User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
10 devastating consequences of psychotic relapses
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
‘Canceling’ obsolete terms
I wanted to thank Dr. Nasrallah for his most important editorial, “Let’s ‘cancel’ these obsolete terms in DSM” (From the Editor,
Robert Barris, MD
Nassau University Medical Center
East Meadow, New York
How sad! This is my reaction to reading Dr. Nasrallah’s January 2021 editorial. Although biological psychiatry is synonymous with brain neurotransmitters and psychopharmacology, absent from this perspective is the visible biology of the human organism, specifically Sigmund Freud’s discovery of the psychosexual development of the infant and child and Wilhelm Reich’s discovery of characterological and muscular armor. Medicine, a natural science, is founded and grounded in observation. Psychiatry, having ignored and eliminated (“canceled”) recognition of these readily observable phenomena essential to understanding psychiatric disorders, including neurosis and schizophrenia, allows Dr. Nasrallah to suggest we “cancel” what should be at the heart of psychiatric diagnosis and treatment. Sadly, this heart has been lost for decades.
Howard Chavis, MD
New York, New York
Dr. Nasrallah responds
Psychiatry, like all medical and scientific disciplines, must go through an ongoing renewal, including the update of its terminology, with or without a change in its concepts or principles. Anxiety is a more accurate description of clinical symptoms than neurosis, and psychosis spectrum is more accurate than schizophrenia. Besides the accuracy issue, “neurotic” and “schizophrenic” have unfortunately devolved into pejorative and stigmatizing terms. The lexicon of psychiatry has gone through seismic changes over the past several decades, as I described in a previous editorial.1 Psychiatry is a vibrant, constantly evolving biopsychosocial/clinical neuroscience, not a static descriptive discipline.
Reference
1. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
I found myself having difficulty with Dr. Nasrallah’s editorial about canceling “obsolete” terms. I agree that making a diagnosis of borderline or narcissistic personality disorder can be pejorative if the clinician is using it to manage their own unprocessed countertransference. While all behavior is brain-mediated, human behavior is influenced by psychological events great and small. I am concerned that you seem to be reducing personality trait disturbances to biological abnormality, pure and simple. Losing psychological understanding of patients while overexplaining behavior as pathological brain dysfunction risks losing why patients see us in the first place.
Michael Friedman, DO
Cherry Hill, New Jersey
Dr. Nasrallah responds
The renaming I suggest goes beyond countertransference. It has to do with scientific validity of the diagnostic construct. And yes, personality traits are heavily genetic, but with some modulation by environmental factors. I suggest reading the seminal works of Thomas J. Bouchard Jr., PhD, and Kenneth S. Kendler, MD, on identical twins reared together or apart for more details about the genetics of personality traits.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
I wanted to thank Dr. Nasrallah for his most important editorial, “Let’s ‘cancel’ these obsolete terms in DSM” (From the Editor,
Robert Barris, MD
Nassau University Medical Center
East Meadow, New York
How sad! This is my reaction to reading Dr. Nasrallah’s January 2021 editorial. Although biological psychiatry is synonymous with brain neurotransmitters and psychopharmacology, absent from this perspective is the visible biology of the human organism, specifically Sigmund Freud’s discovery of the psychosexual development of the infant and child and Wilhelm Reich’s discovery of characterological and muscular armor. Medicine, a natural science, is founded and grounded in observation. Psychiatry, having ignored and eliminated (“canceled”) recognition of these readily observable phenomena essential to understanding psychiatric disorders, including neurosis and schizophrenia, allows Dr. Nasrallah to suggest we “cancel” what should be at the heart of psychiatric diagnosis and treatment. Sadly, this heart has been lost for decades.
Howard Chavis, MD
New York, New York
Dr. Nasrallah responds
Psychiatry, like all medical and scientific disciplines, must go through an ongoing renewal, including the update of its terminology, with or without a change in its concepts or principles. Anxiety is a more accurate description of clinical symptoms than neurosis, and psychosis spectrum is more accurate than schizophrenia. Besides the accuracy issue, “neurotic” and “schizophrenic” have unfortunately devolved into pejorative and stigmatizing terms. The lexicon of psychiatry has gone through seismic changes over the past several decades, as I described in a previous editorial.1 Psychiatry is a vibrant, constantly evolving biopsychosocial/clinical neuroscience, not a static descriptive discipline.
Reference
1. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
I found myself having difficulty with Dr. Nasrallah’s editorial about canceling “obsolete” terms. I agree that making a diagnosis of borderline or narcissistic personality disorder can be pejorative if the clinician is using it to manage their own unprocessed countertransference. While all behavior is brain-mediated, human behavior is influenced by psychological events great and small. I am concerned that you seem to be reducing personality trait disturbances to biological abnormality, pure and simple. Losing psychological understanding of patients while overexplaining behavior as pathological brain dysfunction risks losing why patients see us in the first place.
Michael Friedman, DO
Cherry Hill, New Jersey
Dr. Nasrallah responds
The renaming I suggest goes beyond countertransference. It has to do with scientific validity of the diagnostic construct. And yes, personality traits are heavily genetic, but with some modulation by environmental factors. I suggest reading the seminal works of Thomas J. Bouchard Jr., PhD, and Kenneth S. Kendler, MD, on identical twins reared together or apart for more details about the genetics of personality traits.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
I wanted to thank Dr. Nasrallah for his most important editorial, “Let’s ‘cancel’ these obsolete terms in DSM” (From the Editor,
Robert Barris, MD
Nassau University Medical Center
East Meadow, New York
How sad! This is my reaction to reading Dr. Nasrallah’s January 2021 editorial. Although biological psychiatry is synonymous with brain neurotransmitters and psychopharmacology, absent from this perspective is the visible biology of the human organism, specifically Sigmund Freud’s discovery of the psychosexual development of the infant and child and Wilhelm Reich’s discovery of characterological and muscular armor. Medicine, a natural science, is founded and grounded in observation. Psychiatry, having ignored and eliminated (“canceled”) recognition of these readily observable phenomena essential to understanding psychiatric disorders, including neurosis and schizophrenia, allows Dr. Nasrallah to suggest we “cancel” what should be at the heart of psychiatric diagnosis and treatment. Sadly, this heart has been lost for decades.
Howard Chavis, MD
New York, New York
Dr. Nasrallah responds
Psychiatry, like all medical and scientific disciplines, must go through an ongoing renewal, including the update of its terminology, with or without a change in its concepts or principles. Anxiety is a more accurate description of clinical symptoms than neurosis, and psychosis spectrum is more accurate than schizophrenia. Besides the accuracy issue, “neurotic” and “schizophrenic” have unfortunately devolved into pejorative and stigmatizing terms. The lexicon of psychiatry has gone through seismic changes over the past several decades, as I described in a previous editorial.1 Psychiatry is a vibrant, constantly evolving biopsychosocial/clinical neuroscience, not a static descriptive discipline.
Reference
1. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
I found myself having difficulty with Dr. Nasrallah’s editorial about canceling “obsolete” terms. I agree that making a diagnosis of borderline or narcissistic personality disorder can be pejorative if the clinician is using it to manage their own unprocessed countertransference. While all behavior is brain-mediated, human behavior is influenced by psychological events great and small. I am concerned that you seem to be reducing personality trait disturbances to biological abnormality, pure and simple. Losing psychological understanding of patients while overexplaining behavior as pathological brain dysfunction risks losing why patients see us in the first place.
Michael Friedman, DO
Cherry Hill, New Jersey
Dr. Nasrallah responds
The renaming I suggest goes beyond countertransference. It has to do with scientific validity of the diagnostic construct. And yes, personality traits are heavily genetic, but with some modulation by environmental factors. I suggest reading the seminal works of Thomas J. Bouchard Jr., PhD, and Kenneth S. Kendler, MD, on identical twins reared together or apart for more details about the genetics of personality traits.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
Bright light therapy for bipolar depression: A review of 6 studies
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).
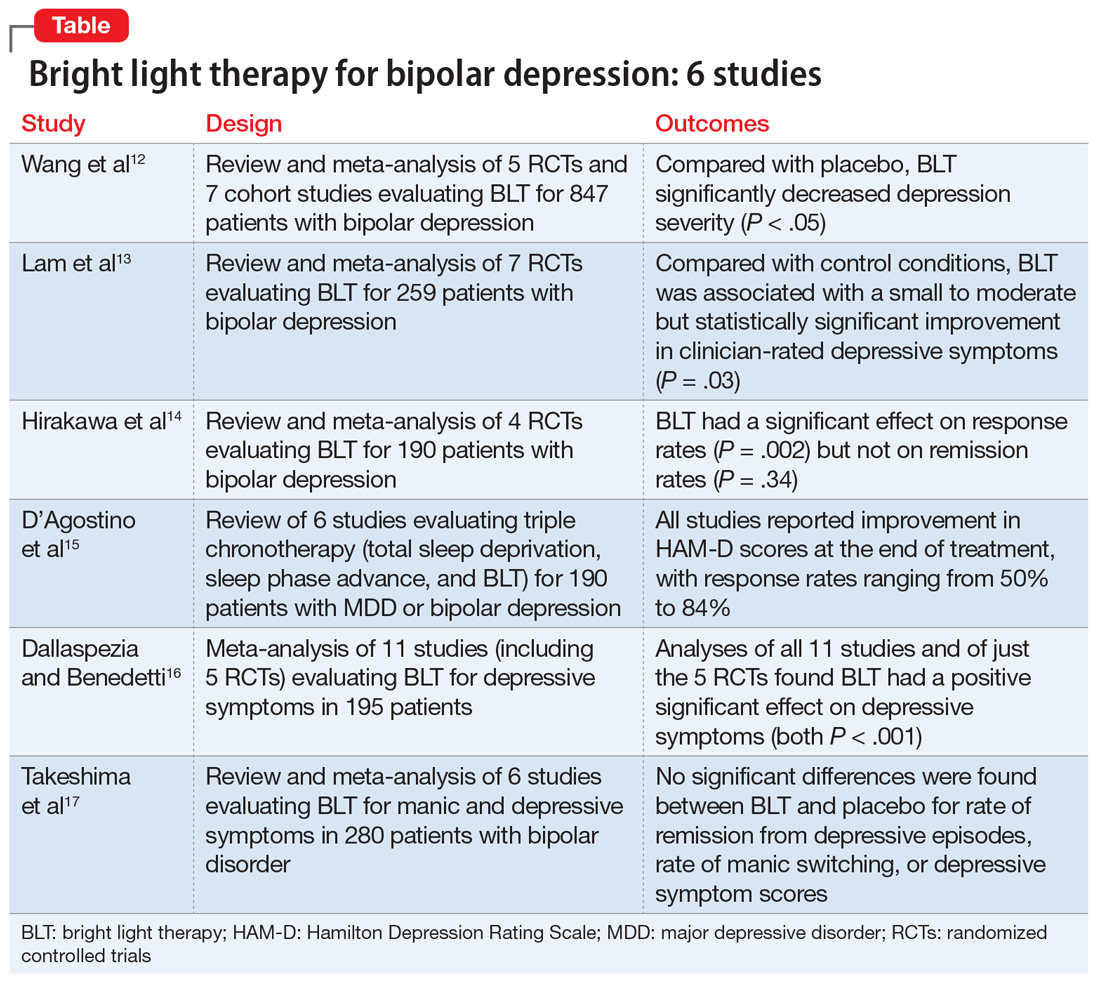
1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).

1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).

1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
ARISE to supportive psychotherapy
Supportive psychotherapy is a common type of therapy that often is used in combination with other modalities. By focusing on improving symptoms and accepting the patient’s limitations, it is particularly helpful for individuals who might have difficulty engaging in insight-oriented psychotherapies, such as those struggling with external stressors, including exposure to trauma, bereavement, physical disabilities, or socioeconomic challenges. Personal limitations, including severe personality disorder or intellectual disabilities, might also limit a patient’s ability to self-reflect on subconscious issues, which can lead to choosing a supportive modality.
While being supportive in the vernacular sense can be helpful, formal supportive psychotherapy employs well-defined goals and techniques.1 A therapist can facilitate progress by explicitly referring to these goals and techniques. The acronym ARISE can help therapists and other clinicians to use and appraise therapeutic progress toward these goals.
Alliance-building. The therapeutic alliance is an important predictor of the success of psychotherapy.2 Warmly encourage positive transference toward the therapist. The patient’s appreciation of the therapist’s empathic interactions can further the alliance. Paraphrasing the patient’s words can demonstrate and enhance empathy. Doing so allows clarification of the patient’s thoughts and helps the patient feel understood. Formulate and partner around shared therapeutic goals. Monitor the strength of the alliance and intervene if it is threatened. For example, if you misunderstand your patient and inadvertently offend them, apologizing may be helpful. In the face of disagreement between the patient and therapist, reorienting back to shared goals reinforces common ground.
