User login
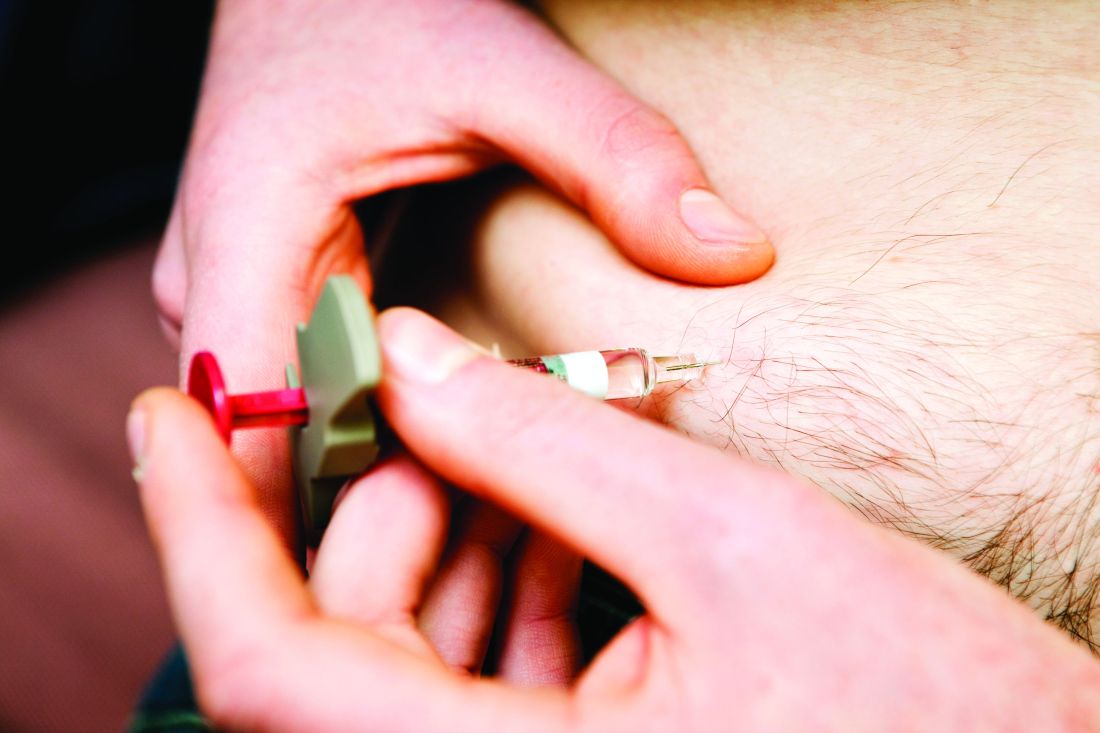
PsA: Phase 3 confirms rapid and significant response of secukinumab on synovitis
Key clinical point: Evaluation with power Doppler ultrasound revealed a rapid and significant reduction of synovitis in patients with psoriatic arthritis (PsA) upon treatment with secukinumab.
Major finding: At week 12, the adjusted mean change in the global European League Against Rheumatism (EULAR) and the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) (EULAR-OMERACT) synovitis score was significantly higher in secukinumab vs. placebo group (−9 vs. −6; difference −3; P = .004), with the effect of secukinumab evident as early as week 1 of treatment initiation. The incidence of treatment-emergent adverse events in secukinumab vs. placebo group was 58% vs. 57%.
Study details: Findings are 12-week results from ULTIMATE, a phase 3 study including 166 biologic-naive patients with concomitant PsA and synovitis who failed conventional synthetic disease-modifying antirheumatic drugs. Patients were randomly assigned to receive subcutaneous secukinumab (300 mg or 150 mg) or placebo weekly until week 4, followed by 4-weekly dosing until week 52.
Disclosures: This study was funded by Novartis. Some of the authors reported ties with various sources including Novartis. A Duggan, P Goyanka, and C Gaillez declared being employees of or owning stock in Novartis.
Source: D'Agostino MA et al. Rheumatology. 2021 Sep 16. doi: 10.1093/rheumatology/keab628.
Key clinical point: Evaluation with power Doppler ultrasound revealed a rapid and significant reduction of synovitis in patients with psoriatic arthritis (PsA) upon treatment with secukinumab.
Major finding: At week 12, the adjusted mean change in the global European League Against Rheumatism (EULAR) and the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) (EULAR-OMERACT) synovitis score was significantly higher in secukinumab vs. placebo group (−9 vs. −6; difference −3; P = .004), with the effect of secukinumab evident as early as week 1 of treatment initiation. The incidence of treatment-emergent adverse events in secukinumab vs. placebo group was 58% vs. 57%.
Study details: Findings are 12-week results from ULTIMATE, a phase 3 study including 166 biologic-naive patients with concomitant PsA and synovitis who failed conventional synthetic disease-modifying antirheumatic drugs. Patients were randomly assigned to receive subcutaneous secukinumab (300 mg or 150 mg) or placebo weekly until week 4, followed by 4-weekly dosing until week 52.
Disclosures: This study was funded by Novartis. Some of the authors reported ties with various sources including Novartis. A Duggan, P Goyanka, and C Gaillez declared being employees of or owning stock in Novartis.
Source: D'Agostino MA et al. Rheumatology. 2021 Sep 16. doi: 10.1093/rheumatology/keab628.
Key clinical point: Evaluation with power Doppler ultrasound revealed a rapid and significant reduction of synovitis in patients with psoriatic arthritis (PsA) upon treatment with secukinumab.
Major finding: At week 12, the adjusted mean change in the global European League Against Rheumatism (EULAR) and the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) (EULAR-OMERACT) synovitis score was significantly higher in secukinumab vs. placebo group (−9 vs. −6; difference −3; P = .004), with the effect of secukinumab evident as early as week 1 of treatment initiation. The incidence of treatment-emergent adverse events in secukinumab vs. placebo group was 58% vs. 57%.
Study details: Findings are 12-week results from ULTIMATE, a phase 3 study including 166 biologic-naive patients with concomitant PsA and synovitis who failed conventional synthetic disease-modifying antirheumatic drugs. Patients were randomly assigned to receive subcutaneous secukinumab (300 mg or 150 mg) or placebo weekly until week 4, followed by 4-weekly dosing until week 52.
Disclosures: This study was funded by Novartis. Some of the authors reported ties with various sources including Novartis. A Duggan, P Goyanka, and C Gaillez declared being employees of or owning stock in Novartis.
Source: D'Agostino MA et al. Rheumatology. 2021 Sep 16. doi: 10.1093/rheumatology/keab628.
Psoriasis patients initiating biologics more likely to develop PsA
Key clinical point: Patients with psoriasis who initiated biologics were more likely to develop psoriatic arthritis (PsA) than those who initiated phototherapy or oral therapy.
Major finding: The incidence rate of PsA regardless of exposure to any treatment was 9.75 per 1000 person-years vs. 77.26, 61.99, and 26.11 per 1000 person-years among initiators of biologics, oral therapy, and phototherapy, respectively. Patients receiving biologics were at a significantly higher risk of developing PsA vs. those receiving either oral or phototherapy (hazard ratio 4.48; 95% CI 4.23-4.75).
Study details: Findings are from a retrospective cohort study including 193,709 patients with psoriasis without PsA, of which 14,569 biologic and 20,321 cumulative oral and phototherapy initiations were identified.
Disclosures: R Fitzsimmons and A Ogdie received funding from National Psoriasis Foundation. Dr. Gelfand, T Love, and A Ogdie reported ties with several sources.
Source: Meer E et al. Ann Rheum Dis. 2021 Oct 6. doi: 10.1136/annrheumdis-2021-220761.
Key clinical point: Patients with psoriasis who initiated biologics were more likely to develop psoriatic arthritis (PsA) than those who initiated phototherapy or oral therapy.
Major finding: The incidence rate of PsA regardless of exposure to any treatment was 9.75 per 1000 person-years vs. 77.26, 61.99, and 26.11 per 1000 person-years among initiators of biologics, oral therapy, and phototherapy, respectively. Patients receiving biologics were at a significantly higher risk of developing PsA vs. those receiving either oral or phototherapy (hazard ratio 4.48; 95% CI 4.23-4.75).
Study details: Findings are from a retrospective cohort study including 193,709 patients with psoriasis without PsA, of which 14,569 biologic and 20,321 cumulative oral and phototherapy initiations were identified.
Disclosures: R Fitzsimmons and A Ogdie received funding from National Psoriasis Foundation. Dr. Gelfand, T Love, and A Ogdie reported ties with several sources.
Source: Meer E et al. Ann Rheum Dis. 2021 Oct 6. doi: 10.1136/annrheumdis-2021-220761.
Key clinical point: Patients with psoriasis who initiated biologics were more likely to develop psoriatic arthritis (PsA) than those who initiated phototherapy or oral therapy.
Major finding: The incidence rate of PsA regardless of exposure to any treatment was 9.75 per 1000 person-years vs. 77.26, 61.99, and 26.11 per 1000 person-years among initiators of biologics, oral therapy, and phototherapy, respectively. Patients receiving biologics were at a significantly higher risk of developing PsA vs. those receiving either oral or phototherapy (hazard ratio 4.48; 95% CI 4.23-4.75).
Study details: Findings are from a retrospective cohort study including 193,709 patients with psoriasis without PsA, of which 14,569 biologic and 20,321 cumulative oral and phototherapy initiations were identified.
Disclosures: R Fitzsimmons and A Ogdie received funding from National Psoriasis Foundation. Dr. Gelfand, T Love, and A Ogdie reported ties with several sources.
Source: Meer E et al. Ann Rheum Dis. 2021 Oct 6. doi: 10.1136/annrheumdis-2021-220761.
Disease activity-guided dose optimization of TNF inhibitors safe and effective in PsA
Key clinical point: Disease activity-guided dose optimization (DAGDO) of tumor necrosis factor inhibitor (TNFi) showed no negative effect on disease activity in patients with psoriatic arthritis (PsA).
Major finding: Compared with the full-dose continuation period, the mean disease activity score in 28-joint count C-reactive protein was not significantly different for DAGDO (0.06; P = .44) and stable dose after DAGDO (0.03; P = .72) periods. Moreover, the rate ratio of infections was not different in DAGDO (0.76; P = .57) and stable dose after DAGDO (0.77; P = .60) periods compared to the full-dose continuation period.
Study details: Findings are from 2 controlled, parallel, retrospective cohorts including 153 patients with PsA and 171 patients with axial spondyloarthritis who were doing well taking TNFi and were eligible for DAGDO.
Disclosures: This study did not report any source of funding. Dr. den Broeder A declared receiving consultancy, honoraria, congress invitations, and research grants from various sources.
Source: Michielsens CAJ et al. Rheumatology. 2021 Oct 2. doi: 10.1093/rheumatology/keab741.
Key clinical point: Disease activity-guided dose optimization (DAGDO) of tumor necrosis factor inhibitor (TNFi) showed no negative effect on disease activity in patients with psoriatic arthritis (PsA).
Major finding: Compared with the full-dose continuation period, the mean disease activity score in 28-joint count C-reactive protein was not significantly different for DAGDO (0.06; P = .44) and stable dose after DAGDO (0.03; P = .72) periods. Moreover, the rate ratio of infections was not different in DAGDO (0.76; P = .57) and stable dose after DAGDO (0.77; P = .60) periods compared to the full-dose continuation period.
Study details: Findings are from 2 controlled, parallel, retrospective cohorts including 153 patients with PsA and 171 patients with axial spondyloarthritis who were doing well taking TNFi and were eligible for DAGDO.
Disclosures: This study did not report any source of funding. Dr. den Broeder A declared receiving consultancy, honoraria, congress invitations, and research grants from various sources.
Source: Michielsens CAJ et al. Rheumatology. 2021 Oct 2. doi: 10.1093/rheumatology/keab741.
Key clinical point: Disease activity-guided dose optimization (DAGDO) of tumor necrosis factor inhibitor (TNFi) showed no negative effect on disease activity in patients with psoriatic arthritis (PsA).
Major finding: Compared with the full-dose continuation period, the mean disease activity score in 28-joint count C-reactive protein was not significantly different for DAGDO (0.06; P = .44) and stable dose after DAGDO (0.03; P = .72) periods. Moreover, the rate ratio of infections was not different in DAGDO (0.76; P = .57) and stable dose after DAGDO (0.77; P = .60) periods compared to the full-dose continuation period.
Study details: Findings are from 2 controlled, parallel, retrospective cohorts including 153 patients with PsA and 171 patients with axial spondyloarthritis who were doing well taking TNFi and were eligible for DAGDO.
Disclosures: This study did not report any source of funding. Dr. den Broeder A declared receiving consultancy, honoraria, congress invitations, and research grants from various sources.
Source: Michielsens CAJ et al. Rheumatology. 2021 Oct 2. doi: 10.1093/rheumatology/keab741.
Better COVID-19 outcomes confirmed in TNF inhibitor users
Among patients with immune-mediated inflammatory diseases (IMIDs) who get COVID-19, the risk for hospitalization and death is lower if they are receiving tumor necrosis factor (TNF) inhibitor monotherapy, compared with receiving most other common drugs for these conditions, with or without TNF inhibitors, according to a study published in JAMA Network Open The only combination not associated with an increased risk for hospitalization or death was TNF inhibitor therapy with methotrexate.
“These findings support the continued use of TNF inhibitor monotherapy during the pandemic and warrant further research investigating the association of other biologic therapies with COVID-19 outcomes,” write Zara Izadi, MPharm, of the University of California, San Francisco, and her colleagues. “Treatment with TNF inhibitor combination therapy was associated with a more favorable safety profile when methotrexate rather than azathioprine/6-mercaptopurine was used, suggesting that clinicians would benefit from weighing the risks versus benefits of deescalating treatment or changing medications when a patient is receiving concomitant TNF inhibitors and azathioprine/6-mercaptopurine,” they write.
