User login
Why I like ham
Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary
Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary
Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary Why I like ham, by Gary
A New Year’s Resolution to Crack Down on Opioids?
Sometime early this year, after two years of controversy, the FDA might finally release its revised proposal for more stringent regulations on opioid pain relievers. The following are the key questions—and answers—surrounding new regulations, as well as a look at other recent FDA actions, including increased scrutiny of diabetes and obesity drugs due to heart concerns.
Question: What’s the latest on the FDA release of new regulations for prescription opioids?
Answer: First, the back story: The controversy concerns the creation of a Risk Evaluation and Mitigation Strategy (REMS) for opioid analgesics amid a troubling boom in prescription drug abuse, addiction, and overdose, and an accompanying spike in deaths from opioid overdoses. Under a 2007 law, the FDA can require drug and medical device manufacturers to adopt a REMS to ensure that the benefits outweigh the risks of continued use and that patients are adequately informed. In this case, the strategy could impact how nearly 4 million patients receive long-acting and extended-release opioids annually, according to FDA statistics.
In February 2009, the FDA sent a letter to manufacturers of opioid pain relievers, proposing a classwide REMS that would require certification by a physician or pharmacist and restrict distribution of the drugs, among other provisions. In response, many healthcare providers and medical organizations warned of an increased regulatory burden that would negatively impact patients. Among the concerns: fewer primary-care physicians (PCPs) willing to manage patients with chronic pain, reduced access to medically necessary drugs in underserved communities, undermedicated cancer patients, and increased use of other drugs with less stringent oversight.
In response, the FDA dropped its proposal for a prescriber accreditation program and another for a patient registry. But an FDA advisory panel soundly rejected a revised REMS proposal in July, by a vote of 25-10. The panel criticized the new plans as being too lax, lacking a formal requirement f
Q: What will the new REMS likely include?
A: Based on the criticisms of FDA panelists in July, the revised REMS could mandate a training requirement for all prescribers. The program would likely be created by the FDA and not by the drug industry, as had first been proposed (many doctors have agreed that a centralized, standardized program would be far preferable to dealing with programs set up independently by drug manufacturers). The strategy also might govern immediate-release opioids, in addition to extended-release and long-acting formulations. The FDA could propose linking doctor education to an existing Drug Enforcement Administration registration, but that would require congressional approval.
Q: What was the outcome of safety deliberations over the diabetes drug rosiglitazone (Avandia)?
A: After several years of concern over a heightened risk of heart attacks and other heart problems among patients taking rosiglitazone for type 2 diabetes mellitus, the FDA sharply restricted its availability in September and required the manufacturer, GlaxoSmithKline, to submit a REMS. When implemented, the REMS will limit new prescriptions to patients who cannot achieve glycemic control with other medications and decide not to take the alternative (pioglitazone) for medical reasons.1 Other diabetes drugs have faced similar scrutiny: In October, the FDA rejected an extended-release form of the diabetes drug exenatide (Bydureon) due to safety concerns, citing the need for more studies from manufacturer Eli Lilly about its effect on heart rate.
Q: What’s the upshot of recent FDA actions on weight-loss drugs and supplements?
A: The FDA also cracked down on weight-loss formulations in 2010, declining to approve several new drugs, overseeing the withdrawal of Abbott Laboratory’s Meridia in October, and announcing a December recall of capsules marketed online as Fruta Planta. Safety concerns over the latter two were linked to sibutramine, a drug that is “known to increase blood pressure and/or pulse rate in some patients and may present a serious risk for patients with a history of coronary artery disease, congestive heart failure, arrhythmias or stroke,” according to the FDA. Additionally, the drug can interact “in life-threatening ways” with other medications, reinforcing the idea that hospitalists and other doctors should delve into patients’ recent history of weight-loss drug or supplement use.
Q: What should we expect from the FDA in the next year?
A: Analysts will be keeping an eye on the number of new drugs approved in 2011 to discern any new trends. As reported by The Wall Street Journal and other publications, the FDA gave 21 drugs the go-ahead in 2010, down from 26 cleared in 2009 and 25 approved in 2008. The dip has concerned some drug industry representatives, who warn that an overly cautious approach could lead to more delays in new drugs reaching patients. Of course, the lower number also could reflect a continued dry spell in the pipelines of many pharmaceutical companies. On the flipside, consumer watchdogs have expressed optimism that the FDA might be devoting more attention to safety. In November, for example, the FDA oversaw the pulling of popular painkiller propoxyphene (marketed as Darvon and Darvocet) after evidence accumulated that the medication can cause potentially fatal heart damage and rhythm abnormalities.
Bryn Nelson is a freelance medical writer based in Seattle.
References
1. Woodcock J, Sharfstein JM, Hamburg M. Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. N Engl J Med. 2010;363(16):1489-1491.
Sometime early this year, after two years of controversy, the FDA might finally release its revised proposal for more stringent regulations on opioid pain relievers. The following are the key questions—and answers—surrounding new regulations, as well as a look at other recent FDA actions, including increased scrutiny of diabetes and obesity drugs due to heart concerns.
Question: What’s the latest on the FDA release of new regulations for prescription opioids?
Answer: First, the back story: The controversy concerns the creation of a Risk Evaluation and Mitigation Strategy (REMS) for opioid analgesics amid a troubling boom in prescription drug abuse, addiction, and overdose, and an accompanying spike in deaths from opioid overdoses. Under a 2007 law, the FDA can require drug and medical device manufacturers to adopt a REMS to ensure that the benefits outweigh the risks of continued use and that patients are adequately informed. In this case, the strategy could impact how nearly 4 million patients receive long-acting and extended-release opioids annually, according to FDA statistics.
In February 2009, the FDA sent a letter to manufacturers of opioid pain relievers, proposing a classwide REMS that would require certification by a physician or pharmacist and restrict distribution of the drugs, among other provisions. In response, many healthcare providers and medical organizations warned of an increased regulatory burden that would negatively impact patients. Among the concerns: fewer primary-care physicians (PCPs) willing to manage patients with chronic pain, reduced access to medically necessary drugs in underserved communities, undermedicated cancer patients, and increased use of other drugs with less stringent oversight.
In response, the FDA dropped its proposal for a prescriber accreditation program and another for a patient registry. But an FDA advisory panel soundly rejected a revised REMS proposal in July, by a vote of 25-10. The panel criticized the new plans as being too lax, lacking a formal requirement f
Q: What will the new REMS likely include?
A: Based on the criticisms of FDA panelists in July, the revised REMS could mandate a training requirement for all prescribers. The program would likely be created by the FDA and not by the drug industry, as had first been proposed (many doctors have agreed that a centralized, standardized program would be far preferable to dealing with programs set up independently by drug manufacturers). The strategy also might govern immediate-release opioids, in addition to extended-release and long-acting formulations. The FDA could propose linking doctor education to an existing Drug Enforcement Administration registration, but that would require congressional approval.
Q: What was the outcome of safety deliberations over the diabetes drug rosiglitazone (Avandia)?
A: After several years of concern over a heightened risk of heart attacks and other heart problems among patients taking rosiglitazone for type 2 diabetes mellitus, the FDA sharply restricted its availability in September and required the manufacturer, GlaxoSmithKline, to submit a REMS. When implemented, the REMS will limit new prescriptions to patients who cannot achieve glycemic control with other medications and decide not to take the alternative (pioglitazone) for medical reasons.1 Other diabetes drugs have faced similar scrutiny: In October, the FDA rejected an extended-release form of the diabetes drug exenatide (Bydureon) due to safety concerns, citing the need for more studies from manufacturer Eli Lilly about its effect on heart rate.
Q: What’s the upshot of recent FDA actions on weight-loss drugs and supplements?
A: The FDA also cracked down on weight-loss formulations in 2010, declining to approve several new drugs, overseeing the withdrawal of Abbott Laboratory’s Meridia in October, and announcing a December recall of capsules marketed online as Fruta Planta. Safety concerns over the latter two were linked to sibutramine, a drug that is “known to increase blood pressure and/or pulse rate in some patients and may present a serious risk for patients with a history of coronary artery disease, congestive heart failure, arrhythmias or stroke,” according to the FDA. Additionally, the drug can interact “in life-threatening ways” with other medications, reinforcing the idea that hospitalists and other doctors should delve into patients’ recent history of weight-loss drug or supplement use.
Q: What should we expect from the FDA in the next year?
A: Analysts will be keeping an eye on the number of new drugs approved in 2011 to discern any new trends. As reported by The Wall Street Journal and other publications, the FDA gave 21 drugs the go-ahead in 2010, down from 26 cleared in 2009 and 25 approved in 2008. The dip has concerned some drug industry representatives, who warn that an overly cautious approach could lead to more delays in new drugs reaching patients. Of course, the lower number also could reflect a continued dry spell in the pipelines of many pharmaceutical companies. On the flipside, consumer watchdogs have expressed optimism that the FDA might be devoting more attention to safety. In November, for example, the FDA oversaw the pulling of popular painkiller propoxyphene (marketed as Darvon and Darvocet) after evidence accumulated that the medication can cause potentially fatal heart damage and rhythm abnormalities.
Bryn Nelson is a freelance medical writer based in Seattle.
References
1. Woodcock J, Sharfstein JM, Hamburg M. Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. N Engl J Med. 2010;363(16):1489-1491.
Sometime early this year, after two years of controversy, the FDA might finally release its revised proposal for more stringent regulations on opioid pain relievers. The following are the key questions—and answers—surrounding new regulations, as well as a look at other recent FDA actions, including increased scrutiny of diabetes and obesity drugs due to heart concerns.
Question: What’s the latest on the FDA release of new regulations for prescription opioids?
Answer: First, the back story: The controversy concerns the creation of a Risk Evaluation and Mitigation Strategy (REMS) for opioid analgesics amid a troubling boom in prescription drug abuse, addiction, and overdose, and an accompanying spike in deaths from opioid overdoses. Under a 2007 law, the FDA can require drug and medical device manufacturers to adopt a REMS to ensure that the benefits outweigh the risks of continued use and that patients are adequately informed. In this case, the strategy could impact how nearly 4 million patients receive long-acting and extended-release opioids annually, according to FDA statistics.
In February 2009, the FDA sent a letter to manufacturers of opioid pain relievers, proposing a classwide REMS that would require certification by a physician or pharmacist and restrict distribution of the drugs, among other provisions. In response, many healthcare providers and medical organizations warned of an increased regulatory burden that would negatively impact patients. Among the concerns: fewer primary-care physicians (PCPs) willing to manage patients with chronic pain, reduced access to medically necessary drugs in underserved communities, undermedicated cancer patients, and increased use of other drugs with less stringent oversight.
In response, the FDA dropped its proposal for a prescriber accreditation program and another for a patient registry. But an FDA advisory panel soundly rejected a revised REMS proposal in July, by a vote of 25-10. The panel criticized the new plans as being too lax, lacking a formal requirement f
Q: What will the new REMS likely include?
A: Based on the criticisms of FDA panelists in July, the revised REMS could mandate a training requirement for all prescribers. The program would likely be created by the FDA and not by the drug industry, as had first been proposed (many doctors have agreed that a centralized, standardized program would be far preferable to dealing with programs set up independently by drug manufacturers). The strategy also might govern immediate-release opioids, in addition to extended-release and long-acting formulations. The FDA could propose linking doctor education to an existing Drug Enforcement Administration registration, but that would require congressional approval.
Q: What was the outcome of safety deliberations over the diabetes drug rosiglitazone (Avandia)?
A: After several years of concern over a heightened risk of heart attacks and other heart problems among patients taking rosiglitazone for type 2 diabetes mellitus, the FDA sharply restricted its availability in September and required the manufacturer, GlaxoSmithKline, to submit a REMS. When implemented, the REMS will limit new prescriptions to patients who cannot achieve glycemic control with other medications and decide not to take the alternative (pioglitazone) for medical reasons.1 Other diabetes drugs have faced similar scrutiny: In October, the FDA rejected an extended-release form of the diabetes drug exenatide (Bydureon) due to safety concerns, citing the need for more studies from manufacturer Eli Lilly about its effect on heart rate.
Q: What’s the upshot of recent FDA actions on weight-loss drugs and supplements?
A: The FDA also cracked down on weight-loss formulations in 2010, declining to approve several new drugs, overseeing the withdrawal of Abbott Laboratory’s Meridia in October, and announcing a December recall of capsules marketed online as Fruta Planta. Safety concerns over the latter two were linked to sibutramine, a drug that is “known to increase blood pressure and/or pulse rate in some patients and may present a serious risk for patients with a history of coronary artery disease, congestive heart failure, arrhythmias or stroke,” according to the FDA. Additionally, the drug can interact “in life-threatening ways” with other medications, reinforcing the idea that hospitalists and other doctors should delve into patients’ recent history of weight-loss drug or supplement use.
Q: What should we expect from the FDA in the next year?
A: Analysts will be keeping an eye on the number of new drugs approved in 2011 to discern any new trends. As reported by The Wall Street Journal and other publications, the FDA gave 21 drugs the go-ahead in 2010, down from 26 cleared in 2009 and 25 approved in 2008. The dip has concerned some drug industry representatives, who warn that an overly cautious approach could lead to more delays in new drugs reaching patients. Of course, the lower number also could reflect a continued dry spell in the pipelines of many pharmaceutical companies. On the flipside, consumer watchdogs have expressed optimism that the FDA might be devoting more attention to safety. In November, for example, the FDA oversaw the pulling of popular painkiller propoxyphene (marketed as Darvon and Darvocet) after evidence accumulated that the medication can cause potentially fatal heart damage and rhythm abnormalities.
Bryn Nelson is a freelance medical writer based in Seattle.
References
1. Woodcock J, Sharfstein JM, Hamburg M. Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. N Engl J Med. 2010;363(16):1489-1491.
Hospitalist Mentoring Lacking, Survey Shows
Academic HM group leaders are concerned about the lack of mentorship their physicians receive and often feel viewed as a clinical service, not a pedagogical program, according to a report in this month's Journal of Hospital Medicine.
The cross-sectional e-mail survey of 57 leaders found that respondents agree a lack of mentorship is a worry for both clinician-educator faculty (75%) and research faculty (58%). Six in 10 of those surveyed say their departments of medicine view them through more of a clinical lens, with that number rising to nearly 8 in 10 when the perceived views of other departments are taken into account.
"The division chiefs, the section chiefs have to pay attention to mentoring; they have to pay attention to faculty development, they have to really understand the needs of their people," says study coauthor Rebecca Harrison, MD, FACP, a hospitalist at Oregon Health & Science University in Portland, Ore.
The report is based on a 2007 survey. Dr. Harrison says that as budgets became "more dire" during the economic downturn, academic HM leaders likely grew more frustrated by a perceived lack of resources committed to them.
She suggests that academic leaders look more to the negotiating tactics of their private-physician counterparts, who seek to leverage their involvement in quality programs and their return on investment when pushing for more support, respect, or resources.
Dr. Harrison adds that given that recruitment costs can range up to $400,000 for clinician-educator faculty, hospital executives "need to see the big picture. Hospital medicine is here to say. It's to their advantage; it behooves them not to avoid investing in sustainability."
Academic HM group leaders are concerned about the lack of mentorship their physicians receive and often feel viewed as a clinical service, not a pedagogical program, according to a report in this month's Journal of Hospital Medicine.
The cross-sectional e-mail survey of 57 leaders found that respondents agree a lack of mentorship is a worry for both clinician-educator faculty (75%) and research faculty (58%). Six in 10 of those surveyed say their departments of medicine view them through more of a clinical lens, with that number rising to nearly 8 in 10 when the perceived views of other departments are taken into account.
"The division chiefs, the section chiefs have to pay attention to mentoring; they have to pay attention to faculty development, they have to really understand the needs of their people," says study coauthor Rebecca Harrison, MD, FACP, a hospitalist at Oregon Health & Science University in Portland, Ore.
The report is based on a 2007 survey. Dr. Harrison says that as budgets became "more dire" during the economic downturn, academic HM leaders likely grew more frustrated by a perceived lack of resources committed to them.
She suggests that academic leaders look more to the negotiating tactics of their private-physician counterparts, who seek to leverage their involvement in quality programs and their return on investment when pushing for more support, respect, or resources.
Dr. Harrison adds that given that recruitment costs can range up to $400,000 for clinician-educator faculty, hospital executives "need to see the big picture. Hospital medicine is here to say. It's to their advantage; it behooves them not to avoid investing in sustainability."
Academic HM group leaders are concerned about the lack of mentorship their physicians receive and often feel viewed as a clinical service, not a pedagogical program, according to a report in this month's Journal of Hospital Medicine.
The cross-sectional e-mail survey of 57 leaders found that respondents agree a lack of mentorship is a worry for both clinician-educator faculty (75%) and research faculty (58%). Six in 10 of those surveyed say their departments of medicine view them through more of a clinical lens, with that number rising to nearly 8 in 10 when the perceived views of other departments are taken into account.
"The division chiefs, the section chiefs have to pay attention to mentoring; they have to pay attention to faculty development, they have to really understand the needs of their people," says study coauthor Rebecca Harrison, MD, FACP, a hospitalist at Oregon Health & Science University in Portland, Ore.
The report is based on a 2007 survey. Dr. Harrison says that as budgets became "more dire" during the economic downturn, academic HM leaders likely grew more frustrated by a perceived lack of resources committed to them.
She suggests that academic leaders look more to the negotiating tactics of their private-physician counterparts, who seek to leverage their involvement in quality programs and their return on investment when pushing for more support, respect, or resources.
Dr. Harrison adds that given that recruitment costs can range up to $400,000 for clinician-educator faculty, hospital executives "need to see the big picture. Hospital medicine is here to say. It's to their advantage; it behooves them not to avoid investing in sustainability."
In the Literature: Research You Need to Know
Clinical question: Does full disclosure of medical errors increase or decrease liability and claims?
Background: The University of Michigan Health System (UMHS) actively seeks out medical errors, fully discloses these errors to patients, and offers compensation when at fault. Full disclosure is consistent with patient safety and ethical principles, but whether such programs exacerbate total liability costs remains controversial amongst physicians and malpractice professionals.
Study design: Retrospective before-and-after analysis from 1995 to 2007.
Setting: Public academic medical center and health system.
Synopsis: After the full implementation of a disclosure to offer program in 2001 at UMHS, the average monthly rate of new claims decreased to 4.52 per 100,000 patient encounters from 7.03 per 100,000 patient encounters. Median time to resolution of each claim also decreased to 0.95 years from 1.36 years. Average total liability monthly costs per month for every $1,000 of operating revenue dropped to $4.00 from $8.48 (p<.001), mostly driven by decreased costs for patient compensation and legal costs.
The observation that total liability costs decrease with full disclosure does not prove causality, as the number of reported claims throughout Michigan decreased from 2001 to 2007, and state wide malpractice reform was initiated in 1994. A comparison with 20 physician insurers, however, suggests quicker resolution times and decreasing legal and compensation costs of UMHS compared with its peers. Moreover, the Veterans Affairs Medical Center in Lexington, Ky., also had success after initiation of a similar disclosure-with-offer program, suggesting that the experience of such programs might be generalized to other settings.
Bottom line: Full disclosure of medical errors with offers of compensation is associated with decreased total claims and liability costs.
Citation: Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010:153(4):213-221.
Reviewed for TH eWire by Jill Goldenberg, MD, Alan Briones, MD, Chad Craig, MD, Ramiro Jervis, MD, FHM, Brian Markoff, MD, FHM, Andrew Dunn, MD, FACP, FHM, Division of Hospital Medicine, Mount Sinai School of Medicine, New York City
For more physician reviews of HM-related research, visit our website.
Clinical question: Does full disclosure of medical errors increase or decrease liability and claims?
Background: The University of Michigan Health System (UMHS) actively seeks out medical errors, fully discloses these errors to patients, and offers compensation when at fault. Full disclosure is consistent with patient safety and ethical principles, but whether such programs exacerbate total liability costs remains controversial amongst physicians and malpractice professionals.
Study design: Retrospective before-and-after analysis from 1995 to 2007.
Setting: Public academic medical center and health system.
Synopsis: After the full implementation of a disclosure to offer program in 2001 at UMHS, the average monthly rate of new claims decreased to 4.52 per 100,000 patient encounters from 7.03 per 100,000 patient encounters. Median time to resolution of each claim also decreased to 0.95 years from 1.36 years. Average total liability monthly costs per month for every $1,000 of operating revenue dropped to $4.00 from $8.48 (p<.001), mostly driven by decreased costs for patient compensation and legal costs.
The observation that total liability costs decrease with full disclosure does not prove causality, as the number of reported claims throughout Michigan decreased from 2001 to 2007, and state wide malpractice reform was initiated in 1994. A comparison with 20 physician insurers, however, suggests quicker resolution times and decreasing legal and compensation costs of UMHS compared with its peers. Moreover, the Veterans Affairs Medical Center in Lexington, Ky., also had success after initiation of a similar disclosure-with-offer program, suggesting that the experience of such programs might be generalized to other settings.
Bottom line: Full disclosure of medical errors with offers of compensation is associated with decreased total claims and liability costs.
Citation: Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010:153(4):213-221.
Reviewed for TH eWire by Jill Goldenberg, MD, Alan Briones, MD, Chad Craig, MD, Ramiro Jervis, MD, FHM, Brian Markoff, MD, FHM, Andrew Dunn, MD, FACP, FHM, Division of Hospital Medicine, Mount Sinai School of Medicine, New York City
For more physician reviews of HM-related research, visit our website.
Clinical question: Does full disclosure of medical errors increase or decrease liability and claims?
Background: The University of Michigan Health System (UMHS) actively seeks out medical errors, fully discloses these errors to patients, and offers compensation when at fault. Full disclosure is consistent with patient safety and ethical principles, but whether such programs exacerbate total liability costs remains controversial amongst physicians and malpractice professionals.
Study design: Retrospective before-and-after analysis from 1995 to 2007.
Setting: Public academic medical center and health system.
Synopsis: After the full implementation of a disclosure to offer program in 2001 at UMHS, the average monthly rate of new claims decreased to 4.52 per 100,000 patient encounters from 7.03 per 100,000 patient encounters. Median time to resolution of each claim also decreased to 0.95 years from 1.36 years. Average total liability monthly costs per month for every $1,000 of operating revenue dropped to $4.00 from $8.48 (p<.001), mostly driven by decreased costs for patient compensation and legal costs.
The observation that total liability costs decrease with full disclosure does not prove causality, as the number of reported claims throughout Michigan decreased from 2001 to 2007, and state wide malpractice reform was initiated in 1994. A comparison with 20 physician insurers, however, suggests quicker resolution times and decreasing legal and compensation costs of UMHS compared with its peers. Moreover, the Veterans Affairs Medical Center in Lexington, Ky., also had success after initiation of a similar disclosure-with-offer program, suggesting that the experience of such programs might be generalized to other settings.
Bottom line: Full disclosure of medical errors with offers of compensation is associated with decreased total claims and liability costs.
Citation: Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010:153(4):213-221.
Reviewed for TH eWire by Jill Goldenberg, MD, Alan Briones, MD, Chad Craig, MD, Ramiro Jervis, MD, FHM, Brian Markoff, MD, FHM, Andrew Dunn, MD, FACP, FHM, Division of Hospital Medicine, Mount Sinai School of Medicine, New York City
For more physician reviews of HM-related research, visit our website.
Determining Efficacy of Antibacterial Hand Wash Products
Determining Efficacy of Antibacterial Hand Wash Products
This promotional supplement was supported by American Cleaning Institute® and the Personal Care Products Council.
