User login
What's Eating You? Human Botfly (Dermatobia hominis)
The Use of Moisturizers as an Integral Component of Topical Therapy for Rosacea: Clinical Results Based on the Assessment of Skin Characteristics Study
Wrong Tx for 4 years...Negligence case hinges on penicillin allergy...more
4 years of Tx, but diagnosis was wrong
FOR 4 YEARS, STARTING AT AGE 50, A WOMAN COMPLAINED TO HER INTERNIST of a persistent cough, nasal congestion, muscle and joint pain, and respiratory difficulty on exertion. The doctor treated her with allergy shots, massage therapy, vitamins, and a combination of drugs.
A little more than 4 years after the woman’s first visit to the internist, another physician diagnosed metastatic bone cancer. By then, the disease had spread from the primary mass in the lungs to the brain, legs, liver, and spine. The patient died 2 months later.
PLAINTIFF’S CLAIM The diagnosis should have been made when the patient first visited the internist; prompt treatment could have saved her life.
DOCTOR’S DEFENSE The patient’s respiratory difficulty wasn’t persistent and was judged to arise from seasonal allergies. In addition, the respiratory problems resulted from deconditioning caused by chronic fatigue syndrome.
VERDICT $1.2 million New York verdict.
COMMENT Persistent symptoms should always prompt a reevaluation of the diagnosis.
Negligence case hinges on penicillin allergy
AN 18-MONTH-OLD GIRL WITH AN EAR INFECTION was seen by a pediatrician, who prescribed amoxicillin clavulanate. The next day she developed puffy eyes and a runny nose. Her parents took her to the emergency room, where the physician diagnosed an allergic reaction to amoxicillin clavulanate and changed her medication to azithromycin. The doctor also prescribed diphenhydramine for the allergic reaction and told the parents to bring the child back the next day for follow-up. After the child took azithromycin, the puffiness and redness around her eyes began to go away. It was more prominent on one side than the other.
When the parents and child returned to the ER the following day, the girl was seen by another doctor, who diagnosed orbital cellulitis without reviewing the chart from the previous visit. He ordered intravenous ceftriaxone, a third-generation cephalosporin with a “known” cross-reactivity with penicillin-based drugs.
Despite the note in the chart about the child’s penicillin allergy, the nursing staff administered the drug while the child’s father held her in his arms. Within several minutes, the girl’s eyes were fixed and she wasn’t moving. The mother ran to get the nurses, by which time the child’s face was turning blue and she was limp. Resuscitation efforts failed.
PLAINTIFFS’ CLAIM The ER physician who saw the child on the second day was negligent in failing to note her history of penicillin allergy. Orbital cellulitis was the wrong diagnosis, unsupported by the symptoms. It should have been confirmed with a computed tomography or magnetic resonance imaging scan. The doctor was negligent in prescribing ceftriaxone, which caused an anaphylactic reaction, acute circulatory collapse, and death. The nurse should have asked the doctor to explain the ceftriaxone order before giving the drug to make sure the doctor was aware of the penicillin allergy. Ceftriaxone should have been administered by IV drip rather than gravity. The child should have been given a green allergy ID wrist band when her parents brought her to the ER the second time.
THE DEFENSE No information about the defense is available.
VERDICT $3 million Illinois settlement.
COMMENT A poorly managed handoff with resulting discontinuity of care, alleged misdiagnosis, and a dubious assertion of cross-reactivity between penicillin and ceftriaxone (see www.jfponline.com/Pages.asp?AID=3850&issue=February%202006 for details) make for a $3 million settlement!
Poor follow-up hinders stage 3 cancer Dx
A LUMP IN HER LEFT BREAST prompted a 42-year-old woman to contact her primary care physician. Office staff returned her phone call, advised her to apply warm compresses to the site, and told her that she’d be scheduled for a mammogram and ultrasound examination. The mammogram revealed bilateral asymmetry. An ultrasound wasn’t done. The woman’s primary care physician didn’t perform a physical examination or refer her for surgical consultation.
Eight months after her initial call to her doctor, the woman began to see another physician, who didn’t follow-up on her complaints of a lump and tenderness in her breast or refer her to a surgeon. Six months later, she was diagnosed with stage 3 breast cancer. Her prognosis was poor.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $1 million Massachusetts settlement.
COMMENT Yet another example of inadequate follow-up of a breast mass that turned out to be cancer. It’s critical that physicians establish a tickler file to assure appropriate follow-up of all women with breast masses.
Was lack of regular PSA testing to blame?
A 49-YEAR-OLD MAN HAD A PARTIAL PHYSICAL EXAM and a prostate-specific antigen test. He complained of urinary problems, including frequent urination and a weak stream. The patient didn’t complete the second part of the exam.
Five months later, he scheduled a follow-up and acute care visit, at which time he complained of rectal bleeding. The doctor performed a digital rectal exam, which revealed an enlarged prostate. He didn’t discuss further PSA testing or follow-up on the previous urinary complaints. He referred the patient to a gastroenterologist.
Six months after the second visit, the patient called to ask about some blood work, including a test for diabetes. The physician ordered a fasting blood sugar test. About a year after that, the patient saw his doctor for a sore throat. The doctor ordered lipid panels, thyroid-stimulating hormone tests, and liver enzyme tests. He didn’t order or discuss PSA testing.
Seventeen months later, the patient was diagnosed with stage 4 prostate cancer, which had metastasized to the brain, lungs, spine, and bony extremities. Various treatment protocols failed to help. By the time of arbitration, the patient had been given fewer than 2 weeks to live.
PLAINTIFF’S CLAIM The plaintiff should have had more regular PSA testing.
THE DEFENSE The PSA test done at the time of the initial physical examination was sufficient; even if the patient had been diagnosed at the second doctor visit 5 months later, his chance of survival would have been less than 50%.
VERDICT $3.5 million California arbitration award.
COMMENT Evidence? What evidence? Here is an arbitration award of $3.5 million for failure to perform PSA testing regularly in a 49-year-old. Although this account is incomplete, remember that the courts are sometimes impervious to evidence-based medicine.
4 years of Tx, but diagnosis was wrong
FOR 4 YEARS, STARTING AT AGE 50, A WOMAN COMPLAINED TO HER INTERNIST of a persistent cough, nasal congestion, muscle and joint pain, and respiratory difficulty on exertion. The doctor treated her with allergy shots, massage therapy, vitamins, and a combination of drugs.
A little more than 4 years after the woman’s first visit to the internist, another physician diagnosed metastatic bone cancer. By then, the disease had spread from the primary mass in the lungs to the brain, legs, liver, and spine. The patient died 2 months later.
PLAINTIFF’S CLAIM The diagnosis should have been made when the patient first visited the internist; prompt treatment could have saved her life.
DOCTOR’S DEFENSE The patient’s respiratory difficulty wasn’t persistent and was judged to arise from seasonal allergies. In addition, the respiratory problems resulted from deconditioning caused by chronic fatigue syndrome.
VERDICT $1.2 million New York verdict.
COMMENT Persistent symptoms should always prompt a reevaluation of the diagnosis.
Negligence case hinges on penicillin allergy
AN 18-MONTH-OLD GIRL WITH AN EAR INFECTION was seen by a pediatrician, who prescribed amoxicillin clavulanate. The next day she developed puffy eyes and a runny nose. Her parents took her to the emergency room, where the physician diagnosed an allergic reaction to amoxicillin clavulanate and changed her medication to azithromycin. The doctor also prescribed diphenhydramine for the allergic reaction and told the parents to bring the child back the next day for follow-up. After the child took azithromycin, the puffiness and redness around her eyes began to go away. It was more prominent on one side than the other.
When the parents and child returned to the ER the following day, the girl was seen by another doctor, who diagnosed orbital cellulitis without reviewing the chart from the previous visit. He ordered intravenous ceftriaxone, a third-generation cephalosporin with a “known” cross-reactivity with penicillin-based drugs.
Despite the note in the chart about the child’s penicillin allergy, the nursing staff administered the drug while the child’s father held her in his arms. Within several minutes, the girl’s eyes were fixed and she wasn’t moving. The mother ran to get the nurses, by which time the child’s face was turning blue and she was limp. Resuscitation efforts failed.
PLAINTIFFS’ CLAIM The ER physician who saw the child on the second day was negligent in failing to note her history of penicillin allergy. Orbital cellulitis was the wrong diagnosis, unsupported by the symptoms. It should have been confirmed with a computed tomography or magnetic resonance imaging scan. The doctor was negligent in prescribing ceftriaxone, which caused an anaphylactic reaction, acute circulatory collapse, and death. The nurse should have asked the doctor to explain the ceftriaxone order before giving the drug to make sure the doctor was aware of the penicillin allergy. Ceftriaxone should have been administered by IV drip rather than gravity. The child should have been given a green allergy ID wrist band when her parents brought her to the ER the second time.
THE DEFENSE No information about the defense is available.
VERDICT $3 million Illinois settlement.
COMMENT A poorly managed handoff with resulting discontinuity of care, alleged misdiagnosis, and a dubious assertion of cross-reactivity between penicillin and ceftriaxone (see www.jfponline.com/Pages.asp?AID=3850&issue=February%202006 for details) make for a $3 million settlement!
Poor follow-up hinders stage 3 cancer Dx
A LUMP IN HER LEFT BREAST prompted a 42-year-old woman to contact her primary care physician. Office staff returned her phone call, advised her to apply warm compresses to the site, and told her that she’d be scheduled for a mammogram and ultrasound examination. The mammogram revealed bilateral asymmetry. An ultrasound wasn’t done. The woman’s primary care physician didn’t perform a physical examination or refer her for surgical consultation.
Eight months after her initial call to her doctor, the woman began to see another physician, who didn’t follow-up on her complaints of a lump and tenderness in her breast or refer her to a surgeon. Six months later, she was diagnosed with stage 3 breast cancer. Her prognosis was poor.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $1 million Massachusetts settlement.
COMMENT Yet another example of inadequate follow-up of a breast mass that turned out to be cancer. It’s critical that physicians establish a tickler file to assure appropriate follow-up of all women with breast masses.
Was lack of regular PSA testing to blame?
A 49-YEAR-OLD MAN HAD A PARTIAL PHYSICAL EXAM and a prostate-specific antigen test. He complained of urinary problems, including frequent urination and a weak stream. The patient didn’t complete the second part of the exam.
Five months later, he scheduled a follow-up and acute care visit, at which time he complained of rectal bleeding. The doctor performed a digital rectal exam, which revealed an enlarged prostate. He didn’t discuss further PSA testing or follow-up on the previous urinary complaints. He referred the patient to a gastroenterologist.
Six months after the second visit, the patient called to ask about some blood work, including a test for diabetes. The physician ordered a fasting blood sugar test. About a year after that, the patient saw his doctor for a sore throat. The doctor ordered lipid panels, thyroid-stimulating hormone tests, and liver enzyme tests. He didn’t order or discuss PSA testing.
Seventeen months later, the patient was diagnosed with stage 4 prostate cancer, which had metastasized to the brain, lungs, spine, and bony extremities. Various treatment protocols failed to help. By the time of arbitration, the patient had been given fewer than 2 weeks to live.
PLAINTIFF’S CLAIM The plaintiff should have had more regular PSA testing.
THE DEFENSE The PSA test done at the time of the initial physical examination was sufficient; even if the patient had been diagnosed at the second doctor visit 5 months later, his chance of survival would have been less than 50%.
VERDICT $3.5 million California arbitration award.
COMMENT Evidence? What evidence? Here is an arbitration award of $3.5 million for failure to perform PSA testing regularly in a 49-year-old. Although this account is incomplete, remember that the courts are sometimes impervious to evidence-based medicine.
4 years of Tx, but diagnosis was wrong
FOR 4 YEARS, STARTING AT AGE 50, A WOMAN COMPLAINED TO HER INTERNIST of a persistent cough, nasal congestion, muscle and joint pain, and respiratory difficulty on exertion. The doctor treated her with allergy shots, massage therapy, vitamins, and a combination of drugs.
A little more than 4 years after the woman’s first visit to the internist, another physician diagnosed metastatic bone cancer. By then, the disease had spread from the primary mass in the lungs to the brain, legs, liver, and spine. The patient died 2 months later.
PLAINTIFF’S CLAIM The diagnosis should have been made when the patient first visited the internist; prompt treatment could have saved her life.
DOCTOR’S DEFENSE The patient’s respiratory difficulty wasn’t persistent and was judged to arise from seasonal allergies. In addition, the respiratory problems resulted from deconditioning caused by chronic fatigue syndrome.
VERDICT $1.2 million New York verdict.
COMMENT Persistent symptoms should always prompt a reevaluation of the diagnosis.
Negligence case hinges on penicillin allergy
AN 18-MONTH-OLD GIRL WITH AN EAR INFECTION was seen by a pediatrician, who prescribed amoxicillin clavulanate. The next day she developed puffy eyes and a runny nose. Her parents took her to the emergency room, where the physician diagnosed an allergic reaction to amoxicillin clavulanate and changed her medication to azithromycin. The doctor also prescribed diphenhydramine for the allergic reaction and told the parents to bring the child back the next day for follow-up. After the child took azithromycin, the puffiness and redness around her eyes began to go away. It was more prominent on one side than the other.
When the parents and child returned to the ER the following day, the girl was seen by another doctor, who diagnosed orbital cellulitis without reviewing the chart from the previous visit. He ordered intravenous ceftriaxone, a third-generation cephalosporin with a “known” cross-reactivity with penicillin-based drugs.
Despite the note in the chart about the child’s penicillin allergy, the nursing staff administered the drug while the child’s father held her in his arms. Within several minutes, the girl’s eyes were fixed and she wasn’t moving. The mother ran to get the nurses, by which time the child’s face was turning blue and she was limp. Resuscitation efforts failed.
PLAINTIFFS’ CLAIM The ER physician who saw the child on the second day was negligent in failing to note her history of penicillin allergy. Orbital cellulitis was the wrong diagnosis, unsupported by the symptoms. It should have been confirmed with a computed tomography or magnetic resonance imaging scan. The doctor was negligent in prescribing ceftriaxone, which caused an anaphylactic reaction, acute circulatory collapse, and death. The nurse should have asked the doctor to explain the ceftriaxone order before giving the drug to make sure the doctor was aware of the penicillin allergy. Ceftriaxone should have been administered by IV drip rather than gravity. The child should have been given a green allergy ID wrist band when her parents brought her to the ER the second time.
THE DEFENSE No information about the defense is available.
VERDICT $3 million Illinois settlement.
COMMENT A poorly managed handoff with resulting discontinuity of care, alleged misdiagnosis, and a dubious assertion of cross-reactivity between penicillin and ceftriaxone (see www.jfponline.com/Pages.asp?AID=3850&issue=February%202006 for details) make for a $3 million settlement!
Poor follow-up hinders stage 3 cancer Dx
A LUMP IN HER LEFT BREAST prompted a 42-year-old woman to contact her primary care physician. Office staff returned her phone call, advised her to apply warm compresses to the site, and told her that she’d be scheduled for a mammogram and ultrasound examination. The mammogram revealed bilateral asymmetry. An ultrasound wasn’t done. The woman’s primary care physician didn’t perform a physical examination or refer her for surgical consultation.