Findings mirror those seen in other settings
These findings are in line with what has been found in other settings, according to Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, Philadelphia.
“In the beginning of the pandemic, there was concern about use of immune-modulating treatments, and many patients self-discontinued treatments like TNF inhibitors,” Dr. Gelfand, who was not involved in the study, told this news organization. “This has ultimately proved unnecessary and unfortunately resulted in harm to many patients due to flaring of their underlying disease.”
Dr. Gelfand emphasized the importance of vaccinating patients against COVID-19 as soon as possible and of getting a third dose for those who are already fully vaccinated with the Pfizer or Moderna shots, as recommended by the Centers for Disease Control and Prevention.
“I typically recommend this third dose be taken 6 months after the second dose,” Dr. Gelfand said. “The good news is that TNF inhibitors do not seem to meaningfully impact response to mRNA vaccines.”
Study details
The researchers analyzed data from three international registries of adults with rheumatic diseases, inflammatory bowel disease, and psoriasis who had COVID-19 between March 12, 2020, and Feb. 1, 2021. The registries included the Secure Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry, the Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect), and the physician-reported registry from the Global Rheumatology Alliance (GRA).
The population included 6,077 patients from 74 countries. About half of the cohort (52.9%) were from Europe; more than half were women (58.6%). The average age was 48 years. A little over one-third of the patients (35.3%) had rheumatoid arthritis, 25.3% had Crohn’s disease, 12.5% had ulcerative colitis, 10.3% had spondyloarthritis, and 9.3% had psoriatic arthritis. Smaller percentages had psoriasis (4.9%), another type of arthritis or multiple types (1.7%), or another inflammatory bowel disease (0.6%).
One in five patients (21.3%) were hospitalized, and 3.1% died. The researchers compared outcomes for those who were receiving TNF inhibitor therapy alone to outcomes for those who were taking azathioprine/6-mercaptopurine therapy (alone or with a TNF inhibitor), methotrexate (alone or with a TNF inhibitor), and Janus kinase (JAK) inhibitors. They adjusted their analysis to account for active disease and common comorbidities, as well as geography and the period during the pandemic in which the person was admitted, because treatment regimens and hospitalization indications have varied over time.
All of the therapies except the combination of TNF inhibitors and methotrexate were associated with higher odds of hospitalization and death than TNF inhibitor monotherapy.
The researchers explored several possible explanations for the findings, including the possibility that high serum TNF concentrations may have been associated with more organ damage at the time of COVID-19 admission, owing to interaction with SARS-CoV-2–associated hyperinflammation.
“Therefore, blocking TNF could inhibit this detrimental immune response,” the authors write. “Multiple case series reporting favorable outcomes among patients receiving TNF inhibitor therapy support this assertion.”
Another possibility relates to the effects of taking non–TNF inhibitor medications for immunosuppression. The authors note that thiopurine medications are linked to a greater risk for opportunistic viral infections and that JAK inhibitors may reduce the body’s ability to clear the virus because of its suppression of innate immune response.
The authors also postulate that methotrexate may lower the likelihood of cytokine storm linked to COVID-19, even though methotrexate monotherapy was associated with poorer outcomes. “This association could mean that TNF inhibitor therapy is exerting a protective benefit or that methotrexate therapy is exerting a harmful consequence,” the authors write.
Caution needed in interpreting uncontrolled, registry-based data
The findings were not surprising to Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who was not involved in the research.
“We’ve been monitoring IBD [inflammatory bowel disease] patients through the Secure registry similar to the rheumatologic and dermatologic societies and have not identified a signal of harm from any international groups,” Dr. Hanauer told this news organization. He noted that these registries also have not shown an increased risk for COVID-19 complications among patients receiving TNF inhibitors, antiadhesion therapies, or anti–IL12/23 inhibitors, compared with the general population not taking these therapies.
The study’s size and the diversity of patients strengthen its findings. However, the registries’ use of convenience sampling increases the potential for reporting bias, although the results remained similar after a sensitivity analysis. The study also lacked a control group, and the registries did not collect data uniformly.
“These are databases that rely on reporting from investigators and are not comprehensive prospective studies,” Dr. Hanauer noted as another study limitation.
Dr. Gelfand similarly advised caution in interpreting these findings, inasmuch as the study is a “collection of spontaneous reports” that should be viewed as hypothesis-generating rather than testing.
“Fortunately, more rigorous studies have been conducted, typically in large medical record systems, and have confirmed the hypothesis that TNF inhibitors are associated with a lower risk of poor COVID-19 outcomes, compared to other treatments,” Dr. Gelfand said.
Previous smaller studies similarly found better outcomes among patients taking TNF inhibitors, compared with other therapies, but their participants were predominantly from North America and Europe, noted Licio A. Velloso, MD, PhD, of the University of Campinas, in São Paulo, in an accompanying commentary.
On the basis of the findings of this study, “which included a much larger sample comprising distinct diseases and patients with a multitude of genetic backgrounds, the evidence in favor of the continued use of TNF inhibitor monotherapy for patients with IMIDs during the COVID-19 pandemic has become more substantial,” Dr. Velloso writes. “The finding that maintenance of TNF inhibitor monotherapy is associated with reductions in the risk of severe COVID-19 among patients with IMIDs offers new perspective that may guide health care professionals in the difficult decisions regarding therapeutic approaches among this specific group of patients.”
The research was funded by the American College of Rheumatology, the European Alliance of Associations for Rheumatology, the United Kingdom’s National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Many authors reported receiving grants and/or personal fees from a variety of pharmaceutical companies. Dr. Velloso has disclosed no relevant financial relationships. Dr. Hanauer has served as a consultant to companies that market TNF inhibitors. Dr. Gelfand has consulted for and received research grants from companies that market TNF inhibitors.
A version of this article first appeared on Medscape.com.
Among patients with immune-mediated inflammatory diseases (IMIDs) who get COVID-19, the risk for hospitalization and death is lower if they are receiving tumor necrosis factor (TNF) inhibitor monotherapy, compared with receiving most other common drugs for these conditions, with or without TNF inhibitors, according to a study published in JAMA Network Open The only combination not associated with an increased risk for hospitalization or death was TNF inhibitor therapy with methotrexate.
“These findings support the continued use of TNF inhibitor monotherapy during the pandemic and warrant further research investigating the association of other biologic therapies with COVID-19 outcomes,” write Zara Izadi, MPharm, of the University of California, San Francisco, and her colleagues. “Treatment with TNF inhibitor combination therapy was associated with a more favorable safety profile when methotrexate rather than azathioprine/6-mercaptopurine was used, suggesting that clinicians would benefit from weighing the risks versus benefits of deescalating treatment or changing medications when a patient is receiving concomitant TNF inhibitors and azathioprine/6-mercaptopurine,” they write.
Findings mirror those seen in other settings
These findings are in line with what has been found in other settings, according to Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, Philadelphia.
“In the beginning of the pandemic, there was concern about use of immune-modulating treatments, and many patients self-discontinued treatments like TNF inhibitors,” Dr. Gelfand, who was not involved in the study, told this news organization. “This has ultimately proved unnecessary and unfortunately resulted in harm to many patients due to flaring of their underlying disease.”
Dr. Gelfand emphasized the importance of vaccinating patients against COVID-19 as soon as possible and of getting a third dose for those who are already fully vaccinated with the Pfizer or Moderna shots, as recommended by the Centers for Disease Control and Prevention.
“I typically recommend this third dose be taken 6 months after the second dose,” Dr. Gelfand said. “The good news is that TNF inhibitors do not seem to meaningfully impact response to mRNA vaccines.”
Study details
The researchers analyzed data from three international registries of adults with rheumatic diseases, inflammatory bowel disease, and psoriasis who had COVID-19 between March 12, 2020, and Feb. 1, 2021. The registries included the Secure Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry, the Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect), and the physician-reported registry from the Global Rheumatology Alliance (GRA).
The population included 6,077 patients from 74 countries. About half of the cohort (52.9%) were from Europe; more than half were women (58.6%). The average age was 48 years. A little over one-third of the patients (35.3%) had rheumatoid arthritis, 25.3% had Crohn’s disease, 12.5% had ulcerative colitis, 10.3% had spondyloarthritis, and 9.3% had psoriatic arthritis. Smaller percentages had psoriasis (4.9%), another type of arthritis or multiple types (1.7%), or another inflammatory bowel disease (0.6%).
One in five patients (21.3%) were hospitalized, and 3.1% died. The researchers compared outcomes for those who were receiving TNF inhibitor therapy alone to outcomes for those who were taking azathioprine/6-mercaptopurine therapy (alone or with a TNF inhibitor), methotrexate (alone or with a TNF inhibitor), and Janus kinase (JAK) inhibitors. They adjusted their analysis to account for active disease and common comorbidities, as well as geography and the period during the pandemic in which the person was admitted, because treatment regimens and hospitalization indications have varied over time.
All of the therapies except the combination of TNF inhibitors and methotrexate were associated with higher odds of hospitalization and death than TNF inhibitor monotherapy.
The researchers explored several possible explanations for the findings, including the possibility that high serum TNF concentrations may have been associated with more organ damage at the time of COVID-19 admission, owing to interaction with SARS-CoV-2–associated hyperinflammation.
“Therefore, blocking TNF could inhibit this detrimental immune response,” the authors write. “Multiple case series reporting favorable outcomes among patients receiving TNF inhibitor therapy support this assertion.”
Another possibility relates to the effects of taking non–TNF inhibitor medications for immunosuppression. The authors note that thiopurine medications are linked to a greater risk for opportunistic viral infections and that JAK inhibitors may reduce the body’s ability to clear the virus because of its suppression of innate immune response.
The authors also postulate that methotrexate may lower the likelihood of cytokine storm linked to COVID-19, even though methotrexate monotherapy was associated with poorer outcomes. “This association could mean that TNF inhibitor therapy is exerting a protective benefit or that methotrexate therapy is exerting a harmful consequence,” the authors write.
Caution needed in interpreting uncontrolled, registry-based data
The findings were not surprising to Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who was not involved in the research.
“We’ve been monitoring IBD [inflammatory bowel disease] patients through the Secure registry similar to the rheumatologic and dermatologic societies and have not identified a signal of harm from any international groups,” Dr. Hanauer told this news organization. He noted that these registries also have not shown an increased risk for COVID-19 complications among patients receiving TNF inhibitors, antiadhesion therapies, or anti–IL12/23 inhibitors, compared with the general population not taking these therapies.
The study’s size and the diversity of patients strengthen its findings. However, the registries’ use of convenience sampling increases the potential for reporting bias, although the results remained similar after a sensitivity analysis. The study also lacked a control group, and the registries did not collect data uniformly.
“These are databases that rely on reporting from investigators and are not comprehensive prospective studies,” Dr. Hanauer noted as another study limitation.
Dr. Gelfand similarly advised caution in interpreting these findings, inasmuch as the study is a “collection of spontaneous reports” that should be viewed as hypothesis-generating rather than testing.
“Fortunately, more rigorous studies have been conducted, typically in large medical record systems, and have confirmed the hypothesis that TNF inhibitors are associated with a lower risk of poor COVID-19 outcomes, compared to other treatments,” Dr. Gelfand said.
Previous smaller studies similarly found better outcomes among patients taking TNF inhibitors, compared with other therapies, but their participants were predominantly from North America and Europe, noted Licio A. Velloso, MD, PhD, of the University of Campinas, in São Paulo, in an accompanying commentary.
On the basis of the findings of this study, “which included a much larger sample comprising distinct diseases and patients with a multitude of genetic backgrounds, the evidence in favor of the continued use of TNF inhibitor monotherapy for patients with IMIDs during the COVID-19 pandemic has become more substantial,” Dr. Velloso writes. “The finding that maintenance of TNF inhibitor monotherapy is associated with reductions in the risk of severe COVID-19 among patients with IMIDs offers new perspective that may guide health care professionals in the difficult decisions regarding therapeutic approaches among this specific group of patients.”
The research was funded by the American College of Rheumatology, the European Alliance of Associations for Rheumatology, the United Kingdom’s National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Many authors reported receiving grants and/or personal fees from a variety of pharmaceutical companies. Dr. Velloso has disclosed no relevant financial relationships. Dr. Hanauer has served as a consultant to companies that market TNF inhibitors. Dr. Gelfand has consulted for and received research grants from companies that market TNF inhibitors.
A version of this article first appeared on Medscape.com.
Among patients with immune-mediated inflammatory diseases (IMIDs) who get COVID-19, the risk for hospitalization and death is lower if they are receiving tumor necrosis factor (TNF) inhibitor monotherapy, compared with receiving most other common drugs for these conditions, with or without TNF inhibitors, according to a study published in JAMA Network Open The only combination not associated with an increased risk for hospitalization or death was TNF inhibitor therapy with methotrexate.
“These findings support the continued use of TNF inhibitor monotherapy during the pandemic and warrant further research investigating the association of other biologic therapies with COVID-19 outcomes,” write Zara Izadi, MPharm, of the University of California, San Francisco, and her colleagues. “Treatment with TNF inhibitor combination therapy was associated with a more favorable safety profile when methotrexate rather than azathioprine/6-mercaptopurine was used, suggesting that clinicians would benefit from weighing the risks versus benefits of deescalating treatment or changing medications when a patient is receiving concomitant TNF inhibitors and azathioprine/6-mercaptopurine,” they write.
Findings mirror those seen in other settings
These findings are in line with what has been found in other settings, according to Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, Philadelphia.
