User login
Comparative efficacy of guselkumab, IL-17A, and TNF inhibitors
Key clinical point: In patients with psoriatic arthritis (PsA), efficacy of the novel interleukin (IL)-23p19 inhibitor guselkumab for joint improvement was comparable to IL-17A and subcutaneous tumor necrosis factor (TNF) inhibitors while offering a better efficacy for skin manifestations.
Major finding: Guselkumab 100 mg every 8 weeks and every 4 weeks were comparable to IL-17A and subcutaneous TNF inhibitors for American College of Rheumatology 20 response. Guselkumab showed better Psoriasis Area Severity Index 90 and 75 responses than most of the other agents.
Study details: Data come from a network meta-analysis of 26 phase 3, randomized controlled trials that evaluated 13 targeted therapies among adults with active PsA.
Disclosures: This work was supported by Janssen Research and Development. The authors including the lead author reported receiving grants, consulting fees, speaker fees, and/or honoraria from various sources. S Peterson, A Schubert, SD Chakravarty, CS Karyekar, and S Nair reported being employees of Janssen Pharmaceuticals and shareholder of Johnson & Johnson.
Source: Mease PJ et al. Rheumatology (Oxford). 2021 Mar 24. doi: 10.1093/rheumatology/keab119.
Key clinical point: In patients with psoriatic arthritis (PsA), efficacy of the novel interleukin (IL)-23p19 inhibitor guselkumab for joint improvement was comparable to IL-17A and subcutaneous tumor necrosis factor (TNF) inhibitors while offering a better efficacy for skin manifestations.
Major finding: Guselkumab 100 mg every 8 weeks and every 4 weeks were comparable to IL-17A and subcutaneous TNF inhibitors for American College of Rheumatology 20 response. Guselkumab showed better Psoriasis Area Severity Index 90 and 75 responses than most of the other agents.
Study details: Data come from a network meta-analysis of 26 phase 3, randomized controlled trials that evaluated 13 targeted therapies among adults with active PsA.
Disclosures: This work was supported by Janssen Research and Development. The authors including the lead author reported receiving grants, consulting fees, speaker fees, and/or honoraria from various sources. S Peterson, A Schubert, SD Chakravarty, CS Karyekar, and S Nair reported being employees of Janssen Pharmaceuticals and shareholder of Johnson & Johnson.
Source: Mease PJ et al. Rheumatology (Oxford). 2021 Mar 24. doi: 10.1093/rheumatology/keab119.
Key clinical point: In patients with psoriatic arthritis (PsA), efficacy of the novel interleukin (IL)-23p19 inhibitor guselkumab for joint improvement was comparable to IL-17A and subcutaneous tumor necrosis factor (TNF) inhibitors while offering a better efficacy for skin manifestations.
Major finding: Guselkumab 100 mg every 8 weeks and every 4 weeks were comparable to IL-17A and subcutaneous TNF inhibitors for American College of Rheumatology 20 response. Guselkumab showed better Psoriasis Area Severity Index 90 and 75 responses than most of the other agents.
Study details: Data come from a network meta-analysis of 26 phase 3, randomized controlled trials that evaluated 13 targeted therapies among adults with active PsA.
Disclosures: This work was supported by Janssen Research and Development. The authors including the lead author reported receiving grants, consulting fees, speaker fees, and/or honoraria from various sources. S Peterson, A Schubert, SD Chakravarty, CS Karyekar, and S Nair reported being employees of Janssen Pharmaceuticals and shareholder of Johnson & Johnson.
Source: Mease PJ et al. Rheumatology (Oxford). 2021 Mar 24. doi: 10.1093/rheumatology/keab119.
Guselkumab yields higher enthesitis resolution rates
Key clinical point: Guselkumab resulted in significantly higher proportions of patients with psoriatic arthritis (PsA) with resolved enthesitis than placebo, which continued to improve through 1 year.
Major finding: A significantly higher proportion of patients with enthesitis at baseline achieved resolution by week 24 when treated with guselkumab 100 mg every 4 weeks (Q4W) and guselkumab 100 mg at week 0, 4, and then every 8 weeks (Q8W) than placebo (45% and 50% vs. 29%; P = .0301) which continued to rise in patients who continued guselkumab with 58% achieving resolution by week 52.
Study details: Findings are from a pooled analysis of DISCOVER-1 and DISCOVER-2 phase 3 trials involving patients with active PsA despite standard therapies randomly allocated to subcutaneous guselkumab 100 mg Q4W, guselkumab 100 mg Q8W, or placebo.
Disclosures: The work was supported by Janssen Research & Development, LLC. The authors reported receiving research grants, honoraria, and/or consultation/speaker fees from various sources, including Janssen. Some authors declared being employees of Janssen and owning stocks of Johnson & Johnson.
Source: McGonagle D et al. Rheumatology (Oxford). 2021 Apr 6. doi: 10.1093/rheumatology/keab285.
Key clinical point: Guselkumab resulted in significantly higher proportions of patients with psoriatic arthritis (PsA) with resolved enthesitis than placebo, which continued to improve through 1 year.
Major finding: A significantly higher proportion of patients with enthesitis at baseline achieved resolution by week 24 when treated with guselkumab 100 mg every 4 weeks (Q4W) and guselkumab 100 mg at week 0, 4, and then every 8 weeks (Q8W) than placebo (45% and 50% vs. 29%; P = .0301) which continued to rise in patients who continued guselkumab with 58% achieving resolution by week 52.
Study details: Findings are from a pooled analysis of DISCOVER-1 and DISCOVER-2 phase 3 trials involving patients with active PsA despite standard therapies randomly allocated to subcutaneous guselkumab 100 mg Q4W, guselkumab 100 mg Q8W, or placebo.
Disclosures: The work was supported by Janssen Research & Development, LLC. The authors reported receiving research grants, honoraria, and/or consultation/speaker fees from various sources, including Janssen. Some authors declared being employees of Janssen and owning stocks of Johnson & Johnson.
Source: McGonagle D et al. Rheumatology (Oxford). 2021 Apr 6. doi: 10.1093/rheumatology/keab285.
Key clinical point: Guselkumab resulted in significantly higher proportions of patients with psoriatic arthritis (PsA) with resolved enthesitis than placebo, which continued to improve through 1 year.
Major finding: A significantly higher proportion of patients with enthesitis at baseline achieved resolution by week 24 when treated with guselkumab 100 mg every 4 weeks (Q4W) and guselkumab 100 mg at week 0, 4, and then every 8 weeks (Q8W) than placebo (45% and 50% vs. 29%; P = .0301) which continued to rise in patients who continued guselkumab with 58% achieving resolution by week 52.
Study details: Findings are from a pooled analysis of DISCOVER-1 and DISCOVER-2 phase 3 trials involving patients with active PsA despite standard therapies randomly allocated to subcutaneous guselkumab 100 mg Q4W, guselkumab 100 mg Q8W, or placebo.
Disclosures: The work was supported by Janssen Research & Development, LLC. The authors reported receiving research grants, honoraria, and/or consultation/speaker fees from various sources, including Janssen. Some authors declared being employees of Janssen and owning stocks of Johnson & Johnson.
Source: McGonagle D et al. Rheumatology (Oxford). 2021 Apr 6. doi: 10.1093/rheumatology/keab285.
OPAL Balance trial confirms long-term safety and efficacy of tofacitinib
Key clinical point: Final analysis of OPAL Balance confirms long-term safety and efficacy of tofacitinib in patients with psoriatic arthritis.
Major finding: Only 1 instance of mortality occurred in tofacitinib group during the risk period (incidence, 0.1 patients with events [95% confidence interval, 0.0-0.3] per 100 person-years). The incidences of adverse events for herpes zoster, serious infections, opportunistic infections, adjudicated malignancies, and major adverse cardiovascular events were consistent as reported previously. Efficacy was sustained up to 36 months.
Study details: Findings are from OPAL Balance, a 36-month, long-term extension phase 3 study involving 686 adult patients with active PsA. Eligible patients (n=180) from the open-label phase entered the randomized, double-blind, 12-month methotrexate withdrawal substudy where they received open-label tofacitinib 5 mg twice daily with either masked placebo or masked methotrexate.
Disclosures: OPAL Balance was funded by Pfizer. The authors including the lead author reported receiving grants/consulting fees, speaker fees, and/or honoraria from various sources including Pfizer. Six of the authors reported being employees and shareholders of Pfizer.
Source: Nash P et al. Lancet Rheumatol. 2021 Apr 1. doi: 10.1016/S2665-9913(21)00010-2.
Key clinical point: Final analysis of OPAL Balance confirms long-term safety and efficacy of tofacitinib in patients with psoriatic arthritis.
Major finding: Only 1 instance of mortality occurred in tofacitinib group during the risk period (incidence, 0.1 patients with events [95% confidence interval, 0.0-0.3] per 100 person-years). The incidences of adverse events for herpes zoster, serious infections, opportunistic infections, adjudicated malignancies, and major adverse cardiovascular events were consistent as reported previously. Efficacy was sustained up to 36 months.
Study details: Findings are from OPAL Balance, a 36-month, long-term extension phase 3 study involving 686 adult patients with active PsA. Eligible patients (n=180) from the open-label phase entered the randomized, double-blind, 12-month methotrexate withdrawal substudy where they received open-label tofacitinib 5 mg twice daily with either masked placebo or masked methotrexate.
Disclosures: OPAL Balance was funded by Pfizer. The authors including the lead author reported receiving grants/consulting fees, speaker fees, and/or honoraria from various sources including Pfizer. Six of the authors reported being employees and shareholders of Pfizer.
Source: Nash P et al. Lancet Rheumatol. 2021 Apr 1. doi: 10.1016/S2665-9913(21)00010-2.
Key clinical point: Final analysis of OPAL Balance confirms long-term safety and efficacy of tofacitinib in patients with psoriatic arthritis.
Major finding: Only 1 instance of mortality occurred in tofacitinib group during the risk period (incidence, 0.1 patients with events [95% confidence interval, 0.0-0.3] per 100 person-years). The incidences of adverse events for herpes zoster, serious infections, opportunistic infections, adjudicated malignancies, and major adverse cardiovascular events were consistent as reported previously. Efficacy was sustained up to 36 months.
Study details: Findings are from OPAL Balance, a 36-month, long-term extension phase 3 study involving 686 adult patients with active PsA. Eligible patients (n=180) from the open-label phase entered the randomized, double-blind, 12-month methotrexate withdrawal substudy where they received open-label tofacitinib 5 mg twice daily with either masked placebo or masked methotrexate.
Disclosures: OPAL Balance was funded by Pfizer. The authors including the lead author reported receiving grants/consulting fees, speaker fees, and/or honoraria from various sources including Pfizer. Six of the authors reported being employees and shareholders of Pfizer.
Source: Nash P et al. Lancet Rheumatol. 2021 Apr 1. doi: 10.1016/S2665-9913(21)00010-2.
Safety and efficacy of upadacitinib in patients refractory to nonbiologic DMARDs
Key clinical point: Proportion of patients with psoriatic arthritis with at least 20% improvement in American College of Rheumatology (ACR20) response was significantly higher with upadacitinib than placebo; however, adverse events were more frequent with upadacitinib.
Major finding: The percentage of patients with ACR20 response at week 12 was higher with upadacitinib 15 mg (70.6%) and 30 mg (78.5%) vs. placebo (36.2%; P less than .001). Incidence of serious infections and serious adverse events with upadacitinib 15 mg, 30 mg, and placebo were 1.2%, 2.6%, and 0.9% and 3.3%, 6.1%, and 3.1%, respectively.
Study details: Findings are from SELECT-Psa 1, a phase 3 trial involving 1,704 patients with PsA who had an inadequate response to at least 1 nonbiologic disease-modifying antirheumatic drugs and were randomly allocated to receive either oral upadacitinib 15 or 30 mg once daily, placebo, or subcutaneous adalimumab (40 mg every other week).
Disclosures: The trial was sponsored by Abbvie. The authors reported receiving consulting fees, advisory board fees, lecture fees, travel support, grant support, and/or being an employee of and/or owning stocks in various pharmaceutical companies, including Abbvie.
Source: McInnes IB et al. N Engl J Med. 2021 Apr 1. doi: 10.1056/NEJMoa2022516.
Key clinical point: Proportion of patients with psoriatic arthritis with at least 20% improvement in American College of Rheumatology (ACR20) response was significantly higher with upadacitinib than placebo; however, adverse events were more frequent with upadacitinib.
Major finding: The percentage of patients with ACR20 response at week 12 was higher with upadacitinib 15 mg (70.6%) and 30 mg (78.5%) vs. placebo (36.2%; P less than .001). Incidence of serious infections and serious adverse events with upadacitinib 15 mg, 30 mg, and placebo were 1.2%, 2.6%, and 0.9% and 3.3%, 6.1%, and 3.1%, respectively.
Study details: Findings are from SELECT-Psa 1, a phase 3 trial involving 1,704 patients with PsA who had an inadequate response to at least 1 nonbiologic disease-modifying antirheumatic drugs and were randomly allocated to receive either oral upadacitinib 15 or 30 mg once daily, placebo, or subcutaneous adalimumab (40 mg every other week).
Disclosures: The trial was sponsored by Abbvie. The authors reported receiving consulting fees, advisory board fees, lecture fees, travel support, grant support, and/or being an employee of and/or owning stocks in various pharmaceutical companies, including Abbvie.
