribution to the discussion on immunodeficiencies.
Disclosure
Nothing to report.
A 41‐year‐old woman with dwarfism was referred for evaluation of an isolated elevated alkaline phosphatase (ALP) of 792 U/L (normal value, 3195 U/L) and a gamma‐glutamyl transferase (GGT) of 729 U/L (normal value, 737 U/L), found incidentally on routine laboratory screening. She denied any fevers, chills, weight loss, abdominal pain, nausea, or vomiting.
The presence of an isolated ALP elevation, presumably of hepatobiliary origin given the increase in GGT, in a relatively young woman immediately calls to mind the diagnosis of primary biliary cirrhosis, and I would specifically inquire about pruritus, which occurs commonly in this setting. The absence of abdominal pain argues against the diagnosis of extrahepatic biliary obstruction. Other processes that could result in this asymptomatic presentation include infiltrative diseases such as amyloidosis, sarcoidosis, and other causes of granulomatous hepatitis. The absence of systemic symptoms makes disseminated infection or malignancy with hepatic involvement less likely. I would query whether underlying dwarfism can be associated with metabolic abnormalities that cause infiltrative liver disease, functional or anatomical hepatobiliary abnormalities, or malignancy.
The patient's medical history was notable for chronic constipation, allergic rhinitis, and basal‐cell carcinoma. She had reconstructive surgeries of the left hip and knee 28 years ago without complications. She underwent a right total hip replacement for hip dysplasia 6 months prior, which was complicated by a postoperative joint infection with Enterobacter cloacae. The hardware was retained, and she was treated with incision and drainage and a prolonged fluoroquinolone course. Furthermore, she had a history of immune thrombocytopenic purpura (ITP), which manifested at the age of 20 years. A bone‐marrow biopsy at that time showed no evidence of hematologic malignancy. For her ITP, she had initially received intravenous immunoglobulin (Ig) and cyclosporine without sustained benefit. She underwent a splenectomy at the age of 26 years and was treated intermittently with rituximab over 11 years prior to admission. Her medications included cetirizine. Her parents were nonconsanguineous, of European and Southeast Asian ancestry, and healthy. She was in a long‐term monogamous relationship. The patient had been employed as an educator.
The history of immune‐mediated thrombocytopenia raises the possibility that the present illness may be part of a broader autoimmune diathesis. Other causes of secondary ITP, such as drug‐induced reactions, hematologic malignancies, and viral infections, are unlikely, as her ITP has been persistent for more than 20 years. She has not evolved into a common phenotypic pattern of autoimmune disease such as systemic lupus erythematosus after the appearance of ITP, nor does she endorse a history of thromboembolic complications that would suggest antiphospholipid syndrome.
Ultrasound of the abdomen demonstrated narrowing of the extrahepatic biliary duct in the region of the pancreas without evidence of a mass lesion. Computerized tomography (CT) of the abdomen and pelvis similarly showed mild intrahepatic biliary ductal dilatation with narrowing of the extrahepatic duct in the region of the pancreas without apparent pancreatic mass. Endoscopic retrograde cholangiopancreatography (ERCP) confirmed a stricture in the distal common bile duct and dilatation of the common bile duct. Cytology brushings obtained during ERCP showed groups of overlapping, enlarged cells with pleomorphic irregular nuclei, one or more prominent nucleoli, and focal nuclear molding, leading to a diagnosis of adenocarcinoma (Figure 1).

The absence of jaundice and pruritus indicates incomplete biliary obstruction. Commonbile duct strictures are most commonly seen after manipulation of the biliary tree. Neoplasms including pancreatic cancer, adenocarcinoma of the ampulla of Vater, and cholangiocarcinoma may cause compression and obstruction of the common bile duct, as well as stricture formation mediated by a desmoplastic reaction to the tumor. Occasionally, metastatic malignancy or lymphoma may involve the porta hepatis and cause extrinsic compression of the common bile duct. Other etiologies of strictures include sclerosing cholangitis and opportunistic infections such as Cryptosporidium, cytomegalovirus, and microsporidiosis, which are not supported by this patient's history.
The atypical cells seen on ERCP brushings were interpreted as evidence of cholangiocarcinoma. The patient underwent a pylorus‐sparing Whipple procedure. Examination of the surgical pathology specimens revealed diffuse non‐necrotizing granulomatous inflammation involving the bile duct and gallbladder (Figure 2). There was focal atypia of the bile‐duct epithelial cells, but no evidence of malignancy. There were non‐necrotizing granulomas in numerous lymph nodes, some with significant sclerosis; stains and cultures for acid‐fast bacilli and fungi were negative, and stains for IgG4 and CD1a for Langerhans‐cell histiocytosis were negative.

