User login
Hyperlipidemia management: A calibrated approach
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2
The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
- Use an alternative to the Friedewald equation, such as the Martin–Hopkins equation, to estimate the low-density lipoprotein cholesterol (LDL-C) value; order direct measurement of LDL-C; or calculate non–high-density lipoprotein cholesterol to assess the risk for atherosclerotic cardiovascular disease (ASCVD) in patients who have a low LDL-C or a high triglycerides level. C
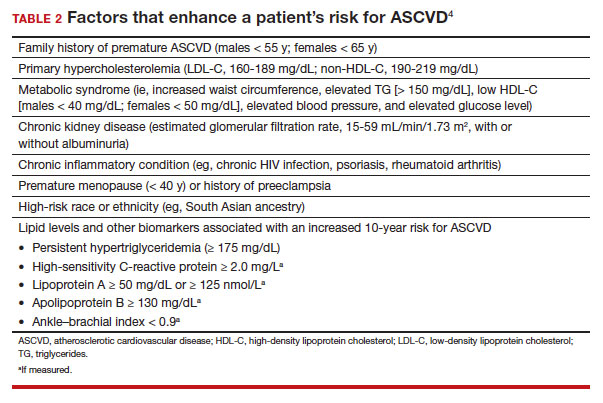
- Consider the impact of ASCVD riskenhancing factors and coronary artery calcium scoring in making a recommendation to begin lipid-lowering therapy in intermediate-risk patients. C
- Add ezetimibe if a statin does not sufficiently lower LDL-C or if a patient cannot tolerate an adequate dosage of the statin. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patientoriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimating risk for ASCVD by ascertaining LDL-C
- The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL),estimation of LDL-C is invalid.
- The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC–HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
- National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
- Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
- Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
- Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Continue to: Applying the estimate of 10-year ASCVD risk...
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
- Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
- Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention: Stratification by age
- 40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12
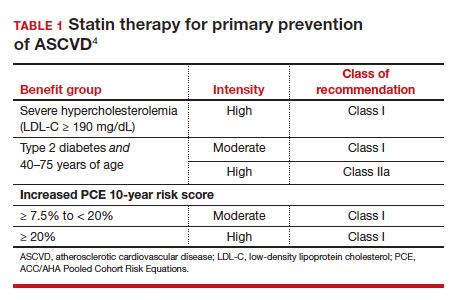
In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).
If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
- 20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
- > 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy...
Options for lipid-lowering pharmacotherapy
- Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4
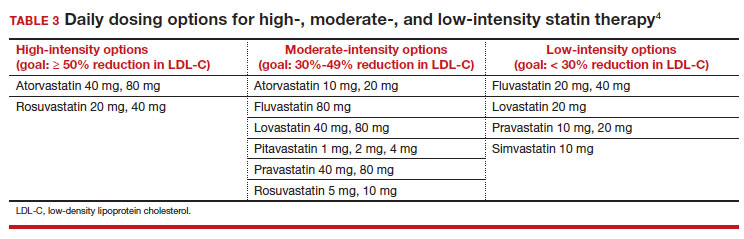
Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
- Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
- PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
- Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
- Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
- Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoicacid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
- Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL)and TG (42 mg/dL).31,32
- Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C< 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4 ●
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; [email protected]
- Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year followup experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
- Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
- Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001 /jama.2013.280532
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/ AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161 /CIR.0000000000000625
- Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
- Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi:10.1161/CIRCOUTCOMES.110.959247
- Framingham Heart Study. Cardiovascular disease (10year risk). Accessed February 14, 2023. www.framing hamheartstudy.org/fhs-risk-functions/cardiovascular -disease-10-year-risk/
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
- Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
- Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a metaanalysis of 60 controlled trials. Am J Clin Nutr. 2003;77:11461155. doi:10.1093/ajcn/77.5.1146
- Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi:10.1001/jama.2016.15450
- Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j. jcmg.2018.04.015
- Valenti V, O Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016 /j.jcmg.2015.01.025
- Armitage J, Baigent C, Barnes E, et al; Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407415. doi: 10.1016/S0140-6736(18)31942-1
- Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161 /CIRCULATIONAHA. 117.028271
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
- Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:23732384. doi: 10.1001/jama.2016.16951
- Sabatine MS, Giugliano RP, Wiviott SD, et al; Open-Label Study of Long-Term Evaluation Against LDL Cholesterol (OSLER) Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
- Robinson JG, Farnier M, Krempf M, et al; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
- Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
- Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
- Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
- Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of highdose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
- Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001 /jamacardio.2021.1157
- US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
- Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
- Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statinintolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
- Landray MJ, Haynes R, Hopewell JC, et al; HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203212. doi: 10.1056/NEJMoa1300955
- Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
- Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001 /jamacardio.2016.4828
- Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056 /NEJMoa1001282
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2
The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
- Use an alternative to the Friedewald equation, such as the Martin–Hopkins equation, to estimate the low-density lipoprotein cholesterol (LDL-C) value; order direct measurement of LDL-C; or calculate non–high-density lipoprotein cholesterol to assess the risk for atherosclerotic cardiovascular disease (ASCVD) in patients who have a low LDL-C or a high triglycerides level. C
- Consider the impact of ASCVD riskenhancing factors and coronary artery calcium scoring in making a recommendation to begin lipid-lowering therapy in intermediate-risk patients. C
- Add ezetimibe if a statin does not sufficiently lower LDL-C or if a patient cannot tolerate an adequate dosage of the statin. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patientoriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimating risk for ASCVD by ascertaining LDL-C
- The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL),estimation of LDL-C is invalid.
- The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC–HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
- National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
- Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
- Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
- Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Continue to: Applying the estimate of 10-year ASCVD risk...
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
- Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
- Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention: Stratification by age
- 40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12

In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).

If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
- 20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
- > 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy...
Options for lipid-lowering pharmacotherapy
- Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4

Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
- Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
- PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
- Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
- Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
- Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoicacid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
- Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL)and TG (42 mg/dL).31,32
- Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C< 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4 ●
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; [email protected]
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2
The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
- Use an alternative to the Friedewald equation, such as the Martin–Hopkins equation, to estimate the low-density lipoprotein cholesterol (LDL-C) value; order direct measurement of LDL-C; or calculate non–high-density lipoprotein cholesterol to assess the risk for atherosclerotic cardiovascular disease (ASCVD) in patients who have a low LDL-C or a high triglycerides level. C
- Consider the impact of ASCVD riskenhancing factors and coronary artery calcium scoring in making a recommendation to begin lipid-lowering therapy in intermediate-risk patients. C
- Add ezetimibe if a statin does not sufficiently lower LDL-C or if a patient cannot tolerate an adequate dosage of the statin. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patientoriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimating risk for ASCVD by ascertaining LDL-C
- The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL),estimation of LDL-C is invalid.
- The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC–HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
- National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
- Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
- Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
- Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Continue to: Applying the estimate of 10-year ASCVD risk...
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
- Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
- Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention: Stratification by age
- 40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12

In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).

If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
- 20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
- > 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy...
Options for lipid-lowering pharmacotherapy
- Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4

Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
- Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
- PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
- Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
- Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
- Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoicacid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
- Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL)and TG (42 mg/dL).31,32
- Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C< 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4 ●
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; [email protected]
- Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year followup experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
- Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
- Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001 /jama.2013.280532
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/ AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161 /CIR.0000000000000625
- Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
- Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi:10.1161/CIRCOUTCOMES.110.959247
- Framingham Heart Study. Cardiovascular disease (10year risk). Accessed February 14, 2023. www.framing hamheartstudy.org/fhs-risk-functions/cardiovascular -disease-10-year-risk/
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
- Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
- Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a metaanalysis of 60 controlled trials. Am J Clin Nutr. 2003;77:11461155. doi:10.1093/ajcn/77.5.1146
- Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi:10.1001/jama.2016.15450
- Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j. jcmg.2018.04.015
- Valenti V, O Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016 /j.jcmg.2015.01.025
- Armitage J, Baigent C, Barnes E, et al; Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407415. doi: 10.1016/S0140-6736(18)31942-1
- Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161 /CIRCULATIONAHA. 117.028271
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
- Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:23732384. doi: 10.1001/jama.2016.16951
- Sabatine MS, Giugliano RP, Wiviott SD, et al; Open-Label Study of Long-Term Evaluation Against LDL Cholesterol (OSLER) Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
- Robinson JG, Farnier M, Krempf M, et al; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
- Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
- Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
- Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
- Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of highdose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
- Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001 /jamacardio.2021.1157
- US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
- Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
- Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statinintolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
- Landray MJ, Haynes R, Hopewell JC, et al; HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203212. doi: 10.1056/NEJMoa1300955
- Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
- Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001 /jamacardio.2016.4828
- Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056 /NEJMoa1001282
- Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year followup experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
- Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
- Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001 /jama.2013.280532
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/ AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161 /CIR.0000000000000625
- Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
- Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi:10.1161/CIRCOUTCOMES.110.959247
- Framingham Heart Study. Cardiovascular disease (10year risk). Accessed February 14, 2023. www.framing hamheartstudy.org/fhs-risk-functions/cardiovascular -disease-10-year-risk/
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
- Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
- Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a metaanalysis of 60 controlled trials. Am J Clin Nutr. 2003;77:11461155. doi:10.1093/ajcn/77.5.1146
- Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi:10.1001/jama.2016.15450
- Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j. jcmg.2018.04.015
- Valenti V, O Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016 /j.jcmg.2015.01.025
- Armitage J, Baigent C, Barnes E, et al; Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407415. doi: 10.1016/S0140-6736(18)31942-1
- Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161 /CIRCULATIONAHA. 117.028271
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
- Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:23732384. doi: 10.1001/jama.2016.16951
- Sabatine MS, Giugliano RP, Wiviott SD, et al; Open-Label Study of Long-Term Evaluation Against LDL Cholesterol (OSLER) Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
- Robinson JG, Farnier M, Krempf M, et al; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
- Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
- Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
- Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
- Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of highdose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
- Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001 /jamacardio.2021.1157
- US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
- Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
- Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statinintolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
- Landray MJ, Haynes R, Hopewell JC, et al; HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203212. doi: 10.1056/NEJMoa1300955
- Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
- Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001 /jamacardio.2016.4828
- Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056 /NEJMoa1001282
First prospective study finds pregnancies with Sjögren’s to be largely safe
Women with Sjögren’s syndrome have pregnancy outcomes similar to those of the general population, according to the first study to prospectively track pregnancy outcomes among people with the autoimmune condition.
“Most early studies of pregnancy in rheumatic disease patients were retrospective and included only small numbers, making it difficult to know how generalizable the reported results were,” said Lisa Sammaritano, MD, a rheumatologist at Hospital for Special Surgery in New York, in an email interview with this news organization. She was not involved with the research.
Most of these previous studies suggested an increased risk of adverse outcomes, such as miscarriages, preterm deliveries, and small-for-gestational-age birth weight. But in addition to small patient numbers, retrospective studies “are subject to greater reporting bias, which may predispose patients with negative outcomes being more likely to be included because they were followed more closely,” Dr. Sammaritano said.
“This prospective study has several advantages over the earlier retrospective reports: The same data were collected in the same way for all the patients, the patients were recruited at similar time points, and – due to the multicenter nature of the cohort – numbers are larger than in prior studies. All these factors make the results stronger and more generalizable to the Sjögren’s patients we see in our practices,” she added.
In the study, published May 8 in The Lancet Rheumatology, first author Grégoire Martin de Frémont, MD, of the rheumatology service at Bicêtre Hospital, Paris-Saclay University and colleagues used the GR2 registry, an observational database of pregnancies of women with systemic autoimmune diseases managed at 76 participating centers in France, to identify pregnant women with primary Sjögren’s syndrome. To avoid bias, only women who entered the database before 18 weeks’ gestation were included. The final cohort included 106 pregnancies in 96 women with primary Sjögren’s syndrome and 420 control pregnancies that were matched from the general population.
Adverse pregnancy outcomes, including preterm delivery (< 37 weeks of gestation), intrauterine growth retardation, and low birth weight occurred in nine pregnancies (9%) in the Sjögren’s syndrome group and in 28 pregnancies in the control group (7%). Adverse pregnancy outcomes were not significantly associated with Sjögren’s syndrome (P = .52). Researchers found that there were more adverse pregnancy outcomes among women with Sjögren’s syndrome with antiphospholipid (aPL) antibodies. Negative outcomes also increased among those with anti-RNP antibodies, but this association was not statistically significant.
“The main message – based on strong data from a well-designed study – is that patients with Sjögren’s overall do as well as the general population in terms of standard adverse pregnancy outcomes. The rate of flare of Sjögren’s disease was relatively low during the second and third trimesters, also reassuring,” Dr. Sammaritano said. She noted that the association between adverse pregnancy outcomes and aPL antibodies was not unexpected, given that they are a known risk factor.
The study authors recommend that patients with Sjögren’s syndrome be screened for aPL and anti-RNP antibodies prior to conception because of the potential increased risk for complications and that patients with positive screens be closely monitored during their pregnancy.
Dr. Sammaritano noted that there are other health problems to keep in mind. “It is important to remember that Sjögren’s patients – more than any other rheumatic disease patients – have the additional risk for neonatal lupus and complete heart block in their infant, since about two-thirds of Sjögren’s patients are positive for anti-Ro/SSA antibody,” she said. “This is a distinct issue related to the presence of this antibody alone and not specifically related to the underlying diagnosis. In clinical practice, positive anti-Ro/SSA antibody is often the main reason for counseling, monitoring, and even recommending therapy (hydroxychloroquine) in these patients.”
The study received funding from Lupus France, the France Association of Scleroderma, and the Association Gougerot Sjögren, among others. Dr. Sammaritano reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women with Sjögren’s syndrome have pregnancy outcomes similar to those of the general population, according to the first study to prospectively track pregnancy outcomes among people with the autoimmune condition.
“Most early studies of pregnancy in rheumatic disease patients were retrospective and included only small numbers, making it difficult to know how generalizable the reported results were,” said Lisa Sammaritano, MD, a rheumatologist at Hospital for Special Surgery in New York, in an email interview with this news organization. She was not involved with the research.
Most of these previous studies suggested an increased risk of adverse outcomes, such as miscarriages, preterm deliveries, and small-for-gestational-age birth weight. But in addition to small patient numbers, retrospective studies “are subject to greater reporting bias, which may predispose patients with negative outcomes being more likely to be included because they were followed more closely,” Dr. Sammaritano said.
“This prospective study has several advantages over the earlier retrospective reports: The same data were collected in the same way for all the patients, the patients were recruited at similar time points, and – due to the multicenter nature of the cohort – numbers are larger than in prior studies. All these factors make the results stronger and more generalizable to the Sjögren’s patients we see in our practices,” she added.
In the study, published May 8 in The Lancet Rheumatology, first author Grégoire Martin de Frémont, MD, of the rheumatology service at Bicêtre Hospital, Paris-Saclay University and colleagues used the GR2 registry, an observational database of pregnancies of women with systemic autoimmune diseases managed at 76 participating centers in France, to identify pregnant women with primary Sjögren’s syndrome. To avoid bias, only women who entered the database before 18 weeks’ gestation were included. The final cohort included 106 pregnancies in 96 women with primary Sjögren’s syndrome and 420 control pregnancies that were matched from the general population.
Adverse pregnancy outcomes, including preterm delivery (< 37 weeks of gestation), intrauterine growth retardation, and low birth weight occurred in nine pregnancies (9%) in the Sjögren’s syndrome group and in 28 pregnancies in the control group (7%). Adverse pregnancy outcomes were not significantly associated with Sjögren’s syndrome (P = .52). Researchers found that there were more adverse pregnancy outcomes among women with Sjögren’s syndrome with antiphospholipid (aPL) antibodies. Negative outcomes also increased among those with anti-RNP antibodies, but this association was not statistically significant.
“The main message – based on strong data from a well-designed study – is that patients with Sjögren’s overall do as well as the general population in terms of standard adverse pregnancy outcomes. The rate of flare of Sjögren’s disease was relatively low during the second and third trimesters, also reassuring,” Dr. Sammaritano said. She noted that the association between adverse pregnancy outcomes and aPL antibodies was not unexpected, given that they are a known risk factor.
The study authors recommend that patients with Sjögren’s syndrome be screened for aPL and anti-RNP antibodies prior to conception because of the potential increased risk for complications and that patients with positive screens be closely monitored during their pregnancy.
Dr. Sammaritano noted that there are other health problems to keep in mind. “It is important to remember that Sjögren’s patients – more than any other rheumatic disease patients – have the additional risk for neonatal lupus and complete heart block in their infant, since about two-thirds of Sjögren’s patients are positive for anti-Ro/SSA antibody,” she said. “This is a distinct issue related to the presence of this antibody alone and not specifically related to the underlying diagnosis. In clinical practice, positive anti-Ro/SSA antibody is often the main reason for counseling, monitoring, and even recommending therapy (hydroxychloroquine) in these patients.”
The study received funding from Lupus France, the France Association of Scleroderma, and the Association Gougerot Sjögren, among others. Dr. Sammaritano reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women with Sjögren’s syndrome have pregnancy outcomes similar to those of the general population, according to the first study to prospectively track pregnancy outcomes among people with the autoimmune condition.
“Most early studies of pregnancy in rheumatic disease patients were retrospective and included only small numbers, making it difficult to know how generalizable the reported results were,” said Lisa Sammaritano, MD, a rheumatologist at Hospital for Special Surgery in New York, in an email interview with this news organization. She was not involved with the research.
Most of these previous studies suggested an increased risk of adverse outcomes, such as miscarriages, preterm deliveries, and small-for-gestational-age birth weight. But in addition to small patient numbers, retrospective studies “are subject to greater reporting bias, which may predispose patients with negative outcomes being more likely to be included because they were followed more closely,” Dr. Sammaritano said.
“This prospective study has several advantages over the earlier retrospective reports: The same data were collected in the same way for all the patients, the patients were recruited at similar time points, and – due to the multicenter nature of the cohort – numbers are larger than in prior studies. All these factors make the results stronger and more generalizable to the Sjögren’s patients we see in our practices,” she added.
In the study, published May 8 in The Lancet Rheumatology, first author Grégoire Martin de Frémont, MD, of the rheumatology service at Bicêtre Hospital, Paris-Saclay University and colleagues used the GR2 registry, an observational database of pregnancies of women with systemic autoimmune diseases managed at 76 participating centers in France, to identify pregnant women with primary Sjögren’s syndrome. To avoid bias, only women who entered the database before 18 weeks’ gestation were included. The final cohort included 106 pregnancies in 96 women with primary Sjögren’s syndrome and 420 control pregnancies that were matched from the general population.
Adverse pregnancy outcomes, including preterm delivery (< 37 weeks of gestation), intrauterine growth retardation, and low birth weight occurred in nine pregnancies (9%) in the Sjögren’s syndrome group and in 28 pregnancies in the control group (7%). Adverse pregnancy outcomes were not significantly associated with Sjögren’s syndrome (P = .52). Researchers found that there were more adverse pregnancy outcomes among women with Sjögren’s syndrome with antiphospholipid (aPL) antibodies. Negative outcomes also increased among those with anti-RNP antibodies, but this association was not statistically significant.
“The main message – based on strong data from a well-designed study – is that patients with Sjögren’s overall do as well as the general population in terms of standard adverse pregnancy outcomes. The rate of flare of Sjögren’s disease was relatively low during the second and third trimesters, also reassuring,” Dr. Sammaritano said. She noted that the association between adverse pregnancy outcomes and aPL antibodies was not unexpected, given that they are a known risk factor.
The study authors recommend that patients with Sjögren’s syndrome be screened for aPL and anti-RNP antibodies prior to conception because of the potential increased risk for complications and that patients with positive screens be closely monitored during their pregnancy.
Dr. Sammaritano noted that there are other health problems to keep in mind. “It is important to remember that Sjögren’s patients – more than any other rheumatic disease patients – have the additional risk for neonatal lupus and complete heart block in their infant, since about two-thirds of Sjögren’s patients are positive for anti-Ro/SSA antibody,” she said. “This is a distinct issue related to the presence of this antibody alone and not specifically related to the underlying diagnosis. In clinical practice, positive anti-Ro/SSA antibody is often the main reason for counseling, monitoring, and even recommending therapy (hydroxychloroquine) in these patients.”
The study received funding from Lupus France, the France Association of Scleroderma, and the Association Gougerot Sjögren, among others. Dr. Sammaritano reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
Endobronchial valves: Sustained improvement in emphysema
WASHINGTON – based on data from 174 individuals.
One-way endobronchial valves demonstrated benefits for patients with severe emphysema over a 12-month period in the EMPROVE trial, according to Gerard J. Criner, MD, of Temple University, Philadelphia, and colleagues.
Five-year results from the EMPROVE study were presented in a poster session at the American Thoracic Society’s international conference.
The initial EMPROVE trial demonstrated safety and efficacy of the Spiration Valve System (SVS) over 12 months. However, data on the long-term benefits of one-way endobronchial values are limited, the researchers wrote.
The valve was designed for use in selected areas of the bronchial airways and features a flexible umbrella that allows air and mucus to clear from treated airways while blocking inspired air flow to areas of the lungs affected by disease, the researchers explained in the poster.
Dr. Criner and colleagues assessed 172 patients who were randomly assigned to treatment with a one-way valve system (113 patients) or a control group (59 patients).
Participants were evaluated at 1, 3, 6, and 12 months, then annually for 5 years.
The primary efficacy outcome was lung function, measured by forced expiratory volume per second (FEV1). At five years, the FEV1 values improved by 0.1098 liters in the treatment group (P < .001). Treated patients and controls experienced decreased FEV1 at a rate of 0.0440 liters per year from baseline, a significant difference (P < .001). Assuming a steady rate of disease progression, “the treatment group gained approximately 2.5 years of FEV1 improvement immediately following SVS treatment, which was maintained, compared to controls,” the researchers noted in their abstract.
Serious adverse events were assessed from 6 months to 5 years (352.7 patient-years) for treated patients and from 6 months to 2 years (72.9 patient-years) for controls.
Overall, 210 SAEs occurred in the treatment group and 35 occurred in controls, for rates of 0.60 and 0.48, respectively (P = .201). The most common SAEs in the treatment and control groups were COPD exacerbations, pneumothorax, and death.
The results suggest that the FEV1 improvements seen in patients with severe emphysema after one-way endobronchial value placement compared with usual care are enduring after 5 years, with no significant changes in safety, the researchers concluded.
The original EMPROVE study was supported by Olympus Respiratory America, a part of Olympus Corporation and the developer of the Spiration Valve System. Results of the original study were published in the American Journal of Respiratory and Critical Care Medicine. Dr. Criner is associate editor of the American Journal of Respiratory and Critical Care Medicine. His participation complies with American Thoracic Society requirements for recusal from review and decisions for authored works.
A version of this article first appeared on Medscape.com.
WASHINGTON – based on data from 174 individuals.
One-way endobronchial valves demonstrated benefits for patients with severe emphysema over a 12-month period in the EMPROVE trial, according to Gerard J. Criner, MD, of Temple University, Philadelphia, and colleagues.
Five-year results from the EMPROVE study were presented in a poster session at the American Thoracic Society’s international conference.
The initial EMPROVE trial demonstrated safety and efficacy of the Spiration Valve System (SVS) over 12 months. However, data on the long-term benefits of one-way endobronchial values are limited, the researchers wrote.
The valve was designed for use in selected areas of the bronchial airways and features a flexible umbrella that allows air and mucus to clear from treated airways while blocking inspired air flow to areas of the lungs affected by disease, the researchers explained in the poster.
Dr. Criner and colleagues assessed 172 patients who were randomly assigned to treatment with a one-way valve system (113 patients) or a control group (59 patients).
Participants were evaluated at 1, 3, 6, and 12 months, then annually for 5 years.
The primary efficacy outcome was lung function, measured by forced expiratory volume per second (FEV1). At five years, the FEV1 values improved by 0.1098 liters in the treatment group (P < .001). Treated patients and controls experienced decreased FEV1 at a rate of 0.0440 liters per year from baseline, a significant difference (P < .001). Assuming a steady rate of disease progression, “the treatment group gained approximately 2.5 years of FEV1 improvement immediately following SVS treatment, which was maintained, compared to controls,” the researchers noted in their abstract.
Serious adverse events were assessed from 6 months to 5 years (352.7 patient-years) for treated patients and from 6 months to 2 years (72.9 patient-years) for controls.
Overall, 210 SAEs occurred in the treatment group and 35 occurred in controls, for rates of 0.60 and 0.48, respectively (P = .201). The most common SAEs in the treatment and control groups were COPD exacerbations, pneumothorax, and death.
The results suggest that the FEV1 improvements seen in patients with severe emphysema after one-way endobronchial value placement compared with usual care are enduring after 5 years, with no significant changes in safety, the researchers concluded.
The original EMPROVE study was supported by Olympus Respiratory America, a part of Olympus Corporation and the developer of the Spiration Valve System. Results of the original study were published in the American Journal of Respiratory and Critical Care Medicine. Dr. Criner is associate editor of the American Journal of Respiratory and Critical Care Medicine. His participation complies with American Thoracic Society requirements for recusal from review and decisions for authored works.
A version of this article first appeared on Medscape.com.
WASHINGTON – based on data from 174 individuals.
One-way endobronchial valves demonstrated benefits for patients with severe emphysema over a 12-month period in the EMPROVE trial, according to Gerard J. Criner, MD, of Temple University, Philadelphia, and colleagues.
Five-year results from the EMPROVE study were presented in a poster session at the American Thoracic Society’s international conference.
The initial EMPROVE trial demonstrated safety and efficacy of the Spiration Valve System (SVS) over 12 months. However, data on the long-term benefits of one-way endobronchial values are limited, the researchers wrote.
The valve was designed for use in selected areas of the bronchial airways and features a flexible umbrella that allows air and mucus to clear from treated airways while blocking inspired air flow to areas of the lungs affected by disease, the researchers explained in the poster.
Dr. Criner and colleagues assessed 172 patients who were randomly assigned to treatment with a one-way valve system (113 patients) or a control group (59 patients).
Participants were evaluated at 1, 3, 6, and 12 months, then annually for 5 years.
The primary efficacy outcome was lung function, measured by forced expiratory volume per second (FEV1). At five years, the FEV1 values improved by 0.1098 liters in the treatment group (P < .001). Treated patients and controls experienced decreased FEV1 at a rate of 0.0440 liters per year from baseline, a significant difference (P < .001). Assuming a steady rate of disease progression, “the treatment group gained approximately 2.5 years of FEV1 improvement immediately following SVS treatment, which was maintained, compared to controls,” the researchers noted in their abstract.
Serious adverse events were assessed from 6 months to 5 years (352.7 patient-years) for treated patients and from 6 months to 2 years (72.9 patient-years) for controls.
Overall, 210 SAEs occurred in the treatment group and 35 occurred in controls, for rates of 0.60 and 0.48, respectively (P = .201). The most common SAEs in the treatment and control groups were COPD exacerbations, pneumothorax, and death.
The results suggest that the FEV1 improvements seen in patients with severe emphysema after one-way endobronchial value placement compared with usual care are enduring after 5 years, with no significant changes in safety, the researchers concluded.
The original EMPROVE study was supported by Olympus Respiratory America, a part of Olympus Corporation and the developer of the Spiration Valve System. Results of the original study were published in the American Journal of Respiratory and Critical Care Medicine. Dr. Criner is associate editor of the American Journal of Respiratory and Critical Care Medicine. His participation complies with American Thoracic Society requirements for recusal from review and decisions for authored works.
A version of this article first appeared on Medscape.com.
AT ATS 2023
Standard measure may underestimate OSA in Black patients
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
FROM ATS 2023
States move to curb insurers’ prior authorization requirements as federal reforms lag
Amid growing criticism of health insurers’ onerous prior authorization practices, lawmakers in 30 states have introduced bills this year that aim to rein in insurer gatekeeping and improve patient care.
“This is something that goes on in every doctor’s office every day; the frustrations, the delays, and the use of office staff time are just unbelievable,” said Steven Orland, MD, a board-certified urologist and president of the Medical Society of New Jersey.
The bills, which cover private health plans and insurers that states regulate, may provide some relief for physicians as federal efforts to streamline prior authorization for some Medicare patients have lagged.
Last year, Congress failed to pass the Improving Seniors’ Timely Access to Care Act of 2021, despite 326 co-sponsors. The bill would have compelled insurers covering Medicare Advantage enrollees to speed up prior authorizations, make the process more transparent, and remove obstacles such as requiring fax machine submissions.
Last month, however, the Centers for Medicare & Medicaid Services issued a final rule that will improve some aspects of prior authorizations in Medicare Advantage insurance plans and ensure that enrollees have the same access to necessary care as traditional Medicare enrollees.
The insurance industry has long defended prior authorization requirements and opposed legislation that would limit them.
America’s Health Insurance Plans (AHIP) and the Blue Cross Blue Shield Association said in a 2019 letter to a congressional committee when the federal legislation was first introduced, “Prior authorizations enforce best practices and guidelines for care management and help physicians identify and avoid care techniques that would harm patient outcomes, such as designating prescriptions that could feed into an opioid addiction.” AHIP didn’t respond to repeated requests for comment.
But some major insurers now appear willing to compromise and voluntarily reduce the volume of prior authorizations they require. Days before the federal final rule was released, three major insurers – United HealthCare, Cigna, and Aetna CVS Health – announced they plan to drop some prior authorization requirements and automate processes.
United HealthCare said it will eliminate almost 20% of its prior authorizations for some nonurgent surgeries and procedures starting this summer. It also will create a national Gold Card program in 2024 for physicians who meet its eligibility requirements, which would eliminate prior authorization requirements for most procedures. Both initiatives will apply to commercial, Medicare Advantage, and Medicaid businesses, said the insurer in a statement.
However, United HealthCare also announced that in June it will start requiring prior authorization for diagnostic (not screening) gastrointestinal endoscopies for its nearly 27 million privately insured patients, citing data it says shows potentially harmful overuse of scopes. Physician groups have publicly criticized the move, saying it could delay lifesaving treatment, and have asked the insurer to reconsider.