Reduce anxiety and negative affect. In contrast to the caricature of the stiff psychoanalyst, the supportive therapist adopts an engaged conversational style to help the patient feel relaxed and to diminish the power differential between therapist and patient. If the patient appears uncomfortable with silence, maintaining the flow of conversation may reduce discomfort.1 Minimize your patient’s discomfort by approaching uncomfortable topics in manageable portions. Seek permission before introducing a subject that induces anxiety. Explain the reasoning behind approaching such topics.3 Reassurance and encouragement can further reduce anxiety.4 When not incongruous to the discussion, appropriate use of warm affect (eg, a smile) or even humor can elicit positive affect.
Increase awareness. Use psychoeducation and psychological interpretation (whether cognitive-behavioral or psychodynamic) to expand your patient’s awareness and help them understand their social contacts’ point of view. Clarification, gentle confrontation, and interpretation can make patients aware of biopsychosocial precipitants of distress.4
Strengthen coping mechanisms. Reinforce adaptive defense mechanisms, such as mature humor or suppression. Educating patients on practical organizational skills, problem-solving, relaxation techniques, and other relevant skills, can help them cope more effectively. Give advice only in limited circumstances, and when doing so, back up your advice with a rationale derived from your professional expertise. Because it is important for patients to realize that their life choices are their own, usually it is best to help the patient understand how they might come to their own decisions rather than to prescribe life choices in the form of advice.
Enhance self-esteem. Many patients in distress suffer from low self-esteem.5,6 Active encouragement and honest praise can nurture your patient’s ability to correct a distorted self-image and challenge self-reproach. Praise should not be false but reality-based. Praise can address preexisting strengths, highlight the patient’s willingness to express challenging material, or provide reinforcement on progress made toward treatment goals.
1. Rothe, EM. Supportive psychotherapy in everyday clinical practice: it’s like riding a bicycle. Psychiatric Times. Published May 24, 2017. Accessed April 12, 2021. https://www.psychiatrictimes.com/view/supportive-psychotherapy-everyday-clinical-practice-its-riding-bicycle
2. Flückiger C, Del Re AC, Wampold BE, et al. The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic). 2018;55(4):316-340.
3. Pine F. The interpretive moment. Variations on classical themes. Bull Menninger Clin. 1984;48(1), 54-71.
4. Grover S, Avasthi A, Jagiwala M. Clinical practice guidelines for practice of supportive psychotherapy. Indian J Psychiatry. 2020;62(Suppl 2):S173-S182.
5. Leary MR, Schreindorfer LS, Haupt AL. The role of low self-esteem in emotional and behavioral problems: why is low self-esteem dysfunctional? J Soc Clin Psychol. 1995;14(3):297-314.
6. Zahn R, Lythe KE, Gethin JA, et al. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J Affect Disord. 2015;186:337-341.
Supportive psychotherapy is a common type of therapy that often is used in combination with other modalities. By focusing on improving symptoms and accepting the patient’s limitations, it is particularly helpful for individuals who might have difficulty engaging in insight-oriented psychotherapies, such as those struggling with external stressors, including exposure to trauma, bereavement, physical disabilities, or socioeconomic challenges. Personal limitations, including severe personality disorder or intellectual disabilities, might also limit a patient’s ability to self-reflect on subconscious issues, which can lead to choosing a supportive modality.
While being supportive in the vernacular sense can be helpful, formal supportive psychotherapy employs well-defined goals and techniques.1 A therapist can facilitate progress by explicitly referring to these goals and techniques. The acronym ARISE can help therapists and other clinicians to use and appraise therapeutic progress toward these goals.
Alliance-building. The therapeutic alliance is an important predictor of the success of psychotherapy.2 Warmly encourage positive transference toward the therapist. The patient’s appreciation of the therapist’s empathic interactions can further the alliance. Paraphrasing the patient’s words can demonstrate and enhance empathy. Doing so allows clarification of the patient’s thoughts and helps the patient feel understood. Formulate and partner around shared therapeutic goals. Monitor the strength of the alliance and intervene if it is threatened. For example, if you misunderstand your patient and inadvertently offend them, apologizing may be helpful. In the face of disagreement between the patient and therapist, reorienting back to shared goals reinforces common ground.
Reduce anxiety and negative affect. In contrast to the caricature of the stiff psychoanalyst, the supportive therapist adopts an engaged conversational style to help the patient feel relaxed and to diminish the power differential between therapist and patient. If the patient appears uncomfortable with silence, maintaining the flow of conversation may reduce discomfort.1 Minimize your patient’s discomfort by approaching uncomfortable topics in manageable portions. Seek permission before introducing a subject that induces anxiety. Explain the reasoning behind approaching such topics.3 Reassurance and encouragement can further reduce anxiety.4 When not incongruous to the discussion, appropriate use of warm affect (eg, a smile) or even humor can elicit positive affect.
Increase awareness. Use psychoeducation and psychological interpretation (whether cognitive-behavioral or psychodynamic) to expand your patient’s awareness and help them understand their social contacts’ point of view. Clarification, gentle confrontation, and interpretation can make patients aware of biopsychosocial precipitants of distress.4
Strengthen coping mechanisms. Reinforce adaptive defense mechanisms, such as mature humor or suppression. Educating patients on practical organizational skills, problem-solving, relaxation techniques, and other relevant skills, can help them cope more effectively. Give advice only in limited circumstances, and when doing so, back up your advice with a rationale derived from your professional expertise. Because it is important for patients to realize that their life choices are their own, usually it is best to help the patient understand how they might come to their own decisions rather than to prescribe life choices in the form of advice.
Enhance self-esteem. Many patients in distress suffer from low self-esteem.5,6 Active encouragement and honest praise can nurture your patient’s ability to correct a distorted self-image and challenge self-reproach. Praise should not be false but reality-based. Praise can address preexisting strengths, highlight the patient’s willingness to express challenging material, or provide reinforcement on progress made toward treatment goals.
Supportive psychotherapy is a common type of therapy that often is used in combination with other modalities. By focusing on improving symptoms and accepting the patient’s limitations, it is particularly helpful for individuals who might have difficulty engaging in insight-oriented psychotherapies, such as those struggling with external stressors, including exposure to trauma, bereavement, physical disabilities, or socioeconomic challenges. Personal limitations, including severe personality disorder or intellectual disabilities, might also limit a patient’s ability to self-reflect on subconscious issues, which can lead to choosing a supportive modality.
While being supportive in the vernacular sense can be helpful, formal supportive psychotherapy employs well-defined goals and techniques.1 A therapist can facilitate progress by explicitly referring to these goals and techniques. The acronym ARISE can help therapists and other clinicians to use and appraise therapeutic progress toward these goals.
Alliance-building. The therapeutic alliance is an important predictor of the success of psychotherapy.2 Warmly encourage positive transference toward the therapist. The patient’s appreciation of the therapist’s empathic interactions can further the alliance. Paraphrasing the patient’s words can demonstrate and enhance empathy. Doing so allows clarification of the patient’s thoughts and helps the patient feel understood. Formulate and partner around shared therapeutic goals. Monitor the strength of the alliance and intervene if it is threatened. For example, if you misunderstand your patient and inadvertently offend them, apologizing may be helpful. In the face of disagreement between the patient and therapist, reorienting back to shared goals reinforces common ground.
Reduce anxiety and negative affect. In contrast to the caricature of the stiff psychoanalyst, the supportive therapist adopts an engaged conversational style to help the patient feel relaxed and to diminish the power differential between therapist and patient. If the patient appears uncomfortable with silence, maintaining the flow of conversation may reduce discomfort.1 Minimize your patient’s discomfort by approaching uncomfortable topics in manageable portions. Seek permission before introducing a subject that induces anxiety. Explain the reasoning behind approaching such topics.3 Reassurance and encouragement can further reduce anxiety.4 When not incongruous to the discussion, appropriate use of warm affect (eg, a smile) or even humor can elicit positive affect.
Increase awareness. Use psychoeducation and psychological interpretation (whether cognitive-behavioral or psychodynamic) to expand your patient’s awareness and help them understand their social contacts’ point of view. Clarification, gentle confrontation, and interpretation can make patients aware of biopsychosocial precipitants of distress.4
Strengthen coping mechanisms. Reinforce adaptive defense mechanisms, such as mature humor or suppression. Educating patients on practical organizational skills, problem-solving, relaxation techniques, and other relevant skills, can help them cope more effectively. Give advice only in limited circumstances, and when doing so, back up your advice with a rationale derived from your professional expertise. Because it is important for patients to realize that their life choices are their own, usually it is best to help the patient understand how they might come to their own decisions rather than to prescribe life choices in the form of advice.
Enhance self-esteem. Many patients in distress suffer from low self-esteem.5,6 Active encouragement and honest praise can nurture your patient’s ability to correct a distorted self-image and challenge self-reproach. Praise should not be false but reality-based. Praise can address preexisting strengths, highlight the patient’s willingness to express challenging material, or provide reinforcement on progress made toward treatment goals.
1. Rothe, EM. Supportive psychotherapy in everyday clinical practice: it’s like riding a bicycle. Psychiatric Times. Published May 24, 2017. Accessed April 12, 2021. https://www.psychiatrictimes.com/view/supportive-psychotherapy-everyday-clinical-practice-its-riding-bicycle
2. Flückiger C, Del Re AC, Wampold BE, et al. The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic). 2018;55(4):316-340.
3. Pine F. The interpretive moment. Variations on classical themes. Bull Menninger Clin. 1984;48(1), 54-71.
4. Grover S, Avasthi A, Jagiwala M. Clinical practice guidelines for practice of supportive psychotherapy. Indian J Psychiatry. 2020;62(Suppl 2):S173-S182.
5. Leary MR, Schreindorfer LS, Haupt AL. The role of low self-esteem in emotional and behavioral problems: why is low self-esteem dysfunctional? J Soc Clin Psychol. 1995;14(3):297-314.
6. Zahn R, Lythe KE, Gethin JA, et al. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J Affect Disord. 2015;186:337-341.
1. Rothe, EM. Supportive psychotherapy in everyday clinical practice: it’s like riding a bicycle. Psychiatric Times. Published May 24, 2017. Accessed April 12, 2021. https://www.psychiatrictimes.com/view/supportive-psychotherapy-everyday-clinical-practice-its-riding-bicycle
2. Flückiger C, Del Re AC, Wampold BE, et al. The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic). 2018;55(4):316-340.
3. Pine F. The interpretive moment. Variations on classical themes. Bull Menninger Clin. 1984;48(1), 54-71.
4. Grover S, Avasthi A, Jagiwala M. Clinical practice guidelines for practice of supportive psychotherapy. Indian J Psychiatry. 2020;62(Suppl 2):S173-S182.
5. Leary MR, Schreindorfer LS, Haupt AL. The role of low self-esteem in emotional and behavioral problems: why is low self-esteem dysfunctional? J Soc Clin Psychol. 1995;14(3):297-314.
6. Zahn R, Lythe KE, Gethin JA, et al. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J Affect Disord. 2015;186:337-341.
Nonpharma approach a potential ‘game changer’ in schizophrenia?
Cognitive remediation (CR), a therapy that encompasses nonpharmacologic approaches to improving cognitive function for patients with severe mental illness, may lead to significant improvement for patients with schizophrenia, new research suggests.
A systematic review of 130 worldwide studies that included almost 9,000 participants showed that CR significantly improved global cognition and global functioning. In addition, investigators identified key patient characteristics that flagged ideal candidates for the therapy.
“Because pharmacological treatment exerts limited effects on cognitive deficits, and clinical remission does not necessarily result in functional recovery, widespread implementation of CR could be a game-changer for achieving the patient’s personal recovery goals,” the researchers wrote.
“We hope that this systematic review could help clinicians understand how to make CR even more effective and even more personalized,” lead author Antonio Vita, MD, PhD, department of clinical and experimental sciences, University of Brescia, Italy, said in an interview.
Dr. Vita noted that he would also encourage clinicians to consider “proposing it for clinical practice.”
The findings were presented at the virtual congress of the Schizophrenia International Research Society (SIRS 2021) and were published simultaneously in JAMA Psychiatry.
Resistance continues
Cognition “should be a focus of treatment because most of the disability and functional consequences of the disease are related to ... neurocognitive impairment and impairment of social cognition,” Dr. Vita said.
He noted that treatments that focus on cognition are crucial for the recovery of patients with schizophrenia.
However, despite a “solid body of evidence” supporting the efficacy of CR and guideline recommendations that CR be included in psychiatric services, reluctance remains, the investigators noted.
The study’s goal was to determine optimal candidates for CR and to assess outcomes of the therapy and its four core elements:
- The presence of an active and trained therapist.
- Repeated practice of cognitive exercises.
- Structured development of cognitive strategies.
- Techniques to improve the transfer of cognitive gains to the real world, such as integrated psychosocial rehabilitation.
The investigators conducted a systematic literature search of the PubMed, Scopus, and PsychInfo databases to find relevant studies of CR published between January 2011 and February 2020. They also “hand-searched” meta-analyses, reviews, and reference lists.
Ultimately, the analysis included 130 randomized clinical trials comparing CR with a control condition in 8,851 patients with schizophrenia spectrum disorders.
Of these studies, 57 were conducted in Europe, 38 in the United States, 22 in Asia, 4 in Canada, 4 in Middle Eastern countries, 3 in Australia, and 2 in Brazil.
