User login
FDA approves Yuflyma as ninth adalimumab biosimilar
The U.S. Food and Drug Administration has approved the biosimilar adalimumab-aaty (Yuflyma) in a citrate-free, high-concentration formulation, the manufacturer, Celltrion USA, announced today. It is the ninth biosimilar of adalimumab (Humira) to be approved in the United States.
Yuflyma is approved for the treatment of adult patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa. It is also approved for polyarticular juvenile idiopathic arthritis for patients aged 2 years or older, as well as for Crohn’s disease in adults and in pediatric patients aged 6 years or older.
The formulation was approved on the basis of a comprehensive data package of analytic, preclinical, and clinical studies, according to Celltrion USA, “demonstrating that Yuflyma is comparable to the reference product Humira in terms of efficacy, safety, pharmacokinetics, and immunogenicity up to 24 weeks and 1 year following treatment.”
The company conducted a double-blind, randomized phase 3 trial that compared switching from reference adalimumab to Yuflyma with continuing either reference adalimumab or Yuflyma for patients with active rheumatoid arthritis. In that trial, the efficacy, pharmacokinetics, safety, and immunogenicity of Yuflyma and reference adalimumab were comparable after 1 year of treatment, including after switching from reference adalimumab to Yuflyma.
“Currently, more than 80% of patients treated with Humira in the United States rely on a high-concentration and citrate-free formulation of this medication. The availability of a high-concentration and citrate-free formulation adalimumab biosimilar provides an important treatment option for patients with inflammatory diseases who benefit from this effective therapy,” said Jonathan Kay, MD, of the University of Massachusetts, Worcester, in the press release.
The citrate-free formulation is thought to lead to less pain on injection.
Yuflyma will be available in prefilled syringe and autoinjector administration options.
Celltrion USA plans to market the drug in the United States in July 2023. Following the initial launch of 40 mg/0.4 mL, the company plans to launch dose forms of 80 mg/0.8 mL and 20 mg/0.2 mL.
Celltrion USA is also seeking an interchangeability designation from the FDA following the completion of an interchangeability trial of 366 patients with chronic plaque psoriasis. The interchangeability designation would mean that patients successfully switched from Humira to Yuflyma multiple times in the trial. The interchangeability designation would allow pharmacists to autosubstitute Humira with Yuflyma. In these cases, individual state laws control how and whether physicians will be notified of this switch.
If interchangeability is approved for Yuflyma, which the company tentatively expects in the fourth quarter of 2024, it would be just the third interchangeable biosimilar approved by the FDA overall and the second adalimumab biosimilar to be designated as such, after adalimumab-adbm (Cyltezo) in October 2021.
Yuflyma was approved in Canada in December 2021 for 10 indications: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, adult ulcerative colitis, hidradenitis suppurativa, plaque psoriasis, adult uveitis, and pediatric uveitis.
In February 2022, the European Commission granted marketing authorization for Yuflyma across those 10 indications, as well as for nonradiographic axial spondyloarthritis, pediatric plaque psoriasis, and pediatric Crohn’s disease.
In April 2022, Celltrion USA signed a licensing agreement with AbbVie, the manufacturer of Humira. Under that agreement, Celltrion will pay royalties to AbbVie on sales of their individual biosimilars, and AbbVie agreed to drop all patent litigation.
The full prescribing information for Yuflyma is available here.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the biosimilar adalimumab-aaty (Yuflyma) in a citrate-free, high-concentration formulation, the manufacturer, Celltrion USA, announced today. It is the ninth biosimilar of adalimumab (Humira) to be approved in the United States.
Yuflyma is approved for the treatment of adult patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa. It is also approved for polyarticular juvenile idiopathic arthritis for patients aged 2 years or older, as well as for Crohn’s disease in adults and in pediatric patients aged 6 years or older.
The formulation was approved on the basis of a comprehensive data package of analytic, preclinical, and clinical studies, according to Celltrion USA, “demonstrating that Yuflyma is comparable to the reference product Humira in terms of efficacy, safety, pharmacokinetics, and immunogenicity up to 24 weeks and 1 year following treatment.”
The company conducted a double-blind, randomized phase 3 trial that compared switching from reference adalimumab to Yuflyma with continuing either reference adalimumab or Yuflyma for patients with active rheumatoid arthritis. In that trial, the efficacy, pharmacokinetics, safety, and immunogenicity of Yuflyma and reference adalimumab were comparable after 1 year of treatment, including after switching from reference adalimumab to Yuflyma.
“Currently, more than 80% of patients treated with Humira in the United States rely on a high-concentration and citrate-free formulation of this medication. The availability of a high-concentration and citrate-free formulation adalimumab biosimilar provides an important treatment option for patients with inflammatory diseases who benefit from this effective therapy,” said Jonathan Kay, MD, of the University of Massachusetts, Worcester, in the press release.
The citrate-free formulation is thought to lead to less pain on injection.
Yuflyma will be available in prefilled syringe and autoinjector administration options.
Celltrion USA plans to market the drug in the United States in July 2023. Following the initial launch of 40 mg/0.4 mL, the company plans to launch dose forms of 80 mg/0.8 mL and 20 mg/0.2 mL.
Celltrion USA is also seeking an interchangeability designation from the FDA following the completion of an interchangeability trial of 366 patients with chronic plaque psoriasis. The interchangeability designation would mean that patients successfully switched from Humira to Yuflyma multiple times in the trial. The interchangeability designation would allow pharmacists to autosubstitute Humira with Yuflyma. In these cases, individual state laws control how and whether physicians will be notified of this switch.
If interchangeability is approved for Yuflyma, which the company tentatively expects in the fourth quarter of 2024, it would be just the third interchangeable biosimilar approved by the FDA overall and the second adalimumab biosimilar to be designated as such, after adalimumab-adbm (Cyltezo) in October 2021.
Yuflyma was approved in Canada in December 2021 for 10 indications: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, adult ulcerative colitis, hidradenitis suppurativa, plaque psoriasis, adult uveitis, and pediatric uveitis.
In February 2022, the European Commission granted marketing authorization for Yuflyma across those 10 indications, as well as for nonradiographic axial spondyloarthritis, pediatric plaque psoriasis, and pediatric Crohn’s disease.
In April 2022, Celltrion USA signed a licensing agreement with AbbVie, the manufacturer of Humira. Under that agreement, Celltrion will pay royalties to AbbVie on sales of their individual biosimilars, and AbbVie agreed to drop all patent litigation.
The full prescribing information for Yuflyma is available here.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the biosimilar adalimumab-aaty (Yuflyma) in a citrate-free, high-concentration formulation, the manufacturer, Celltrion USA, announced today. It is the ninth biosimilar of adalimumab (Humira) to be approved in the United States.
Yuflyma is approved for the treatment of adult patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa. It is also approved for polyarticular juvenile idiopathic arthritis for patients aged 2 years or older, as well as for Crohn’s disease in adults and in pediatric patients aged 6 years or older.
The formulation was approved on the basis of a comprehensive data package of analytic, preclinical, and clinical studies, according to Celltrion USA, “demonstrating that Yuflyma is comparable to the reference product Humira in terms of efficacy, safety, pharmacokinetics, and immunogenicity up to 24 weeks and 1 year following treatment.”
The company conducted a double-blind, randomized phase 3 trial that compared switching from reference adalimumab to Yuflyma with continuing either reference adalimumab or Yuflyma for patients with active rheumatoid arthritis. In that trial, the efficacy, pharmacokinetics, safety, and immunogenicity of Yuflyma and reference adalimumab were comparable after 1 year of treatment, including after switching from reference adalimumab to Yuflyma.
“Currently, more than 80% of patients treated with Humira in the United States rely on a high-concentration and citrate-free formulation of this medication. The availability of a high-concentration and citrate-free formulation adalimumab biosimilar provides an important treatment option for patients with inflammatory diseases who benefit from this effective therapy,” said Jonathan Kay, MD, of the University of Massachusetts, Worcester, in the press release.
The citrate-free formulation is thought to lead to less pain on injection.
Yuflyma will be available in prefilled syringe and autoinjector administration options.
Celltrion USA plans to market the drug in the United States in July 2023. Following the initial launch of 40 mg/0.4 mL, the company plans to launch dose forms of 80 mg/0.8 mL and 20 mg/0.2 mL.
Celltrion USA is also seeking an interchangeability designation from the FDA following the completion of an interchangeability trial of 366 patients with chronic plaque psoriasis. The interchangeability designation would mean that patients successfully switched from Humira to Yuflyma multiple times in the trial. The interchangeability designation would allow pharmacists to autosubstitute Humira with Yuflyma. In these cases, individual state laws control how and whether physicians will be notified of this switch.
If interchangeability is approved for Yuflyma, which the company tentatively expects in the fourth quarter of 2024, it would be just the third interchangeable biosimilar approved by the FDA overall and the second adalimumab biosimilar to be designated as such, after adalimumab-adbm (Cyltezo) in October 2021.
Yuflyma was approved in Canada in December 2021 for 10 indications: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, adult ulcerative colitis, hidradenitis suppurativa, plaque psoriasis, adult uveitis, and pediatric uveitis.
In February 2022, the European Commission granted marketing authorization for Yuflyma across those 10 indications, as well as for nonradiographic axial spondyloarthritis, pediatric plaque psoriasis, and pediatric Crohn’s disease.
In April 2022, Celltrion USA signed a licensing agreement with AbbVie, the manufacturer of Humira. Under that agreement, Celltrion will pay royalties to AbbVie on sales of their individual biosimilars, and AbbVie agreed to drop all patent litigation.
The full prescribing information for Yuflyma is available here.
A version of this article first appeared on Medscape.com.
Sublingual immunotherapy stops onset and worsening of asthma
PARIS – The EfficAPSI study showed with real-world data that sublingual immunotherapy (SLIT) reduces the risks for asthma onset and the worsening of asthma symptoms for patients with allergic rhinitis. The research was presented at the 18th French-language allergy conference.
These results confirm that allergen immunotherapy, or “desensitization,” is indeed an etiologic treatment of this allergic condition.
SLIT encompasses personalized solutions created for an individual specifically for allergies to dust mites, grass, birch, cats, and so on. These preparations are commonly used by allergy specialists when establishing an AIT treatment plan.
In 2017, the French Health Authority published a report indicating that there was insufficient clinical proof regarding the efficacy of SLIT. It subsequently removed injectable forms of these allergen extracts from the list of drugs reimbursed by the state and reduced state reimbursement of sublingual SLIT preparations from 30% to 15%, a step it confirmed in March 2018 and that led to outrage from allergy specialists. The chair of the French allergy society at the time, Jocelyne Just, MD, PhD, argued that conducting double-blind, placebo-controlled studies for all types (grass pollen, birch pollen, dust mites, asthma, allergic rhinitis, subcutaneous injections, sublingual treatments, tablets, liquid preparations) would take decades. Furthermore, meta-analyses on the subject, despite being heterogeneous and unable to answer all questions, are indeed pointing to the effectiveness of SLIT. To supplement existing data and to answer the queries raised by the HAS, several studies have been launched, including EfficAPSI.
The pharmacoepidemiologic EfficAPSI study is the largest retrospective, real-world, longitudinal cohort study ever carried out regarding liquid SLIT using data stored in the French National Health Data System (SNDS). The primary objective of the study was to evaluate the real-world impact of liquid SLIT on the onset and worsening of asthma for patients with allergic rhinitis and to evaluate the impact of sublingual treatments on public health.
A cohort analysis of patients treated with SLIT and control patients treated for allergic rhinitis with or without treatment for asthma was carried out. The patients treated with SLIT for at least 2 consecutive years were anonymously selected from the SNDS using the Stallergenes Greer prescription database.
In all, 99,538 patients who received SLIT were compared with 333,082 control patients (those who had received treatment for allergic rhinitis without taking SLIT). Participants were stratified according to their treatment history for asthma and were paired using a propensity score to minimize comparison bias.
The main definition of the onset of asthma included the first prescription of an asthma medication, hospital admission for asthma, or a diagnosis of chronic asthma. The secondary definition omitted the prescription of any treatment, and the third (sensitive and specific) took into consideration an initial prescription of omalizumab or a prescription of three inhaled corticosteroids (ICSs) associated with or without a long-acting beta-2 agonist (LABA) for a period of 1 year, admission to the hospital, or chronic asthma.
Asthma risk reduced
Among patients with allergic rhinitis without preexisting asthma, liquid SLIT was associated with a significantly lower risk of asthma onset in comparison with the control group (primary hazard ratio: 0.77; secondary HR: 0.66; and tertiary HR: 0.62).
The risk reductions were significant and were consistent regardless of the allergens analyzed (tertiary HR, dust mites: 0.57; grass: 0.52) for all age groups. These new results that were based on the tertiary definition corroborate the results from the primary and secondary definitions.
said study co-author Philippe Devillier, MD, PhD, research director at the respiratory tract diseases center of Foch Hospital, Paris. “These results are consistent with previous studies in the same French health care database, as well as in a German database with SLIT preparations in tablet form. This not only confirms the soundness of the methodology but also the benefit of liquid SLIT as an etiological treatment of respiratory allergies.”
Risk for worsening
Furthermore, in the same study, liquid SLIT treatment was associated with a 27% reduced risk for worsening asthma and a 36% reduced risk for severe asthma. Among patients with allergic rhinitis and preexisting asthma, liquid SLIT was associated with a significantly lower risk for worsening of asthma, compared with the control group (primary HR: 0.73; secondary HR: 0.61; and tertiary HR: 0.64). The primary definition was an initial prescription of an ICS-LABA combination in a patient treated with ICS alone, severe exacerbation of asthma symptoms, hospital admission, or a diagnosis of chronic asthma.
“The risk reductions were significant and consistent for the allergens analyzed,” said study co-author Pascal Demoly, MD, PhD, head of pulmonology at Montpellier University Hospital, France (tertiary HR, dust mites: 0.66; grass: 0.59; birch: 0.34; and cats: 0.77). “This was across all age groups,” he added.
“The results of the EfficAPSI real-world study on health data from the SNDS are consistent with outcomes from clinical trials, suggestive of a reduced risk of asthma onset in patients with allergic rhinitis receiving liquid SLIT, as well as a reduced risk of worsening of preexisting asthma,” said Devillier. “SLIT, in this case in the form of a liquid, thus appears to be an effective etiological treatment, since the use of symptomatic drugs, in particular preventer inhalers, but also reliever inhalers, is lower in patients treated with SLIT over at least two consecutive years, compared with paired control subjects. And it’s the same for the risk of treating asthma in nonasthmatic patients at the start of the study. EfficAPSI is the largest study using data from a comprehensive state drug reimbursement database, allowing us to assess the impact of liquid SLIT on public health. These results, also obtained with other allergen preparations, particularly in tablet form in French and German studies using data from health care databases, demonstrate the consistency of the data regarding the efficacy of SLIT.”
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
PARIS – The EfficAPSI study showed with real-world data that sublingual immunotherapy (SLIT) reduces the risks for asthma onset and the worsening of asthma symptoms for patients with allergic rhinitis. The research was presented at the 18th French-language allergy conference.
These results confirm that allergen immunotherapy, or “desensitization,” is indeed an etiologic treatment of this allergic condition.
SLIT encompasses personalized solutions created for an individual specifically for allergies to dust mites, grass, birch, cats, and so on. These preparations are commonly used by allergy specialists when establishing an AIT treatment plan.
In 2017, the French Health Authority published a report indicating that there was insufficient clinical proof regarding the efficacy of SLIT. It subsequently removed injectable forms of these allergen extracts from the list of drugs reimbursed by the state and reduced state reimbursement of sublingual SLIT preparations from 30% to 15%, a step it confirmed in March 2018 and that led to outrage from allergy specialists. The chair of the French allergy society at the time, Jocelyne Just, MD, PhD, argued that conducting double-blind, placebo-controlled studies for all types (grass pollen, birch pollen, dust mites, asthma, allergic rhinitis, subcutaneous injections, sublingual treatments, tablets, liquid preparations) would take decades. Furthermore, meta-analyses on the subject, despite being heterogeneous and unable to answer all questions, are indeed pointing to the effectiveness of SLIT. To supplement existing data and to answer the queries raised by the HAS, several studies have been launched, including EfficAPSI.
The pharmacoepidemiologic EfficAPSI study is the largest retrospective, real-world, longitudinal cohort study ever carried out regarding liquid SLIT using data stored in the French National Health Data System (SNDS). The primary objective of the study was to evaluate the real-world impact of liquid SLIT on the onset and worsening of asthma for patients with allergic rhinitis and to evaluate the impact of sublingual treatments on public health.
A cohort analysis of patients treated with SLIT and control patients treated for allergic rhinitis with or without treatment for asthma was carried out. The patients treated with SLIT for at least 2 consecutive years were anonymously selected from the SNDS using the Stallergenes Greer prescription database.
In all, 99,538 patients who received SLIT were compared with 333,082 control patients (those who had received treatment for allergic rhinitis without taking SLIT). Participants were stratified according to their treatment history for asthma and were paired using a propensity score to minimize comparison bias.
The main definition of the onset of asthma included the first prescription of an asthma medication, hospital admission for asthma, or a diagnosis of chronic asthma. The secondary definition omitted the prescription of any treatment, and the third (sensitive and specific) took into consideration an initial prescription of omalizumab or a prescription of three inhaled corticosteroids (ICSs) associated with or without a long-acting beta-2 agonist (LABA) for a period of 1 year, admission to the hospital, or chronic asthma.
Asthma risk reduced
Among patients with allergic rhinitis without preexisting asthma, liquid SLIT was associated with a significantly lower risk of asthma onset in comparison with the control group (primary hazard ratio: 0.77; secondary HR: 0.66; and tertiary HR: 0.62).
The risk reductions were significant and were consistent regardless of the allergens analyzed (tertiary HR, dust mites: 0.57; grass: 0.52) for all age groups. These new results that were based on the tertiary definition corroborate the results from the primary and secondary definitions.
said study co-author Philippe Devillier, MD, PhD, research director at the respiratory tract diseases center of Foch Hospital, Paris. “These results are consistent with previous studies in the same French health care database, as well as in a German database with SLIT preparations in tablet form. This not only confirms the soundness of the methodology but also the benefit of liquid SLIT as an etiological treatment of respiratory allergies.”
Risk for worsening
Furthermore, in the same study, liquid SLIT treatment was associated with a 27% reduced risk for worsening asthma and a 36% reduced risk for severe asthma. Among patients with allergic rhinitis and preexisting asthma, liquid SLIT was associated with a significantly lower risk for worsening of asthma, compared with the control group (primary HR: 0.73; secondary HR: 0.61; and tertiary HR: 0.64). The primary definition was an initial prescription of an ICS-LABA combination in a patient treated with ICS alone, severe exacerbation of asthma symptoms, hospital admission, or a diagnosis of chronic asthma.
“The risk reductions were significant and consistent for the allergens analyzed,” said study co-author Pascal Demoly, MD, PhD, head of pulmonology at Montpellier University Hospital, France (tertiary HR, dust mites: 0.66; grass: 0.59; birch: 0.34; and cats: 0.77). “This was across all age groups,” he added.
“The results of the EfficAPSI real-world study on health data from the SNDS are consistent with outcomes from clinical trials, suggestive of a reduced risk of asthma onset in patients with allergic rhinitis receiving liquid SLIT, as well as a reduced risk of worsening of preexisting asthma,” said Devillier. “SLIT, in this case in the form of a liquid, thus appears to be an effective etiological treatment, since the use of symptomatic drugs, in particular preventer inhalers, but also reliever inhalers, is lower in patients treated with SLIT over at least two consecutive years, compared with paired control subjects. And it’s the same for the risk of treating asthma in nonasthmatic patients at the start of the study. EfficAPSI is the largest study using data from a comprehensive state drug reimbursement database, allowing us to assess the impact of liquid SLIT on public health. These results, also obtained with other allergen preparations, particularly in tablet form in French and German studies using data from health care databases, demonstrate the consistency of the data regarding the efficacy of SLIT.”
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
PARIS – The EfficAPSI study showed with real-world data that sublingual immunotherapy (SLIT) reduces the risks for asthma onset and the worsening of asthma symptoms for patients with allergic rhinitis. The research was presented at the 18th French-language allergy conference.
These results confirm that allergen immunotherapy, or “desensitization,” is indeed an etiologic treatment of this allergic condition.
SLIT encompasses personalized solutions created for an individual specifically for allergies to dust mites, grass, birch, cats, and so on. These preparations are commonly used by allergy specialists when establishing an AIT treatment plan.
In 2017, the French Health Authority published a report indicating that there was insufficient clinical proof regarding the efficacy of SLIT. It subsequently removed injectable forms of these allergen extracts from the list of drugs reimbursed by the state and reduced state reimbursement of sublingual SLIT preparations from 30% to 15%, a step it confirmed in March 2018 and that led to outrage from allergy specialists. The chair of the French allergy society at the time, Jocelyne Just, MD, PhD, argued that conducting double-blind, placebo-controlled studies for all types (grass pollen, birch pollen, dust mites, asthma, allergic rhinitis, subcutaneous injections, sublingual treatments, tablets, liquid preparations) would take decades. Furthermore, meta-analyses on the subject, despite being heterogeneous and unable to answer all questions, are indeed pointing to the effectiveness of SLIT. To supplement existing data and to answer the queries raised by the HAS, several studies have been launched, including EfficAPSI.
The pharmacoepidemiologic EfficAPSI study is the largest retrospective, real-world, longitudinal cohort study ever carried out regarding liquid SLIT using data stored in the French National Health Data System (SNDS). The primary objective of the study was to evaluate the real-world impact of liquid SLIT on the onset and worsening of asthma for patients with allergic rhinitis and to evaluate the impact of sublingual treatments on public health.
A cohort analysis of patients treated with SLIT and control patients treated for allergic rhinitis with or without treatment for asthma was carried out. The patients treated with SLIT for at least 2 consecutive years were anonymously selected from the SNDS using the Stallergenes Greer prescription database.
In all, 99,538 patients who received SLIT were compared with 333,082 control patients (those who had received treatment for allergic rhinitis without taking SLIT). Participants were stratified according to their treatment history for asthma and were paired using a propensity score to minimize comparison bias.
