User login
Air travel and venous thromboembolism: Minimizing the risk
Editor’s Note: The views expressed in this article are solely those of the authors and do not reflect the official policy or position of the Department of State or the United States Government. This version of the article was peer-reviewed.
Venous thromboembolism (VTE) associated with travel has emerged as an important public health concern over the past decade. Numerous epidemiologic and case control studies have reported air travel as a risk factor for the development of VTE and have attempted to determine who is at risk and which precautions need to be taken to prevent this potentially fatal event.1–7 Often referred to as “traveler’s thrombosis” or “flight-related deep vein thrombosis,” VTE can also develop after long trips by automobile, bus, or train.8,9 Although the absolute risk is very low, this threat appears to be about three times higher in travelers and increases with longer trips.3
See related patient information material
This article focuses on defining VTE and recognizing its clinical features, as well as providing recommendations and guidelines to prevent, diagnose, and treat this complication in people who travel.
WHAT IS VENOUS THROMBOEMBOLISM?
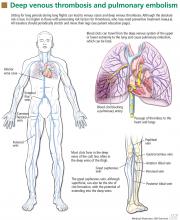
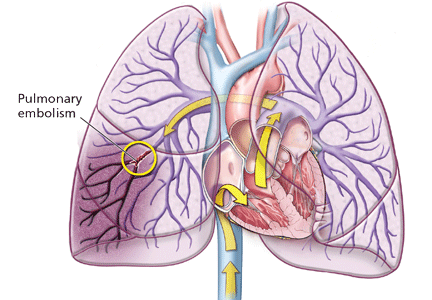
Deep vein thrombosis and pulmonary embolism represent different manifestations of the same clinical entity, ie, VTE. VTE is a common, lethal disease that affects hospitalized and nonhospitalized patients, frequently recurs, is often overlooked, may be asymptomatic, and may result in long-term complications that include pulmonary hypertension and the postthrombotic syndrome.
Deep vein thrombosis of the upper extremities is generally related to an indwelling venous catheter or a central line being used for long-term administration of antibiotics, chemotherapy, or nutrition. A condition known as Paget-Schroetter syndrome or “effort thrombosis” may be seen in younger or athletic people who have a history of strenuous or unusual arm exercise.
RISK FACTORS FOR VTE
Common inherited risk factors include:
- Factor V Leiden mutation
- Prothrombin gene mutation G20210A
- Hyperhomocysteinemia
- Deficiency of the natural anticoagulant proteins C, S, or antithrombin
- Elevated levels of factor VIII (may be inherited or acquired).
Acquired risk factors include:
- Older age
- Immobilization or stasis (such as sitting for long periods of time while traveling)
- Surgery (most notably orthopedic procedures including hip and knee replacement and repair of a hip fracture)
- Trauma
- Stroke
- Acute medical illness (including congestive heart failure, chronic obstructive pulmonary disease, pneumonia)
- The antiphospholipid syndrome (consisting of a lupus anticoagulant, anticardiolipin antibodies, or both)
- Pregnancy and the postpartum state
- Use of oral contraceptives or hormone replacement therapy
- Cancer (including the myeloproliferative disorders) and certain chemotherapeutic agents
- Obesity (a body mass index > 30 kg/m2, see www.nhlbisupport.com/bmi/)
- Inflammatory bowel disease
- Previous VTE
- A central venous catheter or pacemaker
- Nephrotic syndrome.
In addition, emerging risk factors more recently recognized include male sex, persistence of elevated factor VIII levels, and the continued presence of an elevated D-dimer level or deep vein thrombosis on duplex ultrasonography once anticoagulation treatment is completed. There is also evidence of an association between VTE and risk factors for atherosclerotic arterial disease such as smoking, hypertension, hyperlipidemia, and diabetes.
CLINICAL MANIFESTATIONS OF VTE
Patients with deep vein thrombosis may complain of pain, swelling, or both in the leg or arm. Physical examination may reveal increased warmth, tenderness, erythema, edema, or dilated (collateral) veins, most notable on the upper thigh or calf (for deep vein thrombosis in the lower extremity) or the chest wall (for upper-extremity deep vein thrombosis). The examiner may also observe a tender, palpable cord, which represents a superficial vein thrombosis involving the great and small saphenous veins (Figure 1). In extreme situations, the limb may be cyanotic or gangrenous.
DIAGNOSIS OF VTE
Clinical examination alone is generally insufficient to confirm a diagnosis of deep vein thrombosis or pulmonary embolism. Venous duplex ultrasonography is the most dependable investigation for deep vein thrombosis, but other tests include D-dimer and imaging studies such as computed tomographic venography or magnetic resonance venography of the lower extremities. A more invasive approach is venography; formerly considered the gold standard, it is now generally used only when the diagnosis is in doubt after noninvasive testing. The diagnosis of acute pulmonary embolism is best made by spiral computed tomography.
Other studies that may prove helpful include a ventilation-perfusion lung scan for patients who cannot undergo computed tomography due to a contrast allergy or renal insufficiency. Pulmonary angiography, while the gold standard, is less commonly used today, given the specificity and sensitivity of computed tomography.
Echocardiography at the bedside may be useful for patients too sick to move, although the study may not be diagnostic unless thrombi are seen in the heart or pulmonary arteries.
TREATMENT OF VTE
For acute deep venous thrombosis
Acute deep vein thrombosis is now treated on an outpatient basis under most circumstances.
Unfractionated heparin is given intravenously for patients who need to be hospitalized, or subcutaneously in full dose for inpatient or outpatient treatment.
Low-molecular-weight heparins are available in subcutaneous preparations and can be given on an outpatient basis.
Fondaparinux (Arixtra), a factor Xa inhibitor, can also be given subcutaneously on an outpatient basis. Equivalent products are available outside the United States.
Warfarin (Coumadin), an oral vitamin K inhibitor, is the agent of choice for long-term management of deep vein thrombosis.
Other oral agents are available outside the United States.
For pulmonary embolism
Outpatient treatment of pulmonary embolism is not yet advised: an initial hospitalization is necessary. The same anticoagulants used for deep vein thrombosis are also used for acute pulmonary embolism.
Empiric treatment in underdeveloped countries
VTE may be an even greater concern on an outbound trip to a remote area, where medical care capabilities may be less than ideal and diagnostic and treatment options may be limited.
If there is a high pretest probability of acute VTE (Table 2, Table 3) and no diagnostic methods are available, empiric treatment with any of the parenteral anticoagulant agents listed in Table 4 is an option until the diagnosis can be confirmed. Caveats:
- Care must be taken to be certain there is not a strong contraindication to the use of anticoagulation, such as bleeding or a drug allergy.
- Neither unfractionated heparin nor any of the low-molecular-weight heparins should be given to a patient who has a history of heparin-induced thrombocytopenia.
- In patients who have chronic kidney disease (creatinine clearance less than 30 mL/minute), the dosage of low-molecular-weight heparins must be adjusted and factor Xa inhibitors avoided. Both of these types of anticoagulants should be avoided in patients on hemodialysis.
More aggressive therapy
Under select circumstances a more aggressive approach to the treatment of VTE may be necessary. These options are usually indicated for a patient with a massive deep vein thrombosis of a lower extremity and for certain patients with an upper extremity deep vein thrombosis. Treatments include catheter-directed thrombolytic therapy and endovenous or surgical thrombectomy.
Thrombolytic therapy is recommended for a patient with an acute pulmonary embolism who is clinically unstable (systolic blood pressure lower than 90 mm Hg), if there is no contraindication to its use (bleeding risk or recent stroke or surgery). Thrombolytic therapy is also an option for those at low risk of bleeding with an acute pulmonary embolism who have signs and symptoms of right heart failure proven by echocardiography.
Surgical pulmonary embolectomy for acute massive pulmonary embolism and mechanical thrombectomy for extensive deep vein thrombosis are generally available only at highly sophisticated tertiary care centers.
An inferior vena cava filter is advised in patients with acute deep vein thrombosis or pulmonary embolism who cannot be fully anticoagulated, to prevent the clot from migrating from the lower extremities to the lungs. These filters are available as either permanent or temporary implants. Some temporary versions can remain in place for up to 150 days after insertion.
PREVENTION OF VTE
Prevention is the standard of care for all patients admitted to the hospital and in select individuals as outpatients who are at high risk of VTE.
Mechanical compression (graduated compression stockings, intermittent pneumatic compression devices) has proven effective in reducing the incidence of deep vein thrombosis and pulmonary embolism postoperatively in patients who cannot take anticoagulants. One study has demonstrated that compression stockings may also be effective in preventing VTE during travel.12
ABSOLUTE RISK IS LOW
Over the past decade, special attention has been paid to travel as a risk factor for developing VTE.13 Traveler’s thrombosis has become an important public health concern. Numerous publications and epidemiologic studies have targeted air travel in an attempt to determine who is at risk and what precautions are necessary to prevent this complication.1–7,9
The incidence of VTE following air travel is reported to be 3.2 per 1,000 person-years.4 While this incidence is relatively low, it is still 3.2 times higher than in the healthy population that is not flying.
The more serious complication of VTE, ie, acute pulmonary embolism, occurs less often. In three studies, the reported incidence ranged from 1.65 per million patients in flights longer than 8 hours to a high of 4.8 per million patients in flights longer than 12 hours or distances exceeding 10,000 km (6,200 miles).5,14,15 For the 400 passengers on the average long-haul flight of 12 hours, there is at most a 0.2% chance that somebody on the plane will have a symptomatic VTE).
RISK FACTORS IN LONG-DISTANCE TRAVELERS
The risk of traveler’s thrombosis has recently attracted the attention of passengers and the airline industry. Airlines are now openly discussing the risk and providing reminders such as exercises that should be undertaken in-flight (see the patient information page that accompanies this article). Some airlines are recommending that all patients consult their doctor to assess their personal risk of deep vein thrombosis before flying.
The most common risk factors for VTE in travelers are well established and are additive (Table 1). The extent of the additive risk, however, is not entirely clear.
What is clear is that when VTE occurs it is a life-altering and life-threatening event. If it occurs on an outbound trip, the local resources and capabilities available at the destination may not be adequate for optimal treatment. If a traveler experiences a VTE event on an outbound trip, an emergency return trip to the continental United States or a regional center of expertise may be required. There is an additive risk with this subsequent travel event if the patient is not given immediate treatment first (Table 4). Hence, treatment prior to evacuation should be strongly considered.
The traveler must also be aware that VTE can be recognized up to 2 months after a long-haul flight, though it is especially a concern within the first 2 weeks after travel.2,4,16,17
RECOMMENDATIONS FOR LONG-DISTANCE AIR TRAVELERS
Each person should be evaluated on a case-by-case basis for his or her need for VTE prophylaxis. Medical guidelines for airline passengers have been published by the Aerospace Medical Association and the American College of Chest Physicians (ACCP).18,19 In general, travelers should:
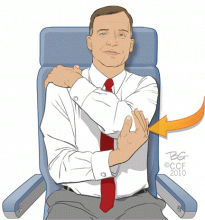
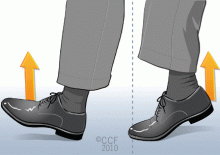
- Exercise the legs by flexing and extending the ankles at regular intervals while seated (see the patient information material that accompanies this article) and frequently contracting the calf muscles.
- Walk about the cabin periodically, 5 minutes for every hour on longer-duration flights (over 4 hours) and when flight conditions permit.
- Drink adequate amounts of water and fruit juices to maintain good hydration.17
- Avoid alcohol and caffeinated beverages, which are dehydrating.
- Be careful about eating too much during the flight.
- Request an aisle seat if you are at risk
- Do not place baggage underneath the seat in front of you, because that reduces the ability to move the legs.
- Do not sleep in a cramped position, and avoid the use of any type of sleep aid.
- Avoid wearing constrictive clothing around the lower extremities or waist.
We recommend that all airplane passengers take the steps listed above to reduce venous stasis and avoid dehydration, even though these measures have not been proven effective in clinical trials.19
The ACCP further advises that decisions about pharmacologic prophylaxis of VTE for airplane passengers at high risk should be made on an individual basis, considering that there are potential adverse effects of prophylaxis and that these may outweigh the benefits. For long-distance travelers with additional risk factors for VTE, we suggest the following:
- Use of properly fitted, below-the-knee graduated compression stockings providing 15 to 30 mm Hg of pressure at the ankle (particularly when large varicosities or leg edema is present)
- For people at very high risk, a single prophylactic dose of a low-molecular-weight heparin or a factor Xa inhibitor injected just before departure (Table 5)
- Aspirin is not recommended as it is not effective for the prevention of VTE.20
SUMMARY FOR THE AIR TRAVELER
All travelers on long flights should perform standard VTE prophylaxis exercises (see the patient information pages accompanying this article). Although VTE is uncommon, people with additional risk factors who travel frequently either on multiple flights in a short period of time or on very long flights should be evaluated on a case-by-case basis for a more aggressive approach to prevention (compression support hose or prophylactic administration of a low-molecular-weight heparin or a factor Xa inhibitor).
Should a VTE event occur during travel, the patient should seek medical care immediately. The standard evaluation of a patient with a suspected VTE should include an estimation of the pretest probability of disease (Table 2, Table 3), followed by duplex ultrasonography of the upper or lower extremity to detect a deep vein thrombosis. If symptoms dictate, then spiral computed tomography, ventilation-perfusion lung scan, or pulmonary angiography (where available) should be ordered to diagnose acute pulmonary embolism. A positive D-dimer blood test alone is not diagnostic and may not be available in more remote locations. A negative D-dimer test result is most helpful to exclude VTE.
Standard therapy for VTE is immediate treatment with one of the anticoagulants listed in Table 4, unless the patient has a contraindication to treatment, such as bleeding or allergy. Immediate evacuation is recommended if the patient has a life-threatening pulmonary embolism, defined as hemodynamic instability (hypotension with a blood pressure under 90 mm Hg systolic or signs of right heart failure) that cannot be treated at a local facility. An air ambulance should be used to transport these patients. If the patient has an iliofemoral deep vein thrombosis, it is also advisable that he or she be considered for evacuation if severe symptoms are present, such as pain, swelling, or cyanosis. Unless contraindicated, all patients should be given either full-dose intravenous or full-dose subcutaneous heparin or subcutaneous injection of a readily available low-molecular-weight heparin preparations or factor Xa inhibitor at once.21
- Brenner B. Interventions to prevent venous thrombosis after air travel, are they necessary? Yes. J Thromb Haemost 2006; 4:2302–2305.
- Cannegieter SC, Doggen CJM, van Houwellingen HC, et al. Travel-related venous thrombosis: results from a large population-based case control study (MEGA Study). PLoS Med 2006; 3:1258–1265.
- Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med 2009; 151:180–190.
- Kuipers S, Cannegieter SC, Middeldorp S, et al. The absolute risk of venous thrombosis after air travel: a cohort study of 8,755 employees of international organizations. PLoS Med 2007; 4:1508–1514.
- Kuipers S, Schreijer AJM, Cannegieter SC, et al. Travel and venous thrombosis: a systematic review. J Intern Med 2007; 262:615–634.
- Lehmann R, Suess C, Leus M, et al. Incidence, clinical characteristics, and long-term prognosis of travel-associated pulmonary embolism. Eur Heart J 2009; 30:233–241.
- Philbrick JT, Shumate R, Siadaty MS, et al. Air travel and venous thromboembolism: a systematic review. J Gen Intern Med 2007; 22:107–114.
- Cruickshank JM, Gorlin R, Jennett B. Air travel and thrombotic episodes: the economy class syndrome. Lancet 1988; 2:497–498.
- Bagshaw M. Traveler’s thrombosis: a review of deep vein thrombosis associated with travel. Air Transport Medicine Committee, Aerospace Medical Association. Aviat Space Environ Med 2001; 72:848–851.
- Wells PS, Owens C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Arnason T, Wells PS, Forester AJ. Appropriateness of diagnostic strategies for evaluating suspected venous thromboembolism. Thromb Haemost 2007; 97:195–201.
- Clarke M, Hopewell S, Juszcak E, Eisinga A, Kjeldstrøm M. Compression stockings in preventing deep vein thrombosis in airline passengers. Cochrane Database of Syst Rev 2006; Apr 19( 2):CD004002. DOI: 10.1002/14651858.
- Kuipers S, Cannegieter SC, Middeldorp S, et al. Use of preventive measures for travel-related venous thrombosis in professionals who attend medical conferences. J Thromb Haemost 2006; 4:2373–2376.
- Perez-Rodriguez E, Jimenez D, Diaz G, et al. Incidence of air travel-related pulmonary embolism in the Madrid-Barajas Airport. Arch Intern Med 2003; 163:2766–2770.
- Lapostolle F, Surget V, Borron SW, et al. Severe pulmonary embolism associated with air travel. N Engl J Med 2001; 345:779–783.
- Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ 2003; 327:1072–1076.
- Eklof B, Kistner RL, Masuda EM, et al. Venous thromboembolism in association with prolonged air travel. Dermatol Surg 1996; 22:637–641.
- Moyle J. Medical guidelines for airline travel. Aviat Space Environ Med 2003: 74:1009.
- Geerts WH, Bergqvist B, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2008; 133:381S–453S.
- Rosendaal FR. Interventions to prevent venous thrombosis after air travel: are they necessary? No. J Thromb Haemost 2006; 4:2306–2307.
- Kearon C, Ginsberg JS, Julian JA, et al; Fixed-Dose Heparin (FIDO) Investigators. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA 2006; 296:935–942.
Editor’s Note: The views expressed in this article are solely those of the authors and do not reflect the official policy or position of the Department of State or the United States Government. This version of the article was peer-reviewed.
Venous thromboembolism (VTE) associated with travel has emerged as an important public health concern over the past decade. Numerous epidemiologic and case control studies have reported air travel as a risk factor for the development of VTE and have attempted to determine who is at risk and which precautions need to be taken to prevent this potentially fatal event.1–7 Often referred to as “traveler’s thrombosis” or “flight-related deep vein thrombosis,” VTE can also develop after long trips by automobile, bus, or train.8,9 Although the absolute risk is very low, this threat appears to be about three times higher in travelers and increases with longer trips.3
See related patient information material
This article focuses on defining VTE and recognizing its clinical features, as well as providing recommendations and guidelines to prevent, diagnose, and treat this complication in people who travel.
WHAT IS VENOUS THROMBOEMBOLISM?
Deep vein thrombosis and pulmonary embolism represent different manifestations of the same clinical entity, ie, VTE. VTE is a common, lethal disease that affects hospitalized and nonhospitalized patients, frequently recurs, is often overlooked, may be asymptomatic, and may result in long-term complications that include pulmonary hypertension and the postthrombotic syndrome.
Deep vein thrombosis of the upper extremities is generally related to an indwelling venous catheter or a central line being used for long-term administration of antibiotics, chemotherapy, or nutrition. A condition known as Paget-Schroetter syndrome or “effort thrombosis” may be seen in younger or athletic people who have a history of strenuous or unusual arm exercise.
RISK FACTORS FOR VTE
Common inherited risk factors include:
- Factor V Leiden mutation
- Prothrombin gene mutation G20210A
- Hyperhomocysteinemia
- Deficiency of the natural anticoagulant proteins C, S, or antithrombin
- Elevated levels of factor VIII (may be inherited or acquired).
Acquired risk factors include:
- Older age
- Immobilization or stasis (such as sitting for long periods of time while traveling)
- Surgery (most notably orthopedic procedures including hip and knee replacement and repair of a hip fracture)
- Trauma
- Stroke
- Acute medical illness (including congestive heart failure, chronic obstructive pulmonary disease, pneumonia)
- The antiphospholipid syndrome (consisting of a lupus anticoagulant, anticardiolipin antibodies, or both)
- Pregnancy and the postpartum state
- Use of oral contraceptives or hormone replacement therapy
- Cancer (including the myeloproliferative disorders) and certain chemotherapeutic agents
- Obesity (a body mass index > 30 kg/m2, see www.nhlbisupport.com/bmi/)
- Inflammatory bowel disease
- Previous VTE
- A central venous catheter or pacemaker
- Nephrotic syndrome.
In addition, emerging risk factors more recently recognized include male sex, persistence of elevated factor VIII levels, and the continued presence of an elevated D-dimer level or deep vein thrombosis on duplex ultrasonography once anticoagulation treatment is completed. There is also evidence of an association between VTE and risk factors for atherosclerotic arterial disease such as smoking, hypertension, hyperlipidemia, and diabetes.
CLINICAL MANIFESTATIONS OF VTE
Patients with deep vein thrombosis may complain of pain, swelling, or both in the leg or arm. Physical examination may reveal increased warmth, tenderness, erythema, edema, or dilated (collateral) veins, most notable on the upper thigh or calf (for deep vein thrombosis in the lower extremity) or the chest wall (for upper-extremity deep vein thrombosis). The examiner may also observe a tender, palpable cord, which represents a superficial vein thrombosis involving the great and small saphenous veins (Figure 1). In extreme situations, the limb may be cyanotic or gangrenous.
DIAGNOSIS OF VTE
Clinical examination alone is generally insufficient to confirm a diagnosis of deep vein thrombosis or pulmonary embolism. Venous duplex ultrasonography is the most dependable investigation for deep vein thrombosis, but other tests include D-dimer and imaging studies such as computed tomographic venography or magnetic resonance venography of the lower extremities. A more invasive approach is venography; formerly considered the gold standard, it is now generally used only when the diagnosis is in doubt after noninvasive testing. The diagnosis of acute pulmonary embolism is best made by spiral computed tomography.
Other studies that may prove helpful include a ventilation-perfusion lung scan for patients who cannot undergo computed tomography due to a contrast allergy or renal insufficiency. Pulmonary angiography, while the gold standard, is less commonly used today, given the specificity and sensitivity of computed tomography.
Echocardiography at the bedside may be useful for patients too sick to move, although the study may not be diagnostic unless thrombi are seen in the heart or pulmonary arteries.
TREATMENT OF VTE
For acute deep venous thrombosis
Acute deep vein thrombosis is now treated on an outpatient basis under most circumstances.
Unfractionated heparin is given intravenously for patients who need to be hospitalized, or subcutaneously in full dose for inpatient or outpatient treatment.
Low-molecular-weight heparins are available in subcutaneous preparations and can be given on an outpatient basis.
Fondaparinux (Arixtra), a factor Xa inhibitor, can also be given subcutaneously on an outpatient basis. Equivalent products are available outside the United States.
Warfarin (Coumadin), an oral vitamin K inhibitor, is the agent of choice for long-term management of deep vein thrombosis.
Other oral agents are available outside the United States.
For pulmonary embolism
Outpatient treatment of pulmonary embolism is not yet advised: an initial hospitalization is necessary. The same anticoagulants used for deep vein thrombosis are also used for acute pulmonary embolism.
Empiric treatment in underdeveloped countries
VTE may be an even greater concern on an outbound trip to a remote area, where medical care capabilities may be less than ideal and diagnostic and treatment options may be limited.
If there is a high pretest probability of acute VTE (Table 2, Table 3) and no diagnostic methods are available, empiric treatment with any of the parenteral anticoagulant agents listed in Table 4 is an option until the diagnosis can be confirmed. Caveats:
- Care must be taken to be certain there is not a strong contraindication to the use of anticoagulation, such as bleeding or a drug allergy.
- Neither unfractionated heparin nor any of the low-molecular-weight heparins should be given to a patient who has a history of heparin-induced thrombocytopenia.
- In patients who have chronic kidney disease (creatinine clearance less than 30 mL/minute), the dosage of low-molecular-weight heparins must be adjusted and factor Xa inhibitors avoided. Both of these types of anticoagulants should be avoided in patients on hemodialysis.
More aggressive therapy
Under select circumstances a more aggressive approach to the treatment of VTE may be necessary. These options are usually indicated for a patient with a massive deep vein thrombosis of a lower extremity and for certain patients with an upper extremity deep vein thrombosis. Treatments include catheter-directed thrombolytic therapy and endovenous or surgical thrombectomy.
Thrombolytic therapy is recommended for a patient with an acute pulmonary embolism who is clinically unstable (systolic blood pressure lower than 90 mm Hg), if there is no contraindication to its use (bleeding risk or recent stroke or surgery). Thrombolytic therapy is also an option for those at low risk of bleeding with an acute pulmonary embolism who have signs and symptoms of right heart failure proven by echocardiography.
Surgical pulmonary embolectomy for acute massive pulmonary embolism and mechanical thrombectomy for extensive deep vein thrombosis are generally available only at highly sophisticated tertiary care centers.
An inferior vena cava filter is advised in patients with acute deep vein thrombosis or pulmonary embolism who cannot be fully anticoagulated, to prevent the clot from migrating from the lower extremities to the lungs. These filters are available as either permanent or temporary implants. Some temporary versions can remain in place for up to 150 days after insertion.
PREVENTION OF VTE
Prevention is the standard of care for all patients admitted to the hospital and in select individuals as outpatients who are at high risk of VTE.
Mechanical compression (graduated compression stockings, intermittent pneumatic compression devices) has proven effective in reducing the incidence of deep vein thrombosis and pulmonary embolism postoperatively in patients who cannot take anticoagulants. One study has demonstrated that compression stockings may also be effective in preventing VTE during travel.12
ABSOLUTE RISK IS LOW
Over the past decade, special attention has been paid to travel as a risk factor for developing VTE.13 Traveler’s thrombosis has become an important public health concern. Numerous publications and epidemiologic studies have targeted air travel in an attempt to determine who is at risk and what precautions are necessary to prevent this complication.1–7,9
The incidence of VTE following air travel is reported to be 3.2 per 1,000 person-years.4 While this incidence is relatively low, it is still 3.2 times higher than in the healthy population that is not flying.
The more serious complication of VTE, ie, acute pulmonary embolism, occurs less often. In three studies, the reported incidence ranged from 1.65 per million patients in flights longer than 8 hours to a high of 4.8 per million patients in flights longer than 12 hours or distances exceeding 10,000 km (6,200 miles).5,14,15 For the 400 passengers on the average long-haul flight of 12 hours, there is at most a 0.2% chance that somebody on the plane will have a symptomatic VTE).
RISK FACTORS IN LONG-DISTANCE TRAVELERS
The risk of traveler’s thrombosis has recently attracted the attention of passengers and the airline industry. Airlines are now openly discussing the risk and providing reminders such as exercises that should be undertaken in-flight (see the patient information page that accompanies this article). Some airlines are recommending that all patients consult their doctor to assess their personal risk of deep vein thrombosis before flying.
The most common risk factors for VTE in travelers are well established and are additive (Table 1). The extent of the additive risk, however, is not entirely clear.
What is clear is that when VTE occurs it is a life-altering and life-threatening event. If it occurs on an outbound trip, the local resources and capabilities available at the destination may not be adequate for optimal treatment. If a traveler experiences a VTE event on an outbound trip, an emergency return trip to the continental United States or a regional center of expertise may be required. There is an additive risk with this subsequent travel event if the patient is not given immediate treatment first (Table 4). Hence, treatment prior to evacuation should be strongly considered.
The traveler must also be aware that VTE can be recognized up to 2 months after a long-haul flight, though it is especially a concern within the first 2 weeks after travel.2,4,16,17
RECOMMENDATIONS FOR LONG-DISTANCE AIR TRAVELERS
Each person should be evaluated on a case-by-case basis for his or her need for VTE prophylaxis. Medical guidelines for airline passengers have been published by the Aerospace Medical Association and the American College of Chest Physicians (ACCP).18,19 In general, travelers should:
- Exercise the legs by flexing and extending the ankles at regular intervals while seated (see the patient information material that accompanies this article) and frequently contracting the calf muscles.
- Walk about the cabin periodically, 5 minutes for every hour on longer-duration flights (over 4 hours) and when flight conditions permit.
- Drink adequate amounts of water and fruit juices to maintain good hydration.17
- Avoid alcohol and caffeinated beverages, which are dehydrating.
- Be careful about eating too much during the flight.
- Request an aisle seat if you are at risk
- Do not place baggage underneath the seat in front of you, because that reduces the ability to move the legs.
- Do not sleep in a cramped position, and avoid the use of any type of sleep aid.
- Avoid wearing constrictive clothing around the lower extremities or waist.
We recommend that all airplane passengers take the steps listed above to reduce venous stasis and avoid dehydration, even though these measures have not been proven effective in clinical trials.19
The ACCP further advises that decisions about pharmacologic prophylaxis of VTE for airplane passengers at high risk should be made on an individual basis, considering that there are potential adverse effects of prophylaxis and that these may outweigh the benefits. For long-distance travelers with additional risk factors for VTE, we suggest the following:
- Use of properly fitted, below-the-knee graduated compression stockings providing 15 to 30 mm Hg of pressure at the ankle (particularly when large varicosities or leg edema is present)
- For people at very high risk, a single prophylactic dose of a low-molecular-weight heparin or a factor Xa inhibitor injected just before departure (Table 5)
- Aspirin is not recommended as it is not effective for the prevention of VTE.20
SUMMARY FOR THE AIR TRAVELER
All travelers on long flights should perform standard VTE prophylaxis exercises (see the patient information pages accompanying this article). Although VTE is uncommon, people with additional risk factors who travel frequently either on multiple flights in a short period of time or on very long flights should be evaluated on a case-by-case basis for a more aggressive approach to prevention (compression support hose or prophylactic administration of a low-molecular-weight heparin or a factor Xa inhibitor).
