User login
The Journal at 80 years: ‘Same as it ever was’—sort of
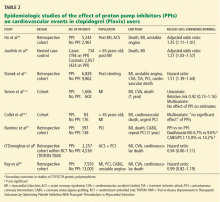
The Journal is currently received by more than 100,000 general internists, cardiologists, hospitalists, and medical subspecialists. It is fully peer-reviewed, listed in MEDLINE, and freely available in complete format at www.ccjm.org. Cleveland Clinic supports the production and distribution of the Journal and provides free CME credits linked to selected articles in an effort to enhance the delivery of high-quality medical care to patients everywhere. The Journal is housed within Cleveland Clinic’s Education Institute, distinct from any direct influence of our marketing or public relations departments—a distinction that I, as editor-in-chief, take extremely seriously. Our primary editorial goal is, and has been, to provide relevant and useful clinical knowledge to the medical community.
The Journal began as the Cleveland Clinic Bulletin in 1931, morphing into the Cleveland Clinic Quarterly the following year and into the Cleveland Clinic Journal of Medicine in 1987. The Quarterly published reprints of papers published elsewhere, as well as case reports and scholarly work presented by Clinic physicians at their staff meetings. Perhaps the latter content was intended to compete with that published in the Proceedings of the Staff Meetings of the Mayo Clinic (first appearing in 1926). Dr. George Crile, one of the founders of Cleveland Clinic, was intent on putting the medical and scientific work of the Clinic in the limelight of American medicine. He felt back in 1931 the same as we feel now, 80 years later, that the Journal contributes to the three pillar missions of the Clinic: better care of the sick, investigation of their problems, and further education of those who serve.
In 1934, the Quarterly ceased publishing reprints from other journals, and Clinic staff were encouraged to publish their case reports, topic reviews, and clinical series in the Quarterly. Over the years, some articles reflected practice at the Clinic and included a description of 1,000 consecutive patients with irritable colon (1941), a description of 10,744 patients who underwent coronary revascularization (1976), and a report of cardiac complications in 951 patients who underwent peripheral vascular surgery (1982). Also notable was the description of the “LE prep test” for the diagnosis of systemic lupus erythematosus (1949).
I am the eighth physician editor-in-chief of the Journal. Three of us have been rheumatologists (perhaps we read more, and cost less). We each have had the opportunity, along with input from the Journal editorial staff, to change the appearance, the content, and sometimes the editorial direction of the Journal. Following the lead of my preceding editor Dr. John Clough and former publisher Linda K. Hengstler, we publish monthly, continue to expand our online content, and publish only peer-reviewed teaching articles and reviews—no longer case reports or original research. We try to address the practical challenges faced by our readers as they strive to deliver quality medical care. We continue to expand our CME offerings, linking with our CME center and working with Dr. Tim Gilligan (our deputy editor) to enhance the educational quality of the Journal-related CME activities.
And always, we emphasize the need for articles to be accurate, timely, relevant to our readership, and perhaps most important, readable. To accomplish the last, our editorial staff includes several talented medical editors and writers—notably Ray Borazanian, Phil Canuto, and Dave Huddleston—who work with our authors on every article we publish. Art directors Joe Pangrace and Ross Papalardo, our medical illustration department, and our production manager Bruce Marich continue to provide the high-quality images that enhance the written word. Our authors and our peer-reviewers include both experienced Clinic staff and nationally recognized clinical content experts.
The Journal in 2011 faces challenges. Advertising income, which has supported a significant portion of our expenses, has decreased, as it has for almost all medical journals. The complicated relationships between industry, academia, physicians, and medical education companies at times strain our ability to provide full disclosure and adequate peer review. The time constraints of authors and the widespread availability of cut-and-paste-ready electronic publications have led us to utilize duplication-recognition software in an effort to limit plagiarism and duplicate publication. The costs and complexity of production and publication of online and print versions of the Journal continue to rise. At the same time, the advances in technology offer the possibility of increased interactivity between reader and content, and we welcome this opportunity. But despite all the challenges, the spirit of the Clinic’s mission to further the education of those who serve is maintained, the same as it ever was.
In this 80th anniversary issue, we kick off a series of articles on the overall care of patients with cancer. Coincidentally, the 1931 seminal issue of the Cleveland Clinic Bulletin started with an article on page 1 by Dr. George Crile entitled, “Treatment of malignancy.” Fortunately, the care of patients with cancer in 2011 is not the same as it was in 1931.
As we start our 80th anniversary year, I offer our sincere wishes to all of you, our readers, for a year of peace and good health.
“Once in a Lifetime” is a song by Talking Heads, from their album Remain in Light. Written by David Byrne, Brian Eno, Chris Frantz, Jerry Harrison, and Tina Weymouth, it was named one of the 100 most important American musical works of the 20th century by National Public Radio. It made #14 in the UK charts and #31 in the Netherlands (Wikipedia).
The Journal is currently received by more than 100,000 general internists, cardiologists, hospitalists, and medical subspecialists. It is fully peer-reviewed, listed in MEDLINE, and freely available in complete format at www.ccjm.org. Cleveland Clinic supports the production and distribution of the Journal and provides free CME credits linked to selected articles in an effort to enhance the delivery of high-quality medical care to patients everywhere. The Journal is housed within Cleveland Clinic’s Education Institute, distinct from any direct influence of our marketing or public relations departments—a distinction that I, as editor-in-chief, take extremely seriously. Our primary editorial goal is, and has been, to provide relevant and useful clinical knowledge to the medical community.
The Journal began as the Cleveland Clinic Bulletin in 1931, morphing into the Cleveland Clinic Quarterly the following year and into the Cleveland Clinic Journal of Medicine in 1987. The Quarterly published reprints of papers published elsewhere, as well as case reports and scholarly work presented by Clinic physicians at their staff meetings. Perhaps the latter content was intended to compete with that published in the Proceedings of the Staff Meetings of the Mayo Clinic (first appearing in 1926). Dr. George Crile, one of the founders of Cleveland Clinic, was intent on putting the medical and scientific work of the Clinic in the limelight of American medicine. He felt back in 1931 the same as we feel now, 80 years later, that the Journal contributes to the three pillar missions of the Clinic: better care of the sick, investigation of their problems, and further education of those who serve.
In 1934, the Quarterly ceased publishing reprints from other journals, and Clinic staff were encouraged to publish their case reports, topic reviews, and clinical series in the Quarterly. Over the years, some articles reflected practice at the Clinic and included a description of 1,000 consecutive patients with irritable colon (1941), a description of 10,744 patients who underwent coronary revascularization (1976), and a report of cardiac complications in 951 patients who underwent peripheral vascular surgery (1982). Also notable was the description of the “LE prep test” for the diagnosis of systemic lupus erythematosus (1949).
I am the eighth physician editor-in-chief of the Journal. Three of us have been rheumatologists (perhaps we read more, and cost less). We each have had the opportunity, along with input from the Journal editorial staff, to change the appearance, the content, and sometimes the editorial direction of the Journal. Following the lead of my preceding editor Dr. John Clough and former publisher Linda K. Hengstler, we publish monthly, continue to expand our online content, and publish only peer-reviewed teaching articles and reviews—no longer case reports or original research. We try to address the practical challenges faced by our readers as they strive to deliver quality medical care. We continue to expand our CME offerings, linking with our CME center and working with Dr. Tim Gilligan (our deputy editor) to enhance the educational quality of the Journal-related CME activities.
And always, we emphasize the need for articles to be accurate, timely, relevant to our readership, and perhaps most important, readable. To accomplish the last, our editorial staff includes several talented medical editors and writers—notably Ray Borazanian, Phil Canuto, and Dave Huddleston—who work with our authors on every article we publish. Art directors Joe Pangrace and Ross Papalardo, our medical illustration department, and our production manager Bruce Marich continue to provide the high-quality images that enhance the written word. Our authors and our peer-reviewers include both experienced Clinic staff and nationally recognized clinical content experts.
The Journal in 2011 faces challenges. Advertising income, which has supported a significant portion of our expenses, has decreased, as it has for almost all medical journals. The complicated relationships between industry, academia, physicians, and medical education companies at times strain our ability to provide full disclosure and adequate peer review. The time constraints of authors and the widespread availability of cut-and-paste-ready electronic publications have led us to utilize duplication-recognition software in an effort to limit plagiarism and duplicate publication. The costs and complexity of production and publication of online and print versions of the Journal continue to rise. At the same time, the advances in technology offer the possibility of increased interactivity between reader and content, and we welcome this opportunity. But despite all the challenges, the spirit of the Clinic’s mission to further the education of those who serve is maintained, the same as it ever was.
In this 80th anniversary issue, we kick off a series of articles on the overall care of patients with cancer. Coincidentally, the 1931 seminal issue of the Cleveland Clinic Bulletin started with an article on page 1 by Dr. George Crile entitled, “Treatment of malignancy.” Fortunately, the care of patients with cancer in 2011 is not the same as it was in 1931.
As we start our 80th anniversary year, I offer our sincere wishes to all of you, our readers, for a year of peace and good health.
“Once in a Lifetime” is a song by Talking Heads, from their album Remain in Light. Written by David Byrne, Brian Eno, Chris Frantz, Jerry Harrison, and Tina Weymouth, it was named one of the 100 most important American musical works of the 20th century by National Public Radio. It made #14 in the UK charts and #31 in the Netherlands (Wikipedia).
The Journal is currently received by more than 100,000 general internists, cardiologists, hospitalists, and medical subspecialists. It is fully peer-reviewed, listed in MEDLINE, and freely available in complete format at www.ccjm.org. Cleveland Clinic supports the production and distribution of the Journal and provides free CME credits linked to selected articles in an effort to enhance the delivery of high-quality medical care to patients everywhere. The Journal is housed within Cleveland Clinic’s Education Institute, distinct from any direct influence of our marketing or public relations departments—a distinction that I, as editor-in-chief, take extremely seriously. Our primary editorial goal is, and has been, to provide relevant and useful clinical knowledge to the medical community.
The Journal began as the Cleveland Clinic Bulletin in 1931, morphing into the Cleveland Clinic Quarterly the following year and into the Cleveland Clinic Journal of Medicine in 1987. The Quarterly published reprints of papers published elsewhere, as well as case reports and scholarly work presented by Clinic physicians at their staff meetings. Perhaps the latter content was intended to compete with that published in the Proceedings of the Staff Meetings of the Mayo Clinic (first appearing in 1926). Dr. George Crile, one of the founders of Cleveland Clinic, was intent on putting the medical and scientific work of the Clinic in the limelight of American medicine. He felt back in 1931 the same as we feel now, 80 years later, that the Journal contributes to the three pillar missions of the Clinic: better care of the sick, investigation of their problems, and further education of those who serve.
In 1934, the Quarterly ceased publishing reprints from other journals, and Clinic staff were encouraged to publish their case reports, topic reviews, and clinical series in the Quarterly. Over the years, some articles reflected practice at the Clinic and included a description of 1,000 consecutive patients with irritable colon (1941), a description of 10,744 patients who underwent coronary revascularization (1976), and a report of cardiac complications in 951 patients who underwent peripheral vascular surgery (1982). Also notable was the description of the “LE prep test” for the diagnosis of systemic lupus erythematosus (1949).
I am the eighth physician editor-in-chief of the Journal. Three of us have been rheumatologists (perhaps we read more, and cost less). We each have had the opportunity, along with input from the Journal editorial staff, to change the appearance, the content, and sometimes the editorial direction of the Journal. Following the lead of my preceding editor Dr. John Clough and former publisher Linda K. Hengstler, we publish monthly, continue to expand our online content, and publish only peer-reviewed teaching articles and reviews—no longer case reports or original research. We try to address the practical challenges faced by our readers as they strive to deliver quality medical care. We continue to expand our CME offerings, linking with our CME center and working with Dr. Tim Gilligan (our deputy editor) to enhance the educational quality of the Journal-related CME activities.
And always, we emphasize the need for articles to be accurate, timely, relevant to our readership, and perhaps most important, readable. To accomplish the last, our editorial staff includes several talented medical editors and writers—notably Ray Borazanian, Phil Canuto, and Dave Huddleston—who work with our authors on every article we publish. Art directors Joe Pangrace and Ross Papalardo, our medical illustration department, and our production manager Bruce Marich continue to provide the high-quality images that enhance the written word. Our authors and our peer-reviewers include both experienced Clinic staff and nationally recognized clinical content experts.
The Journal in 2011 faces challenges. Advertising income, which has supported a significant portion of our expenses, has decreased, as it has for almost all medical journals. The complicated relationships between industry, academia, physicians, and medical education companies at times strain our ability to provide full disclosure and adequate peer review. The time constraints of authors and the widespread availability of cut-and-paste-ready electronic publications have led us to utilize duplication-recognition software in an effort to limit plagiarism and duplicate publication. The costs and complexity of production and publication of online and print versions of the Journal continue to rise. At the same time, the advances in technology offer the possibility of increased interactivity between reader and content, and we welcome this opportunity. But despite all the challenges, the spirit of the Clinic’s mission to further the education of those who serve is maintained, the same as it ever was.
In this 80th anniversary issue, we kick off a series of articles on the overall care of patients with cancer. Coincidentally, the 1931 seminal issue of the Cleveland Clinic Bulletin started with an article on page 1 by Dr. George Crile entitled, “Treatment of malignancy.” Fortunately, the care of patients with cancer in 2011 is not the same as it was in 1931.
As we start our 80th anniversary year, I offer our sincere wishes to all of you, our readers, for a year of peace and good health.
“Once in a Lifetime” is a song by Talking Heads, from their album Remain in Light. Written by David Byrne, Brian Eno, Chris Frantz, Jerry Harrison, and Tina Weymouth, it was named one of the 100 most important American musical works of the 20th century by National Public Radio. It made #14 in the UK charts and #31 in the Netherlands (Wikipedia).
To better manage cancer symptoms
A striking change in cancer medicine over the past several decades has been the rising amplitude of the voices of cancer patients and survivors and their loved ones. Increasingly, they have organized as advocates for better cancer treatment, better research, and better attention to the experience of those directly affected by cancer.
At the same time, despite a somewhat delirious period in the 1970s when it was expected that cancer could be cured, or at least that the mortality rate could be cut in half, it has become clear that progress in eliminating the disease is a long, slow slog with no guarantee of success. Nowhere is this lack of major progress clearer than in the US Food and Drug Administration’s decision a few years ago to approve a drug for pancreatic cancer based on a 10-day increase in median survival.1
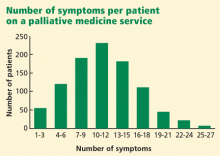
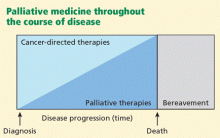
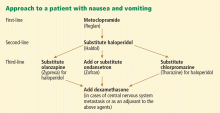
These two factors, the rising voices of those affected by cancer and the failure of cancer research to deliver a cure, have helped fuel a dramatic increase in the attention paid to the symptoms caused by cancer and its treatments. Improving quality of life has become recognized as an additional important goal worthy of rigorous study. Alleviating symptoms is often the most we have to offer patients with advanced cancer, and palliative medicine services are now found at many if not all major cancer centers. To help ensure a supply of well-trained palliative medicine doctors, accredited palliative medicine fellowships have been started at institutions around the country. These programs produce physicians with a higher level of expertise in managing pain, nausea, constipation, fatigue, psychosocial distress, dyspnea, and a wide variety of other symptoms. And while the needs of cancer patients have helped accelerate the growth of palliative medicine, the specialty has a role to play with almost any patient with intractable symptoms, regardless of the nature of the underlying disease.
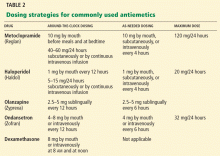
With the growing recognition of a need to better manage cancer patients’ symptoms, research in palliative care has grown rapidly, and an evidence-based approach to symptom management has become possible. Meanwhile, a variety of substantial advances has occurred. For instance, modern antiemetics have dramatically reduced chemotherapy-related vomiting, and long-acting narcotics have allowed patients to achieve better pain control with milder side effects.
In other areas such as cancer-related fatigue or chemotherapy-induced neuropathy, there is very limited evidence that our interventions are effective at alleviating symptoms or improving quality of life. In these and a number of other areas, more research and better treatments are urgently needed.
In order to keep our readers up to date on the progress that is being made in palliative medicine for cancer patients, the Cleveland Clinic Journal of Medicine is presenting a series of articles on the topic. The series begins this issue with an article on the principles of symptom management and a review of the current state of knowledge about alleviating fatigue, nausea, constipation, and dyspnea. Future articles will focus on pain and bowel obstruction.
Our goal is to give our readers practical information that will help them provide better symptom management for their patients, particularly their cancer patients. This series will deal with one of the most important problems of cancer medicine.
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25:1960–1966.
A striking change in cancer medicine over the past several decades has been the rising amplitude of the voices of cancer patients and survivors and their loved ones. Increasingly, they have organized as advocates for better cancer treatment, better research, and better attention to the experience of those directly affected by cancer.
At the same time, despite a somewhat delirious period in the 1970s when it was expected that cancer could be cured, or at least that the mortality rate could be cut in half, it has become clear that progress in eliminating the disease is a long, slow slog with no guarantee of success. Nowhere is this lack of major progress clearer than in the US Food and Drug Administration’s decision a few years ago to approve a drug for pancreatic cancer based on a 10-day increase in median survival.1
These two factors, the rising voices of those affected by cancer and the failure of cancer research to deliver a cure, have helped fuel a dramatic increase in the attention paid to the symptoms caused by cancer and its treatments. Improving quality of life has become recognized as an additional important goal worthy of rigorous study. Alleviating symptoms is often the most we have to offer patients with advanced cancer, and palliative medicine services are now found at many if not all major cancer centers. To help ensure a supply of well-trained palliative medicine doctors, accredited palliative medicine fellowships have been started at institutions around the country. These programs produce physicians with a higher level of expertise in managing pain, nausea, constipation, fatigue, psychosocial distress, dyspnea, and a wide variety of other symptoms. And while the needs of cancer patients have helped accelerate the growth of palliative medicine, the specialty has a role to play with almost any patient with intractable symptoms, regardless of the nature of the underlying disease.
With the growing recognition of a need to better manage cancer patients’ symptoms, research in palliative care has grown rapidly, and an evidence-based approach to symptom management has become possible. Meanwhile, a variety of substantial advances has occurred. For instance, modern antiemetics have dramatically reduced chemotherapy-related vomiting, and long-acting narcotics have allowed patients to achieve better pain control with milder side effects.
In other areas such as cancer-related fatigue or chemotherapy-induced neuropathy, there is very limited evidence that our interventions are effective at alleviating symptoms or improving quality of life. In these and a number of other areas, more research and better treatments are urgently needed.
In order to keep our readers up to date on the progress that is being made in palliative medicine for cancer patients, the Cleveland Clinic Journal of Medicine is presenting a series of articles on the topic. The series begins this issue with an article on the principles of symptom management and a review of the current state of knowledge about alleviating fatigue, nausea, constipation, and dyspnea. Future articles will focus on pain and bowel obstruction.
Our goal is to give our readers practical information that will help them provide better symptom management for their patients, particularly their cancer patients. This series will deal with one of the most important problems of cancer medicine.
A striking change in cancer medicine over the past several decades has been the rising amplitude of the voices of cancer patients and survivors and their loved ones. Increasingly, they have organized as advocates for better cancer treatment, better research, and better attention to the experience of those directly affected by cancer.
At the same time, despite a somewhat delirious period in the 1970s when it was expected that cancer could be cured, or at least that the mortality rate could be cut in half, it has become clear that progress in eliminating the disease is a long, slow slog with no guarantee of success. Nowhere is this lack of major progress clearer than in the US Food and Drug Administration’s decision a few years ago to approve a drug for pancreatic cancer based on a 10-day increase in median survival.1
These two factors, the rising voices of those affected by cancer and the failure of cancer research to deliver a cure, have helped fuel a dramatic increase in the attention paid to the symptoms caused by cancer and its treatments. Improving quality of life has become recognized as an additional important goal worthy of rigorous study. Alleviating symptoms is often the most we have to offer patients with advanced cancer, and palliative medicine services are now found at many if not all major cancer centers. To help ensure a supply of well-trained palliative medicine doctors, accredited palliative medicine fellowships have been started at institutions around the country. These programs produce physicians with a higher level of expertise in managing pain, nausea, constipation, fatigue, psychosocial distress, dyspnea, and a wide variety of other symptoms. And while the needs of cancer patients have helped accelerate the growth of palliative medicine, the specialty has a role to play with almost any patient with intractable symptoms, regardless of the nature of the underlying disease.
With the growing recognition of a need to better manage cancer patients’ symptoms, research in palliative care has grown rapidly, and an evidence-based approach to symptom management has become possible. Meanwhile, a variety of substantial advances has occurred. For instance, modern antiemetics have dramatically reduced chemotherapy-related vomiting, and long-acting narcotics have allowed patients to achieve better pain control with milder side effects.
In other areas such as cancer-related fatigue or chemotherapy-induced neuropathy, there is very limited evidence that our interventions are effective at alleviating symptoms or improving quality of life. In these and a number of other areas, more research and better treatments are urgently needed.
In order to keep our readers up to date on the progress that is being made in palliative medicine for cancer patients, the Cleveland Clinic Journal of Medicine is presenting a series of articles on the topic. The series begins this issue with an article on the principles of symptom management and a review of the current state of knowledge about alleviating fatigue, nausea, constipation, and dyspnea. Future articles will focus on pain and bowel obstruction.
Our goal is to give our readers practical information that will help them provide better symptom management for their patients, particularly their cancer patients. This series will deal with one of the most important problems of cancer medicine.
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25:1960–1966.
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25:1960–1966.
Do patients with prosthetic joints require dental antimicrobial prophylaxis?
We believe the available evidence does not support routine antimicrobial prophylaxis before dental procedures in patients who have undergone total joint replacement, even though the practice is very common1 and even though professional societies recommend it in patients at high risk,2 or even in all patients.3
On the other hand, good oral hygiene prevents dental disease and decreases the frequency of bacteremia from routine daily activities, and thus should be especially encouraged in patients with prosthetic joints or in those undergoing total joint arthroplasty.
AN UNCOMMON BUT SERIOUS PROBLEM
By 2030, an estimated 4 million total hip or total knee replacements per year will be performed in the United States.4 Most patients have a satisfactory outcome, but in a small percentage the prosthesis fails prematurely.
Prosthetic joint infection is the second most common cause of prosthetic failure leading to loss of joint function, after aseptic loosening.5 Its treatment often requires removal of the infected prosthesis and prolonged intravenous antimicrobial therapy. The cost incurred with each episode of prosthetic joint infection is estimated to exceed $50,000.1
Because of the morbidity and substantial cost associated with managing this condition, investigators have focused on identifying preventable risk factors for it.
RISK FACTORS FOR PROSTHETIC JOINT INFECTION
Factors associated with a higher risk of prosthetic joint infection include prior joint surgery, failure to give antimicrobial prophylaxis during surgery, immunosuppression, perioperative wound complications, a high American Society of Anesthesiologists (ASA) score, prolonged operative time, and a history of prosthetic joint infection.6,7 The primary predisposing factors are related to the foreign body itself and to the opportunities for and the degree of exposure of the prosthesis to microorganisms during surgery. Bacteremia, especially with Staphylococcus aureus, has been recognized as a risk factor for hematogenous prosthetic joint infection.6
Whether dental procedures pose a risk of prosthetic joint infection has been debated for decades. Common daily activities such as toothbrushing and chewing can cause transient bacteremia in up to 40% of episodes.8
Extrapolating from the guidelines for preventing endocarditis, the American Dental Association (ADA)2 and the American Academy of Orthopaedic Surgeons (AAOS)3 have issued guidelines favoring antimicrobial prophylaxis in patients with prosthetic joints. However, given the significant differences in the pathophysiology, microbiology, and anatomy of infection between infective endocarditis and prosthetic joint infection, extrapolating the recommendations may not be valid.
MICROBIOLOGY OF PROSTHETIC JOINT INFECTION AND DENTAL FLORA
Staphylococci, the most common cause of prosthetic joint infection, are uncommon commensals of the oral flora and have been rarely implicated in bacteremia occurring after dental procedures.9 In contrast, viridans-group streptococci constitute most of the facultative oral flora and are the most common cause of transient bacteremia after dental procedures that result in trauma to the gingival or oral mucosa.10 However, viridans-group streptococci account for only 2% of all hematogenous prosthetic joint infections.9
DO DENTAL PROCEDURES INCREASE THE RISK OF PROSTHETIC JOINT INFECTION?
Prolonged or high-grade bacteremia is associated with prosthetic joint infection. On the other hand, data are scant on the association between low-grade or transient bacteremia and prosthetic joint infection.
After dental procedures, bacteria can be found in the blood, but at much lower levels (< 104 cfu/mL) than that needed for hematogenous seeding of prostheses in animal studies (3–5 × 108 cfu/mL).11 Transient, low-grade bacteremia occurs not only after dental procedures but also, as mentioned, after common activities such as chewing, brushing, or flossing.1 The cumulative exposure to transient bacteremia through these daily activities is several times higher than the single exposure that a person is subjected to during dental procedures.12
WHAT IS THE EVIDENCE?
Most of the current evidence linking dental procedures or dental manipulation to prosthetic joint infection is based on reports of single cases of infections that occurred after dental procedures.
In two retrospective reviews, late hematogenous prosthetic joint infection associated with a dental source occurred after 0.2% of primary knee arthroplasties11 and 6% of primary hip arthroplasties.13
Ainscow and Denham14 followed 1,000 patients who underwent total joint replacement over 6 years. Of these, 226 subsequently underwent dental procedures without receiving antimicrobial prophylaxis, and none developed a prosthetic joint infection.
In a recently published case-control study,1 our group assessed 339 patients with prosthetic joint infection and 339 patients with prosthetic joints that did not become infected. In this study, neither low-risk nor high-risk dental procedures were associated with an increased risk of prosthetic knee or hip infections (odds ratio [OR] 0.8; 95% confidence interval [CI] 0.4–1.6). Moreover, prophylactic use of antimicrobials before dental procedures was not associated with a lower risk.
However, a factor that was associated with a lower risk of prosthetic joint infection was good oral hygiene (OR 0.7; 95% CI 0.5–1.03). Good oral hygiene and prevention of dental disease could potentially decrease the frequency of bacteremia from daily activities and may even protect against prosthetic joint infection. Further study of the association of poor dental health and the risk of prosthetic joint infection is warranted.
GUIDELINES AND RECOMMENDATIONS
Despite the lack of evidence suggesting an association between prosthetic joint infection and dental procedure, surveys of orthopedists, dentists, infectious disease specialists, and other health care professionals show that a significant number of them recommend antimicrobial prophylaxis for patients with a prosthetic joint prior to a dental procedure.1
In 2003, a consensus panel of the AAOS and the ADA recommended routine consideration of antimicrobial prophylaxis in patients at high risk due to both patient factors and the type of dental procedure.2 Patient factors thought to confer high risk are immunosuppression, diabetes, malnourishment, human immunodeficiency virus infection, prior prosthetic joint infection, hemophilia, malignancy, and a prosthesis less than 2 years old. High-risk dental procedures are tooth extractions, periodontal procedures, root canal surgery, and dental cleaning in which bleeding is anticipated.
In a recent statement, the AAOS recommended antimicrobial prophylaxis in all patients with prosthetic joints.3
Concerns about promoting antimicrobial resistance and about adverse reactions from antimicrobial use may outweigh any hypothetic benefit related to prevention of prosthetic joint infection. Analyses of cost, risks, and benefits argue against this practice.3
In summary, the current evidence does not support the use of antimicrobial therapy to prevent prosthetic joint infection in patients with total joint replacement undergoing dental procedures. However, good oral hygiene should be encouraged to prevent dental disease and to decrease the frequency of bacteremia from routine daily activities in patients who have undergone or will be undergoing total joint arthroplasty.
- Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case-control study. Clin Infect Dis 2010; 50:8–16.
- American Dental Association. Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc 2003; 134:895–899.
- American Academy of Orthopaedic Surgeons. Information statement: antibiotic prophylaxis for bacteremia in patients with joint replacements. http://www.aaos.org/about/papers/advistmt/1033.asp. Accessed October 28, 2010.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89:780–785.
- Roberts VI, Esler CN, Harper WM. A 15-year follow-up study of 4606 primary total knee replacements. J Bone Joint Surg Br 2007; 89:1452–1456.
- Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med 2009; 361:787–794.
- Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis 1998; 27:1247–1254.
- Durack DT. Prevention of infective endocarditis. N Engl J Med 1995; 332:38–44.
- Deacon JM, Pagliaro AJ, Zelicof SB, Horowitz HW. Prophylactic use of antibiotics for procedures after total joint replacement. Bone Joint Surg Am 1996; 78:1755–1770.
- Kaye D. Infective endocarditis. In:Rose LF, Kaye D, editors. Internal Medicine for Dentistry, 2nd ed. Mosby: St. Louis, MO; 1990:156–161.
- Waldman BJ, Mont MA, Hungerford DS. Total knee arthroplasty infections associated with dental procedures. Clin Orthop Relat Res 1997; 343:164–172.
- Guntheroth WG. How important are dental procedures as a cause of infective endocarditis? Am J Cardiol 1984; 54:797–801.
- LaPorte DM, Waldman BJ, Mont MA, Hungerford DS. Infections associated with dental procedures in total hip arthroplasty. J Bone Joint Surg Br 1999; 81:56–59.
- Ainscow DA, Denham RA. The risk of haematogenous infection in total joint replacements. J Bone Joint Surg Br 1984; 66:580–582.
We believe the available evidence does not support routine antimicrobial prophylaxis before dental procedures in patients who have undergone total joint replacement, even though the practice is very common1 and even though professional societies recommend it in patients at high risk,2 or even in all patients.3
On the other hand, good oral hygiene prevents dental disease and decreases the frequency of bacteremia from routine daily activities, and thus should be especially encouraged in patients with prosthetic joints or in those undergoing total joint arthroplasty.
AN UNCOMMON BUT SERIOUS PROBLEM
By 2030, an estimated 4 million total hip or total knee replacements per year will be performed in the United States.4 Most patients have a satisfactory outcome, but in a small percentage the prosthesis fails prematurely.
Prosthetic joint infection is the second most common cause of prosthetic failure leading to loss of joint function, after aseptic loosening.5 Its treatment often requires removal of the infected prosthesis and prolonged intravenous antimicrobial therapy. The cost incurred with each episode of prosthetic joint infection is estimated to exceed $50,000.1
Because of the morbidity and substantial cost associated with managing this condition, investigators have focused on identifying preventable risk factors for it.
RISK FACTORS FOR PROSTHETIC JOINT INFECTION
Factors associated with a higher risk of prosthetic joint infection include prior joint surgery, failure to give antimicrobial prophylaxis during surgery, immunosuppression, perioperative wound complications, a high American Society of Anesthesiologists (ASA) score, prolonged operative time, and a history of prosthetic joint infection.6,7 The primary predisposing factors are related to the foreign body itself and to the opportunities for and the degree of exposure of the prosthesis to microorganisms during surgery. Bacteremia, especially with Staphylococcus aureus, has been recognized as a risk factor for hematogenous prosthetic joint infection.6
Whether dental procedures pose a risk of prosthetic joint infection has been debated for decades. Common daily activities such as toothbrushing and chewing can cause transient bacteremia in up to 40% of episodes.8
Extrapolating from the guidelines for preventing endocarditis, the American Dental Association (ADA)2 and the American Academy of Orthopaedic Surgeons (AAOS)3 have issued guidelines favoring antimicrobial prophylaxis in patients with prosthetic joints. However, given the significant differences in the pathophysiology, microbiology, and anatomy of infection between infective endocarditis and prosthetic joint infection, extrapolating the recommendations may not be valid.
MICROBIOLOGY OF PROSTHETIC JOINT INFECTION AND DENTAL FLORA
Staphylococci, the most common cause of prosthetic joint infection, are uncommon commensals of the oral flora and have been rarely implicated in bacteremia occurring after dental procedures.9 In contrast, viridans-group streptococci constitute most of the facultative oral flora and are the most common cause of transient bacteremia after dental procedures that result in trauma to the gingival or oral mucosa.10 However, viridans-group streptococci account for only 2% of all hematogenous prosthetic joint infections.9
DO DENTAL PROCEDURES INCREASE THE RISK OF PROSTHETIC JOINT INFECTION?
Prolonged or high-grade bacteremia is associated with prosthetic joint infection. On the other hand, data are scant on the association between low-grade or transient bacteremia and prosthetic joint infection.
After dental procedures, bacteria can be found in the blood, but at much lower levels (< 104 cfu/mL) than that needed for hematogenous seeding of prostheses in animal studies (3–5 × 108 cfu/mL).11 Transient, low-grade bacteremia occurs not only after dental procedures but also, as mentioned, after common activities such as chewing, brushing, or flossing.1 The cumulative exposure to transient bacteremia through these daily activities is several times higher than the single exposure that a person is subjected to during dental procedures.12
WHAT IS THE EVIDENCE?
Most of the current evidence linking dental procedures or dental manipulation to prosthetic joint infection is based on reports of single cases of infections that occurred after dental procedures.
In two retrospective reviews, late hematogenous prosthetic joint infection associated with a dental source occurred after 0.2% of primary knee arthroplasties11 and 6% of primary hip arthroplasties.13
Ainscow and Denham14 followed 1,000 patients who underwent total joint replacement over 6 years. Of these, 226 subsequently underwent dental procedures without receiving antimicrobial prophylaxis, and none developed a prosthetic joint infection.
In a recently published case-control study,1 our group assessed 339 patients with prosthetic joint infection and 339 patients with prosthetic joints that did not become infected. In this study, neither low-risk nor high-risk dental procedures were associated with an increased risk of prosthetic knee or hip infections (odds ratio [OR] 0.8; 95% confidence interval [CI] 0.4–1.6). Moreover, prophylactic use of antimicrobials before dental procedures was not associated with a lower risk.
However, a factor that was associated with a lower risk of prosthetic joint infection was good oral hygiene (OR 0.7; 95% CI 0.5–1.03). Good oral hygiene and prevention of dental disease could potentially decrease the frequency of bacteremia from daily activities and may even protect against prosthetic joint infection. Further study of the association of poor dental health and the risk of prosthetic joint infection is warranted.
GUIDELINES AND RECOMMENDATIONS
Despite the lack of evidence suggesting an association between prosthetic joint infection and dental procedure, surveys of orthopedists, dentists, infectious disease specialists, and other health care professionals show that a significant number of them recommend antimicrobial prophylaxis for patients with a prosthetic joint prior to a dental procedure.1
In 2003, a consensus panel of the AAOS and the ADA recommended routine consideration of antimicrobial prophylaxis in patients at high risk due to both patient factors and the type of dental procedure.2 Patient factors thought to confer high risk are immunosuppression, diabetes, malnourishment, human immunodeficiency virus infection, prior prosthetic joint infection, hemophilia, malignancy, and a prosthesis less than 2 years old. High-risk dental procedures are tooth extractions, periodontal procedures, root canal surgery, and dental cleaning in which bleeding is anticipated.
In a recent statement, the AAOS recommended antimicrobial prophylaxis in all patients with prosthetic joints.3
Concerns about promoting antimicrobial resistance and about adverse reactions from antimicrobial use may outweigh any hypothetic benefit related to prevention of prosthetic joint infection. Analyses of cost, risks, and benefits argue against this practice.3
In summary, the current evidence does not support the use of antimicrobial therapy to prevent prosthetic joint infection in patients with total joint replacement undergoing dental procedures. However, good oral hygiene should be encouraged to prevent dental disease and to decrease the frequency of bacteremia from routine daily activities in patients who have undergone or will be undergoing total joint arthroplasty.
We believe the available evidence does not support routine antimicrobial prophylaxis before dental procedures in patients who have undergone total joint replacement, even though the practice is very common1 and even though professional societies recommend it in patients at high risk,2 or even in all patients.3
On the other hand, good oral hygiene prevents dental disease and decreases the frequency of bacteremia from routine daily activities, and thus should be especially encouraged in patients with prosthetic joints or in those undergoing total joint arthroplasty.
AN UNCOMMON BUT SERIOUS PROBLEM
By 2030, an estimated 4 million total hip or total knee replacements per year will be performed in the United States.4 Most patients have a satisfactory outcome, but in a small percentage the prosthesis fails prematurely.
Prosthetic joint infection is the second most common cause of prosthetic failure leading to loss of joint function, after aseptic loosening.5 Its treatment often requires removal of the infected prosthesis and prolonged intravenous antimicrobial therapy. The cost incurred with each episode of prosthetic joint infection is estimated to exceed $50,000.1
Because of the morbidity and substantial cost associated with managing this condition, investigators have focused on identifying preventable risk factors for it.
RISK FACTORS FOR PROSTHETIC JOINT INFECTION
Factors associated with a higher risk of prosthetic joint infection include prior joint surgery, failure to give antimicrobial prophylaxis during surgery, immunosuppression, perioperative wound complications, a high American Society of Anesthesiologists (ASA) score, prolonged operative time, and a history of prosthetic joint infection.6,7 The primary predisposing factors are related to the foreign body itself and to the opportunities for and the degree of exposure of the prosthesis to microorganisms during surgery. Bacteremia, especially with Staphylococcus aureus, has been recognized as a risk factor for hematogenous prosthetic joint infection.6
Whether dental procedures pose a risk of prosthetic joint infection has been debated for decades. Common daily activities such as toothbrushing and chewing can cause transient bacteremia in up to 40% of episodes.8
Extrapolating from the guidelines for preventing endocarditis, the American Dental Association (ADA)2 and the American Academy of Orthopaedic Surgeons (AAOS)3 have issued guidelines favoring antimicrobial prophylaxis in patients with prosthetic joints. However, given the significant differences in the pathophysiology, microbiology, and anatomy of infection between infective endocarditis and prosthetic joint infection, extrapolating the recommendations may not be valid.
MICROBIOLOGY OF PROSTHETIC JOINT INFECTION AND DENTAL FLORA
Staphylococci, the most common cause of prosthetic joint infection, are uncommon commensals of the oral flora and have been rarely implicated in bacteremia occurring after dental procedures.9 In contrast, viridans-group streptococci constitute most of the facultative oral flora and are the most common cause of transient bacteremia after dental procedures that result in trauma to the gingival or oral mucosa.10 However, viridans-group streptococci account for only 2% of all hematogenous prosthetic joint infections.9
DO DENTAL PROCEDURES INCREASE THE RISK OF PROSTHETIC JOINT INFECTION?
Prolonged or high-grade bacteremia is associated with prosthetic joint infection. On the other hand, data are scant on the association between low-grade or transient bacteremia and prosthetic joint infection.
After dental procedures, bacteria can be found in the blood, but at much lower levels (< 104 cfu/mL) than that needed for hematogenous seeding of prostheses in animal studies (3–5 × 108 cfu/mL).11 Transient, low-grade bacteremia occurs not only after dental procedures but also, as mentioned, after common activities such as chewing, brushing, or flossing.1 The cumulative exposure to transient bacteremia through these daily activities is several times higher than the single exposure that a person is subjected to during dental procedures.12
WHAT IS THE EVIDENCE?
Most of the current evidence linking dental procedures or dental manipulation to prosthetic joint infection is based on reports of single cases of infections that occurred after dental procedures.
In two retrospective reviews, late hematogenous prosthetic joint infection associated with a dental source occurred after 0.2% of primary knee arthroplasties11 and 6% of primary hip arthroplasties.13
Ainscow and Denham14 followed 1,000 patients who underwent total joint replacement over 6 years. Of these, 226 subsequently underwent dental procedures without receiving antimicrobial prophylaxis, and none developed a prosthetic joint infection.
In a recently published case-control study,1 our group assessed 339 patients with prosthetic joint infection and 339 patients with prosthetic joints that did not become infected. In this study, neither low-risk nor high-risk dental procedures were associated with an increased risk of prosthetic knee or hip infections (odds ratio [OR] 0.8; 95% confidence interval [CI] 0.4–1.6). Moreover, prophylactic use of antimicrobials before dental procedures was not associated with a lower risk.
However, a factor that was associated with a lower risk of prosthetic joint infection was good oral hygiene (OR 0.7; 95% CI 0.5–1.03). Good oral hygiene and prevention of dental disease could potentially decrease the frequency of bacteremia from daily activities and may even protect against prosthetic joint infection. Further study of the association of poor dental health and the risk of prosthetic joint infection is warranted.
GUIDELINES AND RECOMMENDATIONS
Despite the lack of evidence suggesting an association between prosthetic joint infection and dental procedure, surveys of orthopedists, dentists, infectious disease specialists, and other health care professionals show that a significant number of them recommend antimicrobial prophylaxis for patients with a prosthetic joint prior to a dental procedure.1
In 2003, a consensus panel of the AAOS and the ADA recommended routine consideration of antimicrobial prophylaxis in patients at high risk due to both patient factors and the type of dental procedure.2 Patient factors thought to confer high risk are immunosuppression, diabetes, malnourishment, human immunodeficiency virus infection, prior prosthetic joint infection, hemophilia, malignancy, and a prosthesis less than 2 years old. High-risk dental procedures are tooth extractions, periodontal procedures, root canal surgery, and dental cleaning in which bleeding is anticipated.
In a recent statement, the AAOS recommended antimicrobial prophylaxis in all patients with prosthetic joints.3
Concerns about promoting antimicrobial resistance and about adverse reactions from antimicrobial use may outweigh any hypothetic benefit related to prevention of prosthetic joint infection. Analyses of cost, risks, and benefits argue against this practice.3
In summary, the current evidence does not support the use of antimicrobial therapy to prevent prosthetic joint infection in patients with total joint replacement undergoing dental procedures. However, good oral hygiene should be encouraged to prevent dental disease and to decrease the frequency of bacteremia from routine daily activities in patients who have undergone or will be undergoing total joint arthroplasty.
- Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case-control study. Clin Infect Dis 2010; 50:8–16.
- American Dental Association. Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc 2003; 134:895–899.
- American Academy of Orthopaedic Surgeons. Information statement: antibiotic prophylaxis for bacteremia in patients with joint replacements. http://www.aaos.org/about/papers/advistmt/1033.asp. Accessed October 28, 2010.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89:780–785.
- Roberts VI, Esler CN, Harper WM. A 15-year follow-up study of 4606 primary total knee replacements. J Bone Joint Surg Br 2007; 89:1452–1456.
- Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med 2009; 361:787–794.
- Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis 1998; 27:1247–1254.
- Durack DT. Prevention of infective endocarditis. N Engl J Med 1995; 332:38–44.
- Deacon JM, Pagliaro AJ, Zelicof SB, Horowitz HW. Prophylactic use of antibiotics for procedures after total joint replacement. Bone Joint Surg Am 1996; 78:1755–1770.
- Kaye D. Infective endocarditis. In:Rose LF, Kaye D, editors. Internal Medicine for Dentistry, 2nd ed. Mosby: St. Louis, MO; 1990:156–161.
- Waldman BJ, Mont MA, Hungerford DS. Total knee arthroplasty infections associated with dental procedures. Clin Orthop Relat Res 1997; 343:164–172.
- Guntheroth WG. How important are dental procedures as a cause of infective endocarditis? Am J Cardiol 1984; 54:797–801.
- LaPorte DM, Waldman BJ, Mont MA, Hungerford DS. Infections associated with dental procedures in total hip arthroplasty. J Bone Joint Surg Br 1999; 81:56–59.
- Ainscow DA, Denham RA. The risk of haematogenous infection in total joint replacements. J Bone Joint Surg Br 1984; 66:580–582.
- Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case-control study. Clin Infect Dis 2010; 50:8–16.
- American Dental Association. Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc 2003; 134:895–899.
- American Academy of Orthopaedic Surgeons. Information statement: antibiotic prophylaxis for bacteremia in patients with joint replacements. http://www.aaos.org/about/papers/advistmt/1033.asp. Accessed October 28, 2010.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89:780–785.
- Roberts VI, Esler CN, Harper WM. A 15-year follow-up study of 4606 primary total knee replacements. J Bone Joint Surg Br 2007; 89:1452–1456.
- Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med 2009; 361:787–794.
- Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis 1998; 27:1247–1254.
- Durack DT. Prevention of infective endocarditis. N Engl J Med 1995; 332:38–44.
- Deacon JM, Pagliaro AJ, Zelicof SB, Horowitz HW. Prophylactic use of antibiotics for procedures after total joint replacement. Bone Joint Surg Am 1996; 78:1755–1770.
- Kaye D. Infective endocarditis. In:Rose LF, Kaye D, editors. Internal Medicine for Dentistry, 2nd ed. Mosby: St. Louis, MO; 1990:156–161.
- Waldman BJ, Mont MA, Hungerford DS. Total knee arthroplasty infections associated with dental procedures. Clin Orthop Relat Res 1997; 343:164–172.
- Guntheroth WG. How important are dental procedures as a cause of infective endocarditis? Am J Cardiol 1984; 54:797–801.
- LaPorte DM, Waldman BJ, Mont MA, Hungerford DS. Infections associated with dental procedures in total hip arthroplasty. J Bone Joint Surg Br 1999; 81:56–59.
- Ainscow DA, Denham RA. The risk of haematogenous infection in total joint replacements. J Bone Joint Surg Br 1984; 66:580–582.
Managing bloodstream infections in patients who have short-term central venous catheters
Vascular catheters are very common in everyday inpatient and, increasingly, outpatient care. Nearly 300 million catheters are estimated to be used annually in the United States, and approximately 3 million of these are central venous catheters (CVCs).1
Although significant gains have been made in preventing CVC-related bloodstream infections, these infections continue to occur, with estimated rates ranging from 1.3 per 1,000 catheter days on inpatient medical-surgical wards to 5.6 per 1,000 catheter days in intensive care burn units.2
CVCs are classified as either long-term or short-term. Long-term CVCs are surgically implanted or tunneled and used for prolonged chemotherapy, home infusion therapy, or hemodialysis. Short-term CVCs do not require surgical implantation. They are more common than long-term CVCs and account for most CVC-related bloodstream infections. Given the frequency of short-term CVC use, a growing number of health care providers from mid-level practitioners to intensivists are faced with deciding how to manage bloodstream infection related to short-term CVCs.
At baseline, management decisions about bloodstream infections from short-term CVCs can be challenging. Questions that regularly arise include:
- Should a potentially infected catheter be removed?
- Which empiric antibiotic therapy should be started pending a microbiologic diagnosis?
- How should therapy be tailored (eg, antibiotic choice and course and whether to remove or retain the catheter) based on the specific pathogen identified?
Adding to the complexity of these decisions are increasingly resistant microorganisms, heterogeneity of affected patient populations, and variability in the quality and availability of evidence.
This review provides a concise guide to managing bloodstream infections related to short-term CVCs in adults, based on updated guidelines from the Infectious Diseases Society of America (IDSA).3
DEFINITION AND DIAGNOSTIC CRITERIA
We have adapted the following definition and diagnostic criteria from the general definition and diagnostic criteria for catheter-related bloodstream infections proposed by the IDSA.
Bloodstream infection related to a short-term CVC is defined as bacteremia or fungemia in a patient with the CVC in place, clinical manifestations of infection (eg, fever, chills, hypotension), and no apparent source of the bloodstream infection aside from the catheter. At least one of the three diagnostic criteria should be met:
- Cultures of the catheter tip and of the peripheral blood grow the same organism. Catheter tip culture should be quantitative, with more than 102 colony-forming units (cfu) per catheter segment, or semiquantitative, with more than 15 cfu per catheter segment.
- Blood drawn from the catheter lumen grows the same organism as blood drawn from a peripheral vein (or less optimally, a different lumen), but at three times the amount by quantitative culture.
