User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Managing metabolic syndrome in patients with schizophrenia
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.
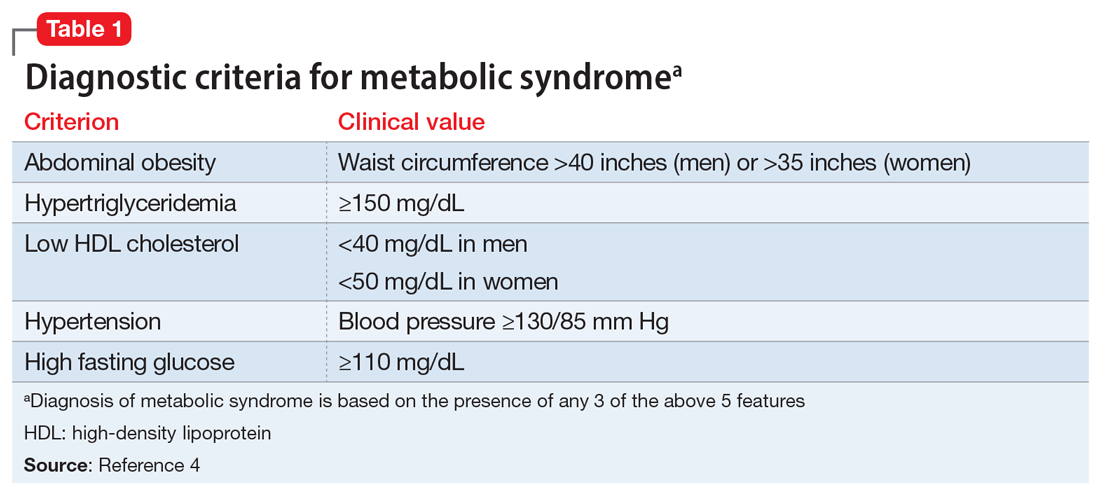
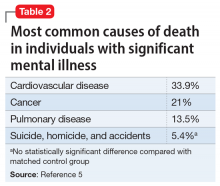
Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.

Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.

Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
What the Biden-Harris COVID-19 Advisory Board is missing
On Nov. 9, the Biden-Harris administration announced the members of its COVID-19 Advisory Board. Among them were many esteemed infectious disease and public health experts – encouraging, given that, for now, the COVID-19 pandemic shows no signs of slowing down. Not among them was a mental health professional.
As psychiatrists, we did not find this omission surprising, given the sidelined role our specialty too often plays among medical professionals. But we did find it disappointing. Not having a single behavioral health provider on the advisory board will prove to be a mistake that could affect millions of Americans.
Studies continue to roll in showing that patients with COVID-19 can present during and after infection with neuropsychiatric symptoms, including delirium, psychosis, and anxiety. In July, a meta-analysis published in The Lancet regarding the neuropsychological outcomes of earlier diseases caused by coronaviruses – severe acute respiratory syndrome and Middle East respiratory syndrome – suggested that, in the short term, close to one-quarter of patients experienced confusion representative of delirium. In the long term, following recovery, respondents frequently reported emotional lability, impaired concentration, and traumatic memories. Additionally, more recent research published in The Lancet suggests that rates of psychiatric disorders, dementia, and insomnia are significantly higher among survivors of COVID-19. This study echoes the findings of an article in JAMA from September that reported that, among patients who were hospitalized for COVID-19, mortality rates were higher for those who had previously been diagnosed with a psychiatric condition. And overall, the pandemic has been associated with significantly increased rates of anxiety and depression symptoms.
Although this research is preliminary,
This is especially true when you consider the following:
- It is very difficult to diagnose and treat mental health symptoms in a primary care setting that is already overburdened. Doing so results in delayed treatment and increased costs.
- In the long term, COVID-19 survivors will overburden the already underfunded mental healthcare system.
- Additional unforeseen psychological outcomes stem from the myriad traumas of events in 2020 (eg, racial unrest, children out of school, loss of jobs, the recent election).
Psychiatric disorders are notoriously difficult to diagnose and treat in the outpatient primary care setting, which is why mental health professionals will need to be a more integral part of the postpandemic treatment model and should be represented on the advisory board. Each year in the United States, there are more than 8 million doctors’ visits for depression, and more than half of these are in the primary care setting. Yet fewer than half of those patients leave with a diagnosis of depression or are treated for it.
Historically, screening for depression in the primary care setting is difficult given its broad presentation of symptoms, which include nonspecific physical complaints, such as digestive problems, headaches, insomnia, or general aches and pains. These shortcomings exist despite multiple changes in guidelines, such as regarding the use of self-screening tools and general screening for specific populations, such as postpartum women.
But screening alone has not been an effective strategy, especially when certain groups are less likely to be screened. These include older adults, Black persons, and men, all of whom are at higher risk for mortality after COVID-19. There is a failure to consistently apply standards of universal screening across all patient groups, and even if it occurs, there is a failure to establish reliable treatment and follow-up regimens. As clinicians, imagine how challenging diagnosis and treatment of more complicated psychiatric syndromes, such as somatoform disorder, will be in the primary care setting after the pandemic.
When almost two-thirds of symptoms in primary care are already “medically unexplained,” how do we expect primary care doctors to differentiate between those presenting with vague coronavirus-related “brain fog,” the run of the mill worrywart, and the 16%-34% with legitimate hypochondriasis of somatoform disorder who won’t improve without the involvement of a mental health provider?
