User login
Firing an Employee Is Never Easy
My recent column on good hiring practices, which stressed the importance of replacing marginal employees with excellent ones, triggered an interesting round of discussion. "Isn’t it true," asked a colleague, "that most physicians tolerate marginal employees because it’s less painful than firing them?"
Indeed it is. Firing someone is never easy, and it is particularly tough on physicians. But sometimes it is unavoidable to preserve the efficiency and morale of yourself and your other employees.
Before you do it, however, be sure that you have legitimate grounds, and assemble as much documentation as possible. Record all terminatable transgressions in the employee’s permanent record, and document all verbal and written warnings. This is essential; you must be prepared to prove that your reasons for termination were legal.
Former employees will sometimes charge that their civil rights were violated. For example, federal law prohibits you from firing anyone because of race, gender, national origin, disability, religion, or age. You cannot fire a woman because she is pregnant, or recently gave birth. Other illegal reasons include assertion of antidiscrimination rights, refusing to take a lie detector test, and reporting OSHA (Occupational Safety and Health Administration) violations.
You also can’t terminate someone for refusing to commit an illegal act, such as filing false insurance claims; or for exercising a legal right, such as voting or participating in a political demonstration.
And you cannot fire an alcohol abuser unless he or she is caught drinking at work; but many forms of illegal drug use are legitimate cause for termination. Other laws may apply, depending on where you live. When in doubt, contact your state labor department or fair employment office.
If a fired employee alleges that he or she was fired for any of these illegal reasons, and you do not have convincing documentation to counter the charge, you may find yourself defending your actions in court.
As I’ve mentioned in the past, consider adding employment practices liability insurance (EPLI) to your umbrella policy, since a wrongful termination lawsuit is always a possibility despite your best efforts to prevent it.
Once you have all your legal ducks in a row, don’t procrastinate. Get it over with, first thing on Monday morning. If you wait until Friday afternoon you will worry about the dreaded task all week long, and the fired employee will stew about it all weekend.
Explain the performance you have expected, the steps you have taken to help correct the problems you have seen, and the fact that the problems persist. Try to limit the conversation to a minute or two, have the final paycheck ready, and make it clear that the decision has already been made, so begging and pleading will not change anything.
I’ve been asked to share exactly what I say; so for what it’s worth, here it is: "I have called you in to discuss a difficult issue. You know that we have not been happy with your performance. We are still not happy with it, despite all the discussions we have had, and we feel that you can do better elsewhere. So today we will part company, and I wish you the best of luck in your future endeavors. Here is your severance check. I hope there are no hard feelings."
There will, of course be hard feelings, but that cannot be helped. The point is to be quick, firm, and decisive. Get it over with and allow everyone to move on.
Be sure to get all your office keys back – or change the locks if you cannot. Back up all important computer files, and change all your passwords. Most employees know more of them than you would ever suspect.
Finally, call the staff together and explain what you have done. They should hear the story from you, not a distorted version through the rumor mill. You don’t have to explain your reasoning or divulge every detail, but do explain how the termination will affect everyone else. Responsibilities will need to be shifted until a replacement can be hired, and all employees should understand that.
If you are asked in the future to give a reference or write a letter of recommendation for the terminated employee, be sure that everything you say is truthful and well documented.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J.
My recent column on good hiring practices, which stressed the importance of replacing marginal employees with excellent ones, triggered an interesting round of discussion. "Isn’t it true," asked a colleague, "that most physicians tolerate marginal employees because it’s less painful than firing them?"
Indeed it is. Firing someone is never easy, and it is particularly tough on physicians. But sometimes it is unavoidable to preserve the efficiency and morale of yourself and your other employees.
Before you do it, however, be sure that you have legitimate grounds, and assemble as much documentation as possible. Record all terminatable transgressions in the employee’s permanent record, and document all verbal and written warnings. This is essential; you must be prepared to prove that your reasons for termination were legal.
Former employees will sometimes charge that their civil rights were violated. For example, federal law prohibits you from firing anyone because of race, gender, national origin, disability, religion, or age. You cannot fire a woman because she is pregnant, or recently gave birth. Other illegal reasons include assertion of antidiscrimination rights, refusing to take a lie detector test, and reporting OSHA (Occupational Safety and Health Administration) violations.
You also can’t terminate someone for refusing to commit an illegal act, such as filing false insurance claims; or for exercising a legal right, such as voting or participating in a political demonstration.
And you cannot fire an alcohol abuser unless he or she is caught drinking at work; but many forms of illegal drug use are legitimate cause for termination. Other laws may apply, depending on where you live. When in doubt, contact your state labor department or fair employment office.
If a fired employee alleges that he or she was fired for any of these illegal reasons, and you do not have convincing documentation to counter the charge, you may find yourself defending your actions in court.
As I’ve mentioned in the past, consider adding employment practices liability insurance (EPLI) to your umbrella policy, since a wrongful termination lawsuit is always a possibility despite your best efforts to prevent it.
Once you have all your legal ducks in a row, don’t procrastinate. Get it over with, first thing on Monday morning. If you wait until Friday afternoon you will worry about the dreaded task all week long, and the fired employee will stew about it all weekend.
Explain the performance you have expected, the steps you have taken to help correct the problems you have seen, and the fact that the problems persist. Try to limit the conversation to a minute or two, have the final paycheck ready, and make it clear that the decision has already been made, so begging and pleading will not change anything.
I’ve been asked to share exactly what I say; so for what it’s worth, here it is: "I have called you in to discuss a difficult issue. You know that we have not been happy with your performance. We are still not happy with it, despite all the discussions we have had, and we feel that you can do better elsewhere. So today we will part company, and I wish you the best of luck in your future endeavors. Here is your severance check. I hope there are no hard feelings."
There will, of course be hard feelings, but that cannot be helped. The point is to be quick, firm, and decisive. Get it over with and allow everyone to move on.
Be sure to get all your office keys back – or change the locks if you cannot. Back up all important computer files, and change all your passwords. Most employees know more of them than you would ever suspect.
Finally, call the staff together and explain what you have done. They should hear the story from you, not a distorted version through the rumor mill. You don’t have to explain your reasoning or divulge every detail, but do explain how the termination will affect everyone else. Responsibilities will need to be shifted until a replacement can be hired, and all employees should understand that.
If you are asked in the future to give a reference or write a letter of recommendation for the terminated employee, be sure that everything you say is truthful and well documented.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J.
My recent column on good hiring practices, which stressed the importance of replacing marginal employees with excellent ones, triggered an interesting round of discussion. "Isn’t it true," asked a colleague, "that most physicians tolerate marginal employees because it’s less painful than firing them?"
Indeed it is. Firing someone is never easy, and it is particularly tough on physicians. But sometimes it is unavoidable to preserve the efficiency and morale of yourself and your other employees.
Before you do it, however, be sure that you have legitimate grounds, and assemble as much documentation as possible. Record all terminatable transgressions in the employee’s permanent record, and document all verbal and written warnings. This is essential; you must be prepared to prove that your reasons for termination were legal.
Former employees will sometimes charge that their civil rights were violated. For example, federal law prohibits you from firing anyone because of race, gender, national origin, disability, religion, or age. You cannot fire a woman because she is pregnant, or recently gave birth. Other illegal reasons include assertion of antidiscrimination rights, refusing to take a lie detector test, and reporting OSHA (Occupational Safety and Health Administration) violations.
You also can’t terminate someone for refusing to commit an illegal act, such as filing false insurance claims; or for exercising a legal right, such as voting or participating in a political demonstration.
And you cannot fire an alcohol abuser unless he or she is caught drinking at work; but many forms of illegal drug use are legitimate cause for termination. Other laws may apply, depending on where you live. When in doubt, contact your state labor department or fair employment office.
If a fired employee alleges that he or she was fired for any of these illegal reasons, and you do not have convincing documentation to counter the charge, you may find yourself defending your actions in court.
As I’ve mentioned in the past, consider adding employment practices liability insurance (EPLI) to your umbrella policy, since a wrongful termination lawsuit is always a possibility despite your best efforts to prevent it.
Once you have all your legal ducks in a row, don’t procrastinate. Get it over with, first thing on Monday morning. If you wait until Friday afternoon you will worry about the dreaded task all week long, and the fired employee will stew about it all weekend.
Explain the performance you have expected, the steps you have taken to help correct the problems you have seen, and the fact that the problems persist. Try to limit the conversation to a minute or two, have the final paycheck ready, and make it clear that the decision has already been made, so begging and pleading will not change anything.
I’ve been asked to share exactly what I say; so for what it’s worth, here it is: "I have called you in to discuss a difficult issue. You know that we have not been happy with your performance. We are still not happy with it, despite all the discussions we have had, and we feel that you can do better elsewhere. So today we will part company, and I wish you the best of luck in your future endeavors. Here is your severance check. I hope there are no hard feelings."
There will, of course be hard feelings, but that cannot be helped. The point is to be quick, firm, and decisive. Get it over with and allow everyone to move on.
Be sure to get all your office keys back – or change the locks if you cannot. Back up all important computer files, and change all your passwords. Most employees know more of them than you would ever suspect.
Finally, call the staff together and explain what you have done. They should hear the story from you, not a distorted version through the rumor mill. You don’t have to explain your reasoning or divulge every detail, but do explain how the termination will affect everyone else. Responsibilities will need to be shifted until a replacement can be hired, and all employees should understand that.
If you are asked in the future to give a reference or write a letter of recommendation for the terminated employee, be sure that everything you say is truthful and well documented.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J.
Tofacitinib Lowered Disease Activity in Ulcerative Colitis
Tofacitinib was associated with greater clinical response and remission rates than was placebo in a double-blind, phase II trial of patients with moderately or severely active ulcerative colitis.