- Blood drawn simultaneously from the catheter lumen and from a peripheral vein (or less optimally, a different lumen) grows the same organism, and growth from the CVC lumen sample is detected (by automated blood culture system) at least 2 hours before growth from the peripheral vein sample.
MANAGING BLOODSTREAM INFECTIONS IN PATIENTS WITH SHORT-TERM CVCs
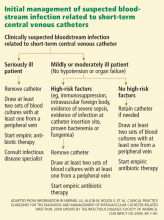
When to remove a potentially infected short-term CVC
In a nonneutropenic intensive care population, Bouza et al4 found that, of 204 episodes of clinically suspected bloodstream infection from a short-term CVC, only 28 (14%) were confirmed to be catheter-related, 27 (13%) were bloodstream infections that were not catheter-related, 36 (18%) involved catheter-tip colonization with negative blood cultures, and the remainder were cases with negative catheter-tip and blood cultures.
Rijnders et al,5 in a study of 100 adult medical-surgical intensive care patients with a clinically suspected bloodstream infection related to a short-term CVC, found only three confirmed cases.
A randomized clinical trial comparing early removal of short-term CVCs and watchful waiting in an adult intensive care population with clinically suspected bloodstream infections showed no difference between treatment groups in length of stay in the intensive care unit or in the mortality rate.6 This trial included a low-risk subset of adult medical-surgical intensive care patients (ie, immunocompetent, no intravascular foreign body, no evidence of severe sepsis or septic shock, no evidence of infection at the catheter insertion site, no proven bacteremia or fungemia). These results suggest that a similar subset of patients can be safely monitored without catheter removal while being assessed for possible catheter-related bloodstream infection.
Empiric catheter removal vs watchful waiting has not and likely will not be studied in higher-risk populations. In this group, clinical judgment should outweigh any specific management algorithm. In patients who are in shock or who are otherwise hemodynamically unstable, early catheter removal should be a priority; however, in some circumstances the risks of immediate catheter removal (eg, coagulopathy with risk of bleeding diathesis, or lack of site to replace the catheter) may outweigh the potential benefits.
Empiric antibiotic therapy for bloodstream infection from a short-term CVC
In order of prevalence, the four most common pathogens are coagulase-negative staphylococci, Staphylococcus aureus, Candida species, and enteric gram-negative bacilli.7
Gram-positive pathogens. A recent randomized clinical trial comparing vancomycin and linezolid (Zyvox) treatment for CVC-related bloodstream infections showed that 89 (57%) of 157 S aureus isolates and 95 (80%) of 119 coagulase-negative staphylococcal isolates were resistant to methicillin.8 Given the prevalence of gram-positive infections and the regularity of methicillin-resistant isolates, vancomycin should be started empirically in cases of suspected bloodstream infection related to short-term CVCs. In institutions where methicillin-resistant S aureus (MRSA) isolates regularly have a vancomycin minimum inhibitory concentration (MIC) of greater than 2 μg/mL, an alternative agent such as daptomycin (Cubicin) should be used.9,10
Gram-negative pathogens. Infections due to resistant gram-negative pathogens have become more common in the past 10 years.11,12 Prospective cohort studies have shown that resistant gram-negative infections and inadequate empiric antimicrobial therapy of bloodstream infections independently predict the risk of death.13,14 Risk factors for resistant gram-negative infections include critical illness, neutropenia, prior antibiotic therapy, and femoral insertion of the CVC.15–18 Patients with these risk factors should receive empiric antibiotic therapy for gram-negative bacilli.
No randomized controlled trial has been done to guide the choice of empiric gram-negative antibiotic coverage. The initial choice should be based on local antimicrobial patterns and susceptibility data and on the severity of the patient’s illness. Initial options include fourth-generation cephalosporins, carbapenems, or combined beta-lactam and beta-lactamase inhibitors. Patients with neutropenia, severe sepsis, or known multiple-drug-resistant gram-negative bacilli colonization or prior infection should receive empiric combination therapy with two different classes of antibiotics.
Candida. Risk factors for CVC-related bloodstream infections due to Candida species include total parenteral nutrition, prolonged use of broad-spectrum antibiotics, hematologic malignancy, solid organ or bone marrow transplantation, colonization with Candida species at multiple sites, and femoral catheter insertion. Empiric treatment with an echinocandin is recommended for patients with these risk factors. Fluconazole (Diflucan) can be substituted for an echinocandin in patients without azole exposure in the previous 3 months and in settings where the prevalence of Candida krusei and Candida glabrata is low.
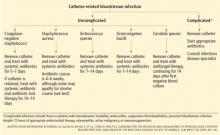
PATHOGEN-SPECIFIC MANAGEMENT: RECOMMENDATIONS
Coagulase-negative staphylococci
Most patients with coagulase-negative staphylococcal infections have a benign clinical course.
Although no randomized trial has evaluated different treatment approaches, most experts recommend removing the catheter and giving a short course of antibiotics (ie, 5–7 days). Longer courses of antibiotics may be required for patients with endovascular hardware in place or persistent fever or bacteremia after catheter removal. The IDSA guidelines recommend 5 to 7 days of antibiotic therapy if the catheter is removed, and 10 to 14 days of systemic antibiotic therapy in combination with “antibiotic lock therapy” if the catheter is retained. Antibiotic lock therapy involves instilling a high concentration of an antibiotic to which the organism is susceptible into the catheter lumen and allowing it to dwell.
Not all patients are good candidates for antibiotic lock therapy, and neither are all organisms. In general, patients should be at low risk (immunocompetent, without hardware in place), and organisms should have a low risk of causing metastatic infection.
Staphylococcus lugdunensis can cause endocarditis and metastatic infections similar to those caused by S aureus and so should be managed similarly to S aureus.19
Staphylococcus aureus
Short-term CVCs infected with S aureus should be removed immediately. Removal of vascular catheters infected with S aureus has been associated with more rapid clinical response and higher cure rates compared with catheter retention.20–23S aureus bacteremia results in hematogenous complications in 20% to 30% of patients, and failure to remove or a delay in removing the catheter increases the risk of complications.21,24–27
There are no data from randomized clinical trials on the optimal duration of antibiotic therapy for S aureus bloodstream infections related to short-term CVCs. Traditionally, 4 weeks have been recommended out of concern for the risk of infective endocarditis,28,29 and the IDSA recommends 4 to 6 weeks unless patients meet certain low-risk criteria.
Factors associated with a higher risk of hematogenous complications include the presence of a retained foreign body, an intravascular prosthetic device, retained catheter, immune suppression, diabetes, persistent bacteremia at 72 hours despite catheter removal and appropriate antibiotics, skin changes consistent with septic emboli, or evidence of endocarditis or suppurative thrombophlebitis on transesophageal echocardiography (TEE) or ultrasonography, respectively.21,25–27 TEE is superior to transthoracic echocardiography and is most sensitive when performed 5 to 7 days after the onset of bacteremia.28,30 Patients who have had the catheter removed and who do not have any of these risk factors, and in whom TEE performed 5 to 7 days after the onset of bacteremia is negative, can be considered for a shorter duration of therapy (but a minimum of 14 days).
Patients with catheters colonized with S aureus (ie, those with positive catheter-tip cultures and negative blood cultures) are at risk of subsequent bacteremia. This risk may be reduced with anti-staphylococcal therapy started within 24 hours of catheter removal.31,32 Therapy should be continued for 5 to 7 days, and patients should be closely monitored for signs or symptoms of ongoing infection.
Oxacillin or nafcillin should be the first-line therapy for susceptible S aureus isolates. Vancomycin should be used to treat MRSA. Patients with MRSA isolates with a vancomycin MIC greater than 2 μg/mL should receive daptomycin or linezolid, depending on susceptibility data.
Enterococcal species
Up to 10% of nosocomially acquired bloodstream infections are due to enterococci, and many are related to intravascular catheters.33,34 Although the risk of endocarditis as a complication of enterococcal CVC-related bloodstream infection is relatively low, estimated at 1.5% in a multicenter prospective study, enterococcal bacteremia lasting longer than 4 days has been independently associated with risk of death.35,36 These observational data support routine removal of short-term CVCs infected with enterococci.
The choice of antibiotics for enterococcal infections depends on the susceptibility of the isolate. Sixty percent of Enterococcus faecium isolates and 2% of Enterococcus faecalis isolates are vancomycin-resistant, and reports of resistance to newer agents, including linezolid, have been published.34,37,38 Ampicillin is the preferred antibiotic for treatment of ampicillin-susceptible enterococci. Vancomycin should be used if the pathogen is ampicillin-resistant and vancomycin-susceptible. Enterococci resistant to both ampicillin and vancomycin can be treated with linezolid or daptomycin, based on susceptibility data.
For combination therapy with an aminoglycoside, the data are mixed. Retrospective observational studies have shown no difference in outcomes in uncomplicated enterococcal bacteremia with combination therapy vs monotherapy.39,40 However, in a large series of patients with enterococcal infections in which the catheter was retained, the combination of gentamicin and ampicillin was more effective than monotherapy.41
No controlled trial has been done to define the optimal duration of antibiotic therapy for enterococcal bloodstream infections related to short-term CVCs, but the IDSA recommends 7 to 14 days. If catheter salvage is attempted, concurrent antimicrobial lock therapy is recommended based on expert opinion. Catheters should be removed if complications arise (eg, insertion site or pocket infection, suppurative thrombophlebitis, sepsis, endocarditis, persistent bacteremia, metastatic infection). Signs and symptoms of endocarditis, persistent bacteremia, or the presence of a prosthetic heart valve should prompt evaluation with TEE.42,43
Gram-negative bacilli
Given the propensity of many gram-negative bacilli to form a biofilm, a number of studies have advocated removing CVCs infected with gram-negative bacilli.15,16,44 Recent studies examining the role of combination systemic antibiotic therapy and antibiotic lock therapy of gram-negative infections have found high success rates.45,46
The IDSA recommends routine removal of short-term CVCs infected with gram-negative bacilli and 7 to 14 days of systemic antibiotic therapy based on microbial susceptibility data. Antibiotic options generally include fourth-generation cephalosporins, carbapenems, or a combination beta-lactam and beta-lactamase inhibitor. The first-line treatment for Stenotrophomonas maltophilia and Burkholderia cepacia is trimethoprim-sulfamethoxazole (Bactrim). Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli should not be treated with cephalosporins or piperacillin-tazobactam (Zosyn) even if the organisms are susceptible in vitro, as doing so has been associated with poor clinical outcomes.11,47
There is growing concern over multiple-drug-resistant gram-negative bacilli with carbapenemases that confer resistance to carbapenems. No controlled study has evaluated treatment of multiple-drug-resistant gram-negative bacilli that require therapy with polymyxin (Colistin).
Candida species
The benefit of removing the CVC in the setting of candidemia is supported by six prospective studies.48–53 Patients with catheter-related bloodstream infections due to Candida species should have the catheter removed. C albicans and azole-susceptible candidal strains can be effectively treated with fluconazole at a dosage of 400 mg daily, continued for 14 days following the first negative blood culture.54 Echinocandins as first-line therapy and lipid formulations of amphotericin B (Abelcet) as an alternative are both highly effective for the treatment of Candida species with decreased susceptibility to azoles (eg, C glabrata and C krusei).55–57
Other gram-positive microorganisms
The isolation of Corynebacterium, Bacillus, and Micrococcus species from a single blood culture does not prove bloodstream infection, and confirmation requires at least two positive results drawn from different sites. CVC infections with these organisms are difficult to treat unless the infected catheter is removed.58,59
ADDITIONAL RECOMMENDATIONS
Infectious disease consultation should be considered for patients with complicated bloodstream infection related to a short-term CVC. Complicated cases include catheter infections in patients with hemodynamic instability, endocarditis, suppurative thrombophlebitis, persistent bloodstream infection despite 72 hours of appropriate antimicrobial therapy, osteomyelitis, active malignancy, or immunosuppression.
Infectious disease consultation should also be sought for assistance with determining if a patient is a candidate for antibiotic lock therapy; for management, dosing, and course of antibiotic lock therapy; for assistance with antibiotic choice and course for multiple-drug-resistant gram-negative bacilli; and for recommendations on management of infections due to uncommon pathogens (eg, Corynebacterium jeikeium, Chryseobacterium species, Malassezia furfur, and Mycobacterium species).
- Edgeworth J. Intravascular catheter infections. J Hosp Infect 2009; 73:323–330.
- Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 2009; 37:783–805.
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45.
- Bouza E, Alvarado N, Alcalá L, Pérez MJ, Rincón C, Muñoz P. A randomized and prospective study of 3 procedures for the diagnosis of catheter-related bloodstream infection without catheter withdrawal. Clin Infect Dis 2007; 44:820–826.
- Rijnders BJ, Verwaest C, Peetermans WE, et al. Difference in time to positivity of hub-blood versus nonhub-blood cultures is not useful for the diagnosis of catheter-related bloodstream infection in critically ill patients. Crit Care Med 2001; 29:1399–1403.
- Rijnders BJ, Peetermans WE, Verwaest C, Wilmer A, Van Wijngaerden E. Watchful waiting versus immediate catheter removal in ICU patients with suspected catheter-related infection: a randomized trial. Intensive Care Med 2004; 30:1073–1080.
- Safdar A, Mermel LA, Maki DG. The epidemiology of catheter-related infection in the critically ill. In:O’Grady NP, Pittet D, editors. Catheter-Related Infections in the Critically Ill. Boston: Kluwer, 2004:1–22.
- Wilcox MH, Tack KJ, Bouza E, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis 2009; 48:203–212.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2007; 51:2582–2586.
- Jacoby GA, Munoz-Price LS. The new beta-lactamases. N Engl J Med 2005; 352:380–391.
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004; 32:470–485.
- Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000; 118:146–155.
- Raymond DP, Pelletier SJ, Crabtree TD, Evans HL, Pruett TL, Sawyer RG. Impact of antibiotic-resistant Gram-negative bacilli infections on outcome in hospitalized patients. Crit Care Med 2003; 31:1035–1041.
- Friedman ND, Korman TM, Fairley CK, Franklin JC, Spelman DW. Bacteraemia due to Stenotrophomonas maltophilia: an analysis of 45 episodes. J Infect 2002; 45:47–53.
- Seifert H, Strate A, Pulverer G. Nosocomial bacteremia due to Acinetobacter baumannii. Clinical features, epidemiology, and predictors of mortality. Medicine (Baltimore) 1995; 74:340–349.
- Seifert H. Catheter-related infections due to gram-negative bacilli. In:Seifert H, Jansen B, Farr BM, editors. Catheter-Related infections. New York: M. Dekker, 1997:111–138.
- Lorente L, Jiménez A, Santana M, et al. Microorganisms responsible for intravascular catheter-related bloodstream infection according to the catheter site. Crit Care Med 2007; 35:2424–2427.
- Zinkernagel AS, Zinkernagel MS, Elzi MV, et al. Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection 2008; 36:314–321.
- Malanoski GJ, Samore MH, Pefanis A, Karchmer AW. Staphylococcus aureus catheter-associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters. Arch Intern Med 1995; 155:1161–1166.
- Fowler VG, Justice A, Moore C, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 2005; 40:695–703.
- Fowler VG, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27:478–486.
- Reilly JJ, Steed DL, Ritter PS. Indwelling venous access catheters in patients with acute leukemia. Cancer 1984; 53:219–223.
- Abraham J, Mansour C, Veledar E, Khan B, Lerakis S. Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am Heart J 2004; 147:536–539.
- Fowler VG, Miro JM, Hoen B, et al; ICE Investigators. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293:3012–3021.
- Fowler VG, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–2072.
- Chang FY, MacDonald BB, Peacock JE, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003; 82:322–332.
- Rosen AB, Fowler VG, Corey GR, et al. Cost-effectiveness of transesophageal echocardiography to determine the duration of therapy for intravascular catheter-associated Staphylococcus aureus bacteremia. Ann Intern Med 1999; 130:810–820.
- Pigrau C, Rodríguez D, Planes AM, et al. Management of catheter-related Staphylococcus aureus bacteremia: when may sonographic study be unnecessary? Eur J Clin Microbiol Infect Dis 2003; 22:713–719.
- Sochowski RA, Chan KL. Implication of negative results on a monoplane transesophageal echocardiographic study in patients with suspected infective endocarditis. J Am Coll Cardiol 1993; 21:216–221.
- Koh DB, Gowardman JR, Rickard CM, Robertson IK, Brown A. Prospective study of peripheral arterial catheter infection and comparison with concurrently sited central venous catheters. Crit Care Med 2008; 36:397–402.
- Ruhe JJ, Menon A. Clinical significance of isolated Staphylococcus aureus central venous catheter tip cultures. Clin Microbiol Infect 2006; 12:933–936.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–317.
- Jones RN, Marshall SA, Pfaller MA, et al. Nosocomial enterococcal blood stream infections in the SCOPE Program: antimicrobial resistance, species occurrence, molecular testing results, and laboratory testing accuracy. SCOPE Hospital Study Group. Diagn Microbiol Infect Dis 1997; 29:95–102.
- DiazGranados CA, Jernigan JA. Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. J Infect Dis 2005; 191:588–595.
- Bhavnani SM, Drake JA, Forrest A, et al. A nationwide, multicenter, case-control study comparing risk factors, treatment, and outcome for vancomycin-resistant and -susceptible enterococcal bacteremia. Diagn Microbiol Infect Dis 2000; 36:145–158.
- Gonzales RD, Schreckenberger PC, Graham MB, Kelkar S, DenBesten K, Quinn JP. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 2001; 357:1179.
- Kanafani ZA, Federspiel JJ, Fowler VG. Infective endocarditis caused by daptomycin-resistant Enterococcus faecalis: a case report. Scand J Infect Dis 2007; 39:75–77.
- Maki DG, Agger WA. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 1988; 67:248–269.
- Gray J, Marsh PJ, Stewart D, Pedler SJ. Enterococcal bacteraemia: a prospective study of 125 episodes. J Hosp Infect 1994; 27:179–186.
- Sandoe JA, Witherden IR, Au-Yeung HK, Kite P, Kerr KG, Wilcox MH. Enterococcal intravascular catheter-related bloodstream infection: management and outcome of 61 consecutive cases. J Antimicrob Chemother 2002; 50:577–582.
- Anderson DJ, Murdoch DR, Sexton DJ, et al. Risk factors for infective endocarditis in patients with enterococcal bacteremia: a case-control study. Infection 2004; 32:72–77.
- Fernández-Guerrero ML, Herrero L, Bellver M, Gadea I, Roblas RF, de Górgolas M. Nosocomial enterococcal endocarditis: a serious hazard for hospitalized patients with enterococcal bacteraemia. J Intern Med 2002; 252:510–515.
- Elting LS, Bodey GP. Septicemia due to Xanthomonas species and non-aeruginosa Pseudomonas species: increasing incidence of catheter-related infections. Medicine (Baltimore) 1990; 69:296–306.
- Fernandez-Hidalgo N, Almirante B, Calleja R, et al. Antibiotic-lock therapy for long-term intravascular catheter-related bacteraemia: results of an open, non-comparative study. J Antimicrob Chemother 2006; 57:1172–1180.
- Poole CV, Carlton D, Bimbo L, Allon M. Treatment of catheter-related bacteraemia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol Dial Transplant 2004; 19:1237–1244.
- Paterson DL, Ko WC, Von Gottberg A, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol 2001; 39:2206–2212.
- Nguyen MH, Peacock JE, Tanner DC, et al. Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Arch Intern Med 1995; 155:2429–2435.
- Hung CC, Chen YC, Chang SC, Luh KT, Hsieh WC. Nosocomial candidemia in a university hospital in Taiwan. J Formos Med Assoc 1996; 95:19–28.
- Rex JH, Bennett JE, Sugar AM, et al. Intravascular catheter exchange and duration of candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Clin Infect Dis 1995; 21:994–996.
- Karlowicz MG, Hashimoto LN, Kelly RE, Buescher ES. Should central venous catheters be removed as soon as candidemia is detected in neonates? Pediatrics 2000; 106:E63.
- Nucci M, Colombo AL, Silveira F, et al. Risk factors for death in patients with candidemia. Infect Control Hosp Epidemiol 1998; 19:846–850.
- Almirante B, Rodríguez D, Park BJ, et al; Barcelona Candidemia Project Study Group. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 2005; 43:1829–1835.
- Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med 1994; 331:1325–1330.
- Kuse ER, Chetchotisakd P, da Cunha CA, et al; Micafungin Invasive Candidiasis Working Group. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369:1519–1527.
- Reboli AC, Rotstein C, Pappas PG, et al; Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472–2482.
- Mora-Duarte J, Betts R, Rotstein C, et al; Caspofungin Invasive Candidiasis Study Group. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 2002; 347:2020–2029.
- Peces R, Gago E, Tejada F, Laures AS, Alvarez-Grande J. Relapsing bacteraemia due to Micrococcus luteus in a haemodialysis patient with a Perm-Cath catheter. Nephrol Dial Transplant 1997; 12:2428–2429.
- Cotton DJ, Gill VJ, Marshall DJ, Gress J, Thaler M, Pizzo PA. Clinical features and therapeutic interventions in 17 cases of Bacillus bacteremia in an immunosuppressed patient population. J Clin Microbiol 1987; 25:672–674.
Vascular catheters are very common in everyday inpatient and, increasingly, outpatient care. Nearly 300 million catheters are estimated to be used annually in the United States, and approximately 3 million of these are central venous catheters (CVCs).1
Although significant gains have been made in preventing CVC-related bloodstream infections, these infections continue to occur, with estimated rates ranging from 1.3 per 1,000 catheter days on inpatient medical-surgical wards to 5.6 per 1,000 catheter days in intensive care burn units.2
CVCs are classified as either long-term or short-term. Long-term CVCs are surgically implanted or tunneled and used for prolonged chemotherapy, home infusion therapy, or hemodialysis. Short-term CVCs do not require surgical implantation. They are more common than long-term CVCs and account for most CVC-related bloodstream infections. Given the frequency of short-term CVC use, a growing number of health care providers from mid-level practitioners to intensivists are faced with deciding how to manage bloodstream infection related to short-term CVCs.
At baseline, management decisions about bloodstream infections from short-term CVCs can be challenging. Questions that regularly arise include:
- Should a potentially infected catheter be removed?
- Which empiric antibiotic therapy should be started pending a microbiologic diagnosis?
- How should therapy be tailored (eg, antibiotic choice and course and whether to remove or retain the catheter) based on the specific pathogen identified?
Adding to the complexity of these decisions are increasingly resistant microorganisms, heterogeneity of affected patient populations, and variability in the quality and availability of evidence.
This review provides a concise guide to managing bloodstream infections related to short-term CVCs in adults, based on updated guidelines from the Infectious Diseases Society of America (IDSA).3
DEFINITION AND DIAGNOSTIC CRITERIA
We have adapted the following definition and diagnostic criteria from the general definition and diagnostic criteria for catheter-related bloodstream infections proposed by the IDSA.
Bloodstream infection related to a short-term CVC is defined as bacteremia or fungemia in a patient with the CVC in place, clinical manifestations of infection (eg, fever, chills, hypotension), and no apparent source of the bloodstream infection aside from the catheter. At least one of the three diagnostic criteria should be met:
- Cultures of the catheter tip and of the peripheral blood grow the same organism. Catheter tip culture should be quantitative, with more than 102 colony-forming units (cfu) per catheter segment, or semiquantitative, with more than 15 cfu per catheter segment.
- Blood drawn from the catheter lumen grows the same organism as blood drawn from a peripheral vein (or less optimally, a different lumen), but at three times the amount by quantitative culture.
- Blood drawn simultaneously from the catheter lumen and from a peripheral vein (or less optimally, a different lumen) grows the same organism, and growth from the CVC lumen sample is detected (by automated blood culture system) at least 2 hours before growth from the peripheral vein sample.
MANAGING BLOODSTREAM INFECTIONS IN PATIENTS WITH SHORT-TERM CVCs
When to remove a potentially infected short-term CVC
In a nonneutropenic intensive care population, Bouza et al4 found that, of 204 episodes of clinically suspected bloodstream infection from a short-term CVC, only 28 (14%) were confirmed to be catheter-related, 27 (13%) were bloodstream infections that were not catheter-related, 36 (18%) involved catheter-tip colonization with negative blood cultures, and the remainder were cases with negative catheter-tip and blood cultures.
Rijnders et al,5 in a study of 100 adult medical-surgical intensive care patients with a clinically suspected bloodstream infection related to a short-term CVC, found only three confirmed cases.
A randomized clinical trial comparing early removal of short-term CVCs and watchful waiting in an adult intensive care population with clinically suspected bloodstream infections showed no difference between treatment groups in length of stay in the intensive care unit or in the mortality rate.6 This trial included a low-risk subset of adult medical-surgical intensive care patients (ie, immunocompetent, no intravascular foreign body, no evidence of severe sepsis or septic shock, no evidence of infection at the catheter insertion site, no proven bacteremia or fungemia). These results suggest that a similar subset of patients can be safely monitored without catheter removal while being assessed for possible catheter-related bloodstream infection.
Empiric catheter removal vs watchful waiting has not and likely will not be studied in higher-risk populations. In this group, clinical judgment should outweigh any specific management algorithm. In patients who are in shock or who are otherwise hemodynamically unstable, early catheter removal should be a priority; however, in some circumstances the risks of immediate catheter removal (eg, coagulopathy with risk of bleeding diathesis, or lack of site to replace the catheter) may outweigh the potential benefits.
Empiric antibiotic therapy for bloodstream infection from a short-term CVC
In order of prevalence, the four most common pathogens are coagulase-negative staphylococci, Staphylococcus aureus, Candida species, and enteric gram-negative bacilli.7
Gram-positive pathogens. A recent randomized clinical trial comparing vancomycin and linezolid (Zyvox) treatment for CVC-related bloodstream infections showed that 89 (57%) of 157 S aureus isolates and 95 (80%) of 119 coagulase-negative staphylococcal isolates were resistant to methicillin.8 Given the prevalence of gram-positive infections and the regularity of methicillin-resistant isolates, vancomycin should be started empirically in cases of suspected bloodstream infection related to short-term CVCs. In institutions where methicillin-resistant S aureus (MRSA) isolates regularly have a vancomycin minimum inhibitory concentration (MIC) of greater than 2 μg/mL, an alternative agent such as daptomycin (Cubicin) should be used.9,10
Gram-negative pathogens. Infections due to resistant gram-negative pathogens have become more common in the past 10 years.11,12 Prospective cohort studies have shown that resistant gram-negative infections and inadequate empiric antimicrobial therapy of bloodstream infections independently predict the risk of death.13,14 Risk factors for resistant gram-negative infections include critical illness, neutropenia, prior antibiotic therapy, and femoral insertion of the CVC.15–18 Patients with these risk factors should receive empiric antibiotic therapy for gram-negative bacilli.
No randomized controlled trial has been done to guide the choice of empiric gram-negative antibiotic coverage. The initial choice should be based on local antimicrobial patterns and susceptibility data and on the severity of the patient’s illness. Initial options include fourth-generation cephalosporins, carbapenems, or combined beta-lactam and beta-lactamase inhibitors. Patients with neutropenia, severe sepsis, or known multiple-drug-resistant gram-negative bacilli colonization or prior infection should receive empiric combination therapy with two different classes of antibiotics.
Candida. Risk factors for CVC-related bloodstream infections due to Candida species include total parenteral nutrition, prolonged use of broad-spectrum antibiotics, hematologic malignancy, solid organ or bone marrow transplantation, colonization with Candida species at multiple sites, and femoral catheter insertion. Empiric treatment with an echinocandin is recommended for patients with these risk factors. Fluconazole (Diflucan) can be substituted for an echinocandin in patients without azole exposure in the previous 3 months and in settings where the prevalence of Candida krusei and Candida glabrata is low.
PATHOGEN-SPECIFIC MANAGEMENT: RECOMMENDATIONS
Coagulase-negative staphylococci
Most patients with coagulase-negative staphylococcal infections have a benign clinical course.
Although no randomized trial has evaluated different treatment approaches, most experts recommend removing the catheter and giving a short course of antibiotics (ie, 5–7 days). Longer courses of antibiotics may be required for patients with endovascular hardware in place or persistent fever or bacteremia after catheter removal. The IDSA guidelines recommend 5 to 7 days of antibiotic therapy if the catheter is removed, and 10 to 14 days of systemic antibiotic therapy in combination with “antibiotic lock therapy” if the catheter is retained. Antibiotic lock therapy involves instilling a high concentration of an antibiotic to which the organism is susceptible into the catheter lumen and allowing it to dwell.
Not all patients are good candidates for antibiotic lock therapy, and neither are all organisms. In general, patients should be at low risk (immunocompetent, without hardware in place), and organisms should have a low risk of causing metastatic infection.
Staphylococcus lugdunensis can cause endocarditis and metastatic infections similar to those caused by S aureus and so should be managed similarly to S aureus.19
Staphylococcus aureus
Short-term CVCs infected with S aureus should be removed immediately. Removal of vascular catheters infected with S aureus has been associated with more rapid clinical response and higher cure rates compared with catheter retention.20–23S aureus bacteremia results in hematogenous complications in 20% to 30% of patients, and failure to remove or a delay in removing the catheter increases the risk of complications.21,24–27
There are no data from randomized clinical trials on the optimal duration of antibiotic therapy for S aureus bloodstream infections related to short-term CVCs. Traditionally, 4 weeks have been recommended out of concern for the risk of infective endocarditis,28,29 and the IDSA recommends 4 to 6 weeks unless patients meet certain low-risk criteria.
Factors associated with a higher risk of hematogenous complications include the presence of a retained foreign body, an intravascular prosthetic device, retained catheter, immune suppression, diabetes, persistent bacteremia at 72 hours despite catheter removal and appropriate antibiotics, skin changes consistent with septic emboli, or evidence of endocarditis or suppurative thrombophlebitis on transesophageal echocardiography (TEE) or ultrasonography, respectively.21,25–27 TEE is superior to transthoracic echocardiography and is most sensitive when performed 5 to 7 days after the onset of bacteremia.28,30 Patients who have had the catheter removed and who do not have any of these risk factors, and in whom TEE performed 5 to 7 days after the onset of bacteremia is negative, can be considered for a shorter duration of therapy (but a minimum of 14 days).
Patients with catheters colonized with S aureus (ie, those with positive catheter-tip cultures and negative blood cultures) are at risk of subsequent bacteremia. This risk may be reduced with anti-staphylococcal therapy started within 24 hours of catheter removal.31,32 Therapy should be continued for 5 to 7 days, and patients should be closely monitored for signs or symptoms of ongoing infection.
Oxacillin or nafcillin should be the first-line therapy for susceptible S aureus isolates. Vancomycin should be used to treat MRSA. Patients with MRSA isolates with a vancomycin MIC greater than 2 μg/mL should receive daptomycin or linezolid, depending on susceptibility data.
Enterococcal species
Up to 10% of nosocomially acquired bloodstream infections are due to enterococci, and many are related to intravascular catheters.33,34 Although the risk of endocarditis as a complication of enterococcal CVC-related bloodstream infection is relatively low, estimated at 1.5% in a multicenter prospective study, enterococcal bacteremia lasting longer than 4 days has been independently associated with risk of death.35,36 These observational data support routine removal of short-term CVCs infected with enterococci.
The choice of antibiotics for enterococcal infections depends on the susceptibility of the isolate. Sixty percent of Enterococcus faecium isolates and 2% of Enterococcus faecalis isolates are vancomycin-resistant, and reports of resistance to newer agents, including linezolid, have been published.34,37,38 Ampicillin is the preferred antibiotic for treatment of ampicillin-susceptible enterococci. Vancomycin should be used if the pathogen is ampicillin-resistant and vancomycin-susceptible. Enterococci resistant to both ampicillin and vancomycin can be treated with linezolid or daptomycin, based on susceptibility data.
For combination therapy with an aminoglycoside, the data are mixed. Retrospective observational studies have shown no difference in outcomes in uncomplicated enterococcal bacteremia with combination therapy vs monotherapy.39,40 However, in a large series of patients with enterococcal infections in which the catheter was retained, the combination of gentamicin and ampicillin was more effective than monotherapy.41
No controlled trial has been done to define the optimal duration of antibiotic therapy for enterococcal bloodstream infections related to short-term CVCs, but the IDSA recommends 7 to 14 days. If catheter salvage is attempted, concurrent antimicrobial lock therapy is recommended based on expert opinion. Catheters should be removed if complications arise (eg, insertion site or pocket infection, suppurative thrombophlebitis, sepsis, endocarditis, persistent bacteremia, metastatic infection). Signs and symptoms of endocarditis, persistent bacteremia, or the presence of a prosthetic heart valve should prompt evaluation with TEE.42,43
Gram-negative bacilli
Given the propensity of many gram-negative bacilli to form a biofilm, a number of studies have advocated removing CVCs infected with gram-negative bacilli.15,16,44 Recent studies examining the role of combination systemic antibiotic therapy and antibiotic lock therapy of gram-negative infections have found high success rates.45,46
The IDSA recommends routine removal of short-term CVCs infected with gram-negative bacilli and 7 to 14 days of systemic antibiotic therapy based on microbial susceptibility data. Antibiotic options generally include fourth-generation cephalosporins, carbapenems, or a combination beta-lactam and beta-lactamase inhibitor. The first-line treatment for Stenotrophomonas maltophilia and Burkholderia cepacia is trimethoprim-sulfamethoxazole (Bactrim). Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli should not be treated with cephalosporins or piperacillin-tazobactam (Zosyn) even if the organisms are susceptible in vitro, as doing so has been associated with poor clinical outcomes.11,47
There is growing concern over multiple-drug-resistant gram-negative bacilli with carbapenemases that confer resistance to carbapenems. No controlled study has evaluated treatment of multiple-drug-resistant gram-negative bacilli that require therapy with polymyxin (Colistin).
Candida species
The benefit of removing the CVC in the setting of candidemia is supported by six prospective studies.48–53 Patients with catheter-related bloodstream infections due to Candida species should have the catheter removed. C albicans and azole-susceptible candidal strains can be effectively treated with fluconazole at a dosage of 400 mg daily, continued for 14 days following the first negative blood culture.54 Echinocandins as first-line therapy and lipid formulations of amphotericin B (Abelcet) as an alternative are both highly effective for the treatment of Candida species with decreased susceptibility to azoles (eg, C glabrata and C krusei).55–57
Other gram-positive microorganisms
The isolation of Corynebacterium, Bacillus, and Micrococcus species from a single blood culture does not prove bloodstream infection, and confirmation requires at least two positive results drawn from different sites. CVC infections with these organisms are difficult to treat unless the infected catheter is removed.58,59
ADDITIONAL RECOMMENDATIONS
Infectious disease consultation should be considered for patients with complicated bloodstream infection related to a short-term CVC. Complicated cases include catheter infections in patients with hemodynamic instability, endocarditis, suppurative thrombophlebitis, persistent bloodstream infection despite 72 hours of appropriate antimicrobial therapy, osteomyelitis, active malignancy, or immunosuppression.
Infectious disease consultation should also be sought for assistance with determining if a patient is a candidate for antibiotic lock therapy; for management, dosing, and course of antibiotic lock therapy; for assistance with antibiotic choice and course for multiple-drug-resistant gram-negative bacilli; and for recommendations on management of infections due to uncommon pathogens (eg, Corynebacterium jeikeium, Chryseobacterium species, Malassezia furfur, and Mycobacterium species).
Vascular catheters are very common in everyday inpatient and, increasingly, outpatient care. Nearly 300 million catheters are estimated to be used annually in the United States, and approximately 3 million of these are central venous catheters (CVCs).1
Although significant gains have been made in preventing CVC-related bloodstream infections, these infections continue to occur, with estimated rates ranging from 1.3 per 1,000 catheter days on inpatient medical-surgical wards to 5.6 per 1,000 catheter days in intensive care burn units.2
CVCs are classified as either long-term or short-term. Long-term CVCs are surgically implanted or tunneled and used for prolonged chemotherapy, home infusion therapy, or hemodialysis. Short-term CVCs do not require surgical implantation. They are more common than long-term CVCs and account for most CVC-related bloodstream infections. Given the frequency of short-term CVC use, a growing number of health care providers from mid-level practitioners to intensivists are faced with deciding how to manage bloodstream infection related to short-term CVCs.
At baseline, management decisions about bloodstream infections from short-term CVCs can be challenging. Questions that regularly arise include:
- Should a potentially infected catheter be removed?
- Which empiric antibiotic therapy should be started pending a microbiologic diagnosis?
- How should therapy be tailored (eg, antibiotic choice and course and whether to remove or retain the catheter) based on the specific pathogen identified?
Adding to the complexity of these decisions are increasingly resistant microorganisms, heterogeneity of affected patient populations, and variability in the quality and availability of evidence.
This review provides a concise guide to managing bloodstream infections related to short-term CVCs in adults, based on updated guidelines from the Infectious Diseases Society of America (IDSA).3
DEFINITION AND DIAGNOSTIC CRITERIA
We have adapted the following definition and diagnostic criteria from the general definition and diagnostic criteria for catheter-related bloodstream infections proposed by the IDSA.
Bloodstream infection related to a short-term CVC is defined as bacteremia or fungemia in a patient with the CVC in place, clinical manifestations of infection (eg, fever, chills, hypotension), and no apparent source of the bloodstream infection aside from the catheter. At least one of the three diagnostic criteria should be met:
- Cultures of the catheter tip and of the peripheral blood grow the same organism. Catheter tip culture should be quantitative, with more than 102 colony-forming units (cfu) per catheter segment, or semiquantitative, with more than 15 cfu per catheter segment.
- Blood drawn from the catheter lumen grows the same organism as blood drawn from a peripheral vein (or less optimally, a different lumen), but at three times the amount by quantitative culture.
- Blood drawn simultaneously from the catheter lumen and from a peripheral vein (or less optimally, a different lumen) grows the same organism, and growth from the CVC lumen sample is detected (by automated blood culture system) at least 2 hours before growth from the peripheral vein sample.
MANAGING BLOODSTREAM INFECTIONS IN PATIENTS WITH SHORT-TERM CVCs
When to remove a potentially infected short-term CVC
In a nonneutropenic intensive care population, Bouza et al4 found that, of 204 episodes of clinically suspected bloodstream infection from a short-term CVC, only 28 (14%) were confirmed to be catheter-related, 27 (13%) were bloodstream infections that were not catheter-related, 36 (18%) involved catheter-tip colonization with negative blood cultures, and the remainder were cases with negative catheter-tip and blood cultures.
Rijnders et al,5 in a study of 100 adult medical-surgical intensive care patients with a clinically suspected bloodstream infection related to a short-term CVC, found only three confirmed cases.
A randomized clinical trial comparing early removal of short-term CVCs and watchful waiting in an adult intensive care population with clinically suspected bloodstream infections showed no difference between treatment groups in length of stay in the intensive care unit or in the mortality rate.6 This trial included a low-risk subset of adult medical-surgical intensive care patients (ie, immunocompetent, no intravascular foreign body, no evidence of severe sepsis or septic shock, no evidence of infection at the catheter insertion site, no proven bacteremia or fungemia). These results suggest that a similar subset of patients can be safely monitored without catheter removal while being assessed for possible catheter-related bloodstream infection.
Empiric catheter removal vs watchful waiting has not and likely will not be studied in higher-risk populations. In this group, clinical judgment should outweigh any specific management algorithm. In patients who are in shock or who are otherwise hemodynamically unstable, early catheter removal should be a priority; however, in some circumstances the risks of immediate catheter removal (eg, coagulopathy with risk of bleeding diathesis, or lack of site to replace the catheter) may outweigh the potential benefits.
Empiric antibiotic therapy for bloodstream infection from a short-term CVC
In order of prevalence, the four most common pathogens are coagulase-negative staphylococci, Staphylococcus aureus, Candida species, and enteric gram-negative bacilli.7
Gram-positive pathogens. A recent randomized clinical trial comparing vancomycin and linezolid (Zyvox) treatment for CVC-related bloodstream infections showed that 89 (57%) of 157 S aureus isolates and 95 (80%) of 119 coagulase-negative staphylococcal isolates were resistant to methicillin.8 Given the prevalence of gram-positive infections and the regularity of methicillin-resistant isolates, vancomycin should be started empirically in cases of suspected bloodstream infection related to short-term CVCs. In institutions where methicillin-resistant S aureus (MRSA) isolates regularly have a vancomycin minimum inhibitory concentration (MIC) of greater than 2 μg/mL, an alternative agent such as daptomycin (Cubicin) should be used.9,10
Gram-negative pathogens. Infections due to resistant gram-negative pathogens have become more common in the past 10 years.11,12 Prospective cohort studies have shown that resistant gram-negative infections and inadequate empiric antimicrobial therapy of bloodstream infections independently predict the risk of death.13,14 Risk factors for resistant gram-negative infections include critical illness, neutropenia, prior antibiotic therapy, and femoral insertion of the CVC.15–18 Patients with these risk factors should receive empiric antibiotic therapy for gram-negative bacilli.
No randomized controlled trial has been done to guide the choice of empiric gram-negative antibiotic coverage. The initial choice should be based on local antimicrobial patterns and susceptibility data and on the severity of the patient’s illness. Initial options include fourth-generation cephalosporins, carbapenems, or combined beta-lactam and beta-lactamase inhibitors. Patients with neutropenia, severe sepsis, or known multiple-drug-resistant gram-negative bacilli colonization or prior infection should receive empiric combination therapy with two different classes of antibiotics.
Candida. Risk factors for CVC-related bloodstream infections due to Candida species include total parenteral nutrition, prolonged use of broad-spectrum antibiotics, hematologic malignancy, solid organ or bone marrow transplantation, colonization with Candida species at multiple sites, and femoral catheter insertion. Empiric treatment with an echinocandin is recommended for patients with these risk factors. Fluconazole (Diflucan) can be substituted for an echinocandin in patients without azole exposure in the previous 3 months and in settings where the prevalence of Candida krusei and Candida glabrata is low.
PATHOGEN-SPECIFIC MANAGEMENT: RECOMMENDATIONS
Coagulase-negative staphylococci
Most patients with coagulase-negative staphylococcal infections have a benign clinical course.
Although no randomized trial has evaluated different treatment approaches, most experts recommend removing the catheter and giving a short course of antibiotics (ie, 5–7 days). Longer courses of antibiotics may be required for patients with endovascular hardware in place or persistent fever or bacteremia after catheter removal. The IDSA guidelines recommend 5 to 7 days of antibiotic therapy if the catheter is removed, and 10 to 14 days of systemic antibiotic therapy in combination with “antibiotic lock therapy” if the catheter is retained. Antibiotic lock therapy involves instilling a high concentration of an antibiotic to which the organism is susceptible into the catheter lumen and allowing it to dwell.
Not all patients are good candidates for antibiotic lock therapy, and neither are all organisms. In general, patients should be at low risk (immunocompetent, without hardware in place), and organisms should have a low risk of causing metastatic infection.
Staphylococcus lugdunensis can cause endocarditis and metastatic infections similar to those caused by S aureus and so should be managed similarly to S aureus.19
Staphylococcus aureus
Short-term CVCs infected with S aureus should be removed immediately. Removal of vascular catheters infected with S aureus has been associated with more rapid clinical response and higher cure rates compared with catheter retention.20–23S aureus bacteremia results in hematogenous complications in 20% to 30% of patients, and failure to remove or a delay in removing the catheter increases the risk of complications.21,24–27
There are no data from randomized clinical trials on the optimal duration of antibiotic therapy for S aureus bloodstream infections related to short-term CVCs. Traditionally, 4 weeks have been recommended out of concern for the risk of infective endocarditis,28,29 and the IDSA recommends 4 to 6 weeks unless patients meet certain low-risk criteria.
Factors associated with a higher risk of hematogenous complications include the presence of a retained foreign body, an intravascular prosthetic device, retained catheter, immune suppression, diabetes, persistent bacteremia at 72 hours despite catheter removal and appropriate antibiotics, skin changes consistent with septic emboli, or evidence of endocarditis or suppurative thrombophlebitis on transesophageal echocardiography (TEE) or ultrasonography, respectively.21,25–27 TEE is superior to transthoracic echocardiography and is most sensitive when performed 5 to 7 days after the onset of bacteremia.28,30 Patients who have had the catheter removed and who do not have any of these risk factors, and in whom TEE performed 5 to 7 days after the onset of bacteremia is negative, can be considered for a shorter duration of therapy (but a minimum of 14 days).
Patients with catheters colonized with S aureus (ie, those with positive catheter-tip cultures and negative blood cultures) are at risk of subsequent bacteremia. This risk may be reduced with anti-staphylococcal therapy started within 24 hours of catheter removal.31,32 Therapy should be continued for 5 to 7 days, and patients should be closely monitored for signs or symptoms of ongoing infection.
Oxacillin or nafcillin should be the first-line therapy for susceptible S aureus isolates. Vancomycin should be used to treat MRSA. Patients with MRSA isolates with a vancomycin MIC greater than 2 μg/mL should receive daptomycin or linezolid, depending on susceptibility data.
Enterococcal species
Up to 10% of nosocomially acquired bloodstream infections are due to enterococci, and many are related to intravascular catheters.33,34 Although the risk of endocarditis as a complication of enterococcal CVC-related bloodstream infection is relatively low, estimated at 1.5% in a multicenter prospective study, enterococcal bacteremia lasting longer than 4 days has been independently associated with risk of death.35,36 These observational data support routine removal of short-term CVCs infected with enterococci.
The choice of antibiotics for enterococcal infections depends on the susceptibility of the isolate. Sixty percent of Enterococcus faecium isolates and 2% of Enterococcus faecalis isolates are vancomycin-resistant, and reports of resistance to newer agents, including linezolid, have been published.34,37,38 Ampicillin is the preferred antibiotic for treatment of ampicillin-susceptible enterococci. Vancomycin should be used if the pathogen is ampicillin-resistant and vancomycin-susceptible. Enterococci resistant to both ampicillin and vancomycin can be treated with linezolid or daptomycin, based on susceptibility data.
For combination therapy with an aminoglycoside, the data are mixed. Retrospective observational studies have shown no difference in outcomes in uncomplicated enterococcal bacteremia with combination therapy vs monotherapy.39,40 However, in a large series of patients with enterococcal infections in which the catheter was retained, the combination of gentamicin and ampicillin was more effective than monotherapy.41
No controlled trial has been done to define the optimal duration of antibiotic therapy for enterococcal bloodstream infections related to short-term CVCs, but the IDSA recommends 7 to 14 days. If catheter salvage is attempted, concurrent antimicrobial lock therapy is recommended based on expert opinion. Catheters should be removed if complications arise (eg, insertion site or pocket infection, suppurative thrombophlebitis, sepsis, endocarditis, persistent bacteremia, metastatic infection). Signs and symptoms of endocarditis, persistent bacteremia, or the presence of a prosthetic heart valve should prompt evaluation with TEE.42,43
Gram-negative bacilli
Given the propensity of many gram-negative bacilli to form a biofilm, a number of studies have advocated removing CVCs infected with gram-negative bacilli.15,16,44 Recent studies examining the role of combination systemic antibiotic therapy and antibiotic lock therapy of gram-negative infections have found high success rates.45,46
The IDSA recommends routine removal of short-term CVCs infected with gram-negative bacilli and 7 to 14 days of systemic antibiotic therapy based on microbial susceptibility data. Antibiotic options generally include fourth-generation cephalosporins, carbapenems, or a combination beta-lactam and beta-lactamase inhibitor. The first-line treatment for Stenotrophomonas maltophilia and Burkholderia cepacia is trimethoprim-sulfamethoxazole (Bactrim). Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli should not be treated with cephalosporins or piperacillin-tazobactam (Zosyn) even if the organisms are susceptible in vitro, as doing so has been associated with poor clinical outcomes.11,47
There is growing concern over multiple-drug-resistant gram-negative bacilli with carbapenemases that confer resistance to carbapenems. No controlled study has evaluated treatment of multiple-drug-resistant gram-negative bacilli that require therapy with polymyxin (Colistin).
