User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Watchdog group demands removal of FDA leaders after aducanumab approval
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
Incorporating self-care, wellness into routines can prevent doctors’ burnout
Gradually, we are emerging from the chaos, isolation, and anxiety of COVID-19. As the Centers for Disease Control and Prevention adjusts its recommendations and vaccinations become more widely available, our communities are beginning to return to normalcy. We are encouraged to put aside our masks if vaccinated and rejoin society, to venture out with less hesitancy and anxiety. As family and friends reunite, memories of confusion, frustration, and fear are beginning to fade to black. Despite the prevailing belief that we should move on, look forward, and remember the past to safeguard our future, remnants of the pandemic remain.
Unvaccinated individuals, notably children under the age of 12, are quite significant in number. The use of telehealth is now standard practice.
For several years, we were warned about the looming “mental health crisis.” The past year has demonstrated that a crisis no longer looms – it has arrived. Our patients can reveal the vulnerability COVID-19 has wrought – from the devastation of lives lost, supply shortages, loss of employment and financial stability – to a lack of access to computers and thereby, the risk of educational decline. Those factors, coupled with isolation and uncertainty about the future, have led to an influx of individuals with anxiety, depression, and other mood disorders seeking mental health treatment.
Doctors, others suffering
As result of a medical culture guided by the sacred oath to which care, compassion, and dedication held as true in ancient Greece as it does today, the focus centers on those around us – while signs of our own weariness are waved away as “a bad day.” Even though several support groups are readily available to offer a listening ear and mental health physicians who focus on the treatment of health care professionals are becoming more ubiquitous, the vestiges of past doctrine remain.
In this modern age of medical training, there is often as much sacrifice as there is attainment of knowledge. This philosophy is so ingrained that throughout training and practice one may come across colleagues experiencing an abundance of guilt when leave is needed for personal reasons. We are quick to recommend such steps for our patients, family, and friends, but hesitant to consider such for ourselves. Yet, of all the lessons this past year has wrought, the importance of mental health and self-care cannot be overstated. This raises the question:
It is vital to accept our humanity as something not to repair, treat, or overcome but to understand. There is strength and power in vulnerability. If we do not perceive and validate this process within ourselves, how can we do so for others? In other words, the oxygen mask must be placed on us first before we can place it on anyone else – patients or otherwise.
Chiefly and above all else, the importance of identifying individual signs of stress is essential. Where do you hold tension? Are you prone to GI distress or headaches when taxed? Do you tend toward irritability, apathy, or exhaustion?
Once this is determined, it is important to assess your stress on a numerical scale, such as those used for pain. Are you a 5 or an 8? Finally, are there identifiable triggers or reliable alleviators? Is there a time of day or day of the week that is most difficult to manage? Can you anticipate potential stressors? Understanding your triggers, listening to your body, and practicing the language of self is the first step toward wellness.
Following introspection and observation, the next step is inventory. Take stock of your reserves. What replenishes? What depletes? What brings joy? What brings dread? Are there certain activities that mitigate stress? If so, how much time do they entail? Identify your number on a scale and associate that number with specific strategies or techniques. Remember that decompression for a 6 might be excessive for a 4. Furthermore, what is the duration of these feelings? Chronic stressors may incur gradual change verses sudden impact if acute. Through identifying personal signs, devising and using a scale, as well as escalating or de-escalating factors, individuals become more in tune with their bodies and therefore, more likely to intervene before burnout takes hold.
With this process well integrated, one can now consider stylized approaches for stress management. For example, those inclined toward mindfulness practices may find yoga, meditation, and relaxation exercises beneficial. Others may thrive on positive affirmations, gratitude, and thankfulness. While some might find relief in physical activity, be it strenuous or casual, the creative arts might appeal to those who find joy in painting, writing, or doing crafts. In addition, baking, reading, dancing, and/or listening to music might help lift stress.
Along with those discoveries, or in some cases, rediscoveries, basic needs such as dietary habits and nutrition, hydration, and sleep are vital toward emotional regulation, physiological homeostasis, and stress modulation. Remember HALT: Hungry, Angry, Lonely, Tired, Too hot, Too cold, Sad or Stressed. Those strategies are meant to guide self-care and highlight the importance of allowing time for self-awareness. Imagine yourself as if you are meeting a new patient. Establish rapport, identify symptoms, and explore options for treatment. When we give time to ourselves, we can give time more freely to others. With this in mind, try following the 5-minute wellness check that I formulated:
1. How am I feeling? What am I feeling?
2. Assess HALTS.
3. Identify the number on your scale.
4. Methods of quick de-escalation:
- Designate and schedule personal time.
- Write down daily goals.
- Repeat positive affirmations or write down words of gratitude.
- Use deep breathing exercises.
- Stretch or take a brief walk.
- Engage in mindfulness practices, such as meditation.
Once we develop a habit of monitoring, assessing, and practicing self-care, the process becomes more efficient and effective. Think of the way a seasoned attending can manage workflow with ease, compared with an intern. Recognizing signs and using these strategies routinely can become a quick daily measure of well-being.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
Gradually, we are emerging from the chaos, isolation, and anxiety of COVID-19. As the Centers for Disease Control and Prevention adjusts its recommendations and vaccinations become more widely available, our communities are beginning to return to normalcy. We are encouraged to put aside our masks if vaccinated and rejoin society, to venture out with less hesitancy and anxiety. As family and friends reunite, memories of confusion, frustration, and fear are beginning to fade to black. Despite the prevailing belief that we should move on, look forward, and remember the past to safeguard our future, remnants of the pandemic remain.
Unvaccinated individuals, notably children under the age of 12, are quite significant in number. The use of telehealth is now standard practice.
For several years, we were warned about the looming “mental health crisis.” The past year has demonstrated that a crisis no longer looms – it has arrived. Our patients can reveal the vulnerability COVID-19 has wrought – from the devastation of lives lost, supply shortages, loss of employment and financial stability – to a lack of access to computers and thereby, the risk of educational decline. Those factors, coupled with isolation and uncertainty about the future, have led to an influx of individuals with anxiety, depression, and other mood disorders seeking mental health treatment.
Doctors, others suffering
As result of a medical culture guided by the sacred oath to which care, compassion, and dedication held as true in ancient Greece as it does today, the focus centers on those around us – while signs of our own weariness are waved away as “a bad day.” Even though several support groups are readily available to offer a listening ear and mental health physicians who focus on the treatment of health care professionals are becoming more ubiquitous, the vestiges of past doctrine remain.
In this modern age of medical training, there is often as much sacrifice as there is attainment of knowledge. This philosophy is so ingrained that throughout training and practice one may come across colleagues experiencing an abundance of guilt when leave is needed for personal reasons. We are quick to recommend such steps for our patients, family, and friends, but hesitant to consider such for ourselves. Yet, of all the lessons this past year has wrought, the importance of mental health and self-care cannot be overstated. This raises the question:
It is vital to accept our humanity as something not to repair, treat, or overcome but to understand. There is strength and power in vulnerability. If we do not perceive and validate this process within ourselves, how can we do so for others? In other words, the oxygen mask must be placed on us first before we can place it on anyone else – patients or otherwise.
Chiefly and above all else, the importance of identifying individual signs of stress is essential. Where do you hold tension? Are you prone to GI distress or headaches when taxed? Do you tend toward irritability, apathy, or exhaustion?
Once this is determined, it is important to assess your stress on a numerical scale, such as those used for pain. Are you a 5 or an 8? Finally, are there identifiable triggers or reliable alleviators? Is there a time of day or day of the week that is most difficult to manage? Can you anticipate potential stressors? Understanding your triggers, listening to your body, and practicing the language of self is the first step toward wellness.
Following introspection and observation, the next step is inventory. Take stock of your reserves. What replenishes? What depletes? What brings joy? What brings dread? Are there certain activities that mitigate stress? If so, how much time do they entail? Identify your number on a scale and associate that number with specific strategies or techniques. Remember that decompression for a 6 might be excessive for a 4. Furthermore, what is the duration of these feelings? Chronic stressors may incur gradual change verses sudden impact if acute. Through identifying personal signs, devising and using a scale, as well as escalating or de-escalating factors, individuals become more in tune with their bodies and therefore, more likely to intervene before burnout takes hold.
With this process well integrated, one can now consider stylized approaches for stress management. For example, those inclined toward mindfulness practices may find yoga, meditation, and relaxation exercises beneficial. Others may thrive on positive affirmations, gratitude, and thankfulness. While some might find relief in physical activity, be it strenuous or casual, the creative arts might appeal to those who find joy in painting, writing, or doing crafts. In addition, baking, reading, dancing, and/or listening to music might help lift stress.
Along with those discoveries, or in some cases, rediscoveries, basic needs such as dietary habits and nutrition, hydration, and sleep are vital toward emotional regulation, physiological homeostasis, and stress modulation. Remember HALT: Hungry, Angry, Lonely, Tired, Too hot, Too cold, Sad or Stressed. Those strategies are meant to guide self-care and highlight the importance of allowing time for self-awareness. Imagine yourself as if you are meeting a new patient. Establish rapport, identify symptoms, and explore options for treatment. When we give time to ourselves, we can give time more freely to others. With this in mind, try following the 5-minute wellness check that I formulated:
1. How am I feeling? What am I feeling?
2. Assess HALTS.
3. Identify the number on your scale.
4. Methods of quick de-escalation:
- Designate and schedule personal time.
- Write down daily goals.
- Repeat positive affirmations or write down words of gratitude.
- Use deep breathing exercises.
- Stretch or take a brief walk.
- Engage in mindfulness practices, such as meditation.
Once we develop a habit of monitoring, assessing, and practicing self-care, the process becomes more efficient and effective. Think of the way a seasoned attending can manage workflow with ease, compared with an intern. Recognizing signs and using these strategies routinely can become a quick daily measure of well-being.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
Gradually, we are emerging from the chaos, isolation, and anxiety of COVID-19. As the Centers for Disease Control and Prevention adjusts its recommendations and vaccinations become more widely available, our communities are beginning to return to normalcy. We are encouraged to put aside our masks if vaccinated and rejoin society, to venture out with less hesitancy and anxiety. As family and friends reunite, memories of confusion, frustration, and fear are beginning to fade to black. Despite the prevailing belief that we should move on, look forward, and remember the past to safeguard our future, remnants of the pandemic remain.
Unvaccinated individuals, notably children under the age of 12, are quite significant in number. The use of telehealth is now standard practice.
For several years, we were warned about the looming “mental health crisis.” The past year has demonstrated that a crisis no longer looms – it has arrived. Our patients can reveal the vulnerability COVID-19 has wrought – from the devastation of lives lost, supply shortages, loss of employment and financial stability – to a lack of access to computers and thereby, the risk of educational decline. Those factors, coupled with isolation and uncertainty about the future, have led to an influx of individuals with anxiety, depression, and other mood disorders seeking mental health treatment.
Doctors, others suffering
As result of a medical culture guided by the sacred oath to which care, compassion, and dedication held as true in ancient Greece as it does today, the focus centers on those around us – while signs of our own weariness are waved away as “a bad day.” Even though several support groups are readily available to offer a listening ear and mental health physicians who focus on the treatment of health care professionals are becoming more ubiquitous, the vestiges of past doctrine remain.
In this modern age of medical training, there is often as much sacrifice as there is attainment of knowledge. This philosophy is so ingrained that throughout training and practice one may come across colleagues experiencing an abundance of guilt when leave is needed for personal reasons. We are quick to recommend such steps for our patients, family, and friends, but hesitant to consider such for ourselves. Yet, of all the lessons this past year has wrought, the importance of mental health and self-care cannot be overstated. This raises the question:
It is vital to accept our humanity as something not to repair, treat, or overcome but to understand. There is strength and power in vulnerability. If we do not perceive and validate this process within ourselves, how can we do so for others? In other words, the oxygen mask must be placed on us first before we can place it on anyone else – patients or otherwise.
Chiefly and above all else, the importance of identifying individual signs of stress is essential. Where do you hold tension? Are you prone to GI distress or headaches when taxed? Do you tend toward irritability, apathy, or exhaustion?
Once this is determined, it is important to assess your stress on a numerical scale, such as those used for pain. Are you a 5 or an 8? Finally, are there identifiable triggers or reliable alleviators? Is there a time of day or day of the week that is most difficult to manage? Can you anticipate potential stressors? Understanding your triggers, listening to your body, and practicing the language of self is the first step toward wellness.
Following introspection and observation, the next step is inventory. Take stock of your reserves. What replenishes? What depletes? What brings joy? What brings dread? Are there certain activities that mitigate stress? If so, how much time do they entail? Identify your number on a scale and associate that number with specific strategies or techniques. Remember that decompression for a 6 might be excessive for a 4. Furthermore, what is the duration of these feelings? Chronic stressors may incur gradual change verses sudden impact if acute. Through identifying personal signs, devising and using a scale, as well as escalating or de-escalating factors, individuals become more in tune with their bodies and therefore, more likely to intervene before burnout takes hold.
With this process well integrated, one can now consider stylized approaches for stress management. For example, those inclined toward mindfulness practices may find yoga, meditation, and relaxation exercises beneficial. Others may thrive on positive affirmations, gratitude, and thankfulness. While some might find relief in physical activity, be it strenuous or casual, the creative arts might appeal to those who find joy in painting, writing, or doing crafts. In addition, baking, reading, dancing, and/or listening to music might help lift stress.
Along with those discoveries, or in some cases, rediscoveries, basic needs such as dietary habits and nutrition, hydration, and sleep are vital toward emotional regulation, physiological homeostasis, and stress modulation. Remember HALT: Hungry, Angry, Lonely, Tired, Too hot, Too cold, Sad or Stressed. Those strategies are meant to guide self-care and highlight the importance of allowing time for self-awareness. Imagine yourself as if you are meeting a new patient. Establish rapport, identify symptoms, and explore options for treatment. When we give time to ourselves, we can give time more freely to others. With this in mind, try following the 5-minute wellness check that I formulated:
1. How am I feeling? What am I feeling?
2. Assess HALTS.
3. Identify the number on your scale.
4. Methods of quick de-escalation:
- Designate and schedule personal time.