Current treatment of ulcerative colitis with mesalamine, glucocorticoids, azathioprine, and anti–tumor necrosis factor (anti-TNF) agents such as infliximab and adalimumab is not always effective, and may be associated with serious toxic effects (Gastroenterology 2006;130:940-87). Thus, "additional treatments are needed," said Dr. William J. Sandborn, chief of the division of gastroenterology and professor of medicine at the University of California, San Diego and his associates (N. Engl. J. Med. 2012;367:616-24).
Tofacitinib is a selective oral inhibitor of the Janus kinase (JAK) family of enzymes that includes JAK1 and JAK3, which mediate signal-transduction activity for multiple cytokines, including several that are integral to lymphocyte activation, function, and proliferation. Although the importance of the JAK family in the pathogenesis of ulcerative colitis is unclear, tofacitinib has shown efficacy against other immune-mediated conditions, including organ allograft rejection, rheumatoid arthritis, and psoriasis.
The 8-week trial was conducted at 51 centers in 17 countries from January 2009 through September 2010 with funding from Pfizer. Subjects had to be at least 18 years of age, with a confirmed diagnosis of ulcerative colitis of 3 months’ duration or longer, a score of 6-12 on the Mayo risk calculator for ulcerative colitis,and evidence on sigmoidoscopic examination of moderately or severely active disease. In most of the patients, the use of conventional therapy (mesalamine, glucocorticoids, immunosuppressants, or anti-TNF agents used as monotherapy or in some combination) had failed, according to the investigators.
During the study, patients could receive oral mesalamine or oral prednisone at a stable dosage of 30 mg or less per day. However, patients receiving azathioprine, 6-mercaptopurine, and methotrexate discontinued them immediately before initiating therapy with tofacitinib, and patients who had previously received anti-TNF therapy were required to discontinue it for at least 8 weeks before study entry.
Patients were randomly assigned to receive twice-daily oral tofacitinib doses of 0.5 mg, 3 mg, 10 mg, or 15 mg or placebo for 8 weeks, and were then followed for 4 weeks through 12 weeks. Of the 195 patients randomized, 194 received at least one dose of the study drug or placebo and 157 completed the full 8 weeks of treatment. Across all treatment groups, 131 patients (67.5%) received concomitant aminosalicylates, and 85 (43.8%) received concomitant glucocorticoids at some point during the study, they noted.
Clinical response was defined as a decrease from baseline in the total Mayo score (defined as an absolute decrease by at least 3 points and a relative decrease by at least 30%) with an accompanying decrease in the rectal bleeding subscore of at least 1 point or an absolute rectal bleeding subscore of 0 or 1.
The primary end point, clinical response at 8 weeks, occurred in 42% (20) of the patients who received placebo. The response was significantly greater than that seen only among the patients who received the highest doses of tofacitinib, 78% of those receiving 15 mg (P less than .001). For the lower doses, the clinical response rates were not significantly different from those with placebo (61%, 48% and 32%, respectively, for 10 mg, 3 mg and 0.5 mg).
Clinical remission, a secondary end point, was defined as a total Mayo score of 0-2, with no individual subscore exceeding 1. Clinical remission at 8 weeks occurred in 10% (5) of the placebo patients. Here, the difference from placebo with tofacitinib was significant for doses of 3 mg (33%; P = .01), 10 mg (48%; P less than .001), and 15 mg (41%; P less than .001).
Another secondary end point, endoscopic response, was defined as a decrease from baseline in the endoscopy subscore by at least 1.The secondary end point of endoscopic remission was defined as an endoscopy subscore of 0.
An endoscopic response at 8 weeks occurred in 46% with placebo, significantly lower than the responses with tofacitinib doses of 10 mg (67%; P = .07) and 15mg (78%; P = .001). Endoscopic remission at 8 weeks occurred in just 2% of the placebo group, vs. significantly greater proportions of 18% with 3 mg (P = .01), 30% with 10 mg (P less than .001), and 27% with 15 mg (P less than .001).
Rates of overall and serious adverse events, as well as adverse events from infection, were similar among the groups. Two patients receiving 10 mg of tofacitinib twice daily had serious adverse events from infection (a postoperative abscess in one and anal abscess in the other). There was a dose-dependent increase in both LDL and HDL cholesterol concentrations at 8 weeks with tofacitinib, which reversed after discontinuation of the study drug . During the study period, the absolute neutrophil count was less than 1,500 cells per cubic millimeter in three patients receiving tofacitinib, but was not less than 1,000 cells per cubic millimeter in any patient, Dr. Sandborn and his associates reported.
In previous studies of patients with rheumatoid arthritis, tofacitinib has been associated with increases in LDL cholesterol and serum creatinine, and decreases in absolute neutrophil count, whereas increased infection risk has been seen with the 15-mg, twice-daily dose. The small size and short duration of the current trial did not allow for a comprehensive assessment of the safety and tolerability of the drug in patients with ulcerative colitis, they noted.
This study was funded by Pfizer, from whom Dr. Sandborn has received grants and consulting fees. He has also served as a consultant and has additional ties to numerous other companies.
Tofacitinib was associated with greater clinical response and remission rates than was placebo in a double-blind, phase II trial of patients with moderately or severely active ulcerative colitis.
Current treatment of ulcerative colitis with mesalamine, glucocorticoids, azathioprine, and anti–tumor necrosis factor (anti-TNF) agents such as infliximab and adalimumab is not always effective, and may be associated with serious toxic effects (Gastroenterology 2006;130:940-87). Thus, "additional treatments are needed," said Dr. William J. Sandborn, chief of the division of gastroenterology and professor of medicine at the University of California, San Diego and his associates (N. Engl. J. Med. 2012;367:616-24).
Tofacitinib is a selective oral inhibitor of the Janus kinase (JAK) family of enzymes that includes JAK1 and JAK3, which mediate signal-transduction activity for multiple cytokines, including several that are integral to lymphocyte activation, function, and proliferation. Although the importance of the JAK family in the pathogenesis of ulcerative colitis is unclear, tofacitinib has shown efficacy against other immune-mediated conditions, including organ allograft rejection, rheumatoid arthritis, and psoriasis.
The 8-week trial was conducted at 51 centers in 17 countries from January 2009 through September 2010 with funding from Pfizer. Subjects had to be at least 18 years of age, with a confirmed diagnosis of ulcerative colitis of 3 months’ duration or longer, a score of 6-12 on the Mayo risk calculator for ulcerative colitis,and evidence on sigmoidoscopic examination of moderately or severely active disease. In most of the patients, the use of conventional therapy (mesalamine, glucocorticoids, immunosuppressants, or anti-TNF agents used as monotherapy or in some combination) had failed, according to the investigators.
During the study, patients could receive oral mesalamine or oral prednisone at a stable dosage of 30 mg or less per day. However, patients receiving azathioprine, 6-mercaptopurine, and methotrexate discontinued them immediately before initiating therapy with tofacitinib, and patients who had previously received anti-TNF therapy were required to discontinue it for at least 8 weeks before study entry.
Patients were randomly assigned to receive twice-daily oral tofacitinib doses of 0.5 mg, 3 mg, 10 mg, or 15 mg or placebo for 8 weeks, and were then followed for 4 weeks through 12 weeks. Of the 195 patients randomized, 194 received at least one dose of the study drug or placebo and 157 completed the full 8 weeks of treatment. Across all treatment groups, 131 patients (67.5%) received concomitant aminosalicylates, and 85 (43.8%) received concomitant glucocorticoids at some point during the study, they noted.
Clinical response was defined as a decrease from baseline in the total Mayo score (defined as an absolute decrease by at least 3 points and a relative decrease by at least 30%) with an accompanying decrease in the rectal bleeding subscore of at least 1 point or an absolute rectal bleeding subscore of 0 or 1.
The primary end point, clinical response at 8 weeks, occurred in 42% (20) of the patients who received placebo. The response was significantly greater than that seen only among the patients who received the highest doses of tofacitinib, 78% of those receiving 15 mg (P less than .001). For the lower doses, the clinical response rates were not significantly different from those with placebo (61%, 48% and 32%, respectively, for 10 mg, 3 mg and 0.5 mg).
Clinical remission, a secondary end point, was defined as a total Mayo score of 0-2, with no individual subscore exceeding 1. Clinical remission at 8 weeks occurred in 10% (5) of the placebo patients. Here, the difference from placebo with tofacitinib was significant for doses of 3 mg (33%; P = .01), 10 mg (48%; P less than .001), and 15 mg (41%; P less than .001).
Another secondary end point, endoscopic response, was defined as a decrease from baseline in the endoscopy subscore by at least 1.The secondary end point of endoscopic remission was defined as an endoscopy subscore of 0.
An endoscopic response at 8 weeks occurred in 46% with placebo, significantly lower than the responses with tofacitinib doses of 10 mg (67%; P = .07) and 15mg (78%; P = .001). Endoscopic remission at 8 weeks occurred in just 2% of the placebo group, vs. significantly greater proportions of 18% with 3 mg (P = .01), 30% with 10 mg (P less than .001), and 27% with 15 mg (P less than .001).
Rates of overall and serious adverse events, as well as adverse events from infection, were similar among the groups. Two patients receiving 10 mg of tofacitinib twice daily had serious adverse events from infection (a postoperative abscess in one and anal abscess in the other). There was a dose-dependent increase in both LDL and HDL cholesterol concentrations at 8 weeks with tofacitinib, which reversed after discontinuation of the study drug . During the study period, the absolute neutrophil count was less than 1,500 cells per cubic millimeter in three patients receiving tofacitinib, but was not less than 1,000 cells per cubic millimeter in any patient, Dr. Sandborn and his associates reported.
In previous studies of patients with rheumatoid arthritis, tofacitinib has been associated with increases in LDL cholesterol and serum creatinine, and decreases in absolute neutrophil count, whereas increased infection risk has been seen with the 15-mg, twice-daily dose. The small size and short duration of the current trial did not allow for a comprehensive assessment of the safety and tolerability of the drug in patients with ulcerative colitis, they noted.
