User login
Staging System
A revolutionary new staging system for lung cancer will unite clinicians from different specialties and nations in characterizing tumor characteristics, node involvement, and metastasis, says Dr. Peter Goldstraw. Betsy Bates of the Global Medical News Network (GMNN) reports from the World Conference on Lung Cancer in San Francisco.
A revolutionary new staging system for lung cancer will unite clinicians from different specialties and nations in characterizing tumor characteristics, node involvement, and metastasis, says Dr. Peter Goldstraw. Betsy Bates of the Global Medical News Network (GMNN) reports from the World Conference on Lung Cancer in San Francisco.
A revolutionary new staging system for lung cancer will unite clinicians from different specialties and nations in characterizing tumor characteristics, node involvement, and metastasis, says Dr. Peter Goldstraw. Betsy Bates of the Global Medical News Network (GMNN) reports from the World Conference on Lung Cancer in San Francisco.
Social Work
Editors’ note: “Alliances” is a new series written about the relationships that hospitalists have with members of the clinical care team—from the team members’ points of view. It’s our hope that each installment of “Alliances” will provide valuable, revealing feedback that hospitalists can use to continually improve their intrateam relationships and, ultimately, patient care.
Social workers are a natural fit with hospitalists and the hospitalist’s strongest allies and staunchest supporters, wrote Bradley Flansbaum, DO, MPH, in his Nov./Dec. 2003 article in The Hospitalist. What makes this collaboration such a positive one and what can members of these two professions learn from each other?
Dr. Flansbaum, a hospitalist and internist with the Division of Internal Medicine/Primary Care at Lenox Hill Hospital, Bronx, N.Y., and a former SHM board member, recently reiterated the benefits of the hospitalist-social worker relationship. In general, he believes that hospitalists provide a unique history-taking perspective that is useful to social workers in their work. Foremost, social workers bring a rich understanding of the available resources that patients need after discharge and a view of the patient’s nonmedical circumstances. Together, the two professionals’ daily interactions generate more effective discharge planning as a part of the multidisciplinary team.
ALWAYS THERE
Amy Lingg, MS, MPA, works on the general medicine unit at Greenwich Hospital (Conn). She says the role of the hospitalist is fairly new at Greenwich. In fact Sabitha Rajan, MD, MS, was the first one at Greenwich Hospital.
In Lingg’s view, nothing can replace the availability of the hospitalist to discuss patient cases, not only with the social worker but also as a team with the patient and family.
“[Attendings] are not there for the moment-by-moment events that happen on the unit, including availability when families are here,” says Lingg. “If I need to speak with a family and the physician’s input is important there, I can just page the hospitalist, she’s here. Whereas with an attending you have to make an appointment; you have to schedule around them. It can become difficult.”
Lingg, who works with hospitalist Dr. Rajan, director of hospitalist services at Greenwich Hospital, cites an example of the benefits of hospitalists’ 24/7 availability: “We had a fairly young woman in her mid-40s who was the divorced mother of a 17-year-old son. The father was not in the picture, and the woman was dying of alcoholic cirrhosis and liver failure. She was Dr. Rajan’s patient. One of the issues was the fact that there was no adult guardian for the son although he was going to be 18 in two months.
“So it involved a lot of talking with friends of the woman, who were sort of stepping in as surrogate guardians to him,” Lingg continues. “There were a lot of logistics [regarding] what would happen with him. We were trying to call the grandfather who was estranged. It was a very, very sensitive, very, very tricky case. It went on for days and days. … Dr. Rajan and I could work on this together on a dayto-day basis, [including] … the counseling, relaying medical knowledge to the family, what was going on clinically, trying to deal with that in a way where she was talking in one way to [the] adults and in a different, more appropriate [for the boy’s age] way to the 17-yearold son. And I can be there to help with that process.”
The situation was resolved to the satisfaction of the mother, the son, the friends, and the providers. “It was really pretty extraordinary,” she said. “I’ve talked about that a couple of times, including at a staff meetings when we were talking about getting new hospitalists. That is something I’ve described because, really, it was very special.”
TRUE TEAMWORK
Although everyone on a multidisciplinary team can bring something to the discussion that makes the team work better, social workers and hospitalists collaborate well in painting a more comprehensive picture of the patient’s lifestyle, living habits, and needs.
“In many hospitals … there’s a pattern that develops [whereby at] some time in the morning the hospitalist and social worker will get together and talk,” says Dr. Flansbaum “The hospitalist speaks the language of the social worker and knows what to tell them and how to direct them rather than just saying, ‘the patient’s homeless or the patient needs help at home.’”
After working regularly with social workers and recognizing what they need to know, he says, “the hospitalist is more likely to say, ‘the patient has Medicaid,’ or ‘the patient has this insurance,’ [or] ‘the patient has a home-health [caregiver] four hours a day and needs six or eight hours a day,’ or ‘the patient’s going to need a subacute nursing facility.’ … I think our insights are different from voluntary physicians and our face-time with social workers is more efficient.”
Sylvia Krafcik, MSSA, LISW, with MetroHealth Medical Center, Cleveland, says hospitalists are “great to work with because they’re very dedicated to the population they’re caring for, because this is their whole responsibility; they don’t have a private caseload.”
But in her view most hospitalists are focused on patients’ medical conditions and some of them are not as tuned in to all the other aspects of the patient, such as all the psychosocial dynamics.
“A lot of them are, but some aren’t,” Krafcik says. “Especially at MetroHealth, we’re a county hospital. So many of the patients that come here are poor. A lot of them are alcoholics or drug abusers. They’re homeless. They live on the streets. They don’t have a primary doctor. They’re usually not compliant with their medications.”
“Here at Metro we have a lot of patients who have extreme social circumstances that affect their medical issues so much,” says Sara Dunson, MSW, LSW, who also works as a social worker at Metro-Health. “I think the hospitalist has greater insight into the person’s environment and all the social structures that they have at home and that are going on in their life [than other physicians might].”
But there is always room for improvement.
“We had one patient who wasn’t able to read, and he never told anybody this,” says Dunson. “And as social workers, we have more of a way of finding that kind of stuff out from patients than the doctors might. And he kept coming in and coming in and was noncompliant with his medication. We eventually determined that this was why he was noncompliant and was causing all these medical issues. The doctors finally [understood] why this gentleman kept coming in with the same problems and he wasn’t taking care of himself. It wasn’t that he didn’t want to, it was just that he was having problems reading all the medications and all the discharge paperwork, and he was too ashamed to tell anybody. [Once the social workers questioned him and got this] out in the open, we were able to get him help with that.”
The doctors focused on what he was or wasn’t doing, but they hadn’t looked at why he wasn’t adherent, explains Dunson. If hospitalists do that more often, she thinks, they could save time and get better outcomes sooner.
COMMUNICATING WITH PATIENTS AND FAMILIES
“I think where hospitalists are coming from is a whole different mindset than a physician who has mainly an office practice,” said Lingg. “The office practice comes first [for them]. Some of our physicians have huge practices in town. And they’ll visit the hospital very early in the morning or in the evening. ... So if I need something in a case like that, if there was not a hospitalist involved, it would have been separate meetings for the family with the physician … and [with] me at another time.”
To hospitalists, a social worker can serve as an important adjunct in talking to the patient and family. “For example, if [social workers] are giving bad news, they warn the physician first,” says Dr. Rajan. “If they’re going to go in and tell the patient that they’re not going to qualify for any home services, they tell the physician as well so that [the hospitalist will not later be] meeting an angry patient.” In addition, she says, “for critically ill or long-term patients, social workers [can] help family members cope. Sometimes as physicians we don’t have the time or we don’t have the resources to do that.”
But this doesn’t let doctors off the hook in regard to addressing the whole person’s needs. Especially if someone has multiple medical problems, the social worker needs to know the availability and level of support for which the family can be counted.
“Social workers will ask questions such as: Are the families involved? or Is there any family?” says Krafcik. “Do they need to go in a nursing home or do they need 24-hour care at home? Is the family able to provide that? [E]very morning we meet to have team rounds. And the [team] go[es] over every patient on the floor, and then I will ask those questions if the doctor hasn’t given me that information.”
Social workers appreciate and would like hospitalists to do more listening to the patient and family for the aspects of the history and psychosocial status that the social worker will need to know.
TEACHING POINTS
In the course of their interactions, what do hospitalists and social workers teach each other that could lead to working a case more effectively and to the greater satisfaction of all involved?
Most of those we interviewed seem to think that the greatest service hospitalists provide is to teach the social worker the medical components that go along with what the social worker does every day.
“[Social workers] get a better understanding of [whether] someone comes in with heart failure or a fall or a stroke, just by repetition and also education; they get to understand after a while what’s needed for individual medical diagnoses,” says Dr. Flansbaum.
“When I know [better] what the medical condition is,” says Krafcik, “I have an idea of how much help [the patient] would need at home and their ability to function. And I would make sure that the patient gets physical therapy or occupational therapy referral or speech therapy.”
Again, perhaps the area where the social worker most teaches the hospitalist regards available resources to solve problems over and above the purely medical. “They know the social system and the needs of different forms and eligibility and what different patients are entitled to and what the system will provide,” says Dr. Flansbaum.
Dunson believes hospitalists are perceived as being more involved in a holistic way with the patient. “I always stress that it is so important to look at the whole person and not just the medical aspects,” she says. “It’s hard for the doctor sometimes to realize that this person might not be able to afford this medication and that’s why they’re noncompliant and all the other issues. So I think is important to open up to the other aspects of a person’s life and not just the medical aspects.”
CONCLUSION
Social workers’ knowledge of medical and nonmedical resources, both locally and nationally, offer hospitalists essential information that leads to designing more appropriate and effective post-discharge plans. Hospitalists can best team with social workers by consistently keeping in mind the patient’s overall circumstances and informing their colleagues of the medical information that can help social workers do their best work. TH
Writer Andrea Sattinger will write about the effect of poor communication skills in the November issue of The Hospitalist.
Editors’ note: “Alliances” is a new series written about the relationships that hospitalists have with members of the clinical care team—from the team members’ points of view. It’s our hope that each installment of “Alliances” will provide valuable, revealing feedback that hospitalists can use to continually improve their intrateam relationships and, ultimately, patient care.
Social workers are a natural fit with hospitalists and the hospitalist’s strongest allies and staunchest supporters, wrote Bradley Flansbaum, DO, MPH, in his Nov./Dec. 2003 article in The Hospitalist. What makes this collaboration such a positive one and what can members of these two professions learn from each other?
Dr. Flansbaum, a hospitalist and internist with the Division of Internal Medicine/Primary Care at Lenox Hill Hospital, Bronx, N.Y., and a former SHM board member, recently reiterated the benefits of the hospitalist-social worker relationship. In general, he believes that hospitalists provide a unique history-taking perspective that is useful to social workers in their work. Foremost, social workers bring a rich understanding of the available resources that patients need after discharge and a view of the patient’s nonmedical circumstances. Together, the two professionals’ daily interactions generate more effective discharge planning as a part of the multidisciplinary team.
ALWAYS THERE
Amy Lingg, MS, MPA, works on the general medicine unit at Greenwich Hospital (Conn). She says the role of the hospitalist is fairly new at Greenwich. In fact Sabitha Rajan, MD, MS, was the first one at Greenwich Hospital.
In Lingg’s view, nothing can replace the availability of the hospitalist to discuss patient cases, not only with the social worker but also as a team with the patient and family.
“[Attendings] are not there for the moment-by-moment events that happen on the unit, including availability when families are here,” says Lingg. “If I need to speak with a family and the physician’s input is important there, I can just page the hospitalist, she’s here. Whereas with an attending you have to make an appointment; you have to schedule around them. It can become difficult.”
Lingg, who works with hospitalist Dr. Rajan, director of hospitalist services at Greenwich Hospital, cites an example of the benefits of hospitalists’ 24/7 availability: “We had a fairly young woman in her mid-40s who was the divorced mother of a 17-year-old son. The father was not in the picture, and the woman was dying of alcoholic cirrhosis and liver failure. She was Dr. Rajan’s patient. One of the issues was the fact that there was no adult guardian for the son although he was going to be 18 in two months.
“So it involved a lot of talking with friends of the woman, who were sort of stepping in as surrogate guardians to him,” Lingg continues. “There were a lot of logistics [regarding] what would happen with him. We were trying to call the grandfather who was estranged. It was a very, very sensitive, very, very tricky case. It went on for days and days. … Dr. Rajan and I could work on this together on a dayto-day basis, [including] … the counseling, relaying medical knowledge to the family, what was going on clinically, trying to deal with that in a way where she was talking in one way to [the] adults and in a different, more appropriate [for the boy’s age] way to the 17-yearold son. And I can be there to help with that process.”
The situation was resolved to the satisfaction of the mother, the son, the friends, and the providers. “It was really pretty extraordinary,” she said. “I’ve talked about that a couple of times, including at a staff meetings when we were talking about getting new hospitalists. That is something I’ve described because, really, it was very special.”
TRUE TEAMWORK
Although everyone on a multidisciplinary team can bring something to the discussion that makes the team work better, social workers and hospitalists collaborate well in painting a more comprehensive picture of the patient’s lifestyle, living habits, and needs.
“In many hospitals … there’s a pattern that develops [whereby at] some time in the morning the hospitalist and social worker will get together and talk,” says Dr. Flansbaum “The hospitalist speaks the language of the social worker and knows what to tell them and how to direct them rather than just saying, ‘the patient’s homeless or the patient needs help at home.’”
After working regularly with social workers and recognizing what they need to know, he says, “the hospitalist is more likely to say, ‘the patient has Medicaid,’ or ‘the patient has this insurance,’ [or] ‘the patient has a home-health [caregiver] four hours a day and needs six or eight hours a day,’ or ‘the patient’s going to need a subacute nursing facility.’ … I think our insights are different from voluntary physicians and our face-time with social workers is more efficient.”
Sylvia Krafcik, MSSA, LISW, with MetroHealth Medical Center, Cleveland, says hospitalists are “great to work with because they’re very dedicated to the population they’re caring for, because this is their whole responsibility; they don’t have a private caseload.”
But in her view most hospitalists are focused on patients’ medical conditions and some of them are not as tuned in to all the other aspects of the patient, such as all the psychosocial dynamics.
“A lot of them are, but some aren’t,” Krafcik says. “Especially at MetroHealth, we’re a county hospital. So many of the patients that come here are poor. A lot of them are alcoholics or drug abusers. They’re homeless. They live on the streets. They don’t have a primary doctor. They’re usually not compliant with their medications.”
“Here at Metro we have a lot of patients who have extreme social circumstances that affect their medical issues so much,” says Sara Dunson, MSW, LSW, who also works as a social worker at Metro-Health. “I think the hospitalist has greater insight into the person’s environment and all the social structures that they have at home and that are going on in their life [than other physicians might].”
But there is always room for improvement.
“We had one patient who wasn’t able to read, and he never told anybody this,” says Dunson. “And as social workers, we have more of a way of finding that kind of stuff out from patients than the doctors might. And he kept coming in and coming in and was noncompliant with his medication. We eventually determined that this was why he was noncompliant and was causing all these medical issues. The doctors finally [understood] why this gentleman kept coming in with the same problems and he wasn’t taking care of himself. It wasn’t that he didn’t want to, it was just that he was having problems reading all the medications and all the discharge paperwork, and he was too ashamed to tell anybody. [Once the social workers questioned him and got this] out in the open, we were able to get him help with that.”
The doctors focused on what he was or wasn’t doing, but they hadn’t looked at why he wasn’t adherent, explains Dunson. If hospitalists do that more often, she thinks, they could save time and get better outcomes sooner.
COMMUNICATING WITH PATIENTS AND FAMILIES
“I think where hospitalists are coming from is a whole different mindset than a physician who has mainly an office practice,” said Lingg. “The office practice comes first [for them]. Some of our physicians have huge practices in town. And they’ll visit the hospital very early in the morning or in the evening. ... So if I need something in a case like that, if there was not a hospitalist involved, it would have been separate meetings for the family with the physician … and [with] me at another time.”
To hospitalists, a social worker can serve as an important adjunct in talking to the patient and family. “For example, if [social workers] are giving bad news, they warn the physician first,” says Dr. Rajan. “If they’re going to go in and tell the patient that they’re not going to qualify for any home services, they tell the physician as well so that [the hospitalist will not later be] meeting an angry patient.” In addition, she says, “for critically ill or long-term patients, social workers [can] help family members cope. Sometimes as physicians we don’t have the time or we don’t have the resources to do that.”
But this doesn’t let doctors off the hook in regard to addressing the whole person’s needs. Especially if someone has multiple medical problems, the social worker needs to know the availability and level of support for which the family can be counted.
“Social workers will ask questions such as: Are the families involved? or Is there any family?” says Krafcik. “Do they need to go in a nursing home or do they need 24-hour care at home? Is the family able to provide that? [E]very morning we meet to have team rounds. And the [team] go[es] over every patient on the floor, and then I will ask those questions if the doctor hasn’t given me that information.”
Social workers appreciate and would like hospitalists to do more listening to the patient and family for the aspects of the history and psychosocial status that the social worker will need to know.
TEACHING POINTS
In the course of their interactions, what do hospitalists and social workers teach each other that could lead to working a case more effectively and to the greater satisfaction of all involved?
Most of those we interviewed seem to think that the greatest service hospitalists provide is to teach the social worker the medical components that go along with what the social worker does every day.
“[Social workers] get a better understanding of [whether] someone comes in with heart failure or a fall or a stroke, just by repetition and also education; they get to understand after a while what’s needed for individual medical diagnoses,” says Dr. Flansbaum.
“When I know [better] what the medical condition is,” says Krafcik, “I have an idea of how much help [the patient] would need at home and their ability to function. And I would make sure that the patient gets physical therapy or occupational therapy referral or speech therapy.”
Again, perhaps the area where the social worker most teaches the hospitalist regards available resources to solve problems over and above the purely medical. “They know the social system and the needs of different forms and eligibility and what different patients are entitled to and what the system will provide,” says Dr. Flansbaum.
Dunson believes hospitalists are perceived as being more involved in a holistic way with the patient. “I always stress that it is so important to look at the whole person and not just the medical aspects,” she says. “It’s hard for the doctor sometimes to realize that this person might not be able to afford this medication and that’s why they’re noncompliant and all the other issues. So I think is important to open up to the other aspects of a person’s life and not just the medical aspects.”
CONCLUSION
Social workers’ knowledge of medical and nonmedical resources, both locally and nationally, offer hospitalists essential information that leads to designing more appropriate and effective post-discharge plans. Hospitalists can best team with social workers by consistently keeping in mind the patient’s overall circumstances and informing their colleagues of the medical information that can help social workers do their best work. TH
Writer Andrea Sattinger will write about the effect of poor communication skills in the November issue of The Hospitalist.
Editors’ note: “Alliances” is a new series written about the relationships that hospitalists have with members of the clinical care team—from the team members’ points of view. It’s our hope that each installment of “Alliances” will provide valuable, revealing feedback that hospitalists can use to continually improve their intrateam relationships and, ultimately, patient care.
Social workers are a natural fit with hospitalists and the hospitalist’s strongest allies and staunchest supporters, wrote Bradley Flansbaum, DO, MPH, in his Nov./Dec. 2003 article in The Hospitalist. What makes this collaboration such a positive one and what can members of these two professions learn from each other?
Dr. Flansbaum, a hospitalist and internist with the Division of Internal Medicine/Primary Care at Lenox Hill Hospital, Bronx, N.Y., and a former SHM board member, recently reiterated the benefits of the hospitalist-social worker relationship. In general, he believes that hospitalists provide a unique history-taking perspective that is useful to social workers in their work. Foremost, social workers bring a rich understanding of the available resources that patients need after discharge and a view of the patient’s nonmedical circumstances. Together, the two professionals’ daily interactions generate more effective discharge planning as a part of the multidisciplinary team.
ALWAYS THERE
Amy Lingg, MS, MPA, works on the general medicine unit at Greenwich Hospital (Conn). She says the role of the hospitalist is fairly new at Greenwich. In fact Sabitha Rajan, MD, MS, was the first one at Greenwich Hospital.
In Lingg’s view, nothing can replace the availability of the hospitalist to discuss patient cases, not only with the social worker but also as a team with the patient and family.
“[Attendings] are not there for the moment-by-moment events that happen on the unit, including availability when families are here,” says Lingg. “If I need to speak with a family and the physician’s input is important there, I can just page the hospitalist, she’s here. Whereas with an attending you have to make an appointment; you have to schedule around them. It can become difficult.”
Lingg, who works with hospitalist Dr. Rajan, director of hospitalist services at Greenwich Hospital, cites an example of the benefits of hospitalists’ 24/7 availability: “We had a fairly young woman in her mid-40s who was the divorced mother of a 17-year-old son. The father was not in the picture, and the woman was dying of alcoholic cirrhosis and liver failure. She was Dr. Rajan’s patient. One of the issues was the fact that there was no adult guardian for the son although he was going to be 18 in two months.
“So it involved a lot of talking with friends of the woman, who were sort of stepping in as surrogate guardians to him,” Lingg continues. “There were a lot of logistics [regarding] what would happen with him. We were trying to call the grandfather who was estranged. It was a very, very sensitive, very, very tricky case. It went on for days and days. … Dr. Rajan and I could work on this together on a dayto-day basis, [including] … the counseling, relaying medical knowledge to the family, what was going on clinically, trying to deal with that in a way where she was talking in one way to [the] adults and in a different, more appropriate [for the boy’s age] way to the 17-yearold son. And I can be there to help with that process.”
The situation was resolved to the satisfaction of the mother, the son, the friends, and the providers. “It was really pretty extraordinary,” she said. “I’ve talked about that a couple of times, including at a staff meetings when we were talking about getting new hospitalists. That is something I’ve described because, really, it was very special.”
TRUE TEAMWORK
Although everyone on a multidisciplinary team can bring something to the discussion that makes the team work better, social workers and hospitalists collaborate well in painting a more comprehensive picture of the patient’s lifestyle, living habits, and needs.
“In many hospitals … there’s a pattern that develops [whereby at] some time in the morning the hospitalist and social worker will get together and talk,” says Dr. Flansbaum “The hospitalist speaks the language of the social worker and knows what to tell them and how to direct them rather than just saying, ‘the patient’s homeless or the patient needs help at home.’”
After working regularly with social workers and recognizing what they need to know, he says, “the hospitalist is more likely to say, ‘the patient has Medicaid,’ or ‘the patient has this insurance,’ [or] ‘the patient has a home-health [caregiver] four hours a day and needs six or eight hours a day,’ or ‘the patient’s going to need a subacute nursing facility.’ … I think our insights are different from voluntary physicians and our face-time with social workers is more efficient.”
Sylvia Krafcik, MSSA, LISW, with MetroHealth Medical Center, Cleveland, says hospitalists are “great to work with because they’re very dedicated to the population they’re caring for, because this is their whole responsibility; they don’t have a private caseload.”
But in her view most hospitalists are focused on patients’ medical conditions and some of them are not as tuned in to all the other aspects of the patient, such as all the psychosocial dynamics.
“A lot of them are, but some aren’t,” Krafcik says. “Especially at MetroHealth, we’re a county hospital. So many of the patients that come here are poor. A lot of them are alcoholics or drug abusers. They’re homeless. They live on the streets. They don’t have a primary doctor. They’re usually not compliant with their medications.”
“Here at Metro we have a lot of patients who have extreme social circumstances that affect their medical issues so much,” says Sara Dunson, MSW, LSW, who also works as a social worker at Metro-Health. “I think the hospitalist has greater insight into the person’s environment and all the social structures that they have at home and that are going on in their life [than other physicians might].”
But there is always room for improvement.
“We had one patient who wasn’t able to read, and he never told anybody this,” says Dunson. “And as social workers, we have more of a way of finding that kind of stuff out from patients than the doctors might. And he kept coming in and coming in and was noncompliant with his medication. We eventually determined that this was why he was noncompliant and was causing all these medical issues. The doctors finally [understood] why this gentleman kept coming in with the same problems and he wasn’t taking care of himself. It wasn’t that he didn’t want to, it was just that he was having problems reading all the medications and all the discharge paperwork, and he was too ashamed to tell anybody. [Once the social workers questioned him and got this] out in the open, we were able to get him help with that.”
The doctors focused on what he was or wasn’t doing, but they hadn’t looked at why he wasn’t adherent, explains Dunson. If hospitalists do that more often, she thinks, they could save time and get better outcomes sooner.
COMMUNICATING WITH PATIENTS AND FAMILIES
“I think where hospitalists are coming from is a whole different mindset than a physician who has mainly an office practice,” said Lingg. “The office practice comes first [for them]. Some of our physicians have huge practices in town. And they’ll visit the hospital very early in the morning or in the evening. ... So if I need something in a case like that, if there was not a hospitalist involved, it would have been separate meetings for the family with the physician … and [with] me at another time.”
To hospitalists, a social worker can serve as an important adjunct in talking to the patient and family. “For example, if [social workers] are giving bad news, they warn the physician first,” says Dr. Rajan. “If they’re going to go in and tell the patient that they’re not going to qualify for any home services, they tell the physician as well so that [the hospitalist will not later be] meeting an angry patient.” In addition, she says, “for critically ill or long-term patients, social workers [can] help family members cope. Sometimes as physicians we don’t have the time or we don’t have the resources to do that.”
But this doesn’t let doctors off the hook in regard to addressing the whole person’s needs. Especially if someone has multiple medical problems, the social worker needs to know the availability and level of support for which the family can be counted.
“Social workers will ask questions such as: Are the families involved? or Is there any family?” says Krafcik. “Do they need to go in a nursing home or do they need 24-hour care at home? Is the family able to provide that? [E]very morning we meet to have team rounds. And the [team] go[es] over every patient on the floor, and then I will ask those questions if the doctor hasn’t given me that information.”
Social workers appreciate and would like hospitalists to do more listening to the patient and family for the aspects of the history and psychosocial status that the social worker will need to know.
TEACHING POINTS
In the course of their interactions, what do hospitalists and social workers teach each other that could lead to working a case more effectively and to the greater satisfaction of all involved?
Most of those we interviewed seem to think that the greatest service hospitalists provide is to teach the social worker the medical components that go along with what the social worker does every day.
“[Social workers] get a better understanding of [whether] someone comes in with heart failure or a fall or a stroke, just by repetition and also education; they get to understand after a while what’s needed for individual medical diagnoses,” says Dr. Flansbaum.
“When I know [better] what the medical condition is,” says Krafcik, “I have an idea of how much help [the patient] would need at home and their ability to function. And I would make sure that the patient gets physical therapy or occupational therapy referral or speech therapy.”
Again, perhaps the area where the social worker most teaches the hospitalist regards available resources to solve problems over and above the purely medical. “They know the social system and the needs of different forms and eligibility and what different patients are entitled to and what the system will provide,” says Dr. Flansbaum.
Dunson believes hospitalists are perceived as being more involved in a holistic way with the patient. “I always stress that it is so important to look at the whole person and not just the medical aspects,” she says. “It’s hard for the doctor sometimes to realize that this person might not be able to afford this medication and that’s why they’re noncompliant and all the other issues. So I think is important to open up to the other aspects of a person’s life and not just the medical aspects.”
CONCLUSION
Social workers’ knowledge of medical and nonmedical resources, both locally and nationally, offer hospitalists essential information that leads to designing more appropriate and effective post-discharge plans. Hospitalists can best team with social workers by consistently keeping in mind the patient’s overall circumstances and informing their colleagues of the medical information that can help social workers do their best work. TH
Writer Andrea Sattinger will write about the effect of poor communication skills in the November issue of The Hospitalist.
Leadership Lessons
In more than 30 years as a healthcare industry consultant, Jack Silversin, DMD, DrPH, has watched as hospitals have evolved into complex organizations that emphasize efficiency, teamwork, and cost-effectiveness—three things absent from most medical training programs, he says.
“Doctors have been trained to be autonomous, but the new organizational structure is to be collective,” says Dr. Silversin, CEO of the Boston-based consulting firm Amicus Inc.
The changes doctors and HM groups are being asked to make are challenging their way of life and their work, Dr. Silversin says, and leading a group of independent-thinking hospitalists is no easy task.
“It’s a very challenging thing to be a leader. … It’s having the confidence and mind-set to engage people and make decisions,” says Dr. Silversin, who plans to address such issues during his daylong seminar at SHM’s Leadership Academy Sept. 14-17 in Miami.
Part of an expert faculty that teaches skills and concepts on beginner and advanced tracks, Dr. Silversin says Leadership Academy attendees learn how to define their roles and how to present their expectations to their groups.
“You go back to your hospital and see things in a different light,” he says. “You need to have the answers, but you need to balance that with relationships.”
The next Leadership Academy is Jan. 25-28 in Scottsdale, Ariz. For complete faculty bios and more information on participating, visit SHM’s events Web site.
In more than 30 years as a healthcare industry consultant, Jack Silversin, DMD, DrPH, has watched as hospitals have evolved into complex organizations that emphasize efficiency, teamwork, and cost-effectiveness—three things absent from most medical training programs, he says.
“Doctors have been trained to be autonomous, but the new organizational structure is to be collective,” says Dr. Silversin, CEO of the Boston-based consulting firm Amicus Inc.
The changes doctors and HM groups are being asked to make are challenging their way of life and their work, Dr. Silversin says, and leading a group of independent-thinking hospitalists is no easy task.
“It’s a very challenging thing to be a leader. … It’s having the confidence and mind-set to engage people and make decisions,” says Dr. Silversin, who plans to address such issues during his daylong seminar at SHM’s Leadership Academy Sept. 14-17 in Miami.
Part of an expert faculty that teaches skills and concepts on beginner and advanced tracks, Dr. Silversin says Leadership Academy attendees learn how to define their roles and how to present their expectations to their groups.
“You go back to your hospital and see things in a different light,” he says. “You need to have the answers, but you need to balance that with relationships.”
The next Leadership Academy is Jan. 25-28 in Scottsdale, Ariz. For complete faculty bios and more information on participating, visit SHM’s events Web site.
In more than 30 years as a healthcare industry consultant, Jack Silversin, DMD, DrPH, has watched as hospitals have evolved into complex organizations that emphasize efficiency, teamwork, and cost-effectiveness—three things absent from most medical training programs, he says.
“Doctors have been trained to be autonomous, but the new organizational structure is to be collective,” says Dr. Silversin, CEO of the Boston-based consulting firm Amicus Inc.
The changes doctors and HM groups are being asked to make are challenging their way of life and their work, Dr. Silversin says, and leading a group of independent-thinking hospitalists is no easy task.
“It’s a very challenging thing to be a leader. … It’s having the confidence and mind-set to engage people and make decisions,” says Dr. Silversin, who plans to address such issues during his daylong seminar at SHM’s Leadership Academy Sept. 14-17 in Miami.
Part of an expert faculty that teaches skills and concepts on beginner and advanced tracks, Dr. Silversin says Leadership Academy attendees learn how to define their roles and how to present their expectations to their groups.
“You go back to your hospital and see things in a different light,” he says. “You need to have the answers, but you need to balance that with relationships.”
The next Leadership Academy is Jan. 25-28 in Scottsdale, Ariz. For complete faculty bios and more information on participating, visit SHM’s events Web site.
The Happiness Factor
HM groups are built—in part—on the theory of work-life balance. But what about work-work balance?
A study published this spring found that faculty physicians at academic medical centers might be more satisfied if they spend at least one day per week on the part of their job that is most meaningful to them (Arch Intern Med, 2009;169(10):990-995).
“The notion of ‘job fit’ is clearly important,” says Noah Harris, MD, FHM, a hospitalist at Presbyterian Hospital in Albuquerque, N.M., and a member of SHM’s Career Satisfaction Task Force. “Since most physicians are drawn to medicine for the notion of patient care, the other activities may be troublesome for many of us.”
To improve employees’ job satisfaction, Dr. Harris and Chad Whelan, MD, FHM, chair of SHM’s career task force, suggest HM leaders do the following:
- Understand what your group has to offer. Let physicians explore parts of the practice unfamiliar to them—and if they find something they have a passion for, encourage it.
- Identify hospitalists who are at risk for burnout and guide them to potential opportunities. Be proactive before dissatisfaction sets in.
- Don’t push people into leadership roles they don’t want. Some people want clinical posts, while others want to be medical directors who meet with administration daily.
- Recognize the importance of flexibility. As HM groups evolve, there are chances to offer new schedules or build in new clinical and nonclinical initiatives.
- Support staff members via mentoring and professional development to make them feel as if they’re doing work they want to do.
“A common mistake, though, is to simply pay people a stipend for doing more,” says Dr. Whelan, associate professor of medicine and director of the division of hospital medicine at Loyola University Chicago Stritch School of Medicine. “If their professional time is already fully taken with other activities, a stipend will not provide time to appropriately pursue those meaningful activities.”
HM groups are built—in part—on the theory of work-life balance. But what about work-work balance?
A study published this spring found that faculty physicians at academic medical centers might be more satisfied if they spend at least one day per week on the part of their job that is most meaningful to them (Arch Intern Med, 2009;169(10):990-995).
“The notion of ‘job fit’ is clearly important,” says Noah Harris, MD, FHM, a hospitalist at Presbyterian Hospital in Albuquerque, N.M., and a member of SHM’s Career Satisfaction Task Force. “Since most physicians are drawn to medicine for the notion of patient care, the other activities may be troublesome for many of us.”
To improve employees’ job satisfaction, Dr. Harris and Chad Whelan, MD, FHM, chair of SHM’s career task force, suggest HM leaders do the following:
- Understand what your group has to offer. Let physicians explore parts of the practice unfamiliar to them—and if they find something they have a passion for, encourage it.
- Identify hospitalists who are at risk for burnout and guide them to potential opportunities. Be proactive before dissatisfaction sets in.
- Don’t push people into leadership roles they don’t want. Some people want clinical posts, while others want to be medical directors who meet with administration daily.
- Recognize the importance of flexibility. As HM groups evolve, there are chances to offer new schedules or build in new clinical and nonclinical initiatives.
- Support staff members via mentoring and professional development to make them feel as if they’re doing work they want to do.
“A common mistake, though, is to simply pay people a stipend for doing more,” says Dr. Whelan, associate professor of medicine and director of the division of hospital medicine at Loyola University Chicago Stritch School of Medicine. “If their professional time is already fully taken with other activities, a stipend will not provide time to appropriately pursue those meaningful activities.”
HM groups are built—in part—on the theory of work-life balance. But what about work-work balance?
A study published this spring found that faculty physicians at academic medical centers might be more satisfied if they spend at least one day per week on the part of their job that is most meaningful to them (Arch Intern Med, 2009;169(10):990-995).
“The notion of ‘job fit’ is clearly important,” says Noah Harris, MD, FHM, a hospitalist at Presbyterian Hospital in Albuquerque, N.M., and a member of SHM’s Career Satisfaction Task Force. “Since most physicians are drawn to medicine for the notion of patient care, the other activities may be troublesome for many of us.”
To improve employees’ job satisfaction, Dr. Harris and Chad Whelan, MD, FHM, chair of SHM’s career task force, suggest HM leaders do the following:
- Understand what your group has to offer. Let physicians explore parts of the practice unfamiliar to them—and if they find something they have a passion for, encourage it.
- Identify hospitalists who are at risk for burnout and guide them to potential opportunities. Be proactive before dissatisfaction sets in.
- Don’t push people into leadership roles they don’t want. Some people want clinical posts, while others want to be medical directors who meet with administration daily.
- Recognize the importance of flexibility. As HM groups evolve, there are chances to offer new schedules or build in new clinical and nonclinical initiatives.
- Support staff members via mentoring and professional development to make them feel as if they’re doing work they want to do.
“A common mistake, though, is to simply pay people a stipend for doing more,” says Dr. Whelan, associate professor of medicine and director of the division of hospital medicine at Loyola University Chicago Stritch School of Medicine. “If their professional time is already fully taken with other activities, a stipend will not provide time to appropriately pursue those meaningful activities.”
Antibiotics for MDR Pathogens
Case 1
A 53‐year‐old woman with a history of hemodialysis‐dependent end‐stage renal disease presents with left lower extremity pain and redness for the past 3 days. On physical examination, her temperature is 102.3F. Erythema, induration, and warmth are noted over her left lower leg and foot. Her history is remarkable for a line‐related bloodstream infection due to methicillin‐resistant Staphylococcus aureus (MRSA) 4 weeks ago. The infected line was removed and replaced with a right‐sided subclavian catheter. You note that the new line site is clean, not erythematous, and not tender. In the emergency department, the patient receives a dose of vancomycin for presumed MRSA cellulitis. Your patient wants to know if there are alternative agents for her infection so she does not require hospitalization.
Unfortunately, MRSA has become commonplace to the hospital setting. Among intensive care units in 2003, 64.4% of healthcare‐associated Staphylococcus aureus infections were caused by MRSA, compared with only 35.9% in 1992; a 3.1% increase per year.1, 2 Increased MRSA rates are not without consequence; a recent review suggests that MRSA infections kill nearly 19,000 hospitalized American patients annually.3 Of note, MRSA infection rates have also increased among previously healthy individuals. These community‐associated isolates (CA‐MRSA) often manifest as pyogenic skin and soft‐tissue infections (SSTIs). In a recent multicenter study, CA‐MRSA accounted for 59% of SSTIs among patients presenting to emergency rooms in the United States.4 In cases of SSTI, oral agents such as clindamycin, doxycycline, and trimethoprim‐sulfamethoxazole have proven successful. For invasive MRSA, vancomycin is still considered the standard treatment; however, several alternatives have emerged in recent years. The advantages and disadvantages of linezolid, daptomycin, tigecycline, and dalbavancin in the treatment of MRSA are described below.
Linezolid
Linezolid (Zyvox), an oxazolidinone approved in 2000, has been touted for its oral bioavailability, twice‐daily dosing, gram‐positive coverage, and unique mechanism of action. Like several other antimicrobials, linezolid inhibits bacterial protein synthesis. The drug binds to the 50S ribosomal subunit near its site of interaction with the 30S subunit, preventing formation of the 70S initiation complex.5 This site of action on the 50S subunit is unique to linezolid; as a result, cross‐resistance between linezolid and other antimicrobials that act at the 50S subunit (eg, chloramphenicol, macrolides, aminoglycosides, and tetracycline) does not occur.6
The oxazolidinones have excellent bacteriostatic activity against all pathogenic gram‐positive bacteria. The U.S. Food and Drug Administration (FDA) approved linezolid for the treatment of serious infections due to vancomycin‐resistant enterococci (VRE), including bacteremia, complicated skin and soft‐tissue infections (cSSTIs) due to Staphylococcus aureus (including MRSA), and nosocomial pneumonia due to Staphylococcus aureus (including MRSA) or penicillin‐susceptible Streptococcus pneumoniae (Table 1).
| Activity | Agent | FDA‐Approved Indications | Limitations in Use | Side Effects |
|---|---|---|---|---|
| ||||
| Gram‐positive | Daptomycin | cSSTIs; MSSA/MRSA bacteremia; MSSA/MRSA endocarditis | Not indicated for pneumonia (inhibited by pulmonary surfactant) | Reversible myopathy may be exacerbated by use with other medications |
| Quinupristin‐dalfopristin | Vancomycin‐resistant E. faecium; group A streptococci or MSSA cSSTIs | Myalgias and arthralgias; infusion site reaction;* thrombophlebitis;* liver enzyme elevation; inhibition of cytochrome p450 34a | ||
| Linezolid | Serious infections due to VRE; MSSA/MRSA cSSTIs; MSSA/MRSA nosocomial pneumonia; pneumonia due to penicillin‐sensitive S. pneumoniae | Not indicated for catheter‐related bloodstream infections or catheter site infections | Myelosuppression; serotonin syndrome; tyramine reaction; peripheral neuropathy; optic neuropathy | |
| Dalbavancin | Approval pending for cSSTIs | Not indicated for pneumonia bone and joint infection | Unknown | |
| Gram‐negative | Colistin | Gram‐negative bacteria that have demonstrated sensitivity to the drug | Not indicated for Proteus spp, Providencia spp, or Serratia spp | Acute tubular necrosis; neurotoxicity∥; bronchospasm |
| Gram‐positive and Gram‐negative | Ertapenem | Complicated intraabdominal infections#; cSSTIs; acute pelvic infections; complicated UTIs; community‐acquired pneumonia; prophylaxis of SSI following colorectal surgery in adult patients | Not indicated for Pseudomonas, Acinetobacter, S. maltophilia | Cross‐reactivity with penicillin; cross‐reactivity with cephalosporins; caution use if history of seizures |
| Doripenem | Complicated intraabdominal infections# and complicated UTIs, including pyelonephritis | Cross‐reactivity with penicillin; cross‐reactivity with cephalosporins; caution use if history of seizures | ||
| Tigecycline | cSSTIs (including those due to MRSA) complicated intraabdominal infections# | Nausea and vomiting; tooth discoloration in children | ||
In retrospective analyses of SSTIs due to MRSA, linezolid was as effective as vancomycin, resulting in higher clinical cure rates and shorter hospitalizations.7 As a result, linezolid has established a role in the treatment of community‐acquired MRSA SSTIs. Evidence limited to case reports and case series suggest that linezolid may also have a role in the treatment of bone and joint infections. In these cases, linezolid was often used because treatment with other agents had failed, the administration of other antibiotics was not indicated due to resistance patterns, the patient refused intravenous therapy, or the patient did not tolerate vancomycin. When such conditions exist, linezolid may be a consideration in cases of osteomyelitis or prosthetic joint infection.8
Potential side effects of linezolid may limit its use, especially for patients who require prolonged therapy (Table 1). Of note, as a reversible, relatively weak nonselective inhibitor of monoamine oxidase, linezolid may interact with adrenergic and serotonergic agents. Concomitant of a serotonin agent such as a selective serotonin‐reuptake inhibitor (SSRI) and linezolid should be approached with caution. Subsequent serotonin syndrome is characterized by autonomic dysfunction (eg, diaphoresis, tachycardia, hypertension) and neuromuscular hyperactivity (eg, muscle rigidity, clonus, hyperreflexia). Though infrequent, cases of reversible myelosuppression have been reported with linezolid use.9 Patients who will receive this drug for more than 2 weeks should be monitored for myelosuppression with a weekly complete blood count. Isolated reports suggest that the prolonged administration of linezolid (>28 days) may be associated with peripheral neuropathy and optic neuropathy. While prompt discontinuation of the drug often results in resolution of symptoms, peripheral or optic nerve injury can be permanent. The mechanism of injury is unclear, though mitochondrial toxicity is suspected.10
Daptomycin
Daptomycin (Cubicin), a cyclic lipopeptide, was discovered in the early 1980s, but skeletal muscle toxicity led to the discontinuation of early clinical trials. When a change from twice‐daily to once‐daily dosing in 2003 resulted in fewer adverse events, the FDA approved daptomycin to treat complicated skin and skin‐structure infections.11 Daptomycin binds to the cell membrane via a calcium‐dependent process, eventually disrupting the cell membrane potential. The bactericidal effect is limited to gram‐positive organisms.12
Daptomycin is effective against almost all gram‐positive organisms including methicillin‐susceptible Staphylococcus aureus (MSSA), MRSA, and VRE.12 As a result, it has FDA approval for the treatment of cSSTIs. While beta‐lactams remain the standard of care for MSSA bacteremia, daptomycin has FDA approval for bloodstream infections and right‐sided endocarditis due to MSSA or MRSA (Table 1).13 Daptomycin has poor penetration into alveolar fluid14 and is inhibited by pulmonary surfactants; as a consequence, it is not indicated for patients with pneumonia.15
Of note, daptomycin is mainly excreted via the kidneys and should be dose‐adjusted for patients with a creatinine clearance 30 mL/minute. A reversible myopathy may occur with daptomycin, requiring intermittent monitoring of creatinine kinase if prolonged use is anticipated. Caution should be used with the coadministration of medications that can also cause a myopathy, such as statins.
