User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Lithium and kidney disease: Understand the risks
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.
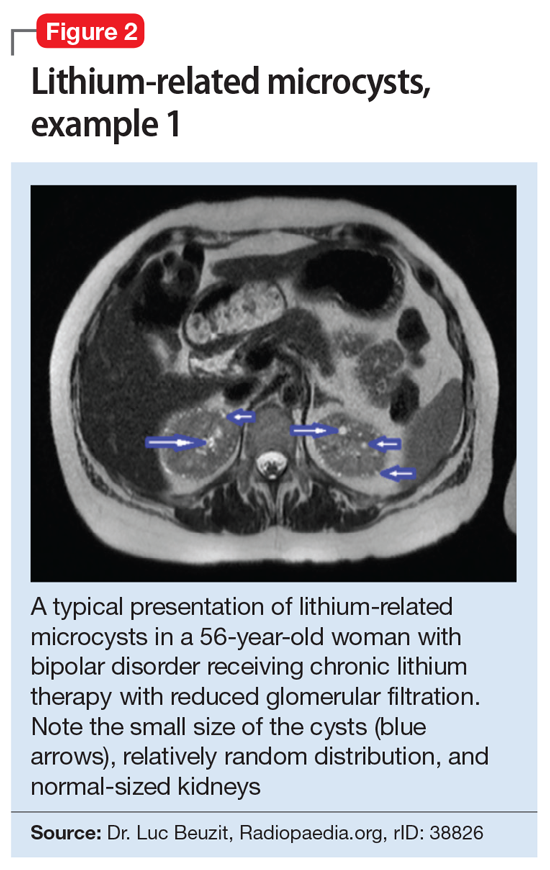
Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37
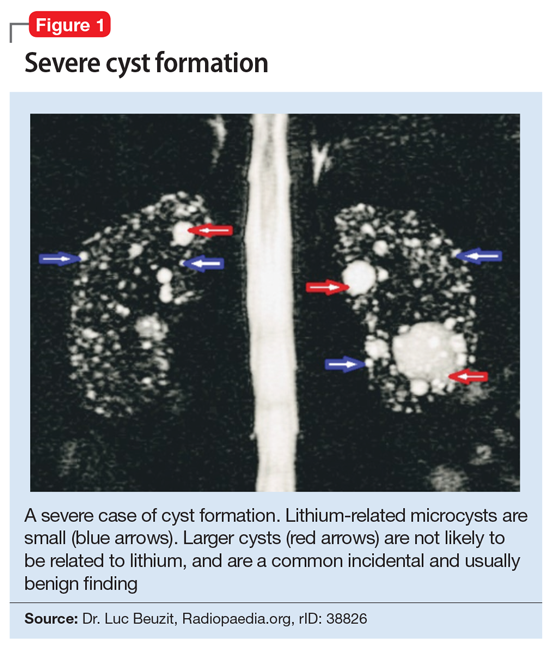
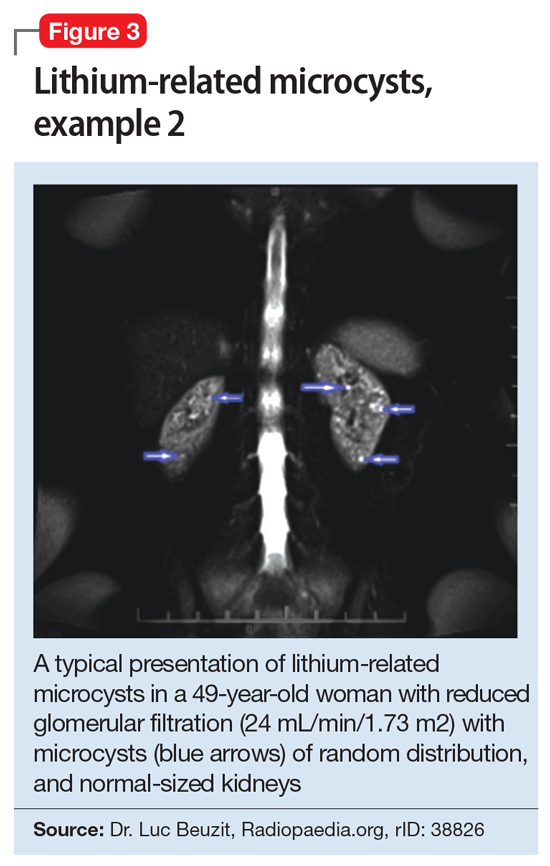
This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).
Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.

Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37

This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).

Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.

Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37

This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).

Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
Storing patients’ credit card information: Keep it safe
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
Harassment of health care workers: A survey
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?
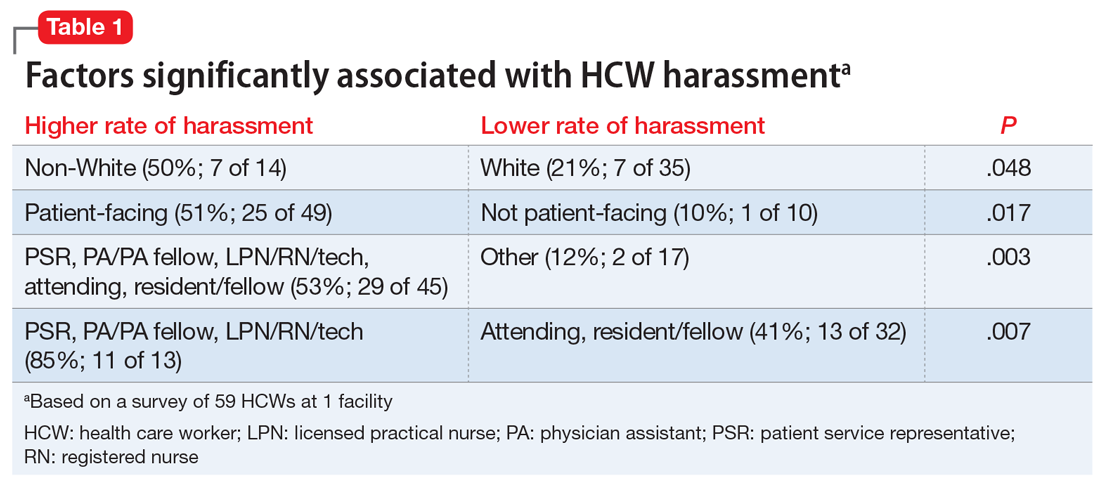
I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).
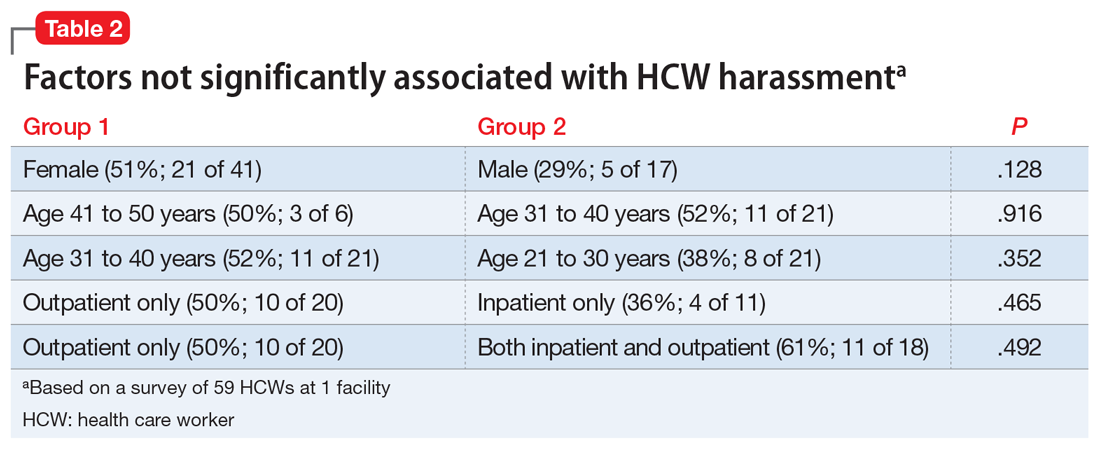
In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?

I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).

In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?

I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).

In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
Private practice: The basics for psychiatry trainees
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
Efficacy and safety of high-dose antipsychotic therapy
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).
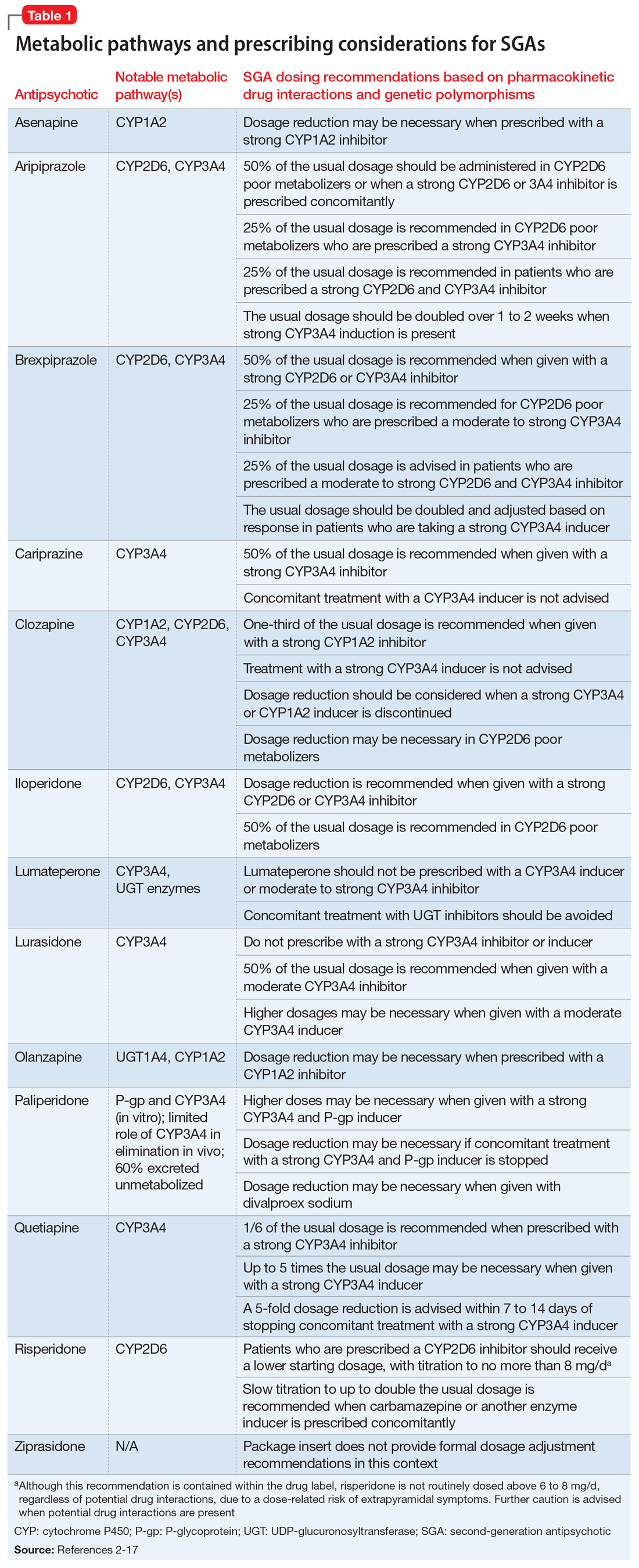
Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.