A specialty in short supply
The mental health system we have now is inadequate for those who are currently diagnosed with mental disorders. Before the pandemic, emergency departments were boarding increasing numbers of patients with psychiatric illness because beds on inpatient units were unavailable. Individuals with insurance faced difficulty finding psychiatrists or psychotherapists who took insurance or who were availabile to accept new patients, given the growing shortage of providers in general. Community health centers continued to grapple with decreases in federal and state funding despite public political support for parity. Individuals with substance use faced few options for the outpatient, residential, or pharmacologic treatment that many needed to maintain sobriety.
Since the pandemic, we have seen rates of anxiety, depression, and suicidal thinking increase among adults and youth while many clinics have been forced to lay off employees, reduce services, or close their doors. As psychiatrists, we not only see the lack of treatment options for our patients but are forced to find creative solutions to meet their needs. How are we supposed to adapt (or feel confident) when individuals with or without previous mental illness face downstream consequences after COVID-19 when not one of our own is represented in the advisory board? How can we feel confident that downstream solutions acknowledge and address the intricacy of the behavioral health system that we, as mental health providers, know so intimately?
And what about the cumulative impact of everything else that has happened in 2020 in addition to the pandemic?! Although cataloging the various negative events that have happened this year is beyond the scope of this discussion, such lists have been compiled by the mainstream media and include the Australian brush fires, the crisis in Armenia, racial protests, economic uncertainties, and the run-up to and occurrence of the 2020 presidential election. Research is solid in its assertion that chronic stress can disturb our immune and cardiovascular systems, as well as mental health, leading to depression or anxiety. As a result of the pandemic itself, plus the events of this year, mental health providers are already warning not only of the current trauma underlying our day-to-day lives but also that of years to come.
More importantly, healthcare providers, both those represented by members of the advisory board and those who are not, are not immune to these issues. Before the pandemic, rates of suicide among doctors were already above average compared with other professions. After witnessing death repeatedly, self-isolation, the risk for infection to family, and dealing with the continued resistance to wearing masks, who knows what the eventual psychological toll our medical workforce will be?
Mental health providers have stepped up to the plate to provide care outside of traditional models to meet the needs that patients have now. One survey found that 81% of behavioral health providers began using telehealth for the first time in the past 6 months, owing to the COVID-19 pandemic. If not for the sake of the mental health of the Biden-Harris advisory board members themselves, who as doctors are likely to downplay the impact when struggling with mental health concerns in their own lives, a mental health provider deserves a seat at the table.
Plus, the outcomes speak for themselves when behavioral health providers collaborate with primary care providers to give treatment or when mental health experts are members of health crisis teams. Why wouldn’t the same be true for the Biden-Harris advisory board?
Kali Cyrus, MD, MPH, is an assistant professor of psychiatry and behavioral medicine at the Johns Hopkins School of Medicine, Baltimore, Maryland. She sees patients in private practice and offers consultation services in diversity strategy. Ranna Parekh, MD, MPH, is past deputy medical director and director of diversity and health equity for the American Psychiatric Association. She is currently a consultant psychiatrist at the Massachusetts General Hospital, Boston, and the chief diversity and inclusion officer at the American College of Cardiology.
A version of this article originally appeared on Medscape.com.
On Nov. 9, the Biden-Harris administration announced the members of its COVID-19 Advisory Board. Among them were many esteemed infectious disease and public health experts – encouraging, given that, for now, the COVID-19 pandemic shows no signs of slowing down. Not among them was a mental health professional.
As psychiatrists, we did not find this omission surprising, given the sidelined role our specialty too often plays among medical professionals. But we did find it disappointing. Not having a single behavioral health provider on the advisory board will prove to be a mistake that could affect millions of Americans.
Studies continue to roll in showing that patients with COVID-19 can present during and after infection with neuropsychiatric symptoms, including delirium, psychosis, and anxiety. In July, a meta-analysis published in The Lancet regarding the neuropsychological outcomes of earlier diseases caused by coronaviruses – severe acute respiratory syndrome and Middle East respiratory syndrome – suggested that, in the short term, close to one-quarter of patients experienced confusion representative of delirium. In the long term, following recovery, respondents frequently reported emotional lability, impaired concentration, and traumatic memories. Additionally, more recent research published in The Lancet suggests that rates of psychiatric disorders, dementia, and insomnia are significantly higher among survivors of COVID-19. This study echoes the findings of an article in JAMA from September that reported that, among patients who were hospitalized for COVID-19, mortality rates were higher for those who had previously been diagnosed with a psychiatric condition. And overall, the pandemic has been associated with significantly increased rates of anxiety and depression symptoms.
Although this research is preliminary,
This is especially true when you consider the following:
- It is very difficult to diagnose and treat mental health symptoms in a primary care setting that is already overburdened. Doing so results in delayed treatment and increased costs.
- In the long term, COVID-19 survivors will overburden the already underfunded mental healthcare system.
- Additional unforeseen psychological outcomes stem from the myriad traumas of events in 2020 (eg, racial unrest, children out of school, loss of jobs, the recent election).
Psychiatric disorders are notoriously difficult to diagnose and treat in the outpatient primary care setting, which is why mental health professionals will need to be a more integral part of the postpandemic treatment model and should be represented on the advisory board. Each year in the United States, there are more than 8 million doctors’ visits for depression, and more than half of these are in the primary care setting. Yet fewer than half of those patients leave with a diagnosis of depression or are treated for it.