Eight months after her initial call to her doctor, the woman began to see another physician, who didn’t follow-up on her complaints of a lump and tenderness in her breast or refer her to a surgeon. Six months later, she was diagnosed with stage 3 breast cancer. Her prognosis was poor.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $1 million Massachusetts settlement.
COMMENT Yet another example of inadequate follow-up of a breast mass that turned out to be cancer. It’s critical that physicians establish a tickler file to assure appropriate follow-up of all women with breast masses.
Was lack of regular PSA testing to blame?
A 49-YEAR-OLD MAN HAD A PARTIAL PHYSICAL EXAM and a prostate-specific antigen test. He complained of urinary problems, including frequent urination and a weak stream. The patient didn’t complete the second part of the exam.
Five months later, he scheduled a follow-up and acute care visit, at which time he complained of rectal bleeding. The doctor performed a digital rectal exam, which revealed an enlarged prostate. He didn’t discuss further PSA testing or follow-up on the previous urinary complaints. He referred the patient to a gastroenterologist.
Six months after the second visit, the patient called to ask about some blood work, including a test for diabetes. The physician ordered a fasting blood sugar test. About a year after that, the patient saw his doctor for a sore throat. The doctor ordered lipid panels, thyroid-stimulating hormone tests, and liver enzyme tests. He didn’t order or discuss PSA testing.
Seventeen months later, the patient was diagnosed with stage 4 prostate cancer, which had metastasized to the brain, lungs, spine, and bony extremities. Various treatment protocols failed to help. By the time of arbitration, the patient had been given fewer than 2 weeks to live.
PLAINTIFF’S CLAIM The plaintiff should have had more regular PSA testing.
THE DEFENSE The PSA test done at the time of the initial physical examination was sufficient; even if the patient had been diagnosed at the second doctor visit 5 months later, his chance of survival would have been less than 50%.
VERDICT $3.5 million California arbitration award.
COMMENT Evidence? What evidence? Here is an arbitration award of $3.5 million for failure to perform PSA testing regularly in a 49-year-old. Although this account is incomplete, remember that the courts are sometimes impervious to evidence-based medicine.
What’s growing on your stethoscope? (And what you can do about it)
Background Studies have shown that rubbing alcohol pads on stethoscope diaphragms can reduce bacterial colonization, but alcohol pads are used infrequently used and not always available.
Methods We conducted a prospective, single-blinded study to investigate whether simultaneously scrubbing hands and stethoscope head with alcohol-based hand foam would significantly reduce bacterial counts on the stethoscope. Using their own stethoscope, participants imprinted the stethoscope head onto a chocolate agar plate, then used alcohol-based hand foam to cleanse their hands while simultaneously rubbing the stethoscope head. Once the stethoscope heads were dry, the participants imprinted their stethoscope heads onto a second plate. After 48 hours’ incubation, we determined the bacterial counts for the prewash and post-wash plates, and compared the 2.
Results We analyzed a total of 184 cultures (from 92 stethoscopes). Both the mean (28 prewash vs 3 post-wash, P=.001) and median (11 prewash vs 1 post-wash, P=.001) colony counts were significantly greater before being cleansed. Three methicillin-resistant Staphylococcus aureus (MRSA) colonies were identified in the prewash period; all were destroyed by the foam. The estimated number of hand washes needed to prevent 1 MRSA colony is 31 (95% confidence interval [CI], 18-89).
Conclusion Simultaneously using hand foam to clean hands and stethoscope heads reduces bacterial counts on stethoscopes. Further research is needed to determine whether this intervention can reduce morbidity and mortality associated with bacterial infection.
More than 160 years after a Hungarian physician introduced a protocol of strict handwashing and instrument sterilization to hospital wards,1 many clinicians still don’t wash their hands regularly or properly sterilize their medical equipment.2,3 The lack of stringent infection control, both in inpatient and office settings, is exacerbated by the rise in antibiotic-resistant bacteria. Methicillin-resistant Staphylococcus aureus (MRSA), in particular, including community-acquired MRSA, accounts for infections ranging from severe skin lesions to sepsis, and an estimated 18,650 deaths annually.4,5
Waterless hand cleansers, such as alcohol-based foams and gels, improve handwashing compliance.6-8 These products are effective in reducing both bacterial and viral agents, are convenient to use, and may even be good for caregivers’ skin.9 But would they work on stethoscopes? Our study was designed to find out.
An often-neglected source of bacteria
Infection can spread from patient to patient, not only on hands, but also via fomites such as ventilators, computer keyboards, pagers, and stethoscopes.10-14 Antimicrobial stethoscope covers, including those impregnated with silver ions, do not decrease bacterial colonization; evidence suggests that their use may actually increase it.15 Studies indicate that rubbing alcohol pads on stethoscope diaphragms can reduce bacterial colonization, and it has been suggested that cleansing of stethoscopes daily may be as effective as more frequent cleaning.16 Unfortunately, many clinicians do not clean their stethoscopes on a regular basis.17 In addition, alcohol pads are not always available, and using them requires an extra step and produces waste.
An earlier study by a member of our research team (A.S., unpublished data, 2007) indicated that rubbing stethoscopes exposed to nonpathogenic Staphylococcus epidermidis with alcohol-based hand foam was comparable to using alcohol wipes in reducing bacterial counts. The primary objective of this study was to determine whether clinicians can simultaneously reduce bacteria on stethoscope heads and clean their hands with alcohol-based foam.
Methods
This study was a prospective, single-blinded, “before-and-after” trial—a design in which each participant served as his or her own control and used foam that was already available on site. The study was conducted at 1 community-based hospital and 1 satellite family health center; the study was approved by the hospital Institutional Review Board. A grant from St. Margaret’s Foundation covered the cost of the agar plates.
We began by asking the attending physicians, faculty, nurses, residents, and medical students who attended a grand rounds program to participate; we visited the satellite health facility to recruit participants, as well. We started with 93 participants, but 1 stethoscope was damaged during the study, so we ended up with 92 participants and 184 cultures.
Interventions
In the prewash, or “before” portion of the study, all participants imprinted the head of their stethoscope onto a chocolate agar plate. The clinicians then used a 62.5% ethyl alcohol-based foam to cleanse their hands, simultaneously rubbing the stethoscope head between their hands. After a brief drying time, the clinicians imprinted their stethoscope head onto a separate agar plate (the post-wash, or “after” component).
We did not tell participants how to wash their hands or for how long. We simply told them to cleanse their hands as they normally would and to rub the foam onto the stethoscope head, as well.
Randomization and measurement
Prior to data collection, randomly assigned ID numbers were recorded on the bottom of 200 agar plates, which were then placed in a box. One member of our research team gave each clinician 2 plates. Participants imprinted their stethoscope head onto the first plate and handed it to another investigator, who recorded the prewash ID numbers. Participants then performed the handwashing and stethoscope rub and repeated the imprinting procedure with the second plate. This time, the investigator recorded the professional role of each participant (eg, resident, attending, nurse, faculty) as well as the post-wash ID numbers.
After 48 hours at 35°C incubation, the plates were arranged in numerical order. A member of the research team then counted the number and identified the type of bacterial colonies on each plate and recorded the findings on a data sheet by ID number.
Validation
In order to validate the bacterial counts, the supervisor of the hospital laboratory—who had 20 years’ experience in examining cultures and served as the gold standard—independently examined a random sample of plates. We agreed in advance that any count that deviated by more than 7 (approximately half the effect the study was powered to detect) from the gold standard would require another investigator to intervene. This proved unnecessary as no such deviation was found.
Coagulase studies were performed on all plates with bacterial isolates, and gram staining was performed on selected plates, along with identification of gram-negative stains, using the Microscan (Siemens, New York, NY). An “honest broker”—the only person authorized to match the plates with the stethoscopes’ ID numbers—then matched the prewash and post-wash data by stethoscope and type of health care provider. Another investigator analyzed the final data sheet for accuracy.
Power and sample size
A pilot study was performed to obtain estimates of the average and variance of the bacterial counts in a control group of stethoscopes and to determine whether the act of imprinting the stethoscope itself would significantly reduce the colony counts. The results established that there was no statistical change in either the summary statistics or the distribution of the bacterial counts over the course of multiple imprinting.
Estimates obtained from the pilot study indicated that 58 stethoscopes would be sufficient to yield 80% power (alpha=0.05, 2-tailed) for detecting an average difference of 15 colony counts between the prewash and post-wash samples. Seventy-eight stethoscopes would increase the power to 90%. We ultimately tested 92 stethoscopes.
Statistical analysis
Descriptive statistical measures were calculated to examine the bacterial counts. Linear regression analysis was used to compute the correlation in the validation data. This before-and-after design results in “paired data,” and both parametric and nonparametric statistical tests were used. We used a paired t-test to test the mean difference in bacterial counts between the pre- and post-wash samples, and a random effects model to estimate the individual components of variance. The difference in the median bacterial counts was tested using the signed rank test. We used various diagnostic measures to examine the assumptions of the statistical tests; and means, medians, 95% confidence intervals (CIs), and P-values (using P<.05 as statistical significance) to report the results. The Bonferroni multiple comparisons procedure was used to determine whether the bacterial counts were statistically different among subgroups of health care providers. All statistical analyses were performed using SAS (Cary, NC) software.
Results
A total of 184 culture plates showing before and after samples for 92 stethoscopes were analyzed. The provider breakdown of the sample consisted of nurses (39%), residents (30%), attending physicians (15%), faculty (13%), and medical students (3%). Thirty-five (approximately 1 in 6) of the 184 plates were randomly sampled for validation. There was a high degree of reliability between the investigator’s bacterial counts and the bacterial counts of the gold standard (r=+0.98, P<.001).
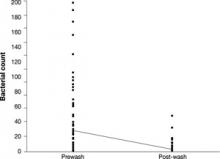
Bacterial counts. The distribution of the bacterial colony counts skewed right in both the prewash (0-198) and post-wash (0-48) samples. The FIGURE shows the skewed distributions in the actual bacterial counts for the 92 pairs of plates before and after hand and stethoscope washing. In the prewash sample, the mean bacterial count was 28.4 (95% CI, 20.2-36.6), vs a post-wash mean of 3.2 (95% CI, 1.8-4.6; P<.001). This resulted in an estimated difference in mean bacterial counts of 25.2 (95% CI, 17.2-33.3). The difference in the medians was also significant, with a prewash median of 11.5 and a post-wash median of 1.0 (P<.001). The difference between the pre- and post-wash periods remained significant even after using various transformations to normalize the data. Random effects modeling showed that very little (<5%) of the total variation was related to the type of health care provider.
Types of bacteria. The TABLE gives the breakdown and frequency of the various types of bacteria that we identified on the stethoscopes. Many were of low pathogenic potential, such as coagulase-negative staph species, which would not cause disease in healthy individuals. However, in hospitalized or immunocompromised patients, they could well induce illness. There were also several clearly pathogenic bacterial isolates, including 3 MRSA colonies (each on a different stethoscope), as well as Pseudomonas and Klebsiella. All of these isolates were killed by scrubbing with foam.
Considering only the MRSA colonies, the number needed to treat is 31 (95% CI, 18-89), indicating that for approximately every 31 hand- and stethoscope-washings with the alcohol-based foam, 1 MRSA colony could potentially be eliminated from a stethoscope head.
FIGURE
Bacterial counts: Prewash and post-wash
The line connects the mean values.
TABLE
What we found on the stethoscopes
| BACTERIA | TOTAL NUMBER OF ISOLATES |
|---|---|
| Coagulase-negative Staphylococcus | 100 |
| Bacillus | 51 |
| Micrococcus | 24 |
| Nonfermenting gram-negative bacteria | 17 |
| MRSA | 3 |
| Coagulase-positive Staphylococcus (non-MRSA) | 2 |
| Lactobacillus | 2 |
| Pseudomonas | 2 |
| Acinetobacter | 1 |
| Enterobacter | 1 |
| Klebsiella | 1 |
| Streptococcus | 1 |
| Zygomycetes | 1 |
| MRSA, methicillin-resistant Staphylococcus aureus. | |
Discussion
The findings of this study suggest that the use of alcohol-based hand foam to simultaneously sterilize the hands and a stethoscope head significantly reduces the number of bacterial colonies, including MRSA. The quantifiable risk of clinical infection with MRSA in patients through brief contact with a contaminated fomite such as a stethoscope is unknown. However, the transmission of the bacteria itself from contaminated surfaces and hands through brief contacts has been well established.11,12
A new standard for cleaning stethoscopes?
Swiping stethoscopes with alcohol pads is currently the gold standard for cleaning these instruments, but physicians do not consistently use alcohol pads for this purpose. Moreover, the pads must be purchased and available for use, require an extra step, and produce waste that must be disposed of—and clinicians still have to cleanse their hands, often using alcohol-based hand foam. Using the foam to cleanse the stethoscope while cleaning hands requires no added cost or additional time, and may reduce or prevent serious nosocomial and community-based infections.
Limitations of the study
One limitation of this study was the lack of control of the washing procedure. But because our goal was to see how the technique fared in actual use among all participants, uniform technique was not required. Knowing they were in a study may have altered the way the participants washed their hands and stethoscopes. If this were true, however, we would expect a much larger proportion of the total variation to be due to differences among clinicians than the 5% that was found.
This technique does not eliminate all bacteria—for instance, sporulating organisms such as Clostridium difficile are not killed by alcohol products.18 Yet friction alone has been found to reduce the number of these pathogens (A.S., unpublished data, 2007).
This study utilized alcohol-based hand foam because it was available at the study institution, so we cannot make any claims for nonalcohol-based products. It does appear, however, that alcohol-based foam may not be susceptible to bacterial resistance, as had previously been found in triclosan-containing products.19
It is not known whether the alcohol-based foam will damage stethoscope diaphragms. Previous studies have suggested that alcohol pads do cause damage to the rubber components of stethoscopes,16 but the foam studied here, like most similar products, contains emollients that may or may not have a protective effect. Another study would be necessary to fully assess this question.
While it is impossible to destroy all bacteria or eliminate all infections by simultaneous hand and stethoscope cleansing, many infections could potentially be prevented with this simple component of a comprehensive infection control program. Alcohol-based hand foam is already in use for hand cleansing between patients in many inpatient and outpatient settings, and this procedure requires no added cost and no additional time. Further research is necessary to determine whether the reduction of bacterial growth also corresponds to a reduction in clinically related disease. The results of this study provide evidence that hand foam, when used to simultaneously sterilize the hands and stethoscope, can significantly reduce the number of bacterial colonies on stethoscopes.
CORRESPONDENCE
Maryellen A. Schroeder, MD, MPH, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15201; [email protected]
1. Semmelweis IP. Etiology, Concept and Prophylaxis of Childbed Fever. Trans. K C. Carter. Madison: University of Wisconsin; 1983.
2. Chandra PN, Milind K. Lapses in measures recommended for preventing hospital-acquired infection. J Hosp Infect. 2001;47:218-222.
3. Cohen HA, Amir J, Matalon A, et al. Stethoscopes and otoscopes—a potential vector of infection? Fam Pract. 1997;14:446-449.