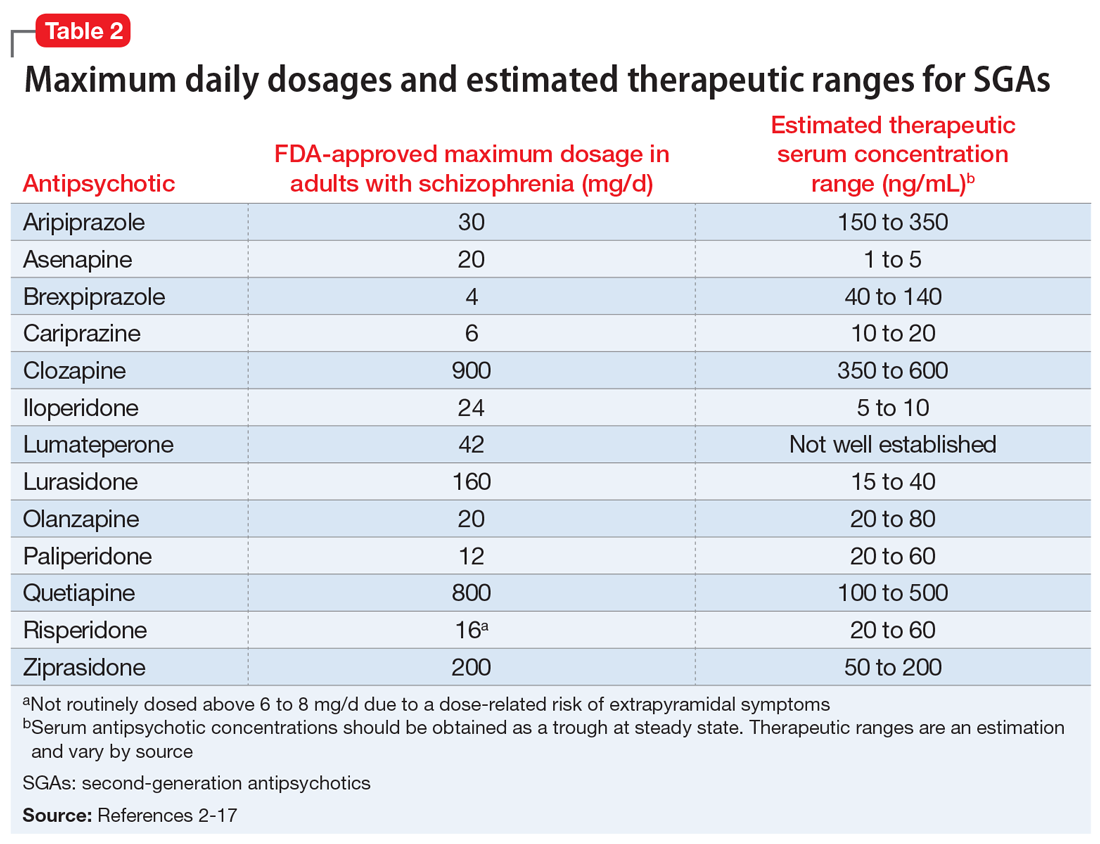
SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).

Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.

SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).

Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.

SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
FDA fast tracks testing of schizophrenia drug for impaired cognition
The U.S. Food and Drug Administration has granted breakthrough therapy designation for Boehringer Ingelheim’s experimental agent for the treatment of cognitive impairment associated with schizophrenia (CIAS).
The drug, known as BI 425809, is a novel glycine transporter-1 inhibitor.
The company announced it will start the CONNEX phase 3 clinical trial program to assess the safety and efficacy of the drug for improving cognition for adults with schizophrenia.
The breakthrough therapy designation and the initiation of phase 3 testing are based on results from a phase 2 clinical trial published in The Lancet Psychiatry.
In the phase 2 trial, oral BI 425809, taken once daily, improved cognition after 12 weeks for patients with schizophrenia; doses of 10 mg and 25 mg showed the largest separation from placebo.
Impairment of cognitive function is a major burden for people with schizophrenia, and no pharmacologic treatments are currently approved for CIAS.
“Cognition is a fundamental aspect of everyday life, including problem solving, memory, and attention, which is why finding solutions for cognitive impairment is a key area of Boehringer Ingelheim mental health research,” Vikas Mohan Sharma, MS, with Boehringer Ingelheim, said in a news release.
“This breakthrough therapy designation further highlights the urgent need for novel treatments for people living with schizophrenia. By combining traditional treatment approaches with new and innovative technologies, we are developing targeted therapies that will help to ease the burden of mental health conditions and enable people living with these conditions to create more meaningful connections to their lives, loved ones, and society,” said Mr. Sharma.
The CONNEX clinical trial program is composed of three clinical trials – CONNEX-1, CONNEX-2, and CONNEX-3. All are phase 3 randomized, double-blind, placebo-controlled parallel group trials that will examine the efficacy and safety of BI 425809 taken once daily over a 26-week period for patients with schizophrenia.
The CONNEX trial program will use VeraSci’s Pathway electronic clinical outcome assessment platform, including VeraSci’s Virtual Reality Functional Capacity Assessment Tool (VRFCAT), which simulates key instrumental activities of daily living in a realistic interactive virtual environment, VeraSci explains in a news release announcing the partnership with Boehringer Ingelheim.
The VRFCAT is sensitive to functional capacity deficits and has been accepted into the FDA’s Clinical Outcome Assessment Qualification Program.
The CONNEX trials will also utilize speech biomarker technology from Aural Analytics, which will provide a “richer picture of trial participants’ cognition alongside more conventional clinical outcome measures,” Boehringer Ingelheim says.
“Several of the symptoms of schizophrenia are generated by cognitive and emotional processes that can be identified through disruptions in the outward flow of speech. Using innovative speech analytics may help to objectively assess the downstream consequences of these disruptions,” said Daniel Jones, Aural Analytics co-founder and CEO.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted breakthrough therapy designation for Boehringer Ingelheim’s experimental agent for the treatment of cognitive impairment associated with schizophrenia (CIAS).
The drug, known as BI 425809, is a novel glycine transporter-1 inhibitor.
The company announced it will start the CONNEX phase 3 clinical trial program to assess the safety and efficacy of the drug for improving cognition for adults with schizophrenia.
The breakthrough therapy designation and the initiation of phase 3 testing are based on results from a phase 2 clinical trial published in The Lancet Psychiatry.
In the phase 2 trial, oral BI 425809, taken once daily, improved cognition after 12 weeks for patients with schizophrenia; doses of 10 mg and 25 mg showed the largest separation from placebo.
Impairment of cognitive function is a major burden for people with schizophrenia, and no pharmacologic treatments are currently approved for CIAS.
“Cognition is a fundamental aspect of everyday life, including problem solving, memory, and attention, which is why finding solutions for cognitive impairment is a key area of Boehringer Ingelheim mental health research,” Vikas Mohan Sharma, MS, with Boehringer Ingelheim, said in a news release.
“This breakthrough therapy designation further highlights the urgent need for novel treatments for people living with schizophrenia. By combining traditional treatment approaches with new and innovative technologies, we are developing targeted therapies that will help to ease the burden of mental health conditions and enable people living with these conditions to create more meaningful connections to their lives, loved ones, and society,” said Mr. Sharma.
The CONNEX clinical trial program is composed of three clinical trials – CONNEX-1, CONNEX-2, and CONNEX-3. All are phase 3 randomized, double-blind, placebo-controlled parallel group trials that will examine the efficacy and safety of BI 425809 taken once daily over a 26-week period for patients with schizophrenia.
The CONNEX trial program will use VeraSci’s Pathway electronic clinical outcome assessment platform, including VeraSci’s Virtual Reality Functional Capacity Assessment Tool (VRFCAT), which simulates key instrumental activities of daily living in a realistic interactive virtual environment, VeraSci explains in a news release announcing the partnership with Boehringer Ingelheim.
The VRFCAT is sensitive to functional capacity deficits and has been accepted into the FDA’s Clinical Outcome Assessment Qualification Program.
The CONNEX trials will also utilize speech biomarker technology from Aural Analytics, which will provide a “richer picture of trial participants’ cognition alongside more conventional clinical outcome measures,” Boehringer Ingelheim says.
“Several of the symptoms of schizophrenia are generated by cognitive and emotional processes that can be identified through disruptions in the outward flow of speech. Using innovative speech analytics may help to objectively assess the downstream consequences of these disruptions,” said Daniel Jones, Aural Analytics co-founder and CEO.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted breakthrough therapy designation for Boehringer Ingelheim’s experimental agent for the treatment of cognitive impairment associated with schizophrenia (CIAS).
The drug, known as BI 425809, is a novel glycine transporter-1 inhibitor.
The company announced it will start the CONNEX phase 3 clinical trial program to assess the safety and efficacy of the drug for improving cognition for adults with schizophrenia.
The breakthrough therapy designation and the initiation of phase 3 testing are based on results from a phase 2 clinical trial published in The Lancet Psychiatry.
In the phase 2 trial, oral BI 425809, taken once daily, improved cognition after 12 weeks for patients with schizophrenia; doses of 10 mg and 25 mg showed the largest separation from placebo.
Impairment of cognitive function is a major burden for people with schizophrenia, and no pharmacologic treatments are currently approved for CIAS.
“Cognition is a fundamental aspect of everyday life, including problem solving, memory, and attention, which is why finding solutions for cognitive impairment is a key area of Boehringer Ingelheim mental health research,” Vikas Mohan Sharma, MS, with Boehringer Ingelheim, said in a news release.
“This breakthrough therapy designation further highlights the urgent need for novel treatments for people living with schizophrenia. By combining traditional treatment approaches with new and innovative technologies, we are developing targeted therapies that will help to ease the burden of mental health conditions and enable people living with these conditions to create more meaningful connections to their lives, loved ones, and society,” said Mr. Sharma.
The CONNEX clinical trial program is composed of three clinical trials – CONNEX-1, CONNEX-2, and CONNEX-3. All are phase 3 randomized, double-blind, placebo-controlled parallel group trials that will examine the efficacy and safety of BI 425809 taken once daily over a 26-week period for patients with schizophrenia.
The CONNEX trial program will use VeraSci’s Pathway electronic clinical outcome assessment platform, including VeraSci’s Virtual Reality Functional Capacity Assessment Tool (VRFCAT), which simulates key instrumental activities of daily living in a realistic interactive virtual environment, VeraSci explains in a news release announcing the partnership with Boehringer Ingelheim.
The VRFCAT is sensitive to functional capacity deficits and has been accepted into the FDA’s Clinical Outcome Assessment Qualification Program.
The CONNEX trials will also utilize speech biomarker technology from Aural Analytics, which will provide a “richer picture of trial participants’ cognition alongside more conventional clinical outcome measures,” Boehringer Ingelheim says.
“Several of the symptoms of schizophrenia are generated by cognitive and emotional processes that can be identified through disruptions in the outward flow of speech. Using innovative speech analytics may help to objectively assess the downstream consequences of these disruptions,” said Daniel Jones, Aural Analytics co-founder and CEO.
A version of this article first appeared on Medscape.com.
Bill seeks to streamline prior authorization in Medicare Advantage plans
A group of bipartisan lawmakers intends to compel insurers to streamline prior authorization processes for Medicare Advantage plans, including a bid to end the use of faxes and develop systems that can allow for real-time decisions.
Rep. Suzan DelBene (D-Wash.); Rep. Mike Kelly (R-Pa.); Rep. Ami Bera, MD (D-Calif.); and Rep. Larry Bucshon, MD, (R-Ind.) on May 13 introduced a bill that would task federal officials with refining standards regarding prior authorization for Medicare Advantage. Titled the Improving Seniors’ Timely Access to Care Act of 2021, the bill would direct the Department of Health & Human Services to create rules intended to make prior authorization more transparent and speedy for the insurer-run Medicare plans. Known as Medicare Advantage, these plans cover about 24.1 million people of the 62 million enrolled in the giant federal health program, according to the nonprofit Kaiser Family Foundation.
These revamped prior authorization systems could not rely on faxes nor could they employ proprietary payer portals that did not meet HHS’ standards, says the text of the bill released by Rep. DelBene. Insurers would also have to report to the Centers for Medicare & Medicaid Services about the extent of their use of prior authorization and the rate of approvals or denials. The bill seeks to encourage plans to adopt prior authorization programs that adhere to evidence-based medical guidelines in consultation with physicians.
There were several reasons for focusing on Medicare Advantage plans, although prior authorization concerns extend more broadly in the U.S. health care system, said Susan Bailey, MD, president of the American Medical Association.
There’s an ample body of research about issues seen in the Medicare Advantage plans. Dr. Bailey also said that, in her experience, Medicare Advantage plans have had some of the most restrictive policies. And, by starting with Medicare Advantage, there’s a potential for a ripple effect in the industry, easing this issue when physicians work with other insurers as well.
“When Medicare adopts a policy whether it be a payment policy or a coverage policy, private insurers typically follow along,” she said.
Strong support among health care groups
There’s strong support for streamlining prior authorization both in the medical community and in Congress.
The bill has the support of about 70 health care organizations, including the AMA and the American Academy of Family Physicians, according to its sponsors. As of May 17, the bill had attracted the backing of 97 members of the House of Representatives, roughly evenly split among Democrats and Republicans.
Rep. DelBene’s previous version of this bill, the Improving Seniors’ Timely Access to Care Act of 2019, attracted 143 Democratic cosponsors and 137 Republican ones, or more than half of the members of the House. This bill was not completed during the previous session of Congress (January 2019–January 2021) because of the more urgent needs of pandemic response, said Rep. Bucshon, who practiced cardiothoracic surgery before joining Congress.