Cigna and Aetna also have moved to pare back prior authorization processes. Scott Josephs, national medical officer for Cigna, told Healthcare Dive that Cigna has removed prior authorization reviews from nearly 500 services since 2020.
An Aetna spokesperson told Healthcare Dive that the CVS-owned payer has implemented a gold card program and rolled back prior authorization requirements on cataract surgeries, video EEGs, and home infusion for some drugs, according to Healthcare Dive.
Cigna has faced increased scrutiny from some state regulators since a ProPublica/The Capitol Forum article revealed in March that its doctors were denying claims without opening patients’ files, contrary to what insurance laws and regulations require in many states.
Over a period of 2 months last year, Cigna doctors denied over 300,000 requests for payments using this method, spending an average of 1.2 seconds on each case, the investigation found. In a written response, Cigna said the reporting by ProPublica and The Capitol Forum was “biased and incomplete.”
States aim to reduce prior authorization volume
The American Medical Association said it has been tracking nearly 90 prior authorization reform bills in 30 states. More than a dozen bills are still being considered in this legislative session, including in Arkansas, California, New Jersey, North Carolina, Maryland, and Washington, D.C.
“The groundswell of activity in the states reflects how big a problem this is,” said an AMA legislative expert. “The issue used to be ‘how can we automate and streamline processes’; now the issue is focused on reducing the volume of prior authorizations and the harm that can cause patients.”
The state bills use different strategies to reduce excessive prior authorization requirements. Maryland’s proposed bill, for example, would require just one prior authorization to stay on a prescription drug, if the insurer has previously approved the drug and the patient continues to successfully be treated by the drug.
Washington, D.C. and New Jersey have introduced comprehensive reform bills that include a “grace period” of 60 days, to ensure continuity of care when a patient switches health plans. They also would eliminate repeat authorizations for chronic and long-term conditions, set explicit timelines for insurers to respond to prior authorization requests and appeals, and require that practicing physicians review denials that are appealed.
Many state bills also would require insurers to be more transparent by posting information on their websites about which services and drugs require prior authorization and what their approval rates are for them, said AMA’s legislative expert.
“There’s a black hole of information that insurers have access to. We would really like to know how many prior authorization requests are denied, the time it takes to deny them, and the reasons for denial,” said Josh Bengal, JD, the director of government relations for the Medical Society of New Jersey.
The legislation in New Jersey and other states faces stiff opposition from the insurance lobby, especially state associations of health plans affiliated with AHIP. The California Association of Health Plans, for example, opposes a “gold card” bill (SB 598), introduced in February, that would allow a select group of high-performing doctors to skip prior authorizations for 1 year.
The CAHP states, “Californians deserve safe, high quality, high-value health care. Yet SB 598 will derail the progress we have made in our health care system by lowering the value and safety that Californians should expect from their health care providers,” according to a fact sheet.
The fact-sheet defines “low-value care” as medical services for which there is little to no benefit and poses potential physical or financial harm to patients, such as unnecessary CT scans or MRIs for uncomplicated conditions.
California is one of about a dozen states that have introduced gold card legislation this year. If enacted, they would join five states with gold card laws: West Virginia, Texas, Vermont, Michigan, and Louisiana.
How do gold cards work?
Physicians who achieve a high approval rate of prior authorizations from insurers for 1 year are eligible to be exempted from obtaining prior authorizations the following year.
The approval rate is at least 90% for a certain number of eligible health services, but the number of prior authorizations required to qualify can range from 5 to 30, depending on the state law.
Gold card legislation typically also gives the treating physician the right to have an appeal of a prior authorization denial by a physician peer of the same or similar specialty.
California’s bill would also apply to all covered health services, which is broader than what United HealthCare has proposed for its gold card exemption. The bill would also require a plan or insurer to annually monitor rates of prior authorization approval, modification, appeal, and denial, and to discontinue services, items, and supplies that are approved 95% of the time.
“These are important reforms that will help ensure that patients can receive the care they need, when they need it,” said CMA president Donaldo Hernandez, MD.
However, it’s not clear how many physicians will meet “gold card” status based on Texas’ recent experience with its own “gold card” law.
The Texas Department of Insurance estimated that only 3.3% of licensed physicians in the state have met “gold card” status since the bill became law in 2021, said Zeke Silva, MD, an interventional radiologist who serves on the Council of Legislation for the Texas Medical Association.
He noted that the legislation has had a limited effect for several reasons. Commercial health plans only make up only about 20% of all health plans in Texas. Also, the final regulations didn’t go into effect until last May and physicians are evaluated by health plans for “gold card” status every 6 months, said Dr. Silva.
In addition, physicians must have at least five prior authorizations approved for the same health service, which the law left up to the health plans to define, said Dr. Silva.
Now, the Texas Medical Association is lobbying for legislative improvements. “We want to reduce the number of eligible services that health plans require for prior authorizations and have more oversight of prior authorization denials by the Texas Department of Insurance and the Texas Medical Board,” said Dr. Silva.
He’s optimistic that if the bill becomes law, the number of physicians eligible for gold cards may increase.
Meanwhile, the AMA’s legislative expert, who declined to be identified because of organization policy, acknowledged the possibility that some prior authorization bills will die in state legislatures this year.
“We remain hopeful, but it’s an uphill battle. The state medical associations face a lot of opposition from health plans who don’t want to see these reforms become law.”
A version of this article originally appeared on Medscape.com.
Amid growing criticism of health insurers’ onerous prior authorization practices, lawmakers in 30 states have introduced bills this year that aim to rein in insurer gatekeeping and improve patient care.
“This is something that goes on in every doctor’s office every day; the frustrations, the delays, and the use of office staff time are just unbelievable,” said Steven Orland, MD, a board-certified urologist and president of the Medical Society of New Jersey.
The bills, which cover private health plans and insurers that states regulate, may provide some relief for physicians as federal efforts to streamline prior authorization for some Medicare patients have lagged.
Last year, Congress failed to pass the Improving Seniors’ Timely Access to Care Act of 2021, despite 326 co-sponsors. The bill would have compelled insurers covering Medicare Advantage enrollees to speed up prior authorizations, make the process more transparent, and remove obstacles such as requiring fax machine submissions.
Last month, however, the Centers for Medicare & Medicaid Services issued a final rule that will improve some aspects of prior authorizations in Medicare Advantage insurance plans and ensure that enrollees have the same access to necessary care as traditional Medicare enrollees.
The insurance industry has long defended prior authorization requirements and opposed legislation that would limit them.
America’s Health Insurance Plans (AHIP) and the Blue Cross Blue Shield Association said in a 2019 letter to a congressional committee when the federal legislation was first introduced, “Prior authorizations enforce best practices and guidelines for care management and help physicians identify and avoid care techniques that would harm patient outcomes, such as designating prescriptions that could feed into an opioid addiction.” AHIP didn’t respond to repeated requests for comment.
But some major insurers now appear willing to compromise and voluntarily reduce the volume of prior authorizations they require. Days before the federal final rule was released, three major insurers – United HealthCare, Cigna, and Aetna CVS Health – announced they plan to drop some prior authorization requirements and automate processes.
United HealthCare said it will eliminate almost 20% of its prior authorizations for some nonurgent surgeries and procedures starting this summer. It also will create a national Gold Card program in 2024 for physicians who meet its eligibility requirements, which would eliminate prior authorization requirements for most procedures. Both initiatives will apply to commercial, Medicare Advantage, and Medicaid businesses, said the insurer in a statement.
However, United HealthCare also announced that in June it will start requiring prior authorization for diagnostic (not screening) gastrointestinal endoscopies for its nearly 27 million privately insured patients, citing data it says shows potentially harmful overuse of scopes. Physician groups have publicly criticized the move, saying it could delay lifesaving treatment, and have asked the insurer to reconsider.
Cigna and Aetna also have moved to pare back prior authorization processes. Scott Josephs, national medical officer for Cigna, told Healthcare Dive that Cigna has removed prior authorization reviews from nearly 500 services since 2020.
An Aetna spokesperson told Healthcare Dive that the CVS-owned payer has implemented a gold card program and rolled back prior authorization requirements on cataract surgeries, video EEGs, and home infusion for some drugs, according to Healthcare Dive.
Cigna has faced increased scrutiny from some state regulators since a ProPublica/The Capitol Forum article revealed in March that its doctors were denying claims without opening patients’ files, contrary to what insurance laws and regulations require in many states.
Over a period of 2 months last year, Cigna doctors denied over 300,000 requests for payments using this method, spending an average of 1.2 seconds on each case, the investigation found. In a written response, Cigna said the reporting by ProPublica and The Capitol Forum was “biased and incomplete.”
States aim to reduce prior authorization volume
The American Medical Association said it has been tracking nearly 90 prior authorization reform bills in 30 states. More than a dozen bills are still being considered in this legislative session, including in Arkansas, California, New Jersey, North Carolina, Maryland, and Washington, D.C.
“The groundswell of activity in the states reflects how big a problem this is,” said an AMA legislative expert. “The issue used to be ‘how can we automate and streamline processes’; now the issue is focused on reducing the volume of prior authorizations and the harm that can cause patients.”
The state bills use different strategies to reduce excessive prior authorization requirements. Maryland’s proposed bill, for example, would require just one prior authorization to stay on a prescription drug, if the insurer has previously approved the drug and the patient continues to successfully be treated by the drug.
Washington, D.C. and New Jersey have introduced comprehensive reform bills that include a “grace period” of 60 days, to ensure continuity of care when a patient switches health plans. They also would eliminate repeat authorizations for chronic and long-term conditions, set explicit timelines for insurers to respond to prior authorization requests and appeals, and require that practicing physicians review denials that are appealed.
Many state bills also would require insurers to be more transparent by posting information on their websites about which services and drugs require prior authorization and what their approval rates are for them, said AMA’s legislative expert.
“There’s a black hole of information that insurers have access to. We would really like to know how many prior authorization requests are denied, the time it takes to deny them, and the reasons for denial,” said Josh Bengal, JD, the director of government relations for the Medical Society of New Jersey.
The legislation in New Jersey and other states faces stiff opposition from the insurance lobby, especially state associations of health plans affiliated with AHIP. The California Association of Health Plans, for example, opposes a “gold card” bill (SB 598), introduced in February, that would allow a select group of high-performing doctors to skip prior authorizations for 1 year.
The CAHP states, “Californians deserve safe, high quality, high-value health care. Yet SB 598 will derail the progress we have made in our health care system by lowering the value and safety that Californians should expect from their health care providers,” according to a fact sheet.
The fact-sheet defines “low-value care” as medical services for which there is little to no benefit and poses potential physical or financial harm to patients, such as unnecessary CT scans or MRIs for uncomplicated conditions.
California is one of about a dozen states that have introduced gold card legislation this year. If enacted, they would join five states with gold card laws: West Virginia, Texas, Vermont, Michigan, and Louisiana.
How do gold cards work?
Physicians who achieve a high approval rate of prior authorizations from insurers for 1 year are eligible to be exempted from obtaining prior authorizations the following year.
The approval rate is at least 90% for a certain number of eligible health services, but the number of prior authorizations required to qualify can range from 5 to 30, depending on the state law.
Gold card legislation typically also gives the treating physician the right to have an appeal of a prior authorization denial by a physician peer of the same or similar specialty.
California’s bill would also apply to all covered health services, which is broader than what United HealthCare has proposed for its gold card exemption. The bill would also require a plan or insurer to annually monitor rates of prior authorization approval, modification, appeal, and denial, and to discontinue services, items, and supplies that are approved 95% of the time.
“These are important reforms that will help ensure that patients can receive the care they need, when they need it,” said CMA president Donaldo Hernandez, MD.
However, it’s not clear how many physicians will meet “gold card” status based on Texas’ recent experience with its own “gold card” law.
The Texas Department of Insurance estimated that only 3.3% of licensed physicians in the state have met “gold card” status since the bill became law in 2021, said Zeke Silva, MD, an interventional radiologist who serves on the Council of Legislation for the Texas Medical Association.
He noted that the legislation has had a limited effect for several reasons. Commercial health plans only make up only about 20% of all health plans in Texas. Also, the final regulations didn’t go into effect until last May and physicians are evaluated by health plans for “gold card” status every 6 months, said Dr. Silva.
In addition, physicians must have at least five prior authorizations approved for the same health service, which the law left up to the health plans to define, said Dr. Silva.
Now, the Texas Medical Association is lobbying for legislative improvements. “We want to reduce the number of eligible services that health plans require for prior authorizations and have more oversight of prior authorization denials by the Texas Department of Insurance and the Texas Medical Board,” said Dr. Silva.
He’s optimistic that if the bill becomes law, the number of physicians eligible for gold cards may increase.
Meanwhile, the AMA’s legislative expert, who declined to be identified because of organization policy, acknowledged the possibility that some prior authorization bills will die in state legislatures this year.
“We remain hopeful, but it’s an uphill battle. The state medical associations face a lot of opposition from health plans who don’t want to see these reforms become law.”
A version of this article originally appeared on Medscape.com.
Amid growing criticism of health insurers’ onerous prior authorization practices, lawmakers in 30 states have introduced bills this year that aim to rein in insurer gatekeeping and improve patient care.
“This is something that goes on in every doctor’s office every day; the frustrations, the delays, and the use of office staff time are just unbelievable,” said Steven Orland, MD, a board-certified urologist and president of the Medical Society of New Jersey.
The bills, which cover private health plans and insurers that states regulate, may provide some relief for physicians as federal efforts to streamline prior authorization for some Medicare patients have lagged.
Last year, Congress failed to pass the Improving Seniors’ Timely Access to Care Act of 2021, despite 326 co-sponsors. The bill would have compelled insurers covering Medicare Advantage enrollees to speed up prior authorizations, make the process more transparent, and remove obstacles such as requiring fax machine submissions.
Last month, however, the Centers for Medicare & Medicaid Services issued a final rule that will improve some aspects of prior authorizations in Medicare Advantage insurance plans and ensure that enrollees have the same access to necessary care as traditional Medicare enrollees.
The insurance industry has long defended prior authorization requirements and opposed legislation that would limit them.
America’s Health Insurance Plans (AHIP) and the Blue Cross Blue Shield Association said in a 2019 letter to a congressional committee when the federal legislation was first introduced, “Prior authorizations enforce best practices and guidelines for care management and help physicians identify and avoid care techniques that would harm patient outcomes, such as designating prescriptions that could feed into an opioid addiction.” AHIP didn’t respond to repeated requests for comment.
But some major insurers now appear willing to compromise and voluntarily reduce the volume of prior authorizations they require. Days before the federal final rule was released, three major insurers – United HealthCare, Cigna, and Aetna CVS Health – announced they plan to drop some prior authorization requirements and automate processes.
United HealthCare said it will eliminate almost 20% of its prior authorizations for some nonurgent surgeries and procedures starting this summer. It also will create a national Gold Card program in 2024 for physicians who meet its eligibility requirements, which would eliminate prior authorization requirements for most procedures. Both initiatives will apply to commercial, Medicare Advantage, and Medicaid businesses, said the insurer in a statement.
However, United HealthCare also announced that in June it will start requiring prior authorization for diagnostic (not screening) gastrointestinal endoscopies for its nearly 27 million privately insured patients, citing data it says shows potentially harmful overuse of scopes. Physician groups have publicly criticized the move, saying it could delay lifesaving treatment, and have asked the insurer to reconsider.
Cigna and Aetna also have moved to pare back prior authorization processes. Scott Josephs, national medical officer for Cigna, told Healthcare Dive that Cigna has removed prior authorization reviews from nearly 500 services since 2020.
An Aetna spokesperson told Healthcare Dive that the CVS-owned payer has implemented a gold card program and rolled back prior authorization requirements on cataract surgeries, video EEGs, and home infusion for some drugs, according to Healthcare Dive.
Cigna has faced increased scrutiny from some state regulators since a ProPublica/The Capitol Forum article revealed in March that its doctors were denying claims without opening patients’ files, contrary to what insurance laws and regulations require in many states.
Over a period of 2 months last year, Cigna doctors denied over 300,000 requests for payments using this method, spending an average of 1.2 seconds on each case, the investigation found. In a written response, Cigna said the reporting by ProPublica and The Capitol Forum was “biased and incomplete.”
States aim to reduce prior authorization volume
The American Medical Association said it has been tracking nearly 90 prior authorization reform bills in 30 states. More than a dozen bills are still being considered in this legislative session, including in Arkansas, California, New Jersey, North Carolina, Maryland, and Washington, D.C.
“The groundswell of activity in the states reflects how big a problem this is,” said an AMA legislative expert. “The issue used to be ‘how can we automate and streamline processes’; now the issue is focused on reducing the volume of prior authorizations and the harm that can cause patients.”
The state bills use different strategies to reduce excessive prior authorization requirements. Maryland’s proposed bill, for example, would require just one prior authorization to stay on a prescription drug, if the insurer has previously approved the drug and the patient continues to successfully be treated by the drug.
Washington, D.C. and New Jersey have introduced comprehensive reform bills that include a “grace period” of 60 days, to ensure continuity of care when a patient switches health plans. They also would eliminate repeat authorizations for chronic and long-term conditions, set explicit timelines for insurers to respond to prior authorization requests and appeals, and require that practicing physicians review denials that are appealed.
Many state bills also would require insurers to be more transparent by posting information on their websites about which services and drugs require prior authorization and what their approval rates are for them, said AMA’s legislative expert.
“There’s a black hole of information that insurers have access to. We would really like to know how many prior authorization requests are denied, the time it takes to deny them, and the reasons for denial,” said Josh Bengal, JD, the director of government relations for the Medical Society of New Jersey.
The legislation in New Jersey and other states faces stiff opposition from the insurance lobby, especially state associations of health plans affiliated with AHIP. The California Association of Health Plans, for example, opposes a “gold card” bill (SB 598), introduced in February, that would allow a select group of high-performing doctors to skip prior authorizations for 1 year.
The CAHP states, “Californians deserve safe, high quality, high-value health care. Yet SB 598 will derail the progress we have made in our health care system by lowering the value and safety that Californians should expect from their health care providers,” according to a fact sheet.
The fact-sheet defines “low-value care” as medical services for which there is little to no benefit and poses potential physical or financial harm to patients, such as unnecessary CT scans or MRIs for uncomplicated conditions.
California is one of about a dozen states that have introduced gold card legislation this year. If enacted, they would join five states with gold card laws: West Virginia, Texas, Vermont, Michigan, and Louisiana.
How do gold cards work?
Physicians who achieve a high approval rate of prior authorizations from insurers for 1 year are eligible to be exempted from obtaining prior authorizations the following year.
The approval rate is at least 90% for a certain number of eligible health services, but the number of prior authorizations required to qualify can range from 5 to 30, depending on the state law.
Gold card legislation typically also gives the treating physician the right to have an appeal of a prior authorization denial by a physician peer of the same or similar specialty.
California’s bill would also apply to all covered health services, which is broader than what United HealthCare has proposed for its gold card exemption. The bill would also require a plan or insurer to annually monitor rates of prior authorization approval, modification, appeal, and denial, and to discontinue services, items, and supplies that are approved 95% of the time.
“These are important reforms that will help ensure that patients can receive the care they need, when they need it,” said CMA president Donaldo Hernandez, MD.
However, it’s not clear how many physicians will meet “gold card” status based on Texas’ recent experience with its own “gold card” law.
The Texas Department of Insurance estimated that only 3.3% of licensed physicians in the state have met “gold card” status since the bill became law in 2021, said Zeke Silva, MD, an interventional radiologist who serves on the Council of Legislation for the Texas Medical Association.
He noted that the legislation has had a limited effect for several reasons. Commercial health plans only make up only about 20% of all health plans in Texas. Also, the final regulations didn’t go into effect until last May and physicians are evaluated by health plans for “gold card” status every 6 months, said Dr. Silva.
In addition, physicians must have at least five prior authorizations approved for the same health service, which the law left up to the health plans to define, said Dr. Silva.
Now, the Texas Medical Association is lobbying for legislative improvements. “We want to reduce the number of eligible services that health plans require for prior authorizations and have more oversight of prior authorization denials by the Texas Department of Insurance and the Texas Medical Board,” said Dr. Silva.
He’s optimistic that if the bill becomes law, the number of physicians eligible for gold cards may increase.
Meanwhile, the AMA’s legislative expert, who declined to be identified because of organization policy, acknowledged the possibility that some prior authorization bills will die in state legislatures this year.
“We remain hopeful, but it’s an uphill battle. The state medical associations face a lot of opposition from health plans who don’t want to see these reforms become law.”
A version of this article originally appeared on Medscape.com.
Common fracture risk predictors often fail for women of any race
according to a study published in JAMA Internal Medicine.
One of the screenings, the U.S. Fracture Risk Assessment Tool (FRAX), proved relatively ineffective at identifying women who developed osteoporosis. The other screening, the Osteoporosis Self-Assessment Tool (OST), excelled at identifying osteoporosis for women in every racial and ethnic group, but also failed at identifying who was most likely to experience a fracture. Osteoporosis experts say that primary care physicians should test for the condition in anyone with any risk factor for it, even if a screening tool suggests doing so is unnecessary.
The United States Preventive Services Task Force (USPSTF) recommends routine testing of bone mineral density in women age 65 years and older to detect risk of developing osteoporosis, which in turn leads to an increased risk for fractures of the hip, spine, shoulder, or forearm. For women aged 50-64, whether bone mineral density accurately reflects who will develop osteoporosis is less clear. In this age range, the USPSTF recommends using either FRAX or OST rather than routine bone mineral density tests.
“I have the utmost respect for the United States Preventive Services Task Force, which lists both of these as valid screening tools for younger postmenopausal women. What I hope this study does is to inform the next iteration of the screening guidelines,” by maintaining the recommendation to use the OST while not keeping FRAX, said Carolyn J. Crandall, MD, MS, an internal medicine physician and health services researcher at University of California, Los Angeles, who helped conduct the research.
The U.S. version of FRAX requires identifying someone’s race, height, and weight, then answering whether they have different risk factors for a fracture such as a previous fracture, rheumatoid arthritis, or smoking. The result was thought to indicate a cumulative risk for major fracture over the next 10 years. Patients at significant risk should then undergo a bone density test.
The tool can also incorporate information about bone mineral density, if available, but the FRAX analyses in Dr. Crandall’s study did not include those data because the study aimed to test the measure’s predictive ability in the absence of a bone scan.
The OST includes only two variables – weight and age – to calculate risk for osteoporosis, and generally takes seconds to complete. It does not include race. As with FRAX, anyone deemed at significant risk for developing osteoporosis should undergo a bone density test.
“OST is really simple; that makes it very appealing,” Dr. Crandall said. “OST could probably be automatically calculated in the electronic medical record.”
Using data from the Women’s Health Initiative, Dr. Crandall and colleagues tracked more than 67,000 women aged 50-64 years for 10 years following enrollment in the study to see who experienced a fracture or developed osteoporosis over that decade. The investigators found that neither FRAX nor OST was particularly good at predicting who went on to experience a fracture.
The accuracy of FRAX at fracture prediction peaked at 65% for Asian women (area under the receiver operating curve, 0.65; 95% confidence interval, 0.58-0.71), and was lowest for Black women (AUC 0.55; 95% CI, 0.52-0.59). OST also was most accurate for Asian women, but only up to 62% (AUC 0.62; 95% CI, 0.56-0.69), and was again lowest for Black women (AUC 0.53; 95% CI, 0.50 - 0.57)
“It is just very hard to predict fractures in this age group,” Dr. Crandall said, noting that more evidence exists about risk for fracture in people older than 65.
The story diverges with predicting risk of osteoporosis in the neck. The OST did this roughly 80% of the time, for all racial groups. That figure proved better than FRAX, without including race.
Treatment gap
“This evidence supports using OST instead of FRAX” for selecting younger postmenopausal women who should undergo a bone mineral density exam, said E. Michael Lewiecki, MD, director of the New Mexico Clinical Research & Osteoporosis Center in Albuquerque.