4. Klevens RM, Morrison MA, Nadle J, et al. Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763-1771.
5. Zeller JL. MRSA Infections. JAMA. 2007;298:1733.-
6. Langley JM. Commentary: waterless hand hygiene: if there’s a will, there’s a way. Pediatr Infect Dis J. 2002;21:496-497.
7. Harbarth S, Didier P, Grady L, et al. Interventional study to evaluate the impact of an alcohol-based hand gel in improving hand hygiene compliance. Pediatr Infect Dis J. 2002;21:489-495.
8. Seal LA, Rizer RL, Maas-Irslinger R. A unique water optional health care personnel handwash provides antimicrobial persistence and residual effects while decreasing the need for additional products. Am J Infect Control. 2005;33:207-216.
9. Larson EL, Aiello AE, Bastyr J, et al. Assessment of two hand hygiene regimens for intensive care unit personnel. Crit Care Med. 2001;29:944-951.
10. Devine J, Cooke RP, Wright EP. Is methicillin-resistant Staphylococcus aureus (MRSA) contamination of ward-based computer terminals a surrogate marker for nosocomial MRSA transmission and handwashing compliance? J Hosp Infect. 2001;48:72-75.
11. Maluf ME, Maldonado AF, Bercial ME, et al. Stethoscope: a friend or an enemy? Sao Paulo Med J. 2002;120:13-15.
12. Oie S, Hosokawa I, Kamiya A. Contamination of room door handles by methicillin-sensitive/methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2002;51:140-143.
13. Zachary KC, Bayne PS, Morrison VJ, et al. Contamination of gowns, gloves, and stethoscopes with vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 2001;22:560-564.
14. Singh D, Kaur H, Gardner WG, et al. Bacterial contamination of hospital pagers. Infect Control Hosp Epidemiol. 2002;23:274-276.
15. Wood MW, Lund RC, Stevenson KB. Bacterial contamination of stethoscopes with antimicrobial diaphragm covers. Am J Infect Control. 2007;35:263-266.
16. Parmar RC, Valvi CC, Sira P, et al. A prospective, randomized, double-blind study of comparative efficacy of immediate versus daily cleaning of stethoscope using 66% ethyl alcohol. Indian J Med Sci. 2004;58:423-430.
17. Hill C, King T, Day R. A strategy to reduce MRSA colonization of stethoscopes. J Hosp Infect. 2006;62:122-123.
18. Weber DJ, Sickbert-Bennett E, Gergen MF, et al. Efficacy of selected hand hygiene agents used to remove Bacillus atrophaeus (a surrogate of Bacillus anthracis) from contaminated hands. JAMA. 2003;289:1274-1277.
19. Levy SB. Antibacterial household products: cause for concern. Emerging Infect Dis. 2001;7(3 suppl):512-515.
Background Studies have shown that rubbing alcohol pads on stethoscope diaphragms can reduce bacterial colonization, but alcohol pads are used infrequently used and not always available.
Methods We conducted a prospective, single-blinded study to investigate whether simultaneously scrubbing hands and stethoscope head with alcohol-based hand foam would significantly reduce bacterial counts on the stethoscope. Using their own stethoscope, participants imprinted the stethoscope head onto a chocolate agar plate, then used alcohol-based hand foam to cleanse their hands while simultaneously rubbing the stethoscope head. Once the stethoscope heads were dry, the participants imprinted their stethoscope heads onto a second plate. After 48 hours’ incubation, we determined the bacterial counts for the prewash and post-wash plates, and compared the 2.
Results We analyzed a total of 184 cultures (from 92 stethoscopes). Both the mean (28 prewash vs 3 post-wash, P=.001) and median (11 prewash vs 1 post-wash, P=.001) colony counts were significantly greater before being cleansed. Three methicillin-resistant Staphylococcus aureus (MRSA) colonies were identified in the prewash period; all were destroyed by the foam. The estimated number of hand washes needed to prevent 1 MRSA colony is 31 (95% confidence interval [CI], 18-89).
Conclusion Simultaneously using hand foam to clean hands and stethoscope heads reduces bacterial counts on stethoscopes. Further research is needed to determine whether this intervention can reduce morbidity and mortality associated with bacterial infection.
More than 160 years after a Hungarian physician introduced a protocol of strict handwashing and instrument sterilization to hospital wards,1 many clinicians still don’t wash their hands regularly or properly sterilize their medical equipment.2,3 The lack of stringent infection control, both in inpatient and office settings, is exacerbated by the rise in antibiotic-resistant bacteria. Methicillin-resistant Staphylococcus aureus (MRSA), in particular, including community-acquired MRSA, accounts for infections ranging from severe skin lesions to sepsis, and an estimated 18,650 deaths annually.4,5
Waterless hand cleansers, such as alcohol-based foams and gels, improve handwashing compliance.6-8 These products are effective in reducing both bacterial and viral agents, are convenient to use, and may even be good for caregivers’ skin.9 But would they work on stethoscopes? Our study was designed to find out.
An often-neglected source of bacteria
Infection can spread from patient to patient, not only on hands, but also via fomites such as ventilators, computer keyboards, pagers, and stethoscopes.10-14 Antimicrobial stethoscope covers, including those impregnated with silver ions, do not decrease bacterial colonization; evidence suggests that their use may actually increase it.15 Studies indicate that rubbing alcohol pads on stethoscope diaphragms can reduce bacterial colonization, and it has been suggested that cleansing of stethoscopes daily may be as effective as more frequent cleaning.16 Unfortunately, many clinicians do not clean their stethoscopes on a regular basis.17 In addition, alcohol pads are not always available, and using them requires an extra step and produces waste.
An earlier study by a member of our research team (A.S., unpublished data, 2007) indicated that rubbing stethoscopes exposed to nonpathogenic Staphylococcus epidermidis with alcohol-based hand foam was comparable to using alcohol wipes in reducing bacterial counts. The primary objective of this study was to determine whether clinicians can simultaneously reduce bacteria on stethoscope heads and clean their hands with alcohol-based foam.
Methods
This study was a prospective, single-blinded, “before-and-after” trial—a design in which each participant served as his or her own control and used foam that was already available on site. The study was conducted at 1 community-based hospital and 1 satellite family health center; the study was approved by the hospital Institutional Review Board. A grant from St. Margaret’s Foundation covered the cost of the agar plates.
We began by asking the attending physicians, faculty, nurses, residents, and medical students who attended a grand rounds program to participate; we visited the satellite health facility to recruit participants, as well. We started with 93 participants, but 1 stethoscope was damaged during the study, so we ended up with 92 participants and 184 cultures.
Interventions
In the prewash, or “before” portion of the study, all participants imprinted the head of their stethoscope onto a chocolate agar plate. The clinicians then used a 62.5% ethyl alcohol-based foam to cleanse their hands, simultaneously rubbing the stethoscope head between their hands. After a brief drying time, the clinicians imprinted their stethoscope head onto a separate agar plate (the post-wash, or “after” component).
We did not tell participants how to wash their hands or for how long. We simply told them to cleanse their hands as they normally would and to rub the foam onto the stethoscope head, as well.
Randomization and measurement
Prior to data collection, randomly assigned ID numbers were recorded on the bottom of 200 agar plates, which were then placed in a box. One member of our research team gave each clinician 2 plates. Participants imprinted their stethoscope head onto the first plate and handed it to another investigator, who recorded the prewash ID numbers. Participants then performed the handwashing and stethoscope rub and repeated the imprinting procedure with the second plate. This time, the investigator recorded the professional role of each participant (eg, resident, attending, nurse, faculty) as well as the post-wash ID numbers.
After 48 hours at 35°C incubation, the plates were arranged in numerical order. A member of the research team then counted the number and identified the type of bacterial colonies on each plate and recorded the findings on a data sheet by ID number.
Validation
In order to validate the bacterial counts, the supervisor of the hospital laboratory—who had 20 years’ experience in examining cultures and served as the gold standard—independently examined a random sample of plates. We agreed in advance that any count that deviated by more than 7 (approximately half the effect the study was powered to detect) from the gold standard would require another investigator to intervene. This proved unnecessary as no such deviation was found.
Coagulase studies were performed on all plates with bacterial isolates, and gram staining was performed on selected plates, along with identification of gram-negative stains, using the Microscan (Siemens, New York, NY). An “honest broker”—the only person authorized to match the plates with the stethoscopes’ ID numbers—then matched the prewash and post-wash data by stethoscope and type of health care provider. Another investigator analyzed the final data sheet for accuracy.
Power and sample size
A pilot study was performed to obtain estimates of the average and variance of the bacterial counts in a control group of stethoscopes and to determine whether the act of imprinting the stethoscope itself would significantly reduce the colony counts. The results established that there was no statistical change in either the summary statistics or the distribution of the bacterial counts over the course of multiple imprinting.
Estimates obtained from the pilot study indicated that 58 stethoscopes would be sufficient to yield 80% power (alpha=0.05, 2-tailed) for detecting an average difference of 15 colony counts between the prewash and post-wash samples. Seventy-eight stethoscopes would increase the power to 90%. We ultimately tested 92 stethoscopes.
Statistical analysis
Descriptive statistical measures were calculated to examine the bacterial counts. Linear regression analysis was used to compute the correlation in the validation data. This before-and-after design results in “paired data,” and both parametric and nonparametric statistical tests were used. We used a paired t-test to test the mean difference in bacterial counts between the pre- and post-wash samples, and a random effects model to estimate the individual components of variance. The difference in the median bacterial counts was tested using the signed rank test. We used various diagnostic measures to examine the assumptions of the statistical tests; and means, medians, 95% confidence intervals (CIs), and P-values (using P<.05 as statistical significance) to report the results. The Bonferroni multiple comparisons procedure was used to determine whether the bacterial counts were statistically different among subgroups of health care providers. All statistical analyses were performed using SAS (Cary, NC) software.
Results
A total of 184 culture plates showing before and after samples for 92 stethoscopes were analyzed. The provider breakdown of the sample consisted of nurses (39%), residents (30%), attending physicians (15%), faculty (13%), and medical students (3%). Thirty-five (approximately 1 in 6) of the 184 plates were randomly sampled for validation. There was a high degree of reliability between the investigator’s bacterial counts and the bacterial counts of the gold standard (r=+0.98, P<.001).
Bacterial counts. The distribution of the bacterial colony counts skewed right in both the prewash (0-198) and post-wash (0-48) samples. The FIGURE shows the skewed distributions in the actual bacterial counts for the 92 pairs of plates before and after hand and stethoscope washing. In the prewash sample, the mean bacterial count was 28.4 (95% CI, 20.2-36.6), vs a post-wash mean of 3.2 (95% CI, 1.8-4.6; P<.001). This resulted in an estimated difference in mean bacterial counts of 25.2 (95% CI, 17.2-33.3). The difference in the medians was also significant, with a prewash median of 11.5 and a post-wash median of 1.0 (P<.001). The difference between the pre- and post-wash periods remained significant even after using various transformations to normalize the data. Random effects modeling showed that very little (<5%) of the total variation was related to the type of health care provider.
Types of bacteria. The TABLE gives the breakdown and frequency of the various types of bacteria that we identified on the stethoscopes. Many were of low pathogenic potential, such as coagulase-negative staph species, which would not cause disease in healthy individuals. However, in hospitalized or immunocompromised patients, they could well induce illness. There were also several clearly pathogenic bacterial isolates, including 3 MRSA colonies (each on a different stethoscope), as well as Pseudomonas and Klebsiella. All of these isolates were killed by scrubbing with foam.
Considering only the MRSA colonies, the number needed to treat is 31 (95% CI, 18-89), indicating that for approximately every 31 hand- and stethoscope-washings with the alcohol-based foam, 1 MRSA colony could potentially be eliminated from a stethoscope head.
FIGURE
Bacterial counts: Prewash and post-wash
The line connects the mean values.
TABLE
What we found on the stethoscopes
| BACTERIA | TOTAL NUMBER OF ISOLATES |
|---|---|
| Coagulase-negative Staphylococcus | 100 |
| Bacillus | 51 |
| Micrococcus | 24 |
| Nonfermenting gram-negative bacteria | 17 |
| MRSA | 3 |
| Coagulase-positive Staphylococcus (non-MRSA) | 2 |
| Lactobacillus | 2 |
| Pseudomonas | 2 |
| Acinetobacter | 1 |
| Enterobacter | 1 |
| Klebsiella | 1 |
| Streptococcus | 1 |
| Zygomycetes | 1 |
| MRSA, methicillin-resistant Staphylococcus aureus. | |
Discussion
The findings of this study suggest that the use of alcohol-based hand foam to simultaneously sterilize the hands and a stethoscope head significantly reduces the number of bacterial colonies, including MRSA. The quantifiable risk of clinical infection with MRSA in patients through brief contact with a contaminated fomite such as a stethoscope is unknown. However, the transmission of the bacteria itself from contaminated surfaces and hands through brief contacts has been well established.11,12
A new standard for cleaning stethoscopes?
Swiping stethoscopes with alcohol pads is currently the gold standard for cleaning these instruments, but physicians do not consistently use alcohol pads for this purpose. Moreover, the pads must be purchased and available for use, require an extra step, and produce waste that must be disposed of—and clinicians still have to cleanse their hands, often using alcohol-based hand foam. Using the foam to cleanse the stethoscope while cleaning hands requires no added cost or additional time, and may reduce or prevent serious nosocomial and community-based infections.
Limitations of the study
One limitation of this study was the lack of control of the washing procedure. But because our goal was to see how the technique fared in actual use among all participants, uniform technique was not required. Knowing they were in a study may have altered the way the participants washed their hands and stethoscopes. If this were true, however, we would expect a much larger proportion of the total variation to be due to differences among clinicians than the 5% that was found.
This technique does not eliminate all bacteria—for instance, sporulating organisms such as Clostridium difficile are not killed by alcohol products.18 Yet friction alone has been found to reduce the number of these pathogens (A.S., unpublished data, 2007).
This study utilized alcohol-based hand foam because it was available at the study institution, so we cannot make any claims for nonalcohol-based products. It does appear, however, that alcohol-based foam may not be susceptible to bacterial resistance, as had previously been found in triclosan-containing products.19
It is not known whether the alcohol-based foam will damage stethoscope diaphragms. Previous studies have suggested that alcohol pads do cause damage to the rubber components of stethoscopes,16 but the foam studied here, like most similar products, contains emollients that may or may not have a protective effect. Another study would be necessary to fully assess this question.
While it is impossible to destroy all bacteria or eliminate all infections by simultaneous hand and stethoscope cleansing, many infections could potentially be prevented with this simple component of a comprehensive infection control program. Alcohol-based hand foam is already in use for hand cleansing between patients in many inpatient and outpatient settings, and this procedure requires no added cost and no additional time. Further research is necessary to determine whether the reduction of bacterial growth also corresponds to a reduction in clinically related disease. The results of this study provide evidence that hand foam, when used to simultaneously sterilize the hands and stethoscope, can significantly reduce the number of bacterial colonies on stethoscopes.
CORRESPONDENCE
Maryellen A. Schroeder, MD, MPH, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15201; [email protected]
Background Studies have shown that rubbing alcohol pads on stethoscope diaphragms can reduce bacterial colonization, but alcohol pads are used infrequently used and not always available.