Candida species
The benefit of removing the CVC in the setting of candidemia is supported by six prospective studies.48–53 Patients with catheter-related bloodstream infections due to Candida species should have the catheter removed. C albicans and azole-susceptible candidal strains can be effectively treated with fluconazole at a dosage of 400 mg daily, continued for 14 days following the first negative blood culture.54 Echinocandins as first-line therapy and lipid formulations of amphotericin B (Abelcet) as an alternative are both highly effective for the treatment of Candida species with decreased susceptibility to azoles (eg, C glabrata and C krusei).55–57
Other gram-positive microorganisms
The isolation of Corynebacterium, Bacillus, and Micrococcus species from a single blood culture does not prove bloodstream infection, and confirmation requires at least two positive results drawn from different sites. CVC infections with these organisms are difficult to treat unless the infected catheter is removed.58,59
ADDITIONAL RECOMMENDATIONS
Infectious disease consultation should be considered for patients with complicated bloodstream infection related to a short-term CVC. Complicated cases include catheter infections in patients with hemodynamic instability, endocarditis, suppurative thrombophlebitis, persistent bloodstream infection despite 72 hours of appropriate antimicrobial therapy, osteomyelitis, active malignancy, or immunosuppression.
Infectious disease consultation should also be sought for assistance with determining if a patient is a candidate for antibiotic lock therapy; for management, dosing, and course of antibiotic lock therapy; for assistance with antibiotic choice and course for multiple-drug-resistant gram-negative bacilli; and for recommendations on management of infections due to uncommon pathogens (eg, Corynebacterium jeikeium, Chryseobacterium species, Malassezia furfur, and Mycobacterium species).
- Edgeworth J. Intravascular catheter infections. J Hosp Infect 2009; 73:323–330.
- Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 2009; 37:783–805.
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45.
- Bouza E, Alvarado N, Alcalá L, Pérez MJ, Rincón C, Muñoz P. A randomized and prospective study of 3 procedures for the diagnosis of catheter-related bloodstream infection without catheter withdrawal. Clin Infect Dis 2007; 44:820–826.
- Rijnders BJ, Verwaest C, Peetermans WE, et al. Difference in time to positivity of hub-blood versus nonhub-blood cultures is not useful for the diagnosis of catheter-related bloodstream infection in critically ill patients. Crit Care Med 2001; 29:1399–1403.
- Rijnders BJ, Peetermans WE, Verwaest C, Wilmer A, Van Wijngaerden E. Watchful waiting versus immediate catheter removal in ICU patients with suspected catheter-related infection: a randomized trial. Intensive Care Med 2004; 30:1073–1080.
- Safdar A, Mermel LA, Maki DG. The epidemiology of catheter-related infection in the critically ill. In:O’Grady NP, Pittet D, editors. Catheter-Related Infections in the Critically Ill. Boston: Kluwer, 2004:1–22.
- Wilcox MH, Tack KJ, Bouza E, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis 2009; 48:203–212.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2007; 51:2582–2586.
- Jacoby GA, Munoz-Price LS. The new beta-lactamases. N Engl J Med 2005; 352:380–391.
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004; 32:470–485.
- Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000; 118:146–155.
- Raymond DP, Pelletier SJ, Crabtree TD, Evans HL, Pruett TL, Sawyer RG. Impact of antibiotic-resistant Gram-negative bacilli infections on outcome in hospitalized patients. Crit Care Med 2003; 31:1035–1041.
- Friedman ND, Korman TM, Fairley CK, Franklin JC, Spelman DW. Bacteraemia due to Stenotrophomonas maltophilia: an analysis of 45 episodes. J Infect 2002; 45:47–53.
- Seifert H, Strate A, Pulverer G. Nosocomial bacteremia due to Acinetobacter baumannii. Clinical features, epidemiology, and predictors of mortality. Medicine (Baltimore) 1995; 74:340–349.
- Seifert H. Catheter-related infections due to gram-negative bacilli. In:Seifert H, Jansen B, Farr BM, editors. Catheter-Related infections. New York: M. Dekker, 1997:111–138.
- Lorente L, Jiménez A, Santana M, et al. Microorganisms responsible for intravascular catheter-related bloodstream infection according to the catheter site. Crit Care Med 2007; 35:2424–2427.
- Zinkernagel AS, Zinkernagel MS, Elzi MV, et al. Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection 2008; 36:314–321.
- Malanoski GJ, Samore MH, Pefanis A, Karchmer AW. Staphylococcus aureus catheter-associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters. Arch Intern Med 1995; 155:1161–1166.
- Fowler VG, Justice A, Moore C, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 2005; 40:695–703.
- Fowler VG, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27:478–486.
- Reilly JJ, Steed DL, Ritter PS. Indwelling venous access catheters in patients with acute leukemia. Cancer 1984; 53:219–223.
- Abraham J, Mansour C, Veledar E, Khan B, Lerakis S. Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am Heart J 2004; 147:536–539.
- Fowler VG, Miro JM, Hoen B, et al; ICE Investigators. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293:3012–3021.
- Fowler VG, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–2072.
- Chang FY, MacDonald BB, Peacock JE, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003; 82:322–332.
- Rosen AB, Fowler VG, Corey GR, et al. Cost-effectiveness of transesophageal echocardiography to determine the duration of therapy for intravascular catheter-associated Staphylococcus aureus bacteremia. Ann Intern Med 1999; 130:810–820.
- Pigrau C, Rodríguez D, Planes AM, et al. Management of catheter-related Staphylococcus aureus bacteremia: when may sonographic study be unnecessary? Eur J Clin Microbiol Infect Dis 2003; 22:713–719.
- Sochowski RA, Chan KL. Implication of negative results on a monoplane transesophageal echocardiographic study in patients with suspected infective endocarditis. J Am Coll Cardiol 1993; 21:216–221.
- Koh DB, Gowardman JR, Rickard CM, Robertson IK, Brown A. Prospective study of peripheral arterial catheter infection and comparison with concurrently sited central venous catheters. Crit Care Med 2008; 36:397–402.
- Ruhe JJ, Menon A. Clinical significance of isolated Staphylococcus aureus central venous catheter tip cultures. Clin Microbiol Infect 2006; 12:933–936.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–317.
- Jones RN, Marshall SA, Pfaller MA, et al. Nosocomial enterococcal blood stream infections in the SCOPE Program: antimicrobial resistance, species occurrence, molecular testing results, and laboratory testing accuracy. SCOPE Hospital Study Group. Diagn Microbiol Infect Dis 1997; 29:95–102.
- DiazGranados CA, Jernigan JA. Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. J Infect Dis 2005; 191:588–595.
- Bhavnani SM, Drake JA, Forrest A, et al. A nationwide, multicenter, case-control study comparing risk factors, treatment, and outcome for vancomycin-resistant and -susceptible enterococcal bacteremia. Diagn Microbiol Infect Dis 2000; 36:145–158.
- Gonzales RD, Schreckenberger PC, Graham MB, Kelkar S, DenBesten K, Quinn JP. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 2001; 357:1179.
- Kanafani ZA, Federspiel JJ, Fowler VG. Infective endocarditis caused by daptomycin-resistant Enterococcus faecalis: a case report. Scand J Infect Dis 2007; 39:75–77.
- Maki DG, Agger WA. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 1988; 67:248–269.
- Gray J, Marsh PJ, Stewart D, Pedler SJ. Enterococcal bacteraemia: a prospective study of 125 episodes. J Hosp Infect 1994; 27:179–186.
- Sandoe JA, Witherden IR, Au-Yeung HK, Kite P, Kerr KG, Wilcox MH. Enterococcal intravascular catheter-related bloodstream infection: management and outcome of 61 consecutive cases. J Antimicrob Chemother 2002; 50:577–582.
- Anderson DJ, Murdoch DR, Sexton DJ, et al. Risk factors for infective endocarditis in patients with enterococcal bacteremia: a case-control study. Infection 2004; 32:72–77.
- Fernández-Guerrero ML, Herrero L, Bellver M, Gadea I, Roblas RF, de Górgolas M. Nosocomial enterococcal endocarditis: a serious hazard for hospitalized patients with enterococcal bacteraemia. J Intern Med 2002; 252:510–515.
- Elting LS, Bodey GP. Septicemia due to Xanthomonas species and non-aeruginosa Pseudomonas species: increasing incidence of catheter-related infections. Medicine (Baltimore) 1990; 69:296–306.
- Fernandez-Hidalgo N, Almirante B, Calleja R, et al. Antibiotic-lock therapy for long-term intravascular catheter-related bacteraemia: results of an open, non-comparative study. J Antimicrob Chemother 2006; 57:1172–1180.
- Poole CV, Carlton D, Bimbo L, Allon M. Treatment of catheter-related bacteraemia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol Dial Transplant 2004; 19:1237–1244.
- Paterson DL, Ko WC, Von Gottberg A, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol 2001; 39:2206–2212.
- Nguyen MH, Peacock JE, Tanner DC, et al. Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Arch Intern Med 1995; 155:2429–2435.
- Hung CC, Chen YC, Chang SC, Luh KT, Hsieh WC. Nosocomial candidemia in a university hospital in Taiwan. J Formos Med Assoc 1996; 95:19–28.
- Rex JH, Bennett JE, Sugar AM, et al. Intravascular catheter exchange and duration of candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Clin Infect Dis 1995; 21:994–996.
- Karlowicz MG, Hashimoto LN, Kelly RE, Buescher ES. Should central venous catheters be removed as soon as candidemia is detected in neonates? Pediatrics 2000; 106:E63.
- Nucci M, Colombo AL, Silveira F, et al. Risk factors for death in patients with candidemia. Infect Control Hosp Epidemiol 1998; 19:846–850.
- Almirante B, Rodríguez D, Park BJ, et al; Barcelona Candidemia Project Study Group. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 2005; 43:1829–1835.
- Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med 1994; 331:1325–1330.
- Kuse ER, Chetchotisakd P, da Cunha CA, et al; Micafungin Invasive Candidiasis Working Group. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369:1519–1527.
- Reboli AC, Rotstein C, Pappas PG, et al; Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472–2482.
- Mora-Duarte J, Betts R, Rotstein C, et al; Caspofungin Invasive Candidiasis Study Group. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 2002; 347:2020–2029.
- Peces R, Gago E, Tejada F, Laures AS, Alvarez-Grande J. Relapsing bacteraemia due to Micrococcus luteus in a haemodialysis patient with a Perm-Cath catheter. Nephrol Dial Transplant 1997; 12:2428–2429.
- Cotton DJ, Gill VJ, Marshall DJ, Gress J, Thaler M, Pizzo PA. Clinical features and therapeutic interventions in 17 cases of Bacillus bacteremia in an immunosuppressed patient population. J Clin Microbiol 1987; 25:672–674.
- Edgeworth J. Intravascular catheter infections. J Hosp Infect 2009; 73:323–330.
- Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 2009; 37:783–805.
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45.
- Bouza E, Alvarado N, Alcalá L, Pérez MJ, Rincón C, Muñoz P. A randomized and prospective study of 3 procedures for the diagnosis of catheter-related bloodstream infection without catheter withdrawal. Clin Infect Dis 2007; 44:820–826.
- Rijnders BJ, Verwaest C, Peetermans WE, et al. Difference in time to positivity of hub-blood versus nonhub-blood cultures is not useful for the diagnosis of catheter-related bloodstream infection in critically ill patients. Crit Care Med 2001; 29:1399–1403.
- Rijnders BJ, Peetermans WE, Verwaest C, Wilmer A, Van Wijngaerden E. Watchful waiting versus immediate catheter removal in ICU patients with suspected catheter-related infection: a randomized trial. Intensive Care Med 2004; 30:1073–1080.
- Safdar A, Mermel LA, Maki DG. The epidemiology of catheter-related infection in the critically ill. In:O’Grady NP, Pittet D, editors. Catheter-Related Infections in the Critically Ill. Boston: Kluwer, 2004:1–22.
- Wilcox MH, Tack KJ, Bouza E, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis 2009; 48:203–212.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2007; 51:2582–2586.
- Jacoby GA, Munoz-Price LS. The new beta-lactamases. N Engl J Med 2005; 352:380–391.
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004; 32:470–485.
- Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000; 118:146–155.
- Raymond DP, Pelletier SJ, Crabtree TD, Evans HL, Pruett TL, Sawyer RG. Impact of antibiotic-resistant Gram-negative bacilli infections on outcome in hospitalized patients. Crit Care Med 2003; 31:1035–1041.
- Friedman ND, Korman TM, Fairley CK, Franklin JC, Spelman DW. Bacteraemia due to Stenotrophomonas maltophilia: an analysis of 45 episodes. J Infect 2002; 45:47–53.
- Seifert H, Strate A, Pulverer G. Nosocomial bacteremia due to Acinetobacter baumannii. Clinical features, epidemiology, and predictors of mortality. Medicine (Baltimore) 1995; 74:340–349.
- Seifert H. Catheter-related infections due to gram-negative bacilli. In:Seifert H, Jansen B, Farr BM, editors. Catheter-Related infections. New York: M. Dekker, 1997:111–138.
- Lorente L, Jiménez A, Santana M, et al. Microorganisms responsible for intravascular catheter-related bloodstream infection according to the catheter site. Crit Care Med 2007; 35:2424–2427.
- Zinkernagel AS, Zinkernagel MS, Elzi MV, et al. Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection 2008; 36:314–321.
- Malanoski GJ, Samore MH, Pefanis A, Karchmer AW. Staphylococcus aureus catheter-associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters. Arch Intern Med 1995; 155:1161–1166.
- Fowler VG, Justice A, Moore C, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 2005; 40:695–703.
- Fowler VG, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27:478–486.
- Reilly JJ, Steed DL, Ritter PS. Indwelling venous access catheters in patients with acute leukemia. Cancer 1984; 53:219–223.
- Abraham J, Mansour C, Veledar E, Khan B, Lerakis S. Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am Heart J 2004; 147:536–539.
- Fowler VG, Miro JM, Hoen B, et al; ICE Investigators. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293:3012–3021.
- Fowler VG, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–2072.
- Chang FY, MacDonald BB, Peacock JE, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003; 82:322–332.
- Rosen AB, Fowler VG, Corey GR, et al. Cost-effectiveness of transesophageal echocardiography to determine the duration of therapy for intravascular catheter-associated Staphylococcus aureus bacteremia. Ann Intern Med 1999; 130:810–820.
- Pigrau C, Rodríguez D, Planes AM, et al. Management of catheter-related Staphylococcus aureus bacteremia: when may sonographic study be unnecessary? Eur J Clin Microbiol Infect Dis 2003; 22:713–719.
- Sochowski RA, Chan KL. Implication of negative results on a monoplane transesophageal echocardiographic study in patients with suspected infective endocarditis. J Am Coll Cardiol 1993; 21:216–221.
- Koh DB, Gowardman JR, Rickard CM, Robertson IK, Brown A. Prospective study of peripheral arterial catheter infection and comparison with concurrently sited central venous catheters. Crit Care Med 2008; 36:397–402.
- Ruhe JJ, Menon A. Clinical significance of isolated Staphylococcus aureus central venous catheter tip cultures. Clin Microbiol Infect 2006; 12:933–936.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–317.
- Jones RN, Marshall SA, Pfaller MA, et al. Nosocomial enterococcal blood stream infections in the SCOPE Program: antimicrobial resistance, species occurrence, molecular testing results, and laboratory testing accuracy. SCOPE Hospital Study Group. Diagn Microbiol Infect Dis 1997; 29:95–102.
- DiazGranados CA, Jernigan JA. Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. J Infect Dis 2005; 191:588–595.
- Bhavnani SM, Drake JA, Forrest A, et al. A nationwide, multicenter, case-control study comparing risk factors, treatment, and outcome for vancomycin-resistant and -susceptible enterococcal bacteremia. Diagn Microbiol Infect Dis 2000; 36:145–158.
- Gonzales RD, Schreckenberger PC, Graham MB, Kelkar S, DenBesten K, Quinn JP. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 2001; 357:1179.
- Kanafani ZA, Federspiel JJ, Fowler VG. Infective endocarditis caused by daptomycin-resistant Enterococcus faecalis: a case report. Scand J Infect Dis 2007; 39:75–77.
- Maki DG, Agger WA. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 1988; 67:248–269.
- Gray J, Marsh PJ, Stewart D, Pedler SJ. Enterococcal bacteraemia: a prospective study of 125 episodes. J Hosp Infect 1994; 27:179–186.
- Sandoe JA, Witherden IR, Au-Yeung HK, Kite P, Kerr KG, Wilcox MH. Enterococcal intravascular catheter-related bloodstream infection: management and outcome of 61 consecutive cases. J Antimicrob Chemother 2002; 50:577–582.
- Anderson DJ, Murdoch DR, Sexton DJ, et al. Risk factors for infective endocarditis in patients with enterococcal bacteremia: a case-control study. Infection 2004; 32:72–77.
- Fernández-Guerrero ML, Herrero L, Bellver M, Gadea I, Roblas RF, de Górgolas M. Nosocomial enterococcal endocarditis: a serious hazard for hospitalized patients with enterococcal bacteraemia. J Intern Med 2002; 252:510–515.
- Elting LS, Bodey GP. Septicemia due to Xanthomonas species and non-aeruginosa Pseudomonas species: increasing incidence of catheter-related infections. Medicine (Baltimore) 1990; 69:296–306.
- Fernandez-Hidalgo N, Almirante B, Calleja R, et al. Antibiotic-lock therapy for long-term intravascular catheter-related bacteraemia: results of an open, non-comparative study. J Antimicrob Chemother 2006; 57:1172–1180.
- Poole CV, Carlton D, Bimbo L, Allon M. Treatment of catheter-related bacteraemia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol Dial Transplant 2004; 19:1237–1244.
- Paterson DL, Ko WC, Von Gottberg A, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol 2001; 39:2206–2212.
- Nguyen MH, Peacock JE, Tanner DC, et al. Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Arch Intern Med 1995; 155:2429–2435.
- Hung CC, Chen YC, Chang SC, Luh KT, Hsieh WC. Nosocomial candidemia in a university hospital in Taiwan. J Formos Med Assoc 1996; 95:19–28.
- Rex JH, Bennett JE, Sugar AM, et al. Intravascular catheter exchange and duration of candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Clin Infect Dis 1995; 21:994–996.
- Karlowicz MG, Hashimoto LN, Kelly RE, Buescher ES. Should central venous catheters be removed as soon as candidemia is detected in neonates? Pediatrics 2000; 106:E63.
- Nucci M, Colombo AL, Silveira F, et al. Risk factors for death in patients with candidemia. Infect Control Hosp Epidemiol 1998; 19:846–850.
- Almirante B, Rodríguez D, Park BJ, et al; Barcelona Candidemia Project Study Group. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 2005; 43:1829–1835.
- Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med 1994; 331:1325–1330.
- Kuse ER, Chetchotisakd P, da Cunha CA, et al; Micafungin Invasive Candidiasis Working Group. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369:1519–1527.
- Reboli AC, Rotstein C, Pappas PG, et al; Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472–2482.
- Mora-Duarte J, Betts R, Rotstein C, et al; Caspofungin Invasive Candidiasis Study Group. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 2002; 347:2020–2029.
- Peces R, Gago E, Tejada F, Laures AS, Alvarez-Grande J. Relapsing bacteraemia due to Micrococcus luteus in a haemodialysis patient with a Perm-Cath catheter. Nephrol Dial Transplant 1997; 12:2428–2429.
- Cotton DJ, Gill VJ, Marshall DJ, Gress J, Thaler M, Pizzo PA. Clinical features and therapeutic interventions in 17 cases of Bacillus bacteremia in an immunosuppressed patient population. J Clin Microbiol 1987; 25:672–674.
KEY POINTS
- Most bloodstream infections related to central venous catheters occur in patients with short-term central venous catheters; these infections result in significant morbidity and health care costs.
- Initial management of suspected cases requires decisions about whether to retain or remove the catheter and the choice of empiric antibiotic therapy.
- Management should be based on the specific pathogen isolated.
- An infectious disease specialist should be consulted in complicated cases or when multidrug-resistant bacteria or uncommon pathogens are isolated.
Proton pump inhibitor side effects and drug interactions: Much ado about nothing?
The development and introduction of the first proton pump inhibitor (PPI), omeprazole (Prilosec), for the management of acid-peptic disorders marks one of the great success stories in gastroenterology. Until the latter part of the 20th century, complications of acid-peptic disease were among the most common problems faced in gastroenterology. Severe peptic strictures were once a highly prevalent cause of dysphagia, and operations for peptic ulcer disease were routinely learned by surgical trainees.
The success of these drugs, with sales total-ling $13.6 billion worldwide in 2009,1 is not just a result of their potency and effectiveness in improving symptoms and complications of acid-peptic disease. Their safety among pharmacologic agents has been unparalleled. When the drugs were first introduced, their use was limited to short courses out of concern that gastric carcinoids could develop, but decades of use have not shown this issue to be of clinical relevance. Serious, acute adverse effects are also exceedingly uncommon.
However, recent reports have questioned the long-term safety of PPIs. Furthermore, these drugs are too often used in patients who have no valid indication for them,2,3 exposing these patients to unnecessary risks.
The goals of this review are to analyze the recent literature about the risks of PPIs and to provide a rational approach for managing patients on PPI therapy in light of these concerns.
DO PPIs REDUCE THE EFFECT OF CLOPIDOGREL?
Clopidogrel (Plavix) is a potent antiplatelet agent commonly used in patients with atherosclerotic cardiac or cerebrovascular disease, sometimes in combination with aspirin. Because of the risk of significant gastrointestinal bleeding, a 2008 multisociety task force recommended prescribing a PPI when both clopidogrel and aspirin are used as dual antiplatelet therapy.4
Studies of clopidogrel plus PPIs: Discrepant results
To date, only one prospective randomized controlled trial has specifically investigated the effect of PPIs on cardiovascular outcomes in patients using clopidogrel. In this trial, patients on dual antiplatelet therapy with clopidogrel and aspirin were randomized to receive either omeprazole 20 mg or placebo. Analysis of the data revealed no significant increase in the composite end point of cardiovascular events (hazard ratio [HR] 0.99, 95% confidence interval [CI] 0.68–1.44, P = .96), but a statistically significant decrease in composite gastrointestinal events (HR 0.34, 95% CI 0.18–0.63, P < .001).17
Unfortunately, this trial had to be terminated before the prespecified sample size and duration of follow-up were reached because the study sponsor declared bankruptcy.
One additional recent retrospective cohort study16 likewise found no significant risk of serious cardiovascular disease related to PPI use in clopidogrel users. It also found that the adjusted incidence of hospitalization for upper gastrointestinal bleeding was 50% lower in patients who used PPIs than in those who did not (HR 0.50, 95% CI 0.39–0.65).
Do factors other than PPIs account for the higher risk in some of the studies?
The discrepant results of these studies suggest that the higher risk of cardiovascular events may be due, either completely or in part, to a factor other than the pharmacologic interaction of PPIs and clopidogrel. It is difficult to infer causality from the available data. In situations in which no randomized controlled trials exist, one looks to observational (case-control or cohort) studies to try to obtain the best estimate of the actual risk. With PPIs and clopidogrel, a randomized controlled trial was performed but terminated before patient enrollment was complete.
The increased risk found in some of these studies may be real, may be due to chance, or may even represent an increased risk from PPIs alone (although data do not support this possibility).18 However, the major concern in observational studies is the inability to account for unmeasured confounders, a problem virtually eliminated by randomization strategies in prospective studies.
In the studies that found a higher risk with the combination of omeprazole plus clopidogrel, the principal concern is confounding by indication, in which distortions of the risk estimates arise from an imbalance in prognostic factors between compared treatment groups that remains unmeasured.19 Stated another way, physicians who believed some patients to be “sicker” or to have a higher risk of serious events may have treated them with a PPI on the basis of factors that remained unaccounted for in the epidemiologic investigation.
This possibility has been reinforced by findings from a nonrandomized subgroup analysis of a randomized controlled trial in which patients who had been receiving a PPI had a higher rate of cardiovascular events whether they received clopidogrel or placebo.20
FDA alert: Avoid using omeprazole or esomeprazole with clopidogrel
Nonetheless, on November 17, 2009, the US Food and Drug Administration (FDA) issued an alert to health care professionals and the public about the potential interaction between clopidogrel and omeprazole.21 In this alert, the FDA stated that the use of omeprazole or esomeprazole (Nexium) with clopidogrel should be avoided.
An algorithm to use when considering clopidogrel plus a PPI
Physicians are now left in a bind between the minimal, if any, pooled risk seen in the available data and the FDA recommendation. What is the best action to take?
First, assess the need for dual antiplatelet therapy. If dual antiplatelet therapy (clopidogrel plus aspirin) is required, then a PPI is warranted for gastric protection because the risk of life-threatening bleeding outweighs any increased risk of cardiovascular events.4
If antiplatelet monotherapy (clopidogrel alone) is required, then assess the reason for antisecretory therapy.
For complicated disease, such as gastroesophageal reflux disease with Barrett esophagus or peptic strictures, PPI therapy is warranted to prevent progression or recurrence of complications. If the antisecretory therapy is being provided for noncomplicated symptomatic disorders such as nonerosive gastroesophageal reflux disease or dyspepsia, then one should try to “step down” the therapy by lowering the PPI dose as much as possible while still controlling symptoms to the patient’s tolerance, then possibly stepping further by substituting a histamine-2-receptor antagonist, an antacid, or “on-demand” use of PPIs.22,23
However, if the rationale for antisecretory therapy is simply for gastrointestinal protection, then further risk stratification for gastro intestinal bleeding should be undertaken.4 For patients with a high risk of future gastrointestinal bleeding, such as those with prior episodes of bleeding or concurrent use of nonsteroidal anti-inflammatory drugs, antisecretory therapy is still recommended. Therefore, if a patient is on monotherapy with clopidogrel, has no complicated or symptomatic gastrointestinal disorder, and does not have a high risk of gastrointestinal bleeding, then therapy with a PPI should be reconsidered.
There are no strong data to indicate that one particular PPI should be used or avoided if one of the above criteria indicates the concurrent need for clopidogrel and a PPI. In their health alert about the potential interaction, the FDA did not issue the same warning for PPIs other than omeprazole and esomeprazole, but fell short of recommending a change to another PPI because of a lack of data to support or refute a similar interaction.
Because the half-lives of clopidogrel and PPIs are short, separating their administration could in theory decrease or eliminate the risk of competitive inhibition. The PPI could be given in the morning before breakfast and the clopidogrel could be given at night, or the clopidogrel could be given at lunchtime and the PPI before dinner. Although the FDA does not believe this strategy will reduce this interaction,21 one expert in the field has suggested it.18
DO PPIs CAUSE OSTEOPOROSIS, FRACTURES?
In a widely publicized paper published in 2006, Yang and colleagues25 reported the results of a large nested case-control study in the United Kingdom. The risk of hip fracture was significantly greater in patients who had been using PPIs for at least 1 year than in those who had not. The risk appeared to increase with longer use and higher doses of PPIs.
A similar risk of hip fracture was seen in a larger Danish case-control study published the same year.26 This study also found an increased odds ratio for PPI use in patients with spine fractures as well as in patients with any type of fracture. Interestingly, this study found a lower risk of fracture in patients using a histamine-2-receptor antagonist instead of a PPI.
Targownik et al27 found that the risk of hip fracture was not significantly higher until after 5 years of PPI exposure, with an even stronger risk after 7 years.
However, the data on both association and causal relationship are not uniform.
The Women’s Health Initiative,30 with more than 1 million person-years of followup, found no association between PPI use and hip fracture, but a modest association between PPI use and spine, arm, and wrist fractures, as well as total fractures.
A study in the United Kingdom found that patients without any major risk factors for hip fracture (defined by a risk ratio > 2) accounted for only 25% of cases but 53% of controls. When only these two average-risk groups were compared, the risk of hip fracture was similar in cases and controls.31
Corley et al32 also found that the risk of fracture associated with PPI use was only significant in the presence of another risk factor. These findings suggest that residual confounding may be to blame, at least in part, for the estimates of increased risk in the prior studies.
Another way to interpret these data is that PPIs increase the risk in patients at high risk to begin with, but not in those at average risk. This is an example of interaction (or effect modification) in which the risk is unequally distributed across groups with different characteristics.
A recently published study refutes the theory that impaired calcium absorption is responsible for the increase in fractures.33 In this study, investigators queried the Manitoba Bone Mineral Density Database to determine the relationship between antisecretory therapy with PPIs and osteoporosis or loss of bone mineral density—and they found none. This study may support the theory that residual confounding is the reason for the finding of an increased risk, but it also leaves open the possibility that PPIs induce other changes in bone microstructure that could increase the risk of fracture.
FDA labeling: Possible risk of fracture with PPIs
Based on the data so far, it appears possible that there is a small, albeit statistically significant, association between PPI use and fracture risk. The association is indeed biologically plausible, but it remains to be seen if this association is clinically significant, as the risk is relatively low. Even though the studies had methodologic limitations, on May 25, 2010, the FDA announced a change in the required labeling information for PPIs to indicate a possible risk of fracture with these drugs.34
Reassess the need for chronic PPI therapy
Although patients may worry that they will develop osteoporosis and fractures if they take PPIs, the data do not support a strong risk. Nevertheless, when faced with a patient on chronic PPI therapy, especially with a high dose, providers should use the opportunity to reassess the indication for the PPI to decide if chronic therapy is required, in a matter similar to the algorithm provided for PPI-clopidogrel cotherapy (FIGURE 2). Providers should educate patients about the data, and limit new and recurring PPI prescriptions to patients who require a PPI for appropriate indications, at the lowest dose, and for the shortest time possible.
DO PPIs INCREASE THE RISK OF PNEUMONIA?
Several recent studies have also raised concern about an association between PPI use and pneumonia.
Normally, the stomach remains free of bacteria (except for Helicobacter pylori) because its acidic milieu destroys nearly all bacteria swallowed. If the stomach becomes less acidic, it loses this protective mechanism, and ingested organisms can survive and proliferate.35 In theory, when gastroesophageal reflux occurs, these bacteria could be carried up to the hypopharynx where microaspiration into the lower airways could lead to pneumonia, especially in patients with compromised oropharyngeal protective reflexes (eg, patients on mechanical ventilation).
This possible association came to the attention of the general medical community when a Dutch study,36 in which 5,551 cases of community-acquired pneumonia developed in 364,683 people, found that the incidence of pneumonia was about 4.5 times higher in people exposed to acid-suppressive drugs (both PPIs and histamine-2-receptor antagonists) than in unexposed individuals. Patients who developed pneumonia also had higher odds of significant comorbid conditions, including heart failure and chronic obstructive pulmonary disease. The authors calculated that about one case of pneumonia per 226 patients treated with a PPI would be attributable to the PPI. A major limitation of this study, however, was that only 18% of the patients diagnosed with pneumonia actually had radiologic or microbiologic confirmation of pneumonia.
Other studies later examined the relationship between PPIs and community-acquired pneumonia,37–41 and most have revealed a modestly higher risk of community-acquired pneumonia in patients exposed to PPIs.
This risk was confirmed in a recent metaanalysis, which found a higher risk of community-acquired pneumonia with PPI use (odds ratio 1.36, 95% CI 1.12–1.65).42 However, the authors refrained from drawing definitive conclusions from these data because of significant heterogeneity between the studies. One study37 found that recent onset of use (within 7 days) had a much stronger association with community-acquired pneumonia than longer-term use, which is contradictory to a causal association, since longer-term use should lead to more cases of pneumonia.
Another study investigated the association between acid-suppressive drugs and hospital-acquired pneumonia in nonventilated patients.43 In a 4-year period, there were 63,878 admissions in 42,093 unique patients. Acid-suppressive drugs were prescribed in 32,922 admissions (52%); the drugs included PPIs in 83% of these. Hospital-acquired pneumonia occurred in 2,219 admissions (3.5%), with a higher incidence in patients exposed to acid-suppressive drugs than in the unexposed group (4.6% vs 2.0%). The adjusted odds ratio for pneumonia was 1.3 (95% CI 1.1–1.4) in the exposed group. Subgroup analysis revealed that the association remained significant for PPIs but not for histamine-2-receptor antagonists.
Adequate studies of mechanically ventilated patients in the current era of intravenous PPI use are lacking. Older studies in this group of patients may not be generalizable to current practice because of the reduction in gastric volume with intravenous PPIs that may offset the theoretical risk of aspiration.35
Although the data supporting the association are not exceedingly strong, the relationship is biologically plausible. If there is a risk, it seems to be greatest in the sickest patients, who can least afford to develop pneumonia. Therefore, prudent prescribing should be the rule for both inpatients and outpatients, especially in patients with comorbidities, in whom pneumonia could have serious consequences.
PPIs AND ENTERIC INFECTIONS
Traditionally, gastric acid was not believed to be important in protecting against Clostridium difficile infection because acid-resistant spores were presumed to be the principal vector of transmission.44 Recently, this thought has been challenged, as several studies have found a higher risk of C difficile infection in PPI users. In theory, PPIs may increase the risk of C difficile infection by increasing the ability of the spore to convert to the vegetative form and to survive intraluminally.
A recent meta-analysis of 11 papers, including nearly 127,000 patients, found a significant relationship between PPI use and C difficile infection, with an odds ratio of 2.05 (95% CI 1.47–2.85).45 Further supporting the hypothesis of a direct causative association, a recent study found a significant dose-response, with more aggressive acid-suppression associated with higher odds ratios.46 In view of this association, patients using PPIs who develop diarrhea should be evaluated for C difficile, perhaps even in the absence of other risk factors.
Other enteric infections have been found to be associated with PPIs.44,45 Small intestinal bacterial overgrowth, a condition that is associated with bloating, diarrhea, and malabsorption, has recently been associated with PPI use, although the significance of the association is uncertain.47
Based on a change in the intestinal flora, recent reports have additionally implied that there is a relationship between PPI use and the development of spontaneous bacterial peritonitis in hospitalized cirrhotic patients with ascites. One study found a strong association (odds ratio 4.3, 95% CI 1.3–11.7) between PPIs and spontaneous bacterial pneumonitis,48 whereas another study found no significant association (odds ratio 1.0, 95% CI 0.4–2.6).49
Both studies were small case-control studies of hospitalized patients. No firm conclusion can be drawn about the relevance of this association from these investigations at this point.
PPIs AND ACUTE INTERSTITIAL NEPHRITIS
Several case reports have implicated PPIs as a cause of acute interstitial nephritis.
A systematic review from 2007 found 64 cases documented in the literature, 12 of which were considered certainly associated, and 9 of which were probably associated.50 Initial symptoms were nonspecific and included nausea, malaise, and fever. With such extensive use worldwide as the denominator, the authors concluded that acute interstitial nephritis was a rare, idiosyncratic occurrence related to PPI use, but did not find enough evidence to support a causative relationship. Despite the rarity of the syndrome, they recommended maintaining a high level of clinical suspicion to detect acute interstitial nephritis early in its course, especially soon after the initiation of PPI therapy.
POSSIBLE ASSOCIATIONS WITH IRON AND B12 DEFICIENCIES
Long-term PPI therapy has been thought to be associated with micronutrient deficiencies, especially of iron and vitamin B12. Hydrochloric acid in the stomach assists in the dissociation of iron salts from food and the reduction of ferric iron to the more soluble ferrous iron.51 Gastric acid also facilitates the release of vitamin B12 bound to proteins within ingested foodstuffs to permit binding to R-proteins for eventual absorption in the terminal ileum.51,52
Despite the biologic plausibility of these deficiencies, there is currently little evidence to support a clinically relevant association to recommend a change in current practice.
NO THERAPY IS COMPLETELY WITHOUT RISK
Although concerns have been raised about the long-term safety of PPIs, the preponderance of the evidence does not strongly support the apprehensions publicized over the last few years. When translating these studies into the routine management of patients, it is important to recall some very basic tenets of good patient care.
No therapy is completely without risk—whether pharmacologic, surgical, or psychological, and no matter how benign or straightforward. Consequently, no drug, procedure, or treatment plan should be ordered without a valid indication. Even with an indication, the risk-benefit ratio of the therapy prescribed should always be considered. If the indication for the PPI is weak or uncertain, then even a slight risk tips the balance away from the drug, and the drug should be discontinued.
When seeing patients in long-term care, the indication and necessity for all drugs, including PPIs, should be reviewed. The algorithm proposed in Figure 2 can be adapted for virtually any of the possible associations.
Consider the indication for the PPI. Was the PPI started during a hospitalization and then routinely continued after discharge? This is one situation in which the use of a PPI could potentially be discontinued.2
For complicated acid-peptic disease, dose reduction or cessation of PPI therapy may not be possible.
If the PPI was prescribed only for symptom relief, as in cases of dyspepsia or nonerosive gastroesophageal reflux disease, reduce the dose of PPI to as low as possible to maintain symptom control. Should chronic therapy still be required, no specific monitoring is recommended, apart from routine monitoring that takes place in the course of patient care.
Lastly, because of the media attention that several of these concerns have garnered, patients may still harbor significant concerns about PPIs, even their short-term use. In such cases, the prescriber should take the opportunity to communicate the reason for the decision to prescribe the PPI, as well as the best available data about the risks PPIs may pose. None of these outcomes is very common in the absence of PPIs, with the possible exception of recurrent cardiovascular events, and the risks provided in all of these studies are relative to the baseline risk. Even if the risk of a particular outcome doubles with long-term PPI use, twice a small risk remains a small risk.
- Gatyas G. IMS Health reports U.S. prescription sales grew 5.1 percent in 2009, to $300.3 Billion. IMS Health. http://www.imshealth.com/portal/site/imshealth/menuitem.a46c6d4df3db4b3d88f611019418c22a/?vgnextoid=d690a27e9d5b7210VgnVCM100000ed152ca2RCRD&vgnextfmt=default. Accessed 10/7/2010.
- Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005; 21:1203–1209.
- Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol 2006; 101:2200–2205.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118:1894–1909.
- Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol 2004; 95:2–8.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008; 51:256–260.
- Small DS, Farid NA, Payne CD, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Sibbing D, Morath T, Stegherr J, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost 2009; 101:714–719.
- O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009; 180:713–718.
- Stanek EJ, Aubert RE, Flockhart DA, et al. A national study of the effect of individual proton pump inhibitors on cardiovascular outcomes in patients treated with clopidogrel following coronary stenting: the Clopidogrel Medco Outcomes Study. Program and abstracts of the 32nd Annual SCAI Scientific Sessions May 6, 2009; Las Vegas, Nevada.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Ramirez JF, Selzer F, Chakaprani R, et al. Proton pump inhibitor and clopidogrel combination is not associated with adverse clinical outcomes after PCI: the NHLBI dynamic registry (abstract). J Am Coll Cardiol 2009; 53(suppl 1):A27.
- Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med 2010; 152:337–345.
- Bhatt DL, Cryer B, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol 2010; 105:34–41.
- Walker AM. Confounding by indication. Epidemiology 1996; 7:335–336.
- Dunn SP, Macaulay TE, Brennan DM, et al. Baseline proton pump inhibitor use is associated with increased cardiovascular events with and without the use of clopidogrel in the CREDO trial (abstract). Circulation 2008; 118:S815.
- US Food and Drug Administration. Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). U.S. Department of Health and Human Services, 11/17/2009. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm. Accessed 9/23/2010.
- Inadomi JM, Jamal R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology 2001; 121:1095–1100.
- Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol 2003; 98:1940–1944.
- O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 2005; 118:778–781.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 2006; 79:76–83.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008; 179:319–326.
- Roux C, Briot K, Gossec L, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int 2009; 84:13–19.
- Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int 2008; 83:251–259.
- Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med 2010; 170:765–771.
- Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 2008; 28:951–959.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 2010; 138:896–904.
- US Food and Drug Administration. FDA Drug Safety Communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. U.S. Department of Health and Human Services, 5/25/2010. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed 12/7/2010.
- Vakil N. Acid inhibition and infections outside the gastrointestinal tract. Am J Gastroenterol 2009; 104(suppl 2):S17–S20.
- Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004; 292:1955–1960.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008; 149:391–398.
- Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community-acquired pneumonia and the use of statins, ACE inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf 2009; 18:269–275.
- Rodríguez LA, Ruigómez A, Wallander MA, Johansson S. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology 2009; 20:800–806.
- Eurich DT, Sadowski CA, Simpson SH, Marrie TJ, Majumdar SR. Recurrent community-acquired pneumonia in patients starting acid-suppressing drugs. Am J Med 2010; 123:47–53.
- Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther 2010; 31:1165–1177.
- Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA 2009; 301:2120–2128.
- Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol 2009; 104(suppl 2):S10–S16.
- Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007; 102:2047–2056.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2010; 8:504–508.
- Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol 2009; 104:1130–1134.
- Campbell MS, Obstein K, Reddy KR, Yang YX. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci 2008; 53:394–398.
- Sierra F, Suarez M, Rey M, Vela MF. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther 2007; 26:545–553.
- McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol 2009; 104(suppl 2):S5–S9.
- Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med 2009; 122:896–903.
The development and introduction of the first proton pump inhibitor (PPI), omeprazole (Prilosec), for the management of acid-peptic disorders marks one of the great success stories in gastroenterology. Until the latter part of the 20th century, complications of acid-peptic disease were among the most common problems faced in gastroenterology. Severe peptic strictures were once a highly prevalent cause of dysphagia, and operations for peptic ulcer disease were routinely learned by surgical trainees.
The success of these drugs, with sales total-ling $13.6 billion worldwide in 2009,1 is not just a result of their potency and effectiveness in improving symptoms and complications of acid-peptic disease. Their safety among pharmacologic agents has been unparalleled. When the drugs were first introduced, their use was limited to short courses out of concern that gastric carcinoids could develop, but decades of use have not shown this issue to be of clinical relevance. Serious, acute adverse effects are also exceedingly uncommon.
However, recent reports have questioned the long-term safety of PPIs. Furthermore, these drugs are too often used in patients who have no valid indication for them,2,3 exposing these patients to unnecessary risks.
The goals of this review are to analyze the recent literature about the risks of PPIs and to provide a rational approach for managing patients on PPI therapy in light of these concerns.
DO PPIs REDUCE THE EFFECT OF CLOPIDOGREL?
Clopidogrel (Plavix) is a potent antiplatelet agent commonly used in patients with atherosclerotic cardiac or cerebrovascular disease, sometimes in combination with aspirin. Because of the risk of significant gastrointestinal bleeding, a 2008 multisociety task force recommended prescribing a PPI when both clopidogrel and aspirin are used as dual antiplatelet therapy.4
Studies of clopidogrel plus PPIs: Discrepant results
To date, only one prospective randomized controlled trial has specifically investigated the effect of PPIs on cardiovascular outcomes in patients using clopidogrel. In this trial, patients on dual antiplatelet therapy with clopidogrel and aspirin were randomized to receive either omeprazole 20 mg or placebo. Analysis of the data revealed no significant increase in the composite end point of cardiovascular events (hazard ratio [HR] 0.99, 95% confidence interval [CI] 0.68–1.44, P = .96), but a statistically significant decrease in composite gastrointestinal events (HR 0.34, 95% CI 0.18–0.63, P < .001).17
Unfortunately, this trial had to be terminated before the prespecified sample size and duration of follow-up were reached because the study sponsor declared bankruptcy.
One additional recent retrospective cohort study16 likewise found no significant risk of serious cardiovascular disease related to PPI use in clopidogrel users. It also found that the adjusted incidence of hospitalization for upper gastrointestinal bleeding was 50% lower in patients who used PPIs than in those who did not (HR 0.50, 95% CI 0.39–0.65).
Do factors other than PPIs account for the higher risk in some of the studies?
The discrepant results of these studies suggest that the higher risk of cardiovascular events may be due, either completely or in part, to a factor other than the pharmacologic interaction of PPIs and clopidogrel. It is difficult to infer causality from the available data. In situations in which no randomized controlled trials exist, one looks to observational (case-control or cohort) studies to try to obtain the best estimate of the actual risk. With PPIs and clopidogrel, a randomized controlled trial was performed but terminated before patient enrollment was complete.
The increased risk found in some of these studies may be real, may be due to chance, or may even represent an increased risk from PPIs alone (although data do not support this possibility).18 However, the major concern in observational studies is the inability to account for unmeasured confounders, a problem virtually eliminated by randomization strategies in prospective studies.
In the studies that found a higher risk with the combination of omeprazole plus clopidogrel, the principal concern is confounding by indication, in which distortions of the risk estimates arise from an imbalance in prognostic factors between compared treatment groups that remains unmeasured.19 Stated another way, physicians who believed some patients to be “sicker” or to have a higher risk of serious events may have treated them with a PPI on the basis of factors that remained unaccounted for in the epidemiologic investigation.
This possibility has been reinforced by findings from a nonrandomized subgroup analysis of a randomized controlled trial in which patients who had been receiving a PPI had a higher rate of cardiovascular events whether they received clopidogrel or placebo.20
FDA alert: Avoid using omeprazole or esomeprazole with clopidogrel
Nonetheless, on November 17, 2009, the US Food and Drug Administration (FDA) issued an alert to health care professionals and the public about the potential interaction between clopidogrel and omeprazole.21 In this alert, the FDA stated that the use of omeprazole or esomeprazole (Nexium) with clopidogrel should be avoided.
An algorithm to use when considering clopidogrel plus a PPI
Physicians are now left in a bind between the minimal, if any, pooled risk seen in the available data and the FDA recommendation. What is the best action to take?
First, assess the need for dual antiplatelet therapy. If dual antiplatelet therapy (clopidogrel plus aspirin) is required, then a PPI is warranted for gastric protection because the risk of life-threatening bleeding outweighs any increased risk of cardiovascular events.4
If antiplatelet monotherapy (clopidogrel alone) is required, then assess the reason for antisecretory therapy.
For complicated disease, such as gastroesophageal reflux disease with Barrett esophagus or peptic strictures, PPI therapy is warranted to prevent progression or recurrence of complications. If the antisecretory therapy is being provided for noncomplicated symptomatic disorders such as nonerosive gastroesophageal reflux disease or dyspepsia, then one should try to “step down” the therapy by lowering the PPI dose as much as possible while still controlling symptoms to the patient’s tolerance, then possibly stepping further by substituting a histamine-2-receptor antagonist, an antacid, or “on-demand” use of PPIs.22,23
However, if the rationale for antisecretory therapy is simply for gastrointestinal protection, then further risk stratification for gastro intestinal bleeding should be undertaken.4 For patients with a high risk of future gastrointestinal bleeding, such as those with prior episodes of bleeding or concurrent use of nonsteroidal anti-inflammatory drugs, antisecretory therapy is still recommended. Therefore, if a patient is on monotherapy with clopidogrel, has no complicated or symptomatic gastrointestinal disorder, and does not have a high risk of gastrointestinal bleeding, then therapy with a PPI should be reconsidered.
There are no strong data to indicate that one particular PPI should be used or avoided if one of the above criteria indicates the concurrent need for clopidogrel and a PPI. In their health alert about the potential interaction, the FDA did not issue the same warning for PPIs other than omeprazole and esomeprazole, but fell short of recommending a change to another PPI because of a lack of data to support or refute a similar interaction.
Because the half-lives of clopidogrel and PPIs are short, separating their administration could in theory decrease or eliminate the risk of competitive inhibition. The PPI could be given in the morning before breakfast and the clopidogrel could be given at night, or the clopidogrel could be given at lunchtime and the PPI before dinner. Although the FDA does not believe this strategy will reduce this interaction,21 one expert in the field has suggested it.18
DO PPIs CAUSE OSTEOPOROSIS, FRACTURES?