Should a VTE event occur during travel, the patient should seek medical care immediately. The standard evaluation of a patient with a suspected VTE should include an estimation of the pretest probability of disease (Table 2, Table 3), followed by duplex ultrasonography of the upper or lower extremity to detect a deep vein thrombosis. If symptoms dictate, then spiral computed tomography, ventilation-perfusion lung scan, or pulmonary angiography (where available) should be ordered to diagnose acute pulmonary embolism. A positive D-dimer blood test alone is not diagnostic and may not be available in more remote locations. A negative D-dimer test result is most helpful to exclude VTE.
Standard therapy for VTE is immediate treatment with one of the anticoagulants listed in Table 4, unless the patient has a contraindication to treatment, such as bleeding or allergy. Immediate evacuation is recommended if the patient has a life-threatening pulmonary embolism, defined as hemodynamic instability (hypotension with a blood pressure under 90 mm Hg systolic or signs of right heart failure) that cannot be treated at a local facility. An air ambulance should be used to transport these patients. If the patient has an iliofemoral deep vein thrombosis, it is also advisable that he or she be considered for evacuation if severe symptoms are present, such as pain, swelling, or cyanosis. Unless contraindicated, all patients should be given either full-dose intravenous or full-dose subcutaneous heparin or subcutaneous injection of a readily available low-molecular-weight heparin preparations or factor Xa inhibitor at once.21
Editor’s Note: The views expressed in this article are solely those of the authors and do not reflect the official policy or position of the Department of State or the United States Government. This version of the article was peer-reviewed.
Venous thromboembolism (VTE) associated with travel has emerged as an important public health concern over the past decade. Numerous epidemiologic and case control studies have reported air travel as a risk factor for the development of VTE and have attempted to determine who is at risk and which precautions need to be taken to prevent this potentially fatal event.1–7 Often referred to as “traveler’s thrombosis” or “flight-related deep vein thrombosis,” VTE can also develop after long trips by automobile, bus, or train.8,9 Although the absolute risk is very low, this threat appears to be about three times higher in travelers and increases with longer trips.3
See related patient information material
This article focuses on defining VTE and recognizing its clinical features, as well as providing recommendations and guidelines to prevent, diagnose, and treat this complication in people who travel.
WHAT IS VENOUS THROMBOEMBOLISM?
Deep vein thrombosis and pulmonary embolism represent different manifestations of the same clinical entity, ie, VTE. VTE is a common, lethal disease that affects hospitalized and nonhospitalized patients, frequently recurs, is often overlooked, may be asymptomatic, and may result in long-term complications that include pulmonary hypertension and the postthrombotic syndrome.
Deep vein thrombosis of the upper extremities is generally related to an indwelling venous catheter or a central line being used for long-term administration of antibiotics, chemotherapy, or nutrition. A condition known as Paget-Schroetter syndrome or “effort thrombosis” may be seen in younger or athletic people who have a history of strenuous or unusual arm exercise.
RISK FACTORS FOR VTE
Common inherited risk factors include:
- Factor V Leiden mutation
- Prothrombin gene mutation G20210A
- Hyperhomocysteinemia
- Deficiency of the natural anticoagulant proteins C, S, or antithrombin
- Elevated levels of factor VIII (may be inherited or acquired).
Acquired risk factors include:
- Older age
- Immobilization or stasis (such as sitting for long periods of time while traveling)
- Surgery (most notably orthopedic procedures including hip and knee replacement and repair of a hip fracture)
- Trauma
- Stroke
- Acute medical illness (including congestive heart failure, chronic obstructive pulmonary disease, pneumonia)
- The antiphospholipid syndrome (consisting of a lupus anticoagulant, anticardiolipin antibodies, or both)
- Pregnancy and the postpartum state
- Use of oral contraceptives or hormone replacement therapy
- Cancer (including the myeloproliferative disorders) and certain chemotherapeutic agents
- Obesity (a body mass index > 30 kg/m2, see www.nhlbisupport.com/bmi/)
- Inflammatory bowel disease
- Previous VTE
- A central venous catheter or pacemaker
- Nephrotic syndrome.
In addition, emerging risk factors more recently recognized include male sex, persistence of elevated factor VIII levels, and the continued presence of an elevated D-dimer level or deep vein thrombosis on duplex ultrasonography once anticoagulation treatment is completed. There is also evidence of an association between VTE and risk factors for atherosclerotic arterial disease such as smoking, hypertension, hyperlipidemia, and diabetes.
CLINICAL MANIFESTATIONS OF VTE
Patients with deep vein thrombosis may complain of pain, swelling, or both in the leg or arm. Physical examination may reveal increased warmth, tenderness, erythema, edema, or dilated (collateral) veins, most notable on the upper thigh or calf (for deep vein thrombosis in the lower extremity) or the chest wall (for upper-extremity deep vein thrombosis). The examiner may also observe a tender, palpable cord, which represents a superficial vein thrombosis involving the great and small saphenous veins (Figure 1). In extreme situations, the limb may be cyanotic or gangrenous.
DIAGNOSIS OF VTE
Clinical examination alone is generally insufficient to confirm a diagnosis of deep vein thrombosis or pulmonary embolism. Venous duplex ultrasonography is the most dependable investigation for deep vein thrombosis, but other tests include D-dimer and imaging studies such as computed tomographic venography or magnetic resonance venography of the lower extremities. A more invasive approach is venography; formerly considered the gold standard, it is now generally used only when the diagnosis is in doubt after noninvasive testing. The diagnosis of acute pulmonary embolism is best made by spiral computed tomography.
Other studies that may prove helpful include a ventilation-perfusion lung scan for patients who cannot undergo computed tomography due to a contrast allergy or renal insufficiency. Pulmonary angiography, while the gold standard, is less commonly used today, given the specificity and sensitivity of computed tomography.
Echocardiography at the bedside may be useful for patients too sick to move, although the study may not be diagnostic unless thrombi are seen in the heart or pulmonary arteries.
TREATMENT OF VTE
For acute deep venous thrombosis
Acute deep vein thrombosis is now treated on an outpatient basis under most circumstances.
Unfractionated heparin is given intravenously for patients who need to be hospitalized, or subcutaneously in full dose for inpatient or outpatient treatment.
Low-molecular-weight heparins are available in subcutaneous preparations and can be given on an outpatient basis.
Fondaparinux (Arixtra), a factor Xa inhibitor, can also be given subcutaneously on an outpatient basis. Equivalent products are available outside the United States.
Warfarin (Coumadin), an oral vitamin K inhibitor, is the agent of choice for long-term management of deep vein thrombosis.
Other oral agents are available outside the United States.
For pulmonary embolism
Outpatient treatment of pulmonary embolism is not yet advised: an initial hospitalization is necessary. The same anticoagulants used for deep vein thrombosis are also used for acute pulmonary embolism.
Empiric treatment in underdeveloped countries
VTE may be an even greater concern on an outbound trip to a remote area, where medical care capabilities may be less than ideal and diagnostic and treatment options may be limited.
If there is a high pretest probability of acute VTE (Table 2, Table 3) and no diagnostic methods are available, empiric treatment with any of the parenteral anticoagulant agents listed in Table 4 is an option until the diagnosis can be confirmed. Caveats:
- Care must be taken to be certain there is not a strong contraindication to the use of anticoagulation, such as bleeding or a drug allergy.
- Neither unfractionated heparin nor any of the low-molecular-weight heparins should be given to a patient who has a history of heparin-induced thrombocytopenia.
- In patients who have chronic kidney disease (creatinine clearance less than 30 mL/minute), the dosage of low-molecular-weight heparins must be adjusted and factor Xa inhibitors avoided. Both of these types of anticoagulants should be avoided in patients on hemodialysis.
More aggressive therapy
Under select circumstances a more aggressive approach to the treatment of VTE may be necessary. These options are usually indicated for a patient with a massive deep vein thrombosis of a lower extremity and for certain patients with an upper extremity deep vein thrombosis. Treatments include catheter-directed thrombolytic therapy and endovenous or surgical thrombectomy.
Thrombolytic therapy is recommended for a patient with an acute pulmonary embolism who is clinically unstable (systolic blood pressure lower than 90 mm Hg), if there is no contraindication to its use (bleeding risk or recent stroke or surgery). Thrombolytic therapy is also an option for those at low risk of bleeding with an acute pulmonary embolism who have signs and symptoms of right heart failure proven by echocardiography.
Surgical pulmonary embolectomy for acute massive pulmonary embolism and mechanical thrombectomy for extensive deep vein thrombosis are generally available only at highly sophisticated tertiary care centers.
An inferior vena cava filter is advised in patients with acute deep vein thrombosis or pulmonary embolism who cannot be fully anticoagulated, to prevent the clot from migrating from the lower extremities to the lungs. These filters are available as either permanent or temporary implants. Some temporary versions can remain in place for up to 150 days after insertion.
PREVENTION OF VTE
Prevention is the standard of care for all patients admitted to the hospital and in select individuals as outpatients who are at high risk of VTE.
Mechanical compression (graduated compression stockings, intermittent pneumatic compression devices) has proven effective in reducing the incidence of deep vein thrombosis and pulmonary embolism postoperatively in patients who cannot take anticoagulants. One study has demonstrated that compression stockings may also be effective in preventing VTE during travel.12
ABSOLUTE RISK IS LOW
Over the past decade, special attention has been paid to travel as a risk factor for developing VTE.13 Traveler’s thrombosis has become an important public health concern. Numerous publications and epidemiologic studies have targeted air travel in an attempt to determine who is at risk and what precautions are necessary to prevent this complication.1–7,9
The incidence of VTE following air travel is reported to be 3.2 per 1,000 person-years.4 While this incidence is relatively low, it is still 3.2 times higher than in the healthy population that is not flying.
The more serious complication of VTE, ie, acute pulmonary embolism, occurs less often. In three studies, the reported incidence ranged from 1.65 per million patients in flights longer than 8 hours to a high of 4.8 per million patients in flights longer than 12 hours or distances exceeding 10,000 km (6,200 miles).5,14,15 For the 400 passengers on the average long-haul flight of 12 hours, there is at most a 0.2% chance that somebody on the plane will have a symptomatic VTE).
RISK FACTORS IN LONG-DISTANCE TRAVELERS
The risk of traveler’s thrombosis has recently attracted the attention of passengers and the airline industry. Airlines are now openly discussing the risk and providing reminders such as exercises that should be undertaken in-flight (see the patient information page that accompanies this article). Some airlines are recommending that all patients consult their doctor to assess their personal risk of deep vein thrombosis before flying.
The most common risk factors for VTE in travelers are well established and are additive (Table 1). The extent of the additive risk, however, is not entirely clear.
What is clear is that when VTE occurs it is a life-altering and life-threatening event. If it occurs on an outbound trip, the local resources and capabilities available at the destination may not be adequate for optimal treatment. If a traveler experiences a VTE event on an outbound trip, an emergency return trip to the continental United States or a regional center of expertise may be required. There is an additive risk with this subsequent travel event if the patient is not given immediate treatment first (Table 4). Hence, treatment prior to evacuation should be strongly considered.
The traveler must also be aware that VTE can be recognized up to 2 months after a long-haul flight, though it is especially a concern within the first 2 weeks after travel.2,4,16,17
RECOMMENDATIONS FOR LONG-DISTANCE AIR TRAVELERS
Each person should be evaluated on a case-by-case basis for his or her need for VTE prophylaxis. Medical guidelines for airline passengers have been published by the Aerospace Medical Association and the American College of Chest Physicians (ACCP).18,19 In general, travelers should:
- Exercise the legs by flexing and extending the ankles at regular intervals while seated (see the patient information material that accompanies this article) and frequently contracting the calf muscles.
- Walk about the cabin periodically, 5 minutes for every hour on longer-duration flights (over 4 hours) and when flight conditions permit.
- Drink adequate amounts of water and fruit juices to maintain good hydration.17
- Avoid alcohol and caffeinated beverages, which are dehydrating.
- Be careful about eating too much during the flight.
- Request an aisle seat if you are at risk
- Do not place baggage underneath the seat in front of you, because that reduces the ability to move the legs.
- Do not sleep in a cramped position, and avoid the use of any type of sleep aid.
- Avoid wearing constrictive clothing around the lower extremities or waist.
We recommend that all airplane passengers take the steps listed above to reduce venous stasis and avoid dehydration, even though these measures have not been proven effective in clinical trials.19
The ACCP further advises that decisions about pharmacologic prophylaxis of VTE for airplane passengers at high risk should be made on an individual basis, considering that there are potential adverse effects of prophylaxis and that these may outweigh the benefits. For long-distance travelers with additional risk factors for VTE, we suggest the following:
- Use of properly fitted, below-the-knee graduated compression stockings providing 15 to 30 mm Hg of pressure at the ankle (particularly when large varicosities or leg edema is present)
- For people at very high risk, a single prophylactic dose of a low-molecular-weight heparin or a factor Xa inhibitor injected just before departure (Table 5)
- Aspirin is not recommended as it is not effective for the prevention of VTE.20
SUMMARY FOR THE AIR TRAVELER
All travelers on long flights should perform standard VTE prophylaxis exercises (see the patient information pages accompanying this article). Although VTE is uncommon, people with additional risk factors who travel frequently either on multiple flights in a short period of time or on very long flights should be evaluated on a case-by-case basis for a more aggressive approach to prevention (compression support hose or prophylactic administration of a low-molecular-weight heparin or a factor Xa inhibitor).
Should a VTE event occur during travel, the patient should seek medical care immediately. The standard evaluation of a patient with a suspected VTE should include an estimation of the pretest probability of disease (Table 2, Table 3), followed by duplex ultrasonography of the upper or lower extremity to detect a deep vein thrombosis. If symptoms dictate, then spiral computed tomography, ventilation-perfusion lung scan, or pulmonary angiography (where available) should be ordered to diagnose acute pulmonary embolism. A positive D-dimer blood test alone is not diagnostic and may not be available in more remote locations. A negative D-dimer test result is most helpful to exclude VTE.
Standard therapy for VTE is immediate treatment with one of the anticoagulants listed in Table 4, unless the patient has a contraindication to treatment, such as bleeding or allergy. Immediate evacuation is recommended if the patient has a life-threatening pulmonary embolism, defined as hemodynamic instability (hypotension with a blood pressure under 90 mm Hg systolic or signs of right heart failure) that cannot be treated at a local facility. An air ambulance should be used to transport these patients. If the patient has an iliofemoral deep vein thrombosis, it is also advisable that he or she be considered for evacuation if severe symptoms are present, such as pain, swelling, or cyanosis. Unless contraindicated, all patients should be given either full-dose intravenous or full-dose subcutaneous heparin or subcutaneous injection of a readily available low-molecular-weight heparin preparations or factor Xa inhibitor at once.21
- Brenner B. Interventions to prevent venous thrombosis after air travel, are they necessary? Yes. J Thromb Haemost 2006; 4:2302–2305.
- Cannegieter SC, Doggen CJM, van Houwellingen HC, et al. Travel-related venous thrombosis: results from a large population-based case control study (MEGA Study). PLoS Med 2006; 3:1258–1265.
- Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med 2009; 151:180–190.
- Kuipers S, Cannegieter SC, Middeldorp S, et al. The absolute risk of venous thrombosis after air travel: a cohort study of 8,755 employees of international organizations. PLoS Med 2007; 4:1508–1514.
- Kuipers S, Schreijer AJM, Cannegieter SC, et al. Travel and venous thrombosis: a systematic review. J Intern Med 2007; 262:615–634.
- Lehmann R, Suess C, Leus M, et al. Incidence, clinical characteristics, and long-term prognosis of travel-associated pulmonary embolism. Eur Heart J 2009; 30:233–241.
- Philbrick JT, Shumate R, Siadaty MS, et al. Air travel and venous thromboembolism: a systematic review. J Gen Intern Med 2007; 22:107–114.
- Cruickshank JM, Gorlin R, Jennett B. Air travel and thrombotic episodes: the economy class syndrome. Lancet 1988; 2:497–498.
- Bagshaw M. Traveler’s thrombosis: a review of deep vein thrombosis associated with travel. Air Transport Medicine Committee, Aerospace Medical Association. Aviat Space Environ Med 2001; 72:848–851.
- Wells PS, Owens C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Arnason T, Wells PS, Forester AJ. Appropriateness of diagnostic strategies for evaluating suspected venous thromboembolism. Thromb Haemost 2007; 97:195–201.
- Clarke M, Hopewell S, Juszcak E, Eisinga A, Kjeldstrøm M. Compression stockings in preventing deep vein thrombosis in airline passengers. Cochrane Database of Syst Rev 2006; Apr 19( 2):CD004002. DOI: 10.1002/14651858.
- Kuipers S, Cannegieter SC, Middeldorp S, et al. Use of preventive measures for travel-related venous thrombosis in professionals who attend medical conferences. J Thromb Haemost 2006; 4:2373–2376.
- Perez-Rodriguez E, Jimenez D, Diaz G, et al. Incidence of air travel-related pulmonary embolism in the Madrid-Barajas Airport. Arch Intern Med 2003; 163:2766–2770.
- Lapostolle F, Surget V, Borron SW, et al. Severe pulmonary embolism associated with air travel. N Engl J Med 2001; 345:779–783.
- Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ 2003; 327:1072–1076.
- Eklof B, Kistner RL, Masuda EM, et al. Venous thromboembolism in association with prolonged air travel. Dermatol Surg 1996; 22:637–641.
- Moyle J. Medical guidelines for airline travel. Aviat Space Environ Med 2003: 74:1009.
- Geerts WH, Bergqvist B, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2008; 133:381S–453S.
- Rosendaal FR. Interventions to prevent venous thrombosis after air travel: are they necessary? No. J Thromb Haemost 2006; 4:2306–2307.
- Kearon C, Ginsberg JS, Julian JA, et al; Fixed-Dose Heparin (FIDO) Investigators. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA 2006; 296:935–942.
- Brenner B. Interventions to prevent venous thrombosis after air travel, are they necessary? Yes. J Thromb Haemost 2006; 4:2302–2305.
- Cannegieter SC, Doggen CJM, van Houwellingen HC, et al. Travel-related venous thrombosis: results from a large population-based case control study (MEGA Study). PLoS Med 2006; 3:1258–1265.
- Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med 2009; 151:180–190.
- Kuipers S, Cannegieter SC, Middeldorp S, et al. The absolute risk of venous thrombosis after air travel: a cohort study of 8,755 employees of international organizations. PLoS Med 2007; 4:1508–1514.
- Kuipers S, Schreijer AJM, Cannegieter SC, et al. Travel and venous thrombosis: a systematic review. J Intern Med 2007; 262:615–634.
- Lehmann R, Suess C, Leus M, et al. Incidence, clinical characteristics, and long-term prognosis of travel-associated pulmonary embolism. Eur Heart J 2009; 30:233–241.
- Philbrick JT, Shumate R, Siadaty MS, et al. Air travel and venous thromboembolism: a systematic review. J Gen Intern Med 2007; 22:107–114.
- Cruickshank JM, Gorlin R, Jennett B. Air travel and thrombotic episodes: the economy class syndrome. Lancet 1988; 2:497–498.
- Bagshaw M. Traveler’s thrombosis: a review of deep vein thrombosis associated with travel. Air Transport Medicine Committee, Aerospace Medical Association. Aviat Space Environ Med 2001; 72:848–851.
- Wells PS, Owens C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Arnason T, Wells PS, Forester AJ. Appropriateness of diagnostic strategies for evaluating suspected venous thromboembolism. Thromb Haemost 2007; 97:195–201.
- Clarke M, Hopewell S, Juszcak E, Eisinga A, Kjeldstrøm M. Compression stockings in preventing deep vein thrombosis in airline passengers. Cochrane Database of Syst Rev 2006; Apr 19( 2):CD004002. DOI: 10.1002/14651858.
- Kuipers S, Cannegieter SC, Middeldorp S, et al. Use of preventive measures for travel-related venous thrombosis in professionals who attend medical conferences. J Thromb Haemost 2006; 4:2373–2376.
- Perez-Rodriguez E, Jimenez D, Diaz G, et al. Incidence of air travel-related pulmonary embolism in the Madrid-Barajas Airport. Arch Intern Med 2003; 163:2766–2770.
- Lapostolle F, Surget V, Borron SW, et al. Severe pulmonary embolism associated with air travel. N Engl J Med 2001; 345:779–783.
- Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ 2003; 327:1072–1076.
- Eklof B, Kistner RL, Masuda EM, et al. Venous thromboembolism in association with prolonged air travel. Dermatol Surg 1996; 22:637–641.
- Moyle J. Medical guidelines for airline travel. Aviat Space Environ Med 2003: 74:1009.
- Geerts WH, Bergqvist B, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2008; 133:381S–453S.
- Rosendaal FR. Interventions to prevent venous thrombosis after air travel: are they necessary? No. J Thromb Haemost 2006; 4:2306–2307.
- Kearon C, Ginsberg JS, Julian JA, et al; Fixed-Dose Heparin (FIDO) Investigators. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA 2006; 296:935–942.
KEY POINTS
- The risk of VTE is about three times higher in passengers on long-distance flights than in the general population, although the absolute risk is still low.
- All long-distance air passengers should perform stretching exercises once an hour while in flight to prevent VTE. They should also stay hydrated.
- For patients at higher risk due to hypercoagulable conditions, physicians can consider prescribing compression stockings or an anticoagulant drug (a low-molecular-weight heparin or a factor Xa inhibitor) to be taken before the flight, or both.
- The evaluation of a patient with suspected VTE should include an estimation of the pretest probability of disease. If symptoms dictate, duplex ultrasonography of the upper or lower extremity to detect deep vein thrombosis or spiral computed tomography, ventilation-perfusion lung scan, or pulmonary angiography (where available) to diagnose an acute pulmonary embolism should be ordered.
Goal-directed antihypertensive therapy: Lower may not always be better
A 50-year-old African American woman with type 2 diabetes mellitus, hypertension, hyperlipidemia, and chronic kidney disease presents for a follow-up visit. The patient had been treated with hydrochlorothiazide 25 mg/day and enalapril (Vasotec) 20 mg twice daily until 6 weeks ago. At that time her blood pressure was 160/85 mm Hg, and amlodipine (Norvasc) 10 mg/day was added to her regimen. Her other medications include glipizide (Glucotrol), metformin (Glucophage), lovastatin (Mevacor), fish oils, aspirin, calcium, and vitamin D. Her current blood pressure is 145/80 mm Hg; her serum creatinine level is 1.5 mg/dL, and her urine albumin-to-creatinine ratio is 180 mg/g.
In hypertensive patients who have diabetes or chronic kidney disease, guidelines1 call for intensification of antihypertensive therapy to reach a goal blood pressure of less than 130/80 mm Hg. What data exist to support these guidelines? And what should the clinician do?
IS MORE-INTENSE THERAPY IN THE PATIENT’S BEST INTEREST?
Often, clinicians are faced with hypertensive patients whose blood pressure, despite treatment, is higher than the accepted goal. Often, these patients are elderly and are already taking multiple medications that are costly and have significant potential adverse effects. The dilemma is whether to try to reach a target blood pressure listed in a guideline (by increasing the dosage of the current drugs or by adding a drug of a different class) or to “do no harm,” accept the patient’s blood pressure, and keep the regimen the same.1,2
The current goal blood pressure is less than 140/90 mm Hg for all but the very elderly, with more intense control recommended for patients at high risk, ie, those with diabetes mellitus, chronic kidney disease, or atherosclerotic cardiovascular disease.1
While it appears to be in the patient’s best interests to follow such guidelines, review of available data indicates that this it not necessarily so, and may even be harmful.
OBSERVATIONAL DATA AND EARLY RANDOMIZED TRIALS
Many observational studies have found that the higher one’s blood pressure, the greater one’s risk of cardiovascular events and death. Indeed, meta-analyses of these trials, which involved more than 1.5 million people, demonstrate a strong, positive, log-linear relationship between blood pressure and the incidence of cardiovascular disease and death.3–5
Further, there is no evidence of a threshold pressure below which the risk is not lower (ie, a “J-point”), starting with 115/75 mm Hg. A J-point may exist for diastolic blood pressure in elderly patients with isolated systolic hypertension6 and in patients with coronary artery disease.7 Otherwise, the observation is clear: the lower the blood pressure the better. For every 20 mm Hg lower systolic blood pressure or 10 mm Hg lower diastolic blood pressure, the risk of a cardiovascular event is about 50% less.4,5
Observational analyses also show a strong, graded relationship between blood pressure and future end-stage renal disease.8,9 Post hoc analyses indicate that chronic kidney disease progresses more slowly with lower achieved blood pressures, especially in those with higher degrees of proteinuria.10–12
However, observational data do not prove cause and effect, nor do they guarantee similar results with treatment. This requires randomized controlled trials.
RANDOMIZED TRIALS OF HYPERTENSION TREATMENT
Initial trials were aimed at determining whether hypertension should even be treated. A 1997 meta-analysis of 18 such trials comparing either low-dose diuretic therapy, high-dose diuretic therapy, or beta-blocker therapy with placebo involved 48,000 patients who were followed for an average of 5 years.13 The rates of stroke and congestive heart failure were consistently reduced, although only low-dose diuretic therapy reduced the risk of coronary heart disease and death from any cause.
More recent trials enrolled people not considered hypertensive who were randomized to receive either active drugs or placebo, or no treatment. Other trials attempted to assess non-pressure-related effects of specific agents, using other antihypertensive agents in the control group. Still other randomized controlled trials compared one agent or agents with other agents while attempting to attain equivalent blood pressure between groups. Frequently, however, there was some blood pressure difference.
Meta-analyses of most of these trials conclude that the major benefit of antihypertensive therapy—reducing rates of cardiovascular morbidity and mortality—comes from a lower attained blood pressure, irrespective of which agent is used.14–18 Exceptions exist, however. For example, specific drug classes are indicated after myocardial infarction, and in congestive heart failure and proteinuric chronic kidney disease.10,19–21
16 TRIALS OF DIFFERENT BLOOD PRESSURE TARGETS
The overriding theme of these observational data is that a lower blood pressure, whether attained naturally or with treatment, is better than a higher one from both the cardiovascular and the renal perspective.
What remains unclear is what blood pressure should be aimed for in a particular patient or group of patients. Is it a specific pressure (eg, 140/90 mm Hg), or does the change from baseline count more? Should other factors such as age or comorbidity alter this number?
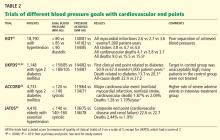
Several randomized controlled trials have addressed these questions by targeting different levels of blood pressure. We are aware of at least 16 such trials in adults, including 13 with renal or cardiovascular primary end points and three with surrogate primary end points.
An unavoidable design flaw of all of these trials is their unblinded nature. Consequently, nearly all of them carry a Jadad score (a measure of quality, based on randomization and blinding)22 of 3 on a scale of 5.
NINE TRIALS WITH RENAL PRIMARY END POINTS
African American Study of Kidney Disease and Hypertension (AASK)23
Patients: 1,094 African Americans with presumed hypertensive renal disease and a measured glomerular filtration rate between 20 and 65 mL/min/1.73 m2.
Randomized blood pressure goals. Mean arterial pressure 92 mm Hg or less vs 102 to 107 mm Hg.
Results. At 4 years, the two groups had average blood pressures of 128/78 and 141/85 mm Hg, respectively. The groups did not differ in the rates of the primary end points—ie, the rate of change in the measured glomerular filtration rate over time or the composite of a 50% reduction in glomerular filtration rate, the onset of end-stage renal disease, or death.
Comments. Several issues have been raised about the internal validity of this trial.
So-called hypertensive kidney disease in African Americans (as opposed to European Americans) may be a genetic disorder related to polymorphisms of one or more genes on chromosome 22q. Initial data implicated the MYH9 gene, which encodes non-muscle myosin heavy chain II.24,25 More recent data implicate the nearby APOL1 gene encoding apolipoprotein L126 as more relevant. These polymorphisms have a much greater prevalence in African Americans and appear responsible for the higher risk of idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy in this population.24–26 Therefore, in African Americans, hypertension may in fact be the result of the kidney disease and not its primary cause, which may explain why in this and other African American populations stricter control of blood pressure did not produce a renal benefit.27,28
Also, there is the possibility of misclassification bias. A secondary analysis of data obtained by ambulatory monitoring showed that of the 377 participants whose blood pressure appeared to be under control when measured in the clinic, 70% actually had masked hypertension, ie, uncontrolled hypertension outside the clinic.29 The real difference in blood pressure between groups may well have been different than that determined in the clinic.