“It wasn’t quite on the radar as much as it might have been if we didn’t have COVID,” Rep. Bucshon said.
Rep. Bucshon added that he expects strong Senate support for a companion measure of the House bill, which could make the difference for efforts to pass it this year.
Insurers have become more aggressive over time in denying payments through prior authorization systems for services that physicians say their patients need, according to Rep. Bucshon. There may be some “bad actors” in medicine who would order unnecessary procedures, Rep. Bucshon allowed, but in most cases, the cumbersome prior authorization processes only put a hurdle for patients seeking needed treatments, he said.
“The premise is that it controls health care costs but actually what it does is it helps insurance company’s bottom line,” Rep. Bucshon said.
In a prepared statement, former Pennsylvania representative Allyson Y. Schwartz, now CEO of the Better Medicare Alliance, said her group had spoken with sponsors of this legislation and appreciates “their receptiveness to feedback in this process.”
“Prior authorization ensures beneficiaries receive clinically appropriate care and reduces exposures to duplicative and unnecessary services,” Ms. Schwartz said. “We share an interest in ensuring prior authorization works as smoothly and effectively as possible for beneficiaries while protecting its essential function of facilitating safe, evidenced-based care.”
The Better Medicare Alliance said its funders include UnitedHealth, Humana, and CVS Health/Aetna, which run Advantage plans. The group also lists as its partners many medical organizations.
“Rationing care by hassling”
Like Rep. Bucshon, Dr. Bailey sees a different motivation in insurers’ persistence in keeping the prior authorization process cumbersome.
Phone calls and faxes remain the key methods for handling prior authorization for medical services, according to the results of a survey done by the AMA in December. Phone calls were always or often required for prior authorization for medical services (59%), with faxes the second-most common approach (46%), followed by health plans’ online portals (39%), electronic health records and practice management systems (29%), and email or U.S. mail (26%), according to the AMA’s report on the survey.
“It seems like every step in the process is designed to make the patient less likely to get the therapy that the doctor thinks that the patient needs,” Dr. Bailey said. “It’s almost like rationing care by hassling the patient and the physician.”
The findings of an investigation by HHS’ internal watchdog unit appear to support Dr. Bailey’s view, showing that insurer-run Medicare plans had a pattern of often walking back their initial rejections.
In 2018, the Office of the Inspector General for HHS reported that Medicare Advantage organizations (MAOs) overturned 75% of their own denials during 2014-16. In addition, independent reviewers within the appeals process overturned additional denials in favor of patients and clinicians, OIG said.
“The high number of overturned denials raises concerns that some Medicare Advantage beneficiaries and providers were initially denied services and payments that should have been provided,” the OIG said in the report. “This is especially concerning because beneficiaries and providers rarely used the appeals process, which is designed to ensure access to care and payment.”
During 2014-2016, patients and clinicians appealed only 1% of denials to the first level of appeal, OIG said. In the report, the watchdog group noted that CMS audits had highlighted “widespread and persistent MAO performance problems related to denials of care and payment.” In 2015, for example, CMS cited 56% of audited contracts for making inappropriate denials.
Dr. Bailey also said in an interview that she routinely encounters problems with prior authorization in her own practice as an allergist and immunologist in Fort Worth, Tex.
In late May, for example, a Medicare Advantage plan made a patient whose chronic asthma had been stable for years change to a new inhaler that resulted in him developing a yeast infection in his mouth, Dr. Bailey said.
“We treated the yeast infection, made some changes in the way he uses his inhaler, so hopefully he would tolerate it better,” Dr. Bailey said. “He had a reaction to the medication to treat the yeast infection and ended up in the hospital. How is that helping anyone? It certainly hasn’t helped my patient.”
Dr. Bailey said insurers have also asked to seek prior authorization to prescribe medications that have been generic for years and have used the process to challenge her on cases of what seem to be common sense in medical practice. This included a bid to have Dr. Bailey prescribe a medication in pill form for a 6-month-old baby who had no teeth.
“Every doctor has got absurd stories like that, but unfortunately, every doctor is going to have tragic stories where prior authorization has resulted in death and harm to the patients,” Dr. Bailey said.
Some physicians leave it to the patient to try to overcome insurers’ decisions on prior authorization, seeing this task as falling outside of their duties, Dr. Bailey said.
“I don’t do that. I fight. I spend a lot of time fighting. I don’t like to lose. I don’t like my patients to lose, so I will go to the mat for them,” Dr. Bailey said. “But I’m blessed to be in a specialty where I’ve got loads more control over my schedule than many other specialties do.”
A version of this article first appeared on Medscape.com.
A group of bipartisan lawmakers intends to compel insurers to streamline prior authorization processes for Medicare Advantage plans, including a bid to end the use of faxes and develop systems that can allow for real-time decisions.
Rep. Suzan DelBene (D-Wash.); Rep. Mike Kelly (R-Pa.); Rep. Ami Bera, MD (D-Calif.); and Rep. Larry Bucshon, MD, (R-Ind.) on May 13 introduced a bill that would task federal officials with refining standards regarding prior authorization for Medicare Advantage. Titled the Improving Seniors’ Timely Access to Care Act of 2021, the bill would direct the Department of Health & Human Services to create rules intended to make prior authorization more transparent and speedy for the insurer-run Medicare plans. Known as Medicare Advantage, these plans cover about 24.1 million people of the 62 million enrolled in the giant federal health program, according to the nonprofit Kaiser Family Foundation.
These revamped prior authorization systems could not rely on faxes nor could they employ proprietary payer portals that did not meet HHS’ standards, says the text of the bill released by Rep. DelBene. Insurers would also have to report to the Centers for Medicare & Medicaid Services about the extent of their use of prior authorization and the rate of approvals or denials. The bill seeks to encourage plans to adopt prior authorization programs that adhere to evidence-based medical guidelines in consultation with physicians.
There were several reasons for focusing on Medicare Advantage plans, although prior authorization concerns extend more broadly in the U.S. health care system, said Susan Bailey, MD, president of the American Medical Association.
There’s an ample body of research about issues seen in the Medicare Advantage plans. Dr. Bailey also said that, in her experience, Medicare Advantage plans have had some of the most restrictive policies. And, by starting with Medicare Advantage, there’s a potential for a ripple effect in the industry, easing this issue when physicians work with other insurers as well.
“When Medicare adopts a policy whether it be a payment policy or a coverage policy, private insurers typically follow along,” she said.
Strong support among health care groups
There’s strong support for streamlining prior authorization both in the medical community and in Congress.
The bill has the support of about 70 health care organizations, including the AMA and the American Academy of Family Physicians, according to its sponsors. As of May 17, the bill had attracted the backing of 97 members of the House of Representatives, roughly evenly split among Democrats and Republicans.
Rep. DelBene’s previous version of this bill, the Improving Seniors’ Timely Access to Care Act of 2019, attracted 143 Democratic cosponsors and 137 Republican ones, or more than half of the members of the House. This bill was not completed during the previous session of Congress (January 2019–January 2021) because of the more urgent needs of pandemic response, said Rep. Bucshon, who practiced cardiothoracic surgery before joining Congress.
“It wasn’t quite on the radar as much as it might have been if we didn’t have COVID,” Rep. Bucshon said.
Rep. Bucshon added that he expects strong Senate support for a companion measure of the House bill, which could make the difference for efforts to pass it this year.
Insurers have become more aggressive over time in denying payments through prior authorization systems for services that physicians say their patients need, according to Rep. Bucshon. There may be some “bad actors” in medicine who would order unnecessary procedures, Rep. Bucshon allowed, but in most cases, the cumbersome prior authorization processes only put a hurdle for patients seeking needed treatments, he said.
“The premise is that it controls health care costs but actually what it does is it helps insurance company’s bottom line,” Rep. Bucshon said.
In a prepared statement, former Pennsylvania representative Allyson Y. Schwartz, now CEO of the Better Medicare Alliance, said her group had spoken with sponsors of this legislation and appreciates “their receptiveness to feedback in this process.”
“Prior authorization ensures beneficiaries receive clinically appropriate care and reduces exposures to duplicative and unnecessary services,” Ms. Schwartz said. “We share an interest in ensuring prior authorization works as smoothly and effectively as possible for beneficiaries while protecting its essential function of facilitating safe, evidenced-based care.”
The Better Medicare Alliance said its funders include UnitedHealth, Humana, and CVS Health/Aetna, which run Advantage plans. The group also lists as its partners many medical organizations.
“Rationing care by hassling”
Like Rep. Bucshon, Dr. Bailey sees a different motivation in insurers’ persistence in keeping the prior authorization process cumbersome.
Phone calls and faxes remain the key methods for handling prior authorization for medical services, according to the results of a survey done by the AMA in December. Phone calls were always or often required for prior authorization for medical services (59%), with faxes the second-most common approach (46%), followed by health plans’ online portals (39%), electronic health records and practice management systems (29%), and email or U.S. mail (26%), according to the AMA’s report on the survey.
“It seems like every step in the process is designed to make the patient less likely to get the therapy that the doctor thinks that the patient needs,” Dr. Bailey said. “It’s almost like rationing care by hassling the patient and the physician.”
The findings of an investigation by HHS’ internal watchdog unit appear to support Dr. Bailey’s view, showing that insurer-run Medicare plans had a pattern of often walking back their initial rejections.
In 2018, the Office of the Inspector General for HHS reported that Medicare Advantage organizations (MAOs) overturned 75% of their own denials during 2014-16. In addition, independent reviewers within the appeals process overturned additional denials in favor of patients and clinicians, OIG said.
“The high number of overturned denials raises concerns that some Medicare Advantage beneficiaries and providers were initially denied services and payments that should have been provided,” the OIG said in the report. “This is especially concerning because beneficiaries and providers rarely used the appeals process, which is designed to ensure access to care and payment.”
During 2014-2016, patients and clinicians appealed only 1% of denials to the first level of appeal, OIG said. In the report, the watchdog group noted that CMS audits had highlighted “widespread and persistent MAO performance problems related to denials of care and payment.” In 2015, for example, CMS cited 56% of audited contracts for making inappropriate denials.
Dr. Bailey also said in an interview that she routinely encounters problems with prior authorization in her own practice as an allergist and immunologist in Fort Worth, Tex.
In late May, for example, a Medicare Advantage plan made a patient whose chronic asthma had been stable for years change to a new inhaler that resulted in him developing a yeast infection in his mouth, Dr. Bailey said.
“We treated the yeast infection, made some changes in the way he uses his inhaler, so hopefully he would tolerate it better,” Dr. Bailey said. “He had a reaction to the medication to treat the yeast infection and ended up in the hospital. How is that helping anyone? It certainly hasn’t helped my patient.”
Dr. Bailey said insurers have also asked to seek prior authorization to prescribe medications that have been generic for years and have used the process to challenge her on cases of what seem to be common sense in medical practice. This included a bid to have Dr. Bailey prescribe a medication in pill form for a 6-month-old baby who had no teeth.
“Every doctor has got absurd stories like that, but unfortunately, every doctor is going to have tragic stories where prior authorization has resulted in death and harm to the patients,” Dr. Bailey said.
Some physicians leave it to the patient to try to overcome insurers’ decisions on prior authorization, seeing this task as falling outside of their duties, Dr. Bailey said.
“I don’t do that. I fight. I spend a lot of time fighting. I don’t like to lose. I don’t like my patients to lose, so I will go to the mat for them,” Dr. Bailey said. “But I’m blessed to be in a specialty where I’ve got loads more control over my schedule than many other specialties do.”
A version of this article first appeared on Medscape.com.
A group of bipartisan lawmakers intends to compel insurers to streamline prior authorization processes for Medicare Advantage plans, including a bid to end the use of faxes and develop systems that can allow for real-time decisions.
Rep. Suzan DelBene (D-Wash.); Rep. Mike Kelly (R-Pa.); Rep. Ami Bera, MD (D-Calif.); and Rep. Larry Bucshon, MD, (R-Ind.) on May 13 introduced a bill that would task federal officials with refining standards regarding prior authorization for Medicare Advantage. Titled the Improving Seniors’ Timely Access to Care Act of 2021, the bill would direct the Department of Health & Human Services to create rules intended to make prior authorization more transparent and speedy for the insurer-run Medicare plans. Known as Medicare Advantage, these plans cover about 24.1 million people of the 62 million enrolled in the giant federal health program, according to the nonprofit Kaiser Family Foundation.
