User login
Clinical trials: Top priority for long COVID
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
Colchicine’s 2010 price spike had major impact on gout care
A large price increase for colchicine in 2010 led to a significant falloff in its use for gout that persisted for the next decade while emergency and rheumatology visits for gout rose, suggesting poorer disease control, a retrospective cohort study reported.
The price of colchicine, commonly prescribed for acute gout attacks, climbed from $11.25 per prescription in 2009 to $190.49 in 2011, with the average out-of-pocket cost more than quadrupling, from $7.37 to $29.42, the study noted. Colchicine prescriptions for gout declined 27% over the next decade, according to adjusted analyses that the study authors performed.
“A roughly 16-fold increase in colchicine prices appeared to have lowered colchicine use over the next decade,” senior author Zirui Song, MD, PhD, an associate professor of health care policy and medicine at Harvard Medical School and an internist at Massachusetts General Hospital in Boston, told this news organization in written comments. “Over the same period, patients with gout used more of other medications that could treat gout. They also had more emergency department visits for gout and rheumatologist visits for gout, which potentially signals poorer disease control.”
The study, published online in JAMA Internal Medicine, examined MarketScan data from a longitudinal cohort of patients who had employer-sponsored health insurance and a diagnosis of gout from 2007 to 2019. MarketScan is an IBM database of medical and drug data from employers and health plans. The study examined more than 2.7 million patient-year observations over the 13-year period.
How the price increase happened
After 2011, a large percentage of patients shifted to less effective but more affordable drugs to treat gout. Prescriptions for allopurinol increased 32% (P < .001) and oral corticosteroids 8.3% over the decade. “These are imperfect substitutes,” Dr. Song said. “Allopurinol is used to prevent gout, while oral corticosteroids can be used to treat a gout flare.”
At the same time, visits for gout-related complaints to emergency departments and rheumatology offices increased through the ensuing years: 39.8% and 10.5% on an adjusted analysis, respectively (P < .001 for both).
Colchicine is actually a drug that predates the creation of the U.S. Food and Drug Administration in 1938 and had been grandfathered under its Unapproved Drug Initiative. Then in 2009, the FDA determined that colchicine was effective for treating arthritis-related gout flares after the manufacturer, URL Pharma, presented results of a randomized, controlled trial of 185 patients with gout.
The next year, the FDA granted URL Pharma 3 years of market exclusivity for the drug under the brand name Colcrys, now trademarked by Takeda Pharmaceuticals.
The latest study noted that longer-term analysis of the impact of the FDA’s decision had been lacking. The goal, said Dr. Song, was “to better understand the long-run implications of large drug price increases in the U.S. by studying the case of colchicine.”
He added, “For drugs that lack competition, large price increases can have large economic and clinical consequences over many years.”
Absorbing the cost
Lead author Dan P. Ly, MD, PhD, MPP, assistant professor at the University of California, Los Angeles, added, “Our study has large implications [for] when generic medications or other medications experience large price increases. Use of the medication in question drops or patients have to pay more out of pocket, and patient health can suffer as a result.”
The dropoff in colchicine use in this patient population could have been worse, Dr. Song said. “Despite colchicine use decreasing by 27% over nearly a decade, the fact that it did not decline more suggests that for patients with gout, the large price increase was mostly absorbed by their insurers, employers, or themselves – e.g., passed through to higher premiums, lower wages, or higher cost-sharing.”
Aaron Kesselheim, MD, JD, MPH, a professor at Harvard Medical School, Boston, reported previously on the price consequences of colchicine early on after the FDA granted the manufacturer market exclusivity.
“In our past research, we looked at how the massive increase in the price of colchicine increased spending on the drug and reduced use in a relatively short time period after the price hike,” said Dr. Kesselheim, who was not involved in this current study by Dr. Ly, Dr. Song, and Mia Giuriato, BBA, MA, from Harvard Medical School. “This study evaluated the experiences of patients with gout over multiple years and showed that the reductions in use persisted and were associated with increases in ED and rheumatology visits, suggesting worsening control of gout due to the relative inaccessibility of the drug at the new high price.”
The latest findings have public policy implications, Dr. Kesselheim said. “In the case of colchicine, the FDA made a bad pitch, leading to a home run for the manufacturer and a shutout for patients.”
“The FDA needs to make sure to take into account the quite predictable patient effects that can result from disruptions to competition when it considers taking steps like it did in the colchicine case to disrupt the market and create an artificial monopoly, even if the FDA acted in the best of intentions in this case,” Dr. Kesselheim added.
Dr. Song received funding for the study from the National Institutes of Health and Arnold Ventures. He also disclosed receiving personal fees from the Research Triangle Institute, Google Ventures, VBID Health, and the International Foundation of Employee Benefit Plans. Dr. Ly, Ms. Giuriato, and Dr. Kesselheim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large price increase for colchicine in 2010 led to a significant falloff in its use for gout that persisted for the next decade while emergency and rheumatology visits for gout rose, suggesting poorer disease control, a retrospective cohort study reported.
The price of colchicine, commonly prescribed for acute gout attacks, climbed from $11.25 per prescription in 2009 to $190.49 in 2011, with the average out-of-pocket cost more than quadrupling, from $7.37 to $29.42, the study noted. Colchicine prescriptions for gout declined 27% over the next decade, according to adjusted analyses that the study authors performed.
“A roughly 16-fold increase in colchicine prices appeared to have lowered colchicine use over the next decade,” senior author Zirui Song, MD, PhD, an associate professor of health care policy and medicine at Harvard Medical School and an internist at Massachusetts General Hospital in Boston, told this news organization in written comments. “Over the same period, patients with gout used more of other medications that could treat gout. They also had more emergency department visits for gout and rheumatologist visits for gout, which potentially signals poorer disease control.”
The study, published online in JAMA Internal Medicine, examined MarketScan data from a longitudinal cohort of patients who had employer-sponsored health insurance and a diagnosis of gout from 2007 to 2019. MarketScan is an IBM database of medical and drug data from employers and health plans. The study examined more than 2.7 million patient-year observations over the 13-year period.
How the price increase happened
After 2011, a large percentage of patients shifted to less effective but more affordable drugs to treat gout. Prescriptions for allopurinol increased 32% (P < .001) and oral corticosteroids 8.3% over the decade. “These are imperfect substitutes,” Dr. Song said. “Allopurinol is used to prevent gout, while oral corticosteroids can be used to treat a gout flare.”
At the same time, visits for gout-related complaints to emergency departments and rheumatology offices increased through the ensuing years: 39.8% and 10.5% on an adjusted analysis, respectively (P < .001 for both).
Colchicine is actually a drug that predates the creation of the U.S. Food and Drug Administration in 1938 and had been grandfathered under its Unapproved Drug Initiative. Then in 2009, the FDA determined that colchicine was effective for treating arthritis-related gout flares after the manufacturer, URL Pharma, presented results of a randomized, controlled trial of 185 patients with gout.
The next year, the FDA granted URL Pharma 3 years of market exclusivity for the drug under the brand name Colcrys, now trademarked by Takeda Pharmaceuticals.
The latest study noted that longer-term analysis of the impact of the FDA’s decision had been lacking. The goal, said Dr. Song, was “to better understand the long-run implications of large drug price increases in the U.S. by studying the case of colchicine.”
He added, “For drugs that lack competition, large price increases can have large economic and clinical consequences over many years.”
Absorbing the cost
Lead author Dan P. Ly, MD, PhD, MPP, assistant professor at the University of California, Los Angeles, added, “Our study has large implications [for] when generic medications or other medications experience large price increases. Use of the medication in question drops or patients have to pay more out of pocket, and patient health can suffer as a result.”
The dropoff in colchicine use in this patient population could have been worse, Dr. Song said. “Despite colchicine use decreasing by 27% over nearly a decade, the fact that it did not decline more suggests that for patients with gout, the large price increase was mostly absorbed by their insurers, employers, or themselves – e.g., passed through to higher premiums, lower wages, or higher cost-sharing.”
Aaron Kesselheim, MD, JD, MPH, a professor at Harvard Medical School, Boston, reported previously on the price consequences of colchicine early on after the FDA granted the manufacturer market exclusivity.
“In our past research, we looked at how the massive increase in the price of colchicine increased spending on the drug and reduced use in a relatively short time period after the price hike,” said Dr. Kesselheim, who was not involved in this current study by Dr. Ly, Dr. Song, and Mia Giuriato, BBA, MA, from Harvard Medical School. “This study evaluated the experiences of patients with gout over multiple years and showed that the reductions in use persisted and were associated with increases in ED and rheumatology visits, suggesting worsening control of gout due to the relative inaccessibility of the drug at the new high price.”
The latest findings have public policy implications, Dr. Kesselheim said. “In the case of colchicine, the FDA made a bad pitch, leading to a home run for the manufacturer and a shutout for patients.”
“The FDA needs to make sure to take into account the quite predictable patient effects that can result from disruptions to competition when it considers taking steps like it did in the colchicine case to disrupt the market and create an artificial monopoly, even if the FDA acted in the best of intentions in this case,” Dr. Kesselheim added.
Dr. Song received funding for the study from the National Institutes of Health and Arnold Ventures. He also disclosed receiving personal fees from the Research Triangle Institute, Google Ventures, VBID Health, and the International Foundation of Employee Benefit Plans. Dr. Ly, Ms. Giuriato, and Dr. Kesselheim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large price increase for colchicine in 2010 led to a significant falloff in its use for gout that persisted for the next decade while emergency and rheumatology visits for gout rose, suggesting poorer disease control, a retrospective cohort study reported.
The price of colchicine, commonly prescribed for acute gout attacks, climbed from $11.25 per prescription in 2009 to $190.49 in 2011, with the average out-of-pocket cost more than quadrupling, from $7.37 to $29.42, the study noted. Colchicine prescriptions for gout declined 27% over the next decade, according to adjusted analyses that the study authors performed.
“A roughly 16-fold increase in colchicine prices appeared to have lowered colchicine use over the next decade,” senior author Zirui Song, MD, PhD, an associate professor of health care policy and medicine at Harvard Medical School and an internist at Massachusetts General Hospital in Boston, told this news organization in written comments. “Over the same period, patients with gout used more of other medications that could treat gout. They also had more emergency department visits for gout and rheumatologist visits for gout, which potentially signals poorer disease control.”
The study, published online in JAMA Internal Medicine, examined MarketScan data from a longitudinal cohort of patients who had employer-sponsored health insurance and a diagnosis of gout from 2007 to 2019. MarketScan is an IBM database of medical and drug data from employers and health plans. The study examined more than 2.7 million patient-year observations over the 13-year period.
How the price increase happened
After 2011, a large percentage of patients shifted to less effective but more affordable drugs to treat gout. Prescriptions for allopurinol increased 32% (P < .001) and oral corticosteroids 8.3% over the decade. “These are imperfect substitutes,” Dr. Song said. “Allopurinol is used to prevent gout, while oral corticosteroids can be used to treat a gout flare.”
At the same time, visits for gout-related complaints to emergency departments and rheumatology offices increased through the ensuing years: 39.8% and 10.5% on an adjusted analysis, respectively (P < .001 for both).
Colchicine is actually a drug that predates the creation of the U.S. Food and Drug Administration in 1938 and had been grandfathered under its Unapproved Drug Initiative. Then in 2009, the FDA determined that colchicine was effective for treating arthritis-related gout flares after the manufacturer, URL Pharma, presented results of a randomized, controlled trial of 185 patients with gout.
The next year, the FDA granted URL Pharma 3 years of market exclusivity for the drug under the brand name Colcrys, now trademarked by Takeda Pharmaceuticals.
The latest study noted that longer-term analysis of the impact of the FDA’s decision had been lacking. The goal, said Dr. Song, was “to better understand the long-run implications of large drug price increases in the U.S. by studying the case of colchicine.”
He added, “For drugs that lack competition, large price increases can have large economic and clinical consequences over many years.”
Absorbing the cost
Lead author Dan P. Ly, MD, PhD, MPP, assistant professor at the University of California, Los Angeles, added, “Our study has large implications [for] when generic medications or other medications experience large price increases. Use of the medication in question drops or patients have to pay more out of pocket, and patient health can suffer as a result.”
The dropoff in colchicine use in this patient population could have been worse, Dr. Song said. “Despite colchicine use decreasing by 27% over nearly a decade, the fact that it did not decline more suggests that for patients with gout, the large price increase was mostly absorbed by their insurers, employers, or themselves – e.g., passed through to higher premiums, lower wages, or higher cost-sharing.”
Aaron Kesselheim, MD, JD, MPH, a professor at Harvard Medical School, Boston, reported previously on the price consequences of colchicine early on after the FDA granted the manufacturer market exclusivity.
“In our past research, we looked at how the massive increase in the price of colchicine increased spending on the drug and reduced use in a relatively short time period after the price hike,” said Dr. Kesselheim, who was not involved in this current study by Dr. Ly, Dr. Song, and Mia Giuriato, BBA, MA, from Harvard Medical School. “This study evaluated the experiences of patients with gout over multiple years and showed that the reductions in use persisted and were associated with increases in ED and rheumatology visits, suggesting worsening control of gout due to the relative inaccessibility of the drug at the new high price.”
The latest findings have public policy implications, Dr. Kesselheim said. “In the case of colchicine, the FDA made a bad pitch, leading to a home run for the manufacturer and a shutout for patients.”
“The FDA needs to make sure to take into account the quite predictable patient effects that can result from disruptions to competition when it considers taking steps like it did in the colchicine case to disrupt the market and create an artificial monopoly, even if the FDA acted in the best of intentions in this case,” Dr. Kesselheim added.
Dr. Song received funding for the study from the National Institutes of Health and Arnold Ventures. He also disclosed receiving personal fees from the Research Triangle Institute, Google Ventures, VBID Health, and the International Foundation of Employee Benefit Plans. Dr. Ly, Ms. Giuriato, and Dr. Kesselheim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Experience With Adaptive Servo-Ventilation Among Veterans in the Post-SERVE-HF Era
Sleep apnea is a heterogeneous group of conditions that may be attributable to a wide array of underlying conditions, with varying contributions of obstructive or central sleep-disordered breathing. The spectrum from obstructive sleep apnea (OSA) to central sleep apnea (CSA) includes mixed sleep apnea, treatment-emergent CSA (TECSA), and Cheyne-Stokes respiration (CSR).1 The pathophysiologic causes of CSA can be attributed to delayed cardiopulmonary circulation in heart failure, decreased brainstem ventilatory response due to stroke, blunting of central chemoreceptors in chronic opioid use, and/or stimulation of the Hering-Breuer reflex from activation of pulmonary stretch receptors after initiating positive airway pressure (PAP) for treatment of OSA.2,3 Medications are commonly implicated in many forms of sleep-disordered breathing; importantly, opioids and benzodiazepines may blunt the respiratory drive, leading to CSA, and/or impair upper airway patency, resulting in or worsening OSA.
Continuous positive airway pressure (CPAP) therapy is largely ineffective in correcting CSA or improving outcomes and is often poorly tolerated in these patients.4 Adaptive servo-ventilation (ASV) is a form of bilevel PAP (BPAP) therapy that delivers variable adjusting pressure support, primarily to treat CSA. PAP also may relieve upper airway obstructions, thereby effectively treating any comorbid obstructive component. ASV has been well documented to improve sleep-related disorders and improve apnea-hypopnea index (AHI) in patients with CSA. However, longitudinal data have demonstrated increased mortality in patients with heart failure with reduced ejection fraction (HFrEF) who were treated with ASV.5 Since the SERVE-HF trial results came to light in 2015, there has been no consensus regarding the optimal use, if any, of ASV therapy.6-8 This is partly related to the inability to fully explain the study’s major findings, which were unexpected at the time, and partly due to the absence of similar relevant mortality data in patients with CSA but without HFrEF.
TECSA may present in some patients with OSA who are new to PAP therapy. These events are frequently seen during PAP titration sleep studies, though patients can also experience significant TECSA shortly after initiating home PAP therapy. TECSA is felt to result from a combination of stimulating pulmonary stretch receptors and lowering arterial carbon dioxide below the apneic threshold. Chemoreceptors located in the medulla respond by attenuating the respiratory drive.9 Previous studies have shown most cases of mild TECSA resolve over time with CPAP treatment. However, in patients with persistent or worsening TECSA, ASV may be considered as an alternative to CPAP.
The prevalence of OSA in the veteran population is estimated to be as high as 60%, considerably higher than the general population estimation.10 Patients with more significant comorbidities may also experience a higher frequency of central events. Patients with CSA have also been shown to have a higher risk for cardiac-related hospital admissions, providing plausible justification for correcting CSA.10
In the current study, we aim to characterize the group of patients using ASV therapy in the modern era. We will assess the objective efficacy and adherence of ASV therapy in patients with primarily CSA compared with those having primarily OSA (ie, TECSA). Secondarily, we aim to identify baseline clinical and polysomnographic features that may be predictive of ASV adherence, as a surrogate for subjective benefit.11 In the wake of the SERVE-HF study, the sleep medicine community has paused prescribing ASV therapy for CSA. We hope to provide more perspective on the treatment of veterans with CSA and identify the patient groups that would benefit most from ASV therapy.
Methods
This retrospective chart review examined patients prescribed ASV therapy at the Hampton Veterans Affairs Medical Center (HVAMC) in Virginia who had therapy data between January 1, 2015, and April 30, 2020. The start date was chosen to approximate the phase-in of wireless PAP devices at HVAMC and to correspond with the release of preliminary results from the SERVE-HF trial.
Patients were initially identified through a query into commercial wireless PAP management databases and cross-referenced with HVAMC patients. Adherence and efficacy data were obtained from the most recent clinical PAP data, which allowed for the evaluation of patients who discontinued therapy for reasons other than intolerance. Clinical, demographic, and polysomnography (PSG) data were obtained from the electronic health record. One patient, identified through the database query but not found in the electronic health record, was excluded. In cases of missing PSG data, especially AHI or similar values, all attempts were made to calculate the data with other provided values. This study was determined to be exempt by the HVAMC Institutional Review Board (protocol #20-01).
Statistics
Statistical analyses were designed to compare clinical characteristics and adherence to therapy of those with primarily CSA on PSG and those with primarily OSA. Because it was not currently known how many patients would fit into each of these categories, we also planned secondary comparisons of the clinical and PSG characteristics of those patients who were adherent with therapy and those who were not. Adherence with ASV therapy was defined as device use for ≥ 4 hours for ≥ 70% of nights.
Comparisons between the means of 2 normally distributed groups were performed with an unpaired t test. Comparisons between 2 nonnormally distributed groups and groups of dates were done with the Mann-Whitney U test. The normality of a group distribution was determined using D’Agostino-Pearson omnibus normality test. Two groups of dichotomous variables were compared with the Fisher exact test. P value < .05 was considered statistically significant.
Results
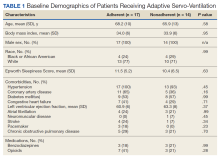
Thirty-one patients were prescribed ASV therapy and had follow-up at HVAMC since 2015. All patients were male. The mean (SD) age was 67.2 (11.4) years, mean body mass index (BMI) was 34.0 (5.9), and the mean (SD) Epworth Sleepiness Scale (ESS) score was 10.9 (5.8). Patient comorbidities included 30 (97%) with hypertension, 17 (55%) with diabetes mellitus, 16 (52%) with coronary artery disease, and 11 (35%) with congestive heart failure. Three patients had no echocardiogram or other documentation of left ventricular ejection fraction (LVEF). One of these patients had voluntarily stopped using PAP therapy, another had been erroneously started on ASV (ordered for fixed BPAP), and the third had since been retitrated to CPAP. In the 28 patients with documented LVEF, the mean (SD) LVEF was 61.8% (6.9). Ten patients (32%) had opioids documented on their medication lists and 6 (19%) had benzodiazepines.
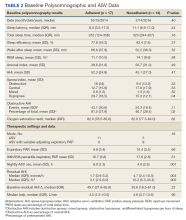
The median date of diagnostic sleep testing was January 9, 2015, and testing was completed after the release of the initial field safety notice regarding the SERVE-HF trial preliminary findings May 13, 2015, for 14 patients (45%).12 On diagnostic sleep testing, the mean (SD) AHI was 47.3 (25.6) events/h and the median (IQR) oxygen saturation (SpO2) nadir was 82% (78-84). Three patients (10%) were initially diagnosed with CSA, 19 (61%) with OSA, and 9 (29%) with both. Sixteen patients (52%) had ASV with fixed expiratory PAP (EPAP), and 15 (48%) had variable adjusting EPAP. Mean (SD) usage of ASV was 6.5 (2.6) hours and 66.0% (34.2) of nights for ≥ 4 hours. Mean (SD) titrated EPAP (set or 90th/95th percentile autotitrated) was 10.1 (3.4) cm H2O and inspiratory PAP (IPAP) (90th/95th percentile) was 17.1 (3.3) cm H2O. The median (IQR) residual AHI on ASV was 2.7 events/h (1.1-5.1), apnea index (AI) was 0.4 (0.1-1.0), and hypopnea index (HI) was 1.4 (1.0-3.2); the residual central and obstructive events were not available in most cases.
Adherence
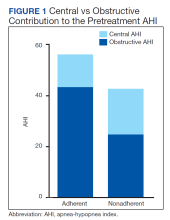
There were no significant differences between the proportions of patients on ASV with set EPAP or the titrated EPAP and IPAP. The median (IQR) residual AHI was lower in the adherent group compared with the nonadherent group, both in absolute values (1.7 [0.9-3.2] events/h vs 4.7 [2.4-10.3] events/h, respectively [P = .004]), and as a percentage of the pretreatment AHI (3.1% [2.5-6.0] vs 10.2% [5.3-34.4], respectively; P = .002) (Figure 2).
Primarily Obstructive Sleep Apnea
Sleep apnea was a mixed picture of obstructive and central events in many patients. Only 3 patients had “pure” CSA. Thus, we were unable to define discrete comparison groups based on the sleep-disordered breathing phenotype. We identified 19 patients with primarily OSA (ie, initially diagnosed with OSA, OSA with TECSA, or complex sleep apnea). The mean (SD) age was 66.1 (12.8) years, BMI was 36.2 (4.7), and ESS was 11.4 (5.6). The mean (SD) baseline AHI was 46.9 (29.5), obstructive AHI was 40.5 (30.4), and central AHI was 0.4 (1.2); the median (IQR) SpO2 nadir was 81% (78%-84%). The mean (SD) titrated EPAP was 10.2 (3.5) cm H2O, and the 90th/95th percentile IPAP was 17.9 (3.5) cm H2O. The mean (SD) usage of ASV was 7.9 (5.3) hours with 11 patients (58%) meeting the minimum standard for adherence to ASV therapy.
No significant differences were seen between the adherent and nonadherent groups in clinical or demographic characteristics or date of diagnostic sleep testing (eAppendix, available online at doi:10.12788/fp.0374). In baseline sleep studies the mean (SD) HI was 32.3 (15.8) in the adherent group compared with 14.7 (8.8) in the nonadherent group (P = .049). In contrast, obstructive AHI was not significantly lower in the adherent group: 51.9 (30.9) in the adherent group compared with 22.2 (20.6) in the nonadherent group (P = .09). The median (IQR) residual AHI on ASV as a percentage of the pretreatment AHI was 3.0% (2.4%-6.5%) in the adherent group compared with 11.3% (5.4%-89.1%) in the nonadherent group, a statistically significant difference (P = .01). No other significant differences were seen between the groups.
Discussion
This study describes a real-world cohort of patients using ASV therapy and the characteristics associated with benefit from therapy. The patients that were prescribed and started ASV therapy most often had a significant degree of obstructive component to sleep-disordered breathing, whether primary OSA with TECSA or comorbid OSA and CSA. Moreover, we found that a higher obstructive AHI on the baseline PSG was associated with adherence to ASV therapy. Another important finding was that a lower residual AHI on ASV as a proportion of the baseline was associated with PAP adherence. Adherent patients had similar clinical characteristics as the nonadherent patients, including comorbidities, severity of sleep-disordered breathing, and obesity.
Though the results of the SERVE-HF trial have dampened the enthusiasm somewhat, ASV therapy has long been considered an effective and well-tolerated treatment for many types of CSA.13 In fact, treatments that can eliminate the central AHI are fairly limited.4,14 Our data suggest that ASV is also effective and tolerated in OSA with TECSA and/or comorbid CSA. Recent studies suggest that CSA resolves spontaneously in a majority of TECSA patients within 2 to 3 months of regular CPAP use.15 Other estimates suggest that persistent TESCA may be present in 2% of patients with OSA on treatment.16
Given the high and rising prevalence of OSA, many people are at risk for clinically significant TESCA. Another retrospective case series found that 72% of patients that failed treatment with CPAP or BPAP during PSG, met diagnostic criteria (at the time) for CSA; ASV was objectively beneficial in these patients.17 ASV can be an especially useful modality to treat OSA in patients with CSA that either prevents tolerance of standard therapies or causes clinical consequences, presuming the patient does not also have HFrEF.18 The long-term outcomes of treatment with ASV therapy remain a matter of debate.
The SERVE-HF trial remains among the only studies that have assessed the mortality effects of CSA treatments, with unfavorable findings. Treatment of OSA has been associated with favorable chronic health benefits, though recent studies have questioned the degree of benefit attributable to OSA treatment.19-24 Similar studies have not been done for comorbidities represented by our study cohort (ie, OSA with TECSA and/or comorbid CSA).
The lack of CSA diagnosis alone in our cohort may be partially attributable to changing practice patterns following the SERVE-HF trial, though it is not clear from these data why a higher baseline obstructive AHI was associated with adherence to ASV therapy. Our data in this regard are somewhat at odds with the preliminary results of the ADVENT-HF trial. In that study, adherence to ASV therapy in patients with predominantly OSA declined significantly more than in patients with predominantly CSA.25 Most of our patients were diagnosed with predominantly OSA, so a direct comparison with the CSA group is problematic; additionally, the primary brand and the pressure adjustments algorithm used in our study differed from the ADVENT-HF trial.
OSA and CSA may present with similar clinical symptoms, including sleep fragmentation, insomnia, and excessive daytime sleepiness; however, the degree of symptomatology, especially daytime sleepiness, and the response to treatment, may be less in CSA.2,26 Both the subjective report of symptoms (ESS) and PSG measures of sleep fragmentation were similar in our patients, again likely explained by the predominance of obstructive events.
The pathophysiology of CSA is more varied than OSA, which is probably relevant in this case. ASV was originally designed for the management of CSA with CSR, accomplishing this goal by stabilizing the periods of central apnea and hyperpnea characteristic of CSR.27 Although other forms of CSA demonstrate breathing patterns distinct from CSR, ASV has become an accepted treatment for most of these. It is plausible that the long-term subjective benefit and tolerance of ASV in CSA without CSR is less than for CSA with CSR or OSA. None of the patients in our study had CSA with CSR.
Ultimately, it may be the objective treatment effect that lends to adherence, as has been shown previously in OSA patients; our group of adherent patients showed a greater improvement in AHI, relative to baseline, than the nonadherent patients did.28 The technology behind ASV therapy can greatly reduce the frequencies of central apneas, yet this same treatment effectively splints the upper airway and even more effectively eliminates obstructive apneas and hypopneas. Variable adjusting EPAP devices would plausibly provide even more benefit in these patients, as has been shown in prior studies.29 To the contrary, our small sample of patients with TESCA showed a nonsignificant trend toward adherence with fixed EPAP ASV.
Opioid use was substantial in our population, without significant differences between the groups. CPAP therapy is ineffective in improving opioid-associated CSA. In a recent study, 20 patients on opioid therapy with CSA were treated with CPAP therapy; after several weeks, the average therapeutic use was 4 to 5 hours per night and CPAP was abandoned in favor of ASV therapy due to persistent central apnea. ASV treatment was associated with a considerable reduction in central apnea index, AHI, arousal index, and oxygen desaturations in a remarkable improvement over CPAP.30
Limitations and Future Directions
This retrospective, single-center study may have limited applicability to other populations. Adherence was used as a surrogate for subjective benefit from treatment, though benefit was not confirmed by the patients directly. Only patients seen in follow-up for documentation of the ASV download were identified for inclusion and data analysis. As a single center, we risk homogeneity in the treatment algorithms, though sleep medicine treatments are often decided at the time of the sleep studies. Studies and treatment recommendations were made at a variety of sites, including our sleep center, other US Department of Veterans Affairs hospitals, in the community network, and at US Department of Defense centers. Our population was homogenous in some ways; notably, 100% of our group was male, which is substantially higher than both the veteran population and the general population. Risk factors for OSA and CSA are more common in male patients, which may partially explain this anomaly. Lastly, with our small sample size, there is increased risk that the results seen occurred by chance.
There are several areas for further study. A larger multicenter study may permit these results to be generalized to the population and should include subjective measures of benefit. Patients with primarily CSA were largely absent in our group and may be the focus of future studies; data on predictors of treatment adherence in CSA are lacking. With the availability of consistent older adherence data, comparisons may be made between the efficacies of clinical practice habits, including treatment efficacy, before and after the results of the SERVE-HF trial became known.
Conclusions
In selected patients with preserved LVEF, ASV therapy appears especially effective in patients with OSA combined with CSA. Adherence to ASV treatment was associated with higher obstructive AHI during the baseline PSG and with a greater reduction in the AHI. This understanding may help guide sleep specialists in personalizing treatments for sleep-disordered breathing. Because objective efficacy appears to be important for therapy adherence, clinicians should be able to consistently determine the obstructive and central components of the residual AHI, thus taking all information into account when optimizing the treatment. Additionally, both OSA and CSA pressure requirements should be considered when developing ASV devices.
Acknowledgments
We thank Martha Harper, RRT, of Hampton Veterans Affairs Medical Center (HVAMC) for helping to identify our patients and assisting with data collection. This material is the result of work supported with resources and the use of HVAMC facilities.
1. Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468-475. doi:10.1093/sleep/30.4.468
2. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595-607. doi:10.1378/chest.06.2287
3. Verbraecken J. Complex sleep apnoea syndrome. Breathe. 2013;9(5):372-380. doi:10.1183/20734735.042412
4. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025-2033. doi:10.1056/NEJMoa051001
5. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105. doi:10.1056/NEJMoa1506459
6. Imamura T, Kinugawa K. What is the optimal strategy for adaptive servo-ventilation therapy? Int Heart J. 2018;59(4):683-688. doi:10.1536/ihj.17-429
7. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149(4):900-904. doi:10.1016/j.chest.2015.12.021
8. Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest. 2015;148(4):848-851. doi:10.1378/chest.15-1536
9. Nigam G, Riaz M, Chang E, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
10. Ratz D, Wiitala W, Badr MS, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zsy058. doi:10.1093/sleep/zsy058
11. Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365-369. doi:10.1155/2008/534372
12. Special Safety Notice: ASV therapy for central sleep apnea patients with heart failure. American Academy of Sleep Medicine. May 15, 2015. Accessed February 13, 2023. https://aasm.org/special-safety-notice-asv-therapy-for-central-sleep-apnea-patients-with-heart-failure/
13. Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337-342. doi:10.1136/hrt.2005.060038
14. Randerath W, Deleanu OC, Schiza S, Pepin J-L. Central sleep apnoea and periodic breathing in heart failure: prognostic significance and treatment options. Eur Respir Rev. 2019;28(153):190084. Published 2019 Oct 11. doi:10.1183/16000617.0084-2019
15. Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med. 2008;4(5):403-405.
16. American Academy of Sleep Medicine. International Classification of Sleep Disorders - Third Edition (ICSD-3). 3rd ed. American Academy of Sleep Medicine; 2014.
17. Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187-195.
18. Aurora RN, Bista SR, Casey KR, et al. Updated Adaptive Servo-Ventilation Recommendations for the 2012 AASM Guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757-761. doi:10.5664/jcsm.5812
19. Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36-41. doi:10.1164/rccm.200808-1341OC
20. Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909-916. doi:10.1164/rccm.201203-0448OC
21. Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. Published 2013 Nov 25. doi:10.1161/JAHA.113.000421
22. Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3):858-865. doi:10.1164/ajrccm.157.3.9709042
23. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi:10.1056/NEJMoa1606599
24. Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166. doi:10.1001/jama.2017.7967
25. Perger E, Lyons OD, Inami T, et al. Predictors of 1-year compliance with adaptive servoventilation in patients with heart failure and sleep disordered breathing: preliminary data from the ADVENT-HF trial. Eur Resp J. 2019;53(2):1801626. doi:10.1183/13993003.01626-2018
26. Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579-587. doi:10.1002/ejhf.790
27. Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614-619. doi:10.1164/ajrccm.164.4.9908114
28. Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21(4):419-426. doi:10.1111/j.1365-2869.2011.00969.x
29. Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep. 2010;33(2):267-271. doi:10.1093/sleep/33.2.267
30. Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637-643. Published 2014 Jun 15. doi:10.5664/jcsm.3788
Sleep apnea is a heterogeneous group of conditions that may be attributable to a wide array of underlying conditions, with varying contributions of obstructive or central sleep-disordered breathing. The spectrum from obstructive sleep apnea (OSA) to central sleep apnea (CSA) includes mixed sleep apnea, treatment-emergent CSA (TECSA), and Cheyne-Stokes respiration (CSR).1 The pathophysiologic causes of CSA can be attributed to delayed cardiopulmonary circulation in heart failure, decreased brainstem ventilatory response due to stroke, blunting of central chemoreceptors in chronic opioid use, and/or stimulation of the Hering-Breuer reflex from activation of pulmonary stretch receptors after initiating positive airway pressure (PAP) for treatment of OSA.2,3 Medications are commonly implicated in many forms of sleep-disordered breathing; importantly, opioids and benzodiazepines may blunt the respiratory drive, leading to CSA, and/or impair upper airway patency, resulting in or worsening OSA.
Continuous positive airway pressure (CPAP) therapy is largely ineffective in correcting CSA or improving outcomes and is often poorly tolerated in these patients.4 Adaptive servo-ventilation (ASV) is a form of bilevel PAP (BPAP) therapy that delivers variable adjusting pressure support, primarily to treat CSA. PAP also may relieve upper airway obstructions, thereby effectively treating any comorbid obstructive component. ASV has been well documented to improve sleep-related disorders and improve apnea-hypopnea index (AHI) in patients with CSA. However, longitudinal data have demonstrated increased mortality in patients with heart failure with reduced ejection fraction (HFrEF) who were treated with ASV.5 Since the SERVE-HF trial results came to light in 2015, there has been no consensus regarding the optimal use, if any, of ASV therapy.6-8 This is partly related to the inability to fully explain the study’s major findings, which were unexpected at the time, and partly due to the absence of similar relevant mortality data in patients with CSA but without HFrEF.
TECSA may present in some patients with OSA who are new to PAP therapy. These events are frequently seen during PAP titration sleep studies, though patients can also experience significant TECSA shortly after initiating home PAP therapy. TECSA is felt to result from a combination of stimulating pulmonary stretch receptors and lowering arterial carbon dioxide below the apneic threshold. Chemoreceptors located in the medulla respond by attenuating the respiratory drive.9 Previous studies have shown most cases of mild TECSA resolve over time with CPAP treatment. However, in patients with persistent or worsening TECSA, ASV may be considered as an alternative to CPAP.
The prevalence of OSA in the veteran population is estimated to be as high as 60%, considerably higher than the general population estimation.10 Patients with more significant comorbidities may also experience a higher frequency of central events. Patients with CSA have also been shown to have a higher risk for cardiac-related hospital admissions, providing plausible justification for correcting CSA.10
In the current study, we aim to characterize the group of patients using ASV therapy in the modern era. We will assess the objective efficacy and adherence of ASV therapy in patients with primarily CSA compared with those having primarily OSA (ie, TECSA). Secondarily, we aim to identify baseline clinical and polysomnographic features that may be predictive of ASV adherence, as a surrogate for subjective benefit.11 In the wake of the SERVE-HF study, the sleep medicine community has paused prescribing ASV therapy for CSA. We hope to provide more perspective on the treatment of veterans with CSA and identify the patient groups that would benefit most from ASV therapy.
Methods
This retrospective chart review examined patients prescribed ASV therapy at the Hampton Veterans Affairs Medical Center (HVAMC) in Virginia who had therapy data between January 1, 2015, and April 30, 2020. The start date was chosen to approximate the phase-in of wireless PAP devices at HVAMC and to correspond with the release of preliminary results from the SERVE-HF trial.
Patients were initially identified through a query into commercial wireless PAP management databases and cross-referenced with HVAMC patients. Adherence and efficacy data were obtained from the most recent clinical PAP data, which allowed for the evaluation of patients who discontinued therapy for reasons other than intolerance. Clinical, demographic, and polysomnography (PSG) data were obtained from the electronic health record. One patient, identified through the database query but not found in the electronic health record, was excluded. In cases of missing PSG data, especially AHI or similar values, all attempts were made to calculate the data with other provided values. This study was determined to be exempt by the HVAMC Institutional Review Board (protocol #20-01).
Statistics
Statistical analyses were designed to compare clinical characteristics and adherence to therapy of those with primarily CSA on PSG and those with primarily OSA. Because it was not currently known how many patients would fit into each of these categories, we also planned secondary comparisons of the clinical and PSG characteristics of those patients who were adherent with therapy and those who were not. Adherence with ASV therapy was defined as device use for ≥ 4 hours for ≥ 70% of nights.
Comparisons between the means of 2 normally distributed groups were performed with an unpaired t test. Comparisons between 2 nonnormally distributed groups and groups of dates were done with the Mann-Whitney U test. The normality of a group distribution was determined using D’Agostino-Pearson omnibus normality test. Two groups of dichotomous variables were compared with the Fisher exact test. P value < .05 was considered statistically significant.
Results
Thirty-one patients were prescribed ASV therapy and had follow-up at HVAMC since 2015. All patients were male. The mean (SD) age was 67.2 (11.4) years, mean body mass index (BMI) was 34.0 (5.9), and the mean (SD) Epworth Sleepiness Scale (ESS) score was 10.9 (5.8). Patient comorbidities included 30 (97%) with hypertension, 17 (55%) with diabetes mellitus, 16 (52%) with coronary artery disease, and 11 (35%) with congestive heart failure. Three patients had no echocardiogram or other documentation of left ventricular ejection fraction (LVEF). One of these patients had voluntarily stopped using PAP therapy, another had been erroneously started on ASV (ordered for fixed BPAP), and the third had since been retitrated to CPAP. In the 28 patients with documented LVEF, the mean (SD) LVEF was 61.8% (6.9). Ten patients (32%) had opioids documented on their medication lists and 6 (19%) had benzodiazepines.
The median date of diagnostic sleep testing was January 9, 2015, and testing was completed after the release of the initial field safety notice regarding the SERVE-HF trial preliminary findings May 13, 2015, for 14 patients (45%).12 On diagnostic sleep testing, the mean (SD) AHI was 47.3 (25.6) events/h and the median (IQR) oxygen saturation (SpO2) nadir was 82% (78-84). Three patients (10%) were initially diagnosed with CSA, 19 (61%) with OSA, and 9 (29%) with both. Sixteen patients (52%) had ASV with fixed expiratory PAP (EPAP), and 15 (48%) had variable adjusting EPAP. Mean (SD) usage of ASV was 6.5 (2.6) hours and 66.0% (34.2) of nights for ≥ 4 hours. Mean (SD) titrated EPAP (set or 90th/95th percentile autotitrated) was 10.1 (3.4) cm H2O and inspiratory PAP (IPAP) (90th/95th percentile) was 17.1 (3.3) cm H2O. The median (IQR) residual AHI on ASV was 2.7 events/h (1.1-5.1), apnea index (AI) was 0.4 (0.1-1.0), and hypopnea index (HI) was 1.4 (1.0-3.2); the residual central and obstructive events were not available in most cases.
Adherence
There were no significant differences between the proportions of patients on ASV with set EPAP or the titrated EPAP and IPAP. The median (IQR) residual AHI was lower in the adherent group compared with the nonadherent group, both in absolute values (1.7 [0.9-3.2] events/h vs 4.7 [2.4-10.3] events/h, respectively [P = .004]), and as a percentage of the pretreatment AHI (3.1% [2.5-6.0] vs 10.2% [5.3-34.4], respectively; P = .002) (Figure 2).
Primarily Obstructive Sleep Apnea
Sleep apnea was a mixed picture of obstructive and central events in many patients. Only 3 patients had “pure” CSA. Thus, we were unable to define discrete comparison groups based on the sleep-disordered breathing phenotype. We identified 19 patients with primarily OSA (ie, initially diagnosed with OSA, OSA with TECSA, or complex sleep apnea). The mean (SD) age was 66.1 (12.8) years, BMI was 36.2 (4.7), and ESS was 11.4 (5.6). The mean (SD) baseline AHI was 46.9 (29.5), obstructive AHI was 40.5 (30.4), and central AHI was 0.4 (1.2); the median (IQR) SpO2 nadir was 81% (78%-84%). The mean (SD) titrated EPAP was 10.2 (3.5) cm H2O, and the 90th/95th percentile IPAP was 17.9 (3.5) cm H2O. The mean (SD) usage of ASV was 7.9 (5.3) hours with 11 patients (58%) meeting the minimum standard for adherence to ASV therapy.
No significant differences were seen between the adherent and nonadherent groups in clinical or demographic characteristics or date of diagnostic sleep testing (eAppendix, available online at doi:10.12788/fp.0374). In baseline sleep studies the mean (SD) HI was 32.3 (15.8) in the adherent group compared with 14.7 (8.8) in the nonadherent group (P = .049). In contrast, obstructive AHI was not significantly lower in the adherent group: 51.9 (30.9) in the adherent group compared with 22.2 (20.6) in the nonadherent group (P = .09). The median (IQR) residual AHI on ASV as a percentage of the pretreatment AHI was 3.0% (2.4%-6.5%) in the adherent group compared with 11.3% (5.4%-89.1%) in the nonadherent group, a statistically significant difference (P = .01). No other significant differences were seen between the groups.
Discussion
This study describes a real-world cohort of patients using ASV therapy and the characteristics associated with benefit from therapy. The patients that were prescribed and started ASV therapy most often had a significant degree of obstructive component to sleep-disordered breathing, whether primary OSA with TECSA or comorbid OSA and CSA. Moreover, we found that a higher obstructive AHI on the baseline PSG was associated with adherence to ASV therapy. Another important finding was that a lower residual AHI on ASV as a proportion of the baseline was associated with PAP adherence. Adherent patients had similar clinical characteristics as the nonadherent patients, including comorbidities, severity of sleep-disordered breathing, and obesity.
Though the results of the SERVE-HF trial have dampened the enthusiasm somewhat, ASV therapy has long been considered an effective and well-tolerated treatment for many types of CSA.13 In fact, treatments that can eliminate the central AHI are fairly limited.4,14 Our data suggest that ASV is also effective and tolerated in OSA with TECSA and/or comorbid CSA. Recent studies suggest that CSA resolves spontaneously in a majority of TECSA patients within 2 to 3 months of regular CPAP use.15 Other estimates suggest that persistent TESCA may be present in 2% of patients with OSA on treatment.16
Given the high and rising prevalence of OSA, many people are at risk for clinically significant TESCA. Another retrospective case series found that 72% of patients that failed treatment with CPAP or BPAP during PSG, met diagnostic criteria (at the time) for CSA; ASV was objectively beneficial in these patients.17 ASV can be an especially useful modality to treat OSA in patients with CSA that either prevents tolerance of standard therapies or causes clinical consequences, presuming the patient does not also have HFrEF.18 The long-term outcomes of treatment with ASV therapy remain a matter of debate.
The SERVE-HF trial remains among the only studies that have assessed the mortality effects of CSA treatments, with unfavorable findings. Treatment of OSA has been associated with favorable chronic health benefits, though recent studies have questioned the degree of benefit attributable to OSA treatment.19-24 Similar studies have not been done for comorbidities represented by our study cohort (ie, OSA with TECSA and/or comorbid CSA).
The lack of CSA diagnosis alone in our cohort may be partially attributable to changing practice patterns following the SERVE-HF trial, though it is not clear from these data why a higher baseline obstructive AHI was associated with adherence to ASV therapy. Our data in this regard are somewhat at odds with the preliminary results of the ADVENT-HF trial. In that study, adherence to ASV therapy in patients with predominantly OSA declined significantly more than in patients with predominantly CSA.25 Most of our patients were diagnosed with predominantly OSA, so a direct comparison with the CSA group is problematic; additionally, the primary brand and the pressure adjustments algorithm used in our study differed from the ADVENT-HF trial.
OSA and CSA may present with similar clinical symptoms, including sleep fragmentation, insomnia, and excessive daytime sleepiness; however, the degree of symptomatology, especially daytime sleepiness, and the response to treatment, may be less in CSA.2,26 Both the subjective report of symptoms (ESS) and PSG measures of sleep fragmentation were similar in our patients, again likely explained by the predominance of obstructive events.
The pathophysiology of CSA is more varied than OSA, which is probably relevant in this case. ASV was originally designed for the management of CSA with CSR, accomplishing this goal by stabilizing the periods of central apnea and hyperpnea characteristic of CSR.27 Although other forms of CSA demonstrate breathing patterns distinct from CSR, ASV has become an accepted treatment for most of these. It is plausible that the long-term subjective benefit and tolerance of ASV in CSA without CSR is less than for CSA with CSR or OSA. None of the patients in our study had CSA with CSR.
Ultimately, it may be the objective treatment effect that lends to adherence, as has been shown previously in OSA patients; our group of adherent patients showed a greater improvement in AHI, relative to baseline, than the nonadherent patients did.28 The technology behind ASV therapy can greatly reduce the frequencies of central apneas, yet this same treatment effectively splints the upper airway and even more effectively eliminates obstructive apneas and hypopneas. Variable adjusting EPAP devices would plausibly provide even more benefit in these patients, as has been shown in prior studies.29 To the contrary, our small sample of patients with TESCA showed a nonsignificant trend toward adherence with fixed EPAP ASV.
Opioid use was substantial in our population, without significant differences between the groups. CPAP therapy is ineffective in improving opioid-associated CSA. In a recent study, 20 patients on opioid therapy with CSA were treated with CPAP therapy; after several weeks, the average therapeutic use was 4 to 5 hours per night and CPAP was abandoned in favor of ASV therapy due to persistent central apnea. ASV treatment was associated with a considerable reduction in central apnea index, AHI, arousal index, and oxygen desaturations in a remarkable improvement over CPAP.30
Limitations and Future Directions
This retrospective, single-center study may have limited applicability to other populations. Adherence was used as a surrogate for subjective benefit from treatment, though benefit was not confirmed by the patients directly. Only patients seen in follow-up for documentation of the ASV download were identified for inclusion and data analysis. As a single center, we risk homogeneity in the treatment algorithms, though sleep medicine treatments are often decided at the time of the sleep studies. Studies and treatment recommendations were made at a variety of sites, including our sleep center, other US Department of Veterans Affairs hospitals, in the community network, and at US Department of Defense centers. Our population was homogenous in some ways; notably, 100% of our group was male, which is substantially higher than both the veteran population and the general population. Risk factors for OSA and CSA are more common in male patients, which may partially explain this anomaly. Lastly, with our small sample size, there is increased risk that the results seen occurred by chance.
There are several areas for further study. A larger multicenter study may permit these results to be generalized to the population and should include subjective measures of benefit. Patients with primarily CSA were largely absent in our group and may be the focus of future studies; data on predictors of treatment adherence in CSA are lacking. With the availability of consistent older adherence data, comparisons may be made between the efficacies of clinical practice habits, including treatment efficacy, before and after the results of the SERVE-HF trial became known.
Conclusions
In selected patients with preserved LVEF, ASV therapy appears especially effective in patients with OSA combined with CSA. Adherence to ASV treatment was associated with higher obstructive AHI during the baseline PSG and with a greater reduction in the AHI. This understanding may help guide sleep specialists in personalizing treatments for sleep-disordered breathing. Because objective efficacy appears to be important for therapy adherence, clinicians should be able to consistently determine the obstructive and central components of the residual AHI, thus taking all information into account when optimizing the treatment. Additionally, both OSA and CSA pressure requirements should be considered when developing ASV devices.
Acknowledgments
We thank Martha Harper, RRT, of Hampton Veterans Affairs Medical Center (HVAMC) for helping to identify our patients and assisting with data collection. This material is the result of work supported with resources and the use of HVAMC facilities.
Sleep apnea is a heterogeneous group of conditions that may be attributable to a wide array of underlying conditions, with varying contributions of obstructive or central sleep-disordered breathing. The spectrum from obstructive sleep apnea (OSA) to central sleep apnea (CSA) includes mixed sleep apnea, treatment-emergent CSA (TECSA), and Cheyne-Stokes respiration (CSR).1 The pathophysiologic causes of CSA can be attributed to delayed cardiopulmonary circulation in heart failure, decreased brainstem ventilatory response due to stroke, blunting of central chemoreceptors in chronic opioid use, and/or stimulation of the Hering-Breuer reflex from activation of pulmonary stretch receptors after initiating positive airway pressure (PAP) for treatment of OSA.2,3 Medications are commonly implicated in many forms of sleep-disordered breathing; importantly, opioids and benzodiazepines may blunt the respiratory drive, leading to CSA, and/or impair upper airway patency, resulting in or worsening OSA.
Continuous positive airway pressure (CPAP) therapy is largely ineffective in correcting CSA or improving outcomes and is often poorly tolerated in these patients.4 Adaptive servo-ventilation (ASV) is a form of bilevel PAP (BPAP) therapy that delivers variable adjusting pressure support, primarily to treat CSA. PAP also may relieve upper airway obstructions, thereby effectively treating any comorbid obstructive component. ASV has been well documented to improve sleep-related disorders and improve apnea-hypopnea index (AHI) in patients with CSA. However, longitudinal data have demonstrated increased mortality in patients with heart failure with reduced ejection fraction (HFrEF) who were treated with ASV.5 Since the SERVE-HF trial results came to light in 2015, there has been no consensus regarding the optimal use, if any, of ASV therapy.6-8 This is partly related to the inability to fully explain the study’s major findings, which were unexpected at the time, and partly due to the absence of similar relevant mortality data in patients with CSA but without HFrEF.
TECSA may present in some patients with OSA who are new to PAP therapy. These events are frequently seen during PAP titration sleep studies, though patients can also experience significant TECSA shortly after initiating home PAP therapy. TECSA is felt to result from a combination of stimulating pulmonary stretch receptors and lowering arterial carbon dioxide below the apneic threshold. Chemoreceptors located in the medulla respond by attenuating the respiratory drive.9 Previous studies have shown most cases of mild TECSA resolve over time with CPAP treatment. However, in patients with persistent or worsening TECSA, ASV may be considered as an alternative to CPAP.
The prevalence of OSA in the veteran population is estimated to be as high as 60%, considerably higher than the general population estimation.10 Patients with more significant comorbidities may also experience a higher frequency of central events. Patients with CSA have also been shown to have a higher risk for cardiac-related hospital admissions, providing plausible justification for correcting CSA.10
In the current study, we aim to characterize the group of patients using ASV therapy in the modern era. We will assess the objective efficacy and adherence of ASV therapy in patients with primarily CSA compared with those having primarily OSA (ie, TECSA). Secondarily, we aim to identify baseline clinical and polysomnographic features that may be predictive of ASV adherence, as a surrogate for subjective benefit.11 In the wake of the SERVE-HF study, the sleep medicine community has paused prescribing ASV therapy for CSA. We hope to provide more perspective on the treatment of veterans with CSA and identify the patient groups that would benefit most from ASV therapy.
Methods
This retrospective chart review examined patients prescribed ASV therapy at the Hampton Veterans Affairs Medical Center (HVAMC) in Virginia who had therapy data between January 1, 2015, and April 30, 2020. The start date was chosen to approximate the phase-in of wireless PAP devices at HVAMC and to correspond with the release of preliminary results from the SERVE-HF trial.
Patients were initially identified through a query into commercial wireless PAP management databases and cross-referenced with HVAMC patients. Adherence and efficacy data were obtained from the most recent clinical PAP data, which allowed for the evaluation of patients who discontinued therapy for reasons other than intolerance. Clinical, demographic, and polysomnography (PSG) data were obtained from the electronic health record. One patient, identified through the database query but not found in the electronic health record, was excluded. In cases of missing PSG data, especially AHI or similar values, all attempts were made to calculate the data with other provided values. This study was determined to be exempt by the HVAMC Institutional Review Board (protocol #20-01).
Statistics
Statistical analyses were designed to compare clinical characteristics and adherence to therapy of those with primarily CSA on PSG and those with primarily OSA. Because it was not currently known how many patients would fit into each of these categories, we also planned secondary comparisons of the clinical and PSG characteristics of those patients who were adherent with therapy and those who were not. Adherence with ASV therapy was defined as device use for ≥ 4 hours for ≥ 70% of nights.
Comparisons between the means of 2 normally distributed groups were performed with an unpaired t test. Comparisons between 2 nonnormally distributed groups and groups of dates were done with the Mann-Whitney U test. The normality of a group distribution was determined using D’Agostino-Pearson omnibus normality test. Two groups of dichotomous variables were compared with the Fisher exact test. P value < .05 was considered statistically significant.
Results
Thirty-one patients were prescribed ASV therapy and had follow-up at HVAMC since 2015. All patients were male. The mean (SD) age was 67.2 (11.4) years, mean body mass index (BMI) was 34.0 (5.9), and the mean (SD) Epworth Sleepiness Scale (ESS) score was 10.9 (5.8). Patient comorbidities included 30 (97%) with hypertension, 17 (55%) with diabetes mellitus, 16 (52%) with coronary artery disease, and 11 (35%) with congestive heart failure. Three patients had no echocardiogram or other documentation of left ventricular ejection fraction (LVEF). One of these patients had voluntarily stopped using PAP therapy, another had been erroneously started on ASV (ordered for fixed BPAP), and the third had since been retitrated to CPAP. In the 28 patients with documented LVEF, the mean (SD) LVEF was 61.8% (6.9). Ten patients (32%) had opioids documented on their medication lists and 6 (19%) had benzodiazepines.
The median date of diagnostic sleep testing was January 9, 2015, and testing was completed after the release of the initial field safety notice regarding the SERVE-HF trial preliminary findings May 13, 2015, for 14 patients (45%).12 On diagnostic sleep testing, the mean (SD) AHI was 47.3 (25.6) events/h and the median (IQR) oxygen saturation (SpO2) nadir was 82% (78-84). Three patients (10%) were initially diagnosed with CSA, 19 (61%) with OSA, and 9 (29%) with both. Sixteen patients (52%) had ASV with fixed expiratory PAP (EPAP), and 15 (48%) had variable adjusting EPAP. Mean (SD) usage of ASV was 6.5 (2.6) hours and 66.0% (34.2) of nights for ≥ 4 hours. Mean (SD) titrated EPAP (set or 90th/95th percentile autotitrated) was 10.1 (3.4) cm H2O and inspiratory PAP (IPAP) (90th/95th percentile) was 17.1 (3.3) cm H2O. The median (IQR) residual AHI on ASV was 2.7 events/h (1.1-5.1), apnea index (AI) was 0.4 (0.1-1.0), and hypopnea index (HI) was 1.4 (1.0-3.2); the residual central and obstructive events were not available in most cases.
Adherence
There were no significant differences between the proportions of patients on ASV with set EPAP or the titrated EPAP and IPAP. The median (IQR) residual AHI was lower in the adherent group compared with the nonadherent group, both in absolute values (1.7 [0.9-3.2] events/h vs 4.7 [2.4-10.3] events/h, respectively [P = .004]), and as a percentage of the pretreatment AHI (3.1% [2.5-6.0] vs 10.2% [5.3-34.4], respectively; P = .002) (Figure 2).
Primarily Obstructive Sleep Apnea
Sleep apnea was a mixed picture of obstructive and central events in many patients. Only 3 patients had “pure” CSA. Thus, we were unable to define discrete comparison groups based on the sleep-disordered breathing phenotype. We identified 19 patients with primarily OSA (ie, initially diagnosed with OSA, OSA with TECSA, or complex sleep apnea). The mean (SD) age was 66.1 (12.8) years, BMI was 36.2 (4.7), and ESS was 11.4 (5.6). The mean (SD) baseline AHI was 46.9 (29.5), obstructive AHI was 40.5 (30.4), and central AHI was 0.4 (1.2); the median (IQR) SpO2 nadir was 81% (78%-84%). The mean (SD) titrated EPAP was 10.2 (3.5) cm H2O, and the 90th/95th percentile IPAP was 17.9 (3.5) cm H2O. The mean (SD) usage of ASV was 7.9 (5.3) hours with 11 patients (58%) meeting the minimum standard for adherence to ASV therapy.
No significant differences were seen between the adherent and nonadherent groups in clinical or demographic characteristics or date of diagnostic sleep testing (eAppendix, available online at doi:10.12788/fp.0374). In baseline sleep studies the mean (SD) HI was 32.3 (15.8) in the adherent group compared with 14.7 (8.8) in the nonadherent group (P = .049). In contrast, obstructive AHI was not significantly lower in the adherent group: 51.9 (30.9) in the adherent group compared with 22.2 (20.6) in the nonadherent group (P = .09). The median (IQR) residual AHI on ASV as a percentage of the pretreatment AHI was 3.0% (2.4%-6.5%) in the adherent group compared with 11.3% (5.4%-89.1%) in the nonadherent group, a statistically significant difference (P = .01). No other significant differences were seen between the groups.
Discussion
This study describes a real-world cohort of patients using ASV therapy and the characteristics associated with benefit from therapy. The patients that were prescribed and started ASV therapy most often had a significant degree of obstructive component to sleep-disordered breathing, whether primary OSA with TECSA or comorbid OSA and CSA. Moreover, we found that a higher obstructive AHI on the baseline PSG was associated with adherence to ASV therapy. Another important finding was that a lower residual AHI on ASV as a proportion of the baseline was associated with PAP adherence. Adherent patients had similar clinical characteristics as the nonadherent patients, including comorbidities, severity of sleep-disordered breathing, and obesity.
Though the results of the SERVE-HF trial have dampened the enthusiasm somewhat, ASV therapy has long been considered an effective and well-tolerated treatment for many types of CSA.13 In fact, treatments that can eliminate the central AHI are fairly limited.4,14 Our data suggest that ASV is also effective and tolerated in OSA with TECSA and/or comorbid CSA. Recent studies suggest that CSA resolves spontaneously in a majority of TECSA patients within 2 to 3 months of regular CPAP use.15 Other estimates suggest that persistent TESCA may be present in 2% of patients with OSA on treatment.16
Given the high and rising prevalence of OSA, many people are at risk for clinically significant TESCA. Another retrospective case series found that 72% of patients that failed treatment with CPAP or BPAP during PSG, met diagnostic criteria (at the time) for CSA; ASV was objectively beneficial in these patients.17 ASV can be an especially useful modality to treat OSA in patients with CSA that either prevents tolerance of standard therapies or causes clinical consequences, presuming the patient does not also have HFrEF.18 The long-term outcomes of treatment with ASV therapy remain a matter of debate.
The SERVE-HF trial remains among the only studies that have assessed the mortality effects of CSA treatments, with unfavorable findings. Treatment of OSA has been associated with favorable chronic health benefits, though recent studies have questioned the degree of benefit attributable to OSA treatment.19-24 Similar studies have not been done for comorbidities represented by our study cohort (ie, OSA with TECSA and/or comorbid CSA).
The lack of CSA diagnosis alone in our cohort may be partially attributable to changing practice patterns following the SERVE-HF trial, though it is not clear from these data why a higher baseline obstructive AHI was associated with adherence to ASV therapy. Our data in this regard are somewhat at odds with the preliminary results of the ADVENT-HF trial. In that study, adherence to ASV therapy in patients with predominantly OSA declined significantly more than in patients with predominantly CSA.25 Most of our patients were diagnosed with predominantly OSA, so a direct comparison with the CSA group is problematic; additionally, the primary brand and the pressure adjustments algorithm used in our study differed from the ADVENT-HF trial.
OSA and CSA may present with similar clinical symptoms, including sleep fragmentation, insomnia, and excessive daytime sleepiness; however, the degree of symptomatology, especially daytime sleepiness, and the response to treatment, may be less in CSA.2,26 Both the subjective report of symptoms (ESS) and PSG measures of sleep fragmentation were similar in our patients, again likely explained by the predominance of obstructive events.