•Topics
•Faculty/Faculty Disclosures
To view the supplement, click the image above.
- 1994 FDA-Proposed Antiseptic Hand Wash Efficacy Criteria
- Need for Link Between Bacterial Log Reduction and Disease
- New Model for Efficacy Evaluation of Antibacterial Hand Washes
- New Scientific Model Links Bacterial Reduction with Reduction in Disease
- Expert Panel Review of the New Model for Efficacy Evaluation
- Expert Panel Consensus Statement
- Expanding the Scope of the New Scientific Model to Other Health Care Settings
- Moving Forward
Francis H. Kruszewski, PhD, DABT
Director, Human Health and Safety
American Cleaning Institute® (formerly the Soap and Detergent Association)
Washington, DC
Francis H. Kruszewski, PhD, DABT is employed by American Cleaning Institute® (formerly the Soap and Detergent Association)
John F. Krowka, PhD
Senior Environmental Scientist
The Personal Care Products Council
Washington, DC
John F. Krowka, PhD is employed by The Personal Care Products Council
Copyright © 2011 Elsevier Inc.
Determining Efficacy of Antibacterial Hand Wash Products
This promotional supplement was supported by American Cleaning Institute® and the Personal Care Products Council.
•Topics
•Faculty/Faculty Disclosures
To view the supplement, click the image above.
- 1994 FDA-Proposed Antiseptic Hand Wash Efficacy Criteria
- Need for Link Between Bacterial Log Reduction and Disease
- New Model for Efficacy Evaluation of Antibacterial Hand Washes
- New Scientific Model Links Bacterial Reduction with Reduction in Disease
- Expert Panel Review of the New Model for Efficacy Evaluation
- Expert Panel Consensus Statement
- Expanding the Scope of the New Scientific Model to Other Health Care Settings
- Moving Forward
Francis H. Kruszewski, PhD, DABT
Director, Human Health and Safety
American Cleaning Institute® (formerly the Soap and Detergent Association)
Washington, DC
Francis H. Kruszewski, PhD, DABT is employed by American Cleaning Institute® (formerly the Soap and Detergent Association)
John F. Krowka, PhD
Senior Environmental Scientist
The Personal Care Products Council
Washington, DC
John F. Krowka, PhD is employed by The Personal Care Products Council
Copyright © 2011 Elsevier Inc.
Determining Efficacy of Antibacterial Hand Wash Products
This promotional supplement was supported by American Cleaning Institute® and the Personal Care Products Council.
•Topics
•Faculty/Faculty Disclosures
To view the supplement, click the image above.
- 1994 FDA-Proposed Antiseptic Hand Wash Efficacy Criteria
- Need for Link Between Bacterial Log Reduction and Disease
- New Model for Efficacy Evaluation of Antibacterial Hand Washes
- New Scientific Model Links Bacterial Reduction with Reduction in Disease
- Expert Panel Review of the New Model for Efficacy Evaluation
- Expert Panel Consensus Statement
- Expanding the Scope of the New Scientific Model to Other Health Care Settings
- Moving Forward
Francis H. Kruszewski, PhD, DABT
Director, Human Health and Safety
American Cleaning Institute® (formerly the Soap and Detergent Association)
Washington, DC
Francis H. Kruszewski, PhD, DABT is employed by American Cleaning Institute® (formerly the Soap and Detergent Association)
John F. Krowka, PhD
Senior Environmental Scientist
The Personal Care Products Council
Washington, DC
John F. Krowka, PhD is employed by The Personal Care Products Council
Copyright © 2011 Elsevier Inc.
Survey of Academic Hospitalist Leaders
Hospitalists are hospital‐based physicians whose primary professional focus is patient care, education, research, and administrative activities related to hospital medicine.1 Initially, community‐based hospitals were far more likely to employ hospitalists than academic centers. However, today most academic centers employ hospitalist models and it is now a fully recognized entity in academic settings.2
While much has been written about the structure, business operations, and potential benefits of nonteaching (clinical) hospitalist programs,3, 4 there is little known about the current state of academic hospitalist programs or their challenges. For example, who are the leaders of academic hospitalist medicine groups? Given the youth of the field, are academic hospitalists receiving adequate mentorship and are they advancing academically? What are future directions and goals for academic hospitalist groups?
To better understand academic hospitalist programs, we surveyed division chiefs and academic hospitalist leaders to explore existing business models and operations, the status of mentorship, and key issues in growth and retention.
Methods
Sites and Subjects
We targeted potential hospital medicine group leaders by identifying academic medical centers using Association of American Medical Colleges (AAMC), the Accreditation Council for Graduate Medical Education (ACGME), the Association of Chiefs of General Internal Medicine (ACGIM), and the Society of Hospital Medicine (SHM) lists of sites with teaching missions. We then used publicly available data (eg, from websites maintained by the sites) to identify physician leaders who: 1) self identified as a leader of a hospitalist group at an academic medical center (or a Chief of Division of General Internal Medicine which managed a hospitalist group) in the SHM database, 2) were listed as such on the website, or 3) were members of ACGIM and listed as a hospitalist group leader at a university based medical center.
Survey Development
Our survey was based on questions used in previous research by the authors,5 with additional questions regarding operations of academic hospitalist programs, growth and retention of hospitalists, and mentorship developed by the study authors. Questions were pretested among a selected group of members of the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force and the SHM Academic Hospitalist Interest Group, after which the survey was refined and converted into its electronic form.
Survey Methods
The email survey process began in April 2007 with an initial survey sent to those physicians identified using preexisting data, as described. Our survey asked first if recipients were directly responsible for the oversight of a hospitalist group (eg, the division chief or director of the hospital medicine group) and if they practiced at an academic medical center. Only respondents who answered yes to both of these criteria were invited to respond to our survey. Those who felt the survey did not apply to them were invited to forward the email survey on to the appropriate person at their site or respond that their hospital had no hospital medicine service. Subsequent reminder emails were sent to nonrespondents at 10‐day intervals up to a total of four times. This survey was granted exempt status from the UCSF Institutional Review Board.
Statistical Methods
Response rates and frequencies and distribution of survey responses were analyzed using univariable statistics.
Results
Characteristics of Responding Sites
We received responses from 57 (40%) of the academic sites identified as having an academic hospital medicine group. Hospitalist group leaders at responding sites had been in their current position 3.8 years, graduated medical school approximately 15 years prior, and were either Assistant (40%), Associate (32%), or Full Professors (23%). Group leaders reported that the vast majority (91%) of group full‐time members were in junior faculty positions (Instructor or Assistant Professor), who were working full‐time. On average, responding programs were 6 years old (formed in 2001) and currently had 10.0 total full time equivalents (FTEs). A total of 38 of the groups (67%) were part of the larger Division of General Internal Medicine, whereas 9 groups (16 %) were their own division within the Department of Medicine. The remaining 17% were part of another division.
Mentorship Practices In Academic Hospital Medicine Groups
As one mechanism of mentorship, annual performance reviews were offered in most programs (88%). These were usually performed by the general medicine division chief or hospitalist leader. Mentoring relationships for clinician investigators (CI) were most often from personnel outside the hospitalist group, whereas clinician‐educators (CE) most often were mentored by faculty inside the group.
Hospitalist Leaders' Priorities and Impressions of Growth, Opportunities, Career Development and Barriers
Hospitalist leaders reported the highest priorities for hospitalist leaders were developing research and teaching programs, and minimizing turnover. Other priorities included achieving financial stability, applying for extramural funding, and reducing clinical workload (Table 2). Only 14% of respondents noted that becoming a separate division was a priority.0
| Characteristic | n (%) |
|---|---|
| |
| Group leader characteristics | |
| Academic rank | |
| Assistant professor/other | 26 (45) |
| Associate professor | 18 (32) |
| Full professor | 13 (23) |
| Years in position (mean, range) | 3.8 (2.07.0) |
| Group characteristics | |
| Hospital medicine place in school of medicine | |
| Within the department of medicine | 55 (98) |
| Separate division | 9 (16) |
| Within division of general medicine | 38 (67) |
| Other | 9 (16) |
| Not in the department of medicine | 1 (2) |
| Program size (mean, range) | |
| Number of hospitalists in program | 10 (718) |
| Number of FTE | 11 (3.512) |
| FTE's hired in past 2 years (July 2005 to survey date) | 4.0 (2.27.0) |
| Hospitalist activities | |
| Medicine consultation | 52 (91) |
| Quality improvement projects | 52 (91) |
| Nonteaching attending | 44 (77) |
| Comanagement of surgical patients | 44 (77) |
| 24‐hour coverage | 24 (61) |
| Manage patient transfer requests | 32 (56) |
| Peer review/morbidity and mortality | 31 (54) |
| Education program leadership | 29 (51) |
| Medical student program leadership | 29 (51) |
| Palliative care program | 23 (40) |
| Preoperative clinic | 23 (40) |
| Emergency department triage | 14 (25) |
| Post discharge follow‐up clinic | 13 (23) |
| Skill nursing facility coverage | 4 (7) |
| Other | 15 (26) |
| Mentorship Activity | n (%) |
|---|---|
| Programs performing annual reviews with faculty | 50 (88) |
| Who performs the annual review? | |
| General Medicine Division Chief | 9 (18) |
| Hospitalist leader | 18 (36) |
| Both | 13 (26) |
| Other (eg, Department Chair, Chief Medical Officer) | 10 (20) |
| Who is the primary source of mentorship for clinician‐educators? | |
| Senior faculty within the group | 43 (77) |
| Generalist faculty outside the group, but within the institution | 6 (11) |
| Subspecialty Internal Medicine faculty outside the group, but within the institution | 3 (5) |
| Non‐Internal Medicine (eg, surgeon, epidemiologist) outside the group, but within the institution | 0 (0) |
| Faculty from another institution | 0 (0) |
| Don't know | 4 (7) |
| Who is the primary source of mentorship for clinician‐investigators? | |
| Senior faculty within the group | 6 (12) |
| Generalist faculty outside the group, but within the institution | 13 (25) |
| Subspecialty internal medicine faculty outside the group, but within the institution | 6 (12) |
| Non‐Internal Medicine (eg, surgeon, epidemiologist) outside the group, but within the institution | 2 (4) |
| Faculty from another institution | 3 (6) |
| Don't know | 2 (4) |
| Not applicable; no clinician investigators | 20 (38) |
| Highest Priority, n (%) | Intermediate Priority, n (%) | Lowest Priority, n (%) | Not a Priority, n (%) | NA, n (%) | |
|---|---|---|---|---|---|
| Reducing individual faculty clinical workload | 9 (16) | 22 (3) | 11 (2) | 14 (2) | 0 (0) |
| Achieving financial stability | 13 (24) | 30 (55) | 6 (11) | 6 (11) | 0 (0) |
| Minimizing turnover | 22 (39) | 27 (48) | 6 (11) | 1 (2) | 0 (0) |
| Developing teaching programs | 22 (39) | 29 (52) | 3 (5) | 2 (4) | 0 (0) |
| Becoming a separate division | 3 (5) | 5 (9) | 11 (20) | 23 (41) | 14 (25) |
| Developing research | 25 (45) | 18 (32) | 5 (9) | 6 (11) | 2 (4) |
| Applying for extramural funding | 10 (18) | 24 (43) | 10 (18) | 8 (14) | 4 (7) |
| Strongly Agree, n (%) | Agree, n (%) | Neutral, n (%) | Disagree, n (%) | Strongly Disagree, n (%) | NA, n (%) | |
|---|---|---|---|---|---|---|
| Growth and sustainability | ||||||
| Availability of funds is limiting expansion of academic functions (eg, education and research) | 20 (36) | 21 (38) | 5 (9) | 7 (12) | 3 (5) | 0 (0) |
| Availability of funds is limiting expansion of clinical functions (eg, development of new services) | 11 (20) | 17 (30) | 14 (25) | 10 (18) | 4 (7) | 0 (0) |
| My faculty are developing sustainable nonclinical activities | 9 (16) | 23 (41) | 12 (21) | 9 (16) | 3 (5) | 0 (0) |
| Career development | ||||||
| Mentorship is a major issue for my clinician‐educator faculty | 14 (25) | 28 (50) | 7 (12) | 4 (7) | 1 (2) | 2 (4) |
| Mentorship is a major issue for my research faculty | 22 (40) | 10 (18) | 4 (7) | 3 (5) | 2 (4) | 14 (25) |
| External support for hospital medicine group | ||||||
| There is investment in the development of academic functions of our hospitalist program from my hospital | 4 (7) | 12 (21) | 10 (18) | 22 (39) | 8 (14) | 0 (0) |
| There is investment in the development of academic functions of our hospitalist program from the Department of Medicine | 22 (40) | 17 (31) | 8 (15) | 4 (7) | 2 (4) | 2 (4) |
In general, academic hospitalist leaders reported that Departments of Medicine and Divisions of General Medicine (where applicable) were invested in the development of their academic functions. Yet, more than half of program directors reported that hospitals were not supportive. Moreover, lack of funds limited the expansion of their academic or clinical functions (Table 3). Additionally, while the majority either strongly agree or agree that their faculty are developing sustainable nonclinical activities (57%), they perceive that they are at risk for burnout (69%), and that lack of mentorship is a major issue for both CE (75%) and research faculty (58%). Lastly, while program directors strongly agree or agree (71%) that their hospitalist groups are respected by other academic physicians, they additionally strongly agree or agree that their Departments of Medicine (58%) and other Divisions (78%) view their hospitalist program as a clinical service rather than an academic program.
Discussion
Our survey provides a unique snapshot of academic hospitalist groups, highlighting a perceived lack of support and respect for their programs, a need to increase education and scholarly activities, and a desire to better prepare faculty for academic promotion.
Academic hospitalist groups and leaders reflected what one would expect from a field that is just over a decade old. Program leaders were relatively new to their position, as were their division group members. As a result, it is not surprising that most of the academic hospitalist leaders identified mentorship as a major issue. We were encouraged to see that most programs were offering annual reviews. However, the majority of these annual reviews were performed by the group leaders, many of whom are relatively junior (40% Assistant Professors) and may not be experienced in mentoring and performing annual reviews. Importantly, the absence of a mentor (or a high‐quality, experienced one) among physicians, and specifically hospitalists, may result in fewer peer‐reviewed first author and non‐peer‐reviewed publications, and less experience leading a teaching session at a national meeting.6 Research suggests that effective mentoring may help faculty increase career satisfaction and productivity and reduce their risk for burn‐out.7 Hospitalist groups might benefit nationally from focusing specifically on finding adequate mentorship either within or outside their groups. In addition, national organizations such as the SHM and the SGIM could potentially help these groups and individual hospitalists in creating mentorship networks and a mentoring infrastructure.
Academic hospitalist leaders were concerned about the ability of their faculty to develop sustainable nonclinical activities and scholarship. Notably, more than 40% of surveyed leaders agreed or strongly agreed that their faculty were not developing sustainable nonclinical activities. For individual faculty, the inability to develop scholarly activities and engage in academic pursuits may create challenges in getting promoted by traditional academic pathways. Some have recognized this issue and tried to develop practical solutions.2 In addition, academic hospitalists often engage in nonclinical activities such as quality improvement or patient safety which do not fit in the traditional tripartite mission of academics (clinical care, education, and research). In this survey, more than 90% of groups were engaged in quality improvement projects and over half in peer review exercises (Table 1). As many of these scholarly activities require innovation, sophisticated data analysis, and can have far‐reaching and substantial impacts on healthcare, some have argued these should be considered as part of the promotion process.8 Notably, the SGIM Academic Hospitalist Taskforce has created the Quality Portfolio, a structured adjunct to promotions packets to organize and document work in quality improvement and patient safety.9
While there were few CI in the divisions surveyed, building CI programs was a major priority of programs. In programs reporting the presence of CI's, they report limited access to research support. This highlights the potential role and benefit of post residency training in designing and conducting clinical research whether in a traditional general internal medicine fellowship or in 1 of the many growing hospital medicine fellowships.10 There also appears to be a need for funding to support the research careers of junior hospitalists. While access to effective mentorship is integrally linked to achieving increased academic accomplishments, there is certainly an ample call for research in the areas of quality improvement, patient safety; systems‐based practice, hospital efficiency, transitions of care,11 perioperative medicine,12 and education.2, 13, 14 While providing lower costs per admission and lower lengths of stay, hospitalists seem well‐positioned to spearhead active research in cost‐effectiveness in the hospital.14 Additionally, a quality portfolio, documenting such quality improvement projects, has been suggested as an effective means to provide a record of this work for academic promotion.9
The diverse activities of today's hospitalists are transforming the traditional view of academic work and are critical to the growth of hospitals, patient care, and development of the field of hospital medicine itself. Until these areas are fully embraced as legitimate areas of academic productivity and scholarship, the academic advancement of hospitalists will be slow.
It is unclear from our survey if academic hospitalist programs are truly getting the support they need to succeed. On one hand, there was general agreement that the Departments of Medicine and Divisions of General and Hospital Medicine were invested in the development of the academic accomplishments. Yet, the majority of program directors believed that they are viewed by the Department or Division as a clinical rather than an academic program. Moreover, over half of program directors report that their hospital was not supportive and therefore have limited the expansion of their hospitalist groups' educational and research activities. Lastly, for a large majority of programs, unavailable funding also acted to limit growth and expansion of academic functions. In a mere 2 decades, Emergency Medicine has become one of the largest US specialties and yet research and funding in the field have been lagging and are limiting academic expansion. Junior faculty seeking research careers struggled to find support and mentorship within their emergency medicine divisions.15 Challenges faced by academic emergency medicine provide important historical perspective for the even more rapidly growing field of academic hospital medicine. Learning from the Academic Emergency Medicine experience, academic hospitalists should proactively identify scholarship and research opportunities unique to hospitalist and fitting the needs of academic institutions. Involvement in national medical organizations, such as SGIM‐SHM‐ACGIM Academic Hospitalist Academy, or the SGIM Academic Hospitalist Task Force, where skill and career development is the focus, will undoubtedly promote the success of academic hospitalist. Expanding valuable niches of expertise, such as quality control, perioperative medicine and care transitions, create an indispensible component of hospital care. Lastly mentoring programs for academic hospitalist within SHM and SGIM are also essential for networking and career development. There are several limitations to our study. Our response rate of 40% was relatively low, and our results may not be representative of all academic hospitalist division chiefs and their programs, may be overstating the perceived difficulties of the survey sample, or conversely missing a large portion too overwhelmed by current duties who lacked the time to complete the survey. Having said this, our survey methodology targeted sites where we could identify potentialnot confirmedhospitalist groups and hospitalist group leaders. For this reason, our response rate could be higher (if some of our contacts were in error). Our results are a cross‐sectional survey based on self report and are subject to recall bias. In addition, our study was carried out in 2007, and while issues such as mentorship may remain important, our results regarding financial arrangements may not be applicable to the current economic climate. Finally, while improving mentorship was identified as a principle objective for program leaders, we did not explore the existing quality of mentorship, nor perceived shortfalls. This should be the subject of future exploration.
The vast majority of academic hospital medicine programs continue to view inadequate support, expanding research, mentorship, and academic promotion as critical issues for the future. Thus, further understanding of these features, and interventions to allow for success, are of crucial importance in the continued development of academic hospitalists. Our study supports the need for mentoring and career development programs, targeting academic hospitalists and their leaders. In addition, attention should be paid to activities that support career fit, creating sustainable and viable job descriptions for academic hospitalists, and preventing burnout.16 At the same time we must expand the traditional view of scholarship and training and advocate for promotion criteria that value the unique contributions of hospitalists to become in line with the broad areas that hospitalists work.
- Anonymous. Definition of a hospitalist.2009. Society of Hospital Medicine Homepage/General Information. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Hospitalist_Definition4:240–246.
- ,,,,.Resource‐based relative value scale analysis between teaching and nonteaching hospitalist services.Health Care Management.2009;1(28):81–85.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service.Ann Intern Med.2002;137:866–874.
- ,,,.Hospitalists and the practice of inpatient medicine.Ann Intern Med.1999;130:343–349.
- ,,,,,.Rates, predictors and consequences of low career satisfaction and burnout in academic hospital medicine.J Hosp Med.2009;4(S1):24–25.
- ,.Mentoring faculty in academic medicine: a new paradigm?.J Gen Intern Med.2005;20(9):866–870.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- ,,,.Academic hospitalist taskforce quality portfolio rationale and development. 02/23/2009; Quality portfolio introduction. Available at:http://www.sgim.org/index.cfm?pageId=846. Accessed July 2010.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72e1–72e7.
- ,,,.Promoting effective care transitions of hospital discharge. A review of key issues for hospitalists.J. Hosp Med.2007;2:314–323.
- ,.Hospitalists and anesthesiologists as perioperative physicians: are their roles complementary?Proc (Bayl Univ Med Cent).2007;20(2):140–142.
- .A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalist.Mayo Clin Proc.2009;84(3):248–254.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- .Anyone, Anything, Anytime A History of Emergency Medicine.1st ed.Philadelphia, PA:Mosby‐Elsevier;2006.
- ,,, et al.Career fit and burnout among academic faculty.Arch Intern Med.2009;169(10):990–995.
Hospitalists are hospital‐based physicians whose primary professional focus is patient care, education, research, and administrative activities related to hospital medicine.1 Initially, community‐based hospitals were far more likely to employ hospitalists than academic centers. However, today most academic centers employ hospitalist models and it is now a fully recognized entity in academic settings.2
While much has been written about the structure, business operations, and potential benefits of nonteaching (clinical) hospitalist programs,3, 4 there is little known about the current state of academic hospitalist programs or their challenges. For example, who are the leaders of academic hospitalist medicine groups? Given the youth of the field, are academic hospitalists receiving adequate mentorship and are they advancing academically? What are future directions and goals for academic hospitalist groups?
To better understand academic hospitalist programs, we surveyed division chiefs and academic hospitalist leaders to explore existing business models and operations, the status of mentorship, and key issues in growth and retention.
Methods
Sites and Subjects
We targeted potential hospital medicine group leaders by identifying academic medical centers using Association of American Medical Colleges (AAMC), the Accreditation Council for Graduate Medical Education (ACGME), the Association of Chiefs of General Internal Medicine (ACGIM), and the Society of Hospital Medicine (SHM) lists of sites with teaching missions. We then used publicly available data (eg, from websites maintained by the sites) to identify physician leaders who: 1) self identified as a leader of a hospitalist group at an academic medical center (or a Chief of Division of General Internal Medicine which managed a hospitalist group) in the SHM database, 2) were listed as such on the website, or 3) were members of ACGIM and listed as a hospitalist group leader at a university based medical center.
Survey Development
Our survey was based on questions used in previous research by the authors,5 with additional questions regarding operations of academic hospitalist programs, growth and retention of hospitalists, and mentorship developed by the study authors. Questions were pretested among a selected group of members of the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force and the SHM Academic Hospitalist Interest Group, after which the survey was refined and converted into its electronic form.
Survey Methods
The email survey process began in April 2007 with an initial survey sent to those physicians identified using preexisting data, as described. Our survey asked first if recipients were directly responsible for the oversight of a hospitalist group (eg, the division chief or director of the hospital medicine group) and if they practiced at an academic medical center. Only respondents who answered yes to both of these criteria were invited to respond to our survey. Those who felt the survey did not apply to them were invited to forward the email survey on to the appropriate person at their site or respond that their hospital had no hospital medicine service. Subsequent reminder emails were sent to nonrespondents at 10‐day intervals up to a total of four times. This survey was granted exempt status from the UCSF Institutional Review Board.