Dr. Lewiecki, who was not involved in the new study, noted that the U.S. version of FRAX specifies race because of some clinical evidence that different races have different rates of fracture. But he and Dr. Crandall said the validity of race-based algorithms to guide clinical care is a controversial and evolving topic in medicine. Dr. Lewiecki said the Canadian version of FRAX, which is similarly applied to a diverse population as in the United States, omits race and works as well as the U.S. version. Future iterations of the instrument in the United States may not include race, Dr. Lewiecki said.
“The study is perfectly valid as far as it goes. But the big gorilla in the room is that most patients who need a bone density test are not getting it,” Dr. Lewiecki added. Sometimes a patient might break a bone in their wrist, for example, and tell their primary care provider that anyone would have broken that bone because the fall was so hard. Even if that’s true, Dr. Lewiecki said, any woman older than 45 who has broken a bone should undergo a bone density test to determine if they have osteoporosis, even if it seems like there are other possible reasons for why the break occurred.
“Most of the clinical practice guidelines that are used by physicians recommend getting a bone density test in postmenopausal women under the age of 65 who have a risk factor for fracture,” Dr. Lewiecki said, with a primary risk factor being a prior fracture. Dr. Lewiecki said he would rather that anyone who could benefit from a bone density test receive it, rather than someone foregoing a scan based on a screening tool that may be flawed.
“Most patients – men and women – who have osteoporosis are currently not being identified. Even when they are being identified, they are commonly not being treated. And when they are started on treatment, many patients discontinue treatment before they’ve taken it long enough to benefit,” Dr. Lewiecki said.
Dr. Crandall and Dr. Lewiecki report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study published in JAMA Internal Medicine.
One of the screenings, the U.S. Fracture Risk Assessment Tool (FRAX), proved relatively ineffective at identifying women who developed osteoporosis. The other screening, the Osteoporosis Self-Assessment Tool (OST), excelled at identifying osteoporosis for women in every racial and ethnic group, but also failed at identifying who was most likely to experience a fracture. Osteoporosis experts say that primary care physicians should test for the condition in anyone with any risk factor for it, even if a screening tool suggests doing so is unnecessary.
The United States Preventive Services Task Force (USPSTF) recommends routine testing of bone mineral density in women age 65 years and older to detect risk of developing osteoporosis, which in turn leads to an increased risk for fractures of the hip, spine, shoulder, or forearm. For women aged 50-64, whether bone mineral density accurately reflects who will develop osteoporosis is less clear. In this age range, the USPSTF recommends using either FRAX or OST rather than routine bone mineral density tests.
“I have the utmost respect for the United States Preventive Services Task Force, which lists both of these as valid screening tools for younger postmenopausal women. What I hope this study does is to inform the next iteration of the screening guidelines,” by maintaining the recommendation to use the OST while not keeping FRAX, said Carolyn J. Crandall, MD, MS, an internal medicine physician and health services researcher at University of California, Los Angeles, who helped conduct the research.
The U.S. version of FRAX requires identifying someone’s race, height, and weight, then answering whether they have different risk factors for a fracture such as a previous fracture, rheumatoid arthritis, or smoking. The result was thought to indicate a cumulative risk for major fracture over the next 10 years. Patients at significant risk should then undergo a bone density test.
The tool can also incorporate information about bone mineral density, if available, but the FRAX analyses in Dr. Crandall’s study did not include those data because the study aimed to test the measure’s predictive ability in the absence of a bone scan.
The OST includes only two variables – weight and age – to calculate risk for osteoporosis, and generally takes seconds to complete. It does not include race. As with FRAX, anyone deemed at significant risk for developing osteoporosis should undergo a bone density test.
“OST is really simple; that makes it very appealing,” Dr. Crandall said. “OST could probably be automatically calculated in the electronic medical record.”
Using data from the Women’s Health Initiative, Dr. Crandall and colleagues tracked more than 67,000 women aged 50-64 years for 10 years following enrollment in the study to see who experienced a fracture or developed osteoporosis over that decade. The investigators found that neither FRAX nor OST was particularly good at predicting who went on to experience a fracture.
The accuracy of FRAX at fracture prediction peaked at 65% for Asian women (area under the receiver operating curve, 0.65; 95% confidence interval, 0.58-0.71), and was lowest for Black women (AUC 0.55; 95% CI, 0.52-0.59). OST also was most accurate for Asian women, but only up to 62% (AUC 0.62; 95% CI, 0.56-0.69), and was again lowest for Black women (AUC 0.53; 95% CI, 0.50 - 0.57)
“It is just very hard to predict fractures in this age group,” Dr. Crandall said, noting that more evidence exists about risk for fracture in people older than 65.
The story diverges with predicting risk of osteoporosis in the neck. The OST did this roughly 80% of the time, for all racial groups. That figure proved better than FRAX, without including race.
Treatment gap
“This evidence supports using OST instead of FRAX” for selecting younger postmenopausal women who should undergo a bone mineral density exam, said E. Michael Lewiecki, MD, director of the New Mexico Clinical Research & Osteoporosis Center in Albuquerque.

