User login
ACOs Present HM Risk/Reward Opportunity
As the healthcare industry digests the Centers for Medicare & Medicaid Services’ (CMS) proposed regulations on accountable care organizations (ACOs), a leading hospitalist wants to ensure that physicians are duly compensated for risk in the process.
An ACO is a type of healthcare delivery model being piloted by CMS in which a group of providers band together to coordinate the care of beneficiaries. Reimbursement is shared by the group and is tied to the quality of care provided.
Under rules released March 31 and published in the Federal Register (PDF) last week, ACOs can enter a shared savings or a shared savings/losses model. According to Becker's Hospital Review, in the shared savings model, also called a "one-sided model," an ACO that creates at least 2% savings is then entitled to 50% of the revenue above that amount. The shared savings/losses construct, known as a "two-sided model," entitles an ACO to 60% of the threshold, but also penalizes them if the model increase costs, the review says.
"You can certainly start by taking a lower amount of risk, just upside risk," says Ron Greeno, MD, FCCP, SFHM, chief medical officer for Brentwood, Tenn.-based Cogent Healthcare and a senior member of SHM's Public Policy Committee. "But your plan should be not to stay there. Your plan should be to take more and more risk as soon as you can, as soon as you're capable."
By the third year of the program, all ACOs would become responsible for losses.
"I didn't see a lot with capitated risk," Dr. Greeno says. "That's where the opportunity is for providers. That's the opportunity to create the most savings in Medicare."
CMS will take comments on the proposed regulations until the first week of June. The program is set to go live Jan. 1, 2012.
As the healthcare industry digests the Centers for Medicare & Medicaid Services’ (CMS) proposed regulations on accountable care organizations (ACOs), a leading hospitalist wants to ensure that physicians are duly compensated for risk in the process.
An ACO is a type of healthcare delivery model being piloted by CMS in which a group of providers band together to coordinate the care of beneficiaries. Reimbursement is shared by the group and is tied to the quality of care provided.
Under rules released March 31 and published in the Federal Register (PDF) last week, ACOs can enter a shared savings or a shared savings/losses model. According to Becker's Hospital Review, in the shared savings model, also called a "one-sided model," an ACO that creates at least 2% savings is then entitled to 50% of the revenue above that amount. The shared savings/losses construct, known as a "two-sided model," entitles an ACO to 60% of the threshold, but also penalizes them if the model increase costs, the review says.
"You can certainly start by taking a lower amount of risk, just upside risk," says Ron Greeno, MD, FCCP, SFHM, chief medical officer for Brentwood, Tenn.-based Cogent Healthcare and a senior member of SHM's Public Policy Committee. "But your plan should be not to stay there. Your plan should be to take more and more risk as soon as you can, as soon as you're capable."
By the third year of the program, all ACOs would become responsible for losses.
"I didn't see a lot with capitated risk," Dr. Greeno says. "That's where the opportunity is for providers. That's the opportunity to create the most savings in Medicare."
CMS will take comments on the proposed regulations until the first week of June. The program is set to go live Jan. 1, 2012.
As the healthcare industry digests the Centers for Medicare & Medicaid Services’ (CMS) proposed regulations on accountable care organizations (ACOs), a leading hospitalist wants to ensure that physicians are duly compensated for risk in the process.
An ACO is a type of healthcare delivery model being piloted by CMS in which a group of providers band together to coordinate the care of beneficiaries. Reimbursement is shared by the group and is tied to the quality of care provided.
Under rules released March 31 and published in the Federal Register (PDF) last week, ACOs can enter a shared savings or a shared savings/losses model. According to Becker's Hospital Review, in the shared savings model, also called a "one-sided model," an ACO that creates at least 2% savings is then entitled to 50% of the revenue above that amount. The shared savings/losses construct, known as a "two-sided model," entitles an ACO to 60% of the threshold, but also penalizes them if the model increase costs, the review says.
"You can certainly start by taking a lower amount of risk, just upside risk," says Ron Greeno, MD, FCCP, SFHM, chief medical officer for Brentwood, Tenn.-based Cogent Healthcare and a senior member of SHM's Public Policy Committee. "But your plan should be not to stay there. Your plan should be to take more and more risk as soon as you can, as soon as you're capable."
By the third year of the program, all ACOs would become responsible for losses.
"I didn't see a lot with capitated risk," Dr. Greeno says. "That's where the opportunity is for providers. That's the opportunity to create the most savings in Medicare."
CMS will take comments on the proposed regulations until the first week of June. The program is set to go live Jan. 1, 2012.
In the Literature: Research You Need to Know
Clinical question: What is the in-hospital mortality risk associated with hospital-acquired Clostridium difficile infection after accounting for time to infection and baseline mortality risk at admission?
Background: Hospital-acquired C. diff infection (CDI) has been shown to be associated with a higher mortality rate and longer length of stay and cost. Previous studies have demonstrated an independent association of mortality with CDI, but have not incorporated time to infection and baseline mortality risk in the analyses.
Study design: Retrospective observational study.
Setting: Single-center, tertiary-care teaching hospital.
Synopsis: Patients who were hospitalized for more than three days were eligible. A baseline in-hospital mortality risk was estimated for each patient using an internally validated tool. A total of 136,877 admissions were identified. Mean baseline mortality risk was 1.8%. Overall rate of CDI was 1.02%.
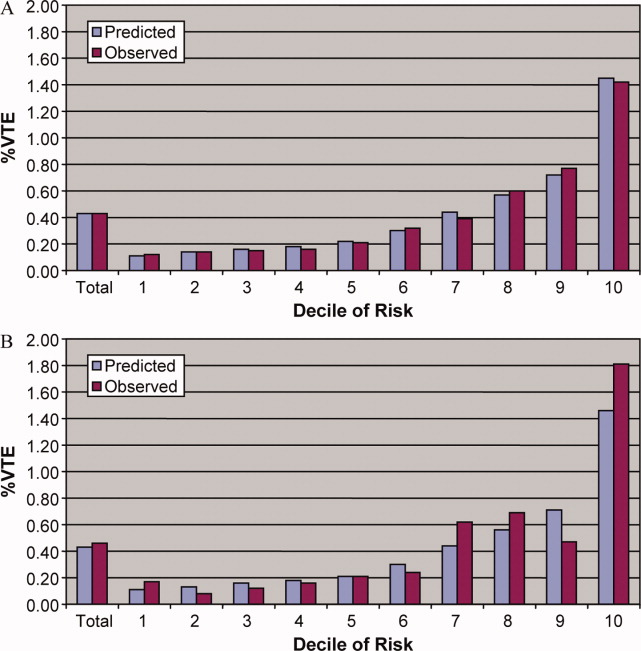
Patients in the highest decile of baseline mortality risk had a higher rate of CDI than patients in the lowest decile (2.6% vs. 0.2%). Median time to diagnosis was 12 days. CDI was associated with an unadjusted fourfold higher risk of in-hospital death. When baseline mortality risk was included, the RR of death with CDI was 1.99 (95% CI 1.81-2.19).
Patients in the lowest decile of mortality risk had the highest risk of death (RR 45.70, 95% CI 11.35-183.98) compared with those in the highest decile (RR 1.29, 95% CI 1.11-1.50). Cox modeling estimated a threefold increase in death.
This study is limited by being single-site and the mortality risk model has not been validated externally. Results are also estimated from a small number of cases in the lower deciles.
Bottom line: CDI is associated with threefold higher in-hospital mortality. Patients with higher baseline mortality risk have a higher risk of CDI but have a lesser risk of dying compared with patients with lower baseline mortality risk. Hospitals should continue their efforts to reduce rates of CDI.
Citation: Oake N, Taljaard M, van Walraven C, Wilson K, Roth V, Forster AJ. The effect of hospital-acquired C. diff infection on in-hospital mortality. Arch Intern Med. 2010;170(20):1804-1810.
For more physician reviews of HM-related research, visit our website.
Clinical question: What is the in-hospital mortality risk associated with hospital-acquired Clostridium difficile infection after accounting for time to infection and baseline mortality risk at admission?
Background: Hospital-acquired C. diff infection (CDI) has been shown to be associated with a higher mortality rate and longer length of stay and cost. Previous studies have demonstrated an independent association of mortality with CDI, but have not incorporated time to infection and baseline mortality risk in the analyses.
Study design: Retrospective observational study.
Setting: Single-center, tertiary-care teaching hospital.
Synopsis: Patients who were hospitalized for more than three days were eligible. A baseline in-hospital mortality risk was estimated for each patient using an internally validated tool. A total of 136,877 admissions were identified. Mean baseline mortality risk was 1.8%. Overall rate of CDI was 1.02%.
Patients in the highest decile of baseline mortality risk had a higher rate of CDI than patients in the lowest decile (2.6% vs. 0.2%). Median time to diagnosis was 12 days. CDI was associated with an unadjusted fourfold higher risk of in-hospital death. When baseline mortality risk was included, the RR of death with CDI was 1.99 (95% CI 1.81-2.19).
Patients in the lowest decile of mortality risk had the highest risk of death (RR 45.70, 95% CI 11.35-183.98) compared with those in the highest decile (RR 1.29, 95% CI 1.11-1.50). Cox modeling estimated a threefold increase in death.
This study is limited by being single-site and the mortality risk model has not been validated externally. Results are also estimated from a small number of cases in the lower deciles.
Bottom line: CDI is associated with threefold higher in-hospital mortality. Patients with higher baseline mortality risk have a higher risk of CDI but have a lesser risk of dying compared with patients with lower baseline mortality risk. Hospitals should continue their efforts to reduce rates of CDI.
Citation: Oake N, Taljaard M, van Walraven C, Wilson K, Roth V, Forster AJ. The effect of hospital-acquired C. diff infection on in-hospital mortality. Arch Intern Med. 2010;170(20):1804-1810.
For more physician reviews of HM-related research, visit our website.
Clinical question: What is the in-hospital mortality risk associated with hospital-acquired Clostridium difficile infection after accounting for time to infection and baseline mortality risk at admission?
Background: Hospital-acquired C. diff infection (CDI) has been shown to be associated with a higher mortality rate and longer length of stay and cost. Previous studies have demonstrated an independent association of mortality with CDI, but have not incorporated time to infection and baseline mortality risk in the analyses.
Study design: Retrospective observational study.
Setting: Single-center, tertiary-care teaching hospital.
Synopsis: Patients who were hospitalized for more than three days were eligible. A baseline in-hospital mortality risk was estimated for each patient using an internally validated tool. A total of 136,877 admissions were identified. Mean baseline mortality risk was 1.8%. Overall rate of CDI was 1.02%.
Patients in the highest decile of baseline mortality risk had a higher rate of CDI than patients in the lowest decile (2.6% vs. 0.2%). Median time to diagnosis was 12 days. CDI was associated with an unadjusted fourfold higher risk of in-hospital death. When baseline mortality risk was included, the RR of death with CDI was 1.99 (95% CI 1.81-2.19).
Patients in the lowest decile of mortality risk had the highest risk of death (RR 45.70, 95% CI 11.35-183.98) compared with those in the highest decile (RR 1.29, 95% CI 1.11-1.50). Cox modeling estimated a threefold increase in death.
This study is limited by being single-site and the mortality risk model has not been validated externally. Results are also estimated from a small number of cases in the lower deciles.
Bottom line: CDI is associated with threefold higher in-hospital mortality. Patients with higher baseline mortality risk have a higher risk of CDI but have a lesser risk of dying compared with patients with lower baseline mortality risk. Hospitals should continue their efforts to reduce rates of CDI.
Citation: Oake N, Taljaard M, van Walraven C, Wilson K, Roth V, Forster AJ. The effect of hospital-acquired C. diff infection on in-hospital mortality. Arch Intern Med. 2010;170(20):1804-1810.
For more physician reviews of HM-related research, visit our website.
ASCO, NCCN Recommend EGFR Testing in Advanced Lung Cancer
Testing for epidermal growth factor receptor mutations is an important step in the evaluation process for systemic therapy in patients with metastatic or recurrent non–small cell lung cancer according to updated recommendations issued by the American Society of Clinical Oncology and the National Comprehensive Cancer Network.
ASCO issued a provisional clinical opinion (PCO) on April 7 that patients with advanced non–small cell lung cancer (NSCLC) who are being considered for treatment with one of the tyrosine kinase inhibitors (TKIs) that target the epidermal growth factor receptor (EGFR) should undergo EGFR-mutation testing.
Oncologists have learned that NSCLC is "really a collection of genetically distinct diseases," ASCO’s PCO panel cochair Dr. Vicki L. Keedy of Vanderbilt-Ingram Cancer Center in Nashville, Tenn., said in a press release. The goal is to "treat patients with drugs that target the molecular drivers of their specific tumors rather than using a one-size-fits-all approach."
The NCCN earlier updated its clinical management guidelines to include a category 1 recommendation that EGFR testing should be undertaken after histologic diagnosis of adenocarcinoma, large cell carcinoma, or undifferentiated carcinoma.
The NCCN recommendation does not extend to patients with squamous cell lung cancer, because the incidence of EGFR mutation in this patient subgroup is less than 3.6%, Dr. David S. Ettinger said in March at the organization’s annual conference.
Both groups based their endorsements on studies demonstrating that mutations in two regions of EGFR gene appear to predict tumor response to chemotherapy in general, and to TKIs specifically.
Among the research priorities that were identified by ASCO, Dr. Keedy noted the trials that are designed to discern whether first-line treatment with a TKI in EGFR mutation–negative patients delays chemotherapy or affects outcome; whether chemotherapy prior to TKI treatment in EGFR mutation–positive patients affects outcome; and whether there are clinically significant differences between erlotinib (Tarceva) and gefitinib (Iressa) among EGFR mutation–positive patients.
The last question is of particular interest, because gefitinib is not Food and Drug Administration approved outside a special program in the United States, whereas erlotinib is currently approved as second-line therapy, she said.
Dr. Ettinger, chair of the NCCN’s NSCLC guideline panel and professor of oncology at Johns Hopkins University in Baltimore, cited findings from the landmark IPASS (Iressa Pan-Asia Study) investigation that compared progression-free and overall survival in 1,217 East Asian patients with advanced NSCLC that was treated with the gefitinib or standard carboplatin and paclitaxel chemotherapy.
IPASS demonstrated that EGFR mutation strongly predicted a lower risk of progression on gefitinib vs. chemotherapy (hazard ratio, 0.48), whereas wild-type EGFR predicted a higher risk of progression on gefitinib relative to chemotherapy (HR, 2.85) (N. Engl. J. Med. 2009;361:947-57).
Similarly, in a pooled analysis of clinical outcomes of NSCLC patients who were treated with erlotinib, EGFR mutations were associated with a median progression-free survival of 13.2 vs. 5.9 months (J. Cell. Mol. Med. 2010;14:51-69). Neither study demonstrated a difference in overall survival among treated patients with and without EGFR mutations, Dr. Ettinger said.
The updated NCCN guidelines also state that the sequencing of KRAS (a G protein involved in the EGFR-related signal transmission) could be useful for the selection of patients as candidates for TKI therapy. The KRAS gene can harbor oncogenic mutations that may render a tumor resistant to EGFR-targeting agents, Dr. Ettinger explained, noting that studies have shown that a KRAS mutation in patients with NSCLC "confers a high level of resistance" to TKIs.
Although the data – which primarily come from retrospective reviews with small sample sizes – are insufficient to make a determination about an association between KRAS mutation status and survival, he said, they are sufficient to warrant a category 2A recommendation for sequencing, as well as a recommendation that patients with a known KRAS mutation should undergo first-line therapy with an agent other than a TKI.
Individuals who test negative for EGFR and KRAS should also be screened for a mutation of the anaplastic lymphoma kinase (ALK) fusion gene, Dr. Ettinger said. "Patients who screen positive may not benefit from EGFR TKIs, but they may be good candidates for an ALK-targeted therapy," he said, noting that the investigational ALK-targeting drug crizotinib, in particular, has demonstrated positive results in early studies of NSCLC patients with echinoderm microtubule-associated proteinlike 4 (EML4)-ALK translocations (N. Engl. J. Med. 2010;363:1693-703).
With respect to first-line systemic therapy, patients with adenocarcinoma, large cell carcinoma, or NSCLC "not otherwise specified" who have an Eastern Cooperative Oncology Group/World Health Organization performance status grade of 0-4 and who test positive for the EGFR mutation prior to first-line therapy should be treated with erlotinib, according to the NCCN guidelines. Alternatively, the guidelines state that gefitinib can be used in place of erlotinib "in areas of the world where it is available."
For patients in whom the EGFR mutation is discovered during chemotherapy, the guidelines recommend either adding erlotinib to the current chemotherapy protocol or switching to erlotinib as maintenance treatment."
For patients whose EGFR status is negative or unknown, even in the presence of clinical characteristics that might be suggestive of a mutation (for example, female, nonsmoker, Asian race), conventional chemotherapy is recommended, Dr. Ettinger said.
The updated NCCN guidelines for NSCLC are posted at www.nccn.org.
The guidelines take a conservative stance on the National Lung Screening Trial finding that screening with low-dose helical CT was associated with a 20% reduction in lung cancer deaths vs. screening with standard chest x-ray. Despite this positive finding, "the NCCN panel does not recommend the routine use of screening CT as a standard clinical practice," said Dr. Ettinger; more conclusive data from ongoing national trials are needed to define the associated risks and benefits. "High-risk patients should participate in a clinical trial evaluating CT screening or go to a center of excellence to discuss the potential risks and benefits of a screening CT," Dr. Ettinger said.
Other notable updates include the following:
• The addition of EBUS (endobronchial ultrasound) as a work-up recommendation.
• The recommendation that bevacizumab (Avastin) and chemotherapy or chemotherapy alone is indicated in performance status 0-1 patients with advanced or recurrent NSCLC, and that bevacizumab should be given until disease progression.
• The recommendation against systemic chemotherapy in performance status 3-4 NSCLC patients.
• The guidance that chemoradiation is better than chemotherapy alone in locally advanced NSCLC, and that concurrent chemoradiation is better than sequential chemoradiation.
• The addition of denosumab (Xgeva) as a treatment option for patients with bone metastases.
• The recommendation favoring cisplatin/pemetrexed (Alimta) vs. cisplatin/gemcitabine (Gemzar) in patients with nonsquamous histology.
• The recommendation against adding a third cytotoxic drug, with the exception of bevacizumab or cetuximab (Erbitux), in treatment-naive performance status 0-1 NSCLC patients.
• The guidance that cisplatin-based combinations are better than best supportive care in advanced, incurable disease, with improvement in median survival and 1-year survival rates.
The ASCO PCO is available online.
In an editorial that accompanied ASCO’s PCO announcement, Dr. Paul A. Bunn Jr. and Dr. Robert C. Doebele of the University of Colorado Cancer Center in Aurora wrote that the growing clinical importance of molecularly defined subgroups of adenocarcinoma signals a "new era of personalized medicine for patients with advanced lung cancer, in which it will be imperative to match the specific mutations of a patient’s tumor with a specific therapy."
The implementation of routine, simultaneous testing of multiple markers will likely be conducted on all patients prior to treatment initiation, regardless of clinical features, they stated, acknowledging certain procedural challenges, including obtaining adequate tumor material at the time of diagnostic biopsy and developing testing platforms "that simultaneously analyze for the presence of somatic mutations, gene fusions, or other genetic challenges."
Dr. Ettinger has consultancy agreements with the following companies: Biodesix, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Genentech, Merck, Novartis Pharmaceuticals, Poniard Pharmaceuticals, Prometheus Laboratories, Shin Nippon Biomedical Laboratories, and Telik. Dr. Keedy receives commercial research support from Ariad Pharmaceuticals, Ziopharm Oncology, and Amgen Oncology Therapeutics. Dr. Bunn has a consultant or advisory role with Amgen, AstraZeneca, Abraxis, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, GlaxoSmithKline, Syndax, Biodesix, Allos Therapeutics, Novartis, OSI/Genentech/Roche, Poniard, and Sanofi-Aventis. Dr. Doebele disclosed research funding from Lilly, ImClone Systems, and Pfizer.
Testing for epidermal growth factor receptor mutations is an important step in the evaluation process for systemic therapy in patients with metastatic or recurrent non–small cell lung cancer according to updated recommendations issued by the American Society of Clinical Oncology and the National Comprehensive Cancer Network.
ASCO issued a provisional clinical opinion (PCO) on April 7 that patients with advanced non–small cell lung cancer (NSCLC) who are being considered for treatment with one of the tyrosine kinase inhibitors (TKIs) that target the epidermal growth factor receptor (EGFR) should undergo EGFR-mutation testing.
Oncologists have learned that NSCLC is "really a collection of genetically distinct diseases," ASCO’s PCO panel cochair Dr. Vicki L. Keedy of Vanderbilt-Ingram Cancer Center in Nashville, Tenn., said in a press release. The goal is to "treat patients with drugs that target the molecular drivers of their specific tumors rather than using a one-size-fits-all approach."
The NCCN earlier updated its clinical management guidelines to include a category 1 recommendation that EGFR testing should be undertaken after histologic diagnosis of adenocarcinoma, large cell carcinoma, or undifferentiated carcinoma.
The NCCN recommendation does not extend to patients with squamous cell lung cancer, because the incidence of EGFR mutation in this patient subgroup is less than 3.6%, Dr. David S. Ettinger said in March at the organization’s annual conference.
Both groups based their endorsements on studies demonstrating that mutations in two regions of EGFR gene appear to predict tumor response to chemotherapy in general, and to TKIs specifically.
Among the research priorities that were identified by ASCO, Dr. Keedy noted the trials that are designed to discern whether first-line treatment with a TKI in EGFR mutation–negative patients delays chemotherapy or affects outcome; whether chemotherapy prior to TKI treatment in EGFR mutation–positive patients affects outcome; and whether there are clinically significant differences between erlotinib (Tarceva) and gefitinib (Iressa) among EGFR mutation–positive patients.
The last question is of particular interest, because gefitinib is not Food and Drug Administration approved outside a special program in the United States, whereas erlotinib is currently approved as second-line therapy, she said.
Dr. Ettinger, chair of the NCCN’s NSCLC guideline panel and professor of oncology at Johns Hopkins University in Baltimore, cited findings from the landmark IPASS (Iressa Pan-Asia Study) investigation that compared progression-free and overall survival in 1,217 East Asian patients with advanced NSCLC that was treated with the gefitinib or standard carboplatin and paclitaxel chemotherapy.
IPASS demonstrated that EGFR mutation strongly predicted a lower risk of progression on gefitinib vs. chemotherapy (hazard ratio, 0.48), whereas wild-type EGFR predicted a higher risk of progression on gefitinib relative to chemotherapy (HR, 2.85) (N. Engl. J. Med. 2009;361:947-57).
Similarly, in a pooled analysis of clinical outcomes of NSCLC patients who were treated with erlotinib, EGFR mutations were associated with a median progression-free survival of 13.2 vs. 5.9 months (J. Cell. Mol. Med. 2010;14:51-69). Neither study demonstrated a difference in overall survival among treated patients with and without EGFR mutations, Dr. Ettinger said.
The updated NCCN guidelines also state that the sequencing of KRAS (a G protein involved in the EGFR-related signal transmission) could be useful for the selection of patients as candidates for TKI therapy. The KRAS gene can harbor oncogenic mutations that may render a tumor resistant to EGFR-targeting agents, Dr. Ettinger explained, noting that studies have shown that a KRAS mutation in patients with NSCLC "confers a high level of resistance" to TKIs.
Although the data – which primarily come from retrospective reviews with small sample sizes – are insufficient to make a determination about an association between KRAS mutation status and survival, he said, they are sufficient to warrant a category 2A recommendation for sequencing, as well as a recommendation that patients with a known KRAS mutation should undergo first-line therapy with an agent other than a TKI.
Individuals who test negative for EGFR and KRAS should also be screened for a mutation of the anaplastic lymphoma kinase (ALK) fusion gene, Dr. Ettinger said. "Patients who screen positive may not benefit from EGFR TKIs, but they may be good candidates for an ALK-targeted therapy," he said, noting that the investigational ALK-targeting drug crizotinib, in particular, has demonstrated positive results in early studies of NSCLC patients with echinoderm microtubule-associated proteinlike 4 (EML4)-ALK translocations (N. Engl. J. Med. 2010;363:1693-703).
With respect to first-line systemic therapy, patients with adenocarcinoma, large cell carcinoma, or NSCLC "not otherwise specified" who have an Eastern Cooperative Oncology Group/World Health Organization performance status grade of 0-4 and who test positive for the EGFR mutation prior to first-line therapy should be treated with erlotinib, according to the NCCN guidelines. Alternatively, the guidelines state that gefitinib can be used in place of erlotinib "in areas of the world where it is available."
For patients in whom the EGFR mutation is discovered during chemotherapy, the guidelines recommend either adding erlotinib to the current chemotherapy protocol or switching to erlotinib as maintenance treatment."
For patients whose EGFR status is negative or unknown, even in the presence of clinical characteristics that might be suggestive of a mutation (for example, female, nonsmoker, Asian race), conventional chemotherapy is recommended, Dr. Ettinger said.
The updated NCCN guidelines for NSCLC are posted at www.nccn.org.
The guidelines take a conservative stance on the National Lung Screening Trial finding that screening with low-dose helical CT was associated with a 20% reduction in lung cancer deaths vs. screening with standard chest x-ray. Despite this positive finding, "the NCCN panel does not recommend the routine use of screening CT as a standard clinical practice," said Dr. Ettinger; more conclusive data from ongoing national trials are needed to define the associated risks and benefits. "High-risk patients should participate in a clinical trial evaluating CT screening or go to a center of excellence to discuss the potential risks and benefits of a screening CT," Dr. Ettinger said.
Other notable updates include the following:
• The addition of EBUS (endobronchial ultrasound) as a work-up recommendation.
• The recommendation that bevacizumab (Avastin) and chemotherapy or chemotherapy alone is indicated in performance status 0-1 patients with advanced or recurrent NSCLC, and that bevacizumab should be given until disease progression.
• The recommendation against systemic chemotherapy in performance status 3-4 NSCLC patients.
• The guidance that chemoradiation is better than chemotherapy alone in locally advanced NSCLC, and that concurrent chemoradiation is better than sequential chemoradiation.
• The addition of denosumab (Xgeva) as a treatment option for patients with bone metastases.
• The recommendation favoring cisplatin/pemetrexed (Alimta) vs. cisplatin/gemcitabine (Gemzar) in patients with nonsquamous histology.
• The recommendation against adding a third cytotoxic drug, with the exception of bevacizumab or cetuximab (Erbitux), in treatment-naive performance status 0-1 NSCLC patients.
• The guidance that cisplatin-based combinations are better than best supportive care in advanced, incurable disease, with improvement in median survival and 1-year survival rates.
The ASCO PCO is available online.
In an editorial that accompanied ASCO’s PCO announcement, Dr. Paul A. Bunn Jr. and Dr. Robert C. Doebele of the University of Colorado Cancer Center in Aurora wrote that the growing clinical importance of molecularly defined subgroups of adenocarcinoma signals a "new era of personalized medicine for patients with advanced lung cancer, in which it will be imperative to match the specific mutations of a patient’s tumor with a specific therapy."
The implementation of routine, simultaneous testing of multiple markers will likely be conducted on all patients prior to treatment initiation, regardless of clinical features, they stated, acknowledging certain procedural challenges, including obtaining adequate tumor material at the time of diagnostic biopsy and developing testing platforms "that simultaneously analyze for the presence of somatic mutations, gene fusions, or other genetic challenges."
Dr. Ettinger has consultancy agreements with the following companies: Biodesix, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Genentech, Merck, Novartis Pharmaceuticals, Poniard Pharmaceuticals, Prometheus Laboratories, Shin Nippon Biomedical Laboratories, and Telik. Dr. Keedy receives commercial research support from Ariad Pharmaceuticals, Ziopharm Oncology, and Amgen Oncology Therapeutics. Dr. Bunn has a consultant or advisory role with Amgen, AstraZeneca, Abraxis, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, GlaxoSmithKline, Syndax, Biodesix, Allos Therapeutics, Novartis, OSI/Genentech/Roche, Poniard, and Sanofi-Aventis. Dr. Doebele disclosed research funding from Lilly, ImClone Systems, and Pfizer.
Testing for epidermal growth factor receptor mutations is an important step in the evaluation process for systemic therapy in patients with metastatic or recurrent non–small cell lung cancer according to updated recommendations issued by the American Society of Clinical Oncology and the National Comprehensive Cancer Network.
ASCO issued a provisional clinical opinion (PCO) on April 7 that patients with advanced non–small cell lung cancer (NSCLC) who are being considered for treatment with one of the tyrosine kinase inhibitors (TKIs) that target the epidermal growth factor receptor (EGFR) should undergo EGFR-mutation testing.
Oncologists have learned that NSCLC is "really a collection of genetically distinct diseases," ASCO’s PCO panel cochair Dr. Vicki L. Keedy of Vanderbilt-Ingram Cancer Center in Nashville, Tenn., said in a press release. The goal is to "treat patients with drugs that target the molecular drivers of their specific tumors rather than using a one-size-fits-all approach."
The NCCN earlier updated its clinical management guidelines to include a category 1 recommendation that EGFR testing should be undertaken after histologic diagnosis of adenocarcinoma, large cell carcinoma, or undifferentiated carcinoma.
The NCCN recommendation does not extend to patients with squamous cell lung cancer, because the incidence of EGFR mutation in this patient subgroup is less than 3.6%, Dr. David S. Ettinger said in March at the organization’s annual conference.
Both groups based their endorsements on studies demonstrating that mutations in two regions of EGFR gene appear to predict tumor response to chemotherapy in general, and to TKIs specifically.
Among the research priorities that were identified by ASCO, Dr. Keedy noted the trials that are designed to discern whether first-line treatment with a TKI in EGFR mutation–negative patients delays chemotherapy or affects outcome; whether chemotherapy prior to TKI treatment in EGFR mutation–positive patients affects outcome; and whether there are clinically significant differences between erlotinib (Tarceva) and gefitinib (Iressa) among EGFR mutation–positive patients.
The last question is of particular interest, because gefitinib is not Food and Drug Administration approved outside a special program in the United States, whereas erlotinib is currently approved as second-line therapy, she said.
Dr. Ettinger, chair of the NCCN’s NSCLC guideline panel and professor of oncology at Johns Hopkins University in Baltimore, cited findings from the landmark IPASS (Iressa Pan-Asia Study) investigation that compared progression-free and overall survival in 1,217 East Asian patients with advanced NSCLC that was treated with the gefitinib or standard carboplatin and paclitaxel chemotherapy.
IPASS demonstrated that EGFR mutation strongly predicted a lower risk of progression on gefitinib vs. chemotherapy (hazard ratio, 0.48), whereas wild-type EGFR predicted a higher risk of progression on gefitinib relative to chemotherapy (HR, 2.85) (N. Engl. J. Med. 2009;361:947-57).
Similarly, in a pooled analysis of clinical outcomes of NSCLC patients who were treated with erlotinib, EGFR mutations were associated with a median progression-free survival of 13.2 vs. 5.9 months (J. Cell. Mol. Med. 2010;14:51-69). Neither study demonstrated a difference in overall survival among treated patients with and without EGFR mutations, Dr. Ettinger said.
The updated NCCN guidelines also state that the sequencing of KRAS (a G protein involved in the EGFR-related signal transmission) could be useful for the selection of patients as candidates for TKI therapy. The KRAS gene can harbor oncogenic mutations that may render a tumor resistant to EGFR-targeting agents, Dr. Ettinger explained, noting that studies have shown that a KRAS mutation in patients with NSCLC "confers a high level of resistance" to TKIs.
Although the data – which primarily come from retrospective reviews with small sample sizes – are insufficient to make a determination about an association between KRAS mutation status and survival, he said, they are sufficient to warrant a category 2A recommendation for sequencing, as well as a recommendation that patients with a known KRAS mutation should undergo first-line therapy with an agent other than a TKI.
Individuals who test negative for EGFR and KRAS should also be screened for a mutation of the anaplastic lymphoma kinase (ALK) fusion gene, Dr. Ettinger said. "Patients who screen positive may not benefit from EGFR TKIs, but they may be good candidates for an ALK-targeted therapy," he said, noting that the investigational ALK-targeting drug crizotinib, in particular, has demonstrated positive results in early studies of NSCLC patients with echinoderm microtubule-associated proteinlike 4 (EML4)-ALK translocations (N. Engl. J. Med. 2010;363:1693-703).
With respect to first-line systemic therapy, patients with adenocarcinoma, large cell carcinoma, or NSCLC "not otherwise specified" who have an Eastern Cooperative Oncology Group/World Health Organization performance status grade of 0-4 and who test positive for the EGFR mutation prior to first-line therapy should be treated with erlotinib, according to the NCCN guidelines. Alternatively, the guidelines state that gefitinib can be used in place of erlotinib "in areas of the world where it is available."
For patients in whom the EGFR mutation is discovered during chemotherapy, the guidelines recommend either adding erlotinib to the current chemotherapy protocol or switching to erlotinib as maintenance treatment."
For patients whose EGFR status is negative or unknown, even in the presence of clinical characteristics that might be suggestive of a mutation (for example, female, nonsmoker, Asian race), conventional chemotherapy is recommended, Dr. Ettinger said.
The updated NCCN guidelines for NSCLC are posted at www.nccn.org.
The guidelines take a conservative stance on the National Lung Screening Trial finding that screening with low-dose helical CT was associated with a 20% reduction in lung cancer deaths vs. screening with standard chest x-ray. Despite this positive finding, "the NCCN panel does not recommend the routine use of screening CT as a standard clinical practice," said Dr. Ettinger; more conclusive data from ongoing national trials are needed to define the associated risks and benefits. "High-risk patients should participate in a clinical trial evaluating CT screening or go to a center of excellence to discuss the potential risks and benefits of a screening CT," Dr. Ettinger said.
Other notable updates include the following:
• The addition of EBUS (endobronchial ultrasound) as a work-up recommendation.
• The recommendation that bevacizumab (Avastin) and chemotherapy or chemotherapy alone is indicated in performance status 0-1 patients with advanced or recurrent NSCLC, and that bevacizumab should be given until disease progression.
• The recommendation against systemic chemotherapy in performance status 3-4 NSCLC patients.
• The guidance that chemoradiation is better than chemotherapy alone in locally advanced NSCLC, and that concurrent chemoradiation is better than sequential chemoradiation.
• The addition of denosumab (Xgeva) as a treatment option for patients with bone metastases.
• The recommendation favoring cisplatin/pemetrexed (Alimta) vs. cisplatin/gemcitabine (Gemzar) in patients with nonsquamous histology.
• The recommendation against adding a third cytotoxic drug, with the exception of bevacizumab or cetuximab (Erbitux), in treatment-naive performance status 0-1 NSCLC patients.
• The guidance that cisplatin-based combinations are better than best supportive care in advanced, incurable disease, with improvement in median survival and 1-year survival rates.
The ASCO PCO is available online.
In an editorial that accompanied ASCO’s PCO announcement, Dr. Paul A. Bunn Jr. and Dr. Robert C. Doebele of the University of Colorado Cancer Center in Aurora wrote that the growing clinical importance of molecularly defined subgroups of adenocarcinoma signals a "new era of personalized medicine for patients with advanced lung cancer, in which it will be imperative to match the specific mutations of a patient’s tumor with a specific therapy."
The implementation of routine, simultaneous testing of multiple markers will likely be conducted on all patients prior to treatment initiation, regardless of clinical features, they stated, acknowledging certain procedural challenges, including obtaining adequate tumor material at the time of diagnostic biopsy and developing testing platforms "that simultaneously analyze for the presence of somatic mutations, gene fusions, or other genetic challenges."