Statistical Methods
Response rates and frequencies and distribution of survey responses were analyzed using univariable statistics.
Results
Characteristics of Responding Sites
We received responses from 57 (40%) of the academic sites identified as having an academic hospital medicine group. Hospitalist group leaders at responding sites had been in their current position 3.8 years, graduated medical school approximately 15 years prior, and were either Assistant (40%), Associate (32%), or Full Professors (23%). Group leaders reported that the vast majority (91%) of group full‐time members were in junior faculty positions (Instructor or Assistant Professor), who were working full‐time. On average, responding programs were 6 years old (formed in 2001) and currently had 10.0 total full time equivalents (FTEs). A total of 38 of the groups (67%) were part of the larger Division of General Internal Medicine, whereas 9 groups (16 %) were their own division within the Department of Medicine. The remaining 17% were part of another division.
Mentorship Practices In Academic Hospital Medicine Groups
As one mechanism of mentorship, annual performance reviews were offered in most programs (88%). These were usually performed by the general medicine division chief or hospitalist leader. Mentoring relationships for clinician investigators (CI) were most often from personnel outside the hospitalist group, whereas clinician‐educators (CE) most often were mentored by faculty inside the group.
Hospitalist Leaders' Priorities and Impressions of Growth, Opportunities, Career Development and Barriers
Hospitalist leaders reported the highest priorities for hospitalist leaders were developing research and teaching programs, and minimizing turnover. Other priorities included achieving financial stability, applying for extramural funding, and reducing clinical workload (Table 2). Only 14% of respondents noted that becoming a separate division was a priority.0
| Characteristic | n (%) |
|---|---|
| |
| Group leader characteristics | |
| Academic rank | |
| Assistant professor/other | 26 (45) |
| Associate professor | 18 (32) |
| Full professor | 13 (23) |
| Years in position (mean, range) | 3.8 (2.07.0) |
| Group characteristics | |
| Hospital medicine place in school of medicine | |
| Within the department of medicine | 55 (98) |
| Separate division | 9 (16) |
| Within division of general medicine | 38 (67) |
| Other | 9 (16) |
| Not in the department of medicine | 1 (2) |
| Program size (mean, range) | |
| Number of hospitalists in program | 10 (718) |
| Number of FTE | 11 (3.512) |
| FTE's hired in past 2 years (July 2005 to survey date) | 4.0 (2.27.0) |
| Hospitalist activities | |
| Medicine consultation | 52 (91) |
| Quality improvement projects | 52 (91) |
| Nonteaching attending | 44 (77) |
| Comanagement of surgical patients | 44 (77) |
| 24‐hour coverage | 24 (61) |
| Manage patient transfer requests | 32 (56) |
| Peer review/morbidity and mortality | 31 (54) |
| Education program leadership | 29 (51) |
| Medical student program leadership | 29 (51) |
| Palliative care program | 23 (40) |
| Preoperative clinic | 23 (40) |
| Emergency department triage | 14 (25) |
| Post discharge follow‐up clinic | 13 (23) |
| Skill nursing facility coverage | 4 (7) |
| Other | 15 (26) |
| Mentorship Activity | n (%) |
|---|---|
| Programs performing annual reviews with faculty | 50 (88) |
| Who performs the annual review? | |
| General Medicine Division Chief | 9 (18) |
| Hospitalist leader | 18 (36) |
| Both | 13 (26) |
| Other (eg, Department Chair, Chief Medical Officer) | 10 (20) |
| Who is the primary source of mentorship for clinician‐educators? | |
| Senior faculty within the group | 43 (77) |
| Generalist faculty outside the group, but within the institution | 6 (11) |
| Subspecialty Internal Medicine faculty outside the group, but within the institution | 3 (5) |
| Non‐Internal Medicine (eg, surgeon, epidemiologist) outside the group, but within the institution | 0 (0) |
| Faculty from another institution | 0 (0) |
| Don't know | 4 (7) |
| Who is the primary source of mentorship for clinician‐investigators? | |
| Senior faculty within the group | 6 (12) |
| Generalist faculty outside the group, but within the institution | 13 (25) |
| Subspecialty internal medicine faculty outside the group, but within the institution | 6 (12) |
| Non‐Internal Medicine (eg, surgeon, epidemiologist) outside the group, but within the institution | 2 (4) |
| Faculty from another institution | 3 (6) |
| Don't know | 2 (4) |
| Not applicable; no clinician investigators | 20 (38) |
| Highest Priority, n (%) | Intermediate Priority, n (%) | Lowest Priority, n (%) | Not a Priority, n (%) | NA, n (%) | |
|---|---|---|---|---|---|
| Reducing individual faculty clinical workload | 9 (16) | 22 (3) | 11 (2) | 14 (2) | 0 (0) |
| Achieving financial stability | 13 (24) | 30 (55) | 6 (11) | 6 (11) | 0 (0) |
| Minimizing turnover | 22 (39) | 27 (48) | 6 (11) | 1 (2) | 0 (0) |
| Developing teaching programs | 22 (39) | 29 (52) | 3 (5) | 2 (4) | 0 (0) |
| Becoming a separate division | 3 (5) | 5 (9) | 11 (20) | 23 (41) | 14 (25) |
| Developing research | 25 (45) | 18 (32) | 5 (9) | 6 (11) | 2 (4) |
| Applying for extramural funding | 10 (18) | 24 (43) | 10 (18) | 8 (14) | 4 (7) |
| Strongly Agree, n (%) | Agree, n (%) | Neutral, n (%) | Disagree, n (%) | Strongly Disagree, n (%) | NA, n (%) | |
|---|---|---|---|---|---|---|
| Growth and sustainability | ||||||
| Availability of funds is limiting expansion of academic functions (eg, education and research) | 20 (36) | 21 (38) | 5 (9) | 7 (12) | 3 (5) | 0 (0) |
| Availability of funds is limiting expansion of clinical functions (eg, development of new services) | 11 (20) | 17 (30) | 14 (25) | 10 (18) | 4 (7) | 0 (0) |
| My faculty are developing sustainable nonclinical activities | 9 (16) | 23 (41) | 12 (21) | 9 (16) | 3 (5) | 0 (0) |
| Career development | ||||||
| Mentorship is a major issue for my clinician‐educator faculty | 14 (25) | 28 (50) | 7 (12) | 4 (7) | 1 (2) | 2 (4) |
| Mentorship is a major issue for my research faculty | 22 (40) | 10 (18) | 4 (7) | 3 (5) | 2 (4) | 14 (25) |
| External support for hospital medicine group | ||||||
| There is investment in the development of academic functions of our hospitalist program from my hospital | 4 (7) | 12 (21) | 10 (18) | 22 (39) | 8 (14) | 0 (0) |
| There is investment in the development of academic functions of our hospitalist program from the Department of Medicine | 22 (40) | 17 (31) | 8 (15) | 4 (7) | 2 (4) | 2 (4) |
In general, academic hospitalist leaders reported that Departments of Medicine and Divisions of General Medicine (where applicable) were invested in the development of their academic functions. Yet, more than half of program directors reported that hospitals were not supportive. Moreover, lack of funds limited the expansion of their academic or clinical functions (Table 3). Additionally, while the majority either strongly agree or agree that their faculty are developing sustainable nonclinical activities (57%), they perceive that they are at risk for burnout (69%), and that lack of mentorship is a major issue for both CE (75%) and research faculty (58%). Lastly, while program directors strongly agree or agree (71%) that their hospitalist groups are respected by other academic physicians, they additionally strongly agree or agree that their Departments of Medicine (58%) and other Divisions (78%) view their hospitalist program as a clinical service rather than an academic program.
Discussion
Our survey provides a unique snapshot of academic hospitalist groups, highlighting a perceived lack of support and respect for their programs, a need to increase education and scholarly activities, and a desire to better prepare faculty for academic promotion.
Academic hospitalist groups and leaders reflected what one would expect from a field that is just over a decade old. Program leaders were relatively new to their position, as were their division group members. As a result, it is not surprising that most of the academic hospitalist leaders identified mentorship as a major issue. We were encouraged to see that most programs were offering annual reviews. However, the majority of these annual reviews were performed by the group leaders, many of whom are relatively junior (40% Assistant Professors) and may not be experienced in mentoring and performing annual reviews. Importantly, the absence of a mentor (or a high‐quality, experienced one) among physicians, and specifically hospitalists, may result in fewer peer‐reviewed first author and non‐peer‐reviewed publications, and less experience leading a teaching session at a national meeting.6 Research suggests that effective mentoring may help faculty increase career satisfaction and productivity and reduce their risk for burn‐out.7 Hospitalist groups might benefit nationally from focusing specifically on finding adequate mentorship either within or outside their groups. In addition, national organizations such as the SHM and the SGIM could potentially help these groups and individual hospitalists in creating mentorship networks and a mentoring infrastructure.
Academic hospitalist leaders were concerned about the ability of their faculty to develop sustainable nonclinical activities and scholarship. Notably, more than 40% of surveyed leaders agreed or strongly agreed that their faculty were not developing sustainable nonclinical activities. For individual faculty, the inability to develop scholarly activities and engage in academic pursuits may create challenges in getting promoted by traditional academic pathways. Some have recognized this issue and tried to develop practical solutions.2 In addition, academic hospitalists often engage in nonclinical activities such as quality improvement or patient safety which do not fit in the traditional tripartite mission of academics (clinical care, education, and research). In this survey, more than 90% of groups were engaged in quality improvement projects and over half in peer review exercises (Table 1). As many of these scholarly activities require innovation, sophisticated data analysis, and can have far‐reaching and substantial impacts on healthcare, some have argued these should be considered as part of the promotion process.8 Notably, the SGIM Academic Hospitalist Taskforce has created the Quality Portfolio, a structured adjunct to promotions packets to organize and document work in quality improvement and patient safety.9
While there were few CI in the divisions surveyed, building CI programs was a major priority of programs. In programs reporting the presence of CI's, they report limited access to research support. This highlights the potential role and benefit of post residency training in designing and conducting clinical research whether in a traditional general internal medicine fellowship or in 1 of the many growing hospital medicine fellowships.10 There also appears to be a need for funding to support the research careers of junior hospitalists. While access to effective mentorship is integrally linked to achieving increased academic accomplishments, there is certainly an ample call for research in the areas of quality improvement, patient safety; systems‐based practice, hospital efficiency, transitions of care,11 perioperative medicine,12 and education.2, 13, 14 While providing lower costs per admission and lower lengths of stay, hospitalists seem well‐positioned to spearhead active research in cost‐effectiveness in the hospital.14 Additionally, a quality portfolio, documenting such quality improvement projects, has been suggested as an effective means to provide a record of this work for academic promotion.9
The diverse activities of today's hospitalists are transforming the traditional view of academic work and are critical to the growth of hospitals, patient care, and development of the field of hospital medicine itself. Until these areas are fully embraced as legitimate areas of academic productivity and scholarship, the academic advancement of hospitalists will be slow.
It is unclear from our survey if academic hospitalist programs are truly getting the support they need to succeed. On one hand, there was general agreement that the Departments of Medicine and Divisions of General and Hospital Medicine were invested in the development of the academic accomplishments. Yet, the majority of program directors believed that they are viewed by the Department or Division as a clinical rather than an academic program. Moreover, over half of program directors report that their hospital was not supportive and therefore have limited the expansion of their hospitalist groups' educational and research activities. Lastly, for a large majority of programs, unavailable funding also acted to limit growth and expansion of academic functions. In a mere 2 decades, Emergency Medicine has become one of the largest US specialties and yet research and funding in the field have been lagging and are limiting academic expansion. Junior faculty seeking research careers struggled to find support and mentorship within their emergency medicine divisions.15 Challenges faced by academic emergency medicine provide important historical perspective for the even more rapidly growing field of academic hospital medicine. Learning from the Academic Emergency Medicine experience, academic hospitalists should proactively identify scholarship and research opportunities unique to hospitalist and fitting the needs of academic institutions. Involvement in national medical organizations, such as SGIM‐SHM‐ACGIM Academic Hospitalist Academy, or the SGIM Academic Hospitalist Task Force, where skill and career development is the focus, will undoubtedly promote the success of academic hospitalist. Expanding valuable niches of expertise, such as quality control, perioperative medicine and care transitions, create an indispensible component of hospital care. Lastly mentoring programs for academic hospitalist within SHM and SGIM are also essential for networking and career development. There are several limitations to our study. Our response rate of 40% was relatively low, and our results may not be representative of all academic hospitalist division chiefs and their programs, may be overstating the perceived difficulties of the survey sample, or conversely missing a large portion too overwhelmed by current duties who lacked the time to complete the survey. Having said this, our survey methodology targeted sites where we could identify potentialnot confirmedhospitalist groups and hospitalist group leaders. For this reason, our response rate could be higher (if some of our contacts were in error). Our results are a cross‐sectional survey based on self report and are subject to recall bias. In addition, our study was carried out in 2007, and while issues such as mentorship may remain important, our results regarding financial arrangements may not be applicable to the current economic climate. Finally, while improving mentorship was identified as a principle objective for program leaders, we did not explore the existing quality of mentorship, nor perceived shortfalls. This should be the subject of future exploration.
The vast majority of academic hospital medicine programs continue to view inadequate support, expanding research, mentorship, and academic promotion as critical issues for the future. Thus, further understanding of these features, and interventions to allow for success, are of crucial importance in the continued development of academic hospitalists. Our study supports the need for mentoring and career development programs, targeting academic hospitalists and their leaders. In addition, attention should be paid to activities that support career fit, creating sustainable and viable job descriptions for academic hospitalists, and preventing burnout.16 At the same time we must expand the traditional view of scholarship and training and advocate for promotion criteria that value the unique contributions of hospitalists to become in line with the broad areas that hospitalists work.
Hospitalists are hospital‐based physicians whose primary professional focus is patient care, education, research, and administrative activities related to hospital medicine.1 Initially, community‐based hospitals were far more likely to employ hospitalists than academic centers. However, today most academic centers employ hospitalist models and it is now a fully recognized entity in academic settings.2
While much has been written about the structure, business operations, and potential benefits of nonteaching (clinical) hospitalist programs,3, 4 there is little known about the current state of academic hospitalist programs or their challenges. For example, who are the leaders of academic hospitalist medicine groups? Given the youth of the field, are academic hospitalists receiving adequate mentorship and are they advancing academically? What are future directions and goals for academic hospitalist groups?
To better understand academic hospitalist programs, we surveyed division chiefs and academic hospitalist leaders to explore existing business models and operations, the status of mentorship, and key issues in growth and retention.
Methods
Sites and Subjects
We targeted potential hospital medicine group leaders by identifying academic medical centers using Association of American Medical Colleges (AAMC), the Accreditation Council for Graduate Medical Education (ACGME), the Association of Chiefs of General Internal Medicine (ACGIM), and the Society of Hospital Medicine (SHM) lists of sites with teaching missions. We then used publicly available data (eg, from websites maintained by the sites) to identify physician leaders who: 1) self identified as a leader of a hospitalist group at an academic medical center (or a Chief of Division of General Internal Medicine which managed a hospitalist group) in the SHM database, 2) were listed as such on the website, or 3) were members of ACGIM and listed as a hospitalist group leader at a university based medical center.
Survey Development
Our survey was based on questions used in previous research by the authors,5 with additional questions regarding operations of academic hospitalist programs, growth and retention of hospitalists, and mentorship developed by the study authors. Questions were pretested among a selected group of members of the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force and the SHM Academic Hospitalist Interest Group, after which the survey was refined and converted into its electronic form.
Survey Methods
The email survey process began in April 2007 with an initial survey sent to those physicians identified using preexisting data, as described. Our survey asked first if recipients were directly responsible for the oversight of a hospitalist group (eg, the division chief or director of the hospital medicine group) and if they practiced at an academic medical center. Only respondents who answered yes to both of these criteria were invited to respond to our survey. Those who felt the survey did not apply to them were invited to forward the email survey on to the appropriate person at their site or respond that their hospital had no hospital medicine service. Subsequent reminder emails were sent to nonrespondents at 10‐day intervals up to a total of four times. This survey was granted exempt status from the UCSF Institutional Review Board.
Statistical Methods
Response rates and frequencies and distribution of survey responses were analyzed using univariable statistics.
Results
Characteristics of Responding Sites
We received responses from 57 (40%) of the academic sites identified as having an academic hospital medicine group. Hospitalist group leaders at responding sites had been in their current position 3.8 years, graduated medical school approximately 15 years prior, and were either Assistant (40%), Associate (32%), or Full Professors (23%). Group leaders reported that the vast majority (91%) of group full‐time members were in junior faculty positions (Instructor or Assistant Professor), who were working full‐time. On average, responding programs were 6 years old (formed in 2001) and currently had 10.0 total full time equivalents (FTEs). A total of 38 of the groups (67%) were part of the larger Division of General Internal Medicine, whereas 9 groups (16 %) were their own division within the Department of Medicine. The remaining 17% were part of another division.
Mentorship Practices In Academic Hospital Medicine Groups
As one mechanism of mentorship, annual performance reviews were offered in most programs (88%). These were usually performed by the general medicine division chief or hospitalist leader. Mentoring relationships for clinician investigators (CI) were most often from personnel outside the hospitalist group, whereas clinician‐educators (CE) most often were mentored by faculty inside the group.
Hospitalist Leaders' Priorities and Impressions of Growth, Opportunities, Career Development and Barriers
Hospitalist leaders reported the highest priorities for hospitalist leaders were developing research and teaching programs, and minimizing turnover. Other priorities included achieving financial stability, applying for extramural funding, and reducing clinical workload (Table 2). Only 14% of respondents noted that becoming a separate division was a priority.0
| Characteristic | n (%) |
|---|---|
| |
| Group leader characteristics | |
| Academic rank | |
| Assistant professor/other | 26 (45) |
| Associate professor | 18 (32) |
| Full professor | 13 (23) |
| Years in position (mean, range) | 3.8 (2.07.0) |
| Group characteristics | |
| Hospital medicine place in school of medicine | |
| Within the department of medicine | 55 (98) |
| Separate division | 9 (16) |
| Within division of general medicine | 38 (67) |
| Other | 9 (16) |
| Not in the department of medicine | 1 (2) |
| Program size (mean, range) | |
| Number of hospitalists in program | 10 (718) |
| Number of FTE | 11 (3.512) |
| FTE's hired in past 2 years (July 2005 to survey date) | 4.0 (2.27.0) |
| Hospitalist activities | |
| Medicine consultation | 52 (91) |
| Quality improvement projects | 52 (91) |
| Nonteaching attending | 44 (77) |
| Comanagement of surgical patients | 44 (77) |
| 24‐hour coverage | 24 (61) |
| Manage patient transfer requests | 32 (56) |
| Peer review/morbidity and mortality | 31 (54) |
| Education program leadership | 29 (51) |
| Medical student program leadership | 29 (51) |
| Palliative care program | 23 (40) |
| Preoperative clinic | 23 (40) |
| Emergency department triage | 14 (25) |
| Post discharge follow‐up clinic | 13 (23) |
| Skill nursing facility coverage | 4 (7) |
| Other | 15 (26) |
| Mentorship Activity | n (%) |
|---|---|
| Programs performing annual reviews with faculty | 50 (88) |
| Who performs the annual review? | |
| General Medicine Division Chief | 9 (18) |
| Hospitalist leader | 18 (36) |
| Both | 13 (26) |
| Other (eg, Department Chair, Chief Medical Officer) | 10 (20) |
| Who is the primary source of mentorship for clinician‐educators? | |
| Senior faculty within the group | 43 (77) |
| Generalist faculty outside the group, but within the institution | 6 (11) |
| Subspecialty Internal Medicine faculty outside the group, but within the institution | 3 (5) |
| Non‐Internal Medicine (eg, surgeon, epidemiologist) outside the group, but within the institution | 0 (0) |
| Faculty from another institution | 0 (0) |
| Don't know | 4 (7) |
| Who is the primary source of mentorship for clinician‐investigators? | |
| Senior faculty within the group | 6 (12) |
| Generalist faculty outside the group, but within the institution | 13 (25) |
| Subspecialty internal medicine faculty outside the group, but within the institution | 6 (12) |
| Non‐Internal Medicine (eg, surgeon, epidemiologist) outside the group, but within the institution | 2 (4) |
| Faculty from another institution | 3 (6) |
| Don't know | 2 (4) |
| Not applicable; no clinician investigators | 20 (38) |
| Highest Priority, n (%) | Intermediate Priority, n (%) | Lowest Priority, n (%) | Not a Priority, n (%) | NA, n (%) | |
|---|---|---|---|---|---|
| Reducing individual faculty clinical workload | 9 (16) | 22 (3) | 11 (2) | 14 (2) | 0 (0) |
| Achieving financial stability | 13 (24) | 30 (55) | 6 (11) | 6 (11) | 0 (0) |
| Minimizing turnover | 22 (39) | 27 (48) | 6 (11) | 1 (2) | 0 (0) |
| Developing teaching programs | 22 (39) | 29 (52) | 3 (5) | 2 (4) | 0 (0) |
| Becoming a separate division | 3 (5) | 5 (9) | 11 (20) | 23 (41) | 14 (25) |
| Developing research | 25 (45) | 18 (32) | 5 (9) | 6 (11) | 2 (4) |
| Applying for extramural funding | 10 (18) | 24 (43) | 10 (18) | 8 (14) | 4 (7) |
| Strongly Agree, n (%) | Agree, n (%) | Neutral, n (%) | Disagree, n (%) | Strongly Disagree, n (%) | NA, n (%) | |
|---|---|---|---|---|---|---|
| Growth and sustainability | ||||||
| Availability of funds is limiting expansion of academic functions (eg, education and research) | 20 (36) | 21 (38) | 5 (9) | 7 (12) | 3 (5) | 0 (0) |
| Availability of funds is limiting expansion of clinical functions (eg, development of new services) | 11 (20) | 17 (30) | 14 (25) | 10 (18) | 4 (7) | 0 (0) |
| My faculty are developing sustainable nonclinical activities | 9 (16) | 23 (41) | 12 (21) | 9 (16) | 3 (5) | 0 (0) |
| Career development | ||||||
| Mentorship is a major issue for my clinician‐educator faculty | 14 (25) | 28 (50) | 7 (12) | 4 (7) | 1 (2) | 2 (4) |
| Mentorship is a major issue for my research faculty | 22 (40) | 10 (18) | 4 (7) | 3 (5) | 2 (4) | 14 (25) |
| External support for hospital medicine group | ||||||
| There is investment in the development of academic functions of our hospitalist program from my hospital | 4 (7) | 12 (21) | 10 (18) | 22 (39) | 8 (14) | 0 (0) |
| There is investment in the development of academic functions of our hospitalist program from the Department of Medicine | 22 (40) | 17 (31) | 8 (15) | 4 (7) | 2 (4) | 2 (4) |
In general, academic hospitalist leaders reported that Departments of Medicine and Divisions of General Medicine (where applicable) were invested in the development of their academic functions. Yet, more than half of program directors reported that hospitals were not supportive. Moreover, lack of funds limited the expansion of their academic or clinical functions (Table 3). Additionally, while the majority either strongly agree or agree that their faculty are developing sustainable nonclinical activities (57%), they perceive that they are at risk for burnout (69%), and that lack of mentorship is a major issue for both CE (75%) and research faculty (58%). Lastly, while program directors strongly agree or agree (71%) that their hospitalist groups are respected by other academic physicians, they additionally strongly agree or agree that their Departments of Medicine (58%) and other Divisions (78%) view their hospitalist program as a clinical service rather than an academic program.