This study was funded by Pfizer, from whom Dr. Sandborn has received grants and consulting fees. He has also served as a consultant and has additional ties to numerous other companies.
Tofacitinib was associated with greater clinical response and remission rates than was placebo in a double-blind, phase II trial of patients with moderately or severely active ulcerative colitis.
Current treatment of ulcerative colitis with mesalamine, glucocorticoids, azathioprine, and anti–tumor necrosis factor (anti-TNF) agents such as infliximab and adalimumab is not always effective, and may be associated with serious toxic effects (Gastroenterology 2006;130:940-87). Thus, "additional treatments are needed," said Dr. William J. Sandborn, chief of the division of gastroenterology and professor of medicine at the University of California, San Diego and his associates (N. Engl. J. Med. 2012;367:616-24).
Tofacitinib is a selective oral inhibitor of the Janus kinase (JAK) family of enzymes that includes JAK1 and JAK3, which mediate signal-transduction activity for multiple cytokines, including several that are integral to lymphocyte activation, function, and proliferation. Although the importance of the JAK family in the pathogenesis of ulcerative colitis is unclear, tofacitinib has shown efficacy against other immune-mediated conditions, including organ allograft rejection, rheumatoid arthritis, and psoriasis.
The 8-week trial was conducted at 51 centers in 17 countries from January 2009 through September 2010 with funding from Pfizer. Subjects had to be at least 18 years of age, with a confirmed diagnosis of ulcerative colitis of 3 months’ duration or longer, a score of 6-12 on the Mayo risk calculator for ulcerative colitis,and evidence on sigmoidoscopic examination of moderately or severely active disease. In most of the patients, the use of conventional therapy (mesalamine, glucocorticoids, immunosuppressants, or anti-TNF agents used as monotherapy or in some combination) had failed, according to the investigators.
During the study, patients could receive oral mesalamine or oral prednisone at a stable dosage of 30 mg or less per day. However, patients receiving azathioprine, 6-mercaptopurine, and methotrexate discontinued them immediately before initiating therapy with tofacitinib, and patients who had previously received anti-TNF therapy were required to discontinue it for at least 8 weeks before study entry.
Patients were randomly assigned to receive twice-daily oral tofacitinib doses of 0.5 mg, 3 mg, 10 mg, or 15 mg or placebo for 8 weeks, and were then followed for 4 weeks through 12 weeks. Of the 195 patients randomized, 194 received at least one dose of the study drug or placebo and 157 completed the full 8 weeks of treatment. Across all treatment groups, 131 patients (67.5%) received concomitant aminosalicylates, and 85 (43.8%) received concomitant glucocorticoids at some point during the study, they noted.
Clinical response was defined as a decrease from baseline in the total Mayo score (defined as an absolute decrease by at least 3 points and a relative decrease by at least 30%) with an accompanying decrease in the rectal bleeding subscore of at least 1 point or an absolute rectal bleeding subscore of 0 or 1.
The primary end point, clinical response at 8 weeks, occurred in 42% (20) of the patients who received placebo. The response was significantly greater than that seen only among the patients who received the highest doses of tofacitinib, 78% of those receiving 15 mg (P less than .001). For the lower doses, the clinical response rates were not significantly different from those with placebo (61%, 48% and 32%, respectively, for 10 mg, 3 mg and 0.5 mg).
Clinical remission, a secondary end point, was defined as a total Mayo score of 0-2, with no individual subscore exceeding 1. Clinical remission at 8 weeks occurred in 10% (5) of the placebo patients. Here, the difference from placebo with tofacitinib was significant for doses of 3 mg (33%; P = .01), 10 mg (48%; P less than .001), and 15 mg (41%; P less than .001).
Another secondary end point, endoscopic response, was defined as a decrease from baseline in the endoscopy subscore by at least 1.The secondary end point of endoscopic remission was defined as an endoscopy subscore of 0.
An endoscopic response at 8 weeks occurred in 46% with placebo, significantly lower than the responses with tofacitinib doses of 10 mg (67%; P = .07) and 15mg (78%; P = .001). Endoscopic remission at 8 weeks occurred in just 2% of the placebo group, vs. significantly greater proportions of 18% with 3 mg (P = .01), 30% with 10 mg (P less than .001), and 27% with 15 mg (P less than .001).
Rates of overall and serious adverse events, as well as adverse events from infection, were similar among the groups. Two patients receiving 10 mg of tofacitinib twice daily had serious adverse events from infection (a postoperative abscess in one and anal abscess in the other). There was a dose-dependent increase in both LDL and HDL cholesterol concentrations at 8 weeks with tofacitinib, which reversed after discontinuation of the study drug . During the study period, the absolute neutrophil count was less than 1,500 cells per cubic millimeter in three patients receiving tofacitinib, but was not less than 1,000 cells per cubic millimeter in any patient, Dr. Sandborn and his associates reported.
In previous studies of patients with rheumatoid arthritis, tofacitinib has been associated with increases in LDL cholesterol and serum creatinine, and decreases in absolute neutrophil count, whereas increased infection risk has been seen with the 15-mg, twice-daily dose. The small size and short duration of the current trial did not allow for a comprehensive assessment of the safety and tolerability of the drug in patients with ulcerative colitis, they noted.
This study was funded by Pfizer, from whom Dr. Sandborn has received grants and consulting fees. He has also served as a consultant and has additional ties to numerous other companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major Finding: The primary end point, clinical response at 8 weeks, occurred in 42% (20) of the patients who received placebo. The response was significantly greater than that among the patients who received the highest doses of tofacitinib, 78% of those receiving 15 mg (P less than .001).
Data Source: The findings come from a randomized, double-blind phase II trial of 194 patients with moderately or severely active ulcerative colitis, most of whom had failed current therapies.
Disclosures: The study was funded by Pfizer, from whom Dr. Sandborn has received grants and consulting fees. He has also served as a consultant and has additional ties to numerous other companies.
FDA Warns About Pediatric Codeine-Related Deaths
The Food and Drug Administration issued a warning on Aug. 15 about the risk of death in children who receive codeine for postoperative pain, particularly after tonsillectomy and/or adenoidectomy, based on three deaths and a nonfatal case of life-threatening respiratory depression.
The three children who died had evidence of being ultrarapid metabolizers of codeine, and the fourth case was in a child with evidence of being an "extensive" metabolizer, the FDA said in a statement announcing the warning. All four children, aged 2-5 years, underwent a tonsillectomy and/or adenoidectomy for treating obstructive sleep apnea syndrome, and received codeine within the typical dose range.
"The FDA is currently conducting a review of adverse event reports and other information to determine if there are additional cases of inadvertent overdose or death in children taking codeine, and if these adverse events occur during treatment of other kinds of pain, such as postoperative pain following other types of surgery or procedures," Dr. Bob Rappaport, director of the Division of Anesthesia, Analgesia, and Addiction Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
For now, the FDA is advising health care professionals and parents to be aware of the risks of codeine in children, "particularly in those who have undergone tonsillectomy and/or adenoidectomy for obstructive sleep apnea syndrome," and – when they prescribe medications that contain codeine to children – to use "the lowest effective dose for the shortest time on an as-needed basis." Parents and caregivers should stop codeine and get immediate medical attention if a child who has been receiving codeine after surgery has any symptoms of an overdose.
CYP2D6 (cytochrome P450 2D6) metabolizes codeine to morphine. Ultrametabolizers of codeine have a genetic variation in this enzyme and are more likely to have abnormally high levels of morphine after taking codeine, and therefore are at greater risk of related adverse events and death.
The four children started to show signs of morphine toxicity within 1-2 days of starting the codeine, and in the three children who died, postmortem morphine levels were "substantially higher" than the normal therapeutic range, according to the FDA. The four cases were also described in Pediatrics (2012;129:e1343-7).
An estimated 1%-7% of the general population are ultrarapid metabolizers, but the rate is much higher in certain ethnic groups, with the highest prevalence (29%) reported for people of Ethiopian descent. The reported prevalence is 6% in the Greek population, 3.4%-6.5% among the African American population, 3.6% in whites, and 1%-2% in northern Europeans.
When the FDA review is completed, the agency plans to provide an update.
The risk of morphine overdose in nursing infants whose mothers are taking codeine and who are ultrarapid metabolizers was recognized several years ago. In 2007, a year after the first case report was described in the Lancet (2006;368:704), the FDA issued a warning about this risk.
The FDA has also posted a consumer update on its website.
The full alert is available, and possible cases should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch.
The Food and Drug Administration issued a warning on Aug. 15 about the risk of death in children who receive codeine for postoperative pain, particularly after tonsillectomy and/or adenoidectomy, based on three deaths and a nonfatal case of life-threatening respiratory depression.
The three children who died had evidence of being ultrarapid metabolizers of codeine, and the fourth case was in a child with evidence of being an "extensive" metabolizer, the FDA said in a statement announcing the warning. All four children, aged 2-5 years, underwent a tonsillectomy and/or adenoidectomy for treating obstructive sleep apnea syndrome, and received codeine within the typical dose range.
"The FDA is currently conducting a review of adverse event reports and other information to determine if there are additional cases of inadvertent overdose or death in children taking codeine, and if these adverse events occur during treatment of other kinds of pain, such as postoperative pain following other types of surgery or procedures," Dr. Bob Rappaport, director of the Division of Anesthesia, Analgesia, and Addiction Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
For now, the FDA is advising health care professionals and parents to be aware of the risks of codeine in children, "particularly in those who have undergone tonsillectomy and/or adenoidectomy for obstructive sleep apnea syndrome," and – when they prescribe medications that contain codeine to children – to use "the lowest effective dose for the shortest time on an as-needed basis." Parents and caregivers should stop codeine and get immediate medical attention if a child who has been receiving codeine after surgery has any symptoms of an overdose.