Dr. Ettinger has consultancy agreements with the following companies: Biodesix, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Genentech, Merck, Novartis Pharmaceuticals, Poniard Pharmaceuticals, Prometheus Laboratories, Shin Nippon Biomedical Laboratories, and Telik. Dr. Keedy receives commercial research support from Ariad Pharmaceuticals, Ziopharm Oncology, and Amgen Oncology Therapeutics. Dr. Bunn has a consultant or advisory role with Amgen, AstraZeneca, Abraxis, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, GlaxoSmithKline, Syndax, Biodesix, Allos Therapeutics, Novartis, OSI/Genentech/Roche, Poniard, and Sanofi-Aventis. Dr. Doebele disclosed research funding from Lilly, ImClone Systems, and Pfizer.
FDA Keeping an Eye on New Malignancy Concerns With Lenalidomide
The Food and Drug Administration has alerted the public that the agency is currently reviewing all available information on the potential for increased risk of new malignancies associated with lenalidomide in patients treated for multiple myeloma or myelodysplatic syndromes.
The agency plans to communicate any new recommendations once it has completed its review of existing data, according to a safety announcement released on April 8, 2011. "At this time, [the] FDA recommends that patients continue their Revlimid [lenalidomide] treatment as prescribed by their health care provider," it said.
The concerns appear to be based in part on results from the phase III Cancer and Leukemia Group B (CALGB) 100104 trial of 460 patients with stage I-III multiple myeloma. In the trial, the estimated time to progression reached 42.3 months with lenalidomide maintenance following transplant vs. 21.8 months with placebo. (The results were reported at the 2010 annual meeting of the American Society of Hematology.)
As of late 2010, though, 25 patients had new malignancies: 15 patients in the lenalidomide group, 6 on placebo, and 4 who developed these before randomization. The second cancers included five cases of acute myeloid leukemia or myelodysplastic syndrome, three of which occurred in patients on lenalidomide maintenance.
Lenalidomide, a less-toxic thalidomide analogue, is one of the more important new therapies in multiple myeloma. In addition to the CALGB trial, results from the Intergroupe Francophone du Myélome (IFM) 2005-02 trial also support maintenance lenalidomide.
Lenalidomide is indicated for the treatment of multiple myeloma, in combination with dexamethasone, in patients who have received at least one prior therapy. It is also indicated for patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities.
"At this time, there is no recommendation to delay, modify, or restrict the use of Revlimid for patients being treated according to the FDA-approved indications," the agency noted. "[The] FDA believes the benefits of Revlimid continue to outweigh the potential risks."
The FDA is also currently reviewing all available information on this potential risk for thalidomide.
Physicians are encouraged to report adverse events involving lenalidomide to the FDA MedWatch program.
Cancer and Leukemia Group B, CALGB,
The Food and Drug Administration has alerted the public that the agency is currently reviewing all available information on the potential for increased risk of new malignancies associated with lenalidomide in patients treated for multiple myeloma or myelodysplatic syndromes.
The agency plans to communicate any new recommendations once it has completed its review of existing data, according to a safety announcement released on April 8, 2011. "At this time, [the] FDA recommends that patients continue their Revlimid [lenalidomide] treatment as prescribed by their health care provider," it said.
The concerns appear to be based in part on results from the phase III Cancer and Leukemia Group B (CALGB) 100104 trial of 460 patients with stage I-III multiple myeloma. In the trial, the estimated time to progression reached 42.3 months with lenalidomide maintenance following transplant vs. 21.8 months with placebo. (The results were reported at the 2010 annual meeting of the American Society of Hematology.)
As of late 2010, though, 25 patients had new malignancies: 15 patients in the lenalidomide group, 6 on placebo, and 4 who developed these before randomization. The second cancers included five cases of acute myeloid leukemia or myelodysplastic syndrome, three of which occurred in patients on lenalidomide maintenance.
Lenalidomide, a less-toxic thalidomide analogue, is one of the more important new therapies in multiple myeloma. In addition to the CALGB trial, results from the Intergroupe Francophone du Myélome (IFM) 2005-02 trial also support maintenance lenalidomide.
Lenalidomide is indicated for the treatment of multiple myeloma, in combination with dexamethasone, in patients who have received at least one prior therapy. It is also indicated for patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities.
"At this time, there is no recommendation to delay, modify, or restrict the use of Revlimid for patients being treated according to the FDA-approved indications," the agency noted. "[The] FDA believes the benefits of Revlimid continue to outweigh the potential risks."
The FDA is also currently reviewing all available information on this potential risk for thalidomide.
Physicians are encouraged to report adverse events involving lenalidomide to the FDA MedWatch program.
The Food and Drug Administration has alerted the public that the agency is currently reviewing all available information on the potential for increased risk of new malignancies associated with lenalidomide in patients treated for multiple myeloma or myelodysplatic syndromes.
The agency plans to communicate any new recommendations once it has completed its review of existing data, according to a safety announcement released on April 8, 2011. "At this time, [the] FDA recommends that patients continue their Revlimid [lenalidomide] treatment as prescribed by their health care provider," it said.
The concerns appear to be based in part on results from the phase III Cancer and Leukemia Group B (CALGB) 100104 trial of 460 patients with stage I-III multiple myeloma. In the trial, the estimated time to progression reached 42.3 months with lenalidomide maintenance following transplant vs. 21.8 months with placebo. (The results were reported at the 2010 annual meeting of the American Society of Hematology.)
As of late 2010, though, 25 patients had new malignancies: 15 patients in the lenalidomide group, 6 on placebo, and 4 who developed these before randomization. The second cancers included five cases of acute myeloid leukemia or myelodysplastic syndrome, three of which occurred in patients on lenalidomide maintenance.
Lenalidomide, a less-toxic thalidomide analogue, is one of the more important new therapies in multiple myeloma. In addition to the CALGB trial, results from the Intergroupe Francophone du Myélome (IFM) 2005-02 trial also support maintenance lenalidomide.
Lenalidomide is indicated for the treatment of multiple myeloma, in combination with dexamethasone, in patients who have received at least one prior therapy. It is also indicated for patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities.
"At this time, there is no recommendation to delay, modify, or restrict the use of Revlimid for patients being treated according to the FDA-approved indications," the agency noted. "[The] FDA believes the benefits of Revlimid continue to outweigh the potential risks."
The FDA is also currently reviewing all available information on this potential risk for thalidomide.
Physicians are encouraged to report adverse events involving lenalidomide to the FDA MedWatch program.
Cancer and Leukemia Group B, CALGB,
Cancer and Leukemia Group B, CALGB,
Serum Sickness with Clarithromycin
Serum sickness is an immunological condition characterized by fever, rash, arthralgia/arthritis, myalgia, edema, and localized lymphadenopathy. Historically, this syndrome was seen as an immunologic response to heterologous protein components administered for therapeutic purposes, such as in the treatment of diphtheria and scarlet fever. Following the decline in use of such heterologous proteins, this same condition is now seen with equine antitoxins, monoclonal antibodies, and some drugs.13 Specifically, the immunologic response to these drugs is referred to as serum sickness‐like reaction (SSLR). The classic serum sickness is described as a prototype Gell and Coombs type III or immune complex‐mediated hypersensitivity disease.4 When a foreign protein antitoxin is administered into human serum, immune system recognition and antibody production occurs. Antibodies become attached to antigens and, when there are sufficient antibody/antigen bonds, a lattice‐like aggregate called the immune complex forms. Normally these immune complexes are cleared from the blood by the reticulo‐endothelial system, but if the system is defective, or the complexes are in a sufficiently large quantity, then deposition into various tissues like the internal elastic lamina of arteries, perivascular regions, synovia, and glomeruli occurs. Following deposition, complement is activated, causing inflammation in these same tissues, resulting in fever, rash, arthralgia, and myalgia.5 A similar reaction has been seen with certain drug exposures as well. The mechanism for this reaction is less clear, but thought to be similar to haptens attaching to plasma proteins and inciting the immunological response.6
Case
A 57‐year‐old white female presented with rash and generalized body aches. She had no significant past medical history, except for sinusitis several years ago; she was prescribed clarithromycin but did not report any problem with this medication at that time. The patient was diagnosed with acute sinusitis 4 days before this presentation. She had visited a primary care physician for her sinusitis and had been prescribed clarithromycin 500 mg twice daily for 7 days. The patient did not use any prescribed or nonprescribed medications in the last 6 months, except the current use of clarithromycin. She used the medication for 3 days as directed, when she developed a generalized rash. The rash first developed on both arms and then migrated to involve the rest of the body within 1 day. The following day, she developed generalized weakness, muscle aches, and symmetric joint pain in the wrists, arms, fingers, and knees. She stopped taking the medication after her sixth dose because she thought her symptoms might be related to its use. Her rash began to fade away slightly. On the 4th day, her myalgias and arthralgias acutely worsened, limiting her normal activities. She developed shortness of breath, ultimately prompting her visit to the emergency department. On presentation, her temperature was 98F, pulse 76, blood pressure 115/73, and oxygen saturation 99% on room air. She was in no acute distress, had no signs of acute airway compromise, and was comfortable at rest. On examination, she had a pruritic morbilliform rash which was most prominent on her upper extremities. There was no muscular tenderness elicited on her body. The joint examination was entirely normal. Ear, nose, and throat examination was normal; there was no lip swelling, erythema, or swelling in the oral cavity or stridor. The chest was clear to auscultation, and the heart examination was normal. Pertinent labs (and normal ranges) included: C3, 83 mg/dL (79‐152 mg/dL); C4, 11 mg/dL (16‐38 mg/dL); total complement, 24 mg/dL (30‐75 mg/dL); erythrocyte sedimentation rate (ESR), 21 mm/hr (20 mm/hr); and C‐reactive protein (CRP), 0.8 mg/dL (normal, 0.8 mg/dL). Basic chemistries were unremarkable. Serum creatinine was 0.8 mg/dL, and blood urea nitrogen was 11 mg/dL. Creatine phosphokinase was 54 U/L. Liver function tests were normal. Complete blood count with differential showed: Hb, 12.5 g/dL; platelets, 228,000/mm3; polymorphonuclear cells, 76%; lymphocytes, 15%; and eosinophils, 5%. Given the history, the temporal association of symptoms with medication use, physical examination findings, low complement level, and elevated ESR, the diagnosis of serum sickness‐like reaction was made. The patient received intravenous dexamethasone 4 mg once and, following an observation period in the emergency department, was discharged on an oral prednisone taper, with diphenhydramine to use as needed. The patient responded well, and recovered uneventfully.
Discussion
Serum sickness‐like reaction has been described for many drugs, especially antibiotics.7 A clarithromycin‐associated reaction has not been reported previously. Diagnosis of SSLR in this case was suggested by several factors, including the temporal association between clarithromycin ingestion, as well as consistent physical examination and laboratory findings. The patient's past history of clarithromycin use caused the reaction to occur within 36 hours of drug ingestion. Important diagnoses that were considered included angioedema, systemic lupus erythematosus, StevensJohnson syndrome or other drug eruptions, viral exanthemata, reactive arthritis, and acute rheumatic fever. However, the typical morbilliform skin eruptions with mucosal sparing made both lupus and StevensJohnson syndrome unlikely. Without facial or lip edema, angioedema also seemed less probable. Typical features of viral exanthem were also not seen in this patient. The lack of a prior history of a similar reaction and prompt recovery with antiinflammatories also supported a diagnosis of SSLR. Clarithromycin is a very commonly prescribed antibiotic for the treatment of upper respiratory tract infections; this case emphasizes that clinicians should remain aware that its use may rarely be associated with SSLR.
- ,,,.Serum sickness‐like reactions in patients receiving intravenous infliximab.J Emerg Med.2006;30(1):41–44.
- ,,,.Severe serum sickness reaction to oral and intramuscular penicillin.Pharmacotherapy.2006;26(5):705–708.
- ,,,.Serum sickness‐like reactions to amoxicillin, cefaclor, cephalexin, and trimethoprim‐sulfamethoxazole.J Infect Dis.1988;158(2):474–477.
- ,,, et al.A prospective clinical and immunologic analysis of patients with serum sickness.N Engl J Med.1984;311(22):1407–1413.
- ,.Severe adverse cutaneous reactions to drugs.N Engl J Med.1994;331(19):1272–1285.
- ,,.Idiosyncratic drug reactions: the reactive metabolite syndromes.Lancet2000;356(9241):1587–1591.
- ,,,,.Cefaclor‐associated serum sickness‐like disease: eight cases and review of the literature.Ann Pharmacother.1992;26(7–8):910–914.
Serum sickness is an immunological condition characterized by fever, rash, arthralgia/arthritis, myalgia, edema, and localized lymphadenopathy. Historically, this syndrome was seen as an immunologic response to heterologous protein components administered for therapeutic purposes, such as in the treatment of diphtheria and scarlet fever. Following the decline in use of such heterologous proteins, this same condition is now seen with equine antitoxins, monoclonal antibodies, and some drugs.13 Specifically, the immunologic response to these drugs is referred to as serum sickness‐like reaction (SSLR). The classic serum sickness is described as a prototype Gell and Coombs type III or immune complex‐mediated hypersensitivity disease.4 When a foreign protein antitoxin is administered into human serum, immune system recognition and antibody production occurs. Antibodies become attached to antigens and, when there are sufficient antibody/antigen bonds, a lattice‐like aggregate called the immune complex forms. Normally these immune complexes are cleared from the blood by the reticulo‐endothelial system, but if the system is defective, or the complexes are in a sufficiently large quantity, then deposition into various tissues like the internal elastic lamina of arteries, perivascular regions, synovia, and glomeruli occurs. Following deposition, complement is activated, causing inflammation in these same tissues, resulting in fever, rash, arthralgia, and myalgia.5 A similar reaction has been seen with certain drug exposures as well. The mechanism for this reaction is less clear, but thought to be similar to haptens attaching to plasma proteins and inciting the immunological response.6
Case
A 57‐year‐old white female presented with rash and generalized body aches. She had no significant past medical history, except for sinusitis several years ago; she was prescribed clarithromycin but did not report any problem with this medication at that time. The patient was diagnosed with acute sinusitis 4 days before this presentation. She had visited a primary care physician for her sinusitis and had been prescribed clarithromycin 500 mg twice daily for 7 days. The patient did not use any prescribed or nonprescribed medications in the last 6 months, except the current use of clarithromycin. She used the medication for 3 days as directed, when she developed a generalized rash. The rash first developed on both arms and then migrated to involve the rest of the body within 1 day. The following day, she developed generalized weakness, muscle aches, and symmetric joint pain in the wrists, arms, fingers, and knees. She stopped taking the medication after her sixth dose because she thought her symptoms might be related to its use. Her rash began to fade away slightly. On the 4th day, her myalgias and arthralgias acutely worsened, limiting her normal activities. She developed shortness of breath, ultimately prompting her visit to the emergency department. On presentation, her temperature was 98F, pulse 76, blood pressure 115/73, and oxygen saturation 99% on room air. She was in no acute distress, had no signs of acute airway compromise, and was comfortable at rest. On examination, she had a pruritic morbilliform rash which was most prominent on her upper extremities. There was no muscular tenderness elicited on her body. The joint examination was entirely normal. Ear, nose, and throat examination was normal; there was no lip swelling, erythema, or swelling in the oral cavity or stridor. The chest was clear to auscultation, and the heart examination was normal. Pertinent labs (and normal ranges) included: C3, 83 mg/dL (79‐152 mg/dL); C4, 11 mg/dL (16‐38 mg/dL); total complement, 24 mg/dL (30‐75 mg/dL); erythrocyte sedimentation rate (ESR), 21 mm/hr (20 mm/hr); and C‐reactive protein (CRP), 0.8 mg/dL (normal, 0.8 mg/dL). Basic chemistries were unremarkable. Serum creatinine was 0.8 mg/dL, and blood urea nitrogen was 11 mg/dL. Creatine phosphokinase was 54 U/L. Liver function tests were normal. Complete blood count with differential showed: Hb, 12.5 g/dL; platelets, 228,000/mm3; polymorphonuclear cells, 76%; lymphocytes, 15%; and eosinophils, 5%. Given the history, the temporal association of symptoms with medication use, physical examination findings, low complement level, and elevated ESR, the diagnosis of serum sickness‐like reaction was made. The patient received intravenous dexamethasone 4 mg once and, following an observation period in the emergency department, was discharged on an oral prednisone taper, with diphenhydramine to use as needed. The patient responded well, and recovered uneventfully.
Discussion
Serum sickness‐like reaction has been described for many drugs, especially antibiotics.7 A clarithromycin‐associated reaction has not been reported previously. Diagnosis of SSLR in this case was suggested by several factors, including the temporal association between clarithromycin ingestion, as well as consistent physical examination and laboratory findings. The patient's past history of clarithromycin use caused the reaction to occur within 36 hours of drug ingestion. Important diagnoses that were considered included angioedema, systemic lupus erythematosus, StevensJohnson syndrome or other drug eruptions, viral exanthemata, reactive arthritis, and acute rheumatic fever. However, the typical morbilliform skin eruptions with mucosal sparing made both lupus and StevensJohnson syndrome unlikely. Without facial or lip edema, angioedema also seemed less probable. Typical features of viral exanthem were also not seen in this patient. The lack of a prior history of a similar reaction and prompt recovery with antiinflammatories also supported a diagnosis of SSLR. Clarithromycin is a very commonly prescribed antibiotic for the treatment of upper respiratory tract infections; this case emphasizes that clinicians should remain aware that its use may rarely be associated with SSLR.
Serum sickness is an immunological condition characterized by fever, rash, arthralgia/arthritis, myalgia, edema, and localized lymphadenopathy. Historically, this syndrome was seen as an immunologic response to heterologous protein components administered for therapeutic purposes, such as in the treatment of diphtheria and scarlet fever. Following the decline in use of such heterologous proteins, this same condition is now seen with equine antitoxins, monoclonal antibodies, and some drugs.13 Specifically, the immunologic response to these drugs is referred to as serum sickness‐like reaction (SSLR). The classic serum sickness is described as a prototype Gell and Coombs type III or immune complex‐mediated hypersensitivity disease.4 When a foreign protein antitoxin is administered into human serum, immune system recognition and antibody production occurs. Antibodies become attached to antigens and, when there are sufficient antibody/antigen bonds, a lattice‐like aggregate called the immune complex forms. Normally these immune complexes are cleared from the blood by the reticulo‐endothelial system, but if the system is defective, or the complexes are in a sufficiently large quantity, then deposition into various tissues like the internal elastic lamina of arteries, perivascular regions, synovia, and glomeruli occurs. Following deposition, complement is activated, causing inflammation in these same tissues, resulting in fever, rash, arthralgia, and myalgia.5 A similar reaction has been seen with certain drug exposures as well. The mechanism for this reaction is less clear, but thought to be similar to haptens attaching to plasma proteins and inciting the immunological response.6
Case
A 57‐year‐old white female presented with rash and generalized body aches. She had no significant past medical history, except for sinusitis several years ago; she was prescribed clarithromycin but did not report any problem with this medication at that time. The patient was diagnosed with acute sinusitis 4 days before this presentation. She had visited a primary care physician for her sinusitis and had been prescribed clarithromycin 500 mg twice daily for 7 days. The patient did not use any prescribed or nonprescribed medications in the last 6 months, except the current use of clarithromycin. She used the medication for 3 days as directed, when she developed a generalized rash. The rash first developed on both arms and then migrated to involve the rest of the body within 1 day. The following day, she developed generalized weakness, muscle aches, and symmetric joint pain in the wrists, arms, fingers, and knees. She stopped taking the medication after her sixth dose because she thought her symptoms might be related to its use. Her rash began to fade away slightly. On the 4th day, her myalgias and arthralgias acutely worsened, limiting her normal activities. She developed shortness of breath, ultimately prompting her visit to the emergency department. On presentation, her temperature was 98F, pulse 76, blood pressure 115/73, and oxygen saturation 99% on room air. She was in no acute distress, had no signs of acute airway compromise, and was comfortable at rest. On examination, she had a pruritic morbilliform rash which was most prominent on her upper extremities. There was no muscular tenderness elicited on her body. The joint examination was entirely normal. Ear, nose, and throat examination was normal; there was no lip swelling, erythema, or swelling in the oral cavity or stridor. The chest was clear to auscultation, and the heart examination was normal. Pertinent labs (and normal ranges) included: C3, 83 mg/dL (79‐152 mg/dL); C4, 11 mg/dL (16‐38 mg/dL); total complement, 24 mg/dL (30‐75 mg/dL); erythrocyte sedimentation rate (ESR), 21 mm/hr (20 mm/hr); and C‐reactive protein (CRP), 0.8 mg/dL (normal, 0.8 mg/dL). Basic chemistries were unremarkable. Serum creatinine was 0.8 mg/dL, and blood urea nitrogen was 11 mg/dL. Creatine phosphokinase was 54 U/L. Liver function tests were normal. Complete blood count with differential showed: Hb, 12.5 g/dL; platelets, 228,000/mm3; polymorphonuclear cells, 76%; lymphocytes, 15%; and eosinophils, 5%. Given the history, the temporal association of symptoms with medication use, physical examination findings, low complement level, and elevated ESR, the diagnosis of serum sickness‐like reaction was made. The patient received intravenous dexamethasone 4 mg once and, following an observation period in the emergency department, was discharged on an oral prednisone taper, with diphenhydramine to use as needed. The patient responded well, and recovered uneventfully.
Discussion
Serum sickness‐like reaction has been described for many drugs, especially antibiotics.7 A clarithromycin‐associated reaction has not been reported previously. Diagnosis of SSLR in this case was suggested by several factors, including the temporal association between clarithromycin ingestion, as well as consistent physical examination and laboratory findings. The patient's past history of clarithromycin use caused the reaction to occur within 36 hours of drug ingestion. Important diagnoses that were considered included angioedema, systemic lupus erythematosus, StevensJohnson syndrome or other drug eruptions, viral exanthemata, reactive arthritis, and acute rheumatic fever. However, the typical morbilliform skin eruptions with mucosal sparing made both lupus and StevensJohnson syndrome unlikely. Without facial or lip edema, angioedema also seemed less probable. Typical features of viral exanthem were also not seen in this patient. The lack of a prior history of a similar reaction and prompt recovery with antiinflammatories also supported a diagnosis of SSLR. Clarithromycin is a very commonly prescribed antibiotic for the treatment of upper respiratory tract infections; this case emphasizes that clinicians should remain aware that its use may rarely be associated with SSLR.
- ,,,.Serum sickness‐like reactions in patients receiving intravenous infliximab.J Emerg Med.2006;30(1):41–44.
- ,,,.Severe serum sickness reaction to oral and intramuscular penicillin.Pharmacotherapy.2006;26(5):705–708.
- ,,,.Serum sickness‐like reactions to amoxicillin, cefaclor, cephalexin, and trimethoprim‐sulfamethoxazole.J Infect Dis.1988;158(2):474–477.
- ,,, et al.A prospective clinical and immunologic analysis of patients with serum sickness.N Engl J Med.1984;311(22):1407–1413.
- ,.Severe adverse cutaneous reactions to drugs.N Engl J Med.1994;331(19):1272–1285.
- ,,.Idiosyncratic drug reactions: the reactive metabolite syndromes.Lancet2000;356(9241):1587–1591.
- ,,,,.Cefaclor‐associated serum sickness‐like disease: eight cases and review of the literature.Ann Pharmacother.1992;26(7–8):910–914.
- ,,,.Serum sickness‐like reactions in patients receiving intravenous infliximab.J Emerg Med.2006;30(1):41–44.
- ,,,.Severe serum sickness reaction to oral and intramuscular penicillin.Pharmacotherapy.2006;26(5):705–708.
- ,,,.Serum sickness‐like reactions to amoxicillin, cefaclor, cephalexin, and trimethoprim‐sulfamethoxazole.J Infect Dis.1988;158(2):474–477.
- ,,, et al.A prospective clinical and immunologic analysis of patients with serum sickness.N Engl J Med.1984;311(22):1407–1413.
- ,.Severe adverse cutaneous reactions to drugs.N Engl J Med.1994;331(19):1272–1285.
- ,,.Idiosyncratic drug reactions: the reactive metabolite syndromes.Lancet2000;356(9241):1587–1591.
- ,,,,.Cefaclor‐associated serum sickness‐like disease: eight cases and review of the literature.Ann Pharmacother.1992;26(7–8):910–914.
Journal of Hospital Medicine Because It Matters
An old man and a young boy walk along a beach, with the old man throwing stranded starfish back into the ocean. The boy asks, Why bother tossing them back when you know they'll just keep washing up? To which the old man replies, after casting another starfish into the water, Because it matters to that one.
The Starfish Story, based upon an essay by Loren Eiseley
Most patients seem not to notice it. Occasionally I'll have a patient who, with comedic license, overlooks the stipples and wavy edges and jokingly asks, What are you, a 1‐star general?
I'm referring to the gold starfish pin I wear on the left lapel of my white coat. Where I trained, all Medicine interns receive one on the first day of internship, in tribute of the much‐celebrated story above. The purpose of the pin is simple: to remind us that no matter how tired, frustrated, or overwhelmed we sometimes feel in medicine, we can always make a difference in the life of the patient in front of us.
The older I get the more I've come to appreciate that the starfish tale ‐ inspiring as it is ‐ reminds us of only half the story. As we all know, patients don't exist in isolation. They have brothers and sisters, mothers and fathers, sons and daughters, friends and lovers. In the middle of the night, or from under a pile of paperwork, it can be even harder to remember that what we as healthcare professionals do for our patients matters to these people, too. For this latter reminder, I turn to my hospital chapel.
For those who know me this might come as a surprise. I'm spiritual, but not very religious, and while I often reflect on what's going on in my life or the lives of my loved ones, I usually don't feel the need to go to a sanctuary to do it. I'm not sure why I visited the chapel that first time.
Located behind 2 unadorned double doors off the main hallway, my hospital chapel looks from the outside like any other room in the building. Similarly, on the inside it looks like any other chapel ‐ dimly lit, with an altar, a Bible, pews, and an electric organ. What makes this space special for me is the volume that rests on a podium opposite the altar, under a bright fluorescent lamp and a sign that reads, This book is for your prayer requests. Please write down whatever helps you.
On the days when I feel overwhelmed with patient care, in addition to glancing at the starfish pin on my lapel I think of the prayer request book. Open for all to read, the book is a place where people can share their hopes and fears, about themselves or loved ones, with others who may or may not be complete strangers. Most people write about ill loved ones. What they have to say is profoundly moving and can only be done justice in their own words (with names and other details altered to protect confidentiality).
Some people write about letting go, about redirecting care from attempts at cure to comfort. Their anguish, like the indentation from their pen, is palpable. Dear God, our mom is almost 91 years old, says one person. She's been sick and hospitalized about 7 times or so. We really don't want her to leave us, but[w]e know she has had enough pain and wants to join our dad. Another person reflects, Dear God, my sister Pat is on the 4th floor. I know today I will take her off the vent. Please take her hand. Show her the way if that's Your will.
Others write about specific procedures or illnesses. In the jagged hand of a 10‐year‐old, a child prays for my mother, Mary, because she is getting a spinal tap right now and I want her to get well, which he then signs with a large heart and Love you, Mom. Someone else writes, Dear God, I need Your healing touch for my dad, who has lung cancer. Then, as if an afterthought, Also for me, because I have to have a colonoscopy this weekend.
Although many of the entries are addressed to God, a considerable number are directed toward anyone reading the book. Please pray for my dad, implores one person in an earnest hand. He was in a bad accident. I just want him to get better. He makes everything better. I just want my dad back. Is that selfish? Please pray for him. One of the most heart‐wrenching requests is from a new mother hoping for a second chance: My newborn son is here in the NICU. Please pray he is alrightand that they (the social workers) give me the next 18 or so years to make up for what I've done to him. Please, I want another chance to be the good mother I know he needs.
Every few months, the prayer request book fills up with hopes and fears just like these, including ‐ if it's not refreshed quickly enough ‐ the inside of the front and back cover. More meaningful than anything I could ever pin to my white coat, each entry is a powerful reminder of how we as healthcare providers affect more than just our patients. Indeed, for better or for worse, the stakes are much higher than that. What we do also matters to the people to whom our patients matter.
The people who penned the preceding entries are among those I see walking down the hall, riding with me in the elevator, standing in line next to me in the cafeteria, and sitting at my patients' bedsides. They could be anyone, and so they are everyone. In honor of them all I share this entry of my own: Thank you for opening up your hearts. Thank you for helping me remember how privileged I am to be a physician, and how, through helping one, I help more than one.
An old man and a young boy walk along a beach, with the old man throwing stranded starfish back into the ocean. The boy asks, Why bother tossing them back when you know they'll just keep washing up? To which the old man replies, after casting another starfish into the water, Because it matters to that one.
The Starfish Story, based upon an essay by Loren Eiseley
Most patients seem not to notice it. Occasionally I'll have a patient who, with comedic license, overlooks the stipples and wavy edges and jokingly asks, What are you, a 1‐star general?
I'm referring to the gold starfish pin I wear on the left lapel of my white coat. Where I trained, all Medicine interns receive one on the first day of internship, in tribute of the much‐celebrated story above. The purpose of the pin is simple: to remind us that no matter how tired, frustrated, or overwhelmed we sometimes feel in medicine, we can always make a difference in the life of the patient in front of us.
The older I get the more I've come to appreciate that the starfish tale ‐ inspiring as it is ‐ reminds us of only half the story. As we all know, patients don't exist in isolation. They have brothers and sisters, mothers and fathers, sons and daughters, friends and lovers. In the middle of the night, or from under a pile of paperwork, it can be even harder to remember that what we as healthcare professionals do for our patients matters to these people, too. For this latter reminder, I turn to my hospital chapel.
For those who know me this might come as a surprise. I'm spiritual, but not very religious, and while I often reflect on what's going on in my life or the lives of my loved ones, I usually don't feel the need to go to a sanctuary to do it. I'm not sure why I visited the chapel that first time.
Located behind 2 unadorned double doors off the main hallway, my hospital chapel looks from the outside like any other room in the building. Similarly, on the inside it looks like any other chapel ‐ dimly lit, with an altar, a Bible, pews, and an electric organ. What makes this space special for me is the volume that rests on a podium opposite the altar, under a bright fluorescent lamp and a sign that reads, This book is for your prayer requests. Please write down whatever helps you.
On the days when I feel overwhelmed with patient care, in addition to glancing at the starfish pin on my lapel I think of the prayer request book. Open for all to read, the book is a place where people can share their hopes and fears, about themselves or loved ones, with others who may or may not be complete strangers. Most people write about ill loved ones. What they have to say is profoundly moving and can only be done justice in their own words (with names and other details altered to protect confidentiality).
Some people write about letting go, about redirecting care from attempts at cure to comfort. Their anguish, like the indentation from their pen, is palpable. Dear God, our mom is almost 91 years old, says one person. She's been sick and hospitalized about 7 times or so. We really don't want her to leave us, but[w]e know she has had enough pain and wants to join our dad. Another person reflects, Dear God, my sister Pat is on the 4th floor. I know today I will take her off the vent. Please take her hand. Show her the way if that's Your will.
Others write about specific procedures or illnesses. In the jagged hand of a 10‐year‐old, a child prays for my mother, Mary, because she is getting a spinal tap right now and I want her to get well, which he then signs with a large heart and Love you, Mom. Someone else writes, Dear God, I need Your healing touch for my dad, who has lung cancer. Then, as if an afterthought, Also for me, because I have to have a colonoscopy this weekend.
Although many of the entries are addressed to God, a considerable number are directed toward anyone reading the book. Please pray for my dad, implores one person in an earnest hand. He was in a bad accident. I just want him to get better. He makes everything better. I just want my dad back. Is that selfish? Please pray for him. One of the most heart‐wrenching requests is from a new mother hoping for a second chance: My newborn son is here in the NICU. Please pray he is alrightand that they (the social workers) give me the next 18 or so years to make up for what I've done to him. Please, I want another chance to be the good mother I know he needs.
Every few months, the prayer request book fills up with hopes and fears just like these, including ‐ if it's not refreshed quickly enough ‐ the inside of the front and back cover. More meaningful than anything I could ever pin to my white coat, each entry is a powerful reminder of how we as healthcare providers affect more than just our patients. Indeed, for better or for worse, the stakes are much higher than that. What we do also matters to the people to whom our patients matter.
The people who penned the preceding entries are among those I see walking down the hall, riding with me in the elevator, standing in line next to me in the cafeteria, and sitting at my patients' bedsides. They could be anyone, and so they are everyone. In honor of them all I share this entry of my own: Thank you for opening up your hearts. Thank you for helping me remember how privileged I am to be a physician, and how, through helping one, I help more than one.
An old man and a young boy walk along a beach, with the old man throwing stranded starfish back into the ocean. The boy asks, Why bother tossing them back when you know they'll just keep washing up? To which the old man replies, after casting another starfish into the water, Because it matters to that one.
The Starfish Story, based upon an essay by Loren Eiseley
Most patients seem not to notice it. Occasionally I'll have a patient who, with comedic license, overlooks the stipples and wavy edges and jokingly asks, What are you, a 1‐star general?
I'm referring to the gold starfish pin I wear on the left lapel of my white coat. Where I trained, all Medicine interns receive one on the first day of internship, in tribute of the much‐celebrated story above. The purpose of the pin is simple: to remind us that no matter how tired, frustrated, or overwhelmed we sometimes feel in medicine, we can always make a difference in the life of the patient in front of us.
The older I get the more I've come to appreciate that the starfish tale ‐ inspiring as it is ‐ reminds us of only half the story. As we all know, patients don't exist in isolation. They have brothers and sisters, mothers and fathers, sons and daughters, friends and lovers. In the middle of the night, or from under a pile of paperwork, it can be even harder to remember that what we as healthcare professionals do for our patients matters to these people, too. For this latter reminder, I turn to my hospital chapel.
For those who know me this might come as a surprise. I'm spiritual, but not very religious, and while I often reflect on what's going on in my life or the lives of my loved ones, I usually don't feel the need to go to a sanctuary to do it. I'm not sure why I visited the chapel that first time.
Located behind 2 unadorned double doors off the main hallway, my hospital chapel looks from the outside like any other room in the building. Similarly, on the inside it looks like any other chapel ‐ dimly lit, with an altar, a Bible, pews, and an electric organ. What makes this space special for me is the volume that rests on a podium opposite the altar, under a bright fluorescent lamp and a sign that reads, This book is for your prayer requests. Please write down whatever helps you.
On the days when I feel overwhelmed with patient care, in addition to glancing at the starfish pin on my lapel I think of the prayer request book. Open for all to read, the book is a place where people can share their hopes and fears, about themselves or loved ones, with others who may or may not be complete strangers. Most people write about ill loved ones. What they have to say is profoundly moving and can only be done justice in their own words (with names and other details altered to protect confidentiality).
Some people write about letting go, about redirecting care from attempts at cure to comfort. Their anguish, like the indentation from their pen, is palpable. Dear God, our mom is almost 91 years old, says one person. She's been sick and hospitalized about 7 times or so. We really don't want her to leave us, but[w]e know she has had enough pain and wants to join our dad. Another person reflects, Dear God, my sister Pat is on the 4th floor. I know today I will take her off the vent. Please take her hand. Show her the way if that's Your will.
Others write about specific procedures or illnesses. In the jagged hand of a 10‐year‐old, a child prays for my mother, Mary, because she is getting a spinal tap right now and I want her to get well, which he then signs with a large heart and Love you, Mom. Someone else writes, Dear God, I need Your healing touch for my dad, who has lung cancer. Then, as if an afterthought, Also for me, because I have to have a colonoscopy this weekend.
Although many of the entries are addressed to God, a considerable number are directed toward anyone reading the book. Please pray for my dad, implores one person in an earnest hand. He was in a bad accident. I just want him to get better. He makes everything better. I just want my dad back. Is that selfish? Please pray for him. One of the most heart‐wrenching requests is from a new mother hoping for a second chance: My newborn son is here in the NICU. Please pray he is alrightand that they (the social workers) give me the next 18 or so years to make up for what I've done to him. Please, I want another chance to be the good mother I know he needs.
Every few months, the prayer request book fills up with hopes and fears just like these, including ‐ if it's not refreshed quickly enough ‐ the inside of the front and back cover. More meaningful than anything I could ever pin to my white coat, each entry is a powerful reminder of how we as healthcare providers affect more than just our patients. Indeed, for better or for worse, the stakes are much higher than that. What we do also matters to the people to whom our patients matter.