Historically, screening for depression in the primary care setting is difficult given its broad presentation of symptoms, which include nonspecific physical complaints, such as digestive problems, headaches, insomnia, or general aches and pains. These shortcomings exist despite multiple changes in guidelines, such as regarding the use of self-screening tools and general screening for specific populations, such as postpartum women.
But screening alone has not been an effective strategy, especially when certain groups are less likely to be screened. These include older adults, Black persons, and men, all of whom are at higher risk for mortality after COVID-19. There is a failure to consistently apply standards of universal screening across all patient groups, and even if it occurs, there is a failure to establish reliable treatment and follow-up regimens. As clinicians, imagine how challenging diagnosis and treatment of more complicated psychiatric syndromes, such as somatoform disorder, will be in the primary care setting after the pandemic.
When almost two-thirds of symptoms in primary care are already “medically unexplained,” how do we expect primary care doctors to differentiate between those presenting with vague coronavirus-related “brain fog,” the run of the mill worrywart, and the 16%-34% with legitimate hypochondriasis of somatoform disorder who won’t improve without the involvement of a mental health provider?
A specialty in short supply
The mental health system we have now is inadequate for those who are currently diagnosed with mental disorders. Before the pandemic, emergency departments were boarding increasing numbers of patients with psychiatric illness because beds on inpatient units were unavailable. Individuals with insurance faced difficulty finding psychiatrists or psychotherapists who took insurance or who were availabile to accept new patients, given the growing shortage of providers in general. Community health centers continued to grapple with decreases in federal and state funding despite public political support for parity. Individuals with substance use faced few options for the outpatient, residential, or pharmacologic treatment that many needed to maintain sobriety.
Since the pandemic, we have seen rates of anxiety, depression, and suicidal thinking increase among adults and youth while many clinics have been forced to lay off employees, reduce services, or close their doors. As psychiatrists, we not only see the lack of treatment options for our patients but are forced to find creative solutions to meet their needs. How are we supposed to adapt (or feel confident) when individuals with or without previous mental illness face downstream consequences after COVID-19 when not one of our own is represented in the advisory board? How can we feel confident that downstream solutions acknowledge and address the intricacy of the behavioral health system that we, as mental health providers, know so intimately?
And what about the cumulative impact of everything else that has happened in 2020 in addition to the pandemic?! Although cataloging the various negative events that have happened this year is beyond the scope of this discussion, such lists have been compiled by the mainstream media and include the Australian brush fires, the crisis in Armenia, racial protests, economic uncertainties, and the run-up to and occurrence of the 2020 presidential election. Research is solid in its assertion that chronic stress can disturb our immune and cardiovascular systems, as well as mental health, leading to depression or anxiety. As a result of the pandemic itself, plus the events of this year, mental health providers are already warning not only of the current trauma underlying our day-to-day lives but also that of years to come.
More importantly, healthcare providers, both those represented by members of the advisory board and those who are not, are not immune to these issues. Before the pandemic, rates of suicide among doctors were already above average compared with other professions. After witnessing death repeatedly, self-isolation, the risk for infection to family, and dealing with the continued resistance to wearing masks, who knows what the eventual psychological toll our medical workforce will be?
Mental health providers have stepped up to the plate to provide care outside of traditional models to meet the needs that patients have now. One survey found that 81% of behavioral health providers began using telehealth for the first time in the past 6 months, owing to the COVID-19 pandemic. If not for the sake of the mental health of the Biden-Harris advisory board members themselves, who as doctors are likely to downplay the impact when struggling with mental health concerns in their own lives, a mental health provider deserves a seat at the table.
Plus, the outcomes speak for themselves when behavioral health providers collaborate with primary care providers to give treatment or when mental health experts are members of health crisis teams. Why wouldn’t the same be true for the Biden-Harris advisory board?
Kali Cyrus, MD, MPH, is an assistant professor of psychiatry and behavioral medicine at the Johns Hopkins School of Medicine, Baltimore, Maryland. She sees patients in private practice and offers consultation services in diversity strategy. Ranna Parekh, MD, MPH, is past deputy medical director and director of diversity and health equity for the American Psychiatric Association. She is currently a consultant psychiatrist at the Massachusetts General Hospital, Boston, and the chief diversity and inclusion officer at the American College of Cardiology.
A version of this article originally appeared on Medscape.com.
On Nov. 9, the Biden-Harris administration announced the members of its COVID-19 Advisory Board. Among them were many esteemed infectious disease and public health experts – encouraging, given that, for now, the COVID-19 pandemic shows no signs of slowing down. Not among them was a mental health professional.
As psychiatrists, we did not find this omission surprising, given the sidelined role our specialty too often plays among medical professionals. But we did find it disappointing. Not having a single behavioral health provider on the advisory board will prove to be a mistake that could affect millions of Americans.
Studies continue to roll in showing that patients with COVID-19 can present during and after infection with neuropsychiatric symptoms, including delirium, psychosis, and anxiety. In July, a meta-analysis published in The Lancet regarding the neuropsychological outcomes of earlier diseases caused by coronaviruses – severe acute respiratory syndrome and Middle East respiratory syndrome – suggested that, in the short term, close to one-quarter of patients experienced confusion representative of delirium. In the long term, following recovery, respondents frequently reported emotional lability, impaired concentration, and traumatic memories. Additionally, more recent research published in The Lancet suggests that rates of psychiatric disorders, dementia, and insomnia are significantly higher among survivors of COVID-19. This study echoes the findings of an article in JAMA from September that reported that, among patients who were hospitalized for COVID-19, mortality rates were higher for those who had previously been diagnosed with a psychiatric condition. And overall, the pandemic has been associated with significantly increased rates of anxiety and depression symptoms.