Discussion
Our survey provides a unique snapshot of academic hospitalist groups, highlighting a perceived lack of support and respect for their programs, a need to increase education and scholarly activities, and a desire to better prepare faculty for academic promotion.
Academic hospitalist groups and leaders reflected what one would expect from a field that is just over a decade old. Program leaders were relatively new to their position, as were their division group members. As a result, it is not surprising that most of the academic hospitalist leaders identified mentorship as a major issue. We were encouraged to see that most programs were offering annual reviews. However, the majority of these annual reviews were performed by the group leaders, many of whom are relatively junior (40% Assistant Professors) and may not be experienced in mentoring and performing annual reviews. Importantly, the absence of a mentor (or a high‐quality, experienced one) among physicians, and specifically hospitalists, may result in fewer peer‐reviewed first author and non‐peer‐reviewed publications, and less experience leading a teaching session at a national meeting.6 Research suggests that effective mentoring may help faculty increase career satisfaction and productivity and reduce their risk for burn‐out.7 Hospitalist groups might benefit nationally from focusing specifically on finding adequate mentorship either within or outside their groups. In addition, national organizations such as the SHM and the SGIM could potentially help these groups and individual hospitalists in creating mentorship networks and a mentoring infrastructure.
Academic hospitalist leaders were concerned about the ability of their faculty to develop sustainable nonclinical activities and scholarship. Notably, more than 40% of surveyed leaders agreed or strongly agreed that their faculty were not developing sustainable nonclinical activities. For individual faculty, the inability to develop scholarly activities and engage in academic pursuits may create challenges in getting promoted by traditional academic pathways. Some have recognized this issue and tried to develop practical solutions.2 In addition, academic hospitalists often engage in nonclinical activities such as quality improvement or patient safety which do not fit in the traditional tripartite mission of academics (clinical care, education, and research). In this survey, more than 90% of groups were engaged in quality improvement projects and over half in peer review exercises (Table 1). As many of these scholarly activities require innovation, sophisticated data analysis, and can have far‐reaching and substantial impacts on healthcare, some have argued these should be considered as part of the promotion process.8 Notably, the SGIM Academic Hospitalist Taskforce has created the Quality Portfolio, a structured adjunct to promotions packets to organize and document work in quality improvement and patient safety.9
While there were few CI in the divisions surveyed, building CI programs was a major priority of programs. In programs reporting the presence of CI's, they report limited access to research support. This highlights the potential role and benefit of post residency training in designing and conducting clinical research whether in a traditional general internal medicine fellowship or in 1 of the many growing hospital medicine fellowships.10 There also appears to be a need for funding to support the research careers of junior hospitalists. While access to effective mentorship is integrally linked to achieving increased academic accomplishments, there is certainly an ample call for research in the areas of quality improvement, patient safety; systems‐based practice, hospital efficiency, transitions of care,11 perioperative medicine,12 and education.2, 13, 14 While providing lower costs per admission and lower lengths of stay, hospitalists seem well‐positioned to spearhead active research in cost‐effectiveness in the hospital.14 Additionally, a quality portfolio, documenting such quality improvement projects, has been suggested as an effective means to provide a record of this work for academic promotion.9
The diverse activities of today's hospitalists are transforming the traditional view of academic work and are critical to the growth of hospitals, patient care, and development of the field of hospital medicine itself. Until these areas are fully embraced as legitimate areas of academic productivity and scholarship, the academic advancement of hospitalists will be slow.
It is unclear from our survey if academic hospitalist programs are truly getting the support they need to succeed. On one hand, there was general agreement that the Departments of Medicine and Divisions of General and Hospital Medicine were invested in the development of the academic accomplishments. Yet, the majority of program directors believed that they are viewed by the Department or Division as a clinical rather than an academic program. Moreover, over half of program directors report that their hospital was not supportive and therefore have limited the expansion of their hospitalist groups' educational and research activities. Lastly, for a large majority of programs, unavailable funding also acted to limit growth and expansion of academic functions. In a mere 2 decades, Emergency Medicine has become one of the largest US specialties and yet research and funding in the field have been lagging and are limiting academic expansion. Junior faculty seeking research careers struggled to find support and mentorship within their emergency medicine divisions.15 Challenges faced by academic emergency medicine provide important historical perspective for the even more rapidly growing field of academic hospital medicine. Learning from the Academic Emergency Medicine experience, academic hospitalists should proactively identify scholarship and research opportunities unique to hospitalist and fitting the needs of academic institutions. Involvement in national medical organizations, such as SGIM‐SHM‐ACGIM Academic Hospitalist Academy, or the SGIM Academic Hospitalist Task Force, where skill and career development is the focus, will undoubtedly promote the success of academic hospitalist. Expanding valuable niches of expertise, such as quality control, perioperative medicine and care transitions, create an indispensible component of hospital care. Lastly mentoring programs for academic hospitalist within SHM and SGIM are also essential for networking and career development. There are several limitations to our study. Our response rate of 40% was relatively low, and our results may not be representative of all academic hospitalist division chiefs and their programs, may be overstating the perceived difficulties of the survey sample, or conversely missing a large portion too overwhelmed by current duties who lacked the time to complete the survey. Having said this, our survey methodology targeted sites where we could identify potentialnot confirmedhospitalist groups and hospitalist group leaders. For this reason, our response rate could be higher (if some of our contacts were in error). Our results are a cross‐sectional survey based on self report and are subject to recall bias. In addition, our study was carried out in 2007, and while issues such as mentorship may remain important, our results regarding financial arrangements may not be applicable to the current economic climate. Finally, while improving mentorship was identified as a principle objective for program leaders, we did not explore the existing quality of mentorship, nor perceived shortfalls. This should be the subject of future exploration.
The vast majority of academic hospital medicine programs continue to view inadequate support, expanding research, mentorship, and academic promotion as critical issues for the future. Thus, further understanding of these features, and interventions to allow for success, are of crucial importance in the continued development of academic hospitalists. Our study supports the need for mentoring and career development programs, targeting academic hospitalists and their leaders. In addition, attention should be paid to activities that support career fit, creating sustainable and viable job descriptions for academic hospitalists, and preventing burnout.16 At the same time we must expand the traditional view of scholarship and training and advocate for promotion criteria that value the unique contributions of hospitalists to become in line with the broad areas that hospitalists work.
- Anonymous. Definition of a hospitalist.2009. Society of Hospital Medicine Homepage/General Information. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Hospitalist_Definition4:240–246.
- ,,,,.Resource‐based relative value scale analysis between teaching and nonteaching hospitalist services.Health Care Management.2009;1(28):81–85.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service.Ann Intern Med.2002;137:866–874.
- ,,,.Hospitalists and the practice of inpatient medicine.Ann Intern Med.1999;130:343–349.
- ,,,,,.Rates, predictors and consequences of low career satisfaction and burnout in academic hospital medicine.J Hosp Med.2009;4(S1):24–25.
- ,.Mentoring faculty in academic medicine: a new paradigm?.J Gen Intern Med.2005;20(9):866–870.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- ,,,.Academic hospitalist taskforce quality portfolio rationale and development. 02/23/2009; Quality portfolio introduction. Available at:http://www.sgim.org/index.cfm?pageId=846. Accessed July 2010.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72e1–72e7.
- ,,,.Promoting effective care transitions of hospital discharge. A review of key issues for hospitalists.J. Hosp Med.2007;2:314–323.
- ,.Hospitalists and anesthesiologists as perioperative physicians: are their roles complementary?Proc (Bayl Univ Med Cent).2007;20(2):140–142.
- .A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalist.Mayo Clin Proc.2009;84(3):248–254.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- .Anyone, Anything, Anytime A History of Emergency Medicine.1st ed.Philadelphia, PA:Mosby‐Elsevier;2006.
- ,,, et al.Career fit and burnout among academic faculty.Arch Intern Med.2009;169(10):990–995.
- Anonymous. Definition of a hospitalist.2009. Society of Hospital Medicine Homepage/General Information. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Hospitalist_Definition4:240–246.
- ,,,,.Resource‐based relative value scale analysis between teaching and nonteaching hospitalist services.Health Care Management.2009;1(28):81–85.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service.Ann Intern Med.2002;137:866–874.
- ,,,.Hospitalists and the practice of inpatient medicine.Ann Intern Med.1999;130:343–349.
- ,,,,,.Rates, predictors and consequences of low career satisfaction and burnout in academic hospital medicine.J Hosp Med.2009;4(S1):24–25.
- ,.Mentoring faculty in academic medicine: a new paradigm?.J Gen Intern Med.2005;20(9):866–870.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- ,,,.Academic hospitalist taskforce quality portfolio rationale and development. 02/23/2009; Quality portfolio introduction. Available at:http://www.sgim.org/index.cfm?pageId=846. Accessed July 2010.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72e1–72e7.
- ,,,.Promoting effective care transitions of hospital discharge. A review of key issues for hospitalists.J. Hosp Med.2007;2:314–323.
- ,.Hospitalists and anesthesiologists as perioperative physicians: are their roles complementary?Proc (Bayl Univ Med Cent).2007;20(2):140–142.
- .A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalist.Mayo Clin Proc.2009;84(3):248–254.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- .Anyone, Anything, Anytime A History of Emergency Medicine.1st ed.Philadelphia, PA:Mosby‐Elsevier;2006.
- ,,, et al.Career fit and burnout among academic faculty.Arch Intern Med.2009;169(10):990–995.
Copyright © 2011 Society of Hospital Medicine
RIP Conference Provides Peer Mentoring
The research‐in‐progress (RIP) conference is commonplace in academia, but there are no studies that objectively characterize its value. Bringing faculty together away from revenue‐generating activities carries a significant cost. As such, measuring the success of such gatherings is necessary.
Mentors are an invaluable influence on the careers of junior faculty members, helping them to produce high‐quality research.13 Unfortunately, some divisions lack mentorship to support the academic needs of less experienced faculty.1 Peer mentorship may be a solution. RIP sessions represent an opportunity to intentionally formalize peer mentoring. Further, these sessions can facilitate collaborations as individuals become aware of colleagues' interests. The goal of this study was to assess the value of the research‐in‐progress conference initiated within the hospitalist division at our institution.
Methods
Study Design
This cohort study was conducted to evaluate the value of the RIP conference among hospitalists in our division and the academic outcomes of the projects.
Setting and Participants
The study took place at Johns Hopkins Bayview Medical Center (JHBMC), a 335‐bed university‐affiliated medical center in Baltimore, Maryland. The hospitalist division consists of faculty physicians, nurse practitioners, and physician assistants (20.06 FTE physicians and 7.41 FTE midlevel providers). Twelve (54%) of our faculty members are female, and the mean age of providers is 35.7 years. The providers have been practicing hospitalist medicine for 3.0 years on average; 2 (9%) are clinical associates, 16 (73%) are instructors, and 3 (14%) are assistant professors.
All faculty members presenting at the RIP session were members of the division. A senior faculty member (a professor in the Division of General Internal Medicine) helps to coordinate the conference. The group's research assistant was present at the sessions and was charged with data collection and collation.
The Johns Hopkins University institutional review board approved the study.
The Research in Progress Conference
During the 2009 academic year, our division held 15 RIP sessions. At each session, 1 faculty member presented a research proposal. The goal of each session was to provide a forum where faculty members could share their research ideas (specific aims, hypotheses, planned design, outcome measures, analytic plans, and preliminary results [if applicable]) in order to receive feedback. The senior faculty member met with the presenter prior to each session in order to: (1) ensure that half the RIP time was reserved for discussion and (2) review the presenter's goals so these would be made explicit to peers. The coordinator of the RIP conference facilitated the discussion, solicited input from all attendees, and encouraged constructive criticism.
Evaluation, Data Collection, and Analysis
At the end of each session, attendees (who were exclusively members of the hospitalist division) were asked to complete an anonymous survey. The 1‐page instrument was designed (1) with input from curriculum development experts4 and (2) after a review of the literature about RIP conferences. These steps conferred content validity to the instrument, which assessed perceptions about the session's quality and what was learned. Five‐point Likert scales were used to characterize the conference's success in several areas, including being intellectually/professionally stimulating and keeping them apprised of their colleagues' interests. The survey also assessed the participatory nature of the conference (balance of presentation vs discussion), its climate (extremely critical vs extremely supportive), and how the conference assisted the presenter. The presenters completed a distinct survey related to how helpful the conference was in improving/enhancing their projects. A final open‐ended section invited additional comments. The instrument was piloted and iteratively revised before its use in this study.
For the projects presented, we assessed the percentage that resulted in a peer‐reviewed publication or a presentation at a national meeting.
Results
The mean number of attendees at the RIP sessions was 9.6 persons. A total of 143 evaluations were completed. All 15 presenters (100%) completed their assessments. The research ideas presented spanned a breadth of topics in clinical research, quality improvement, policy, and professional development (Table 1).
| Session | Date | Presenter | Topic | Evaluations Completed |
|---|---|---|---|---|
| 1 | 7/2008 | Dr. CS | Hospital medicine in Canada versus the United States | 7 |
| 2 | 7/2008 | Dr. RT | Procedures by hospitalists | 9 |
| 3 | 8/2008 | Dr. MA | Clostridium difficile treatment in the hospital | 11 |
| 4 | 8/2008 | Dr. EH | Active bed management | 6 |
| 5 | 9/2008 | Dr. AS | Medication reconciliation for geriatric inpatients | 10 |
| 6 | 9/2008 | Dr. DT | Time‐motion study of hospitalists | 10 |
| 7 | 10/2008 | Dr. KV | e‐Triage pilot | 16 |
| 8 | 11/2008 | Dr. EH | Assessing clinical performance of hospitalists | 7 |
| 9 | 12/2008 | Dr. SC | Trends and implications of hospitalists' morale | 8 |
| 10 | 1/2009 | Dr. TB | Lessons learned: tracking urinary catheter use at Bayview | 11 |
| 11 | 2/2009 | Dr. FK | Utilizing audit and feedback to improve performance in tobacco dependence counseling | 12 |
| 12 | 3/2009 | Dr. MK | Survivorship care plans | 7 |
| 13 | 4/2009 | Dr. DK | Outpatient provider preference for discharge summary format/style/length | 7 |
| 14 | 5/2009 | Dr. RW | Comparing preoperative consults done by hospitalists and cardiologists | 11 |
| 15 | 6/2009 | Dr. AK | Development of Web‐based messaging tool for providers | 12 |
Presenter Perspective
All 15 presenters (100%) felt a lot or tremendously supported during their sessions. Thirteen physicians (86%) believed that the sessions were a lot or tremendously helpful in advancing their projects. The presenters believed that the guidance and discussions related to their research ideas, aims, hypotheses, and plans were most helpful for advancing their projects (Table 2).
| Not at All, n (%) | A Little, n (%) | Some, n (%) | A Lot, n (%) | Tremendously, n (%) | |
|---|---|---|---|---|---|
| General questions: | |||||
| Intellectually/professionally stimulating | 0 (0) | 0 (0) | 0 (0) | 5 (33) | 10 (66) |
| Feeling supported by your colleagues in your scholarly pursuits | 0 (0) | 0 (0) | 0 (0) | 4 (27) | 11 (73) |
| Session helpful in the following areas: | |||||
| Advancing your project | 0 (0) | 0 (0) | 2 (13) | 5 (33) | 8 (53) |
| Generated new hypotheses | 1 (6) | 3 (20) | 5 (33) | 5 (33) | 1 (6) |
| Clarification of research questions | 0 (0) | 2 (13) | 4 (27) | 7 (47) | 2 (13) |
| Ideas for alternate methods | 1 (6) | 1 (6) | 2 (13) | 7 (47) | 4 (27) |
| New outcomes suggested | 1 (6) | 2 (13) | 2 (13) | 5 (33) | 5 (33) |
| Strategies to improve or enhance data collection | 0 (0) | 2 (13) | 0 (0) | 8 (53) | 5 (33) |
| Suggestions for alternate analyses or analytical strategies | 1 (1) | 1 (6) | 4 (27) | 5 (33) | 4 (27) |
| Input into what is most novel/emnteresting about this work | 0 (0) | 2 (13) | 3 (20) | 6 (40) | 4 (27) |
| Guidance about the implications of the work | 1 (6) | 2 (13) | 1 (6) | 7 (47) | 4 (27) |
| Ideas about next steps or future direction/studies | 0 (0) | 0 (0) | 3 (21) | 8 (57) | 3 (21) |
Examples of the written comments are:
I was overwhelmed by how engaged people were in my project.
The process of preparing for the session and then the discussion both helped my thinking. Colleagues were very supportive.
I am so glad I heard these comments and received this feedback now, rather than from peer reviewers selected by a journal to review my study. It would have been a much more difficult situation to fix at that later time.
Attendee Perspective
The majority of attendees (123 of 143, 86%) found the sessions to be a lot or extremely stimulating, and almost all (96%) were a lot or extremely satisfied with how the RIP sessions kept them abreast of their colleagues' academic interests. In addition, 92% judged the session's climate to be a lot or extremely supportive, and 88% deemed the balance of presentation to discussion to be just right. Attendees believed that they were most helpful to the presenter in terms of conceiving ideas for alternative methods to be used to answer the research question and in providing strategies to improve data collection (Table 3).
| Insight Offered | n (%) |
|---|---|
| Ideas for alternate methods | 92 (64%) |
| Strategies to improve data collection | 85 (59.4%) |
| New hypotheses generated | 84 (58.7%) |
| Ideas for next steps/future direction/studies | 83 (58%) |
| New outcomes suggested that should be considered | 69 (48%) |
| Clarification of the research questions | 61 (43%) |
| Input about what is most novel/emnteresting about the work | 60 (42%) |
| Guidance about the real implications of the work | 59 (41%) |
| Suggestions for alternate analyses or analytical strategies | 51 (36%) |
The free text comments primarily addressed how the presenters' research ideas were helped by the session:
There were great ideas for improvementincluding practical approaches for recruitment.
The session made me think of the daily routine things that we do that could be studied.
There were some great ideas to help Dr. A make the study more simple, doable, and practical. There were also some good ideas regarding potential sources of funding.
Academic Success
Of the 15 projects, 6 have been published in peer‐reviewed journals as first‐ or senior‐authored publications.510 Of these, 3 were presented at national meetings prior to publication. Four additional projects have been presented at a national society's annual meeting, all of which are being prepared for publication. Of the remaining 5 presentations, 4 were terminated because of the low likelihood of academic success. The remaining project is ongoing.
Comparatively, scholarly output in the prior year by the 24 physicians in the hospitalist group was 4 first‐ or senior‐authored publications in peer‐reviewed journals and 3 presentations at national meetings.
Discussion
In this article, we report our experience with the RIP conference. The sessions were perceived to be intellectually stimulating and supportive, whereas the discussions proved helpful in advancing project ideas. Ample discussion time and good attendance were thought to be critical to the success.
To our knowledge, this is the first article gathering feedback from attendees and presenters at a RIP conference and to track academic outcomes. Several types of meetings have been established within faculty and trainee groups to support and encourage scholarly activities.11, 12 The benefits of peer collaboration and peer mentoring have been described in the literature.13, 14 For example, Edwards described the success of shortstop meetings among small groups of faculty members every 4‐6 weeks in which discussions of research projects and mutual feedback would occur.15 Santucci described peer‐mentored research development meetings, with increased research productivity.12
Mentoring is critically important for academic success in medicine.1619 When divisions have limited senior mentors available, peer mentoring has proven to be indispensable as a mechanism to support faculty members.2022 The RIP conference provided a forum for peer mentoring and provided a partial solution to the limited resource of experienced research mentors in the division. The RIP sessions appear to have helped to bring the majority of presented ideas to academic fruition. Perhaps even more important, the sessions were able to terminate studies judged to have low academic promise before the faculty had invested significant time.
Several limitations of our study should be considered. First, this study involved a research‐in‐progress conference coordinated for a group of hospitalist physicians at 1 institution, and the results may not be generalizable. Second, although attendance was good at each conference, some faculty members did not come to many sessions. It is possible that those not attending may have rated the sessions differently. Session evaluations were anonymous, and we do not know whether specific attendees rated all sessions highly, thereby resulting in some degree of clustering. Third, this study did not compare the effectiveness of the RIP conference with other peer‐mentorship models. Finally, our study was uncontrolled. Although it would not be possible to restrict specific faculty from presenting at or attending the RIP conference, we intend to more carefully collect attendance data to see whether there might be a dose‐response effect with respect to participation in this conference and academic success.
In conclusion, our RIP conference was perceived as valuable by our group and was associated with academic success. In our division, the RIP conference serves as a way to operationalize peer mentoring. Our findings may help other groups to refine either the focus or format of their RIP sessions and those wishing to initiate such a conference.
- ,,, et al.Junior faculty members' mentoring relationships and their professional development in US medical schools.Acad Med.1998;73:318–323.
- ,.Mentors, Advisors and Role Models in Graduate and Professional Education.Washington, DC:Association of Academic Health Centers;1996.
- ,.Characteristics of the successful researcher and implications for faculty development.J Med Educ.1986;61:22–31.
- ,,.Curriculum Development for Medical Education: A Six‐Step Approach.2nd ed.Baltimore, MD:The Johns Hopkins University Press;2009.
- ,,, et al.Characteristics of hospitalists and hospitalist programs in the United States and Canada.J Clin Outcomes Meas.2009;16:69–74
- ,,, et al.Procedures performed by hospitalist and non‐hospitalist general internists.J Gen Intern Med.2010;25:448–452.
- ,,, et al.Intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis: an observational study and review of the literature [review].J Hosp Med.2010;5:E1–E9.
- ,,, et al.Active bed management by hospitalists and emergency department throughput.Ann Intern Med.2008;149:804–811.
- ,,, et al.Transitioning to breast cancer survivorship: perspectives of patients, cancer specialists, and primary care providers.J Gen Intern Med.2009;24(Suppl 2):S459–S466.
- ,,, et al.Utilizing audit and feedback to improve hospitalists' performance in tobacco dependence counseling.Nicotine Tob Res.2010;12:797–800.
- ,,, et al.An internal medicine interest group research program can improve scholarly productivity of medical students and foster mentoring relationships with internists.Teach Learn Med.2008;20:163–167.
- ,,, et al.Peer‐mentored research development meeting: a model for successful peer mentoring among junior level researchers.Acad Psychiatry.2008;32:493–497.
- ,,, et al.Mentoring junior faculty in geriatric oncology: report from the cancer and aging research group.J Clin Oncol.2008;26:3125–3127.
- ,,the Canadian Critical Care Trials Group.Investigator‐led clinical research consortia: the Canadian Critical Care Trials Group.Crit Care Med.2009;37(1):S165–S172.
- .“Short stops”: peer support of scholarly activity.Acad Med.2002;77:939.
- ,,,,,,.Mentorship in academic general internal medicine. Results of a survey of mentors.J Gen Intern Med.2005;20:1014–1018.
- ,,, et al.Making the most of mentors: a guide for mentees.Acad Med.2009;84:140–144.
- ,,.Mentoring in academic medicine: a systematic review.JAMA.2006;296:1103–1115.
- ,,, et al.Assessing the role of influential mentors in the research development of primary care fellows.Acad Med.2004;79:865–872.
- ,,.Peer group mentoring of junior faculty.Acad Psychiatry.2008;32:230–235.
- ,,,.Facilitated peer mentorship: a pilot program for academic advancement of female medical faculty.J Womens Health.2008;17:1009–1015.
- ,Mentoring faculty in academic medicine. A new paradigm?J Gen Intern Med.2005;20:866–870.