Tigecycline
Tigecycline (Tygacil) was approved for use by the FDA in 2005. The first in a class of new tetracycline analogs, the glycylcyclines, tigecycline is notable for its activity against several multidrug‐resistant (MDR) organisms, including MRSA, VRE, and Enterobacteriaceae carrying extended‐spectrum beta‐lactamases (ESBL). Tigecycline impairs bacterial protein synthesis by binding to the 30S ribosomal subunit. Due to steric hindrance from an N‐alkyl‐glycylamido group at position 9, tigecycline cannot be removed by most bacterial efflux mechanisms.16
Tigecycline has been approved for the therapy of cSSTIs, including those due to MSSA and MRSA. In a pooled analysis of 2 international, multicenter, phase III randomized, double‐blind trials, tigecycline was not inferior to vancomycin plus aztreonam in the treatment of cSSTIs. Of note, MRSA eradication rates were similar between patients treated with tigecycline and vancomycin plus aztreonam (78.1% and 75.8%, respectively).17
Dalbavancin
Dalbavancin (Zeven), a new, semisynthetic lipoglycopeptide, was approved by the FDA in late 2007; however, it has not been cleared for marketing. Though dalbavancin is derived from teicoplanin, its lipophilic anchor to the bacterial cell membrane makes the drug more potent than its predecessor. Dalbavancin interferes with bacterial cell wall synthesis by binding to the C‐terminal D‐alanyl‐D alanine of the growing peptidoglycan chains.18 Enhanced pharmacokinetic properties of dalbavancin (half‐life 149‐250 hours) allow it to be dosed once‐weekly, a novel concept in antimicrobial use.19
Like other glycopeptides, dalbavancin maintains in vitro activity against most gram‐positive aerobic organisms, including MRSA and penicillin‐susceptible and penicillin‐resistant strains of Streptococcus pneumoniae. Notably, when compared to vancomycin in vitro, the agent is more active against Enterococcus faecium and Enterococcus faecalis isolates. In a recent phase III double‐blind trial, dalbavancin was compared to linezolid for the treatment of cSSTIs. Dalbavancin was not inferior to linezolid (clinical success rate 90% vs. 92%). Of note, 51% of study patients with SSTI had infection due to MRSA. Microbiological response to dalbavancin paralleled the clinical success rate; MRSA eradication rates after dalbavancin and linezolid were 91% and 89%, respectively.20
Given its once‐weekly dosing, dalbavancin may be an attractive agent in the outpatient treatment of gram‐positive bacteremia. In a phase II study, dalbavancin administered as a single 1‐g dose, followed by a 500‐mg dose 1 week later, was comparable to 14 days of vancomycin for the treatment of catheter‐related bloodstream infections (CRBSI) due to coagulase‐negative staphylococci or S. aureus (including MRSA).21 Phase III studies are underway. At present, there is no evidence to support the use of dalbavancin for the treatment of pneumonia or bone and joint infections.
Despite the administration of vancomycin, the patient continues to experience fever and chills. Blood cultures drawn in the emergency department are now growing Enterococcus species. You review the patient's medical record and notice that she was colonized with VRE on a prior admission. You consider the antibiotic options for serious infections due to VRE.
Though rates of VRE have remained fairly stable in recent years,22 the pathogen continues to present a challenge to hospital epidemiologists. A national survey in 2004 suggested that nearly 30% of enterococci in U.S. intensive care units display vancomycin resistance.1 Additional U.S. surveillance data reveals that VRE accounts for 10% to 26% of enterococci hospital‐wide.23, 24 In 2005, a meta‐analysis noted that bloodstream infections due to VRE resulted in higher mortality rates than those due to vancomycin‐susceptible enterococci.25 This discrepancy is most evident among neutropenia patients.26 Unfortunately, the options for the treatment of serious infections due to VRE are limited. The advantages and disadvantages of linezolid, quinupristin‐dalfopristin, tigecycline, and daptomycin in the treatment for VRE are discussed below.
Linezolid
Currently, linezolid is the only oral drug that is FDA‐approved for the treatment of infections due to VRE, including bacteremia. Notably, linezolid therapy resulted in the cure of 77% of 22 cases of vancomycin‐resistant enterococcal endocarditis.27 Current guidelines by the Infectious Disease Society of America (IDSA) support the use of linezolid in cases of endocarditis due to ampicillin‐resistant and vancomycin‐resistant Enterococcus faecium.28 Unfortunately, recent reports highlight the emergence of linezolid‐resistant VRE,29 suggesting use of this drug should be limited to circumstances in which other alternatives do not exist.
Quinupristin‐Dalfopristin
Quinupristin‐dalfopristin (Synercid) was approved by the FDA in 1999. It is used in the treatment of infections caused by gram‐positive organisms and is a combination of 2 semisynthetic pristinamycin derivatives. They diffuse into bacteria and bind to different areas on the 50S ribosomal subunit, thereby inhibiting protein synthesis. Individually, quinupristin and dalfopristin are bacteriostatic but together they are bactericidal.30
Quinupristin‐dalfopristin has activity against Staphylococcus aureus (including MRSA), Streptococcus pneumoniae, gram‐positive anaerobes, and vancomycin‐sensitive and resistant Enterococcus faecium. It has little activity against Enterococcus faecalis.31 FDA‐approved uses of quinupristin‐dalfopristin are limited, but include the treatment of serious infections caused by vancomycin‐resistant E. faecium (VREF).32 In a study of 396 patients with VREF the clinical success rate of quinupristin‐dalfopristin was 73.6%.33 The drug also has FDA approval for the use in cSSTIs due to group A streptococci or MSSA.32 The use of this agent is limited due to its toxicity profile. In cases of serious VRE‐related infection, quinupristin‐dalfopristin is often only utilized if linezolid cannot be tolerated.
Daptomycin
In vitro studies suggest that daptomycin is active against enterococci, including vancomycin‐resistant isolates.34 However, clinical data on the use of this agent in the treatment of infections due to VRE are lacking. FDA approval for the use of daptomycin in cSSTI included the treatment of 45 patients infected with Enterococcus faecalis.13 In addition, several reports have detailed the successful treatment of VRE bloodstream infections with daptomycin,35, 36 including a case series of VRE endocarditis.37 To determine the role of this agent in the treatment of invasive infections due to VRE, further study is needed.
You decide to discontinue vancomycin and administer linezolid. The patient's vascular catheter is removed; catheter‐tip cultures grow >1000 colonies of VRE. Blood cultures the following day are negative and a new catheter is placed. You ask the patient to continue oral linezolid to complete a 2‐week course. A review of her medication list reveals that she is not taking SSRIs or monoamine oxidase inhibitors (MAOIs).
While linezolid has retained its FDA indication for VRE bacteremia, empiric use in suspected cases of CRBSI or catheter site infection is not advised. In an open‐label trial among seriously ill patients with intravascular catheter‐related infections, linezolid use was associated with a higher mortality when compared to vancomycin/oxacillin. Interestingly, mortality among linezolid‐treated patients included those with CRBSI due to gram‐negative pathogens, due to both gram‐negative and gram‐positive pathogens, or due to an identifiable pathogen; mortality rates did not differ among patients with gram‐positive infections only.38
Case 2
A 27‐year‐old male with a history of T10 paraplegia following a motor vehicle accident presents with abdominal pain, fever, and chills. He notes that he experiences these symptoms when he has a urinary tract infection (UTI), a frequent complication of his chronic indwelling suprapubic catheter. You review his medical record and notice that he has had prior UTIs with multiple gram‐negative rods over the past 2 years, including MDR Pseudomonas and Acinetobacter. When his urine culture grows >100,000 colonies of gram‐negative rods, you initiate meropenem and consider the options for treatment of these MDR pathogens.
According to national U.S. surveillance in 2001, 22% of Pseudomonas aeruginosa were resistant to imipenem, an increase of 32% from 1997.39 More alarming is the recent development of MDR P. aeruginosa, a pathogen resistant not only to the beta‐lactams (including the carbapenems) but to the fluoroquinolones and aminoglycosides as well.40 MDR P. aeruginosa is virulent, and has been associated with higher rates of mortality, longer hospital stays, and greater cost.41
Already equipped with intrinsic resistance to the aminopenicillins and first‐generation and second‐generation cephalosporins, A. baumannii has gained recent notoriety with acquired resistance to beta‐lactams, aminoglycosides, fluoroquinolones, and tetracyclines. Most notably, carbapenem‐resistant A. baumannii has emerged due to enzymes capable of hydrolyzing imipenem. Like MDR P. aeruginosa, MDR A. baumannii infection has led to longer hospital stays42 and increased patient mortality43 when compared to infections with more susceptible strains.
Therapeutic options for these MDR gram‐negative pathogens remain limited, but the advent of doripenem and the return of colistin may play a role in treatment. The use of these 2 agents and tigecycline in the treatment of MDR P. aeruginosa and/or A. baumannii are described below.
Doripenem
In October 2007, the FDA approved the use of doripenem (Doribax), a much‐anticipated carbapenem. In structure, doripenem resembles meropenem and does not require a renal dehydropeptidase I inhibitor (eg, cilastatin).44 Similar to other beta‐lactams, doripenem binds to penicillin‐binding proteins (PBPs), inhibiting PBP‐directed cell wall synthesis.
Like imipenem and meropenem, doripenem has broad‐spectrum antimicrobial activity. It demonstrates in vitro activity against most gram‐positive pathogens including MSSA and ampicillin‐sensitive enterococci. Doripenem also has in vitro activity against most gram‐negative pathogens (including ESBL‐producing Enterobacteriaceae) and most anaerobes, including Bacteriodes fragilis. Most notably, when compared to other carbapenems, doripenem has demonstrated better in vitro activity against Pseudomonas aeruginosa.45 However, clinical implications of this in vitro activity are unclear.
When compared to meropenem or levofloxacin for the treatment of complicated UTIs, doripenem is an effective alternative. Clinical response rates among affected patients were 95% to 96% with doripenem, 89% with meropenem, and 90% with levofloxacin.46, 47 Doripenem was not inferior to meropenem in patients with serious lower respiratory tract infections, and comparable to imipenem‐cilastin and pipercillin‐tazobactam for the treatment of nosocomial or ventilator‐associated pneumonia (VAP).48, 49 Finally, for the treatment of complicated intraabdominal infections, doripenem was not inferior to meropenem; both drugs achieved microbiologic cure rates of >84%.50
Currently, doripenem is FDA‐approved for the treatment of complicated intraabdominal infections (eg, appendicitis, pancreatitis, cholecystitis, peritonitis) and complicated lower UTIs or pyelonephritis (Table 1). Given its expanded spectrum of activity, use of doripenem should be limited to circumstances in which a MDR pathogen is highly suspected or confirmed.
Colistin
Colistin (Coly‐Mycin M) falls within the family of polymyxin antibiotics, which were discovered in 1947. Colistin has been available for almost 50 years for the treatment of infections caused by gram‐negative bacteria, including Pseudomonas spp. However, early use of colistin was associated with significant nephrotoxicity. Its use decreased markedly with the advent of new antibiotics that had the same antimicrobial spectrum and a better side effect profile. With the emergence of MDR gram‐negative bacteria, colistin has returned to limited clinical use.51 As a polymyxin, colistin is a cell membrane detergent. It disrupts the cell membrane, causing leakage of bacterial cell content and ultimately cell death.52
Colistin has bactericidal activity against most gram‐negative bacteria including Acinetobacter spp, and members of the family Enterobacteriaceae (eg, Klebsiella spp, Escherichia coli, Enterobacter spp), including those producing ESBLs.53 Colistin is not active against several predominant gram‐negative pathogens including Proteus spp, Providencia spp, or Serratia spp (Table 1).
In 2007, several studies suggested that colistin monotherapy was effective for patients with VAP due to MDR P. aeruginosa or A. baumannii isolate.54, 55 A third trial that year suggested that colistin may have a role in the treatment of MDR P. aeruginosa among neutropenic patients. In that study, infected patients receiving colistin monotherapy experienced higher rates of clinical and microbiologic response than those receiving other antipseudomonal agents (eg, beta‐lactams or fluoroquinolones if active against the isolate).56 While uncontrolled studies suggest that the use of colistin in combination with other antimicrobials (including carbapenems, ampicillin‐sulbactam, aminoglycosides, and rifampin) may have some success in the treatment of VAP due to MDR A. baumannii,57, 58 further trials are needed.
Currently, colistin has FDA approval only for the treatment of acute infections due to gram‐negative bacteria that have demonstrated susceptibility to the drug and is therefore administered on a case by case basis. Although it has been used via the inhalation route to treat infections in cystic fibrosis patients, colistin does not have FDA approval for this indication.
Tigecycline
Tigecycline is approved for the treatment of complicated intraabdominal infections based on the results of 2 international, multicenter, phase III, randomized, double‐blind trials. In this pooled analysis, tigecycline was as effective and as safe as imipenem/cilastatin. Notably, study patients were not severely ill (baseline APACHE II score of 6.0).59 FDA approval suggests tigecycline use be focused on intraabdominal infections due to members of the family Enterobacteriaceae (eg, Klebsiella spp, Escherichia coli, Enterobacter spp), including those producing ESBLs, vancomycin‐sensitive enterococci, and/or MSSA. Notably, tigecycline lacks significant in vitro activity against Pseudomonas spp, Proteus spp, or Providencia spp. It has demonstrated in vitro activity against MDR strains of Acinetobacter spp (Table 1).
Given its bacteriostatic activity, tigecycline's effectiveness in the treatment bacteremia is unclear.
In addition, as no published studies have addressed its activity among seriously ill patients, tigecycline is considered a second‐line or third‐line agent for SSTI and complicated intraabdominal infections. Evidence for use of tigecycline for the treatment of UTIs is lacking and, as a rule, its use should be limited to scenarios in which alternatives for the proven or suspected pathogens do not exist.
The urine isolate is identified as Escherichia coli. You review the susceptibility profile and determine that this isolate is an ESBL‐producing strain. In addition, the patient's isolate demonstrates resistance to the fluoroquinolones and trimethoprim‐sulfamethoxazole. You consider other options for treatment of this ESBL‐producing E. coli.
According to national surveillance data, more than 20% of Klebsiella isolates in U.S. intensive care units produced ESBLs in 2003, a 47% increase when compared to 1998.39 Bloodstream infections due to ESBL‐producing isolates have led to increased length of hospital stay,60, 61 increased hospital costs,4 improper antibiotic use,5 and, most notably, increased mortality.61‐63 Of concern, ESBLs have been demonstrated within community Enterobacteriaceae isolates, most notably due to CTX‐M beta‐lactamase production among E. coli. In addition to ESBL production, these community E. coli isolates tend to express fluoroquinolone and trimethoprim‐sulfamethoxazole resistance.64 Carbapenems remain the mainstay of therapy for serious infections due to ESBL‐producing organisms. The once‐daily dosing of ertapenem makes this agent an attractive alternative for outpatient management.
Ertapenem
Ertapenem (Invanz) obtained FDA approval for use in the United States in 2001 and in the European Union in 2002.65 Similar to doripenem, ertapenem blocks cell wall synthesis by binding to specific penicillin‐binding proteins (PBPs).
Ertapenem has activity against numerous gram‐positive and gram‐negative bacteria as well as some anaerobic microorganisms. The FDA‐approved indications include complicated intraabdominal infections, cSSTIs, acute pelvic infections, complicated UTIs, and community‐acquired pneumonias (Table 1).66 Of note, in contrast to other carabapenems, ertapenem does not have activity against Pseudomonas aeruginosa or Acinetobacter spp.67
Ertapenem is approved as a single daily dose of 1 g and can be administered intravenously or intramuscularly. Changes in dosing must also be considered for critically ill patients. When administered to patients with VAP, ertapenem achieved a lower maximum concentration and area under the curve.68 In such patients, it is recommended that the dosage interval be decreased or that a continuous infusion of ertapenem be administered.
The patient's symptoms improve on meropenem. A peripherally‐inserted central catheter is placed for the administration of intravenous antibiotics at home. You prescribe ertapenem (1 g/day) for the remainder of a 14‐day course.
Conclusions
MDR bacteria continue to present a clinical challenge to hospitalists. Proper treatment of patients infected with these organisms is necessary, as inappropriate antibiotic use for MDR bacterial infections has been associated with longer hospital stays, greater cost, and, in some cases, increased mortality. Unfortunately, antibiotic production and development has declined steadily in the past 25 years. To minimize the rate of antimicrobial resistance, physicians must take care to prescribe antibiotics appropriately. While these promising new agents for resistant gram‐positive and gram‐negative infections may aid in battling MDR infections, these antibiotics must be used judiciously to maintain their clinical utility. Hospitalists will continue to play an important role in ensuring that hospitalized patients receive the most effective antimicrobial therapy to both treat the infection and prevent the development of resistance.
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004.Am J Infect Control.2004;32:470–485.
- ,,,,,.Changes in the epidemiology of methicillin‐resistant Staphylococcus aureus in intensive care units in US hospitals, 1992‐2003.Clin Infect Dis.2006;42:389–391.
- ,,, et al.Invasive methicillin‐resistant Staphylococcus aureus infections in the United States.JAMA.2007;298:1763–1771.
- ,,,,,.Emergence of community‐acquired methicillin‐resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft tissue infections.Ann Intern Med.2006;144:309–317.
- ,,,.The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria.Antimicrob Agents Chemother.1998;42:3251–3255.
- ,.Activity of linezolid against gram‐positive cocci possessing genes conferring resistance to protein synthesis inhibitors.J Antimicrob Chemother.2000;45:797–802.
- ,,.Clinical and economic outcomes of oral linezolid versus intravenous vancomycin in the treatment of MRSA‐complicated, lower‐extremity skin and soft‐tissue infections caused by methicillin‐resistant Staphylococcus aureus.Am J Surg.2005;189:425–428.
- ,,,.Linezolid for the treatment of adults with bone and joint infections.Intern J Antimicrob Agents.2007;29:233–239.
- .Efficacy and safety of linezolid in the treatment of skin and soft tissue infections.Eur J Clin Microbiol Infect Dis.2002;21:491–498.
- ,,.Linezolid‐associated peripheral and optic neuropathy, lactic acidosis, and serotonin syndrome.Pharmacotherapy.2007;27(8):1189–1197.
- ,.Development of daptomycin for gram‐positive infections.J Antimicrob Chemother.2000;46(4):523–526.
- .Daptomycin and tigecycline: a review of clinical efficacy in the antimicrobial era.Expert Opin Pharmacother.2007;8(14):2279–2292.
- ,,, et al.Daptomycin verses standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus.N Engl J Med.2006:355(7):653–665.
- .Lipopeptides, focusing on daptomycin, for the treatment of gram‐positive infections.Expert Opin Invest Drugs.2004;13:1159–1169.
- .Alternatives to vancomycin for the treatment of methicillin‐resistant Staphylococcus aureus infections.Clin Infect Dis.2007;45(suppl 3):S184–S190.
- .Tigecycline: a new glycylcycline for treatment of serious infections.Clin Infect Dis.2005;41(suppl 5):S303–S314.
- ,,, et al.The efficacy and safety of tigecycline in the treatment of skin and skin‐structure infections: results of 2 double‐blind phase 3 comparison studies with vancomycin‐aztreonam.Clin Infect Dis.2005;41(suppl 5):S341–S353.
- ,.Origin, structure, and activity in vitro and in vivo of dalbavancin.J Antimicrob Chemother2005;55(suppl S2):ii15–ii20.
- ,.Dalbavancin: a novel lipoglycopeptide antibacterial.Pharmacotherapy2006;26:908–918.
- ,,, et al.Randomized, double‐blind comparison of a once‐weekly dalbavancin versus twice‐daily linezolid therapy for the treatment of complicated skin and skin structure infections.Clin Infect Dis.2005;41:1407–1415.
- ,,, et al.Efficacy and safety of weekly dalbavancin therapy for catheter‐related bloodstream infection caused by gram‐positive pathogens.Clin Infect Dis.2005;40:374–380.
- ,.Vancomycin‐resistant staphylococci and enterococci: epidemiology and control.Curr Opin Infect Dis.2005;18:300–305.
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992‐June 2001, issued August 2001.Am J Infect Control.2001;29:404–421.
- ,,,,, et al.Antimicrobial resistance trends and outbreak frequency in United States hospitals.Clin Infect Dis.2004;38:78–85.
- ,,,.Comparison of mortality associated with vancomycin‐resistant and vancomycin‐susceptible enterococcal bloodstream infections: a meta‐analysis.Clin Infect Dis.2005;41:327–333.
- ,.Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection.J Infect Dis.2005;191(4):588–595.
- ,,,,,.Linezolid for the treatment of multidrug‐resistant gram positive infections: experience from a compassionate‐use program.Clin Infect Dis.2003;36:159–168.
- ,,, et al.Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America.Circulation.2005;111(23):e394–e434.
- ,,.Nosocomial spread of linezolid‐resistant, vancomycin‐resistant Enterococcus faecium.N Engl J Med.2002;346:867–869.
- ,.Novel antibacterial agents for skin and skin structure infections.J Am Acad Dermatol.2004;50(3):331–340.
- ,,.New antimicrobial agents as therapy for resistant gram‐positive cocci.Eur J Clin Microbiol Infect Dis.2008;27(1):3–15.
- .Quinupristin‐dalfopristin and linezolid: evidence and opinion.Clin Infect Dis.2003;36(4):473–481.
- ,,,,,.The efficacy and safety of quinupristin/dalfopristin for the treatment of infections caused by vancomycin‐resistant Enterococcus faecium. Synercid Emergency‐Use Study Group.J Antimicrob Chemother.1999:44(2):251–261.
- ,,.Evaluation of the in vitro activity of daptomycin against 19615 clinical isolates of gram‐positive cocci collected in North American hospitals (2002‐2005).Diagn Microbiol Infect Dis.2007;57(4):459–465.
- ,,,,.Daptomycin in the treatment of vancomycin‐resistant Enterococcus faecium bacteremia in neutropenic patients.J Infect.2007;54(6):567–571.
- ,,,,.Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia.Scand J Infect Dis.2006;38:290–292.
- ,,.Daptomycin for the treatment of gram‐positive bacteremia and infective endocarditis: a retrospective case series of 31 patients.Pharmacotherapy.2006;26(3):347–352.
- Pfizer Pharmacia and Upjohn Company. United States Pharmacopeia. Zyvox. Available at: http://media.pfizer.com/files/products/uspi_zyvox.pdf. Accessed April 2009.
- NNIS System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2003, issued August 2003.Am J Infect Control.2003;31(8):481–498.
- .Resistance in nonfermenting gram‐negative bacteria: multidrug resistance to the maximum.Am J Med.2006;119:S29–S36.
- ,,, et al.Emergence of antibiotic‐resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents.Antimicrob Agents Chemother.1999;43(6):1379–1382.
- ,,, et al.Multidrug‐resistant Acinetobacter infection mortality rate and length of hospitalization.Emerg Infect Dis.2007;13:97–103.
- ,,, et al.Bloodstream infections due to Acinetobacter spp: epidemiology, risk factors, and impact of multi‐drug resistance.Eur J Clin Microbiol Infect Dis.2008;27(7):607–612.
- ,,,,.Doripenem (S‐4661), a novel carbapenem: comparative activity against contemporary pathogens including bactericidal action and preliminary in vitro methods evaluation.J Antimicrob Chemother.2004;54:144–154.
- ,,.Antimicrobial activity of doripenem (S‐4661): a global surveillance report.Clin Microbiol Infect.2005;11:974–984.
- ,,, et al.Intravenous therapy with. doripenem versus levofloxacin with an option for oral step‐down therapy in the treatment of complicated urinary tract infections and pyelonephritis. 17th European Congress of Clinical Microbiology and Infectious Diseases and the 25th International Congress of Chemotherapy. Munich, Germany. March 31‐April 3, 2007. Abstract no. 833 plus poster.
- .New uses for older antibiotics: nitrofurantoin, amikacin, colistin, polymyxin B, doxycyline, and minocycline revisited.Med Clin North Am.2006;90(6):1089–1107.
- ,,, et al.Efficacy and safety of doripenem versus piperacillin/tazobactam in nosocomial pneumonia: a randomized, open‐label, multicenter study.Curr Med Res Opin.2008;24(7):2113–2126.
- ,,, et al.Efficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator‐associated pneumonia: a multicenter, randomized study.Crit Care Med.2008;36(4):1089–1096.
- ,,, et al.Efficacy and tolerability of IV doripenem versus meropenem in adults with complicated intra‐abdominal infection: a phase III, prospective, multicenter, randomized, double‐blind, noninferiority study.Clin Ther.2008;30(5):868–883.
- ,,,,.Evaluation of colistin as an agent against multi‐resistant Gram‐negative bacteria.Int J Antimicrob Agents.2005;25(1):11–25.
- .New uses for older antibiotics: nitrofurantoin, amikacin, colistin, polymyxin B, doxycycline, and minocycline revisited.Med Clin North Am.2006;90(6):1089–1107.
- ,.Colistin: the revival of polymyxins for the management of multidrug‐resistant gram‐negative bacterial infections.Clin Infect Dis.2005;40(9):1333–1341.
- ,,, et al.Ventilator‐associated pneumonia (VAP) due to susceptible only to colistin microorganisms.Eur Respir J.2007;30(2):307–313.
- ,,, et al.Safety and efficacy of colistin compared with imipenem in the treatment of ventilator‐associated pneumonia: a matched case‐control study.Intensive Care Med.2007;33(7):1162–1167.
- ,,, et al.Colistin is effective in treatment of infections caused by multidrug‐resistant Pseudomonas aeruginosa in cancer patients.Antimicrob Agents Chemother.2007;51(6):1905–1911.
- ,,,,,.Combination therapy with intravenous colistin for management of infections due to multidrug‐resistant gram‐negative bacteria in patients without cystic fibrosis.Antimicrob Agents Chemother.2005;49:3136–3146.
- ,,, et al.Combined colistin and rifampicin therapy for carbapenem‐resistant Acinetobacter baumannii infections: clinical outcome and adverse events.Clin Microbiol Infect.2005;11:682–683.
- ,,, et al.The efficacy and safety of tigecycline for the treatment of complicated intra‐abdominal infections: analysis of pooled clinical trial data.Clin Infect Dis.2005;41(suppl 5):S354–S367.
- ,,,,.Clinical implications of extended‐spectrum beta‐lactamase‐producing Klebsiella pneumoniae bacteraemia.J Hosp Infect.2002;52:99–106.
- ,,,,,.Clinical and economic impact of bacteremia with extended spectrum beta‐lactamase–producing Enterobacteriaceae.Antimicrob Agents Chemother.2006;50:1257–1262.
- ,,, et al.Ceftazidime‐resistant Klebsiella pneumoniae bloodstream infection in children with febrile neutropenia.Int J Infect Dis.2000;4:21–25.
- ,,, et al.Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended‐ spectrum beta‐lactamases.Clin Infect Dis.2004;39:31–37.
- ,.Extended‐spectrum beta‐lactamase‐producing Enterobacteriaceae: an emerging public‐health concern.Lancet Infect Dis.2008;8(3):159–166.
- ,.Ertapenem, the first of a new group of carbapenems.J Antimicrob Chemother.2003;52(4):538–542.
- Merck 2006.
- ,,.Ertapenem: the new carbapenem 5 years after first FDA licensing for clinical practice.Expert Opin Pharmacother.2007;8(2):237–256.
- ,,, et al.Ertapenem in critically ill patients with early‐onset ventilator‐associated pneumonia: pharmacokinetics with special consideration of free‐drug concentration.J Antimicrob Chemother.2007;59(2):277–284.
- ,.Quinupristin/dalfopristin: a therapeutic review.Clin Ther.2001;23(1):24–44.
Case 1
A 53‐year‐old woman with a history of hemodialysis‐dependent end‐stage renal disease presents with left lower extremity pain and redness for the past 3 days. On physical examination, her temperature is 102.3F. Erythema, induration, and warmth are noted over her left lower leg and foot. Her history is remarkable for a line‐related bloodstream infection due to methicillin‐resistant Staphylococcus aureus (MRSA) 4 weeks ago. The infected line was removed and replaced with a right‐sided subclavian catheter. You note that the new line site is clean, not erythematous, and not tender. In the emergency department, the patient receives a dose of vancomycin for presumed MRSA cellulitis. Your patient wants to know if there are alternative agents for her infection so she does not require hospitalization.
Unfortunately, MRSA has become commonplace to the hospital setting. Among intensive care units in 2003, 64.4% of healthcare‐associated Staphylococcus aureus infections were caused by MRSA, compared with only 35.9% in 1992; a 3.1% increase per year.1, 2 Increased MRSA rates are not without consequence; a recent review suggests that MRSA infections kill nearly 19,000 hospitalized American patients annually.3 Of note, MRSA infection rates have also increased among previously healthy individuals. These community‐associated isolates (CA‐MRSA) often manifest as pyogenic skin and soft‐tissue infections (SSTIs). In a recent multicenter study, CA‐MRSA accounted for 59% of SSTIs among patients presenting to emergency rooms in the United States.4 In cases of SSTI, oral agents such as clindamycin, doxycycline, and trimethoprim‐sulfamethoxazole have proven successful. For invasive MRSA, vancomycin is still considered the standard treatment; however, several alternatives have emerged in recent years. The advantages and disadvantages of linezolid, daptomycin, tigecycline, and dalbavancin in the treatment of MRSA are described below.
Linezolid
Linezolid (Zyvox), an oxazolidinone approved in 2000, has been touted for its oral bioavailability, twice‐daily dosing, gram‐positive coverage, and unique mechanism of action. Like several other antimicrobials, linezolid inhibits bacterial protein synthesis. The drug binds to the 50S ribosomal subunit near its site of interaction with the 30S subunit, preventing formation of the 70S initiation complex.5 This site of action on the 50S subunit is unique to linezolid; as a result, cross‐resistance between linezolid and other antimicrobials that act at the 50S subunit (eg, chloramphenicol, macrolides, aminoglycosides, and tetracycline) does not occur.6
The oxazolidinones have excellent bacteriostatic activity against all pathogenic gram‐positive bacteria. The U.S. Food and Drug Administration (FDA) approved linezolid for the treatment of serious infections due to vancomycin‐resistant enterococci (VRE), including bacteremia, complicated skin and soft‐tissue infections (cSSTIs) due to Staphylococcus aureus (including MRSA), and nosocomial pneumonia due to Staphylococcus aureus (including MRSA) or penicillin‐susceptible Streptococcus pneumoniae (Table 1).
| Activity | Agent | FDA‐Approved Indications | Limitations in Use | Side Effects |
|---|---|---|---|---|
| ||||
| Gram‐positive | Daptomycin | cSSTIs; MSSA/MRSA bacteremia; MSSA/MRSA endocarditis | Not indicated for pneumonia (inhibited by pulmonary surfactant) | Reversible myopathy may be exacerbated by use with other medications |
| Quinupristin‐dalfopristin | Vancomycin‐resistant E. faecium; group A streptococci or MSSA cSSTIs | Myalgias and arthralgias; infusion site reaction;* thrombophlebitis;* liver enzyme elevation; inhibition of cytochrome p450 34a | ||
| Linezolid | Serious infections due to VRE; MSSA/MRSA cSSTIs; MSSA/MRSA nosocomial pneumonia; pneumonia due to penicillin‐sensitive S. pneumoniae | Not indicated for catheter‐related bloodstream infections or catheter site infections | Myelosuppression; serotonin syndrome; tyramine reaction; peripheral neuropathy; optic neuropathy | |
| Dalbavancin | Approval pending for cSSTIs | Not indicated for pneumonia bone and joint infection | Unknown | |
| Gram‐negative | Colistin | Gram‐negative bacteria that have demonstrated sensitivity to the drug | Not indicated for Proteus spp, Providencia spp, or Serratia spp | Acute tubular necrosis; neurotoxicity∥; bronchospasm |
| Gram‐positive and Gram‐negative | Ertapenem | Complicated intraabdominal infections#; cSSTIs; acute pelvic infections; complicated UTIs; community‐acquired pneumonia; prophylaxis of SSI following colorectal surgery in adult patients | Not indicated for Pseudomonas, Acinetobacter, S. maltophilia | Cross‐reactivity with penicillin; cross‐reactivity with cephalosporins; caution use if history of seizures |
| Doripenem | Complicated intraabdominal infections# and complicated UTIs, including pyelonephritis | Cross‐reactivity with penicillin; cross‐reactivity with cephalosporins; caution use if history of seizures | ||
| Tigecycline | cSSTIs (including those due to MRSA) complicated intraabdominal infections# | Nausea and vomiting; tooth discoloration in children | ||
In retrospective analyses of SSTIs due to MRSA, linezolid was as effective as vancomycin, resulting in higher clinical cure rates and shorter hospitalizations.7 As a result, linezolid has established a role in the treatment of community‐acquired MRSA SSTIs. Evidence limited to case reports and case series suggest that linezolid may also have a role in the treatment of bone and joint infections. In these cases, linezolid was often used because treatment with other agents had failed, the administration of other antibiotics was not indicated due to resistance patterns, the patient refused intravenous therapy, or the patient did not tolerate vancomycin. When such conditions exist, linezolid may be a consideration in cases of osteomyelitis or prosthetic joint infection.8
Potential side effects of linezolid may limit its use, especially for patients who require prolonged therapy (Table 1). Of note, as a reversible, relatively weak nonselective inhibitor of monoamine oxidase, linezolid may interact with adrenergic and serotonergic agents. Concomitant of a serotonin agent such as a selective serotonin‐reuptake inhibitor (SSRI) and linezolid should be approached with caution. Subsequent serotonin syndrome is characterized by autonomic dysfunction (eg, diaphoresis, tachycardia, hypertension) and neuromuscular hyperactivity (eg, muscle rigidity, clonus, hyperreflexia). Though infrequent, cases of reversible myelosuppression have been reported with linezolid use.9 Patients who will receive this drug for more than 2 weeks should be monitored for myelosuppression with a weekly complete blood count. Isolated reports suggest that the prolonged administration of linezolid (>28 days) may be associated with peripheral neuropathy and optic neuropathy. While prompt discontinuation of the drug often results in resolution of symptoms, peripheral or optic nerve injury can be permanent. The mechanism of injury is unclear, though mitochondrial toxicity is suspected.10
Daptomycin
Daptomycin (Cubicin), a cyclic lipopeptide, was discovered in the early 1980s, but skeletal muscle toxicity led to the discontinuation of early clinical trials. When a change from twice‐daily to once‐daily dosing in 2003 resulted in fewer adverse events, the FDA approved daptomycin to treat complicated skin and skin‐structure infections.11 Daptomycin binds to the cell membrane via a calcium‐dependent process, eventually disrupting the cell membrane potential. The bactericidal effect is limited to gram‐positive organisms.12
Daptomycin is effective against almost all gram‐positive organisms including methicillin‐susceptible Staphylococcus aureus (MSSA), MRSA, and VRE.12 As a result, it has FDA approval for the treatment of cSSTIs. While beta‐lactams remain the standard of care for MSSA bacteremia, daptomycin has FDA approval for bloodstream infections and right‐sided endocarditis due to MSSA or MRSA (Table 1).13 Daptomycin has poor penetration into alveolar fluid14 and is inhibited by pulmonary surfactants; as a consequence, it is not indicated for patients with pneumonia.15
Of note, daptomycin is mainly excreted via the kidneys and should be dose‐adjusted for patients with a creatinine clearance 30 mL/minute. A reversible myopathy may occur with daptomycin, requiring intermittent monitoring of creatinine kinase if prolonged use is anticipated. Caution should be used with the coadministration of medications that can also cause a myopathy, such as statins.
Tigecycline
Tigecycline (Tygacil) was approved for use by the FDA in 2005. The first in a class of new tetracycline analogs, the glycylcyclines, tigecycline is notable for its activity against several multidrug‐resistant (MDR) organisms, including MRSA, VRE, and Enterobacteriaceae carrying extended‐spectrum beta‐lactamases (ESBL). Tigecycline impairs bacterial protein synthesis by binding to the 30S ribosomal subunit. Due to steric hindrance from an N‐alkyl‐glycylamido group at position 9, tigecycline cannot be removed by most bacterial efflux mechanisms.16
Tigecycline has been approved for the therapy of cSSTIs, including those due to MSSA and MRSA. In a pooled analysis of 2 international, multicenter, phase III randomized, double‐blind trials, tigecycline was not inferior to vancomycin plus aztreonam in the treatment of cSSTIs. Of note, MRSA eradication rates were similar between patients treated with tigecycline and vancomycin plus aztreonam (78.1% and 75.8%, respectively).17
Dalbavancin
Dalbavancin (Zeven), a new, semisynthetic lipoglycopeptide, was approved by the FDA in late 2007; however, it has not been cleared for marketing. Though dalbavancin is derived from teicoplanin, its lipophilic anchor to the bacterial cell membrane makes the drug more potent than its predecessor. Dalbavancin interferes with bacterial cell wall synthesis by binding to the C‐terminal D‐alanyl‐D alanine of the growing peptidoglycan chains.18 Enhanced pharmacokinetic properties of dalbavancin (half‐life 149‐250 hours) allow it to be dosed once‐weekly, a novel concept in antimicrobial use.19
Like other glycopeptides, dalbavancin maintains in vitro activity against most gram‐positive aerobic organisms, including MRSA and penicillin‐susceptible and penicillin‐resistant strains of Streptococcus pneumoniae. Notably, when compared to vancomycin in vitro, the agent is more active against Enterococcus faecium and Enterococcus faecalis isolates. In a recent phase III double‐blind trial, dalbavancin was compared to linezolid for the treatment of cSSTIs. Dalbavancin was not inferior to linezolid (clinical success rate 90% vs. 92%). Of note, 51% of study patients with SSTI had infection due to MRSA. Microbiological response to dalbavancin paralleled the clinical success rate; MRSA eradication rates after dalbavancin and linezolid were 91% and 89%, respectively.20
Given its once‐weekly dosing, dalbavancin may be an attractive agent in the outpatient treatment of gram‐positive bacteremia. In a phase II study, dalbavancin administered as a single 1‐g dose, followed by a 500‐mg dose 1 week later, was comparable to 14 days of vancomycin for the treatment of catheter‐related bloodstream infections (CRBSI) due to coagulase‐negative staphylococci or S. aureus (including MRSA).21 Phase III studies are underway. At present, there is no evidence to support the use of dalbavancin for the treatment of pneumonia or bone and joint infections.
Despite the administration of vancomycin, the patient continues to experience fever and chills. Blood cultures drawn in the emergency department are now growing Enterococcus species. You review the patient's medical record and notice that she was colonized with VRE on a prior admission. You consider the antibiotic options for serious infections due to VRE.
Though rates of VRE have remained fairly stable in recent years,22 the pathogen continues to present a challenge to hospital epidemiologists. A national survey in 2004 suggested that nearly 30% of enterococci in U.S. intensive care units display vancomycin resistance.1 Additional U.S. surveillance data reveals that VRE accounts for 10% to 26% of enterococci hospital‐wide.23, 24 In 2005, a meta‐analysis noted that bloodstream infections due to VRE resulted in higher mortality rates than those due to vancomycin‐susceptible enterococci.25 This discrepancy is most evident among neutropenia patients.26 Unfortunately, the options for the treatment of serious infections due to VRE are limited. The advantages and disadvantages of linezolid, quinupristin‐dalfopristin, tigecycline, and daptomycin in the treatment for VRE are discussed below.
Linezolid
Currently, linezolid is the only oral drug that is FDA‐approved for the treatment of infections due to VRE, including bacteremia. Notably, linezolid therapy resulted in the cure of 77% of 22 cases of vancomycin‐resistant enterococcal endocarditis.27 Current guidelines by the Infectious Disease Society of America (IDSA) support the use of linezolid in cases of endocarditis due to ampicillin‐resistant and vancomycin‐resistant Enterococcus faecium.28 Unfortunately, recent reports highlight the emergence of linezolid‐resistant VRE,29 suggesting use of this drug should be limited to circumstances in which other alternatives do not exist.
Quinupristin‐Dalfopristin
Quinupristin‐dalfopristin (Synercid) was approved by the FDA in 1999. It is used in the treatment of infections caused by gram‐positive organisms and is a combination of 2 semisynthetic pristinamycin derivatives. They diffuse into bacteria and bind to different areas on the 50S ribosomal subunit, thereby inhibiting protein synthesis. Individually, quinupristin and dalfopristin are bacteriostatic but together they are bactericidal.30
Quinupristin‐dalfopristin has activity against Staphylococcus aureus (including MRSA), Streptococcus pneumoniae, gram‐positive anaerobes, and vancomycin‐sensitive and resistant Enterococcus faecium. It has little activity against Enterococcus faecalis.31 FDA‐approved uses of quinupristin‐dalfopristin are limited, but include the treatment of serious infections caused by vancomycin‐resistant E. faecium (VREF).32 In a study of 396 patients with VREF the clinical success rate of quinupristin‐dalfopristin was 73.6%.33 The drug also has FDA approval for the use in cSSTIs due to group A streptococci or MSSA.32 The use of this agent is limited due to its toxicity profile. In cases of serious VRE‐related infection, quinupristin‐dalfopristin is often only utilized if linezolid cannot be tolerated.
Daptomycin
In vitro studies suggest that daptomycin is active against enterococci, including vancomycin‐resistant isolates.34 However, clinical data on the use of this agent in the treatment of infections due to VRE are lacking. FDA approval for the use of daptomycin in cSSTI included the treatment of 45 patients infected with Enterococcus faecalis.13 In addition, several reports have detailed the successful treatment of VRE bloodstream infections with daptomycin,35, 36 including a case series of VRE endocarditis.37 To determine the role of this agent in the treatment of invasive infections due to VRE, further study is needed.
You decide to discontinue vancomycin and administer linezolid. The patient's vascular catheter is removed; catheter‐tip cultures grow >1000 colonies of VRE. Blood cultures the following day are negative and a new catheter is placed. You ask the patient to continue oral linezolid to complete a 2‐week course. A review of her medication list reveals that she is not taking SSRIs or monoamine oxidase inhibitors (MAOIs).