Source: McInnes IB et al. N Engl J Med. 2021 Apr 1. doi: 10.1056/NEJMoa2022516.
Key clinical point: Proportion of patients with psoriatic arthritis with at least 20% improvement in American College of Rheumatology (ACR20) response was significantly higher with upadacitinib than placebo; however, adverse events were more frequent with upadacitinib.
Major finding: The percentage of patients with ACR20 response at week 12 was higher with upadacitinib 15 mg (70.6%) and 30 mg (78.5%) vs. placebo (36.2%; P less than .001). Incidence of serious infections and serious adverse events with upadacitinib 15 mg, 30 mg, and placebo were 1.2%, 2.6%, and 0.9% and 3.3%, 6.1%, and 3.1%, respectively.
Study details: Findings are from SELECT-Psa 1, a phase 3 trial involving 1,704 patients with PsA who had an inadequate response to at least 1 nonbiologic disease-modifying antirheumatic drugs and were randomly allocated to receive either oral upadacitinib 15 or 30 mg once daily, placebo, or subcutaneous adalimumab (40 mg every other week).
Disclosures: The trial was sponsored by Abbvie. The authors reported receiving consulting fees, advisory board fees, lecture fees, travel support, grant support, and/or being an employee of and/or owning stocks in various pharmaceutical companies, including Abbvie.
Source: McInnes IB et al. N Engl J Med. 2021 Apr 1. doi: 10.1056/NEJMoa2022516.
Psoriasis associated with an increased risk of COVID-19 in real-world study
in patients, compared with those on topical therapy, a new study finds.
“Our study results suggest that psoriasis is an independent risk factor for COVID-19 illness,” study coauthor Jeffrey Liu, a medical student at the University of Southern California, Los Angeles, said in an interview after he presented the findings at the American Academy of Dermatology Virtual Meeting Experience. “And our findings are consistent with the hypothesis that certain systemic agents may confer a protective effect against COVID-19 illness.”
Mr. Liu and coinvestigators used a Symphony Health dataset to analyze the health records of 167,027 U.S. patients diagnosed with psoriasis and a control group of 1,002,162 patients. The participants, all at least 20 years old, had been treated for psoriasis or psoriatic arthritis from May 2019 through Jan. 1, 2020, and were tracked until Nov. 11, 2020.
The ages and races of peoples in the two groups were roughly similar. Overall, 55% were women and 75% were White, and their average age was 58 years. Type 2 diabetes was more common in the psoriasis group than the control group (23% vs. 16%), as was obesity (27% vs. 15%). Of the patients with psoriasis, 60% were on topical treatments, 19% were on oral therapies, and 22% were on biologic therapy, with only a few taking both oral and biologic therapies.
After adjustment for age and gender, patients with psoriasis were 33% more likely than the control group to develop COVID-19 (adjusted incidence rate ratio, 1.33; 95% confidence interval, 1.23-1.38; P < .0001).
In a separate analysis, the gap persisted after adjustment for demographics and comorbidities: Patients with psoriasis had a higher rate of COVID-19 infection vs. controls (adjusted odds ratio, 1.18; 95% CI, 1.13-1.23; P < .0001). Among all patients, non-White race, older age, and comorbidities were all linked to higher risk of COVID-19 (all P < .0001).
Psoriasis might make patients more vulnerable to COVID-19 because the presence of up-regulated genes in psoriatic skin “may lead to systemic hyperinflammation and sensitization of patients with psoriasis to proinflammatory cytokine storm,” Mr. Liu said. This, in turn, may trigger more severe symptomatic disease that requires medical treatment, he said.
Reduced risk, compared with topical therapies
After adjustment for age and gender, those treated with TNF-alpha inhibitors, methotrexate, and apremilast (Otezla) all had statistically lower risks of COVID-19 vs. those on topical therapy (aIRR, 0.82; 95% CI, 0.69-0.95; P < .0029 for TNF-alpha inhibitors; aIRR, 0.75; 95% CI, 0.67-0.86; P < .0001 for methotrexate; and aIRR, 0.69; 95% CI, 0.55-0.85; P < .0006 for apremilast).
Reduced risk held true for those in the separate analysis after adjustment for comorbidities and demographics (respectively, aOR, 0.87; 95% CI, 0.77-1.00; P < .0469; aOR, 0.81; 95% CI, 0.71-0.92; P < .0011; and aOR, 0.70; 95% CI, 0.57-0.87; P < .0014).
Apremilast and methotrexate may boost protection against COVID-19 by inhibiting the body’s production of cytokines, Mr. Liu said.
One message of the study is that “dermatologists should not be scared of prescribing biologics or oral therapies for psoriasis,” the study’s lead author Jashin J. Wu, MD, of the Dermatology Research and Education Foundation in Irvine, Calif., said in an interview.
However, the results on the effects of systemic therapies were not all positive. Interleukin (IL)–17 inhibitors were an outlier: After adjustment for age and gender, patients treated with this class of drugs were 36% more likely to develop COVID-19 than those on oral agents (aIRR, 1.36; 95% CI, 1.13-1.63; P < .0009).
Among patients on biologics, those taking IL-17 inhibitors had the highest risk of COVID-19, Mr. Liu said. “The risk was higher in this class regardless of reference group – general population, the topical cohort, and the oral cohort,” he said. “This may relate to the observation that this biologic class exerts more broad immunosuppressive effects on antiviral host immunity. Notably, large meta-estimates of pivotal trials have observed increased risk of respiratory tract infections for patients on IL-17 inhibitors.”
In an interview, Erica Dommasch, MD, MPH, of the department of dermatology at Beth Israel Deaconess Medical Center, Boston, cautioned that “the data from this study is very hard to interpret.”
It’s likely that some patients with psoriasis on systemic medications “may have been the most careful about limiting exposures,” she said. “Thus, it’s hard to account for behavioral changes in individuals that may have led to the decreased incidence in psoriasis in patients on systemic agents versus topical therapy alone.”
Patients with psoriasis may also be tested more often for COVID-19, and unmeasured comorbidities like chronic kidney disease may play a role too, she said. Still, she added, “it’s reassuring that the authors did not find an increased rate of COVID among psoriasis patients on systemic agents versus topicals alone.” And she agreed with Dr. Wu about the importance of treating psoriasis with therapy beyond topical treatments during the pandemic: “Providers should feel comfortable prescribing systemic medications to psoriasis patients when otherwise appropriate.”
As for the next steps, Dr. Wu said, “we will be exploring more about the prognosis of COVID-19 infection in psoriasis patients. In addition, we will be exploring the relationship of COVID-19 infection with other inflammatory skin diseases, such as atopic dermatitis.”
No study funding is reported. Dr. Wu discloses investigator, consultant, or speaker relationships with AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, Dr. Reddy’s Laboratories, Eli Lilly, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Regeneron, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, Valeant Pharmaceuticals North America, and Zerigo Health. Mr. Liu and Dr. Dommasch have no disclosures.
in patients, compared with those on topical therapy, a new study finds.
“Our study results suggest that psoriasis is an independent risk factor for COVID-19 illness,” study coauthor Jeffrey Liu, a medical student at the University of Southern California, Los Angeles, said in an interview after he presented the findings at the American Academy of Dermatology Virtual Meeting Experience. “And our findings are consistent with the hypothesis that certain systemic agents may confer a protective effect against COVID-19 illness.”
Mr. Liu and coinvestigators used a Symphony Health dataset to analyze the health records of 167,027 U.S. patients diagnosed with psoriasis and a control group of 1,002,162 patients. The participants, all at least 20 years old, had been treated for psoriasis or psoriatic arthritis from May 2019 through Jan. 1, 2020, and were tracked until Nov. 11, 2020.
The ages and races of peoples in the two groups were roughly similar. Overall, 55% were women and 75% were White, and their average age was 58 years. Type 2 diabetes was more common in the psoriasis group than the control group (23% vs. 16%), as was obesity (27% vs. 15%). Of the patients with psoriasis, 60% were on topical treatments, 19% were on oral therapies, and 22% were on biologic therapy, with only a few taking both oral and biologic therapies.
After adjustment for age and gender, patients with psoriasis were 33% more likely than the control group to develop COVID-19 (adjusted incidence rate ratio, 1.33; 95% confidence interval, 1.23-1.38; P < .0001).
In a separate analysis, the gap persisted after adjustment for demographics and comorbidities: Patients with psoriasis had a higher rate of COVID-19 infection vs. controls (adjusted odds ratio, 1.18; 95% CI, 1.13-1.23; P < .0001). Among all patients, non-White race, older age, and comorbidities were all linked to higher risk of COVID-19 (all P < .0001).
Psoriasis might make patients more vulnerable to COVID-19 because the presence of up-regulated genes in psoriatic skin “may lead to systemic hyperinflammation and sensitization of patients with psoriasis to proinflammatory cytokine storm,” Mr. Liu said. This, in turn, may trigger more severe symptomatic disease that requires medical treatment, he said.
Reduced risk, compared with topical therapies
After adjustment for age and gender, those treated with TNF-alpha inhibitors, methotrexate, and apremilast (Otezla) all had statistically lower risks of COVID-19 vs. those on topical therapy (aIRR, 0.82; 95% CI, 0.69-0.95; P < .0029 for TNF-alpha inhibitors; aIRR, 0.75; 95% CI, 0.67-0.86; P < .0001 for methotrexate; and aIRR, 0.69; 95% CI, 0.55-0.85; P < .0006 for apremilast).
Reduced risk held true for those in the separate analysis after adjustment for comorbidities and demographics (respectively, aOR, 0.87; 95% CI, 0.77-1.00; P < .0469; aOR, 0.81; 95% CI, 0.71-0.92; P < .0011; and aOR, 0.70; 95% CI, 0.57-0.87; P < .0014).
Apremilast and methotrexate may boost protection against COVID-19 by inhibiting the body’s production of cytokines, Mr. Liu said.
One message of the study is that “dermatologists should not be scared of prescribing biologics or oral therapies for psoriasis,” the study’s lead author Jashin J. Wu, MD, of the Dermatology Research and Education Foundation in Irvine, Calif., said in an interview.
However, the results on the effects of systemic therapies were not all positive. Interleukin (IL)–17 inhibitors were an outlier: After adjustment for age and gender, patients treated with this class of drugs were 36% more likely to develop COVID-19 than those on oral agents (aIRR, 1.36; 95% CI, 1.13-1.63; P < .0009).
Among patients on biologics, those taking IL-17 inhibitors had the highest risk of COVID-19, Mr. Liu said. “The risk was higher in this class regardless of reference group – general population, the topical cohort, and the oral cohort,” he said. “This may relate to the observation that this biologic class exerts more broad immunosuppressive effects on antiviral host immunity. Notably, large meta-estimates of pivotal trials have observed increased risk of respiratory tract infections for patients on IL-17 inhibitors.”
In an interview, Erica Dommasch, MD, MPH, of the department of dermatology at Beth Israel Deaconess Medical Center, Boston, cautioned that “the data from this study is very hard to interpret.”
It’s likely that some patients with psoriasis on systemic medications “may have been the most careful about limiting exposures,” she said. “Thus, it’s hard to account for behavioral changes in individuals that may have led to the decreased incidence in psoriasis in patients on systemic agents versus topical therapy alone.”
Patients with psoriasis may also be tested more often for COVID-19, and unmeasured comorbidities like chronic kidney disease may play a role too, she said. Still, she added, “it’s reassuring that the authors did not find an increased rate of COVID among psoriasis patients on systemic agents versus topicals alone.” And she agreed with Dr. Wu about the importance of treating psoriasis with therapy beyond topical treatments during the pandemic: “Providers should feel comfortable prescribing systemic medications to psoriasis patients when otherwise appropriate.”
As for the next steps, Dr. Wu said, “we will be exploring more about the prognosis of COVID-19 infection in psoriasis patients. In addition, we will be exploring the relationship of COVID-19 infection with other inflammatory skin diseases, such as atopic dermatitis.”
No study funding is reported. Dr. Wu discloses investigator, consultant, or speaker relationships with AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, Dr. Reddy’s Laboratories, Eli Lilly, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Regeneron, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, Valeant Pharmaceuticals North America, and Zerigo Health. Mr. Liu and Dr. Dommasch have no disclosures.
in patients, compared with those on topical therapy, a new study finds.
“Our study results suggest that psoriasis is an independent risk factor for COVID-19 illness,” study coauthor Jeffrey Liu, a medical student at the University of Southern California, Los Angeles, said in an interview after he presented the findings at the American Academy of Dermatology Virtual Meeting Experience. “And our findings are consistent with the hypothesis that certain systemic agents may confer a protective effect against COVID-19 illness.”
Mr. Liu and coinvestigators used a Symphony Health dataset to analyze the health records of 167,027 U.S. patients diagnosed with psoriasis and a control group of 1,002,162 patients. The participants, all at least 20 years old, had been treated for psoriasis or psoriatic arthritis from May 2019 through Jan. 1, 2020, and were tracked until Nov. 11, 2020.
The ages and races of peoples in the two groups were roughly similar. Overall, 55% were women and 75% were White, and their average age was 58 years. Type 2 diabetes was more common in the psoriasis group than the control group (23% vs. 16%), as was obesity (27% vs. 15%). Of the patients with psoriasis, 60% were on topical treatments, 19% were on oral therapies, and 22% were on biologic therapy, with only a few taking both oral and biologic therapies.