These revamped prior authorization systems could not rely on faxes nor could they employ proprietary payer portals that did not meet HHS’ standards, says the text of the bill released by Rep. DelBene. Insurers would also have to report to the Centers for Medicare & Medicaid Services about the extent of their use of prior authorization and the rate of approvals or denials. The bill seeks to encourage plans to adopt prior authorization programs that adhere to evidence-based medical guidelines in consultation with physicians.
There were several reasons for focusing on Medicare Advantage plans, although prior authorization concerns extend more broadly in the U.S. health care system, said Susan Bailey, MD, president of the American Medical Association.
There’s an ample body of research about issues seen in the Medicare Advantage plans. Dr. Bailey also said that, in her experience, Medicare Advantage plans have had some of the most restrictive policies. And, by starting with Medicare Advantage, there’s a potential for a ripple effect in the industry, easing this issue when physicians work with other insurers as well.
“When Medicare adopts a policy whether it be a payment policy or a coverage policy, private insurers typically follow along,” she said.
Strong support among health care groups
There’s strong support for streamlining prior authorization both in the medical community and in Congress.
The bill has the support of about 70 health care organizations, including the AMA and the American Academy of Family Physicians, according to its sponsors. As of May 17, the bill had attracted the backing of 97 members of the House of Representatives, roughly evenly split among Democrats and Republicans.
Rep. DelBene’s previous version of this bill, the Improving Seniors’ Timely Access to Care Act of 2019, attracted 143 Democratic cosponsors and 137 Republican ones, or more than half of the members of the House. This bill was not completed during the previous session of Congress (January 2019–January 2021) because of the more urgent needs of pandemic response, said Rep. Bucshon, who practiced cardiothoracic surgery before joining Congress.
“It wasn’t quite on the radar as much as it might have been if we didn’t have COVID,” Rep. Bucshon said.
Rep. Bucshon added that he expects strong Senate support for a companion measure of the House bill, which could make the difference for efforts to pass it this year.
Insurers have become more aggressive over time in denying payments through prior authorization systems for services that physicians say their patients need, according to Rep. Bucshon. There may be some “bad actors” in medicine who would order unnecessary procedures, Rep. Bucshon allowed, but in most cases, the cumbersome prior authorization processes only put a hurdle for patients seeking needed treatments, he said.
“The premise is that it controls health care costs but actually what it does is it helps insurance company’s bottom line,” Rep. Bucshon said.
In a prepared statement, former Pennsylvania representative Allyson Y. Schwartz, now CEO of the Better Medicare Alliance, said her group had spoken with sponsors of this legislation and appreciates “their receptiveness to feedback in this process.”
“Prior authorization ensures beneficiaries receive clinically appropriate care and reduces exposures to duplicative and unnecessary services,” Ms. Schwartz said. “We share an interest in ensuring prior authorization works as smoothly and effectively as possible for beneficiaries while protecting its essential function of facilitating safe, evidenced-based care.”
The Better Medicare Alliance said its funders include UnitedHealth, Humana, and CVS Health/Aetna, which run Advantage plans. The group also lists as its partners many medical organizations.
“Rationing care by hassling”
Like Rep. Bucshon, Dr. Bailey sees a different motivation in insurers’ persistence in keeping the prior authorization process cumbersome.
Phone calls and faxes remain the key methods for handling prior authorization for medical services, according to the results of a survey done by the AMA in December. Phone calls were always or often required for prior authorization for medical services (59%), with faxes the second-most common approach (46%), followed by health plans’ online portals (39%), electronic health records and practice management systems (29%), and email or U.S. mail (26%), according to the AMA’s report on the survey.
“It seems like every step in the process is designed to make the patient less likely to get the therapy that the doctor thinks that the patient needs,” Dr. Bailey said. “It’s almost like rationing care by hassling the patient and the physician.”
The findings of an investigation by HHS’ internal watchdog unit appear to support Dr. Bailey’s view, showing that insurer-run Medicare plans had a pattern of often walking back their initial rejections.
In 2018, the Office of the Inspector General for HHS reported that Medicare Advantage organizations (MAOs) overturned 75% of their own denials during 2014-16. In addition, independent reviewers within the appeals process overturned additional denials in favor of patients and clinicians, OIG said.
“The high number of overturned denials raises concerns that some Medicare Advantage beneficiaries and providers were initially denied services and payments that should have been provided,” the OIG said in the report. “This is especially concerning because beneficiaries and providers rarely used the appeals process, which is designed to ensure access to care and payment.”
During 2014-2016, patients and clinicians appealed only 1% of denials to the first level of appeal, OIG said. In the report, the watchdog group noted that CMS audits had highlighted “widespread and persistent MAO performance problems related to denials of care and payment.” In 2015, for example, CMS cited 56% of audited contracts for making inappropriate denials.
Dr. Bailey also said in an interview that she routinely encounters problems with prior authorization in her own practice as an allergist and immunologist in Fort Worth, Tex.
In late May, for example, a Medicare Advantage plan made a patient whose chronic asthma had been stable for years change to a new inhaler that resulted in him developing a yeast infection in his mouth, Dr. Bailey said.
“We treated the yeast infection, made some changes in the way he uses his inhaler, so hopefully he would tolerate it better,” Dr. Bailey said. “He had a reaction to the medication to treat the yeast infection and ended up in the hospital. How is that helping anyone? It certainly hasn’t helped my patient.”
Dr. Bailey said insurers have also asked to seek prior authorization to prescribe medications that have been generic for years and have used the process to challenge her on cases of what seem to be common sense in medical practice. This included a bid to have Dr. Bailey prescribe a medication in pill form for a 6-month-old baby who had no teeth.
“Every doctor has got absurd stories like that, but unfortunately, every doctor is going to have tragic stories where prior authorization has resulted in death and harm to the patients,” Dr. Bailey said.
Some physicians leave it to the patient to try to overcome insurers’ decisions on prior authorization, seeing this task as falling outside of their duties, Dr. Bailey said.
“I don’t do that. I fight. I spend a lot of time fighting. I don’t like to lose. I don’t like my patients to lose, so I will go to the mat for them,” Dr. Bailey said. “But I’m blessed to be in a specialty where I’ve got loads more control over my schedule than many other specialties do.”
A version of this article first appeared on Medscape.com.
What brought me back from the brink of suicide: A physician’s story
William Lynes, MD, had a flourishing medical practice and a fulfilling family life with three children when he first attempted suicide in 1999 at age 45. By 2003, depression and two more suicide attempts led to his early retirement.
In a session at the recent virtual American Psychiatric Association (APA) 2021 annual meeting, Dr. Lynes talked about the challenges of dealing with depression while managing the stresses of a career in medicine. The session in which he spoke was called, “The Suicidal Physician: Narratives From a Physician Who Survived and the Physician Widow of One Who Did Not.”
By writing and speaking about his experiences, he says, he has been able to retain his identity as a physician and avoid obsessive thoughts about suicide. He hopes conversations like these help other physicians feel less alone and enable them to push past stigmas to get the help they need. He suspects they do. More than 600 people joined the APA session, and Dr. Lynes received dozens of thankful messages afterward.
“I love medicine, but intrinsically, the practice of medicine is stressful, and you can’t get away,” said Dr. Lynes, a retired urologist in Temecula, Calif. “As far as feedback, it made me feel like it’s something I should continue to do.”
A way to heal
For Dr. Lynes, his “downward spiral into darkness” began with a series of catastrophic medical events starting in 1998, when he came home from a family vacation in Mexico feeling unwell. He didn’t bother to do anything about it – typical of a physician, he says. Then one night he woke up shaking with chills and fever. Soon he was in the hospital with respiratory failure from septic shock.
Dr. Lynes spent 6 weeks in the intensive care unit, including 4 weeks on a ventilator. He underwent a tracheostomy. He lost 40 pounds and experienced ICU-related delirium. It was a terrifying time, he said. When he tried to return to work 10 months later, he didn’t feel as though he could function normally.
Having once been a driven doctor who worked long hours, he now doubted himself and dreaded giving patients bad news. Spontaneously, he tried to take his own life.
Afterward, he concealed what had happened from everyone except his wife and managed to resume his practice. However, he was unable to regain the enthusiasm he had once had for his work. Although he had experienced depression before, this time it was unrelenting.
He sought help from a psychiatrist, received a diagnosis of bipolar disorder, and began taking medication. Still, he struggled to fulfill his responsibilities. Then in April 2002, he had a snowboarding accident that caused multiple facial fractures and required five operations. When he returned to work this time, he felt like a failure but resisted asking colleagues for help.
A few months later, Dr. Lynes again attempted suicide, which led to another stay in the ICU and more time on a ventilator. Doctors told his family they didn’t think he would survive. When he recovered, he spent time as an inpatient in a psychiatric ward, where he received the first of a series of electroconvulsive therapy sessions. Compounding his anxiety and depression was the inability to come to terms with his life if he were not able to practice medicine.
The next fall, in September 2003, his third suicide attempt took place in his office on a weekend when no one was around. After locking the door, he looked at his reflection in the frame of his medical school diploma. The glass was cracked. “It was dark, it was black, it was cold,” he said. “I can remember seeing my reflection and thinking how disgusted I was.”
For years after that, Dr. Lynes struggled with his sense of self-worth. He hid from the medical system and dreaded doctors’ appointments. Finally in 2016, he found new meaning at a writing conference, where he met a fellow physician whose story was similar to his. She encouraged him to write about his experience. His essay was published in Annals of Internal Medicine that year. “Then I started speaking, and I feel like I’m a physician again,” he said. “That has really healed me quite a bit.”
Why physicians die by suicide
Working in health care can be extremely stressful, even in the best of times, said Michael Myers, MD, a psychiatrist at State University of New York, Brooklyn, and author of the book, “Why Physicians Die By Suicide: Lessons Learned From Their Families and Others Who Cared.”
Years of school and training culminate in a career in which demands are relentless. Societal expectations are high. Many doctors are perfectionists by nature, and physicians tend to feel intense pressure to compete for coveted positions.
Stress starts early in a medical career. A 2016 systematic review and meta-analysis of 183 studies from 43 countries showed that nearly 30% of medical students experienced symptoms of depression and that 11% reported suicidal thoughts, but only 15% sought help.
A 2015 review of 31 studies that involved residents showed that rates of depression remained close to 30% and that about three-quarters of trainees meet criteria for burnout, a type of emotional exhaustion and sense of inadequacy that can result from chronic stress at work.
The stress of medical training appears to be a direct cause of mental health struggles. Rates of depression are higher among those working to become physicians than among their peers of the same age, research shows. In addition, symptoms become more prevalent as people progress through their training.
The COVID-19 pandemic has added stress to an already stressful job. Of more than 2,300 physicians surveyed in August 2020 by the Physicians Foundation, a physicians advocacy organization, 50% indicated that they experienced excessive anger, tearfulness, or anxiety because of the way the pandemic affected their work; 30% felt hopeless or lacking purpose; and 8% had thoughts of self-harm related to the pandemic. Rates of burnout had risen from 40% in 2018 to 58%.
Those problems might be even more acute in places experiencing other types of crises. A 2020 study of 154 emergency department (ED) physicians in Libya, which is in the midst of a civil war, found that 65% were experiencing anxiety, 73% were showing signs of depression, and 68% felt emotionally exhausted.
Every story is different
It is unclear how common suicide is among physicians. One often-repeated estimate is that 300-400 physicians die by suicide each year, but no one is certain how that number was determined, said Dr. Myers, who organized the APA panel.
Studies on suicide are inconsistent, and trends are hard to pinpoint. Anecdotally, he has received just as many calls about physician suicides in the past year as he did before the pandemic started.
Every person is different, and so is every death. Sometimes, career problems have nothing to do with a physician’s suicide, Dr. Myers said. When job stress does play a role, factors are often varied and complex.
After a 35-year career as a double board certified ED physician, Matthew Seaman, MD, retired in January 2017. The same month, a patient filed a complaint against him with the Washington State medical board, which led to an investigation and a lawsuit.
The case was hard on Dr. Seaman, who had continued to work night shifts throughout his career and had won a Hero Award from the American Board of Emergency Medicine, said his wife, Linda Seaman, MD, a family practitioner in Yakima, Wash., who also spoke on the APA panel.
Dr. Seaman said that 2 years after the investigation started, her husband was growing increasingly depressed. In 2019, he testified in a deposition. She said the plaintiff’s attorney “tried every way he could to shame Matt, humiliate Matt, make him believe he was a very bad doctor.” Three days later, he died by suicide at age 62.