“In the beginning of the pandemic, there was concern about use of immune-modulating treatments, and many patients self-discontinued treatments like TNF inhibitors,” Dr. Gelfand, who was not involved in the study, told this news organization. “This has ultimately proved unnecessary and unfortunately resulted in harm to many patients due to flaring of their underlying disease.”
Dr. Gelfand emphasized the importance of vaccinating patients against COVID-19 as soon as possible and of getting a third dose for those who are already fully vaccinated with the Pfizer or Moderna shots, as recommended by the Centers for Disease Control and Prevention.
“I typically recommend this third dose be taken 6 months after the second dose,” Dr. Gelfand said. “The good news is that TNF inhibitors do not seem to meaningfully impact response to mRNA vaccines.”
Study details
The researchers analyzed data from three international registries of adults with rheumatic diseases, inflammatory bowel disease, and psoriasis who had COVID-19 between March 12, 2020, and Feb. 1, 2021. The registries included the Secure Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry, the Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect), and the physician-reported registry from the Global Rheumatology Alliance (GRA).
The population included 6,077 patients from 74 countries. About half of the cohort (52.9%) were from Europe; more than half were women (58.6%). The average age was 48 years. A little over one-third of the patients (35.3%) had rheumatoid arthritis, 25.3% had Crohn’s disease, 12.5% had ulcerative colitis, 10.3% had spondyloarthritis, and 9.3% had psoriatic arthritis. Smaller percentages had psoriasis (4.9%), another type of arthritis or multiple types (1.7%), or another inflammatory bowel disease (0.6%).
One in five patients (21.3%) were hospitalized, and 3.1% died. The researchers compared outcomes for those who were receiving TNF inhibitor therapy alone to outcomes for those who were taking azathioprine/6-mercaptopurine therapy (alone or with a TNF inhibitor), methotrexate (alone or with a TNF inhibitor), and Janus kinase (JAK) inhibitors. They adjusted their analysis to account for active disease and common comorbidities, as well as geography and the period during the pandemic in which the person was admitted, because treatment regimens and hospitalization indications have varied over time.
All of the therapies except the combination of TNF inhibitors and methotrexate were associated with higher odds of hospitalization and death than TNF inhibitor monotherapy.
The researchers explored several possible explanations for the findings, including the possibility that high serum TNF concentrations may have been associated with more organ damage at the time of COVID-19 admission, owing to interaction with SARS-CoV-2–associated hyperinflammation.
“Therefore, blocking TNF could inhibit this detrimental immune response,” the authors write. “Multiple case series reporting favorable outcomes among patients receiving TNF inhibitor therapy support this assertion.”
Another possibility relates to the effects of taking non–TNF inhibitor medications for immunosuppression. The authors note that thiopurine medications are linked to a greater risk for opportunistic viral infections and that JAK inhibitors may reduce the body’s ability to clear the virus because of its suppression of innate immune response.
The authors also postulate that methotrexate may lower the likelihood of cytokine storm linked to COVID-19, even though methotrexate monotherapy was associated with poorer outcomes. “This association could mean that TNF inhibitor therapy is exerting a protective benefit or that methotrexate therapy is exerting a harmful consequence,” the authors write.
Caution needed in interpreting uncontrolled, registry-based data
The findings were not surprising to Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who was not involved in the research.
“We’ve been monitoring IBD [inflammatory bowel disease] patients through the Secure registry similar to the rheumatologic and dermatologic societies and have not identified a signal of harm from any international groups,” Dr. Hanauer told this news organization. He noted that these registries also have not shown an increased risk for COVID-19 complications among patients receiving TNF inhibitors, antiadhesion therapies, or anti–IL12/23 inhibitors, compared with the general population not taking these therapies.
The study’s size and the diversity of patients strengthen its findings. However, the registries’ use of convenience sampling increases the potential for reporting bias, although the results remained similar after a sensitivity analysis. The study also lacked a control group, and the registries did not collect data uniformly.
“These are databases that rely on reporting from investigators and are not comprehensive prospective studies,” Dr. Hanauer noted as another study limitation.
Dr. Gelfand similarly advised caution in interpreting these findings, inasmuch as the study is a “collection of spontaneous reports” that should be viewed as hypothesis-generating rather than testing.
“Fortunately, more rigorous studies have been conducted, typically in large medical record systems, and have confirmed the hypothesis that TNF inhibitors are associated with a lower risk of poor COVID-19 outcomes, compared to other treatments,” Dr. Gelfand said.
Previous smaller studies similarly found better outcomes among patients taking TNF inhibitors, compared with other therapies, but their participants were predominantly from North America and Europe, noted Licio A. Velloso, MD, PhD, of the University of Campinas, in São Paulo, in an accompanying commentary.
On the basis of the findings of this study, “which included a much larger sample comprising distinct diseases and patients with a multitude of genetic backgrounds, the evidence in favor of the continued use of TNF inhibitor monotherapy for patients with IMIDs during the COVID-19 pandemic has become more substantial,” Dr. Velloso writes. “The finding that maintenance of TNF inhibitor monotherapy is associated with reductions in the risk of severe COVID-19 among patients with IMIDs offers new perspective that may guide health care professionals in the difficult decisions regarding therapeutic approaches among this specific group of patients.”
The research was funded by the American College of Rheumatology, the European Alliance of Associations for Rheumatology, the United Kingdom’s National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Many authors reported receiving grants and/or personal fees from a variety of pharmaceutical companies. Dr. Velloso has disclosed no relevant financial relationships. Dr. Hanauer has served as a consultant to companies that market TNF inhibitors. Dr. Gelfand has consulted for and received research grants from companies that market TNF inhibitors.
A version of this article first appeared on Medscape.com.
Tramadol linked to higher risk of mortality, compared with codeine
Tramadol is increasingly used to manage chronic noncancer pain, but as compared with opioids, it appears to be linked to a higher risk for adverse outcomes, according to new data.
Among a cohort of patients who received a prescription for either tramadol or codeine for orthopedic-related pain, tramadol was significantly associated with a higher risk of mortality, cardiovascular events, and fractures.
However, there was no significant difference in the risk of falls, delirium, constipation, opioid abuse/dependence, or sleep disorders between the two drugs.
“However, this is a retrospective cohort study, and despite it providing information that would otherwise be impossible to gather – such as from randomized controlled trials – clinicians should not solely base their decision on this study,” cautioned lead author Carlen Reyes, MD, PhD, of the Institut Universitari d’Investigació en Atenció Primària (IDIAP Jordi Gol), Barcelona.
Dr. Reyes noted that the intake of tramadol and codeine was analyzed using the number of “packages” that were dispensed, as an approximation of the real intake. “Logically we could think that the more packages dispensed of one drug, the more dose the patient is taking, but this is not always true given the availability of different doses commercialized of tramadol and different doses prescribed,” she said. “Given that we did not account for the real dose prescribed, we can only suspect an increased risk of these outcomes and reinforce the need for further prospective studies with more specific dose-response analysis comparing tramadol and codeine.”
The paper was published Oct. 19 in JAMA.
Tramadol has been considered to be a relatively safe opioid and was even strongly recommended by the American Academy of Orthopaedic Surgeons for patients experiencing symptomatic knee osteoarthritis. The authors point out that studies looking at opioid use from 2019 to 2020 show that tramadol was the most prescribed opioid in England, the Netherlands, and Spain.
In the United States, the age-adjusted rate of drug overdose deaths from synthetic opioids rose from 1.0 per 100 000 in 2013 to 11.4 in 2019. Most of these deaths were attributable to fentanyl but some were also related to tramadol.
But despite its wide use in managing chronic noncancer pain, results of recent studies suggest adverse outcomes as compared with other agents. Last year, one study found that older patients who received tramadol had a significant increase in the risk of hip fracture vs. those using NSAIDs or codeine. Another study, also published in 2020, showed that patients with osteoarthritis who were treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs.
In the current paper, Dr. Reyes and colleagues evaluated the association of tramadol with mortality and other adverse clinical outcomes in outpatient settings, compared with codeine.
They conducted a retrospective, population-based, propensity score–matched cohort study using a primary care database that routinely collects medical records and pharmacy dispensations for more than 80% of the population of Catalonia, Spain. The cohort included people 18 years or older who had been prescribed tramadol or codeine from 2007 to 2017 and were followed up to Dec. 31, 2017.
After propensity score matching, the final analysis included 368,960 participants: 184,480 in the tramadol arm and 184,480 in the codeine arm.
The mean age of patients was 52.7 years in the tramadol arm and 53.5 years in the codeine arm, and the prevalence of cancer was 3.2% and 3.3%, respectively. The most common diagnoses in this cohort were back pain (47.5% vs. 48.5%), neck/shoulder pain (28.6% vs. 29.5%), and osteoarthritis (15.3% vs. 15.5%). The most commonly used drugs were ibuprofen (34.4% vs. 34.3%) and paracetamol/acetaminophen (37.1% vs. 36.8%)
Higher risk of adverse outcomes
As compared with codeine, tramadol use was significantly associated with a higher risk of mortality (13.00 vs. 5.61 per 1,000 person-years; hazard ratio, 2.31; 95% confidence interval, 2.08-2.56); absolute rate differences (7.37 per 1,000 person-years; 95% CI, 6.09-8.78), cardiovascular events (10.03 vs. 8.67 per 1,000 person-years; HR, 1.15; 95% CI, 1.05-1.27; ARD, 1.36 per 1,000 person-years; 95% CI, 0.45-2.36), and fractures (12.26 vs. 8.13 per 1,000 person-years; HR, 1.50; 95% CI, 1.37-1.65; ARD, 4.10 per 1,000 person-years; 95% CI, 3.02-5.29).
A subgroup and sensitivity analysis showed that the increased mortality risk associated with tramadol was significantly higher in younger persons vs. older ones (HR, 3.14; 95% CI, 1.82-5.41 vs. 2.39; 95% CI, 2.20-2.60]; P < .001 for interaction). In addition, women had a significantly greater risk of cardiovascular events versus men (HR, 1.32; 95% CI, 1.19-1.46] vs. 1.03; 95% CI, 0.9-1.13]; P < .001 for interaction).
Potential for confounding
Weighing in on the data, Daniel Solomon, MD, MPH, chief of clinical sciences, division of rheumatology, Brigham and Women’s Hospital, and professor of medicine, Harvard Medical School, Boston, noted that because it is extremely unlikely that anyone will ever conduct a large, head-to-head safety trial comparing different opioids, the results of this paper are important to consider.
“However, as the authors appropriately caution, this type of analysis is limited by the strong potential for residual confounding,” he said. “In other words, even though the authors used state-of-the-art methods to limit imbalances between the patients initiating tramadol versus codeine, there is strong reason to believe that imbalances that may account for the differences in adverse events exist.”
For example, he noted that if one looks at the distribution of comorbid conditions in the before-matching group, tramadol initiators demonstrate a higher frequency of chronic kidney disease, diabetes, and overall chronic comorbid diseases. “This suggests to me that prescribers apply selection criteria when choosing who to prescribe which opioid,” Dr. Solomon explained.
“While the authors’ use of propensity score matching limits confounding, it only can improve balance for measured confounders,” he said. “Other factors not measured in this type of data set – blood pressure, pain, physical activity, tobacco use, body mass index – may still demonstrate imbalances even after matching.”
But after these limitations are taken into consideration, the results remain concerning, Dr. Solomon emphasized, particularly the all-cause mortality excess of tramadol versus codeine users. “This study did not include cause of death, which would help the reader understand why users of tramadol were dying more frequently,” he added. “It also might help in understanding whether this is a true biologic effect or residual confounding.”
Perceived safety
In an accompanying editorial, Howard S. Kim, MD, MS, and colleagues from Northwestern University, Chicago, write that the greatest risk of tramadol may involve the perception that it is “inherently safer than other opioids.”
“In actuality, the mechanisms of action and variable metabolism of tramadol in a given population create considerable therapeutic uncertainty and introduce additional risk exposure,” they say, as demonstrated in the current study.
Therefore, when clinicians determine that an opioid is needed for pain relief, it may be a better option to select a pure opioid agonist that has a more predictable therapeutic effect and known adverse effect profile, such as morphine or hydrocodone. “This would allow clinicians and patients to more properly weigh the risks and benefits of initiating opioid therapy through shared decision-making and prompt the level of counseling on safe use, storage, and disposal practices that all opioids deserve,” write the editorialists.
The study was funded by the Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina. The research was supported by the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Reyes has disclosed no relevant financial relationships. Dr. Solomon disclosed salary support from research contracts to his hospital from Amgen, AbbVie, Moderna, the Rheumatology Research Foundation, and National Institutes of Health; and royalties from UpToDate. Dr. Kim reported unrelated grant support from the Agency for Healthcare Research and Quality.
A version of this article first appeared on Medscape.com.
Tramadol is increasingly used to manage chronic noncancer pain, but as compared with opioids, it appears to be linked to a higher risk for adverse outcomes, according to new data.
Among a cohort of patients who received a prescription for either tramadol or codeine for orthopedic-related pain, tramadol was significantly associated with a higher risk of mortality, cardiovascular events, and fractures.
However, there was no significant difference in the risk of falls, delirium, constipation, opioid abuse/dependence, or sleep disorders between the two drugs.
“However, this is a retrospective cohort study, and despite it providing information that would otherwise be impossible to gather – such as from randomized controlled trials – clinicians should not solely base their decision on this study,” cautioned lead author Carlen Reyes, MD, PhD, of the Institut Universitari d’Investigació en Atenció Primària (IDIAP Jordi Gol), Barcelona.
Dr. Reyes noted that the intake of tramadol and codeine was analyzed using the number of “packages” that were dispensed, as an approximation of the real intake. “Logically we could think that the more packages dispensed of one drug, the more dose the patient is taking, but this is not always true given the availability of different doses commercialized of tramadol and different doses prescribed,” she said. “Given that we did not account for the real dose prescribed, we can only suspect an increased risk of these outcomes and reinforce the need for further prospective studies with more specific dose-response analysis comparing tramadol and codeine.”