After adjustment for age and gender, patients with psoriasis were 33% more likely than the control group to develop COVID-19 (adjusted incidence rate ratio, 1.33; 95% confidence interval, 1.23-1.38; P < .0001).
In a separate analysis, the gap persisted after adjustment for demographics and comorbidities: Patients with psoriasis had a higher rate of COVID-19 infection vs. controls (adjusted odds ratio, 1.18; 95% CI, 1.13-1.23; P < .0001). Among all patients, non-White race, older age, and comorbidities were all linked to higher risk of COVID-19 (all P < .0001).
Psoriasis might make patients more vulnerable to COVID-19 because the presence of up-regulated genes in psoriatic skin “may lead to systemic hyperinflammation and sensitization of patients with psoriasis to proinflammatory cytokine storm,” Mr. Liu said. This, in turn, may trigger more severe symptomatic disease that requires medical treatment, he said.
Reduced risk, compared with topical therapies
After adjustment for age and gender, those treated with TNF-alpha inhibitors, methotrexate, and apremilast (Otezla) all had statistically lower risks of COVID-19 vs. those on topical therapy (aIRR, 0.82; 95% CI, 0.69-0.95; P < .0029 for TNF-alpha inhibitors; aIRR, 0.75; 95% CI, 0.67-0.86; P < .0001 for methotrexate; and aIRR, 0.69; 95% CI, 0.55-0.85; P < .0006 for apremilast).
Reduced risk held true for those in the separate analysis after adjustment for comorbidities and demographics (respectively, aOR, 0.87; 95% CI, 0.77-1.00; P < .0469; aOR, 0.81; 95% CI, 0.71-0.92; P < .0011; and aOR, 0.70; 95% CI, 0.57-0.87; P < .0014).
Apremilast and methotrexate may boost protection against COVID-19 by inhibiting the body’s production of cytokines, Mr. Liu said.
One message of the study is that “dermatologists should not be scared of prescribing biologics or oral therapies for psoriasis,” the study’s lead author Jashin J. Wu, MD, of the Dermatology Research and Education Foundation in Irvine, Calif., said in an interview.
However, the results on the effects of systemic therapies were not all positive. Interleukin (IL)–17 inhibitors were an outlier: After adjustment for age and gender, patients treated with this class of drugs were 36% more likely to develop COVID-19 than those on oral agents (aIRR, 1.36; 95% CI, 1.13-1.63; P < .0009).
Among patients on biologics, those taking IL-17 inhibitors had the highest risk of COVID-19, Mr. Liu said. “The risk was higher in this class regardless of reference group – general population, the topical cohort, and the oral cohort,” he said. “This may relate to the observation that this biologic class exerts more broad immunosuppressive effects on antiviral host immunity. Notably, large meta-estimates of pivotal trials have observed increased risk of respiratory tract infections for patients on IL-17 inhibitors.”
In an interview, Erica Dommasch, MD, MPH, of the department of dermatology at Beth Israel Deaconess Medical Center, Boston, cautioned that “the data from this study is very hard to interpret.”
It’s likely that some patients with psoriasis on systemic medications “may have been the most careful about limiting exposures,” she said. “Thus, it’s hard to account for behavioral changes in individuals that may have led to the decreased incidence in psoriasis in patients on systemic agents versus topical therapy alone.”
Patients with psoriasis may also be tested more often for COVID-19, and unmeasured comorbidities like chronic kidney disease may play a role too, she said. Still, she added, “it’s reassuring that the authors did not find an increased rate of COVID among psoriasis patients on systemic agents versus topicals alone.” And she agreed with Dr. Wu about the importance of treating psoriasis with therapy beyond topical treatments during the pandemic: “Providers should feel comfortable prescribing systemic medications to psoriasis patients when otherwise appropriate.”
As for the next steps, Dr. Wu said, “we will be exploring more about the prognosis of COVID-19 infection in psoriasis patients. In addition, we will be exploring the relationship of COVID-19 infection with other inflammatory skin diseases, such as atopic dermatitis.”
No study funding is reported. Dr. Wu discloses investigator, consultant, or speaker relationships with AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, Dr. Reddy’s Laboratories, Eli Lilly, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Regeneron, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, Valeant Pharmaceuticals North America, and Zerigo Health. Mr. Liu and Dr. Dommasch have no disclosures.
FROM AAD VMX 2021
Debate: Should biologics be used for milder cases of psoriasis?
The issue was tackled in a debate at the American Academy of Dermatology Virtual Meeting Experience.
Taking the con side, Kenneth Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee, argued that, with the high cost of biologics, availability of many alternatives, and other issues, “we should just say no. ... There is no good reason that we need to expand the use of biologics in patients with limited disease.”
On the pro side, Richard Langley, MD, professor of dermatology at Dalhousie University Halifax, N.S, argued for a nuanced approach. He noted that patients with smaller patches of disease can be just as miserable as patients who hit traditional benchmarks of increased severity, such as high body surface area involvement – especially if those small areas are in sensitive locations like the scalp, palms, or genitals.
The decision to use a biologic should hinge on how badly patients and their quality of life are affected, not on “some artificial and limiting definition” of severity, Dr. Langley said.
Dr. Gordon didn’t disagree, noting that current use criteria include objective measures as well as disease in sensitive areas and failure of alternative treatments.
Rather, he was concerned about “expanding the definition of who is eligible beyond these criteria ... to chase every last bit of” disease. “I don’t think we have” a good rationale for that approach, he said.
Cost is the most important issue, Dr. Gordon said.
With more biologics on the way and prices continuing to go up, “there is going to a be a huge challenge to our use of these expensive medicines over the next few years” from payers. “It is important that we use them smartly in order to make sure we are able to use them for people with severe disease” who really need them. If “we start using biologics for all our patients with psoriasis,” it will be a “cost disaster,” Dr. Gordon said.
In addition, topicals and home phototherapy can be effective as long as patients adhere to them, as can alternative systemic agents, such as methotrexate and apremilast.
Often with biologics, “the issue is mainly convenience” rather than a fundamental problem with the alternatives, and despite the good safety record in trials, “chasing the last bit” of psoriasis with a biologic “is not necessarily” without risk for the patient, Dr. Gordon said.
Still, there can be a “pretty significant disconnect” between how patients perceive their psoriasis and “what physicians are thinking and prescribing” for it based on objective measures, Dr. Langley noted. Sometimes patients who have limited disease but are in significant distress aren’t even receiving treatment or are only given another cream to add to their collection of ones that haven’t worked.
One problem with traditional severity classifications is that they don’t generally take patients’ subjective experience into account, he added. There’s also been a lack of standardization to the point that dermatologists, researchers, and payers can sometimes disagree over severity in a given patient.
There’s movement toward better incorporation of patient experience into severity considerations, but for now at least, a designation of mild psoriasis can underestimate the true severity of disease, Dr. Langley said.
Dr. Gordon and Dr. Langley reported receiving honoraria and/or research support from many pharmaceutical companies, including AbbVie, Pfizer, and Lilly.
A version of this article first appeared on Medscape.com.
The issue was tackled in a debate at the American Academy of Dermatology Virtual Meeting Experience.
Taking the con side, Kenneth Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee, argued that, with the high cost of biologics, availability of many alternatives, and other issues, “we should just say no. ... There is no good reason that we need to expand the use of biologics in patients with limited disease.”
On the pro side, Richard Langley, MD, professor of dermatology at Dalhousie University Halifax, N.S, argued for a nuanced approach. He noted that patients with smaller patches of disease can be just as miserable as patients who hit traditional benchmarks of increased severity, such as high body surface area involvement – especially if those small areas are in sensitive locations like the scalp, palms, or genitals.
The decision to use a biologic should hinge on how badly patients and their quality of life are affected, not on “some artificial and limiting definition” of severity, Dr. Langley said.
Dr. Gordon didn’t disagree, noting that current use criteria include objective measures as well as disease in sensitive areas and failure of alternative treatments.
Rather, he was concerned about “expanding the definition of who is eligible beyond these criteria ... to chase every last bit of” disease. “I don’t think we have” a good rationale for that approach, he said.
Cost is the most important issue, Dr. Gordon said.
With more biologics on the way and prices continuing to go up, “there is going to a be a huge challenge to our use of these expensive medicines over the next few years” from payers. “It is important that we use them smartly in order to make sure we are able to use them for people with severe disease” who really need them. If “we start using biologics for all our patients with psoriasis,” it will be a “cost disaster,” Dr. Gordon said.
In addition, topicals and home phototherapy can be effective as long as patients adhere to them, as can alternative systemic agents, such as methotrexate and apremilast.
Often with biologics, “the issue is mainly convenience” rather than a fundamental problem with the alternatives, and despite the good safety record in trials, “chasing the last bit” of psoriasis with a biologic “is not necessarily” without risk for the patient, Dr. Gordon said.
Still, there can be a “pretty significant disconnect” between how patients perceive their psoriasis and “what physicians are thinking and prescribing” for it based on objective measures, Dr. Langley noted. Sometimes patients who have limited disease but are in significant distress aren’t even receiving treatment or are only given another cream to add to their collection of ones that haven’t worked.
One problem with traditional severity classifications is that they don’t generally take patients’ subjective experience into account, he added. There’s also been a lack of standardization to the point that dermatologists, researchers, and payers can sometimes disagree over severity in a given patient.
There’s movement toward better incorporation of patient experience into severity considerations, but for now at least, a designation of mild psoriasis can underestimate the true severity of disease, Dr. Langley said.
Dr. Gordon and Dr. Langley reported receiving honoraria and/or research support from many pharmaceutical companies, including AbbVie, Pfizer, and Lilly.
A version of this article first appeared on Medscape.com.
The issue was tackled in a debate at the American Academy of Dermatology Virtual Meeting Experience.
Taking the con side, Kenneth Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee, argued that, with the high cost of biologics, availability of many alternatives, and other issues, “we should just say no. ... There is no good reason that we need to expand the use of biologics in patients with limited disease.”
On the pro side, Richard Langley, MD, professor of dermatology at Dalhousie University Halifax, N.S, argued for a nuanced approach. He noted that patients with smaller patches of disease can be just as miserable as patients who hit traditional benchmarks of increased severity, such as high body surface area involvement – especially if those small areas are in sensitive locations like the scalp, palms, or genitals.
The decision to use a biologic should hinge on how badly patients and their quality of life are affected, not on “some artificial and limiting definition” of severity, Dr. Langley said.
Dr. Gordon didn’t disagree, noting that current use criteria include objective measures as well as disease in sensitive areas and failure of alternative treatments.
Rather, he was concerned about “expanding the definition of who is eligible beyond these criteria ... to chase every last bit of” disease. “I don’t think we have” a good rationale for that approach, he said.
Cost is the most important issue, Dr. Gordon said.
With more biologics on the way and prices continuing to go up, “there is going to a be a huge challenge to our use of these expensive medicines over the next few years” from payers. “It is important that we use them smartly in order to make sure we are able to use them for people with severe disease” who really need them. If “we start using biologics for all our patients with psoriasis,” it will be a “cost disaster,” Dr. Gordon said.
In addition, topicals and home phototherapy can be effective as long as patients adhere to them, as can alternative systemic agents, such as methotrexate and apremilast.
Often with biologics, “the issue is mainly convenience” rather than a fundamental problem with the alternatives, and despite the good safety record in trials, “chasing the last bit” of psoriasis with a biologic “is not necessarily” without risk for the patient, Dr. Gordon said.
Still, there can be a “pretty significant disconnect” between how patients perceive their psoriasis and “what physicians are thinking and prescribing” for it based on objective measures, Dr. Langley noted. Sometimes patients who have limited disease but are in significant distress aren’t even receiving treatment or are only given another cream to add to their collection of ones that haven’t worked.
One problem with traditional severity classifications is that they don’t generally take patients’ subjective experience into account, he added. There’s also been a lack of standardization to the point that dermatologists, researchers, and payers can sometimes disagree over severity in a given patient.
There’s movement toward better incorporation of patient experience into severity considerations, but for now at least, a designation of mild psoriasis can underestimate the true severity of disease, Dr. Langley said.
Dr. Gordon and Dr. Langley reported receiving honoraria and/or research support from many pharmaceutical companies, including AbbVie, Pfizer, and Lilly.
A version of this article first appeared on Medscape.com.
Boosting the presence of darker skin in rheumatology education
Studies are flagging racial and ethnic disparities in rheumatology training materials, pointing to a need to boost representation of darker skin tones and better educate physicians in evaluating this cohort.
Not enough is known about these disparities in rheumatology education, despite the fact that minorities make up 40% of the population in the United States.
The problem starts with books and references used in medical schools, Lynn McKinley-Grant, MD, immediate past president of the Skin of Color Society and associate professor of dermatology at Howard University, Washington, said in an interview. “In the medical literature there has been a dearth of images in skin of color in all specialties,” she said. With an increased diversity in the U.S. population, there is a need for health care providers to be able to recognize disease patterns in all skin types.” If a physician is training at an institution where there are not many patients of color in the community, the rheumatologists are even more limited in terms of their clinical experience.