- Write down daily goals.
- Repeat positive affirmations or write down words of gratitude.
- Use deep breathing exercises.
- Stretch or take a brief walk.
- Engage in mindfulness practices, such as meditation.
Once we develop a habit of monitoring, assessing, and practicing self-care, the process becomes more efficient and effective. Think of the way a seasoned attending can manage workflow with ease, compared with an intern. Recognizing signs and using these strategies routinely can become a quick daily measure of well-being.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
AMA acknowledges medical education racism of past, vows better future
The report received overwhelming support at the House of Delegates, the AMA’s legislative policy making body, during an online meeting held June 13.
The Council on Medical Education’s report recommends that the AMA acknowledge the harm caused by the Flexner Report, which was issued in 1910 and has since shaped medical education. The Flexner Report caused harm not only to historically Black medical schools, but also to physician workforce diversity and to the clinical outcomes of minority and marginalized patients, according to the medical education advisory body.
The council also recommended conducting a study on medical education with a focus on health equity and racial justice, improving diversity among healthcare workers, and fixing inequitable outcomes from minorities and marginalized patient populations.
The report comes on the heels of the resignation of JAMA editor-in-chief Howard Bauchner, MD, and another high-ranking editor following a February podcast on systemic racism in medicine. The AMA has since released a strategic plan addressing racism and health inequity that has divided membership.
Flexner Report’s effect on physician diversity
The Council on Medical Education’s report observed that as a result of the Flexner Report’s recommendations, 89 medical schools, including 5 of the 7 existing medical schools training Black physicians, were closed because they didn’t meet the report’s standards. In addition, the report created a limited role for Black physicians while “hint[ing] that Black physicians possessed less potential and ability than their White counterparts,” read the Council’s report.
In addition to consigning the role of the Black physician to “educating the [Black] race to know and to practice fundamental hygienic principles,” the Flexner Report also observed that “a well-taught negro sanitarian will be immensely useful,” per the Council’s report.
The impact of the closure of medical schools training Black physicians was dramatic. According to the Council’s report, in 1964, 93% of medical students in the United States were men and 97% of those students were non-Hispanic White.
Today, 56% of physicians identify as White, 17% as Asian, 6% as Hispanic, and 5% as Black or African American, per the Association of American Medical Colleges; nearly 14% of active physicians didn’t report their race in the survey. By means of contrast, the U.S. population in 2019 was 60% White, 19% Latino/Hispanic, 13% Black or African American, and 6% Asian American, according to the Brookings Institute.
Abraham Flexner, who wrote the Flexner Report, is often referred to as the “father of modern medical education,” according to the AAMC. In November, the AAMC observed that the Flexner Report contained racist and sexist ideas and that his work contributed to the closure of historically Black medical schools. Both statements were included in AAMC’s announcement about the removal of Flexner’s name from its most prestigious award. As of January, the award is now called the AAMC Award for Excellence in Medical Education.
Pathway programs can increase diversity
Pathway programs, which leverage targeted milestones along the journey to becoming a physician in order to increase diversity, were an area of focus in the council’s report. These programs “can exert a meaningful, positive effect on student outcomes and increase diversity across various levels of educational settings,” according to its report.
Centers of Excellence, which provides grants for mentorship and training programs, is one of many pathway programs. During the 2018-2019 academic year, Centers of Excellence supported more than 1,300 trainees – 99% of them were underrepresented minorities and 64% came from financially or educationally disadvantaged backgrounds. In 2006, federal funding was cut to these programs and the number of Centers of Excellence fell.
Still, the report cites the passage of federal funding in 2020 of $50 million for public institutions of higher education that train physicians; educational institutions in states with a projected primary care shortage in 2025 are given priority in the grant-funding process.
AMA council’s report garners support from delegates
Delegates voiced overwhelming support of the council’s report during the June 13 meeting. Lou Edje, MD, a Perrysburgh, Ohio–based family physician, voiced strong support for the council’s report, in particular its recommendations that recognize the harm caused by the Flexner Report. Dr. Edje observed that the Flexner Report, with its elimination of five of seven Black medical schools, “[set] back admissions of Black students into medicine by 50 years.”
“Empathy is what we are called to have as physicians. I implore you to simply substitute your ethnicity into these quotes to help understand the historic need for health equity in medicine today. This CME report is part of the antidote to Flexner. We support [it] fully,” concluded Dr. Edje, who spoke for the Great Lakes States Coalition of the AMA.
Rohan Khazanchi, a medical student at the University of Nebraska, Omaha, and a member of the council, said, “Our broad attempt with this report was twofold: to fill gaps in AMA policy with evidence-based recommendations which could improve diversity in our health workforce and, second, to enhance our organization’s vision for truth, reconciliation, and healing to redress the historic marginalization of minoritized physicians in medicine.”
According to an AMA spokesperson, the House of Delegates will vote on this and other policies this week, after which the policies are considered final.
A version of this article first appeared on Medscape.com.
The report received overwhelming support at the House of Delegates, the AMA’s legislative policy making body, during an online meeting held June 13.
The Council on Medical Education’s report recommends that the AMA acknowledge the harm caused by the Flexner Report, which was issued in 1910 and has since shaped medical education. The Flexner Report caused harm not only to historically Black medical schools, but also to physician workforce diversity and to the clinical outcomes of minority and marginalized patients, according to the medical education advisory body.
The council also recommended conducting a study on medical education with a focus on health equity and racial justice, improving diversity among healthcare workers, and fixing inequitable outcomes from minorities and marginalized patient populations.
The report comes on the heels of the resignation of JAMA editor-in-chief Howard Bauchner, MD, and another high-ranking editor following a February podcast on systemic racism in medicine. The AMA has since released a strategic plan addressing racism and health inequity that has divided membership.
Flexner Report’s effect on physician diversity
The Council on Medical Education’s report observed that as a result of the Flexner Report’s recommendations, 89 medical schools, including 5 of the 7 existing medical schools training Black physicians, were closed because they didn’t meet the report’s standards. In addition, the report created a limited role for Black physicians while “hint[ing] that Black physicians possessed less potential and ability than their White counterparts,” read the Council’s report.
In addition to consigning the role of the Black physician to “educating the [Black] race to know and to practice fundamental hygienic principles,” the Flexner Report also observed that “a well-taught negro sanitarian will be immensely useful,” per the Council’s report.
The impact of the closure of medical schools training Black physicians was dramatic. According to the Council’s report, in 1964, 93% of medical students in the United States were men and 97% of those students were non-Hispanic White.
Today, 56% of physicians identify as White, 17% as Asian, 6% as Hispanic, and 5% as Black or African American, per the Association of American Medical Colleges; nearly 14% of active physicians didn’t report their race in the survey. By means of contrast, the U.S. population in 2019 was 60% White, 19% Latino/Hispanic, 13% Black or African American, and 6% Asian American, according to the Brookings Institute.
Abraham Flexner, who wrote the Flexner Report, is often referred to as the “father of modern medical education,” according to the AAMC. In November, the AAMC observed that the Flexner Report contained racist and sexist ideas and that his work contributed to the closure of historically Black medical schools. Both statements were included in AAMC’s announcement about the removal of Flexner’s name from its most prestigious award. As of January, the award is now called the AAMC Award for Excellence in Medical Education.
Pathway programs can increase diversity
Pathway programs, which leverage targeted milestones along the journey to becoming a physician in order to increase diversity, were an area of focus in the council’s report. These programs “can exert a meaningful, positive effect on student outcomes and increase diversity across various levels of educational settings,” according to its report.
Centers of Excellence, which provides grants for mentorship and training programs, is one of many pathway programs. During the 2018-2019 academic year, Centers of Excellence supported more than 1,300 trainees – 99% of them were underrepresented minorities and 64% came from financially or educationally disadvantaged backgrounds. In 2006, federal funding was cut to these programs and the number of Centers of Excellence fell.
Still, the report cites the passage of federal funding in 2020 of $50 million for public institutions of higher education that train physicians; educational institutions in states with a projected primary care shortage in 2025 are given priority in the grant-funding process.
AMA council’s report garners support from delegates
Delegates voiced overwhelming support of the council’s report during the June 13 meeting. Lou Edje, MD, a Perrysburgh, Ohio–based family physician, voiced strong support for the council’s report, in particular its recommendations that recognize the harm caused by the Flexner Report. Dr. Edje observed that the Flexner Report, with its elimination of five of seven Black medical schools, “[set] back admissions of Black students into medicine by 50 years.”
“Empathy is what we are called to have as physicians. I implore you to simply substitute your ethnicity into these quotes to help understand the historic need for health equity in medicine today. This CME report is part of the antidote to Flexner. We support [it] fully,” concluded Dr. Edje, who spoke for the Great Lakes States Coalition of the AMA.
Rohan Khazanchi, a medical student at the University of Nebraska, Omaha, and a member of the council, said, “Our broad attempt with this report was twofold: to fill gaps in AMA policy with evidence-based recommendations which could improve diversity in our health workforce and, second, to enhance our organization’s vision for truth, reconciliation, and healing to redress the historic marginalization of minoritized physicians in medicine.”
According to an AMA spokesperson, the House of Delegates will vote on this and other policies this week, after which the policies are considered final.
A version of this article first appeared on Medscape.com.
The report received overwhelming support at the House of Delegates, the AMA’s legislative policy making body, during an online meeting held June 13.
The Council on Medical Education’s report recommends that the AMA acknowledge the harm caused by the Flexner Report, which was issued in 1910 and has since shaped medical education. The Flexner Report caused harm not only to historically Black medical schools, but also to physician workforce diversity and to the clinical outcomes of minority and marginalized patients, according to the medical education advisory body.
The council also recommended conducting a study on medical education with a focus on health equity and racial justice, improving diversity among healthcare workers, and fixing inequitable outcomes from minorities and marginalized patient populations.
The report comes on the heels of the resignation of JAMA editor-in-chief Howard Bauchner, MD, and another high-ranking editor following a February podcast on systemic racism in medicine. The AMA has since released a strategic plan addressing racism and health inequity that has divided membership.
Flexner Report’s effect on physician diversity
The Council on Medical Education’s report observed that as a result of the Flexner Report’s recommendations, 89 medical schools, including 5 of the 7 existing medical schools training Black physicians, were closed because they didn’t meet the report’s standards. In addition, the report created a limited role for Black physicians while “hint[ing] that Black physicians possessed less potential and ability than their White counterparts,” read the Council’s report.
In addition to consigning the role of the Black physician to “educating the [Black] race to know and to practice fundamental hygienic principles,” the Flexner Report also observed that “a well-taught negro sanitarian will be immensely useful,” per the Council’s report.
The impact of the closure of medical schools training Black physicians was dramatic. According to the Council’s report, in 1964, 93% of medical students in the United States were men and 97% of those students were non-Hispanic White.
Today, 56% of physicians identify as White, 17% as Asian, 6% as Hispanic, and 5% as Black or African American, per the Association of American Medical Colleges; nearly 14% of active physicians didn’t report their race in the survey. By means of contrast, the U.S. population in 2019 was 60% White, 19% Latino/Hispanic, 13% Black or African American, and 6% Asian American, according to the Brookings Institute.
Abraham Flexner, who wrote the Flexner Report, is often referred to as the “father of modern medical education,” according to the AAMC. In November, the AAMC observed that the Flexner Report contained racist and sexist ideas and that his work contributed to the closure of historically Black medical schools. Both statements were included in AAMC’s announcement about the removal of Flexner’s name from its most prestigious award. As of January, the award is now called the AAMC Award for Excellence in Medical Education.
Pathway programs can increase diversity
Pathway programs, which leverage targeted milestones along the journey to becoming a physician in order to increase diversity, were an area of focus in the council’s report. These programs “can exert a meaningful, positive effect on student outcomes and increase diversity across various levels of educational settings,” according to its report.
Centers of Excellence, which provides grants for mentorship and training programs, is one of many pathway programs. During the 2018-2019 academic year, Centers of Excellence supported more than 1,300 trainees – 99% of them were underrepresented minorities and 64% came from financially or educationally disadvantaged backgrounds. In 2006, federal funding was cut to these programs and the number of Centers of Excellence fell.
Still, the report cites the passage of federal funding in 2020 of $50 million for public institutions of higher education that train physicians; educational institutions in states with a projected primary care shortage in 2025 are given priority in the grant-funding process.
AMA council’s report garners support from delegates
Delegates voiced overwhelming support of the council’s report during the June 13 meeting. Lou Edje, MD, a Perrysburgh, Ohio–based family physician, voiced strong support for the council’s report, in particular its recommendations that recognize the harm caused by the Flexner Report. Dr. Edje observed that the Flexner Report, with its elimination of five of seven Black medical schools, “[set] back admissions of Black students into medicine by 50 years.”
“Empathy is what we are called to have as physicians. I implore you to simply substitute your ethnicity into these quotes to help understand the historic need for health equity in medicine today. This CME report is part of the antidote to Flexner. We support [it] fully,” concluded Dr. Edje, who spoke for the Great Lakes States Coalition of the AMA.
Rohan Khazanchi, a medical student at the University of Nebraska, Omaha, and a member of the council, said, “Our broad attempt with this report was twofold: to fill gaps in AMA policy with evidence-based recommendations which could improve diversity in our health workforce and, second, to enhance our organization’s vision for truth, reconciliation, and healing to redress the historic marginalization of minoritized physicians in medicine.”
According to an AMA spokesperson, the House of Delegates will vote on this and other policies this week, after which the policies are considered final.
A version of this article first appeared on Medscape.com.
The aducanumab revolution
The approval was hailed by advocacy groups and some practitioners as a victory for patients and families, as the drug – the first anti-Alzheimer’s agent to reach the market in 18 years – is a potentially disease-modifying therapy, which acts to clear amyloid plaques from the brain.