CYP2D6 (cytochrome P450 2D6) metabolizes codeine to morphine. Ultrametabolizers of codeine have a genetic variation in this enzyme and are more likely to have abnormally high levels of morphine after taking codeine, and therefore are at greater risk of related adverse events and death.
The four children started to show signs of morphine toxicity within 1-2 days of starting the codeine, and in the three children who died, postmortem morphine levels were "substantially higher" than the normal therapeutic range, according to the FDA. The four cases were also described in Pediatrics (2012;129:e1343-7).
An estimated 1%-7% of the general population are ultrarapid metabolizers, but the rate is much higher in certain ethnic groups, with the highest prevalence (29%) reported for people of Ethiopian descent. The reported prevalence is 6% in the Greek population, 3.4%-6.5% among the African American population, 3.6% in whites, and 1%-2% in northern Europeans.
When the FDA review is completed, the agency plans to provide an update.
The risk of morphine overdose in nursing infants whose mothers are taking codeine and who are ultrarapid metabolizers was recognized several years ago. In 2007, a year after the first case report was described in the Lancet (2006;368:704), the FDA issued a warning about this risk.
The FDA has also posted a consumer update on its website.
The full alert is available, and possible cases should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch.
The Food and Drug Administration issued a warning on Aug. 15 about the risk of death in children who receive codeine for postoperative pain, particularly after tonsillectomy and/or adenoidectomy, based on three deaths and a nonfatal case of life-threatening respiratory depression.
The three children who died had evidence of being ultrarapid metabolizers of codeine, and the fourth case was in a child with evidence of being an "extensive" metabolizer, the FDA said in a statement announcing the warning. All four children, aged 2-5 years, underwent a tonsillectomy and/or adenoidectomy for treating obstructive sleep apnea syndrome, and received codeine within the typical dose range.
"The FDA is currently conducting a review of adverse event reports and other information to determine if there are additional cases of inadvertent overdose or death in children taking codeine, and if these adverse events occur during treatment of other kinds of pain, such as postoperative pain following other types of surgery or procedures," Dr. Bob Rappaport, director of the Division of Anesthesia, Analgesia, and Addiction Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
For now, the FDA is advising health care professionals and parents to be aware of the risks of codeine in children, "particularly in those who have undergone tonsillectomy and/or adenoidectomy for obstructive sleep apnea syndrome," and – when they prescribe medications that contain codeine to children – to use "the lowest effective dose for the shortest time on an as-needed basis." Parents and caregivers should stop codeine and get immediate medical attention if a child who has been receiving codeine after surgery has any symptoms of an overdose.
CYP2D6 (cytochrome P450 2D6) metabolizes codeine to morphine. Ultrametabolizers of codeine have a genetic variation in this enzyme and are more likely to have abnormally high levels of morphine after taking codeine, and therefore are at greater risk of related adverse events and death.
The four children started to show signs of morphine toxicity within 1-2 days of starting the codeine, and in the three children who died, postmortem morphine levels were "substantially higher" than the normal therapeutic range, according to the FDA. The four cases were also described in Pediatrics (2012;129:e1343-7).
An estimated 1%-7% of the general population are ultrarapid metabolizers, but the rate is much higher in certain ethnic groups, with the highest prevalence (29%) reported for people of Ethiopian descent. The reported prevalence is 6% in the Greek population, 3.4%-6.5% among the African American population, 3.6% in whites, and 1%-2% in northern Europeans.
When the FDA review is completed, the agency plans to provide an update.
The risk of morphine overdose in nursing infants whose mothers are taking codeine and who are ultrarapid metabolizers was recognized several years ago. In 2007, a year after the first case report was described in the Lancet (2006;368:704), the FDA issued a warning about this risk.
The FDA has also posted a consumer update on its website.
The full alert is available, and possible cases should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch.
BEST PRACTICES IN: Acne Management
Medical Education Library
A Best Practices Supplement to Skin & Allergy News®. This supplement was sponsored by Valeant Dermatology.
- Introduction
- CL/BPO 2.5%
- CL/BPO 2.5% In Clinical Practice
- Conclusion
Faculty/Faculty Disclosure
Julie Harper, MD
Physician/Speaker
The Dermatology and Skin Care Center of Birmingham, PC
Birmingham, AL
Dr Harper received an honorarium for writing this article.
Copyright © 2012 Elsevier Inc.
Medical Education Library
A Best Practices Supplement to Skin & Allergy News®. This supplement was sponsored by Valeant Dermatology.
- Introduction
- CL/BPO 2.5%
- CL/BPO 2.5% In Clinical Practice
- Conclusion
Faculty/Faculty Disclosure
Julie Harper, MD
Physician/Speaker
The Dermatology and Skin Care Center of Birmingham, PC
Birmingham, AL
Dr Harper received an honorarium for writing this article.
Copyright © 2012 Elsevier Inc.
Medical Education Library
A Best Practices Supplement to Skin & Allergy News®. This supplement was sponsored by Valeant Dermatology.
- Introduction
- CL/BPO 2.5%
- CL/BPO 2.5% In Clinical Practice
- Conclusion
Faculty/Faculty Disclosure
Julie Harper, MD
Physician/Speaker
The Dermatology and Skin Care Center of Birmingham, PC
Birmingham, AL
Dr Harper received an honorarium for writing this article.
Copyright © 2012 Elsevier Inc.
Discordant Antibiotic Use in Pediatric UTIs Associated with Higher LOS
Discordant antibiotic therapy for urinary tract infections (UTIs) is common and associated with higher length of stay (LOS) in hospitalized children, according to a study published online last month in the Journal of Hospital Medicine. But lead author Karen Jerardi, MD, division of hospital medicine at Cincinnati Children's Hospital Medical Center says the reason might be related to physicians, not their patients.
"First, use our knowledge of local resistance patterns and patient factors to select an antibiotic likely to be concordant," she says. "The second thing is [that] we probably need to analyze our practice a little bit more and try to figure out if we are just keeping patients in the hospital because we want to see them be on the concordant antibiotic for X number of hours before we send them home. Does that benefit the patient more, or are we keeping them in the hospital longer for our own peace of mind?"
The report, "Discordant Antibiotic Therapy and Length of Stay in Children Hospitalized for Urinary Tract Infection," found that discordant therapy occurred in 10% of cases in which patients had laboratory-confirmed UTIs and, in adjusted analyses, was associated with a 1.8-day increase in LOS.
Dr. Jerardi says that future studies are needed to determine whether pediatric hospitalists are extending LOS by keeping patients longer than absolutely necessary. She cautions, though, that how long a child is kept in the hospital should be determined by case-specific circumstances.
"Hopefully, this will make people analyze how they do things," she adds, "and think to themselves, 'Would I keep that patient an extra day longer because I had to switch their antibiotic—even if their fever went away, they were drinking great, and Mom and Dad were ready to go home—just for my peace of mind?'"
Discordant antibiotic therapy for urinary tract infections (UTIs) is common and associated with higher length of stay (LOS) in hospitalized children, according to a study published online last month in the Journal of Hospital Medicine. But lead author Karen Jerardi, MD, division of hospital medicine at Cincinnati Children's Hospital Medical Center says the reason might be related to physicians, not their patients.
"First, use our knowledge of local resistance patterns and patient factors to select an antibiotic likely to be concordant," she says. "The second thing is [that] we probably need to analyze our practice a little bit more and try to figure out if we are just keeping patients in the hospital because we want to see them be on the concordant antibiotic for X number of hours before we send them home. Does that benefit the patient more, or are we keeping them in the hospital longer for our own peace of mind?"
The report, "Discordant Antibiotic Therapy and Length of Stay in Children Hospitalized for Urinary Tract Infection," found that discordant therapy occurred in 10% of cases in which patients had laboratory-confirmed UTIs and, in adjusted analyses, was associated with a 1.8-day increase in LOS.
Dr. Jerardi says that future studies are needed to determine whether pediatric hospitalists are extending LOS by keeping patients longer than absolutely necessary. She cautions, though, that how long a child is kept in the hospital should be determined by case-specific circumstances.
"Hopefully, this will make people analyze how they do things," she adds, "and think to themselves, 'Would I keep that patient an extra day longer because I had to switch their antibiotic—even if their fever went away, they were drinking great, and Mom and Dad were ready to go home—just for my peace of mind?'"
Discordant antibiotic therapy for urinary tract infections (UTIs) is common and associated with higher length of stay (LOS) in hospitalized children, according to a study published online last month in the Journal of Hospital Medicine. But lead author Karen Jerardi, MD, division of hospital medicine at Cincinnati Children's Hospital Medical Center says the reason might be related to physicians, not their patients.
"First, use our knowledge of local resistance patterns and patient factors to select an antibiotic likely to be concordant," she says. "The second thing is [that] we probably need to analyze our practice a little bit more and try to figure out if we are just keeping patients in the hospital because we want to see them be on the concordant antibiotic for X number of hours before we send them home. Does that benefit the patient more, or are we keeping them in the hospital longer for our own peace of mind?"
The report, "Discordant Antibiotic Therapy and Length of Stay in Children Hospitalized for Urinary Tract Infection," found that discordant therapy occurred in 10% of cases in which patients had laboratory-confirmed UTIs and, in adjusted analyses, was associated with a 1.8-day increase in LOS.
Dr. Jerardi says that future studies are needed to determine whether pediatric hospitalists are extending LOS by keeping patients longer than absolutely necessary. She cautions, though, that how long a child is kept in the hospital should be determined by case-specific circumstances.
"Hopefully, this will make people analyze how they do things," she adds, "and think to themselves, 'Would I keep that patient an extra day longer because I had to switch their antibiotic—even if their fever went away, they were drinking great, and Mom and Dad were ready to go home—just for my peace of mind?'"