The people who penned the preceding entries are among those I see walking down the hall, riding with me in the elevator, standing in line next to me in the cafeteria, and sitting at my patients' bedsides. They could be anyone, and so they are everyone. In honor of them all I share this entry of my own: Thank you for opening up your hearts. Thank you for helping me remember how privileged I am to be a physician, and how, through helping one, I help more than one.
Erratum: Investing in the future: Building an academic hospitalist faculty development program
The disclosure statement for the following article, Investing in the Future: Building an Academic Hospitalist Faculty Development Program, by Niraj L. Sehgal, MD, MPH, Bradley A. Sharpe, MD, Andrew A. Auerbach, MD, MPH, Robert M. Wachter, MD, that published in Volume 6, Issue 3 pages 161166 of the Journal of Hospital Medicine, was incorrect. The correct disclosure statement is: All authors report no relevant conflicts of interest. The publisher regrets this error.
The disclosure statement for the following article, Investing in the Future: Building an Academic Hospitalist Faculty Development Program, by Niraj L. Sehgal, MD, MPH, Bradley A. Sharpe, MD, Andrew A. Auerbach, MD, MPH, Robert M. Wachter, MD, that published in Volume 6, Issue 3 pages 161166 of the Journal of Hospital Medicine, was incorrect. The correct disclosure statement is: All authors report no relevant conflicts of interest. The publisher regrets this error.
The disclosure statement for the following article, Investing in the Future: Building an Academic Hospitalist Faculty Development Program, by Niraj L. Sehgal, MD, MPH, Bradley A. Sharpe, MD, Andrew A. Auerbach, MD, MPH, Robert M. Wachter, MD, that published in Volume 6, Issue 3 pages 161166 of the Journal of Hospital Medicine, was incorrect. The correct disclosure statement is: All authors report no relevant conflicts of interest. The publisher regrets this error.
Risk Model for VTE
Venous thromboembolism (VTE) is a major source of morbidity and mortality for hospitalized patients. Among medical patients at the highest risk, as many as 15% can be expected to develop a VTE during their hospital stay1, 2; however, among general medical patients, the incidence of symptomatic VTE is less than 1%,1 and potentially as low as 0.3%.3 Thromboprophylaxis with subcutaneous heparin reduces the risk of VTE by approximately 50%,4 and is therefore recommended for medical patients at high risk. However, heparin also increases the risk of bleeding and thrombocytopenia and thus should be avoided for patients at low risk of VTE. Consequently, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) recommends that all hospitalized medical patients receive a risk assessment for VTE.5
Certain disease states, including stroke, acute myocardial infarction, heart failure, respiratory disease, sepsis, and cancer, have been associated with increased risk for VTE, and, based on the inclusion criteria of several randomized trials, current American College of Chest Physicians (ACCP) guidelines recommend thromboprophylaxis for patients hospitalized with these diagnoses.2 However, evidence that these factors actually increase a patient's risk for VTE comes from studies of ambulatory patients and is often weak or conflicting. Existing risk‐stratification tools,6, 7 as well as the ACCP guidelines, have not been validated, and accordingly JCAHO does not specify how risk assessment should be conducted. In order to help clinicians better estimate the risk of VTE in medical patients and therefore to provide more targeted thromboprophylaxis, we examined a large cohort of patients with high‐risk diagnoses and created a risk stratification model.
Methods
Setting and Patients
We identified a retrospective cohort of patients discharged between January 1, 2004 and June 30, 2005 from 374 acute care facilities in the US that participated in Premier's Perspective, a database developed for measuring quality and healthcare utilization. Participating hospitals represent all regions of the US, and are generally similar in composition to US hospitals; however, in comparison to information contained in the American Hospital Association annual survey, Perspective hospitals are more likely to be located in the South and in urban areas. Available data elements include those derived from the uniform billing 04 form, such as sociodemographic information about each patient, their International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis and procedure codes, as well as hospital and physician information. This information is supplemented with a date‐stamped log of all items and services billed to the patient or insurer, including diagnostic tests, medications, and other treatments. Permission to conduct the study was obtained from the Institutional Review Board at Baystate Medical Center.
We included all patients age 18 years at moderate‐to‐high risk of VTE according to the ACCP recommendations,8 based on a principal diagnosis of pneumonia, septicemia or respiratory failure with pneumonia, heart failure, chronic obstructive pulmonary disease (COPD), stroke, and urinary tract infection. Diagnoses were assessed using ICD‐9‐CM codes. Patients who were prescribed warfarin or therapeutic doses of heparin on hospital day 1 or 2, and those who received >1 therapeutic dose of heparin but otherwise did not fulfill criteria for VTE, were excluded because we could not evaluate whether they experienced a VTE event during hospitalization. We also excluded patients whose length of stay was <3 days, because our definition of hospital‐acquired VTE required treatment begun on day 3 or later, and those with an indication for anticoagulation other than VTE (eg, prosthetic cardiac valve or atrial fibrillation), because we could not reliably distinguish treatment for VTE from treatment of the underlying condition.
Risk Factors
For each patient, we extracted age, gender, race/ethnicity, and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9‐CM secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser et al.9 We also assessed risk factors which have been previously linked to VTE: paralysis, cancer (metastatic, solid tumor, and lymphoma), chemotherapy/radiation, prior VTE, use of estrogens and estrogen modulators, inflammatory bowel disease, nephrotic syndrome, myeloproliferative disorders, obesity, smoking, central venous catheter, inherited or acquired thrombophilia, steroid use, mechanical ventilation, urinary catheter, decubitus ulcer, HMGco‐A reductase inhibitors, restraints, diabetes, varicose veins, and length‐of‐stay 6 days. These additional comorbidities were defined based on the presence of specific ICD‐9 codes, while use of HMG‐co‐A reductase inhibitors were identified from medication charge files. We also noted whether patients received anticoagulants, the dosages and days of administration, as well as intermittent pneumatic compression devices.
Identification of VTE
Because the presence of a secondary diagnosis of VTE in medical patients is not a reliable way of differentiating hospital‐acquired VTE from those present at the time of admission,10 subjects were considered to have experienced a hospital‐acquired VTE only if they underwent a diagnostic test for VTE (lower extremity ultrasound, venography, CT angiogram, ventilation‐perfusion scan, or pulmonary angiogram) on hospital day 3 or later, received treatment for VTE for at least 50% of the remaining hospital stay, or until initiation of warfarin or appearance of a complication (eg, transfusion or treatment for heparin‐induced thrombocytopenia) and were given a secondary diagnosis of VTE (ICD‐9 diagnoses 453.4, 453.40, 453.41, 453.42, 453.8, 453.9, 415.1, 415.11, 415.19). We considered the following to be treatments for VTE: intravenous unfractionated heparin, >60 mg of enoxaparin, 7500 mg of dalteparin, or placement of an inferior vena cava filter. In addition, patients who were readmitted within 30 days of discharge with a primary diagnosis of VTE were also considered to have developed a VTE as a complication of their previous hospital stay.
Statistical Analysis
Univariate predictors of VTE were assessed using chi‐square tests. We developed a multivariable logistic regression model for VTE on an 80% randomly selected subset of the eligible admissions (the derivation cohort) using all measured risk factors for VTE and selected interaction terms. Generalized estimating equations (GEE) models with a logit link (SAS PROC GENMOD) were used to account for the clustering of patients within hospitals. Initial models were stratified on VTE prophylaxis. Factors significant at P < 0.05 were retained. Parameter estimates derived from the model were used to compute individual VTE risk in the remaining 20% of the admissions (the validation cohort). Discrimination in the validation model was assessed by the c‐statistic, as well as the expected/observed ratio. Both cohorts were categorized by decile of risk, based on the probability distribution in the derivation cohort, and observed VTE events compared to those predicted by the model. All analyses were performed using the Statistical Analysis System (version 9.1, SAS Institute, Inc., Cary, NC).
Role of the Funding Source
This study was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation. The funding source had no role in the study design, analysis, or interpretation of the data.
Results
Our sample contained 242,738 patients, 194,198 (80%) assigned to the derivation set and 48,540 (20%) to the validation set. Patient characteristics were similar in both sets (Supporting Information Appendix Table 1). Most patients were over age 65, 59% were female, and 64% were white (Table 1). The most common primary diagnoses were pneumonia (33%) and congestive heart failure (19%). The most common comorbidities were hypertension (50%), diabetes (31%), chronic pulmonary disease (30%), and anemia (20%). Most patients were cared for by internists (54%) or family practitioners (21%), and 30% received some form of anticoagulant VTE prophylaxis (Table 2). Of patients with an ICD‐9 code for VTE during hospitalization, just over half lacked either diagnostic testing, treatment, or both, leaving 612 (0.25%) patients who fulfilled our criteria for VTE; an additional 440 (0.18%) were readmitted for VTE, for an overall incidence of 0.43%. Patients with a length of stay 6 days had an incidence of 0.79% vs 0.19% for patients with shorter stays.
| Total | No VTE | VTE | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | P‐Value |
| Total | 242,738 | 100 | 241,686 | 100.0 | 1,052 | 100.0 | |
| Demographics | |||||||
| Age | 0.20 | ||||||
| 18‐49 | 31,065 | 12.8 | 30,952 | 12.8 | 113 | 10.7 | |
| 50‐64 | 51,309 | 21.1 | 51,083 | 21.1 | 226 | 21.5 | |
| 65‐74 | 51,230 | 21.1 | 50,993 | 21.1 | 237 | 22.5 | |
| 75+ | 109,134 | 45.0 | 108,658 | 45.0 | 476 | 45.2 | |
| Female | 142,910 | 58.9 | 142,330 | 58.9 | 580 | 55.1 | 0.01 |
| Race/ethnicity | 0.49 | ||||||
| White | 155,866 | 64.2 | 155,189 | 64.2 | 677 | 64.4 | |
| Black | 41,556 | 17.1 | 41,374 | 17.1 | 182 | 17.3 | |
| Hispanic | 9,809 | 4.0 | 9,776 | 4.0 | 33 | 3.1 | |
| Other | 35,507 | 14.6 | 35,347 | 14.6 | 160 | 15.2 | |
| Marital status | 0.28 | ||||||
| Married/life partner | 88,035 | 36.3 | 87,627 | 36.3 | 408 | 38.8 | |
| Single | 39,254 | 16.2 | 39,103 | 16.2 | 151 | 14.4 | |
| Separated/divorced | 23,492 | 9.7 | 23,394 | 9.7 | 98 | 9.3 | |
| Widowed | 58,669 | 24.2 | 58,426 | 24.2 | 243 | 23.1 | |
| Other | 33,288 | 13.7 | 33,136 | 13.7 | 152 | 14.4 | |
| Admission characteristics | |||||||
| Primary diagnosis | <0.001 | ||||||
| Community‐acquired pneumonia | 81,171 | 33.4 | 80,792 | 33.4 | 379 | 36.0 | |
| Septicemia | 7,643 | 3.2 | 7,568 | 3.1 | 75 | 7.1 | |
| Chronic obstructive pulmonary disease | 35,116 | 14.5 | 35,027 | 14.5 | 89 | 8.5 | |
| Respiratory failure | 7,098 | 2.9 | 7,012 | 2.9 | 86 | 8.2 | |
| Congestive heart failure | 46,503 | 19.2 | 46,336 | 19.2 | 167 | 15.9 | |
| Cardiovascular disease | 33,044 | 13.6 | 32,931 | 13.6 | 113 | 10.7 | |
| Urinary tract infection | 32,163 | 13.3 | 32,020 | 13.2 | 143 | 13.6 | |
| Insurance payer | 0.93 | ||||||
| Medicare traditional | 157,609 | 64.9 | 156,927 | 64.9 | 682 | 64.8 | |
| Medicare managed care | 10,649 | 4.4 | 10,597 | 4.4 | 52 | 4.9 | |
| Medicaid | 17,796 | 7.3 | 17,720 | 7.3 | 76 | 7.2 | |
| Private | 44,858 | 18.5 | 44,665 | 18.5 | 193 | 18.3 | |
| Self‐pay/uninsured/other | 11,826 | 4.9 | 11,777 | 4.9 | 49 | 4.7 | |
| Admitted from skilled nursing facility | 3,003 | 1.2 | 2,980 | 1.2 | 23 | 2.2 | 0.005 |
| Risk factors | |||||||
| Any VTE prophylaxis | 72,558 | 29.9 | 72,164 | 29.9 | 394 | 37.5 | <0.001 |
| Length of stay 6 days | 99,463 | 41.0 | 98,680 | 40.8 | 783 | 74.4 | <0.001 |
| Paralysis | 16,764 | 6.9 | 16,689 | 6.9 | 75 | 7.1 | 0.77 |
| Metastatic cancer | 5,013 | 2.1 | 4,928 | 2.0 | 85 | 8.1 | <0.001 |
| Solid tumor without metastasis | 25,127 | 10.4 | 24,995 | 10.3 | 132 | 12.5 | 0.02 |
| Lymphoma | 3,026 | 1.2 | 2,995 | 1.2 | 31 | 2.9 | <0.001 |
| Cancer chemotherapy/radiation | 1,254 | 0.5 | 1,231 | 0.5 | 23 | 2.2 | <0.001 |
| Prior venous thromboembolism | 2,945 | 1.2 | 2,926 | 1.2 | 19 | 1.8 | 0.08 |
| Estrogens | 4,819 | 2.0 | 4,807 | 2.0 | 12 | 1.1 | 0.05 |
| Estrogen modulators | 2,102 | 0.9 | 2,091 | 0.9 | 11 | 1.0 | 0.53 |
| Inflammatory bowel disease | 814 | 0.3 | 803 | 0.3 | 11 | 1.0 | <0.001 |
| Nephrotic syndrome | 520 | 0.2 | 517 | 0.2 | 3 | 0.3 | 0.62 |
| Myeloproliferative disorder | 1,983 | 0.8 | 1,973 | 0.8 | 10 | 1.0 | 0.63 |
| Obesity | 16,938 | 7.0 | 16,856 | 7.0 | 82 | 7.8 | 0.30 |
| Smoking | 35,386 | 14.6 | 35,284 | 14.6 | 102 | 9.7 | <0.001 |
| Central venous catheter | 14,754 | 6.1 | 14,525 | 6.0 | 229 | 21.8 | <0.001 |
| Inherited or acquired thrombophilia | 114 | 0.1 | 108 | 0.0 | 6 | 0.6 | <0.001 |
| Steroids | 82,606 | 34.0 | 82,185 | 34.0 | 421 | 40.0 | <0.001 |
| Mechanical ventilation | 13,347 | 5.5 | 13,167 | 5.4 | 180 | 17.1 | <0.001 |
| Urinary catheter | 39,080 | 16.1 | 38,816 | 16.1 | 264 | 25.1 | <0.001 |
| Decubitus ulcer | 6,829 | 2.8 | 6,776 | 2.8 | 53 | 5.0 | <0.001 |
| Statins use | 57,282 | 23.6 | 57,068 | 23.6 | 214 | 20.3 | 0.01 |
| Use of restraints | 5,970 | 2.5 | 5,914 | 2.4 | 56 | 5.3 | <0.001 |
| Diabetes mellitus | 75,103 | 30.9 | 74,799 | 30.9 | 304 | 28.9 | 0.15 |
| Varicose veins | 166 | 0.1 | 165 | 0.1 | 1 | 0.1 | 0.74 |
| Comorbidities | |||||||
| Hypertension | 120,606 | 49.7 | 120,126 | 49.7 | 480 | 45.6 | 0.008 |
| Congestive heart failure | 18,900 | 7.8 | 18,793 | 7.8 | 107 | 10.2 | 0.004 |
| Peripheral vascular disease | 16,705 | 6.9 | 16,639 | 6.9 | 66 | 6.3 | 0.43 |
| Valvular disease | 13,683 | 5.6 | 13,628 | 5.6 | 55 | 5.2 | 0.56 |
| Pulmonary circulation disease | 5,530 | 2.3 | 5,492 | 2.3 | 38 | 3.6 | 0.004 |
| Chronic pulmonary disease | 72,028 | 29.7 | 71,698 | 29.7 | 330 | 31.4 | 0.23 |
| Respiratory failure second diagnosis | 13,027 | 5.4 | 12,893 | 5.3 | 134 | 12.7 | <0.001 |
| Rheumatoid arthritis/collagen vascular disease | 7,090 | 2.9 | 7,050 | 2.9 | 40 | 3.8 | 0.09 |
| Deficiency anemias | 49,605 | 20.4 | 49,352 | 20.4 | 253 | 24.0 | 0.004 |
| Weight loss | 8,810 | 3.6 | 8,714 | 3.6 | 96 | 9.1 | <0.001 |
| Peptic ulcer disease bleeding | 4,736 | 2.0 | 4,723 | 2.0 | 13 | 1.2 | 0.09 |
| Chronic blood loss anemia | 2,354 | 1.0 | 2,338 | 1.0 | 16 | 1.5 | 0.07 |
| Hypothyroidism | 28,773 | 11.9 | 28,668 | 11.9 | 105 | 10.0 | 0.06 |
| Renal failure | 19,768 | 8.1 | 19,669 | 8.1 | 99 | 9.4 | 0.13 |
| Liver disease | 4,682 | 1.9 | 4,657 | 1.9 | 25 | 2.4 | 0.29 |
| Other neurological disorders | 33,094 | 13.6 | 32,905 | 13.6 | 189 | 18.0 | <0.001 |
| Psychoses | 9,330 | 3.8 | 9,283 | 3.8 | 47 | 4.5 | 0.29 |
| Depression | 25,561 | 10.5 | 25,442 | 10.5 | 119 | 11.3 | 0.41 |
| Alcohol abuse | 7,756 | 3.2 | 7,727 | 3.2 | 29 | 2.8 | 0.42 |
| Drug abuse | 4,336 | 1.8 | 4,318 | 1.8 | 18 | 1.7 | 0.85 |
| Acquired immune deficiency syndrome | 1,048 | 0.4 | 1,045 | 0.4 | 3 | 0.3 | 0.47 |
| Total | Derivation | Validation | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | P‐Value |
| |||||||
| Total | 242,738 | 100 | 194,198 | 100 | 48,540 | 100 | |
| VTE prophylaxis | 0.97 | ||||||
| No prophylaxis | 170,180 | 70.1 | 136,153 | 70.1 | 34,027 | 70.1 | |
| Any prophylaxis | 72,558 | 29.9 | 58,045 | 29.9 | 14,513 | 29.9 | |
| Outcomes | |||||||
| ICD‐9 code for VTE | 1,304 | 0.5 | 1,025 | 0.5 | 279 | 0.6 | 0.21 |
| ICD‐9 code + diagnostic test | 989 | 0.4 | 777 | 0.4 | 212 | 0.4 | 0.26 |
| ICD‐9 code + diagnostic test + treatment for VTE | 612 | 0.3 | 471 | 0.2 | 141 | 0.3 | 0.06 |
| Readmission for VTE within 30 days | 446 | 0.2 | 363 | 0.2 | 83 | 0.2 | 0.46 |
| Total hospital‐acquired VTE | 1,052 | 0.4 | 829 | 0.4 | 223 | 0.5 | 0.33 |
| In‐hospital mortality | 8,019 | 3.3 | 6,403 | 3.3 | 1,616 | 3.3 | 0.72 |
| Any readmission within 30 days | 28,664 | 11.8 | 22,885 | 11.8 | 5,779 | 11.9 | 0.46 |
Risk factors for VTE
A large number of patient and hospital factors were associated with the development of VTE (Table 1). Due to the large sample size, even weak associations appear highly statistically significant. Compared to patients without VTE, those with VTE were more likely to have received VTE prophylaxis (37% vs 30%, P < 0.001). However, models of patients receiving prophylaxis and of patients not receiving prophylaxis produced similar odds ratios for the various risk factors (Supporting Information Appendix Table 2); therefore, the final model includes both patients who did, and did not, receive VTE prophylaxis. In the multivariable model (Supporting Information Appendix Table 3), age, length of stay, gender, primary diagnosis, cancer, inflammatory bowel disease, obesity, central venous catheter, inherited thrombophilia, steroid use, mechanical ventilation, active chemotherapy, and urinary catheters were all associated with VTE (Table 3). The strongest risk factors were length of stay 6 days (OR 3.22, 95% CI 2.73, 3.79), central venous catheter (OR 1.87, 95% CI 1.52, 2.29), inflammatory bowel disease (OR 3.11, 95% CI 1.59, 6.08), and inherited thrombophilia (OR 4.00, 95% CI 0.98, 16.40). In addition, there were important interactions between age and cancer; cancer was a strong risk factor among younger patients, but is not as strong a risk factor among older patients (OR compared to young patients without cancer was 4.62 (95% CI 2.72, 7.87) for those age 1849 years, and 3.64 (95% CI 2.52, 5.25) for those aged 5064 years).
| Risk Factor | OR | 95% CI |
|---|---|---|
| ||
| Any prophylaxis | 0.98 | (0.84, 1.14) |
| Female | 0.85 | (0.74, 0.98) |
| Length of stay 6 days | 3.22 | (2.73, 3.79) |
| Age* | ||
| 18‐49 years | 1 | Referent |
| 50‐64 years | 1.15 | (0.86, 1.56) |
| >65 years | 1.51 | (1.17, 1.96) |
| Primary diagnosis | ||
| Pneumonia | 1 | Referent |
| Chronic obstructive pulmonary disease | 0.57 | (0.44, 0.75) |
| Stroke | 0.84 | (0.66, 1.08) |
| Congestive heart failure | 0.86 | (0.70, 1.06) |
| Urinary tract infection | 1.19 | (0.95, 1.50) |
| Respiratory failure | 1.15 | (0.85, 1.55) |
| Septicemia | 1.11 | (0.82, 1.50) |
| Comorbidities | ||
| Inflammatory bowel disease | 3.11 | (1.59, 6.08) |
| Obesity | 1.28 | (0.99, 1.66) |
| Inherited thrombophilia | 4.00 | (0.98, 16.40) |
| Cancer | ||
| 18‐49 years | 4.62 | (2.72, 7.87) |
| 50‐64 years | 3.64 | (2.52, 5.25) |
| >65 years | 2.17 | (1.61, 2.92) |
| Treatments | ||
| Central venous catheter | 1.87 | (1.52, 2.29) |
| Mechanical ventilation | 1.61 | (1.27, 2.05) |
| Urinary catheter | 1.17 | (0.99, 1.38) |
| Chemotherapy | 1.71 | (1.03, 2.83) |
| Steroids | 1.22 | (1.04, 1.43) |
In the derivation set, the multivariable model produced deciles of mean predicted risk from 0.11% to 1.45%, while mean observed risk over the same deciles ranged from 0.12% to 1.42% (Figure 1). Within the validation cohort, the observed rate of VTE was 0.46% (223 cases among 48,543 subjects). The expected rate according to the model was 0.43% (expected/observed ratio: 0.93 [95% CI 0.82, 1.06]). Model discrimination measured by the c‐statistic in the validation set was 0.75 (95% CI 0.71, 0.78). The model produced deciles of mean predicted risk from 0.11% to 1.46%, with mean observed risk over the same deciles from 0.17% to 1.81%. Risk gradient was relatively flat across the first 6 deciles, began to rise at the seventh decile, and rose sharply in the highest one. Using a risk threshold of 1%, the model had a sensitivity of 28% and a specificity of 93%. In the validation set, this translated into a positive predictive value of 2.2% and a negative predictive value of 99.7%. Assuming that VTE prophylaxis has an efficacy of 50%, the number‐needed‐to‐treat to prevent one VTE among high‐risk patients (predicted risk >1%) would be 91. In contrast, providing prophylaxis to the entire validation sample would result in a number‐needed‐to‐treat of 435. Using a lower treatment threshold of 0.4% produced a positive predictive value of 1% and a negative predictive value of 99.8%. At this threshold, the model would detect 73% of patients with VTE and the number‐needed‐to‐treat to prevent one VTE would be 200.
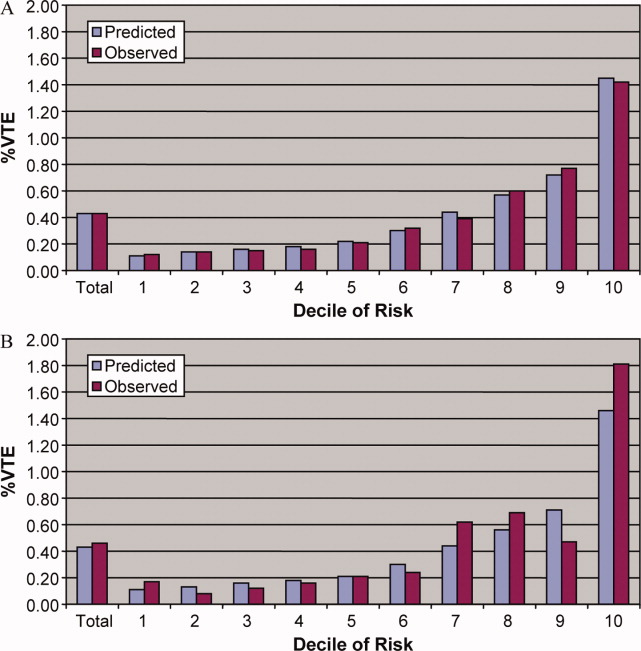
Discussion
In a representative sample of 243,000 hospitalized medical patients with at least one major risk factor for VTE, we found that symptomatic VTE was an uncommon event, occurring in approximately 1 in 231 patients. We identified a number of factors that were associated with an increased risk of VTE, but many previously cited risk factors did not show an association in multivariable models. In particular, patients with a primary diagnosis of COPD appeared not to share the same high risk of VTE as patients with the other diagnoses we examined, a finding reported by others.11 The risk model we developed accurately stratifies patients across a wide range of VTE probabilities, but even among those with the highest predicted rates, symptomatic VTE occurred in less than 2%.
VTE is often described as a frequent complication of hospitalization for medical illness and one of the most common potentially preventable causes of death. Indeed, rates of asymptomatic VTE have been demonstrated to be 3.7% to 26%.12 Although some of these might have fatal consequences, most are distal vein thromboses and their significance is unknown. In contrast, symptomatic events are uncommon, with previous estimates among general medical patients in observational studies in the range of 0.3%3 to 0.8%,12 similar to the rate observed in our study. Symptomatic event rates among control patients in landmark randomized trials have ranged from 0.86%13 to 2.3%,14 but these studies enrolled only very high‐risk patients with more extended hospitalizations, and may involve follow‐up periods of a month or more.
Because it is unlikely that our diagnostic algorithm was 100% sensitive, and because 30% of our patients received chemoprophylaxis, it is probable that we have underestimated the true rate of VTE in our sample. Among the patients who received prophylaxis, the observed rate of VTE was 0.54%. If we assume that prophylaxis is 50% effective, then had these patients not received prophylaxis, their rate of VTE would have been 1.08% (vs 0.39% among those patients who received no prophylaxis) and the overall rate of VTE for the sample would have been 0.60% (1.08 0.30 + 0.39 0.70). If we further assume that our algorithm was only 80% sensitive and 100% specific, the true underlying rate of symptomatic VTE could have been as high as 0.75%, still less than half that seen in randomized trials.
Prophylaxis with heparin has been shown to decrease the rate of both asymptomatic and symptomatic events, but because of the low prevalence, the number‐needed‐to‐treat to prevent one symptomatic pulmonary embolism has been estimated at 345, and prophylaxis has not been shown to affect all‐cause mortality.4, 15 At the same time, prophylaxis costs money, is uncomfortable, and carries a small risk of bleeding and heparin‐induced thrombocytopenia. Given the generally low incidence of symptomatic VTE, it therefore makes sense to reserve prophylaxis for patients at higher risk of thromboembolism.
To decide whether prophylaxis is appropriate for a given patient, it is necessary to quantify the patient's risk and then apply an appropriate threshold for treatment. The National Quality Forum (NQF) recommends,16 and JCAHO has adopted, that a clinician must evaluate each patient upon admission, and regularly thereafter, for the risk of developing DVT [deep vein thrombosis]/VTE. Until now, however, there has been no widely accepted, validated method to risk stratify medical patients. The ACCP recommendations cite just three studies of VTE risk factors in hospitalized medical patients.11, 17, 18 Together they examined 477 cases and 1197 controls, identifying congestive heart failure, pneumonia, cancer, and previous VTE as risk factors. Predictive models based on these factors17, 1921 have not been subjected to validation or have performed poorly.18 Acknowledging this lack of standardized risk assessment, JCAHO leaves the means of assessment to individual hospitals. A quality improvement guide published by the Agency for Healthcare Research and Quality goes one step further, stating that In a typical hospital, it is estimated that fewer than 5% of medical patients could be considered at low risk by most VTE risk stratification methods.22 The guide recommends near universal VTE prophylaxis.
In light of the JCAHO requirements, our model should be welcomed by hospitalists. Rather than assuming that all patients over 40 years of age are at high risk, our model will enable clinicians to risk stratify patients from a low of 0.1% to >1.4% (>10‐fold increase in risk). Moreover, the model was derived from more than 800 episodes of symptomatic VTE among almost 190,000 general medical patients and validated on almost 50,000 more. The observed patients were cared for in clinical practice at a nationally representative group of US hospitals, not in a highly selected clinical trial, increasing the generalizability of our findings. Finally, the model includes ten common risk factors that can easily be entered into decision support software or extracted automatically from the electronic medical record. Electronic reminder systems have already been shown to increase use of VTE prophylaxis, and prevent VTE, especially among cancer patients.23
A more challenging task is defining the appropriate risk threshold to initiate VTE prophylaxis. The Thromboembolic Risk Factors (THRIFT) Consensus Group classified patients according to risk of proximal DVT as low (<1%), moderate (1%‐10%), and high (>10%).21 They recommended heparin prophylaxis for all patients at moderate risk or higher. Although the patients included in our study all had a diagnosis that warranted prophylaxis according to the ACCP guidelines, using the THRIFT threshold for moderate‐to‐high risk, only 7% of our patients should have received prophylaxis. The recommendation not to offer heparin prophylaxis to patients with less than 1% chance of developing symptomatic VTE seems reasonable, given the large number‐needed‐to‐treat, but formal decision analyses should be conducted to better define this threshold. Many hospitalists, however, may feel uncomfortable using the 1% threshold, because our model failed to identify almost three out of four patients who ultimately experienced symptomatic VTE. At that threshold, it would seem that hospital‐acquired VTE is not a preventable complication in most medical patients, as others have pointed out.3, 24 Alternatively, if the threshold were lowered to 0.4%, our model could reduce the use of prophylaxis by 60%, while still identifying three‐fourths of all VTE cases. Further research is needed to know whether such a threshold is reasonable.
Our study has a number of important limitations. First, we relied on claims data, not chart review. We do not know for certain which patients experienced VTE, although our definition of VTE required diagnosis codes plus charges for both diagnosis and treatment. Moreover, our rates are similar to those observed in other trials where symptomatic events were confirmed. Second, about 30% of our patients received at least some VTE prophylaxis, and this may have prevented as many as half of the VTEs in that group. Without prophylaxis, rates might have been 20%30% higher. Similarly, we could not detect patients who were diagnosed after discharge but not admitted to hospital. While we believe this number to be small, it would again increase the rate slightly. Third, we could not assess certain clinical circumstances that are not associated with hospital charges or diagnosis codes, especially prolonged bed rest. Other risk factors, such as the urinary catheter, were probably surrogate markers for immobilization rather than true risk factors. Fourth, we included length of stay in our prediction model. We did this because most randomized trials of VTE prophylaxis included only patients with an expected length of stay 6 days. Physicians' estimates about probable length of stay may be less accurate than actual length of stay as a predictor of VTE. Moreover, the relationship may have been confounded if hospital‐acquired VTE led to longer lengths of stay. We think this unlikely since many of the events were discovered on readmission. Fifth, we studied only patients carrying high‐risk diagnoses, and therefore do not know the baseline risk for patients with less risky conditions, although it should be lower than what we observed. It seems probable that COPD, rather than being protective, as it appears in our model, actually represents the baseline risk for low‐risk diagnoses. It should be noted that we did include a number of other high‐risk diagnoses, such as cancer and inflammatory bowel disease, as secondary diagnoses. A larger, more inclusive study should be conducted to validate our model in other populations. Finally, we cannot know who died of undiagnosed VTE, either in the hospital or after discharge. Such an outcome would be important, but those events are likely to be rare, and VTE prophylaxis has not been shown to affect mortality.
VTE remains a daunting problem in hospitalized medical patients. Although VTE is responsible for a large number of hospital deaths each year, identifying patients at high risk for clinically important VTE is challenging, and may contribute to the persistently low rates of VTE prophylaxis seen in hospitals.25 Current efforts to treat nearly all patients are likely to lead to unnecessary cost, discomfort, and side effects. We present a simple logistic regression model that can easily identify patients at moderate‐to‐high risk (>1%) of developing symptomatic VTE. Future studies should focus on prospectively validating the model in a wider spectrum of medical illness, and better defining the appropriate risk cutoff for general prophylaxis.
Acknowledgements
The authors thank Aruna Priya, MS, for her help with some of the statistical analyses.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133(6 suppl):381S–453S.
- ,,.Thrombosis prophylaxis in hospitalised medical patients: does prophylaxis in all patients make sense?Neth J Med.2000;56(5):171–176.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- The Joint Commission on the Accreditation of Healthcare Organizations. Venous thromboembolism (VTE) core measure set. Available at: http://www. jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm. Accessed June 1,2009.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 suppl 5):12–19.
- ,,, et al.Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients.Thromb Haemost.2005;94(4):750–759.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(3 suppl):338S–400S.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36(1):8–27.
- ,,,,,.Identification of in‐hospital complications from claims data. Is it valid?Med Care.2000;38(8):785–795.
- ,,, et al.Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study.Arch Intern Med.2004;164(9):963–968.
- ,,.The magnitude of an iatrogenic disorder: a systematic review of the incidence of venous thromboembolism for general medical inpatients.Thromb Haemost.2006;95(5):758–762.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum.National Voluntary Consensus Standards for Prevention and Care of Venous Thromboembolism: Policy, Preferred Practices, and Initial Performance Measures.Washington, DC;2006.
- ,,, et al.Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case‐control multicenter study.J Am Geriatr Soc.2004;52(8):1299–1304.
- ,,.Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score.J Thromb Haemost.2004;2(12):2156–2161.
- ,,,,,.Venous thromboembolism prophylaxis and risk assessment in medical patients.Semin Thromb Hemost.1991;17(suppl 3):313–318.
- ,,, et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151(5):933–938.
- Thromboembolic Risk Factors (THRIFT) Consensus Group.Risk of and prophylaxis for venous thromboembolism in hospital patients.BMJ.1992;305(6853):567–574.
- ,.Preventing Hospital‐Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement. AHRQ Publication No. 08–0075.Rockville, MD:Agency for Healthcare Research and Quality;2008.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
- ,,.Prophylaxis against venous thromboembolism.BMJ.1992;305(6862):1156.
- ,.Prevention of in‐hospital VTE: why can't we do better?Lancet.2008;371(9610):361–362.
Venous thromboembolism (VTE) is a major source of morbidity and mortality for hospitalized patients. Among medical patients at the highest risk, as many as 15% can be expected to develop a VTE during their hospital stay1, 2; however, among general medical patients, the incidence of symptomatic VTE is less than 1%,1 and potentially as low as 0.3%.3 Thromboprophylaxis with subcutaneous heparin reduces the risk of VTE by approximately 50%,4 and is therefore recommended for medical patients at high risk. However, heparin also increases the risk of bleeding and thrombocytopenia and thus should be avoided for patients at low risk of VTE. Consequently, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) recommends that all hospitalized medical patients receive a risk assessment for VTE.5
Certain disease states, including stroke, acute myocardial infarction, heart failure, respiratory disease, sepsis, and cancer, have been associated with increased risk for VTE, and, based on the inclusion criteria of several randomized trials, current American College of Chest Physicians (ACCP) guidelines recommend thromboprophylaxis for patients hospitalized with these diagnoses.2 However, evidence that these factors actually increase a patient's risk for VTE comes from studies of ambulatory patients and is often weak or conflicting. Existing risk‐stratification tools,6, 7 as well as the ACCP guidelines, have not been validated, and accordingly JCAHO does not specify how risk assessment should be conducted. In order to help clinicians better estimate the risk of VTE in medical patients and therefore to provide more targeted thromboprophylaxis, we examined a large cohort of patients with high‐risk diagnoses and created a risk stratification model.
