User login
Ready to be a Fellow in Hospital Medicine?
If you’re ready to demonstrate your commitment to HM and hospitalized patients, you still have time to submit your SHM fellowship application.
The deadline for 2013 applications is Jan. 18. To apply online or learn more, visit www.hospitalmedicine.org/fellows.
The class of 2013 Fellows will be inducted during a plenary session at SHM’s annual meeting in May in National Harbor, Md.
This year’s class will reach a milestone—not just for hospital medicine, but for all of healthcare. SHM has expanded eligibility in its Fellowship in Hospital Medicine program to include nurse practitioners (NPs), physician assistants (PAs), and HM practice administrators. By opening the designation to nonphysicians, SHM becomes the only medical society to offer a singular designation to the entire care team.
SHM members who meet eligibility criteria are recognized as Fellows each year at the annual meeting. Based on current membership, SHM estimates that more than 300 NPs, PAs, and administrators are eligible immediately; thousands more will be eligible after they meet the three-year membership requirement for fellow status.
“We are proud to be able to recognize excellence within the specialty and contributions to the field by nurse practitioners, physician assistants, and practice administrators,” says SHM President Shaun Frost, MD, SFHM. “The standards by which SHM fellows are measured promote the highest quality of patient care and systems efficiency. And they can be equally applied to physicians, NPs, PAs, and administrators within the hospital medicine specialty.”
SHM’s Fellows program is rooted in the society’s Core Competencies in Hospital Medicine, and those who earn the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation have demonstrated a commitment to hospital medicine, system change, and quality-improvement (QI) principles.
All candidates for the designation are required to submit applications that demonstrate experience, organizational teamwork and leadership, and a dedication to lifelong learning. Applicants must receive endorsement from practitioners in the field and are subject to committee review.
“Hospital medicine was built on the principle that caregivers must act as a team,” Dr. Frost says. “We are honored to recognize more members of that team today through our Fellows designation.”
Brendon Shank is associate vice president of communications for SHM.
If you’re ready to demonstrate your commitment to HM and hospitalized patients, you still have time to submit your SHM fellowship application.
The deadline for 2013 applications is Jan. 18. To apply online or learn more, visit www.hospitalmedicine.org/fellows.
The class of 2013 Fellows will be inducted during a plenary session at SHM’s annual meeting in May in National Harbor, Md.
This year’s class will reach a milestone—not just for hospital medicine, but for all of healthcare. SHM has expanded eligibility in its Fellowship in Hospital Medicine program to include nurse practitioners (NPs), physician assistants (PAs), and HM practice administrators. By opening the designation to nonphysicians, SHM becomes the only medical society to offer a singular designation to the entire care team.
SHM members who meet eligibility criteria are recognized as Fellows each year at the annual meeting. Based on current membership, SHM estimates that more than 300 NPs, PAs, and administrators are eligible immediately; thousands more will be eligible after they meet the three-year membership requirement for fellow status.
“We are proud to be able to recognize excellence within the specialty and contributions to the field by nurse practitioners, physician assistants, and practice administrators,” says SHM President Shaun Frost, MD, SFHM. “The standards by which SHM fellows are measured promote the highest quality of patient care and systems efficiency. And they can be equally applied to physicians, NPs, PAs, and administrators within the hospital medicine specialty.”
SHM’s Fellows program is rooted in the society’s Core Competencies in Hospital Medicine, and those who earn the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation have demonstrated a commitment to hospital medicine, system change, and quality-improvement (QI) principles.
All candidates for the designation are required to submit applications that demonstrate experience, organizational teamwork and leadership, and a dedication to lifelong learning. Applicants must receive endorsement from practitioners in the field and are subject to committee review.
“Hospital medicine was built on the principle that caregivers must act as a team,” Dr. Frost says. “We are honored to recognize more members of that team today through our Fellows designation.”
Brendon Shank is associate vice president of communications for SHM.
If you’re ready to demonstrate your commitment to HM and hospitalized patients, you still have time to submit your SHM fellowship application.
The deadline for 2013 applications is Jan. 18. To apply online or learn more, visit www.hospitalmedicine.org/fellows.
The class of 2013 Fellows will be inducted during a plenary session at SHM’s annual meeting in May in National Harbor, Md.
This year’s class will reach a milestone—not just for hospital medicine, but for all of healthcare. SHM has expanded eligibility in its Fellowship in Hospital Medicine program to include nurse practitioners (NPs), physician assistants (PAs), and HM practice administrators. By opening the designation to nonphysicians, SHM becomes the only medical society to offer a singular designation to the entire care team.
SHM members who meet eligibility criteria are recognized as Fellows each year at the annual meeting. Based on current membership, SHM estimates that more than 300 NPs, PAs, and administrators are eligible immediately; thousands more will be eligible after they meet the three-year membership requirement for fellow status.
“We are proud to be able to recognize excellence within the specialty and contributions to the field by nurse practitioners, physician assistants, and practice administrators,” says SHM President Shaun Frost, MD, SFHM. “The standards by which SHM fellows are measured promote the highest quality of patient care and systems efficiency. And they can be equally applied to physicians, NPs, PAs, and administrators within the hospital medicine specialty.”
SHM’s Fellows program is rooted in the society’s Core Competencies in Hospital Medicine, and those who earn the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation have demonstrated a commitment to hospital medicine, system change, and quality-improvement (QI) principles.
All candidates for the designation are required to submit applications that demonstrate experience, organizational teamwork and leadership, and a dedication to lifelong learning. Applicants must receive endorsement from practitioners in the field and are subject to committee review.
“Hospital medicine was built on the principle that caregivers must act as a team,” Dr. Frost says. “We are honored to recognize more members of that team today through our Fellows designation.”
Brendon Shank is associate vice president of communications for SHM.
Accuracy Matters When Compensation for Hospitalists Is at Stake
Not long ago, I received an email from a hospitalist group leader who was working with her CMO on a new compensation plan. The CMO, wanting to ensure that the proposed compensation per unit of work was appropriate, had taken the MGMA national median annual compensation for internal-medicine hospitalists ($234,437) and divided it by the national median annual work RVUs (4,185) to arrive at a targeted compensation per wRVU of $56.01.
The hospitalist leader, however, had the benefit of referring to her 2012 State of Hospital Medicine report, in which Table 6.30 reported an MGMA median compensation per wRVU for internal-medicine hospitalists of $58.28. That variance of more than two dollars per wRVU could mean an additional $8,000 or so in annual compensation to her and her colleagues, so she was seeking to understand why the report has a different number than the one calculated by her CMO.