In addition, a prespecified secondary analysis showed no difference in the rates of cardiovascular events and death between the groups.30 However, the study was not designed to have the statistical power to detect a difference in cardiovascular events. Moreover fewer cardiovascular events occurred than expected, further reducing the study’s power to detect a difference.
Toto et al31
Toto et al reported similar results in an earlier trial in 87 hypertensive patients (77 randomized), predominantly African American, and similar concerns apply.
Lewis et al32
Patients: 129 patients with type 1 diabetes.
Randomized blood pressure goals. A mean arterial pressure of either no higher than 92 mm Hg or 100 to 107 mm Hg.
Results. At 2 years, despite a difference of 6 mm Hg in mean arterial pressure, the glomerular filtration rate (measured) had declined by the same amount in the two groups. The study was underpowered for this end point. Patients in the group with the lower goal pressure were excreting significantly less protein than those in the other group, but they were received higher doses of an angiotensin-converting enzyme (ACE) inhibitor—in this case, ramipril (Altace).
The Appropriate Blood Pressure Control in Diabetes (ABCD) trials33–35
Patients: 950 patients with type 2 diabetes mellitus and either normal or high blood pressure.
Randomized blood pressure goals. Either intensive or moderate therapy (see Table 1).
Results. At 5 years, creatinine clearance (estimated) had declined by the same amount in the two groups. However, fewer of the hypertensive patients had died in the intensive-therapy group.34 Similarly, normotensive patients had less progression of albuminuria if treated intensively.33
In the ABCD Part 2 with Valsartan (ABCD-2V) trial in normotensive patients,35 therapy with valsartan (Diovan) did not affect creatinine clearance but did reduce albuminuria. However, 75% of the patients in the moderate-treatment group were untreated.
Schrier et al36
Patients. 75 hypertensive patients with autosomal-dominant polycystic kidney disease and left ventricular hypertrophy.
Randomized blood pressure targets. Less than 120/80 mm Hg vs 135/85 to 140/90 mm Hg.
Results. After 7 years, despite a difference in average mean arterial pressure of 11 mm Hg between the groups (90 vs 101 mm Hg), there was no difference in the rate of decline of creatinine clearance. The left ventricular mass index decreased by 21% in the lower-target group and by 35% in the higher-target group (P < .01).
Modification of Diet in Renal Disease (MDRD) trial37,38
Patients: 840 patients whose measured glomerular filtration rate was between 13 and 55 mL/min/1.73 m2.
Randomized blood pressure targets. A target mean arterial pressure of less than 92 mm Hg vs less than 107 mm Hg.11,37
Results. After 2.2 years, the mean difference in mean arterial pressure was 4.7 mm Hg. There was, however, no difference in the rate of decline in the glomerular filtration rate.
In a 6-year follow-up, significantly fewer patients in the lower-blood-pressure group reached the end point of end-stage renal disease or the combined end point of end-stage renal disease or death.38 The rate of death, however, was nearly twice as high in the lower-blood-pressure group (10% vs 6%). The blood pressure and treatment during follow-up were not reported.
Comments. Internal validity is an issue, since the blood pressure and therapy during follow-up were unknown, and more patients received ACE inhibitors in the lower-blood-pressure group during the trial. Further, the higher death rate in the lower-blood-pressure group is worrisome.
The Ramipril Efficacy in Nephropathy (REIN)-2 trial39
Patients: 338 nondiabetic patients who had proteinuria and reduced creatinine clearance.
Treatment and blood pressure goals. All were treated with ramipril and randomized to intensive (< 130/80 mm Hg) vs standard control (diastolic blood pressure < 90 mm Hg) with therapy based on felodipine (Plendil).
Results. The study was terminated early because of futility. Despite a mean difference of 4.1 mm Hg systolic and 2.8 mm Hg diastolic, the groups did not differ in the rate of progression to end-stage renal disease (23% with intensive therapy vs 20% with standard therapy) or in the rate of decline of the measured glomerular filtration rate (0.22 vs 0.24 mL/min/1.73 m2/month).
Comment. The internal validity of this study can be questioned because of the low separation of achieved blood pressure and because of its early termination.
No benefit from a lower blood pressure goal in preserving kidney function
To summarize, these trials all showed no significant benefit from either targeting or achieving lower blood pressure in terms of slowing the decline of kidney function. Overall, they do not define a target and offer little support that a lower goal blood pressure is indicated with respect to the rate of loss of glomerular filtration rate in chronic kidney disease.
However, post hoc analysis of the MDRD trial indicates a statistical interaction between targeted blood pressure and degree of baseline proteinuria. At higher levels of proteinuria (≥ 1 g/day), the group with the lower blood pressure target had better outcomes.
In addition, long-term follow-up (mean of 12.2 years) of the AASK trial, including a 7-year cohort phase with nearly similar blood pressures in both groups, also indicated an interaction with targeted blood pressure and baseline proteinuria.40 Although the overall analysis was negative, there was a significant reduction in the primary end point in the group originally assigned the low target when analysis was restricted to those in the highest tertile of proteinuria. These and other data10 suggest that patients with chronic kidney disease and proteinuria may represent a distinct subset of chronic kidney disease patients who benefit from more intensive blood-pressure-lowering. However, patients in the REIN-2 trial34 and the macroalbuminuric patients in the ABCD hypertensive trial35 did not benefit from a lower targeted blood pressure despite significant proteinuria.
FOUR TRIALS WITH CARDIOVASCULAR END POINTS
The Hypertension Optimal Treatment (HOT) trial41
Patients: 18,790 patients with diastolic blood pressure between 100 and 115 mm Hg.
Randomized blood pressure goals. Diastolic pressure of equal to or less than 80, 85, or 90 mm Hg.
Results. At an average of 3.8 years, the average blood pressures in the three groups were approximately 140/81, 141/83, and 144/85 mm Hg, respectively. There was no difference between the groups in the rate of the composite primary end point of all myocardial infarctions, all strokes, and cardiovascular death. Any conclusions from this trial were compromised by the small difference in achieved blood pressures between groups.
In the 1,501 patients with diabetes, the incidence of the primary end point was 50% lower with a goal of 80 mm Hg or less than with a goal of 90 mm Hg or less.
The UK Prospective Diabetes Study (UKPDS)42,43
Patients: 1,148 hypertensive patients with type 2 diabetes mellitus.
Randomized blood pressure goals. Either “tight control” (aiming for < 150/85 mm Hg) or “less tight control” (aiming for < 180/105 mm Hg).
Results. At a median follow-up of 8.4 years, the attained blood pressures were 144/82 vs 154/87 mm Hg. The difference produced significant benefits, including a 24% lower rate of any diabetes-related end point, a 32% lower rate of death due to diabetes, and a nonsignificant 18% lower rate of total mortality—all co-primary end points.
The less-tight-control group had many patients with initial blood pressures below 180/105 mm Hg; hence, over 50% of patients received no antihypertensive therapy at the start of the trial. By the end of the trial 9 years later, 20% had still not been treated. This compares with only 5% of patients in the tight-control group who were not treated with antihypertensives throughout the trial. Therefore, this trial serves as better evidence for treating vs not treating, rather than defining a specific goal.
During a 10-year follow-up, blood pressure differences disappeared within 2 years.43 There was no legacy effect, as the significant differences noted during the trial were no longer present 10 years later.
Action to Control Cardiovascular Risk in Diabetes (ACCORD)44
Patients: 4,733 patients with type 2 diabetes.
Randomized blood pressure goals. Systolic blood pressure lower than either 120 or 140 mm Hg.
Results. At 4.7 years, despite a significant difference in mean systolic blood pressure of 14.2 mm Hg after the first year (119.3 vs 133.5 mm Hg), there was no difference in the primary end point of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. There were fewer strokes in the lower-pressure group but no difference in myocardial infarctions, which were five times more common than strokes. Serious adverse events attributed to antihypertensive treatment occurred more frequently in the intensive-therapy group (3.3% vs 1.3%, P < .001).
Comment. There were fewer events than expected, possibly limiting the trial’s ability to detect a statistical difference. Compared with both the UKPDS and the diabetic population of HOT, ACCORD is much larger and more internally valid (unlike in UKPDS, nearly all patients in both groups were treated, and compared with HOT there was much greater separation of achieved pressure). It is more recent and better reflects current overall practice. It indicates that when specifically aiming for a target blood pressure, lower is not always better and comes at a price (more severe adverse events).
Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)45
Patients: 4,418 patients, age 65 to 85 years, with a pretreatment systolic blood pressure above 160 mm Hg.
Randomized blood pressure goals. Systolic pressure either lower than 140 mm Hg or 140 to 160 mm Hg.
Results. At 2 years, despite a difference of 9.7/3.3 mm Hg, there was no difference in the primary end point (the combined incidence of cerebrovascular disease, cardiac and vascular disease, and renal failure). Fifty-four patients had died in the strict-treatment group and 42 in the mild-treatment group; the difference was not statistically significant.
Three other trials
Three other trials46–48 had surrogate end points, but only one of them reported a composite cardiovascular secondary end point.46 We will not discuss the other two.47,48
Cardio-Sis. In the Studio Italiano Sugli Effetti Cardiovascolari del Controllo della Pressione Arteriosa Sistolica (Cardio-Sis) trial,46 1,111 people without diabetes with systolic pressure higher than 150 mm Hg were randomized to tight control (systolic pressure < 130 mm Hg) vs usual control (systolic pressure < 140 mm Hg) and followed for 2 years with electrocardiography to detect left ventricular hypertrophy.
At a median of 2 years, the systolic blood pressure had declined by an average of 3.8 mm Hg more in the tight-control group than in the usual-control group, and the diastolic pressure by an average of 1.5 mm Hg. There was significantly less left ventricular hypertrophy in the tight-control group. The incidence of the secondary end point of a composite of cardiovascular and renal events was also significantly lower. There was no difference individually in the rates of myocardial infarction, stroke, transient ischemic attack, admission for congestive heart failure, or death.
DISCUSSION: THE DILEMMA OF TREATING AN INDIVIDUAL PATIENT
These data illustrate the dilemma of treating an individual patient whose blood pressure is not at the currently accepted goal while on multiple antihypertensive medications. According to guidelines, therapy should be intensified in this situation. Observational data show a strong graded relationship between blood pressure and cardiovascular events and death, starting with a blood pressure of 115/75 mm Hg. The observational data relating blood pressure to kidney disease are similar. These data support the guideline recommendations that additional medications should be added to reach the promulgated target. Unfortunately, the targeting trials do not define a target, nor do they support the concept that lower is better.
Possible explanations for the negative results
Why does targeting a lower blood pressure not produce the benefit that the observational data lead us to expect?
One possibility is that blood pressure is merely a marker of cardiovascular risk, not a cause of it. This is unlikely, given the temporal relationship, reproducibility, and biologic plausibility that is supported by a very large body of experimental data. However, blood pressure is only one of multiple factors involved in the pathogenesis of vascular and renal disease, and perhaps better attention to other factors such as lipids and smoking may have made the targeting trials underpowered.
Another possibility is that these trials had such strict inclusion and exclusion criteria that they do not represent the general hypertensive population, reducing their external validity.49 However, the trials generally enrolled populations at higher risk, in which end points were more likely to occur. This would have enhanced the chance to show a positive effect rather than mask it.
It is possible that antihypertensive medications themselves have unwanted side effects that offset their potential benefit. Medication-related side effects could directly contribute to vascular disease despite their beneficial effect of lowering pressure. There could also be reduced tissue perfusion due to lower blood pressure per se in the face of a diseased vasculature, with the lower pressure directly contributing to organ dysfunction.
Finally, these trials measured brachial pressures to monitor blood pressure. Brachial pressure does not always correlate with central aortic pressure, which is probably a better marker of the overall pressure burden.50 It is possible that in these targeting trials, the peripheral blood pressure did not reflect the true central blood pressure and, therefore, significant separation of blood pressures may not have actually occurred.
Targeted vs achieved blood pressures: Analogies with other markers
This contradiction is not an exceptional circumstance in medicine.
For example, in chronic kidney disease, a graded observational relationship exists between decreasing levels of hemoglobin and various adverse outcomes.51–53 However, targeting a more normal level of hemoglobin compared with a lower one has been shown to be detrimental.54–57 This implies either that anemia is merely a marker of higher risk or, more likely, that the actual measures used to raise the hemoglobin to higher levels are the culprit. Notably, although targeting a higher hemoglobin concentration vs a lower one was detrimental, achieving a higher hemoglobin was beneficial within each targeted group.54,58
Another example of harm caused by targeting goals based on observational data is tight glucose control, both acutely in the critically ill59 and chronically in patients with type 2 diabetes.60 In both cases higher mortality rates ensued.
The same concept may apply to lowering blood pressure. While achieving a lower blood pressure may be more beneficial, targeting a specific goal may be harmful. Given that perhaps 20% of those labeled as hypertensive have resistant hypertension,61 millions of patients are susceptible to potential harm from targeting a specific goal based solely on observational data. If lower is always better, the randomized trials outlined above should have had more positive outcomes.
It becomes problematic to assign a specific goal for all patients or even groups of patients. The targeting trials do not provide the answer. Based on the observational data it would be optimal to have a blood pressure less than 120/80 mm Hg. This is an observation, not a recommendation. Patients should be assessed on an individual basis, taking into consideration their starting blood pressure, age, medication burden (antihypertensive and otherwise), comorbidities, and ability to comply with a regimen. Given the available data, it is hard to be more specific. In the future it may be possible to identify specific blood pressure targets based on the patient’s genetic makeup, but today that is not possible. Even patients with lower initial blood pressure may benefit from therapy,62,63 and some experts have advocated blood-pressure-lowering in all, irrespective of the baseline value.14
Avoid misclassification
The first step in treating hypertension should be to avoid misclassification. Make sure the clinic blood pressure is measured correctly, using an appropriately sized cuff, positioning the patient properly, and following all the other recommendations.64
However, the clinic blood pressure may not reflect true blood pressure load in up to one-third of all patients.65 We recommend 24-hour ambulatory blood pressure monitoring66 or home self-measurement, or both,67 to better assess true blood pressure burden in several circumstances, including in patients with resistant hypertension (any patient who has not achieved acceptable clinic blood pressure on three or more antihypertensive medications including a diuretic or who requires four or more medications for adequate control), suspicion of white-coat hypertension (or effect), and any patient who has achieved acceptable clinic blood pressure but either has symptoms of hypotension or progressive end-organ damage.
Currently, we base therapy on out-of-office blood pressure (self-measured or by ambulatory monitoring) whenever there is a discrepancy with clinic blood pressure.
Whether therapy should be altered by other less traditional measures of blood pressure such as assessment of central aortic pressure by radial applanation tonometry,68,69 or 24-hour ambulatory monitoring to assess nighttime blood pressures (specifically, “dipping”),70 morning surge,71 or blood pressure variability72,73 remains unclear and in need of randomized controlled trials.
In any patient requiring blood-pressure-lowering, we recommend lifestyle modifications.1,2 These include exercise, weight loss, salt and alcohol restriction, evaluation for sleep apnea, and avoidance of medications known to elevate blood pressure such as nonsteroidal anti-inflammatory drugs and sympathomimetic decongestants.
Much needs to be learned
For the individual patient with unacceptably high blood pressure who is already taking multiple antihypertensive medications of different classes, it is unclear what to do. This type of patient with resistant hypertension would be an excellent candidate for a future targeting trial. Other cardiovascular risk factors should be appropriately addressed, including obesity, lipids, smoking, and poor glycemic control.74 Each patient should be individually assessed with consideration of both global cardiovascular risk and quality-of-life issues.
Much still needs to be learned about the treatment of hypertension. The facts demonstrate that blood pressure is a strong modifiable risk factor of cardiovascular morbidity and mortality. Lowering it clearly produces benefits. It is unclear what treatment goals should be promulgated by official guidelines for large groups of patients. The resistant case remains a therapeutic dilemma with the potential for harm from overly aggressive treatment. The truly optimal level for an individual patient remains difficult to define. We anxiously await results of ongoing and future targeting trials.
CASE REVISITED
Regarding the initial case vignette, the patient is clearly not at her recommended goal blood pressure, especially given her high-risk status (diabetes mellitus and chronic kidney disease). Observational data support intensification of therapy, whereas targeting trials are essentially negative and indicate the potential for harm with overly aggressive treatment. Thus, we remain uncertain about what is correct or incorrect in terms of a targeted blood pressure, especially when applied to the individual patient.
Our approach would be to emphasize lifestyle modifications, to ensure accurate determination of her true blood pressure load (self-measurement at home or ambulatory blood pressure monitoring), to consider secondary causes of hypertension, and to educate the patient about the benefits and consequences of intensifying therapy with the aim of involving her in the decision.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Mancia G, Laurent S, Agabiti-Rosei E, et al; European Society of Hypertension. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009; 27:2121–2158.
- MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335:765–774.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Lawes CM, Rodgers A, Bennett DA, et al; Asia Pacific Cohort Studies Collaboration. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens 2003; 21:707–716.
- Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000; 355:865–872.
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006; 144:884–893.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension 2003; 41:1341–1345.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 1995; 123:754–762.
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16:3027–3037.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338:b1665.
- Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003; 362:1527–1535.
- Blood Pressure Lowering Treatment Trialists’ Collaboration; Turnbull F, Neal B, Ninomiya T, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ 2008; 336:1121–1123.
- Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. J Hypertens 2003; 21:1055–1076.
- Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA 2003; 289:2534–2544.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society.—Summary Article. Circulation 2005; 112:e154–e235.
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12.
- Wright JT, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002; 288:2421–2431.
- Freedman BI, Hicks PJ, Bostrom MA, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int 2009; 75:736–745.
- Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 2008; 40:1175–1184.
- Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 2010; 21:1422–1426.
- Rostand SG, Brown G, Kirk KA, Rutsky EA, Dustan HP. Renal insufficiency in treated essential hypertension. N Engl J Med 1989; 320:684–688.
- Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. The MRFIT Research Group. JAMA 1992; 268:3085–3091.
- Pogue V, Rahman M, Lipkowitz M, et al; African American Study of Kidney Disease and Hypertension Collaborative Research Group. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009; 53:20–27.
- Norris K, Bourgoigne J, Gassman J, et al; AASK Study Group. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis 2006; 48:739–751.
- Toto RD, Mitchell HC, Smith RD, Lee HC, McIntire D, Pettinger WA. “Strict” blood pressure control and progression of renal disease in hypertensive nephrosclerosis. Kidney Int 1995; 48:851–859.
- Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ. Effect of intensive blood pressure control on the course of type 1 diabetic nephropathy. Collaborative Study Group. Am J Kidney Dis 1999; 34:809–817.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000; 23( suppl 2):B54–B64.
- Estacio RO, Coll JR, Tran ZV, Schrier RW. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. Am J Hypertens 2006; 19:1241–1248.
- Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol 2002; 13:1733–1739.
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330:877–884.
- Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med 2005; 142:342–351.
- Ruggenenti P, Perna A, Loriga G, et al; REIN-2 Study Group. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 2005; 365:939–946.
- Appel LJ, Wright JT, Greene T, et al; AASK Collaborative Research Group. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317:703–713.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Verdecchia P, Staessen JA, Angeli F, et al; Cardio-Sis investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet 2009; 374:525–533.
- Solomon SD, Verma A, Desai A, et al; Exforge Intensive Control of Hypertension to Evaluate Efficacy in Diastolic Dysfunction Investigators. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension 2010; 55:241–248.
- Ichihara A, Hayashi M, Koura Y, Tada Y, Hirota N, Saruta T. Long-term effects of intensive blood-pressure lowering on arterial wall stiffness in hypertensive patients. Am J Hypertens 2003; 16:959–965.
- Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet 2005; 365:82–93.
- Townsend RR, Roman MJ, Najjar SS, Cockcroft JR, Feig PU, Stockbridge NL. Central blood pressure measurements-an opportunity for efficacy and safety in drug development? J Am Soc Hypertens 2010; 4:211–214.
- Xia H, Ebben J, Ma JZ, Collins AJ. Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol 1999; 10:1309–1316.
- Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 1999; 10:610–619.
- Ofsthun N, Labrecque J, Lacson E, Keen M, Lazarus JM. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int 2003; 63:1908–1914.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339:584–590.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 2008; 74:791–798.
- Finfer S, Chittock DR, Su SY, et al; NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Nissen SE, Tuzcu EM, Libby P, et al; CAMELOT Investigators. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study, a randomized controlled trial. JAMA 2004; 292:2217–2225.
- Patel A; ADVANCE Collaborative Group; MacMahon S, Chalmers J, Neal B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716.
- Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens 2007; 25:2193–2198.
- Pickering TG, White WB, Giles TD, et al. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens 2010; 4:56–61.
- Hänninen MR, Niiranen TJ, Puukka PJ, Jula AM. Comparison of home and ambulatory blood pressure measurement in the diagnosis of masked hypertension. J Hypertens 2010; 28:709–714.
- Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007; 50:197–203.
- Williams B, Lacy PS, Thom SM, et al; CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113:1213–1225.
- Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension 2007; 49:1235–1241.
- Li Y, Thijs L, Hansen TW, et al; International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension 2010; 55:1040–1048.
- Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 2010; 375:938–948.
- Hansen TW, Thijs L, Li Y, et al; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension 2010; 55:1049–1057.
- Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet 2005; 365:434–441.
A 50-year-old African American woman with type 2 diabetes mellitus, hypertension, hyperlipidemia, and chronic kidney disease presents for a follow-up visit. The patient had been treated with hydrochlorothiazide 25 mg/day and enalapril (Vasotec) 20 mg twice daily until 6 weeks ago. At that time her blood pressure was 160/85 mm Hg, and amlodipine (Norvasc) 10 mg/day was added to her regimen. Her other medications include glipizide (Glucotrol), metformin (Glucophage), lovastatin (Mevacor), fish oils, aspirin, calcium, and vitamin D. Her current blood pressure is 145/80 mm Hg; her serum creatinine level is 1.5 mg/dL, and her urine albumin-to-creatinine ratio is 180 mg/g.
In hypertensive patients who have diabetes or chronic kidney disease, guidelines1 call for intensification of antihypertensive therapy to reach a goal blood pressure of less than 130/80 mm Hg. What data exist to support these guidelines? And what should the clinician do?
IS MORE-INTENSE THERAPY IN THE PATIENT’S BEST INTEREST?
Often, clinicians are faced with hypertensive patients whose blood pressure, despite treatment, is higher than the accepted goal. Often, these patients are elderly and are already taking multiple medications that are costly and have significant potential adverse effects. The dilemma is whether to try to reach a target blood pressure listed in a guideline (by increasing the dosage of the current drugs or by adding a drug of a different class) or to “do no harm,” accept the patient’s blood pressure, and keep the regimen the same.1,2
The current goal blood pressure is less than 140/90 mm Hg for all but the very elderly, with more intense control recommended for patients at high risk, ie, those with diabetes mellitus, chronic kidney disease, or atherosclerotic cardiovascular disease.1
While it appears to be in the patient’s best interests to follow such guidelines, review of available data indicates that this it not necessarily so, and may even be harmful.
OBSERVATIONAL DATA AND EARLY RANDOMIZED TRIALS
Many observational studies have found that the higher one’s blood pressure, the greater one’s risk of cardiovascular events and death. Indeed, meta-analyses of these trials, which involved more than 1.5 million people, demonstrate a strong, positive, log-linear relationship between blood pressure and the incidence of cardiovascular disease and death.3–5
Further, there is no evidence of a threshold pressure below which the risk is not lower (ie, a “J-point”), starting with 115/75 mm Hg. A J-point may exist for diastolic blood pressure in elderly patients with isolated systolic hypertension6 and in patients with coronary artery disease.7 Otherwise, the observation is clear: the lower the blood pressure the better. For every 20 mm Hg lower systolic blood pressure or 10 mm Hg lower diastolic blood pressure, the risk of a cardiovascular event is about 50% less.4,5
Observational analyses also show a strong, graded relationship between blood pressure and future end-stage renal disease.8,9 Post hoc analyses indicate that chronic kidney disease progresses more slowly with lower achieved blood pressures, especially in those with higher degrees of proteinuria.10–12
However, observational data do not prove cause and effect, nor do they guarantee similar results with treatment. This requires randomized controlled trials.
RANDOMIZED TRIALS OF HYPERTENSION TREATMENT
Initial trials were aimed at determining whether hypertension should even be treated. A 1997 meta-analysis of 18 such trials comparing either low-dose diuretic therapy, high-dose diuretic therapy, or beta-blocker therapy with placebo involved 48,000 patients who were followed for an average of 5 years.13 The rates of stroke and congestive heart failure were consistently reduced, although only low-dose diuretic therapy reduced the risk of coronary heart disease and death from any cause.
More recent trials enrolled people not considered hypertensive who were randomized to receive either active drugs or placebo, or no treatment. Other trials attempted to assess non-pressure-related effects of specific agents, using other antihypertensive agents in the control group. Still other randomized controlled trials compared one agent or agents with other agents while attempting to attain equivalent blood pressure between groups. Frequently, however, there was some blood pressure difference.
Meta-analyses of most of these trials conclude that the major benefit of antihypertensive therapy—reducing rates of cardiovascular morbidity and mortality—comes from a lower attained blood pressure, irrespective of which agent is used.14–18 Exceptions exist, however. For example, specific drug classes are indicated after myocardial infarction, and in congestive heart failure and proteinuric chronic kidney disease.10,19–21
16 TRIALS OF DIFFERENT BLOOD PRESSURE TARGETS
The overriding theme of these observational data is that a lower blood pressure, whether attained naturally or with treatment, is better than a higher one from both the cardiovascular and the renal perspective.
What remains unclear is what blood pressure should be aimed for in a particular patient or group of patients. Is it a specific pressure (eg, 140/90 mm Hg), or does the change from baseline count more? Should other factors such as age or comorbidity alter this number?
Several randomized controlled trials have addressed these questions by targeting different levels of blood pressure. We are aware of at least 16 such trials in adults, including 13 with renal or cardiovascular primary end points and three with surrogate primary end points.
An unavoidable design flaw of all of these trials is their unblinded nature. Consequently, nearly all of them carry a Jadad score (a measure of quality, based on randomization and blinding)22 of 3 on a scale of 5.
NINE TRIALS WITH RENAL PRIMARY END POINTS
African American Study of Kidney Disease and Hypertension (AASK)23
Patients: 1,094 African Americans with presumed hypertensive renal disease and a measured glomerular filtration rate between 20 and 65 mL/min/1.73 m2.
Randomized blood pressure goals. Mean arterial pressure 92 mm Hg or less vs 102 to 107 mm Hg.
Results. At 4 years, the two groups had average blood pressures of 128/78 and 141/85 mm Hg, respectively. The groups did not differ in the rates of the primary end points—ie, the rate of change in the measured glomerular filtration rate over time or the composite of a 50% reduction in glomerular filtration rate, the onset of end-stage renal disease, or death.
Comments. Several issues have been raised about the internal validity of this trial.
So-called hypertensive kidney disease in African Americans (as opposed to European Americans) may be a genetic disorder related to polymorphisms of one or more genes on chromosome 22q. Initial data implicated the MYH9 gene, which encodes non-muscle myosin heavy chain II.24,25 More recent data implicate the nearby APOL1 gene encoding apolipoprotein L126 as more relevant. These polymorphisms have a much greater prevalence in African Americans and appear responsible for the higher risk of idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy in this population.24–26 Therefore, in African Americans, hypertension may in fact be the result of the kidney disease and not its primary cause, which may explain why in this and other African American populations stricter control of blood pressure did not produce a renal benefit.27,28
Also, there is the possibility of misclassification bias. A secondary analysis of data obtained by ambulatory monitoring showed that of the 377 participants whose blood pressure appeared to be under control when measured in the clinic, 70% actually had masked hypertension, ie, uncontrolled hypertension outside the clinic.29 The real difference in blood pressure between groups may well have been different than that determined in the clinic.
In addition, a prespecified secondary analysis showed no difference in the rates of cardiovascular events and death between the groups.30 However, the study was not designed to have the statistical power to detect a difference in cardiovascular events. Moreover fewer cardiovascular events occurred than expected, further reducing the study’s power to detect a difference.
Toto et al31
Toto et al reported similar results in an earlier trial in 87 hypertensive patients (77 randomized), predominantly African American, and similar concerns apply.
Lewis et al32
Patients: 129 patients with type 1 diabetes.
Randomized blood pressure goals. A mean arterial pressure of either no higher than 92 mm Hg or 100 to 107 mm Hg.
Results. At 2 years, despite a difference of 6 mm Hg in mean arterial pressure, the glomerular filtration rate (measured) had declined by the same amount in the two groups. The study was underpowered for this end point. Patients in the group with the lower goal pressure were excreting significantly less protein than those in the other group, but they were received higher doses of an angiotensin-converting enzyme (ACE) inhibitor—in this case, ramipril (Altace).
The Appropriate Blood Pressure Control in Diabetes (ABCD) trials33–35
Patients: 950 patients with type 2 diabetes mellitus and either normal or high blood pressure.
