User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Preterm and early term birth linked to an increased risk of autism
Preterm and early birth is associated with an increased risk of autism independent of genetic or environmental factors, according to new research published in Pediatrics.
Although previous studies have linked preterm births to an increased risk of autism – one 2017 study published in Cerebral Cortex found that 27.4% of the children born extremely preterm were diagnosed with autism – Casey Crump, MD, PhD, said potential causality, sex-specific differences, and association with early-term births were still unclear.
“Preterm birth had previously been linked with higher risk of autism; however, several important questions remained unanswered,” said Dr. Crump, professor and vice chair for research at the department of family medicine and community health and professor of epidemiology in the department of population health science and policy at Icahn School of Medicine at Mount Sinai New York. “To our knowledge, [our study] is the largest to date of gestational age at birth in relation to autism, and one of the first to investigate sex-specific differences, early term birth, or the influence of shared familial factors.”
Dr. Crump and colleagues examined data from more than 4 million infants born in Sweden between 1973 and 2013 who were followed-up for autism spectrum disorder identified from nationwide outpatient and inpatient diagnoses through December 2015. Children born between 22 and 27 weeks were considered extremely preterm, those born between 28 and 33 week were characterized as very to moderate preterm, and those born between 34 and 36 weeks were considered late preterm. Early-term births are characterized as infants born between 37 and 38 weeks and children born between 39 and 41 weeks were considered term births.
They found that 6.1% of those born extremely preterm were diagnosed with autism. Meanwhile, autism spectrum disorder prevalences were 2.6% for very to moderate preterm, 1.9% for late preterm, 2.1% for all preterm, and 1.6% for early term, compared with 1.4% for term birth.
The researchers’ analysis showed that preterm and early birth were associated with a significantly increased risk of autism in males and females. Children who were born extremely preterm had an approximately fourfold increased risk of autism. Researchers also found that each additional week of gestation was associated with a 5% lower prevalence of autism spectrum disorder (ASD) on average.
“The elevated risk even in [late preterm] infants is not completely surprising because a number of investigators have shown higher incidences of early cognitive, language motor and impairment, and school problems ... and psychiatric disorders, some of which may extend to adulthood,” Elisabeth McGowan, MD, who was not involved in the study, said in a solicited editorial commentary about the study.
Dr. Crump believes the association between preterm birth and autism may be because of increased inflammatory marker levels. A 2009 study published in Reproductive Sciences found that increased proinflammatory cytokine levels have been associated with the timing and initiation of preterm birth, and also have been detected in the brain and cerebrospinal fluid of individuals with autism “and may play a key role in its pathogenesis,” Dr. Crump said.
“Inflammatory-driven alteration of neuronal connections during critical periods of brain development may be central to the development of autism,” Dr. Crump explained.
However, Dr. Crump said that, although the relative risks of autism were higher in those born preterm, the absolute risk of the condition is low.
“The report by Crump is in many ways a definitive accounting of the elevated rates of ASD in preterm infants,” said Dr. McGowan, associate professor of pediatrics at the Women and Infants Hospital, Providence, R.I. “And although the impact of prematurity on brain development may be part of the causal chain resulting in ASD (or other neurodevelopmental outcomes), these factors are operating in a complex biological landscape, with pathways to ASD outcomes that can be expected to be heterogeneous.”
ASD is a developmental condition that affects about 1 in 54 children, according to the Centers for Disease Control and Prevention. Many children are not diagnosed with ASD until later in childhood, which in some cases delays treatment and early intervention. ASD may be detected as early as 18 months, but the average age of diagnosis for ASD is 4.3 years, according to the CDC.
“Children born prematurely need early evaluation and long-term follow-up to facilitate timely detection and treatment of autism, especially those born at the earliest gestational ages,” Dr. Crump said in an interview. “In patients of all ages, gestational age at birth should be routinely included in history-taking and medical records to help identify in clinical practice those born preterm or early term. Such information can provide additional valuable context for understanding patients’ health and may facilitate earlier evaluation for autism and other neurodevelopmental conditions in those born prematurely.”
Dr. Crump and colleagues said more research is needed to understand the biologic mechanisms linking preterm birth with higher risks of autism, which “may reveal new targets for intervention at critical windows of neurodevelopment to improve the disease trajectory.”
Experts interviewed did not disclose any relevant financial relationships.
Preterm and early birth is associated with an increased risk of autism independent of genetic or environmental factors, according to new research published in Pediatrics.
Although previous studies have linked preterm births to an increased risk of autism – one 2017 study published in Cerebral Cortex found that 27.4% of the children born extremely preterm were diagnosed with autism – Casey Crump, MD, PhD, said potential causality, sex-specific differences, and association with early-term births were still unclear.
“Preterm birth had previously been linked with higher risk of autism; however, several important questions remained unanswered,” said Dr. Crump, professor and vice chair for research at the department of family medicine and community health and professor of epidemiology in the department of population health science and policy at Icahn School of Medicine at Mount Sinai New York. “To our knowledge, [our study] is the largest to date of gestational age at birth in relation to autism, and one of the first to investigate sex-specific differences, early term birth, or the influence of shared familial factors.”
Dr. Crump and colleagues examined data from more than 4 million infants born in Sweden between 1973 and 2013 who were followed-up for autism spectrum disorder identified from nationwide outpatient and inpatient diagnoses through December 2015. Children born between 22 and 27 weeks were considered extremely preterm, those born between 28 and 33 week were characterized as very to moderate preterm, and those born between 34 and 36 weeks were considered late preterm. Early-term births are characterized as infants born between 37 and 38 weeks and children born between 39 and 41 weeks were considered term births.
They found that 6.1% of those born extremely preterm were diagnosed with autism. Meanwhile, autism spectrum disorder prevalences were 2.6% for very to moderate preterm, 1.9% for late preterm, 2.1% for all preterm, and 1.6% for early term, compared with 1.4% for term birth.
The researchers’ analysis showed that preterm and early birth were associated with a significantly increased risk of autism in males and females. Children who were born extremely preterm had an approximately fourfold increased risk of autism. Researchers also found that each additional week of gestation was associated with a 5% lower prevalence of autism spectrum disorder (ASD) on average.
“The elevated risk even in [late preterm] infants is not completely surprising because a number of investigators have shown higher incidences of early cognitive, language motor and impairment, and school problems ... and psychiatric disorders, some of which may extend to adulthood,” Elisabeth McGowan, MD, who was not involved in the study, said in a solicited editorial commentary about the study.
Dr. Crump believes the association between preterm birth and autism may be because of increased inflammatory marker levels. A 2009 study published in Reproductive Sciences found that increased proinflammatory cytokine levels have been associated with the timing and initiation of preterm birth, and also have been detected in the brain and cerebrospinal fluid of individuals with autism “and may play a key role in its pathogenesis,” Dr. Crump said.
“Inflammatory-driven alteration of neuronal connections during critical periods of brain development may be central to the development of autism,” Dr. Crump explained.
However, Dr. Crump said that, although the relative risks of autism were higher in those born preterm, the absolute risk of the condition is low.
“The report by Crump is in many ways a definitive accounting of the elevated rates of ASD in preterm infants,” said Dr. McGowan, associate professor of pediatrics at the Women and Infants Hospital, Providence, R.I. “And although the impact of prematurity on brain development may be part of the causal chain resulting in ASD (or other neurodevelopmental outcomes), these factors are operating in a complex biological landscape, with pathways to ASD outcomes that can be expected to be heterogeneous.”
ASD is a developmental condition that affects about 1 in 54 children, according to the Centers for Disease Control and Prevention. Many children are not diagnosed with ASD until later in childhood, which in some cases delays treatment and early intervention. ASD may be detected as early as 18 months, but the average age of diagnosis for ASD is 4.3 years, according to the CDC.
“Children born prematurely need early evaluation and long-term follow-up to facilitate timely detection and treatment of autism, especially those born at the earliest gestational ages,” Dr. Crump said in an interview. “In patients of all ages, gestational age at birth should be routinely included in history-taking and medical records to help identify in clinical practice those born preterm or early term. Such information can provide additional valuable context for understanding patients’ health and may facilitate earlier evaluation for autism and other neurodevelopmental conditions in those born prematurely.”
Dr. Crump and colleagues said more research is needed to understand the biologic mechanisms linking preterm birth with higher risks of autism, which “may reveal new targets for intervention at critical windows of neurodevelopment to improve the disease trajectory.”
Experts interviewed did not disclose any relevant financial relationships.
Preterm and early birth is associated with an increased risk of autism independent of genetic or environmental factors, according to new research published in Pediatrics.
Although previous studies have linked preterm births to an increased risk of autism – one 2017 study published in Cerebral Cortex found that 27.4% of the children born extremely preterm were diagnosed with autism – Casey Crump, MD, PhD, said potential causality, sex-specific differences, and association with early-term births were still unclear.
“Preterm birth had previously been linked with higher risk of autism; however, several important questions remained unanswered,” said Dr. Crump, professor and vice chair for research at the department of family medicine and community health and professor of epidemiology in the department of population health science and policy at Icahn School of Medicine at Mount Sinai New York. “To our knowledge, [our study] is the largest to date of gestational age at birth in relation to autism, and one of the first to investigate sex-specific differences, early term birth, or the influence of shared familial factors.”
Dr. Crump and colleagues examined data from more than 4 million infants born in Sweden between 1973 and 2013 who were followed-up for autism spectrum disorder identified from nationwide outpatient and inpatient diagnoses through December 2015. Children born between 22 and 27 weeks were considered extremely preterm, those born between 28 and 33 week were characterized as very to moderate preterm, and those born between 34 and 36 weeks were considered late preterm. Early-term births are characterized as infants born between 37 and 38 weeks and children born between 39 and 41 weeks were considered term births.
They found that 6.1% of those born extremely preterm were diagnosed with autism. Meanwhile, autism spectrum disorder prevalences were 2.6% for very to moderate preterm, 1.9% for late preterm, 2.1% for all preterm, and 1.6% for early term, compared with 1.4% for term birth.
The researchers’ analysis showed that preterm and early birth were associated with a significantly increased risk of autism in males and females. Children who were born extremely preterm had an approximately fourfold increased risk of autism. Researchers also found that each additional week of gestation was associated with a 5% lower prevalence of autism spectrum disorder (ASD) on average.
“The elevated risk even in [late preterm] infants is not completely surprising because a number of investigators have shown higher incidences of early cognitive, language motor and impairment, and school problems ... and psychiatric disorders, some of which may extend to adulthood,” Elisabeth McGowan, MD, who was not involved in the study, said in a solicited editorial commentary about the study.
Dr. Crump believes the association between preterm birth and autism may be because of increased inflammatory marker levels. A 2009 study published in Reproductive Sciences found that increased proinflammatory cytokine levels have been associated with the timing and initiation of preterm birth, and also have been detected in the brain and cerebrospinal fluid of individuals with autism “and may play a key role in its pathogenesis,” Dr. Crump said.
“Inflammatory-driven alteration of neuronal connections during critical periods of brain development may be central to the development of autism,” Dr. Crump explained.
However, Dr. Crump said that, although the relative risks of autism were higher in those born preterm, the absolute risk of the condition is low.
“The report by Crump is in many ways a definitive accounting of the elevated rates of ASD in preterm infants,” said Dr. McGowan, associate professor of pediatrics at the Women and Infants Hospital, Providence, R.I. “And although the impact of prematurity on brain development may be part of the causal chain resulting in ASD (or other neurodevelopmental outcomes), these factors are operating in a complex biological landscape, with pathways to ASD outcomes that can be expected to be heterogeneous.”
ASD is a developmental condition that affects about 1 in 54 children, according to the Centers for Disease Control and Prevention. Many children are not diagnosed with ASD until later in childhood, which in some cases delays treatment and early intervention. ASD may be detected as early as 18 months, but the average age of diagnosis for ASD is 4.3 years, according to the CDC.
“Children born prematurely need early evaluation and long-term follow-up to facilitate timely detection and treatment of autism, especially those born at the earliest gestational ages,” Dr. Crump said in an interview. “In patients of all ages, gestational age at birth should be routinely included in history-taking and medical records to help identify in clinical practice those born preterm or early term. Such information can provide additional valuable context for understanding patients’ health and may facilitate earlier evaluation for autism and other neurodevelopmental conditions in those born prematurely.”
Dr. Crump and colleagues said more research is needed to understand the biologic mechanisms linking preterm birth with higher risks of autism, which “may reveal new targets for intervention at critical windows of neurodevelopment to improve the disease trajectory.”
Experts interviewed did not disclose any relevant financial relationships.
FROM PEDIATRICS
‘Reassuring’ findings for second-generation antipsychotics during pregnancy
Second-generation antipsychotics (SGAs) taken by pregnant women are linked to a low rate of adverse effects in their children, new research suggests.
Data from a large registry study of almost 2,000 women showed that 2.5% of the live births in a group that had been exposed to antipsychotics had confirmed major malformations compared with 2% of the live births in a non-exposed group. This translated into an estimated odds ratio of 1.5 for major malformations.