But several prominent Alzheimer’s researchers lambasted the agency’s decision, citing unclear evidence of benefit, trials that did not meet their primary endpoints, and reliance on a post hoc analysis of a high-dose subgroup of patients in a halted trial to argue that aducanumab (Aduhelm, Biogen, and Eisai), slowed cognitive and functional decline by 22% on one measure. In November 2020, 10 of 11 members of an independent FDA advisory committee voted against aducanumab’s approval, citing holes in the data and concerns about the quality of the evidence. After the agency went on to approve anyway, three members of that committee resigned in protest.
The FDA decision on aducanumab was made using the agency’s accelerated approval pathway, which allows for the use of a surrogate endpoint – in this case imaging that showed amyloid clearance from the brain – to predict clinical benefit. But amyloid clearance, which a number of experimental antiamyloid antibodies have been shown capable of, has not been definitively linked to clinical benefit. Aducanumab, which is delivered by monthly intravenous infusion, will be marketed pending results from a phase 4 clinical trial, which the manufacturer has nearly a decade to complete. The drug’s price was announced at $56,000 per year, underscoring concern over its modest-at-best benefits.
Clinicians prescribing aducanumab must obtain magnetic resonance imaging at baseline and repeatedly during the course of treatment to detect brain edema and microhemorrhages, which occurred in a third of high-dose patients in clinical trials. Beyond this, there are few restrictions. The FDA label allows for its use in any patient deemed to have Alzheimer’s disease, without stipulations as to disease stage or evidence of brain amyloid. Payers, of course, are likely to restrict use to certain patient groups, and to require evidence of amyloid positivity. The FDA offered no guidance on when treatment should be ceased, leaving payers to make that call as well. Whatever aducanumab’s value and role turns out to be, the first-in-class treatment for Alzheimer’s disease is likely to have a major impact on how patients are assessed and treated in the coming years, and embolden manufactures of similar agents to seek FDA approval.
This news organization reached out to researchers, advocates, and specialists in the community to learn how they see this change playing out.
Fielding broad interest
Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association, which was a strong proponent of aducanumab’s approval, acknowledged in an interview that the months to come are likely to be confusing for practitioners and families alike as the drug makes its way into community practices.
“We understand that off the bat millions of Americans will not have access to this tomorrow, but over time that will build. And the physician community, the specialists most likely to be prescribing this, over the next few years will even expand further,” Dr. Carrillo said.
For now, those specialists are mostly just struggling to respond responsibly to a deluge of inquiries from patients and their families.
“I’ve gotten like 20 calls in the just the past 2 days,” said neurologist Philip R. Delio, MD, who practices in Santa Barbara, Calif. “This is a longstanding issue that physicians have with patients’ access to information. Patients are getting information about a drug which isn’t available yet. They don’t know that it’s not ready to be sold. They don’t necessarily realize that a biopharma company won’t go into production until the FDA approves the drug.”
Many patients, Dr. Delio said, are aware of the controversy surrounding aducanumab and eager to hear their neurologist’s opinion. “I have tried to let them know that I want to see the trial data and to better understand the FDA’s rationale in approving it. I always caution patients that the devil will be in the details.”
While aducanumab’s label gives physicians remarkably wide latitude in whom to treat, clinicians say that until payers weigh in, the label is all but meaningless. Neurologist Douglas Scharre, MD, of the Ohio State University Wexner Medical Center, and a site investigator on a trial of aducanumab, said that he and his colleagues at the university’s memory center have tried to anticipate who might be deemed eligible by triaging calls.
Dr. Scharre and colleagues have been working under the assumption that payers will support aducanumab only for patients like those who seemed to benefit in the trials – people with mild cognitive impairment (MCI) or in the earliest stages of dementia with evidence of brain amyloid.
“I don’t want to fill up our new patient slots with people who are not even appropriate for this drug,” Dr. Scharre said. “We have a call center, and we have a few triage questions. After that a nurse practitioner collects some more data, and there’s a review process. Only then do we decide whether that person could be a candidate. If we deem that they are, we will want them in and to order an amyloid PET” – a type of brain scan that is seldom used outside research settings and not reimbursed by Medicare.
Dr. Scharre predicts that regardless of payer limitations, “there will be people hounding for the drug who are not appropriate for the drug. There will be very wealthy people who will want to pay for tests and get it no matter what.” Another concern, he said, was that having poorly selected patients on the drug could make definitive trial results even more elusive.
“The label the way it’s written is not going to help the drug in phase 4 trials,” he said. “It’s good to have real-world patient data, but if you have all these people in your cohort who are too early or too late, you won’t have good results.”
The challenge of delivery
Intravenous infusions are new to Alzheimer’s disease and pose all sorts of logistical hurdles. The Alzheimer’s Association’s Dr. Carrillo described the situation as “manageable,” noting that infusions are standard of care for many diseases, and that neurologists now have more than 15 years’ experience with them for multiple sclerosis.
Still, most clinicians treating Alzheimer’s disease in the community – neurologists, geriatricians, psychiatrists, and primary care physicians – do not have infusion centers in their practices. Virtually none have experience with or access to PET-amyloid, or with screening for amyloid-related imaging abnormalities–edema (ARIA-e) on MRI, as required by the FDA.
“I contacted the hospital infusion center we use and said I could end up sending five or six patients a week, can you handle this? They only have so many chairs,” Dr. Delio said. “I am one neurologist in a local community, and I might have 50 candidates for this drug. That’s a lot for them.” Patients with cognitive impairment are also difficult to infuse and may need to be treated at home, he noted.
“MRIs are easy enough to do,” Dr. Delio said. “But do we know what ARIA-e looks like on imaging? You’d have to talk to the radiologists – this is another element of uncertainty. Do we even know what we’re looking for with these scans? Will we recognize this?”
Neurologist Jeffrey L. Cummings, MD, ScD, of the University of Nevada, Las Vegas, a vocal proponent of aducanumab and lead author of a May 2021 paper defending the evidence for it, acknowledged that the field was unprepared for a wide-scale adoption of infusions in dementia treatment, pointing to a Rand Corporation study from 2017 that warned that screening, diagnosis, and availability of infusion chairs would have to be drastically scaled up to meet demand.
“There are few clinicians who know how to identify MCI, too few imaging centers, too few radiologists who know how to identify ARIA-e on MRI, so all of these things will be required to be put into place. The label doesn’t specify any of this, but good clinical practice will require that, and getting this up and running will take 18 to 24 months,” Dr. Cummings said.
Neurologist David S. Knopman, MD, of the Mayo Clinic in Rochester, Minn., a leading critic of the evidence for aducanumab who recently resigned his position on the independent committee that advises the FDA on neurology drugs, said that for large research institutions like his that have served as trial sites, the transition to offering PET-amyloid, MRI, and infusions in clinical practice will be easier.
“We have all this because this is what we do every day. And we have a very extensive understanding of MCI and mild dementia staging,” Dr. Knopman said. “But the amount of infrastructure that is implied by this, and all the extra steps it would take, would be a real challenge for people in general neurology practice.”
In addition to routine use of PET-amyloid and MRI screening for ARIA-e, Dr. Knopman said, clinicians will have to provide genetic screening and counseling before administering aducanumab, as clinical trials showed that treated patients have a higher risk of developing ARIA-e if they have APOE4, a risk variant for Alzheimer’s disease. “And that has real implications for the families and the children of patients,” he said.
Uncertainty over costs
Aducanumab’s true costs, to patients and to taxpayers, remain unknown. The $56,000 per year currently cited by its manufacturer “doesn’t count the PET scans and MRIs,” Dr. Knopman noted. “We’re probably pushing $100,00 a year for the first year of treatment.”
Most of that expense will likely be borne by Medicare, he said, and if not, “that will exacerbate existing health care disparities. People who can pay out of pocket are a pretty limited group.”
Dr. Scharre agreed that the costs of treatment were concerning, and that “at least you should be able to narrow it down and hopefully just use health care dollars for people who might stand to benefit,” he said – namely patients in an earlier stage of disease.
The Alzheimer’s Association’s Dr. Carrillo declined to address the high price of aducanumab or its implications, saying only that the association is “very invested in all aspects of access including covering costs associated with the drug and the rest of treatment.”
Access also means “infrastructure, access to physicians to diagnose, access to diagnostics,” Dr. Carrillo said.
Dr. Cummings said aducanumab’s price would likely come down through negotiations with the Centers for Medicare & Medicaid Services, copayments, and bulk purchases.
The FDA has offered no guidance on how long treatment with aducanumab should last, or what should prompt withdrawal of treatment, meaning that patients could, in theory, stay on it to the end of their lives – raising costs further.
Critics have also noted that a built-in financial incentive under Medicare Part B, which covers infusion drugs, could result in overprescription of aducanumab. Under Medicare Part B, prescribing physicians are reimbursed 6% of a drug’s average sales price.
Geriatricians wary
On social media and in the lay press, geriatricians have been among the most outspoken opponents of the FDA decision and the Alzheimer’s Association’s advocacy of aducanumab.
Eric Widera, MD, a geriatrician at the University of California, San Francisco, said that the specialty might be less likely than others to embrace aducanumab. “I think part of the reasons geriatricians don’t make a lot of money is they have strong commitment to their values,” Dr. Widera said.
The American Geriatrics Society opposed the drug’s approval, citing concerns about evidence, side effects, and cost. “Additional considerations are the unintended consequences of overstressing Medicare’s limited financial reserves, and of challenging health care systems … to divert precious resources to an expensive treatment of uncertain value,” the society’s president, Peter Hollmann, MD, and chief executive officer, Nancy E. Lundebjerg, wrote in a June 2 letter to the FDA.
Dr. Widera said the approval was likely to undermine confidence in the FDA and in the Alzheimer’s Association, which receives significant funding from drug manufacturers, including Biogen and Eisai. “There’s a lot of reasons that the Geriatrics Society could have done what the Alzheimer’s Association did, and yet they came out against it, which I applaud.”
Dr. Widera pointed to a study showing that dementia patients were less likely to be on an antidementia drug if they were treated by a geriatrician, compared with a psychiatrist or a neurologist. But whether the specialty will prove as cautious with aducanumab remains to be seen. Some geriatricians will be tempted to open lucrative infusion centers, he predicted.
What is especially worrisome, Dr. Widera said, is that aducanumab’s label offers no guidance as to when to withdraw treatment. “We’ll probably see something similar to what happened with the cholinesterase inhibitors” – the class of marginally effective antidementia drugs that includes donepezil (Aricept, Pfizer) and rivastigmine (Exelon, Novartis). “No one thinks about deprescribing them. People are prescribed them even in their last months of life. There is no reason to think these infusions won’t be continued for a very long time, well beyond how long people were dosed in the trials.”
“Taking care of someone with dementia is hard enough,” Dr. Widera added. “We can’t even get normal support in the home for someone with dementia. But we are more than happy to throw money to Biogen for a drug they have not yet showed benefit for. Hopefully in 5 years we’ll have a drug that actually works,” Dr. Widera said. “After 5 years of giving this to people at $50,000 a year.”
A fractured research community
Ever since October 2019, when Biogen and Eisai announced that despite two trials halted for futility, they would go ahead and seek FDA approval for aducanumab, the Alzheimer’s research community has been bitterly divided over the drug and the FDA’s accelerated approval process.
Top researchers published critical editorials in journals, with some eventually taking their case to major newspapers as well. The Alzheimer’s Association’s position on the drug has clashed with that of many researchers whose work it supports.
“The Alzheimer’s community has been wonderfully collegial – we all have a common purpose,” Dr. Cummings said. “Now we have people taking extreme positions and I’m hoping this will not result in a permanent fracturing of the community.”
Chief among the critics’ concerns is that the FDA decision ratified the use of antiamyloid therapies based on biomarker evidence, opening the door for makers of similar drugs – those still under development or even those whose development has been halted – to seek approval on weak evidence of clinical benefit.
Whether the approval will chill research into drugs targeting pathways other than amyloid is uncertain.
Dr. Cummings said he felt that while the aducanumab decision would spur other manufacturers of antiamyloid drugs to seek accelerated approval, other classes of Alzheimer’s therapies in development also stand to get a boost. Many Alzheimer’s experts believe that a combination of drugs targeting different elements of the disease pathway – not just amyloid – will be needed in the long run.
Dr. Scharre said that the buzz over aducanumab’s approval will have at least one concrete benefit: people getting into doctors’ offices sooner.
“The people who come into our memory centers represent only a fraction of people walking around with MCI – there are people out there who may have heard that it’s normal aging; they have decreased insight; there’s denial, there’s embarrassment – there’s hundreds of reasons people avoid getting seen,” he said.
“Perhaps they come in and learn that they don’t have any degenerative process but their thyroid is out of whack, or there’s something else causing cognitive impairment. And if they do have a degenerative process, they’ll have time to start [aducanumab], and hopefully get to see a reduction in the decline.”
Dr. Knopman was a site investigator for the Biogen aducanumab trials and has consulted for Samus Therapeutics, Third Rock, Roche, and Alzeca Biosciences. A former member of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee, he was recused from the Nov. 6, 2020, meeting that voted against aducanumab. Dr. Cummings has consulted for Biogen, Eisai, and other manufacturers. Dr. Scharre reports financial relationships with Biogen, Brain Test, Acadia, and Vascular Scientific. Dr. Widera has no disclosures. Dr. Delio is a speaker for Gore Medical, Allergan, and Biohaven Pharmaceuticals.
The approval was hailed by advocacy groups and some practitioners as a victory for patients and families, as the drug – the first anti-Alzheimer’s agent to reach the market in 18 years – is a potentially disease-modifying therapy, which acts to clear amyloid plaques from the brain.
But several prominent Alzheimer’s researchers lambasted the agency’s decision, citing unclear evidence of benefit, trials that did not meet their primary endpoints, and reliance on a post hoc analysis of a high-dose subgroup of patients in a halted trial to argue that aducanumab (Aduhelm, Biogen, and Eisai), slowed cognitive and functional decline by 22% on one measure. In November 2020, 10 of 11 members of an independent FDA advisory committee voted against aducanumab’s approval, citing holes in the data and concerns about the quality of the evidence. After the agency went on to approve anyway, three members of that committee resigned in protest.