While linezolid has retained its FDA indication for VRE bacteremia, empiric use in suspected cases of CRBSI or catheter site infection is not advised. In an open‐label trial among seriously ill patients with intravascular catheter‐related infections, linezolid use was associated with a higher mortality when compared to vancomycin/oxacillin. Interestingly, mortality among linezolid‐treated patients included those with CRBSI due to gram‐negative pathogens, due to both gram‐negative and gram‐positive pathogens, or due to an identifiable pathogen; mortality rates did not differ among patients with gram‐positive infections only.38
Case 2
A 27‐year‐old male with a history of T10 paraplegia following a motor vehicle accident presents with abdominal pain, fever, and chills. He notes that he experiences these symptoms when he has a urinary tract infection (UTI), a frequent complication of his chronic indwelling suprapubic catheter. You review his medical record and notice that he has had prior UTIs with multiple gram‐negative rods over the past 2 years, including MDR Pseudomonas and Acinetobacter. When his urine culture grows >100,000 colonies of gram‐negative rods, you initiate meropenem and consider the options for treatment of these MDR pathogens.
According to national U.S. surveillance in 2001, 22% of Pseudomonas aeruginosa were resistant to imipenem, an increase of 32% from 1997.39 More alarming is the recent development of MDR P. aeruginosa, a pathogen resistant not only to the beta‐lactams (including the carbapenems) but to the fluoroquinolones and aminoglycosides as well.40 MDR P. aeruginosa is virulent, and has been associated with higher rates of mortality, longer hospital stays, and greater cost.41
Already equipped with intrinsic resistance to the aminopenicillins and first‐generation and second‐generation cephalosporins, A. baumannii has gained recent notoriety with acquired resistance to beta‐lactams, aminoglycosides, fluoroquinolones, and tetracyclines. Most notably, carbapenem‐resistant A. baumannii has emerged due to enzymes capable of hydrolyzing imipenem. Like MDR P. aeruginosa, MDR A. baumannii infection has led to longer hospital stays42 and increased patient mortality43 when compared to infections with more susceptible strains.
Therapeutic options for these MDR gram‐negative pathogens remain limited, but the advent of doripenem and the return of colistin may play a role in treatment. The use of these 2 agents and tigecycline in the treatment of MDR P. aeruginosa and/or A. baumannii are described below.
Doripenem
In October 2007, the FDA approved the use of doripenem (Doribax), a much‐anticipated carbapenem. In structure, doripenem resembles meropenem and does not require a renal dehydropeptidase I inhibitor (eg, cilastatin).44 Similar to other beta‐lactams, doripenem binds to penicillin‐binding proteins (PBPs), inhibiting PBP‐directed cell wall synthesis.
Like imipenem and meropenem, doripenem has broad‐spectrum antimicrobial activity. It demonstrates in vitro activity against most gram‐positive pathogens including MSSA and ampicillin‐sensitive enterococci. Doripenem also has in vitro activity against most gram‐negative pathogens (including ESBL‐producing Enterobacteriaceae) and most anaerobes, including Bacteriodes fragilis. Most notably, when compared to other carbapenems, doripenem has demonstrated better in vitro activity against Pseudomonas aeruginosa.45 However, clinical implications of this in vitro activity are unclear.
When compared to meropenem or levofloxacin for the treatment of complicated UTIs, doripenem is an effective alternative. Clinical response rates among affected patients were 95% to 96% with doripenem, 89% with meropenem, and 90% with levofloxacin.46, 47 Doripenem was not inferior to meropenem in patients with serious lower respiratory tract infections, and comparable to imipenem‐cilastin and pipercillin‐tazobactam for the treatment of nosocomial or ventilator‐associated pneumonia (VAP).48, 49 Finally, for the treatment of complicated intraabdominal infections, doripenem was not inferior to meropenem; both drugs achieved microbiologic cure rates of >84%.50
Currently, doripenem is FDA‐approved for the treatment of complicated intraabdominal infections (eg, appendicitis, pancreatitis, cholecystitis, peritonitis) and complicated lower UTIs or pyelonephritis (Table 1). Given its expanded spectrum of activity, use of doripenem should be limited to circumstances in which a MDR pathogen is highly suspected or confirmed.
Colistin
Colistin (Coly‐Mycin M) falls within the family of polymyxin antibiotics, which were discovered in 1947. Colistin has been available for almost 50 years for the treatment of infections caused by gram‐negative bacteria, including Pseudomonas spp. However, early use of colistin was associated with significant nephrotoxicity. Its use decreased markedly with the advent of new antibiotics that had the same antimicrobial spectrum and a better side effect profile. With the emergence of MDR gram‐negative bacteria, colistin has returned to limited clinical use.51 As a polymyxin, colistin is a cell membrane detergent. It disrupts the cell membrane, causing leakage of bacterial cell content and ultimately cell death.52
Colistin has bactericidal activity against most gram‐negative bacteria including Acinetobacter spp, and members of the family Enterobacteriaceae (eg, Klebsiella spp, Escherichia coli, Enterobacter spp), including those producing ESBLs.53 Colistin is not active against several predominant gram‐negative pathogens including Proteus spp, Providencia spp, or Serratia spp (Table 1).
In 2007, several studies suggested that colistin monotherapy was effective for patients with VAP due to MDR P. aeruginosa or A. baumannii isolate.54, 55 A third trial that year suggested that colistin may have a role in the treatment of MDR P. aeruginosa among neutropenic patients. In that study, infected patients receiving colistin monotherapy experienced higher rates of clinical and microbiologic response than those receiving other antipseudomonal agents (eg, beta‐lactams or fluoroquinolones if active against the isolate).56 While uncontrolled studies suggest that the use of colistin in combination with other antimicrobials (including carbapenems, ampicillin‐sulbactam, aminoglycosides, and rifampin) may have some success in the treatment of VAP due to MDR A. baumannii,57, 58 further trials are needed.
Currently, colistin has FDA approval only for the treatment of acute infections due to gram‐negative bacteria that have demonstrated susceptibility to the drug and is therefore administered on a case by case basis. Although it has been used via the inhalation route to treat infections in cystic fibrosis patients, colistin does not have FDA approval for this indication.
Tigecycline
Tigecycline is approved for the treatment of complicated intraabdominal infections based on the results of 2 international, multicenter, phase III, randomized, double‐blind trials. In this pooled analysis, tigecycline was as effective and as safe as imipenem/cilastatin. Notably, study patients were not severely ill (baseline APACHE II score of 6.0).59 FDA approval suggests tigecycline use be focused on intraabdominal infections due to members of the family Enterobacteriaceae (eg, Klebsiella spp, Escherichia coli, Enterobacter spp), including those producing ESBLs, vancomycin‐sensitive enterococci, and/or MSSA. Notably, tigecycline lacks significant in vitro activity against Pseudomonas spp, Proteus spp, or Providencia spp. It has demonstrated in vitro activity against MDR strains of Acinetobacter spp (Table 1).
Given its bacteriostatic activity, tigecycline's effectiveness in the treatment bacteremia is unclear.
In addition, as no published studies have addressed its activity among seriously ill patients, tigecycline is considered a second‐line or third‐line agent for SSTI and complicated intraabdominal infections. Evidence for use of tigecycline for the treatment of UTIs is lacking and, as a rule, its use should be limited to scenarios in which alternatives for the proven or suspected pathogens do not exist.
The urine isolate is identified as Escherichia coli. You review the susceptibility profile and determine that this isolate is an ESBL‐producing strain. In addition, the patient's isolate demonstrates resistance to the fluoroquinolones and trimethoprim‐sulfamethoxazole. You consider other options for treatment of this ESBL‐producing E. coli.
According to national surveillance data, more than 20% of Klebsiella isolates in U.S. intensive care units produced ESBLs in 2003, a 47% increase when compared to 1998.39 Bloodstream infections due to ESBL‐producing isolates have led to increased length of hospital stay,60, 61 increased hospital costs,4 improper antibiotic use,5 and, most notably, increased mortality.61‐63 Of concern, ESBLs have been demonstrated within community Enterobacteriaceae isolates, most notably due to CTX‐M beta‐lactamase production among E. coli. In addition to ESBL production, these community E. coli isolates tend to express fluoroquinolone and trimethoprim‐sulfamethoxazole resistance.64 Carbapenems remain the mainstay of therapy for serious infections due to ESBL‐producing organisms. The once‐daily dosing of ertapenem makes this agent an attractive alternative for outpatient management.
Ertapenem
Ertapenem (Invanz) obtained FDA approval for use in the United States in 2001 and in the European Union in 2002.65 Similar to doripenem, ertapenem blocks cell wall synthesis by binding to specific penicillin‐binding proteins (PBPs).
Ertapenem has activity against numerous gram‐positive and gram‐negative bacteria as well as some anaerobic microorganisms. The FDA‐approved indications include complicated intraabdominal infections, cSSTIs, acute pelvic infections, complicated UTIs, and community‐acquired pneumonias (Table 1).66 Of note, in contrast to other carabapenems, ertapenem does not have activity against Pseudomonas aeruginosa or Acinetobacter spp.67
Ertapenem is approved as a single daily dose of 1 g and can be administered intravenously or intramuscularly. Changes in dosing must also be considered for critically ill patients. When administered to patients with VAP, ertapenem achieved a lower maximum concentration and area under the curve.68 In such patients, it is recommended that the dosage interval be decreased or that a continuous infusion of ertapenem be administered.
The patient's symptoms improve on meropenem. A peripherally‐inserted central catheter is placed for the administration of intravenous antibiotics at home. You prescribe ertapenem (1 g/day) for the remainder of a 14‐day course.
Conclusions
MDR bacteria continue to present a clinical challenge to hospitalists. Proper treatment of patients infected with these organisms is necessary, as inappropriate antibiotic use for MDR bacterial infections has been associated with longer hospital stays, greater cost, and, in some cases, increased mortality. Unfortunately, antibiotic production and development has declined steadily in the past 25 years. To minimize the rate of antimicrobial resistance, physicians must take care to prescribe antibiotics appropriately. While these promising new agents for resistant gram‐positive and gram‐negative infections may aid in battling MDR infections, these antibiotics must be used judiciously to maintain their clinical utility. Hospitalists will continue to play an important role in ensuring that hospitalized patients receive the most effective antimicrobial therapy to both treat the infection and prevent the development of resistance.
Case 1
A 53‐year‐old woman with a history of hemodialysis‐dependent end‐stage renal disease presents with left lower extremity pain and redness for the past 3 days. On physical examination, her temperature is 102.3F. Erythema, induration, and warmth are noted over her left lower leg and foot. Her history is remarkable for a line‐related bloodstream infection due to methicillin‐resistant Staphylococcus aureus (MRSA) 4 weeks ago. The infected line was removed and replaced with a right‐sided subclavian catheter. You note that the new line site is clean, not erythematous, and not tender. In the emergency department, the patient receives a dose of vancomycin for presumed MRSA cellulitis. Your patient wants to know if there are alternative agents for her infection so she does not require hospitalization.
Unfortunately, MRSA has become commonplace to the hospital setting. Among intensive care units in 2003, 64.4% of healthcare‐associated Staphylococcus aureus infections were caused by MRSA, compared with only 35.9% in 1992; a 3.1% increase per year.1, 2 Increased MRSA rates are not without consequence; a recent review suggests that MRSA infections kill nearly 19,000 hospitalized American patients annually.3 Of note, MRSA infection rates have also increased among previously healthy individuals. These community‐associated isolates (CA‐MRSA) often manifest as pyogenic skin and soft‐tissue infections (SSTIs). In a recent multicenter study, CA‐MRSA accounted for 59% of SSTIs among patients presenting to emergency rooms in the United States.4 In cases of SSTI, oral agents such as clindamycin, doxycycline, and trimethoprim‐sulfamethoxazole have proven successful. For invasive MRSA, vancomycin is still considered the standard treatment; however, several alternatives have emerged in recent years. The advantages and disadvantages of linezolid, daptomycin, tigecycline, and dalbavancin in the treatment of MRSA are described below.
Linezolid
Linezolid (Zyvox), an oxazolidinone approved in 2000, has been touted for its oral bioavailability, twice‐daily dosing, gram‐positive coverage, and unique mechanism of action. Like several other antimicrobials, linezolid inhibits bacterial protein synthesis. The drug binds to the 50S ribosomal subunit near its site of interaction with the 30S subunit, preventing formation of the 70S initiation complex.5 This site of action on the 50S subunit is unique to linezolid; as a result, cross‐resistance between linezolid and other antimicrobials that act at the 50S subunit (eg, chloramphenicol, macrolides, aminoglycosides, and tetracycline) does not occur.6
The oxazolidinones have excellent bacteriostatic activity against all pathogenic gram‐positive bacteria. The U.S. Food and Drug Administration (FDA) approved linezolid for the treatment of serious infections due to vancomycin‐resistant enterococci (VRE), including bacteremia, complicated skin and soft‐tissue infections (cSSTIs) due to Staphylococcus aureus (including MRSA), and nosocomial pneumonia due to Staphylococcus aureus (including MRSA) or penicillin‐susceptible Streptococcus pneumoniae (Table 1).
| Activity | Agent | FDA‐Approved Indications | Limitations in Use | Side Effects |
|---|---|---|---|---|
| ||||
| Gram‐positive | Daptomycin | cSSTIs; MSSA/MRSA bacteremia; MSSA/MRSA endocarditis | Not indicated for pneumonia (inhibited by pulmonary surfactant) | Reversible myopathy may be exacerbated by use with other medications |
| Quinupristin‐dalfopristin | Vancomycin‐resistant E. faecium; group A streptococci or MSSA cSSTIs | Myalgias and arthralgias; infusion site reaction;* thrombophlebitis;* liver enzyme elevation; inhibition of cytochrome p450 34a | ||
| Linezolid | Serious infections due to VRE; MSSA/MRSA cSSTIs; MSSA/MRSA nosocomial pneumonia; pneumonia due to penicillin‐sensitive S. pneumoniae | Not indicated for catheter‐related bloodstream infections or catheter site infections | Myelosuppression; serotonin syndrome; tyramine reaction; peripheral neuropathy; optic neuropathy | |
| Dalbavancin | Approval pending for cSSTIs | Not indicated for pneumonia bone and joint infection | Unknown | |
| Gram‐negative | Colistin | Gram‐negative bacteria that have demonstrated sensitivity to the drug | Not indicated for Proteus spp, Providencia spp, or Serratia spp | Acute tubular necrosis; neurotoxicity∥; bronchospasm |
| Gram‐positive and Gram‐negative | Ertapenem | Complicated intraabdominal infections#; cSSTIs; acute pelvic infections; complicated UTIs; community‐acquired pneumonia; prophylaxis of SSI following colorectal surgery in adult patients | Not indicated for Pseudomonas, Acinetobacter, S. maltophilia | Cross‐reactivity with penicillin; cross‐reactivity with cephalosporins; caution use if history of seizures |
| Doripenem | Complicated intraabdominal infections# and complicated UTIs, including pyelonephritis | Cross‐reactivity with penicillin; cross‐reactivity with cephalosporins; caution use if history of seizures | ||
| Tigecycline | cSSTIs (including those due to MRSA) complicated intraabdominal infections# | Nausea and vomiting; tooth discoloration in children | ||
In retrospective analyses of SSTIs due to MRSA, linezolid was as effective as vancomycin, resulting in higher clinical cure rates and shorter hospitalizations.7 As a result, linezolid has established a role in the treatment of community‐acquired MRSA SSTIs. Evidence limited to case reports and case series suggest that linezolid may also have a role in the treatment of bone and joint infections. In these cases, linezolid was often used because treatment with other agents had failed, the administration of other antibiotics was not indicated due to resistance patterns, the patient refused intravenous therapy, or the patient did not tolerate vancomycin. When such conditions exist, linezolid may be a consideration in cases of osteomyelitis or prosthetic joint infection.8
Potential side effects of linezolid may limit its use, especially for patients who require prolonged therapy (Table 1). Of note, as a reversible, relatively weak nonselective inhibitor of monoamine oxidase, linezolid may interact with adrenergic and serotonergic agents. Concomitant of a serotonin agent such as a selective serotonin‐reuptake inhibitor (SSRI) and linezolid should be approached with caution. Subsequent serotonin syndrome is characterized by autonomic dysfunction (eg, diaphoresis, tachycardia, hypertension) and neuromuscular hyperactivity (eg, muscle rigidity, clonus, hyperreflexia). Though infrequent, cases of reversible myelosuppression have been reported with linezolid use.9 Patients who will receive this drug for more than 2 weeks should be monitored for myelosuppression with a weekly complete blood count. Isolated reports suggest that the prolonged administration of linezolid (>28 days) may be associated with peripheral neuropathy and optic neuropathy. While prompt discontinuation of the drug often results in resolution of symptoms, peripheral or optic nerve injury can be permanent. The mechanism of injury is unclear, though mitochondrial toxicity is suspected.10
Daptomycin
Daptomycin (Cubicin), a cyclic lipopeptide, was discovered in the early 1980s, but skeletal muscle toxicity led to the discontinuation of early clinical trials. When a change from twice‐daily to once‐daily dosing in 2003 resulted in fewer adverse events, the FDA approved daptomycin to treat complicated skin and skin‐structure infections.11 Daptomycin binds to the cell membrane via a calcium‐dependent process, eventually disrupting the cell membrane potential. The bactericidal effect is limited to gram‐positive organisms.12
Daptomycin is effective against almost all gram‐positive organisms including methicillin‐susceptible Staphylococcus aureus (MSSA), MRSA, and VRE.12 As a result, it has FDA approval for the treatment of cSSTIs. While beta‐lactams remain the standard of care for MSSA bacteremia, daptomycin has FDA approval for bloodstream infections and right‐sided endocarditis due to MSSA or MRSA (Table 1).13 Daptomycin has poor penetration into alveolar fluid14 and is inhibited by pulmonary surfactants; as a consequence, it is not indicated for patients with pneumonia.15
Of note, daptomycin is mainly excreted via the kidneys and should be dose‐adjusted for patients with a creatinine clearance 30 mL/minute. A reversible myopathy may occur with daptomycin, requiring intermittent monitoring of creatinine kinase if prolonged use is anticipated. Caution should be used with the coadministration of medications that can also cause a myopathy, such as statins.
Tigecycline
Tigecycline (Tygacil) was approved for use by the FDA in 2005. The first in a class of new tetracycline analogs, the glycylcyclines, tigecycline is notable for its activity against several multidrug‐resistant (MDR) organisms, including MRSA, VRE, and Enterobacteriaceae carrying extended‐spectrum beta‐lactamases (ESBL). Tigecycline impairs bacterial protein synthesis by binding to the 30S ribosomal subunit. Due to steric hindrance from an N‐alkyl‐glycylamido group at position 9, tigecycline cannot be removed by most bacterial efflux mechanisms.16
Tigecycline has been approved for the therapy of cSSTIs, including those due to MSSA and MRSA. In a pooled analysis of 2 international, multicenter, phase III randomized, double‐blind trials, tigecycline was not inferior to vancomycin plus aztreonam in the treatment of cSSTIs. Of note, MRSA eradication rates were similar between patients treated with tigecycline and vancomycin plus aztreonam (78.1% and 75.8%, respectively).17
Dalbavancin
Dalbavancin (Zeven), a new, semisynthetic lipoglycopeptide, was approved by the FDA in late 2007; however, it has not been cleared for marketing. Though dalbavancin is derived from teicoplanin, its lipophilic anchor to the bacterial cell membrane makes the drug more potent than its predecessor. Dalbavancin interferes with bacterial cell wall synthesis by binding to the C‐terminal D‐alanyl‐D alanine of the growing peptidoglycan chains.18 Enhanced pharmacokinetic properties of dalbavancin (half‐life 149‐250 hours) allow it to be dosed once‐weekly, a novel concept in antimicrobial use.19
Like other glycopeptides, dalbavancin maintains in vitro activity against most gram‐positive aerobic organisms, including MRSA and penicillin‐susceptible and penicillin‐resistant strains of Streptococcus pneumoniae. Notably, when compared to vancomycin in vitro, the agent is more active against Enterococcus faecium and Enterococcus faecalis isolates. In a recent phase III double‐blind trial, dalbavancin was compared to linezolid for the treatment of cSSTIs. Dalbavancin was not inferior to linezolid (clinical success rate 90% vs. 92%). Of note, 51% of study patients with SSTI had infection due to MRSA. Microbiological response to dalbavancin paralleled the clinical success rate; MRSA eradication rates after dalbavancin and linezolid were 91% and 89%, respectively.20
Given its once‐weekly dosing, dalbavancin may be an attractive agent in the outpatient treatment of gram‐positive bacteremia. In a phase II study, dalbavancin administered as a single 1‐g dose, followed by a 500‐mg dose 1 week later, was comparable to 14 days of vancomycin for the treatment of catheter‐related bloodstream infections (CRBSI) due to coagulase‐negative staphylococci or S. aureus (including MRSA).21 Phase III studies are underway. At present, there is no evidence to support the use of dalbavancin for the treatment of pneumonia or bone and joint infections.
Despite the administration of vancomycin, the patient continues to experience fever and chills. Blood cultures drawn in the emergency department are now growing Enterococcus species. You review the patient's medical record and notice that she was colonized with VRE on a prior admission. You consider the antibiotic options for serious infections due to VRE.
Though rates of VRE have remained fairly stable in recent years,22 the pathogen continues to present a challenge to hospital epidemiologists. A national survey in 2004 suggested that nearly 30% of enterococci in U.S. intensive care units display vancomycin resistance.1 Additional U.S. surveillance data reveals that VRE accounts for 10% to 26% of enterococci hospital‐wide.23, 24 In 2005, a meta‐analysis noted that bloodstream infections due to VRE resulted in higher mortality rates than those due to vancomycin‐susceptible enterococci.25 This discrepancy is most evident among neutropenia patients.26 Unfortunately, the options for the treatment of serious infections due to VRE are limited. The advantages and disadvantages of linezolid, quinupristin‐dalfopristin, tigecycline, and daptomycin in the treatment for VRE are discussed below.
Linezolid
Currently, linezolid is the only oral drug that is FDA‐approved for the treatment of infections due to VRE, including bacteremia. Notably, linezolid therapy resulted in the cure of 77% of 22 cases of vancomycin‐resistant enterococcal endocarditis.27 Current guidelines by the Infectious Disease Society of America (IDSA) support the use of linezolid in cases of endocarditis due to ampicillin‐resistant and vancomycin‐resistant Enterococcus faecium.28 Unfortunately, recent reports highlight the emergence of linezolid‐resistant VRE,29 suggesting use of this drug should be limited to circumstances in which other alternatives do not exist.
Quinupristin‐Dalfopristin
Quinupristin‐dalfopristin (Synercid) was approved by the FDA in 1999. It is used in the treatment of infections caused by gram‐positive organisms and is a combination of 2 semisynthetic pristinamycin derivatives. They diffuse into bacteria and bind to different areas on the 50S ribosomal subunit, thereby inhibiting protein synthesis. Individually, quinupristin and dalfopristin are bacteriostatic but together they are bactericidal.30
Quinupristin‐dalfopristin has activity against Staphylococcus aureus (including MRSA), Streptococcus pneumoniae, gram‐positive anaerobes, and vancomycin‐sensitive and resistant Enterococcus faecium. It has little activity against Enterococcus faecalis.31 FDA‐approved uses of quinupristin‐dalfopristin are limited, but include the treatment of serious infections caused by vancomycin‐resistant E. faecium (VREF).32 In a study of 396 patients with VREF the clinical success rate of quinupristin‐dalfopristin was 73.6%.33 The drug also has FDA approval for the use in cSSTIs due to group A streptococci or MSSA.32 The use of this agent is limited due to its toxicity profile. In cases of serious VRE‐related infection, quinupristin‐dalfopristin is often only utilized if linezolid cannot be tolerated.
Daptomycin
In vitro studies suggest that daptomycin is active against enterococci, including vancomycin‐resistant isolates.34 However, clinical data on the use of this agent in the treatment of infections due to VRE are lacking. FDA approval for the use of daptomycin in cSSTI included the treatment of 45 patients infected with Enterococcus faecalis.13 In addition, several reports have detailed the successful treatment of VRE bloodstream infections with daptomycin,35, 36 including a case series of VRE endocarditis.37 To determine the role of this agent in the treatment of invasive infections due to VRE, further study is needed.
You decide to discontinue vancomycin and administer linezolid. The patient's vascular catheter is removed; catheter‐tip cultures grow >1000 colonies of VRE. Blood cultures the following day are negative and a new catheter is placed. You ask the patient to continue oral linezolid to complete a 2‐week course. A review of her medication list reveals that she is not taking SSRIs or monoamine oxidase inhibitors (MAOIs).
While linezolid has retained its FDA indication for VRE bacteremia, empiric use in suspected cases of CRBSI or catheter site infection is not advised. In an open‐label trial among seriously ill patients with intravascular catheter‐related infections, linezolid use was associated with a higher mortality when compared to vancomycin/oxacillin. Interestingly, mortality among linezolid‐treated patients included those with CRBSI due to gram‐negative pathogens, due to both gram‐negative and gram‐positive pathogens, or due to an identifiable pathogen; mortality rates did not differ among patients with gram‐positive infections only.38
Case 2
A 27‐year‐old male with a history of T10 paraplegia following a motor vehicle accident presents with abdominal pain, fever, and chills. He notes that he experiences these symptoms when he has a urinary tract infection (UTI), a frequent complication of his chronic indwelling suprapubic catheter. You review his medical record and notice that he has had prior UTIs with multiple gram‐negative rods over the past 2 years, including MDR Pseudomonas and Acinetobacter. When his urine culture grows >100,000 colonies of gram‐negative rods, you initiate meropenem and consider the options for treatment of these MDR pathogens.
According to national U.S. surveillance in 2001, 22% of Pseudomonas aeruginosa were resistant to imipenem, an increase of 32% from 1997.39 More alarming is the recent development of MDR P. aeruginosa, a pathogen resistant not only to the beta‐lactams (including the carbapenems) but to the fluoroquinolones and aminoglycosides as well.40 MDR P. aeruginosa is virulent, and has been associated with higher rates of mortality, longer hospital stays, and greater cost.41
Already equipped with intrinsic resistance to the aminopenicillins and first‐generation and second‐generation cephalosporins, A. baumannii has gained recent notoriety with acquired resistance to beta‐lactams, aminoglycosides, fluoroquinolones, and tetracyclines. Most notably, carbapenem‐resistant A. baumannii has emerged due to enzymes capable of hydrolyzing imipenem. Like MDR P. aeruginosa, MDR A. baumannii infection has led to longer hospital stays42 and increased patient mortality43 when compared to infections with more susceptible strains.
Therapeutic options for these MDR gram‐negative pathogens remain limited, but the advent of doripenem and the return of colistin may play a role in treatment. The use of these 2 agents and tigecycline in the treatment of MDR P. aeruginosa and/or A. baumannii are described below.
Doripenem
In October 2007, the FDA approved the use of doripenem (Doribax), a much‐anticipated carbapenem. In structure, doripenem resembles meropenem and does not require a renal dehydropeptidase I inhibitor (eg, cilastatin).44 Similar to other beta‐lactams, doripenem binds to penicillin‐binding proteins (PBPs), inhibiting PBP‐directed cell wall synthesis.
Like imipenem and meropenem, doripenem has broad‐spectrum antimicrobial activity. It demonstrates in vitro activity against most gram‐positive pathogens including MSSA and ampicillin‐sensitive enterococci. Doripenem also has in vitro activity against most gram‐negative pathogens (including ESBL‐producing Enterobacteriaceae) and most anaerobes, including Bacteriodes fragilis. Most notably, when compared to other carbapenems, doripenem has demonstrated better in vitro activity against Pseudomonas aeruginosa.45 However, clinical implications of this in vitro activity are unclear.
When compared to meropenem or levofloxacin for the treatment of complicated UTIs, doripenem is an effective alternative. Clinical response rates among affected patients were 95% to 96% with doripenem, 89% with meropenem, and 90% with levofloxacin.46, 47 Doripenem was not inferior to meropenem in patients with serious lower respiratory tract infections, and comparable to imipenem‐cilastin and pipercillin‐tazobactam for the treatment of nosocomial or ventilator‐associated pneumonia (VAP).48, 49 Finally, for the treatment of complicated intraabdominal infections, doripenem was not inferior to meropenem; both drugs achieved microbiologic cure rates of >84%.50
Currently, doripenem is FDA‐approved for the treatment of complicated intraabdominal infections (eg, appendicitis, pancreatitis, cholecystitis, peritonitis) and complicated lower UTIs or pyelonephritis (Table 1). Given its expanded spectrum of activity, use of doripenem should be limited to circumstances in which a MDR pathogen is highly suspected or confirmed.
Colistin
Colistin (Coly‐Mycin M) falls within the family of polymyxin antibiotics, which were discovered in 1947. Colistin has been available for almost 50 years for the treatment of infections caused by gram‐negative bacteria, including Pseudomonas spp. However, early use of colistin was associated with significant nephrotoxicity. Its use decreased markedly with the advent of new antibiotics that had the same antimicrobial spectrum and a better side effect profile. With the emergence of MDR gram‐negative bacteria, colistin has returned to limited clinical use.51 As a polymyxin, colistin is a cell membrane detergent. It disrupts the cell membrane, causing leakage of bacterial cell content and ultimately cell death.52
Colistin has bactericidal activity against most gram‐negative bacteria including Acinetobacter spp, and members of the family Enterobacteriaceae (eg, Klebsiella spp, Escherichia coli, Enterobacter spp), including those producing ESBLs.53 Colistin is not active against several predominant gram‐negative pathogens including Proteus spp, Providencia spp, or Serratia spp (Table 1).
In 2007, several studies suggested that colistin monotherapy was effective for patients with VAP due to MDR P. aeruginosa or A. baumannii isolate.54, 55 A third trial that year suggested that colistin may have a role in the treatment of MDR P. aeruginosa among neutropenic patients. In that study, infected patients receiving colistin monotherapy experienced higher rates of clinical and microbiologic response than those receiving other antipseudomonal agents (eg, beta‐lactams or fluoroquinolones if active against the isolate).56 While uncontrolled studies suggest that the use of colistin in combination with other antimicrobials (including carbapenems, ampicillin‐sulbactam, aminoglycosides, and rifampin) may have some success in the treatment of VAP due to MDR A. baumannii,57, 58 further trials are needed.
Currently, colistin has FDA approval only for the treatment of acute infections due to gram‐negative bacteria that have demonstrated susceptibility to the drug and is therefore administered on a case by case basis. Although it has been used via the inhalation route to treat infections in cystic fibrosis patients, colistin does not have FDA approval for this indication.
Tigecycline
Tigecycline is approved for the treatment of complicated intraabdominal infections based on the results of 2 international, multicenter, phase III, randomized, double‐blind trials. In this pooled analysis, tigecycline was as effective and as safe as imipenem/cilastatin. Notably, study patients were not severely ill (baseline APACHE II score of 6.0).59 FDA approval suggests tigecycline use be focused on intraabdominal infections due to members of the family Enterobacteriaceae (eg, Klebsiella spp, Escherichia coli, Enterobacter spp), including those producing ESBLs, vancomycin‐sensitive enterococci, and/or MSSA. Notably, tigecycline lacks significant in vitro activity against Pseudomonas spp, Proteus spp, or Providencia spp. It has demonstrated in vitro activity against MDR strains of Acinetobacter spp (Table 1).
Given its bacteriostatic activity, tigecycline's effectiveness in the treatment bacteremia is unclear.
In addition, as no published studies have addressed its activity among seriously ill patients, tigecycline is considered a second‐line or third‐line agent for SSTI and complicated intraabdominal infections. Evidence for use of tigecycline for the treatment of UTIs is lacking and, as a rule, its use should be limited to scenarios in which alternatives for the proven or suspected pathogens do not exist.
The urine isolate is identified as Escherichia coli. You review the susceptibility profile and determine that this isolate is an ESBL‐producing strain. In addition, the patient's isolate demonstrates resistance to the fluoroquinolones and trimethoprim‐sulfamethoxazole. You consider other options for treatment of this ESBL‐producing E. coli.
According to national surveillance data, more than 20% of Klebsiella isolates in U.S. intensive care units produced ESBLs in 2003, a 47% increase when compared to 1998.39 Bloodstream infections due to ESBL‐producing isolates have led to increased length of hospital stay,60, 61 increased hospital costs,4 improper antibiotic use,5 and, most notably, increased mortality.61‐63 Of concern, ESBLs have been demonstrated within community Enterobacteriaceae isolates, most notably due to CTX‐M beta‐lactamase production among E. coli. In addition to ESBL production, these community E. coli isolates tend to express fluoroquinolone and trimethoprim‐sulfamethoxazole resistance.64 Carbapenems remain the mainstay of therapy for serious infections due to ESBL‐producing organisms. The once‐daily dosing of ertapenem makes this agent an attractive alternative for outpatient management.
Ertapenem
Ertapenem (Invanz) obtained FDA approval for use in the United States in 2001 and in the European Union in 2002.65 Similar to doripenem, ertapenem blocks cell wall synthesis by binding to specific penicillin‐binding proteins (PBPs).
Ertapenem has activity against numerous gram‐positive and gram‐negative bacteria as well as some anaerobic microorganisms. The FDA‐approved indications include complicated intraabdominal infections, cSSTIs, acute pelvic infections, complicated UTIs, and community‐acquired pneumonias (Table 1).66 Of note, in contrast to other carabapenems, ertapenem does not have activity against Pseudomonas aeruginosa or Acinetobacter spp.67
Ertapenem is approved as a single daily dose of 1 g and can be administered intravenously or intramuscularly. Changes in dosing must also be considered for critically ill patients. When administered to patients with VAP, ertapenem achieved a lower maximum concentration and area under the curve.68 In such patients, it is recommended that the dosage interval be decreased or that a continuous infusion of ertapenem be administered.
The patient's symptoms improve on meropenem. A peripherally‐inserted central catheter is placed for the administration of intravenous antibiotics at home. You prescribe ertapenem (1 g/day) for the remainder of a 14‐day course.
Conclusions
MDR bacteria continue to present a clinical challenge to hospitalists. Proper treatment of patients infected with these organisms is necessary, as inappropriate antibiotic use for MDR bacterial infections has been associated with longer hospital stays, greater cost, and, in some cases, increased mortality. Unfortunately, antibiotic production and development has declined steadily in the past 25 years. To minimize the rate of antimicrobial resistance, physicians must take care to prescribe antibiotics appropriately. While these promising new agents for resistant gram‐positive and gram‐negative infections may aid in battling MDR infections, these antibiotics must be used judiciously to maintain their clinical utility. Hospitalists will continue to play an important role in ensuring that hospitalized patients receive the most effective antimicrobial therapy to both treat the infection and prevent the development of resistance.
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004.Am J Infect Control.2004;32:470–485.
- ,,,,,.Changes in the epidemiology of methicillin‐resistant Staphylococcus aureus in intensive care units in US hospitals, 1992‐2003.Clin Infect Dis.2006;42:389–391.
- ,,, et al.Invasive methicillin‐resistant Staphylococcus aureus infections in the United States.JAMA.2007;298:1763–1771.
- ,,,,,.Emergence of community‐acquired methicillin‐resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft tissue infections.Ann Intern Med.2006;144:309–317.
- ,,,.The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria.Antimicrob Agents Chemother.1998;42:3251–3255.
- ,.Activity of linezolid against gram‐positive cocci possessing genes conferring resistance to protein synthesis inhibitors.J Antimicrob Chemother.2000;45:797–802.
- ,,.Clinical and economic outcomes of oral linezolid versus intravenous vancomycin in the treatment of MRSA‐complicated, lower‐extremity skin and soft‐tissue infections caused by methicillin‐resistant Staphylococcus aureus.Am J Surg.2005;189:425–428.
- ,,,.Linezolid for the treatment of adults with bone and joint infections.Intern J Antimicrob Agents.2007;29:233–239.
- .Efficacy and safety of linezolid in the treatment of skin and soft tissue infections.Eur J Clin Microbiol Infect Dis.2002;21:491–498.
- ,,.Linezolid‐associated peripheral and optic neuropathy, lactic acidosis, and serotonin syndrome.Pharmacotherapy.2007;27(8):1189–1197.
- ,.Development of daptomycin for gram‐positive infections.J Antimicrob Chemother.2000;46(4):523–526.
- .Daptomycin and tigecycline: a review of clinical efficacy in the antimicrobial era.Expert Opin Pharmacother.2007;8(14):2279–2292.
- ,,, et al.Daptomycin verses standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus.N Engl J Med.2006:355(7):653–665.
- .Lipopeptides, focusing on daptomycin, for the treatment of gram‐positive infections.Expert Opin Invest Drugs.2004;13:1159–1169.
- .Alternatives to vancomycin for the treatment of methicillin‐resistant Staphylococcus aureus infections.Clin Infect Dis.2007;45(suppl 3):S184–S190.
- .Tigecycline: a new glycylcycline for treatment of serious infections.Clin Infect Dis.2005;41(suppl 5):S303–S314.
- ,,, et al.The efficacy and safety of tigecycline in the treatment of skin and skin‐structure infections: results of 2 double‐blind phase 3 comparison studies with vancomycin‐aztreonam.Clin Infect Dis.2005;41(suppl 5):S341–S353.
- ,.Origin, structure, and activity in vitro and in vivo of dalbavancin.J Antimicrob Chemother2005;55(suppl S2):ii15–ii20.
- ,.Dalbavancin: a novel lipoglycopeptide antibacterial.Pharmacotherapy2006;26:908–918.
- ,,, et al.Randomized, double‐blind comparison of a once‐weekly dalbavancin versus twice‐daily linezolid therapy for the treatment of complicated skin and skin structure infections.Clin Infect Dis.2005;41:1407–1415.
- ,,, et al.Efficacy and safety of weekly dalbavancin therapy for catheter‐related bloodstream infection caused by gram‐positive pathogens.Clin Infect Dis.2005;40:374–380.
- ,.Vancomycin‐resistant staphylococci and enterococci: epidemiology and control.Curr Opin Infect Dis.2005;18:300–305.
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992‐June 2001, issued August 2001.Am J Infect Control.2001;29:404–421.
- ,,,,, et al.Antimicrobial resistance trends and outbreak frequency in United States hospitals.Clin Infect Dis.2004;38:78–85.
- ,,,.Comparison of mortality associated with vancomycin‐resistant and vancomycin‐susceptible enterococcal bloodstream infections: a meta‐analysis.Clin Infect Dis.2005;41:327–333.
- ,.Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection.J Infect Dis.2005;191(4):588–595.
- ,,,,,.Linezolid for the treatment of multidrug‐resistant gram positive infections: experience from a compassionate‐use program.Clin Infect Dis.2003;36:159–168.
- ,,, et al.Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America.Circulation.2005;111(23):e394–e434.
- ,,.Nosocomial spread of linezolid‐resistant, vancomycin‐resistant Enterococcus faecium.N Engl J Med.2002;346:867–869.
- ,.Novel antibacterial agents for skin and skin structure infections.J Am Acad Dermatol.2004;50(3):331–340.
- ,,.New antimicrobial agents as therapy for resistant gram‐positive cocci.Eur J Clin Microbiol Infect Dis.2008;27(1):3–15.
- .Quinupristin‐dalfopristin and linezolid: evidence and opinion.Clin Infect Dis.2003;36(4):473–481.
- ,,,,,.The efficacy and safety of quinupristin/dalfopristin for the treatment of infections caused by vancomycin‐resistant Enterococcus faecium. Synercid Emergency‐Use Study Group.J Antimicrob Chemother.1999:44(2):251–261.
- ,,.Evaluation of the in vitro activity of daptomycin against 19615 clinical isolates of gram‐positive cocci collected in North American hospitals (2002‐2005).Diagn Microbiol Infect Dis.2007;57(4):459–465.
- ,,,,.Daptomycin in the treatment of vancomycin‐resistant Enterococcus faecium bacteremia in neutropenic patients.J Infect.2007;54(6):567–571.
- ,,,,.Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia.Scand J Infect Dis.2006;38:290–292.
- ,,.Daptomycin for the treatment of gram‐positive bacteremia and infective endocarditis: a retrospective case series of 31 patients.Pharmacotherapy.2006;26(3):347–352.
- Pfizer Pharmacia and Upjohn Company. United States Pharmacopeia. Zyvox. Available at: http://media.pfizer.com/files/products/uspi_zyvox.pdf. Accessed April 2009.
- NNIS System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2003, issued August 2003.Am J Infect Control.2003;31(8):481–498.
- .Resistance in nonfermenting gram‐negative bacteria: multidrug resistance to the maximum.Am J Med.2006;119:S29–S36.
- ,,, et al.Emergence of antibiotic‐resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents.Antimicrob Agents Chemother.1999;43(6):1379–1382.
- ,,, et al.Multidrug‐resistant Acinetobacter infection mortality rate and length of hospitalization.Emerg Infect Dis.2007;13:97–103.
- ,,, et al.Bloodstream infections due to Acinetobacter spp: epidemiology, risk factors, and impact of multi‐drug resistance.Eur J Clin Microbiol Infect Dis.2008;27(7):607–612.
- ,,,,.Doripenem (S‐4661), a novel carbapenem: comparative activity against contemporary pathogens including bactericidal action and preliminary in vitro methods evaluation.J Antimicrob Chemother.2004;54:144–154.
- ,,.Antimicrobial activity of doripenem (S‐4661): a global surveillance report.Clin Microbiol Infect.2005;11:974–984.
- ,,, et al.Intravenous therapy with. doripenem versus levofloxacin with an option for oral step‐down therapy in the treatment of complicated urinary tract infections and pyelonephritis. 17th European Congress of Clinical Microbiology and Infectious Diseases and the 25th International Congress of Chemotherapy. Munich, Germany. March 31‐April 3, 2007. Abstract no. 833 plus poster.
- .New uses for older antibiotics: nitrofurantoin, amikacin, colistin, polymyxin B, doxycyline, and minocycline revisited.Med Clin North Am.2006;90(6):1089–1107.
- ,,, et al.Efficacy and safety of doripenem versus piperacillin/tazobactam in nosocomial pneumonia: a randomized, open‐label, multicenter study.Curr Med Res Opin.2008;24(7):2113–2126.
- ,,, et al.Efficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator‐associated pneumonia: a multicenter, randomized study.Crit Care Med.2008;36(4):1089–1096.
- ,,, et al.Efficacy and tolerability of IV doripenem versus meropenem in adults with complicated intra‐abdominal infection: a phase III, prospective, multicenter, randomized, double‐blind, noninferiority study.Clin Ther.2008;30(5):868–883.
- ,,,,.Evaluation of colistin as an agent against multi‐resistant Gram‐negative bacteria.Int J Antimicrob Agents.2005;25(1):11–25.