Methods
Setting and Patients
We identified a retrospective cohort of patients discharged between January 1, 2004 and June 30, 2005 from 374 acute care facilities in the US that participated in Premier's Perspective, a database developed for measuring quality and healthcare utilization. Participating hospitals represent all regions of the US, and are generally similar in composition to US hospitals; however, in comparison to information contained in the American Hospital Association annual survey, Perspective hospitals are more likely to be located in the South and in urban areas. Available data elements include those derived from the uniform billing 04 form, such as sociodemographic information about each patient, their International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis and procedure codes, as well as hospital and physician information. This information is supplemented with a date‐stamped log of all items and services billed to the patient or insurer, including diagnostic tests, medications, and other treatments. Permission to conduct the study was obtained from the Institutional Review Board at Baystate Medical Center.
We included all patients age 18 years at moderate‐to‐high risk of VTE according to the ACCP recommendations,8 based on a principal diagnosis of pneumonia, septicemia or respiratory failure with pneumonia, heart failure, chronic obstructive pulmonary disease (COPD), stroke, and urinary tract infection. Diagnoses were assessed using ICD‐9‐CM codes. Patients who were prescribed warfarin or therapeutic doses of heparin on hospital day 1 or 2, and those who received >1 therapeutic dose of heparin but otherwise did not fulfill criteria for VTE, were excluded because we could not evaluate whether they experienced a VTE event during hospitalization. We also excluded patients whose length of stay was <3 days, because our definition of hospital‐acquired VTE required treatment begun on day 3 or later, and those with an indication for anticoagulation other than VTE (eg, prosthetic cardiac valve or atrial fibrillation), because we could not reliably distinguish treatment for VTE from treatment of the underlying condition.
Risk Factors
For each patient, we extracted age, gender, race/ethnicity, and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9‐CM secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser et al.9 We also assessed risk factors which have been previously linked to VTE: paralysis, cancer (metastatic, solid tumor, and lymphoma), chemotherapy/radiation, prior VTE, use of estrogens and estrogen modulators, inflammatory bowel disease, nephrotic syndrome, myeloproliferative disorders, obesity, smoking, central venous catheter, inherited or acquired thrombophilia, steroid use, mechanical ventilation, urinary catheter, decubitus ulcer, HMGco‐A reductase inhibitors, restraints, diabetes, varicose veins, and length‐of‐stay 6 days. These additional comorbidities were defined based on the presence of specific ICD‐9 codes, while use of HMG‐co‐A reductase inhibitors were identified from medication charge files. We also noted whether patients received anticoagulants, the dosages and days of administration, as well as intermittent pneumatic compression devices.
Identification of VTE
Because the presence of a secondary diagnosis of VTE in medical patients is not a reliable way of differentiating hospital‐acquired VTE from those present at the time of admission,10 subjects were considered to have experienced a hospital‐acquired VTE only if they underwent a diagnostic test for VTE (lower extremity ultrasound, venography, CT angiogram, ventilation‐perfusion scan, or pulmonary angiogram) on hospital day 3 or later, received treatment for VTE for at least 50% of the remaining hospital stay, or until initiation of warfarin or appearance of a complication (eg, transfusion or treatment for heparin‐induced thrombocytopenia) and were given a secondary diagnosis of VTE (ICD‐9 diagnoses 453.4, 453.40, 453.41, 453.42, 453.8, 453.9, 415.1, 415.11, 415.19). We considered the following to be treatments for VTE: intravenous unfractionated heparin, >60 mg of enoxaparin, 7500 mg of dalteparin, or placement of an inferior vena cava filter. In addition, patients who were readmitted within 30 days of discharge with a primary diagnosis of VTE were also considered to have developed a VTE as a complication of their previous hospital stay.
Statistical Analysis
Univariate predictors of VTE were assessed using chi‐square tests. We developed a multivariable logistic regression model for VTE on an 80% randomly selected subset of the eligible admissions (the derivation cohort) using all measured risk factors for VTE and selected interaction terms. Generalized estimating equations (GEE) models with a logit link (SAS PROC GENMOD) were used to account for the clustering of patients within hospitals. Initial models were stratified on VTE prophylaxis. Factors significant at P < 0.05 were retained. Parameter estimates derived from the model were used to compute individual VTE risk in the remaining 20% of the admissions (the validation cohort). Discrimination in the validation model was assessed by the c‐statistic, as well as the expected/observed ratio. Both cohorts were categorized by decile of risk, based on the probability distribution in the derivation cohort, and observed VTE events compared to those predicted by the model. All analyses were performed using the Statistical Analysis System (version 9.1, SAS Institute, Inc., Cary, NC).
Role of the Funding Source
This study was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation. The funding source had no role in the study design, analysis, or interpretation of the data.
Results
Our sample contained 242,738 patients, 194,198 (80%) assigned to the derivation set and 48,540 (20%) to the validation set. Patient characteristics were similar in both sets (Supporting Information Appendix Table 1). Most patients were over age 65, 59% were female, and 64% were white (Table 1). The most common primary diagnoses were pneumonia (33%) and congestive heart failure (19%). The most common comorbidities were hypertension (50%), diabetes (31%), chronic pulmonary disease (30%), and anemia (20%). Most patients were cared for by internists (54%) or family practitioners (21%), and 30% received some form of anticoagulant VTE prophylaxis (Table 2). Of patients with an ICD‐9 code for VTE during hospitalization, just over half lacked either diagnostic testing, treatment, or both, leaving 612 (0.25%) patients who fulfilled our criteria for VTE; an additional 440 (0.18%) were readmitted for VTE, for an overall incidence of 0.43%. Patients with a length of stay 6 days had an incidence of 0.79% vs 0.19% for patients with shorter stays.
| Total | No VTE | VTE | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | P‐Value |
| Total | 242,738 | 100 | 241,686 | 100.0 | 1,052 | 100.0 | |
| Demographics | |||||||
| Age | 0.20 | ||||||
| 18‐49 | 31,065 | 12.8 | 30,952 | 12.8 | 113 | 10.7 | |
| 50‐64 | 51,309 | 21.1 | 51,083 | 21.1 | 226 | 21.5 | |
| 65‐74 | 51,230 | 21.1 | 50,993 | 21.1 | 237 | 22.5 | |
| 75+ | 109,134 | 45.0 | 108,658 | 45.0 | 476 | 45.2 | |
| Female | 142,910 | 58.9 | 142,330 | 58.9 | 580 | 55.1 | 0.01 |
| Race/ethnicity | 0.49 | ||||||
| White | 155,866 | 64.2 | 155,189 | 64.2 | 677 | 64.4 | |
| Black | 41,556 | 17.1 | 41,374 | 17.1 | 182 | 17.3 | |
| Hispanic | 9,809 | 4.0 | 9,776 | 4.0 | 33 | 3.1 | |
| Other | 35,507 | 14.6 | 35,347 | 14.6 | 160 | 15.2 | |
| Marital status | 0.28 | ||||||
| Married/life partner | 88,035 | 36.3 | 87,627 | 36.3 | 408 | 38.8 | |
| Single | 39,254 | 16.2 | 39,103 | 16.2 | 151 | 14.4 | |
| Separated/divorced | 23,492 | 9.7 | 23,394 | 9.7 | 98 | 9.3 | |
| Widowed | 58,669 | 24.2 | 58,426 | 24.2 | 243 | 23.1 | |
| Other | 33,288 | 13.7 | 33,136 | 13.7 | 152 | 14.4 | |
| Admission characteristics | |||||||
| Primary diagnosis | <0.001 | ||||||
| Community‐acquired pneumonia | 81,171 | 33.4 | 80,792 | 33.4 | 379 | 36.0 | |
| Septicemia | 7,643 | 3.2 | 7,568 | 3.1 | 75 | 7.1 | |
| Chronic obstructive pulmonary disease | 35,116 | 14.5 | 35,027 | 14.5 | 89 | 8.5 | |
| Respiratory failure | 7,098 | 2.9 | 7,012 | 2.9 | 86 | 8.2 | |
| Congestive heart failure | 46,503 | 19.2 | 46,336 | 19.2 | 167 | 15.9 | |
| Cardiovascular disease | 33,044 | 13.6 | 32,931 | 13.6 | 113 | 10.7 | |
| Urinary tract infection | 32,163 | 13.3 | 32,020 | 13.2 | 143 | 13.6 | |
| Insurance payer | 0.93 | ||||||
| Medicare traditional | 157,609 | 64.9 | 156,927 | 64.9 | 682 | 64.8 | |
| Medicare managed care | 10,649 | 4.4 | 10,597 | 4.4 | 52 | 4.9 | |
| Medicaid | 17,796 | 7.3 | 17,720 | 7.3 | 76 | 7.2 | |
| Private | 44,858 | 18.5 | 44,665 | 18.5 | 193 | 18.3 | |
| Self‐pay/uninsured/other | 11,826 | 4.9 | 11,777 | 4.9 | 49 | 4.7 | |
| Admitted from skilled nursing facility | 3,003 | 1.2 | 2,980 | 1.2 | 23 | 2.2 | 0.005 |
| Risk factors | |||||||
| Any VTE prophylaxis | 72,558 | 29.9 | 72,164 | 29.9 | 394 | 37.5 | <0.001 |
| Length of stay 6 days | 99,463 | 41.0 | 98,680 | 40.8 | 783 | 74.4 | <0.001 |
| Paralysis | 16,764 | 6.9 | 16,689 | 6.9 | 75 | 7.1 | 0.77 |
| Metastatic cancer | 5,013 | 2.1 | 4,928 | 2.0 | 85 | 8.1 | <0.001 |
| Solid tumor without metastasis | 25,127 | 10.4 | 24,995 | 10.3 | 132 | 12.5 | 0.02 |
| Lymphoma | 3,026 | 1.2 | 2,995 | 1.2 | 31 | 2.9 | <0.001 |
| Cancer chemotherapy/radiation | 1,254 | 0.5 | 1,231 | 0.5 | 23 | 2.2 | <0.001 |
| Prior venous thromboembolism | 2,945 | 1.2 | 2,926 | 1.2 | 19 | 1.8 | 0.08 |
| Estrogens | 4,819 | 2.0 | 4,807 | 2.0 | 12 | 1.1 | 0.05 |
| Estrogen modulators | 2,102 | 0.9 | 2,091 | 0.9 | 11 | 1.0 | 0.53 |
| Inflammatory bowel disease | 814 | 0.3 | 803 | 0.3 | 11 | 1.0 | <0.001 |
| Nephrotic syndrome | 520 | 0.2 | 517 | 0.2 | 3 | 0.3 | 0.62 |
| Myeloproliferative disorder | 1,983 | 0.8 | 1,973 | 0.8 | 10 | 1.0 | 0.63 |
| Obesity | 16,938 | 7.0 | 16,856 | 7.0 | 82 | 7.8 | 0.30 |
| Smoking | 35,386 | 14.6 | 35,284 | 14.6 | 102 | 9.7 | <0.001 |
| Central venous catheter | 14,754 | 6.1 | 14,525 | 6.0 | 229 | 21.8 | <0.001 |
| Inherited or acquired thrombophilia | 114 | 0.1 | 108 | 0.0 | 6 | 0.6 | <0.001 |
| Steroids | 82,606 | 34.0 | 82,185 | 34.0 | 421 | 40.0 | <0.001 |
| Mechanical ventilation | 13,347 | 5.5 | 13,167 | 5.4 | 180 | 17.1 | <0.001 |
| Urinary catheter | 39,080 | 16.1 | 38,816 | 16.1 | 264 | 25.1 | <0.001 |
| Decubitus ulcer | 6,829 | 2.8 | 6,776 | 2.8 | 53 | 5.0 | <0.001 |
| Statins use | 57,282 | 23.6 | 57,068 | 23.6 | 214 | 20.3 | 0.01 |
| Use of restraints | 5,970 | 2.5 | 5,914 | 2.4 | 56 | 5.3 | <0.001 |
| Diabetes mellitus | 75,103 | 30.9 | 74,799 | 30.9 | 304 | 28.9 | 0.15 |
| Varicose veins | 166 | 0.1 | 165 | 0.1 | 1 | 0.1 | 0.74 |
| Comorbidities | |||||||
| Hypertension | 120,606 | 49.7 | 120,126 | 49.7 | 480 | 45.6 | 0.008 |
| Congestive heart failure | 18,900 | 7.8 | 18,793 | 7.8 | 107 | 10.2 | 0.004 |
| Peripheral vascular disease | 16,705 | 6.9 | 16,639 | 6.9 | 66 | 6.3 | 0.43 |
| Valvular disease | 13,683 | 5.6 | 13,628 | 5.6 | 55 | 5.2 | 0.56 |
| Pulmonary circulation disease | 5,530 | 2.3 | 5,492 | 2.3 | 38 | 3.6 | 0.004 |
| Chronic pulmonary disease | 72,028 | 29.7 | 71,698 | 29.7 | 330 | 31.4 | 0.23 |
| Respiratory failure second diagnosis | 13,027 | 5.4 | 12,893 | 5.3 | 134 | 12.7 | <0.001 |
| Rheumatoid arthritis/collagen vascular disease | 7,090 | 2.9 | 7,050 | 2.9 | 40 | 3.8 | 0.09 |
| Deficiency anemias | 49,605 | 20.4 | 49,352 | 20.4 | 253 | 24.0 | 0.004 |
| Weight loss | 8,810 | 3.6 | 8,714 | 3.6 | 96 | 9.1 | <0.001 |
| Peptic ulcer disease bleeding | 4,736 | 2.0 | 4,723 | 2.0 | 13 | 1.2 | 0.09 |
| Chronic blood loss anemia | 2,354 | 1.0 | 2,338 | 1.0 | 16 | 1.5 | 0.07 |
| Hypothyroidism | 28,773 | 11.9 | 28,668 | 11.9 | 105 | 10.0 | 0.06 |
| Renal failure | 19,768 | 8.1 | 19,669 | 8.1 | 99 | 9.4 | 0.13 |
| Liver disease | 4,682 | 1.9 | 4,657 | 1.9 | 25 | 2.4 | 0.29 |
| Other neurological disorders | 33,094 | 13.6 | 32,905 | 13.6 | 189 | 18.0 | <0.001 |
| Psychoses | 9,330 | 3.8 | 9,283 | 3.8 | 47 | 4.5 | 0.29 |
| Depression | 25,561 | 10.5 | 25,442 | 10.5 | 119 | 11.3 | 0.41 |
| Alcohol abuse | 7,756 | 3.2 | 7,727 | 3.2 | 29 | 2.8 | 0.42 |
| Drug abuse | 4,336 | 1.8 | 4,318 | 1.8 | 18 | 1.7 | 0.85 |
| Acquired immune deficiency syndrome | 1,048 | 0.4 | 1,045 | 0.4 | 3 | 0.3 | 0.47 |
| Total | Derivation | Validation | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | P‐Value |
| |||||||
| Total | 242,738 | 100 | 194,198 | 100 | 48,540 | 100 | |
| VTE prophylaxis | 0.97 | ||||||
| No prophylaxis | 170,180 | 70.1 | 136,153 | 70.1 | 34,027 | 70.1 | |
| Any prophylaxis | 72,558 | 29.9 | 58,045 | 29.9 | 14,513 | 29.9 | |
| Outcomes | |||||||
| ICD‐9 code for VTE | 1,304 | 0.5 | 1,025 | 0.5 | 279 | 0.6 | 0.21 |
| ICD‐9 code + diagnostic test | 989 | 0.4 | 777 | 0.4 | 212 | 0.4 | 0.26 |
| ICD‐9 code + diagnostic test + treatment for VTE | 612 | 0.3 | 471 | 0.2 | 141 | 0.3 | 0.06 |
| Readmission for VTE within 30 days | 446 | 0.2 | 363 | 0.2 | 83 | 0.2 | 0.46 |
| Total hospital‐acquired VTE | 1,052 | 0.4 | 829 | 0.4 | 223 | 0.5 | 0.33 |
| In‐hospital mortality | 8,019 | 3.3 | 6,403 | 3.3 | 1,616 | 3.3 | 0.72 |
| Any readmission within 30 days | 28,664 | 11.8 | 22,885 | 11.8 | 5,779 | 11.9 | 0.46 |
Risk factors for VTE
A large number of patient and hospital factors were associated with the development of VTE (Table 1). Due to the large sample size, even weak associations appear highly statistically significant. Compared to patients without VTE, those with VTE were more likely to have received VTE prophylaxis (37% vs 30%, P < 0.001). However, models of patients receiving prophylaxis and of patients not receiving prophylaxis produced similar odds ratios for the various risk factors (Supporting Information Appendix Table 2); therefore, the final model includes both patients who did, and did not, receive VTE prophylaxis. In the multivariable model (Supporting Information Appendix Table 3), age, length of stay, gender, primary diagnosis, cancer, inflammatory bowel disease, obesity, central venous catheter, inherited thrombophilia, steroid use, mechanical ventilation, active chemotherapy, and urinary catheters were all associated with VTE (Table 3). The strongest risk factors were length of stay 6 days (OR 3.22, 95% CI 2.73, 3.79), central venous catheter (OR 1.87, 95% CI 1.52, 2.29), inflammatory bowel disease (OR 3.11, 95% CI 1.59, 6.08), and inherited thrombophilia (OR 4.00, 95% CI 0.98, 16.40). In addition, there were important interactions between age and cancer; cancer was a strong risk factor among younger patients, but is not as strong a risk factor among older patients (OR compared to young patients without cancer was 4.62 (95% CI 2.72, 7.87) for those age 1849 years, and 3.64 (95% CI 2.52, 5.25) for those aged 5064 years).
| Risk Factor | OR | 95% CI |
|---|---|---|
| ||
| Any prophylaxis | 0.98 | (0.84, 1.14) |
| Female | 0.85 | (0.74, 0.98) |
| Length of stay 6 days | 3.22 | (2.73, 3.79) |
| Age* | ||
| 18‐49 years | 1 | Referent |
| 50‐64 years | 1.15 | (0.86, 1.56) |
| >65 years | 1.51 | (1.17, 1.96) |
| Primary diagnosis | ||
| Pneumonia | 1 | Referent |
| Chronic obstructive pulmonary disease | 0.57 | (0.44, 0.75) |
| Stroke | 0.84 | (0.66, 1.08) |
| Congestive heart failure | 0.86 | (0.70, 1.06) |
| Urinary tract infection | 1.19 | (0.95, 1.50) |
| Respiratory failure | 1.15 | (0.85, 1.55) |
| Septicemia | 1.11 | (0.82, 1.50) |
| Comorbidities | ||
| Inflammatory bowel disease | 3.11 | (1.59, 6.08) |
| Obesity | 1.28 | (0.99, 1.66) |
| Inherited thrombophilia | 4.00 | (0.98, 16.40) |
| Cancer | ||
| 18‐49 years | 4.62 | (2.72, 7.87) |
| 50‐64 years | 3.64 | (2.52, 5.25) |
| >65 years | 2.17 | (1.61, 2.92) |
| Treatments | ||
| Central venous catheter | 1.87 | (1.52, 2.29) |
| Mechanical ventilation | 1.61 | (1.27, 2.05) |
| Urinary catheter | 1.17 | (0.99, 1.38) |
| Chemotherapy | 1.71 | (1.03, 2.83) |
| Steroids | 1.22 | (1.04, 1.43) |
In the derivation set, the multivariable model produced deciles of mean predicted risk from 0.11% to 1.45%, while mean observed risk over the same deciles ranged from 0.12% to 1.42% (Figure 1). Within the validation cohort, the observed rate of VTE was 0.46% (223 cases among 48,543 subjects). The expected rate according to the model was 0.43% (expected/observed ratio: 0.93 [95% CI 0.82, 1.06]). Model discrimination measured by the c‐statistic in the validation set was 0.75 (95% CI 0.71, 0.78). The model produced deciles of mean predicted risk from 0.11% to 1.46%, with mean observed risk over the same deciles from 0.17% to 1.81%. Risk gradient was relatively flat across the first 6 deciles, began to rise at the seventh decile, and rose sharply in the highest one. Using a risk threshold of 1%, the model had a sensitivity of 28% and a specificity of 93%. In the validation set, this translated into a positive predictive value of 2.2% and a negative predictive value of 99.7%. Assuming that VTE prophylaxis has an efficacy of 50%, the number‐needed‐to‐treat to prevent one VTE among high‐risk patients (predicted risk >1%) would be 91. In contrast, providing prophylaxis to the entire validation sample would result in a number‐needed‐to‐treat of 435. Using a lower treatment threshold of 0.4% produced a positive predictive value of 1% and a negative predictive value of 99.8%. At this threshold, the model would detect 73% of patients with VTE and the number‐needed‐to‐treat to prevent one VTE would be 200.

Discussion
In a representative sample of 243,000 hospitalized medical patients with at least one major risk factor for VTE, we found that symptomatic VTE was an uncommon event, occurring in approximately 1 in 231 patients. We identified a number of factors that were associated with an increased risk of VTE, but many previously cited risk factors did not show an association in multivariable models. In particular, patients with a primary diagnosis of COPD appeared not to share the same high risk of VTE as patients with the other diagnoses we examined, a finding reported by others.11 The risk model we developed accurately stratifies patients across a wide range of VTE probabilities, but even among those with the highest predicted rates, symptomatic VTE occurred in less than 2%.
VTE is often described as a frequent complication of hospitalization for medical illness and one of the most common potentially preventable causes of death. Indeed, rates of asymptomatic VTE have been demonstrated to be 3.7% to 26%.12 Although some of these might have fatal consequences, most are distal vein thromboses and their significance is unknown. In contrast, symptomatic events are uncommon, with previous estimates among general medical patients in observational studies in the range of 0.3%3 to 0.8%,12 similar to the rate observed in our study. Symptomatic event rates among control patients in landmark randomized trials have ranged from 0.86%13 to 2.3%,14 but these studies enrolled only very high‐risk patients with more extended hospitalizations, and may involve follow‐up periods of a month or more.
Because it is unlikely that our diagnostic algorithm was 100% sensitive, and because 30% of our patients received chemoprophylaxis, it is probable that we have underestimated the true rate of VTE in our sample. Among the patients who received prophylaxis, the observed rate of VTE was 0.54%. If we assume that prophylaxis is 50% effective, then had these patients not received prophylaxis, their rate of VTE would have been 1.08% (vs 0.39% among those patients who received no prophylaxis) and the overall rate of VTE for the sample would have been 0.60% (1.08 0.30 + 0.39 0.70). If we further assume that our algorithm was only 80% sensitive and 100% specific, the true underlying rate of symptomatic VTE could have been as high as 0.75%, still less than half that seen in randomized trials.
Prophylaxis with heparin has been shown to decrease the rate of both asymptomatic and symptomatic events, but because of the low prevalence, the number‐needed‐to‐treat to prevent one symptomatic pulmonary embolism has been estimated at 345, and prophylaxis has not been shown to affect all‐cause mortality.4, 15 At the same time, prophylaxis costs money, is uncomfortable, and carries a small risk of bleeding and heparin‐induced thrombocytopenia. Given the generally low incidence of symptomatic VTE, it therefore makes sense to reserve prophylaxis for patients at higher risk of thromboembolism.
To decide whether prophylaxis is appropriate for a given patient, it is necessary to quantify the patient's risk and then apply an appropriate threshold for treatment. The National Quality Forum (NQF) recommends,16 and JCAHO has adopted, that a clinician must evaluate each patient upon admission, and regularly thereafter, for the risk of developing DVT [deep vein thrombosis]/VTE. Until now, however, there has been no widely accepted, validated method to risk stratify medical patients. The ACCP recommendations cite just three studies of VTE risk factors in hospitalized medical patients.11, 17, 18 Together they examined 477 cases and 1197 controls, identifying congestive heart failure, pneumonia, cancer, and previous VTE as risk factors. Predictive models based on these factors17, 1921 have not been subjected to validation or have performed poorly.18 Acknowledging this lack of standardized risk assessment, JCAHO leaves the means of assessment to individual hospitals. A quality improvement guide published by the Agency for Healthcare Research and Quality goes one step further, stating that In a typical hospital, it is estimated that fewer than 5% of medical patients could be considered at low risk by most VTE risk stratification methods.22 The guide recommends near universal VTE prophylaxis.
In light of the JCAHO requirements, our model should be welcomed by hospitalists. Rather than assuming that all patients over 40 years of age are at high risk, our model will enable clinicians to risk stratify patients from a low of 0.1% to >1.4% (>10‐fold increase in risk). Moreover, the model was derived from more than 800 episodes of symptomatic VTE among almost 190,000 general medical patients and validated on almost 50,000 more. The observed patients were cared for in clinical practice at a nationally representative group of US hospitals, not in a highly selected clinical trial, increasing the generalizability of our findings. Finally, the model includes ten common risk factors that can easily be entered into decision support software or extracted automatically from the electronic medical record. Electronic reminder systems have already been shown to increase use of VTE prophylaxis, and prevent VTE, especially among cancer patients.23
A more challenging task is defining the appropriate risk threshold to initiate VTE prophylaxis. The Thromboembolic Risk Factors (THRIFT) Consensus Group classified patients according to risk of proximal DVT as low (<1%), moderate (1%‐10%), and high (>10%).21 They recommended heparin prophylaxis for all patients at moderate risk or higher. Although the patients included in our study all had a diagnosis that warranted prophylaxis according to the ACCP guidelines, using the THRIFT threshold for moderate‐to‐high risk, only 7% of our patients should have received prophylaxis. The recommendation not to offer heparin prophylaxis to patients with less than 1% chance of developing symptomatic VTE seems reasonable, given the large number‐needed‐to‐treat, but formal decision analyses should be conducted to better define this threshold. Many hospitalists, however, may feel uncomfortable using the 1% threshold, because our model failed to identify almost three out of four patients who ultimately experienced symptomatic VTE. At that threshold, it would seem that hospital‐acquired VTE is not a preventable complication in most medical patients, as others have pointed out.3, 24 Alternatively, if the threshold were lowered to 0.4%, our model could reduce the use of prophylaxis by 60%, while still identifying three‐fourths of all VTE cases. Further research is needed to know whether such a threshold is reasonable.
Our study has a number of important limitations. First, we relied on claims data, not chart review. We do not know for certain which patients experienced VTE, although our definition of VTE required diagnosis codes plus charges for both diagnosis and treatment. Moreover, our rates are similar to those observed in other trials where symptomatic events were confirmed. Second, about 30% of our patients received at least some VTE prophylaxis, and this may have prevented as many as half of the VTEs in that group. Without prophylaxis, rates might have been 20%30% higher. Similarly, we could not detect patients who were diagnosed after discharge but not admitted to hospital. While we believe this number to be small, it would again increase the rate slightly. Third, we could not assess certain clinical circumstances that are not associated with hospital charges or diagnosis codes, especially prolonged bed rest. Other risk factors, such as the urinary catheter, were probably surrogate markers for immobilization rather than true risk factors. Fourth, we included length of stay in our prediction model. We did this because most randomized trials of VTE prophylaxis included only patients with an expected length of stay 6 days. Physicians' estimates about probable length of stay may be less accurate than actual length of stay as a predictor of VTE. Moreover, the relationship may have been confounded if hospital‐acquired VTE led to longer lengths of stay. We think this unlikely since many of the events were discovered on readmission. Fifth, we studied only patients carrying high‐risk diagnoses, and therefore do not know the baseline risk for patients with less risky conditions, although it should be lower than what we observed. It seems probable that COPD, rather than being protective, as it appears in our model, actually represents the baseline risk for low‐risk diagnoses. It should be noted that we did include a number of other high‐risk diagnoses, such as cancer and inflammatory bowel disease, as secondary diagnoses. A larger, more inclusive study should be conducted to validate our model in other populations. Finally, we cannot know who died of undiagnosed VTE, either in the hospital or after discharge. Such an outcome would be important, but those events are likely to be rare, and VTE prophylaxis has not been shown to affect mortality.
VTE remains a daunting problem in hospitalized medical patients. Although VTE is responsible for a large number of hospital deaths each year, identifying patients at high risk for clinically important VTE is challenging, and may contribute to the persistently low rates of VTE prophylaxis seen in hospitals.25 Current efforts to treat nearly all patients are likely to lead to unnecessary cost, discomfort, and side effects. We present a simple logistic regression model that can easily identify patients at moderate‐to‐high risk (>1%) of developing symptomatic VTE. Future studies should focus on prospectively validating the model in a wider spectrum of medical illness, and better defining the appropriate risk cutoff for general prophylaxis.
Acknowledgements
The authors thank Aruna Priya, MS, for her help with some of the statistical analyses.
Venous thromboembolism (VTE) is a major source of morbidity and mortality for hospitalized patients. Among medical patients at the highest risk, as many as 15% can be expected to develop a VTE during their hospital stay1, 2; however, among general medical patients, the incidence of symptomatic VTE is less than 1%,1 and potentially as low as 0.3%.3 Thromboprophylaxis with subcutaneous heparin reduces the risk of VTE by approximately 50%,4 and is therefore recommended for medical patients at high risk. However, heparin also increases the risk of bleeding and thrombocytopenia and thus should be avoided for patients at low risk of VTE. Consequently, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) recommends that all hospitalized medical patients receive a risk assessment for VTE.5
Certain disease states, including stroke, acute myocardial infarction, heart failure, respiratory disease, sepsis, and cancer, have been associated with increased risk for VTE, and, based on the inclusion criteria of several randomized trials, current American College of Chest Physicians (ACCP) guidelines recommend thromboprophylaxis for patients hospitalized with these diagnoses.2 However, evidence that these factors actually increase a patient's risk for VTE comes from studies of ambulatory patients and is often weak or conflicting. Existing risk‐stratification tools,6, 7 as well as the ACCP guidelines, have not been validated, and accordingly JCAHO does not specify how risk assessment should be conducted. In order to help clinicians better estimate the risk of VTE in medical patients and therefore to provide more targeted thromboprophylaxis, we examined a large cohort of patients with high‐risk diagnoses and created a risk stratification model.
Methods
Setting and Patients
We identified a retrospective cohort of patients discharged between January 1, 2004 and June 30, 2005 from 374 acute care facilities in the US that participated in Premier's Perspective, a database developed for measuring quality and healthcare utilization. Participating hospitals represent all regions of the US, and are generally similar in composition to US hospitals; however, in comparison to information contained in the American Hospital Association annual survey, Perspective hospitals are more likely to be located in the South and in urban areas. Available data elements include those derived from the uniform billing 04 form, such as sociodemographic information about each patient, their International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis and procedure codes, as well as hospital and physician information. This information is supplemented with a date‐stamped log of all items and services billed to the patient or insurer, including diagnostic tests, medications, and other treatments. Permission to conduct the study was obtained from the Institutional Review Board at Baystate Medical Center.
We included all patients age 18 years at moderate‐to‐high risk of VTE according to the ACCP recommendations,8 based on a principal diagnosis of pneumonia, septicemia or respiratory failure with pneumonia, heart failure, chronic obstructive pulmonary disease (COPD), stroke, and urinary tract infection. Diagnoses were assessed using ICD‐9‐CM codes. Patients who were prescribed warfarin or therapeutic doses of heparin on hospital day 1 or 2, and those who received >1 therapeutic dose of heparin but otherwise did not fulfill criteria for VTE, were excluded because we could not evaluate whether they experienced a VTE event during hospitalization. We also excluded patients whose length of stay was <3 days, because our definition of hospital‐acquired VTE required treatment begun on day 3 or later, and those with an indication for anticoagulation other than VTE (eg, prosthetic cardiac valve or atrial fibrillation), because we could not reliably distinguish treatment for VTE from treatment of the underlying condition.
Risk Factors
For each patient, we extracted age, gender, race/ethnicity, and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9‐CM secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser et al.9 We also assessed risk factors which have been previously linked to VTE: paralysis, cancer (metastatic, solid tumor, and lymphoma), chemotherapy/radiation, prior VTE, use of estrogens and estrogen modulators, inflammatory bowel disease, nephrotic syndrome, myeloproliferative disorders, obesity, smoking, central venous catheter, inherited or acquired thrombophilia, steroid use, mechanical ventilation, urinary catheter, decubitus ulcer, HMGco‐A reductase inhibitors, restraints, diabetes, varicose veins, and length‐of‐stay 6 days. These additional comorbidities were defined based on the presence of specific ICD‐9 codes, while use of HMG‐co‐A reductase inhibitors were identified from medication charge files. We also noted whether patients received anticoagulants, the dosages and days of administration, as well as intermittent pneumatic compression devices.
Identification of VTE
Because the presence of a secondary diagnosis of VTE in medical patients is not a reliable way of differentiating hospital‐acquired VTE from those present at the time of admission,10 subjects were considered to have experienced a hospital‐acquired VTE only if they underwent a diagnostic test for VTE (lower extremity ultrasound, venography, CT angiogram, ventilation‐perfusion scan, or pulmonary angiogram) on hospital day 3 or later, received treatment for VTE for at least 50% of the remaining hospital stay, or until initiation of warfarin or appearance of a complication (eg, transfusion or treatment for heparin‐induced thrombocytopenia) and were given a secondary diagnosis of VTE (ICD‐9 diagnoses 453.4, 453.40, 453.41, 453.42, 453.8, 453.9, 415.1, 415.11, 415.19). We considered the following to be treatments for VTE: intravenous unfractionated heparin, >60 mg of enoxaparin, 7500 mg of dalteparin, or placement of an inferior vena cava filter. In addition, patients who were readmitted within 30 days of discharge with a primary diagnosis of VTE were also considered to have developed a VTE as a complication of their previous hospital stay.
Statistical Analysis
Univariate predictors of VTE were assessed using chi‐square tests. We developed a multivariable logistic regression model for VTE on an 80% randomly selected subset of the eligible admissions (the derivation cohort) using all measured risk factors for VTE and selected interaction terms. Generalized estimating equations (GEE) models with a logit link (SAS PROC GENMOD) were used to account for the clustering of patients within hospitals. Initial models were stratified on VTE prophylaxis. Factors significant at P < 0.05 were retained. Parameter estimates derived from the model were used to compute individual VTE risk in the remaining 20% of the admissions (the validation cohort). Discrimination in the validation model was assessed by the c‐statistic, as well as the expected/observed ratio. Both cohorts were categorized by decile of risk, based on the probability distribution in the derivation cohort, and observed VTE events compared to those predicted by the model. All analyses were performed using the Statistical Analysis System (version 9.1, SAS Institute, Inc., Cary, NC).
Role of the Funding Source
This study was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation. The funding source had no role in the study design, analysis, or interpretation of the data.