Methods We conducted a prospective, single-blinded study to investigate whether simultaneously scrubbing hands and stethoscope head with alcohol-based hand foam would significantly reduce bacterial counts on the stethoscope. Using their own stethoscope, participants imprinted the stethoscope head onto a chocolate agar plate, then used alcohol-based hand foam to cleanse their hands while simultaneously rubbing the stethoscope head. Once the stethoscope heads were dry, the participants imprinted their stethoscope heads onto a second plate. After 48 hours’ incubation, we determined the bacterial counts for the prewash and post-wash plates, and compared the 2.
Results We analyzed a total of 184 cultures (from 92 stethoscopes). Both the mean (28 prewash vs 3 post-wash, P=.001) and median (11 prewash vs 1 post-wash, P=.001) colony counts were significantly greater before being cleansed. Three methicillin-resistant Staphylococcus aureus (MRSA) colonies were identified in the prewash period; all were destroyed by the foam. The estimated number of hand washes needed to prevent 1 MRSA colony is 31 (95% confidence interval [CI], 18-89).
Conclusion Simultaneously using hand foam to clean hands and stethoscope heads reduces bacterial counts on stethoscopes. Further research is needed to determine whether this intervention can reduce morbidity and mortality associated with bacterial infection.
More than 160 years after a Hungarian physician introduced a protocol of strict handwashing and instrument sterilization to hospital wards,1 many clinicians still don’t wash their hands regularly or properly sterilize their medical equipment.2,3 The lack of stringent infection control, both in inpatient and office settings, is exacerbated by the rise in antibiotic-resistant bacteria. Methicillin-resistant Staphylococcus aureus (MRSA), in particular, including community-acquired MRSA, accounts for infections ranging from severe skin lesions to sepsis, and an estimated 18,650 deaths annually.4,5
Waterless hand cleansers, such as alcohol-based foams and gels, improve handwashing compliance.6-8 These products are effective in reducing both bacterial and viral agents, are convenient to use, and may even be good for caregivers’ skin.9 But would they work on stethoscopes? Our study was designed to find out.
An often-neglected source of bacteria
Infection can spread from patient to patient, not only on hands, but also via fomites such as ventilators, computer keyboards, pagers, and stethoscopes.10-14 Antimicrobial stethoscope covers, including those impregnated with silver ions, do not decrease bacterial colonization; evidence suggests that their use may actually increase it.15 Studies indicate that rubbing alcohol pads on stethoscope diaphragms can reduce bacterial colonization, and it has been suggested that cleansing of stethoscopes daily may be as effective as more frequent cleaning.16 Unfortunately, many clinicians do not clean their stethoscopes on a regular basis.17 In addition, alcohol pads are not always available, and using them requires an extra step and produces waste.
An earlier study by a member of our research team (A.S., unpublished data, 2007) indicated that rubbing stethoscopes exposed to nonpathogenic Staphylococcus epidermidis with alcohol-based hand foam was comparable to using alcohol wipes in reducing bacterial counts. The primary objective of this study was to determine whether clinicians can simultaneously reduce bacteria on stethoscope heads and clean their hands with alcohol-based foam.
Methods
This study was a prospective, single-blinded, “before-and-after” trial—a design in which each participant served as his or her own control and used foam that was already available on site. The study was conducted at 1 community-based hospital and 1 satellite family health center; the study was approved by the hospital Institutional Review Board. A grant from St. Margaret’s Foundation covered the cost of the agar plates.
We began by asking the attending physicians, faculty, nurses, residents, and medical students who attended a grand rounds program to participate; we visited the satellite health facility to recruit participants, as well. We started with 93 participants, but 1 stethoscope was damaged during the study, so we ended up with 92 participants and 184 cultures.
Interventions
In the prewash, or “before” portion of the study, all participants imprinted the head of their stethoscope onto a chocolate agar plate. The clinicians then used a 62.5% ethyl alcohol-based foam to cleanse their hands, simultaneously rubbing the stethoscope head between their hands. After a brief drying time, the clinicians imprinted their stethoscope head onto a separate agar plate (the post-wash, or “after” component).
We did not tell participants how to wash their hands or for how long. We simply told them to cleanse their hands as they normally would and to rub the foam onto the stethoscope head, as well.
Randomization and measurement
Prior to data collection, randomly assigned ID numbers were recorded on the bottom of 200 agar plates, which were then placed in a box. One member of our research team gave each clinician 2 plates. Participants imprinted their stethoscope head onto the first plate and handed it to another investigator, who recorded the prewash ID numbers. Participants then performed the handwashing and stethoscope rub and repeated the imprinting procedure with the second plate. This time, the investigator recorded the professional role of each participant (eg, resident, attending, nurse, faculty) as well as the post-wash ID numbers.
After 48 hours at 35°C incubation, the plates were arranged in numerical order. A member of the research team then counted the number and identified the type of bacterial colonies on each plate and recorded the findings on a data sheet by ID number.
Validation
In order to validate the bacterial counts, the supervisor of the hospital laboratory—who had 20 years’ experience in examining cultures and served as the gold standard—independently examined a random sample of plates. We agreed in advance that any count that deviated by more than 7 (approximately half the effect the study was powered to detect) from the gold standard would require another investigator to intervene. This proved unnecessary as no such deviation was found.
Coagulase studies were performed on all plates with bacterial isolates, and gram staining was performed on selected plates, along with identification of gram-negative stains, using the Microscan (Siemens, New York, NY). An “honest broker”—the only person authorized to match the plates with the stethoscopes’ ID numbers—then matched the prewash and post-wash data by stethoscope and type of health care provider. Another investigator analyzed the final data sheet for accuracy.
Power and sample size
A pilot study was performed to obtain estimates of the average and variance of the bacterial counts in a control group of stethoscopes and to determine whether the act of imprinting the stethoscope itself would significantly reduce the colony counts. The results established that there was no statistical change in either the summary statistics or the distribution of the bacterial counts over the course of multiple imprinting.
Estimates obtained from the pilot study indicated that 58 stethoscopes would be sufficient to yield 80% power (alpha=0.05, 2-tailed) for detecting an average difference of 15 colony counts between the prewash and post-wash samples. Seventy-eight stethoscopes would increase the power to 90%. We ultimately tested 92 stethoscopes.
Statistical analysis
Descriptive statistical measures were calculated to examine the bacterial counts. Linear regression analysis was used to compute the correlation in the validation data. This before-and-after design results in “paired data,” and both parametric and nonparametric statistical tests were used. We used a paired t-test to test the mean difference in bacterial counts between the pre- and post-wash samples, and a random effects model to estimate the individual components of variance. The difference in the median bacterial counts was tested using the signed rank test. We used various diagnostic measures to examine the assumptions of the statistical tests; and means, medians, 95% confidence intervals (CIs), and P-values (using P<.05 as statistical significance) to report the results. The Bonferroni multiple comparisons procedure was used to determine whether the bacterial counts were statistically different among subgroups of health care providers. All statistical analyses were performed using SAS (Cary, NC) software.
Results
A total of 184 culture plates showing before and after samples for 92 stethoscopes were analyzed. The provider breakdown of the sample consisted of nurses (39%), residents (30%), attending physicians (15%), faculty (13%), and medical students (3%). Thirty-five (approximately 1 in 6) of the 184 plates were randomly sampled for validation. There was a high degree of reliability between the investigator’s bacterial counts and the bacterial counts of the gold standard (r=+0.98, P<.001).
Bacterial counts. The distribution of the bacterial colony counts skewed right in both the prewash (0-198) and post-wash (0-48) samples. The FIGURE shows the skewed distributions in the actual bacterial counts for the 92 pairs of plates before and after hand and stethoscope washing. In the prewash sample, the mean bacterial count was 28.4 (95% CI, 20.2-36.6), vs a post-wash mean of 3.2 (95% CI, 1.8-4.6; P<.001). This resulted in an estimated difference in mean bacterial counts of 25.2 (95% CI, 17.2-33.3). The difference in the medians was also significant, with a prewash median of 11.5 and a post-wash median of 1.0 (P<.001). The difference between the pre- and post-wash periods remained significant even after using various transformations to normalize the data. Random effects modeling showed that very little (<5%) of the total variation was related to the type of health care provider.
Types of bacteria. The TABLE gives the breakdown and frequency of the various types of bacteria that we identified on the stethoscopes. Many were of low pathogenic potential, such as coagulase-negative staph species, which would not cause disease in healthy individuals. However, in hospitalized or immunocompromised patients, they could well induce illness. There were also several clearly pathogenic bacterial isolates, including 3 MRSA colonies (each on a different stethoscope), as well as Pseudomonas and Klebsiella. All of these isolates were killed by scrubbing with foam.
Considering only the MRSA colonies, the number needed to treat is 31 (95% CI, 18-89), indicating that for approximately every 31 hand- and stethoscope-washings with the alcohol-based foam, 1 MRSA colony could potentially be eliminated from a stethoscope head.
FIGURE
Bacterial counts: Prewash and post-wash
The line connects the mean values.
TABLE
What we found on the stethoscopes
| BACTERIA | TOTAL NUMBER OF ISOLATES |
|---|---|
| Coagulase-negative Staphylococcus | 100 |
| Bacillus | 51 |
| Micrococcus | 24 |
| Nonfermenting gram-negative bacteria | 17 |
| MRSA | 3 |
| Coagulase-positive Staphylococcus (non-MRSA) | 2 |
| Lactobacillus | 2 |
| Pseudomonas | 2 |
| Acinetobacter | 1 |
| Enterobacter | 1 |
| Klebsiella | 1 |
| Streptococcus | 1 |
| Zygomycetes | 1 |
| MRSA, methicillin-resistant Staphylococcus aureus. | |
Discussion
The findings of this study suggest that the use of alcohol-based hand foam to simultaneously sterilize the hands and a stethoscope head significantly reduces the number of bacterial colonies, including MRSA. The quantifiable risk of clinical infection with MRSA in patients through brief contact with a contaminated fomite such as a stethoscope is unknown. However, the transmission of the bacteria itself from contaminated surfaces and hands through brief contacts has been well established.11,12
A new standard for cleaning stethoscopes?
Swiping stethoscopes with alcohol pads is currently the gold standard for cleaning these instruments, but physicians do not consistently use alcohol pads for this purpose. Moreover, the pads must be purchased and available for use, require an extra step, and produce waste that must be disposed of—and clinicians still have to cleanse their hands, often using alcohol-based hand foam. Using the foam to cleanse the stethoscope while cleaning hands requires no added cost or additional time, and may reduce or prevent serious nosocomial and community-based infections.
Limitations of the study
One limitation of this study was the lack of control of the washing procedure. But because our goal was to see how the technique fared in actual use among all participants, uniform technique was not required. Knowing they were in a study may have altered the way the participants washed their hands and stethoscopes. If this were true, however, we would expect a much larger proportion of the total variation to be due to differences among clinicians than the 5% that was found.
This technique does not eliminate all bacteria—for instance, sporulating organisms such as Clostridium difficile are not killed by alcohol products.18 Yet friction alone has been found to reduce the number of these pathogens (A.S., unpublished data, 2007).
This study utilized alcohol-based hand foam because it was available at the study institution, so we cannot make any claims for nonalcohol-based products. It does appear, however, that alcohol-based foam may not be susceptible to bacterial resistance, as had previously been found in triclosan-containing products.19
It is not known whether the alcohol-based foam will damage stethoscope diaphragms. Previous studies have suggested that alcohol pads do cause damage to the rubber components of stethoscopes,16 but the foam studied here, like most similar products, contains emollients that may or may not have a protective effect. Another study would be necessary to fully assess this question.
While it is impossible to destroy all bacteria or eliminate all infections by simultaneous hand and stethoscope cleansing, many infections could potentially be prevented with this simple component of a comprehensive infection control program. Alcohol-based hand foam is already in use for hand cleansing between patients in many inpatient and outpatient settings, and this procedure requires no added cost and no additional time. Further research is necessary to determine whether the reduction of bacterial growth also corresponds to a reduction in clinically related disease. The results of this study provide evidence that hand foam, when used to simultaneously sterilize the hands and stethoscope, can significantly reduce the number of bacterial colonies on stethoscopes.
CORRESPONDENCE
Maryellen A. Schroeder, MD, MPH, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15201; [email protected]
1. Semmelweis IP. Etiology, Concept and Prophylaxis of Childbed Fever. Trans. K C. Carter. Madison: University of Wisconsin; 1983.
2. Chandra PN, Milind K. Lapses in measures recommended for preventing hospital-acquired infection. J Hosp Infect. 2001;47:218-222.
3. Cohen HA, Amir J, Matalon A, et al. Stethoscopes and otoscopes—a potential vector of infection? Fam Pract. 1997;14:446-449.
4. Klevens RM, Morrison MA, Nadle J, et al. Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763-1771.
5. Zeller JL. MRSA Infections. JAMA. 2007;298:1733.-
6. Langley JM. Commentary: waterless hand hygiene: if there’s a will, there’s a way. Pediatr Infect Dis J. 2002;21:496-497.
7. Harbarth S, Didier P, Grady L, et al. Interventional study to evaluate the impact of an alcohol-based hand gel in improving hand hygiene compliance. Pediatr Infect Dis J. 2002;21:489-495.
8. Seal LA, Rizer RL, Maas-Irslinger R. A unique water optional health care personnel handwash provides antimicrobial persistence and residual effects while decreasing the need for additional products. Am J Infect Control. 2005;33:207-216.
9. Larson EL, Aiello AE, Bastyr J, et al. Assessment of two hand hygiene regimens for intensive care unit personnel. Crit Care Med. 2001;29:944-951.
10. Devine J, Cooke RP, Wright EP. Is methicillin-resistant Staphylococcus aureus (MRSA) contamination of ward-based computer terminals a surrogate marker for nosocomial MRSA transmission and handwashing compliance? J Hosp Infect. 2001;48:72-75.
11. Maluf ME, Maldonado AF, Bercial ME, et al. Stethoscope: a friend or an enemy? Sao Paulo Med J. 2002;120:13-15.
12. Oie S, Hosokawa I, Kamiya A. Contamination of room door handles by methicillin-sensitive/methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2002;51:140-143.
13. Zachary KC, Bayne PS, Morrison VJ, et al. Contamination of gowns, gloves, and stethoscopes with vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 2001;22:560-564.
14. Singh D, Kaur H, Gardner WG, et al. Bacterial contamination of hospital pagers. Infect Control Hosp Epidemiol. 2002;23:274-276.
15. Wood MW, Lund RC, Stevenson KB. Bacterial contamination of stethoscopes with antimicrobial diaphragm covers. Am J Infect Control. 2007;35:263-266.
16. Parmar RC, Valvi CC, Sira P, et al. A prospective, randomized, double-blind study of comparative efficacy of immediate versus daily cleaning of stethoscope using 66% ethyl alcohol. Indian J Med Sci. 2004;58:423-430.