The FDA decision on aducanumab was made using the agency’s accelerated approval pathway, which allows for the use of a surrogate endpoint – in this case imaging that showed amyloid clearance from the brain – to predict clinical benefit. But amyloid clearance, which a number of experimental antiamyloid antibodies have been shown capable of, has not been definitively linked to clinical benefit. Aducanumab, which is delivered by monthly intravenous infusion, will be marketed pending results from a phase 4 clinical trial, which the manufacturer has nearly a decade to complete. The drug’s price was announced at $56,000 per year, underscoring concern over its modest-at-best benefits.
Clinicians prescribing aducanumab must obtain magnetic resonance imaging at baseline and repeatedly during the course of treatment to detect brain edema and microhemorrhages, which occurred in a third of high-dose patients in clinical trials. Beyond this, there are few restrictions. The FDA label allows for its use in any patient deemed to have Alzheimer’s disease, without stipulations as to disease stage or evidence of brain amyloid. Payers, of course, are likely to restrict use to certain patient groups, and to require evidence of amyloid positivity. The FDA offered no guidance on when treatment should be ceased, leaving payers to make that call as well. Whatever aducanumab’s value and role turns out to be, the first-in-class treatment for Alzheimer’s disease is likely to have a major impact on how patients are assessed and treated in the coming years, and embolden manufactures of similar agents to seek FDA approval.
This news organization reached out to researchers, advocates, and specialists in the community to learn how they see this change playing out.
Fielding broad interest
Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association, which was a strong proponent of aducanumab’s approval, acknowledged in an interview that the months to come are likely to be confusing for practitioners and families alike as the drug makes its way into community practices.
“We understand that off the bat millions of Americans will not have access to this tomorrow, but over time that will build. And the physician community, the specialists most likely to be prescribing this, over the next few years will even expand further,” Dr. Carrillo said.
For now, those specialists are mostly just struggling to respond responsibly to a deluge of inquiries from patients and their families.
“I’ve gotten like 20 calls in the just the past 2 days,” said neurologist Philip R. Delio, MD, who practices in Santa Barbara, Calif. “This is a longstanding issue that physicians have with patients’ access to information. Patients are getting information about a drug which isn’t available yet. They don’t know that it’s not ready to be sold. They don’t necessarily realize that a biopharma company won’t go into production until the FDA approves the drug.”
Many patients, Dr. Delio said, are aware of the controversy surrounding aducanumab and eager to hear their neurologist’s opinion. “I have tried to let them know that I want to see the trial data and to better understand the FDA’s rationale in approving it. I always caution patients that the devil will be in the details.”
While aducanumab’s label gives physicians remarkably wide latitude in whom to treat, clinicians say that until payers weigh in, the label is all but meaningless. Neurologist Douglas Scharre, MD, of the Ohio State University Wexner Medical Center, and a site investigator on a trial of aducanumab, said that he and his colleagues at the university’s memory center have tried to anticipate who might be deemed eligible by triaging calls.
Dr. Scharre and colleagues have been working under the assumption that payers will support aducanumab only for patients like those who seemed to benefit in the trials – people with mild cognitive impairment (MCI) or in the earliest stages of dementia with evidence of brain amyloid.
“I don’t want to fill up our new patient slots with people who are not even appropriate for this drug,” Dr. Scharre said. “We have a call center, and we have a few triage questions. After that a nurse practitioner collects some more data, and there’s a review process. Only then do we decide whether that person could be a candidate. If we deem that they are, we will want them in and to order an amyloid PET” – a type of brain scan that is seldom used outside research settings and not reimbursed by Medicare.
Dr. Scharre predicts that regardless of payer limitations, “there will be people hounding for the drug who are not appropriate for the drug. There will be very wealthy people who will want to pay for tests and get it no matter what.” Another concern, he said, was that having poorly selected patients on the drug could make definitive trial results even more elusive.
“The label the way it’s written is not going to help the drug in phase 4 trials,” he said. “It’s good to have real-world patient data, but if you have all these people in your cohort who are too early or too late, you won’t have good results.”
The challenge of delivery
Intravenous infusions are new to Alzheimer’s disease and pose all sorts of logistical hurdles. The Alzheimer’s Association’s Dr. Carrillo described the situation as “manageable,” noting that infusions are standard of care for many diseases, and that neurologists now have more than 15 years’ experience with them for multiple sclerosis.
Still, most clinicians treating Alzheimer’s disease in the community – neurologists, geriatricians, psychiatrists, and primary care physicians – do not have infusion centers in their practices. Virtually none have experience with or access to PET-amyloid, or with screening for amyloid-related imaging abnormalities–edema (ARIA-e) on MRI, as required by the FDA.
“I contacted the hospital infusion center we use and said I could end up sending five or six patients a week, can you handle this? They only have so many chairs,” Dr. Delio said. “I am one neurologist in a local community, and I might have 50 candidates for this drug. That’s a lot for them.” Patients with cognitive impairment are also difficult to infuse and may need to be treated at home, he noted.
“MRIs are easy enough to do,” Dr. Delio said. “But do we know what ARIA-e looks like on imaging? You’d have to talk to the radiologists – this is another element of uncertainty. Do we even know what we’re looking for with these scans? Will we recognize this?”
Neurologist Jeffrey L. Cummings, MD, ScD, of the University of Nevada, Las Vegas, a vocal proponent of aducanumab and lead author of a May 2021 paper defending the evidence for it, acknowledged that the field was unprepared for a wide-scale adoption of infusions in dementia treatment, pointing to a Rand Corporation study from 2017 that warned that screening, diagnosis, and availability of infusion chairs would have to be drastically scaled up to meet demand.
“There are few clinicians who know how to identify MCI, too few imaging centers, too few radiologists who know how to identify ARIA-e on MRI, so all of these things will be required to be put into place. The label doesn’t specify any of this, but good clinical practice will require that, and getting this up and running will take 18 to 24 months,” Dr. Cummings said.
Neurologist David S. Knopman, MD, of the Mayo Clinic in Rochester, Minn., a leading critic of the evidence for aducanumab who recently resigned his position on the independent committee that advises the FDA on neurology drugs, said that for large research institutions like his that have served as trial sites, the transition to offering PET-amyloid, MRI, and infusions in clinical practice will be easier.
“We have all this because this is what we do every day. And we have a very extensive understanding of MCI and mild dementia staging,” Dr. Knopman said. “But the amount of infrastructure that is implied by this, and all the extra steps it would take, would be a real challenge for people in general neurology practice.”
In addition to routine use of PET-amyloid and MRI screening for ARIA-e, Dr. Knopman said, clinicians will have to provide genetic screening and counseling before administering aducanumab, as clinical trials showed that treated patients have a higher risk of developing ARIA-e if they have APOE4, a risk variant for Alzheimer’s disease. “And that has real implications for the families and the children of patients,” he said.
Uncertainty over costs
Aducanumab’s true costs, to patients and to taxpayers, remain unknown. The $56,000 per year currently cited by its manufacturer “doesn’t count the PET scans and MRIs,” Dr. Knopman noted. “We’re probably pushing $100,00 a year for the first year of treatment.”
Most of that expense will likely be borne by Medicare, he said, and if not, “that will exacerbate existing health care disparities. People who can pay out of pocket are a pretty limited group.”
Dr. Scharre agreed that the costs of treatment were concerning, and that “at least you should be able to narrow it down and hopefully just use health care dollars for people who might stand to benefit,” he said – namely patients in an earlier stage of disease.
The Alzheimer’s Association’s Dr. Carrillo declined to address the high price of aducanumab or its implications, saying only that the association is “very invested in all aspects of access including covering costs associated with the drug and the rest of treatment.”
Access also means “infrastructure, access to physicians to diagnose, access to diagnostics,” Dr. Carrillo said.
Dr. Cummings said aducanumab’s price would likely come down through negotiations with the Centers for Medicare & Medicaid Services, copayments, and bulk purchases.
The FDA has offered no guidance on how long treatment with aducanumab should last, or what should prompt withdrawal of treatment, meaning that patients could, in theory, stay on it to the end of their lives – raising costs further.
Critics have also noted that a built-in financial incentive under Medicare Part B, which covers infusion drugs, could result in overprescription of aducanumab. Under Medicare Part B, prescribing physicians are reimbursed 6% of a drug’s average sales price.
Geriatricians wary
On social media and in the lay press, geriatricians have been among the most outspoken opponents of the FDA decision and the Alzheimer’s Association’s advocacy of aducanumab.
Eric Widera, MD, a geriatrician at the University of California, San Francisco, said that the specialty might be less likely than others to embrace aducanumab. “I think part of the reasons geriatricians don’t make a lot of money is they have strong commitment to their values,” Dr. Widera said.
The American Geriatrics Society opposed the drug’s approval, citing concerns about evidence, side effects, and cost. “Additional considerations are the unintended consequences of overstressing Medicare’s limited financial reserves, and of challenging health care systems … to divert precious resources to an expensive treatment of uncertain value,” the society’s president, Peter Hollmann, MD, and chief executive officer, Nancy E. Lundebjerg, wrote in a June 2 letter to the FDA.
Dr. Widera said the approval was likely to undermine confidence in the FDA and in the Alzheimer’s Association, which receives significant funding from drug manufacturers, including Biogen and Eisai. “There’s a lot of reasons that the Geriatrics Society could have done what the Alzheimer’s Association did, and yet they came out against it, which I applaud.”
Dr. Widera pointed to a study showing that dementia patients were less likely to be on an antidementia drug if they were treated by a geriatrician, compared with a psychiatrist or a neurologist. But whether the specialty will prove as cautious with aducanumab remains to be seen. Some geriatricians will be tempted to open lucrative infusion centers, he predicted.
What is especially worrisome, Dr. Widera said, is that aducanumab’s label offers no guidance as to when to withdraw treatment. “We’ll probably see something similar to what happened with the cholinesterase inhibitors” – the class of marginally effective antidementia drugs that includes donepezil (Aricept, Pfizer) and rivastigmine (Exelon, Novartis). “No one thinks about deprescribing them. People are prescribed them even in their last months of life. There is no reason to think these infusions won’t be continued for a very long time, well beyond how long people were dosed in the trials.”
“Taking care of someone with dementia is hard enough,” Dr. Widera added. “We can’t even get normal support in the home for someone with dementia. But we are more than happy to throw money to Biogen for a drug they have not yet showed benefit for. Hopefully in 5 years we’ll have a drug that actually works,” Dr. Widera said. “After 5 years of giving this to people at $50,000 a year.”
A fractured research community
Ever since October 2019, when Biogen and Eisai announced that despite two trials halted for futility, they would go ahead and seek FDA approval for aducanumab, the Alzheimer’s research community has been bitterly divided over the drug and the FDA’s accelerated approval process.
Top researchers published critical editorials in journals, with some eventually taking their case to major newspapers as well. The Alzheimer’s Association’s position on the drug has clashed with that of many researchers whose work it supports.
“The Alzheimer’s community has been wonderfully collegial – we all have a common purpose,” Dr. Cummings said. “Now we have people taking extreme positions and I’m hoping this will not result in a permanent fracturing of the community.”
Chief among the critics’ concerns is that the FDA decision ratified the use of antiamyloid therapies based on biomarker evidence, opening the door for makers of similar drugs – those still under development or even those whose development has been halted – to seek approval on weak evidence of clinical benefit.
Whether the approval will chill research into drugs targeting pathways other than amyloid is uncertain.
Dr. Cummings said he felt that while the aducanumab decision would spur other manufacturers of antiamyloid drugs to seek accelerated approval, other classes of Alzheimer’s therapies in development also stand to get a boost. Many Alzheimer’s experts believe that a combination of drugs targeting different elements of the disease pathway – not just amyloid – will be needed in the long run.
Dr. Scharre said that the buzz over aducanumab’s approval will have at least one concrete benefit: people getting into doctors’ offices sooner.
“The people who come into our memory centers represent only a fraction of people walking around with MCI – there are people out there who may have heard that it’s normal aging; they have decreased insight; there’s denial, there’s embarrassment – there’s hundreds of reasons people avoid getting seen,” he said.
“Perhaps they come in and learn that they don’t have any degenerative process but their thyroid is out of whack, or there’s something else causing cognitive impairment. And if they do have a degenerative process, they’ll have time to start [aducanumab], and hopefully get to see a reduction in the decline.”
Dr. Knopman was a site investigator for the Biogen aducanumab trials and has consulted for Samus Therapeutics, Third Rock, Roche, and Alzeca Biosciences. A former member of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee, he was recused from the Nov. 6, 2020, meeting that voted against aducanumab. Dr. Cummings has consulted for Biogen, Eisai, and other manufacturers. Dr. Scharre reports financial relationships with Biogen, Brain Test, Acadia, and Vascular Scientific. Dr. Widera has no disclosures. Dr. Delio is a speaker for Gore Medical, Allergan, and Biohaven Pharmaceuticals.
The approval was hailed by advocacy groups and some practitioners as a victory for patients and families, as the drug – the first anti-Alzheimer’s agent to reach the market in 18 years – is a potentially disease-modifying therapy, which acts to clear amyloid plaques from the brain.
But several prominent Alzheimer’s researchers lambasted the agency’s decision, citing unclear evidence of benefit, trials that did not meet their primary endpoints, and reliance on a post hoc analysis of a high-dose subgroup of patients in a halted trial to argue that aducanumab (Aduhelm, Biogen, and Eisai), slowed cognitive and functional decline by 22% on one measure. In November 2020, 10 of 11 members of an independent FDA advisory committee voted against aducanumab’s approval, citing holes in the data and concerns about the quality of the evidence. After the agency went on to approve anyway, three members of that committee resigned in protest.
The FDA decision on aducanumab was made using the agency’s accelerated approval pathway, which allows for the use of a surrogate endpoint – in this case imaging that showed amyloid clearance from the brain – to predict clinical benefit. But amyloid clearance, which a number of experimental antiamyloid antibodies have been shown capable of, has not been definitively linked to clinical benefit. Aducanumab, which is delivered by monthly intravenous infusion, will be marketed pending results from a phase 4 clinical trial, which the manufacturer has nearly a decade to complete. The drug’s price was announced at $56,000 per year, underscoring concern over its modest-at-best benefits.