“The 2.5% absolute risk for major malformations is consistent with the estimates of the Centers for Disease Control and Prevention’s national baseline rate of major malformations in the general population,” lead author Adele Viguera, MD, MPH, director of research for women’s mental health, Cleveland Clinic Neurological Institute, told this news organization.
“Our results are reassuring and suggest that second-generation antipsychotics, as a class, do not substantially increase the risk of major malformations,” Dr. Viguera said.
The findings were published online August 3 in the Journal of Clinical Psychiatry.
Safety data scarce
Despite the increasing use of SGAs to treat a “spectrum of psychiatric disorders,” relatively little data are available on the reproductive safety of these agents, Dr. Viguera said.
The National Pregnancy Registry for Atypical Antipsychotics (NPRAA) was established in 2008 to determine risk for major malformation among infants exposed to these medications during the first trimester, relative to a comparison group of unexposed infants of mothers with histories of psychiatric morbidity.
The NPRAA follows pregnant women (aged 18 to 45 years) with psychiatric illness who are exposed or unexposed to SGAs during pregnancy. Participants are recruited through nationwide provider referral, self-referral, and advertisement through the Massachusetts General Hospital Center for Women’s Mental Health website.
Specific data collected are shown in the following table.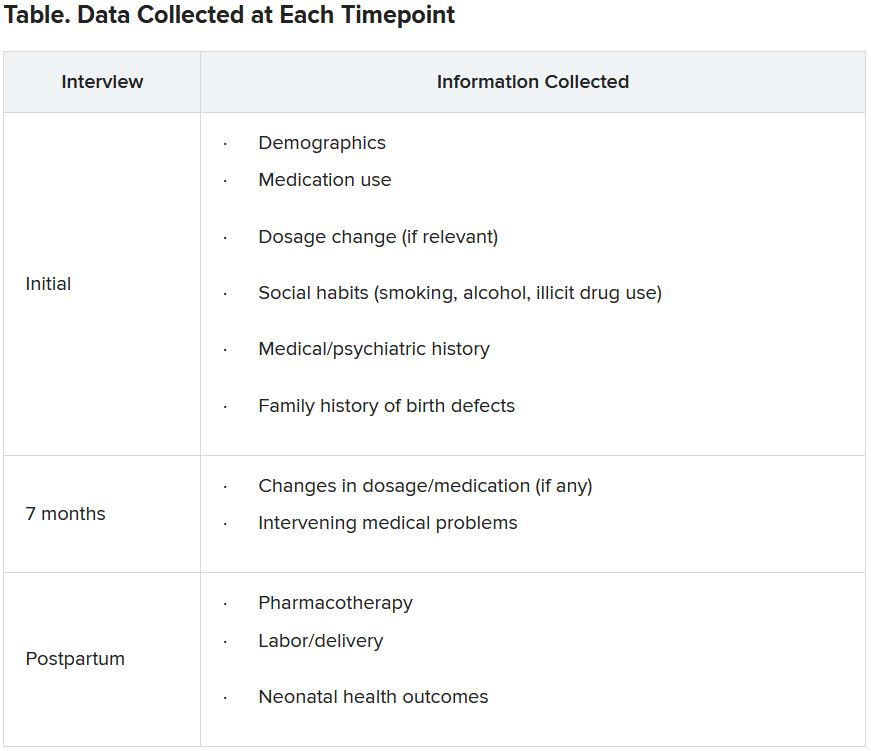
Since publication of the first results in 2015, the sample size for the trial has increased – and the absolute and relative risk for major malformations observed in the study population are “more precise,” the investigators note. The current study presented updated previous findings.
Demographic differences
Of the 1,906 women who enrolled as of April 2020, 1,311 (mean age, 32.6 years; 81.3% White) completed the study and were eligible for inclusion in the analysis.
Although the groups had a virtually identical mean age, fewer women in the exposure group were married compared with those in the non-exposure group (77% vs. 90%, respectively) and fewer had a college education (71.2% vs. 87.8%). There was also a higher percentage of first-trimester cigarette smokers in the exposure group (18.4% vs. 5.1%).
On the other hand, more women in the non-exposure group used alcohol than in the exposure group (28.6% vs. 21.4%, respectively).
The most frequent psychiatric disorder in the exposure group was bipolar disorder (63.9%), followed by major depression (12.9%), anxiety (5.8%), and schizophrenia (4.5%). Only 11.4% of women in the non-exposure group were diagnosed with bipolar disorder, whereas 34.1% were diagnosed with major depression, 31.3% with anxiety, and none with schizophrenia.
Notably, a large percentage of women in both groups had a history of postpartum depression and/or psychosis (41.4% and 35.5%, respectively).
The most frequently used SGAs in the exposure group were quetiapine (Seroquel), aripiprazole (Abilify), and lurasidone (Latuda).
Participants in the exposure group had a higher age at initial onset of primary psychiatric diagnosis and a lower proportion of lifetime illness compared with those in the non-exposure group.
Major clinical implication?
Among 640 live births in the exposure group, which included 17 twin pregnancies and 1 triplet pregnancy, 2.5% reported major malformations. Among 704 live births in the control group, which included 14 twin pregnancies, 1.99% reported major malformations.
The estimated OR for major malformations comparing exposed and unexposed infants was 1.48 (95% confidence interval, 0.625-3.517).
The authors note that their findings were consistent with one of the largest studies to date, which included a nationwide sample of more than 1 million women. Its results showed that, among infants exposed to SGAs versus those who were not exposed, the estimated risk ratio after adjusting for psychiatric conditions was 1.05 (95% CI, 0.96-1.16).
Additionally, “a hallmark of a teratogen is that it tends to cause a specific type or pattern of malformations, and we found no preponderance of one single type of major malformation or specific pattern of malformations among the exposed and unexposed groups,” Dr. Viguera said
“A major clinical implication of these findings is that for women with major mood and/or psychotic disorders, treatment with an atypical antipsychotic during pregnancy may be the most prudent clinical decision, much as continued treatment is recommended for pregnant women with other serious and chronic medical conditions, such as epilepsy,” she added.
The concept of ‘satisficing’
Commenting on the study, Vivien Burt, MD, PhD, founder and director/consultant of the Women’s Life Center at the Resnick University of California, Los Angeles (UCLA) Neuropsychiatric Hospital, called the findings “reassuring.”
The results “support the conclusion that in pregnant women with serious psychiatric illnesses, the use of SGAs is often a better option than avoiding these medications and exposing both the women and their offspring to the adverse consequences of maternal mental illness,” she said.
An accompanying editorial co-authored by Dr. Burt and colleague Sonya Rasminsky, MD, introduced the concept of “satisficing” – a term coined by Herbert Simon, a behavioral economist and Nobel Laureate. “Satisficing” is a “decision-making strategy that aims for a satisfactory (‘good enough’) outcome rather than a perfect one.”
The concept applies to decision-making beyond the field of economics “and is critical to how physicians help patients make decisions when they are faced with multiple treatment options,” said Dr. Burt, a professor emeritus of psychiatry at UCLA.
“The goal of ‘satisficing’ is to plan for the most satisfactory outcome, knowing that there are always unknowns, so in an uncertain world, clinicians should carefully help their patients make decisions that will allow them to achieve an outcome they can best live with,” she noted.
The investigators note that their findings may not be generalizable to the larger population of women taking SGAs, given that their participants were “overwhelmingly White, married, and well-educated women.”
They add that enrollment into the NPRAA registry is ongoing and larger sample sizes will “further narrow the confidence interval around the risk estimates and allow for adjustment of likely sources of confounding.”
The NPRAA is supported by Alkermes, Johnson & Johnson/Janssen Pharmaceuticals, Otsuka America Pharmaceutical, Sunovion Pharmaceuticals, SAGE Therapeutics, Teva Pharmaceuticals, and Aurobindo Pharma. Past sponsors of the NPRAA are listed in the original paper. Dr. Viguera receives research support from the NPRAA, Alkermes Biopharmaceuticals, Aurobindo Pharma, Janssen Pharmaceuticals, Otsuka Pharmaceutical, Sunovion Pharmaceuticals, Teva Pharmaceuticals, and SAGE Therapeutics and receives adviser/consulting fees from Up-to-Date. Dr. Burt has been a consultant/speaker for Sage Therapeutics. Dr. Rasminsky has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Second-generation antipsychotics (SGAs) taken by pregnant women are linked to a low rate of adverse effects in their children, new research suggests.
Data from a large registry study of almost 2,000 women showed that 2.5% of the live births in a group that had been exposed to antipsychotics had confirmed major malformations compared with 2% of the live births in a non-exposed group. This translated into an estimated odds ratio of 1.5 for major malformations.
“The 2.5% absolute risk for major malformations is consistent with the estimates of the Centers for Disease Control and Prevention’s national baseline rate of major malformations in the general population,” lead author Adele Viguera, MD, MPH, director of research for women’s mental health, Cleveland Clinic Neurological Institute, told this news organization.
“Our results are reassuring and suggest that second-generation antipsychotics, as a class, do not substantially increase the risk of major malformations,” Dr. Viguera said.
The findings were published online August 3 in the Journal of Clinical Psychiatry.
Safety data scarce
Despite the increasing use of SGAs to treat a “spectrum of psychiatric disorders,” relatively little data are available on the reproductive safety of these agents, Dr. Viguera said.
The National Pregnancy Registry for Atypical Antipsychotics (NPRAA) was established in 2008 to determine risk for major malformation among infants exposed to these medications during the first trimester, relative to a comparison group of unexposed infants of mothers with histories of psychiatric morbidity.
The NPRAA follows pregnant women (aged 18 to 45 years) with psychiatric illness who are exposed or unexposed to SGAs during pregnancy. Participants are recruited through nationwide provider referral, self-referral, and advertisement through the Massachusetts General Hospital Center for Women’s Mental Health website.
Specific data collected are shown in the following table.
Since publication of the first results in 2015, the sample size for the trial has increased – and the absolute and relative risk for major malformations observed in the study population are “more precise,” the investigators note. The current study presented updated previous findings.
Demographic differences
Of the 1,906 women who enrolled as of April 2020, 1,311 (mean age, 32.6 years; 81.3% White) completed the study and were eligible for inclusion in the analysis.
Although the groups had a virtually identical mean age, fewer women in the exposure group were married compared with those in the non-exposure group (77% vs. 90%, respectively) and fewer had a college education (71.2% vs. 87.8%). There was also a higher percentage of first-trimester cigarette smokers in the exposure group (18.4% vs. 5.1%).
On the other hand, more women in the non-exposure group used alcohol than in the exposure group (28.6% vs. 21.4%, respectively).
The most frequent psychiatric disorder in the exposure group was bipolar disorder (63.9%), followed by major depression (12.9%), anxiety (5.8%), and schizophrenia (4.5%). Only 11.4% of women in the non-exposure group were diagnosed with bipolar disorder, whereas 34.1% were diagnosed with major depression, 31.3% with anxiety, and none with schizophrenia.
Notably, a large percentage of women in both groups had a history of postpartum depression and/or psychosis (41.4% and 35.5%, respectively).
The most frequently used SGAs in the exposure group were quetiapine (Seroquel), aripiprazole (Abilify), and lurasidone (Latuda).
Participants in the exposure group had a higher age at initial onset of primary psychiatric diagnosis and a lower proportion of lifetime illness compared with those in the non-exposure group.
Major clinical implication?
Among 640 live births in the exposure group, which included 17 twin pregnancies and 1 triplet pregnancy, 2.5% reported major malformations. Among 704 live births in the control group, which included 14 twin pregnancies, 1.99% reported major malformations.
The estimated OR for major malformations comparing exposed and unexposed infants was 1.48 (95% confidence interval, 0.625-3.517).
The authors note that their findings were consistent with one of the largest studies to date, which included a nationwide sample of more than 1 million women. Its results showed that, among infants exposed to SGAs versus those who were not exposed, the estimated risk ratio after adjusting for psychiatric conditions was 1.05 (95% CI, 0.96-1.16).
Additionally, “a hallmark of a teratogen is that it tends to cause a specific type or pattern of malformations, and we found no preponderance of one single type of major malformation or specific pattern of malformations among the exposed and unexposed groups,” Dr. Viguera said
“A major clinical implication of these findings is that for women with major mood and/or psychotic disorders, treatment with an atypical antipsychotic during pregnancy may be the most prudent clinical decision, much as continued treatment is recommended for pregnant women with other serious and chronic medical conditions, such as epilepsy,” she added.
The concept of ‘satisficing’
Commenting on the study, Vivien Burt, MD, PhD, founder and director/consultant of the Women’s Life Center at the Resnick University of California, Los Angeles (UCLA) Neuropsychiatric Hospital, called the findings “reassuring.”
The results “support the conclusion that in pregnant women with serious psychiatric illnesses, the use of SGAs is often a better option than avoiding these medications and exposing both the women and their offspring to the adverse consequences of maternal mental illness,” she said.