This lack of training in diagnosis of disease has serious clinical repercussions, as seen in COVID cases, Dr. McKinley-Grant noted. “You end up not being able to recognize early erythema, jaundice, anemia, or hypoxemia because those conditions are a different color or pattern in the darker skin types. This can lead to errors in treatment, diagnosis, and medical care, resulting in increased morbidity and mortality.”
Studies point to education gaps
A team of researchers from Washington University in St. Louis called attention to this issue at the American College of Rhematology’s Convergence 2020 conference.
“Patients of color with lupus are especially vulnerable as they often carry a greater disease burden, yet studies show that individuals with darker skin tones are underrepresented in medical educational materials,” Vijay Kannuthurai, MD, and colleagues wrote in their study abstract. The team surveyed 132 providers in St. Louis, Mo., on their confidence in evaluating any rash, and rashes in patients with lupus and varied skin tones.
Participating clinicians, mostly rheumatologists, dermatologists, or internists, had a higher confidence level in diagnosing any rash versus lupus rashes, but were considerably less confident in diagnosing lupus rash on darker skin, compared with those on fair skin. This represents “a disparity between provider confidence and the patient population lupus traditionally affects,” the investigators concluded.
Another recent study found evidence of disparities in clinical education resources. “The lack of dark skin representation among rheumatology educational materials contributes to the implicit bias and structural racism present in medical education by promoting White-only models of disease,” lead author Adrienne Strait, a medical student at the University of California, San Francisco, said in an interview. “Given that rheumatic diseases disproportionately impact racial and ethnic minorities, we felt it was important to examine the representation of these groups within rheumatology training resources.”
She and her colleagues gathered images of rheumatic diseases from four major databases: the American College of Rheumatology’s Image Library, UpToDate, the New England Journal of Medicine Images in Clinical Medicine and Clinical Cases filtered by “Rheumatology,” and the 9th edition of Kelley’s Textbook of Rheumatology. They used Fitzpatrick’s skin phototypes to independently code images depicting skin as “light” (skin types I-IV), “dark” (skin types V-VI), or “indeterminate,” focusing on systemic lupus erythematosus (SLE) and rheumatoid arthritis, two conditions with a known connection to racial and ethnic health disparities.
Taking into account the high incidence of sarcoidosis and SLE in Black patients when compared with White patients, the investigators did a secondary analysis that excluded these cases.
Among 1,043 patient images studied, just 13.4% represented dark skin, compared with 84% that represented light skin. More than 2% represented an indeterminate skin color. Comparing dark-skin representation in the clinical images and SLE images with the representation of Asian, Native American, and Black individuals in the United States and within lupus cases nationally, the investigators found significant underrepresentation of dark skin.
Only 4.2% of RA images had dark-skin representation, making RA one of the diseases with the lowest representation in the study, along with juvenile idiopathic arthritis, the spondyloarthropathies, and Kawasaki disease. “Representation of dark skin in SLE was also lower than the proportion of Black individuals in SLE studies,” the investigators noted. Overall, representation of dark skin in SLE images was just 22.6%. Sarcoidosis comparatively had the largest representation of dark-skin images (69.6%, n = 32).
“Excluding sarcoidosis and SLE images, the overall representation of dark skin was 9.4% (n = 84), which was significantly lower than the proportion of Asian, Native American, and Black individuals within the U.S. Census population,” according to Ms. Strait and her associates. UpToDate contained the largest proportion of images of dark skin respective to other databases, whereas Kelley’s Textbook had the smallest.
Actionable steps
Many physicians are willing to improve upon their skills in identifying conditions on darker skin, as the study by Dr. Kannuthurai and associates suggests. Overall, 93% of the survey’s participants wanted to learn more about rashes in patients of color. “Future educational interventions may help practitioners improve their confidence when diagnosing rashes in lupus patients” with darker skin, they suggested.
Ms. Strait and her colleagues recommended a series of actionable steps to improve diversity and equity of dark skin tone representation in rheumatology curricula.
Editors of educational resources, for example, should make image diversity a priority for those diseases that are most commonly associated with cutaneous manifestations, such as SLE, vasculitis, inflammatory myopathies, systemic sclerosis, sarcoidosis, and psoriasis. They also called for educators in academic rheumatology programs to collaborate to improve diversity in resources used at the undergraduate and graduate medical education level.
Efforts should take place at the local, regional, and national level to publicly discuss and educate clinicians about rheumatic diseases in individuals of color. Speakers at rheumatology conferences should strive to educate learners about presentations of rheumatic diseases in individuals of color. The ACR in the meantime could establish a task force to enhance racial and ethnic diversity in their image library and other published resources.
“These steps may improve provider recognition and diagnosis of rheumatic disease manifestations in skin of color, which may in turn reduce health disparities among racial and ethnic minority groups,” Ms. Strait said.
Beth L. Jonas, MD, chair of the ACR’s Committee on Rheumatology Training and Workforce Issues, called the findings of this study “timely and important.” The researchers highlighted a deficiency in rheumatology training materials that needs addressing, she said in an interview. “I definitely agree that ACR needs to be mindful of this. There’s no doubt that we need to take these recommendations and move along these lines.”
The ACR took a first step in 2020 with the creation of a diversity, equity, and inclusion committee. “We are undergoing a college-wide look at what we do, with an eye toward inclusion. There is a strong interest in addressing health disparities and being an equitable and inclusive community of rheumatology health care professionals,” said Dr. Jonas, chief of the University of North Carolina at Chapel Hill’s division of rheumatology, allergy, and immunology.
The American Academy of Dermatology is also working to improve the image library with images of disease in skin of color. “Everyone’s jumping on this now,” Dr. McKinley-Grant observed. The medical profession can’t afford not to. It’s a life-threatening issue when rheumatoid arthritis and other diseases in people of color aren’t diagnosed early and correctly, she added.
Technologies seek to reduce bias
While many organizations are taking steps to improve representation of darker skin images, VisualDx has taken the lead on this, she said. “They’ve been doing this for years now. There are over 14,000 images of disease in skin of color, including all the rheumatologic diseases. There’s a mobile app and desktop decision support system, and it is very popular. A majority of medical schools have this as a library resource, and hospital systems license it for EHR integration.” Doctors can also get it individually. This enables them to share images and handouts of a diagnosis and select images of patients of color, said Dr. McKinley-Grant, who uses the VisualDx smartphone app DermExpert, which is an app for nondermatologists that features an image library of skin lesions, including darker-skin images.
ProjectIMPACT, powered by VisualDx, is another effort to support reducing health care bias in darker skin. The project is a collaboration between the New England Journal of Medicine Group and the Skin Of Color Society. According to Dr. McKinley-Grant, the organizers are building awareness of the importance of reducing the educational and clinical gaps in diagnosing patients of color and trying to get students and educators to pledge to take meaningful steps and to have real-world impact.
This isn’t just exclusive to dermatology and rheumatology – it involves all medical specialties, she stressed.
ProjectIMPACT isn’t just a resource for physicians, she continued. Librarians can also use it to develop more resources on skin of color.
The Skin Of Color Society and VisualDx have also partnered with the NEJM Group to develop a comprehensive virtual series on the impact of skin color and ethnicity on clinical research. The four-part series addresses structural racism and racial bias in medicine, hair disorders in people of color, pigmentary disorders, keloids, COVID-19 comorbidities, and cutaneous manifestations of systemic diseases in children and adults.
Nuances of recognizing disease
As a medical student, Dr. McKinley-Grant said she was fortunate to attend the Albert Schweitzer Hospital in Lambarene, Gabon, on a fellowship. For 3 months, she gained a wealth of experience examining only African patients with brown skin.
In her other training in medicine, “I’ve been at institutions with diverse populations, in Boston, New York, and Washington,” learning more about all different skin pigments.
This type of training should be more widely available, especially now, with COVID-19 producing new manifestations of skin lesions, she emphasized. Such efforts involve a diversification of images physicians are being trained on so that they can recognize the same disease in a person of color.
“Doctors have to be able to recognize different colors, different shades of brown and shades of white. Not all white skin is the same color,” she noted. In looking at a rash or lesion, “you have to learn how to discern differences in the background color of the skin, which is determined by melanin in the skin (Fitzpatrick skin types I-VI) and by what’s going on in the blood, such as how much oxygen and hemoglobin the patient has in their blood.” Inflammation and infection (erythema) will appear more violaceous in IV-VI skin types, for example.
At the University of North Carolina at Chapel Hill, a group of students and faculty have created a dermatology image library to address the deficiency in the availability of images for teaching purposes. “Our medical students recognized the gap and started this,” Dr. Jonas said. Julie Mervak, MD, assistant professor of dermatology, is spearheading this effort, with students Linnea Westerkam and Anuj Pranav Sanghvi.
“I understand that others around the country are working on similar initiatives,” Dr. Jonas said.
None of the sources for this story had any relevant disclosures.
Studies are flagging racial and ethnic disparities in rheumatology training materials, pointing to a need to boost representation of darker skin tones and better educate physicians in evaluating this cohort.
Not enough is known about these disparities in rheumatology education, despite the fact that minorities make up 40% of the population in the United States.
The problem starts with books and references used in medical schools, Lynn McKinley-Grant, MD, immediate past president of the Skin of Color Society and associate professor of dermatology at Howard University, Washington, said in an interview. “In the medical literature there has been a dearth of images in skin of color in all specialties,” she said. With an increased diversity in the U.S. population, there is a need for health care providers to be able to recognize disease patterns in all skin types.” If a physician is training at an institution where there are not many patients of color in the community, the rheumatologists are even more limited in terms of their clinical experience.
This lack of training in diagnosis of disease has serious clinical repercussions, as seen in COVID cases, Dr. McKinley-Grant noted. “You end up not being able to recognize early erythema, jaundice, anemia, or hypoxemia because those conditions are a different color or pattern in the darker skin types. This can lead to errors in treatment, diagnosis, and medical care, resulting in increased morbidity and mortality.”
Studies point to education gaps
A team of researchers from Washington University in St. Louis called attention to this issue at the American College of Rhematology’s Convergence 2020 conference.
“Patients of color with lupus are especially vulnerable as they often carry a greater disease burden, yet studies show that individuals with darker skin tones are underrepresented in medical educational materials,” Vijay Kannuthurai, MD, and colleagues wrote in their study abstract. The team surveyed 132 providers in St. Louis, Mo., on their confidence in evaluating any rash, and rashes in patients with lupus and varied skin tones.
Participating clinicians, mostly rheumatologists, dermatologists, or internists, had a higher confidence level in diagnosing any rash versus lupus rashes, but were considerably less confident in diagnosing lupus rash on darker skin, compared with those on fair skin. This represents “a disparity between provider confidence and the patient population lupus traditionally affects,” the investigators concluded.
Another recent study found evidence of disparities in clinical education resources. “The lack of dark skin representation among rheumatology educational materials contributes to the implicit bias and structural racism present in medical education by promoting White-only models of disease,” lead author Adrienne Strait, a medical student at the University of California, San Francisco, said in an interview. “Given that rheumatic diseases disproportionately impact racial and ethnic minorities, we felt it was important to examine the representation of these groups within rheumatology training resources.”
She and her colleagues gathered images of rheumatic diseases from four major databases: the American College of Rheumatology’s Image Library, UpToDate, the New England Journal of Medicine Images in Clinical Medicine and Clinical Cases filtered by “Rheumatology,” and the 9th edition of Kelley’s Textbook of Rheumatology. They used Fitzpatrick’s skin phototypes to independently code images depicting skin as “light” (skin types I-IV), “dark” (skin types V-VI), or “indeterminate,” focusing on systemic lupus erythematosus (SLE) and rheumatoid arthritis, two conditions with a known connection to racial and ethnic health disparities.
Taking into account the high incidence of sarcoidosis and SLE in Black patients when compared with White patients, the investigators did a secondary analysis that excluded these cases.
Among 1,043 patient images studied, just 13.4% represented dark skin, compared with 84% that represented light skin. More than 2% represented an indeterminate skin color. Comparing dark-skin representation in the clinical images and SLE images with the representation of Asian, Native American, and Black individuals in the United States and within lupus cases nationally, the investigators found significant underrepresentation of dark skin.
Only 4.2% of RA images had dark-skin representation, making RA one of the diseases with the lowest representation in the study, along with juvenile idiopathic arthritis, the spondyloarthropathies, and Kawasaki disease. “Representation of dark skin in SLE was also lower than the proportion of Black individuals in SLE studies,” the investigators noted. Overall, representation of dark skin in SLE images was just 22.6%. Sarcoidosis comparatively had the largest representation of dark-skin images (69.6%, n = 32).
“Excluding sarcoidosis and SLE images, the overall representation of dark skin was 9.4% (n = 84), which was significantly lower than the proportion of Asian, Native American, and Black individuals within the U.S. Census population,” according to Ms. Strait and her associates. UpToDate contained the largest proportion of images of dark skin respective to other databases, whereas Kelley’s Textbook had the smallest.
Actionable steps
Many physicians are willing to improve upon their skills in identifying conditions on darker skin, as the study by Dr. Kannuthurai and associates suggests. Overall, 93% of the survey’s participants wanted to learn more about rashes in patients of color. “Future educational interventions may help practitioners improve their confidence when diagnosing rashes in lupus patients” with darker skin, they suggested.