Clinicians prescribing aducanumab must obtain magnetic resonance imaging at baseline and repeatedly during the course of treatment to detect brain edema and microhemorrhages, which occurred in a third of high-dose patients in clinical trials. Beyond this, there are few restrictions. The FDA label allows for its use in any patient deemed to have Alzheimer’s disease, without stipulations as to disease stage or evidence of brain amyloid. Payers, of course, are likely to restrict use to certain patient groups, and to require evidence of amyloid positivity. The FDA offered no guidance on when treatment should be ceased, leaving payers to make that call as well. Whatever aducanumab’s value and role turns out to be, the first-in-class treatment for Alzheimer’s disease is likely to have a major impact on how patients are assessed and treated in the coming years, and embolden manufactures of similar agents to seek FDA approval.
This news organization reached out to researchers, advocates, and specialists in the community to learn how they see this change playing out.
Fielding broad interest
Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association, which was a strong proponent of aducanumab’s approval, acknowledged in an interview that the months to come are likely to be confusing for practitioners and families alike as the drug makes its way into community practices.
“We understand that off the bat millions of Americans will not have access to this tomorrow, but over time that will build. And the physician community, the specialists most likely to be prescribing this, over the next few years will even expand further,” Dr. Carrillo said.
For now, those specialists are mostly just struggling to respond responsibly to a deluge of inquiries from patients and their families.
“I’ve gotten like 20 calls in the just the past 2 days,” said neurologist Philip R. Delio, MD, who practices in Santa Barbara, Calif. “This is a longstanding issue that physicians have with patients’ access to information. Patients are getting information about a drug which isn’t available yet. They don’t know that it’s not ready to be sold. They don’t necessarily realize that a biopharma company won’t go into production until the FDA approves the drug.”
Many patients, Dr. Delio said, are aware of the controversy surrounding aducanumab and eager to hear their neurologist’s opinion. “I have tried to let them know that I want to see the trial data and to better understand the FDA’s rationale in approving it. I always caution patients that the devil will be in the details.”
While aducanumab’s label gives physicians remarkably wide latitude in whom to treat, clinicians say that until payers weigh in, the label is all but meaningless. Neurologist Douglas Scharre, MD, of the Ohio State University Wexner Medical Center, and a site investigator on a trial of aducanumab, said that he and his colleagues at the university’s memory center have tried to anticipate who might be deemed eligible by triaging calls.
Dr. Scharre and colleagues have been working under the assumption that payers will support aducanumab only for patients like those who seemed to benefit in the trials – people with mild cognitive impairment (MCI) or in the earliest stages of dementia with evidence of brain amyloid.
“I don’t want to fill up our new patient slots with people who are not even appropriate for this drug,” Dr. Scharre said. “We have a call center, and we have a few triage questions. After that a nurse practitioner collects some more data, and there’s a review process. Only then do we decide whether that person could be a candidate. If we deem that they are, we will want them in and to order an amyloid PET” – a type of brain scan that is seldom used outside research settings and not reimbursed by Medicare.
Dr. Scharre predicts that regardless of payer limitations, “there will be people hounding for the drug who are not appropriate for the drug. There will be very wealthy people who will want to pay for tests and get it no matter what.” Another concern, he said, was that having poorly selected patients on the drug could make definitive trial results even more elusive.
“The label the way it’s written is not going to help the drug in phase 4 trials,” he said. “It’s good to have real-world patient data, but if you have all these people in your cohort who are too early or too late, you won’t have good results.”
The challenge of delivery
Intravenous infusions are new to Alzheimer’s disease and pose all sorts of logistical hurdles. The Alzheimer’s Association’s Dr. Carrillo described the situation as “manageable,” noting that infusions are standard of care for many diseases, and that neurologists now have more than 15 years’ experience with them for multiple sclerosis.
Still, most clinicians treating Alzheimer’s disease in the community – neurologists, geriatricians, psychiatrists, and primary care physicians – do not have infusion centers in their practices. Virtually none have experience with or access to PET-amyloid, or with screening for amyloid-related imaging abnormalities–edema (ARIA-e) on MRI, as required by the FDA.
“I contacted the hospital infusion center we use and said I could end up sending five or six patients a week, can you handle this? They only have so many chairs,” Dr. Delio said. “I am one neurologist in a local community, and I might have 50 candidates for this drug. That’s a lot for them.” Patients with cognitive impairment are also difficult to infuse and may need to be treated at home, he noted.
“MRIs are easy enough to do,” Dr. Delio said. “But do we know what ARIA-e looks like on imaging? You’d have to talk to the radiologists – this is another element of uncertainty. Do we even know what we’re looking for with these scans? Will we recognize this?”
Neurologist Jeffrey L. Cummings, MD, ScD, of the University of Nevada, Las Vegas, a vocal proponent of aducanumab and lead author of a May 2021 paper defending the evidence for it, acknowledged that the field was unprepared for a wide-scale adoption of infusions in dementia treatment, pointing to a Rand Corporation study from 2017 that warned that screening, diagnosis, and availability of infusion chairs would have to be drastically scaled up to meet demand.
“There are few clinicians who know how to identify MCI, too few imaging centers, too few radiologists who know how to identify ARIA-e on MRI, so all of these things will be required to be put into place. The label doesn’t specify any of this, but good clinical practice will require that, and getting this up and running will take 18 to 24 months,” Dr. Cummings said.
Neurologist David S. Knopman, MD, of the Mayo Clinic in Rochester, Minn., a leading critic of the evidence for aducanumab who recently resigned his position on the independent committee that advises the FDA on neurology drugs, said that for large research institutions like his that have served as trial sites, the transition to offering PET-amyloid, MRI, and infusions in clinical practice will be easier.
“We have all this because this is what we do every day. And we have a very extensive understanding of MCI and mild dementia staging,” Dr. Knopman said. “But the amount of infrastructure that is implied by this, and all the extra steps it would take, would be a real challenge for people in general neurology practice.”
In addition to routine use of PET-amyloid and MRI screening for ARIA-e, Dr. Knopman said, clinicians will have to provide genetic screening and counseling before administering aducanumab, as clinical trials showed that treated patients have a higher risk of developing ARIA-e if they have APOE4, a risk variant for Alzheimer’s disease. “And that has real implications for the families and the children of patients,” he said.
Uncertainty over costs
Aducanumab’s true costs, to patients and to taxpayers, remain unknown. The $56,000 per year currently cited by its manufacturer “doesn’t count the PET scans and MRIs,” Dr. Knopman noted. “We’re probably pushing $100,00 a year for the first year of treatment.”
Most of that expense will likely be borne by Medicare, he said, and if not, “that will exacerbate existing health care disparities. People who can pay out of pocket are a pretty limited group.”
Dr. Scharre agreed that the costs of treatment were concerning, and that “at least you should be able to narrow it down and hopefully just use health care dollars for people who might stand to benefit,” he said – namely patients in an earlier stage of disease.
The Alzheimer’s Association’s Dr. Carrillo declined to address the high price of aducanumab or its implications, saying only that the association is “very invested in all aspects of access including covering costs associated with the drug and the rest of treatment.”
Access also means “infrastructure, access to physicians to diagnose, access to diagnostics,” Dr. Carrillo said.
Dr. Cummings said aducanumab’s price would likely come down through negotiations with the Centers for Medicare & Medicaid Services, copayments, and bulk purchases.
The FDA has offered no guidance on how long treatment with aducanumab should last, or what should prompt withdrawal of treatment, meaning that patients could, in theory, stay on it to the end of their lives – raising costs further.
Critics have also noted that a built-in financial incentive under Medicare Part B, which covers infusion drugs, could result in overprescription of aducanumab. Under Medicare Part B, prescribing physicians are reimbursed 6% of a drug’s average sales price.
Geriatricians wary
On social media and in the lay press, geriatricians have been among the most outspoken opponents of the FDA decision and the Alzheimer’s Association’s advocacy of aducanumab.
Eric Widera, MD, a geriatrician at the University of California, San Francisco, said that the specialty might be less likely than others to embrace aducanumab. “I think part of the reasons geriatricians don’t make a lot of money is they have strong commitment to their values,” Dr. Widera said.
The American Geriatrics Society opposed the drug’s approval, citing concerns about evidence, side effects, and cost. “Additional considerations are the unintended consequences of overstressing Medicare’s limited financial reserves, and of challenging health care systems … to divert precious resources to an expensive treatment of uncertain value,” the society’s president, Peter Hollmann, MD, and chief executive officer, Nancy E. Lundebjerg, wrote in a June 2 letter to the FDA.
Dr. Widera said the approval was likely to undermine confidence in the FDA and in the Alzheimer’s Association, which receives significant funding from drug manufacturers, including Biogen and Eisai. “There’s a lot of reasons that the Geriatrics Society could have done what the Alzheimer’s Association did, and yet they came out against it, which I applaud.”
Dr. Widera pointed to a study showing that dementia patients were less likely to be on an antidementia drug if they were treated by a geriatrician, compared with a psychiatrist or a neurologist. But whether the specialty will prove as cautious with aducanumab remains to be seen. Some geriatricians will be tempted to open lucrative infusion centers, he predicted.
What is especially worrisome, Dr. Widera said, is that aducanumab’s label offers no guidance as to when to withdraw treatment. “We’ll probably see something similar to what happened with the cholinesterase inhibitors” – the class of marginally effective antidementia drugs that includes donepezil (Aricept, Pfizer) and rivastigmine (Exelon, Novartis). “No one thinks about deprescribing them. People are prescribed them even in their last months of life. There is no reason to think these infusions won’t be continued for a very long time, well beyond how long people were dosed in the trials.”
“Taking care of someone with dementia is hard enough,” Dr. Widera added. “We can’t even get normal support in the home for someone with dementia. But we are more than happy to throw money to Biogen for a drug they have not yet showed benefit for. Hopefully in 5 years we’ll have a drug that actually works,” Dr. Widera said. “After 5 years of giving this to people at $50,000 a year.”
A fractured research community
Ever since October 2019, when Biogen and Eisai announced that despite two trials halted for futility, they would go ahead and seek FDA approval for aducanumab, the Alzheimer’s research community has been bitterly divided over the drug and the FDA’s accelerated approval process.
Top researchers published critical editorials in journals, with some eventually taking their case to major newspapers as well. The Alzheimer’s Association’s position on the drug has clashed with that of many researchers whose work it supports.
“The Alzheimer’s community has been wonderfully collegial – we all have a common purpose,” Dr. Cummings said. “Now we have people taking extreme positions and I’m hoping this will not result in a permanent fracturing of the community.”
Chief among the critics’ concerns is that the FDA decision ratified the use of antiamyloid therapies based on biomarker evidence, opening the door for makers of similar drugs – those still under development or even those whose development has been halted – to seek approval on weak evidence of clinical benefit.
Whether the approval will chill research into drugs targeting pathways other than amyloid is uncertain.
Dr. Cummings said he felt that while the aducanumab decision would spur other manufacturers of antiamyloid drugs to seek accelerated approval, other classes of Alzheimer’s therapies in development also stand to get a boost. Many Alzheimer’s experts believe that a combination of drugs targeting different elements of the disease pathway – not just amyloid – will be needed in the long run.
Dr. Scharre said that the buzz over aducanumab’s approval will have at least one concrete benefit: people getting into doctors’ offices sooner.
“The people who come into our memory centers represent only a fraction of people walking around with MCI – there are people out there who may have heard that it’s normal aging; they have decreased insight; there’s denial, there’s embarrassment – there’s hundreds of reasons people avoid getting seen,” he said.
“Perhaps they come in and learn that they don’t have any degenerative process but their thyroid is out of whack, or there’s something else causing cognitive impairment. And if they do have a degenerative process, they’ll have time to start [aducanumab], and hopefully get to see a reduction in the decline.”
Dr. Knopman was a site investigator for the Biogen aducanumab trials and has consulted for Samus Therapeutics, Third Rock, Roche, and Alzeca Biosciences. A former member of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee, he was recused from the Nov. 6, 2020, meeting that voted against aducanumab. Dr. Cummings has consulted for Biogen, Eisai, and other manufacturers. Dr. Scharre reports financial relationships with Biogen, Brain Test, Acadia, and Vascular Scientific. Dr. Widera has no disclosures. Dr. Delio is a speaker for Gore Medical, Allergan, and Biohaven Pharmaceuticals.
AMA: ‘Excited delirium’ not a legitimate medical diagnosis
Current evidence does not support use of “excited delirium” or “excited delirium syndrome” as a medical diagnosis, the American Medical Association said June 14, and the term should not be used unless clear diagnostic criteria are validated.
The term is disproportionately applied to people of color, “for whom inappropriate and excessive pharmacotherapy continues to be the norm instead of behavioral deescalation,” the report by the AMA’s Council on Science and Public Health stated, and is therefore indicative of systemic racism.
That conclusion was one of many included in CSAPH Report 2, which was adopted June 14 at the special meeting of the AMA House of Delegates.
The AMA also opposes “use of sedative/hypnotic and dissociative agents, including ketamine, as a pharmacologic intervention for agitated individuals in the out-of-hospital setting, when done solely for a law enforcement purpose.”
Medications typically used for restraint include dissociative ketamine, benzodiazepine sedatives such as midazolam, and antipsychotic medications including olanzapine or haloperidol, alone or in combination.
Kenneth Certa, MD, from the American Psychiatric Association, speaking on behalf of the section council on psychiatry, said in a reference committee hearing: “We have been very concerned over the years with the development of the inexact diagnosis of ‘agitated delirium’ or ‘excited delirium,’ especially after having had a number of individuals, more than what’s reported in the press, die by the use of ketamine in the field for this inexact diagnosis.”
Tamaan Osbourne-Roberts, MD, a delegate and CSAPH member, said the diagnosis lacks scientific evidence and is “disproportionately applied to otherwise healthy Black men in their mid-30s and these men are most likely to die from resulting first-responder actions.”