Dr. Lewiecki, who was not involved in the new study, noted that the U.S. version of FRAX specifies race because of some clinical evidence that different races have different rates of fracture. But he and Dr. Crandall said the validity of race-based algorithms to guide clinical care is a controversial and evolving topic in medicine. Dr. Lewiecki said the Canadian version of FRAX, which is similarly applied to a diverse population as in the United States, omits race and works as well as the U.S. version. Future iterations of the instrument in the United States may not include race, Dr. Lewiecki said.
“The study is perfectly valid as far as it goes. But the big gorilla in the room is that most patients who need a bone density test are not getting it,” Dr. Lewiecki added. Sometimes a patient might break a bone in their wrist, for example, and tell their primary care provider that anyone would have broken that bone because the fall was so hard. Even if that’s true, Dr. Lewiecki said, any woman older than 45 who has broken a bone should undergo a bone density test to determine if they have osteoporosis, even if it seems like there are other possible reasons for why the break occurred.
“Most of the clinical practice guidelines that are used by physicians recommend getting a bone density test in postmenopausal women under the age of 65 who have a risk factor for fracture,” Dr. Lewiecki said, with a primary risk factor being a prior fracture. Dr. Lewiecki said he would rather that anyone who could benefit from a bone density test receive it, rather than someone foregoing a scan based on a screening tool that may be flawed.
“Most patients – men and women – who have osteoporosis are currently not being identified. Even when they are being identified, they are commonly not being treated. And when they are started on treatment, many patients discontinue treatment before they’ve taken it long enough to benefit,” Dr. Lewiecki said.
Dr. Crandall and Dr. Lewiecki report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study published in JAMA Internal Medicine.
One of the screenings, the U.S. Fracture Risk Assessment Tool (FRAX), proved relatively ineffective at identifying women who developed osteoporosis. The other screening, the Osteoporosis Self-Assessment Tool (OST), excelled at identifying osteoporosis for women in every racial and ethnic group, but also failed at identifying who was most likely to experience a fracture. Osteoporosis experts say that primary care physicians should test for the condition in anyone with any risk factor for it, even if a screening tool suggests doing so is unnecessary.
The United States Preventive Services Task Force (USPSTF) recommends routine testing of bone mineral density in women age 65 years and older to detect risk of developing osteoporosis, which in turn leads to an increased risk for fractures of the hip, spine, shoulder, or forearm. For women aged 50-64, whether bone mineral density accurately reflects who will develop osteoporosis is less clear. In this age range, the USPSTF recommends using either FRAX or OST rather than routine bone mineral density tests.
“I have the utmost respect for the United States Preventive Services Task Force, which lists both of these as valid screening tools for younger postmenopausal women. What I hope this study does is to inform the next iteration of the screening guidelines,” by maintaining the recommendation to use the OST while not keeping FRAX, said Carolyn J. Crandall, MD, MS, an internal medicine physician and health services researcher at University of California, Los Angeles, who helped conduct the research.
The U.S. version of FRAX requires identifying someone’s race, height, and weight, then answering whether they have different risk factors for a fracture such as a previous fracture, rheumatoid arthritis, or smoking. The result was thought to indicate a cumulative risk for major fracture over the next 10 years. Patients at significant risk should then undergo a bone density test.
The tool can also incorporate information about bone mineral density, if available, but the FRAX analyses in Dr. Crandall’s study did not include those data because the study aimed to test the measure’s predictive ability in the absence of a bone scan.
The OST includes only two variables – weight and age – to calculate risk for osteoporosis, and generally takes seconds to complete. It does not include race. As with FRAX, anyone deemed at significant risk for developing osteoporosis should undergo a bone density test.
“OST is really simple; that makes it very appealing,” Dr. Crandall said. “OST could probably be automatically calculated in the electronic medical record.”
Using data from the Women’s Health Initiative, Dr. Crandall and colleagues tracked more than 67,000 women aged 50-64 years for 10 years following enrollment in the study to see who experienced a fracture or developed osteoporosis over that decade. The investigators found that neither FRAX nor OST was particularly good at predicting who went on to experience a fracture.
The accuracy of FRAX at fracture prediction peaked at 65% for Asian women (area under the receiver operating curve, 0.65; 95% confidence interval, 0.58-0.71), and was lowest for Black women (AUC 0.55; 95% CI, 0.52-0.59). OST also was most accurate for Asian women, but only up to 62% (AUC 0.62; 95% CI, 0.56-0.69), and was again lowest for Black women (AUC 0.53; 95% CI, 0.50 - 0.57)
“It is just very hard to predict fractures in this age group,” Dr. Crandall said, noting that more evidence exists about risk for fracture in people older than 65.
The story diverges with predicting risk of osteoporosis in the neck. The OST did this roughly 80% of the time, for all racial groups. That figure proved better than FRAX, without including race.
Treatment gap
“This evidence supports using OST instead of FRAX” for selecting younger postmenopausal women who should undergo a bone mineral density exam, said E. Michael Lewiecki, MD, director of the New Mexico Clinical Research & Osteoporosis Center in Albuquerque.