Ms. Strait and her colleagues recommended a series of actionable steps to improve diversity and equity of dark skin tone representation in rheumatology curricula.
Editors of educational resources, for example, should make image diversity a priority for those diseases that are most commonly associated with cutaneous manifestations, such as SLE, vasculitis, inflammatory myopathies, systemic sclerosis, sarcoidosis, and psoriasis. They also called for educators in academic rheumatology programs to collaborate to improve diversity in resources used at the undergraduate and graduate medical education level.
Efforts should take place at the local, regional, and national level to publicly discuss and educate clinicians about rheumatic diseases in individuals of color. Speakers at rheumatology conferences should strive to educate learners about presentations of rheumatic diseases in individuals of color. The ACR in the meantime could establish a task force to enhance racial and ethnic diversity in their image library and other published resources.
“These steps may improve provider recognition and diagnosis of rheumatic disease manifestations in skin of color, which may in turn reduce health disparities among racial and ethnic minority groups,” Ms. Strait said.
Beth L. Jonas, MD, chair of the ACR’s Committee on Rheumatology Training and Workforce Issues, called the findings of this study “timely and important.” The researchers highlighted a deficiency in rheumatology training materials that needs addressing, she said in an interview. “I definitely agree that ACR needs to be mindful of this. There’s no doubt that we need to take these recommendations and move along these lines.”
The ACR took a first step in 2020 with the creation of a diversity, equity, and inclusion committee. “We are undergoing a college-wide look at what we do, with an eye toward inclusion. There is a strong interest in addressing health disparities and being an equitable and inclusive community of rheumatology health care professionals,” said Dr. Jonas, chief of the University of North Carolina at Chapel Hill’s division of rheumatology, allergy, and immunology.
The American Academy of Dermatology is also working to improve the image library with images of disease in skin of color. “Everyone’s jumping on this now,” Dr. McKinley-Grant observed. The medical profession can’t afford not to. It’s a life-threatening issue when rheumatoid arthritis and other diseases in people of color aren’t diagnosed early and correctly, she added.
Technologies seek to reduce bias
While many organizations are taking steps to improve representation of darker skin images, VisualDx has taken the lead on this, she said. “They’ve been doing this for years now. There are over 14,000 images of disease in skin of color, including all the rheumatologic diseases. There’s a mobile app and desktop decision support system, and it is very popular. A majority of medical schools have this as a library resource, and hospital systems license it for EHR integration.” Doctors can also get it individually. This enables them to share images and handouts of a diagnosis and select images of patients of color, said Dr. McKinley-Grant, who uses the VisualDx smartphone app DermExpert, which is an app for nondermatologists that features an image library of skin lesions, including darker-skin images.
ProjectIMPACT, powered by VisualDx, is another effort to support reducing health care bias in darker skin. The project is a collaboration between the New England Journal of Medicine Group and the Skin Of Color Society. According to Dr. McKinley-Grant, the organizers are building awareness of the importance of reducing the educational and clinical gaps in diagnosing patients of color and trying to get students and educators to pledge to take meaningful steps and to have real-world impact.
This isn’t just exclusive to dermatology and rheumatology – it involves all medical specialties, she stressed.
ProjectIMPACT isn’t just a resource for physicians, she continued. Librarians can also use it to develop more resources on skin of color.
The Skin Of Color Society and VisualDx have also partnered with the NEJM Group to develop a comprehensive virtual series on the impact of skin color and ethnicity on clinical research. The four-part series addresses structural racism and racial bias in medicine, hair disorders in people of color, pigmentary disorders, keloids, COVID-19 comorbidities, and cutaneous manifestations of systemic diseases in children and adults.
Nuances of recognizing disease
As a medical student, Dr. McKinley-Grant said she was fortunate to attend the Albert Schweitzer Hospital in Lambarene, Gabon, on a fellowship. For 3 months, she gained a wealth of experience examining only African patients with brown skin.
In her other training in medicine, “I’ve been at institutions with diverse populations, in Boston, New York, and Washington,” learning more about all different skin pigments.
This type of training should be more widely available, especially now, with COVID-19 producing new manifestations of skin lesions, she emphasized. Such efforts involve a diversification of images physicians are being trained on so that they can recognize the same disease in a person of color.
“Doctors have to be able to recognize different colors, different shades of brown and shades of white. Not all white skin is the same color,” she noted. In looking at a rash or lesion, “you have to learn how to discern differences in the background color of the skin, which is determined by melanin in the skin (Fitzpatrick skin types I-VI) and by what’s going on in the blood, such as how much oxygen and hemoglobin the patient has in their blood.” Inflammation and infection (erythema) will appear more violaceous in IV-VI skin types, for example.
At the University of North Carolina at Chapel Hill, a group of students and faculty have created a dermatology image library to address the deficiency in the availability of images for teaching purposes. “Our medical students recognized the gap and started this,” Dr. Jonas said. Julie Mervak, MD, assistant professor of dermatology, is spearheading this effort, with students Linnea Westerkam and Anuj Pranav Sanghvi.
“I understand that others around the country are working on similar initiatives,” Dr. Jonas said.
None of the sources for this story had any relevant disclosures.
Studies are flagging racial and ethnic disparities in rheumatology training materials, pointing to a need to boost representation of darker skin tones and better educate physicians in evaluating this cohort.
Not enough is known about these disparities in rheumatology education, despite the fact that minorities make up 40% of the population in the United States.
The problem starts with books and references used in medical schools, Lynn McKinley-Grant, MD, immediate past president of the Skin of Color Society and associate professor of dermatology at Howard University, Washington, said in an interview. “In the medical literature there has been a dearth of images in skin of color in all specialties,” she said. With an increased diversity in the U.S. population, there is a need for health care providers to be able to recognize disease patterns in all skin types.” If a physician is training at an institution where there are not many patients of color in the community, the rheumatologists are even more limited in terms of their clinical experience.
This lack of training in diagnosis of disease has serious clinical repercussions, as seen in COVID cases, Dr. McKinley-Grant noted. “You end up not being able to recognize early erythema, jaundice, anemia, or hypoxemia because those conditions are a different color or pattern in the darker skin types. This can lead to errors in treatment, diagnosis, and medical care, resulting in increased morbidity and mortality.”
Studies point to education gaps
A team of researchers from Washington University in St. Louis called attention to this issue at the American College of Rhematology’s Convergence 2020 conference.
“Patients of color with lupus are especially vulnerable as they often carry a greater disease burden, yet studies show that individuals with darker skin tones are underrepresented in medical educational materials,” Vijay Kannuthurai, MD, and colleagues wrote in their study abstract. The team surveyed 132 providers in St. Louis, Mo., on their confidence in evaluating any rash, and rashes in patients with lupus and varied skin tones.
Participating clinicians, mostly rheumatologists, dermatologists, or internists, had a higher confidence level in diagnosing any rash versus lupus rashes, but were considerably less confident in diagnosing lupus rash on darker skin, compared with those on fair skin. This represents “a disparity between provider confidence and the patient population lupus traditionally affects,” the investigators concluded.
Another recent study found evidence of disparities in clinical education resources. “The lack of dark skin representation among rheumatology educational materials contributes to the implicit bias and structural racism present in medical education by promoting White-only models of disease,” lead author Adrienne Strait, a medical student at the University of California, San Francisco, said in an interview. “Given that rheumatic diseases disproportionately impact racial and ethnic minorities, we felt it was important to examine the representation of these groups within rheumatology training resources.”
She and her colleagues gathered images of rheumatic diseases from four major databases: the American College of Rheumatology’s Image Library, UpToDate, the New England Journal of Medicine Images in Clinical Medicine and Clinical Cases filtered by “Rheumatology,” and the 9th edition of Kelley’s Textbook of Rheumatology. They used Fitzpatrick’s skin phototypes to independently code images depicting skin as “light” (skin types I-IV), “dark” (skin types V-VI), or “indeterminate,” focusing on systemic lupus erythematosus (SLE) and rheumatoid arthritis, two conditions with a known connection to racial and ethnic health disparities.
Taking into account the high incidence of sarcoidosis and SLE in Black patients when compared with White patients, the investigators did a secondary analysis that excluded these cases.
Among 1,043 patient images studied, just 13.4% represented dark skin, compared with 84% that represented light skin. More than 2% represented an indeterminate skin color. Comparing dark-skin representation in the clinical images and SLE images with the representation of Asian, Native American, and Black individuals in the United States and within lupus cases nationally, the investigators found significant underrepresentation of dark skin.
Only 4.2% of RA images had dark-skin representation, making RA one of the diseases with the lowest representation in the study, along with juvenile idiopathic arthritis, the spondyloarthropathies, and Kawasaki disease. “Representation of dark skin in SLE was also lower than the proportion of Black individuals in SLE studies,” the investigators noted. Overall, representation of dark skin in SLE images was just 22.6%. Sarcoidosis comparatively had the largest representation of dark-skin images (69.6%, n = 32).
“Excluding sarcoidosis and SLE images, the overall representation of dark skin was 9.4% (n = 84), which was significantly lower than the proportion of Asian, Native American, and Black individuals within the U.S. Census population,” according to Ms. Strait and her associates. UpToDate contained the largest proportion of images of dark skin respective to other databases, whereas Kelley’s Textbook had the smallest.
Actionable steps
Many physicians are willing to improve upon their skills in identifying conditions on darker skin, as the study by Dr. Kannuthurai and associates suggests. Overall, 93% of the survey’s participants wanted to learn more about rashes in patients of color. “Future educational interventions may help practitioners improve their confidence when diagnosing rashes in lupus patients” with darker skin, they suggested.
Ms. Strait and her colleagues recommended a series of actionable steps to improve diversity and equity of dark skin tone representation in rheumatology curricula.
Editors of educational resources, for example, should make image diversity a priority for those diseases that are most commonly associated with cutaneous manifestations, such as SLE, vasculitis, inflammatory myopathies, systemic sclerosis, sarcoidosis, and psoriasis. They also called for educators in academic rheumatology programs to collaborate to improve diversity in resources used at the undergraduate and graduate medical education level.
Efforts should take place at the local, regional, and national level to publicly discuss and educate clinicians about rheumatic diseases in individuals of color. Speakers at rheumatology conferences should strive to educate learners about presentations of rheumatic diseases in individuals of color. The ACR in the meantime could establish a task force to enhance racial and ethnic diversity in their image library and other published resources.
“These steps may improve provider recognition and diagnosis of rheumatic disease manifestations in skin of color, which may in turn reduce health disparities among racial and ethnic minority groups,” Ms. Strait said.
Beth L. Jonas, MD, chair of the ACR’s Committee on Rheumatology Training and Workforce Issues, called the findings of this study “timely and important.” The researchers highlighted a deficiency in rheumatology training materials that needs addressing, she said in an interview. “I definitely agree that ACR needs to be mindful of this. There’s no doubt that we need to take these recommendations and move along these lines.”
The ACR took a first step in 2020 with the creation of a diversity, equity, and inclusion committee. “We are undergoing a college-wide look at what we do, with an eye toward inclusion. There is a strong interest in addressing health disparities and being an equitable and inclusive community of rheumatology health care professionals,” said Dr. Jonas, chief of the University of North Carolina at Chapel Hill’s division of rheumatology, allergy, and immunology.
The American Academy of Dermatology is also working to improve the image library with images of disease in skin of color. “Everyone’s jumping on this now,” Dr. McKinley-Grant observed. The medical profession can’t afford not to. It’s a life-threatening issue when rheumatoid arthritis and other diseases in people of color aren’t diagnosed early and correctly, she added.
Technologies seek to reduce bias
While many organizations are taking steps to improve representation of darker skin images, VisualDx has taken the lead on this, she said. “They’ve been doing this for years now. There are over 14,000 images of disease in skin of color, including all the rheumatologic diseases. There’s a mobile app and desktop decision support system, and it is very popular. A majority of medical schools have this as a library resource, and hospital systems license it for EHR integration.” Doctors can also get it individually. This enables them to share images and handouts of a diagnosis and select images of patients of color, said Dr. McKinley-Grant, who uses the VisualDx smartphone app DermExpert, which is an app for nondermatologists that features an image library of skin lesions, including darker-skin images.
ProjectIMPACT, powered by VisualDx, is another effort to support reducing health care bias in darker skin. The project is a collaboration between the New England Journal of Medicine Group and the Skin Of Color Society. According to Dr. McKinley-Grant, the organizers are building awareness of the importance of reducing the educational and clinical gaps in diagnosing patients of color and trying to get students and educators to pledge to take meaningful steps and to have real-world impact.
This isn’t just exclusive to dermatology and rheumatology – it involves all medical specialties, she stressed.
ProjectIMPACT isn’t just a resource for physicians, she continued. Librarians can also use it to develop more resources on skin of color.
The Skin Of Color Society and VisualDx have also partnered with the NEJM Group to develop a comprehensive virtual series on the impact of skin color and ethnicity on clinical research. The four-part series addresses structural racism and racial bias in medicine, hair disorders in people of color, pigmentary disorders, keloids, COVID-19 comorbidities, and cutaneous manifestations of systemic diseases in children and adults.
Nuances of recognizing disease
As a medical student, Dr. McKinley-Grant said she was fortunate to attend the Albert Schweitzer Hospital in Lambarene, Gabon, on a fellowship. For 3 months, she gained a wealth of experience examining only African patients with brown skin.