The pathophysiology of CSA is more varied than OSA, which is probably relevant in this case. ASV was originally designed for the management of CSA with CSR, accomplishing this goal by stabilizing the periods of central apnea and hyperpnea characteristic of CSR.27 Although other forms of CSA demonstrate breathing patterns distinct from CSR, ASV has become an accepted treatment for most of these. It is plausible that the long-term subjective benefit and tolerance of ASV in CSA without CSR is less than for CSA with CSR or OSA. None of the patients in our study had CSA with CSR.
Ultimately, it may be the objective treatment effect that lends to adherence, as has been shown previously in OSA patients; our group of adherent patients showed a greater improvement in AHI, relative to baseline, than the nonadherent patients did.28 The technology behind ASV therapy can greatly reduce the frequencies of central apneas, yet this same treatment effectively splints the upper airway and even more effectively eliminates obstructive apneas and hypopneas. Variable adjusting EPAP devices would plausibly provide even more benefit in these patients, as has been shown in prior studies.29 To the contrary, our small sample of patients with TESCA showed a nonsignificant trend toward adherence with fixed EPAP ASV.
Opioid use was substantial in our population, without significant differences between the groups. CPAP therapy is ineffective in improving opioid-associated CSA. In a recent study, 20 patients on opioid therapy with CSA were treated with CPAP therapy; after several weeks, the average therapeutic use was 4 to 5 hours per night and CPAP was abandoned in favor of ASV therapy due to persistent central apnea. ASV treatment was associated with a considerable reduction in central apnea index, AHI, arousal index, and oxygen desaturations in a remarkable improvement over CPAP.30
Limitations and Future Directions
This retrospective, single-center study may have limited applicability to other populations. Adherence was used as a surrogate for subjective benefit from treatment, though benefit was not confirmed by the patients directly. Only patients seen in follow-up for documentation of the ASV download were identified for inclusion and data analysis. As a single center, we risk homogeneity in the treatment algorithms, though sleep medicine treatments are often decided at the time of the sleep studies. Studies and treatment recommendations were made at a variety of sites, including our sleep center, other US Department of Veterans Affairs hospitals, in the community network, and at US Department of Defense centers. Our population was homogenous in some ways; notably, 100% of our group was male, which is substantially higher than both the veteran population and the general population. Risk factors for OSA and CSA are more common in male patients, which may partially explain this anomaly. Lastly, with our small sample size, there is increased risk that the results seen occurred by chance.
There are several areas for further study. A larger multicenter study may permit these results to be generalized to the population and should include subjective measures of benefit. Patients with primarily CSA were largely absent in our group and may be the focus of future studies; data on predictors of treatment adherence in CSA are lacking. With the availability of consistent older adherence data, comparisons may be made between the efficacies of clinical practice habits, including treatment efficacy, before and after the results of the SERVE-HF trial became known.
Conclusions
In selected patients with preserved LVEF, ASV therapy appears especially effective in patients with OSA combined with CSA. Adherence to ASV treatment was associated with higher obstructive AHI during the baseline PSG and with a greater reduction in the AHI. This understanding may help guide sleep specialists in personalizing treatments for sleep-disordered breathing. Because objective efficacy appears to be important for therapy adherence, clinicians should be able to consistently determine the obstructive and central components of the residual AHI, thus taking all information into account when optimizing the treatment. Additionally, both OSA and CSA pressure requirements should be considered when developing ASV devices.
Acknowledgments
We thank Martha Harper, RRT, of Hampton Veterans Affairs Medical Center (HVAMC) for helping to identify our patients and assisting with data collection. This material is the result of work supported with resources and the use of HVAMC facilities.
1. Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468-475. doi:10.1093/sleep/30.4.468
2. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595-607. doi:10.1378/chest.06.2287
3. Verbraecken J. Complex sleep apnoea syndrome. Breathe. 2013;9(5):372-380. doi:10.1183/20734735.042412
4. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025-2033. doi:10.1056/NEJMoa051001
5. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105. doi:10.1056/NEJMoa1506459
6. Imamura T, Kinugawa K. What is the optimal strategy for adaptive servo-ventilation therapy? Int Heart J. 2018;59(4):683-688. doi:10.1536/ihj.17-429
7. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149(4):900-904. doi:10.1016/j.chest.2015.12.021
8. Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest. 2015;148(4):848-851. doi:10.1378/chest.15-1536
9. Nigam G, Riaz M, Chang E, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
10. Ratz D, Wiitala W, Badr MS, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zsy058. doi:10.1093/sleep/zsy058
11. Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365-369. doi:10.1155/2008/534372
12. Special Safety Notice: ASV therapy for central sleep apnea patients with heart failure. American Academy of Sleep Medicine. May 15, 2015. Accessed February 13, 2023. https://aasm.org/special-safety-notice-asv-therapy-for-central-sleep-apnea-patients-with-heart-failure/
13. Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337-342. doi:10.1136/hrt.2005.060038
14. Randerath W, Deleanu OC, Schiza S, Pepin J-L. Central sleep apnoea and periodic breathing in heart failure: prognostic significance and treatment options. Eur Respir Rev. 2019;28(153):190084. Published 2019 Oct 11. doi:10.1183/16000617.0084-2019
15. Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med. 2008;4(5):403-405.
16. American Academy of Sleep Medicine. International Classification of Sleep Disorders - Third Edition (ICSD-3). 3rd ed. American Academy of Sleep Medicine; 2014.
17. Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187-195.
18. Aurora RN, Bista SR, Casey KR, et al. Updated Adaptive Servo-Ventilation Recommendations for the 2012 AASM Guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757-761. doi:10.5664/jcsm.5812
19. Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36-41. doi:10.1164/rccm.200808-1341OC
20. Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909-916. doi:10.1164/rccm.201203-0448OC
21. Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. Published 2013 Nov 25. doi:10.1161/JAHA.113.000421
22. Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3):858-865. doi:10.1164/ajrccm.157.3.9709042
23. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi:10.1056/NEJMoa1606599
24. Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166. doi:10.1001/jama.2017.7967
25. Perger E, Lyons OD, Inami T, et al. Predictors of 1-year compliance with adaptive servoventilation in patients with heart failure and sleep disordered breathing: preliminary data from the ADVENT-HF trial. Eur Resp J. 2019;53(2):1801626. doi:10.1183/13993003.01626-2018
26. Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579-587. doi:10.1002/ejhf.790
27. Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614-619. doi:10.1164/ajrccm.164.4.9908114
28. Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21(4):419-426. doi:10.1111/j.1365-2869.2011.00969.x
29. Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep. 2010;33(2):267-271. doi:10.1093/sleep/33.2.267
30. Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637-643. Published 2014 Jun 15. doi:10.5664/jcsm.3788
1. Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468-475. doi:10.1093/sleep/30.4.468
2. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595-607. doi:10.1378/chest.06.2287
3. Verbraecken J. Complex sleep apnoea syndrome. Breathe. 2013;9(5):372-380. doi:10.1183/20734735.042412
4. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025-2033. doi:10.1056/NEJMoa051001
5. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105. doi:10.1056/NEJMoa1506459
6. Imamura T, Kinugawa K. What is the optimal strategy for adaptive servo-ventilation therapy? Int Heart J. 2018;59(4):683-688. doi:10.1536/ihj.17-429
7. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149(4):900-904. doi:10.1016/j.chest.2015.12.021
8. Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest. 2015;148(4):848-851. doi:10.1378/chest.15-1536
9. Nigam G, Riaz M, Chang E, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
10. Ratz D, Wiitala W, Badr MS, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zsy058. doi:10.1093/sleep/zsy058
11. Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365-369. doi:10.1155/2008/534372
12. Special Safety Notice: ASV therapy for central sleep apnea patients with heart failure. American Academy of Sleep Medicine. May 15, 2015. Accessed February 13, 2023. https://aasm.org/special-safety-notice-asv-therapy-for-central-sleep-apnea-patients-with-heart-failure/
13. Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337-342. doi:10.1136/hrt.2005.060038
14. Randerath W, Deleanu OC, Schiza S, Pepin J-L. Central sleep apnoea and periodic breathing in heart failure: prognostic significance and treatment options. Eur Respir Rev. 2019;28(153):190084. Published 2019 Oct 11. doi:10.1183/16000617.0084-2019
15. Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med. 2008;4(5):403-405.
16. American Academy of Sleep Medicine. International Classification of Sleep Disorders - Third Edition (ICSD-3). 3rd ed. American Academy of Sleep Medicine; 2014.
17. Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187-195.
18. Aurora RN, Bista SR, Casey KR, et al. Updated Adaptive Servo-Ventilation Recommendations for the 2012 AASM Guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757-761. doi:10.5664/jcsm.5812
19. Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36-41. doi:10.1164/rccm.200808-1341OC
20. Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909-916. doi:10.1164/rccm.201203-0448OC
21. Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. Published 2013 Nov 25. doi:10.1161/JAHA.113.000421
22. Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3):858-865. doi:10.1164/ajrccm.157.3.9709042
23. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi:10.1056/NEJMoa1606599
24. Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166. doi:10.1001/jama.2017.7967
25. Perger E, Lyons OD, Inami T, et al. Predictors of 1-year compliance with adaptive servoventilation in patients with heart failure and sleep disordered breathing: preliminary data from the ADVENT-HF trial. Eur Resp J. 2019;53(2):1801626. doi:10.1183/13993003.01626-2018
26. Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579-587. doi:10.1002/ejhf.790
27. Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614-619. doi:10.1164/ajrccm.164.4.9908114
28. Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21(4):419-426. doi:10.1111/j.1365-2869.2011.00969.x
29. Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep. 2010;33(2):267-271. doi:10.1093/sleep/33.2.267
30. Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637-643. Published 2014 Jun 15. doi:10.5664/jcsm.3788
Pharmacist-Led Antimicrobial Stewardship and Antibiotic Use in Hospitalized Patients With COVID-19
The inappropriate use of antibiotics is associated with an increased risk of antibiotic resistance, health care costs, and risk of adverse drug reactions.1 According to the Centers for Disease Control and Prevention (CDC), a 10% decrease in overall antibiotic use across different wards was associated with a 34% decrease in Clostridioides difficile (C difficile) infections.2 In addition, antimicrobial resistance accounts for > 2.8 million infections and > 35,000 deaths each year.3 The estimated total economic costs of antibiotic resistance to the US economy have ranged as high as $20 billion in excess direct health care costs.4 A main goal of an antimicrobial stewardship program (ASP) is to optimize antibiotic use to prevent the adverse consequences of inappropriate antibiotic prescribing.
During the COVID-19 pandemic, increased use of empiric antibiotic therapy has been observed. According to the CDC, almost 80% of patients hospitalized with COVID-19 received an antibiotic from March 2020 to October 2020.5 Studies were conducted to investigate the prevalence of bacterial coinfection in patients with COVID-19 and whether antibiotics were indicated in this patient population. A United Kingdom multicenter, prospective cohort study showed a high proportion of patients hospitalized with COVID-19 received antimicrobials despite microbiologically confirmed bacterial infections being rare and more likely to be secondary infections.6
Many other studies have reported similar findings. Langord and colleagues found the prevalence of bacterial coinfection in patients with COVID-19 was 3.5% but that 71.9% received antibiotics.7 Coenen and colleagues identified 12.4% of the patients with possible and 1.1% of patients with probable bacterial coinfection, while 81% of the study population and 78% of patients were classified as unlikely bacterial coinfection received antibiotics.8
At Veterans Affairs Southern Nevada Healthcare System (VASNHS), an ASP team consisting of an infectious disease (ID) physician and 2 pharmacists provide daily prospective audits with intervention and feedback along with other interventions, such as providing restricted order menus, institutional treatment guidelines, and staff education to help improve antibiotic prescribing. The ASP pharmacists have a scope of practice to make changes to anti-infective therapies. The purpose of this study was to describe antibiotic prescribing in patients hospitalized with COVID-19 from November 1, 2020, to January 31, 2021, in an ASP setting led by pharmacists.
Methods
This retrospective descriptive study included patients who were hospitalized for the treatment of laboratory-confirmed COVID-19 infection. The Theradoc clinical surveillance system was used to retrieve a list of patients who were admitted to VASNHS from November 1, 2020, to January 31, 2021, and tested positive for COVID-19. Patients with incidental positive COVID-19 test results or those who received antibiotics for extrapulmonary indications on hospital admission were excluded.
Each patient chart was reviewed and data, including clinical presentations, procalcitonin (PCT), the requirement of supplemental oxygen, vital signs, imaging findings, antibiotic orders on admission, ASP interventions such as discontinuation or changes to antibiotic therapy during the first 72 hours of hospital admission, clinical outcomes, culture results, and readmission rate, defined as any hospital admission related to COVID-19 or respiratory tract infection within 30 days from the previous discharge, were collected.
The primary objective of the study was to describe antibiotic prescribing in patients hospitalized with COVID-19. The secondary outcomes included the prevalence of bacterial coinfection and nosocomial bacterial infection in patients hospitalized with COVID-19.
Results
A total of 199 patients were admitted to the hospital for laboratory-confirmed COVID-19 infection from November 1, 2020, to January 31, 2021. Sixty-one patients (31%) received at least 1 antibiotic on hospital admission. Among those patients who received empiric antibiotic treatment, 29 patients (48%) met the Systemic Inflammatory Response Syndrome (SIRS) criteria. Fifty-six patients (92%) had ≥ 1 PCT level obtained, and 26 of those (46%) presented with elevated PCT levels (PCT > 0.25). Fifty patients (82%) required oxygen supplement and 49 (80%) presented with remarkable imaging findings. Of 138 patients who did not receive empiric antibiotic therapy within 72 hours of hospital admission, 56 (41%) met the SIRS criteria, 31 (29%) had elevated PCT levels, 100 (72%) required oxygen supplement, and 79 (59%) presented with remarkable imaging findings.
Antibiotic Prescribing
Forty-six of 61 patients (75%) received antibiotic treatment for community-acquired pneumonia (CAP) that included ceftriaxone and azithromycin. Three patients (5%) received ≥ 1 broad-spectrum antibiotic (4th generation cephalosporin [cefepime] or piperacillin-tazobactam), 2 (3%) received vancomycin, and 1 (2%) received a fluoroquinolone (levofloxacin) on admission.
Among 61 patients who received empiric antibiotics, the readmission rate was 6%. The mortality rate was 20%, and the mean (SD) duration of hospital stay was 13.1 (12.5) days.
Six of 199 patients (3%) had microbiologically confirmed bacterial coinfection on hospital admission: 3 were Pseudomonas aeruginosa (P aeruginosa) and 2 were Klebsiella oxytoca (Table 1).
Discussion
Prospective audit and feedback and preauthorization are recommended in guidelines as “core components of any stewardship program.”9 At VASNHS, the ASP performs daily prospective audits with intervention and feedback. Efforts have been made to maintain daily ASP activities during the pandemic. This study aimed to describe antibiotic prescribing for patients hospitalized with COVID-19 in a pharmacist-led ASP setting. It was found that up to 31% of the patients received ≥ 1 antibiotic on admission for empiric treatment of bacterial coinfection. About half of these patients met the SIRS criteria. Most of these patients received ceftriaxone and azithromycin for concern of CAP. ASP discontinued antibiotics within 72 hours in most of the patients. Chart review and discussion with ID physicians and/or hospitalists determined the probability of bacterial coinfection as well as any potential complication or patient-specific risk factor. It is important to note that most patients who received antibiotics on admission had ≥ 1 PCT level and up to 46% of them had a PCT level > 0.25. However, according to Relph and colleagues, PCT may not be a reliable indicator of bacterial infection in severe viral diseases with raised interleukin-6 levels.10 An elevated PCT level should not be the sole indicator for empiric antibiotic treatment.
Study findings confirmed the low prevalence of bacterial coinfection in patients hospitalized with COVID-19. The overuse of empiric antibiotics in a patient population unlikely to present with bacterial coinfection is concerning. It is essential to continue promoting antimicrobial stewardship during the COVID-19 pandemic to ensure appropriate and responsible antimicrobial prescribing. A thorough clinical assessment consisting of comorbidities, clinical symptoms, radiologic and microbiologic findings, as well as other relevant workup or biomarker results is crucial to determine whether the antibiotic is strongly indicated in patients hospitalized with COVID-19. Empiric antibiotic therapy should be considered only in patients with clinical findings suggestive of bacterial coinfection.
Limitations
Limitations of our study included the study design (single-center, retrospective review, lack of comparative group) and small sample size with a 3-month study period. In addition, respiratory cultures are not commonly obtained in patients who present with mild-to-moderate CAP. Using culture results solely to confirm bacterial coinfection in patients with COVID-19 could have underestimated the prevalence of bacterial infection. Developing diagnostic criteria that include clinical signs and symptoms, imaging findings, and laboratory results as well as culture results would help to better assess the presence of bacterial coinfection in this patient population.
Conclusions
The study findings showed that up to 30% of patients hospitalized for COVID-19 infection received empiric antibiotic treatment for concern of bacterial coinfection. A pharmacist-led ASP provided interventions, including early discontinuation of antibiotics in 77% of these patients.
A low prevalence of bacterial coinfection (3%) in patients hospitalized with COVID-19 also was reported. A thorough clinical workup to determine the risk of bacterial coinfection in patients with COVID-19 is important before starting empiric antibiotic therapy. Continuing to promote the ASP during the COVID-19 pandemic to ensure responsible antibiotic use and prevent antimicrobial resistance is essential.
1. Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC grand rounds: getting smart about antibiotics. MMWR Morb Mortal Wkly Rep. 2015;64(32):871-873. doi:10.15585/mmwr.mm6432a3
2. Nearly half a million Americans suffered from Clostridium difficile infections in a single year. Centers for Disease Control and Prevention. Updated March 22, 2017. Accessed March 21, 2023. https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html
3. Centers for Disease Control and Prevention. About antimicrobial resistance. Updated October 5, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/about.html
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
5. Centers for Disease Control and Prevention. COVID-19 & antibiotic resistance. Updated February 25, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/covid19.html
6. Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2(8):e354-e365. doi:10.1016/S2666-5247(21)00090-2
7. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622-1629. doi:10.1016/j.cmi.2020.07.016
8. Coenen S, de la Court JR, Buis DTP, et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10(1):155. doi:10.1186/s13756-021-01024-4
9. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs: 2019. Accessed March 21, 2023. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
10. Relph KA, Russell CD, Fairfield CJ, et al; International Severe Acute Respiratory and Emerging Infections Consortium; Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators. Procalcitonin is not a reliable biomarker of bacterial coinfection in people with Coronavirus Disease 2019 undergoing microbiological investigation at the time of hospital admission. Open Forum Infect Dis. 2022;9(5):ofac179. doi:10.1093/ofid/ofac179
The inappropriate use of antibiotics is associated with an increased risk of antibiotic resistance, health care costs, and risk of adverse drug reactions.1 According to the Centers for Disease Control and Prevention (CDC), a 10% decrease in overall antibiotic use across different wards was associated with a 34% decrease in Clostridioides difficile (C difficile) infections.2 In addition, antimicrobial resistance accounts for > 2.8 million infections and > 35,000 deaths each year.3 The estimated total economic costs of antibiotic resistance to the US economy have ranged as high as $20 billion in excess direct health care costs.4 A main goal of an antimicrobial stewardship program (ASP) is to optimize antibiotic use to prevent the adverse consequences of inappropriate antibiotic prescribing.
During the COVID-19 pandemic, increased use of empiric antibiotic therapy has been observed. According to the CDC, almost 80% of patients hospitalized with COVID-19 received an antibiotic from March 2020 to October 2020.5 Studies were conducted to investigate the prevalence of bacterial coinfection in patients with COVID-19 and whether antibiotics were indicated in this patient population. A United Kingdom multicenter, prospective cohort study showed a high proportion of patients hospitalized with COVID-19 received antimicrobials despite microbiologically confirmed bacterial infections being rare and more likely to be secondary infections.6
Many other studies have reported similar findings. Langord and colleagues found the prevalence of bacterial coinfection in patients with COVID-19 was 3.5% but that 71.9% received antibiotics.7 Coenen and colleagues identified 12.4% of the patients with possible and 1.1% of patients with probable bacterial coinfection, while 81% of the study population and 78% of patients were classified as unlikely bacterial coinfection received antibiotics.8
At Veterans Affairs Southern Nevada Healthcare System (VASNHS), an ASP team consisting of an infectious disease (ID) physician and 2 pharmacists provide daily prospective audits with intervention and feedback along with other interventions, such as providing restricted order menus, institutional treatment guidelines, and staff education to help improve antibiotic prescribing. The ASP pharmacists have a scope of practice to make changes to anti-infective therapies. The purpose of this study was to describe antibiotic prescribing in patients hospitalized with COVID-19 from November 1, 2020, to January 31, 2021, in an ASP setting led by pharmacists.
Methods
This retrospective descriptive study included patients who were hospitalized for the treatment of laboratory-confirmed COVID-19 infection. The Theradoc clinical surveillance system was used to retrieve a list of patients who were admitted to VASNHS from November 1, 2020, to January 31, 2021, and tested positive for COVID-19. Patients with incidental positive COVID-19 test results or those who received antibiotics for extrapulmonary indications on hospital admission were excluded.
Each patient chart was reviewed and data, including clinical presentations, procalcitonin (PCT), the requirement of supplemental oxygen, vital signs, imaging findings, antibiotic orders on admission, ASP interventions such as discontinuation or changes to antibiotic therapy during the first 72 hours of hospital admission, clinical outcomes, culture results, and readmission rate, defined as any hospital admission related to COVID-19 or respiratory tract infection within 30 days from the previous discharge, were collected.
The primary objective of the study was to describe antibiotic prescribing in patients hospitalized with COVID-19. The secondary outcomes included the prevalence of bacterial coinfection and nosocomial bacterial infection in patients hospitalized with COVID-19.
Results
A total of 199 patients were admitted to the hospital for laboratory-confirmed COVID-19 infection from November 1, 2020, to January 31, 2021. Sixty-one patients (31%) received at least 1 antibiotic on hospital admission. Among those patients who received empiric antibiotic treatment, 29 patients (48%) met the Systemic Inflammatory Response Syndrome (SIRS) criteria. Fifty-six patients (92%) had ≥ 1 PCT level obtained, and 26 of those (46%) presented with elevated PCT levels (PCT > 0.25). Fifty patients (82%) required oxygen supplement and 49 (80%) presented with remarkable imaging findings. Of 138 patients who did not receive empiric antibiotic therapy within 72 hours of hospital admission, 56 (41%) met the SIRS criteria, 31 (29%) had elevated PCT levels, 100 (72%) required oxygen supplement, and 79 (59%) presented with remarkable imaging findings.
Antibiotic Prescribing
Forty-six of 61 patients (75%) received antibiotic treatment for community-acquired pneumonia (CAP) that included ceftriaxone and azithromycin. Three patients (5%) received ≥ 1 broad-spectrum antibiotic (4th generation cephalosporin [cefepime] or piperacillin-tazobactam), 2 (3%) received vancomycin, and 1 (2%) received a fluoroquinolone (levofloxacin) on admission.
Among 61 patients who received empiric antibiotics, the readmission rate was 6%. The mortality rate was 20%, and the mean (SD) duration of hospital stay was 13.1 (12.5) days.
Six of 199 patients (3%) had microbiologically confirmed bacterial coinfection on hospital admission: 3 were Pseudomonas aeruginosa (P aeruginosa) and 2 were Klebsiella oxytoca (Table 1).
Discussion
Prospective audit and feedback and preauthorization are recommended in guidelines as “core components of any stewardship program.”9 At VASNHS, the ASP performs daily prospective audits with intervention and feedback. Efforts have been made to maintain daily ASP activities during the pandemic. This study aimed to describe antibiotic prescribing for patients hospitalized with COVID-19 in a pharmacist-led ASP setting. It was found that up to 31% of the patients received ≥ 1 antibiotic on admission for empiric treatment of bacterial coinfection. About half of these patients met the SIRS criteria. Most of these patients received ceftriaxone and azithromycin for concern of CAP. ASP discontinued antibiotics within 72 hours in most of the patients. Chart review and discussion with ID physicians and/or hospitalists determined the probability of bacterial coinfection as well as any potential complication or patient-specific risk factor. It is important to note that most patients who received antibiotics on admission had ≥ 1 PCT level and up to 46% of them had a PCT level > 0.25. However, according to Relph and colleagues, PCT may not be a reliable indicator of bacterial infection in severe viral diseases with raised interleukin-6 levels.10 An elevated PCT level should not be the sole indicator for empiric antibiotic treatment.
Study findings confirmed the low prevalence of bacterial coinfection in patients hospitalized with COVID-19. The overuse of empiric antibiotics in a patient population unlikely to present with bacterial coinfection is concerning. It is essential to continue promoting antimicrobial stewardship during the COVID-19 pandemic to ensure appropriate and responsible antimicrobial prescribing. A thorough clinical assessment consisting of comorbidities, clinical symptoms, radiologic and microbiologic findings, as well as other relevant workup or biomarker results is crucial to determine whether the antibiotic is strongly indicated in patients hospitalized with COVID-19. Empiric antibiotic therapy should be considered only in patients with clinical findings suggestive of bacterial coinfection.
Limitations
Limitations of our study included the study design (single-center, retrospective review, lack of comparative group) and small sample size with a 3-month study period. In addition, respiratory cultures are not commonly obtained in patients who present with mild-to-moderate CAP. Using culture results solely to confirm bacterial coinfection in patients with COVID-19 could have underestimated the prevalence of bacterial infection. Developing diagnostic criteria that include clinical signs and symptoms, imaging findings, and laboratory results as well as culture results would help to better assess the presence of bacterial coinfection in this patient population.
Conclusions
The study findings showed that up to 30% of patients hospitalized for COVID-19 infection received empiric antibiotic treatment for concern of bacterial coinfection. A pharmacist-led ASP provided interventions, including early discontinuation of antibiotics in 77% of these patients.
A low prevalence of bacterial coinfection (3%) in patients hospitalized with COVID-19 also was reported. A thorough clinical workup to determine the risk of bacterial coinfection in patients with COVID-19 is important before starting empiric antibiotic therapy. Continuing to promote the ASP during the COVID-19 pandemic to ensure responsible antibiotic use and prevent antimicrobial resistance is essential.
The inappropriate use of antibiotics is associated with an increased risk of antibiotic resistance, health care costs, and risk of adverse drug reactions.1 According to the Centers for Disease Control and Prevention (CDC), a 10% decrease in overall antibiotic use across different wards was associated with a 34% decrease in Clostridioides difficile (C difficile) infections.2 In addition, antimicrobial resistance accounts for > 2.8 million infections and > 35,000 deaths each year.3 The estimated total economic costs of antibiotic resistance to the US economy have ranged as high as $20 billion in excess direct health care costs.4 A main goal of an antimicrobial stewardship program (ASP) is to optimize antibiotic use to prevent the adverse consequences of inappropriate antibiotic prescribing.
During the COVID-19 pandemic, increased use of empiric antibiotic therapy has been observed. According to the CDC, almost 80% of patients hospitalized with COVID-19 received an antibiotic from March 2020 to October 2020.5 Studies were conducted to investigate the prevalence of bacterial coinfection in patients with COVID-19 and whether antibiotics were indicated in this patient population. A United Kingdom multicenter, prospective cohort study showed a high proportion of patients hospitalized with COVID-19 received antimicrobials despite microbiologically confirmed bacterial infections being rare and more likely to be secondary infections.6
Many other studies have reported similar findings. Langord and colleagues found the prevalence of bacterial coinfection in patients with COVID-19 was 3.5% but that 71.9% received antibiotics.7 Coenen and colleagues identified 12.4% of the patients with possible and 1.1% of patients with probable bacterial coinfection, while 81% of the study population and 78% of patients were classified as unlikely bacterial coinfection received antibiotics.8
At Veterans Affairs Southern Nevada Healthcare System (VASNHS), an ASP team consisting of an infectious disease (ID) physician and 2 pharmacists provide daily prospective audits with intervention and feedback along with other interventions, such as providing restricted order menus, institutional treatment guidelines, and staff education to help improve antibiotic prescribing. The ASP pharmacists have a scope of practice to make changes to anti-infective therapies. The purpose of this study was to describe antibiotic prescribing in patients hospitalized with COVID-19 from November 1, 2020, to January 31, 2021, in an ASP setting led by pharmacists.
Methods
This retrospective descriptive study included patients who were hospitalized for the treatment of laboratory-confirmed COVID-19 infection. The Theradoc clinical surveillance system was used to retrieve a list of patients who were admitted to VASNHS from November 1, 2020, to January 31, 2021, and tested positive for COVID-19. Patients with incidental positive COVID-19 test results or those who received antibiotics for extrapulmonary indications on hospital admission were excluded.
Each patient chart was reviewed and data, including clinical presentations, procalcitonin (PCT), the requirement of supplemental oxygen, vital signs, imaging findings, antibiotic orders on admission, ASP interventions such as discontinuation or changes to antibiotic therapy during the first 72 hours of hospital admission, clinical outcomes, culture results, and readmission rate, defined as any hospital admission related to COVID-19 or respiratory tract infection within 30 days from the previous discharge, were collected.
The primary objective of the study was to describe antibiotic prescribing in patients hospitalized with COVID-19. The secondary outcomes included the prevalence of bacterial coinfection and nosocomial bacterial infection in patients hospitalized with COVID-19.
Results
A total of 199 patients were admitted to the hospital for laboratory-confirmed COVID-19 infection from November 1, 2020, to January 31, 2021. Sixty-one patients (31%) received at least 1 antibiotic on hospital admission. Among those patients who received empiric antibiotic treatment, 29 patients (48%) met the Systemic Inflammatory Response Syndrome (SIRS) criteria. Fifty-six patients (92%) had ≥ 1 PCT level obtained, and 26 of those (46%) presented with elevated PCT levels (PCT > 0.25). Fifty patients (82%) required oxygen supplement and 49 (80%) presented with remarkable imaging findings. Of 138 patients who did not receive empiric antibiotic therapy within 72 hours of hospital admission, 56 (41%) met the SIRS criteria, 31 (29%) had elevated PCT levels, 100 (72%) required oxygen supplement, and 79 (59%) presented with remarkable imaging findings.
Antibiotic Prescribing
Forty-six of 61 patients (75%) received antibiotic treatment for community-acquired pneumonia (CAP) that included ceftriaxone and azithromycin. Three patients (5%) received ≥ 1 broad-spectrum antibiotic (4th generation cephalosporin [cefepime] or piperacillin-tazobactam), 2 (3%) received vancomycin, and 1 (2%) received a fluoroquinolone (levofloxacin) on admission.