Randomized blood pressure goals. Either intensive or moderate therapy (see Table 1).
Results. At 5 years, creatinine clearance (estimated) had declined by the same amount in the two groups. However, fewer of the hypertensive patients had died in the intensive-therapy group.34 Similarly, normotensive patients had less progression of albuminuria if treated intensively.33
In the ABCD Part 2 with Valsartan (ABCD-2V) trial in normotensive patients,35 therapy with valsartan (Diovan) did not affect creatinine clearance but did reduce albuminuria. However, 75% of the patients in the moderate-treatment group were untreated.
Schrier et al36
Patients. 75 hypertensive patients with autosomal-dominant polycystic kidney disease and left ventricular hypertrophy.
Randomized blood pressure targets. Less than 120/80 mm Hg vs 135/85 to 140/90 mm Hg.
Results. After 7 years, despite a difference in average mean arterial pressure of 11 mm Hg between the groups (90 vs 101 mm Hg), there was no difference in the rate of decline of creatinine clearance. The left ventricular mass index decreased by 21% in the lower-target group and by 35% in the higher-target group (P < .01).
Modification of Diet in Renal Disease (MDRD) trial37,38
Patients: 840 patients whose measured glomerular filtration rate was between 13 and 55 mL/min/1.73 m2.
Randomized blood pressure targets. A target mean arterial pressure of less than 92 mm Hg vs less than 107 mm Hg.11,37
Results. After 2.2 years, the mean difference in mean arterial pressure was 4.7 mm Hg. There was, however, no difference in the rate of decline in the glomerular filtration rate.
In a 6-year follow-up, significantly fewer patients in the lower-blood-pressure group reached the end point of end-stage renal disease or the combined end point of end-stage renal disease or death.38 The rate of death, however, was nearly twice as high in the lower-blood-pressure group (10% vs 6%). The blood pressure and treatment during follow-up were not reported.
Comments. Internal validity is an issue, since the blood pressure and therapy during follow-up were unknown, and more patients received ACE inhibitors in the lower-blood-pressure group during the trial. Further, the higher death rate in the lower-blood-pressure group is worrisome.
The Ramipril Efficacy in Nephropathy (REIN)-2 trial39
Patients: 338 nondiabetic patients who had proteinuria and reduced creatinine clearance.
Treatment and blood pressure goals. All were treated with ramipril and randomized to intensive (< 130/80 mm Hg) vs standard control (diastolic blood pressure < 90 mm Hg) with therapy based on felodipine (Plendil).
Results. The study was terminated early because of futility. Despite a mean difference of 4.1 mm Hg systolic and 2.8 mm Hg diastolic, the groups did not differ in the rate of progression to end-stage renal disease (23% with intensive therapy vs 20% with standard therapy) or in the rate of decline of the measured glomerular filtration rate (0.22 vs 0.24 mL/min/1.73 m2/month).
Comment. The internal validity of this study can be questioned because of the low separation of achieved blood pressure and because of its early termination.
No benefit from a lower blood pressure goal in preserving kidney function
To summarize, these trials all showed no significant benefit from either targeting or achieving lower blood pressure in terms of slowing the decline of kidney function. Overall, they do not define a target and offer little support that a lower goal blood pressure is indicated with respect to the rate of loss of glomerular filtration rate in chronic kidney disease.
However, post hoc analysis of the MDRD trial indicates a statistical interaction between targeted blood pressure and degree of baseline proteinuria. At higher levels of proteinuria (≥ 1 g/day), the group with the lower blood pressure target had better outcomes.
In addition, long-term follow-up (mean of 12.2 years) of the AASK trial, including a 7-year cohort phase with nearly similar blood pressures in both groups, also indicated an interaction with targeted blood pressure and baseline proteinuria.40 Although the overall analysis was negative, there was a significant reduction in the primary end point in the group originally assigned the low target when analysis was restricted to those in the highest tertile of proteinuria. These and other data10 suggest that patients with chronic kidney disease and proteinuria may represent a distinct subset of chronic kidney disease patients who benefit from more intensive blood-pressure-lowering. However, patients in the REIN-2 trial34 and the macroalbuminuric patients in the ABCD hypertensive trial35 did not benefit from a lower targeted blood pressure despite significant proteinuria.
FOUR TRIALS WITH CARDIOVASCULAR END POINTS
The Hypertension Optimal Treatment (HOT) trial41
Patients: 18,790 patients with diastolic blood pressure between 100 and 115 mm Hg.
Randomized blood pressure goals. Diastolic pressure of equal to or less than 80, 85, or 90 mm Hg.
Results. At an average of 3.8 years, the average blood pressures in the three groups were approximately 140/81, 141/83, and 144/85 mm Hg, respectively. There was no difference between the groups in the rate of the composite primary end point of all myocardial infarctions, all strokes, and cardiovascular death. Any conclusions from this trial were compromised by the small difference in achieved blood pressures between groups.
In the 1,501 patients with diabetes, the incidence of the primary end point was 50% lower with a goal of 80 mm Hg or less than with a goal of 90 mm Hg or less.
The UK Prospective Diabetes Study (UKPDS)42,43
Patients: 1,148 hypertensive patients with type 2 diabetes mellitus.
Randomized blood pressure goals. Either “tight control” (aiming for < 150/85 mm Hg) or “less tight control” (aiming for < 180/105 mm Hg).
Results. At a median follow-up of 8.4 years, the attained blood pressures were 144/82 vs 154/87 mm Hg. The difference produced significant benefits, including a 24% lower rate of any diabetes-related end point, a 32% lower rate of death due to diabetes, and a nonsignificant 18% lower rate of total mortality—all co-primary end points.
The less-tight-control group had many patients with initial blood pressures below 180/105 mm Hg; hence, over 50% of patients received no antihypertensive therapy at the start of the trial. By the end of the trial 9 years later, 20% had still not been treated. This compares with only 5% of patients in the tight-control group who were not treated with antihypertensives throughout the trial. Therefore, this trial serves as better evidence for treating vs not treating, rather than defining a specific goal.
During a 10-year follow-up, blood pressure differences disappeared within 2 years.43 There was no legacy effect, as the significant differences noted during the trial were no longer present 10 years later.
Action to Control Cardiovascular Risk in Diabetes (ACCORD)44
Patients: 4,733 patients with type 2 diabetes.
Randomized blood pressure goals. Systolic blood pressure lower than either 120 or 140 mm Hg.
Results. At 4.7 years, despite a significant difference in mean systolic blood pressure of 14.2 mm Hg after the first year (119.3 vs 133.5 mm Hg), there was no difference in the primary end point of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. There were fewer strokes in the lower-pressure group but no difference in myocardial infarctions, which were five times more common than strokes. Serious adverse events attributed to antihypertensive treatment occurred more frequently in the intensive-therapy group (3.3% vs 1.3%, P < .001).
Comment. There were fewer events than expected, possibly limiting the trial’s ability to detect a statistical difference. Compared with both the UKPDS and the diabetic population of HOT, ACCORD is much larger and more internally valid (unlike in UKPDS, nearly all patients in both groups were treated, and compared with HOT there was much greater separation of achieved pressure). It is more recent and better reflects current overall practice. It indicates that when specifically aiming for a target blood pressure, lower is not always better and comes at a price (more severe adverse events).
Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)45
Patients: 4,418 patients, age 65 to 85 years, with a pretreatment systolic blood pressure above 160 mm Hg.
Randomized blood pressure goals. Systolic pressure either lower than 140 mm Hg or 140 to 160 mm Hg.
Results. At 2 years, despite a difference of 9.7/3.3 mm Hg, there was no difference in the primary end point (the combined incidence of cerebrovascular disease, cardiac and vascular disease, and renal failure). Fifty-four patients had died in the strict-treatment group and 42 in the mild-treatment group; the difference was not statistically significant.
Three other trials
Three other trials46–48 had surrogate end points, but only one of them reported a composite cardiovascular secondary end point.46 We will not discuss the other two.47,48
Cardio-Sis. In the Studio Italiano Sugli Effetti Cardiovascolari del Controllo della Pressione Arteriosa Sistolica (Cardio-Sis) trial,46 1,111 people without diabetes with systolic pressure higher than 150 mm Hg were randomized to tight control (systolic pressure < 130 mm Hg) vs usual control (systolic pressure < 140 mm Hg) and followed for 2 years with electrocardiography to detect left ventricular hypertrophy.
At a median of 2 years, the systolic blood pressure had declined by an average of 3.8 mm Hg more in the tight-control group than in the usual-control group, and the diastolic pressure by an average of 1.5 mm Hg. There was significantly less left ventricular hypertrophy in the tight-control group. The incidence of the secondary end point of a composite of cardiovascular and renal events was also significantly lower. There was no difference individually in the rates of myocardial infarction, stroke, transient ischemic attack, admission for congestive heart failure, or death.
DISCUSSION: THE DILEMMA OF TREATING AN INDIVIDUAL PATIENT
These data illustrate the dilemma of treating an individual patient whose blood pressure is not at the currently accepted goal while on multiple antihypertensive medications. According to guidelines, therapy should be intensified in this situation. Observational data show a strong graded relationship between blood pressure and cardiovascular events and death, starting with a blood pressure of 115/75 mm Hg. The observational data relating blood pressure to kidney disease are similar. These data support the guideline recommendations that additional medications should be added to reach the promulgated target. Unfortunately, the targeting trials do not define a target, nor do they support the concept that lower is better.
Possible explanations for the negative results
Why does targeting a lower blood pressure not produce the benefit that the observational data lead us to expect?
One possibility is that blood pressure is merely a marker of cardiovascular risk, not a cause of it. This is unlikely, given the temporal relationship, reproducibility, and biologic plausibility that is supported by a very large body of experimental data. However, blood pressure is only one of multiple factors involved in the pathogenesis of vascular and renal disease, and perhaps better attention to other factors such as lipids and smoking may have made the targeting trials underpowered.
Another possibility is that these trials had such strict inclusion and exclusion criteria that they do not represent the general hypertensive population, reducing their external validity.49 However, the trials generally enrolled populations at higher risk, in which end points were more likely to occur. This would have enhanced the chance to show a positive effect rather than mask it.
It is possible that antihypertensive medications themselves have unwanted side effects that offset their potential benefit. Medication-related side effects could directly contribute to vascular disease despite their beneficial effect of lowering pressure. There could also be reduced tissue perfusion due to lower blood pressure per se in the face of a diseased vasculature, with the lower pressure directly contributing to organ dysfunction.
Finally, these trials measured brachial pressures to monitor blood pressure. Brachial pressure does not always correlate with central aortic pressure, which is probably a better marker of the overall pressure burden.50 It is possible that in these targeting trials, the peripheral blood pressure did not reflect the true central blood pressure and, therefore, significant separation of blood pressures may not have actually occurred.
Targeted vs achieved blood pressures: Analogies with other markers
This contradiction is not an exceptional circumstance in medicine.
For example, in chronic kidney disease, a graded observational relationship exists between decreasing levels of hemoglobin and various adverse outcomes.51–53 However, targeting a more normal level of hemoglobin compared with a lower one has been shown to be detrimental.54–57 This implies either that anemia is merely a marker of higher risk or, more likely, that the actual measures used to raise the hemoglobin to higher levels are the culprit. Notably, although targeting a higher hemoglobin concentration vs a lower one was detrimental, achieving a higher hemoglobin was beneficial within each targeted group.54,58
Another example of harm caused by targeting goals based on observational data is tight glucose control, both acutely in the critically ill59 and chronically in patients with type 2 diabetes.60 In both cases higher mortality rates ensued.
The same concept may apply to lowering blood pressure. While achieving a lower blood pressure may be more beneficial, targeting a specific goal may be harmful. Given that perhaps 20% of those labeled as hypertensive have resistant hypertension,61 millions of patients are susceptible to potential harm from targeting a specific goal based solely on observational data. If lower is always better, the randomized trials outlined above should have had more positive outcomes.
It becomes problematic to assign a specific goal for all patients or even groups of patients. The targeting trials do not provide the answer. Based on the observational data it would be optimal to have a blood pressure less than 120/80 mm Hg. This is an observation, not a recommendation. Patients should be assessed on an individual basis, taking into consideration their starting blood pressure, age, medication burden (antihypertensive and otherwise), comorbidities, and ability to comply with a regimen. Given the available data, it is hard to be more specific. In the future it may be possible to identify specific blood pressure targets based on the patient’s genetic makeup, but today that is not possible. Even patients with lower initial blood pressure may benefit from therapy,62,63 and some experts have advocated blood-pressure-lowering in all, irrespective of the baseline value.14
Avoid misclassification
The first step in treating hypertension should be to avoid misclassification. Make sure the clinic blood pressure is measured correctly, using an appropriately sized cuff, positioning the patient properly, and following all the other recommendations.64
However, the clinic blood pressure may not reflect true blood pressure load in up to one-third of all patients.65 We recommend 24-hour ambulatory blood pressure monitoring66 or home self-measurement, or both,67 to better assess true blood pressure burden in several circumstances, including in patients with resistant hypertension (any patient who has not achieved acceptable clinic blood pressure on three or more antihypertensive medications including a diuretic or who requires four or more medications for adequate control), suspicion of white-coat hypertension (or effect), and any patient who has achieved acceptable clinic blood pressure but either has symptoms of hypotension or progressive end-organ damage.
Currently, we base therapy on out-of-office blood pressure (self-measured or by ambulatory monitoring) whenever there is a discrepancy with clinic blood pressure.
Whether therapy should be altered by other less traditional measures of blood pressure such as assessment of central aortic pressure by radial applanation tonometry,68,69 or 24-hour ambulatory monitoring to assess nighttime blood pressures (specifically, “dipping”),70 morning surge,71 or blood pressure variability72,73 remains unclear and in need of randomized controlled trials.
In any patient requiring blood-pressure-lowering, we recommend lifestyle modifications.1,2 These include exercise, weight loss, salt and alcohol restriction, evaluation for sleep apnea, and avoidance of medications known to elevate blood pressure such as nonsteroidal anti-inflammatory drugs and sympathomimetic decongestants.
Much needs to be learned
For the individual patient with unacceptably high blood pressure who is already taking multiple antihypertensive medications of different classes, it is unclear what to do. This type of patient with resistant hypertension would be an excellent candidate for a future targeting trial. Other cardiovascular risk factors should be appropriately addressed, including obesity, lipids, smoking, and poor glycemic control.74 Each patient should be individually assessed with consideration of both global cardiovascular risk and quality-of-life issues.
Much still needs to be learned about the treatment of hypertension. The facts demonstrate that blood pressure is a strong modifiable risk factor of cardiovascular morbidity and mortality. Lowering it clearly produces benefits. It is unclear what treatment goals should be promulgated by official guidelines for large groups of patients. The resistant case remains a therapeutic dilemma with the potential for harm from overly aggressive treatment. The truly optimal level for an individual patient remains difficult to define. We anxiously await results of ongoing and future targeting trials.
CASE REVISITED
Regarding the initial case vignette, the patient is clearly not at her recommended goal blood pressure, especially given her high-risk status (diabetes mellitus and chronic kidney disease). Observational data support intensification of therapy, whereas targeting trials are essentially negative and indicate the potential for harm with overly aggressive treatment. Thus, we remain uncertain about what is correct or incorrect in terms of a targeted blood pressure, especially when applied to the individual patient.
Our approach would be to emphasize lifestyle modifications, to ensure accurate determination of her true blood pressure load (self-measurement at home or ambulatory blood pressure monitoring), to consider secondary causes of hypertension, and to educate the patient about the benefits and consequences of intensifying therapy with the aim of involving her in the decision.
A 50-year-old African American woman with type 2 diabetes mellitus, hypertension, hyperlipidemia, and chronic kidney disease presents for a follow-up visit. The patient had been treated with hydrochlorothiazide 25 mg/day and enalapril (Vasotec) 20 mg twice daily until 6 weeks ago. At that time her blood pressure was 160/85 mm Hg, and amlodipine (Norvasc) 10 mg/day was added to her regimen. Her other medications include glipizide (Glucotrol), metformin (Glucophage), lovastatin (Mevacor), fish oils, aspirin, calcium, and vitamin D. Her current blood pressure is 145/80 mm Hg; her serum creatinine level is 1.5 mg/dL, and her urine albumin-to-creatinine ratio is 180 mg/g.
In hypertensive patients who have diabetes or chronic kidney disease, guidelines1 call for intensification of antihypertensive therapy to reach a goal blood pressure of less than 130/80 mm Hg. What data exist to support these guidelines? And what should the clinician do?
IS MORE-INTENSE THERAPY IN THE PATIENT’S BEST INTEREST?
Often, clinicians are faced with hypertensive patients whose blood pressure, despite treatment, is higher than the accepted goal. Often, these patients are elderly and are already taking multiple medications that are costly and have significant potential adverse effects. The dilemma is whether to try to reach a target blood pressure listed in a guideline (by increasing the dosage of the current drugs or by adding a drug of a different class) or to “do no harm,” accept the patient’s blood pressure, and keep the regimen the same.1,2
The current goal blood pressure is less than 140/90 mm Hg for all but the very elderly, with more intense control recommended for patients at high risk, ie, those with diabetes mellitus, chronic kidney disease, or atherosclerotic cardiovascular disease.1
While it appears to be in the patient’s best interests to follow such guidelines, review of available data indicates that this it not necessarily so, and may even be harmful.
OBSERVATIONAL DATA AND EARLY RANDOMIZED TRIALS
Many observational studies have found that the higher one’s blood pressure, the greater one’s risk of cardiovascular events and death. Indeed, meta-analyses of these trials, which involved more than 1.5 million people, demonstrate a strong, positive, log-linear relationship between blood pressure and the incidence of cardiovascular disease and death.3–5
Further, there is no evidence of a threshold pressure below which the risk is not lower (ie, a “J-point”), starting with 115/75 mm Hg. A J-point may exist for diastolic blood pressure in elderly patients with isolated systolic hypertension6 and in patients with coronary artery disease.7 Otherwise, the observation is clear: the lower the blood pressure the better. For every 20 mm Hg lower systolic blood pressure or 10 mm Hg lower diastolic blood pressure, the risk of a cardiovascular event is about 50% less.4,5
Observational analyses also show a strong, graded relationship between blood pressure and future end-stage renal disease.8,9 Post hoc analyses indicate that chronic kidney disease progresses more slowly with lower achieved blood pressures, especially in those with higher degrees of proteinuria.10–12
However, observational data do not prove cause and effect, nor do they guarantee similar results with treatment. This requires randomized controlled trials.
RANDOMIZED TRIALS OF HYPERTENSION TREATMENT
Initial trials were aimed at determining whether hypertension should even be treated. A 1997 meta-analysis of 18 such trials comparing either low-dose diuretic therapy, high-dose diuretic therapy, or beta-blocker therapy with placebo involved 48,000 patients who were followed for an average of 5 years.13 The rates of stroke and congestive heart failure were consistently reduced, although only low-dose diuretic therapy reduced the risk of coronary heart disease and death from any cause.
More recent trials enrolled people not considered hypertensive who were randomized to receive either active drugs or placebo, or no treatment. Other trials attempted to assess non-pressure-related effects of specific agents, using other antihypertensive agents in the control group. Still other randomized controlled trials compared one agent or agents with other agents while attempting to attain equivalent blood pressure between groups. Frequently, however, there was some blood pressure difference.
Meta-analyses of most of these trials conclude that the major benefit of antihypertensive therapy—reducing rates of cardiovascular morbidity and mortality—comes from a lower attained blood pressure, irrespective of which agent is used.14–18 Exceptions exist, however. For example, specific drug classes are indicated after myocardial infarction, and in congestive heart failure and proteinuric chronic kidney disease.10,19–21
16 TRIALS OF DIFFERENT BLOOD PRESSURE TARGETS
The overriding theme of these observational data is that a lower blood pressure, whether attained naturally or with treatment, is better than a higher one from both the cardiovascular and the renal perspective.
What remains unclear is what blood pressure should be aimed for in a particular patient or group of patients. Is it a specific pressure (eg, 140/90 mm Hg), or does the change from baseline count more? Should other factors such as age or comorbidity alter this number?
Several randomized controlled trials have addressed these questions by targeting different levels of blood pressure. We are aware of at least 16 such trials in adults, including 13 with renal or cardiovascular primary end points and three with surrogate primary end points.
An unavoidable design flaw of all of these trials is their unblinded nature. Consequently, nearly all of them carry a Jadad score (a measure of quality, based on randomization and blinding)22 of 3 on a scale of 5.
NINE TRIALS WITH RENAL PRIMARY END POINTS
African American Study of Kidney Disease and Hypertension (AASK)23
Patients: 1,094 African Americans with presumed hypertensive renal disease and a measured glomerular filtration rate between 20 and 65 mL/min/1.73 m2.
Randomized blood pressure goals. Mean arterial pressure 92 mm Hg or less vs 102 to 107 mm Hg.
Results. At 4 years, the two groups had average blood pressures of 128/78 and 141/85 mm Hg, respectively. The groups did not differ in the rates of the primary end points—ie, the rate of change in the measured glomerular filtration rate over time or the composite of a 50% reduction in glomerular filtration rate, the onset of end-stage renal disease, or death.
Comments. Several issues have been raised about the internal validity of this trial.
So-called hypertensive kidney disease in African Americans (as opposed to European Americans) may be a genetic disorder related to polymorphisms of one or more genes on chromosome 22q. Initial data implicated the MYH9 gene, which encodes non-muscle myosin heavy chain II.24,25 More recent data implicate the nearby APOL1 gene encoding apolipoprotein L126 as more relevant. These polymorphisms have a much greater prevalence in African Americans and appear responsible for the higher risk of idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy in this population.24–26 Therefore, in African Americans, hypertension may in fact be the result of the kidney disease and not its primary cause, which may explain why in this and other African American populations stricter control of blood pressure did not produce a renal benefit.27,28
Also, there is the possibility of misclassification bias. A secondary analysis of data obtained by ambulatory monitoring showed that of the 377 participants whose blood pressure appeared to be under control when measured in the clinic, 70% actually had masked hypertension, ie, uncontrolled hypertension outside the clinic.29 The real difference in blood pressure between groups may well have been different than that determined in the clinic.
In addition, a prespecified secondary analysis showed no difference in the rates of cardiovascular events and death between the groups.30 However, the study was not designed to have the statistical power to detect a difference in cardiovascular events. Moreover fewer cardiovascular events occurred than expected, further reducing the study’s power to detect a difference.
Toto et al31
Toto et al reported similar results in an earlier trial in 87 hypertensive patients (77 randomized), predominantly African American, and similar concerns apply.
Lewis et al32
Patients: 129 patients with type 1 diabetes.
Randomized blood pressure goals. A mean arterial pressure of either no higher than 92 mm Hg or 100 to 107 mm Hg.
Results. At 2 years, despite a difference of 6 mm Hg in mean arterial pressure, the glomerular filtration rate (measured) had declined by the same amount in the two groups. The study was underpowered for this end point. Patients in the group with the lower goal pressure were excreting significantly less protein than those in the other group, but they were received higher doses of an angiotensin-converting enzyme (ACE) inhibitor—in this case, ramipril (Altace).
The Appropriate Blood Pressure Control in Diabetes (ABCD) trials33–35
Patients: 950 patients with type 2 diabetes mellitus and either normal or high blood pressure.
Randomized blood pressure goals. Either intensive or moderate therapy (see Table 1).
Results. At 5 years, creatinine clearance (estimated) had declined by the same amount in the two groups. However, fewer of the hypertensive patients had died in the intensive-therapy group.34 Similarly, normotensive patients had less progression of albuminuria if treated intensively.33
In the ABCD Part 2 with Valsartan (ABCD-2V) trial in normotensive patients,35 therapy with valsartan (Diovan) did not affect creatinine clearance but did reduce albuminuria. However, 75% of the patients in the moderate-treatment group were untreated.
Schrier et al36
Patients. 75 hypertensive patients with autosomal-dominant polycystic kidney disease and left ventricular hypertrophy.
Randomized blood pressure targets. Less than 120/80 mm Hg vs 135/85 to 140/90 mm Hg.
Results. After 7 years, despite a difference in average mean arterial pressure of 11 mm Hg between the groups (90 vs 101 mm Hg), there was no difference in the rate of decline of creatinine clearance. The left ventricular mass index decreased by 21% in the lower-target group and by 35% in the higher-target group (P < .01).
Modification of Diet in Renal Disease (MDRD) trial37,38
Patients: 840 patients whose measured glomerular filtration rate was between 13 and 55 mL/min/1.73 m2.
Randomized blood pressure targets. A target mean arterial pressure of less than 92 mm Hg vs less than 107 mm Hg.11,37
Results. After 2.2 years, the mean difference in mean arterial pressure was 4.7 mm Hg. There was, however, no difference in the rate of decline in the glomerular filtration rate.
In a 6-year follow-up, significantly fewer patients in the lower-blood-pressure group reached the end point of end-stage renal disease or the combined end point of end-stage renal disease or death.38 The rate of death, however, was nearly twice as high in the lower-blood-pressure group (10% vs 6%). The blood pressure and treatment during follow-up were not reported.
Comments. Internal validity is an issue, since the blood pressure and therapy during follow-up were unknown, and more patients received ACE inhibitors in the lower-blood-pressure group during the trial. Further, the higher death rate in the lower-blood-pressure group is worrisome.
The Ramipril Efficacy in Nephropathy (REIN)-2 trial39
Patients: 338 nondiabetic patients who had proteinuria and reduced creatinine clearance.
Treatment and blood pressure goals. All were treated with ramipril and randomized to intensive (< 130/80 mm Hg) vs standard control (diastolic blood pressure < 90 mm Hg) with therapy based on felodipine (Plendil).
Results. The study was terminated early because of futility. Despite a mean difference of 4.1 mm Hg systolic and 2.8 mm Hg diastolic, the groups did not differ in the rate of progression to end-stage renal disease (23% with intensive therapy vs 20% with standard therapy) or in the rate of decline of the measured glomerular filtration rate (0.22 vs 0.24 mL/min/1.73 m2/month).
Comment. The internal validity of this study can be questioned because of the low separation of achieved blood pressure and because of its early termination.
No benefit from a lower blood pressure goal in preserving kidney function
To summarize, these trials all showed no significant benefit from either targeting or achieving lower blood pressure in terms of slowing the decline of kidney function. Overall, they do not define a target and offer little support that a lower goal blood pressure is indicated with respect to the rate of loss of glomerular filtration rate in chronic kidney disease.
However, post hoc analysis of the MDRD trial indicates a statistical interaction between targeted blood pressure and degree of baseline proteinuria. At higher levels of proteinuria (≥ 1 g/day), the group with the lower blood pressure target had better outcomes.
In addition, long-term follow-up (mean of 12.2 years) of the AASK trial, including a 7-year cohort phase with nearly similar blood pressures in both groups, also indicated an interaction with targeted blood pressure and baseline proteinuria.40 Although the overall analysis was negative, there was a significant reduction in the primary end point in the group originally assigned the low target when analysis was restricted to those in the highest tertile of proteinuria. These and other data10 suggest that patients with chronic kidney disease and proteinuria may represent a distinct subset of chronic kidney disease patients who benefit from more intensive blood-pressure-lowering. However, patients in the REIN-2 trial34 and the macroalbuminuric patients in the ABCD hypertensive trial35 did not benefit from a lower targeted blood pressure despite significant proteinuria.
FOUR TRIALS WITH CARDIOVASCULAR END POINTS
The Hypertension Optimal Treatment (HOT) trial41
Patients: 18,790 patients with diastolic blood pressure between 100 and 115 mm Hg.
Randomized blood pressure goals. Diastolic pressure of equal to or less than 80, 85, or 90 mm Hg.
Results. At an average of 3.8 years, the average blood pressures in the three groups were approximately 140/81, 141/83, and 144/85 mm Hg, respectively. There was no difference between the groups in the rate of the composite primary end point of all myocardial infarctions, all strokes, and cardiovascular death. Any conclusions from this trial were compromised by the small difference in achieved blood pressures between groups.
In the 1,501 patients with diabetes, the incidence of the primary end point was 50% lower with a goal of 80 mm Hg or less than with a goal of 90 mm Hg or less.
The UK Prospective Diabetes Study (UKPDS)42,43
Patients: 1,148 hypertensive patients with type 2 diabetes mellitus.
Randomized blood pressure goals. Either “tight control” (aiming for < 150/85 mm Hg) or “less tight control” (aiming for < 180/105 mm Hg).
Results. At a median follow-up of 8.4 years, the attained blood pressures were 144/82 vs 154/87 mm Hg. The difference produced significant benefits, including a 24% lower rate of any diabetes-related end point, a 32% lower rate of death due to diabetes, and a nonsignificant 18% lower rate of total mortality—all co-primary end points.
The less-tight-control group had many patients with initial blood pressures below 180/105 mm Hg; hence, over 50% of patients received no antihypertensive therapy at the start of the trial. By the end of the trial 9 years later, 20% had still not been treated. This compares with only 5% of patients in the tight-control group who were not treated with antihypertensives throughout the trial. Therefore, this trial serves as better evidence for treating vs not treating, rather than defining a specific goal.