Granulomatous inflammation may be caused by a variety of intracellular infections, environmental and occupational exposures, and drug hypersensitivity, or may be associated with malignancy such as lymphoma. In the absence of an alternative explanation, the presence of non‐necrotizing granulomas in multiple organs suggests the diagnosis of sarcoidosis, even if classic intrathoracic involvement is not present. Hepatic involvement with sarcoidosis is common but rarely symptomatic, whereas biliary disease is distinctly uncommon. Interestingly, there is an association between both primary biliary cirrhosis and sclerosing cholangitis with sarcoidosis. The pathologic findings could indicate an autoimmune process that has led to widespread granulomas with this unusual distribution. Disseminated infections such as mycobacterial or fungal diseases seem much less plausible in this woman, who had no prior systemic complaints. The atypical cells seen on the ERCP brushings were almost certainly caused by inflammation and a fibroproliferative response rather than malignancy.
On further questioning, the patient endorsed a history of multiple childhood ear infections that required bilateral myringotomy tubes, and multiple episodes of sinusitis, but both problems improved in adulthood. She had experienced 2 episodes of dermatomal zoster in her lifetime. She also noted frequent vaginal yeast infections. She denied any history of pneumonias or thrush. In her second decade of life, she developed allergic rhinitis and eczema. She denied any chemical or environmental exposures. She had had negative tuberculin skin tests as part of her occupational screening and denied any recent travel.
The additional history of recurrent upper‐respiratory infections early in life and subsequent episodes of dermatomal zoster and candidal infections increases the likelihood that this patient has a primary immunodeficiency. A combined cellular and humoral immunodeficiency would predispose to both bacterial sinopulmonary infections, generally a result of Ig isotype or IgG subclass deficiencies, and recurrent zoster and candidal infection. Any evaluation of her Igs at this time may be confounded by her receipt of anti‐CD20 monoclonal antibody therapy, which may decrease serum Ig levels.
The relatively benign course in terms of infection is consistent with the heterogeneous immunodeficiencies classified as combined immunodeficiency (CID), a less‐penetrant phenotype of severe combined immunodeficiency (SCID), or common variable immunodeficiency (CVID). Autoimmunity is a frequent manifestation of CID and CVID, and affected patients have an increased risk of lymphoma and other malignancies. Granulomatous disease may also be a manifestation of both CID and CVID.
Postoperatively, she developed progressive abdominal distension and pain. A CT of the abdomen and pelvis showed colonic dilatation consistent with Ogilvie pseudo‐obstruction. On postoperative day 9, she developed fevers. On physical examination, her temperature was 38.5C, the blood pressure was 104/56 mm Hg, and the heart rate was 131 beats per minute. Her oxygen saturation was 95% on room air. Her height was 105 cm. She had diffuse alopecia without scarring. She did not have a malar rash or oral ulcerations. Both lungs were clear to auscultation. A cardiac examination showed tachycardia with a regular rhythm, normal heart sounds, and no murmurs. Her musculoskeletal exam was notable for short limbs and phalanges, without synovitis. Bilateral hip exam demonstrated internal and external range of motion without abnormalities. No rashes were present. Her abdominal exam revealed diffuse tenderness with postoperative drains in place. She had nonbloody loose stools.
Although autoimmune diseases such as sarcoidosis can rarely manifest with fevers, evaluation of postoperative fever in this patient should focus first on common processes that also occur in immunocompetent patients. Since she has had a splenectomy and we are now suspicious of an underlying immunodeficiency, appropriate cultures should be obtained and broad‐spectrum intravenous antibiotics should be initiated without delay. The presence of nonscarring alopecia could either represent autoimmune alopecia, if the onset was recent, or it could be part of this patient's underlying skeletal dysplasia syndrome.
Piperacillin/tazobactam and oral metronidazole were started for presumed intra‐abdominal infection. The white cell count was 20,500/mm3 with 96% neutrophils, 1.4% lymphocytes with an absolute lymphocyte count 0.33 109/L (normal value, >1.0 109/L), and 2.6% monocytes. The hematocrit was 27.8% with a mean corpuscular volume of 95 fL. The platelet count was 323,000/mm3. Serum aminotransferase and total bilirubin levels were normal, and ALP was 904 U/L. The serum albumin was 1.2 g/dL (normal value, 3.54.8 g/dL) and prealbumin was 6 mg/dL (normal value, 2037 mg/dL).
Blood cultures returned positive for E. cloacae. Clostridium difficile toxin assay was negative. Piperacillin/tazobactam was switched to meroperem, and metronidazole was discontinued. She continued to have fevers, and on postoperative day 16, repeat blood cultures and urine cultures grew Candida albicans; caspofungin was initiated.
In addition to the neutrophilic leukocytosis in response to gram‐negative bacteremia, there is marked lymphopenia. Although sepsis may cause transient declines in the total lymphocyte count, I do not believe that this entirely accounts for such severe lymphopenia. The albumin is also profoundly low. Her catabolic postsurgical state might explain part of this abnormality, but taken together with her prior gastrointestinal symptoms, these findings could be consistent with intestinal malabsorption or a protein‐losing enteropathy, which can also be associated with primary immunodeficiency.
Serum angiotensin‐converting enzyme was 32 U/L (normal value, 967 U/L). A CT of the chest was performed and did not reveal mediastinal lymphadenopathy, nodules, or consolidations. Antinuclear, antismooth muscle, and antimitochondrial antibodies were negative. Human immunodeficiency virus antibody was negative. Serum quantitative Igs, including IgG, IgM, IgA, and IgE, were undetectable.
Serum lymphocyte subset analysis revealed a CD3 T‐cell count of 101 106/L (normal value, >690 106/L), CD4 T cells 46 106/L (normal value, >410 106/L), CD8 T cells 55 106/L (normal value, >190 106/L), CD19 B cells undetectable at 2 106/L (normal value, >90 106/L), CD16 CD56 NK cells 134 106/L (normal value, >90 106/L). T‐cell lymphocyte proliferation assay showed a completely absent response to candida and tetanus antigens, and a very low response to mitogens.
The immunologic evaluation is confounded by her critical illness and by the prior administration of anti‐CD20 monoclonal antibody. Despite these caveats, the results of these studies are profoundly abnormal and suggest a combined B‐cell and T‐cell immunodeficiency that is more severe from a laboratory standpoint than her history prior to surgery has suggested. Low T lymphocyte numbers, with or without functional abnormalities, are a hallmark of CID and can be also be seen in CVID. The extremely low Ig levels in the presence of severe infections warrant replacement with intravenous Ig.
Combined immunodeficiency and CVID may be associated with a number of mutations; elucidating the genetics and molecular mechanism of immunodeficiency may be important in identifying patients whose immunodeficiency may be cured by stem‐cell transplantation.
Intravenous Ig was administered. Her serum was sent for sequencing of the RMRP gene, mutations of which are found in patients who have cartilage‐hair hypoplasia (CHH), a rare autosomal recessive skeletal dysplasia characterized by short‐limbed dwarfism; fine, sparse hair; and variable degrees of immunodeficiency. She was found to have 2 RMRP mutations, a 126 CT transition and a 218 AC transversion.
The patient developed multiple abdominal abscesses, which were drained and grew vancomycin‐resistant enterococcus (VRE) and C. albicans. Blood cultures also turned positive for VRE. A colonoscopy was performed because of radiographic evidence suggestive of colitis. Biopsies taken from the colonoscopy were negative for cytomegalovirus or other infections, but did reveal rare non‐necrotizing granulomas. The patient developed progressive multiorgan failure requiring mechanical ventilation and continuous venovenous hemofiltration. On postoperative day 36, the patient was transitioned to comfort care, and she expired the next day. A unifying diagnosis of CHH‐related immunodeficiency and disseminated granulomatous disease, complicated by postoperative sepsis, was made. An autopsy was declined.
COMMENTARY
Evaluation of abnormal liver tests is a frequent diagnostic challenge faced by clinicians in both ambulatory and inpatient settings. Identifying the pattern of liver injuryhepatocellular, cholestatic, or infiltrativemay guide the initial workup. This patient's presentation of a normal bilirubin and transaminases with elevations in ALP was consistent with infiltrative hepatic disease. The radiographic finding of extrahepatic biliary strictures, on the other hand, raised concern for an obstructive etiology and prompted an ERCP. Brush cytology has high specificity for malignancy, but interpretation of atypical cells can rarely be inconclusive or be associated with false positives.[1]
The suspicion for infiltrative hepatitis was supported postoperatively by the discovery of diffuse hepatobiliary granulomatous disease, which can be associated with a spectrum of disease states including sarcoidosis, autoimmune disorders, intracellular infections, immunodeficiency, malignancy, environmental or occupational exposures, and drug reactions.[2, 3] During the patient's hospital course and case presentation to the discussant, the possibility of sarcoidosis was raised based on the operative findings. Additional history‐taking was essential to evaluate other etiologies of granulomatous inflammation, and this clinical correlation prevented a second erroneous pathologic diagnosis.
Multiple elements of this patient's presentation led to recognition of an underlying primary immunodeficiency. Her prior history of recurrent childhood infections, dermatomal zoster, and vaginal infections suggested a congenital immunodeficiency. The additional features of refractory autoimmune cytopenias (ie, ITP), granulomatous inflammation, undetectable serum Igs, and low T‐cell and B‐cell counts, were consistent with CID or CVID. By definition, CID involves defects in both B and T cells; CVID represents a predominantly B‐cell disorder characterized by abnormalities in Ig production, though concomitant T‐cell dysfunction may also be found.[4] It is worth noting that although this patient had previously received anti‐CD20 monoclonal antibody, which depletes CD20‐positive B lymphocytes, Ig levels are not typically depleted by anti‐CD20 unless there is preexisting antibody deficiency.[5]
We were able to make the unifying diagnosis of CHH to explain her constellation of physical findings, laboratory abnormalities, and histopathology. Also known as McKusick type metaphyseal chondrodysplasia, CHH has a relatively high carrier frequency in the Amish (1:19) and Finnish (1:76) populations.[6, 7] Additional clinical features can include gastrointestinal disorders, poorly pigmented skin and hair, and joint disorders. Dysregulation of immunity is a particular challenge and can be manifested by malignancy, lymphoproliferative disease, cytopenias, or primary immunodeficiencies. Combined immunodeficiency and T cellmediated defects are most common, although there are case reports of CHH associated with severe humoral defects.[8, 9] Primary immunodeficiency, if severe and recognized early, can be treated with bone‐marrow transplantation.[10, 11] Granulomatous inflammation also has been described in CHH.[12]
Although tissue biopsy is often viewed as the gold standard for establishing a definitive diagnosis, this case highlights the significance of applying clinical context to pathologic interpretation and medical decision‐making. Prior to any diagnostic procedure, the patient's history of dwarfism, recurrent infections, and refractory ITP provided clues to an immunodeficiency syndrome, CHH. Knowledge of this immunodeficiency might have better informed the initial pathologic interpretation of atypical cells, which were misread as adenocarcinoma. Furthermore, awareness of the patient's profound immunodeficiency would have given pause to proceeding with invasive surgery without prior Ig and antibiotic support and may have averted a fatal outcome.
KEY TEACHING POINTS
- Infiltrative hepatobiliary diseases may manifest with isolated elevations in ALP.
- Granulomas and autoimmune cytopenias may be features of primary immunodeficiency states.
- A history of recurrent childhood infections should raise suspicion for congenital immunodeficiencies.
- Unique medical complications, including immunodeficiency, can be associated with dwarfism subtypes.
Acknowledgements
The authors thank Jennifer M. Puck, MD, from the University of California San Francisco, Departments of Immunology and Pediatrics, for her invaluable contribution to the discussion on immunodeficiencies.
Disclosure
Nothing to report.
- , , , . Brush cytology of ductal strictures during ERCP. Acta Gastroenterol Belg. 2000;63:254–259.
- , . Granulomatous lung disease: an approach to the differential diagnosis. Arch Pathol Lab Med. 2010;134;667–690.
- James DG, Zumla A, eds. The Granulomatous Disorders. Cambridge, UK: Cambridge University Press; 1999:17–27.
- , , , et al. Unraveling the complexity of T cell abnormalities in common variable immunodeficiency. J Immunol. 2007;178:3932–3943.
- , , , et al. Does rituximab aggravate pre‐existing hypogammaglobulinaemia? J Clin Pathol. 2010;63:275–277.
- . Cartilage‐hair hypoplasia in Finland: epidemiological and genetic aspects of 107 patients. J Med Genet. 1992;29:652–655.
- , , , et al. High‐resolution genetic mapping of the cartilage‐hair hypoplasia (CHH) gene in Amish and Finnish families. Genomics. 1994;20:347–353.
- , , , et al. Combined immunodeficiency and vaccine‐related poliomyelitis in a child with cartilage‐hair hypoplasia. J Pediatr. 1975;86:868–872.
- , , . Deficiency of humoral immunity in cartilage‐hair hypoplasia. J Pediatr. 2000;137:487–492.
- , , , , . Bone marrow transplantation for cartilage‐hair hypoplasia. Bone Marrow Transplant. 2006;38:751–756.
- , , , et al. Clinical and immunologic outcome of patients with cartilage hair hypoplasia after hematopoietic stem cell transplantation [published corrections appear in Blood. 2010;116:2402 and Blood. 2011;117:2077]. Blood. 2010;116:27–35.
- , , , et al. Granulomatous inflammation in cartilage‐hair hypoplasia: risks and benefits of anti‐TNF‐α mAbs. J Allergy Clin Immunol. 2011;128:847–853.
- , , , . Brush cytology of ductal strictures during ERCP. Acta Gastroenterol Belg. 2000;63:254–259.
- , . Granulomatous lung disease: an approach to the differential diagnosis. Arch Pathol Lab Med. 2010;134;667–690.
- James DG, Zumla A, eds. The Granulomatous Disorders. Cambridge, UK: Cambridge University Press; 1999:17–27.
- , , , et al. Unraveling the complexity of T cell abnormalities in common variable immunodeficiency. J Immunol. 2007;178:3932–3943.
- , , , et al. Does rituximab aggravate pre‐existing hypogammaglobulinaemia? J Clin Pathol. 2010;63:275–277.
- . Cartilage‐hair hypoplasia in Finland: epidemiological and genetic aspects of 107 patients. J Med Genet. 1992;29:652–655.
- , , , et al. High‐resolution genetic mapping of the cartilage‐hair hypoplasia (CHH) gene in Amish and Finnish families. Genomics. 1994;20:347–353.
- , , , et al. Combined immunodeficiency and vaccine‐related poliomyelitis in a child with cartilage‐hair hypoplasia. J Pediatr. 1975;86:868–872.
- , , . Deficiency of humoral immunity in cartilage‐hair hypoplasia. J Pediatr. 2000;137:487–492.
- , , , , . Bone marrow transplantation for cartilage‐hair hypoplasia. Bone Marrow Transplant. 2006;38:751–756.
- , , , et al. Clinical and immunologic outcome of patients with cartilage hair hypoplasia after hematopoietic stem cell transplantation [published corrections appear in Blood. 2010;116:2402 and Blood. 2011;117:2077]. Blood. 2010;116:27–35.
- , , , et al. Granulomatous inflammation in cartilage‐hair hypoplasia: risks and benefits of anti‐TNF‐α mAbs. J Allergy Clin Immunol. 2011;128:847–853.
Teamwork Key to Effective Interdisciplinary Rounds
A new study in the Journal of Hospital Medicine is among the first to assess and characterize the effectiveness of teamwork in interdisciplinary rounds (IDR). The upshot: Varied performance on rounds suggests a need to improve the consistency of teamwork.
The report, "Assessment of Teamwork During Structured Interdisciplinary Rounds on Medical Units," adapted the Observational Teamwork Assessment for Surgery (OTAS) behavioral rating scale tool to evaluate and characterize teamwork of hospitalists. Mark Williams, MD, FACP, MHM, professor and chief of the division of hospital medicine at Northwestern University’s Feinberg School of Medicine in Chicago, says the review shows that mere implementation of IDR is not enough. Physician leaders must occasionally check how the rounds operate to ensure against such roadblocks as a team member who dominates discussions, or the formation of hierarchal relationships that not everyone is comfortable participating in, he says.
"You can't just say, 'Oh, we're practicing teamwork, we have structured interdisciplinary rounds,'" says Dr. Williams, who credits the research to lead author Kevin O'Leary, MD, MS, also of Feinberg. "You need to ensure that it’s occurring."
The paper fills a gap in research, the authors write, as much of the prior work on IDR has focused on patient outcomes, cost, and length of stay. But Dr. Williams says he doesn't expect community hospital medicine groups to conduct similar research because of their busy schedules. Still, he hopes group leaders and administrators consider the research an impetus to periodically check those rounds.
"Even in an institution [like Northwestern] that has strong buy-in to this [teamwork], you need to go back and check," Dr. Williams adds. "We saw variation in performance and we realized we needed to do some retraining."
Visit our website for more information about interdisciplinary rounds.
A new study in the Journal of Hospital Medicine is among the first to assess and characterize the effectiveness of teamwork in interdisciplinary rounds (IDR). The upshot: Varied performance on rounds suggests a need to improve the consistency of teamwork.
The report, "Assessment of Teamwork During Structured Interdisciplinary Rounds on Medical Units," adapted the Observational Teamwork Assessment for Surgery (OTAS) behavioral rating scale tool to evaluate and characterize teamwork of hospitalists. Mark Williams, MD, FACP, MHM, professor and chief of the division of hospital medicine at Northwestern University’s Feinberg School of Medicine in Chicago, says the review shows that mere implementation of IDR is not enough. Physician leaders must occasionally check how the rounds operate to ensure against such roadblocks as a team member who dominates discussions, or the formation of hierarchal relationships that not everyone is comfortable participating in, he says.
"You can't just say, 'Oh, we're practicing teamwork, we have structured interdisciplinary rounds,'" says Dr. Williams, who credits the research to lead author Kevin O'Leary, MD, MS, also of Feinberg. "You need to ensure that it’s occurring."
The paper fills a gap in research, the authors write, as much of the prior work on IDR has focused on patient outcomes, cost, and length of stay. But Dr. Williams says he doesn't expect community hospital medicine groups to conduct similar research because of their busy schedules. Still, he hopes group leaders and administrators consider the research an impetus to periodically check those rounds.
"Even in an institution [like Northwestern] that has strong buy-in to this [teamwork], you need to go back and check," Dr. Williams adds. "We saw variation in performance and we realized we needed to do some retraining."
Visit our website for more information about interdisciplinary rounds.
A new study in the Journal of Hospital Medicine is among the first to assess and characterize the effectiveness of teamwork in interdisciplinary rounds (IDR). The upshot: Varied performance on rounds suggests a need to improve the consistency of teamwork.
The report, "Assessment of Teamwork During Structured Interdisciplinary Rounds on Medical Units," adapted the Observational Teamwork Assessment for Surgery (OTAS) behavioral rating scale tool to evaluate and characterize teamwork of hospitalists. Mark Williams, MD, FACP, MHM, professor and chief of the division of hospital medicine at Northwestern University’s Feinberg School of Medicine in Chicago, says the review shows that mere implementation of IDR is not enough. Physician leaders must occasionally check how the rounds operate to ensure against such roadblocks as a team member who dominates discussions, or the formation of hierarchal relationships that not everyone is comfortable participating in, he says.
"You can't just say, 'Oh, we're practicing teamwork, we have structured interdisciplinary rounds,'" says Dr. Williams, who credits the research to lead author Kevin O'Leary, MD, MS, also of Feinberg. "You need to ensure that it’s occurring."
The paper fills a gap in research, the authors write, as much of the prior work on IDR has focused on patient outcomes, cost, and length of stay. But Dr. Williams says he doesn't expect community hospital medicine groups to conduct similar research because of their busy schedules. Still, he hopes group leaders and administrators consider the research an impetus to periodically check those rounds.
"Even in an institution [like Northwestern] that has strong buy-in to this [teamwork], you need to go back and check," Dr. Williams adds. "We saw variation in performance and we realized we needed to do some retraining."
Visit our website for more information about interdisciplinary rounds.
ITL: Physician Reviews of HM-Relevant Research
Clinical question: Is there a difference between aspirin and warfarin in preventing thromboembolic complications and risk of bleeding in patients with chronic kidney disease (CKD) and nonvalvular atrial fibrillation (NVAF)?
Background: Data are lacking on risks and benefits of aspirin and warfarin in CKD, as this group of patients largely has been excluded from anticoagulation therapy trials for NVAF. This study examined the risks and benefits of aspirin and warfarin in patients with CKD with NVAF.
Study design: Retrospective, observational cohort study.
Setting: Danish National Registries.
Synopsis: Of 132,372 patients with NVAF, 2.7% had CKD and 0.7% had end-stage renal disease (ESRD). Compared to patients with no CKD, there was increased risk of stroke or systemic thromboembolism in patients with ESRD (HR, 1.83; 95% CI, 1.57-2.14) and with non-end-stage CKD (HR 1.49; 95% CI 1.38-1.59).
In patients with CKD, warfarin significantly reduced stroke risk (HR, 0.76; 95% CI, 0.64-0.91) and significantly increased bleeding risk (HR, 1.33; 95% CI, 1.16-1.53); aspirin significantly increased bleeding risk (HR, 1.17; 95% CI, 1.02-1.34), with no reduction in stroke risk.
Bottom line: CKD was associated with an increased risk of stroke among NVAF patients. While both aspirin and warfarin were associated with increased risk of bleeding, there was a reduction in the risk of stroke with warfarin, but not with aspirin.
Citation: Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625-635.
Click here for more physician reviews of HM-relevant literature.
Clinical question: Is there a difference between aspirin and warfarin in preventing thromboembolic complications and risk of bleeding in patients with chronic kidney disease (CKD) and nonvalvular atrial fibrillation (NVAF)?
Background: Data are lacking on risks and benefits of aspirin and warfarin in CKD, as this group of patients largely has been excluded from anticoagulation therapy trials for NVAF. This study examined the risks and benefits of aspirin and warfarin in patients with CKD with NVAF.
Study design: Retrospective, observational cohort study.
Setting: Danish National Registries.
Synopsis: Of 132,372 patients with NVAF, 2.7% had CKD and 0.7% had end-stage renal disease (ESRD). Compared to patients with no CKD, there was increased risk of stroke or systemic thromboembolism in patients with ESRD (HR, 1.83; 95% CI, 1.57-2.14) and with non-end-stage CKD (HR 1.49; 95% CI 1.38-1.59).
In patients with CKD, warfarin significantly reduced stroke risk (HR, 0.76; 95% CI, 0.64-0.91) and significantly increased bleeding risk (HR, 1.33; 95% CI, 1.16-1.53); aspirin significantly increased bleeding risk (HR, 1.17; 95% CI, 1.02-1.34), with no reduction in stroke risk.
Bottom line: CKD was associated with an increased risk of stroke among NVAF patients. While both aspirin and warfarin were associated with increased risk of bleeding, there was a reduction in the risk of stroke with warfarin, but not with aspirin.
Citation: Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625-635.
Click here for more physician reviews of HM-relevant literature.
Clinical question: Is there a difference between aspirin and warfarin in preventing thromboembolic complications and risk of bleeding in patients with chronic kidney disease (CKD) and nonvalvular atrial fibrillation (NVAF)?
Background: Data are lacking on risks and benefits of aspirin and warfarin in CKD, as this group of patients largely has been excluded from anticoagulation therapy trials for NVAF. This study examined the risks and benefits of aspirin and warfarin in patients with CKD with NVAF.
Study design: Retrospective, observational cohort study.
Setting: Danish National Registries.
Synopsis: Of 132,372 patients with NVAF, 2.7% had CKD and 0.7% had end-stage renal disease (ESRD). Compared to patients with no CKD, there was increased risk of stroke or systemic thromboembolism in patients with ESRD (HR, 1.83; 95% CI, 1.57-2.14) and with non-end-stage CKD (HR 1.49; 95% CI 1.38-1.59).
In patients with CKD, warfarin significantly reduced stroke risk (HR, 0.76; 95% CI, 0.64-0.91) and significantly increased bleeding risk (HR, 1.33; 95% CI, 1.16-1.53); aspirin significantly increased bleeding risk (HR, 1.17; 95% CI, 1.02-1.34), with no reduction in stroke risk.
Bottom line: CKD was associated with an increased risk of stroke among NVAF patients. While both aspirin and warfarin were associated with increased risk of bleeding, there was a reduction in the risk of stroke with warfarin, but not with aspirin.
Citation: Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625-635.
Click here for more physician reviews of HM-relevant literature.
Quality Improvement Project Helps Hospital Patients Get Needed Prescriptions
A quality-improvement (QI) project to give high-risk patients ready access to prescribed medications at the time of hospital discharge achieved an 86% success rate, according to an abstract poster presented at HM12 in San Diego last April.1
Lead author Elizabeth Le, MD, then a resident at the University of California at San Francisco Medical Center (UCSF) and now a practicing hospitalist at the Veterans Administration Medical Center in Palo Alto, Calif., says the multidisciplinary “brown bag medications” project involved training house staff to recognize patients at risk. Staff meetings and rounds were used to identify appropriate candidates—those with limited mobility or cognitive issues, lacking insurance coverage or financial resources, a history of medication noncompliance, or leaving the hospital against medical advice—as well as those prescribed medications with a greater urgency for administration on schedule, such as anticoagulants or antibiotics.
About one-quarter of patients on the unit where this approach was first tested were found to need the service, which involved faxing prescriptions to an outpatient pharmacy across the street from the hospital for either pick-up by the family or delivery to the patient’s hospital room. For those with financial impediments, hospital social workers and case managers explored other options, including the social work department’s discretionary use fund, to pay for the drugs.
Dr. Le believes the project could be replicated in other facilities that lack access to in-house pharmacy services at discharge. She recommends involving social workers and case managers in the planning.
At UCSF, recent EHR implementation has automated the ordering of medications, but the challenge of recognizing who could benefit from extra help in obtaining their discharge medications remains a critical issue for hospitals trying to bring readmissions under control.
For more information about the brown bag medications program, contact Dr. Le at [email protected].
References
A quality-improvement (QI) project to give high-risk patients ready access to prescribed medications at the time of hospital discharge achieved an 86% success rate, according to an abstract poster presented at HM12 in San Diego last April.1
Lead author Elizabeth Le, MD, then a resident at the University of California at San Francisco Medical Center (UCSF) and now a practicing hospitalist at the Veterans Administration Medical Center in Palo Alto, Calif., says the multidisciplinary “brown bag medications” project involved training house staff to recognize patients at risk. Staff meetings and rounds were used to identify appropriate candidates—those with limited mobility or cognitive issues, lacking insurance coverage or financial resources, a history of medication noncompliance, or leaving the hospital against medical advice—as well as those prescribed medications with a greater urgency for administration on schedule, such as anticoagulants or antibiotics.
About one-quarter of patients on the unit where this approach was first tested were found to need the service, which involved faxing prescriptions to an outpatient pharmacy across the street from the hospital for either pick-up by the family or delivery to the patient’s hospital room. For those with financial impediments, hospital social workers and case managers explored other options, including the social work department’s discretionary use fund, to pay for the drugs.
Dr. Le believes the project could be replicated in other facilities that lack access to in-house pharmacy services at discharge. She recommends involving social workers and case managers in the planning.
At UCSF, recent EHR implementation has automated the ordering of medications, but the challenge of recognizing who could benefit from extra help in obtaining their discharge medications remains a critical issue for hospitals trying to bring readmissions under control.
For more information about the brown bag medications program, contact Dr. Le at [email protected].
References
A quality-improvement (QI) project to give high-risk patients ready access to prescribed medications at the time of hospital discharge achieved an 86% success rate, according to an abstract poster presented at HM12 in San Diego last April.1
Lead author Elizabeth Le, MD, then a resident at the University of California at San Francisco Medical Center (UCSF) and now a practicing hospitalist at the Veterans Administration Medical Center in Palo Alto, Calif., says the multidisciplinary “brown bag medications” project involved training house staff to recognize patients at risk. Staff meetings and rounds were used to identify appropriate candidates—those with limited mobility or cognitive issues, lacking insurance coverage or financial resources, a history of medication noncompliance, or leaving the hospital against medical advice—as well as those prescribed medications with a greater urgency for administration on schedule, such as anticoagulants or antibiotics.
About one-quarter of patients on the unit where this approach was first tested were found to need the service, which involved faxing prescriptions to an outpatient pharmacy across the street from the hospital for either pick-up by the family or delivery to the patient’s hospital room. For those with financial impediments, hospital social workers and case managers explored other options, including the social work department’s discretionary use fund, to pay for the drugs.
Dr. Le believes the project could be replicated in other facilities that lack access to in-house pharmacy services at discharge. She recommends involving social workers and case managers in the planning.
At UCSF, recent EHR implementation has automated the ordering of medications, but the challenge of recognizing who could benefit from extra help in obtaining their discharge medications remains a critical issue for hospitals trying to bring readmissions under control.
For more information about the brown bag medications program, contact Dr. Le at [email protected].
References
12 Things Hospitalists Need to Know About Billing and Coding
Documentation, CPT codes, modifiers—it’s not glamorous, but it’s an integral part of a 21st-century physician’s job description. The Hospitalist queried more than a handful of billing and coding experts about the advice they would dispense to clinicians navigating the reimbursement maze.
“Physicians often do more than what is reflected in the documentation,” says Barb Pierce, CCS-P, ACS-EM, a national coding consultant based in West Des Moines, Iowa, and CODE-H faculty. “They can’t always bill for everything they do, but they certainly can document and code to obtain the appropriate levels of service.”
Meanwhile, hospitalists have to be careful they aren’t excessive in their billing practices. “The name of the game isn’t just to bill higher,” Pierce adds, “but to make sure that your documentation supports the service being billed, and Medicare is watching. They’re doing a lot of focused audits.”
Some hospitalists might opt for a lower level of service, suspecting they’re less likely to be audited. Other hospitalists might seek reimbursement for more of their time and efforts.
“You have both ends of the spectrum,” says Raemarie Jimenez, CPC, CPMA, CPC-I, CANPC, CRHC, director of education for AAPC, formerly known as the American Academy of Professional Coders. “There are a lot of factors that would go into why a provider would code something incorrectly.”
Here’s how to land somewhere in the middle.
1 Be thorough in documenting the initial hospital visit.
When selecting the level of service for an initial hospital visit, the documentation consists of three key components: history, physical examination, and medical decision-making. The history includes the chief complaint as well as the review of systems. This is “an inventory of the patient’s organ systems.” Both the complaint and the systems review are often incorporated in the history of present illness, says Mary Mulholland, MHA, BSN, RN, CPC, senior coding and education specialist in the Department of Medicine at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
A patient’s family history is commonly overlooked in a hospitalist’s notes, primarily when they know the patient from previous admissions for chronic diseases and when the family history will likely not have an impact on treatment. “If they do not document a complete review of systems or miss one of the histories, the service will definitely be down-coded,” Mulholland says, “no matter how complete the exam and medical decision-making documentation.”
2 Familiarize yourself with Medicare reimbursement rules in the state where you practice.
In some states, Medicare contractors require providers to document the status of each organ system reviewed individually. In other states, it’s acceptable to document a system review with pertinent findings, “whether positive or negative,” and the statement of “all other systems negative,” Mulholland says.
The auditor will give credit for the review based on the number of organ systems documented. “If you miss one system review, it will take down what otherwise would be a Level Three hospital admission to a Level One,” she says. “So there would be a significant financial impact.”
Medicare reimbursement for a Level Three initial visit in Mulholland’s area of practice—Philadelphia County in Pennsylvania—is $206.57, compared with $104.69 for a Level One. During this visit, each of the key components—history, exam, and medical decision-making—need to be documented completely for the provider to receive the highest level of reimbursement.
3 Ask about a patient’s social history.
Social history can be obtained by querying the patient about smoking, drug and alcohol use, his or her occupation, marital status, and type of living arrangement.
“Knowing the social history helps the hospitalist understand the home situation or social circumstances that may have contributed to the hospitalization or may complicate the discharge plan,” Mulholland says.
This is particularly important in decision-making that involves elderly patients. The clinician should “think down the road” as to where the patient will be discharged and if a social worker’s assistance will be needed. It’s about “seeing the whole patient,” she says, “not just the disease.”
4 Remember to include the actual diagnosis.
“As coders, we can see all the clinical indicators of a particular diagnosis,” says Kathryn DeVault, RHIA, CCS, CCS-P, a director at HIM Solutions at the American Health Management Association. However, “unless [physicians] write down the diagnosis, we can’t code it.”
Documents without a diagnosis are more common than one would expect. For example, if a patient has pain when urinating, the hospitalist typically orders a culture. If the result is positive, the hospitalist prescribes an antibiotic for the infection, and too often “the story ends there.” From experience, DeVault can decipher that the patient is being treated for a urinary tract infection, but she can’t assign a code without querying the physician. Hospitalists, she suggests, should try to “close the loop in their documentation.”
5 Be specific in your written assessment of the patient’s condition.
“The main thing that we see is missing documentation,” says Angie Comfort, RHIT, CCS, a director at HIM Solutions. For instance, if a hospitalist documents congestive heart failure, it’s important to indicate whether the condition is chronic or acute and systolic or diastolic.
In the case of a diabetic patient, the notes should specify the type of diabetes. Not doing so “could be a reimbursement-changer,” Comfort says. In contrast, documenting such specifics could result in higher reimbursement, especially if a patient has complications from Type 1 diabetes.
6 Note the severity of the patient’s case.
Hospitalists’ documentation doesn’t always capture everything they’re evaluating for patients. “I’ve seen notes to the extent of ‘patient doing well; waiting on test results,’” the AAPC’s Jimenez says. “If they’re doing certain tests, why are they doing them? What are they trying to diagnose for the patient? What treatment are they considering?”
The reasons for the tests need to be explained. When a provider is monitoring someone in the hospital, the documentation should elaborate on the patient’s response to a treatment, and whether the patient’s condition is better, stable, or worse. This information helps put the severity in perspective.
“A diabetic could be a diabetic out of control. It could be a diabetic who’s not responding or who has comorbidities,” Jimenez says. “No one diagnosis is the same for every patient.”