Among 61 patients who received empiric antibiotics, the readmission rate was 6%. The mortality rate was 20%, and the mean (SD) duration of hospital stay was 13.1 (12.5) days.
Six of 199 patients (3%) had microbiologically confirmed bacterial coinfection on hospital admission: 3 were Pseudomonas aeruginosa (P aeruginosa) and 2 were Klebsiella oxytoca (Table 1).
Discussion
Prospective audit and feedback and preauthorization are recommended in guidelines as “core components of any stewardship program.”9 At VASNHS, the ASP performs daily prospective audits with intervention and feedback. Efforts have been made to maintain daily ASP activities during the pandemic. This study aimed to describe antibiotic prescribing for patients hospitalized with COVID-19 in a pharmacist-led ASP setting. It was found that up to 31% of the patients received ≥ 1 antibiotic on admission for empiric treatment of bacterial coinfection. About half of these patients met the SIRS criteria. Most of these patients received ceftriaxone and azithromycin for concern of CAP. ASP discontinued antibiotics within 72 hours in most of the patients. Chart review and discussion with ID physicians and/or hospitalists determined the probability of bacterial coinfection as well as any potential complication or patient-specific risk factor. It is important to note that most patients who received antibiotics on admission had ≥ 1 PCT level and up to 46% of them had a PCT level > 0.25. However, according to Relph and colleagues, PCT may not be a reliable indicator of bacterial infection in severe viral diseases with raised interleukin-6 levels.10 An elevated PCT level should not be the sole indicator for empiric antibiotic treatment.
Study findings confirmed the low prevalence of bacterial coinfection in patients hospitalized with COVID-19. The overuse of empiric antibiotics in a patient population unlikely to present with bacterial coinfection is concerning. It is essential to continue promoting antimicrobial stewardship during the COVID-19 pandemic to ensure appropriate and responsible antimicrobial prescribing. A thorough clinical assessment consisting of comorbidities, clinical symptoms, radiologic and microbiologic findings, as well as other relevant workup or biomarker results is crucial to determine whether the antibiotic is strongly indicated in patients hospitalized with COVID-19. Empiric antibiotic therapy should be considered only in patients with clinical findings suggestive of bacterial coinfection.
Limitations
Limitations of our study included the study design (single-center, retrospective review, lack of comparative group) and small sample size with a 3-month study period. In addition, respiratory cultures are not commonly obtained in patients who present with mild-to-moderate CAP. Using culture results solely to confirm bacterial coinfection in patients with COVID-19 could have underestimated the prevalence of bacterial infection. Developing diagnostic criteria that include clinical signs and symptoms, imaging findings, and laboratory results as well as culture results would help to better assess the presence of bacterial coinfection in this patient population.
Conclusions
The study findings showed that up to 30% of patients hospitalized for COVID-19 infection received empiric antibiotic treatment for concern of bacterial coinfection. A pharmacist-led ASP provided interventions, including early discontinuation of antibiotics in 77% of these patients.
A low prevalence of bacterial coinfection (3%) in patients hospitalized with COVID-19 also was reported. A thorough clinical workup to determine the risk of bacterial coinfection in patients with COVID-19 is important before starting empiric antibiotic therapy. Continuing to promote the ASP during the COVID-19 pandemic to ensure responsible antibiotic use and prevent antimicrobial resistance is essential.
1. Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC grand rounds: getting smart about antibiotics. MMWR Morb Mortal Wkly Rep. 2015;64(32):871-873. doi:10.15585/mmwr.mm6432a3
2. Nearly half a million Americans suffered from Clostridium difficile infections in a single year. Centers for Disease Control and Prevention. Updated March 22, 2017. Accessed March 21, 2023. https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html
3. Centers for Disease Control and Prevention. About antimicrobial resistance. Updated October 5, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/about.html
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
5. Centers for Disease Control and Prevention. COVID-19 & antibiotic resistance. Updated February 25, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/covid19.html
6. Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2(8):e354-e365. doi:10.1016/S2666-5247(21)00090-2
7. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622-1629. doi:10.1016/j.cmi.2020.07.016
8. Coenen S, de la Court JR, Buis DTP, et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10(1):155. doi:10.1186/s13756-021-01024-4
9. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs: 2019. Accessed March 21, 2023. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
10. Relph KA, Russell CD, Fairfield CJ, et al; International Severe Acute Respiratory and Emerging Infections Consortium; Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators. Procalcitonin is not a reliable biomarker of bacterial coinfection in people with Coronavirus Disease 2019 undergoing microbiological investigation at the time of hospital admission. Open Forum Infect Dis. 2022;9(5):ofac179. doi:10.1093/ofid/ofac179
1. Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC grand rounds: getting smart about antibiotics. MMWR Morb Mortal Wkly Rep. 2015;64(32):871-873. doi:10.15585/mmwr.mm6432a3
2. Nearly half a million Americans suffered from Clostridium difficile infections in a single year. Centers for Disease Control and Prevention. Updated March 22, 2017. Accessed March 21, 2023. https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html
3. Centers for Disease Control and Prevention. About antimicrobial resistance. Updated October 5, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/about.html
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
5. Centers for Disease Control and Prevention. COVID-19 & antibiotic resistance. Updated February 25, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/covid19.html
6. Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2(8):e354-e365. doi:10.1016/S2666-5247(21)00090-2
7. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622-1629. doi:10.1016/j.cmi.2020.07.016
8. Coenen S, de la Court JR, Buis DTP, et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10(1):155. doi:10.1186/s13756-021-01024-4
9. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs: 2019. Accessed March 21, 2023. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
10. Relph KA, Russell CD, Fairfield CJ, et al; International Severe Acute Respiratory and Emerging Infections Consortium; Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators. Procalcitonin is not a reliable biomarker of bacterial coinfection in people with Coronavirus Disease 2019 undergoing microbiological investigation at the time of hospital admission. Open Forum Infect Dis. 2022;9(5):ofac179. doi:10.1093/ofid/ofac179
Prevalence of Antibiotic Allergy at a Spinal Cord Injury Center
Infectious diseases are the most common reason for rehospitalization among patients with spinal cord injuries (SCI), regardless of the number of years postinjury.1 The appropriate use and selection of antibiotics for properly diagnosed infectious diseases is especially important for this population. This principle helps to avoid the development of drug-resistant organisms and reduces the risk of recurrent infections, aligning with antibiotic stewardship.
Antibiotics are the most common class of drug allergies in the general population, and penicillin is the most frequently reported allergen (up to 10%).2 Prescription drug–induced anaphylaxis is severe and life threatening with a reported frequency of 1.1%. Penicillin and sulfonamide (46 and 15 per 10,000 patients, respectively) are the most common allergens.3 Although there is a significant difference between an adverse drug reaction (ADR) and true hypersensitivity, once documented in the electronic health record (EHR) as an allergy, this information deters use of the listed drugs.
Genitourinary, skin, and respiratory diseases are the leading causes for rehospitalization in patients with SCI.1 A large proportion of these are infectious in etiology and require antibiotic treatment. In fact, persons with SCI are at high risk for antibiotic overuse and hospital-acquired infection due to chronic bacteriuria, frequent health care exposure, implanted medical devices, and other factors.4 Concurrently, there is a crisis of antibiotic-resistant bacteria proliferation, described as a threat to patient safety and public health.5,6 Its severity is illustrated by the report that 38% of the cultures from patients with spinal cord injury are multidrug resistant gram-negative organisms.7
The SCI center at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, serves a high concentration of active-duty military members and veterans with SCI. A study that reviews the exact frequency of antibiotic drug allergies listed on the EHR would be a key first step to identify the magnitude of this issue. The results could guide investigation into differentiating true allergies from ADRs, thereby widening the options for potentially life-saving antibiotic treatment.
Methods
We performed a retrospective chart review of patients included in the local SCI registry between October 1, 2015, and September 30, 2017. We collected data on patient demographics (age, sex, race and ethnicity) and a description of patients’ injuries (International Standards for Neurological Classification of Spinal Cord Injury [ISNCSCI] and etiology of injury [traumatic vs atraumatic]). The outcomes included antibiotic allergy and ADRs.
In the EHR, allergies can be listed toward an antibiotic class or a specific antibiotic. An allergy to each specific antibiotic would be recorded separately; however, overlap among antibiotic classes was not duplicated. For example, if a subject has a listed antibiotic allergy to ceftriaxone and cefepime with listed reactions, we would record allergies to each of these antibiotics but would only report a single allergy to the cephalosporin subclass.
Since we did not differentiate hypersensitivity reactions (HSRs) from other ADRs, the reported reactions were grouped by signs and symptoms. There is a variety of terms used to report similar reactions, and best efforts were made to record the data as accurately as possible. Patient-reported history for risk stratification is a tool we used to group these historical reactions into high- vs low-risk for severe reactions. High-risk signs are those listed as anaphylaxis; anaphylactic reactions; angioedema presenting as swelling of mouth, eyes, lips, or tongue; blisters or ulcers involving the lips, mouth, eyes, urethra, vagina, or peeling skin; respiratory changes; shortness of breath; dyspnea; hypotension; or organ involvement (kidneys, lungs, liver).6
Inclusion criteria were all veterans who were diagnosed with tetraplegia or paraplegia and received annual evaluation between October 1, 2015, and September 30, 2017. We chose this period because it was the beginning of a financial year at the JAHVH SCI department using the SCI registry. The SCI annual evaluation is a routine practitioner encounter with the veteran, along with appropriate laboratory testing and imaging to follow up potential chronic health issues specific to patients with SCI. Annual evaluations provide an opportunity to maintain routine health screening and preventive care. Patients who had significant portions of data missing or missing elements of primary outcomes were excluded from analysis. The study was reviewed and approved by the University of South Florida Institutional Review Board (VA IRBNet #1573370-4 on September 9, 2019).
Results
Of 1866 patients reviewed, 207 (11.1%) were excluded due to missing data, resulting in 1659 records that were analyzed. Mean age was 64 years, and male to female ratio was about 10 to 1. Most of the SCI or diseases were classified as incomplete (n = 1249) per ISNCSCI (absence of sensory and motor function in the lowest sacral segments) compared with 373 classified as complete.
Of the 1659 patients, 494 (29.8%) had a recorded allergy to antibiotics. The most frequently recorded were 217 penicillin (13.1%), 159 sulfa drugs (9.6%), 75 fluoroquinolone (4.5%), 66 cephalosporin (4.0%), and 44 vancomycin (2.7%) allergies.
Discussion
In this study, we evaluated the frequency and characteristics of antibiotic allergies at a single SCI center to better identify potential areas for quality improvement when recording drug allergies. A study in the general population used self-reported methods to collect such information found about a 15% prevalence of antibiotic allergy, which was lower than the 29.8% prevalence noted in our study.8
Regarding the most common antibiotic allergies, one study reported allergy to penicillin in the EHR in 12.8% of patients at a major US regional health care system, while 13.1% of patients with SCI had documented allergy to penicillin in our study.9 Regarding the other antibiotic classes, the percentage of allergies were higher than those reported in the general population: sulfonamide (9.6% vs 7.4%), fluoroquinolones (4.5% vs 1.3%), and cephalosporins (4.0% vs 1.7%).10 The EHR appears to capture a much higher rate of antibiotic allergies than that in self-reported studies, such as a study of self-reported allergy in the general adult population in Portugal, where only 4.5% of patients reported allergy to any β-lactam medications.10
The prevalence of an antibiotic allergy could be affected by the health care setting and sex distribution. For example, the Zhou and colleagues’ study conducted in the Greater Boston area showed higher reported antibiotic rates than those in a study from a Southern California medical group. The higher proportion of tertiary referral patients in that specific network was suggested to be the cause of the difference.8,9 Our results in the SCI population are more comparable to that in a tertiary setting. This is consistent with the fact that persons with SCI generally have more exposure to antibiotics and consequently a higher reported rate of allergic reactions to antibiotics.
Similarly, the same study in Southern California noted that female patients use more antibiotics than do male patients, thus potentially contributing to higher rates of reported allergy toward all classes of antibiotics.8 Our study did not investigate antibiotic allergy by sex; however, the significantly higher proportion of male sex among the veteran population would have impacted these results.
Limitations
Our study was limited as a single-center retrospective study. However, our center is one of the major SCI specialty hubs, and the results should be somewhat reflective of those in the veterans with SCI population. Veterans under the US Department of Veterans Affairs (VA) medical care have the option to seek care or procedures in non-VA facilities. If allergies to antibiotics occurred outside of the VA system, there is no mechanism to automatically merge with the VA EHR allergy list, unless they are later recorded and added to the VA EHR. Thus, there is potential for underreporting.
Drug anaphylaxis incidence was noted to change over time.4,8,9 For example, a downtrend of reported antibiotic allergy was reported between 1990 and 2013.10 Our study only reflects an overall prevalence of a single cohort, without demonstration of relationship to time.
Lastly, this study did not aim to differentiate HSRs from other ADRs. This is exactly the point of the study, which investigated the frequency of EHR-recorded antibiotic allergies in our SCI population and reflects the issue with indiscriminate recording of ADRs and HSRs under the umbrella of allergy in the EHR. Further diagnosing true allergies should be considered in the SCI population after weighing the risks and benefits of assessment, aligning with the wishes of the veteran, obtaining informed consent, and addressing the cost-effectiveness of specific tests. We suggest that primary care practitioners work closely with allergy specialists to formulate a mechanism to diagnose various antibiotic allergic reactions, including serum tryptase, epicutaneous skin testing, intradermal skin testing, patch testing, delayed intradermal testing, and drug challenge as appropriate. It is also possible that in cases where very mild reactions/adverse effects of antibiotics were recorded in the EHR, the clinicians and veterans may discuss reintroducing the same antibiotics or proceeding with further testing if necessary. In contrast, the 12% of those with a high risk of severe allergic reactions to penicillin in our study would benefit from allergist evaluation and access to epinephrine auto-injectors at all times. Differentiating true allergy is the only clear way to deter unnecessary avoidance of first-line therapies for antibiotic treatment and avoid promotion of antibiotic resistance.
Future studies can analyze antibiotic allergy based on demographics, including sex and age difference, as well as exploring outpatient vs inpatient settings. Aside from prevalence, we hope to demonstrate antibiotic allergy over time, especially after integration of diagnostic allergy testing, to evaluate the impact to EHR-recorded allergies.
Conclusions
Almost 30% of patients with SCI had a recorded allergy to at least 1 antibiotic. The most common allergy was to penicillin, which is similar to what has previously been reported for the general adult US population. However, only 12% of those with a penicillin allergy were considered high risk of true allergic reactions. Consequently, there are opportunities to examine whether approaches to confirm true reactions (such as skin testing) would help to mitigate unnecessary avoidance of certain antibiotic classes due to mild ADRs, rather than a true allergy, in persons with SCI. This would be an important effort to combat both individual safety concerns and the public health crisis of antibiotic resistance. Given the available evidence, it is reasonable for SCI health care practitioners to discuss the potential risks and benefits of allergy testing with patients with SCI; this maintains a patient-centered approach that can ensure judicious use of antibiotics when necessary.
Acknowledgments
This material is based on work supported (or supported in part) with resources and the use of facilities at the James A. Haley Veterans’ Hospital
References
1. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Model Systems. 2016 Annual Report –Complete Public Version. University of Alabama at Birmingham. Accessed March 20, 2023. https://www.nscisc.uab.edu/Public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf
2. Macy E, Richter PK, Falkoff R, Zeiger R. Skin testing with penicilloate and penilloate prepared by an improved method: amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. J Allergy Clin Immunol. 1997;100(5):586-591. doi:10.1016/s0091-6749(97)70159-3 3. Dhopeshwarkar N, Sheikh A, Doan R, et al. Drug-induced anaphylaxis documented in electronic health records. J Allergy Clin Immunol Pract. 2019;7(1):103-111. doi:10.1016/j.jaip.2018.06.010
4. Evans CT, LaVela SL, Weaver FM, et al. Epidemiology of hospital-acquired infections in veterans with spinal cord injury and disorder. Infect Control Hosp Epidemiol. 2008;29(3):234-242. doi:10.1086/527509
5. Evans CT, Jump RL, Krein SL, et al. Setting a research agenda in prevention of healthcare-associated infections (HAIs) and multidrug-resistant organisms (MDROs) outside of acute care settings. Infect Control Hosp Epidemiol. 2018;39(2):210-213. doi:10.1017/ice.2017.291
6. Blumenthal KG, Peter JG, Trubiano JA, Phllips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183-198. doi:10.1016/S0140-6736(18)32218-9 7. Evans CT, Fitzpatrick MA, Jones MM, et al. Prevalence and factors associated with multidrug-resistant gram-negative organisms in patients with spinal cord injury. Infect Control Hosp Epidemiol. 2017;38(12):1464-1471. doi:10.1017/ice.2017.238 8. Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7. doi:10.1016/j.amjmed.2009.01.034
9. Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
10. Gomes E, Cardoso MF, Praça F, Gomes L, Mariño E, Demoly P. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy. 2004;34(10):1597-1601. doi:10.1111/j.1365-2222.2004.02070.x
Infectious diseases are the most common reason for rehospitalization among patients with spinal cord injuries (SCI), regardless of the number of years postinjury.1 The appropriate use and selection of antibiotics for properly diagnosed infectious diseases is especially important for this population. This principle helps to avoid the development of drug-resistant organisms and reduces the risk of recurrent infections, aligning with antibiotic stewardship.
Antibiotics are the most common class of drug allergies in the general population, and penicillin is the most frequently reported allergen (up to 10%).2 Prescription drug–induced anaphylaxis is severe and life threatening with a reported frequency of 1.1%. Penicillin and sulfonamide (46 and 15 per 10,000 patients, respectively) are the most common allergens.3 Although there is a significant difference between an adverse drug reaction (ADR) and true hypersensitivity, once documented in the electronic health record (EHR) as an allergy, this information deters use of the listed drugs.
Genitourinary, skin, and respiratory diseases are the leading causes for rehospitalization in patients with SCI.1 A large proportion of these are infectious in etiology and require antibiotic treatment. In fact, persons with SCI are at high risk for antibiotic overuse and hospital-acquired infection due to chronic bacteriuria, frequent health care exposure, implanted medical devices, and other factors.4 Concurrently, there is a crisis of antibiotic-resistant bacteria proliferation, described as a threat to patient safety and public health.5,6 Its severity is illustrated by the report that 38% of the cultures from patients with spinal cord injury are multidrug resistant gram-negative organisms.7
The SCI center at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, serves a high concentration of active-duty military members and veterans with SCI. A study that reviews the exact frequency of antibiotic drug allergies listed on the EHR would be a key first step to identify the magnitude of this issue. The results could guide investigation into differentiating true allergies from ADRs, thereby widening the options for potentially life-saving antibiotic treatment.
Methods
We performed a retrospective chart review of patients included in the local SCI registry between October 1, 2015, and September 30, 2017. We collected data on patient demographics (age, sex, race and ethnicity) and a description of patients’ injuries (International Standards for Neurological Classification of Spinal Cord Injury [ISNCSCI] and etiology of injury [traumatic vs atraumatic]). The outcomes included antibiotic allergy and ADRs.
In the EHR, allergies can be listed toward an antibiotic class or a specific antibiotic. An allergy to each specific antibiotic would be recorded separately; however, overlap among antibiotic classes was not duplicated. For example, if a subject has a listed antibiotic allergy to ceftriaxone and cefepime with listed reactions, we would record allergies to each of these antibiotics but would only report a single allergy to the cephalosporin subclass.
Since we did not differentiate hypersensitivity reactions (HSRs) from other ADRs, the reported reactions were grouped by signs and symptoms. There is a variety of terms used to report similar reactions, and best efforts were made to record the data as accurately as possible. Patient-reported history for risk stratification is a tool we used to group these historical reactions into high- vs low-risk for severe reactions. High-risk signs are those listed as anaphylaxis; anaphylactic reactions; angioedema presenting as swelling of mouth, eyes, lips, or tongue; blisters or ulcers involving the lips, mouth, eyes, urethra, vagina, or peeling skin; respiratory changes; shortness of breath; dyspnea; hypotension; or organ involvement (kidneys, lungs, liver).6
Inclusion criteria were all veterans who were diagnosed with tetraplegia or paraplegia and received annual evaluation between October 1, 2015, and September 30, 2017. We chose this period because it was the beginning of a financial year at the JAHVH SCI department using the SCI registry. The SCI annual evaluation is a routine practitioner encounter with the veteran, along with appropriate laboratory testing and imaging to follow up potential chronic health issues specific to patients with SCI. Annual evaluations provide an opportunity to maintain routine health screening and preventive care. Patients who had significant portions of data missing or missing elements of primary outcomes were excluded from analysis. The study was reviewed and approved by the University of South Florida Institutional Review Board (VA IRBNet #1573370-4 on September 9, 2019).
Results
Of 1866 patients reviewed, 207 (11.1%) were excluded due to missing data, resulting in 1659 records that were analyzed. Mean age was 64 years, and male to female ratio was about 10 to 1. Most of the SCI or diseases were classified as incomplete (n = 1249) per ISNCSCI (absence of sensory and motor function in the lowest sacral segments) compared with 373 classified as complete.
Of the 1659 patients, 494 (29.8%) had a recorded allergy to antibiotics. The most frequently recorded were 217 penicillin (13.1%), 159 sulfa drugs (9.6%), 75 fluoroquinolone (4.5%), 66 cephalosporin (4.0%), and 44 vancomycin (2.7%) allergies.
Discussion
In this study, we evaluated the frequency and characteristics of antibiotic allergies at a single SCI center to better identify potential areas for quality improvement when recording drug allergies. A study in the general population used self-reported methods to collect such information found about a 15% prevalence of antibiotic allergy, which was lower than the 29.8% prevalence noted in our study.8
Regarding the most common antibiotic allergies, one study reported allergy to penicillin in the EHR in 12.8% of patients at a major US regional health care system, while 13.1% of patients with SCI had documented allergy to penicillin in our study.9 Regarding the other antibiotic classes, the percentage of allergies were higher than those reported in the general population: sulfonamide (9.6% vs 7.4%), fluoroquinolones (4.5% vs 1.3%), and cephalosporins (4.0% vs 1.7%).10 The EHR appears to capture a much higher rate of antibiotic allergies than that in self-reported studies, such as a study of self-reported allergy in the general adult population in Portugal, where only 4.5% of patients reported allergy to any β-lactam medications.10
The prevalence of an antibiotic allergy could be affected by the health care setting and sex distribution. For example, the Zhou and colleagues’ study conducted in the Greater Boston area showed higher reported antibiotic rates than those in a study from a Southern California medical group. The higher proportion of tertiary referral patients in that specific network was suggested to be the cause of the difference.8,9 Our results in the SCI population are more comparable to that in a tertiary setting. This is consistent with the fact that persons with SCI generally have more exposure to antibiotics and consequently a higher reported rate of allergic reactions to antibiotics.
Similarly, the same study in Southern California noted that female patients use more antibiotics than do male patients, thus potentially contributing to higher rates of reported allergy toward all classes of antibiotics.8 Our study did not investigate antibiotic allergy by sex; however, the significantly higher proportion of male sex among the veteran population would have impacted these results.
Limitations
Our study was limited as a single-center retrospective study. However, our center is one of the major SCI specialty hubs, and the results should be somewhat reflective of those in the veterans with SCI population. Veterans under the US Department of Veterans Affairs (VA) medical care have the option to seek care or procedures in non-VA facilities. If allergies to antibiotics occurred outside of the VA system, there is no mechanism to automatically merge with the VA EHR allergy list, unless they are later recorded and added to the VA EHR. Thus, there is potential for underreporting.
Drug anaphylaxis incidence was noted to change over time.4,8,9 For example, a downtrend of reported antibiotic allergy was reported between 1990 and 2013.10 Our study only reflects an overall prevalence of a single cohort, without demonstration of relationship to time.
Lastly, this study did not aim to differentiate HSRs from other ADRs. This is exactly the point of the study, which investigated the frequency of EHR-recorded antibiotic allergies in our SCI population and reflects the issue with indiscriminate recording of ADRs and HSRs under the umbrella of allergy in the EHR. Further diagnosing true allergies should be considered in the SCI population after weighing the risks and benefits of assessment, aligning with the wishes of the veteran, obtaining informed consent, and addressing the cost-effectiveness of specific tests. We suggest that primary care practitioners work closely with allergy specialists to formulate a mechanism to diagnose various antibiotic allergic reactions, including serum tryptase, epicutaneous skin testing, intradermal skin testing, patch testing, delayed intradermal testing, and drug challenge as appropriate. It is also possible that in cases where very mild reactions/adverse effects of antibiotics were recorded in the EHR, the clinicians and veterans may discuss reintroducing the same antibiotics or proceeding with further testing if necessary. In contrast, the 12% of those with a high risk of severe allergic reactions to penicillin in our study would benefit from allergist evaluation and access to epinephrine auto-injectors at all times. Differentiating true allergy is the only clear way to deter unnecessary avoidance of first-line therapies for antibiotic treatment and avoid promotion of antibiotic resistance.
Future studies can analyze antibiotic allergy based on demographics, including sex and age difference, as well as exploring outpatient vs inpatient settings. Aside from prevalence, we hope to demonstrate antibiotic allergy over time, especially after integration of diagnostic allergy testing, to evaluate the impact to EHR-recorded allergies.
Conclusions
Almost 30% of patients with SCI had a recorded allergy to at least 1 antibiotic. The most common allergy was to penicillin, which is similar to what has previously been reported for the general adult US population. However, only 12% of those with a penicillin allergy were considered high risk of true allergic reactions. Consequently, there are opportunities to examine whether approaches to confirm true reactions (such as skin testing) would help to mitigate unnecessary avoidance of certain antibiotic classes due to mild ADRs, rather than a true allergy, in persons with SCI. This would be an important effort to combat both individual safety concerns and the public health crisis of antibiotic resistance. Given the available evidence, it is reasonable for SCI health care practitioners to discuss the potential risks and benefits of allergy testing with patients with SCI; this maintains a patient-centered approach that can ensure judicious use of antibiotics when necessary.
Acknowledgments
This material is based on work supported (or supported in part) with resources and the use of facilities at the James A. Haley Veterans’ Hospital
Infectious diseases are the most common reason for rehospitalization among patients with spinal cord injuries (SCI), regardless of the number of years postinjury.1 The appropriate use and selection of antibiotics for properly diagnosed infectious diseases is especially important for this population. This principle helps to avoid the development of drug-resistant organisms and reduces the risk of recurrent infections, aligning with antibiotic stewardship.
Antibiotics are the most common class of drug allergies in the general population, and penicillin is the most frequently reported allergen (up to 10%).2 Prescription drug–induced anaphylaxis is severe and life threatening with a reported frequency of 1.1%. Penicillin and sulfonamide (46 and 15 per 10,000 patients, respectively) are the most common allergens.3 Although there is a significant difference between an adverse drug reaction (ADR) and true hypersensitivity, once documented in the electronic health record (EHR) as an allergy, this information deters use of the listed drugs.
Genitourinary, skin, and respiratory diseases are the leading causes for rehospitalization in patients with SCI.1 A large proportion of these are infectious in etiology and require antibiotic treatment. In fact, persons with SCI are at high risk for antibiotic overuse and hospital-acquired infection due to chronic bacteriuria, frequent health care exposure, implanted medical devices, and other factors.4 Concurrently, there is a crisis of antibiotic-resistant bacteria proliferation, described as a threat to patient safety and public health.5,6 Its severity is illustrated by the report that 38% of the cultures from patients with spinal cord injury are multidrug resistant gram-negative organisms.7
The SCI center at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, serves a high concentration of active-duty military members and veterans with SCI. A study that reviews the exact frequency of antibiotic drug allergies listed on the EHR would be a key first step to identify the magnitude of this issue. The results could guide investigation into differentiating true allergies from ADRs, thereby widening the options for potentially life-saving antibiotic treatment.
Methods
We performed a retrospective chart review of patients included in the local SCI registry between October 1, 2015, and September 30, 2017. We collected data on patient demographics (age, sex, race and ethnicity) and a description of patients’ injuries (International Standards for Neurological Classification of Spinal Cord Injury [ISNCSCI] and etiology of injury [traumatic vs atraumatic]). The outcomes included antibiotic allergy and ADRs.
In the EHR, allergies can be listed toward an antibiotic class or a specific antibiotic. An allergy to each specific antibiotic would be recorded separately; however, overlap among antibiotic classes was not duplicated. For example, if a subject has a listed antibiotic allergy to ceftriaxone and cefepime with listed reactions, we would record allergies to each of these antibiotics but would only report a single allergy to the cephalosporin subclass.
Since we did not differentiate hypersensitivity reactions (HSRs) from other ADRs, the reported reactions were grouped by signs and symptoms. There is a variety of terms used to report similar reactions, and best efforts were made to record the data as accurately as possible. Patient-reported history for risk stratification is a tool we used to group these historical reactions into high- vs low-risk for severe reactions. High-risk signs are those listed as anaphylaxis; anaphylactic reactions; angioedema presenting as swelling of mouth, eyes, lips, or tongue; blisters or ulcers involving the lips, mouth, eyes, urethra, vagina, or peeling skin; respiratory changes; shortness of breath; dyspnea; hypotension; or organ involvement (kidneys, lungs, liver).6
Inclusion criteria were all veterans who were diagnosed with tetraplegia or paraplegia and received annual evaluation between October 1, 2015, and September 30, 2017. We chose this period because it was the beginning of a financial year at the JAHVH SCI department using the SCI registry. The SCI annual evaluation is a routine practitioner encounter with the veteran, along with appropriate laboratory testing and imaging to follow up potential chronic health issues specific to patients with SCI. Annual evaluations provide an opportunity to maintain routine health screening and preventive care. Patients who had significant portions of data missing or missing elements of primary outcomes were excluded from analysis. The study was reviewed and approved by the University of South Florida Institutional Review Board (VA IRBNet #1573370-4 on September 9, 2019).
Results
Of 1866 patients reviewed, 207 (11.1%) were excluded due to missing data, resulting in 1659 records that were analyzed. Mean age was 64 years, and male to female ratio was about 10 to 1. Most of the SCI or diseases were classified as incomplete (n = 1249) per ISNCSCI (absence of sensory and motor function in the lowest sacral segments) compared with 373 classified as complete.