Results
Our sample contained 242,738 patients, 194,198 (80%) assigned to the derivation set and 48,540 (20%) to the validation set. Patient characteristics were similar in both sets (Supporting Information Appendix Table 1). Most patients were over age 65, 59% were female, and 64% were white (Table 1). The most common primary diagnoses were pneumonia (33%) and congestive heart failure (19%). The most common comorbidities were hypertension (50%), diabetes (31%), chronic pulmonary disease (30%), and anemia (20%). Most patients were cared for by internists (54%) or family practitioners (21%), and 30% received some form of anticoagulant VTE prophylaxis (Table 2). Of patients with an ICD‐9 code for VTE during hospitalization, just over half lacked either diagnostic testing, treatment, or both, leaving 612 (0.25%) patients who fulfilled our criteria for VTE; an additional 440 (0.18%) were readmitted for VTE, for an overall incidence of 0.43%. Patients with a length of stay 6 days had an incidence of 0.79% vs 0.19% for patients with shorter stays.
| Total | No VTE | VTE | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | P‐Value |
| Total | 242,738 | 100 | 241,686 | 100.0 | 1,052 | 100.0 | |
| Demographics | |||||||
| Age | 0.20 | ||||||
| 18‐49 | 31,065 | 12.8 | 30,952 | 12.8 | 113 | 10.7 | |
| 50‐64 | 51,309 | 21.1 | 51,083 | 21.1 | 226 | 21.5 | |
| 65‐74 | 51,230 | 21.1 | 50,993 | 21.1 | 237 | 22.5 | |
| 75+ | 109,134 | 45.0 | 108,658 | 45.0 | 476 | 45.2 | |
| Female | 142,910 | 58.9 | 142,330 | 58.9 | 580 | 55.1 | 0.01 |
| Race/ethnicity | 0.49 | ||||||
| White | 155,866 | 64.2 | 155,189 | 64.2 | 677 | 64.4 | |
| Black | 41,556 | 17.1 | 41,374 | 17.1 | 182 | 17.3 | |
| Hispanic | 9,809 | 4.0 | 9,776 | 4.0 | 33 | 3.1 | |
| Other | 35,507 | 14.6 | 35,347 | 14.6 | 160 | 15.2 | |
| Marital status | 0.28 | ||||||
| Married/life partner | 88,035 | 36.3 | 87,627 | 36.3 | 408 | 38.8 | |
| Single | 39,254 | 16.2 | 39,103 | 16.2 | 151 | 14.4 | |
| Separated/divorced | 23,492 | 9.7 | 23,394 | 9.7 | 98 | 9.3 | |
| Widowed | 58,669 | 24.2 | 58,426 | 24.2 | 243 | 23.1 | |
| Other | 33,288 | 13.7 | 33,136 | 13.7 | 152 | 14.4 | |
| Admission characteristics | |||||||
| Primary diagnosis | <0.001 | ||||||
| Community‐acquired pneumonia | 81,171 | 33.4 | 80,792 | 33.4 | 379 | 36.0 | |
| Septicemia | 7,643 | 3.2 | 7,568 | 3.1 | 75 | 7.1 | |
| Chronic obstructive pulmonary disease | 35,116 | 14.5 | 35,027 | 14.5 | 89 | 8.5 | |
| Respiratory failure | 7,098 | 2.9 | 7,012 | 2.9 | 86 | 8.2 | |
| Congestive heart failure | 46,503 | 19.2 | 46,336 | 19.2 | 167 | 15.9 | |
| Cardiovascular disease | 33,044 | 13.6 | 32,931 | 13.6 | 113 | 10.7 | |
| Urinary tract infection | 32,163 | 13.3 | 32,020 | 13.2 | 143 | 13.6 | |
| Insurance payer | 0.93 | ||||||
| Medicare traditional | 157,609 | 64.9 | 156,927 | 64.9 | 682 | 64.8 | |
| Medicare managed care | 10,649 | 4.4 | 10,597 | 4.4 | 52 | 4.9 | |
| Medicaid | 17,796 | 7.3 | 17,720 | 7.3 | 76 | 7.2 | |
| Private | 44,858 | 18.5 | 44,665 | 18.5 | 193 | 18.3 | |
| Self‐pay/uninsured/other | 11,826 | 4.9 | 11,777 | 4.9 | 49 | 4.7 | |
| Admitted from skilled nursing facility | 3,003 | 1.2 | 2,980 | 1.2 | 23 | 2.2 | 0.005 |
| Risk factors | |||||||
| Any VTE prophylaxis | 72,558 | 29.9 | 72,164 | 29.9 | 394 | 37.5 | <0.001 |
| Length of stay 6 days | 99,463 | 41.0 | 98,680 | 40.8 | 783 | 74.4 | <0.001 |
| Paralysis | 16,764 | 6.9 | 16,689 | 6.9 | 75 | 7.1 | 0.77 |
| Metastatic cancer | 5,013 | 2.1 | 4,928 | 2.0 | 85 | 8.1 | <0.001 |
| Solid tumor without metastasis | 25,127 | 10.4 | 24,995 | 10.3 | 132 | 12.5 | 0.02 |
| Lymphoma | 3,026 | 1.2 | 2,995 | 1.2 | 31 | 2.9 | <0.001 |
| Cancer chemotherapy/radiation | 1,254 | 0.5 | 1,231 | 0.5 | 23 | 2.2 | <0.001 |
| Prior venous thromboembolism | 2,945 | 1.2 | 2,926 | 1.2 | 19 | 1.8 | 0.08 |
| Estrogens | 4,819 | 2.0 | 4,807 | 2.0 | 12 | 1.1 | 0.05 |
| Estrogen modulators | 2,102 | 0.9 | 2,091 | 0.9 | 11 | 1.0 | 0.53 |
| Inflammatory bowel disease | 814 | 0.3 | 803 | 0.3 | 11 | 1.0 | <0.001 |
| Nephrotic syndrome | 520 | 0.2 | 517 | 0.2 | 3 | 0.3 | 0.62 |
| Myeloproliferative disorder | 1,983 | 0.8 | 1,973 | 0.8 | 10 | 1.0 | 0.63 |
| Obesity | 16,938 | 7.0 | 16,856 | 7.0 | 82 | 7.8 | 0.30 |
| Smoking | 35,386 | 14.6 | 35,284 | 14.6 | 102 | 9.7 | <0.001 |
| Central venous catheter | 14,754 | 6.1 | 14,525 | 6.0 | 229 | 21.8 | <0.001 |
| Inherited or acquired thrombophilia | 114 | 0.1 | 108 | 0.0 | 6 | 0.6 | <0.001 |
| Steroids | 82,606 | 34.0 | 82,185 | 34.0 | 421 | 40.0 | <0.001 |
| Mechanical ventilation | 13,347 | 5.5 | 13,167 | 5.4 | 180 | 17.1 | <0.001 |
| Urinary catheter | 39,080 | 16.1 | 38,816 | 16.1 | 264 | 25.1 | <0.001 |
| Decubitus ulcer | 6,829 | 2.8 | 6,776 | 2.8 | 53 | 5.0 | <0.001 |
| Statins use | 57,282 | 23.6 | 57,068 | 23.6 | 214 | 20.3 | 0.01 |
| Use of restraints | 5,970 | 2.5 | 5,914 | 2.4 | 56 | 5.3 | <0.001 |
| Diabetes mellitus | 75,103 | 30.9 | 74,799 | 30.9 | 304 | 28.9 | 0.15 |
| Varicose veins | 166 | 0.1 | 165 | 0.1 | 1 | 0.1 | 0.74 |
| Comorbidities | |||||||
| Hypertension | 120,606 | 49.7 | 120,126 | 49.7 | 480 | 45.6 | 0.008 |
| Congestive heart failure | 18,900 | 7.8 | 18,793 | 7.8 | 107 | 10.2 | 0.004 |
| Peripheral vascular disease | 16,705 | 6.9 | 16,639 | 6.9 | 66 | 6.3 | 0.43 |
| Valvular disease | 13,683 | 5.6 | 13,628 | 5.6 | 55 | 5.2 | 0.56 |
| Pulmonary circulation disease | 5,530 | 2.3 | 5,492 | 2.3 | 38 | 3.6 | 0.004 |
| Chronic pulmonary disease | 72,028 | 29.7 | 71,698 | 29.7 | 330 | 31.4 | 0.23 |
| Respiratory failure second diagnosis | 13,027 | 5.4 | 12,893 | 5.3 | 134 | 12.7 | <0.001 |
| Rheumatoid arthritis/collagen vascular disease | 7,090 | 2.9 | 7,050 | 2.9 | 40 | 3.8 | 0.09 |
| Deficiency anemias | 49,605 | 20.4 | 49,352 | 20.4 | 253 | 24.0 | 0.004 |
| Weight loss | 8,810 | 3.6 | 8,714 | 3.6 | 96 | 9.1 | <0.001 |
| Peptic ulcer disease bleeding | 4,736 | 2.0 | 4,723 | 2.0 | 13 | 1.2 | 0.09 |
| Chronic blood loss anemia | 2,354 | 1.0 | 2,338 | 1.0 | 16 | 1.5 | 0.07 |
| Hypothyroidism | 28,773 | 11.9 | 28,668 | 11.9 | 105 | 10.0 | 0.06 |
| Renal failure | 19,768 | 8.1 | 19,669 | 8.1 | 99 | 9.4 | 0.13 |
| Liver disease | 4,682 | 1.9 | 4,657 | 1.9 | 25 | 2.4 | 0.29 |
| Other neurological disorders | 33,094 | 13.6 | 32,905 | 13.6 | 189 | 18.0 | <0.001 |
| Psychoses | 9,330 | 3.8 | 9,283 | 3.8 | 47 | 4.5 | 0.29 |
| Depression | 25,561 | 10.5 | 25,442 | 10.5 | 119 | 11.3 | 0.41 |
| Alcohol abuse | 7,756 | 3.2 | 7,727 | 3.2 | 29 | 2.8 | 0.42 |
| Drug abuse | 4,336 | 1.8 | 4,318 | 1.8 | 18 | 1.7 | 0.85 |
| Acquired immune deficiency syndrome | 1,048 | 0.4 | 1,045 | 0.4 | 3 | 0.3 | 0.47 |
| Total | Derivation | Validation | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | P‐Value |
| |||||||
| Total | 242,738 | 100 | 194,198 | 100 | 48,540 | 100 | |
| VTE prophylaxis | 0.97 | ||||||
| No prophylaxis | 170,180 | 70.1 | 136,153 | 70.1 | 34,027 | 70.1 | |
| Any prophylaxis | 72,558 | 29.9 | 58,045 | 29.9 | 14,513 | 29.9 | |
| Outcomes | |||||||
| ICD‐9 code for VTE | 1,304 | 0.5 | 1,025 | 0.5 | 279 | 0.6 | 0.21 |
| ICD‐9 code + diagnostic test | 989 | 0.4 | 777 | 0.4 | 212 | 0.4 | 0.26 |
| ICD‐9 code + diagnostic test + treatment for VTE | 612 | 0.3 | 471 | 0.2 | 141 | 0.3 | 0.06 |
| Readmission for VTE within 30 days | 446 | 0.2 | 363 | 0.2 | 83 | 0.2 | 0.46 |
| Total hospital‐acquired VTE | 1,052 | 0.4 | 829 | 0.4 | 223 | 0.5 | 0.33 |
| In‐hospital mortality | 8,019 | 3.3 | 6,403 | 3.3 | 1,616 | 3.3 | 0.72 |
| Any readmission within 30 days | 28,664 | 11.8 | 22,885 | 11.8 | 5,779 | 11.9 | 0.46 |
Risk factors for VTE
A large number of patient and hospital factors were associated with the development of VTE (Table 1). Due to the large sample size, even weak associations appear highly statistically significant. Compared to patients without VTE, those with VTE were more likely to have received VTE prophylaxis (37% vs 30%, P < 0.001). However, models of patients receiving prophylaxis and of patients not receiving prophylaxis produced similar odds ratios for the various risk factors (Supporting Information Appendix Table 2); therefore, the final model includes both patients who did, and did not, receive VTE prophylaxis. In the multivariable model (Supporting Information Appendix Table 3), age, length of stay, gender, primary diagnosis, cancer, inflammatory bowel disease, obesity, central venous catheter, inherited thrombophilia, steroid use, mechanical ventilation, active chemotherapy, and urinary catheters were all associated with VTE (Table 3). The strongest risk factors were length of stay 6 days (OR 3.22, 95% CI 2.73, 3.79), central venous catheter (OR 1.87, 95% CI 1.52, 2.29), inflammatory bowel disease (OR 3.11, 95% CI 1.59, 6.08), and inherited thrombophilia (OR 4.00, 95% CI 0.98, 16.40). In addition, there were important interactions between age and cancer; cancer was a strong risk factor among younger patients, but is not as strong a risk factor among older patients (OR compared to young patients without cancer was 4.62 (95% CI 2.72, 7.87) for those age 1849 years, and 3.64 (95% CI 2.52, 5.25) for those aged 5064 years).
| Risk Factor | OR | 95% CI |
|---|---|---|
| ||
| Any prophylaxis | 0.98 | (0.84, 1.14) |
| Female | 0.85 | (0.74, 0.98) |
| Length of stay 6 days | 3.22 | (2.73, 3.79) |
| Age* | ||
| 18‐49 years | 1 | Referent |
| 50‐64 years | 1.15 | (0.86, 1.56) |
| >65 years | 1.51 | (1.17, 1.96) |
| Primary diagnosis | ||
| Pneumonia | 1 | Referent |
| Chronic obstructive pulmonary disease | 0.57 | (0.44, 0.75) |
| Stroke | 0.84 | (0.66, 1.08) |
| Congestive heart failure | 0.86 | (0.70, 1.06) |
| Urinary tract infection | 1.19 | (0.95, 1.50) |
| Respiratory failure | 1.15 | (0.85, 1.55) |
| Septicemia | 1.11 | (0.82, 1.50) |
| Comorbidities | ||
| Inflammatory bowel disease | 3.11 | (1.59, 6.08) |
| Obesity | 1.28 | (0.99, 1.66) |
| Inherited thrombophilia | 4.00 | (0.98, 16.40) |
| Cancer | ||
| 18‐49 years | 4.62 | (2.72, 7.87) |
| 50‐64 years | 3.64 | (2.52, 5.25) |
| >65 years | 2.17 | (1.61, 2.92) |
| Treatments | ||
| Central venous catheter | 1.87 | (1.52, 2.29) |
| Mechanical ventilation | 1.61 | (1.27, 2.05) |
| Urinary catheter | 1.17 | (0.99, 1.38) |
| Chemotherapy | 1.71 | (1.03, 2.83) |
| Steroids | 1.22 | (1.04, 1.43) |
In the derivation set, the multivariable model produced deciles of mean predicted risk from 0.11% to 1.45%, while mean observed risk over the same deciles ranged from 0.12% to 1.42% (Figure 1). Within the validation cohort, the observed rate of VTE was 0.46% (223 cases among 48,543 subjects). The expected rate according to the model was 0.43% (expected/observed ratio: 0.93 [95% CI 0.82, 1.06]). Model discrimination measured by the c‐statistic in the validation set was 0.75 (95% CI 0.71, 0.78). The model produced deciles of mean predicted risk from 0.11% to 1.46%, with mean observed risk over the same deciles from 0.17% to 1.81%. Risk gradient was relatively flat across the first 6 deciles, began to rise at the seventh decile, and rose sharply in the highest one. Using a risk threshold of 1%, the model had a sensitivity of 28% and a specificity of 93%. In the validation set, this translated into a positive predictive value of 2.2% and a negative predictive value of 99.7%. Assuming that VTE prophylaxis has an efficacy of 50%, the number‐needed‐to‐treat to prevent one VTE among high‐risk patients (predicted risk >1%) would be 91. In contrast, providing prophylaxis to the entire validation sample would result in a number‐needed‐to‐treat of 435. Using a lower treatment threshold of 0.4% produced a positive predictive value of 1% and a negative predictive value of 99.8%. At this threshold, the model would detect 73% of patients with VTE and the number‐needed‐to‐treat to prevent one VTE would be 200.

Discussion
In a representative sample of 243,000 hospitalized medical patients with at least one major risk factor for VTE, we found that symptomatic VTE was an uncommon event, occurring in approximately 1 in 231 patients. We identified a number of factors that were associated with an increased risk of VTE, but many previously cited risk factors did not show an association in multivariable models. In particular, patients with a primary diagnosis of COPD appeared not to share the same high risk of VTE as patients with the other diagnoses we examined, a finding reported by others.11 The risk model we developed accurately stratifies patients across a wide range of VTE probabilities, but even among those with the highest predicted rates, symptomatic VTE occurred in less than 2%.
VTE is often described as a frequent complication of hospitalization for medical illness and one of the most common potentially preventable causes of death. Indeed, rates of asymptomatic VTE have been demonstrated to be 3.7% to 26%.12 Although some of these might have fatal consequences, most are distal vein thromboses and their significance is unknown. In contrast, symptomatic events are uncommon, with previous estimates among general medical patients in observational studies in the range of 0.3%3 to 0.8%,12 similar to the rate observed in our study. Symptomatic event rates among control patients in landmark randomized trials have ranged from 0.86%13 to 2.3%,14 but these studies enrolled only very high‐risk patients with more extended hospitalizations, and may involve follow‐up periods of a month or more.
Because it is unlikely that our diagnostic algorithm was 100% sensitive, and because 30% of our patients received chemoprophylaxis, it is probable that we have underestimated the true rate of VTE in our sample. Among the patients who received prophylaxis, the observed rate of VTE was 0.54%. If we assume that prophylaxis is 50% effective, then had these patients not received prophylaxis, their rate of VTE would have been 1.08% (vs 0.39% among those patients who received no prophylaxis) and the overall rate of VTE for the sample would have been 0.60% (1.08 0.30 + 0.39 0.70). If we further assume that our algorithm was only 80% sensitive and 100% specific, the true underlying rate of symptomatic VTE could have been as high as 0.75%, still less than half that seen in randomized trials.
Prophylaxis with heparin has been shown to decrease the rate of both asymptomatic and symptomatic events, but because of the low prevalence, the number‐needed‐to‐treat to prevent one symptomatic pulmonary embolism has been estimated at 345, and prophylaxis has not been shown to affect all‐cause mortality.4, 15 At the same time, prophylaxis costs money, is uncomfortable, and carries a small risk of bleeding and heparin‐induced thrombocytopenia. Given the generally low incidence of symptomatic VTE, it therefore makes sense to reserve prophylaxis for patients at higher risk of thromboembolism.
To decide whether prophylaxis is appropriate for a given patient, it is necessary to quantify the patient's risk and then apply an appropriate threshold for treatment. The National Quality Forum (NQF) recommends,16 and JCAHO has adopted, that a clinician must evaluate each patient upon admission, and regularly thereafter, for the risk of developing DVT [deep vein thrombosis]/VTE. Until now, however, there has been no widely accepted, validated method to risk stratify medical patients. The ACCP recommendations cite just three studies of VTE risk factors in hospitalized medical patients.11, 17, 18 Together they examined 477 cases and 1197 controls, identifying congestive heart failure, pneumonia, cancer, and previous VTE as risk factors. Predictive models based on these factors17, 1921 have not been subjected to validation or have performed poorly.18 Acknowledging this lack of standardized risk assessment, JCAHO leaves the means of assessment to individual hospitals. A quality improvement guide published by the Agency for Healthcare Research and Quality goes one step further, stating that In a typical hospital, it is estimated that fewer than 5% of medical patients could be considered at low risk by most VTE risk stratification methods.22 The guide recommends near universal VTE prophylaxis.
In light of the JCAHO requirements, our model should be welcomed by hospitalists. Rather than assuming that all patients over 40 years of age are at high risk, our model will enable clinicians to risk stratify patients from a low of 0.1% to >1.4% (>10‐fold increase in risk). Moreover, the model was derived from more than 800 episodes of symptomatic VTE among almost 190,000 general medical patients and validated on almost 50,000 more. The observed patients were cared for in clinical practice at a nationally representative group of US hospitals, not in a highly selected clinical trial, increasing the generalizability of our findings. Finally, the model includes ten common risk factors that can easily be entered into decision support software or extracted automatically from the electronic medical record. Electronic reminder systems have already been shown to increase use of VTE prophylaxis, and prevent VTE, especially among cancer patients.23
A more challenging task is defining the appropriate risk threshold to initiate VTE prophylaxis. The Thromboembolic Risk Factors (THRIFT) Consensus Group classified patients according to risk of proximal DVT as low (<1%), moderate (1%‐10%), and high (>10%).21 They recommended heparin prophylaxis for all patients at moderate risk or higher. Although the patients included in our study all had a diagnosis that warranted prophylaxis according to the ACCP guidelines, using the THRIFT threshold for moderate‐to‐high risk, only 7% of our patients should have received prophylaxis. The recommendation not to offer heparin prophylaxis to patients with less than 1% chance of developing symptomatic VTE seems reasonable, given the large number‐needed‐to‐treat, but formal decision analyses should be conducted to better define this threshold. Many hospitalists, however, may feel uncomfortable using the 1% threshold, because our model failed to identify almost three out of four patients who ultimately experienced symptomatic VTE. At that threshold, it would seem that hospital‐acquired VTE is not a preventable complication in most medical patients, as others have pointed out.3, 24 Alternatively, if the threshold were lowered to 0.4%, our model could reduce the use of prophylaxis by 60%, while still identifying three‐fourths of all VTE cases. Further research is needed to know whether such a threshold is reasonable.
Our study has a number of important limitations. First, we relied on claims data, not chart review. We do not know for certain which patients experienced VTE, although our definition of VTE required diagnosis codes plus charges for both diagnosis and treatment. Moreover, our rates are similar to those observed in other trials where symptomatic events were confirmed. Second, about 30% of our patients received at least some VTE prophylaxis, and this may have prevented as many as half of the VTEs in that group. Without prophylaxis, rates might have been 20%30% higher. Similarly, we could not detect patients who were diagnosed after discharge but not admitted to hospital. While we believe this number to be small, it would again increase the rate slightly. Third, we could not assess certain clinical circumstances that are not associated with hospital charges or diagnosis codes, especially prolonged bed rest. Other risk factors, such as the urinary catheter, were probably surrogate markers for immobilization rather than true risk factors. Fourth, we included length of stay in our prediction model. We did this because most randomized trials of VTE prophylaxis included only patients with an expected length of stay 6 days. Physicians' estimates about probable length of stay may be less accurate than actual length of stay as a predictor of VTE. Moreover, the relationship may have been confounded if hospital‐acquired VTE led to longer lengths of stay. We think this unlikely since many of the events were discovered on readmission. Fifth, we studied only patients carrying high‐risk diagnoses, and therefore do not know the baseline risk for patients with less risky conditions, although it should be lower than what we observed. It seems probable that COPD, rather than being protective, as it appears in our model, actually represents the baseline risk for low‐risk diagnoses. It should be noted that we did include a number of other high‐risk diagnoses, such as cancer and inflammatory bowel disease, as secondary diagnoses. A larger, more inclusive study should be conducted to validate our model in other populations. Finally, we cannot know who died of undiagnosed VTE, either in the hospital or after discharge. Such an outcome would be important, but those events are likely to be rare, and VTE prophylaxis has not been shown to affect mortality.
VTE remains a daunting problem in hospitalized medical patients. Although VTE is responsible for a large number of hospital deaths each year, identifying patients at high risk for clinically important VTE is challenging, and may contribute to the persistently low rates of VTE prophylaxis seen in hospitals.25 Current efforts to treat nearly all patients are likely to lead to unnecessary cost, discomfort, and side effects. We present a simple logistic regression model that can easily identify patients at moderate‐to‐high risk (>1%) of developing symptomatic VTE. Future studies should focus on prospectively validating the model in a wider spectrum of medical illness, and better defining the appropriate risk cutoff for general prophylaxis.
Acknowledgements
The authors thank Aruna Priya, MS, for her help with some of the statistical analyses.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133(6 suppl):381S–453S.
- ,,.Thrombosis prophylaxis in hospitalised medical patients: does prophylaxis in all patients make sense?Neth J Med.2000;56(5):171–176.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- The Joint Commission on the Accreditation of Healthcare Organizations. Venous thromboembolism (VTE) core measure set. Available at: http://www. jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm. Accessed June 1,2009.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 suppl 5):12–19.
- ,,, et al.Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients.Thromb Haemost.2005;94(4):750–759.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(3 suppl):338S–400S.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36(1):8–27.
- ,,,,,.Identification of in‐hospital complications from claims data. Is it valid?Med Care.2000;38(8):785–795.
- ,,, et al.Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study.Arch Intern Med.2004;164(9):963–968.
- ,,.The magnitude of an iatrogenic disorder: a systematic review of the incidence of venous thromboembolism for general medical inpatients.Thromb Haemost.2006;95(5):758–762.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum.National Voluntary Consensus Standards for Prevention and Care of Venous Thromboembolism: Policy, Preferred Practices, and Initial Performance Measures.Washington, DC;2006.
- ,,, et al.Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case‐control multicenter study.J Am Geriatr Soc.2004;52(8):1299–1304.
- ,,.Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score.J Thromb Haemost.2004;2(12):2156–2161.
- ,,,,,.Venous thromboembolism prophylaxis and risk assessment in medical patients.Semin Thromb Hemost.1991;17(suppl 3):313–318.
- ,,, et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151(5):933–938.
- Thromboembolic Risk Factors (THRIFT) Consensus Group.Risk of and prophylaxis for venous thromboembolism in hospital patients.BMJ.1992;305(6853):567–574.
- ,.Preventing Hospital‐Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement. AHRQ Publication No. 08–0075.Rockville, MD:Agency for Healthcare Research and Quality;2008.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
- ,,.Prophylaxis against venous thromboembolism.BMJ.1992;305(6862):1156.
- ,.Prevention of in‐hospital VTE: why can't we do better?Lancet.2008;371(9610):361–362.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133(6 suppl):381S–453S.
- ,,.Thrombosis prophylaxis in hospitalised medical patients: does prophylaxis in all patients make sense?Neth J Med.2000;56(5):171–176.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- The Joint Commission on the Accreditation of Healthcare Organizations. Venous thromboembolism (VTE) core measure set. Available at: http://www. jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm. Accessed June 1,2009.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 suppl 5):12–19.
- ,,, et al.Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients.Thromb Haemost.2005;94(4):750–759.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(3 suppl):338S–400S.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36(1):8–27.
- ,,,,,.Identification of in‐hospital complications from claims data. Is it valid?Med Care.2000;38(8):785–795.
- ,,, et al.Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study.Arch Intern Med.2004;164(9):963–968.
- ,,.The magnitude of an iatrogenic disorder: a systematic review of the incidence of venous thromboembolism for general medical inpatients.Thromb Haemost.2006;95(5):758–762.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum.National Voluntary Consensus Standards for Prevention and Care of Venous Thromboembolism: Policy, Preferred Practices, and Initial Performance Measures.Washington, DC;2006.
- ,,, et al.Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case‐control multicenter study.J Am Geriatr Soc.2004;52(8):1299–1304.
- ,,.Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score.J Thromb Haemost.2004;2(12):2156–2161.
- ,,,,,.Venous thromboembolism prophylaxis and risk assessment in medical patients.Semin Thromb Hemost.1991;17(suppl 3):313–318.
- ,,, et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151(5):933–938.
- Thromboembolic Risk Factors (THRIFT) Consensus Group.Risk of and prophylaxis for venous thromboembolism in hospital patients.BMJ.1992;305(6853):567–574.
- ,.Preventing Hospital‐Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement. AHRQ Publication No. 08–0075.Rockville, MD:Agency for Healthcare Research and Quality;2008.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
- ,,.Prophylaxis against venous thromboembolism.BMJ.1992;305(6862):1156.
- ,.Prevention of in‐hospital VTE: why can't we do better?Lancet.2008;371(9610):361–362.
Copyright © 2011 Society of Hospital Medicine
The Wells Rule and VTE Prophylaxis
Symptoms, signs, chest radiograms, electrocardiograms and laboratory data have a low specificity for the diagnosis of pulmonary embolism (PE) when used in isolation, but when used in combination they can accurately identify patients with an increased likelihood of having a PE.17 The Wells score combines multiple variables into a prediction tool (Table 1). The original model identified three categories of patients with increasing likelihoods of having a PE,6 but a simpler, dichotomous version was subsequently proposed.7 A sequential diagnostic strategy combining the dichotomous Wells rule with a serum d‐dimer test has been validated against contrast‐enhanced spiral computed tomography (CTPE) on cohorts comprised largely of ambulatory outpatient and emergency room patients.815 This method, however, has never been tested in hospitalized patients who were receiving heparin in doses designed to prevent the development of venous thromboembolism (VTE). The purpose of this study was to evaluate the utility of the modified Wells score to predict the presence or absence of PE in hospitalized patients who were receiving prophylactic heparin.
| |
| Symptoms and signs of deep‐vein thrombosis | 3.0 |
| Heart rate >100 beats per minute | 1.5 |
| Recent immobilization or surgery (<4 weeks) | 1.5 |
| Previous VTE | 1.5 |
| Hemoptysis | 1.0 |
| Active cancer | 1.0 |
| PE more likely than alternate diagnosis | 3.0 |
Methods
We screened consecutive patients who underwent CTPE studies from January 2006 through December 2007 at Denver Health, a university‐affiliated public hospital. Inclusion criteria were patients between 18 and 89 years of age who underwent CTPE imaging 2 or more days after being hospitalized, and had been receiving fractionated or unfractionated heparin in doses appropriate for preventing the development of deep venous thrombosis from the time of admission. Patients were excluded if they had signs or symptoms that were consistent with a diagnosis of PE at the time of admission, if they had a contraindication to prophylactic anticoagulation or if their prophylactic heparin therapy had been interrupted for any reason from the time prior to when the CTPE was ordered.
Patients were grouped depending on the service or location of their admission (ie, Medicine, Surgery, Orthopedics, Medical or Surgical Intensive Care Units). The objective elements of the Wells score were obtained by reviewing each patient's history and physical examination, progress notes and discharge summary. Patients were considered to have an alternate diagnosis of equal or greater likelihood than a PE if a d‐dimer was ordered, or if such a possibility was suggested by the treating clinician in the computerized order for the CTPE. The modified Wells score was used to classify patients into PE‐likely (total score 4) or PE‐unlikely (total score <4).7 Fisher's exact test was used to analyze the 2 2 table. P< 0.05 was taken to represent significance.
The Colorado Multiple Institutional Review Board approved this study with a waiver of informed consent.
Results
Of 446 patients who had CTPEs during the study period 286 (64%) met the inclusion criteria (Figure 1). Those who were excluded included 131 who did not receive continuous prophylactic anticoagulation from the time they were admitted to the time of the CT, 18 who had preexisting signs or symptoms and signs consistent with a diagnosis of PE at the time of admission, and 11 who were receiving therapeutic anticoagulation. The patients were hospitalized on different units and on a number of different services (Table 2).
| Total Patients | PE | PE Likely | |
|---|---|---|---|
| |||
| Medicine | 89 | 7 (8%) | 59 (66%) |
| Surgery | 55 | 0 (0%) | 43 (78%) |
| Orthopedics | 57 | 6 (11%) | 43 (75%) |
| MICU | 24 | 3 (13%) | 20 (83%) |
| SICU | 61 | 4 (7%) | 47 (77%) |
| Total | 286 | 20 (7%) | 212 (74%) |
Low molecular weight heparin was given to 165 patients (dalteparin, 5000 units, once daily), unfractionated heparin to 120 patients (104 receiving 5000 units twice daily and 16 receiving 5000 units 3 times a day) and 1 patient was given a Factor Xa inhibitor (fondaparinux 2.5 mg once daily) due to a history of heparin induced thrombocytopenia.
Hypoxia and tachycardia were the most common reasons for requesting a CTPE in instances in which an indication for CT imaging was documented. In almost 28% of patients, however, the reason for suspecting PE was not apparent on chart review (Table 3).
| Patients (%) | |
|---|---|
| |
| Hypoxia | 118 (41) |
| Hypoxia + tachycardia | 45 (16) |
| Tachycardia | 32 (11) |
| Chest pain | 10 (3) |
| Hemoptysis | 1 (0.3) |
| Not specified | 80 (28) |
| Total | 286 (100) |
The prevalence of PE was 20/286 (7.0%, 95% CI (confidence interval): 4.0‐10.0). On the basis of the Wells score 212 patients (74%) were classified as PE‐likely and 74 (26%) as PE‐unlikely. Immobility or recent surgery, tachycardia and the absence of a more plausible diagnosis were the most common contributors to the final score (Table 4).
| n (%) | |
|---|---|
| |
| Symptoms and signs of deep‐vein thrombosis | 12 (6) |
| Heart rate >100 beats per minute | 119 (60) |
| Recent immobilization or surgery (<4 weeks) | 179 (90) |
| Previous VTE | 10 (5) |
| Hemoptysis | 1 (<1) |
| Active cancer | 18 (9) |
| PE more likely than alternate diagnosis | 131 (66) |
Nineteen of the 20 patients (95%) who had PE diagnosed on the basis of a positive CTPE were risk‐stratified on the basis of the Wells score into the PE‐likely category, and 1 (5%) was classified as PE‐unlikely. Of the 266 patients whose CTPEs were negative 193 (73%) were classified as PE‐likely and 73 (27%) as PE‐unlikely (P < 0.03). Accordingly, the modified Wells score was 95% sensitive for having a diagnosis of PE confirmed on CTPE, the specificity was only 27%, the positive predictive value was only 9% and the negative predictive value was 99%(Table 5) with negative likelihood ratio of 0.19.
| Wells Rule | CTPE | Total | |
|---|---|---|---|
| Positive | Negative | ||
| |||
| PE likely | 19 | 193 | 272 |
| PE unlikely | 1 | 73 | 74 |
| Total | 20 | 266 | 286 |
| Sensitivity | 0.95 | ||
| Specificity | 0.27 | ||
| Positive predictive value | 0.09 | ||
| Negative predictive value | 0.99 | ||
| Positive likelihood ratio | 1.31 | ||
| Negative likelihood ratio | 0.18 | ||
| Two‐sided P value | 0.03 | ||
A d‐dimer was ordered for 70 of the 74 patients (95%) who were classified as PE‐unlikely. In 67 of these (96%) the test was positive, and in all but 1 the result was falsely positive. D‐dimer testing was also obtained in 8 of 212 (4%) of patients classified as PE‐likely and was positive in all 8.
Discussion
This retrospective cohort study demonstrated that in hospitalized patients who were receiving prophylactic doses of fractionated or unfractionated heparin and underwent CTPE studies for the clinical suspicion of PE, the prevalence of PE was very low, the modified Wells rule classified 26% of the patients as PE‐unlikely, and the PE‐unlikely category was associated with an extremely high negative predictive value and low negative likelihood ratio for PE. We also confirmed that the prevalence of a positive d‐dimer was so high in this population that the test did not add to the ability to risk‐stratify patients for the likelihood of having a PE. These findings lead to the conclusion that CTPE studies were performed excessively in this cohort of patients.
Previous studies validating the Wells score enrolled combinations of inpatients and outpatients813 or outpatients exclusively.14, 15 To our knowledge the present study is the first to validate the utility of the scoring system in inpatients receiving prophylactic anticoagulation. As would be expected, the prevalence of PE in our population was lower than the 9% to 30% that has previously been reported in patients not receiving prophylactic anticoagulation,815 consistent with the 68% to 76% reduction in the risk of deep venous thrombosis that occurs with use of low‐dose heparin or low molecular weight heparin.16
Similar to the findings of Arnason et al.17 a large proportion of this inpatient cohort was classified as PE‐likely on the basis of only 3 of the 7 variablestachycardia, immobility or previous surgery, and the absence of a more likely competing diagnosis.
The d‐dimer was elevated above the upper limit of normal in nearly all the cases in which it was tested (96%). Bounameaux et al.18 first suggested that conditions other than VTE could increase the plasma d‐dimer level. D‐dimer levels above the cutoff that excludes thrombosis have been documented in absence of thrombosis in the elderly and in patients with numerous other conditions including infections, cancer, coronary, cerebral and peripheral arterial vascular disease, heart failure, rheumatologic diseases, surgery, trauma burns, and pregnancy.1821 Van Beek et al.22 and Miron et al.23 demonstrated that d‐dimer testing was not useful in hospitalized patients. Kabrhel et al.24 reported similar results in an Emergency Department cohort and concluded that d‐dimer testing increased the percent of patients who were investigated for PE and the percent that were sent for pulmonary vascular imaging without increasing the percent of patients diagnosed as having a PE. In our cohort, 74 patients (26%) were classified as PE‐unlikely, and we theorize that 67 (90%) of these underwent CTPE studies solely on the basis of having a positive d‐dimer. All but one of the CTPEs in the patients with positive d‐dimers were negative for PE confirming the that the low specificity of d‐dimer testing in hospitalized patients also applies to those receiving prophylactic anticoagulation.