An accompanying editorial co-authored by Dr. Burt and colleague Sonya Rasminsky, MD, introduced the concept of “satisficing” – a term coined by Herbert Simon, a behavioral economist and Nobel Laureate. “Satisficing” is a “decision-making strategy that aims for a satisfactory (‘good enough’) outcome rather than a perfect one.”
The concept applies to decision-making beyond the field of economics “and is critical to how physicians help patients make decisions when they are faced with multiple treatment options,” said Dr. Burt, a professor emeritus of psychiatry at UCLA.
“The goal of ‘satisficing’ is to plan for the most satisfactory outcome, knowing that there are always unknowns, so in an uncertain world, clinicians should carefully help their patients make decisions that will allow them to achieve an outcome they can best live with,” she noted.
The investigators note that their findings may not be generalizable to the larger population of women taking SGAs, given that their participants were “overwhelmingly White, married, and well-educated women.”
They add that enrollment into the NPRAA registry is ongoing and larger sample sizes will “further narrow the confidence interval around the risk estimates and allow for adjustment of likely sources of confounding.”
The NPRAA is supported by Alkermes, Johnson & Johnson/Janssen Pharmaceuticals, Otsuka America Pharmaceutical, Sunovion Pharmaceuticals, SAGE Therapeutics, Teva Pharmaceuticals, and Aurobindo Pharma. Past sponsors of the NPRAA are listed in the original paper. Dr. Viguera receives research support from the NPRAA, Alkermes Biopharmaceuticals, Aurobindo Pharma, Janssen Pharmaceuticals, Otsuka Pharmaceutical, Sunovion Pharmaceuticals, Teva Pharmaceuticals, and SAGE Therapeutics and receives adviser/consulting fees from Up-to-Date. Dr. Burt has been a consultant/speaker for Sage Therapeutics. Dr. Rasminsky has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Second-generation antipsychotics (SGAs) taken by pregnant women are linked to a low rate of adverse effects in their children, new research suggests.
Data from a large registry study of almost 2,000 women showed that 2.5% of the live births in a group that had been exposed to antipsychotics had confirmed major malformations compared with 2% of the live births in a non-exposed group. This translated into an estimated odds ratio of 1.5 for major malformations.
“The 2.5% absolute risk for major malformations is consistent with the estimates of the Centers for Disease Control and Prevention’s national baseline rate of major malformations in the general population,” lead author Adele Viguera, MD, MPH, director of research for women’s mental health, Cleveland Clinic Neurological Institute, told this news organization.
“Our results are reassuring and suggest that second-generation antipsychotics, as a class, do not substantially increase the risk of major malformations,” Dr. Viguera said.
The findings were published online August 3 in the Journal of Clinical Psychiatry.
Safety data scarce
Despite the increasing use of SGAs to treat a “spectrum of psychiatric disorders,” relatively little data are available on the reproductive safety of these agents, Dr. Viguera said.
The National Pregnancy Registry for Atypical Antipsychotics (NPRAA) was established in 2008 to determine risk for major malformation among infants exposed to these medications during the first trimester, relative to a comparison group of unexposed infants of mothers with histories of psychiatric morbidity.
The NPRAA follows pregnant women (aged 18 to 45 years) with psychiatric illness who are exposed or unexposed to SGAs during pregnancy. Participants are recruited through nationwide provider referral, self-referral, and advertisement through the Massachusetts General Hospital Center for Women’s Mental Health website.
Specific data collected are shown in the following table.
Since publication of the first results in 2015, the sample size for the trial has increased – and the absolute and relative risk for major malformations observed in the study population are “more precise,” the investigators note. The current study presented updated previous findings.
Demographic differences
Of the 1,906 women who enrolled as of April 2020, 1,311 (mean age, 32.6 years; 81.3% White) completed the study and were eligible for inclusion in the analysis.
Although the groups had a virtually identical mean age, fewer women in the exposure group were married compared with those in the non-exposure group (77% vs. 90%, respectively) and fewer had a college education (71.2% vs. 87.8%). There was also a higher percentage of first-trimester cigarette smokers in the exposure group (18.4% vs. 5.1%).
On the other hand, more women in the non-exposure group used alcohol than in the exposure group (28.6% vs. 21.4%, respectively).
The most frequent psychiatric disorder in the exposure group was bipolar disorder (63.9%), followed by major depression (12.9%), anxiety (5.8%), and schizophrenia (4.5%). Only 11.4% of women in the non-exposure group were diagnosed with bipolar disorder, whereas 34.1% were diagnosed with major depression, 31.3% with anxiety, and none with schizophrenia.
Notably, a large percentage of women in both groups had a history of postpartum depression and/or psychosis (41.4% and 35.5%, respectively).
The most frequently used SGAs in the exposure group were quetiapine (Seroquel), aripiprazole (Abilify), and lurasidone (Latuda).
Participants in the exposure group had a higher age at initial onset of primary psychiatric diagnosis and a lower proportion of lifetime illness compared with those in the non-exposure group.
Major clinical implication?
Among 640 live births in the exposure group, which included 17 twin pregnancies and 1 triplet pregnancy, 2.5% reported major malformations. Among 704 live births in the control group, which included 14 twin pregnancies, 1.99% reported major malformations.
The estimated OR for major malformations comparing exposed and unexposed infants was 1.48 (95% confidence interval, 0.625-3.517).
The authors note that their findings were consistent with one of the largest studies to date, which included a nationwide sample of more than 1 million women. Its results showed that, among infants exposed to SGAs versus those who were not exposed, the estimated risk ratio after adjusting for psychiatric conditions was 1.05 (95% CI, 0.96-1.16).
Additionally, “a hallmark of a teratogen is that it tends to cause a specific type or pattern of malformations, and we found no preponderance of one single type of major malformation or specific pattern of malformations among the exposed and unexposed groups,” Dr. Viguera said
“A major clinical implication of these findings is that for women with major mood and/or psychotic disorders, treatment with an atypical antipsychotic during pregnancy may be the most prudent clinical decision, much as continued treatment is recommended for pregnant women with other serious and chronic medical conditions, such as epilepsy,” she added.
The concept of ‘satisficing’
Commenting on the study, Vivien Burt, MD, PhD, founder and director/consultant of the Women’s Life Center at the Resnick University of California, Los Angeles (UCLA) Neuropsychiatric Hospital, called the findings “reassuring.”
The results “support the conclusion that in pregnant women with serious psychiatric illnesses, the use of SGAs is often a better option than avoiding these medications and exposing both the women and their offspring to the adverse consequences of maternal mental illness,” she said.
An accompanying editorial co-authored by Dr. Burt and colleague Sonya Rasminsky, MD, introduced the concept of “satisficing” – a term coined by Herbert Simon, a behavioral economist and Nobel Laureate. “Satisficing” is a “decision-making strategy that aims for a satisfactory (‘good enough’) outcome rather than a perfect one.”
The concept applies to decision-making beyond the field of economics “and is critical to how physicians help patients make decisions when they are faced with multiple treatment options,” said Dr. Burt, a professor emeritus of psychiatry at UCLA.
“The goal of ‘satisficing’ is to plan for the most satisfactory outcome, knowing that there are always unknowns, so in an uncertain world, clinicians should carefully help their patients make decisions that will allow them to achieve an outcome they can best live with,” she noted.
The investigators note that their findings may not be generalizable to the larger population of women taking SGAs, given that their participants were “overwhelmingly White, married, and well-educated women.”
They add that enrollment into the NPRAA registry is ongoing and larger sample sizes will “further narrow the confidence interval around the risk estimates and allow for adjustment of likely sources of confounding.”
The NPRAA is supported by Alkermes, Johnson & Johnson/Janssen Pharmaceuticals, Otsuka America Pharmaceutical, Sunovion Pharmaceuticals, SAGE Therapeutics, Teva Pharmaceuticals, and Aurobindo Pharma. Past sponsors of the NPRAA are listed in the original paper. Dr. Viguera receives research support from the NPRAA, Alkermes Biopharmaceuticals, Aurobindo Pharma, Janssen Pharmaceuticals, Otsuka Pharmaceutical, Sunovion Pharmaceuticals, Teva Pharmaceuticals, and SAGE Therapeutics and receives adviser/consulting fees from Up-to-Date. Dr. Burt has been a consultant/speaker for Sage Therapeutics. Dr. Rasminsky has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shedding the super-doctor myth requires an honest look at systemic racism
An overwhelmingly loud and high-pitched screech rattles against your hip. You startle and groan into the pillow as your thoughts settle into conscious awareness. It is 3 a.m. You are a 2nd-year resident trudging through the night shift, alerted to the presence of a new patient awaiting an emergency assessment. You are the only in-house physician. Walking steadfastly toward the emergency unit, you enter and greet the patient. Immediately, you observe a look of surprise followed immediately by a scowl.
You extend a hand, but your greeting is abruptly cut short with: “I want to see a doctor!” You pace your breaths to quell annoyance and resume your introduction, asserting that you are a doctor and indeed the only doctor on duty. After moments of deep sighs and questions regarding your credentials, you persuade the patient to start the interview.
It is now 8 a.m. The frustration of the night starts to ease as you prepare to leave. While gathering your things, a visitor is overheard inquiring the whereabouts of a hospital unit. Volunteering as a guide, you walk the person toward the opposite end of the hospital. Bleary eyed, muscle laxed, and bone weary, you point out the entrance, then turn to leave. The steady rhythm of your steps suddenly halts as you hear from behind: “Thank you! You speak English really well!” Blankly, you stare. Your voice remains mute while your brain screams: “What is that supposed to mean?” But you do not utter a sound, because intuitively, you know the answer.
While reading this scenario, what did you feel? Pride in knowing that the physician was able to successfully navigate a busy night? Relief in the physician’s ability to maintain a professional demeanor despite belittling microaggressions? Are you angry? Would you replay those moments like reruns of a bad TV show? Can you imagine entering your home and collapsing onto the bed as your tears of fury pool over your rumpled sheets?
The emotional release of that morning is seared into my memory. Over the years, I questioned my reactions. Was I too passive? Should I have schooled them on their ignorance? Had I done so, would I have incurred reprimands? Would standing up for myself cause years of hard work to fall away? Moreover, had I defended myself, would I forever have been viewed as “The Angry Black Woman?”
This story is more than a vignette. For me, it is another reminder that, despite how far we have come, we have much further to go. As a Black woman in a professional sphere, I stand upon the shoulders of those who sacrificed for a dream, a greater purpose. My foremothers and forefathers fought bravely and tirelessly so that we could attain levels of success that were only once but a dream. Despite this progress, a grimace, carelessly spoken words, or a mindless gesture remind me that, no matter how much I toil and what levels of success I achieve, when I meet someone for the first time or encounter someone from my past, I find myself wondering whether I am remembered for me or because I am “The Black One.”
Honest look at medicine is imperative
It is important to consider multiple facets of the super-doctor myth. We are dedicated, fearless, authoritative, ambitious individuals. We do not yield to sickness, family obligations, or fatigue. Medicine is a calling, and the patient deserves the utmost respect and professional behavior. Impervious to ethnicity, race, nationality, or creed, we are unbiased and always in service of the greater good. Often, however, I wonder how the expectations of patient-focused, patient-centered care can prevail without an honest look at the vicissitudes facing medicine.
We find ourselves amid a tumultuous year overshadowed by a devastating pandemic that skews heavily toward Black and Brown communities, in addition to political turmoil and racial reckoning that sprang forth from fear, anger, and determination ignited by the murders of Breonna Taylor and George Floyd – communities united in outrage lamenting the cries of Black Lives Matter.
I remember the tears briskly falling upon my blouse as I watched Mr. Floyd’s life violently ripped from this Earth. Shortly thereafter, I remember the phone calls, emails, and texts from close friends, acquaintances, and colleagues offering support, listening ears, pledging to learn and endeavoring to understand the struggle for recognition and the fight for human rights. Even so, the deafening support was clouded by the preternatural silence of some medical organizations. Within the Black physician community, outrage was palpable. We reflected upon years of sacrifice and perseverance despite the challenge of bigotry, ignorance, and racism – not only from patients and their families – but also colleagues and administrators. Yet, in our time of horror and need, in those moments of vulnerability ... silence. Eventually, lengthy proclamations of support were expressed through various media. However, it felt too safe, too corporate, and too generic and inauthentic. As a result, an exodus of Black physicians from leadership positions and academic medicine took hold as the blatant continuation of rhetoric – coupled with ineffective outreach and support – finally took its toll.
Frequently, I question how the obstacles of medical school, residency, and beyond are expected to be traversed while living in a world that consistently affords additional challenges to those who look, act, or speak in a manner that varies from the perceived standard. In a culture where the myth of the super doctor reigns, how do we reconcile attainment of a false and detrimental narrative while the overarching pressure acutely felt by Black physicians magnifies in the setting of stereotypes, sociopolitical turbulence, bigotry, and racism? How can one sacrifice for an entity that is unwilling to acknowledge the psychological implications of that sacrifice?