In her other training in medicine, “I’ve been at institutions with diverse populations, in Boston, New York, and Washington,” learning more about all different skin pigments.
This type of training should be more widely available, especially now, with COVID-19 producing new manifestations of skin lesions, she emphasized. Such efforts involve a diversification of images physicians are being trained on so that they can recognize the same disease in a person of color.
“Doctors have to be able to recognize different colors, different shades of brown and shades of white. Not all white skin is the same color,” she noted. In looking at a rash or lesion, “you have to learn how to discern differences in the background color of the skin, which is determined by melanin in the skin (Fitzpatrick skin types I-VI) and by what’s going on in the blood, such as how much oxygen and hemoglobin the patient has in their blood.” Inflammation and infection (erythema) will appear more violaceous in IV-VI skin types, for example.
At the University of North Carolina at Chapel Hill, a group of students and faculty have created a dermatology image library to address the deficiency in the availability of images for teaching purposes. “Our medical students recognized the gap and started this,” Dr. Jonas said. Julie Mervak, MD, assistant professor of dermatology, is spearheading this effort, with students Linnea Westerkam and Anuj Pranav Sanghvi.
“I understand that others around the country are working on similar initiatives,” Dr. Jonas said.
None of the sources for this story had any relevant disclosures.
Most patients with chronic inflammatory diseases have sufficient response to COVID-19 vaccination
Glucocorticoids and B-cell–depleting therapies are trouble spots
Although most patients with chronic inflammatory diseases mounted immune responses after two doses of mRNA-based COVID-19 vaccines, glucocorticoids and B-cell–depleting therapies markedly reduced the response, according to a recently published preprint of a new study.
The study, published on MedRxiv and not yet peer reviewed, involved a prospective look at 133 patients with chronic inflammatory disease (CID) and 53 patients with healthy immune systems at Washington University, St. Louis, and the University of California, San Francisco. It is regarded as the largest and most detailed study yet in how vaccines perform in people with immune-mediated inflammatory disease. The patients were enrolled between December 2020 and March 2021, and the most common diseases were inflammatory bowel disease (32%), rheumatoid arthritis (29%), spondyloarthritis (15%), and systemic lupus erythematosus (11%).
A ‘modest’ reduction in antibody response
Senior author Alfred Kim, MD, PhD, of the department of medicine at Washington University, said the overall results so far are encouraging.
“Most patients with an autoimmune disease that are on immunosuppression can mount antibody responses,” he said. “We’re seeing the majority of our subjects respond.”
The immune-healthy controls and most of the patients with CID had a robust immune response against the spike protein, although the CID group had a mean reduction in antibody titers that was three times lower than the controls (P = .0092). The CID group similarly had a 2.7-fold reduction in preventing neutralization, or halting the virus’ ability to infect (P < .0001), researchers reported.
This reduction in response is “modest,” he said.
“Is the level of reduction going to be detrimental for protection? Time will tell,” he said, adding that researchers anticipate that it won’t have a critical effect on protection because responses tended to be within the range of the immunocompetent controls, who themselves had wildly varied antibody titers across a 20-fold range. “ ‘Optimal’ isn’t necessarily the same as ‘sufficient.’ ”
Type of medication has big impact on antibody titers
But there was a wide variety of effects on the immune response depending on the medication. Glucorticoids resulted in a response that was 10 times lower than the immune-healthy controls, as well as fewer circulating plasmablasts after vaccination. Researchers found that 98% of controls were seropositive for antibody, compared with 92% of those with CID who were not taking prednisone, and 65% of CID patients on prednisone (P = .0006 and .0115, respectively). Prevention of neutralization of the virus was similarly reduced in those groups, compared with the controls. Dr. Kim noted this was a small sample size, with about 15 patients. These effects were seen regardless of the dose.
“We would’ve anticipated this would have been dose dependent, so this was a little bit surprising,” Dr. Kim said.
B-cell–depleting therapies, such as rituximab (Rituxan) and ocrelizumab (Ocrevus), reduced antibody titers by 36 times, compared with controls (P < .0001), with a similar reduction in preventing infection (P = .0066), the researchers found. The reduction in antibody titers was the most pronounced among those who had received B-cell–depleting therapies within the previous 6 months. Dr. Kim noted this was a small sample size, with about 10 patients.
CID study subjects taking an antimetabolite, including methotrexate, had an average of a two- to threefold reduction in antibody titers and in neutralization (P = .0006). This reduction was greatest with methotrexate, researchers found (P = .0027).
JAK inhibitors also significantly reduced antibody titers (P = .0066), but the reduction in neutralization of the virus was not significant. In addition, researchers found a reduction in antibody titers, the prevention of viral infection, and circulating plasmablasts among those on tumor necrosis factor (TNF) inhibitors, compared with controls, but these were insignificant statistically except for virus neutralization.
Dr. Kim said he hopes the glucocorticoid data spur physicians to try harder to wean patients off the drugs, when possible, in keeping with recommendations already in place.
“The general culture in rheumatology has been very lax about the need to reduce glucocorticoids,” he said. “This reinvigorates that call.” Questions about possible drug holidays from glucocorticoids remain, regarding how long a holiday would be needed, he said. He noted that many patients on glucocorticoids nonetheless mounted responses.
Those on B-cell–depleting therapies present a “much more difficult” question, he said. Some patients possibly could wait a bit longer than their normal, every-6-month schedule, but it’s an individual decision, he said. Since a booster of influenza vaccine has been found to enhance the response even within the 6-month window among ocrelizumab patients, a booster of COVID-19 vaccine might also help, although this remains to be studied.
The study group has already increased its sample size and is looking at adverse reactions and long-term immune responses, Dr. Kim said.
Encouraging, rather than discouraging, results
Leonard Calabrese, DO, professor of medicine at the Cleveland Clinic in Ohio, said the findings shouldn’t discourage clinicians from encouraging vaccination.
“There’s still a preponderance of people who will develop a robust antibody vaccine response,” he said.
He cautioned that the findings look only at antibodies to the spike protein and at plasmablasts. The reduction in these titers is “of concern,” he said, but “we don’t really know with certainty what are the effects of these drugs, and these data are on the overall biologic protective effect of the vaccine. There’s much more to a vaccine response than anti–spike protein and plasmablasts,” including cell-mediated immune response.
For an individual patient, the findings “mean a lot,” he said.
“I think that people who are on significant prednisone and B-cell–depleting agents, I think you have to share with them that there’s a reasonable chance that you’re not going to be making a response similar to healthy people,” he said. “Thus, even with your vaccine, we’re not going to cut you loose to do things that are violating social distancing and group settings. … Should you be hugging your grandchildren if you’re a rituximab vaccine recipient? I think I would wait until we have a little bit more data.”
Kevin Winthrop, MD, MPH, professor of ophthalmology at Oregon Health & Science University, Portland, where he studies vaccinations in the immunocompromised, said that glucocorticoids tend to have little effect on vaccinations generally at low doses.
When effects are seen they can be difficult to interpret, he said.
“It’s hard to extricate that from the effect of the underlying disease,” he said. The drug can be a proxy for worse disease control.
Although it’s a small study, it’s reassuring that overall the responses were similar to healthy controls.
For B-cell–depleting therapies, his usual guidance is to not give vaccine until a patient is at least 3 months out from their last dose, and not to restart until at least 2 weeks after vaccination.
“It’s not surprising that some of these DMARDs [disease-modifying antirheumatic drugs] do negatively affect vaccine response, particularly B-cell–depletion therapy. We need to do some studies to find a way to overcome that, or optimize delivery of the vaccine.”
Dr. Kim reported participating in consulting, advisory board, or speaker’s bureau for Alexion, Aurinia, Annexon Biosciences, Exagen Diagnostics, and GlaxoSmithKline, and receiving funding under a sponsored research agreement unrelated to the data in the paper from GlaxoSmithKline. Dr. Winthrop reported receiving consulting fees from Pfizer, AbbVie, UCB, Eli Lilly, Galapagos, GlaxoSmithKline, Roche, Gilead, Bristol-Myers Squibb, Regeneron, Sanofi, AstraZeneca, Novartis, and research grants from Bristol-Myers Squibb and Pfizer. Dr. Calabrese reported no relevant disclosures.
Glucocorticoids and B-cell–depleting therapies are trouble spots
Glucocorticoids and B-cell–depleting therapies are trouble spots
Although most patients with chronic inflammatory diseases mounted immune responses after two doses of mRNA-based COVID-19 vaccines, glucocorticoids and B-cell–depleting therapies markedly reduced the response, according to a recently published preprint of a new study.
The study, published on MedRxiv and not yet peer reviewed, involved a prospective look at 133 patients with chronic inflammatory disease (CID) and 53 patients with healthy immune systems at Washington University, St. Louis, and the University of California, San Francisco. It is regarded as the largest and most detailed study yet in how vaccines perform in people with immune-mediated inflammatory disease. The patients were enrolled between December 2020 and March 2021, and the most common diseases were inflammatory bowel disease (32%), rheumatoid arthritis (29%), spondyloarthritis (15%), and systemic lupus erythematosus (11%).
A ‘modest’ reduction in antibody response
Senior author Alfred Kim, MD, PhD, of the department of medicine at Washington University, said the overall results so far are encouraging.
“Most patients with an autoimmune disease that are on immunosuppression can mount antibody responses,” he said. “We’re seeing the majority of our subjects respond.”
The immune-healthy controls and most of the patients with CID had a robust immune response against the spike protein, although the CID group had a mean reduction in antibody titers that was three times lower than the controls (P = .0092). The CID group similarly had a 2.7-fold reduction in preventing neutralization, or halting the virus’ ability to infect (P < .0001), researchers reported.
This reduction in response is “modest,” he said.
“Is the level of reduction going to be detrimental for protection? Time will tell,” he said, adding that researchers anticipate that it won’t have a critical effect on protection because responses tended to be within the range of the immunocompetent controls, who themselves had wildly varied antibody titers across a 20-fold range. “ ‘Optimal’ isn’t necessarily the same as ‘sufficient.’ ”
Type of medication has big impact on antibody titers
But there was a wide variety of effects on the immune response depending on the medication. Glucorticoids resulted in a response that was 10 times lower than the immune-healthy controls, as well as fewer circulating plasmablasts after vaccination. Researchers found that 98% of controls were seropositive for antibody, compared with 92% of those with CID who were not taking prednisone, and 65% of CID patients on prednisone (P = .0006 and .0115, respectively). Prevention of neutralization of the virus was similarly reduced in those groups, compared with the controls. Dr. Kim noted this was a small sample size, with about 15 patients. These effects were seen regardless of the dose.
“We would’ve anticipated this would have been dose dependent, so this was a little bit surprising,” Dr. Kim said.
B-cell–depleting therapies, such as rituximab (Rituxan) and ocrelizumab (Ocrevus), reduced antibody titers by 36 times, compared with controls (P < .0001), with a similar reduction in preventing infection (P = .0066), the researchers found. The reduction in antibody titers was the most pronounced among those who had received B-cell–depleting therapies within the previous 6 months. Dr. Kim noted this was a small sample size, with about 10 patients.
CID study subjects taking an antimetabolite, including methotrexate, had an average of a two- to threefold reduction in antibody titers and in neutralization (P = .0006). This reduction was greatest with methotrexate, researchers found (P = .0027).
JAK inhibitors also significantly reduced antibody titers (P = .0066), but the reduction in neutralization of the virus was not significant. In addition, researchers found a reduction in antibody titers, the prevention of viral infection, and circulating plasmablasts among those on tumor necrosis factor (TNF) inhibitors, compared with controls, but these were insignificant statistically except for virus neutralization.
Dr. Kim said he hopes the glucocorticoid data spur physicians to try harder to wean patients off the drugs, when possible, in keeping with recommendations already in place.
“The general culture in rheumatology has been very lax about the need to reduce glucocorticoids,” he said. “This reinvigorates that call.” Questions about possible drug holidays from glucocorticoids remain, regarding how long a holiday would be needed, he said. He noted that many patients on glucocorticoids nonetheless mounted responses.
Those on B-cell–depleting therapies present a “much more difficult” question, he said. Some patients possibly could wait a bit longer than their normal, every-6-month schedule, but it’s an individual decision, he said. Since a booster of influenza vaccine has been found to enhance the response even within the 6-month window among ocrelizumab patients, a booster of COVID-19 vaccine might also help, although this remains to be studied.
The study group has already increased its sample size and is looking at adverse reactions and long-term immune responses, Dr. Kim said.
Encouraging, rather than discouraging, results
Leonard Calabrese, DO, professor of medicine at the Cleveland Clinic in Ohio, said the findings shouldn’t discourage clinicians from encouraging vaccination.
“There’s still a preponderance of people who will develop a robust antibody vaccine response,” he said.
He cautioned that the findings look only at antibodies to the spike protein and at plasmablasts. The reduction in these titers is “of concern,” he said, but “we don’t really know with certainty what are the effects of these drugs, and these data are on the overall biologic protective effect of the vaccine. There’s much more to a vaccine response than anti–spike protein and plasmablasts,” including cell-mediated immune response.
For an individual patient, the findings “mean a lot,” he said.