The main definition of the onset of asthma included the first prescription of an asthma medication, hospital admission for asthma, or a diagnosis of chronic asthma. The secondary definition omitted the prescription of any treatment, and the third (sensitive and specific) took into consideration an initial prescription of omalizumab or a prescription of three inhaled corticosteroids (ICSs) associated with or without a long-acting beta-2 agonist (LABA) for a period of 1 year, admission to the hospital, or chronic asthma.
Asthma risk reduced
Among patients with allergic rhinitis without preexisting asthma, liquid SLIT was associated with a significantly lower risk of asthma onset in comparison with the control group (primary hazard ratio: 0.77; secondary HR: 0.66; and tertiary HR: 0.62).
The risk reductions were significant and were consistent regardless of the allergens analyzed (tertiary HR, dust mites: 0.57; grass: 0.52) for all age groups. These new results that were based on the tertiary definition corroborate the results from the primary and secondary definitions.
said study co-author Philippe Devillier, MD, PhD, research director at the respiratory tract diseases center of Foch Hospital, Paris. “These results are consistent with previous studies in the same French health care database, as well as in a German database with SLIT preparations in tablet form. This not only confirms the soundness of the methodology but also the benefit of liquid SLIT as an etiological treatment of respiratory allergies.”
Risk for worsening
Furthermore, in the same study, liquid SLIT treatment was associated with a 27% reduced risk for worsening asthma and a 36% reduced risk for severe asthma. Among patients with allergic rhinitis and preexisting asthma, liquid SLIT was associated with a significantly lower risk for worsening of asthma, compared with the control group (primary HR: 0.73; secondary HR: 0.61; and tertiary HR: 0.64). The primary definition was an initial prescription of an ICS-LABA combination in a patient treated with ICS alone, severe exacerbation of asthma symptoms, hospital admission, or a diagnosis of chronic asthma.
“The risk reductions were significant and consistent for the allergens analyzed,” said study co-author Pascal Demoly, MD, PhD, head of pulmonology at Montpellier University Hospital, France (tertiary HR, dust mites: 0.66; grass: 0.59; birch: 0.34; and cats: 0.77). “This was across all age groups,” he added.
“The results of the EfficAPSI real-world study on health data from the SNDS are consistent with outcomes from clinical trials, suggestive of a reduced risk of asthma onset in patients with allergic rhinitis receiving liquid SLIT, as well as a reduced risk of worsening of preexisting asthma,” said Devillier. “SLIT, in this case in the form of a liquid, thus appears to be an effective etiological treatment, since the use of symptomatic drugs, in particular preventer inhalers, but also reliever inhalers, is lower in patients treated with SLIT over at least two consecutive years, compared with paired control subjects. And it’s the same for the risk of treating asthma in nonasthmatic patients at the start of the study. EfficAPSI is the largest study using data from a comprehensive state drug reimbursement database, allowing us to assess the impact of liquid SLIT on public health. These results, also obtained with other allergen preparations, particularly in tablet form in French and German studies using data from health care databases, demonstrate the consistency of the data regarding the efficacy of SLIT.”
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Circulating tumor DNA may predict poor prognosis in breast cancer
a new meta-analysis and systematic review found.
“Circulating tumor DNA (ctDNA) has been extensively studied as a prognostic biomarker in early breast cancer. However, there is a significant heterogeneity in the study results, which is probably related to the fact that each individual study included different patient populations, collected blood at different time points, and used different methods (assays) for ctDNA analysis,” said Guilherme Nader Marta, MD, of the Institut Jules Bordet, Anderlecht, Belgium, in an interview.
“The aim of our study was to summarize the available evidence that has been presented so far on this topic by performing a systematic review and meta-analysis including studies that reported the association between ctDNA detection and long-term outcomes,” said Dr. Nader Marta, who coauthored the new research, which was presented as a poster (Poster 26P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
Methods and results
The authors identified 57 studies including data from 5,729 individuals with early breast cancer. The 44.5% for whom stages were reported consisted of 18.3% with stage I disease, 60.0% with stage II, and 21.5% with stage III. Patients’ ctDNA collection was divided into three groups: baseline, after neoadjuvant therapy (End-of-NAT), and during follow-up care; ctDNA assays were classified as tumor-informed or non–tumor-informed.
The detection of ctDNA at any time point during diagnosis and treatment was associated with worse disease-free survival (DFS) and overall survival (OS), compared with no ctDNA. The association was stronger in tumor-informed assays, the researchers said.
For disease-free survival, the overall multivariate hazard ratios were 2.5, 5.5, and 7.2 for ctDNA detection at baseline, End-of-NAT, and follow-up, respectively.
For overall survival, the overall multivariate hazard ratios were 3.0, 12.9, and 5.6, for ctDNA detection at baseline, End-of-NAT, and follow-up, respectively.
The pooled hazard ratios were numerically higher for both DFS and OS when ctDNA was detected at either End-of-NAT or follow-up.
In addition, detection of ctDNA was associated with a high degree of specificity (from 0.7 to 1.0) for breast cancer relapse; sensitivity ranged from 0.31 to 1.0, the researchers noted. The mean lead time from ctDNA detection to breast cancer recurrence in these cases was approximately 10 months.
Results show ctDNA detection is associated with worse survival
“Our study results demonstrate that ctDNA detection is associated with worse disease-free survival and overall survival in patients with early breast cancer, particularly when measured after treatment with tumor-informed assays,” Dr. Nader Marta said in an interview.
“As next steps, we need to build on this evidence to bring the potential benefits of this powerful prognostic tool to our patients,” said Dr. Nader Marta. “Ongoing studies exploring different management strategies based on serial ctDNA assessments will help us understand the exact role of this technology in our clinical practice.”
The study received no outside funding. Dr. Nader Marta disclosed relationships with companies including Roche and Bayer.
a new meta-analysis and systematic review found.
“Circulating tumor DNA (ctDNA) has been extensively studied as a prognostic biomarker in early breast cancer. However, there is a significant heterogeneity in the study results, which is probably related to the fact that each individual study included different patient populations, collected blood at different time points, and used different methods (assays) for ctDNA analysis,” said Guilherme Nader Marta, MD, of the Institut Jules Bordet, Anderlecht, Belgium, in an interview.
“The aim of our study was to summarize the available evidence that has been presented so far on this topic by performing a systematic review and meta-analysis including studies that reported the association between ctDNA detection and long-term outcomes,” said Dr. Nader Marta, who coauthored the new research, which was presented as a poster (Poster 26P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
Methods and results
The authors identified 57 studies including data from 5,729 individuals with early breast cancer. The 44.5% for whom stages were reported consisted of 18.3% with stage I disease, 60.0% with stage II, and 21.5% with stage III. Patients’ ctDNA collection was divided into three groups: baseline, after neoadjuvant therapy (End-of-NAT), and during follow-up care; ctDNA assays were classified as tumor-informed or non–tumor-informed.
The detection of ctDNA at any time point during diagnosis and treatment was associated with worse disease-free survival (DFS) and overall survival (OS), compared with no ctDNA. The association was stronger in tumor-informed assays, the researchers said.
For disease-free survival, the overall multivariate hazard ratios were 2.5, 5.5, and 7.2 for ctDNA detection at baseline, End-of-NAT, and follow-up, respectively.
For overall survival, the overall multivariate hazard ratios were 3.0, 12.9, and 5.6, for ctDNA detection at baseline, End-of-NAT, and follow-up, respectively.
The pooled hazard ratios were numerically higher for both DFS and OS when ctDNA was detected at either End-of-NAT or follow-up.
In addition, detection of ctDNA was associated with a high degree of specificity (from 0.7 to 1.0) for breast cancer relapse; sensitivity ranged from 0.31 to 1.0, the researchers noted. The mean lead time from ctDNA detection to breast cancer recurrence in these cases was approximately 10 months.
Results show ctDNA detection is associated with worse survival
“Our study results demonstrate that ctDNA detection is associated with worse disease-free survival and overall survival in patients with early breast cancer, particularly when measured after treatment with tumor-informed assays,” Dr. Nader Marta said in an interview.
“As next steps, we need to build on this evidence to bring the potential benefits of this powerful prognostic tool to our patients,” said Dr. Nader Marta. “Ongoing studies exploring different management strategies based on serial ctDNA assessments will help us understand the exact role of this technology in our clinical practice.”
The study received no outside funding. Dr. Nader Marta disclosed relationships with companies including Roche and Bayer.
a new meta-analysis and systematic review found.
“Circulating tumor DNA (ctDNA) has been extensively studied as a prognostic biomarker in early breast cancer. However, there is a significant heterogeneity in the study results, which is probably related to the fact that each individual study included different patient populations, collected blood at different time points, and used different methods (assays) for ctDNA analysis,” said Guilherme Nader Marta, MD, of the Institut Jules Bordet, Anderlecht, Belgium, in an interview.
“The aim of our study was to summarize the available evidence that has been presented so far on this topic by performing a systematic review and meta-analysis including studies that reported the association between ctDNA detection and long-term outcomes,” said Dr. Nader Marta, who coauthored the new research, which was presented as a poster (Poster 26P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
Methods and results
The authors identified 57 studies including data from 5,729 individuals with early breast cancer. The 44.5% for whom stages were reported consisted of 18.3% with stage I disease, 60.0% with stage II, and 21.5% with stage III. Patients’ ctDNA collection was divided into three groups: baseline, after neoadjuvant therapy (End-of-NAT), and during follow-up care; ctDNA assays were classified as tumor-informed or non–tumor-informed.
The detection of ctDNA at any time point during diagnosis and treatment was associated with worse disease-free survival (DFS) and overall survival (OS), compared with no ctDNA. The association was stronger in tumor-informed assays, the researchers said.
For disease-free survival, the overall multivariate hazard ratios were 2.5, 5.5, and 7.2 for ctDNA detection at baseline, End-of-NAT, and follow-up, respectively.
For overall survival, the overall multivariate hazard ratios were 3.0, 12.9, and 5.6, for ctDNA detection at baseline, End-of-NAT, and follow-up, respectively.
The pooled hazard ratios were numerically higher for both DFS and OS when ctDNA was detected at either End-of-NAT or follow-up.
In addition, detection of ctDNA was associated with a high degree of specificity (from 0.7 to 1.0) for breast cancer relapse; sensitivity ranged from 0.31 to 1.0, the researchers noted. The mean lead time from ctDNA detection to breast cancer recurrence in these cases was approximately 10 months.
Results show ctDNA detection is associated with worse survival
“Our study results demonstrate that ctDNA detection is associated with worse disease-free survival and overall survival in patients with early breast cancer, particularly when measured after treatment with tumor-informed assays,” Dr. Nader Marta said in an interview.
“As next steps, we need to build on this evidence to bring the potential benefits of this powerful prognostic tool to our patients,” said Dr. Nader Marta. “Ongoing studies exploring different management strategies based on serial ctDNA assessments will help us understand the exact role of this technology in our clinical practice.”
The study received no outside funding. Dr. Nader Marta disclosed relationships with companies including Roche and Bayer.
ESMO BREAST CANCER 2023
Breast cancer outcomes are worse for Black men
A new study finds that racial disparities in male breast cancer are persisting in the United States.
From 2000 to 2019, Black men were diagnosed at later ages than White males (median ages, 69 and 63 years, respectively) and were more likely to die from the disease (22.4% vs. 16.8%, respectively). Male breast cancer (MBC) was more likely to kill Black men in rural vs. urban areas (hazard ratio = 1.4; 95% confidence interval, 1.0-2.1; P less than .05). Among White males, in contrast, there was no difference on that front, according to the research, which was presented in a poster (Abstract No. 87P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
It’s not clear why the disparities exist, said lead author Lekha Yadukumar, MBBS, an internal medicine resident at the Wright Center for Graduate Medical Education in Scranton, Penn., in an interview.
“Several potential factors may contribute to the higher rate of breast cancer diagnosis in older [Black] men, including the pathology of the disease, limited awareness about breast cancer, and potential barriers to accessibility,” she said. “The increased mortality among [Black men] may be linked to variations in tumor pathology and molecular biology. Social factors may also potentially impact survival rates, including [having] limited access to health care in rural areas and inadequate social support.”
Male breast cancer is rare, accounting for less than 1% of all breast cancer cases in the United States, according to the Breast Cancer Research Foundation. An estimated 2,700 men are diagnosed each year, and about 530 will die. Previous research has suggested Black men have worse outcomes than White men, but the data covered earlier years than the new study.
Methods and results
Dr. Yadukumar and colleagues retrospectively analyzed statistics from the Surveillance, Epidemiology, and End Results database for patients diagnosed with primary male breast cancer from 2000 to 2019 (n = 8,373; Black men, 1,111 [13.26%]; White men, 6,817 [81.41%]).
Median income didn’t affect mortality, whereas men in both racial groups were less likely to die if they were married vs. single/divorced (hazard ratio = 0.6; P less than .05).
Other studies have shown that “[Black American] men diagnosed with breast cancer experience longer time intervals before receiving treatment, encounter more severe disease manifestations, and exhibit lower rates of survivorship,” Dr. Yadukumar said. “Despite these findings, there remains a scarcity of genetic studies aimed at comprehending the underlying causes of these disparities. Moreover, there is a dearth of research investigating other factors that may influence survival outcomes among men with breast cancer.”
Findings reflect the disparities in female breast cancer
In an interview, Duke University, Durham, N.C., oncologist Arif Kamal, MD, MBA, MHS, the chief patient officer at the American Cancer Society, said the study is impressive since the number of patients is large for a rare cancer and the population is diverse. Plus, the findings reflect the disparities in female breast cancer, he noted.
“We know that Black women’s mortality is worse vs. White women in breast cancer, and we believe that most of that has nothing to do with cancer screening,” said Dr. Kamal, who was not involved in the new study. “When the clock starts from diagnosis onwards, you start to see less introduction to clinical trials and standard care medications and more time to treatment, surgery, and radiation,” he said.
“You see similar disparities as related to mortality in Black vs. White men,” he noted.
The new findings about higher death rates for Black men, especially in rural areas, suggest that “distance matters, and race matters,” he said. In rural areas, it can be hard to access pathologists, radiologists, and surgeons with more experience with breast cancer, he said.
But, he noted, the study finds that income doesn’t appear to be a factor.
In the big picture, he said, the results suggest that when it comes to barriers to better outcomes, “things that are systemic don’t make exceptions because you are a man vs. a woman.”
No study funding was reported. The study authors and Dr. Kamal have no relevant financial disclosures.
A new study finds that racial disparities in male breast cancer are persisting in the United States.
From 2000 to 2019, Black men were diagnosed at later ages than White males (median ages, 69 and 63 years, respectively) and were more likely to die from the disease (22.4% vs. 16.8%, respectively). Male breast cancer (MBC) was more likely to kill Black men in rural vs. urban areas (hazard ratio = 1.4; 95% confidence interval, 1.0-2.1; P less than .05). Among White males, in contrast, there was no difference on that front, according to the research, which was presented in a poster (Abstract No. 87P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
It’s not clear why the disparities exist, said lead author Lekha Yadukumar, MBBS, an internal medicine resident at the Wright Center for Graduate Medical Education in Scranton, Penn., in an interview.
“Several potential factors may contribute to the higher rate of breast cancer diagnosis in older [Black] men, including the pathology of the disease, limited awareness about breast cancer, and potential barriers to accessibility,” she said. “The increased mortality among [Black men] may be linked to variations in tumor pathology and molecular biology. Social factors may also potentially impact survival rates, including [having] limited access to health care in rural areas and inadequate social support.”
Male breast cancer is rare, accounting for less than 1% of all breast cancer cases in the United States, according to the Breast Cancer Research Foundation. An estimated 2,700 men are diagnosed each year, and about 530 will die. Previous research has suggested Black men have worse outcomes than White men, but the data covered earlier years than the new study.
Methods and results
Dr. Yadukumar and colleagues retrospectively analyzed statistics from the Surveillance, Epidemiology, and End Results database for patients diagnosed with primary male breast cancer from 2000 to 2019 (n = 8,373; Black men, 1,111 [13.26%]; White men, 6,817 [81.41%]).
Median income didn’t affect mortality, whereas men in both racial groups were less likely to die if they were married vs. single/divorced (hazard ratio = 0.6; P less than .05).
Other studies have shown that “[Black American] men diagnosed with breast cancer experience longer time intervals before receiving treatment, encounter more severe disease manifestations, and exhibit lower rates of survivorship,” Dr. Yadukumar said. “Despite these findings, there remains a scarcity of genetic studies aimed at comprehending the underlying causes of these disparities. Moreover, there is a dearth of research investigating other factors that may influence survival outcomes among men with breast cancer.”
Findings reflect the disparities in female breast cancer
In an interview, Duke University, Durham, N.C., oncologist Arif Kamal, MD, MBA, MHS, the chief patient officer at the American Cancer Society, said the study is impressive since the number of patients is large for a rare cancer and the population is diverse. Plus, the findings reflect the disparities in female breast cancer, he noted.
“We know that Black women’s mortality is worse vs. White women in breast cancer, and we believe that most of that has nothing to do with cancer screening,” said Dr. Kamal, who was not involved in the new study. “When the clock starts from diagnosis onwards, you start to see less introduction to clinical trials and standard care medications and more time to treatment, surgery, and radiation,” he said.
“You see similar disparities as related to mortality in Black vs. White men,” he noted.
The new findings about higher death rates for Black men, especially in rural areas, suggest that “distance matters, and race matters,” he said. In rural areas, it can be hard to access pathologists, radiologists, and surgeons with more experience with breast cancer, he said.
But, he noted, the study finds that income doesn’t appear to be a factor.
In the big picture, he said, the results suggest that when it comes to barriers to better outcomes, “things that are systemic don’t make exceptions because you are a man vs. a woman.”
No study funding was reported. The study authors and Dr. Kamal have no relevant financial disclosures.
A new study finds that racial disparities in male breast cancer are persisting in the United States.
From 2000 to 2019, Black men were diagnosed at later ages than White males (median ages, 69 and 63 years, respectively) and were more likely to die from the disease (22.4% vs. 16.8%, respectively). Male breast cancer (MBC) was more likely to kill Black men in rural vs. urban areas (hazard ratio = 1.4; 95% confidence interval, 1.0-2.1; P less than .05). Among White males, in contrast, there was no difference on that front, according to the research, which was presented in a poster (Abstract No. 87P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
It’s not clear why the disparities exist, said lead author Lekha Yadukumar, MBBS, an internal medicine resident at the Wright Center for Graduate Medical Education in Scranton, Penn., in an interview.
“Several potential factors may contribute to the higher rate of breast cancer diagnosis in older [Black] men, including the pathology of the disease, limited awareness about breast cancer, and potential barriers to accessibility,” she said. “The increased mortality among [Black men] may be linked to variations in tumor pathology and molecular biology. Social factors may also potentially impact survival rates, including [having] limited access to health care in rural areas and inadequate social support.”
Male breast cancer is rare, accounting for less than 1% of all breast cancer cases in the United States, according to the Breast Cancer Research Foundation. An estimated 2,700 men are diagnosed each year, and about 530 will die. Previous research has suggested Black men have worse outcomes than White men, but the data covered earlier years than the new study.
Methods and results
Dr. Yadukumar and colleagues retrospectively analyzed statistics from the Surveillance, Epidemiology, and End Results database for patients diagnosed with primary male breast cancer from 2000 to 2019 (n = 8,373; Black men, 1,111 [13.26%]; White men, 6,817 [81.41%]).
Median income didn’t affect mortality, whereas men in both racial groups were less likely to die if they were married vs. single/divorced (hazard ratio = 0.6; P less than .05).
Other studies have shown that “[Black American] men diagnosed with breast cancer experience longer time intervals before receiving treatment, encounter more severe disease manifestations, and exhibit lower rates of survivorship,” Dr. Yadukumar said. “Despite these findings, there remains a scarcity of genetic studies aimed at comprehending the underlying causes of these disparities. Moreover, there is a dearth of research investigating other factors that may influence survival outcomes among men with breast cancer.”
Findings reflect the disparities in female breast cancer
In an interview, Duke University, Durham, N.C., oncologist Arif Kamal, MD, MBA, MHS, the chief patient officer at the American Cancer Society, said the study is impressive since the number of patients is large for a rare cancer and the population is diverse. Plus, the findings reflect the disparities in female breast cancer, he noted.
“We know that Black women’s mortality is worse vs. White women in breast cancer, and we believe that most of that has nothing to do with cancer screening,” said Dr. Kamal, who was not involved in the new study. “When the clock starts from diagnosis onwards, you start to see less introduction to clinical trials and standard care medications and more time to treatment, surgery, and radiation,” he said.
“You see similar disparities as related to mortality in Black vs. White men,” he noted.
The new findings about higher death rates for Black men, especially in rural areas, suggest that “distance matters, and race matters,” he said. In rural areas, it can be hard to access pathologists, radiologists, and surgeons with more experience with breast cancer, he said.
But, he noted, the study finds that income doesn’t appear to be a factor.
In the big picture, he said, the results suggest that when it comes to barriers to better outcomes, “things that are systemic don’t make exceptions because you are a man vs. a woman.”
No study funding was reported. The study authors and Dr. Kamal have no relevant financial disclosures.
FROM ESMO BREAST CANCER 2023
Eating disorder apps fall short when it comes to privacy
SAN FRANCISCO –
Federal laws require those handling sensitive health information to have policies and security safeguards in place to protect such information, whether it’s stored on paper or electronically.
“As it stands right now, there’s not enough evidence to support using these apps as an adjunct to clinical care,” study author Theodora O’Leary, a 4th-year medical student at Tufts University, Boston, said in an interview. “We need more research on the efficacy of these apps because right now not enough of them meet HIPAA [standards] and don’t have privacy and security measures.”
The findings were presented at the annual meeting of the American Psychiatric Association.
Eating disorders (EDs) are a common mental health condition affecting almost 1 in 10 Americans over their lifetime. Yet only about a third of patients with an eating disorder receive adequate treatment.
The pandemic saw a rise in eating disorders and in the use of mental health apps “to kind of fill the gap because people couldn’t be seen in person,” said Ms. O’Leary.
Inexpensive and accessible
Smartphone apps have a lot of advantages for patients with an ED. For one thing, they’re relatively inexpensive and accessible; most Americans already own one or more devices on which these apps can be used.