Dr. Osbourne-Roberts testified that deescalation training should be more widely used and that crisis intervention team models in which behavioral health specialists are first deployed to respond to behavioral health emergencies should be more prevalent.
Andrew Rudawsky, MD, an assistant medical director of two emergency departments and delegate from Ohio, speaking as an individual, testified: “I can tell you from first-hand experience that ‘excited delirium’ is very real. These acutely ill, unstable patients have an emergency medical condition best cared for by an emergency medicine physician.”
The report recognizes that drugs used outside a hospital setting by nonphysicians come with significant risks, particularly for those with underlying conditions and in terms of drug–drug interactions.
“I completely agree that medicine should not be practiced by law enforcement,” Dr. Rudawsky said. “I’m gravely concerned by the legal ramifications of stating that this condition doesn’t exist.”
He said he is optimistic that the Diagnostic and Statistical Manual of Mental Disorders (DSM) will be updated to include “excited delirium.”
in medical and mental health emergencies in local communities.
Additionally, the report urges that “administration of any pharmacologic treatments in the out-of-hospital setting be done equitably, in an evidence-based, antiracist, and stigma-free way.”
The report calls on law enforcement and frontline emergency medical service personnel, who are a part of the “dual response” in emergency situations, to engage in training overseen by EMS medical directors. “The training should minimally include deescalation techniques and the appropriate use of pharmacologic intervention for agitated individuals in the out-of-hospital setting,” the report states.
Recommendation on oversight draws controversy
Several commenters were emergency physicians and medical directors who expressed concern that investigation of potential cases of inappropriate pharmacologic intervention would be overseen by nonphysicians.
The CSAPH authors write that independent investigators are appropriate, whereas those in emergency medicine say EMS medical directors should lead oversight.
Stephen Epstein, MD, chair of the section council on emergency medicine, speaking on behalf of the section council, had moved for referral of the portion of the report that deals with oversight of EMS.
“We’re concerned that recommendation 6, by calling for independent investigators, would put nonphysicians in the position of supervising the practice of medicine of a board-approved specialty. This would set an unfortunate precedent for our AMA,” he said.
Dr. Epstein also said the American College of Emergency Physicians will soon release a report on “excited delirium,” which will add key information for debating the issue.
He added that a new report on the safety of ketamine in out-of-hospital use was published just last week in the Annals of Emergency Medicine. The authors reviewed more than 11,000 cases of the pharmacologic intervention over the past 2 years.
“We believe this information may add substantively to the recommendation in this report,” Dr. Epstein said.
Recommendation 6 was referred to the AMA Board for a decision, but the rest of the report was overwhelmingly adopted.
Dr. Certa, Dr. Osbourne-Roberts, Dr. Rudawsky, and Dr. Epstein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Current evidence does not support use of “excited delirium” or “excited delirium syndrome” as a medical diagnosis, the American Medical Association said June 14, and the term should not be used unless clear diagnostic criteria are validated.
The term is disproportionately applied to people of color, “for whom inappropriate and excessive pharmacotherapy continues to be the norm instead of behavioral deescalation,” the report by the AMA’s Council on Science and Public Health stated, and is therefore indicative of systemic racism.
That conclusion was one of many included in CSAPH Report 2, which was adopted June 14 at the special meeting of the AMA House of Delegates.
The AMA also opposes “use of sedative/hypnotic and dissociative agents, including ketamine, as a pharmacologic intervention for agitated individuals in the out-of-hospital setting, when done solely for a law enforcement purpose.”
Medications typically used for restraint include dissociative ketamine, benzodiazepine sedatives such as midazolam, and antipsychotic medications including olanzapine or haloperidol, alone or in combination.
Kenneth Certa, MD, from the American Psychiatric Association, speaking on behalf of the section council on psychiatry, said in a reference committee hearing: “We have been very concerned over the years with the development of the inexact diagnosis of ‘agitated delirium’ or ‘excited delirium,’ especially after having had a number of individuals, more than what’s reported in the press, die by the use of ketamine in the field for this inexact diagnosis.”
Tamaan Osbourne-Roberts, MD, a delegate and CSAPH member, said the diagnosis lacks scientific evidence and is “disproportionately applied to otherwise healthy Black men in their mid-30s and these men are most likely to die from resulting first-responder actions.”
Dr. Osbourne-Roberts testified that deescalation training should be more widely used and that crisis intervention team models in which behavioral health specialists are first deployed to respond to behavioral health emergencies should be more prevalent.
Andrew Rudawsky, MD, an assistant medical director of two emergency departments and delegate from Ohio, speaking as an individual, testified: “I can tell you from first-hand experience that ‘excited delirium’ is very real. These acutely ill, unstable patients have an emergency medical condition best cared for by an emergency medicine physician.”
The report recognizes that drugs used outside a hospital setting by nonphysicians come with significant risks, particularly for those with underlying conditions and in terms of drug–drug interactions.
“I completely agree that medicine should not be practiced by law enforcement,” Dr. Rudawsky said. “I’m gravely concerned by the legal ramifications of stating that this condition doesn’t exist.”
He said he is optimistic that the Diagnostic and Statistical Manual of Mental Disorders (DSM) will be updated to include “excited delirium.”
in medical and mental health emergencies in local communities.
Additionally, the report urges that “administration of any pharmacologic treatments in the out-of-hospital setting be done equitably, in an evidence-based, antiracist, and stigma-free way.”
The report calls on law enforcement and frontline emergency medical service personnel, who are a part of the “dual response” in emergency situations, to engage in training overseen by EMS medical directors. “The training should minimally include deescalation techniques and the appropriate use of pharmacologic intervention for agitated individuals in the out-of-hospital setting,” the report states.
Recommendation on oversight draws controversy
Several commenters were emergency physicians and medical directors who expressed concern that investigation of potential cases of inappropriate pharmacologic intervention would be overseen by nonphysicians.
The CSAPH authors write that independent investigators are appropriate, whereas those in emergency medicine say EMS medical directors should lead oversight.
Stephen Epstein, MD, chair of the section council on emergency medicine, speaking on behalf of the section council, had moved for referral of the portion of the report that deals with oversight of EMS.
“We’re concerned that recommendation 6, by calling for independent investigators, would put nonphysicians in the position of supervising the practice of medicine of a board-approved specialty. This would set an unfortunate precedent for our AMA,” he said.
Dr. Epstein also said the American College of Emergency Physicians will soon release a report on “excited delirium,” which will add key information for debating the issue.
He added that a new report on the safety of ketamine in out-of-hospital use was published just last week in the Annals of Emergency Medicine. The authors reviewed more than 11,000 cases of the pharmacologic intervention over the past 2 years.
“We believe this information may add substantively to the recommendation in this report,” Dr. Epstein said.
Recommendation 6 was referred to the AMA Board for a decision, but the rest of the report was overwhelmingly adopted.
Dr. Certa, Dr. Osbourne-Roberts, Dr. Rudawsky, and Dr. Epstein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Current evidence does not support use of “excited delirium” or “excited delirium syndrome” as a medical diagnosis, the American Medical Association said June 14, and the term should not be used unless clear diagnostic criteria are validated.
The term is disproportionately applied to people of color, “for whom inappropriate and excessive pharmacotherapy continues to be the norm instead of behavioral deescalation,” the report by the AMA’s Council on Science and Public Health stated, and is therefore indicative of systemic racism.
That conclusion was one of many included in CSAPH Report 2, which was adopted June 14 at the special meeting of the AMA House of Delegates.
The AMA also opposes “use of sedative/hypnotic and dissociative agents, including ketamine, as a pharmacologic intervention for agitated individuals in the out-of-hospital setting, when done solely for a law enforcement purpose.”
Medications typically used for restraint include dissociative ketamine, benzodiazepine sedatives such as midazolam, and antipsychotic medications including olanzapine or haloperidol, alone or in combination.
Kenneth Certa, MD, from the American Psychiatric Association, speaking on behalf of the section council on psychiatry, said in a reference committee hearing: “We have been very concerned over the years with the development of the inexact diagnosis of ‘agitated delirium’ or ‘excited delirium,’ especially after having had a number of individuals, more than what’s reported in the press, die by the use of ketamine in the field for this inexact diagnosis.”
Tamaan Osbourne-Roberts, MD, a delegate and CSAPH member, said the diagnosis lacks scientific evidence and is “disproportionately applied to otherwise healthy Black men in their mid-30s and these men are most likely to die from resulting first-responder actions.”
Dr. Osbourne-Roberts testified that deescalation training should be more widely used and that crisis intervention team models in which behavioral health specialists are first deployed to respond to behavioral health emergencies should be more prevalent.
Andrew Rudawsky, MD, an assistant medical director of two emergency departments and delegate from Ohio, speaking as an individual, testified: “I can tell you from first-hand experience that ‘excited delirium’ is very real. These acutely ill, unstable patients have an emergency medical condition best cared for by an emergency medicine physician.”
The report recognizes that drugs used outside a hospital setting by nonphysicians come with significant risks, particularly for those with underlying conditions and in terms of drug–drug interactions.
“I completely agree that medicine should not be practiced by law enforcement,” Dr. Rudawsky said. “I’m gravely concerned by the legal ramifications of stating that this condition doesn’t exist.”
He said he is optimistic that the Diagnostic and Statistical Manual of Mental Disorders (DSM) will be updated to include “excited delirium.”
in medical and mental health emergencies in local communities.
Additionally, the report urges that “administration of any pharmacologic treatments in the out-of-hospital setting be done equitably, in an evidence-based, antiracist, and stigma-free way.”
The report calls on law enforcement and frontline emergency medical service personnel, who are a part of the “dual response” in emergency situations, to engage in training overseen by EMS medical directors. “The training should minimally include deescalation techniques and the appropriate use of pharmacologic intervention for agitated individuals in the out-of-hospital setting,” the report states.
Recommendation on oversight draws controversy
Several commenters were emergency physicians and medical directors who expressed concern that investigation of potential cases of inappropriate pharmacologic intervention would be overseen by nonphysicians.
The CSAPH authors write that independent investigators are appropriate, whereas those in emergency medicine say EMS medical directors should lead oversight.
Stephen Epstein, MD, chair of the section council on emergency medicine, speaking on behalf of the section council, had moved for referral of the portion of the report that deals with oversight of EMS.
“We’re concerned that recommendation 6, by calling for independent investigators, would put nonphysicians in the position of supervising the practice of medicine of a board-approved specialty. This would set an unfortunate precedent for our AMA,” he said.
Dr. Epstein also said the American College of Emergency Physicians will soon release a report on “excited delirium,” which will add key information for debating the issue.
He added that a new report on the safety of ketamine in out-of-hospital use was published just last week in the Annals of Emergency Medicine. The authors reviewed more than 11,000 cases of the pharmacologic intervention over the past 2 years.
“We believe this information may add substantively to the recommendation in this report,” Dr. Epstein said.
Recommendation 6 was referred to the AMA Board for a decision, but the rest of the report was overwhelmingly adopted.
Dr. Certa, Dr. Osbourne-Roberts, Dr. Rudawsky, and Dr. Epstein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Minnesota named best place to practice in 2021
For physicians who are just starting out or thinking about moving, the “Land of 10,000 Lakes” could be the land of opportunity, according to a recent Medscape analysis.
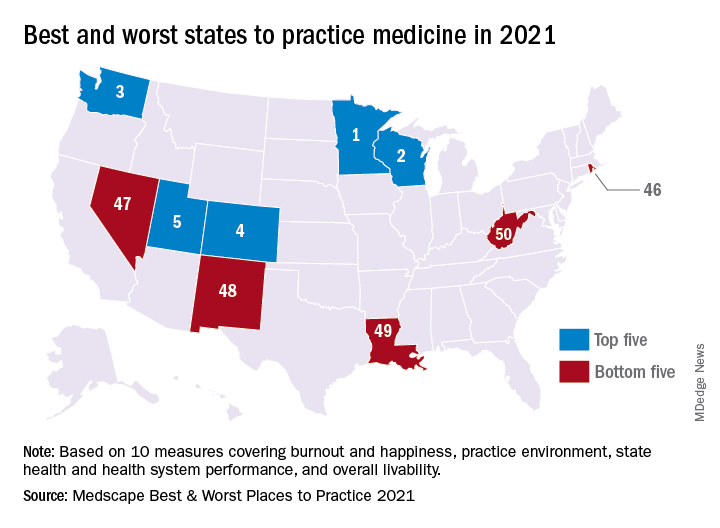
In a ranking of the 50 states, Minnesota “claimed top marks for livability, low incidence of adverse actions against doctors, and the performance of its health system,” Shelly Reese wrote in Medscape’s “Best & Worst Places to Practice 2021.”
Minnesota is below average where it’s good to be below average – share of physicians reporting burnout and/or depression – but above average in the share of physicians who say they’re “very happy” outside of work, Medscape said in the annual report.
and adverse actions and a high level of livability. Third place went to Washington (called the most livable state in the country by U.S. News and World Report), fourth to Colorado (physicians happy at and outside of work, high retention rate for residents), and fifth to Utah (low crime rate, high quality of life), Medscape said.
At the bottom of the list for 2021 is West Virginia, where physicians “may confront a bevy of challenges” in the form of low livability, a high rate of adverse actions, and relatively high malpractice payouts, Ms. Reese noted in the report.
State number 49 is Louisiana, where livability is low, malpractice payouts are high, and more than half of physicians say that they’re burned out and/or depressed. New Mexico is 48th (very high rate of adverse actions, poor resident retention), Nevada is 47th (low marks for avoidable hospital use and disparity in care), and Rhode Island is 46th (high malpractice payouts, low physician compensation), Medscape said.
Continuing with the group-of-five theme, America’s three most populous states finished in the top half of the ranking – California 16th, Texas 11th, and Florida 21st – but New York and Pennsylvania, numbers four and five by population size, did not.
The rankings are based on states’ performance in 10 different measures, three of which were sourced from Medscape surveys – happiness at work, happiness outside of work, and burnout/depression – and seven from other organizations: adverse actions against physicians, malpractice payouts, compensation (adjusted for cost of living), overall health, health system performance, overall livability, resident retention.