In a widely publicized paper published in 2006, Yang and colleagues25 reported the results of a large nested case-control study in the United Kingdom. The risk of hip fracture was significantly greater in patients who had been using PPIs for at least 1 year than in those who had not. The risk appeared to increase with longer use and higher doses of PPIs.
A similar risk of hip fracture was seen in a larger Danish case-control study published the same year.26 This study also found an increased odds ratio for PPI use in patients with spine fractures as well as in patients with any type of fracture. Interestingly, this study found a lower risk of fracture in patients using a histamine-2-receptor antagonist instead of a PPI.
Targownik et al27 found that the risk of hip fracture was not significantly higher until after 5 years of PPI exposure, with an even stronger risk after 7 years.
However, the data on both association and causal relationship are not uniform.
The Women’s Health Initiative,30 with more than 1 million person-years of followup, found no association between PPI use and hip fracture, but a modest association between PPI use and spine, arm, and wrist fractures, as well as total fractures.
A study in the United Kingdom found that patients without any major risk factors for hip fracture (defined by a risk ratio > 2) accounted for only 25% of cases but 53% of controls. When only these two average-risk groups were compared, the risk of hip fracture was similar in cases and controls.31
Corley et al32 also found that the risk of fracture associated with PPI use was only significant in the presence of another risk factor. These findings suggest that residual confounding may be to blame, at least in part, for the estimates of increased risk in the prior studies.
Another way to interpret these data is that PPIs increase the risk in patients at high risk to begin with, but not in those at average risk. This is an example of interaction (or effect modification) in which the risk is unequally distributed across groups with different characteristics.
A recently published study refutes the theory that impaired calcium absorption is responsible for the increase in fractures.33 In this study, investigators queried the Manitoba Bone Mineral Density Database to determine the relationship between antisecretory therapy with PPIs and osteoporosis or loss of bone mineral density—and they found none. This study may support the theory that residual confounding is the reason for the finding of an increased risk, but it also leaves open the possibility that PPIs induce other changes in bone microstructure that could increase the risk of fracture.
FDA labeling: Possible risk of fracture with PPIs
Based on the data so far, it appears possible that there is a small, albeit statistically significant, association between PPI use and fracture risk. The association is indeed biologically plausible, but it remains to be seen if this association is clinically significant, as the risk is relatively low. Even though the studies had methodologic limitations, on May 25, 2010, the FDA announced a change in the required labeling information for PPIs to indicate a possible risk of fracture with these drugs.34
Reassess the need for chronic PPI therapy
Although patients may worry that they will develop osteoporosis and fractures if they take PPIs, the data do not support a strong risk. Nevertheless, when faced with a patient on chronic PPI therapy, especially with a high dose, providers should use the opportunity to reassess the indication for the PPI to decide if chronic therapy is required, in a matter similar to the algorithm provided for PPI-clopidogrel cotherapy (FIGURE 2). Providers should educate patients about the data, and limit new and recurring PPI prescriptions to patients who require a PPI for appropriate indications, at the lowest dose, and for the shortest time possible.
DO PPIs INCREASE THE RISK OF PNEUMONIA?
Several recent studies have also raised concern about an association between PPI use and pneumonia.
Normally, the stomach remains free of bacteria (except for Helicobacter pylori) because its acidic milieu destroys nearly all bacteria swallowed. If the stomach becomes less acidic, it loses this protective mechanism, and ingested organisms can survive and proliferate.35 In theory, when gastroesophageal reflux occurs, these bacteria could be carried up to the hypopharynx where microaspiration into the lower airways could lead to pneumonia, especially in patients with compromised oropharyngeal protective reflexes (eg, patients on mechanical ventilation).
This possible association came to the attention of the general medical community when a Dutch study,36 in which 5,551 cases of community-acquired pneumonia developed in 364,683 people, found that the incidence of pneumonia was about 4.5 times higher in people exposed to acid-suppressive drugs (both PPIs and histamine-2-receptor antagonists) than in unexposed individuals. Patients who developed pneumonia also had higher odds of significant comorbid conditions, including heart failure and chronic obstructive pulmonary disease. The authors calculated that about one case of pneumonia per 226 patients treated with a PPI would be attributable to the PPI. A major limitation of this study, however, was that only 18% of the patients diagnosed with pneumonia actually had radiologic or microbiologic confirmation of pneumonia.
Other studies later examined the relationship between PPIs and community-acquired pneumonia,37–41 and most have revealed a modestly higher risk of community-acquired pneumonia in patients exposed to PPIs.
This risk was confirmed in a recent metaanalysis, which found a higher risk of community-acquired pneumonia with PPI use (odds ratio 1.36, 95% CI 1.12–1.65).42 However, the authors refrained from drawing definitive conclusions from these data because of significant heterogeneity between the studies. One study37 found that recent onset of use (within 7 days) had a much stronger association with community-acquired pneumonia than longer-term use, which is contradictory to a causal association, since longer-term use should lead to more cases of pneumonia.
Another study investigated the association between acid-suppressive drugs and hospital-acquired pneumonia in nonventilated patients.43 In a 4-year period, there were 63,878 admissions in 42,093 unique patients. Acid-suppressive drugs were prescribed in 32,922 admissions (52%); the drugs included PPIs in 83% of these. Hospital-acquired pneumonia occurred in 2,219 admissions (3.5%), with a higher incidence in patients exposed to acid-suppressive drugs than in the unexposed group (4.6% vs 2.0%). The adjusted odds ratio for pneumonia was 1.3 (95% CI 1.1–1.4) in the exposed group. Subgroup analysis revealed that the association remained significant for PPIs but not for histamine-2-receptor antagonists.
Adequate studies of mechanically ventilated patients in the current era of intravenous PPI use are lacking. Older studies in this group of patients may not be generalizable to current practice because of the reduction in gastric volume with intravenous PPIs that may offset the theoretical risk of aspiration.35
Although the data supporting the association are not exceedingly strong, the relationship is biologically plausible. If there is a risk, it seems to be greatest in the sickest patients, who can least afford to develop pneumonia. Therefore, prudent prescribing should be the rule for both inpatients and outpatients, especially in patients with comorbidities, in whom pneumonia could have serious consequences.
PPIs AND ENTERIC INFECTIONS
Traditionally, gastric acid was not believed to be important in protecting against Clostridium difficile infection because acid-resistant spores were presumed to be the principal vector of transmission.44 Recently, this thought has been challenged, as several studies have found a higher risk of C difficile infection in PPI users. In theory, PPIs may increase the risk of C difficile infection by increasing the ability of the spore to convert to the vegetative form and to survive intraluminally.
A recent meta-analysis of 11 papers, including nearly 127,000 patients, found a significant relationship between PPI use and C difficile infection, with an odds ratio of 2.05 (95% CI 1.47–2.85).45 Further supporting the hypothesis of a direct causative association, a recent study found a significant dose-response, with more aggressive acid-suppression associated with higher odds ratios.46 In view of this association, patients using PPIs who develop diarrhea should be evaluated for C difficile, perhaps even in the absence of other risk factors.
Other enteric infections have been found to be associated with PPIs.44,45 Small intestinal bacterial overgrowth, a condition that is associated with bloating, diarrhea, and malabsorption, has recently been associated with PPI use, although the significance of the association is uncertain.47
Based on a change in the intestinal flora, recent reports have additionally implied that there is a relationship between PPI use and the development of spontaneous bacterial peritonitis in hospitalized cirrhotic patients with ascites. One study found a strong association (odds ratio 4.3, 95% CI 1.3–11.7) between PPIs and spontaneous bacterial pneumonitis,48 whereas another study found no significant association (odds ratio 1.0, 95% CI 0.4–2.6).49
Both studies were small case-control studies of hospitalized patients. No firm conclusion can be drawn about the relevance of this association from these investigations at this point.
PPIs AND ACUTE INTERSTITIAL NEPHRITIS
Several case reports have implicated PPIs as a cause of acute interstitial nephritis.
A systematic review from 2007 found 64 cases documented in the literature, 12 of which were considered certainly associated, and 9 of which were probably associated.50 Initial symptoms were nonspecific and included nausea, malaise, and fever. With such extensive use worldwide as the denominator, the authors concluded that acute interstitial nephritis was a rare, idiosyncratic occurrence related to PPI use, but did not find enough evidence to support a causative relationship. Despite the rarity of the syndrome, they recommended maintaining a high level of clinical suspicion to detect acute interstitial nephritis early in its course, especially soon after the initiation of PPI therapy.
POSSIBLE ASSOCIATIONS WITH IRON AND B12 DEFICIENCIES
Long-term PPI therapy has been thought to be associated with micronutrient deficiencies, especially of iron and vitamin B12. Hydrochloric acid in the stomach assists in the dissociation of iron salts from food and the reduction of ferric iron to the more soluble ferrous iron.51 Gastric acid also facilitates the release of vitamin B12 bound to proteins within ingested foodstuffs to permit binding to R-proteins for eventual absorption in the terminal ileum.51,52
Despite the biologic plausibility of these deficiencies, there is currently little evidence to support a clinically relevant association to recommend a change in current practice.
NO THERAPY IS COMPLETELY WITHOUT RISK
Although concerns have been raised about the long-term safety of PPIs, the preponderance of the evidence does not strongly support the apprehensions publicized over the last few years. When translating these studies into the routine management of patients, it is important to recall some very basic tenets of good patient care.
No therapy is completely without risk—whether pharmacologic, surgical, or psychological, and no matter how benign or straightforward. Consequently, no drug, procedure, or treatment plan should be ordered without a valid indication. Even with an indication, the risk-benefit ratio of the therapy prescribed should always be considered. If the indication for the PPI is weak or uncertain, then even a slight risk tips the balance away from the drug, and the drug should be discontinued.
When seeing patients in long-term care, the indication and necessity for all drugs, including PPIs, should be reviewed. The algorithm proposed in Figure 2 can be adapted for virtually any of the possible associations.
Consider the indication for the PPI. Was the PPI started during a hospitalization and then routinely continued after discharge? This is one situation in which the use of a PPI could potentially be discontinued.2
For complicated acid-peptic disease, dose reduction or cessation of PPI therapy may not be possible.
If the PPI was prescribed only for symptom relief, as in cases of dyspepsia or nonerosive gastroesophageal reflux disease, reduce the dose of PPI to as low as possible to maintain symptom control. Should chronic therapy still be required, no specific monitoring is recommended, apart from routine monitoring that takes place in the course of patient care.
Lastly, because of the media attention that several of these concerns have garnered, patients may still harbor significant concerns about PPIs, even their short-term use. In such cases, the prescriber should take the opportunity to communicate the reason for the decision to prescribe the PPI, as well as the best available data about the risks PPIs may pose. None of these outcomes is very common in the absence of PPIs, with the possible exception of recurrent cardiovascular events, and the risks provided in all of these studies are relative to the baseline risk. Even if the risk of a particular outcome doubles with long-term PPI use, twice a small risk remains a small risk.
The development and introduction of the first proton pump inhibitor (PPI), omeprazole (Prilosec), for the management of acid-peptic disorders marks one of the great success stories in gastroenterology. Until the latter part of the 20th century, complications of acid-peptic disease were among the most common problems faced in gastroenterology. Severe peptic strictures were once a highly prevalent cause of dysphagia, and operations for peptic ulcer disease were routinely learned by surgical trainees.
The success of these drugs, with sales total-ling $13.6 billion worldwide in 2009,1 is not just a result of their potency and effectiveness in improving symptoms and complications of acid-peptic disease. Their safety among pharmacologic agents has been unparalleled. When the drugs were first introduced, their use was limited to short courses out of concern that gastric carcinoids could develop, but decades of use have not shown this issue to be of clinical relevance. Serious, acute adverse effects are also exceedingly uncommon.
However, recent reports have questioned the long-term safety of PPIs. Furthermore, these drugs are too often used in patients who have no valid indication for them,2,3 exposing these patients to unnecessary risks.
The goals of this review are to analyze the recent literature about the risks of PPIs and to provide a rational approach for managing patients on PPI therapy in light of these concerns.
DO PPIs REDUCE THE EFFECT OF CLOPIDOGREL?
Clopidogrel (Plavix) is a potent antiplatelet agent commonly used in patients with atherosclerotic cardiac or cerebrovascular disease, sometimes in combination with aspirin. Because of the risk of significant gastrointestinal bleeding, a 2008 multisociety task force recommended prescribing a PPI when both clopidogrel and aspirin are used as dual antiplatelet therapy.4
Studies of clopidogrel plus PPIs: Discrepant results
To date, only one prospective randomized controlled trial has specifically investigated the effect of PPIs on cardiovascular outcomes in patients using clopidogrel. In this trial, patients on dual antiplatelet therapy with clopidogrel and aspirin were randomized to receive either omeprazole 20 mg or placebo. Analysis of the data revealed no significant increase in the composite end point of cardiovascular events (hazard ratio [HR] 0.99, 95% confidence interval [CI] 0.68–1.44, P = .96), but a statistically significant decrease in composite gastrointestinal events (HR 0.34, 95% CI 0.18–0.63, P < .001).17
Unfortunately, this trial had to be terminated before the prespecified sample size and duration of follow-up were reached because the study sponsor declared bankruptcy.
One additional recent retrospective cohort study16 likewise found no significant risk of serious cardiovascular disease related to PPI use in clopidogrel users. It also found that the adjusted incidence of hospitalization for upper gastrointestinal bleeding was 50% lower in patients who used PPIs than in those who did not (HR 0.50, 95% CI 0.39–0.65).
Do factors other than PPIs account for the higher risk in some of the studies?
The discrepant results of these studies suggest that the higher risk of cardiovascular events may be due, either completely or in part, to a factor other than the pharmacologic interaction of PPIs and clopidogrel. It is difficult to infer causality from the available data. In situations in which no randomized controlled trials exist, one looks to observational (case-control or cohort) studies to try to obtain the best estimate of the actual risk. With PPIs and clopidogrel, a randomized controlled trial was performed but terminated before patient enrollment was complete.
The increased risk found in some of these studies may be real, may be due to chance, or may even represent an increased risk from PPIs alone (although data do not support this possibility).18 However, the major concern in observational studies is the inability to account for unmeasured confounders, a problem virtually eliminated by randomization strategies in prospective studies.
In the studies that found a higher risk with the combination of omeprazole plus clopidogrel, the principal concern is confounding by indication, in which distortions of the risk estimates arise from an imbalance in prognostic factors between compared treatment groups that remains unmeasured.19 Stated another way, physicians who believed some patients to be “sicker” or to have a higher risk of serious events may have treated them with a PPI on the basis of factors that remained unaccounted for in the epidemiologic investigation.
This possibility has been reinforced by findings from a nonrandomized subgroup analysis of a randomized controlled trial in which patients who had been receiving a PPI had a higher rate of cardiovascular events whether they received clopidogrel or placebo.20
FDA alert: Avoid using omeprazole or esomeprazole with clopidogrel
Nonetheless, on November 17, 2009, the US Food and Drug Administration (FDA) issued an alert to health care professionals and the public about the potential interaction between clopidogrel and omeprazole.21 In this alert, the FDA stated that the use of omeprazole or esomeprazole (Nexium) with clopidogrel should be avoided.
An algorithm to use when considering clopidogrel plus a PPI
Physicians are now left in a bind between the minimal, if any, pooled risk seen in the available data and the FDA recommendation. What is the best action to take?
First, assess the need for dual antiplatelet therapy. If dual antiplatelet therapy (clopidogrel plus aspirin) is required, then a PPI is warranted for gastric protection because the risk of life-threatening bleeding outweighs any increased risk of cardiovascular events.4
If antiplatelet monotherapy (clopidogrel alone) is required, then assess the reason for antisecretory therapy.
For complicated disease, such as gastroesophageal reflux disease with Barrett esophagus or peptic strictures, PPI therapy is warranted to prevent progression or recurrence of complications. If the antisecretory therapy is being provided for noncomplicated symptomatic disorders such as nonerosive gastroesophageal reflux disease or dyspepsia, then one should try to “step down” the therapy by lowering the PPI dose as much as possible while still controlling symptoms to the patient’s tolerance, then possibly stepping further by substituting a histamine-2-receptor antagonist, an antacid, or “on-demand” use of PPIs.22,23
However, if the rationale for antisecretory therapy is simply for gastrointestinal protection, then further risk stratification for gastro intestinal bleeding should be undertaken.4 For patients with a high risk of future gastrointestinal bleeding, such as those with prior episodes of bleeding or concurrent use of nonsteroidal anti-inflammatory drugs, antisecretory therapy is still recommended. Therefore, if a patient is on monotherapy with clopidogrel, has no complicated or symptomatic gastrointestinal disorder, and does not have a high risk of gastrointestinal bleeding, then therapy with a PPI should be reconsidered.
There are no strong data to indicate that one particular PPI should be used or avoided if one of the above criteria indicates the concurrent need for clopidogrel and a PPI. In their health alert about the potential interaction, the FDA did not issue the same warning for PPIs other than omeprazole and esomeprazole, but fell short of recommending a change to another PPI because of a lack of data to support or refute a similar interaction.
Because the half-lives of clopidogrel and PPIs are short, separating their administration could in theory decrease or eliminate the risk of competitive inhibition. The PPI could be given in the morning before breakfast and the clopidogrel could be given at night, or the clopidogrel could be given at lunchtime and the PPI before dinner. Although the FDA does not believe this strategy will reduce this interaction,21 one expert in the field has suggested it.18
DO PPIs CAUSE OSTEOPOROSIS, FRACTURES?
In a widely publicized paper published in 2006, Yang and colleagues25 reported the results of a large nested case-control study in the United Kingdom. The risk of hip fracture was significantly greater in patients who had been using PPIs for at least 1 year than in those who had not. The risk appeared to increase with longer use and higher doses of PPIs.
A similar risk of hip fracture was seen in a larger Danish case-control study published the same year.26 This study also found an increased odds ratio for PPI use in patients with spine fractures as well as in patients with any type of fracture. Interestingly, this study found a lower risk of fracture in patients using a histamine-2-receptor antagonist instead of a PPI.
Targownik et al27 found that the risk of hip fracture was not significantly higher until after 5 years of PPI exposure, with an even stronger risk after 7 years.
However, the data on both association and causal relationship are not uniform.
The Women’s Health Initiative,30 with more than 1 million person-years of followup, found no association between PPI use and hip fracture, but a modest association between PPI use and spine, arm, and wrist fractures, as well as total fractures.
A study in the United Kingdom found that patients without any major risk factors for hip fracture (defined by a risk ratio > 2) accounted for only 25% of cases but 53% of controls. When only these two average-risk groups were compared, the risk of hip fracture was similar in cases and controls.31
Corley et al32 also found that the risk of fracture associated with PPI use was only significant in the presence of another risk factor. These findings suggest that residual confounding may be to blame, at least in part, for the estimates of increased risk in the prior studies.
Another way to interpret these data is that PPIs increase the risk in patients at high risk to begin with, but not in those at average risk. This is an example of interaction (or effect modification) in which the risk is unequally distributed across groups with different characteristics.
A recently published study refutes the theory that impaired calcium absorption is responsible for the increase in fractures.33 In this study, investigators queried the Manitoba Bone Mineral Density Database to determine the relationship between antisecretory therapy with PPIs and osteoporosis or loss of bone mineral density—and they found none. This study may support the theory that residual confounding is the reason for the finding of an increased risk, but it also leaves open the possibility that PPIs induce other changes in bone microstructure that could increase the risk of fracture.
FDA labeling: Possible risk of fracture with PPIs
Based on the data so far, it appears possible that there is a small, albeit statistically significant, association between PPI use and fracture risk. The association is indeed biologically plausible, but it remains to be seen if this association is clinically significant, as the risk is relatively low. Even though the studies had methodologic limitations, on May 25, 2010, the FDA announced a change in the required labeling information for PPIs to indicate a possible risk of fracture with these drugs.34
Reassess the need for chronic PPI therapy
Although patients may worry that they will develop osteoporosis and fractures if they take PPIs, the data do not support a strong risk. Nevertheless, when faced with a patient on chronic PPI therapy, especially with a high dose, providers should use the opportunity to reassess the indication for the PPI to decide if chronic therapy is required, in a matter similar to the algorithm provided for PPI-clopidogrel cotherapy (FIGURE 2). Providers should educate patients about the data, and limit new and recurring PPI prescriptions to patients who require a PPI for appropriate indications, at the lowest dose, and for the shortest time possible.
DO PPIs INCREASE THE RISK OF PNEUMONIA?
Several recent studies have also raised concern about an association between PPI use and pneumonia.
Normally, the stomach remains free of bacteria (except for Helicobacter pylori) because its acidic milieu destroys nearly all bacteria swallowed. If the stomach becomes less acidic, it loses this protective mechanism, and ingested organisms can survive and proliferate.35 In theory, when gastroesophageal reflux occurs, these bacteria could be carried up to the hypopharynx where microaspiration into the lower airways could lead to pneumonia, especially in patients with compromised oropharyngeal protective reflexes (eg, patients on mechanical ventilation).
This possible association came to the attention of the general medical community when a Dutch study,36 in which 5,551 cases of community-acquired pneumonia developed in 364,683 people, found that the incidence of pneumonia was about 4.5 times higher in people exposed to acid-suppressive drugs (both PPIs and histamine-2-receptor antagonists) than in unexposed individuals. Patients who developed pneumonia also had higher odds of significant comorbid conditions, including heart failure and chronic obstructive pulmonary disease. The authors calculated that about one case of pneumonia per 226 patients treated with a PPI would be attributable to the PPI. A major limitation of this study, however, was that only 18% of the patients diagnosed with pneumonia actually had radiologic or microbiologic confirmation of pneumonia.
Other studies later examined the relationship between PPIs and community-acquired pneumonia,37–41 and most have revealed a modestly higher risk of community-acquired pneumonia in patients exposed to PPIs.
This risk was confirmed in a recent metaanalysis, which found a higher risk of community-acquired pneumonia with PPI use (odds ratio 1.36, 95% CI 1.12–1.65).42 However, the authors refrained from drawing definitive conclusions from these data because of significant heterogeneity between the studies. One study37 found that recent onset of use (within 7 days) had a much stronger association with community-acquired pneumonia than longer-term use, which is contradictory to a causal association, since longer-term use should lead to more cases of pneumonia.
Another study investigated the association between acid-suppressive drugs and hospital-acquired pneumonia in nonventilated patients.43 In a 4-year period, there were 63,878 admissions in 42,093 unique patients. Acid-suppressive drugs were prescribed in 32,922 admissions (52%); the drugs included PPIs in 83% of these. Hospital-acquired pneumonia occurred in 2,219 admissions (3.5%), with a higher incidence in patients exposed to acid-suppressive drugs than in the unexposed group (4.6% vs 2.0%). The adjusted odds ratio for pneumonia was 1.3 (95% CI 1.1–1.4) in the exposed group. Subgroup analysis revealed that the association remained significant for PPIs but not for histamine-2-receptor antagonists.
Adequate studies of mechanically ventilated patients in the current era of intravenous PPI use are lacking. Older studies in this group of patients may not be generalizable to current practice because of the reduction in gastric volume with intravenous PPIs that may offset the theoretical risk of aspiration.35
Although the data supporting the association are not exceedingly strong, the relationship is biologically plausible. If there is a risk, it seems to be greatest in the sickest patients, who can least afford to develop pneumonia. Therefore, prudent prescribing should be the rule for both inpatients and outpatients, especially in patients with comorbidities, in whom pneumonia could have serious consequences.
PPIs AND ENTERIC INFECTIONS
Traditionally, gastric acid was not believed to be important in protecting against Clostridium difficile infection because acid-resistant spores were presumed to be the principal vector of transmission.44 Recently, this thought has been challenged, as several studies have found a higher risk of C difficile infection in PPI users. In theory, PPIs may increase the risk of C difficile infection by increasing the ability of the spore to convert to the vegetative form and to survive intraluminally.
A recent meta-analysis of 11 papers, including nearly 127,000 patients, found a significant relationship between PPI use and C difficile infection, with an odds ratio of 2.05 (95% CI 1.47–2.85).45 Further supporting the hypothesis of a direct causative association, a recent study found a significant dose-response, with more aggressive acid-suppression associated with higher odds ratios.46 In view of this association, patients using PPIs who develop diarrhea should be evaluated for C difficile, perhaps even in the absence of other risk factors.
Other enteric infections have been found to be associated with PPIs.44,45 Small intestinal bacterial overgrowth, a condition that is associated with bloating, diarrhea, and malabsorption, has recently been associated with PPI use, although the significance of the association is uncertain.47
Based on a change in the intestinal flora, recent reports have additionally implied that there is a relationship between PPI use and the development of spontaneous bacterial peritonitis in hospitalized cirrhotic patients with ascites. One study found a strong association (odds ratio 4.3, 95% CI 1.3–11.7) between PPIs and spontaneous bacterial pneumonitis,48 whereas another study found no significant association (odds ratio 1.0, 95% CI 0.4–2.6).49
Both studies were small case-control studies of hospitalized patients. No firm conclusion can be drawn about the relevance of this association from these investigations at this point.
PPIs AND ACUTE INTERSTITIAL NEPHRITIS
Several case reports have implicated PPIs as a cause of acute interstitial nephritis.
A systematic review from 2007 found 64 cases documented in the literature, 12 of which were considered certainly associated, and 9 of which were probably associated.50 Initial symptoms were nonspecific and included nausea, malaise, and fever. With such extensive use worldwide as the denominator, the authors concluded that acute interstitial nephritis was a rare, idiosyncratic occurrence related to PPI use, but did not find enough evidence to support a causative relationship. Despite the rarity of the syndrome, they recommended maintaining a high level of clinical suspicion to detect acute interstitial nephritis early in its course, especially soon after the initiation of PPI therapy.
POSSIBLE ASSOCIATIONS WITH IRON AND B12 DEFICIENCIES
Long-term PPI therapy has been thought to be associated with micronutrient deficiencies, especially of iron and vitamin B12. Hydrochloric acid in the stomach assists in the dissociation of iron salts from food and the reduction of ferric iron to the more soluble ferrous iron.51 Gastric acid also facilitates the release of vitamin B12 bound to proteins within ingested foodstuffs to permit binding to R-proteins for eventual absorption in the terminal ileum.51,52
Despite the biologic plausibility of these deficiencies, there is currently little evidence to support a clinically relevant association to recommend a change in current practice.
NO THERAPY IS COMPLETELY WITHOUT RISK
Although concerns have been raised about the long-term safety of PPIs, the preponderance of the evidence does not strongly support the apprehensions publicized over the last few years. When translating these studies into the routine management of patients, it is important to recall some very basic tenets of good patient care.
No therapy is completely without risk—whether pharmacologic, surgical, or psychological, and no matter how benign or straightforward. Consequently, no drug, procedure, or treatment plan should be ordered without a valid indication. Even with an indication, the risk-benefit ratio of the therapy prescribed should always be considered. If the indication for the PPI is weak or uncertain, then even a slight risk tips the balance away from the drug, and the drug should be discontinued.
When seeing patients in long-term care, the indication and necessity for all drugs, including PPIs, should be reviewed. The algorithm proposed in Figure 2 can be adapted for virtually any of the possible associations.
Consider the indication for the PPI. Was the PPI started during a hospitalization and then routinely continued after discharge? This is one situation in which the use of a PPI could potentially be discontinued.2
For complicated acid-peptic disease, dose reduction or cessation of PPI therapy may not be possible.
If the PPI was prescribed only for symptom relief, as in cases of dyspepsia or nonerosive gastroesophageal reflux disease, reduce the dose of PPI to as low as possible to maintain symptom control. Should chronic therapy still be required, no specific monitoring is recommended, apart from routine monitoring that takes place in the course of patient care.
Lastly, because of the media attention that several of these concerns have garnered, patients may still harbor significant concerns about PPIs, even their short-term use. In such cases, the prescriber should take the opportunity to communicate the reason for the decision to prescribe the PPI, as well as the best available data about the risks PPIs may pose. None of these outcomes is very common in the absence of PPIs, with the possible exception of recurrent cardiovascular events, and the risks provided in all of these studies are relative to the baseline risk. Even if the risk of a particular outcome doubles with long-term PPI use, twice a small risk remains a small risk.
- Gatyas G. IMS Health reports U.S. prescription sales grew 5.1 percent in 2009, to $300.3 Billion. IMS Health. http://www.imshealth.com/portal/site/imshealth/menuitem.a46c6d4df3db4b3d88f611019418c22a/?vgnextoid=d690a27e9d5b7210VgnVCM100000ed152ca2RCRD&vgnextfmt=default. Accessed 10/7/2010.
- Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005; 21:1203–1209.
- Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol 2006; 101:2200–2205.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118:1894–1909.
- Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol 2004; 95:2–8.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008; 51:256–260.
- Small DS, Farid NA, Payne CD, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Sibbing D, Morath T, Stegherr J, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost 2009; 101:714–719.
- O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009; 180:713–718.
- Stanek EJ, Aubert RE, Flockhart DA, et al. A national study of the effect of individual proton pump inhibitors on cardiovascular outcomes in patients treated with clopidogrel following coronary stenting: the Clopidogrel Medco Outcomes Study. Program and abstracts of the 32nd Annual SCAI Scientific Sessions May 6, 2009; Las Vegas, Nevada.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Ramirez JF, Selzer F, Chakaprani R, et al. Proton pump inhibitor and clopidogrel combination is not associated with adverse clinical outcomes after PCI: the NHLBI dynamic registry (abstract). J Am Coll Cardiol 2009; 53(suppl 1):A27.
- Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med 2010; 152:337–345.
- Bhatt DL, Cryer B, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol 2010; 105:34–41.
- Walker AM. Confounding by indication. Epidemiology 1996; 7:335–336.
- Dunn SP, Macaulay TE, Brennan DM, et al. Baseline proton pump inhibitor use is associated with increased cardiovascular events with and without the use of clopidogrel in the CREDO trial (abstract). Circulation 2008; 118:S815.
- US Food and Drug Administration. Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). U.S. Department of Health and Human Services, 11/17/2009. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm. Accessed 9/23/2010.
- Inadomi JM, Jamal R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology 2001; 121:1095–1100.
- Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol 2003; 98:1940–1944.
- O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 2005; 118:778–781.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 2006; 79:76–83.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008; 179:319–326.
- Roux C, Briot K, Gossec L, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int 2009; 84:13–19.
- Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int 2008; 83:251–259.
- Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med 2010; 170:765–771.
- Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 2008; 28:951–959.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 2010; 138:896–904.
- US Food and Drug Administration. FDA Drug Safety Communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. U.S. Department of Health and Human Services, 5/25/2010. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed 12/7/2010.
- Vakil N. Acid inhibition and infections outside the gastrointestinal tract. Am J Gastroenterol 2009; 104(suppl 2):S17–S20.
- Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004; 292:1955–1960.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008; 149:391–398.
- Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community-acquired pneumonia and the use of statins, ACE inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf 2009; 18:269–275.
- Rodríguez LA, Ruigómez A, Wallander MA, Johansson S. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology 2009; 20:800–806.
- Eurich DT, Sadowski CA, Simpson SH, Marrie TJ, Majumdar SR. Recurrent community-acquired pneumonia in patients starting acid-suppressing drugs. Am J Med 2010; 123:47–53.
- Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther 2010; 31:1165–1177.
- Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA 2009; 301:2120–2128.
- Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol 2009; 104(suppl 2):S10–S16.
- Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007; 102:2047–2056.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2010; 8:504–508.
- Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol 2009; 104:1130–1134.
- Campbell MS, Obstein K, Reddy KR, Yang YX. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci 2008; 53:394–398.
- Sierra F, Suarez M, Rey M, Vela MF. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther 2007; 26:545–553.
- McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol 2009; 104(suppl 2):S5–S9.
- Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med 2009; 122:896–903.
- Gatyas G. IMS Health reports U.S. prescription sales grew 5.1 percent in 2009, to $300.3 Billion. IMS Health. http://www.imshealth.com/portal/site/imshealth/menuitem.a46c6d4df3db4b3d88f611019418c22a/?vgnextoid=d690a27e9d5b7210VgnVCM100000ed152ca2RCRD&vgnextfmt=default. Accessed 10/7/2010.
- Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005; 21:1203–1209.
- Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol 2006; 101:2200–2205.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118:1894–1909.
- Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol 2004; 95:2–8.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008; 51:256–260.
- Small DS, Farid NA, Payne CD, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Sibbing D, Morath T, Stegherr J, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost 2009; 101:714–719.
- O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009; 180:713–718.
- Stanek EJ, Aubert RE, Flockhart DA, et al. A national study of the effect of individual proton pump inhibitors on cardiovascular outcomes in patients treated with clopidogrel following coronary stenting: the Clopidogrel Medco Outcomes Study. Program and abstracts of the 32nd Annual SCAI Scientific Sessions May 6, 2009; Las Vegas, Nevada.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Ramirez JF, Selzer F, Chakaprani R, et al. Proton pump inhibitor and clopidogrel combination is not associated with adverse clinical outcomes after PCI: the NHLBI dynamic registry (abstract). J Am Coll Cardiol 2009; 53(suppl 1):A27.
- Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med 2010; 152:337–345.
- Bhatt DL, Cryer B, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol 2010; 105:34–41.
- Walker AM. Confounding by indication. Epidemiology 1996; 7:335–336.
- Dunn SP, Macaulay TE, Brennan DM, et al. Baseline proton pump inhibitor use is associated with increased cardiovascular events with and without the use of clopidogrel in the CREDO trial (abstract). Circulation 2008; 118:S815.
- US Food and Drug Administration. Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). U.S. Department of Health and Human Services, 11/17/2009. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm. Accessed 9/23/2010.
- Inadomi JM, Jamal R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology 2001; 121:1095–1100.
- Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol 2003; 98:1940–1944.
- O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 2005; 118:778–781.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 2006; 79:76–83.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008; 179:319–326.
- Roux C, Briot K, Gossec L, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int 2009; 84:13–19.
- Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int 2008; 83:251–259.
- Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med 2010; 170:765–771.
- Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 2008; 28:951–959.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 2010; 138:896–904.
- US Food and Drug Administration. FDA Drug Safety Communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. U.S. Department of Health and Human Services, 5/25/2010. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed 12/7/2010.
- Vakil N. Acid inhibition and infections outside the gastrointestinal tract. Am J Gastroenterol 2009; 104(suppl 2):S17–S20.
- Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004; 292:1955–1960.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008; 149:391–398.
- Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community-acquired pneumonia and the use of statins, ACE inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf 2009; 18:269–275.
- Rodríguez LA, Ruigómez A, Wallander MA, Johansson S. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology 2009; 20:800–806.
- Eurich DT, Sadowski CA, Simpson SH, Marrie TJ, Majumdar SR. Recurrent community-acquired pneumonia in patients starting acid-suppressing drugs. Am J Med 2010; 123:47–53.
- Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther 2010; 31:1165–1177.
- Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA 2009; 301:2120–2128.
- Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol 2009; 104(suppl 2):S10–S16.
- Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007; 102:2047–2056.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2010; 8:504–508.
- Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol 2009; 104:1130–1134.
- Campbell MS, Obstein K, Reddy KR, Yang YX. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci 2008; 53:394–398.
- Sierra F, Suarez M, Rey M, Vela MF. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther 2007; 26:545–553.
- McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol 2009; 104(suppl 2):S5–S9.
- Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med 2009; 122:896–903.
KEY POINTS
- The US Food and Drug Administration has issued alerts that PPIs may increase the rate of osteoporosis-related fractures and may decrease the effectiveness of clopidogrel (Plavix) for preventing serious cardiovascular events.
- Other concerns include increased rates of pneumonia, Clostridium difficile infection, and other infections.
- A prudent approach to managing these concerns in day-to-day practice is required: PPIs, like any other drugs, should be prescribed only if indicated.
Premenopausal osteoporosis, an overlooked consequence of anorexia nervosa
Among the devastating effects of anorexia nervosa, and one that is easily overlooked, is its impact on bone.
Probably more than half of young women with anorexia nervosa develop osteoporosis, and relatively quickly. Baker et al1 obtained bone scans in a series of 56 young women, mean age 27 years, who had had an eating disorder for a mean of about 10 years, and found that the bone mineral density in the femur was below the critical fracture threshold in 42 (75%).
Osteoporosis is particularly common and worrisome in female athletes (and is becoming increasingly common in male athletes as well). Female athletes have a much higher incidence of disordered eating than their peers2 and therefore are at a much higher risk of fractures.
This review summarizes the factors affecting the development of osteoporosis in these patients and discusses potential targets for intervention.
ANOREXIA AND BONE HEALTH: A COMPLEX RELATIONSHIP
Anorexia nervosa is characterized by an intense fear of gaining weight, a body weight less than 85% of expected, a distorted self-image, and, in women, missing three consecutive menstrual periods.3 The lifetime prevalence in women is about 0.5%; it is much lower in men.3 The prevalence of eating disorders in female athletes is much higher, estimated at 15% to 62%.2
The etiology of osteoporosis in patients with anorexia nervosa is complex and multifaceted. In these patients, bone resorption is increased without a concomitant increase in bone formation, resulting in a net loss of bone.4 Thus, markers of bone resorption such as N-teleopeptide and deoxypyridoline are elevated, but markers of bone formation such as osteocalcin are not.4
The loss of bone may be rapid and can occur relatively early in the disease. Some studies suggest that an illness duration longer than 12 months predicts significant loss of bone density.5 Thus, early diagnosis and intervention are important to minimize bone loss.
Women gain from 40% to 60% of their bone mass during the pubertal growth spurt in ages 11 to 14, the time when anorexia nervosa is most prevalent.6 Peak bone mass is attained by the third decade of life, but the rate of growth of bone mass is highest during adolescence and early adulthood.7 Hence, it is important to optimize bone mass during this time, as small differences in bone density can have significant clinical implications later in life: a 5% increase in bone density significantly decreases fracture risk, whereas a 10% decrease in adult bone mineral density is associated with a two to three times higher risk of fracture (reviewed by Rome and Ammerman6).
What is the role of amenorrhea in the development of osteoporosis in premenopausal patients?
Given that two of the most characteristic manifestations of anorexia nervosa are low body weight and the absence of menses, these factors have been hypothesized to be potential causes of osteoporosis.
In general, young women who present with amenorrhea should be evaluated to determine if the amenorrhea is primary or secondary. Primary amenorrhea is the absence of menarche by age 16; secondary amenorrhea is the absence of menses for more than three cycles or more than 6 months in someone who previously had had menses. The most common causes of secondary amenorrhea are ovarian disease, hypothalamic or pituitary disease, and uterine disease. Anorexia nervosa causes hypothalamic dysfunction and is a cause of secondary amenorrhea. In clinical practice, it is also important to remember that pregnancy can occur even in the setting of amenorrhea.
Amenorrhea in patients with anorexia nervosa is related to hypothalamic suppression of the release of gonadotropin-releasing hormone, resulting in lower levels of follicle-stimulating hormone and luteinizing hormone and a resultant prepubertal low-estrogen state.
In a study of 73 women with anorexia nervosa and a mean age of 17.2 years,8 20 months of amenorrhea was the threshold above which the most severe osteopenia was seen, implying that the duration of amenorrhea affects bone health.
Which factors besides amenorrhea influence bone density in premenopausal women?
Undernutrition. Body weight has been suggested to have an independent effect on bone mineral density, and density has been found to increase following weight gain, even before the return of menses.1 Once a regular menstrual cycle has been restored, significant increases in trabecular and cortical bone have been detected.1
Deficiency of insulin-like growth factor 1 (IGF-1). Anorexia nervosa is associated with decreased hepatic synthesis of IGF-I.9 Low levels of IGF-I reduce the levels of osteocalcin, a marker of bone formation, and cause abnormalities in osteoblast function.10 This deficiency is associated with the development of osteopenia in patients with anorexia nervosa.11
Low androgen levels are present in patients with anorexia nervosa, and levels appear to be further reduced by oral contraceptives.12 It remains to be determined whether the further reduction in androgens in women with anorexia nervosa using oral contraceptives is harmful to skeletal health. Low testosterone levels in boys with anorexia nervosa have been associated with lower libido, fewer erections, and potentially lower bone density.13
Hypercortisolemia. Elevated levels of total and free serum cortisol and high 24-hour urinary free cortisol excretion have been noted in anorectic patients. Levels of cortisol are inversely related to levels of osteocalcin, and hypercortisolism has been shown to be associated with osteoporosis.14,15 However, no study has yet shown causality in this population.
Osteoprotegerin has been recognized as an important regulator of bone resorption. Osteoprotegerin inhibits osteoclast differentiation and activation and stimulates osteoclast apoptosis, helping to preserve bone density.
Misra et al16 showed that adolescent girls with anorexia nervosa have higher serum osteoprotegerin levels than controls and that osteoprotegerin levels correlate inversely with markers of nutritional status and lumbar bone density Z scores.16 They and other investigators17 postulate that osteoprotegerin may be released as a compensatory response to the bone loss seen in these patients in an attempt to preserve bone health.
Leptin is an adipocyte-derived hormone that acts on receptors in the hypothalamus, decreasing food intake and increasing energy expenditure. Low leptin levels are a key endocrinologic feature of anorexia nervosa.18 Leptin helps to induce weight loss by stimulating neurons in the hypothalamus that express “weight-loss-inducing” neuropeptides such as pro-opiomelanocortin and inhibiting “weight-gain-inducing” peptides such as neuropeptide Y.19
Although leptin was first believed to be a hormone released to counteract obesity, recent studies19,20 suggest that it is part of a major signaling system that controls adaptation to starvation. These studies have shown that the body senses its corporeal fat through leptin and inhibits ovulation when fat reserves are low.19 In addition, luteinizing hormone and leptin levels have been shown to increase in parallel in patients with anorexia nervosa when weight is restored.20 Thus, rising leptin levels correlate with the resumption of menses in women with anorexia nervosa and in turn have potential consequences for bone health.
Not enough ghrelin, too much obestatin? Ghrelin, a gastric hormone, acts as a natural antagonist to leptin, resulting in an increase in food intake and body weight.19 Circulating ghrelin levels are higher in illness-induced anorexia as well as in anorexia nervosa, and they normalize with weight gain, perhaps as an adaptive mechanism to compensate for a negative energy balance.21
Several in vitro studies suggest that ghrelin directly promotes osteoblast proliferation and differentiation.22 However, human studies of ghrelin’s effects on bone are limited. In a study of healthy younger women, healthy boys, and anorexia nervosa patients, plasma ghrelin levels were only weakly associated with bone mineral density.23
The effects of obestatin, another gastric hormone, are still being investigated. Obestatin was initially shown to oppose the effects of ghrelin by decreasing appetite and weight gain. When given with ghrelin, obestatin appears to work with ghrelin at the hypothalamic level to modulate food intake and growth hormone secretion.24
Interestingly, obestatin and the ratio of ghrelin to obestatin are decreased in patients with anorexia nervosa, but the ratio is unchanged in thin women who have an equivalent body mass index but no eating disorder.25 It has been hypothesized that the ghrelin-obestatin ratio may be the key to explaining the eating restriction and reduced motivation to eat despite high ghrelin levels seen in anorexia nervosa.26 Further studies are needed to determine the role of obestatin and the ghrelin-obestatin ratio in the bone health of women with anorexia nervosa.
HOW SHOULD WE DIAGNOSE OSTEOPOROSIS IN PREMENOPAUSAL PATIENTS?
Our approach to screening for and diagnosing osteoporosis is still largely based on measuring bone mineral density, although density by itself is not a perfect tool for predicting who will or will not experience a fracture, particularly in premenopausal women.26,27 Most premenopausal women with low bone mineral density but no other risk factors for fracture such as previous fractures or glucocorticoid therapy are at very low short-term risk of fracture.26
For these reasons, in premenopausal women and adolescents, the International Society for Clinical Densitometry28 advises against diagnosing osteoporosis on the basis of bone mineral density alone. Instead, it should be diagnosed in this population only if the bone mineral density is low (defined as a Z score below −2.0) and the patient has risk factors that suggest a higher short-term risk of bone mineral loss and fracture. Risk factors include chronic malnutrition, eating disorders, hypogonadism, glucocorticoid exposure, and previous fracture.29
A pitfall in interpreting low bone mineral density in premenopausal women younger than age 30 is the possibility that they may not yet have reached their peak bone mass. In addition, small stature and body size (and therefore bone size) also influence the results of dual-energy x-ray absorptiometry. This test may not be able to distinguish bone that is small but of normal density from bone that is of low density.26
Despite its limitations, until newer risk assessment tools are available for this patient population, measuring bone mineral density is still recommended in addition to assessing clinical risk factors to diagnose osteoporosis. Also, changes in bone mineral density over time can help to assess risk and guide treatment.
When should a patient with anorexia be screened for osteoporosis?
Because bone loss may begin early in the course of anorexia and progress rapidly (potentially inexorably), baseline screening is recommended for all patients who have had anorexia nervosa or amenorrhea for more than 6 to 12 months.30 The National Osteoporosis Foundation recommends screening in women under age 65 who have a low body weight.31 The American College of Sports Medicine recommends screening for osteoporosis in athletes with a history of hypoestrogenism or disordered eating for a cumulative total of 6 months or more, or with a history of stress fracture or fracture from minimal trauma.32
Knowledge of low bone mineral density and fracture risk can often guide treatment and prompt behavioral change. Given that most osteoporosis treatments do not lead to detectable changes in bone density until 18 months to 2 years, it is reasonable to repeat testing at this interval.33
NEW AND OLD TREATMENTS FOR LOW BONE DENSITY IN ANOREXIA NERVOSA
Weight restoration is the cornerstone
Restoration of body weight and nutritional rehabilitation remain the cornerstones of treatment. All patients with anorexia nervosa should be referred to a nutritionist to develop a meal plan that is adjusted for the amount of energy expended. The challenges lie in managing the complications of refeeding and the high relapse rate. The treatment goals in disordered eating are to optimize the overall nutritional status, normalize eating behavior, modify unhealthy thought processes that maintain the disorder, and treat possible emotional issues that help create or maintain the disorder.
The younger the patient, the more the family’s involvement is recommended. In addition to nutritional counseling, the care team should include a psychotherapist, a psychiatrist, and a primary care physician to assist with management and screening of medical complications.
Vitamin D for all
Low vitamin D levels have long been associated with low bone mineral density and risk of hip fracture.34
Vitamin D insufficiency is very common. More than 90% of blacks, Hispanics, and Asians and nearly 75% of whites have insufficient levels of vitamin D (25-hydroxyvitamin D3 level < 30 ng/mL).35 In a study of 307 healthy adolescents, vitamin D insufficiency (a 25-hydroxyvitamin D3 level < 20 ng/mL) was found in 42% and vitamin D deficiency (a level < 15 ng/mL) in 24.1%.36 In addition, this study confirmed an inverse correlation between body mass index and serum 25-hydroxyvitamin D3 concentration.
Therefore, while vitamin D supplementation has not been consistently shown to improve bone loss in anorectic patients,9 given the prevalence of vitamin D deficiency and insufficiency, supplementation is almost universally recommended.
There is no consensus as to the amount of supplementation to recommend for women with anorexia nervosa. The American College of Sports Medicine recommends a total daily intake of 800 IU of vitamin D (ie, from diet and supplements). Therapy should be titrated to doses that result in normocalcemia and a serum 25-hydroxyvitamin D3 concentration of at least 30 ng/mL.37,38
Does hormone treatment improve bone density?
In postmenopausal osteoporosis, estrogen therapy maintains or improves bone density and appears to reduce the rate of vertebral fractures.39,40 Perhaps not so with premenopausal osteoporosis due to anorexia nervosa.