They’re also a feasible means of delivering psychological interventions, which are often recommended for EDs. Among these interventions, cognitive-behavioral therapy (CBT) has the largest evidence base for this condition.
Also, as many individuals with an ED may be reluctant to seek treatment because of stigma and shame, the anonymity afforded by an app could increase access to the help they seek.
But Ms. O’Leary warned the Food and Drug Administration does not regulate these apps, and people are sharing their personal health information on them.
The researchers conducted a review of commercially available eating disorder apps by searching the Apple and Google play stores using key phrases such as “eating disorder,” “anorexia,” and “binge eating disorder.”
They found 16 relevant apps that they added to the publicly available apps already in a database at Tufts, for a total of about 36 that were evaluated in the study (the number fluctuates as apps are deleted.)
They then reviewed the apps using the 105 questions based on the APA’s app evaluation model, which covers categories such as efficacy, privacy, accessibility, and clinical applicability. And they used filters to group apps by characteristics such as function, cost, and features.
The vast majority were self-help apps, which include things like journaling, meditation, and information on CBT. Others were reference apps that provide related definitions and sometimes include surveys to determine, for example, if the user has an eating disorder.
About 44% of the apps track mood, and 53% track symptoms. Some 56% include journaling, 42% mindfulness, 53% goal setting, and 42% psychoeducation.
Hybrid care
Only 5% of apps allow for “hybrid” care. This, explained Ms. O’Leary, is when clinicians use their own app to access patients’ apps, allowing them to track food restrictions and therapies, and make comments.
“The hybrid is viewed as the best form of app”, she said. “It’s almost like an adjunct to clinical care.”
Hybrid apps also tend to have patient safety features, she added. And these apps meet HIPAA standards, which only 11% of the apps in the study did.
Only 15% of apps advised patients to take steps in case of an emergency, and 11% had supporting studies. And where there was supporting research, much of it was funded by the app creators, said Ms. O’Leary.
For example, an app provided by Noom (the weight loss program that promises to help change habits and mindsets around food) “has a bunch of feasibility studies but they’re all funded by Noom”, which can introduce bias, she explained.
None of the apps were created by an accredited health care institution, she noted. “And I think only one app out of all the eating disorder apps we looked at was from a nonprofit.”
About 17% of the apps offer help with a “coach” or “expert”. However, these apps often fail to disclose the definition of a coach or state they’re not a replacement for medical care.
Coaching apps more expensive
Additionally, these coaching apps are often some of the most expensive, said Ms. O’Leary.
Daniel E. Gih, MD, associate professor at the University of Nebraska Medical Center, Omaha, helped start an eating disorders program at the University of Michigan and continues to treat patients with eating disorders. He said the increase in the use of eating disorder apps isn’t surprising as the incidence of these disorders increased in the wake of pandemic restrictions, especially among young people.
“Patients are likely doing more research on their medical conditions and trying to crowdsource information or self-treat,” Dr. Gih said.
It’s unclear whether “shame or just the general lack of specialized eating disorder professionals,” including physicians, is driving some of the interest in these apps, he added.
Dr. Gih stressed eating disorder apps should not only include screening for suicidality, but also explicitly tell patients to seek immediate attention if they show certain signs – for example, fainting, chest pain, or blood in emesis.
“The apps may be giving patients false hope or delaying medical care,” he said. “Apps are likely not sufficient enough to replace a multidisciplinary team with experience and expertise in eating disorders.”
Ms. O’Leary and Dr. Gih report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO –
Federal laws require those handling sensitive health information to have policies and security safeguards in place to protect such information, whether it’s stored on paper or electronically.
“As it stands right now, there’s not enough evidence to support using these apps as an adjunct to clinical care,” study author Theodora O’Leary, a 4th-year medical student at Tufts University, Boston, said in an interview. “We need more research on the efficacy of these apps because right now not enough of them meet HIPAA [standards] and don’t have privacy and security measures.”
The findings were presented at the annual meeting of the American Psychiatric Association.
Eating disorders (EDs) are a common mental health condition affecting almost 1 in 10 Americans over their lifetime. Yet only about a third of patients with an eating disorder receive adequate treatment.
The pandemic saw a rise in eating disorders and in the use of mental health apps “to kind of fill the gap because people couldn’t be seen in person,” said Ms. O’Leary.
Inexpensive and accessible
Smartphone apps have a lot of advantages for patients with an ED. For one thing, they’re relatively inexpensive and accessible; most Americans already own one or more devices on which these apps can be used.
They’re also a feasible means of delivering psychological interventions, which are often recommended for EDs. Among these interventions, cognitive-behavioral therapy (CBT) has the largest evidence base for this condition.
Also, as many individuals with an ED may be reluctant to seek treatment because of stigma and shame, the anonymity afforded by an app could increase access to the help they seek.
But Ms. O’Leary warned the Food and Drug Administration does not regulate these apps, and people are sharing their personal health information on them.
The researchers conducted a review of commercially available eating disorder apps by searching the Apple and Google play stores using key phrases such as “eating disorder,” “anorexia,” and “binge eating disorder.”
They found 16 relevant apps that they added to the publicly available apps already in a database at Tufts, for a total of about 36 that were evaluated in the study (the number fluctuates as apps are deleted.)
They then reviewed the apps using the 105 questions based on the APA’s app evaluation model, which covers categories such as efficacy, privacy, accessibility, and clinical applicability. And they used filters to group apps by characteristics such as function, cost, and features.
The vast majority were self-help apps, which include things like journaling, meditation, and information on CBT. Others were reference apps that provide related definitions and sometimes include surveys to determine, for example, if the user has an eating disorder.
About 44% of the apps track mood, and 53% track symptoms. Some 56% include journaling, 42% mindfulness, 53% goal setting, and 42% psychoeducation.
Hybrid care
Only 5% of apps allow for “hybrid” care. This, explained Ms. O’Leary, is when clinicians use their own app to access patients’ apps, allowing them to track food restrictions and therapies, and make comments.
“The hybrid is viewed as the best form of app”, she said. “It’s almost like an adjunct to clinical care.”
Hybrid apps also tend to have patient safety features, she added. And these apps meet HIPAA standards, which only 11% of the apps in the study did.
Only 15% of apps advised patients to take steps in case of an emergency, and 11% had supporting studies. And where there was supporting research, much of it was funded by the app creators, said Ms. O’Leary.
For example, an app provided by Noom (the weight loss program that promises to help change habits and mindsets around food) “has a bunch of feasibility studies but they’re all funded by Noom”, which can introduce bias, she explained.
None of the apps were created by an accredited health care institution, she noted. “And I think only one app out of all the eating disorder apps we looked at was from a nonprofit.”
About 17% of the apps offer help with a “coach” or “expert”. However, these apps often fail to disclose the definition of a coach or state they’re not a replacement for medical care.
Coaching apps more expensive
Additionally, these coaching apps are often some of the most expensive, said Ms. O’Leary.
Daniel E. Gih, MD, associate professor at the University of Nebraska Medical Center, Omaha, helped start an eating disorders program at the University of Michigan and continues to treat patients with eating disorders. He said the increase in the use of eating disorder apps isn’t surprising as the incidence of these disorders increased in the wake of pandemic restrictions, especially among young people.
“Patients are likely doing more research on their medical conditions and trying to crowdsource information or self-treat,” Dr. Gih said.
It’s unclear whether “shame or just the general lack of specialized eating disorder professionals,” including physicians, is driving some of the interest in these apps, he added.
Dr. Gih stressed eating disorder apps should not only include screening for suicidality, but also explicitly tell patients to seek immediate attention if they show certain signs – for example, fainting, chest pain, or blood in emesis.
“The apps may be giving patients false hope or delaying medical care,” he said. “Apps are likely not sufficient enough to replace a multidisciplinary team with experience and expertise in eating disorders.”
Ms. O’Leary and Dr. Gih report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO –
Federal laws require those handling sensitive health information to have policies and security safeguards in place to protect such information, whether it’s stored on paper or electronically.
“As it stands right now, there’s not enough evidence to support using these apps as an adjunct to clinical care,” study author Theodora O’Leary, a 4th-year medical student at Tufts University, Boston, said in an interview. “We need more research on the efficacy of these apps because right now not enough of them meet HIPAA [standards] and don’t have privacy and security measures.”
The findings were presented at the annual meeting of the American Psychiatric Association.
Eating disorders (EDs) are a common mental health condition affecting almost 1 in 10 Americans over their lifetime. Yet only about a third of patients with an eating disorder receive adequate treatment.
The pandemic saw a rise in eating disorders and in the use of mental health apps “to kind of fill the gap because people couldn’t be seen in person,” said Ms. O’Leary.
Inexpensive and accessible
Smartphone apps have a lot of advantages for patients with an ED. For one thing, they’re relatively inexpensive and accessible; most Americans already own one or more devices on which these apps can be used.
They’re also a feasible means of delivering psychological interventions, which are often recommended for EDs. Among these interventions, cognitive-behavioral therapy (CBT) has the largest evidence base for this condition.
Also, as many individuals with an ED may be reluctant to seek treatment because of stigma and shame, the anonymity afforded by an app could increase access to the help they seek.
But Ms. O’Leary warned the Food and Drug Administration does not regulate these apps, and people are sharing their personal health information on them.
The researchers conducted a review of commercially available eating disorder apps by searching the Apple and Google play stores using key phrases such as “eating disorder,” “anorexia,” and “binge eating disorder.”
They found 16 relevant apps that they added to the publicly available apps already in a database at Tufts, for a total of about 36 that were evaluated in the study (the number fluctuates as apps are deleted.)
They then reviewed the apps using the 105 questions based on the APA’s app evaluation model, which covers categories such as efficacy, privacy, accessibility, and clinical applicability. And they used filters to group apps by characteristics such as function, cost, and features.
The vast majority were self-help apps, which include things like journaling, meditation, and information on CBT. Others were reference apps that provide related definitions and sometimes include surveys to determine, for example, if the user has an eating disorder.
About 44% of the apps track mood, and 53% track symptoms. Some 56% include journaling, 42% mindfulness, 53% goal setting, and 42% psychoeducation.
Hybrid care
Only 5% of apps allow for “hybrid” care. This, explained Ms. O’Leary, is when clinicians use their own app to access patients’ apps, allowing them to track food restrictions and therapies, and make comments.
“The hybrid is viewed as the best form of app”, she said. “It’s almost like an adjunct to clinical care.”
Hybrid apps also tend to have patient safety features, she added. And these apps meet HIPAA standards, which only 11% of the apps in the study did.
Only 15% of apps advised patients to take steps in case of an emergency, and 11% had supporting studies. And where there was supporting research, much of it was funded by the app creators, said Ms. O’Leary.
For example, an app provided by Noom (the weight loss program that promises to help change habits and mindsets around food) “has a bunch of feasibility studies but they’re all funded by Noom”, which can introduce bias, she explained.
None of the apps were created by an accredited health care institution, she noted. “And I think only one app out of all the eating disorder apps we looked at was from a nonprofit.”
About 17% of the apps offer help with a “coach” or “expert”. However, these apps often fail to disclose the definition of a coach or state they’re not a replacement for medical care.
Coaching apps more expensive
Additionally, these coaching apps are often some of the most expensive, said Ms. O’Leary.
Daniel E. Gih, MD, associate professor at the University of Nebraska Medical Center, Omaha, helped start an eating disorders program at the University of Michigan and continues to treat patients with eating disorders. He said the increase in the use of eating disorder apps isn’t surprising as the incidence of these disorders increased in the wake of pandemic restrictions, especially among young people.
“Patients are likely doing more research on their medical conditions and trying to crowdsource information or self-treat,” Dr. Gih said.
It’s unclear whether “shame or just the general lack of specialized eating disorder professionals,” including physicians, is driving some of the interest in these apps, he added.
Dr. Gih stressed eating disorder apps should not only include screening for suicidality, but also explicitly tell patients to seek immediate attention if they show certain signs – for example, fainting, chest pain, or blood in emesis.
“The apps may be giving patients false hope or delaying medical care,” he said. “Apps are likely not sufficient enough to replace a multidisciplinary team with experience and expertise in eating disorders.”
Ms. O’Leary and Dr. Gih report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT APA 2023
Tweaking food delivery apps can lower calories purchased
DUBLIN – , show three new randomized trials from the United Kingdom.
The prominent positioning of low-calorie menu items, and restaurants with low-calorie main meals, on a food app emerged as the most promising approach to promote healthier eating, followed by preselecting smaller portions by default, and finally calorie labels, Anna Keleher, MPA, a behavioral scientist at Nesta, London, reported at the European Congress on Obesity (ECO) meeting.
“Many out-of-home meals have more calories than meals cooked in-home and using delivery apps is linked with a higher risk of becoming overweight or obese,” she remarked. “We’re interested in understanding more about delivery apps because they can be modified at scale easily and can reach millions of people with interventions to promote healthier and more nutritious options in these settings.”
Food delivery apps have surged in use in the United Kingdom with a 55% increase since 2015; examples include Uber Eats, Just Eat, and Deliveroo. “This trend is similar in the United States, with more and more consumers using delivery apps to buy food,” said Ms. Keleher, a senior adviser at the Behavioral Insights Team, New York.
Emma Boyland, PhD, an obesity psychologist from Liverpool (England) University, said: “Apps are an increasingly popular way for people to buy food and the virtual food environment is becoming as prominent as the physical food environment in how we go about obtaining meals.”
She highlighted the need to understand more about how food apps change the way we purchase and eat, but noted that “the work presented today” showed that “moving the position of food choices and information, as well as the brand name and imagery, influences what people end up buying and consuming.
“I think there’s a place for interventions that challenge these things and improve dietary health,” said Dr. Boyland, who chaired the session during which Ms. Keleher presented her results. “However, as we’ve seen with calorie labeling, they don’t always have the biggest effect on their own, so as is often the case, we need to take multiple actions, incorporating all the elements of the environment to make a meaningful difference.”
Three trials changing displays on simulated food delivery apps
“Delivery apps could reach millions of people and help us select healthier food options, and yet there is very little research looking at what works to promote healthier and more nutritious options in these settings,” Filippo Bianchi, MD, a colleague working with Ms. Keleher, said in a press release issued by ECO.
So the research team carried out a proof-of-concept testing of health-promoting interventions by developing a simulated food delivery app and asking 23,783 adults who typically use such services to choose a meal for themselves as if it were a real-life food delivery order.
“As a first step, we developed a simulated online food delivery platform to generate evidence on the effectiveness of our interventions,” Ms. Keleher explained, noting that the simulated platform included 21 restaurants and almost 600 food and drink items to choose from.
The research evaluated 14 interventions across three randomized controlled trials, displaying various food-ordering options that promoted lower-calorie options against a control. The trials investigated default choices (promoting the selection of small portion sizes through defaults, n = 6,000); positioning (promoting the selection of less calorie-dense options through positioning, n = 9,003); and labeling (promoting the selection of less calorific options through calorie labels, n = 8,780).
The primary outcome was the total number of calories in the basket at checkout. The results were adjusted for potentially confounding factors, such as body mass index, age, gender, and income.
For the trial that promoted smaller portions by default, “all of our interventions significantly reduced calorie purchases, with each additional intervention element increasing the effect sizes, which ranged from a 6% to 13% reduction in calories [–5.5% to –12.5% kcal/order; P < .05],” reported Ms. Keleher.
The second trial varied the position of both items on the menu and the order of restaurants – effectively, lower-calorie menu options were more prominent, and restaurant options with lower-calorie main meals were placed at the top of the restaurant selection page.
Ms. Keleher noted that there have been some concerns about whether this strategy would negatively affect restaurant business, so the research team counteracted this by also incorporating an option where low-calorie but high-price options were placed near the top of the display to promote healthier options but without loss of income for participating restaurants. This last intervention with low-calorie/high-price options placed near the top also led to reduced calorie intake.
“This showed that promoting low-calorie options does not necessarily mean damaging business revenue,” she said. “We hope that the industry can evolve to meet the widely recognized needs of society and consumers.”
Repositioning restaurants emerged as more effective than repositioning foods on the menu, while all interventions significantly reduced calorie purchases. “Effect sizes ranged from 6% to 15% reductions in calories purchased per order [P < .05],” reported Ms. Keleher.
The last trial tested seven calorie labels: four that changed the font size and location of the label, two that added a switch on/off filter for calorie label display, and one that was a calorie summary at checkout.
“All these standard calorie labels directionally reduced the number of excess calories with two [options] reaching statistical significance. Five out of seven labels significantly reduced calorie purchases with effect sizes ranging from 4.3% to –7.8% kcal/order (P < .05),” reported Ms. Keleher.
“This research is important for policymakers so they can understand the best way for companies to display calorie labels and what to include in regulations and guidelines,” she summarized.
Qualitative think-aloud study explored views around food delivery apps
Another piece of research, the think-aloud study, by the same authors, was presented at ECO, and explored how best to enhance the effectiveness and acceptability of calorie labels in food delivery apps in consultation with 20 adult delivery app users in the United Kingdom.
Researchers tried to document the range of views people have about calorie labels, including variation both between people and within an individual.
“For example, on a weekend, people might not want to engage with calories at all because they are more concerned to treat themselves, whereas at a mid-week lunch that same person might really want the ability to check the calorie content of their food,” Ms. Keleher reported.
She said that considerations varied significantly between people such that they described different ways in which calorie labeling impacted their food-ordering experience.
“Some people felt labels supported their existing intentions, whereas others felt labels built their knowledge. Still others felt calorie labels were insufficient to support their health and wanted more information, such as on macronutrients,” said Ms. Keleher, quoting one participant: “There’s no situation in which I would look at [calories]. I look at nutrients. I prefer the traffic light system [color-coding salt, fat, and sugar content],” she relayed.
The key recommendations based on the think-aloud study included providing a filter that allows users to switch calorie labels on and off; communicating recommended energy intake per meal (that is, 600 kcal) and not just per day (that is, 2,000 kcal); and avoiding framing calorie label messaging or formatting as judgmental (for example, red fonts).
“These studies provide encouraging proof-of-concept evidence that small tweaks in delivery apps could help many people to identify and select healthier foods. Testing similar initiatives with real restaurants and delivery apps will be important to assess the long-term impact of these interventions in the real world. Further research should also explore the best way to balance desired health impacts while minimizing effects on businesses and on cost-of-living concerns for consumers,” concluded Dr. Bianchi.
A version of this article first appeared on Medscape.com.
DUBLIN – , show three new randomized trials from the United Kingdom.
The prominent positioning of low-calorie menu items, and restaurants with low-calorie main meals, on a food app emerged as the most promising approach to promote healthier eating, followed by preselecting smaller portions by default, and finally calorie labels, Anna Keleher, MPA, a behavioral scientist at Nesta, London, reported at the European Congress on Obesity (ECO) meeting.
“Many out-of-home meals have more calories than meals cooked in-home and using delivery apps is linked with a higher risk of becoming overweight or obese,” she remarked. “We’re interested in understanding more about delivery apps because they can be modified at scale easily and can reach millions of people with interventions to promote healthier and more nutritious options in these settings.”
Food delivery apps have surged in use in the United Kingdom with a 55% increase since 2015; examples include Uber Eats, Just Eat, and Deliveroo. “This trend is similar in the United States, with more and more consumers using delivery apps to buy food,” said Ms. Keleher, a senior adviser at the Behavioral Insights Team, New York.
Emma Boyland, PhD, an obesity psychologist from Liverpool (England) University, said: “Apps are an increasingly popular way for people to buy food and the virtual food environment is becoming as prominent as the physical food environment in how we go about obtaining meals.”
She highlighted the need to understand more about how food apps change the way we purchase and eat, but noted that “the work presented today” showed that “moving the position of food choices and information, as well as the brand name and imagery, influences what people end up buying and consuming.
“I think there’s a place for interventions that challenge these things and improve dietary health,” said Dr. Boyland, who chaired the session during which Ms. Keleher presented her results. “However, as we’ve seen with calorie labeling, they don’t always have the biggest effect on their own, so as is often the case, we need to take multiple actions, incorporating all the elements of the environment to make a meaningful difference.”
Three trials changing displays on simulated food delivery apps
“Delivery apps could reach millions of people and help us select healthier food options, and yet there is very little research looking at what works to promote healthier and more nutritious options in these settings,” Filippo Bianchi, MD, a colleague working with Ms. Keleher, said in a press release issued by ECO.
So the research team carried out a proof-of-concept testing of health-promoting interventions by developing a simulated food delivery app and asking 23,783 adults who typically use such services to choose a meal for themselves as if it were a real-life food delivery order.
“As a first step, we developed a simulated online food delivery platform to generate evidence on the effectiveness of our interventions,” Ms. Keleher explained, noting that the simulated platform included 21 restaurants and almost 600 food and drink items to choose from.