The paper was published Oct. 19 in JAMA.
Tramadol has been considered to be a relatively safe opioid and was even strongly recommended by the American Academy of Orthopaedic Surgeons for patients experiencing symptomatic knee osteoarthritis. The authors point out that studies looking at opioid use from 2019 to 2020 show that tramadol was the most prescribed opioid in England, the Netherlands, and Spain.
In the United States, the age-adjusted rate of drug overdose deaths from synthetic opioids rose from 1.0 per 100 000 in 2013 to 11.4 in 2019. Most of these deaths were attributable to fentanyl but some were also related to tramadol.
But despite its wide use in managing chronic noncancer pain, results of recent studies suggest adverse outcomes as compared with other agents. Last year, one study found that older patients who received tramadol had a significant increase in the risk of hip fracture vs. those using NSAIDs or codeine. Another study, also published in 2020, showed that patients with osteoarthritis who were treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs.
In the current paper, Dr. Reyes and colleagues evaluated the association of tramadol with mortality and other adverse clinical outcomes in outpatient settings, compared with codeine.
They conducted a retrospective, population-based, propensity score–matched cohort study using a primary care database that routinely collects medical records and pharmacy dispensations for more than 80% of the population of Catalonia, Spain. The cohort included people 18 years or older who had been prescribed tramadol or codeine from 2007 to 2017 and were followed up to Dec. 31, 2017.
After propensity score matching, the final analysis included 368,960 participants: 184,480 in the tramadol arm and 184,480 in the codeine arm.
The mean age of patients was 52.7 years in the tramadol arm and 53.5 years in the codeine arm, and the prevalence of cancer was 3.2% and 3.3%, respectively. The most common diagnoses in this cohort were back pain (47.5% vs. 48.5%), neck/shoulder pain (28.6% vs. 29.5%), and osteoarthritis (15.3% vs. 15.5%). The most commonly used drugs were ibuprofen (34.4% vs. 34.3%) and paracetamol/acetaminophen (37.1% vs. 36.8%)
Higher risk of adverse outcomes
As compared with codeine, tramadol use was significantly associated with a higher risk of mortality (13.00 vs. 5.61 per 1,000 person-years; hazard ratio, 2.31; 95% confidence interval, 2.08-2.56); absolute rate differences (7.37 per 1,000 person-years; 95% CI, 6.09-8.78), cardiovascular events (10.03 vs. 8.67 per 1,000 person-years; HR, 1.15; 95% CI, 1.05-1.27; ARD, 1.36 per 1,000 person-years; 95% CI, 0.45-2.36), and fractures (12.26 vs. 8.13 per 1,000 person-years; HR, 1.50; 95% CI, 1.37-1.65; ARD, 4.10 per 1,000 person-years; 95% CI, 3.02-5.29).
A subgroup and sensitivity analysis showed that the increased mortality risk associated with tramadol was significantly higher in younger persons vs. older ones (HR, 3.14; 95% CI, 1.82-5.41 vs. 2.39; 95% CI, 2.20-2.60]; P < .001 for interaction). In addition, women had a significantly greater risk of cardiovascular events versus men (HR, 1.32; 95% CI, 1.19-1.46] vs. 1.03; 95% CI, 0.9-1.13]; P < .001 for interaction).
Potential for confounding
Weighing in on the data, Daniel Solomon, MD, MPH, chief of clinical sciences, division of rheumatology, Brigham and Women’s Hospital, and professor of medicine, Harvard Medical School, Boston, noted that because it is extremely unlikely that anyone will ever conduct a large, head-to-head safety trial comparing different opioids, the results of this paper are important to consider.
“However, as the authors appropriately caution, this type of analysis is limited by the strong potential for residual confounding,” he said. “In other words, even though the authors used state-of-the-art methods to limit imbalances between the patients initiating tramadol versus codeine, there is strong reason to believe that imbalances that may account for the differences in adverse events exist.”
For example, he noted that if one looks at the distribution of comorbid conditions in the before-matching group, tramadol initiators demonstrate a higher frequency of chronic kidney disease, diabetes, and overall chronic comorbid diseases. “This suggests to me that prescribers apply selection criteria when choosing who to prescribe which opioid,” Dr. Solomon explained.
“While the authors’ use of propensity score matching limits confounding, it only can improve balance for measured confounders,” he said. “Other factors not measured in this type of data set – blood pressure, pain, physical activity, tobacco use, body mass index – may still demonstrate imbalances even after matching.”
But after these limitations are taken into consideration, the results remain concerning, Dr. Solomon emphasized, particularly the all-cause mortality excess of tramadol versus codeine users. “This study did not include cause of death, which would help the reader understand why users of tramadol were dying more frequently,” he added. “It also might help in understanding whether this is a true biologic effect or residual confounding.”
Perceived safety
In an accompanying editorial, Howard S. Kim, MD, MS, and colleagues from Northwestern University, Chicago, write that the greatest risk of tramadol may involve the perception that it is “inherently safer than other opioids.”
“In actuality, the mechanisms of action and variable metabolism of tramadol in a given population create considerable therapeutic uncertainty and introduce additional risk exposure,” they say, as demonstrated in the current study.
Therefore, when clinicians determine that an opioid is needed for pain relief, it may be a better option to select a pure opioid agonist that has a more predictable therapeutic effect and known adverse effect profile, such as morphine or hydrocodone. “This would allow clinicians and patients to more properly weigh the risks and benefits of initiating opioid therapy through shared decision-making and prompt the level of counseling on safe use, storage, and disposal practices that all opioids deserve,” write the editorialists.
The study was funded by the Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina. The research was supported by the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Reyes has disclosed no relevant financial relationships. Dr. Solomon disclosed salary support from research contracts to his hospital from Amgen, AbbVie, Moderna, the Rheumatology Research Foundation, and National Institutes of Health; and royalties from UpToDate. Dr. Kim reported unrelated grant support from the Agency for Healthcare Research and Quality.
A version of this article first appeared on Medscape.com.
Tramadol is increasingly used to manage chronic noncancer pain, but as compared with opioids, it appears to be linked to a higher risk for adverse outcomes, according to new data.
Among a cohort of patients who received a prescription for either tramadol or codeine for orthopedic-related pain, tramadol was significantly associated with a higher risk of mortality, cardiovascular events, and fractures.
However, there was no significant difference in the risk of falls, delirium, constipation, opioid abuse/dependence, or sleep disorders between the two drugs.
“However, this is a retrospective cohort study, and despite it providing information that would otherwise be impossible to gather – such as from randomized controlled trials – clinicians should not solely base their decision on this study,” cautioned lead author Carlen Reyes, MD, PhD, of the Institut Universitari d’Investigació en Atenció Primària (IDIAP Jordi Gol), Barcelona.
Dr. Reyes noted that the intake of tramadol and codeine was analyzed using the number of “packages” that were dispensed, as an approximation of the real intake. “Logically we could think that the more packages dispensed of one drug, the more dose the patient is taking, but this is not always true given the availability of different doses commercialized of tramadol and different doses prescribed,” she said. “Given that we did not account for the real dose prescribed, we can only suspect an increased risk of these outcomes and reinforce the need for further prospective studies with more specific dose-response analysis comparing tramadol and codeine.”
The paper was published Oct. 19 in JAMA.
Tramadol has been considered to be a relatively safe opioid and was even strongly recommended by the American Academy of Orthopaedic Surgeons for patients experiencing symptomatic knee osteoarthritis. The authors point out that studies looking at opioid use from 2019 to 2020 show that tramadol was the most prescribed opioid in England, the Netherlands, and Spain.
In the United States, the age-adjusted rate of drug overdose deaths from synthetic opioids rose from 1.0 per 100 000 in 2013 to 11.4 in 2019. Most of these deaths were attributable to fentanyl but some were also related to tramadol.
But despite its wide use in managing chronic noncancer pain, results of recent studies suggest adverse outcomes as compared with other agents. Last year, one study found that older patients who received tramadol had a significant increase in the risk of hip fracture vs. those using NSAIDs or codeine. Another study, also published in 2020, showed that patients with osteoarthritis who were treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs.
In the current paper, Dr. Reyes and colleagues evaluated the association of tramadol with mortality and other adverse clinical outcomes in outpatient settings, compared with codeine.
They conducted a retrospective, population-based, propensity score–matched cohort study using a primary care database that routinely collects medical records and pharmacy dispensations for more than 80% of the population of Catalonia, Spain. The cohort included people 18 years or older who had been prescribed tramadol or codeine from 2007 to 2017 and were followed up to Dec. 31, 2017.
After propensity score matching, the final analysis included 368,960 participants: 184,480 in the tramadol arm and 184,480 in the codeine arm.
The mean age of patients was 52.7 years in the tramadol arm and 53.5 years in the codeine arm, and the prevalence of cancer was 3.2% and 3.3%, respectively. The most common diagnoses in this cohort were back pain (47.5% vs. 48.5%), neck/shoulder pain (28.6% vs. 29.5%), and osteoarthritis (15.3% vs. 15.5%). The most commonly used drugs were ibuprofen (34.4% vs. 34.3%) and paracetamol/acetaminophen (37.1% vs. 36.8%)
Higher risk of adverse outcomes
As compared with codeine, tramadol use was significantly associated with a higher risk of mortality (13.00 vs. 5.61 per 1,000 person-years; hazard ratio, 2.31; 95% confidence interval, 2.08-2.56); absolute rate differences (7.37 per 1,000 person-years; 95% CI, 6.09-8.78), cardiovascular events (10.03 vs. 8.67 per 1,000 person-years; HR, 1.15; 95% CI, 1.05-1.27; ARD, 1.36 per 1,000 person-years; 95% CI, 0.45-2.36), and fractures (12.26 vs. 8.13 per 1,000 person-years; HR, 1.50; 95% CI, 1.37-1.65; ARD, 4.10 per 1,000 person-years; 95% CI, 3.02-5.29).
A subgroup and sensitivity analysis showed that the increased mortality risk associated with tramadol was significantly higher in younger persons vs. older ones (HR, 3.14; 95% CI, 1.82-5.41 vs. 2.39; 95% CI, 2.20-2.60]; P < .001 for interaction). In addition, women had a significantly greater risk of cardiovascular events versus men (HR, 1.32; 95% CI, 1.19-1.46] vs. 1.03; 95% CI, 0.9-1.13]; P < .001 for interaction).
Potential for confounding
Weighing in on the data, Daniel Solomon, MD, MPH, chief of clinical sciences, division of rheumatology, Brigham and Women’s Hospital, and professor of medicine, Harvard Medical School, Boston, noted that because it is extremely unlikely that anyone will ever conduct a large, head-to-head safety trial comparing different opioids, the results of this paper are important to consider.
“However, as the authors appropriately caution, this type of analysis is limited by the strong potential for residual confounding,” he said. “In other words, even though the authors used state-of-the-art methods to limit imbalances between the patients initiating tramadol versus codeine, there is strong reason to believe that imbalances that may account for the differences in adverse events exist.”
For example, he noted that if one looks at the distribution of comorbid conditions in the before-matching group, tramadol initiators demonstrate a higher frequency of chronic kidney disease, diabetes, and overall chronic comorbid diseases. “This suggests to me that prescribers apply selection criteria when choosing who to prescribe which opioid,” Dr. Solomon explained.
“While the authors’ use of propensity score matching limits confounding, it only can improve balance for measured confounders,” he said. “Other factors not measured in this type of data set – blood pressure, pain, physical activity, tobacco use, body mass index – may still demonstrate imbalances even after matching.”
But after these limitations are taken into consideration, the results remain concerning, Dr. Solomon emphasized, particularly the all-cause mortality excess of tramadol versus codeine users. “This study did not include cause of death, which would help the reader understand why users of tramadol were dying more frequently,” he added. “It also might help in understanding whether this is a true biologic effect or residual confounding.”
Perceived safety
In an accompanying editorial, Howard S. Kim, MD, MS, and colleagues from Northwestern University, Chicago, write that the greatest risk of tramadol may involve the perception that it is “inherently safer than other opioids.”
“In actuality, the mechanisms of action and variable metabolism of tramadol in a given population create considerable therapeutic uncertainty and introduce additional risk exposure,” they say, as demonstrated in the current study.
Therefore, when clinicians determine that an opioid is needed for pain relief, it may be a better option to select a pure opioid agonist that has a more predictable therapeutic effect and known adverse effect profile, such as morphine or hydrocodone. “This would allow clinicians and patients to more properly weigh the risks and benefits of initiating opioid therapy through shared decision-making and prompt the level of counseling on safe use, storage, and disposal practices that all opioids deserve,” write the editorialists.
The study was funded by the Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina. The research was supported by the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Reyes has disclosed no relevant financial relationships. Dr. Solomon disclosed salary support from research contracts to his hospital from Amgen, AbbVie, Moderna, the Rheumatology Research Foundation, and National Institutes of Health; and royalties from UpToDate. Dr. Kim reported unrelated grant support from the Agency for Healthcare Research and Quality.
A version of this article first appeared on Medscape.com.
U.S. arthritis prevalence continues steady rise; activity limitations grow more rapidly
Nearly a quarter of adults in the United States have been diagnosed with various forms of arthritis, new federal estimates report. The disorders limit the activities of 43.9% of them. Researchers also report that adults with poorer mental or physical health and those who are more disadvantaged socially are most vulnerable to arthritis.