During a 10-year follow-up, blood pressure differences disappeared within 2 years.43 There was no legacy effect, as the significant differences noted during the trial were no longer present 10 years later.
Action to Control Cardiovascular Risk in Diabetes (ACCORD)44
Patients: 4,733 patients with type 2 diabetes.
Randomized blood pressure goals. Systolic blood pressure lower than either 120 or 140 mm Hg.
Results. At 4.7 years, despite a significant difference in mean systolic blood pressure of 14.2 mm Hg after the first year (119.3 vs 133.5 mm Hg), there was no difference in the primary end point of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. There were fewer strokes in the lower-pressure group but no difference in myocardial infarctions, which were five times more common than strokes. Serious adverse events attributed to antihypertensive treatment occurred more frequently in the intensive-therapy group (3.3% vs 1.3%, P < .001).
Comment. There were fewer events than expected, possibly limiting the trial’s ability to detect a statistical difference. Compared with both the UKPDS and the diabetic population of HOT, ACCORD is much larger and more internally valid (unlike in UKPDS, nearly all patients in both groups were treated, and compared with HOT there was much greater separation of achieved pressure). It is more recent and better reflects current overall practice. It indicates that when specifically aiming for a target blood pressure, lower is not always better and comes at a price (more severe adverse events).
Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)45
Patients: 4,418 patients, age 65 to 85 years, with a pretreatment systolic blood pressure above 160 mm Hg.
Randomized blood pressure goals. Systolic pressure either lower than 140 mm Hg or 140 to 160 mm Hg.
Results. At 2 years, despite a difference of 9.7/3.3 mm Hg, there was no difference in the primary end point (the combined incidence of cerebrovascular disease, cardiac and vascular disease, and renal failure). Fifty-four patients had died in the strict-treatment group and 42 in the mild-treatment group; the difference was not statistically significant.
Three other trials
Three other trials46–48 had surrogate end points, but only one of them reported a composite cardiovascular secondary end point.46 We will not discuss the other two.47,48
Cardio-Sis. In the Studio Italiano Sugli Effetti Cardiovascolari del Controllo della Pressione Arteriosa Sistolica (Cardio-Sis) trial,46 1,111 people without diabetes with systolic pressure higher than 150 mm Hg were randomized to tight control (systolic pressure < 130 mm Hg) vs usual control (systolic pressure < 140 mm Hg) and followed for 2 years with electrocardiography to detect left ventricular hypertrophy.
At a median of 2 years, the systolic blood pressure had declined by an average of 3.8 mm Hg more in the tight-control group than in the usual-control group, and the diastolic pressure by an average of 1.5 mm Hg. There was significantly less left ventricular hypertrophy in the tight-control group. The incidence of the secondary end point of a composite of cardiovascular and renal events was also significantly lower. There was no difference individually in the rates of myocardial infarction, stroke, transient ischemic attack, admission for congestive heart failure, or death.
DISCUSSION: THE DILEMMA OF TREATING AN INDIVIDUAL PATIENT
These data illustrate the dilemma of treating an individual patient whose blood pressure is not at the currently accepted goal while on multiple antihypertensive medications. According to guidelines, therapy should be intensified in this situation. Observational data show a strong graded relationship between blood pressure and cardiovascular events and death, starting with a blood pressure of 115/75 mm Hg. The observational data relating blood pressure to kidney disease are similar. These data support the guideline recommendations that additional medications should be added to reach the promulgated target. Unfortunately, the targeting trials do not define a target, nor do they support the concept that lower is better.
Possible explanations for the negative results
Why does targeting a lower blood pressure not produce the benefit that the observational data lead us to expect?
One possibility is that blood pressure is merely a marker of cardiovascular risk, not a cause of it. This is unlikely, given the temporal relationship, reproducibility, and biologic plausibility that is supported by a very large body of experimental data. However, blood pressure is only one of multiple factors involved in the pathogenesis of vascular and renal disease, and perhaps better attention to other factors such as lipids and smoking may have made the targeting trials underpowered.
Another possibility is that these trials had such strict inclusion and exclusion criteria that they do not represent the general hypertensive population, reducing their external validity.49 However, the trials generally enrolled populations at higher risk, in which end points were more likely to occur. This would have enhanced the chance to show a positive effect rather than mask it.
It is possible that antihypertensive medications themselves have unwanted side effects that offset their potential benefit. Medication-related side effects could directly contribute to vascular disease despite their beneficial effect of lowering pressure. There could also be reduced tissue perfusion due to lower blood pressure per se in the face of a diseased vasculature, with the lower pressure directly contributing to organ dysfunction.
Finally, these trials measured brachial pressures to monitor blood pressure. Brachial pressure does not always correlate with central aortic pressure, which is probably a better marker of the overall pressure burden.50 It is possible that in these targeting trials, the peripheral blood pressure did not reflect the true central blood pressure and, therefore, significant separation of blood pressures may not have actually occurred.
Targeted vs achieved blood pressures: Analogies with other markers
This contradiction is not an exceptional circumstance in medicine.
For example, in chronic kidney disease, a graded observational relationship exists between decreasing levels of hemoglobin and various adverse outcomes.51–53 However, targeting a more normal level of hemoglobin compared with a lower one has been shown to be detrimental.54–57 This implies either that anemia is merely a marker of higher risk or, more likely, that the actual measures used to raise the hemoglobin to higher levels are the culprit. Notably, although targeting a higher hemoglobin concentration vs a lower one was detrimental, achieving a higher hemoglobin was beneficial within each targeted group.54,58
Another example of harm caused by targeting goals based on observational data is tight glucose control, both acutely in the critically ill59 and chronically in patients with type 2 diabetes.60 In both cases higher mortality rates ensued.
The same concept may apply to lowering blood pressure. While achieving a lower blood pressure may be more beneficial, targeting a specific goal may be harmful. Given that perhaps 20% of those labeled as hypertensive have resistant hypertension,61 millions of patients are susceptible to potential harm from targeting a specific goal based solely on observational data. If lower is always better, the randomized trials outlined above should have had more positive outcomes.
It becomes problematic to assign a specific goal for all patients or even groups of patients. The targeting trials do not provide the answer. Based on the observational data it would be optimal to have a blood pressure less than 120/80 mm Hg. This is an observation, not a recommendation. Patients should be assessed on an individual basis, taking into consideration their starting blood pressure, age, medication burden (antihypertensive and otherwise), comorbidities, and ability to comply with a regimen. Given the available data, it is hard to be more specific. In the future it may be possible to identify specific blood pressure targets based on the patient’s genetic makeup, but today that is not possible. Even patients with lower initial blood pressure may benefit from therapy,62,63 and some experts have advocated blood-pressure-lowering in all, irrespective of the baseline value.14
Avoid misclassification
The first step in treating hypertension should be to avoid misclassification. Make sure the clinic blood pressure is measured correctly, using an appropriately sized cuff, positioning the patient properly, and following all the other recommendations.64
However, the clinic blood pressure may not reflect true blood pressure load in up to one-third of all patients.65 We recommend 24-hour ambulatory blood pressure monitoring66 or home self-measurement, or both,67 to better assess true blood pressure burden in several circumstances, including in patients with resistant hypertension (any patient who has not achieved acceptable clinic blood pressure on three or more antihypertensive medications including a diuretic or who requires four or more medications for adequate control), suspicion of white-coat hypertension (or effect), and any patient who has achieved acceptable clinic blood pressure but either has symptoms of hypotension or progressive end-organ damage.
Currently, we base therapy on out-of-office blood pressure (self-measured or by ambulatory monitoring) whenever there is a discrepancy with clinic blood pressure.
Whether therapy should be altered by other less traditional measures of blood pressure such as assessment of central aortic pressure by radial applanation tonometry,68,69 or 24-hour ambulatory monitoring to assess nighttime blood pressures (specifically, “dipping”),70 morning surge,71 or blood pressure variability72,73 remains unclear and in need of randomized controlled trials.
In any patient requiring blood-pressure-lowering, we recommend lifestyle modifications.1,2 These include exercise, weight loss, salt and alcohol restriction, evaluation for sleep apnea, and avoidance of medications known to elevate blood pressure such as nonsteroidal anti-inflammatory drugs and sympathomimetic decongestants.
Much needs to be learned
For the individual patient with unacceptably high blood pressure who is already taking multiple antihypertensive medications of different classes, it is unclear what to do. This type of patient with resistant hypertension would be an excellent candidate for a future targeting trial. Other cardiovascular risk factors should be appropriately addressed, including obesity, lipids, smoking, and poor glycemic control.74 Each patient should be individually assessed with consideration of both global cardiovascular risk and quality-of-life issues.
Much still needs to be learned about the treatment of hypertension. The facts demonstrate that blood pressure is a strong modifiable risk factor of cardiovascular morbidity and mortality. Lowering it clearly produces benefits. It is unclear what treatment goals should be promulgated by official guidelines for large groups of patients. The resistant case remains a therapeutic dilemma with the potential for harm from overly aggressive treatment. The truly optimal level for an individual patient remains difficult to define. We anxiously await results of ongoing and future targeting trials.
CASE REVISITED
Regarding the initial case vignette, the patient is clearly not at her recommended goal blood pressure, especially given her high-risk status (diabetes mellitus and chronic kidney disease). Observational data support intensification of therapy, whereas targeting trials are essentially negative and indicate the potential for harm with overly aggressive treatment. Thus, we remain uncertain about what is correct or incorrect in terms of a targeted blood pressure, especially when applied to the individual patient.
Our approach would be to emphasize lifestyle modifications, to ensure accurate determination of her true blood pressure load (self-measurement at home or ambulatory blood pressure monitoring), to consider secondary causes of hypertension, and to educate the patient about the benefits and consequences of intensifying therapy with the aim of involving her in the decision.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Mancia G, Laurent S, Agabiti-Rosei E, et al; European Society of Hypertension. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009; 27:2121–2158.
- MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335:765–774.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Lawes CM, Rodgers A, Bennett DA, et al; Asia Pacific Cohort Studies Collaboration. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens 2003; 21:707–716.
- Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000; 355:865–872.
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006; 144:884–893.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension 2003; 41:1341–1345.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 1995; 123:754–762.
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16:3027–3037.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338:b1665.
- Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003; 362:1527–1535.
- Blood Pressure Lowering Treatment Trialists’ Collaboration; Turnbull F, Neal B, Ninomiya T, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ 2008; 336:1121–1123.
- Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. J Hypertens 2003; 21:1055–1076.
- Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA 2003; 289:2534–2544.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society.—Summary Article. Circulation 2005; 112:e154–e235.
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12.
- Wright JT, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002; 288:2421–2431.
- Freedman BI, Hicks PJ, Bostrom MA, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int 2009; 75:736–745.
- Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 2008; 40:1175–1184.
- Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 2010; 21:1422–1426.
- Rostand SG, Brown G, Kirk KA, Rutsky EA, Dustan HP. Renal insufficiency in treated essential hypertension. N Engl J Med 1989; 320:684–688.
- Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. The MRFIT Research Group. JAMA 1992; 268:3085–3091.
- Pogue V, Rahman M, Lipkowitz M, et al; African American Study of Kidney Disease and Hypertension Collaborative Research Group. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009; 53:20–27.
- Norris K, Bourgoigne J, Gassman J, et al; AASK Study Group. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis 2006; 48:739–751.
- Toto RD, Mitchell HC, Smith RD, Lee HC, McIntire D, Pettinger WA. “Strict” blood pressure control and progression of renal disease in hypertensive nephrosclerosis. Kidney Int 1995; 48:851–859.
- Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ. Effect of intensive blood pressure control on the course of type 1 diabetic nephropathy. Collaborative Study Group. Am J Kidney Dis 1999; 34:809–817.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000; 23( suppl 2):B54–B64.
- Estacio RO, Coll JR, Tran ZV, Schrier RW. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. Am J Hypertens 2006; 19:1241–1248.
- Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol 2002; 13:1733–1739.
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330:877–884.
- Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med 2005; 142:342–351.
- Ruggenenti P, Perna A, Loriga G, et al; REIN-2 Study Group. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 2005; 365:939–946.
- Appel LJ, Wright JT, Greene T, et al; AASK Collaborative Research Group. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317:703–713.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Verdecchia P, Staessen JA, Angeli F, et al; Cardio-Sis investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet 2009; 374:525–533.
- Solomon SD, Verma A, Desai A, et al; Exforge Intensive Control of Hypertension to Evaluate Efficacy in Diastolic Dysfunction Investigators. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension 2010; 55:241–248.
- Ichihara A, Hayashi M, Koura Y, Tada Y, Hirota N, Saruta T. Long-term effects of intensive blood-pressure lowering on arterial wall stiffness in hypertensive patients. Am J Hypertens 2003; 16:959–965.
- Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet 2005; 365:82–93.
- Townsend RR, Roman MJ, Najjar SS, Cockcroft JR, Feig PU, Stockbridge NL. Central blood pressure measurements-an opportunity for efficacy and safety in drug development? J Am Soc Hypertens 2010; 4:211–214.
- Xia H, Ebben J, Ma JZ, Collins AJ. Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol 1999; 10:1309–1316.
- Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 1999; 10:610–619.
- Ofsthun N, Labrecque J, Lacson E, Keen M, Lazarus JM. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int 2003; 63:1908–1914.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339:584–590.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 2008; 74:791–798.
- Finfer S, Chittock DR, Su SY, et al; NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Nissen SE, Tuzcu EM, Libby P, et al; CAMELOT Investigators. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study, a randomized controlled trial. JAMA 2004; 292:2217–2225.
- Patel A; ADVANCE Collaborative Group; MacMahon S, Chalmers J, Neal B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716.
- Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens 2007; 25:2193–2198.
- Pickering TG, White WB, Giles TD, et al. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens 2010; 4:56–61.
- Hänninen MR, Niiranen TJ, Puukka PJ, Jula AM. Comparison of home and ambulatory blood pressure measurement in the diagnosis of masked hypertension. J Hypertens 2010; 28:709–714.
- Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007; 50:197–203.
- Williams B, Lacy PS, Thom SM, et al; CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113:1213–1225.
- Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension 2007; 49:1235–1241.
- Li Y, Thijs L, Hansen TW, et al; International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension 2010; 55:1040–1048.
- Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 2010; 375:938–948.
- Hansen TW, Thijs L, Li Y, et al; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension 2010; 55:1049–1057.
- Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet 2005; 365:434–441.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Mancia G, Laurent S, Agabiti-Rosei E, et al; European Society of Hypertension. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009; 27:2121–2158.
- MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335:765–774.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Lawes CM, Rodgers A, Bennett DA, et al; Asia Pacific Cohort Studies Collaboration. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens 2003; 21:707–716.
- Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000; 355:865–872.
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006; 144:884–893.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension 2003; 41:1341–1345.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 1995; 123:754–762.
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16:3027–3037.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338:b1665.
- Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003; 362:1527–1535.
- Blood Pressure Lowering Treatment Trialists’ Collaboration; Turnbull F, Neal B, Ninomiya T, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ 2008; 336:1121–1123.
- Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. J Hypertens 2003; 21:1055–1076.
- Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA 2003; 289:2534–2544.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society.—Summary Article. Circulation 2005; 112:e154–e235.
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12.
- Wright JT, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002; 288:2421–2431.
- Freedman BI, Hicks PJ, Bostrom MA, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int 2009; 75:736–745.
- Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 2008; 40:1175–1184.
- Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 2010; 21:1422–1426.
- Rostand SG, Brown G, Kirk KA, Rutsky EA, Dustan HP. Renal insufficiency in treated essential hypertension. N Engl J Med 1989; 320:684–688.
- Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. The MRFIT Research Group. JAMA 1992; 268:3085–3091.
- Pogue V, Rahman M, Lipkowitz M, et al; African American Study of Kidney Disease and Hypertension Collaborative Research Group. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009; 53:20–27.
- Norris K, Bourgoigne J, Gassman J, et al; AASK Study Group. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis 2006; 48:739–751.
- Toto RD, Mitchell HC, Smith RD, Lee HC, McIntire D, Pettinger WA. “Strict” blood pressure control and progression of renal disease in hypertensive nephrosclerosis. Kidney Int 1995; 48:851–859.
- Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ. Effect of intensive blood pressure control on the course of type 1 diabetic nephropathy. Collaborative Study Group. Am J Kidney Dis 1999; 34:809–817.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000; 23( suppl 2):B54–B64.
- Estacio RO, Coll JR, Tran ZV, Schrier RW. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. Am J Hypertens 2006; 19:1241–1248.
- Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol 2002; 13:1733–1739.
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330:877–884.
- Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med 2005; 142:342–351.
- Ruggenenti P, Perna A, Loriga G, et al; REIN-2 Study Group. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 2005; 365:939–946.
- Appel LJ, Wright JT, Greene T, et al; AASK Collaborative Research Group. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317:703–713.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Verdecchia P, Staessen JA, Angeli F, et al; Cardio-Sis investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet 2009; 374:525–533.
- Solomon SD, Verma A, Desai A, et al; Exforge Intensive Control of Hypertension to Evaluate Efficacy in Diastolic Dysfunction Investigators. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension 2010; 55:241–248.
- Ichihara A, Hayashi M, Koura Y, Tada Y, Hirota N, Saruta T. Long-term effects of intensive blood-pressure lowering on arterial wall stiffness in hypertensive patients. Am J Hypertens 2003; 16:959–965.
- Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet 2005; 365:82–93.
- Townsend RR, Roman MJ, Najjar SS, Cockcroft JR, Feig PU, Stockbridge NL. Central blood pressure measurements-an opportunity for efficacy and safety in drug development? J Am Soc Hypertens 2010; 4:211–214.
- Xia H, Ebben J, Ma JZ, Collins AJ. Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol 1999; 10:1309–1316.
- Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 1999; 10:610–619.
- Ofsthun N, Labrecque J, Lacson E, Keen M, Lazarus JM. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int 2003; 63:1908–1914.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339:584–590.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 2008; 74:791–798.
- Finfer S, Chittock DR, Su SY, et al; NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Nissen SE, Tuzcu EM, Libby P, et al; CAMELOT Investigators. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study, a randomized controlled trial. JAMA 2004; 292:2217–2225.
- Patel A; ADVANCE Collaborative Group; MacMahon S, Chalmers J, Neal B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716.
- Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens 2007; 25:2193–2198.
- Pickering TG, White WB, Giles TD, et al. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens 2010; 4:56–61.
- Hänninen MR, Niiranen TJ, Puukka PJ, Jula AM. Comparison of home and ambulatory blood pressure measurement in the diagnosis of masked hypertension. J Hypertens 2010; 28:709–714.
- Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007; 50:197–203.
- Williams B, Lacy PS, Thom SM, et al; CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113:1213–1225.
- Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension 2007; 49:1235–1241.
- Li Y, Thijs L, Hansen TW, et al; International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension 2010; 55:1040–1048.
- Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 2010; 375:938–948.
- Hansen TW, Thijs L, Li Y, et al; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension 2010; 55:1049–1057.
- Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet 2005; 365:434–441.
KEY POINTS
- Observational data indicate that lower blood pressure is better than higher, and many trials have confirmed that treatment of hypertension is beneficial. Guidelines have set specific goals based on the observational data.
- Surprisingly, randomized controlled trials have not shown a lower target to offer significant clinical benefit, and suggest the potential for harm with overly aggressive therapy.
- The optimal blood pressure on treatment for an individual patient remains unclear.
Seek and treat: HIV update 2011
With early treatment of human immunodeficiency virus (HIV) infection, we can now expect patients to live a much longer life and, in some situations, have a near-normal lifespan.1 Unfortunately, in screening for HIV infection, the United States lags behind many regions of the world, and infection is often not diagnosed until patients present with advanced disease, ie, the acquired immunodeficiency syndrome (AIDS). In this country there is a critical need to make HIV screening a routine part of medical care in all health settings in order to give patients their best chance for a healthy life, to prevent mother-to-child transmission, and to reduce the spread of HIV in the community.
HIV infection meets the criteria that justify routine screening, as laid out by the World Health Organization2:
- It is a serious health disorder that can be detected before symptoms develop
- Treatment is more beneficial if begun before symptoms develop
- Reliable, inexpensive, and acceptable screening tests exist
- The costs of screening are reasonable in relation to the anticipated benefits.
This article will review the epidemiology of the HIV epidemic, present the benefits of early treatment, and make the case for widely expanding screening for HIV infection in the US health care system.
HIV INFECTION CONTINUES TO BE A LARGE BURDEN
In 2008, an estimated 33.4 million people worldwide were HIV-positive. The vast majority of infected people—more than 22 million—live in sub-Saharan Africa.3
The United States has approximately 1.2 million cases.4 Although this is a small proportion of cases worldwide, it still represents a significant health care burden. In this country, the number of AIDS cases peaked in 1993, and the rate of deaths from AIDS began to decrease over the ensuing years as adequate therapy for HIV was developed. Standard therapy then and now consists of at least three drugs from two different classes.
Unfortunately, we have made little progress on the incidence of this disease. The estimated number of new HIV infections in the United States in 2008 was 56,000 and had remained about the same over the previous 15 years.5,6 Because of improved rates of survival, the prevalence has risen steadily since the mid-1990s to the current estimate of 1.2 million persons living with HIV/AIDS in the US.
About 25% of people infected with HIV are unaware of it. This group accounts for more than half of all new infections annually, which highlights the importance of enhanced screening. Once people know they are infected, they tend to change their behavior and are less likely to spread the disease.7
HIV disproportionately affects minority populations and gay men
Cases of HIV infection are reported among all age groups, although most patients tend to have been infected as young adults. Currently, the largest age group living with HIV is middle-aged. As this cohort grows older, an increasing burden of comorbidities due to aging can be expected. In 5 years, about half of the people with HIV in this country are expected to be 50 years of age or older. Although survival rates have steadily increased due to better treatment, survival tends to be shorter for older people newly diagnosed with HIV.
Worldwide, about an equal number of men and women are infected with HIV, but in the United States infected men outnumber women. In this country, about half the cases of HIV transmission among adults are by male-to-male sexual contact, about 30% are by high-risk heterosexual contact (ie, with a partner known to be HIV-infected or at high risk for being infected), and about 10% are by injection drug use.
In the United States, AIDS is predominantly and disproportionately a disease of minorities and those who live in poverty. African Americans account for the largest number of cases, followed by whites and then by Hispanics. Combined, African Americans and Hispanics account for two-thirds to three-fourths of all new cases, although they make up less than one-fourth of the US population. The incidence rate is nearly 137 per 100,000 for African Americans, 56 per 100,000 for Hispanics, and 19 per 100,000 for whites. The incidence is highest in New York and in the southeast, the geographic areas where the greatest number of minorities and people living in poverty reside. These groups also often lack access to health care.
HIV TREATMENT IS MORE EFFECTIVE IF STARTED EARLY
Treatment guidelines from the US Department of Health and Human Services (DHHS) have changed over the years. When effective medications were first introduced in the 1990s, the trend was to treat everyone as soon as they were diagnosed. As the burden of therapy began to unfold (side effects, cost, adherence, and drug resistance), the consensus was to wait until the CD4 T-cell count dropped to a lower level. As the medications have improved and have become better tolerated, the pendulum has swung back to treating earlier in the course of the disease. Currently, the DHHS recommends that therapy be started at CD4 counts of 350 cells/mL or lower (level of evidence: A1).8 It also recommends therapy for CD4 counts between 350 and 500 cells/mL, but the level of evidence is lower.8
The CD4 T cell is the prime target of the HIV virus and also an important marker of the health of the immune system. The lower the CD4 count at the start of therapy, the more challenging it is to normalize.9 If HIV infection is diagnosed early and therapy is started early, the likelihood is higher of normalizing the CD4 count and preserving immune function.
Progress is being made toward diagnosing HIV earlier. The CD4 count at presentation is increasing, but patients in the United States still present for care later than in other countries. In 1997, the median CD4 count at presentation was 234 cells/mL; in 2007, it was 327 (normal is about 550–1,000). Although this is a significant improvement, more than 50% of patients still have fewer than 350 cells/mL at presentation, which is the current threshold for beginning therapy, according to the most recent guidelines.10
Before triple therapy was available, almost all HIV-infected patients died of AIDS-related diseases. Now, about half of treated HIV-infected patients in Europe and North America die of other causes.11 However, many diseases not previously attributed to AIDS are now also known to be exacerbated by HIV infection.
Cancer risk increases with lower CD4 counts
The cumulative incidence of AIDS-defining cancers (Kaposi sarcoma, non-Hodgkin lymphoma, cervical carcinoma) has decreased steadily from 8.7% in the 1980s to 6.4% during the years 1990 to 1995, and to 2.1% between 1996 and 2006. This is attributable to improved immune function as a result of treatment success with antiviral therapy.12
But the incidence of non-AIDS-defining cancers (Hodgkin disease, anal cancer, oral and respiratory cancers) has increased.11 As therapy has regenerated the immune system, patients are surviving longer and are developing the more common cancers but with higher rates than in the general population.
Higher cancer risk is attributed to reduced immune surveillance. Many of these cancers are associated with viruses, such as human papillomavirus (anal and oral or pharyngeal cancers) and Epstein-Barr virus (Hodgkin disease), which can usually be controlled by a fully functioning immune system. The lower the CD4 count, the higher the risk of cancer, which highlights the need to diagnose HIV and start treatment early.13
Cardiovascular disease increases with lower CD4 counts
Associations have recently been identified between coronary disease and HIV as well as with HIV medications. Protease inhibitors tend to raise the levels of triglycerides, low-density lipoprotein cholesterol, and total cholesterol and increase the risk of heart attack.14
Regardless of therapy, HIV appears to be an independent risk factor for coronary disease. Arterial stiffness, as measured by carotid femoral pulse-wave velocity, was found to be increased among a sample of 80 HIV-infected men. This was associated with the usual risk factors of increasing age, blood pressure, and diabetes, as well as with lower nadir CD4 count.15
Fractures and neurocognitive disorders increase with HIV
Osteoporotic fractures are also more common in patients with HIV than in the general population. Risk factors include the traditional risks of older age, hepatitis C infection, diabetes, and substance abuse, but also nadir CD4 count less than 200.16
The risk of neurocognitive disorders is also associated with lower nadir CD4 counts. The lower the CD4 count, the higher the risk of developing neurocognitive deficits.17 The potential benefits of earlier diagnosis and treatment are obvious based upon the multiple recent findings outlined above.
CLINICAL PRESENTATION OF PRIMARY HIV INFECTION
During primary HIV infection, when patients are first infected, 50% to 90% are symptomatic. Symptoms usually appear in the first 6 weeks. The viral load tends to be highest at this time. Higher viral loads appear directly correlated with the degree of infectivity, highlighting the urgency of finding and treating new infections promptly to help avoid transmission to others.18
The clinical picture during primary infection is similar to that of acute mononucleosis. Signs and symptoms include fever, fatigue, rash, headache, lymphadenopathy, sore throat, and muscle aches. Although this presentation is common to many viral infections, questioning the patient about high-risk behavior (unprotected sex, multiple partners, intravenous drug use) will lead the astute physician to the correct testing and diagnosis.
Other early manifestations include mucocutaneous signs, such as seborrheic dermatitis, psoriasis, folliculitis, and thrush. Laboratory test results demonstrating leukopenia, thrombocytopenia, elevated total protein levels, proteinuria, and transaminitis are also suggestive of HIV infection.
THE CASE FOR INCREASED TESTING AND TREATMENT
The estimated prevalence of HIV in the United States is approximately 0.3%. However, its prevalence in Washington, DC, is 3%, which rivals rates in some areas of the developing world. From 2004 to 2008, health officials made a concerted effort in Washington, DC, to screen more people, particularly those at high risk. The number of publicly funded HIV tests performed increased by a factor of 3.7, and the number of newly reported cases increased by 17%. There was also a significant increase in the median CD4 count at the time of HIV diagnosis and a significant delay in time to progression to AIDS after HIV diagnosis.19
A study in British Columbia expanded access to highly active antiretroviral therapy during 2004 through 2009. High-risk individuals were targeted for increased screening. All those diagnosed with HIV were provided free medication. This resulted in a 50% reduction in new diagnoses of HIV infection throughout the community, especially among injectable drug users, a usually marginalized population. The proportion of patients with HIV-1 RNA levels above 1,500 copies/mL fell from about 50% to about 20%, indicating that the viral load—a measure of infectivity throughout the community—was reduced. Interestingly, this trend occurred during a time of increased rates of gonorrhea, syphilis, and other sexually transmitted diseases known to be associated with enhanced HIV transmission.20
In Africa, antiretroviral therapy was offered to discordant couples (one partner was infected with HIV and the other was not). Among those who chose therapy, the rate of HIV transmission was 92% lower than in those not receiving antiretroviral drugs,21 once again demonstrating that control of HIV by treatment can lead to decreased transmission.