The mean age of the participants was 36.7 years, and 68% were men. The average age at the time of schizophrenia onset was 23.3 years, and the mean duration of illness was 13.8 years.
The average duration of CR treatment was 15.2 weeks. The four elements were well represented; each was offered to at least 71% of patients.
The comparator therapy was treatment as usual (TAU), in 34.3% of cases, or active TAU with multidisciplinary rehabilitation, in 15.2% of cases. The remaining interventions were either nonspecific (30.8%) or were devised specifically for the study (19.9%).
Results showed that CR had a significant, albeit moderate, effect on global cognition (Cohen’s d effect size, 0.29; P < .001) and global functioning (effect size, 0.22; P < .01).
Having an active and trained therapist had a significant impact on cognition and functioning (P = .04 for both), as did the structured development of cognitive strategies (P = .002 for cognition; P = .004 for functioning).
The integration of psychosocial rehabilitation also had a significant effect on functioning (P = .003).
Interventions that included all of the core elements had a “highly significant” association with global cognition (P = .02) and global functioning (P < .001), the investigators reported. Longer treatments were significantly associated with greater functional improvement (P = .006).
The investigators found that improvements were greater among patients who had fewer years of education (P = .03 for cognition; P = .02 for functioning), lower premorbid IQ scores (P = .04 for functioning), and more severe symptoms at baseline (P = .005 for cognition).
The researchers noted that CR should become more widely available because it has the “potential to be an element of standard care rather than an optional intervention targeting selected individuals.”
An overlooked treatment option
Commenting on the findings for this news organization, Alice Medalia, PhD, director of the Lieber Recovery Clinic at Columbia University Irving Medical Center, New York, noted that this study is the second large-scale analysis of the use of CR for patients with schizophrenia to come out this year. The other was published in Schizophrenia Bulletin.
“So this is a banner year for large reviews,” she said. “It’s great to have two studies like this [that] tell a very consistent story.”
Dr. Medalia, who was not involved with the research, said individuals “don’t really talk about cognition very much.”
CR, she added, is “one of an array of services that one should be providing, and the bigger picture is that every single person should have their cognitive health needs addressed.
“If someone is having problems, and it’s getting in the way of them being the kind of person they want to be and doing want they want to do, we need to intervene. How we intervene should always be in the least disruptive and intense way,” she said.
These measures could include examining sleep hygiene, adjusting medications, or introducing exercise.
“But there really does come a time for some people where cognitive remediation is going to be helpful, so it should be more available,” Dr. Medalia said.
Although increased availability is partially dependent on having enough trained therapists, the main reason CR is not more widely available is because “people just don’t think about cognition and they don’t know how to talk about it,” she noted. In addition, she said, even when it is available, clinicians don’t refer patients.
“That tells you something. The solution here is not to put a cognitive remediation program everywhere but ... to get people comfortable talking about cognition and identifying when an intervention is needed,” said Dr. Medalia.
One study author received grants from the National Institute for Health Research during the conduct of the study and is the creator of CIRCuiTs, a cognitive remediation software program. The other investigators and Dr. Medalia have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cognitive remediation (CR), a therapy that encompasses nonpharmacologic approaches to improving cognitive function for patients with severe mental illness, may lead to significant improvement for patients with schizophrenia, new research suggests.
A systematic review of 130 worldwide studies that included almost 9,000 participants showed that CR significantly improved global cognition and global functioning. In addition, investigators identified key patient characteristics that flagged ideal candidates for the therapy.
“Because pharmacological treatment exerts limited effects on cognitive deficits, and clinical remission does not necessarily result in functional recovery, widespread implementation of CR could be a game-changer for achieving the patient’s personal recovery goals,” the researchers wrote.
“We hope that this systematic review could help clinicians understand how to make CR even more effective and even more personalized,” lead author Antonio Vita, MD, PhD, department of clinical and experimental sciences, University of Brescia, Italy, said in an interview.
Dr. Vita noted that he would also encourage clinicians to consider “proposing it for clinical practice.”
The findings were presented at the virtual congress of the Schizophrenia International Research Society (SIRS 2021) and were published simultaneously in JAMA Psychiatry.
Resistance continues
Cognition “should be a focus of treatment because most of the disability and functional consequences of the disease are related to ... neurocognitive impairment and impairment of social cognition,” Dr. Vita said.
He noted that treatments that focus on cognition are crucial for the recovery of patients with schizophrenia.
However, despite a “solid body of evidence” supporting the efficacy of CR and guideline recommendations that CR be included in psychiatric services, reluctance remains, the investigators noted.
The study’s goal was to determine optimal candidates for CR and to assess outcomes of the therapy and its four core elements:
- The presence of an active and trained therapist.
- Repeated practice of cognitive exercises.
- Structured development of cognitive strategies.
- Techniques to improve the transfer of cognitive gains to the real world, such as integrated psychosocial rehabilitation.
The investigators conducted a systematic literature search of the PubMed, Scopus, and PsychInfo databases to find relevant studies of CR published between January 2011 and February 2020. They also “hand-searched” meta-analyses, reviews, and reference lists.
Ultimately, the analysis included 130 randomized clinical trials comparing CR with a control condition in 8,851 patients with schizophrenia spectrum disorders.
Of these studies, 57 were conducted in Europe, 38 in the United States, 22 in Asia, 4 in Canada, 4 in Middle Eastern countries, 3 in Australia, and 2 in Brazil.
The mean age of the participants was 36.7 years, and 68% were men. The average age at the time of schizophrenia onset was 23.3 years, and the mean duration of illness was 13.8 years.
The average duration of CR treatment was 15.2 weeks. The four elements were well represented; each was offered to at least 71% of patients.
The comparator therapy was treatment as usual (TAU), in 34.3% of cases, or active TAU with multidisciplinary rehabilitation, in 15.2% of cases. The remaining interventions were either nonspecific (30.8%) or were devised specifically for the study (19.9%).
Results showed that CR had a significant, albeit moderate, effect on global cognition (Cohen’s d effect size, 0.29; P < .001) and global functioning (effect size, 0.22; P < .01).
Having an active and trained therapist had a significant impact on cognition and functioning (P = .04 for both), as did the structured development of cognitive strategies (P = .002 for cognition; P = .004 for functioning).
The integration of psychosocial rehabilitation also had a significant effect on functioning (P = .003).
Interventions that included all of the core elements had a “highly significant” association with global cognition (P = .02) and global functioning (P < .001), the investigators reported. Longer treatments were significantly associated with greater functional improvement (P = .006).
The investigators found that improvements were greater among patients who had fewer years of education (P = .03 for cognition; P = .02 for functioning), lower premorbid IQ scores (P = .04 for functioning), and more severe symptoms at baseline (P = .005 for cognition).
The researchers noted that CR should become more widely available because it has the “potential to be an element of standard care rather than an optional intervention targeting selected individuals.”
An overlooked treatment option
Commenting on the findings for this news organization, Alice Medalia, PhD, director of the Lieber Recovery Clinic at Columbia University Irving Medical Center, New York, noted that this study is the second large-scale analysis of the use of CR for patients with schizophrenia to come out this year. The other was published in Schizophrenia Bulletin.
“So this is a banner year for large reviews,” she said. “It’s great to have two studies like this [that] tell a very consistent story.”
Dr. Medalia, who was not involved with the research, said individuals “don’t really talk about cognition very much.”
CR, she added, is “one of an array of services that one should be providing, and the bigger picture is that every single person should have their cognitive health needs addressed.
“If someone is having problems, and it’s getting in the way of them being the kind of person they want to be and doing want they want to do, we need to intervene. How we intervene should always be in the least disruptive and intense way,” she said.
These measures could include examining sleep hygiene, adjusting medications, or introducing exercise.
“But there really does come a time for some people where cognitive remediation is going to be helpful, so it should be more available,” Dr. Medalia said.
Although increased availability is partially dependent on having enough trained therapists, the main reason CR is not more widely available is because “people just don’t think about cognition and they don’t know how to talk about it,” she noted. In addition, she said, even when it is available, clinicians don’t refer patients.
“That tells you something. The solution here is not to put a cognitive remediation program everywhere but ... to get people comfortable talking about cognition and identifying when an intervention is needed,” said Dr. Medalia.
One study author received grants from the National Institute for Health Research during the conduct of the study and is the creator of CIRCuiTs, a cognitive remediation software program. The other investigators and Dr. Medalia have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cognitive remediation (CR), a therapy that encompasses nonpharmacologic approaches to improving cognitive function for patients with severe mental illness, may lead to significant improvement for patients with schizophrenia, new research suggests.
A systematic review of 130 worldwide studies that included almost 9,000 participants showed that CR significantly improved global cognition and global functioning. In addition, investigators identified key patient characteristics that flagged ideal candidates for the therapy.
“Because pharmacological treatment exerts limited effects on cognitive deficits, and clinical remission does not necessarily result in functional recovery, widespread implementation of CR could be a game-changer for achieving the patient’s personal recovery goals,” the researchers wrote.
“We hope that this systematic review could help clinicians understand how to make CR even more effective and even more personalized,” lead author Antonio Vita, MD, PhD, department of clinical and experimental sciences, University of Brescia, Italy, said in an interview.
Dr. Vita noted that he would also encourage clinicians to consider “proposing it for clinical practice.”
The findings were presented at the virtual congress of the Schizophrenia International Research Society (SIRS 2021) and were published simultaneously in JAMA Psychiatry.
Resistance continues
Cognition “should be a focus of treatment because most of the disability and functional consequences of the disease are related to ... neurocognitive impairment and impairment of social cognition,” Dr. Vita said.
He noted that treatments that focus on cognition are crucial for the recovery of patients with schizophrenia.
However, despite a “solid body of evidence” supporting the efficacy of CR and guideline recommendations that CR be included in psychiatric services, reluctance remains, the investigators noted.
The study’s goal was to determine optimal candidates for CR and to assess outcomes of the therapy and its four core elements:
- The presence of an active and trained therapist.
- Repeated practice of cognitive exercises.
- Structured development of cognitive strategies.
- Techniques to improve the transfer of cognitive gains to the real world, such as integrated psychosocial rehabilitation.
The investigators conducted a systematic literature search of the PubMed, Scopus, and PsychInfo databases to find relevant studies of CR published between January 2011 and February 2020. They also “hand-searched” meta-analyses, reviews, and reference lists.
Ultimately, the analysis included 130 randomized clinical trials comparing CR with a control condition in 8,851 patients with schizophrenia spectrum disorders.
Of these studies, 57 were conducted in Europe, 38 in the United States, 22 in Asia, 4 in Canada, 4 in Middle Eastern countries, 3 in Australia, and 2 in Brazil.
The mean age of the participants was 36.7 years, and 68% were men. The average age at the time of schizophrenia onset was 23.3 years, and the mean duration of illness was 13.8 years.
The average duration of CR treatment was 15.2 weeks. The four elements were well represented; each was offered to at least 71% of patients.
The comparator therapy was treatment as usual (TAU), in 34.3% of cases, or active TAU with multidisciplinary rehabilitation, in 15.2% of cases. The remaining interventions were either nonspecific (30.8%) or were devised specifically for the study (19.9%).
Results showed that CR had a significant, albeit moderate, effect on global cognition (Cohen’s d effect size, 0.29; P < .001) and global functioning (effect size, 0.22; P < .01).
Having an active and trained therapist had a significant impact on cognition and functioning (P = .04 for both), as did the structured development of cognitive strategies (P = .002 for cognition; P = .004 for functioning).
The integration of psychosocial rehabilitation also had a significant effect on functioning (P = .003).
Interventions that included all of the core elements had a “highly significant” association with global cognition (P = .02) and global functioning (P < .001), the investigators reported. Longer treatments were significantly associated with greater functional improvement (P = .006).
The investigators found that improvements were greater among patients who had fewer years of education (P = .03 for cognition; P = .02 for functioning), lower premorbid IQ scores (P = .04 for functioning), and more severe symptoms at baseline (P = .005 for cognition).
The researchers noted that CR should become more widely available because it has the “potential to be an element of standard care rather than an optional intervention targeting selected individuals.”
An overlooked treatment option
Commenting on the findings for this news organization, Alice Medalia, PhD, director of the Lieber Recovery Clinic at Columbia University Irving Medical Center, New York, noted that this study is the second large-scale analysis of the use of CR for patients with schizophrenia to come out this year. The other was published in Schizophrenia Bulletin.
“So this is a banner year for large reviews,” she said. “It’s great to have two studies like this [that] tell a very consistent story.”
Dr. Medalia, who was not involved with the research, said individuals “don’t really talk about cognition very much.”
CR, she added, is “one of an array of services that one should be providing, and the bigger picture is that every single person should have their cognitive health needs addressed.
“If someone is having problems, and it’s getting in the way of them being the kind of person they want to be and doing want they want to do, we need to intervene. How we intervene should always be in the least disruptive and intense way,” she said.
These measures could include examining sleep hygiene, adjusting medications, or introducing exercise.
“But there really does come a time for some people where cognitive remediation is going to be helpful, so it should be more available,” Dr. Medalia said.