—Mary Mulholland, MHA, BSN, RN, CPC, senior coding and education specialist, department of medicine, University of Pennsylvania, Philadelphia
7 Indicate which aspect of the patient’s condition you are treating.
When multiple providers are involved in a hospitalized patient’s care, it’s important to document your specific role apart from the services rendered by specialists, Jimenez says. The codes billed must be supported by the documentation for each service. Many providers contribute to the inpatient documentation, so it must be clear what each clinician personally performs.
Only report the diagnosis you are treating or the diagnoses that affect the ones you are managing. If a specialist has been brought in to take over treatment for a specific condition, a hospitalist would not bill for that diagnosis code.

—Raemarie Jimenez, CPC, CPMA, CPC-I, CANPC, CRHC, director of education, AAPC Salt Lake City
8 Note your personal review of medical records and reports from other clinicians.
Hospitalists should document their review of lab data or radiology reports, discussion of the case with other providers, or collection of the history from someone other than the patient. It’s also helpful to document your personal review of any images, such as a chest X-ray or MRI. Examining the images yourself might lead to higher reimbursement, Mulholland says.
Providers also should note when they request or review old records, and they should include a short synopsis of the information obtained and how it contributed to the current treatment plan.
9 Learn the correct coding for patients being transferred.
A transfer can occur either from a different facility or from a hospital floor to a rehabilitation unit. Either way, the patient is seen twice in one day, with each visit covered by the same hospitalist practice.
“Both physicians often report a separate independent visit. However, because these services occurred on the same day, it is not appropriate to bill for two separate subsequent or initial hospital codes,” says Sherri Dumford, MBA, CHBME, director of operations and past president of the Healthcare Billing and Management Association. “Often what will happen is both services will be reported and get through the billing system. The second claim is just written off as a denied service, when, in fact, you could combine the elements of service of both visits and possibly bill for a single higher level of visit.”
10 Consider delegating to a coding expert.
While smaller hospitalist groups can turn to a coding consultant on an as-needed basis, larger groups might consider bringing a certified coder on staff. This person would inform physicians about proper coding, review their documentation, and “give real-time feedback,” Pierce says.
An internal audit would show if the documentation meets selected evaluation management codes. Also, it usually takes a coding professional to determine whether prolonged services are an option for the team on any given date of service. Someone would need to internally “add together” multiple services on one date to see if there is sufficient time documented to allow billing for these add-on codes, Pierce says. Similarly, critical-care time needs to be accumulated during a date of service.