“There is a substantial unmet need for existing, evidence-based, arthritis-appropriate interventions for people with arthritis to minimize activity limitations,” study coauthor and Centers for Disease Control and Prevention epidemiologist Kristina Theis, PhD, MPH, told this news organization. “Our findings show that interventions addressing self-management, education, physical activity, workplace accommodations, and mental health, among other areas, are all indicated for people with arthritis.”
The CDC report was published Oct. 8 in Morbidity and Mortality Weekly Report. Researchers estimated the number of arthritis cases on the basis of in-person interviews conducted with tens of thousands of U.S. adults as part of the National Health Interview Survey during 2016-2018. In the report, the researchers considered arthritis to include general arthritis, rheumatoid arthritis, gout, lupus, and fibromyalgia.
Activity limitations rose faster than predicted
According to the report, an estimated 58.5 million U.S. adults (23.7%; 21.5% age-standardized) told interviewers that they had been diagnosed with arthritis conditions. Of those, 25.7 million (43.9%; 40.8% age-standardized) had arthritis-attributable activity limitations (AAALs), which represents 10.4% of all adults.
The number of adults who reported having arthritis rose by 4.1 million from previous estimates for the years 2013-2015, a number that’s on pace with predictions. The number in the AAAL category rose by 2 million, a jump that’s higher than what had been predicted.
“The aging of the population is one factor in the increasing number of people with arthritis, even though arthritis is not an inevitable part of aging,” Dr. Theis said. “Individual factors, such as body mass index or other health conditions, and societal factors, such as educational and economic opportunities, likely play a role.”
Arthritis was especially common among those aged ≥ 65 years (50.4%), those who were unable to work or were disabled (52.3%), and those who self-reported fair/poor health (51.2%) or joint symptoms in the past 30 days (52.2%). The rate of arthritis was also high among those whose activities of daily living (ADL) were limited (54.8%) and those whose instrumental activities of daily living (IADL) were limited (55.9%).
The researchers report that the percentage of AAAL was also high among the following groups: “adults with joint symptoms in the past 30 days (51.6%), adults who were unable to work or disabled (54.7%), adults of other/multiple races (54.5%) or non-Hispanic American Indian or Alaska Natives (60.7%), adults with low income (53.3%) or poor/near poor income-to-poverty ratios (63.3%), or with moderate psychological distress (59.5%). AAAL was reported by a high proportion of adults with arthritis who had an ADL disability (82.6%), IADL disability (80.4%), serious psychological distress (76.3%), or fair/poor self-rated health (72.6%).”
The researchers found that among all adults with arthritis, the percentage of adults with arthritis was high among women (59.3%), those with obesity or overweight (74.2%), and those who weren’t sufficiently active (58%).
Comments on latest findings
Michael LaValley, PhD, biostatistician at the Boston University School of Public Health, who has studied arthritis statistics, told this news organization that the findings “fall right in line with the trends that have been observed in arthritis over the past 20 years. The prevalence is increasing, which certainly seems to be influenced by the aging population in the U.S.”
As for specific conditions, he said the rate of osteoarthritis may be influenced by older Americans and by those with obesity and sedentary behavior. “There is also some thinking that there may be environmental factors increasing the risk for some types of arthritis, but nothing conclusive. There also may be more clinical attention paid to arthritic conditions, leading to more people being diagnosed or even just suspecting that they have arthritis.”
It’s difficult to disentangle connections between arthritis and risk factors such as poverty, he said. “There almost certainly are occupational exposures that put people at risk of osteoarthritis – having to kneel, stoop, and lift heavy things – or other musculoskeletal conditions like lower back pain. These exposures are most likely in jobs that would predominantly go to people with few other options because of lower levels of income and education. People in these jobs would also be more likely to have financial stresses that lead to increased psychological distress and less time to take care of their health.”
Also, he said, “There is probably some reverse causation with the occupational results, self-related health, and psychological distress. These could all be affected by a person’s arthritis. Having arthritis may interfere with getting a better-paying job, and arthritis could certainly reduce someone’s self-reported health and induce psychological distress.”
The authors and LaValley have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nearly a quarter of adults in the United States have been diagnosed with various forms of arthritis, new federal estimates report. The disorders limit the activities of 43.9% of them. Researchers also report that adults with poorer mental or physical health and those who are more disadvantaged socially are most vulnerable to arthritis.
“There is a substantial unmet need for existing, evidence-based, arthritis-appropriate interventions for people with arthritis to minimize activity limitations,” study coauthor and Centers for Disease Control and Prevention epidemiologist Kristina Theis, PhD, MPH, told this news organization. “Our findings show that interventions addressing self-management, education, physical activity, workplace accommodations, and mental health, among other areas, are all indicated for people with arthritis.”
The CDC report was published Oct. 8 in Morbidity and Mortality Weekly Report. Researchers estimated the number of arthritis cases on the basis of in-person interviews conducted with tens of thousands of U.S. adults as part of the National Health Interview Survey during 2016-2018. In the report, the researchers considered arthritis to include general arthritis, rheumatoid arthritis, gout, lupus, and fibromyalgia.
Activity limitations rose faster than predicted
According to the report, an estimated 58.5 million U.S. adults (23.7%; 21.5% age-standardized) told interviewers that they had been diagnosed with arthritis conditions. Of those, 25.7 million (43.9%; 40.8% age-standardized) had arthritis-attributable activity limitations (AAALs), which represents 10.4% of all adults.
The number of adults who reported having arthritis rose by 4.1 million from previous estimates for the years 2013-2015, a number that’s on pace with predictions. The number in the AAAL category rose by 2 million, a jump that’s higher than what had been predicted.
“The aging of the population is one factor in the increasing number of people with arthritis, even though arthritis is not an inevitable part of aging,” Dr. Theis said. “Individual factors, such as body mass index or other health conditions, and societal factors, such as educational and economic opportunities, likely play a role.”
Arthritis was especially common among those aged ≥ 65 years (50.4%), those who were unable to work or were disabled (52.3%), and those who self-reported fair/poor health (51.2%) or joint symptoms in the past 30 days (52.2%). The rate of arthritis was also high among those whose activities of daily living (ADL) were limited (54.8%) and those whose instrumental activities of daily living (IADL) were limited (55.9%).
The researchers report that the percentage of AAAL was also high among the following groups: “adults with joint symptoms in the past 30 days (51.6%), adults who were unable to work or disabled (54.7%), adults of other/multiple races (54.5%) or non-Hispanic American Indian or Alaska Natives (60.7%), adults with low income (53.3%) or poor/near poor income-to-poverty ratios (63.3%), or with moderate psychological distress (59.5%). AAAL was reported by a high proportion of adults with arthritis who had an ADL disability (82.6%), IADL disability (80.4%), serious psychological distress (76.3%), or fair/poor self-rated health (72.6%).”
The researchers found that among all adults with arthritis, the percentage of adults with arthritis was high among women (59.3%), those with obesity or overweight (74.2%), and those who weren’t sufficiently active (58%).
Comments on latest findings
Michael LaValley, PhD, biostatistician at the Boston University School of Public Health, who has studied arthritis statistics, told this news organization that the findings “fall right in line with the trends that have been observed in arthritis over the past 20 years. The prevalence is increasing, which certainly seems to be influenced by the aging population in the U.S.”
As for specific conditions, he said the rate of osteoarthritis may be influenced by older Americans and by those with obesity and sedentary behavior. “There is also some thinking that there may be environmental factors increasing the risk for some types of arthritis, but nothing conclusive. There also may be more clinical attention paid to arthritic conditions, leading to more people being diagnosed or even just suspecting that they have arthritis.”
It’s difficult to disentangle connections between arthritis and risk factors such as poverty, he said. “There almost certainly are occupational exposures that put people at risk of osteoarthritis – having to kneel, stoop, and lift heavy things – or other musculoskeletal conditions like lower back pain. These exposures are most likely in jobs that would predominantly go to people with few other options because of lower levels of income and education. People in these jobs would also be more likely to have financial stresses that lead to increased psychological distress and less time to take care of their health.”
Also, he said, “There is probably some reverse causation with the occupational results, self-related health, and psychological distress. These could all be affected by a person’s arthritis. Having arthritis may interfere with getting a better-paying job, and arthritis could certainly reduce someone’s self-reported health and induce psychological distress.”
The authors and LaValley have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nearly a quarter of adults in the United States have been diagnosed with various forms of arthritis, new federal estimates report. The disorders limit the activities of 43.9% of them. Researchers also report that adults with poorer mental or physical health and those who are more disadvantaged socially are most vulnerable to arthritis.
“There is a substantial unmet need for existing, evidence-based, arthritis-appropriate interventions for people with arthritis to minimize activity limitations,” study coauthor and Centers for Disease Control and Prevention epidemiologist Kristina Theis, PhD, MPH, told this news organization. “Our findings show that interventions addressing self-management, education, physical activity, workplace accommodations, and mental health, among other areas, are all indicated for people with arthritis.”
The CDC report was published Oct. 8 in Morbidity and Mortality Weekly Report. Researchers estimated the number of arthritis cases on the basis of in-person interviews conducted with tens of thousands of U.S. adults as part of the National Health Interview Survey during 2016-2018. In the report, the researchers considered arthritis to include general arthritis, rheumatoid arthritis, gout, lupus, and fibromyalgia.
Activity limitations rose faster than predicted
According to the report, an estimated 58.5 million U.S. adults (23.7%; 21.5% age-standardized) told interviewers that they had been diagnosed with arthritis conditions. Of those, 25.7 million (43.9%; 40.8% age-standardized) had arthritis-attributable activity limitations (AAALs), which represents 10.4% of all adults.
The number of adults who reported having arthritis rose by 4.1 million from previous estimates for the years 2013-2015, a number that’s on pace with predictions. The number in the AAAL category rose by 2 million, a jump that’s higher than what had been predicted.
“The aging of the population is one factor in the increasing number of people with arthritis, even though arthritis is not an inevitable part of aging,” Dr. Theis said. “Individual factors, such as body mass index or other health conditions, and societal factors, such as educational and economic opportunities, likely play a role.”
Arthritis was especially common among those aged ≥ 65 years (50.4%), those who were unable to work or were disabled (52.3%), and those who self-reported fair/poor health (51.2%) or joint symptoms in the past 30 days (52.2%). The rate of arthritis was also high among those whose activities of daily living (ADL) were limited (54.8%) and those whose instrumental activities of daily living (IADL) were limited (55.9%).
The researchers report that the percentage of AAAL was also high among the following groups: “adults with joint symptoms in the past 30 days (51.6%), adults who were unable to work or disabled (54.7%), adults of other/multiple races (54.5%) or non-Hispanic American Indian or Alaska Natives (60.7%), adults with low income (53.3%) or poor/near poor income-to-poverty ratios (63.3%), or with moderate psychological distress (59.5%). AAAL was reported by a high proportion of adults with arthritis who had an ADL disability (82.6%), IADL disability (80.4%), serious psychological distress (76.3%), or fair/poor self-rated health (72.6%).”
The researchers found that among all adults with arthritis, the percentage of adults with arthritis was high among women (59.3%), those with obesity or overweight (74.2%), and those who weren’t sufficiently active (58%).
Comments on latest findings
Michael LaValley, PhD, biostatistician at the Boston University School of Public Health, who has studied arthritis statistics, told this news organization that the findings “fall right in line with the trends that have been observed in arthritis over the past 20 years. The prevalence is increasing, which certainly seems to be influenced by the aging population in the U.S.”
As for specific conditions, he said the rate of osteoarthritis may be influenced by older Americans and by those with obesity and sedentary behavior. “There is also some thinking that there may be environmental factors increasing the risk for some types of arthritis, but nothing conclusive. There also may be more clinical attention paid to arthritic conditions, leading to more people being diagnosed or even just suspecting that they have arthritis.”
It’s difficult to disentangle connections between arthritis and risk factors such as poverty, he said. “There almost certainly are occupational exposures that put people at risk of osteoarthritis – having to kneel, stoop, and lift heavy things – or other musculoskeletal conditions like lower back pain. These exposures are most likely in jobs that would predominantly go to people with few other options because of lower levels of income and education. People in these jobs would also be more likely to have financial stresses that lead to increased psychological distress and less time to take care of their health.”
Also, he said, “There is probably some reverse causation with the occupational results, self-related health, and psychological distress. These could all be affected by a person’s arthritis. Having arthritis may interfere with getting a better-paying job, and arthritis could certainly reduce someone’s self-reported health and induce psychological distress.”
The authors and LaValley have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Few JAK inhibitor users have diminished immune response to COVID-19 vaccines
Patients who are being treated with Janus kinase (JAK) inhibitors overall show a high immune response rate to COVID-19 vaccination, one that matches the rates seen in patients on other immunosuppressants, a new study has found.
The patients taking a JAK inhibitor who are most at risk of a diminished response may be those on upadacitinib (Rinvoq) and anyone 65 years or older, wrote Raphaèle Seror, MD, PhD, of Paris-Saclay (France) University and coauthors. The study was published in The Lancet Rheumatology.
To gauge the effectiveness of COVID-19 vaccines in this subset of immunosuppressed patients, the researchers analyzed 113 participants in the MAJIK-SFR Registry, a multicenter study of French patients with rheumatoid or psoriatic arthritis. The participants were treated at 13 centers throughout France; their mean age was 61.8 years (standard deviation, 12.5), and 72% were female. A total of 56 were taking baricitinib (Olumiant), 30 were taking tofacitinib (Xeljanz), and 27 were taking upadacitinib.
Serologic assessment was performed an average of 8.7 weeks (SD, 5.2) after the last dose of vaccine. The overall response rate – defined as the proportion of patients with detectable anti-spike antibodies per manufacturer’s cutoff values – was 88% (100 of 113). The nonresponse rate was higher with upadacitinib (7 of 27 patients, 26%) than with baricitinib (5 of 56, 9%) or tofacitinib (1 of 30, 3%). The only nonresponders who were not age 65 or older were four of the seven who received upadacitinib. The interval between the last vaccine dose and serologic assessment was somewhat longer in nonresponders (11.3 weeks) than in responders (8.3 weeks).