The Wells rule was associated with a high negative predictive value (99%) and a corresponding low negative likelihood ratio of 0.19, with both of these parameters likely being strongly influenced by the low prevalence of PE in this cohort.
In most longitudinal controlled studies of heparin‐based prophylaxis the incidence of VTE in all medical and most surgical patients approximates 5%.25,26 If this were taken to represent the pre‐test probability of VTE in patients on prophylaxis in whom the question of PE arises, then according to Bayesian theory, a PE‐unlikely classification with a negative likelihood ratio of 0.19 would result in a post‐test probability of less than 1%. This is well below the threshold at which diagnostic imaging delivers no benefit and in fact, may cause harm. Accordingly, PE can be safely excluded in those who are risk‐stratified to PE‐unlikely, with or without an accompanying negative d‐dimer. The average charge for a CTPE at our institution is $1800 and the 2009 cost/charge ratio was 54%. Accordingly, the cost savings to our hospital if CTPEs were not done on the 74 patients classified as PE‐unlikely would exceed $66,000/year.
Our study has a number of potential limitations. Because the data came from a single university‐affiliated public hospital the results might not generalize to other hospitals (teaching or nonteaching). Despite finding a very low prevalence of PE in patients receiving prophylactic heparin, the true prevalence of PE might have been overestimated since our sample size was small and Denver Health is a regional level I trauma center and has a busy joint arthroplasty service, i.e., services known to have an increased prevalence of venous thrombosis.16 If the prevalence of PE were indeed lower than what we observed, however, it would decrease the number of true positive and false negative CTPEs which would, in turn, further strengthen the conclusion that CTPEs are being overused in hospitalized patients receiving prophylactic heparin who are risk‐stratified to a PE‐unlikely category. Similarly, because our sample size was small we may have underestimated the prevalence of PE. Our narrow CIs, and the fact that the prevalence we observed is consistent with the effect of prophylaxic heparin on the incidence of VTE suggest that, if an error were made, it would not be large enough to alter our conclusions.
Our analysis did not include patients in whom PE was excluded without performing CTPE testing. If these patients had CTPEs the large majority would be negative because of a very low pretest probability and risk‐stratification would have placed them in a PE‐unlikely category (ie, true negatives), thereby also increasing the negative predictive value of the Wells score used in this setting.
We calculated the Wells score retrospectively as was previously done in studies by Chagnon et al.,11 Righini et al.,14 and Ranji et al.27 (although the methods used in these studies were not described in detail). We assumed that whenever a d‐dimer test was ordered the treating physician thought that PE was less likely than an alternate diagnosis reasoning that, if they thought PE were the most likely diagnosis, d‐dimers should not have been obtained as, in this circumstance, they are not recommended as part of the diagnostic algorithm.8 Conversely, we assumed that for patients who did not get d‐dimer testing, the treating physician thought that PE was the most likely diagnosis. Alternatively, the physicians might not have ordered a d‐dimer because they recognized that the test is of limited clinical utility in hospitalized patients. In this latter circumstance, the number of PE‐likely patients would be overestimated and the number of PE‐unlikely would be underestimated, reducing the strength of our conclusions or potentially invalidating them. Since the accuracy of prediction rules mirrors that of implicit clinical judgment, however, we suggest that, for most of the patients who had CTPEs performed without d‐dimers, the ordering physician had a high suspicion of PE28, 29 and that the large majority of PE‐likely patients were correctly classified.
In summary, we found that CTPE testing is frequently performed in hospitalized patients receiving prophylactic heparin despite there being a very low prevalence of PE in this cohort, and that risk‐stratifying patients into the PE‐unlikely category using the modified Wells score accurately excludes the diagnosis of PE. The problem of overuse of CTPEs is compounded by the well‐recognized misuse of d‐dimer testing in hospitalized patients. On the basis of our findings we recommend that, when hospitalized patients who are receiving heparin prophylaxis to prevent VTE develop signs or symptoms suggestive of PE they should be risk‐stratified using the modified Wells criteria. In those classified as PE‐unlikely PE can be safely excluded without further testing. Using this approach 26% of CTPEs done on the cohort of hospitalized patients we studied, and all d‐dimers could have been avoided. If the results of our study are duplicated in other centers these recommendations should be included in future guidelines summarizing the most cost‐effective ways to evaluate patients for possible PE.
Acknowledgements
Ms. Angela Keniston assisted in this study by identifying the initial population by using the hospital's computerized data warehouse.
- ,Accuracy of the clinical diagnosis of pulmonary embolism.JAMA.1967;202(7):115–118.
- ,,, et al.Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no preexisting cardiac or pulmonary disease.Chest.1991;100(3):598–603.
- ,,,Chest Radiographs in acute pulmonary embolism. Results from the International Cooperative Pulmonary Embolism Registry.Chest.2000;118(1):33–38.
- ,,Alveolar‐arterial gradient in the assessment of acute pulmonary embolism.Chest.1995;107(1):139–143.
- ,,, et al.Diagnostic value of the electrocardiogram in suspected pulmonary embolism.Am J Cardiol.2000;86(7):807–809.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129(12):997–1005.
- ,,, et al.Derivation of a simple clinical model to categorize patient's probability of pulmonary embolism: increasing the models utility with the simpliRed D‐dimer.Thromb Haemost.2000;83(3):416–420.
- ,,, et al.Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, d‐dimer testing and computer tomography.JAMA.2006;295(2):172–179.
- ,,,, et al.Comparison of the revised Geneva score with the Wells rule for assessing clinical probability of pulmonary embolism.J Thromb Haemost.2008;6(1):40–44.
- ,,, et al.Further validation and simplification of the Wells clinical decision rule in pulmonary embolism.J Thromb Haemost.2008;99(1):229–234.
- ,,, et al.Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism.Am J Med.2002;113(4):269–275.
- ,,,A prospective reassessment of the utility of the Wells score in identifying pulmonary embolism.Med J Aust.2007;187(6):333–336.
- ,,,et al.Performance of the Wells and Revised Geneva scores for predicting pulmonary embolism.Eur J Emerg Med.2009;16(1):49–52.
- ,,,Clinical probability assessment of pulmonary embolism by the Wells' score: is the easiest the best?J Thromb Haemost.2006;4(3):702–704
- ,,, et al.Simple and safe exclusion of pulmonary embolism in outpatients using quantitative D‐dimer and Wells' simplified decision rule.J Thromb Haemost.2007;97:146–150.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119:132S–175S.
- ,,Appropriateness of diagnostic strategies for evaluating suspected pulmonary embolism.J Thromb Haemost.2007;97(2):195–201.
- ,,, et al.Measurement of plasma D‐dimer for diagnosis of deep venous thrombosis.Am J Clin Path.1989;91(1):82–85.
- ,,,Plasma D‐dimer levels in elderly patients with suspected pulmonary embolism.Thromb Res.2000;98(6):577–579.
- ,,, et al.D‐dimer plasma concentration in various clinical conditions: implication for the use of this test in the diagnostic approach of venous thromboembolism.Thromb Res.1993;69(1):125–130.
- ,,,D‐dimer for venous thromboembolism diagnosis: 20 years later.J Thromb Haemost.2008;6(7):1059–1071.
- ,,, et al.The role of plasma D‐dimer concentration in the exclusion of pulmonary embolism.Brit J Haematol.1996;92(3):725–732.
- ,,, et al.Contribution of non‐invasive evaluation to the diagnosis of pulmonary embolism in hospitalized patients.Eur Respir J.1999;13(6):1365–1370.
- ,,A highly sensitive ELISA D‐dimer increases testing but not diagnosis of pulmonary embolism.Acad Emerg Med2006;13:519–524.
- ,,A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,The incidence of symptomatic venous thromboembolism after enoxaparin prophylaxis in lower extremity arthroplasty: a cohort study of 1,984 patients.Canadian Collaborative Group.1998;114:115S–118S.
- ,,,,Impact of reliance on CT pulmonary angiography on diagnosis of pulmonary embolism: a Bayesian analysis.J Hosp Med.2006;1:81–87.
- The PIOPED investigators: Value of the ventilation‐perfusion scan in acute pulmonary embolism.JAMA.1990;263:2753–2759.
- ,,, et al.Non‐invasive diagnosis of venous thromboembolism in outpatients.Lancet.353:190–195.
Symptoms, signs, chest radiograms, electrocardiograms and laboratory data have a low specificity for the diagnosis of pulmonary embolism (PE) when used in isolation, but when used in combination they can accurately identify patients with an increased likelihood of having a PE.17 The Wells score combines multiple variables into a prediction tool (Table 1). The original model identified three categories of patients with increasing likelihoods of having a PE,6 but a simpler, dichotomous version was subsequently proposed.7 A sequential diagnostic strategy combining the dichotomous Wells rule with a serum d‐dimer test has been validated against contrast‐enhanced spiral computed tomography (CTPE) on cohorts comprised largely of ambulatory outpatient and emergency room patients.815 This method, however, has never been tested in hospitalized patients who were receiving heparin in doses designed to prevent the development of venous thromboembolism (VTE). The purpose of this study was to evaluate the utility of the modified Wells score to predict the presence or absence of PE in hospitalized patients who were receiving prophylactic heparin.
| |
| Symptoms and signs of deep‐vein thrombosis | 3.0 |
| Heart rate >100 beats per minute | 1.5 |
| Recent immobilization or surgery (<4 weeks) | 1.5 |
| Previous VTE | 1.5 |
| Hemoptysis | 1.0 |
| Active cancer | 1.0 |
| PE more likely than alternate diagnosis | 3.0 |
Methods
We screened consecutive patients who underwent CTPE studies from January 2006 through December 2007 at Denver Health, a university‐affiliated public hospital. Inclusion criteria were patients between 18 and 89 years of age who underwent CTPE imaging 2 or more days after being hospitalized, and had been receiving fractionated or unfractionated heparin in doses appropriate for preventing the development of deep venous thrombosis from the time of admission. Patients were excluded if they had signs or symptoms that were consistent with a diagnosis of PE at the time of admission, if they had a contraindication to prophylactic anticoagulation or if their prophylactic heparin therapy had been interrupted for any reason from the time prior to when the CTPE was ordered.
Patients were grouped depending on the service or location of their admission (ie, Medicine, Surgery, Orthopedics, Medical or Surgical Intensive Care Units). The objective elements of the Wells score were obtained by reviewing each patient's history and physical examination, progress notes and discharge summary. Patients were considered to have an alternate diagnosis of equal or greater likelihood than a PE if a d‐dimer was ordered, or if such a possibility was suggested by the treating clinician in the computerized order for the CTPE. The modified Wells score was used to classify patients into PE‐likely (total score 4) or PE‐unlikely (total score <4).7 Fisher's exact test was used to analyze the 2 2 table. P< 0.05 was taken to represent significance.
The Colorado Multiple Institutional Review Board approved this study with a waiver of informed consent.
Results
Of 446 patients who had CTPEs during the study period 286 (64%) met the inclusion criteria (Figure 1). Those who were excluded included 131 who did not receive continuous prophylactic anticoagulation from the time they were admitted to the time of the CT, 18 who had preexisting signs or symptoms and signs consistent with a diagnosis of PE at the time of admission, and 11 who were receiving therapeutic anticoagulation. The patients were hospitalized on different units and on a number of different services (Table 2).

| Total Patients | PE | PE Likely | |
|---|---|---|---|
| |||
| Medicine | 89 | 7 (8%) | 59 (66%) |
| Surgery | 55 | 0 (0%) | 43 (78%) |
| Orthopedics | 57 | 6 (11%) | 43 (75%) |
| MICU | 24 | 3 (13%) | 20 (83%) |
| SICU | 61 | 4 (7%) | 47 (77%) |
| Total | 286 | 20 (7%) | 212 (74%) |
Low molecular weight heparin was given to 165 patients (dalteparin, 5000 units, once daily), unfractionated heparin to 120 patients (104 receiving 5000 units twice daily and 16 receiving 5000 units 3 times a day) and 1 patient was given a Factor Xa inhibitor (fondaparinux 2.5 mg once daily) due to a history of heparin induced thrombocytopenia.
Hypoxia and tachycardia were the most common reasons for requesting a CTPE in instances in which an indication for CT imaging was documented. In almost 28% of patients, however, the reason for suspecting PE was not apparent on chart review (Table 3).
| Patients (%) | |
|---|---|
| |
| Hypoxia | 118 (41) |
| Hypoxia + tachycardia | 45 (16) |
| Tachycardia | 32 (11) |
| Chest pain | 10 (3) |
| Hemoptysis | 1 (0.3) |
| Not specified | 80 (28) |
| Total | 286 (100) |
The prevalence of PE was 20/286 (7.0%, 95% CI (confidence interval): 4.0‐10.0). On the basis of the Wells score 212 patients (74%) were classified as PE‐likely and 74 (26%) as PE‐unlikely. Immobility or recent surgery, tachycardia and the absence of a more plausible diagnosis were the most common contributors to the final score (Table 4).
| n (%) | |
|---|---|
| |
| Symptoms and signs of deep‐vein thrombosis | 12 (6) |
| Heart rate >100 beats per minute | 119 (60) |
| Recent immobilization or surgery (<4 weeks) | 179 (90) |
| Previous VTE | 10 (5) |
| Hemoptysis | 1 (<1) |
| Active cancer | 18 (9) |
| PE more likely than alternate diagnosis | 131 (66) |
Nineteen of the 20 patients (95%) who had PE diagnosed on the basis of a positive CTPE were risk‐stratified on the basis of the Wells score into the PE‐likely category, and 1 (5%) was classified as PE‐unlikely. Of the 266 patients whose CTPEs were negative 193 (73%) were classified as PE‐likely and 73 (27%) as PE‐unlikely (P < 0.03). Accordingly, the modified Wells score was 95% sensitive for having a diagnosis of PE confirmed on CTPE, the specificity was only 27%, the positive predictive value was only 9% and the negative predictive value was 99%(Table 5) with negative likelihood ratio of 0.19.
| Wells Rule | CTPE | Total | |
|---|---|---|---|
| Positive | Negative | ||
| |||
| PE likely | 19 | 193 | 272 |
| PE unlikely | 1 | 73 | 74 |
| Total | 20 | 266 | 286 |
| Sensitivity | 0.95 | ||
| Specificity | 0.27 | ||
| Positive predictive value | 0.09 | ||
| Negative predictive value | 0.99 | ||
| Positive likelihood ratio | 1.31 | ||
| Negative likelihood ratio | 0.18 | ||
| Two‐sided P value | 0.03 | ||
A d‐dimer was ordered for 70 of the 74 patients (95%) who were classified as PE‐unlikely. In 67 of these (96%) the test was positive, and in all but 1 the result was falsely positive. D‐dimer testing was also obtained in 8 of 212 (4%) of patients classified as PE‐likely and was positive in all 8.
Discussion
This retrospective cohort study demonstrated that in hospitalized patients who were receiving prophylactic doses of fractionated or unfractionated heparin and underwent CTPE studies for the clinical suspicion of PE, the prevalence of PE was very low, the modified Wells rule classified 26% of the patients as PE‐unlikely, and the PE‐unlikely category was associated with an extremely high negative predictive value and low negative likelihood ratio for PE. We also confirmed that the prevalence of a positive d‐dimer was so high in this population that the test did not add to the ability to risk‐stratify patients for the likelihood of having a PE. These findings lead to the conclusion that CTPE studies were performed excessively in this cohort of patients.
Previous studies validating the Wells score enrolled combinations of inpatients and outpatients813 or outpatients exclusively.14, 15 To our knowledge the present study is the first to validate the utility of the scoring system in inpatients receiving prophylactic anticoagulation. As would be expected, the prevalence of PE in our population was lower than the 9% to 30% that has previously been reported in patients not receiving prophylactic anticoagulation,815 consistent with the 68% to 76% reduction in the risk of deep venous thrombosis that occurs with use of low‐dose heparin or low molecular weight heparin.16
Similar to the findings of Arnason et al.17 a large proportion of this inpatient cohort was classified as PE‐likely on the basis of only 3 of the 7 variablestachycardia, immobility or previous surgery, and the absence of a more likely competing diagnosis.
The d‐dimer was elevated above the upper limit of normal in nearly all the cases in which it was tested (96%). Bounameaux et al.18 first suggested that conditions other than VTE could increase the plasma d‐dimer level. D‐dimer levels above the cutoff that excludes thrombosis have been documented in absence of thrombosis in the elderly and in patients with numerous other conditions including infections, cancer, coronary, cerebral and peripheral arterial vascular disease, heart failure, rheumatologic diseases, surgery, trauma burns, and pregnancy.1821 Van Beek et al.22 and Miron et al.23 demonstrated that d‐dimer testing was not useful in hospitalized patients. Kabrhel et al.24 reported similar results in an Emergency Department cohort and concluded that d‐dimer testing increased the percent of patients who were investigated for PE and the percent that were sent for pulmonary vascular imaging without increasing the percent of patients diagnosed as having a PE. In our cohort, 74 patients (26%) were classified as PE‐unlikely, and we theorize that 67 (90%) of these underwent CTPE studies solely on the basis of having a positive d‐dimer. All but one of the CTPEs in the patients with positive d‐dimers were negative for PE confirming the that the low specificity of d‐dimer testing in hospitalized patients also applies to those receiving prophylactic anticoagulation.
The Wells rule was associated with a high negative predictive value (99%) and a corresponding low negative likelihood ratio of 0.19, with both of these parameters likely being strongly influenced by the low prevalence of PE in this cohort.
In most longitudinal controlled studies of heparin‐based prophylaxis the incidence of VTE in all medical and most surgical patients approximates 5%.25,26 If this were taken to represent the pre‐test probability of VTE in patients on prophylaxis in whom the question of PE arises, then according to Bayesian theory, a PE‐unlikely classification with a negative likelihood ratio of 0.19 would result in a post‐test probability of less than 1%. This is well below the threshold at which diagnostic imaging delivers no benefit and in fact, may cause harm. Accordingly, PE can be safely excluded in those who are risk‐stratified to PE‐unlikely, with or without an accompanying negative d‐dimer. The average charge for a CTPE at our institution is $1800 and the 2009 cost/charge ratio was 54%. Accordingly, the cost savings to our hospital if CTPEs were not done on the 74 patients classified as PE‐unlikely would exceed $66,000/year.
Our study has a number of potential limitations. Because the data came from a single university‐affiliated public hospital the results might not generalize to other hospitals (teaching or nonteaching). Despite finding a very low prevalence of PE in patients receiving prophylactic heparin, the true prevalence of PE might have been overestimated since our sample size was small and Denver Health is a regional level I trauma center and has a busy joint arthroplasty service, i.e., services known to have an increased prevalence of venous thrombosis.16 If the prevalence of PE were indeed lower than what we observed, however, it would decrease the number of true positive and false negative CTPEs which would, in turn, further strengthen the conclusion that CTPEs are being overused in hospitalized patients receiving prophylactic heparin who are risk‐stratified to a PE‐unlikely category. Similarly, because our sample size was small we may have underestimated the prevalence of PE. Our narrow CIs, and the fact that the prevalence we observed is consistent with the effect of prophylaxic heparin on the incidence of VTE suggest that, if an error were made, it would not be large enough to alter our conclusions.
Our analysis did not include patients in whom PE was excluded without performing CTPE testing. If these patients had CTPEs the large majority would be negative because of a very low pretest probability and risk‐stratification would have placed them in a PE‐unlikely category (ie, true negatives), thereby also increasing the negative predictive value of the Wells score used in this setting.
We calculated the Wells score retrospectively as was previously done in studies by Chagnon et al.,11 Righini et al.,14 and Ranji et al.27 (although the methods used in these studies were not described in detail). We assumed that whenever a d‐dimer test was ordered the treating physician thought that PE was less likely than an alternate diagnosis reasoning that, if they thought PE were the most likely diagnosis, d‐dimers should not have been obtained as, in this circumstance, they are not recommended as part of the diagnostic algorithm.8 Conversely, we assumed that for patients who did not get d‐dimer testing, the treating physician thought that PE was the most likely diagnosis. Alternatively, the physicians might not have ordered a d‐dimer because they recognized that the test is of limited clinical utility in hospitalized patients. In this latter circumstance, the number of PE‐likely patients would be overestimated and the number of PE‐unlikely would be underestimated, reducing the strength of our conclusions or potentially invalidating them. Since the accuracy of prediction rules mirrors that of implicit clinical judgment, however, we suggest that, for most of the patients who had CTPEs performed without d‐dimers, the ordering physician had a high suspicion of PE28, 29 and that the large majority of PE‐likely patients were correctly classified.
In summary, we found that CTPE testing is frequently performed in hospitalized patients receiving prophylactic heparin despite there being a very low prevalence of PE in this cohort, and that risk‐stratifying patients into the PE‐unlikely category using the modified Wells score accurately excludes the diagnosis of PE. The problem of overuse of CTPEs is compounded by the well‐recognized misuse of d‐dimer testing in hospitalized patients. On the basis of our findings we recommend that, when hospitalized patients who are receiving heparin prophylaxis to prevent VTE develop signs or symptoms suggestive of PE they should be risk‐stratified using the modified Wells criteria. In those classified as PE‐unlikely PE can be safely excluded without further testing. Using this approach 26% of CTPEs done on the cohort of hospitalized patients we studied, and all d‐dimers could have been avoided. If the results of our study are duplicated in other centers these recommendations should be included in future guidelines summarizing the most cost‐effective ways to evaluate patients for possible PE.
Acknowledgements
Ms. Angela Keniston assisted in this study by identifying the initial population by using the hospital's computerized data warehouse.
Symptoms, signs, chest radiograms, electrocardiograms and laboratory data have a low specificity for the diagnosis of pulmonary embolism (PE) when used in isolation, but when used in combination they can accurately identify patients with an increased likelihood of having a PE.17 The Wells score combines multiple variables into a prediction tool (Table 1). The original model identified three categories of patients with increasing likelihoods of having a PE,6 but a simpler, dichotomous version was subsequently proposed.7 A sequential diagnostic strategy combining the dichotomous Wells rule with a serum d‐dimer test has been validated against contrast‐enhanced spiral computed tomography (CTPE) on cohorts comprised largely of ambulatory outpatient and emergency room patients.815 This method, however, has never been tested in hospitalized patients who were receiving heparin in doses designed to prevent the development of venous thromboembolism (VTE). The purpose of this study was to evaluate the utility of the modified Wells score to predict the presence or absence of PE in hospitalized patients who were receiving prophylactic heparin.
| |
| Symptoms and signs of deep‐vein thrombosis | 3.0 |
| Heart rate >100 beats per minute | 1.5 |
| Recent immobilization or surgery (<4 weeks) | 1.5 |
| Previous VTE | 1.5 |
| Hemoptysis | 1.0 |
| Active cancer | 1.0 |
| PE more likely than alternate diagnosis | 3.0 |
Methods
We screened consecutive patients who underwent CTPE studies from January 2006 through December 2007 at Denver Health, a university‐affiliated public hospital. Inclusion criteria were patients between 18 and 89 years of age who underwent CTPE imaging 2 or more days after being hospitalized, and had been receiving fractionated or unfractionated heparin in doses appropriate for preventing the development of deep venous thrombosis from the time of admission. Patients were excluded if they had signs or symptoms that were consistent with a diagnosis of PE at the time of admission, if they had a contraindication to prophylactic anticoagulation or if their prophylactic heparin therapy had been interrupted for any reason from the time prior to when the CTPE was ordered.
Patients were grouped depending on the service or location of their admission (ie, Medicine, Surgery, Orthopedics, Medical or Surgical Intensive Care Units). The objective elements of the Wells score were obtained by reviewing each patient's history and physical examination, progress notes and discharge summary. Patients were considered to have an alternate diagnosis of equal or greater likelihood than a PE if a d‐dimer was ordered, or if such a possibility was suggested by the treating clinician in the computerized order for the CTPE. The modified Wells score was used to classify patients into PE‐likely (total score 4) or PE‐unlikely (total score <4).7 Fisher's exact test was used to analyze the 2 2 table. P< 0.05 was taken to represent significance.
The Colorado Multiple Institutional Review Board approved this study with a waiver of informed consent.
Results
Of 446 patients who had CTPEs during the study period 286 (64%) met the inclusion criteria (Figure 1). Those who were excluded included 131 who did not receive continuous prophylactic anticoagulation from the time they were admitted to the time of the CT, 18 who had preexisting signs or symptoms and signs consistent with a diagnosis of PE at the time of admission, and 11 who were receiving therapeutic anticoagulation. The patients were hospitalized on different units and on a number of different services (Table 2).

| Total Patients | PE | PE Likely | |
|---|---|---|---|
| |||
| Medicine | 89 | 7 (8%) | 59 (66%) |
| Surgery | 55 | 0 (0%) | 43 (78%) |
| Orthopedics | 57 | 6 (11%) | 43 (75%) |
| MICU | 24 | 3 (13%) | 20 (83%) |
| SICU | 61 | 4 (7%) | 47 (77%) |
| Total | 286 | 20 (7%) | 212 (74%) |
Low molecular weight heparin was given to 165 patients (dalteparin, 5000 units, once daily), unfractionated heparin to 120 patients (104 receiving 5000 units twice daily and 16 receiving 5000 units 3 times a day) and 1 patient was given a Factor Xa inhibitor (fondaparinux 2.5 mg once daily) due to a history of heparin induced thrombocytopenia.
Hypoxia and tachycardia were the most common reasons for requesting a CTPE in instances in which an indication for CT imaging was documented. In almost 28% of patients, however, the reason for suspecting PE was not apparent on chart review (Table 3).
| Patients (%) | |
|---|---|
| |
| Hypoxia | 118 (41) |
| Hypoxia + tachycardia | 45 (16) |
| Tachycardia | 32 (11) |
| Chest pain | 10 (3) |
| Hemoptysis | 1 (0.3) |
| Not specified | 80 (28) |
| Total | 286 (100) |
The prevalence of PE was 20/286 (7.0%, 95% CI (confidence interval): 4.0‐10.0). On the basis of the Wells score 212 patients (74%) were classified as PE‐likely and 74 (26%) as PE‐unlikely. Immobility or recent surgery, tachycardia and the absence of a more plausible diagnosis were the most common contributors to the final score (Table 4).
| n (%) | |
|---|---|
| |
| Symptoms and signs of deep‐vein thrombosis | 12 (6) |
| Heart rate >100 beats per minute | 119 (60) |
| Recent immobilization or surgery (<4 weeks) | 179 (90) |
| Previous VTE | 10 (5) |
| Hemoptysis | 1 (<1) |
| Active cancer | 18 (9) |
| PE more likely than alternate diagnosis | 131 (66) |
Nineteen of the 20 patients (95%) who had PE diagnosed on the basis of a positive CTPE were risk‐stratified on the basis of the Wells score into the PE‐likely category, and 1 (5%) was classified as PE‐unlikely. Of the 266 patients whose CTPEs were negative 193 (73%) were classified as PE‐likely and 73 (27%) as PE‐unlikely (P < 0.03). Accordingly, the modified Wells score was 95% sensitive for having a diagnosis of PE confirmed on CTPE, the specificity was only 27%, the positive predictive value was only 9% and the negative predictive value was 99%(Table 5) with negative likelihood ratio of 0.19.
| Wells Rule | CTPE | Total | |
|---|---|---|---|
| Positive | Negative | ||
| |||
| PE likely | 19 | 193 | 272 |
| PE unlikely | 1 | 73 | 74 |
| Total | 20 | 266 | 286 |
| Sensitivity | 0.95 | ||
| Specificity | 0.27 | ||
| Positive predictive value | 0.09 | ||
| Negative predictive value | 0.99 | ||
| Positive likelihood ratio | 1.31 | ||
| Negative likelihood ratio | 0.18 | ||
| Two‐sided P value | 0.03 | ||
A d‐dimer was ordered for 70 of the 74 patients (95%) who were classified as PE‐unlikely. In 67 of these (96%) the test was positive, and in all but 1 the result was falsely positive. D‐dimer testing was also obtained in 8 of 212 (4%) of patients classified as PE‐likely and was positive in all 8.
Discussion
This retrospective cohort study demonstrated that in hospitalized patients who were receiving prophylactic doses of fractionated or unfractionated heparin and underwent CTPE studies for the clinical suspicion of PE, the prevalence of PE was very low, the modified Wells rule classified 26% of the patients as PE‐unlikely, and the PE‐unlikely category was associated with an extremely high negative predictive value and low negative likelihood ratio for PE. We also confirmed that the prevalence of a positive d‐dimer was so high in this population that the test did not add to the ability to risk‐stratify patients for the likelihood of having a PE. These findings lead to the conclusion that CTPE studies were performed excessively in this cohort of patients.
Previous studies validating the Wells score enrolled combinations of inpatients and outpatients813 or outpatients exclusively.14, 15 To our knowledge the present study is the first to validate the utility of the scoring system in inpatients receiving prophylactic anticoagulation. As would be expected, the prevalence of PE in our population was lower than the 9% to 30% that has previously been reported in patients not receiving prophylactic anticoagulation,815 consistent with the 68% to 76% reduction in the risk of deep venous thrombosis that occurs with use of low‐dose heparin or low molecular weight heparin.16
Similar to the findings of Arnason et al.17 a large proportion of this inpatient cohort was classified as PE‐likely on the basis of only 3 of the 7 variablestachycardia, immobility or previous surgery, and the absence of a more likely competing diagnosis.
The d‐dimer was elevated above the upper limit of normal in nearly all the cases in which it was tested (96%). Bounameaux et al.18 first suggested that conditions other than VTE could increase the plasma d‐dimer level. D‐dimer levels above the cutoff that excludes thrombosis have been documented in absence of thrombosis in the elderly and in patients with numerous other conditions including infections, cancer, coronary, cerebral and peripheral arterial vascular disease, heart failure, rheumatologic diseases, surgery, trauma burns, and pregnancy.1821 Van Beek et al.22 and Miron et al.23 demonstrated that d‐dimer testing was not useful in hospitalized patients. Kabrhel et al.24 reported similar results in an Emergency Department cohort and concluded that d‐dimer testing increased the percent of patients who were investigated for PE and the percent that were sent for pulmonary vascular imaging without increasing the percent of patients diagnosed as having a PE. In our cohort, 74 patients (26%) were classified as PE‐unlikely, and we theorize that 67 (90%) of these underwent CTPE studies solely on the basis of having a positive d‐dimer. All but one of the CTPEs in the patients with positive d‐dimers were negative for PE confirming the that the low specificity of d‐dimer testing in hospitalized patients also applies to those receiving prophylactic anticoagulation.
The Wells rule was associated with a high negative predictive value (99%) and a corresponding low negative likelihood ratio of 0.19, with both of these parameters likely being strongly influenced by the low prevalence of PE in this cohort.
In most longitudinal controlled studies of heparin‐based prophylaxis the incidence of VTE in all medical and most surgical patients approximates 5%.25,26 If this were taken to represent the pre‐test probability of VTE in patients on prophylaxis in whom the question of PE arises, then according to Bayesian theory, a PE‐unlikely classification with a negative likelihood ratio of 0.19 would result in a post‐test probability of less than 1%. This is well below the threshold at which diagnostic imaging delivers no benefit and in fact, may cause harm. Accordingly, PE can be safely excluded in those who are risk‐stratified to PE‐unlikely, with or without an accompanying negative d‐dimer. The average charge for a CTPE at our institution is $1800 and the 2009 cost/charge ratio was 54%. Accordingly, the cost savings to our hospital if CTPEs were not done on the 74 patients classified as PE‐unlikely would exceed $66,000/year.
Our study has a number of potential limitations. Because the data came from a single university‐affiliated public hospital the results might not generalize to other hospitals (teaching or nonteaching). Despite finding a very low prevalence of PE in patients receiving prophylactic heparin, the true prevalence of PE might have been overestimated since our sample size was small and Denver Health is a regional level I trauma center and has a busy joint arthroplasty service, i.e., services known to have an increased prevalence of venous thrombosis.16 If the prevalence of PE were indeed lower than what we observed, however, it would decrease the number of true positive and false negative CTPEs which would, in turn, further strengthen the conclusion that CTPEs are being overused in hospitalized patients receiving prophylactic heparin who are risk‐stratified to a PE‐unlikely category. Similarly, because our sample size was small we may have underestimated the prevalence of PE. Our narrow CIs, and the fact that the prevalence we observed is consistent with the effect of prophylaxic heparin on the incidence of VTE suggest that, if an error were made, it would not be large enough to alter our conclusions.
Our analysis did not include patients in whom PE was excluded without performing CTPE testing. If these patients had CTPEs the large majority would be negative because of a very low pretest probability and risk‐stratification would have placed them in a PE‐unlikely category (ie, true negatives), thereby also increasing the negative predictive value of the Wells score used in this setting.
We calculated the Wells score retrospectively as was previously done in studies by Chagnon et al.,11 Righini et al.,14 and Ranji et al.27 (although the methods used in these studies were not described in detail). We assumed that whenever a d‐dimer test was ordered the treating physician thought that PE was less likely than an alternate diagnosis reasoning that, if they thought PE were the most likely diagnosis, d‐dimers should not have been obtained as, in this circumstance, they are not recommended as part of the diagnostic algorithm.8 Conversely, we assumed that for patients who did not get d‐dimer testing, the treating physician thought that PE was the most likely diagnosis. Alternatively, the physicians might not have ordered a d‐dimer because they recognized that the test is of limited clinical utility in hospitalized patients. In this latter circumstance, the number of PE‐likely patients would be overestimated and the number of PE‐unlikely would be underestimated, reducing the strength of our conclusions or potentially invalidating them. Since the accuracy of prediction rules mirrors that of implicit clinical judgment, however, we suggest that, for most of the patients who had CTPEs performed without d‐dimers, the ordering physician had a high suspicion of PE28, 29 and that the large majority of PE‐likely patients were correctly classified.
In summary, we found that CTPE testing is frequently performed in hospitalized patients receiving prophylactic heparin despite there being a very low prevalence of PE in this cohort, and that risk‐stratifying patients into the PE‐unlikely category using the modified Wells score accurately excludes the diagnosis of PE. The problem of overuse of CTPEs is compounded by the well‐recognized misuse of d‐dimer testing in hospitalized patients. On the basis of our findings we recommend that, when hospitalized patients who are receiving heparin prophylaxis to prevent VTE develop signs or symptoms suggestive of PE they should be risk‐stratified using the modified Wells criteria. In those classified as PE‐unlikely PE can be safely excluded without further testing. Using this approach 26% of CTPEs done on the cohort of hospitalized patients we studied, and all d‐dimers could have been avoided. If the results of our study are duplicated in other centers these recommendations should be included in future guidelines summarizing the most cost‐effective ways to evaluate patients for possible PE.
Acknowledgements
Ms. Angela Keniston assisted in this study by identifying the initial population by using the hospital's computerized data warehouse.
- ,Accuracy of the clinical diagnosis of pulmonary embolism.JAMA.1967;202(7):115–118.
- ,,, et al.Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no preexisting cardiac or pulmonary disease.Chest.1991;100(3):598–603.
- ,,,Chest Radiographs in acute pulmonary embolism. Results from the International Cooperative Pulmonary Embolism Registry.Chest.2000;118(1):33–38.
- ,,Alveolar‐arterial gradient in the assessment of acute pulmonary embolism.Chest.1995;107(1):139–143.
- ,,, et al.Diagnostic value of the electrocardiogram in suspected pulmonary embolism.Am J Cardiol.2000;86(7):807–809.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129(12):997–1005.
- ,,, et al.Derivation of a simple clinical model to categorize patient's probability of pulmonary embolism: increasing the models utility with the simpliRed D‐dimer.Thromb Haemost.2000;83(3):416–420.
- ,,, et al.Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, d‐dimer testing and computer tomography.JAMA.2006;295(2):172–179.
- ,,,, et al.Comparison of the revised Geneva score with the Wells rule for assessing clinical probability of pulmonary embolism.J Thromb Haemost.2008;6(1):40–44.