The research evaluated 14 interventions across three randomized controlled trials, displaying various food-ordering options that promoted lower-calorie options against a control. The trials investigated default choices (promoting the selection of small portion sizes through defaults, n = 6,000); positioning (promoting the selection of less calorie-dense options through positioning, n = 9,003); and labeling (promoting the selection of less calorific options through calorie labels, n = 8,780).
The primary outcome was the total number of calories in the basket at checkout. The results were adjusted for potentially confounding factors, such as body mass index, age, gender, and income.
For the trial that promoted smaller portions by default, “all of our interventions significantly reduced calorie purchases, with each additional intervention element increasing the effect sizes, which ranged from a 6% to 13% reduction in calories [–5.5% to –12.5% kcal/order; P < .05],” reported Ms. Keleher.
The second trial varied the position of both items on the menu and the order of restaurants – effectively, lower-calorie menu options were more prominent, and restaurant options with lower-calorie main meals were placed at the top of the restaurant selection page.
Ms. Keleher noted that there have been some concerns about whether this strategy would negatively affect restaurant business, so the research team counteracted this by also incorporating an option where low-calorie but high-price options were placed near the top of the display to promote healthier options but without loss of income for participating restaurants. This last intervention with low-calorie/high-price options placed near the top also led to reduced calorie intake.
“This showed that promoting low-calorie options does not necessarily mean damaging business revenue,” she said. “We hope that the industry can evolve to meet the widely recognized needs of society and consumers.”
Repositioning restaurants emerged as more effective than repositioning foods on the menu, while all interventions significantly reduced calorie purchases. “Effect sizes ranged from 6% to 15% reductions in calories purchased per order [P < .05],” reported Ms. Keleher.
The last trial tested seven calorie labels: four that changed the font size and location of the label, two that added a switch on/off filter for calorie label display, and one that was a calorie summary at checkout.
“All these standard calorie labels directionally reduced the number of excess calories with two [options] reaching statistical significance. Five out of seven labels significantly reduced calorie purchases with effect sizes ranging from 4.3% to –7.8% kcal/order (P < .05),” reported Ms. Keleher.
“This research is important for policymakers so they can understand the best way for companies to display calorie labels and what to include in regulations and guidelines,” she summarized.
Qualitative think-aloud study explored views around food delivery apps
Another piece of research, the think-aloud study, by the same authors, was presented at ECO, and explored how best to enhance the effectiveness and acceptability of calorie labels in food delivery apps in consultation with 20 adult delivery app users in the United Kingdom.
Researchers tried to document the range of views people have about calorie labels, including variation both between people and within an individual.
“For example, on a weekend, people might not want to engage with calories at all because they are more concerned to treat themselves, whereas at a mid-week lunch that same person might really want the ability to check the calorie content of their food,” Ms. Keleher reported.
She said that considerations varied significantly between people such that they described different ways in which calorie labeling impacted their food-ordering experience.
“Some people felt labels supported their existing intentions, whereas others felt labels built their knowledge. Still others felt calorie labels were insufficient to support their health and wanted more information, such as on macronutrients,” said Ms. Keleher, quoting one participant: “There’s no situation in which I would look at [calories]. I look at nutrients. I prefer the traffic light system [color-coding salt, fat, and sugar content],” she relayed.
The key recommendations based on the think-aloud study included providing a filter that allows users to switch calorie labels on and off; communicating recommended energy intake per meal (that is, 600 kcal) and not just per day (that is, 2,000 kcal); and avoiding framing calorie label messaging or formatting as judgmental (for example, red fonts).
“These studies provide encouraging proof-of-concept evidence that small tweaks in delivery apps could help many people to identify and select healthier foods. Testing similar initiatives with real restaurants and delivery apps will be important to assess the long-term impact of these interventions in the real world. Further research should also explore the best way to balance desired health impacts while minimizing effects on businesses and on cost-of-living concerns for consumers,” concluded Dr. Bianchi.
A version of this article first appeared on Medscape.com.
DUBLIN – , show three new randomized trials from the United Kingdom.
The prominent positioning of low-calorie menu items, and restaurants with low-calorie main meals, on a food app emerged as the most promising approach to promote healthier eating, followed by preselecting smaller portions by default, and finally calorie labels, Anna Keleher, MPA, a behavioral scientist at Nesta, London, reported at the European Congress on Obesity (ECO) meeting.
“Many out-of-home meals have more calories than meals cooked in-home and using delivery apps is linked with a higher risk of becoming overweight or obese,” she remarked. “We’re interested in understanding more about delivery apps because they can be modified at scale easily and can reach millions of people with interventions to promote healthier and more nutritious options in these settings.”
Food delivery apps have surged in use in the United Kingdom with a 55% increase since 2015; examples include Uber Eats, Just Eat, and Deliveroo. “This trend is similar in the United States, with more and more consumers using delivery apps to buy food,” said Ms. Keleher, a senior adviser at the Behavioral Insights Team, New York.
Emma Boyland, PhD, an obesity psychologist from Liverpool (England) University, said: “Apps are an increasingly popular way for people to buy food and the virtual food environment is becoming as prominent as the physical food environment in how we go about obtaining meals.”
She highlighted the need to understand more about how food apps change the way we purchase and eat, but noted that “the work presented today” showed that “moving the position of food choices and information, as well as the brand name and imagery, influences what people end up buying and consuming.
“I think there’s a place for interventions that challenge these things and improve dietary health,” said Dr. Boyland, who chaired the session during which Ms. Keleher presented her results. “However, as we’ve seen with calorie labeling, they don’t always have the biggest effect on their own, so as is often the case, we need to take multiple actions, incorporating all the elements of the environment to make a meaningful difference.”
Three trials changing displays on simulated food delivery apps
“Delivery apps could reach millions of people and help us select healthier food options, and yet there is very little research looking at what works to promote healthier and more nutritious options in these settings,” Filippo Bianchi, MD, a colleague working with Ms. Keleher, said in a press release issued by ECO.
So the research team carried out a proof-of-concept testing of health-promoting interventions by developing a simulated food delivery app and asking 23,783 adults who typically use such services to choose a meal for themselves as if it were a real-life food delivery order.
“As a first step, we developed a simulated online food delivery platform to generate evidence on the effectiveness of our interventions,” Ms. Keleher explained, noting that the simulated platform included 21 restaurants and almost 600 food and drink items to choose from.
The research evaluated 14 interventions across three randomized controlled trials, displaying various food-ordering options that promoted lower-calorie options against a control. The trials investigated default choices (promoting the selection of small portion sizes through defaults, n = 6,000); positioning (promoting the selection of less calorie-dense options through positioning, n = 9,003); and labeling (promoting the selection of less calorific options through calorie labels, n = 8,780).
The primary outcome was the total number of calories in the basket at checkout. The results were adjusted for potentially confounding factors, such as body mass index, age, gender, and income.
For the trial that promoted smaller portions by default, “all of our interventions significantly reduced calorie purchases, with each additional intervention element increasing the effect sizes, which ranged from a 6% to 13% reduction in calories [–5.5% to –12.5% kcal/order; P < .05],” reported Ms. Keleher.
The second trial varied the position of both items on the menu and the order of restaurants – effectively, lower-calorie menu options were more prominent, and restaurant options with lower-calorie main meals were placed at the top of the restaurant selection page.
Ms. Keleher noted that there have been some concerns about whether this strategy would negatively affect restaurant business, so the research team counteracted this by also incorporating an option where low-calorie but high-price options were placed near the top of the display to promote healthier options but without loss of income for participating restaurants. This last intervention with low-calorie/high-price options placed near the top also led to reduced calorie intake.
“This showed that promoting low-calorie options does not necessarily mean damaging business revenue,” she said. “We hope that the industry can evolve to meet the widely recognized needs of society and consumers.”
Repositioning restaurants emerged as more effective than repositioning foods on the menu, while all interventions significantly reduced calorie purchases. “Effect sizes ranged from 6% to 15% reductions in calories purchased per order [P < .05],” reported Ms. Keleher.
The last trial tested seven calorie labels: four that changed the font size and location of the label, two that added a switch on/off filter for calorie label display, and one that was a calorie summary at checkout.
“All these standard calorie labels directionally reduced the number of excess calories with two [options] reaching statistical significance. Five out of seven labels significantly reduced calorie purchases with effect sizes ranging from 4.3% to –7.8% kcal/order (P < .05),” reported Ms. Keleher.
“This research is important for policymakers so they can understand the best way for companies to display calorie labels and what to include in regulations and guidelines,” she summarized.
Qualitative think-aloud study explored views around food delivery apps
Another piece of research, the think-aloud study, by the same authors, was presented at ECO, and explored how best to enhance the effectiveness and acceptability of calorie labels in food delivery apps in consultation with 20 adult delivery app users in the United Kingdom.
Researchers tried to document the range of views people have about calorie labels, including variation both between people and within an individual.
“For example, on a weekend, people might not want to engage with calories at all because they are more concerned to treat themselves, whereas at a mid-week lunch that same person might really want the ability to check the calorie content of their food,” Ms. Keleher reported.
She said that considerations varied significantly between people such that they described different ways in which calorie labeling impacted their food-ordering experience.
“Some people felt labels supported their existing intentions, whereas others felt labels built their knowledge. Still others felt calorie labels were insufficient to support their health and wanted more information, such as on macronutrients,” said Ms. Keleher, quoting one participant: “There’s no situation in which I would look at [calories]. I look at nutrients. I prefer the traffic light system [color-coding salt, fat, and sugar content],” she relayed.
The key recommendations based on the think-aloud study included providing a filter that allows users to switch calorie labels on and off; communicating recommended energy intake per meal (that is, 600 kcal) and not just per day (that is, 2,000 kcal); and avoiding framing calorie label messaging or formatting as judgmental (for example, red fonts).
“These studies provide encouraging proof-of-concept evidence that small tweaks in delivery apps could help many people to identify and select healthier foods. Testing similar initiatives with real restaurants and delivery apps will be important to assess the long-term impact of these interventions in the real world. Further research should also explore the best way to balance desired health impacts while minimizing effects on businesses and on cost-of-living concerns for consumers,” concluded Dr. Bianchi.
A version of this article first appeared on Medscape.com.
AT ECO 2023
Lupus landmark study aims for personalized medicine goals
A new prospective, observational study from the Lupus Research Alliance (LRA) aims to enroll 3,500 patients in an effort to accelerate the development of personalized treatments for individuals living with systemic lupus erythematosus (SLE).
The LRA on May 23 announced the launch of the Lupus Landmark Study (LLS). The study will be conducted in partnership with Lupus Therapeutics, the clinical research affiliate of the LRA.
The study will be a key feature of the Lupus Nexus, a unique combination of lupus patient registry, biorepository, and portal for data sharing and analysis.
“The aim of the Lupus Nexus is to transform lupus research and drug development through unprecedented information exchange capabilities,” according to the LRA press release.
“SLE is a debilitating autoimmune disease that disproportionately impacts women and people from minority groups, but the cause of lupus is unknown, and no single laboratory test can definitively identify lupus,” lead investigator S. Sam Lim, MD, of Emory University, Atlanta, told this news organization.
“Nevertheless, early detection and treatment can often lessen the progression and severity of the disease. Although there are numerous contributing factors to the lag in research discoveries and new treatments for patients with lupus, limited access to standardized, high-quality biological samples and natural history data provides a significant roadblock to advancing lupus research,” Dr. Lim said.
“Existing registry and biorepository resources in the lupus field are largely siloed, mostly limited to relatively small or discrete patient populations, and frequently not designed for broad sharing across all stakeholders of the research community,” Dr. Lim said. The LRA and its affiliate Lupus Therapeutics are committed to developing Lupus Nexus, a first-of-its-kind registry and biorepository, to serve as a collaborative research platform for lupus and a leading source of prospective, longitudinal patient data and biological samples for the research community, Dr. Lim added.
“The Lupus Landmark Study will form the foundation of this registry and biorepository and will provide a critical resource to enable the understanding of lupus heterogeneity at the molecular level,” Dr. Lim said. The molecular data can be linked to clinical phenotypes, he explained, “while providing an opportunity to better understand the holistic experience of patients with lupus, thus helping patients address the daily life challenges they face.”
The Lupus Accelerating Breakthroughs Consortium (Lupus ABC) was announced earlier this spring by the LRA. It represents a collaboration between the U.S. Food and Drug Administration and the lupus community to improve and accelerate the development of safer and more effective treatments for people with lupus, Dr. Lim said. “Data and other results from the LLS will inform this collaboration,” he said.
“The LLS will provide greater insight into the pathogenesis and evolution of the condition, providing much needed information and guidance to clinicians so that the disease can be detected and treated earlier and with better precision,” Dr. Lim said. “The partnership with patients will ensure that advances will not only be meaningful to clinicians but their patients and caregivers as well,” he added.
Individuals living with lupus were essential to the development of the Lupus Nexus, and patients will continue to be engaged through participation in the LLS, which will not only generate data to promote patient-centered treatments but will also give participants more insight into their health data, according to the LRA press release.
The clinical coordinating center and biorepository elements of the Lupus Nexus will be managed by Embleema and Azenta Life Sciences, respectively, according the LRA.
Biomarker analysis will be conducted by DxTerity Diagnostics via the company’s proprietary DxCollection MicroCollection Device and Modular Immune Profile platform.
The LLS is scheduled to begin enrolling patients through select academic medical centers in the Lupus Therapeutics Lupus Clinical Investigators Network later in 2023, with an expanded roll-out in 2024, according to the press release. More information about the Lupus Landmark Study is available from Lupus Nexus at [email protected].
A version of this article first appeared on Medscape.com.
A new prospective, observational study from the Lupus Research Alliance (LRA) aims to enroll 3,500 patients in an effort to accelerate the development of personalized treatments for individuals living with systemic lupus erythematosus (SLE).
The LRA on May 23 announced the launch of the Lupus Landmark Study (LLS). The study will be conducted in partnership with Lupus Therapeutics, the clinical research affiliate of the LRA.
The study will be a key feature of the Lupus Nexus, a unique combination of lupus patient registry, biorepository, and portal for data sharing and analysis.
“The aim of the Lupus Nexus is to transform lupus research and drug development through unprecedented information exchange capabilities,” according to the LRA press release.
“SLE is a debilitating autoimmune disease that disproportionately impacts women and people from minority groups, but the cause of lupus is unknown, and no single laboratory test can definitively identify lupus,” lead investigator S. Sam Lim, MD, of Emory University, Atlanta, told this news organization.
“Nevertheless, early detection and treatment can often lessen the progression and severity of the disease. Although there are numerous contributing factors to the lag in research discoveries and new treatments for patients with lupus, limited access to standardized, high-quality biological samples and natural history data provides a significant roadblock to advancing lupus research,” Dr. Lim said.
“Existing registry and biorepository resources in the lupus field are largely siloed, mostly limited to relatively small or discrete patient populations, and frequently not designed for broad sharing across all stakeholders of the research community,” Dr. Lim said. The LRA and its affiliate Lupus Therapeutics are committed to developing Lupus Nexus, a first-of-its-kind registry and biorepository, to serve as a collaborative research platform for lupus and a leading source of prospective, longitudinal patient data and biological samples for the research community, Dr. Lim added.
“The Lupus Landmark Study will form the foundation of this registry and biorepository and will provide a critical resource to enable the understanding of lupus heterogeneity at the molecular level,” Dr. Lim said. The molecular data can be linked to clinical phenotypes, he explained, “while providing an opportunity to better understand the holistic experience of patients with lupus, thus helping patients address the daily life challenges they face.”
The Lupus Accelerating Breakthroughs Consortium (Lupus ABC) was announced earlier this spring by the LRA. It represents a collaboration between the U.S. Food and Drug Administration and the lupus community to improve and accelerate the development of safer and more effective treatments for people with lupus, Dr. Lim said. “Data and other results from the LLS will inform this collaboration,” he said.
“The LLS will provide greater insight into the pathogenesis and evolution of the condition, providing much needed information and guidance to clinicians so that the disease can be detected and treated earlier and with better precision,” Dr. Lim said. “The partnership with patients will ensure that advances will not only be meaningful to clinicians but their patients and caregivers as well,” he added.
Individuals living with lupus were essential to the development of the Lupus Nexus, and patients will continue to be engaged through participation in the LLS, which will not only generate data to promote patient-centered treatments but will also give participants more insight into their health data, according to the LRA press release.
The clinical coordinating center and biorepository elements of the Lupus Nexus will be managed by Embleema and Azenta Life Sciences, respectively, according the LRA.
Biomarker analysis will be conducted by DxTerity Diagnostics via the company’s proprietary DxCollection MicroCollection Device and Modular Immune Profile platform.
The LLS is scheduled to begin enrolling patients through select academic medical centers in the Lupus Therapeutics Lupus Clinical Investigators Network later in 2023, with an expanded roll-out in 2024, according to the press release. More information about the Lupus Landmark Study is available from Lupus Nexus at [email protected].
A version of this article first appeared on Medscape.com.
A new prospective, observational study from the Lupus Research Alliance (LRA) aims to enroll 3,500 patients in an effort to accelerate the development of personalized treatments for individuals living with systemic lupus erythematosus (SLE).
The LRA on May 23 announced the launch of the Lupus Landmark Study (LLS). The study will be conducted in partnership with Lupus Therapeutics, the clinical research affiliate of the LRA.
The study will be a key feature of the Lupus Nexus, a unique combination of lupus patient registry, biorepository, and portal for data sharing and analysis.
“The aim of the Lupus Nexus is to transform lupus research and drug development through unprecedented information exchange capabilities,” according to the LRA press release.
“SLE is a debilitating autoimmune disease that disproportionately impacts women and people from minority groups, but the cause of lupus is unknown, and no single laboratory test can definitively identify lupus,” lead investigator S. Sam Lim, MD, of Emory University, Atlanta, told this news organization.
“Nevertheless, early detection and treatment can often lessen the progression and severity of the disease. Although there are numerous contributing factors to the lag in research discoveries and new treatments for patients with lupus, limited access to standardized, high-quality biological samples and natural history data provides a significant roadblock to advancing lupus research,” Dr. Lim said.
“Existing registry and biorepository resources in the lupus field are largely siloed, mostly limited to relatively small or discrete patient populations, and frequently not designed for broad sharing across all stakeholders of the research community,” Dr. Lim said. The LRA and its affiliate Lupus Therapeutics are committed to developing Lupus Nexus, a first-of-its-kind registry and biorepository, to serve as a collaborative research platform for lupus and a leading source of prospective, longitudinal patient data and biological samples for the research community, Dr. Lim added.
“The Lupus Landmark Study will form the foundation of this registry and biorepository and will provide a critical resource to enable the understanding of lupus heterogeneity at the molecular level,” Dr. Lim said. The molecular data can be linked to clinical phenotypes, he explained, “while providing an opportunity to better understand the holistic experience of patients with lupus, thus helping patients address the daily life challenges they face.”
The Lupus Accelerating Breakthroughs Consortium (Lupus ABC) was announced earlier this spring by the LRA. It represents a collaboration between the U.S. Food and Drug Administration and the lupus community to improve and accelerate the development of safer and more effective treatments for people with lupus, Dr. Lim said. “Data and other results from the LLS will inform this collaboration,” he said.
“The LLS will provide greater insight into the pathogenesis and evolution of the condition, providing much needed information and guidance to clinicians so that the disease can be detected and treated earlier and with better precision,” Dr. Lim said. “The partnership with patients will ensure that advances will not only be meaningful to clinicians but their patients and caregivers as well,” he added.
Individuals living with lupus were essential to the development of the Lupus Nexus, and patients will continue to be engaged through participation in the LLS, which will not only generate data to promote patient-centered treatments but will also give participants more insight into their health data, according to the LRA press release.
The clinical coordinating center and biorepository elements of the Lupus Nexus will be managed by Embleema and Azenta Life Sciences, respectively, according the LRA.
Biomarker analysis will be conducted by DxTerity Diagnostics via the company’s proprietary DxCollection MicroCollection Device and Modular Immune Profile platform.
The LLS is scheduled to begin enrolling patients through select academic medical centers in the Lupus Therapeutics Lupus Clinical Investigators Network later in 2023, with an expanded roll-out in 2024, according to the press release. More information about the Lupus Landmark Study is available from Lupus Nexus at [email protected].
A version of this article first appeared on Medscape.com.
Chondrodermatitis Nodularis Helicis After Mohs Micrographic Surgery and Radiation Therapy
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.
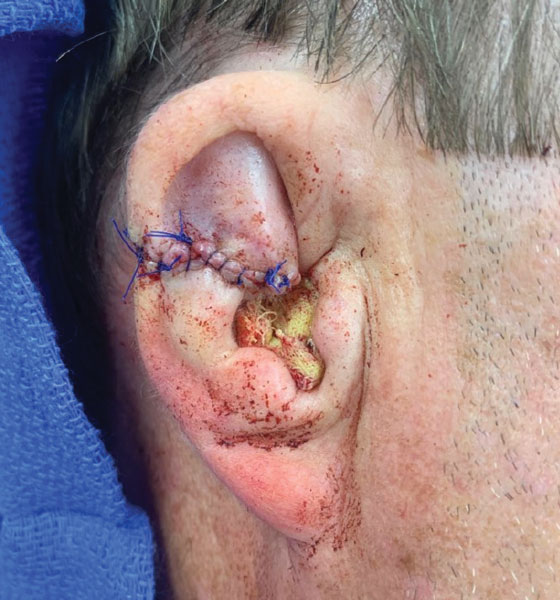
A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.
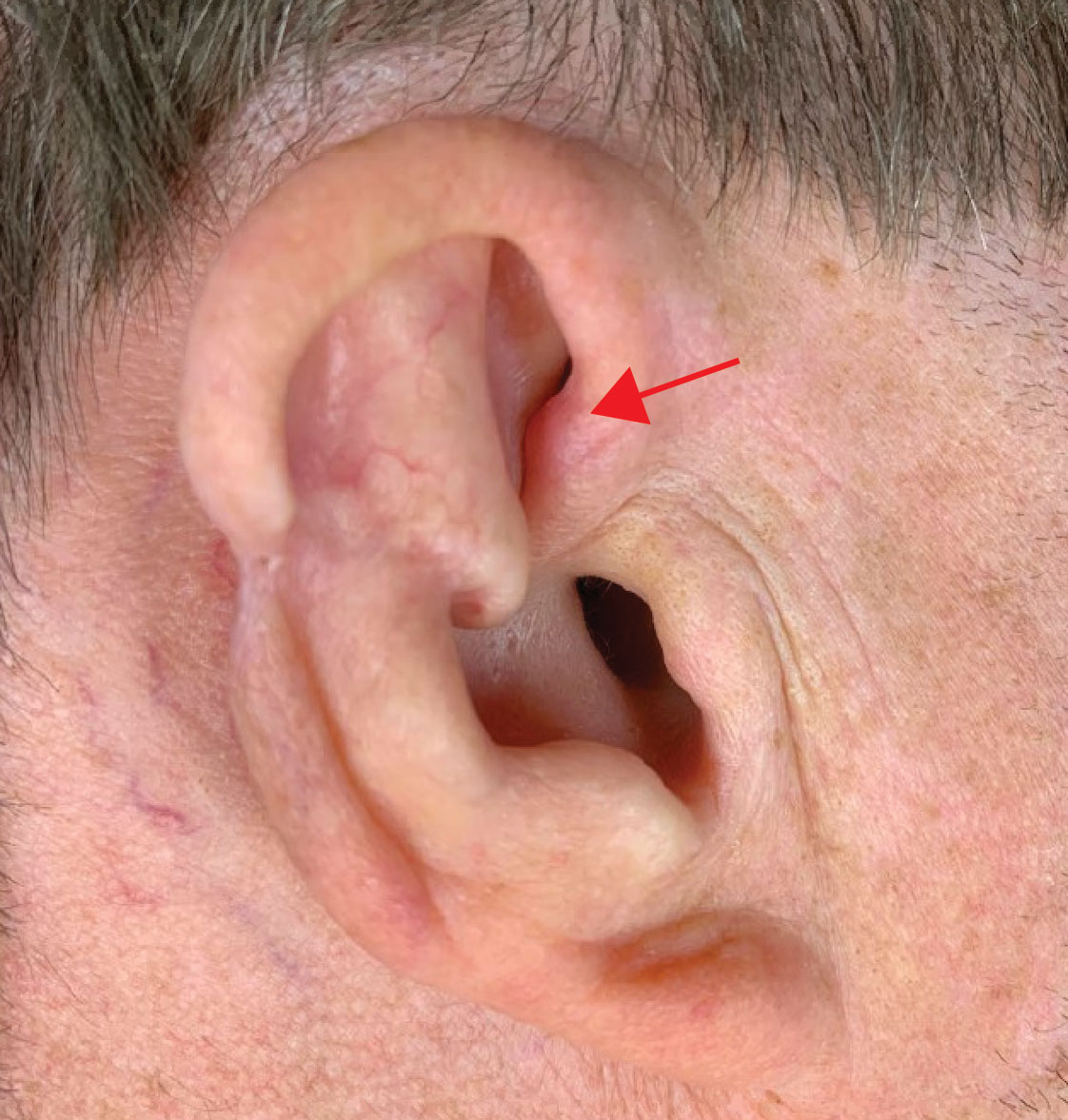
Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).
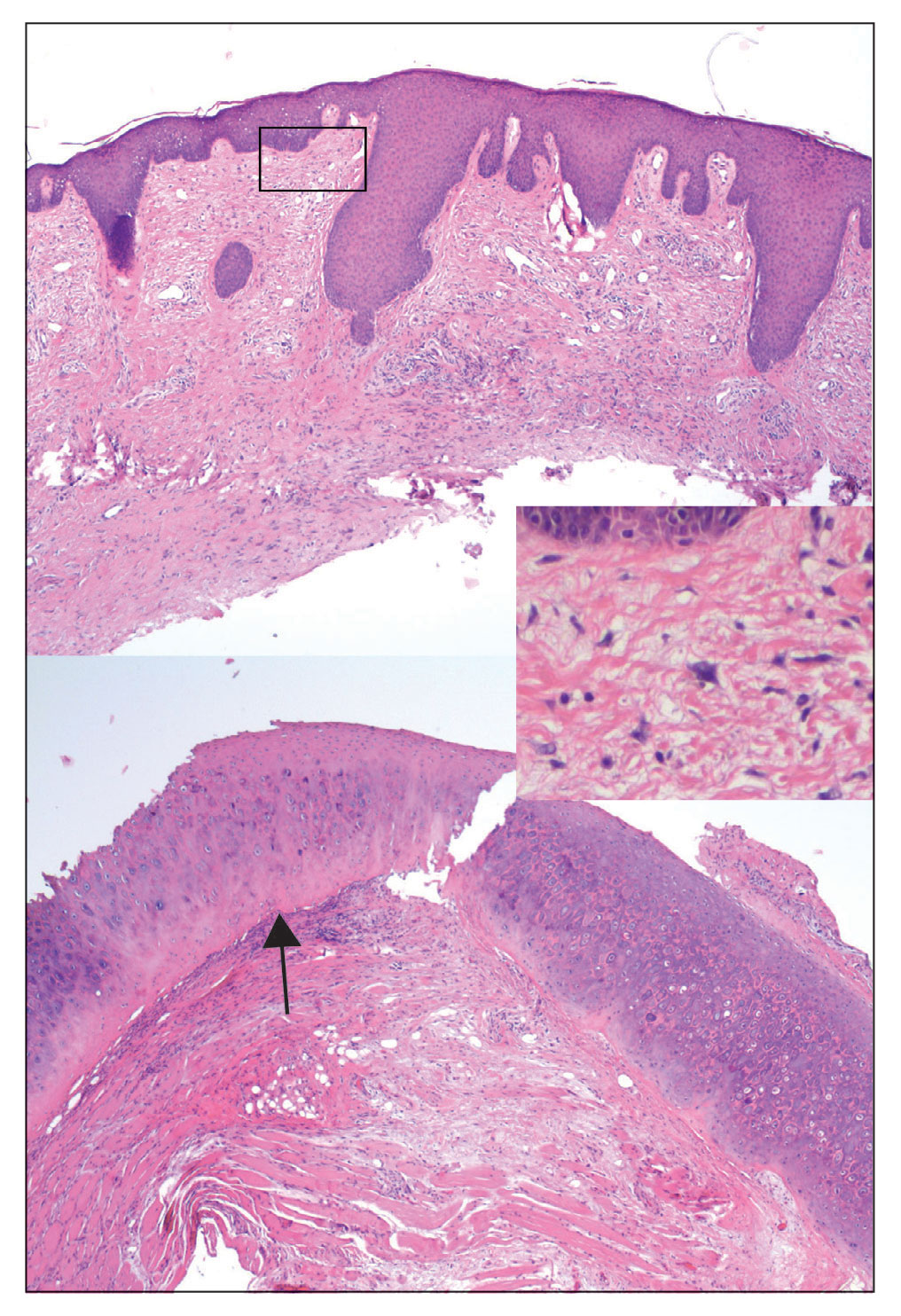
Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.