Although this research is preliminary,
This is especially true when you consider the following:
- It is very difficult to diagnose and treat mental health symptoms in a primary care setting that is already overburdened. Doing so results in delayed treatment and increased costs.
- In the long term, COVID-19 survivors will overburden the already underfunded mental healthcare system.
- Additional unforeseen psychological outcomes stem from the myriad traumas of events in 2020 (eg, racial unrest, children out of school, loss of jobs, the recent election).
Psychiatric disorders are notoriously difficult to diagnose and treat in the outpatient primary care setting, which is why mental health professionals will need to be a more integral part of the postpandemic treatment model and should be represented on the advisory board. Each year in the United States, there are more than 8 million doctors’ visits for depression, and more than half of these are in the primary care setting. Yet fewer than half of those patients leave with a diagnosis of depression or are treated for it.
Historically, screening for depression in the primary care setting is difficult given its broad presentation of symptoms, which include nonspecific physical complaints, such as digestive problems, headaches, insomnia, or general aches and pains. These shortcomings exist despite multiple changes in guidelines, such as regarding the use of self-screening tools and general screening for specific populations, such as postpartum women.
But screening alone has not been an effective strategy, especially when certain groups are less likely to be screened. These include older adults, Black persons, and men, all of whom are at higher risk for mortality after COVID-19. There is a failure to consistently apply standards of universal screening across all patient groups, and even if it occurs, there is a failure to establish reliable treatment and follow-up regimens. As clinicians, imagine how challenging diagnosis and treatment of more complicated psychiatric syndromes, such as somatoform disorder, will be in the primary care setting after the pandemic.
When almost two-thirds of symptoms in primary care are already “medically unexplained,” how do we expect primary care doctors to differentiate between those presenting with vague coronavirus-related “brain fog,” the run of the mill worrywart, and the 16%-34% with legitimate hypochondriasis of somatoform disorder who won’t improve without the involvement of a mental health provider?
A specialty in short supply
The mental health system we have now is inadequate for those who are currently diagnosed with mental disorders. Before the pandemic, emergency departments were boarding increasing numbers of patients with psychiatric illness because beds on inpatient units were unavailable. Individuals with insurance faced difficulty finding psychiatrists or psychotherapists who took insurance or who were availabile to accept new patients, given the growing shortage of providers in general. Community health centers continued to grapple with decreases in federal and state funding despite public political support for parity. Individuals with substance use faced few options for the outpatient, residential, or pharmacologic treatment that many needed to maintain sobriety.
Since the pandemic, we have seen rates of anxiety, depression, and suicidal thinking increase among adults and youth while many clinics have been forced to lay off employees, reduce services, or close their doors. As psychiatrists, we not only see the lack of treatment options for our patients but are forced to find creative solutions to meet their needs. How are we supposed to adapt (or feel confident) when individuals with or without previous mental illness face downstream consequences after COVID-19 when not one of our own is represented in the advisory board? How can we feel confident that downstream solutions acknowledge and address the intricacy of the behavioral health system that we, as mental health providers, know so intimately?
And what about the cumulative impact of everything else that has happened in 2020 in addition to the pandemic?! Although cataloging the various negative events that have happened this year is beyond the scope of this discussion, such lists have been compiled by the mainstream media and include the Australian brush fires, the crisis in Armenia, racial protests, economic uncertainties, and the run-up to and occurrence of the 2020 presidential election. Research is solid in its assertion that chronic stress can disturb our immune and cardiovascular systems, as well as mental health, leading to depression or anxiety. As a result of the pandemic itself, plus the events of this year, mental health providers are already warning not only of the current trauma underlying our day-to-day lives but also that of years to come.
More importantly, healthcare providers, both those represented by members of the advisory board and those who are not, are not immune to these issues. Before the pandemic, rates of suicide among doctors were already above average compared with other professions. After witnessing death repeatedly, self-isolation, the risk for infection to family, and dealing with the continued resistance to wearing masks, who knows what the eventual psychological toll our medical workforce will be?
Mental health providers have stepped up to the plate to provide care outside of traditional models to meet the needs that patients have now. One survey found that 81% of behavioral health providers began using telehealth for the first time in the past 6 months, owing to the COVID-19 pandemic. If not for the sake of the mental health of the Biden-Harris advisory board members themselves, who as doctors are likely to downplay the impact when struggling with mental health concerns in their own lives, a mental health provider deserves a seat at the table.
Plus, the outcomes speak for themselves when behavioral health providers collaborate with primary care providers to give treatment or when mental health experts are members of health crisis teams. Why wouldn’t the same be true for the Biden-Harris advisory board?
Kali Cyrus, MD, MPH, is an assistant professor of psychiatry and behavioral medicine at the Johns Hopkins School of Medicine, Baltimore, Maryland. She sees patients in private practice and offers consultation services in diversity strategy. Ranna Parekh, MD, MPH, is past deputy medical director and director of diversity and health equity for the American Psychiatric Association. She is currently a consultant psychiatrist at the Massachusetts General Hospital, Boston, and the chief diversity and inclusion officer at the American College of Cardiology.