17. Hill C, King T, Day R. A strategy to reduce MRSA colonization of stethoscopes. J Hosp Infect. 2006;62:122-123.
18. Weber DJ, Sickbert-Bennett E, Gergen MF, et al. Efficacy of selected hand hygiene agents used to remove Bacillus atrophaeus (a surrogate of Bacillus anthracis) from contaminated hands. JAMA. 2003;289:1274-1277.
19. Levy SB. Antibacterial household products: cause for concern. Emerging Infect Dis. 2001;7(3 suppl):512-515.
1. Semmelweis IP. Etiology, Concept and Prophylaxis of Childbed Fever. Trans. K C. Carter. Madison: University of Wisconsin; 1983.
2. Chandra PN, Milind K. Lapses in measures recommended for preventing hospital-acquired infection. J Hosp Infect. 2001;47:218-222.
3. Cohen HA, Amir J, Matalon A, et al. Stethoscopes and otoscopes—a potential vector of infection? Fam Pract. 1997;14:446-449.
4. Klevens RM, Morrison MA, Nadle J, et al. Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763-1771.
5. Zeller JL. MRSA Infections. JAMA. 2007;298:1733.-
6. Langley JM. Commentary: waterless hand hygiene: if there’s a will, there’s a way. Pediatr Infect Dis J. 2002;21:496-497.
7. Harbarth S, Didier P, Grady L, et al. Interventional study to evaluate the impact of an alcohol-based hand gel in improving hand hygiene compliance. Pediatr Infect Dis J. 2002;21:489-495.
8. Seal LA, Rizer RL, Maas-Irslinger R. A unique water optional health care personnel handwash provides antimicrobial persistence and residual effects while decreasing the need for additional products. Am J Infect Control. 2005;33:207-216.
9. Larson EL, Aiello AE, Bastyr J, et al. Assessment of two hand hygiene regimens for intensive care unit personnel. Crit Care Med. 2001;29:944-951.
10. Devine J, Cooke RP, Wright EP. Is methicillin-resistant Staphylococcus aureus (MRSA) contamination of ward-based computer terminals a surrogate marker for nosocomial MRSA transmission and handwashing compliance? J Hosp Infect. 2001;48:72-75.
11. Maluf ME, Maldonado AF, Bercial ME, et al. Stethoscope: a friend or an enemy? Sao Paulo Med J. 2002;120:13-15.
12. Oie S, Hosokawa I, Kamiya A. Contamination of room door handles by methicillin-sensitive/methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2002;51:140-143.
13. Zachary KC, Bayne PS, Morrison VJ, et al. Contamination of gowns, gloves, and stethoscopes with vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 2001;22:560-564.
14. Singh D, Kaur H, Gardner WG, et al. Bacterial contamination of hospital pagers. Infect Control Hosp Epidemiol. 2002;23:274-276.
15. Wood MW, Lund RC, Stevenson KB. Bacterial contamination of stethoscopes with antimicrobial diaphragm covers. Am J Infect Control. 2007;35:263-266.
16. Parmar RC, Valvi CC, Sira P, et al. A prospective, randomized, double-blind study of comparative efficacy of immediate versus daily cleaning of stethoscope using 66% ethyl alcohol. Indian J Med Sci. 2004;58:423-430.
17. Hill C, King T, Day R. A strategy to reduce MRSA colonization of stethoscopes. J Hosp Infect. 2006;62:122-123.
18. Weber DJ, Sickbert-Bennett E, Gergen MF, et al. Efficacy of selected hand hygiene agents used to remove Bacillus atrophaeus (a surrogate of Bacillus anthracis) from contaminated hands. JAMA. 2003;289:1274-1277.
19. Levy SB. Antibacterial household products: cause for concern. Emerging Infect Dis. 2001;7(3 suppl):512-515.
Sex with former patients: OK after retirement?
Dear Dr. Mossman,
A psychiatrist retires from practice and goes into some other line of work—perhaps managing a restaurant. He then has an “affair” with a former patient whom he had not treated for several years. Could the retired psychiatrist’s conduct be the basis of a successful lawsuit?—Submitted by “Dr. D”
Evidence tells us that the retired psychiatrist’s behavior likely could do emotional harm to his former patient. If the former patient suffers some injury, a successful suit could follow—if not on grounds of malpractice, then on other grounds. In this article we’ll see why by looking at:
- rates of doctor-patient sex
- potential harm from doctor-patient sex
- ethical bans on sex with former patients
- possible legal actions.
Sex with patients: Rates and risk
Doctors and patients often develop erotic thoughts about each other.1,2 But as Sigmund Freud noted almost a century ago, an actual love relationship between a doctor and a psychotherapy patient can cause a “complete defeat for the treatment” and destroy the patient’s chance for recovery.3
More than 5 decades later, surveys of medical professionals supplemented Freud’s observations with data about the frequency and impact of doctor-patient sex. In a 1973 survey, 11% of physicians said they had erotic contact with patients, and 5% reported intercourse.4 In a 1986 survey of psychiatrists, 3% of women and 7% of men acknowledged having sexual contact with patients.5 In a 1992 study of 10,000 nonpsychiatric physicians, 9% of respondents reported having sex with patients.6 Actual rates of doctor-patient sex probably are much higher than reported because physicians may be reluctant to admit to having erotic contact with patients, even in anonymous surveys.7 The typical therapist-patient sex scenario involves a male doctor and an adult female patient, but same-sex encounters and sexual contact with minors occur, too.8
Sex between a therapist and a patient is likely to cause emotional injury. For example, a 1991 study found that 90% of psychotherapy patients who had sexual involvement with a prior therapist had been harmed by the experience.9 Books, articles, and Web sites offer vivid individual accounts of harm patients have suffered ( Table ). Doctors who have sex with patients could face public opprobrium, civil lawsuits, actions against their medical licenses, and prosecution in states that make sex with psychiatric patients a criminal offense.10
Table
How sexual relationships can harm patients
| Type of harm | Explanation |
|---|---|
| Ambivalence | Psychological paralysis regarding whether to protect or take action against the abusive therapist |
| Cognitive dysfunction | Impaired memory and concentration, intrusive thoughts, flashbacks |
| Emotional lability | Unpredictable emotional responses, abrupt changes in mood, severe disruption of the patient’s typical way of feeling |
| Emptiness, isolation | Lost sense of self, feeling cut off from others |
| Guilt | Irrational self-blame for causing the sexual contact |
| Impaired trust | Fear of being taken advantage of, used, or abused in future therapy |
| Suicide | 14% of patients who had sex with a therapist attempt suicide; approximately 1% commit suicide |
| Role confusion | Treatment sessions and the therapeutic relationship serve the therapist’s needs rather than the patient’s; this perception may generalize to later therapies and other relationships |
| Sexual confusion | Examples include disgust with sexual feelings, uncertainty about sexual orientation, belief that self-worth comes from gratifying others’ sexual desires |
| Confusion about anger | Rage at self, self-loathing, need to suppress angry feelings, mistaken beliefs that others are angry at you |
| Source: Adapted from reference 8 | |
What about former patients?
Sex between providers and current patients is opposed by all major healthcare organizations, including the American Psychiatric Association (APA),11 American Medical Association,12 and American Psychological Association.13 The last 2 groups strongly discourage sex with former patients, but the APA’s ethics code states that such activity is always unethical.
The APA’s position reflects 2 general truths of psychiatric practice:
- Psychiatric patients often return for care years after initial treatment has ended. “Former patients” are really “possible future patients.” Improper relationships with former patients disrupt the doctor’s obligation to remain available for future care.
- Even if a patient never returns to treatment, intense feelings about a doctor can last for years. A psychiatrist who engages in sex with a former patient may evoke and manipulate feelings “left over” from therapy.
Psychiatrists therefore “have only one kind of relationship with a patient—that is, a doctor-patient relationship.”14 Moreover, as Simon and Shuman observe, “[N]o patient [is] strong enough, no pause is long enough, and no love is true enough to justify compromising this boundary.”15
Legal actions
If a physician no longer practices medicine, can any of his activities—including with a former patient—be malpractice? In fact, sex between practicing doctors and current patients might not always be malpractice. If a psychiatrist gains sexual access to a patient by saying that the sex will be therapeutic, the psychiatrist has perpetrated fraud and this intentional action might not be covered by malpractice insurance.16
In several cases involving nonpsychiatric physicians,17 courts have held that consensual doctor-patient sex is not malpractice, though it might represent some other form of wrongdoing. The argument is that sex with a patient is an intentional act that is never a professional service, whereas malpractice by definition arises unintentionally from negligence while rendering professional services. Other courts, however, have held that doctor-patient sex can be malpractice because it breaches the physician’s fiduciary relationship and can constitute an abuse of power.18
After retirement, physicians still have responsibilities to former patients: to protect records, to respect confidentiality, and to release information upon proper requests. Some fiduciary duties to patients survive the conclusion of treatment, and behavior that breaches those responsibilities can bring legal action.
Psychiatrists should realize that many former patients remain vulnerable because of feelings “left over” from therapy. Therefore, potential civil actions against a retired psychiatrist might include:
A suit for intentional infliction of emotional distress. This tort action requires proving more than mere insults or indignities; it occurs only when someone “by extreme and outrageous conduct intentionally or recklessly causes severe emotional distress to another.”19 Initiating sex with a former patient is strongly disapproved and meets the legal criterion of having a high probability of causing mental distress.20
A suit for negligent infliction of emotional distress. Modern law permits recourse for negligently inflicted emotional distress when harm occurs in “the course of specified categories of activities, undertakings, or relationships in which the negligent conduct is especially likely to cause emotional disturbance.”21
Suits for exploitation. Some jurisdictions allow suits against therapists who have sex with former patients, irrespective of therapists’ license status. For example, Minnesota allows lawsuits for “sexual exploitation” if the former patient’s capacity to consent was impaired by emotional dependence on the psychotherapist.22
Actions by licensing boards. Many retired practitioners maintain their medical licenses. Retired-but-still-licensed psychiatrists can be subject to professional disciplinary actions.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
1. Pope KS, Keith-Spiegel P, Tabachnick BG. Sexual attraction to clients. The human therapist and the (sometimes) inhuman training system. Am Psychol. 1986;4:147-158.
2. Golden GA, Brennan M. Managing erotic feelings in the physician-patient relationship. CMAJ. 1995;153:1241-1245.
3. Freud S. Observations on transference-love. In: Strachey J, ed. Complete psychological works of Sigmund Freud, standard edition, vol 12. London, UK: Hogarth Press; 1958:157-173.
4. Kardener SH, Fuller M, Mensh IN. A survey of physicians’ attitudes and practice regarding erotic and non-erotic contact with patients. Am J Psychiatry. 1973;130:1077-1081.
5. Gartrell N, Herman J, Olarte S, et al. Psychiatrist-patient sexual contact: results of a national survey, 1: prevalence. Am J Psychiatry. 1986;143:1126-1131.
6. Gartrell N, Milliken N, Goodson WH, et al. Physician-patient sexual contact—prevalence and problems. West J Med. 1992;157:139-143.
7. Roman B, Kay J. Residency education on the prevention of physician-patient sexual misconduct. Acad Psychiatry. 1997;21:26-34.
8. Pope KS. Sex between therapists and clients. In: Worrell J, ed. Encyclopedia of women and gender: sex similarities and differences and the impact of society on gender. New York, NY: Academic Press; 2001;955-962.
9. Pope KS, Vetter VA. Prior therapist-patient sexual involvement among patients seen by psychologists. Psychotherapy. 1991;28:429-438.
10. Simon RI. Clinical psychiatry and the law, 2nd edition. Arlington, VA: American Psychiatric Publishing, Inc.; 2003.
11. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Available at: http://www.psych.org/MainMenu/PsychiatricPractice/Ethics/
ResourcesStandards/PrinciplesofMedicalEthics.aspx. Accessed May 4, 2009.
12. American Medical Association Council on Ethical and Judicial Affairs. Sexual misconduct in the practice of medicine. JAMA. 1991;266:2741-2745.
13. American Psychological Association. Ethical principles of psychologists and code of conduct. Available at: http://www.apa.org/ethics/code2002.html. Accessed May 4, 2009.
14. Gruenberg PB. Boundary violations. In: Ethics primer of the American Psychiatric Association. Washington, DC: American Psychiatric Association; 2001;1-9.
15. Simon RI, Shuman DW. Clinical manual of psychiatry and the law. Arlington, VA: American Psychiatric Publishing, Inc.; 2007.
16. Sadock BJ, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry, 10th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.
17. Clemente v Roth, 171 Fed. Appx. 999 (4th Cir. Md. 2006).
18. Hoopes v Hammargren, 102 Nev. 425, 725 P.2d 238 (1986).
19. Restatement (Third) of Torts: Liability for Physical Harm, ch 8, §45 (2007 draft).
20. Prosser WL, Keeton WP, Dobbs DB, et al. Prosser and Keeton on torts, 5th ed. St. Paul, MN: West Publishing Co.; 1984.
21. Restatement (Third) of Torts: Liability for Physical Harm, ch 8, §46 (2007 draft).
22. Minnesota Statutes §148A (2008).
Dear Dr. Mossman,
A psychiatrist retires from practice and goes into some other line of work—perhaps managing a restaurant. He then has an “affair” with a former patient whom he had not treated for several years. Could the retired psychiatrist’s conduct be the basis of a successful lawsuit?—Submitted by “Dr. D”
Evidence tells us that the retired psychiatrist’s behavior likely could do emotional harm to his former patient. If the former patient suffers some injury, a successful suit could follow—if not on grounds of malpractice, then on other grounds. In this article we’ll see why by looking at:
- rates of doctor-patient sex
- potential harm from doctor-patient sex
- ethical bans on sex with former patients
- possible legal actions.
Sex with patients: Rates and risk
Doctors and patients often develop erotic thoughts about each other.1,2 But as Sigmund Freud noted almost a century ago, an actual love relationship between a doctor and a psychotherapy patient can cause a “complete defeat for the treatment” and destroy the patient’s chance for recovery.3
More than 5 decades later, surveys of medical professionals supplemented Freud’s observations with data about the frequency and impact of doctor-patient sex. In a 1973 survey, 11% of physicians said they had erotic contact with patients, and 5% reported intercourse.4 In a 1986 survey of psychiatrists, 3% of women and 7% of men acknowledged having sexual contact with patients.5 In a 1992 study of 10,000 nonpsychiatric physicians, 9% of respondents reported having sex with patients.6 Actual rates of doctor-patient sex probably are much higher than reported because physicians may be reluctant to admit to having erotic contact with patients, even in anonymous surveys.7 The typical therapist-patient sex scenario involves a male doctor and an adult female patient, but same-sex encounters and sexual contact with minors occur, too.8
Sex between a therapist and a patient is likely to cause emotional injury. For example, a 1991 study found that 90% of psychotherapy patients who had sexual involvement with a prior therapist had been harmed by the experience.9 Books, articles, and Web sites offer vivid individual accounts of harm patients have suffered ( Table ). Doctors who have sex with patients could face public opprobrium, civil lawsuits, actions against their medical licenses, and prosecution in states that make sex with psychiatric patients a criminal offense.10
Table
How sexual relationships can harm patients
| Type of harm | Explanation |
|---|---|
| Ambivalence | Psychological paralysis regarding whether to protect or take action against the abusive therapist |
| Cognitive dysfunction | Impaired memory and concentration, intrusive thoughts, flashbacks |
| Emotional lability | Unpredictable emotional responses, abrupt changes in mood, severe disruption of the patient’s typical way of feeling |
| Emptiness, isolation | Lost sense of self, feeling cut off from others |
| Guilt | Irrational self-blame for causing the sexual contact |
| Impaired trust | Fear of being taken advantage of, used, or abused in future therapy |
| Suicide | 14% of patients who had sex with a therapist attempt suicide; approximately 1% commit suicide |
| Role confusion | Treatment sessions and the therapeutic relationship serve the therapist’s needs rather than the patient’s; this perception may generalize to later therapies and other relationships |
| Sexual confusion | Examples include disgust with sexual feelings, uncertainty about sexual orientation, belief that self-worth comes from gratifying others’ sexual desires |
| Confusion about anger | Rage at self, self-loathing, need to suppress angry feelings, mistaken beliefs that others are angry at you |
| Source: Adapted from reference 8 | |
What about former patients?