“I think that people who are on significant prednisone and B-cell–depleting agents, I think you have to share with them that there’s a reasonable chance that you’re not going to be making a response similar to healthy people,” he said. “Thus, even with your vaccine, we’re not going to cut you loose to do things that are violating social distancing and group settings. … Should you be hugging your grandchildren if you’re a rituximab vaccine recipient? I think I would wait until we have a little bit more data.”
Kevin Winthrop, MD, MPH, professor of ophthalmology at Oregon Health & Science University, Portland, where he studies vaccinations in the immunocompromised, said that glucocorticoids tend to have little effect on vaccinations generally at low doses.
When effects are seen they can be difficult to interpret, he said.
“It’s hard to extricate that from the effect of the underlying disease,” he said. The drug can be a proxy for worse disease control.
Although it’s a small study, it’s reassuring that overall the responses were similar to healthy controls.
For B-cell–depleting therapies, his usual guidance is to not give vaccine until a patient is at least 3 months out from their last dose, and not to restart until at least 2 weeks after vaccination.
“It’s not surprising that some of these DMARDs [disease-modifying antirheumatic drugs] do negatively affect vaccine response, particularly B-cell–depletion therapy. We need to do some studies to find a way to overcome that, or optimize delivery of the vaccine.”
Dr. Kim reported participating in consulting, advisory board, or speaker’s bureau for Alexion, Aurinia, Annexon Biosciences, Exagen Diagnostics, and GlaxoSmithKline, and receiving funding under a sponsored research agreement unrelated to the data in the paper from GlaxoSmithKline. Dr. Winthrop reported receiving consulting fees from Pfizer, AbbVie, UCB, Eli Lilly, Galapagos, GlaxoSmithKline, Roche, Gilead, Bristol-Myers Squibb, Regeneron, Sanofi, AstraZeneca, Novartis, and research grants from Bristol-Myers Squibb and Pfizer. Dr. Calabrese reported no relevant disclosures.
Although most patients with chronic inflammatory diseases mounted immune responses after two doses of mRNA-based COVID-19 vaccines, glucocorticoids and B-cell–depleting therapies markedly reduced the response, according to a recently published preprint of a new study.
The study, published on MedRxiv and not yet peer reviewed, involved a prospective look at 133 patients with chronic inflammatory disease (CID) and 53 patients with healthy immune systems at Washington University, St. Louis, and the University of California, San Francisco. It is regarded as the largest and most detailed study yet in how vaccines perform in people with immune-mediated inflammatory disease. The patients were enrolled between December 2020 and March 2021, and the most common diseases were inflammatory bowel disease (32%), rheumatoid arthritis (29%), spondyloarthritis (15%), and systemic lupus erythematosus (11%).
A ‘modest’ reduction in antibody response
Senior author Alfred Kim, MD, PhD, of the department of medicine at Washington University, said the overall results so far are encouraging.
“Most patients with an autoimmune disease that are on immunosuppression can mount antibody responses,” he said. “We’re seeing the majority of our subjects respond.”
The immune-healthy controls and most of the patients with CID had a robust immune response against the spike protein, although the CID group had a mean reduction in antibody titers that was three times lower than the controls (P = .0092). The CID group similarly had a 2.7-fold reduction in preventing neutralization, or halting the virus’ ability to infect (P < .0001), researchers reported.
This reduction in response is “modest,” he said.
“Is the level of reduction going to be detrimental for protection? Time will tell,” he said, adding that researchers anticipate that it won’t have a critical effect on protection because responses tended to be within the range of the immunocompetent controls, who themselves had wildly varied antibody titers across a 20-fold range. “ ‘Optimal’ isn’t necessarily the same as ‘sufficient.’ ”
Type of medication has big impact on antibody titers
But there was a wide variety of effects on the immune response depending on the medication. Glucorticoids resulted in a response that was 10 times lower than the immune-healthy controls, as well as fewer circulating plasmablasts after vaccination. Researchers found that 98% of controls were seropositive for antibody, compared with 92% of those with CID who were not taking prednisone, and 65% of CID patients on prednisone (P = .0006 and .0115, respectively). Prevention of neutralization of the virus was similarly reduced in those groups, compared with the controls. Dr. Kim noted this was a small sample size, with about 15 patients. These effects were seen regardless of the dose.
“We would’ve anticipated this would have been dose dependent, so this was a little bit surprising,” Dr. Kim said.
B-cell–depleting therapies, such as rituximab (Rituxan) and ocrelizumab (Ocrevus), reduced antibody titers by 36 times, compared with controls (P < .0001), with a similar reduction in preventing infection (P = .0066), the researchers found. The reduction in antibody titers was the most pronounced among those who had received B-cell–depleting therapies within the previous 6 months. Dr. Kim noted this was a small sample size, with about 10 patients.
CID study subjects taking an antimetabolite, including methotrexate, had an average of a two- to threefold reduction in antibody titers and in neutralization (P = .0006). This reduction was greatest with methotrexate, researchers found (P = .0027).
JAK inhibitors also significantly reduced antibody titers (P = .0066), but the reduction in neutralization of the virus was not significant. In addition, researchers found a reduction in antibody titers, the prevention of viral infection, and circulating plasmablasts among those on tumor necrosis factor (TNF) inhibitors, compared with controls, but these were insignificant statistically except for virus neutralization.
Dr. Kim said he hopes the glucocorticoid data spur physicians to try harder to wean patients off the drugs, when possible, in keeping with recommendations already in place.
“The general culture in rheumatology has been very lax about the need to reduce glucocorticoids,” he said. “This reinvigorates that call.” Questions about possible drug holidays from glucocorticoids remain, regarding how long a holiday would be needed, he said. He noted that many patients on glucocorticoids nonetheless mounted responses.
Those on B-cell–depleting therapies present a “much more difficult” question, he said. Some patients possibly could wait a bit longer than their normal, every-6-month schedule, but it’s an individual decision, he said. Since a booster of influenza vaccine has been found to enhance the response even within the 6-month window among ocrelizumab patients, a booster of COVID-19 vaccine might also help, although this remains to be studied.
The study group has already increased its sample size and is looking at adverse reactions and long-term immune responses, Dr. Kim said.
Encouraging, rather than discouraging, results
Leonard Calabrese, DO, professor of medicine at the Cleveland Clinic in Ohio, said the findings shouldn’t discourage clinicians from encouraging vaccination.
“There’s still a preponderance of people who will develop a robust antibody vaccine response,” he said.
He cautioned that the findings look only at antibodies to the spike protein and at plasmablasts. The reduction in these titers is “of concern,” he said, but “we don’t really know with certainty what are the effects of these drugs, and these data are on the overall biologic protective effect of the vaccine. There’s much more to a vaccine response than anti–spike protein and plasmablasts,” including cell-mediated immune response.
For an individual patient, the findings “mean a lot,” he said.
“I think that people who are on significant prednisone and B-cell–depleting agents, I think you have to share with them that there’s a reasonable chance that you’re not going to be making a response similar to healthy people,” he said. “Thus, even with your vaccine, we’re not going to cut you loose to do things that are violating social distancing and group settings. … Should you be hugging your grandchildren if you’re a rituximab vaccine recipient? I think I would wait until we have a little bit more data.”
Kevin Winthrop, MD, MPH, professor of ophthalmology at Oregon Health & Science University, Portland, where he studies vaccinations in the immunocompromised, said that glucocorticoids tend to have little effect on vaccinations generally at low doses.
When effects are seen they can be difficult to interpret, he said.
“It’s hard to extricate that from the effect of the underlying disease,” he said. The drug can be a proxy for worse disease control.
Although it’s a small study, it’s reassuring that overall the responses were similar to healthy controls.
For B-cell–depleting therapies, his usual guidance is to not give vaccine until a patient is at least 3 months out from their last dose, and not to restart until at least 2 weeks after vaccination.
“It’s not surprising that some of these DMARDs [disease-modifying antirheumatic drugs] do negatively affect vaccine response, particularly B-cell–depletion therapy. We need to do some studies to find a way to overcome that, or optimize delivery of the vaccine.”
Dr. Kim reported participating in consulting, advisory board, or speaker’s bureau for Alexion, Aurinia, Annexon Biosciences, Exagen Diagnostics, and GlaxoSmithKline, and receiving funding under a sponsored research agreement unrelated to the data in the paper from GlaxoSmithKline. Dr. Winthrop reported receiving consulting fees from Pfizer, AbbVie, UCB, Eli Lilly, Galapagos, GlaxoSmithKline, Roche, Gilead, Bristol-Myers Squibb, Regeneron, Sanofi, AstraZeneca, Novartis, and research grants from Bristol-Myers Squibb and Pfizer. Dr. Calabrese reported no relevant disclosures.
FROM MEDRXIV
Rheumatology clinics find success with smoking cessation referral program
A new protocol designed to help patients in rheumatology clinics quit smoking proved both efficient and effective in referring willing participants to free tobacco quit lines.
“Rheumatology visits provide a unique opportunity to address smoking as a chronic modifiable risk factor in populations at high risk for cardiovascular disease, pulmonary disease, and rheumatic disease progression,” wrote Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, and colleagues. The study was published in Arthritis Care & Research.
To assess the effectiveness of implementing a smoking cessation protocol for patients with rheumatic diseases, the researchers launched a quasi-experimental cohort study in which their Quit Connect protocol was tested at three rheumatology clinics. Adapting the Ask, Advise, Connect primary care protocol to a new setting, nurses and medical assistants were trained to use electronic health record (EHR) prompts that would check if patients who smoked were ready to quit within 30 days, advise them to do so, and then use electronic referrals to connect them to state-run tobacco quit lines. An extended baseline period – October 2012 to March 2016 – was compared to a 6-month intervention period from April to October 2016.
Across 54,090 pre- and postimplementation rheumatology clinic visits, 4,601 were with current smokers. Demographics were similar across both periods: The mean age of the patients was 51 years, about two-thirds were female, and 85% were White.
Clinicians’ assessment of tobacco use before and after implementation of the program stayed steady at 96% of patient visits, but the percentage of tobacco users’ visits that included checking for readiness to quit within the next 30 days rose from 3% (135 of 4,078) to 80% (421 of 523).
Before the implementation of the program, 0.6% of eligible visits with current smokers included a quit-line referral offer. After implementation, 93 (18%) of the 523 smokers who visited – 122 of whom said they were ready to quit – were offered referrals, a 26-fold increase. Of the 93 offered referrals, 66 (71%) accepted and 16 set a quit date or reported having quit; 11 accepted counseling services and nicotine replacement.
Although clinic staff reported encountering several obstacles, such as the need to craft nonthreatening language for challenging patients, they also contributed their own talking points that were included in the EHR tools and desktop brochures. On average, the protocol took less than 90 seconds to perform.
Rheumatologists can make headway on patients quitting smoking
“While smoking cessation programs require time and resources to implement, this study suggests a role for evidence-based protocols within rheumatology centers,” Medha Barbhaiya, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. “Given that current smokers are at an increased risk of developing more severe rheumatic disease and cardiovascular disease, and patients often visit their rheumatologist multiple times yearly, rheumatologists may be well-positioned to address smoking cessation with patients.”
In regard to next steps, she noted that “while future large studies in diverse cohorts are needed to confirm these findings, implementing a formal smoking cessation protocol within rheumatology centers may provide a unique opportunity for rheumatologists to directly help patients modify their disease risk, leading to improved health outcomes.”
The authors acknowledged their study’s limitations, including the fact that it was a prepost design and not a randomized trial. They also recognized that many tobacco users require 8-10 attempts before permanently quitting, likely lessening the lasting impact of the short-term study. They did cite expert analysis, however, that says “connecting patients to evidence-based resources makes them more likely to permanently quit.”
The study was supported in part by Pfizer’s office of Independent Grants for Learning and Change and by a grant collaboration from the University of Wisconsin Clinical and Translational Science Award and the University of Wisconsin School of Medicine and Public Health’s Wisconsin Partnership Program, through the NIH National Center for Advancing Translational Sciences.
A new protocol designed to help patients in rheumatology clinics quit smoking proved both efficient and effective in referring willing participants to free tobacco quit lines.
“Rheumatology visits provide a unique opportunity to address smoking as a chronic modifiable risk factor in populations at high risk for cardiovascular disease, pulmonary disease, and rheumatic disease progression,” wrote Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, and colleagues. The study was published in Arthritis Care & Research.
To assess the effectiveness of implementing a smoking cessation protocol for patients with rheumatic diseases, the researchers launched a quasi-experimental cohort study in which their Quit Connect protocol was tested at three rheumatology clinics. Adapting the Ask, Advise, Connect primary care protocol to a new setting, nurses and medical assistants were trained to use electronic health record (EHR) prompts that would check if patients who smoked were ready to quit within 30 days, advise them to do so, and then use electronic referrals to connect them to state-run tobacco quit lines. An extended baseline period – October 2012 to March 2016 – was compared to a 6-month intervention period from April to October 2016.
Across 54,090 pre- and postimplementation rheumatology clinic visits, 4,601 were with current smokers. Demographics were similar across both periods: The mean age of the patients was 51 years, about two-thirds were female, and 85% were White.
Clinicians’ assessment of tobacco use before and after implementation of the program stayed steady at 96% of patient visits, but the percentage of tobacco users’ visits that included checking for readiness to quit within the next 30 days rose from 3% (135 of 4,078) to 80% (421 of 523).