US HIV testing is inadequate
The current state of HIV testing in the United States needs to be improved. Testing is not performed routinely, leading to delayed diagnosis when patients present with symptomatic, advanced disease. Patients who are tested late (within 12 months before being diagnosed with AIDS) tend to be younger and less educated and are more likely to be heterosexual and either African American or Hispanic than patients who are tested earlier.22 When retrospectively evaluated, these patients often have been in the health care system but not tested. Routine universal screening and targeted testing could lead to a much earlier diagnosis and potential better long-term outcomes.
A 1996 survey of 95 academic emergency departments found that for patients with suspected sexually transmitted infections, 93% of physicians said they screen for gonorrhea, 88% for Chlamydia infection, 58% for syphilis, but only 3% for HIV.23 Sexually transmitted infections and HIV are often transmitted together.
A similar 2002 survey of 154 emergency department providers who saw an average of 13 patients with sexually transmitted infections per week found that only 10% always recommend HIV testing to these patients. Reasons given for not testing were concern about follow-up (51%), not having a “certified” counselor (45%), HIV testing being too time-consuming (19%), and HIV testing being unavailable (27%).24
Although most HIV tests are given by private doctors and health maintenance organizations, the likelihood of finding patients with HIV is greatest in hospitals, emergency departments, outpatient clinics, and public community clinics.
The Advancing HIV Prevention initiative of the US Centers for Disease Control and Prevention (CDC) has four priorities:
- To make voluntary HIV testing a routine part of medical care
- To implement new models for diagnosing HIV infection outside medical settings
- To prevent HIV infection by working with patients with HIV and their partners
- To further decrease the rate of perinatal HIV transmission.
Rapid tests for HIV are available
There is a public health need to have rapid HIV testing available in all health care settings. With standard HIV tests, which can take 48 to 72 hours to run, about one-third of patients do not return for results. Subsequently locating them can be a huge challenge and is sometimes impossible. The ability to have rapid test results can improve this situation. It is especially important in prenatal care settings, where the mother can be immediately treated to reduce the risk of transmission to the child. Rapid testing increases the feasibility of testing in multiple venues, particularly acute-care settings with almost immediate results and linkage to care.
Several rapid tests are available and can be performed on whole blood, serum, plasma, and oral fluid. The tests provide reliable results in minutes, with 99% sensitivity and specificity. Positive results must be confirmed by subsequent two-stage laboratory testing, enzyme-linked immunosorbent assay, and Western blot. Patients who have negative or have indeterminate results on Western blot testing should be tested again after 4 weeks.
The cost-effectiveness of routine screening for HIV, even in populations with a low prevalence, is similar to that of commonly accepted interventions.25 In populations with a 1% prevalence of HIV, the cost is $15,078 per quality-adjusted life-year.26 Even if the prevalence is less than 0.05%, the cost is less than $50,000 per quality-adjusted life-year, which is normally the cutoff for acceptability for screening tests.25,26
‘OPT-OUT’ TESTING
In the past, patients were asked if they would like to have HIV testing (“opt-in” testing). It is now recommended that physicians request testing to be performed (“opt-out” testing). This still allows the patient to decline but also conveys a “matter of fact” nonjudgmental message, indicative of a routine procedure no different than other screening tests. When testing was done on an opt-in basis, only 35% of pregnant women agreed to be tested. Some women felt that accepting an HIV test indicated that they engage in high-risk behavior. When testing was instead offered as routine but with an opportunity to decline, 88% accepted testing, and they were significantly less anxious about testing.27
CDC RECOMMENDATIONS
The CDC now recommends that routine, voluntary HIV screening be done for all persons ages 13 to 64 in health care settings, regardless of risk.28 Screening should be repeated at least annually in persons with known risk. Screening should be done on an opt-out basis, with the opportunity to ask questions and the option to decline. Consent for HIV testing should be included with general consent for care. A separate signed informed consent is not recommended, and verbal consent can merely be documented in the medical record. Prevention counseling in conjunction with HIV screening in health care settings is not required.
Testing should be done in all health care settings, including primary care settings, inpatient services, emergency departments, urgent care clinics, and sexually transmitted disease clinics. Test results should be communicated in the same manner as other diagnostic and screening care. Clinical HIV care should be available onsite or reliable referral to qualified providers should be established.
For pregnant women, the CDC recommends universal opt-out HIV screening, with HIV testing as part of the routine panel of prenatal screening tests. The consent for prenatal care includes HIV testing, with notification and the option to decline. Women should be tested again in the third trimester if they are known to be at risk for HIV, and in areas and health care facilities in which the prevalence of HIV is high.
In women whose HIV status is undocumented in labor and delivery, opt-out rapid testing should be performed, and antiretroviral prophylaxis should be given on the basis of the rapid test result. Rapid testing of the newborn is recommended if the mother’s status is unknown at delivery, and antiretroviral prophylaxis should be started within 12 hours of birth on the basis of the rapid test result.
Widespread routine screening and earlier treatment could significantly reduce the incidence and improve the outcomes of HIV in this country. Health care providers are encouraged to adopt these practices.
- Van Sighem A, Gras L, Reiss P, Brinkman K, de Wolf F, and ATHENA Natl Observational Cohort Study. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 526.
- World Health Organization. Principles and Practice of Screening for Disease. WHO Public Health Paper, 1968.
- Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO). Global Facts & Figures 09. http://data.unaids.org/pub/FactSheet/2009/20091124_FS_global_en.pdf. Accessed 1/4/2011.
- World Health Organization. Epidemiological Fact Sheet on HIV and AIDS. Core data on epidemiology and response. United States of America. 2008 Update. http://apps.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_US.pdf. Accessed 1/4/2011.
- US Centers for Disease Control and Prevention. HIV Surveillance Report, 2008; vol. 20. http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Published June 2010. Accessed 8/7/2010.
- Hall HI, Song R, Rhodes P, et al; HIV Incidence Surveillance Group. Estimation of HIV incidence in the United States. JAMA 2008; 300:520–529.
- Marks G, Crepaz N, Janssen RS. Estimated sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006; 20:1447–1450.
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. December 1, 2009;1–161. http://www.aidsinfo.nih.gov/ContentFiles/AdultsandAdolescentGL.pdf. Accessed 1/4/2011.
- Palella F, Armon C, Buchacz , et al; the HOPS Investigators. CD4 at HAART initiation predicts long term CD4 responses and mortality from AIDS and non-AIDS causes in the HIV Outpatient Study (HOPS). Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 983.
- Althoff K, Gange S, Klein M, et al; the North American-AIDS Cohort Collaboration on Res and Design. Late presentation for HIV care in the United States and Canada. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 982.
- Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis 2010; 50:1387–1396.
- Simard E, Pfeiffer R, Engels E. Cancer incidence and cancer-attributable mortality among persons with AIDS in the United States. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 27.
- Silverberg M, Xu L, Chao C, et al. Immunodeficiency, HIV RNA levels, and risk of non-AIDS-defining cancers. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 28.
- DAD Study Group, Friis-Møller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–1735.
- Ho J, Deeks S, Hecht F, et al. Earlier initiation of antiretroviral therapy in HIV-infected individuals is associated with reduced arterial stiffness. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 707.
- Dao C, Young B, Buchacz K, Baker R, Brooks J, and the HIV Outpatient Study Investigators. Higher and increasing rates of fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared to the general US population 1994 to 2008. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 128.
- Ellis R, Heaton R, Letendre S, et al; the CHARTER Group. Higher CD4 nadir is associated with reduced rates of HIV-associated neurocognitive disorders in the CHARTER study: potential implications for early treatment initiation. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 429.
- Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med 1996; 125:257–264.
- Castel A, Samala R, Griffin A, et al. Monitoring the impact of expanded HIV testing in the District of Columbia using population-based HIV/AIDS surveillance data. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 34.
- Montaner J, Wood E, Kerr T, et al. Association of expanded HAART coverage with a decrease in new HIV diagnoses, particularly mong injection drug users in British Columbia, Canada. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 88LB.
- Donnell D, Kiarie J, Thomas K, et al. ART and risk of heterosexual HIV-1 transmissin in HIV-1 serodiscordant African couples: a multinational prospective study. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 136.
- Centers for Disease Control and Prevention. Late versus early testing of HIV—16 sites, United States, 2000–2003. MMWR Morb Mortal Wkly Rep 2003; Jun 27; 52( 25):581–586.
- Wilson SR, Mitchell C, Bradbury DR, Chavez J. Testing for HIV: current practies in the academic ED. Am J Emerg Med 1999; 17:346–356.
- Fincher-Mergi M, Cartone KJ, Mischler J, Pasieka P, Lerner EB, Billittier AJ. Assessment of emergency department heatlh care professionals’ behaviors regaridng HIV testing and referral for patients with STDs. AIDS Patient Care STDs 2002; 16:549–553.
- Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med 2005; 352:586–595.
- Sanders GD, Gayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med 2005; 352:570–585.
- Simpson WM, Johnstone FD, Goldberg DJ, Gormley SM, Hart GJ. Antenatal HIV testing: assessment of a routine voluntary approach. BMJ 1999; 318:1660–1661.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55(RR-14):1–17.
With early treatment of human immunodeficiency virus (HIV) infection, we can now expect patients to live a much longer life and, in some situations, have a near-normal lifespan.1 Unfortunately, in screening for HIV infection, the United States lags behind many regions of the world, and infection is often not diagnosed until patients present with advanced disease, ie, the acquired immunodeficiency syndrome (AIDS). In this country there is a critical need to make HIV screening a routine part of medical care in all health settings in order to give patients their best chance for a healthy life, to prevent mother-to-child transmission, and to reduce the spread of HIV in the community.
HIV infection meets the criteria that justify routine screening, as laid out by the World Health Organization2:
- It is a serious health disorder that can be detected before symptoms develop
- Treatment is more beneficial if begun before symptoms develop
- Reliable, inexpensive, and acceptable screening tests exist
- The costs of screening are reasonable in relation to the anticipated benefits.
This article will review the epidemiology of the HIV epidemic, present the benefits of early treatment, and make the case for widely expanding screening for HIV infection in the US health care system.
HIV INFECTION CONTINUES TO BE A LARGE BURDEN
In 2008, an estimated 33.4 million people worldwide were HIV-positive. The vast majority of infected people—more than 22 million—live in sub-Saharan Africa.3
The United States has approximately 1.2 million cases.4 Although this is a small proportion of cases worldwide, it still represents a significant health care burden. In this country, the number of AIDS cases peaked in 1993, and the rate of deaths from AIDS began to decrease over the ensuing years as adequate therapy for HIV was developed. Standard therapy then and now consists of at least three drugs from two different classes.
Unfortunately, we have made little progress on the incidence of this disease. The estimated number of new HIV infections in the United States in 2008 was 56,000 and had remained about the same over the previous 15 years.5,6 Because of improved rates of survival, the prevalence has risen steadily since the mid-1990s to the current estimate of 1.2 million persons living with HIV/AIDS in the US.
About 25% of people infected with HIV are unaware of it. This group accounts for more than half of all new infections annually, which highlights the importance of enhanced screening. Once people know they are infected, they tend to change their behavior and are less likely to spread the disease.7
HIV disproportionately affects minority populations and gay men
Cases of HIV infection are reported among all age groups, although most patients tend to have been infected as young adults. Currently, the largest age group living with HIV is middle-aged. As this cohort grows older, an increasing burden of comorbidities due to aging can be expected. In 5 years, about half of the people with HIV in this country are expected to be 50 years of age or older. Although survival rates have steadily increased due to better treatment, survival tends to be shorter for older people newly diagnosed with HIV.
Worldwide, about an equal number of men and women are infected with HIV, but in the United States infected men outnumber women. In this country, about half the cases of HIV transmission among adults are by male-to-male sexual contact, about 30% are by high-risk heterosexual contact (ie, with a partner known to be HIV-infected or at high risk for being infected), and about 10% are by injection drug use.
In the United States, AIDS is predominantly and disproportionately a disease of minorities and those who live in poverty. African Americans account for the largest number of cases, followed by whites and then by Hispanics. Combined, African Americans and Hispanics account for two-thirds to three-fourths of all new cases, although they make up less than one-fourth of the US population. The incidence rate is nearly 137 per 100,000 for African Americans, 56 per 100,000 for Hispanics, and 19 per 100,000 for whites. The incidence is highest in New York and in the southeast, the geographic areas where the greatest number of minorities and people living in poverty reside. These groups also often lack access to health care.
HIV TREATMENT IS MORE EFFECTIVE IF STARTED EARLY
Treatment guidelines from the US Department of Health and Human Services (DHHS) have changed over the years. When effective medications were first introduced in the 1990s, the trend was to treat everyone as soon as they were diagnosed. As the burden of therapy began to unfold (side effects, cost, adherence, and drug resistance), the consensus was to wait until the CD4 T-cell count dropped to a lower level. As the medications have improved and have become better tolerated, the pendulum has swung back to treating earlier in the course of the disease. Currently, the DHHS recommends that therapy be started at CD4 counts of 350 cells/mL or lower (level of evidence: A1).8 It also recommends therapy for CD4 counts between 350 and 500 cells/mL, but the level of evidence is lower.8
The CD4 T cell is the prime target of the HIV virus and also an important marker of the health of the immune system. The lower the CD4 count at the start of therapy, the more challenging it is to normalize.9 If HIV infection is diagnosed early and therapy is started early, the likelihood is higher of normalizing the CD4 count and preserving immune function.
Progress is being made toward diagnosing HIV earlier. The CD4 count at presentation is increasing, but patients in the United States still present for care later than in other countries. In 1997, the median CD4 count at presentation was 234 cells/mL; in 2007, it was 327 (normal is about 550–1,000). Although this is a significant improvement, more than 50% of patients still have fewer than 350 cells/mL at presentation, which is the current threshold for beginning therapy, according to the most recent guidelines.10
Before triple therapy was available, almost all HIV-infected patients died of AIDS-related diseases. Now, about half of treated HIV-infected patients in Europe and North America die of other causes.11 However, many diseases not previously attributed to AIDS are now also known to be exacerbated by HIV infection.
Cancer risk increases with lower CD4 counts
The cumulative incidence of AIDS-defining cancers (Kaposi sarcoma, non-Hodgkin lymphoma, cervical carcinoma) has decreased steadily from 8.7% in the 1980s to 6.4% during the years 1990 to 1995, and to 2.1% between 1996 and 2006. This is attributable to improved immune function as a result of treatment success with antiviral therapy.12
But the incidence of non-AIDS-defining cancers (Hodgkin disease, anal cancer, oral and respiratory cancers) has increased.11 As therapy has regenerated the immune system, patients are surviving longer and are developing the more common cancers but with higher rates than in the general population.
Higher cancer risk is attributed to reduced immune surveillance. Many of these cancers are associated with viruses, such as human papillomavirus (anal and oral or pharyngeal cancers) and Epstein-Barr virus (Hodgkin disease), which can usually be controlled by a fully functioning immune system. The lower the CD4 count, the higher the risk of cancer, which highlights the need to diagnose HIV and start treatment early.13
Cardiovascular disease increases with lower CD4 counts
Associations have recently been identified between coronary disease and HIV as well as with HIV medications. Protease inhibitors tend to raise the levels of triglycerides, low-density lipoprotein cholesterol, and total cholesterol and increase the risk of heart attack.14
Regardless of therapy, HIV appears to be an independent risk factor for coronary disease. Arterial stiffness, as measured by carotid femoral pulse-wave velocity, was found to be increased among a sample of 80 HIV-infected men. This was associated with the usual risk factors of increasing age, blood pressure, and diabetes, as well as with lower nadir CD4 count.15
Fractures and neurocognitive disorders increase with HIV
Osteoporotic fractures are also more common in patients with HIV than in the general population. Risk factors include the traditional risks of older age, hepatitis C infection, diabetes, and substance abuse, but also nadir CD4 count less than 200.16
The risk of neurocognitive disorders is also associated with lower nadir CD4 counts. The lower the CD4 count, the higher the risk of developing neurocognitive deficits.17 The potential benefits of earlier diagnosis and treatment are obvious based upon the multiple recent findings outlined above.
CLINICAL PRESENTATION OF PRIMARY HIV INFECTION
During primary HIV infection, when patients are first infected, 50% to 90% are symptomatic. Symptoms usually appear in the first 6 weeks. The viral load tends to be highest at this time. Higher viral loads appear directly correlated with the degree of infectivity, highlighting the urgency of finding and treating new infections promptly to help avoid transmission to others.18
The clinical picture during primary infection is similar to that of acute mononucleosis. Signs and symptoms include fever, fatigue, rash, headache, lymphadenopathy, sore throat, and muscle aches. Although this presentation is common to many viral infections, questioning the patient about high-risk behavior (unprotected sex, multiple partners, intravenous drug use) will lead the astute physician to the correct testing and diagnosis.
Other early manifestations include mucocutaneous signs, such as seborrheic dermatitis, psoriasis, folliculitis, and thrush. Laboratory test results demonstrating leukopenia, thrombocytopenia, elevated total protein levels, proteinuria, and transaminitis are also suggestive of HIV infection.
THE CASE FOR INCREASED TESTING AND TREATMENT
The estimated prevalence of HIV in the United States is approximately 0.3%. However, its prevalence in Washington, DC, is 3%, which rivals rates in some areas of the developing world. From 2004 to 2008, health officials made a concerted effort in Washington, DC, to screen more people, particularly those at high risk. The number of publicly funded HIV tests performed increased by a factor of 3.7, and the number of newly reported cases increased by 17%. There was also a significant increase in the median CD4 count at the time of HIV diagnosis and a significant delay in time to progression to AIDS after HIV diagnosis.19
A study in British Columbia expanded access to highly active antiretroviral therapy during 2004 through 2009. High-risk individuals were targeted for increased screening. All those diagnosed with HIV were provided free medication. This resulted in a 50% reduction in new diagnoses of HIV infection throughout the community, especially among injectable drug users, a usually marginalized population. The proportion of patients with HIV-1 RNA levels above 1,500 copies/mL fell from about 50% to about 20%, indicating that the viral load—a measure of infectivity throughout the community—was reduced. Interestingly, this trend occurred during a time of increased rates of gonorrhea, syphilis, and other sexually transmitted diseases known to be associated with enhanced HIV transmission.20
In Africa, antiretroviral therapy was offered to discordant couples (one partner was infected with HIV and the other was not). Among those who chose therapy, the rate of HIV transmission was 92% lower than in those not receiving antiretroviral drugs,21 once again demonstrating that control of HIV by treatment can lead to decreased transmission.
US HIV testing is inadequate
The current state of HIV testing in the United States needs to be improved. Testing is not performed routinely, leading to delayed diagnosis when patients present with symptomatic, advanced disease. Patients who are tested late (within 12 months before being diagnosed with AIDS) tend to be younger and less educated and are more likely to be heterosexual and either African American or Hispanic than patients who are tested earlier.22 When retrospectively evaluated, these patients often have been in the health care system but not tested. Routine universal screening and targeted testing could lead to a much earlier diagnosis and potential better long-term outcomes.
A 1996 survey of 95 academic emergency departments found that for patients with suspected sexually transmitted infections, 93% of physicians said they screen for gonorrhea, 88% for Chlamydia infection, 58% for syphilis, but only 3% for HIV.23 Sexually transmitted infections and HIV are often transmitted together.
A similar 2002 survey of 154 emergency department providers who saw an average of 13 patients with sexually transmitted infections per week found that only 10% always recommend HIV testing to these patients. Reasons given for not testing were concern about follow-up (51%), not having a “certified” counselor (45%), HIV testing being too time-consuming (19%), and HIV testing being unavailable (27%).24
Although most HIV tests are given by private doctors and health maintenance organizations, the likelihood of finding patients with HIV is greatest in hospitals, emergency departments, outpatient clinics, and public community clinics.
The Advancing HIV Prevention initiative of the US Centers for Disease Control and Prevention (CDC) has four priorities:
- To make voluntary HIV testing a routine part of medical care
- To implement new models for diagnosing HIV infection outside medical settings
- To prevent HIV infection by working with patients with HIV and their partners
- To further decrease the rate of perinatal HIV transmission.
Rapid tests for HIV are available
There is a public health need to have rapid HIV testing available in all health care settings. With standard HIV tests, which can take 48 to 72 hours to run, about one-third of patients do not return for results. Subsequently locating them can be a huge challenge and is sometimes impossible. The ability to have rapid test results can improve this situation. It is especially important in prenatal care settings, where the mother can be immediately treated to reduce the risk of transmission to the child. Rapid testing increases the feasibility of testing in multiple venues, particularly acute-care settings with almost immediate results and linkage to care.
Several rapid tests are available and can be performed on whole blood, serum, plasma, and oral fluid. The tests provide reliable results in minutes, with 99% sensitivity and specificity. Positive results must be confirmed by subsequent two-stage laboratory testing, enzyme-linked immunosorbent assay, and Western blot. Patients who have negative or have indeterminate results on Western blot testing should be tested again after 4 weeks.
The cost-effectiveness of routine screening for HIV, even in populations with a low prevalence, is similar to that of commonly accepted interventions.25 In populations with a 1% prevalence of HIV, the cost is $15,078 per quality-adjusted life-year.26 Even if the prevalence is less than 0.05%, the cost is less than $50,000 per quality-adjusted life-year, which is normally the cutoff for acceptability for screening tests.25,26
‘OPT-OUT’ TESTING
In the past, patients were asked if they would like to have HIV testing (“opt-in” testing). It is now recommended that physicians request testing to be performed (“opt-out” testing). This still allows the patient to decline but also conveys a “matter of fact” nonjudgmental message, indicative of a routine procedure no different than other screening tests. When testing was done on an opt-in basis, only 35% of pregnant women agreed to be tested. Some women felt that accepting an HIV test indicated that they engage in high-risk behavior. When testing was instead offered as routine but with an opportunity to decline, 88% accepted testing, and they were significantly less anxious about testing.27
CDC RECOMMENDATIONS
The CDC now recommends that routine, voluntary HIV screening be done for all persons ages 13 to 64 in health care settings, regardless of risk.28 Screening should be repeated at least annually in persons with known risk. Screening should be done on an opt-out basis, with the opportunity to ask questions and the option to decline. Consent for HIV testing should be included with general consent for care. A separate signed informed consent is not recommended, and verbal consent can merely be documented in the medical record. Prevention counseling in conjunction with HIV screening in health care settings is not required.
Testing should be done in all health care settings, including primary care settings, inpatient services, emergency departments, urgent care clinics, and sexually transmitted disease clinics. Test results should be communicated in the same manner as other diagnostic and screening care. Clinical HIV care should be available onsite or reliable referral to qualified providers should be established.
For pregnant women, the CDC recommends universal opt-out HIV screening, with HIV testing as part of the routine panel of prenatal screening tests. The consent for prenatal care includes HIV testing, with notification and the option to decline. Women should be tested again in the third trimester if they are known to be at risk for HIV, and in areas and health care facilities in which the prevalence of HIV is high.
In women whose HIV status is undocumented in labor and delivery, opt-out rapid testing should be performed, and antiretroviral prophylaxis should be given on the basis of the rapid test result. Rapid testing of the newborn is recommended if the mother’s status is unknown at delivery, and antiretroviral prophylaxis should be started within 12 hours of birth on the basis of the rapid test result.
Widespread routine screening and earlier treatment could significantly reduce the incidence and improve the outcomes of HIV in this country. Health care providers are encouraged to adopt these practices.
With early treatment of human immunodeficiency virus (HIV) infection, we can now expect patients to live a much longer life and, in some situations, have a near-normal lifespan.1 Unfortunately, in screening for HIV infection, the United States lags behind many regions of the world, and infection is often not diagnosed until patients present with advanced disease, ie, the acquired immunodeficiency syndrome (AIDS). In this country there is a critical need to make HIV screening a routine part of medical care in all health settings in order to give patients their best chance for a healthy life, to prevent mother-to-child transmission, and to reduce the spread of HIV in the community.
HIV infection meets the criteria that justify routine screening, as laid out by the World Health Organization2:
- It is a serious health disorder that can be detected before symptoms develop
- Treatment is more beneficial if begun before symptoms develop
- Reliable, inexpensive, and acceptable screening tests exist
- The costs of screening are reasonable in relation to the anticipated benefits.
This article will review the epidemiology of the HIV epidemic, present the benefits of early treatment, and make the case for widely expanding screening for HIV infection in the US health care system.
HIV INFECTION CONTINUES TO BE A LARGE BURDEN
In 2008, an estimated 33.4 million people worldwide were HIV-positive. The vast majority of infected people—more than 22 million—live in sub-Saharan Africa.3
The United States has approximately 1.2 million cases.4 Although this is a small proportion of cases worldwide, it still represents a significant health care burden. In this country, the number of AIDS cases peaked in 1993, and the rate of deaths from AIDS began to decrease over the ensuing years as adequate therapy for HIV was developed. Standard therapy then and now consists of at least three drugs from two different classes.
Unfortunately, we have made little progress on the incidence of this disease. The estimated number of new HIV infections in the United States in 2008 was 56,000 and had remained about the same over the previous 15 years.5,6 Because of improved rates of survival, the prevalence has risen steadily since the mid-1990s to the current estimate of 1.2 million persons living with HIV/AIDS in the US.
About 25% of people infected with HIV are unaware of it. This group accounts for more than half of all new infections annually, which highlights the importance of enhanced screening. Once people know they are infected, they tend to change their behavior and are less likely to spread the disease.7
HIV disproportionately affects minority populations and gay men
Cases of HIV infection are reported among all age groups, although most patients tend to have been infected as young adults. Currently, the largest age group living with HIV is middle-aged. As this cohort grows older, an increasing burden of comorbidities due to aging can be expected. In 5 years, about half of the people with HIV in this country are expected to be 50 years of age or older. Although survival rates have steadily increased due to better treatment, survival tends to be shorter for older people newly diagnosed with HIV.
Worldwide, about an equal number of men and women are infected with HIV, but in the United States infected men outnumber women. In this country, about half the cases of HIV transmission among adults are by male-to-male sexual contact, about 30% are by high-risk heterosexual contact (ie, with a partner known to be HIV-infected or at high risk for being infected), and about 10% are by injection drug use.
In the United States, AIDS is predominantly and disproportionately a disease of minorities and those who live in poverty. African Americans account for the largest number of cases, followed by whites and then by Hispanics. Combined, African Americans and Hispanics account for two-thirds to three-fourths of all new cases, although they make up less than one-fourth of the US population. The incidence rate is nearly 137 per 100,000 for African Americans, 56 per 100,000 for Hispanics, and 19 per 100,000 for whites. The incidence is highest in New York and in the southeast, the geographic areas where the greatest number of minorities and people living in poverty reside. These groups also often lack access to health care.
HIV TREATMENT IS MORE EFFECTIVE IF STARTED EARLY
Treatment guidelines from the US Department of Health and Human Services (DHHS) have changed over the years. When effective medications were first introduced in the 1990s, the trend was to treat everyone as soon as they were diagnosed. As the burden of therapy began to unfold (side effects, cost, adherence, and drug resistance), the consensus was to wait until the CD4 T-cell count dropped to a lower level. As the medications have improved and have become better tolerated, the pendulum has swung back to treating earlier in the course of the disease. Currently, the DHHS recommends that therapy be started at CD4 counts of 350 cells/mL or lower (level of evidence: A1).8 It also recommends therapy for CD4 counts between 350 and 500 cells/mL, but the level of evidence is lower.8
The CD4 T cell is the prime target of the HIV virus and also an important marker of the health of the immune system. The lower the CD4 count at the start of therapy, the more challenging it is to normalize.9 If HIV infection is diagnosed early and therapy is started early, the likelihood is higher of normalizing the CD4 count and preserving immune function.
Progress is being made toward diagnosing HIV earlier. The CD4 count at presentation is increasing, but patients in the United States still present for care later than in other countries. In 1997, the median CD4 count at presentation was 234 cells/mL; in 2007, it was 327 (normal is about 550–1,000). Although this is a significant improvement, more than 50% of patients still have fewer than 350 cells/mL at presentation, which is the current threshold for beginning therapy, according to the most recent guidelines.10
Before triple therapy was available, almost all HIV-infected patients died of AIDS-related diseases. Now, about half of treated HIV-infected patients in Europe and North America die of other causes.11 However, many diseases not previously attributed to AIDS are now also known to be exacerbated by HIV infection.
Cancer risk increases with lower CD4 counts
The cumulative incidence of AIDS-defining cancers (Kaposi sarcoma, non-Hodgkin lymphoma, cervical carcinoma) has decreased steadily from 8.7% in the 1980s to 6.4% during the years 1990 to 1995, and to 2.1% between 1996 and 2006. This is attributable to improved immune function as a result of treatment success with antiviral therapy.12
But the incidence of non-AIDS-defining cancers (Hodgkin disease, anal cancer, oral and respiratory cancers) has increased.11 As therapy has regenerated the immune system, patients are surviving longer and are developing the more common cancers but with higher rates than in the general population.