A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.

Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).

Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.

A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.

Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).

Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
Practice Points
- Although chondrodermatitis nodularis helicis (CNH) is benign by nature, it can mimic tumor recurrence when it presents close to the site of prior Mohs micrographic surgery (MMS). Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Skin lesions in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis.
An Evaluation of Spin in the Abstracts of Systematic Reviews and Meta-analyses on the Treatment of Psoriasis: A Cross-sectional Analysis
Psoriasis is an inflammatory autoimmune skin condition that affects approximately 125 million individuals worldwide, with approximately 8 million patients in the United States.1 Psoriasis not only involves a cosmetic component but also comprises other comorbidities, such as psoriatic arthritis, cardiovascular disease, and psychiatric disorders, that can influence patient quality of life.2-4 In addition, the costs associated with psoriasis are substantial, with an estimated economic burden of $35.2 billion in the United States in 2015.5 Given the prevalence of psoriasis and its many effects on patients, it is important that providers have high-quality evidence regarding efficacious treatment options.
Systematic reviews, which compile all available evidence on a subject to answer a specific question, represent the gold standard of research.6 However, studies have demonstrated that when referencing research literature, physicians tend to read only the abstract of a study rather than the entire article.7,8 A study by Marcelo et al8 showed that residents at a tertiary care center answered clinical questions using only the abstract of a paper 69% of the time. Based on these findings, it is imperative that the results of systematic reviews be accurately reported in their abstracts because they can influence patient care.
Referencing only the abstracts of systematic reviews can be problematic if the abstract contains spin. Spin is a form of reporting that inappropriately highlights the benefits of a treatment with greater emphasis than what is shown by the results.9 Research has identified the presence of spin in the abstracts of randomized controlled trials.10-12 For example, Cooper et al10 found that 70% (33/47) of abstracts in otolaryngology randomized controlled trials contained spin. Additionally, Arthur et al11 and Austin et al12 had similar findings within abstracts of orthopedic and obesity trials, where 44.8% (112/250) and 46.7% (21/45) contained spin, respectively. Ottwell et al13 found that the presence of spin in abstracts is not limited to randomized controlled trials; they demonstrated that the abstracts of nearly one-third (31% [11/36]) of systematic reviews focused on the treatment of acne vulgaris contained spin.
In our study, we aimed to evaluate the presence of spin in the abstracts of systematic reviews focused on the treatment of psoriasis.
Methods
Reproducibility and Reporting—Our study did not meet the regulatory definition for human subjects research per the US Code of Federal Regulations because the study did not involve human research subjects. The study also was not subject to review by the institutional review board. Our protocol, data set, analysis scripts, extraction forms, and other material related to the study have been placed on Open Science Framework to provide transparency and ensure reproducibility. To further allow for analytic reproducibility, our data set was given to an independent laboratory and reanalyzed with a masked approach. Our study was carried out alongside other studies assessing spin in systematic reviews regarding different specialties and disease states. Because these studies were similar in design, this methodology also has been reported elsewhere. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)14 and the guidelines for meta-epidemiological studies developed by Murad and Wang15 were used in drafting this article.
Search Strategy—The search strategies for the MEDLINE (Ovid) and Embase (Ovid) databases were created by a systematic review librarian (D.N.W.) to identify systematic reviews and meta-analyses regarding treatments for psoriasis (Figure 1). The searches were performed on June 2, 2020, and uploaded to Rayyan, a systematic review screening platform.16 After duplicates were removed, the records were screened for eligibility by 2 authors (C.H. and A.L.) using the titles and abstracts. Screening was conducted independently while each of these authors was masked to the other’s results; disagreements were resolved through discussion.
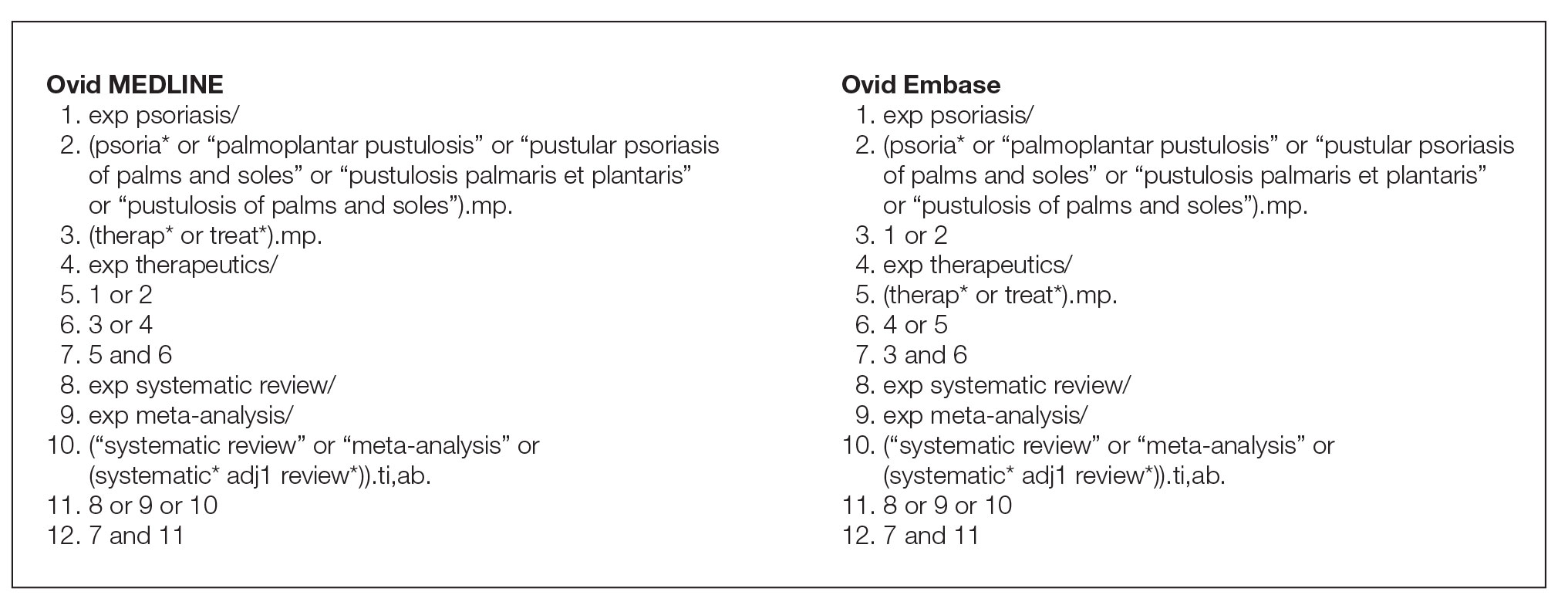
Eligibility Criteria—An article had to meet the following criteria for inclusion in our study: (1) be a systematic review with or without a meta-analysis; (2) relate to the treatment of psoriasis; and (3) be written in English and include human patients only. The PRISMA definition of systematic reviews and meta-analyses was applied.17
Training—Various training occurred throughout our study to ensure understanding of each step and mitigate subjectivity. Before beginning screening, 2 investigators (C.H. and A.L.) completed the Introduction to Systematic Review and Meta-Analysis course offered by Johns Hopkins University.18 They also underwent 2 days of online and in-person training on the definition and interpretation of the 9 most severe types of spin found in the abstracts of systematic reviews as defined by Yavchitz et al.9 Finally, they were trained to use A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) to appraise the methodological quality of each systematic review. Our protocol contained an outline of all training modules used.
Data Extraction—The investigators (C.H. and A.L.) analyzed included abstracts for the 9 most severe types of spin (Table 1). Data were extracted in a masked duplicate fashion using the Google form. AMSTAR-2 was used to assess systematic reviews for methodological quality. AMSTAR-2 is an appraisal tool consisting of a 16-item checklist for systematic reviews or meta-analyses. Scores range from critically low to high based on the methodological quality of the review. Interrater reliability of AMSTAR-2 scores has been moderate to high across studies. Construct validity coefficients have been high with the original AMSTAR instrument (r=0.91) and the Risk of Bias in Systematic Reviews instrument (r=0.84).19
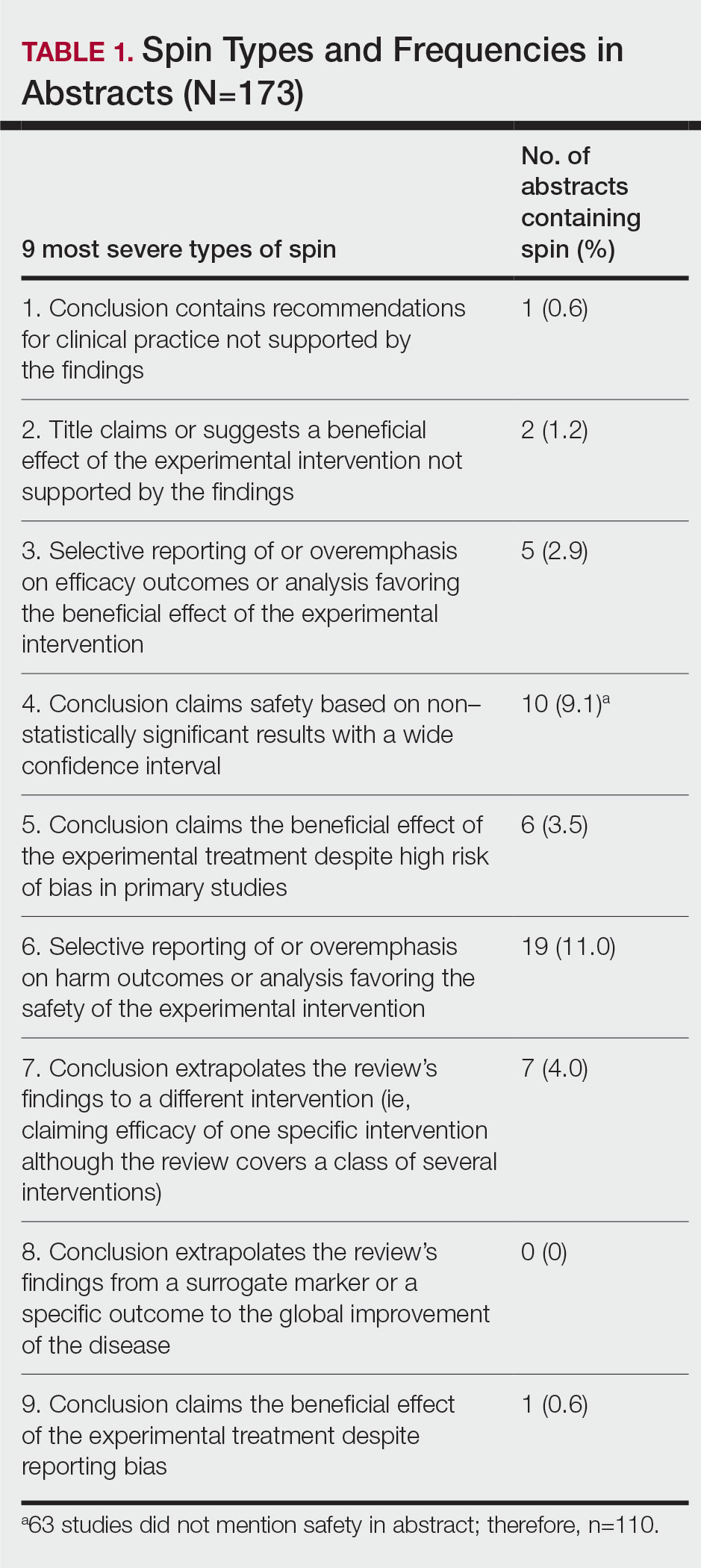
During data extraction from each included systematic review, the following additional items were obtained: (1) the date the review was received; (2) intervention type (ie, pharmacologic, nonpharmacologic, surgery, light therapy, mixed); (3) the funding source(s) for each systematic review (ie, industry, private, public, none, not mentioned, hospital, a combination of funding not including industry, a combination of funding including industry, other); (4) whether the journal submission guidelines suggested adherence to PRISMA guidelines; (5) whether the review discussed adherence to PRISMA14 or PRISMA for Abstracts20 (PRISMA-A); (6) the publishing journal’s 5-year impact factor; and (6) the country of the systematic review’s origin. When data extraction was complete, investigators (C.H. and A.L.) were unmasked and met to resolve any disagreements by discussion. Two authors (R.O. or M.V.) served as arbiters in the case that an agreement between C.H. and A.L. could not be reached.
Statistical Analysis—Frequencies and percentages were calculated to evaluate the most common types of spin found within systematic reviews and meta-analyses. One author (M.H.) prespecified the possibility of a binary logistic regression and calculated a power analysis to determine sample size, as stated in our protocol. Our final sample size of 173 was not powered to perform the multivariable logistic regression; therefore, we calculated unadjusted odds ratios to enable assessing relationships between the presence of spin in abstracts and the various study characteristics. We used Stata 16.1 for all analyses, and all analytic decisions can be found in our protocol.
Results
General Characteristics—Our systematic search of MEDLINE and Embase returned 3200 articles, of which 665 were duplicates that were removed. An additional 2253 articles were excluded during initial abstract and title screening, and full-text screening led to the exclusion of another 109 articles. In total, 173 systematic reviews were included for data extraction. Figure 2 illustrates the screening process with the rationale for all exclusions.
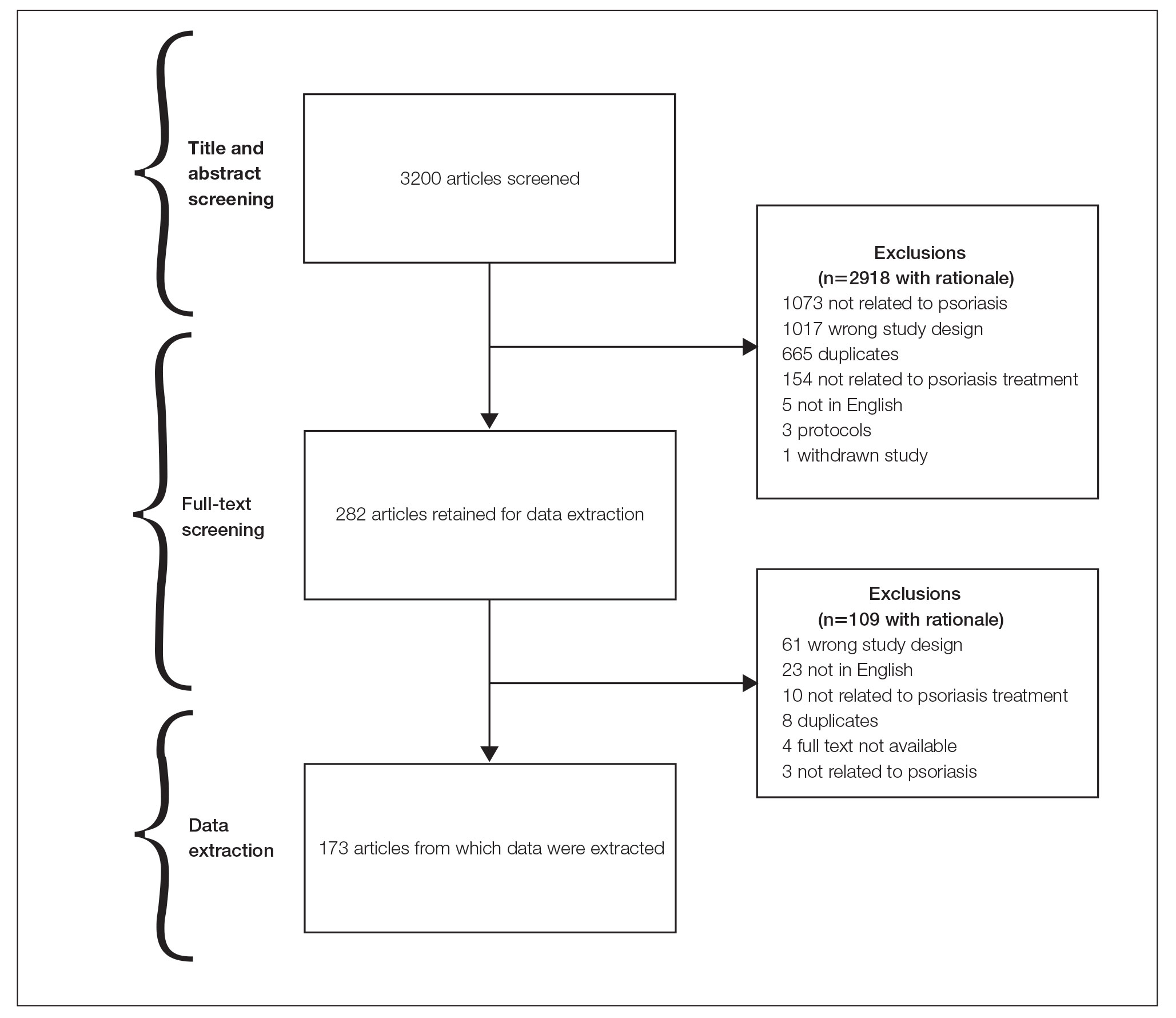
Of the 173 included systematic reviews and meta-analyses, 150 (86.7%) focused on pharmacologic interventions. The majority of studies did not mention adhering to PRISMA guidelines (125/173 [72.3%]), and the publishing journals recommended their authors adhere to PRISMA for only 66 (38.2%) of the included articles. For the articles that received funding (90/173 [52.0%]), industry sources were the most common funding source (40/90 [44.4%]), followed by private (27/90 [30%]) and public funding sources (23/90 [25.6%]). Of the remaining studies, 46 articles did not include a funding statement (46/83 [55.4%]), and 37 studies were not funded (37/83 [44.6%]). The average (SD) 5-year impact factor of our included journals was 4.68 (4.64). Systematic reviews were from 31 different countries. All studies were received by their respective journals between the years 2000 and 2020 (Table 2).
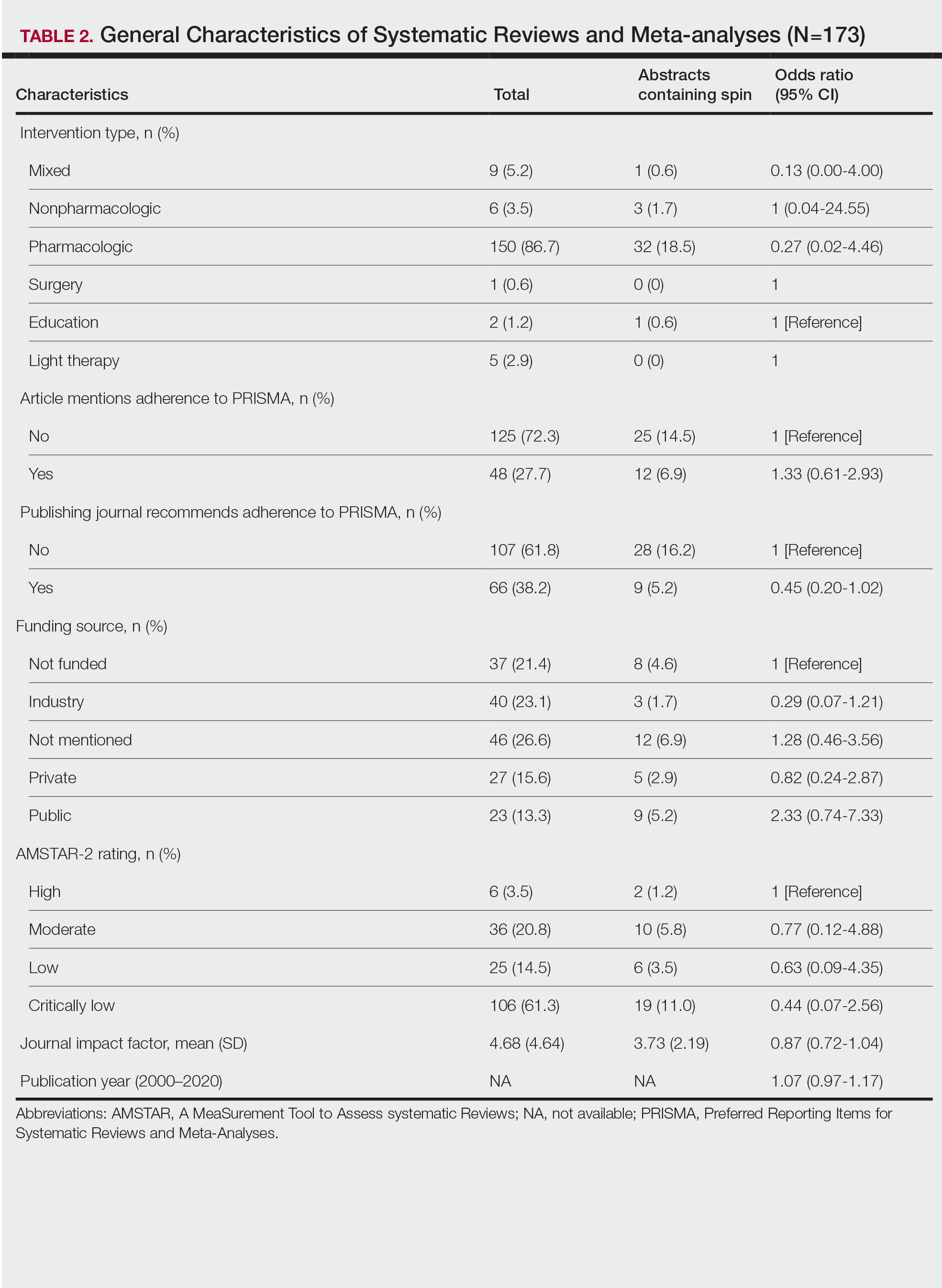
Abstracts Containing Spin—We found that 37 (21.4%) of the abstracts of systematic reviews focused on psoriasis treatments contained at least 1 type of spin. Some abstracts had more than 1 type; thus, a total of 51 different instances of spin were detected. Spin type 6—selective reporting of or overemphasis on harm outcomes or analysis favoring the safety of the experimental intervention—was the most common type ofspin, found in 19 of 173 abstracts (11.0%). The most severe type of spin—type 1 (conclusion contains recommendations for clinical practice not supported by the findings)—occurred in only 1 abstract (0.6%). Spin type 8 did not occur in any of the abstracts (Table 1). There was no statistically significant association between the presence of spin and any of the study characteristics (Table 2).
AMSTAR Ratings—After using AMSTAR-2 to appraise the included systematic reviews, we found that 6 (3.5%) of the 173 studies could be rated as high; 36 (20.8%) as moderate; 25 (14.5%) as low; and 106 (61.3%) as critically low. Of the 37 abstracts containing spin, 2 (5.4%) had an AMSTAR-2 rating of high, 10 (27%) had a rating of moderate, 6 (16.2%) had a rating of low, and 19 (51.4%) had a rating of critically low (Table 2). No statistically significant associations were seen between abstracts found to have spin and the AMSTAR-2 rating of the review.
Nearly all (160/173 [92.5%]) of the included reviews were compliant with the inclusion of Population, Intervention, Comparison, and Outcome (PICO) method. Only 17 of 173 (9.8%) reviews reported funding sources for the studies included. See Table 3 for all AMSTAR-2 items.
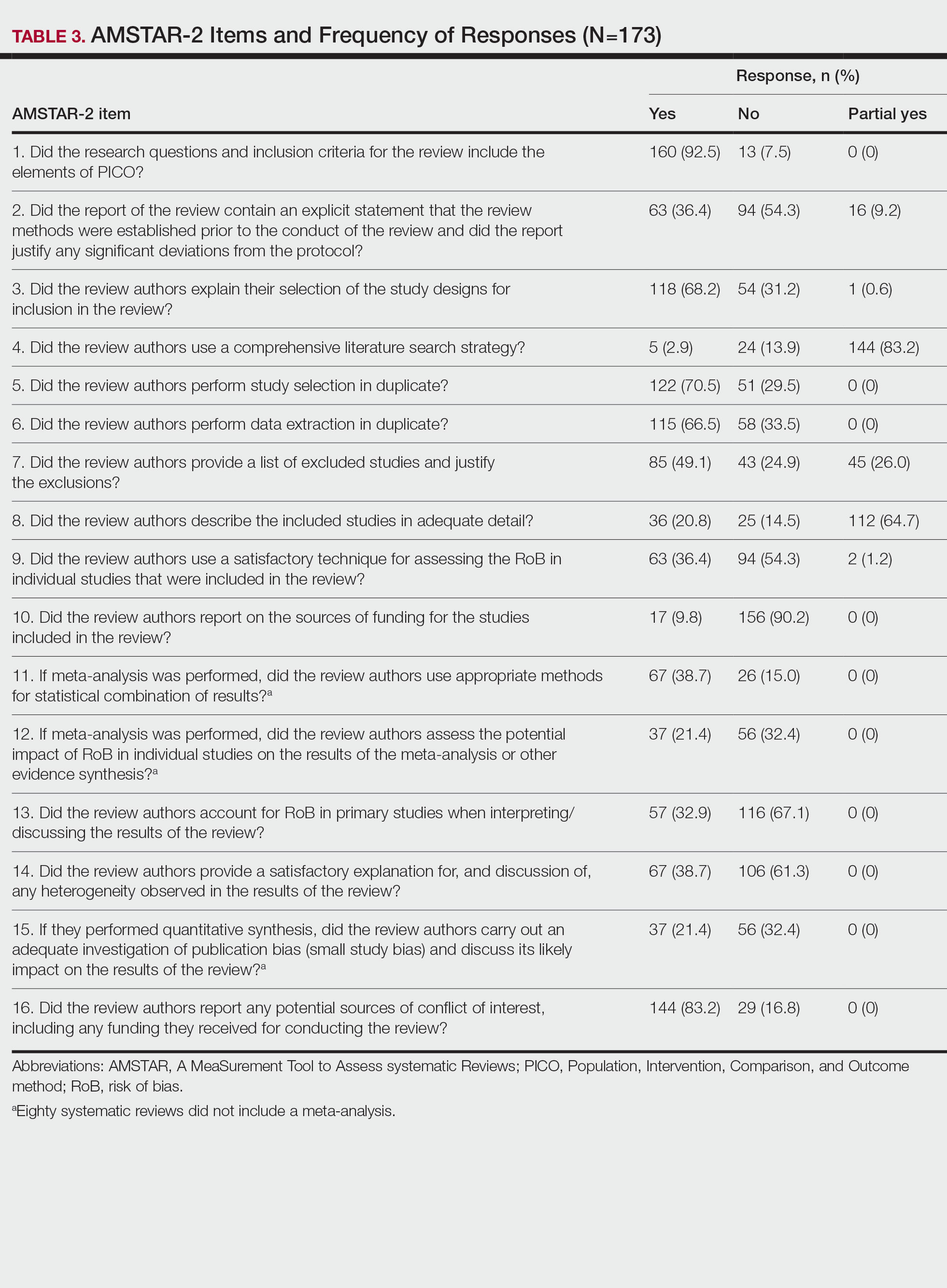
Comment
Primary Findings—We evaluated the abstracts of systematic reviews for the treatment of psoriasis and found that more than one-fifth of them contained spin. Our study contributes to the existing literature surrounding spin. Spin in randomized controlled trials is well documented across several fields of medicine, including otolaryngology,10 obesity medicine,12 dermatology,21 anesthesiology,22 psychiatry,23 orthopedics,24 emergency medicine,25 oncology,26 and cardiology.27 More recently, studies have emerged evaluating the presence of spin in systematic reviews. Specific to dermatology, one study found that 74% (84/113) of systematic reviews related to atopic dermatitis treatment contained spin.28 Additionally, Ottwell et al13 identified spin in 31% (11/36) of the systematic reviews related to the treatment of acne vulgaris, which is similar to our results for systematic reviews focused on psoriasis treatments. When comparing the presence of spin in abstracts of systematic reviews from the field of dermatology with other specialties, dermatology-focused systematic reviews appear to contain more spin in the abstract than systematic reviews focused on tinnitus and glaucoma therapies.29,30 However, systematic reviews from the field of dermatology appear to contain less spin than systematic reviews focused on therapies for lower back pain.31 For example, Nascimento et al31 found that 80% (53/66) of systematic reviews focused on low-back pain treatments contained spin.
Examples of Spin—The most common type of spin found in our study was type 6.9 An example of spin type 6 can be found in an article by Bai et al32 that investigated the short-term efficacy and safety of multiple interleukin inhibitors for the treatment of plaque psoriasis. The conclusion of the abstract states, “Risankizumab appeared to have relatively high efficacy and low risk.” However, in the results section, the authors showed that risankizumab had the highest risk of serious adverse events and was ranked highest for discontinuation because of adverse events when compared with other interleukin inhibitors. Here, the presence of spin in the abstract may mislead the reader to accept the “low risk” of risankizumab without understanding the study’s full results.32
Another example of selective reporting of harm outcomes in a systematic review can be found in the article by Wu et al,33 which focused on assessing IL-17 antagonists for the treatment of plaque psoriasis. The conclusion of the abstract indicated that IL-17 antagonists should be accepted as safe; however, in the results section, the authors discussed serious safety concerns with brodalumab, including the death of 4 patients from suicide.33 This example of spin type 6 highlights how the overgeneralization of a drug’s safety profile neglects serious harm outcomes that are critical to patient safety. In fact, against the safety claims of Wu et al,33 brodalumab later received a boxed warning from the US Food and Drug Administration after 6 patients died from suicide while receiving the drug, which led to early discontinuation of the trials.34,35 Although studies suggest this relationship is not causal,34-36 the purpose of our study was not to investigate this association but to highlight the importance of this finding. Thus, with this example of spin in mind, we offer recommendations that we believe will improve reporting in abstracts as well as quality of patient care.
Recommendations for Reporting in Abstracts—Regarding the boxed warning37 for brodalumab because of suicidal ideation and behavior, the US Food and Drug Administration recommends that prior to prescribing brodalumab, clinicians consider the potential benefits and risks in patients with a history of depression and/or suicidal ideation or behavior. However, a clinician would not adequately assess the full risks and benefits when an abstract, such as that for the article by Wu et al,33 contains spin through selectively reporting harm outcomes. Arguably, clinicians could just read the full text; however, research confirms that abstracts often are utilized by clinicians and commonly are used to guide clinical decisions.7,38 It is reasonable that clinicians would use abstracts in this fashion because they provide a quick synopsis of the full article’s findings and are widely available to clinicians who may not have access to article databases. Initiatives are in place to improve the quality of reporting in an abstract, such as PRISMA-A,20 but even this fails to address spin. In fact, it may suggest spin because checklist item 10 of PRISMA-A advises authors of systematic reviews to provide a “general interpretation of the results and important implications.” This item is concerning because it suggests that the authors interpret importance rather than the clinician who prescribes the drug and is ultimately responsible for patient safety. Therefore, we recommend a reform to abstract reporting and an update to PRISMA-A that leads authors to report all benefits and risks encountered instead of reporting what the authors define as important.
Strengths and Limitations—Our study has several strengths as well as limitations. One of these strengths is that our protocol was strictly adhered to; any deviations were noted and added as an amendment. Our protocol, data, and all study artifacts were made freely available online on the Open Science Framework to strengthen reproducibility (https://osf.io/zrxh8/). Investigators underwent training to ensure comprehension of spin and systematic review designs. All data were extracted in masked duplicate fashion per the Cochrane Handbook for Systematic Reviews of Interventions.39
Regarding limitations, only 2 databases were searched—MEDLINE and Embase. Therefore, our screening process may not have included every available systematic review on the treatment of psoriasis. Journal impact factors may be inaccurate for the systematic reviews that were published earlier in our data date range; however, we attempted to negate this limitation by using a 5-year average. Our study characteristic regarding PRISMA adherence did not account for studies published before the PRISMA statement release; we also could not access prior submission guidelines to determine when a journal began recommending PRISMA adherence. Another limitation of our study was the intrinsic subjectivity behind spin. Some may disagree with our classifications. Finally, our cross-sectional design should not be generalized to study types that are not systematic reviews or published in other journals during different periods.
Conclusion
Evidence of spin was present in many of the abstracts of systematic reviews pertaining to the treatment of psoriasis. Future clinical research should investigate any reporting of spin and search for ways to better reduce spin within literature. Continued research is necessary to evaluate the presence of spin within dermatology and other specialties.
- Psoriasis statistics. National Psoriasis Foundation. Updated December 21, 2022. Accessed March 6, 2023. https://www.psoriasis.org/content/statistics
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
- Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18:2211.
- Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18:613-620.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta‑analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2:9-14.
- Barry HC, Ebell MH, Shaughnessy AF, et al. Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract. 2001;14:437-442.
- Marcelo A, Gavino A, Isip-Tan IT, et al. A comparison of the accuracy of clinical decisions based on full-text articles and on journal abstracts alone: a study among residents in a tertiary care hospital. Evid Based Med. 2013;18:48-53.
- Yavchitz A, Ravaud P, Altman DG, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56-65.
- Cooper CM, Gray HM, Ross AE, et al. Evaluation of spin in the abstracts of otolaryngology randomized controlled trials. Laryngoscope. 2019;129:2036-2040.
- Arthur W, Zaaza Z, Checketts JX, et al. Analyzing spin in abstracts of orthopaedic randomized controlled trials with statistically insignificant primary endpoints. Arthroscopy. 2020;36:1443-1450.
- Austin J, Smith C, Natarajan K, et al. Evaluation of spin within abstracts in obesity randomized clinical trials: a cross-sectional review. Clin Obes. 2019;9:E12292.
- Ottwell R, Rogers TC, Michael Anderson J, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on the treatment of acne vulgaris: cross-sectional analysis. JMIR Dermatol. 2020;3:E16978.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:E1000100.
- Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22:139-142.
- Rayyan QCRI. Accessed September 10, 2019. https://rayyan.qcri.org/reviews/81224
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Coursera. Introduction to systematic review and meta-analysis. Accessed May 18, 2023. https://www.coursera.org/learn/systematic-review
- Lorenz RC, Matthias K, Pieper D, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 2019;114:133-140.
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:E1001419.
- Motosko CC, Ault AK, Kimberly LL, et al. Analysis of spin in the reporting of studies of topical treatments of photoaged skin. J Am Acad Dermatol. 2019;80:516-522.e12.
- Kinder NC, Weaver MD, Wayant C, et al. Presence of “spin” in the abstracts and titles of anaesthesiology randomised controlled trials. Br J Anaesth. 2019;122:E13-E14.
- Jellison S, Roberts W, Bowers A, et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2019;5:178-181.
- Checketts JX, Riddle J, Zaaza Z, et al. An evaluation of spin in lower extremity joint trials. J Arthroplasty. 2019;34:1008-1012.
- Reynolds-Vaughn V, Riddle J, Brown J, et al. Evaluation of spin in the abstracts of emergency medicine randomized controlled trials. Ann Emerg Med. 2019;14:423-431.
- Wayant C, Margalski D, Vaughn K, et al. Evaluation of spin in oncology clinical trials. Crit Rev Oncol Hematol. 2019;144:102821.
- Khan MS, Lateef N, Siddiqi TJ, et al. Level and prevalence of spin in published cardiovascular randomized clinical trial reports with statistically nonsignificant primary outcomes: a systematic review. JAMA Netw Open. 2019;2:E192622.
- Lin V, Patel R, Wirtz A, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of atopic dermatitis treatments and interventions. Dermatology. 2021;237:496-505.
- Rucker B, Umbarger E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on tinnitus. Otol Neurotol. 2021;10:1237-1244.
- Okonya O, Lai E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of treatments for glaucoma. J Glaucoma. 2021;30:235-241.
- Nascimento DP, Gonzalez GZ, Araujo AC, et al. Eight out of every ten abstracts of low back pain systematic reviews presented spin and inconsistencies with the full text: an analysis of 66 systematic reviews. J Orthop Sports Phys Ther. 2020;50:17-23.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-1003.
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23:1-3.
- Rodrigeuz-Bolanos F, Gooderham M, Papp K. A closer look at the data regarding suicidal ideation and behavior in psoriasis patients: the case of brodalumab. Skin Therapy Lett. 2019;24:1-4.
- Danesh MJ, Kimball AB. Brodalumab and suicidal ideation in the context of a recent economic crisis in the United States. J Am Acad Dermatol. 2016;74:190-192.
- Siliq. Prescribing information. Valeant Pharmaceuticals North America LLC; 2017. Accessed May 18, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Johnson HL, Fontelo P, Olsen CH, et al. Family nurse practitioner student perception of journal abstract usefulness in clinical decision making: a randomized controlled trial. J Am Assoc Nurse Pract. 2013;25:597-603.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
Psoriasis is an inflammatory autoimmune skin condition that affects approximately 125 million individuals worldwide, with approximately 8 million patients in the United States.1 Psoriasis not only involves a cosmetic component but also comprises other comorbidities, such as psoriatic arthritis, cardiovascular disease, and psychiatric disorders, that can influence patient quality of life.2-4 In addition, the costs associated with psoriasis are substantial, with an estimated economic burden of $35.2 billion in the United States in 2015.5 Given the prevalence of psoriasis and its many effects on patients, it is important that providers have high-quality evidence regarding efficacious treatment options.
Systematic reviews, which compile all available evidence on a subject to answer a specific question, represent the gold standard of research.6 However, studies have demonstrated that when referencing research literature, physicians tend to read only the abstract of a study rather than the entire article.7,8 A study by Marcelo et al8 showed that residents at a tertiary care center answered clinical questions using only the abstract of a paper 69% of the time. Based on these findings, it is imperative that the results of systematic reviews be accurately reported in their abstracts because they can influence patient care.
Referencing only the abstracts of systematic reviews can be problematic if the abstract contains spin. Spin is a form of reporting that inappropriately highlights the benefits of a treatment with greater emphasis than what is shown by the results.9 Research has identified the presence of spin in the abstracts of randomized controlled trials.10-12 For example, Cooper et al10 found that 70% (33/47) of abstracts in otolaryngology randomized controlled trials contained spin. Additionally, Arthur et al11 and Austin et al12 had similar findings within abstracts of orthopedic and obesity trials, where 44.8% (112/250) and 46.7% (21/45) contained spin, respectively. Ottwell et al13 found that the presence of spin in abstracts is not limited to randomized controlled trials; they demonstrated that the abstracts of nearly one-third (31% [11/36]) of systematic reviews focused on the treatment of acne vulgaris contained spin.
In our study, we aimed to evaluate the presence of spin in the abstracts of systematic reviews focused on the treatment of psoriasis.
Methods
Reproducibility and Reporting—Our study did not meet the regulatory definition for human subjects research per the US Code of Federal Regulations because the study did not involve human research subjects. The study also was not subject to review by the institutional review board. Our protocol, data set, analysis scripts, extraction forms, and other material related to the study have been placed on Open Science Framework to provide transparency and ensure reproducibility. To further allow for analytic reproducibility, our data set was given to an independent laboratory and reanalyzed with a masked approach. Our study was carried out alongside other studies assessing spin in systematic reviews regarding different specialties and disease states. Because these studies were similar in design, this methodology also has been reported elsewhere. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)14 and the guidelines for meta-epidemiological studies developed by Murad and Wang15 were used in drafting this article.
Search Strategy—The search strategies for the MEDLINE (Ovid) and Embase (Ovid) databases were created by a systematic review librarian (D.N.W.) to identify systematic reviews and meta-analyses regarding treatments for psoriasis (Figure 1). The searches were performed on June 2, 2020, and uploaded to Rayyan, a systematic review screening platform.16 After duplicates were removed, the records were screened for eligibility by 2 authors (C.H. and A.L.) using the titles and abstracts. Screening was conducted independently while each of these authors was masked to the other’s results; disagreements were resolved through discussion.