—Barb Pierce, CCS-P, ACS-EM, national coding consultant, West Des Moines, Iowa
11 Indicate the number of minutes spent arranging for a patient’s discharge.
Discharging a patient involves various steps, says Peter Thompson, MD, chief of clinical operations at the Phoenix headquarters of Apogee Physicians, a hospitalist management company that employs about 750 hospitalists across the country. Hospitalists discuss the hospital stay with the patient and family members, prescribe medications, issue discharge recommendations, set up follow-up care, and coordinate with the case manager, specialists, and primary-care physician.
“It generally is one sequential event after the other,” lasting between 20 and 40 minutes and leading up to discharge, Thompson says. Reimbursement for a high-level discharge constitutes more than 30 minutes. However, without proper documentation, he cautions, the claim could be downgraded or denied.
12 Don’t forget to sign, date, and time your progress note.
Last but not least, when it comes to reimbursement, your signature really does matter.
“For an illegible signature, Medicare and the insurance companies have the option of not paying for the service,” Mulholland says. “They’re trying to establish or authenticate who provided the service.”
And they want to know when the hospitalist saw the patient, so it’s a good idea to indicate the exact time of your visit.
Susan Kreimer is a freelance medical writer in New York.
Documentation, CPT codes, modifiers—it’s not glamorous, but it’s an integral part of a 21st-century physician’s job description. The Hospitalist queried more than a handful of billing and coding experts about the advice they would dispense to clinicians navigating the reimbursement maze.
“Physicians often do more than what is reflected in the documentation,” says Barb Pierce, CCS-P, ACS-EM, a national coding consultant based in West Des Moines, Iowa, and CODE-H faculty. “They can’t always bill for everything they do, but they certainly can document and code to obtain the appropriate levels of service.”
Meanwhile, hospitalists have to be careful they aren’t excessive in their billing practices. “The name of the game isn’t just to bill higher,” Pierce adds, “but to make sure that your documentation supports the service being billed, and Medicare is watching. They’re doing a lot of focused audits.”
Some hospitalists might opt for a lower level of service, suspecting they’re less likely to be audited. Other hospitalists might seek reimbursement for more of their time and efforts.
“You have both ends of the spectrum,” says Raemarie Jimenez, CPC, CPMA, CPC-I, CANPC, CRHC, director of education for AAPC, formerly known as the American Academy of Professional Coders. “There are a lot of factors that would go into why a provider would code something incorrectly.”
Here’s how to land somewhere in the middle.
1 Be thorough in documenting the initial hospital visit.
When selecting the level of service for an initial hospital visit, the documentation consists of three key components: history, physical examination, and medical decision-making. The history includes the chief complaint as well as the review of systems. This is “an inventory of the patient’s organ systems.” Both the complaint and the systems review are often incorporated in the history of present illness, says Mary Mulholland, MHA, BSN, RN, CPC, senior coding and education specialist in the Department of Medicine at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
A patient’s family history is commonly overlooked in a hospitalist’s notes, primarily when they know the patient from previous admissions for chronic diseases and when the family history will likely not have an impact on treatment. “If they do not document a complete review of systems or miss one of the histories, the service will definitely be down-coded,” Mulholland says, “no matter how complete the exam and medical decision-making documentation.”
2 Familiarize yourself with Medicare reimbursement rules in the state where you practice.
In some states, Medicare contractors require providers to document the status of each organ system reviewed individually. In other states, it’s acceptable to document a system review with pertinent findings, “whether positive or negative,” and the statement of “all other systems negative,” Mulholland says.
The auditor will give credit for the review based on the number of organ systems documented. “If you miss one system review, it will take down what otherwise would be a Level Three hospital admission to a Level One,” she says. “So there would be a significant financial impact.”
Medicare reimbursement for a Level Three initial visit in Mulholland’s area of practice—Philadelphia County in Pennsylvania—is $206.57, compared with $104.69 for a Level One. During this visit, each of the key components—history, exam, and medical decision-making—need to be documented completely for the provider to receive the highest level of reimbursement.
3 Ask about a patient’s social history.
Social history can be obtained by querying the patient about smoking, drug and alcohol use, his or her occupation, marital status, and type of living arrangement.
“Knowing the social history helps the hospitalist understand the home situation or social circumstances that may have contributed to the hospitalization or may complicate the discharge plan,” Mulholland says.
This is particularly important in decision-making that involves elderly patients. The clinician should “think down the road” as to where the patient will be discharged and if a social worker’s assistance will be needed. It’s about “seeing the whole patient,” she says, “not just the disease.”
4 Remember to include the actual diagnosis.
“As coders, we can see all the clinical indicators of a particular diagnosis,” says Kathryn DeVault, RHIA, CCS, CCS-P, a director at HIM Solutions at the American Health Management Association. However, “unless [physicians] write down the diagnosis, we can’t code it.”
Documents without a diagnosis are more common than one would expect. For example, if a patient has pain when urinating, the hospitalist typically orders a culture. If the result is positive, the hospitalist prescribes an antibiotic for the infection, and too often “the story ends there.” From experience, DeVault can decipher that the patient is being treated for a urinary tract infection, but she can’t assign a code without querying the physician. Hospitalists, she suggests, should try to “close the loop in their documentation.”
5 Be specific in your written assessment of the patient’s condition.
“The main thing that we see is missing documentation,” says Angie Comfort, RHIT, CCS, a director at HIM Solutions. For instance, if a hospitalist documents congestive heart failure, it’s important to indicate whether the condition is chronic or acute and systolic or diastolic.
In the case of a diabetic patient, the notes should specify the type of diabetes. Not doing so “could be a reimbursement-changer,” Comfort says. In contrast, documenting such specifics could result in higher reimbursement, especially if a patient has complications from Type 1 diabetes.
6 Note the severity of the patient’s case.
Hospitalists’ documentation doesn’t always capture everything they’re evaluating for patients. “I’ve seen notes to the extent of ‘patient doing well; waiting on test results,’” the AAPC’s Jimenez says. “If they’re doing certain tests, why are they doing them? What are they trying to diagnose for the patient? What treatment are they considering?”
The reasons for the tests need to be explained. When a provider is monitoring someone in the hospital, the documentation should elaborate on the patient’s response to a treatment, and whether the patient’s condition is better, stable, or worse. This information helps put the severity in perspective.
“A diabetic could be a diabetic out of control. It could be a diabetic who’s not responding or who has comorbidities,” Jimenez says. “No one diagnosis is the same for every patient.”

—Mary Mulholland, MHA, BSN, RN, CPC, senior coding and education specialist, department of medicine, University of Pennsylvania, Philadelphia
7 Indicate which aspect of the patient’s condition you are treating.
When multiple providers are involved in a hospitalized patient’s care, it’s important to document your specific role apart from the services rendered by specialists, Jimenez says. The codes billed must be supported by the documentation for each service. Many providers contribute to the inpatient documentation, so it must be clear what each clinician personally performs.
Only report the diagnosis you are treating or the diagnoses that affect the ones you are managing. If a specialist has been brought in to take over treatment for a specific condition, a hospitalist would not bill for that diagnosis code.

—Raemarie Jimenez, CPC, CPMA, CPC-I, CANPC, CRHC, director of education, AAPC Salt Lake City
8 Note your personal review of medical records and reports from other clinicians.
Hospitalists should document their review of lab data or radiology reports, discussion of the case with other providers, or collection of the history from someone other than the patient. It’s also helpful to document your personal review of any images, such as a chest X-ray or MRI. Examining the images yourself might lead to higher reimbursement, Mulholland says.
Providers also should note when they request or review old records, and they should include a short synopsis of the information obtained and how it contributed to the current treatment plan.
9 Learn the correct coding for patients being transferred.
A transfer can occur either from a different facility or from a hospital floor to a rehabilitation unit. Either way, the patient is seen twice in one day, with each visit covered by the same hospitalist practice.
“Both physicians often report a separate independent visit. However, because these services occurred on the same day, it is not appropriate to bill for two separate subsequent or initial hospital codes,” says Sherri Dumford, MBA, CHBME, director of operations and past president of the Healthcare Billing and Management Association. “Often what will happen is both services will be reported and get through the billing system. The second claim is just written off as a denied service, when, in fact, you could combine the elements of service of both visits and possibly bill for a single higher level of visit.”
10 Consider delegating to a coding expert.
While smaller hospitalist groups can turn to a coding consultant on an as-needed basis, larger groups might consider bringing a certified coder on staff. This person would inform physicians about proper coding, review their documentation, and “give real-time feedback,” Pierce says.
An internal audit would show if the documentation meets selected evaluation management codes. Also, it usually takes a coding professional to determine whether prolonged services are an option for the team on any given date of service. Someone would need to internally “add together” multiple services on one date to see if there is sufficient time documented to allow billing for these add-on codes, Pierce says. Similarly, critical-care time needs to be accumulated during a date of service.

—Barb Pierce, CCS-P, ACS-EM, national coding consultant, West Des Moines, Iowa
11 Indicate the number of minutes spent arranging for a patient’s discharge.
Discharging a patient involves various steps, says Peter Thompson, MD, chief of clinical operations at the Phoenix headquarters of Apogee Physicians, a hospitalist management company that employs about 750 hospitalists across the country. Hospitalists discuss the hospital stay with the patient and family members, prescribe medications, issue discharge recommendations, set up follow-up care, and coordinate with the case manager, specialists, and primary-care physician.
“It generally is one sequential event after the other,” lasting between 20 and 40 minutes and leading up to discharge, Thompson says. Reimbursement for a high-level discharge constitutes more than 30 minutes. However, without proper documentation, he cautions, the claim could be downgraded or denied.
12 Don’t forget to sign, date, and time your progress note.
Last but not least, when it comes to reimbursement, your signature really does matter.
“For an illegible signature, Medicare and the insurance companies have the option of not paying for the service,” Mulholland says. “They’re trying to establish or authenticate who provided the service.”
And they want to know when the hospitalist saw the patient, so it’s a good idea to indicate the exact time of your visit.
Susan Kreimer is a freelance medical writer in New York.
Documentation, CPT codes, modifiers—it’s not glamorous, but it’s an integral part of a 21st-century physician’s job description. The Hospitalist queried more than a handful of billing and coding experts about the advice they would dispense to clinicians navigating the reimbursement maze.
“Physicians often do more than what is reflected in the documentation,” says Barb Pierce, CCS-P, ACS-EM, a national coding consultant based in West Des Moines, Iowa, and CODE-H faculty. “They can’t always bill for everything they do, but they certainly can document and code to obtain the appropriate levels of service.”
Meanwhile, hospitalists have to be careful they aren’t excessive in their billing practices. “The name of the game isn’t just to bill higher,” Pierce adds, “but to make sure that your documentation supports the service being billed, and Medicare is watching. They’re doing a lot of focused audits.”
Some hospitalists might opt for a lower level of service, suspecting they’re less likely to be audited. Other hospitalists might seek reimbursement for more of their time and efforts.
“You have both ends of the spectrum,” says Raemarie Jimenez, CPC, CPMA, CPC-I, CANPC, CRHC, director of education for AAPC, formerly known as the American Academy of Professional Coders. “There are a lot of factors that would go into why a provider would code something incorrectly.”
Here’s how to land somewhere in the middle.
1 Be thorough in documenting the initial hospital visit.
When selecting the level of service for an initial hospital visit, the documentation consists of three key components: history, physical examination, and medical decision-making. The history includes the chief complaint as well as the review of systems. This is “an inventory of the patient’s organ systems.” Both the complaint and the systems review are often incorporated in the history of present illness, says Mary Mulholland, MHA, BSN, RN, CPC, senior coding and education specialist in the Department of Medicine at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
A patient’s family history is commonly overlooked in a hospitalist’s notes, primarily when they know the patient from previous admissions for chronic diseases and when the family history will likely not have an impact on treatment. “If they do not document a complete review of systems or miss one of the histories, the service will definitely be down-coded,” Mulholland says, “no matter how complete the exam and medical decision-making documentation.”
2 Familiarize yourself with Medicare reimbursement rules in the state where you practice.
In some states, Medicare contractors require providers to document the status of each organ system reviewed individually. In other states, it’s acceptable to document a system review with pertinent findings, “whether positive or negative,” and the statement of “all other systems negative,” Mulholland says.
The auditor will give credit for the review based on the number of organ systems documented. “If you miss one system review, it will take down what otherwise would be a Level Three hospital admission to a Level One,” she says. “So there would be a significant financial impact.”
Medicare reimbursement for a Level Three initial visit in Mulholland’s area of practice—Philadelphia County in Pennsylvania—is $206.57, compared with $104.69 for a Level One. During this visit, each of the key components—history, exam, and medical decision-making—need to be documented completely for the provider to receive the highest level of reimbursement.
3 Ask about a patient’s social history.
Social history can be obtained by querying the patient about smoking, drug and alcohol use, his or her occupation, marital status, and type of living arrangement.
“Knowing the social history helps the hospitalist understand the home situation or social circumstances that may have contributed to the hospitalization or may complicate the discharge plan,” Mulholland says.
This is particularly important in decision-making that involves elderly patients. The clinician should “think down the road” as to where the patient will be discharged and if a social worker’s assistance will be needed. It’s about “seeing the whole patient,” she says, “not just the disease.”
4 Remember to include the actual diagnosis.
“As coders, we can see all the clinical indicators of a particular diagnosis,” says Kathryn DeVault, RHIA, CCS, CCS-P, a director at HIM Solutions at the American Health Management Association. However, “unless [physicians] write down the diagnosis, we can’t code it.”
Documents without a diagnosis are more common than one would expect. For example, if a patient has pain when urinating, the hospitalist typically orders a culture. If the result is positive, the hospitalist prescribes an antibiotic for the infection, and too often “the story ends there.” From experience, DeVault can decipher that the patient is being treated for a urinary tract infection, but she can’t assign a code without querying the physician. Hospitalists, she suggests, should try to “close the loop in their documentation.”
5 Be specific in your written assessment of the patient’s condition.
“The main thing that we see is missing documentation,” says Angie Comfort, RHIT, CCS, a director at HIM Solutions. For instance, if a hospitalist documents congestive heart failure, it’s important to indicate whether the condition is chronic or acute and systolic or diastolic.
In the case of a diabetic patient, the notes should specify the type of diabetes. Not doing so “could be a reimbursement-changer,” Comfort says. In contrast, documenting such specifics could result in higher reimbursement, especially if a patient has complications from Type 1 diabetes.
6 Note the severity of the patient’s case.
Hospitalists’ documentation doesn’t always capture everything they’re evaluating for patients. “I’ve seen notes to the extent of ‘patient doing well; waiting on test results,’” the AAPC’s Jimenez says. “If they’re doing certain tests, why are they doing them? What are they trying to diagnose for the patient? What treatment are they considering?”
The reasons for the tests need to be explained. When a provider is monitoring someone in the hospital, the documentation should elaborate on the patient’s response to a treatment, and whether the patient’s condition is better, stable, or worse. This information helps put the severity in perspective.
“A diabetic could be a diabetic out of control. It could be a diabetic who’s not responding or who has comorbidities,” Jimenez says. “No one diagnosis is the same for every patient.”