Higher cancer risk is attributed to reduced immune surveillance. Many of these cancers are associated with viruses, such as human papillomavirus (anal and oral or pharyngeal cancers) and Epstein-Barr virus (Hodgkin disease), which can usually be controlled by a fully functioning immune system. The lower the CD4 count, the higher the risk of cancer, which highlights the need to diagnose HIV and start treatment early.13
Cardiovascular disease increases with lower CD4 counts
Associations have recently been identified between coronary disease and HIV as well as with HIV medications. Protease inhibitors tend to raise the levels of triglycerides, low-density lipoprotein cholesterol, and total cholesterol and increase the risk of heart attack.14
Regardless of therapy, HIV appears to be an independent risk factor for coronary disease. Arterial stiffness, as measured by carotid femoral pulse-wave velocity, was found to be increased among a sample of 80 HIV-infected men. This was associated with the usual risk factors of increasing age, blood pressure, and diabetes, as well as with lower nadir CD4 count.15
Fractures and neurocognitive disorders increase with HIV
Osteoporotic fractures are also more common in patients with HIV than in the general population. Risk factors include the traditional risks of older age, hepatitis C infection, diabetes, and substance abuse, but also nadir CD4 count less than 200.16
The risk of neurocognitive disorders is also associated with lower nadir CD4 counts. The lower the CD4 count, the higher the risk of developing neurocognitive deficits.17 The potential benefits of earlier diagnosis and treatment are obvious based upon the multiple recent findings outlined above.
CLINICAL PRESENTATION OF PRIMARY HIV INFECTION
During primary HIV infection, when patients are first infected, 50% to 90% are symptomatic. Symptoms usually appear in the first 6 weeks. The viral load tends to be highest at this time. Higher viral loads appear directly correlated with the degree of infectivity, highlighting the urgency of finding and treating new infections promptly to help avoid transmission to others.18
The clinical picture during primary infection is similar to that of acute mononucleosis. Signs and symptoms include fever, fatigue, rash, headache, lymphadenopathy, sore throat, and muscle aches. Although this presentation is common to many viral infections, questioning the patient about high-risk behavior (unprotected sex, multiple partners, intravenous drug use) will lead the astute physician to the correct testing and diagnosis.
Other early manifestations include mucocutaneous signs, such as seborrheic dermatitis, psoriasis, folliculitis, and thrush. Laboratory test results demonstrating leukopenia, thrombocytopenia, elevated total protein levels, proteinuria, and transaminitis are also suggestive of HIV infection.
THE CASE FOR INCREASED TESTING AND TREATMENT
The estimated prevalence of HIV in the United States is approximately 0.3%. However, its prevalence in Washington, DC, is 3%, which rivals rates in some areas of the developing world. From 2004 to 2008, health officials made a concerted effort in Washington, DC, to screen more people, particularly those at high risk. The number of publicly funded HIV tests performed increased by a factor of 3.7, and the number of newly reported cases increased by 17%. There was also a significant increase in the median CD4 count at the time of HIV diagnosis and a significant delay in time to progression to AIDS after HIV diagnosis.19
A study in British Columbia expanded access to highly active antiretroviral therapy during 2004 through 2009. High-risk individuals were targeted for increased screening. All those diagnosed with HIV were provided free medication. This resulted in a 50% reduction in new diagnoses of HIV infection throughout the community, especially among injectable drug users, a usually marginalized population. The proportion of patients with HIV-1 RNA levels above 1,500 copies/mL fell from about 50% to about 20%, indicating that the viral load—a measure of infectivity throughout the community—was reduced. Interestingly, this trend occurred during a time of increased rates of gonorrhea, syphilis, and other sexually transmitted diseases known to be associated with enhanced HIV transmission.20
In Africa, antiretroviral therapy was offered to discordant couples (one partner was infected with HIV and the other was not). Among those who chose therapy, the rate of HIV transmission was 92% lower than in those not receiving antiretroviral drugs,21 once again demonstrating that control of HIV by treatment can lead to decreased transmission.
US HIV testing is inadequate
The current state of HIV testing in the United States needs to be improved. Testing is not performed routinely, leading to delayed diagnosis when patients present with symptomatic, advanced disease. Patients who are tested late (within 12 months before being diagnosed with AIDS) tend to be younger and less educated and are more likely to be heterosexual and either African American or Hispanic than patients who are tested earlier.22 When retrospectively evaluated, these patients often have been in the health care system but not tested. Routine universal screening and targeted testing could lead to a much earlier diagnosis and potential better long-term outcomes.
A 1996 survey of 95 academic emergency departments found that for patients with suspected sexually transmitted infections, 93% of physicians said they screen for gonorrhea, 88% for Chlamydia infection, 58% for syphilis, but only 3% for HIV.23 Sexually transmitted infections and HIV are often transmitted together.
A similar 2002 survey of 154 emergency department providers who saw an average of 13 patients with sexually transmitted infections per week found that only 10% always recommend HIV testing to these patients. Reasons given for not testing were concern about follow-up (51%), not having a “certified” counselor (45%), HIV testing being too time-consuming (19%), and HIV testing being unavailable (27%).24
Although most HIV tests are given by private doctors and health maintenance organizations, the likelihood of finding patients with HIV is greatest in hospitals, emergency departments, outpatient clinics, and public community clinics.
The Advancing HIV Prevention initiative of the US Centers for Disease Control and Prevention (CDC) has four priorities:
- To make voluntary HIV testing a routine part of medical care
- To implement new models for diagnosing HIV infection outside medical settings
- To prevent HIV infection by working with patients with HIV and their partners
- To further decrease the rate of perinatal HIV transmission.
Rapid tests for HIV are available
There is a public health need to have rapid HIV testing available in all health care settings. With standard HIV tests, which can take 48 to 72 hours to run, about one-third of patients do not return for results. Subsequently locating them can be a huge challenge and is sometimes impossible. The ability to have rapid test results can improve this situation. It is especially important in prenatal care settings, where the mother can be immediately treated to reduce the risk of transmission to the child. Rapid testing increases the feasibility of testing in multiple venues, particularly acute-care settings with almost immediate results and linkage to care.
Several rapid tests are available and can be performed on whole blood, serum, plasma, and oral fluid. The tests provide reliable results in minutes, with 99% sensitivity and specificity. Positive results must be confirmed by subsequent two-stage laboratory testing, enzyme-linked immunosorbent assay, and Western blot. Patients who have negative or have indeterminate results on Western blot testing should be tested again after 4 weeks.
The cost-effectiveness of routine screening for HIV, even in populations with a low prevalence, is similar to that of commonly accepted interventions.25 In populations with a 1% prevalence of HIV, the cost is $15,078 per quality-adjusted life-year.26 Even if the prevalence is less than 0.05%, the cost is less than $50,000 per quality-adjusted life-year, which is normally the cutoff for acceptability for screening tests.25,26
‘OPT-OUT’ TESTING
In the past, patients were asked if they would like to have HIV testing (“opt-in” testing). It is now recommended that physicians request testing to be performed (“opt-out” testing). This still allows the patient to decline but also conveys a “matter of fact” nonjudgmental message, indicative of a routine procedure no different than other screening tests. When testing was done on an opt-in basis, only 35% of pregnant women agreed to be tested. Some women felt that accepting an HIV test indicated that they engage in high-risk behavior. When testing was instead offered as routine but with an opportunity to decline, 88% accepted testing, and they were significantly less anxious about testing.27
CDC RECOMMENDATIONS
The CDC now recommends that routine, voluntary HIV screening be done for all persons ages 13 to 64 in health care settings, regardless of risk.28 Screening should be repeated at least annually in persons with known risk. Screening should be done on an opt-out basis, with the opportunity to ask questions and the option to decline. Consent for HIV testing should be included with general consent for care. A separate signed informed consent is not recommended, and verbal consent can merely be documented in the medical record. Prevention counseling in conjunction with HIV screening in health care settings is not required.
Testing should be done in all health care settings, including primary care settings, inpatient services, emergency departments, urgent care clinics, and sexually transmitted disease clinics. Test results should be communicated in the same manner as other diagnostic and screening care. Clinical HIV care should be available onsite or reliable referral to qualified providers should be established.
For pregnant women, the CDC recommends universal opt-out HIV screening, with HIV testing as part of the routine panel of prenatal screening tests. The consent for prenatal care includes HIV testing, with notification and the option to decline. Women should be tested again in the third trimester if they are known to be at risk for HIV, and in areas and health care facilities in which the prevalence of HIV is high.
In women whose HIV status is undocumented in labor and delivery, opt-out rapid testing should be performed, and antiretroviral prophylaxis should be given on the basis of the rapid test result. Rapid testing of the newborn is recommended if the mother’s status is unknown at delivery, and antiretroviral prophylaxis should be started within 12 hours of birth on the basis of the rapid test result.
Widespread routine screening and earlier treatment could significantly reduce the incidence and improve the outcomes of HIV in this country. Health care providers are encouraged to adopt these practices.
- Van Sighem A, Gras L, Reiss P, Brinkman K, de Wolf F, and ATHENA Natl Observational Cohort Study. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 526.
- World Health Organization. Principles and Practice of Screening for Disease. WHO Public Health Paper, 1968.
- Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO). Global Facts & Figures 09. http://data.unaids.org/pub/FactSheet/2009/20091124_FS_global_en.pdf. Accessed 1/4/2011.
- World Health Organization. Epidemiological Fact Sheet on HIV and AIDS. Core data on epidemiology and response. United States of America. 2008 Update. http://apps.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_US.pdf. Accessed 1/4/2011.
- US Centers for Disease Control and Prevention. HIV Surveillance Report, 2008; vol. 20. http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Published June 2010. Accessed 8/7/2010.
- Hall HI, Song R, Rhodes P, et al; HIV Incidence Surveillance Group. Estimation of HIV incidence in the United States. JAMA 2008; 300:520–529.
- Marks G, Crepaz N, Janssen RS. Estimated sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006; 20:1447–1450.
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. December 1, 2009;1–161. http://www.aidsinfo.nih.gov/ContentFiles/AdultsandAdolescentGL.pdf. Accessed 1/4/2011.
- Palella F, Armon C, Buchacz , et al; the HOPS Investigators. CD4 at HAART initiation predicts long term CD4 responses and mortality from AIDS and non-AIDS causes in the HIV Outpatient Study (HOPS). Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 983.
- Althoff K, Gange S, Klein M, et al; the North American-AIDS Cohort Collaboration on Res and Design. Late presentation for HIV care in the United States and Canada. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 982.
- Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis 2010; 50:1387–1396.
- Simard E, Pfeiffer R, Engels E. Cancer incidence and cancer-attributable mortality among persons with AIDS in the United States. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 27.
- Silverberg M, Xu L, Chao C, et al. Immunodeficiency, HIV RNA levels, and risk of non-AIDS-defining cancers. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 28.
- DAD Study Group, Friis-Møller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–1735.
- Ho J, Deeks S, Hecht F, et al. Earlier initiation of antiretroviral therapy in HIV-infected individuals is associated with reduced arterial stiffness. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 707.
- Dao C, Young B, Buchacz K, Baker R, Brooks J, and the HIV Outpatient Study Investigators. Higher and increasing rates of fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared to the general US population 1994 to 2008. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 128.
- Ellis R, Heaton R, Letendre S, et al; the CHARTER Group. Higher CD4 nadir is associated with reduced rates of HIV-associated neurocognitive disorders in the CHARTER study: potential implications for early treatment initiation. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 429.
- Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med 1996; 125:257–264.
- Castel A, Samala R, Griffin A, et al. Monitoring the impact of expanded HIV testing in the District of Columbia using population-based HIV/AIDS surveillance data. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 34.
- Montaner J, Wood E, Kerr T, et al. Association of expanded HAART coverage with a decrease in new HIV diagnoses, particularly mong injection drug users in British Columbia, Canada. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 88LB.
- Donnell D, Kiarie J, Thomas K, et al. ART and risk of heterosexual HIV-1 transmissin in HIV-1 serodiscordant African couples: a multinational prospective study. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 136.
- Centers for Disease Control and Prevention. Late versus early testing of HIV—16 sites, United States, 2000–2003. MMWR Morb Mortal Wkly Rep 2003; Jun 27; 52( 25):581–586.
- Wilson SR, Mitchell C, Bradbury DR, Chavez J. Testing for HIV: current practies in the academic ED. Am J Emerg Med 1999; 17:346–356.
- Fincher-Mergi M, Cartone KJ, Mischler J, Pasieka P, Lerner EB, Billittier AJ. Assessment of emergency department heatlh care professionals’ behaviors regaridng HIV testing and referral for patients with STDs. AIDS Patient Care STDs 2002; 16:549–553.
- Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med 2005; 352:586–595.
- Sanders GD, Gayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med 2005; 352:570–585.
- Simpson WM, Johnstone FD, Goldberg DJ, Gormley SM, Hart GJ. Antenatal HIV testing: assessment of a routine voluntary approach. BMJ 1999; 318:1660–1661.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55(RR-14):1–17.
- Van Sighem A, Gras L, Reiss P, Brinkman K, de Wolf F, and ATHENA Natl Observational Cohort Study. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 526.
- World Health Organization. Principles and Practice of Screening for Disease. WHO Public Health Paper, 1968.
- Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO). Global Facts & Figures 09. http://data.unaids.org/pub/FactSheet/2009/20091124_FS_global_en.pdf. Accessed 1/4/2011.
- World Health Organization. Epidemiological Fact Sheet on HIV and AIDS. Core data on epidemiology and response. United States of America. 2008 Update. http://apps.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_US.pdf. Accessed 1/4/2011.
- US Centers for Disease Control and Prevention. HIV Surveillance Report, 2008; vol. 20. http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Published June 2010. Accessed 8/7/2010.
- Hall HI, Song R, Rhodes P, et al; HIV Incidence Surveillance Group. Estimation of HIV incidence in the United States. JAMA 2008; 300:520–529.
- Marks G, Crepaz N, Janssen RS. Estimated sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006; 20:1447–1450.
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. December 1, 2009;1–161. http://www.aidsinfo.nih.gov/ContentFiles/AdultsandAdolescentGL.pdf. Accessed 1/4/2011.
- Palella F, Armon C, Buchacz , et al; the HOPS Investigators. CD4 at HAART initiation predicts long term CD4 responses and mortality from AIDS and non-AIDS causes in the HIV Outpatient Study (HOPS). Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 983.
- Althoff K, Gange S, Klein M, et al; the North American-AIDS Cohort Collaboration on Res and Design. Late presentation for HIV care in the United States and Canada. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 982.
- Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis 2010; 50:1387–1396.
- Simard E, Pfeiffer R, Engels E. Cancer incidence and cancer-attributable mortality among persons with AIDS in the United States. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 27.
- Silverberg M, Xu L, Chao C, et al. Immunodeficiency, HIV RNA levels, and risk of non-AIDS-defining cancers. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 28.
- DAD Study Group, Friis-Møller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–1735.
- Ho J, Deeks S, Hecht F, et al. Earlier initiation of antiretroviral therapy in HIV-infected individuals is associated with reduced arterial stiffness. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 707.
- Dao C, Young B, Buchacz K, Baker R, Brooks J, and the HIV Outpatient Study Investigators. Higher and increasing rates of fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared to the general US population 1994 to 2008. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 128.
- Ellis R, Heaton R, Letendre S, et al; the CHARTER Group. Higher CD4 nadir is associated with reduced rates of HIV-associated neurocognitive disorders in the CHARTER study: potential implications for early treatment initiation. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 429.
- Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med 1996; 125:257–264.
- Castel A, Samala R, Griffin A, et al. Monitoring the impact of expanded HIV testing in the District of Columbia using population-based HIV/AIDS surveillance data. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 34.
- Montaner J, Wood E, Kerr T, et al. Association of expanded HAART coverage with a decrease in new HIV diagnoses, particularly mong injection drug users in British Columbia, Canada. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 88LB.
- Donnell D, Kiarie J, Thomas K, et al. ART and risk of heterosexual HIV-1 transmissin in HIV-1 serodiscordant African couples: a multinational prospective study. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 136.
- Centers for Disease Control and Prevention. Late versus early testing of HIV—16 sites, United States, 2000–2003. MMWR Morb Mortal Wkly Rep 2003; Jun 27; 52( 25):581–586.
- Wilson SR, Mitchell C, Bradbury DR, Chavez J. Testing for HIV: current practies in the academic ED. Am J Emerg Med 1999; 17:346–356.
- Fincher-Mergi M, Cartone KJ, Mischler J, Pasieka P, Lerner EB, Billittier AJ. Assessment of emergency department heatlh care professionals’ behaviors regaridng HIV testing and referral for patients with STDs. AIDS Patient Care STDs 2002; 16:549–553.
- Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med 2005; 352:586–595.
- Sanders GD, Gayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med 2005; 352:570–585.
- Simpson WM, Johnstone FD, Goldberg DJ, Gormley SM, Hart GJ. Antenatal HIV testing: assessment of a routine voluntary approach. BMJ 1999; 318:1660–1661.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55(RR-14):1–17.
KEY POINTS
- Recommendations from the US Centers for Disease Control and Prevention call for routine HIV screening for all people ages 13 to 64 at least once regardless of their risk profile, and annual testing for people with known risk factors for acquiring HIV.
- Early treatment of HIV infection may reduce the risk of cancer, cardiovascular disease, neurocognitive disorders, and osteoporotic fractures and improve the rate of survival compared with patients treated late in the course of HIV infection.
- Finding and treating patients early in the course of infection has the potential to reduce infectivity in the community.
- Reliable rapid testing is now available to screen for HIV in community settings, emergency departments, and public health clinics, and during labor for those not tested in the prenatal period. It is also useful when follow-up is uncertain.
Exercises for air travel
These exercises should be repeated every hour on a flight when you are awake.
Neck roll
With your shoulders and arms relaxed and hanging down, tilt your head to your left, hold for a few seconds, then slowly roll your head toward your back and hold for a few seconds, then slowly roll your head toward your right shoulder and hold for a few seconds, and then slowly roll your head toward your chest and hold for a few seconds. Repeat this exercise for a total of five times clockwise and then five times counterclockwise.
Shoulder roll
While in your seat with your arms on the arm rests, move both shoulders in a circular motion from front to back five times and then repeat in the opposite direction.
Shoulder stretch
While in your seat, put your left hand on your right shoulder. With your right hand, grasp your elbow and pull your left elbow toward your right side. Hold this position for 15 seconds and then switch arms and repeat the stretch with the opposite side. Repeat these stretches five times with each arm.
Knee-to-chest stretch
While in your seat, lean forward slightly and grab your knee just below the joint. Slowly pull your knee toward your chest and hold for 15 seconds. Repeat the stretch with your other knee. Repeat the cycle five times.
Ankle circles
Raise your feet off the floor and rotate them in a circular motion five times clockwise and then five times counterclockwise.
Foot pumps
With your heels on the floor, first raise your toes as high as you can and hold that position for 10 seconds. Then lower your toes until they touch the floor, and then raise your heels off the floor as much as you can, keeping your toes on the floor, and hold that position for 10 seconds. Repeat this exercise five times.
Adapted with permission from Continental Airlines
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints are available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Web site, www.clevelandclinic.org/health
These exercises should be repeated every hour on a flight when you are awake.
Neck roll
With your shoulders and arms relaxed and hanging down, tilt your head to your left, hold for a few seconds, then slowly roll your head toward your back and hold for a few seconds, then slowly roll your head toward your right shoulder and hold for a few seconds, and then slowly roll your head toward your chest and hold for a few seconds. Repeat this exercise for a total of five times clockwise and then five times counterclockwise.
Shoulder roll
While in your seat with your arms on the arm rests, move both shoulders in a circular motion from front to back five times and then repeat in the opposite direction.
Shoulder stretch
While in your seat, put your left hand on your right shoulder. With your right hand, grasp your elbow and pull your left elbow toward your right side. Hold this position for 15 seconds and then switch arms and repeat the stretch with the opposite side. Repeat these stretches five times with each arm.
Knee-to-chest stretch
While in your seat, lean forward slightly and grab your knee just below the joint. Slowly pull your knee toward your chest and hold for 15 seconds. Repeat the stretch with your other knee. Repeat the cycle five times.
Ankle circles
Raise your feet off the floor and rotate them in a circular motion five times clockwise and then five times counterclockwise.
Foot pumps
With your heels on the floor, first raise your toes as high as you can and hold that position for 10 seconds. Then lower your toes until they touch the floor, and then raise your heels off the floor as much as you can, keeping your toes on the floor, and hold that position for 10 seconds. Repeat this exercise five times.
Adapted with permission from Continental Airlines
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints are available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Web site, www.clevelandclinic.org/health
These exercises should be repeated every hour on a flight when you are awake.
Neck roll
With your shoulders and arms relaxed and hanging down, tilt your head to your left, hold for a few seconds, then slowly roll your head toward your back and hold for a few seconds, then slowly roll your head toward your right shoulder and hold for a few seconds, and then slowly roll your head toward your chest and hold for a few seconds. Repeat this exercise for a total of five times clockwise and then five times counterclockwise.
Shoulder roll
While in your seat with your arms on the arm rests, move both shoulders in a circular motion from front to back five times and then repeat in the opposite direction.
Shoulder stretch
While in your seat, put your left hand on your right shoulder. With your right hand, grasp your elbow and pull your left elbow toward your right side. Hold this position for 15 seconds and then switch arms and repeat the stretch with the opposite side. Repeat these stretches five times with each arm.
Knee-to-chest stretch
While in your seat, lean forward slightly and grab your knee just below the joint. Slowly pull your knee toward your chest and hold for 15 seconds. Repeat the stretch with your other knee. Repeat the cycle five times.
Ankle circles
Raise your feet off the floor and rotate them in a circular motion five times clockwise and then five times counterclockwise.
Foot pumps
With your heels on the floor, first raise your toes as high as you can and hold that position for 10 seconds. Then lower your toes until they touch the floor, and then raise your heels off the floor as much as you can, keeping your toes on the floor, and hold that position for 10 seconds. Repeat this exercise five times.
Adapted with permission from Continental Airlines
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints are available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Web site, www.clevelandclinic.org/health
REV, Metras Beat Rastelli for TGA, VSD, and LVOTO
Optimal surgical management of patients with transposition of the great arteries, ventricular septal defect, and left ventricular outflow obstruction is still considered controversial. Although the Rastelli operation is the most commonly performed procedure, the Réparation à l'Etage Ventriculaire procedure and Metras modification yielded the best long-term results for both survival and event-free survival, according to a retrospective study of 146 patients who underwent surgery from 1980 to 2008 in eight European hospitals.
The multicenter study compared use and outcomes of several different surgical operations for transposition of the great arteries (TGA), ventricular septal defect (VSD), and left ventricular outflow obstruction (LVOTO), according to a report published in the European Journal of Cardio-thoracic Surgery.
A total of 141 patients had TGA, VSD, and LVOTO; 5 patients had the TGA type of double-outlet right ventricle (DORV) with LVOTO. Only those patients for whom the surgical method chosen was equivalent to those for TGA, VSD, and LVOTO were included in the study; all other DORV types were excluded, according to Dr. Mark Gerard Hazekamp of Leids Universitair Medisch Centrum, Leiden, the Netherlands, and his colleagues from various European universities on behalf of the European Congenital Heart Surgeons Association.
The procedures investigated were the Rastelli (82 patients), arterial (24) and atrial (5) switch operation with relief of LVOTO, Reparation l'Etage Ventriculaire (REV) procedure (7), and Metras modification (24), as well as the Nikaidoh (4). The type of surgery used has traditionally been different in different countries, they said, with the REV procedure and Metras modification mainly in France and the Rastelli procedure being the norm in most other countries.
Patients had a median age at operation of 21.5 months (range 0.2-165.1 months) and a median weight of 10 kg (range 2.0-41.0 kg). Pulmonary stenosis was found in 119 patients, while 27 had pulmonary atresia. LVOTO was solely valvar in 24% of the patients, only subvalvar in 37% of patients, and multilevel in 39%.
The location of the most important VSD was known in 143 patients, with outlet septum in 102, inlet septum in 14, trabecular septum in 3, and a combination of the three in 24 patients. The great majority of the 140 patients for whom data were available had great artery commitment of the biggest VSD: to the aorta in 60, the pulmonary artery in 32, and doubly committed to both in 19. Only 29 patients had noncommitment of one of the great arteries to the VSD.
Overall postoperative survival was 92% at 1 month, 88% at 1 year, 88% at 10 years, and 58% at 20 years. Events were followed as an outcome and were defined as death, reoperation, transcatheter intervention, or cardiac transplantation. The frequent necessity of reintervention (40.7% over follow-up) caused the overall event-free survival to be lower: 85% at 1 month, 80% at 1 year, 45% at 10 years, and 26% at 20 years (Euro. J. Cardiothorac. Surg. 2010;38:699-706).
There were 41 surgical reinterventions and 20 percutaneous procedures, with the most frequent cause of reoperation being RVOT obstruction, including conduit failure (25.0%), followed by LVOT obstruction (7.9%), residual VSD closure (7.1%), and pulmonary artery plasty (4.3%).
In multivariate analysis, age at the corrective surgery, year of the operation, and type of operation were significant predictors for reoperation and trans-catheter intervention, in general, as well as for RVOT reoperation/intervention. The younger the patient at the time of operation, the higher the risk of later reoperation, leading the researchers to speculate that the more recent the surgery, the less the probability that a patient would undergo reoperation.
Reoperation for RVOTO was most common in patients with a Rastelli operation, according to the authors.
"Although there are some differences between Rastelli outcomes among different groups, the all-over rates of freedom from reoperation and, especially, event-free survival, are not satisfactory with event-free survival rates at 10 years that vary from 24% to 49%," they said.
"The Rastelli procedure was a significant independent risk factor for re-operation, with the REV/Metras and the Nikaidoh having the lowest re-intervention rates," they wrote.
They indicated more patients need to be studied with longer follow-up, especially for the Nikaidoh technique.
The authors had no disclosures.
Optimal surgical management of patients with transposition of the great arteries, ventricular septal defect, and left ventricular outflow obstruction is still considered controversial. Although the Rastelli operation is the most commonly performed procedure, the Réparation à l'Etage Ventriculaire procedure and Metras modification yielded the best long-term results for both survival and event-free survival, according to a retrospective study of 146 patients who underwent surgery from 1980 to 2008 in eight European hospitals.
The multicenter study compared use and outcomes of several different surgical operations for transposition of the great arteries (TGA), ventricular septal defect (VSD), and left ventricular outflow obstruction (LVOTO), according to a report published in the European Journal of Cardio-thoracic Surgery.
A total of 141 patients had TGA, VSD, and LVOTO; 5 patients had the TGA type of double-outlet right ventricle (DORV) with LVOTO. Only those patients for whom the surgical method chosen was equivalent to those for TGA, VSD, and LVOTO were included in the study; all other DORV types were excluded, according to Dr. Mark Gerard Hazekamp of Leids Universitair Medisch Centrum, Leiden, the Netherlands, and his colleagues from various European universities on behalf of the European Congenital Heart Surgeons Association.
The procedures investigated were the Rastelli (82 patients), arterial (24) and atrial (5) switch operation with relief of LVOTO, Reparation l'Etage Ventriculaire (REV) procedure (7), and Metras modification (24), as well as the Nikaidoh (4). The type of surgery used has traditionally been different in different countries, they said, with the REV procedure and Metras modification mainly in France and the Rastelli procedure being the norm in most other countries.
Patients had a median age at operation of 21.5 months (range 0.2-165.1 months) and a median weight of 10 kg (range 2.0-41.0 kg). Pulmonary stenosis was found in 119 patients, while 27 had pulmonary atresia. LVOTO was solely valvar in 24% of the patients, only subvalvar in 37% of patients, and multilevel in 39%.
The location of the most important VSD was known in 143 patients, with outlet septum in 102, inlet septum in 14, trabecular septum in 3, and a combination of the three in 24 patients. The great majority of the 140 patients for whom data were available had great artery commitment of the biggest VSD: to the aorta in 60, the pulmonary artery in 32, and doubly committed to both in 19. Only 29 patients had noncommitment of one of the great arteries to the VSD.
Overall postoperative survival was 92% at 1 month, 88% at 1 year, 88% at 10 years, and 58% at 20 years. Events were followed as an outcome and were defined as death, reoperation, transcatheter intervention, or cardiac transplantation. The frequent necessity of reintervention (40.7% over follow-up) caused the overall event-free survival to be lower: 85% at 1 month, 80% at 1 year, 45% at 10 years, and 26% at 20 years (Euro. J. Cardiothorac. Surg. 2010;38:699-706).
There were 41 surgical reinterventions and 20 percutaneous procedures, with the most frequent cause of reoperation being RVOT obstruction, including conduit failure (25.0%), followed by LVOT obstruction (7.9%), residual VSD closure (7.1%), and pulmonary artery plasty (4.3%).
In multivariate analysis, age at the corrective surgery, year of the operation, and type of operation were significant predictors for reoperation and trans-catheter intervention, in general, as well as for RVOT reoperation/intervention. The younger the patient at the time of operation, the higher the risk of later reoperation, leading the researchers to speculate that the more recent the surgery, the less the probability that a patient would undergo reoperation.