For physicians who are just starting out or thinking about moving, the “Land of 10,000 Lakes” could be the land of opportunity, according to a recent Medscape analysis.

In a ranking of the 50 states, Minnesota “claimed top marks for livability, low incidence of adverse actions against doctors, and the performance of its health system,” Shelly Reese wrote in Medscape’s “Best & Worst Places to Practice 2021.”
Minnesota is below average where it’s good to be below average – share of physicians reporting burnout and/or depression – but above average in the share of physicians who say they’re “very happy” outside of work, Medscape said in the annual report.
and adverse actions and a high level of livability. Third place went to Washington (called the most livable state in the country by U.S. News and World Report), fourth to Colorado (physicians happy at and outside of work, high retention rate for residents), and fifth to Utah (low crime rate, high quality of life), Medscape said.
At the bottom of the list for 2021 is West Virginia, where physicians “may confront a bevy of challenges” in the form of low livability, a high rate of adverse actions, and relatively high malpractice payouts, Ms. Reese noted in the report.
State number 49 is Louisiana, where livability is low, malpractice payouts are high, and more than half of physicians say that they’re burned out and/or depressed. New Mexico is 48th (very high rate of adverse actions, poor resident retention), Nevada is 47th (low marks for avoidable hospital use and disparity in care), and Rhode Island is 46th (high malpractice payouts, low physician compensation), Medscape said.
Continuing with the group-of-five theme, America’s three most populous states finished in the top half of the ranking – California 16th, Texas 11th, and Florida 21st – but New York and Pennsylvania, numbers four and five by population size, did not.
The rankings are based on states’ performance in 10 different measures, three of which were sourced from Medscape surveys – happiness at work, happiness outside of work, and burnout/depression – and seven from other organizations: adverse actions against physicians, malpractice payouts, compensation (adjusted for cost of living), overall health, health system performance, overall livability, resident retention.
For physicians who are just starting out or thinking about moving, the “Land of 10,000 Lakes” could be the land of opportunity, according to a recent Medscape analysis.

In a ranking of the 50 states, Minnesota “claimed top marks for livability, low incidence of adverse actions against doctors, and the performance of its health system,” Shelly Reese wrote in Medscape’s “Best & Worst Places to Practice 2021.”
Minnesota is below average where it’s good to be below average – share of physicians reporting burnout and/or depression – but above average in the share of physicians who say they’re “very happy” outside of work, Medscape said in the annual report.
and adverse actions and a high level of livability. Third place went to Washington (called the most livable state in the country by U.S. News and World Report), fourth to Colorado (physicians happy at and outside of work, high retention rate for residents), and fifth to Utah (low crime rate, high quality of life), Medscape said.
At the bottom of the list for 2021 is West Virginia, where physicians “may confront a bevy of challenges” in the form of low livability, a high rate of adverse actions, and relatively high malpractice payouts, Ms. Reese noted in the report.
State number 49 is Louisiana, where livability is low, malpractice payouts are high, and more than half of physicians say that they’re burned out and/or depressed. New Mexico is 48th (very high rate of adverse actions, poor resident retention), Nevada is 47th (low marks for avoidable hospital use and disparity in care), and Rhode Island is 46th (high malpractice payouts, low physician compensation), Medscape said.
Continuing with the group-of-five theme, America’s three most populous states finished in the top half of the ranking – California 16th, Texas 11th, and Florida 21st – but New York and Pennsylvania, numbers four and five by population size, did not.
The rankings are based on states’ performance in 10 different measures, three of which were sourced from Medscape surveys – happiness at work, happiness outside of work, and burnout/depression – and seven from other organizations: adverse actions against physicians, malpractice payouts, compensation (adjusted for cost of living), overall health, health system performance, overall livability, resident retention.
The Cures Act: Is the “cure” worse than the disease?
There is a sudden spill of icy anxiety down your spine as you pick up your phone in your shaking hands. It’s 6 p.m.; your doctor’s office is closed. You open the message, and your worst fears are confirmed ... the cancer is back.
Or is it? You’re not sure. The biopsy sure sounds bad. But you’re an English teacher, not a doctor, and you spend the rest of the night Googling words like “tubulovillous” and “high-grade dysplasia.” You sit awake, terrified in front of the computer screen desperately trying to make sense of the possibly life-changing results. You wish you knew someone who could help you understand; you consider calling your doctor’s emergency line, or your cousin who is an ophthalmologist – anybody who can help you make sense of the results.
Or imagine another scenario: you’re a trans teen who has asked your doctor to refer to you by your preferred pronouns. You’re still presenting as your birth sex, in part because your family would disown you if they knew, and you’re not financially or emotionally ready for that step. You feel proud of yourself for advocating for your needs to your long-time physician, and excited about the resources they’ve included in your after visit summary and the referrals they’d made to gender-confirming specialists.
When you get home, you are confronted with a terrible reality that your doctor’s notes, orders, and recommendations are immediately viewable to anybody with your MyChart login – your parents knew the second your doctor signed the note. They received the notification, logged on as your guardians, and you have effectively been “outed” by the physician who took and oath to care for you and who you trusted implicitly.
How the Cures Act is affecting patients
While these examples may sound extreme, they are becoming more and more commonplace thanks to a recently enacted 21st Century Cures Act. The act was originally written to improve communication between physicians and patients. Part of the act stipulates that nearly all medical information – from notes to biopsies to lab results – must be available within 24 hours, published to a patient portal and a notification be sent to the patient by phone.
Oftentimes, this occurs before the ordering physician has even seen the results, much less interpreted them and made a plan for the patient. What happens now, not long after its enactment date, when it has become clear that the Cures Act is causing extreme harm to our patients?
Take, for example, the real example of a physician whose patient found out about her own intrauterine fetal demise by way of an EMR text message alert of “new imaging results!” sent directly to her phone. Or a physician colleague who witnessed firsthand the intrusive unhelpfulness of the Cures Act when she was informed via patient portal releasing her imaging information that she had a large, possibly malignant breast mass. “No phone call,” she said. “No human being for questions or comfort. Just a notification on my phone.”
The stories about the impact of the Cures Act across the medical community are an endless stream of anxiety, hurt, and broken trust. The relationship between a physician and a patient should be sacred, bolstered by communication and mutual respect.
In many ways, the new act feels like a third party to the patient-physician relationship – a digital imposter, oftentimes blurting out personal and life-altering medical information without any of the finesse, context, and perspective of an experienced physician.
Breaking ‘bad news’ to a patient
In training, some residents are taught how to “break bad news” to a patient. Some good practices for doing this are to have information available for the patient, provide emotional support, have a plan for their next steps already formulated, and call the appropriate specialist ahead of time if you can.
Above all, it’s most important to let the patient be the one to direct their own care. Give them time to ask questions and answer them honestly and clearly. Ask them how much they want to know and help them to understand the complex change in their usual state of health.
Now, unless physicians are keeping a very close eye on their inbox, results are slipping out to patients in a void. The bad news conversations aren’t happening at all, or if they are, they’re happening at 8 p.m. on a phone call after an exhausted physician ends their shift but has to slog through their results bin, calling all the patients who shouldn’t have to find out their results in solitude.
Reaching out to these patients immediately is an honorable, kind thing to, but for a physician, knowing they need to beat the patient to opening an email creates anxiety. Plus, making these calls at whatever hour the results are released to a patient is another burden added to doctors’ already-full plates.
Interpreting results
None of us want to harm our patients. All of us want to be there for them. But this act stands in the way of delivering quality, humanizing medical care.
It is true that patients have a right to access their own medical information. It is also true that waiting anxiously on results can cause undue harm to a patient. But the across-the-board, breakneck speed of information release mandated in this act causes irreparable harm not only to patients, but to the patient-physician relationship.
No patient should find out their cancer recurred while checking their emails at their desk. No patient should first learn of a life-altering diagnosis by way of scrolling through their smartphone in bed. The role of a physician is more than just a healer – we should also be educators, interpreters, partners and, first and foremost, advocates for our patients’ needs.
Our patients are depending on us to stand up and speak out about necessary changes to this act. Result releases should be delayed until they are viewed by a physician. Our patients deserve the dignity and opportunity of a conversation with their medical provider about their test results, and physicians deserve the chance to interpret results and frame the conversation in a way which is conducive to patient understanding and healing.
Dr. Persampiere is a first-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece. You can contact them at [email protected].
There is a sudden spill of icy anxiety down your spine as you pick up your phone in your shaking hands. It’s 6 p.m.; your doctor’s office is closed. You open the message, and your worst fears are confirmed ... the cancer is back.
Or is it? You’re not sure. The biopsy sure sounds bad. But you’re an English teacher, not a doctor, and you spend the rest of the night Googling words like “tubulovillous” and “high-grade dysplasia.” You sit awake, terrified in front of the computer screen desperately trying to make sense of the possibly life-changing results. You wish you knew someone who could help you understand; you consider calling your doctor’s emergency line, or your cousin who is an ophthalmologist – anybody who can help you make sense of the results.
Or imagine another scenario: you’re a trans teen who has asked your doctor to refer to you by your preferred pronouns. You’re still presenting as your birth sex, in part because your family would disown you if they knew, and you’re not financially or emotionally ready for that step. You feel proud of yourself for advocating for your needs to your long-time physician, and excited about the resources they’ve included in your after visit summary and the referrals they’d made to gender-confirming specialists.
When you get home, you are confronted with a terrible reality that your doctor’s notes, orders, and recommendations are immediately viewable to anybody with your MyChart login – your parents knew the second your doctor signed the note. They received the notification, logged on as your guardians, and you have effectively been “outed” by the physician who took and oath to care for you and who you trusted implicitly.
How the Cures Act is affecting patients
While these examples may sound extreme, they are becoming more and more commonplace thanks to a recently enacted 21st Century Cures Act. The act was originally written to improve communication between physicians and patients. Part of the act stipulates that nearly all medical information – from notes to biopsies to lab results – must be available within 24 hours, published to a patient portal and a notification be sent to the patient by phone.
Oftentimes, this occurs before the ordering physician has even seen the results, much less interpreted them and made a plan for the patient. What happens now, not long after its enactment date, when it has become clear that the Cures Act is causing extreme harm to our patients?
Take, for example, the real example of a physician whose patient found out about her own intrauterine fetal demise by way of an EMR text message alert of “new imaging results!” sent directly to her phone. Or a physician colleague who witnessed firsthand the intrusive unhelpfulness of the Cures Act when she was informed via patient portal releasing her imaging information that she had a large, possibly malignant breast mass. “No phone call,” she said. “No human being for questions or comfort. Just a notification on my phone.”
The stories about the impact of the Cures Act across the medical community are an endless stream of anxiety, hurt, and broken trust. The relationship between a physician and a patient should be sacred, bolstered by communication and mutual respect.
In many ways, the new act feels like a third party to the patient-physician relationship – a digital imposter, oftentimes blurting out personal and life-altering medical information without any of the finesse, context, and perspective of an experienced physician.
Breaking ‘bad news’ to a patient
In training, some residents are taught how to “break bad news” to a patient. Some good practices for doing this are to have information available for the patient, provide emotional support, have a plan for their next steps already formulated, and call the appropriate specialist ahead of time if you can.
Above all, it’s most important to let the patient be the one to direct their own care. Give them time to ask questions and answer them honestly and clearly. Ask them how much they want to know and help them to understand the complex change in their usual state of health.
Now, unless physicians are keeping a very close eye on their inbox, results are slipping out to patients in a void. The bad news conversations aren’t happening at all, or if they are, they’re happening at 8 p.m. on a phone call after an exhausted physician ends their shift but has to slog through their results bin, calling all the patients who shouldn’t have to find out their results in solitude.
Reaching out to these patients immediately is an honorable, kind thing to, but for a physician, knowing they need to beat the patient to opening an email creates anxiety. Plus, making these calls at whatever hour the results are released to a patient is another burden added to doctors’ already-full plates.
Interpreting results
None of us want to harm our patients. All of us want to be there for them. But this act stands in the way of delivering quality, humanizing medical care.
It is true that patients have a right to access their own medical information. It is also true that waiting anxiously on results can cause undue harm to a patient. But the across-the-board, breakneck speed of information release mandated in this act causes irreparable harm not only to patients, but to the patient-physician relationship.
No patient should find out their cancer recurred while checking their emails at their desk. No patient should first learn of a life-altering diagnosis by way of scrolling through their smartphone in bed. The role of a physician is more than just a healer – we should also be educators, interpreters, partners and, first and foremost, advocates for our patients’ needs.
Our patients are depending on us to stand up and speak out about necessary changes to this act. Result releases should be delayed until they are viewed by a physician. Our patients deserve the dignity and opportunity of a conversation with their medical provider about their test results, and physicians deserve the chance to interpret results and frame the conversation in a way which is conducive to patient understanding and healing.
Dr. Persampiere is a first-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece. You can contact them at [email protected].
There is a sudden spill of icy anxiety down your spine as you pick up your phone in your shaking hands. It’s 6 p.m.; your doctor’s office is closed. You open the message, and your worst fears are confirmed ... the cancer is back.
Or is it? You’re not sure. The biopsy sure sounds bad. But you’re an English teacher, not a doctor, and you spend the rest of the night Googling words like “tubulovillous” and “high-grade dysplasia.” You sit awake, terrified in front of the computer screen desperately trying to make sense of the possibly life-changing results. You wish you knew someone who could help you understand; you consider calling your doctor’s emergency line, or your cousin who is an ophthalmologist – anybody who can help you make sense of the results.
Or imagine another scenario: you’re a trans teen who has asked your doctor to refer to you by your preferred pronouns. You’re still presenting as your birth sex, in part because your family would disown you if they knew, and you’re not financially or emotionally ready for that step. You feel proud of yourself for advocating for your needs to your long-time physician, and excited about the resources they’ve included in your after visit summary and the referrals they’d made to gender-confirming specialists.