—Mary Mulholland, MHA, BSN, RN, CPC, senior coding and education specialist, department of medicine, University of Pennsylvania, Philadelphia
7 Indicate which aspect of the patient’s condition you are treating.
When multiple providers are involved in a hospitalized patient’s care, it’s important to document your specific role apart from the services rendered by specialists, Jimenez says. The codes billed must be supported by the documentation for each service. Many providers contribute to the inpatient documentation, so it must be clear what each clinician personally performs.
Only report the diagnosis you are treating or the diagnoses that affect the ones you are managing. If a specialist has been brought in to take over treatment for a specific condition, a hospitalist would not bill for that diagnosis code.

—Raemarie Jimenez, CPC, CPMA, CPC-I, CANPC, CRHC, director of education, AAPC Salt Lake City
8 Note your personal review of medical records and reports from other clinicians.
Hospitalists should document their review of lab data or radiology reports, discussion of the case with other providers, or collection of the history from someone other than the patient. It’s also helpful to document your personal review of any images, such as a chest X-ray or MRI. Examining the images yourself might lead to higher reimbursement, Mulholland says.
Providers also should note when they request or review old records, and they should include a short synopsis of the information obtained and how it contributed to the current treatment plan.
9 Learn the correct coding for patients being transferred.
A transfer can occur either from a different facility or from a hospital floor to a rehabilitation unit. Either way, the patient is seen twice in one day, with each visit covered by the same hospitalist practice.
“Both physicians often report a separate independent visit. However, because these services occurred on the same day, it is not appropriate to bill for two separate subsequent or initial hospital codes,” says Sherri Dumford, MBA, CHBME, director of operations and past president of the Healthcare Billing and Management Association. “Often what will happen is both services will be reported and get through the billing system. The second claim is just written off as a denied service, when, in fact, you could combine the elements of service of both visits and possibly bill for a single higher level of visit.”
10 Consider delegating to a coding expert.
While smaller hospitalist groups can turn to a coding consultant on an as-needed basis, larger groups might consider bringing a certified coder on staff. This person would inform physicians about proper coding, review their documentation, and “give real-time feedback,” Pierce says.
An internal audit would show if the documentation meets selected evaluation management codes. Also, it usually takes a coding professional to determine whether prolonged services are an option for the team on any given date of service. Someone would need to internally “add together” multiple services on one date to see if there is sufficient time documented to allow billing for these add-on codes, Pierce says. Similarly, critical-care time needs to be accumulated during a date of service.

—Barb Pierce, CCS-P, ACS-EM, national coding consultant, West Des Moines, Iowa
11 Indicate the number of minutes spent arranging for a patient’s discharge.
Discharging a patient involves various steps, says Peter Thompson, MD, chief of clinical operations at the Phoenix headquarters of Apogee Physicians, a hospitalist management company that employs about 750 hospitalists across the country. Hospitalists discuss the hospital stay with the patient and family members, prescribe medications, issue discharge recommendations, set up follow-up care, and coordinate with the case manager, specialists, and primary-care physician.
“It generally is one sequential event after the other,” lasting between 20 and 40 minutes and leading up to discharge, Thompson says. Reimbursement for a high-level discharge constitutes more than 30 minutes. However, without proper documentation, he cautions, the claim could be downgraded or denied.
12 Don’t forget to sign, date, and time your progress note.
Last but not least, when it comes to reimbursement, your signature really does matter.
“For an illegible signature, Medicare and the insurance companies have the option of not paying for the service,” Mulholland says. “They’re trying to establish or authenticate who provided the service.”
And they want to know when the hospitalist saw the patient, so it’s a good idea to indicate the exact time of your visit.
Susan Kreimer is a freelance medical writer in New York.
Bloodsteam Infections in ICU Patients Plummet
Reduction in bloodstream infection rates resulting from a simple intervention: bathing all ICU patients daily with antimicrobial chlorhexidine soap rather than the widely mandated practice of screening ICU patients to determine which ones harbor methicillin-resistant Staphylococcus aureus (MRSA) and then implementing an MRSA treatment protocol for them.
According to data on 75,000 patients at hospitals in 16 states presented in October at the Infectious Diseases Society of America annual meeting, there also was a 37% reduction in patients with MRSA.
Lead researcher Susan Huang, MD, an infectious-disease specialist at the University of California at Irvine, says the results show the benefits of this preventive approach, which included applying an antibiotic ointment to the patient’s nasal passage, and could make ICU screening for drug-resistant organisms, such as MRSA, unnecessary.
Reduction in bloodstream infection rates resulting from a simple intervention: bathing all ICU patients daily with antimicrobial chlorhexidine soap rather than the widely mandated practice of screening ICU patients to determine which ones harbor methicillin-resistant Staphylococcus aureus (MRSA) and then implementing an MRSA treatment protocol for them.
According to data on 75,000 patients at hospitals in 16 states presented in October at the Infectious Diseases Society of America annual meeting, there also was a 37% reduction in patients with MRSA.
Lead researcher Susan Huang, MD, an infectious-disease specialist at the University of California at Irvine, says the results show the benefits of this preventive approach, which included applying an antibiotic ointment to the patient’s nasal passage, and could make ICU screening for drug-resistant organisms, such as MRSA, unnecessary.
Reduction in bloodstream infection rates resulting from a simple intervention: bathing all ICU patients daily with antimicrobial chlorhexidine soap rather than the widely mandated practice of screening ICU patients to determine which ones harbor methicillin-resistant Staphylococcus aureus (MRSA) and then implementing an MRSA treatment protocol for them.
According to data on 75,000 patients at hospitals in 16 states presented in October at the Infectious Diseases Society of America annual meeting, there also was a 37% reduction in patients with MRSA.
Lead researcher Susan Huang, MD, an infectious-disease specialist at the University of California at Irvine, says the results show the benefits of this preventive approach, which included applying an antibiotic ointment to the patient’s nasal passage, and could make ICU screening for drug-resistant organisms, such as MRSA, unnecessary.
Accountability Hits Home for Hospitalists
Russell Cowles III, MD, lead hospitalist at Bergan Mercy Medical Center in Omaha, Neb., recalls the shock on the faces of hospitalists who attended his presentation to SHM’s Nebraska Area chapter meeting last spring. Dr. Cowles and co-presenter Eric Rice, MD, MMM, SFHM, chapter president and assistant medical director of Alegent Creighton Hospital Medicine Services, were introducing their fellow hospitalists to a forthcoming Medicare initiative called the Physician Feedback/Value-Based Payment Modifier (VBPM) program.
“And everyone in the audience was completely stunned,” Dr. Cowles says. “They had never even dreamed that any of this would come down to the physician level.”
They’re not alone.
“Unless you work in administration or you’re leading a group, I don’t think very many people know this exists,” Dr. Cowles says. “Your average practicing physician, I think, has no clue that this measurement is going on behind the scenes.”
Authorized by the Affordable Care Act, the budget-neutral scheme ties future Medicare reimbursements to measures of quality and efficiency, and grades physicians on a curve. The Physician Quality Reporting System (PQRS), in place since 2007, forms the foundation of the new program, with feedback arriving in the form of a Quality and Resource Use Report (QRUR), a confidential report card sent to providers. The VBPM program then uses those reports as the basis for a financial reward or penalty.
In principle, SHM and hospitalist leaders have supported the concept of quality measurements as a way to hold doctors more accountable and to help the Centers for Medicare & Medicaid Services (CMS) take a more proactive role in improving quality of care while containing costs. And, in theory, HM leaders say hospitalists might be better able to adapt to the added responsibility of performance measurement and reporting due to their central role in the like-minded hospital value-based purchasing (VBP) program that began Oct. 1.
“If the expectation is that we will be involved in some of these initiatives and help the hospitals gain revenue, now we can actually see some dollars for those efforts,” says Julia Wright, MD, SFHM, FACP, president of the MidAtlantic Business Unit for Brentwood, Tenn.-based Cogent HMG. But the inverse is also true: If hospitals are going to have dollars at risk for performance, she says, CMS believes physicians should share in that risk as the providers of healthcare.
On that score, Dr. Rice says, hospitalists might have an advantage due to their focus on teamwork and their role in transitioning patients between inpatient and outpatient settings. In fact, he sees the VBPM as an “enormous opportunity” for hospitalists to demonstrate their leadership in helping to shape how organizations and institutions adapt to a quickly evolving healthcare environment.
But first, hospitalists will need to fully engage. In 2010, CMS found that only about 1 in 4 eligible physicians were participating in the voluntary PQRS and earning a reporting bonus of what is now 0.5% of allowable Medicare charges (roughly $800 for the average hospitalist). The stakes will grow when the PQRS transforms into a negative incentive program in 2015, with a 1.5% penalty for doctors who do not meet its reporting requirements. In 2016 and thereafter, the assessed penalty grows to 2% (about $3,200 for the average hospitalist).
“I think the unfolding timeline has really provided the potential for lulling us into complacency and procrastination,” says Patrick Torcson, MD, MMM, FACP, SFHM, director of hospital medicine at St. Tammany Parish Hospital in Covington, La., and chair of SHM’s Performance Measurement and Reporting Committee.
According to CMS, “physician groups can avoid all negative adjustments simply by participating in the PQRS.” Nonparticipants, however, could get hit with a double whammy. With no quality data, CMS would have no way to assess groups’ performances and would automatically deduct an extra 1% of Medicare reimbursements under the VBPM program. For groups of 100 eligible providers or more, that combined PQRS-VBPM penalty could amount to 2.5% in 2015.
PQRS participants have more leeway and a smaller downside. Starting January 2015, eligible provider groups who meet the reporting requirements can choose either to have no adjustments at all or to compete in the VBPM program for a performance-based bonus or a penalty of 1%, based on cost and quality scores. In January 2017, the program is expected to expand to include all providers, whether in individual or group practice.
A Measure of Relevance
Based on the first QRURs, sent out in March 2012 to providers in four pilot states, SHM wrote a letter to CMS that offered a detailed analysis of several additional concerns. The society followed up with a second letter that provided a more expansive critique of the proposed 2013 Physician Fee Schedule.
One worry is whether the physician feedback/VBPM program has included enough performance measures that are relevant to hospitalists. A Public Policy column in The Hospitalist (“Metric Accountability,” November 2012, p. 18) counted only 10 PQRS measures that apply routinely to HM providers out of a list of more than 200. Even those 10 aren’t always applicable.
“I work at a teaching hospital that’s large enough to have a neurology program, so most acute-stroke patients are admitted by the neurologists,” says Gregory Seymann, MD, SFHM, chief of the division of hospital medicine at the University of California at San Diego and a member of SHM’s Performance Measurement and Reporting Committee. “Five of the 10 measures are related to stroke patients, but my group rarely admits stroke patients.” That means only five PQRS measures remain relevant to him.
On paper, the issue might be readily resolved by expanding the number of measures to better reflect HM responsibilities—such as four measures proposed by SHM that relate to transitions of care and medication reconciliation.

—Patrick Torcson, MD, MMM, FACP, SFHM, director, hospital medicine, St. Tammany Parish Hospital, Covington, La., chair, SHM’s Performance Measurement and Reporting Committee
Other groups, though, have their own ideas. A letter to CMS signed by 28 patient and healthcare payor groups calls for the elimination of almost two dozen PQRS measures deemed unnecessary, duplicative, or uninformative, and for the addition of nine others that might better assess patient outcomes and quality of care. Jennifer Eames Huff, director of the Consumer-Purchaser Disclosure Project at San Francisco-based Pacific Business Group on Health, one of the letter’s signatories, says some of those potential measures might be more applicable to hospitalists as well.
But therein lies the rub. Although process measures might not always be strong indicators of quality of care, the introduction of outcome measures often makes providers nervous, says Gary Young, JD, PhD, director of the Center for Health Policy and Healthcare Research at Northeastern University in Boston. “Most providers feel that their patients are sicker and more vulnerable to poorer outcomes, and they don’t want to be judged poorly because they have sicker patients,” he says. Reaching an agreement on the best collection of measures may require some intense negotiations, he says.

–Win Whitcomb, MD, MHM, medical director of healthcare quality, Baystate Medical Center, Springfield, Mass.
Fairer Comparisons
Dr. Cowles cites two de-identified QRURs received by Alegent Creighton Health back in March—one for a hospitalist and one for an office-based general internist—to illustrate another major concern shared by many HM providers. The reports broke down each doctor’s relative healthcare contributions, using predetermined percentages of the total care and costs to conclude whether that doctor directed, influenced, or contributed to a patient’s care.
Hospitalists, by the nature of their jobs, seldom direct the care of any patient. But because their influence or contribution is almost always within the inpatient environment, HM providers account for proportionately higher costs than office-based physicians. The result can be a rather ugly curve: For healthcare costs incurred, the general internist was at the 65th percentile, while the hospitalist was at the 96th percentile.
The point, Dr. Cowles says, is that hospitalists and clinic-based physicians see patients with remarkably different acuities. “We just need to make sure that we’re comparing apples to apples, that you’re going to compare someone who sees a high-acuity patient with someone else who sees a high-acuity patient,” he says.
One silver lining could be increased momentum toward establishing HM as its own Medicare-recognized specialty. Hospitalist leaders who say the process is likely to be difficult but not impossible cite the successful effort to win recognition of HM as a focused practice by the American Board of Internal Medicine.
“We’re going to have to think outside the box in terms of working toward an identifier for hospitalists,” says Win Whitcomb, MD, MHM, medical director of healthcare quality at Baystate Medical Center in Springfield, Mass., and a member of SHM’s Performance and Measurement Reporting Committee. “But that’s going to happen—it’s not a matter of if, it’s a matter of when and how.”
As one potential interim solution, SHM has suggested a self-identification designation by which hospitalists would distinguish themselves from the larger, general internal-medicine category and thereby avoid unfair comparisons.
A Question of Attribution
Of the concerns raised by SHM, the question of attribution might be among the thorniest. Dr. Young says the “big-time issue” is pitting many consumer groups, payors, and employers against healthcare providers. The consumer groups want accountability at the individual provider level, while the providers strongly prefer group accountability, setting up a major clash over how responsibility will be parceled out.
Hospitalists have been taught to embrace responsibility while viewing healthcare delivery as a team sport. And the contributions of individual HM providers aren’t easily untangled. “If somebody has a bad outcome and they’ve been under the care of three different hospitalists, it’s virtually impossible to attribute that outcome to one of those three hospitalists,” Dr. Whitcomb says. “We really need to think about attribution differently, and it’s going to need to be across groups of hospitalists.”
SHM has suggested that CMS include an option for group rather than individual evaluation. “You’re just making it explicit that you can’t assign some of these measures to individual physicians. We can assign some of these measures to groups,” Dr. Whitcomb says.