Before the implementation of the program, 0.6% of eligible visits with current smokers included a quit-line referral offer. After implementation, 93 (18%) of the 523 smokers who visited – 122 of whom said they were ready to quit – were offered referrals, a 26-fold increase. Of the 93 offered referrals, 66 (71%) accepted and 16 set a quit date or reported having quit; 11 accepted counseling services and nicotine replacement.
Although clinic staff reported encountering several obstacles, such as the need to craft nonthreatening language for challenging patients, they also contributed their own talking points that were included in the EHR tools and desktop brochures. On average, the protocol took less than 90 seconds to perform.
Rheumatologists can make headway on patients quitting smoking
“While smoking cessation programs require time and resources to implement, this study suggests a role for evidence-based protocols within rheumatology centers,” Medha Barbhaiya, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. “Given that current smokers are at an increased risk of developing more severe rheumatic disease and cardiovascular disease, and patients often visit their rheumatologist multiple times yearly, rheumatologists may be well-positioned to address smoking cessation with patients.”
In regard to next steps, she noted that “while future large studies in diverse cohorts are needed to confirm these findings, implementing a formal smoking cessation protocol within rheumatology centers may provide a unique opportunity for rheumatologists to directly help patients modify their disease risk, leading to improved health outcomes.”
The authors acknowledged their study’s limitations, including the fact that it was a prepost design and not a randomized trial. They also recognized that many tobacco users require 8-10 attempts before permanently quitting, likely lessening the lasting impact of the short-term study. They did cite expert analysis, however, that says “connecting patients to evidence-based resources makes them more likely to permanently quit.”
The study was supported in part by Pfizer’s office of Independent Grants for Learning and Change and by a grant collaboration from the University of Wisconsin Clinical and Translational Science Award and the University of Wisconsin School of Medicine and Public Health’s Wisconsin Partnership Program, through the NIH National Center for Advancing Translational Sciences.
A new protocol designed to help patients in rheumatology clinics quit smoking proved both efficient and effective in referring willing participants to free tobacco quit lines.
“Rheumatology visits provide a unique opportunity to address smoking as a chronic modifiable risk factor in populations at high risk for cardiovascular disease, pulmonary disease, and rheumatic disease progression,” wrote Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, and colleagues. The study was published in Arthritis Care & Research.
To assess the effectiveness of implementing a smoking cessation protocol for patients with rheumatic diseases, the researchers launched a quasi-experimental cohort study in which their Quit Connect protocol was tested at three rheumatology clinics. Adapting the Ask, Advise, Connect primary care protocol to a new setting, nurses and medical assistants were trained to use electronic health record (EHR) prompts that would check if patients who smoked were ready to quit within 30 days, advise them to do so, and then use electronic referrals to connect them to state-run tobacco quit lines. An extended baseline period – October 2012 to March 2016 – was compared to a 6-month intervention period from April to October 2016.
Across 54,090 pre- and postimplementation rheumatology clinic visits, 4,601 were with current smokers. Demographics were similar across both periods: The mean age of the patients was 51 years, about two-thirds were female, and 85% were White.
Clinicians’ assessment of tobacco use before and after implementation of the program stayed steady at 96% of patient visits, but the percentage of tobacco users’ visits that included checking for readiness to quit within the next 30 days rose from 3% (135 of 4,078) to 80% (421 of 523).
Before the implementation of the program, 0.6% of eligible visits with current smokers included a quit-line referral offer. After implementation, 93 (18%) of the 523 smokers who visited – 122 of whom said they were ready to quit – were offered referrals, a 26-fold increase. Of the 93 offered referrals, 66 (71%) accepted and 16 set a quit date or reported having quit; 11 accepted counseling services and nicotine replacement.
Although clinic staff reported encountering several obstacles, such as the need to craft nonthreatening language for challenging patients, they also contributed their own talking points that were included in the EHR tools and desktop brochures. On average, the protocol took less than 90 seconds to perform.
Rheumatologists can make headway on patients quitting smoking
“While smoking cessation programs require time and resources to implement, this study suggests a role for evidence-based protocols within rheumatology centers,” Medha Barbhaiya, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. “Given that current smokers are at an increased risk of developing more severe rheumatic disease and cardiovascular disease, and patients often visit their rheumatologist multiple times yearly, rheumatologists may be well-positioned to address smoking cessation with patients.”
In regard to next steps, she noted that “while future large studies in diverse cohorts are needed to confirm these findings, implementing a formal smoking cessation protocol within rheumatology centers may provide a unique opportunity for rheumatologists to directly help patients modify their disease risk, leading to improved health outcomes.”
The authors acknowledged their study’s limitations, including the fact that it was a prepost design and not a randomized trial. They also recognized that many tobacco users require 8-10 attempts before permanently quitting, likely lessening the lasting impact of the short-term study. They did cite expert analysis, however, that says “connecting patients to evidence-based resources makes them more likely to permanently quit.”
The study was supported in part by Pfizer’s office of Independent Grants for Learning and Change and by a grant collaboration from the University of Wisconsin Clinical and Translational Science Award and the University of Wisconsin School of Medicine and Public Health’s Wisconsin Partnership Program, through the NIH National Center for Advancing Translational Sciences.
FROM ARTHRITIS CARE & RESEARCH
Apremilast Uses and Relevance to the Military
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.
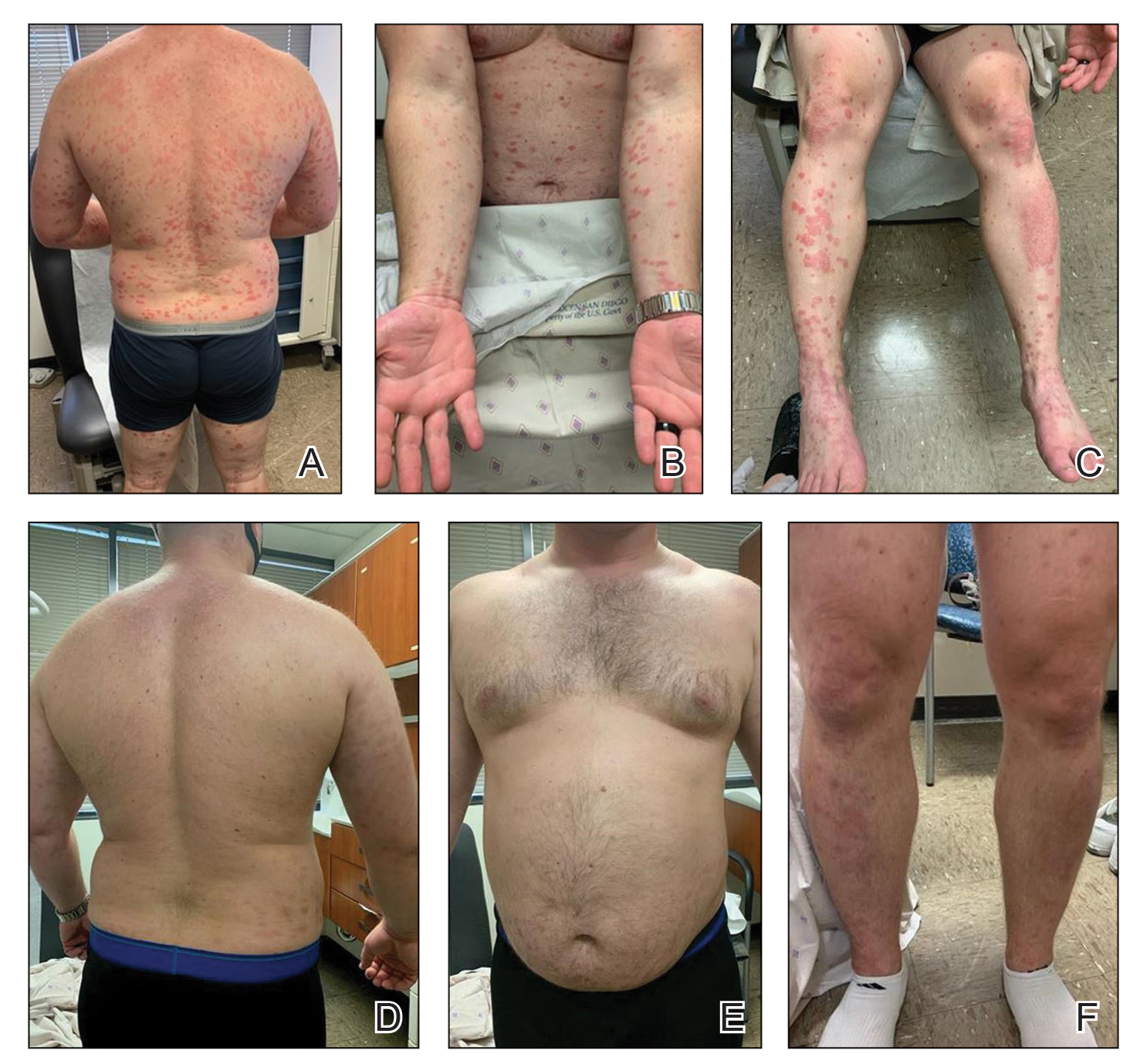
A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32
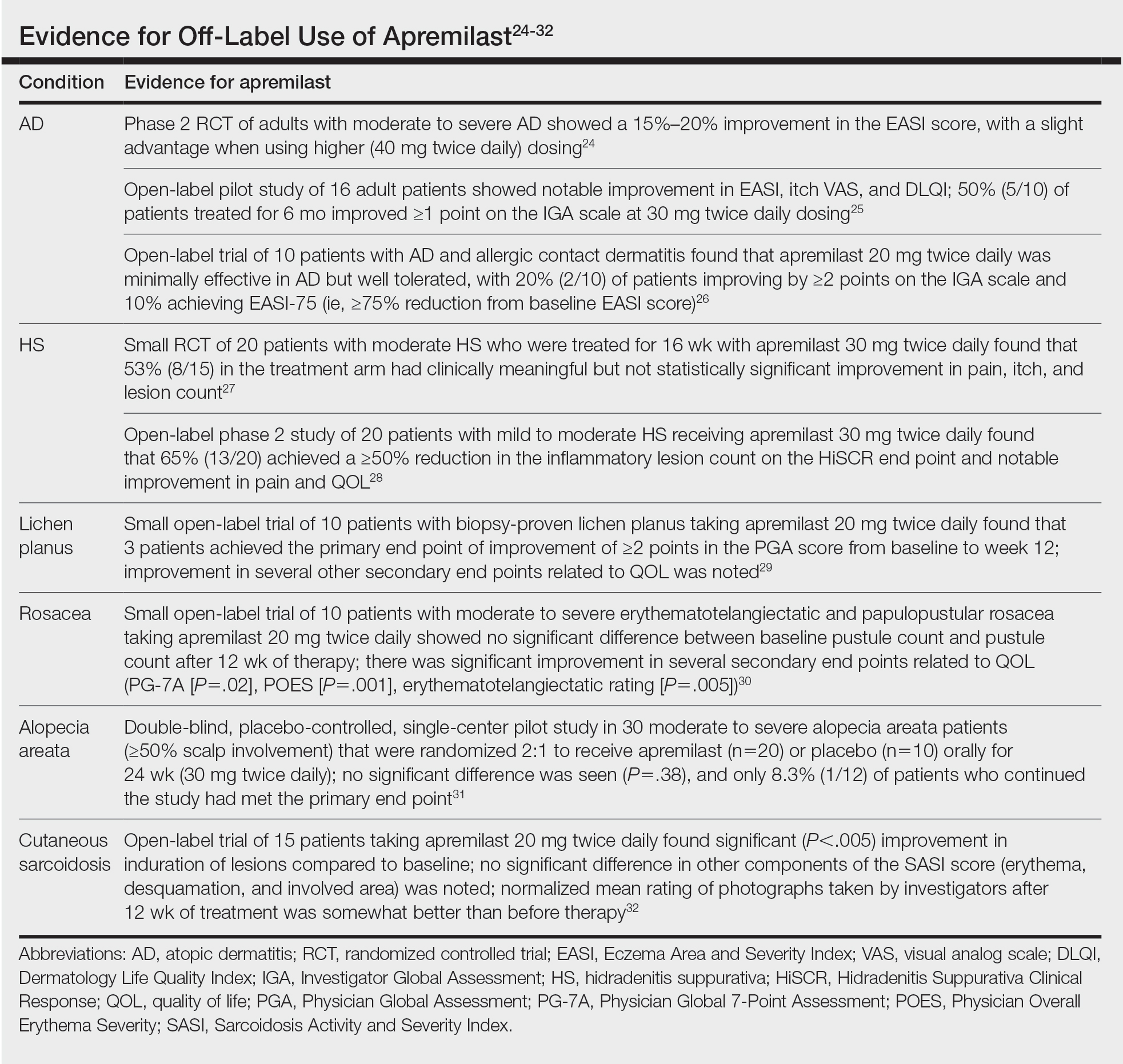
Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.

A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32

Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.

A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32

Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
Practice Points
- Apremilast is a versatile and easy-to-use therapeutic option for treatment of psoriasis and psoriatic arthritis.
- Ease of transport and storage as well as lack of necessary laboratory monitoring have made apremilast a compelling treatment option for psoriasis and psoriatic arthritis in military populations with high operational tempos.
- Dermatologists should consider apremilast for treatment in populations that work for prolonged periods in austere or resource-limited environments.