When you get home, you are confronted with a terrible reality that your doctor’s notes, orders, and recommendations are immediately viewable to anybody with your MyChart login – your parents knew the second your doctor signed the note. They received the notification, logged on as your guardians, and you have effectively been “outed” by the physician who took and oath to care for you and who you trusted implicitly.
How the Cures Act is affecting patients
While these examples may sound extreme, they are becoming more and more commonplace thanks to a recently enacted 21st Century Cures Act. The act was originally written to improve communication between physicians and patients. Part of the act stipulates that nearly all medical information – from notes to biopsies to lab results – must be available within 24 hours, published to a patient portal and a notification be sent to the patient by phone.
Oftentimes, this occurs before the ordering physician has even seen the results, much less interpreted them and made a plan for the patient. What happens now, not long after its enactment date, when it has become clear that the Cures Act is causing extreme harm to our patients?
Take, for example, the real example of a physician whose patient found out about her own intrauterine fetal demise by way of an EMR text message alert of “new imaging results!” sent directly to her phone. Or a physician colleague who witnessed firsthand the intrusive unhelpfulness of the Cures Act when she was informed via patient portal releasing her imaging information that she had a large, possibly malignant breast mass. “No phone call,” she said. “No human being for questions or comfort. Just a notification on my phone.”
The stories about the impact of the Cures Act across the medical community are an endless stream of anxiety, hurt, and broken trust. The relationship between a physician and a patient should be sacred, bolstered by communication and mutual respect.
In many ways, the new act feels like a third party to the patient-physician relationship – a digital imposter, oftentimes blurting out personal and life-altering medical information without any of the finesse, context, and perspective of an experienced physician.
Breaking ‘bad news’ to a patient
In training, some residents are taught how to “break bad news” to a patient. Some good practices for doing this are to have information available for the patient, provide emotional support, have a plan for their next steps already formulated, and call the appropriate specialist ahead of time if you can.
Above all, it’s most important to let the patient be the one to direct their own care. Give them time to ask questions and answer them honestly and clearly. Ask them how much they want to know and help them to understand the complex change in their usual state of health.
Now, unless physicians are keeping a very close eye on their inbox, results are slipping out to patients in a void. The bad news conversations aren’t happening at all, or if they are, they’re happening at 8 p.m. on a phone call after an exhausted physician ends their shift but has to slog through their results bin, calling all the patients who shouldn’t have to find out their results in solitude.
Reaching out to these patients immediately is an honorable, kind thing to, but for a physician, knowing they need to beat the patient to opening an email creates anxiety. Plus, making these calls at whatever hour the results are released to a patient is another burden added to doctors’ already-full plates.
Interpreting results
None of us want to harm our patients. All of us want to be there for them. But this act stands in the way of delivering quality, humanizing medical care.
It is true that patients have a right to access their own medical information. It is also true that waiting anxiously on results can cause undue harm to a patient. But the across-the-board, breakneck speed of information release mandated in this act causes irreparable harm not only to patients, but to the patient-physician relationship.
No patient should find out their cancer recurred while checking their emails at their desk. No patient should first learn of a life-altering diagnosis by way of scrolling through their smartphone in bed. The role of a physician is more than just a healer – we should also be educators, interpreters, partners and, first and foremost, advocates for our patients’ needs.
Our patients are depending on us to stand up and speak out about necessary changes to this act. Result releases should be delayed until they are viewed by a physician. Our patients deserve the dignity and opportunity of a conversation with their medical provider about their test results, and physicians deserve the chance to interpret results and frame the conversation in a way which is conducive to patient understanding and healing.
Dr. Persampiere is a first-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece. You can contact them at [email protected].
Judge tosses hospital staff suit over vaccine mandate
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
OSHA issues new rules on COVID-19 safety for health care workers
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
COVID-19 death toll higher for international medical graduates
researchers report.
“I’ve always felt that international medical graduates [IMGs] in America are largely invisible,” said senior author Abraham Verghese, MD, MFA, an infectious disease specialist at Stanford (Calif.) University. “Everyone is aware that there are foreign doctors, but very few are aware of how many there are and also how vital they are to providing health care in America.”
IMGs made up 25% of all U.S. physicians in 2020 but accounted for 45% of those whose deaths had been attributed to COVID-19 through Nov. 23, 2020, Deendayal Dinakarpandian, MD, PhD, clinical associate professor of medicine at Stanford (Calif.) University, and colleagues report in JAMA Network Open.
IMGs are more likely to work in places where the incidence of COVID-19 is high and in facilities with fewer resources, Dr. Verghese said in an interview. “So, it’s not surprising that they were on the front lines when this thing came along,” he said.
To see whether their vulnerability affected their risk for death, Dr. Dinakarpandian and colleagues collected data from Nov. 23, 2020, from three sources of information regarding deaths among physicians: MedPage Today, which used investigative and voluntary reporting; Medscape, which used voluntary reporting of verifiable information; and a collaboration of The Guardian and Kaiser Health News, which used investigative reporting.
The Medscape project was launched on April 1, 2020. The MedPage Today and The Guardian/Kaiser Health News projects were launched on April 8, 2020.
Dr. Verghese and colleagues researched obituaries and news articles referenced by the three projects to verify their data. They used DocInfo to ascertain the deceased physicians’ medical schools.
After eliminating duplications from the lists, the researchers counted 132 physician deaths in 28 states. Of these, 59 physicians had graduated from medical schools outside the United States, a death toll 1.8 times higher than the proportion of IMGs among U.S. physicians (95% confidence interval, 1.52-2.21; P < .001).
New York, New Jersey, and Florida accounted for 66% of the deaths among IMGs but for only 45% of the deaths among U.S. medical school graduates.
Within each state, the proportion of IMGs among deceased physicians was not statistically different from their proportion among physicians in those states, with the exception of New York.
Two-thirds of the physicians’ deaths occurred in states where IMGs make up a larger proportion of physicians than in the nation as a whole. In these states, the incidence of COVID-19 was high at the start of the pandemic.
In New York, IMGs accounted for 60% of physician deaths, which was 1.62 times higher (95% CI, 1.26-2.09; P = .005) than the 37% among New York physicians overall.
Physicians who were trained abroad frequently can’t get into the most prestigious residency programs or into the highest paid specialties and are more likely to serve in primary care, Dr. Verghese said. Overall, 60% of the physicians who died of COVID-19 worked in primary care.
IMGs often staff hospitals serving low-income communities and communities of color, which were hardest hit by the pandemic and where personal protective equipment was hard to obtain, said Dr. Verghese.
In addition to these risks, IMGs sometimes endure racism, said Dr. Verghese, who obtained his medical degree at Madras Medical College, Chennai, India. “We’ve actually seen in the COVID era, in keeping with the sort of political tone that was set in Washington, that there’s been a lot more abuses of both foreign physicians and foreign looking physicians – even if they’re not foreign trained – and nurses by patients who have been given license. And I want to acknowledge the heroism of all these physicians.”
The study was partially funded by the Presence Center at Stanford. Dr. Verghese is a regular contributor to Medscape. He served on the advisory board for Gilead Sciences, serves as a speaker or a member of a speakers bureau for Leigh Bureau, and receives royalties from Penguin Random House and Simon & Schuster.
A version of this article first appeared on Medscape.com.
researchers report.
“I’ve always felt that international medical graduates [IMGs] in America are largely invisible,” said senior author Abraham Verghese, MD, MFA, an infectious disease specialist at Stanford (Calif.) University. “Everyone is aware that there are foreign doctors, but very few are aware of how many there are and also how vital they are to providing health care in America.”
IMGs made up 25% of all U.S. physicians in 2020 but accounted for 45% of those whose deaths had been attributed to COVID-19 through Nov. 23, 2020, Deendayal Dinakarpandian, MD, PhD, clinical associate professor of medicine at Stanford (Calif.) University, and colleagues report in JAMA Network Open.
IMGs are more likely to work in places where the incidence of COVID-19 is high and in facilities with fewer resources, Dr. Verghese said in an interview. “So, it’s not surprising that they were on the front lines when this thing came along,” he said.
To see whether their vulnerability affected their risk for death, Dr. Dinakarpandian and colleagues collected data from Nov. 23, 2020, from three sources of information regarding deaths among physicians: MedPage Today, which used investigative and voluntary reporting; Medscape, which used voluntary reporting of verifiable information; and a collaboration of The Guardian and Kaiser Health News, which used investigative reporting.
The Medscape project was launched on April 1, 2020. The MedPage Today and The Guardian/Kaiser Health News projects were launched on April 8, 2020.
Dr. Verghese and colleagues researched obituaries and news articles referenced by the three projects to verify their data. They used DocInfo to ascertain the deceased physicians’ medical schools.
After eliminating duplications from the lists, the researchers counted 132 physician deaths in 28 states. Of these, 59 physicians had graduated from medical schools outside the United States, a death toll 1.8 times higher than the proportion of IMGs among U.S. physicians (95% confidence interval, 1.52-2.21; P < .001).
New York, New Jersey, and Florida accounted for 66% of the deaths among IMGs but for only 45% of the deaths among U.S. medical school graduates.
Within each state, the proportion of IMGs among deceased physicians was not statistically different from their proportion among physicians in those states, with the exception of New York.
Two-thirds of the physicians’ deaths occurred in states where IMGs make up a larger proportion of physicians than in the nation as a whole. In these states, the incidence of COVID-19 was high at the start of the pandemic.
In New York, IMGs accounted for 60% of physician deaths, which was 1.62 times higher (95% CI, 1.26-2.09; P = .005) than the 37% among New York physicians overall.
Physicians who were trained abroad frequently can’t get into the most prestigious residency programs or into the highest paid specialties and are more likely to serve in primary care, Dr. Verghese said. Overall, 60% of the physicians who died of COVID-19 worked in primary care.
IMGs often staff hospitals serving low-income communities and communities of color, which were hardest hit by the pandemic and where personal protective equipment was hard to obtain, said Dr. Verghese.
In addition to these risks, IMGs sometimes endure racism, said Dr. Verghese, who obtained his medical degree at Madras Medical College, Chennai, India. “We’ve actually seen in the COVID era, in keeping with the sort of political tone that was set in Washington, that there’s been a lot more abuses of both foreign physicians and foreign looking physicians – even if they’re not foreign trained – and nurses by patients who have been given license. And I want to acknowledge the heroism of all these physicians.”
The study was partially funded by the Presence Center at Stanford. Dr. Verghese is a regular contributor to Medscape. He served on the advisory board for Gilead Sciences, serves as a speaker or a member of a speakers bureau for Leigh Bureau, and receives royalties from Penguin Random House and Simon & Schuster.
A version of this article first appeared on Medscape.com.
researchers report.
“I’ve always felt that international medical graduates [IMGs] in America are largely invisible,” said senior author Abraham Verghese, MD, MFA, an infectious disease specialist at Stanford (Calif.) University. “Everyone is aware that there are foreign doctors, but very few are aware of how many there are and also how vital they are to providing health care in America.”
IMGs made up 25% of all U.S. physicians in 2020 but accounted for 45% of those whose deaths had been attributed to COVID-19 through Nov. 23, 2020, Deendayal Dinakarpandian, MD, PhD, clinical associate professor of medicine at Stanford (Calif.) University, and colleagues report in JAMA Network Open.
IMGs are more likely to work in places where the incidence of COVID-19 is high and in facilities with fewer resources, Dr. Verghese said in an interview. “So, it’s not surprising that they were on the front lines when this thing came along,” he said.
To see whether their vulnerability affected their risk for death, Dr. Dinakarpandian and colleagues collected data from Nov. 23, 2020, from three sources of information regarding deaths among physicians: MedPage Today, which used investigative and voluntary reporting; Medscape, which used voluntary reporting of verifiable information; and a collaboration of The Guardian and Kaiser Health News, which used investigative reporting.
The Medscape project was launched on April 1, 2020. The MedPage Today and The Guardian/Kaiser Health News projects were launched on April 8, 2020.
Dr. Verghese and colleagues researched obituaries and news articles referenced by the three projects to verify their data. They used DocInfo to ascertain the deceased physicians’ medical schools.
After eliminating duplications from the lists, the researchers counted 132 physician deaths in 28 states. Of these, 59 physicians had graduated from medical schools outside the United States, a death toll 1.8 times higher than the proportion of IMGs among U.S. physicians (95% confidence interval, 1.52-2.21; P < .001).
New York, New Jersey, and Florida accounted for 66% of the deaths among IMGs but for only 45% of the deaths among U.S. medical school graduates.
Within each state, the proportion of IMGs among deceased physicians was not statistically different from their proportion among physicians in those states, with the exception of New York.
Two-thirds of the physicians’ deaths occurred in states where IMGs make up a larger proportion of physicians than in the nation as a whole. In these states, the incidence of COVID-19 was high at the start of the pandemic.
In New York, IMGs accounted for 60% of physician deaths, which was 1.62 times higher (95% CI, 1.26-2.09; P = .005) than the 37% among New York physicians overall.
Physicians who were trained abroad frequently can’t get into the most prestigious residency programs or into the highest paid specialties and are more likely to serve in primary care, Dr. Verghese said. Overall, 60% of the physicians who died of COVID-19 worked in primary care.
IMGs often staff hospitals serving low-income communities and communities of color, which were hardest hit by the pandemic and where personal protective equipment was hard to obtain, said Dr. Verghese.
In addition to these risks, IMGs sometimes endure racism, said Dr. Verghese, who obtained his medical degree at Madras Medical College, Chennai, India. “We’ve actually seen in the COVID era, in keeping with the sort of political tone that was set in Washington, that there’s been a lot more abuses of both foreign physicians and foreign looking physicians – even if they’re not foreign trained – and nurses by patients who have been given license. And I want to acknowledge the heroism of all these physicians.”
The study was partially funded by the Presence Center at Stanford. Dr. Verghese is a regular contributor to Medscape. He served on the advisory board for Gilead Sciences, serves as a speaker or a member of a speakers bureau for Leigh Bureau, and receives royalties from Penguin Random House and Simon & Schuster.
A version of this article first appeared on Medscape.com.