The answer is that the CMO got caught in a common error of logic: The CMO assumed that the compensation median and the wRVU median were derived from exactly the same population, failing to consider that the underlying data sets might be different. Here’s what happened: Compensation data were reported for 3,192 internal-medicine hospitalists, but wRVUs were reported for only 2,389 of those hospitalists. So the analysis of compensation per wRVU can be accurately calculated only for those 2,389 hospitalists for whom both compensation and wRVUs were reported. The other 803 hospitalists for whom no wRVUs were reported had to be excluded from the ratio calculation. The CMO’s error was to calculate a ratio of two medians based on different data sets, rather than calculating the individual comp-to-wRVU ratios, then determining the median for that smaller data set.
A similar thing has happened over the years with nocturnist data. In SHM’s 2007-2008 compensation and productivity survey, and again in the 2011 SHM/MGMA State of Hospital Medicine report, the median compensation reported for nocturnists actually was lower than that reported for all adult hospitalists. In my work with hospitalist practices across the country, I’ve only run into one or two where the nocturnists earned less than the daytime doctors, so I was flummoxed by this finding. Turns out, I was making the same mistake of assuming I was looking at “nocturnist” and “all adult hospitalist” compensation for the same hospitalist groups. But the adult medicine groups using nocturnists are actually a small subset of all adult medicine groups, and the nocturnist data likely included at least a few pediatric hospitalist nocturnists. Because the underlying data sets are different, the two medians aren’t directly comparable.
When all is said and done, we don’t really care whether the average nocturnist earns more or less than the average non-nocturnist hospitalist. What we really want to know is, Do the nocturnists in a given group earn more than the non-nocturnists in the same group? That’s why this year SHM asked groups to report the average percent compensation differential between nocturnists and non-nocturnists in their groups. It turns out that groups serving adults only reported a median of 15% higher compensation for nocturnists, a far different result than users of previous surveys inferred.
The bottom line: Make sure you understand how the State of Hospital Medicine survey results are calculated. Many of the formulas used are described in Appendix B of the report, and if you have questions about others, feel free to contact SHM and ask.
Leslie Flores is a partner in Nelson Flores Hospital Medicine Consultants.
Not long ago, I received an email from a hospitalist group leader who was working with her CMO on a new compensation plan. The CMO, wanting to ensure that the proposed compensation per unit of work was appropriate, had taken the MGMA national median annual compensation for internal-medicine hospitalists ($234,437) and divided it by the national median annual work RVUs (4,185) to arrive at a targeted compensation per wRVU of $56.01.
The hospitalist leader, however, had the benefit of referring to her 2012 State of Hospital Medicine report, in which Table 6.30 reported an MGMA median compensation per wRVU for internal-medicine hospitalists of $58.28. That variance of more than two dollars per wRVU could mean an additional $8,000 or so in annual compensation to her and her colleagues, so she was seeking to understand why the report has a different number than the one calculated by her CMO.
The answer is that the CMO got caught in a common error of logic: The CMO assumed that the compensation median and the wRVU median were derived from exactly the same population, failing to consider that the underlying data sets might be different. Here’s what happened: Compensation data were reported for 3,192 internal-medicine hospitalists, but wRVUs were reported for only 2,389 of those hospitalists. So the analysis of compensation per wRVU can be accurately calculated only for those 2,389 hospitalists for whom both compensation and wRVUs were reported. The other 803 hospitalists for whom no wRVUs were reported had to be excluded from the ratio calculation. The CMO’s error was to calculate a ratio of two medians based on different data sets, rather than calculating the individual comp-to-wRVU ratios, then determining the median for that smaller data set.
A similar thing has happened over the years with nocturnist data. In SHM’s 2007-2008 compensation and productivity survey, and again in the 2011 SHM/MGMA State of Hospital Medicine report, the median compensation reported for nocturnists actually was lower than that reported for all adult hospitalists. In my work with hospitalist practices across the country, I’ve only run into one or two where the nocturnists earned less than the daytime doctors, so I was flummoxed by this finding. Turns out, I was making the same mistake of assuming I was looking at “nocturnist” and “all adult hospitalist” compensation for the same hospitalist groups. But the adult medicine groups using nocturnists are actually a small subset of all adult medicine groups, and the nocturnist data likely included at least a few pediatric hospitalist nocturnists. Because the underlying data sets are different, the two medians aren’t directly comparable.
When all is said and done, we don’t really care whether the average nocturnist earns more or less than the average non-nocturnist hospitalist. What we really want to know is, Do the nocturnists in a given group earn more than the non-nocturnists in the same group? That’s why this year SHM asked groups to report the average percent compensation differential between nocturnists and non-nocturnists in their groups. It turns out that groups serving adults only reported a median of 15% higher compensation for nocturnists, a far different result than users of previous surveys inferred.
The bottom line: Make sure you understand how the State of Hospital Medicine survey results are calculated. Many of the formulas used are described in Appendix B of the report, and if you have questions about others, feel free to contact SHM and ask.
Leslie Flores is a partner in Nelson Flores Hospital Medicine Consultants.
Not long ago, I received an email from a hospitalist group leader who was working with her CMO on a new compensation plan. The CMO, wanting to ensure that the proposed compensation per unit of work was appropriate, had taken the MGMA national median annual compensation for internal-medicine hospitalists ($234,437) and divided it by the national median annual work RVUs (4,185) to arrive at a targeted compensation per wRVU of $56.01.
The hospitalist leader, however, had the benefit of referring to her 2012 State of Hospital Medicine report, in which Table 6.30 reported an MGMA median compensation per wRVU for internal-medicine hospitalists of $58.28. That variance of more than two dollars per wRVU could mean an additional $8,000 or so in annual compensation to her and her colleagues, so she was seeking to understand why the report has a different number than the one calculated by her CMO.
The answer is that the CMO got caught in a common error of logic: The CMO assumed that the compensation median and the wRVU median were derived from exactly the same population, failing to consider that the underlying data sets might be different. Here’s what happened: Compensation data were reported for 3,192 internal-medicine hospitalists, but wRVUs were reported for only 2,389 of those hospitalists. So the analysis of compensation per wRVU can be accurately calculated only for those 2,389 hospitalists for whom both compensation and wRVUs were reported. The other 803 hospitalists for whom no wRVUs were reported had to be excluded from the ratio calculation. The CMO’s error was to calculate a ratio of two medians based on different data sets, rather than calculating the individual comp-to-wRVU ratios, then determining the median for that smaller data set.