The research‐in‐progress (RIP) conference is commonplace in academia, but there are no studies that objectively characterize its value. Bringing faculty together away from revenue‐generating activities carries a significant cost. As such, measuring the success of such gatherings is necessary.
Mentors are an invaluable influence on the careers of junior faculty members, helping them to produce high‐quality research.13 Unfortunately, some divisions lack mentorship to support the academic needs of less experienced faculty.1 Peer mentorship may be a solution. RIP sessions represent an opportunity to intentionally formalize peer mentoring. Further, these sessions can facilitate collaborations as individuals become aware of colleagues' interests. The goal of this study was to assess the value of the research‐in‐progress conference initiated within the hospitalist division at our institution.
Methods
Study Design
This cohort study was conducted to evaluate the value of the RIP conference among hospitalists in our division and the academic outcomes of the projects.
Setting and Participants
The study took place at Johns Hopkins Bayview Medical Center (JHBMC), a 335‐bed university‐affiliated medical center in Baltimore, Maryland. The hospitalist division consists of faculty physicians, nurse practitioners, and physician assistants (20.06 FTE physicians and 7.41 FTE midlevel providers). Twelve (54%) of our faculty members are female, and the mean age of providers is 35.7 years. The providers have been practicing hospitalist medicine for 3.0 years on average; 2 (9%) are clinical associates, 16 (73%) are instructors, and 3 (14%) are assistant professors.
All faculty members presenting at the RIP session were members of the division. A senior faculty member (a professor in the Division of General Internal Medicine) helps to coordinate the conference. The group's research assistant was present at the sessions and was charged with data collection and collation.
The Johns Hopkins University institutional review board approved the study.
The Research in Progress Conference
During the 2009 academic year, our division held 15 RIP sessions. At each session, 1 faculty member presented a research proposal. The goal of each session was to provide a forum where faculty members could share their research ideas (specific aims, hypotheses, planned design, outcome measures, analytic plans, and preliminary results [if applicable]) in order to receive feedback. The senior faculty member met with the presenter prior to each session in order to: (1) ensure that half the RIP time was reserved for discussion and (2) review the presenter's goals so these would be made explicit to peers. The coordinator of the RIP conference facilitated the discussion, solicited input from all attendees, and encouraged constructive criticism.
Evaluation, Data Collection, and Analysis
At the end of each session, attendees (who were exclusively members of the hospitalist division) were asked to complete an anonymous survey. The 1‐page instrument was designed (1) with input from curriculum development experts4 and (2) after a review of the literature about RIP conferences. These steps conferred content validity to the instrument, which assessed perceptions about the session's quality and what was learned. Five‐point Likert scales were used to characterize the conference's success in several areas, including being intellectually/professionally stimulating and keeping them apprised of their colleagues' interests. The survey also assessed the participatory nature of the conference (balance of presentation vs discussion), its climate (extremely critical vs extremely supportive), and how the conference assisted the presenter. The presenters completed a distinct survey related to how helpful the conference was in improving/enhancing their projects. A final open‐ended section invited additional comments. The instrument was piloted and iteratively revised before its use in this study.
For the projects presented, we assessed the percentage that resulted in a peer‐reviewed publication or a presentation at a national meeting.
Results
The mean number of attendees at the RIP sessions was 9.6 persons. A total of 143 evaluations were completed. All 15 presenters (100%) completed their assessments. The research ideas presented spanned a breadth of topics in clinical research, quality improvement, policy, and professional development (Table 1).
| Session | Date | Presenter | Topic | Evaluations Completed |
|---|---|---|---|---|
| 1 | 7/2008 | Dr. CS | Hospital medicine in Canada versus the United States | 7 |
| 2 | 7/2008 | Dr. RT | Procedures by hospitalists | 9 |
| 3 | 8/2008 | Dr. MA | Clostridium difficile treatment in the hospital | 11 |
| 4 | 8/2008 | Dr. EH | Active bed management | 6 |
| 5 | 9/2008 | Dr. AS | Medication reconciliation for geriatric inpatients | 10 |
| 6 | 9/2008 | Dr. DT | Time‐motion study of hospitalists | 10 |
| 7 | 10/2008 | Dr. KV | e‐Triage pilot | 16 |
| 8 | 11/2008 | Dr. EH | Assessing clinical performance of hospitalists | 7 |
| 9 | 12/2008 | Dr. SC | Trends and implications of hospitalists' morale | 8 |
| 10 | 1/2009 | Dr. TB | Lessons learned: tracking urinary catheter use at Bayview | 11 |
| 11 | 2/2009 | Dr. FK | Utilizing audit and feedback to improve performance in tobacco dependence counseling | 12 |
| 12 | 3/2009 | Dr. MK | Survivorship care plans | 7 |
| 13 | 4/2009 | Dr. DK | Outpatient provider preference for discharge summary format/style/length | 7 |
| 14 | 5/2009 | Dr. RW | Comparing preoperative consults done by hospitalists and cardiologists | 11 |
| 15 | 6/2009 | Dr. AK | Development of Web‐based messaging tool for providers | 12 |
Presenter Perspective
All 15 presenters (100%) felt a lot or tremendously supported during their sessions. Thirteen physicians (86%) believed that the sessions were a lot or tremendously helpful in advancing their projects. The presenters believed that the guidance and discussions related to their research ideas, aims, hypotheses, and plans were most helpful for advancing their projects (Table 2).
| Not at All, n (%) | A Little, n (%) | Some, n (%) | A Lot, n (%) | Tremendously, n (%) | |
|---|---|---|---|---|---|
| General questions: | |||||
| Intellectually/professionally stimulating | 0 (0) | 0 (0) | 0 (0) | 5 (33) | 10 (66) |
| Feeling supported by your colleagues in your scholarly pursuits | 0 (0) | 0 (0) | 0 (0) | 4 (27) | 11 (73) |
| Session helpful in the following areas: | |||||
| Advancing your project | 0 (0) | 0 (0) | 2 (13) | 5 (33) | 8 (53) |
| Generated new hypotheses | 1 (6) | 3 (20) | 5 (33) | 5 (33) | 1 (6) |
| Clarification of research questions | 0 (0) | 2 (13) | 4 (27) | 7 (47) | 2 (13) |
| Ideas for alternate methods | 1 (6) | 1 (6) | 2 (13) | 7 (47) | 4 (27) |
| New outcomes suggested | 1 (6) | 2 (13) | 2 (13) | 5 (33) | 5 (33) |
| Strategies to improve or enhance data collection | 0 (0) | 2 (13) | 0 (0) | 8 (53) | 5 (33) |
| Suggestions for alternate analyses or analytical strategies | 1 (1) | 1 (6) | 4 (27) | 5 (33) | 4 (27) |
| Input into what is most novel/emnteresting about this work | 0 (0) | 2 (13) | 3 (20) | 6 (40) | 4 (27) |
| Guidance about the implications of the work | 1 (6) | 2 (13) | 1 (6) | 7 (47) | 4 (27) |
| Ideas about next steps or future direction/studies | 0 (0) | 0 (0) | 3 (21) | 8 (57) | 3 (21) |
Examples of the written comments are:
I was overwhelmed by how engaged people were in my project.
The process of preparing for the session and then the discussion both helped my thinking. Colleagues were very supportive.
I am so glad I heard these comments and received this feedback now, rather than from peer reviewers selected by a journal to review my study. It would have been a much more difficult situation to fix at that later time.
Attendee Perspective
The majority of attendees (123 of 143, 86%) found the sessions to be a lot or extremely stimulating, and almost all (96%) were a lot or extremely satisfied with how the RIP sessions kept them abreast of their colleagues' academic interests. In addition, 92% judged the session's climate to be a lot or extremely supportive, and 88% deemed the balance of presentation to discussion to be just right. Attendees believed that they were most helpful to the presenter in terms of conceiving ideas for alternative methods to be used to answer the research question and in providing strategies to improve data collection (Table 3).
| Insight Offered | n (%) |
|---|---|
| Ideas for alternate methods | 92 (64%) |
| Strategies to improve data collection | 85 (59.4%) |
| New hypotheses generated | 84 (58.7%) |
| Ideas for next steps/future direction/studies | 83 (58%) |
| New outcomes suggested that should be considered | 69 (48%) |
| Clarification of the research questions | 61 (43%) |
| Input about what is most novel/emnteresting about the work | 60 (42%) |
| Guidance about the real implications of the work | 59 (41%) |
| Suggestions for alternate analyses or analytical strategies | 51 (36%) |
The free text comments primarily addressed how the presenters' research ideas were helped by the session:
There were great ideas for improvementincluding practical approaches for recruitment.
The session made me think of the daily routine things that we do that could be studied.
There were some great ideas to help Dr. A make the study more simple, doable, and practical. There were also some good ideas regarding potential sources of funding.
Academic Success
Of the 15 projects, 6 have been published in peer‐reviewed journals as first‐ or senior‐authored publications.510 Of these, 3 were presented at national meetings prior to publication. Four additional projects have been presented at a national society's annual meeting, all of which are being prepared for publication. Of the remaining 5 presentations, 4 were terminated because of the low likelihood of academic success. The remaining project is ongoing.
Comparatively, scholarly output in the prior year by the 24 physicians in the hospitalist group was 4 first‐ or senior‐authored publications in peer‐reviewed journals and 3 presentations at national meetings.
Discussion
In this article, we report our experience with the RIP conference. The sessions were perceived to be intellectually stimulating and supportive, whereas the discussions proved helpful in advancing project ideas. Ample discussion time and good attendance were thought to be critical to the success.
To our knowledge, this is the first article gathering feedback from attendees and presenters at a RIP conference and to track academic outcomes. Several types of meetings have been established within faculty and trainee groups to support and encourage scholarly activities.11, 12 The benefits of peer collaboration and peer mentoring have been described in the literature.13, 14 For example, Edwards described the success of shortstop meetings among small groups of faculty members every 4‐6 weeks in which discussions of research projects and mutual feedback would occur.15 Santucci described peer‐mentored research development meetings, with increased research productivity.12
Mentoring is critically important for academic success in medicine.1619 When divisions have limited senior mentors available, peer mentoring has proven to be indispensable as a mechanism to support faculty members.2022 The RIP conference provided a forum for peer mentoring and provided a partial solution to the limited resource of experienced research mentors in the division. The RIP sessions appear to have helped to bring the majority of presented ideas to academic fruition. Perhaps even more important, the sessions were able to terminate studies judged to have low academic promise before the faculty had invested significant time.
Several limitations of our study should be considered. First, this study involved a research‐in‐progress conference coordinated for a group of hospitalist physicians at 1 institution, and the results may not be generalizable. Second, although attendance was good at each conference, some faculty members did not come to many sessions. It is possible that those not attending may have rated the sessions differently. Session evaluations were anonymous, and we do not know whether specific attendees rated all sessions highly, thereby resulting in some degree of clustering. Third, this study did not compare the effectiveness of the RIP conference with other peer‐mentorship models. Finally, our study was uncontrolled. Although it would not be possible to restrict specific faculty from presenting at or attending the RIP conference, we intend to more carefully collect attendance data to see whether there might be a dose‐response effect with respect to participation in this conference and academic success.
In conclusion, our RIP conference was perceived as valuable by our group and was associated with academic success. In our division, the RIP conference serves as a way to operationalize peer mentoring. Our findings may help other groups to refine either the focus or format of their RIP sessions and those wishing to initiate such a conference.
The research‐in‐progress (RIP) conference is commonplace in academia, but there are no studies that objectively characterize its value. Bringing faculty together away from revenue‐generating activities carries a significant cost. As such, measuring the success of such gatherings is necessary.
Mentors are an invaluable influence on the careers of junior faculty members, helping them to produce high‐quality research.13 Unfortunately, some divisions lack mentorship to support the academic needs of less experienced faculty.1 Peer mentorship may be a solution. RIP sessions represent an opportunity to intentionally formalize peer mentoring. Further, these sessions can facilitate collaborations as individuals become aware of colleagues' interests. The goal of this study was to assess the value of the research‐in‐progress conference initiated within the hospitalist division at our institution.
Methods
Study Design
This cohort study was conducted to evaluate the value of the RIP conference among hospitalists in our division and the academic outcomes of the projects.
Setting and Participants
The study took place at Johns Hopkins Bayview Medical Center (JHBMC), a 335‐bed university‐affiliated medical center in Baltimore, Maryland. The hospitalist division consists of faculty physicians, nurse practitioners, and physician assistants (20.06 FTE physicians and 7.41 FTE midlevel providers). Twelve (54%) of our faculty members are female, and the mean age of providers is 35.7 years. The providers have been practicing hospitalist medicine for 3.0 years on average; 2 (9%) are clinical associates, 16 (73%) are instructors, and 3 (14%) are assistant professors.
All faculty members presenting at the RIP session were members of the division. A senior faculty member (a professor in the Division of General Internal Medicine) helps to coordinate the conference. The group's research assistant was present at the sessions and was charged with data collection and collation.
The Johns Hopkins University institutional review board approved the study.
The Research in Progress Conference
During the 2009 academic year, our division held 15 RIP sessions. At each session, 1 faculty member presented a research proposal. The goal of each session was to provide a forum where faculty members could share their research ideas (specific aims, hypotheses, planned design, outcome measures, analytic plans, and preliminary results [if applicable]) in order to receive feedback. The senior faculty member met with the presenter prior to each session in order to: (1) ensure that half the RIP time was reserved for discussion and (2) review the presenter's goals so these would be made explicit to peers. The coordinator of the RIP conference facilitated the discussion, solicited input from all attendees, and encouraged constructive criticism.
Evaluation, Data Collection, and Analysis
At the end of each session, attendees (who were exclusively members of the hospitalist division) were asked to complete an anonymous survey. The 1‐page instrument was designed (1) with input from curriculum development experts4 and (2) after a review of the literature about RIP conferences. These steps conferred content validity to the instrument, which assessed perceptions about the session's quality and what was learned. Five‐point Likert scales were used to characterize the conference's success in several areas, including being intellectually/professionally stimulating and keeping them apprised of their colleagues' interests. The survey also assessed the participatory nature of the conference (balance of presentation vs discussion), its climate (extremely critical vs extremely supportive), and how the conference assisted the presenter. The presenters completed a distinct survey related to how helpful the conference was in improving/enhancing their projects. A final open‐ended section invited additional comments. The instrument was piloted and iteratively revised before its use in this study.
For the projects presented, we assessed the percentage that resulted in a peer‐reviewed publication or a presentation at a national meeting.
Results
The mean number of attendees at the RIP sessions was 9.6 persons. A total of 143 evaluations were completed. All 15 presenters (100%) completed their assessments. The research ideas presented spanned a breadth of topics in clinical research, quality improvement, policy, and professional development (Table 1).
| Session | Date | Presenter | Topic | Evaluations Completed |
|---|---|---|---|---|
| 1 | 7/2008 | Dr. CS | Hospital medicine in Canada versus the United States | 7 |
| 2 | 7/2008 | Dr. RT | Procedures by hospitalists | 9 |
| 3 | 8/2008 | Dr. MA | Clostridium difficile treatment in the hospital | 11 |
| 4 | 8/2008 | Dr. EH | Active bed management | 6 |
| 5 | 9/2008 | Dr. AS | Medication reconciliation for geriatric inpatients | 10 |
| 6 | 9/2008 | Dr. DT | Time‐motion study of hospitalists | 10 |
| 7 | 10/2008 | Dr. KV | e‐Triage pilot | 16 |
| 8 | 11/2008 | Dr. EH | Assessing clinical performance of hospitalists | 7 |
| 9 | 12/2008 | Dr. SC | Trends and implications of hospitalists' morale | 8 |
| 10 | 1/2009 | Dr. TB | Lessons learned: tracking urinary catheter use at Bayview | 11 |
| 11 | 2/2009 | Dr. FK | Utilizing audit and feedback to improve performance in tobacco dependence counseling | 12 |
| 12 | 3/2009 | Dr. MK | Survivorship care plans | 7 |
| 13 | 4/2009 | Dr. DK | Outpatient provider preference for discharge summary format/style/length | 7 |
| 14 | 5/2009 | Dr. RW | Comparing preoperative consults done by hospitalists and cardiologists | 11 |
| 15 | 6/2009 | Dr. AK | Development of Web‐based messaging tool for providers | 12 |
Presenter Perspective
All 15 presenters (100%) felt a lot or tremendously supported during their sessions. Thirteen physicians (86%) believed that the sessions were a lot or tremendously helpful in advancing their projects. The presenters believed that the guidance and discussions related to their research ideas, aims, hypotheses, and plans were most helpful for advancing their projects (Table 2).
| Not at All, n (%) | A Little, n (%) | Some, n (%) | A Lot, n (%) | Tremendously, n (%) | |
|---|---|---|---|---|---|
| General questions: | |||||
| Intellectually/professionally stimulating | 0 (0) | 0 (0) | 0 (0) | 5 (33) | 10 (66) |
| Feeling supported by your colleagues in your scholarly pursuits | 0 (0) | 0 (0) | 0 (0) | 4 (27) | 11 (73) |
| Session helpful in the following areas: | |||||
| Advancing your project | 0 (0) | 0 (0) | 2 (13) | 5 (33) | 8 (53) |
| Generated new hypotheses | 1 (6) | 3 (20) | 5 (33) | 5 (33) | 1 (6) |
| Clarification of research questions | 0 (0) | 2 (13) | 4 (27) | 7 (47) | 2 (13) |
| Ideas for alternate methods | 1 (6) | 1 (6) | 2 (13) | 7 (47) | 4 (27) |
| New outcomes suggested | 1 (6) | 2 (13) | 2 (13) | 5 (33) | 5 (33) |
| Strategies to improve or enhance data collection | 0 (0) | 2 (13) | 0 (0) | 8 (53) | 5 (33) |
| Suggestions for alternate analyses or analytical strategies | 1 (1) | 1 (6) | 4 (27) | 5 (33) | 4 (27) |
| Input into what is most novel/emnteresting about this work | 0 (0) | 2 (13) | 3 (20) | 6 (40) | 4 (27) |
| Guidance about the implications of the work | 1 (6) | 2 (13) | 1 (6) | 7 (47) | 4 (27) |
| Ideas about next steps or future direction/studies | 0 (0) | 0 (0) | 3 (21) | 8 (57) | 3 (21) |
Examples of the written comments are:
I was overwhelmed by how engaged people were in my project.
The process of preparing for the session and then the discussion both helped my thinking. Colleagues were very supportive.
I am so glad I heard these comments and received this feedback now, rather than from peer reviewers selected by a journal to review my study. It would have been a much more difficult situation to fix at that later time.
Attendee Perspective
The majority of attendees (123 of 143, 86%) found the sessions to be a lot or extremely stimulating, and almost all (96%) were a lot or extremely satisfied with how the RIP sessions kept them abreast of their colleagues' academic interests. In addition, 92% judged the session's climate to be a lot or extremely supportive, and 88% deemed the balance of presentation to discussion to be just right. Attendees believed that they were most helpful to the presenter in terms of conceiving ideas for alternative methods to be used to answer the research question and in providing strategies to improve data collection (Table 3).
| Insight Offered | n (%) |
|---|---|
| Ideas for alternate methods | 92 (64%) |
| Strategies to improve data collection | 85 (59.4%) |
| New hypotheses generated | 84 (58.7%) |
| Ideas for next steps/future direction/studies | 83 (58%) |
| New outcomes suggested that should be considered | 69 (48%) |
| Clarification of the research questions | 61 (43%) |
| Input about what is most novel/emnteresting about the work | 60 (42%) |
| Guidance about the real implications of the work | 59 (41%) |
| Suggestions for alternate analyses or analytical strategies | 51 (36%) |
The free text comments primarily addressed how the presenters' research ideas were helped by the session:
There were great ideas for improvementincluding practical approaches for recruitment.
The session made me think of the daily routine things that we do that could be studied.
There were some great ideas to help Dr. A make the study more simple, doable, and practical. There were also some good ideas regarding potential sources of funding.
Academic Success
Of the 15 projects, 6 have been published in peer‐reviewed journals as first‐ or senior‐authored publications.510 Of these, 3 were presented at national meetings prior to publication. Four additional projects have been presented at a national society's annual meeting, all of which are being prepared for publication. Of the remaining 5 presentations, 4 were terminated because of the low likelihood of academic success. The remaining project is ongoing.
Comparatively, scholarly output in the prior year by the 24 physicians in the hospitalist group was 4 first‐ or senior‐authored publications in peer‐reviewed journals and 3 presentations at national meetings.
Discussion
In this article, we report our experience with the RIP conference. The sessions were perceived to be intellectually stimulating and supportive, whereas the discussions proved helpful in advancing project ideas. Ample discussion time and good attendance were thought to be critical to the success.
To our knowledge, this is the first article gathering feedback from attendees and presenters at a RIP conference and to track academic outcomes. Several types of meetings have been established within faculty and trainee groups to support and encourage scholarly activities.11, 12 The benefits of peer collaboration and peer mentoring have been described in the literature.13, 14 For example, Edwards described the success of shortstop meetings among small groups of faculty members every 4‐6 weeks in which discussions of research projects and mutual feedback would occur.15 Santucci described peer‐mentored research development meetings, with increased research productivity.12
Mentoring is critically important for academic success in medicine.1619 When divisions have limited senior mentors available, peer mentoring has proven to be indispensable as a mechanism to support faculty members.2022 The RIP conference provided a forum for peer mentoring and provided a partial solution to the limited resource of experienced research mentors in the division. The RIP sessions appear to have helped to bring the majority of presented ideas to academic fruition. Perhaps even more important, the sessions were able to terminate studies judged to have low academic promise before the faculty had invested significant time.
Several limitations of our study should be considered. First, this study involved a research‐in‐progress conference coordinated for a group of hospitalist physicians at 1 institution, and the results may not be generalizable. Second, although attendance was good at each conference, some faculty members did not come to many sessions. It is possible that those not attending may have rated the sessions differently. Session evaluations were anonymous, and we do not know whether specific attendees rated all sessions highly, thereby resulting in some degree of clustering. Third, this study did not compare the effectiveness of the RIP conference with other peer‐mentorship models. Finally, our study was uncontrolled. Although it would not be possible to restrict specific faculty from presenting at or attending the RIP conference, we intend to more carefully collect attendance data to see whether there might be a dose‐response effect with respect to participation in this conference and academic success.
In conclusion, our RIP conference was perceived as valuable by our group and was associated with academic success. In our division, the RIP conference serves as a way to operationalize peer mentoring. Our findings may help other groups to refine either the focus or format of their RIP sessions and those wishing to initiate such a conference.
- ,,, et al.Junior faculty members' mentoring relationships and their professional development in US medical schools.Acad Med.1998;73:318–323.
- ,.Mentors, Advisors and Role Models in Graduate and Professional Education.Washington, DC:Association of Academic Health Centers;1996.
- ,.Characteristics of the successful researcher and implications for faculty development.J Med Educ.1986;61:22–31.
- ,,.Curriculum Development for Medical Education: A Six‐Step Approach.2nd ed.Baltimore, MD:The Johns Hopkins University Press;2009.
- ,,, et al.Characteristics of hospitalists and hospitalist programs in the United States and Canada.J Clin Outcomes Meas.2009;16:69–74
- ,,, et al.Procedures performed by hospitalist and non‐hospitalist general internists.J Gen Intern Med.2010;25:448–452.
- ,,, et al.Intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis: an observational study and review of the literature [review].J Hosp Med.2010;5:E1–E9.
- ,,, et al.Active bed management by hospitalists and emergency department throughput.Ann Intern Med.2008;149:804–811.