—Julia Wright, MD, SFHM, FACP, president, MidAtlantic Business Unit, Cogent HMG, Brentwood, Tenn.
In its 2013 Medicare Physician Fee Schedule final rule, CMS opted to alter the doctor comparison methodology used for upcoming QRURs and the 2015 application of the VBPM. The agency also agreed to consider hospitalists’ concerns about fair attribution, relevant measures, and proper designation as it develops future proposals. Regardless of how those issues are ironed out, Dr. Torcson says, it’s clear to him that sitting on the sidelines is no longer an option for any physician group. Nor is it acceptable “to say this won’t work for me. We’re having to come up with proactive proposals for what will work to be part of the CMS quality agenda.”
SHM’s thorough analysis and realistic feedback, he says, has been well received by Medicare officials, raising hopes that many of the remaining differences can be resolved. “I am very confident that self-reporting or self-nomination as a hospitalist is going to be in place by the time those negative incentives kick in,” Dr. Torcson says. “And I’m also very confident that we’re going to have other, very creative options for quality measurement and performance reporting.”
One idea under consideration by CMS would allow hospitalists or other doctors to designate their hospitals’ quality data as a surrogate measure of their own performance. “I think that’s going to be a really great option for hospitalists who self-nominate,” Dr. Torcson says.
For many hospitalists, the option would effectively get around the issue of individual versus group attribution and instead align doctors’ fates with that of their institutions. SHM, Dr. Torcson says, has endorsed the proposal and offered to work with CMS to help institute it. He’s also confident that the reporting requirements for multiple, overlapping CMS programs will be more streamlined over time.
Some health professionals believe that hospitals and doctors already are devoting too much time and energy to measuring and recording the proliferating set of mandatory metrics. But Dr. Whitcomb says payors and patients are unlikely to have much sympathy.
“We as a profession are accountable to society at large. And that argument, that there are too many measurements and that we shouldn’t be held accountable as physicians for our performance, is a nonstarter when you’re trying to explain that to consumers,” he says. “The status quo is not tenable, and so it’s going to be a long journey and we need to be able to move in that direction.”
Bryn Nelson is a freelance medical writer in Seattle.
Russell Cowles III, MD, lead hospitalist at Bergan Mercy Medical Center in Omaha, Neb., recalls the shock on the faces of hospitalists who attended his presentation to SHM’s Nebraska Area chapter meeting last spring. Dr. Cowles and co-presenter Eric Rice, MD, MMM, SFHM, chapter president and assistant medical director of Alegent Creighton Hospital Medicine Services, were introducing their fellow hospitalists to a forthcoming Medicare initiative called the Physician Feedback/Value-Based Payment Modifier (VBPM) program.
“And everyone in the audience was completely stunned,” Dr. Cowles says. “They had never even dreamed that any of this would come down to the physician level.”
They’re not alone.
“Unless you work in administration or you’re leading a group, I don’t think very many people know this exists,” Dr. Cowles says. “Your average practicing physician, I think, has no clue that this measurement is going on behind the scenes.”
Authorized by the Affordable Care Act, the budget-neutral scheme ties future Medicare reimbursements to measures of quality and efficiency, and grades physicians on a curve. The Physician Quality Reporting System (PQRS), in place since 2007, forms the foundation of the new program, with feedback arriving in the form of a Quality and Resource Use Report (QRUR), a confidential report card sent to providers. The VBPM program then uses those reports as the basis for a financial reward or penalty.
In principle, SHM and hospitalist leaders have supported the concept of quality measurements as a way to hold doctors more accountable and to help the Centers for Medicare & Medicaid Services (CMS) take a more proactive role in improving quality of care while containing costs. And, in theory, HM leaders say hospitalists might be better able to adapt to the added responsibility of performance measurement and reporting due to their central role in the like-minded hospital value-based purchasing (VBP) program that began Oct. 1.
“If the expectation is that we will be involved in some of these initiatives and help the hospitals gain revenue, now we can actually see some dollars for those efforts,” says Julia Wright, MD, SFHM, FACP, president of the MidAtlantic Business Unit for Brentwood, Tenn.-based Cogent HMG. But the inverse is also true: If hospitals are going to have dollars at risk for performance, she says, CMS believes physicians should share in that risk as the providers of healthcare.
On that score, Dr. Rice says, hospitalists might have an advantage due to their focus on teamwork and their role in transitioning patients between inpatient and outpatient settings. In fact, he sees the VBPM as an “enormous opportunity” for hospitalists to demonstrate their leadership in helping to shape how organizations and institutions adapt to a quickly evolving healthcare environment.
But first, hospitalists will need to fully engage. In 2010, CMS found that only about 1 in 4 eligible physicians were participating in the voluntary PQRS and earning a reporting bonus of what is now 0.5% of allowable Medicare charges (roughly $800 for the average hospitalist). The stakes will grow when the PQRS transforms into a negative incentive program in 2015, with a 1.5% penalty for doctors who do not meet its reporting requirements. In 2016 and thereafter, the assessed penalty grows to 2% (about $3,200 for the average hospitalist).
“I think the unfolding timeline has really provided the potential for lulling us into complacency and procrastination,” says Patrick Torcson, MD, MMM, FACP, SFHM, director of hospital medicine at St. Tammany Parish Hospital in Covington, La., and chair of SHM’s Performance Measurement and Reporting Committee.
According to CMS, “physician groups can avoid all negative adjustments simply by participating in the PQRS.” Nonparticipants, however, could get hit with a double whammy. With no quality data, CMS would have no way to assess groups’ performances and would automatically deduct an extra 1% of Medicare reimbursements under the VBPM program. For groups of 100 eligible providers or more, that combined PQRS-VBPM penalty could amount to 2.5% in 2015.
PQRS participants have more leeway and a smaller downside. Starting January 2015, eligible provider groups who meet the reporting requirements can choose either to have no adjustments at all or to compete in the VBPM program for a performance-based bonus or a penalty of 1%, based on cost and quality scores. In January 2017, the program is expected to expand to include all providers, whether in individual or group practice.
A Measure of Relevance
Based on the first QRURs, sent out in March 2012 to providers in four pilot states, SHM wrote a letter to CMS that offered a detailed analysis of several additional concerns. The society followed up with a second letter that provided a more expansive critique of the proposed 2013 Physician Fee Schedule.
One worry is whether the physician feedback/VBPM program has included enough performance measures that are relevant to hospitalists. A Public Policy column in The Hospitalist (“Metric Accountability,” November 2012, p. 18) counted only 10 PQRS measures that apply routinely to HM providers out of a list of more than 200. Even those 10 aren’t always applicable.
“I work at a teaching hospital that’s large enough to have a neurology program, so most acute-stroke patients are admitted by the neurologists,” says Gregory Seymann, MD, SFHM, chief of the division of hospital medicine at the University of California at San Diego and a member of SHM’s Performance Measurement and Reporting Committee. “Five of the 10 measures are related to stroke patients, but my group rarely admits stroke patients.” That means only five PQRS measures remain relevant to him.
On paper, the issue might be readily resolved by expanding the number of measures to better reflect HM responsibilities—such as four measures proposed by SHM that relate to transitions of care and medication reconciliation.

—Patrick Torcson, MD, MMM, FACP, SFHM, director, hospital medicine, St. Tammany Parish Hospital, Covington, La., chair, SHM’s Performance Measurement and Reporting Committee
Other groups, though, have their own ideas. A letter to CMS signed by 28 patient and healthcare payor groups calls for the elimination of almost two dozen PQRS measures deemed unnecessary, duplicative, or uninformative, and for the addition of nine others that might better assess patient outcomes and quality of care. Jennifer Eames Huff, director of the Consumer-Purchaser Disclosure Project at San Francisco-based Pacific Business Group on Health, one of the letter’s signatories, says some of those potential measures might be more applicable to hospitalists as well.
But therein lies the rub. Although process measures might not always be strong indicators of quality of care, the introduction of outcome measures often makes providers nervous, says Gary Young, JD, PhD, director of the Center for Health Policy and Healthcare Research at Northeastern University in Boston. “Most providers feel that their patients are sicker and more vulnerable to poorer outcomes, and they don’t want to be judged poorly because they have sicker patients,” he says. Reaching an agreement on the best collection of measures may require some intense negotiations, he says.

–Win Whitcomb, MD, MHM, medical director of healthcare quality, Baystate Medical Center, Springfield, Mass.
Fairer Comparisons
Dr. Cowles cites two de-identified QRURs received by Alegent Creighton Health back in March—one for a hospitalist and one for an office-based general internist—to illustrate another major concern shared by many HM providers. The reports broke down each doctor’s relative healthcare contributions, using predetermined percentages of the total care and costs to conclude whether that doctor directed, influenced, or contributed to a patient’s care.
Hospitalists, by the nature of their jobs, seldom direct the care of any patient. But because their influence or contribution is almost always within the inpatient environment, HM providers account for proportionately higher costs than office-based physicians. The result can be a rather ugly curve: For healthcare costs incurred, the general internist was at the 65th percentile, while the hospitalist was at the 96th percentile.
The point, Dr. Cowles says, is that hospitalists and clinic-based physicians see patients with remarkably different acuities. “We just need to make sure that we’re comparing apples to apples, that you’re going to compare someone who sees a high-acuity patient with someone else who sees a high-acuity patient,” he says.
One silver lining could be increased momentum toward establishing HM as its own Medicare-recognized specialty. Hospitalist leaders who say the process is likely to be difficult but not impossible cite the successful effort to win recognition of HM as a focused practice by the American Board of Internal Medicine.
“We’re going to have to think outside the box in terms of working toward an identifier for hospitalists,” says Win Whitcomb, MD, MHM, medical director of healthcare quality at Baystate Medical Center in Springfield, Mass., and a member of SHM’s Performance and Measurement Reporting Committee. “But that’s going to happen—it’s not a matter of if, it’s a matter of when and how.”
As one potential interim solution, SHM has suggested a self-identification designation by which hospitalists would distinguish themselves from the larger, general internal-medicine category and thereby avoid unfair comparisons.
A Question of Attribution
Of the concerns raised by SHM, the question of attribution might be among the thorniest. Dr. Young says the “big-time issue” is pitting many consumer groups, payors, and employers against healthcare providers. The consumer groups want accountability at the individual provider level, while the providers strongly prefer group accountability, setting up a major clash over how responsibility will be parceled out.
Hospitalists have been taught to embrace responsibility while viewing healthcare delivery as a team sport. And the contributions of individual HM providers aren’t easily untangled. “If somebody has a bad outcome and they’ve been under the care of three different hospitalists, it’s virtually impossible to attribute that outcome to one of those three hospitalists,” Dr. Whitcomb says. “We really need to think about attribution differently, and it’s going to need to be across groups of hospitalists.”
SHM has suggested that CMS include an option for group rather than individual evaluation. “You’re just making it explicit that you can’t assign some of these measures to individual physicians. We can assign some of these measures to groups,” Dr. Whitcomb says.