Earlier this year, the American College of Rheumatology recommended withholding JAK inhibitors for 1 week after each vaccine dose because of “concern related to the effects of this medication class on interferon signaling that may result in a diminished vaccine response Only two patients in the study had treatment with JAK inhibitors stopped before or after vaccination.
Questions about antibody levels remain difficult to answer
“This study does further confirm a big point,” said Alfred Kim, MD, PhD, of Washington University, St. Louis, in an interview. “Most people on any sort of immunosuppression, with rare exceptions, can mount responses to COVID-19 vaccination.”
“What level of response is going to be sufficient, of course, is not clear,” he added. “Even though most people generate responses, at the population level those responses seem lower than those in nonimmunosuppressed people. Particularly for those on upadacitinib, which is lower than patients on the other JAK inhibitors. Is that problematic? We don’t know yet.”
Dr. Kim, who was part of a separate, earlier study that assessed vaccine response in patients with chronic inflammatory disease who were being treated with immunosuppressive medications, noted that many of the questions patients are asking about their antibody levels cannot yet be answered.
“It’s kind of the Wild West of serologic testing out there right now,” he said. “Even though we’re recommending that people still don’t check their antibody levels because their results are largely inactionable, everyone is still getting them anyway. But each of these tests are slightly different, and the results and the interpretation are further clouded because of those slight performance differences between each platform.”
Dr. Kim highlighted the number of different tests as one of this study’s notable limitations: 11 different assays were used to determine patients’ immune responses. “The authors made the argument that these tests are FDA approved, and that’s true, but that doesn’t necessarily mean much. Approval does translate to technical reliability but not to comparisons between the tests.”
As for next steps, both the authors and Dr. Kim recognized the need for a prospective trial. “To do a vaccine effectiveness–type study and show clinical protection against either infection or hospitalization – those are going to take a while, simply because of the nature of how many people you need for each of these studies,” he said. “Time will tell whether or not the data that are being presented here will translate literally into protective outcomes downstream.”
The MAJIK Registry is supported by the French Rheumatology Society. The authors acknowledged numerous potential conflicts of interest, including receiving consulting fees, research support, and honoraria from various pharmaceutical companies.
Patients who are being treated with Janus kinase (JAK) inhibitors overall show a high immune response rate to COVID-19 vaccination, one that matches the rates seen in patients on other immunosuppressants, a new study has found.
The patients taking a JAK inhibitor who are most at risk of a diminished response may be those on upadacitinib (Rinvoq) and anyone 65 years or older, wrote Raphaèle Seror, MD, PhD, of Paris-Saclay (France) University and coauthors. The study was published in The Lancet Rheumatology.
To gauge the effectiveness of COVID-19 vaccines in this subset of immunosuppressed patients, the researchers analyzed 113 participants in the MAJIK-SFR Registry, a multicenter study of French patients with rheumatoid or psoriatic arthritis. The participants were treated at 13 centers throughout France; their mean age was 61.8 years (standard deviation, 12.5), and 72% were female. A total of 56 were taking baricitinib (Olumiant), 30 were taking tofacitinib (Xeljanz), and 27 were taking upadacitinib.
Serologic assessment was performed an average of 8.7 weeks (SD, 5.2) after the last dose of vaccine. The overall response rate – defined as the proportion of patients with detectable anti-spike antibodies per manufacturer’s cutoff values – was 88% (100 of 113). The nonresponse rate was higher with upadacitinib (7 of 27 patients, 26%) than with baricitinib (5 of 56, 9%) or tofacitinib (1 of 30, 3%). The only nonresponders who were not age 65 or older were four of the seven who received upadacitinib. The interval between the last vaccine dose and serologic assessment was somewhat longer in nonresponders (11.3 weeks) than in responders (8.3 weeks).
Earlier this year, the American College of Rheumatology recommended withholding JAK inhibitors for 1 week after each vaccine dose because of “concern related to the effects of this medication class on interferon signaling that may result in a diminished vaccine response Only two patients in the study had treatment with JAK inhibitors stopped before or after vaccination.
Questions about antibody levels remain difficult to answer
“This study does further confirm a big point,” said Alfred Kim, MD, PhD, of Washington University, St. Louis, in an interview. “Most people on any sort of immunosuppression, with rare exceptions, can mount responses to COVID-19 vaccination.”
“What level of response is going to be sufficient, of course, is not clear,” he added. “Even though most people generate responses, at the population level those responses seem lower than those in nonimmunosuppressed people. Particularly for those on upadacitinib, which is lower than patients on the other JAK inhibitors. Is that problematic? We don’t know yet.”
Dr. Kim, who was part of a separate, earlier study that assessed vaccine response in patients with chronic inflammatory disease who were being treated with immunosuppressive medications, noted that many of the questions patients are asking about their antibody levels cannot yet be answered.
“It’s kind of the Wild West of serologic testing out there right now,” he said. “Even though we’re recommending that people still don’t check their antibody levels because their results are largely inactionable, everyone is still getting them anyway. But each of these tests are slightly different, and the results and the interpretation are further clouded because of those slight performance differences between each platform.”
Dr. Kim highlighted the number of different tests as one of this study’s notable limitations: 11 different assays were used to determine patients’ immune responses. “The authors made the argument that these tests are FDA approved, and that’s true, but that doesn’t necessarily mean much. Approval does translate to technical reliability but not to comparisons between the tests.”
As for next steps, both the authors and Dr. Kim recognized the need for a prospective trial. “To do a vaccine effectiveness–type study and show clinical protection against either infection or hospitalization – those are going to take a while, simply because of the nature of how many people you need for each of these studies,” he said. “Time will tell whether or not the data that are being presented here will translate literally into protective outcomes downstream.”
The MAJIK Registry is supported by the French Rheumatology Society. The authors acknowledged numerous potential conflicts of interest, including receiving consulting fees, research support, and honoraria from various pharmaceutical companies.
Patients who are being treated with Janus kinase (JAK) inhibitors overall show a high immune response rate to COVID-19 vaccination, one that matches the rates seen in patients on other immunosuppressants, a new study has found.
The patients taking a JAK inhibitor who are most at risk of a diminished response may be those on upadacitinib (Rinvoq) and anyone 65 years or older, wrote Raphaèle Seror, MD, PhD, of Paris-Saclay (France) University and coauthors. The study was published in The Lancet Rheumatology.
To gauge the effectiveness of COVID-19 vaccines in this subset of immunosuppressed patients, the researchers analyzed 113 participants in the MAJIK-SFR Registry, a multicenter study of French patients with rheumatoid or psoriatic arthritis. The participants were treated at 13 centers throughout France; their mean age was 61.8 years (standard deviation, 12.5), and 72% were female. A total of 56 were taking baricitinib (Olumiant), 30 were taking tofacitinib (Xeljanz), and 27 were taking upadacitinib.
Serologic assessment was performed an average of 8.7 weeks (SD, 5.2) after the last dose of vaccine. The overall response rate – defined as the proportion of patients with detectable anti-spike antibodies per manufacturer’s cutoff values – was 88% (100 of 113). The nonresponse rate was higher with upadacitinib (7 of 27 patients, 26%) than with baricitinib (5 of 56, 9%) or tofacitinib (1 of 30, 3%). The only nonresponders who were not age 65 or older were four of the seven who received upadacitinib. The interval between the last vaccine dose and serologic assessment was somewhat longer in nonresponders (11.3 weeks) than in responders (8.3 weeks).
Earlier this year, the American College of Rheumatology recommended withholding JAK inhibitors for 1 week after each vaccine dose because of “concern related to the effects of this medication class on interferon signaling that may result in a diminished vaccine response Only two patients in the study had treatment with JAK inhibitors stopped before or after vaccination.
Questions about antibody levels remain difficult to answer
“This study does further confirm a big point,” said Alfred Kim, MD, PhD, of Washington University, St. Louis, in an interview. “Most people on any sort of immunosuppression, with rare exceptions, can mount responses to COVID-19 vaccination.”
“What level of response is going to be sufficient, of course, is not clear,” he added. “Even though most people generate responses, at the population level those responses seem lower than those in nonimmunosuppressed people. Particularly for those on upadacitinib, which is lower than patients on the other JAK inhibitors. Is that problematic? We don’t know yet.”
Dr. Kim, who was part of a separate, earlier study that assessed vaccine response in patients with chronic inflammatory disease who were being treated with immunosuppressive medications, noted that many of the questions patients are asking about their antibody levels cannot yet be answered.
“It’s kind of the Wild West of serologic testing out there right now,” he said. “Even though we’re recommending that people still don’t check their antibody levels because their results are largely inactionable, everyone is still getting them anyway. But each of these tests are slightly different, and the results and the interpretation are further clouded because of those slight performance differences between each platform.”
Dr. Kim highlighted the number of different tests as one of this study’s notable limitations: 11 different assays were used to determine patients’ immune responses. “The authors made the argument that these tests are FDA approved, and that’s true, but that doesn’t necessarily mean much. Approval does translate to technical reliability but not to comparisons between the tests.”
As for next steps, both the authors and Dr. Kim recognized the need for a prospective trial. “To do a vaccine effectiveness–type study and show clinical protection against either infection or hospitalization – those are going to take a while, simply because of the nature of how many people you need for each of these studies,” he said. “Time will tell whether or not the data that are being presented here will translate literally into protective outcomes downstream.”
The MAJIK Registry is supported by the French Rheumatology Society. The authors acknowledged numerous potential conflicts of interest, including receiving consulting fees, research support, and honoraria from various pharmaceutical companies.
FROM THE LANCET RHEUMATOLOGY
Adalimumab biosimilar Cyltezo gets interchangeability designation
The Food and Drug Administration approved a supplement to the biologics license application of the adalimumab biosimilar drug Cyltezo (adalimumab-adbm) that makes it the first interchangeable biosimilar with Humira (adalimumab), the original branded version of the drug, its manufacturer Boehringer Ingelheim announced Oct. 15.
The FDA originally approved Cyltezo in 2017 for the treatment of multiple chronic inflammatory diseases, including seven of Humira’s nine indications for adults and pediatric patients: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis.
The interchangeability designation means that Cyltezo was tested in an additional clinical trial in which patients were successfully switched back and forth multiple times from Humira to Cyltezo and allows pharmacists to autosubstitute Humira with Cyltezo. In these cases, individual state laws control how and whether physicians will be notified of this switch.
Cyltezo is just the second biosimilar to be designated as interchangeable with its originator biologic product. The first approval, announced July 28, was for the interchangeability of Semglee (insulin glargine-yfgn) with the originator Lantus.
The agency based its decision on positive data from the VOLTAIRE-X study of 238 patients with moderate to severe chronic plaque psoriasis in which Cyltezo had no meaningful clinical differences from Humira in pharmacokinetics, efficacy, immunogenicity, and safety between the switching and continuous treatment groups.
Cyltezo will not be commercially available in the United States until July 1, 2023, according to Boehringer Ingelheim.
The Food and Drug Administration approved a supplement to the biologics license application of the adalimumab biosimilar drug Cyltezo (adalimumab-adbm) that makes it the first interchangeable biosimilar with Humira (adalimumab), the original branded version of the drug, its manufacturer Boehringer Ingelheim announced Oct. 15.
The FDA originally approved Cyltezo in 2017 for the treatment of multiple chronic inflammatory diseases, including seven of Humira’s nine indications for adults and pediatric patients: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis.
The interchangeability designation means that Cyltezo was tested in an additional clinical trial in which patients were successfully switched back and forth multiple times from Humira to Cyltezo and allows pharmacists to autosubstitute Humira with Cyltezo. In these cases, individual state laws control how and whether physicians will be notified of this switch.
Cyltezo is just the second biosimilar to be designated as interchangeable with its originator biologic product. The first approval, announced July 28, was for the interchangeability of Semglee (insulin glargine-yfgn) with the originator Lantus.
The agency based its decision on positive data from the VOLTAIRE-X study of 238 patients with moderate to severe chronic plaque psoriasis in which Cyltezo had no meaningful clinical differences from Humira in pharmacokinetics, efficacy, immunogenicity, and safety between the switching and continuous treatment groups.
Cyltezo will not be commercially available in the United States until July 1, 2023, according to Boehringer Ingelheim.
The Food and Drug Administration approved a supplement to the biologics license application of the adalimumab biosimilar drug Cyltezo (adalimumab-adbm) that makes it the first interchangeable biosimilar with Humira (adalimumab), the original branded version of the drug, its manufacturer Boehringer Ingelheim announced Oct. 15.
The FDA originally approved Cyltezo in 2017 for the treatment of multiple chronic inflammatory diseases, including seven of Humira’s nine indications for adults and pediatric patients: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis.
The interchangeability designation means that Cyltezo was tested in an additional clinical trial in which patients were successfully switched back and forth multiple times from Humira to Cyltezo and allows pharmacists to autosubstitute Humira with Cyltezo. In these cases, individual state laws control how and whether physicians will be notified of this switch.
Cyltezo is just the second biosimilar to be designated as interchangeable with its originator biologic product. The first approval, announced July 28, was for the interchangeability of Semglee (insulin glargine-yfgn) with the originator Lantus.
The agency based its decision on positive data from the VOLTAIRE-X study of 238 patients with moderate to severe chronic plaque psoriasis in which Cyltezo had no meaningful clinical differences from Humira in pharmacokinetics, efficacy, immunogenicity, and safety between the switching and continuous treatment groups.
Cyltezo will not be commercially available in the United States until July 1, 2023, according to Boehringer Ingelheim.