Looking back at the year leading up to her husband’s death, Dr. Seaman recognizes multiple obstacles that interfered with her husband’s ability to get help, including frustrating interactions with psychiatrists and the couple’s insurance company.
His identity and experience as a physician also played a role. A couple of months before he died, she tried unsuccessfully to reach his psychiatrist, whose office suggested he go to the ED. However, because he worked as an ED doctor in their small town, he wouldn’t go. Dr. Seaman suspects he was wary of the stigma.
Burnout likely set him up to cave in after decades of work on the front lines, she added. Working in the ED exposes providers to horrific, traumatic cases every day, she said. Physicians learn to suppress their own emotions to deal with what they encounter. Stuffing their feelings can lead to posttraumatic stress. “You just perform,” she said. “You learn to do that.”
A real gift
Whenever Dr. Myers hears stories about doctors who died by suicide or who have written about their mental health struggles to help others, he contacts them. One goal of his own writing and of the conference sessions he organizes is to make it easier for others to share their own stories.
“I tell them, first of all, their courage and honesty is a real gift, and they’re saving lives,” he said. “There are so many suffering doctors out there who think that they’re the only one.”
Public conversations such as those that occurred in the APA session also offer opportunities to share advice, including Dr. Myers’ recommendation that doctors be sure they have a primary care physician of their own.
Many don’t, he says, because they say they are too busy, they can treat their own symptoms, or they can self-refer to specialists when needed. But physicians don’t always recognize symptoms of depression in themselves, and when mental health problems arise, they may not seek help or treat themselves appropriately.
A primary care physician can be the first person to recognize a mental health problem and refer a patient for mental health care, said Dr. Myers, whose latest book, “Becoming a Doctors’ Doctor: A Memoir,” explores his experiences treating doctors with burnout and other mental health problems.
Whether they have a primary care doctor or not, he suggests that physicians talk to anyone they trust – a social worker, a religious leader, or a family member who can then help them find the right sort of care.
In the United States, around-the-clock help is available through the National Suicide Prevention Lifeline at 800-273-8255. A psychiatrist-run hotline specifically for physicians is available at 888-409-0141. “Reach out and get some help,” Dr. Myers said. “Just don’t do it alone.”
Dr. Lynes advocates setting boundaries between life and work. He has also benefited from writing about his experiences. A blog or a diary can help physicians process their feelings, he said. His 2016 essay marked a major turning point in his life, giving his life meaning in helping others.
“Since I wrote that article, I can’t tell you how much better I am,” he said. “Now, I’m not embarrassed to be around physicians. I actually consider myself a physician. I didn’t for many, many years. So, I’m doing pretty well.”
A version of this article first appeared on Medscape.com.
William Lynes, MD, had a flourishing medical practice and a fulfilling family life with three children when he first attempted suicide in 1999 at age 45. By 2003, depression and two more suicide attempts led to his early retirement.
In a session at the recent virtual American Psychiatric Association (APA) 2021 annual meeting, Dr. Lynes talked about the challenges of dealing with depression while managing the stresses of a career in medicine. The session in which he spoke was called, “The Suicidal Physician: Narratives From a Physician Who Survived and the Physician Widow of One Who Did Not.”
By writing and speaking about his experiences, he says, he has been able to retain his identity as a physician and avoid obsessive thoughts about suicide. He hopes conversations like these help other physicians feel less alone and enable them to push past stigmas to get the help they need. He suspects they do. More than 600 people joined the APA session, and Dr. Lynes received dozens of thankful messages afterward.
“I love medicine, but intrinsically, the practice of medicine is stressful, and you can’t get away,” said Dr. Lynes, a retired urologist in Temecula, Calif. “As far as feedback, it made me feel like it’s something I should continue to do.”
A way to heal
For Dr. Lynes, his “downward spiral into darkness” began with a series of catastrophic medical events starting in 1998, when he came home from a family vacation in Mexico feeling unwell. He didn’t bother to do anything about it – typical of a physician, he says. Then one night he woke up shaking with chills and fever. Soon he was in the hospital with respiratory failure from septic shock.
Dr. Lynes spent 6 weeks in the intensive care unit, including 4 weeks on a ventilator. He underwent a tracheostomy. He lost 40 pounds and experienced ICU-related delirium. It was a terrifying time, he said. When he tried to return to work 10 months later, he didn’t feel as though he could function normally.
Having once been a driven doctor who worked long hours, he now doubted himself and dreaded giving patients bad news. Spontaneously, he tried to take his own life.
Afterward, he concealed what had happened from everyone except his wife and managed to resume his practice. However, he was unable to regain the enthusiasm he had once had for his work. Although he had experienced depression before, this time it was unrelenting.
He sought help from a psychiatrist, received a diagnosis of bipolar disorder, and began taking medication. Still, he struggled to fulfill his responsibilities. Then in April 2002, he had a snowboarding accident that caused multiple facial fractures and required five operations. When he returned to work this time, he felt like a failure but resisted asking colleagues for help.
A few months later, Dr. Lynes again attempted suicide, which led to another stay in the ICU and more time on a ventilator. Doctors told his family they didn’t think he would survive. When he recovered, he spent time as an inpatient in a psychiatric ward, where he received the first of a series of electroconvulsive therapy sessions. Compounding his anxiety and depression was the inability to come to terms with his life if he were not able to practice medicine.
The next fall, in September 2003, his third suicide attempt took place in his office on a weekend when no one was around. After locking the door, he looked at his reflection in the frame of his medical school diploma. The glass was cracked. “It was dark, it was black, it was cold,” he said. “I can remember seeing my reflection and thinking how disgusted I was.”
For years after that, Dr. Lynes struggled with his sense of self-worth. He hid from the medical system and dreaded doctors’ appointments. Finally in 2016, he found new meaning at a writing conference, where he met a fellow physician whose story was similar to his. She encouraged him to write about his experience. His essay was published in Annals of Internal Medicine that year. “Then I started speaking, and I feel like I’m a physician again,” he said. “That has really healed me quite a bit.”
Why physicians die by suicide
Working in health care can be extremely stressful, even in the best of times, said Michael Myers, MD, a psychiatrist at State University of New York, Brooklyn, and author of the book, “Why Physicians Die By Suicide: Lessons Learned From Their Families and Others Who Cared.”
Years of school and training culminate in a career in which demands are relentless. Societal expectations are high. Many doctors are perfectionists by nature, and physicians tend to feel intense pressure to compete for coveted positions.
Stress starts early in a medical career. A 2016 systematic review and meta-analysis of 183 studies from 43 countries showed that nearly 30% of medical students experienced symptoms of depression and that 11% reported suicidal thoughts, but only 15% sought help.
A 2015 review of 31 studies that involved residents showed that rates of depression remained close to 30% and that about three-quarters of trainees meet criteria for burnout, a type of emotional exhaustion and sense of inadequacy that can result from chronic stress at work.
The stress of medical training appears to be a direct cause of mental health struggles. Rates of depression are higher among those working to become physicians than among their peers of the same age, research shows. In addition, symptoms become more prevalent as people progress through their training.
The COVID-19 pandemic has added stress to an already stressful job. Of more than 2,300 physicians surveyed in August 2020 by the Physicians Foundation, a physicians advocacy organization, 50% indicated that they experienced excessive anger, tearfulness, or anxiety because of the way the pandemic affected their work; 30% felt hopeless or lacking purpose; and 8% had thoughts of self-harm related to the pandemic. Rates of burnout had risen from 40% in 2018 to 58%.
Those problems might be even more acute in places experiencing other types of crises. A 2020 study of 154 emergency department (ED) physicians in Libya, which is in the midst of a civil war, found that 65% were experiencing anxiety, 73% were showing signs of depression, and 68% felt emotionally exhausted.
Every story is different
It is unclear how common suicide is among physicians. One often-repeated estimate is that 300-400 physicians die by suicide each year, but no one is certain how that number was determined, said Dr. Myers, who organized the APA panel.
Studies on suicide are inconsistent, and trends are hard to pinpoint. Anecdotally, he has received just as many calls about physician suicides in the past year as he did before the pandemic started.
Every person is different, and so is every death. Sometimes, career problems have nothing to do with a physician’s suicide, Dr. Myers said. When job stress does play a role, factors are often varied and complex.
After a 35-year career as a double board certified ED physician, Matthew Seaman, MD, retired in January 2017. The same month, a patient filed a complaint against him with the Washington State medical board, which led to an investigation and a lawsuit.
The case was hard on Dr. Seaman, who had continued to work night shifts throughout his career and had won a Hero Award from the American Board of Emergency Medicine, said his wife, Linda Seaman, MD, a family practitioner in Yakima, Wash., who also spoke on the APA panel.
Dr. Seaman said that 2 years after the investigation started, her husband was growing increasingly depressed. In 2019, he testified in a deposition. She said the plaintiff’s attorney “tried every way he could to shame Matt, humiliate Matt, make him believe he was a very bad doctor.” Three days later, he died by suicide at age 62.
Looking back at the year leading up to her husband’s death, Dr. Seaman recognizes multiple obstacles that interfered with her husband’s ability to get help, including frustrating interactions with psychiatrists and the couple’s insurance company.
His identity and experience as a physician also played a role. A couple of months before he died, she tried unsuccessfully to reach his psychiatrist, whose office suggested he go to the ED. However, because he worked as an ED doctor in their small town, he wouldn’t go. Dr. Seaman suspects he was wary of the stigma.
Burnout likely set him up to cave in after decades of work on the front lines, she added. Working in the ED exposes providers to horrific, traumatic cases every day, she said. Physicians learn to suppress their own emotions to deal with what they encounter. Stuffing their feelings can lead to posttraumatic stress. “You just perform,” she said. “You learn to do that.”
A real gift
Whenever Dr. Myers hears stories about doctors who died by suicide or who have written about their mental health struggles to help others, he contacts them. One goal of his own writing and of the conference sessions he organizes is to make it easier for others to share their own stories.
“I tell them, first of all, their courage and honesty is a real gift, and they’re saving lives,” he said. “There are so many suffering doctors out there who think that they’re the only one.”
Public conversations such as those that occurred in the APA session also offer opportunities to share advice, including Dr. Myers’ recommendation that doctors be sure they have a primary care physician of their own.
Many don’t, he says, because they say they are too busy, they can treat their own symptoms, or they can self-refer to specialists when needed. But physicians don’t always recognize symptoms of depression in themselves, and when mental health problems arise, they may not seek help or treat themselves appropriately.
A primary care physician can be the first person to recognize a mental health problem and refer a patient for mental health care, said Dr. Myers, whose latest book, “Becoming a Doctors’ Doctor: A Memoir,” explores his experiences treating doctors with burnout and other mental health problems.
Whether they have a primary care doctor or not, he suggests that physicians talk to anyone they trust – a social worker, a religious leader, or a family member who can then help them find the right sort of care.
In the United States, around-the-clock help is available through the National Suicide Prevention Lifeline at 800-273-8255. A psychiatrist-run hotline specifically for physicians is available at 888-409-0141. “Reach out and get some help,” Dr. Myers said. “Just don’t do it alone.”
Dr. Lynes advocates setting boundaries between life and work. He has also benefited from writing about his experiences. A blog or a diary can help physicians process their feelings, he said. His 2016 essay marked a major turning point in his life, giving his life meaning in helping others.
“Since I wrote that article, I can’t tell you how much better I am,” he said. “Now, I’m not embarrassed to be around physicians. I actually consider myself a physician. I didn’t for many, many years. So, I’m doing pretty well.”
A version of this article first appeared on Medscape.com.
William Lynes, MD, had a flourishing medical practice and a fulfilling family life with three children when he first attempted suicide in 1999 at age 45. By 2003, depression and two more suicide attempts led to his early retirement.
In a session at the recent virtual American Psychiatric Association (APA) 2021 annual meeting, Dr. Lynes talked about the challenges of dealing with depression while managing the stresses of a career in medicine. The session in which he spoke was called, “The Suicidal Physician: Narratives From a Physician Who Survived and the Physician Widow of One Who Did Not.”
By writing and speaking about his experiences, he says, he has been able to retain his identity as a physician and avoid obsessive thoughts about suicide. He hopes conversations like these help other physicians feel less alone and enable them to push past stigmas to get the help they need. He suspects they do. More than 600 people joined the APA session, and Dr. Lynes received dozens of thankful messages afterward.
“I love medicine, but intrinsically, the practice of medicine is stressful, and you can’t get away,” said Dr. Lynes, a retired urologist in Temecula, Calif. “As far as feedback, it made me feel like it’s something I should continue to do.”