Sex between providers and current patients is opposed by all major healthcare organizations, including the American Psychiatric Association (APA),11 American Medical Association,12 and American Psychological Association.13 The last 2 groups strongly discourage sex with former patients, but the APA’s ethics code states that such activity is always unethical.
The APA’s position reflects 2 general truths of psychiatric practice:
- Psychiatric patients often return for care years after initial treatment has ended. “Former patients” are really “possible future patients.” Improper relationships with former patients disrupt the doctor’s obligation to remain available for future care.
- Even if a patient never returns to treatment, intense feelings about a doctor can last for years. A psychiatrist who engages in sex with a former patient may evoke and manipulate feelings “left over” from therapy.
Psychiatrists therefore “have only one kind of relationship with a patient—that is, a doctor-patient relationship.”14 Moreover, as Simon and Shuman observe, “[N]o patient [is] strong enough, no pause is long enough, and no love is true enough to justify compromising this boundary.”15
Legal actions
If a physician no longer practices medicine, can any of his activities—including with a former patient—be malpractice? In fact, sex between practicing doctors and current patients might not always be malpractice. If a psychiatrist gains sexual access to a patient by saying that the sex will be therapeutic, the psychiatrist has perpetrated fraud and this intentional action might not be covered by malpractice insurance.16
In several cases involving nonpsychiatric physicians,17 courts have held that consensual doctor-patient sex is not malpractice, though it might represent some other form of wrongdoing. The argument is that sex with a patient is an intentional act that is never a professional service, whereas malpractice by definition arises unintentionally from negligence while rendering professional services. Other courts, however, have held that doctor-patient sex can be malpractice because it breaches the physician’s fiduciary relationship and can constitute an abuse of power.18
After retirement, physicians still have responsibilities to former patients: to protect records, to respect confidentiality, and to release information upon proper requests. Some fiduciary duties to patients survive the conclusion of treatment, and behavior that breaches those responsibilities can bring legal action.
Psychiatrists should realize that many former patients remain vulnerable because of feelings “left over” from therapy. Therefore, potential civil actions against a retired psychiatrist might include:
A suit for intentional infliction of emotional distress. This tort action requires proving more than mere insults or indignities; it occurs only when someone “by extreme and outrageous conduct intentionally or recklessly causes severe emotional distress to another.”19 Initiating sex with a former patient is strongly disapproved and meets the legal criterion of having a high probability of causing mental distress.20
A suit for negligent infliction of emotional distress. Modern law permits recourse for negligently inflicted emotional distress when harm occurs in “the course of specified categories of activities, undertakings, or relationships in which the negligent conduct is especially likely to cause emotional disturbance.”21
Suits for exploitation. Some jurisdictions allow suits against therapists who have sex with former patients, irrespective of therapists’ license status. For example, Minnesota allows lawsuits for “sexual exploitation” if the former patient’s capacity to consent was impaired by emotional dependence on the psychotherapist.22
Actions by licensing boards. Many retired practitioners maintain their medical licenses. Retired-but-still-licensed psychiatrists can be subject to professional disciplinary actions.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
Dear Dr. Mossman,
A psychiatrist retires from practice and goes into some other line of work—perhaps managing a restaurant. He then has an “affair” with a former patient whom he had not treated for several years. Could the retired psychiatrist’s conduct be the basis of a successful lawsuit?—Submitted by “Dr. D”
Evidence tells us that the retired psychiatrist’s behavior likely could do emotional harm to his former patient. If the former patient suffers some injury, a successful suit could follow—if not on grounds of malpractice, then on other grounds. In this article we’ll see why by looking at:
- rates of doctor-patient sex
- potential harm from doctor-patient sex
- ethical bans on sex with former patients
- possible legal actions.
Sex with patients: Rates and risk
Doctors and patients often develop erotic thoughts about each other.1,2 But as Sigmund Freud noted almost a century ago, an actual love relationship between a doctor and a psychotherapy patient can cause a “complete defeat for the treatment” and destroy the patient’s chance for recovery.3
More than 5 decades later, surveys of medical professionals supplemented Freud’s observations with data about the frequency and impact of doctor-patient sex. In a 1973 survey, 11% of physicians said they had erotic contact with patients, and 5% reported intercourse.4 In a 1986 survey of psychiatrists, 3% of women and 7% of men acknowledged having sexual contact with patients.5 In a 1992 study of 10,000 nonpsychiatric physicians, 9% of respondents reported having sex with patients.6 Actual rates of doctor-patient sex probably are much higher than reported because physicians may be reluctant to admit to having erotic contact with patients, even in anonymous surveys.7 The typical therapist-patient sex scenario involves a male doctor and an adult female patient, but same-sex encounters and sexual contact with minors occur, too.8
Sex between a therapist and a patient is likely to cause emotional injury. For example, a 1991 study found that 90% of psychotherapy patients who had sexual involvement with a prior therapist had been harmed by the experience.9 Books, articles, and Web sites offer vivid individual accounts of harm patients have suffered ( Table ). Doctors who have sex with patients could face public opprobrium, civil lawsuits, actions against their medical licenses, and prosecution in states that make sex with psychiatric patients a criminal offense.10
Table
How sexual relationships can harm patients
| Type of harm | Explanation |
|---|---|
| Ambivalence | Psychological paralysis regarding whether to protect or take action against the abusive therapist |
| Cognitive dysfunction | Impaired memory and concentration, intrusive thoughts, flashbacks |
| Emotional lability | Unpredictable emotional responses, abrupt changes in mood, severe disruption of the patient’s typical way of feeling |
| Emptiness, isolation | Lost sense of self, feeling cut off from others |
| Guilt | Irrational self-blame for causing the sexual contact |
| Impaired trust | Fear of being taken advantage of, used, or abused in future therapy |
| Suicide | 14% of patients who had sex with a therapist attempt suicide; approximately 1% commit suicide |
| Role confusion | Treatment sessions and the therapeutic relationship serve the therapist’s needs rather than the patient’s; this perception may generalize to later therapies and other relationships |
| Sexual confusion | Examples include disgust with sexual feelings, uncertainty about sexual orientation, belief that self-worth comes from gratifying others’ sexual desires |
| Confusion about anger | Rage at self, self-loathing, need to suppress angry feelings, mistaken beliefs that others are angry at you |
| Source: Adapted from reference 8 | |
What about former patients?
Sex between providers and current patients is opposed by all major healthcare organizations, including the American Psychiatric Association (APA),11 American Medical Association,12 and American Psychological Association.13 The last 2 groups strongly discourage sex with former patients, but the APA’s ethics code states that such activity is always unethical.
The APA’s position reflects 2 general truths of psychiatric practice:
- Psychiatric patients often return for care years after initial treatment has ended. “Former patients” are really “possible future patients.” Improper relationships with former patients disrupt the doctor’s obligation to remain available for future care.
- Even if a patient never returns to treatment, intense feelings about a doctor can last for years. A psychiatrist who engages in sex with a former patient may evoke and manipulate feelings “left over” from therapy.
Psychiatrists therefore “have only one kind of relationship with a patient—that is, a doctor-patient relationship.”14 Moreover, as Simon and Shuman observe, “[N]o patient [is] strong enough, no pause is long enough, and no love is true enough to justify compromising this boundary.”15
Legal actions
If a physician no longer practices medicine, can any of his activities—including with a former patient—be malpractice? In fact, sex between practicing doctors and current patients might not always be malpractice. If a psychiatrist gains sexual access to a patient by saying that the sex will be therapeutic, the psychiatrist has perpetrated fraud and this intentional action might not be covered by malpractice insurance.16
In several cases involving nonpsychiatric physicians,17 courts have held that consensual doctor-patient sex is not malpractice, though it might represent some other form of wrongdoing. The argument is that sex with a patient is an intentional act that is never a professional service, whereas malpractice by definition arises unintentionally from negligence while rendering professional services. Other courts, however, have held that doctor-patient sex can be malpractice because it breaches the physician’s fiduciary relationship and can constitute an abuse of power.18
After retirement, physicians still have responsibilities to former patients: to protect records, to respect confidentiality, and to release information upon proper requests. Some fiduciary duties to patients survive the conclusion of treatment, and behavior that breaches those responsibilities can bring legal action.
Psychiatrists should realize that many former patients remain vulnerable because of feelings “left over” from therapy. Therefore, potential civil actions against a retired psychiatrist might include:
A suit for intentional infliction of emotional distress. This tort action requires proving more than mere insults or indignities; it occurs only when someone “by extreme and outrageous conduct intentionally or recklessly causes severe emotional distress to another.”19 Initiating sex with a former patient is strongly disapproved and meets the legal criterion of having a high probability of causing mental distress.20
A suit for negligent infliction of emotional distress. Modern law permits recourse for negligently inflicted emotional distress when harm occurs in “the course of specified categories of activities, undertakings, or relationships in which the negligent conduct is especially likely to cause emotional disturbance.”21
Suits for exploitation. Some jurisdictions allow suits against therapists who have sex with former patients, irrespective of therapists’ license status. For example, Minnesota allows lawsuits for “sexual exploitation” if the former patient’s capacity to consent was impaired by emotional dependence on the psychotherapist.22
Actions by licensing boards. Many retired practitioners maintain their medical licenses. Retired-but-still-licensed psychiatrists can be subject to professional disciplinary actions.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
1. Pope KS, Keith-Spiegel P, Tabachnick BG. Sexual attraction to clients. The human therapist and the (sometimes) inhuman training system. Am Psychol. 1986;4:147-158.
2. Golden GA, Brennan M. Managing erotic feelings in the physician-patient relationship. CMAJ. 1995;153:1241-1245.
3. Freud S. Observations on transference-love. In: Strachey J, ed. Complete psychological works of Sigmund Freud, standard edition, vol 12. London, UK: Hogarth Press; 1958:157-173.
4. Kardener SH, Fuller M, Mensh IN. A survey of physicians’ attitudes and practice regarding erotic and non-erotic contact with patients. Am J Psychiatry. 1973;130:1077-1081.
5. Gartrell N, Herman J, Olarte S, et al. Psychiatrist-patient sexual contact: results of a national survey, 1: prevalence. Am J Psychiatry. 1986;143:1126-1131.
6. Gartrell N, Milliken N, Goodson WH, et al. Physician-patient sexual contact—prevalence and problems. West J Med. 1992;157:139-143.
7. Roman B, Kay J. Residency education on the prevention of physician-patient sexual misconduct. Acad Psychiatry. 1997;21:26-34.
8. Pope KS. Sex between therapists and clients. In: Worrell J, ed. Encyclopedia of women and gender: sex similarities and differences and the impact of society on gender. New York, NY: Academic Press; 2001;955-962.
9. Pope KS, Vetter VA. Prior therapist-patient sexual involvement among patients seen by psychologists. Psychotherapy. 1991;28:429-438.
10. Simon RI. Clinical psychiatry and the law, 2nd edition. Arlington, VA: American Psychiatric Publishing, Inc.; 2003.
11. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Available at: http://www.psych.org/MainMenu/PsychiatricPractice/Ethics/
ResourcesStandards/PrinciplesofMedicalEthics.aspx. Accessed May 4, 2009.
12. American Medical Association Council on Ethical and Judicial Affairs. Sexual misconduct in the practice of medicine. JAMA. 1991;266:2741-2745.
13. American Psychological Association. Ethical principles of psychologists and code of conduct. Available at: http://www.apa.org/ethics/code2002.html. Accessed May 4, 2009.
14. Gruenberg PB. Boundary violations. In: Ethics primer of the American Psychiatric Association. Washington, DC: American Psychiatric Association; 2001;1-9.
15. Simon RI, Shuman DW. Clinical manual of psychiatry and the law. Arlington, VA: American Psychiatric Publishing, Inc.; 2007.
16. Sadock BJ, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry, 10th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.
17. Clemente v Roth, 171 Fed. Appx. 999 (4th Cir. Md. 2006).
18. Hoopes v Hammargren, 102 Nev. 425, 725 P.2d 238 (1986).
19. Restatement (Third) of Torts: Liability for Physical Harm, ch 8, §45 (2007 draft).
20. Prosser WL, Keeton WP, Dobbs DB, et al. Prosser and Keeton on torts, 5th ed. St. Paul, MN: West Publishing Co.; 1984.
21. Restatement (Third) of Torts: Liability for Physical Harm, ch 8, §46 (2007 draft).
22. Minnesota Statutes §148A (2008).
1. Pope KS, Keith-Spiegel P, Tabachnick BG. Sexual attraction to clients. The human therapist and the (sometimes) inhuman training system. Am Psychol. 1986;4:147-158.
2. Golden GA, Brennan M. Managing erotic feelings in the physician-patient relationship. CMAJ. 1995;153:1241-1245.
3. Freud S. Observations on transference-love. In: Strachey J, ed. Complete psychological works of Sigmund Freud, standard edition, vol 12. London, UK: Hogarth Press; 1958:157-173.
4. Kardener SH, Fuller M, Mensh IN. A survey of physicians’ attitudes and practice regarding erotic and non-erotic contact with patients. Am J Psychiatry. 1973;130:1077-1081.
5. Gartrell N, Herman J, Olarte S, et al. Psychiatrist-patient sexual contact: results of a national survey, 1: prevalence. Am J Psychiatry. 1986;143:1126-1131.
6. Gartrell N, Milliken N, Goodson WH, et al. Physician-patient sexual contact—prevalence and problems. West J Med. 1992;157:139-143.
7. Roman B, Kay J. Residency education on the prevention of physician-patient sexual misconduct. Acad Psychiatry. 1997;21:26-34.
8. Pope KS. Sex between therapists and clients. In: Worrell J, ed. Encyclopedia of women and gender: sex similarities and differences and the impact of society on gender. New York, NY: Academic Press; 2001;955-962.
9. Pope KS, Vetter VA. Prior therapist-patient sexual involvement among patients seen by psychologists. Psychotherapy. 1991;28:429-438.
10. Simon RI. Clinical psychiatry and the law, 2nd edition. Arlington, VA: American Psychiatric Publishing, Inc.; 2003.
11. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Available at: http://www.psych.org/MainMenu/PsychiatricPractice/Ethics/
ResourcesStandards/PrinciplesofMedicalEthics.aspx. Accessed May 4, 2009.