A similar thing has happened over the years with nocturnist data. In SHM’s 2007-2008 compensation and productivity survey, and again in the 2011 SHM/MGMA State of Hospital Medicine report, the median compensation reported for nocturnists actually was lower than that reported for all adult hospitalists. In my work with hospitalist practices across the country, I’ve only run into one or two where the nocturnists earned less than the daytime doctors, so I was flummoxed by this finding. Turns out, I was making the same mistake of assuming I was looking at “nocturnist” and “all adult hospitalist” compensation for the same hospitalist groups. But the adult medicine groups using nocturnists are actually a small subset of all adult medicine groups, and the nocturnist data likely included at least a few pediatric hospitalist nocturnists. Because the underlying data sets are different, the two medians aren’t directly comparable.
When all is said and done, we don’t really care whether the average nocturnist earns more or less than the average non-nocturnist hospitalist. What we really want to know is, Do the nocturnists in a given group earn more than the non-nocturnists in the same group? That’s why this year SHM asked groups to report the average percent compensation differential between nocturnists and non-nocturnists in their groups. It turns out that groups serving adults only reported a median of 15% higher compensation for nocturnists, a far different result than users of previous surveys inferred.
The bottom line: Make sure you understand how the State of Hospital Medicine survey results are calculated. Many of the formulas used are described in Appendix B of the report, and if you have questions about others, feel free to contact SHM and ask.
Leslie Flores is a partner in Nelson Flores Hospital Medicine Consultants.
Win Whitcomb: Hospitalists Central to Helping Hospitals Meet Performance Goals, Avoid Financial Penalities
After a long wait, the time where quality really matters to the finance department has arrived. Why? Because now a lot of money is on the line based on hospitals’ ability to demonstrate performance on quality, patient safety, and patient satisfaction measures. And there is no physician group more central to the hospital’s performance on these measures than hospitalists.
To make this point, I will discuss the financial implications of three programs that are part of the Affordable Care Act: hospital value-based purchasing (HVBP), readmission penalties, and hospital-acquired conditions (HACs). Although many have found fault with these programs, especially the ones that only penalize hospitals (HACs and readmissions*), the dollars at risk can represent a new business case for hospitalist programs. High-performing hospitalist programs can positively impact their institution’s income statement.
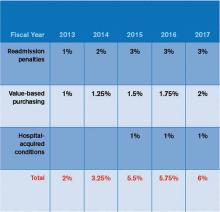
In October 2012, readmissions penalties and VBP payments/penalties went into effect. In October 2014, a 1% penalty for groups in the worst-performing quartile in the HACs will be in force. Taken together, as Table 1 demonstrates, the total payments at risk will grow such that by fiscal-year 2017, 6% of a hospital’s inpatient payments from Medicare will be at risk. To put this in perspective, Table 2 models the dollars at risk for each of the five years beginning in 2013 for the three programs in a hypothetical, 327-bed hospital. While the risk in 2013 is a modest $1.7 million, by 2017, this hospital has more than $5 million at risk.
So where should hospitalists be focusing their efforts with these three programs and the monies that accompany them? First, for those designing incentive compensation for hospitalists, the incentives should address the applicable elements of these programs. Second, the components of VBP will evolve. For example, 2013 VPB payments are based on 70% process measures (a subset of the core measure set for heart failure, myocardial infarction, pneumonia, and surgery) and 30% patient satisfaction measures. In 2015, VBP will add such outcomes measures as central-line-associated bloodstream infections, catheter UTIs, mortality rates, and the new efficiency measure, Medicare spending per beneficiary. Third, readmissions penalties, while encompassing heart failure, myocardial infarction, and pneumonia for fiscal-year 2013 payments, will expand in fiscal-year 2015 to include chronic obstructive pulmonary disease, coronary artery bypass grafting, percutaneous coronary intervention, and other vascular conditions.
While all this can be hard to keep track of, not to mention address in the course of daily patient care, I suggest hospitalists set the following priorities to enable high performance for their hospitals under these programs:
- Catheter UTIs. Work with nursing, the ED, and other areas to ensure that catheters are indicated, insertion is sterile, there is a mechanism for their prompt removal, specimens are collected and handled appropriately, and that “present on admission” is documented if appropriate.
- Central-line-associated bloodstream infections. Ensure your hospital has the systems in place to support the central-line insertion bundle, and that the bundle elements are followed and documented.
- Readmissions. Focus on heart failure, pneumonia, myocardial infarction, and COPD; work with nursing and case management to identify those at high risk for readmission; perform targeted interventions based on that risk (e.g. palliative care or clinical pharmacy consultation); prioritize medication reconciliation; provide timely communication of discharge summary to the next provider of care; and contact the patient soon after discharge to ensure they are following their plan of care.
- Patient satisfaction. Have a system for high performance on the questions comprising the “doctor communication” domain. These are “How often did doctors treat you with courtesy and respect/listen carefully to you/explain things in a way you could understand?”
Medicare’s Message
Clearly, the financial impacts of hospital quality, satisfaction, and safety are growing, conveying the message from the Centers for Medicare & Medicaid Services (CMS) that quality matters, making a business case for quality. Our focus as leaders in hospital systems improvement will only sharpen as we see hospital payments increasingly affected as a direct consequence of our efforts. If that doesn’t get the attention of the finance department, what will?
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is a co-founder and past president of SHM. Email him at [email protected].
After a long wait, the time where quality really matters to the finance department has arrived. Why? Because now a lot of money is on the line based on hospitals’ ability to demonstrate performance on quality, patient safety, and patient satisfaction measures. And there is no physician group more central to the hospital’s performance on these measures than hospitalists.
To make this point, I will discuss the financial implications of three programs that are part of the Affordable Care Act: hospital value-based purchasing (HVBP), readmission penalties, and hospital-acquired conditions (HACs). Although many have found fault with these programs, especially the ones that only penalize hospitals (HACs and readmissions*), the dollars at risk can represent a new business case for hospitalist programs. High-performing hospitalist programs can positively impact their institution’s income statement.
In October 2012, readmissions penalties and VBP payments/penalties went into effect. In October 2014, a 1% penalty for groups in the worst-performing quartile in the HACs will be in force. Taken together, as Table 1 demonstrates, the total payments at risk will grow such that by fiscal-year 2017, 6% of a hospital’s inpatient payments from Medicare will be at risk. To put this in perspective, Table 2 models the dollars at risk for each of the five years beginning in 2013 for the three programs in a hypothetical, 327-bed hospital. While the risk in 2013 is a modest $1.7 million, by 2017, this hospital has more than $5 million at risk.