For instance, while in medical school, I transitioned my hair to its natural state but was counseled against doing so because of the risk of losing residency opportunities as a direct result of my “unprofessional” appearance. Throughout residency, multiple incidents come to mind, including frequent demands to see my hospital badge despite the same not being of asked of my White cohorts; denial of entry into physician entrance within the residency building because, despite my professional attire, I was presumed to be a member of the custodial staff; and patients being confused and asking for a doctor despite my long white coat and clear introductions.
Furthermore, the fluency of my speech and the absence of regional dialect or vernacular are quite often lauded by patients. Inquiries to touch my hair as well as hypotheses regarding my nationality or degree of “blackness” with respect to the shape of my nose, eyes, and lips are openly questioned. Unfortunately, those uncomfortable incidents have not been limited to patient encounters.
In one instance, while presenting a patient in the presence of my attending and a 3rd-year medical student, I was sternly admonished for disclosing the race of the patient. I sat still and resolute as this doctor spoke on increased risk of bias in diagnosis and treatment when race is identified. Outwardly, I projected patience but inside, I seethed. In that moment, I realized that I would never have the luxury of ignorance or denial. Although I desire to be valued for my prowess in medicine, the mythical status was not created with my skin color in mind. For is avoidance not but a reflection of denial?
In these chaotic and uncertain times, how can we continue to promote a pathological ideal when the roads traveled are so fundamentally skewed? If a White physician faces a belligerent and argumentative patient, there is opportunity for debriefing both individually and among a larger cohort via classes, conferences, and supervisions. Conversely, when a Black physician is derided with racist sentiment, will they have the same opportunity for reflection and support? Despite identical expectations of professionalism and growth, how can one be successful in a system that either directly or indirectly encourages the opposite?
As we try to shed the super-doctor myth, we must recognize that this unattainable and detrimental persona hinders progress. This myth undermines our ability to understand our fragility, the limitations of our capabilities, and the strength of our vulnerability. We must take an honest look at the manner in which our individual biases and the deeply ingrained (and potentially unconscious) systemic biases are counterintuitive to the success and support of physicians of color.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
An overwhelmingly loud and high-pitched screech rattles against your hip. You startle and groan into the pillow as your thoughts settle into conscious awareness. It is 3 a.m. You are a 2nd-year resident trudging through the night shift, alerted to the presence of a new patient awaiting an emergency assessment. You are the only in-house physician. Walking steadfastly toward the emergency unit, you enter and greet the patient. Immediately, you observe a look of surprise followed immediately by a scowl.
You extend a hand, but your greeting is abruptly cut short with: “I want to see a doctor!” You pace your breaths to quell annoyance and resume your introduction, asserting that you are a doctor and indeed the only doctor on duty. After moments of deep sighs and questions regarding your credentials, you persuade the patient to start the interview.
It is now 8 a.m. The frustration of the night starts to ease as you prepare to leave. While gathering your things, a visitor is overheard inquiring the whereabouts of a hospital unit. Volunteering as a guide, you walk the person toward the opposite end of the hospital. Bleary eyed, muscle laxed, and bone weary, you point out the entrance, then turn to leave. The steady rhythm of your steps suddenly halts as you hear from behind: “Thank you! You speak English really well!” Blankly, you stare. Your voice remains mute while your brain screams: “What is that supposed to mean?” But you do not utter a sound, because intuitively, you know the answer.
While reading this scenario, what did you feel? Pride in knowing that the physician was able to successfully navigate a busy night? Relief in the physician’s ability to maintain a professional demeanor despite belittling microaggressions? Are you angry? Would you replay those moments like reruns of a bad TV show? Can you imagine entering your home and collapsing onto the bed as your tears of fury pool over your rumpled sheets?
The emotional release of that morning is seared into my memory. Over the years, I questioned my reactions. Was I too passive? Should I have schooled them on their ignorance? Had I done so, would I have incurred reprimands? Would standing up for myself cause years of hard work to fall away? Moreover, had I defended myself, would I forever have been viewed as “The Angry Black Woman?”
This story is more than a vignette. For me, it is another reminder that, despite how far we have come, we have much further to go. As a Black woman in a professional sphere, I stand upon the shoulders of those who sacrificed for a dream, a greater purpose. My foremothers and forefathers fought bravely and tirelessly so that we could attain levels of success that were only once but a dream. Despite this progress, a grimace, carelessly spoken words, or a mindless gesture remind me that, no matter how much I toil and what levels of success I achieve, when I meet someone for the first time or encounter someone from my past, I find myself wondering whether I am remembered for me or because I am “The Black One.”
Honest look at medicine is imperative
It is important to consider multiple facets of the super-doctor myth. We are dedicated, fearless, authoritative, ambitious individuals. We do not yield to sickness, family obligations, or fatigue. Medicine is a calling, and the patient deserves the utmost respect and professional behavior. Impervious to ethnicity, race, nationality, or creed, we are unbiased and always in service of the greater good. Often, however, I wonder how the expectations of patient-focused, patient-centered care can prevail without an honest look at the vicissitudes facing medicine.
We find ourselves amid a tumultuous year overshadowed by a devastating pandemic that skews heavily toward Black and Brown communities, in addition to political turmoil and racial reckoning that sprang forth from fear, anger, and determination ignited by the murders of Breonna Taylor and George Floyd – communities united in outrage lamenting the cries of Black Lives Matter.
I remember the tears briskly falling upon my blouse as I watched Mr. Floyd’s life violently ripped from this Earth. Shortly thereafter, I remember the phone calls, emails, and texts from close friends, acquaintances, and colleagues offering support, listening ears, pledging to learn and endeavoring to understand the struggle for recognition and the fight for human rights. Even so, the deafening support was clouded by the preternatural silence of some medical organizations. Within the Black physician community, outrage was palpable. We reflected upon years of sacrifice and perseverance despite the challenge of bigotry, ignorance, and racism – not only from patients and their families – but also colleagues and administrators. Yet, in our time of horror and need, in those moments of vulnerability ... silence. Eventually, lengthy proclamations of support were expressed through various media. However, it felt too safe, too corporate, and too generic and inauthentic. As a result, an exodus of Black physicians from leadership positions and academic medicine took hold as the blatant continuation of rhetoric – coupled with ineffective outreach and support – finally took its toll.
Frequently, I question how the obstacles of medical school, residency, and beyond are expected to be traversed while living in a world that consistently affords additional challenges to those who look, act, or speak in a manner that varies from the perceived standard. In a culture where the myth of the super doctor reigns, how do we reconcile attainment of a false and detrimental narrative while the overarching pressure acutely felt by Black physicians magnifies in the setting of stereotypes, sociopolitical turbulence, bigotry, and racism? How can one sacrifice for an entity that is unwilling to acknowledge the psychological implications of that sacrifice?
For instance, while in medical school, I transitioned my hair to its natural state but was counseled against doing so because of the risk of losing residency opportunities as a direct result of my “unprofessional” appearance. Throughout residency, multiple incidents come to mind, including frequent demands to see my hospital badge despite the same not being of asked of my White cohorts; denial of entry into physician entrance within the residency building because, despite my professional attire, I was presumed to be a member of the custodial staff; and patients being confused and asking for a doctor despite my long white coat and clear introductions.
Furthermore, the fluency of my speech and the absence of regional dialect or vernacular are quite often lauded by patients. Inquiries to touch my hair as well as hypotheses regarding my nationality or degree of “blackness” with respect to the shape of my nose, eyes, and lips are openly questioned. Unfortunately, those uncomfortable incidents have not been limited to patient encounters.
In one instance, while presenting a patient in the presence of my attending and a 3rd-year medical student, I was sternly admonished for disclosing the race of the patient. I sat still and resolute as this doctor spoke on increased risk of bias in diagnosis and treatment when race is identified. Outwardly, I projected patience but inside, I seethed. In that moment, I realized that I would never have the luxury of ignorance or denial. Although I desire to be valued for my prowess in medicine, the mythical status was not created with my skin color in mind. For is avoidance not but a reflection of denial?
In these chaotic and uncertain times, how can we continue to promote a pathological ideal when the roads traveled are so fundamentally skewed? If a White physician faces a belligerent and argumentative patient, there is opportunity for debriefing both individually and among a larger cohort via classes, conferences, and supervisions. Conversely, when a Black physician is derided with racist sentiment, will they have the same opportunity for reflection and support? Despite identical expectations of professionalism and growth, how can one be successful in a system that either directly or indirectly encourages the opposite?
As we try to shed the super-doctor myth, we must recognize that this unattainable and detrimental persona hinders progress. This myth undermines our ability to understand our fragility, the limitations of our capabilities, and the strength of our vulnerability. We must take an honest look at the manner in which our individual biases and the deeply ingrained (and potentially unconscious) systemic biases are counterintuitive to the success and support of physicians of color.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
An overwhelmingly loud and high-pitched screech rattles against your hip. You startle and groan into the pillow as your thoughts settle into conscious awareness. It is 3 a.m. You are a 2nd-year resident trudging through the night shift, alerted to the presence of a new patient awaiting an emergency assessment. You are the only in-house physician. Walking steadfastly toward the emergency unit, you enter and greet the patient. Immediately, you observe a look of surprise followed immediately by a scowl.
You extend a hand, but your greeting is abruptly cut short with: “I want to see a doctor!” You pace your breaths to quell annoyance and resume your introduction, asserting that you are a doctor and indeed the only doctor on duty. After moments of deep sighs and questions regarding your credentials, you persuade the patient to start the interview.
It is now 8 a.m. The frustration of the night starts to ease as you prepare to leave. While gathering your things, a visitor is overheard inquiring the whereabouts of a hospital unit. Volunteering as a guide, you walk the person toward the opposite end of the hospital. Bleary eyed, muscle laxed, and bone weary, you point out the entrance, then turn to leave. The steady rhythm of your steps suddenly halts as you hear from behind: “Thank you! You speak English really well!” Blankly, you stare. Your voice remains mute while your brain screams: “What is that supposed to mean?” But you do not utter a sound, because intuitively, you know the answer.
While reading this scenario, what did you feel? Pride in knowing that the physician was able to successfully navigate a busy night? Relief in the physician’s ability to maintain a professional demeanor despite belittling microaggressions? Are you angry? Would you replay those moments like reruns of a bad TV show? Can you imagine entering your home and collapsing onto the bed as your tears of fury pool over your rumpled sheets?
The emotional release of that morning is seared into my memory. Over the years, I questioned my reactions. Was I too passive? Should I have schooled them on their ignorance? Had I done so, would I have incurred reprimands? Would standing up for myself cause years of hard work to fall away? Moreover, had I defended myself, would I forever have been viewed as “The Angry Black Woman?”
This story is more than a vignette. For me, it is another reminder that, despite how far we have come, we have much further to go. As a Black woman in a professional sphere, I stand upon the shoulders of those who sacrificed for a dream, a greater purpose. My foremothers and forefathers fought bravely and tirelessly so that we could attain levels of success that were only once but a dream. Despite this progress, a grimace, carelessly spoken words, or a mindless gesture remind me that, no matter how much I toil and what levels of success I achieve, when I meet someone for the first time or encounter someone from my past, I find myself wondering whether I am remembered for me or because I am “The Black One.”
Honest look at medicine is imperative
It is important to consider multiple facets of the super-doctor myth. We are dedicated, fearless, authoritative, ambitious individuals. We do not yield to sickness, family obligations, or fatigue. Medicine is a calling, and the patient deserves the utmost respect and professional behavior. Impervious to ethnicity, race, nationality, or creed, we are unbiased and always in service of the greater good. Often, however, I wonder how the expectations of patient-focused, patient-centered care can prevail without an honest look at the vicissitudes facing medicine.
We find ourselves amid a tumultuous year overshadowed by a devastating pandemic that skews heavily toward Black and Brown communities, in addition to political turmoil and racial reckoning that sprang forth from fear, anger, and determination ignited by the murders of Breonna Taylor and George Floyd – communities united in outrage lamenting the cries of Black Lives Matter.
I remember the tears briskly falling upon my blouse as I watched Mr. Floyd’s life violently ripped from this Earth. Shortly thereafter, I remember the phone calls, emails, and texts from close friends, acquaintances, and colleagues offering support, listening ears, pledging to learn and endeavoring to understand the struggle for recognition and the fight for human rights. Even so, the deafening support was clouded by the preternatural silence of some medical organizations. Within the Black physician community, outrage was palpable. We reflected upon years of sacrifice and perseverance despite the challenge of bigotry, ignorance, and racism – not only from patients and their families – but also colleagues and administrators. Yet, in our time of horror and need, in those moments of vulnerability ... silence. Eventually, lengthy proclamations of support were expressed through various media. However, it felt too safe, too corporate, and too generic and inauthentic. As a result, an exodus of Black physicians from leadership positions and academic medicine took hold as the blatant continuation of rhetoric – coupled with ineffective outreach and support – finally took its toll.