Why should this be? In postmenopausal women, estrogen therapy appears to work by impairing osteoclast-mediated bone resorption, but it has only limited effects on bone formation. In premenopausal anorexia, however, bone loss appears to be due to a unique uncoupling of osteoblastic and osteoclastic functions, resulting in both reduced bone formation and increased bone resorption, which estrogen therapy may not improve.5
Despite the documented association between anorexia nervosa and estrogen deficiency and the strong correlation between osteoporosis and the duration of amenorrhea, most studies have found no improvement in bone mass with hormonal therapy.9 In particular, three randomized, placebo-controlled trials have been published to date, and not one showed a significant improvement in bone mineral density with estrogen therapy compared with placebo in patients with anorexia nervosa.41–43
Klibanski et al,41 in the first of these trials, found no significant difference in spinal bone mineral density between treated patients and controls. However, estrogen-treated patients whose initial body weight was very low (< 70% of expected) had a significant increase in their bone mineral density, whereas those in the control group lost bone density.
Baker et al44 suggest that hormone therapy might protect bone mass in athletes with amenorrhea, citing a study that found that women with a history of stress fractures were less likely to have used oral contraceptives previously than athletes without fractures.45 However, no prospective randomized study to date has established that hormone therapy effectively preserves bone mass in athletes with amenorrhea.
Based on the data presented above, we have little evidence for using estrogen to treat or prevent premenopausal osteoporosis.
The American College of Sports Medicine32 recommends consideration of estrogen therapy if there is evidence of a decline in bone mineral density in an athlete over the age of 16 with persistent functional hypothalamic amenorrhea despite adequate nutritional intake and weight. However, it acknowledges that restoring regular menstrual cycles with oral contraceptive pills will not normalize the metabolic factors that impair bone formation, health, and performance and is not likely to fully reverse low bone mineral density in this population.
What is the effect of exercise on bone health in these patients?
Several studies have examined the effect of weight-bearing exercise on bone density.
Young et al46 compared normal teenagers, ballet dancers, and young women with anorexia nervosa and found that weight-bearing exercise protected against osteoporosis, but only at weight-bearing sites. Athletes in weight-bearing sports had a 5% to 15% higher bone mineral density in weight-bearing sites (ie, the femur) compared with nonathletes, but had lower bone mineral density in the spine.
Therefore, a Z score below −1.0 in an athlete, especially in a distal site, warrants further investigation and treatment.32 In general, exercise does not necessarily protect against osteoporosis in this patient population, and it can sometimes mask underlying bone loss. In addition, keep in mind that many of these patients exercise compulsively, using it as a form of purging.
Insulin-like growth factor-1: More study needed
IGF-1 contributes to bone growth by stimulating osteoblasts, and patients with anorexia nervosa have been shown to have low levels of IGF-1.9
Grinspoon et al10 randomized 60 patients with anorexia nervosa to receive IGF-1 alone, IGF-1 plus an oral contraceptive, an oral contraceptive alone, or placebo. All patients were given calcium and vitamin D and were followed for 9 months. Total bone mass increased significantly in those taking IGF-1 compared with those taking placebo. Those taking an IGF-1 and an oral contraceptive had a significant increase in spinal bone mineral density compared with those on placebo group. At other skeletal sites, however, IGF-1 plus an oral contraceptive and IGF-1 alone failed to produce significant increases in bone mineral density compared with placebo.
Further study is needed to determine the role of IGF-1 in treating low bone mineral density in anorexia nervosa.
Bisphosphonates: Not approved for this indication
In premenopausal women, bisphosphonates are approved by the US Food and Drug Administration (FDA) for use only in those taking glucocorticoids. Although bisphosphonates have been shown to significantly increase bone mineral density in young women with anorexia nervosa,26 they should be used with caution in patients of childbearing age because they are teratogenic. Bisphosphonates have a long half-life and may continue to affect bone turnover for up to 2 years after they are discontinued.47 In addition, they are not recommended in patients with a history of purging via vomiting, due to a risk of esophageal ulceration.
Parathyroid hormone therapy: Studies ongoing
The parathyroid hormone fragment teriparatide (Forteo) is widely used for treating postmenopausal osteoporosis.
Before teriparatide was approved, there was concern that it might increase the risk of osteosarcoma, as almost 45% of rats treated with this drug at the highest-tested dose level developed this aggressive form of bone cancer.48 Balancing the proven benefits of teriparatide shown by clinical trials with the theoretic risk of teriparatide-induced osteosarcoma, the FDA mandated a “black-box” warning about this potential effect.
Studies of parathyroid hormone treatment in anorexia nervosa and other premenopausal patients are ongoing.26
Leptin: More study needed
Leptin is a potent stimulator of bone growth and has been shown to increase bone mineral density in vitro and in vivo.19 However, concerns have been raised about giving supra-physiologic doses of leptin to patients with anorexia nervosa, as this may increase the risk of further weight loss and relapse.
More work is needed to determine the role of leptin for the treatment of osteoporosis in anorexia nervosa.
Ghrelin: Probably not effective as a single agent
Pharmacologic use of ghrelin increases food intake in healthy humans,49 and it has been proposed as a treatment for weight restoration and bone health in anorexia nervosa. Preliminary studies have not shown it to increase appetite or weight gain,50 but it did increase slow-wave sleep.
Based on these studies, it is unlikely that ghrelin will be effective as a single agent to stimulate appetite, but it may be helpful in conjunction with other therapies.
Cannabinoids: Little ongoing research
Cannabinoids have been proposed as a treatment for anorexia nervosa in the hope that they would induce weight gain and in turn prevent osteoporosis.
Interest in their use in anorexia nervosa stems from the discovery of two cannabinoid receptors (CB1 and CB2) located in the brain and peripheral organ systems. Anorexia nervosa has been associated with different alleles of the CB1 gene,51 but the therapeutic implications of this are far from clear.
Cannabinoids appear to regulate eating behavior at several levels within the brain and periphery: the hypothalamus and hindbrain (integrative functions), the limbic system (for hedonic evaluation of foods), the intestinal system, and adipose tissue.52 At each of these levels, the endocannabinoid system interacts with a number of better known peptides involved in appetite regulation, including leptin, ghrelin, and the melanocortins. In mouse studies, genetic leptin deficiency is associated with elevated hypothalamic endocannabinoid levels.
Appetite stimulation by cannabinoids has been studied for several decades, particularly in relation to cachexia and malnutrition associated with cancer. Very few trials have studied cannabinoids for anorexia nervosa.
In a 4-week crossover trial in 11 patients with anorexia nervosa,53 tetrahydrocannabinol (THC) treatment resulted in an increase in sleep disturbances and interpersonal sensitivity, but it had no significant effect on weight gain compared with diazepam treatment.53
Another pilot study of nine outpatients with anorexia nervosa treated with THC showed a significant improvement in depression and perfectionism scores without any significant weight gain.19
Although this research was once promising, the risk was felt to outweigh the benefit, as cannabinoids may induce dependency in this patient group, who may already be at high risk of drug addiction, and very few have continued this line of investigation.
WHAT CAN WE DO FOR NOW?
- Weight restoration and nutritional rehabilitation remain the keys to treatment of low bone density to reduce the risk of osteoporosis in patients with anorexia nervosa. However, as many as one-third of patients with anorexia nervosa relapse during their lifetime, and other treatments are needed to stabilize and prevent bone loss.
- Vitamin D deficiency is clearly associated with a risk of osteoporosis and fracture, and patients with vitamin D deficiency should be treated with supplementation.
- Standard therapies in postmenopausal patients (such as bisphosphonates and teriparatide) should be used with caution in premenopausal anorexia nervosa patients because of potential long-term health risks.
- As we learn more about hormonal factors in anorexia nervosa, we hope to identify interventions that will help restore weight and decrease the risk of osteoporosis. A summary of potential treatment strategies and targets for prevention of osteoporosis in anorexia nervosa is presented in Table 2.
Acknowledgment: The author thanks the General Internal Medicine Works in Progress Group for its editorial comments, and Dr. Ellen Rome for her mentorship and support.
- Baker D, Roberts R, Towell T. Factors predictive of bone mineral density in eating-disordered women: a longitudinal study. Int J Eat Disord 2000; 27:29–35.
- Rome ES. Eating disorders. Obstet Gynecol Clin North Am 2003; 30:353–377.
- First MB, editor. Diagnostic and Statistical Manual of Mental Disorders—4th edition. Washington, DC: American Psychiatric Association, 2000.
- Mehler PS, MacKenzie TD. Treatment of osteopenia and osteoporosis in anorexia nervosa: a systematic review of the literature. Int J Eat Disord 2009; 42:195–201.
- Wong JC, Lewindon P, Mortimer R, Shepherd R. Bone mineral density in adolescent females with recently diagnosed anorexia nervosa. Int J Eat Disord 2001; 29:11–16.
- Rome ES, Ammerman S. Medical complications of eating disorders: an update. J Adolesc Health 2003; 33:418–426.
- Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women. JAMA 1992; 268:2403–2408.
- Sterling WM, Golden NH, Jacobson MS, Ornstein RM, Hertz SM. Metabolic assessment of menstruating and nonmenstruating normal weight adolescents. Int J Eat Disord 2009; 42:658–663.
- Legroux-Gerot I, Vignau J, Collier F, Cortet B. Bone loss associated with anorexia nervosa. Joint Bone Spine 2005; 72:489–495.
- Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab 2002; 87:2883–2891.
- Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab 1999; 84:4489–4496.
- Miller KK, Lawson EA, Mathur V, et al. Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab 2007; 92:1334–1339.
- Misra M, Katzman DK, Cord J, et al. Bone metabolism in adolescent boys with anorexia nervosa. J Clin Endocrinol Metab 2008; 93:3029–3036.
- Misra M, Miller KK, Almazan C, et al. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab 2004; 89:4972–4980.
- Chiodini I, Mascia ML, Muscarella S, et al. Subclinical hypercortisolism among outpatients referred for osteoporosis. Ann Intern Med 2007; 147:541–548.
- Misra M, Soyka LA, Miller KK, et al. Serum osteoprotegerin in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 2003; 88:3816–3822.
- Ohwada R, Hotta M, Sato K, Shibasaki T, Takano K. The relationship between serum levels of estradiol and osteoprotegerin in patients with anorexia nervosa. Endocr J 2007; 54:953–959.
- Müller TD, Föcker M, Holtkamp K, Herpertz-Dahlmann B, Hebebrand J. Leptin-mediated neuroendocrine alterations in anorexia nervosa: somatic and behavioral implications. Child Adolesc Psychiatr Clin N Am 2009; 18:117–129.
- Støving RK, Andries A, Brixen K, Flyvbjerg A, Hørder K, Frystyk J. Leptin, ghrelin, and endocannabinoids: potential therapeutic targets in anorexia nervosa. J Psychiatr Res 2009; 43:671–679.
- Audi L, Mantzoros CS, Vidal-Puig A, Vargas D, Gussinye M, Carrascosa A. Leptin in relation to resumption of menses in women with anorexia nervosa. Mol Psychiatry 1998; 3:544–547.
- Wong IP, Baldock PA, Herzog H. Gastrointestinal peptides and bone health. Curr Opin Endocrinol Diabetes Obes 2010; 17:44–50.
- Fukushima N, Hanada R, Teranishi H, et al. Ghrelin directly regulates bone formation. J Bone Miner Res 2005; 20:790–798.
- Makovey J, Naganathan V, Seibel M, Sambrook P. Gender differences in plasma ghrelin and its relations to body composition and bone—an opposite-sex twin study. Clin Endocrinol (Oxf) 2007; 66:530–537.
- Hassouna R, Zizzari P, Tolle V. The ghrelin/obestatin balance in the physiological and pathological control of growth hormone secretion, body composition and food intake. J Neuroendocrinol 2010; 22:793–804.
- Germain N, Galusca B, Grouselle D, et al. Ghrelin/obestatin ratio in two populations with low body weight: constitutional thinness and anorexia nervosa. Psychoneuroendocrinology 2009; 34:413–419.
- Cohen A, Shane E. Treatment of premenopausal women with low bone mineral density. Curr Osteoporos Rep 2008; 6:39–46.
- Licata A. Bone density vs bone quality: what’s a clinician to do? Cleve Clin J Med 2009; 76:331–336.
- Bianchi ML, Baim S, Bishop NJ, et al. Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatr Nephrol 2010; 25:37–47.
- Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin North Am 2010; 39:155–167.
- Mehler PS, Krantz M. Anorexia nervosa medical issues. J Womens Health (Larchmt) 2003; 12:331–340.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National Osteoporosis Foundation 2008 Clinician’s Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J Clin Densitom 2008; 11:473–477.
- Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 2007; 39:1867–1882.
- Cummings SR, Palermo L, Browner W, et al. Monitoring osteoporosis therapy with bone densitometry: misleading changes and regression to the mean. Fracture Intervention Trial Research Group. JAMA 2000; 283:1318–1321.
- LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA 1999; 281:1505–1511.
- Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab 2010; 95:471–478.
- Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 2004; 158:531–537.
- Hofbauer LC, Hamann C, Ebeling PR. Approach to the patient with secondary osteoporosis. Eur J Endocrinol 2010; 162:1009–1020.
- Stoffman N, Gordon CM. Vitamin D and adolescents: what do we know? Curr Opin Pediatr 2009; 21:465–471.
- Bone HG, Greenspan SL, McKeever C, et al. Alendronate and estrogen effects in postmenopausal women with low bone mineral density. Alendronate/Estrogen Study Group. J Clin Endocrinol Metab 2000; 85:720–726.
- Barrett-Connor E, Wenger NK, Grady D, et al. Hormone and nonhormone therapy for the maintenance of postmenopausal health: the need for randomized controlled trials of estrogen and raloxifene. J Womens Health 1998; 7:839–847.
- Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 1995; 80:898–904.
- Stokosch GR, Friedman AJ, Wu SC, Kamin M. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in adolescent females with anorexia nervosa: double-blind, placebo-controlled study. J Adolesc Health 2006; 39:819–827.
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15:135–143.
- Robinson E, Bachrach LK, Katzman DK. Use of hormone replacement therapy to reduce the risk of osteopenia in adolescent girls with anorexia nervosa. J Adolesc Health 2000; 26:343–348.
- Myburgh KH, Hutchins J, Fataar AB, et al. Low bone density is an etiologic factor for stress fractures in athletes. Ann Intern Med 1990; 113:754–759.
- Young N, Formica C, Szmukler G, Seeman E. Bone density at weight-bearing and nonweight-bearing sites in ballet dancers: the effects of exercise, hypogonadism, and body weight. J Clin Endocrinol Metab 1994; 78:449–454.
- Stock JL, Bell NH, Chesnut CH, et al. Increments in bone mineral density of the lumbar spine and hip and suppression of bone turnover are maintained after discontinuation of alendronate in postmenopausal women. Am J Med 1997; 103:291–297.
- Subbiah V, Madsen VS, Raymond AK, Benjamin RS, Ludwig JA. Of mice and men: divergent risks of teriparatide-induced osteosarcoma. Osteoporos Int 2010; 21:1041–1045.
- Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001; 86:5992.
- Miljic D, Pekic S, Djurovic M, et al. Ghrelin has partial or no effect on appetite, growth hormone, prolactin, and cortisol release in patients with anorexia nervosa. J Clin Endocrinol Metab 2006; 914:1491–1495.
- Siegfried Z, Kanyas K, Latzer Y, et al. Association study of cannabinoid receptor gene (CNR1) alleles and anorexia nervosa: differences between restricting and binging/purging subtypes. Am J Med Genet B Neuropsychiatr Genet 2004; 125B:126–130.
- Cota D, Marsicano G, Lutz B, et al. Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord 2003; 27:289–301.
- Gross H, Ebert MH, Faden VB, et al. A double-blind trial of delta 9-tetrahydrocannabinol in primary anorexia nervosa. J Clin Psychopharmacol 1983; 3:165–171.
Among the devastating effects of anorexia nervosa, and one that is easily overlooked, is its impact on bone.
Probably more than half of young women with anorexia nervosa develop osteoporosis, and relatively quickly. Baker et al1 obtained bone scans in a series of 56 young women, mean age 27 years, who had had an eating disorder for a mean of about 10 years, and found that the bone mineral density in the femur was below the critical fracture threshold in 42 (75%).
Osteoporosis is particularly common and worrisome in female athletes (and is becoming increasingly common in male athletes as well). Female athletes have a much higher incidence of disordered eating than their peers2 and therefore are at a much higher risk of fractures.
This review summarizes the factors affecting the development of osteoporosis in these patients and discusses potential targets for intervention.
ANOREXIA AND BONE HEALTH: A COMPLEX RELATIONSHIP
Anorexia nervosa is characterized by an intense fear of gaining weight, a body weight less than 85% of expected, a distorted self-image, and, in women, missing three consecutive menstrual periods.3 The lifetime prevalence in women is about 0.5%; it is much lower in men.3 The prevalence of eating disorders in female athletes is much higher, estimated at 15% to 62%.2
The etiology of osteoporosis in patients with anorexia nervosa is complex and multifaceted. In these patients, bone resorption is increased without a concomitant increase in bone formation, resulting in a net loss of bone.4 Thus, markers of bone resorption such as N-teleopeptide and deoxypyridoline are elevated, but markers of bone formation such as osteocalcin are not.4
The loss of bone may be rapid and can occur relatively early in the disease. Some studies suggest that an illness duration longer than 12 months predicts significant loss of bone density.5 Thus, early diagnosis and intervention are important to minimize bone loss.
Women gain from 40% to 60% of their bone mass during the pubertal growth spurt in ages 11 to 14, the time when anorexia nervosa is most prevalent.6 Peak bone mass is attained by the third decade of life, but the rate of growth of bone mass is highest during adolescence and early adulthood.7 Hence, it is important to optimize bone mass during this time, as small differences in bone density can have significant clinical implications later in life: a 5% increase in bone density significantly decreases fracture risk, whereas a 10% decrease in adult bone mineral density is associated with a two to three times higher risk of fracture (reviewed by Rome and Ammerman6).
What is the role of amenorrhea in the development of osteoporosis in premenopausal patients?
Given that two of the most characteristic manifestations of anorexia nervosa are low body weight and the absence of menses, these factors have been hypothesized to be potential causes of osteoporosis.
In general, young women who present with amenorrhea should be evaluated to determine if the amenorrhea is primary or secondary. Primary amenorrhea is the absence of menarche by age 16; secondary amenorrhea is the absence of menses for more than three cycles or more than 6 months in someone who previously had had menses. The most common causes of secondary amenorrhea are ovarian disease, hypothalamic or pituitary disease, and uterine disease. Anorexia nervosa causes hypothalamic dysfunction and is a cause of secondary amenorrhea. In clinical practice, it is also important to remember that pregnancy can occur even in the setting of amenorrhea.
Amenorrhea in patients with anorexia nervosa is related to hypothalamic suppression of the release of gonadotropin-releasing hormone, resulting in lower levels of follicle-stimulating hormone and luteinizing hormone and a resultant prepubertal low-estrogen state.
In a study of 73 women with anorexia nervosa and a mean age of 17.2 years,8 20 months of amenorrhea was the threshold above which the most severe osteopenia was seen, implying that the duration of amenorrhea affects bone health.
Which factors besides amenorrhea influence bone density in premenopausal women?
Undernutrition. Body weight has been suggested to have an independent effect on bone mineral density, and density has been found to increase following weight gain, even before the return of menses.1 Once a regular menstrual cycle has been restored, significant increases in trabecular and cortical bone have been detected.1
Deficiency of insulin-like growth factor 1 (IGF-1). Anorexia nervosa is associated with decreased hepatic synthesis of IGF-I.9 Low levels of IGF-I reduce the levels of osteocalcin, a marker of bone formation, and cause abnormalities in osteoblast function.10 This deficiency is associated with the development of osteopenia in patients with anorexia nervosa.11
Low androgen levels are present in patients with anorexia nervosa, and levels appear to be further reduced by oral contraceptives.12 It remains to be determined whether the further reduction in androgens in women with anorexia nervosa using oral contraceptives is harmful to skeletal health. Low testosterone levels in boys with anorexia nervosa have been associated with lower libido, fewer erections, and potentially lower bone density.13
Hypercortisolemia. Elevated levels of total and free serum cortisol and high 24-hour urinary free cortisol excretion have been noted in anorectic patients. Levels of cortisol are inversely related to levels of osteocalcin, and hypercortisolism has been shown to be associated with osteoporosis.14,15 However, no study has yet shown causality in this population.
Osteoprotegerin has been recognized as an important regulator of bone resorption. Osteoprotegerin inhibits osteoclast differentiation and activation and stimulates osteoclast apoptosis, helping to preserve bone density.
Misra et al16 showed that adolescent girls with anorexia nervosa have higher serum osteoprotegerin levels than controls and that osteoprotegerin levels correlate inversely with markers of nutritional status and lumbar bone density Z scores.16 They and other investigators17 postulate that osteoprotegerin may be released as a compensatory response to the bone loss seen in these patients in an attempt to preserve bone health.
Leptin is an adipocyte-derived hormone that acts on receptors in the hypothalamus, decreasing food intake and increasing energy expenditure. Low leptin levels are a key endocrinologic feature of anorexia nervosa.18 Leptin helps to induce weight loss by stimulating neurons in the hypothalamus that express “weight-loss-inducing” neuropeptides such as pro-opiomelanocortin and inhibiting “weight-gain-inducing” peptides such as neuropeptide Y.19
Although leptin was first believed to be a hormone released to counteract obesity, recent studies19,20 suggest that it is part of a major signaling system that controls adaptation to starvation. These studies have shown that the body senses its corporeal fat through leptin and inhibits ovulation when fat reserves are low.19 In addition, luteinizing hormone and leptin levels have been shown to increase in parallel in patients with anorexia nervosa when weight is restored.20 Thus, rising leptin levels correlate with the resumption of menses in women with anorexia nervosa and in turn have potential consequences for bone health.
Not enough ghrelin, too much obestatin? Ghrelin, a gastric hormone, acts as a natural antagonist to leptin, resulting in an increase in food intake and body weight.19 Circulating ghrelin levels are higher in illness-induced anorexia as well as in anorexia nervosa, and they normalize with weight gain, perhaps as an adaptive mechanism to compensate for a negative energy balance.21
Several in vitro studies suggest that ghrelin directly promotes osteoblast proliferation and differentiation.22 However, human studies of ghrelin’s effects on bone are limited. In a study of healthy younger women, healthy boys, and anorexia nervosa patients, plasma ghrelin levels were only weakly associated with bone mineral density.23
The effects of obestatin, another gastric hormone, are still being investigated. Obestatin was initially shown to oppose the effects of ghrelin by decreasing appetite and weight gain. When given with ghrelin, obestatin appears to work with ghrelin at the hypothalamic level to modulate food intake and growth hormone secretion.24
Interestingly, obestatin and the ratio of ghrelin to obestatin are decreased in patients with anorexia nervosa, but the ratio is unchanged in thin women who have an equivalent body mass index but no eating disorder.25 It has been hypothesized that the ghrelin-obestatin ratio may be the key to explaining the eating restriction and reduced motivation to eat despite high ghrelin levels seen in anorexia nervosa.26 Further studies are needed to determine the role of obestatin and the ghrelin-obestatin ratio in the bone health of women with anorexia nervosa.
HOW SHOULD WE DIAGNOSE OSTEOPOROSIS IN PREMENOPAUSAL PATIENTS?
Our approach to screening for and diagnosing osteoporosis is still largely based on measuring bone mineral density, although density by itself is not a perfect tool for predicting who will or will not experience a fracture, particularly in premenopausal women.26,27 Most premenopausal women with low bone mineral density but no other risk factors for fracture such as previous fractures or glucocorticoid therapy are at very low short-term risk of fracture.26
For these reasons, in premenopausal women and adolescents, the International Society for Clinical Densitometry28 advises against diagnosing osteoporosis on the basis of bone mineral density alone. Instead, it should be diagnosed in this population only if the bone mineral density is low (defined as a Z score below −2.0) and the patient has risk factors that suggest a higher short-term risk of bone mineral loss and fracture. Risk factors include chronic malnutrition, eating disorders, hypogonadism, glucocorticoid exposure, and previous fracture.29
A pitfall in interpreting low bone mineral density in premenopausal women younger than age 30 is the possibility that they may not yet have reached their peak bone mass. In addition, small stature and body size (and therefore bone size) also influence the results of dual-energy x-ray absorptiometry. This test may not be able to distinguish bone that is small but of normal density from bone that is of low density.26
Despite its limitations, until newer risk assessment tools are available for this patient population, measuring bone mineral density is still recommended in addition to assessing clinical risk factors to diagnose osteoporosis. Also, changes in bone mineral density over time can help to assess risk and guide treatment.
When should a patient with anorexia be screened for osteoporosis?
Because bone loss may begin early in the course of anorexia and progress rapidly (potentially inexorably), baseline screening is recommended for all patients who have had anorexia nervosa or amenorrhea for more than 6 to 12 months.30 The National Osteoporosis Foundation recommends screening in women under age 65 who have a low body weight.31 The American College of Sports Medicine recommends screening for osteoporosis in athletes with a history of hypoestrogenism or disordered eating for a cumulative total of 6 months or more, or with a history of stress fracture or fracture from minimal trauma.32
Knowledge of low bone mineral density and fracture risk can often guide treatment and prompt behavioral change. Given that most osteoporosis treatments do not lead to detectable changes in bone density until 18 months to 2 years, it is reasonable to repeat testing at this interval.33
NEW AND OLD TREATMENTS FOR LOW BONE DENSITY IN ANOREXIA NERVOSA
Weight restoration is the cornerstone
Restoration of body weight and nutritional rehabilitation remain the cornerstones of treatment. All patients with anorexia nervosa should be referred to a nutritionist to develop a meal plan that is adjusted for the amount of energy expended. The challenges lie in managing the complications of refeeding and the high relapse rate. The treatment goals in disordered eating are to optimize the overall nutritional status, normalize eating behavior, modify unhealthy thought processes that maintain the disorder, and treat possible emotional issues that help create or maintain the disorder.
The younger the patient, the more the family’s involvement is recommended. In addition to nutritional counseling, the care team should include a psychotherapist, a psychiatrist, and a primary care physician to assist with management and screening of medical complications.
Vitamin D for all
Low vitamin D levels have long been associated with low bone mineral density and risk of hip fracture.34
Vitamin D insufficiency is very common. More than 90% of blacks, Hispanics, and Asians and nearly 75% of whites have insufficient levels of vitamin D (25-hydroxyvitamin D3 level < 30 ng/mL).35 In a study of 307 healthy adolescents, vitamin D insufficiency (a 25-hydroxyvitamin D3 level < 20 ng/mL) was found in 42% and vitamin D deficiency (a level < 15 ng/mL) in 24.1%.36 In addition, this study confirmed an inverse correlation between body mass index and serum 25-hydroxyvitamin D3 concentration.
Therefore, while vitamin D supplementation has not been consistently shown to improve bone loss in anorectic patients,9 given the prevalence of vitamin D deficiency and insufficiency, supplementation is almost universally recommended.
There is no consensus as to the amount of supplementation to recommend for women with anorexia nervosa. The American College of Sports Medicine recommends a total daily intake of 800 IU of vitamin D (ie, from diet and supplements). Therapy should be titrated to doses that result in normocalcemia and a serum 25-hydroxyvitamin D3 concentration of at least 30 ng/mL.37,38
Does hormone treatment improve bone density?
In postmenopausal osteoporosis, estrogen therapy maintains or improves bone density and appears to reduce the rate of vertebral fractures.39,40 Perhaps not so with premenopausal osteoporosis due to anorexia nervosa.
Why should this be? In postmenopausal women, estrogen therapy appears to work by impairing osteoclast-mediated bone resorption, but it has only limited effects on bone formation. In premenopausal anorexia, however, bone loss appears to be due to a unique uncoupling of osteoblastic and osteoclastic functions, resulting in both reduced bone formation and increased bone resorption, which estrogen therapy may not improve.5
Despite the documented association between anorexia nervosa and estrogen deficiency and the strong correlation between osteoporosis and the duration of amenorrhea, most studies have found no improvement in bone mass with hormonal therapy.9 In particular, three randomized, placebo-controlled trials have been published to date, and not one showed a significant improvement in bone mineral density with estrogen therapy compared with placebo in patients with anorexia nervosa.41–43
Klibanski et al,41 in the first of these trials, found no significant difference in spinal bone mineral density between treated patients and controls. However, estrogen-treated patients whose initial body weight was very low (< 70% of expected) had a significant increase in their bone mineral density, whereas those in the control group lost bone density.
Baker et al44 suggest that hormone therapy might protect bone mass in athletes with amenorrhea, citing a study that found that women with a history of stress fractures were less likely to have used oral contraceptives previously than athletes without fractures.45 However, no prospective randomized study to date has established that hormone therapy effectively preserves bone mass in athletes with amenorrhea.
Based on the data presented above, we have little evidence for using estrogen to treat or prevent premenopausal osteoporosis.
The American College of Sports Medicine32 recommends consideration of estrogen therapy if there is evidence of a decline in bone mineral density in an athlete over the age of 16 with persistent functional hypothalamic amenorrhea despite adequate nutritional intake and weight. However, it acknowledges that restoring regular menstrual cycles with oral contraceptive pills will not normalize the metabolic factors that impair bone formation, health, and performance and is not likely to fully reverse low bone mineral density in this population.
What is the effect of exercise on bone health in these patients?
Several studies have examined the effect of weight-bearing exercise on bone density.
Young et al46 compared normal teenagers, ballet dancers, and young women with anorexia nervosa and found that weight-bearing exercise protected against osteoporosis, but only at weight-bearing sites. Athletes in weight-bearing sports had a 5% to 15% higher bone mineral density in weight-bearing sites (ie, the femur) compared with nonathletes, but had lower bone mineral density in the spine.
Therefore, a Z score below −1.0 in an athlete, especially in a distal site, warrants further investigation and treatment.32 In general, exercise does not necessarily protect against osteoporosis in this patient population, and it can sometimes mask underlying bone loss. In addition, keep in mind that many of these patients exercise compulsively, using it as a form of purging.
Insulin-like growth factor-1: More study needed
IGF-1 contributes to bone growth by stimulating osteoblasts, and patients with anorexia nervosa have been shown to have low levels of IGF-1.9
Grinspoon et al10 randomized 60 patients with anorexia nervosa to receive IGF-1 alone, IGF-1 plus an oral contraceptive, an oral contraceptive alone, or placebo. All patients were given calcium and vitamin D and were followed for 9 months. Total bone mass increased significantly in those taking IGF-1 compared with those taking placebo. Those taking an IGF-1 and an oral contraceptive had a significant increase in spinal bone mineral density compared with those on placebo group. At other skeletal sites, however, IGF-1 plus an oral contraceptive and IGF-1 alone failed to produce significant increases in bone mineral density compared with placebo.
Further study is needed to determine the role of IGF-1 in treating low bone mineral density in anorexia nervosa.
Bisphosphonates: Not approved for this indication
In premenopausal women, bisphosphonates are approved by the US Food and Drug Administration (FDA) for use only in those taking glucocorticoids. Although bisphosphonates have been shown to significantly increase bone mineral density in young women with anorexia nervosa,26 they should be used with caution in patients of childbearing age because they are teratogenic. Bisphosphonates have a long half-life and may continue to affect bone turnover for up to 2 years after they are discontinued.47 In addition, they are not recommended in patients with a history of purging via vomiting, due to a risk of esophageal ulceration.
Parathyroid hormone therapy: Studies ongoing
The parathyroid hormone fragment teriparatide (Forteo) is widely used for treating postmenopausal osteoporosis.
Before teriparatide was approved, there was concern that it might increase the risk of osteosarcoma, as almost 45% of rats treated with this drug at the highest-tested dose level developed this aggressive form of bone cancer.48 Balancing the proven benefits of teriparatide shown by clinical trials with the theoretic risk of teriparatide-induced osteosarcoma, the FDA mandated a “black-box” warning about this potential effect.
Studies of parathyroid hormone treatment in anorexia nervosa and other premenopausal patients are ongoing.26
Leptin: More study needed
Leptin is a potent stimulator of bone growth and has been shown to increase bone mineral density in vitro and in vivo.19 However, concerns have been raised about giving supra-physiologic doses of leptin to patients with anorexia nervosa, as this may increase the risk of further weight loss and relapse.
More work is needed to determine the role of leptin for the treatment of osteoporosis in anorexia nervosa.
Ghrelin: Probably not effective as a single agent
Pharmacologic use of ghrelin increases food intake in healthy humans,49 and it has been proposed as a treatment for weight restoration and bone health in anorexia nervosa. Preliminary studies have not shown it to increase appetite or weight gain,50 but it did increase slow-wave sleep.
Based on these studies, it is unlikely that ghrelin will be effective as a single agent to stimulate appetite, but it may be helpful in conjunction with other therapies.
Cannabinoids: Little ongoing research
Cannabinoids have been proposed as a treatment for anorexia nervosa in the hope that they would induce weight gain and in turn prevent osteoporosis.
Interest in their use in anorexia nervosa stems from the discovery of two cannabinoid receptors (CB1 and CB2) located in the brain and peripheral organ systems. Anorexia nervosa has been associated with different alleles of the CB1 gene,51 but the therapeutic implications of this are far from clear.
Cannabinoids appear to regulate eating behavior at several levels within the brain and periphery: the hypothalamus and hindbrain (integrative functions), the limbic system (for hedonic evaluation of foods), the intestinal system, and adipose tissue.52 At each of these levels, the endocannabinoid system interacts with a number of better known peptides involved in appetite regulation, including leptin, ghrelin, and the melanocortins. In mouse studies, genetic leptin deficiency is associated with elevated hypothalamic endocannabinoid levels.
Appetite stimulation by cannabinoids has been studied for several decades, particularly in relation to cachexia and malnutrition associated with cancer. Very few trials have studied cannabinoids for anorexia nervosa.
In a 4-week crossover trial in 11 patients with anorexia nervosa,53 tetrahydrocannabinol (THC) treatment resulted in an increase in sleep disturbances and interpersonal sensitivity, but it had no significant effect on weight gain compared with diazepam treatment.53
Another pilot study of nine outpatients with anorexia nervosa treated with THC showed a significant improvement in depression and perfectionism scores without any significant weight gain.19
Although this research was once promising, the risk was felt to outweigh the benefit, as cannabinoids may induce dependency in this patient group, who may already be at high risk of drug addiction, and very few have continued this line of investigation.
WHAT CAN WE DO FOR NOW?
- Weight restoration and nutritional rehabilitation remain the keys to treatment of low bone density to reduce the risk of osteoporosis in patients with anorexia nervosa. However, as many as one-third of patients with anorexia nervosa relapse during their lifetime, and other treatments are needed to stabilize and prevent bone loss.
- Vitamin D deficiency is clearly associated with a risk of osteoporosis and fracture, and patients with vitamin D deficiency should be treated with supplementation.
- Standard therapies in postmenopausal patients (such as bisphosphonates and teriparatide) should be used with caution in premenopausal anorexia nervosa patients because of potential long-term health risks.
- As we learn more about hormonal factors in anorexia nervosa, we hope to identify interventions that will help restore weight and decrease the risk of osteoporosis. A summary of potential treatment strategies and targets for prevention of osteoporosis in anorexia nervosa is presented in Table 2.
Acknowledgment: The author thanks the General Internal Medicine Works in Progress Group for its editorial comments, and Dr. Ellen Rome for her mentorship and support.
Among the devastating effects of anorexia nervosa, and one that is easily overlooked, is its impact on bone.
Probably more than half of young women with anorexia nervosa develop osteoporosis, and relatively quickly. Baker et al1 obtained bone scans in a series of 56 young women, mean age 27 years, who had had an eating disorder for a mean of about 10 years, and found that the bone mineral density in the femur was below the critical fracture threshold in 42 (75%).
Osteoporosis is particularly common and worrisome in female athletes (and is becoming increasingly common in male athletes as well). Female athletes have a much higher incidence of disordered eating than their peers2 and therefore are at a much higher risk of fractures.
This review summarizes the factors affecting the development of osteoporosis in these patients and discusses potential targets for intervention.
ANOREXIA AND BONE HEALTH: A COMPLEX RELATIONSHIP
Anorexia nervosa is characterized by an intense fear of gaining weight, a body weight less than 85% of expected, a distorted self-image, and, in women, missing three consecutive menstrual periods.3 The lifetime prevalence in women is about 0.5%; it is much lower in men.3 The prevalence of eating disorders in female athletes is much higher, estimated at 15% to 62%.2
The etiology of osteoporosis in patients with anorexia nervosa is complex and multifaceted. In these patients, bone resorption is increased without a concomitant increase in bone formation, resulting in a net loss of bone.4 Thus, markers of bone resorption such as N-teleopeptide and deoxypyridoline are elevated, but markers of bone formation such as osteocalcin are not.4
The loss of bone may be rapid and can occur relatively early in the disease. Some studies suggest that an illness duration longer than 12 months predicts significant loss of bone density.5 Thus, early diagnosis and intervention are important to minimize bone loss.
Women gain from 40% to 60% of their bone mass during the pubertal growth spurt in ages 11 to 14, the time when anorexia nervosa is most prevalent.6 Peak bone mass is attained by the third decade of life, but the rate of growth of bone mass is highest during adolescence and early adulthood.7 Hence, it is important to optimize bone mass during this time, as small differences in bone density can have significant clinical implications later in life: a 5% increase in bone density significantly decreases fracture risk, whereas a 10% decrease in adult bone mineral density is associated with a two to three times higher risk of fracture (reviewed by Rome and Ammerman6).
What is the role of amenorrhea in the development of osteoporosis in premenopausal patients?
Given that two of the most characteristic manifestations of anorexia nervosa are low body weight and the absence of menses, these factors have been hypothesized to be potential causes of osteoporosis.
In general, young women who present with amenorrhea should be evaluated to determine if the amenorrhea is primary or secondary. Primary amenorrhea is the absence of menarche by age 16; secondary amenorrhea is the absence of menses for more than three cycles or more than 6 months in someone who previously had had menses. The most common causes of secondary amenorrhea are ovarian disease, hypothalamic or pituitary disease, and uterine disease. Anorexia nervosa causes hypothalamic dysfunction and is a cause of secondary amenorrhea. In clinical practice, it is also important to remember that pregnancy can occur even in the setting of amenorrhea.
Amenorrhea in patients with anorexia nervosa is related to hypothalamic suppression of the release of gonadotropin-releasing hormone, resulting in lower levels of follicle-stimulating hormone and luteinizing hormone and a resultant prepubertal low-estrogen state.
In a study of 73 women with anorexia nervosa and a mean age of 17.2 years,8 20 months of amenorrhea was the threshold above which the most severe osteopenia was seen, implying that the duration of amenorrhea affects bone health.
Which factors besides amenorrhea influence bone density in premenopausal women?
Undernutrition. Body weight has been suggested to have an independent effect on bone mineral density, and density has been found to increase following weight gain, even before the return of menses.1 Once a regular menstrual cycle has been restored, significant increases in trabecular and cortical bone have been detected.1
Deficiency of insulin-like growth factor 1 (IGF-1). Anorexia nervosa is associated with decreased hepatic synthesis of IGF-I.9 Low levels of IGF-I reduce the levels of osteocalcin, a marker of bone formation, and cause abnormalities in osteoblast function.10 This deficiency is associated with the development of osteopenia in patients with anorexia nervosa.11
Low androgen levels are present in patients with anorexia nervosa, and levels appear to be further reduced by oral contraceptives.12 It remains to be determined whether the further reduction in androgens in women with anorexia nervosa using oral contraceptives is harmful to skeletal health. Low testosterone levels in boys with anorexia nervosa have been associated with lower libido, fewer erections, and potentially lower bone density.13
Hypercortisolemia. Elevated levels of total and free serum cortisol and high 24-hour urinary free cortisol excretion have been noted in anorectic patients. Levels of cortisol are inversely related to levels of osteocalcin, and hypercortisolism has been shown to be associated with osteoporosis.14,15 However, no study has yet shown causality in this population.
Osteoprotegerin has been recognized as an important regulator of bone resorption. Osteoprotegerin inhibits osteoclast differentiation and activation and stimulates osteoclast apoptosis, helping to preserve bone density.
Misra et al16 showed that adolescent girls with anorexia nervosa have higher serum osteoprotegerin levels than controls and that osteoprotegerin levels correlate inversely with markers of nutritional status and lumbar bone density Z scores.16 They and other investigators17 postulate that osteoprotegerin may be released as a compensatory response to the bone loss seen in these patients in an attempt to preserve bone health.
Leptin is an adipocyte-derived hormone that acts on receptors in the hypothalamus, decreasing food intake and increasing energy expenditure. Low leptin levels are a key endocrinologic feature of anorexia nervosa.18 Leptin helps to induce weight loss by stimulating neurons in the hypothalamus that express “weight-loss-inducing” neuropeptides such as pro-opiomelanocortin and inhibiting “weight-gain-inducing” peptides such as neuropeptide Y.19
Although leptin was first believed to be a hormone released to counteract obesity, recent studies19,20 suggest that it is part of a major signaling system that controls adaptation to starvation. These studies have shown that the body senses its corporeal fat through leptin and inhibits ovulation when fat reserves are low.19 In addition, luteinizing hormone and leptin levels have been shown to increase in parallel in patients with anorexia nervosa when weight is restored.20 Thus, rising leptin levels correlate with the resumption of menses in women with anorexia nervosa and in turn have potential consequences for bone health.
Not enough ghrelin, too much obestatin? Ghrelin, a gastric hormone, acts as a natural antagonist to leptin, resulting in an increase in food intake and body weight.19 Circulating ghrelin levels are higher in illness-induced anorexia as well as in anorexia nervosa, and they normalize with weight gain, perhaps as an adaptive mechanism to compensate for a negative energy balance.21
Several in vitro studies suggest that ghrelin directly promotes osteoblast proliferation and differentiation.22 However, human studies of ghrelin’s effects on bone are limited. In a study of healthy younger women, healthy boys, and anorexia nervosa patients, plasma ghrelin levels were only weakly associated with bone mineral density.23
The effects of obestatin, another gastric hormone, are still being investigated. Obestatin was initially shown to oppose the effects of ghrelin by decreasing appetite and weight gain. When given with ghrelin, obestatin appears to work with ghrelin at the hypothalamic level to modulate food intake and growth hormone secretion.24
Interestingly, obestatin and the ratio of ghrelin to obestatin are decreased in patients with anorexia nervosa, but the ratio is unchanged in thin women who have an equivalent body mass index but no eating disorder.25 It has been hypothesized that the ghrelin-obestatin ratio may be the key to explaining the eating restriction and reduced motivation to eat despite high ghrelin levels seen in anorexia nervosa.26 Further studies are needed to determine the role of obestatin and the ghrelin-obestatin ratio in the bone health of women with anorexia nervosa.
HOW SHOULD WE DIAGNOSE OSTEOPOROSIS IN PREMENOPAUSAL PATIENTS?
Our approach to screening for and diagnosing osteoporosis is still largely based on measuring bone mineral density, although density by itself is not a perfect tool for predicting who will or will not experience a fracture, particularly in premenopausal women.26,27 Most premenopausal women with low bone mineral density but no other risk factors for fracture such as previous fractures or glucocorticoid therapy are at very low short-term risk of fracture.26
For these reasons, in premenopausal women and adolescents, the International Society for Clinical Densitometry28 advises against diagnosing osteoporosis on the basis of bone mineral density alone. Instead, it should be diagnosed in this population only if the bone mineral density is low (defined as a Z score below −2.0) and the patient has risk factors that suggest a higher short-term risk of bone mineral loss and fracture. Risk factors include chronic malnutrition, eating disorders, hypogonadism, glucocorticoid exposure, and previous fracture.29
A pitfall in interpreting low bone mineral density in premenopausal women younger than age 30 is the possibility that they may not yet have reached their peak bone mass. In addition, small stature and body size (and therefore bone size) also influence the results of dual-energy x-ray absorptiometry. This test may not be able to distinguish bone that is small but of normal density from bone that is of low density.26
Despite its limitations, until newer risk assessment tools are available for this patient population, measuring bone mineral density is still recommended in addition to assessing clinical risk factors to diagnose osteoporosis. Also, changes in bone mineral density over time can help to assess risk and guide treatment.
When should a patient with anorexia be screened for osteoporosis?
Because bone loss may begin early in the course of anorexia and progress rapidly (potentially inexorably), baseline screening is recommended for all patients who have had anorexia nervosa or amenorrhea for more than 6 to 12 months.30 The National Osteoporosis Foundation recommends screening in women under age 65 who have a low body weight.31 The American College of Sports Medicine recommends screening for osteoporosis in athletes with a history of hypoestrogenism or disordered eating for a cumulative total of 6 months or more, or with a history of stress fracture or fracture from minimal trauma.32
Knowledge of low bone mineral density and fracture risk can often guide treatment and prompt behavioral change. Given that most osteoporosis treatments do not lead to detectable changes in bone density until 18 months to 2 years, it is reasonable to repeat testing at this interval.33
NEW AND OLD TREATMENTS FOR LOW BONE DENSITY IN ANOREXIA NERVOSA
Weight restoration is the cornerstone
Restoration of body weight and nutritional rehabilitation remain the cornerstones of treatment. All patients with anorexia nervosa should be referred to a nutritionist to develop a meal plan that is adjusted for the amount of energy expended. The challenges lie in managing the complications of refeeding and the high relapse rate. The treatment goals in disordered eating are to optimize the overall nutritional status, normalize eating behavior, modify unhealthy thought processes that maintain the disorder, and treat possible emotional issues that help create or maintain the disorder.
The younger the patient, the more the family’s involvement is recommended. In addition to nutritional counseling, the care team should include a psychotherapist, a psychiatrist, and a primary care physician to assist with management and screening of medical complications.
Vitamin D for all
Low vitamin D levels have long been associated with low bone mineral density and risk of hip fracture.34
Vitamin D insufficiency is very common. More than 90% of blacks, Hispanics, and Asians and nearly 75% of whites have insufficient levels of vitamin D (25-hydroxyvitamin D3 level < 30 ng/mL).35 In a study of 307 healthy adolescents, vitamin D insufficiency (a 25-hydroxyvitamin D3 level < 20 ng/mL) was found in 42% and vitamin D deficiency (a level < 15 ng/mL) in 24.1%.36 In addition, this study confirmed an inverse correlation between body mass index and serum 25-hydroxyvitamin D3 concentration.
Therefore, while vitamin D supplementation has not been consistently shown to improve bone loss in anorectic patients,9 given the prevalence of vitamin D deficiency and insufficiency, supplementation is almost universally recommended.
There is no consensus as to the amount of supplementation to recommend for women with anorexia nervosa. The American College of Sports Medicine recommends a total daily intake of 800 IU of vitamin D (ie, from diet and supplements). Therapy should be titrated to doses that result in normocalcemia and a serum 25-hydroxyvitamin D3 concentration of at least 30 ng/mL.37,38
Does hormone treatment improve bone density?
In postmenopausal osteoporosis, estrogen therapy maintains or improves bone density and appears to reduce the rate of vertebral fractures.39,40 Perhaps not so with premenopausal osteoporosis due to anorexia nervosa.
Why should this be? In postmenopausal women, estrogen therapy appears to work by impairing osteoclast-mediated bone resorption, but it has only limited effects on bone formation. In premenopausal anorexia, however, bone loss appears to be due to a unique uncoupling of osteoblastic and osteoclastic functions, resulting in both reduced bone formation and increased bone resorption, which estrogen therapy may not improve.5
Despite the documented association between anorexia nervosa and estrogen deficiency and the strong correlation between osteoporosis and the duration of amenorrhea, most studies have found no improvement in bone mass with hormonal therapy.9 In particular, three randomized, placebo-controlled trials have been published to date, and not one showed a significant improvement in bone mineral density with estrogen therapy compared with placebo in patients with anorexia nervosa.41–43
Klibanski et al,41 in the first of these trials, found no significant difference in spinal bone mineral density between treated patients and controls. However, estrogen-treated patients whose initial body weight was very low (< 70% of expected) had a significant increase in their bone mineral density, whereas those in the control group lost bone density.