Eligibility Criteria—An article had to meet the following criteria for inclusion in our study: (1) be a systematic review with or without a meta-analysis; (2) relate to the treatment of psoriasis; and (3) be written in English and include human patients only. The PRISMA definition of systematic reviews and meta-analyses was applied.17
Training—Various training occurred throughout our study to ensure understanding of each step and mitigate subjectivity. Before beginning screening, 2 investigators (C.H. and A.L.) completed the Introduction to Systematic Review and Meta-Analysis course offered by Johns Hopkins University.18 They also underwent 2 days of online and in-person training on the definition and interpretation of the 9 most severe types of spin found in the abstracts of systematic reviews as defined by Yavchitz et al.9 Finally, they were trained to use A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) to appraise the methodological quality of each systematic review. Our protocol contained an outline of all training modules used.
Data Extraction—The investigators (C.H. and A.L.) analyzed included abstracts for the 9 most severe types of spin (Table 1). Data were extracted in a masked duplicate fashion using the Google form. AMSTAR-2 was used to assess systematic reviews for methodological quality. AMSTAR-2 is an appraisal tool consisting of a 16-item checklist for systematic reviews or meta-analyses. Scores range from critically low to high based on the methodological quality of the review. Interrater reliability of AMSTAR-2 scores has been moderate to high across studies. Construct validity coefficients have been high with the original AMSTAR instrument (r=0.91) and the Risk of Bias in Systematic Reviews instrument (r=0.84).19

During data extraction from each included systematic review, the following additional items were obtained: (1) the date the review was received; (2) intervention type (ie, pharmacologic, nonpharmacologic, surgery, light therapy, mixed); (3) the funding source(s) for each systematic review (ie, industry, private, public, none, not mentioned, hospital, a combination of funding not including industry, a combination of funding including industry, other); (4) whether the journal submission guidelines suggested adherence to PRISMA guidelines; (5) whether the review discussed adherence to PRISMA14 or PRISMA for Abstracts20 (PRISMA-A); (6) the publishing journal’s 5-year impact factor; and (6) the country of the systematic review’s origin. When data extraction was complete, investigators (C.H. and A.L.) were unmasked and met to resolve any disagreements by discussion. Two authors (R.O. or M.V.) served as arbiters in the case that an agreement between C.H. and A.L. could not be reached.
Statistical Analysis—Frequencies and percentages were calculated to evaluate the most common types of spin found within systematic reviews and meta-analyses. One author (M.H.) prespecified the possibility of a binary logistic regression and calculated a power analysis to determine sample size, as stated in our protocol. Our final sample size of 173 was not powered to perform the multivariable logistic regression; therefore, we calculated unadjusted odds ratios to enable assessing relationships between the presence of spin in abstracts and the various study characteristics. We used Stata 16.1 for all analyses, and all analytic decisions can be found in our protocol.
Results
General Characteristics—Our systematic search of MEDLINE and Embase returned 3200 articles, of which 665 were duplicates that were removed. An additional 2253 articles were excluded during initial abstract and title screening, and full-text screening led to the exclusion of another 109 articles. In total, 173 systematic reviews were included for data extraction. Figure 2 illustrates the screening process with the rationale for all exclusions.

Of the 173 included systematic reviews and meta-analyses, 150 (86.7%) focused on pharmacologic interventions. The majority of studies did not mention adhering to PRISMA guidelines (125/173 [72.3%]), and the publishing journals recommended their authors adhere to PRISMA for only 66 (38.2%) of the included articles. For the articles that received funding (90/173 [52.0%]), industry sources were the most common funding source (40/90 [44.4%]), followed by private (27/90 [30%]) and public funding sources (23/90 [25.6%]). Of the remaining studies, 46 articles did not include a funding statement (46/83 [55.4%]), and 37 studies were not funded (37/83 [44.6%]). The average (SD) 5-year impact factor of our included journals was 4.68 (4.64). Systematic reviews were from 31 different countries. All studies were received by their respective journals between the years 2000 and 2020 (Table 2).

Abstracts Containing Spin—We found that 37 (21.4%) of the abstracts of systematic reviews focused on psoriasis treatments contained at least 1 type of spin. Some abstracts had more than 1 type; thus, a total of 51 different instances of spin were detected. Spin type 6—selective reporting of or overemphasis on harm outcomes or analysis favoring the safety of the experimental intervention—was the most common type ofspin, found in 19 of 173 abstracts (11.0%). The most severe type of spin—type 1 (conclusion contains recommendations for clinical practice not supported by the findings)—occurred in only 1 abstract (0.6%). Spin type 8 did not occur in any of the abstracts (Table 1). There was no statistically significant association between the presence of spin and any of the study characteristics (Table 2).
AMSTAR Ratings—After using AMSTAR-2 to appraise the included systematic reviews, we found that 6 (3.5%) of the 173 studies could be rated as high; 36 (20.8%) as moderate; 25 (14.5%) as low; and 106 (61.3%) as critically low. Of the 37 abstracts containing spin, 2 (5.4%) had an AMSTAR-2 rating of high, 10 (27%) had a rating of moderate, 6 (16.2%) had a rating of low, and 19 (51.4%) had a rating of critically low (Table 2). No statistically significant associations were seen between abstracts found to have spin and the AMSTAR-2 rating of the review.
Nearly all (160/173 [92.5%]) of the included reviews were compliant with the inclusion of Population, Intervention, Comparison, and Outcome (PICO) method. Only 17 of 173 (9.8%) reviews reported funding sources for the studies included. See Table 3 for all AMSTAR-2 items.