- ,,, et al.Further validation and simplification of the Wells clinical decision rule in pulmonary embolism.J Thromb Haemost.2008;99(1):229–234.
- ,,, et al.Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism.Am J Med.2002;113(4):269–275.
- ,,,A prospective reassessment of the utility of the Wells score in identifying pulmonary embolism.Med J Aust.2007;187(6):333–336.
- ,,,et al.Performance of the Wells and Revised Geneva scores for predicting pulmonary embolism.Eur J Emerg Med.2009;16(1):49–52.
- ,,,Clinical probability assessment of pulmonary embolism by the Wells' score: is the easiest the best?J Thromb Haemost.2006;4(3):702–704
- ,,, et al.Simple and safe exclusion of pulmonary embolism in outpatients using quantitative D‐dimer and Wells' simplified decision rule.J Thromb Haemost.2007;97:146–150.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119:132S–175S.
- ,,Appropriateness of diagnostic strategies for evaluating suspected pulmonary embolism.J Thromb Haemost.2007;97(2):195–201.
- ,,, et al.Measurement of plasma D‐dimer for diagnosis of deep venous thrombosis.Am J Clin Path.1989;91(1):82–85.
- ,,,Plasma D‐dimer levels in elderly patients with suspected pulmonary embolism.Thromb Res.2000;98(6):577–579.
- ,,, et al.D‐dimer plasma concentration in various clinical conditions: implication for the use of this test in the diagnostic approach of venous thromboembolism.Thromb Res.1993;69(1):125–130.
- ,,,D‐dimer for venous thromboembolism diagnosis: 20 years later.J Thromb Haemost.2008;6(7):1059–1071.
- ,,, et al.The role of plasma D‐dimer concentration in the exclusion of pulmonary embolism.Brit J Haematol.1996;92(3):725–732.
- ,,, et al.Contribution of non‐invasive evaluation to the diagnosis of pulmonary embolism in hospitalized patients.Eur Respir J.1999;13(6):1365–1370.
- ,,A highly sensitive ELISA D‐dimer increases testing but not diagnosis of pulmonary embolism.Acad Emerg Med2006;13:519–524.
- ,,A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,The incidence of symptomatic venous thromboembolism after enoxaparin prophylaxis in lower extremity arthroplasty: a cohort study of 1,984 patients.Canadian Collaborative Group.1998;114:115S–118S.
- ,,,,Impact of reliance on CT pulmonary angiography on diagnosis of pulmonary embolism: a Bayesian analysis.J Hosp Med.2006;1:81–87.
- The PIOPED investigators: Value of the ventilation‐perfusion scan in acute pulmonary embolism.JAMA.1990;263:2753–2759.
- ,,, et al.Non‐invasive diagnosis of venous thromboembolism in outpatients.Lancet.353:190–195.
- ,Accuracy of the clinical diagnosis of pulmonary embolism.JAMA.1967;202(7):115–118.
- ,,, et al.Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no preexisting cardiac or pulmonary disease.Chest.1991;100(3):598–603.
- ,,,Chest Radiographs in acute pulmonary embolism. Results from the International Cooperative Pulmonary Embolism Registry.Chest.2000;118(1):33–38.
- ,,Alveolar‐arterial gradient in the assessment of acute pulmonary embolism.Chest.1995;107(1):139–143.
- ,,, et al.Diagnostic value of the electrocardiogram in suspected pulmonary embolism.Am J Cardiol.2000;86(7):807–809.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129(12):997–1005.
- ,,, et al.Derivation of a simple clinical model to categorize patient's probability of pulmonary embolism: increasing the models utility with the simpliRed D‐dimer.Thromb Haemost.2000;83(3):416–420.
- ,,, et al.Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, d‐dimer testing and computer tomography.JAMA.2006;295(2):172–179.
- ,,,, et al.Comparison of the revised Geneva score with the Wells rule for assessing clinical probability of pulmonary embolism.J Thromb Haemost.2008;6(1):40–44.
- ,,, et al.Further validation and simplification of the Wells clinical decision rule in pulmonary embolism.J Thromb Haemost.2008;99(1):229–234.
- ,,, et al.Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism.Am J Med.2002;113(4):269–275.
- ,,,A prospective reassessment of the utility of the Wells score in identifying pulmonary embolism.Med J Aust.2007;187(6):333–336.
- ,,,et al.Performance of the Wells and Revised Geneva scores for predicting pulmonary embolism.Eur J Emerg Med.2009;16(1):49–52.
- ,,,Clinical probability assessment of pulmonary embolism by the Wells' score: is the easiest the best?J Thromb Haemost.2006;4(3):702–704
- ,,, et al.Simple and safe exclusion of pulmonary embolism in outpatients using quantitative D‐dimer and Wells' simplified decision rule.J Thromb Haemost.2007;97:146–150.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119:132S–175S.
- ,,Appropriateness of diagnostic strategies for evaluating suspected pulmonary embolism.J Thromb Haemost.2007;97(2):195–201.
- ,,, et al.Measurement of plasma D‐dimer for diagnosis of deep venous thrombosis.Am J Clin Path.1989;91(1):82–85.
- ,,,Plasma D‐dimer levels in elderly patients with suspected pulmonary embolism.Thromb Res.2000;98(6):577–579.
- ,,, et al.D‐dimer plasma concentration in various clinical conditions: implication for the use of this test in the diagnostic approach of venous thromboembolism.Thromb Res.1993;69(1):125–130.
- ,,,D‐dimer for venous thromboembolism diagnosis: 20 years later.J Thromb Haemost.2008;6(7):1059–1071.
- ,,, et al.The role of plasma D‐dimer concentration in the exclusion of pulmonary embolism.Brit J Haematol.1996;92(3):725–732.
- ,,, et al.Contribution of non‐invasive evaluation to the diagnosis of pulmonary embolism in hospitalized patients.Eur Respir J.1999;13(6):1365–1370.
- ,,A highly sensitive ELISA D‐dimer increases testing but not diagnosis of pulmonary embolism.Acad Emerg Med2006;13:519–524.
- ,,A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,The incidence of symptomatic venous thromboembolism after enoxaparin prophylaxis in lower extremity arthroplasty: a cohort study of 1,984 patients.Canadian Collaborative Group.1998;114:115S–118S.
- ,,,,Impact of reliance on CT pulmonary angiography on diagnosis of pulmonary embolism: a Bayesian analysis.J Hosp Med.2006;1:81–87.
- The PIOPED investigators: Value of the ventilation‐perfusion scan in acute pulmonary embolism.JAMA.1990;263:2753–2759.
- ,,, et al.Non‐invasive diagnosis of venous thromboembolism in outpatients.Lancet.353:190–195.
Copyright © 2011 Society of Hospital Medicine
Patient Satisfaction With Procedural Care
In order to improve resident supervision and timeliness of invasive bedside procedures such as paracentesis, thoracentesis, and lumbar puncture, some academic medical centers have implemented procedure services that focus on providing high‐quality procedural care.1, 2
Procedure services have the potential to affect patient satisfaction, a key indicator in quality of care measurment.3 Having senior physicians present increases patient comfort during outpatient case presentations4 and improves patient satisfaction with explanations of tests and medications.5 However, we had concerns that teaching during a procedure may heighten patient anxiety. Patients are reluctant to be the first patient of a resident or medical student for a procedure,68 and patients are more likely to refuse consent to have a resident perform complex procedures.8 In previous studies, patient satisfaction with gynecological exams and flexible sigmoidoscopy performed by residents was comparable to satisfaction with those performed by staff physicians,9, 10 though in the case of flexible sigmoidoscopy, procedure duration was slightly longer.10 Few, if any, data describe bedside teaching or patient impressions of physician communication during procedures.
We carried out a prospective study of patient perceptions of the University of California San Francisco (UCSF) Hospitalist Procedure Service (HPS). Our study had the primary goal of understanding how our modelwhich involves bedside procedural teaching and feedback in real time (eg, as the procedure is performed)is perceived by patients.
Patients and Methods
Site
Our survey was carried out at UCSF Moffitt‐Long Hospital, a 560‐bed university teaching hospital and the primary university hospital for the University of California San Francisco. This study was reviewed and approved by the Committee on Human Research at UCSF.
Procedure Service
The HPS is composed of two interns who rotate for 2 weeks on a mandatory rotation performing the majority of the procedures done by the service. Every procedure is supervised by an attending hospitalist who has received extended training from interventional radiologists and emergency department ultrasound faculty. Patients are referred to the service by their primary admitting team. Interns receive procedure‐specific didactics, demonstration, and practice with procedure kits, supplemental readings, computer‐based procedure modules, and evidence‐based summaries of procedure‐related considerations. All interns also attend a half‐day procedure simulation session to review procedural and ultrasound techniques.
While interns obtain informed consent and prepare the patient for the procedure, the attending and intern team communicate the following points with each patient: 1) identification as the dedicated procedure team, separate from the primary team caring for the patient; 2) attending self‐identification as the supervisor; 3) attention to stepwise communication with the patient during the procedure; 4) attention to patient comfort throughout the procedure; 5) emphasis on patient safety through the use of time‐outs, sterile technique, and ultrasound when appropriate; and 6) the intention to discuss best practice and teach during the procedure.
All paracentesis and thoracentesis sites are marked by using bedside ultrasound (S‐Cath, SonoSite, Bothell, WA) guidance prior to and, if needed, during the procedure. Ultrasound is occasionally used for marking joint aspiration and lumbar puncture.11 Interns are responsible for making an initial site marking, which is then confirmed by the attending physician. Although not systematized, our service encourages the intern and attending to communicate about proper technique during the procedure itself. For example, attendings ask questions about technique based on evidence in the literature (eg, Why do you replace the stylet in a lumbar puncture needle prior to removal?) or about trouble shooting (eg, What would you do if the flow of ascites stops during this paracentesis?) and also correct any errors in technique (Recall the angle you intended to use based on the ultrasound view).
Patients
Patients are referred to the procedure service by their primary team; referrals are accepted for patients on all services at all levels of care, including the emergency department (ED) and the intensive care unit (ICU). Participants in this study were referred for one of our target procedures (paracentesis, thoracentesis, or lumbar puncture) between November 2008 and July 2009. Patients gave written consent for the supplemental survey independent of consent for the procedure. All consents and procedures were performed in a patient's hospital room and one family member was allowed to stay in the room if desired by the patient. After the completion of the procedure, the attending on the procedure service at the time, which included study authors D.S. and M.M., approached consecutive patients who spoke and read English and were deemed to have capacity to consent for their own procedure to be surveyed. Patients were considered to have capacity to consent based on commonly accepted criteria described in the literature.12, 13 Patients were also excluded if their procedure was performed by the attending alone, if they had repeated procedures done by the service, or if they were too altered or critically ill to participate in the survey.
Survey
Our survey was developed through identification of items reported in the literature,1421 as well as items newly developed for purposes of examining our primary aims. Newly developed questions focused on patients' satisfaction with major aspects of procedure performance as well as the quality and impact of communication with the patient and between members of the team. Two open‐text questions were included to allow patients to share what went well with the procedure as well as areas for improvement. The research team developed a pool of question items for potential inclusion in a patient satisfaction questionnaire. These items were then shown to a group of research‐oriented health professionals, who meet regularly to review academic research protocols. The group provided their opinions about the content and comprehension of the questions, and the written survey employed was a result of their revisions (see Appendix in Supporting Information online).
Written surveys were distributed to patients by the hospitalist attending on service following the procedure as permitted by patients' severity of illness and availability. Surveys were anonymous and self‐administered by the patient or a family member who was in the room for the procedure; all questions were voluntary. A nurse was made responsible for collecting the survey when possible. Survey results were entered into a database without identifiers, with limited demographic information; patient gender, age, and procedure type were included by the attending hospitalist at the end of the survey. A separate and more detailed procedure database was kept of all procedures performed and was used to record patient consent or reason for not consenting as well as documented receipt of a completed survey. This non‐anonymous database contained detailed supplemental information including patient age, level of care, referring service, presence of bloody fluid at any point during the procedure, and physician‐reported immediate complications at the bedside in free text.
Analysis
Reported immediate complications were classified into major and minor based on reported definitions in the literature.2226 Similar to previous studies, major immediate complications were defined as those requiring further procedural intervention, medical therapy, or both.27 Major complications were defined as: bleeding requiring transfusion, pneumothorax requiring a chest tube, respiratory failure, bowel perforation, cerebral herniation or shock, cerebrospinal fluid (CSF) leak requiring intervention, and transfer to a higher level of care. For patients receiving a thoracentesis, chart review was performed to determine the presence of a follow‐up chest x‐ray, the presence of a pneumothorax, or clinical evidence for re‐expansion pulmonary edema. We analyzed differences between respondents and non‐respondents using Chi‐square tests for categorical variables (gender, level of care, referring service, procedure type, bloody fluid, and immediate reported complications) and independent t tests for continuous variables (age).
After review of the open‐ended fields, responses were classified into the following categories: pain control, physician skill, professionalism, communication, symptom relief, procedure duration, and miscellaneous comments. Responses regarding patient perceptions of physician communication were dichotomized into positive (1 = Strongly Agree, 2 = Agree) and negative (3 = Neutral, 4 = Disagree, and 5 = Strongly Disagree), and independent t tests were used to determine the contribution of factors, such as age, while Chi‐square tests were used for the contribution of gender and procedure type. All statistical tests were performed by using the SAS statistical application program (version 9.2).
Results
Respondent Characteristics
Of 324 procedures performed by the HPS during the study period, 95 (29%) were eligible for consent. Of the 229 patients not eligible for consent, 32 (10%) were excluded because the procedure was performed by the attending alone, 76 (23%) lacked English proficiency or literacy, 66 (20%) had altered mental status, 32 (10%) were intubated and/or had severe illness precluding consent, and 23 (7%) were repeat procedures on patients who had previously completed the survey. Only two patients specifically requested an attending to perform the procedure after an introduction to the service. Of the 95 patients eligible for consent, 89 were consented for the survey, and 65 (68%) completed the survey. Of the six eligible, non‐consented patients, all were leaving the floor immediately following the procedure, and time did not allow for consent and survey distribution. There were no differences between eligible responders and nonresponders in age, gender, procedure, requesting service, presence of bloody fluid, or physician‐reported immediate complications (Table 1).
| Demographics | Respondera (n = 65) | Nonresponder (n = 24) |
|---|---|---|
| ||
| Age, y [mean (SD)] | 55.4 (15.7) | 50.4 (17.4) |
| Male gender, n (%) male | 41 (63.1) | 11 (45.8) |
| Procedure, n (%) | ||
| Paracentesis | 31 (47.7) | 10 (41.7) |
| Thoracentesis | 17 (25.8) | 6 (25.0) |
| Lumbar puncture | 15 (22.7) | 7 (29.2) |
| Arthrocentesis | 2 (3.0) | 1 (4.2) |
| Patient location, n (%) | ||
| Floor | 47 (72.3) | 19 (79.2) |
| Step down/telemetry | 17 (26.1) | 3 (12.5) |
| Intensive care unit | 1 (1.5) | 2 (8.3) |
| Service requesting, n (%) | ||
| Medicine | 29 (44.6) | 10 (41.7) |
| Cardiology | 6 (9.1) | 3 (12.5) |
| Liver transplant | 20 (30.3) | 7 (29.2) |
| Bone marrow transplant | 7 (10.6) | 1 (4.2) |
| Surgery | 0 | 1 (4.2) |
| Neurosurgery | 1 (1.5) | 1 (4.2) |
| Other | 2 (3.0) | 1 (4.2) |
| Reported presence of bloody fluid at any point in the procedure, n (%) | 9 (13.6) | 4 (16.7) |
| Other reported immediate complications | ||
| Equipment malfunction | 2 (3.0) | 1 (4.2) |
| Significant cough/pleuritic pain | 1 (1.5) | 1 (4.2) |
| Transient oxygen desaturation | 1 (1.5) | 0 |
| Ascites leak | 0 | 0 |
| Hematoma | 0 | 0 |
| Persistent bleeding | 0 | 0 |
| Transfer to a higher level of care | 0 | 0 |
Complications
As complications would likely play a role in procedure satisfaction, we describe immediate complications for the study population. Of the 324 procedures performed during the study period, no patient had predefined major immediate complications. Upon further chart review of the 96 patients that had a thoracentesis performed, all had a follow‐up chest x‐ray and none suffered an iatrogenic pneumothorax or re‐expansion pulmonary edema. Minor immediate complications for the 324 procedures were reported as follows: postprocedure pain in four patients (1.2%), cough in nine patients (2.8%), five equipment malfunctions (1.5%), four ascites leaks (1.2%), and one incisional bleed requiring a suture for hemostasis (0.3%). There was no significant difference in complications between those consented for the survey and the total study population.
Procedure Satisfaction
More than 90% of patients were satisfied or very satisfied with most aspects of the procedure, including the informed consent process, pain control, expertise, and courtesy of physicians (Table 2). The percentage of patients satisfied with the duration of procedure (88%) was lower than for other measures of satisfaction. Of the 38 patients receiving therapeutic procedures, 34 (89%) were satisfied or highly satisfied with the improvement in symptoms following the procedure.
| Very Satisfied and Satisfied No. (%) | Neutral No. (%) | Dissatisfied and Very Dissatisfied No. (%) | N/A No. (%) | |
|---|---|---|---|---|
| Your overall procedure experience | 65 (100) | 0 (0) | 0 (0) | 0 (0) |
| Explanation of the procedure, risks, and benefits before the procedure | 64 (99) | 1 (2) | 0 (0) | 0 (0) |
| Pain control during the procedure | 60 (92) | 5 (8) | 0 (0) | 0 (0) |
| Expertise/skill of the physicians performing your procedure | 62 (95) | 3 (5) | 0 (0) | 0 (0) |
| Courtesy and bedside manner of the physicians performing your procedure | 65 (100) | 0 (0) | 0 (0) | 0 (0) |
| The time it took to perform your procedure | 57 (88) | 6 (9) | 0 (0) | 2 (3) |
| Improvement in your symptoms following this procedure, if applicable | 34 (52) | 7 (11) | 0 (0) | 24 (37) |
When asked what went well with the procedure, 59 (91%) respondents provided additional comments and feedback. Each response was classified as described in the Methods section. Of the free text responses, 8 of the 59 patients (14%) commented on the attention to pain control (eg, The caring and attention to my pain was most important to me), 5 (8%) on the skills of the operators (Great examination of the entire stomach region with the ultrasound to ensure the best position of the catheter), 6 (10%) on the courtesy and professionalism of the team (eg, Courteous, team‐feeling, addressed my concerns), 9 (15%) on their communication with the team (eg, The doctors made me feel very comfortable before the procedure by laying out the plan and explaining each part of the procedure), and 8 (14%) on relief of their symptoms (eg, There was an almost immediate and significant improvement in my breathing, bloating, and pain). Twenty‐three of the 59 comments (39%) were categorized as miscellaneous (eg, All went great. I fell asleep).
When asked areas for improvement, 55 (85%) patients responded. Thirty‐three patients (60%) reported that nothing could be improved or they instructed the team to just keep doing what you are doing, while 22 (40%) patients expressed a concern. Responses were categorized in a similar fashion to the positive responses. Five of the 22 negative comments (23%) reported that the procedure took too long (eg, Procedure could have been shorter. I got tired sitting up), 4 (18%) commented on pain control (eg, The poke for marking my skin hurt more than the anesthetic. I was surprised), 6 (27%) felt communication was a problem (eg, Discuss the steps with the patient audibly, no whispering, speak clearly), and 7 (32%) had miscellaneous concerns (eg, Try not to do this procedure right after another one).
Physician Communication
Sixty‐four patients (98%) reported that the physicians performing their procedure communicated with each other during the procedure (Table 3). Although one patient did not feel that the physicians communicated with each other, he or she still answered the follow‐up questions regarding perceptions of physician communication. We excluded this patient from our analysis as his or her answers may not be reliable. The majority of patients (84%) reported this communication as reassuring and felt it was a normal part of procedure performance (94%). Those that did not agree that physician communication was reassuring did not differ in average age (P = 0.307), gender (P = 0.511), or procedure type (P = 0.562).
| Strongly Agree and Agree No. (%) | Neutral No. (%) | Disagree, and Strongly Disagree No. (%) | |
|---|---|---|---|
| I felt that the physicians talking to each other about my procedure was reassuring to me | 54 (84) | 10 (16) | 0 (0) |
| Physicians talking to each other while doing a procedure is a normal part of doing a procedure | 60 (94) | 4 (6) | 0 (0) |
Of all positive and negative comments, five specifically addressed communication between physicians. Most (four) reflected satisfaction with bedside teaching (eg, They discussed the procedure in a professional manner and eased my mind at all times) and with having an expert in the room (eg, [The team] discussed things like needle placement, which was nice because there was a second opinion right there in the room). Patients also felt that it was good to experience the teaching, with one patient reporting that the best part of the procedure was watching doctors learn from each other. Patients did not express specific reservations about bedside teaching, resident technique, or fear of complications in free text.
Discussion
Even though novice interns performed procedures and simultaneous bedside teaching, patient satisfaction with a teaching procedure service was high, and reported complication rates were low. In addition, a majority of patients found discussions related to teaching activities reassuring and potentially important to their perception of care quality. Analogous studies examining patient satisfaction with endoscopic care found similar rates of patient satisfaction with endoscopists' bedside manner, technical skills, and pain control, but these studies included sedated patients.21 Our results are unique, as we evaluated awake patients with attention to perception of bedside teaching with novice interns.
Our findings offer an alternative strategy for bedside procedural teaching that employs transparency in the use of an expert and a trainee to introduce patients to bedside teaching by experts, which is not common at many academic medical centers.28 Patients may have been reassured by a clear explanation of the role of the service and the providers involved as well as an assurance of expertise and attention to patient comfort and safety. In addition to patient satisfaction, this model has the potential to impact both the safety of bedside procedures and housestaff education around procedure performance. For example, pneumothorax rates using our procedure service model are lower than those published (0% vs. 4% for ultrasound‐guided thoracentesis and 8.5% for thoracentesis by less experienced clinicians).29
Providers may be reluctant to teach at the bedside of awake patients for fear of heightening patient anxiety over trainee inexperience. In the 1960s similar fears were raised over the concern for patient anxiety with bedside rounding,30 but later studies revealed these concerns to be largely unfounded. Instead, bedside rounds have been shown to positively influence patients' feelings about their hospital experience and their relationships with their physicians compared with patients whose case presentations were made in a conference room.31, 32 Given the opportunity to comment on areas for improvement, patients in our study specifically elaborated regarding pain control, communication, and efficiency problems. Although 16% of patients did not find the communication of physicians reassuring, none of the negative comments reflected problems with bedside teaching, but rather concepts such as desiring a better explanation of steps throughout the procedure. Specifically, patients desire better communication for unanticipated pain.
There are several limitations to this study. Lack of patient satisfaction data from a control group of patients whose procedures were performed by attendings or housestaff alone limits our ability to draw conclusions about our satisfaction scores. The scarce applicable literature offers only imperfect comparison data. Because hospitalists were not blinded to the survey, attending behavior may have been subject to a Hawthorne effect.33 Consenting patients after the procedure could have provided hospitalists with an opportunity to exclude patients who appeared less satisfied with their procedure; however, attempts were made to prevent this behavior by requiring strict accounting of why a patient was not consented for the study. Use of alternative personnel for consent such as nurses was explored, but was found not to be feasible due to limited resources. These data are only applicable to English‐speaking patients who are literate and well enough to complete a survey. It is not clear whether the experience for other patients would reflect the same outcomes. It is plausible that non‐English‐speaking patients might have more concerns about incomprehensible conversations taking place during their procedure. Although the surveys were anonymous and patients were told that the proceduralists would not see individual responses, responses may have been biased out of patient concern that their response might affect their care. Hospitalists obtaining consent, however, were careful to stress anonymity and the distinction between the primary team and the procedure team.
Academic hospitals are struggling with providing quality procedural care while balancing housestaff education and experience.28 With hospitalists playing an increasingly prominent role in housestaff education and patient satisfaction initiatives, the supervision of housestaff by trained hospitalist faculty may help meet both aims in the performance of invasive bedside procedures, particularly at institutions where simulation training resources are limited. Although concern may exist for potential patient anxiety with bedside teaching, our data demonstrate high levels of patient satisfaction with a hospitalist procedure service despite novice procedure performers and an emphasis on teaching during the procedure.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19(5 Pt 2):510–513.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: A firm‐based trial.J Hosp Med.2007;2(3):143–149.
- Hospital Care Quality Information from the Consumer Perspective (HCAHPS).Quality Assurance Guidelines.Baltimore, MD:Centers for Medicare 113(8):657–662.
- ,,,,.The effect of bedside case presentations on patients' perceptions of their medical care.N Engl J Med.1997;336(16):1150–1155.
- ,,,.‘Sorry, it's my first time!’ Will patients consent to medical students learning procedures?Med Educ.2005;39(4):365–369.
- ,.Ethical considerations surrounding first time procedures: a study and analysis of patient attitudes toward spinal taps by students.Kennedy Inst Ethics J.1992;2(3):217–231.
- ,,,.Patients' willingness to allow residents to learn to practice medical procedures.Acad Med.2004;79(2):144–147.
- ,,.Patient satisfaction with gynecologic care provided by family practice resident physicians.Fam Pract Res J.1991;11(4):421–428.
- ,,.Resident participation in flexible sigmoidoscopy does not affect patient satisfaction.Am J Gastroenterol.2000;95(6):1563–1566.
- ,.Bedside ultrasound for difficult lumbar puncture.J Emerg Med.2005;28(2):197–200.
- ,.Conducting the Assessment. In:Assessing Competence to Consent to Treatment: A Guide for Physicians and Other Health Professionals.First Edition ed.New York, NY:Oxford University Press;1998:80–91.
- ,.Care of Ill, Socially Complicated Patients. In:Medical Management of Vulnerable 2007:407–418.
- ,,,,.Interventional radiologic procedures: patient anxiety, perception of pain, understanding of procedure, and satisfaction with medication‐‐a prospective study.Radiology.2000;215(3):684–688.
- ,,,,.Improving the assessment of (in)patients' satisfaction with hospital care.Med Care.2001;39(3):270–283.
- ,,,.Factors determining inpatient satisfaction with care.Soc Sci Med.2002;54(4):493–504.
- ,,,.Reliability and validity of the Satisfaction with Hospital Care Questionnaire.Int J Qual Health Care.2002;14(6):471–482.
- ,,,,.A randomized trial of four patient satisfaction questionnaires.Med Care.2003;41(12):1343–1352.
- ,,, et al.Development and validation of an in‐patient satisfaction questionnaire.Int J Qual Health Care.2005;17(6):465–472.
- ,,,.Central venous port catheters: evaluation of patients' satisfaction with implantation under local anesthesia.J Vasc Access.2009;10(1):27–32.
- ,,,.Factors influencing patient satisfaction when undergoing endoscopic procedures.Gastrointest Endosc.2009;69(4):883–91, quiz 891.e1.
- ,,, et al.Complications associated with thoracentesis. A prospective, randomized study comparing three different methods.Arch Intern Med.1990;150(4):873–877.
- ,,, et al.Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study.Clin Gastroenterol Hepatol.2009;7(8):906–909.
- ,,, et al.Performance standards for therapeutic abdominal paracentesis.Hepatology.2004;40(2):484–488.
- ,,,,.Lumbar puncture: its indications, contraindications, complications and technique.Rev Neurol.2007;45(7):433–436.
- .How to perform a lumbar puncture with the patient in the seated position.Br J Hosp Med (Lond).2006;67(3):M46–7.
- ,,.Are commonly used resident measurements associated with procedural skills in internal medicine residency training?J Gen Intern Med.2007;22(3):357–361.
- ,,,MERN Group,.Supervising the Supervisors‐Procedural Training and Supervision in Internal Medicine Residency.J Gen Intern Med.2010.
- ,,,.Pneumothorax following thoracentesis: a systematic review and meta‐analysis.Arch Intern Med.2010;170(4):332–339.
- ,,.The emotional impact of ward rounds.J Mt Sinai Hosp NY.1956;23(6):782–803.
- ,,,.The physiologic and psychological effects of the bedside presentation.N Engl J Med.1989;321(18):1273–1275.
- ,,,,.The effect of bedside case presentations on patients' perceptions of their medical care.N Engl J Med.1997;336(16):1150–1155.
- .Hawthorne effects and research into professional practice.J Eval Clin Pract.2001;7(1):65–70.
In order to improve resident supervision and timeliness of invasive bedside procedures such as paracentesis, thoracentesis, and lumbar puncture, some academic medical centers have implemented procedure services that focus on providing high‐quality procedural care.1, 2
Procedure services have the potential to affect patient satisfaction, a key indicator in quality of care measurment.3 Having senior physicians present increases patient comfort during outpatient case presentations4 and improves patient satisfaction with explanations of tests and medications.5 However, we had concerns that teaching during a procedure may heighten patient anxiety. Patients are reluctant to be the first patient of a resident or medical student for a procedure,68 and patients are more likely to refuse consent to have a resident perform complex procedures.8 In previous studies, patient satisfaction with gynecological exams and flexible sigmoidoscopy performed by residents was comparable to satisfaction with those performed by staff physicians,9, 10 though in the case of flexible sigmoidoscopy, procedure duration was slightly longer.10 Few, if any, data describe bedside teaching or patient impressions of physician communication during procedures.
We carried out a prospective study of patient perceptions of the University of California San Francisco (UCSF) Hospitalist Procedure Service (HPS). Our study had the primary goal of understanding how our modelwhich involves bedside procedural teaching and feedback in real time (eg, as the procedure is performed)is perceived by patients.
Patients and Methods
Site
Our survey was carried out at UCSF Moffitt‐Long Hospital, a 560‐bed university teaching hospital and the primary university hospital for the University of California San Francisco. This study was reviewed and approved by the Committee on Human Research at UCSF.
Procedure Service
The HPS is composed of two interns who rotate for 2 weeks on a mandatory rotation performing the majority of the procedures done by the service. Every procedure is supervised by an attending hospitalist who has received extended training from interventional radiologists and emergency department ultrasound faculty. Patients are referred to the service by their primary admitting team. Interns receive procedure‐specific didactics, demonstration, and practice with procedure kits, supplemental readings, computer‐based procedure modules, and evidence‐based summaries of procedure‐related considerations. All interns also attend a half‐day procedure simulation session to review procedural and ultrasound techniques.
While interns obtain informed consent and prepare the patient for the procedure, the attending and intern team communicate the following points with each patient: 1) identification as the dedicated procedure team, separate from the primary team caring for the patient; 2) attending self‐identification as the supervisor; 3) attention to stepwise communication with the patient during the procedure; 4) attention to patient comfort throughout the procedure; 5) emphasis on patient safety through the use of time‐outs, sterile technique, and ultrasound when appropriate; and 6) the intention to discuss best practice and teach during the procedure.
All paracentesis and thoracentesis sites are marked by using bedside ultrasound (S‐Cath, SonoSite, Bothell, WA) guidance prior to and, if needed, during the procedure. Ultrasound is occasionally used for marking joint aspiration and lumbar puncture.11 Interns are responsible for making an initial site marking, which is then confirmed by the attending physician. Although not systematized, our service encourages the intern and attending to communicate about proper technique during the procedure itself. For example, attendings ask questions about technique based on evidence in the literature (eg, Why do you replace the stylet in a lumbar puncture needle prior to removal?) or about trouble shooting (eg, What would you do if the flow of ascites stops during this paracentesis?) and also correct any errors in technique (Recall the angle you intended to use based on the ultrasound view).
Patients
Patients are referred to the procedure service by their primary team; referrals are accepted for patients on all services at all levels of care, including the emergency department (ED) and the intensive care unit (ICU). Participants in this study were referred for one of our target procedures (paracentesis, thoracentesis, or lumbar puncture) between November 2008 and July 2009. Patients gave written consent for the supplemental survey independent of consent for the procedure. All consents and procedures were performed in a patient's hospital room and one family member was allowed to stay in the room if desired by the patient. After the completion of the procedure, the attending on the procedure service at the time, which included study authors D.S. and M.M., approached consecutive patients who spoke and read English and were deemed to have capacity to consent for their own procedure to be surveyed. Patients were considered to have capacity to consent based on commonly accepted criteria described in the literature.12, 13 Patients were also excluded if their procedure was performed by the attending alone, if they had repeated procedures done by the service, or if they were too altered or critically ill to participate in the survey.
Survey
Our survey was developed through identification of items reported in the literature,1421 as well as items newly developed for purposes of examining our primary aims. Newly developed questions focused on patients' satisfaction with major aspects of procedure performance as well as the quality and impact of communication with the patient and between members of the team. Two open‐text questions were included to allow patients to share what went well with the procedure as well as areas for improvement. The research team developed a pool of question items for potential inclusion in a patient satisfaction questionnaire. These items were then shown to a group of research‐oriented health professionals, who meet regularly to review academic research protocols. The group provided their opinions about the content and comprehension of the questions, and the written survey employed was a result of their revisions (see Appendix in Supporting Information online).
Written surveys were distributed to patients by the hospitalist attending on service following the procedure as permitted by patients' severity of illness and availability. Surveys were anonymous and self‐administered by the patient or a family member who was in the room for the procedure; all questions were voluntary. A nurse was made responsible for collecting the survey when possible. Survey results were entered into a database without identifiers, with limited demographic information; patient gender, age, and procedure type were included by the attending hospitalist at the end of the survey. A separate and more detailed procedure database was kept of all procedures performed and was used to record patient consent or reason for not consenting as well as documented receipt of a completed survey. This non‐anonymous database contained detailed supplemental information including patient age, level of care, referring service, presence of bloody fluid at any point during the procedure, and physician‐reported immediate complications at the bedside in free text.
Analysis
Reported immediate complications were classified into major and minor based on reported definitions in the literature.2226 Similar to previous studies, major immediate complications were defined as those requiring further procedural intervention, medical therapy, or both.27 Major complications were defined as: bleeding requiring transfusion, pneumothorax requiring a chest tube, respiratory failure, bowel perforation, cerebral herniation or shock, cerebrospinal fluid (CSF) leak requiring intervention, and transfer to a higher level of care. For patients receiving a thoracentesis, chart review was performed to determine the presence of a follow‐up chest x‐ray, the presence of a pneumothorax, or clinical evidence for re‐expansion pulmonary edema. We analyzed differences between respondents and non‐respondents using Chi‐square tests for categorical variables (gender, level of care, referring service, procedure type, bloody fluid, and immediate reported complications) and independent t tests for continuous variables (age).
After review of the open‐ended fields, responses were classified into the following categories: pain control, physician skill, professionalism, communication, symptom relief, procedure duration, and miscellaneous comments. Responses regarding patient perceptions of physician communication were dichotomized into positive (1 = Strongly Agree, 2 = Agree) and negative (3 = Neutral, 4 = Disagree, and 5 = Strongly Disagree), and independent t tests were used to determine the contribution of factors, such as age, while Chi‐square tests were used for the contribution of gender and procedure type. All statistical tests were performed by using the SAS statistical application program (version 9.2).