A version of this article originally appeared on Medscape.com.
Moderna filing for FDA emergency COVID-19 vaccine approval, reports 94.1% efficacy
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
Approval of COVID-19 vaccines will change nature of clinical trials
While stressing the urgent need to vaccinate the whole U.S. population, infectious disease experts and medical ethicists are raising questions about the clinical trials needed to answer important questions about the new COVID-19 vaccines.
In a statement released on Nov. 20, Barbara Alexander, MD, president of the Infectious Diseases Society of America (IDSA) and a professor at Duke University, Durham, N.C., commented on Pfizer and BioNTech’s application to the Food and Drug Administration for an emergency use authorization (EUA) for its COVID-19 vaccine. Besides emphasizing the need for a transparent review of the companies’ trial data prior to the FDA’s granting an EUA, she said, “If emergency use authorization is granted, clinical trials and data collection must continue.”
In an interview, Dr. Alexander said she is convinced that both Pfizer and Moderna, which is also expected to seek an EUA soon, will continue their clinical trials to monitor the long-term safety and efficacy of their vaccines.
“The EUA guidance for COVID vaccine authorization is very clear that clinical trials will move forward,” she said. “Any EUA request would have to include a strategy to ensure that the long-term safety and efficacy of a vaccine could be monitored. I see no evidence that either Pfizer or Moderna is not prepared to follow those regulations.”
Eventually, she added, the drug makers will have to seek full FDA approval to replace an EUA, which as its name signifies, is designed for public health emergencies. “The EUA is a tool to help us get the vaccine into circulation and have it start working as quickly as possible in the current health crisis,” she said. “But once the crisis is over, if the sponsors want to continue to market their vaccines, they have to go forward and get full approval.”
Medical ethicists, however, point out there may be ethical and practical dilemmas involved in continuing or initiating clinical trials once a vaccine has been approved for use even on an emergency basis.
In a commentary in Annals of Internal Medicine, Rafael Dal-Re, MD, PhD, Arthur L. Caplan, PhD, and two other ethicists stipulated that the pandemic requires early licensing and deployment of COVID-19 vaccines. Nevertheless, they noted, additional months of data are required to establish the long-term efficacy and safety of the vaccines. “Moreover, early deployment could interfere with the acquisition of long-term data,” both on these vaccines and on others coming through the pipeline, they wrote.
In countries where an approved vaccine is deployed, the ethicists noted, investigators must inform participants in an ongoing trial about the approved vaccine’s status and ask if they want to continue in the study. If enough participants decline, the trial might have to be terminated early. At that point, researchers may not have sufficient long-term data to identify late-term safety issues, determine how long efficacy lasts, determine whether waning immunity is associated with reduced levels of antibodies, or identify the level of neutralizing antibodies that correlates with immunity.
Moreover, they observed, long-term trials are especially important for vaccines that use mRNA technology, because less is known about them than about traditional kinds of vaccines.
The authors also pointed out that early licensing of any vaccine might make it harder to evaluate vaccines that haven’t yet been approved. “Once a vaccine is licensed, new placebo-controlled RCTs [randomized controlled trials] of other vaccines will not be acceptable ethically, and noninferiority RCTs will be the most likely alternative.
“The goal of noninferiority trials will be to demonstrate that the immune response (that is, neutralizing antibody titers or levels) of the candidate vaccine is not inferior to that of the approved vaccine within a prespecified margin, which the FDA has established as less than 10% for COVID-19 vaccines,” the authors noted.
More data with more study designs
Dial Hewlett Jr., MD, medical director for disease control services, Westchester County Department of Health, White Plains, N.Y., said in an interview that the ethicists raise important issues that have been discussed in other forums, including a recent webinar of the National Academy of Medicine.
“As the authors point out, once you have a vaccine that has been shown to be effective and safe, it’s no longer ethical to enroll people in placebo trials,” he said.
Therefore, he said, Pfizer and Moderna will undoubtedly offer their vaccines to the people in their studies’ placebo groups after the vaccines receive an EUA. Then they will follow everyone who has been vaccinated for 2 years to determine long-term safety. Efficacy will also continue to be measured as an adjunct of safety, he said.
With regard to the difficulty of reconsenting individuals to enter a new clinical trial after a vaccine has been approved, he said, “I’d agree that trying to get all the same participants to come into another study would be a challenge. You can, however, design studies that will allow you to obtain the same information. You will have a large number of people out there who haven’t been vaccinated, and you can do single-arm longitudinal studies and measure a number of things in the individuals who are enrolled in those studies,” he said.
“You can look at the immunologic markers, both antibody and T-cell. You can follow these individuals longitudinally to see if they do develop disease over a period of time. If they do, you can determine what their levels of response were,” he added. “So there are opportunities to design studies that would give you some of the same information, although it would not be in the same population that was in the randomized trials.”
For newer vaccines that have yet to be tested, he said, developers can compare “historical controls” from the trials of approved vaccines, i.e., data from the unvaccinated participants in those studies, with the data from inoculating people with the novel agents. The historical data can be sex- and age-matched, among other things, to individuals in the new trials. Moreover, because the study protocols have been harmonized for all trials under Operation Warp Speed, it doesn’t matter what kind of vaccine they’re testing, he said.