So where should hospitalists be focusing their efforts with these three programs and the monies that accompany them? First, for those designing incentive compensation for hospitalists, the incentives should address the applicable elements of these programs. Second, the components of VBP will evolve. For example, 2013 VPB payments are based on 70% process measures (a subset of the core measure set for heart failure, myocardial infarction, pneumonia, and surgery) and 30% patient satisfaction measures. In 2015, VBP will add such outcomes measures as central-line-associated bloodstream infections, catheter UTIs, mortality rates, and the new efficiency measure, Medicare spending per beneficiary. Third, readmissions penalties, while encompassing heart failure, myocardial infarction, and pneumonia for fiscal-year 2013 payments, will expand in fiscal-year 2015 to include chronic obstructive pulmonary disease, coronary artery bypass grafting, percutaneous coronary intervention, and other vascular conditions.
While all this can be hard to keep track of, not to mention address in the course of daily patient care, I suggest hospitalists set the following priorities to enable high performance for their hospitals under these programs:
- Catheter UTIs. Work with nursing, the ED, and other areas to ensure that catheters are indicated, insertion is sterile, there is a mechanism for their prompt removal, specimens are collected and handled appropriately, and that “present on admission” is documented if appropriate.
- Central-line-associated bloodstream infections. Ensure your hospital has the systems in place to support the central-line insertion bundle, and that the bundle elements are followed and documented.
- Readmissions. Focus on heart failure, pneumonia, myocardial infarction, and COPD; work with nursing and case management to identify those at high risk for readmission; perform targeted interventions based on that risk (e.g. palliative care or clinical pharmacy consultation); prioritize medication reconciliation; provide timely communication of discharge summary to the next provider of care; and contact the patient soon after discharge to ensure they are following their plan of care.
- Patient satisfaction. Have a system for high performance on the questions comprising the “doctor communication” domain. These are “How often did doctors treat you with courtesy and respect/listen carefully to you/explain things in a way you could understand?”
Medicare’s Message
Clearly, the financial impacts of hospital quality, satisfaction, and safety are growing, conveying the message from the Centers for Medicare & Medicaid Services (CMS) that quality matters, making a business case for quality. Our focus as leaders in hospital systems improvement will only sharpen as we see hospital payments increasingly affected as a direct consequence of our efforts. If that doesn’t get the attention of the finance department, what will?
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is a co-founder and past president of SHM. Email him at [email protected].
After a long wait, the time where quality really matters to the finance department has arrived. Why? Because now a lot of money is on the line based on hospitals’ ability to demonstrate performance on quality, patient safety, and patient satisfaction measures. And there is no physician group more central to the hospital’s performance on these measures than hospitalists.
To make this point, I will discuss the financial implications of three programs that are part of the Affordable Care Act: hospital value-based purchasing (HVBP), readmission penalties, and hospital-acquired conditions (HACs). Although many have found fault with these programs, especially the ones that only penalize hospitals (HACs and readmissions*), the dollars at risk can represent a new business case for hospitalist programs. High-performing hospitalist programs can positively impact their institution’s income statement.
In October 2012, readmissions penalties and VBP payments/penalties went into effect. In October 2014, a 1% penalty for groups in the worst-performing quartile in the HACs will be in force. Taken together, as Table 1 demonstrates, the total payments at risk will grow such that by fiscal-year 2017, 6% of a hospital’s inpatient payments from Medicare will be at risk. To put this in perspective, Table 2 models the dollars at risk for each of the five years beginning in 2013 for the three programs in a hypothetical, 327-bed hospital. While the risk in 2013 is a modest $1.7 million, by 2017, this hospital has more than $5 million at risk.
So where should hospitalists be focusing their efforts with these three programs and the monies that accompany them? First, for those designing incentive compensation for hospitalists, the incentives should address the applicable elements of these programs. Second, the components of VBP will evolve. For example, 2013 VPB payments are based on 70% process measures (a subset of the core measure set for heart failure, myocardial infarction, pneumonia, and surgery) and 30% patient satisfaction measures. In 2015, VBP will add such outcomes measures as central-line-associated bloodstream infections, catheter UTIs, mortality rates, and the new efficiency measure, Medicare spending per beneficiary. Third, readmissions penalties, while encompassing heart failure, myocardial infarction, and pneumonia for fiscal-year 2013 payments, will expand in fiscal-year 2015 to include chronic obstructive pulmonary disease, coronary artery bypass grafting, percutaneous coronary intervention, and other vascular conditions.
While all this can be hard to keep track of, not to mention address in the course of daily patient care, I suggest hospitalists set the following priorities to enable high performance for their hospitals under these programs:
- Catheter UTIs. Work with nursing, the ED, and other areas to ensure that catheters are indicated, insertion is sterile, there is a mechanism for their prompt removal, specimens are collected and handled appropriately, and that “present on admission” is documented if appropriate.
- Central-line-associated bloodstream infections. Ensure your hospital has the systems in place to support the central-line insertion bundle, and that the bundle elements are followed and documented.
- Readmissions. Focus on heart failure, pneumonia, myocardial infarction, and COPD; work with nursing and case management to identify those at high risk for readmission; perform targeted interventions based on that risk (e.g. palliative care or clinical pharmacy consultation); prioritize medication reconciliation; provide timely communication of discharge summary to the next provider of care; and contact the patient soon after discharge to ensure they are following their plan of care.
- Patient satisfaction. Have a system for high performance on the questions comprising the “doctor communication” domain. These are “How often did doctors treat you with courtesy and respect/listen carefully to you/explain things in a way you could understand?”
Medicare’s Message
Clearly, the financial impacts of hospital quality, satisfaction, and safety are growing, conveying the message from the Centers for Medicare & Medicaid Services (CMS) that quality matters, making a business case for quality. Our focus as leaders in hospital systems improvement will only sharpen as we see hospital payments increasingly affected as a direct consequence of our efforts. If that doesn’t get the attention of the finance department, what will?
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is a co-founder and past president of SHM. Email him at [email protected].
Value-Based Purchasing Model for Medicare Reimbursement Extends to Physicians
What: The program, authorized by 2010’s Affordable Care Act, extends CMS’ concept of value-based purchasing to the individual physician level. That means a portion of doctors’ Medicare reimbursements will be contingent on scores designed to measure the quality and efficiency of the healthcare they deliver.