Comment
Primary Findings—We evaluated the abstracts of systematic reviews for the treatment of psoriasis and found that more than one-fifth of them contained spin. Our study contributes to the existing literature surrounding spin. Spin in randomized controlled trials is well documented across several fields of medicine, including otolaryngology,10 obesity medicine,12 dermatology,21 anesthesiology,22 psychiatry,23 orthopedics,24 emergency medicine,25 oncology,26 and cardiology.27 More recently, studies have emerged evaluating the presence of spin in systematic reviews. Specific to dermatology, one study found that 74% (84/113) of systematic reviews related to atopic dermatitis treatment contained spin.28 Additionally, Ottwell et al13 identified spin in 31% (11/36) of the systematic reviews related to the treatment of acne vulgaris, which is similar to our results for systematic reviews focused on psoriasis treatments. When comparing the presence of spin in abstracts of systematic reviews from the field of dermatology with other specialties, dermatology-focused systematic reviews appear to contain more spin in the abstract than systematic reviews focused on tinnitus and glaucoma therapies.29,30 However, systematic reviews from the field of dermatology appear to contain less spin than systematic reviews focused on therapies for lower back pain.31 For example, Nascimento et al31 found that 80% (53/66) of systematic reviews focused on low-back pain treatments contained spin.
Examples of Spin—The most common type of spin found in our study was type 6.9 An example of spin type 6 can be found in an article by Bai et al32 that investigated the short-term efficacy and safety of multiple interleukin inhibitors for the treatment of plaque psoriasis. The conclusion of the abstract states, “Risankizumab appeared to have relatively high efficacy and low risk.” However, in the results section, the authors showed that risankizumab had the highest risk of serious adverse events and was ranked highest for discontinuation because of adverse events when compared with other interleukin inhibitors. Here, the presence of spin in the abstract may mislead the reader to accept the “low risk” of risankizumab without understanding the study’s full results.32
Another example of selective reporting of harm outcomes in a systematic review can be found in the article by Wu et al,33 which focused on assessing IL-17 antagonists for the treatment of plaque psoriasis. The conclusion of the abstract indicated that IL-17 antagonists should be accepted as safe; however, in the results section, the authors discussed serious safety concerns with brodalumab, including the death of 4 patients from suicide.33 This example of spin type 6 highlights how the overgeneralization of a drug’s safety profile neglects serious harm outcomes that are critical to patient safety. In fact, against the safety claims of Wu et al,33 brodalumab later received a boxed warning from the US Food and Drug Administration after 6 patients died from suicide while receiving the drug, which led to early discontinuation of the trials.34,35 Although studies suggest this relationship is not causal,34-36 the purpose of our study was not to investigate this association but to highlight the importance of this finding. Thus, with this example of spin in mind, we offer recommendations that we believe will improve reporting in abstracts as well as quality of patient care.
Recommendations for Reporting in Abstracts—Regarding the boxed warning37 for brodalumab because of suicidal ideation and behavior, the US Food and Drug Administration recommends that prior to prescribing brodalumab, clinicians consider the potential benefits and risks in patients with a history of depression and/or suicidal ideation or behavior. However, a clinician would not adequately assess the full risks and benefits when an abstract, such as that for the article by Wu et al,33 contains spin through selectively reporting harm outcomes. Arguably, clinicians could just read the full text; however, research confirms that abstracts often are utilized by clinicians and commonly are used to guide clinical decisions.7,38 It is reasonable that clinicians would use abstracts in this fashion because they provide a quick synopsis of the full article’s findings and are widely available to clinicians who may not have access to article databases. Initiatives are in place to improve the quality of reporting in an abstract, such as PRISMA-A,20 but even this fails to address spin. In fact, it may suggest spin because checklist item 10 of PRISMA-A advises authors of systematic reviews to provide a “general interpretation of the results and important implications.” This item is concerning because it suggests that the authors interpret importance rather than the clinician who prescribes the drug and is ultimately responsible for patient safety. Therefore, we recommend a reform to abstract reporting and an update to PRISMA-A that leads authors to report all benefits and risks encountered instead of reporting what the authors define as important.
Strengths and Limitations—Our study has several strengths as well as limitations. One of these strengths is that our protocol was strictly adhered to; any deviations were noted and added as an amendment. Our protocol, data, and all study artifacts were made freely available online on the Open Science Framework to strengthen reproducibility (https://osf.io/zrxh8/). Investigators underwent training to ensure comprehension of spin and systematic review designs. All data were extracted in masked duplicate fashion per the Cochrane Handbook for Systematic Reviews of Interventions.39
Regarding limitations, only 2 databases were searched—MEDLINE and Embase. Therefore, our screening process may not have included every available systematic review on the treatment of psoriasis. Journal impact factors may be inaccurate for the systematic reviews that were published earlier in our data date range; however, we attempted to negate this limitation by using a 5-year average. Our study characteristic regarding PRISMA adherence did not account for studies published before the PRISMA statement release; we also could not access prior submission guidelines to determine when a journal began recommending PRISMA adherence. Another limitation of our study was the intrinsic subjectivity behind spin. Some may disagree with our classifications. Finally, our cross-sectional design should not be generalized to study types that are not systematic reviews or published in other journals during different periods.
Conclusion
Evidence of spin was present in many of the abstracts of systematic reviews pertaining to the treatment of psoriasis. Future clinical research should investigate any reporting of spin and search for ways to better reduce spin within literature. Continued research is necessary to evaluate the presence of spin within dermatology and other specialties.
Psoriasis is an inflammatory autoimmune skin condition that affects approximately 125 million individuals worldwide, with approximately 8 million patients in the United States.1 Psoriasis not only involves a cosmetic component but also comprises other comorbidities, such as psoriatic arthritis, cardiovascular disease, and psychiatric disorders, that can influence patient quality of life.2-4 In addition, the costs associated with psoriasis are substantial, with an estimated economic burden of $35.2 billion in the United States in 2015.5 Given the prevalence of psoriasis and its many effects on patients, it is important that providers have high-quality evidence regarding efficacious treatment options.
Systematic reviews, which compile all available evidence on a subject to answer a specific question, represent the gold standard of research.6 However, studies have demonstrated that when referencing research literature, physicians tend to read only the abstract of a study rather than the entire article.7,8 A study by Marcelo et al8 showed that residents at a tertiary care center answered clinical questions using only the abstract of a paper 69% of the time. Based on these findings, it is imperative that the results of systematic reviews be accurately reported in their abstracts because they can influence patient care.
Referencing only the abstracts of systematic reviews can be problematic if the abstract contains spin. Spin is a form of reporting that inappropriately highlights the benefits of a treatment with greater emphasis than what is shown by the results.9 Research has identified the presence of spin in the abstracts of randomized controlled trials.10-12 For example, Cooper et al10 found that 70% (33/47) of abstracts in otolaryngology randomized controlled trials contained spin. Additionally, Arthur et al11 and Austin et al12 had similar findings within abstracts of orthopedic and obesity trials, where 44.8% (112/250) and 46.7% (21/45) contained spin, respectively. Ottwell et al13 found that the presence of spin in abstracts is not limited to randomized controlled trials; they demonstrated that the abstracts of nearly one-third (31% [11/36]) of systematic reviews focused on the treatment of acne vulgaris contained spin.
In our study, we aimed to evaluate the presence of spin in the abstracts of systematic reviews focused on the treatment of psoriasis.
Methods
Reproducibility and Reporting—Our study did not meet the regulatory definition for human subjects research per the US Code of Federal Regulations because the study did not involve human research subjects. The study also was not subject to review by the institutional review board. Our protocol, data set, analysis scripts, extraction forms, and other material related to the study have been placed on Open Science Framework to provide transparency and ensure reproducibility. To further allow for analytic reproducibility, our data set was given to an independent laboratory and reanalyzed with a masked approach. Our study was carried out alongside other studies assessing spin in systematic reviews regarding different specialties and disease states. Because these studies were similar in design, this methodology also has been reported elsewhere. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)14 and the guidelines for meta-epidemiological studies developed by Murad and Wang15 were used in drafting this article.
Search Strategy—The search strategies for the MEDLINE (Ovid) and Embase (Ovid) databases were created by a systematic review librarian (D.N.W.) to identify systematic reviews and meta-analyses regarding treatments for psoriasis (Figure 1). The searches were performed on June 2, 2020, and uploaded to Rayyan, a systematic review screening platform.16 After duplicates were removed, the records were screened for eligibility by 2 authors (C.H. and A.L.) using the titles and abstracts. Screening was conducted independently while each of these authors was masked to the other’s results; disagreements were resolved through discussion.

Eligibility Criteria—An article had to meet the following criteria for inclusion in our study: (1) be a systematic review with or without a meta-analysis; (2) relate to the treatment of psoriasis; and (3) be written in English and include human patients only. The PRISMA definition of systematic reviews and meta-analyses was applied.17
Training—Various training occurred throughout our study to ensure understanding of each step and mitigate subjectivity. Before beginning screening, 2 investigators (C.H. and A.L.) completed the Introduction to Systematic Review and Meta-Analysis course offered by Johns Hopkins University.18 They also underwent 2 days of online and in-person training on the definition and interpretation of the 9 most severe types of spin found in the abstracts of systematic reviews as defined by Yavchitz et al.9 Finally, they were trained to use A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) to appraise the methodological quality of each systematic review. Our protocol contained an outline of all training modules used.
Data Extraction—The investigators (C.H. and A.L.) analyzed included abstracts for the 9 most severe types of spin (Table 1). Data were extracted in a masked duplicate fashion using the Google form. AMSTAR-2 was used to assess systematic reviews for methodological quality. AMSTAR-2 is an appraisal tool consisting of a 16-item checklist for systematic reviews or meta-analyses. Scores range from critically low to high based on the methodological quality of the review. Interrater reliability of AMSTAR-2 scores has been moderate to high across studies. Construct validity coefficients have been high with the original AMSTAR instrument (r=0.91) and the Risk of Bias in Systematic Reviews instrument (r=0.84).19

During data extraction from each included systematic review, the following additional items were obtained: (1) the date the review was received; (2) intervention type (ie, pharmacologic, nonpharmacologic, surgery, light therapy, mixed); (3) the funding source(s) for each systematic review (ie, industry, private, public, none, not mentioned, hospital, a combination of funding not including industry, a combination of funding including industry, other); (4) whether the journal submission guidelines suggested adherence to PRISMA guidelines; (5) whether the review discussed adherence to PRISMA14 or PRISMA for Abstracts20 (PRISMA-A); (6) the publishing journal’s 5-year impact factor; and (6) the country of the systematic review’s origin. When data extraction was complete, investigators (C.H. and A.L.) were unmasked and met to resolve any disagreements by discussion. Two authors (R.O. or M.V.) served as arbiters in the case that an agreement between C.H. and A.L. could not be reached.
Statistical Analysis—Frequencies and percentages were calculated to evaluate the most common types of spin found within systematic reviews and meta-analyses. One author (M.H.) prespecified the possibility of a binary logistic regression and calculated a power analysis to determine sample size, as stated in our protocol. Our final sample size of 173 was not powered to perform the multivariable logistic regression; therefore, we calculated unadjusted odds ratios to enable assessing relationships between the presence of spin in abstracts and the various study characteristics. We used Stata 16.1 for all analyses, and all analytic decisions can be found in our protocol.
Results
General Characteristics—Our systematic search of MEDLINE and Embase returned 3200 articles, of which 665 were duplicates that were removed. An additional 2253 articles were excluded during initial abstract and title screening, and full-text screening led to the exclusion of another 109 articles. In total, 173 systematic reviews were included for data extraction. Figure 2 illustrates the screening process with the rationale for all exclusions.

Of the 173 included systematic reviews and meta-analyses, 150 (86.7%) focused on pharmacologic interventions. The majority of studies did not mention adhering to PRISMA guidelines (125/173 [72.3%]), and the publishing journals recommended their authors adhere to PRISMA for only 66 (38.2%) of the included articles. For the articles that received funding (90/173 [52.0%]), industry sources were the most common funding source (40/90 [44.4%]), followed by private (27/90 [30%]) and public funding sources (23/90 [25.6%]). Of the remaining studies, 46 articles did not include a funding statement (46/83 [55.4%]), and 37 studies were not funded (37/83 [44.6%]). The average (SD) 5-year impact factor of our included journals was 4.68 (4.64). Systematic reviews were from 31 different countries. All studies were received by their respective journals between the years 2000 and 2020 (Table 2).

Abstracts Containing Spin—We found that 37 (21.4%) of the abstracts of systematic reviews focused on psoriasis treatments contained at least 1 type of spin. Some abstracts had more than 1 type; thus, a total of 51 different instances of spin were detected. Spin type 6—selective reporting of or overemphasis on harm outcomes or analysis favoring the safety of the experimental intervention—was the most common type ofspin, found in 19 of 173 abstracts (11.0%). The most severe type of spin—type 1 (conclusion contains recommendations for clinical practice not supported by the findings)—occurred in only 1 abstract (0.6%). Spin type 8 did not occur in any of the abstracts (Table 1). There was no statistically significant association between the presence of spin and any of the study characteristics (Table 2).
AMSTAR Ratings—After using AMSTAR-2 to appraise the included systematic reviews, we found that 6 (3.5%) of the 173 studies could be rated as high; 36 (20.8%) as moderate; 25 (14.5%) as low; and 106 (61.3%) as critically low. Of the 37 abstracts containing spin, 2 (5.4%) had an AMSTAR-2 rating of high, 10 (27%) had a rating of moderate, 6 (16.2%) had a rating of low, and 19 (51.4%) had a rating of critically low (Table 2). No statistically significant associations were seen between abstracts found to have spin and the AMSTAR-2 rating of the review.
Nearly all (160/173 [92.5%]) of the included reviews were compliant with the inclusion of Population, Intervention, Comparison, and Outcome (PICO) method. Only 17 of 173 (9.8%) reviews reported funding sources for the studies included. See Table 3 for all AMSTAR-2 items.