Frequently, I question how the obstacles of medical school, residency, and beyond are expected to be traversed while living in a world that consistently affords additional challenges to those who look, act, or speak in a manner that varies from the perceived standard. In a culture where the myth of the super doctor reigns, how do we reconcile attainment of a false and detrimental narrative while the overarching pressure acutely felt by Black physicians magnifies in the setting of stereotypes, sociopolitical turbulence, bigotry, and racism? How can one sacrifice for an entity that is unwilling to acknowledge the psychological implications of that sacrifice?
For instance, while in medical school, I transitioned my hair to its natural state but was counseled against doing so because of the risk of losing residency opportunities as a direct result of my “unprofessional” appearance. Throughout residency, multiple incidents come to mind, including frequent demands to see my hospital badge despite the same not being of asked of my White cohorts; denial of entry into physician entrance within the residency building because, despite my professional attire, I was presumed to be a member of the custodial staff; and patients being confused and asking for a doctor despite my long white coat and clear introductions.
Furthermore, the fluency of my speech and the absence of regional dialect or vernacular are quite often lauded by patients. Inquiries to touch my hair as well as hypotheses regarding my nationality or degree of “blackness” with respect to the shape of my nose, eyes, and lips are openly questioned. Unfortunately, those uncomfortable incidents have not been limited to patient encounters.
In one instance, while presenting a patient in the presence of my attending and a 3rd-year medical student, I was sternly admonished for disclosing the race of the patient. I sat still and resolute as this doctor spoke on increased risk of bias in diagnosis and treatment when race is identified. Outwardly, I projected patience but inside, I seethed. In that moment, I realized that I would never have the luxury of ignorance or denial. Although I desire to be valued for my prowess in medicine, the mythical status was not created with my skin color in mind. For is avoidance not but a reflection of denial?
In these chaotic and uncertain times, how can we continue to promote a pathological ideal when the roads traveled are so fundamentally skewed? If a White physician faces a belligerent and argumentative patient, there is opportunity for debriefing both individually and among a larger cohort via classes, conferences, and supervisions. Conversely, when a Black physician is derided with racist sentiment, will they have the same opportunity for reflection and support? Despite identical expectations of professionalism and growth, how can one be successful in a system that either directly or indirectly encourages the opposite?
As we try to shed the super-doctor myth, we must recognize that this unattainable and detrimental persona hinders progress. This myth undermines our ability to understand our fragility, the limitations of our capabilities, and the strength of our vulnerability. We must take an honest look at the manner in which our individual biases and the deeply ingrained (and potentially unconscious) systemic biases are counterintuitive to the success and support of physicians of color.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
CDC officially endorses third dose of mRNA vaccines for immunocompromised
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Colorectal cancer screening, 2021: An update
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.
Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).
In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.

Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).

In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.

Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).

In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
CDC reports Burkholderia cepacia and B. pseudomallei outbreaks
The Centers for Disease Control and Prevention and Food and Drug Administration have announced an outbreak of at least 15 Burkholderia cepacia infections associated with contaminated ultrasound gel used to guide invasive procedures as well as an unrelated outbreak of Burkholderia pseudomallei that caused two deaths.
The procedures involved in the B. cepacia outbreak included placement of both central and peripheral intravenous catheters and paracentesis (removal of peritoneal fluid from the abdominal cavity). Cases have occurred in several states.
Further testing has shown the presence of Burkholderia stabilis, a member of B. cepacia complex (Bcc), in four lots of unopened bottles of MediChoice M500812 ultrasound gel. Eco-Med Pharmaceuticals of Etobicoke, Ont., the parent manufacturer, has issued a recall of MediChoice M500812 or Eco-Gel 200 with the following lot numbers: B029, B030, B031, B032, B040, B041, B048, B055. A similar outbreak occurred in Canada.
Some of these cases resulted in bloodstream infections. Further details are not yet available. Bcc infections have ranged from asymptomatic to life-threatening pneumonias, particularly in patients with cystic fibrosis. Other risk factors include immunosuppression, mechanical ventilation, and the use of other invasive venous or urinary catheters.
Kiran M. Perkins, MD, MPH, outbreak lead with the CDC’s Prevention Research Branch, said in an interview via email that automated systems such as Vitek might have trouble identifying the organism as “the system may only reveal the microbial species at the genus level but not at the species level, and/or it may have difficulty distinguishing between members of closely related group members.”
In the CDC’s experience, “most facilities do not conduct further species identification.” The agency added that it cannot tell if there has been any increase in cases associated with COVID-19, as they are not notifiable diseases and the “CDC does not systematically collect information on B. cepacia complex infections.”
Rodney Rohde, PhD, professor of clinical laboratory science and chair of the clinical laboratory science program, Texas State University, San Marcos, told this news organization via email that Burkholderia’s “detection in the manufacturing process is difficult, and product recalls are frequent.” He added, “A recent review by the Food and Drug Administration in the U.S. found that almost 40% of contamination reports in both sterile and nonsterile pharmaceutical products were caused by Bcc bacteria.” Another problem is that they often create biofilms, so “they are tenacious environmental colonizers of medical equipment and surfaces in general.”
There have been many other outbreaks as a result to B. cepacia complex. Because it is often in the water supply used in pharmaceutical manufacturing and is resistant to preservatives, the FDA cautions that it poses a risk of contamination in all nonsterile, water-based drug products.
Recalls have included contaminated antiseptics, such as povidone iodine, benzalkonium chloride, and chlorhexidine gluconate. Contamination in manufacturing may not be uniform, and only some samples may be affected. Antiseptic mouthwashes have also been affected. So have nonbacterial soaps and docusate (a stool softener) solutions, and various personal care products, including nasal sprays, lotions, simethicone gas relief drops (Mylicon), and baby wipes.
Although Bcc are considered “objectionable organisms,” there have been no strong or consistent standards for their detection from the U.S. Pharmacopeia, and some manufacturers reportedly underestimate the consequences of contamination. The FDA issued a guidance to manufacturers in 2017 on quality assurance and cleaning procedures. This is particularly important since preservatives are ineffective against Bcc, and sterility has to be insured at each step of production.
Burkholderia isolates are generally resistant to commonly used antibiotics. Treatment might therefore include a combination of two drugs (to try to limit the emergence of more resistance) such as ceftazidime, piperacillin, meropenem with trimethoprim-sulfamethoxazole, or a beta-lactam plus aminoglycoside.
Interestingly, an outbreak of Burkholderia pseudomallei was just reported by the CDC as well. This is a related gram-negative bacillus which is quite uncommon in the United States. It causes melioidosis, usually a tropical infection, which presents with nonspecific symptoms or serious pneumonia, abscesses, or bloodstream infections.
Four cases have been identified this year in Georgia, Kansas, Minnesota, and Texas, two of them fatal. It is usually acquired from soil or water. By genomic analysis, the four cases are felt to be related, but no common source of exposure has been identified. They also appear to be closely related to South Asian strains, although none of the patients had traveled internationally. Prolonged antibiotic therapy with ceftazidime or meropenem, followed by 3-6 months of trimethoprim-sulfamethoxazole, is often required.
In his email, Dr. Rohde stated, “Melioidosis causes cough, chest pain, high fever, headache or unexplained weight loss, but it may take 2-3 weeks for symptoms of melioidosis to appear after a person’s initial exposure to the bacteria. So, one could see how this might be overlooked as COVID per symptoms and per the limitations of laboratory identification.”
It’s essential for clinicians to recognize that automated microbiology identification systems can misidentify B. pseudomallei as B. cepacia and to ask the lab for more specialized molecular diagnostics, particularly when relatively unusual organisms are isolated.
Candice Hoffmann, a public affairs specialist at the CDC, told this news organization that “clinicians should consider melioidosis as a differential diagnosis in both adult and pediatric patients who are suspected to have a bacterial infection (pneumonia, sepsis, meningitis, wound) and are not responding to antibacterial treatment, even if they have not traveled outside of the continental United States.”
Dr. Rohde has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and Food and Drug Administration have announced an outbreak of at least 15 Burkholderia cepacia infections associated with contaminated ultrasound gel used to guide invasive procedures as well as an unrelated outbreak of Burkholderia pseudomallei that caused two deaths.
The procedures involved in the B. cepacia outbreak included placement of both central and peripheral intravenous catheters and paracentesis (removal of peritoneal fluid from the abdominal cavity). Cases have occurred in several states.
Further testing has shown the presence of Burkholderia stabilis, a member of B. cepacia complex (Bcc), in four lots of unopened bottles of MediChoice M500812 ultrasound gel. Eco-Med Pharmaceuticals of Etobicoke, Ont., the parent manufacturer, has issued a recall of MediChoice M500812 or Eco-Gel 200 with the following lot numbers: B029, B030, B031, B032, B040, B041, B048, B055. A similar outbreak occurred in Canada.
Some of these cases resulted in bloodstream infections. Further details are not yet available. Bcc infections have ranged from asymptomatic to life-threatening pneumonias, particularly in patients with cystic fibrosis. Other risk factors include immunosuppression, mechanical ventilation, and the use of other invasive venous or urinary catheters.
Kiran M. Perkins, MD, MPH, outbreak lead with the CDC’s Prevention Research Branch, said in an interview via email that automated systems such as Vitek might have trouble identifying the organism as “the system may only reveal the microbial species at the genus level but not at the species level, and/or it may have difficulty distinguishing between members of closely related group members.”
In the CDC’s experience, “most facilities do not conduct further species identification.” The agency added that it cannot tell if there has been any increase in cases associated with COVID-19, as they are not notifiable diseases and the “CDC does not systematically collect information on B. cepacia complex infections.”
Rodney Rohde, PhD, professor of clinical laboratory science and chair of the clinical laboratory science program, Texas State University, San Marcos, told this news organization via email that Burkholderia’s “detection in the manufacturing process is difficult, and product recalls are frequent.” He added, “A recent review by the Food and Drug Administration in the U.S. found that almost 40% of contamination reports in both sterile and nonsterile pharmaceutical products were caused by Bcc bacteria.” Another problem is that they often create biofilms, so “they are tenacious environmental colonizers of medical equipment and surfaces in general.”
There have been many other outbreaks as a result to B. cepacia complex. Because it is often in the water supply used in pharmaceutical manufacturing and is resistant to preservatives, the FDA cautions that it poses a risk of contamination in all nonsterile, water-based drug products.
Recalls have included contaminated antiseptics, such as povidone iodine, benzalkonium chloride, and chlorhexidine gluconate. Contamination in manufacturing may not be uniform, and only some samples may be affected. Antiseptic mouthwashes have also been affected. So have nonbacterial soaps and docusate (a stool softener) solutions, and various personal care products, including nasal sprays, lotions, simethicone gas relief drops (Mylicon), and baby wipes.
Although Bcc are considered “objectionable organisms,” there have been no strong or consistent standards for their detection from the U.S. Pharmacopeia, and some manufacturers reportedly underestimate the consequences of contamination. The FDA issued a guidance to manufacturers in 2017 on quality assurance and cleaning procedures. This is particularly important since preservatives are ineffective against Bcc, and sterility has to be insured at each step of production.
Burkholderia isolates are generally resistant to commonly used antibiotics. Treatment might therefore include a combination of two drugs (to try to limit the emergence of more resistance) such as ceftazidime, piperacillin, meropenem with trimethoprim-sulfamethoxazole, or a beta-lactam plus aminoglycoside.
Interestingly, an outbreak of Burkholderia pseudomallei was just reported by the CDC as well. This is a related gram-negative bacillus which is quite uncommon in the United States. It causes melioidosis, usually a tropical infection, which presents with nonspecific symptoms or serious pneumonia, abscesses, or bloodstream infections.
Four cases have been identified this year in Georgia, Kansas, Minnesota, and Texas, two of them fatal. It is usually acquired from soil or water. By genomic analysis, the four cases are felt to be related, but no common source of exposure has been identified. They also appear to be closely related to South Asian strains, although none of the patients had traveled internationally. Prolonged antibiotic therapy with ceftazidime or meropenem, followed by 3-6 months of trimethoprim-sulfamethoxazole, is often required.
In his email, Dr. Rohde stated, “Melioidosis causes cough, chest pain, high fever, headache or unexplained weight loss, but it may take 2-3 weeks for symptoms of melioidosis to appear after a person’s initial exposure to the bacteria. So, one could see how this might be overlooked as COVID per symptoms and per the limitations of laboratory identification.”
It’s essential for clinicians to recognize that automated microbiology identification systems can misidentify B. pseudomallei as B. cepacia and to ask the lab for more specialized molecular diagnostics, particularly when relatively unusual organisms are isolated.
Candice Hoffmann, a public affairs specialist at the CDC, told this news organization that “clinicians should consider melioidosis as a differential diagnosis in both adult and pediatric patients who are suspected to have a bacterial infection (pneumonia, sepsis, meningitis, wound) and are not responding to antibacterial treatment, even if they have not traveled outside of the continental United States.”
Dr. Rohde has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and Food and Drug Administration have announced an outbreak of at least 15 Burkholderia cepacia infections associated with contaminated ultrasound gel used to guide invasive procedures as well as an unrelated outbreak of Burkholderia pseudomallei that caused two deaths.