- ,,, et al.Transitioning to breast cancer survivorship: perspectives of patients, cancer specialists, and primary care providers.J Gen Intern Med.2009;24(Suppl 2):S459–S466.
- ,,, et al.Utilizing audit and feedback to improve hospitalists' performance in tobacco dependence counseling.Nicotine Tob Res.2010;12:797–800.
- ,,, et al.An internal medicine interest group research program can improve scholarly productivity of medical students and foster mentoring relationships with internists.Teach Learn Med.2008;20:163–167.
- ,,, et al.Peer‐mentored research development meeting: a model for successful peer mentoring among junior level researchers.Acad Psychiatry.2008;32:493–497.
- ,,, et al.Mentoring junior faculty in geriatric oncology: report from the cancer and aging research group.J Clin Oncol.2008;26:3125–3127.
- ,,the Canadian Critical Care Trials Group.Investigator‐led clinical research consortia: the Canadian Critical Care Trials Group.Crit Care Med.2009;37(1):S165–S172.
- .“Short stops”: peer support of scholarly activity.Acad Med.2002;77:939.
- ,,,,,,.Mentorship in academic general internal medicine. Results of a survey of mentors.J Gen Intern Med.2005;20:1014–1018.
- ,,, et al.Making the most of mentors: a guide for mentees.Acad Med.2009;84:140–144.
- ,,.Mentoring in academic medicine: a systematic review.JAMA.2006;296:1103–1115.
- ,,, et al.Assessing the role of influential mentors in the research development of primary care fellows.Acad Med.2004;79:865–872.
- ,,.Peer group mentoring of junior faculty.Acad Psychiatry.2008;32:230–235.
- ,,,.Facilitated peer mentorship: a pilot program for academic advancement of female medical faculty.J Womens Health.2008;17:1009–1015.
- ,Mentoring faculty in academic medicine. A new paradigm?J Gen Intern Med.2005;20:866–870.
- ,,, et al.Junior faculty members' mentoring relationships and their professional development in US medical schools.Acad Med.1998;73:318–323.
- ,.Mentors, Advisors and Role Models in Graduate and Professional Education.Washington, DC:Association of Academic Health Centers;1996.
- ,.Characteristics of the successful researcher and implications for faculty development.J Med Educ.1986;61:22–31.
- ,,.Curriculum Development for Medical Education: A Six‐Step Approach.2nd ed.Baltimore, MD:The Johns Hopkins University Press;2009.
- ,,, et al.Characteristics of hospitalists and hospitalist programs in the United States and Canada.J Clin Outcomes Meas.2009;16:69–74
- ,,, et al.Procedures performed by hospitalist and non‐hospitalist general internists.J Gen Intern Med.2010;25:448–452.
- ,,, et al.Intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis: an observational study and review of the literature [review].J Hosp Med.2010;5:E1–E9.
- ,,, et al.Active bed management by hospitalists and emergency department throughput.Ann Intern Med.2008;149:804–811.
- ,,, et al.Transitioning to breast cancer survivorship: perspectives of patients, cancer specialists, and primary care providers.J Gen Intern Med.2009;24(Suppl 2):S459–S466.
- ,,, et al.Utilizing audit and feedback to improve hospitalists' performance in tobacco dependence counseling.Nicotine Tob Res.2010;12:797–800.
- ,,, et al.An internal medicine interest group research program can improve scholarly productivity of medical students and foster mentoring relationships with internists.Teach Learn Med.2008;20:163–167.
- ,,, et al.Peer‐mentored research development meeting: a model for successful peer mentoring among junior level researchers.Acad Psychiatry.2008;32:493–497.
- ,,, et al.Mentoring junior faculty in geriatric oncology: report from the cancer and aging research group.J Clin Oncol.2008;26:3125–3127.
- ,,the Canadian Critical Care Trials Group.Investigator‐led clinical research consortia: the Canadian Critical Care Trials Group.Crit Care Med.2009;37(1):S165–S172.
- .“Short stops”: peer support of scholarly activity.Acad Med.2002;77:939.
- ,,,,,,.Mentorship in academic general internal medicine. Results of a survey of mentors.J Gen Intern Med.2005;20:1014–1018.
- ,,, et al.Making the most of mentors: a guide for mentees.Acad Med.2009;84:140–144.
- ,,.Mentoring in academic medicine: a systematic review.JAMA.2006;296:1103–1115.
- ,,, et al.Assessing the role of influential mentors in the research development of primary care fellows.Acad Med.2004;79:865–872.
- ,,.Peer group mentoring of junior faculty.Acad Psychiatry.2008;32:230–235.
- ,,,.Facilitated peer mentorship: a pilot program for academic advancement of female medical faculty.J Womens Health.2008;17:1009–1015.
- ,Mentoring faculty in academic medicine. A new paradigm?J Gen Intern Med.2005;20:866–870.
Copyright © 2011 Society of Hospital Medicine
Off‐hours care: not so off
The word housestaff came from an era when you moved into the hospital (house) for your internship (staff). In those days, call every other night meant you missed half the learning opportunities. In those days, supervision was as remote as it could get. My, have things changed.
In 2003, the Accreditation Council for Graduate Medical Education (ACGME) implemented duty hour standards that called for restricting resident duty hours to no more than 80 hours per week, and no more than 24 continuous hours with an additional 6 hours for transfer of care.1 The expectation was that limiting work hours would have positive effects on outcomes, such as resident fatigue, the educational experience, and patient safety. A recent systematic review commissioned by the ACGME reviewed three decades worth of research on duty hours and patient safety. It reviewed laboratory studies of resident performance on clinical simulations, and tests of cognitive and fine motor skills under conditions of sleep deprivation. The results of these studies imply that limiting residents' duty hours should have positive effects on patient safety. However, that assumption has not been borne out. Paradoxically, examination of studies of alternative duty hour schedules, as well as the follow‐up studies on the impact of the New York duty hour rules and ACGME duty hour regulations have documented few measurable effects on patient safety.2 Thus, the simplistic view that fewer hours worked equals better patient care deserves a second‐look. In fact, a cause‐and‐effect relationship between resident fatigue and medical errors has yet to be proven.
The hospitalist movement was in full swing by 2003 and had already transformed the care of hospitalized patients. The early studies of hospitalists focused on cost and comparing outcomes with those of other providers. As many studies showed the economic benefit of hospitalists, residency programs began redesigning their inpatient services with hospitalists at the helm. Institutions sponsoring graduate medical education programs saw hospitalists as a necessary resource to enable and maintain compliance with duty hour reform. Some institutions created hospitalist‐only services with no housestaff (nonteaching services). Others created hospitalist teaching services that used indirect supervision at night from a hospitalist on‐call from home, while others had on‐site hospitalists at night that provided direct supervision of housestaff. It is clear that hospitalists are a versatile and valuable resource.
In this issue of the Journal of Hospital Medicine, Khanna and colleagues3 describe a retrospective medical record review at a large, urban academic hospital that evaluates the potential association between night admissions by night‐shiftbased hospitalists and a resident night float system, and hospitalization‐relevant outcomes. The outcomes included length of stay, hospital charges, Intensive Care Unit (ICU) transfers, Emergency Department visits within 30 days of discharge, 30‐day readmission rates, and poor outcomes within the first 24 hours of admission. Their results showed that night or weekend admission was not associated with worse hospitalization‐relevant outcomes. The extension of on‐site faculty supervision to nights, as well as awards for nursing excellence, likely had a strong positive impact on these outcomes. In some cases, night or weekend admission was associated with better outcomes, particularly in terms of ICU transfer during hospitalization and hospital charges. Maybe off‐hours care is not so off after all.
I am not sure where we are going, but the duty hour standards have been revised again. The new duty hour standards limit continuous duty hours for 1st‐year residents to 16, and all others to 24. Upper‐level residents can stay an additional 4 hours for transfer of care.4 Functionally, this proposed standard eliminates overnight call for 1st‐year residents. Although I would never advocate returning to the days of yore where autonomy and independence were the rule, I mourn the loss of extended shifts for 1st‐year residents. The loss of the extended shift will come at the expense of overnight call in many programs. I worry about the loss of some unique teaching moments for all residents, and the increased number of transitions in a patients' care. The hospital is a different place at night. It is less chaotic with fewer visitors, and there are less interruptions in patient care. There is time for teaching and learning because the work intensity is lower. The bedside teaching among senior and junior residents, during an acute patient episode in the middle of the night, is an experience that many of us would recount as an invaluable part of our training. It is also a time when on‐site faculty supervision and nurse staffing ratios are typically reduced. In other words, the entire system of care is different at night. This dichotomy in day versus night medicine has spurred research into whether patient outcomes differed between the two. Various investigators have looked at the differences in care, and there is some data to support that patient care outcomes are worse at night than during the day.58
The results from Khanna and colleagues3 are welcome news, since graduate medical education is undergoing a paradigm shift with further reductions in duty hours. Hospital‐based medicine is a 24‐hour operation, 7 days a week. We don't get to pick when patients are admitted or need care. In fact, this study demonstrates that 58% of the admissions came after 7 PM. It turns out that this pattern of admissions is not atypical.9 In light of the new standards, programs are left deciding where to deploy their 1st‐year resident resources. There are essentially two types of models to provide patient care off‐hours in the absence of an extended shift option: night float and night medicine. Night float implies that a trainee, usually a resident, comes from a different site or rotation to provide nocturnal care of a group of patients. That resident may do one isolated night of coverage or several nights in a row. The resident may perform cross‐cover functions, admit patients, or do both. Night medicine refers to having teams of housestaff at night along with an educational program as part of a rotation. The latter implies that trainees will get the full educational experience whether working days or nights. If one designs a model of care that puts 1st‐year residents where the volume is, they would come in late and leave early in the morning the next day. Both create more handoffs in patient care, which critics of these models have said lead to worse patient outcomes. This research challenges such earlier work and should make us all feel more comfortable that care at night does not need to be different if the proper systems are in place to manage it.
The graduate medical education community needs to take a step back and ask: Where are we going? As a long‐standing program director, I don't know anymore. As training has become competency‐based, and milestones are being developed for each level of training, we need to ask if reducing hours further is in the best interests of graduate medical education and, ultimately, the general public. I have long been interested in work intensity as a contributor to resident fatigue, burnout, and errors. A systematic review of resident workload and work activity looked at 21 studies examining resident time allocation and suggests that at least one‐third of resident time is spent on activities of limited educational value. Eight of the nine studies evaluating the impact of resident workload on patient care showed a negative impact on outcomes, including length of stay, mortality, patient satisfaction, medication errors, and lab utilization.10 If we are going to further restrict duty hours, then we need to transform the learning environment in a similar fashion. It does not seem logical to restrict duty hours further without a major redesign of the learning environment. I fear that all we have done is create more work compression because the system of care will be further strained in its weakest areas.
Hospitalists are uniquely poised to mitigate some of these concerns by being available for direct and indirect supervision, as well as being highly skilled at working in our complex systems. They are experts in systems‐based practice. The extension of direct faculty supervision to nights may be a major factor in eliminating the variances in care between nights and days. Not every program or institution will have the ability to employ night hospitalists, let alone have them in a supervisory and teaching role. Those that do can take great comfort in this research. Those that don't must challenge themselves to think about how to get there.
We all share the collective goal of producing the next generation of well‐trained physicians who will practice safe and effective care. As we begin to embrace competency‐based training, the prism through which we view patient safety must extend beyond duty hours and include learning environment reform. While the Khanna et al.3 study supports that off‐hours care is no longer off, we need to be careful that we don't drift too far towards shift work as the fundamental structure of medical training. In the beginning, residents lived in the hospital, thus the term. As we move through these stormy seas, we don't want to trade patient safety for transient trainees. There is a middle ground here that will be informed by future research. There is something to be said for living in your house.
- ACGME.Duty hour requirements. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed August 16,2010.
- ,,, et al.Systematic review of the literature on the impact of variation in residents' duty hour schedules on patient safety. Available at: http://acgme‐2010standards.org/pdf/Jefferson_Medical_College_Duty_Hours_Review.pdf. Accessed August 18,2010.
- ,,, et al.The association between night or weekend admission and hospitalization‐relevant patient outcomes.J Hosp Med2011;6:10–14.
- ACGME.Proposed duty hour standards. Available at: http://acgme‐2010standards.org/pdf/Proposed_Standards.pdf. Accessed August 16,2010.
- ,.Mortality among patients admitted to hospitals on weekends as compared with weekdays.N Engl J Med.2001;345(9):663–668.
- ,,, et al.Survival from in‐hospital cardiac arrest during nights and weekends.JAMA.2008;299(7):785–792.
- ,,,,.What effect does inpatient physician specialty and experience have on clinical outcomes and resource utilization on a general medicine service?J Gen Intern Med.2004;19:395–401.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- .Like night and day—shedding light on off‐hours care.N Engl J Med.2008;358(20):2091–2093.
- ,,.Systematic review of resident workload and work activity: impact on resident education and patient care. Available at: http://acgme‐2010standards.org/pdf/Resident_Duty_Hours_and_Related_Topics.pdf. Accessed on August 16,2010.
The word housestaff came from an era when you moved into the hospital (house) for your internship (staff). In those days, call every other night meant you missed half the learning opportunities. In those days, supervision was as remote as it could get. My, have things changed.
In 2003, the Accreditation Council for Graduate Medical Education (ACGME) implemented duty hour standards that called for restricting resident duty hours to no more than 80 hours per week, and no more than 24 continuous hours with an additional 6 hours for transfer of care.1 The expectation was that limiting work hours would have positive effects on outcomes, such as resident fatigue, the educational experience, and patient safety. A recent systematic review commissioned by the ACGME reviewed three decades worth of research on duty hours and patient safety. It reviewed laboratory studies of resident performance on clinical simulations, and tests of cognitive and fine motor skills under conditions of sleep deprivation. The results of these studies imply that limiting residents' duty hours should have positive effects on patient safety. However, that assumption has not been borne out. Paradoxically, examination of studies of alternative duty hour schedules, as well as the follow‐up studies on the impact of the New York duty hour rules and ACGME duty hour regulations have documented few measurable effects on patient safety.2 Thus, the simplistic view that fewer hours worked equals better patient care deserves a second‐look. In fact, a cause‐and‐effect relationship between resident fatigue and medical errors has yet to be proven.
The hospitalist movement was in full swing by 2003 and had already transformed the care of hospitalized patients. The early studies of hospitalists focused on cost and comparing outcomes with those of other providers. As many studies showed the economic benefit of hospitalists, residency programs began redesigning their inpatient services with hospitalists at the helm. Institutions sponsoring graduate medical education programs saw hospitalists as a necessary resource to enable and maintain compliance with duty hour reform. Some institutions created hospitalist‐only services with no housestaff (nonteaching services). Others created hospitalist teaching services that used indirect supervision at night from a hospitalist on‐call from home, while others had on‐site hospitalists at night that provided direct supervision of housestaff. It is clear that hospitalists are a versatile and valuable resource.
In this issue of the Journal of Hospital Medicine, Khanna and colleagues3 describe a retrospective medical record review at a large, urban academic hospital that evaluates the potential association between night admissions by night‐shiftbased hospitalists and a resident night float system, and hospitalization‐relevant outcomes. The outcomes included length of stay, hospital charges, Intensive Care Unit (ICU) transfers, Emergency Department visits within 30 days of discharge, 30‐day readmission rates, and poor outcomes within the first 24 hours of admission. Their results showed that night or weekend admission was not associated with worse hospitalization‐relevant outcomes. The extension of on‐site faculty supervision to nights, as well as awards for nursing excellence, likely had a strong positive impact on these outcomes. In some cases, night or weekend admission was associated with better outcomes, particularly in terms of ICU transfer during hospitalization and hospital charges. Maybe off‐hours care is not so off after all.
I am not sure where we are going, but the duty hour standards have been revised again. The new duty hour standards limit continuous duty hours for 1st‐year residents to 16, and all others to 24. Upper‐level residents can stay an additional 4 hours for transfer of care.4 Functionally, this proposed standard eliminates overnight call for 1st‐year residents. Although I would never advocate returning to the days of yore where autonomy and independence were the rule, I mourn the loss of extended shifts for 1st‐year residents. The loss of the extended shift will come at the expense of overnight call in many programs. I worry about the loss of some unique teaching moments for all residents, and the increased number of transitions in a patients' care. The hospital is a different place at night. It is less chaotic with fewer visitors, and there are less interruptions in patient care. There is time for teaching and learning because the work intensity is lower. The bedside teaching among senior and junior residents, during an acute patient episode in the middle of the night, is an experience that many of us would recount as an invaluable part of our training. It is also a time when on‐site faculty supervision and nurse staffing ratios are typically reduced. In other words, the entire system of care is different at night. This dichotomy in day versus night medicine has spurred research into whether patient outcomes differed between the two. Various investigators have looked at the differences in care, and there is some data to support that patient care outcomes are worse at night than during the day.58
The results from Khanna and colleagues3 are welcome news, since graduate medical education is undergoing a paradigm shift with further reductions in duty hours. Hospital‐based medicine is a 24‐hour operation, 7 days a week. We don't get to pick when patients are admitted or need care. In fact, this study demonstrates that 58% of the admissions came after 7 PM. It turns out that this pattern of admissions is not atypical.9 In light of the new standards, programs are left deciding where to deploy their 1st‐year resident resources. There are essentially two types of models to provide patient care off‐hours in the absence of an extended shift option: night float and night medicine. Night float implies that a trainee, usually a resident, comes from a different site or rotation to provide nocturnal care of a group of patients. That resident may do one isolated night of coverage or several nights in a row. The resident may perform cross‐cover functions, admit patients, or do both. Night medicine refers to having teams of housestaff at night along with an educational program as part of a rotation. The latter implies that trainees will get the full educational experience whether working days or nights. If one designs a model of care that puts 1st‐year residents where the volume is, they would come in late and leave early in the morning the next day. Both create more handoffs in patient care, which critics of these models have said lead to worse patient outcomes. This research challenges such earlier work and should make us all feel more comfortable that care at night does not need to be different if the proper systems are in place to manage it.
The graduate medical education community needs to take a step back and ask: Where are we going? As a long‐standing program director, I don't know anymore. As training has become competency‐based, and milestones are being developed for each level of training, we need to ask if reducing hours further is in the best interests of graduate medical education and, ultimately, the general public. I have long been interested in work intensity as a contributor to resident fatigue, burnout, and errors. A systematic review of resident workload and work activity looked at 21 studies examining resident time allocation and suggests that at least one‐third of resident time is spent on activities of limited educational value. Eight of the nine studies evaluating the impact of resident workload on patient care showed a negative impact on outcomes, including length of stay, mortality, patient satisfaction, medication errors, and lab utilization.10 If we are going to further restrict duty hours, then we need to transform the learning environment in a similar fashion. It does not seem logical to restrict duty hours further without a major redesign of the learning environment. I fear that all we have done is create more work compression because the system of care will be further strained in its weakest areas.
Hospitalists are uniquely poised to mitigate some of these concerns by being available for direct and indirect supervision, as well as being highly skilled at working in our complex systems. They are experts in systems‐based practice. The extension of direct faculty supervision to nights may be a major factor in eliminating the variances in care between nights and days. Not every program or institution will have the ability to employ night hospitalists, let alone have them in a supervisory and teaching role. Those that do can take great comfort in this research. Those that don't must challenge themselves to think about how to get there.
We all share the collective goal of producing the next generation of well‐trained physicians who will practice safe and effective care. As we begin to embrace competency‐based training, the prism through which we view patient safety must extend beyond duty hours and include learning environment reform. While the Khanna et al.3 study supports that off‐hours care is no longer off, we need to be careful that we don't drift too far towards shift work as the fundamental structure of medical training. In the beginning, residents lived in the hospital, thus the term. As we move through these stormy seas, we don't want to trade patient safety for transient trainees. There is a middle ground here that will be informed by future research. There is something to be said for living in your house.
The word housestaff came from an era when you moved into the hospital (house) for your internship (staff). In those days, call every other night meant you missed half the learning opportunities. In those days, supervision was as remote as it could get. My, have things changed.
In 2003, the Accreditation Council for Graduate Medical Education (ACGME) implemented duty hour standards that called for restricting resident duty hours to no more than 80 hours per week, and no more than 24 continuous hours with an additional 6 hours for transfer of care.1 The expectation was that limiting work hours would have positive effects on outcomes, such as resident fatigue, the educational experience, and patient safety. A recent systematic review commissioned by the ACGME reviewed three decades worth of research on duty hours and patient safety. It reviewed laboratory studies of resident performance on clinical simulations, and tests of cognitive and fine motor skills under conditions of sleep deprivation. The results of these studies imply that limiting residents' duty hours should have positive effects on patient safety. However, that assumption has not been borne out. Paradoxically, examination of studies of alternative duty hour schedules, as well as the follow‐up studies on the impact of the New York duty hour rules and ACGME duty hour regulations have documented few measurable effects on patient safety.2 Thus, the simplistic view that fewer hours worked equals better patient care deserves a second‐look. In fact, a cause‐and‐effect relationship between resident fatigue and medical errors has yet to be proven.
The hospitalist movement was in full swing by 2003 and had already transformed the care of hospitalized patients. The early studies of hospitalists focused on cost and comparing outcomes with those of other providers. As many studies showed the economic benefit of hospitalists, residency programs began redesigning their inpatient services with hospitalists at the helm. Institutions sponsoring graduate medical education programs saw hospitalists as a necessary resource to enable and maintain compliance with duty hour reform. Some institutions created hospitalist‐only services with no housestaff (nonteaching services). Others created hospitalist teaching services that used indirect supervision at night from a hospitalist on‐call from home, while others had on‐site hospitalists at night that provided direct supervision of housestaff. It is clear that hospitalists are a versatile and valuable resource.
In this issue of the Journal of Hospital Medicine, Khanna and colleagues3 describe a retrospective medical record review at a large, urban academic hospital that evaluates the potential association between night admissions by night‐shiftbased hospitalists and a resident night float system, and hospitalization‐relevant outcomes. The outcomes included length of stay, hospital charges, Intensive Care Unit (ICU) transfers, Emergency Department visits within 30 days of discharge, 30‐day readmission rates, and poor outcomes within the first 24 hours of admission. Their results showed that night or weekend admission was not associated with worse hospitalization‐relevant outcomes. The extension of on‐site faculty supervision to nights, as well as awards for nursing excellence, likely had a strong positive impact on these outcomes. In some cases, night or weekend admission was associated with better outcomes, particularly in terms of ICU transfer during hospitalization and hospital charges. Maybe off‐hours care is not so off after all.