Of the 1659 patients, 494 (29.8%) had a recorded allergy to antibiotics. The most frequently recorded were 217 penicillin (13.1%), 159 sulfa drugs (9.6%), 75 fluoroquinolone (4.5%), 66 cephalosporin (4.0%), and 44 vancomycin (2.7%) allergies.
Discussion
In this study, we evaluated the frequency and characteristics of antibiotic allergies at a single SCI center to better identify potential areas for quality improvement when recording drug allergies. A study in the general population used self-reported methods to collect such information found about a 15% prevalence of antibiotic allergy, which was lower than the 29.8% prevalence noted in our study.8
Regarding the most common antibiotic allergies, one study reported allergy to penicillin in the EHR in 12.8% of patients at a major US regional health care system, while 13.1% of patients with SCI had documented allergy to penicillin in our study.9 Regarding the other antibiotic classes, the percentage of allergies were higher than those reported in the general population: sulfonamide (9.6% vs 7.4%), fluoroquinolones (4.5% vs 1.3%), and cephalosporins (4.0% vs 1.7%).10 The EHR appears to capture a much higher rate of antibiotic allergies than that in self-reported studies, such as a study of self-reported allergy in the general adult population in Portugal, where only 4.5% of patients reported allergy to any β-lactam medications.10
The prevalence of an antibiotic allergy could be affected by the health care setting and sex distribution. For example, the Zhou and colleagues’ study conducted in the Greater Boston area showed higher reported antibiotic rates than those in a study from a Southern California medical group. The higher proportion of tertiary referral patients in that specific network was suggested to be the cause of the difference.8,9 Our results in the SCI population are more comparable to that in a tertiary setting. This is consistent with the fact that persons with SCI generally have more exposure to antibiotics and consequently a higher reported rate of allergic reactions to antibiotics.
Similarly, the same study in Southern California noted that female patients use more antibiotics than do male patients, thus potentially contributing to higher rates of reported allergy toward all classes of antibiotics.8 Our study did not investigate antibiotic allergy by sex; however, the significantly higher proportion of male sex among the veteran population would have impacted these results.
Limitations
Our study was limited as a single-center retrospective study. However, our center is one of the major SCI specialty hubs, and the results should be somewhat reflective of those in the veterans with SCI population. Veterans under the US Department of Veterans Affairs (VA) medical care have the option to seek care or procedures in non-VA facilities. If allergies to antibiotics occurred outside of the VA system, there is no mechanism to automatically merge with the VA EHR allergy list, unless they are later recorded and added to the VA EHR. Thus, there is potential for underreporting.
Drug anaphylaxis incidence was noted to change over time.4,8,9 For example, a downtrend of reported antibiotic allergy was reported between 1990 and 2013.10 Our study only reflects an overall prevalence of a single cohort, without demonstration of relationship to time.
Lastly, this study did not aim to differentiate HSRs from other ADRs. This is exactly the point of the study, which investigated the frequency of EHR-recorded antibiotic allergies in our SCI population and reflects the issue with indiscriminate recording of ADRs and HSRs under the umbrella of allergy in the EHR. Further diagnosing true allergies should be considered in the SCI population after weighing the risks and benefits of assessment, aligning with the wishes of the veteran, obtaining informed consent, and addressing the cost-effectiveness of specific tests. We suggest that primary care practitioners work closely with allergy specialists to formulate a mechanism to diagnose various antibiotic allergic reactions, including serum tryptase, epicutaneous skin testing, intradermal skin testing, patch testing, delayed intradermal testing, and drug challenge as appropriate. It is also possible that in cases where very mild reactions/adverse effects of antibiotics were recorded in the EHR, the clinicians and veterans may discuss reintroducing the same antibiotics or proceeding with further testing if necessary. In contrast, the 12% of those with a high risk of severe allergic reactions to penicillin in our study would benefit from allergist evaluation and access to epinephrine auto-injectors at all times. Differentiating true allergy is the only clear way to deter unnecessary avoidance of first-line therapies for antibiotic treatment and avoid promotion of antibiotic resistance.
Future studies can analyze antibiotic allergy based on demographics, including sex and age difference, as well as exploring outpatient vs inpatient settings. Aside from prevalence, we hope to demonstrate antibiotic allergy over time, especially after integration of diagnostic allergy testing, to evaluate the impact to EHR-recorded allergies.
Conclusions
Almost 30% of patients with SCI had a recorded allergy to at least 1 antibiotic. The most common allergy was to penicillin, which is similar to what has previously been reported for the general adult US population. However, only 12% of those with a penicillin allergy were considered high risk of true allergic reactions. Consequently, there are opportunities to examine whether approaches to confirm true reactions (such as skin testing) would help to mitigate unnecessary avoidance of certain antibiotic classes due to mild ADRs, rather than a true allergy, in persons with SCI. This would be an important effort to combat both individual safety concerns and the public health crisis of antibiotic resistance. Given the available evidence, it is reasonable for SCI health care practitioners to discuss the potential risks and benefits of allergy testing with patients with SCI; this maintains a patient-centered approach that can ensure judicious use of antibiotics when necessary.
Acknowledgments
This material is based on work supported (or supported in part) with resources and the use of facilities at the James A. Haley Veterans’ Hospital
References
1. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Model Systems. 2016 Annual Report –Complete Public Version. University of Alabama at Birmingham. Accessed March 20, 2023. https://www.nscisc.uab.edu/Public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf
2. Macy E, Richter PK, Falkoff R, Zeiger R. Skin testing with penicilloate and penilloate prepared by an improved method: amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. J Allergy Clin Immunol. 1997;100(5):586-591. doi:10.1016/s0091-6749(97)70159-3 3. Dhopeshwarkar N, Sheikh A, Doan R, et al. Drug-induced anaphylaxis documented in electronic health records. J Allergy Clin Immunol Pract. 2019;7(1):103-111. doi:10.1016/j.jaip.2018.06.010
4. Evans CT, LaVela SL, Weaver FM, et al. Epidemiology of hospital-acquired infections in veterans with spinal cord injury and disorder. Infect Control Hosp Epidemiol. 2008;29(3):234-242. doi:10.1086/527509
5. Evans CT, Jump RL, Krein SL, et al. Setting a research agenda in prevention of healthcare-associated infections (HAIs) and multidrug-resistant organisms (MDROs) outside of acute care settings. Infect Control Hosp Epidemiol. 2018;39(2):210-213. doi:10.1017/ice.2017.291
6. Blumenthal KG, Peter JG, Trubiano JA, Phllips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183-198. doi:10.1016/S0140-6736(18)32218-9 7. Evans CT, Fitzpatrick MA, Jones MM, et al. Prevalence and factors associated with multidrug-resistant gram-negative organisms in patients with spinal cord injury. Infect Control Hosp Epidemiol. 2017;38(12):1464-1471. doi:10.1017/ice.2017.238 8. Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7. doi:10.1016/j.amjmed.2009.01.034
9. Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
10. Gomes E, Cardoso MF, Praça F, Gomes L, Mariño E, Demoly P. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy. 2004;34(10):1597-1601. doi:10.1111/j.1365-2222.2004.02070.x
References
1. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Model Systems. 2016 Annual Report –Complete Public Version. University of Alabama at Birmingham. Accessed March 20, 2023. https://www.nscisc.uab.edu/Public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf
2. Macy E, Richter PK, Falkoff R, Zeiger R. Skin testing with penicilloate and penilloate prepared by an improved method: amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. J Allergy Clin Immunol. 1997;100(5):586-591. doi:10.1016/s0091-6749(97)70159-3 3. Dhopeshwarkar N, Sheikh A, Doan R, et al. Drug-induced anaphylaxis documented in electronic health records. J Allergy Clin Immunol Pract. 2019;7(1):103-111. doi:10.1016/j.jaip.2018.06.010
4. Evans CT, LaVela SL, Weaver FM, et al. Epidemiology of hospital-acquired infections in veterans with spinal cord injury and disorder. Infect Control Hosp Epidemiol. 2008;29(3):234-242. doi:10.1086/527509
5. Evans CT, Jump RL, Krein SL, et al. Setting a research agenda in prevention of healthcare-associated infections (HAIs) and multidrug-resistant organisms (MDROs) outside of acute care settings. Infect Control Hosp Epidemiol. 2018;39(2):210-213. doi:10.1017/ice.2017.291
6. Blumenthal KG, Peter JG, Trubiano JA, Phllips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183-198. doi:10.1016/S0140-6736(18)32218-9 7. Evans CT, Fitzpatrick MA, Jones MM, et al. Prevalence and factors associated with multidrug-resistant gram-negative organisms in patients with spinal cord injury. Infect Control Hosp Epidemiol. 2017;38(12):1464-1471. doi:10.1017/ice.2017.238 8. Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7. doi:10.1016/j.amjmed.2009.01.034
9. Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
10. Gomes E, Cardoso MF, Praça F, Gomes L, Mariño E, Demoly P. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy. 2004;34(10):1597-1601. doi:10.1111/j.1365-2222.2004.02070.x
Open Clinical Trials for Patients With Cancer
Prostate Cancer
18F-DCFPyL PET/CT Impact on Treatment Strategies for Patients With Prostate Cancer (PROSPYL)
The main purpose of this phase II trial study is to determine whether a positron emission tomography (PET)/computed tomography (CT) scan using 18F-DCFPyL affects the clinical management plan in veterans. In this study, the management plan prior to and after 18F-DCFPyL PET/CT will be recorded by specific questionnaires and corresponding changes in management will be analyzed. The scan will be used to see how the disease has spread. Both the treatment strategies and probable disease outcomes as relevant to clinical endpoints will be assessed. This study is open to veterans only.
ID: NCT04390880
Sponsor: VA Greater Los Angeles Healthcare System
Location: VA Greater Los Angeles Healthcare System
Patient Decision-Making About Precision Oncology in Veterans With Advanced Prostate Cancer
This project proposes to understand and improve veterans’ decision-making in precision oncology (germline testing, somatic tumor testing, and targeted therapy) for advanced prostate cancer. As precision oncology expands, a comprehensive strategy to support patient informed decision-making has not been developed.
ID: NCT05396872
Sponsor; Collaborator: University of California, San Francisco; US Department of Defense
Location: San Francisco VA Medical Center
Intramuscular Mechanisms of Androgen Deprivation-Related Sarcopenia
Prostate cancer is the most common cancer among men and is even more common in the military and veteran population. For patients with advanced prostate cancer, the most common treatment includes lowering the levels of the hormone testosterone as much as possible, which is called androgen deprivation therapy (ADT). Unfortunately, ADT also causes patients to be fatigued, weak, and to lose muscle. This is often referred to as sarcopenia, and it leads to falls, poor quality of life, and higher risk of death. Currently, there is no treatment for sarcopenia because investigators do not understand the mechanisms that cause it. The mitochondria is the part of the cells responsible for providing energy to muscles but to date the investigators do not know if it is affected in prostate cancer patients with sarcopenia due to ADT. The overall goal of this proposal is to establish if the mitochondria is responsible for sarcopenia in patients with prostate cancer receiving ADT. The investigators will measure mitochondrial function, muscle mass and strength, and feelings of fatigue and quality of life in patients with prostate cancer before starting and after 6 months of ADT.
ID: NCT03867357
Sponsor; Collaborator: Seattle Institute for Biomedical and Clinical Research; US Department of Defense
Location: VA Puget Sound Health Care System
VA Seamless Phase II/III Randomized Trial of Standard Systemic Therapy With or Without PET-Directed Local Therapy for OligoRecurrent Prostate Cancer (VA STARPORT)
The primary goal of this study is to determine if adding PET-directed local therapy improves disease control compared to standard systemic therapy alone (SST) in veterans with oligorecurrent prostate cancer on PET/CT. The investigators will conduct a multi-institutional phase II/III randomized trial comparing SST with or without PET-directed local therapy using radiation or surgery to all metastases and if a local recurrence is present.
ID: NCT04787744
Sponsor: VA Office of Research and Development
Locations: VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VA Medical Center, VA Boston Healthcare System Jamaica Plain Campus, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Kansas City VA Medical Center, St. Louis VA Medical Center John Cochran Division, East Orange Campus of the VA New Jersey Health Care System, Durham VA Medical Center, Louis Stokes VA Medical Center, Michael E. DeBakey VA Medical Center, Hunter Holmes McGuire VA Medical Center, Clement J. Zablocki VA Medical Center
Standard Systemic Therapy With or Without Definitive Treatment in Treating Participants With Metastatic Prostate Cancer
This phase III trial studies how well standard systemic therapy with or without definitive treatment (prostate removal surgery or radiation therapy) works in treating participants with prostate cancer that has spread to other places in the body. The addition of prostate removal surgery or radiation therapy to standard systemic therapy for prostate cancer may lower the chance of the cancer growing or spreading.
ID: NCT03678025
Sponsor; Collaborator: Southwest Oncology Group; National Cancer Institute (NCI)
Locations: 328 sites, including Tibor Rubin VA Medical Center, Atlanta VA Medical Center, James J. Peters VA Medical Center, Michael E. DeBakey VA Medical Center, and Audie L. Murphy VA Hospital
A Clinical Study Evaluating the Benefit of Adding Rucaparib to Enzalutamide for Men With Metastatic Prostate Cancer That Has Become Resistant to Testosterone-Deprivation Therapy (CASPAR)
This randomized, placebo-controlled, phase III trial is evaluating the benefit of rucaparib and enzalutamide combination therapy vs enzalutamide alone for the treatment of men with prostate cancer that has spread to other places in the body (metastatic) and has become resistant to testosterone-deprivation therapy (castration-resistant). Enzalutamide helps fight prostate cancer by blocking the use of testosterone by the tumor cells for growth. Poly adenosine diphosphate (ADP)-ribose polymerase (PARP) inhibitors, such as rucaparib, fight prostate cancer by prevent tumor cells from repairing their DNA. Giving enzalutamide and rucaparib may make patients live longer or prevent their cancer from growing or spreading for a longer time, or both. It may also help doctors learn if a mutation in any of the homologous recombination DNA repair genes is helpful to decide which treatment is best for the patient.
ID: NCT04455750
Sponsor; Collaborator: Alliance for Clinical Trials in Oncology; National Cancer Institute (NCI)
Locations: 413 sites
Digitally Captured Activity Data and PROs to Monitor Physical Function in Prostate Cancer Patients (DigiPRO)
Physical function is a known predictor of quality of life in advanced prostate cancer patients and key measure of treatment tolerability. While treatment with androgen deprivation therapy (ADT) improves survival, it is associated with significant toxicities that lead to physical function (PF) decline. The average age of incident prostate cancer is 66 years, and in this older group of men, chronic comorbid conditions often co-occur with diagnosis, further adding to the risk for PF decline. With over 2.9 million prostate cancer survivors in the US, there is an increasing demand for adequate symptom monitoring and PF assessment throughout cancer care. However, there are currently no validated methods to systematically evaluate and predict PF decline. Thus, the overarching objective of this proposal is to determine whether the use of wearable technology to monitor objective daily activity combined with routine symptom reporting can predict PF decline. To accomplish this, we propose a mixed-methods approach that will provide quantitative information to help identify PC survivors at higher risk for PF decline as well as a qualitative aim gain a deeper understanding of the perceived relationships that PC survivors have with their physical activity levels and treatment symptoms.
ID: NCT04575402
Sponsor; Collaborator: Cedars-Sinai Medical Center; US Department of Defense
Location: Cedars Sinai Medical Center
The BurnAlong Pilot Study for Adolescent and Young Adult Cancer Survivors
The purpose of this prospective, interventional, single-arm pilot study is to evaluate whether virtually delivered group-based physical activity is feasible for adolescent and young adult (AYA) cancer survivors. AYAs who were diagnosed with cancer and have completed cancer treatment will be recruited for this study. This study will enroll 20 participants in total and will last approximately 3 months.
ID: NCT05131815
Sponsor; Collaborator: Cedars-Sinai Medical Center; Walter Reed National Military Medical Center
Location: Cedars-Sinai Medical Center
Lung Cancer
DECAMP 1 PLUS: Prediction of Lung Cancer Using Noninvasive Biomarkers
The Detection of Early lung Cancer Among Military Personnel (DECAMP) consortium is a multidisciplinary and translational research program for lung cancer early detection. DECAMP 1 PLUS aims to improve the efficiency of the diagnostic evaluation of patients with indeterminate pulmonary nodules (8-25 mm). Molecular biomarkers for lung cancer diagnosis measured in minimally invasive and noninvasive biospecimens may be able to distinguish between malignant or benign indeterminate pulmonary nodules in high-risk smokers. Ultimately, this study aims to validate molecular as well as clinical and imaging biomarkers of lung cancer in individuals with indeterminate lung nodules.
ID: NCT04165564
Sponsor: Boston University
Locations: 3 VA medical centers (VA Greater LA Healthcare System, VA Boston Healthcare System, and VA Tennessee Valley Healthcare System), 3 military treatment facilities (Naval Medical Center San Diego, Walter Reed National Military Medical Center, and Naval Medical Center Portsmouth) and 12 academic hospitals
DECAMP-2: Screening of Patients With Early Stage Lung Cancer or at High Risk for Developing Lung Cancer (DECAMP-2)
The goal of this project is to improve lung cancer screening in high-risk individuals by identifying biomarkers of preclinical disease and disease risk that are measured in minimally invasive and noninvasive biospecimens. Existing biomarkers for lung cancer diagnosis as well as new biomarkers discovered specifically in this clinical setting will be examined. Biomarkers that identify individuals at highest risk for being diagnosed with lung cancer prior to the appearance of concerning symptoms could increase the utility of lung cancer surveillance and the efficiency of lung cancer chemoprevention clinical trials. Achieving these goals would improve the detection and treatment of early-stage and incipient lung cancer, while restricting the risk of these procedures to those individuals who currently exhibit the early molecular warning signs of impending disease.
ID: NCT02504697
Sponsor: Boston University
Locations: VA medical centers (including Los Angeles VA Healthcare System, Boston VA Research Institute, Inc, Philadelphia VA Medical Center, Veterans Research Foundation of Pittsburgh, and VA North Texas Health Care System), 4 military treatment facilities (Naval Medical Center San Diego, Walter Reed National Military Medical Center, San Antonio Military Medical Center, and Naval Medical Center Portsmouth), and 4 academic hospitals
Improving Decision-Making Encounters in Lung Cancer Using a Low-Literacy Conversation Tool (iDECIDE)
This clinical trial evaluates the effectiveness of a conversation tool on patient-centered health and decision-making outcomes in patients with lung cancer making treatment decisions. This research is being conducted to help doctors understand the information patients need to participate in shared decision-making about their lung cancer treatment options. The focus of this research is to study how patients choose lung cancer treatment options and the information needed to make that choice, with a focus on patients with lower health literacy.
ID: NCT05407168
Sponsor: Oregon Health & Science University Knight Cancer Institute
Locations: Portland VA Medical Center and Oregon Health & Science University Knight Cancer Institute
VA Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR)
The standard of care for stage I non–small cell lung cancer has historically been surgical resection in patients who are medically fit to tolerate an operation. Recent data now suggest that stereotactic radiotherapy may be a suitable alternative. This includes the results from a pooled analysis of 2 incomplete phase III studies that reported a 15% overall survival advantage with stereotactic radiotherapy at 3 years. While these data are promising, the median follow-up period was short, the results underpowered, and the findings were in contradiction to multiple retrospective studies that demonstrate the outcomes with surgery are likely equal or superior. Therefore, the herein trial aims to evaluate these 2 treatments in a prospective randomized fashion with a goal to compare the overall survival beyond 5 years. It has been designed to enroll patients who have a long life expectancy and are fit enough to tolerate an anatomic pulmonary resection with intraoperative lymph node sampling.
This study is designed to open at VA medical centers with expertise in both treatments. The recruitment process includes shared decision making and multidisciplinary evaluations with lung cancer specialists. Mandatory evaluations before randomization include tissue confirmation of NSCLC, staging with FDG-PET/CT, and biopsies of all hilar and/or mediastinal lymph nodes > 10 mm that have a SUV > 2.5. Prerandomization elective lymph node sampling is strongly encouraged, but not required. Following treatment, patients will be followed for a minimum of 5 years.
ID: NCT02984761
Sponsor: VA Office of Research and Development
Locations: 17 VA medical centers, including VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Miami VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VA Medical Center, Baltimore VA Medical Center, VA Boston Healthcare System Jamaica Plain Campus, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Durham VA Medical Center, Louis Stokes VA Medical Center, Corporal Micheal J. Crescenz VA Medical Center, VA Pittsburgh Healthcare System University Drive Division, Michael E. DeBakey VA Medical Center, Hunter Holmes McGuire VA Medical Center, and Clement J. Zablocki VA Medical Center
Utility of CAML as Diagnostic for Early Stage Lung Cancer
The primary objective of this study is to determine the prevalence of cancer associated macrophage-like cells (CAMLS) in patients with pulmonary nodules. Secondary objectives include the following: determine the positive and negative predictive value of CAMLS in patients with pulmonary nodules who undergo biopsy; model combinations of clinical factors with the presence/absence of CAMLS to refine strategies for assessment of patients with pulmonary nodules; and evaluate whether these measures result in enhanced T-cell activity and/or natural killer cell function and number.
ID: NCT03992183
Sponsor; Collaborators: Fox Chase Cancer Center; US Department of Defense
Locations: Corporal Michael J. Crescenz VA Medical Center and Fox Chase Cancer Center
PROSPECT - Profiling of Resistance Patterns & Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax and Therapeutic Target Identification
This study will use therapeutic target-focused (TTF) profiling, genome-wide mRNA profiling, and assessments of tumor phosphopeptides and DNA that are shed into the bloodstream to define how various molecular factors alone and in combination relate to resistance to therapy, to prognosis, and to metastatic patterns at relapse. This study will examine how the presence of factors that drive cell growth, antagonize apoptosis, or confer resistance in other ways may counter the effect of systemic therapies and/or promote rapid tumor recurrence. In this way, the investigators will identify new, previously unappreciated potential therapeutic targets while also identifying which targets are most likely to increase resistance to therapy and worsen prognosis.
ID: NCT05049837
Sponsor; Collaborators: MD Anderson Cancer Center; US Department of Defense, National Institutes of Health (NIH), and National Cancer Institute (NCI)
Location: MD Anderson Cancer Center
Tribally Engaged Approaches to Lung Screening (TEALS)
Lung cancer is the leading cause of cancer mortality among American Indians and Alaska Natives (AI/AN), and AI/AN have worse lung cancer incidence rates, survival, and death compared to the general population. Because lung cancer screening (LCS) with low-dose computed tomography (LDCT) has been shown to reduce lung cancer mortality by roughly 20%, the US Preventive Services Task Force now recommends LCS for persons aged 55 to 80 years who meet specific eligibility criteria (grade-B evidence), and subsequently the Center for Medicare and Medicaid Services (CMS) opted to cover this test. However, the uptake of LCS implementation has been slow in most health care systems, and LCS implementation among AI/AN has never been studied.
To address this knowledge, the Tribally Engaged Approaches to Lung Screening (TEALS) study, a collaborative effort between the Choctaw Nation of Oklahoma, the Stephenson Cancer Center, and the University of Oklahoma Health Sciences Center, will address the following over the course of 5 years: conduct focus groups and semistructured interviews with Choctaw Nation Health Services Authority (CNHSA) patients, clinicians, and health administrators to elucidate individual- and system-level barriers and facilitators that affect the implementation of LCS; develop an LCS care coordination intervention that will identify eligible persons for LCS, help these patients navigate the screening process, and link them with smoking cessation services, when applicable; measure the impact of the TEALS intervention on the receipt of screening and a set of patient- and practice-level outcomes by conducting a cluster-randomized clinical trial of LCS implementation; and disseminate the TEALS program to other researchers and healthcare systems that serve AI/AN patients. TEALS will bridge the gap between evidence and clinical practice for LCS in a high-need, low-resource setting by intervening at the level of the healthcare system.
System-level interventions for guideline implementation tend to be understudied compared to those evaluating individual-level, behavioral interventions. However, the careful development and evaluation of an LCS screening program at the level of the healthcare system would be critical to ensure that more patients can receive LCS. Our research will create a critically needed platform from which future studies could be launched that will examine how to tailor the application of the LCS guideline to the individual preferences of AI/AN patients. TEALS will establish an effective LCS program in a tribal system and thus provide a direct benefit to the Choctaw Nation by increasing LCS participation. TEALS will serve as a blueprint for establishing a sustainable and accessible infrastructure for LCS in AI/AN and other community health systems. By increasing screening for early stage lung cancer, TEALS could ultimately reduce lung cancer mortality in AI/AN communities.
ID: NCT04948060
Sponsor; Collaborator: University of Oklahoma; Choctaw Nation of Oklahoma
Location: University of Oklahoma Health Sciences Center
Prostate Cancer
18F-DCFPyL PET/CT Impact on Treatment Strategies for Patients With Prostate Cancer (PROSPYL)
The main purpose of this phase II trial study is to determine whether a positron emission tomography (PET)/computed tomography (CT) scan using 18F-DCFPyL affects the clinical management plan in veterans. In this study, the management plan prior to and after 18F-DCFPyL PET/CT will be recorded by specific questionnaires and corresponding changes in management will be analyzed. The scan will be used to see how the disease has spread. Both the treatment strategies and probable disease outcomes as relevant to clinical endpoints will be assessed. This study is open to veterans only.
ID: NCT04390880
Sponsor: VA Greater Los Angeles Healthcare System
Location: VA Greater Los Angeles Healthcare System
Patient Decision-Making About Precision Oncology in Veterans With Advanced Prostate Cancer
This project proposes to understand and improve veterans’ decision-making in precision oncology (germline testing, somatic tumor testing, and targeted therapy) for advanced prostate cancer. As precision oncology expands, a comprehensive strategy to support patient informed decision-making has not been developed.
ID: NCT05396872
Sponsor; Collaborator: University of California, San Francisco; US Department of Defense
Location: San Francisco VA Medical Center
Intramuscular Mechanisms of Androgen Deprivation-Related Sarcopenia
Prostate cancer is the most common cancer among men and is even more common in the military and veteran population. For patients with advanced prostate cancer, the most common treatment includes lowering the levels of the hormone testosterone as much as possible, which is called androgen deprivation therapy (ADT). Unfortunately, ADT also causes patients to be fatigued, weak, and to lose muscle. This is often referred to as sarcopenia, and it leads to falls, poor quality of life, and higher risk of death. Currently, there is no treatment for sarcopenia because investigators do not understand the mechanisms that cause it. The mitochondria is the part of the cells responsible for providing energy to muscles but to date the investigators do not know if it is affected in prostate cancer patients with sarcopenia due to ADT. The overall goal of this proposal is to establish if the mitochondria is responsible for sarcopenia in patients with prostate cancer receiving ADT. The investigators will measure mitochondrial function, muscle mass and strength, and feelings of fatigue and quality of life in patients with prostate cancer before starting and after 6 months of ADT.
ID: NCT03867357
Sponsor; Collaborator: Seattle Institute for Biomedical and Clinical Research; US Department of Defense
Location: VA Puget Sound Health Care System
VA Seamless Phase II/III Randomized Trial of Standard Systemic Therapy With or Without PET-Directed Local Therapy for OligoRecurrent Prostate Cancer (VA STARPORT)
The primary goal of this study is to determine if adding PET-directed local therapy improves disease control compared to standard systemic therapy alone (SST) in veterans with oligorecurrent prostate cancer on PET/CT. The investigators will conduct a multi-institutional phase II/III randomized trial comparing SST with or without PET-directed local therapy using radiation or surgery to all metastases and if a local recurrence is present.
ID: NCT04787744
Sponsor: VA Office of Research and Development
Locations: VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VA Medical Center, VA Boston Healthcare System Jamaica Plain Campus, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Kansas City VA Medical Center, St. Louis VA Medical Center John Cochran Division, East Orange Campus of the VA New Jersey Health Care System, Durham VA Medical Center, Louis Stokes VA Medical Center, Michael E. DeBakey VA Medical Center, Hunter Holmes McGuire VA Medical Center, Clement J. Zablocki VA Medical Center
Standard Systemic Therapy With or Without Definitive Treatment in Treating Participants With Metastatic Prostate Cancer
This phase III trial studies how well standard systemic therapy with or without definitive treatment (prostate removal surgery or radiation therapy) works in treating participants with prostate cancer that has spread to other places in the body. The addition of prostate removal surgery or radiation therapy to standard systemic therapy for prostate cancer may lower the chance of the cancer growing or spreading.
ID: NCT03678025
Sponsor; Collaborator: Southwest Oncology Group; National Cancer Institute (NCI)
Locations: 328 sites, including Tibor Rubin VA Medical Center, Atlanta VA Medical Center, James J. Peters VA Medical Center, Michael E. DeBakey VA Medical Center, and Audie L. Murphy VA Hospital
A Clinical Study Evaluating the Benefit of Adding Rucaparib to Enzalutamide for Men With Metastatic Prostate Cancer That Has Become Resistant to Testosterone-Deprivation Therapy (CASPAR)
This randomized, placebo-controlled, phase III trial is evaluating the benefit of rucaparib and enzalutamide combination therapy vs enzalutamide alone for the treatment of men with prostate cancer that has spread to other places in the body (metastatic) and has become resistant to testosterone-deprivation therapy (castration-resistant). Enzalutamide helps fight prostate cancer by blocking the use of testosterone by the tumor cells for growth. Poly adenosine diphosphate (ADP)-ribose polymerase (PARP) inhibitors, such as rucaparib, fight prostate cancer by prevent tumor cells from repairing their DNA. Giving enzalutamide and rucaparib may make patients live longer or prevent their cancer from growing or spreading for a longer time, or both. It may also help doctors learn if a mutation in any of the homologous recombination DNA repair genes is helpful to decide which treatment is best for the patient.