Although increased availability is partially dependent on having enough trained therapists, the main reason CR is not more widely available is because “people just don’t think about cognition and they don’t know how to talk about it,” she noted. In addition, she said, even when it is available, clinicians don’t refer patients.
“That tells you something. The solution here is not to put a cognitive remediation program everywhere but ... to get people comfortable talking about cognition and identifying when an intervention is needed,” said Dr. Medalia.
One study author received grants from the National Institute for Health Research during the conduct of the study and is the creator of CIRCuiTs, a cognitive remediation software program. The other investigators and Dr. Medalia have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID lockdowns linked to PTSD in patients with eating disorders
COVID-19 and its resulting lockdowns are linked to posttraumatic stress disorder symptoms and other adverse outcomes among patients with eating disorders (EDs), two new studies show.
Results of the first study show that patients with EDs had more stress, anxiety, depression, and PTSD-related symptoms during the lockdowns than their mentally healthy peers.
In the second study, treatment-related symptom improvement among patients with bulimia nervosa (BN) slowed following lockdown. In addition, patients with BN or anorexia nervosa (AN) experienced significant worsening of disorder-specific behaviors, including binge eating and overexercising.
Because of the strict lockdown measures introduced by the Italian government to contain the COVID-19 pandemic, “everyday life of all citizens was disrupted,” Veronica Nisticò, MS, Università Degli Studi Di Milano, who led the first study, told delegates attending the virtual European Psychiatric Association 2021 Congress.
In addition to difficulties in accessing health care, “it became difficult to go to the supermarket, to the gym, and to have the social support we were all used to,” all of which had a well-documented impact on mental health, added Ms. Nisticò, who is also affiliated with Aldo Ravelli Research Center for Neurotechnology and Experimental Brain Therapeutics.
Loss of control
Previous research suggests that individuals with EDs experience high levels of anxiety and an increase in binge eating, exercise, and purging behaviors, said Ms. Nisticò.
To investigate further, the researchers conducted a longitudinal study of the changes in prevalence of adverse outcomes. In the study, two assessments were conducted.
The second group served as the control group.
Participants completed an online survey that included several standardized depression and anxiety scales, as well as an ad hoc survey adapted from the Eating Disorder Examination Questionnaire. This assessed changes in restrictive dieting, control over food, body image, and psychological well-being in comparison with prepandemic levels.
The results, which were also recently published online in Eating and Weight Disorders – Studies on Anorexia, Bulimia and Obesity, showed that patients with EDs experienced significantly more stress, anxiety, depression, and PTSD-related symptoms in comparison with control persons (P < .05 for all).
In addition, the investigators found that those with EDs were more fearful of losing control over their eating behavior, spent more time thinking about food and their body, and became more uncomfortable seeing their body than before the lockdown in comparison with those without EDs (P < .05).
Clinical implications
A second assessment, which occurred in June 2020, after lockdown restrictions were lifted, included 40 patients with EDs who had taken part in the first assessment. This time, participants were asked to compare their current eating behavior with their eating behavior during the lockdown.
Although the lifting of lockdown restrictions was associated with significant improvement in PTSD-related symptoms, the impact on stress, anxiety, and depression persisted.
These findings, said Ms. Nisticó, support the hypothesis that specific conditions that occurred during the lockdown had a direct effect on specific ED symptoms.
These findings, she added, should be considered when developing interventions for EDs in the context of individual psychotherapy and when designing large, preventive interventions.
In the second study, Eleonora Rossi, MD, psychiatric unit, department of health sciences, University of Florence (Italy), and colleagues examined the longitudinal impact of the pandemic on individuals with EDs.
They examined 74 patients with AN or BN who had undergone baseline assessments and had completed a number of questionnaires in the first months of 2019 in conjunction with being enrolled in another study.
Participants were treated with enhanced cognitive-behavioral therapy and were reevaluated between November 2019 and January 2020. They were then compared with 97 healthy individuals.
Bulimia patients more vulnerable
After the outbreak of the pandemic, most treatment was administered online, so patients were able to continue therapy, Dr. Rossi said during her presentation.
All participants were assessed again in April 2020, 6 weeks after the start of Italy’s lockdown.
The results, which were published in the International Journal of Eating Disorders, show that the patients with EDs “underwent a significant improvement in terms of general and eating disorder specific psychopathology” during the first treatment period, Dr. Rossi reported. In addition, among those with AN, body mass index increased significantly (P < .05 for all).
Patients with AN continued to improve during the lockdown when therapy was administered online. However, improvements that had occurred among those with BN slowed, Dr. Rossi noted.
In addition, both groups of patients with EDs experienced a worsening of their pathological eating behaviors during the lockdown, in particular, objective binge eating and compensatory physical exercise (P < .05).
“Indeed, the positive trajectory of improvement observed before lockdown was clearly interrupted during the pandemic period,” Dr. Rossi said. This could “represent a possible hint of an imminent exacerbation of the disease.”
The results also suggest that the occurrence of arguments within the household and fear regarding the safety of loved ones predicted an increase in symptoms during the lockdown, she added.
In addition, patients with BN reported more severe COVID-related PTSD symptoms than did those with AN and the control group. This increase in severity of symptoms was more prevalent among patients who had a history of childhood trauma and among those with insecure attachment, suggesting that such patients may be more vulnerable.
Evidence of recovery
Commenting on the studies, David Spiegel, MD, associate chair of psychiatry, Stanford (Calif.) University, noted that EDs commonly occur after physical or sexual trauma earlier in life.
“It’s a standard thing with trauma-related disorders that any other, even relatively minor, traumatic experience can exacerbate PTSD symptoms,” said Dr. Spiegel, who was not involved in the studies. In addition, the trauma of the COVID pandemic “was not minor.
“The relative isolation and the lack of outside contact may focus many people with eating disorders even more on their struggles with how they are taking care of their bodies,” said Dr. Spiegel.
“It struck me that the anorexia nervosa group were more impervious than the bulimia nervosa group, but I think that’s the case with the disorder. In some ways it’s more severe, obviously a more life-threatening disorder,” he added.
The “hopeful thing is that there seemed to be some evidence of recovery and improvement, particularly with the posttraumatic stress exacerbation, as time went on,” Dr. Spiegel said, “and that’s a good thing.”
The study authors and Dr. Spiegel reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 and its resulting lockdowns are linked to posttraumatic stress disorder symptoms and other adverse outcomes among patients with eating disorders (EDs), two new studies show.
Results of the first study show that patients with EDs had more stress, anxiety, depression, and PTSD-related symptoms during the lockdowns than their mentally healthy peers.
In the second study, treatment-related symptom improvement among patients with bulimia nervosa (BN) slowed following lockdown. In addition, patients with BN or anorexia nervosa (AN) experienced significant worsening of disorder-specific behaviors, including binge eating and overexercising.
Because of the strict lockdown measures introduced by the Italian government to contain the COVID-19 pandemic, “everyday life of all citizens was disrupted,” Veronica Nisticò, MS, Università Degli Studi Di Milano, who led the first study, told delegates attending the virtual European Psychiatric Association 2021 Congress.
In addition to difficulties in accessing health care, “it became difficult to go to the supermarket, to the gym, and to have the social support we were all used to,” all of which had a well-documented impact on mental health, added Ms. Nisticò, who is also affiliated with Aldo Ravelli Research Center for Neurotechnology and Experimental Brain Therapeutics.
Loss of control
Previous research suggests that individuals with EDs experience high levels of anxiety and an increase in binge eating, exercise, and purging behaviors, said Ms. Nisticò.
To investigate further, the researchers conducted a longitudinal study of the changes in prevalence of adverse outcomes. In the study, two assessments were conducted.
The second group served as the control group.
Participants completed an online survey that included several standardized depression and anxiety scales, as well as an ad hoc survey adapted from the Eating Disorder Examination Questionnaire. This assessed changes in restrictive dieting, control over food, body image, and psychological well-being in comparison with prepandemic levels.
The results, which were also recently published online in Eating and Weight Disorders – Studies on Anorexia, Bulimia and Obesity, showed that patients with EDs experienced significantly more stress, anxiety, depression, and PTSD-related symptoms in comparison with control persons (P < .05 for all).
In addition, the investigators found that those with EDs were more fearful of losing control over their eating behavior, spent more time thinking about food and their body, and became more uncomfortable seeing their body than before the lockdown in comparison with those without EDs (P < .05).
Clinical implications
A second assessment, which occurred in June 2020, after lockdown restrictions were lifted, included 40 patients with EDs who had taken part in the first assessment. This time, participants were asked to compare their current eating behavior with their eating behavior during the lockdown.
Although the lifting of lockdown restrictions was associated with significant improvement in PTSD-related symptoms, the impact on stress, anxiety, and depression persisted.
These findings, said Ms. Nisticó, support the hypothesis that specific conditions that occurred during the lockdown had a direct effect on specific ED symptoms.
These findings, she added, should be considered when developing interventions for EDs in the context of individual psychotherapy and when designing large, preventive interventions.
In the second study, Eleonora Rossi, MD, psychiatric unit, department of health sciences, University of Florence (Italy), and colleagues examined the longitudinal impact of the pandemic on individuals with EDs.
They examined 74 patients with AN or BN who had undergone baseline assessments and had completed a number of questionnaires in the first months of 2019 in conjunction with being enrolled in another study.
Participants were treated with enhanced cognitive-behavioral therapy and were reevaluated between November 2019 and January 2020. They were then compared with 97 healthy individuals.
Bulimia patients more vulnerable
After the outbreak of the pandemic, most treatment was administered online, so patients were able to continue therapy, Dr. Rossi said during her presentation.
All participants were assessed again in April 2020, 6 weeks after the start of Italy’s lockdown.
The results, which were published in the International Journal of Eating Disorders, show that the patients with EDs “underwent a significant improvement in terms of general and eating disorder specific psychopathology” during the first treatment period, Dr. Rossi reported. In addition, among those with AN, body mass index increased significantly (P < .05 for all).
Patients with AN continued to improve during the lockdown when therapy was administered online. However, improvements that had occurred among those with BN slowed, Dr. Rossi noted.
In addition, both groups of patients with EDs experienced a worsening of their pathological eating behaviors during the lockdown, in particular, objective binge eating and compensatory physical exercise (P < .05).
“Indeed, the positive trajectory of improvement observed before lockdown was clearly interrupted during the pandemic period,” Dr. Rossi said. This could “represent a possible hint of an imminent exacerbation of the disease.”
The results also suggest that the occurrence of arguments within the household and fear regarding the safety of loved ones predicted an increase in symptoms during the lockdown, she added.
In addition, patients with BN reported more severe COVID-related PTSD symptoms than did those with AN and the control group. This increase in severity of symptoms was more prevalent among patients who had a history of childhood trauma and among those with insecure attachment, suggesting that such patients may be more vulnerable.
Evidence of recovery
Commenting on the studies, David Spiegel, MD, associate chair of psychiatry, Stanford (Calif.) University, noted that EDs commonly occur after physical or sexual trauma earlier in life.
“It’s a standard thing with trauma-related disorders that any other, even relatively minor, traumatic experience can exacerbate PTSD symptoms,” said Dr. Spiegel, who was not involved in the studies. In addition, the trauma of the COVID pandemic “was not minor.
“The relative isolation and the lack of outside contact may focus many people with eating disorders even more on their struggles with how they are taking care of their bodies,” said Dr. Spiegel.
“It struck me that the anorexia nervosa group were more impervious than the bulimia nervosa group, but I think that’s the case with the disorder. In some ways it’s more severe, obviously a more life-threatening disorder,” he added.
The “hopeful thing is that there seemed to be some evidence of recovery and improvement, particularly with the posttraumatic stress exacerbation, as time went on,” Dr. Spiegel said, “and that’s a good thing.”
The study authors and Dr. Spiegel reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 and its resulting lockdowns are linked to posttraumatic stress disorder symptoms and other adverse outcomes among patients with eating disorders (EDs), two new studies show.
Results of the first study show that patients with EDs had more stress, anxiety, depression, and PTSD-related symptoms during the lockdowns than their mentally healthy peers.
In the second study, treatment-related symptom improvement among patients with bulimia nervosa (BN) slowed following lockdown. In addition, patients with BN or anorexia nervosa (AN) experienced significant worsening of disorder-specific behaviors, including binge eating and overexercising.
Because of the strict lockdown measures introduced by the Italian government to contain the COVID-19 pandemic, “everyday life of all citizens was disrupted,” Veronica Nisticò, MS, Università Degli Studi Di Milano, who led the first study, told delegates attending the virtual European Psychiatric Association 2021 Congress.
In addition to difficulties in accessing health care, “it became difficult to go to the supermarket, to the gym, and to have the social support we were all used to,” all of which had a well-documented impact on mental health, added Ms. Nisticò, who is also affiliated with Aldo Ravelli Research Center for Neurotechnology and Experimental Brain Therapeutics.
Loss of control
Previous research suggests that individuals with EDs experience high levels of anxiety and an increase in binge eating, exercise, and purging behaviors, said Ms. Nisticò.
To investigate further, the researchers conducted a longitudinal study of the changes in prevalence of adverse outcomes. In the study, two assessments were conducted.
The second group served as the control group.