Reoperation for RVOTO was most common in patients with a Rastelli operation, according to the authors.
"Although there are some differences between Rastelli outcomes among different groups, the all-over rates of freedom from reoperation and, especially, event-free survival, are not satisfactory with event-free survival rates at 10 years that vary from 24% to 49%," they said.
"The Rastelli procedure was a significant independent risk factor for re-operation, with the REV/Metras and the Nikaidoh having the lowest re-intervention rates," they wrote.
They indicated more patients need to be studied with longer follow-up, especially for the Nikaidoh technique.
The authors had no disclosures.
Optimal surgical management of patients with transposition of the great arteries, ventricular septal defect, and left ventricular outflow obstruction is still considered controversial. Although the Rastelli operation is the most commonly performed procedure, the Réparation à l'Etage Ventriculaire procedure and Metras modification yielded the best long-term results for both survival and event-free survival, according to a retrospective study of 146 patients who underwent surgery from 1980 to 2008 in eight European hospitals.
The multicenter study compared use and outcomes of several different surgical operations for transposition of the great arteries (TGA), ventricular septal defect (VSD), and left ventricular outflow obstruction (LVOTO), according to a report published in the European Journal of Cardio-thoracic Surgery.
A total of 141 patients had TGA, VSD, and LVOTO; 5 patients had the TGA type of double-outlet right ventricle (DORV) with LVOTO. Only those patients for whom the surgical method chosen was equivalent to those for TGA, VSD, and LVOTO were included in the study; all other DORV types were excluded, according to Dr. Mark Gerard Hazekamp of Leids Universitair Medisch Centrum, Leiden, the Netherlands, and his colleagues from various European universities on behalf of the European Congenital Heart Surgeons Association.
The procedures investigated were the Rastelli (82 patients), arterial (24) and atrial (5) switch operation with relief of LVOTO, Reparation l'Etage Ventriculaire (REV) procedure (7), and Metras modification (24), as well as the Nikaidoh (4). The type of surgery used has traditionally been different in different countries, they said, with the REV procedure and Metras modification mainly in France and the Rastelli procedure being the norm in most other countries.
Patients had a median age at operation of 21.5 months (range 0.2-165.1 months) and a median weight of 10 kg (range 2.0-41.0 kg). Pulmonary stenosis was found in 119 patients, while 27 had pulmonary atresia. LVOTO was solely valvar in 24% of the patients, only subvalvar in 37% of patients, and multilevel in 39%.
The location of the most important VSD was known in 143 patients, with outlet septum in 102, inlet septum in 14, trabecular septum in 3, and a combination of the three in 24 patients. The great majority of the 140 patients for whom data were available had great artery commitment of the biggest VSD: to the aorta in 60, the pulmonary artery in 32, and doubly committed to both in 19. Only 29 patients had noncommitment of one of the great arteries to the VSD.
Overall postoperative survival was 92% at 1 month, 88% at 1 year, 88% at 10 years, and 58% at 20 years. Events were followed as an outcome and were defined as death, reoperation, transcatheter intervention, or cardiac transplantation. The frequent necessity of reintervention (40.7% over follow-up) caused the overall event-free survival to be lower: 85% at 1 month, 80% at 1 year, 45% at 10 years, and 26% at 20 years (Euro. J. Cardiothorac. Surg. 2010;38:699-706).
There were 41 surgical reinterventions and 20 percutaneous procedures, with the most frequent cause of reoperation being RVOT obstruction, including conduit failure (25.0%), followed by LVOT obstruction (7.9%), residual VSD closure (7.1%), and pulmonary artery plasty (4.3%).
In multivariate analysis, age at the corrective surgery, year of the operation, and type of operation were significant predictors for reoperation and trans-catheter intervention, in general, as well as for RVOT reoperation/intervention. The younger the patient at the time of operation, the higher the risk of later reoperation, leading the researchers to speculate that the more recent the surgery, the less the probability that a patient would undergo reoperation.
Reoperation for RVOTO was most common in patients with a Rastelli operation, according to the authors.
"Although there are some differences between Rastelli outcomes among different groups, the all-over rates of freedom from reoperation and, especially, event-free survival, are not satisfactory with event-free survival rates at 10 years that vary from 24% to 49%," they said.
"The Rastelli procedure was a significant independent risk factor for re-operation, with the REV/Metras and the Nikaidoh having the lowest re-intervention rates," they wrote.
They indicated more patients need to be studied with longer follow-up, especially for the Nikaidoh technique.
The authors had no disclosures.
Neutropenia and the White Blood Cells
VA Launches Program to Assist Dying Veterans
Anomalous Motor Learning May Be Specific to Children With Autism
Children with autism rely heavily on proprioception, unlike children with other developmental motor impairments or typically developing children.
PROVIDENCE, RI—Children with autism spectrum disorder form a representation of internal models that places an unusually strong reliance on proprioception, according to research presented at the 39th National Meeting of the Child Neurology Society.
“This anomalous motor learning is specific to autism spectrum disorder, rather than a general deficit of all populations with developmental motor impairments, as children with ADHD did not generalize differently than typically developing children,” reported Stewart H. Mostofsky, MD, research scientist at the Kennedy Krieger Institute and Associate Professor of Neurology, Johns Hopkins University School of Medicine, Baltimore, and colleagues. “Our results suggest that autism-associated impairment in understanding actions of others may be a consequence of the fact that in learning to perform actions, children with autism place a greater than normal reliance on their own proprioception while discounting the visual consequences of their actions.”
The researchers analyzed 25 children with autism (mean age, 10.31), 16 with ADHD (mean age, 10.66), and 39 typically developing children (mean age, 10.82). As part of a game, each child held the handle of a robotic arm, trying to capture animals that had escaped from a zoo. An animal would appear at a target location 8 cm away; if the child reached the target in time, the animal was captured and the child was given a point.
“Analyses revealed that all groups were able to effectively adapt their arm movement,” stated Dr. Mostofsky’s group. “However, generalization patterns were markedly different. There was a significant interaction between diagnostic group and relative generalization to targets 2 and 3. Posthoc analyses revealed this difference was due to significantly greater generalization of the autism group in the intrinsic (proprioceptive) coordinate system as compared to typically developing children. In contrast, there was no significant difference in generalization between ADHD and typically developing children.”
Regression analyses revealed that among all groups, generalization in the intrinsic (proprioceptive) coordinate system (ie, to target 3) was a significant predictor of social ability, such that greater social impairment was predicted by increased force for target 3, noted Dr. Mostofsky and colleagues. “Further,” the researchers concluded, “for the children with autism, increased force for target 3 predicted impaired social interaction. In addition, increased generalization to target 3 also predicted impaired imitation ability, as assessed on a praxis examination, as well as impairment in motor control.”
Children with autism rely heavily on proprioception, unlike children with other developmental motor impairments or typically developing children.
PROVIDENCE, RI—Children with autism spectrum disorder form a representation of internal models that places an unusually strong reliance on proprioception, according to research presented at the 39th National Meeting of the Child Neurology Society.
“This anomalous motor learning is specific to autism spectrum disorder, rather than a general deficit of all populations with developmental motor impairments, as children with ADHD did not generalize differently than typically developing children,” reported Stewart H. Mostofsky, MD, research scientist at the Kennedy Krieger Institute and Associate Professor of Neurology, Johns Hopkins University School of Medicine, Baltimore, and colleagues. “Our results suggest that autism-associated impairment in understanding actions of others may be a consequence of the fact that in learning to perform actions, children with autism place a greater than normal reliance on their own proprioception while discounting the visual consequences of their actions.”
The researchers analyzed 25 children with autism (mean age, 10.31), 16 with ADHD (mean age, 10.66), and 39 typically developing children (mean age, 10.82). As part of a game, each child held the handle of a robotic arm, trying to capture animals that had escaped from a zoo. An animal would appear at a target location 8 cm away; if the child reached the target in time, the animal was captured and the child was given a point.
“Analyses revealed that all groups were able to effectively adapt their arm movement,” stated Dr. Mostofsky’s group. “However, generalization patterns were markedly different. There was a significant interaction between diagnostic group and relative generalization to targets 2 and 3. Posthoc analyses revealed this difference was due to significantly greater generalization of the autism group in the intrinsic (proprioceptive) coordinate system as compared to typically developing children. In contrast, there was no significant difference in generalization between ADHD and typically developing children.”
Regression analyses revealed that among all groups, generalization in the intrinsic (proprioceptive) coordinate system (ie, to target 3) was a significant predictor of social ability, such that greater social impairment was predicted by increased force for target 3, noted Dr. Mostofsky and colleagues. “Further,” the researchers concluded, “for the children with autism, increased force for target 3 predicted impaired social interaction. In addition, increased generalization to target 3 also predicted impaired imitation ability, as assessed on a praxis examination, as well as impairment in motor control.”
Children with autism rely heavily on proprioception, unlike children with other developmental motor impairments or typically developing children.
PROVIDENCE, RI—Children with autism spectrum disorder form a representation of internal models that places an unusually strong reliance on proprioception, according to research presented at the 39th National Meeting of the Child Neurology Society.
“This anomalous motor learning is specific to autism spectrum disorder, rather than a general deficit of all populations with developmental motor impairments, as children with ADHD did not generalize differently than typically developing children,” reported Stewart H. Mostofsky, MD, research scientist at the Kennedy Krieger Institute and Associate Professor of Neurology, Johns Hopkins University School of Medicine, Baltimore, and colleagues. “Our results suggest that autism-associated impairment in understanding actions of others may be a consequence of the fact that in learning to perform actions, children with autism place a greater than normal reliance on their own proprioception while discounting the visual consequences of their actions.”
The researchers analyzed 25 children with autism (mean age, 10.31), 16 with ADHD (mean age, 10.66), and 39 typically developing children (mean age, 10.82). As part of a game, each child held the handle of a robotic arm, trying to capture animals that had escaped from a zoo. An animal would appear at a target location 8 cm away; if the child reached the target in time, the animal was captured and the child was given a point.
“Analyses revealed that all groups were able to effectively adapt their arm movement,” stated Dr. Mostofsky’s group. “However, generalization patterns were markedly different. There was a significant interaction between diagnostic group and relative generalization to targets 2 and 3. Posthoc analyses revealed this difference was due to significantly greater generalization of the autism group in the intrinsic (proprioceptive) coordinate system as compared to typically developing children. In contrast, there was no significant difference in generalization between ADHD and typically developing children.”
Regression analyses revealed that among all groups, generalization in the intrinsic (proprioceptive) coordinate system (ie, to target 3) was a significant predictor of social ability, such that greater social impairment was predicted by increased force for target 3, noted Dr. Mostofsky and colleagues. “Further,” the researchers concluded, “for the children with autism, increased force for target 3 predicted impaired social interaction. In addition, increased generalization to target 3 also predicted impaired imitation ability, as assessed on a praxis examination, as well as impairment in motor control.”
Self-Monitoring of Glucose in Diabetes
Despite therapeutic advances in diabetes management, the majority of patients with diabetes are unable to achieve glycemic targets proven to reduce the burden of the disease. This burden not only involves the quality of life of patients with diabetes who experience the complications of this disease; it also includes the burden to society. One out of every five health care dollars is spent on caring for someone with diabetes—the majority on treating the complications.1
Major barriers to patients’ ability to achieve glycemic goals include the need to make behavioral changes, lack of awareness of glycemic levels, and fear of hypoglycemia.2
Q: Is self-monitoring of blood glucose worthwhile in diabetes?
Studies have shown a benefit from self-monitoring of blood glucose (SMBG) in patients using insulin but not in those taking oral antidiabetic drugs. However, the American Diabetes Association recommends that patients with diabetes monitor their glucose once daily if they are being treated with noninsulin therapy and at least three times daily if they are taking insulin.3
Guidelines from the American Association of Clinical Endocrinologists (AACE) state that patients taking noninsulin or once-daily insulin therapy who have not achieved A1C targets should monitor at least twice daily, while those at target should monitor at least once daily. Those taking multiple daily injections should perform SMBG at least three times per day. If patients experience frequent hypoglycemia, AACE suggests monitoring glucose more often.4
The A1C test provides the “big picture,” the average daily glucose level during the previous 90 to 120 days, and correlates with end-organ impact. It does not identify glycemic variability, hypoglycemia, or hyperglycemia.
By contrast, SMBG patterns provide day-to-day data that can be used to select and manage glucose control programs and ultimately optimize a patient’s A1C. SMBG provides a measure of the specific pharmacologic impact of medications and, through feedback, allows design and implementation of physiologic insulin-replacement programs.
One example of SMBG is to have patients monitor glucose in pairs (ie, pick a meal each day and do a premeal and two-hour postmeal reading) and ask them to keep a log or download the data from their meter in the office. This type of monitoring can be enlightening and self-empowering for the patient in that it can provide valuable information regarding the glycemic response to the particular meal.
Intensive glycemic management has been shown to reduce the incidence and progression of diabetic complications. However, it is associated with an increase in severe hypoglycemia. This is worrisome for both patients and providers, as severe hypoglycemia has been associated with an increase in risk for mortality. SMBG can assist patients in understanding how their lifestyle affects their diabetes, as well as identifying hypoglycemia for those who may have hypoglycemia unawareness (ie, who lack the relevant symptoms).
Q: What is continuous glucose monitoring (CGM)?
CGM devices give real-time readouts of current glucose levels. They utilize a subcutaneous sensor that is inserted in the abdomen and worn for 3 to 7 days (depending on which device is used). The sensor sends an electronic signal to a receiver worn by the patient.
There are three major CGM devices that have been approved by the FDA and are available for both personal and professional use. Health care providers can purchase the units and have patients wear them for retrospective analysis; this is a reimbursable expense. All available CGM devices measure glucose values in the interstitial fluid. The sensor reads electrical current produced by the same glucose-oxidase reaction that is utilized by glucose meters that patients use to perform fingersticks for home monitoring.
Currently available CGM systems need to be calibrated at least twice daily. Sensor calibration entails the pairing of the fingerstick value with the sensor value from the interstitial space. Calibration confirms sensor accuracy during various points by “teaching” the sensor the glucose value that corresponds with the electrical current signal.
There is a known physiologic lag time that occurs between fingerstick and sensor values. This lag time is typically up to 15 minutes but is increased with rapidly changing glucose values.
Q: What are the benefits of CGM?
Recent studies have shown CGM can improve A1C without increasing the incidence of hypoglycemia.5
CGM systems have both low and high glucose threshold alarms that can be set to alert once the threshold is reached. The newest generation devices can also predict hypoglycemia or hyperglycemia by tracking rate of change, and users can be alerted to a potential event. This would then allow them to take appropriate action, such as consuming food or carbohydrates or taking insulin as necessary. (Before taking any action, the glucose should first be confirmed by SMBG.)
Software programs allow for review of glucose data, which can assist in identifying trends not appreciated by typical SMBG testing (such as nocturnal hypoglycemia and meal-time excursions). This allows for adjustment of insulin regimens to reduce the incidence of these events.
Q: Can CGM replace SMBG?
While CGM can provide much more detail regarding glucose trends and patterns, it is not a replacement for SMBG. CGM should not be used as a replacement for SMBG to dose insulin for meal- or activity-related adjustments. All dosing decisions should be based on the SMBG.
Currently, CGM is indicated for patients 18 or older, in conjunction with SMBG for the purpose of improving glycemic control:
• to identify and aid in management of glycemic patterns not recognized with typical SMBG
• to prevent glycemic excursions of hypoglycemia and hyperglycemia.
Its use is supported by ADA and AACE guidelines for glucose monitoring.
Q: Who would benefit from CGM?
Suitable candidates for CGM include those with a high degree of glycemic variability, those with hypoglycemic unawareness, shift workers, patients who use insulin pumps, athletes, and women who are planning to become or are pregnant. Patients should work closely with their health care team and perform regular SMBG.
It has been suggested that patients need comprehensive training and follow-up visits to fully understand the large amount of data that they can be confronted with, in order to fully benefit from these devices.6 While the accuracy is improving, there are a few limitations to this technology, including false alarms. Studies have also shown a positive correlation between sensor wear time (hours per week) and greater reductions in A1C.5
Conclusion
Glucose monitoring is a necessary tool—for patients as well as providers—that assists in identifying how patients’ lifestyles affect their diabetes.
1. American Diabetes Association. Economic costs of diabetes in the US in 2007. Diabetes Care. 2008;31(3):596-615.
2. Hirsch IB, Armstrong D, Bergenstal RM, et al. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM). Diabetes Technol Ther. 2008;10(4):232-246.
3. American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2010;34(suppl 1):S11-S61.
4. American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the management of diabetes mellitus. Endocrin Prac. 2007;13(suppl 1):1-68.
5. Bergenstal RM, Tamberlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320.
6. Fabiato K, Buse J, Duclos M, et al. Clinical experience with continuous glucose monitoring in adults. Diabetes Technol Ther. 2009;11(suppl 1):S93-S103.
Despite therapeutic advances in diabetes management, the majority of patients with diabetes are unable to achieve glycemic targets proven to reduce the burden of the disease. This burden not only involves the quality of life of patients with diabetes who experience the complications of this disease; it also includes the burden to society. One out of every five health care dollars is spent on caring for someone with diabetes—the majority on treating the complications.1
Major barriers to patients’ ability to achieve glycemic goals include the need to make behavioral changes, lack of awareness of glycemic levels, and fear of hypoglycemia.2
Q: Is self-monitoring of blood glucose worthwhile in diabetes?
Studies have shown a benefit from self-monitoring of blood glucose (SMBG) in patients using insulin but not in those taking oral antidiabetic drugs. However, the American Diabetes Association recommends that patients with diabetes monitor their glucose once daily if they are being treated with noninsulin therapy and at least three times daily if they are taking insulin.3
Guidelines from the American Association of Clinical Endocrinologists (AACE) state that patients taking noninsulin or once-daily insulin therapy who have not achieved A1C targets should monitor at least twice daily, while those at target should monitor at least once daily. Those taking multiple daily injections should perform SMBG at least three times per day. If patients experience frequent hypoglycemia, AACE suggests monitoring glucose more often.4
The A1C test provides the “big picture,” the average daily glucose level during the previous 90 to 120 days, and correlates with end-organ impact. It does not identify glycemic variability, hypoglycemia, or hyperglycemia.
By contrast, SMBG patterns provide day-to-day data that can be used to select and manage glucose control programs and ultimately optimize a patient’s A1C. SMBG provides a measure of the specific pharmacologic impact of medications and, through feedback, allows design and implementation of physiologic insulin-replacement programs.
One example of SMBG is to have patients monitor glucose in pairs (ie, pick a meal each day and do a premeal and two-hour postmeal reading) and ask them to keep a log or download the data from their meter in the office. This type of monitoring can be enlightening and self-empowering for the patient in that it can provide valuable information regarding the glycemic response to the particular meal.
Intensive glycemic management has been shown to reduce the incidence and progression of diabetic complications. However, it is associated with an increase in severe hypoglycemia. This is worrisome for both patients and providers, as severe hypoglycemia has been associated with an increase in risk for mortality. SMBG can assist patients in understanding how their lifestyle affects their diabetes, as well as identifying hypoglycemia for those who may have hypoglycemia unawareness (ie, who lack the relevant symptoms).
Q: What is continuous glucose monitoring (CGM)?
CGM devices give real-time readouts of current glucose levels. They utilize a subcutaneous sensor that is inserted in the abdomen and worn for 3 to 7 days (depending on which device is used). The sensor sends an electronic signal to a receiver worn by the patient.
There are three major CGM devices that have been approved by the FDA and are available for both personal and professional use. Health care providers can purchase the units and have patients wear them for retrospective analysis; this is a reimbursable expense. All available CGM devices measure glucose values in the interstitial fluid. The sensor reads electrical current produced by the same glucose-oxidase reaction that is utilized by glucose meters that patients use to perform fingersticks for home monitoring.
Currently available CGM systems need to be calibrated at least twice daily. Sensor calibration entails the pairing of the fingerstick value with the sensor value from the interstitial space. Calibration confirms sensor accuracy during various points by “teaching” the sensor the glucose value that corresponds with the electrical current signal.
There is a known physiologic lag time that occurs between fingerstick and sensor values. This lag time is typically up to 15 minutes but is increased with rapidly changing glucose values.
Q: What are the benefits of CGM?
Recent studies have shown CGM can improve A1C without increasing the incidence of hypoglycemia.5
CGM systems have both low and high glucose threshold alarms that can be set to alert once the threshold is reached. The newest generation devices can also predict hypoglycemia or hyperglycemia by tracking rate of change, and users can be alerted to a potential event. This would then allow them to take appropriate action, such as consuming food or carbohydrates or taking insulin as necessary. (Before taking any action, the glucose should first be confirmed by SMBG.)
Software programs allow for review of glucose data, which can assist in identifying trends not appreciated by typical SMBG testing (such as nocturnal hypoglycemia and meal-time excursions). This allows for adjustment of insulin regimens to reduce the incidence of these events.
Q: Can CGM replace SMBG?
While CGM can provide much more detail regarding glucose trends and patterns, it is not a replacement for SMBG. CGM should not be used as a replacement for SMBG to dose insulin for meal- or activity-related adjustments. All dosing decisions should be based on the SMBG.
Currently, CGM is indicated for patients 18 or older, in conjunction with SMBG for the purpose of improving glycemic control:
• to identify and aid in management of glycemic patterns not recognized with typical SMBG
• to prevent glycemic excursions of hypoglycemia and hyperglycemia.
Its use is supported by ADA and AACE guidelines for glucose monitoring.
Q: Who would benefit from CGM?
Suitable candidates for CGM include those with a high degree of glycemic variability, those with hypoglycemic unawareness, shift workers, patients who use insulin pumps, athletes, and women who are planning to become or are pregnant. Patients should work closely with their health care team and perform regular SMBG.
It has been suggested that patients need comprehensive training and follow-up visits to fully understand the large amount of data that they can be confronted with, in order to fully benefit from these devices.6 While the accuracy is improving, there are a few limitations to this technology, including false alarms. Studies have also shown a positive correlation between sensor wear time (hours per week) and greater reductions in A1C.5
Conclusion
Glucose monitoring is a necessary tool—for patients as well as providers—that assists in identifying how patients’ lifestyles affect their diabetes.
Despite therapeutic advances in diabetes management, the majority of patients with diabetes are unable to achieve glycemic targets proven to reduce the burden of the disease. This burden not only involves the quality of life of patients with diabetes who experience the complications of this disease; it also includes the burden to society. One out of every five health care dollars is spent on caring for someone with diabetes—the majority on treating the complications.1
Major barriers to patients’ ability to achieve glycemic goals include the need to make behavioral changes, lack of awareness of glycemic levels, and fear of hypoglycemia.2
Q: Is self-monitoring of blood glucose worthwhile in diabetes?
Studies have shown a benefit from self-monitoring of blood glucose (SMBG) in patients using insulin but not in those taking oral antidiabetic drugs. However, the American Diabetes Association recommends that patients with diabetes monitor their glucose once daily if they are being treated with noninsulin therapy and at least three times daily if they are taking insulin.3
Guidelines from the American Association of Clinical Endocrinologists (AACE) state that patients taking noninsulin or once-daily insulin therapy who have not achieved A1C targets should monitor at least twice daily, while those at target should monitor at least once daily. Those taking multiple daily injections should perform SMBG at least three times per day. If patients experience frequent hypoglycemia, AACE suggests monitoring glucose more often.4
The A1C test provides the “big picture,” the average daily glucose level during the previous 90 to 120 days, and correlates with end-organ impact. It does not identify glycemic variability, hypoglycemia, or hyperglycemia.
By contrast, SMBG patterns provide day-to-day data that can be used to select and manage glucose control programs and ultimately optimize a patient’s A1C. SMBG provides a measure of the specific pharmacologic impact of medications and, through feedback, allows design and implementation of physiologic insulin-replacement programs.
One example of SMBG is to have patients monitor glucose in pairs (ie, pick a meal each day and do a premeal and two-hour postmeal reading) and ask them to keep a log or download the data from their meter in the office. This type of monitoring can be enlightening and self-empowering for the patient in that it can provide valuable information regarding the glycemic response to the particular meal.
Intensive glycemic management has been shown to reduce the incidence and progression of diabetic complications. However, it is associated with an increase in severe hypoglycemia. This is worrisome for both patients and providers, as severe hypoglycemia has been associated with an increase in risk for mortality. SMBG can assist patients in understanding how their lifestyle affects their diabetes, as well as identifying hypoglycemia for those who may have hypoglycemia unawareness (ie, who lack the relevant symptoms).
Q: What is continuous glucose monitoring (CGM)?
CGM devices give real-time readouts of current glucose levels. They utilize a subcutaneous sensor that is inserted in the abdomen and worn for 3 to 7 days (depending on which device is used). The sensor sends an electronic signal to a receiver worn by the patient.
There are three major CGM devices that have been approved by the FDA and are available for both personal and professional use. Health care providers can purchase the units and have patients wear them for retrospective analysis; this is a reimbursable expense. All available CGM devices measure glucose values in the interstitial fluid. The sensor reads electrical current produced by the same glucose-oxidase reaction that is utilized by glucose meters that patients use to perform fingersticks for home monitoring.
Currently available CGM systems need to be calibrated at least twice daily. Sensor calibration entails the pairing of the fingerstick value with the sensor value from the interstitial space. Calibration confirms sensor accuracy during various points by “teaching” the sensor the glucose value that corresponds with the electrical current signal.
There is a known physiologic lag time that occurs between fingerstick and sensor values. This lag time is typically up to 15 minutes but is increased with rapidly changing glucose values.
Q: What are the benefits of CGM?
Recent studies have shown CGM can improve A1C without increasing the incidence of hypoglycemia.5
CGM systems have both low and high glucose threshold alarms that can be set to alert once the threshold is reached. The newest generation devices can also predict hypoglycemia or hyperglycemia by tracking rate of change, and users can be alerted to a potential event. This would then allow them to take appropriate action, such as consuming food or carbohydrates or taking insulin as necessary. (Before taking any action, the glucose should first be confirmed by SMBG.)
Software programs allow for review of glucose data, which can assist in identifying trends not appreciated by typical SMBG testing (such as nocturnal hypoglycemia and meal-time excursions). This allows for adjustment of insulin regimens to reduce the incidence of these events.
Q: Can CGM replace SMBG?
While CGM can provide much more detail regarding glucose trends and patterns, it is not a replacement for SMBG. CGM should not be used as a replacement for SMBG to dose insulin for meal- or activity-related adjustments. All dosing decisions should be based on the SMBG.
Currently, CGM is indicated for patients 18 or older, in conjunction with SMBG for the purpose of improving glycemic control:
• to identify and aid in management of glycemic patterns not recognized with typical SMBG
• to prevent glycemic excursions of hypoglycemia and hyperglycemia.
Its use is supported by ADA and AACE guidelines for glucose monitoring.
Q: Who would benefit from CGM?
Suitable candidates for CGM include those with a high degree of glycemic variability, those with hypoglycemic unawareness, shift workers, patients who use insulin pumps, athletes, and women who are planning to become or are pregnant. Patients should work closely with their health care team and perform regular SMBG.
It has been suggested that patients need comprehensive training and follow-up visits to fully understand the large amount of data that they can be confronted with, in order to fully benefit from these devices.6 While the accuracy is improving, there are a few limitations to this technology, including false alarms. Studies have also shown a positive correlation between sensor wear time (hours per week) and greater reductions in A1C.5
Conclusion
Glucose monitoring is a necessary tool—for patients as well as providers—that assists in identifying how patients’ lifestyles affect their diabetes.
1. American Diabetes Association. Economic costs of diabetes in the US in 2007. Diabetes Care. 2008;31(3):596-615.
2. Hirsch IB, Armstrong D, Bergenstal RM, et al. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM). Diabetes Technol Ther. 2008;10(4):232-246.
3. American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2010;34(suppl 1):S11-S61.
4. American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the management of diabetes mellitus. Endocrin Prac. 2007;13(suppl 1):1-68.
5. Bergenstal RM, Tamberlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320.
6. Fabiato K, Buse J, Duclos M, et al. Clinical experience with continuous glucose monitoring in adults. Diabetes Technol Ther. 2009;11(suppl 1):S93-S103.
1. American Diabetes Association. Economic costs of diabetes in the US in 2007. Diabetes Care. 2008;31(3):596-615.
2. Hirsch IB, Armstrong D, Bergenstal RM, et al. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM). Diabetes Technol Ther. 2008;10(4):232-246.
3. American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2010;34(suppl 1):S11-S61.
4. American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the management of diabetes mellitus. Endocrin Prac. 2007;13(suppl 1):1-68.
5. Bergenstal RM, Tamberlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320.
6. Fabiato K, Buse J, Duclos M, et al. Clinical experience with continuous glucose monitoring in adults. Diabetes Technol Ther. 2009;11(suppl 1):S93-S103.