How: The program is designed to be budget-neutral, meaning that doctors will be graded on a curve; some will lose a percentage of Medicare reimbursements, while others will gain increased payments. A prerequisite for any bonus will be participation in the Physician Quality Reporting System (PQRS), which carries its own nonparticipation penalties. The PQRS forms the core of the program, with a beefed-up Physician Compare website and the Physician Feedback Program providing public and private report cards, respectively. The latter will consist of confidential Quality and Resource Use Reports (QRURs), based on information from Medicare claims and the PQRS.
Who: At first, the VBPM program will apply to groups of 100 or more eligible professionals, currently defined as physicians, practitioners, and therapists. By 2017, however, all physicians will participate. Those in rural practices, critical-access hospitals, and federally qualified health centers are exempt; ACO participants are exempt for 2015 and 2016.
What: The program, authorized by 2010’s Affordable Care Act, extends CMS’ concept of value-based purchasing to the individual physician level. That means a portion of doctors’ Medicare reimbursements will be contingent on scores designed to measure the quality and efficiency of the healthcare they deliver.
How: The program is designed to be budget-neutral, meaning that doctors will be graded on a curve; some will lose a percentage of Medicare reimbursements, while others will gain increased payments. A prerequisite for any bonus will be participation in the Physician Quality Reporting System (PQRS), which carries its own nonparticipation penalties. The PQRS forms the core of the program, with a beefed-up Physician Compare website and the Physician Feedback Program providing public and private report cards, respectively. The latter will consist of confidential Quality and Resource Use Reports (QRURs), based on information from Medicare claims and the PQRS.
Who: At first, the VBPM program will apply to groups of 100 or more eligible professionals, currently defined as physicians, practitioners, and therapists. By 2017, however, all physicians will participate. Those in rural practices, critical-access hospitals, and federally qualified health centers are exempt; ACO participants are exempt for 2015 and 2016.
What: The program, authorized by 2010’s Affordable Care Act, extends CMS’ concept of value-based purchasing to the individual physician level. That means a portion of doctors’ Medicare reimbursements will be contingent on scores designed to measure the quality and efficiency of the healthcare they deliver.
How: The program is designed to be budget-neutral, meaning that doctors will be graded on a curve; some will lose a percentage of Medicare reimbursements, while others will gain increased payments. A prerequisite for any bonus will be participation in the Physician Quality Reporting System (PQRS), which carries its own nonparticipation penalties. The PQRS forms the core of the program, with a beefed-up Physician Compare website and the Physician Feedback Program providing public and private report cards, respectively. The latter will consist of confidential Quality and Resource Use Reports (QRURs), based on information from Medicare claims and the PQRS.
Who: At first, the VBPM program will apply to groups of 100 or more eligible professionals, currently defined as physicians, practitioners, and therapists. By 2017, however, all physicians will participate. Those in rural practices, critical-access hospitals, and federally qualified health centers are exempt; ACO participants are exempt for 2015 and 2016.
Paying Attention to Detail Critical in Medical Coding
Documentation demands attention to detail. For a patient with abdominal pain, be sure to ask: How long has the patient experienced pain? Is it generalized or in a particular quadrant? Sharp or dull? And does it radiate? And jot down the answers.
“Try to use adjectives that would give specifics regarding the complaint,” says Mary Mulholland, MHA, BSN, RN, CPC, senior coding and education specialist at the University of Pennsylvania’s department of medicine. She also suggests hospitalists find out which medications a patient is taking or has taken and indicate whether symptoms have improved or deteriorated. Here’s how Mulholland would document such a case:
Initial hospital admission, level of service:
Code 99223
83-year-old male admitted from the emergency room, complaining of intermittent crampy lower abdominal pain (severe at times), blood in stool and increased weakness for three weeks, worse when getting up or standing. Patient has decreased appetite and progressive shortness of breath. His review of systems is otherwise negative.
Past medical history: Coronary artery disease and hypertension
Family history: Mother with Type 2 diabetes
Social history: Quit smoking 20 years ago
Alert: Blood pressure (90/68), pulse (88), and respiratory (24)
Eyes: Non-icteric
ENT: Dry oral mucosa
Lymphatic: Palpable nodes—right auxilla and right inguinal areas
Lungs: Clear
Cardio: Slight tachycardia, no murmurs, rubs or gallops
Abdomen: Slightly distended, tender on palpation
Skin: Slightly diaphoretic, no rashes or bruising
Neurologic: Cranial nerves intact, alert and conversant
Psychiatric: Anxious
Lab results: Blood in stool, hemoglobin (6.7), serum blood glucose (120), serum sodium (132), serum potassium (4.3), chest X-ray clear (my interpretation). Old records requested.
Assessment: Gastrointestional bleeding, tachycardia, and mild dehydration
Treatment plan: Check hemoglobin and hematocrit every six hours. Also check prothrombin time and partial prothrombin time. Repeat electrolytes in the morning. Order an X-ray of the lower gastrointestinal tract. Type and screen for 2 units of packed red blood cells, and transfuse pending repeat hemoglobin and hematocrit values. Infuse intravenous fluids at 80 cc per minute. Check electrocardiogram. Consult GI regarding endoscopy.
—Susan Kreimer
Documentation demands attention to detail. For a patient with abdominal pain, be sure to ask: How long has the patient experienced pain? Is it generalized or in a particular quadrant? Sharp or dull? And does it radiate? And jot down the answers.
“Try to use adjectives that would give specifics regarding the complaint,” says Mary Mulholland, MHA, BSN, RN, CPC, senior coding and education specialist at the University of Pennsylvania’s department of medicine. She also suggests hospitalists find out which medications a patient is taking or has taken and indicate whether symptoms have improved or deteriorated. Here’s how Mulholland would document such a case:
Initial hospital admission, level of service:
Code 99223
83-year-old male admitted from the emergency room, complaining of intermittent crampy lower abdominal pain (severe at times), blood in stool and increased weakness for three weeks, worse when getting up or standing. Patient has decreased appetite and progressive shortness of breath. His review of systems is otherwise negative.
Past medical history: Coronary artery disease and hypertension
Family history: Mother with Type 2 diabetes
Social history: Quit smoking 20 years ago
Alert: Blood pressure (90/68), pulse (88), and respiratory (24)
Eyes: Non-icteric
ENT: Dry oral mucosa
Lymphatic: Palpable nodes—right auxilla and right inguinal areas
Lungs: Clear
Cardio: Slight tachycardia, no murmurs, rubs or gallops
Abdomen: Slightly distended, tender on palpation
Skin: Slightly diaphoretic, no rashes or bruising
Neurologic: Cranial nerves intact, alert and conversant
Psychiatric: Anxious
Lab results: Blood in stool, hemoglobin (6.7), serum blood glucose (120), serum sodium (132), serum potassium (4.3), chest X-ray clear (my interpretation). Old records requested.