Baker et al44 suggest that hormone therapy might protect bone mass in athletes with amenorrhea, citing a study that found that women with a history of stress fractures were less likely to have used oral contraceptives previously than athletes without fractures.45 However, no prospective randomized study to date has established that hormone therapy effectively preserves bone mass in athletes with amenorrhea.
Based on the data presented above, we have little evidence for using estrogen to treat or prevent premenopausal osteoporosis.
The American College of Sports Medicine32 recommends consideration of estrogen therapy if there is evidence of a decline in bone mineral density in an athlete over the age of 16 with persistent functional hypothalamic amenorrhea despite adequate nutritional intake and weight. However, it acknowledges that restoring regular menstrual cycles with oral contraceptive pills will not normalize the metabolic factors that impair bone formation, health, and performance and is not likely to fully reverse low bone mineral density in this population.
What is the effect of exercise on bone health in these patients?
Several studies have examined the effect of weight-bearing exercise on bone density.
Young et al46 compared normal teenagers, ballet dancers, and young women with anorexia nervosa and found that weight-bearing exercise protected against osteoporosis, but only at weight-bearing sites. Athletes in weight-bearing sports had a 5% to 15% higher bone mineral density in weight-bearing sites (ie, the femur) compared with nonathletes, but had lower bone mineral density in the spine.
Therefore, a Z score below −1.0 in an athlete, especially in a distal site, warrants further investigation and treatment.32 In general, exercise does not necessarily protect against osteoporosis in this patient population, and it can sometimes mask underlying bone loss. In addition, keep in mind that many of these patients exercise compulsively, using it as a form of purging.
Insulin-like growth factor-1: More study needed
IGF-1 contributes to bone growth by stimulating osteoblasts, and patients with anorexia nervosa have been shown to have low levels of IGF-1.9
Grinspoon et al10 randomized 60 patients with anorexia nervosa to receive IGF-1 alone, IGF-1 plus an oral contraceptive, an oral contraceptive alone, or placebo. All patients were given calcium and vitamin D and were followed for 9 months. Total bone mass increased significantly in those taking IGF-1 compared with those taking placebo. Those taking an IGF-1 and an oral contraceptive had a significant increase in spinal bone mineral density compared with those on placebo group. At other skeletal sites, however, IGF-1 plus an oral contraceptive and IGF-1 alone failed to produce significant increases in bone mineral density compared with placebo.
Further study is needed to determine the role of IGF-1 in treating low bone mineral density in anorexia nervosa.
Bisphosphonates: Not approved for this indication
In premenopausal women, bisphosphonates are approved by the US Food and Drug Administration (FDA) for use only in those taking glucocorticoids. Although bisphosphonates have been shown to significantly increase bone mineral density in young women with anorexia nervosa,26 they should be used with caution in patients of childbearing age because they are teratogenic. Bisphosphonates have a long half-life and may continue to affect bone turnover for up to 2 years after they are discontinued.47 In addition, they are not recommended in patients with a history of purging via vomiting, due to a risk of esophageal ulceration.
Parathyroid hormone therapy: Studies ongoing
The parathyroid hormone fragment teriparatide (Forteo) is widely used for treating postmenopausal osteoporosis.
Before teriparatide was approved, there was concern that it might increase the risk of osteosarcoma, as almost 45% of rats treated with this drug at the highest-tested dose level developed this aggressive form of bone cancer.48 Balancing the proven benefits of teriparatide shown by clinical trials with the theoretic risk of teriparatide-induced osteosarcoma, the FDA mandated a “black-box” warning about this potential effect.
Studies of parathyroid hormone treatment in anorexia nervosa and other premenopausal patients are ongoing.26
Leptin: More study needed
Leptin is a potent stimulator of bone growth and has been shown to increase bone mineral density in vitro and in vivo.19 However, concerns have been raised about giving supra-physiologic doses of leptin to patients with anorexia nervosa, as this may increase the risk of further weight loss and relapse.
More work is needed to determine the role of leptin for the treatment of osteoporosis in anorexia nervosa.
Ghrelin: Probably not effective as a single agent
Pharmacologic use of ghrelin increases food intake in healthy humans,49 and it has been proposed as a treatment for weight restoration and bone health in anorexia nervosa. Preliminary studies have not shown it to increase appetite or weight gain,50 but it did increase slow-wave sleep.
Based on these studies, it is unlikely that ghrelin will be effective as a single agent to stimulate appetite, but it may be helpful in conjunction with other therapies.
Cannabinoids: Little ongoing research
Cannabinoids have been proposed as a treatment for anorexia nervosa in the hope that they would induce weight gain and in turn prevent osteoporosis.
Interest in their use in anorexia nervosa stems from the discovery of two cannabinoid receptors (CB1 and CB2) located in the brain and peripheral organ systems. Anorexia nervosa has been associated with different alleles of the CB1 gene,51 but the therapeutic implications of this are far from clear.
Cannabinoids appear to regulate eating behavior at several levels within the brain and periphery: the hypothalamus and hindbrain (integrative functions), the limbic system (for hedonic evaluation of foods), the intestinal system, and adipose tissue.52 At each of these levels, the endocannabinoid system interacts with a number of better known peptides involved in appetite regulation, including leptin, ghrelin, and the melanocortins. In mouse studies, genetic leptin deficiency is associated with elevated hypothalamic endocannabinoid levels.
Appetite stimulation by cannabinoids has been studied for several decades, particularly in relation to cachexia and malnutrition associated with cancer. Very few trials have studied cannabinoids for anorexia nervosa.
In a 4-week crossover trial in 11 patients with anorexia nervosa,53 tetrahydrocannabinol (THC) treatment resulted in an increase in sleep disturbances and interpersonal sensitivity, but it had no significant effect on weight gain compared with diazepam treatment.53
Another pilot study of nine outpatients with anorexia nervosa treated with THC showed a significant improvement in depression and perfectionism scores without any significant weight gain.19
Although this research was once promising, the risk was felt to outweigh the benefit, as cannabinoids may induce dependency in this patient group, who may already be at high risk of drug addiction, and very few have continued this line of investigation.
WHAT CAN WE DO FOR NOW?
- Weight restoration and nutritional rehabilitation remain the keys to treatment of low bone density to reduce the risk of osteoporosis in patients with anorexia nervosa. However, as many as one-third of patients with anorexia nervosa relapse during their lifetime, and other treatments are needed to stabilize and prevent bone loss.
- Vitamin D deficiency is clearly associated with a risk of osteoporosis and fracture, and patients with vitamin D deficiency should be treated with supplementation.
- Standard therapies in postmenopausal patients (such as bisphosphonates and teriparatide) should be used with caution in premenopausal anorexia nervosa patients because of potential long-term health risks.
- As we learn more about hormonal factors in anorexia nervosa, we hope to identify interventions that will help restore weight and decrease the risk of osteoporosis. A summary of potential treatment strategies and targets for prevention of osteoporosis in anorexia nervosa is presented in Table 2.
Acknowledgment: The author thanks the General Internal Medicine Works in Progress Group for its editorial comments, and Dr. Ellen Rome for her mentorship and support.
- Baker D, Roberts R, Towell T. Factors predictive of bone mineral density in eating-disordered women: a longitudinal study. Int J Eat Disord 2000; 27:29–35.
- Rome ES. Eating disorders. Obstet Gynecol Clin North Am 2003; 30:353–377.
- First MB, editor. Diagnostic and Statistical Manual of Mental Disorders—4th edition. Washington, DC: American Psychiatric Association, 2000.
- Mehler PS, MacKenzie TD. Treatment of osteopenia and osteoporosis in anorexia nervosa: a systematic review of the literature. Int J Eat Disord 2009; 42:195–201.
- Wong JC, Lewindon P, Mortimer R, Shepherd R. Bone mineral density in adolescent females with recently diagnosed anorexia nervosa. Int J Eat Disord 2001; 29:11–16.
- Rome ES, Ammerman S. Medical complications of eating disorders: an update. J Adolesc Health 2003; 33:418–426.
- Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women. JAMA 1992; 268:2403–2408.
- Sterling WM, Golden NH, Jacobson MS, Ornstein RM, Hertz SM. Metabolic assessment of menstruating and nonmenstruating normal weight adolescents. Int J Eat Disord 2009; 42:658–663.
- Legroux-Gerot I, Vignau J, Collier F, Cortet B. Bone loss associated with anorexia nervosa. Joint Bone Spine 2005; 72:489–495.
- Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab 2002; 87:2883–2891.
- Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab 1999; 84:4489–4496.
- Miller KK, Lawson EA, Mathur V, et al. Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab 2007; 92:1334–1339.
- Misra M, Katzman DK, Cord J, et al. Bone metabolism in adolescent boys with anorexia nervosa. J Clin Endocrinol Metab 2008; 93:3029–3036.
- Misra M, Miller KK, Almazan C, et al. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab 2004; 89:4972–4980.
- Chiodini I, Mascia ML, Muscarella S, et al. Subclinical hypercortisolism among outpatients referred for osteoporosis. Ann Intern Med 2007; 147:541–548.
- Misra M, Soyka LA, Miller KK, et al. Serum osteoprotegerin in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 2003; 88:3816–3822.
- Ohwada R, Hotta M, Sato K, Shibasaki T, Takano K. The relationship between serum levels of estradiol and osteoprotegerin in patients with anorexia nervosa. Endocr J 2007; 54:953–959.
- Müller TD, Föcker M, Holtkamp K, Herpertz-Dahlmann B, Hebebrand J. Leptin-mediated neuroendocrine alterations in anorexia nervosa: somatic and behavioral implications. Child Adolesc Psychiatr Clin N Am 2009; 18:117–129.
- Støving RK, Andries A, Brixen K, Flyvbjerg A, Hørder K, Frystyk J. Leptin, ghrelin, and endocannabinoids: potential therapeutic targets in anorexia nervosa. J Psychiatr Res 2009; 43:671–679.
- Audi L, Mantzoros CS, Vidal-Puig A, Vargas D, Gussinye M, Carrascosa A. Leptin in relation to resumption of menses in women with anorexia nervosa. Mol Psychiatry 1998; 3:544–547.
- Wong IP, Baldock PA, Herzog H. Gastrointestinal peptides and bone health. Curr Opin Endocrinol Diabetes Obes 2010; 17:44–50.
- Fukushima N, Hanada R, Teranishi H, et al. Ghrelin directly regulates bone formation. J Bone Miner Res 2005; 20:790–798.
- Makovey J, Naganathan V, Seibel M, Sambrook P. Gender differences in plasma ghrelin and its relations to body composition and bone—an opposite-sex twin study. Clin Endocrinol (Oxf) 2007; 66:530–537.
- Hassouna R, Zizzari P, Tolle V. The ghrelin/obestatin balance in the physiological and pathological control of growth hormone secretion, body composition and food intake. J Neuroendocrinol 2010; 22:793–804.
- Germain N, Galusca B, Grouselle D, et al. Ghrelin/obestatin ratio in two populations with low body weight: constitutional thinness and anorexia nervosa. Psychoneuroendocrinology 2009; 34:413–419.
- Cohen A, Shane E. Treatment of premenopausal women with low bone mineral density. Curr Osteoporos Rep 2008; 6:39–46.
- Licata A. Bone density vs bone quality: what’s a clinician to do? Cleve Clin J Med 2009; 76:331–336.
- Bianchi ML, Baim S, Bishop NJ, et al. Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatr Nephrol 2010; 25:37–47.
- Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin North Am 2010; 39:155–167.
- Mehler PS, Krantz M. Anorexia nervosa medical issues. J Womens Health (Larchmt) 2003; 12:331–340.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National Osteoporosis Foundation 2008 Clinician’s Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J Clin Densitom 2008; 11:473–477.
- Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 2007; 39:1867–1882.
- Cummings SR, Palermo L, Browner W, et al. Monitoring osteoporosis therapy with bone densitometry: misleading changes and regression to the mean. Fracture Intervention Trial Research Group. JAMA 2000; 283:1318–1321.
- LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA 1999; 281:1505–1511.
- Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab 2010; 95:471–478.
- Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 2004; 158:531–537.
- Hofbauer LC, Hamann C, Ebeling PR. Approach to the patient with secondary osteoporosis. Eur J Endocrinol 2010; 162:1009–1020.
- Stoffman N, Gordon CM. Vitamin D and adolescents: what do we know? Curr Opin Pediatr 2009; 21:465–471.
- Bone HG, Greenspan SL, McKeever C, et al. Alendronate and estrogen effects in postmenopausal women with low bone mineral density. Alendronate/Estrogen Study Group. J Clin Endocrinol Metab 2000; 85:720–726.
- Barrett-Connor E, Wenger NK, Grady D, et al. Hormone and nonhormone therapy for the maintenance of postmenopausal health: the need for randomized controlled trials of estrogen and raloxifene. J Womens Health 1998; 7:839–847.
- Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 1995; 80:898–904.
- Stokosch GR, Friedman AJ, Wu SC, Kamin M. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in adolescent females with anorexia nervosa: double-blind, placebo-controlled study. J Adolesc Health 2006; 39:819–827.
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15:135–143.
- Robinson E, Bachrach LK, Katzman DK. Use of hormone replacement therapy to reduce the risk of osteopenia in adolescent girls with anorexia nervosa. J Adolesc Health 2000; 26:343–348.
- Myburgh KH, Hutchins J, Fataar AB, et al. Low bone density is an etiologic factor for stress fractures in athletes. Ann Intern Med 1990; 113:754–759.
- Young N, Formica C, Szmukler G, Seeman E. Bone density at weight-bearing and nonweight-bearing sites in ballet dancers: the effects of exercise, hypogonadism, and body weight. J Clin Endocrinol Metab 1994; 78:449–454.
- Stock JL, Bell NH, Chesnut CH, et al. Increments in bone mineral density of the lumbar spine and hip and suppression of bone turnover are maintained after discontinuation of alendronate in postmenopausal women. Am J Med 1997; 103:291–297.
- Subbiah V, Madsen VS, Raymond AK, Benjamin RS, Ludwig JA. Of mice and men: divergent risks of teriparatide-induced osteosarcoma. Osteoporos Int 2010; 21:1041–1045.
- Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001; 86:5992.
- Miljic D, Pekic S, Djurovic M, et al. Ghrelin has partial or no effect on appetite, growth hormone, prolactin, and cortisol release in patients with anorexia nervosa. J Clin Endocrinol Metab 2006; 914:1491–1495.
- Siegfried Z, Kanyas K, Latzer Y, et al. Association study of cannabinoid receptor gene (CNR1) alleles and anorexia nervosa: differences between restricting and binging/purging subtypes. Am J Med Genet B Neuropsychiatr Genet 2004; 125B:126–130.
- Cota D, Marsicano G, Lutz B, et al. Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord 2003; 27:289–301.
- Gross H, Ebert MH, Faden VB, et al. A double-blind trial of delta 9-tetrahydrocannabinol in primary anorexia nervosa. J Clin Psychopharmacol 1983; 3:165–171.
- Baker D, Roberts R, Towell T. Factors predictive of bone mineral density in eating-disordered women: a longitudinal study. Int J Eat Disord 2000; 27:29–35.
- Rome ES. Eating disorders. Obstet Gynecol Clin North Am 2003; 30:353–377.
- First MB, editor. Diagnostic and Statistical Manual of Mental Disorders—4th edition. Washington, DC: American Psychiatric Association, 2000.
- Mehler PS, MacKenzie TD. Treatment of osteopenia and osteoporosis in anorexia nervosa: a systematic review of the literature. Int J Eat Disord 2009; 42:195–201.
- Wong JC, Lewindon P, Mortimer R, Shepherd R. Bone mineral density in adolescent females with recently diagnosed anorexia nervosa. Int J Eat Disord 2001; 29:11–16.
- Rome ES, Ammerman S. Medical complications of eating disorders: an update. J Adolesc Health 2003; 33:418–426.
- Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women. JAMA 1992; 268:2403–2408.
- Sterling WM, Golden NH, Jacobson MS, Ornstein RM, Hertz SM. Metabolic assessment of menstruating and nonmenstruating normal weight adolescents. Int J Eat Disord 2009; 42:658–663.
- Legroux-Gerot I, Vignau J, Collier F, Cortet B. Bone loss associated with anorexia nervosa. Joint Bone Spine 2005; 72:489–495.
- Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab 2002; 87:2883–2891.
- Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab 1999; 84:4489–4496.
- Miller KK, Lawson EA, Mathur V, et al. Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab 2007; 92:1334–1339.
- Misra M, Katzman DK, Cord J, et al. Bone metabolism in adolescent boys with anorexia nervosa. J Clin Endocrinol Metab 2008; 93:3029–3036.
- Misra M, Miller KK, Almazan C, et al. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab 2004; 89:4972–4980.
- Chiodini I, Mascia ML, Muscarella S, et al. Subclinical hypercortisolism among outpatients referred for osteoporosis. Ann Intern Med 2007; 147:541–548.
- Misra M, Soyka LA, Miller KK, et al. Serum osteoprotegerin in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 2003; 88:3816–3822.
- Ohwada R, Hotta M, Sato K, Shibasaki T, Takano K. The relationship between serum levels of estradiol and osteoprotegerin in patients with anorexia nervosa. Endocr J 2007; 54:953–959.
- Müller TD, Föcker M, Holtkamp K, Herpertz-Dahlmann B, Hebebrand J. Leptin-mediated neuroendocrine alterations in anorexia nervosa: somatic and behavioral implications. Child Adolesc Psychiatr Clin N Am 2009; 18:117–129.
- Støving RK, Andries A, Brixen K, Flyvbjerg A, Hørder K, Frystyk J. Leptin, ghrelin, and endocannabinoids: potential therapeutic targets in anorexia nervosa. J Psychiatr Res 2009; 43:671–679.
- Audi L, Mantzoros CS, Vidal-Puig A, Vargas D, Gussinye M, Carrascosa A. Leptin in relation to resumption of menses in women with anorexia nervosa. Mol Psychiatry 1998; 3:544–547.
- Wong IP, Baldock PA, Herzog H. Gastrointestinal peptides and bone health. Curr Opin Endocrinol Diabetes Obes 2010; 17:44–50.
- Fukushima N, Hanada R, Teranishi H, et al. Ghrelin directly regulates bone formation. J Bone Miner Res 2005; 20:790–798.
- Makovey J, Naganathan V, Seibel M, Sambrook P. Gender differences in plasma ghrelin and its relations to body composition and bone—an opposite-sex twin study. Clin Endocrinol (Oxf) 2007; 66:530–537.
- Hassouna R, Zizzari P, Tolle V. The ghrelin/obestatin balance in the physiological and pathological control of growth hormone secretion, body composition and food intake. J Neuroendocrinol 2010; 22:793–804.
- Germain N, Galusca B, Grouselle D, et al. Ghrelin/obestatin ratio in two populations with low body weight: constitutional thinness and anorexia nervosa. Psychoneuroendocrinology 2009; 34:413–419.
- Cohen A, Shane E. Treatment of premenopausal women with low bone mineral density. Curr Osteoporos Rep 2008; 6:39–46.
- Licata A. Bone density vs bone quality: what’s a clinician to do? Cleve Clin J Med 2009; 76:331–336.
- Bianchi ML, Baim S, Bishop NJ, et al. Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatr Nephrol 2010; 25:37–47.
- Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin North Am 2010; 39:155–167.
- Mehler PS, Krantz M. Anorexia nervosa medical issues. J Womens Health (Larchmt) 2003; 12:331–340.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National Osteoporosis Foundation 2008 Clinician’s Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J Clin Densitom 2008; 11:473–477.
- Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 2007; 39:1867–1882.
- Cummings SR, Palermo L, Browner W, et al. Monitoring osteoporosis therapy with bone densitometry: misleading changes and regression to the mean. Fracture Intervention Trial Research Group. JAMA 2000; 283:1318–1321.
- LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA 1999; 281:1505–1511.
- Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab 2010; 95:471–478.
- Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 2004; 158:531–537.
- Hofbauer LC, Hamann C, Ebeling PR. Approach to the patient with secondary osteoporosis. Eur J Endocrinol 2010; 162:1009–1020.
- Stoffman N, Gordon CM. Vitamin D and adolescents: what do we know? Curr Opin Pediatr 2009; 21:465–471.
- Bone HG, Greenspan SL, McKeever C, et al. Alendronate and estrogen effects in postmenopausal women with low bone mineral density. Alendronate/Estrogen Study Group. J Clin Endocrinol Metab 2000; 85:720–726.
- Barrett-Connor E, Wenger NK, Grady D, et al. Hormone and nonhormone therapy for the maintenance of postmenopausal health: the need for randomized controlled trials of estrogen and raloxifene. J Womens Health 1998; 7:839–847.
- Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 1995; 80:898–904.
- Stokosch GR, Friedman AJ, Wu SC, Kamin M. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in adolescent females with anorexia nervosa: double-blind, placebo-controlled study. J Adolesc Health 2006; 39:819–827.
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15:135–143.
- Robinson E, Bachrach LK, Katzman DK. Use of hormone replacement therapy to reduce the risk of osteopenia in adolescent girls with anorexia nervosa. J Adolesc Health 2000; 26:343–348.
- Myburgh KH, Hutchins J, Fataar AB, et al. Low bone density is an etiologic factor for stress fractures in athletes. Ann Intern Med 1990; 113:754–759.
- Young N, Formica C, Szmukler G, Seeman E. Bone density at weight-bearing and nonweight-bearing sites in ballet dancers: the effects of exercise, hypogonadism, and body weight. J Clin Endocrinol Metab 1994; 78:449–454.
- Stock JL, Bell NH, Chesnut CH, et al. Increments in bone mineral density of the lumbar spine and hip and suppression of bone turnover are maintained after discontinuation of alendronate in postmenopausal women. Am J Med 1997; 103:291–297.
- Subbiah V, Madsen VS, Raymond AK, Benjamin RS, Ludwig JA. Of mice and men: divergent risks of teriparatide-induced osteosarcoma. Osteoporos Int 2010; 21:1041–1045.
- Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001; 86:5992.
- Miljic D, Pekic S, Djurovic M, et al. Ghrelin has partial or no effect on appetite, growth hormone, prolactin, and cortisol release in patients with anorexia nervosa. J Clin Endocrinol Metab 2006; 914:1491–1495.
- Siegfried Z, Kanyas K, Latzer Y, et al. Association study of cannabinoid receptor gene (CNR1) alleles and anorexia nervosa: differences between restricting and binging/purging subtypes. Am J Med Genet B Neuropsychiatr Genet 2004; 125B:126–130.
- Cota D, Marsicano G, Lutz B, et al. Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord 2003; 27:289–301.
- Gross H, Ebert MH, Faden VB, et al. A double-blind trial of delta 9-tetrahydrocannabinol in primary anorexia nervosa. J Clin Psychopharmacol 1983; 3:165–171.
KEY POINTS
- Women gain 40% to 60% of their bone mass during adolescence, a time coinciding with the peak incidence of anorexia nervosa, and they attain their peak bone mass by the time they are in their 20s.
- The etiology of osteoporosis in anorexia nervosa is complex and multifaceted. Early detection and treatment are critical.
- Osteoporosis in premenopausal patients is defined as low bone mineral density (a Z score below −2.0) in combination with risk factors such as chronic malnutrition, eating disorders, hypogonadism, glucocorticoid exposure, and previous fractures.
- Restoring body weight is the key treatment. Vitamin D should be supplemented if low. Estrogen therapy has not been shown to be effective, and exercise may be counterproductive. Bisphosphonates and teriparatide should be used with caution, if at all.
Symptom management: An important part of cancer care
Cancer patients experience many distressing symptoms during the course of their illness. In addition to pain, they commonly suffer from fatigue, anorexia, constipation, dyspnea, nausea, and vomiting.1
Although it is important to diagnose and manage the cancer itself, it is also the physician’s duty to recognize and effectively treat associated symptoms, regardless of the outcome of the underlying disease.
Some of the symptoms are due to the underlying disease, but some are iatrogenic, as many medical interventions have predictable adverse effects, such as nausea and vomiting with chemotherapy or constipation with opioids.
Symptoms of advanced cancer become chronic, and patients usually rate them as moderate or severe.1 Unrelieved suffering causes demoralization and may quickly impair quality of life.2
Understanding the principles of symptom management may help optimize palliation and improve quality of life. In this paper, we outline an approach to the management of cancer-related symptoms.
A HEAVY BURDEN OF SYMPTOMS
In patients with advanced cancer, the prevalence rates of various symptoms are approximately as follows1,3:
- Pain 89%
- Fatigue 69%
- Weakness 66%
- Anorexia 66%
- Lack of energy 61%
- Nausea 60%
- Dry mouth 57%
- Constipation 52%
- Early satiety 51%
- Dyspnea 50%
- Vomiting 30%.
Furthermore, patients with advanced cancer typically have multiple concurrent symptoms. In a survey of patients in a palliative medicine service at our hospital,4 we found that the median number of symptoms per patient was 10 (range 0–25) (Figure 1).
PRINCIPLES OF SYMPTOM MANAGEMENT
Show an interest in the patient’s symptoms. Many patients with advanced cancer believe that suffering is an inevitable part of the disease or of its treatment.
Ask patients about their symptoms in a positive and detailed fashion, starting with open-ended questions and following up with specific questions. Patients may underreport their symptoms or may not mention them if not asked directly. In the survey of palliative care patients at our hospital mentioned above,4 the median number of volunteered symptoms was only 1 (range 0–6), whereas a median of 10 were found by systematic assessment.
The examiner should clarify when necessary and recognize that a layperson’s language may not directly translate to medical language. For example, a patient may not understand the term “anorexia.” Furthermore, “loss of appetite” may mean nausea, vomiting, constipation, or early satiety. “Numbness” may mean a loss of sensation or a pins-and-needles sensation. Symptoms should also be quantified using a consistent measure (ie, numerical or categorical) to facilitate monitoring.
Prioritize the symptoms. Advanced cancer is accompanied by multiple symptoms. Assess which ones are most bothersome, and where therapy should be directed first.
Try to understand the pathophysiology behind the symptom. When possible, choose a drug treatment that targets the likely underlying cause. Nausea and vomiting, for example, can be secondary to gastric outlet obstruction, hypercalcemia, increased intracranial pressure, esophagitis, opioid use, or constipation.
Be specific about the drug, dosing, timing, and route, and keep it simple. If a regimen is cumbersome, compliance suffers. It is better to start one medication for the most bothersome symptom or symptoms and make some progress than it is to overwhelm the patient with a complex list of drugs. Sustained-release formulations are often useful. It is unrealistic to expect most patients to take a medication every 4 hours around the clock. Try the most cost-effective remedies first, and attempt to use one drug that may address multiple symptoms. For example, dexamethasone may have positive effects on energy, pain, and appetite.
Use ‘rescue dosing.’ Rescue drugs are important for expected symptom exacerbations in those on sustained medication. This approach increases efficacy and minimizes adverse effects. In most cases, the rescue medication should be the same as the regularly scheduled one. For example, a prescription to treat nausea may read “metoclopramide (Reglan) 10 mg by mouth before meals and at bedtime and every 4 hours as needed to treat nausea or vomiting.”
Consider the patient’s age and fragility, the cost of the drug, and anticipated adverse effects. Oral or transdermal preparations are preferable to parenteral ones with regard to convenience and compliance, although many transdermal preparations are costly. If parenteral dosing is necessary, the subcutaneous route is an alternative to the intravenous route.
Discontinue drugs that are ineffective or unnecessary. This may help compliance and diminish adverse effects.
Make one change at a time so the response to that change is clear. Titrate one drug to its effective dose, to its maximum dose, or to a level of intolerability before considering another. If one drug of a class is ineffective, another drug in the same class may work.
Reassess often. A follow-up phone call or office visit in 1 to 2 weeks is appropriate. The symptoms of advanced cancer are often progressive, so regular evaluation is important, even if symptoms are controlled on stable drug regimens. Instructions should be both verbal and written and should be communicated to patients and any involved caregiver to ensure compliance. Have a “plan B” if the first plan is ineffective.
A challenging and important part of symptom management is to assess the goals of care. Every intervention is not appropriate for every patient. Which therapies are used depends on the stage of the disease, the available disease-modifying treatments, and the patient’s condition and preferences. Patients and their loved ones should be engaged in discussions about goals of care early in the disease and should be included in medical decision-making. Both curative treatment and palliative treatment are important, but palliation plays a bigger role towards the later stages of advanced cancer (Figure 2).
CANCER-RELATED FATIGUE: COMMON BUT NOT INEVITABLE
Most cancer patients report fatigue. Although it is one of the most common symptoms in advanced cancer,5 it is not necessarily inevitable or untreatable.6
Cancer-related fatigue is multidimensional and develops over time, diminishing energy, mental capacity, and psychological condition.7 Patients may report feeling tired or being unable to complete their activities of daily living. People who were previously very active may be frustrated by their inability to participate in favorite leisure activities, which has a big impact on quality of life. Fatigue can be physical, emotional, or mental. It is important to distinguish physical weakness from dyspnea on exertion, which is commonly reported as fatigue. Depression may also cause or exacerbate fatigue.
Unlike fatigue in the general population, cancer-related fatigue does not improve with rest, and patients often report large amounts of unrestorative sleep.
Look for reversible causes of fatigue
First, conduct a thorough assessment to identify any reversible causes, such as:
- Anemia
- Insomnia, sleep disturbance
- Malnutrition
- Pain
- Depression
- Medical comorbidities: renal, cardiac, or pulmonary disease
- Hypothyroidism
- Hypogonadism.
In many cases, however, a reversible cause cannot be found.
Treating cancer-related fatigue
Nonpharmacologic interventions have been evaluated for this application, but evidence of efficacy is limited and mixed. The National Cancer Comprehensive Network guidelines8 suggest that energy conservation and education about cancer-related fatigue are central to management. Patients should be advised that fatigue has a fluctuating course and that they have a limited pool of energy, which they should conserve and use judiciously.
In a meta-analysis by Schmitz et al,9 physical activity interventions were found to be beneficial. Sixty-three percent of those studied were undergoing active treatment, so whether this population reflects advanced cancer is unclear. A small pilot study in advanced cancer found a trend toward benefit with exercise.10
Comment. The strategies of rest and exercise are complementary. The key point is to plan them per personal preference.
Psychostimulants include methylphenidate (Ritalin). A randomized placebo-controlled trial in patients with acquired immunodeficiency syndrome (AIDS) found methylphenidate 15 to 60 mg/day to have a positive effect.11 Prospective studies have shown similar results in cancer patients,12 and a Cochrane review in 2008 showed a small but significant benefit in cancer-related fatigue.13
Methylphenidate is usually started at a dose of 5 mg given at 8:00 am and at noon, and then titrated. Benefit, when experienced, is typically noted within 24 to 48 hours. Possible adverse effects include anorexia, insomnia, anxiety, confusion, tremor, and tachycardia.
Stimulants should be used with caution in patients with cardiac disease or delirium.
Modafinil (Provigil), a nonstimulant agent, has been less studied, but it may also help.14,15 The usual dosage is 50 to 200 mg daily.
Corticosteroids may have a role in advanced cancer, as suggested by anecdotal reports.16 They should be used judiciously, as their adverse effects (insomnia, muscle wasting, edema) are themselves burdensome and may outweigh their benefits.
ANOREXIA CAN BE DISTRESSING TO THE FAMILY AND THE PATIENT
Most patients with advanced cancer experience anorexia, which is a marker of poor prognosis.1
Appetite loss may occur in isolation or as a part of the anorexia-cachexia syndrome. This syndrome is a wasting state seen in chronic, advanced diseases including cancer, AIDS, chronic obstructive pulmonary disease, chronic renal insufficiency, and congestive heart failure.17 The associated weight loss is involuntary and includes both muscle and fat.
Appetite loss alone is usually not bothersome. In fact, anorexia frequently causes more distress to the family than to the patient.18 The ramifications of decreased appetite, on the other hand, can be devastating. Decreased caloric intake coupled with the hypermetabolic state of malignancy leads to rapid, dramatic changes in body habitus. This outward sign of the ravages of cancer can be psychologically damaging to patients and their loved ones as they contemplate advanced disease and limited life expectancy. They may be concerned about starvation, in which case education about and attempts to normalize the anorexia-cachexia syndrome are essential.
Look for reversible causes of anorexia
The first step in the management of anorexia is to identify any reversible causes, such as:
- Stomatitis
- Constipation
- Uncontrolled severe symptoms such as pain or dyspnea
- Delirium
- Nausea, vomiting
- Depression
- Gastroparesis.
Managing cancer-related anorexia
Nonpharmacologic measures include nutritional counseling and increased physical activity. Patients may be counseled to eat calorie-dense foods and supplemental high-calorie, high-protein, high-fat drinks. Some may be able to take advantage of a diurnal variation in appetite, usually an increased appetite in the morning.
Megestrol acetate (Megace) improved appetite and induced weight gain when used in a dosage of 800 mg daily in a randomized controlled trial in AIDS patients.19 Case studies have shown doses as low as 80 to 160 mg daily to be beneficial.20 Most of the added weight is fat, not lean muscle mass. Unfortunately, the addition of testosterone to megestrol did not increase the accumulation of lean muscle mass in another randomized trial.21 But the addition of olanzapine (Zyprexa) to megestrol was associated with improved appetite and weight gain in a significant percentage of advanced cancer patients.22 Rates of adverse effects with megestrol are low; the most significant adverse effect is thromboembolism.
Corticosteroids. While much of the support for corticosteroids is anecdotal, a prospective study of dexamethasone 4 to 16 mg daily showed improvement in several symptoms, including appetite.23 Because of the multiple adverse effects of corticosteroids, careful attention to dose, duration, and tolerability is essential. Corticosteroids should be discontinued if the desired positive effects are not observed within 3 to 5 days. If prolonged survival is expected, wean to the lowest effective dose.
Cannabinoids. Dronabinol (Marinol), a synthetic formulation of delta-9-tetrahydrocannabinol (THC), the active agent of marijuana, has been beneficial in AIDS anorexia. Fewer studies have been done in advanced cancer.
In a small, open-label case series, doses of 7.5 to 15 mg of dronabinol daily improved appetite and were well tolerated.24 On the other hand, in a multicenter, randomized, double-blind, placebo-controlled trial, neither cannabis extract nor THC (5 mg daily) significantly improved appetite over a 6-week period.25
A large randomized study found megestrol acetate 800 mg to be superior to dronabinol 5 mg daily for treating anorexia.26
Neurotoxicity, anxiety, nervousness, dizziness, euphoria, and somnolence from dronabinol can be severe and intolerable for some.
Enteral tube feeding and parenteral nutrition do not improve survival or comfort in terminally ill patients.27 On the contrary, they are associated with complications, including aspiration pneumonia, sepsis, abdominal pain, vomiting, and diarrhea. Nevertheless, in some patients with mechanical impediments to nutrition (eg, esophageal fistula, obstruction, or proximal small bowel obstruction) or in those who are hungry and unable to take food by mouth, tube feeding may be appropriate.
CONSTIPATION SHOULD BE ANTICIPATED, AND PREVENTED IF POSSIBLE
Constipation is variably defined by patients and health care professionals, but it usually includes components of the Rome II criteria, ie, two or more of the following symptoms28:
- Straining at least 25% of the time
- Hard stools at least 25% of the time
- Incomplete evacuation at least 25% of the time
- Two or fewer bowel movements per week.
These criteria were intended to describe functional constipation in a healthy population.27
More than 50% of patients with advanced cancer report constipation,1 and in those on opioids, the scope of the problem is larger. In addition to binding central nervous system receptors to mediate pain perception, opioids bind systemic receptors including those in the gut. As a result, opioids interfere with smooth muscle tone and contractility, lengthen transit time, promote dry stools, and increase anal sphincter tone.29 A nursing study found that when patients taking opioids were screened for constipation, 95% identified it as the major adverse effect of their pain regimen.30
Multiple causes of constipation
Factors that can cause or contribute to constipation include:
- Dietary factors such as a generally low intake of food, and specifically of fiber
- Inactivity
- Confusion
- Dehydration
- Intestinal obstruction
- Cormorbidities such as diabetes mellitus, hypothyroidism, hypercalcemia
- Uncomfortable toilet arrangements
- Drugs such as opioids (as noted above), anticholinergics, antihypertensives, antacids, diuretics, and iron supplements.
Take a proactive approach to constipation
Constipation is expected in a number of clinical scenarios, such as with the use of opioids or with limited mobility. Patients often attribute constipation to diminished oral intake. But despite low oral intake, regular, smaller-caliber bowel movements are important to ensure that sloughed bowel endothelium and bacteria are eliminated.
Although little evidence supports the use of one standard bowel regimen, prevention is essential. The goal is a soft bowel movement every 1 to 2 days. Constipation prophylaxis should be started at the initiation of any regular opioid regimen. Encouraging physical activity and oral fluid intake and creating a favorable environment for elimination may also help manage constipation.
In our practice, we use a softening agent such as docusate sodium (Colace) 100 mg twice daily, and add a laxative agent such as senna (Senokot) or a magnesium-based osmotic agent as needed. Bulking agents such as over-the-counter fiber supplements should be used with caution in opioid-related constipation. If there has been no bowel movement for 48 hours, a rectal suppository or enema is used. Suppositories or enemas can be scheduled regularly for bedbound patients with chronic constipation.
Methylnaltrexone (Relistor), a mu-opioid antagonist, is a new agent that blocks peripheral opioid receptors in the gut. In a randomized study of 133 patients, methylnaltrexone produced laxation within 4 hours of administration in 48%.31 This methylated, charged compound does not significantly cross the blood-brain barrier and therefore does not interfere with analgesia or cause opioid withdrawal. The dose is 8 mg or 12 mg subcutaneously (based on weight), which can be repeated in 48 hours. If laxation does not occur after one to three doses, other causes of constipation should be explored.
Methylnaltrexone is contraindicated in patients with bowel obstruction, even if the obstruction is thought to be secondary to opioids. Adverse effects include abdominal pain, flatulence, and nausea.
NAUSEA AND VOMITING: NOT ALWAYS DUE TO CHEMOTHERAPY
Nausea (the sensation of the need to vomit) and vomiting (the forceful expulsion of gastric contents) are common symptoms in advanced cancer and are not necessarily related to chemotherapy or radiation therapy. About 60% of cancer patients have nausea, and about 30% vomit.32 Both symptoms are very distressing and diminish quality of life.
Look for potentially reversible causes of nausea and vomiting
Identifying the cause, which is sometimes reversible, may help direct treatment. Potentially reversible causes include:
- Drugs
- Uremia
- Infection
- Anxiety
- Constipation
- Gastric irritation
- Proximal gastrointestinal obstruction.
In a prospective study of 121 patients with advanced cancer, the most common causes of nausea and vomiting were impaired gastric emptying, chemical and metabolic factors (drugs, organ failure, electrolyte disturbance, infection), and bowel obstruction.33–35
Management of nausea and vomiting
Management of nausea and vomiting may require multiple antiemetics, which may need to be given intravenously or subcutaneously.33
The choice of drugs depends on the cause of the nausea
The evidence-based choice of drugs for nausea depends on the cause33–35:
- Nausea due to chemical or metabolic factors: haloperidol (Haldol), levomepromazine (another antipsychotic drug, not available in the United States), cyclizine (Marezine)
- Nausea due to gastric stasis, outlet obstruction: metoclopramide, domperidone (a similar drug, not available in the United States), levomepromazine
- Nausea due to regurgitation: metoclopramide, cyclizine, haloperidol, levomepromazine
- Nausea due to bowel obstruction: metoclopramide (if obstruction is not complete), domperidone, cyclizine, levomepromazine, octreotide (Sandostatin), hyoscyamine (Levsin)
- Nausea due to cranial disease: cyclizine, levomepromazine
- Movement-related nausea: cyclizine, levomepromazine, hyoscyamine
- Cause unclear or multiple causes: cyclizine, haloperidol, levomepromazine
- Cortical nausea: lorazepam (Ativan).
Metoclopramide. If complete bowel obstruction is not suspected, oral metoclopramide, a dopamine antagonist, is our choice for first-line drug therapy.32 Adverse effects include abdominal pain, diarrhea, and sedation.
Haloperidol, another dopamine antagonist, can also be used.32 Haloperidol may cause sedation and is associated with a prolonged QTc interval. Care should be taken in those at risk for dysrhythmia or arrhythmia.
Olanzapine (Zyprexa) is an alternative antipsychotic for patients who cannot tolerate or do not respond to metoclopramide and haloperidol.
Ondansetron (Zofran), a serotonin 5-HT3 receptor antagonist, is usually reserved for nausea and vomiting associated with chemotherapy or radiation, but it can be used in advanced cancer if the above agents fail.37
DYSPNEA IS COMMON, EVEN WITHOUT LUNG DISEASE
Dyspnea is the subjective perception of impaired breathing, which may include the sensation of breathlessness, chest tightness, air hunger, suffocation, or increased work of breathing.
At least half of patients with advanced cancer complain of dyspnea.1 Most have primary pulmonary malignancies or metastatic lung disease, but almost 25% have no documented lung involvement or underlying cardiopulmonary diagnosis to which to attribute it.38
Dyspnea is often very distressing. Palliative sedation is used more frequently for the relief of intractable dyspnea than for pain.39
Opioids are effective but underutilized for dyspnea
Although opioids are effective in both oral and parenteral formulations for the symptomatic management of dyspnea,40 the exact mechanism by which they improve dyspnea is unknown. Central control of respiration occurs in the medulla, and perception of dyspnea is mediated by the sensory cortex.
Opioids are underutilized by physicians other than palliative medicine specialists because of concern about respiratory depression. Appropriately titrated, opioids are safe and do not cause clinically significant respiratory depression.41
Allen et al42 showed that an opioid in low doses (diamorphine 2.5 mg subcutaneously) was effective and well tolerated in elderly patients with advanced pulmonary fibrosis who had not received opioids before.
Start low and go slow. An appropriate starting dose for a patient who has not been on opioids before may be morphine sulfate 2 mg intravenously (or a 5-mg immediate-release tablet by mouth) every 2 hours as needed for dyspnea. After 24 to 48 hours of an as-needed regimen, one can evaluate the patient’s response, tolerance, and dose requirement. If needed, parenteral infusion or a long-acting opioid preparation can be started with continued as-needed bolus dosing for breakthrough dyspnea.
We do not recommend writing opioid infusion orders with a “titrate to comfort” clause in the terminally ill. Increasing the rate of a continuous infusion does not provide the prompt symptomatic relief a bolus dose delivers. Dose accumulation and adverse effects are more likely when opioids are titrated in this fashion.
A Cochrane review showed that nebulized opioids are ineffective for dyspnea.43
Oxygen paradoxically does not improve dyspnea
Oxygen is commonly prescribed, although the literature does not indicate that it improves the sensation of breathlessness.44
A study by Clemens et al45 showed no correlation between dyspnea and oxygen saturation. It also found morphine to be superior to oxygen in subjective dyspnea, even in hypoxia.
A double-blind crossover study showed that ambient air delivered via nasal cannula was as effective as oxygen for dyspnea.46 The inexpensive and simple practice of a fan to blow ambient air on the patient’s face may help relieve dyspnea.
- Donnelly S, Walsh D. The symptoms of advanced cancer. Semin Oncol 1995; 22(2 suppl 3):67–72.
- Walsh D, Rybicki L, Nelson KA, Donnelly S. Symptoms and prognosis in advanced cancer. Support Care Cancer 2002; 10:385–388.
- Komurcu S, Nelson KA, Walsh D, Donnelly SM, Homsi J, Abdullah O. Common symptoms in advanced cancer. Semin Oncol 2000; 27:24–33.
- Homsi J, Walsh D, Rivera N, et al. Symptom evaluation in palliative medicine: patient report vs systematic assessment. Support Care Cancer 2006; 14:444–453.
- Donnelly S. Quality-of-life assessment in advanced cancer. Curr Oncol Rep 2000; 2:338–342.
- Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N. Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann Oncol 2000; 11:971–975.
- Portenoy RK, Itri LM. Cancer-related fatigue: guidelines for evaluation and management. Oncologist 1999; 4:1–10.
- Berger AM, Abernethy AP, Atkinson A, et al. NCCN Clinical Practice Guidelines in Oncology Cancer-related fatigue—v.1.2010. www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf. Accessed November 15, 2010.
- Schmitz KH, Holtzman J, Courneya KS, Mâsse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2005; 14:1588–1595.
- Porock D, Kristjanson LJ, Tinnelly K, Duke T, Blight J. An exercise intervention for advanced cancer patients experiencing fatigue: a pilot study. J Palliat Care 2000 Autumn; 16:30–36.
- Breitbart W, Rosenfeld B, Kaim M, Funesti-Esch J. A randomized, double-blind, placebo-controlled trial of psychostimulants for the treatment of fatigue in ambulatory patients with human immunodeficiency virus disease. Arch Intern Med 2001; 161:411–420.
- Sarhill N, Walsh D, Nelson KA, Homsi J, LeGrand S, Davis MP. Methylphenidate for fatigue in advanced cancer: a prospective open-label pilot study. Am J Hosp Palliat Care 2001; 18:187–192.
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst 2008; 100:1155–1166.
- Kaleita TA, Wellisch DK, Graham CA, et al. Pilot study of modafinil for treatment of neurobehavioral dysfunction and fatigue in adult patients with brain tumors (abstract). J Clin Oncol 2006; 24(suppl):58s.
- Morrow GR, Jean-Pierre P, Roscoe JA, et al. A phase III randomized, placebo-controlled, double-blind trial of a eugeroic agent in 642 cancer patients reporting fatigue during chemotherapy: a URCC CCOP study (abstract). J Clin Oncol 2008; 26(suppl):504s.
- Tannock I, Gospodarowicz M, Meakin W, Panzarella T, Stewart L, Rider W. Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol 1989; 7:590–597.
- Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr 2006; 83:735–743.
- Poole K, Froggatt K. Loss of weight and loss of appetite in advanced cancer: a problem for the patient, the carer, or the health professional? Palliat Med 2002; 16:499–506.
- Von Roenn JH. Randomized trials of megestrol acetate for AIDS-associated anorexia and cachexia. Oncology 1994; 51(suppl 1):19–24.
- Donnelly S, Walsh TD. Low-dose megestrol acetate for appetite stimulation in advanced cancer. J Pain Symptom Manage 1995; 10:182–183.
- Mulligan K, Zackin R, Von Roenn JH, et al; ACTG 313 Study Team. Testosterone supplementation of megestrol therapy does not enhance lean tissue accrual in men with human immunodeficiency virus-associated weight loss: a randomized, double-blind, placebo-controlled, multicenter trial. J Clin Endocrinol Metab 2007; 92:563–570.
- Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 2010; 18:951–956.
- Mercadante S, Fulfaro F, Casuccio A. The use of corticosteroids in home palliative care. Support Care Cancer 2001; 9:386–389.
- Walsh D, Kirkova J, Davis MP. The efficacy and tolerability of long-term use of dronabinol in cancer-related anorexia: a case series. J Pain Symptom Manage 2005; 30:493–495.
- Cannabis-In-Cachexia-Study-Group; Strasser F, Luftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol 2006; 24:3394–3400.
- Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol 2002; 20:567–573.
- Winter SM. Terminal nutrition: framing the debate for the withdrawal of nutritional support in terminally ill patients. Am J Med 2000; 109:723–726.
- Drossman DA, Sandler RS, McKee DC, Lovitz AJ. Bowel patterns among subjects not seeking health care. Use of a questionnaire to identify a population with bowel dysfunction. Gastroenterology 1982; 83:529–534.
- McMillan SC. Assessing and managing opiate-induced constipation in adults with cancer. Cancer Control 2004; 11(suppl 3):3–9.