A way to heal
For Dr. Lynes, his “downward spiral into darkness” began with a series of catastrophic medical events starting in 1998, when he came home from a family vacation in Mexico feeling unwell. He didn’t bother to do anything about it – typical of a physician, he says. Then one night he woke up shaking with chills and fever. Soon he was in the hospital with respiratory failure from septic shock.
Dr. Lynes spent 6 weeks in the intensive care unit, including 4 weeks on a ventilator. He underwent a tracheostomy. He lost 40 pounds and experienced ICU-related delirium. It was a terrifying time, he said. When he tried to return to work 10 months later, he didn’t feel as though he could function normally.
Having once been a driven doctor who worked long hours, he now doubted himself and dreaded giving patients bad news. Spontaneously, he tried to take his own life.
Afterward, he concealed what had happened from everyone except his wife and managed to resume his practice. However, he was unable to regain the enthusiasm he had once had for his work. Although he had experienced depression before, this time it was unrelenting.
He sought help from a psychiatrist, received a diagnosis of bipolar disorder, and began taking medication. Still, he struggled to fulfill his responsibilities. Then in April 2002, he had a snowboarding accident that caused multiple facial fractures and required five operations. When he returned to work this time, he felt like a failure but resisted asking colleagues for help.
A few months later, Dr. Lynes again attempted suicide, which led to another stay in the ICU and more time on a ventilator. Doctors told his family they didn’t think he would survive. When he recovered, he spent time as an inpatient in a psychiatric ward, where he received the first of a series of electroconvulsive therapy sessions. Compounding his anxiety and depression was the inability to come to terms with his life if he were not able to practice medicine.
The next fall, in September 2003, his third suicide attempt took place in his office on a weekend when no one was around. After locking the door, he looked at his reflection in the frame of his medical school diploma. The glass was cracked. “It was dark, it was black, it was cold,” he said. “I can remember seeing my reflection and thinking how disgusted I was.”
For years after that, Dr. Lynes struggled with his sense of self-worth. He hid from the medical system and dreaded doctors’ appointments. Finally in 2016, he found new meaning at a writing conference, where he met a fellow physician whose story was similar to his. She encouraged him to write about his experience. His essay was published in Annals of Internal Medicine that year. “Then I started speaking, and I feel like I’m a physician again,” he said. “That has really healed me quite a bit.”
Why physicians die by suicide
Working in health care can be extremely stressful, even in the best of times, said Michael Myers, MD, a psychiatrist at State University of New York, Brooklyn, and author of the book, “Why Physicians Die By Suicide: Lessons Learned From Their Families and Others Who Cared.”
Years of school and training culminate in a career in which demands are relentless. Societal expectations are high. Many doctors are perfectionists by nature, and physicians tend to feel intense pressure to compete for coveted positions.
Stress starts early in a medical career. A 2016 systematic review and meta-analysis of 183 studies from 43 countries showed that nearly 30% of medical students experienced symptoms of depression and that 11% reported suicidal thoughts, but only 15% sought help.
A 2015 review of 31 studies that involved residents showed that rates of depression remained close to 30% and that about three-quarters of trainees meet criteria for burnout, a type of emotional exhaustion and sense of inadequacy that can result from chronic stress at work.
The stress of medical training appears to be a direct cause of mental health struggles. Rates of depression are higher among those working to become physicians than among their peers of the same age, research shows. In addition, symptoms become more prevalent as people progress through their training.
The COVID-19 pandemic has added stress to an already stressful job. Of more than 2,300 physicians surveyed in August 2020 by the Physicians Foundation, a physicians advocacy organization, 50% indicated that they experienced excessive anger, tearfulness, or anxiety because of the way the pandemic affected their work; 30% felt hopeless or lacking purpose; and 8% had thoughts of self-harm related to the pandemic. Rates of burnout had risen from 40% in 2018 to 58%.
Those problems might be even more acute in places experiencing other types of crises. A 2020 study of 154 emergency department (ED) physicians in Libya, which is in the midst of a civil war, found that 65% were experiencing anxiety, 73% were showing signs of depression, and 68% felt emotionally exhausted.
Every story is different
It is unclear how common suicide is among physicians. One often-repeated estimate is that 300-400 physicians die by suicide each year, but no one is certain how that number was determined, said Dr. Myers, who organized the APA panel.
Studies on suicide are inconsistent, and trends are hard to pinpoint. Anecdotally, he has received just as many calls about physician suicides in the past year as he did before the pandemic started.
Every person is different, and so is every death. Sometimes, career problems have nothing to do with a physician’s suicide, Dr. Myers said. When job stress does play a role, factors are often varied and complex.
After a 35-year career as a double board certified ED physician, Matthew Seaman, MD, retired in January 2017. The same month, a patient filed a complaint against him with the Washington State medical board, which led to an investigation and a lawsuit.
The case was hard on Dr. Seaman, who had continued to work night shifts throughout his career and had won a Hero Award from the American Board of Emergency Medicine, said his wife, Linda Seaman, MD, a family practitioner in Yakima, Wash., who also spoke on the APA panel.
Dr. Seaman said that 2 years after the investigation started, her husband was growing increasingly depressed. In 2019, he testified in a deposition. She said the plaintiff’s attorney “tried every way he could to shame Matt, humiliate Matt, make him believe he was a very bad doctor.” Three days later, he died by suicide at age 62.
Looking back at the year leading up to her husband’s death, Dr. Seaman recognizes multiple obstacles that interfered with her husband’s ability to get help, including frustrating interactions with psychiatrists and the couple’s insurance company.
His identity and experience as a physician also played a role. A couple of months before he died, she tried unsuccessfully to reach his psychiatrist, whose office suggested he go to the ED. However, because he worked as an ED doctor in their small town, he wouldn’t go. Dr. Seaman suspects he was wary of the stigma.
Burnout likely set him up to cave in after decades of work on the front lines, she added. Working in the ED exposes providers to horrific, traumatic cases every day, she said. Physicians learn to suppress their own emotions to deal with what they encounter. Stuffing their feelings can lead to posttraumatic stress. “You just perform,” she said. “You learn to do that.”
A real gift
Whenever Dr. Myers hears stories about doctors who died by suicide or who have written about their mental health struggles to help others, he contacts them. One goal of his own writing and of the conference sessions he organizes is to make it easier for others to share their own stories.
“I tell them, first of all, their courage and honesty is a real gift, and they’re saving lives,” he said. “There are so many suffering doctors out there who think that they’re the only one.”
Public conversations such as those that occurred in the APA session also offer opportunities to share advice, including Dr. Myers’ recommendation that doctors be sure they have a primary care physician of their own.
Many don’t, he says, because they say they are too busy, they can treat their own symptoms, or they can self-refer to specialists when needed. But physicians don’t always recognize symptoms of depression in themselves, and when mental health problems arise, they may not seek help or treat themselves appropriately.
A primary care physician can be the first person to recognize a mental health problem and refer a patient for mental health care, said Dr. Myers, whose latest book, “Becoming a Doctors’ Doctor: A Memoir,” explores his experiences treating doctors with burnout and other mental health problems.
Whether they have a primary care doctor or not, he suggests that physicians talk to anyone they trust – a social worker, a religious leader, or a family member who can then help them find the right sort of care.
In the United States, around-the-clock help is available through the National Suicide Prevention Lifeline at 800-273-8255. A psychiatrist-run hotline specifically for physicians is available at 888-409-0141. “Reach out and get some help,” Dr. Myers said. “Just don’t do it alone.”
Dr. Lynes advocates setting boundaries between life and work. He has also benefited from writing about his experiences. A blog or a diary can help physicians process their feelings, he said. His 2016 essay marked a major turning point in his life, giving his life meaning in helping others.
“Since I wrote that article, I can’t tell you how much better I am,” he said. “Now, I’m not embarrassed to be around physicians. I actually consider myself a physician. I didn’t for many, many years. So, I’m doing pretty well.”
A version of this article first appeared on Medscape.com.
APA, AMA, others move to stop insurer from overturning mental health claims ruling
The American Psychiatric Association has joined with the American Medical Association and other medical societies to oppose United Behavioral Health’s (UBH) request that a court throw out a ruling that found the insurer unfairly denied tens of thousands of claims for mental health and substance use disorder services.
Wit v. United Behavioral Health, in litigation since 2014, is being closely watched by clinicians, patients, providers, and attorneys.
Reena Kapoor, MD, chair of the APA’s Committee on Judicial Action, said in an interview that the APA is hopeful that “whatever the court says about UBH should be applicable to all insurance companies that are providing employer-sponsored health benefits.”
In a friend of the court (amicus curiae) brief, the APA, AMA, the California Medical Association, Southern California Psychiatric Society, Northern California Psychiatric Society, Orange County Psychiatric Society, Central California Psychiatric Society, and San Diego Psychiatric Society argue that “despite the availability of professionally developed, evidence-based guidelines embodying generally accepted standards of care for mental health and substance use disorders, managed care organizations commonly base coverage decisions on internally developed ‘level of care guidelines’ that are inappropriately restrictive.”
The guidelines “may lead to denial of coverage for treatment that is recommended by a patient’s physician and even cut off coverage when treatment is already being delivered,” said the groups.
The U.S. Department of Labor also filed a brief in support of the plaintiffs who are suing UBH. Those individuals suffered injury when they were denied coverage, said the federal agency, which regulates employer-sponsored insurance plans.
California Attorney General Rob Bonta also made an amicus filing supporting the plaintiffs.
“When insurers limit access to this critical care, they leave Californians who need it feeling as if they have no other option than to try to cope alone,” said Mr. Bonta in a statement.
‘Discrimination must end’
Mr. Bonta said he agreed with a 2019 ruling by the U.S. District Court for the Northern District of California that UBH had violated its fiduciary duties by wrongfully using its internally developed coverage determination guidelines and level of care guidelines to deny care.
The court also found that UBH’s medically necessary criteria meant that only “acute” episodes would be covered. Instead, said the court last November, chronic and comorbid conditions should always be treated, according to Maureen Gammon and Kathleen Rosenow of Willis Towers Watson, a risk advisor.
In November, the same Northern California District Court ruled on the remedies it would require of United, including that the insurer reprocess more than 67,000 claims. UBH was also barred indefinitely from using any of its guidelines to make coverage determinations. Instead, it was ordered to make determinations “consistent with generally accepted standards of care,” and consistent with state laws.
The District Court denied a request by UBH to put a hold on the claims reprocessing until it appealed the overall case. But the Ninth Circuit Court of Appeals in February granted that request.
Then, in March, United appealed the District Court’s overall ruling, claiming that the plaintiffs had not proven harm.
The U.S. Chamber of Commerce has filed a brief in support of United, agreeing with its arguments.
However, the APA and other clinician groups said there is no question of harm.
“Failure to provide appropriate levels of care for treatment of mental illness and substance use disorders leads to relapse, overdose, transmission of infectious diseases, and death,” said APA CEO and Medical Director Saul Levin, MD, MPA, in a statement.
APA President Vivian Pender, MD, said guidelines that “are overly focused on stabilizing acute symptoms of mental health and substance use disorders” are not treating the underlying disease. “When the injury is physical, insurers treat the underlying disease and not just the symptoms. Discrimination against patients with mental illness must end,” she said.
No court has ever recognized the type of claims reprocessing ordered by the District Court judge, said attorneys Nathaniel Cohen and Joseph Laska of Manatt, Phelps & Phillips, in an analysis of the case.
Mr. Cohen and Mr. Laska write. “Practitioners, health plans, and health insurers would be wise to track UBH’s long-awaited appeal to the Ninth Circuit.”
This article first appeared on Medscape.com.
The American Psychiatric Association has joined with the American Medical Association and other medical societies to oppose United Behavioral Health’s (UBH) request that a court throw out a ruling that found the insurer unfairly denied tens of thousands of claims for mental health and substance use disorder services.
Wit v. United Behavioral Health, in litigation since 2014, is being closely watched by clinicians, patients, providers, and attorneys.
Reena Kapoor, MD, chair of the APA’s Committee on Judicial Action, said in an interview that the APA is hopeful that “whatever the court says about UBH should be applicable to all insurance companies that are providing employer-sponsored health benefits.”
In a friend of the court (amicus curiae) brief, the APA, AMA, the California Medical Association, Southern California Psychiatric Society, Northern California Psychiatric Society, Orange County Psychiatric Society, Central California Psychiatric Society, and San Diego Psychiatric Society argue that “despite the availability of professionally developed, evidence-based guidelines embodying generally accepted standards of care for mental health and substance use disorders, managed care organizations commonly base coverage decisions on internally developed ‘level of care guidelines’ that are inappropriately restrictive.”