It may be necessary to do additional studies to find out how long immunity lasts after people have been vaccinated, Dr. Hewlett pointed out.
“You may have a different trial design. You don’t need a control arm to determine how long immunity lasts. You’re just comparing the patients who were vaccinated to nothing,” he said. “So you could have a single-arm trial on a group of people who consent to be immunized and followed. You can see what their antibody levels are and other surrogate markers, and you can see when they might develop disease, if they do. You’d need a large sample, but you can do that.”
Dr. Hewlett noted that additional studies will be required to determine whether the new vaccines stop transmission of the coronavirus or just prevent symptoms of COVID-19. Until it’s established that a vaccine halts transmission or the country achieves herd immunity, he said, “we’ll still have to wear masks and take other precautions, because a significant portion of people will still be at risk.”
‘A lot of redundancy’
Dr. Alexander emphasized that any safety or efficacy issues with the first COVID-19 vaccines must be identified before the vaccine is offered to a large portion of the U.S. population.
“While the data from the Pfizer and Moderna trials are said to be favorable, we at IDSA want to make sure that whatever vaccine comes to market is safe,” she said. “Having an unsafe vaccine on the market would be worse than no vaccine, because you’re compromising the public confidence. We have to make sure the public trusts the process and that sufficient data have been evaluated to ensure the vaccine is safe and efficacious.
“I believe the FDA is being very careful and thoughtful in [its] response,” Dr. Alexander said. “They realize how important it is to get a vaccine and save lives. While they’re doing things differently and moving much faster than before, they’re still trying to be thoughtful and reasonable. They don’t seem to be putting people at risk or circumventing the regulatory standards.”
Moreover, she pointed out, the FDA’s Vaccines and Related Biological Products Advisory Committee, which is expected to meet on Dec. 10, will review the trial data before the agency grants an EUA to Pfizer or Moderna. Then the FDA will post the data publicly.
The next step is for the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention to look at the data and decide who in the United States should receive the vaccine first, she pointed out. And both Pfizer and Moderna have shown their data to advisory panels of outside experts.
“There’s a lot of redundancy, and a lot of people are looking at the data,” Dr. Alexander said. “So I don’t think we’re cutting corners to get it out there more quickly.”
A version of this article originally appeared on Medscape.com.
While stressing the urgent need to vaccinate the whole U.S. population, infectious disease experts and medical ethicists are raising questions about the clinical trials needed to answer important questions about the new COVID-19 vaccines.
In a statement released on Nov. 20, Barbara Alexander, MD, president of the Infectious Diseases Society of America (IDSA) and a professor at Duke University, Durham, N.C., commented on Pfizer and BioNTech’s application to the Food and Drug Administration for an emergency use authorization (EUA) for its COVID-19 vaccine. Besides emphasizing the need for a transparent review of the companies’ trial data prior to the FDA’s granting an EUA, she said, “If emergency use authorization is granted, clinical trials and data collection must continue.”
In an interview, Dr. Alexander said she is convinced that both Pfizer and Moderna, which is also expected to seek an EUA soon, will continue their clinical trials to monitor the long-term safety and efficacy of their vaccines.
“The EUA guidance for COVID vaccine authorization is very clear that clinical trials will move forward,” she said. “Any EUA request would have to include a strategy to ensure that the long-term safety and efficacy of a vaccine could be monitored. I see no evidence that either Pfizer or Moderna is not prepared to follow those regulations.”
Eventually, she added, the drug makers will have to seek full FDA approval to replace an EUA, which as its name signifies, is designed for public health emergencies. “The EUA is a tool to help us get the vaccine into circulation and have it start working as quickly as possible in the current health crisis,” she said. “But once the crisis is over, if the sponsors want to continue to market their vaccines, they have to go forward and get full approval.”
Medical ethicists, however, point out there may be ethical and practical dilemmas involved in continuing or initiating clinical trials once a vaccine has been approved for use even on an emergency basis.
In a commentary in Annals of Internal Medicine, Rafael Dal-Re, MD, PhD, Arthur L. Caplan, PhD, and two other ethicists stipulated that the pandemic requires early licensing and deployment of COVID-19 vaccines. Nevertheless, they noted, additional months of data are required to establish the long-term efficacy and safety of the vaccines. “Moreover, early deployment could interfere with the acquisition of long-term data,” both on these vaccines and on others coming through the pipeline, they wrote.
In countries where an approved vaccine is deployed, the ethicists noted, investigators must inform participants in an ongoing trial about the approved vaccine’s status and ask if they want to continue in the study. If enough participants decline, the trial might have to be terminated early. At that point, researchers may not have sufficient long-term data to identify late-term safety issues, determine how long efficacy lasts, determine whether waning immunity is associated with reduced levels of antibodies, or identify the level of neutralizing antibodies that correlates with immunity.
Moreover, they observed, long-term trials are especially important for vaccines that use mRNA technology, because less is known about them than about traditional kinds of vaccines.
The authors also pointed out that early licensing of any vaccine might make it harder to evaluate vaccines that haven’t yet been approved. “Once a vaccine is licensed, new placebo-controlled RCTs [randomized controlled trials] of other vaccines will not be acceptable ethically, and noninferiority RCTs will be the most likely alternative.
“The goal of noninferiority trials will be to demonstrate that the immune response (that is, neutralizing antibody titers or levels) of the candidate vaccine is not inferior to that of the approved vaccine within a prespecified margin, which the FDA has established as less than 10% for COVID-19 vaccines,” the authors noted.