Participants completed an online survey that included several standardized depression and anxiety scales, as well as an ad hoc survey adapted from the Eating Disorder Examination Questionnaire. This assessed changes in restrictive dieting, control over food, body image, and psychological well-being in comparison with prepandemic levels.
The results, which were also recently published online in Eating and Weight Disorders – Studies on Anorexia, Bulimia and Obesity, showed that patients with EDs experienced significantly more stress, anxiety, depression, and PTSD-related symptoms in comparison with control persons (P < .05 for all).
In addition, the investigators found that those with EDs were more fearful of losing control over their eating behavior, spent more time thinking about food and their body, and became more uncomfortable seeing their body than before the lockdown in comparison with those without EDs (P < .05).
Clinical implications
A second assessment, which occurred in June 2020, after lockdown restrictions were lifted, included 40 patients with EDs who had taken part in the first assessment. This time, participants were asked to compare their current eating behavior with their eating behavior during the lockdown.
Although the lifting of lockdown restrictions was associated with significant improvement in PTSD-related symptoms, the impact on stress, anxiety, and depression persisted.
These findings, said Ms. Nisticó, support the hypothesis that specific conditions that occurred during the lockdown had a direct effect on specific ED symptoms.
These findings, she added, should be considered when developing interventions for EDs in the context of individual psychotherapy and when designing large, preventive interventions.
In the second study, Eleonora Rossi, MD, psychiatric unit, department of health sciences, University of Florence (Italy), and colleagues examined the longitudinal impact of the pandemic on individuals with EDs.
They examined 74 patients with AN or BN who had undergone baseline assessments and had completed a number of questionnaires in the first months of 2019 in conjunction with being enrolled in another study.
Participants were treated with enhanced cognitive-behavioral therapy and were reevaluated between November 2019 and January 2020. They were then compared with 97 healthy individuals.
Bulimia patients more vulnerable
After the outbreak of the pandemic, most treatment was administered online, so patients were able to continue therapy, Dr. Rossi said during her presentation.
All participants were assessed again in April 2020, 6 weeks after the start of Italy’s lockdown.
The results, which were published in the International Journal of Eating Disorders, show that the patients with EDs “underwent a significant improvement in terms of general and eating disorder specific psychopathology” during the first treatment period, Dr. Rossi reported. In addition, among those with AN, body mass index increased significantly (P < .05 for all).
Patients with AN continued to improve during the lockdown when therapy was administered online. However, improvements that had occurred among those with BN slowed, Dr. Rossi noted.
In addition, both groups of patients with EDs experienced a worsening of their pathological eating behaviors during the lockdown, in particular, objective binge eating and compensatory physical exercise (P < .05).
“Indeed, the positive trajectory of improvement observed before lockdown was clearly interrupted during the pandemic period,” Dr. Rossi said. This could “represent a possible hint of an imminent exacerbation of the disease.”
The results also suggest that the occurrence of arguments within the household and fear regarding the safety of loved ones predicted an increase in symptoms during the lockdown, she added.
In addition, patients with BN reported more severe COVID-related PTSD symptoms than did those with AN and the control group. This increase in severity of symptoms was more prevalent among patients who had a history of childhood trauma and among those with insecure attachment, suggesting that such patients may be more vulnerable.
Evidence of recovery
Commenting on the studies, David Spiegel, MD, associate chair of psychiatry, Stanford (Calif.) University, noted that EDs commonly occur after physical or sexual trauma earlier in life.
“It’s a standard thing with trauma-related disorders that any other, even relatively minor, traumatic experience can exacerbate PTSD symptoms,” said Dr. Spiegel, who was not involved in the studies. In addition, the trauma of the COVID pandemic “was not minor.
“The relative isolation and the lack of outside contact may focus many people with eating disorders even more on their struggles with how they are taking care of their bodies,” said Dr. Spiegel.
“It struck me that the anorexia nervosa group were more impervious than the bulimia nervosa group, but I think that’s the case with the disorder. In some ways it’s more severe, obviously a more life-threatening disorder,” he added.
The “hopeful thing is that there seemed to be some evidence of recovery and improvement, particularly with the posttraumatic stress exacerbation, as time went on,” Dr. Spiegel said, “and that’s a good thing.”
The study authors and Dr. Spiegel reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Marijuana vaping more common among Hispanic teens
Hispanic adolescents were more likely to use e-cigarettes to vape marijuana than were their Black and White counterparts in 2020, according to a recent study conducted by the Centers for Disease Control and Prevention and published in JAMA Pediatrics.
Researchers found that 25.6% of Hispanic students reported vaping marijuana, compared to 19.4% of Black students and 18.2% of White students. The study, which is an analysis of 2017, 2018, and 2020 results from the National Youth Tobacco Survey, also revealed that increases in this recreational practice occurred among all racial and ethnic groups within those 3 years, with Hispanic students having the largest percent increase, 11.6%, followed by Black students at 8.8% and White students at 7.4%.
“The initial motivation [to do this study] was to gain a better understanding of the prevalence of use of marijuana in e-cigarettes among youth, particularly given the context of the 2019 outbreak of e-cigarette, or vaping, product use–associated lung injury (EVALI),” study author Christina Vaughan Watson, DrPH, health scientist at the CDC’s National Center for Chronic Disease Prevention and Health Promotion, said in an interview.
The findings could help clinicians and physicians understand demographic variations among marijuana vapers and help inform targeted interventions for specific populations.
“Understanding demographic variations among those who are using marijuana in e-cigarettes can help inform evidenced-based interventions that may resonate with specific populations,” Dr. Watson explained.
Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin in Milwaukee, who was not involved in the study, said in an interview that the findings were “eye opening” and revealed a pattern she hasn’t seen before in her adolescent clinic.
“I would have thought African-American or non-Hispanic Blacks would’ve been a higher group of use, because when we screen kids that’s what we tend to get from the population we see here,” Ms. Thew said.
Ms. Thew said the findings also had made her reconsider her clinic’s approach to screening adolescents for marijuana use as well as address possible language barriers.
“We are probably missing access to some of the kids that we may need to seek out,” she explained. “I also thought it sends a good message that we need to direct some of our education probably a little differently, especially if it’s a Hispanic population and English may not be the primary language.”
Dr. Watson said more research is needed to assess why differences in marijuana use in e-cigarettes exist among youth.
Marijuana use in e-cigarettes has become increasingly popular among U.S. teens, with one in five students in grades 10 and 12 reporting vaping marijuana within the past year in a 2019 study conducted by the National Institute on Drug Abuse.
Dr. Watson and colleagues also found statistically significant increases in vaping marijuana, with 19.5% of students reporting smoking marijuana via e-cigarettes in 2020, compared to 11.1% of them vaping the drug in 2017. They believe the rise in marijuana vaping among youth may be attributed to states increasingly legalizing adult marijuana sales, which could impact ease of access and social acceptance.
Ms. Thew believes the rise in marijuana vaping among youth can be attributed to the legalization of marijuana, which may send “a message to adolescents that it must be safe for them to use,” as well as the increasing popularity of e-cigarettes.
In fact, as of April 2021, marijuana is legal for adults in 16 states and the District of Columbia. Meanwhile, medical marijuana is legal in 36 states, according to the National Conference of State Legislatures.
“I mean, there’s just definitely been a lot more use of [e-cigarettes]. Vaping and things like that definitely took off between 2019 and 2020,” Ms. Thew explained. “And I think marijuana use in itself is going up tremendously, I think more kids who would have used alcohol in the past use weed.”
Although public attitudes toward marijuana have relaxed, previous studies have linked it to memory dysfunction, as well as long-term cognitive effects that can interfere with perception of time and motor function. However, studies also have shown that cannabis use can combat age-related cognitive decline and help with pain reduction.
However, when it comes to adolescents, Dr. Watson and colleagues said e-cigarette use among youth and young adults is unsafe, regardless of the substances used in these products, including marijuana. Furthermore, they said marijuana use can lead to higher risks of more problematic use later in life, adding that evidence-based strategies to reduce marijuana use in e-cigarettes are important for protecting young people.
The study author and experts disclosed no relevant financial relationships.
Hispanic adolescents were more likely to use e-cigarettes to vape marijuana than were their Black and White counterparts in 2020, according to a recent study conducted by the Centers for Disease Control and Prevention and published in JAMA Pediatrics.
Researchers found that 25.6% of Hispanic students reported vaping marijuana, compared to 19.4% of Black students and 18.2% of White students. The study, which is an analysis of 2017, 2018, and 2020 results from the National Youth Tobacco Survey, also revealed that increases in this recreational practice occurred among all racial and ethnic groups within those 3 years, with Hispanic students having the largest percent increase, 11.6%, followed by Black students at 8.8% and White students at 7.4%.
“The initial motivation [to do this study] was to gain a better understanding of the prevalence of use of marijuana in e-cigarettes among youth, particularly given the context of the 2019 outbreak of e-cigarette, or vaping, product use–associated lung injury (EVALI),” study author Christina Vaughan Watson, DrPH, health scientist at the CDC’s National Center for Chronic Disease Prevention and Health Promotion, said in an interview.
The findings could help clinicians and physicians understand demographic variations among marijuana vapers and help inform targeted interventions for specific populations.
“Understanding demographic variations among those who are using marijuana in e-cigarettes can help inform evidenced-based interventions that may resonate with specific populations,” Dr. Watson explained.
Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin in Milwaukee, who was not involved in the study, said in an interview that the findings were “eye opening” and revealed a pattern she hasn’t seen before in her adolescent clinic.
“I would have thought African-American or non-Hispanic Blacks would’ve been a higher group of use, because when we screen kids that’s what we tend to get from the population we see here,” Ms. Thew said.
Ms. Thew said the findings also had made her reconsider her clinic’s approach to screening adolescents for marijuana use as well as address possible language barriers.
“We are probably missing access to some of the kids that we may need to seek out,” she explained. “I also thought it sends a good message that we need to direct some of our education probably a little differently, especially if it’s a Hispanic population and English may not be the primary language.”
Dr. Watson said more research is needed to assess why differences in marijuana use in e-cigarettes exist among youth.
Marijuana use in e-cigarettes has become increasingly popular among U.S. teens, with one in five students in grades 10 and 12 reporting vaping marijuana within the past year in a 2019 study conducted by the National Institute on Drug Abuse.
Dr. Watson and colleagues also found statistically significant increases in vaping marijuana, with 19.5% of students reporting smoking marijuana via e-cigarettes in 2020, compared to 11.1% of them vaping the drug in 2017. They believe the rise in marijuana vaping among youth may be attributed to states increasingly legalizing adult marijuana sales, which could impact ease of access and social acceptance.
Ms. Thew believes the rise in marijuana vaping among youth can be attributed to the legalization of marijuana, which may send “a message to adolescents that it must be safe for them to use,” as well as the increasing popularity of e-cigarettes.
In fact, as of April 2021, marijuana is legal for adults in 16 states and the District of Columbia. Meanwhile, medical marijuana is legal in 36 states, according to the National Conference of State Legislatures.
“I mean, there’s just definitely been a lot more use of [e-cigarettes]. Vaping and things like that definitely took off between 2019 and 2020,” Ms. Thew explained. “And I think marijuana use in itself is going up tremendously, I think more kids who would have used alcohol in the past use weed.”
Although public attitudes toward marijuana have relaxed, previous studies have linked it to memory dysfunction, as well as long-term cognitive effects that can interfere with perception of time and motor function. However, studies also have shown that cannabis use can combat age-related cognitive decline and help with pain reduction.
However, when it comes to adolescents, Dr. Watson and colleagues said e-cigarette use among youth and young adults is unsafe, regardless of the substances used in these products, including marijuana. Furthermore, they said marijuana use can lead to higher risks of more problematic use later in life, adding that evidence-based strategies to reduce marijuana use in e-cigarettes are important for protecting young people.
The study author and experts disclosed no relevant financial relationships.
Hispanic adolescents were more likely to use e-cigarettes to vape marijuana than were their Black and White counterparts in 2020, according to a recent study conducted by the Centers for Disease Control and Prevention and published in JAMA Pediatrics.
Researchers found that 25.6% of Hispanic students reported vaping marijuana, compared to 19.4% of Black students and 18.2% of White students. The study, which is an analysis of 2017, 2018, and 2020 results from the National Youth Tobacco Survey, also revealed that increases in this recreational practice occurred among all racial and ethnic groups within those 3 years, with Hispanic students having the largest percent increase, 11.6%, followed by Black students at 8.8% and White students at 7.4%.
“The initial motivation [to do this study] was to gain a better understanding of the prevalence of use of marijuana in e-cigarettes among youth, particularly given the context of the 2019 outbreak of e-cigarette, or vaping, product use–associated lung injury (EVALI),” study author Christina Vaughan Watson, DrPH, health scientist at the CDC’s National Center for Chronic Disease Prevention and Health Promotion, said in an interview.
The findings could help clinicians and physicians understand demographic variations among marijuana vapers and help inform targeted interventions for specific populations.
“Understanding demographic variations among those who are using marijuana in e-cigarettes can help inform evidenced-based interventions that may resonate with specific populations,” Dr. Watson explained.
Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin in Milwaukee, who was not involved in the study, said in an interview that the findings were “eye opening” and revealed a pattern she hasn’t seen before in her adolescent clinic.
“I would have thought African-American or non-Hispanic Blacks would’ve been a higher group of use, because when we screen kids that’s what we tend to get from the population we see here,” Ms. Thew said.
Ms. Thew said the findings also had made her reconsider her clinic’s approach to screening adolescents for marijuana use as well as address possible language barriers.
“We are probably missing access to some of the kids that we may need to seek out,” she explained. “I also thought it sends a good message that we need to direct some of our education probably a little differently, especially if it’s a Hispanic population and English may not be the primary language.”
Dr. Watson said more research is needed to assess why differences in marijuana use in e-cigarettes exist among youth.
Marijuana use in e-cigarettes has become increasingly popular among U.S. teens, with one in five students in grades 10 and 12 reporting vaping marijuana within the past year in a 2019 study conducted by the National Institute on Drug Abuse.
Dr. Watson and colleagues also found statistically significant increases in vaping marijuana, with 19.5% of students reporting smoking marijuana via e-cigarettes in 2020, compared to 11.1% of them vaping the drug in 2017. They believe the rise in marijuana vaping among youth may be attributed to states increasingly legalizing adult marijuana sales, which could impact ease of access and social acceptance.
Ms. Thew believes the rise in marijuana vaping among youth can be attributed to the legalization of marijuana, which may send “a message to adolescents that it must be safe for them to use,” as well as the increasing popularity of e-cigarettes.
In fact, as of April 2021, marijuana is legal for adults in 16 states and the District of Columbia. Meanwhile, medical marijuana is legal in 36 states, according to the National Conference of State Legislatures.
“I mean, there’s just definitely been a lot more use of [e-cigarettes]. Vaping and things like that definitely took off between 2019 and 2020,” Ms. Thew explained. “And I think marijuana use in itself is going up tremendously, I think more kids who would have used alcohol in the past use weed.”
Although public attitudes toward marijuana have relaxed, previous studies have linked it to memory dysfunction, as well as long-term cognitive effects that can interfere with perception of time and motor function. However, studies also have shown that cannabis use can combat age-related cognitive decline and help with pain reduction.
However, when it comes to adolescents, Dr. Watson and colleagues said e-cigarette use among youth and young adults is unsafe, regardless of the substances used in these products, including marijuana. Furthermore, they said marijuana use can lead to higher risks of more problematic use later in life, adding that evidence-based strategies to reduce marijuana use in e-cigarettes are important for protecting young people.
The study author and experts disclosed no relevant financial relationships.
NfL beats T-tau as a prognostic marker of cognitive decline
, new research suggests. In certain contexts, T-tau improves cross-sectional analyses of these outcomes, but adding T-tau measurements to NfL measurements does not improve the predictive power of NfL, results of a longitudinal analysis show.
“The major distinction, for cognition at least, was that NfL cross-sectionally was associated with most cognitive outcomes, and longitudinally, higher NfL at baseline was associated with cognitive decline in every domain,” said study investigator Jordan Marks, an MD/PhD student at the Mayo Medical School, Rochester, Minn.
The findings were presented at the American Academy of Neurology’s 2021 annual meeting.
New tool for dementia diagnosis?
In recent years, researchers have studied NfL and T-tau as potential blood-based biomarkers of neurodegeneration. In cross-sectional and longitudinal studies, NfL and T-tau have been associated with worse cognition and with neuroimaging measures of cortical thickness, cortical atrophy, white-matter hyperintensity, and white-matter integrity. However, no previous research has directly compared the prognostic ability of these two biomarkers.
The study included 995 participants without dementia in the Mayo Clinic Study on Aging. All participants underwent measurement of NfL and T-tau and assessment of cognitive status, as well as neuroimaging. The investigators measured NfL and T-tau on the Simoa HD-1 platform. They reexamined patients approximately every 15 months. The median follow-up time was 6.2 years.
To examine associations between baseline plasma NfL or T-tau and cognitive or neuroimaging outcomes, the researchers conducted data analyses using linear mixed effects models and adjusted the data for age, sex, and education. They replicated these analyses using data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). For these analyses, they selected 387 participants without dementia who had been followed for a median of 3.0 years.
In all analyses, baseline plasma NfL was more strongly associated with cognitive and neuroimaging outcomes than T-tau. “Baseline plasma NfL was associated with cognitive decline in all domains measured, while T-tau was not associated with cognitive decline,” said Mr. Marks.
Plasma NfL was more strongly associated with decreases in cortical thickness over time than T-tau was. NfL was also more strongly associated with declining hippocampal volume and white-matter changes.
However, in cross-sectional analysis, the combination of elevated NfL levels and elevated T-tau levels at baseline was more strongly associated with decreased global cognition and memory, compared with elevated NfL levels alone. The combination also was more strongly associated with neuroimaging measures, such as temporal cortex thickness and increased number of infarcts. However, in longitudinal analyses, T-tau did not add to the predictive value of NfL.
The analyses using ADNI data yielded similar results. Overall, the results suggest that NfL is a better prognostic marker of neurodegeneration in general, said Mr. Marks.
These findings, he said, may have implications for screening and diagnosis. “I’m definitely hopeful that NfL will be useful in a clinical setting to screen for those at risk of dementia and will be helpful, along with other modalities, like cognitive testing, for dementia diagnosis,” said Mr. Marks.
Future research should examine how changes in these biomarkers are associated with cognitive and neuroimaging outcomes over time.
“We used plasma levels at one point in time in this study, but we need a better sense of how to interpret, for example, what a rise in plasma NfL over a certain time period means for someone’s risk of developing neurodegenerative disease,” Mr. Marks added.
An ‘exciting’ prospect
Commenting on the study, Glen R. Finney, MD, director of the Memory and Cognition Program for Geisinger Health in Wilkes-Barre, Pa., said the findings add to neurologists’ ability to screen for brain diseases. “Evidence of neurodegeneration is part of the modern diagnosis of several disorders. While brain imaging can also provide that and may be needed for other reasons, this could provide an easy, potentially inexpensive way to screen for damage to the brain, giving us an added tool,” said Dr. Finney.
The prospect of using blood plasma markers to explore disease of the brain is exciting, Dr. Finney added. “I would like to see ongoing refinement of this approach and would like to see if there’s other markers in blood that could be used to find what specifically may be causing the damage,” he said.
The study was funded by the National Institutes of Health, the National Institute on Aging, and the GHR Foundation. Mr. Marks and Dr. Finney have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. In certain contexts, T-tau improves cross-sectional analyses of these outcomes, but adding T-tau measurements to NfL measurements does not improve the predictive power of NfL, results of a longitudinal analysis show.
“The major distinction, for cognition at least, was that NfL cross-sectionally was associated with most cognitive outcomes, and longitudinally, higher NfL at baseline was associated with cognitive decline in every domain,” said study investigator Jordan Marks, an MD/PhD student at the Mayo Medical School, Rochester, Minn.
The findings were presented at the American Academy of Neurology’s 2021 annual meeting.
New tool for dementia diagnosis?
In recent years, researchers have studied NfL and T-tau as potential blood-based biomarkers of neurodegeneration. In cross-sectional and longitudinal studies, NfL and T-tau have been associated with worse cognition and with neuroimaging measures of cortical thickness, cortical atrophy, white-matter hyperintensity, and white-matter integrity. However, no previous research has directly compared the prognostic ability of these two biomarkers.
The study included 995 participants without dementia in the Mayo Clinic Study on Aging. All participants underwent measurement of NfL and T-tau and assessment of cognitive status, as well as neuroimaging. The investigators measured NfL and T-tau on the Simoa HD-1 platform. They reexamined patients approximately every 15 months. The median follow-up time was 6.2 years.
To examine associations between baseline plasma NfL or T-tau and cognitive or neuroimaging outcomes, the researchers conducted data analyses using linear mixed effects models and adjusted the data for age, sex, and education. They replicated these analyses using data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). For these analyses, they selected 387 participants without dementia who had been followed for a median of 3.0 years.
In all analyses, baseline plasma NfL was more strongly associated with cognitive and neuroimaging outcomes than T-tau. “Baseline plasma NfL was associated with cognitive decline in all domains measured, while T-tau was not associated with cognitive decline,” said Mr. Marks.
Plasma NfL was more strongly associated with decreases in cortical thickness over time than T-tau was. NfL was also more strongly associated with declining hippocampal volume and white-matter changes.
However, in cross-sectional analysis, the combination of elevated NfL levels and elevated T-tau levels at baseline was more strongly associated with decreased global cognition and memory, compared with elevated NfL levels alone. The combination also was more strongly associated with neuroimaging measures, such as temporal cortex thickness and increased number of infarcts. However, in longitudinal analyses, T-tau did not add to the predictive value of NfL.
The analyses using ADNI data yielded similar results. Overall, the results suggest that NfL is a better prognostic marker of neurodegeneration in general, said Mr. Marks.
These findings, he said, may have implications for screening and diagnosis. “I’m definitely hopeful that NfL will be useful in a clinical setting to screen for those at risk of dementia and will be helpful, along with other modalities, like cognitive testing, for dementia diagnosis,” said Mr. Marks.
Future research should examine how changes in these biomarkers are associated with cognitive and neuroimaging outcomes over time.
“We used plasma levels at one point in time in this study, but we need a better sense of how to interpret, for example, what a rise in plasma NfL over a certain time period means for someone’s risk of developing neurodegenerative disease,” Mr. Marks added.
An ‘exciting’ prospect
Commenting on the study, Glen R. Finney, MD, director of the Memory and Cognition Program for Geisinger Health in Wilkes-Barre, Pa., said the findings add to neurologists’ ability to screen for brain diseases. “Evidence of neurodegeneration is part of the modern diagnosis of several disorders. While brain imaging can also provide that and may be needed for other reasons, this could provide an easy, potentially inexpensive way to screen for damage to the brain, giving us an added tool,” said Dr. Finney.
The prospect of using blood plasma markers to explore disease of the brain is exciting, Dr. Finney added. “I would like to see ongoing refinement of this approach and would like to see if there’s other markers in blood that could be used to find what specifically may be causing the damage,” he said.
The study was funded by the National Institutes of Health, the National Institute on Aging, and the GHR Foundation. Mr. Marks and Dr. Finney have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. In certain contexts, T-tau improves cross-sectional analyses of these outcomes, but adding T-tau measurements to NfL measurements does not improve the predictive power of NfL, results of a longitudinal analysis show.
“The major distinction, for cognition at least, was that NfL cross-sectionally was associated with most cognitive outcomes, and longitudinally, higher NfL at baseline was associated with cognitive decline in every domain,” said study investigator Jordan Marks, an MD/PhD student at the Mayo Medical School, Rochester, Minn.
The findings were presented at the American Academy of Neurology’s 2021 annual meeting.
New tool for dementia diagnosis?
In recent years, researchers have studied NfL and T-tau as potential blood-based biomarkers of neurodegeneration. In cross-sectional and longitudinal studies, NfL and T-tau have been associated with worse cognition and with neuroimaging measures of cortical thickness, cortical atrophy, white-matter hyperintensity, and white-matter integrity. However, no previous research has directly compared the prognostic ability of these two biomarkers.
The study included 995 participants without dementia in the Mayo Clinic Study on Aging. All participants underwent measurement of NfL and T-tau and assessment of cognitive status, as well as neuroimaging. The investigators measured NfL and T-tau on the Simoa HD-1 platform. They reexamined patients approximately every 15 months. The median follow-up time was 6.2 years.
To examine associations between baseline plasma NfL or T-tau and cognitive or neuroimaging outcomes, the researchers conducted data analyses using linear mixed effects models and adjusted the data for age, sex, and education. They replicated these analyses using data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). For these analyses, they selected 387 participants without dementia who had been followed for a median of 3.0 years.
In all analyses, baseline plasma NfL was more strongly associated with cognitive and neuroimaging outcomes than T-tau. “Baseline plasma NfL was associated with cognitive decline in all domains measured, while T-tau was not associated with cognitive decline,” said Mr. Marks.
Plasma NfL was more strongly associated with decreases in cortical thickness over time than T-tau was. NfL was also more strongly associated with declining hippocampal volume and white-matter changes.
However, in cross-sectional analysis, the combination of elevated NfL levels and elevated T-tau levels at baseline was more strongly associated with decreased global cognition and memory, compared with elevated NfL levels alone. The combination also was more strongly associated with neuroimaging measures, such as temporal cortex thickness and increased number of infarcts. However, in longitudinal analyses, T-tau did not add to the predictive value of NfL.
The analyses using ADNI data yielded similar results. Overall, the results suggest that NfL is a better prognostic marker of neurodegeneration in general, said Mr. Marks.
These findings, he said, may have implications for screening and diagnosis. “I’m definitely hopeful that NfL will be useful in a clinical setting to screen for those at risk of dementia and will be helpful, along with other modalities, like cognitive testing, for dementia diagnosis,” said Mr. Marks.
Future research should examine how changes in these biomarkers are associated with cognitive and neuroimaging outcomes over time.