- .New uses for older antibiotics: nitrofurantoin, amikacin, colistin, polymyxin B, doxycycline, and minocycline revisited.Med Clin North Am.2006;90(6):1089–1107.
- ,.Colistin: the revival of polymyxins for the management of multidrug‐resistant gram‐negative bacterial infections.Clin Infect Dis.2005;40(9):1333–1341.
- ,,, et al.Ventilator‐associated pneumonia (VAP) due to susceptible only to colistin microorganisms.Eur Respir J.2007;30(2):307–313.
- ,,, et al.Safety and efficacy of colistin compared with imipenem in the treatment of ventilator‐associated pneumonia: a matched case‐control study.Intensive Care Med.2007;33(7):1162–1167.
- ,,, et al.Colistin is effective in treatment of infections caused by multidrug‐resistant Pseudomonas aeruginosa in cancer patients.Antimicrob Agents Chemother.2007;51(6):1905–1911.
- ,,,,,.Combination therapy with intravenous colistin for management of infections due to multidrug‐resistant gram‐negative bacteria in patients without cystic fibrosis.Antimicrob Agents Chemother.2005;49:3136–3146.
- ,,, et al.Combined colistin and rifampicin therapy for carbapenem‐resistant Acinetobacter baumannii infections: clinical outcome and adverse events.Clin Microbiol Infect.2005;11:682–683.
- ,,, et al.The efficacy and safety of tigecycline for the treatment of complicated intra‐abdominal infections: analysis of pooled clinical trial data.Clin Infect Dis.2005;41(suppl 5):S354–S367.
- ,,,,.Clinical implications of extended‐spectrum beta‐lactamase‐producing Klebsiella pneumoniae bacteraemia.J Hosp Infect.2002;52:99–106.
- ,,,,,.Clinical and economic impact of bacteremia with extended spectrum beta‐lactamase–producing Enterobacteriaceae.Antimicrob Agents Chemother.2006;50:1257–1262.
- ,,, et al.Ceftazidime‐resistant Klebsiella pneumoniae bloodstream infection in children with febrile neutropenia.Int J Infect Dis.2000;4:21–25.
- ,,, et al.Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended‐ spectrum beta‐lactamases.Clin Infect Dis.2004;39:31–37.
- ,.Extended‐spectrum beta‐lactamase‐producing Enterobacteriaceae: an emerging public‐health concern.Lancet Infect Dis.2008;8(3):159–166.
- ,.Ertapenem, the first of a new group of carbapenems.J Antimicrob Chemother.2003;52(4):538–542.
- Merck 2006.
- ,,.Ertapenem: the new carbapenem 5 years after first FDA licensing for clinical practice.Expert Opin Pharmacother.2007;8(2):237–256.
- ,,, et al.Ertapenem in critically ill patients with early‐onset ventilator‐associated pneumonia: pharmacokinetics with special consideration of free‐drug concentration.J Antimicrob Chemother.2007;59(2):277–284.
- ,.Quinupristin/dalfopristin: a therapeutic review.Clin Ther.2001;23(1):24–44.
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004.Am J Infect Control.2004;32:470–485.
- ,,,,,.Changes in the epidemiology of methicillin‐resistant Staphylococcus aureus in intensive care units in US hospitals, 1992‐2003.Clin Infect Dis.2006;42:389–391.
- ,,, et al.Invasive methicillin‐resistant Staphylococcus aureus infections in the United States.JAMA.2007;298:1763–1771.
- ,,,,,.Emergence of community‐acquired methicillin‐resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft tissue infections.Ann Intern Med.2006;144:309–317.
- ,,,.The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria.Antimicrob Agents Chemother.1998;42:3251–3255.
- ,.Activity of linezolid against gram‐positive cocci possessing genes conferring resistance to protein synthesis inhibitors.J Antimicrob Chemother.2000;45:797–802.
- ,,.Clinical and economic outcomes of oral linezolid versus intravenous vancomycin in the treatment of MRSA‐complicated, lower‐extremity skin and soft‐tissue infections caused by methicillin‐resistant Staphylococcus aureus.Am J Surg.2005;189:425–428.
- ,,,.Linezolid for the treatment of adults with bone and joint infections.Intern J Antimicrob Agents.2007;29:233–239.
- .Efficacy and safety of linezolid in the treatment of skin and soft tissue infections.Eur J Clin Microbiol Infect Dis.2002;21:491–498.
- ,,.Linezolid‐associated peripheral and optic neuropathy, lactic acidosis, and serotonin syndrome.Pharmacotherapy.2007;27(8):1189–1197.
- ,.Development of daptomycin for gram‐positive infections.J Antimicrob Chemother.2000;46(4):523–526.
- .Daptomycin and tigecycline: a review of clinical efficacy in the antimicrobial era.Expert Opin Pharmacother.2007;8(14):2279–2292.
- ,,, et al.Daptomycin verses standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus.N Engl J Med.2006:355(7):653–665.
- .Lipopeptides, focusing on daptomycin, for the treatment of gram‐positive infections.Expert Opin Invest Drugs.2004;13:1159–1169.
- .Alternatives to vancomycin for the treatment of methicillin‐resistant Staphylococcus aureus infections.Clin Infect Dis.2007;45(suppl 3):S184–S190.
- .Tigecycline: a new glycylcycline for treatment of serious infections.Clin Infect Dis.2005;41(suppl 5):S303–S314.
- ,,, et al.The efficacy and safety of tigecycline in the treatment of skin and skin‐structure infections: results of 2 double‐blind phase 3 comparison studies with vancomycin‐aztreonam.Clin Infect Dis.2005;41(suppl 5):S341–S353.
- ,.Origin, structure, and activity in vitro and in vivo of dalbavancin.J Antimicrob Chemother2005;55(suppl S2):ii15–ii20.
- ,.Dalbavancin: a novel lipoglycopeptide antibacterial.Pharmacotherapy2006;26:908–918.
- ,,, et al.Randomized, double‐blind comparison of a once‐weekly dalbavancin versus twice‐daily linezolid therapy for the treatment of complicated skin and skin structure infections.Clin Infect Dis.2005;41:1407–1415.
- ,,, et al.Efficacy and safety of weekly dalbavancin therapy for catheter‐related bloodstream infection caused by gram‐positive pathogens.Clin Infect Dis.2005;40:374–380.
- ,.Vancomycin‐resistant staphylococci and enterococci: epidemiology and control.Curr Opin Infect Dis.2005;18:300–305.
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992‐June 2001, issued August 2001.Am J Infect Control.2001;29:404–421.
- ,,,,, et al.Antimicrobial resistance trends and outbreak frequency in United States hospitals.Clin Infect Dis.2004;38:78–85.
- ,,,.Comparison of mortality associated with vancomycin‐resistant and vancomycin‐susceptible enterococcal bloodstream infections: a meta‐analysis.Clin Infect Dis.2005;41:327–333.
- ,.Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection.J Infect Dis.2005;191(4):588–595.
- ,,,,,.Linezolid for the treatment of multidrug‐resistant gram positive infections: experience from a compassionate‐use program.Clin Infect Dis.2003;36:159–168.
- ,,, et al.Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America.Circulation.2005;111(23):e394–e434.
- ,,.Nosocomial spread of linezolid‐resistant, vancomycin‐resistant Enterococcus faecium.N Engl J Med.2002;346:867–869.
- ,.Novel antibacterial agents for skin and skin structure infections.J Am Acad Dermatol.2004;50(3):331–340.
- ,,.New antimicrobial agents as therapy for resistant gram‐positive cocci.Eur J Clin Microbiol Infect Dis.2008;27(1):3–15.
- .Quinupristin‐dalfopristin and linezolid: evidence and opinion.Clin Infect Dis.2003;36(4):473–481.
- ,,,,,.The efficacy and safety of quinupristin/dalfopristin for the treatment of infections caused by vancomycin‐resistant Enterococcus faecium. Synercid Emergency‐Use Study Group.J Antimicrob Chemother.1999:44(2):251–261.
- ,,.Evaluation of the in vitro activity of daptomycin against 19615 clinical isolates of gram‐positive cocci collected in North American hospitals (2002‐2005).Diagn Microbiol Infect Dis.2007;57(4):459–465.
- ,,,,.Daptomycin in the treatment of vancomycin‐resistant Enterococcus faecium bacteremia in neutropenic patients.J Infect.2007;54(6):567–571.
- ,,,,.Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia.Scand J Infect Dis.2006;38:290–292.
- ,,.Daptomycin for the treatment of gram‐positive bacteremia and infective endocarditis: a retrospective case series of 31 patients.Pharmacotherapy.2006;26(3):347–352.
- Pfizer Pharmacia and Upjohn Company. United States Pharmacopeia. Zyvox. Available at: http://media.pfizer.com/files/products/uspi_zyvox.pdf. Accessed April 2009.
- NNIS System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2003, issued August 2003.Am J Infect Control.2003;31(8):481–498.
- .Resistance in nonfermenting gram‐negative bacteria: multidrug resistance to the maximum.Am J Med.2006;119:S29–S36.
- ,,, et al.Emergence of antibiotic‐resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents.Antimicrob Agents Chemother.1999;43(6):1379–1382.
- ,,, et al.Multidrug‐resistant Acinetobacter infection mortality rate and length of hospitalization.Emerg Infect Dis.2007;13:97–103.
- ,,, et al.Bloodstream infections due to Acinetobacter spp: epidemiology, risk factors, and impact of multi‐drug resistance.Eur J Clin Microbiol Infect Dis.2008;27(7):607–612.
- ,,,,.Doripenem (S‐4661), a novel carbapenem: comparative activity against contemporary pathogens including bactericidal action and preliminary in vitro methods evaluation.J Antimicrob Chemother.2004;54:144–154.
- ,,.Antimicrobial activity of doripenem (S‐4661): a global surveillance report.Clin Microbiol Infect.2005;11:974–984.
- ,,, et al.Intravenous therapy with. doripenem versus levofloxacin with an option for oral step‐down therapy in the treatment of complicated urinary tract infections and pyelonephritis. 17th European Congress of Clinical Microbiology and Infectious Diseases and the 25th International Congress of Chemotherapy. Munich, Germany. March 31‐April 3, 2007. Abstract no. 833 plus poster.
- .New uses for older antibiotics: nitrofurantoin, amikacin, colistin, polymyxin B, doxycyline, and minocycline revisited.Med Clin North Am.2006;90(6):1089–1107.
- ,,, et al.Efficacy and safety of doripenem versus piperacillin/tazobactam in nosocomial pneumonia: a randomized, open‐label, multicenter study.Curr Med Res Opin.2008;24(7):2113–2126.
- ,,, et al.Efficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator‐associated pneumonia: a multicenter, randomized study.Crit Care Med.2008;36(4):1089–1096.
- ,,, et al.Efficacy and tolerability of IV doripenem versus meropenem in adults with complicated intra‐abdominal infection: a phase III, prospective, multicenter, randomized, double‐blind, noninferiority study.Clin Ther.2008;30(5):868–883.
- ,,,,.Evaluation of colistin as an agent against multi‐resistant Gram‐negative bacteria.Int J Antimicrob Agents.2005;25(1):11–25.
- .New uses for older antibiotics: nitrofurantoin, amikacin, colistin, polymyxin B, doxycycline, and minocycline revisited.Med Clin North Am.2006;90(6):1089–1107.
- ,.Colistin: the revival of polymyxins for the management of multidrug‐resistant gram‐negative bacterial infections.Clin Infect Dis.2005;40(9):1333–1341.
- ,,, et al.Ventilator‐associated pneumonia (VAP) due to susceptible only to colistin microorganisms.Eur Respir J.2007;30(2):307–313.
- ,,, et al.Safety and efficacy of colistin compared with imipenem in the treatment of ventilator‐associated pneumonia: a matched case‐control study.Intensive Care Med.2007;33(7):1162–1167.
- ,,, et al.Colistin is effective in treatment of infections caused by multidrug‐resistant Pseudomonas aeruginosa in cancer patients.Antimicrob Agents Chemother.2007;51(6):1905–1911.
- ,,,,,.Combination therapy with intravenous colistin for management of infections due to multidrug‐resistant gram‐negative bacteria in patients without cystic fibrosis.Antimicrob Agents Chemother.2005;49:3136–3146.
- ,,, et al.Combined colistin and rifampicin therapy for carbapenem‐resistant Acinetobacter baumannii infections: clinical outcome and adverse events.Clin Microbiol Infect.2005;11:682–683.
- ,,, et al.The efficacy and safety of tigecycline for the treatment of complicated intra‐abdominal infections: analysis of pooled clinical trial data.Clin Infect Dis.2005;41(suppl 5):S354–S367.
- ,,,,.Clinical implications of extended‐spectrum beta‐lactamase‐producing Klebsiella pneumoniae bacteraemia.J Hosp Infect.2002;52:99–106.
- ,,,,,.Clinical and economic impact of bacteremia with extended spectrum beta‐lactamase–producing Enterobacteriaceae.Antimicrob Agents Chemother.2006;50:1257–1262.
- ,,, et al.Ceftazidime‐resistant Klebsiella pneumoniae bloodstream infection in children with febrile neutropenia.Int J Infect Dis.2000;4:21–25.
- ,,, et al.Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended‐ spectrum beta‐lactamases.Clin Infect Dis.2004;39:31–37.
- ,.Extended‐spectrum beta‐lactamase‐producing Enterobacteriaceae: an emerging public‐health concern.Lancet Infect Dis.2008;8(3):159–166.
- ,.Ertapenem, the first of a new group of carbapenems.J Antimicrob Chemother.2003;52(4):538–542.
- Merck 2006.
- ,,.Ertapenem: the new carbapenem 5 years after first FDA licensing for clinical practice.Expert Opin Pharmacother.2007;8(2):237–256.
- ,,, et al.Ertapenem in critically ill patients with early‐onset ventilator‐associated pneumonia: pharmacokinetics with special consideration of free‐drug concentration.J Antimicrob Chemother.2007;59(2):277–284.
- ,.Quinupristin/dalfopristin: a therapeutic review.Clin Ther.2001;23(1):24–44.
A SAFE DC
Homeless patients are admitted to the hospital more frequently for both medical and psychiatric conditions as compared with domiciled but otherwise similar patients.13 They are also more likely to be hospitalized for conditions usually managed in the outpatient setting, such as cellulitis and respiratory infections.35 Physicians have reported a lower threshold for admission of patients whose conditions will worsen on the streets.4 Homeless inpatients are typically younger and may be hospitalized for longer than comparable patients with housing, often at higher cost.4, 5 These patients suffer from an average of 8 to 9 active medical problems6 and markedly increased mortality,710 with an average life expectancy of 45 years.7 Many homeless patients are uninsured or underinsured11, 12 and receive no ambulatory medical care.11 These patients are often cared for by hospitalists.
A general understanding of the unique needs of the homeless population is paramount for the hospitalist who strives to provide high‐quality care. The most commonly referenced definition of homelessness from the McKinney‐Vento Homeless Assistance Act defines a homeless person as an individual who lacks a fixed, regular, and adequate nighttime residence or a person who resides in a shelter, welfare hotel, transitional program, or place not ordinarily used as regular sleeping accommodations, such as streets, cars, movie theaters, abandoned buildings, or on the streets.13 This definition is often extended to include those who are occasionally but unstably housed with family or friends.14 Undomiciled and unstably housed patients face many barriers in obtaining healthcare, including cognitive or developmental impairment, cultural or linguistic issues, unreliable means of transportation, inability to pay for medications and supplies, and addiction and substance abuse. Systemic barriers include inadequate health insurance, limited access to health services, and provider bias or ignorance toward the issues of homelessness.
A hospitalist working with homeless patients may be discouraged by perceived inability to arrange reliable follow‐up or may be frustrated by hospital readmission resulting from patient noncompliance. Commonly, crisis management takes precedence over addressing the fundamental issues of homelessness.15, 16 Managing transitions of care at discharge, a vulnerable time for all hospitalized patients,17 is often particularly difficult when a patient has no place to go. We present here a review of selected literature that may inform care of the hospitalized homeless inpatient, providing background information on burden of disease, and supplementing this with evidence‐based and consensus‐based recommendations for adaptations of care. Additionally, we propose a simple mnemonic checklist, A SAFE DC, and discuss systems‐based approaches to the challenges of providing care to this population.
A SAFE DC: A Conceptual Framework for Care of the Homeless Inpatient
The mnemonic checklist A SAFE DC is an acronym for the 7 parts of a conceptual framework for care of the homeless inpatient (Table 1).
| A = assess housing status |
| S = screening and prevention |
| A = address primary care issues |
| F = follow‐up care |
| E = end of life discussions |
| D = discharge instructions, simple and realistic |
| C = communication method after discharge |
A: Assess Housing Situation
Hospitals are not required to collect homelessness data. Where such data are collected, they are often inaccurate and internally inconsistent. In 1 survey of inpatients at a public hospital, over 25% of inpatients met strict criteria for homelessness.18 Effective discharge planning begins on admission.15 Hospitalists should ask specifically about housing status at the onset of hospitalization.19 This should be done in a direct, yet sensitive, manner. Given the recent economic downturn, increasing numbers of individuals and families are marginally housed; these patients may not show outward signs of homelessness and may not volunteer this information during the initial encounter. Be aware that some patients may become homeless during hospitalization,18 often as a result of inability to work or attend to financial matters during an inpatient stay. Resultant medical debt is a common cause of personal bankruptcy and homelessness following discharge.
Although it is accepted that a patient should be medically stable prior to discharge and that the decision to discharge should be based on medical, not financial considerations,20, 21 other standards for discharge vary from provider to provider. Hospitalists may be more cautious in discharging a patient without a stable home,4 yet facilitating outpatient follow‐up care or arranging transfer to a sheltered, structured environment can lengthen the hospital stay. Many cities offer formal medical respite care in a number of forms well described in the literature, including free‐standing2225 or shelter‐based units,25, 26 or skilled nursing facilities that contract directly with hospitals for short stays. One innovative model is the hoptel, or hospital hotel,27 a temporary housing facility proximate to the hospital to which self‐sufficient homeless patients may be discharged for recuperation. Some hospitals distribute motel vouchers at discharge.22, 25 All of these options provide opportunities for rest and recovery. Some facilities are staffed with a nurse who can check vital signs and provide wound care. Respite discharge may decrease early readmission and death rates23 and decrease repeat hospitalizations,24 particularly in human immunodeficiency virus (HIV) patients.
The National Health Care for the Homeless Council (NHCHC) maintains a national map and directory of respite care programs and services (see Table 2). Hospital providers should develop familiarity with all programs offered in a given geographic area and work closely with case managers and social workers to ensure that a homeless patient is considered for all programs for which he or she is eligible.
|
| NHCHC ( |
| Clinical Practice Guidelines ( |
| Cardiovascular disease |
| HIV/AIDS |
| Otitis media |
| Asthma |
| Chlamydial and gonococcal infections |
| Reproductive healthcare |
| Diabetes mellitus (wallet‐sized personal health history available for homeless patients) |
| Clinical Practice Resources ( |
| Shelter Health Fact Sheets for patients (in English and Spanish) ( |
| NHCHC Clinicians' Network ( |
| Respite resources, including Introduction to Medical Respite Care ( |
| Discharge Planning resources ( |
| National Coalition for the Homeless ( |
| Directory of local homeless service organizations by state ( |
| National housing database for the homeless and low‐income ( |
| Homeless Health Care Los Angeles ( |
| Representative programs: Hospital Discharge Planning Training seminar ( |
S: Screening and Prevention
In addition to treating the presenting condition, a hospitalist should evaluate homeless patients for disease processes common in indigence. A full physical examination, preferably unclothed, is also recommended.28 Homelessness markedly increases an individual's risk of chronic medical conditions. Reactive airway disease and chronic obstructive pulmonary disease (COPD) occur at higher rates as a result of tobacco and inhalational drug abuse. Diabetes mellitus, hypertension, and chronic liver and renal disease may remain undetected for years, with end‐organ effects commonly seen at presentation. Peripheral vascular disease is 10 to 15 times more frequent than in the general population.16, 28, 29 Tuberculosis, with prevalence rates greater than 30 times the national average,30 and other communicable diseases, including HIV, hepatitis B, and hepatitis C,16 are exceedingly prevalent and in some cases endemic.12 Infestations are also common. One out of 5 Health Care for the Homeless clients has an infectious or communicable disease.16 Up to two‐thirds of homeless individuals are HIV‐positive, with younger, Hispanic, and black populations at highest risk.29 Systemic infections may be traced to poor dentition, common in this population. Poor vision and skin conditions, also rampant,30 are easily overlooked in acute care encounters. The rate of drug and alcohol abuse in the homeless population may be as high as 8 times that of the general population.31 In 1 survey of homeless adults, the majority identified substance abuse as a major factor in ongoing homelessness.32 Mental illness prevalence in the may be as high as 80% to 95%33 and street violence is commonplace; more than 50% of homeless women have been sexually assaulted.11
There is a paucity of data on the effectiveness of inpatient health interventions for the homeless. In a 2005 systematic review of 45 studies evaluating the impact of various programs on homeless health, only 1 targeted an inpatient population.34 Furthermore, the literature suggests that street‐based or shelter‐based delivery of preventative services is most effective for undomiciled patients.35 Understanding these limitations, inpatient admission remains an opportunity to offer services that may decrease morbidity.
Evidence‐based preventative measures (Table 3) include vaccination against hepatitis A and hepatitis B in the intravenous drugusing homeless population. An accelerated hepatitis B vaccine administration schedule, with doses at 0, 7, and 21 days and a booster at 12 months, has been shown to increase completion rates.36 Drug users should be advised to utilize needle exchange programs and avoid sharing equipment. Sexually active homeless patients should be counseled regarding safe sexual practices and condom use. Consider tuberculosis screening with purified protein derivative (PPD) testing and spot sputum check, which have been shown in a shelter‐based intervention to detect an infection rate of 3.1%.37 Notably, within that cohort, symptom‐based screening was not found to be helpful. Influenza, diphtheria, tetanus, and pneumococcal vaccinations are also recommended, but have not been studied in regard to secondary decrease of infection rates in the homeless.
|
| Vaccines: hepatitis A and B, influenza, Pneumococcus, Td |
| Tobacco abuse: cessation counseling and resources |
| Substance abuse: information regarding needle exchange programs, social work consultation for treatment options |
| Tuberculosis: consider screening with PPD (spot sputum for AFB) |
| Sexual behavior: counseling on safer sex practices and STD risk |
| Domestic and street violence: social work consultation for counseling and resources |
| Mental health: depression screening, MMSE |
Admission to the hospital should be considered a treatable moment for substance abuse. In focus groups of homeless smokers, 76% of participants expressed intention to quit within 6 months and all were interested in using pharmacotherapy and behavioral treatments.38 In another study comparing admitted homeless vs. domiciled substance‐using adults, a higher percentage of the homeless patients were found to be in the action stage of change, as compared with the precontemplative or contemplative stage.39 When ongoing use is likely, recommended strategies include advocating for safer routes or patterns or use and praising small successes on the continuum to abstinence.40 Where such services are available, the hospitalist should coordinate with primary care providers (PCPs) and social workers to refer patients for drug treatment and rehabilitation. Likewise, mental health follow‐up should be confirmed and ongoing care coordinated with the patient's mental health case worker, if one exists.
A: Address Primary Care Issues
The inpatient setting is often a homeless patient's only ongoing source of medical care, but may not meet all of his or her healthcare needs. During an admission for congestive heart failure (CHF), for example, he or she may receive diuresis and afterload reduction but not outpatient interventions such lipid and blood pressure management. Chronic diagnoses, such as malignancy, may be viewed as secondary and remain unaddressed. Questions about extent of a hospitalist's obligations to provide primary care arise in cases where a patient has failed to establish (and the system failed to provide) an outpatient medical home.
Just as emergency department physicians have become de facto primary care providers for underserved patients, hospitalists can expect to provide routine care for patients facing homelessness. Some interventions traditionally considered outpatient services, such as pneumococcal vaccination or counseling regarding smoking cessation, are now identified as inpatient core quality measures. Whether sexually transmitted disease or colon cancer screening or evaluation of cardiac risk status, for example, should become inpatient services for medically indigent patients is open for debate. Whenever possible, our goal is to facilitate screening and specialty consultations in the inpatient setting when this will not unnecessarily prolong hospitalization.
F: Follow‐Up Care
Ideally, transfer of care occurs smoothly between the hospitalist and a PCP or specialist who will provide a patient's ongoing medical care. Because many homeless patients lack or cannot identify a consistent outpatient provider, they may require additional assistance to ensure they receive medical care after discharge. If the patient has a PCP, the hospitalist should initiate contact with this individual at admission and discharge, forwarding relevant records in a timely fashion, including a faxed or electronic discharge summary. We often provide patients with a hard copy of the discharge summary and ask them to hand‐carry it to any follow‐up appointments. When a patient has no PCP, the hospitalist should attempt to expedite establishment of primary care. Unfortunately, many communities have limited primary care availability for patients who lack health insurance, posing challenges for hospital providers and patients.
At our institution, follow‐up appointments are often made by a clerk or nurse who later relays the appointment date and time to the patient. Some clinics collect contact information and call the patient themselves. There are frequent lapses in this scheduling system; some patients never receive a follow‐up appointment because they have no means of contact. Providing a scheduled follow‐up date and time prior to discharge may circumvent this problem.41
It is also optimal if some options for follow‐up care do not require a previously scheduled appointment. At our institution, a postdischarge aftercare clinic fills this need for patients without an established PCP, until such a relationship can be established. Aftercare appointments are designed to address specific, time‐critical, clinical issues (eg, assessing response to antibiotics, follow‐up creatinine in patient on diuretics, etc). To the degree that it is possible, selecting a site for follow‐up care that minimizes transportation (eg, a shelter‐based clinic) may improve the likelihood of follow‐up. It is wise to ask the patient when and where he or she would prefer to be seen. Consider that evening appointments may be best for day workers.28 Some authors have advocated that providers consider dispensing fewer numbers of medications at any given time, in order to enhance compliance with the follow‐up appointments,28 even if this may not reflect optimal medical management.
Careful consideration should be given before ordering tests for which results may not be available prior to anticipated discharge. These may include microbiological cultures, pathology reports, or sexually transmitted disease screening, including HIV testing. Note that even when a patient does have an established PCP, the hospitalist's liability for medical care may persist after hospital discharge. Emergency room physicians, for example, have been found liable for lack of postdischarge communication of radiologic findings.42
Timely and thorough documentation is critical. In many cases, a hospitalist is the only physician aware of a homeless patient's active medical issues. On admission, records should be thoroughly reviewed to ensure that pressing concerns, even those not traditionally requiring hospitalization, are addressed in a timely fashion. Detailed discharge documentation helps to ensure that ongoing issues are not lost during follow‐up. It may be useful to provide a given patient with a portable summary of his or her medical history for self‐reference and facilitation of ongoing care, particularly for those with a history of seeking healthcare at multiple facilities.28
E: End‐of‐Life Discussions
Given the increased mortality and decreased life expectancy of the homeless population, an acute care hospitalization provides an excellent opportunity to discuss end‐of‐life preferences, particularly if the patient does not have an established PCP. Focus groups have noted little difference in the range of end‐of‐life preferences of the homeless as compared with the general population, yet a common fear among the homeless is that of an anonymous death, or a life without remembrance.43 Many homeless patients believe that physicians would use deceit in withdrawing life‐sustaining support or that their body might be disposed of without consent. They identify advance directives as a way to regain control over their lives.44 It is important to obtain and update emergency contacts for friends and family on each admission. Notably, homeless people often designate an unrelated friend or associate as their decision maker, rather than family, and express that it is less important to have family present at their death as it is to be cared for compassionately and respectfully by those who are present.44
D: Discharge Instructions Simple and Realistic
Health illiteracy profoundly affects homeless patients. In the predischarge narratives of 21 low‐income urban medical inpatients, almost one‐half believed it would be impossible to follow medical advice at discharge.45 Healthcare providers may overestimate a patient's ability to understand discharge instructions46 and to provide self‐care at the time of discharge.47 Homeless patients are at high risk for disease relapse following discharge, given chaotic living conditions and lack of social support.1 The presence of community support has been shown to decrease the likelihood of rehospitalization.48
Medication compliance poses a particular challenge. In 1 study, one‐third of homeless patients reported inability to comply with medications.2 Cost, storage capability, and complexity of regimen are common obstacles. Side effects should be considered when medications are selected, since common side effects like gastrointestinal upset or diarrhea, or desired effects like diuresis, may be intolerable if a patient cannot reliably access a restroom. Physicians should also weigh the possibility that discharge medications and supplies may be abused or stolen on the streets. Difficulty accessing routine meals can be particularly problematic in homeless patients with diabetes, who must eat on a regular schedule in order to avoid hypoglycemia. Diabetic goals may be adjusted accordingly to minimize risk. Diet may also be an issue if a patient must take a medication with food, as with some antiretrovirals. The physician must anticipate an erratic diet and, whenever possible, dose medications accordingly. Directly observed therapy for diseases such as tuberculosis is optimal if the ability to comply is in question.28 The NHCHC has developed guidelines for adaptations of care in homeless patients with a variety of clinical conditions, including diabetes mellitus, HIV, cardiovascular disease, and asthma; these are available for reference and download on their website (Table 2).
Illiteracy and low educational level also impact compliance. In 1 sample of indigent psychiatric patients, 76% read at or below the seventh grade level.49 Aftercare instructions should be easy to understand by those with lower levels of education (fourth grade level or less), written down in simple language, and reviewed verbally by nurse, pharmacist, and physician. Consider initiating projects within your hospital to streamline discharge instruction forms.50 The use of pictorial or video‐assisted discharge instructions for common diagnoses is an area of promise.17, 51, 52
Of note, there is no body of literature addressing the extent to which hospitalists or other inpatient physicians alter treatment goals at the time of discharge for homeless patients, but this may be a common occurrence and warrants further study.
C: Communication Methods After Discharge
Before discharge, clarify how a patient can be contacted for additional test results or information regarding follow‐up appointments. Although some homeless patients maintain mobile phones, telephone‐based methods used by some hospitalists for postdischarge follow‐up53, 54 may be unreliable in this population. Some shelters or respite facilities will accept messages for clients who reside there; others will provide clients with access to voicemail or e‐mail. For those patients who are technologically savvy, free e‐mail accounts can readily be obtained and accessed at public facilities, such as the public library. Contact information for a case manager can also be very useful. We occasionally ask patients to return to the hospital to retrieve test results or a message from their physician at a predetermined time and place. Where safe and appropriate, providing patients with direct physician contact information (rather than general hospital information) may minimize communication barriers.
The Big Picture: Systems‐Based Approaches to the Discharge of Homeless Patients
The discharge of homeless patients is suited to a comprehensive, interdisciplinary approach. There are many challenges to effective discharge planning: lack of time, lack of process ownership at the institutional level, financial constraints, and perhaps most significantly, lack of consensus regarding best practices.19 There is growing acknowledgement of the need to develop policies and standardize practice in this area. Hospitalists are uniquely situated to contribute to the development of new initiatives at the institutional, local, and national level.
Interventions (Table 4) may be as simple as the identification of a dedicated social worker for all homeless discharges15, 21, 55 or creation of a hospital‐wide discharge planning committee or inpatient homeless consultation service.15, 21 The distribution of discharge planning guides for patients and resource lists to providers is also gaining in popularity.21, 56 Some innovations specifically target clinicians, such as training seminars that teach communication skills and motivational interviewing and build familiarity with safety‐net services within the community.21, 57 Community‐based programs include medical respite care services, previously discussed, and the facilitation of preferred provider relationships directed by hospitals toward skilled nursing facilities willing to accept homeless and other challenging clients.15
|
| Discharge planning training seminars for the clinician |
| SWAT team for difficult discharges |
| Hospital‐wide discharge planning committee |
| Inpatient homeless consult service |
| Dedicated social worker for homeless discharges |
| Preferred provider status for skilled nursing facilities |
| Medical respite care |
| Discharge planning guide or resource list for homeless patients |
Homelessness has also been identified as an area of focus by state governments, with many states funding initiatives to improve training and assistance to homeless providers, policies for discharge planning from public institutions, and homeless needs assessments. Some states have gone so far as to determine that discharge to an emergency shelter is not appropriate.19 On the national level, large advocacy organizations such as the NHCHC and National Coalition for the Homeless have spearheaded Housing First efforts on behalf of homeless patients and providers throughout the country. Such programs have been shown to decrease healthcare expenditures, emergency department visits, and hospitalizations in certain homeless populations.58, 59 Check the NHCHC website for consolidated discharge planning program development resources for healthcare institutions (
Commentary
Homeless patients frequently require more energy and services at the time of service in order to achieve standard medical care. Optimally, a patient assumes full responsibility for his or her health, but there may be limits to this responsibility for selected patients,60 especially in light of limited access to primary care. Understandably, homeless patients may focus more on immediate physical needs (eg, food, shelter, safety) than on chronic medical problems. In addition, they may experience a sense of unwelcomeness from healthcare providers that they perceive as discrimination; this may dissuade them from seeking care.61 The inpatient physician should aim to build trust with each encounter. As suggested by 1 author, it is important to promise only what can be delivered and deliver what is promised.62 Involving the patient in care and decision‐making is the most important first step in accomplishing this goal.
It is important in caring for homeless patients to reframe one's notion of a successful outcome.16 Ideally, on resolution of his or her acute medical issues, a homeless patient would be discharged to permanent housing with substance abuse and mental health treatment. This scenario is unfortunately rare. The hospitalist often has little ability to arrange stable, on‐demand housing at discharge. He or she is best advised to focus on optimizing acute care delivery at the point of care and maximize opportunities for future health.
It has been suggested that the discontinuity inherent in the hospitalist model may confer a special obligation on hospital medicine providers to abide by a more rigorous standard of care42; one might argue that this obligation becomes even more compelling when applied to this vulnerable population. In 1 study, a disturbing 27% of an American cohort of homeless adults had no healthcare contacts in the year prior to death, underscoring this group's underutilization of health services. Armed with this knowledge, hospitalists should seize every healthcare interaction as an opportunity to offer therapies with potential for longer‐term benefit.
- ,,,,.Mental health and social characteristics of the homeless: a survey of mission users.Am J Public Health.1986;76:519–524.
- ,,.Factors associated with the health care utilization of homeless persons.JAMA.2001;285(2):200–206.
- ,,,,,.Hospitalization in an urban homeless population: the Honolulu urban homeless project.Ann Int Med.1992:116:299–303.
- ,,,,.Hospitalization costs associated with homelessness in New York City.N Engl J Med.1997;338:1734–1740.
- ,.Homelessness; health service use and related costs.Med Care.1998;38:1256–1264.
- ,,, et al.Health and mental problems of homeless men and women in Baltimore.JAMA.1989;262:1352–1357.
- ,,, et al.Mortality in a cohort of homeless adults in Philadelphia.N Engl J Med.1994;331:304–309.
- . Utilization and costs of medical services by homeless persons: a review of the literature and implications for the future. National Health Care for the Homeless Council website. Published April 1999. Available at: http://www.nhchc.org/Publications/utilization.htm. Accessed Month Year.
- ,,,,.Causes of death in homeless adults in Boston.Ann Intern Med.1997;126:625–628.
- ,.Risk of death among homeless women; a cohort study and review of the literature.CMAJ.2004;170(8):1243–1237.
- ,.Health care for homeless persons.N Engl J Med.2004;350:2329–2332.
- .Homelessness and health.CMAJ.2001;164:229–233.
- U.S. Congress..McKinney Homeless Assistance Act. Publ. No. 100–77, 101 Stat. 484.Washington, DC:U.S. Congress;1987.
- ,.How can health services effectively meet the health needs of homeless people?Br J Gen Pract.2006;56:286–293.
- Homeless Health Care Los Angeles. Homelessness: An Overview and Effective Strategies for Discharge Planning of Homeless Patients. Available at: http://www.nhchc.org/Publications/utilizations.html. Accessed Month Year.
- ,,. Balancing act: clinical practices that respond to the needs of homeless people. 1998 National Symposium on Homelessness Research. U.S. Health and Human Services. Available at: http://aspe.hhs.gov/ProgSys/homeless/symposium/8‐Clinical.htm. Accessed June2009.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,.Identifying homelessness at an urban public hospital: a moving target?J Health Care Poor Underserved.2005;16:297–307.
- ,,.The role of effective discharge planning in preventing homelessness.J Prim Prev.2007;28:229–243.
- ,.Saying goodbye: ethical issues in the stewardship of bed spaces.J Clin Ethics.2005;16:170–175.
- National Health Care for the Homeless Council.Tools to help clinicians achieve effective discharge planning.Healing Hands2008;12:1–6. Available at: http://www.nhchc.org/Network/HealingHands/2008/Oct2008Healing Hands.pdf. Accessed Juneyear="2009"2009.
- ,,, et al.It takes a village: a multidisciplinary model of the acute illness aftercare of individuals experiencing homelessness.J Health Care Poor Underserved.2005;16:257–272.
- ,,, et al. Hospital discharge to a homeless medical respite program prevents readmission [Abstract]. Boston Health Care for the Homeless Program. Published 2005. Available at: http://www.nhchc.org/Respite/RespiteResearcUpdateSept05.ppt. Accessed June2009.
- ,,,.The effects of respite care for homeless patients: a cohort study.Am J Public Health.2006:96:1278–1281.
- . Medical Respite Services for Homeless People: Practical Models. National Health Care for the Homeless Council. Published 1999. Available at: http://www.nhchc.org/Publications/MedicalRespiteServices.pdf. Accessed June2009.
- ,,,.Shelter‐based convalescence for homeless adults.Can J Public Health.2006;97:379–383.
- ,.Hoptel equalizes length of stay for homeless and domiciled inpatients.Med Care.2000;38:1003–1010.
- .The homeless in America: adapting your practice.Am Fam Physician.2006;74:1132–1138.
- . A Preliminary Review of Literature: Chronic Medical Illness and Homeless Individuals, Nashville, TN. National Health Care for the Homeless Council. Published April 2002. Available at: http://www.nhchc.org/Publications/literaturereview_chronicillness.pdf. Accessed June2009.
- ,.Diagnostic patterns in hospital use by an urban homeless population.West J Med.1989;151:472–478.
- ,,.Drug use disorders and treatment contact among homeless adults in Alameda County, California.Am J Public Health.1997;87:221–228.
- ,,,,,.Self‐reported changes in drug and alcohol use after becoming homeless.Am J Public Health.2004;94:830–835.
- .A review of physical and mental health in homeless persons.Public Health Rev.2001;29:13–33.
- ,,,.Interventions to improve the health of the homeless; a systematic review.Am J Prev Med.2005;29:311–319.
- ,,.Preventing and controlling emerging and reemerging transmissible diseases in the homeless.Emerg Infect Dis.2008;14:1353–1359.
- ,,.Comparison of conventional and accelerated hepatitis B immunisation schedules for homeless drug users.Commun Dis Public Health.2002;5:324–326.
- ,,,,,.Spot sputum screening: evaluation of an intervention in two homeless shelters.Int J Tuberc Lung Dis.1999;3:613–619.
- ,,, et al.Homelessness and smoking cessation: insights from focus groups.Nicotine Tob Res.2006;8:287–296.
- ,,,.The health encounter as a treatable moment for homeless substance‐using adults: the role of homelessness, health‐seeking behavior, readiness for behavior change and motivation for treatment.Addict Behav.2008;33:1239–1243.
- ,. To dance with grace: outreach and engagement to persons on the street. 1998 National Symposium on Homelessness Research. U.S. Health and Human Services. Available at: http://aspe.hhs.gov/ProgSys/homeless/symposium/6‐Outreach.htm. Accessed June2009.
- .The best way to improve emergency department follow‐up is actually to give the patient a specific appointment.J Gen Intern Med.2006;21:398.
- .Key legal principles for hospitalists.Am J Med.2001;11:5s–9s.
- ,,.Attitudes, experiences and beliefs affecting end of life decision‐making among homeless individuals.J Palliat Med.2005;8:36–48.
- ,,,,,.Dying on the streets; homeless person's concerns and desires about end of life care.J Gen Intern Med.2007;22:435–441.
- ,,.Understanding rehospitalization risk: can hospital discharge be modified to reduce recurrent hospitalization.J Hosp Med.2007:2:297–304.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,,,,,.Discharge planning: comparison of patients and nurses' perceptions of patients following hospital discharge.Image J Nurs Sch.1996;28:143–147.
- ,,,.Predicting health services utilization among homeless adults: a prospective analysis.J Health Care Poor Underserved.2000:11:212–230.
- ,.The prevalence of low literacy in an indigent psychiatric population.Psychiatr Serv.1999;50:262–263.
- Majority of emergency patients don't understand discharge instructions.ED Manag.2008;20:97–98.
- ,.Communicating information to patients: the use of cartoon illustrations to improve comprehension of instructions.Acad Emerg Med.1996;3:264–270.
- ,,,.The effectiveness of mobile discharge instruction videos (MDIVs) in communicating discharge instructions to patients with lacerations or sprains.South Med J.2009;102:239–247.
- .The importance of postdischarge telephone follow up from hospitalists: a view from the trenches.Am J Med.2001;111:43s–44s.
- ,.Using an interactive voice response system to improve patient safety following hospital discharge.Eval Clin Pract.2007;13:346–351.
- Resource Guide for Service Providers. Homeless Health Care Los Angeles. Available at: http://www.hhcla.org/training/pdf‐docs/2007%20RESOURCE%20GUIDE.pdf. Accessed June2009.
- Hospital Discharge Planning Training Workshop. Homeless Health Care Los Angeles. Available at: http://www.hhcla.org/discharge.htm. Accessed June2009.
- ,,, et al.Health care and public service use and costs before and after provision of housing for chronically homeless persons with severe alcohol problems.JAMA.2009;301:1349–1357.
- ,,,.Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults.JAMA.2009;301:1771–1778.
- .Limits on patient responsibility.J Med Phil.2005;30:189–206.
- ,,.Homeless people's perceptions of welcomeness and unwelcomeness in healthcare encounters.J Gen Intern Med.2007;22:1011–1017.
- .Increasing competency in the care of homeless patients [Teaching Tips].J Contin Educ Nurs.2008;39:153–154.
- ,,, et al.Health care utilization among homeless adults prior to death.J Health Care Poor Underserved.1999;12:50–58.
Homeless patients are admitted to the hospital more frequently for both medical and psychiatric conditions as compared with domiciled but otherwise similar patients.13 They are also more likely to be hospitalized for conditions usually managed in the outpatient setting, such as cellulitis and respiratory infections.35 Physicians have reported a lower threshold for admission of patients whose conditions will worsen on the streets.4 Homeless inpatients are typically younger and may be hospitalized for longer than comparable patients with housing, often at higher cost.4, 5 These patients suffer from an average of 8 to 9 active medical problems6 and markedly increased mortality,710 with an average life expectancy of 45 years.7 Many homeless patients are uninsured or underinsured11, 12 and receive no ambulatory medical care.11 These patients are often cared for by hospitalists.