Comment
Primary Findings—We evaluated the abstracts of systematic reviews for the treatment of psoriasis and found that more than one-fifth of them contained spin. Our study contributes to the existing literature surrounding spin. Spin in randomized controlled trials is well documented across several fields of medicine, including otolaryngology,10 obesity medicine,12 dermatology,21 anesthesiology,22 psychiatry,23 orthopedics,24 emergency medicine,25 oncology,26 and cardiology.27 More recently, studies have emerged evaluating the presence of spin in systematic reviews. Specific to dermatology, one study found that 74% (84/113) of systematic reviews related to atopic dermatitis treatment contained spin.28 Additionally, Ottwell et al13 identified spin in 31% (11/36) of the systematic reviews related to the treatment of acne vulgaris, which is similar to our results for systematic reviews focused on psoriasis treatments. When comparing the presence of spin in abstracts of systematic reviews from the field of dermatology with other specialties, dermatology-focused systematic reviews appear to contain more spin in the abstract than systematic reviews focused on tinnitus and glaucoma therapies.29,30 However, systematic reviews from the field of dermatology appear to contain less spin than systematic reviews focused on therapies for lower back pain.31 For example, Nascimento et al31 found that 80% (53/66) of systematic reviews focused on low-back pain treatments contained spin.
Examples of Spin—The most common type of spin found in our study was type 6.9 An example of spin type 6 can be found in an article by Bai et al32 that investigated the short-term efficacy and safety of multiple interleukin inhibitors for the treatment of plaque psoriasis. The conclusion of the abstract states, “Risankizumab appeared to have relatively high efficacy and low risk.” However, in the results section, the authors showed that risankizumab had the highest risk of serious adverse events and was ranked highest for discontinuation because of adverse events when compared with other interleukin inhibitors. Here, the presence of spin in the abstract may mislead the reader to accept the “low risk” of risankizumab without understanding the study’s full results.32
Another example of selective reporting of harm outcomes in a systematic review can be found in the article by Wu et al,33 which focused on assessing IL-17 antagonists for the treatment of plaque psoriasis. The conclusion of the abstract indicated that IL-17 antagonists should be accepted as safe; however, in the results section, the authors discussed serious safety concerns with brodalumab, including the death of 4 patients from suicide.33 This example of spin type 6 highlights how the overgeneralization of a drug’s safety profile neglects serious harm outcomes that are critical to patient safety. In fact, against the safety claims of Wu et al,33 brodalumab later received a boxed warning from the US Food and Drug Administration after 6 patients died from suicide while receiving the drug, which led to early discontinuation of the trials.34,35 Although studies suggest this relationship is not causal,34-36 the purpose of our study was not to investigate this association but to highlight the importance of this finding. Thus, with this example of spin in mind, we offer recommendations that we believe will improve reporting in abstracts as well as quality of patient care.
Recommendations for Reporting in Abstracts—Regarding the boxed warning37 for brodalumab because of suicidal ideation and behavior, the US Food and Drug Administration recommends that prior to prescribing brodalumab, clinicians consider the potential benefits and risks in patients with a history of depression and/or suicidal ideation or behavior. However, a clinician would not adequately assess the full risks and benefits when an abstract, such as that for the article by Wu et al,33 contains spin through selectively reporting harm outcomes. Arguably, clinicians could just read the full text; however, research confirms that abstracts often are utilized by clinicians and commonly are used to guide clinical decisions.7,38 It is reasonable that clinicians would use abstracts in this fashion because they provide a quick synopsis of the full article’s findings and are widely available to clinicians who may not have access to article databases. Initiatives are in place to improve the quality of reporting in an abstract, such as PRISMA-A,20 but even this fails to address spin. In fact, it may suggest spin because checklist item 10 of PRISMA-A advises authors of systematic reviews to provide a “general interpretation of the results and important implications.” This item is concerning because it suggests that the authors interpret importance rather than the clinician who prescribes the drug and is ultimately responsible for patient safety. Therefore, we recommend a reform to abstract reporting and an update to PRISMA-A that leads authors to report all benefits and risks encountered instead of reporting what the authors define as important.
Strengths and Limitations—Our study has several strengths as well as limitations. One of these strengths is that our protocol was strictly adhered to; any deviations were noted and added as an amendment. Our protocol, data, and all study artifacts were made freely available online on the Open Science Framework to strengthen reproducibility (https://osf.io/zrxh8/). Investigators underwent training to ensure comprehension of spin and systematic review designs. All data were extracted in masked duplicate fashion per the Cochrane Handbook for Systematic Reviews of Interventions.39
Regarding limitations, only 2 databases were searched—MEDLINE and Embase. Therefore, our screening process may not have included every available systematic review on the treatment of psoriasis. Journal impact factors may be inaccurate for the systematic reviews that were published earlier in our data date range; however, we attempted to negate this limitation by using a 5-year average. Our study characteristic regarding PRISMA adherence did not account for studies published before the PRISMA statement release; we also could not access prior submission guidelines to determine when a journal began recommending PRISMA adherence. Another limitation of our study was the intrinsic subjectivity behind spin. Some may disagree with our classifications. Finally, our cross-sectional design should not be generalized to study types that are not systematic reviews or published in other journals during different periods.
Conclusion
Evidence of spin was present in many of the abstracts of systematic reviews pertaining to the treatment of psoriasis. Future clinical research should investigate any reporting of spin and search for ways to better reduce spin within literature. Continued research is necessary to evaluate the presence of spin within dermatology and other specialties.
- Psoriasis statistics. National Psoriasis Foundation. Updated December 21, 2022. Accessed March 6, 2023. https://www.psoriasis.org/content/statistics
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
- Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18:2211.
- Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18:613-620.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta‑analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2:9-14.
- Barry HC, Ebell MH, Shaughnessy AF, et al. Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract. 2001;14:437-442.
- Marcelo A, Gavino A, Isip-Tan IT, et al. A comparison of the accuracy of clinical decisions based on full-text articles and on journal abstracts alone: a study among residents in a tertiary care hospital. Evid Based Med. 2013;18:48-53.
- Yavchitz A, Ravaud P, Altman DG, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56-65.
- Cooper CM, Gray HM, Ross AE, et al. Evaluation of spin in the abstracts of otolaryngology randomized controlled trials. Laryngoscope. 2019;129:2036-2040.
- Arthur W, Zaaza Z, Checketts JX, et al. Analyzing spin in abstracts of orthopaedic randomized controlled trials with statistically insignificant primary endpoints. Arthroscopy. 2020;36:1443-1450.
- Austin J, Smith C, Natarajan K, et al. Evaluation of spin within abstracts in obesity randomized clinical trials: a cross-sectional review. Clin Obes. 2019;9:E12292.
- Ottwell R, Rogers TC, Michael Anderson J, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on the treatment of acne vulgaris: cross-sectional analysis. JMIR Dermatol. 2020;3:E16978.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:E1000100.
- Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22:139-142.
- Rayyan QCRI. Accessed September 10, 2019. https://rayyan.qcri.org/reviews/81224
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Coursera. Introduction to systematic review and meta-analysis. Accessed May 18, 2023. https://www.coursera.org/learn/systematic-review
- Lorenz RC, Matthias K, Pieper D, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 2019;114:133-140.
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:E1001419.
- Motosko CC, Ault AK, Kimberly LL, et al. Analysis of spin in the reporting of studies of topical treatments of photoaged skin. J Am Acad Dermatol. 2019;80:516-522.e12.
- Kinder NC, Weaver MD, Wayant C, et al. Presence of “spin” in the abstracts and titles of anaesthesiology randomised controlled trials. Br J Anaesth. 2019;122:E13-E14.
- Jellison S, Roberts W, Bowers A, et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2019;5:178-181.
- Checketts JX, Riddle J, Zaaza Z, et al. An evaluation of spin in lower extremity joint trials. J Arthroplasty. 2019;34:1008-1012.
- Reynolds-Vaughn V, Riddle J, Brown J, et al. Evaluation of spin in the abstracts of emergency medicine randomized controlled trials. Ann Emerg Med. 2019;14:423-431.
- Wayant C, Margalski D, Vaughn K, et al. Evaluation of spin in oncology clinical trials. Crit Rev Oncol Hematol. 2019;144:102821.
- Khan MS, Lateef N, Siddiqi TJ, et al. Level and prevalence of spin in published cardiovascular randomized clinical trial reports with statistically nonsignificant primary outcomes: a systematic review. JAMA Netw Open. 2019;2:E192622.
- Lin V, Patel R, Wirtz A, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of atopic dermatitis treatments and interventions. Dermatology. 2021;237:496-505.
- Rucker B, Umbarger E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on tinnitus. Otol Neurotol. 2021;10:1237-1244.
- Okonya O, Lai E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of treatments for glaucoma. J Glaucoma. 2021;30:235-241.
- Nascimento DP, Gonzalez GZ, Araujo AC, et al. Eight out of every ten abstracts of low back pain systematic reviews presented spin and inconsistencies with the full text: an analysis of 66 systematic reviews. J Orthop Sports Phys Ther. 2020;50:17-23.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-1003.
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23:1-3.
- Rodrigeuz-Bolanos F, Gooderham M, Papp K. A closer look at the data regarding suicidal ideation and behavior in psoriasis patients: the case of brodalumab. Skin Therapy Lett. 2019;24:1-4.
- Danesh MJ, Kimball AB. Brodalumab and suicidal ideation in the context of a recent economic crisis in the United States. J Am Acad Dermatol. 2016;74:190-192.
- Siliq. Prescribing information. Valeant Pharmaceuticals North America LLC; 2017. Accessed May 18, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Johnson HL, Fontelo P, Olsen CH, et al. Family nurse practitioner student perception of journal abstract usefulness in clinical decision making: a randomized controlled trial. J Am Assoc Nurse Pract. 2013;25:597-603.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
- Psoriasis statistics. National Psoriasis Foundation. Updated December 21, 2022. Accessed March 6, 2023. https://www.psoriasis.org/content/statistics
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
- Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18:2211.
- Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18:613-620.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta‑analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2:9-14.
- Barry HC, Ebell MH, Shaughnessy AF, et al. Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract. 2001;14:437-442.
- Marcelo A, Gavino A, Isip-Tan IT, et al. A comparison of the accuracy of clinical decisions based on full-text articles and on journal abstracts alone: a study among residents in a tertiary care hospital. Evid Based Med. 2013;18:48-53.
- Yavchitz A, Ravaud P, Altman DG, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56-65.
- Cooper CM, Gray HM, Ross AE, et al. Evaluation of spin in the abstracts of otolaryngology randomized controlled trials. Laryngoscope. 2019;129:2036-2040.
- Arthur W, Zaaza Z, Checketts JX, et al. Analyzing spin in abstracts of orthopaedic randomized controlled trials with statistically insignificant primary endpoints. Arthroscopy. 2020;36:1443-1450.
- Austin J, Smith C, Natarajan K, et al. Evaluation of spin within abstracts in obesity randomized clinical trials: a cross-sectional review. Clin Obes. 2019;9:E12292.
- Ottwell R, Rogers TC, Michael Anderson J, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on the treatment of acne vulgaris: cross-sectional analysis. JMIR Dermatol. 2020;3:E16978.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:E1000100.
- Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22:139-142.
- Rayyan QCRI. Accessed September 10, 2019. https://rayyan.qcri.org/reviews/81224
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Coursera. Introduction to systematic review and meta-analysis. Accessed May 18, 2023. https://www.coursera.org/learn/systematic-review
- Lorenz RC, Matthias K, Pieper D, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 2019;114:133-140.
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:E1001419.
- Motosko CC, Ault AK, Kimberly LL, et al. Analysis of spin in the reporting of studies of topical treatments of photoaged skin. J Am Acad Dermatol. 2019;80:516-522.e12.
- Kinder NC, Weaver MD, Wayant C, et al. Presence of “spin” in the abstracts and titles of anaesthesiology randomised controlled trials. Br J Anaesth. 2019;122:E13-E14.
- Jellison S, Roberts W, Bowers A, et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2019;5:178-181.
- Checketts JX, Riddle J, Zaaza Z, et al. An evaluation of spin in lower extremity joint trials. J Arthroplasty. 2019;34:1008-1012.
- Reynolds-Vaughn V, Riddle J, Brown J, et al. Evaluation of spin in the abstracts of emergency medicine randomized controlled trials. Ann Emerg Med. 2019;14:423-431.
- Wayant C, Margalski D, Vaughn K, et al. Evaluation of spin in oncology clinical trials. Crit Rev Oncol Hematol. 2019;144:102821.
- Khan MS, Lateef N, Siddiqi TJ, et al. Level and prevalence of spin in published cardiovascular randomized clinical trial reports with statistically nonsignificant primary outcomes: a systematic review. JAMA Netw Open. 2019;2:E192622.
- Lin V, Patel R, Wirtz A, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of atopic dermatitis treatments and interventions. Dermatology. 2021;237:496-505.
- Rucker B, Umbarger E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on tinnitus. Otol Neurotol. 2021;10:1237-1244.
- Okonya O, Lai E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of treatments for glaucoma. J Glaucoma. 2021;30:235-241.
- Nascimento DP, Gonzalez GZ, Araujo AC, et al. Eight out of every ten abstracts of low back pain systematic reviews presented spin and inconsistencies with the full text: an analysis of 66 systematic reviews. J Orthop Sports Phys Ther. 2020;50:17-23.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-1003.
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23:1-3.
- Rodrigeuz-Bolanos F, Gooderham M, Papp K. A closer look at the data regarding suicidal ideation and behavior in psoriasis patients: the case of brodalumab. Skin Therapy Lett. 2019;24:1-4.
- Danesh MJ, Kimball AB. Brodalumab and suicidal ideation in the context of a recent economic crisis in the United States. J Am Acad Dermatol. 2016;74:190-192.
- Siliq. Prescribing information. Valeant Pharmaceuticals North America LLC; 2017. Accessed May 18, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Johnson HL, Fontelo P, Olsen CH, et al. Family nurse practitioner student perception of journal abstract usefulness in clinical decision making: a randomized controlled trial. J Am Assoc Nurse Pract. 2013;25:597-603.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
Practice Points
- Spin is defined as the intentional or unintentional misrepresentation of findings and can inappropriately highlight results and disregard results of equal importance.
- Our findings show that more than 20% of systematic reviews focused on the treatment of psoriasis contained some form of spin within the abstract.
- Because spin has the potential to misrepresent findings and distort a reader’s perception of psoriasis therapies, efforts are needed to prevent its occurrence.
First in utero cerebrovascular surgery success
The team from Boston Children’s Hospital and Brigham and Women’s Hospital used ultrasound guidance to repair the vein of Galen malformation, which causes excessively high blood flow, resulting in both neurologic and cardiac complications.
The surgery was performed in a fetus at 34 weeks’ gestational age, with remarkable results. Since birth, the baby girl, who was identified in utero as being at high risk of suffering serious complications of the malformation, has required no medication to treat heart failure and no postnatal surgery.
Repeated echocardiograms after birth displayed marked improvement in cardiac output, and brain MRI showed no brain injury and a normal neurologic exam.
“This is incredibly exciting. The hope is that this baby, and others with this condition who receive this in utero surgery in future, will go on to have a normal life,” lead researcher Darren B. Orbach, MD, PhD, said in an interview.
“We were thrilled to see that the aggressive decline usually seen after birth simply did not appear. We are pleased to report that at 6 weeks, the infant is progressing remarkably well, on no medications, eating normally, gaining weight and is back home. There are no signs of any negative effects on the brain,” he added.
Dr. Orbach, codirector of the Cerebrovascular Surgery & Interventions Center at Boston Children’s Hospital, and colleagues described this first case report of the in utero vein of Galen malformation repair in a research letter, published online in the journal Stroke.
Vein of Galen malformation
Dr. Orbach explained that vein of Galen malformation, which occurs in around 1 in every 60,000 births, is a cerebrovascular anomaly in which the arterial system is directly connected to the venous system rather than to capillaries that are necessary to slow blood flow and deliver oxygen to surrounding brain tissue.
“The arterial and venous systems are fundamentally very different. The arterial system is high pressure, high flow; while the venous system is low pressure, low flow. They shouldn’t be directly connected,” he noted.
The vein of Galen malformation is the most extreme version of such an anomaly. Developing in early gestation, it is associated with a large increase in blood flow through the brain which grows over time and can sometimes result in twice the total cardiac output of the body or even more, Dr. Orbach said.
The placenta is believed to be protective as most babies don’t have overt physiologic problems in utero, but they can run into crisis after birth, with the abnormally high blood flow causing an immense stress to the heart.
Babies typically present with heart failure as their first major symptom soon after birth, Dr. Orbach said. “Although the anatomical problem is in the brain, the clinical manifestation is high-output heart failure. The heart is trying to do double its normal work, pumping the blood to the malformation and immediately back to the heart and that blood is not performing any useful function.
“These newborns can get very sick. They need multiple medications to support their cardiovascular system and we need to do procedures to try and reduce the blood flow,” he explained.
Brain injury is also a common problem. “The brain circulation is very abnormal. The blood is being shunted through the malformation rather than circulating through the brain tissue which can become ischemic,” Dr. Orbach commented.
“The babies who get sick would have a very high mortality (up to 90%) without expert care. Even those who do receive expert care at a specialty center have a mortality rate of 30% to 40% and those who survive have a high risk of neurologic and cognitive impairment,” he added.
The current treatment for babies born with the condition involves transarterial embolization, by which a catheter is inserted into the arterial system to enable the malformation to be occluded by various techniques.
But Dr. Orbach pointed out that some babies are born too sick to have the postnatal intervention. “The heart failure and brain injury is so overwhelming that no matter what we do, we cannot reverse it, and these babies normally do not survive. What we are doing with the fetal surgery is trying to help those babies who cannot be treated with the current postnatal approach,” he said.
The first stage of this research involved trying to identify these very-high-risk babies in utero, and the researchers found that on fetal MRI a particular measurement of one of the venous sinuses that drains the main malformation was a good predictor of how the baby would fare after birth. The babies predicted to do poorly from this test are the targets for the fetal surgery.
The technique used for the postnatal intervention is too technically challenging to perform in utero. “So we have developed a different approach for the in utero surgery that involves navigating into the accepting vein in the malformation with a needle under ultrasound guidance, and then packing the vein with metal coils to dramatically reduce the blood flow,” Dr. Orbach explained.
This procedure was performed in this first patient on March 15. The surgery was part of a clinical trial that is planned to include 20 cases in total.
“The immediate goal is to see whether we can transform those fetuses who are at very high risk of getting sick after birth into babies who do well in the [neonatal] ICU and are able to be sent home for elective treatment at a few months of age,” Dr. Orbach noted. “The study is continuing as it is vital that we continue and show efficacy and safety in other patients as well,” he added.
Dr. Orbach said the results of this first case were extremely encouraging. “Each stage was exciting – the technical success of the procedure, and then seeing the [blood] flow diminish on the ultrasound right there during the procedure; then the next day we did a fetal echocardiogram, and we could see that the abnormal cardiac output was dramatically reduced, and a fetal MRI scan also showed the malformation was already coming down in size.”
The baby was born prematurely 2 days after the procedure because of ruptured membranes with a birth weight of 1.9 kg (4.2 lb). She has not required any cardiovascular support or postnatal embolization.
“We were waiting with bated breath until the baby was born to see how she did clinically. I was trying to be conservative in my expectations, but it was quickly apparent that she was going to do great,” he said. Now at home, she has some oxygen treatment for the first few weeks, “but right now her neurological status is completely intact and essentially she looks like any other baby,” Dr. Orbach commented.
It is not yet known whether the infant will need any additional procedures. “We will follow her closely and make a decision on whether further treatment is needed based on whether the malformation is growing or not,” Dr. Orbach said. Longer term follow-up will also assess secondary problems sometimes seen, such as learning problems and seizures.
Although other fetal surgeries are now routinely performed, this is believed to be the first in utero surgery aimed at the cerebrovascular system.
“There were a lot of uncertainties,” Dr. Orbach said. “We didn’t even know if we would be able to see our instruments on ultrasound.” To model the procedure, the researchers had a phantom fetal skull and brain constructed with a vein of Galen malformation, which was key to obtaining Food and Drug Administration approval for the study.
If the study shows success in the other patients too, the technique could be rolled out to other centers. “There definitely needs to be fetal surgery and neurointerventional teams familiar with vein of Galen malformation in place, and ready to manage complications after delivery regardless of outcome. But we are not the only center with those capabilities, so if our trial pans out, yes, the hope is that other teams in specialist children’s hospitals around the world could do this too,” he added.
Pioneering work
Commenting on the case report in an American Heart Association press release, Colin Derdeyn, MD, a neurointerventional radiologist at University of Iowa Health Care, Iowa City, who performs vein of Galen malformation embolizations on neonates, said: “The key advance here is to intervene before the physiologic events of birth can cause life-threatening heart failure.”
Dr. Derdeyn, who is a past chair of the American Heart Association’s Stroke Council, cautioned that one successful case is not enough experience to conclude that the risks of this procedure are worth the benefits.
But, he added: “The positive hemodynamic changes that they observed in utero and after birth – reduction in flow, reduction in size of the draining vein, reversal of the abnormal reversed flow in the aorta – are really encouraging. These are some of the most exciting and surprising aspects of this case report. This is pioneering work being done in a very careful and responsible way.”
The study was funded by a grant from the Sage Schermerhorn Chair for Image-Guided Therapy.
A version of this article first appeared on Medscape.com.
The team from Boston Children’s Hospital and Brigham and Women’s Hospital used ultrasound guidance to repair the vein of Galen malformation, which causes excessively high blood flow, resulting in both neurologic and cardiac complications.
The surgery was performed in a fetus at 34 weeks’ gestational age, with remarkable results. Since birth, the baby girl, who was identified in utero as being at high risk of suffering serious complications of the malformation, has required no medication to treat heart failure and no postnatal surgery.
Repeated echocardiograms after birth displayed marked improvement in cardiac output, and brain MRI showed no brain injury and a normal neurologic exam.
“This is incredibly exciting. The hope is that this baby, and others with this condition who receive this in utero surgery in future, will go on to have a normal life,” lead researcher Darren B. Orbach, MD, PhD, said in an interview.
“We were thrilled to see that the aggressive decline usually seen after birth simply did not appear. We are pleased to report that at 6 weeks, the infant is progressing remarkably well, on no medications, eating normally, gaining weight and is back home. There are no signs of any negative effects on the brain,” he added.
Dr. Orbach, codirector of the Cerebrovascular Surgery & Interventions Center at Boston Children’s Hospital, and colleagues described this first case report of the in utero vein of Galen malformation repair in a research letter, published online in the journal Stroke.
Vein of Galen malformation
Dr. Orbach explained that vein of Galen malformation, which occurs in around 1 in every 60,000 births, is a cerebrovascular anomaly in which the arterial system is directly connected to the venous system rather than to capillaries that are necessary to slow blood flow and deliver oxygen to surrounding brain tissue.
“The arterial and venous systems are fundamentally very different. The arterial system is high pressure, high flow; while the venous system is low pressure, low flow. They shouldn’t be directly connected,” he noted.
The vein of Galen malformation is the most extreme version of such an anomaly. Developing in early gestation, it is associated with a large increase in blood flow through the brain which grows over time and can sometimes result in twice the total cardiac output of the body or even more, Dr. Orbach said.
The placenta is believed to be protective as most babies don’t have overt physiologic problems in utero, but they can run into crisis after birth, with the abnormally high blood flow causing an immense stress to the heart.
Babies typically present with heart failure as their first major symptom soon after birth, Dr. Orbach said. “Although the anatomical problem is in the brain, the clinical manifestation is high-output heart failure. The heart is trying to do double its normal work, pumping the blood to the malformation and immediately back to the heart and that blood is not performing any useful function.
“These newborns can get very sick. They need multiple medications to support their cardiovascular system and we need to do procedures to try and reduce the blood flow,” he explained.
Brain injury is also a common problem. “The brain circulation is very abnormal. The blood is being shunted through the malformation rather than circulating through the brain tissue which can become ischemic,” Dr. Orbach commented.
“The babies who get sick would have a very high mortality (up to 90%) without expert care. Even those who do receive expert care at a specialty center have a mortality rate of 30% to 40% and those who survive have a high risk of neurologic and cognitive impairment,” he added.
The current treatment for babies born with the condition involves transarterial embolization, by which a catheter is inserted into the arterial system to enable the malformation to be occluded by various techniques.
But Dr. Orbach pointed out that some babies are born too sick to have the postnatal intervention. “The heart failure and brain injury is so overwhelming that no matter what we do, we cannot reverse it, and these babies normally do not survive. What we are doing with the fetal surgery is trying to help those babies who cannot be treated with the current postnatal approach,” he said.
The first stage of this research involved trying to identify these very-high-risk babies in utero, and the researchers found that on fetal MRI a particular measurement of one of the venous sinuses that drains the main malformation was a good predictor of how the baby would fare after birth. The babies predicted to do poorly from this test are the targets for the fetal surgery.
The technique used for the postnatal intervention is too technically challenging to perform in utero. “So we have developed a different approach for the in utero surgery that involves navigating into the accepting vein in the malformation with a needle under ultrasound guidance, and then packing the vein with metal coils to dramatically reduce the blood flow,” Dr. Orbach explained.
This procedure was performed in this first patient on March 15. The surgery was part of a clinical trial that is planned to include 20 cases in total.
“The immediate goal is to see whether we can transform those fetuses who are at very high risk of getting sick after birth into babies who do well in the [neonatal] ICU and are able to be sent home for elective treatment at a few months of age,” Dr. Orbach noted. “The study is continuing as it is vital that we continue and show efficacy and safety in other patients as well,” he added.
Dr. Orbach said the results of this first case were extremely encouraging. “Each stage was exciting – the technical success of the procedure, and then seeing the [blood] flow diminish on the ultrasound right there during the procedure; then the next day we did a fetal echocardiogram, and we could see that the abnormal cardiac output was dramatically reduced, and a fetal MRI scan also showed the malformation was already coming down in size.”
The baby was born prematurely 2 days after the procedure because of ruptured membranes with a birth weight of 1.9 kg (4.2 lb). She has not required any cardiovascular support or postnatal embolization.
“We were waiting with bated breath until the baby was born to see how she did clinically. I was trying to be conservative in my expectations, but it was quickly apparent that she was going to do great,” he said. Now at home, she has some oxygen treatment for the first few weeks, “but right now her neurological status is completely intact and essentially she looks like any other baby,” Dr. Orbach commented.
It is not yet known whether the infant will need any additional procedures. “We will follow her closely and make a decision on whether further treatment is needed based on whether the malformation is growing or not,” Dr. Orbach said. Longer term follow-up will also assess secondary problems sometimes seen, such as learning problems and seizures.
Although other fetal surgeries are now routinely performed, this is believed to be the first in utero surgery aimed at the cerebrovascular system.
“There were a lot of uncertainties,” Dr. Orbach said. “We didn’t even know if we would be able to see our instruments on ultrasound.” To model the procedure, the researchers had a phantom fetal skull and brain constructed with a vein of Galen malformation, which was key to obtaining Food and Drug Administration approval for the study.
If the study shows success in the other patients too, the technique could be rolled out to other centers. “There definitely needs to be fetal surgery and neurointerventional teams familiar with vein of Galen malformation in place, and ready to manage complications after delivery regardless of outcome. But we are not the only center with those capabilities, so if our trial pans out, yes, the hope is that other teams in specialist children’s hospitals around the world could do this too,” he added.
Pioneering work
Commenting on the case report in an American Heart Association press release, Colin Derdeyn, MD, a neurointerventional radiologist at University of Iowa Health Care, Iowa City, who performs vein of Galen malformation embolizations on neonates, said: “The key advance here is to intervene before the physiologic events of birth can cause life-threatening heart failure.”
Dr. Derdeyn, who is a past chair of the American Heart Association’s Stroke Council, cautioned that one successful case is not enough experience to conclude that the risks of this procedure are worth the benefits.
But, he added: “The positive hemodynamic changes that they observed in utero and after birth – reduction in flow, reduction in size of the draining vein, reversal of the abnormal reversed flow in the aorta – are really encouraging. These are some of the most exciting and surprising aspects of this case report. This is pioneering work being done in a very careful and responsible way.”
The study was funded by a grant from the Sage Schermerhorn Chair for Image-Guided Therapy.
A version of this article first appeared on Medscape.com.
The team from Boston Children’s Hospital and Brigham and Women’s Hospital used ultrasound guidance to repair the vein of Galen malformation, which causes excessively high blood flow, resulting in both neurologic and cardiac complications.
The surgery was performed in a fetus at 34 weeks’ gestational age, with remarkable results. Since birth, the baby girl, who was identified in utero as being at high risk of suffering serious complications of the malformation, has required no medication to treat heart failure and no postnatal surgery.
Repeated echocardiograms after birth displayed marked improvement in cardiac output, and brain MRI showed no brain injury and a normal neurologic exam.
“This is incredibly exciting. The hope is that this baby, and others with this condition who receive this in utero surgery in future, will go on to have a normal life,” lead researcher Darren B. Orbach, MD, PhD, said in an interview.
“We were thrilled to see that the aggressive decline usually seen after birth simply did not appear. We are pleased to report that at 6 weeks, the infant is progressing remarkably well, on no medications, eating normally, gaining weight and is back home. There are no signs of any negative effects on the brain,” he added.
Dr. Orbach, codirector of the Cerebrovascular Surgery & Interventions Center at Boston Children’s Hospital, and colleagues described this first case report of the in utero vein of Galen malformation repair in a research letter, published online in the journal Stroke.
Vein of Galen malformation
Dr. Orbach explained that vein of Galen malformation, which occurs in around 1 in every 60,000 births, is a cerebrovascular anomaly in which the arterial system is directly connected to the venous system rather than to capillaries that are necessary to slow blood flow and deliver oxygen to surrounding brain tissue.
“The arterial and venous systems are fundamentally very different. The arterial system is high pressure, high flow; while the venous system is low pressure, low flow. They shouldn’t be directly connected,” he noted.
The vein of Galen malformation is the most extreme version of such an anomaly. Developing in early gestation, it is associated with a large increase in blood flow through the brain which grows over time and can sometimes result in twice the total cardiac output of the body or even more, Dr. Orbach said.
The placenta is believed to be protective as most babies don’t have overt physiologic problems in utero, but they can run into crisis after birth, with the abnormally high blood flow causing an immense stress to the heart.
Babies typically present with heart failure as their first major symptom soon after birth, Dr. Orbach said. “Although the anatomical problem is in the brain, the clinical manifestation is high-output heart failure. The heart is trying to do double its normal work, pumping the blood to the malformation and immediately back to the heart and that blood is not performing any useful function.
“These newborns can get very sick. They need multiple medications to support their cardiovascular system and we need to do procedures to try and reduce the blood flow,” he explained.
Brain injury is also a common problem. “The brain circulation is very abnormal. The blood is being shunted through the malformation rather than circulating through the brain tissue which can become ischemic,” Dr. Orbach commented.
“The babies who get sick would have a very high mortality (up to 90%) without expert care. Even those who do receive expert care at a specialty center have a mortality rate of 30% to 40% and those who survive have a high risk of neurologic and cognitive impairment,” he added.
The current treatment for babies born with the condition involves transarterial embolization, by which a catheter is inserted into the arterial system to enable the malformation to be occluded by various techniques.
But Dr. Orbach pointed out that some babies are born too sick to have the postnatal intervention. “The heart failure and brain injury is so overwhelming that no matter what we do, we cannot reverse it, and these babies normally do not survive. What we are doing with the fetal surgery is trying to help those babies who cannot be treated with the current postnatal approach,” he said.
The first stage of this research involved trying to identify these very-high-risk babies in utero, and the researchers found that on fetal MRI a particular measurement of one of the venous sinuses that drains the main malformation was a good predictor of how the baby would fare after birth. The babies predicted to do poorly from this test are the targets for the fetal surgery.
The technique used for the postnatal intervention is too technically challenging to perform in utero. “So we have developed a different approach for the in utero surgery that involves navigating into the accepting vein in the malformation with a needle under ultrasound guidance, and then packing the vein with metal coils to dramatically reduce the blood flow,” Dr. Orbach explained.
This procedure was performed in this first patient on March 15. The surgery was part of a clinical trial that is planned to include 20 cases in total.
“The immediate goal is to see whether we can transform those fetuses who are at very high risk of getting sick after birth into babies who do well in the [neonatal] ICU and are able to be sent home for elective treatment at a few months of age,” Dr. Orbach noted. “The study is continuing as it is vital that we continue and show efficacy and safety in other patients as well,” he added.
Dr. Orbach said the results of this first case were extremely encouraging. “Each stage was exciting – the technical success of the procedure, and then seeing the [blood] flow diminish on the ultrasound right there during the procedure; then the next day we did a fetal echocardiogram, and we could see that the abnormal cardiac output was dramatically reduced, and a fetal MRI scan also showed the malformation was already coming down in size.”
The baby was born prematurely 2 days after the procedure because of ruptured membranes with a birth weight of 1.9 kg (4.2 lb). She has not required any cardiovascular support or postnatal embolization.
“We were waiting with bated breath until the baby was born to see how she did clinically. I was trying to be conservative in my expectations, but it was quickly apparent that she was going to do great,” he said. Now at home, she has some oxygen treatment for the first few weeks, “but right now her neurological status is completely intact and essentially she looks like any other baby,” Dr. Orbach commented.
It is not yet known whether the infant will need any additional procedures. “We will follow her closely and make a decision on whether further treatment is needed based on whether the malformation is growing or not,” Dr. Orbach said. Longer term follow-up will also assess secondary problems sometimes seen, such as learning problems and seizures.
Although other fetal surgeries are now routinely performed, this is believed to be the first in utero surgery aimed at the cerebrovascular system.
“There were a lot of uncertainties,” Dr. Orbach said. “We didn’t even know if we would be able to see our instruments on ultrasound.” To model the procedure, the researchers had a phantom fetal skull and brain constructed with a vein of Galen malformation, which was key to obtaining Food and Drug Administration approval for the study.
If the study shows success in the other patients too, the technique could be rolled out to other centers. “There definitely needs to be fetal surgery and neurointerventional teams familiar with vein of Galen malformation in place, and ready to manage complications after delivery regardless of outcome. But we are not the only center with those capabilities, so if our trial pans out, yes, the hope is that other teams in specialist children’s hospitals around the world could do this too,” he added.
Pioneering work
Commenting on the case report in an American Heart Association press release, Colin Derdeyn, MD, a neurointerventional radiologist at University of Iowa Health Care, Iowa City, who performs vein of Galen malformation embolizations on neonates, said: “The key advance here is to intervene before the physiologic events of birth can cause life-threatening heart failure.”
Dr. Derdeyn, who is a past chair of the American Heart Association’s Stroke Council, cautioned that one successful case is not enough experience to conclude that the risks of this procedure are worth the benefits.
But, he added: “The positive hemodynamic changes that they observed in utero and after birth – reduction in flow, reduction in size of the draining vein, reversal of the abnormal reversed flow in the aorta – are really encouraging. These are some of the most exciting and surprising aspects of this case report. This is pioneering work being done in a very careful and responsible way.”
The study was funded by a grant from the Sage Schermerhorn Chair for Image-Guided Therapy.
A version of this article first appeared on Medscape.com.
FROM STROKE