- Robinson CB, Fritch M, Hullett L, et al. Development of a protocol to prevent opioid-induced constipation in patients with cancer: a research utilization project. Clin J Oncol Nurs 2000; 4:79–84.
- Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med 2008; 358:2332–2343.
- Davis MP, Walsh D. Treatment of nausea and vomiting in advanced cancer. Support Care Cancer 2000; 8:444–452.
- Stephenson J, Davies A. An assessment of aetiology-based guidelines for the management of nausea and vomiting in patients with advanced cancer. Support Care Cancer 2006; 14:348–353.
- Lichter I. Results of antiemetic management in terminal illness. J Palliat Care 1993; 9:19–21.
- Bentley A, Boyd K. Use of clinical pictures in the management of nausea and vomiting: a prospective audit. Palliat Med 2001; 15:247–253.
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Support Care Cancer 2004; 12:432–440.
- Currow DC, Coughlan M, Fardell B, Cooney NJ. Use of ondansetron in palliative medicine. J Pain Symptom Manage 1997; 13:302–307.
- Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest 1986; 89:234–236.
- Fainsinger RL, Waller A, Bercovici M, et al. A multicentre international study of sedation for uncontrolled symptoms in terminally ill patients. Palliat Med 2000; 14:257–265.
- Jennings AL, Davies AN, Higgins JP, Broadley K. Opioids for the palliation of breathlessness in terminal illness. Cochrane Database Syst Rev 2001;CD002066.
- Estfan B, Mahmoud F, Shaheen P, et al. Respiratory function during parenteral opioid titration for cancer pain. Palliat Med 2007; 21:81–86.
- Allen S, Raut S, Woollard J, Vassallo M. Low dose diamorphine reduces breathlessness without causing a fall in oxygen saturation in elderly patients with end-stage idiopathic pulmonary fibrosis. Palliat Med 2005; 19:128–130.
- Polosa R, Simidchiev A, Walters EH. Nebulised morphine for severe interstitial lung disease. Cochrane Database Syst Rev 2002;CD002872.
- Currow DC, Agar M, Smith J, Abernethy AP. Does palliative home oxygen improve dyspnoea? A consecutive cohort study. Palliat Med 2009; 23:309–316.
- Clemens KE, Quednau I, Klaschik E. Use of oxygen and opioids in the palliation of dyspnoea in hypoxic and non-hypoxic palliative care patients: a prospective study. Support Care Cancer 2009; 17:367–377.
- Philip J, Gold M, Milner A, Di Iulio J, Miller B, Spruyt O. A randomized, double-blind, crossover trial of the effect of oxygen on dyspnea in patients with advanced cancer. J Pain Symptom Manage 2006; 32:541–550.
Cancer patients experience many distressing symptoms during the course of their illness. In addition to pain, they commonly suffer from fatigue, anorexia, constipation, dyspnea, nausea, and vomiting.1
Although it is important to diagnose and manage the cancer itself, it is also the physician’s duty to recognize and effectively treat associated symptoms, regardless of the outcome of the underlying disease.
Some of the symptoms are due to the underlying disease, but some are iatrogenic, as many medical interventions have predictable adverse effects, such as nausea and vomiting with chemotherapy or constipation with opioids.
Symptoms of advanced cancer become chronic, and patients usually rate them as moderate or severe.1 Unrelieved suffering causes demoralization and may quickly impair quality of life.2
Understanding the principles of symptom management may help optimize palliation and improve quality of life. In this paper, we outline an approach to the management of cancer-related symptoms.
A HEAVY BURDEN OF SYMPTOMS
In patients with advanced cancer, the prevalence rates of various symptoms are approximately as follows1,3:
- Pain 89%
- Fatigue 69%
- Weakness 66%
- Anorexia 66%
- Lack of energy 61%
- Nausea 60%
- Dry mouth 57%
- Constipation 52%
- Early satiety 51%
- Dyspnea 50%
- Vomiting 30%.
Furthermore, patients with advanced cancer typically have multiple concurrent symptoms. In a survey of patients in a palliative medicine service at our hospital,4 we found that the median number of symptoms per patient was 10 (range 0–25) (Figure 1).
PRINCIPLES OF SYMPTOM MANAGEMENT
Show an interest in the patient’s symptoms. Many patients with advanced cancer believe that suffering is an inevitable part of the disease or of its treatment.
Ask patients about their symptoms in a positive and detailed fashion, starting with open-ended questions and following up with specific questions. Patients may underreport their symptoms or may not mention them if not asked directly. In the survey of palliative care patients at our hospital mentioned above,4 the median number of volunteered symptoms was only 1 (range 0–6), whereas a median of 10 were found by systematic assessment.
The examiner should clarify when necessary and recognize that a layperson’s language may not directly translate to medical language. For example, a patient may not understand the term “anorexia.” Furthermore, “loss of appetite” may mean nausea, vomiting, constipation, or early satiety. “Numbness” may mean a loss of sensation or a pins-and-needles sensation. Symptoms should also be quantified using a consistent measure (ie, numerical or categorical) to facilitate monitoring.
Prioritize the symptoms. Advanced cancer is accompanied by multiple symptoms. Assess which ones are most bothersome, and where therapy should be directed first.
Try to understand the pathophysiology behind the symptom. When possible, choose a drug treatment that targets the likely underlying cause. Nausea and vomiting, for example, can be secondary to gastric outlet obstruction, hypercalcemia, increased intracranial pressure, esophagitis, opioid use, or constipation.
Be specific about the drug, dosing, timing, and route, and keep it simple. If a regimen is cumbersome, compliance suffers. It is better to start one medication for the most bothersome symptom or symptoms and make some progress than it is to overwhelm the patient with a complex list of drugs. Sustained-release formulations are often useful. It is unrealistic to expect most patients to take a medication every 4 hours around the clock. Try the most cost-effective remedies first, and attempt to use one drug that may address multiple symptoms. For example, dexamethasone may have positive effects on energy, pain, and appetite.
Use ‘rescue dosing.’ Rescue drugs are important for expected symptom exacerbations in those on sustained medication. This approach increases efficacy and minimizes adverse effects. In most cases, the rescue medication should be the same as the regularly scheduled one. For example, a prescription to treat nausea may read “metoclopramide (Reglan) 10 mg by mouth before meals and at bedtime and every 4 hours as needed to treat nausea or vomiting.”
Consider the patient’s age and fragility, the cost of the drug, and anticipated adverse effects. Oral or transdermal preparations are preferable to parenteral ones with regard to convenience and compliance, although many transdermal preparations are costly. If parenteral dosing is necessary, the subcutaneous route is an alternative to the intravenous route.
Discontinue drugs that are ineffective or unnecessary. This may help compliance and diminish adverse effects.
Make one change at a time so the response to that change is clear. Titrate one drug to its effective dose, to its maximum dose, or to a level of intolerability before considering another. If one drug of a class is ineffective, another drug in the same class may work.
Reassess often. A follow-up phone call or office visit in 1 to 2 weeks is appropriate. The symptoms of advanced cancer are often progressive, so regular evaluation is important, even if symptoms are controlled on stable drug regimens. Instructions should be both verbal and written and should be communicated to patients and any involved caregiver to ensure compliance. Have a “plan B” if the first plan is ineffective.
A challenging and important part of symptom management is to assess the goals of care. Every intervention is not appropriate for every patient. Which therapies are used depends on the stage of the disease, the available disease-modifying treatments, and the patient’s condition and preferences. Patients and their loved ones should be engaged in discussions about goals of care early in the disease and should be included in medical decision-making. Both curative treatment and palliative treatment are important, but palliation plays a bigger role towards the later stages of advanced cancer (Figure 2).
CANCER-RELATED FATIGUE: COMMON BUT NOT INEVITABLE
Most cancer patients report fatigue. Although it is one of the most common symptoms in advanced cancer,5 it is not necessarily inevitable or untreatable.6
Cancer-related fatigue is multidimensional and develops over time, diminishing energy, mental capacity, and psychological condition.7 Patients may report feeling tired or being unable to complete their activities of daily living. People who were previously very active may be frustrated by their inability to participate in favorite leisure activities, which has a big impact on quality of life. Fatigue can be physical, emotional, or mental. It is important to distinguish physical weakness from dyspnea on exertion, which is commonly reported as fatigue. Depression may also cause or exacerbate fatigue.
Unlike fatigue in the general population, cancer-related fatigue does not improve with rest, and patients often report large amounts of unrestorative sleep.
Look for reversible causes of fatigue
First, conduct a thorough assessment to identify any reversible causes, such as:
- Anemia
- Insomnia, sleep disturbance
- Malnutrition
- Pain
- Depression
- Medical comorbidities: renal, cardiac, or pulmonary disease
- Hypothyroidism
- Hypogonadism.
In many cases, however, a reversible cause cannot be found.
Treating cancer-related fatigue
Nonpharmacologic interventions have been evaluated for this application, but evidence of efficacy is limited and mixed. The National Cancer Comprehensive Network guidelines8 suggest that energy conservation and education about cancer-related fatigue are central to management. Patients should be advised that fatigue has a fluctuating course and that they have a limited pool of energy, which they should conserve and use judiciously.
In a meta-analysis by Schmitz et al,9 physical activity interventions were found to be beneficial. Sixty-three percent of those studied were undergoing active treatment, so whether this population reflects advanced cancer is unclear. A small pilot study in advanced cancer found a trend toward benefit with exercise.10
Comment. The strategies of rest and exercise are complementary. The key point is to plan them per personal preference.
Psychostimulants include methylphenidate (Ritalin). A randomized placebo-controlled trial in patients with acquired immunodeficiency syndrome (AIDS) found methylphenidate 15 to 60 mg/day to have a positive effect.11 Prospective studies have shown similar results in cancer patients,12 and a Cochrane review in 2008 showed a small but significant benefit in cancer-related fatigue.13
Methylphenidate is usually started at a dose of 5 mg given at 8:00 am and at noon, and then titrated. Benefit, when experienced, is typically noted within 24 to 48 hours. Possible adverse effects include anorexia, insomnia, anxiety, confusion, tremor, and tachycardia.
Stimulants should be used with caution in patients with cardiac disease or delirium.
Modafinil (Provigil), a nonstimulant agent, has been less studied, but it may also help.14,15 The usual dosage is 50 to 200 mg daily.
Corticosteroids may have a role in advanced cancer, as suggested by anecdotal reports.16 They should be used judiciously, as their adverse effects (insomnia, muscle wasting, edema) are themselves burdensome and may outweigh their benefits.
ANOREXIA CAN BE DISTRESSING TO THE FAMILY AND THE PATIENT
Most patients with advanced cancer experience anorexia, which is a marker of poor prognosis.1
Appetite loss may occur in isolation or as a part of the anorexia-cachexia syndrome. This syndrome is a wasting state seen in chronic, advanced diseases including cancer, AIDS, chronic obstructive pulmonary disease, chronic renal insufficiency, and congestive heart failure.17 The associated weight loss is involuntary and includes both muscle and fat.
Appetite loss alone is usually not bothersome. In fact, anorexia frequently causes more distress to the family than to the patient.18 The ramifications of decreased appetite, on the other hand, can be devastating. Decreased caloric intake coupled with the hypermetabolic state of malignancy leads to rapid, dramatic changes in body habitus. This outward sign of the ravages of cancer can be psychologically damaging to patients and their loved ones as they contemplate advanced disease and limited life expectancy. They may be concerned about starvation, in which case education about and attempts to normalize the anorexia-cachexia syndrome are essential.
Look for reversible causes of anorexia
The first step in the management of anorexia is to identify any reversible causes, such as:
- Stomatitis
- Constipation
- Uncontrolled severe symptoms such as pain or dyspnea
- Delirium
- Nausea, vomiting
- Depression
- Gastroparesis.
Managing cancer-related anorexia
Nonpharmacologic measures include nutritional counseling and increased physical activity. Patients may be counseled to eat calorie-dense foods and supplemental high-calorie, high-protein, high-fat drinks. Some may be able to take advantage of a diurnal variation in appetite, usually an increased appetite in the morning.
Megestrol acetate (Megace) improved appetite and induced weight gain when used in a dosage of 800 mg daily in a randomized controlled trial in AIDS patients.19 Case studies have shown doses as low as 80 to 160 mg daily to be beneficial.20 Most of the added weight is fat, not lean muscle mass. Unfortunately, the addition of testosterone to megestrol did not increase the accumulation of lean muscle mass in another randomized trial.21 But the addition of olanzapine (Zyprexa) to megestrol was associated with improved appetite and weight gain in a significant percentage of advanced cancer patients.22 Rates of adverse effects with megestrol are low; the most significant adverse effect is thromboembolism.
Corticosteroids. While much of the support for corticosteroids is anecdotal, a prospective study of dexamethasone 4 to 16 mg daily showed improvement in several symptoms, including appetite.23 Because of the multiple adverse effects of corticosteroids, careful attention to dose, duration, and tolerability is essential. Corticosteroids should be discontinued if the desired positive effects are not observed within 3 to 5 days. If prolonged survival is expected, wean to the lowest effective dose.
Cannabinoids. Dronabinol (Marinol), a synthetic formulation of delta-9-tetrahydrocannabinol (THC), the active agent of marijuana, has been beneficial in AIDS anorexia. Fewer studies have been done in advanced cancer.
In a small, open-label case series, doses of 7.5 to 15 mg of dronabinol daily improved appetite and were well tolerated.24 On the other hand, in a multicenter, randomized, double-blind, placebo-controlled trial, neither cannabis extract nor THC (5 mg daily) significantly improved appetite over a 6-week period.25
A large randomized study found megestrol acetate 800 mg to be superior to dronabinol 5 mg daily for treating anorexia.26
Neurotoxicity, anxiety, nervousness, dizziness, euphoria, and somnolence from dronabinol can be severe and intolerable for some.
Enteral tube feeding and parenteral nutrition do not improve survival or comfort in terminally ill patients.27 On the contrary, they are associated with complications, including aspiration pneumonia, sepsis, abdominal pain, vomiting, and diarrhea. Nevertheless, in some patients with mechanical impediments to nutrition (eg, esophageal fistula, obstruction, or proximal small bowel obstruction) or in those who are hungry and unable to take food by mouth, tube feeding may be appropriate.
CONSTIPATION SHOULD BE ANTICIPATED, AND PREVENTED IF POSSIBLE
Constipation is variably defined by patients and health care professionals, but it usually includes components of the Rome II criteria, ie, two or more of the following symptoms28:
- Straining at least 25% of the time
- Hard stools at least 25% of the time
- Incomplete evacuation at least 25% of the time
- Two or fewer bowel movements per week.
These criteria were intended to describe functional constipation in a healthy population.27
More than 50% of patients with advanced cancer report constipation,1 and in those on opioids, the scope of the problem is larger. In addition to binding central nervous system receptors to mediate pain perception, opioids bind systemic receptors including those in the gut. As a result, opioids interfere with smooth muscle tone and contractility, lengthen transit time, promote dry stools, and increase anal sphincter tone.29 A nursing study found that when patients taking opioids were screened for constipation, 95% identified it as the major adverse effect of their pain regimen.30
Multiple causes of constipation
Factors that can cause or contribute to constipation include:
- Dietary factors such as a generally low intake of food, and specifically of fiber
- Inactivity
- Confusion
- Dehydration
- Intestinal obstruction
- Cormorbidities such as diabetes mellitus, hypothyroidism, hypercalcemia
- Uncomfortable toilet arrangements
- Drugs such as opioids (as noted above), anticholinergics, antihypertensives, antacids, diuretics, and iron supplements.
Take a proactive approach to constipation
Constipation is expected in a number of clinical scenarios, such as with the use of opioids or with limited mobility. Patients often attribute constipation to diminished oral intake. But despite low oral intake, regular, smaller-caliber bowel movements are important to ensure that sloughed bowel endothelium and bacteria are eliminated.
Although little evidence supports the use of one standard bowel regimen, prevention is essential. The goal is a soft bowel movement every 1 to 2 days. Constipation prophylaxis should be started at the initiation of any regular opioid regimen. Encouraging physical activity and oral fluid intake and creating a favorable environment for elimination may also help manage constipation.
In our practice, we use a softening agent such as docusate sodium (Colace) 100 mg twice daily, and add a laxative agent such as senna (Senokot) or a magnesium-based osmotic agent as needed. Bulking agents such as over-the-counter fiber supplements should be used with caution in opioid-related constipation. If there has been no bowel movement for 48 hours, a rectal suppository or enema is used. Suppositories or enemas can be scheduled regularly for bedbound patients with chronic constipation.
Methylnaltrexone (Relistor), a mu-opioid antagonist, is a new agent that blocks peripheral opioid receptors in the gut. In a randomized study of 133 patients, methylnaltrexone produced laxation within 4 hours of administration in 48%.31 This methylated, charged compound does not significantly cross the blood-brain barrier and therefore does not interfere with analgesia or cause opioid withdrawal. The dose is 8 mg or 12 mg subcutaneously (based on weight), which can be repeated in 48 hours. If laxation does not occur after one to three doses, other causes of constipation should be explored.
Methylnaltrexone is contraindicated in patients with bowel obstruction, even if the obstruction is thought to be secondary to opioids. Adverse effects include abdominal pain, flatulence, and nausea.
NAUSEA AND VOMITING: NOT ALWAYS DUE TO CHEMOTHERAPY
Nausea (the sensation of the need to vomit) and vomiting (the forceful expulsion of gastric contents) are common symptoms in advanced cancer and are not necessarily related to chemotherapy or radiation therapy. About 60% of cancer patients have nausea, and about 30% vomit.32 Both symptoms are very distressing and diminish quality of life.
Look for potentially reversible causes of nausea and vomiting
Identifying the cause, which is sometimes reversible, may help direct treatment. Potentially reversible causes include:
- Drugs
- Uremia
- Infection
- Anxiety
- Constipation
- Gastric irritation
- Proximal gastrointestinal obstruction.
In a prospective study of 121 patients with advanced cancer, the most common causes of nausea and vomiting were impaired gastric emptying, chemical and metabolic factors (drugs, organ failure, electrolyte disturbance, infection), and bowel obstruction.33–35
Management of nausea and vomiting
Management of nausea and vomiting may require multiple antiemetics, which may need to be given intravenously or subcutaneously.33
The choice of drugs depends on the cause of the nausea
The evidence-based choice of drugs for nausea depends on the cause33–35:
- Nausea due to chemical or metabolic factors: haloperidol (Haldol), levomepromazine (another antipsychotic drug, not available in the United States), cyclizine (Marezine)
- Nausea due to gastric stasis, outlet obstruction: metoclopramide, domperidone (a similar drug, not available in the United States), levomepromazine
- Nausea due to regurgitation: metoclopramide, cyclizine, haloperidol, levomepromazine
- Nausea due to bowel obstruction: metoclopramide (if obstruction is not complete), domperidone, cyclizine, levomepromazine, octreotide (Sandostatin), hyoscyamine (Levsin)
- Nausea due to cranial disease: cyclizine, levomepromazine
- Movement-related nausea: cyclizine, levomepromazine, hyoscyamine
- Cause unclear or multiple causes: cyclizine, haloperidol, levomepromazine
- Cortical nausea: lorazepam (Ativan).
Metoclopramide. If complete bowel obstruction is not suspected, oral metoclopramide, a dopamine antagonist, is our choice for first-line drug therapy.32 Adverse effects include abdominal pain, diarrhea, and sedation.
Haloperidol, another dopamine antagonist, can also be used.32 Haloperidol may cause sedation and is associated with a prolonged QTc interval. Care should be taken in those at risk for dysrhythmia or arrhythmia.
Olanzapine (Zyprexa) is an alternative antipsychotic for patients who cannot tolerate or do not respond to metoclopramide and haloperidol.
Ondansetron (Zofran), a serotonin 5-HT3 receptor antagonist, is usually reserved for nausea and vomiting associated with chemotherapy or radiation, but it can be used in advanced cancer if the above agents fail.37
DYSPNEA IS COMMON, EVEN WITHOUT LUNG DISEASE
Dyspnea is the subjective perception of impaired breathing, which may include the sensation of breathlessness, chest tightness, air hunger, suffocation, or increased work of breathing.
At least half of patients with advanced cancer complain of dyspnea.1 Most have primary pulmonary malignancies or metastatic lung disease, but almost 25% have no documented lung involvement or underlying cardiopulmonary diagnosis to which to attribute it.38
Dyspnea is often very distressing. Palliative sedation is used more frequently for the relief of intractable dyspnea than for pain.39
Opioids are effective but underutilized for dyspnea
Although opioids are effective in both oral and parenteral formulations for the symptomatic management of dyspnea,40 the exact mechanism by which they improve dyspnea is unknown. Central control of respiration occurs in the medulla, and perception of dyspnea is mediated by the sensory cortex.
Opioids are underutilized by physicians other than palliative medicine specialists because of concern about respiratory depression. Appropriately titrated, opioids are safe and do not cause clinically significant respiratory depression.41
Allen et al42 showed that an opioid in low doses (diamorphine 2.5 mg subcutaneously) was effective and well tolerated in elderly patients with advanced pulmonary fibrosis who had not received opioids before.
Start low and go slow. An appropriate starting dose for a patient who has not been on opioids before may be morphine sulfate 2 mg intravenously (or a 5-mg immediate-release tablet by mouth) every 2 hours as needed for dyspnea. After 24 to 48 hours of an as-needed regimen, one can evaluate the patient’s response, tolerance, and dose requirement. If needed, parenteral infusion or a long-acting opioid preparation can be started with continued as-needed bolus dosing for breakthrough dyspnea.
We do not recommend writing opioid infusion orders with a “titrate to comfort” clause in the terminally ill. Increasing the rate of a continuous infusion does not provide the prompt symptomatic relief a bolus dose delivers. Dose accumulation and adverse effects are more likely when opioids are titrated in this fashion.
A Cochrane review showed that nebulized opioids are ineffective for dyspnea.43
Oxygen paradoxically does not improve dyspnea
Oxygen is commonly prescribed, although the literature does not indicate that it improves the sensation of breathlessness.44
A study by Clemens et al45 showed no correlation between dyspnea and oxygen saturation. It also found morphine to be superior to oxygen in subjective dyspnea, even in hypoxia.
A double-blind crossover study showed that ambient air delivered via nasal cannula was as effective as oxygen for dyspnea.46 The inexpensive and simple practice of a fan to blow ambient air on the patient’s face may help relieve dyspnea.
Cancer patients experience many distressing symptoms during the course of their illness. In addition to pain, they commonly suffer from fatigue, anorexia, constipation, dyspnea, nausea, and vomiting.1
Although it is important to diagnose and manage the cancer itself, it is also the physician’s duty to recognize and effectively treat associated symptoms, regardless of the outcome of the underlying disease.
Some of the symptoms are due to the underlying disease, but some are iatrogenic, as many medical interventions have predictable adverse effects, such as nausea and vomiting with chemotherapy or constipation with opioids.
Symptoms of advanced cancer become chronic, and patients usually rate them as moderate or severe.1 Unrelieved suffering causes demoralization and may quickly impair quality of life.2
Understanding the principles of symptom management may help optimize palliation and improve quality of life. In this paper, we outline an approach to the management of cancer-related symptoms.
A HEAVY BURDEN OF SYMPTOMS
In patients with advanced cancer, the prevalence rates of various symptoms are approximately as follows1,3:
- Pain 89%
- Fatigue 69%
- Weakness 66%
- Anorexia 66%
- Lack of energy 61%
- Nausea 60%
- Dry mouth 57%
- Constipation 52%
- Early satiety 51%
- Dyspnea 50%
- Vomiting 30%.
Furthermore, patients with advanced cancer typically have multiple concurrent symptoms. In a survey of patients in a palliative medicine service at our hospital,4 we found that the median number of symptoms per patient was 10 (range 0–25) (Figure 1).
PRINCIPLES OF SYMPTOM MANAGEMENT
Show an interest in the patient’s symptoms. Many patients with advanced cancer believe that suffering is an inevitable part of the disease or of its treatment.
Ask patients about their symptoms in a positive and detailed fashion, starting with open-ended questions and following up with specific questions. Patients may underreport their symptoms or may not mention them if not asked directly. In the survey of palliative care patients at our hospital mentioned above,4 the median number of volunteered symptoms was only 1 (range 0–6), whereas a median of 10 were found by systematic assessment.
The examiner should clarify when necessary and recognize that a layperson’s language may not directly translate to medical language. For example, a patient may not understand the term “anorexia.” Furthermore, “loss of appetite” may mean nausea, vomiting, constipation, or early satiety. “Numbness” may mean a loss of sensation or a pins-and-needles sensation. Symptoms should also be quantified using a consistent measure (ie, numerical or categorical) to facilitate monitoring.
Prioritize the symptoms. Advanced cancer is accompanied by multiple symptoms. Assess which ones are most bothersome, and where therapy should be directed first.
Try to understand the pathophysiology behind the symptom. When possible, choose a drug treatment that targets the likely underlying cause. Nausea and vomiting, for example, can be secondary to gastric outlet obstruction, hypercalcemia, increased intracranial pressure, esophagitis, opioid use, or constipation.
Be specific about the drug, dosing, timing, and route, and keep it simple. If a regimen is cumbersome, compliance suffers. It is better to start one medication for the most bothersome symptom or symptoms and make some progress than it is to overwhelm the patient with a complex list of drugs. Sustained-release formulations are often useful. It is unrealistic to expect most patients to take a medication every 4 hours around the clock. Try the most cost-effective remedies first, and attempt to use one drug that may address multiple symptoms. For example, dexamethasone may have positive effects on energy, pain, and appetite.
Use ‘rescue dosing.’ Rescue drugs are important for expected symptom exacerbations in those on sustained medication. This approach increases efficacy and minimizes adverse effects. In most cases, the rescue medication should be the same as the regularly scheduled one. For example, a prescription to treat nausea may read “metoclopramide (Reglan) 10 mg by mouth before meals and at bedtime and every 4 hours as needed to treat nausea or vomiting.”
Consider the patient’s age and fragility, the cost of the drug, and anticipated adverse effects. Oral or transdermal preparations are preferable to parenteral ones with regard to convenience and compliance, although many transdermal preparations are costly. If parenteral dosing is necessary, the subcutaneous route is an alternative to the intravenous route.
Discontinue drugs that are ineffective or unnecessary. This may help compliance and diminish adverse effects.
Make one change at a time so the response to that change is clear. Titrate one drug to its effective dose, to its maximum dose, or to a level of intolerability before considering another. If one drug of a class is ineffective, another drug in the same class may work.
Reassess often. A follow-up phone call or office visit in 1 to 2 weeks is appropriate. The symptoms of advanced cancer are often progressive, so regular evaluation is important, even if symptoms are controlled on stable drug regimens. Instructions should be both verbal and written and should be communicated to patients and any involved caregiver to ensure compliance. Have a “plan B” if the first plan is ineffective.
A challenging and important part of symptom management is to assess the goals of care. Every intervention is not appropriate for every patient. Which therapies are used depends on the stage of the disease, the available disease-modifying treatments, and the patient’s condition and preferences. Patients and their loved ones should be engaged in discussions about goals of care early in the disease and should be included in medical decision-making. Both curative treatment and palliative treatment are important, but palliation plays a bigger role towards the later stages of advanced cancer (Figure 2).
CANCER-RELATED FATIGUE: COMMON BUT NOT INEVITABLE
Most cancer patients report fatigue. Although it is one of the most common symptoms in advanced cancer,5 it is not necessarily inevitable or untreatable.6
Cancer-related fatigue is multidimensional and develops over time, diminishing energy, mental capacity, and psychological condition.7 Patients may report feeling tired or being unable to complete their activities of daily living. People who were previously very active may be frustrated by their inability to participate in favorite leisure activities, which has a big impact on quality of life. Fatigue can be physical, emotional, or mental. It is important to distinguish physical weakness from dyspnea on exertion, which is commonly reported as fatigue. Depression may also cause or exacerbate fatigue.
Unlike fatigue in the general population, cancer-related fatigue does not improve with rest, and patients often report large amounts of unrestorative sleep.
Look for reversible causes of fatigue
First, conduct a thorough assessment to identify any reversible causes, such as:
- Anemia
- Insomnia, sleep disturbance
- Malnutrition
- Pain
- Depression
- Medical comorbidities: renal, cardiac, or pulmonary disease
- Hypothyroidism
- Hypogonadism.
In many cases, however, a reversible cause cannot be found.
Treating cancer-related fatigue
Nonpharmacologic interventions have been evaluated for this application, but evidence of efficacy is limited and mixed. The National Cancer Comprehensive Network guidelines8 suggest that energy conservation and education about cancer-related fatigue are central to management. Patients should be advised that fatigue has a fluctuating course and that they have a limited pool of energy, which they should conserve and use judiciously.
In a meta-analysis by Schmitz et al,9 physical activity interventions were found to be beneficial. Sixty-three percent of those studied were undergoing active treatment, so whether this population reflects advanced cancer is unclear. A small pilot study in advanced cancer found a trend toward benefit with exercise.10
Comment. The strategies of rest and exercise are complementary. The key point is to plan them per personal preference.
Psychostimulants include methylphenidate (Ritalin). A randomized placebo-controlled trial in patients with acquired immunodeficiency syndrome (AIDS) found methylphenidate 15 to 60 mg/day to have a positive effect.11 Prospective studies have shown similar results in cancer patients,12 and a Cochrane review in 2008 showed a small but significant benefit in cancer-related fatigue.13
Methylphenidate is usually started at a dose of 5 mg given at 8:00 am and at noon, and then titrated. Benefit, when experienced, is typically noted within 24 to 48 hours. Possible adverse effects include anorexia, insomnia, anxiety, confusion, tremor, and tachycardia.
Stimulants should be used with caution in patients with cardiac disease or delirium.
Modafinil (Provigil), a nonstimulant agent, has been less studied, but it may also help.14,15 The usual dosage is 50 to 200 mg daily.
Corticosteroids may have a role in advanced cancer, as suggested by anecdotal reports.16 They should be used judiciously, as their adverse effects (insomnia, muscle wasting, edema) are themselves burdensome and may outweigh their benefits.
ANOREXIA CAN BE DISTRESSING TO THE FAMILY AND THE PATIENT
Most patients with advanced cancer experience anorexia, which is a marker of poor prognosis.1
Appetite loss may occur in isolation or as a part of the anorexia-cachexia syndrome. This syndrome is a wasting state seen in chronic, advanced diseases including cancer, AIDS, chronic obstructive pulmonary disease, chronic renal insufficiency, and congestive heart failure.17 The associated weight loss is involuntary and includes both muscle and fat.
Appetite loss alone is usually not bothersome. In fact, anorexia frequently causes more distress to the family than to the patient.18 The ramifications of decreased appetite, on the other hand, can be devastating. Decreased caloric intake coupled with the hypermetabolic state of malignancy leads to rapid, dramatic changes in body habitus. This outward sign of the ravages of cancer can be psychologically damaging to patients and their loved ones as they contemplate advanced disease and limited life expectancy. They may be concerned about starvation, in which case education about and attempts to normalize the anorexia-cachexia syndrome are essential.
Look for reversible causes of anorexia
The first step in the management of anorexia is to identify any reversible causes, such as:
- Stomatitis
- Constipation
- Uncontrolled severe symptoms such as pain or dyspnea
- Delirium
- Nausea, vomiting
- Depression
- Gastroparesis.
Managing cancer-related anorexia
Nonpharmacologic measures include nutritional counseling and increased physical activity. Patients may be counseled to eat calorie-dense foods and supplemental high-calorie, high-protein, high-fat drinks. Some may be able to take advantage of a diurnal variation in appetite, usually an increased appetite in the morning.
Megestrol acetate (Megace) improved appetite and induced weight gain when used in a dosage of 800 mg daily in a randomized controlled trial in AIDS patients.19 Case studies have shown doses as low as 80 to 160 mg daily to be beneficial.20 Most of the added weight is fat, not lean muscle mass. Unfortunately, the addition of testosterone to megestrol did not increase the accumulation of lean muscle mass in another randomized trial.21 But the addition of olanzapine (Zyprexa) to megestrol was associated with improved appetite and weight gain in a significant percentage of advanced cancer patients.22 Rates of adverse effects with megestrol are low; the most significant adverse effect is thromboembolism.
Corticosteroids. While much of the support for corticosteroids is anecdotal, a prospective study of dexamethasone 4 to 16 mg daily showed improvement in several symptoms, including appetite.23 Because of the multiple adverse effects of corticosteroids, careful attention to dose, duration, and tolerability is essential. Corticosteroids should be discontinued if the desired positive effects are not observed within 3 to 5 days. If prolonged survival is expected, wean to the lowest effective dose.
Cannabinoids. Dronabinol (Marinol), a synthetic formulation of delta-9-tetrahydrocannabinol (THC), the active agent of marijuana, has been beneficial in AIDS anorexia. Fewer studies have been done in advanced cancer.
In a small, open-label case series, doses of 7.5 to 15 mg of dronabinol daily improved appetite and were well tolerated.24 On the other hand, in a multicenter, randomized, double-blind, placebo-controlled trial, neither cannabis extract nor THC (5 mg daily) significantly improved appetite over a 6-week period.25
A large randomized study found megestrol acetate 800 mg to be superior to dronabinol 5 mg daily for treating anorexia.26
Neurotoxicity, anxiety, nervousness, dizziness, euphoria, and somnolence from dronabinol can be severe and intolerable for some.
Enteral tube feeding and parenteral nutrition do not improve survival or comfort in terminally ill patients.27 On the contrary, they are associated with complications, including aspiration pneumonia, sepsis, abdominal pain, vomiting, and diarrhea. Nevertheless, in some patients with mechanical impediments to nutrition (eg, esophageal fistula, obstruction, or proximal small bowel obstruction) or in those who are hungry and unable to take food by mouth, tube feeding may be appropriate.
CONSTIPATION SHOULD BE ANTICIPATED, AND PREVENTED IF POSSIBLE
Constipation is variably defined by patients and health care professionals, but it usually includes components of the Rome II criteria, ie, two or more of the following symptoms28:
- Straining at least 25% of the time
- Hard stools at least 25% of the time
- Incomplete evacuation at least 25% of the time
- Two or fewer bowel movements per week.
These criteria were intended to describe functional constipation in a healthy population.27
More than 50% of patients with advanced cancer report constipation,1 and in those on opioids, the scope of the problem is larger. In addition to binding central nervous system receptors to mediate pain perception, opioids bind systemic receptors including those in the gut. As a result, opioids interfere with smooth muscle tone and contractility, lengthen transit time, promote dry stools, and increase anal sphincter tone.29 A nursing study found that when patients taking opioids were screened for constipation, 95% identified it as the major adverse effect of their pain regimen.30
Multiple causes of constipation
Factors that can cause or contribute to constipation include:
- Dietary factors such as a generally low intake of food, and specifically of fiber
- Inactivity
- Confusion
- Dehydration
- Intestinal obstruction
- Cormorbidities such as diabetes mellitus, hypothyroidism, hypercalcemia
- Uncomfortable toilet arrangements
- Drugs such as opioids (as noted above), anticholinergics, antihypertensives, antacids, diuretics, and iron supplements.
Take a proactive approach to constipation
Constipation is expected in a number of clinical scenarios, such as with the use of opioids or with limited mobility. Patients often attribute constipation to diminished oral intake. But despite low oral intake, regular, smaller-caliber bowel movements are important to ensure that sloughed bowel endothelium and bacteria are eliminated.
Although little evidence supports the use of one standard bowel regimen, prevention is essential. The goal is a soft bowel movement every 1 to 2 days. Constipation prophylaxis should be started at the initiation of any regular opioid regimen. Encouraging physical activity and oral fluid intake and creating a favorable environment for elimination may also help manage constipation.
In our practice, we use a softening agent such as docusate sodium (Colace) 100 mg twice daily, and add a laxative agent such as senna (Senokot) or a magnesium-based osmotic agent as needed. Bulking agents such as over-the-counter fiber supplements should be used with caution in opioid-related constipation. If there has been no bowel movement for 48 hours, a rectal suppository or enema is used. Suppositories or enemas can be scheduled regularly for bedbound patients with chronic constipation.
Methylnaltrexone (Relistor), a mu-opioid antagonist, is a new agent that blocks peripheral opioid receptors in the gut. In a randomized study of 133 patients, methylnaltrexone produced laxation within 4 hours of administration in 48%.31 This methylated, charged compound does not significantly cross the blood-brain barrier and therefore does not interfere with analgesia or cause opioid withdrawal. The dose is 8 mg or 12 mg subcutaneously (based on weight), which can be repeated in 48 hours. If laxation does not occur after one to three doses, other causes of constipation should be explored.
Methylnaltrexone is contraindicated in patients with bowel obstruction, even if the obstruction is thought to be secondary to opioids. Adverse effects include abdominal pain, flatulence, and nausea.
NAUSEA AND VOMITING: NOT ALWAYS DUE TO CHEMOTHERAPY
Nausea (the sensation of the need to vomit) and vomiting (the forceful expulsion of gastric contents) are common symptoms in advanced cancer and are not necessarily related to chemotherapy or radiation therapy. About 60% of cancer patients have nausea, and about 30% vomit.32 Both symptoms are very distressing and diminish quality of life.
Look for potentially reversible causes of nausea and vomiting
Identifying the cause, which is sometimes reversible, may help direct treatment. Potentially reversible causes include:
- Drugs
- Uremia
- Infection
- Anxiety
- Constipation
- Gastric irritation
- Proximal gastrointestinal obstruction.
In a prospective study of 121 patients with advanced cancer, the most common causes of nausea and vomiting were impaired gastric emptying, chemical and metabolic factors (drugs, organ failure, electrolyte disturbance, infection), and bowel obstruction.33–35
Management of nausea and vomiting
Management of nausea and vomiting may require multiple antiemetics, which may need to be given intravenously or subcutaneously.33
The choice of drugs depends on the cause of the nausea
The evidence-based choice of drugs for nausea depends on the cause33–35:
- Nausea due to chemical or metabolic factors: haloperidol (Haldol), levomepromazine (another antipsychotic drug, not available in the United States), cyclizine (Marezine)
- Nausea due to gastric stasis, outlet obstruction: metoclopramide, domperidone (a similar drug, not available in the United States), levomepromazine
- Nausea due to regurgitation: metoclopramide, cyclizine, haloperidol, levomepromazine
- Nausea due to bowel obstruction: metoclopramide (if obstruction is not complete), domperidone, cyclizine, levomepromazine, octreotide (Sandostatin), hyoscyamine (Levsin)
- Nausea due to cranial disease: cyclizine, levomepromazine
- Movement-related nausea: cyclizine, levomepromazine, hyoscyamine
- Cause unclear or multiple causes: cyclizine, haloperidol, levomepromazine
- Cortical nausea: lorazepam (Ativan).
Metoclopramide. If complete bowel obstruction is not suspected, oral metoclopramide, a dopamine antagonist, is our choice for first-line drug therapy.32 Adverse effects include abdominal pain, diarrhea, and sedation.
Haloperidol, another dopamine antagonist, can also be used.32 Haloperidol may cause sedation and is associated with a prolonged QTc interval. Care should be taken in those at risk for dysrhythmia or arrhythmia.
Olanzapine (Zyprexa) is an alternative antipsychotic for patients who cannot tolerate or do not respond to metoclopramide and haloperidol.
Ondansetron (Zofran), a serotonin 5-HT3 receptor antagonist, is usually reserved for nausea and vomiting associated with chemotherapy or radiation, but it can be used in advanced cancer if the above agents fail.37
DYSPNEA IS COMMON, EVEN WITHOUT LUNG DISEASE
Dyspnea is the subjective perception of impaired breathing, which may include the sensation of breathlessness, chest tightness, air hunger, suffocation, or increased work of breathing.
At least half of patients with advanced cancer complain of dyspnea.1 Most have primary pulmonary malignancies or metastatic lung disease, but almost 25% have no documented lung involvement or underlying cardiopulmonary diagnosis to which to attribute it.38
Dyspnea is often very distressing. Palliative sedation is used more frequently for the relief of intractable dyspnea than for pain.39
Opioids are effective but underutilized for dyspnea
Although opioids are effective in both oral and parenteral formulations for the symptomatic management of dyspnea,40 the exact mechanism by which they improve dyspnea is unknown. Central control of respiration occurs in the medulla, and perception of dyspnea is mediated by the sensory cortex.
Opioids are underutilized by physicians other than palliative medicine specialists because of concern about respiratory depression. Appropriately titrated, opioids are safe and do not cause clinically significant respiratory depression.41
Allen et al42 showed that an opioid in low doses (diamorphine 2.5 mg subcutaneously) was effective and well tolerated in elderly patients with advanced pulmonary fibrosis who had not received opioids before.
Start low and go slow. An appropriate starting dose for a patient who has not been on opioids before may be morphine sulfate 2 mg intravenously (or a 5-mg immediate-release tablet by mouth) every 2 hours as needed for dyspnea. After 24 to 48 hours of an as-needed regimen, one can evaluate the patient’s response, tolerance, and dose requirement. If needed, parenteral infusion or a long-acting opioid preparation can be started with continued as-needed bolus dosing for breakthrough dyspnea.
We do not recommend writing opioid infusion orders with a “titrate to comfort” clause in the terminally ill. Increasing the rate of a continuous infusion does not provide the prompt symptomatic relief a bolus dose delivers. Dose accumulation and adverse effects are more likely when opioids are titrated in this fashion.
A Cochrane review showed that nebulized opioids are ineffective for dyspnea.43
Oxygen paradoxically does not improve dyspnea
Oxygen is commonly prescribed, although the literature does not indicate that it improves the sensation of breathlessness.44
A study by Clemens et al45 showed no correlation between dyspnea and oxygen saturation. It also found morphine to be superior to oxygen in subjective dyspnea, even in hypoxia.
A double-blind crossover study showed that ambient air delivered via nasal cannula was as effective as oxygen for dyspnea.46 The inexpensive and simple practice of a fan to blow ambient air on the patient’s face may help relieve dyspnea.
- Donnelly S, Walsh D. The symptoms of advanced cancer. Semin Oncol 1995; 22(2 suppl 3):67–72.
- Walsh D, Rybicki L, Nelson KA, Donnelly S. Symptoms and prognosis in advanced cancer. Support Care Cancer 2002; 10:385–388.
- Komurcu S, Nelson KA, Walsh D, Donnelly SM, Homsi J, Abdullah O. Common symptoms in advanced cancer. Semin Oncol 2000; 27:24–33.
- Homsi J, Walsh D, Rivera N, et al. Symptom evaluation in palliative medicine: patient report vs systematic assessment. Support Care Cancer 2006; 14:444–453.
- Donnelly S. Quality-of-life assessment in advanced cancer. Curr Oncol Rep 2000; 2:338–342.
- Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N. Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann Oncol 2000; 11:971–975.
- Portenoy RK, Itri LM. Cancer-related fatigue: guidelines for evaluation and management. Oncologist 1999; 4:1–10.
- Berger AM, Abernethy AP, Atkinson A, et al. NCCN Clinical Practice Guidelines in Oncology Cancer-related fatigue—v.1.2010. www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf. Accessed November 15, 2010.
- Schmitz KH, Holtzman J, Courneya KS, Mâsse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2005; 14:1588–1595.
- Porock D, Kristjanson LJ, Tinnelly K, Duke T, Blight J. An exercise intervention for advanced cancer patients experiencing fatigue: a pilot study. J Palliat Care 2000 Autumn; 16:30–36.
- Breitbart W, Rosenfeld B, Kaim M, Funesti-Esch J. A randomized, double-blind, placebo-controlled trial of psychostimulants for the treatment of fatigue in ambulatory patients with human immunodeficiency virus disease. Arch Intern Med 2001; 161:411–420.
- Sarhill N, Walsh D, Nelson KA, Homsi J, LeGrand S, Davis MP. Methylphenidate for fatigue in advanced cancer: a prospective open-label pilot study. Am J Hosp Palliat Care 2001; 18:187–192.
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst 2008; 100:1155–1166.
- Kaleita TA, Wellisch DK, Graham CA, et al. Pilot study of modafinil for treatment of neurobehavioral dysfunction and fatigue in adult patients with brain tumors (abstract). J Clin Oncol 2006; 24(suppl):58s.
- Morrow GR, Jean-Pierre P, Roscoe JA, et al. A phase III randomized, placebo-controlled, double-blind trial of a eugeroic agent in 642 cancer patients reporting fatigue during chemotherapy: a URCC CCOP study (abstract). J Clin Oncol 2008; 26(suppl):504s.
- Tannock I, Gospodarowicz M, Meakin W, Panzarella T, Stewart L, Rider W. Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol 1989; 7:590–597.
- Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr 2006; 83:735–743.
- Poole K, Froggatt K. Loss of weight and loss of appetite in advanced cancer: a problem for the patient, the carer, or the health professional? Palliat Med 2002; 16:499–506.
- Von Roenn JH. Randomized trials of megestrol acetate for AIDS-associated anorexia and cachexia. Oncology 1994; 51(suppl 1):19–24.
- Donnelly S, Walsh TD. Low-dose megestrol acetate for appetite stimulation in advanced cancer. J Pain Symptom Manage 1995; 10:182–183.
- Mulligan K, Zackin R, Von Roenn JH, et al; ACTG 313 Study Team. Testosterone supplementation of megestrol therapy does not enhance lean tissue accrual in men with human immunodeficiency virus-associated weight loss: a randomized, double-blind, placebo-controlled, multicenter trial. J Clin Endocrinol Metab 2007; 92:563–570.
- Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 2010; 18:951–956.
- Mercadante S, Fulfaro F, Casuccio A. The use of corticosteroids in home palliative care. Support Care Cancer 2001; 9:386–389.
- Walsh D, Kirkova J, Davis MP. The efficacy and tolerability of long-term use of dronabinol in cancer-related anorexia: a case series. J Pain Symptom Manage 2005; 30:493–495.
- Cannabis-In-Cachexia-Study-Group; Strasser F, Luftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol 2006; 24:3394–3400.
- Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol 2002; 20:567–573.
- Winter SM. Terminal nutrition: framing the debate for the withdrawal of nutritional support in terminally ill patients. Am J Med 2000; 109:723–726.
- Drossman DA, Sandler RS, McKee DC, Lovitz AJ. Bowel patterns among subjects not seeking health care. Use of a questionnaire to identify a population with bowel dysfunction. Gastroenterology 1982; 83:529–534.
- McMillan SC. Assessing and managing opiate-induced constipation in adults with cancer. Cancer Control 2004; 11(suppl 3):3–9.
- Robinson CB, Fritch M, Hullett L, et al. Development of a protocol to prevent opioid-induced constipation in patients with cancer: a research utilization project. Clin J Oncol Nurs 2000; 4:79–84.
- Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med 2008; 358:2332–2343.
- Davis MP, Walsh D. Treatment of nausea and vomiting in advanced cancer. Support Care Cancer 2000; 8:444–452.
- Stephenson J, Davies A. An assessment of aetiology-based guidelines for the management of nausea and vomiting in patients with advanced cancer. Support Care Cancer 2006; 14:348–353.
- Lichter I. Results of antiemetic management in terminal illness. J Palliat Care 1993; 9:19–21.
- Bentley A, Boyd K. Use of clinical pictures in the management of nausea and vomiting: a prospective audit. Palliat Med 2001; 15:247–253.
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Support Care Cancer 2004; 12:432–440.
- Currow DC, Coughlan M, Fardell B, Cooney NJ. Use of ondansetron in palliative medicine. J Pain Symptom Manage 1997; 13:302–307.
- Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest 1986; 89:234–236.
- Fainsinger RL, Waller A, Bercovici M, et al. A multicentre international study of sedation for uncontrolled symptoms in terminally ill patients. Palliat Med 2000; 14:257–265.
- Jennings AL, Davies AN, Higgins JP, Broadley K. Opioids for the palliation of breathlessness in terminal illness. Cochrane Database Syst Rev 2001;CD002066.