AHRQ's Director Looks to Hospitalists to Help Reduce Readmissions
Although a recently released study of Medicare data uncovers little progress in reducing hospital readmissions, and the Oct. 1 deadline to implement CMS’ Hospital Readmissions Reduction Program looms, Carolyn Clancy, MD, director of the Agency for Healthcare Research and Quality (AHRQ), says she's not worried about the ability of America’s hospitalists to rise to the occasion and get a handle on the problem.
Dr. Clancy recently wrote a commentary outlining the government's approach to controlling readmissions, stating that taking aim at readmissions is 1) an integral component of its value-based purchasing program and 2) is an opportunity for improving hospital quality and patient safety.
"Hospitalists are often on the receiving end of hospitalizations resulting from poor coordination of care. I think it would be very exciting to be part of the solution," Dr. Clancy says. She says she observed firsthand during a recent hospital stay how hospitalists helped her to think about how she should care for herself after returning home. But her father suffered a needless rehospitalization when important information (how much Coumadin to take) was miscommunicated in a post-discharge follow-up phone call, causing him to start bleeding.
"Hospitalists who want to embrace the challenge will find a phenomenal amount of information on Innovations Exchange, where people from all over America are sharing their clinical innovations."
Dr. Clancy says she hopes AHRQ-supported tools and studies "will make it easier for hospitals to do the right thing."
Although a recently released study of Medicare data uncovers little progress in reducing hospital readmissions, and the Oct. 1 deadline to implement CMS’ Hospital Readmissions Reduction Program looms, Carolyn Clancy, MD, director of the Agency for Healthcare Research and Quality (AHRQ), says she's not worried about the ability of America’s hospitalists to rise to the occasion and get a handle on the problem.
Dr. Clancy recently wrote a commentary outlining the government's approach to controlling readmissions, stating that taking aim at readmissions is 1) an integral component of its value-based purchasing program and 2) is an opportunity for improving hospital quality and patient safety.
"Hospitalists are often on the receiving end of hospitalizations resulting from poor coordination of care. I think it would be very exciting to be part of the solution," Dr. Clancy says. She says she observed firsthand during a recent hospital stay how hospitalists helped her to think about how she should care for herself after returning home. But her father suffered a needless rehospitalization when important information (how much Coumadin to take) was miscommunicated in a post-discharge follow-up phone call, causing him to start bleeding.
"Hospitalists who want to embrace the challenge will find a phenomenal amount of information on Innovations Exchange, where people from all over America are sharing their clinical innovations."
Dr. Clancy says she hopes AHRQ-supported tools and studies "will make it easier for hospitals to do the right thing."
Although a recently released study of Medicare data uncovers little progress in reducing hospital readmissions, and the Oct. 1 deadline to implement CMS’ Hospital Readmissions Reduction Program looms, Carolyn Clancy, MD, director of the Agency for Healthcare Research and Quality (AHRQ), says she's not worried about the ability of America’s hospitalists to rise to the occasion and get a handle on the problem.
Dr. Clancy recently wrote a commentary outlining the government's approach to controlling readmissions, stating that taking aim at readmissions is 1) an integral component of its value-based purchasing program and 2) is an opportunity for improving hospital quality and patient safety.
"Hospitalists are often on the receiving end of hospitalizations resulting from poor coordination of care. I think it would be very exciting to be part of the solution," Dr. Clancy says. She says she observed firsthand during a recent hospital stay how hospitalists helped her to think about how she should care for herself after returning home. But her father suffered a needless rehospitalization when important information (how much Coumadin to take) was miscommunicated in a post-discharge follow-up phone call, causing him to start bleeding.
"Hospitalists who want to embrace the challenge will find a phenomenal amount of information on Innovations Exchange, where people from all over America are sharing their clinical innovations."
Dr. Clancy says she hopes AHRQ-supported tools and studies "will make it easier for hospitals to do the right thing."
Some Sports Injuries Greater for Girls
STANFORD, CALIF. – Help girls avoid the sports injuries that are more common for them than for boys by clearly explaining energy requirements, recommending prevention training programs, and educating about concussions.
Brains and knees face extra risk from sports injuries in girls than in boys, and only girls develop the Female Athlete Triad – disordered eating, amenorrhea and osteoporosis.
In gender-compatible sports such as soccer or basketball, girls have higher concussion rates than do boys and more postconcussion symptoms. Anterior cruciate ligament (ACL) injuries are two to eight times more common in females than in males. Approximately 1% of female high school athletes meet all three criteria of the Female Athlete Triad, 6% may meet two criteria, and approximately one in five may meet one of the criteria, Dr. Jennifer L. Carlson said at a pediatric update sponsored by Stanford University.
• Female athlete triad. The key to prevention is education. Dr. Carlson explains to patients that they should consume 2,200-2,400 kcal/day (depending on their age) if they are "active," meaning their daily activity is the equivalent of walking more than 3 miles/day at 3-4 mph. If they do more, they’re "very active" and need 2,500-4,000 kcal/day, depending on the sport and the number of hours spent training.
"Many have no idea that that’s what they need to be taking in," said Dr. Carlson of the university.
Girls also need to hear that losing one’s period is not a sign of fitness. And coaches may need to be asked to de-emphasize weight goals and abandon harmful weight-loss practices.
"I’ve had athletes in non–weight-class sports where the coach monitors weight" and even asks them to get a bone density scan, for no good reason, Dr. Carlson said.
One study of 170 female athletes in six Southern California high schools found that 1% met the three criteria for female athlete triad and 6% met two criteria. "But any one of the three criteria is pretty significant," Dr. Carlson said, and 18%-24% in the study had one of the individual criteria (Arch. Pediatr. Adolesc. Med. 2006;160:137-42).
The three criteria of disordered eating, amenorrhea, and osteoporosis that were identified in 1992 have evolved, and today would be described as low energy availability, menstrual disturbances, and low bone mineral density, she said. These can lead to fatigue, difficulty concentrating, emotional lability, impaired athletic performance, stress reactions, and fractures.
The triad is most likely in sports such as gymnastics in which the athlete is scored partly on aesthetics, endurance sports such as cross-country running that favor low body weight for better performance, sports like wrestling or crew that have different weight classes, or any sport in which clothing reveals body contours.
Physicians can find helpful resources about the syndrome from the Female Athlete Triad Coalition website, she said.
• ACL injuries. In females, ACL injuries most commonly come from noncontact maneuvers in sports involving sudden stopping and changing of direction, known as "cutting." The injuries range from small, mild tears to completely torn ligaments. Prevention focuses on awareness of risk factors and specific training programs.
Prevention training programs focus on minimizing risky positions (such as landing from a jump in an upright position instead of crouched), increasing balance and knee stiffness, and decreasing ACL strain. Training programs significantly reduced the risk for ACL injuries by 60% in a meta-analysis of six studies (Am. J. Sports Med. 2006;34:490-8).
The elite and collegiate-level sports programs incorporate prevention training programs. More and more experts advocate for them to be integrated into sports programs for prepubertal age groups, she said.
Greater friction on playing fields increases the risk of ACL injury. Generally, artificial turf is thought to be riskier, and wet surfaces may decrease risk of an ACL tear. Females may have higher risk because of neuromuscular or anatomic factors (such as wider hips) or less core stability, some think.
Biomechanical differences contribute to risk, compared with males. Hormones play a role, too. The peak time of female ACL injury is in the first phase of the menstrual cycle, and oral contraceptives seem to be protective, probably because of the action on neuromuscular junctions that hormones affect, Dr. Carlson said.
• Concussion. Higher rates of concussions in girls playing gender-comparable sports may be caused by reporting bias if boys are more reluctant than girls to report the injury, some speculate. In a recent study of 296 athletes, however, females had worse visual memory scores and more concussion symptoms than did males after a concussion, and neurocognitive impairments persisted as long as 10-21 days for high schoolers and 5-7 days for college athletes (Am. J. Sports Med. 2012;40:1303-12).
Possible reasons for these sex differences may include female sex hormones: rat studies show that estrogen has both protective and exacerbative effects, Dr. Carlson said. Cerebral blood flow and basal rate of glucose metabolism are higher in females than in males, and perhaps an increase in either of these after injury may cause concussion symptoms to persist or be more severe, she speculated.
There’s nothing gender specific about concussion-prevention efforts, except perhaps how they’re applied. Boys playing lacrosse are required to wear helmets, for example, but girls are not, she said. Concerns about universal use of headgear leading to more aggressive play and more collisions come from studies of boys, she added.
Regulations may play an increasing role in preventing concussions. A 2011 California law requires that players with suspected concussion must be pulled from school-based sports and not allowed to return without clearance from a health care profession, and they must get yearly head-trauma clearance.
Female participation in high school sports increased 900% in the past 40 years and increased 500% in collegiate sports. It’s not just the older girls who are at risk, though. Recent trends of increased participation in organized sports and greater specialization in sports at younger ages may contribute to overuse and sport-specific injuries at younger ages, Dr. Carlson said.
"My 3-year-old already gets flyers for soccer teams," she said.
Dr. Carlson reported having no financial disclosures.
STANFORD, CALIF. – Help girls avoid the sports injuries that are more common for them than for boys by clearly explaining energy requirements, recommending prevention training programs, and educating about concussions.
Brains and knees face extra risk from sports injuries in girls than in boys, and only girls develop the Female Athlete Triad – disordered eating, amenorrhea and osteoporosis.
In gender-compatible sports such as soccer or basketball, girls have higher concussion rates than do boys and more postconcussion symptoms. Anterior cruciate ligament (ACL) injuries are two to eight times more common in females than in males. Approximately 1% of female high school athletes meet all three criteria of the Female Athlete Triad, 6% may meet two criteria, and approximately one in five may meet one of the criteria, Dr. Jennifer L. Carlson said at a pediatric update sponsored by Stanford University.