Assessment: Gastrointestional bleeding, tachycardia, and mild dehydration
Treatment plan: Check hemoglobin and hematocrit every six hours. Also check prothrombin time and partial prothrombin time. Repeat electrolytes in the morning. Order an X-ray of the lower gastrointestinal tract. Type and screen for 2 units of packed red blood cells, and transfuse pending repeat hemoglobin and hematocrit values. Infuse intravenous fluids at 80 cc per minute. Check electrocardiogram. Consult GI regarding endoscopy.
—Susan Kreimer
Documentation demands attention to detail. For a patient with abdominal pain, be sure to ask: How long has the patient experienced pain? Is it generalized or in a particular quadrant? Sharp or dull? And does it radiate? And jot down the answers.
“Try to use adjectives that would give specifics regarding the complaint,” says Mary Mulholland, MHA, BSN, RN, CPC, senior coding and education specialist at the University of Pennsylvania’s department of medicine. She also suggests hospitalists find out which medications a patient is taking or has taken and indicate whether symptoms have improved or deteriorated. Here’s how Mulholland would document such a case:
Initial hospital admission, level of service:
Code 99223
83-year-old male admitted from the emergency room, complaining of intermittent crampy lower abdominal pain (severe at times), blood in stool and increased weakness for three weeks, worse when getting up or standing. Patient has decreased appetite and progressive shortness of breath. His review of systems is otherwise negative.
Past medical history: Coronary artery disease and hypertension
Family history: Mother with Type 2 diabetes
Social history: Quit smoking 20 years ago
Alert: Blood pressure (90/68), pulse (88), and respiratory (24)
Eyes: Non-icteric
ENT: Dry oral mucosa
Lymphatic: Palpable nodes—right auxilla and right inguinal areas
Lungs: Clear
Cardio: Slight tachycardia, no murmurs, rubs or gallops
Abdomen: Slightly distended, tender on palpation
Skin: Slightly diaphoretic, no rashes or bruising
Neurologic: Cranial nerves intact, alert and conversant
Psychiatric: Anxious
Lab results: Blood in stool, hemoglobin (6.7), serum blood glucose (120), serum sodium (132), serum potassium (4.3), chest X-ray clear (my interpretation). Old records requested.
Assessment: Gastrointestional bleeding, tachycardia, and mild dehydration
Treatment plan: Check hemoglobin and hematocrit every six hours. Also check prothrombin time and partial prothrombin time. Repeat electrolytes in the morning. Order an X-ray of the lower gastrointestinal tract. Type and screen for 2 units of packed red blood cells, and transfuse pending repeat hemoglobin and hematocrit values. Infuse intravenous fluids at 80 cc per minute. Check electrocardiogram. Consult GI regarding endoscopy.
—Susan Kreimer
2013: The Year of Quality Improvement
Years ago, hospitalists were introduced as new, efficient options for staffing hospitals. Today, they are known as the quarterbacks for patient care and quality improvement (QI) within the hospital.
This year, hospitalists everywhere can help their hospitals resolve to make 2013 a landmark year for QI. And making time for quality improvement need not be a major investment, nor do you have to “reinvent the wheel” and create your own programs.
SHM’s full menu of QI options gives hospitalists and other caregivers the confidence that their program is field-tested as well as the ability to choose the level of involvement that’s right for them.
For more information, visit www.hospitalmedicine.org/qi.
Years ago, hospitalists were introduced as new, efficient options for staffing hospitals. Today, they are known as the quarterbacks for patient care and quality improvement (QI) within the hospital.
This year, hospitalists everywhere can help their hospitals resolve to make 2013 a landmark year for QI. And making time for quality improvement need not be a major investment, nor do you have to “reinvent the wheel” and create your own programs.
SHM’s full menu of QI options gives hospitalists and other caregivers the confidence that their program is field-tested as well as the ability to choose the level of involvement that’s right for them.
For more information, visit www.hospitalmedicine.org/qi.
Years ago, hospitalists were introduced as new, efficient options for staffing hospitals. Today, they are known as the quarterbacks for patient care and quality improvement (QI) within the hospital.
This year, hospitalists everywhere can help their hospitals resolve to make 2013 a landmark year for QI. And making time for quality improvement need not be a major investment, nor do you have to “reinvent the wheel” and create your own programs.
SHM’s full menu of QI options gives hospitalists and other caregivers the confidence that their program is field-tested as well as the ability to choose the level of involvement that’s right for them.
For more information, visit www.hospitalmedicine.org/qi.
Fellow in Hospital Medicine Spotlight: Amir Jaffer, MD, SFHM
Undergraduate education: Boston University, College of Liberal Arts.
Medical school: Boston University School of Medicine.
Notable: Dr. Jaffer is a pioneer in demonstrating how hospitalists can perform preoperative evaluations. He was the medical director of the IMPACT (Internal Medicine Preoperative Assessment, Consultation, and Treatment) Center at Cleveland Clinic. He is an editor of the recently published “Perioperative Medicine: Medical Consultation and Co-Management”, the first comprehensive reference text focused on perioperative medicine created specifically for hospitalists. Dr. Jaffer’s focus is on perioperative medicine, and he is passionate about anticoagulation and thrombosis; he has served on the panel of the 8th and 9th edition of the American College of Chest Physician’s Antithrombotic Guidelines. In 2010, he received the SHM Award of Excellence in Teaching and was HM10 course director.
FYI: Dr. Jaffer loves to travel with his family. In recent years he’s visited Spain, Australia, and Turkey. He has become more health-conscious and works out four to five times a week.
Quotable: “Being a senior fellow means being a role model for other leaders, hospitalists, residents, and students, and highlighting for them how one can be a leader in education, teamwork, quality, and systems improvement.”
Undergraduate education: Boston University, College of Liberal Arts.
Medical school: Boston University School of Medicine.