- Estfan B, Mahmoud F, Shaheen P, et al. Respiratory function during parenteral opioid titration for cancer pain. Palliat Med 2007; 21:81–86.
- Allen S, Raut S, Woollard J, Vassallo M. Low dose diamorphine reduces breathlessness without causing a fall in oxygen saturation in elderly patients with end-stage idiopathic pulmonary fibrosis. Palliat Med 2005; 19:128–130.
- Polosa R, Simidchiev A, Walters EH. Nebulised morphine for severe interstitial lung disease. Cochrane Database Syst Rev 2002;CD002872.
- Currow DC, Agar M, Smith J, Abernethy AP. Does palliative home oxygen improve dyspnoea? A consecutive cohort study. Palliat Med 2009; 23:309–316.
- Clemens KE, Quednau I, Klaschik E. Use of oxygen and opioids in the palliation of dyspnoea in hypoxic and non-hypoxic palliative care patients: a prospective study. Support Care Cancer 2009; 17:367–377.
- Philip J, Gold M, Milner A, Di Iulio J, Miller B, Spruyt O. A randomized, double-blind, crossover trial of the effect of oxygen on dyspnea in patients with advanced cancer. J Pain Symptom Manage 2006; 32:541–550.
- Donnelly S, Walsh D. The symptoms of advanced cancer. Semin Oncol 1995; 22(2 suppl 3):67–72.
- Walsh D, Rybicki L, Nelson KA, Donnelly S. Symptoms and prognosis in advanced cancer. Support Care Cancer 2002; 10:385–388.
- Komurcu S, Nelson KA, Walsh D, Donnelly SM, Homsi J, Abdullah O. Common symptoms in advanced cancer. Semin Oncol 2000; 27:24–33.
- Homsi J, Walsh D, Rivera N, et al. Symptom evaluation in palliative medicine: patient report vs systematic assessment. Support Care Cancer 2006; 14:444–453.
- Donnelly S. Quality-of-life assessment in advanced cancer. Curr Oncol Rep 2000; 2:338–342.
- Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N. Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann Oncol 2000; 11:971–975.
- Portenoy RK, Itri LM. Cancer-related fatigue: guidelines for evaluation and management. Oncologist 1999; 4:1–10.
- Berger AM, Abernethy AP, Atkinson A, et al. NCCN Clinical Practice Guidelines in Oncology Cancer-related fatigue—v.1.2010. www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf. Accessed November 15, 2010.
- Schmitz KH, Holtzman J, Courneya KS, Mâsse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2005; 14:1588–1595.
- Porock D, Kristjanson LJ, Tinnelly K, Duke T, Blight J. An exercise intervention for advanced cancer patients experiencing fatigue: a pilot study. J Palliat Care 2000 Autumn; 16:30–36.
- Breitbart W, Rosenfeld B, Kaim M, Funesti-Esch J. A randomized, double-blind, placebo-controlled trial of psychostimulants for the treatment of fatigue in ambulatory patients with human immunodeficiency virus disease. Arch Intern Med 2001; 161:411–420.
- Sarhill N, Walsh D, Nelson KA, Homsi J, LeGrand S, Davis MP. Methylphenidate for fatigue in advanced cancer: a prospective open-label pilot study. Am J Hosp Palliat Care 2001; 18:187–192.
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst 2008; 100:1155–1166.
- Kaleita TA, Wellisch DK, Graham CA, et al. Pilot study of modafinil for treatment of neurobehavioral dysfunction and fatigue in adult patients with brain tumors (abstract). J Clin Oncol 2006; 24(suppl):58s.
- Morrow GR, Jean-Pierre P, Roscoe JA, et al. A phase III randomized, placebo-controlled, double-blind trial of a eugeroic agent in 642 cancer patients reporting fatigue during chemotherapy: a URCC CCOP study (abstract). J Clin Oncol 2008; 26(suppl):504s.
- Tannock I, Gospodarowicz M, Meakin W, Panzarella T, Stewart L, Rider W. Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol 1989; 7:590–597.
- Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr 2006; 83:735–743.
- Poole K, Froggatt K. Loss of weight and loss of appetite in advanced cancer: a problem for the patient, the carer, or the health professional? Palliat Med 2002; 16:499–506.
- Von Roenn JH. Randomized trials of megestrol acetate for AIDS-associated anorexia and cachexia. Oncology 1994; 51(suppl 1):19–24.
- Donnelly S, Walsh TD. Low-dose megestrol acetate for appetite stimulation in advanced cancer. J Pain Symptom Manage 1995; 10:182–183.
- Mulligan K, Zackin R, Von Roenn JH, et al; ACTG 313 Study Team. Testosterone supplementation of megestrol therapy does not enhance lean tissue accrual in men with human immunodeficiency virus-associated weight loss: a randomized, double-blind, placebo-controlled, multicenter trial. J Clin Endocrinol Metab 2007; 92:563–570.
- Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 2010; 18:951–956.
- Mercadante S, Fulfaro F, Casuccio A. The use of corticosteroids in home palliative care. Support Care Cancer 2001; 9:386–389.
- Walsh D, Kirkova J, Davis MP. The efficacy and tolerability of long-term use of dronabinol in cancer-related anorexia: a case series. J Pain Symptom Manage 2005; 30:493–495.
- Cannabis-In-Cachexia-Study-Group; Strasser F, Luftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol 2006; 24:3394–3400.
- Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol 2002; 20:567–573.
- Winter SM. Terminal nutrition: framing the debate for the withdrawal of nutritional support in terminally ill patients. Am J Med 2000; 109:723–726.
- Drossman DA, Sandler RS, McKee DC, Lovitz AJ. Bowel patterns among subjects not seeking health care. Use of a questionnaire to identify a population with bowel dysfunction. Gastroenterology 1982; 83:529–534.
- McMillan SC. Assessing and managing opiate-induced constipation in adults with cancer. Cancer Control 2004; 11(suppl 3):3–9.
- Robinson CB, Fritch M, Hullett L, et al. Development of a protocol to prevent opioid-induced constipation in patients with cancer: a research utilization project. Clin J Oncol Nurs 2000; 4:79–84.
- Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med 2008; 358:2332–2343.
- Davis MP, Walsh D. Treatment of nausea and vomiting in advanced cancer. Support Care Cancer 2000; 8:444–452.
- Stephenson J, Davies A. An assessment of aetiology-based guidelines for the management of nausea and vomiting in patients with advanced cancer. Support Care Cancer 2006; 14:348–353.
- Lichter I. Results of antiemetic management in terminal illness. J Palliat Care 1993; 9:19–21.
- Bentley A, Boyd K. Use of clinical pictures in the management of nausea and vomiting: a prospective audit. Palliat Med 2001; 15:247–253.
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Support Care Cancer 2004; 12:432–440.
- Currow DC, Coughlan M, Fardell B, Cooney NJ. Use of ondansetron in palliative medicine. J Pain Symptom Manage 1997; 13:302–307.
- Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest 1986; 89:234–236.
- Fainsinger RL, Waller A, Bercovici M, et al. A multicentre international study of sedation for uncontrolled symptoms in terminally ill patients. Palliat Med 2000; 14:257–265.
- Jennings AL, Davies AN, Higgins JP, Broadley K. Opioids for the palliation of breathlessness in terminal illness. Cochrane Database Syst Rev 2001;CD002066.
- Estfan B, Mahmoud F, Shaheen P, et al. Respiratory function during parenteral opioid titration for cancer pain. Palliat Med 2007; 21:81–86.
- Allen S, Raut S, Woollard J, Vassallo M. Low dose diamorphine reduces breathlessness without causing a fall in oxygen saturation in elderly patients with end-stage idiopathic pulmonary fibrosis. Palliat Med 2005; 19:128–130.
- Polosa R, Simidchiev A, Walters EH. Nebulised morphine for severe interstitial lung disease. Cochrane Database Syst Rev 2002;CD002872.
- Currow DC, Agar M, Smith J, Abernethy AP. Does palliative home oxygen improve dyspnoea? A consecutive cohort study. Palliat Med 2009; 23:309–316.
- Clemens KE, Quednau I, Klaschik E. Use of oxygen and opioids in the palliation of dyspnoea in hypoxic and non-hypoxic palliative care patients: a prospective study. Support Care Cancer 2009; 17:367–377.
- Philip J, Gold M, Milner A, Di Iulio J, Miller B, Spruyt O. A randomized, double-blind, crossover trial of the effect of oxygen on dyspnea in patients with advanced cancer. J Pain Symptom Manage 2006; 32:541–550.
KEY POINTS
- Patients with advanced cancer typically suffer from multiple concurrent symptoms, which they rate as moderate or severe.
- The principles of symptom management include taking an aggressive detailed approach, prioritizing, and identifying symptom pathophysiology.
- Prescribed regimens should be specific and simple; physicians should consider the patient’s age and fragility, the cost of the treatment, and anticipated drug side effects.
- To ensure optimal palliation with the fewest possible adverse effects, reassess frequently, make one change at a time, and use rescue doses.
A 31-year-old man with abdominal pain and a rectal nodule
A 31-year-old man presents to the emergency department with abdominal pain and diarrhea, which began 4 days ago. The pain is in both of the lower quadrants, is crampy and persistent, and is relieved with bowel movements. He has been having watery stools five to six times per day, without frank blood.
He reports no fevers, chills, nausea, or vomiting, and he has never travelled outside the country. He underwent laparotomy 6 months ago for a gunshot wound. He takes no prescription drugs. He smokes and he drinks alcohol, and he says he has used heroin and oxycodone recreationally.
His blood pressure is 134/74 mm Hg, and he is afebrile. An abdominal examination reveals no mass or tenderness.
Results of a complete blood count, serum chemistry panel, and serum amylase level are normal. His lipase level is slightly elevated at 80 U/L (reference range 12–70). His stool is negative for Clostridium difficile toxin on enzyme immunoassay.
Computed tomography of the abdomen reveals diffuse pericolonic hyperemia and possible thickening of the rectosigmoid colon, raising the concern that he might have infectious or inflammatory colitis. The patient is admitted for further evaluation.
Colonoscopy to evaluate the abnormalities on computed tomography finds only a 5-mm submucosal nodule in the rectum (Figure 1). Biopsy of the nodule shows it to be a well-differentiated neuroendocrine neoplasm (carcinoid tumor). Random colon biopsy samples are normal.
The patient’s symptoms resolve over the next 24 hours without any treatment.
WHAT EXPLAINS THE PATIENT’S SYMPTOMS?
1. Which of the following best explains the patient’s clinical presentation?
- Narcotic withdrawal
- Carcinoid syndrome
- Viral gastroenteritis
- Acute pancreatitis
Viral gastroenteritis is common and affects people of all ages. The very young and the elderly are at higher risk of adverse outcomes, but few people die of it in the United States.
Our patient’s symptoms were consistent with viral gastroenteritis that resolved spontaneously while he received only supportive care.
Narcotic withdrawal can also cause watery stools and abdominal pain. However, this patient lacked other signs and symptoms of withdrawal, and his symptoms improved without any detoxification or maintenance treatment.
Pancreatitis. Although the patient had a mildly elevated lipase level, his lack of nausea and vomiting and the location of the pain were not consistent with acute pancreatitis.
Carcinoid syndrome. Carcinoid tumors are rare, typically indolent neuroendocrine neoplasms. The carcinoid syndrome consists of cutaneous flushing, gut hypermotility with diarrhea, and bronchospasm.1–5 Our patient did not have the full range of these symptoms. However, the presentation of carcinoid tumors varies broadly depending on the location, morphology, or biology of the tumor.6 Although our patient had diarrhea, his symptoms improved without any specific treatment. Rectal carcinoid tumors rarely cause diarrhea, and therefore the tumor noted on colonoscopy was almost certainly an incidental finding unrelated to his clinical presentation.
The classic symptoms are caused by production of 5-hydroxyindoleacetic acid, typically by a carcinoid tumor of the small bowel. Rectal carcinoids do not produce the 5-hydroxyindoleacetic acid responsible for this “malignant” serotonin-driven syndrome and are typically asymptomatic. When rectal carcinoid tumors are symptomatic, patients may have symptoms of local irritation or obstruction, such as hematochezia, constipation, other changes in bowel habits, rectal pain, pruritis ani, or weight loss.2,7
Nearly 50% of rectal carcinoid tumors are discovered incidentally. The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry database documented a 10-fold increase in the incidence of rectal carcinoids in the last 35 years, attributed in part to an increase in screening colonoscopy.8 Furthermore, although studies of large national or multicenter databases have found that 65% to 80% of all rectal carcinoid tumors are smaller than 1.0 cm, 93.3% to 100% of those discovered on screening endoscopy were 1.0 cm or smaller.8
Rectal carcinoid tumors have a characteristic feel on digital examination, with a hard, “buckshot” consistency, and are freely mobile.5 They have also been described as firm, nodular, rubbery, yellow, submucosal, and polypoid.8
WHERE DO CARCINOID TUMORS TEND TO ARISE?
2. Which of the following sites is the most commonly recognized site of a primary carcinoid tumor?
- Small bowel
- Lung
- Liver
- Pancreas
- Rectum
The small bowel is the most common site.
Carcinoid tumors derive from neoplastic proliferation of cells of the diffuse neuroendocrine system. Therefore, they can be found anywhere neuroendocrine cells are present, commonly in the gastrointestinal tract, urogenital tract, and the bronchial epithelium.
Traditionally, neuroendocrine tumors were classified by their embryologic origin: foregut (including the respiratory tract, thymus, stomach, and pancreas), midgut (including the small intestine, appendix, and right colon), and hindgut (including the transverse, descending, and sigmoid colon and rectum). Functionally, this was sensible, as each class of tumors presented similarly due to the similar hormonal secretory products.2,3,9
A 2004 population-based review of the SEER database10 classified incidence rates of carcinoid tumors and their distribution throughout the body. Most (54.5%) were discovered in the gastrointestinal tract, and of these, 44.7% were in the small intestine, 19.6% were in the rectum, 16.7% were in the appendix, 10.6% were in the colon, 7.2% were in the stomach, and the remaining 1.2% were at other gastrointestinal sites. Nongastrointestinal sites included the lungs and bronchi (30.1%), pancreas (2.3%), female reproductive tract and ovaries (1.2%), biliary system (1.1%), and head and neck (0.4%).10
The incidence rates have increased and the distribution of sites in the body has changed over time. For example, the appendix was once considered the site of highest incidence, with tumors often discovered incidentally during surgical resection. However, these data were based on anecdotal or single-institution reports and so may have been subject to reporting bias. According to the SEER data, the small intestine is now the leading site, perhaps because of increased awareness or improved diagnostic technology and imaging.10,11
The liver is a common site of metastasis, but it is an exceptionally rare location for a primary tumor.
HOW SHOULD THIS PATIENT BE MANAGED?
3. What is the appropriate management of rectal carcinoid in this patient?
- Since the nodule is 1.0 cm or smaller, watchful waiting is acceptable
- Since the nodule is 1.0 cm or smaller, local excision is appropriate, and no follow-up is required
- Because all carcinoid tumors are potentially malignant, radical resection (eg, abdominal perineal resection) is appropriate
- Because all carcinoid tumors are potentially malignant, radical resection with chemotherapy with 5-fluorouracil (Adrucil) and doxorubicin (Adriamycin) is required
Since the nodule is 1.0 cm or smaller, local excision is appropriate, and no follow-up will be required. Rectal carcinoid tumors generally have a favorable prognosis, with a 5-year survival rate of 87.5%.10
PROGNOSIS DEPENDS ON TUMOR SIZE, OTHER FACTORS
Many studies have examined risk factors contributing to poor prognosis, and this is an area of active study. Early research categorized rectal carcinoid risk in terms of tumor diameter, and this is still widely used to guide management. As early as 1959, Hanley et al5 recognized that tumors that were likely to metastasize were often larger than 1 cm, had infiltrated the muscularis, or were ulcerated. Today, it is understood that only 3% to 10% of rectal carcinoids smaller than 1 cm metastasize, whereas 17% to 42% of those 1 to 2 cm and 60% to 80% of those larger than 2 cm do.2,8,12,13
However, size is not the only consideration. Wang et al12 showed that muscular invasion is an independent risk factor for survival, and that tumor diameter is a significant predictor of invasion and metastasis. Similarly, a metaanalysis by Mani et al13 recognized tumor size and muscularis invasion as the most important predictors of malignancy in these neoplasms.
To aid in predicting prognosis, staging systems have been developed from institutional or national registries. Landry et al14 developed a TNM (tumor, node, metastasis) staging system for rectal carcinoids, in which the T value was based on tumor size and degree of invasion. A group at Memorial-Sloan Kettering Cancer Center15 developed a system for risk stratification of carcinoid of the rectum that is based on tumor size, muscularis invasion, lymphovascular invasion, and the mitotic rate.
TREATMENT IS BY EXCISION
Despite these new prognostic systems, there is no new guidance on therapeutic management. Surgical therapy is still largely guided by tumor size.
Lesions smaller than 1 cm are resected endoscopically or by another local transanal technique.2,3,15,16 Standard endoscopic mucosal resection is performed, and recent studies have suggested that endoscopic submucosal dissection is as effective17 or even preferred, because it resects to the deeper submucosa (as the name suggests).18 This en bloc technique may be appropriate for lesions with evidence of local invasion.18 Other situations may call for deeper resection, such as transanal resection for higher lesions and full-thickness mucosal-muscularis resection.
Tumors 1 to 2 cm are currently evaluated for other factors such as ulceration and umbilication, which influence the choice of local vs radical resection. Otherwise, there is little guidance for tumors of 1 to 2 cm.
Tumors larger than 2 cm have a high risk of muscularis invasion and metastasis, and hence they are resected with wide margins and imaging is then used to evaluate for metastasis.8,19 In cases of metastasis, local resection is often palliative, providing local symptom relief.19
AN INCIDENTALLY DISCOVERED CASE; PATIENT LOST TO FOLLOW-UP
Our patient’s case is typical of rectal carcinoid in that it was discovered incidentally during colonoscopy. His clinical presentation was likely unrelated to his carcinoid tumor, and he improved without specific treatment. His symptoms resolved within 24 hours with supportive treatment and he was discharged.
Pathologic confirmation of carcinoid tumor occurred after his discharge. Despite persistent attempts to contact the patient, he never returned for a follow-up appointment.
TAKE-HOME POINTS
- Carcinoid tumors are rare neoplasms of neuroendocrine origin.
- Rectal carcinoids are the third most common carcinoid of the gastrointestinal tract.
- Most rectal carcinoids are asymptomatic.
- Diagnosis is most often incidental and histologic.
- Treatment is by excision.
- Prognosis is favorable for smaller carcinoids and depends on size (and therefore, invasion).
- Thorson A, Biorck G, Bjorkman G, Waldenstrom J. Malignant carcinoid of the small intestine with metastases to the liver, valvular disease of the right side of the heart (pulmonary stenosis and tricuspid regurgitation without septal defects), peripheral vasomotor symptoms, bronchoconstriction, and an unusual type of cyanosis; a clinical and pathologic syndrome. Am Heart J 1954; 47:795–817.
- Wang AY, Ahmad NA. Rectal carcinoids. Curr Opin Gastroenterol 2006; 22:529–535.
- Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology 2005; 128:1717–1751.
- Aggarwal G, Obideen K, Wehbi M. Carcinoid tumors: what should increase our suspicion? Cleve Clin J Med 2008; 75:849–855.
- Hanley PH, Hines MO, Ray J, Armstrong R. Carcinoid tumors of the rectum. Experience with 26 cases. Proc R Soc Med 1959; 52(suppl):113–117.
- Pasieka JL. Carcinoid tumors. Surg Clin North Am 2009; 89:1123–1137.
- Jetmore AB, Ray JE, Gathright JB, McMullen KM, Hicks TC, Timmcke AE. Rectal carcinoids: the most frequent carcinoid tumor. Dis Colon Rectum 1992; 35:717–725.
- Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy 2009; 41:162–165.
- Wilander E, Lundqvist M, Oberg K. Gastrointestinal carcinoid tumours. Histogenetic, histochemical, immunohistochemical, clinical and therapeutic aspects. Prog Histochem Cytochem 1989; 19:1–88.
- Maggard MA, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg 2004; 240:117–122.
- Modlin IM, Sandor A. An analyisis of 8,305 cases of carcinoid tumors. Cancer 1997; 79:813–829.
- Wang M, Peng J, Yang W, Chen W, Mo S, Cai S. Prognostic analysis for carcinoid tumors of the rectum: a single institutional analysis of 106 cases. Colorectal Dis 2009; Epub ahead of print.
- Mani S, Modlin IM, Ballantyne G, Ahlman H, West B. Carcinoids of the rectum. J Am Coll Surg 1994; 179:231–248.
- Landry CS, Brock G, Scoggins CR, McMasters KM, Martin RC. A proposed staging system for rectal carcinoid tumors based on an analysis of 4701 patients. Surgery 2008; 144:460–466.
- Fahy BN, Tang LH, Klimstra D, et al. Carcinoid of the rectum risk stratification (CaRRs): a strategy for preoperative outcome assessment. Ann Surg Oncol 2007; 14:1735–1743.
- Shirouzu K, Isomoto H, Kakegawa T, Morimatsu M. Treatment of rectal carcinoid tumors. Am J Surg 1990; 160:262–265.
- Baek IH. Endoscopic submucosal dissection or conventional endoscopic mucosal resection is an effective and safe treatment for rectal carcinoid tumors: a retrospective study. J Laparoendosc Adv Surg Tech A 2010; 20:329–331.
- Yamaguchi N, Isomoto H, Nishiyama H, et al. Endoscopic submucosal dissection for rectal carcinoid tumors. Surg Endosc 2010; 24:504–508.
- Ramage JK, Goretzki PE, Manfredi R, et al; Frascati Consensus Conference participants. Consensus guidelines for the management of patients with digestive neuroendocrine tumours: well-differentiated colon and rectum tumour/carcinoma. Neuroendocrinology 2008; 87:31–39.
A 31-year-old man presents to the emergency department with abdominal pain and diarrhea, which began 4 days ago. The pain is in both of the lower quadrants, is crampy and persistent, and is relieved with bowel movements. He has been having watery stools five to six times per day, without frank blood.
He reports no fevers, chills, nausea, or vomiting, and he has never travelled outside the country. He underwent laparotomy 6 months ago for a gunshot wound. He takes no prescription drugs. He smokes and he drinks alcohol, and he says he has used heroin and oxycodone recreationally.
His blood pressure is 134/74 mm Hg, and he is afebrile. An abdominal examination reveals no mass or tenderness.
Results of a complete blood count, serum chemistry panel, and serum amylase level are normal. His lipase level is slightly elevated at 80 U/L (reference range 12–70). His stool is negative for Clostridium difficile toxin on enzyme immunoassay.
Computed tomography of the abdomen reveals diffuse pericolonic hyperemia and possible thickening of the rectosigmoid colon, raising the concern that he might have infectious or inflammatory colitis. The patient is admitted for further evaluation.
Colonoscopy to evaluate the abnormalities on computed tomography finds only a 5-mm submucosal nodule in the rectum (Figure 1). Biopsy of the nodule shows it to be a well-differentiated neuroendocrine neoplasm (carcinoid tumor). Random colon biopsy samples are normal.
The patient’s symptoms resolve over the next 24 hours without any treatment.
WHAT EXPLAINS THE PATIENT’S SYMPTOMS?
1. Which of the following best explains the patient’s clinical presentation?
- Narcotic withdrawal
- Carcinoid syndrome
- Viral gastroenteritis
- Acute pancreatitis
Viral gastroenteritis is common and affects people of all ages. The very young and the elderly are at higher risk of adverse outcomes, but few people die of it in the United States.
Our patient’s symptoms were consistent with viral gastroenteritis that resolved spontaneously while he received only supportive care.
Narcotic withdrawal can also cause watery stools and abdominal pain. However, this patient lacked other signs and symptoms of withdrawal, and his symptoms improved without any detoxification or maintenance treatment.
Pancreatitis. Although the patient had a mildly elevated lipase level, his lack of nausea and vomiting and the location of the pain were not consistent with acute pancreatitis.
Carcinoid syndrome. Carcinoid tumors are rare, typically indolent neuroendocrine neoplasms. The carcinoid syndrome consists of cutaneous flushing, gut hypermotility with diarrhea, and bronchospasm.1–5 Our patient did not have the full range of these symptoms. However, the presentation of carcinoid tumors varies broadly depending on the location, morphology, or biology of the tumor.6 Although our patient had diarrhea, his symptoms improved without any specific treatment. Rectal carcinoid tumors rarely cause diarrhea, and therefore the tumor noted on colonoscopy was almost certainly an incidental finding unrelated to his clinical presentation.
The classic symptoms are caused by production of 5-hydroxyindoleacetic acid, typically by a carcinoid tumor of the small bowel. Rectal carcinoids do not produce the 5-hydroxyindoleacetic acid responsible for this “malignant” serotonin-driven syndrome and are typically asymptomatic. When rectal carcinoid tumors are symptomatic, patients may have symptoms of local irritation or obstruction, such as hematochezia, constipation, other changes in bowel habits, rectal pain, pruritis ani, or weight loss.2,7
Nearly 50% of rectal carcinoid tumors are discovered incidentally. The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry database documented a 10-fold increase in the incidence of rectal carcinoids in the last 35 years, attributed in part to an increase in screening colonoscopy.8 Furthermore, although studies of large national or multicenter databases have found that 65% to 80% of all rectal carcinoid tumors are smaller than 1.0 cm, 93.3% to 100% of those discovered on screening endoscopy were 1.0 cm or smaller.8
Rectal carcinoid tumors have a characteristic feel on digital examination, with a hard, “buckshot” consistency, and are freely mobile.5 They have also been described as firm, nodular, rubbery, yellow, submucosal, and polypoid.8
WHERE DO CARCINOID TUMORS TEND TO ARISE?
2. Which of the following sites is the most commonly recognized site of a primary carcinoid tumor?
- Small bowel
- Lung
- Liver
- Pancreas
- Rectum
The small bowel is the most common site.
Carcinoid tumors derive from neoplastic proliferation of cells of the diffuse neuroendocrine system. Therefore, they can be found anywhere neuroendocrine cells are present, commonly in the gastrointestinal tract, urogenital tract, and the bronchial epithelium.
Traditionally, neuroendocrine tumors were classified by their embryologic origin: foregut (including the respiratory tract, thymus, stomach, and pancreas), midgut (including the small intestine, appendix, and right colon), and hindgut (including the transverse, descending, and sigmoid colon and rectum). Functionally, this was sensible, as each class of tumors presented similarly due to the similar hormonal secretory products.2,3,9
A 2004 population-based review of the SEER database10 classified incidence rates of carcinoid tumors and their distribution throughout the body. Most (54.5%) were discovered in the gastrointestinal tract, and of these, 44.7% were in the small intestine, 19.6% were in the rectum, 16.7% were in the appendix, 10.6% were in the colon, 7.2% were in the stomach, and the remaining 1.2% were at other gastrointestinal sites. Nongastrointestinal sites included the lungs and bronchi (30.1%), pancreas (2.3%), female reproductive tract and ovaries (1.2%), biliary system (1.1%), and head and neck (0.4%).10
The incidence rates have increased and the distribution of sites in the body has changed over time. For example, the appendix was once considered the site of highest incidence, with tumors often discovered incidentally during surgical resection. However, these data were based on anecdotal or single-institution reports and so may have been subject to reporting bias. According to the SEER data, the small intestine is now the leading site, perhaps because of increased awareness or improved diagnostic technology and imaging.10,11
The liver is a common site of metastasis, but it is an exceptionally rare location for a primary tumor.
HOW SHOULD THIS PATIENT BE MANAGED?
3. What is the appropriate management of rectal carcinoid in this patient?
- Since the nodule is 1.0 cm or smaller, watchful waiting is acceptable
- Since the nodule is 1.0 cm or smaller, local excision is appropriate, and no follow-up is required
- Because all carcinoid tumors are potentially malignant, radical resection (eg, abdominal perineal resection) is appropriate
- Because all carcinoid tumors are potentially malignant, radical resection with chemotherapy with 5-fluorouracil (Adrucil) and doxorubicin (Adriamycin) is required
Since the nodule is 1.0 cm or smaller, local excision is appropriate, and no follow-up will be required. Rectal carcinoid tumors generally have a favorable prognosis, with a 5-year survival rate of 87.5%.10
PROGNOSIS DEPENDS ON TUMOR SIZE, OTHER FACTORS
Many studies have examined risk factors contributing to poor prognosis, and this is an area of active study. Early research categorized rectal carcinoid risk in terms of tumor diameter, and this is still widely used to guide management. As early as 1959, Hanley et al5 recognized that tumors that were likely to metastasize were often larger than 1 cm, had infiltrated the muscularis, or were ulcerated. Today, it is understood that only 3% to 10% of rectal carcinoids smaller than 1 cm metastasize, whereas 17% to 42% of those 1 to 2 cm and 60% to 80% of those larger than 2 cm do.2,8,12,13
However, size is not the only consideration. Wang et al12 showed that muscular invasion is an independent risk factor for survival, and that tumor diameter is a significant predictor of invasion and metastasis. Similarly, a metaanalysis by Mani et al13 recognized tumor size and muscularis invasion as the most important predictors of malignancy in these neoplasms.
To aid in predicting prognosis, staging systems have been developed from institutional or national registries. Landry et al14 developed a TNM (tumor, node, metastasis) staging system for rectal carcinoids, in which the T value was based on tumor size and degree of invasion. A group at Memorial-Sloan Kettering Cancer Center15 developed a system for risk stratification of carcinoid of the rectum that is based on tumor size, muscularis invasion, lymphovascular invasion, and the mitotic rate.
TREATMENT IS BY EXCISION
Despite these new prognostic systems, there is no new guidance on therapeutic management. Surgical therapy is still largely guided by tumor size.
Lesions smaller than 1 cm are resected endoscopically or by another local transanal technique.2,3,15,16 Standard endoscopic mucosal resection is performed, and recent studies have suggested that endoscopic submucosal dissection is as effective17 or even preferred, because it resects to the deeper submucosa (as the name suggests).18 This en bloc technique may be appropriate for lesions with evidence of local invasion.18 Other situations may call for deeper resection, such as transanal resection for higher lesions and full-thickness mucosal-muscularis resection.
Tumors 1 to 2 cm are currently evaluated for other factors such as ulceration and umbilication, which influence the choice of local vs radical resection. Otherwise, there is little guidance for tumors of 1 to 2 cm.
Tumors larger than 2 cm have a high risk of muscularis invasion and metastasis, and hence they are resected with wide margins and imaging is then used to evaluate for metastasis.8,19 In cases of metastasis, local resection is often palliative, providing local symptom relief.19
AN INCIDENTALLY DISCOVERED CASE; PATIENT LOST TO FOLLOW-UP
Our patient’s case is typical of rectal carcinoid in that it was discovered incidentally during colonoscopy. His clinical presentation was likely unrelated to his carcinoid tumor, and he improved without specific treatment. His symptoms resolved within 24 hours with supportive treatment and he was discharged.
Pathologic confirmation of carcinoid tumor occurred after his discharge. Despite persistent attempts to contact the patient, he never returned for a follow-up appointment.
TAKE-HOME POINTS
- Carcinoid tumors are rare neoplasms of neuroendocrine origin.
- Rectal carcinoids are the third most common carcinoid of the gastrointestinal tract.
- Most rectal carcinoids are asymptomatic.
- Diagnosis is most often incidental and histologic.
- Treatment is by excision.
- Prognosis is favorable for smaller carcinoids and depends on size (and therefore, invasion).
A 31-year-old man presents to the emergency department with abdominal pain and diarrhea, which began 4 days ago. The pain is in both of the lower quadrants, is crampy and persistent, and is relieved with bowel movements. He has been having watery stools five to six times per day, without frank blood.
He reports no fevers, chills, nausea, or vomiting, and he has never travelled outside the country. He underwent laparotomy 6 months ago for a gunshot wound. He takes no prescription drugs. He smokes and he drinks alcohol, and he says he has used heroin and oxycodone recreationally.
His blood pressure is 134/74 mm Hg, and he is afebrile. An abdominal examination reveals no mass or tenderness.
Results of a complete blood count, serum chemistry panel, and serum amylase level are normal. His lipase level is slightly elevated at 80 U/L (reference range 12–70). His stool is negative for Clostridium difficile toxin on enzyme immunoassay.
Computed tomography of the abdomen reveals diffuse pericolonic hyperemia and possible thickening of the rectosigmoid colon, raising the concern that he might have infectious or inflammatory colitis. The patient is admitted for further evaluation.
Colonoscopy to evaluate the abnormalities on computed tomography finds only a 5-mm submucosal nodule in the rectum (Figure 1). Biopsy of the nodule shows it to be a well-differentiated neuroendocrine neoplasm (carcinoid tumor). Random colon biopsy samples are normal.
The patient’s symptoms resolve over the next 24 hours without any treatment.
WHAT EXPLAINS THE PATIENT’S SYMPTOMS?
1. Which of the following best explains the patient’s clinical presentation?
- Narcotic withdrawal
- Carcinoid syndrome
- Viral gastroenteritis
- Acute pancreatitis
Viral gastroenteritis is common and affects people of all ages. The very young and the elderly are at higher risk of adverse outcomes, but few people die of it in the United States.
Our patient’s symptoms were consistent with viral gastroenteritis that resolved spontaneously while he received only supportive care.
Narcotic withdrawal can also cause watery stools and abdominal pain. However, this patient lacked other signs and symptoms of withdrawal, and his symptoms improved without any detoxification or maintenance treatment.
Pancreatitis. Although the patient had a mildly elevated lipase level, his lack of nausea and vomiting and the location of the pain were not consistent with acute pancreatitis.
Carcinoid syndrome. Carcinoid tumors are rare, typically indolent neuroendocrine neoplasms. The carcinoid syndrome consists of cutaneous flushing, gut hypermotility with diarrhea, and bronchospasm.1–5 Our patient did not have the full range of these symptoms. However, the presentation of carcinoid tumors varies broadly depending on the location, morphology, or biology of the tumor.6 Although our patient had diarrhea, his symptoms improved without any specific treatment. Rectal carcinoid tumors rarely cause diarrhea, and therefore the tumor noted on colonoscopy was almost certainly an incidental finding unrelated to his clinical presentation.
The classic symptoms are caused by production of 5-hydroxyindoleacetic acid, typically by a carcinoid tumor of the small bowel. Rectal carcinoids do not produce the 5-hydroxyindoleacetic acid responsible for this “malignant” serotonin-driven syndrome and are typically asymptomatic. When rectal carcinoid tumors are symptomatic, patients may have symptoms of local irritation or obstruction, such as hematochezia, constipation, other changes in bowel habits, rectal pain, pruritis ani, or weight loss.2,7
Nearly 50% of rectal carcinoid tumors are discovered incidentally. The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry database documented a 10-fold increase in the incidence of rectal carcinoids in the last 35 years, attributed in part to an increase in screening colonoscopy.8 Furthermore, although studies of large national or multicenter databases have found that 65% to 80% of all rectal carcinoid tumors are smaller than 1.0 cm, 93.3% to 100% of those discovered on screening endoscopy were 1.0 cm or smaller.8
Rectal carcinoid tumors have a characteristic feel on digital examination, with a hard, “buckshot” consistency, and are freely mobile.5 They have also been described as firm, nodular, rubbery, yellow, submucosal, and polypoid.8
WHERE DO CARCINOID TUMORS TEND TO ARISE?
2. Which of the following sites is the most commonly recognized site of a primary carcinoid tumor?
- Small bowel
- Lung
- Liver
- Pancreas
- Rectum
The small bowel is the most common site.
Carcinoid tumors derive from neoplastic proliferation of cells of the diffuse neuroendocrine system. Therefore, they can be found anywhere neuroendocrine cells are present, commonly in the gastrointestinal tract, urogenital tract, and the bronchial epithelium.
Traditionally, neuroendocrine tumors were classified by their embryologic origin: foregut (including the respiratory tract, thymus, stomach, and pancreas), midgut (including the small intestine, appendix, and right colon), and hindgut (including the transverse, descending, and sigmoid colon and rectum). Functionally, this was sensible, as each class of tumors presented similarly due to the similar hormonal secretory products.2,3,9
A 2004 population-based review of the SEER database10 classified incidence rates of carcinoid tumors and their distribution throughout the body. Most (54.5%) were discovered in the gastrointestinal tract, and of these, 44.7% were in the small intestine, 19.6% were in the rectum, 16.7% were in the appendix, 10.6% were in the colon, 7.2% were in the stomach, and the remaining 1.2% were at other gastrointestinal sites. Nongastrointestinal sites included the lungs and bronchi (30.1%), pancreas (2.3%), female reproductive tract and ovaries (1.2%), biliary system (1.1%), and head and neck (0.4%).10
The incidence rates have increased and the distribution of sites in the body has changed over time. For example, the appendix was once considered the site of highest incidence, with tumors often discovered incidentally during surgical resection. However, these data were based on anecdotal or single-institution reports and so may have been subject to reporting bias. According to the SEER data, the small intestine is now the leading site, perhaps because of increased awareness or improved diagnostic technology and imaging.10,11
The liver is a common site of metastasis, but it is an exceptionally rare location for a primary tumor.
HOW SHOULD THIS PATIENT BE MANAGED?
3. What is the appropriate management of rectal carcinoid in this patient?
- Since the nodule is 1.0 cm or smaller, watchful waiting is acceptable
- Since the nodule is 1.0 cm or smaller, local excision is appropriate, and no follow-up is required
- Because all carcinoid tumors are potentially malignant, radical resection (eg, abdominal perineal resection) is appropriate
- Because all carcinoid tumors are potentially malignant, radical resection with chemotherapy with 5-fluorouracil (Adrucil) and doxorubicin (Adriamycin) is required
Since the nodule is 1.0 cm or smaller, local excision is appropriate, and no follow-up will be required. Rectal carcinoid tumors generally have a favorable prognosis, with a 5-year survival rate of 87.5%.10
PROGNOSIS DEPENDS ON TUMOR SIZE, OTHER FACTORS
Many studies have examined risk factors contributing to poor prognosis, and this is an area of active study. Early research categorized rectal carcinoid risk in terms of tumor diameter, and this is still widely used to guide management. As early as 1959, Hanley et al5 recognized that tumors that were likely to metastasize were often larger than 1 cm, had infiltrated the muscularis, or were ulcerated. Today, it is understood that only 3% to 10% of rectal carcinoids smaller than 1 cm metastasize, whereas 17% to 42% of those 1 to 2 cm and 60% to 80% of those larger than 2 cm do.2,8,12,13
However, size is not the only consideration. Wang et al12 showed that muscular invasion is an independent risk factor for survival, and that tumor diameter is a significant predictor of invasion and metastasis. Similarly, a metaanalysis by Mani et al13 recognized tumor size and muscularis invasion as the most important predictors of malignancy in these neoplasms.
To aid in predicting prognosis, staging systems have been developed from institutional or national registries. Landry et al14 developed a TNM (tumor, node, metastasis) staging system for rectal carcinoids, in which the T value was based on tumor size and degree of invasion. A group at Memorial-Sloan Kettering Cancer Center15 developed a system for risk stratification of carcinoid of the rectum that is based on tumor size, muscularis invasion, lymphovascular invasion, and the mitotic rate.
TREATMENT IS BY EXCISION
Despite these new prognostic systems, there is no new guidance on therapeutic management. Surgical therapy is still largely guided by tumor size.
Lesions smaller than 1 cm are resected endoscopically or by another local transanal technique.2,3,15,16 Standard endoscopic mucosal resection is performed, and recent studies have suggested that endoscopic submucosal dissection is as effective17 or even preferred, because it resects to the deeper submucosa (as the name suggests).18 This en bloc technique may be appropriate for lesions with evidence of local invasion.18 Other situations may call for deeper resection, such as transanal resection for higher lesions and full-thickness mucosal-muscularis resection.
Tumors 1 to 2 cm are currently evaluated for other factors such as ulceration and umbilication, which influence the choice of local vs radical resection. Otherwise, there is little guidance for tumors of 1 to 2 cm.
Tumors larger than 2 cm have a high risk of muscularis invasion and metastasis, and hence they are resected with wide margins and imaging is then used to evaluate for metastasis.8,19 In cases of metastasis, local resection is often palliative, providing local symptom relief.19
AN INCIDENTALLY DISCOVERED CASE; PATIENT LOST TO FOLLOW-UP
Our patient’s case is typical of rectal carcinoid in that it was discovered incidentally during colonoscopy. His clinical presentation was likely unrelated to his carcinoid tumor, and he improved without specific treatment. His symptoms resolved within 24 hours with supportive treatment and he was discharged.
Pathologic confirmation of carcinoid tumor occurred after his discharge. Despite persistent attempts to contact the patient, he never returned for a follow-up appointment.
TAKE-HOME POINTS
- Carcinoid tumors are rare neoplasms of neuroendocrine origin.
- Rectal carcinoids are the third most common carcinoid of the gastrointestinal tract.
- Most rectal carcinoids are asymptomatic.
- Diagnosis is most often incidental and histologic.
- Treatment is by excision.
- Prognosis is favorable for smaller carcinoids and depends on size (and therefore, invasion).
- Thorson A, Biorck G, Bjorkman G, Waldenstrom J. Malignant carcinoid of the small intestine with metastases to the liver, valvular disease of the right side of the heart (pulmonary stenosis and tricuspid regurgitation without septal defects), peripheral vasomotor symptoms, bronchoconstriction, and an unusual type of cyanosis; a clinical and pathologic syndrome. Am Heart J 1954; 47:795–817.
- Wang AY, Ahmad NA. Rectal carcinoids. Curr Opin Gastroenterol 2006; 22:529–535.
- Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology 2005; 128:1717–1751.
- Aggarwal G, Obideen K, Wehbi M. Carcinoid tumors: what should increase our suspicion? Cleve Clin J Med 2008; 75:849–855.
- Hanley PH, Hines MO, Ray J, Armstrong R. Carcinoid tumors of the rectum. Experience with 26 cases. Proc R Soc Med 1959; 52(suppl):113–117.
- Pasieka JL. Carcinoid tumors. Surg Clin North Am 2009; 89:1123–1137.
- Jetmore AB, Ray JE, Gathright JB, McMullen KM, Hicks TC, Timmcke AE. Rectal carcinoids: the most frequent carcinoid tumor. Dis Colon Rectum 1992; 35:717–725.
- Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy 2009; 41:162–165.
- Wilander E, Lundqvist M, Oberg K. Gastrointestinal carcinoid tumours. Histogenetic, histochemical, immunohistochemical, clinical and therapeutic aspects. Prog Histochem Cytochem 1989; 19:1–88.
- Maggard MA, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg 2004; 240:117–122.
- Modlin IM, Sandor A. An analyisis of 8,305 cases of carcinoid tumors. Cancer 1997; 79:813–829.
- Wang M, Peng J, Yang W, Chen W, Mo S, Cai S. Prognostic analysis for carcinoid tumors of the rectum: a single institutional analysis of 106 cases. Colorectal Dis 2009; Epub ahead of print.
- Mani S, Modlin IM, Ballantyne G, Ahlman H, West B. Carcinoids of the rectum. J Am Coll Surg 1994; 179:231–248.
- Landry CS, Brock G, Scoggins CR, McMasters KM, Martin RC. A proposed staging system for rectal carcinoid tumors based on an analysis of 4701 patients. Surgery 2008; 144:460–466.
- Fahy BN, Tang LH, Klimstra D, et al. Carcinoid of the rectum risk stratification (CaRRs): a strategy for preoperative outcome assessment. Ann Surg Oncol 2007; 14:1735–1743.
- Shirouzu K, Isomoto H, Kakegawa T, Morimatsu M. Treatment of rectal carcinoid tumors. Am J Surg 1990; 160:262–265.
- Baek IH. Endoscopic submucosal dissection or conventional endoscopic mucosal resection is an effective and safe treatment for rectal carcinoid tumors: a retrospective study. J Laparoendosc Adv Surg Tech A 2010; 20:329–331.
- Yamaguchi N, Isomoto H, Nishiyama H, et al. Endoscopic submucosal dissection for rectal carcinoid tumors. Surg Endosc 2010; 24:504–508.
- Ramage JK, Goretzki PE, Manfredi R, et al; Frascati Consensus Conference participants. Consensus guidelines for the management of patients with digestive neuroendocrine tumours: well-differentiated colon and rectum tumour/carcinoma. Neuroendocrinology 2008; 87:31–39.
- Thorson A, Biorck G, Bjorkman G, Waldenstrom J. Malignant carcinoid of the small intestine with metastases to the liver, valvular disease of the right side of the heart (pulmonary stenosis and tricuspid regurgitation without septal defects), peripheral vasomotor symptoms, bronchoconstriction, and an unusual type of cyanosis; a clinical and pathologic syndrome. Am Heart J 1954; 47:795–817.
- Wang AY, Ahmad NA. Rectal carcinoids. Curr Opin Gastroenterol 2006; 22:529–535.
- Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology 2005; 128:1717–1751.
- Aggarwal G, Obideen K, Wehbi M. Carcinoid tumors: what should increase our suspicion? Cleve Clin J Med 2008; 75:849–855.
- Hanley PH, Hines MO, Ray J, Armstrong R. Carcinoid tumors of the rectum. Experience with 26 cases. Proc R Soc Med 1959; 52(suppl):113–117.
- Pasieka JL. Carcinoid tumors. Surg Clin North Am 2009; 89:1123–1137.
- Jetmore AB, Ray JE, Gathright JB, McMullen KM, Hicks TC, Timmcke AE. Rectal carcinoids: the most frequent carcinoid tumor. Dis Colon Rectum 1992; 35:717–725.
- Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy 2009; 41:162–165.
- Wilander E, Lundqvist M, Oberg K. Gastrointestinal carcinoid tumours. Histogenetic, histochemical, immunohistochemical, clinical and therapeutic aspects. Prog Histochem Cytochem 1989; 19:1–88.
- Maggard MA, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg 2004; 240:117–122.
- Modlin IM, Sandor A. An analyisis of 8,305 cases of carcinoid tumors. Cancer 1997; 79:813–829.
- Wang M, Peng J, Yang W, Chen W, Mo S, Cai S. Prognostic analysis for carcinoid tumors of the rectum: a single institutional analysis of 106 cases. Colorectal Dis 2009; Epub ahead of print.
- Mani S, Modlin IM, Ballantyne G, Ahlman H, West B. Carcinoids of the rectum. J Am Coll Surg 1994; 179:231–248.
- Landry CS, Brock G, Scoggins CR, McMasters KM, Martin RC. A proposed staging system for rectal carcinoid tumors based on an analysis of 4701 patients. Surgery 2008; 144:460–466.
- Fahy BN, Tang LH, Klimstra D, et al. Carcinoid of the rectum risk stratification (CaRRs): a strategy for preoperative outcome assessment. Ann Surg Oncol 2007; 14:1735–1743.
- Shirouzu K, Isomoto H, Kakegawa T, Morimatsu M. Treatment of rectal carcinoid tumors. Am J Surg 1990; 160:262–265.
- Baek IH. Endoscopic submucosal dissection or conventional endoscopic mucosal resection is an effective and safe treatment for rectal carcinoid tumors: a retrospective study. J Laparoendosc Adv Surg Tech A 2010; 20:329–331.
- Yamaguchi N, Isomoto H, Nishiyama H, et al. Endoscopic submucosal dissection for rectal carcinoid tumors. Surg Endosc 2010; 24:504–508.
- Ramage JK, Goretzki PE, Manfredi R, et al; Frascati Consensus Conference participants. Consensus guidelines for the management of patients with digestive neuroendocrine tumours: well-differentiated colon and rectum tumour/carcinoma. Neuroendocrinology 2008; 87:31–39.