ID: NCT04455750
Sponsor; Collaborator: Alliance for Clinical Trials in Oncology; National Cancer Institute (NCI)
Locations: 413 sites
Digitally Captured Activity Data and PROs to Monitor Physical Function in Prostate Cancer Patients (DigiPRO)
Physical function is a known predictor of quality of life in advanced prostate cancer patients and key measure of treatment tolerability. While treatment with androgen deprivation therapy (ADT) improves survival, it is associated with significant toxicities that lead to physical function (PF) decline. The average age of incident prostate cancer is 66 years, and in this older group of men, chronic comorbid conditions often co-occur with diagnosis, further adding to the risk for PF decline. With over 2.9 million prostate cancer survivors in the US, there is an increasing demand for adequate symptom monitoring and PF assessment throughout cancer care. However, there are currently no validated methods to systematically evaluate and predict PF decline. Thus, the overarching objective of this proposal is to determine whether the use of wearable technology to monitor objective daily activity combined with routine symptom reporting can predict PF decline. To accomplish this, we propose a mixed-methods approach that will provide quantitative information to help identify PC survivors at higher risk for PF decline as well as a qualitative aim gain a deeper understanding of the perceived relationships that PC survivors have with their physical activity levels and treatment symptoms.
ID: NCT04575402
Sponsor; Collaborator: Cedars-Sinai Medical Center; US Department of Defense
Location: Cedars Sinai Medical Center
The BurnAlong Pilot Study for Adolescent and Young Adult Cancer Survivors
The purpose of this prospective, interventional, single-arm pilot study is to evaluate whether virtually delivered group-based physical activity is feasible for adolescent and young adult (AYA) cancer survivors. AYAs who were diagnosed with cancer and have completed cancer treatment will be recruited for this study. This study will enroll 20 participants in total and will last approximately 3 months.
ID: NCT05131815
Sponsor; Collaborator: Cedars-Sinai Medical Center; Walter Reed National Military Medical Center
Location: Cedars-Sinai Medical Center
Lung Cancer
DECAMP 1 PLUS: Prediction of Lung Cancer Using Noninvasive Biomarkers
The Detection of Early lung Cancer Among Military Personnel (DECAMP) consortium is a multidisciplinary and translational research program for lung cancer early detection. DECAMP 1 PLUS aims to improve the efficiency of the diagnostic evaluation of patients with indeterminate pulmonary nodules (8-25 mm). Molecular biomarkers for lung cancer diagnosis measured in minimally invasive and noninvasive biospecimens may be able to distinguish between malignant or benign indeterminate pulmonary nodules in high-risk smokers. Ultimately, this study aims to validate molecular as well as clinical and imaging biomarkers of lung cancer in individuals with indeterminate lung nodules.
ID: NCT04165564
Sponsor: Boston University
Locations: 3 VA medical centers (VA Greater LA Healthcare System, VA Boston Healthcare System, and VA Tennessee Valley Healthcare System), 3 military treatment facilities (Naval Medical Center San Diego, Walter Reed National Military Medical Center, and Naval Medical Center Portsmouth) and 12 academic hospitals
DECAMP-2: Screening of Patients With Early Stage Lung Cancer or at High Risk for Developing Lung Cancer (DECAMP-2)
The goal of this project is to improve lung cancer screening in high-risk individuals by identifying biomarkers of preclinical disease and disease risk that are measured in minimally invasive and noninvasive biospecimens. Existing biomarkers for lung cancer diagnosis as well as new biomarkers discovered specifically in this clinical setting will be examined. Biomarkers that identify individuals at highest risk for being diagnosed with lung cancer prior to the appearance of concerning symptoms could increase the utility of lung cancer surveillance and the efficiency of lung cancer chemoprevention clinical trials. Achieving these goals would improve the detection and treatment of early-stage and incipient lung cancer, while restricting the risk of these procedures to those individuals who currently exhibit the early molecular warning signs of impending disease.
ID: NCT02504697
Sponsor: Boston University
Locations: VA medical centers (including Los Angeles VA Healthcare System, Boston VA Research Institute, Inc, Philadelphia VA Medical Center, Veterans Research Foundation of Pittsburgh, and VA North Texas Health Care System), 4 military treatment facilities (Naval Medical Center San Diego, Walter Reed National Military Medical Center, San Antonio Military Medical Center, and Naval Medical Center Portsmouth), and 4 academic hospitals
Improving Decision-Making Encounters in Lung Cancer Using a Low-Literacy Conversation Tool (iDECIDE)
This clinical trial evaluates the effectiveness of a conversation tool on patient-centered health and decision-making outcomes in patients with lung cancer making treatment decisions. This research is being conducted to help doctors understand the information patients need to participate in shared decision-making about their lung cancer treatment options. The focus of this research is to study how patients choose lung cancer treatment options and the information needed to make that choice, with a focus on patients with lower health literacy.
ID: NCT05407168
Sponsor: Oregon Health & Science University Knight Cancer Institute
Locations: Portland VA Medical Center and Oregon Health & Science University Knight Cancer Institute
VA Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR)
The standard of care for stage I non–small cell lung cancer has historically been surgical resection in patients who are medically fit to tolerate an operation. Recent data now suggest that stereotactic radiotherapy may be a suitable alternative. This includes the results from a pooled analysis of 2 incomplete phase III studies that reported a 15% overall survival advantage with stereotactic radiotherapy at 3 years. While these data are promising, the median follow-up period was short, the results underpowered, and the findings were in contradiction to multiple retrospective studies that demonstrate the outcomes with surgery are likely equal or superior. Therefore, the herein trial aims to evaluate these 2 treatments in a prospective randomized fashion with a goal to compare the overall survival beyond 5 years. It has been designed to enroll patients who have a long life expectancy and are fit enough to tolerate an anatomic pulmonary resection with intraoperative lymph node sampling.
This study is designed to open at VA medical centers with expertise in both treatments. The recruitment process includes shared decision making and multidisciplinary evaluations with lung cancer specialists. Mandatory evaluations before randomization include tissue confirmation of NSCLC, staging with FDG-PET/CT, and biopsies of all hilar and/or mediastinal lymph nodes > 10 mm that have a SUV > 2.5. Prerandomization elective lymph node sampling is strongly encouraged, but not required. Following treatment, patients will be followed for a minimum of 5 years.
ID: NCT02984761
Sponsor: VA Office of Research and Development
Locations: 17 VA medical centers, including VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Miami VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VA Medical Center, Baltimore VA Medical Center, VA Boston Healthcare System Jamaica Plain Campus, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Durham VA Medical Center, Louis Stokes VA Medical Center, Corporal Micheal J. Crescenz VA Medical Center, VA Pittsburgh Healthcare System University Drive Division, Michael E. DeBakey VA Medical Center, Hunter Holmes McGuire VA Medical Center, and Clement J. Zablocki VA Medical Center
Utility of CAML as Diagnostic for Early Stage Lung Cancer
The primary objective of this study is to determine the prevalence of cancer associated macrophage-like cells (CAMLS) in patients with pulmonary nodules. Secondary objectives include the following: determine the positive and negative predictive value of CAMLS in patients with pulmonary nodules who undergo biopsy; model combinations of clinical factors with the presence/absence of CAMLS to refine strategies for assessment of patients with pulmonary nodules; and evaluate whether these measures result in enhanced T-cell activity and/or natural killer cell function and number.
ID: NCT03992183
Sponsor; Collaborators: Fox Chase Cancer Center; US Department of Defense
Locations: Corporal Michael J. Crescenz VA Medical Center and Fox Chase Cancer Center
PROSPECT - Profiling of Resistance Patterns & Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax and Therapeutic Target Identification
This study will use therapeutic target-focused (TTF) profiling, genome-wide mRNA profiling, and assessments of tumor phosphopeptides and DNA that are shed into the bloodstream to define how various molecular factors alone and in combination relate to resistance to therapy, to prognosis, and to metastatic patterns at relapse. This study will examine how the presence of factors that drive cell growth, antagonize apoptosis, or confer resistance in other ways may counter the effect of systemic therapies and/or promote rapid tumor recurrence. In this way, the investigators will identify new, previously unappreciated potential therapeutic targets while also identifying which targets are most likely to increase resistance to therapy and worsen prognosis.
ID: NCT05049837
Sponsor; Collaborators: MD Anderson Cancer Center; US Department of Defense, National Institutes of Health (NIH), and National Cancer Institute (NCI)
Location: MD Anderson Cancer Center
Tribally Engaged Approaches to Lung Screening (TEALS)
Lung cancer is the leading cause of cancer mortality among American Indians and Alaska Natives (AI/AN), and AI/AN have worse lung cancer incidence rates, survival, and death compared to the general population. Because lung cancer screening (LCS) with low-dose computed tomography (LDCT) has been shown to reduce lung cancer mortality by roughly 20%, the US Preventive Services Task Force now recommends LCS for persons aged 55 to 80 years who meet specific eligibility criteria (grade-B evidence), and subsequently the Center for Medicare and Medicaid Services (CMS) opted to cover this test. However, the uptake of LCS implementation has been slow in most health care systems, and LCS implementation among AI/AN has never been studied.
To address this knowledge, the Tribally Engaged Approaches to Lung Screening (TEALS) study, a collaborative effort between the Choctaw Nation of Oklahoma, the Stephenson Cancer Center, and the University of Oklahoma Health Sciences Center, will address the following over the course of 5 years: conduct focus groups and semistructured interviews with Choctaw Nation Health Services Authority (CNHSA) patients, clinicians, and health administrators to elucidate individual- and system-level barriers and facilitators that affect the implementation of LCS; develop an LCS care coordination intervention that will identify eligible persons for LCS, help these patients navigate the screening process, and link them with smoking cessation services, when applicable; measure the impact of the TEALS intervention on the receipt of screening and a set of patient- and practice-level outcomes by conducting a cluster-randomized clinical trial of LCS implementation; and disseminate the TEALS program to other researchers and healthcare systems that serve AI/AN patients. TEALS will bridge the gap between evidence and clinical practice for LCS in a high-need, low-resource setting by intervening at the level of the healthcare system.
System-level interventions for guideline implementation tend to be understudied compared to those evaluating individual-level, behavioral interventions. However, the careful development and evaluation of an LCS screening program at the level of the healthcare system would be critical to ensure that more patients can receive LCS. Our research will create a critically needed platform from which future studies could be launched that will examine how to tailor the application of the LCS guideline to the individual preferences of AI/AN patients. TEALS will establish an effective LCS program in a tribal system and thus provide a direct benefit to the Choctaw Nation by increasing LCS participation. TEALS will serve as a blueprint for establishing a sustainable and accessible infrastructure for LCS in AI/AN and other community health systems. By increasing screening for early stage lung cancer, TEALS could ultimately reduce lung cancer mortality in AI/AN communities.
ID: NCT04948060
Sponsor; Collaborator: University of Oklahoma; Choctaw Nation of Oklahoma
Location: University of Oklahoma Health Sciences Center
Prostate Cancer
18F-DCFPyL PET/CT Impact on Treatment Strategies for Patients With Prostate Cancer (PROSPYL)
The main purpose of this phase II trial study is to determine whether a positron emission tomography (PET)/computed tomography (CT) scan using 18F-DCFPyL affects the clinical management plan in veterans. In this study, the management plan prior to and after 18F-DCFPyL PET/CT will be recorded by specific questionnaires and corresponding changes in management will be analyzed. The scan will be used to see how the disease has spread. Both the treatment strategies and probable disease outcomes as relevant to clinical endpoints will be assessed. This study is open to veterans only.
ID: NCT04390880
Sponsor: VA Greater Los Angeles Healthcare System
Location: VA Greater Los Angeles Healthcare System
Patient Decision-Making About Precision Oncology in Veterans With Advanced Prostate Cancer
This project proposes to understand and improve veterans’ decision-making in precision oncology (germline testing, somatic tumor testing, and targeted therapy) for advanced prostate cancer. As precision oncology expands, a comprehensive strategy to support patient informed decision-making has not been developed.
ID: NCT05396872
Sponsor; Collaborator: University of California, San Francisco; US Department of Defense
Location: San Francisco VA Medical Center
Intramuscular Mechanisms of Androgen Deprivation-Related Sarcopenia
Prostate cancer is the most common cancer among men and is even more common in the military and veteran population. For patients with advanced prostate cancer, the most common treatment includes lowering the levels of the hormone testosterone as much as possible, which is called androgen deprivation therapy (ADT). Unfortunately, ADT also causes patients to be fatigued, weak, and to lose muscle. This is often referred to as sarcopenia, and it leads to falls, poor quality of life, and higher risk of death. Currently, there is no treatment for sarcopenia because investigators do not understand the mechanisms that cause it. The mitochondria is the part of the cells responsible for providing energy to muscles but to date the investigators do not know if it is affected in prostate cancer patients with sarcopenia due to ADT. The overall goal of this proposal is to establish if the mitochondria is responsible for sarcopenia in patients with prostate cancer receiving ADT. The investigators will measure mitochondrial function, muscle mass and strength, and feelings of fatigue and quality of life in patients with prostate cancer before starting and after 6 months of ADT.
ID: NCT03867357
Sponsor; Collaborator: Seattle Institute for Biomedical and Clinical Research; US Department of Defense
Location: VA Puget Sound Health Care System
VA Seamless Phase II/III Randomized Trial of Standard Systemic Therapy With or Without PET-Directed Local Therapy for OligoRecurrent Prostate Cancer (VA STARPORT)
The primary goal of this study is to determine if adding PET-directed local therapy improves disease control compared to standard systemic therapy alone (SST) in veterans with oligorecurrent prostate cancer on PET/CT. The investigators will conduct a multi-institutional phase II/III randomized trial comparing SST with or without PET-directed local therapy using radiation or surgery to all metastases and if a local recurrence is present.
ID: NCT04787744
Sponsor: VA Office of Research and Development
Locations: VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VA Medical Center, VA Boston Healthcare System Jamaica Plain Campus, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Kansas City VA Medical Center, St. Louis VA Medical Center John Cochran Division, East Orange Campus of the VA New Jersey Health Care System, Durham VA Medical Center, Louis Stokes VA Medical Center, Michael E. DeBakey VA Medical Center, Hunter Holmes McGuire VA Medical Center, Clement J. Zablocki VA Medical Center
Standard Systemic Therapy With or Without Definitive Treatment in Treating Participants With Metastatic Prostate Cancer
This phase III trial studies how well standard systemic therapy with or without definitive treatment (prostate removal surgery or radiation therapy) works in treating participants with prostate cancer that has spread to other places in the body. The addition of prostate removal surgery or radiation therapy to standard systemic therapy for prostate cancer may lower the chance of the cancer growing or spreading.
ID: NCT03678025
Sponsor; Collaborator: Southwest Oncology Group; National Cancer Institute (NCI)
Locations: 328 sites, including Tibor Rubin VA Medical Center, Atlanta VA Medical Center, James J. Peters VA Medical Center, Michael E. DeBakey VA Medical Center, and Audie L. Murphy VA Hospital
A Clinical Study Evaluating the Benefit of Adding Rucaparib to Enzalutamide for Men With Metastatic Prostate Cancer That Has Become Resistant to Testosterone-Deprivation Therapy (CASPAR)
This randomized, placebo-controlled, phase III trial is evaluating the benefit of rucaparib and enzalutamide combination therapy vs enzalutamide alone for the treatment of men with prostate cancer that has spread to other places in the body (metastatic) and has become resistant to testosterone-deprivation therapy (castration-resistant). Enzalutamide helps fight prostate cancer by blocking the use of testosterone by the tumor cells for growth. Poly adenosine diphosphate (ADP)-ribose polymerase (PARP) inhibitors, such as rucaparib, fight prostate cancer by prevent tumor cells from repairing their DNA. Giving enzalutamide and rucaparib may make patients live longer or prevent their cancer from growing or spreading for a longer time, or both. It may also help doctors learn if a mutation in any of the homologous recombination DNA repair genes is helpful to decide which treatment is best for the patient.
ID: NCT04455750
Sponsor; Collaborator: Alliance for Clinical Trials in Oncology; National Cancer Institute (NCI)
Locations: 413 sites
Digitally Captured Activity Data and PROs to Monitor Physical Function in Prostate Cancer Patients (DigiPRO)
Physical function is a known predictor of quality of life in advanced prostate cancer patients and key measure of treatment tolerability. While treatment with androgen deprivation therapy (ADT) improves survival, it is associated with significant toxicities that lead to physical function (PF) decline. The average age of incident prostate cancer is 66 years, and in this older group of men, chronic comorbid conditions often co-occur with diagnosis, further adding to the risk for PF decline. With over 2.9 million prostate cancer survivors in the US, there is an increasing demand for adequate symptom monitoring and PF assessment throughout cancer care. However, there are currently no validated methods to systematically evaluate and predict PF decline. Thus, the overarching objective of this proposal is to determine whether the use of wearable technology to monitor objective daily activity combined with routine symptom reporting can predict PF decline. To accomplish this, we propose a mixed-methods approach that will provide quantitative information to help identify PC survivors at higher risk for PF decline as well as a qualitative aim gain a deeper understanding of the perceived relationships that PC survivors have with their physical activity levels and treatment symptoms.
ID: NCT04575402
Sponsor; Collaborator: Cedars-Sinai Medical Center; US Department of Defense
Location: Cedars Sinai Medical Center
The BurnAlong Pilot Study for Adolescent and Young Adult Cancer Survivors
The purpose of this prospective, interventional, single-arm pilot study is to evaluate whether virtually delivered group-based physical activity is feasible for adolescent and young adult (AYA) cancer survivors. AYAs who were diagnosed with cancer and have completed cancer treatment will be recruited for this study. This study will enroll 20 participants in total and will last approximately 3 months.
ID: NCT05131815
Sponsor; Collaborator: Cedars-Sinai Medical Center; Walter Reed National Military Medical Center
Location: Cedars-Sinai Medical Center
Lung Cancer
DECAMP 1 PLUS: Prediction of Lung Cancer Using Noninvasive Biomarkers
The Detection of Early lung Cancer Among Military Personnel (DECAMP) consortium is a multidisciplinary and translational research program for lung cancer early detection. DECAMP 1 PLUS aims to improve the efficiency of the diagnostic evaluation of patients with indeterminate pulmonary nodules (8-25 mm). Molecular biomarkers for lung cancer diagnosis measured in minimally invasive and noninvasive biospecimens may be able to distinguish between malignant or benign indeterminate pulmonary nodules in high-risk smokers. Ultimately, this study aims to validate molecular as well as clinical and imaging biomarkers of lung cancer in individuals with indeterminate lung nodules.
ID: NCT04165564
Sponsor: Boston University
Locations: 3 VA medical centers (VA Greater LA Healthcare System, VA Boston Healthcare System, and VA Tennessee Valley Healthcare System), 3 military treatment facilities (Naval Medical Center San Diego, Walter Reed National Military Medical Center, and Naval Medical Center Portsmouth) and 12 academic hospitals
DECAMP-2: Screening of Patients With Early Stage Lung Cancer or at High Risk for Developing Lung Cancer (DECAMP-2)
The goal of this project is to improve lung cancer screening in high-risk individuals by identifying biomarkers of preclinical disease and disease risk that are measured in minimally invasive and noninvasive biospecimens. Existing biomarkers for lung cancer diagnosis as well as new biomarkers discovered specifically in this clinical setting will be examined. Biomarkers that identify individuals at highest risk for being diagnosed with lung cancer prior to the appearance of concerning symptoms could increase the utility of lung cancer surveillance and the efficiency of lung cancer chemoprevention clinical trials. Achieving these goals would improve the detection and treatment of early-stage and incipient lung cancer, while restricting the risk of these procedures to those individuals who currently exhibit the early molecular warning signs of impending disease.
ID: NCT02504697
Sponsor: Boston University
Locations: VA medical centers (including Los Angeles VA Healthcare System, Boston VA Research Institute, Inc, Philadelphia VA Medical Center, Veterans Research Foundation of Pittsburgh, and VA North Texas Health Care System), 4 military treatment facilities (Naval Medical Center San Diego, Walter Reed National Military Medical Center, San Antonio Military Medical Center, and Naval Medical Center Portsmouth), and 4 academic hospitals
Improving Decision-Making Encounters in Lung Cancer Using a Low-Literacy Conversation Tool (iDECIDE)
This clinical trial evaluates the effectiveness of a conversation tool on patient-centered health and decision-making outcomes in patients with lung cancer making treatment decisions. This research is being conducted to help doctors understand the information patients need to participate in shared decision-making about their lung cancer treatment options. The focus of this research is to study how patients choose lung cancer treatment options and the information needed to make that choice, with a focus on patients with lower health literacy.
ID: NCT05407168
Sponsor: Oregon Health & Science University Knight Cancer Institute
Locations: Portland VA Medical Center and Oregon Health & Science University Knight Cancer Institute
VA Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR)
The standard of care for stage I non–small cell lung cancer has historically been surgical resection in patients who are medically fit to tolerate an operation. Recent data now suggest that stereotactic radiotherapy may be a suitable alternative. This includes the results from a pooled analysis of 2 incomplete phase III studies that reported a 15% overall survival advantage with stereotactic radiotherapy at 3 years. While these data are promising, the median follow-up period was short, the results underpowered, and the findings were in contradiction to multiple retrospective studies that demonstrate the outcomes with surgery are likely equal or superior. Therefore, the herein trial aims to evaluate these 2 treatments in a prospective randomized fashion with a goal to compare the overall survival beyond 5 years. It has been designed to enroll patients who have a long life expectancy and are fit enough to tolerate an anatomic pulmonary resection with intraoperative lymph node sampling.
This study is designed to open at VA medical centers with expertise in both treatments. The recruitment process includes shared decision making and multidisciplinary evaluations with lung cancer specialists. Mandatory evaluations before randomization include tissue confirmation of NSCLC, staging with FDG-PET/CT, and biopsies of all hilar and/or mediastinal lymph nodes > 10 mm that have a SUV > 2.5. Prerandomization elective lymph node sampling is strongly encouraged, but not required. Following treatment, patients will be followed for a minimum of 5 years.
ID: NCT02984761
Sponsor: VA Office of Research and Development
Locations: 17 VA medical centers, including VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Miami VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VA Medical Center, Baltimore VA Medical Center, VA Boston Healthcare System Jamaica Plain Campus, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Durham VA Medical Center, Louis Stokes VA Medical Center, Corporal Micheal J. Crescenz VA Medical Center, VA Pittsburgh Healthcare System University Drive Division, Michael E. DeBakey VA Medical Center, Hunter Holmes McGuire VA Medical Center, and Clement J. Zablocki VA Medical Center
Utility of CAML as Diagnostic for Early Stage Lung Cancer
The primary objective of this study is to determine the prevalence of cancer associated macrophage-like cells (CAMLS) in patients with pulmonary nodules. Secondary objectives include the following: determine the positive and negative predictive value of CAMLS in patients with pulmonary nodules who undergo biopsy; model combinations of clinical factors with the presence/absence of CAMLS to refine strategies for assessment of patients with pulmonary nodules; and evaluate whether these measures result in enhanced T-cell activity and/or natural killer cell function and number.
ID: NCT03992183
Sponsor; Collaborators: Fox Chase Cancer Center; US Department of Defense
Locations: Corporal Michael J. Crescenz VA Medical Center and Fox Chase Cancer Center
PROSPECT - Profiling of Resistance Patterns & Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax and Therapeutic Target Identification
This study will use therapeutic target-focused (TTF) profiling, genome-wide mRNA profiling, and assessments of tumor phosphopeptides and DNA that are shed into the bloodstream to define how various molecular factors alone and in combination relate to resistance to therapy, to prognosis, and to metastatic patterns at relapse. This study will examine how the presence of factors that drive cell growth, antagonize apoptosis, or confer resistance in other ways may counter the effect of systemic therapies and/or promote rapid tumor recurrence. In this way, the investigators will identify new, previously unappreciated potential therapeutic targets while also identifying which targets are most likely to increase resistance to therapy and worsen prognosis.
ID: NCT05049837
Sponsor; Collaborators: MD Anderson Cancer Center; US Department of Defense, National Institutes of Health (NIH), and National Cancer Institute (NCI)
Location: MD Anderson Cancer Center
Tribally Engaged Approaches to Lung Screening (TEALS)
Lung cancer is the leading cause of cancer mortality among American Indians and Alaska Natives (AI/AN), and AI/AN have worse lung cancer incidence rates, survival, and death compared to the general population. Because lung cancer screening (LCS) with low-dose computed tomography (LDCT) has been shown to reduce lung cancer mortality by roughly 20%, the US Preventive Services Task Force now recommends LCS for persons aged 55 to 80 years who meet specific eligibility criteria (grade-B evidence), and subsequently the Center for Medicare and Medicaid Services (CMS) opted to cover this test. However, the uptake of LCS implementation has been slow in most health care systems, and LCS implementation among AI/AN has never been studied.
To address this knowledge, the Tribally Engaged Approaches to Lung Screening (TEALS) study, a collaborative effort between the Choctaw Nation of Oklahoma, the Stephenson Cancer Center, and the University of Oklahoma Health Sciences Center, will address the following over the course of 5 years: conduct focus groups and semistructured interviews with Choctaw Nation Health Services Authority (CNHSA) patients, clinicians, and health administrators to elucidate individual- and system-level barriers and facilitators that affect the implementation of LCS; develop an LCS care coordination intervention that will identify eligible persons for LCS, help these patients navigate the screening process, and link them with smoking cessation services, when applicable; measure the impact of the TEALS intervention on the receipt of screening and a set of patient- and practice-level outcomes by conducting a cluster-randomized clinical trial of LCS implementation; and disseminate the TEALS program to other researchers and healthcare systems that serve AI/AN patients. TEALS will bridge the gap between evidence and clinical practice for LCS in a high-need, low-resource setting by intervening at the level of the healthcare system.
System-level interventions for guideline implementation tend to be understudied compared to those evaluating individual-level, behavioral interventions. However, the careful development and evaluation of an LCS screening program at the level of the healthcare system would be critical to ensure that more patients can receive LCS. Our research will create a critically needed platform from which future studies could be launched that will examine how to tailor the application of the LCS guideline to the individual preferences of AI/AN patients. TEALS will establish an effective LCS program in a tribal system and thus provide a direct benefit to the Choctaw Nation by increasing LCS participation. TEALS will serve as a blueprint for establishing a sustainable and accessible infrastructure for LCS in AI/AN and other community health systems. By increasing screening for early stage lung cancer, TEALS could ultimately reduce lung cancer mortality in AI/AN communities.
ID: NCT04948060
Sponsor; Collaborator: University of Oklahoma; Choctaw Nation of Oklahoma
Location: University of Oklahoma Health Sciences Center
Oropharyngeal Squamous Cell Carcinoma Outcomes by p16 INK4a Antigen Status in a Veteran Population
Since 1983, the correlation between head and neck squamous cell carcinoma (SCC) and human papillomavirus (HPV) has been of great interest to head and neck oncologists.1 In 1998, Smith and colleagues provided evidence of HPV as an independent risk factor for the development of head and neck SCC.2 HPV-associated head and neck SCC accounts for between 30% and 64% of oropharyngeal SCC, depending on the published study; tonsil primaries account for the majority of these cancers.3,4
The presence of HPV E6 and E7 oncoproteins leads to the inactivation of p53 and pRb tumor suppressors. Furthermore, Ragin and colleagues discussed a distinct molecular pathway specific to HPV-associated head and neck SCC, which was different from non–HPV-associated head and neck SCC, involving genetic mutations in CDKN2A/p16.5
Current methods in correlating the presence of HPV infection in head and neck SCC have centered on p16INK4a (p16) immunohistochemistry (IHC) staining and DNA in situ hybridization (ISH) for specific HPV DNA types. IHC staining for p16 involves a monoclonal antibody specific to p16. The usefulness of this test relies on p16 overexpression due to the inactivation of pRb by the HPV E7 oncoprotein. This test is readily performed on archived tissue and has a documented sensitivity and specificity of 100% and 79%, respectively, as reported by Singhi and Westra in 2010.6 HPV DNA fluorescence in situ hybridization is the gold standard for determining the presence of specific types of HPV DNA; however, p16 IHC can serve as a rapid, less costly means of studying archived tissue, lending its utility to retrospective population-based studies.
METHODS
A retrospective study was designed to determine the proportion of HPV-associated oropharyngeal SCC in a US Department of Veterans Affairs (VA) population, using p16 antigen IHC on paraffin-embedded tissue as the surrogate marker for the presence of HPV infection. Patients consisted of veterans who were treated for oropharyngeal SCC at Veterans Affairs Memphis Healthcare System (VAMHS) in Tennessee between January 1, 2000, and December 31, 2008. This data range allowed for at least 5 years of follow-up. Patients were excluded who lacked enough tissue specimens for analysis. Measurement outcomes included p16 expression, with subset analysis by race and ethnicity, degree of tobacco and alcohol use, tumor location, stage, age at diagnosis, and survival outcome. Microsoft Excel was used to calculate Fisher exact test, Student t test, and χ2 statistics. Significance was set at P < .05. This study received institutional review board approval from the University of Tennessee Health Science Center and the VAMHS.
RESULTS
We identified 66 total cases of oropharyngeal SCC; 19 cases (29%) were positive for p16. The mean age at diagnosis for the p16-positive cohort was 59 years vs 61 years for the p16-negative cohort (P = .22; Table 1).
Although the tonsil was the most common site of tumor origin in both the p16-positive and negative cohorts (63% vs 51%, respectively), our analysis showed no statistically significant difference in sites of origin (P = .69) (Table 2).
DISCUSSION
The VAMHS population in our study had a lower proportion of HPV-associated oropharyngeal SCC compared with studies on nonveteran populations (29% vs 40%-80%, respectively).5,6 This disparity may indicate a true difference in these populations or may be related to a decreased prevalence of HPV infection in the population served by the VAMHS. This single-institution population did not completely correlate with previous population studies. Specifically, age at presentation (equivalent to patients with p16-negative status rather than earlier age at onset), disease stage at presentation (lower stage for patients with p16-positive status), and disease-specific survival (not improved compared with patients with p16-negative status in other studies) were dissimilar to previous investigations.2,3
The increased age and staging at presentation could be related in these patients with p16-positive status, which may further account for the lack of improved survival. Furthermore, both groups tended to use alcohol at a high proportion; whereas other populations have had a lesser degree of alcohol intake with p16 positivity.1-4 These differences may be due to variations in the habits and behavior of VA patients compared with non-VA patients.3,4
HPV-associated oropharyngeal SCC in published data has been associated with high-risk sexual behavior, lower age, and less tobacco and alcohol use.5,6 No difference was noted in tumor site predilection; however, the small size of our study could explain the lack of finding site preference shown in previous studies.2,3Other veteran-specific factors are absent in the at-large population, such as Agent Orange exposure. More than 8 million veterans (22%) from the Vietnam era self-reported Agent Orange exposure.7 Agent Orange exposure significantly predicted developing upper aerodigestive tract cancer. Oropharyngeal, nasopharyngeal, laryngeal, and thyroid cancers were significantly associated with Agent Orange exposure. Interestingly, these patients experienced an improved 10-year survival rate compared with patients not exposed to Agent Orange. This finding contrasts with our patients, who did not experience improved outcomes vs nonveteran patients with head and neck cancer.7
Suicide in veterans with head and neck cancer has been evaluated and was found at an incidence of 0.7%. Survivors of head and neck cancer are almost twice as likely to die by suicide compared with other cancer survivors. These patients have a higher rate of mental health disorders, substance misuse, and use of palliative care services.8 Sixty-five of 66 of our patients died during the 5-year observation period, although none died by suicide.