A general understanding of the unique needs of the homeless population is paramount for the hospitalist who strives to provide high‐quality care. The most commonly referenced definition of homelessness from the McKinney‐Vento Homeless Assistance Act defines a homeless person as an individual who lacks a fixed, regular, and adequate nighttime residence or a person who resides in a shelter, welfare hotel, transitional program, or place not ordinarily used as regular sleeping accommodations, such as streets, cars, movie theaters, abandoned buildings, or on the streets.13 This definition is often extended to include those who are occasionally but unstably housed with family or friends.14 Undomiciled and unstably housed patients face many barriers in obtaining healthcare, including cognitive or developmental impairment, cultural or linguistic issues, unreliable means of transportation, inability to pay for medications and supplies, and addiction and substance abuse. Systemic barriers include inadequate health insurance, limited access to health services, and provider bias or ignorance toward the issues of homelessness.
A hospitalist working with homeless patients may be discouraged by perceived inability to arrange reliable follow‐up or may be frustrated by hospital readmission resulting from patient noncompliance. Commonly, crisis management takes precedence over addressing the fundamental issues of homelessness.15, 16 Managing transitions of care at discharge, a vulnerable time for all hospitalized patients,17 is often particularly difficult when a patient has no place to go. We present here a review of selected literature that may inform care of the hospitalized homeless inpatient, providing background information on burden of disease, and supplementing this with evidence‐based and consensus‐based recommendations for adaptations of care. Additionally, we propose a simple mnemonic checklist, A SAFE DC, and discuss systems‐based approaches to the challenges of providing care to this population.
A SAFE DC: A Conceptual Framework for Care of the Homeless Inpatient
The mnemonic checklist A SAFE DC is an acronym for the 7 parts of a conceptual framework for care of the homeless inpatient (Table 1).
| A = assess housing status |
| S = screening and prevention |
| A = address primary care issues |
| F = follow‐up care |
| E = end of life discussions |
| D = discharge instructions, simple and realistic |
| C = communication method after discharge |
A: Assess Housing Situation
Hospitals are not required to collect homelessness data. Where such data are collected, they are often inaccurate and internally inconsistent. In 1 survey of inpatients at a public hospital, over 25% of inpatients met strict criteria for homelessness.18 Effective discharge planning begins on admission.15 Hospitalists should ask specifically about housing status at the onset of hospitalization.19 This should be done in a direct, yet sensitive, manner. Given the recent economic downturn, increasing numbers of individuals and families are marginally housed; these patients may not show outward signs of homelessness and may not volunteer this information during the initial encounter. Be aware that some patients may become homeless during hospitalization,18 often as a result of inability to work or attend to financial matters during an inpatient stay. Resultant medical debt is a common cause of personal bankruptcy and homelessness following discharge.
Although it is accepted that a patient should be medically stable prior to discharge and that the decision to discharge should be based on medical, not financial considerations,20, 21 other standards for discharge vary from provider to provider. Hospitalists may be more cautious in discharging a patient without a stable home,4 yet facilitating outpatient follow‐up care or arranging transfer to a sheltered, structured environment can lengthen the hospital stay. Many cities offer formal medical respite care in a number of forms well described in the literature, including free‐standing2225 or shelter‐based units,25, 26 or skilled nursing facilities that contract directly with hospitals for short stays. One innovative model is the hoptel, or hospital hotel,27 a temporary housing facility proximate to the hospital to which self‐sufficient homeless patients may be discharged for recuperation. Some hospitals distribute motel vouchers at discharge.22, 25 All of these options provide opportunities for rest and recovery. Some facilities are staffed with a nurse who can check vital signs and provide wound care. Respite discharge may decrease early readmission and death rates23 and decrease repeat hospitalizations,24 particularly in human immunodeficiency virus (HIV) patients.
The National Health Care for the Homeless Council (NHCHC) maintains a national map and directory of respite care programs and services (see Table 2). Hospital providers should develop familiarity with all programs offered in a given geographic area and work closely with case managers and social workers to ensure that a homeless patient is considered for all programs for which he or she is eligible.
|
| NHCHC ( |
| Clinical Practice Guidelines ( |
| Cardiovascular disease |
| HIV/AIDS |
| Otitis media |
| Asthma |
| Chlamydial and gonococcal infections |
| Reproductive healthcare |
| Diabetes mellitus (wallet‐sized personal health history available for homeless patients) |
| Clinical Practice Resources ( |
| Shelter Health Fact Sheets for patients (in English and Spanish) ( |
| NHCHC Clinicians' Network ( |
| Respite resources, including Introduction to Medical Respite Care ( |
| Discharge Planning resources ( |
| National Coalition for the Homeless ( |
| Directory of local homeless service organizations by state ( |
| National housing database for the homeless and low‐income ( |
| Homeless Health Care Los Angeles ( |
| Representative programs: Hospital Discharge Planning Training seminar ( |
S: Screening and Prevention
In addition to treating the presenting condition, a hospitalist should evaluate homeless patients for disease processes common in indigence. A full physical examination, preferably unclothed, is also recommended.28 Homelessness markedly increases an individual's risk of chronic medical conditions. Reactive airway disease and chronic obstructive pulmonary disease (COPD) occur at higher rates as a result of tobacco and inhalational drug abuse. Diabetes mellitus, hypertension, and chronic liver and renal disease may remain undetected for years, with end‐organ effects commonly seen at presentation. Peripheral vascular disease is 10 to 15 times more frequent than in the general population.16, 28, 29 Tuberculosis, with prevalence rates greater than 30 times the national average,30 and other communicable diseases, including HIV, hepatitis B, and hepatitis C,16 are exceedingly prevalent and in some cases endemic.12 Infestations are also common. One out of 5 Health Care for the Homeless clients has an infectious or communicable disease.16 Up to two‐thirds of homeless individuals are HIV‐positive, with younger, Hispanic, and black populations at highest risk.29 Systemic infections may be traced to poor dentition, common in this population. Poor vision and skin conditions, also rampant,30 are easily overlooked in acute care encounters. The rate of drug and alcohol abuse in the homeless population may be as high as 8 times that of the general population.31 In 1 survey of homeless adults, the majority identified substance abuse as a major factor in ongoing homelessness.32 Mental illness prevalence in the may be as high as 80% to 95%33 and street violence is commonplace; more than 50% of homeless women have been sexually assaulted.11
There is a paucity of data on the effectiveness of inpatient health interventions for the homeless. In a 2005 systematic review of 45 studies evaluating the impact of various programs on homeless health, only 1 targeted an inpatient population.34 Furthermore, the literature suggests that street‐based or shelter‐based delivery of preventative services is most effective for undomiciled patients.35 Understanding these limitations, inpatient admission remains an opportunity to offer services that may decrease morbidity.
Evidence‐based preventative measures (Table 3) include vaccination against hepatitis A and hepatitis B in the intravenous drugusing homeless population. An accelerated hepatitis B vaccine administration schedule, with doses at 0, 7, and 21 days and a booster at 12 months, has been shown to increase completion rates.36 Drug users should be advised to utilize needle exchange programs and avoid sharing equipment. Sexually active homeless patients should be counseled regarding safe sexual practices and condom use. Consider tuberculosis screening with purified protein derivative (PPD) testing and spot sputum check, which have been shown in a shelter‐based intervention to detect an infection rate of 3.1%.37 Notably, within that cohort, symptom‐based screening was not found to be helpful. Influenza, diphtheria, tetanus, and pneumococcal vaccinations are also recommended, but have not been studied in regard to secondary decrease of infection rates in the homeless.
|
| Vaccines: hepatitis A and B, influenza, Pneumococcus, Td |
| Tobacco abuse: cessation counseling and resources |
| Substance abuse: information regarding needle exchange programs, social work consultation for treatment options |
| Tuberculosis: consider screening with PPD (spot sputum for AFB) |
| Sexual behavior: counseling on safer sex practices and STD risk |
| Domestic and street violence: social work consultation for counseling and resources |
| Mental health: depression screening, MMSE |
Admission to the hospital should be considered a treatable moment for substance abuse. In focus groups of homeless smokers, 76% of participants expressed intention to quit within 6 months and all were interested in using pharmacotherapy and behavioral treatments.38 In another study comparing admitted homeless vs. domiciled substance‐using adults, a higher percentage of the homeless patients were found to be in the action stage of change, as compared with the precontemplative or contemplative stage.39 When ongoing use is likely, recommended strategies include advocating for safer routes or patterns or use and praising small successes on the continuum to abstinence.40 Where such services are available, the hospitalist should coordinate with primary care providers (PCPs) and social workers to refer patients for drug treatment and rehabilitation. Likewise, mental health follow‐up should be confirmed and ongoing care coordinated with the patient's mental health case worker, if one exists.
A: Address Primary Care Issues
The inpatient setting is often a homeless patient's only ongoing source of medical care, but may not meet all of his or her healthcare needs. During an admission for congestive heart failure (CHF), for example, he or she may receive diuresis and afterload reduction but not outpatient interventions such lipid and blood pressure management. Chronic diagnoses, such as malignancy, may be viewed as secondary and remain unaddressed. Questions about extent of a hospitalist's obligations to provide primary care arise in cases where a patient has failed to establish (and the system failed to provide) an outpatient medical home.
Just as emergency department physicians have become de facto primary care providers for underserved patients, hospitalists can expect to provide routine care for patients facing homelessness. Some interventions traditionally considered outpatient services, such as pneumococcal vaccination or counseling regarding smoking cessation, are now identified as inpatient core quality measures. Whether sexually transmitted disease or colon cancer screening or evaluation of cardiac risk status, for example, should become inpatient services for medically indigent patients is open for debate. Whenever possible, our goal is to facilitate screening and specialty consultations in the inpatient setting when this will not unnecessarily prolong hospitalization.
F: Follow‐Up Care
Ideally, transfer of care occurs smoothly between the hospitalist and a PCP or specialist who will provide a patient's ongoing medical care. Because many homeless patients lack or cannot identify a consistent outpatient provider, they may require additional assistance to ensure they receive medical care after discharge. If the patient has a PCP, the hospitalist should initiate contact with this individual at admission and discharge, forwarding relevant records in a timely fashion, including a faxed or electronic discharge summary. We often provide patients with a hard copy of the discharge summary and ask them to hand‐carry it to any follow‐up appointments. When a patient has no PCP, the hospitalist should attempt to expedite establishment of primary care. Unfortunately, many communities have limited primary care availability for patients who lack health insurance, posing challenges for hospital providers and patients.
At our institution, follow‐up appointments are often made by a clerk or nurse who later relays the appointment date and time to the patient. Some clinics collect contact information and call the patient themselves. There are frequent lapses in this scheduling system; some patients never receive a follow‐up appointment because they have no means of contact. Providing a scheduled follow‐up date and time prior to discharge may circumvent this problem.41
It is also optimal if some options for follow‐up care do not require a previously scheduled appointment. At our institution, a postdischarge aftercare clinic fills this need for patients without an established PCP, until such a relationship can be established. Aftercare appointments are designed to address specific, time‐critical, clinical issues (eg, assessing response to antibiotics, follow‐up creatinine in patient on diuretics, etc). To the degree that it is possible, selecting a site for follow‐up care that minimizes transportation (eg, a shelter‐based clinic) may improve the likelihood of follow‐up. It is wise to ask the patient when and where he or she would prefer to be seen. Consider that evening appointments may be best for day workers.28 Some authors have advocated that providers consider dispensing fewer numbers of medications at any given time, in order to enhance compliance with the follow‐up appointments,28 even if this may not reflect optimal medical management.
Careful consideration should be given before ordering tests for which results may not be available prior to anticipated discharge. These may include microbiological cultures, pathology reports, or sexually transmitted disease screening, including HIV testing. Note that even when a patient does have an established PCP, the hospitalist's liability for medical care may persist after hospital discharge. Emergency room physicians, for example, have been found liable for lack of postdischarge communication of radiologic findings.42
Timely and thorough documentation is critical. In many cases, a hospitalist is the only physician aware of a homeless patient's active medical issues. On admission, records should be thoroughly reviewed to ensure that pressing concerns, even those not traditionally requiring hospitalization, are addressed in a timely fashion. Detailed discharge documentation helps to ensure that ongoing issues are not lost during follow‐up. It may be useful to provide a given patient with a portable summary of his or her medical history for self‐reference and facilitation of ongoing care, particularly for those with a history of seeking healthcare at multiple facilities.28
E: End‐of‐Life Discussions
Given the increased mortality and decreased life expectancy of the homeless population, an acute care hospitalization provides an excellent opportunity to discuss end‐of‐life preferences, particularly if the patient does not have an established PCP. Focus groups have noted little difference in the range of end‐of‐life preferences of the homeless as compared with the general population, yet a common fear among the homeless is that of an anonymous death, or a life without remembrance.43 Many homeless patients believe that physicians would use deceit in withdrawing life‐sustaining support or that their body might be disposed of without consent. They identify advance directives as a way to regain control over their lives.44 It is important to obtain and update emergency contacts for friends and family on each admission. Notably, homeless people often designate an unrelated friend or associate as their decision maker, rather than family, and express that it is less important to have family present at their death as it is to be cared for compassionately and respectfully by those who are present.44
D: Discharge Instructions Simple and Realistic
Health illiteracy profoundly affects homeless patients. In the predischarge narratives of 21 low‐income urban medical inpatients, almost one‐half believed it would be impossible to follow medical advice at discharge.45 Healthcare providers may overestimate a patient's ability to understand discharge instructions46 and to provide self‐care at the time of discharge.47 Homeless patients are at high risk for disease relapse following discharge, given chaotic living conditions and lack of social support.1 The presence of community support has been shown to decrease the likelihood of rehospitalization.48
Medication compliance poses a particular challenge. In 1 study, one‐third of homeless patients reported inability to comply with medications.2 Cost, storage capability, and complexity of regimen are common obstacles. Side effects should be considered when medications are selected, since common side effects like gastrointestinal upset or diarrhea, or desired effects like diuresis, may be intolerable if a patient cannot reliably access a restroom. Physicians should also weigh the possibility that discharge medications and supplies may be abused or stolen on the streets. Difficulty accessing routine meals can be particularly problematic in homeless patients with diabetes, who must eat on a regular schedule in order to avoid hypoglycemia. Diabetic goals may be adjusted accordingly to minimize risk. Diet may also be an issue if a patient must take a medication with food, as with some antiretrovirals. The physician must anticipate an erratic diet and, whenever possible, dose medications accordingly. Directly observed therapy for diseases such as tuberculosis is optimal if the ability to comply is in question.28 The NHCHC has developed guidelines for adaptations of care in homeless patients with a variety of clinical conditions, including diabetes mellitus, HIV, cardiovascular disease, and asthma; these are available for reference and download on their website (Table 2).
Illiteracy and low educational level also impact compliance. In 1 sample of indigent psychiatric patients, 76% read at or below the seventh grade level.49 Aftercare instructions should be easy to understand by those with lower levels of education (fourth grade level or less), written down in simple language, and reviewed verbally by nurse, pharmacist, and physician. Consider initiating projects within your hospital to streamline discharge instruction forms.50 The use of pictorial or video‐assisted discharge instructions for common diagnoses is an area of promise.17, 51, 52
Of note, there is no body of literature addressing the extent to which hospitalists or other inpatient physicians alter treatment goals at the time of discharge for homeless patients, but this may be a common occurrence and warrants further study.
C: Communication Methods After Discharge
Before discharge, clarify how a patient can be contacted for additional test results or information regarding follow‐up appointments. Although some homeless patients maintain mobile phones, telephone‐based methods used by some hospitalists for postdischarge follow‐up53, 54 may be unreliable in this population. Some shelters or respite facilities will accept messages for clients who reside there; others will provide clients with access to voicemail or e‐mail. For those patients who are technologically savvy, free e‐mail accounts can readily be obtained and accessed at public facilities, such as the public library. Contact information for a case manager can also be very useful. We occasionally ask patients to return to the hospital to retrieve test results or a message from their physician at a predetermined time and place. Where safe and appropriate, providing patients with direct physician contact information (rather than general hospital information) may minimize communication barriers.
The Big Picture: Systems‐Based Approaches to the Discharge of Homeless Patients
The discharge of homeless patients is suited to a comprehensive, interdisciplinary approach. There are many challenges to effective discharge planning: lack of time, lack of process ownership at the institutional level, financial constraints, and perhaps most significantly, lack of consensus regarding best practices.19 There is growing acknowledgement of the need to develop policies and standardize practice in this area. Hospitalists are uniquely situated to contribute to the development of new initiatives at the institutional, local, and national level.
Interventions (Table 4) may be as simple as the identification of a dedicated social worker for all homeless discharges15, 21, 55 or creation of a hospital‐wide discharge planning committee or inpatient homeless consultation service.15, 21 The distribution of discharge planning guides for patients and resource lists to providers is also gaining in popularity.21, 56 Some innovations specifically target clinicians, such as training seminars that teach communication skills and motivational interviewing and build familiarity with safety‐net services within the community.21, 57 Community‐based programs include medical respite care services, previously discussed, and the facilitation of preferred provider relationships directed by hospitals toward skilled nursing facilities willing to accept homeless and other challenging clients.15
|
| Discharge planning training seminars for the clinician |
| SWAT team for difficult discharges |
| Hospital‐wide discharge planning committee |
| Inpatient homeless consult service |
| Dedicated social worker for homeless discharges |
| Preferred provider status for skilled nursing facilities |
| Medical respite care |
| Discharge planning guide or resource list for homeless patients |
Homelessness has also been identified as an area of focus by state governments, with many states funding initiatives to improve training and assistance to homeless providers, policies for discharge planning from public institutions, and homeless needs assessments. Some states have gone so far as to determine that discharge to an emergency shelter is not appropriate.19 On the national level, large advocacy organizations such as the NHCHC and National Coalition for the Homeless have spearheaded Housing First efforts on behalf of homeless patients and providers throughout the country. Such programs have been shown to decrease healthcare expenditures, emergency department visits, and hospitalizations in certain homeless populations.58, 59 Check the NHCHC website for consolidated discharge planning program development resources for healthcare institutions (
Commentary
Homeless patients frequently require more energy and services at the time of service in order to achieve standard medical care. Optimally, a patient assumes full responsibility for his or her health, but there may be limits to this responsibility for selected patients,60 especially in light of limited access to primary care. Understandably, homeless patients may focus more on immediate physical needs (eg, food, shelter, safety) than on chronic medical problems. In addition, they may experience a sense of unwelcomeness from healthcare providers that they perceive as discrimination; this may dissuade them from seeking care.61 The inpatient physician should aim to build trust with each encounter. As suggested by 1 author, it is important to promise only what can be delivered and deliver what is promised.62 Involving the patient in care and decision‐making is the most important first step in accomplishing this goal.
It is important in caring for homeless patients to reframe one's notion of a successful outcome.16 Ideally, on resolution of his or her acute medical issues, a homeless patient would be discharged to permanent housing with substance abuse and mental health treatment. This scenario is unfortunately rare. The hospitalist often has little ability to arrange stable, on‐demand housing at discharge. He or she is best advised to focus on optimizing acute care delivery at the point of care and maximize opportunities for future health.
It has been suggested that the discontinuity inherent in the hospitalist model may confer a special obligation on hospital medicine providers to abide by a more rigorous standard of care42; one might argue that this obligation becomes even more compelling when applied to this vulnerable population. In 1 study, a disturbing 27% of an American cohort of homeless adults had no healthcare contacts in the year prior to death, underscoring this group's underutilization of health services. Armed with this knowledge, hospitalists should seize every healthcare interaction as an opportunity to offer therapies with potential for longer‐term benefit.
Homeless patients are admitted to the hospital more frequently for both medical and psychiatric conditions as compared with domiciled but otherwise similar patients.13 They are also more likely to be hospitalized for conditions usually managed in the outpatient setting, such as cellulitis and respiratory infections.35 Physicians have reported a lower threshold for admission of patients whose conditions will worsen on the streets.4 Homeless inpatients are typically younger and may be hospitalized for longer than comparable patients with housing, often at higher cost.4, 5 These patients suffer from an average of 8 to 9 active medical problems6 and markedly increased mortality,710 with an average life expectancy of 45 years.7 Many homeless patients are uninsured or underinsured11, 12 and receive no ambulatory medical care.11 These patients are often cared for by hospitalists.
A general understanding of the unique needs of the homeless population is paramount for the hospitalist who strives to provide high‐quality care. The most commonly referenced definition of homelessness from the McKinney‐Vento Homeless Assistance Act defines a homeless person as an individual who lacks a fixed, regular, and adequate nighttime residence or a person who resides in a shelter, welfare hotel, transitional program, or place not ordinarily used as regular sleeping accommodations, such as streets, cars, movie theaters, abandoned buildings, or on the streets.13 This definition is often extended to include those who are occasionally but unstably housed with family or friends.14 Undomiciled and unstably housed patients face many barriers in obtaining healthcare, including cognitive or developmental impairment, cultural or linguistic issues, unreliable means of transportation, inability to pay for medications and supplies, and addiction and substance abuse. Systemic barriers include inadequate health insurance, limited access to health services, and provider bias or ignorance toward the issues of homelessness.
A hospitalist working with homeless patients may be discouraged by perceived inability to arrange reliable follow‐up or may be frustrated by hospital readmission resulting from patient noncompliance. Commonly, crisis management takes precedence over addressing the fundamental issues of homelessness.15, 16 Managing transitions of care at discharge, a vulnerable time for all hospitalized patients,17 is often particularly difficult when a patient has no place to go. We present here a review of selected literature that may inform care of the hospitalized homeless inpatient, providing background information on burden of disease, and supplementing this with evidence‐based and consensus‐based recommendations for adaptations of care. Additionally, we propose a simple mnemonic checklist, A SAFE DC, and discuss systems‐based approaches to the challenges of providing care to this population.
A SAFE DC: A Conceptual Framework for Care of the Homeless Inpatient
The mnemonic checklist A SAFE DC is an acronym for the 7 parts of a conceptual framework for care of the homeless inpatient (Table 1).
| A = assess housing status |
| S = screening and prevention |
| A = address primary care issues |
| F = follow‐up care |
| E = end of life discussions |
| D = discharge instructions, simple and realistic |
| C = communication method after discharge |
A: Assess Housing Situation
Hospitals are not required to collect homelessness data. Where such data are collected, they are often inaccurate and internally inconsistent. In 1 survey of inpatients at a public hospital, over 25% of inpatients met strict criteria for homelessness.18 Effective discharge planning begins on admission.15 Hospitalists should ask specifically about housing status at the onset of hospitalization.19 This should be done in a direct, yet sensitive, manner. Given the recent economic downturn, increasing numbers of individuals and families are marginally housed; these patients may not show outward signs of homelessness and may not volunteer this information during the initial encounter. Be aware that some patients may become homeless during hospitalization,18 often as a result of inability to work or attend to financial matters during an inpatient stay. Resultant medical debt is a common cause of personal bankruptcy and homelessness following discharge.
Although it is accepted that a patient should be medically stable prior to discharge and that the decision to discharge should be based on medical, not financial considerations,20, 21 other standards for discharge vary from provider to provider. Hospitalists may be more cautious in discharging a patient without a stable home,4 yet facilitating outpatient follow‐up care or arranging transfer to a sheltered, structured environment can lengthen the hospital stay. Many cities offer formal medical respite care in a number of forms well described in the literature, including free‐standing2225 or shelter‐based units,25, 26 or skilled nursing facilities that contract directly with hospitals for short stays. One innovative model is the hoptel, or hospital hotel,27 a temporary housing facility proximate to the hospital to which self‐sufficient homeless patients may be discharged for recuperation. Some hospitals distribute motel vouchers at discharge.22, 25 All of these options provide opportunities for rest and recovery. Some facilities are staffed with a nurse who can check vital signs and provide wound care. Respite discharge may decrease early readmission and death rates23 and decrease repeat hospitalizations,24 particularly in human immunodeficiency virus (HIV) patients.
The National Health Care for the Homeless Council (NHCHC) maintains a national map and directory of respite care programs and services (see Table 2). Hospital providers should develop familiarity with all programs offered in a given geographic area and work closely with case managers and social workers to ensure that a homeless patient is considered for all programs for which he or she is eligible.
|
| NHCHC ( |
| Clinical Practice Guidelines ( |
| Cardiovascular disease |
| HIV/AIDS |
| Otitis media |
| Asthma |
| Chlamydial and gonococcal infections |
| Reproductive healthcare |
| Diabetes mellitus (wallet‐sized personal health history available for homeless patients) |
| Clinical Practice Resources ( |
| Shelter Health Fact Sheets for patients (in English and Spanish) ( |
| NHCHC Clinicians' Network ( |
| Respite resources, including Introduction to Medical Respite Care ( |
| Discharge Planning resources ( |
| National Coalition for the Homeless ( |
| Directory of local homeless service organizations by state ( |
| National housing database for the homeless and low‐income ( |
| Homeless Health Care Los Angeles ( |
| Representative programs: Hospital Discharge Planning Training seminar ( |
S: Screening and Prevention
In addition to treating the presenting condition, a hospitalist should evaluate homeless patients for disease processes common in indigence. A full physical examination, preferably unclothed, is also recommended.28 Homelessness markedly increases an individual's risk of chronic medical conditions. Reactive airway disease and chronic obstructive pulmonary disease (COPD) occur at higher rates as a result of tobacco and inhalational drug abuse. Diabetes mellitus, hypertension, and chronic liver and renal disease may remain undetected for years, with end‐organ effects commonly seen at presentation. Peripheral vascular disease is 10 to 15 times more frequent than in the general population.16, 28, 29 Tuberculosis, with prevalence rates greater than 30 times the national average,30 and other communicable diseases, including HIV, hepatitis B, and hepatitis C,16 are exceedingly prevalent and in some cases endemic.12 Infestations are also common. One out of 5 Health Care for the Homeless clients has an infectious or communicable disease.16 Up to two‐thirds of homeless individuals are HIV‐positive, with younger, Hispanic, and black populations at highest risk.29 Systemic infections may be traced to poor dentition, common in this population. Poor vision and skin conditions, also rampant,30 are easily overlooked in acute care encounters. The rate of drug and alcohol abuse in the homeless population may be as high as 8 times that of the general population.31 In 1 survey of homeless adults, the majority identified substance abuse as a major factor in ongoing homelessness.32 Mental illness prevalence in the may be as high as 80% to 95%33 and street violence is commonplace; more than 50% of homeless women have been sexually assaulted.11
There is a paucity of data on the effectiveness of inpatient health interventions for the homeless. In a 2005 systematic review of 45 studies evaluating the impact of various programs on homeless health, only 1 targeted an inpatient population.34 Furthermore, the literature suggests that street‐based or shelter‐based delivery of preventative services is most effective for undomiciled patients.35 Understanding these limitations, inpatient admission remains an opportunity to offer services that may decrease morbidity.
Evidence‐based preventative measures (Table 3) include vaccination against hepatitis A and hepatitis B in the intravenous drugusing homeless population. An accelerated hepatitis B vaccine administration schedule, with doses at 0, 7, and 21 days and a booster at 12 months, has been shown to increase completion rates.36 Drug users should be advised to utilize needle exchange programs and avoid sharing equipment. Sexually active homeless patients should be counseled regarding safe sexual practices and condom use. Consider tuberculosis screening with purified protein derivative (PPD) testing and spot sputum check, which have been shown in a shelter‐based intervention to detect an infection rate of 3.1%.37 Notably, within that cohort, symptom‐based screening was not found to be helpful. Influenza, diphtheria, tetanus, and pneumococcal vaccinations are also recommended, but have not been studied in regard to secondary decrease of infection rates in the homeless.
|
| Vaccines: hepatitis A and B, influenza, Pneumococcus, Td |
| Tobacco abuse: cessation counseling and resources |
| Substance abuse: information regarding needle exchange programs, social work consultation for treatment options |
| Tuberculosis: consider screening with PPD (spot sputum for AFB) |
| Sexual behavior: counseling on safer sex practices and STD risk |
| Domestic and street violence: social work consultation for counseling and resources |
| Mental health: depression screening, MMSE |
Admission to the hospital should be considered a treatable moment for substance abuse. In focus groups of homeless smokers, 76% of participants expressed intention to quit within 6 months and all were interested in using pharmacotherapy and behavioral treatments.38 In another study comparing admitted homeless vs. domiciled substance‐using adults, a higher percentage of the homeless patients were found to be in the action stage of change, as compared with the precontemplative or contemplative stage.39 When ongoing use is likely, recommended strategies include advocating for safer routes or patterns or use and praising small successes on the continuum to abstinence.40 Where such services are available, the hospitalist should coordinate with primary care providers (PCPs) and social workers to refer patients for drug treatment and rehabilitation. Likewise, mental health follow‐up should be confirmed and ongoing care coordinated with the patient's mental health case worker, if one exists.
A: Address Primary Care Issues
The inpatient setting is often a homeless patient's only ongoing source of medical care, but may not meet all of his or her healthcare needs. During an admission for congestive heart failure (CHF), for example, he or she may receive diuresis and afterload reduction but not outpatient interventions such lipid and blood pressure management. Chronic diagnoses, such as malignancy, may be viewed as secondary and remain unaddressed. Questions about extent of a hospitalist's obligations to provide primary care arise in cases where a patient has failed to establish (and the system failed to provide) an outpatient medical home.
Just as emergency department physicians have become de facto primary care providers for underserved patients, hospitalists can expect to provide routine care for patients facing homelessness. Some interventions traditionally considered outpatient services, such as pneumococcal vaccination or counseling regarding smoking cessation, are now identified as inpatient core quality measures. Whether sexually transmitted disease or colon cancer screening or evaluation of cardiac risk status, for example, should become inpatient services for medically indigent patients is open for debate. Whenever possible, our goal is to facilitate screening and specialty consultations in the inpatient setting when this will not unnecessarily prolong hospitalization.
F: Follow‐Up Care
Ideally, transfer of care occurs smoothly between the hospitalist and a PCP or specialist who will provide a patient's ongoing medical care. Because many homeless patients lack or cannot identify a consistent outpatient provider, they may require additional assistance to ensure they receive medical care after discharge. If the patient has a PCP, the hospitalist should initiate contact with this individual at admission and discharge, forwarding relevant records in a timely fashion, including a faxed or electronic discharge summary. We often provide patients with a hard copy of the discharge summary and ask them to hand‐carry it to any follow‐up appointments. When a patient has no PCP, the hospitalist should attempt to expedite establishment of primary care. Unfortunately, many communities have limited primary care availability for patients who lack health insurance, posing challenges for hospital providers and patients.
At our institution, follow‐up appointments are often made by a clerk or nurse who later relays the appointment date and time to the patient. Some clinics collect contact information and call the patient themselves. There are frequent lapses in this scheduling system; some patients never receive a follow‐up appointment because they have no means of contact. Providing a scheduled follow‐up date and time prior to discharge may circumvent this problem.41
It is also optimal if some options for follow‐up care do not require a previously scheduled appointment. At our institution, a postdischarge aftercare clinic fills this need for patients without an established PCP, until such a relationship can be established. Aftercare appointments are designed to address specific, time‐critical, clinical issues (eg, assessing response to antibiotics, follow‐up creatinine in patient on diuretics, etc). To the degree that it is possible, selecting a site for follow‐up care that minimizes transportation (eg, a shelter‐based clinic) may improve the likelihood of follow‐up. It is wise to ask the patient when and where he or she would prefer to be seen. Consider that evening appointments may be best for day workers.28 Some authors have advocated that providers consider dispensing fewer numbers of medications at any given time, in order to enhance compliance with the follow‐up appointments,28 even if this may not reflect optimal medical management.
Careful consideration should be given before ordering tests for which results may not be available prior to anticipated discharge. These may include microbiological cultures, pathology reports, or sexually transmitted disease screening, including HIV testing. Note that even when a patient does have an established PCP, the hospitalist's liability for medical care may persist after hospital discharge. Emergency room physicians, for example, have been found liable for lack of postdischarge communication of radiologic findings.42
Timely and thorough documentation is critical. In many cases, a hospitalist is the only physician aware of a homeless patient's active medical issues. On admission, records should be thoroughly reviewed to ensure that pressing concerns, even those not traditionally requiring hospitalization, are addressed in a timely fashion. Detailed discharge documentation helps to ensure that ongoing issues are not lost during follow‐up. It may be useful to provide a given patient with a portable summary of his or her medical history for self‐reference and facilitation of ongoing care, particularly for those with a history of seeking healthcare at multiple facilities.28
E: End‐of‐Life Discussions
Given the increased mortality and decreased life expectancy of the homeless population, an acute care hospitalization provides an excellent opportunity to discuss end‐of‐life preferences, particularly if the patient does not have an established PCP. Focus groups have noted little difference in the range of end‐of‐life preferences of the homeless as compared with the general population, yet a common fear among the homeless is that of an anonymous death, or a life without remembrance.43 Many homeless patients believe that physicians would use deceit in withdrawing life‐sustaining support or that their body might be disposed of without consent. They identify advance directives as a way to regain control over their lives.44 It is important to obtain and update emergency contacts for friends and family on each admission. Notably, homeless people often designate an unrelated friend or associate as their decision maker, rather than family, and express that it is less important to have family present at their death as it is to be cared for compassionately and respectfully by those who are present.44
D: Discharge Instructions Simple and Realistic
Health illiteracy profoundly affects homeless patients. In the predischarge narratives of 21 low‐income urban medical inpatients, almost one‐half believed it would be impossible to follow medical advice at discharge.45 Healthcare providers may overestimate a patient's ability to understand discharge instructions46 and to provide self‐care at the time of discharge.47 Homeless patients are at high risk for disease relapse following discharge, given chaotic living conditions and lack of social support.1 The presence of community support has been shown to decrease the likelihood of rehospitalization.48
Medication compliance poses a particular challenge. In 1 study, one‐third of homeless patients reported inability to comply with medications.2 Cost, storage capability, and complexity of regimen are common obstacles. Side effects should be considered when medications are selected, since common side effects like gastrointestinal upset or diarrhea, or desired effects like diuresis, may be intolerable if a patient cannot reliably access a restroom. Physicians should also weigh the possibility that discharge medications and supplies may be abused or stolen on the streets. Difficulty accessing routine meals can be particularly problematic in homeless patients with diabetes, who must eat on a regular schedule in order to avoid hypoglycemia. Diabetic goals may be adjusted accordingly to minimize risk. Diet may also be an issue if a patient must take a medication with food, as with some antiretrovirals. The physician must anticipate an erratic diet and, whenever possible, dose medications accordingly. Directly observed therapy for diseases such as tuberculosis is optimal if the ability to comply is in question.28 The NHCHC has developed guidelines for adaptations of care in homeless patients with a variety of clinical conditions, including diabetes mellitus, HIV, cardiovascular disease, and asthma; these are available for reference and download on their website (Table 2).
Illiteracy and low educational level also impact compliance. In 1 sample of indigent psychiatric patients, 76% read at or below the seventh grade level.49 Aftercare instructions should be easy to understand by those with lower levels of education (fourth grade level or less), written down in simple language, and reviewed verbally by nurse, pharmacist, and physician. Consider initiating projects within your hospital to streamline discharge instruction forms.50 The use of pictorial or video‐assisted discharge instructions for common diagnoses is an area of promise.17, 51, 52
Of note, there is no body of literature addressing the extent to which hospitalists or other inpatient physicians alter treatment goals at the time of discharge for homeless patients, but this may be a common occurrence and warrants further study.
C: Communication Methods After Discharge
Before discharge, clarify how a patient can be contacted for additional test results or information regarding follow‐up appointments. Although some homeless patients maintain mobile phones, telephone‐based methods used by some hospitalists for postdischarge follow‐up53, 54 may be unreliable in this population. Some shelters or respite facilities will accept messages for clients who reside there; others will provide clients with access to voicemail or e‐mail. For those patients who are technologically savvy, free e‐mail accounts can readily be obtained and accessed at public facilities, such as the public library. Contact information for a case manager can also be very useful. We occasionally ask patients to return to the hospital to retrieve test results or a message from their physician at a predetermined time and place. Where safe and appropriate, providing patients with direct physician contact information (rather than general hospital information) may minimize communication barriers.
The Big Picture: Systems‐Based Approaches to the Discharge of Homeless Patients
The discharge of homeless patients is suited to a comprehensive, interdisciplinary approach. There are many challenges to effective discharge planning: lack of time, lack of process ownership at the institutional level, financial constraints, and perhaps most significantly, lack of consensus regarding best practices.19 There is growing acknowledgement of the need to develop policies and standardize practice in this area. Hospitalists are uniquely situated to contribute to the development of new initiatives at the institutional, local, and national level.
Interventions (Table 4) may be as simple as the identification of a dedicated social worker for all homeless discharges15, 21, 55 or creation of a hospital‐wide discharge planning committee or inpatient homeless consultation service.15, 21 The distribution of discharge planning guides for patients and resource lists to providers is also gaining in popularity.21, 56 Some innovations specifically target clinicians, such as training seminars that teach communication skills and motivational interviewing and build familiarity with safety‐net services within the community.21, 57 Community‐based programs include medical respite care services, previously discussed, and the facilitation of preferred provider relationships directed by hospitals toward skilled nursing facilities willing to accept homeless and other challenging clients.15
|
| Discharge planning training seminars for the clinician |
| SWAT team for difficult discharges |
| Hospital‐wide discharge planning committee |
| Inpatient homeless consult service |
| Dedicated social worker for homeless discharges |
| Preferred provider status for skilled nursing facilities |
| Medical respite care |
| Discharge planning guide or resource list for homeless patients |
Homelessness has also been identified as an area of focus by state governments, with many states funding initiatives to improve training and assistance to homeless providers, policies for discharge planning from public institutions, and homeless needs assessments. Some states have gone so far as to determine that discharge to an emergency shelter is not appropriate.19 On the national level, large advocacy organizations such as the NHCHC and National Coalition for the Homeless have spearheaded Housing First efforts on behalf of homeless patients and providers throughout the country. Such programs have been shown to decrease healthcare expenditures, emergency department visits, and hospitalizations in certain homeless populations.58, 59 Check the NHCHC website for consolidated discharge planning program development resources for healthcare institutions (
Commentary
Homeless patients frequently require more energy and services at the time of service in order to achieve standard medical care. Optimally, a patient assumes full responsibility for his or her health, but there may be limits to this responsibility for selected patients,60 especially in light of limited access to primary care. Understandably, homeless patients may focus more on immediate physical needs (eg, food, shelter, safety) than on chronic medical problems. In addition, they may experience a sense of unwelcomeness from healthcare providers that they perceive as discrimination; this may dissuade them from seeking care.61 The inpatient physician should aim to build trust with each encounter. As suggested by 1 author, it is important to promise only what can be delivered and deliver what is promised.62 Involving the patient in care and decision‐making is the most important first step in accomplishing this goal.
It is important in caring for homeless patients to reframe one's notion of a successful outcome.16 Ideally, on resolution of his or her acute medical issues, a homeless patient would be discharged to permanent housing with substance abuse and mental health treatment. This scenario is unfortunately rare. The hospitalist often has little ability to arrange stable, on‐demand housing at discharge. He or she is best advised to focus on optimizing acute care delivery at the point of care and maximize opportunities for future health.
It has been suggested that the discontinuity inherent in the hospitalist model may confer a special obligation on hospital medicine providers to abide by a more rigorous standard of care42; one might argue that this obligation becomes even more compelling when applied to this vulnerable population. In 1 study, a disturbing 27% of an American cohort of homeless adults had no healthcare contacts in the year prior to death, underscoring this group's underutilization of health services. Armed with this knowledge, hospitalists should seize every healthcare interaction as an opportunity to offer therapies with potential for longer‐term benefit.
- ,,,,.Mental health and social characteristics of the homeless: a survey of mission users.Am J Public Health.1986;76:519–524.
- ,,.Factors associated with the health care utilization of homeless persons.JAMA.2001;285(2):200–206.
- ,,,,,.Hospitalization in an urban homeless population: the Honolulu urban homeless project.Ann Int Med.1992:116:299–303.
- ,,,,.Hospitalization costs associated with homelessness in New York City.N Engl J Med.1997;338:1734–1740.
- ,.Homelessness; health service use and related costs.Med Care.1998;38:1256–1264.
- ,,, et al.Health and mental problems of homeless men and women in Baltimore.JAMA.1989;262:1352–1357.
- ,,, et al.Mortality in a cohort of homeless adults in Philadelphia.N Engl J Med.1994;331:304–309.
- . Utilization and costs of medical services by homeless persons: a review of the literature and implications for the future. National Health Care for the Homeless Council website. Published April 1999. Available at: http://www.nhchc.org/Publications/utilization.htm. Accessed Month Year.
- ,,,,.Causes of death in homeless adults in Boston.Ann Intern Med.1997;126:625–628.
- ,.Risk of death among homeless women; a cohort study and review of the literature.CMAJ.2004;170(8):1243–1237.
- ,.Health care for homeless persons.N Engl J Med.2004;350:2329–2332.
- .Homelessness and health.CMAJ.2001;164:229–233.
- U.S. Congress..McKinney Homeless Assistance Act. Publ. No. 100–77, 101 Stat. 484.Washington, DC:U.S. Congress;1987.
- ,.How can health services effectively meet the health needs of homeless people?Br J Gen Pract.2006;56:286–293.
- Homeless Health Care Los Angeles. Homelessness: An Overview and Effective Strategies for Discharge Planning of Homeless Patients. Available at: http://www.nhchc.org/Publications/utilizations.html. Accessed Month Year.
- ,,. Balancing act: clinical practices that respond to the needs of homeless people. 1998 National Symposium on Homelessness Research. U.S. Health and Human Services. Available at: http://aspe.hhs.gov/ProgSys/homeless/symposium/8‐Clinical.htm. Accessed June2009.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,.Identifying homelessness at an urban public hospital: a moving target?J Health Care Poor Underserved.2005;16:297–307.
- ,,.The role of effective discharge planning in preventing homelessness.J Prim Prev.2007;28:229–243.
- ,.Saying goodbye: ethical issues in the stewardship of bed spaces.J Clin Ethics.2005;16:170–175.
- National Health Care for the Homeless Council.Tools to help clinicians achieve effective discharge planning.Healing Hands2008;12:1–6. Available at: http://www.nhchc.org/Network/HealingHands/2008/Oct2008Healing Hands.pdf. Accessed Juneyear="2009"2009.
- ,,, et al.It takes a village: a multidisciplinary model of the acute illness aftercare of individuals experiencing homelessness.J Health Care Poor Underserved.2005;16:257–272.
- ,,, et al. Hospital discharge to a homeless medical respite program prevents readmission [Abstract]. Boston Health Care for the Homeless Program. Published 2005. Available at: http://www.nhchc.org/Respite/RespiteResearcUpdateSept05.ppt. Accessed June2009.
- ,,,.The effects of respite care for homeless patients: a cohort study.Am J Public Health.2006:96:1278–1281.