Notable: Dr. Jaffer is a pioneer in demonstrating how hospitalists can perform preoperative evaluations. He was the medical director of the IMPACT (Internal Medicine Preoperative Assessment, Consultation, and Treatment) Center at Cleveland Clinic. He is an editor of the recently published “Perioperative Medicine: Medical Consultation and Co-Management”, the first comprehensive reference text focused on perioperative medicine created specifically for hospitalists. Dr. Jaffer’s focus is on perioperative medicine, and he is passionate about anticoagulation and thrombosis; he has served on the panel of the 8th and 9th edition of the American College of Chest Physician’s Antithrombotic Guidelines. In 2010, he received the SHM Award of Excellence in Teaching and was HM10 course director.
FYI: Dr. Jaffer loves to travel with his family. In recent years he’s visited Spain, Australia, and Turkey. He has become more health-conscious and works out four to five times a week.
Quotable: “Being a senior fellow means being a role model for other leaders, hospitalists, residents, and students, and highlighting for them how one can be a leader in education, teamwork, quality, and systems improvement.”
Undergraduate education: Boston University, College of Liberal Arts.
Medical school: Boston University School of Medicine.
Notable: Dr. Jaffer is a pioneer in demonstrating how hospitalists can perform preoperative evaluations. He was the medical director of the IMPACT (Internal Medicine Preoperative Assessment, Consultation, and Treatment) Center at Cleveland Clinic. He is an editor of the recently published “Perioperative Medicine: Medical Consultation and Co-Management”, the first comprehensive reference text focused on perioperative medicine created specifically for hospitalists. Dr. Jaffer’s focus is on perioperative medicine, and he is passionate about anticoagulation and thrombosis; he has served on the panel of the 8th and 9th edition of the American College of Chest Physician’s Antithrombotic Guidelines. In 2010, he received the SHM Award of Excellence in Teaching and was HM10 course director.
FYI: Dr. Jaffer loves to travel with his family. In recent years he’s visited Spain, Australia, and Turkey. He has become more health-conscious and works out four to five times a week.
Quotable: “Being a senior fellow means being a role model for other leaders, hospitalists, residents, and students, and highlighting for them how one can be a leader in education, teamwork, quality, and systems improvement.”
Society for Hospital Medicine Compiles List of Don'ts for Hospitalists
In hospital medicine, what a hospitalist doesn’t do can be just as important as what he or she does do.
That’s why SHM and hospitalist experts from across the country collaborated with the American Board of Internal Medicine Foundation on its groundbreaking Choosing Wisely campaign to publish 10 procedures that hospitalists should think twice about before conducting. Together, with more than a dozen medical specialties, SHM will announce the list of procedures in Washington, D.C., on Feb. 21.
Of the medical specialties contributing lists to Choosing Wisely, SHM is unique in that it will publish two lists (each with five recommendations): one for adult HM and another for pediatric HM.
Once the recommendations have been made public, hospitalists will have multiple ways of learning about them. SHM will publish the recommendations online, via email, and in The Hospitalist. Details about the unique process of developing the Choosing Wisely lists—and the impact they will have on everyday hospitalist practice—will be published in the Journal of Hospital Medicine.
Others in healthcare, including patients and family members, will have a chance to learn about Choosing Wisely through a partnership with Consumer Reports and the public dialogue that the campaign hopes to generate.
SHM President Shaun Frost, MD, SFHM, has been unequivocal in his support for the campaign and has urged all hospitalists to support it as well. “Attention to care affordability and experience are essential to reforming our broken healthcare system, so let’s lead the charge in these areas and help others who are doing the same,” Dr. Frost wrote in the November 2012 issue of The Hospitalist.
To get more involved with this industry-changing campaign, visit www.choosingwisely.org and check out the upcoming Choosing Wisely pre-course at SHM’s annual meeting at www.hospitalmedicine2013.org.
In hospital medicine, what a hospitalist doesn’t do can be just as important as what he or she does do.
That’s why SHM and hospitalist experts from across the country collaborated with the American Board of Internal Medicine Foundation on its groundbreaking Choosing Wisely campaign to publish 10 procedures that hospitalists should think twice about before conducting. Together, with more than a dozen medical specialties, SHM will announce the list of procedures in Washington, D.C., on Feb. 21.
Of the medical specialties contributing lists to Choosing Wisely, SHM is unique in that it will publish two lists (each with five recommendations): one for adult HM and another for pediatric HM.
Once the recommendations have been made public, hospitalists will have multiple ways of learning about them. SHM will publish the recommendations online, via email, and in The Hospitalist. Details about the unique process of developing the Choosing Wisely lists—and the impact they will have on everyday hospitalist practice—will be published in the Journal of Hospital Medicine.
Others in healthcare, including patients and family members, will have a chance to learn about Choosing Wisely through a partnership with Consumer Reports and the public dialogue that the campaign hopes to generate.
SHM President Shaun Frost, MD, SFHM, has been unequivocal in his support for the campaign and has urged all hospitalists to support it as well. “Attention to care affordability and experience are essential to reforming our broken healthcare system, so let’s lead the charge in these areas and help others who are doing the same,” Dr. Frost wrote in the November 2012 issue of The Hospitalist.
To get more involved with this industry-changing campaign, visit www.choosingwisely.org and check out the upcoming Choosing Wisely pre-course at SHM’s annual meeting at www.hospitalmedicine2013.org.
In hospital medicine, what a hospitalist doesn’t do can be just as important as what he or she does do.
That’s why SHM and hospitalist experts from across the country collaborated with the American Board of Internal Medicine Foundation on its groundbreaking Choosing Wisely campaign to publish 10 procedures that hospitalists should think twice about before conducting. Together, with more than a dozen medical specialties, SHM will announce the list of procedures in Washington, D.C., on Feb. 21.
Of the medical specialties contributing lists to Choosing Wisely, SHM is unique in that it will publish two lists (each with five recommendations): one for adult HM and another for pediatric HM.
Once the recommendations have been made public, hospitalists will have multiple ways of learning about them. SHM will publish the recommendations online, via email, and in The Hospitalist. Details about the unique process of developing the Choosing Wisely lists—and the impact they will have on everyday hospitalist practice—will be published in the Journal of Hospital Medicine.
Others in healthcare, including patients and family members, will have a chance to learn about Choosing Wisely through a partnership with Consumer Reports and the public dialogue that the campaign hopes to generate.
SHM President Shaun Frost, MD, SFHM, has been unequivocal in his support for the campaign and has urged all hospitalists to support it as well. “Attention to care affordability and experience are essential to reforming our broken healthcare system, so let’s lead the charge in these areas and help others who are doing the same,” Dr. Frost wrote in the November 2012 issue of The Hospitalist.