12. American Medical Association Council on Ethical and Judicial Affairs. Sexual misconduct in the practice of medicine. JAMA. 1991;266:2741-2745.
13. American Psychological Association. Ethical principles of psychologists and code of conduct. Available at: http://www.apa.org/ethics/code2002.html. Accessed May 4, 2009.
14. Gruenberg PB. Boundary violations. In: Ethics primer of the American Psychiatric Association. Washington, DC: American Psychiatric Association; 2001;1-9.
15. Simon RI, Shuman DW. Clinical manual of psychiatry and the law. Arlington, VA: American Psychiatric Publishing, Inc.; 2007.
16. Sadock BJ, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry, 10th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.
17. Clemente v Roth, 171 Fed. Appx. 999 (4th Cir. Md. 2006).
18. Hoopes v Hammargren, 102 Nev. 425, 725 P.2d 238 (1986).
19. Restatement (Third) of Torts: Liability for Physical Harm, ch 8, §45 (2007 draft).
20. Prosser WL, Keeton WP, Dobbs DB, et al. Prosser and Keeton on torts, 5th ed. St. Paul, MN: West Publishing Co.; 1984.
21. Restatement (Third) of Torts: Liability for Physical Harm, ch 8, §46 (2007 draft).
22. Minnesota Statutes §148A (2008).
The Child With a Suspicious Cough
A thorough differential diagnosis, primarily based on history and physical examination, is essential when a child presents with a suspicious cough. Certain imaging modalities are also useful for diagnosis.
Identification of an underlying cause is crucial. When doing your history and physical exam, look for something that does not fit a routine presentation. For example, a cough in the presence of a constitutional change, such as weight loss, can indicate a more serious problem. In addition, a cough with a relatively sudden onset or one associated with labored breathing can be worrisome. Also, a choking episode followed by sudden cough, for example, can indicate the presence of a foreign body.
Asthma is the most common cause of chronic cough in the pediatric population, but also consider less common etiologies such as tracheoesophageal fistula, cystic fibrosis (CF), and bronchopulmonary dysplasia. Failure to thrive, clubbing, cardiac signs, and persistent stridor suggest alternative diagnoses.
Patient age offers some guidance in your differential diagnosis. In a neonate (younger than 28 days), persistent cough might suggest an infection or a congenital anomaly such as compression of the esophagus and trachea by a vascular ring. Infectious etiologies include rhinovirus, adenovirus, respiratory syncytial virus, and pertussis.
In preschool children, think upper or lower respiratory tract infection, rhinitis, postnasal drip syndrome, gastroesophageal reflux, an irritant source (such as passive smoking or air pollution), and, of course, asthma.
Among school-age children and adolescents, consider the same possibilities, but add inhalant or other substance abuse to your list of possible irritant causes. In addition, these older children can develop psychogenic or “habit” cough, one that is absent during sleep, distraction, or periods of concentration. Vocal cord dysfunction, also known as laryngeal wheeze, is another possibility in this group.
General pediatricians commonly treat children with a cough that lasts 5-10 days in the context of an upper respiratory tract illness, such as a cold. If a child still coughs incessantly after other cold symptoms have resolved, I would be concerned. This is not necessarily a call to refer the patient to a specialist, but this scenario is a call to do further diagnostic evaluation.
If the child already is diagnosed with asthma and develops a cough, determine whether the patient is taking the appropriate medication and/or is compliant with therapy. Also, ask about the child's environment, particularly the presence of passive smoking, dust, and pets.
In terms of allergy testing, I recommend a radioallergosorbent allergen-specific IgE antibody assay. This is indicated if a child has other lateral symptoms, such as eczema, and/or during peak times for seasonal allergies.
It is helpful when pediatricians do spirometry for a child with a suspicious cough. Nationwide, about 20%-25% of general pediatricians do pulmonary function testing. Pediatric pulmonologists like me would like to see more pediatricians perform these tests. Sinus x-rays also can be helpful, and are within the purview of the general pediatrician. Some might consider this an unnecessary test, however, or one for which you need a high index of suspicion before ordering.
A test that is generally unnecessary is a sweat test for cystic fibrosis. A lot of pediatricians get this test, and I would not tell them not to because often the child with CF has other symptoms that are more diagnostic.
A thorough differential diagnosis, primarily based on history and physical examination, is essential when a child presents with a suspicious cough. Certain imaging modalities are also useful for diagnosis.
Identification of an underlying cause is crucial. When doing your history and physical exam, look for something that does not fit a routine presentation. For example, a cough in the presence of a constitutional change, such as weight loss, can indicate a more serious problem. In addition, a cough with a relatively sudden onset or one associated with labored breathing can be worrisome. Also, a choking episode followed by sudden cough, for example, can indicate the presence of a foreign body.
Asthma is the most common cause of chronic cough in the pediatric population, but also consider less common etiologies such as tracheoesophageal fistula, cystic fibrosis (CF), and bronchopulmonary dysplasia. Failure to thrive, clubbing, cardiac signs, and persistent stridor suggest alternative diagnoses.
Patient age offers some guidance in your differential diagnosis. In a neonate (younger than 28 days), persistent cough might suggest an infection or a congenital anomaly such as compression of the esophagus and trachea by a vascular ring. Infectious etiologies include rhinovirus, adenovirus, respiratory syncytial virus, and pertussis.
In preschool children, think upper or lower respiratory tract infection, rhinitis, postnasal drip syndrome, gastroesophageal reflux, an irritant source (such as passive smoking or air pollution), and, of course, asthma.
Among school-age children and adolescents, consider the same possibilities, but add inhalant or other substance abuse to your list of possible irritant causes. In addition, these older children can develop psychogenic or “habit” cough, one that is absent during sleep, distraction, or periods of concentration. Vocal cord dysfunction, also known as laryngeal wheeze, is another possibility in this group.
General pediatricians commonly treat children with a cough that lasts 5-10 days in the context of an upper respiratory tract illness, such as a cold. If a child still coughs incessantly after other cold symptoms have resolved, I would be concerned. This is not necessarily a call to refer the patient to a specialist, but this scenario is a call to do further diagnostic evaluation.
If the child already is diagnosed with asthma and develops a cough, determine whether the patient is taking the appropriate medication and/or is compliant with therapy. Also, ask about the child's environment, particularly the presence of passive smoking, dust, and pets.
In terms of allergy testing, I recommend a radioallergosorbent allergen-specific IgE antibody assay. This is indicated if a child has other lateral symptoms, such as eczema, and/or during peak times for seasonal allergies.
It is helpful when pediatricians do spirometry for a child with a suspicious cough. Nationwide, about 20%-25% of general pediatricians do pulmonary function testing. Pediatric pulmonologists like me would like to see more pediatricians perform these tests. Sinus x-rays also can be helpful, and are within the purview of the general pediatrician. Some might consider this an unnecessary test, however, or one for which you need a high index of suspicion before ordering.
A test that is generally unnecessary is a sweat test for cystic fibrosis. A lot of pediatricians get this test, and I would not tell them not to because often the child with CF has other symptoms that are more diagnostic.
A thorough differential diagnosis, primarily based on history and physical examination, is essential when a child presents with a suspicious cough. Certain imaging modalities are also useful for diagnosis.
Identification of an underlying cause is crucial. When doing your history and physical exam, look for something that does not fit a routine presentation. For example, a cough in the presence of a constitutional change, such as weight loss, can indicate a more serious problem. In addition, a cough with a relatively sudden onset or one associated with labored breathing can be worrisome. Also, a choking episode followed by sudden cough, for example, can indicate the presence of a foreign body.
Asthma is the most common cause of chronic cough in the pediatric population, but also consider less common etiologies such as tracheoesophageal fistula, cystic fibrosis (CF), and bronchopulmonary dysplasia. Failure to thrive, clubbing, cardiac signs, and persistent stridor suggest alternative diagnoses.
Patient age offers some guidance in your differential diagnosis. In a neonate (younger than 28 days), persistent cough might suggest an infection or a congenital anomaly such as compression of the esophagus and trachea by a vascular ring. Infectious etiologies include rhinovirus, adenovirus, respiratory syncytial virus, and pertussis.
In preschool children, think upper or lower respiratory tract infection, rhinitis, postnasal drip syndrome, gastroesophageal reflux, an irritant source (such as passive smoking or air pollution), and, of course, asthma.
Among school-age children and adolescents, consider the same possibilities, but add inhalant or other substance abuse to your list of possible irritant causes. In addition, these older children can develop psychogenic or “habit” cough, one that is absent during sleep, distraction, or periods of concentration. Vocal cord dysfunction, also known as laryngeal wheeze, is another possibility in this group.
General pediatricians commonly treat children with a cough that lasts 5-10 days in the context of an upper respiratory tract illness, such as a cold. If a child still coughs incessantly after other cold symptoms have resolved, I would be concerned. This is not necessarily a call to refer the patient to a specialist, but this scenario is a call to do further diagnostic evaluation.
If the child already is diagnosed with asthma and develops a cough, determine whether the patient is taking the appropriate medication and/or is compliant with therapy. Also, ask about the child's environment, particularly the presence of passive smoking, dust, and pets.
In terms of allergy testing, I recommend a radioallergosorbent allergen-specific IgE antibody assay. This is indicated if a child has other lateral symptoms, such as eczema, and/or during peak times for seasonal allergies.
It is helpful when pediatricians do spirometry for a child with a suspicious cough. Nationwide, about 20%-25% of general pediatricians do pulmonary function testing. Pediatric pulmonologists like me would like to see more pediatricians perform these tests. Sinus x-rays also can be helpful, and are within the purview of the general pediatrician. Some might consider this an unnecessary test, however, or one for which you need a high index of suspicion before ordering.
A test that is generally unnecessary is a sweat test for cystic fibrosis. A lot of pediatricians get this test, and I would not tell them not to because often the child with CF has other symptoms that are more diagnostic.
Regulated Relationship?
As the American Medical Association (AMA) develops a toolkit to help physicians implement contracts that govern the professional relationship between physicians and non-physician providers (NPPs), one SHM committee member says the trade group should keep an open mind on the pros and cons of such agreements.
The AMA House of Delegates last month tasked its staff to develop the toolkit after several delegates expressed concerns that without the so-called “practice agreements,” NPPs and physicians do not have clear boundaries on the scope of practice responsibilities, according to American Medical News, the society’s newspaper.
Lorraine Britting, MS, NP-C, a member of SHM’s Non-Physician Provider Committee, says the agreements can be a framework for certain practices but might be seen as burdensome to HM groups that have worked for years without the contracts in place.
“In the states that already have practice agreements mandated, you put these regulations in place and they work well. That being said, if new requirements are too restrictive, there is certainly potential for there to be some conflict between the physicians and the nurse practitioners,” says Britting, lead nurse practitioner in the department of cardiology medicine at the Cardiovascular Institute at Beth Israel Deaconess Medical Center in Boston.
Britting notes that many HM groups already have practice agreements in place and that she has never worked without one because Massachusetts requires them. She says professional relationships between doctors and NPPs could be helped by rules on who is responsible for what—but only if those agreements are developed with input from all stakeholders.
“It’s hard to make a blanket statement. Someone who has 10 years’ experience working in hospital medicine versus somebody who just graduated ... their needs are going to be very different,” Britting says. “It has to be individually tailored.”
As the American Medical Association (AMA) develops a toolkit to help physicians implement contracts that govern the professional relationship between physicians and non-physician providers (NPPs), one SHM committee member says the trade group should keep an open mind on the pros and cons of such agreements.
The AMA House of Delegates last month tasked its staff to develop the toolkit after several delegates expressed concerns that without the so-called “practice agreements,” NPPs and physicians do not have clear boundaries on the scope of practice responsibilities, according to American Medical News, the society’s newspaper.
Lorraine Britting, MS, NP-C, a member of SHM’s Non-Physician Provider Committee, says the agreements can be a framework for certain practices but might be seen as burdensome to HM groups that have worked for years without the contracts in place.
“In the states that already have practice agreements mandated, you put these regulations in place and they work well. That being said, if new requirements are too restrictive, there is certainly potential for there to be some conflict between the physicians and the nurse practitioners,” says Britting, lead nurse practitioner in the department of cardiology medicine at the Cardiovascular Institute at Beth Israel Deaconess Medical Center in Boston.
Britting notes that many HM groups already have practice agreements in place and that she has never worked without one because Massachusetts requires them. She says professional relationships between doctors and NPPs could be helped by rules on who is responsible for what—but only if those agreements are developed with input from all stakeholders.
“It’s hard to make a blanket statement. Someone who has 10 years’ experience working in hospital medicine versus somebody who just graduated ... their needs are going to be very different,” Britting says. “It has to be individually tailored.”
As the American Medical Association (AMA) develops a toolkit to help physicians implement contracts that govern the professional relationship between physicians and non-physician providers (NPPs), one SHM committee member says the trade group should keep an open mind on the pros and cons of such agreements.
The AMA House of Delegates last month tasked its staff to develop the toolkit after several delegates expressed concerns that without the so-called “practice agreements,” NPPs and physicians do not have clear boundaries on the scope of practice responsibilities, according to American Medical News, the society’s newspaper.
Lorraine Britting, MS, NP-C, a member of SHM’s Non-Physician Provider Committee, says the agreements can be a framework for certain practices but might be seen as burdensome to HM groups that have worked for years without the contracts in place.
“In the states that already have practice agreements mandated, you put these regulations in place and they work well. That being said, if new requirements are too restrictive, there is certainly potential for there to be some conflict between the physicians and the nurse practitioners,” says Britting, lead nurse practitioner in the department of cardiology medicine at the Cardiovascular Institute at Beth Israel Deaconess Medical Center in Boston.
Britting notes that many HM groups already have practice agreements in place and that she has never worked without one because Massachusetts requires them. She says professional relationships between doctors and NPPs could be helped by rules on who is responsible for what—but only if those agreements are developed with input from all stakeholders.
“It’s hard to make a blanket statement. Someone who has 10 years’ experience working in hospital medicine versus somebody who just graduated ... their needs are going to be very different,” Britting says. “It has to be individually tailored.”
Development Phase
More than 350 pediatric hospitalists convened in Tampa, Fla., last week for Pediatric Hospital Medicine (PHM) 2009, tri-sponsored by SHM, the Academic Pediatric Association (APA), and the American Academy of Pediatrics (AAP). More impressive than the continued growth of the field, however, was the palpable theme of development and maturation.
The theme was immediately evident as Patrick Conway, MD, MSc, took the stage to give the keynote address. Dr. Conway, a card-carrying pediatric hospitalist, has built upon his beginnings in health services research and a White House fellowship to become the chief medical officer in the Department of Health and Human Services (HHS) Office of the Secretary/Assistant Secretary for Planning and Evaluation. He also is the executive director of the Federal Coordinating Council for Comparative Effectiveness Research.
After providing an insider’s view of HHS, comparative effectiveness research, and healthcare reform and policy, he challenged pediatric hospitalists to demonstrate their value to the healthcare system.