Dr. Lewiecki, who was not involved in the new study, noted that the U.S. version of FRAX specifies race because of some clinical evidence that different races have different rates of fracture. But he and Dr. Crandall said the validity of race-based algorithms to guide clinical care is a controversial and evolving topic in medicine. Dr. Lewiecki said the Canadian version of FRAX, which is similarly applied to a diverse population as in the United States, omits race and works as well as the U.S. version. Future iterations of the instrument in the United States may not include race, Dr. Lewiecki said.
“The study is perfectly valid as far as it goes. But the big gorilla in the room is that most patients who need a bone density test are not getting it,” Dr. Lewiecki added. Sometimes a patient might break a bone in their wrist, for example, and tell their primary care provider that anyone would have broken that bone because the fall was so hard. Even if that’s true, Dr. Lewiecki said, any woman older than 45 who has broken a bone should undergo a bone density test to determine if they have osteoporosis, even if it seems like there are other possible reasons for why the break occurred.
“Most of the clinical practice guidelines that are used by physicians recommend getting a bone density test in postmenopausal women under the age of 65 who have a risk factor for fracture,” Dr. Lewiecki said, with a primary risk factor being a prior fracture. Dr. Lewiecki said he would rather that anyone who could benefit from a bone density test receive it, rather than someone foregoing a scan based on a screening tool that may be flawed.
“Most patients – men and women – who have osteoporosis are currently not being identified. Even when they are being identified, they are commonly not being treated. And when they are started on treatment, many patients discontinue treatment before they’ve taken it long enough to benefit,” Dr. Lewiecki said.
Dr. Crandall and Dr. Lewiecki report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICINE
2023 Update on genetics in fetal growth
Whole exome sequencing’s role in diagnosing genetic causes of FGR with and without associated anomalies
Mone F, Mellis R, Gabriel H, et al. Should we offer prenatal exome sequencing for intrauterine growth restriction or short long bones? A systematic review and meta-analysis. Am J Obstet Gynecol. Published online October 7, 2022. doi:10.1016/j.ajog.2022.09.045
Multiple factors can play a role in FGR, including inherent maternal, placental, or fetal factors; the environment; and/or nutrition. However, prenatal diagnosis is an important consideration when exploring the underlying etiology for a growth-restricted fetus, especially in severe or early-onset cases. Many genetic conditions do not result in structural anomalies but can disrupt overall growth. Additionally, phenotyping in the prenatal period is limited and can miss more subtle physical differences that could point to a genetic cause.
When compared with karyotype, chromosomal microarray (CMA) has been shown to increase the diagnostic yield in cases of isolated early FGR by 5%,1,2 and the incidence of chromosomal abnormalities has been reported to be as high as 19% in this population. Let’s explore the data on exome sequencing for prenatal diagnosis in cases of isolated FGR.
Meta-analysis details
In this meta-analysis, the authors reviewed 19 cohort studies or case series that investigated the yield of prenatal sequencing in fetuses with intrauterine growth restriction (IUGR) or short long bones, both in association with and without additional anomalies. All cases had nondiagnostic cytogenetic results. Fetal DNA in most cases was obtained through amniocentesis. Variants classified as likely pathogenic and pathogenic were considered diagnostic. The authors then calculated the incremental yield of prenatal sequencing over cytogenetic studies as a pooled value, comparing the following groups:
- isolated FGR
- growth restriction with associated anomalies
- isolated short long bones
- short long bones with additional skeletal features.
Study outcomes
The total number of cases were as follows: isolated IUGR (n = 71), IUGR associated with additional anomalies (n = 45), isolated short long bones (n = 84), and short long bones associated with additional skeletal findings (n = 252). Causative pathogenic or likely pathogenic variants were identified in 224 (50%) cases. Apparent incremental yields with prenatal sequencing were as follows for the 4 groups (as illustrated in the FIGURE):
- 4% in isolated IUGR (95% confidence interval [CI], -5%–12%)
- 30% in IUGR with additional anomalies (95% CI, 13%–47%)
- 48% in isolated short long bones (95% CI, 26%–70%)
- 68% in short long bones with additional skeletal changes (95% CI, 58%–77%).
Overall, the authors concluded that prenatal sequencing does not improve prenatal diagnosis in cases of isolated IUGR. The majority of these cases were thought to be related to placental insufficiency.
Strengths and limitations
The main limitation of this study with regard to our discussion is the small study populationof isolated growth restriction. The authors indicate that the number of cases of isolated IUGR were too small to draw firm conclusions. Another limitation was the heterogeneity of the isolated FGR population, which was not limited to severe or early-onset cases. However, the authors did demonstrate that growth restriction in association with fetal anomalies has very high genetic yield rates with prenatal sequencing.
Not surprisingly, there is a high yield of diagnosing genetic conditions in pregnancies complicated by isolated or nonisolated short long bones or in cases of growth restriction with multisystem abnormalities. Based on the results of this study, the authors advise against sending for exome sequencing in cases of isolated growth restriction with coexisting evidence of placental insufficiency.
Continue to: Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?...
Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?
Zhou H, Fu F, Wang Y, et al. Genetic causes of isolated and severe fetal growth restriction in normal chromosomal microarray analysis. Int J Gynaecol Obstet. Published online December 10, 2022. doi:10.1002/ijgo.14620
Severe FGR is diagnosed based on an estimated fetal weight (EFW) or abdominal circumference (AC) below the third percentile. As we discussed in the above study by Mone and colleagues, it does not appear that prenatal sequencing significantly improves the diagnostic yield in all isolated FGR cases. However, this has not been previously explored in isolated severe FGR or cases of early-onset FGR (<32 weeks’ gestation). We know that several monogenic conditions are associated with severe and early-onset isolated fetal growth impairment, including but not limited to Cornelia de Lange syndrome, Smith-Lemli-Opitz syndrome, and Meier-Gorlin syndrome. Often, these syndromes can present in the prenatal period without other phenotypic findings. Therefore, this study explored the possibility that prenatal sequencing plays an important role for severe cases of FGR with nondiagnostic CMA and/or karyotype.
Retrospective study details
Zhou and colleagues retrospectively analyzed 51 cases of severe (EFW or AC below the third percentile) isolated FGR with negative CMA who underwent trio whole exome sequencing, which includes submitting fetal cells as well as both parental samples for testing. Patients with abnormal toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus (TORCH) tests; structural anomalies; and multiple gestation were excluded from the analysis. As in the study by Mone et al, variants classified as likely pathogenic and pathogenic were categorized as diagnostic.
Results
Eight of 51 cases (15.7%) with severe isolated FGR had diagnostic findings on trio whole exome sequencing as shown in the TABLE. Another 8 cases (15.7%) were found to have variants of unknown significance, of which 2 were later determined to be novel pathogenic variants. Genetic conditions uncovered in this cohort include Cornelia de Lange syndrome, pyruvate dehydrogenase deficiency, Dent disease, trichohepaticenteric syndrome, achondroplasia, osteogenesis imperfecta, Pendred syndrome, and both autosomal dominant type 3A and autosomal recessive type 1A deafness. All 10 cases with diagnostic whole exome sequencing or identified novel pathogenic variants were affected by early-onset FGR (<32 weeks’ gestation). Of these 10 cases, 7 patients underwent pregnancy termination.
To summarize, a total of 10 cases (19.6%) of severe isolated early-onset FGR with negative cytogenetic studies were subsequently diagnosed with an underlying genetic condition using prenatal trio whole exome sequencing.
Strengths and limitations
This study is retrospective and has a small sample size (n = 51) that was mostly limited to early-onset isolated severe FGR. However, the diagnostic yield (19.6%) of whole exome sequencing after negative CMA testing was noteworthy and shows that monogenic conditions are an important consideration in the evaluation of severe early-onset FGR, even in the absence of structural abnormalities.
As indications for exome sequencing during pregnancy continue to evolve, severe isolated FGR is emerging as a high-yield condition in which a subset of patients may benefit from the described testing strategy. We learned from our look at the prior study (Mone et al) that unselected isolated growth restriction with evident placental insufficiency may not benefit from exome sequencing, but this study differs in its selection of early-onset, severe cases—defined by diagnosis before 32 weeks’ gestation and an EFW or AC below the third percentile. Almost 20% of cases who met the aforementioned criteria received a genetic diagnosis from exome sequencing. We should remember to offer genetic counseling and diagnostic testing to our patients with severe growth restriction, even in the absence of additional structural anomalies.
Could epigenetic mechanisms of placental dysregulation explain low birthweight and future cardiometabolic disease?
Tekola-Ayele F, Zeng X, Chatterjee S, et al. Placental multi-omics integration identifies candidate functional genes for birthweight. Nat Commun. 2022;13:2384.
FGR has been linked to greater mortality in childhood and increased risk for cardiometabolic disease in adulthood. While genomewide associations studies (GWAS) have defined areas of interest linking genetic variants to low birthweight, their relationship to epigenetic changes in the placenta as well as biologic and functional mechanisms are not yet well understood.
Multiomics used to identify candidate functional genes for birthweight
This study analyzed the methylation and gene expression patterns of 291 placental samples, integrating findings into pathways of previously defined GWAS variants. Patient samples were obtained from participants in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies–Singleton cohort. The cohort is ethnically diverse, with 97 Hispanic, 74 White, 71 Black, and 49 Asian participants. Of 286 single nucleotide polymorphisms (SNPs) found to be associated with birthweight, 273 were analyzed as part of the authors’ data set. These were found to have 7,901 unique protein-coding mRNAs (expression quantitative trait loci [eQTL]) and more than 100,000 nearby (within 1 Mb) CpG islands thought to be involved in changes in DNA methylation (methylation quantitative trait loci [mQTL]). Each functionally connected GWAS-eQTL-mQTL association is referred to as a triplet.
The next arm of the study investigatedthe connections and pathways within each triplet. Three possible scenarios were explored for birthweight GWAS SNPs using a causal interference test (CIT):
- the SNP alters placental DNA methylation, which then influences gene expression
- the SNP first alters placental DNA expression, which then influences methylation
- the SNP influences placental DNA expression and methylation independently, with no notable crossover between their pathways.
Triplets were investigated using the Mendelian randomization (MR) Steiger directionality test to validate the directionality of the pathways found by CIT. Lastly, the possibility of linkage disequilibrium was also studied using the moloc test.
Results
Using CIT, a causal relationship was predicted in 88 of 197 triplets, in which 84 (95.5%) indicated DNA methylation influences gene expression, and 4 (4.5%) indicated gene expression influences DNA methylation. The authors also used the MR Steiger test to investigate triplets to identify possible causal pathways. Using the MR Steiger test, only 3 of 45 (7%) triplets were found to have independent gene expression and methylation pathways. Thirty-eight of 45 (84%) triplets indicated that gene expression influences DNA methylation, and 7 (15%)triplets demonstrated that DNA methylation influences gene expression. Consistent predictions between CIT and the MR Steiger test revealed 3 triplets in which DNA methylation influences gene expression for the following genes: WNT3A, CTDNEP1, and RANBP2. Additionally, a strong colocalization signal was found among birthweight, DNA methylation, and gene expression for the following genes: PLEKHA1, FES, PRMT7, and CTDNEP1. Gene set enrichment analysis was performed as well and found that low birthweight is associated in substantial upregulation of genes associated with oxidative stress, immune response, adipogenesis, myogenesis, and the production of pancreatic ß cells.
Study strengths and limitations
The study is one of the first to identify regulatory targets for placental DNA methylation and gene expression in previously identified GWAS loci associated with low birthweight. For example, DNA methylation was found to influence gene expression of WNT3A, CTDNEP1, and RANBP2, which have previously been shown in animal studies to impact the vascularization and development of the placenta, embryogenesis, and fetal growth. The study also identified 4 genes (PLEKHA1, FES, PRMT7, and CTDNEP1) thought to have direct regulatory influence on placental DNA methylation and gene expression.
A limitation of the study is that it could not distinguish between whether the epigenetic changes we outlined have a maternal or fetal origin. Another limitation is that tissue used by the authors for analysis was a small placental biopsy, which does not accurately reflect the genetic heterogeneity of the placenta. Finally, this study does not establish causality between the identified epigenetic pathways and low birthweight. ●
We know that the placenta is critical to in utero development. This study begins to explore the genetic changes and programming in the placenta that may have profound effects on health and well-being both early and later in life.
- Li LS, Li DZ. A genetic approach to the etiologic investigation of isolated intrauterine growth restriction. Am J Obstet Gynecol. 2021;225:695-696. doi: 10.1016/j.ajog.2021 .07.021.
- Borrell A, Grande M, Pauta M, et al. Chromosomal microarray analysis in fetuses with growth restriction and normal karyotype: a systematic review and meta-analysis. Fetal Diagn Ther. 2018;44:1-9. doi: 10.1159/000479506.
Whole exome sequencing’s role in diagnosing genetic causes of FGR with and without associated anomalies
Mone F, Mellis R, Gabriel H, et al. Should we offer prenatal exome sequencing for intrauterine growth restriction or short long bones? A systematic review and meta-analysis. Am J Obstet Gynecol. Published online October 7, 2022. doi:10.1016/j.ajog.2022.09.045
Multiple factors can play a role in FGR, including inherent maternal, placental, or fetal factors; the environment; and/or nutrition. However, prenatal diagnosis is an important consideration when exploring the underlying etiology for a growth-restricted fetus, especially in severe or early-onset cases. Many genetic conditions do not result in structural anomalies but can disrupt overall growth. Additionally, phenotyping in the prenatal period is limited and can miss more subtle physical differences that could point to a genetic cause.
When compared with karyotype, chromosomal microarray (CMA) has been shown to increase the diagnostic yield in cases of isolated early FGR by 5%,1,2 and the incidence of chromosomal abnormalities has been reported to be as high as 19% in this population. Let’s explore the data on exome sequencing for prenatal diagnosis in cases of isolated FGR.
Meta-analysis details
In this meta-analysis, the authors reviewed 19 cohort studies or case series that investigated the yield of prenatal sequencing in fetuses with intrauterine growth restriction (IUGR) or short long bones, both in association with and without additional anomalies. All cases had nondiagnostic cytogenetic results. Fetal DNA in most cases was obtained through amniocentesis. Variants classified as likely pathogenic and pathogenic were considered diagnostic. The authors then calculated the incremental yield of prenatal sequencing over cytogenetic studies as a pooled value, comparing the following groups:
- isolated FGR
- growth restriction with associated anomalies
- isolated short long bones
- short long bones with additional skeletal features.
Study outcomes
The total number of cases were as follows: isolated IUGR (n = 71), IUGR associated with additional anomalies (n = 45), isolated short long bones (n = 84), and short long bones associated with additional skeletal findings (n = 252). Causative pathogenic or likely pathogenic variants were identified in 224 (50%) cases. Apparent incremental yields with prenatal sequencing were as follows for the 4 groups (as illustrated in the FIGURE):

- 4% in isolated IUGR (95% confidence interval [CI], -5%–12%)
- 30% in IUGR with additional anomalies (95% CI, 13%–47%)
- 48% in isolated short long bones (95% CI, 26%–70%)
- 68% in short long bones with additional skeletal changes (95% CI, 58%–77%).
Overall, the authors concluded that prenatal sequencing does not improve prenatal diagnosis in cases of isolated IUGR. The majority of these cases were thought to be related to placental insufficiency.
Strengths and limitations
The main limitation of this study with regard to our discussion is the small study populationof isolated growth restriction. The authors indicate that the number of cases of isolated IUGR were too small to draw firm conclusions. Another limitation was the heterogeneity of the isolated FGR population, which was not limited to severe or early-onset cases. However, the authors did demonstrate that growth restriction in association with fetal anomalies has very high genetic yield rates with prenatal sequencing.
Not surprisingly, there is a high yield of diagnosing genetic conditions in pregnancies complicated by isolated or nonisolated short long bones or in cases of growth restriction with multisystem abnormalities. Based on the results of this study, the authors advise against sending for exome sequencing in cases of isolated growth restriction with coexisting evidence of placental insufficiency.
Continue to: Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?...
Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?
Zhou H, Fu F, Wang Y, et al. Genetic causes of isolated and severe fetal growth restriction in normal chromosomal microarray analysis. Int J Gynaecol Obstet. Published online December 10, 2022. doi:10.1002/ijgo.14620
Severe FGR is diagnosed based on an estimated fetal weight (EFW) or abdominal circumference (AC) below the third percentile. As we discussed in the above study by Mone and colleagues, it does not appear that prenatal sequencing significantly improves the diagnostic yield in all isolated FGR cases. However, this has not been previously explored in isolated severe FGR or cases of early-onset FGR (<32 weeks’ gestation). We know that several monogenic conditions are associated with severe and early-onset isolated fetal growth impairment, including but not limited to Cornelia de Lange syndrome, Smith-Lemli-Opitz syndrome, and Meier-Gorlin syndrome. Often, these syndromes can present in the prenatal period without other phenotypic findings. Therefore, this study explored the possibility that prenatal sequencing plays an important role for severe cases of FGR with nondiagnostic CMA and/or karyotype.
Retrospective study details
Zhou and colleagues retrospectively analyzed 51 cases of severe (EFW or AC below the third percentile) isolated FGR with negative CMA who underwent trio whole exome sequencing, which includes submitting fetal cells as well as both parental samples for testing. Patients with abnormal toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus (TORCH) tests; structural anomalies; and multiple gestation were excluded from the analysis. As in the study by Mone et al, variants classified as likely pathogenic and pathogenic were categorized as diagnostic.
Results
Eight of 51 cases (15.7%) with severe isolated FGR had diagnostic findings on trio whole exome sequencing as shown in the TABLE. Another 8 cases (15.7%) were found to have variants of unknown significance, of which 2 were later determined to be novel pathogenic variants. Genetic conditions uncovered in this cohort include Cornelia de Lange syndrome, pyruvate dehydrogenase deficiency, Dent disease, trichohepaticenteric syndrome, achondroplasia, osteogenesis imperfecta, Pendred syndrome, and both autosomal dominant type 3A and autosomal recessive type 1A deafness. All 10 cases with diagnostic whole exome sequencing or identified novel pathogenic variants were affected by early-onset FGR (<32 weeks’ gestation). Of these 10 cases, 7 patients underwent pregnancy termination.