—Julia Wright, MD, SFHM, FACP, president, MidAtlantic Business Unit, Cogent HMG, Brentwood, Tenn.
In its 2013 Medicare Physician Fee Schedule final rule, CMS opted to alter the doctor comparison methodology used for upcoming QRURs and the 2015 application of the VBPM. The agency also agreed to consider hospitalists’ concerns about fair attribution, relevant measures, and proper designation as it develops future proposals. Regardless of how those issues are ironed out, Dr. Torcson says, it’s clear to him that sitting on the sidelines is no longer an option for any physician group. Nor is it acceptable “to say this won’t work for me. We’re having to come up with proactive proposals for what will work to be part of the CMS quality agenda.”
SHM’s thorough analysis and realistic feedback, he says, has been well received by Medicare officials, raising hopes that many of the remaining differences can be resolved. “I am very confident that self-reporting or self-nomination as a hospitalist is going to be in place by the time those negative incentives kick in,” Dr. Torcson says. “And I’m also very confident that we’re going to have other, very creative options for quality measurement and performance reporting.”
One idea under consideration by CMS would allow hospitalists or other doctors to designate their hospitals’ quality data as a surrogate measure of their own performance. “I think that’s going to be a really great option for hospitalists who self-nominate,” Dr. Torcson says.
For many hospitalists, the option would effectively get around the issue of individual versus group attribution and instead align doctors’ fates with that of their institutions. SHM, Dr. Torcson says, has endorsed the proposal and offered to work with CMS to help institute it. He’s also confident that the reporting requirements for multiple, overlapping CMS programs will be more streamlined over time.
Some health professionals believe that hospitals and doctors already are devoting too much time and energy to measuring and recording the proliferating set of mandatory metrics. But Dr. Whitcomb says payors and patients are unlikely to have much sympathy.
“We as a profession are accountable to society at large. And that argument, that there are too many measurements and that we shouldn’t be held accountable as physicians for our performance, is a nonstarter when you’re trying to explain that to consumers,” he says. “The status quo is not tenable, and so it’s going to be a long journey and we need to be able to move in that direction.”
Bryn Nelson is a freelance medical writer in Seattle.
Russell Cowles III, MD, lead hospitalist at Bergan Mercy Medical Center in Omaha, Neb., recalls the shock on the faces of hospitalists who attended his presentation to SHM’s Nebraska Area chapter meeting last spring. Dr. Cowles and co-presenter Eric Rice, MD, MMM, SFHM, chapter president and assistant medical director of Alegent Creighton Hospital Medicine Services, were introducing their fellow hospitalists to a forthcoming Medicare initiative called the Physician Feedback/Value-Based Payment Modifier (VBPM) program.
“And everyone in the audience was completely stunned,” Dr. Cowles says. “They had never even dreamed that any of this would come down to the physician level.”
They’re not alone.
“Unless you work in administration or you’re leading a group, I don’t think very many people know this exists,” Dr. Cowles says. “Your average practicing physician, I think, has no clue that this measurement is going on behind the scenes.”
Authorized by the Affordable Care Act, the budget-neutral scheme ties future Medicare reimbursements to measures of quality and efficiency, and grades physicians on a curve. The Physician Quality Reporting System (PQRS), in place since 2007, forms the foundation of the new program, with feedback arriving in the form of a Quality and Resource Use Report (QRUR), a confidential report card sent to providers. The VBPM program then uses those reports as the basis for a financial reward or penalty.
In principle, SHM and hospitalist leaders have supported the concept of quality measurements as a way to hold doctors more accountable and to help the Centers for Medicare & Medicaid Services (CMS) take a more proactive role in improving quality of care while containing costs. And, in theory, HM leaders say hospitalists might be better able to adapt to the added responsibility of performance measurement and reporting due to their central role in the like-minded hospital value-based purchasing (VBP) program that began Oct. 1.
“If the expectation is that we will be involved in some of these initiatives and help the hospitals gain revenue, now we can actually see some dollars for those efforts,” says Julia Wright, MD, SFHM, FACP, president of the MidAtlantic Business Unit for Brentwood, Tenn.-based Cogent HMG. But the inverse is also true: If hospitals are going to have dollars at risk for performance, she says, CMS believes physicians should share in that risk as the providers of healthcare.
On that score, Dr. Rice says, hospitalists might have an advantage due to their focus on teamwork and their role in transitioning patients between inpatient and outpatient settings. In fact, he sees the VBPM as an “enormous opportunity” for hospitalists to demonstrate their leadership in helping to shape how organizations and institutions adapt to a quickly evolving healthcare environment.
But first, hospitalists will need to fully engage. In 2010, CMS found that only about 1 in 4 eligible physicians were participating in the voluntary PQRS and earning a reporting bonus of what is now 0.5% of allowable Medicare charges (roughly $800 for the average hospitalist). The stakes will grow when the PQRS transforms into a negative incentive program in 2015, with a 1.5% penalty for doctors who do not meet its reporting requirements. In 2016 and thereafter, the assessed penalty grows to 2% (about $3,200 for the average hospitalist).
“I think the unfolding timeline has really provided the potential for lulling us into complacency and procrastination,” says Patrick Torcson, MD, MMM, FACP, SFHM, director of hospital medicine at St. Tammany Parish Hospital in Covington, La., and chair of SHM’s Performance Measurement and Reporting Committee.
According to CMS, “physician groups can avoid all negative adjustments simply by participating in the PQRS.” Nonparticipants, however, could get hit with a double whammy. With no quality data, CMS would have no way to assess groups’ performances and would automatically deduct an extra 1% of Medicare reimbursements under the VBPM program. For groups of 100 eligible providers or more, that combined PQRS-VBPM penalty could amount to 2.5% in 2015.
PQRS participants have more leeway and a smaller downside. Starting January 2015, eligible provider groups who meet the reporting requirements can choose either to have no adjustments at all or to compete in the VBPM program for a performance-based bonus or a penalty of 1%, based on cost and quality scores. In January 2017, the program is expected to expand to include all providers, whether in individual or group practice.
A Measure of Relevance
Based on the first QRURs, sent out in March 2012 to providers in four pilot states, SHM wrote a letter to CMS that offered a detailed analysis of several additional concerns. The society followed up with a second letter that provided a more expansive critique of the proposed 2013 Physician Fee Schedule.
One worry is whether the physician feedback/VBPM program has included enough performance measures that are relevant to hospitalists. A Public Policy column in The Hospitalist (“Metric Accountability,” November 2012, p. 18) counted only 10 PQRS measures that apply routinely to HM providers out of a list of more than 200. Even those 10 aren’t always applicable.
“I work at a teaching hospital that’s large enough to have a neurology program, so most acute-stroke patients are admitted by the neurologists,” says Gregory Seymann, MD, SFHM, chief of the division of hospital medicine at the University of California at San Diego and a member of SHM’s Performance Measurement and Reporting Committee. “Five of the 10 measures are related to stroke patients, but my group rarely admits stroke patients.” That means only five PQRS measures remain relevant to him.
On paper, the issue might be readily resolved by expanding the number of measures to better reflect HM responsibilities—such as four measures proposed by SHM that relate to transitions of care and medication reconciliation.

—Patrick Torcson, MD, MMM, FACP, SFHM, director, hospital medicine, St. Tammany Parish Hospital, Covington, La., chair, SHM’s Performance Measurement and Reporting Committee
Other groups, though, have their own ideas. A letter to CMS signed by 28 patient and healthcare payor groups calls for the elimination of almost two dozen PQRS measures deemed unnecessary, duplicative, or uninformative, and for the addition of nine others that might better assess patient outcomes and quality of care. Jennifer Eames Huff, director of the Consumer-Purchaser Disclosure Project at San Francisco-based Pacific Business Group on Health, one of the letter’s signatories, says some of those potential measures might be more applicable to hospitalists as well.
But therein lies the rub. Although process measures might not always be strong indicators of quality of care, the introduction of outcome measures often makes providers nervous, says Gary Young, JD, PhD, director of the Center for Health Policy and Healthcare Research at Northeastern University in Boston. “Most providers feel that their patients are sicker and more vulnerable to poorer outcomes, and they don’t want to be judged poorly because they have sicker patients,” he says. Reaching an agreement on the best collection of measures may require some intense negotiations, he says.

–Win Whitcomb, MD, MHM, medical director of healthcare quality, Baystate Medical Center, Springfield, Mass.
Fairer Comparisons
Dr. Cowles cites two de-identified QRURs received by Alegent Creighton Health back in March—one for a hospitalist and one for an office-based general internist—to illustrate another major concern shared by many HM providers. The reports broke down each doctor’s relative healthcare contributions, using predetermined percentages of the total care and costs to conclude whether that doctor directed, influenced, or contributed to a patient’s care.
Hospitalists, by the nature of their jobs, seldom direct the care of any patient. But because their influence or contribution is almost always within the inpatient environment, HM providers account for proportionately higher costs than office-based physicians. The result can be a rather ugly curve: For healthcare costs incurred, the general internist was at the 65th percentile, while the hospitalist was at the 96th percentile.
The point, Dr. Cowles says, is that hospitalists and clinic-based physicians see patients with remarkably different acuities. “We just need to make sure that we’re comparing apples to apples, that you’re going to compare someone who sees a high-acuity patient with someone else who sees a high-acuity patient,” he says.
One silver lining could be increased momentum toward establishing HM as its own Medicare-recognized specialty. Hospitalist leaders who say the process is likely to be difficult but not impossible cite the successful effort to win recognition of HM as a focused practice by the American Board of Internal Medicine.
“We’re going to have to think outside the box in terms of working toward an identifier for hospitalists,” says Win Whitcomb, MD, MHM, medical director of healthcare quality at Baystate Medical Center in Springfield, Mass., and a member of SHM’s Performance and Measurement Reporting Committee. “But that’s going to happen—it’s not a matter of if, it’s a matter of when and how.”
As one potential interim solution, SHM has suggested a self-identification designation by which hospitalists would distinguish themselves from the larger, general internal-medicine category and thereby avoid unfair comparisons.
A Question of Attribution
Of the concerns raised by SHM, the question of attribution might be among the thorniest. Dr. Young says the “big-time issue” is pitting many consumer groups, payors, and employers against healthcare providers. The consumer groups want accountability at the individual provider level, while the providers strongly prefer group accountability, setting up a major clash over how responsibility will be parceled out.
Hospitalists have been taught to embrace responsibility while viewing healthcare delivery as a team sport. And the contributions of individual HM providers aren’t easily untangled. “If somebody has a bad outcome and they’ve been under the care of three different hospitalists, it’s virtually impossible to attribute that outcome to one of those three hospitalists,” Dr. Whitcomb says. “We really need to think about attribution differently, and it’s going to need to be across groups of hospitalists.”
SHM has suggested that CMS include an option for group rather than individual evaluation. “You’re just making it explicit that you can’t assign some of these measures to individual physicians. We can assign some of these measures to groups,” Dr. Whitcomb says.