The procedures involved in the B. cepacia outbreak included placement of both central and peripheral intravenous catheters and paracentesis (removal of peritoneal fluid from the abdominal cavity). Cases have occurred in several states.
Further testing has shown the presence of Burkholderia stabilis, a member of B. cepacia complex (Bcc), in four lots of unopened bottles of MediChoice M500812 ultrasound gel. Eco-Med Pharmaceuticals of Etobicoke, Ont., the parent manufacturer, has issued a recall of MediChoice M500812 or Eco-Gel 200 with the following lot numbers: B029, B030, B031, B032, B040, B041, B048, B055. A similar outbreak occurred in Canada.
Some of these cases resulted in bloodstream infections. Further details are not yet available. Bcc infections have ranged from asymptomatic to life-threatening pneumonias, particularly in patients with cystic fibrosis. Other risk factors include immunosuppression, mechanical ventilation, and the use of other invasive venous or urinary catheters.
Kiran M. Perkins, MD, MPH, outbreak lead with the CDC’s Prevention Research Branch, said in an interview via email that automated systems such as Vitek might have trouble identifying the organism as “the system may only reveal the microbial species at the genus level but not at the species level, and/or it may have difficulty distinguishing between members of closely related group members.”
In the CDC’s experience, “most facilities do not conduct further species identification.” The agency added that it cannot tell if there has been any increase in cases associated with COVID-19, as they are not notifiable diseases and the “CDC does not systematically collect information on B. cepacia complex infections.”
Rodney Rohde, PhD, professor of clinical laboratory science and chair of the clinical laboratory science program, Texas State University, San Marcos, told this news organization via email that Burkholderia’s “detection in the manufacturing process is difficult, and product recalls are frequent.” He added, “A recent review by the Food and Drug Administration in the U.S. found that almost 40% of contamination reports in both sterile and nonsterile pharmaceutical products were caused by Bcc bacteria.” Another problem is that they often create biofilms, so “they are tenacious environmental colonizers of medical equipment and surfaces in general.”
There have been many other outbreaks as a result to B. cepacia complex. Because it is often in the water supply used in pharmaceutical manufacturing and is resistant to preservatives, the FDA cautions that it poses a risk of contamination in all nonsterile, water-based drug products.
Recalls have included contaminated antiseptics, such as povidone iodine, benzalkonium chloride, and chlorhexidine gluconate. Contamination in manufacturing may not be uniform, and only some samples may be affected. Antiseptic mouthwashes have also been affected. So have nonbacterial soaps and docusate (a stool softener) solutions, and various personal care products, including nasal sprays, lotions, simethicone gas relief drops (Mylicon), and baby wipes.
Although Bcc are considered “objectionable organisms,” there have been no strong or consistent standards for their detection from the U.S. Pharmacopeia, and some manufacturers reportedly underestimate the consequences of contamination. The FDA issued a guidance to manufacturers in 2017 on quality assurance and cleaning procedures. This is particularly important since preservatives are ineffective against Bcc, and sterility has to be insured at each step of production.
Burkholderia isolates are generally resistant to commonly used antibiotics. Treatment might therefore include a combination of two drugs (to try to limit the emergence of more resistance) such as ceftazidime, piperacillin, meropenem with trimethoprim-sulfamethoxazole, or a beta-lactam plus aminoglycoside.
Interestingly, an outbreak of Burkholderia pseudomallei was just reported by the CDC as well. This is a related gram-negative bacillus which is quite uncommon in the United States. It causes melioidosis, usually a tropical infection, which presents with nonspecific symptoms or serious pneumonia, abscesses, or bloodstream infections.
Four cases have been identified this year in Georgia, Kansas, Minnesota, and Texas, two of them fatal. It is usually acquired from soil or water. By genomic analysis, the four cases are felt to be related, but no common source of exposure has been identified. They also appear to be closely related to South Asian strains, although none of the patients had traveled internationally. Prolonged antibiotic therapy with ceftazidime or meropenem, followed by 3-6 months of trimethoprim-sulfamethoxazole, is often required.
In his email, Dr. Rohde stated, “Melioidosis causes cough, chest pain, high fever, headache or unexplained weight loss, but it may take 2-3 weeks for symptoms of melioidosis to appear after a person’s initial exposure to the bacteria. So, one could see how this might be overlooked as COVID per symptoms and per the limitations of laboratory identification.”
It’s essential for clinicians to recognize that automated microbiology identification systems can misidentify B. pseudomallei as B. cepacia and to ask the lab for more specialized molecular diagnostics, particularly when relatively unusual organisms are isolated.
Candice Hoffmann, a public affairs specialist at the CDC, told this news organization that “clinicians should consider melioidosis as a differential diagnosis in both adult and pediatric patients who are suspected to have a bacterial infection (pneumonia, sepsis, meningitis, wound) and are not responding to antibacterial treatment, even if they have not traveled outside of the continental United States.”
Dr. Rohde has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heparin’s COVID-19 benefit greatest in moderately ill patients
Critically ill derive no benefit
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
Critically ill derive no benefit
Critically ill derive no benefit
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
One center’s experience delivering monochorionic twins
Between 2005 and 2021, mode of delivery of diamniotic twins at this practice did not significantly differ by chorionicity, researchers affiliated with Maternal Fetal Medicine Associates and the department of obstetrics, gynecology, and reproductive science at Icahn School of Medicine at Mount Sinai, New York reported in Obstetrics & Gynecology.
The study supports a recommendation from the American College of Obstetricians and Gynecologists that vaginal delivery “is a reasonable option in well selected diamniotic twin pregnancies, irrespective of chorionicity, and should be considered, provided that an experienced obstetrician is available,” said Iris Krishna, MD, assistant professor of maternal-fetal medicine at Emory University, Atlanta.
The experience at this practice, however, may not apply to many practices in the United States, said Dr. Krishna, who was not involved in the study.
Of 1,121 diamniotic twin pregnancies included in the analysis, 202 (18%) were monochorionic. The cesarean delivery rate was not significantly different between groups: 61% for monochorionic and 63% for dichorionic pregnancies.
Among women with planned vaginal delivery (101 monochorionic pregnancies and 422 dichorionic pregnancies), the cesarean delivery rate likewise did not significantly differ by chorionicity. Twenty-two percent of the monochorionic pregnancies and 21% of the dichorionic pregnancies in this subgroup had a cesarean delivery.
Among patients with a vaginal delivery of twin A, chorionicity was not associated with mode of delivery for twin B. Combined vaginal-cesarean deliveries occurred less than 1% of the time, and breech extraction of twin B occurred approximately 75% of the time, regardless of chorionicity.
The researchers also compared neonatal outcomes for monochorionic-diamniotic twin pregnancies at or after 34 weeks of gestation, based on the intended mode of delivery (95 women with planned vaginal delivery and 68 with planned cesarean delivery). Neonatal outcomes generally were similar, although the incidence of mechanical ventilation was less common in cases with planned vaginal delivery (7% vs. 21%).
“Our data affirm that an attempt at a vaginal birth for twin pregnancies, without contraindications to vaginal delivery and regardless of chorionicity, is reasonable and achievable,” wrote study author Henry N. Lesser, MD, with the department of obstetrics and gynecology at Sinai Hospital in Baltimore, and colleagues.
The patients with planned cesarean delivery had a contraindication to vaginal delivery or otherwise chose to have a cesarean delivery. The researchers excluded from their analysis pregnancies with intrauterine fetal demise of either twin before labor or planned cesarean delivery.
The study’s reliance on data from a single practice decreases its external validity, the researchers noted. Induction of labor at this center typically occurs at 37 weeks’ gestation for monochorionic twins and at 38 weeks for dichorionic twins, and “senior personnel experienced in intrauterine twin manipulation are always present at delivery,” the study authors said.
The study describes “the experience of a single site with skilled obstetricians following a standardized approach to management of diamniotic twin deliveries,” Dr. Krishna said. “Findings may not be generalizable to many U.S. practices as obstetrics and gynecology residents often lack training in breech extraction or internal podalic version of the second twin. This underscores the importance of a concerted effort by skilled senior physicians to train junior physicians in vaginal delivery of the second twin to improve overall outcomes amongst women with diamniotic twin gestations.”
Michael F. Greene, MD, professor emeritus of obstetrics, gynecology, and reproductive biology at Massachusetts General Hospital, Boston, agreed that the findings are not generalizable to the national population. Approximately 10% of the patients in the study had prepregnancy obesity, whereas doctors practicing in other areas likely encounter higher rates, Dr. Greene said in an interview.
He also wondered about other data points that could be of interest but were not reported, such as the racial or ethnic distribution of the patients, rates of birth defects, the use of instruments to aid delivery, and neonatal outcomes for the dichorionic twins.
Monochorionic pregnancies entail a risk of twin-twin transfusion syndrome and other complications, including an increased likelihood of birth defects.
Dr. Greene is an associate editor with the New England Journal of Medicine, which in 2013 published results from the Twin Birth Study, an international trial where women with dichorionic or monochorionic twins were randomly assigned to planned vaginal delivery or planned cesarean delivery. Outcomes did not significantly differ between groups. In the trial, the rate of cesarean delivery in the group with planned vaginal delivery was 43.8%, and Dr. Greene discussed the implications of the study in an accompanying editorial.
Since then, the obstetrics and gynecology community “has been focusing in recent years on trying to avoid the first cesarean section” when it is safe to do so, Dr. Greene said. “That has become almost a bumper sticker in modern obstetrics.”
And patients should know that it is an option, Dr. Krishna added.
“Women with monochorionic-diamniotic twins should be counseled that with an experienced obstetrician that an attempt at vaginal delivery is not associated with adverse neonatal outcomes when compared with planned cesarean delivery,” Dr. Krishna said.
A study coauthor disclosed serving on the speakers bureau for Natera and Hologic. Dr. Krishna is a member of the editorial advisory board for Ob.Gyn. News.
Between 2005 and 2021, mode of delivery of diamniotic twins at this practice did not significantly differ by chorionicity, researchers affiliated with Maternal Fetal Medicine Associates and the department of obstetrics, gynecology, and reproductive science at Icahn School of Medicine at Mount Sinai, New York reported in Obstetrics & Gynecology.
The study supports a recommendation from the American College of Obstetricians and Gynecologists that vaginal delivery “is a reasonable option in well selected diamniotic twin pregnancies, irrespective of chorionicity, and should be considered, provided that an experienced obstetrician is available,” said Iris Krishna, MD, assistant professor of maternal-fetal medicine at Emory University, Atlanta.
The experience at this practice, however, may not apply to many practices in the United States, said Dr. Krishna, who was not involved in the study.
Of 1,121 diamniotic twin pregnancies included in the analysis, 202 (18%) were monochorionic. The cesarean delivery rate was not significantly different between groups: 61% for monochorionic and 63% for dichorionic pregnancies.
Among women with planned vaginal delivery (101 monochorionic pregnancies and 422 dichorionic pregnancies), the cesarean delivery rate likewise did not significantly differ by chorionicity. Twenty-two percent of the monochorionic pregnancies and 21% of the dichorionic pregnancies in this subgroup had a cesarean delivery.
Among patients with a vaginal delivery of twin A, chorionicity was not associated with mode of delivery for twin B. Combined vaginal-cesarean deliveries occurred less than 1% of the time, and breech extraction of twin B occurred approximately 75% of the time, regardless of chorionicity.
The researchers also compared neonatal outcomes for monochorionic-diamniotic twin pregnancies at or after 34 weeks of gestation, based on the intended mode of delivery (95 women with planned vaginal delivery and 68 with planned cesarean delivery). Neonatal outcomes generally were similar, although the incidence of mechanical ventilation was less common in cases with planned vaginal delivery (7% vs. 21%).
“Our data affirm that an attempt at a vaginal birth for twin pregnancies, without contraindications to vaginal delivery and regardless of chorionicity, is reasonable and achievable,” wrote study author Henry N. Lesser, MD, with the department of obstetrics and gynecology at Sinai Hospital in Baltimore, and colleagues.
The patients with planned cesarean delivery had a contraindication to vaginal delivery or otherwise chose to have a cesarean delivery. The researchers excluded from their analysis pregnancies with intrauterine fetal demise of either twin before labor or planned cesarean delivery.
The study’s reliance on data from a single practice decreases its external validity, the researchers noted. Induction of labor at this center typically occurs at 37 weeks’ gestation for monochorionic twins and at 38 weeks for dichorionic twins, and “senior personnel experienced in intrauterine twin manipulation are always present at delivery,” the study authors said.
The study describes “the experience of a single site with skilled obstetricians following a standardized approach to management of diamniotic twin deliveries,” Dr. Krishna said. “Findings may not be generalizable to many U.S. practices as obstetrics and gynecology residents often lack training in breech extraction or internal podalic version of the second twin. This underscores the importance of a concerted effort by skilled senior physicians to train junior physicians in vaginal delivery of the second twin to improve overall outcomes amongst women with diamniotic twin gestations.”