- . Medical Respite Services for Homeless People: Practical Models. National Health Care for the Homeless Council. Published 1999. Available at: http://www.nhchc.org/Publications/MedicalRespiteServices.pdf. Accessed June2009.
- ,,,.Shelter‐based convalescence for homeless adults.Can J Public Health.2006;97:379–383.
- ,.Hoptel equalizes length of stay for homeless and domiciled inpatients.Med Care.2000;38:1003–1010.
- .The homeless in America: adapting your practice.Am Fam Physician.2006;74:1132–1138.
- . A Preliminary Review of Literature: Chronic Medical Illness and Homeless Individuals, Nashville, TN. National Health Care for the Homeless Council. Published April 2002. Available at: http://www.nhchc.org/Publications/literaturereview_chronicillness.pdf. Accessed June2009.
- ,.Diagnostic patterns in hospital use by an urban homeless population.West J Med.1989;151:472–478.
- ,,.Drug use disorders and treatment contact among homeless adults in Alameda County, California.Am J Public Health.1997;87:221–228.
- ,,,,,.Self‐reported changes in drug and alcohol use after becoming homeless.Am J Public Health.2004;94:830–835.
- .A review of physical and mental health in homeless persons.Public Health Rev.2001;29:13–33.
- ,,,.Interventions to improve the health of the homeless; a systematic review.Am J Prev Med.2005;29:311–319.
- ,,.Preventing and controlling emerging and reemerging transmissible diseases in the homeless.Emerg Infect Dis.2008;14:1353–1359.
- ,,.Comparison of conventional and accelerated hepatitis B immunisation schedules for homeless drug users.Commun Dis Public Health.2002;5:324–326.
- ,,,,,.Spot sputum screening: evaluation of an intervention in two homeless shelters.Int J Tuberc Lung Dis.1999;3:613–619.
- ,,, et al.Homelessness and smoking cessation: insights from focus groups.Nicotine Tob Res.2006;8:287–296.
- ,,,.The health encounter as a treatable moment for homeless substance‐using adults: the role of homelessness, health‐seeking behavior, readiness for behavior change and motivation for treatment.Addict Behav.2008;33:1239–1243.
- ,. To dance with grace: outreach and engagement to persons on the street. 1998 National Symposium on Homelessness Research. U.S. Health and Human Services. Available at: http://aspe.hhs.gov/ProgSys/homeless/symposium/6‐Outreach.htm. Accessed June2009.
- .The best way to improve emergency department follow‐up is actually to give the patient a specific appointment.J Gen Intern Med.2006;21:398.
- .Key legal principles for hospitalists.Am J Med.2001;11:5s–9s.
- ,,.Attitudes, experiences and beliefs affecting end of life decision‐making among homeless individuals.J Palliat Med.2005;8:36–48.
- ,,,,,.Dying on the streets; homeless person's concerns and desires about end of life care.J Gen Intern Med.2007;22:435–441.
- ,,.Understanding rehospitalization risk: can hospital discharge be modified to reduce recurrent hospitalization.J Hosp Med.2007:2:297–304.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,,,,,.Discharge planning: comparison of patients and nurses' perceptions of patients following hospital discharge.Image J Nurs Sch.1996;28:143–147.
- ,,,.Predicting health services utilization among homeless adults: a prospective analysis.J Health Care Poor Underserved.2000:11:212–230.
- ,.The prevalence of low literacy in an indigent psychiatric population.Psychiatr Serv.1999;50:262–263.
- Majority of emergency patients don't understand discharge instructions.ED Manag.2008;20:97–98.
- ,.Communicating information to patients: the use of cartoon illustrations to improve comprehension of instructions.Acad Emerg Med.1996;3:264–270.
- ,,,.The effectiveness of mobile discharge instruction videos (MDIVs) in communicating discharge instructions to patients with lacerations or sprains.South Med J.2009;102:239–247.
- .The importance of postdischarge telephone follow up from hospitalists: a view from the trenches.Am J Med.2001;111:43s–44s.
- ,.Using an interactive voice response system to improve patient safety following hospital discharge.Eval Clin Pract.2007;13:346–351.
- Resource Guide for Service Providers. Homeless Health Care Los Angeles. Available at: http://www.hhcla.org/training/pdf‐docs/2007%20RESOURCE%20GUIDE.pdf. Accessed June2009.
- Hospital Discharge Planning Training Workshop. Homeless Health Care Los Angeles. Available at: http://www.hhcla.org/discharge.htm. Accessed June2009.
- ,,, et al.Health care and public service use and costs before and after provision of housing for chronically homeless persons with severe alcohol problems.JAMA.2009;301:1349–1357.
- ,,,.Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults.JAMA.2009;301:1771–1778.
- .Limits on patient responsibility.J Med Phil.2005;30:189–206.
- ,,.Homeless people's perceptions of welcomeness and unwelcomeness in healthcare encounters.J Gen Intern Med.2007;22:1011–1017.
- .Increasing competency in the care of homeless patients [Teaching Tips].J Contin Educ Nurs.2008;39:153–154.
- ,,, et al.Health care utilization among homeless adults prior to death.J Health Care Poor Underserved.1999;12:50–58.
- ,,,,.Mental health and social characteristics of the homeless: a survey of mission users.Am J Public Health.1986;76:519–524.
- ,,.Factors associated with the health care utilization of homeless persons.JAMA.2001;285(2):200–206.
- ,,,,,.Hospitalization in an urban homeless population: the Honolulu urban homeless project.Ann Int Med.1992:116:299–303.
- ,,,,.Hospitalization costs associated with homelessness in New York City.N Engl J Med.1997;338:1734–1740.
- ,.Homelessness; health service use and related costs.Med Care.1998;38:1256–1264.
- ,,, et al.Health and mental problems of homeless men and women in Baltimore.JAMA.1989;262:1352–1357.
- ,,, et al.Mortality in a cohort of homeless adults in Philadelphia.N Engl J Med.1994;331:304–309.
- . Utilization and costs of medical services by homeless persons: a review of the literature and implications for the future. National Health Care for the Homeless Council website. Published April 1999. Available at: http://www.nhchc.org/Publications/utilization.htm. Accessed Month Year.
- ,,,,.Causes of death in homeless adults in Boston.Ann Intern Med.1997;126:625–628.
- ,.Risk of death among homeless women; a cohort study and review of the literature.CMAJ.2004;170(8):1243–1237.
- ,.Health care for homeless persons.N Engl J Med.2004;350:2329–2332.
- .Homelessness and health.CMAJ.2001;164:229–233.
- U.S. Congress..McKinney Homeless Assistance Act. Publ. No. 100–77, 101 Stat. 484.Washington, DC:U.S. Congress;1987.
- ,.How can health services effectively meet the health needs of homeless people?Br J Gen Pract.2006;56:286–293.
- Homeless Health Care Los Angeles. Homelessness: An Overview and Effective Strategies for Discharge Planning of Homeless Patients. Available at: http://www.nhchc.org/Publications/utilizations.html. Accessed Month Year.
- ,,. Balancing act: clinical practices that respond to the needs of homeless people. 1998 National Symposium on Homelessness Research. U.S. Health and Human Services. Available at: http://aspe.hhs.gov/ProgSys/homeless/symposium/8‐Clinical.htm. Accessed June2009.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,.Identifying homelessness at an urban public hospital: a moving target?J Health Care Poor Underserved.2005;16:297–307.
- ,,.The role of effective discharge planning in preventing homelessness.J Prim Prev.2007;28:229–243.
- ,.Saying goodbye: ethical issues in the stewardship of bed spaces.J Clin Ethics.2005;16:170–175.
- National Health Care for the Homeless Council.Tools to help clinicians achieve effective discharge planning.Healing Hands2008;12:1–6. Available at: http://www.nhchc.org/Network/HealingHands/2008/Oct2008Healing Hands.pdf. Accessed Juneyear="2009"2009.
- ,,, et al.It takes a village: a multidisciplinary model of the acute illness aftercare of individuals experiencing homelessness.J Health Care Poor Underserved.2005;16:257–272.
- ,,, et al. Hospital discharge to a homeless medical respite program prevents readmission [Abstract]. Boston Health Care for the Homeless Program. Published 2005. Available at: http://www.nhchc.org/Respite/RespiteResearcUpdateSept05.ppt. Accessed June2009.
- ,,,.The effects of respite care for homeless patients: a cohort study.Am J Public Health.2006:96:1278–1281.
- . Medical Respite Services for Homeless People: Practical Models. National Health Care for the Homeless Council. Published 1999. Available at: http://www.nhchc.org/Publications/MedicalRespiteServices.pdf. Accessed June2009.
- ,,,.Shelter‐based convalescence for homeless adults.Can J Public Health.2006;97:379–383.
- ,.Hoptel equalizes length of stay for homeless and domiciled inpatients.Med Care.2000;38:1003–1010.
- .The homeless in America: adapting your practice.Am Fam Physician.2006;74:1132–1138.
- . A Preliminary Review of Literature: Chronic Medical Illness and Homeless Individuals, Nashville, TN. National Health Care for the Homeless Council. Published April 2002. Available at: http://www.nhchc.org/Publications/literaturereview_chronicillness.pdf. Accessed June2009.
- ,.Diagnostic patterns in hospital use by an urban homeless population.West J Med.1989;151:472–478.
- ,,.Drug use disorders and treatment contact among homeless adults in Alameda County, California.Am J Public Health.1997;87:221–228.
- ,,,,,.Self‐reported changes in drug and alcohol use after becoming homeless.Am J Public Health.2004;94:830–835.
- .A review of physical and mental health in homeless persons.Public Health Rev.2001;29:13–33.
- ,,,.Interventions to improve the health of the homeless; a systematic review.Am J Prev Med.2005;29:311–319.
- ,,.Preventing and controlling emerging and reemerging transmissible diseases in the homeless.Emerg Infect Dis.2008;14:1353–1359.
- ,,.Comparison of conventional and accelerated hepatitis B immunisation schedules for homeless drug users.Commun Dis Public Health.2002;5:324–326.
- ,,,,,.Spot sputum screening: evaluation of an intervention in two homeless shelters.Int J Tuberc Lung Dis.1999;3:613–619.
- ,,, et al.Homelessness and smoking cessation: insights from focus groups.Nicotine Tob Res.2006;8:287–296.
- ,,,.The health encounter as a treatable moment for homeless substance‐using adults: the role of homelessness, health‐seeking behavior, readiness for behavior change and motivation for treatment.Addict Behav.2008;33:1239–1243.
- ,. To dance with grace: outreach and engagement to persons on the street. 1998 National Symposium on Homelessness Research. U.S. Health and Human Services. Available at: http://aspe.hhs.gov/ProgSys/homeless/symposium/6‐Outreach.htm. Accessed June2009.
- .The best way to improve emergency department follow‐up is actually to give the patient a specific appointment.J Gen Intern Med.2006;21:398.
- .Key legal principles for hospitalists.Am J Med.2001;11:5s–9s.
- ,,.Attitudes, experiences and beliefs affecting end of life decision‐making among homeless individuals.J Palliat Med.2005;8:36–48.
- ,,,,,.Dying on the streets; homeless person's concerns and desires about end of life care.J Gen Intern Med.2007;22:435–441.
- ,,.Understanding rehospitalization risk: can hospital discharge be modified to reduce recurrent hospitalization.J Hosp Med.2007:2:297–304.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,,,,,.Discharge planning: comparison of patients and nurses' perceptions of patients following hospital discharge.Image J Nurs Sch.1996;28:143–147.
- ,,,.Predicting health services utilization among homeless adults: a prospective analysis.J Health Care Poor Underserved.2000:11:212–230.
- ,.The prevalence of low literacy in an indigent psychiatric population.Psychiatr Serv.1999;50:262–263.
- Majority of emergency patients don't understand discharge instructions.ED Manag.2008;20:97–98.
- ,.Communicating information to patients: the use of cartoon illustrations to improve comprehension of instructions.Acad Emerg Med.1996;3:264–270.
- ,,,.The effectiveness of mobile discharge instruction videos (MDIVs) in communicating discharge instructions to patients with lacerations or sprains.South Med J.2009;102:239–247.
- .The importance of postdischarge telephone follow up from hospitalists: a view from the trenches.Am J Med.2001;111:43s–44s.
- ,.Using an interactive voice response system to improve patient safety following hospital discharge.Eval Clin Pract.2007;13:346–351.
- Resource Guide for Service Providers. Homeless Health Care Los Angeles. Available at: http://www.hhcla.org/training/pdf‐docs/2007%20RESOURCE%20GUIDE.pdf. Accessed June2009.
- Hospital Discharge Planning Training Workshop. Homeless Health Care Los Angeles. Available at: http://www.hhcla.org/discharge.htm. Accessed June2009.
- ,,, et al.Health care and public service use and costs before and after provision of housing for chronically homeless persons with severe alcohol problems.JAMA.2009;301:1349–1357.
- ,,,.Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults.JAMA.2009;301:1771–1778.
- .Limits on patient responsibility.J Med Phil.2005;30:189–206.
- ,,.Homeless people's perceptions of welcomeness and unwelcomeness in healthcare encounters.J Gen Intern Med.2007;22:1011–1017.
- .Increasing competency in the care of homeless patients [Teaching Tips].J Contin Educ Nurs.2008;39:153–154.
- ,,, et al.Health care utilization among homeless adults prior to death.J Health Care Poor Underserved.1999;12:50–58.
ICD for Vasospasm‐Induced Polymorphic VT
Arrhythmias are well‐described in patients with vasospastic angina. Coronary vasospasm may occur in the setting of angiographically normal or diseased coronary arteries. Patients with vasospastic angina are at increased risk of sudden death. However, it is unclear which of these patients would benefit from implantable cardioverter defibrillator (ICD) insertion.
We report a case of a young woman who presented with atypical angina. During an episode of chest pain she had a documented run of sustained polymorphic ventricular tachycardia (VT). In addition to medical therapy, she received an ICD to prevent future episodes of sudden cardiac death.
Case Report
A 38‐year‐old woman was admitted with an episode of severe central chest pain. The pain was sharp, localized, occurred at rest, and resolved spontaneously after about 2 hours. She reported intermittent but shorter episodes of similar chest pain for the preceding 5 months. These episodes were associated with palpitation and lightheadedness. She was a smoker with past medical history of hyperlipidemia, asthma, and psoriasis. There was no family history of sudden cardiac death or premature coronary artery disease. On presentation, she was pain‐free, and vitals were stable. Electrocardiogram revealed normal sinus rhythm, no ischemic ST segment changes, and a QTc interval of 479 msec. Serum troponins were normal, serum potassium was 3.5 mmol/L, and serum magnesium was 1.9 mg/dL. While she was being monitored on telemetry, she suddenly experienced chest pain with palpitation. The telemetry recording of the event showed transient ST segment elevation followed by an episode of sustained polymorphic ventricular tachycardia (VT) (Figure 1). She remained hemodynamically stable and did not lose consciousness. The VT was self‐terminating.
Coronary angiogram revealed moderate 2‐vessel disease and spontaneous spasm of the dominant left circumflex artery. Therapy was initiated using both an oral nitrate and calcium channel antagonist. Potassium and magnesium levels were corrected with supplementation. On further questioning, she reported 1 episode of near‐syncope in the past. In view of the above history and a documented episode of spontaneous sustained polymorphic VT, an implantable cardioverter defibrillator (ICD) was implanted. She was strongly advised to quit smoking and was discharged home in stable condition. Three months later, she was admitted with recurrence of similar episodes of chest pain and dizziness, and multiple shocks from her ICD. Interrogation of the ICD revealed 5 episodes of polymorphic VT that were appropriately terminated with ICD discharges. The doses of calcium channel antagonist and oral nitrate were maximized, and she was discharged home in stable condition.
Discussion
Our case highlights an important management dilemma in patients with vasospastic angina. ICD implantation in this group has been reported in patients resuscitated from cardiac arrest. Our patient was recognized to be at high risk of sudden death but had never experienced cardiac arrest.
An increased incidence of sudden cardiac death, VT, and ventricular fibrillation has been observed during episodes of vasospastic angina. In a retrospective multicenter study of 349 patients with vasospastic angina, VT or ventricular fibrillation was noted in 6.5% of patients.1 Sudden death was reported in 2% of the patients (mean follow‐up period, 3.4 years), of whom the majority had ST segment elevation during anginal attacks. Increased ventricular vulnerability has been noted even during symptom‐free periods.2 Some cases of unexplained out‐of‐hospital cardiac arrest and sudden deaths may be secondary to coronary artery spasm.3 In a prospective study of 356 survivors of out‐of‐hospital cardiac arrest, Myerburg et al.4 reported 5 patients with coronary artery spasm who had silent ischemic events associated with life‐threatening ventricular arrhythmias. Interestingly, in 2 of the 5 patients, onset of ventricular arrhythmia correlated with reperfusion, rather than ischemia.
Calcium‐channel antagonists and nitrates are accepted as the first‐line treatment for vasospastic angina. Although this therapy improves prognosis, the risk of ventricular arrhythmia and sudden death is not eliminated.1 The data regarding use of ICDs in patients with coronary vasospasm are limited to case reports. Lacroix et al.5 reported 2 patients with vasospastic angina resuscitated from out‐of‐hospital cardiac arrest who received ICDs. Postimplantation, at 4 months and 11 months, respectively, each of the 2 patients had appropriate ICD discharges. Fuertes et al.6 reported a patient resuscitated from cardiac arrest due to ventricular fibrillation related to an episode of angina. The patient had vasospasm despite intensive medical therapy and had an ICD implanted. The above previously published cases describe ICD implantation for patients resuscitated from cardiac arrest.
However, in patients with coronary vasospasm who have never experienced cardiac arrest, it is unclear which subset would benefit from an ICD. Electrophysiological studies are not always helpful in identifying these patients. In the study by Myerburg et al.,4 only 1 out of the 5 patients with coronary artery spasm had inducible arrhythmia during electrophysiological testing. Clinical features associated with increased risk of sudden death were reported by McAlpin3 in a study of 81 patients with vasospastic angina. The risk of sudden death was tripled by the presence of either a history of angina‐linked syncope or documentation of serious arrhythmia complicating attacks. Paradoxically, the risk was increased by the absence of high‐grade coronary artery stenosis. Some researchers have reported a strong association between cigarette smoking and coronary spasm.7 Patients with known or suspected coronary artery spasm should be strongly discouraged from smoking. Our patient, in addition to being a smoker, had 2 of the 3 risk factors described by McAlpin,3 namely, documented serious arrhythmia and absence of high‐grade coronary stenosis. Considering these risk factors, an ICD was implanted. To our knowledge this is the first reported case of ICD insertion in a patient with vasospasm‐induced VT who had never experienced cardiac arrest.
Conclusions
In summary, patients with vasospastic angina are at increased risk of sudden death, especially during an episode of angina. Some cases of unexplained sudden death and malignant ventricular arrhythmia are probably a consequence of acute myocardial ischemia resulting from coronary arterial spasm. Early recognition and treatment of polymorphic VT is critical in preventing sudden cardiac death. In the absence of myocardial infarction, ST segment elevation preceding an episode of syncope or arrhythmia should raise the suspicion of coronary vasospasm as the underlying etiology. ICD placement is potentially beneficial in patients with coronary spasm who are at high risk of sudden cardiac death. Larger trials with longer follow‐up periods would help clinicians make this decision with greater confidence.
- ,,.Clinical characteristics associated with myocardial infarction, arrhythmias, and sudden death in patients with vasospastic angina.Circulation.1987;75:1110–1116.
- ,,, et al.Induction of polymorphic ventricular tachycardia by programmed ventricular stimulation in vasospastic angina pectoris.Am J Cardiol.1996;77:355–360.
- .Cardiac arrest and sudden unexpected death in variant angina: complications of coronary spasm that can occur in the absence of severe organic coronary stenosis.Am Heart J.1993;125:1011–1017.
- ,,, et al.Life‐threatening ventricular arrhythmias in patients with silent myocardial ischemia due to coronary‐artery spasm.N Engl J Med.1992;326(22):1451–1455.
- ,,.Vasospastic angina without flow‐limiting coronary lesion as a cause for aborted sudden death.Int J Cardiol (Ireland).1994;43:247–249.
- ,,,.Implantable cardioverter defibrillator as a therapeutic option for sudden cardiac death secondary to severe coronary vasospasm.Int J Cardiol.1998;63:181–183.
- ,.Cigarette smoking is a major risk factor for coronary spasm.Circulation.1993;87:76–79.
Arrhythmias are well‐described in patients with vasospastic angina. Coronary vasospasm may occur in the setting of angiographically normal or diseased coronary arteries. Patients with vasospastic angina are at increased risk of sudden death. However, it is unclear which of these patients would benefit from implantable cardioverter defibrillator (ICD) insertion.
We report a case of a young woman who presented with atypical angina. During an episode of chest pain she had a documented run of sustained polymorphic ventricular tachycardia (VT). In addition to medical therapy, she received an ICD to prevent future episodes of sudden cardiac death.
Case Report
A 38‐year‐old woman was admitted with an episode of severe central chest pain. The pain was sharp, localized, occurred at rest, and resolved spontaneously after about 2 hours. She reported intermittent but shorter episodes of similar chest pain for the preceding 5 months. These episodes were associated with palpitation and lightheadedness. She was a smoker with past medical history of hyperlipidemia, asthma, and psoriasis. There was no family history of sudden cardiac death or premature coronary artery disease. On presentation, she was pain‐free, and vitals were stable. Electrocardiogram revealed normal sinus rhythm, no ischemic ST segment changes, and a QTc interval of 479 msec. Serum troponins were normal, serum potassium was 3.5 mmol/L, and serum magnesium was 1.9 mg/dL. While she was being monitored on telemetry, she suddenly experienced chest pain with palpitation. The telemetry recording of the event showed transient ST segment elevation followed by an episode of sustained polymorphic ventricular tachycardia (VT) (Figure 1). She remained hemodynamically stable and did not lose consciousness. The VT was self‐terminating.

Coronary angiogram revealed moderate 2‐vessel disease and spontaneous spasm of the dominant left circumflex artery. Therapy was initiated using both an oral nitrate and calcium channel antagonist. Potassium and magnesium levels were corrected with supplementation. On further questioning, she reported 1 episode of near‐syncope in the past. In view of the above history and a documented episode of spontaneous sustained polymorphic VT, an implantable cardioverter defibrillator (ICD) was implanted. She was strongly advised to quit smoking and was discharged home in stable condition. Three months later, she was admitted with recurrence of similar episodes of chest pain and dizziness, and multiple shocks from her ICD. Interrogation of the ICD revealed 5 episodes of polymorphic VT that were appropriately terminated with ICD discharges. The doses of calcium channel antagonist and oral nitrate were maximized, and she was discharged home in stable condition.
Discussion
Our case highlights an important management dilemma in patients with vasospastic angina. ICD implantation in this group has been reported in patients resuscitated from cardiac arrest. Our patient was recognized to be at high risk of sudden death but had never experienced cardiac arrest.
An increased incidence of sudden cardiac death, VT, and ventricular fibrillation has been observed during episodes of vasospastic angina. In a retrospective multicenter study of 349 patients with vasospastic angina, VT or ventricular fibrillation was noted in 6.5% of patients.1 Sudden death was reported in 2% of the patients (mean follow‐up period, 3.4 years), of whom the majority had ST segment elevation during anginal attacks. Increased ventricular vulnerability has been noted even during symptom‐free periods.2 Some cases of unexplained out‐of‐hospital cardiac arrest and sudden deaths may be secondary to coronary artery spasm.3 In a prospective study of 356 survivors of out‐of‐hospital cardiac arrest, Myerburg et al.4 reported 5 patients with coronary artery spasm who had silent ischemic events associated with life‐threatening ventricular arrhythmias. Interestingly, in 2 of the 5 patients, onset of ventricular arrhythmia correlated with reperfusion, rather than ischemia.
Calcium‐channel antagonists and nitrates are accepted as the first‐line treatment for vasospastic angina. Although this therapy improves prognosis, the risk of ventricular arrhythmia and sudden death is not eliminated.1 The data regarding use of ICDs in patients with coronary vasospasm are limited to case reports. Lacroix et al.5 reported 2 patients with vasospastic angina resuscitated from out‐of‐hospital cardiac arrest who received ICDs. Postimplantation, at 4 months and 11 months, respectively, each of the 2 patients had appropriate ICD discharges. Fuertes et al.6 reported a patient resuscitated from cardiac arrest due to ventricular fibrillation related to an episode of angina. The patient had vasospasm despite intensive medical therapy and had an ICD implanted. The above previously published cases describe ICD implantation for patients resuscitated from cardiac arrest.
However, in patients with coronary vasospasm who have never experienced cardiac arrest, it is unclear which subset would benefit from an ICD. Electrophysiological studies are not always helpful in identifying these patients. In the study by Myerburg et al.,4 only 1 out of the 5 patients with coronary artery spasm had inducible arrhythmia during electrophysiological testing. Clinical features associated with increased risk of sudden death were reported by McAlpin3 in a study of 81 patients with vasospastic angina. The risk of sudden death was tripled by the presence of either a history of angina‐linked syncope or documentation of serious arrhythmia complicating attacks. Paradoxically, the risk was increased by the absence of high‐grade coronary artery stenosis. Some researchers have reported a strong association between cigarette smoking and coronary spasm.7 Patients with known or suspected coronary artery spasm should be strongly discouraged from smoking. Our patient, in addition to being a smoker, had 2 of the 3 risk factors described by McAlpin,3 namely, documented serious arrhythmia and absence of high‐grade coronary stenosis. Considering these risk factors, an ICD was implanted. To our knowledge this is the first reported case of ICD insertion in a patient with vasospasm‐induced VT who had never experienced cardiac arrest.
Conclusions
In summary, patients with vasospastic angina are at increased risk of sudden death, especially during an episode of angina. Some cases of unexplained sudden death and malignant ventricular arrhythmia are probably a consequence of acute myocardial ischemia resulting from coronary arterial spasm. Early recognition and treatment of polymorphic VT is critical in preventing sudden cardiac death. In the absence of myocardial infarction, ST segment elevation preceding an episode of syncope or arrhythmia should raise the suspicion of coronary vasospasm as the underlying etiology. ICD placement is potentially beneficial in patients with coronary spasm who are at high risk of sudden cardiac death. Larger trials with longer follow‐up periods would help clinicians make this decision with greater confidence.
Arrhythmias are well‐described in patients with vasospastic angina. Coronary vasospasm may occur in the setting of angiographically normal or diseased coronary arteries. Patients with vasospastic angina are at increased risk of sudden death. However, it is unclear which of these patients would benefit from implantable cardioverter defibrillator (ICD) insertion.
We report a case of a young woman who presented with atypical angina. During an episode of chest pain she had a documented run of sustained polymorphic ventricular tachycardia (VT). In addition to medical therapy, she received an ICD to prevent future episodes of sudden cardiac death.
Case Report
A 38‐year‐old woman was admitted with an episode of severe central chest pain. The pain was sharp, localized, occurred at rest, and resolved spontaneously after about 2 hours. She reported intermittent but shorter episodes of similar chest pain for the preceding 5 months. These episodes were associated with palpitation and lightheadedness. She was a smoker with past medical history of hyperlipidemia, asthma, and psoriasis. There was no family history of sudden cardiac death or premature coronary artery disease. On presentation, she was pain‐free, and vitals were stable. Electrocardiogram revealed normal sinus rhythm, no ischemic ST segment changes, and a QTc interval of 479 msec. Serum troponins were normal, serum potassium was 3.5 mmol/L, and serum magnesium was 1.9 mg/dL. While she was being monitored on telemetry, she suddenly experienced chest pain with palpitation. The telemetry recording of the event showed transient ST segment elevation followed by an episode of sustained polymorphic ventricular tachycardia (VT) (Figure 1). She remained hemodynamically stable and did not lose consciousness. The VT was self‐terminating.

Coronary angiogram revealed moderate 2‐vessel disease and spontaneous spasm of the dominant left circumflex artery. Therapy was initiated using both an oral nitrate and calcium channel antagonist. Potassium and magnesium levels were corrected with supplementation. On further questioning, she reported 1 episode of near‐syncope in the past. In view of the above history and a documented episode of spontaneous sustained polymorphic VT, an implantable cardioverter defibrillator (ICD) was implanted. She was strongly advised to quit smoking and was discharged home in stable condition. Three months later, she was admitted with recurrence of similar episodes of chest pain and dizziness, and multiple shocks from her ICD. Interrogation of the ICD revealed 5 episodes of polymorphic VT that were appropriately terminated with ICD discharges. The doses of calcium channel antagonist and oral nitrate were maximized, and she was discharged home in stable condition.
Discussion
Our case highlights an important management dilemma in patients with vasospastic angina. ICD implantation in this group has been reported in patients resuscitated from cardiac arrest. Our patient was recognized to be at high risk of sudden death but had never experienced cardiac arrest.
An increased incidence of sudden cardiac death, VT, and ventricular fibrillation has been observed during episodes of vasospastic angina. In a retrospective multicenter study of 349 patients with vasospastic angina, VT or ventricular fibrillation was noted in 6.5% of patients.1 Sudden death was reported in 2% of the patients (mean follow‐up period, 3.4 years), of whom the majority had ST segment elevation during anginal attacks. Increased ventricular vulnerability has been noted even during symptom‐free periods.2 Some cases of unexplained out‐of‐hospital cardiac arrest and sudden deaths may be secondary to coronary artery spasm.3 In a prospective study of 356 survivors of out‐of‐hospital cardiac arrest, Myerburg et al.4 reported 5 patients with coronary artery spasm who had silent ischemic events associated with life‐threatening ventricular arrhythmias. Interestingly, in 2 of the 5 patients, onset of ventricular arrhythmia correlated with reperfusion, rather than ischemia.
Calcium‐channel antagonists and nitrates are accepted as the first‐line treatment for vasospastic angina. Although this therapy improves prognosis, the risk of ventricular arrhythmia and sudden death is not eliminated.1 The data regarding use of ICDs in patients with coronary vasospasm are limited to case reports. Lacroix et al.5 reported 2 patients with vasospastic angina resuscitated from out‐of‐hospital cardiac arrest who received ICDs. Postimplantation, at 4 months and 11 months, respectively, each of the 2 patients had appropriate ICD discharges. Fuertes et al.6 reported a patient resuscitated from cardiac arrest due to ventricular fibrillation related to an episode of angina. The patient had vasospasm despite intensive medical therapy and had an ICD implanted. The above previously published cases describe ICD implantation for patients resuscitated from cardiac arrest.
However, in patients with coronary vasospasm who have never experienced cardiac arrest, it is unclear which subset would benefit from an ICD. Electrophysiological studies are not always helpful in identifying these patients. In the study by Myerburg et al.,4 only 1 out of the 5 patients with coronary artery spasm had inducible arrhythmia during electrophysiological testing. Clinical features associated with increased risk of sudden death were reported by McAlpin3 in a study of 81 patients with vasospastic angina. The risk of sudden death was tripled by the presence of either a history of angina‐linked syncope or documentation of serious arrhythmia complicating attacks. Paradoxically, the risk was increased by the absence of high‐grade coronary artery stenosis. Some researchers have reported a strong association between cigarette smoking and coronary spasm.7 Patients with known or suspected coronary artery spasm should be strongly discouraged from smoking. Our patient, in addition to being a smoker, had 2 of the 3 risk factors described by McAlpin,3 namely, documented serious arrhythmia and absence of high‐grade coronary stenosis. Considering these risk factors, an ICD was implanted. To our knowledge this is the first reported case of ICD insertion in a patient with vasospasm‐induced VT who had never experienced cardiac arrest.
Conclusions
In summary, patients with vasospastic angina are at increased risk of sudden death, especially during an episode of angina. Some cases of unexplained sudden death and malignant ventricular arrhythmia are probably a consequence of acute myocardial ischemia resulting from coronary arterial spasm. Early recognition and treatment of polymorphic VT is critical in preventing sudden cardiac death. In the absence of myocardial infarction, ST segment elevation preceding an episode of syncope or arrhythmia should raise the suspicion of coronary vasospasm as the underlying etiology. ICD placement is potentially beneficial in patients with coronary spasm who are at high risk of sudden cardiac death. Larger trials with longer follow‐up periods would help clinicians make this decision with greater confidence.
- ,,.Clinical characteristics associated with myocardial infarction, arrhythmias, and sudden death in patients with vasospastic angina.Circulation.1987;75:1110–1116.
- ,,, et al.Induction of polymorphic ventricular tachycardia by programmed ventricular stimulation in vasospastic angina pectoris.Am J Cardiol.1996;77:355–360.
- .Cardiac arrest and sudden unexpected death in variant angina: complications of coronary spasm that can occur in the absence of severe organic coronary stenosis.Am Heart J.1993;125:1011–1017.
- ,,, et al.Life‐threatening ventricular arrhythmias in patients with silent myocardial ischemia due to coronary‐artery spasm.N Engl J Med.1992;326(22):1451–1455.
- ,,.Vasospastic angina without flow‐limiting coronary lesion as a cause for aborted sudden death.Int J Cardiol (Ireland).1994;43:247–249.
- ,,,.Implantable cardioverter defibrillator as a therapeutic option for sudden cardiac death secondary to severe coronary vasospasm.Int J Cardiol.1998;63:181–183.
- ,.Cigarette smoking is a major risk factor for coronary spasm.Circulation.1993;87:76–79.
- ,,.Clinical characteristics associated with myocardial infarction, arrhythmias, and sudden death in patients with vasospastic angina.Circulation.1987;75:1110–1116.
- ,,, et al.Induction of polymorphic ventricular tachycardia by programmed ventricular stimulation in vasospastic angina pectoris.Am J Cardiol.1996;77:355–360.
- .Cardiac arrest and sudden unexpected death in variant angina: complications of coronary spasm that can occur in the absence of severe organic coronary stenosis.Am Heart J.1993;125:1011–1017.
- ,,, et al.Life‐threatening ventricular arrhythmias in patients with silent myocardial ischemia due to coronary‐artery spasm.N Engl J Med.1992;326(22):1451–1455.
- ,,.Vasospastic angina without flow‐limiting coronary lesion as a cause for aborted sudden death.Int J Cardiol (Ireland).1994;43:247–249.
- ,,,.Implantable cardioverter defibrillator as a therapeutic option for sudden cardiac death secondary to severe coronary vasospasm.Int J Cardiol.1998;63:181–183.
- ,.Cigarette smoking is a major risk factor for coronary spasm.Circulation.1993;87:76–79.
Safety in Numbers
A lack of communication and accountability among healthcare professionals in general, and physicians in particular, jeopardizes quality and safety for our patients who are transitioning across sites of care.1, 2 Our patients, their family caregivers, and our health care professional colleagues on the receiving end of these transfers are often left flying blind without adequate information or direction to make sound clinical decisions.
Beyond our attempts to ensure effective transitions on a professional level, many of the readers of the Journal of Hospital Medicine likely have struggled to ensure seamless transitions for our families, despite the benefits of our training and experience.3 If some of the nation's most respected healthcare leaders are unable to make this work for their loved ones,48 one can only imagine the challenges faced by those without such advantages.
National and local quality collaboratives aimed at improving communication and collaboration across settings have found physicians difficult to engage as partners in these efforts.9 All too often there is a false expectation that these types of activities are best left to nonphysician healthcare professionals on the sending side of the transfer or to those receiving the transfer.10, 11
In this issue of the Journal, we commend the leadership provided by representatives of 6 of the nation's leading physician professional societies to join forces toward the common purpose of articulating physicians' roles and accountability for care delivered during transitions.12 Ensuring effective care transitions is a team sport, yet rarely do we have a clear understanding of who are the other members of our team, how to interact with them, or a clear delineation of their respective roles. Simply stated, this article is a key step to facilitating teamwork across settings among physicians, our interdisciplinary healthcare professional colleagues, our patients, and their family caregivers. These standards clearly convey the type of care we expect for our loved ones.
Drawing from proven strategies used in nonhealthcare industries, the standards assert that the sending provider or institution retains responsibility for the patient's care until the receiving team confirms receipt of the transfer and assumes responsibility. Further, the receiving team is given the opportunity to ask questions and clarify the proposed care plan in recognition of the fact that communication is more than simply the transfer of information. Rather, such communication involves the need to ensure comprehension and provide an opportunity to have a 2‐way dialog. These standards distinguish between the transmission of information and true communication.
The timing of the release of these standards is ideal. As physicians concentrate their practice within particular settings we can no longer rely on casual random interchanges in hospital parking lots or the hospital's physician lounge. Rather, we need to take a more active and reliable approach to ensuring timely and accurate exchanges. These standards cut to the essence of how we communicate with our physician and nonphysician colleagues alike, and in so doing move us away from nonproductive blame and finger‐pointing.
Although the implications for these standards are far reaching in terms of raising the quality bar, they could reach even further with respect to the types of settings they address. These standards need to extend beyond hospitals and the outpatient arena to include nursing homes, rehabilitation facilities, home care agencies, adult day health centers, and other settings where chronic care services are delivered.
Further, the standards devote considerable focus to the transfer of health information. Even with advances in health information exchange technologies, we must recognize that information is necessary but not sufficient for ensuring safe and high‐quality transfers. Implementing these standards will undoubtedly require that we reconfigure our daily workflows.13 The article in this issue by Graumlich et al.14 emphasizes the challenges of how to introduce technology into our daily clinical routines. The standards also open the door for how we can best ensure not just the transmission of information, but also the comprehension of transfer instructions to our patients with attention to health literacy, cognitive ability, and the patient's level of activation.15 Best and Young16 provide valuable action steps for how to address the needs of diverse and underserved populations.
These standards may serve to uncover the fact that most physicians have not received formal training in executing high‐quality care transitions in the role of either the sender or the receiver. Further, few physicians have a mechanism in place to evaluate their performance. The American Board of Internal Medicine and the American Board of Family Practice has developed Maintenance of Certification Practice Improvement Modules (PIM) on care coordination that provide an excellent opportunity to sharpen our skills. The HMO Care Management Workgroup has also attempted to summarize the essential skills necessary to care for patients during transitions.17
Perhaps the greatest value of these standards is that they lay the framework for actionable improvement. Local, state, and national quality collaboratives can immediately incorporate these recommendations into their overall strategy. These standards will likely influence the design and implementation of the Medical Home.18 As national attention focuses on how to operationalize bundled payment approaches and Accountable Care Organizations,19 these standards provide a clear consensus on communication, accountability, and ensuring patient‐centeredness. The standards are an excellent start and provide a framework for further innovation.
One area in particular may be the opportunity to reinvent the format, content, and medium by which essential information is transferred. For example, one might envision the value of producing a scaled down version of the discharge summary with a limited core set of data elements that could be quickly completed and communicated to the next care team via fax, e‐mail, or text messaging.
Complementing new strategies to improve the exchange of health information are opportunities to reconsider the culture within which this communication occurs. Our profession has a long‐standing tradition of not providing directives to our colleagues on the details of clinical management. Hospitalists develop important insights during a patient's hospital stay and are in an ideal position to anticipate potential developments in the subsequent course after discharge. Contrast this with the 5 to 10 minutes that a primary care physician or specialist may have to come up to speed on the hospital and posthospital events in order to manage the patient in the ambulatory arena. Thus, rather than the traditional historical orientation to a discharge summary, one could envision a more future‐orientated document characterized by a series of if‐then statements that outline a series of possible clinical scenarios that may play out over the weeks after discharge along with recommendations for adjustments to the treatment plan.
At a broader level, the release of these standards demonstrate to our communities and to our nation that physicians can join forces to address a particularly complex and challenging aspect of healthcare. Change can indeed come from within our profession rather than being imposed by outside influences such as government administrators, regulatory bodies, or malpractice attorneys. I applaud such efforts and believe that hospitalists will continue to play a central role in national efforts to improve transitions of care.
- ,,,,,.Deficits in information transfer from inpatient to outpatient physician at hospital discharge: a systematic review.J Gen Intern Med.2004;19(S1):135.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,.It Shouldn't Be This Way: The Failure of Long Term Care.1st ed.Nashville, TN:Vanderbilt University Press;2005.
- .Dismantling Rube Goldberg: cutting through chaos to achieve coordinated care.J Hosp Med.2009;4(4):259–260.
- ,.The experience of an orthopaedic traumatologist when the trauma hits home: observations and suggestions.J Bone Joint Surg Am.2008;90(9):2026–2036.
- .Quality comes home.Ann Intern Med.1996;125(10):839–843.
- .My mother and the medical care ad‐hoc‐racy.Health Aff.2003;22(2):238–242.
- .A hospitalization from hell: a patient's perspective on quality.Ann Intern Med.2003;138:33–39.
- ,,,.The ReACH Collaborative—improving quality home care.Caring.2007;26(8):44–51.
- Next step in care. Available at: http://www.nextstepincare.org. Accessed June2009.
- ,,,,. Health information exchange in post‐acute and long‐term care case study findings: final report. 2007. Office of Disability, Aging and Long‐Term Care Policy; Office of the Assistant Secretary for Planning and Evaluation; U.S. Department of Health and Human Services. Available at: http://aspe.hhs.gov/daltcp/reports/2007/HIEcase.pdf. Accessed June2009.
- .Transitions of Care Consensus Policy Statement. American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine.J Hosp Med.2009;4(6):364–370.
- ,,,.Better transitions: improving comprehension of discharge instructions.Front Health Serv Manag.2009;25(3):11–32.
- ,,,.Patient and physician perceptions after software‐assisted discharge from hospital: cluster randomized trial.J Hosp Med.2009;4(6):356–363.
- Patient Activation Measure. Available at: http://www.insigniahealth.com/products/pam.html. Accessed June2009.
- ,.A SAFE DC: a conceptual framework for care of the homeless inpatient.J Hosp Med2009;4(6):375–381.
- HMO Care Management Workgroup. One patient, many places: managing healthcare transitions. 2004. Available at: http://www.caretransitions. org/documents/One%20Patient%20RWJ%20Report.pdf. Accessed June2009.
- American College of Physicians. Patient‐Centered Medical Home: ACP delivers expanded PCMH resources online. Available at: http://www.acponline.org/advocacy/where_we_stand/medical_home. Accessed June2009.
- American College of Physicians. Accountable Care Organizations. Available at: http://www.acponline.org/advocacy/where_we_stand/medical_ home. Accessed June2009.
A lack of communication and accountability among healthcare professionals in general, and physicians in particular, jeopardizes quality and safety for our patients who are transitioning across sites of care.1, 2 Our patients, their family caregivers, and our health care professional colleagues on the receiving end of these transfers are often left flying blind without adequate information or direction to make sound clinical decisions.
Beyond our attempts to ensure effective transitions on a professional level, many of the readers of the Journal of Hospital Medicine likely have struggled to ensure seamless transitions for our families, despite the benefits of our training and experience.3 If some of the nation's most respected healthcare leaders are unable to make this work for their loved ones,48 one can only imagine the challenges faced by those without such advantages.