More data with more study designs
Dial Hewlett Jr., MD, medical director for disease control services, Westchester County Department of Health, White Plains, N.Y., said in an interview that the ethicists raise important issues that have been discussed in other forums, including a recent webinar of the National Academy of Medicine.
“As the authors point out, once you have a vaccine that has been shown to be effective and safe, it’s no longer ethical to enroll people in placebo trials,” he said.
Therefore, he said, Pfizer and Moderna will undoubtedly offer their vaccines to the people in their studies’ placebo groups after the vaccines receive an EUA. Then they will follow everyone who has been vaccinated for 2 years to determine long-term safety. Efficacy will also continue to be measured as an adjunct of safety, he said.
With regard to the difficulty of reconsenting individuals to enter a new clinical trial after a vaccine has been approved, he said, “I’d agree that trying to get all the same participants to come into another study would be a challenge. You can, however, design studies that will allow you to obtain the same information. You will have a large number of people out there who haven’t been vaccinated, and you can do single-arm longitudinal studies and measure a number of things in the individuals who are enrolled in those studies,” he said.
“You can look at the immunologic markers, both antibody and T-cell. You can follow these individuals longitudinally to see if they do develop disease over a period of time. If they do, you can determine what their levels of response were,” he added. “So there are opportunities to design studies that would give you some of the same information, although it would not be in the same population that was in the randomized trials.”
For newer vaccines that have yet to be tested, he said, developers can compare “historical controls” from the trials of approved vaccines, i.e., data from the unvaccinated participants in those studies, with the data from inoculating people with the novel agents. The historical data can be sex- and age-matched, among other things, to individuals in the new trials. Moreover, because the study protocols have been harmonized for all trials under Operation Warp Speed, it doesn’t matter what kind of vaccine they’re testing, he said.
It may be necessary to do additional studies to find out how long immunity lasts after people have been vaccinated, Dr. Hewlett pointed out.
“You may have a different trial design. You don’t need a control arm to determine how long immunity lasts. You’re just comparing the patients who were vaccinated to nothing,” he said. “So you could have a single-arm trial on a group of people who consent to be immunized and followed. You can see what their antibody levels are and other surrogate markers, and you can see when they might develop disease, if they do. You’d need a large sample, but you can do that.”
Dr. Hewlett noted that additional studies will be required to determine whether the new vaccines stop transmission of the coronavirus or just prevent symptoms of COVID-19. Until it’s established that a vaccine halts transmission or the country achieves herd immunity, he said, “we’ll still have to wear masks and take other precautions, because a significant portion of people will still be at risk.”
‘A lot of redundancy’
Dr. Alexander emphasized that any safety or efficacy issues with the first COVID-19 vaccines must be identified before the vaccine is offered to a large portion of the U.S. population.
“While the data from the Pfizer and Moderna trials are said to be favorable, we at IDSA want to make sure that whatever vaccine comes to market is safe,” she said. “Having an unsafe vaccine on the market would be worse than no vaccine, because you’re compromising the public confidence. We have to make sure the public trusts the process and that sufficient data have been evaluated to ensure the vaccine is safe and efficacious.
“I believe the FDA is being very careful and thoughtful in [its] response,” Dr. Alexander said. “They realize how important it is to get a vaccine and save lives. While they’re doing things differently and moving much faster than before, they’re still trying to be thoughtful and reasonable. They don’t seem to be putting people at risk or circumventing the regulatory standards.”
Moreover, she pointed out, the FDA’s Vaccines and Related Biological Products Advisory Committee, which is expected to meet on Dec. 10, will review the trial data before the agency grants an EUA to Pfizer or Moderna. Then the FDA will post the data publicly.
The next step is for the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention to look at the data and decide who in the United States should receive the vaccine first, she pointed out. And both Pfizer and Moderna have shown their data to advisory panels of outside experts.
“There’s a lot of redundancy, and a lot of people are looking at the data,” Dr. Alexander said. “So I don’t think we’re cutting corners to get it out there more quickly.”
A version of this article originally appeared on Medscape.com.
While stressing the urgent need to vaccinate the whole U.S. population, infectious disease experts and medical ethicists are raising questions about the clinical trials needed to answer important questions about the new COVID-19 vaccines.
In a statement released on Nov. 20, Barbara Alexander, MD, president of the Infectious Diseases Society of America (IDSA) and a professor at Duke University, Durham, N.C., commented on Pfizer and BioNTech’s application to the Food and Drug Administration for an emergency use authorization (EUA) for its COVID-19 vaccine. Besides emphasizing the need for a transparent review of the companies’ trial data prior to the FDA’s granting an EUA, she said, “If emergency use authorization is granted, clinical trials and data collection must continue.”
In an interview, Dr. Alexander said she is convinced that both Pfizer and Moderna, which is also expected to seek an EUA soon, will continue their clinical trials to monitor the long-term safety and efficacy of their vaccines.
“The EUA guidance for COVID vaccine authorization is very clear that clinical trials will move forward,” she said. “Any EUA request would have to include a strategy to ensure that the long-term safety and efficacy of a vaccine could be monitored. I see no evidence that either Pfizer or Moderna is not prepared to follow those regulations.”