Study of biologics’ impact on psoriasis-to-PsA transition contradicts previous findings
Data source likely contributes biases
A new study has found that patients with psoriasis who were treated with biologics were more likely to develop psoriatic arthritis (PsA) than those treated with phototherapy, oral therapy, or no therapy at all, although the authors cautioned readers to consider potential biases when reviewing their findings.
“We do not suggest that these results should be interpreted causally; in other words, biologics likely do not cause PsA,” Elana Meer of the University of Pennsylvania, Philadelphia, and coauthors wrote. The study was published in Annals of the Rheumatic Diseases.
Three studies in dermatology clinic-based populations published this past summer – one from Italy, one from Argentina, and one from Israel – suggested that biologics can decrease a psoriasis patient’s risk of developing PsA. To further assess the impact of treatment with biologics, Ms. Meer and associates retrospectively examined the health records of thousands of patients with psoriasis between the ages of 16 and 90 who were initiating therapy. All told, data from 193,709 patients with psoriasis and without PsA who were treated between 2006 and 2017 were gathered from the OptumInsights Electronic Health Record Database.
A total of 14,569 patients from that cohort initiated biologic therapy while 20,321 patients initiated either oral therapy or phototherapy. The mean age in the biologics group was 45.9 years, compared with 49.8 years in the oral and phototherapy group.
The incidence of PsA across all patients was 9.75 cases per 1,000 person-years, compared with 77.26 among the biologic group, 61.99 among the oral therapy group, 26.11 among the phototherapy group, and 5.85 among those who did not receive therapy. After a multivariable adjustment in which biologics were a time-varying exposure, receiving biologics was associated with a higher incidence of PsA (hazard ratio, 4.48; 95% confidence interval, 4.23-4.75). In a model where time starts at the first use of biologics, the incidence was lower – but still notable – after multivariable adjustment (HR, 2.14; 95% CI, 2.00-2.28) and propensity score matching (HR, 2.17; 95% CI, 2.03-2.33).
Bias likely plays a large role in retrospective PsA study
“We’ve been struggling for the last several years to find a database that allows us to really address this question retrospectively,” study coauthor Christopher T. Ritchlin, MD, of the University of Rochester (N.Y.), said in an interview. “It looks like the model you use for a retrospective analysis heavily influences what you come out with.”
He described the potential biases they identified, including the possibility of protopathic bias indicating that patients being treated with biologics who then report joint pain have developed PsA – and are coded accordingly after visiting a rheumatologist.
“This has convinced us that you have to do a prospective study,” he said. “We’ve known that there were flaws with previous studies in this area. We tried to overcome them with our methodology, but there’s no way you can overcome a coding issue when you’re looking at such a large database.”
He noted another likely bias: The patients who are more likely to develop PsA are the ones with severe psoriasis, and they are also the patients most likely to be prescribed biologics.
“In my clinical experience, I have seen many patients develop psoriatic arthritis while on biologics for their psoriasis,” coauthor Joel M. Gelfand, MD, of the University of Pennsylvania, added in an interview. “Currently, we do not have adequate data to recommend treating psoriasis with a particular modality in order to prevent psoriatic arthritis. This question, however, is very important to patients and clinicians and ultimately is best answered with a large-scale pragmatic trial.”
Dr. Ritchlin reported that a prospective study in which “patients with psoriasis who do not have arthritis but do have certain risk factors and abnormal findings on musculoskeletal ultrasounds” will be treated with either biologic agents or placebo is about to begin, with a goal of “either attenuating or preventing the onset of PsA.”
The authors recognized their study’s additional limitations, including electronic health records being used as the primary data source and the possibility that medications were prescribed but never filled. That said, they did attempt to address the latter by using two prescriptions for a given therapy as the primary analysis, “suggesting a refill was initiated.”
The authors said that no commercial entities provided support for the study. Two of the authors acknowledged receiving funding from the National Psoriasis Foundation, and several authors declared potential conflicts of interests that included consulting and receiving honoraria from various pharmaceutical companies.
Data source likely contributes biases
Data source likely contributes biases
A new study has found that patients with psoriasis who were treated with biologics were more likely to develop psoriatic arthritis (PsA) than those treated with phototherapy, oral therapy, or no therapy at all, although the authors cautioned readers to consider potential biases when reviewing their findings.
“We do not suggest that these results should be interpreted causally; in other words, biologics likely do not cause PsA,” Elana Meer of the University of Pennsylvania, Philadelphia, and coauthors wrote. The study was published in Annals of the Rheumatic Diseases.
Three studies in dermatology clinic-based populations published this past summer – one from Italy, one from Argentina, and one from Israel – suggested that biologics can decrease a psoriasis patient’s risk of developing PsA. To further assess the impact of treatment with biologics, Ms. Meer and associates retrospectively examined the health records of thousands of patients with psoriasis between the ages of 16 and 90 who were initiating therapy. All told, data from 193,709 patients with psoriasis and without PsA who were treated between 2006 and 2017 were gathered from the OptumInsights Electronic Health Record Database.
A total of 14,569 patients from that cohort initiated biologic therapy while 20,321 patients initiated either oral therapy or phototherapy. The mean age in the biologics group was 45.9 years, compared with 49.8 years in the oral and phototherapy group.
The incidence of PsA across all patients was 9.75 cases per 1,000 person-years, compared with 77.26 among the biologic group, 61.99 among the oral therapy group, 26.11 among the phototherapy group, and 5.85 among those who did not receive therapy. After a multivariable adjustment in which biologics were a time-varying exposure, receiving biologics was associated with a higher incidence of PsA (hazard ratio, 4.48; 95% confidence interval, 4.23-4.75). In a model where time starts at the first use of biologics, the incidence was lower – but still notable – after multivariable adjustment (HR, 2.14; 95% CI, 2.00-2.28) and propensity score matching (HR, 2.17; 95% CI, 2.03-2.33).
Bias likely plays a large role in retrospective PsA study
“We’ve been struggling for the last several years to find a database that allows us to really address this question retrospectively,” study coauthor Christopher T. Ritchlin, MD, of the University of Rochester (N.Y.), said in an interview. “It looks like the model you use for a retrospective analysis heavily influences what you come out with.”
He described the potential biases they identified, including the possibility of protopathic bias indicating that patients being treated with biologics who then report joint pain have developed PsA – and are coded accordingly after visiting a rheumatologist.
“This has convinced us that you have to do a prospective study,” he said. “We’ve known that there were flaws with previous studies in this area. We tried to overcome them with our methodology, but there’s no way you can overcome a coding issue when you’re looking at such a large database.”
He noted another likely bias: The patients who are more likely to develop PsA are the ones with severe psoriasis, and they are also the patients most likely to be prescribed biologics.
“In my clinical experience, I have seen many patients develop psoriatic arthritis while on biologics for their psoriasis,” coauthor Joel M. Gelfand, MD, of the University of Pennsylvania, added in an interview. “Currently, we do not have adequate data to recommend treating psoriasis with a particular modality in order to prevent psoriatic arthritis. This question, however, is very important to patients and clinicians and ultimately is best answered with a large-scale pragmatic trial.”
Dr. Ritchlin reported that a prospective study in which “patients with psoriasis who do not have arthritis but do have certain risk factors and abnormal findings on musculoskeletal ultrasounds” will be treated with either biologic agents or placebo is about to begin, with a goal of “either attenuating or preventing the onset of PsA.”
The authors recognized their study’s additional limitations, including electronic health records being used as the primary data source and the possibility that medications were prescribed but never filled. That said, they did attempt to address the latter by using two prescriptions for a given therapy as the primary analysis, “suggesting a refill was initiated.”
The authors said that no commercial entities provided support for the study. Two of the authors acknowledged receiving funding from the National Psoriasis Foundation, and several authors declared potential conflicts of interests that included consulting and receiving honoraria from various pharmaceutical companies.
A new study has found that patients with psoriasis who were treated with biologics were more likely to develop psoriatic arthritis (PsA) than those treated with phototherapy, oral therapy, or no therapy at all, although the authors cautioned readers to consider potential biases when reviewing their findings.
“We do not suggest that these results should be interpreted causally; in other words, biologics likely do not cause PsA,” Elana Meer of the University of Pennsylvania, Philadelphia, and coauthors wrote. The study was published in Annals of the Rheumatic Diseases.
Three studies in dermatology clinic-based populations published this past summer – one from Italy, one from Argentina, and one from Israel – suggested that biologics can decrease a psoriasis patient’s risk of developing PsA. To further assess the impact of treatment with biologics, Ms. Meer and associates retrospectively examined the health records of thousands of patients with psoriasis between the ages of 16 and 90 who were initiating therapy. All told, data from 193,709 patients with psoriasis and without PsA who were treated between 2006 and 2017 were gathered from the OptumInsights Electronic Health Record Database.
A total of 14,569 patients from that cohort initiated biologic therapy while 20,321 patients initiated either oral therapy or phototherapy. The mean age in the biologics group was 45.9 years, compared with 49.8 years in the oral and phototherapy group.
The incidence of PsA across all patients was 9.75 cases per 1,000 person-years, compared with 77.26 among the biologic group, 61.99 among the oral therapy group, 26.11 among the phototherapy group, and 5.85 among those who did not receive therapy. After a multivariable adjustment in which biologics were a time-varying exposure, receiving biologics was associated with a higher incidence of PsA (hazard ratio, 4.48; 95% confidence interval, 4.23-4.75). In a model where time starts at the first use of biologics, the incidence was lower – but still notable – after multivariable adjustment (HR, 2.14; 95% CI, 2.00-2.28) and propensity score matching (HR, 2.17; 95% CI, 2.03-2.33).
Bias likely plays a large role in retrospective PsA study
“We’ve been struggling for the last several years to find a database that allows us to really address this question retrospectively,” study coauthor Christopher T. Ritchlin, MD, of the University of Rochester (N.Y.), said in an interview. “It looks like the model you use for a retrospective analysis heavily influences what you come out with.”
He described the potential biases they identified, including the possibility of protopathic bias indicating that patients being treated with biologics who then report joint pain have developed PsA – and are coded accordingly after visiting a rheumatologist.
“This has convinced us that you have to do a prospective study,” he said. “We’ve known that there were flaws with previous studies in this area. We tried to overcome them with our methodology, but there’s no way you can overcome a coding issue when you’re looking at such a large database.”
He noted another likely bias: The patients who are more likely to develop PsA are the ones with severe psoriasis, and they are also the patients most likely to be prescribed biologics.
“In my clinical experience, I have seen many patients develop psoriatic arthritis while on biologics for their psoriasis,” coauthor Joel M. Gelfand, MD, of the University of Pennsylvania, added in an interview. “Currently, we do not have adequate data to recommend treating psoriasis with a particular modality in order to prevent psoriatic arthritis. This question, however, is very important to patients and clinicians and ultimately is best answered with a large-scale pragmatic trial.”
Dr. Ritchlin reported that a prospective study in which “patients with psoriasis who do not have arthritis but do have certain risk factors and abnormal findings on musculoskeletal ultrasounds” will be treated with either biologic agents or placebo is about to begin, with a goal of “either attenuating or preventing the onset of PsA.”
The authors recognized their study’s additional limitations, including electronic health records being used as the primary data source and the possibility that medications were prescribed but never filled. That said, they did attempt to address the latter by using two prescriptions for a given therapy as the primary analysis, “suggesting a refill was initiated.”
The authors said that no commercial entities provided support for the study. Two of the authors acknowledged receiving funding from the National Psoriasis Foundation, and several authors declared potential conflicts of interests that included consulting and receiving honoraria from various pharmaceutical companies.
FROM ANNALS OF THE RHEUMATIC DISEASES
First-in-class TYK inhibitor shows durable effect for psoriasis
of follow-up, according to late-breaking data from two pivotal trials presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
From benefit reported on the two coprimary endpoints previously reported at 16 weeks, longer follow-up showed further gains out to 24 weeks and then persistent efficacy out to 52 weeks across these and multiple secondary endpoints, reported Richard Warren, MBChB, PhD, professor of dermatology and therapeutics, University of Manchester (England).
“This could be a unique oral therapy and an important treatment option for moderate to severe psoriasis,” Dr. Warren contended.
The multinational double-blind trials, called POETYK PSO-1 and PSO-2, enrolled 666 and 1,020 patients, respectively. The designs were similar. Patients with moderate to severe plaque psoriasis were randomly assigned in a 2:1:1 ratio to deucravacitinib (6 mg once daily), placebo, or apremilast (Otezla; 30 mg twice daily). At 16 weeks, those on placebo were switched to deucravacitinib.
For the coprimary endpoint of PASI 75 (75% clearance on the Psoriasis and Severity Index), the similar rate of response for deucravacitinib in the two studies (58.7%/53.6%) at week 16 was superior to the rates observed on both apremilast (35.1%/40.2%) and placebo (12.7%/9.4%).
By week 24, the proportion of deucravacitinib patients with a PASI 75 response had reached 69.3% and 58.7% in the POETYK PSO-1 and PSO-2 trials, respectively. The proportion of patients on apremilast with PASI 75 at this time point did not increase appreciably in one study and fell modestly in the other.
By week 52, the response rates achieved with deucravacitinib at week 24 were generally unchanged and nearly double those observed on apremilast.
The pattern of relative benefit on the other coprimary endpoint, which was a score of 0 or 1, signifying clear or almost clear skin on the static Physicians Global Assessment (sPGA), followed the same pattern. At week 16, 53.6% of patients had achieved sPGA 0/1. This was significantly higher than that observed on either apremilast or placebo, and this level of response was sustained through week 52.
When patients on placebo were switched to deucravacitinib at week 16, the PASI 75 response climbed quickly. There was complete catch-up by 32 weeks. In both groups, a PASI 75 response rate of about 65% or higher was maintained for the remainder of the study.