The guidelines “may lead to denial of coverage for treatment that is recommended by a patient’s physician and even cut off coverage when treatment is already being delivered,” said the groups.
The U.S. Department of Labor also filed a brief in support of the plaintiffs who are suing UBH. Those individuals suffered injury when they were denied coverage, said the federal agency, which regulates employer-sponsored insurance plans.
California Attorney General Rob Bonta also made an amicus filing supporting the plaintiffs.
“When insurers limit access to this critical care, they leave Californians who need it feeling as if they have no other option than to try to cope alone,” said Mr. Bonta in a statement.
‘Discrimination must end’
Mr. Bonta said he agreed with a 2019 ruling by the U.S. District Court for the Northern District of California that UBH had violated its fiduciary duties by wrongfully using its internally developed coverage determination guidelines and level of care guidelines to deny care.
The court also found that UBH’s medically necessary criteria meant that only “acute” episodes would be covered. Instead, said the court last November, chronic and comorbid conditions should always be treated, according to Maureen Gammon and Kathleen Rosenow of Willis Towers Watson, a risk advisor.
In November, the same Northern California District Court ruled on the remedies it would require of United, including that the insurer reprocess more than 67,000 claims. UBH was also barred indefinitely from using any of its guidelines to make coverage determinations. Instead, it was ordered to make determinations “consistent with generally accepted standards of care,” and consistent with state laws.
The District Court denied a request by UBH to put a hold on the claims reprocessing until it appealed the overall case. But the Ninth Circuit Court of Appeals in February granted that request.
Then, in March, United appealed the District Court’s overall ruling, claiming that the plaintiffs had not proven harm.
The U.S. Chamber of Commerce has filed a brief in support of United, agreeing with its arguments.
However, the APA and other clinician groups said there is no question of harm.
“Failure to provide appropriate levels of care for treatment of mental illness and substance use disorders leads to relapse, overdose, transmission of infectious diseases, and death,” said APA CEO and Medical Director Saul Levin, MD, MPA, in a statement.
APA President Vivian Pender, MD, said guidelines that “are overly focused on stabilizing acute symptoms of mental health and substance use disorders” are not treating the underlying disease. “When the injury is physical, insurers treat the underlying disease and not just the symptoms. Discrimination against patients with mental illness must end,” she said.
No court has ever recognized the type of claims reprocessing ordered by the District Court judge, said attorneys Nathaniel Cohen and Joseph Laska of Manatt, Phelps & Phillips, in an analysis of the case.
Mr. Cohen and Mr. Laska write. “Practitioners, health plans, and health insurers would be wise to track UBH’s long-awaited appeal to the Ninth Circuit.”
This article first appeared on Medscape.com.
The American Psychiatric Association has joined with the American Medical Association and other medical societies to oppose United Behavioral Health’s (UBH) request that a court throw out a ruling that found the insurer unfairly denied tens of thousands of claims for mental health and substance use disorder services.
Wit v. United Behavioral Health, in litigation since 2014, is being closely watched by clinicians, patients, providers, and attorneys.
Reena Kapoor, MD, chair of the APA’s Committee on Judicial Action, said in an interview that the APA is hopeful that “whatever the court says about UBH should be applicable to all insurance companies that are providing employer-sponsored health benefits.”
In a friend of the court (amicus curiae) brief, the APA, AMA, the California Medical Association, Southern California Psychiatric Society, Northern California Psychiatric Society, Orange County Psychiatric Society, Central California Psychiatric Society, and San Diego Psychiatric Society argue that “despite the availability of professionally developed, evidence-based guidelines embodying generally accepted standards of care for mental health and substance use disorders, managed care organizations commonly base coverage decisions on internally developed ‘level of care guidelines’ that are inappropriately restrictive.”
The guidelines “may lead to denial of coverage for treatment that is recommended by a patient’s physician and even cut off coverage when treatment is already being delivered,” said the groups.
The U.S. Department of Labor also filed a brief in support of the plaintiffs who are suing UBH. Those individuals suffered injury when they were denied coverage, said the federal agency, which regulates employer-sponsored insurance plans.
California Attorney General Rob Bonta also made an amicus filing supporting the plaintiffs.
“When insurers limit access to this critical care, they leave Californians who need it feeling as if they have no other option than to try to cope alone,” said Mr. Bonta in a statement.
‘Discrimination must end’
Mr. Bonta said he agreed with a 2019 ruling by the U.S. District Court for the Northern District of California that UBH had violated its fiduciary duties by wrongfully using its internally developed coverage determination guidelines and level of care guidelines to deny care.
The court also found that UBH’s medically necessary criteria meant that only “acute” episodes would be covered. Instead, said the court last November, chronic and comorbid conditions should always be treated, according to Maureen Gammon and Kathleen Rosenow of Willis Towers Watson, a risk advisor.
In November, the same Northern California District Court ruled on the remedies it would require of United, including that the insurer reprocess more than 67,000 claims. UBH was also barred indefinitely from using any of its guidelines to make coverage determinations. Instead, it was ordered to make determinations “consistent with generally accepted standards of care,” and consistent with state laws.
The District Court denied a request by UBH to put a hold on the claims reprocessing until it appealed the overall case. But the Ninth Circuit Court of Appeals in February granted that request.
Then, in March, United appealed the District Court’s overall ruling, claiming that the plaintiffs had not proven harm.
The U.S. Chamber of Commerce has filed a brief in support of United, agreeing with its arguments.
However, the APA and other clinician groups said there is no question of harm.
“Failure to provide appropriate levels of care for treatment of mental illness and substance use disorders leads to relapse, overdose, transmission of infectious diseases, and death,” said APA CEO and Medical Director Saul Levin, MD, MPA, in a statement.
APA President Vivian Pender, MD, said guidelines that “are overly focused on stabilizing acute symptoms of mental health and substance use disorders” are not treating the underlying disease. “When the injury is physical, insurers treat the underlying disease and not just the symptoms. Discrimination against patients with mental illness must end,” she said.
No court has ever recognized the type of claims reprocessing ordered by the District Court judge, said attorneys Nathaniel Cohen and Joseph Laska of Manatt, Phelps & Phillips, in an analysis of the case.
Mr. Cohen and Mr. Laska write. “Practitioners, health plans, and health insurers would be wise to track UBH’s long-awaited appeal to the Ninth Circuit.”
This article first appeared on Medscape.com.
No-cancel culture: How telehealth is making it easier to keep that therapy session
When the COVID-19 pandemic forced behavioral health providers to stop seeing patients in person and instead hold therapy sessions remotely, the switch produced an unintended, positive consequence: Fewer patients skipped appointments.
That had long been a problem in mental health care. Some outpatient programs previously had no-show rates as high as 60%, according to several studies.
Only 9% of psychiatrists reported that all patients kept their appointments before the pandemic, according to an American Psychiatric Association report. Once providers switched to telepsychiatry, that number increased to 32%.
Not only that, but providers and patients say teletherapy has largely been an effective lifeline for people struggling with anxiety, depression, and other psychological issues during an extraordinarily difficult time, even though it created a new set of challenges.
Many providers say they plan to continue offering teletherapy after the pandemic. Some states are making permanent the temporary pandemic rules that allow providers to be reimbursed at the same rates as for in-person visits, which is welcome news to practitioners who take patients’ insurance.
“We are in a mental health crisis right now, so more people are struggling and may be more open to accessing services,” said psychologist Allison Dempsey, PhD, associate professor at University of Colorado at Denver, Aurora. “It’s much easier to connect from your living room.”
The problem for patients who didn’t show up was often as simple as a canceled ride, said Jody Long, a clinical social worker who studied the 60% rate of no-shows or late cancellations at the University of Tennessee Health Science Center psychiatric clinic in Memphis.
But sometimes it was the health problem itself. Mr. Long remembers seeing a first-time patient drive around the parking lot and then exit. The patient later called and told Mr. Long, “I just could not get out of the car; please forgive me and reschedule me.”
Mr. Long, now an assistant professor at Jacksonville (Ala.) State University, said that incident changed his perspective. “I realized when you’re having panic attacks or anxiety attacks or suffering from major depressive disorder, it’s hard,” he said. “It’s like you have built up these walls for protection and then all of a sudden you’re having to let these walls down.”
Absences strain providers whose bosses set billing and productivity expectations and those in private practice who lose billable hours, said Dr. Dempsey, who directs a program to provide mental health care for families of babies with serious medical complications. Psychotherapists often overbooked patients with the expectation that some would not show up.
Now Dr. Dempsey and colleagues no longer need to overbook. When patients don’t show up, staffers can sometimes contact a patient right away and hold the session. Other times, they can reschedule them for later that day or a different day.
And telepsychiatry performs as well as, if not better than, face-to-face delivery of mental health services, according to a World Journal of Psychiatry review of 452 studies.
Virtual visits can also save patients money, because they might not need to travel, take time off work, or pay for child care, said Jay Shore, MD, MPH, chairperson of the American Psychiatric Association’s telepsychiatry committee and a psychiatrist at the University of Colorado.
Dr. Shore started examining the potential of video conferencing to reach rural patients in the late ’90s and concluded that patients and providers can virtually build rapport, which he said is fundamental for effective therapy and medicine management.
But before the pandemic, almost 64% of psychiatrists had never used telehealth, according to the psychiatric association. Amid widespread skepticism, providers then had to do “10 years of implementations in 10 days,” said Dr. Shore, who has consulted with Dr. Dempsey and other providers.
Dr. Dempsey and colleagues faced a steep learning curve. She said she recently held a video therapy session with a mother who “seemed very out of it” before disappearing from the screen while her baby was crying.
She wondered if the patient’s exit was related to the stress of new motherhood or “something more concerning,” like addiction. She thinks she might have better understood the woman’s condition had they been in the same room. The patient called Dr. Dempsey’s team that night and told them she had relapsed into drug use and been taken to the emergency room. The mental health providers directed her to a treatment program, Dr. Dempsey said.
“We spent a lot of time reviewing what happened with that case and thinking about what we need to do differently,” Dr. Dempsey said.
Providers now routinely ask for the name of someone to call if they lose a connection and can no longer reach the patient.
In another session, Dr. Dempsey noticed that a patient seemed guarded and saw her partner hovering in the background. She said she worried about the possibility of domestic violence or “some other form of controlling behavior.”
In such cases, Dr. Dempsey called after the appointments or sent the patients secure messages to their online health portal. She asked if they felt safe and suggested they talk in person.
Such inability to maintain privacy remains a concern.
In a Walmart parking lot recently, psychologist Kristy Keefe, PsyD, of Western Illinois University, Macomb, heard a patient talking with her therapist from her car. Dr. Keefe said she wondered if the patient “had no other safe place to go to.”
To avoid that scenario, Dr. Keefe does 30-minute consultations with patients before their first telehealth appointment. She asks if they have space to talk where no one can overhear them and makes sure they have sufficient internet access and know how to use video conferencing.
To ensure that she, too, was prepared, Dr. Keefe upgraded her WiFi router, purchased two white-noise machines to drown out her conversations, and placed a stop sign on her door during appointments so her 5-year-old son knew she was seeing patients.
Dr. Keefe concluded that audio alone sometimes works better than video, which often lags. Over the phone, she and her psychology students “got really sensitive to tone fluctuations” in a patient’s voice and were better able to “pick up the emotion” than with video conferencing.
With those telehealth visits, her 20% no-show rate evaporated.
Kate Barnes, a 29-year-old middle school teacher in Fayetteville, Ark., who struggles with anxiety and depression, also has found visits easier by phone than by Zoom, because she doesn’t feel like a spotlight is on her.
“I can focus more on what I want to say,” she said.
In one of Dr. Keefe’s video sessions, though, a patient reached out, touched the camera and started to cry as she said how appreciative she was that someone was there, Dr. Keefe recalled.
“I am so very thankful that they had something in this terrible time of loss and trauma and isolation,” said Dr. Keefe.
Demand for mental health services will likely continue even after the lifting of all COVID restrictions. according to data from the U.S. Census Bureau and the National Health Interview Survey.
“That is not going to go away with snapping our fingers,” Dr. Dempsey said.
After the pandemic, Dr. Shore said, providers should review data from the past year and determine when virtual care or in-person care is more effective. He also said the health care industry needs to work to bridge the digital divide that exists because of lack of access to devices and broadband internet.
Even though Ms. Barnes said she did not see teletherapy as less effective than in-person therapy, she would like to return to seeing her therapist in person.