Michael F. Greene, MD, professor emeritus of obstetrics, gynecology, and reproductive biology at Massachusetts General Hospital, Boston, agreed that the findings are not generalizable to the national population. Approximately 10% of the patients in the study had prepregnancy obesity, whereas doctors practicing in other areas likely encounter higher rates, Dr. Greene said in an interview.
He also wondered about other data points that could be of interest but were not reported, such as the racial or ethnic distribution of the patients, rates of birth defects, the use of instruments to aid delivery, and neonatal outcomes for the dichorionic twins.
Monochorionic pregnancies entail a risk of twin-twin transfusion syndrome and other complications, including an increased likelihood of birth defects.
Dr. Greene is an associate editor with the New England Journal of Medicine, which in 2013 published results from the Twin Birth Study, an international trial where women with dichorionic or monochorionic twins were randomly assigned to planned vaginal delivery or planned cesarean delivery. Outcomes did not significantly differ between groups. In the trial, the rate of cesarean delivery in the group with planned vaginal delivery was 43.8%, and Dr. Greene discussed the implications of the study in an accompanying editorial.
Since then, the obstetrics and gynecology community “has been focusing in recent years on trying to avoid the first cesarean section” when it is safe to do so, Dr. Greene said. “That has become almost a bumper sticker in modern obstetrics.”
And patients should know that it is an option, Dr. Krishna added.
“Women with monochorionic-diamniotic twins should be counseled that with an experienced obstetrician that an attempt at vaginal delivery is not associated with adverse neonatal outcomes when compared with planned cesarean delivery,” Dr. Krishna said.
A study coauthor disclosed serving on the speakers bureau for Natera and Hologic. Dr. Krishna is a member of the editorial advisory board for Ob.Gyn. News.
Between 2005 and 2021, mode of delivery of diamniotic twins at this practice did not significantly differ by chorionicity, researchers affiliated with Maternal Fetal Medicine Associates and the department of obstetrics, gynecology, and reproductive science at Icahn School of Medicine at Mount Sinai, New York reported in Obstetrics & Gynecology.
The study supports a recommendation from the American College of Obstetricians and Gynecologists that vaginal delivery “is a reasonable option in well selected diamniotic twin pregnancies, irrespective of chorionicity, and should be considered, provided that an experienced obstetrician is available,” said Iris Krishna, MD, assistant professor of maternal-fetal medicine at Emory University, Atlanta.
The experience at this practice, however, may not apply to many practices in the United States, said Dr. Krishna, who was not involved in the study.
Of 1,121 diamniotic twin pregnancies included in the analysis, 202 (18%) were monochorionic. The cesarean delivery rate was not significantly different between groups: 61% for monochorionic and 63% for dichorionic pregnancies.
Among women with planned vaginal delivery (101 monochorionic pregnancies and 422 dichorionic pregnancies), the cesarean delivery rate likewise did not significantly differ by chorionicity. Twenty-two percent of the monochorionic pregnancies and 21% of the dichorionic pregnancies in this subgroup had a cesarean delivery.
Among patients with a vaginal delivery of twin A, chorionicity was not associated with mode of delivery for twin B. Combined vaginal-cesarean deliveries occurred less than 1% of the time, and breech extraction of twin B occurred approximately 75% of the time, regardless of chorionicity.
The researchers also compared neonatal outcomes for monochorionic-diamniotic twin pregnancies at or after 34 weeks of gestation, based on the intended mode of delivery (95 women with planned vaginal delivery and 68 with planned cesarean delivery). Neonatal outcomes generally were similar, although the incidence of mechanical ventilation was less common in cases with planned vaginal delivery (7% vs. 21%).
“Our data affirm that an attempt at a vaginal birth for twin pregnancies, without contraindications to vaginal delivery and regardless of chorionicity, is reasonable and achievable,” wrote study author Henry N. Lesser, MD, with the department of obstetrics and gynecology at Sinai Hospital in Baltimore, and colleagues.
The patients with planned cesarean delivery had a contraindication to vaginal delivery or otherwise chose to have a cesarean delivery. The researchers excluded from their analysis pregnancies with intrauterine fetal demise of either twin before labor or planned cesarean delivery.
The study’s reliance on data from a single practice decreases its external validity, the researchers noted. Induction of labor at this center typically occurs at 37 weeks’ gestation for monochorionic twins and at 38 weeks for dichorionic twins, and “senior personnel experienced in intrauterine twin manipulation are always present at delivery,” the study authors said.
The study describes “the experience of a single site with skilled obstetricians following a standardized approach to management of diamniotic twin deliveries,” Dr. Krishna said. “Findings may not be generalizable to many U.S. practices as obstetrics and gynecology residents often lack training in breech extraction or internal podalic version of the second twin. This underscores the importance of a concerted effort by skilled senior physicians to train junior physicians in vaginal delivery of the second twin to improve overall outcomes amongst women with diamniotic twin gestations.”
Michael F. Greene, MD, professor emeritus of obstetrics, gynecology, and reproductive biology at Massachusetts General Hospital, Boston, agreed that the findings are not generalizable to the national population. Approximately 10% of the patients in the study had prepregnancy obesity, whereas doctors practicing in other areas likely encounter higher rates, Dr. Greene said in an interview.
He also wondered about other data points that could be of interest but were not reported, such as the racial or ethnic distribution of the patients, rates of birth defects, the use of instruments to aid delivery, and neonatal outcomes for the dichorionic twins.
Monochorionic pregnancies entail a risk of twin-twin transfusion syndrome and other complications, including an increased likelihood of birth defects.
Dr. Greene is an associate editor with the New England Journal of Medicine, which in 2013 published results from the Twin Birth Study, an international trial where women with dichorionic or monochorionic twins were randomly assigned to planned vaginal delivery or planned cesarean delivery. Outcomes did not significantly differ between groups. In the trial, the rate of cesarean delivery in the group with planned vaginal delivery was 43.8%, and Dr. Greene discussed the implications of the study in an accompanying editorial.
Since then, the obstetrics and gynecology community “has been focusing in recent years on trying to avoid the first cesarean section” when it is safe to do so, Dr. Greene said. “That has become almost a bumper sticker in modern obstetrics.”
And patients should know that it is an option, Dr. Krishna added.
“Women with monochorionic-diamniotic twins should be counseled that with an experienced obstetrician that an attempt at vaginal delivery is not associated with adverse neonatal outcomes when compared with planned cesarean delivery,” Dr. Krishna said.
A study coauthor disclosed serving on the speakers bureau for Natera and Hologic. Dr. Krishna is a member of the editorial advisory board for Ob.Gyn. News.
FROM OBSTETRICS & GYNECOLOGY
Tachycardia syndrome may be distinct marker for long COVID
Tachycardia is commonly reported in patients with post-acute COVID-19 syndrome (PACS), also known as long COVID, authors report in a new article. The researchers say tachycardia syndrome should be considered a distinct phenotype.
The study by Marcus Ståhlberg, MD, PhD, of Karolinska University Hospital, Stockholm, and colleagues was published online August 11 in The American Journal of Medicine.
Dr. Ståhlberg told this news organization that although much attention has been paid to cases of clotting and perimyocarditis in patients after COVID, relatively little attention has been paid to tachycardia, despite case reports that show that palpitations are a common complaint.
“We have diagnosed a large number of patients with postural orthostatic tachycardia syndrome [POTS] and other forms of COVID-related tachycardia at our post-COVID outpatient clinic at Karolinska University Hospital and wanted to highlight this phenomenon,” he said.
Between 25% and 50% of patients at the clinic report tachycardia and/or palpitations that last 12 weeks or longer, the authors report.
“Systematic investigations suggest that 9% of Post-acute COVID-19 syndrome patients report palpitations at six months,” the authors write.
The findings also shed light on potential tests and treatments, he said.
“Physicians should be liberal in performing a basic cardiological workup, including an ECG [electrocardiogram], echocardiography, and Holter ECG monitoring in patients complaining of palpitations and/or chest pain,” Dr. Ståhlberg said.
“If orthostatic intolerance is also reported – such as vertigo, nausea, dyspnea – suspicion of POTS should be raised and a head-up tilt test or at least an active standing test should be performed,” he said.
If POTS is confirmed, he said, patients should be offered a heart rate–lowering drug, such as low-dose propranolol or ivabradine. Compression garments, increased fluid intake, and a structured rehabilitation program also help.
“According to our clinical experience, ivabradine can also reduce symptoms in patients with inappropriate sinus tachycardia and post-COVID,” Dr. Ståhlberg said. “Another finding on Holter-ECG to look out for is frequent premature extrasystoles, which could indicate myocarditis and should warrant a cardiac MRI.”
Dr. Ståhlberg said the researchers think the mechanism underlying the tachycardia is autoimmune and that primary SARS-CoV-2 infections trigger an autoimmune response with formation of autoantibodies that can activate receptors regulating blood pressure and heart rate.
Long-lasting symptoms from COVID are prevalent, the authors note, especially in patients who experienced severe forms of the disease.
In the longest follow-up study to date of patients hospitalized with COVID, more than 60% experienced fatigue or muscle weakness 6 months after hospitalization.
PACS should not be considered a single syndrome; the term denotes an array of subsyndromes and phenotypes, the authors write. Typical symptoms include headache, fatigue, dyspnea, and mental fog but can involve multiple organs and systems.
Tachycardia can also be used as a marker to help gauge the severity of long COVID, the authors write.
“[T]achycardia can be considered a universal and easily obtainable quantitative marker of Post-acute COVID-19 syndrome and its severity rather than patient-reported symptoms, blood testing, and thoracic CT-scans,” they write.
An underrecognized complication
Erin D. Michos, MD, MHS, director of women’s cardiovascular health and associate director of preventive cardiology at Johns Hopkins University, Baltimore, said in an interview that she has seen many similar symptoms in the long-COVID patients referred to her practice.
Dr. Michos, who is also an associate professor of medicine and epidemiology, said she’s been receiving a “huge number” of referrals of long-COVID patients with postural tachycardia, inappropriate sinus tachycardia, and POTS.
“I think this is all in the spectrum of autonomic dysfunction that has been recognized a lot since COVID. POTS has been thought to have [a potentially] viral cause that triggers an autoimmune response. Even before COVID, many patients had POTS triggered by a viral infection. The question is whether COVID-related POTS for long COVID is different from other kinds of POTS.”
She says she treats long-COVID patients who complain of elevated heart rates with many of the cardiac workup procedures the authors list and that she treats them in a way similar to the way she treats patients with POTS.
She recommends checking resting oxygen levels and having patients walk the halls and measure their oxygen levels after walking, because their elevated heart rate may be related to ongoing lung injury from COVID.
Eric Adler, MD, a cardiologist with University of San Diego Health, told this news organization that the findings by Dr. Ståhlberg and colleagues are consistent with what he’s seeing in his clinical practice.
Dr. Adler agrees with the authors that tachycardia is an underrecognized complication of long COVID.
He said the article represents further proof that though people may survive COVID, the threat of long-term symptoms, such as heart palpitations, is real and supports the case for vaccinations.
The authors, Dr. Michos, and Dr. Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tachycardia is commonly reported in patients with post-acute COVID-19 syndrome (PACS), also known as long COVID, authors report in a new article. The researchers say tachycardia syndrome should be considered a distinct phenotype.
The study by Marcus Ståhlberg, MD, PhD, of Karolinska University Hospital, Stockholm, and colleagues was published online August 11 in The American Journal of Medicine.
Dr. Ståhlberg told this news organization that although much attention has been paid to cases of clotting and perimyocarditis in patients after COVID, relatively little attention has been paid to tachycardia, despite case reports that show that palpitations are a common complaint.
“We have diagnosed a large number of patients with postural orthostatic tachycardia syndrome [POTS] and other forms of COVID-related tachycardia at our post-COVID outpatient clinic at Karolinska University Hospital and wanted to highlight this phenomenon,” he said.
Between 25% and 50% of patients at the clinic report tachycardia and/or palpitations that last 12 weeks or longer, the authors report.
“Systematic investigations suggest that 9% of Post-acute COVID-19 syndrome patients report palpitations at six months,” the authors write.
The findings also shed light on potential tests and treatments, he said.
“Physicians should be liberal in performing a basic cardiological workup, including an ECG [electrocardiogram], echocardiography, and Holter ECG monitoring in patients complaining of palpitations and/or chest pain,” Dr. Ståhlberg said.
“If orthostatic intolerance is also reported – such as vertigo, nausea, dyspnea – suspicion of POTS should be raised and a head-up tilt test or at least an active standing test should be performed,” he said.
If POTS is confirmed, he said, patients should be offered a heart rate–lowering drug, such as low-dose propranolol or ivabradine. Compression garments, increased fluid intake, and a structured rehabilitation program also help.
“According to our clinical experience, ivabradine can also reduce symptoms in patients with inappropriate sinus tachycardia and post-COVID,” Dr. Ståhlberg said. “Another finding on Holter-ECG to look out for is frequent premature extrasystoles, which could indicate myocarditis and should warrant a cardiac MRI.”