“We used plasma levels at one point in time in this study, but we need a better sense of how to interpret, for example, what a rise in plasma NfL over a certain time period means for someone’s risk of developing neurodegenerative disease,” Mr. Marks added.
An ‘exciting’ prospect
Commenting on the study, Glen R. Finney, MD, director of the Memory and Cognition Program for Geisinger Health in Wilkes-Barre, Pa., said the findings add to neurologists’ ability to screen for brain diseases. “Evidence of neurodegeneration is part of the modern diagnosis of several disorders. While brain imaging can also provide that and may be needed for other reasons, this could provide an easy, potentially inexpensive way to screen for damage to the brain, giving us an added tool,” said Dr. Finney.
The prospect of using blood plasma markers to explore disease of the brain is exciting, Dr. Finney added. “I would like to see ongoing refinement of this approach and would like to see if there’s other markers in blood that could be used to find what specifically may be causing the damage,” he said.
The study was funded by the National Institutes of Health, the National Institute on Aging, and the GHR Foundation. Mr. Marks and Dr. Finney have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From AAN 2021
Doctors more likely to prescribe opioids to COVID ‘long-haulers,’ raising addiction fears
COVID-19 survivors are at risk from a possible second pandemic, this time of opioid addiction, given the high rate of painkillers being prescribed to these patients, health experts say.
A new study in Nature found alarmingly high rates of opioid use among COVID survivors with lingering symptoms at Veterans Affairs facilities. About 10% of COVID survivors develop “long COVID,” struggling with often disabling health problems even 6 months or longer after a diagnosis.
For every 1,000 long-COVID patients, known as “long-haulers,” who were treated at a VA facility, doctors wrote nine more prescriptions for opioids than they otherwise would have, along with 22 additional prescriptions for benzodiazepines, which include Xanax and other addictive pills used to treat anxiety.
Although previous studies have found many COVID survivors experience persistent health problems, the new article is the first to show they’re using more addictive medications, said Ziyad Al-Aly, MD, the paper’s lead author.
He’s concerned that even an apparently small increase in the inappropriate use of addictive pain pills will lead to a resurgence of the prescription opioid crisis, given the large number of COVID survivors. More than 3 million of the 31 million Americans infected with COVID develop long-term symptoms, which can include fatigue, shortness of breath, depression, anxiety, and memory problems known as “brain fog.”
The new study also found many patients have significant muscle and bone pain.
The frequent use of opioids was surprising, given concerns about their potential for addiction, said Dr. Al-Aly, chief of research and education service at the VA St. Louis Health Care System.
“Physicians now are supposed to shy away from prescribing opioids,” said Dr. Al-Aly, who studied more than 73,000 patients in the VA system. When Dr. Al-Aly saw the number of opioids prescriptions, he said, he thought to himself: “Is this really happening all over again?”
Doctors need to act now, before “it’s too late to do something,” Dr. Al-Aly said. “We must act now and ensure that people are getting the care they need. We do not want this to balloon into a suicide crisis or another opioid epidemic.”
As more doctors became aware of their addictive potential, new opioid prescriptions fell, by more than half since 2012. But said Andrew Kolodny, MD, medical director of opioid policy research at Brandeis University, Waltham, Mass.
Some patients who became addicted to prescription painkillers switched to heroin, either because it was cheaper or because they could no longer obtain opioids from their doctors. Overdose deaths surged in recent years as drug dealers began spiking heroin with a powerful synthetic opioid called fentanyl.
More than 88,000 Americans died from overdoses during the 12 months ending in August 2020, according to the Centers for Disease Control and Prevention. Health experts now advise doctors to avoid prescribing opioids for long periods.
The new study “suggests to me that many clinicians still don’t get it,” Dr. Kolodny said. “Many clinicians are under the false impression that opioids are appropriate for chronic pain patients.”
Hospitalized COVID patients often receive a lot of medication to control pain and anxiety, especially in ICUs, said Greg Martin, MD, president of the Society of Critical Care Medicine. Patients placed on ventilators, for example, are often sedated to make them more comfortable.
Martin said he’s concerned by the study’s findings, which suggest patients are unnecessarily continuing medications after leaving the hospital.
“I worry that COVID-19 patients, especially those who are severely and critically ill, receive a lot of medications during the hospitalization, and because they have persistent symptoms, the medications are continued after hospital discharge,” Dr. Martin said.
While some COVID patients are experiencing muscle and bone pain for the first time, others say the illness has intensified their preexisting pain.
Rachael Sunshine Burnett has suffered from chronic pain in her back and feet for 20 years, ever since an accident at a warehouse where she once worked. But Ms. Burnett, who first was diagnosed with COVID in April 2020, said the pain soon became 10 times worse and spread to the area between her shoulders and spine. Although she was already taking long-acting OxyContin twice a day, her doctor prescribed an additional opioid called oxycodone, which relieves pain immediately. She was reinfected with COVID in December.
“It’s been a horrible, horrible year,” said Ms. Burnett, 43, of Coxsackie, N.Y.
Doctors should recognize that pain can be a part of long COVID, Dr. Martin said. “We need to find the proper nonnarcotic treatment for it, just like we do with other forms of chronic pain,” he said.
The CDC recommends a number of alternatives to opioids – from physical therapy to biofeedback, over-the-counter anti-inflammatories, antidepressants, and antiseizure drugs that also relieve nerve pain.
The country also needs an overall strategy to cope with the wave of post-COVID complications, Dr. Al-Aly said.
“It’s better to be prepared than to be caught off guard years from now, when doctors realize: ‘Oh, we have a resurgence in opioids,’ ” Dr. Al-Aly said.
Dr. Al-Aly noted that his study may not capture the full complexity of post-COVID patient needs. Although women make up the majority of long-COVID patients in most studies, most patients in the VA system are men.
The study of VA patients makes it “abundantly clear that we are not prepared to meet the needs of 3 million Americans with long COVID,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego. “We desperately need an intervention that will effectively treat these individuals.”
Dr. Al-Aly said COVID survivors may need care for years.
“That’s going to be a huge, significant burden on the health care system,” Dr. Al-Aly said. “Long COVID will reverberate in the health system for years or even decades to come.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
COVID-19 survivors are at risk from a possible second pandemic, this time of opioid addiction, given the high rate of painkillers being prescribed to these patients, health experts say.
A new study in Nature found alarmingly high rates of opioid use among COVID survivors with lingering symptoms at Veterans Affairs facilities. About 10% of COVID survivors develop “long COVID,” struggling with often disabling health problems even 6 months or longer after a diagnosis.
For every 1,000 long-COVID patients, known as “long-haulers,” who were treated at a VA facility, doctors wrote nine more prescriptions for opioids than they otherwise would have, along with 22 additional prescriptions for benzodiazepines, which include Xanax and other addictive pills used to treat anxiety.
Although previous studies have found many COVID survivors experience persistent health problems, the new article is the first to show they’re using more addictive medications, said Ziyad Al-Aly, MD, the paper’s lead author.
He’s concerned that even an apparently small increase in the inappropriate use of addictive pain pills will lead to a resurgence of the prescription opioid crisis, given the large number of COVID survivors. More than 3 million of the 31 million Americans infected with COVID develop long-term symptoms, which can include fatigue, shortness of breath, depression, anxiety, and memory problems known as “brain fog.”
The new study also found many patients have significant muscle and bone pain.
The frequent use of opioids was surprising, given concerns about their potential for addiction, said Dr. Al-Aly, chief of research and education service at the VA St. Louis Health Care System.
“Physicians now are supposed to shy away from prescribing opioids,” said Dr. Al-Aly, who studied more than 73,000 patients in the VA system. When Dr. Al-Aly saw the number of opioids prescriptions, he said, he thought to himself: “Is this really happening all over again?”
Doctors need to act now, before “it’s too late to do something,” Dr. Al-Aly said. “We must act now and ensure that people are getting the care they need. We do not want this to balloon into a suicide crisis or another opioid epidemic.”
As more doctors became aware of their addictive potential, new opioid prescriptions fell, by more than half since 2012. But said Andrew Kolodny, MD, medical director of opioid policy research at Brandeis University, Waltham, Mass.
Some patients who became addicted to prescription painkillers switched to heroin, either because it was cheaper or because they could no longer obtain opioids from their doctors. Overdose deaths surged in recent years as drug dealers began spiking heroin with a powerful synthetic opioid called fentanyl.
More than 88,000 Americans died from overdoses during the 12 months ending in August 2020, according to the Centers for Disease Control and Prevention. Health experts now advise doctors to avoid prescribing opioids for long periods.
The new study “suggests to me that many clinicians still don’t get it,” Dr. Kolodny said. “Many clinicians are under the false impression that opioids are appropriate for chronic pain patients.”
Hospitalized COVID patients often receive a lot of medication to control pain and anxiety, especially in ICUs, said Greg Martin, MD, president of the Society of Critical Care Medicine. Patients placed on ventilators, for example, are often sedated to make them more comfortable.
Martin said he’s concerned by the study’s findings, which suggest patients are unnecessarily continuing medications after leaving the hospital.
“I worry that COVID-19 patients, especially those who are severely and critically ill, receive a lot of medications during the hospitalization, and because they have persistent symptoms, the medications are continued after hospital discharge,” Dr. Martin said.
While some COVID patients are experiencing muscle and bone pain for the first time, others say the illness has intensified their preexisting pain.
Rachael Sunshine Burnett has suffered from chronic pain in her back and feet for 20 years, ever since an accident at a warehouse where she once worked. But Ms. Burnett, who first was diagnosed with COVID in April 2020, said the pain soon became 10 times worse and spread to the area between her shoulders and spine. Although she was already taking long-acting OxyContin twice a day, her doctor prescribed an additional opioid called oxycodone, which relieves pain immediately. She was reinfected with COVID in December.
“It’s been a horrible, horrible year,” said Ms. Burnett, 43, of Coxsackie, N.Y.
Doctors should recognize that pain can be a part of long COVID, Dr. Martin said. “We need to find the proper nonnarcotic treatment for it, just like we do with other forms of chronic pain,” he said.
The CDC recommends a number of alternatives to opioids – from physical therapy to biofeedback, over-the-counter anti-inflammatories, antidepressants, and antiseizure drugs that also relieve nerve pain.
The country also needs an overall strategy to cope with the wave of post-COVID complications, Dr. Al-Aly said.
“It’s better to be prepared than to be caught off guard years from now, when doctors realize: ‘Oh, we have a resurgence in opioids,’ ” Dr. Al-Aly said.
Dr. Al-Aly noted that his study may not capture the full complexity of post-COVID patient needs. Although women make up the majority of long-COVID patients in most studies, most patients in the VA system are men.
The study of VA patients makes it “abundantly clear that we are not prepared to meet the needs of 3 million Americans with long COVID,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego. “We desperately need an intervention that will effectively treat these individuals.”
Dr. Al-Aly said COVID survivors may need care for years.
“That’s going to be a huge, significant burden on the health care system,” Dr. Al-Aly said. “Long COVID will reverberate in the health system for years or even decades to come.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
COVID-19 survivors are at risk from a possible second pandemic, this time of opioid addiction, given the high rate of painkillers being prescribed to these patients, health experts say.
A new study in Nature found alarmingly high rates of opioid use among COVID survivors with lingering symptoms at Veterans Affairs facilities. About 10% of COVID survivors develop “long COVID,” struggling with often disabling health problems even 6 months or longer after a diagnosis.
For every 1,000 long-COVID patients, known as “long-haulers,” who were treated at a VA facility, doctors wrote nine more prescriptions for opioids than they otherwise would have, along with 22 additional prescriptions for benzodiazepines, which include Xanax and other addictive pills used to treat anxiety.
Although previous studies have found many COVID survivors experience persistent health problems, the new article is the first to show they’re using more addictive medications, said Ziyad Al-Aly, MD, the paper’s lead author.
He’s concerned that even an apparently small increase in the inappropriate use of addictive pain pills will lead to a resurgence of the prescription opioid crisis, given the large number of COVID survivors. More than 3 million of the 31 million Americans infected with COVID develop long-term symptoms, which can include fatigue, shortness of breath, depression, anxiety, and memory problems known as “brain fog.”
The new study also found many patients have significant muscle and bone pain.
The frequent use of opioids was surprising, given concerns about their potential for addiction, said Dr. Al-Aly, chief of research and education service at the VA St. Louis Health Care System.
“Physicians now are supposed to shy away from prescribing opioids,” said Dr. Al-Aly, who studied more than 73,000 patients in the VA system. When Dr. Al-Aly saw the number of opioids prescriptions, he said, he thought to himself: “Is this really happening all over again?”
Doctors need to act now, before “it’s too late to do something,” Dr. Al-Aly said. “We must act now and ensure that people are getting the care they need. We do not want this to balloon into a suicide crisis or another opioid epidemic.”
As more doctors became aware of their addictive potential, new opioid prescriptions fell, by more than half since 2012. But said Andrew Kolodny, MD, medical director of opioid policy research at Brandeis University, Waltham, Mass.
Some patients who became addicted to prescription painkillers switched to heroin, either because it was cheaper or because they could no longer obtain opioids from their doctors. Overdose deaths surged in recent years as drug dealers began spiking heroin with a powerful synthetic opioid called fentanyl.