To get more involved with this industry-changing campaign, visit www.choosingwisely.org and check out the upcoming Choosing Wisely pre-course at SHM’s annual meeting at www.hospitalmedicine2013.org.
Hospitalists Encouraged to Join the Future of Hospital Conversation
This month, hospitalists can play a role in crowdsourcing the future of the community hospital. And one hospitalist is helping to shape the game.
On Jan. 8, the Institute for the Future launched its Future of Hospital foresight game (www.futureofhospital.org). The game will tap the wisdom of healthcare experts, urban planners, technologists, sociologists, local community leaders, social entrepreneurs, and citizens interested in engaging in a thoughtful discussion about future possibilities for community hospitals.
Hospitalist Jason Stein, MD, SFHM, is working with the Institute for the Future to help judge the responses and participation in the online, crowd-sourced game. As an advisor to the Centers for Medicare & Medicaid Services Innovation Center, Dr. Stein is no stranger to new ideas for hospitals. He was one of the co-architects of SHM’s Implementation Guides, which create step-wise approaches for system-level change. He also has been recognized nationally for his work redesigning traditional medical wards into accountable-care units, each featuring unit-based physician teams that conduct structured interdisciplinary bedside rounds (SIBRs).
“This is an exciting way for hospitalists to join a dynamic conversation about how hospitals—and their staff—provide the best care possible to their patients in 21st-century, American healthcare,” Dr. Stein says.
The Future of the Hospital is a game that anyone can play; all that is required is ideas. If others build on those ideas, contributors win points and create award-winning chains of innovation, linking people and their ideas from all over the world.
“Hospitalists have been on the cutting edge of some of the best ideas in healthcare for the last two decades,” Dr. Stein says. “This is an exciting and innovative way for all of us to contribute ideas and leverage our collective experience for the good of our hospitals and patients.”
This month, hospitalists can play a role in crowdsourcing the future of the community hospital. And one hospitalist is helping to shape the game.
On Jan. 8, the Institute for the Future launched its Future of Hospital foresight game (www.futureofhospital.org). The game will tap the wisdom of healthcare experts, urban planners, technologists, sociologists, local community leaders, social entrepreneurs, and citizens interested in engaging in a thoughtful discussion about future possibilities for community hospitals.
Hospitalist Jason Stein, MD, SFHM, is working with the Institute for the Future to help judge the responses and participation in the online, crowd-sourced game. As an advisor to the Centers for Medicare & Medicaid Services Innovation Center, Dr. Stein is no stranger to new ideas for hospitals. He was one of the co-architects of SHM’s Implementation Guides, which create step-wise approaches for system-level change. He also has been recognized nationally for his work redesigning traditional medical wards into accountable-care units, each featuring unit-based physician teams that conduct structured interdisciplinary bedside rounds (SIBRs).
“This is an exciting way for hospitalists to join a dynamic conversation about how hospitals—and their staff—provide the best care possible to their patients in 21st-century, American healthcare,” Dr. Stein says.
The Future of the Hospital is a game that anyone can play; all that is required is ideas. If others build on those ideas, contributors win points and create award-winning chains of innovation, linking people and their ideas from all over the world.
“Hospitalists have been on the cutting edge of some of the best ideas in healthcare for the last two decades,” Dr. Stein says. “This is an exciting and innovative way for all of us to contribute ideas and leverage our collective experience for the good of our hospitals and patients.”
This month, hospitalists can play a role in crowdsourcing the future of the community hospital. And one hospitalist is helping to shape the game.
On Jan. 8, the Institute for the Future launched its Future of Hospital foresight game (www.futureofhospital.org). The game will tap the wisdom of healthcare experts, urban planners, technologists, sociologists, local community leaders, social entrepreneurs, and citizens interested in engaging in a thoughtful discussion about future possibilities for community hospitals.
Hospitalist Jason Stein, MD, SFHM, is working with the Institute for the Future to help judge the responses and participation in the online, crowd-sourced game. As an advisor to the Centers for Medicare & Medicaid Services Innovation Center, Dr. Stein is no stranger to new ideas for hospitals. He was one of the co-architects of SHM’s Implementation Guides, which create step-wise approaches for system-level change. He also has been recognized nationally for his work redesigning traditional medical wards into accountable-care units, each featuring unit-based physician teams that conduct structured interdisciplinary bedside rounds (SIBRs).
“This is an exciting way for hospitalists to join a dynamic conversation about how hospitals—and their staff—provide the best care possible to their patients in 21st-century, American healthcare,” Dr. Stein says.
The Future of the Hospital is a game that anyone can play; all that is required is ideas. If others build on those ideas, contributors win points and create award-winning chains of innovation, linking people and their ideas from all over the world.
“Hospitalists have been on the cutting edge of some of the best ideas in healthcare for the last two decades,” Dr. Stein says. “This is an exciting and innovative way for all of us to contribute ideas and leverage our collective experience for the good of our hospitals and patients.”
HMX Term of the Month: Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS)
HCAHPS is a national, standardized survey of hospital patients created to publicly report patients’ perspective of hospital care. The survey asks a random sample of recently discharged patients about important aspects of their hospital experience. The HCAHPS results posted on Medicare’s Hospital Compare website (www.hospitalcompare.hhs.gov) allow consumers to make fair and objective comparisons of hospitals and individual hospitals to state and national benchmarks.
Contributed by HMX user Dr. Patrick Torcson, MD, MMM, FACP, SFHM, chair of SHM’s Performance and Standards Committee.
HCAHPS is a national, standardized survey of hospital patients created to publicly report patients’ perspective of hospital care. The survey asks a random sample of recently discharged patients about important aspects of their hospital experience. The HCAHPS results posted on Medicare’s Hospital Compare website (www.hospitalcompare.hhs.gov) allow consumers to make fair and objective comparisons of hospitals and individual hospitals to state and national benchmarks.
Contributed by HMX user Dr. Patrick Torcson, MD, MMM, FACP, SFHM, chair of SHM’s Performance and Standards Committee.
HCAHPS is a national, standardized survey of hospital patients created to publicly report patients’ perspective of hospital care. The survey asks a random sample of recently discharged patients about important aspects of their hospital experience. The HCAHPS results posted on Medicare’s Hospital Compare website (www.hospitalcompare.hhs.gov) allow consumers to make fair and objective comparisons of hospitals and individual hospitals to state and national benchmarks.
Contributed by HMX user Dr. Patrick Torcson, MD, MMM, FACP, SFHM, chair of SHM’s Performance and Standards Committee.