“When you are in person with someone, you can pick up on their body language better,” she said. “It’s a lot harder over a video call to do that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
When the COVID-19 pandemic forced behavioral health providers to stop seeing patients in person and instead hold therapy sessions remotely, the switch produced an unintended, positive consequence: Fewer patients skipped appointments.
That had long been a problem in mental health care. Some outpatient programs previously had no-show rates as high as 60%, according to several studies.
Only 9% of psychiatrists reported that all patients kept their appointments before the pandemic, according to an American Psychiatric Association report. Once providers switched to telepsychiatry, that number increased to 32%.
Not only that, but providers and patients say teletherapy has largely been an effective lifeline for people struggling with anxiety, depression, and other psychological issues during an extraordinarily difficult time, even though it created a new set of challenges.
Many providers say they plan to continue offering teletherapy after the pandemic. Some states are making permanent the temporary pandemic rules that allow providers to be reimbursed at the same rates as for in-person visits, which is welcome news to practitioners who take patients’ insurance.
“We are in a mental health crisis right now, so more people are struggling and may be more open to accessing services,” said psychologist Allison Dempsey, PhD, associate professor at University of Colorado at Denver, Aurora. “It’s much easier to connect from your living room.”
The problem for patients who didn’t show up was often as simple as a canceled ride, said Jody Long, a clinical social worker who studied the 60% rate of no-shows or late cancellations at the University of Tennessee Health Science Center psychiatric clinic in Memphis.
But sometimes it was the health problem itself. Mr. Long remembers seeing a first-time patient drive around the parking lot and then exit. The patient later called and told Mr. Long, “I just could not get out of the car; please forgive me and reschedule me.”
Mr. Long, now an assistant professor at Jacksonville (Ala.) State University, said that incident changed his perspective. “I realized when you’re having panic attacks or anxiety attacks or suffering from major depressive disorder, it’s hard,” he said. “It’s like you have built up these walls for protection and then all of a sudden you’re having to let these walls down.”
Absences strain providers whose bosses set billing and productivity expectations and those in private practice who lose billable hours, said Dr. Dempsey, who directs a program to provide mental health care for families of babies with serious medical complications. Psychotherapists often overbooked patients with the expectation that some would not show up.
Now Dr. Dempsey and colleagues no longer need to overbook. When patients don’t show up, staffers can sometimes contact a patient right away and hold the session. Other times, they can reschedule them for later that day or a different day.
And telepsychiatry performs as well as, if not better than, face-to-face delivery of mental health services, according to a World Journal of Psychiatry review of 452 studies.
Virtual visits can also save patients money, because they might not need to travel, take time off work, or pay for child care, said Jay Shore, MD, MPH, chairperson of the American Psychiatric Association’s telepsychiatry committee and a psychiatrist at the University of Colorado.
Dr. Shore started examining the potential of video conferencing to reach rural patients in the late ’90s and concluded that patients and providers can virtually build rapport, which he said is fundamental for effective therapy and medicine management.
But before the pandemic, almost 64% of psychiatrists had never used telehealth, according to the psychiatric association. Amid widespread skepticism, providers then had to do “10 years of implementations in 10 days,” said Dr. Shore, who has consulted with Dr. Dempsey and other providers.
Dr. Dempsey and colleagues faced a steep learning curve. She said she recently held a video therapy session with a mother who “seemed very out of it” before disappearing from the screen while her baby was crying.
She wondered if the patient’s exit was related to the stress of new motherhood or “something more concerning,” like addiction. She thinks she might have better understood the woman’s condition had they been in the same room. The patient called Dr. Dempsey’s team that night and told them she had relapsed into drug use and been taken to the emergency room. The mental health providers directed her to a treatment program, Dr. Dempsey said.
“We spent a lot of time reviewing what happened with that case and thinking about what we need to do differently,” Dr. Dempsey said.
Providers now routinely ask for the name of someone to call if they lose a connection and can no longer reach the patient.
In another session, Dr. Dempsey noticed that a patient seemed guarded and saw her partner hovering in the background. She said she worried about the possibility of domestic violence or “some other form of controlling behavior.”
In such cases, Dr. Dempsey called after the appointments or sent the patients secure messages to their online health portal. She asked if they felt safe and suggested they talk in person.
Such inability to maintain privacy remains a concern.
In a Walmart parking lot recently, psychologist Kristy Keefe, PsyD, of Western Illinois University, Macomb, heard a patient talking with her therapist from her car. Dr. Keefe said she wondered if the patient “had no other safe place to go to.”
To avoid that scenario, Dr. Keefe does 30-minute consultations with patients before their first telehealth appointment. She asks if they have space to talk where no one can overhear them and makes sure they have sufficient internet access and know how to use video conferencing.
To ensure that she, too, was prepared, Dr. Keefe upgraded her WiFi router, purchased two white-noise machines to drown out her conversations, and placed a stop sign on her door during appointments so her 5-year-old son knew she was seeing patients.
Dr. Keefe concluded that audio alone sometimes works better than video, which often lags. Over the phone, she and her psychology students “got really sensitive to tone fluctuations” in a patient’s voice and were better able to “pick up the emotion” than with video conferencing.
With those telehealth visits, her 20% no-show rate evaporated.
Kate Barnes, a 29-year-old middle school teacher in Fayetteville, Ark., who struggles with anxiety and depression, also has found visits easier by phone than by Zoom, because she doesn’t feel like a spotlight is on her.
“I can focus more on what I want to say,” she said.
In one of Dr. Keefe’s video sessions, though, a patient reached out, touched the camera and started to cry as she said how appreciative she was that someone was there, Dr. Keefe recalled.
“I am so very thankful that they had something in this terrible time of loss and trauma and isolation,” said Dr. Keefe.
Demand for mental health services will likely continue even after the lifting of all COVID restrictions. according to data from the U.S. Census Bureau and the National Health Interview Survey.
“That is not going to go away with snapping our fingers,” Dr. Dempsey said.
After the pandemic, Dr. Shore said, providers should review data from the past year and determine when virtual care or in-person care is more effective. He also said the health care industry needs to work to bridge the digital divide that exists because of lack of access to devices and broadband internet.
Even though Ms. Barnes said she did not see teletherapy as less effective than in-person therapy, she would like to return to seeing her therapist in person.
“When you are in person with someone, you can pick up on their body language better,” she said. “It’s a lot harder over a video call to do that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
When the COVID-19 pandemic forced behavioral health providers to stop seeing patients in person and instead hold therapy sessions remotely, the switch produced an unintended, positive consequence: Fewer patients skipped appointments.
That had long been a problem in mental health care. Some outpatient programs previously had no-show rates as high as 60%, according to several studies.
Only 9% of psychiatrists reported that all patients kept their appointments before the pandemic, according to an American Psychiatric Association report. Once providers switched to telepsychiatry, that number increased to 32%.
Not only that, but providers and patients say teletherapy has largely been an effective lifeline for people struggling with anxiety, depression, and other psychological issues during an extraordinarily difficult time, even though it created a new set of challenges.
Many providers say they plan to continue offering teletherapy after the pandemic. Some states are making permanent the temporary pandemic rules that allow providers to be reimbursed at the same rates as for in-person visits, which is welcome news to practitioners who take patients’ insurance.
“We are in a mental health crisis right now, so more people are struggling and may be more open to accessing services,” said psychologist Allison Dempsey, PhD, associate professor at University of Colorado at Denver, Aurora. “It’s much easier to connect from your living room.”
The problem for patients who didn’t show up was often as simple as a canceled ride, said Jody Long, a clinical social worker who studied the 60% rate of no-shows or late cancellations at the University of Tennessee Health Science Center psychiatric clinic in Memphis.
But sometimes it was the health problem itself. Mr. Long remembers seeing a first-time patient drive around the parking lot and then exit. The patient later called and told Mr. Long, “I just could not get out of the car; please forgive me and reschedule me.”
Mr. Long, now an assistant professor at Jacksonville (Ala.) State University, said that incident changed his perspective. “I realized when you’re having panic attacks or anxiety attacks or suffering from major depressive disorder, it’s hard,” he said. “It’s like you have built up these walls for protection and then all of a sudden you’re having to let these walls down.”
Absences strain providers whose bosses set billing and productivity expectations and those in private practice who lose billable hours, said Dr. Dempsey, who directs a program to provide mental health care for families of babies with serious medical complications. Psychotherapists often overbooked patients with the expectation that some would not show up.
Now Dr. Dempsey and colleagues no longer need to overbook. When patients don’t show up, staffers can sometimes contact a patient right away and hold the session. Other times, they can reschedule them for later that day or a different day.
And telepsychiatry performs as well as, if not better than, face-to-face delivery of mental health services, according to a World Journal of Psychiatry review of 452 studies.
Virtual visits can also save patients money, because they might not need to travel, take time off work, or pay for child care, said Jay Shore, MD, MPH, chairperson of the American Psychiatric Association’s telepsychiatry committee and a psychiatrist at the University of Colorado.
Dr. Shore started examining the potential of video conferencing to reach rural patients in the late ’90s and concluded that patients and providers can virtually build rapport, which he said is fundamental for effective therapy and medicine management.
But before the pandemic, almost 64% of psychiatrists had never used telehealth, according to the psychiatric association. Amid widespread skepticism, providers then had to do “10 years of implementations in 10 days,” said Dr. Shore, who has consulted with Dr. Dempsey and other providers.
Dr. Dempsey and colleagues faced a steep learning curve. She said she recently held a video therapy session with a mother who “seemed very out of it” before disappearing from the screen while her baby was crying.
She wondered if the patient’s exit was related to the stress of new motherhood or “something more concerning,” like addiction. She thinks she might have better understood the woman’s condition had they been in the same room. The patient called Dr. Dempsey’s team that night and told them she had relapsed into drug use and been taken to the emergency room. The mental health providers directed her to a treatment program, Dr. Dempsey said.
“We spent a lot of time reviewing what happened with that case and thinking about what we need to do differently,” Dr. Dempsey said.
Providers now routinely ask for the name of someone to call if they lose a connection and can no longer reach the patient.
In another session, Dr. Dempsey noticed that a patient seemed guarded and saw her partner hovering in the background. She said she worried about the possibility of domestic violence or “some other form of controlling behavior.”
In such cases, Dr. Dempsey called after the appointments or sent the patients secure messages to their online health portal. She asked if they felt safe and suggested they talk in person.
Such inability to maintain privacy remains a concern.
In a Walmart parking lot recently, psychologist Kristy Keefe, PsyD, of Western Illinois University, Macomb, heard a patient talking with her therapist from her car. Dr. Keefe said she wondered if the patient “had no other safe place to go to.”
To avoid that scenario, Dr. Keefe does 30-minute consultations with patients before their first telehealth appointment. She asks if they have space to talk where no one can overhear them and makes sure they have sufficient internet access and know how to use video conferencing.
To ensure that she, too, was prepared, Dr. Keefe upgraded her WiFi router, purchased two white-noise machines to drown out her conversations, and placed a stop sign on her door during appointments so her 5-year-old son knew she was seeing patients.
Dr. Keefe concluded that audio alone sometimes works better than video, which often lags. Over the phone, she and her psychology students “got really sensitive to tone fluctuations” in a patient’s voice and were better able to “pick up the emotion” than with video conferencing.
With those telehealth visits, her 20% no-show rate evaporated.
Kate Barnes, a 29-year-old middle school teacher in Fayetteville, Ark., who struggles with anxiety and depression, also has found visits easier by phone than by Zoom, because she doesn’t feel like a spotlight is on her.
“I can focus more on what I want to say,” she said.
In one of Dr. Keefe’s video sessions, though, a patient reached out, touched the camera and started to cry as she said how appreciative she was that someone was there, Dr. Keefe recalled.
“I am so very thankful that they had something in this terrible time of loss and trauma and isolation,” said Dr. Keefe.
Demand for mental health services will likely continue even after the lifting of all COVID restrictions. according to data from the U.S. Census Bureau and the National Health Interview Survey.
“That is not going to go away with snapping our fingers,” Dr. Dempsey said.
After the pandemic, Dr. Shore said, providers should review data from the past year and determine when virtual care or in-person care is more effective. He also said the health care industry needs to work to bridge the digital divide that exists because of lack of access to devices and broadband internet.
Even though Ms. Barnes said she did not see teletherapy as less effective than in-person therapy, she would like to return to seeing her therapist in person.
“When you are in person with someone, you can pick up on their body language better,” she said. “It’s a lot harder over a video call to do that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.