Results
Respondent Characteristics
Of 324 procedures performed by the HPS during the study period, 95 (29%) were eligible for consent. Of the 229 patients not eligible for consent, 32 (10%) were excluded because the procedure was performed by the attending alone, 76 (23%) lacked English proficiency or literacy, 66 (20%) had altered mental status, 32 (10%) were intubated and/or had severe illness precluding consent, and 23 (7%) were repeat procedures on patients who had previously completed the survey. Only two patients specifically requested an attending to perform the procedure after an introduction to the service. Of the 95 patients eligible for consent, 89 were consented for the survey, and 65 (68%) completed the survey. Of the six eligible, non‐consented patients, all were leaving the floor immediately following the procedure, and time did not allow for consent and survey distribution. There were no differences between eligible responders and nonresponders in age, gender, procedure, requesting service, presence of bloody fluid, or physician‐reported immediate complications (Table 1).
| Demographics | Respondera (n = 65) | Nonresponder (n = 24) |
|---|---|---|
| ||
| Age, y [mean (SD)] | 55.4 (15.7) | 50.4 (17.4) |
| Male gender, n (%) male | 41 (63.1) | 11 (45.8) |
| Procedure, n (%) | ||
| Paracentesis | 31 (47.7) | 10 (41.7) |
| Thoracentesis | 17 (25.8) | 6 (25.0) |
| Lumbar puncture | 15 (22.7) | 7 (29.2) |
| Arthrocentesis | 2 (3.0) | 1 (4.2) |
| Patient location, n (%) | ||
| Floor | 47 (72.3) | 19 (79.2) |
| Step down/telemetry | 17 (26.1) | 3 (12.5) |
| Intensive care unit | 1 (1.5) | 2 (8.3) |
| Service requesting, n (%) | ||
| Medicine | 29 (44.6) | 10 (41.7) |
| Cardiology | 6 (9.1) | 3 (12.5) |
| Liver transplant | 20 (30.3) | 7 (29.2) |
| Bone marrow transplant | 7 (10.6) | 1 (4.2) |
| Surgery | 0 | 1 (4.2) |
| Neurosurgery | 1 (1.5) | 1 (4.2) |
| Other | 2 (3.0) | 1 (4.2) |
| Reported presence of bloody fluid at any point in the procedure, n (%) | 9 (13.6) | 4 (16.7) |
| Other reported immediate complications | ||
| Equipment malfunction | 2 (3.0) | 1 (4.2) |
| Significant cough/pleuritic pain | 1 (1.5) | 1 (4.2) |
| Transient oxygen desaturation | 1 (1.5) | 0 |
| Ascites leak | 0 | 0 |
| Hematoma | 0 | 0 |
| Persistent bleeding | 0 | 0 |
| Transfer to a higher level of care | 0 | 0 |
Complications
As complications would likely play a role in procedure satisfaction, we describe immediate complications for the study population. Of the 324 procedures performed during the study period, no patient had predefined major immediate complications. Upon further chart review of the 96 patients that had a thoracentesis performed, all had a follow‐up chest x‐ray and none suffered an iatrogenic pneumothorax or re‐expansion pulmonary edema. Minor immediate complications for the 324 procedures were reported as follows: postprocedure pain in four patients (1.2%), cough in nine patients (2.8%), five equipment malfunctions (1.5%), four ascites leaks (1.2%), and one incisional bleed requiring a suture for hemostasis (0.3%). There was no significant difference in complications between those consented for the survey and the total study population.
Procedure Satisfaction
More than 90% of patients were satisfied or very satisfied with most aspects of the procedure, including the informed consent process, pain control, expertise, and courtesy of physicians (Table 2). The percentage of patients satisfied with the duration of procedure (88%) was lower than for other measures of satisfaction. Of the 38 patients receiving therapeutic procedures, 34 (89%) were satisfied or highly satisfied with the improvement in symptoms following the procedure.
| Very Satisfied and Satisfied No. (%) | Neutral No. (%) | Dissatisfied and Very Dissatisfied No. (%) | N/A No. (%) | |
|---|---|---|---|---|
| Your overall procedure experience | 65 (100) | 0 (0) | 0 (0) | 0 (0) |
| Explanation of the procedure, risks, and benefits before the procedure | 64 (99) | 1 (2) | 0 (0) | 0 (0) |
| Pain control during the procedure | 60 (92) | 5 (8) | 0 (0) | 0 (0) |
| Expertise/skill of the physicians performing your procedure | 62 (95) | 3 (5) | 0 (0) | 0 (0) |
| Courtesy and bedside manner of the physicians performing your procedure | 65 (100) | 0 (0) | 0 (0) | 0 (0) |
| The time it took to perform your procedure | 57 (88) | 6 (9) | 0 (0) | 2 (3) |
| Improvement in your symptoms following this procedure, if applicable | 34 (52) | 7 (11) | 0 (0) | 24 (37) |
When asked what went well with the procedure, 59 (91%) respondents provided additional comments and feedback. Each response was classified as described in the Methods section. Of the free text responses, 8 of the 59 patients (14%) commented on the attention to pain control (eg, The caring and attention to my pain was most important to me), 5 (8%) on the skills of the operators (Great examination of the entire stomach region with the ultrasound to ensure the best position of the catheter), 6 (10%) on the courtesy and professionalism of the team (eg, Courteous, team‐feeling, addressed my concerns), 9 (15%) on their communication with the team (eg, The doctors made me feel very comfortable before the procedure by laying out the plan and explaining each part of the procedure), and 8 (14%) on relief of their symptoms (eg, There was an almost immediate and significant improvement in my breathing, bloating, and pain). Twenty‐three of the 59 comments (39%) were categorized as miscellaneous (eg, All went great. I fell asleep).
When asked areas for improvement, 55 (85%) patients responded. Thirty‐three patients (60%) reported that nothing could be improved or they instructed the team to just keep doing what you are doing, while 22 (40%) patients expressed a concern. Responses were categorized in a similar fashion to the positive responses. Five of the 22 negative comments (23%) reported that the procedure took too long (eg, Procedure could have been shorter. I got tired sitting up), 4 (18%) commented on pain control (eg, The poke for marking my skin hurt more than the anesthetic. I was surprised), 6 (27%) felt communication was a problem (eg, Discuss the steps with the patient audibly, no whispering, speak clearly), and 7 (32%) had miscellaneous concerns (eg, Try not to do this procedure right after another one).
Physician Communication
Sixty‐four patients (98%) reported that the physicians performing their procedure communicated with each other during the procedure (Table 3). Although one patient did not feel that the physicians communicated with each other, he or she still answered the follow‐up questions regarding perceptions of physician communication. We excluded this patient from our analysis as his or her answers may not be reliable. The majority of patients (84%) reported this communication as reassuring and felt it was a normal part of procedure performance (94%). Those that did not agree that physician communication was reassuring did not differ in average age (P = 0.307), gender (P = 0.511), or procedure type (P = 0.562).
| Strongly Agree and Agree No. (%) | Neutral No. (%) | Disagree, and Strongly Disagree No. (%) | |
|---|---|---|---|
| I felt that the physicians talking to each other about my procedure was reassuring to me | 54 (84) | 10 (16) | 0 (0) |
| Physicians talking to each other while doing a procedure is a normal part of doing a procedure | 60 (94) | 4 (6) | 0 (0) |
Of all positive and negative comments, five specifically addressed communication between physicians. Most (four) reflected satisfaction with bedside teaching (eg, They discussed the procedure in a professional manner and eased my mind at all times) and with having an expert in the room (eg, [The team] discussed things like needle placement, which was nice because there was a second opinion right there in the room). Patients also felt that it was good to experience the teaching, with one patient reporting that the best part of the procedure was watching doctors learn from each other. Patients did not express specific reservations about bedside teaching, resident technique, or fear of complications in free text.
Discussion
Even though novice interns performed procedures and simultaneous bedside teaching, patient satisfaction with a teaching procedure service was high, and reported complication rates were low. In addition, a majority of patients found discussions related to teaching activities reassuring and potentially important to their perception of care quality. Analogous studies examining patient satisfaction with endoscopic care found similar rates of patient satisfaction with endoscopists' bedside manner, technical skills, and pain control, but these studies included sedated patients.21 Our results are unique, as we evaluated awake patients with attention to perception of bedside teaching with novice interns.
Our findings offer an alternative strategy for bedside procedural teaching that employs transparency in the use of an expert and a trainee to introduce patients to bedside teaching by experts, which is not common at many academic medical centers.28 Patients may have been reassured by a clear explanation of the role of the service and the providers involved as well as an assurance of expertise and attention to patient comfort and safety. In addition to patient satisfaction, this model has the potential to impact both the safety of bedside procedures and housestaff education around procedure performance. For example, pneumothorax rates using our procedure service model are lower than those published (0% vs. 4% for ultrasound‐guided thoracentesis and 8.5% for thoracentesis by less experienced clinicians).29
Providers may be reluctant to teach at the bedside of awake patients for fear of heightening patient anxiety over trainee inexperience. In the 1960s similar fears were raised over the concern for patient anxiety with bedside rounding,30 but later studies revealed these concerns to be largely unfounded. Instead, bedside rounds have been shown to positively influence patients' feelings about their hospital experience and their relationships with their physicians compared with patients whose case presentations were made in a conference room.31, 32 Given the opportunity to comment on areas for improvement, patients in our study specifically elaborated regarding pain control, communication, and efficiency problems. Although 16% of patients did not find the communication of physicians reassuring, none of the negative comments reflected problems with bedside teaching, but rather concepts such as desiring a better explanation of steps throughout the procedure. Specifically, patients desire better communication for unanticipated pain.
There are several limitations to this study. Lack of patient satisfaction data from a control group of patients whose procedures were performed by attendings or housestaff alone limits our ability to draw conclusions about our satisfaction scores. The scarce applicable literature offers only imperfect comparison data. Because hospitalists were not blinded to the survey, attending behavior may have been subject to a Hawthorne effect.33 Consenting patients after the procedure could have provided hospitalists with an opportunity to exclude patients who appeared less satisfied with their procedure; however, attempts were made to prevent this behavior by requiring strict accounting of why a patient was not consented for the study. Use of alternative personnel for consent such as nurses was explored, but was found not to be feasible due to limited resources. These data are only applicable to English‐speaking patients who are literate and well enough to complete a survey. It is not clear whether the experience for other patients would reflect the same outcomes. It is plausible that non‐English‐speaking patients might have more concerns about incomprehensible conversations taking place during their procedure. Although the surveys were anonymous and patients were told that the proceduralists would not see individual responses, responses may have been biased out of patient concern that their response might affect their care. Hospitalists obtaining consent, however, were careful to stress anonymity and the distinction between the primary team and the procedure team.
Academic hospitals are struggling with providing quality procedural care while balancing housestaff education and experience.28 With hospitalists playing an increasingly prominent role in housestaff education and patient satisfaction initiatives, the supervision of housestaff by trained hospitalist faculty may help meet both aims in the performance of invasive bedside procedures, particularly at institutions where simulation training resources are limited. Although concern may exist for potential patient anxiety with bedside teaching, our data demonstrate high levels of patient satisfaction with a hospitalist procedure service despite novice procedure performers and an emphasis on teaching during the procedure.
In order to improve resident supervision and timeliness of invasive bedside procedures such as paracentesis, thoracentesis, and lumbar puncture, some academic medical centers have implemented procedure services that focus on providing high‐quality procedural care.1, 2
Procedure services have the potential to affect patient satisfaction, a key indicator in quality of care measurment.3 Having senior physicians present increases patient comfort during outpatient case presentations4 and improves patient satisfaction with explanations of tests and medications.5 However, we had concerns that teaching during a procedure may heighten patient anxiety. Patients are reluctant to be the first patient of a resident or medical student for a procedure,68 and patients are more likely to refuse consent to have a resident perform complex procedures.8 In previous studies, patient satisfaction with gynecological exams and flexible sigmoidoscopy performed by residents was comparable to satisfaction with those performed by staff physicians,9, 10 though in the case of flexible sigmoidoscopy, procedure duration was slightly longer.10 Few, if any, data describe bedside teaching or patient impressions of physician communication during procedures.
We carried out a prospective study of patient perceptions of the University of California San Francisco (UCSF) Hospitalist Procedure Service (HPS). Our study had the primary goal of understanding how our modelwhich involves bedside procedural teaching and feedback in real time (eg, as the procedure is performed)is perceived by patients.
Patients and Methods
Site
Our survey was carried out at UCSF Moffitt‐Long Hospital, a 560‐bed university teaching hospital and the primary university hospital for the University of California San Francisco. This study was reviewed and approved by the Committee on Human Research at UCSF.
Procedure Service
The HPS is composed of two interns who rotate for 2 weeks on a mandatory rotation performing the majority of the procedures done by the service. Every procedure is supervised by an attending hospitalist who has received extended training from interventional radiologists and emergency department ultrasound faculty. Patients are referred to the service by their primary admitting team. Interns receive procedure‐specific didactics, demonstration, and practice with procedure kits, supplemental readings, computer‐based procedure modules, and evidence‐based summaries of procedure‐related considerations. All interns also attend a half‐day procedure simulation session to review procedural and ultrasound techniques.
While interns obtain informed consent and prepare the patient for the procedure, the attending and intern team communicate the following points with each patient: 1) identification as the dedicated procedure team, separate from the primary team caring for the patient; 2) attending self‐identification as the supervisor; 3) attention to stepwise communication with the patient during the procedure; 4) attention to patient comfort throughout the procedure; 5) emphasis on patient safety through the use of time‐outs, sterile technique, and ultrasound when appropriate; and 6) the intention to discuss best practice and teach during the procedure.
All paracentesis and thoracentesis sites are marked by using bedside ultrasound (S‐Cath, SonoSite, Bothell, WA) guidance prior to and, if needed, during the procedure. Ultrasound is occasionally used for marking joint aspiration and lumbar puncture.11 Interns are responsible for making an initial site marking, which is then confirmed by the attending physician. Although not systematized, our service encourages the intern and attending to communicate about proper technique during the procedure itself. For example, attendings ask questions about technique based on evidence in the literature (eg, Why do you replace the stylet in a lumbar puncture needle prior to removal?) or about trouble shooting (eg, What would you do if the flow of ascites stops during this paracentesis?) and also correct any errors in technique (Recall the angle you intended to use based on the ultrasound view).
Patients
Patients are referred to the procedure service by their primary team; referrals are accepted for patients on all services at all levels of care, including the emergency department (ED) and the intensive care unit (ICU). Participants in this study were referred for one of our target procedures (paracentesis, thoracentesis, or lumbar puncture) between November 2008 and July 2009. Patients gave written consent for the supplemental survey independent of consent for the procedure. All consents and procedures were performed in a patient's hospital room and one family member was allowed to stay in the room if desired by the patient. After the completion of the procedure, the attending on the procedure service at the time, which included study authors D.S. and M.M., approached consecutive patients who spoke and read English and were deemed to have capacity to consent for their own procedure to be surveyed. Patients were considered to have capacity to consent based on commonly accepted criteria described in the literature.12, 13 Patients were also excluded if their procedure was performed by the attending alone, if they had repeated procedures done by the service, or if they were too altered or critically ill to participate in the survey.
Survey
Our survey was developed through identification of items reported in the literature,1421 as well as items newly developed for purposes of examining our primary aims. Newly developed questions focused on patients' satisfaction with major aspects of procedure performance as well as the quality and impact of communication with the patient and between members of the team. Two open‐text questions were included to allow patients to share what went well with the procedure as well as areas for improvement. The research team developed a pool of question items for potential inclusion in a patient satisfaction questionnaire. These items were then shown to a group of research‐oriented health professionals, who meet regularly to review academic research protocols. The group provided their opinions about the content and comprehension of the questions, and the written survey employed was a result of their revisions (see Appendix in Supporting Information online).
Written surveys were distributed to patients by the hospitalist attending on service following the procedure as permitted by patients' severity of illness and availability. Surveys were anonymous and self‐administered by the patient or a family member who was in the room for the procedure; all questions were voluntary. A nurse was made responsible for collecting the survey when possible. Survey results were entered into a database without identifiers, with limited demographic information; patient gender, age, and procedure type were included by the attending hospitalist at the end of the survey. A separate and more detailed procedure database was kept of all procedures performed and was used to record patient consent or reason for not consenting as well as documented receipt of a completed survey. This non‐anonymous database contained detailed supplemental information including patient age, level of care, referring service, presence of bloody fluid at any point during the procedure, and physician‐reported immediate complications at the bedside in free text.
Analysis
Reported immediate complications were classified into major and minor based on reported definitions in the literature.2226 Similar to previous studies, major immediate complications were defined as those requiring further procedural intervention, medical therapy, or both.27 Major complications were defined as: bleeding requiring transfusion, pneumothorax requiring a chest tube, respiratory failure, bowel perforation, cerebral herniation or shock, cerebrospinal fluid (CSF) leak requiring intervention, and transfer to a higher level of care. For patients receiving a thoracentesis, chart review was performed to determine the presence of a follow‐up chest x‐ray, the presence of a pneumothorax, or clinical evidence for re‐expansion pulmonary edema. We analyzed differences between respondents and non‐respondents using Chi‐square tests for categorical variables (gender, level of care, referring service, procedure type, bloody fluid, and immediate reported complications) and independent t tests for continuous variables (age).
After review of the open‐ended fields, responses were classified into the following categories: pain control, physician skill, professionalism, communication, symptom relief, procedure duration, and miscellaneous comments. Responses regarding patient perceptions of physician communication were dichotomized into positive (1 = Strongly Agree, 2 = Agree) and negative (3 = Neutral, 4 = Disagree, and 5 = Strongly Disagree), and independent t tests were used to determine the contribution of factors, such as age, while Chi‐square tests were used for the contribution of gender and procedure type. All statistical tests were performed by using the SAS statistical application program (version 9.2).
Results
Respondent Characteristics
Of 324 procedures performed by the HPS during the study period, 95 (29%) were eligible for consent. Of the 229 patients not eligible for consent, 32 (10%) were excluded because the procedure was performed by the attending alone, 76 (23%) lacked English proficiency or literacy, 66 (20%) had altered mental status, 32 (10%) were intubated and/or had severe illness precluding consent, and 23 (7%) were repeat procedures on patients who had previously completed the survey. Only two patients specifically requested an attending to perform the procedure after an introduction to the service. Of the 95 patients eligible for consent, 89 were consented for the survey, and 65 (68%) completed the survey. Of the six eligible, non‐consented patients, all were leaving the floor immediately following the procedure, and time did not allow for consent and survey distribution. There were no differences between eligible responders and nonresponders in age, gender, procedure, requesting service, presence of bloody fluid, or physician‐reported immediate complications (Table 1).
| Demographics | Respondera (n = 65) | Nonresponder (n = 24) |
|---|---|---|
| ||
| Age, y [mean (SD)] | 55.4 (15.7) | 50.4 (17.4) |
| Male gender, n (%) male | 41 (63.1) | 11 (45.8) |
| Procedure, n (%) | ||
| Paracentesis | 31 (47.7) | 10 (41.7) |
| Thoracentesis | 17 (25.8) | 6 (25.0) |
| Lumbar puncture | 15 (22.7) | 7 (29.2) |
| Arthrocentesis | 2 (3.0) | 1 (4.2) |
| Patient location, n (%) | ||
| Floor | 47 (72.3) | 19 (79.2) |
| Step down/telemetry | 17 (26.1) | 3 (12.5) |
| Intensive care unit | 1 (1.5) | 2 (8.3) |
| Service requesting, n (%) | ||
| Medicine | 29 (44.6) | 10 (41.7) |
| Cardiology | 6 (9.1) | 3 (12.5) |
| Liver transplant | 20 (30.3) | 7 (29.2) |
| Bone marrow transplant | 7 (10.6) | 1 (4.2) |
| Surgery | 0 | 1 (4.2) |
| Neurosurgery | 1 (1.5) | 1 (4.2) |
| Other | 2 (3.0) | 1 (4.2) |
| Reported presence of bloody fluid at any point in the procedure, n (%) | 9 (13.6) | 4 (16.7) |
| Other reported immediate complications | ||
| Equipment malfunction | 2 (3.0) | 1 (4.2) |
| Significant cough/pleuritic pain | 1 (1.5) | 1 (4.2) |
| Transient oxygen desaturation | 1 (1.5) | 0 |
| Ascites leak | 0 | 0 |
| Hematoma | 0 | 0 |
| Persistent bleeding | 0 | 0 |
| Transfer to a higher level of care | 0 | 0 |
Complications
As complications would likely play a role in procedure satisfaction, we describe immediate complications for the study population. Of the 324 procedures performed during the study period, no patient had predefined major immediate complications. Upon further chart review of the 96 patients that had a thoracentesis performed, all had a follow‐up chest x‐ray and none suffered an iatrogenic pneumothorax or re‐expansion pulmonary edema. Minor immediate complications for the 324 procedures were reported as follows: postprocedure pain in four patients (1.2%), cough in nine patients (2.8%), five equipment malfunctions (1.5%), four ascites leaks (1.2%), and one incisional bleed requiring a suture for hemostasis (0.3%). There was no significant difference in complications between those consented for the survey and the total study population.
Procedure Satisfaction
More than 90% of patients were satisfied or very satisfied with most aspects of the procedure, including the informed consent process, pain control, expertise, and courtesy of physicians (Table 2). The percentage of patients satisfied with the duration of procedure (88%) was lower than for other measures of satisfaction. Of the 38 patients receiving therapeutic procedures, 34 (89%) were satisfied or highly satisfied with the improvement in symptoms following the procedure.
| Very Satisfied and Satisfied No. (%) | Neutral No. (%) | Dissatisfied and Very Dissatisfied No. (%) | N/A No. (%) | |
|---|---|---|---|---|
| Your overall procedure experience | 65 (100) | 0 (0) | 0 (0) | 0 (0) |
| Explanation of the procedure, risks, and benefits before the procedure | 64 (99) | 1 (2) | 0 (0) | 0 (0) |
| Pain control during the procedure | 60 (92) | 5 (8) | 0 (0) | 0 (0) |
| Expertise/skill of the physicians performing your procedure | 62 (95) | 3 (5) | 0 (0) | 0 (0) |
| Courtesy and bedside manner of the physicians performing your procedure | 65 (100) | 0 (0) | 0 (0) | 0 (0) |
| The time it took to perform your procedure | 57 (88) | 6 (9) | 0 (0) | 2 (3) |
| Improvement in your symptoms following this procedure, if applicable | 34 (52) | 7 (11) | 0 (0) | 24 (37) |
When asked what went well with the procedure, 59 (91%) respondents provided additional comments and feedback. Each response was classified as described in the Methods section. Of the free text responses, 8 of the 59 patients (14%) commented on the attention to pain control (eg, The caring and attention to my pain was most important to me), 5 (8%) on the skills of the operators (Great examination of the entire stomach region with the ultrasound to ensure the best position of the catheter), 6 (10%) on the courtesy and professionalism of the team (eg, Courteous, team‐feeling, addressed my concerns), 9 (15%) on their communication with the team (eg, The doctors made me feel very comfortable before the procedure by laying out the plan and explaining each part of the procedure), and 8 (14%) on relief of their symptoms (eg, There was an almost immediate and significant improvement in my breathing, bloating, and pain). Twenty‐three of the 59 comments (39%) were categorized as miscellaneous (eg, All went great. I fell asleep).
When asked areas for improvement, 55 (85%) patients responded. Thirty‐three patients (60%) reported that nothing could be improved or they instructed the team to just keep doing what you are doing, while 22 (40%) patients expressed a concern. Responses were categorized in a similar fashion to the positive responses. Five of the 22 negative comments (23%) reported that the procedure took too long (eg, Procedure could have been shorter. I got tired sitting up), 4 (18%) commented on pain control (eg, The poke for marking my skin hurt more than the anesthetic. I was surprised), 6 (27%) felt communication was a problem (eg, Discuss the steps with the patient audibly, no whispering, speak clearly), and 7 (32%) had miscellaneous concerns (eg, Try not to do this procedure right after another one).
Physician Communication
Sixty‐four patients (98%) reported that the physicians performing their procedure communicated with each other during the procedure (Table 3). Although one patient did not feel that the physicians communicated with each other, he or she still answered the follow‐up questions regarding perceptions of physician communication. We excluded this patient from our analysis as his or her answers may not be reliable. The majority of patients (84%) reported this communication as reassuring and felt it was a normal part of procedure performance (94%). Those that did not agree that physician communication was reassuring did not differ in average age (P = 0.307), gender (P = 0.511), or procedure type (P = 0.562).
| Strongly Agree and Agree No. (%) | Neutral No. (%) | Disagree, and Strongly Disagree No. (%) | |
|---|---|---|---|
| I felt that the physicians talking to each other about my procedure was reassuring to me | 54 (84) | 10 (16) | 0 (0) |
| Physicians talking to each other while doing a procedure is a normal part of doing a procedure | 60 (94) | 4 (6) | 0 (0) |
Of all positive and negative comments, five specifically addressed communication between physicians. Most (four) reflected satisfaction with bedside teaching (eg, They discussed the procedure in a professional manner and eased my mind at all times) and with having an expert in the room (eg, [The team] discussed things like needle placement, which was nice because there was a second opinion right there in the room). Patients also felt that it was good to experience the teaching, with one patient reporting that the best part of the procedure was watching doctors learn from each other. Patients did not express specific reservations about bedside teaching, resident technique, or fear of complications in free text.
Discussion
Even though novice interns performed procedures and simultaneous bedside teaching, patient satisfaction with a teaching procedure service was high, and reported complication rates were low. In addition, a majority of patients found discussions related to teaching activities reassuring and potentially important to their perception of care quality. Analogous studies examining patient satisfaction with endoscopic care found similar rates of patient satisfaction with endoscopists' bedside manner, technical skills, and pain control, but these studies included sedated patients.21 Our results are unique, as we evaluated awake patients with attention to perception of bedside teaching with novice interns.
Our findings offer an alternative strategy for bedside procedural teaching that employs transparency in the use of an expert and a trainee to introduce patients to bedside teaching by experts, which is not common at many academic medical centers.28 Patients may have been reassured by a clear explanation of the role of the service and the providers involved as well as an assurance of expertise and attention to patient comfort and safety. In addition to patient satisfaction, this model has the potential to impact both the safety of bedside procedures and housestaff education around procedure performance. For example, pneumothorax rates using our procedure service model are lower than those published (0% vs. 4% for ultrasound‐guided thoracentesis and 8.5% for thoracentesis by less experienced clinicians).29
Providers may be reluctant to teach at the bedside of awake patients for fear of heightening patient anxiety over trainee inexperience. In the 1960s similar fears were raised over the concern for patient anxiety with bedside rounding,30 but later studies revealed these concerns to be largely unfounded. Instead, bedside rounds have been shown to positively influence patients' feelings about their hospital experience and their relationships with their physicians compared with patients whose case presentations were made in a conference room.31, 32 Given the opportunity to comment on areas for improvement, patients in our study specifically elaborated regarding pain control, communication, and efficiency problems. Although 16% of patients did not find the communication of physicians reassuring, none of the negative comments reflected problems with bedside teaching, but rather concepts such as desiring a better explanation of steps throughout the procedure. Specifically, patients desire better communication for unanticipated pain.
There are several limitations to this study. Lack of patient satisfaction data from a control group of patients whose procedures were performed by attendings or housestaff alone limits our ability to draw conclusions about our satisfaction scores. The scarce applicable literature offers only imperfect comparison data. Because hospitalists were not blinded to the survey, attending behavior may have been subject to a Hawthorne effect.33 Consenting patients after the procedure could have provided hospitalists with an opportunity to exclude patients who appeared less satisfied with their procedure; however, attempts were made to prevent this behavior by requiring strict accounting of why a patient was not consented for the study. Use of alternative personnel for consent such as nurses was explored, but was found not to be feasible due to limited resources. These data are only applicable to English‐speaking patients who are literate and well enough to complete a survey. It is not clear whether the experience for other patients would reflect the same outcomes. It is plausible that non‐English‐speaking patients might have more concerns about incomprehensible conversations taking place during their procedure. Although the surveys were anonymous and patients were told that the proceduralists would not see individual responses, responses may have been biased out of patient concern that their response might affect their care. Hospitalists obtaining consent, however, were careful to stress anonymity and the distinction between the primary team and the procedure team.
Academic hospitals are struggling with providing quality procedural care while balancing housestaff education and experience.28 With hospitalists playing an increasingly prominent role in housestaff education and patient satisfaction initiatives, the supervision of housestaff by trained hospitalist faculty may help meet both aims in the performance of invasive bedside procedures, particularly at institutions where simulation training resources are limited. Although concern may exist for potential patient anxiety with bedside teaching, our data demonstrate high levels of patient satisfaction with a hospitalist procedure service despite novice procedure performers and an emphasis on teaching during the procedure.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19(5 Pt 2):510–513.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: A firm‐based trial.J Hosp Med.2007;2(3):143–149.
- Hospital Care Quality Information from the Consumer Perspective (HCAHPS).Quality Assurance Guidelines.Baltimore, MD:Centers for Medicare 113(8):657–662.
- ,,,,.The effect of bedside case presentations on patients' perceptions of their medical care.N Engl J Med.1997;336(16):1150–1155.
- ,,,.‘Sorry, it's my first time!’ Will patients consent to medical students learning procedures?Med Educ.2005;39(4):365–369.
- ,.Ethical considerations surrounding first time procedures: a study and analysis of patient attitudes toward spinal taps by students.Kennedy Inst Ethics J.1992;2(3):217–231.
- ,,,.Patients' willingness to allow residents to learn to practice medical procedures.Acad Med.2004;79(2):144–147.
- ,,.Patient satisfaction with gynecologic care provided by family practice resident physicians.Fam Pract Res J.1991;11(4):421–428.
- ,,.Resident participation in flexible sigmoidoscopy does not affect patient satisfaction.Am J Gastroenterol.2000;95(6):1563–1566.
- ,.Bedside ultrasound for difficult lumbar puncture.J Emerg Med.2005;28(2):197–200.
- ,.Conducting the Assessment. In:Assessing Competence to Consent to Treatment: A Guide for Physicians and Other Health Professionals.First Edition ed.New York, NY:Oxford University Press;1998:80–91.
- ,.Care of Ill, Socially Complicated Patients. In:Medical Management of Vulnerable 2007:407–418.
- ,,,,.Interventional radiologic procedures: patient anxiety, perception of pain, understanding of procedure, and satisfaction with medication‐‐a prospective study.Radiology.2000;215(3):684–688.
- ,,,,.Improving the assessment of (in)patients' satisfaction with hospital care.Med Care.2001;39(3):270–283.
- ,,,.Factors determining inpatient satisfaction with care.Soc Sci Med.2002;54(4):493–504.
- ,,,.Reliability and validity of the Satisfaction with Hospital Care Questionnaire.Int J Qual Health Care.2002;14(6):471–482.
- ,,,,.A randomized trial of four patient satisfaction questionnaires.Med Care.2003;41(12):1343–1352.
- ,,, et al.Development and validation of an in‐patient satisfaction questionnaire.Int J Qual Health Care.2005;17(6):465–472.
- ,,,.Central venous port catheters: evaluation of patients' satisfaction with implantation under local anesthesia.J Vasc Access.2009;10(1):27–32.
- ,,,.Factors influencing patient satisfaction when undergoing endoscopic procedures.Gastrointest Endosc.2009;69(4):883–91, quiz 891.e1.
- ,,, et al.Complications associated with thoracentesis. A prospective, randomized study comparing three different methods.Arch Intern Med.1990;150(4):873–877.
- ,,, et al.Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study.Clin Gastroenterol Hepatol.2009;7(8):906–909.
- ,,, et al.Performance standards for therapeutic abdominal paracentesis.Hepatology.2004;40(2):484–488.
- ,,,,.Lumbar puncture: its indications, contraindications, complications and technique.Rev Neurol.2007;45(7):433–436.
- .How to perform a lumbar puncture with the patient in the seated position.Br J Hosp Med (Lond).2006;67(3):M46–7.
- ,,.Are commonly used resident measurements associated with procedural skills in internal medicine residency training?J Gen Intern Med.2007;22(3):357–361.
- ,,,MERN Group,.Supervising the Supervisors‐Procedural Training and Supervision in Internal Medicine Residency.J Gen Intern Med.2010.
- ,,,.Pneumothorax following thoracentesis: a systematic review and meta‐analysis.Arch Intern Med.2010;170(4):332–339.
- ,,.The emotional impact of ward rounds.J Mt Sinai Hosp NY.1956;23(6):782–803.
- ,,,.The physiologic and psychological effects of the bedside presentation.N Engl J Med.1989;321(18):1273–1275.
- ,,,,.The effect of bedside case presentations on patients' perceptions of their medical care.N Engl J Med.1997;336(16):1150–1155.
- .Hawthorne effects and research into professional practice.J Eval Clin Pract.2001;7(1):65–70.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19(5 Pt 2):510–513.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: A firm‐based trial.J Hosp Med.2007;2(3):143–149.
- Hospital Care Quality Information from the Consumer Perspective (HCAHPS).Quality Assurance Guidelines.Baltimore, MD:Centers for Medicare 113(8):657–662.
- ,,,,.The effect of bedside case presentations on patients' perceptions of their medical care.N Engl J Med.1997;336(16):1150–1155.
- ,,,.‘Sorry, it's my first time!’ Will patients consent to medical students learning procedures?Med Educ.2005;39(4):365–369.
- ,.Ethical considerations surrounding first time procedures: a study and analysis of patient attitudes toward spinal taps by students.Kennedy Inst Ethics J.1992;2(3):217–231.
- ,,,.Patients' willingness to allow residents to learn to practice medical procedures.Acad Med.2004;79(2):144–147.
- ,,.Patient satisfaction with gynecologic care provided by family practice resident physicians.Fam Pract Res J.1991;11(4):421–428.
- ,,.Resident participation in flexible sigmoidoscopy does not affect patient satisfaction.Am J Gastroenterol.2000;95(6):1563–1566.
- ,.Bedside ultrasound for difficult lumbar puncture.J Emerg Med.2005;28(2):197–200.
- ,.Conducting the Assessment. In:Assessing Competence to Consent to Treatment: A Guide for Physicians and Other Health Professionals.First Edition ed.New York, NY:Oxford University Press;1998:80–91.
- ,.Care of Ill, Socially Complicated Patients. In:Medical Management of Vulnerable 2007:407–418.
- ,,,,.Interventional radiologic procedures: patient anxiety, perception of pain, understanding of procedure, and satisfaction with medication‐‐a prospective study.Radiology.2000;215(3):684–688.
- ,,,,.Improving the assessment of (in)patients' satisfaction with hospital care.Med Care.2001;39(3):270–283.
- ,,,.Factors determining inpatient satisfaction with care.Soc Sci Med.2002;54(4):493–504.
- ,,,.Reliability and validity of the Satisfaction with Hospital Care Questionnaire.Int J Qual Health Care.2002;14(6):471–482.
- ,,,,.A randomized trial of four patient satisfaction questionnaires.Med Care.2003;41(12):1343–1352.
- ,,, et al.Development and validation of an in‐patient satisfaction questionnaire.Int J Qual Health Care.2005;17(6):465–472.
- ,,,.Central venous port catheters: evaluation of patients' satisfaction with implantation under local anesthesia.J Vasc Access.2009;10(1):27–32.
- ,,,.Factors influencing patient satisfaction when undergoing endoscopic procedures.Gastrointest Endosc.2009;69(4):883–91, quiz 891.e1.
- ,,, et al.Complications associated with thoracentesis. A prospective, randomized study comparing three different methods.Arch Intern Med.1990;150(4):873–877.
- ,,, et al.Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study.Clin Gastroenterol Hepatol.2009;7(8):906–909.
- ,,, et al.Performance standards for therapeutic abdominal paracentesis.Hepatology.2004;40(2):484–488.
- ,,,,.Lumbar puncture: its indications, contraindications, complications and technique.Rev Neurol.2007;45(7):433–436.
- .How to perform a lumbar puncture with the patient in the seated position.Br J Hosp Med (Lond).2006;67(3):M46–7.
- ,,.Are commonly used resident measurements associated with procedural skills in internal medicine residency training?J Gen Intern Med.2007;22(3):357–361.
- ,,,MERN Group,.Supervising the Supervisors‐Procedural Training and Supervision in Internal Medicine Residency.J Gen Intern Med.2010.
- ,,,.Pneumothorax following thoracentesis: a systematic review and meta‐analysis.Arch Intern Med.2010;170(4):332–339.
- ,,.The emotional impact of ward rounds.J Mt Sinai Hosp NY.1956;23(6):782–803.
- ,,,.The physiologic and psychological effects of the bedside presentation.N Engl J Med.1989;321(18):1273–1275.
- ,,,,.The effect of bedside case presentations on patients' perceptions of their medical care.N Engl J Med.1997;336(16):1150–1155.
- .Hawthorne effects and research into professional practice.J Eval Clin Pract.2001;7(1):65–70.
Copyright © 2011 Society of Hospital Medicine