Synergistically, this year’s meeting also provided an opportunity for the PHM Roundtable, a strategic planning session of pediatric hospitalist leaders, to fully share its vision for transforming the delivery of hospital care to children. As a manifestation of this vision, collaborative discussions and workgroup plans coalesced amid the enlightening mix of clinical, practice management, academic, and quality and patient safety workshops.
Growth and development are central concepts in pediatrics, and PHM 2009 highlighted the field’s energetic steps towards maturation.
Dr. Shen is pediatric editor of The Hospitalist and medical director of hospital medicine at Dell Children’s Medical Center in Austin, Texas
More than 350 pediatric hospitalists convened in Tampa, Fla., last week for Pediatric Hospital Medicine (PHM) 2009, tri-sponsored by SHM, the Academic Pediatric Association (APA), and the American Academy of Pediatrics (AAP). More impressive than the continued growth of the field, however, was the palpable theme of development and maturation.
The theme was immediately evident as Patrick Conway, MD, MSc, took the stage to give the keynote address. Dr. Conway, a card-carrying pediatric hospitalist, has built upon his beginnings in health services research and a White House fellowship to become the chief medical officer in the Department of Health and Human Services (HHS) Office of the Secretary/Assistant Secretary for Planning and Evaluation. He also is the executive director of the Federal Coordinating Council for Comparative Effectiveness Research.
After providing an insider’s view of HHS, comparative effectiveness research, and healthcare reform and policy, he challenged pediatric hospitalists to demonstrate their value to the healthcare system.
Synergistically, this year’s meeting also provided an opportunity for the PHM Roundtable, a strategic planning session of pediatric hospitalist leaders, to fully share its vision for transforming the delivery of hospital care to children. As a manifestation of this vision, collaborative discussions and workgroup plans coalesced amid the enlightening mix of clinical, practice management, academic, and quality and patient safety workshops.
Growth and development are central concepts in pediatrics, and PHM 2009 highlighted the field’s energetic steps towards maturation.
Dr. Shen is pediatric editor of The Hospitalist and medical director of hospital medicine at Dell Children’s Medical Center in Austin, Texas
More than 350 pediatric hospitalists convened in Tampa, Fla., last week for Pediatric Hospital Medicine (PHM) 2009, tri-sponsored by SHM, the Academic Pediatric Association (APA), and the American Academy of Pediatrics (AAP). More impressive than the continued growth of the field, however, was the palpable theme of development and maturation.
The theme was immediately evident as Patrick Conway, MD, MSc, took the stage to give the keynote address. Dr. Conway, a card-carrying pediatric hospitalist, has built upon his beginnings in health services research and a White House fellowship to become the chief medical officer in the Department of Health and Human Services (HHS) Office of the Secretary/Assistant Secretary for Planning and Evaluation. He also is the executive director of the Federal Coordinating Council for Comparative Effectiveness Research.
After providing an insider’s view of HHS, comparative effectiveness research, and healthcare reform and policy, he challenged pediatric hospitalists to demonstrate their value to the healthcare system.
Synergistically, this year’s meeting also provided an opportunity for the PHM Roundtable, a strategic planning session of pediatric hospitalist leaders, to fully share its vision for transforming the delivery of hospital care to children. As a manifestation of this vision, collaborative discussions and workgroup plans coalesced amid the enlightening mix of clinical, practice management, academic, and quality and patient safety workshops.
Growth and development are central concepts in pediatrics, and PHM 2009 highlighted the field’s energetic steps towards maturation.
Dr. Shen is pediatric editor of The Hospitalist and medical director of hospital medicine at Dell Children’s Medical Center in Austin, Texas
Docs recommend transdermal patch for HRT
Boston—Postmenopausal women on hormone replacement therapy (HRT) are known to be at increased risk of venous thromboembolism (VTE). But whether the route of administration influences the risk was not known until now.
Investigators at Kings College Hospital NHS Foundation Trust in London discovered that VTE risk is increased in women using oral HRT but not in those using a transdermal patch. Catherine N. Bagot, MD, reported these results at the 22nd Congress of the International Society on Thrombosis and Haemostasis (ISTH).
The investigators have thus far recruited 155 women to this ongoing study, 98 on HRT and 57 controls not on HRT. Fifty-four women were on oral HRT and 44 were using the transdermal patch.
Dr Bagot and colleagues used thrombin generation as a marker of thrombotic risk. They found that women taking HRT had significantly higher peak thrombin generation than controls (P=0.0019). They performed a subgroup analysis and the difference was only detectable in women using oral HRT (P<0.0001) and not in women using the transdermal route (P=0.7).
Investigators verified the data further and confirmed that peak thrombin generation was significantly higher in women on oral compared to transdermal HRT (P<0.0001).
The presence of progestogen or testosterone did not have any impact on the results.
These findings indicate that postmenopausal women taking oral HRT are at greater risk for VTE than those using transdermal administration. Dr Bagot suggested that transdermal administration may be safe in women who have had previous VTEs.
The research team also investigated the relationship between estradiol and peak thrombin generation.
They analyzed blood samples of 132 women. Eighty-six women were on HRT, 42 oral and 44 transdermal. The remaining women not on HRT served as controls.
Investigators excluded women whose HRT formulation contained equine estrogens or who had less than 100 pMol/L estradiol levels.
They found that estradiol levels were significantly higher in women using either formulation of HRT than controls. However, the levels were not significantly different between the two HRT groups.
Investigators also found peak thrombin generation to be significantly higher in women on HRT than controls, and this correlated with estradiol concentrations. Women taking oral HRT had a significantly higher peak thrombin generation compared to women using the patch. Investigators observed a correlation between estradiol levels and peak thrombin generation only in women using oral HRT.
Dr Bagot indicated that a limitation of this second study was that there was no way of knowing whether the estradiol levels were at the peak or trough. Nevertheless, this study confirmed further a causal link between oral estrogens, hypercoagulability, and an increased risk of VTE.
The investigators again recommended the transdermal route over oral HRT administration in postmenopausal women to achieve the lowest thrombotic risk. ![]()
Boston—Postmenopausal women on hormone replacement therapy (HRT) are known to be at increased risk of venous thromboembolism (VTE). But whether the route of administration influences the risk was not known until now.
Investigators at Kings College Hospital NHS Foundation Trust in London discovered that VTE risk is increased in women using oral HRT but not in those using a transdermal patch. Catherine N. Bagot, MD, reported these results at the 22nd Congress of the International Society on Thrombosis and Haemostasis (ISTH).
The investigators have thus far recruited 155 women to this ongoing study, 98 on HRT and 57 controls not on HRT. Fifty-four women were on oral HRT and 44 were using the transdermal patch.
Dr Bagot and colleagues used thrombin generation as a marker of thrombotic risk. They found that women taking HRT had significantly higher peak thrombin generation than controls (P=0.0019). They performed a subgroup analysis and the difference was only detectable in women using oral HRT (P<0.0001) and not in women using the transdermal route (P=0.7).
Investigators verified the data further and confirmed that peak thrombin generation was significantly higher in women on oral compared to transdermal HRT (P<0.0001).
The presence of progestogen or testosterone did not have any impact on the results.
These findings indicate that postmenopausal women taking oral HRT are at greater risk for VTE than those using transdermal administration. Dr Bagot suggested that transdermal administration may be safe in women who have had previous VTEs.
The research team also investigated the relationship between estradiol and peak thrombin generation.
They analyzed blood samples of 132 women. Eighty-six women were on HRT, 42 oral and 44 transdermal. The remaining women not on HRT served as controls.
Investigators excluded women whose HRT formulation contained equine estrogens or who had less than 100 pMol/L estradiol levels.
They found that estradiol levels were significantly higher in women using either formulation of HRT than controls. However, the levels were not significantly different between the two HRT groups.
Investigators also found peak thrombin generation to be significantly higher in women on HRT than controls, and this correlated with estradiol concentrations. Women taking oral HRT had a significantly higher peak thrombin generation compared to women using the patch. Investigators observed a correlation between estradiol levels and peak thrombin generation only in women using oral HRT.
Dr Bagot indicated that a limitation of this second study was that there was no way of knowing whether the estradiol levels were at the peak or trough. Nevertheless, this study confirmed further a causal link between oral estrogens, hypercoagulability, and an increased risk of VTE.
The investigators again recommended the transdermal route over oral HRT administration in postmenopausal women to achieve the lowest thrombotic risk. ![]()
Boston—Postmenopausal women on hormone replacement therapy (HRT) are known to be at increased risk of venous thromboembolism (VTE). But whether the route of administration influences the risk was not known until now.
Investigators at Kings College Hospital NHS Foundation Trust in London discovered that VTE risk is increased in women using oral HRT but not in those using a transdermal patch. Catherine N. Bagot, MD, reported these results at the 22nd Congress of the International Society on Thrombosis and Haemostasis (ISTH).
The investigators have thus far recruited 155 women to this ongoing study, 98 on HRT and 57 controls not on HRT. Fifty-four women were on oral HRT and 44 were using the transdermal patch.
Dr Bagot and colleagues used thrombin generation as a marker of thrombotic risk. They found that women taking HRT had significantly higher peak thrombin generation than controls (P=0.0019). They performed a subgroup analysis and the difference was only detectable in women using oral HRT (P<0.0001) and not in women using the transdermal route (P=0.7).
Investigators verified the data further and confirmed that peak thrombin generation was significantly higher in women on oral compared to transdermal HRT (P<0.0001).
The presence of progestogen or testosterone did not have any impact on the results.
These findings indicate that postmenopausal women taking oral HRT are at greater risk for VTE than those using transdermal administration. Dr Bagot suggested that transdermal administration may be safe in women who have had previous VTEs.
The research team also investigated the relationship between estradiol and peak thrombin generation.
They analyzed blood samples of 132 women. Eighty-six women were on HRT, 42 oral and 44 transdermal. The remaining women not on HRT served as controls.
Investigators excluded women whose HRT formulation contained equine estrogens or who had less than 100 pMol/L estradiol levels.
They found that estradiol levels were significantly higher in women using either formulation of HRT than controls. However, the levels were not significantly different between the two HRT groups.
Investigators also found peak thrombin generation to be significantly higher in women on HRT than controls, and this correlated with estradiol concentrations. Women taking oral HRT had a significantly higher peak thrombin generation compared to women using the patch. Investigators observed a correlation between estradiol levels and peak thrombin generation only in women using oral HRT.
Dr Bagot indicated that a limitation of this second study was that there was no way of knowing whether the estradiol levels were at the peak or trough. Nevertheless, this study confirmed further a causal link between oral estrogens, hypercoagulability, and an increased risk of VTE.
The investigators again recommended the transdermal route over oral HRT administration in postmenopausal women to achieve the lowest thrombotic risk. ![]()
Caution Urged in Interpreting Glargine Cancer Risk
A series of studies reported last month by a European health organization has questioned whether the use of insulin glargine, known commercially as Lantus, inflates a patient’s risk of cancer. But according to one source, hospitalists with a large census of diabetic or hypoglycemic patients shouldn’t pull their patients off the treatment just yet.
Four different population-based studies were reported on the Web site for Diabetologia, the journal of the European Association of the Study of Diabetes. A German study of 127,000 patients in an insurance database found that for every 100 patients taking Lantus, there was one more person diagnosed with cancer when compared with 100 patients taking similar doses of human insulin. The risk increased with the dosage, the study reported.
But the American Diabetes Association quickly released a statement calling the studies “conflicting and confusing.”
Hospitalist Jeffrey Schnipper, MD, MPH, FHM, who estimates one-third of his patients are either diabetic or hypoglycemic, agrees, saying that observational studies make it hard to draw any conclusions.
“I tell patients we don’t know enough,” says Dr. Schnipper, director of clinical research and associate physician in the Division of General Medicine at Brigham and Women’s Hospital Hospitalist Service in Boston. “Right now, there’s no good strong evidence that Lantus is worse than any other alternative.”
Dr. Schnipper cautioned fellow hospitalists to not overreact to the reports, noting that without a randomized trial to follow through on the hypotheses raised, there is no resolution to the confounding-by-indication bias that can plague observational studies.
“There’s an adage in medicine that you never want to be the first person or the last person to use a drug,” Dr. Schnipper says. “I would say you should never be the first person or the last person to stop using a drug.”
A series of studies reported last month by a European health organization has questioned whether the use of insulin glargine, known commercially as Lantus, inflates a patient’s risk of cancer. But according to one source, hospitalists with a large census of diabetic or hypoglycemic patients shouldn’t pull their patients off the treatment just yet.
Four different population-based studies were reported on the Web site for Diabetologia, the journal of the European Association of the Study of Diabetes. A German study of 127,000 patients in an insurance database found that for every 100 patients taking Lantus, there was one more person diagnosed with cancer when compared with 100 patients taking similar doses of human insulin. The risk increased with the dosage, the study reported.
But the American Diabetes Association quickly released a statement calling the studies “conflicting and confusing.”
Hospitalist Jeffrey Schnipper, MD, MPH, FHM, who estimates one-third of his patients are either diabetic or hypoglycemic, agrees, saying that observational studies make it hard to draw any conclusions.
“I tell patients we don’t know enough,” says Dr. Schnipper, director of clinical research and associate physician in the Division of General Medicine at Brigham and Women’s Hospital Hospitalist Service in Boston. “Right now, there’s no good strong evidence that Lantus is worse than any other alternative.”
Dr. Schnipper cautioned fellow hospitalists to not overreact to the reports, noting that without a randomized trial to follow through on the hypotheses raised, there is no resolution to the confounding-by-indication bias that can plague observational studies.
“There’s an adage in medicine that you never want to be the first person or the last person to use a drug,” Dr. Schnipper says. “I would say you should never be the first person or the last person to stop using a drug.”
A series of studies reported last month by a European health organization has questioned whether the use of insulin glargine, known commercially as Lantus, inflates a patient’s risk of cancer. But according to one source, hospitalists with a large census of diabetic or hypoglycemic patients shouldn’t pull their patients off the treatment just yet.
Four different population-based studies were reported on the Web site for Diabetologia, the journal of the European Association of the Study of Diabetes. A German study of 127,000 patients in an insurance database found that for every 100 patients taking Lantus, there was one more person diagnosed with cancer when compared with 100 patients taking similar doses of human insulin. The risk increased with the dosage, the study reported.
But the American Diabetes Association quickly released a statement calling the studies “conflicting and confusing.”
Hospitalist Jeffrey Schnipper, MD, MPH, FHM, who estimates one-third of his patients are either diabetic or hypoglycemic, agrees, saying that observational studies make it hard to draw any conclusions.
“I tell patients we don’t know enough,” says Dr. Schnipper, director of clinical research and associate physician in the Division of General Medicine at Brigham and Women’s Hospital Hospitalist Service in Boston. “Right now, there’s no good strong evidence that Lantus is worse than any other alternative.”
Dr. Schnipper cautioned fellow hospitalists to not overreact to the reports, noting that without a randomized trial to follow through on the hypotheses raised, there is no resolution to the confounding-by-indication bias that can plague observational studies.
“There’s an adage in medicine that you never want to be the first person or the last person to use a drug,” Dr. Schnipper says. “I would say you should never be the first person or the last person to stop using a drug.”