• Female athlete triad. The key to prevention is education. Dr. Carlson explains to patients that they should consume 2,200-2,400 kcal/day (depending on their age) if they are "active," meaning their daily activity is the equivalent of walking more than 3 miles/day at 3-4 mph. If they do more, they’re "very active" and need 2,500-4,000 kcal/day, depending on the sport and the number of hours spent training.
"Many have no idea that that’s what they need to be taking in," said Dr. Carlson of the university.
Girls also need to hear that losing one’s period is not a sign of fitness. And coaches may need to be asked to de-emphasize weight goals and abandon harmful weight-loss practices.
"I’ve had athletes in non–weight-class sports where the coach monitors weight" and even asks them to get a bone density scan, for no good reason, Dr. Carlson said.
One study of 170 female athletes in six Southern California high schools found that 1% met the three criteria for female athlete triad and 6% met two criteria. "But any one of the three criteria is pretty significant," Dr. Carlson said, and 18%-24% in the study had one of the individual criteria (Arch. Pediatr. Adolesc. Med. 2006;160:137-42).
The three criteria of disordered eating, amenorrhea, and osteoporosis that were identified in 1992 have evolved, and today would be described as low energy availability, menstrual disturbances, and low bone mineral density, she said. These can lead to fatigue, difficulty concentrating, emotional lability, impaired athletic performance, stress reactions, and fractures.
The triad is most likely in sports such as gymnastics in which the athlete is scored partly on aesthetics, endurance sports such as cross-country running that favor low body weight for better performance, sports like wrestling or crew that have different weight classes, or any sport in which clothing reveals body contours.
Physicians can find helpful resources about the syndrome from the Female Athlete Triad Coalition website, she said.
• ACL injuries. In females, ACL injuries most commonly come from noncontact maneuvers in sports involving sudden stopping and changing of direction, known as "cutting." The injuries range from small, mild tears to completely torn ligaments. Prevention focuses on awareness of risk factors and specific training programs.
Prevention training programs focus on minimizing risky positions (such as landing from a jump in an upright position instead of crouched), increasing balance and knee stiffness, and decreasing ACL strain. Training programs significantly reduced the risk for ACL injuries by 60% in a meta-analysis of six studies (Am. J. Sports Med. 2006;34:490-8).
The elite and collegiate-level sports programs incorporate prevention training programs. More and more experts advocate for them to be integrated into sports programs for prepubertal age groups, she said.
Greater friction on playing fields increases the risk of ACL injury. Generally, artificial turf is thought to be riskier, and wet surfaces may decrease risk of an ACL tear. Females may have higher risk because of neuromuscular or anatomic factors (such as wider hips) or less core stability, some think.
Biomechanical differences contribute to risk, compared with males. Hormones play a role, too. The peak time of female ACL injury is in the first phase of the menstrual cycle, and oral contraceptives seem to be protective, probably because of the action on neuromuscular junctions that hormones affect, Dr. Carlson said.
• Concussion. Higher rates of concussions in girls playing gender-comparable sports may be caused by reporting bias if boys are more reluctant than girls to report the injury, some speculate. In a recent study of 296 athletes, however, females had worse visual memory scores and more concussion symptoms than did males after a concussion, and neurocognitive impairments persisted as long as 10-21 days for high schoolers and 5-7 days for college athletes (Am. J. Sports Med. 2012;40:1303-12).
Possible reasons for these sex differences may include female sex hormones: rat studies show that estrogen has both protective and exacerbative effects, Dr. Carlson said. Cerebral blood flow and basal rate of glucose metabolism are higher in females than in males, and perhaps an increase in either of these after injury may cause concussion symptoms to persist or be more severe, she speculated.
There’s nothing gender specific about concussion-prevention efforts, except perhaps how they’re applied. Boys playing lacrosse are required to wear helmets, for example, but girls are not, she said. Concerns about universal use of headgear leading to more aggressive play and more collisions come from studies of boys, she added.
Regulations may play an increasing role in preventing concussions. A 2011 California law requires that players with suspected concussion must be pulled from school-based sports and not allowed to return without clearance from a health care profession, and they must get yearly head-trauma clearance.
Female participation in high school sports increased 900% in the past 40 years and increased 500% in collegiate sports. It’s not just the older girls who are at risk, though. Recent trends of increased participation in organized sports and greater specialization in sports at younger ages may contribute to overuse and sport-specific injuries at younger ages, Dr. Carlson said.
"My 3-year-old already gets flyers for soccer teams," she said.
Dr. Carlson reported having no financial disclosures.
STANFORD, CALIF. – Help girls avoid the sports injuries that are more common for them than for boys by clearly explaining energy requirements, recommending prevention training programs, and educating about concussions.
Brains and knees face extra risk from sports injuries in girls than in boys, and only girls develop the Female Athlete Triad – disordered eating, amenorrhea and osteoporosis.
In gender-compatible sports such as soccer or basketball, girls have higher concussion rates than do boys and more postconcussion symptoms. Anterior cruciate ligament (ACL) injuries are two to eight times more common in females than in males. Approximately 1% of female high school athletes meet all three criteria of the Female Athlete Triad, 6% may meet two criteria, and approximately one in five may meet one of the criteria, Dr. Jennifer L. Carlson said at a pediatric update sponsored by Stanford University.
• Female athlete triad. The key to prevention is education. Dr. Carlson explains to patients that they should consume 2,200-2,400 kcal/day (depending on their age) if they are "active," meaning their daily activity is the equivalent of walking more than 3 miles/day at 3-4 mph. If they do more, they’re "very active" and need 2,500-4,000 kcal/day, depending on the sport and the number of hours spent training.
"Many have no idea that that’s what they need to be taking in," said Dr. Carlson of the university.
Girls also need to hear that losing one’s period is not a sign of fitness. And coaches may need to be asked to de-emphasize weight goals and abandon harmful weight-loss practices.
"I’ve had athletes in non–weight-class sports where the coach monitors weight" and even asks them to get a bone density scan, for no good reason, Dr. Carlson said.
One study of 170 female athletes in six Southern California high schools found that 1% met the three criteria for female athlete triad and 6% met two criteria. "But any one of the three criteria is pretty significant," Dr. Carlson said, and 18%-24% in the study had one of the individual criteria (Arch. Pediatr. Adolesc. Med. 2006;160:137-42).
The three criteria of disordered eating, amenorrhea, and osteoporosis that were identified in 1992 have evolved, and today would be described as low energy availability, menstrual disturbances, and low bone mineral density, she said. These can lead to fatigue, difficulty concentrating, emotional lability, impaired athletic performance, stress reactions, and fractures.
The triad is most likely in sports such as gymnastics in which the athlete is scored partly on aesthetics, endurance sports such as cross-country running that favor low body weight for better performance, sports like wrestling or crew that have different weight classes, or any sport in which clothing reveals body contours.
Physicians can find helpful resources about the syndrome from the Female Athlete Triad Coalition website, she said.
• ACL injuries. In females, ACL injuries most commonly come from noncontact maneuvers in sports involving sudden stopping and changing of direction, known as "cutting." The injuries range from small, mild tears to completely torn ligaments. Prevention focuses on awareness of risk factors and specific training programs.
Prevention training programs focus on minimizing risky positions (such as landing from a jump in an upright position instead of crouched), increasing balance and knee stiffness, and decreasing ACL strain. Training programs significantly reduced the risk for ACL injuries by 60% in a meta-analysis of six studies (Am. J. Sports Med. 2006;34:490-8).
The elite and collegiate-level sports programs incorporate prevention training programs. More and more experts advocate for them to be integrated into sports programs for prepubertal age groups, she said.
Greater friction on playing fields increases the risk of ACL injury. Generally, artificial turf is thought to be riskier, and wet surfaces may decrease risk of an ACL tear. Females may have higher risk because of neuromuscular or anatomic factors (such as wider hips) or less core stability, some think.
Biomechanical differences contribute to risk, compared with males. Hormones play a role, too. The peak time of female ACL injury is in the first phase of the menstrual cycle, and oral contraceptives seem to be protective, probably because of the action on neuromuscular junctions that hormones affect, Dr. Carlson said.
• Concussion. Higher rates of concussions in girls playing gender-comparable sports may be caused by reporting bias if boys are more reluctant than girls to report the injury, some speculate. In a recent study of 296 athletes, however, females had worse visual memory scores and more concussion symptoms than did males after a concussion, and neurocognitive impairments persisted as long as 10-21 days for high schoolers and 5-7 days for college athletes (Am. J. Sports Med. 2012;40:1303-12).
Possible reasons for these sex differences may include female sex hormones: rat studies show that estrogen has both protective and exacerbative effects, Dr. Carlson said. Cerebral blood flow and basal rate of glucose metabolism are higher in females than in males, and perhaps an increase in either of these after injury may cause concussion symptoms to persist or be more severe, she speculated.
There’s nothing gender specific about concussion-prevention efforts, except perhaps how they’re applied. Boys playing lacrosse are required to wear helmets, for example, but girls are not, she said. Concerns about universal use of headgear leading to more aggressive play and more collisions come from studies of boys, she added.
Regulations may play an increasing role in preventing concussions. A 2011 California law requires that players with suspected concussion must be pulled from school-based sports and not allowed to return without clearance from a health care profession, and they must get yearly head-trauma clearance.
Female participation in high school sports increased 900% in the past 40 years and increased 500% in collegiate sports. It’s not just the older girls who are at risk, though. Recent trends of increased participation in organized sports and greater specialization in sports at younger ages may contribute to overuse and sport-specific injuries at younger ages, Dr. Carlson said.
"My 3-year-old already gets flyers for soccer teams," she said.
Dr. Carlson reported having no financial disclosures.