In a 2022 cohort study by Sun and colleagues, upfront surgical treatment was associated with a 23% reduced risk of stroke compared with definitive chemoradiotherapy in US veterans with oropharyngeal carcinoma.9 In our study, 58 of 66 patients (88%) received concurrent chemoradiation, possibly reflecting the more advanced stage of diagnosis in our study population. This was due to comorbidities and other health and economic factors. In our study, 43 patients (65%) died of factors not related to the disease, reflecting the overall comorbidity burden of this population. Seven patients (11%) in our 5-year study died of a documented stroke. In the study of veterans by Sun and colleagues, the 10-year cumulative incidence of stroke was 12.5% and death was 57.3%.9 Our veteran population experienced a similar incidence of strokes. These findings may need to be included when discussing the risk-benefit aspects of different treatment options with our veteran patients with oropharyngeal cancer.
To understand the influence of HPV infection on the course of oropharyngeal SCC in the VA patient population and to apply this understanding to future individualized treatment paradigms, this study can be expanded to a greater number of VA patients. p16 immunoexpression appears to be a useful surrogate for high-risk HPV infection in oropharyngeal SCC, and its ease of use supports its feasibility in further VA population analysis.10 While realizing that the veteran HPV-associated oropharyngeal SCC population differs from the civilian HPV-associated oropharyngeal SCC population, we also have realized that other unique considerations in the veteran population, such as chemical warfare exposure, mental illness, and vascular disease, complicate treatment decisions in these patients.
CONCLUSIONS
Disparities in racial distribution and tobacco use between patients with p16-positive and p16-negative status are similar to those reported in non-VA populations. In contrast, the frequently reported younger age at presentation and better disease outcomes seen in non-VA patients were not observed, perhaps due to the lower percentage of p16 expression in VA patients with oropharyngeal SCC. Whereas de-intensification of therapy may be considered for many patients with oropharygeal cancer that is HPV-associated because of improved prognosis, this approach should be undertaken with great care in this group of patients. Personalization of therapy for these HPV-associated oropharyngeal SCC in the veteran population must be adapted to mitigate this critical disparity.
1. Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418-424. doi:10.1016/s0300-9785(83)80033-7
2. Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108(7):1098-1103. doi:10.1097/00005537-199807000-00027
3. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi:10.1056/NEJMoa0912217
4. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813-1820. doi:10.1002/ijc.22851
5. Ragin CC, Taioli E, Weissfeld JL, et al. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer. 2006;95(10):1432-1438. doi:10.1038/sj.bjc.6603394
6. Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166-2173. doi:10.1002/cncr.25033
7. Mowery A, Conlin M, Clayburgh D. Increased risk of head and neck cancer in Agent Orange exposed Vietnam Era veterans. Oral Oncol. 2020;100:104483. doi:10.1016/j.oraloncology.2019.104483
8. Nugent SM, Morasco BJ, Handley R, et al. Risk of suicidal self-directed violence among US veteran survivors of head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2021;147(11):981-989. doi:10.1001/jamaoto.2021.2625
9. Sun L, Brody R, Candelieri D, et al. Association between up-front surgery and risk of stroke in US veterans with oropharyngeal carcinoma. JAMA Otolaryngol Head Neck Surg. 2022;148(8):740-747. doi:10.1001/jamaoto.2022.1327
10. El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34(4):459-461. doi:10.1002/hed.21974
Since 1983, the correlation between head and neck squamous cell carcinoma (SCC) and human papillomavirus (HPV) has been of great interest to head and neck oncologists.1 In 1998, Smith and colleagues provided evidence of HPV as an independent risk factor for the development of head and neck SCC.2 HPV-associated head and neck SCC accounts for between 30% and 64% of oropharyngeal SCC, depending on the published study; tonsil primaries account for the majority of these cancers.3,4
The presence of HPV E6 and E7 oncoproteins leads to the inactivation of p53 and pRb tumor suppressors. Furthermore, Ragin and colleagues discussed a distinct molecular pathway specific to HPV-associated head and neck SCC, which was different from non–HPV-associated head and neck SCC, involving genetic mutations in CDKN2A/p16.5
Current methods in correlating the presence of HPV infection in head and neck SCC have centered on p16INK4a (p16) immunohistochemistry (IHC) staining and DNA in situ hybridization (ISH) for specific HPV DNA types. IHC staining for p16 involves a monoclonal antibody specific to p16. The usefulness of this test relies on p16 overexpression due to the inactivation of pRb by the HPV E7 oncoprotein. This test is readily performed on archived tissue and has a documented sensitivity and specificity of 100% and 79%, respectively, as reported by Singhi and Westra in 2010.6 HPV DNA fluorescence in situ hybridization is the gold standard for determining the presence of specific types of HPV DNA; however, p16 IHC can serve as a rapid, less costly means of studying archived tissue, lending its utility to retrospective population-based studies.
METHODS
A retrospective study was designed to determine the proportion of HPV-associated oropharyngeal SCC in a US Department of Veterans Affairs (VA) population, using p16 antigen IHC on paraffin-embedded tissue as the surrogate marker for the presence of HPV infection. Patients consisted of veterans who were treated for oropharyngeal SCC at Veterans Affairs Memphis Healthcare System (VAMHS) in Tennessee between January 1, 2000, and December 31, 2008. This data range allowed for at least 5 years of follow-up. Patients were excluded who lacked enough tissue specimens for analysis. Measurement outcomes included p16 expression, with subset analysis by race and ethnicity, degree of tobacco and alcohol use, tumor location, stage, age at diagnosis, and survival outcome. Microsoft Excel was used to calculate Fisher exact test, Student t test, and χ2 statistics. Significance was set at P < .05. This study received institutional review board approval from the University of Tennessee Health Science Center and the VAMHS.
RESULTS
We identified 66 total cases of oropharyngeal SCC; 19 cases (29%) were positive for p16. The mean age at diagnosis for the p16-positive cohort was 59 years vs 61 years for the p16-negative cohort (P = .22; Table 1).
Although the tonsil was the most common site of tumor origin in both the p16-positive and negative cohorts (63% vs 51%, respectively), our analysis showed no statistically significant difference in sites of origin (P = .69) (Table 2).
DISCUSSION
The VAMHS population in our study had a lower proportion of HPV-associated oropharyngeal SCC compared with studies on nonveteran populations (29% vs 40%-80%, respectively).5,6 This disparity may indicate a true difference in these populations or may be related to a decreased prevalence of HPV infection in the population served by the VAMHS. This single-institution population did not completely correlate with previous population studies. Specifically, age at presentation (equivalent to patients with p16-negative status rather than earlier age at onset), disease stage at presentation (lower stage for patients with p16-positive status), and disease-specific survival (not improved compared with patients with p16-negative status in other studies) were dissimilar to previous investigations.2,3
The increased age and staging at presentation could be related in these patients with p16-positive status, which may further account for the lack of improved survival. Furthermore, both groups tended to use alcohol at a high proportion; whereas other populations have had a lesser degree of alcohol intake with p16 positivity.1-4 These differences may be due to variations in the habits and behavior of VA patients compared with non-VA patients.3,4
HPV-associated oropharyngeal SCC in published data has been associated with high-risk sexual behavior, lower age, and less tobacco and alcohol use.5,6 No difference was noted in tumor site predilection; however, the small size of our study could explain the lack of finding site preference shown in previous studies.2,3Other veteran-specific factors are absent in the at-large population, such as Agent Orange exposure. More than 8 million veterans (22%) from the Vietnam era self-reported Agent Orange exposure.7 Agent Orange exposure significantly predicted developing upper aerodigestive tract cancer. Oropharyngeal, nasopharyngeal, laryngeal, and thyroid cancers were significantly associated with Agent Orange exposure. Interestingly, these patients experienced an improved 10-year survival rate compared with patients not exposed to Agent Orange. This finding contrasts with our patients, who did not experience improved outcomes vs nonveteran patients with head and neck cancer.7
Suicide in veterans with head and neck cancer has been evaluated and was found at an incidence of 0.7%. Survivors of head and neck cancer are almost twice as likely to die by suicide compared with other cancer survivors. These patients have a higher rate of mental health disorders, substance misuse, and use of palliative care services.8 Sixty-five of 66 of our patients died during the 5-year observation period, although none died by suicide.
In a 2022 cohort study by Sun and colleagues, upfront surgical treatment was associated with a 23% reduced risk of stroke compared with definitive chemoradiotherapy in US veterans with oropharyngeal carcinoma.9 In our study, 58 of 66 patients (88%) received concurrent chemoradiation, possibly reflecting the more advanced stage of diagnosis in our study population. This was due to comorbidities and other health and economic factors. In our study, 43 patients (65%) died of factors not related to the disease, reflecting the overall comorbidity burden of this population. Seven patients (11%) in our 5-year study died of a documented stroke. In the study of veterans by Sun and colleagues, the 10-year cumulative incidence of stroke was 12.5% and death was 57.3%.9 Our veteran population experienced a similar incidence of strokes. These findings may need to be included when discussing the risk-benefit aspects of different treatment options with our veteran patients with oropharyngeal cancer.
To understand the influence of HPV infection on the course of oropharyngeal SCC in the VA patient population and to apply this understanding to future individualized treatment paradigms, this study can be expanded to a greater number of VA patients. p16 immunoexpression appears to be a useful surrogate for high-risk HPV infection in oropharyngeal SCC, and its ease of use supports its feasibility in further VA population analysis.10 While realizing that the veteran HPV-associated oropharyngeal SCC population differs from the civilian HPV-associated oropharyngeal SCC population, we also have realized that other unique considerations in the veteran population, such as chemical warfare exposure, mental illness, and vascular disease, complicate treatment decisions in these patients.
CONCLUSIONS
Disparities in racial distribution and tobacco use between patients with p16-positive and p16-negative status are similar to those reported in non-VA populations. In contrast, the frequently reported younger age at presentation and better disease outcomes seen in non-VA patients were not observed, perhaps due to the lower percentage of p16 expression in VA patients with oropharyngeal SCC. Whereas de-intensification of therapy may be considered for many patients with oropharygeal cancer that is HPV-associated because of improved prognosis, this approach should be undertaken with great care in this group of patients. Personalization of therapy for these HPV-associated oropharyngeal SCC in the veteran population must be adapted to mitigate this critical disparity.
Since 1983, the correlation between head and neck squamous cell carcinoma (SCC) and human papillomavirus (HPV) has been of great interest to head and neck oncologists.1 In 1998, Smith and colleagues provided evidence of HPV as an independent risk factor for the development of head and neck SCC.2 HPV-associated head and neck SCC accounts for between 30% and 64% of oropharyngeal SCC, depending on the published study; tonsil primaries account for the majority of these cancers.3,4
The presence of HPV E6 and E7 oncoproteins leads to the inactivation of p53 and pRb tumor suppressors. Furthermore, Ragin and colleagues discussed a distinct molecular pathway specific to HPV-associated head and neck SCC, which was different from non–HPV-associated head and neck SCC, involving genetic mutations in CDKN2A/p16.5
Current methods in correlating the presence of HPV infection in head and neck SCC have centered on p16INK4a (p16) immunohistochemistry (IHC) staining and DNA in situ hybridization (ISH) for specific HPV DNA types. IHC staining for p16 involves a monoclonal antibody specific to p16. The usefulness of this test relies on p16 overexpression due to the inactivation of pRb by the HPV E7 oncoprotein. This test is readily performed on archived tissue and has a documented sensitivity and specificity of 100% and 79%, respectively, as reported by Singhi and Westra in 2010.6 HPV DNA fluorescence in situ hybridization is the gold standard for determining the presence of specific types of HPV DNA; however, p16 IHC can serve as a rapid, less costly means of studying archived tissue, lending its utility to retrospective population-based studies.
METHODS
A retrospective study was designed to determine the proportion of HPV-associated oropharyngeal SCC in a US Department of Veterans Affairs (VA) population, using p16 antigen IHC on paraffin-embedded tissue as the surrogate marker for the presence of HPV infection. Patients consisted of veterans who were treated for oropharyngeal SCC at Veterans Affairs Memphis Healthcare System (VAMHS) in Tennessee between January 1, 2000, and December 31, 2008. This data range allowed for at least 5 years of follow-up. Patients were excluded who lacked enough tissue specimens for analysis. Measurement outcomes included p16 expression, with subset analysis by race and ethnicity, degree of tobacco and alcohol use, tumor location, stage, age at diagnosis, and survival outcome. Microsoft Excel was used to calculate Fisher exact test, Student t test, and χ2 statistics. Significance was set at P < .05. This study received institutional review board approval from the University of Tennessee Health Science Center and the VAMHS.
RESULTS
We identified 66 total cases of oropharyngeal SCC; 19 cases (29%) were positive for p16. The mean age at diagnosis for the p16-positive cohort was 59 years vs 61 years for the p16-negative cohort (P = .22; Table 1).
Although the tonsil was the most common site of tumor origin in both the p16-positive and negative cohorts (63% vs 51%, respectively), our analysis showed no statistically significant difference in sites of origin (P = .69) (Table 2).
DISCUSSION
The VAMHS population in our study had a lower proportion of HPV-associated oropharyngeal SCC compared with studies on nonveteran populations (29% vs 40%-80%, respectively).5,6 This disparity may indicate a true difference in these populations or may be related to a decreased prevalence of HPV infection in the population served by the VAMHS. This single-institution population did not completely correlate with previous population studies. Specifically, age at presentation (equivalent to patients with p16-negative status rather than earlier age at onset), disease stage at presentation (lower stage for patients with p16-positive status), and disease-specific survival (not improved compared with patients with p16-negative status in other studies) were dissimilar to previous investigations.2,3
The increased age and staging at presentation could be related in these patients with p16-positive status, which may further account for the lack of improved survival. Furthermore, both groups tended to use alcohol at a high proportion; whereas other populations have had a lesser degree of alcohol intake with p16 positivity.1-4 These differences may be due to variations in the habits and behavior of VA patients compared with non-VA patients.3,4
HPV-associated oropharyngeal SCC in published data has been associated with high-risk sexual behavior, lower age, and less tobacco and alcohol use.5,6 No difference was noted in tumor site predilection; however, the small size of our study could explain the lack of finding site preference shown in previous studies.2,3Other veteran-specific factors are absent in the at-large population, such as Agent Orange exposure. More than 8 million veterans (22%) from the Vietnam era self-reported Agent Orange exposure.7 Agent Orange exposure significantly predicted developing upper aerodigestive tract cancer. Oropharyngeal, nasopharyngeal, laryngeal, and thyroid cancers were significantly associated with Agent Orange exposure. Interestingly, these patients experienced an improved 10-year survival rate compared with patients not exposed to Agent Orange. This finding contrasts with our patients, who did not experience improved outcomes vs nonveteran patients with head and neck cancer.7
Suicide in veterans with head and neck cancer has been evaluated and was found at an incidence of 0.7%. Survivors of head and neck cancer are almost twice as likely to die by suicide compared with other cancer survivors. These patients have a higher rate of mental health disorders, substance misuse, and use of palliative care services.8 Sixty-five of 66 of our patients died during the 5-year observation period, although none died by suicide.
In a 2022 cohort study by Sun and colleagues, upfront surgical treatment was associated with a 23% reduced risk of stroke compared with definitive chemoradiotherapy in US veterans with oropharyngeal carcinoma.9 In our study, 58 of 66 patients (88%) received concurrent chemoradiation, possibly reflecting the more advanced stage of diagnosis in our study population. This was due to comorbidities and other health and economic factors. In our study, 43 patients (65%) died of factors not related to the disease, reflecting the overall comorbidity burden of this population. Seven patients (11%) in our 5-year study died of a documented stroke. In the study of veterans by Sun and colleagues, the 10-year cumulative incidence of stroke was 12.5% and death was 57.3%.9 Our veteran population experienced a similar incidence of strokes. These findings may need to be included when discussing the risk-benefit aspects of different treatment options with our veteran patients with oropharyngeal cancer.
To understand the influence of HPV infection on the course of oropharyngeal SCC in the VA patient population and to apply this understanding to future individualized treatment paradigms, this study can be expanded to a greater number of VA patients. p16 immunoexpression appears to be a useful surrogate for high-risk HPV infection in oropharyngeal SCC, and its ease of use supports its feasibility in further VA population analysis.10 While realizing that the veteran HPV-associated oropharyngeal SCC population differs from the civilian HPV-associated oropharyngeal SCC population, we also have realized that other unique considerations in the veteran population, such as chemical warfare exposure, mental illness, and vascular disease, complicate treatment decisions in these patients.
CONCLUSIONS
Disparities in racial distribution and tobacco use between patients with p16-positive and p16-negative status are similar to those reported in non-VA populations. In contrast, the frequently reported younger age at presentation and better disease outcomes seen in non-VA patients were not observed, perhaps due to the lower percentage of p16 expression in VA patients with oropharyngeal SCC. Whereas de-intensification of therapy may be considered for many patients with oropharygeal cancer that is HPV-associated because of improved prognosis, this approach should be undertaken with great care in this group of patients. Personalization of therapy for these HPV-associated oropharyngeal SCC in the veteran population must be adapted to mitigate this critical disparity.
1. Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418-424. doi:10.1016/s0300-9785(83)80033-7
2. Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108(7):1098-1103. doi:10.1097/00005537-199807000-00027
3. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi:10.1056/NEJMoa0912217
4. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813-1820. doi:10.1002/ijc.22851
5. Ragin CC, Taioli E, Weissfeld JL, et al. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer. 2006;95(10):1432-1438. doi:10.1038/sj.bjc.6603394
6. Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166-2173. doi:10.1002/cncr.25033
7. Mowery A, Conlin M, Clayburgh D. Increased risk of head and neck cancer in Agent Orange exposed Vietnam Era veterans. Oral Oncol. 2020;100:104483. doi:10.1016/j.oraloncology.2019.104483
8. Nugent SM, Morasco BJ, Handley R, et al. Risk of suicidal self-directed violence among US veteran survivors of head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2021;147(11):981-989. doi:10.1001/jamaoto.2021.2625
9. Sun L, Brody R, Candelieri D, et al. Association between up-front surgery and risk of stroke in US veterans with oropharyngeal carcinoma. JAMA Otolaryngol Head Neck Surg. 2022;148(8):740-747. doi:10.1001/jamaoto.2022.1327
10. El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34(4):459-461. doi:10.1002/hed.21974
1. Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418-424. doi:10.1016/s0300-9785(83)80033-7
2. Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108(7):1098-1103. doi:10.1097/00005537-199807000-00027
3. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi:10.1056/NEJMoa0912217
4. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813-1820. doi:10.1002/ijc.22851
5. Ragin CC, Taioli E, Weissfeld JL, et al. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer. 2006;95(10):1432-1438. doi:10.1038/sj.bjc.6603394
6. Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166-2173. doi:10.1002/cncr.25033
7. Mowery A, Conlin M, Clayburgh D. Increased risk of head and neck cancer in Agent Orange exposed Vietnam Era veterans. Oral Oncol. 2020;100:104483. doi:10.1016/j.oraloncology.2019.104483
8. Nugent SM, Morasco BJ, Handley R, et al. Risk of suicidal self-directed violence among US veteran survivors of head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2021;147(11):981-989. doi:10.1001/jamaoto.2021.2625
9. Sun L, Brody R, Candelieri D, et al. Association between up-front surgery and risk of stroke in US veterans with oropharyngeal carcinoma. JAMA Otolaryngol Head Neck Surg. 2022;148(8):740-747. doi:10.1001/jamaoto.2022.1327
10. El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34(4):459-461. doi:10.1002/hed.21974
Outcomes in Patients With Curative Malignancies Receiving Filgrastim as Primary Prophylaxis
Febrile neutropenia (FN) frequently occurs in patients receiving chemotherapy, with the greatest risk of complications occurring in those who experience profound and prolonged neutropenia. Although granulocyte colony-stimulating factor (G-CSF) prophylaxis has been shown to reduce the risk and duration of chemotherapy-induced neutropenia and FN, there is no well-established optimal regimen.1 The 2022 National Comprehensive Cancer Network guidelines for hematopoietic growth factors recommend prophylaxis with G-CSF in at-risk patients receiving chemotherapy, specifically in chemotherapy regimens considered high risk for FN (incidence > 20%) or intermediate risk for FN (incidence 10%-20%) with additional patient risk factors.2 The incidence of developing FN with at least 1 chemotherapy cycle is estimated at 10% to 50% of patients with solid tumors and > 80% of patients with hematologic malignancies.3 The rate of major complications (eg, hypotension, acute renal, respiratory, or heart failure) in the context of FN is 25% to 30%, and mortality is reported up to 11% in this population.4
Because of the significant consequences of neutropenia and FN, prevention is imperative due to the increase in morbidity and mortality, including chemotherapy delays, increased hospitalizations, chemotherapy dose reductions, and discontinuations that cause delays in care.5 In patients with curative malignancies, these consequences can negatively impact treatment efficacy and overall survival. Additionally, infections occur in 20% to 30% of patients with febrile episodes. Although fever is often the only clinical sign or symptom of infection, patients who are profoundly neutropenic may present with suspected infection and be afebrile or hypothermic.3
For filgrastim, the National Comprehensive Cancer Network guidelines do not specify the total days of required injections but state that a daily dose should be given until the postnadir absolute neutrophil count (ANC) recovers to normal or near normal levels by laboratory standards.2 It is uncommon in clinical practice to track postnadir ANCs due to frequent laboratory monitoring. Clinical trial data suggest an average duration of 11 days of daily filgrastim injections for ANC recovery; however, real-world data exist supporting a range from 4 to 10 days with a median of 7 injections per cycle for prevention of neutropenia or FN.6,7
At the South Texas Veterans Health Care System in San Antonio, daily filgrastim injections are preferred due to cost; patients typically receive a 7-day course for primary prophylaxis for FN.
Electronic health record reviews at the South Texas Veterans Health Care System were performed to identify patients who received filgrastim primary prophylaxis (defined as filgrastim, tbo-filgrastim, or filgrastim-sndz) for a curative cancer diagnosis. Primary prophylaxis refers to the administration of G-CSF in the first cycle of chemotherapy before the onset of neutropenia. Patients received filgrastim prophylaxis if they were undergoing treatment with a chemotherapy regimen with either high risk for FN or a chemotherapy regimen with an intermediate risk for FN and additional patient risk factors. Risk factors for patients included prior chemotherapy or radiation therapy; persistent neutropenia; bone marrow involvement by tumor; recent surgery and/or open wounds; liver dysfunction (defined as total bilirubin > 2 mg/dL); renal dysfunction (defined as creatinine clearance < 50 mL/min); and those aged > 65 years receiving full chemotherapy dose intensity. Neutropenia is defined as a decrease in ANC < 1000 neutrophils/μL, whereas FN is defined as a single temperature of > 38.3 °C or > 38.0 °C for longer than 1 hour with < 500 neutrophils/μL or < 1000 neutrophils/μL predicted to decline to < 500 neutrophils/μL over the next 48 hours. All patients had their filgrastim dispensed for home administration during their chemotherapy appointment.
Descriptive statistics were used to summarize the study population and their health outcomes. Fisher exact test was used to compare FN incidence for high- and intermediate-risk FN groups.
RESULTS
Between September 1, 2015, and September 24, 2020, 381 patients received filgrastim. Of these patients, 59 met the inclusion criteria. Patients receiving filgrastim were excluded due to stem cell transplant mobilization/engraftment (n = 145), a noncurative cancer diagnosis (n = 134), use as a secondary prophylaxis (n = 33), and nononcologic neutropenia (n = 8). Additionally, 2 patients initially received pegfilgrastim and were not included in this data set.
The median (IQR) age was 64 (55-70) years and 42 patients (71%) were male (Table 1).
Ten patients (17%) experienced dose delays despite filgrastim use (Table 2).
Nine patients (15%) had the number of filgrastim injections per chemotherapy cycle extended due to various reasons. Five patients required extended days after hospitalization for FN, 3 patients for dose delays due to neutropenia with the previous cycle, and 1 patient with an undocumented reason outside of the prespecified outcomes. Two of these patients experienced continued neutropenia and dose delays after extending filgrastim from 5 to 7 days or 7 to 10 days. One patient who experienced continued neutropenia after extending filgrastim to 10 days was subsequently transitioned to pegfilgrastim without further episodes of neutropenia. The other patient who still experienced neutropenia after extending filgrastim to 7 days was receiving the last chemotherapy cycle and did not require subsequent doses of filgrastim.
Two additional patients were not included in the hospitalizations. The first was a patient on a chemotherapy regimen with a high risk for FN who presented to the emergency department with documented FN but was never admitted since the patient elected to not be hospitalized. This patient developed oral, anal, and vaginal candidiasis, and it was noted by the oncologist at the next clinic visit that this was likely secondary to grade 4 neutropenia (ANC < 500 neutrophils/μL). The second was a patient on a chemotherapy regimen with an intermediate risk for FN who was already hospitalized but had developed FN and sepsis despite filgrastim use.
Finally, out of the hospitalized patients, 9 (15%) had infections. This included 6 patients (18%) in the high risk for FN group and 3 patients (12%) in the intermediate risk for FN group (P = .72). Six patients transitioned to pegfilgrastim for hospitalization, 2 for neutropenia, and 1 for an unspecified reason. Nine patients (15%) who received filgrastim ended up transitioning to pegfilgrastim; 6 (67%) of these patients were transitioned due to hospitalization for FN. Of all the patients who transitioned to pegfilgrastim, 1 patient on a high risk for FN regimen developed sepsis due to herpes zoster in the setting of neutropenia after the previous cycle of chemotherapy.
Real-world data are limited regarding G-CSF practice patterns; however, available data demonstrate patients may receive suboptimal treatment courses of filgrastim leading to increased complications associated with neutropenia and FN, such as dose delays and hospitalizations.8,9 At the South Texas Veterans Health Care System, 48 patients (81%) received a filgrastim course of ≥ 7 days as an initial course for primary prophylaxis. Multivariate analyses performed by Weycker and colleagues described a decreased risk of hospitalization for neutropenia or FN with each additional day of filgrastim prophylaxis; however, such analysis could not be performed in our data set due to the small sample size.8 In this review, 10 patients (17%) experienced treatment delays due to neutropenia or FN, mirroring previously published data. The hospitalization rate of 25% is higher than the published incidence of 5.2% of cancer-related hospitalizations among adults.7,10 This difference may be explained by a difference in health care access for the veteran population.
As an alternative to daily filgrastim injections, the National Comprehensive Cancer Network also recommends a single dose of pegfilgrastim for primary prevention of FN. Efficacy benefits of pegfilgrastim use include increased patient adherence due to a single injection, a reduction in FN incidence and FN-related hospitalizations, and improved time to ANC recovery compared with filgrastim.11 There are reports suggesting pegfilgrastim significantly reduces neutropenia and FN incidence to a greater extent compared with daily filgrastim injections.6 In patients with breast cancer receiving dose-dense adjuvant chemotherapy, there are data demonstrating that patients who received filgrastim were more likely to experience severe neutropenia, dose reductions, and treatment delays leading to lower dose density compared with pegfilgrastim.12 Of the 19 patients with breast cancer included in our population, 26% experienced one of the previously described outcomes leading to either extensions of daily filgrastim injections or transitions to pegfilgrastim to successfully maintain dose density. In patients with acute myeloid leukemia receiving consolidation chemotherapy, filgrastim was found to be associated with a statistically significant increased risk of hospitalizations compared with pegfilgrastim.13 The one patient with acute myeloid leukemia included in our study did not require additional hospitalizations for neutropenia or FN after transitioning to pegfilgrastim.
Given the cost advantage, the South Texas Veterans Health Care System continues to prefer daily filgrastim injections. A recent survey demonstrated that 73% of patients at 23 sites in the Veterans Health Administration used filgrastim rather than pegfilgrastim for cost savings, although it is recognized that daily filgrastim injections are less convenient for patients.14 This analysis did not review costs associated with hospitalization for FN or the appropriateness of G-CSF use. Cancer-related neutropenia accounts for 8.3% of all cancer-related hospitalization costs among adults; the average hospitalization costs nearly $25,000 per stay and about $2.3 billion among adult patients with cancer annually.10,15
Limitations
This study has limitations that affected the applicability and interpretation of the results. This included the study design since it was a retrospective, single-center, descriptive cohort study. Patient adherence to daily filgrastim injections could not be assessed due to the retrospective nature of the study. The small sample size of 59 patients was prohibitive for utilization of additional analytical tools. Additionally, the predominately male veteran population may make applicability to non-VA populations restrictive.
CONCLUSIONS
Based on the incidence of primary and secondary outcomes associated with using daily filgrastim injections as primary prophylaxis in this study, additional measures such as tracking postnadir ANCs should be performed to ensure patients receive an appropriate number of filgrastim doses to prevent complications associated with neutropenia.
Acknowledgments
We thank Eric Dougherty, PharmD, for assistance in producing granulocyte colony-stimulating factor data.
1. Hanna KS, Mancini R, Wilson D, Zuckerman D. Comparing granulocyte colony-stimulating factors prescribing practices versus guideline recommendations in a large community cancer center. J Hematol Oncol Pharm. 2019;9(3):121-126.
2. Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines insights: hematopoietic growth factors, version 1.2022. J Natl Compr Canc Netw. 2022;20(5):436-442. doi:10.6004/jnccn.2022.0026
3. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. doi:10.1093/cid/cir073
4. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-1453. doi:10.1200/JCO.2017.77.6211
5. Clemons M, Fergusson D, Simos D, et al. A multicentre, randomized trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951-957. doi:10.1016/j.annonc.2020.04.005
6. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. Published 2011 Sep 23. doi:10.1186/1471-2407-11-404
7. Altwairgi A, Hopman W, Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20(3):e171-e179. doi:10.3747/co.20.1306
8. Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402-407. doi:10.1345/aph.1G516
9. Link H, Nietsch J, Kerkmann M, Ortner P; Supportive Care Group (ASORS) of the German Cancer Society (DKG). Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy—a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367-376. doi:10.1007/s00520-015-2779-5
10. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266. doi:10.1002/cncr.21847
11. Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25(11):3295-3304. doi :10.1007/s00520-017-3842-1
12. Kourlaba G, Dimopoulos MA, Pectasides D, et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer. 2015;23(7):2045-2051. doi:10.1007/s00520-014-2555-y
13. Field E, Caimi PF, Cooper B, et al. Comparison of pegfilgrastim and filgrastim to prevent neutropenic fever during consolidation with high dose cytarabine for acute myeloid leukemia. Blood. 2018;132(suppl 1):1404. doi:10.1182/blood-2018-99-118336
14. Knopf K, Hrureshky W, Love BL, Norris L, Bennett CL. Cost-effective use of white blood cell growth factors in the Veterans Administration. Blood. 2018;132(suppl 1):4761. doi:10.1182/blood-2018-99-119724
15. Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-e561. doi:10.1200/JOP.2016.019588
Febrile neutropenia (FN) frequently occurs in patients receiving chemotherapy, with the greatest risk of complications occurring in those who experience profound and prolonged neutropenia. Although granulocyte colony-stimulating factor (G-CSF) prophylaxis has been shown to reduce the risk and duration of chemotherapy-induced neutropenia and FN, there is no well-established optimal regimen.1 The 2022 National Comprehensive Cancer Network guidelines for hematopoietic growth factors recommend prophylaxis with G-CSF in at-risk patients receiving chemotherapy, specifically in chemotherapy regimens considered high risk for FN (incidence > 20%) or intermediate risk for FN (incidence 10%-20%) with additional patient risk factors.2 The incidence of developing FN with at least 1 chemotherapy cycle is estimated at 10% to 50% of patients with solid tumors and > 80% of patients with hematologic malignancies.3 The rate of major complications (eg, hypotension, acute renal, respiratory, or heart failure) in the context of FN is 25% to 30%, and mortality is reported up to 11% in this population.4
Because of the significant consequences of neutropenia and FN, prevention is imperative due to the increase in morbidity and mortality, including chemotherapy delays, increased hospitalizations, chemotherapy dose reductions, and discontinuations that cause delays in care.5 In patients with curative malignancies, these consequences can negatively impact treatment efficacy and overall survival. Additionally, infections occur in 20% to 30% of patients with febrile episodes. Although fever is often the only clinical sign or symptom of infection, patients who are profoundly neutropenic may present with suspected infection and be afebrile or hypothermic.3
For filgrastim, the National Comprehensive Cancer Network guidelines do not specify the total days of required injections but state that a daily dose should be given until the postnadir absolute neutrophil count (ANC) recovers to normal or near normal levels by laboratory standards.2 It is uncommon in clinical practice to track postnadir ANCs due to frequent laboratory monitoring. Clinical trial data suggest an average duration of 11 days of daily filgrastim injections for ANC recovery; however, real-world data exist supporting a range from 4 to 10 days with a median of 7 injections per cycle for prevention of neutropenia or FN.6,7
At the South Texas Veterans Health Care System in San Antonio, daily filgrastim injections are preferred due to cost; patients typically receive a 7-day course for primary prophylaxis for FN.
Electronic health record reviews at the South Texas Veterans Health Care System were performed to identify patients who received filgrastim primary prophylaxis (defined as filgrastim, tbo-filgrastim, or filgrastim-sndz) for a curative cancer diagnosis. Primary prophylaxis refers to the administration of G-CSF in the first cycle of chemotherapy before the onset of neutropenia. Patients received filgrastim prophylaxis if they were undergoing treatment with a chemotherapy regimen with either high risk for FN or a chemotherapy regimen with an intermediate risk for FN and additional patient risk factors. Risk factors for patients included prior chemotherapy or radiation therapy; persistent neutropenia; bone marrow involvement by tumor; recent surgery and/or open wounds; liver dysfunction (defined as total bilirubin > 2 mg/dL); renal dysfunction (defined as creatinine clearance < 50 mL/min); and those aged > 65 years receiving full chemotherapy dose intensity. Neutropenia is defined as a decrease in ANC < 1000 neutrophils/μL, whereas FN is defined as a single temperature of > 38.3 °C or > 38.0 °C for longer than 1 hour with < 500 neutrophils/μL or < 1000 neutrophils/μL predicted to decline to < 500 neutrophils/μL over the next 48 hours. All patients had their filgrastim dispensed for home administration during their chemotherapy appointment.
Descriptive statistics were used to summarize the study population and their health outcomes. Fisher exact test was used to compare FN incidence for high- and intermediate-risk FN groups.
RESULTS
Between September 1, 2015, and September 24, 2020, 381 patients received filgrastim. Of these patients, 59 met the inclusion criteria. Patients receiving filgrastim were excluded due to stem cell transplant mobilization/engraftment (n = 145), a noncurative cancer diagnosis (n = 134), use as a secondary prophylaxis (n = 33), and nononcologic neutropenia (n = 8). Additionally, 2 patients initially received pegfilgrastim and were not included in this data set.
The median (IQR) age was 64 (55-70) years and 42 patients (71%) were male (Table 1).
Ten patients (17%) experienced dose delays despite filgrastim use (Table 2).
Nine patients (15%) had the number of filgrastim injections per chemotherapy cycle extended due to various reasons. Five patients required extended days after hospitalization for FN, 3 patients for dose delays due to neutropenia with the previous cycle, and 1 patient with an undocumented reason outside of the prespecified outcomes. Two of these patients experienced continued neutropenia and dose delays after extending filgrastim from 5 to 7 days or 7 to 10 days. One patient who experienced continued neutropenia after extending filgrastim to 10 days was subsequently transitioned to pegfilgrastim without further episodes of neutropenia. The other patient who still experienced neutropenia after extending filgrastim to 7 days was receiving the last chemotherapy cycle and did not require subsequent doses of filgrastim.
Two additional patients were not included in the hospitalizations. The first was a patient on a chemotherapy regimen with a high risk for FN who presented to the emergency department with documented FN but was never admitted since the patient elected to not be hospitalized. This patient developed oral, anal, and vaginal candidiasis, and it was noted by the oncologist at the next clinic visit that this was likely secondary to grade 4 neutropenia (ANC < 500 neutrophils/μL). The second was a patient on a chemotherapy regimen with an intermediate risk for FN who was already hospitalized but had developed FN and sepsis despite filgrastim use.
Finally, out of the hospitalized patients, 9 (15%) had infections. This included 6 patients (18%) in the high risk for FN group and 3 patients (12%) in the intermediate risk for FN group (P = .72). Six patients transitioned to pegfilgrastim for hospitalization, 2 for neutropenia, and 1 for an unspecified reason. Nine patients (15%) who received filgrastim ended up transitioning to pegfilgrastim; 6 (67%) of these patients were transitioned due to hospitalization for FN. Of all the patients who transitioned to pegfilgrastim, 1 patient on a high risk for FN regimen developed sepsis due to herpes zoster in the setting of neutropenia after the previous cycle of chemotherapy.
Real-world data are limited regarding G-CSF practice patterns; however, available data demonstrate patients may receive suboptimal treatment courses of filgrastim leading to increased complications associated with neutropenia and FN, such as dose delays and hospitalizations.8,9 At the South Texas Veterans Health Care System, 48 patients (81%) received a filgrastim course of ≥ 7 days as an initial course for primary prophylaxis. Multivariate analyses performed by Weycker and colleagues described a decreased risk of hospitalization for neutropenia or FN with each additional day of filgrastim prophylaxis; however, such analysis could not be performed in our data set due to the small sample size.8 In this review, 10 patients (17%) experienced treatment delays due to neutropenia or FN, mirroring previously published data. The hospitalization rate of 25% is higher than the published incidence of 5.2% of cancer-related hospitalizations among adults.7,10 This difference may be explained by a difference in health care access for the veteran population.
As an alternative to daily filgrastim injections, the National Comprehensive Cancer Network also recommends a single dose of pegfilgrastim for primary prevention of FN. Efficacy benefits of pegfilgrastim use include increased patient adherence due to a single injection, a reduction in FN incidence and FN-related hospitalizations, and improved time to ANC recovery compared with filgrastim.11 There are reports suggesting pegfilgrastim significantly reduces neutropenia and FN incidence to a greater extent compared with daily filgrastim injections.6 In patients with breast cancer receiving dose-dense adjuvant chemotherapy, there are data demonstrating that patients who received filgrastim were more likely to experience severe neutropenia, dose reductions, and treatment delays leading to lower dose density compared with pegfilgrastim.12 Of the 19 patients with breast cancer included in our population, 26% experienced one of the previously described outcomes leading to either extensions of daily filgrastim injections or transitions to pegfilgrastim to successfully maintain dose density. In patients with acute myeloid leukemia receiving consolidation chemotherapy, filgrastim was found to be associated with a statistically significant increased risk of hospitalizations compared with pegfilgrastim.13 The one patient with acute myeloid leukemia included in our study did not require additional hospitalizations for neutropenia or FN after transitioning to pegfilgrastim.
Given the cost advantage, the South Texas Veterans Health Care System continues to prefer daily filgrastim injections. A recent survey demonstrated that 73% of patients at 23 sites in the Veterans Health Administration used filgrastim rather than pegfilgrastim for cost savings, although it is recognized that daily filgrastim injections are less convenient for patients.14 This analysis did not review costs associated with hospitalization for FN or the appropriateness of G-CSF use. Cancer-related neutropenia accounts for 8.3% of all cancer-related hospitalization costs among adults; the average hospitalization costs nearly $25,000 per stay and about $2.3 billion among adult patients with cancer annually.10,15
Limitations
This study has limitations that affected the applicability and interpretation of the results. This included the study design since it was a retrospective, single-center, descriptive cohort study. Patient adherence to daily filgrastim injections could not be assessed due to the retrospective nature of the study. The small sample size of 59 patients was prohibitive for utilization of additional analytical tools. Additionally, the predominately male veteran population may make applicability to non-VA populations restrictive.
CONCLUSIONS
Based on the incidence of primary and secondary outcomes associated with using daily filgrastim injections as primary prophylaxis in this study, additional measures such as tracking postnadir ANCs should be performed to ensure patients receive an appropriate number of filgrastim doses to prevent complications associated with neutropenia.
Acknowledgments
We thank Eric Dougherty, PharmD, for assistance in producing granulocyte colony-stimulating factor data.
Febrile neutropenia (FN) frequently occurs in patients receiving chemotherapy, with the greatest risk of complications occurring in those who experience profound and prolonged neutropenia. Although granulocyte colony-stimulating factor (G-CSF) prophylaxis has been shown to reduce the risk and duration of chemotherapy-induced neutropenia and FN, there is no well-established optimal regimen.1 The 2022 National Comprehensive Cancer Network guidelines for hematopoietic growth factors recommend prophylaxis with G-CSF in at-risk patients receiving chemotherapy, specifically in chemotherapy regimens considered high risk for FN (incidence > 20%) or intermediate risk for FN (incidence 10%-20%) with additional patient risk factors.2 The incidence of developing FN with at least 1 chemotherapy cycle is estimated at 10% to 50% of patients with solid tumors and > 80% of patients with hematologic malignancies.3 The rate of major complications (eg, hypotension, acute renal, respiratory, or heart failure) in the context of FN is 25% to 30%, and mortality is reported up to 11% in this population.4
Because of the significant consequences of neutropenia and FN, prevention is imperative due to the increase in morbidity and mortality, including chemotherapy delays, increased hospitalizations, chemotherapy dose reductions, and discontinuations that cause delays in care.5 In patients with curative malignancies, these consequences can negatively impact treatment efficacy and overall survival. Additionally, infections occur in 20% to 30% of patients with febrile episodes. Although fever is often the only clinical sign or symptom of infection, patients who are profoundly neutropenic may present with suspected infection and be afebrile or hypothermic.3
For filgrastim, the National Comprehensive Cancer Network guidelines do not specify the total days of required injections but state that a daily dose should be given until the postnadir absolute neutrophil count (ANC) recovers to normal or near normal levels by laboratory standards.2 It is uncommon in clinical practice to track postnadir ANCs due to frequent laboratory monitoring. Clinical trial data suggest an average duration of 11 days of daily filgrastim injections for ANC recovery; however, real-world data exist supporting a range from 4 to 10 days with a median of 7 injections per cycle for prevention of neutropenia or FN.6,7
At the South Texas Veterans Health Care System in San Antonio, daily filgrastim injections are preferred due to cost; patients typically receive a 7-day course for primary prophylaxis for FN.
Electronic health record reviews at the South Texas Veterans Health Care System were performed to identify patients who received filgrastim primary prophylaxis (defined as filgrastim, tbo-filgrastim, or filgrastim-sndz) for a curative cancer diagnosis. Primary prophylaxis refers to the administration of G-CSF in the first cycle of chemotherapy before the onset of neutropenia. Patients received filgrastim prophylaxis if they were undergoing treatment with a chemotherapy regimen with either high risk for FN or a chemotherapy regimen with an intermediate risk for FN and additional patient risk factors. Risk factors for patients included prior chemotherapy or radiation therapy; persistent neutropenia; bone marrow involvement by tumor; recent surgery and/or open wounds; liver dysfunction (defined as total bilirubin > 2 mg/dL); renal dysfunction (defined as creatinine clearance < 50 mL/min); and those aged > 65 years receiving full chemotherapy dose intensity. Neutropenia is defined as a decrease in ANC < 1000 neutrophils/μL, whereas FN is defined as a single temperature of > 38.3 °C or > 38.0 °C for longer than 1 hour with < 500 neutrophils/μL or < 1000 neutrophils/μL predicted to decline to < 500 neutrophils/μL over the next 48 hours. All patients had their filgrastim dispensed for home administration during their chemotherapy appointment.
Descriptive statistics were used to summarize the study population and their health outcomes. Fisher exact test was used to compare FN incidence for high- and intermediate-risk FN groups.
RESULTS
Between September 1, 2015, and September 24, 2020, 381 patients received filgrastim. Of these patients, 59 met the inclusion criteria. Patients receiving filgrastim were excluded due to stem cell transplant mobilization/engraftment (n = 145), a noncurative cancer diagnosis (n = 134), use as a secondary prophylaxis (n = 33), and nononcologic neutropenia (n = 8). Additionally, 2 patients initially received pegfilgrastim and were not included in this data set.
The median (IQR) age was 64 (55-70) years and 42 patients (71%) were male (Table 1).
Ten patients (17%) experienced dose delays despite filgrastim use (Table 2).
Nine patients (15%) had the number of filgrastim injections per chemotherapy cycle extended due to various reasons. Five patients required extended days after hospitalization for FN, 3 patients for dose delays due to neutropenia with the previous cycle, and 1 patient with an undocumented reason outside of the prespecified outcomes. Two of these patients experienced continued neutropenia and dose delays after extending filgrastim from 5 to 7 days or 7 to 10 days. One patient who experienced continued neutropenia after extending filgrastim to 10 days was subsequently transitioned to pegfilgrastim without further episodes of neutropenia. The other patient who still experienced neutropenia after extending filgrastim to 7 days was receiving the last chemotherapy cycle and did not require subsequent doses of filgrastim.
Two additional patients were not included in the hospitalizations. The first was a patient on a chemotherapy regimen with a high risk for FN who presented to the emergency department with documented FN but was never admitted since the patient elected to not be hospitalized. This patient developed oral, anal, and vaginal candidiasis, and it was noted by the oncologist at the next clinic visit that this was likely secondary to grade 4 neutropenia (ANC < 500 neutrophils/μL). The second was a patient on a chemotherapy regimen with an intermediate risk for FN who was already hospitalized but had developed FN and sepsis despite filgrastim use.
Finally, out of the hospitalized patients, 9 (15%) had infections. This included 6 patients (18%) in the high risk for FN group and 3 patients (12%) in the intermediate risk for FN group (P = .72). Six patients transitioned to pegfilgrastim for hospitalization, 2 for neutropenia, and 1 for an unspecified reason. Nine patients (15%) who received filgrastim ended up transitioning to pegfilgrastim; 6 (67%) of these patients were transitioned due to hospitalization for FN. Of all the patients who transitioned to pegfilgrastim, 1 patient on a high risk for FN regimen developed sepsis due to herpes zoster in the setting of neutropenia after the previous cycle of chemotherapy.
Real-world data are limited regarding G-CSF practice patterns; however, available data demonstrate patients may receive suboptimal treatment courses of filgrastim leading to increased complications associated with neutropenia and FN, such as dose delays and hospitalizations.8,9 At the South Texas Veterans Health Care System, 48 patients (81%) received a filgrastim course of ≥ 7 days as an initial course for primary prophylaxis. Multivariate analyses performed by Weycker and colleagues described a decreased risk of hospitalization for neutropenia or FN with each additional day of filgrastim prophylaxis; however, such analysis could not be performed in our data set due to the small sample size.8 In this review, 10 patients (17%) experienced treatment delays due to neutropenia or FN, mirroring previously published data. The hospitalization rate of 25% is higher than the published incidence of 5.2% of cancer-related hospitalizations among adults.7,10 This difference may be explained by a difference in health care access for the veteran population.
As an alternative to daily filgrastim injections, the National Comprehensive Cancer Network also recommends a single dose of pegfilgrastim for primary prevention of FN. Efficacy benefits of pegfilgrastim use include increased patient adherence due to a single injection, a reduction in FN incidence and FN-related hospitalizations, and improved time to ANC recovery compared with filgrastim.11 There are reports suggesting pegfilgrastim significantly reduces neutropenia and FN incidence to a greater extent compared with daily filgrastim injections.6 In patients with breast cancer receiving dose-dense adjuvant chemotherapy, there are data demonstrating that patients who received filgrastim were more likely to experience severe neutropenia, dose reductions, and treatment delays leading to lower dose density compared with pegfilgrastim.12 Of the 19 patients with breast cancer included in our population, 26% experienced one of the previously described outcomes leading to either extensions of daily filgrastim injections or transitions to pegfilgrastim to successfully maintain dose density. In patients with acute myeloid leukemia receiving consolidation chemotherapy, filgrastim was found to be associated with a statistically significant increased risk of hospitalizations compared with pegfilgrastim.13 The one patient with acute myeloid leukemia included in our study did not require additional hospitalizations for neutropenia or FN after transitioning to pegfilgrastim.
Given the cost advantage, the South Texas Veterans Health Care System continues to prefer daily filgrastim injections. A recent survey demonstrated that 73% of patients at 23 sites in the Veterans Health Administration used filgrastim rather than pegfilgrastim for cost savings, although it is recognized that daily filgrastim injections are less convenient for patients.14 This analysis did not review costs associated with hospitalization for FN or the appropriateness of G-CSF use. Cancer-related neutropenia accounts for 8.3% of all cancer-related hospitalization costs among adults; the average hospitalization costs nearly $25,000 per stay and about $2.3 billion among adult patients with cancer annually.10,15
Limitations
This study has limitations that affected the applicability and interpretation of the results. This included the study design since it was a retrospective, single-center, descriptive cohort study. Patient adherence to daily filgrastim injections could not be assessed due to the retrospective nature of the study. The small sample size of 59 patients was prohibitive for utilization of additional analytical tools. Additionally, the predominately male veteran population may make applicability to non-VA populations restrictive.
CONCLUSIONS
Based on the incidence of primary and secondary outcomes associated with using daily filgrastim injections as primary prophylaxis in this study, additional measures such as tracking postnadir ANCs should be performed to ensure patients receive an appropriate number of filgrastim doses to prevent complications associated with neutropenia.
Acknowledgments
We thank Eric Dougherty, PharmD, for assistance in producing granulocyte colony-stimulating factor data.
1. Hanna KS, Mancini R, Wilson D, Zuckerman D. Comparing granulocyte colony-stimulating factors prescribing practices versus guideline recommendations in a large community cancer center. J Hematol Oncol Pharm. 2019;9(3):121-126.
2. Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines insights: hematopoietic growth factors, version 1.2022. J Natl Compr Canc Netw. 2022;20(5):436-442. doi:10.6004/jnccn.2022.0026
3. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. doi:10.1093/cid/cir073
4. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-1453. doi:10.1200/JCO.2017.77.6211
5. Clemons M, Fergusson D, Simos D, et al. A multicentre, randomized trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951-957. doi:10.1016/j.annonc.2020.04.005
6. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. Published 2011 Sep 23. doi:10.1186/1471-2407-11-404
7. Altwairgi A, Hopman W, Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20(3):e171-e179. doi:10.3747/co.20.1306
8. Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402-407. doi:10.1345/aph.1G516
9. Link H, Nietsch J, Kerkmann M, Ortner P; Supportive Care Group (ASORS) of the German Cancer Society (DKG). Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy—a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367-376. doi:10.1007/s00520-015-2779-5
10. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266. doi:10.1002/cncr.21847
11. Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25(11):3295-3304. doi :10.1007/s00520-017-3842-1
12. Kourlaba G, Dimopoulos MA, Pectasides D, et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer. 2015;23(7):2045-2051. doi:10.1007/s00520-014-2555-y
13. Field E, Caimi PF, Cooper B, et al. Comparison of pegfilgrastim and filgrastim to prevent neutropenic fever during consolidation with high dose cytarabine for acute myeloid leukemia. Blood. 2018;132(suppl 1):1404. doi:10.1182/blood-2018-99-118336
14. Knopf K, Hrureshky W, Love BL, Norris L, Bennett CL. Cost-effective use of white blood cell growth factors in the Veterans Administration. Blood. 2018;132(suppl 1):4761. doi:10.1182/blood-2018-99-119724
15. Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-e561. doi:10.1200/JOP.2016.019588
1. Hanna KS, Mancini R, Wilson D, Zuckerman D. Comparing granulocyte colony-stimulating factors prescribing practices versus guideline recommendations in a large community cancer center. J Hematol Oncol Pharm. 2019;9(3):121-126.
2. Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines insights: hematopoietic growth factors, version 1.2022. J Natl Compr Canc Netw. 2022;20(5):436-442. doi:10.6004/jnccn.2022.0026
3. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. doi:10.1093/cid/cir073
4. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-1453. doi:10.1200/JCO.2017.77.6211
5. Clemons M, Fergusson D, Simos D, et al. A multicentre, randomized trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951-957. doi:10.1016/j.annonc.2020.04.005
6. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. Published 2011 Sep 23. doi:10.1186/1471-2407-11-404
7. Altwairgi A, Hopman W, Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20(3):e171-e179. doi:10.3747/co.20.1306
8. Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402-407. doi:10.1345/aph.1G516
9. Link H, Nietsch J, Kerkmann M, Ortner P; Supportive Care Group (ASORS) of the German Cancer Society (DKG). Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy—a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367-376. doi:10.1007/s00520-015-2779-5
10. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266. doi:10.1002/cncr.21847
11. Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25(11):3295-3304. doi :10.1007/s00520-017-3842-1
12. Kourlaba G, Dimopoulos MA, Pectasides D, et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer. 2015;23(7):2045-2051. doi:10.1007/s00520-014-2555-y
13. Field E, Caimi PF, Cooper B, et al. Comparison of pegfilgrastim and filgrastim to prevent neutropenic fever during consolidation with high dose cytarabine for acute myeloid leukemia. Blood. 2018;132(suppl 1):1404. doi:10.1182/blood-2018-99-118336
14. Knopf K, Hrureshky W, Love BL, Norris L, Bennett CL. Cost-effective use of white blood cell growth factors in the Veterans Administration. Blood. 2018;132(suppl 1):4761. doi:10.1182/blood-2018-99-119724
15. Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-e561. doi:10.1200/JOP.2016.019588
Contralateral Constrictor Dose Predicts Swallowing Function After Radiation for Head and Neck Cancer
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck cancer. 1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia. 4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was. 5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures. 6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume (PTV) for the lowest dose target volume. NRG head and neck trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for head and neck squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as head and neck cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the head and neck.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for head and neck cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy. Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 70.0 Gy in 33 fractions, with elective volumes treated to 54.5 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased dysphagia at 1 year.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for head and neck cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant. An advantage of contralateral constrictor is that it is independent of PTV size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with head and neck cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after intensity-modulated radiotherapy for head and neck cancer.16 Radiation has been shown to decrease pharyngeal function in patients with head and neck cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with head and neck cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with head and neck cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck cancer. 1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia. 4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was. 5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures. 6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume (PTV) for the lowest dose target volume. NRG head and neck trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for head and neck squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as head and neck cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the head and neck.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for head and neck cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy. Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 70.0 Gy in 33 fractions, with elective volumes treated to 54.5 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased dysphagia at 1 year.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for head and neck cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant. An advantage of contralateral constrictor is that it is independent of PTV size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with head and neck cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after intensity-modulated radiotherapy for head and neck cancer.16 Radiation has been shown to decrease pharyngeal function in patients with head and neck cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with head and neck cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with head and neck cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck cancer. 1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia. 4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was. 5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures. 6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume (PTV) for the lowest dose target volume. NRG head and neck trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for head and neck squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as head and neck cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the head and neck.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for head and neck cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy. Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 70.0 Gy in 33 fractions, with elective volumes treated to 54.5 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased dysphagia at 1 year.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for head and neck cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant. An advantage of contralateral constrictor is that it is independent of PTV size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with head and neck cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after intensity-modulated radiotherapy for head and neck cancer.16 Radiation has been shown to decrease pharyngeal function in patients with head and neck cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with head and neck cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with head and neck cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938
Sybil – Prophecies for lung cancer risk prediction?
Thoracic Oncology and Chest Procedures Network
Lung Cancer Section
The mortality benefit associated with lung cancer screening (LCS) using low dose CT (LDCT) relies, in large part, on adherence rates to annual screening of ≥90%. However, the first 1 million “real world” patients screened in the US had very low (22%) annual adherence (Silvestri, et al. Chest. 2023;S0012-3692[23]00175-7). Refining how we estimate future lung cancer risk is an important opportunity for personalized medicine to bolster adherence to follow-up after initial LDCT.
(Mikhael, et al. J Clin Oncol. 2023;JCO2201345). The model was developed, trained, and tested in a total of 14,185 National Lung Screening Trial (NLST) participants including all cancer diagnoses. Within these data, Sybil’s accuracy in predicting 1-year lung cancer risk had AUC 0.92 (95% CI, 0.88-0.95) and at 6 years, AUC 0.75 (95% CI, 0.72-0.78).
The model was validated in two large independent LCS datasets, one in the US and one in Taiwan, where an LDCT can be obtained regardless of a personal smoking history. The cancer prevalence in these datasets was 3.4% and 0.9%, respectively. Reassuringly, Sybil’s performance was similar to the NLST data and was maintained in relevant subgroups such as sex, age and smoking history. Furthermore, Sybil reduced the false positive rate in the NLST to 8% at baseline scan, compared with 14% for Lung-RADS 1.0. Sybil’s algorithm, unlike others, has been made publicly available and hopefully will spur further validation and prospective study.
Robert Smyth, MD
Member-at-Large
Thoracic Oncology and Chest Procedures Network
Lung Cancer Section
The mortality benefit associated with lung cancer screening (LCS) using low dose CT (LDCT) relies, in large part, on adherence rates to annual screening of ≥90%. However, the first 1 million “real world” patients screened in the US had very low (22%) annual adherence (Silvestri, et al. Chest. 2023;S0012-3692[23]00175-7). Refining how we estimate future lung cancer risk is an important opportunity for personalized medicine to bolster adherence to follow-up after initial LDCT.
(Mikhael, et al. J Clin Oncol. 2023;JCO2201345). The model was developed, trained, and tested in a total of 14,185 National Lung Screening Trial (NLST) participants including all cancer diagnoses. Within these data, Sybil’s accuracy in predicting 1-year lung cancer risk had AUC 0.92 (95% CI, 0.88-0.95) and at 6 years, AUC 0.75 (95% CI, 0.72-0.78).
The model was validated in two large independent LCS datasets, one in the US and one in Taiwan, where an LDCT can be obtained regardless of a personal smoking history. The cancer prevalence in these datasets was 3.4% and 0.9%, respectively. Reassuringly, Sybil’s performance was similar to the NLST data and was maintained in relevant subgroups such as sex, age and smoking history. Furthermore, Sybil reduced the false positive rate in the NLST to 8% at baseline scan, compared with 14% for Lung-RADS 1.0. Sybil’s algorithm, unlike others, has been made publicly available and hopefully will spur further validation and prospective study.
Robert Smyth, MD
Member-at-Large
Thoracic Oncology and Chest Procedures Network
Lung Cancer Section
The mortality benefit associated with lung cancer screening (LCS) using low dose CT (LDCT) relies, in large part, on adherence rates to annual screening of ≥90%. However, the first 1 million “real world” patients screened in the US had very low (22%) annual adherence (Silvestri, et al. Chest. 2023;S0012-3692[23]00175-7). Refining how we estimate future lung cancer risk is an important opportunity for personalized medicine to bolster adherence to follow-up after initial LDCT.
(Mikhael, et al. J Clin Oncol. 2023;JCO2201345). The model was developed, trained, and tested in a total of 14,185 National Lung Screening Trial (NLST) participants including all cancer diagnoses. Within these data, Sybil’s accuracy in predicting 1-year lung cancer risk had AUC 0.92 (95% CI, 0.88-0.95) and at 6 years, AUC 0.75 (95% CI, 0.72-0.78).
The model was validated in two large independent LCS datasets, one in the US and one in Taiwan, where an LDCT can be obtained regardless of a personal smoking history. The cancer prevalence in these datasets was 3.4% and 0.9%, respectively. Reassuringly, Sybil’s performance was similar to the NLST data and was maintained in relevant subgroups such as sex, age and smoking history. Furthermore, Sybil reduced the false positive rate in the NLST to 8% at baseline scan, compared with 14% for Lung-RADS 1.0. Sybil’s algorithm, unlike others, has been made publicly available and hopefully will spur further validation and prospective study.
Robert Smyth, MD
Member-at-Large