I am not sure where we are going, but the duty hour standards have been revised again. The new duty hour standards limit continuous duty hours for 1st‐year residents to 16, and all others to 24. Upper‐level residents can stay an additional 4 hours for transfer of care.4 Functionally, this proposed standard eliminates overnight call for 1st‐year residents. Although I would never advocate returning to the days of yore where autonomy and independence were the rule, I mourn the loss of extended shifts for 1st‐year residents. The loss of the extended shift will come at the expense of overnight call in many programs. I worry about the loss of some unique teaching moments for all residents, and the increased number of transitions in a patients' care. The hospital is a different place at night. It is less chaotic with fewer visitors, and there are less interruptions in patient care. There is time for teaching and learning because the work intensity is lower. The bedside teaching among senior and junior residents, during an acute patient episode in the middle of the night, is an experience that many of us would recount as an invaluable part of our training. It is also a time when on‐site faculty supervision and nurse staffing ratios are typically reduced. In other words, the entire system of care is different at night. This dichotomy in day versus night medicine has spurred research into whether patient outcomes differed between the two. Various investigators have looked at the differences in care, and there is some data to support that patient care outcomes are worse at night than during the day.58
The results from Khanna and colleagues3 are welcome news, since graduate medical education is undergoing a paradigm shift with further reductions in duty hours. Hospital‐based medicine is a 24‐hour operation, 7 days a week. We don't get to pick when patients are admitted or need care. In fact, this study demonstrates that 58% of the admissions came after 7 PM. It turns out that this pattern of admissions is not atypical.9 In light of the new standards, programs are left deciding where to deploy their 1st‐year resident resources. There are essentially two types of models to provide patient care off‐hours in the absence of an extended shift option: night float and night medicine. Night float implies that a trainee, usually a resident, comes from a different site or rotation to provide nocturnal care of a group of patients. That resident may do one isolated night of coverage or several nights in a row. The resident may perform cross‐cover functions, admit patients, or do both. Night medicine refers to having teams of housestaff at night along with an educational program as part of a rotation. The latter implies that trainees will get the full educational experience whether working days or nights. If one designs a model of care that puts 1st‐year residents where the volume is, they would come in late and leave early in the morning the next day. Both create more handoffs in patient care, which critics of these models have said lead to worse patient outcomes. This research challenges such earlier work and should make us all feel more comfortable that care at night does not need to be different if the proper systems are in place to manage it.
The graduate medical education community needs to take a step back and ask: Where are we going? As a long‐standing program director, I don't know anymore. As training has become competency‐based, and milestones are being developed for each level of training, we need to ask if reducing hours further is in the best interests of graduate medical education and, ultimately, the general public. I have long been interested in work intensity as a contributor to resident fatigue, burnout, and errors. A systematic review of resident workload and work activity looked at 21 studies examining resident time allocation and suggests that at least one‐third of resident time is spent on activities of limited educational value. Eight of the nine studies evaluating the impact of resident workload on patient care showed a negative impact on outcomes, including length of stay, mortality, patient satisfaction, medication errors, and lab utilization.10 If we are going to further restrict duty hours, then we need to transform the learning environment in a similar fashion. It does not seem logical to restrict duty hours further without a major redesign of the learning environment. I fear that all we have done is create more work compression because the system of care will be further strained in its weakest areas.
Hospitalists are uniquely poised to mitigate some of these concerns by being available for direct and indirect supervision, as well as being highly skilled at working in our complex systems. They are experts in systems‐based practice. The extension of direct faculty supervision to nights may be a major factor in eliminating the variances in care between nights and days. Not every program or institution will have the ability to employ night hospitalists, let alone have them in a supervisory and teaching role. Those that do can take great comfort in this research. Those that don't must challenge themselves to think about how to get there.
We all share the collective goal of producing the next generation of well‐trained physicians who will practice safe and effective care. As we begin to embrace competency‐based training, the prism through which we view patient safety must extend beyond duty hours and include learning environment reform. While the Khanna et al.3 study supports that off‐hours care is no longer off, we need to be careful that we don't drift too far towards shift work as the fundamental structure of medical training. In the beginning, residents lived in the hospital, thus the term. As we move through these stormy seas, we don't want to trade patient safety for transient trainees. There is a middle ground here that will be informed by future research. There is something to be said for living in your house.
- ACGME.Duty hour requirements. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed August 16,2010.
- ,,, et al.Systematic review of the literature on the impact of variation in residents' duty hour schedules on patient safety. Available at: http://acgme‐2010standards.org/pdf/Jefferson_Medical_College_Duty_Hours_Review.pdf. Accessed August 18,2010.
- ,,, et al.The association between night or weekend admission and hospitalization‐relevant patient outcomes.J Hosp Med2011;6:10–14.
- ACGME.Proposed duty hour standards. Available at: http://acgme‐2010standards.org/pdf/Proposed_Standards.pdf. Accessed August 16,2010.
- ,.Mortality among patients admitted to hospitals on weekends as compared with weekdays.N Engl J Med.2001;345(9):663–668.
- ,,, et al.Survival from in‐hospital cardiac arrest during nights and weekends.JAMA.2008;299(7):785–792.
- ,,,,.What effect does inpatient physician specialty and experience have on clinical outcomes and resource utilization on a general medicine service?J Gen Intern Med.2004;19:395–401.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- .Like night and day—shedding light on off‐hours care.N Engl J Med.2008;358(20):2091–2093.
- ,,.Systematic review of resident workload and work activity: impact on resident education and patient care. Available at: http://acgme‐2010standards.org/pdf/Resident_Duty_Hours_and_Related_Topics.pdf. Accessed on August 16,2010.
- ACGME.Duty hour requirements. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed August 16,2010.
- ,,, et al.Systematic review of the literature on the impact of variation in residents' duty hour schedules on patient safety. Available at: http://acgme‐2010standards.org/pdf/Jefferson_Medical_College_Duty_Hours_Review.pdf. Accessed August 18,2010.
- ,,, et al.The association between night or weekend admission and hospitalization‐relevant patient outcomes.J Hosp Med2011;6:10–14.
- ACGME.Proposed duty hour standards. Available at: http://acgme‐2010standards.org/pdf/Proposed_Standards.pdf. Accessed August 16,2010.
- ,.Mortality among patients admitted to hospitals on weekends as compared with weekdays.N Engl J Med.2001;345(9):663–668.
- ,,, et al.Survival from in‐hospital cardiac arrest during nights and weekends.JAMA.2008;299(7):785–792.
- ,,,,.What effect does inpatient physician specialty and experience have on clinical outcomes and resource utilization on a general medicine service?J Gen Intern Med.2004;19:395–401.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- .Like night and day—shedding light on off‐hours care.N Engl J Med.2008;358(20):2091–2093.
- ,,.Systematic review of resident workload and work activity: impact on resident education and patient care. Available at: http://acgme‐2010standards.org/pdf/Resident_Duty_Hours_and_Related_Topics.pdf. Accessed on August 16,2010.
Discharge Summary Completion
Discharge summaries (DS) correlate with rates of rehospitalization1, 2 and adverse events after discharge.3 The Joint Commission on the Accreditation of Healthcare Organizations acknowledges their importance and mandates that certain elements be included.4 Thus far, however, DS are not standardized across institutions and there is no expectation that they be available at postdischarge visits. There have been numerous attempts to improve the quality of DS by using more structured formats or computer generated summaries with positive results in term of comprehensiveness, clarity, and practitioner satisfaction58 but with persistence of serious errors and omissions.9
Postgraduate training is often the first opportunity for physicians to learn information transfer management skills. Unfortunately, DS are created by house staff who have minimal training in this area11 and feel like they have to learn by osmosis,12 resulting in poor quality DS and lack of availability at the point of care.1315
Previous research suggested that individualized feedback sessions for Internal Medicine residents improved the quality of certain aspects of their completed DS.10 We postulated that an audit and feedback educational intervention on DS for first year geriatric medicine fellows would also improve their quality. This technique involves chart or case review of clinical practice behaviors for a specific task followed by recommendation of new behaviors when applicable.16 Audit and feedback incorporates adult learning theory,1719 an essential part of continuous quality improvement that fits within the Accreditation Council for Graduate Medical Education (ACGME) competency of practice based learning and improvement,20 as an educational activity.
Methods
Setting
We conducted a preintervention post intervention study at the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai Medical Center (MSMC) in New York City between July 1, 2006 and June 30, 2007. The study received an exemption from the MSMC Institutional Review Board. First year geriatric medicine fellows at MSMC were required to complete 2 months of inpatient service; the first during the first 6 months of the academic year and the second during the last 6 months of the year. Fellows dictated all DS, which were transcribed and routed for signature to the attending of record. Prior to our study, a discharge summary template consisting of 21 items was developed for clinical use. Template items, agreed upon by an expert internal panel of geriatricians and interprofessional faculty, were selected for their importance in assuring a safe transition of older adults from the inpatient to the outpatient setting.
Participants
All 5 first‐year fellows at the Brookdale Department of Geriatrics and Palliative Medicine at MSMC were invited to participate in the study.
Intervention
Audit #1
All available DS for each fellow's first month of inpatient service were audited for completeness of the 21 item discharge summary template by 1 author (AD). The 21 items were focused on 4 distinct periods of the hospitalization: admission, hospital course, discharge planning, and postdischarge care (Figure 1).
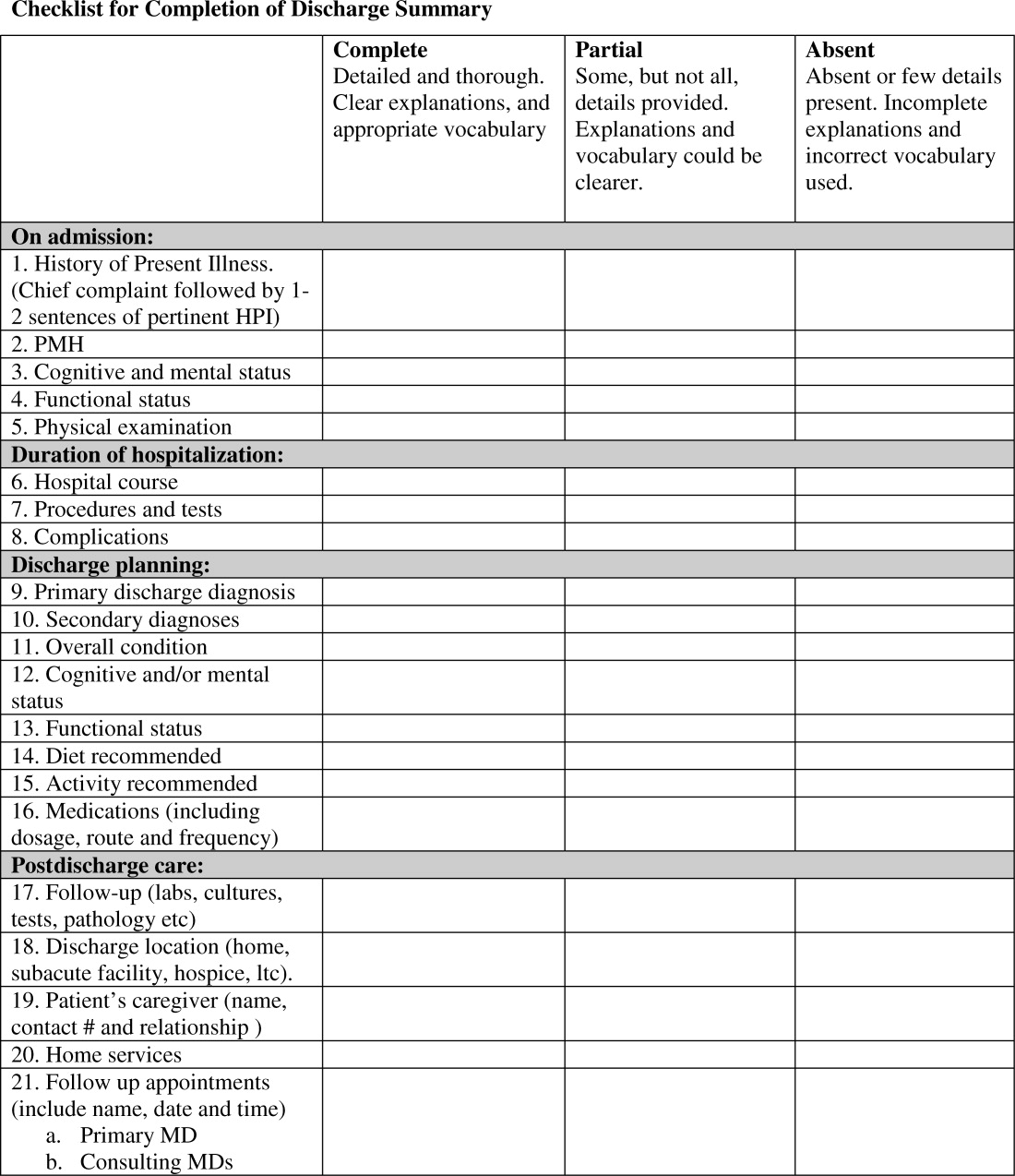
Content under each of the 21 items was classified as complete, partially complete, or absent. An item was considered complete if most information was present and appropriate medical terms were used, partially complete if information was unclear, and absent if no information was present for that area of the DS. To ensure investigator reliability, a random sample of 25% of each fellow's DS was scored by 2 additional investigators (RK and HF) and all disagreements were reviewed and resolved by consensus.
Feedback
Between December 2006 and January 2007, one‐on‐one formative feedback sessions were scheduled. The sessions were approximately 30 minutes long, confidential, performed by 1 of the authors (AD) and followed a written format. During these sessions, each fellow received the results of their discharge summary audit, each partially complete or absent item was discussed, and the importance of DS was emphasized.
Audit #2
All available DS for each fellow's second month of inpatient service were audited for completeness, using the same 21 item assessment tool and the same scoring system.
Statistical Analysis
To determine the impact of our audit and feedback intervention, we compared scores before and after formative feedback sessions, both overall and for the composite discharge summary scores for each of the 4 domains of care: admission, hospital course, discharge‐planning, and postdischarge care. Scores were dichotomized as being complete or partially complete or absent. We used generalized estimating equations to account for the clustering of DS within fellows. Analyses were performed using SAS 9.1 (SAS Institute, Inc., Cary, NC). All statistical tests were 2‐tailed and used a type I error rate of 0.01 to account for multiple comparisons.
Results
Five fellows participated, 4 of whom were women; 2 were in postgraduate year 4, 3 in year 5. A total of 158 DS were audited, 89 prefeedback and 79 postfeedback. Each fellow dictated an average of 17 DS during each inpatient month.
During Audit #1, the 21 item DS were complete among 71%, incomplete among 18%, absent among 11%. Admission items, hospital course items, and discharge planning items were complete among 70%, 78%, and 77% of DS respectively, but postdischarge items were complete among only 57%. Examining individual items, the lowest completion rates were found for test result follow‐up (42%), caregiver information (10%), and home services (64%), as well for assessment at admission and discharge of cognitive and mental status (56% and 53% respectively) and functional status (57% and 40%). Of note, all these items are of particular importance to geriatric care.
After receiving the audit and feedback intervention, fellows were more likely to complete all required discharge summary data when compared to prior‐to‐feedback (91% vs. 71%, P 0.001). Discharge summary completeness improved for all composite outcomes examining the four domains of care: admission (93% vs. 70%, P 0.001), hospital course (93% vs. 78%, P 0.001), discharge planning (93% vs. 77%, P 0.02), and postdischarge care (83% vs. 57%., P 0.001) (Table 1).
| Criteria | Preintervention | Postintervention | P Value* | ||
|---|---|---|---|---|---|
| Complete | Absent | Complete | Absent | ||
| |||||
| Admission composite (5 items) | 70 (3585) | 30 (1565) | 93 (79100) | 7 (021) | 0.001 |
| HPI | 79 (38100) | 21 (1563) | 100 | 0 | 0.001 |
| PMH | 94 (75100) | 5 (025) | 99 (93100) | 1 (07) | 0.001 |
| Cognitive/mental status | 56 (1979) | 44 (2182) | 99 (93100) | 1 (07) | 0.001 |
| Functional status | 57 (2588) | 43 (1375) | 97 (89100) | 2 (010) | 0.001 |
| Physical exam | 63 (19100) | 37 (082) | 72 (0100) | 28 (5100) | 0.27 |
| Hospital course composite (3 items) | 78 (2593) | 22 (775) | 93 (76100) | 7 (023) | 0.001 |
| Hospital course | 84 (25100) | 15 (076) | 99 (93100) | 1 (07) | 0.001 |
| Procedures and tests | 70 (690) | 30 (1094) | 90 (57100) | 10 (043) | 0.001 |
| Complications | 80 (4490) | 20 (556) | 90 (77100) | 10 (023) | 0.07 |
| Discharge planning composite (8 items) | 77 (4989) | 22 (1151) | 93 (64100) | 7 (036) | 0.02 |
| Primary diagnosis | 93 (75100) | 6 (026) | 100 | 0 | 0.03 |
| Secondary diagnosis | 82 (56100) | 18 (044) | 100 | 0 | 0.002 |
| Overall condition | 81 (38100) | 19 (062) | 86 (21100) | 14 (079) | 0.47 |
| Cognitive/mental status | 53 (1380) | 57 (2088) | 97 (93100) | 3 (07) | 0.001 |
| Functional status | 40 (1381) | 50 (1988) | 99 (93100) | 1 (07) | 0.001 |
| Diet | 89 (63100) | 12 (538) | 81 (0100) | 19 (0100) | 0.25 |
| Activity | 89 (69100) | 11 (032) | 82 (0100) | 18 (0100) | 0.49 |
| Medications | 83 (50100) | 17 (050) | 100 | 0 | 0.002 |
| Postdischarge care composite (5 items) | 57 (4183) | 43 (1759) | 83 (6998) | 18 (231) | 0.001 |
| F/U results | 42 (1190) | 58 (1089) | 81 (50100) | 20 (050) | 0.02 |
| Discharge location | 92 (88100) | 8 (012) | 100 | 0 | 0.02 |
| Caregiver info | 10 (025) | 89 (75100) | 48 (795) | 52 (584) | 0.001 |
| Home services | 64 (32100) | 35 (068) | 87 (7195) | 12 (029) | 0.001 |
| F/U appointments | 78 (33100) | 23 (067) | 96 (86100) | 4 (014) | 0.001 |
| Overall composite (21 items) | 71 (4287) | 29 (1358) | 91 (7399) | 9 (227) | 0.001 |
Discussion
Our study found that audit and feedback sessions significantly improved the completeness of DS dictated by geriatric medicine fellows at 1 academic medical center. Before feedback, completeness was high in most traditional areas of the DS including admission data, hospital course, and discharge planning, but was low in other areas critical for safe transitions of older adults such as postdischarge care, test follow‐up, caregiver information, and cognitive and functional status changes. These findings were surprising, as using a template should render a completion rate close to 100%. Notably, during feedback sessions, fellows suggested low completion rates were due to lack of awareness regarding the importance of completing all 21 items of the template and missing documentation in patient medical records.
Feedback sessions dramatically improved overall completeness of subsequent DS and in most of areas of specific importance for geriatric care, although we remain uncertain why all areas did not show improvement (for example, caregiver information completion remained low). One possible explanation is the lack of accurate documentation for all necessary items in the hospital medical record. Moreover, we did not observe completion improvement for other items, ie, diet and activity. Overall, we believe that drawing attention to areas of particular importance to geriatric care transitions and providing learners with individual reports on their performance increased their awareness and motivated changes to their practice, improving discharge summary completion.
Our study has limitations. This study was a pilot intervention without a control group, because of time and budgetary constraints. Also, we were unable to assess for sustainability because the fellows studied for this project graduated after the second audit. Third, we studied discharge summary completion; further research should focus on accuracy of discharge summary content. Finally, while we did not use any advanced technologies or materials, faculty time required to conduct the audit and feedback in this study was estimated at 45 hours. In our opinion this estimate would classify our audit and feedback intervention as a low external cost and moderately‐high human cost intervention, which may represent a potential barrier to generalizability. On the other hand, we believe that even an audit of a small sample of DS done by a physician could provide valuable data for feedback and would involve less faculty time.
Our finding that audit and feedback sessions improved the completeness of DS among house‐staff is important for 2 reasons. First, we were able to demonstrate that focused feedback targeted to areas of particular importance to the transition of older adults changed subsequent behavior and resulted in improved documentation of these areas. Second, our study provides evidence of a programmatic approach to address the ACGME competency of practice‐based learning and improvement. We believe that our intervention can be reproduced by training programs across the country and are hopeful that such interventions will result in improved patient outcomes during critical care transitions such as hospital discharge.
- ,Quality assessment of a discharge summary system.CMAJ.1995;152:1437–1442.
- ,,Accuracy of information on medicines in hospital discharge summaries.Intern Med J.2006;36:221–225.
- ,,,,The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- Available at: http://www.jointcommission.org./. The Joint Commission Requirements/Hospitals/Record of Care/Patient safety. Accessed July2010.
- ,,,,General practitioners' attitudes to computer‐generated surgical discharge letters.Med J Aust.1992;157(6):380–382.
- ,,,Do primary care physicians prefer dictated or computer‐generated discharge summaries?Am J Dis Child.1993;147(9):986–988.
- ,,,,,Evaluation of a computer‐generated discharge summary for patients with acute coronary syndromes.Br J Gen Pract.1998;48(429):1163–1164.
- ,,, et al.Creating a better discharge summary: improvement in quality and timeliness using an electronic dischanrge summary,J Hosp Med.2009;4(4):219–225.
- ,Communication with general practitioners after accident and emergency attendance: computer generated letters are often deficient.Emerg Med J.2003;20(3):256–257.
- ,,Evaluation of electronic discharge summaries: a comparison of documentation in electronic and handwritten discharge summaries.Int J Med Inform.2008;77:613–620.
- ,,,,,Are discharge summaries teachable? The effects of a discharge summary curriculum on the quality of discharge summaries in an internal medicine residency program.Acad Med.2006;81:S5–S8.
- ,,Experience of medical senior house officers in preparing discharge summaries.BMJ.1996;312:350.
- ,,,,,Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,Primary care physician attitudes regarding communication with hospitalists.Dis Mon.2002;48:218–229.
- ,,Improving the continuity of care following discharge of patients hospitalized with heart failure: is the discharge summary adequate?Can J Cardiol.2003;19:365–370.
- Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews.Int J Technol Assess Health Care.2005;21:380–385.
- ,,, et al.Impact of feedback and didactic sessions on the reporting behavior of upper endoscopic findings by physicians and nurses.Clin Gastroenterol Hepatol.2007;5:326–330.
- ,,Prospective assessment of the impact of feedback on colonoscopy performance.Aliment Pharmacol Ther.2006;24:313–318.
- ,,, et al.Inpatient care to community care: improving clinical handover in the private mental health setting.Med J Aust.2009;190(11 Suppl):S144–S149.
- Available at: http://www.Acgme.org, Record of care, Treatment, and Serives, Standard RC.02.04.01. Accessed July2010.
Discharge summaries (DS) correlate with rates of rehospitalization1, 2 and adverse events after discharge.3 The Joint Commission on the Accreditation of Healthcare Organizations acknowledges their importance and mandates that certain elements be included.4 Thus far, however, DS are not standardized across institutions and there is no expectation that they be available at postdischarge visits. There have been numerous attempts to improve the quality of DS by using more structured formats or computer generated summaries with positive results in term of comprehensiveness, clarity, and practitioner satisfaction58 but with persistence of serious errors and omissions.9
Postgraduate training is often the first opportunity for physicians to learn information transfer management skills. Unfortunately, DS are created by house staff who have minimal training in this area11 and feel like they have to learn by osmosis,12 resulting in poor quality DS and lack of availability at the point of care.1315
Previous research suggested that individualized feedback sessions for Internal Medicine residents improved the quality of certain aspects of their completed DS.10 We postulated that an audit and feedback educational intervention on DS for first year geriatric medicine fellows would also improve their quality. This technique involves chart or case review of clinical practice behaviors for a specific task followed by recommendation of new behaviors when applicable.16 Audit and feedback incorporates adult learning theory,1719 an essential part of continuous quality improvement that fits within the Accreditation Council for Graduate Medical Education (ACGME) competency of practice based learning and improvement,20 as an educational activity.