EXPERT ANALYSIS FROM A PEDIATRIC UPDATE SPONSORED BY STANFORD UNIVERSITY
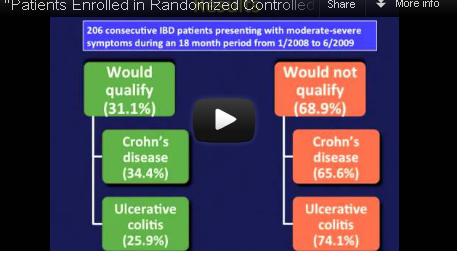
Most IBD Patients Don't Meet Biologics Trial Criteria
Participants in randomized controlled trials of biologics for inflammatory bowel disease do not adequately represent real-world patients because of stringent inclusion criteria for the trials, reported Dr. Christina Ha and her colleagues in the September issue of Clinical Gastroenterology and Hepatology.
Indeed, among 125 Crohn’s disease (CD) patients seen in routine clinical practice at a tertiary care center, only 43 – just 34% – would have qualified for enrollment in at least one of seven randomized controlled trials (RCTs) for biologics, calling into question the generalizability of these trials’ results. The corresponding percentage for ulcerative colitis (UC) patients was only 25%.
Video Source: American Gastroenterological Association
Dr. Ha, of the division of gastroenterology at Johns Hopkins University, Baltimore, and her colleagues reviewed the medical records of 206 patients with moderate to severe CD or UC presenting to the Mount Sinai Medical Center in New York for adjustment of therapy during 2008-2009.
The researchers then looked at seven randomized controlled trials of biologics in CD as well as two trials of biologics in UC, and applied their inclusion and exclusion criteria to her real-world study population (Clin. Gastroenterol. Hepatol. 2012 September [doi: 10.1016/j.cgh.2012.02.004]).
Among the Crohn’s patients, "trial eligibility for the RCTs for infliximab, adalimumab, certolizumab pegol, and natalizumab ranged from 8% for the SONIC [Study of Biologic and Immunomodulator Naïve Patients in Crohn’s Disease] trial to 27% for the CHARM [Crohn’s Trial of the Fully Human Antibody Adalimumab for Remission Maintenance] and PRECISE [Pegylated Antibody Fragment Evaluation in Crohn’s Disease] trials," wrote the investigators.
Similarly, among the 81 UC patients, only 21 (25%) would have qualified for enrollment into the ACT (Active Ulcerative Colitis Trial) 1 or 2 trials.
Among the Crohn’s patients, the most common reasons for ineligibility were symptomatic strictures or abscesses (n = 51, 62.2%), recent exposure or prior nonresponse to anti–tumor necrosis factor drugs (n = 42, 51.2%), use of high-dose steroids (n = 15, 18.3%), and comorbid cardiovascular or pulmonary disease and malignancies (n = 21, 25.6%).
"The most common reason for trial ineligibility for UC was current rectal therapy usage (n = 34, 56.7%)," wrote the authors. Other reasons included steroid naivety (n = 27, 45.0%); new diagnoses of UC (n = 10, 16.7%); or need for colectomy due to age, comorbidity, or concomitant dysplasia found during colonoscopy (n = 9, 15.0%).
Finally, the authors assessed the outcomes of patients who would not have qualified for the biologics trials but initiated biologic therapy nevertheless. In the CD cohort, "ultimately, almost 50% of these ‘trial ineligible’ patients underwent surgery, either as a primary therapy or due to inadequate response to biologics or immunomodulators," wrote the authors. In the UC group, roughly one-quarter of would-be ineligible patients underwent colectomy at 4-12 weeks.
The authors conceded that if patients had been studied longitudinally, "with the inclusion and exclusion criteria applied at the time patients were first being considered for immunomodulators or biologics or at the time of diagnosis, a larger percentage of patients may have been eligible for trial participation."
Nevertheless, Dr. Ha and her colleagues concluded, "pragmatic trials of the major IBD therapeutics would not only serve to validate RCT findings, but provide additional insight regarding medication safety across a broader patient population."
Several of the authors disclosed financial relationships with pharmaceutical companies, including the makers of biologics. The authors stated that this study received no grant support.
Participants in randomized controlled trials of biologics for inflammatory bowel disease do not adequately represent real-world patients because of stringent inclusion criteria for the trials, reported Dr. Christina Ha and her colleagues in the September issue of Clinical Gastroenterology and Hepatology.
Indeed, among 125 Crohn’s disease (CD) patients seen in routine clinical practice at a tertiary care center, only 43 – just 34% – would have qualified for enrollment in at least one of seven randomized controlled trials (RCTs) for biologics, calling into question the generalizability of these trials’ results. The corresponding percentage for ulcerative colitis (UC) patients was only 25%.
Video Source: American Gastroenterological Association
Dr. Ha, of the division of gastroenterology at Johns Hopkins University, Baltimore, and her colleagues reviewed the medical records of 206 patients with moderate to severe CD or UC presenting to the Mount Sinai Medical Center in New York for adjustment of therapy during 2008-2009.
The researchers then looked at seven randomized controlled trials of biologics in CD as well as two trials of biologics in UC, and applied their inclusion and exclusion criteria to her real-world study population (Clin. Gastroenterol. Hepatol. 2012 September [doi: 10.1016/j.cgh.2012.02.004]).
Among the Crohn’s patients, "trial eligibility for the RCTs for infliximab, adalimumab, certolizumab pegol, and natalizumab ranged from 8% for the SONIC [Study of Biologic and Immunomodulator Naïve Patients in Crohn’s Disease] trial to 27% for the CHARM [Crohn’s Trial of the Fully Human Antibody Adalimumab for Remission Maintenance] and PRECISE [Pegylated Antibody Fragment Evaluation in Crohn’s Disease] trials," wrote the investigators.
Similarly, among the 81 UC patients, only 21 (25%) would have qualified for enrollment into the ACT (Active Ulcerative Colitis Trial) 1 or 2 trials.
Among the Crohn’s patients, the most common reasons for ineligibility were symptomatic strictures or abscesses (n = 51, 62.2%), recent exposure or prior nonresponse to anti–tumor necrosis factor drugs (n = 42, 51.2%), use of high-dose steroids (n = 15, 18.3%), and comorbid cardiovascular or pulmonary disease and malignancies (n = 21, 25.6%).
"The most common reason for trial ineligibility for UC was current rectal therapy usage (n = 34, 56.7%)," wrote the authors. Other reasons included steroid naivety (n = 27, 45.0%); new diagnoses of UC (n = 10, 16.7%); or need for colectomy due to age, comorbidity, or concomitant dysplasia found during colonoscopy (n = 9, 15.0%).
Finally, the authors assessed the outcomes of patients who would not have qualified for the biologics trials but initiated biologic therapy nevertheless. In the CD cohort, "ultimately, almost 50% of these ‘trial ineligible’ patients underwent surgery, either as a primary therapy or due to inadequate response to biologics or immunomodulators," wrote the authors. In the UC group, roughly one-quarter of would-be ineligible patients underwent colectomy at 4-12 weeks.
The authors conceded that if patients had been studied longitudinally, "with the inclusion and exclusion criteria applied at the time patients were first being considered for immunomodulators or biologics or at the time of diagnosis, a larger percentage of patients may have been eligible for trial participation."
Nevertheless, Dr. Ha and her colleagues concluded, "pragmatic trials of the major IBD therapeutics would not only serve to validate RCT findings, but provide additional insight regarding medication safety across a broader patient population."
Several of the authors disclosed financial relationships with pharmaceutical companies, including the makers of biologics. The authors stated that this study received no grant support.
Participants in randomized controlled trials of biologics for inflammatory bowel disease do not adequately represent real-world patients because of stringent inclusion criteria for the trials, reported Dr. Christina Ha and her colleagues in the September issue of Clinical Gastroenterology and Hepatology.
Indeed, among 125 Crohn’s disease (CD) patients seen in routine clinical practice at a tertiary care center, only 43 – just 34% – would have qualified for enrollment in at least one of seven randomized controlled trials (RCTs) for biologics, calling into question the generalizability of these trials’ results. The corresponding percentage for ulcerative colitis (UC) patients was only 25%.
Video Source: American Gastroenterological Association
Dr. Ha, of the division of gastroenterology at Johns Hopkins University, Baltimore, and her colleagues reviewed the medical records of 206 patients with moderate to severe CD or UC presenting to the Mount Sinai Medical Center in New York for adjustment of therapy during 2008-2009.
The researchers then looked at seven randomized controlled trials of biologics in CD as well as two trials of biologics in UC, and applied their inclusion and exclusion criteria to her real-world study population (Clin. Gastroenterol. Hepatol. 2012 September [doi: 10.1016/j.cgh.2012.02.004]).
Among the Crohn’s patients, "trial eligibility for the RCTs for infliximab, adalimumab, certolizumab pegol, and natalizumab ranged from 8% for the SONIC [Study of Biologic and Immunomodulator Naïve Patients in Crohn’s Disease] trial to 27% for the CHARM [Crohn’s Trial of the Fully Human Antibody Adalimumab for Remission Maintenance] and PRECISE [Pegylated Antibody Fragment Evaluation in Crohn’s Disease] trials," wrote the investigators.
Similarly, among the 81 UC patients, only 21 (25%) would have qualified for enrollment into the ACT (Active Ulcerative Colitis Trial) 1 or 2 trials.
Among the Crohn’s patients, the most common reasons for ineligibility were symptomatic strictures or abscesses (n = 51, 62.2%), recent exposure or prior nonresponse to anti–tumor necrosis factor drugs (n = 42, 51.2%), use of high-dose steroids (n = 15, 18.3%), and comorbid cardiovascular or pulmonary disease and malignancies (n = 21, 25.6%).
"The most common reason for trial ineligibility for UC was current rectal therapy usage (n = 34, 56.7%)," wrote the authors. Other reasons included steroid naivety (n = 27, 45.0%); new diagnoses of UC (n = 10, 16.7%); or need for colectomy due to age, comorbidity, or concomitant dysplasia found during colonoscopy (n = 9, 15.0%).