More than 88,000 Americans died from overdoses during the 12 months ending in August 2020, according to the Centers for Disease Control and Prevention. Health experts now advise doctors to avoid prescribing opioids for long periods.
The new study “suggests to me that many clinicians still don’t get it,” Dr. Kolodny said. “Many clinicians are under the false impression that opioids are appropriate for chronic pain patients.”
Hospitalized COVID patients often receive a lot of medication to control pain and anxiety, especially in ICUs, said Greg Martin, MD, president of the Society of Critical Care Medicine. Patients placed on ventilators, for example, are often sedated to make them more comfortable.
Martin said he’s concerned by the study’s findings, which suggest patients are unnecessarily continuing medications after leaving the hospital.
“I worry that COVID-19 patients, especially those who are severely and critically ill, receive a lot of medications during the hospitalization, and because they have persistent symptoms, the medications are continued after hospital discharge,” Dr. Martin said.
While some COVID patients are experiencing muscle and bone pain for the first time, others say the illness has intensified their preexisting pain.
Rachael Sunshine Burnett has suffered from chronic pain in her back and feet for 20 years, ever since an accident at a warehouse where she once worked. But Ms. Burnett, who first was diagnosed with COVID in April 2020, said the pain soon became 10 times worse and spread to the area between her shoulders and spine. Although she was already taking long-acting OxyContin twice a day, her doctor prescribed an additional opioid called oxycodone, which relieves pain immediately. She was reinfected with COVID in December.
“It’s been a horrible, horrible year,” said Ms. Burnett, 43, of Coxsackie, N.Y.
Doctors should recognize that pain can be a part of long COVID, Dr. Martin said. “We need to find the proper nonnarcotic treatment for it, just like we do with other forms of chronic pain,” he said.
The CDC recommends a number of alternatives to opioids – from physical therapy to biofeedback, over-the-counter anti-inflammatories, antidepressants, and antiseizure drugs that also relieve nerve pain.
The country also needs an overall strategy to cope with the wave of post-COVID complications, Dr. Al-Aly said.
“It’s better to be prepared than to be caught off guard years from now, when doctors realize: ‘Oh, we have a resurgence in opioids,’ ” Dr. Al-Aly said.
Dr. Al-Aly noted that his study may not capture the full complexity of post-COVID patient needs. Although women make up the majority of long-COVID patients in most studies, most patients in the VA system are men.
The study of VA patients makes it “abundantly clear that we are not prepared to meet the needs of 3 million Americans with long COVID,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego. “We desperately need an intervention that will effectively treat these individuals.”
Dr. Al-Aly said COVID survivors may need care for years.
“That’s going to be a huge, significant burden on the health care system,” Dr. Al-Aly said. “Long COVID will reverberate in the health system for years or even decades to come.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
CDC guidelines coming on long COVID
The Centers for Disease Control and Prevention is finalizing new guidelines to help clinicians diagnose and manage long COVID, or postacute sequelae of SARS-CoV-2 infection.
In a day-long congressional hearing on April 28, John Brooks, MD, a medical epidemiologist at the CDC’s division of HIV/AIDS prevention, testified that the guidelines were going through the clearance process at the agency, but would be forthcoming.
“They should be coming out very shortly,” Dr. Brooks said.
The guidelines, which were developed in collaboration with newly established long-COVID clinics and patient advocacy groups, will “illustrate how to diagnose and begin to pull together what we know about management,” of the complex condition, he said.
For many doctors and patients who are struggling to understand symptoms that persist for months after the initial viral infection, the guidelines can’t come soon enough.
National Institutes of Health Director Francis Collins, MD, PhD, who also testified at the hearing, estimated that as many as 3 million people could be left with chronic health problems after even mild COVID infections.
“I can’t overstate how serious this issue is for the health of our nation,” he said.
Dr. Collins said his estimate was based on studies showing that roughly 10% of people who get COVID could be affected by this and whose “long-term course is uncertain,” he said. So far, more than 32 million Americans are known to have been infected with the new coronavirus.
“We need to make sure we put our arms around them and bring answers and care to them,” said Rep. Anna Eshoo (D-Calif.), chairwoman of the Subcommittee on Health.
Jennifer Possick, MD, who directs the post-COVID recovery program at Yale New Haven (Conn.) Hospital, testified that the tidal wave of patients she and her colleagues were seeing was overwhelming.
“We are a well-resourced program at an academic medical center, but we are swamped by the need in our community. This year, we have seen more patients with post COVID-19 conditions in our clinic alone than we have new cases of asthma and COPD combined,” she said. “The magnitude of the challenge is daunting.”
Dr. Possick estimated that there are “over 60” clinics in the United States that have started to treat long-COVID patients, but said they are grassroots efforts and all very different from each other.
“Whoever had the resources, had the time, [and] was able to take the initiative and forge to the relationships because most of them are multidisciplinary, did so,” she said.
Patients testify
Several representatives shared moving personal stories of loved ones or staffers who remained ill months after a COVID diagnosis.
Rep. Ann Kuster, from New Hampshire, talked about her 34-year-old niece, a member of the U.S. Ski Team, who had COVID just over a year ago and “continues to struggle with everything, even the simplest activities of daily living” she said. “She has to choose between taking a shower or making dinner. I’m so proud of her for hanging in there.”
Long-COVID patients invited to testify by the subcommittee described months of disability that left them with soaring medical bills and no ability to work to pay them.
“I am now a poor, Black, disabled woman, living with long COVID,” said Chimere Smith, who said she had been a school teacher in Baltimore. “Saying it aloud makes it no more easy to accept.”
She said COVID had affected her ability to think clearly and caused debilitating fatigue, which prevented her from working. She said she lost her vision for almost 5 months because doctors misdiagnosed a cataract caused by long COVID as dry eye.
“If I did not have a loving family, I [would] be speaking to you today [from] my car, the only property I now own.”
Ms. Smith said that long-COVID clinics, which are mostly housed within academic medical centers, were not going to be accessible for all long-haulers, who are disproportionately women of color. She has started a clinic, based out of her church, to help other patients from her community.
“No one wants to hear that long COVID has decimated my life or the lives of other black women in less than a year,” Ms. Smith said. “We’ve just been waiting and hoping for compassionate doctors and politicians who would acknowledge us.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention is finalizing new guidelines to help clinicians diagnose and manage long COVID, or postacute sequelae of SARS-CoV-2 infection.
In a day-long congressional hearing on April 28, John Brooks, MD, a medical epidemiologist at the CDC’s division of HIV/AIDS prevention, testified that the guidelines were going through the clearance process at the agency, but would be forthcoming.
“They should be coming out very shortly,” Dr. Brooks said.
The guidelines, which were developed in collaboration with newly established long-COVID clinics and patient advocacy groups, will “illustrate how to diagnose and begin to pull together what we know about management,” of the complex condition, he said.
For many doctors and patients who are struggling to understand symptoms that persist for months after the initial viral infection, the guidelines can’t come soon enough.
National Institutes of Health Director Francis Collins, MD, PhD, who also testified at the hearing, estimated that as many as 3 million people could be left with chronic health problems after even mild COVID infections.
“I can’t overstate how serious this issue is for the health of our nation,” he said.
Dr. Collins said his estimate was based on studies showing that roughly 10% of people who get COVID could be affected by this and whose “long-term course is uncertain,” he said. So far, more than 32 million Americans are known to have been infected with the new coronavirus.
“We need to make sure we put our arms around them and bring answers and care to them,” said Rep. Anna Eshoo (D-Calif.), chairwoman of the Subcommittee on Health.
Jennifer Possick, MD, who directs the post-COVID recovery program at Yale New Haven (Conn.) Hospital, testified that the tidal wave of patients she and her colleagues were seeing was overwhelming.
“We are a well-resourced program at an academic medical center, but we are swamped by the need in our community. This year, we have seen more patients with post COVID-19 conditions in our clinic alone than we have new cases of asthma and COPD combined,” she said. “The magnitude of the challenge is daunting.”
Dr. Possick estimated that there are “over 60” clinics in the United States that have started to treat long-COVID patients, but said they are grassroots efforts and all very different from each other.
“Whoever had the resources, had the time, [and] was able to take the initiative and forge to the relationships because most of them are multidisciplinary, did so,” she said.
Patients testify
Several representatives shared moving personal stories of loved ones or staffers who remained ill months after a COVID diagnosis.
Rep. Ann Kuster, from New Hampshire, talked about her 34-year-old niece, a member of the U.S. Ski Team, who had COVID just over a year ago and “continues to struggle with everything, even the simplest activities of daily living” she said. “She has to choose between taking a shower or making dinner. I’m so proud of her for hanging in there.”
Long-COVID patients invited to testify by the subcommittee described months of disability that left them with soaring medical bills and no ability to work to pay them.
“I am now a poor, Black, disabled woman, living with long COVID,” said Chimere Smith, who said she had been a school teacher in Baltimore. “Saying it aloud makes it no more easy to accept.”
She said COVID had affected her ability to think clearly and caused debilitating fatigue, which prevented her from working. She said she lost her vision for almost 5 months because doctors misdiagnosed a cataract caused by long COVID as dry eye.
“If I did not have a loving family, I [would] be speaking to you today [from] my car, the only property I now own.”
Ms. Smith said that long-COVID clinics, which are mostly housed within academic medical centers, were not going to be accessible for all long-haulers, who are disproportionately women of color. She has started a clinic, based out of her church, to help other patients from her community.
“No one wants to hear that long COVID has decimated my life or the lives of other black women in less than a year,” Ms. Smith said. “We’ve just been waiting and hoping for compassionate doctors and politicians who would acknowledge us.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention is finalizing new guidelines to help clinicians diagnose and manage long COVID, or postacute sequelae of SARS-CoV-2 infection.
In a day-long congressional hearing on April 28, John Brooks, MD, a medical epidemiologist at the CDC’s division of HIV/AIDS prevention, testified that the guidelines were going through the clearance process at the agency, but would be forthcoming.
“They should be coming out very shortly,” Dr. Brooks said.
The guidelines, which were developed in collaboration with newly established long-COVID clinics and patient advocacy groups, will “illustrate how to diagnose and begin to pull together what we know about management,” of the complex condition, he said.
For many doctors and patients who are struggling to understand symptoms that persist for months after the initial viral infection, the guidelines can’t come soon enough.
National Institutes of Health Director Francis Collins, MD, PhD, who also testified at the hearing, estimated that as many as 3 million people could be left with chronic health problems after even mild COVID infections.
“I can’t overstate how serious this issue is for the health of our nation,” he said.
Dr. Collins said his estimate was based on studies showing that roughly 10% of people who get COVID could be affected by this and whose “long-term course is uncertain,” he said. So far, more than 32 million Americans are known to have been infected with the new coronavirus.
“We need to make sure we put our arms around them and bring answers and care to them,” said Rep. Anna Eshoo (D-Calif.), chairwoman of the Subcommittee on Health.
Jennifer Possick, MD, who directs the post-COVID recovery program at Yale New Haven (Conn.) Hospital, testified that the tidal wave of patients she and her colleagues were seeing was overwhelming.
“We are a well-resourced program at an academic medical center, but we are swamped by the need in our community. This year, we have seen more patients with post COVID-19 conditions in our clinic alone than we have new cases of asthma and COPD combined,” she said. “The magnitude of the challenge is daunting.”
Dr. Possick estimated that there are “over 60” clinics in the United States that have started to treat long-COVID patients, but said they are grassroots efforts and all very different from each other.
“Whoever had the resources, had the time, [and] was able to take the initiative and forge to the relationships because most of them are multidisciplinary, did so,” she said.
Patients testify
Several representatives shared moving personal stories of loved ones or staffers who remained ill months after a COVID diagnosis.
Rep. Ann Kuster, from New Hampshire, talked about her 34-year-old niece, a member of the U.S. Ski Team, who had COVID just over a year ago and “continues to struggle with everything, even the simplest activities of daily living” she said. “She has to choose between taking a shower or making dinner. I’m so proud of her for hanging in there.”
Long-COVID patients invited to testify by the subcommittee described months of disability that left them with soaring medical bills and no ability to work to pay them.
“I am now a poor, Black, disabled woman, living with long COVID,” said Chimere Smith, who said she had been a school teacher in Baltimore. “Saying it aloud makes it no more easy to accept.”
She said COVID had affected her ability to think clearly and caused debilitating fatigue, which prevented her from working. She said she lost her vision for almost 5 months because doctors misdiagnosed a cataract caused by long COVID as dry eye.
“If I did not have a loving family, I [would] be speaking to you today [from] my car, the only property I now own.”
Ms. Smith said that long-COVID clinics, which are mostly housed within academic medical centers, were not going to be accessible for all long-haulers, who are disproportionately women of color. She has started a clinic, based out of her church, to help other patients from her community.
“No one wants to hear that long COVID has decimated my life or the lives of other black women in less than a year,” Ms. Smith said. “We’ve just been waiting and hoping for compassionate doctors and politicians who would acknowledge us.”
A version of this article first appeared on Medscape.com.