—Julia Wright, MD, SFHM, FACP, president, MidAtlantic Business Unit, Cogent HMG, Brentwood, Tenn.
In its 2013 Medicare Physician Fee Schedule final rule, CMS opted to alter the doctor comparison methodology used for upcoming QRURs and the 2015 application of the VBPM. The agency also agreed to consider hospitalists’ concerns about fair attribution, relevant measures, and proper designation as it develops future proposals. Regardless of how those issues are ironed out, Dr. Torcson says, it’s clear to him that sitting on the sidelines is no longer an option for any physician group. Nor is it acceptable “to say this won’t work for me. We’re having to come up with proactive proposals for what will work to be part of the CMS quality agenda.”
SHM’s thorough analysis and realistic feedback, he says, has been well received by Medicare officials, raising hopes that many of the remaining differences can be resolved. “I am very confident that self-reporting or self-nomination as a hospitalist is going to be in place by the time those negative incentives kick in,” Dr. Torcson says. “And I’m also very confident that we’re going to have other, very creative options for quality measurement and performance reporting.”
One idea under consideration by CMS would allow hospitalists or other doctors to designate their hospitals’ quality data as a surrogate measure of their own performance. “I think that’s going to be a really great option for hospitalists who self-nominate,” Dr. Torcson says.
For many hospitalists, the option would effectively get around the issue of individual versus group attribution and instead align doctors’ fates with that of their institutions. SHM, Dr. Torcson says, has endorsed the proposal and offered to work with CMS to help institute it. He’s also confident that the reporting requirements for multiple, overlapping CMS programs will be more streamlined over time.
Some health professionals believe that hospitals and doctors already are devoting too much time and energy to measuring and recording the proliferating set of mandatory metrics. But Dr. Whitcomb says payors and patients are unlikely to have much sympathy.
“We as a profession are accountable to society at large. And that argument, that there are too many measurements and that we shouldn’t be held accountable as physicians for our performance, is a nonstarter when you’re trying to explain that to consumers,” he says. “The status quo is not tenable, and so it’s going to be a long journey and we need to be able to move in that direction.”
Bryn Nelson is a freelance medical writer in Seattle.
Hospitalists Should Consider Fall Risks with Sleep Agent
An author of a new study associating the hypnotic zolpidem (Ambien) with higher rates of patient falls says hospitalists should keep the popular drug’s risks front of mind.
The retrospective cohort study in the Journal of Hospital Medicine, “Zolpidem is Independently Associated with Increased Risk of Inpatient Falls,” found that the rate of falls increased nearly six times among patients taking the sleep agent.1 The research team at the Center for Sleep Medicine at the Mayo Clinic in Rochester, N.Y., calculated one additional fall for every 55 admitted patients who were administered the treatment.
“What this says to me is if one is going to use zolpidem, you have to be aware you’re increasing the risk of fall,” says sleep specialist Timothy Morgenthaler, MD, the Mayo Clinic’s chief patient officer. “Knowledgeable of that, one ought to consider whether there are alternatives or whether the risks outweigh the goal in that setting.”
Dr. Morgenthaler says zolpidem is the most commonly prescribed hypnotic at his hospital, and believes it to be the most common treatment in the U.S. He began studying the issue after nurses reported that it appeared patients were falling after taking the agent. In response to the study, Mayo Clinic removed zolpidem from many of its admission order sets and attempted to help improve patient sleep via other methods, including noise reduction.
“We haven’t removed it from our formulary, and I’m not saying it doesn’t have a role in some points,” he says, “but rather than encouraging it as an option in patients being admitted into the patient, we’re choosing instead now to encourage nonpharmacologic sleep enhancements.”
Visit the-hospitalist.org for more information about HM’s approach to patient falls.
Reference
An author of a new study associating the hypnotic zolpidem (Ambien) with higher rates of patient falls says hospitalists should keep the popular drug’s risks front of mind.
The retrospective cohort study in the Journal of Hospital Medicine, “Zolpidem is Independently Associated with Increased Risk of Inpatient Falls,” found that the rate of falls increased nearly six times among patients taking the sleep agent.1 The research team at the Center for Sleep Medicine at the Mayo Clinic in Rochester, N.Y., calculated one additional fall for every 55 admitted patients who were administered the treatment.
“What this says to me is if one is going to use zolpidem, you have to be aware you’re increasing the risk of fall,” says sleep specialist Timothy Morgenthaler, MD, the Mayo Clinic’s chief patient officer. “Knowledgeable of that, one ought to consider whether there are alternatives or whether the risks outweigh the goal in that setting.”
Dr. Morgenthaler says zolpidem is the most commonly prescribed hypnotic at his hospital, and believes it to be the most common treatment in the U.S. He began studying the issue after nurses reported that it appeared patients were falling after taking the agent. In response to the study, Mayo Clinic removed zolpidem from many of its admission order sets and attempted to help improve patient sleep via other methods, including noise reduction.
“We haven’t removed it from our formulary, and I’m not saying it doesn’t have a role in some points,” he says, “but rather than encouraging it as an option in patients being admitted into the patient, we’re choosing instead now to encourage nonpharmacologic sleep enhancements.”
Visit the-hospitalist.org for more information about HM’s approach to patient falls.
Reference
An author of a new study associating the hypnotic zolpidem (Ambien) with higher rates of patient falls says hospitalists should keep the popular drug’s risks front of mind.
The retrospective cohort study in the Journal of Hospital Medicine, “Zolpidem is Independently Associated with Increased Risk of Inpatient Falls,” found that the rate of falls increased nearly six times among patients taking the sleep agent.1 The research team at the Center for Sleep Medicine at the Mayo Clinic in Rochester, N.Y., calculated one additional fall for every 55 admitted patients who were administered the treatment.
“What this says to me is if one is going to use zolpidem, you have to be aware you’re increasing the risk of fall,” says sleep specialist Timothy Morgenthaler, MD, the Mayo Clinic’s chief patient officer. “Knowledgeable of that, one ought to consider whether there are alternatives or whether the risks outweigh the goal in that setting.”
Dr. Morgenthaler says zolpidem is the most commonly prescribed hypnotic at his hospital, and believes it to be the most common treatment in the U.S. He began studying the issue after nurses reported that it appeared patients were falling after taking the agent. In response to the study, Mayo Clinic removed zolpidem from many of its admission order sets and attempted to help improve patient sleep via other methods, including noise reduction.
“We haven’t removed it from our formulary, and I’m not saying it doesn’t have a role in some points,” he says, “but rather than encouraging it as an option in patients being admitted into the patient, we’re choosing instead now to encourage nonpharmacologic sleep enhancements.”
Visit the-hospitalist.org for more information about HM’s approach to patient falls.
Reference
Smartphones Distract Hospital Staff on Rounds
Smartphone use by hospitalists and other hospital staff is becoming ubiquitous, with a recent survey showing 72% of physicians using these devices at work.1 At the same time, concerns are being raised about clinical distractions and threats to patient privacy, even while such benefits as rapid access to colleagues, medical references, and patient records are touted.
In a study published in the Journal of Hospital Medicine, Rachel Katz-Sidlow, MD, of the department of pediatrics at Jacobi Medical Center in Bronx, N.Y., and colleagues surveyed residents’ and attendings’ perceptions of the use of smartphones during inpatient rounds, both their own and observed behaviors of colleagues.2 Fifty-seven percent of residents and 28% of faculty reported using smartphones during inpatient rounds, while significantly higher percentages observed other team members doing so.
The most common smartphone uses were for patient care, but doctors also use them to read and reply to personal texts and emails, as well as for non-patient-care-related Web searches. The authors observe that smartphones “introduce another source of interruption, multitasking, and distraction into the hospital environment,” with potential negative consequences.
Nineteen percent of residents believed they had missed important clinical information because of smartphone distraction during rounds. After seeing the survey results, Jacobi Medical Center instituted a smartphone policy in February 2012, essentially requiring personal mobile communication devices to be silenced at the start of rounds, except for patient care communication or urgent family matters, Dr. Katz-Sidlow wrote in an email to the The Hospitalist.
Confirmation of the spread of communication technology in the hospital toward smartphones and away from traditional pagers comes from data presented at the American Academy of Pediatrics conference in New Orleans in October by Stephanie Kuhlmann, MD, pediatric hospitalist at the University of Kansas at Wichita.3 Dr. Kuhlmann conducted an electronic survey of pediatric hospitalists, with 60% reporting that they receive work-related text messages. Twelve percent sent more than 10 text messages per shift, while 40% expressed concern about HIPAA violations. Most text messages are not encrypted, and many hospitals have yet to implement appropriately secure programs and policies, Dr. Kuhlmann says.
“Hospitals need to be aware of this trend and need to find a way to secure these text messages,” she adds.
Another recent survey by the Orem, Utah-based firm KLAS Research found that while 70% of clinicians report using smartphones or tablets to look up electronic patient records, they are less likely to input information into the EHR on these devices because of the difficulty of entering data on their small screens.4
References
- Dolan B. 72 percent of US physicians use smartphones. Mobi Health News website. Available at: http://mobihealthnews.com/7505/72-percent-of-us-physicians-use-smartphones/. Accessed Dec. 8, 2012.
- Katz-Sidlow RJ, Ludwig A, Millers S, Sidlow R. Smartphone use during inpatient attending rounds: prevalence, patterns and potential for distraction. J Hosp Med. 2012;7(8):595-599.
- Miller NS. Text messages are a growing trend among pediatric hospitalists. Pediatric News Digital Network website. Available at: http://www.pediatricnews.com/news/top-news/single-article/text-messages-are-a-growing-trend-among-pediatric-hospitalists/3dabf7208c75c44d36f368a83221d320.html. Accessed Nov. 1, 2012.
- Westerlind E. Mobile healthcare applications: can enterprise vendors keep up? KLAS website. Available at: http://www.klasresearch.com/KLASreports. Accessed Dec. 8, 2012.
Smartphone use by hospitalists and other hospital staff is becoming ubiquitous, with a recent survey showing 72% of physicians using these devices at work.1 At the same time, concerns are being raised about clinical distractions and threats to patient privacy, even while such benefits as rapid access to colleagues, medical references, and patient records are touted.
In a study published in the Journal of Hospital Medicine, Rachel Katz-Sidlow, MD, of the department of pediatrics at Jacobi Medical Center in Bronx, N.Y., and colleagues surveyed residents’ and attendings’ perceptions of the use of smartphones during inpatient rounds, both their own and observed behaviors of colleagues.2 Fifty-seven percent of residents and 28% of faculty reported using smartphones during inpatient rounds, while significantly higher percentages observed other team members doing so.
The most common smartphone uses were for patient care, but doctors also use them to read and reply to personal texts and emails, as well as for non-patient-care-related Web searches. The authors observe that smartphones “introduce another source of interruption, multitasking, and distraction into the hospital environment,” with potential negative consequences.
Nineteen percent of residents believed they had missed important clinical information because of smartphone distraction during rounds. After seeing the survey results, Jacobi Medical Center instituted a smartphone policy in February 2012, essentially requiring personal mobile communication devices to be silenced at the start of rounds, except for patient care communication or urgent family matters, Dr. Katz-Sidlow wrote in an email to the The Hospitalist.
Confirmation of the spread of communication technology in the hospital toward smartphones and away from traditional pagers comes from data presented at the American Academy of Pediatrics conference in New Orleans in October by Stephanie Kuhlmann, MD, pediatric hospitalist at the University of Kansas at Wichita.3 Dr. Kuhlmann conducted an electronic survey of pediatric hospitalists, with 60% reporting that they receive work-related text messages. Twelve percent sent more than 10 text messages per shift, while 40% expressed concern about HIPAA violations. Most text messages are not encrypted, and many hospitals have yet to implement appropriately secure programs and policies, Dr. Kuhlmann says.
“Hospitals need to be aware of this trend and need to find a way to secure these text messages,” she adds.
Another recent survey by the Orem, Utah-based firm KLAS Research found that while 70% of clinicians report using smartphones or tablets to look up electronic patient records, they are less likely to input information into the EHR on these devices because of the difficulty of entering data on their small screens.4
References
- Dolan B. 72 percent of US physicians use smartphones. Mobi Health News website. Available at: http://mobihealthnews.com/7505/72-percent-of-us-physicians-use-smartphones/. Accessed Dec. 8, 2012.
- Katz-Sidlow RJ, Ludwig A, Millers S, Sidlow R. Smartphone use during inpatient attending rounds: prevalence, patterns and potential for distraction. J Hosp Med. 2012;7(8):595-599.
- Miller NS. Text messages are a growing trend among pediatric hospitalists. Pediatric News Digital Network website. Available at: http://www.pediatricnews.com/news/top-news/single-article/text-messages-are-a-growing-trend-among-pediatric-hospitalists/3dabf7208c75c44d36f368a83221d320.html. Accessed Nov. 1, 2012.
- Westerlind E. Mobile healthcare applications: can enterprise vendors keep up? KLAS website. Available at: http://www.klasresearch.com/KLASreports. Accessed Dec. 8, 2012.
Smartphone use by hospitalists and other hospital staff is becoming ubiquitous, with a recent survey showing 72% of physicians using these devices at work.1 At the same time, concerns are being raised about clinical distractions and threats to patient privacy, even while such benefits as rapid access to colleagues, medical references, and patient records are touted.
In a study published in the Journal of Hospital Medicine, Rachel Katz-Sidlow, MD, of the department of pediatrics at Jacobi Medical Center in Bronx, N.Y., and colleagues surveyed residents’ and attendings’ perceptions of the use of smartphones during inpatient rounds, both their own and observed behaviors of colleagues.2 Fifty-seven percent of residents and 28% of faculty reported using smartphones during inpatient rounds, while significantly higher percentages observed other team members doing so.
The most common smartphone uses were for patient care, but doctors also use them to read and reply to personal texts and emails, as well as for non-patient-care-related Web searches. The authors observe that smartphones “introduce another source of interruption, multitasking, and distraction into the hospital environment,” with potential negative consequences.
Nineteen percent of residents believed they had missed important clinical information because of smartphone distraction during rounds. After seeing the survey results, Jacobi Medical Center instituted a smartphone policy in February 2012, essentially requiring personal mobile communication devices to be silenced at the start of rounds, except for patient care communication or urgent family matters, Dr. Katz-Sidlow wrote in an email to the The Hospitalist.
Confirmation of the spread of communication technology in the hospital toward smartphones and away from traditional pagers comes from data presented at the American Academy of Pediatrics conference in New Orleans in October by Stephanie Kuhlmann, MD, pediatric hospitalist at the University of Kansas at Wichita.3 Dr. Kuhlmann conducted an electronic survey of pediatric hospitalists, with 60% reporting that they receive work-related text messages. Twelve percent sent more than 10 text messages per shift, while 40% expressed concern about HIPAA violations. Most text messages are not encrypted, and many hospitals have yet to implement appropriately secure programs and policies, Dr. Kuhlmann says.
“Hospitals need to be aware of this trend and need to find a way to secure these text messages,” she adds.
Another recent survey by the Orem, Utah-based firm KLAS Research found that while 70% of clinicians report using smartphones or tablets to look up electronic patient records, they are less likely to input information into the EHR on these devices because of the difficulty of entering data on their small screens.4
References
- Dolan B. 72 percent of US physicians use smartphones. Mobi Health News website. Available at: http://mobihealthnews.com/7505/72-percent-of-us-physicians-use-smartphones/. Accessed Dec. 8, 2012.
- Katz-Sidlow RJ, Ludwig A, Millers S, Sidlow R. Smartphone use during inpatient attending rounds: prevalence, patterns and potential for distraction. J Hosp Med. 2012;7(8):595-599.
- Miller NS. Text messages are a growing trend among pediatric hospitalists. Pediatric News Digital Network website. Available at: http://www.pediatricnews.com/news/top-news/single-article/text-messages-are-a-growing-trend-among-pediatric-hospitalists/3dabf7208c75c44d36f368a83221d320.html. Accessed Nov. 1, 2012.
- Westerlind E. Mobile healthcare applications: can enterprise vendors keep up? KLAS website. Available at: http://www.klasresearch.com/KLASreports. Accessed Dec. 8, 2012.
Adherence to CHF Measures Doesn’t Improve Hospital Readmission Rates
A study of the relationship between hospital adherence to congestive heart failure (CHF) quality performance measures and 30-day readmission rates found little association, except for the assessment of left ventricular function, which, if not performed according to guidelines, was associated with higher readmissions.1
Lead author Sula Mazimba, MD, MPH, and colleagues at Kettering Medical Center in Kettering, Ohio, looked at adherence to the performance measures, which in recent years have been adopted by quality organizations and third-party payors as surrogate markers for quality of care. These include documented ordering of angiotensin-converting enzyme (ACE) inhibitors, providing discharge instructions to patients, and counseling on smoking cessation.
The study looked retrospectively at 6,000 CHF patients within a four-hospital healthcare system between 2001 and 2009, at a time when adherence to the performance measures rose to 99.9% from 95.8%. The hospital readmission rate for these patients averaged 19.6%.
Larry Beresford is a freelance writer in Oakland, Calif.
References
A study of the relationship between hospital adherence to congestive heart failure (CHF) quality performance measures and 30-day readmission rates found little association, except for the assessment of left ventricular function, which, if not performed according to guidelines, was associated with higher readmissions.1
Lead author Sula Mazimba, MD, MPH, and colleagues at Kettering Medical Center in Kettering, Ohio, looked at adherence to the performance measures, which in recent years have been adopted by quality organizations and third-party payors as surrogate markers for quality of care. These include documented ordering of angiotensin-converting enzyme (ACE) inhibitors, providing discharge instructions to patients, and counseling on smoking cessation.
The study looked retrospectively at 6,000 CHF patients within a four-hospital healthcare system between 2001 and 2009, at a time when adherence to the performance measures rose to 99.9% from 95.8%. The hospital readmission rate for these patients averaged 19.6%.
Larry Beresford is a freelance writer in Oakland, Calif.
References
A study of the relationship between hospital adherence to congestive heart failure (CHF) quality performance measures and 30-day readmission rates found little association, except for the assessment of left ventricular function, which, if not performed according to guidelines, was associated with higher readmissions.1
Lead author Sula Mazimba, MD, MPH, and colleagues at Kettering Medical Center in Kettering, Ohio, looked at adherence to the performance measures, which in recent years have been adopted by quality organizations and third-party payors as surrogate markers for quality of care. These include documented ordering of angiotensin-converting enzyme (ACE) inhibitors, providing discharge instructions to patients, and counseling on smoking cessation.
The study looked retrospectively at 6,000 CHF patients within a four-hospital healthcare system between 2001 and 2009, at a time when adherence to the performance measures rose to 99.9% from 95.8%. The hospital readmission rate for these patients averaged 19.6%.
Larry Beresford is a freelance writer in Oakland, Calif.