Methods
Setting
We conducted a preintervention post intervention study at the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai Medical Center (MSMC) in New York City between July 1, 2006 and June 30, 2007. The study received an exemption from the MSMC Institutional Review Board. First year geriatric medicine fellows at MSMC were required to complete 2 months of inpatient service; the first during the first 6 months of the academic year and the second during the last 6 months of the year. Fellows dictated all DS, which were transcribed and routed for signature to the attending of record. Prior to our study, a discharge summary template consisting of 21 items was developed for clinical use. Template items, agreed upon by an expert internal panel of geriatricians and interprofessional faculty, were selected for their importance in assuring a safe transition of older adults from the inpatient to the outpatient setting.
Participants
All 5 first‐year fellows at the Brookdale Department of Geriatrics and Palliative Medicine at MSMC were invited to participate in the study.
Intervention
Audit #1
All available DS for each fellow's first month of inpatient service were audited for completeness of the 21 item discharge summary template by 1 author (AD). The 21 items were focused on 4 distinct periods of the hospitalization: admission, hospital course, discharge planning, and postdischarge care (Figure 1).

Content under each of the 21 items was classified as complete, partially complete, or absent. An item was considered complete if most information was present and appropriate medical terms were used, partially complete if information was unclear, and absent if no information was present for that area of the DS. To ensure investigator reliability, a random sample of 25% of each fellow's DS was scored by 2 additional investigators (RK and HF) and all disagreements were reviewed and resolved by consensus.
Feedback
Between December 2006 and January 2007, one‐on‐one formative feedback sessions were scheduled. The sessions were approximately 30 minutes long, confidential, performed by 1 of the authors (AD) and followed a written format. During these sessions, each fellow received the results of their discharge summary audit, each partially complete or absent item was discussed, and the importance of DS was emphasized.
Audit #2
All available DS for each fellow's second month of inpatient service were audited for completeness, using the same 21 item assessment tool and the same scoring system.
Statistical Analysis
To determine the impact of our audit and feedback intervention, we compared scores before and after formative feedback sessions, both overall and for the composite discharge summary scores for each of the 4 domains of care: admission, hospital course, discharge‐planning, and postdischarge care. Scores were dichotomized as being complete or partially complete or absent. We used generalized estimating equations to account for the clustering of DS within fellows. Analyses were performed using SAS 9.1 (SAS Institute, Inc., Cary, NC). All statistical tests were 2‐tailed and used a type I error rate of 0.01 to account for multiple comparisons.
Results
Five fellows participated, 4 of whom were women; 2 were in postgraduate year 4, 3 in year 5. A total of 158 DS were audited, 89 prefeedback and 79 postfeedback. Each fellow dictated an average of 17 DS during each inpatient month.
During Audit #1, the 21 item DS were complete among 71%, incomplete among 18%, absent among 11%. Admission items, hospital course items, and discharge planning items were complete among 70%, 78%, and 77% of DS respectively, but postdischarge items were complete among only 57%. Examining individual items, the lowest completion rates were found for test result follow‐up (42%), caregiver information (10%), and home services (64%), as well for assessment at admission and discharge of cognitive and mental status (56% and 53% respectively) and functional status (57% and 40%). Of note, all these items are of particular importance to geriatric care.
After receiving the audit and feedback intervention, fellows were more likely to complete all required discharge summary data when compared to prior‐to‐feedback (91% vs. 71%, P 0.001). Discharge summary completeness improved for all composite outcomes examining the four domains of care: admission (93% vs. 70%, P 0.001), hospital course (93% vs. 78%, P 0.001), discharge planning (93% vs. 77%, P 0.02), and postdischarge care (83% vs. 57%., P 0.001) (Table 1).
| Criteria | Preintervention | Postintervention | P Value* | ||
|---|---|---|---|---|---|
| Complete | Absent | Complete | Absent | ||
| |||||
| Admission composite (5 items) | 70 (3585) | 30 (1565) | 93 (79100) | 7 (021) | 0.001 |
| HPI | 79 (38100) | 21 (1563) | 100 | 0 | 0.001 |
| PMH | 94 (75100) | 5 (025) | 99 (93100) | 1 (07) | 0.001 |
| Cognitive/mental status | 56 (1979) | 44 (2182) | 99 (93100) | 1 (07) | 0.001 |
| Functional status | 57 (2588) | 43 (1375) | 97 (89100) | 2 (010) | 0.001 |
| Physical exam | 63 (19100) | 37 (082) | 72 (0100) | 28 (5100) | 0.27 |
| Hospital course composite (3 items) | 78 (2593) | 22 (775) | 93 (76100) | 7 (023) | 0.001 |
| Hospital course | 84 (25100) | 15 (076) | 99 (93100) | 1 (07) | 0.001 |
| Procedures and tests | 70 (690) | 30 (1094) | 90 (57100) | 10 (043) | 0.001 |
| Complications | 80 (4490) | 20 (556) | 90 (77100) | 10 (023) | 0.07 |
| Discharge planning composite (8 items) | 77 (4989) | 22 (1151) | 93 (64100) | 7 (036) | 0.02 |
| Primary diagnosis | 93 (75100) | 6 (026) | 100 | 0 | 0.03 |
| Secondary diagnosis | 82 (56100) | 18 (044) | 100 | 0 | 0.002 |
| Overall condition | 81 (38100) | 19 (062) | 86 (21100) | 14 (079) | 0.47 |
| Cognitive/mental status | 53 (1380) | 57 (2088) | 97 (93100) | 3 (07) | 0.001 |
| Functional status | 40 (1381) | 50 (1988) | 99 (93100) | 1 (07) | 0.001 |
| Diet | 89 (63100) | 12 (538) | 81 (0100) | 19 (0100) | 0.25 |
| Activity | 89 (69100) | 11 (032) | 82 (0100) | 18 (0100) | 0.49 |
| Medications | 83 (50100) | 17 (050) | 100 | 0 | 0.002 |
| Postdischarge care composite (5 items) | 57 (4183) | 43 (1759) | 83 (6998) | 18 (231) | 0.001 |
| F/U results | 42 (1190) | 58 (1089) | 81 (50100) | 20 (050) | 0.02 |
| Discharge location | 92 (88100) | 8 (012) | 100 | 0 | 0.02 |
| Caregiver info | 10 (025) | 89 (75100) | 48 (795) | 52 (584) | 0.001 |
| Home services | 64 (32100) | 35 (068) | 87 (7195) | 12 (029) | 0.001 |
| F/U appointments | 78 (33100) | 23 (067) | 96 (86100) | 4 (014) | 0.001 |
| Overall composite (21 items) | 71 (4287) | 29 (1358) | 91 (7399) | 9 (227) | 0.001 |
Discussion
Our study found that audit and feedback sessions significantly improved the completeness of DS dictated by geriatric medicine fellows at 1 academic medical center. Before feedback, completeness was high in most traditional areas of the DS including admission data, hospital course, and discharge planning, but was low in other areas critical for safe transitions of older adults such as postdischarge care, test follow‐up, caregiver information, and cognitive and functional status changes. These findings were surprising, as using a template should render a completion rate close to 100%. Notably, during feedback sessions, fellows suggested low completion rates were due to lack of awareness regarding the importance of completing all 21 items of the template and missing documentation in patient medical records.
Feedback sessions dramatically improved overall completeness of subsequent DS and in most of areas of specific importance for geriatric care, although we remain uncertain why all areas did not show improvement (for example, caregiver information completion remained low). One possible explanation is the lack of accurate documentation for all necessary items in the hospital medical record. Moreover, we did not observe completion improvement for other items, ie, diet and activity. Overall, we believe that drawing attention to areas of particular importance to geriatric care transitions and providing learners with individual reports on their performance increased their awareness and motivated changes to their practice, improving discharge summary completion.
Our study has limitations. This study was a pilot intervention without a control group, because of time and budgetary constraints. Also, we were unable to assess for sustainability because the fellows studied for this project graduated after the second audit. Third, we studied discharge summary completion; further research should focus on accuracy of discharge summary content. Finally, while we did not use any advanced technologies or materials, faculty time required to conduct the audit and feedback in this study was estimated at 45 hours. In our opinion this estimate would classify our audit and feedback intervention as a low external cost and moderately‐high human cost intervention, which may represent a potential barrier to generalizability. On the other hand, we believe that even an audit of a small sample of DS done by a physician could provide valuable data for feedback and would involve less faculty time.
Our finding that audit and feedback sessions improved the completeness of DS among house‐staff is important for 2 reasons. First, we were able to demonstrate that focused feedback targeted to areas of particular importance to the transition of older adults changed subsequent behavior and resulted in improved documentation of these areas. Second, our study provides evidence of a programmatic approach to address the ACGME competency of practice‐based learning and improvement. We believe that our intervention can be reproduced by training programs across the country and are hopeful that such interventions will result in improved patient outcomes during critical care transitions such as hospital discharge.
Discharge summaries (DS) correlate with rates of rehospitalization1, 2 and adverse events after discharge.3 The Joint Commission on the Accreditation of Healthcare Organizations acknowledges their importance and mandates that certain elements be included.4 Thus far, however, DS are not standardized across institutions and there is no expectation that they be available at postdischarge visits. There have been numerous attempts to improve the quality of DS by using more structured formats or computer generated summaries with positive results in term of comprehensiveness, clarity, and practitioner satisfaction58 but with persistence of serious errors and omissions.9
Postgraduate training is often the first opportunity for physicians to learn information transfer management skills. Unfortunately, DS are created by house staff who have minimal training in this area11 and feel like they have to learn by osmosis,12 resulting in poor quality DS and lack of availability at the point of care.1315
Previous research suggested that individualized feedback sessions for Internal Medicine residents improved the quality of certain aspects of their completed DS.10 We postulated that an audit and feedback educational intervention on DS for first year geriatric medicine fellows would also improve their quality. This technique involves chart or case review of clinical practice behaviors for a specific task followed by recommendation of new behaviors when applicable.16 Audit and feedback incorporates adult learning theory,1719 an essential part of continuous quality improvement that fits within the Accreditation Council for Graduate Medical Education (ACGME) competency of practice based learning and improvement,20 as an educational activity.
Methods
Setting
We conducted a preintervention post intervention study at the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai Medical Center (MSMC) in New York City between July 1, 2006 and June 30, 2007. The study received an exemption from the MSMC Institutional Review Board. First year geriatric medicine fellows at MSMC were required to complete 2 months of inpatient service; the first during the first 6 months of the academic year and the second during the last 6 months of the year. Fellows dictated all DS, which were transcribed and routed for signature to the attending of record. Prior to our study, a discharge summary template consisting of 21 items was developed for clinical use. Template items, agreed upon by an expert internal panel of geriatricians and interprofessional faculty, were selected for their importance in assuring a safe transition of older adults from the inpatient to the outpatient setting.
Participants
All 5 first‐year fellows at the Brookdale Department of Geriatrics and Palliative Medicine at MSMC were invited to participate in the study.
Intervention
Audit #1
All available DS for each fellow's first month of inpatient service were audited for completeness of the 21 item discharge summary template by 1 author (AD). The 21 items were focused on 4 distinct periods of the hospitalization: admission, hospital course, discharge planning, and postdischarge care (Figure 1).

Content under each of the 21 items was classified as complete, partially complete, or absent. An item was considered complete if most information was present and appropriate medical terms were used, partially complete if information was unclear, and absent if no information was present for that area of the DS. To ensure investigator reliability, a random sample of 25% of each fellow's DS was scored by 2 additional investigators (RK and HF) and all disagreements were reviewed and resolved by consensus.
Feedback
Between December 2006 and January 2007, one‐on‐one formative feedback sessions were scheduled. The sessions were approximately 30 minutes long, confidential, performed by 1 of the authors (AD) and followed a written format. During these sessions, each fellow received the results of their discharge summary audit, each partially complete or absent item was discussed, and the importance of DS was emphasized.
Audit #2
All available DS for each fellow's second month of inpatient service were audited for completeness, using the same 21 item assessment tool and the same scoring system.
Statistical Analysis
To determine the impact of our audit and feedback intervention, we compared scores before and after formative feedback sessions, both overall and for the composite discharge summary scores for each of the 4 domains of care: admission, hospital course, discharge‐planning, and postdischarge care. Scores were dichotomized as being complete or partially complete or absent. We used generalized estimating equations to account for the clustering of DS within fellows. Analyses were performed using SAS 9.1 (SAS Institute, Inc., Cary, NC). All statistical tests were 2‐tailed and used a type I error rate of 0.01 to account for multiple comparisons.
Results
Five fellows participated, 4 of whom were women; 2 were in postgraduate year 4, 3 in year 5. A total of 158 DS were audited, 89 prefeedback and 79 postfeedback. Each fellow dictated an average of 17 DS during each inpatient month.
During Audit #1, the 21 item DS were complete among 71%, incomplete among 18%, absent among 11%. Admission items, hospital course items, and discharge planning items were complete among 70%, 78%, and 77% of DS respectively, but postdischarge items were complete among only 57%. Examining individual items, the lowest completion rates were found for test result follow‐up (42%), caregiver information (10%), and home services (64%), as well for assessment at admission and discharge of cognitive and mental status (56% and 53% respectively) and functional status (57% and 40%). Of note, all these items are of particular importance to geriatric care.
After receiving the audit and feedback intervention, fellows were more likely to complete all required discharge summary data when compared to prior‐to‐feedback (91% vs. 71%, P 0.001). Discharge summary completeness improved for all composite outcomes examining the four domains of care: admission (93% vs. 70%, P 0.001), hospital course (93% vs. 78%, P 0.001), discharge planning (93% vs. 77%, P 0.02), and postdischarge care (83% vs. 57%., P 0.001) (Table 1).
| Criteria | Preintervention | Postintervention | P Value* | ||
|---|---|---|---|---|---|
| Complete | Absent | Complete | Absent | ||
| |||||
| Admission composite (5 items) | 70 (3585) | 30 (1565) | 93 (79100) | 7 (021) | 0.001 |
| HPI | 79 (38100) | 21 (1563) | 100 | 0 | 0.001 |
| PMH | 94 (75100) | 5 (025) | 99 (93100) | 1 (07) | 0.001 |
| Cognitive/mental status | 56 (1979) | 44 (2182) | 99 (93100) | 1 (07) | 0.001 |
| Functional status | 57 (2588) | 43 (1375) | 97 (89100) | 2 (010) | 0.001 |
| Physical exam | 63 (19100) | 37 (082) | 72 (0100) | 28 (5100) | 0.27 |
| Hospital course composite (3 items) | 78 (2593) | 22 (775) | 93 (76100) | 7 (023) | 0.001 |
| Hospital course | 84 (25100) | 15 (076) | 99 (93100) | 1 (07) | 0.001 |
| Procedures and tests | 70 (690) | 30 (1094) | 90 (57100) | 10 (043) | 0.001 |
| Complications | 80 (4490) | 20 (556) | 90 (77100) | 10 (023) | 0.07 |
| Discharge planning composite (8 items) | 77 (4989) | 22 (1151) | 93 (64100) | 7 (036) | 0.02 |
| Primary diagnosis | 93 (75100) | 6 (026) | 100 | 0 | 0.03 |
| Secondary diagnosis | 82 (56100) | 18 (044) | 100 | 0 | 0.002 |
| Overall condition | 81 (38100) | 19 (062) | 86 (21100) | 14 (079) | 0.47 |
| Cognitive/mental status | 53 (1380) | 57 (2088) | 97 (93100) | 3 (07) | 0.001 |
| Functional status | 40 (1381) | 50 (1988) | 99 (93100) | 1 (07) | 0.001 |
| Diet | 89 (63100) | 12 (538) | 81 (0100) | 19 (0100) | 0.25 |
| Activity | 89 (69100) | 11 (032) | 82 (0100) | 18 (0100) | 0.49 |
| Medications | 83 (50100) | 17 (050) | 100 | 0 | 0.002 |
| Postdischarge care composite (5 items) | 57 (4183) | 43 (1759) | 83 (6998) | 18 (231) | 0.001 |
| F/U results | 42 (1190) | 58 (1089) | 81 (50100) | 20 (050) | 0.02 |
| Discharge location | 92 (88100) | 8 (012) | 100 | 0 | 0.02 |
| Caregiver info | 10 (025) | 89 (75100) | 48 (795) | 52 (584) | 0.001 |
| Home services | 64 (32100) | 35 (068) | 87 (7195) | 12 (029) | 0.001 |
| F/U appointments | 78 (33100) | 23 (067) | 96 (86100) | 4 (014) | 0.001 |
| Overall composite (21 items) | 71 (4287) | 29 (1358) | 91 (7399) | 9 (227) | 0.001 |
Discussion
Our study found that audit and feedback sessions significantly improved the completeness of DS dictated by geriatric medicine fellows at 1 academic medical center. Before feedback, completeness was high in most traditional areas of the DS including admission data, hospital course, and discharge planning, but was low in other areas critical for safe transitions of older adults such as postdischarge care, test follow‐up, caregiver information, and cognitive and functional status changes. These findings were surprising, as using a template should render a completion rate close to 100%. Notably, during feedback sessions, fellows suggested low completion rates were due to lack of awareness regarding the importance of completing all 21 items of the template and missing documentation in patient medical records.
Feedback sessions dramatically improved overall completeness of subsequent DS and in most of areas of specific importance for geriatric care, although we remain uncertain why all areas did not show improvement (for example, caregiver information completion remained low). One possible explanation is the lack of accurate documentation for all necessary items in the hospital medical record. Moreover, we did not observe completion improvement for other items, ie, diet and activity. Overall, we believe that drawing attention to areas of particular importance to geriatric care transitions and providing learners with individual reports on their performance increased their awareness and motivated changes to their practice, improving discharge summary completion.
Our study has limitations. This study was a pilot intervention without a control group, because of time and budgetary constraints. Also, we were unable to assess for sustainability because the fellows studied for this project graduated after the second audit. Third, we studied discharge summary completion; further research should focus on accuracy of discharge summary content. Finally, while we did not use any advanced technologies or materials, faculty time required to conduct the audit and feedback in this study was estimated at 45 hours. In our opinion this estimate would classify our audit and feedback intervention as a low external cost and moderately‐high human cost intervention, which may represent a potential barrier to generalizability. On the other hand, we believe that even an audit of a small sample of DS done by a physician could provide valuable data for feedback and would involve less faculty time.
Our finding that audit and feedback sessions improved the completeness of DS among house‐staff is important for 2 reasons. First, we were able to demonstrate that focused feedback targeted to areas of particular importance to the transition of older adults changed subsequent behavior and resulted in improved documentation of these areas. Second, our study provides evidence of a programmatic approach to address the ACGME competency of practice‐based learning and improvement. We believe that our intervention can be reproduced by training programs across the country and are hopeful that such interventions will result in improved patient outcomes during critical care transitions such as hospital discharge.
- ,Quality assessment of a discharge summary system.CMAJ.1995;152:1437–1442.
- ,,Accuracy of information on medicines in hospital discharge summaries.Intern Med J.2006;36:221–225.
- ,,,,The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- Available at: http://www.jointcommission.org./. The Joint Commission Requirements/Hospitals/Record of Care/Patient safety. Accessed July2010.
- ,,,,General practitioners' attitudes to computer‐generated surgical discharge letters.Med J Aust.1992;157(6):380–382.
- ,,,Do primary care physicians prefer dictated or computer‐generated discharge summaries?Am J Dis Child.1993;147(9):986–988.
- ,,,,,Evaluation of a computer‐generated discharge summary for patients with acute coronary syndromes.Br J Gen Pract.1998;48(429):1163–1164.
- ,,, et al.Creating a better discharge summary: improvement in quality and timeliness using an electronic dischanrge summary,J Hosp Med.2009;4(4):219–225.
- ,Communication with general practitioners after accident and emergency attendance: computer generated letters are often deficient.Emerg Med J.2003;20(3):256–257.
- ,,Evaluation of electronic discharge summaries: a comparison of documentation in electronic and handwritten discharge summaries.Int J Med Inform.2008;77:613–620.
- ,,,,,Are discharge summaries teachable? The effects of a discharge summary curriculum on the quality of discharge summaries in an internal medicine residency program.Acad Med.2006;81:S5–S8.
- ,,Experience of medical senior house officers in preparing discharge summaries.BMJ.1996;312:350.
- ,,,,,Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,Primary care physician attitudes regarding communication with hospitalists.Dis Mon.2002;48:218–229.
- ,,Improving the continuity of care following discharge of patients hospitalized with heart failure: is the discharge summary adequate?Can J Cardiol.2003;19:365–370.
- Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews.Int J Technol Assess Health Care.2005;21:380–385.
- ,,, et al.Impact of feedback and didactic sessions on the reporting behavior of upper endoscopic findings by physicians and nurses.Clin Gastroenterol Hepatol.2007;5:326–330.
- ,,Prospective assessment of the impact of feedback on colonoscopy performance.Aliment Pharmacol Ther.2006;24:313–318.
- ,,, et al.Inpatient care to community care: improving clinical handover in the private mental health setting.Med J Aust.2009;190(11 Suppl):S144–S149.
- Available at: http://www.Acgme.org, Record of care, Treatment, and Serives, Standard RC.02.04.01. Accessed July2010.
- ,Quality assessment of a discharge summary system.CMAJ.1995;152:1437–1442.
- ,,Accuracy of information on medicines in hospital discharge summaries.Intern Med J.2006;36:221–225.
- ,,,,The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- Available at: http://www.jointcommission.org./. The Joint Commission Requirements/Hospitals/Record of Care/Patient safety. Accessed July2010.
- ,,,,General practitioners' attitudes to computer‐generated surgical discharge letters.Med J Aust.1992;157(6):380–382.
- ,,,Do primary care physicians prefer dictated or computer‐generated discharge summaries?Am J Dis Child.1993;147(9):986–988.
- ,,,,,Evaluation of a computer‐generated discharge summary for patients with acute coronary syndromes.Br J Gen Pract.1998;48(429):1163–1164.
- ,,, et al.Creating a better discharge summary: improvement in quality and timeliness using an electronic dischanrge summary,J Hosp Med.2009;4(4):219–225.
- ,Communication with general practitioners after accident and emergency attendance: computer generated letters are often deficient.Emerg Med J.2003;20(3):256–257.
- ,,Evaluation of electronic discharge summaries: a comparison of documentation in electronic and handwritten discharge summaries.Int J Med Inform.2008;77:613–620.
- ,,,,,Are discharge summaries teachable? The effects of a discharge summary curriculum on the quality of discharge summaries in an internal medicine residency program.Acad Med.2006;81:S5–S8.
- ,,Experience of medical senior house officers in preparing discharge summaries.BMJ.1996;312:350.
- ,,,,,Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,Primary care physician attitudes regarding communication with hospitalists.Dis Mon.2002;48:218–229.
- ,,Improving the continuity of care following discharge of patients hospitalized with heart failure: is the discharge summary adequate?Can J Cardiol.2003;19:365–370.
- Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews.Int J Technol Assess Health Care.2005;21:380–385.
- ,,, et al.Impact of feedback and didactic sessions on the reporting behavior of upper endoscopic findings by physicians and nurses.Clin Gastroenterol Hepatol.2007;5:326–330.
- ,,Prospective assessment of the impact of feedback on colonoscopy performance.Aliment Pharmacol Ther.2006;24:313–318.
- ,,, et al.Inpatient care to community care: improving clinical handover in the private mental health setting.Med J Aust.2009;190(11 Suppl):S144–S149.
- Available at: http://www.Acgme.org, Record of care, Treatment, and Serives, Standard RC.02.04.01. Accessed July2010.
Continuing Medical Education Program in
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.