Finally, the authors assessed the outcomes of patients who would not have qualified for the biologics trials but initiated biologic therapy nevertheless. In the CD cohort, "ultimately, almost 50% of these ‘trial ineligible’ patients underwent surgery, either as a primary therapy or due to inadequate response to biologics or immunomodulators," wrote the authors. In the UC group, roughly one-quarter of would-be ineligible patients underwent colectomy at 4-12 weeks.
The authors conceded that if patients had been studied longitudinally, "with the inclusion and exclusion criteria applied at the time patients were first being considered for immunomodulators or biologics or at the time of diagnosis, a larger percentage of patients may have been eligible for trial participation."
Nevertheless, Dr. Ha and her colleagues concluded, "pragmatic trials of the major IBD therapeutics would not only serve to validate RCT findings, but provide additional insight regarding medication safety across a broader patient population."
Several of the authors disclosed financial relationships with pharmaceutical companies, including the makers of biologics. The authors stated that this study received no grant support.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Photoprotection for Preventing Skin Cancer and Premature Skin Aging
Dr. Hale discusses how regular sunscreen use can diminish the chances of developing future skin cancers and can slow the process of premature skin aging. For more information, read Dr. Hale's article in the May 2012 issue, "Sunscreens and Photoaging: An Update."
Dr. Hale discusses how regular sunscreen use can diminish the chances of developing future skin cancers and can slow the process of premature skin aging. For more information, read Dr. Hale's article in the May 2012 issue, "Sunscreens and Photoaging: An Update."
Dr. Hale discusses how regular sunscreen use can diminish the chances of developing future skin cancers and can slow the process of premature skin aging. For more information, read Dr. Hale's article in the May 2012 issue, "Sunscreens and Photoaging: An Update."
From the Mailbag: Patient Engagement and ACOs
Many thanks for the thoughtful e-mails from many of you readers. Some express cautious hope, some skepticism, and all the curiosity and caring that reminds me of how fortunate I am to have been a physician advocate for my legal career.
Here is one reader’s concerns about patient engagement – or lack thereof – in accountable care organizations.
– Reader: "ACOs will never work because, once again, the patient has been left out of the equation."
We hear this quite often, and it is surely true in some cases – but not all. In fact, we are convinced that patient engagement is such an essential element necessary for every successful ACO, that an ACO should not be called an ACO without it.
Patient noncompliance is a problem, especially regarding chronic diseases and lifestyle management. It is difficult to accept a compensation model based on input on improved patient population health when that is dramatically affected by a variable outside of your control: patient adherence. But patient engagement is part of patient-centeredness, which is required by the Affordable Care Act for an ACO to qualify for CMS’ Shared Savings Program.
So, what can an ACO do to engage patients?
Consider the following approaches:
– The patient compact. Some ACOs, such as the Geisinger Clinic, engage the patient through a compact, or agreement. It may involve a written commitment by the patient to be responsible for his or her own wellness or chronic care management, coupled with rewards for so doing, education, tools, self-care modules, and shared decision-making empowerment. The providers will need to embrace the importance of patient involvement and hold up their end of the engagement bargain.
– Benefit differentials for lifestyle choices. The financial impact of many volitional patient lifestyle choices is actuarially measurable. A logical consequence of the patient choice could be a benefit or financial differential reflecting at least partially these avoidable health care costs.
– Stay in contact. A Kaiser Permanente study of more than 35,000 hypertensive and diabetic patients found that the blood pressure and cholesterol levels for those who engaged in secure messaging were better than for those who did not.
– More time with your patients. Develop personal relationships. One ACO saw its results jump when its primary care physicians started using Biosignia’s "Know Your Number," a computer-generated graphic depiction of a patient’s health risks based on lab results and the Framingham Study. It is used by the treating physician at the point of care.
– Patient remote access to test results. This can be achieved with tools such as a web portal with multiple functions.
– Care navigators. We predict that the demands for care navigators, or coordinators, will skyrocket as ACOs take hold. Their use will include home visits. This may be the best patient engagement method.
– Empathetic listening. In curriculum and residency programs of medical schools, the paternalistic model is yielding to empathetic listening and communication skills in physician training.
– Educational materials. This consists of patient-friendly educational material that explains the benefits of being linked to a medical home.
I agree so much with this reader’s assertion that an ACO without patient engagement will fail, that I consider it an essential, almost definitional, element of every successful ACO. It is crucial that we emphasize the role of the patient. It is truly the "other shoe" that must fall for the new outcomes-based health care to succeed.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact Mr. Bobbit at [email protected], or at 919-821-6612.
Many thanks for the thoughtful e-mails from many of you readers. Some express cautious hope, some skepticism, and all the curiosity and caring that reminds me of how fortunate I am to have been a physician advocate for my legal career.
Here is one reader’s concerns about patient engagement – or lack thereof – in accountable care organizations.
– Reader: "ACOs will never work because, once again, the patient has been left out of the equation."
We hear this quite often, and it is surely true in some cases – but not all. In fact, we are convinced that patient engagement is such an essential element necessary for every successful ACO, that an ACO should not be called an ACO without it.
Patient noncompliance is a problem, especially regarding chronic diseases and lifestyle management. It is difficult to accept a compensation model based on input on improved patient population health when that is dramatically affected by a variable outside of your control: patient adherence. But patient engagement is part of patient-centeredness, which is required by the Affordable Care Act for an ACO to qualify for CMS’ Shared Savings Program.
So, what can an ACO do to engage patients?
Consider the following approaches:
– The patient compact. Some ACOs, such as the Geisinger Clinic, engage the patient through a compact, or agreement. It may involve a written commitment by the patient to be responsible for his or her own wellness or chronic care management, coupled with rewards for so doing, education, tools, self-care modules, and shared decision-making empowerment. The providers will need to embrace the importance of patient involvement and hold up their end of the engagement bargain.
– Benefit differentials for lifestyle choices. The financial impact of many volitional patient lifestyle choices is actuarially measurable. A logical consequence of the patient choice could be a benefit or financial differential reflecting at least partially these avoidable health care costs.
– Stay in contact. A Kaiser Permanente study of more than 35,000 hypertensive and diabetic patients found that the blood pressure and cholesterol levels for those who engaged in secure messaging were better than for those who did not.
– More time with your patients. Develop personal relationships. One ACO saw its results jump when its primary care physicians started using Biosignia’s "Know Your Number," a computer-generated graphic depiction of a patient’s health risks based on lab results and the Framingham Study. It is used by the treating physician at the point of care.
– Patient remote access to test results. This can be achieved with tools such as a web portal with multiple functions.
– Care navigators. We predict that the demands for care navigators, or coordinators, will skyrocket as ACOs take hold. Their use will include home visits. This may be the best patient engagement method.
– Empathetic listening. In curriculum and residency programs of medical schools, the paternalistic model is yielding to empathetic listening and communication skills in physician training.
– Educational materials. This consists of patient-friendly educational material that explains the benefits of being linked to a medical home.
I agree so much with this reader’s assertion that an ACO without patient engagement will fail, that I consider it an essential, almost definitional, element of every successful ACO. It is crucial that we emphasize the role of the patient. It is truly the "other shoe" that must fall for the new outcomes-based health care to succeed.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact Mr. Bobbit at [email protected], or at 919-821-6612.
Many thanks for the thoughtful e-mails from many of you readers. Some express cautious hope, some skepticism, and all the curiosity and caring that reminds me of how fortunate I am to have been a physician advocate for my legal career.
Here is one reader’s concerns about patient engagement – or lack thereof – in accountable care organizations.
– Reader: "ACOs will never work because, once again, the patient has been left out of the equation."
We hear this quite often, and it is surely true in some cases – but not all. In fact, we are convinced that patient engagement is such an essential element necessary for every successful ACO, that an ACO should not be called an ACO without it.
Patient noncompliance is a problem, especially regarding chronic diseases and lifestyle management. It is difficult to accept a compensation model based on input on improved patient population health when that is dramatically affected by a variable outside of your control: patient adherence. But patient engagement is part of patient-centeredness, which is required by the Affordable Care Act for an ACO to qualify for CMS’ Shared Savings Program.
So, what can an ACO do to engage patients?
Consider the following approaches:
– The patient compact. Some ACOs, such as the Geisinger Clinic, engage the patient through a compact, or agreement. It may involve a written commitment by the patient to be responsible for his or her own wellness or chronic care management, coupled with rewards for so doing, education, tools, self-care modules, and shared decision-making empowerment. The providers will need to embrace the importance of patient involvement and hold up their end of the engagement bargain.
– Benefit differentials for lifestyle choices. The financial impact of many volitional patient lifestyle choices is actuarially measurable. A logical consequence of the patient choice could be a benefit or financial differential reflecting at least partially these avoidable health care costs.
– Stay in contact. A Kaiser Permanente study of more than 35,000 hypertensive and diabetic patients found that the blood pressure and cholesterol levels for those who engaged in secure messaging were better than for those who did not.
– More time with your patients. Develop personal relationships. One ACO saw its results jump when its primary care physicians started using Biosignia’s "Know Your Number," a computer-generated graphic depiction of a patient’s health risks based on lab results and the Framingham Study. It is used by the treating physician at the point of care.
– Patient remote access to test results. This can be achieved with tools such as a web portal with multiple functions.
– Care navigators. We predict that the demands for care navigators, or coordinators, will skyrocket as ACOs take hold. Their use will include home visits. This may be the best patient engagement method.
– Empathetic listening. In curriculum and residency programs of medical schools, the paternalistic model is yielding to empathetic listening and communication skills in physician training.
– Educational materials. This consists of patient-friendly educational material that explains the benefits of being linked to a medical home.
I agree so much with this reader’s assertion that an ACO without patient engagement will fail, that I consider it an essential, almost definitional, element of every successful ACO. It is crucial that we emphasize the role of the patient. It is truly the "other shoe" that must fall for the new outcomes-based health care to succeed.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact Mr. Bobbit at [email protected], or at 919-821-6612.