To summarize, a total of 10 cases (19.6%) of severe isolated early-onset FGR with negative cytogenetic studies were subsequently diagnosed with an underlying genetic condition using prenatal trio whole exome sequencing.
Strengths and limitations
This study is retrospective and has a small sample size (n = 51) that was mostly limited to early-onset isolated severe FGR. However, the diagnostic yield (19.6%) of whole exome sequencing after negative CMA testing was noteworthy and shows that monogenic conditions are an important consideration in the evaluation of severe early-onset FGR, even in the absence of structural abnormalities.
As indications for exome sequencing during pregnancy continue to evolve, severe isolated FGR is emerging as a high-yield condition in which a subset of patients may benefit from the described testing strategy. We learned from our look at the prior study (Mone et al) that unselected isolated growth restriction with evident placental insufficiency may not benefit from exome sequencing, but this study differs in its selection of early-onset, severe cases—defined by diagnosis before 32 weeks’ gestation and an EFW or AC below the third percentile. Almost 20% of cases who met the aforementioned criteria received a genetic diagnosis from exome sequencing. We should remember to offer genetic counseling and diagnostic testing to our patients with severe growth restriction, even in the absence of additional structural anomalies.
Could epigenetic mechanisms of placental dysregulation explain low birthweight and future cardiometabolic disease?
Tekola-Ayele F, Zeng X, Chatterjee S, et al. Placental multi-omics integration identifies candidate functional genes for birthweight. Nat Commun. 2022;13:2384.
FGR has been linked to greater mortality in childhood and increased risk for cardiometabolic disease in adulthood. While genomewide associations studies (GWAS) have defined areas of interest linking genetic variants to low birthweight, their relationship to epigenetic changes in the placenta as well as biologic and functional mechanisms are not yet well understood.
Multiomics used to identify candidate functional genes for birthweight
This study analyzed the methylation and gene expression patterns of 291 placental samples, integrating findings into pathways of previously defined GWAS variants. Patient samples were obtained from participants in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies–Singleton cohort. The cohort is ethnically diverse, with 97 Hispanic, 74 White, 71 Black, and 49 Asian participants. Of 286 single nucleotide polymorphisms (SNPs) found to be associated with birthweight, 273 were analyzed as part of the authors’ data set. These were found to have 7,901 unique protein-coding mRNAs (expression quantitative trait loci [eQTL]) and more than 100,000 nearby (within 1 Mb) CpG islands thought to be involved in changes in DNA methylation (methylation quantitative trait loci [mQTL]). Each functionally connected GWAS-eQTL-mQTL association is referred to as a triplet.
The next arm of the study investigatedthe connections and pathways within each triplet. Three possible scenarios were explored for birthweight GWAS SNPs using a causal interference test (CIT):
- the SNP alters placental DNA methylation, which then influences gene expression
- the SNP first alters placental DNA expression, which then influences methylation
- the SNP influences placental DNA expression and methylation independently, with no notable crossover between their pathways.
Triplets were investigated using the Mendelian randomization (MR) Steiger directionality test to validate the directionality of the pathways found by CIT. Lastly, the possibility of linkage disequilibrium was also studied using the moloc test.
Results
Using CIT, a causal relationship was predicted in 88 of 197 triplets, in which 84 (95.5%) indicated DNA methylation influences gene expression, and 4 (4.5%) indicated gene expression influences DNA methylation. The authors also used the MR Steiger test to investigate triplets to identify possible causal pathways. Using the MR Steiger test, only 3 of 45 (7%) triplets were found to have independent gene expression and methylation pathways. Thirty-eight of 45 (84%) triplets indicated that gene expression influences DNA methylation, and 7 (15%)triplets demonstrated that DNA methylation influences gene expression. Consistent predictions between CIT and the MR Steiger test revealed 3 triplets in which DNA methylation influences gene expression for the following genes: WNT3A, CTDNEP1, and RANBP2. Additionally, a strong colocalization signal was found among birthweight, DNA methylation, and gene expression for the following genes: PLEKHA1, FES, PRMT7, and CTDNEP1. Gene set enrichment analysis was performed as well and found that low birthweight is associated in substantial upregulation of genes associated with oxidative stress, immune response, adipogenesis, myogenesis, and the production of pancreatic ß cells.
Study strengths and limitations
The study is one of the first to identify regulatory targets for placental DNA methylation and gene expression in previously identified GWAS loci associated with low birthweight. For example, DNA methylation was found to influence gene expression of WNT3A, CTDNEP1, and RANBP2, which have previously been shown in animal studies to impact the vascularization and development of the placenta, embryogenesis, and fetal growth. The study also identified 4 genes (PLEKHA1, FES, PRMT7, and CTDNEP1) thought to have direct regulatory influence on placental DNA methylation and gene expression.
A limitation of the study is that it could not distinguish between whether the epigenetic changes we outlined have a maternal or fetal origin. Another limitation is that tissue used by the authors for analysis was a small placental biopsy, which does not accurately reflect the genetic heterogeneity of the placenta. Finally, this study does not establish causality between the identified epigenetic pathways and low birthweight. ●
We know that the placenta is critical to in utero development. This study begins to explore the genetic changes and programming in the placenta that may have profound effects on health and well-being both early and later in life.
Whole exome sequencing’s role in diagnosing genetic causes of FGR with and without associated anomalies
Mone F, Mellis R, Gabriel H, et al. Should we offer prenatal exome sequencing for intrauterine growth restriction or short long bones? A systematic review and meta-analysis. Am J Obstet Gynecol. Published online October 7, 2022. doi:10.1016/j.ajog.2022.09.045
Multiple factors can play a role in FGR, including inherent maternal, placental, or fetal factors; the environment; and/or nutrition. However, prenatal diagnosis is an important consideration when exploring the underlying etiology for a growth-restricted fetus, especially in severe or early-onset cases. Many genetic conditions do not result in structural anomalies but can disrupt overall growth. Additionally, phenotyping in the prenatal period is limited and can miss more subtle physical differences that could point to a genetic cause.
When compared with karyotype, chromosomal microarray (CMA) has been shown to increase the diagnostic yield in cases of isolated early FGR by 5%,1,2 and the incidence of chromosomal abnormalities has been reported to be as high as 19% in this population. Let’s explore the data on exome sequencing for prenatal diagnosis in cases of isolated FGR.
Meta-analysis details
In this meta-analysis, the authors reviewed 19 cohort studies or case series that investigated the yield of prenatal sequencing in fetuses with intrauterine growth restriction (IUGR) or short long bones, both in association with and without additional anomalies. All cases had nondiagnostic cytogenetic results. Fetal DNA in most cases was obtained through amniocentesis. Variants classified as likely pathogenic and pathogenic were considered diagnostic. The authors then calculated the incremental yield of prenatal sequencing over cytogenetic studies as a pooled value, comparing the following groups:
- isolated FGR
- growth restriction with associated anomalies
- isolated short long bones
- short long bones with additional skeletal features.
Study outcomes
The total number of cases were as follows: isolated IUGR (n = 71), IUGR associated with additional anomalies (n = 45), isolated short long bones (n = 84), and short long bones associated with additional skeletal findings (n = 252). Causative pathogenic or likely pathogenic variants were identified in 224 (50%) cases. Apparent incremental yields with prenatal sequencing were as follows for the 4 groups (as illustrated in the FIGURE):

- 4% in isolated IUGR (95% confidence interval [CI], -5%–12%)
- 30% in IUGR with additional anomalies (95% CI, 13%–47%)
- 48% in isolated short long bones (95% CI, 26%–70%)
- 68% in short long bones with additional skeletal changes (95% CI, 58%–77%).
Overall, the authors concluded that prenatal sequencing does not improve prenatal diagnosis in cases of isolated IUGR. The majority of these cases were thought to be related to placental insufficiency.
Strengths and limitations
The main limitation of this study with regard to our discussion is the small study populationof isolated growth restriction. The authors indicate that the number of cases of isolated IUGR were too small to draw firm conclusions. Another limitation was the heterogeneity of the isolated FGR population, which was not limited to severe or early-onset cases. However, the authors did demonstrate that growth restriction in association with fetal anomalies has very high genetic yield rates with prenatal sequencing.
Not surprisingly, there is a high yield of diagnosing genetic conditions in pregnancies complicated by isolated or nonisolated short long bones or in cases of growth restriction with multisystem abnormalities. Based on the results of this study, the authors advise against sending for exome sequencing in cases of isolated growth restriction with coexisting evidence of placental insufficiency.
Continue to: Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?...
Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?
Zhou H, Fu F, Wang Y, et al. Genetic causes of isolated and severe fetal growth restriction in normal chromosomal microarray analysis. Int J Gynaecol Obstet. Published online December 10, 2022. doi:10.1002/ijgo.14620
Severe FGR is diagnosed based on an estimated fetal weight (EFW) or abdominal circumference (AC) below the third percentile. As we discussed in the above study by Mone and colleagues, it does not appear that prenatal sequencing significantly improves the diagnostic yield in all isolated FGR cases. However, this has not been previously explored in isolated severe FGR or cases of early-onset FGR (<32 weeks’ gestation). We know that several monogenic conditions are associated with severe and early-onset isolated fetal growth impairment, including but not limited to Cornelia de Lange syndrome, Smith-Lemli-Opitz syndrome, and Meier-Gorlin syndrome. Often, these syndromes can present in the prenatal period without other phenotypic findings. Therefore, this study explored the possibility that prenatal sequencing plays an important role for severe cases of FGR with nondiagnostic CMA and/or karyotype.
Retrospective study details
Zhou and colleagues retrospectively analyzed 51 cases of severe (EFW or AC below the third percentile) isolated FGR with negative CMA who underwent trio whole exome sequencing, which includes submitting fetal cells as well as both parental samples for testing. Patients with abnormal toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus (TORCH) tests; structural anomalies; and multiple gestation were excluded from the analysis. As in the study by Mone et al, variants classified as likely pathogenic and pathogenic were categorized as diagnostic.
Results
Eight of 51 cases (15.7%) with severe isolated FGR had diagnostic findings on trio whole exome sequencing as shown in the TABLE. Another 8 cases (15.7%) were found to have variants of unknown significance, of which 2 were later determined to be novel pathogenic variants. Genetic conditions uncovered in this cohort include Cornelia de Lange syndrome, pyruvate dehydrogenase deficiency, Dent disease, trichohepaticenteric syndrome, achondroplasia, osteogenesis imperfecta, Pendred syndrome, and both autosomal dominant type 3A and autosomal recessive type 1A deafness. All 10 cases with diagnostic whole exome sequencing or identified novel pathogenic variants were affected by early-onset FGR (<32 weeks’ gestation). Of these 10 cases, 7 patients underwent pregnancy termination.

To summarize, a total of 10 cases (19.6%) of severe isolated early-onset FGR with negative cytogenetic studies were subsequently diagnosed with an underlying genetic condition using prenatal trio whole exome sequencing.
Strengths and limitations
This study is retrospective and has a small sample size (n = 51) that was mostly limited to early-onset isolated severe FGR. However, the diagnostic yield (19.6%) of whole exome sequencing after negative CMA testing was noteworthy and shows that monogenic conditions are an important consideration in the evaluation of severe early-onset FGR, even in the absence of structural abnormalities.
As indications for exome sequencing during pregnancy continue to evolve, severe isolated FGR is emerging as a high-yield condition in which a subset of patients may benefit from the described testing strategy. We learned from our look at the prior study (Mone et al) that unselected isolated growth restriction with evident placental insufficiency may not benefit from exome sequencing, but this study differs in its selection of early-onset, severe cases—defined by diagnosis before 32 weeks’ gestation and an EFW or AC below the third percentile. Almost 20% of cases who met the aforementioned criteria received a genetic diagnosis from exome sequencing. We should remember to offer genetic counseling and diagnostic testing to our patients with severe growth restriction, even in the absence of additional structural anomalies.
Could epigenetic mechanisms of placental dysregulation explain low birthweight and future cardiometabolic disease?
Tekola-Ayele F, Zeng X, Chatterjee S, et al. Placental multi-omics integration identifies candidate functional genes for birthweight. Nat Commun. 2022;13:2384.
FGR has been linked to greater mortality in childhood and increased risk for cardiometabolic disease in adulthood. While genomewide associations studies (GWAS) have defined areas of interest linking genetic variants to low birthweight, their relationship to epigenetic changes in the placenta as well as biologic and functional mechanisms are not yet well understood.
Multiomics used to identify candidate functional genes for birthweight
This study analyzed the methylation and gene expression patterns of 291 placental samples, integrating findings into pathways of previously defined GWAS variants. Patient samples were obtained from participants in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies–Singleton cohort. The cohort is ethnically diverse, with 97 Hispanic, 74 White, 71 Black, and 49 Asian participants. Of 286 single nucleotide polymorphisms (SNPs) found to be associated with birthweight, 273 were analyzed as part of the authors’ data set. These were found to have 7,901 unique protein-coding mRNAs (expression quantitative trait loci [eQTL]) and more than 100,000 nearby (within 1 Mb) CpG islands thought to be involved in changes in DNA methylation (methylation quantitative trait loci [mQTL]). Each functionally connected GWAS-eQTL-mQTL association is referred to as a triplet.
The next arm of the study investigatedthe connections and pathways within each triplet. Three possible scenarios were explored for birthweight GWAS SNPs using a causal interference test (CIT):
- the SNP alters placental DNA methylation, which then influences gene expression
- the SNP first alters placental DNA expression, which then influences methylation
- the SNP influences placental DNA expression and methylation independently, with no notable crossover between their pathways.
Triplets were investigated using the Mendelian randomization (MR) Steiger directionality test to validate the directionality of the pathways found by CIT. Lastly, the possibility of linkage disequilibrium was also studied using the moloc test.
Results
Using CIT, a causal relationship was predicted in 88 of 197 triplets, in which 84 (95.5%) indicated DNA methylation influences gene expression, and 4 (4.5%) indicated gene expression influences DNA methylation. The authors also used the MR Steiger test to investigate triplets to identify possible causal pathways. Using the MR Steiger test, only 3 of 45 (7%) triplets were found to have independent gene expression and methylation pathways. Thirty-eight of 45 (84%) triplets indicated that gene expression influences DNA methylation, and 7 (15%)triplets demonstrated that DNA methylation influences gene expression. Consistent predictions between CIT and the MR Steiger test revealed 3 triplets in which DNA methylation influences gene expression for the following genes: WNT3A, CTDNEP1, and RANBP2. Additionally, a strong colocalization signal was found among birthweight, DNA methylation, and gene expression for the following genes: PLEKHA1, FES, PRMT7, and CTDNEP1. Gene set enrichment analysis was performed as well and found that low birthweight is associated in substantial upregulation of genes associated with oxidative stress, immune response, adipogenesis, myogenesis, and the production of pancreatic ß cells.
Study strengths and limitations
The study is one of the first to identify regulatory targets for placental DNA methylation and gene expression in previously identified GWAS loci associated with low birthweight. For example, DNA methylation was found to influence gene expression of WNT3A, CTDNEP1, and RANBP2, which have previously been shown in animal studies to impact the vascularization and development of the placenta, embryogenesis, and fetal growth. The study also identified 4 genes (PLEKHA1, FES, PRMT7, and CTDNEP1) thought to have direct regulatory influence on placental DNA methylation and gene expression.
A limitation of the study is that it could not distinguish between whether the epigenetic changes we outlined have a maternal or fetal origin. Another limitation is that tissue used by the authors for analysis was a small placental biopsy, which does not accurately reflect the genetic heterogeneity of the placenta. Finally, this study does not establish causality between the identified epigenetic pathways and low birthweight. ●
We know that the placenta is critical to in utero development. This study begins to explore the genetic changes and programming in the placenta that may have profound effects on health and well-being both early and later in life.
- Li LS, Li DZ. A genetic approach to the etiologic investigation of isolated intrauterine growth restriction. Am J Obstet Gynecol. 2021;225:695-696. doi: 10.1016/j.ajog.2021 .07.021.
- Borrell A, Grande M, Pauta M, et al. Chromosomal microarray analysis in fetuses with growth restriction and normal karyotype: a systematic review and meta-analysis. Fetal Diagn Ther. 2018;44:1-9. doi: 10.1159/000479506.
- Li LS, Li DZ. A genetic approach to the etiologic investigation of isolated intrauterine growth restriction. Am J Obstet Gynecol. 2021;225:695-696. doi: 10.1016/j.ajog.2021 .07.021.
- Borrell A, Grande M, Pauta M, et al. Chromosomal microarray analysis in fetuses with growth restriction and normal karyotype: a systematic review and meta-analysis. Fetal Diagn Ther. 2018;44:1-9. doi: 10.1159/000479506.
The perimenopausal period and the benefits of progestin IUDs
Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.
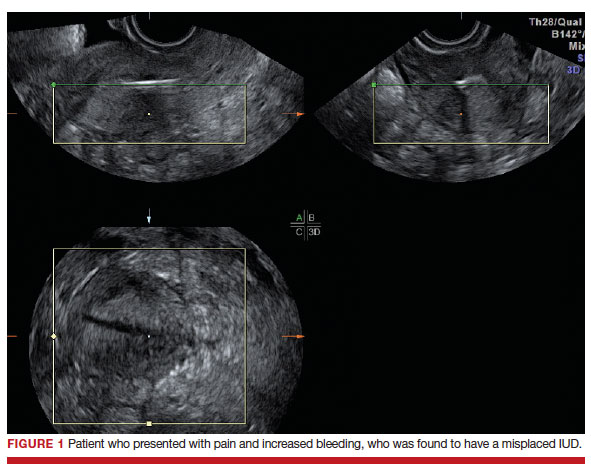
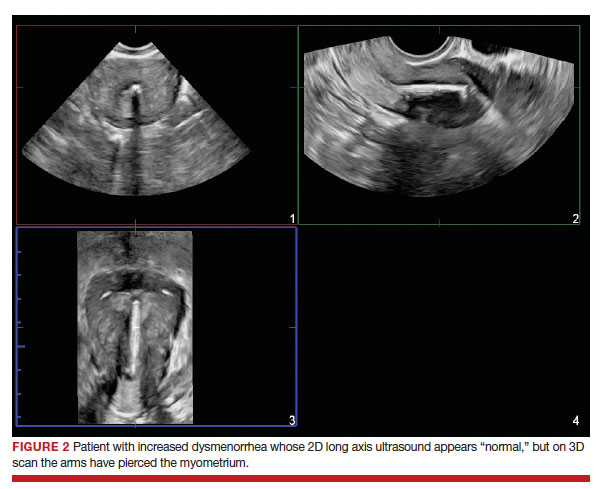
It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.

Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.


It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●

Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.


It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.
Youth-led sexual health program improves teen knowledge, autonomy
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
While the small pilot study focused primarily on assessing feasibility and effectiveness, the results suggest potential for scaling the program up to reach a larger audience and assessing the knowledge disseminated from direct youth participants.
“The good thing about this subject is that not a lot of it has to be context-specific,” Saumya Sao, a clinical researcher in gynecology and obstetrics at the Johns Hopkins University, Baltimore, and the study’s lead author, said in an interview. “A lot of it is just baseline information that everybody needs and doesn’t get.”
Jaime Friedman, MD, a pediatrician and director of marketing at Children’s Primary Care Medical Group in San Diego, was not involved in the study but was impressed with the program’s objectives and results so far.
“While education is massively important, teens don’t always want to hear it from their parents or other adults,” Dr. Friedman said in an interview. “Learning from their peers is one way to overcome this hurdle.”
Given the high rate of sexually transmitted infections and unintended pregnancies in youth, paired with low sexual and reproductive health literacy in this population, the researchers sought to learn whether a program focused on peer-to-peer health education on these topics was feasible. The goal was to increase youth sexual and reproductive health knowledge, self-efficacy, and autonomy using a youth-led intervention.
The researchers hosted nine monthly, interactive, youth-led sessions that lasted 2 hours over Zoom or in person. Incorporated into the meetings were principles from Youth Participatory Action Research (YPAR) and Positive Youth Development (PYD).
The major topics included the following: Use of social media, values and goal-setting, anatomy and menstrual health, risk factors of sexual activities , STI and HIV prevention, contraceptive methods, healthy relationships and consent, practice responding to unhealthy behavior, gender and sexuality, and social media and body image.
The 24 participants were provided with transportation to the study site at the researchers’ institution and received financial compensation for their participation. They were an average 15.8 years old, lived in the greater Baltimore area, and mostly self-identified as female. Eight percent identified as non-binary and half (50%) identified as LGBTQIA+. Just over half the participants (52%) were Black/African American, 28% were Asian/Asian American, 12% were White, and 8% were Hispanic. The participants attended an average 88% of the sessions throughout the full intervention.
For each of the nine sessions, more than 50% of participants reported that they “learned a lot,” and only one participant reported for one session (session 5) that they “didn’t learn” anything. The researchers assessed participants’ knowledge, self-efficacy, and sense of autonomy at baseline and after completion of the intervention. Significant improvements occurred across all areas.
The average score improved by 31% in sexual and reproductive health knowledge (P < .001), 33% in sexual and reproductive health services awareness (P = .002), 46% in advocacy and empowerment (P < .001), 16% in general perceived efficacy (P = .002), and 22% personal sexuality empowerment (P = .006).
Ms. Sao said she was very pleased to see that the improvements were significant in every domain they measured, which she attributed largely to the incorporation of YPAR and PYD into the program.
“We approached it using these two frameworks that really do focus on involving youth in the teaching themselves, so I think that’s what increased their general perceived efficacy and advocacy empowerment without us necessarily having to emphasize, ‘You are advocates,’” Ms. Sao said. “Those frameworks ask the youth for their opinions and then give the youth an opportunity in every single session to be teachers themselves, and I think that lends itself well to all of the domains.”
Ms. Sao was also pleasantly surprised at the high level of retention across the 9 months.
“Every single session was slotted for 2 hours, but they would want to stay for 3 hours. Eventually, we actually started meeting with them twice a month, just adding an extra session,” she said. “As they gained confidence, they were so excited to be peer educators and realized, ‘I can really do this. I can teach my peers. We’re not getting this from anywhere else.’ ”
Ms. Sao and another study author, Maclaine Barré-Quick, an undergraduate research assistant at Johns Hopkins University, said the participants quickly discovered how easy it was to have a non-stigmatizing conversation about many of the topics once a subject was brought up.
“They’re actively looking for that opportunity,” Ms. Barré-Quick said in an interview.
Dr. Friedman agreed that this type of program provides what many adolescents need in a way that they may welcome more than through other methods.
“Adolescents’ bodies are approaching adulthood and function like adults, but their brains are still developing. They don’t have the worldly experience and education of adults, but they think they know everything,” Dr. Friedman said. “They are a population known for their high risk behavior due to their natural impulsivity. This can be a scary combination, especially when it comes to sexual health.”
But if teens don’t want to hear some of the information they need from adults, they may be more open to hearing it from other teens, Dr. Friedman said.
“Using an evidence-based approach ensures the desired outcome of healthier habits, decreased STIs and decreased teen pregnancy,” Dr. Friedman said. “It also adds weight to the argument against abstinence-only education. Teens deserve accurate and evidence-based education about their own bodies.”
Ms. Sao said the next steps will be exploring ways to scale the program up, such as putting the curriculum resources into a bundle available to other educators. They’re also looking at ways to put it into an online platform that’s self-paced, though that requires solving the challenge of having synchronous meetings for youth-led discussion.
“There are certain kinks that we have to work out because there were some activities where I think the students really benefited from having those open discussions with each other, so [we need to determine] how to replicate that in an online format,” Ms. Sao said.
Dr. Friedman agreed that scalability appears to be the biggest challenge, along with funding programs. But if those obstacles can be overcome, such programs would complement and expand on the education she does currently with families.
“I don’t have time for a full sex ed course at each visit,” Dr. Friedman said. “I would like to be able to direct them to a program that I know works and would be easy for them to complete. Even better, this would be an amazing program to ‘sell’ to practices interested in hosting these sessions themselves.”
Ms. Sao said they also hope to assess the impact of the intervention on the participants’ peers to see how well the knowledge and self-efficacy spread through the youths’ teaching.
No external funding was noted. One author reported research support from Hologic and Merck. Dr. Friedman had no disclosures.
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
While the small pilot study focused primarily on assessing feasibility and effectiveness, the results suggest potential for scaling the program up to reach a larger audience and assessing the knowledge disseminated from direct youth participants.
“The good thing about this subject is that not a lot of it has to be context-specific,” Saumya Sao, a clinical researcher in gynecology and obstetrics at the Johns Hopkins University, Baltimore, and the study’s lead author, said in an interview. “A lot of it is just baseline information that everybody needs and doesn’t get.”
Jaime Friedman, MD, a pediatrician and director of marketing at Children’s Primary Care Medical Group in San Diego, was not involved in the study but was impressed with the program’s objectives and results so far.
“While education is massively important, teens don’t always want to hear it from their parents or other adults,” Dr. Friedman said in an interview. “Learning from their peers is one way to overcome this hurdle.”
Given the high rate of sexually transmitted infections and unintended pregnancies in youth, paired with low sexual and reproductive health literacy in this population, the researchers sought to learn whether a program focused on peer-to-peer health education on these topics was feasible. The goal was to increase youth sexual and reproductive health knowledge, self-efficacy, and autonomy using a youth-led intervention.
The researchers hosted nine monthly, interactive, youth-led sessions that lasted 2 hours over Zoom or in person. Incorporated into the meetings were principles from Youth Participatory Action Research (YPAR) and Positive Youth Development (PYD).
The major topics included the following: Use of social media, values and goal-setting, anatomy and menstrual health, risk factors of sexual activities , STI and HIV prevention, contraceptive methods, healthy relationships and consent, practice responding to unhealthy behavior, gender and sexuality, and social media and body image.
The 24 participants were provided with transportation to the study site at the researchers’ institution and received financial compensation for their participation. They were an average 15.8 years old, lived in the greater Baltimore area, and mostly self-identified as female. Eight percent identified as non-binary and half (50%) identified as LGBTQIA+. Just over half the participants (52%) were Black/African American, 28% were Asian/Asian American, 12% were White, and 8% were Hispanic. The participants attended an average 88% of the sessions throughout the full intervention.
For each of the nine sessions, more than 50% of participants reported that they “learned a lot,” and only one participant reported for one session (session 5) that they “didn’t learn” anything. The researchers assessed participants’ knowledge, self-efficacy, and sense of autonomy at baseline and after completion of the intervention. Significant improvements occurred across all areas.
The average score improved by 31% in sexual and reproductive health knowledge (P < .001), 33% in sexual and reproductive health services awareness (P = .002), 46% in advocacy and empowerment (P < .001), 16% in general perceived efficacy (P = .002), and 22% personal sexuality empowerment (P = .006).
Ms. Sao said she was very pleased to see that the improvements were significant in every domain they measured, which she attributed largely to the incorporation of YPAR and PYD into the program.
“We approached it using these two frameworks that really do focus on involving youth in the teaching themselves, so I think that’s what increased their general perceived efficacy and advocacy empowerment without us necessarily having to emphasize, ‘You are advocates,’” Ms. Sao said. “Those frameworks ask the youth for their opinions and then give the youth an opportunity in every single session to be teachers themselves, and I think that lends itself well to all of the domains.”
Ms. Sao was also pleasantly surprised at the high level of retention across the 9 months.
“Every single session was slotted for 2 hours, but they would want to stay for 3 hours. Eventually, we actually started meeting with them twice a month, just adding an extra session,” she said. “As they gained confidence, they were so excited to be peer educators and realized, ‘I can really do this. I can teach my peers. We’re not getting this from anywhere else.’ ”
Ms. Sao and another study author, Maclaine Barré-Quick, an undergraduate research assistant at Johns Hopkins University, said the participants quickly discovered how easy it was to have a non-stigmatizing conversation about many of the topics once a subject was brought up.
“They’re actively looking for that opportunity,” Ms. Barré-Quick said in an interview.
Dr. Friedman agreed that this type of program provides what many adolescents need in a way that they may welcome more than through other methods.
“Adolescents’ bodies are approaching adulthood and function like adults, but their brains are still developing. They don’t have the worldly experience and education of adults, but they think they know everything,” Dr. Friedman said. “They are a population known for their high risk behavior due to their natural impulsivity. This can be a scary combination, especially when it comes to sexual health.”
But if teens don’t want to hear some of the information they need from adults, they may be more open to hearing it from other teens, Dr. Friedman said.
“Using an evidence-based approach ensures the desired outcome of healthier habits, decreased STIs and decreased teen pregnancy,” Dr. Friedman said. “It also adds weight to the argument against abstinence-only education. Teens deserve accurate and evidence-based education about their own bodies.”
Ms. Sao said the next steps will be exploring ways to scale the program up, such as putting the curriculum resources into a bundle available to other educators. They’re also looking at ways to put it into an online platform that’s self-paced, though that requires solving the challenge of having synchronous meetings for youth-led discussion.
“There are certain kinks that we have to work out because there were some activities where I think the students really benefited from having those open discussions with each other, so [we need to determine] how to replicate that in an online format,” Ms. Sao said.
Dr. Friedman agreed that scalability appears to be the biggest challenge, along with funding programs. But if those obstacles can be overcome, such programs would complement and expand on the education she does currently with families.
“I don’t have time for a full sex ed course at each visit,” Dr. Friedman said. “I would like to be able to direct them to a program that I know works and would be easy for them to complete. Even better, this would be an amazing program to ‘sell’ to practices interested in hosting these sessions themselves.”
Ms. Sao said they also hope to assess the impact of the intervention on the participants’ peers to see how well the knowledge and self-efficacy spread through the youths’ teaching.
No external funding was noted. One author reported research support from Hologic and Merck. Dr. Friedman had no disclosures.
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
While the small pilot study focused primarily on assessing feasibility and effectiveness, the results suggest potential for scaling the program up to reach a larger audience and assessing the knowledge disseminated from direct youth participants.
“The good thing about this subject is that not a lot of it has to be context-specific,” Saumya Sao, a clinical researcher in gynecology and obstetrics at the Johns Hopkins University, Baltimore, and the study’s lead author, said in an interview. “A lot of it is just baseline information that everybody needs and doesn’t get.”
Jaime Friedman, MD, a pediatrician and director of marketing at Children’s Primary Care Medical Group in San Diego, was not involved in the study but was impressed with the program’s objectives and results so far.
“While education is massively important, teens don’t always want to hear it from their parents or other adults,” Dr. Friedman said in an interview. “Learning from their peers is one way to overcome this hurdle.”
Given the high rate of sexually transmitted infections and unintended pregnancies in youth, paired with low sexual and reproductive health literacy in this population, the researchers sought to learn whether a program focused on peer-to-peer health education on these topics was feasible. The goal was to increase youth sexual and reproductive health knowledge, self-efficacy, and autonomy using a youth-led intervention.
The researchers hosted nine monthly, interactive, youth-led sessions that lasted 2 hours over Zoom or in person. Incorporated into the meetings were principles from Youth Participatory Action Research (YPAR) and Positive Youth Development (PYD).
The major topics included the following: Use of social media, values and goal-setting, anatomy and menstrual health, risk factors of sexual activities , STI and HIV prevention, contraceptive methods, healthy relationships and consent, practice responding to unhealthy behavior, gender and sexuality, and social media and body image.
The 24 participants were provided with transportation to the study site at the researchers’ institution and received financial compensation for their participation. They were an average 15.8 years old, lived in the greater Baltimore area, and mostly self-identified as female. Eight percent identified as non-binary and half (50%) identified as LGBTQIA+. Just over half the participants (52%) were Black/African American, 28% were Asian/Asian American, 12% were White, and 8% were Hispanic. The participants attended an average 88% of the sessions throughout the full intervention.
For each of the nine sessions, more than 50% of participants reported that they “learned a lot,” and only one participant reported for one session (session 5) that they “didn’t learn” anything. The researchers assessed participants’ knowledge, self-efficacy, and sense of autonomy at baseline and after completion of the intervention. Significant improvements occurred across all areas.
The average score improved by 31% in sexual and reproductive health knowledge (P < .001), 33% in sexual and reproductive health services awareness (P = .002), 46% in advocacy and empowerment (P < .001), 16% in general perceived efficacy (P = .002), and 22% personal sexuality empowerment (P = .006).
Ms. Sao said she was very pleased to see that the improvements were significant in every domain they measured, which she attributed largely to the incorporation of YPAR and PYD into the program.
“We approached it using these two frameworks that really do focus on involving youth in the teaching themselves, so I think that’s what increased their general perceived efficacy and advocacy empowerment without us necessarily having to emphasize, ‘You are advocates,’” Ms. Sao said. “Those frameworks ask the youth for their opinions and then give the youth an opportunity in every single session to be teachers themselves, and I think that lends itself well to all of the domains.”
Ms. Sao was also pleasantly surprised at the high level of retention across the 9 months.
“Every single session was slotted for 2 hours, but they would want to stay for 3 hours. Eventually, we actually started meeting with them twice a month, just adding an extra session,” she said. “As they gained confidence, they were so excited to be peer educators and realized, ‘I can really do this. I can teach my peers. We’re not getting this from anywhere else.’ ”
Ms. Sao and another study author, Maclaine Barré-Quick, an undergraduate research assistant at Johns Hopkins University, said the participants quickly discovered how easy it was to have a non-stigmatizing conversation about many of the topics once a subject was brought up.
“They’re actively looking for that opportunity,” Ms. Barré-Quick said in an interview.
Dr. Friedman agreed that this type of program provides what many adolescents need in a way that they may welcome more than through other methods.
“Adolescents’ bodies are approaching adulthood and function like adults, but their brains are still developing. They don’t have the worldly experience and education of adults, but they think they know everything,” Dr. Friedman said. “They are a population known for their high risk behavior due to their natural impulsivity. This can be a scary combination, especially when it comes to sexual health.”
But if teens don’t want to hear some of the information they need from adults, they may be more open to hearing it from other teens, Dr. Friedman said.
“Using an evidence-based approach ensures the desired outcome of healthier habits, decreased STIs and decreased teen pregnancy,” Dr. Friedman said. “It also adds weight to the argument against abstinence-only education. Teens deserve accurate and evidence-based education about their own bodies.”
Ms. Sao said the next steps will be exploring ways to scale the program up, such as putting the curriculum resources into a bundle available to other educators. They’re also looking at ways to put it into an online platform that’s self-paced, though that requires solving the challenge of having synchronous meetings for youth-led discussion.
“There are certain kinks that we have to work out because there were some activities where I think the students really benefited from having those open discussions with each other, so [we need to determine] how to replicate that in an online format,” Ms. Sao said.
Dr. Friedman agreed that scalability appears to be the biggest challenge, along with funding programs. But if those obstacles can be overcome, such programs would complement and expand on the education she does currently with families.
“I don’t have time for a full sex ed course at each visit,” Dr. Friedman said. “I would like to be able to direct them to a program that I know works and would be easy for them to complete. Even better, this would be an amazing program to ‘sell’ to practices interested in hosting these sessions themselves.”
Ms. Sao said they also hope to assess the impact of the intervention on the participants’ peers to see how well the knowledge and self-efficacy spread through the youths’ teaching.
No external funding was noted. One author reported research support from Hologic and Merck. Dr. Friedman had no disclosures.
AT ACOG 2023
Circulatory support for RV failure caused by pulmonary embolism
A new review article highlights approaches for mechanical circulatory support in patients with high-risk acute pulmonary embolism (PE).
Pulmonary embolism with hemodynamic significance is widely underdiagnosed, and the mortality rate can be as high as 30%, but new therapeutic developments offer promise. “Over the past few years, a renewed interest in mechanical circulatory support (MCS; both percutaneous and surgical) for acute RVF has emerged, increasing viable treatment options for high-risk acute PE,” wrote the authors of the review, which was published online in Interventional Cardiology Clinics.
Poor outcomes are often driven by RVF, which is tricky to diagnose and manage, and it stems from a sudden increase in pulmonary vascular resistance (PVR) following PE. “The mechanism for increased PVR in acute PE is multifactorial, including direct blood flow impedance, local hypoxia-induced vasoconstriction, and platelet/thrombin-induced release of vasoactive peptides. The cascade of events that then leads to RVF includes decreased RV stoke volume, increased RV wall tension, and RV dilation,” the authors wrote.
The authors noted that diuretics help to correct changes to RV geometry and can improve left ventricle filling, which improves hemodynamics. Diuretics can be used in patients who are hypotensive and volume overloaded, but vasopressors should be employed to support blood pressure.
When using mechanical ventilation, strategies such as low tidal volumes, minimization of positive end expiratory pressure, and prevention of hypoxemia and acidemia should be employed to prevent an increase of pulmonary vascular resistance, which can worsen RV failure.
Pulmonary vasodilators aren’t recommended for acute PE, but inhaled pulmonary vasodilators may be considered in hemodynamically unstable patients.
Surgically implanted right ventricle assistance device are generally not used for acute RV failure in high-risk PE, unless the patient has not improved after medical management.
Percutaneous devices
Percutaneous mechanical circulatory support devices can be used for patients experiencing refractory shock. The review highlighted three such devices, including the Impella RP, tandem-heart right ventricular assist devices (TH-RVAD) or Protek Duo, and venoarterial extracorporeal membrane oxygenation (VA-ECMO), but they are not without limitations. “Challenges to using these devices in patients with acute PE include clot dislodgement, vascular complications, infections, device migration, and fracture of individual elements,” the authors wrote.
The Impella RP is easy to deploy and bypasses the RV, but it can’t provide blood oxygenation and may cause bleeding or hemolysis. TH-RVAD oxygenates the blood and bypasses the RV, but suffers from a large sheath size. VA-ECMO oxygenates the blood but may cause bleeding.
There are important differences among the mechanical support devices, according to Jonathan Ludmir, MD, who was asked to comment. “In reality, if someone has a large pulmonary embolism burden, to put in the Impella RP or the Protek Duo would be a little bit risky, because you’d be sometimes putting the device right where the clot is. At least what we do in our institution, when someone is in extremis despite using [intravenous] medications like vasopressors or inotropes, VA-ECMO is kind of the go to. This is both the quickest and probably most effective way to support the patient. I say the quickest because this is a procedure you can do at the bedside.”
Benefits of PERT
One message that the review only briefly mentions, but Dr. Ludmir believes is key, is employing a pulmonary embolism response team. “That’s been looked at extensively, and it’s a really key part of any decision-making. If someone presents to the emergency room or someone inside the hospital has an acute pulmonary embolism, you have a team of people that can respond and help assess the next step. Typically, that involves a cardiologist or an interventional cardiologist, a hematologist, vascular surgeon, often a cardiac surgeon, so it’s a whole slew of people. Based on the patient assessment they can quickly decide, can this patient just be okay with a blood thinner like heparin? Does this patient need something more aggressive, like a thrombectomy? Or is this a serious case where you involve the shock team or the ECMO team, and you have to stabilize the patient on mechanical circulatory support, so you can accomplish what you need to do to get rid of the pulmonary embolism,” said Dr. Ludmir, who is an assistant professor of medicine at Corrigan Minehan Heart Center at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“Every case is individualized, hence the importance of having a team of a variety of different backgrounds and thoughts to approach it. And I think that’s kind of like the key takeaway. Yes, you have to be familiar with all the therapies, but at the end of the day, not every patient is going to fit into the algorithm for how you approach pulmonary embolism,” said Dr. Ludmir.
Dr. Ludmir has no relevant conflicts of interest.
A new review article highlights approaches for mechanical circulatory support in patients with high-risk acute pulmonary embolism (PE).
Pulmonary embolism with hemodynamic significance is widely underdiagnosed, and the mortality rate can be as high as 30%, but new therapeutic developments offer promise. “Over the past few years, a renewed interest in mechanical circulatory support (MCS; both percutaneous and surgical) for acute RVF has emerged, increasing viable treatment options for high-risk acute PE,” wrote the authors of the review, which was published online in Interventional Cardiology Clinics.
Poor outcomes are often driven by RVF, which is tricky to diagnose and manage, and it stems from a sudden increase in pulmonary vascular resistance (PVR) following PE. “The mechanism for increased PVR in acute PE is multifactorial, including direct blood flow impedance, local hypoxia-induced vasoconstriction, and platelet/thrombin-induced release of vasoactive peptides. The cascade of events that then leads to RVF includes decreased RV stoke volume, increased RV wall tension, and RV dilation,” the authors wrote.
The authors noted that diuretics help to correct changes to RV geometry and can improve left ventricle filling, which improves hemodynamics. Diuretics can be used in patients who are hypotensive and volume overloaded, but vasopressors should be employed to support blood pressure.
When using mechanical ventilation, strategies such as low tidal volumes, minimization of positive end expiratory pressure, and prevention of hypoxemia and acidemia should be employed to prevent an increase of pulmonary vascular resistance, which can worsen RV failure.
Pulmonary vasodilators aren’t recommended for acute PE, but inhaled pulmonary vasodilators may be considered in hemodynamically unstable patients.
Surgically implanted right ventricle assistance device are generally not used for acute RV failure in high-risk PE, unless the patient has not improved after medical management.
Percutaneous devices
Percutaneous mechanical circulatory support devices can be used for patients experiencing refractory shock. The review highlighted three such devices, including the Impella RP, tandem-heart right ventricular assist devices (TH-RVAD) or Protek Duo, and venoarterial extracorporeal membrane oxygenation (VA-ECMO), but they are not without limitations. “Challenges to using these devices in patients with acute PE include clot dislodgement, vascular complications, infections, device migration, and fracture of individual elements,” the authors wrote.
The Impella RP is easy to deploy and bypasses the RV, but it can’t provide blood oxygenation and may cause bleeding or hemolysis. TH-RVAD oxygenates the blood and bypasses the RV, but suffers from a large sheath size. VA-ECMO oxygenates the blood but may cause bleeding.
There are important differences among the mechanical support devices, according to Jonathan Ludmir, MD, who was asked to comment. “In reality, if someone has a large pulmonary embolism burden, to put in the Impella RP or the Protek Duo would be a little bit risky, because you’d be sometimes putting the device right where the clot is. At least what we do in our institution, when someone is in extremis despite using [intravenous] medications like vasopressors or inotropes, VA-ECMO is kind of the go to. This is both the quickest and probably most effective way to support the patient. I say the quickest because this is a procedure you can do at the bedside.”
Benefits of PERT
One message that the review only briefly mentions, but Dr. Ludmir believes is key, is employing a pulmonary embolism response team. “That’s been looked at extensively, and it’s a really key part of any decision-making. If someone presents to the emergency room or someone inside the hospital has an acute pulmonary embolism, you have a team of people that can respond and help assess the next step. Typically, that involves a cardiologist or an interventional cardiologist, a hematologist, vascular surgeon, often a cardiac surgeon, so it’s a whole slew of people. Based on the patient assessment they can quickly decide, can this patient just be okay with a blood thinner like heparin? Does this patient need something more aggressive, like a thrombectomy? Or is this a serious case where you involve the shock team or the ECMO team, and you have to stabilize the patient on mechanical circulatory support, so you can accomplish what you need to do to get rid of the pulmonary embolism,” said Dr. Ludmir, who is an assistant professor of medicine at Corrigan Minehan Heart Center at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“Every case is individualized, hence the importance of having a team of a variety of different backgrounds and thoughts to approach it. And I think that’s kind of like the key takeaway. Yes, you have to be familiar with all the therapies, but at the end of the day, not every patient is going to fit into the algorithm for how you approach pulmonary embolism,” said Dr. Ludmir.
Dr. Ludmir has no relevant conflicts of interest.
A new review article highlights approaches for mechanical circulatory support in patients with high-risk acute pulmonary embolism (PE).
Pulmonary embolism with hemodynamic significance is widely underdiagnosed, and the mortality rate can be as high as 30%, but new therapeutic developments offer promise. “Over the past few years, a renewed interest in mechanical circulatory support (MCS; both percutaneous and surgical) for acute RVF has emerged, increasing viable treatment options for high-risk acute PE,” wrote the authors of the review, which was published online in Interventional Cardiology Clinics.
Poor outcomes are often driven by RVF, which is tricky to diagnose and manage, and it stems from a sudden increase in pulmonary vascular resistance (PVR) following PE. “The mechanism for increased PVR in acute PE is multifactorial, including direct blood flow impedance, local hypoxia-induced vasoconstriction, and platelet/thrombin-induced release of vasoactive peptides. The cascade of events that then leads to RVF includes decreased RV stoke volume, increased RV wall tension, and RV dilation,” the authors wrote.
The authors noted that diuretics help to correct changes to RV geometry and can improve left ventricle filling, which improves hemodynamics. Diuretics can be used in patients who are hypotensive and volume overloaded, but vasopressors should be employed to support blood pressure.
When using mechanical ventilation, strategies such as low tidal volumes, minimization of positive end expiratory pressure, and prevention of hypoxemia and acidemia should be employed to prevent an increase of pulmonary vascular resistance, which can worsen RV failure.
Pulmonary vasodilators aren’t recommended for acute PE, but inhaled pulmonary vasodilators may be considered in hemodynamically unstable patients.
Surgically implanted right ventricle assistance device are generally not used for acute RV failure in high-risk PE, unless the patient has not improved after medical management.
Percutaneous devices
Percutaneous mechanical circulatory support devices can be used for patients experiencing refractory shock. The review highlighted three such devices, including the Impella RP, tandem-heart right ventricular assist devices (TH-RVAD) or Protek Duo, and venoarterial extracorporeal membrane oxygenation (VA-ECMO), but they are not without limitations. “Challenges to using these devices in patients with acute PE include clot dislodgement, vascular complications, infections, device migration, and fracture of individual elements,” the authors wrote.
The Impella RP is easy to deploy and bypasses the RV, but it can’t provide blood oxygenation and may cause bleeding or hemolysis. TH-RVAD oxygenates the blood and bypasses the RV, but suffers from a large sheath size. VA-ECMO oxygenates the blood but may cause bleeding.
There are important differences among the mechanical support devices, according to Jonathan Ludmir, MD, who was asked to comment. “In reality, if someone has a large pulmonary embolism burden, to put in the Impella RP or the Protek Duo would be a little bit risky, because you’d be sometimes putting the device right where the clot is. At least what we do in our institution, when someone is in extremis despite using [intravenous] medications like vasopressors or inotropes, VA-ECMO is kind of the go to. This is both the quickest and probably most effective way to support the patient. I say the quickest because this is a procedure you can do at the bedside.”
Benefits of PERT
One message that the review only briefly mentions, but Dr. Ludmir believes is key, is employing a pulmonary embolism response team. “That’s been looked at extensively, and it’s a really key part of any decision-making. If someone presents to the emergency room or someone inside the hospital has an acute pulmonary embolism, you have a team of people that can respond and help assess the next step. Typically, that involves a cardiologist or an interventional cardiologist, a hematologist, vascular surgeon, often a cardiac surgeon, so it’s a whole slew of people. Based on the patient assessment they can quickly decide, can this patient just be okay with a blood thinner like heparin? Does this patient need something more aggressive, like a thrombectomy? Or is this a serious case where you involve the shock team or the ECMO team, and you have to stabilize the patient on mechanical circulatory support, so you can accomplish what you need to do to get rid of the pulmonary embolism,” said Dr. Ludmir, who is an assistant professor of medicine at Corrigan Minehan Heart Center at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“Every case is individualized, hence the importance of having a team of a variety of different backgrounds and thoughts to approach it. And I think that’s kind of like the key takeaway. Yes, you have to be familiar with all the therapies, but at the end of the day, not every patient is going to fit into the algorithm for how you approach pulmonary embolism,” said Dr. Ludmir.
Dr. Ludmir has no relevant conflicts of interest.
FROM INTERVENTIONAL CARDIOLOGY CLINICS