National and local quality collaboratives aimed at improving communication and collaboration across settings have found physicians difficult to engage as partners in these efforts.9 All too often there is a false expectation that these types of activities are best left to nonphysician healthcare professionals on the sending side of the transfer or to those receiving the transfer.10, 11
In this issue of the Journal, we commend the leadership provided by representatives of 6 of the nation's leading physician professional societies to join forces toward the common purpose of articulating physicians' roles and accountability for care delivered during transitions.12 Ensuring effective care transitions is a team sport, yet rarely do we have a clear understanding of who are the other members of our team, how to interact with them, or a clear delineation of their respective roles. Simply stated, this article is a key step to facilitating teamwork across settings among physicians, our interdisciplinary healthcare professional colleagues, our patients, and their family caregivers. These standards clearly convey the type of care we expect for our loved ones.
Drawing from proven strategies used in nonhealthcare industries, the standards assert that the sending provider or institution retains responsibility for the patient's care until the receiving team confirms receipt of the transfer and assumes responsibility. Further, the receiving team is given the opportunity to ask questions and clarify the proposed care plan in recognition of the fact that communication is more than simply the transfer of information. Rather, such communication involves the need to ensure comprehension and provide an opportunity to have a 2‐way dialog. These standards distinguish between the transmission of information and true communication.
The timing of the release of these standards is ideal. As physicians concentrate their practice within particular settings we can no longer rely on casual random interchanges in hospital parking lots or the hospital's physician lounge. Rather, we need to take a more active and reliable approach to ensuring timely and accurate exchanges. These standards cut to the essence of how we communicate with our physician and nonphysician colleagues alike, and in so doing move us away from nonproductive blame and finger‐pointing.
Although the implications for these standards are far reaching in terms of raising the quality bar, they could reach even further with respect to the types of settings they address. These standards need to extend beyond hospitals and the outpatient arena to include nursing homes, rehabilitation facilities, home care agencies, adult day health centers, and other settings where chronic care services are delivered.
Further, the standards devote considerable focus to the transfer of health information. Even with advances in health information exchange technologies, we must recognize that information is necessary but not sufficient for ensuring safe and high‐quality transfers. Implementing these standards will undoubtedly require that we reconfigure our daily workflows.13 The article in this issue by Graumlich et al.14 emphasizes the challenges of how to introduce technology into our daily clinical routines. The standards also open the door for how we can best ensure not just the transmission of information, but also the comprehension of transfer instructions to our patients with attention to health literacy, cognitive ability, and the patient's level of activation.15 Best and Young16 provide valuable action steps for how to address the needs of diverse and underserved populations.
These standards may serve to uncover the fact that most physicians have not received formal training in executing high‐quality care transitions in the role of either the sender or the receiver. Further, few physicians have a mechanism in place to evaluate their performance. The American Board of Internal Medicine and the American Board of Family Practice has developed Maintenance of Certification Practice Improvement Modules (PIM) on care coordination that provide an excellent opportunity to sharpen our skills. The HMO Care Management Workgroup has also attempted to summarize the essential skills necessary to care for patients during transitions.17
Perhaps the greatest value of these standards is that they lay the framework for actionable improvement. Local, state, and national quality collaboratives can immediately incorporate these recommendations into their overall strategy. These standards will likely influence the design and implementation of the Medical Home.18 As national attention focuses on how to operationalize bundled payment approaches and Accountable Care Organizations,19 these standards provide a clear consensus on communication, accountability, and ensuring patient‐centeredness. The standards are an excellent start and provide a framework for further innovation.
One area in particular may be the opportunity to reinvent the format, content, and medium by which essential information is transferred. For example, one might envision the value of producing a scaled down version of the discharge summary with a limited core set of data elements that could be quickly completed and communicated to the next care team via fax, e‐mail, or text messaging.
Complementing new strategies to improve the exchange of health information are opportunities to reconsider the culture within which this communication occurs. Our profession has a long‐standing tradition of not providing directives to our colleagues on the details of clinical management. Hospitalists develop important insights during a patient's hospital stay and are in an ideal position to anticipate potential developments in the subsequent course after discharge. Contrast this with the 5 to 10 minutes that a primary care physician or specialist may have to come up to speed on the hospital and posthospital events in order to manage the patient in the ambulatory arena. Thus, rather than the traditional historical orientation to a discharge summary, one could envision a more future‐orientated document characterized by a series of if‐then statements that outline a series of possible clinical scenarios that may play out over the weeks after discharge along with recommendations for adjustments to the treatment plan.
At a broader level, the release of these standards demonstrate to our communities and to our nation that physicians can join forces to address a particularly complex and challenging aspect of healthcare. Change can indeed come from within our profession rather than being imposed by outside influences such as government administrators, regulatory bodies, or malpractice attorneys. I applaud such efforts and believe that hospitalists will continue to play a central role in national efforts to improve transitions of care.
A lack of communication and accountability among healthcare professionals in general, and physicians in particular, jeopardizes quality and safety for our patients who are transitioning across sites of care.1, 2 Our patients, their family caregivers, and our health care professional colleagues on the receiving end of these transfers are often left flying blind without adequate information or direction to make sound clinical decisions.
Beyond our attempts to ensure effective transitions on a professional level, many of the readers of the Journal of Hospital Medicine likely have struggled to ensure seamless transitions for our families, despite the benefits of our training and experience.3 If some of the nation's most respected healthcare leaders are unable to make this work for their loved ones,48 one can only imagine the challenges faced by those without such advantages.
National and local quality collaboratives aimed at improving communication and collaboration across settings have found physicians difficult to engage as partners in these efforts.9 All too often there is a false expectation that these types of activities are best left to nonphysician healthcare professionals on the sending side of the transfer or to those receiving the transfer.10, 11
In this issue of the Journal, we commend the leadership provided by representatives of 6 of the nation's leading physician professional societies to join forces toward the common purpose of articulating physicians' roles and accountability for care delivered during transitions.12 Ensuring effective care transitions is a team sport, yet rarely do we have a clear understanding of who are the other members of our team, how to interact with them, or a clear delineation of their respective roles. Simply stated, this article is a key step to facilitating teamwork across settings among physicians, our interdisciplinary healthcare professional colleagues, our patients, and their family caregivers. These standards clearly convey the type of care we expect for our loved ones.
Drawing from proven strategies used in nonhealthcare industries, the standards assert that the sending provider or institution retains responsibility for the patient's care until the receiving team confirms receipt of the transfer and assumes responsibility. Further, the receiving team is given the opportunity to ask questions and clarify the proposed care plan in recognition of the fact that communication is more than simply the transfer of information. Rather, such communication involves the need to ensure comprehension and provide an opportunity to have a 2‐way dialog. These standards distinguish between the transmission of information and true communication.
The timing of the release of these standards is ideal. As physicians concentrate their practice within particular settings we can no longer rely on casual random interchanges in hospital parking lots or the hospital's physician lounge. Rather, we need to take a more active and reliable approach to ensuring timely and accurate exchanges. These standards cut to the essence of how we communicate with our physician and nonphysician colleagues alike, and in so doing move us away from nonproductive blame and finger‐pointing.
Although the implications for these standards are far reaching in terms of raising the quality bar, they could reach even further with respect to the types of settings they address. These standards need to extend beyond hospitals and the outpatient arena to include nursing homes, rehabilitation facilities, home care agencies, adult day health centers, and other settings where chronic care services are delivered.
Further, the standards devote considerable focus to the transfer of health information. Even with advances in health information exchange technologies, we must recognize that information is necessary but not sufficient for ensuring safe and high‐quality transfers. Implementing these standards will undoubtedly require that we reconfigure our daily workflows.13 The article in this issue by Graumlich et al.14 emphasizes the challenges of how to introduce technology into our daily clinical routines. The standards also open the door for how we can best ensure not just the transmission of information, but also the comprehension of transfer instructions to our patients with attention to health literacy, cognitive ability, and the patient's level of activation.15 Best and Young16 provide valuable action steps for how to address the needs of diverse and underserved populations.
These standards may serve to uncover the fact that most physicians have not received formal training in executing high‐quality care transitions in the role of either the sender or the receiver. Further, few physicians have a mechanism in place to evaluate their performance. The American Board of Internal Medicine and the American Board of Family Practice has developed Maintenance of Certification Practice Improvement Modules (PIM) on care coordination that provide an excellent opportunity to sharpen our skills. The HMO Care Management Workgroup has also attempted to summarize the essential skills necessary to care for patients during transitions.17
Perhaps the greatest value of these standards is that they lay the framework for actionable improvement. Local, state, and national quality collaboratives can immediately incorporate these recommendations into their overall strategy. These standards will likely influence the design and implementation of the Medical Home.18 As national attention focuses on how to operationalize bundled payment approaches and Accountable Care Organizations,19 these standards provide a clear consensus on communication, accountability, and ensuring patient‐centeredness. The standards are an excellent start and provide a framework for further innovation.
One area in particular may be the opportunity to reinvent the format, content, and medium by which essential information is transferred. For example, one might envision the value of producing a scaled down version of the discharge summary with a limited core set of data elements that could be quickly completed and communicated to the next care team via fax, e‐mail, or text messaging.
Complementing new strategies to improve the exchange of health information are opportunities to reconsider the culture within which this communication occurs. Our profession has a long‐standing tradition of not providing directives to our colleagues on the details of clinical management. Hospitalists develop important insights during a patient's hospital stay and are in an ideal position to anticipate potential developments in the subsequent course after discharge. Contrast this with the 5 to 10 minutes that a primary care physician or specialist may have to come up to speed on the hospital and posthospital events in order to manage the patient in the ambulatory arena. Thus, rather than the traditional historical orientation to a discharge summary, one could envision a more future‐orientated document characterized by a series of if‐then statements that outline a series of possible clinical scenarios that may play out over the weeks after discharge along with recommendations for adjustments to the treatment plan.
At a broader level, the release of these standards demonstrate to our communities and to our nation that physicians can join forces to address a particularly complex and challenging aspect of healthcare. Change can indeed come from within our profession rather than being imposed by outside influences such as government administrators, regulatory bodies, or malpractice attorneys. I applaud such efforts and believe that hospitalists will continue to play a central role in national efforts to improve transitions of care.
- ,,,,,.Deficits in information transfer from inpatient to outpatient physician at hospital discharge: a systematic review.J Gen Intern Med.2004;19(S1):135.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,.It Shouldn't Be This Way: The Failure of Long Term Care.1st ed.Nashville, TN:Vanderbilt University Press;2005.
- .Dismantling Rube Goldberg: cutting through chaos to achieve coordinated care.J Hosp Med.2009;4(4):259–260.
- ,.The experience of an orthopaedic traumatologist when the trauma hits home: observations and suggestions.J Bone Joint Surg Am.2008;90(9):2026–2036.
- .Quality comes home.Ann Intern Med.1996;125(10):839–843.
- .My mother and the medical care ad‐hoc‐racy.Health Aff.2003;22(2):238–242.
- .A hospitalization from hell: a patient's perspective on quality.Ann Intern Med.2003;138:33–39.
- ,,,.The ReACH Collaborative—improving quality home care.Caring.2007;26(8):44–51.
- Next step in care. Available at: http://www.nextstepincare.org. Accessed June2009.
- ,,,,. Health information exchange in post‐acute and long‐term care case study findings: final report. 2007. Office of Disability, Aging and Long‐Term Care Policy; Office of the Assistant Secretary for Planning and Evaluation; U.S. Department of Health and Human Services. Available at: http://aspe.hhs.gov/daltcp/reports/2007/HIEcase.pdf. Accessed June2009.
- .Transitions of Care Consensus Policy Statement. American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine.J Hosp Med.2009;4(6):364–370.
- ,,,.Better transitions: improving comprehension of discharge instructions.Front Health Serv Manag.2009;25(3):11–32.
- ,,,.Patient and physician perceptions after software‐assisted discharge from hospital: cluster randomized trial.J Hosp Med.2009;4(6):356–363.
- Patient Activation Measure. Available at: http://www.insigniahealth.com/products/pam.html. Accessed June2009.
- ,.A SAFE DC: a conceptual framework for care of the homeless inpatient.J Hosp Med2009;4(6):375–381.
- HMO Care Management Workgroup. One patient, many places: managing healthcare transitions. 2004. Available at: http://www.caretransitions. org/documents/One%20Patient%20RWJ%20Report.pdf. Accessed June2009.
- American College of Physicians. Patient‐Centered Medical Home: ACP delivers expanded PCMH resources online. Available at: http://www.acponline.org/advocacy/where_we_stand/medical_home. Accessed June2009.
- American College of Physicians. Accountable Care Organizations. Available at: http://www.acponline.org/advocacy/where_we_stand/medical_ home. Accessed June2009.
- ,,,,,.Deficits in information transfer from inpatient to outpatient physician at hospital discharge: a systematic review.J Gen Intern Med.2004;19(S1):135.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,.It Shouldn't Be This Way: The Failure of Long Term Care.1st ed.Nashville, TN:Vanderbilt University Press;2005.
- .Dismantling Rube Goldberg: cutting through chaos to achieve coordinated care.J Hosp Med.2009;4(4):259–260.
- ,.The experience of an orthopaedic traumatologist when the trauma hits home: observations and suggestions.J Bone Joint Surg Am.2008;90(9):2026–2036.
- .Quality comes home.Ann Intern Med.1996;125(10):839–843.
- .My mother and the medical care ad‐hoc‐racy.Health Aff.2003;22(2):238–242.
- .A hospitalization from hell: a patient's perspective on quality.Ann Intern Med.2003;138:33–39.
- ,,,.The ReACH Collaborative—improving quality home care.Caring.2007;26(8):44–51.
- Next step in care. Available at: http://www.nextstepincare.org. Accessed June2009.
- ,,,,. Health information exchange in post‐acute and long‐term care case study findings: final report. 2007. Office of Disability, Aging and Long‐Term Care Policy; Office of the Assistant Secretary for Planning and Evaluation; U.S. Department of Health and Human Services. Available at: http://aspe.hhs.gov/daltcp/reports/2007/HIEcase.pdf. Accessed June2009.
- .Transitions of Care Consensus Policy Statement. American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine.J Hosp Med.2009;4(6):364–370.
- ,,,.Better transitions: improving comprehension of discharge instructions.Front Health Serv Manag.2009;25(3):11–32.
- ,,,.Patient and physician perceptions after software‐assisted discharge from hospital: cluster randomized trial.J Hosp Med.2009;4(6):356–363.
- Patient Activation Measure. Available at: http://www.insigniahealth.com/products/pam.html. Accessed June2009.
- ,.A SAFE DC: a conceptual framework for care of the homeless inpatient.J Hosp Med2009;4(6):375–381.
- HMO Care Management Workgroup. One patient, many places: managing healthcare transitions. 2004. Available at: http://www.caretransitions. org/documents/One%20Patient%20RWJ%20Report.pdf. Accessed June2009.
- American College of Physicians. Patient‐Centered Medical Home: ACP delivers expanded PCMH resources online. Available at: http://www.acponline.org/advocacy/where_we_stand/medical_home. Accessed June2009.
- American College of Physicians. Accountable Care Organizations. Available at: http://www.acponline.org/advocacy/where_we_stand/medical_ home. Accessed June2009.
Ultrasound Measurement to Estimate CVP
Severe sepsis and septic shock account for more than 750,000 hospital admissions and 215,000 deaths per year.1 Early fluid resuscitation is the cornerstone of treatment, and early goal‐directed therapy (EGDT), which includes a target central venous pressure (CVP) of 8 to 12 mm Hg, has been shown to improve outcomes, including mortality and length of stay.2 This goal allows appropriate initial resuscitation and may decrease the risk of excess fluid administration, which is related to adverse outcomes in critically ill patients.3 However, nonintensivists may not start early aggressive fluid resuscitation because of inability to accurately assess intravascular volume, concerns for inadvertent volume overload, or the difficulty of recognizing insidious illness. Assessment of volume status, primarily from inspection of the internal jugular vein to estimate CVP, is difficult to perform by clinical examination alone, especially if CVP is very low.4 Inspection of the external jugular vein is perhaps easier than inspecting the internal jugular vein and appears to accurately estimate CVP,5 but it does not allow the degree of precision necessary for EGDT. Echocardiography can estimate CVP based on respirophasic variation or collapsibility index, but this technique requires expensive equipment and sonographic expertise. The current gold standard technique for measuring CVP requires an invasive central venous catheter, which can delay timely resuscitation and is associated with complications.6
An alternative technique to guide resuscitation efforts should be accurate, safe, rapid, and easy to perform at the bedside, while providing real‐time measurement results. We hypothesized that CVP can be accurately assessed using noninvasive ultrasound imaging of the internal jugular vein, since jugular venous pressure is essentially equal to CVP.7 Specifically, our study estimated the diagnostic accuracy of ultrasound measurement of the aspect ratio (height/width) of the internal jugular vein compared with the invasively measured CVP target for EGDT. We expected that a lower aspect ratio would correlate with a lower CVP and a higher aspect ratio would correlate with a higher CVP.
Methods
Volunteers were enrolled at Saint Mary's Hospital (Mayo Clinic) in Rochester, MN, from January to March 2006, and patients were enrolled at Saint Mary's Hospital and at Abbott Northwestern Hospital (Allina Hospitals and Clinics) in Minneapolis, MN, from May 2006 to October 2007. The study was approved by the Institutional Review Boards of Mayo Clinic and Allina and had 2 phases. The first phase comprised ultrasound measurements of internal jugular vein aspect ratio and determination of intraobserver and interobserver agreement in healthy volunteers. The second phase involved measurement of internal jugular vein aspect ratio and invasive CVP in a convenience sample of 44 spontaneously breathing patients admitted to medical intensive care units: 9 patients at Saint Marys Hospital and 35 patients at Abbott Northwestern Hospital. Patients were enrolled only when study members were on duty in the intensive care unit and able to perform study measurements. As a result, a high proportion of patients who may have been eligible were not asked to participate.
Each volunteer was deemed euvolemic on the basis of normal orthostatic measurements and normal oral intake with no vomiting or diarrhea in the previous 5 days. Measurements of 19 volunteers were made by 1 author (A.S.K.), with subsequent measurements of 15 of the volunteers made by another author (O.G.) to determine interobserver variability; 4 participants did not undergo a second measurement because of scheduling conflicts.
Inclusion and exclusion criteria for the critically ill patients are provided in Table 1. Recruitment was based on presenting symptoms and test results that led the intensive care unit physicians to decide to place a CVP monitor. All the enrolled patients had invasive CVP measurement performed approximately 30 to 40 minutes after ultrasound measurement of the internal jugular vein; this delay was the time required to place the central line and obtain the measurement. All patients who were invited to participate in the study were included. No patients were excluded on the basis of the exclusion criteria or because of inability to place a central line. No complications related to central line placement occurred.
| Inclusion criteria |
| 1. Aged 18 years or older |
| 2. Admission to the intensive care unit |
| 3. Spontaneously breathing (not intubated/ventilated) |
| 4. Planned insertion of a central venous pressure monitor for therapy |
| Exclusion criteria |
| 1. Known cervical spine injuries or fusion |
| 2. Nonremovable cervical collars |
| 3. Surgical dressings that would prevent visualization of the internal jugular vein |
| 4. Inability of the patient to be properly positioned |
| 5. A code situation |
We followed a prescribed measurement technique (Table 2) to determine the internal jugular vein aspect ratio in all volunteers and patients. Measurements of the volunteers were made with a Site‐Rite 3 Ultrasound System (Bard Access Systems, Inc., Salt Lake City, UT) using a 9.0‐MHz transducer. Measurements of the critically ill patients were made with a SonoSite MicroMaxx ultrasound system (SonoSite, Inc., Bothell, WA) using a 10.5‐MHz transducer. Study team physicians initially were blinded to actual measured CVP. Internal jugular vein aspect ratio and CVP were measured at tidal volume end‐expiration for all patients. One measurement was obtained for each patient, with measurements being made by 1 of 4 physicians (2 intensivists, 1 critical care fellow, and 1 chief medicine resident). With no specific ultrasound training and with only minimal practice, the physicians could obtain the optimal aspect ratio within a few seconds (Figure 1).
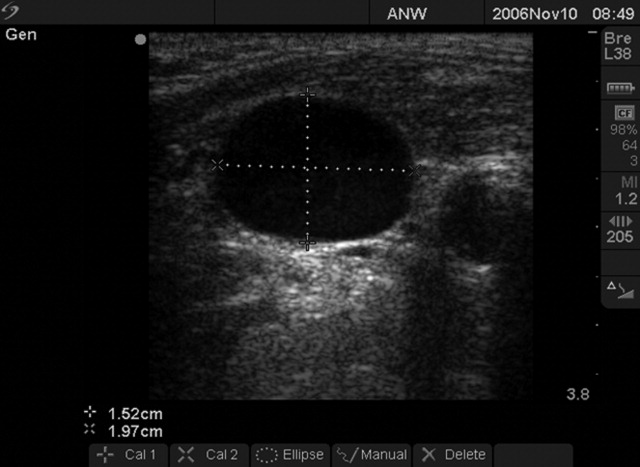
| 1. Position the patient supine (0) with head and legs flat, ensuring overall comfort. A small pillow can be used to help keep head, neck, and trunk aligned |
| 2. Have the patient rotate his or her head slightly to the side (<30) to expose the internal jugular vein |
| 3. Place the transducer transversely on the patient's neck over the expected location of the internal jugular vein. The transducer should be perpendicular to the patient's neck |
| 4. Apply slight pressure to the transducer to locate the internal jugular vein on the view screen. Use the minimum pressure necessary to obtain a good quality ultrasound image |
| 5. Once the internal jugular vein is found, adjust the position of the transducer over the vein to obtain the most circular cross‐sectional image |
| 6. Have the patient breathe normally, then ask him or her to briefly stop breathing at normal (tidal volume) end‐expiration |
| 7. Store the best end‐expiration image (in which the internal jugular vein appears most circular) and have the patient resume normal breathing |
| 8. Measure the height and width of the internal jugular vein using the built‐in cursor function or a ruler |
This was an exploratory prospective study, and all methods of data collection were designed before patient enrollment. However, the ultrasound‐derived aspect ratio of 0.83 (which defined a CVP of 8 mm Hg) was determined post hoc to maximize sensitivity and specificity and was based on the aspect ratio of the euvolemic volunteers and the inflection point of the CVP vs aspect ratio curve for the critically ill patients.
Statistical Analysis
Groups were compared using the 2 test for differences in proportions and the Wilcoxon rank sum test for continuous data. P < 0.05 was considered statistically significant. Bland‐Altman plots were used to describe the bias and variability of the aspect ratio within and between observers.8 This technique compares 2 methods of measurement to determine agreement and repeatability by plotting the mean of the differences (which should be zero) and the upper and lower limits of agreement (1.96 standard deviations [SDs] of those differences above and below the mean). Results were calculated using the available data; there was no adjustment for missing data. Analyses were performed using SPLUS and SAS/STAT software (SAS Institute, Inc., Cary, NC).
Results
We first evaluated 19 white volunteers: 12 women and 7 men. Mean (SD) age was 42 (11) years and mean body mass index was 26.6 (4.5) kg/m2. Mean arterial pressure was 89 (13) mm Hg and mean heart rate was 71 (15) beats/minute. Mean aspect ratio of the right and left internal jugular vein for all volunteers was 0.82 (0.07). There was no difference in aspect ratio between the right (0.83 [0.10]) and left (0.81 [0.13]) vein (P > 0.10). Also, no difference in the aspect ratio was seen between men (0.81 [0.08]) and women (0.83 [0.07]) (P = 0.77). Bland‐Altman analysis indicated moderate intraobserver and interobserver agreement for the aspect ratio measurements (Figure 2).
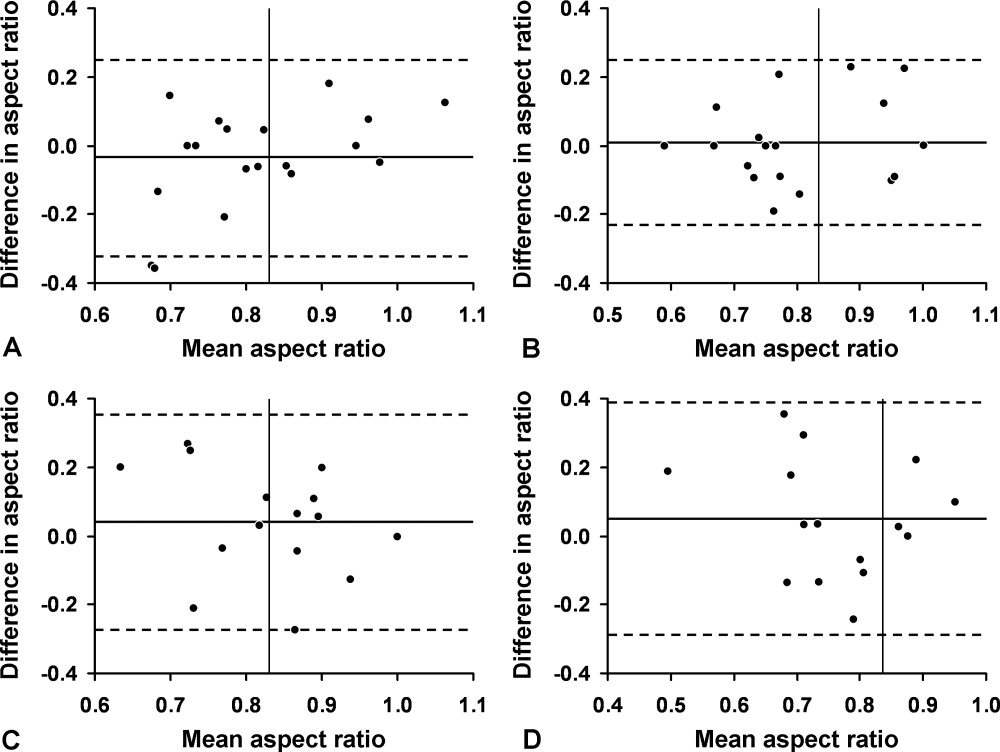
We then compared the aspect ratio measured using ultrasound and CVP measured with an invasive monitor for 44 spontaneously breathing critically ill patients (22 women and 22 men; 38 were white). Mean (SD) age was 66 (14) years and mean body mass index was 28.8 (9.1) kg/m2. Mean arterial pressure (n = 36) was 67 (12) mm Hg and mean heart rate (n = 34) was 92 (22) beats/minute. Systemic inflammatory response syndrome (SIRS) criteria were present in 23 of 40 patients; complete data were unavailable for the other 4 patients. Of these 40 patients, 20 had sepsis, 15 had severe sepsis, and 5 had septic shock. The most common diagnoses were gastrointestinal tract bleeding in 6 patients and congestive heart failure in 4 patients. Acute Physiology and Chronic Health Evaluation (APACHE III) score, available for 8 of the 9 patients at Saint Marys Hospital, was 63 (10).
Figure 3 shows measured aspect ratios vs. invasively measured CVP for the critically ill patients. The curvilinear result is consistent with venous and right ventricular compliance ( volume/ pressure) characteristics. Note that the inflection point (beginning of the increased slope) of the curve corresponds to a CVP of about 8 mm Hg. Furthermore, the aspect ratio (0.8) at this point is the same as that seen in the euvolemic volunteers. These findings suggest that, in spontaneously breathing patients, a CVP of about 8 mm Hg and an aspect ratio of about 0.8 each defines the beginning of the plateau on the cardiac Frank‐Starling curve.
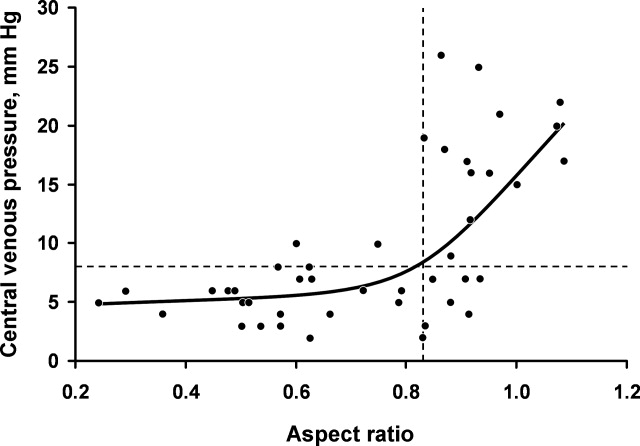
Ultrasound imaging of the internal jugular vein aspect ratio accurately estimated the CVP target of 8 mm Hg based on the area under the receiver operating characteristics curve of 0.84 (95% confidence interval [CI], 0.72‐0.96) (Figure 4). For an invasively measured CVP of less than 8 mm Hg, the likelihood ratio for a positive ultrasound test result (aspect ratio < 0.83) was 3.5 (95% CI, 1.4‐8.4) and for a negative test result (aspect ratio 0.83) was 0.30 (95% CI, 0.14‐0.62). Clinically, this means that patients with a measured aspect ratio of less than 0.83 require further fluid resuscitation, whereas patients with a measured aspect ratio of 0.83 or greater are less likely to benefit from fluid resuscitation.
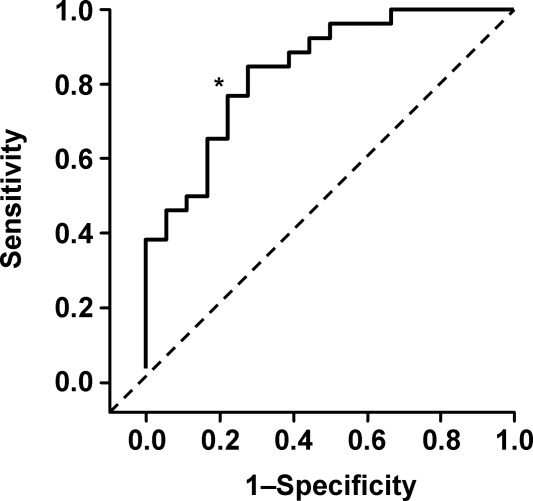
Discussion
This study demonstrated that the EGDT CVP target of 8 to 12 mm Hg can be accurately estimated (referenced to invasive CVP monitoring) using noninvasive ultrasound measurement of the internal jugular vein in spontaneously breathing critically ill patients. The measurement process is simple to perform at the bedside and moderately reliable when performed by different observers; also, the results appear to be equivalent for both sides and for males or females. Images can be stored electronically for serial comparisons and for viewing by other caregivers. Because the aspect ratio is essentially constant over the length of the internal jugular vein, unlike diameter, measurements can be performed anywhere along the vein. Also, ultrasound imaging allows visualization of the internal jugular vein despite anatomic variation.9
Previous attempts at noninvasive hemodynamic monitoring using plethysmography, thoracic electrical bioimpedance, and external Doppler probes have shown these methods to be cumbersome or inaccurate.1013 Other investigators have used echocardiography14, 15 and handheld ultrasound16 to image the diameter of the inferior vena cava in order to assess intravascular volume status, but these techniques require expertise in sonographic imaging. An alternative technique is to measure peripheral venous pressure, which correlates with CVP.17 This method, however, requires technical expertise to zero the monitor and is not yet widely used for critically ill patients. A literature search found 1 letter to the editor suggesting that real‐time ultrasound imaging of the internal jugular vein could be used to qualitatively determine jugular venous pressure18 and 3 studies using ultrasound in conjunction with a pressure transducer or manometer to determine the pressure needed to collapse the vein (either the internal jugular or a peripheral vein), with subsequent correlation to CVP.1921 These latter techniques appear to be cumbersome and require custom equipment that is not readily available in most hospitals.
Any measurement of CVP, including our technique, assumes correlation with volume responsiveness as a surrogate for intravascular volume. However, CVP is governed by multiple physiologic and pathologic factors, including intravascular volume, vascular and ventricular compliance, ventricular function, tricuspid stenosis and regurgitation, cardiac tamponade, and atrioventricular dissociation.22, 23 Therefore, CVP alone may not be an accurate measure of volume responsiveness (intravascular volume). CVP may also have spontaneous variation similar to pulmonary capillary wedge pressure, which can be as high as 7 mm Hg in any given patient.24 Furthermore, invasive CVP monitors also have limitations, and the overall accuracy of the Philips system used at Saint Marys Hospital is 4% of the reading or 4 mm Hg, whichever is greater.25 Nonetheless, the EGDT algorithm that incorporates CVP measurement with a target of 8 to 12 mm Hg in spontaneously breathing patients and 12 mm Hg in mechanically ventilated patients has resulted in decreased mortality among patients with severe sepsis and is recommended by the Surviving Sepsis Campaign guidelines26 and the Institute for Healthcare Improvement.27
These study results are important because nonintensivists such as hospitalists and emergency department physicians can use this technique to provide rapid fluid resuscitation early in the course of severe sepsis and septic shock, when aggressive fluid resuscitation is most effective. Ultrasound imaging of the internal jugular vein is easy to perform without formal training, and the equipment is readily available in most hospitals. Future studies will evaluate outcomes in spontaneously breathing and ventilated patients to determine the accuracy of this measurement technique in volume‐depleted and volume‐overloaded states. If validated in different patient populations, ultrasound measurement of the internal jugular vein could substitute for the EGDT CVP target in critically ill patients and allow early aggressive fluid resuscitation before a central venous catheter is placed.
Limitations
This exploratory study enrolled a small convenience sample of primarily white patients. The convenience sample is potentially prone to selection bias since a majority of patients who may have been eligible were never asked to participate. Also, not all patients had sepsis syndrome; our intention was to measure CVP and aspect ratio for available critically ill patients. Accordingly, results may be different depending on severity of illness. In addition, some of the patients were transferred from outside medical centers or from emergency departments and therefore may have already been partly resuscitated. Another limitation is that the intraobserver and interobserver variability for the healthy volunteers showed only moderate agreement, possibly indicating limited repeatability, although these results could be due to the small sample size. Also, we did not determine intraobserver and interobserver variability for the critically ill patients; results may be different from those of the healthy volunteers. Furthermore, underlying conditions such as tricuspid stenosis or regurgitation and cardiac tamponade may affect measurement results, but we included all patients without formal assessment, since treatment was performed on an urgent/emergent basis as would happen in real clinical settings.
Acknowledgements
The authors dedicate this work to their patients with severe sepsis. They thank Lisa Kirkland, MD, and Murat Yilmaz, MD, for their assistance with this study. They also thank the Mayo Clinic Divisions of General Internal Medicine and Pulmonary and Critical Care Medicine for funding.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29(7):1303–1310.
- ,,, et al.Early Goal‐Directed Therapy Collaborative Group. Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345(19):1368–1377.
- ,.Fluid therapy in resuscitated sepsis: less is more.Chest.2008;133(1):252–263.
- ,.The rational clinical examination: does this patient have abnormal central venous pressure?JAMA.1996;275(8):630–634.
- ,,,,,.Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients.Arch Intern Med.2006;166(19):2132–2137.
- ,.Central venous catheterization.Crit Care Med.2007;35(5):1390–1396.
- .How to use central venous pressure measurements.Curr Opin Crit Care.2005;11(3):264–270.
- ,.Statistical methods for assessing agreement between two methods of clinical measurement.Lancet.1986;1(8476):307–310.
- ,.Anatomical variations of internal jugular vein location: impact on central venous access.Crit Care Med.1991;19(12):1516–1519.
- ,,.Noninvasive measurement of central venous pressure by neck inductive plethysmography.Chest.1991;100(2):371–375.
- ,,, et al.A new noninvasive method to determine central venous pressure.Resuscitation.2006;70(2):238–246.
- .Advances in critical care monitoring.Arch Surg.1997;132(7):734–739.
- ,,, et al.Continuous recording of pulmonary artery diastolic pressure and cardiac output using a novel ultrasound transducer.J Am Soc Echocardiogr.2002;15(11):1381–1386.
- ,,,,.Measurement of anterior‐posterior diameter of inferior vena cava by ultrasonography: a new non‐invasive method to assess acute changes in vascular filling state.Cardiovasc Res.1994;28(8):1269–1272.
- ,,,.Utility of the inferior vena cava diameter as a marker of dry weight in nonoliguric hemodialyzed patients.ASAIO J.2001;47(5):528–532.
- ,,, et al.Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic.Clin J Am Soc Nephrol.2006;1(4):749–753.
- ,,, et al.Comparison of peripheral and central venous pressures in critically ill patients.Anaesth Intensive Care.2003;31(1):34–39.
- .Determination of elevated jugular venous pressure by real‐time ultrasound.Ann Emerg Med.1999;34(1):115.
- ,,,.Ultrasound‐guided noninvasive measurement of a patient's central venous pressure.Conf Proc IEEE Eng Med Biol Soc.2006;1:3843–3849.
- ,,, et al.Noninvasive central venous pressure measurement by controlled compression sonography at the forearm.J Am Coll Cardiol.2007;50(16):1584–1589.
- ,,,,,.Estimation of central venous pressure by ultrasound.Resuscitation.2005;64(2):193–199.
- ,,, et al.Determination of total effective vascular compliance in patients with sepsis syndrome.Am J Respir Crit Care Med.1998;157(1):50–56.
- ,,.Central venous pressure: uses and limitations. In: Pinsky MR, Payen D, eds.Functional Hemodynamic Monitoring.Berlin, Germany:Springer‐Verlag Berlin Heidelberg;2006:101.
- ,.Normal fluctuations in pulmonary artery and pulmonary capillary wedge pressures in acutely ill patients.Heart Lung.1982;11(5):393–398.
- Philips M3012A Data Sheet.Hemodynamic extension to the multi‐measurement server.Amsterdam:Koninklijke Philips Electronics N.V.;2003.
- ,,, et al.Surviving Sepsis Campaign Management Guidelines Committee. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32(3):858–873. [Erratua: Crit Care Med. 2004;32(6):1448. Correction of dosage error in text. Crit Care Med. 2004;32(10):2169–2170.]
- Institute for Healthcare Improvement.Sepsis.Cambridge, MA:Institute for Healthcare Improvement. Available at:http://www.ihi.org/IHI/Topics/CriticalCare/Sepsis. Accessed March 2009.
Severe sepsis and septic shock account for more than 750,000 hospital admissions and 215,000 deaths per year.1 Early fluid resuscitation is the cornerstone of treatment, and early goal‐directed therapy (EGDT), which includes a target central venous pressure (CVP) of 8 to 12 mm Hg, has been shown to improve outcomes, including mortality and length of stay.2 This goal allows appropriate initial resuscitation and may decrease the risk of excess fluid administration, which is related to adverse outcomes in critically ill patients.3 However, nonintensivists may not start early aggressive fluid resuscitation because of inability to accurately assess intravascular volume, concerns for inadvertent volume overload, or the difficulty of recognizing insidious illness. Assessment of volume status, primarily from inspection of the internal jugular vein to estimate CVP, is difficult to perform by clinical examination alone, especially if CVP is very low.4 Inspection of the external jugular vein is perhaps easier than inspecting the internal jugular vein and appears to accurately estimate CVP,5 but it does not allow the degree of precision necessary for EGDT. Echocardiography can estimate CVP based on respirophasic variation or collapsibility index, but this technique requires expensive equipment and sonographic expertise. The current gold standard technique for measuring CVP requires an invasive central venous catheter, which can delay timely resuscitation and is associated with complications.6
An alternative technique to guide resuscitation efforts should be accurate, safe, rapid, and easy to perform at the bedside, while providing real‐time measurement results. We hypothesized that CVP can be accurately assessed using noninvasive ultrasound imaging of the internal jugular vein, since jugular venous pressure is essentially equal to CVP.7 Specifically, our study estimated the diagnostic accuracy of ultrasound measurement of the aspect ratio (height/width) of the internal jugular vein compared with the invasively measured CVP target for EGDT. We expected that a lower aspect ratio would correlate with a lower CVP and a higher aspect ratio would correlate with a higher CVP.
Methods
Volunteers were enrolled at Saint Mary's Hospital (Mayo Clinic) in Rochester, MN, from January to March 2006, and patients were enrolled at Saint Mary's Hospital and at Abbott Northwestern Hospital (Allina Hospitals and Clinics) in Minneapolis, MN, from May 2006 to October 2007. The study was approved by the Institutional Review Boards of Mayo Clinic and Allina and had 2 phases. The first phase comprised ultrasound measurements of internal jugular vein aspect ratio and determination of intraobserver and interobserver agreement in healthy volunteers. The second phase involved measurement of internal jugular vein aspect ratio and invasive CVP in a convenience sample of 44 spontaneously breathing patients admitted to medical intensive care units: 9 patients at Saint Marys Hospital and 35 patients at Abbott Northwestern Hospital. Patients were enrolled only when study members were on duty in the intensive care unit and able to perform study measurements. As a result, a high proportion of patients who may have been eligible were not asked to participate.
Each volunteer was deemed euvolemic on the basis of normal orthostatic measurements and normal oral intake with no vomiting or diarrhea in the previous 5 days. Measurements of 19 volunteers were made by 1 author (A.S.K.), with subsequent measurements of 15 of the volunteers made by another author (O.G.) to determine interobserver variability; 4 participants did not undergo a second measurement because of scheduling conflicts.
Inclusion and exclusion criteria for the critically ill patients are provided in Table 1. Recruitment was based on presenting symptoms and test results that led the intensive care unit physicians to decide to place a CVP monitor. All the enrolled patients had invasive CVP measurement performed approximately 30 to 40 minutes after ultrasound measurement of the internal jugular vein; this delay was the time required to place the central line and obtain the measurement. All patients who were invited to participate in the study were included. No patients were excluded on the basis of the exclusion criteria or because of inability to place a central line. No complications related to central line placement occurred.
| Inclusion criteria |
| 1. Aged 18 years or older |
| 2. Admission to the intensive care unit |
| 3. Spontaneously breathing (not intubated/ventilated) |
| 4. Planned insertion of a central venous pressure monitor for therapy |
| Exclusion criteria |
| 1. Known cervical spine injuries or fusion |
| 2. Nonremovable cervical collars |
| 3. Surgical dressings that would prevent visualization of the internal jugular vein |
| 4. Inability of the patient to be properly positioned |
| 5. A code situation |
We followed a prescribed measurement technique (Table 2) to determine the internal jugular vein aspect ratio in all volunteers and patients. Measurements of the volunteers were made with a Site‐Rite 3 Ultrasound System (Bard Access Systems, Inc., Salt Lake City, UT) using a 9.0‐MHz transducer. Measurements of the critically ill patients were made with a SonoSite MicroMaxx ultrasound system (SonoSite, Inc., Bothell, WA) using a 10.5‐MHz transducer. Study team physicians initially were blinded to actual measured CVP. Internal jugular vein aspect ratio and CVP were measured at tidal volume end‐expiration for all patients. One measurement was obtained for each patient, with measurements being made by 1 of 4 physicians (2 intensivists, 1 critical care fellow, and 1 chief medicine resident). With no specific ultrasound training and with only minimal practice, the physicians could obtain the optimal aspect ratio within a few seconds (Figure 1).