Dr. Ståhlberg said the researchers think the mechanism underlying the tachycardia is autoimmune and that primary SARS-CoV-2 infections trigger an autoimmune response with formation of autoantibodies that can activate receptors regulating blood pressure and heart rate.
Long-lasting symptoms from COVID are prevalent, the authors note, especially in patients who experienced severe forms of the disease.
In the longest follow-up study to date of patients hospitalized with COVID, more than 60% experienced fatigue or muscle weakness 6 months after hospitalization.
PACS should not be considered a single syndrome; the term denotes an array of subsyndromes and phenotypes, the authors write. Typical symptoms include headache, fatigue, dyspnea, and mental fog but can involve multiple organs and systems.
Tachycardia can also be used as a marker to help gauge the severity of long COVID, the authors write.
“[T]achycardia can be considered a universal and easily obtainable quantitative marker of Post-acute COVID-19 syndrome and its severity rather than patient-reported symptoms, blood testing, and thoracic CT-scans,” they write.
An underrecognized complication
Erin D. Michos, MD, MHS, director of women’s cardiovascular health and associate director of preventive cardiology at Johns Hopkins University, Baltimore, said in an interview that she has seen many similar symptoms in the long-COVID patients referred to her practice.
Dr. Michos, who is also an associate professor of medicine and epidemiology, said she’s been receiving a “huge number” of referrals of long-COVID patients with postural tachycardia, inappropriate sinus tachycardia, and POTS.
“I think this is all in the spectrum of autonomic dysfunction that has been recognized a lot since COVID. POTS has been thought to have [a potentially] viral cause that triggers an autoimmune response. Even before COVID, many patients had POTS triggered by a viral infection. The question is whether COVID-related POTS for long COVID is different from other kinds of POTS.”
She says she treats long-COVID patients who complain of elevated heart rates with many of the cardiac workup procedures the authors list and that she treats them in a way similar to the way she treats patients with POTS.
She recommends checking resting oxygen levels and having patients walk the halls and measure their oxygen levels after walking, because their elevated heart rate may be related to ongoing lung injury from COVID.
Eric Adler, MD, a cardiologist with University of San Diego Health, told this news organization that the findings by Dr. Ståhlberg and colleagues are consistent with what he’s seeing in his clinical practice.
Dr. Adler agrees with the authors that tachycardia is an underrecognized complication of long COVID.
He said the article represents further proof that though people may survive COVID, the threat of long-term symptoms, such as heart palpitations, is real and supports the case for vaccinations.
The authors, Dr. Michos, and Dr. Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tachycardia is commonly reported in patients with post-acute COVID-19 syndrome (PACS), also known as long COVID, authors report in a new article. The researchers say tachycardia syndrome should be considered a distinct phenotype.
The study by Marcus Ståhlberg, MD, PhD, of Karolinska University Hospital, Stockholm, and colleagues was published online August 11 in The American Journal of Medicine.
Dr. Ståhlberg told this news organization that although much attention has been paid to cases of clotting and perimyocarditis in patients after COVID, relatively little attention has been paid to tachycardia, despite case reports that show that palpitations are a common complaint.
“We have diagnosed a large number of patients with postural orthostatic tachycardia syndrome [POTS] and other forms of COVID-related tachycardia at our post-COVID outpatient clinic at Karolinska University Hospital and wanted to highlight this phenomenon,” he said.
Between 25% and 50% of patients at the clinic report tachycardia and/or palpitations that last 12 weeks or longer, the authors report.
“Systematic investigations suggest that 9% of Post-acute COVID-19 syndrome patients report palpitations at six months,” the authors write.
The findings also shed light on potential tests and treatments, he said.
“Physicians should be liberal in performing a basic cardiological workup, including an ECG [electrocardiogram], echocardiography, and Holter ECG monitoring in patients complaining of palpitations and/or chest pain,” Dr. Ståhlberg said.
“If orthostatic intolerance is also reported – such as vertigo, nausea, dyspnea – suspicion of POTS should be raised and a head-up tilt test or at least an active standing test should be performed,” he said.
If POTS is confirmed, he said, patients should be offered a heart rate–lowering drug, such as low-dose propranolol or ivabradine. Compression garments, increased fluid intake, and a structured rehabilitation program also help.
“According to our clinical experience, ivabradine can also reduce symptoms in patients with inappropriate sinus tachycardia and post-COVID,” Dr. Ståhlberg said. “Another finding on Holter-ECG to look out for is frequent premature extrasystoles, which could indicate myocarditis and should warrant a cardiac MRI.”
Dr. Ståhlberg said the researchers think the mechanism underlying the tachycardia is autoimmune and that primary SARS-CoV-2 infections trigger an autoimmune response with formation of autoantibodies that can activate receptors regulating blood pressure and heart rate.
Long-lasting symptoms from COVID are prevalent, the authors note, especially in patients who experienced severe forms of the disease.
In the longest follow-up study to date of patients hospitalized with COVID, more than 60% experienced fatigue or muscle weakness 6 months after hospitalization.
PACS should not be considered a single syndrome; the term denotes an array of subsyndromes and phenotypes, the authors write. Typical symptoms include headache, fatigue, dyspnea, and mental fog but can involve multiple organs and systems.
Tachycardia can also be used as a marker to help gauge the severity of long COVID, the authors write.
“[T]achycardia can be considered a universal and easily obtainable quantitative marker of Post-acute COVID-19 syndrome and its severity rather than patient-reported symptoms, blood testing, and thoracic CT-scans,” they write.
An underrecognized complication
Erin D. Michos, MD, MHS, director of women’s cardiovascular health and associate director of preventive cardiology at Johns Hopkins University, Baltimore, said in an interview that she has seen many similar symptoms in the long-COVID patients referred to her practice.
Dr. Michos, who is also an associate professor of medicine and epidemiology, said she’s been receiving a “huge number” of referrals of long-COVID patients with postural tachycardia, inappropriate sinus tachycardia, and POTS.
“I think this is all in the spectrum of autonomic dysfunction that has been recognized a lot since COVID. POTS has been thought to have [a potentially] viral cause that triggers an autoimmune response. Even before COVID, many patients had POTS triggered by a viral infection. The question is whether COVID-related POTS for long COVID is different from other kinds of POTS.”
She says she treats long-COVID patients who complain of elevated heart rates with many of the cardiac workup procedures the authors list and that she treats them in a way similar to the way she treats patients with POTS.
She recommends checking resting oxygen levels and having patients walk the halls and measure their oxygen levels after walking, because their elevated heart rate may be related to ongoing lung injury from COVID.
Eric Adler, MD, a cardiologist with University of San Diego Health, told this news organization that the findings by Dr. Ståhlberg and colleagues are consistent with what he’s seeing in his clinical practice.
Dr. Adler agrees with the authors that tachycardia is an underrecognized complication of long COVID.
He said the article represents further proof that though people may survive COVID, the threat of long-term symptoms, such as heart palpitations, is real and supports the case for vaccinations.
The authors, Dr. Michos, and Dr. Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA authorizes booster shot for immunocompromised Americans
The decision, which came late on Aug. 12, was not unexpected and a Centers for Disease Control and Prevention (CDC) panel meeting Aug. 13 is expected to approve directions to doctors and health care providers on who should receive the booster shot.
“The country has entered yet another wave of the COVID-19 pandemic, and the FDA is especially cognizant that immunocompromised people are particularly at risk for severe disease. After a thorough review of the available data, the FDA determined that this small, vulnerable group may benefit from a third dose of the Pfizer-BioNTech or Moderna Vaccines,” acting FDA Commissioner Janet Woodcock, MD, said in a statement.
Those eligible for a third dose include solid organ transplant recipients, those undergoing cancer treatments, and people with autoimmune diseases that suppress their immune systems.
Meanwhile, White House officials said Aug. 12 they “have supply and are prepared” to give all U.S. residents COVID-19 boosters -- which, as of now, are likely to be authorized first only for immunocompromised people.
“We believe sooner or later you will need a booster,” Anthony Fauci, MD, said at a news briefing Aug. 12. “Right now, we are evaluating this on a day-by-day, week-by-week, month-by-month basis.”
He added: “Right at this moment, apart from the immunocompromised -- elderly or not elderly -- people do not need a booster.” But, he said, “We’re preparing for the eventuality of doing that.”
White House COVID-19 Response Coordinator Jeff Zients said officials “have supply and are prepared” to at some point provide widespread access to boosters.
The immunocompromised population is very small -- less than 3% of adults, said CDC Director Rochelle Walensky, MD.
Meanwhile, COVID-19 rates continue to rise. Dr. Walensky reported that the 7-day average of daily cases is 132,384 -- an increase of 24% from the previous week. Average daily hospitalizations are up 31%, at 9,700, and deaths are up to 452 -- an increase of 22%.
In the past week, Florida has had more COVID-19 cases than the 30 states with the lowest case rates combined, Mr. Zients said. Florida and Texas alone have accounted for nearly 40% of new hospitalizations across the country.
A version of this article first appeared on WebMD.com.
The decision, which came late on Aug. 12, was not unexpected and a Centers for Disease Control and Prevention (CDC) panel meeting Aug. 13 is expected to approve directions to doctors and health care providers on who should receive the booster shot.
“The country has entered yet another wave of the COVID-19 pandemic, and the FDA is especially cognizant that immunocompromised people are particularly at risk for severe disease. After a thorough review of the available data, the FDA determined that this small, vulnerable group may benefit from a third dose of the Pfizer-BioNTech or Moderna Vaccines,” acting FDA Commissioner Janet Woodcock, MD, said in a statement.
Those eligible for a third dose include solid organ transplant recipients, those undergoing cancer treatments, and people with autoimmune diseases that suppress their immune systems.
Meanwhile, White House officials said Aug. 12 they “have supply and are prepared” to give all U.S. residents COVID-19 boosters -- which, as of now, are likely to be authorized first only for immunocompromised people.
“We believe sooner or later you will need a booster,” Anthony Fauci, MD, said at a news briefing Aug. 12. “Right now, we are evaluating this on a day-by-day, week-by-week, month-by-month basis.”
He added: “Right at this moment, apart from the immunocompromised -- elderly or not elderly -- people do not need a booster.” But, he said, “We’re preparing for the eventuality of doing that.”
White House COVID-19 Response Coordinator Jeff Zients said officials “have supply and are prepared” to at some point provide widespread access to boosters.
The immunocompromised population is very small -- less than 3% of adults, said CDC Director Rochelle Walensky, MD.
Meanwhile, COVID-19 rates continue to rise. Dr. Walensky reported that the 7-day average of daily cases is 132,384 -- an increase of 24% from the previous week. Average daily hospitalizations are up 31%, at 9,700, and deaths are up to 452 -- an increase of 22%.
In the past week, Florida has had more COVID-19 cases than the 30 states with the lowest case rates combined, Mr. Zients said. Florida and Texas alone have accounted for nearly 40% of new hospitalizations across the country.
A version of this article first appeared on WebMD.com.
The decision, which came late on Aug. 12, was not unexpected and a Centers for Disease Control and Prevention (CDC) panel meeting Aug. 13 is expected to approve directions to doctors and health care providers on who should receive the booster shot.
“The country has entered yet another wave of the COVID-19 pandemic, and the FDA is especially cognizant that immunocompromised people are particularly at risk for severe disease. After a thorough review of the available data, the FDA determined that this small, vulnerable group may benefit from a third dose of the Pfizer-BioNTech or Moderna Vaccines,” acting FDA Commissioner Janet Woodcock, MD, said in a statement.
Those eligible for a third dose include solid organ transplant recipients, those undergoing cancer treatments, and people with autoimmune diseases that suppress their immune systems.
Meanwhile, White House officials said Aug. 12 they “have supply and are prepared” to give all U.S. residents COVID-19 boosters -- which, as of now, are likely to be authorized first only for immunocompromised people.
“We believe sooner or later you will need a booster,” Anthony Fauci, MD, said at a news briefing Aug. 12. “Right now, we are evaluating this on a day-by-day, week-by-week, month-by-month basis.”
He added: “Right at this moment, apart from the immunocompromised -- elderly or not elderly -- people do not need a booster.” But, he said, “We’re preparing for the eventuality of doing that.”
White House COVID-19 Response Coordinator Jeff Zients said officials “have supply and are prepared” to at some point provide widespread access to boosters.
The immunocompromised population is very small -- less than 3% of adults, said CDC Director Rochelle Walensky, MD.
Meanwhile, COVID-19 rates continue to rise. Dr. Walensky reported that the 7-day average of daily cases is 132,384 -- an increase of 24% from the previous week. Average daily hospitalizations are up 31%, at 9,700, and deaths are up to 452 -- an increase of 22%.
In the past week, Florida has had more COVID-19 cases than the 30 states with the lowest case rates combined, Mr. Zients said. Florida and Texas alone have accounted for nearly 40% of new hospitalizations across the country.
A version of this article first appeared on WebMD.com.