On a prespecified analysis, prior treatment exposure was not associated with any impact on the degree of response with deucravacitinib. This included a comparison between patients exposed to no prior biologic, one prior biologic, or two or more biologics, Dr. Warren reported.
Unlike patients in POETYK PSO-1, those with a PASI 75 response at 16 weeks in the POETYK PSO-2 trial were rerandomized to remain on deucravacitinib or switch to placebo. Designed to evaluate response durability, this analysis showed a relatively gradual decline in disease control.
“The median time to a loss of response was 12 weeks,” Dr. Warren said. He was referring in this case to the PASI 75 response, but the slope of decline was similar for sPGA score 0/1. At the end of 52 weeks, 31.3% of patients who had been rerandomized to placebo still maintained a PASI 75 while 80.4% of those who stayed on deucravacitinib still had PASI 75 clearance.
In the 52-week data from these two trials, several secondary endpoints have already been examined, and Dr. Warren said more analyses are coming. So far, the pattern of response has been similar for all endpoints.
Reporting on one as an example, Dr. Warren said that sPGA 0/1 for scalp psoriasis was achieved at week 16 by 70.3% of those randomly assigned to deucravacitinib versus 17.4% of those in the placebo arm. Among those switched from placebo to deucravacitinib at 16 weeks, the scalp response had caught up to that observed in those initiated on deucravacitinib by week 28. The response was sustained out to 52 weeks in both groups.
In the long-term trials, there have been no new safety concerns, according to Dr. Warren. He described this drug as “well tolerated,” adding that no significant laboratory abnormalities have been observed on long-term treatment. Although there has been a trend for increased risk of viral infections, such as herpes zoster, relative to apremilast, cases have so far been mild.
The Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR) has been approved for psoriatic arthritis, and numerous other JAK inhibitors are now in clinical trials for plaque psoriasis. These agents vary for their relative selectivity for JAK1, 2, and 3 kinases, but deucravacitinib is the first JAK inhibitor to reach clinical trials that target TYK2, which inhibits interleukin-23 and other cytokines implicated in the pathogenesis of plaque psoriasis.
“Deucravacitinib is very distinct from the other JAK inhibitors, and I think we are seeing this in the clinical studies,” Dr. Warren said. As a result of responses in the POETYK PRO trials that rival those achieved with monoclonal antibodies, he expects this drug, if approved, to be an important option for those with moderate to severe disease who prefer oral therapies.
Mark G. Lebwohl, MD, professor of dermatology and dean for clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York, shares this opinion. In an interview, he emphasized the unique mechanism of deucravacitinib and its clinical potential.
“Unlike other less specific JAK inhibitors, deucravacitinib has a unique binding site on TYK2, the regulatory domain of the molecule. This makes deucravacitinib more targeted and therefore safer than other JAK inhibitors,” said Dr. Lebwohl.
“After cyclosporine, which has many side effects, deucravacitinib is the most effective oral therapy we have for psoriasis and one of the safest,” he added.
The POETYK PSO-1 and PSO-2 trials received funding from Bristol-Myers Squibb. Dr. Warren has financial relationships with AbbVie, Almirall, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi, UCB, and Xenoport. Dr. Lebwohl has financial relationships with more than 20 pharmaceutical companies, including Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
of follow-up, according to late-breaking data from two pivotal trials presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
From benefit reported on the two coprimary endpoints previously reported at 16 weeks, longer follow-up showed further gains out to 24 weeks and then persistent efficacy out to 52 weeks across these and multiple secondary endpoints, reported Richard Warren, MBChB, PhD, professor of dermatology and therapeutics, University of Manchester (England).
“This could be a unique oral therapy and an important treatment option for moderate to severe psoriasis,” Dr. Warren contended.
The multinational double-blind trials, called POETYK PSO-1 and PSO-2, enrolled 666 and 1,020 patients, respectively. The designs were similar. Patients with moderate to severe plaque psoriasis were randomly assigned in a 2:1:1 ratio to deucravacitinib (6 mg once daily), placebo, or apremilast (Otezla; 30 mg twice daily). At 16 weeks, those on placebo were switched to deucravacitinib.
For the coprimary endpoint of PASI 75 (75% clearance on the Psoriasis and Severity Index), the similar rate of response for deucravacitinib in the two studies (58.7%/53.6%) at week 16 was superior to the rates observed on both apremilast (35.1%/40.2%) and placebo (12.7%/9.4%).
By week 24, the proportion of deucravacitinib patients with a PASI 75 response had reached 69.3% and 58.7% in the POETYK PSO-1 and PSO-2 trials, respectively. The proportion of patients on apremilast with PASI 75 at this time point did not increase appreciably in one study and fell modestly in the other.
By week 52, the response rates achieved with deucravacitinib at week 24 were generally unchanged and nearly double those observed on apremilast.
The pattern of relative benefit on the other coprimary endpoint, which was a score of 0 or 1, signifying clear or almost clear skin on the static Physicians Global Assessment (sPGA), followed the same pattern. At week 16, 53.6% of patients had achieved sPGA 0/1. This was significantly higher than that observed on either apremilast or placebo, and this level of response was sustained through week 52.
When patients on placebo were switched to deucravacitinib at week 16, the PASI 75 response climbed quickly. There was complete catch-up by 32 weeks. In both groups, a PASI 75 response rate of about 65% or higher was maintained for the remainder of the study.
On a prespecified analysis, prior treatment exposure was not associated with any impact on the degree of response with deucravacitinib. This included a comparison between patients exposed to no prior biologic, one prior biologic, or two or more biologics, Dr. Warren reported.
Unlike patients in POETYK PSO-1, those with a PASI 75 response at 16 weeks in the POETYK PSO-2 trial were rerandomized to remain on deucravacitinib or switch to placebo. Designed to evaluate response durability, this analysis showed a relatively gradual decline in disease control.
“The median time to a loss of response was 12 weeks,” Dr. Warren said. He was referring in this case to the PASI 75 response, but the slope of decline was similar for sPGA score 0/1. At the end of 52 weeks, 31.3% of patients who had been rerandomized to placebo still maintained a PASI 75 while 80.4% of those who stayed on deucravacitinib still had PASI 75 clearance.
In the 52-week data from these two trials, several secondary endpoints have already been examined, and Dr. Warren said more analyses are coming. So far, the pattern of response has been similar for all endpoints.
Reporting on one as an example, Dr. Warren said that sPGA 0/1 for scalp psoriasis was achieved at week 16 by 70.3% of those randomly assigned to deucravacitinib versus 17.4% of those in the placebo arm. Among those switched from placebo to deucravacitinib at 16 weeks, the scalp response had caught up to that observed in those initiated on deucravacitinib by week 28. The response was sustained out to 52 weeks in both groups.
In the long-term trials, there have been no new safety concerns, according to Dr. Warren. He described this drug as “well tolerated,” adding that no significant laboratory abnormalities have been observed on long-term treatment. Although there has been a trend for increased risk of viral infections, such as herpes zoster, relative to apremilast, cases have so far been mild.
The Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR) has been approved for psoriatic arthritis, and numerous other JAK inhibitors are now in clinical trials for plaque psoriasis. These agents vary for their relative selectivity for JAK1, 2, and 3 kinases, but deucravacitinib is the first JAK inhibitor to reach clinical trials that target TYK2, which inhibits interleukin-23 and other cytokines implicated in the pathogenesis of plaque psoriasis.
“Deucravacitinib is very distinct from the other JAK inhibitors, and I think we are seeing this in the clinical studies,” Dr. Warren said. As a result of responses in the POETYK PRO trials that rival those achieved with monoclonal antibodies, he expects this drug, if approved, to be an important option for those with moderate to severe disease who prefer oral therapies.
Mark G. Lebwohl, MD, professor of dermatology and dean for clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York, shares this opinion. In an interview, he emphasized the unique mechanism of deucravacitinib and its clinical potential.
“Unlike other less specific JAK inhibitors, deucravacitinib has a unique binding site on TYK2, the regulatory domain of the molecule. This makes deucravacitinib more targeted and therefore safer than other JAK inhibitors,” said Dr. Lebwohl.
“After cyclosporine, which has many side effects, deucravacitinib is the most effective oral therapy we have for psoriasis and one of the safest,” he added.
The POETYK PSO-1 and PSO-2 trials received funding from Bristol-Myers Squibb. Dr. Warren has financial relationships with AbbVie, Almirall, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi, UCB, and Xenoport. Dr. Lebwohl has financial relationships with more than 20 pharmaceutical companies, including Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
of follow-up, according to late-breaking data from two pivotal trials presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
From benefit reported on the two coprimary endpoints previously reported at 16 weeks, longer follow-up showed further gains out to 24 weeks and then persistent efficacy out to 52 weeks across these and multiple secondary endpoints, reported Richard Warren, MBChB, PhD, professor of dermatology and therapeutics, University of Manchester (England).
“This could be a unique oral therapy and an important treatment option for moderate to severe psoriasis,” Dr. Warren contended.
The multinational double-blind trials, called POETYK PSO-1 and PSO-2, enrolled 666 and 1,020 patients, respectively. The designs were similar. Patients with moderate to severe plaque psoriasis were randomly assigned in a 2:1:1 ratio to deucravacitinib (6 mg once daily), placebo, or apremilast (Otezla; 30 mg twice daily). At 16 weeks, those on placebo were switched to deucravacitinib.
For the coprimary endpoint of PASI 75 (75% clearance on the Psoriasis and Severity Index), the similar rate of response for deucravacitinib in the two studies (58.7%/53.6%) at week 16 was superior to the rates observed on both apremilast (35.1%/40.2%) and placebo (12.7%/9.4%).
By week 24, the proportion of deucravacitinib patients with a PASI 75 response had reached 69.3% and 58.7% in the POETYK PSO-1 and PSO-2 trials, respectively. The proportion of patients on apremilast with PASI 75 at this time point did not increase appreciably in one study and fell modestly in the other.
By week 52, the response rates achieved with deucravacitinib at week 24 were generally unchanged and nearly double those observed on apremilast.
The pattern of relative benefit on the other coprimary endpoint, which was a score of 0 or 1, signifying clear or almost clear skin on the static Physicians Global Assessment (sPGA), followed the same pattern. At week 16, 53.6% of patients had achieved sPGA 0/1. This was significantly higher than that observed on either apremilast or placebo, and this level of response was sustained through week 52.
When patients on placebo were switched to deucravacitinib at week 16, the PASI 75 response climbed quickly. There was complete catch-up by 32 weeks. In both groups, a PASI 75 response rate of about 65% or higher was maintained for the remainder of the study.
On a prespecified analysis, prior treatment exposure was not associated with any impact on the degree of response with deucravacitinib. This included a comparison between patients exposed to no prior biologic, one prior biologic, or two or more biologics, Dr. Warren reported.
Unlike patients in POETYK PSO-1, those with a PASI 75 response at 16 weeks in the POETYK PSO-2 trial were rerandomized to remain on deucravacitinib or switch to placebo. Designed to evaluate response durability, this analysis showed a relatively gradual decline in disease control.
“The median time to a loss of response was 12 weeks,” Dr. Warren said. He was referring in this case to the PASI 75 response, but the slope of decline was similar for sPGA score 0/1. At the end of 52 weeks, 31.3% of patients who had been rerandomized to placebo still maintained a PASI 75 while 80.4% of those who stayed on deucravacitinib still had PASI 75 clearance.
In the 52-week data from these two trials, several secondary endpoints have already been examined, and Dr. Warren said more analyses are coming. So far, the pattern of response has been similar for all endpoints.
Reporting on one as an example, Dr. Warren said that sPGA 0/1 for scalp psoriasis was achieved at week 16 by 70.3% of those randomly assigned to deucravacitinib versus 17.4% of those in the placebo arm. Among those switched from placebo to deucravacitinib at 16 weeks, the scalp response had caught up to that observed in those initiated on deucravacitinib by week 28. The response was sustained out to 52 weeks in both groups.
In the long-term trials, there have been no new safety concerns, according to Dr. Warren. He described this drug as “well tolerated,” adding that no significant laboratory abnormalities have been observed on long-term treatment. Although there has been a trend for increased risk of viral infections, such as herpes zoster, relative to apremilast, cases have so far been mild.
The Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR) has been approved for psoriatic arthritis, and numerous other JAK inhibitors are now in clinical trials for plaque psoriasis. These agents vary for their relative selectivity for JAK1, 2, and 3 kinases, but deucravacitinib is the first JAK inhibitor to reach clinical trials that target TYK2, which inhibits interleukin-23 and other cytokines implicated in the pathogenesis of plaque psoriasis.
“Deucravacitinib is very distinct from the other JAK inhibitors, and I think we are seeing this in the clinical studies,” Dr. Warren said. As a result of responses in the POETYK PRO trials that rival those achieved with monoclonal antibodies, he expects this drug, if approved, to be an important option for those with moderate to severe disease who prefer oral therapies.
Mark G. Lebwohl, MD, professor of dermatology and dean for clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York, shares this opinion. In an interview, he emphasized the unique mechanism of deucravacitinib and its clinical potential.
“Unlike other less specific JAK inhibitors, deucravacitinib has a unique binding site on TYK2, the regulatory domain of the molecule. This makes deucravacitinib more targeted and therefore safer than other JAK inhibitors,” said Dr. Lebwohl.
“After cyclosporine, which has many side effects, deucravacitinib is the most effective oral therapy we have for psoriasis and one of the safest,” he added.
The POETYK PSO-1 and PSO-2 trials received funding from Bristol-Myers Squibb. Dr. Warren has financial relationships with AbbVie, Almirall, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi, UCB, and Xenoport. Dr. Lebwohl has financial relationships with more than 20 pharmaceutical companies, including Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.