| 1. Position the patient supine (0) with head and legs flat, ensuring overall comfort. A small pillow can be used to help keep head, neck, and trunk aligned |
| 2. Have the patient rotate his or her head slightly to the side (<30) to expose the internal jugular vein |
| 3. Place the transducer transversely on the patient's neck over the expected location of the internal jugular vein. The transducer should be perpendicular to the patient's neck |
| 4. Apply slight pressure to the transducer to locate the internal jugular vein on the view screen. Use the minimum pressure necessary to obtain a good quality ultrasound image |
| 5. Once the internal jugular vein is found, adjust the position of the transducer over the vein to obtain the most circular cross‐sectional image |
| 6. Have the patient breathe normally, then ask him or her to briefly stop breathing at normal (tidal volume) end‐expiration |
| 7. Store the best end‐expiration image (in which the internal jugular vein appears most circular) and have the patient resume normal breathing |
| 8. Measure the height and width of the internal jugular vein using the built‐in cursor function or a ruler |
This was an exploratory prospective study, and all methods of data collection were designed before patient enrollment. However, the ultrasound‐derived aspect ratio of 0.83 (which defined a CVP of 8 mm Hg) was determined post hoc to maximize sensitivity and specificity and was based on the aspect ratio of the euvolemic volunteers and the inflection point of the CVP vs aspect ratio curve for the critically ill patients.
Statistical Analysis
Groups were compared using the 2 test for differences in proportions and the Wilcoxon rank sum test for continuous data. P < 0.05 was considered statistically significant. Bland‐Altman plots were used to describe the bias and variability of the aspect ratio within and between observers.8 This technique compares 2 methods of measurement to determine agreement and repeatability by plotting the mean of the differences (which should be zero) and the upper and lower limits of agreement (1.96 standard deviations [SDs] of those differences above and below the mean). Results were calculated using the available data; there was no adjustment for missing data. Analyses were performed using SPLUS and SAS/STAT software (SAS Institute, Inc., Cary, NC).
Results
We first evaluated 19 white volunteers: 12 women and 7 men. Mean (SD) age was 42 (11) years and mean body mass index was 26.6 (4.5) kg/m2. Mean arterial pressure was 89 (13) mm Hg and mean heart rate was 71 (15) beats/minute. Mean aspect ratio of the right and left internal jugular vein for all volunteers was 0.82 (0.07). There was no difference in aspect ratio between the right (0.83 [0.10]) and left (0.81 [0.13]) vein (P > 0.10). Also, no difference in the aspect ratio was seen between men (0.81 [0.08]) and women (0.83 [0.07]) (P = 0.77). Bland‐Altman analysis indicated moderate intraobserver and interobserver agreement for the aspect ratio measurements (Figure 2).

We then compared the aspect ratio measured using ultrasound and CVP measured with an invasive monitor for 44 spontaneously breathing critically ill patients (22 women and 22 men; 38 were white). Mean (SD) age was 66 (14) years and mean body mass index was 28.8 (9.1) kg/m2. Mean arterial pressure (n = 36) was 67 (12) mm Hg and mean heart rate (n = 34) was 92 (22) beats/minute. Systemic inflammatory response syndrome (SIRS) criteria were present in 23 of 40 patients; complete data were unavailable for the other 4 patients. Of these 40 patients, 20 had sepsis, 15 had severe sepsis, and 5 had septic shock. The most common diagnoses were gastrointestinal tract bleeding in 6 patients and congestive heart failure in 4 patients. Acute Physiology and Chronic Health Evaluation (APACHE III) score, available for 8 of the 9 patients at Saint Marys Hospital, was 63 (10).
Figure 3 shows measured aspect ratios vs. invasively measured CVP for the critically ill patients. The curvilinear result is consistent with venous and right ventricular compliance ( volume/ pressure) characteristics. Note that the inflection point (beginning of the increased slope) of the curve corresponds to a CVP of about 8 mm Hg. Furthermore, the aspect ratio (0.8) at this point is the same as that seen in the euvolemic volunteers. These findings suggest that, in spontaneously breathing patients, a CVP of about 8 mm Hg and an aspect ratio of about 0.8 each defines the beginning of the plateau on the cardiac Frank‐Starling curve.

Ultrasound imaging of the internal jugular vein aspect ratio accurately estimated the CVP target of 8 mm Hg based on the area under the receiver operating characteristics curve of 0.84 (95% confidence interval [CI], 0.72‐0.96) (Figure 4). For an invasively measured CVP of less than 8 mm Hg, the likelihood ratio for a positive ultrasound test result (aspect ratio < 0.83) was 3.5 (95% CI, 1.4‐8.4) and for a negative test result (aspect ratio 0.83) was 0.30 (95% CI, 0.14‐0.62). Clinically, this means that patients with a measured aspect ratio of less than 0.83 require further fluid resuscitation, whereas patients with a measured aspect ratio of 0.83 or greater are less likely to benefit from fluid resuscitation.

Discussion
This study demonstrated that the EGDT CVP target of 8 to 12 mm Hg can be accurately estimated (referenced to invasive CVP monitoring) using noninvasive ultrasound measurement of the internal jugular vein in spontaneously breathing critically ill patients. The measurement process is simple to perform at the bedside and moderately reliable when performed by different observers; also, the results appear to be equivalent for both sides and for males or females. Images can be stored electronically for serial comparisons and for viewing by other caregivers. Because the aspect ratio is essentially constant over the length of the internal jugular vein, unlike diameter, measurements can be performed anywhere along the vein. Also, ultrasound imaging allows visualization of the internal jugular vein despite anatomic variation.9
Previous attempts at noninvasive hemodynamic monitoring using plethysmography, thoracic electrical bioimpedance, and external Doppler probes have shown these methods to be cumbersome or inaccurate.1013 Other investigators have used echocardiography14, 15 and handheld ultrasound16 to image the diameter of the inferior vena cava in order to assess intravascular volume status, but these techniques require expertise in sonographic imaging. An alternative technique is to measure peripheral venous pressure, which correlates with CVP.17 This method, however, requires technical expertise to zero the monitor and is not yet widely used for critically ill patients. A literature search found 1 letter to the editor suggesting that real‐time ultrasound imaging of the internal jugular vein could be used to qualitatively determine jugular venous pressure18 and 3 studies using ultrasound in conjunction with a pressure transducer or manometer to determine the pressure needed to collapse the vein (either the internal jugular or a peripheral vein), with subsequent correlation to CVP.1921 These latter techniques appear to be cumbersome and require custom equipment that is not readily available in most hospitals.
Any measurement of CVP, including our technique, assumes correlation with volume responsiveness as a surrogate for intravascular volume. However, CVP is governed by multiple physiologic and pathologic factors, including intravascular volume, vascular and ventricular compliance, ventricular function, tricuspid stenosis and regurgitation, cardiac tamponade, and atrioventricular dissociation.22, 23 Therefore, CVP alone may not be an accurate measure of volume responsiveness (intravascular volume). CVP may also have spontaneous variation similar to pulmonary capillary wedge pressure, which can be as high as 7 mm Hg in any given patient.24 Furthermore, invasive CVP monitors also have limitations, and the overall accuracy of the Philips system used at Saint Marys Hospital is 4% of the reading or 4 mm Hg, whichever is greater.25 Nonetheless, the EGDT algorithm that incorporates CVP measurement with a target of 8 to 12 mm Hg in spontaneously breathing patients and 12 mm Hg in mechanically ventilated patients has resulted in decreased mortality among patients with severe sepsis and is recommended by the Surviving Sepsis Campaign guidelines26 and the Institute for Healthcare Improvement.27
These study results are important because nonintensivists such as hospitalists and emergency department physicians can use this technique to provide rapid fluid resuscitation early in the course of severe sepsis and septic shock, when aggressive fluid resuscitation is most effective. Ultrasound imaging of the internal jugular vein is easy to perform without formal training, and the equipment is readily available in most hospitals. Future studies will evaluate outcomes in spontaneously breathing and ventilated patients to determine the accuracy of this measurement technique in volume‐depleted and volume‐overloaded states. If validated in different patient populations, ultrasound measurement of the internal jugular vein could substitute for the EGDT CVP target in critically ill patients and allow early aggressive fluid resuscitation before a central venous catheter is placed.
Limitations
This exploratory study enrolled a small convenience sample of primarily white patients. The convenience sample is potentially prone to selection bias since a majority of patients who may have been eligible were never asked to participate. Also, not all patients had sepsis syndrome; our intention was to measure CVP and aspect ratio for available critically ill patients. Accordingly, results may be different depending on severity of illness. In addition, some of the patients were transferred from outside medical centers or from emergency departments and therefore may have already been partly resuscitated. Another limitation is that the intraobserver and interobserver variability for the healthy volunteers showed only moderate agreement, possibly indicating limited repeatability, although these results could be due to the small sample size. Also, we did not determine intraobserver and interobserver variability for the critically ill patients; results may be different from those of the healthy volunteers. Furthermore, underlying conditions such as tricuspid stenosis or regurgitation and cardiac tamponade may affect measurement results, but we included all patients without formal assessment, since treatment was performed on an urgent/emergent basis as would happen in real clinical settings.
Acknowledgements
The authors dedicate this work to their patients with severe sepsis. They thank Lisa Kirkland, MD, and Murat Yilmaz, MD, for their assistance with this study. They also thank the Mayo Clinic Divisions of General Internal Medicine and Pulmonary and Critical Care Medicine for funding.
Severe sepsis and septic shock account for more than 750,000 hospital admissions and 215,000 deaths per year.1 Early fluid resuscitation is the cornerstone of treatment, and early goal‐directed therapy (EGDT), which includes a target central venous pressure (CVP) of 8 to 12 mm Hg, has been shown to improve outcomes, including mortality and length of stay.2 This goal allows appropriate initial resuscitation and may decrease the risk of excess fluid administration, which is related to adverse outcomes in critically ill patients.3 However, nonintensivists may not start early aggressive fluid resuscitation because of inability to accurately assess intravascular volume, concerns for inadvertent volume overload, or the difficulty of recognizing insidious illness. Assessment of volume status, primarily from inspection of the internal jugular vein to estimate CVP, is difficult to perform by clinical examination alone, especially if CVP is very low.4 Inspection of the external jugular vein is perhaps easier than inspecting the internal jugular vein and appears to accurately estimate CVP,5 but it does not allow the degree of precision necessary for EGDT. Echocardiography can estimate CVP based on respirophasic variation or collapsibility index, but this technique requires expensive equipment and sonographic expertise. The current gold standard technique for measuring CVP requires an invasive central venous catheter, which can delay timely resuscitation and is associated with complications.6
An alternative technique to guide resuscitation efforts should be accurate, safe, rapid, and easy to perform at the bedside, while providing real‐time measurement results. We hypothesized that CVP can be accurately assessed using noninvasive ultrasound imaging of the internal jugular vein, since jugular venous pressure is essentially equal to CVP.7 Specifically, our study estimated the diagnostic accuracy of ultrasound measurement of the aspect ratio (height/width) of the internal jugular vein compared with the invasively measured CVP target for EGDT. We expected that a lower aspect ratio would correlate with a lower CVP and a higher aspect ratio would correlate with a higher CVP.
Methods
Volunteers were enrolled at Saint Mary's Hospital (Mayo Clinic) in Rochester, MN, from January to March 2006, and patients were enrolled at Saint Mary's Hospital and at Abbott Northwestern Hospital (Allina Hospitals and Clinics) in Minneapolis, MN, from May 2006 to October 2007. The study was approved by the Institutional Review Boards of Mayo Clinic and Allina and had 2 phases. The first phase comprised ultrasound measurements of internal jugular vein aspect ratio and determination of intraobserver and interobserver agreement in healthy volunteers. The second phase involved measurement of internal jugular vein aspect ratio and invasive CVP in a convenience sample of 44 spontaneously breathing patients admitted to medical intensive care units: 9 patients at Saint Marys Hospital and 35 patients at Abbott Northwestern Hospital. Patients were enrolled only when study members were on duty in the intensive care unit and able to perform study measurements. As a result, a high proportion of patients who may have been eligible were not asked to participate.
Each volunteer was deemed euvolemic on the basis of normal orthostatic measurements and normal oral intake with no vomiting or diarrhea in the previous 5 days. Measurements of 19 volunteers were made by 1 author (A.S.K.), with subsequent measurements of 15 of the volunteers made by another author (O.G.) to determine interobserver variability; 4 participants did not undergo a second measurement because of scheduling conflicts.
Inclusion and exclusion criteria for the critically ill patients are provided in Table 1. Recruitment was based on presenting symptoms and test results that led the intensive care unit physicians to decide to place a CVP monitor. All the enrolled patients had invasive CVP measurement performed approximately 30 to 40 minutes after ultrasound measurement of the internal jugular vein; this delay was the time required to place the central line and obtain the measurement. All patients who were invited to participate in the study were included. No patients were excluded on the basis of the exclusion criteria or because of inability to place a central line. No complications related to central line placement occurred.
| Inclusion criteria |
| 1. Aged 18 years or older |
| 2. Admission to the intensive care unit |
| 3. Spontaneously breathing (not intubated/ventilated) |
| 4. Planned insertion of a central venous pressure monitor for therapy |
| Exclusion criteria |
| 1. Known cervical spine injuries or fusion |
| 2. Nonremovable cervical collars |
| 3. Surgical dressings that would prevent visualization of the internal jugular vein |
| 4. Inability of the patient to be properly positioned |
| 5. A code situation |
We followed a prescribed measurement technique (Table 2) to determine the internal jugular vein aspect ratio in all volunteers and patients. Measurements of the volunteers were made with a Site‐Rite 3 Ultrasound System (Bard Access Systems, Inc., Salt Lake City, UT) using a 9.0‐MHz transducer. Measurements of the critically ill patients were made with a SonoSite MicroMaxx ultrasound system (SonoSite, Inc., Bothell, WA) using a 10.5‐MHz transducer. Study team physicians initially were blinded to actual measured CVP. Internal jugular vein aspect ratio and CVP were measured at tidal volume end‐expiration for all patients. One measurement was obtained for each patient, with measurements being made by 1 of 4 physicians (2 intensivists, 1 critical care fellow, and 1 chief medicine resident). With no specific ultrasound training and with only minimal practice, the physicians could obtain the optimal aspect ratio within a few seconds (Figure 1).

| 1. Position the patient supine (0) with head and legs flat, ensuring overall comfort. A small pillow can be used to help keep head, neck, and trunk aligned |
| 2. Have the patient rotate his or her head slightly to the side (<30) to expose the internal jugular vein |
| 3. Place the transducer transversely on the patient's neck over the expected location of the internal jugular vein. The transducer should be perpendicular to the patient's neck |
| 4. Apply slight pressure to the transducer to locate the internal jugular vein on the view screen. Use the minimum pressure necessary to obtain a good quality ultrasound image |
| 5. Once the internal jugular vein is found, adjust the position of the transducer over the vein to obtain the most circular cross‐sectional image |
| 6. Have the patient breathe normally, then ask him or her to briefly stop breathing at normal (tidal volume) end‐expiration |
| 7. Store the best end‐expiration image (in which the internal jugular vein appears most circular) and have the patient resume normal breathing |
| 8. Measure the height and width of the internal jugular vein using the built‐in cursor function or a ruler |
This was an exploratory prospective study, and all methods of data collection were designed before patient enrollment. However, the ultrasound‐derived aspect ratio of 0.83 (which defined a CVP of 8 mm Hg) was determined post hoc to maximize sensitivity and specificity and was based on the aspect ratio of the euvolemic volunteers and the inflection point of the CVP vs aspect ratio curve for the critically ill patients.
Statistical Analysis
Groups were compared using the 2 test for differences in proportions and the Wilcoxon rank sum test for continuous data. P < 0.05 was considered statistically significant. Bland‐Altman plots were used to describe the bias and variability of the aspect ratio within and between observers.8 This technique compares 2 methods of measurement to determine agreement and repeatability by plotting the mean of the differences (which should be zero) and the upper and lower limits of agreement (1.96 standard deviations [SDs] of those differences above and below the mean). Results were calculated using the available data; there was no adjustment for missing data. Analyses were performed using SPLUS and SAS/STAT software (SAS Institute, Inc., Cary, NC).
Results
We first evaluated 19 white volunteers: 12 women and 7 men. Mean (SD) age was 42 (11) years and mean body mass index was 26.6 (4.5) kg/m2. Mean arterial pressure was 89 (13) mm Hg and mean heart rate was 71 (15) beats/minute. Mean aspect ratio of the right and left internal jugular vein for all volunteers was 0.82 (0.07). There was no difference in aspect ratio between the right (0.83 [0.10]) and left (0.81 [0.13]) vein (P > 0.10). Also, no difference in the aspect ratio was seen between men (0.81 [0.08]) and women (0.83 [0.07]) (P = 0.77). Bland‐Altman analysis indicated moderate intraobserver and interobserver agreement for the aspect ratio measurements (Figure 2).

We then compared the aspect ratio measured using ultrasound and CVP measured with an invasive monitor for 44 spontaneously breathing critically ill patients (22 women and 22 men; 38 were white). Mean (SD) age was 66 (14) years and mean body mass index was 28.8 (9.1) kg/m2. Mean arterial pressure (n = 36) was 67 (12) mm Hg and mean heart rate (n = 34) was 92 (22) beats/minute. Systemic inflammatory response syndrome (SIRS) criteria were present in 23 of 40 patients; complete data were unavailable for the other 4 patients. Of these 40 patients, 20 had sepsis, 15 had severe sepsis, and 5 had septic shock. The most common diagnoses were gastrointestinal tract bleeding in 6 patients and congestive heart failure in 4 patients. Acute Physiology and Chronic Health Evaluation (APACHE III) score, available for 8 of the 9 patients at Saint Marys Hospital, was 63 (10).
Figure 3 shows measured aspect ratios vs. invasively measured CVP for the critically ill patients. The curvilinear result is consistent with venous and right ventricular compliance ( volume/ pressure) characteristics. Note that the inflection point (beginning of the increased slope) of the curve corresponds to a CVP of about 8 mm Hg. Furthermore, the aspect ratio (0.8) at this point is the same as that seen in the euvolemic volunteers. These findings suggest that, in spontaneously breathing patients, a CVP of about 8 mm Hg and an aspect ratio of about 0.8 each defines the beginning of the plateau on the cardiac Frank‐Starling curve.

Ultrasound imaging of the internal jugular vein aspect ratio accurately estimated the CVP target of 8 mm Hg based on the area under the receiver operating characteristics curve of 0.84 (95% confidence interval [CI], 0.72‐0.96) (Figure 4). For an invasively measured CVP of less than 8 mm Hg, the likelihood ratio for a positive ultrasound test result (aspect ratio < 0.83) was 3.5 (95% CI, 1.4‐8.4) and for a negative test result (aspect ratio 0.83) was 0.30 (95% CI, 0.14‐0.62). Clinically, this means that patients with a measured aspect ratio of less than 0.83 require further fluid resuscitation, whereas patients with a measured aspect ratio of 0.83 or greater are less likely to benefit from fluid resuscitation.

Discussion
This study demonstrated that the EGDT CVP target of 8 to 12 mm Hg can be accurately estimated (referenced to invasive CVP monitoring) using noninvasive ultrasound measurement of the internal jugular vein in spontaneously breathing critically ill patients. The measurement process is simple to perform at the bedside and moderately reliable when performed by different observers; also, the results appear to be equivalent for both sides and for males or females. Images can be stored electronically for serial comparisons and for viewing by other caregivers. Because the aspect ratio is essentially constant over the length of the internal jugular vein, unlike diameter, measurements can be performed anywhere along the vein. Also, ultrasound imaging allows visualization of the internal jugular vein despite anatomic variation.9
Previous attempts at noninvasive hemodynamic monitoring using plethysmography, thoracic electrical bioimpedance, and external Doppler probes have shown these methods to be cumbersome or inaccurate.1013 Other investigators have used echocardiography14, 15 and handheld ultrasound16 to image the diameter of the inferior vena cava in order to assess intravascular volume status, but these techniques require expertise in sonographic imaging. An alternative technique is to measure peripheral venous pressure, which correlates with CVP.17 This method, however, requires technical expertise to zero the monitor and is not yet widely used for critically ill patients. A literature search found 1 letter to the editor suggesting that real‐time ultrasound imaging of the internal jugular vein could be used to qualitatively determine jugular venous pressure18 and 3 studies using ultrasound in conjunction with a pressure transducer or manometer to determine the pressure needed to collapse the vein (either the internal jugular or a peripheral vein), with subsequent correlation to CVP.1921 These latter techniques appear to be cumbersome and require custom equipment that is not readily available in most hospitals.
Any measurement of CVP, including our technique, assumes correlation with volume responsiveness as a surrogate for intravascular volume. However, CVP is governed by multiple physiologic and pathologic factors, including intravascular volume, vascular and ventricular compliance, ventricular function, tricuspid stenosis and regurgitation, cardiac tamponade, and atrioventricular dissociation.22, 23 Therefore, CVP alone may not be an accurate measure of volume responsiveness (intravascular volume). CVP may also have spontaneous variation similar to pulmonary capillary wedge pressure, which can be as high as 7 mm Hg in any given patient.24 Furthermore, invasive CVP monitors also have limitations, and the overall accuracy of the Philips system used at Saint Marys Hospital is 4% of the reading or 4 mm Hg, whichever is greater.25 Nonetheless, the EGDT algorithm that incorporates CVP measurement with a target of 8 to 12 mm Hg in spontaneously breathing patients and 12 mm Hg in mechanically ventilated patients has resulted in decreased mortality among patients with severe sepsis and is recommended by the Surviving Sepsis Campaign guidelines26 and the Institute for Healthcare Improvement.27
These study results are important because nonintensivists such as hospitalists and emergency department physicians can use this technique to provide rapid fluid resuscitation early in the course of severe sepsis and septic shock, when aggressive fluid resuscitation is most effective. Ultrasound imaging of the internal jugular vein is easy to perform without formal training, and the equipment is readily available in most hospitals. Future studies will evaluate outcomes in spontaneously breathing and ventilated patients to determine the accuracy of this measurement technique in volume‐depleted and volume‐overloaded states. If validated in different patient populations, ultrasound measurement of the internal jugular vein could substitute for the EGDT CVP target in critically ill patients and allow early aggressive fluid resuscitation before a central venous catheter is placed.
Limitations
This exploratory study enrolled a small convenience sample of primarily white patients. The convenience sample is potentially prone to selection bias since a majority of patients who may have been eligible were never asked to participate. Also, not all patients had sepsis syndrome; our intention was to measure CVP and aspect ratio for available critically ill patients. Accordingly, results may be different depending on severity of illness. In addition, some of the patients were transferred from outside medical centers or from emergency departments and therefore may have already been partly resuscitated. Another limitation is that the intraobserver and interobserver variability for the healthy volunteers showed only moderate agreement, possibly indicating limited repeatability, although these results could be due to the small sample size. Also, we did not determine intraobserver and interobserver variability for the critically ill patients; results may be different from those of the healthy volunteers. Furthermore, underlying conditions such as tricuspid stenosis or regurgitation and cardiac tamponade may affect measurement results, but we included all patients without formal assessment, since treatment was performed on an urgent/emergent basis as would happen in real clinical settings.
Acknowledgements
The authors dedicate this work to their patients with severe sepsis. They thank Lisa Kirkland, MD, and Murat Yilmaz, MD, for their assistance with this study. They also thank the Mayo Clinic Divisions of General Internal Medicine and Pulmonary and Critical Care Medicine for funding.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29(7):1303–1310.
- ,,, et al.Early Goal‐Directed Therapy Collaborative Group. Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345(19):1368–1377.
- ,.Fluid therapy in resuscitated sepsis: less is more.Chest.2008;133(1):252–263.
- ,.The rational clinical examination: does this patient have abnormal central venous pressure?JAMA.1996;275(8):630–634.
- ,,,,,.Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients.Arch Intern Med.2006;166(19):2132–2137.
- ,.Central venous catheterization.Crit Care Med.2007;35(5):1390–1396.
- .How to use central venous pressure measurements.Curr Opin Crit Care.2005;11(3):264–270.
- ,.Statistical methods for assessing agreement between two methods of clinical measurement.Lancet.1986;1(8476):307–310.
- ,.Anatomical variations of internal jugular vein location: impact on central venous access.Crit Care Med.1991;19(12):1516–1519.
- ,,.Noninvasive measurement of central venous pressure by neck inductive plethysmography.Chest.1991;100(2):371–375.
- ,,, et al.A new noninvasive method to determine central venous pressure.Resuscitation.2006;70(2):238–246.
- .Advances in critical care monitoring.Arch Surg.1997;132(7):734–739.
- ,,, et al.Continuous recording of pulmonary artery diastolic pressure and cardiac output using a novel ultrasound transducer.J Am Soc Echocardiogr.2002;15(11):1381–1386.
- ,,,,.Measurement of anterior‐posterior diameter of inferior vena cava by ultrasonography: a new non‐invasive method to assess acute changes in vascular filling state.Cardiovasc Res.1994;28(8):1269–1272.
- ,,,.Utility of the inferior vena cava diameter as a marker of dry weight in nonoliguric hemodialyzed patients.ASAIO J.2001;47(5):528–532.
- ,,, et al.Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic.Clin J Am Soc Nephrol.2006;1(4):749–753.
- ,,, et al.Comparison of peripheral and central venous pressures in critically ill patients.Anaesth Intensive Care.2003;31(1):34–39.
- .Determination of elevated jugular venous pressure by real‐time ultrasound.Ann Emerg Med.1999;34(1):115.
- ,,,.Ultrasound‐guided noninvasive measurement of a patient's central venous pressure.Conf Proc IEEE Eng Med Biol Soc.2006;1:3843–3849.
- ,,, et al.Noninvasive central venous pressure measurement by controlled compression sonography at the forearm.J Am Coll Cardiol.2007;50(16):1584–1589.
- ,,,,,.Estimation of central venous pressure by ultrasound.Resuscitation.2005;64(2):193–199.
- ,,, et al.Determination of total effective vascular compliance in patients with sepsis syndrome.Am J Respir Crit Care Med.1998;157(1):50–56.
- ,,.Central venous pressure: uses and limitations. In: Pinsky MR, Payen D, eds.Functional Hemodynamic Monitoring.Berlin, Germany:Springer‐Verlag Berlin Heidelberg;2006:101.
- ,.Normal fluctuations in pulmonary artery and pulmonary capillary wedge pressures in acutely ill patients.Heart Lung.1982;11(5):393–398.
- Philips M3012A Data Sheet.Hemodynamic extension to the multi‐measurement server.Amsterdam:Koninklijke Philips Electronics N.V.;2003.
- ,,, et al.Surviving Sepsis Campaign Management Guidelines Committee. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32(3):858–873. [Erratua: Crit Care Med. 2004;32(6):1448. Correction of dosage error in text. Crit Care Med. 2004;32(10):2169–2170.]
- Institute for Healthcare Improvement.Sepsis.Cambridge, MA:Institute for Healthcare Improvement. Available at:http://www.ihi.org/IHI/Topics/CriticalCare/Sepsis. Accessed March 2009.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29(7):1303–1310.
- ,,, et al.Early Goal‐Directed Therapy Collaborative Group. Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345(19):1368–1377.
- ,.Fluid therapy in resuscitated sepsis: less is more.Chest.2008;133(1):252–263.
- ,.The rational clinical examination: does this patient have abnormal central venous pressure?JAMA.1996;275(8):630–634.
- ,,,,,.Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients.Arch Intern Med.2006;166(19):2132–2137.
- ,.Central venous catheterization.Crit Care Med.2007;35(5):1390–1396.
- .How to use central venous pressure measurements.Curr Opin Crit Care.2005;11(3):264–270.
- ,.Statistical methods for assessing agreement between two methods of clinical measurement.Lancet.1986;1(8476):307–310.
- ,.Anatomical variations of internal jugular vein location: impact on central venous access.Crit Care Med.1991;19(12):1516–1519.
- ,,.Noninvasive measurement of central venous pressure by neck inductive plethysmography.Chest.1991;100(2):371–375.
- ,,, et al.A new noninvasive method to determine central venous pressure.Resuscitation.2006;70(2):238–246.
- .Advances in critical care monitoring.Arch Surg.1997;132(7):734–739.
- ,,, et al.Continuous recording of pulmonary artery diastolic pressure and cardiac output using a novel ultrasound transducer.J Am Soc Echocardiogr.2002;15(11):1381–1386.
- ,,,,.Measurement of anterior‐posterior diameter of inferior vena cava by ultrasonography: a new non‐invasive method to assess acute changes in vascular filling state.Cardiovasc Res.1994;28(8):1269–1272.
- ,,,.Utility of the inferior vena cava diameter as a marker of dry weight in nonoliguric hemodialyzed patients.ASAIO J.2001;47(5):528–532.
- ,,, et al.Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic.Clin J Am Soc Nephrol.2006;1(4):749–753.
- ,,, et al.Comparison of peripheral and central venous pressures in critically ill patients.Anaesth Intensive Care.2003;31(1):34–39.
- .Determination of elevated jugular venous pressure by real‐time ultrasound.Ann Emerg Med.1999;34(1):115.
- ,,,.Ultrasound‐guided noninvasive measurement of a patient's central venous pressure.Conf Proc IEEE Eng Med Biol Soc.2006;1:3843–3849.
- ,,, et al.Noninvasive central venous pressure measurement by controlled compression sonography at the forearm.J Am Coll Cardiol.2007;50(16):1584–1589.
- ,,,,,.Estimation of central venous pressure by ultrasound.Resuscitation.2005;64(2):193–199.
- ,,, et al.Determination of total effective vascular compliance in patients with sepsis syndrome.Am J Respir Crit Care Med.1998;157(1):50–56.
- ,,.Central venous pressure: uses and limitations. In: Pinsky MR, Payen D, eds.Functional Hemodynamic Monitoring.Berlin, Germany:Springer‐Verlag Berlin Heidelberg;2006:101.
- ,.Normal fluctuations in pulmonary artery and pulmonary capillary wedge pressures in acutely ill patients.Heart Lung.1982;11(5):393–398.
- Philips M3012A Data Sheet.Hemodynamic extension to the multi‐measurement server.Amsterdam:Koninklijke Philips Electronics N.V.;2003.
- ,,, et al.Surviving Sepsis Campaign Management Guidelines Committee. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32(3):858–873. [Erratua: Crit Care Med. 2004;32(6):1448. Correction of dosage error in text. Crit Care Med. 2004;32(10):2169–2170.]
- Institute for Healthcare Improvement.Sepsis.Cambridge, MA:Institute for Healthcare Improvement. Available at:http://www.ihi.org/IHI/Topics/CriticalCare/Sepsis. Accessed March 2009.
Copyright © 2009 Society of Hospital Medicine
Pneumomediastinum and Pneumopericardium
A 73‐year‐old male presented with acute congestive heart failure and non‐ST elevation myocardial infarction. His initial chest x‐ray and computed tomography (CT) demonstrated pulmonary vascular congestion and alveolar infiltrates, and he promptly underwent cardiac catheterization with placement of a coronary stent. Subsequently, his respiratory status deteriorated, and repeat films and chest CT demonstrated extensive pneumomediastinum and pneumopericardium (Figures 13). The patient was intubated, and bronchoscopy and upper gastrointestinal (GI) endoscopy were performed, but demonstrated no evidence of perforation that could cause such an air leak. There was no evidence of tamponade, clinically or on echocardiogram. His condition worsened abruptly, and he expired following a cardiac arrest. Postmortem, the team considered that the extensive air leak could have been caused by catheterization, stent placement, central line placement, or mediastinitis or pericarditis causing microscopic fistulae. The patient's tracheal aspirate and biopsy grew Candida albicans but no evidence of invasive candidiasis was found on autopsy. No definitive etiology was found.
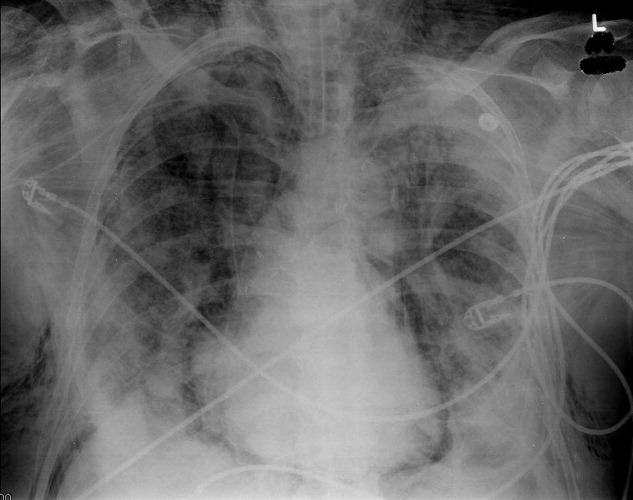
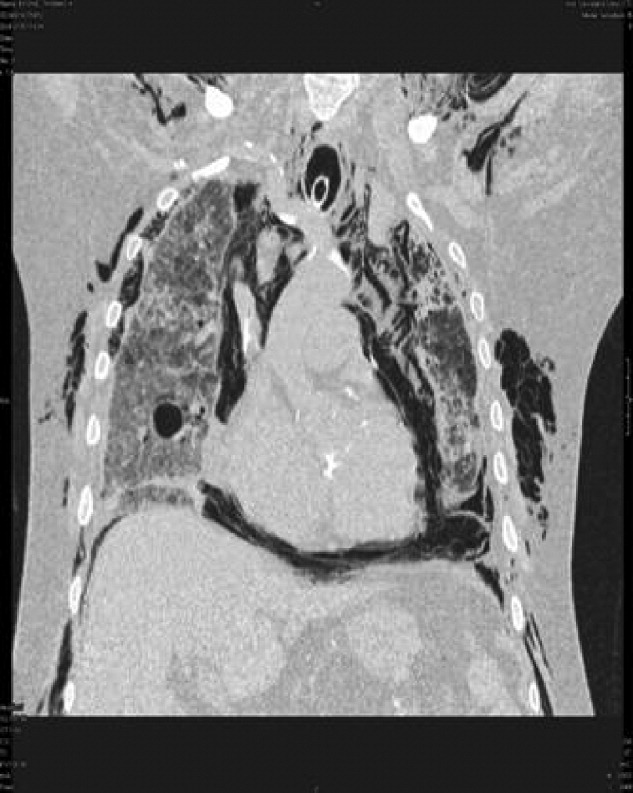
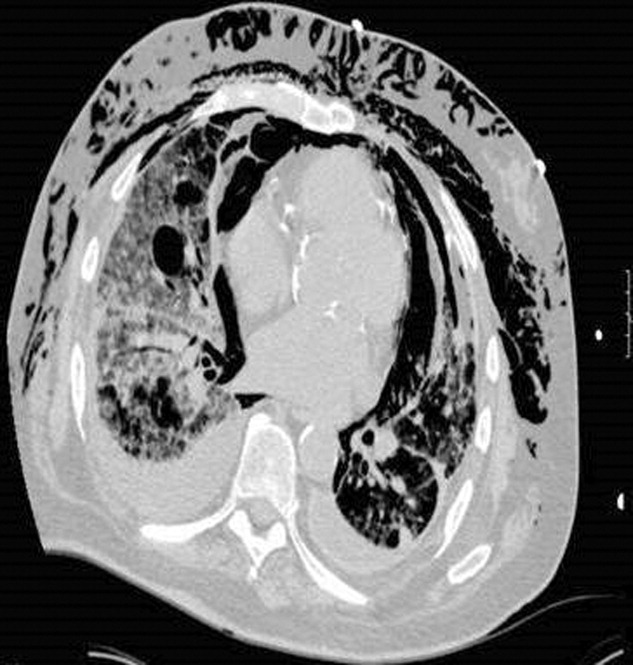
In contrast to pneumomediastinum, pneumopericardium is a rare condition and its pathophysiology is not well understood. Most cases have been reported in newborns receiving mechanical ventilation. In adults, the condition occurs due to chest trauma, or can be iatrogenic secondary to laparoscopy, bronchoscopy, or endotracheal intubation. There have been case reports of pneumopericardium after cardiac catheterization and central line placement.1, 2 Other causes include lung transplant, esophageal perforation, severe asthma, positive pressure ventilation, and pericarditis (eg, histoplasmosis and tuberculosis).3, 4 Clinical findings include distant heart sounds, shifting precordial tympany, and a succussion splash with metallic tinkling (known as mill wheel murmur) in hydropneumopericardium.5 Chest CT can distinguish pneumopericardium from pneumomediastinum: with the former, the air changes position when the patient adopts a supine position.6 Cardiac tamponade can occur in up to 37% of cases, and pericardiocentesis or pericardial tube drainage in these cases can be lifesaving.7
- ,,,,.[Cardiac tamponade and central venous catheterization].Ann Fr Anesth Reanim.1992;11:201–204. [French]
- ,,,.Pneumopericardium as a complication of balloon atrial septostomy.Pediatr Cardiol.1987;8:135–137.
- ,,,,.Continuous left hemidiaphragm sign revisited: a case of spontaneous pneumopericardium and literature review.Heart.2002;88:e5.
- ,.Tension pneumopericardium: a case report and a review of the literature.Am Surg.2006;72:330–331.
- Symptoms and signs of syndromes associated with mill wheel murmurs.NC Med J.1988;49:569–572.
- ,.Pneumomediastinum: old signs and new signs.AJR Am J Roentgenol.1996;166:1041–1048.
- ,,,,.Cardiac tamponade without pericardial effusion after blunt chest trauma.Am Heart J.1996;131:198–200.
A 73‐year‐old male presented with acute congestive heart failure and non‐ST elevation myocardial infarction. His initial chest x‐ray and computed tomography (CT) demonstrated pulmonary vascular congestion and alveolar infiltrates, and he promptly underwent cardiac catheterization with placement of a coronary stent. Subsequently, his respiratory status deteriorated, and repeat films and chest CT demonstrated extensive pneumomediastinum and pneumopericardium (Figures 13). The patient was intubated, and bronchoscopy and upper gastrointestinal (GI) endoscopy were performed, but demonstrated no evidence of perforation that could cause such an air leak. There was no evidence of tamponade, clinically or on echocardiogram. His condition worsened abruptly, and he expired following a cardiac arrest. Postmortem, the team considered that the extensive air leak could have been caused by catheterization, stent placement, central line placement, or mediastinitis or pericarditis causing microscopic fistulae. The patient's tracheal aspirate and biopsy grew Candida albicans but no evidence of invasive candidiasis was found on autopsy. No definitive etiology was found.



In contrast to pneumomediastinum, pneumopericardium is a rare condition and its pathophysiology is not well understood. Most cases have been reported in newborns receiving mechanical ventilation. In adults, the condition occurs due to chest trauma, or can be iatrogenic secondary to laparoscopy, bronchoscopy, or endotracheal intubation. There have been case reports of pneumopericardium after cardiac catheterization and central line placement.1, 2 Other causes include lung transplant, esophageal perforation, severe asthma, positive pressure ventilation, and pericarditis (eg, histoplasmosis and tuberculosis).3, 4 Clinical findings include distant heart sounds, shifting precordial tympany, and a succussion splash with metallic tinkling (known as mill wheel murmur) in hydropneumopericardium.5 Chest CT can distinguish pneumopericardium from pneumomediastinum: with the former, the air changes position when the patient adopts a supine position.6 Cardiac tamponade can occur in up to 37% of cases, and pericardiocentesis or pericardial tube drainage in these cases can be lifesaving.7
A 73‐year‐old male presented with acute congestive heart failure and non‐ST elevation myocardial infarction. His initial chest x‐ray and computed tomography (CT) demonstrated pulmonary vascular congestion and alveolar infiltrates, and he promptly underwent cardiac catheterization with placement of a coronary stent. Subsequently, his respiratory status deteriorated, and repeat films and chest CT demonstrated extensive pneumomediastinum and pneumopericardium (Figures 13). The patient was intubated, and bronchoscopy and upper gastrointestinal (GI) endoscopy were performed, but demonstrated no evidence of perforation that could cause such an air leak. There was no evidence of tamponade, clinically or on echocardiogram. His condition worsened abruptly, and he expired following a cardiac arrest. Postmortem, the team considered that the extensive air leak could have been caused by catheterization, stent placement, central line placement, or mediastinitis or pericarditis causing microscopic fistulae. The patient's tracheal aspirate and biopsy grew Candida albicans but no evidence of invasive candidiasis was found on autopsy. No definitive etiology was found.



In contrast to pneumomediastinum, pneumopericardium is a rare condition and its pathophysiology is not well understood. Most cases have been reported in newborns receiving mechanical ventilation. In adults, the condition occurs due to chest trauma, or can be iatrogenic secondary to laparoscopy, bronchoscopy, or endotracheal intubation. There have been case reports of pneumopericardium after cardiac catheterization and central line placement.1, 2 Other causes include lung transplant, esophageal perforation, severe asthma, positive pressure ventilation, and pericarditis (eg, histoplasmosis and tuberculosis).3, 4 Clinical findings include distant heart sounds, shifting precordial tympany, and a succussion splash with metallic tinkling (known as mill wheel murmur) in hydropneumopericardium.5 Chest CT can distinguish pneumopericardium from pneumomediastinum: with the former, the air changes position when the patient adopts a supine position.6 Cardiac tamponade can occur in up to 37% of cases, and pericardiocentesis or pericardial tube drainage in these cases can be lifesaving.7
- ,,,,.[Cardiac tamponade and central venous catheterization].Ann Fr Anesth Reanim.1992;11:201–204. [French]
- ,,,.Pneumopericardium as a complication of balloon atrial septostomy.Pediatr Cardiol.1987;8:135–137.
- ,,,,.Continuous left hemidiaphragm sign revisited: a case of spontaneous pneumopericardium and literature review.Heart.2002;88:e5.
- ,.Tension pneumopericardium: a case report and a review of the literature.Am Surg.2006;72:330–331.
- Symptoms and signs of syndromes associated with mill wheel murmurs.NC Med J.1988;49:569–572.
- ,.Pneumomediastinum: old signs and new signs.AJR Am J Roentgenol.1996;166:1041–1048.
- ,,,,.Cardiac tamponade without pericardial effusion after blunt chest trauma.Am Heart J.1996;131:198–200.
- ,,,,.[Cardiac tamponade and central venous catheterization].Ann Fr Anesth Reanim.1992;11:201–204. [French]
- ,,,.Pneumopericardium as a complication of balloon atrial septostomy.Pediatr Cardiol.1987;8:135–137.
- ,,,,.Continuous left hemidiaphragm sign revisited: a case of spontaneous pneumopericardium and literature review.Heart.2002;88:e5.
- ,.Tension pneumopericardium: a case report and a review of the literature.Am Surg.2006;72:330–331.
- Symptoms and signs of syndromes associated with mill wheel murmurs.NC Med J.1988;49:569–572.
- ,.Pneumomediastinum: old signs and new signs.AJR Am J Roentgenol.1996;166:1041–1048.
- ,,,,.Cardiac tamponade without pericardial effusion after blunt chest trauma.Am Heart J.1996;131:198–200.