Eventually, she added, the drug makers will have to seek full FDA approval to replace an EUA, which as its name signifies, is designed for public health emergencies. “The EUA is a tool to help us get the vaccine into circulation and have it start working as quickly as possible in the current health crisis,” she said. “But once the crisis is over, if the sponsors want to continue to market their vaccines, they have to go forward and get full approval.”
Medical ethicists, however, point out there may be ethical and practical dilemmas involved in continuing or initiating clinical trials once a vaccine has been approved for use even on an emergency basis.
In a commentary in Annals of Internal Medicine, Rafael Dal-Re, MD, PhD, Arthur L. Caplan, PhD, and two other ethicists stipulated that the pandemic requires early licensing and deployment of COVID-19 vaccines. Nevertheless, they noted, additional months of data are required to establish the long-term efficacy and safety of the vaccines. “Moreover, early deployment could interfere with the acquisition of long-term data,” both on these vaccines and on others coming through the pipeline, they wrote.
In countries where an approved vaccine is deployed, the ethicists noted, investigators must inform participants in an ongoing trial about the approved vaccine’s status and ask if they want to continue in the study. If enough participants decline, the trial might have to be terminated early. At that point, researchers may not have sufficient long-term data to identify late-term safety issues, determine how long efficacy lasts, determine whether waning immunity is associated with reduced levels of antibodies, or identify the level of neutralizing antibodies that correlates with immunity.
Moreover, they observed, long-term trials are especially important for vaccines that use mRNA technology, because less is known about them than about traditional kinds of vaccines.
The authors also pointed out that early licensing of any vaccine might make it harder to evaluate vaccines that haven’t yet been approved. “Once a vaccine is licensed, new placebo-controlled RCTs [randomized controlled trials] of other vaccines will not be acceptable ethically, and noninferiority RCTs will be the most likely alternative.
“The goal of noninferiority trials will be to demonstrate that the immune response (that is, neutralizing antibody titers or levels) of the candidate vaccine is not inferior to that of the approved vaccine within a prespecified margin, which the FDA has established as less than 10% for COVID-19 vaccines,” the authors noted.
More data with more study designs
Dial Hewlett Jr., MD, medical director for disease control services, Westchester County Department of Health, White Plains, N.Y., said in an interview that the ethicists raise important issues that have been discussed in other forums, including a recent webinar of the National Academy of Medicine.
“As the authors point out, once you have a vaccine that has been shown to be effective and safe, it’s no longer ethical to enroll people in placebo trials,” he said.
Therefore, he said, Pfizer and Moderna will undoubtedly offer their vaccines to the people in their studies’ placebo groups after the vaccines receive an EUA. Then they will follow everyone who has been vaccinated for 2 years to determine long-term safety. Efficacy will also continue to be measured as an adjunct of safety, he said.
With regard to the difficulty of reconsenting individuals to enter a new clinical trial after a vaccine has been approved, he said, “I’d agree that trying to get all the same participants to come into another study would be a challenge. You can, however, design studies that will allow you to obtain the same information. You will have a large number of people out there who haven’t been vaccinated, and you can do single-arm longitudinal studies and measure a number of things in the individuals who are enrolled in those studies,” he said.
“You can look at the immunologic markers, both antibody and T-cell. You can follow these individuals longitudinally to see if they do develop disease over a period of time. If they do, you can determine what their levels of response were,” he added. “So there are opportunities to design studies that would give you some of the same information, although it would not be in the same population that was in the randomized trials.”
For newer vaccines that have yet to be tested, he said, developers can compare “historical controls” from the trials of approved vaccines, i.e., data from the unvaccinated participants in those studies, with the data from inoculating people with the novel agents. The historical data can be sex- and age-matched, among other things, to individuals in the new trials. Moreover, because the study protocols have been harmonized for all trials under Operation Warp Speed, it doesn’t matter what kind of vaccine they’re testing, he said.
It may be necessary to do additional studies to find out how long immunity lasts after people have been vaccinated, Dr. Hewlett pointed out.
“You may have a different trial design. You don’t need a control arm to determine how long immunity lasts. You’re just comparing the patients who were vaccinated to nothing,” he said. “So you could have a single-arm trial on a group of people who consent to be immunized and followed. You can see what their antibody levels are and other surrogate markers, and you can see when they might develop disease, if they do. You’d need a large sample, but you can do that.”
Dr. Hewlett noted that additional studies will be required to determine whether the new vaccines stop transmission of the coronavirus or just prevent symptoms of COVID-19. Until it’s established that a vaccine halts transmission or the country achieves herd immunity, he said, “we’ll still have to wear masks and take other precautions, because a significant portion of people will still be at risk.”
‘A lot of redundancy’
Dr. Alexander emphasized that any safety or efficacy issues with the first COVID-19 vaccines must be identified before the vaccine is offered to a large portion of the U.S. population.
“While the data from the Pfizer and Moderna trials are said to be favorable, we at IDSA want to make sure that whatever vaccine comes to market is safe,” she said. “Having an unsafe vaccine on the market would be worse than no vaccine, because you’re compromising the public confidence. We have to make sure the public trusts the process and that sufficient data have been evaluated to ensure the vaccine is safe and efficacious.
“I believe the FDA is being very careful and thoughtful in [its] response,” Dr. Alexander said. “They realize how important it is to get a vaccine and save lives. While they’re doing things differently and moving much faster than before, they’re still trying to be thoughtful and reasonable. They don’t seem to be putting people at risk or circumventing the regulatory standards.”
Moreover, she pointed out, the FDA’s Vaccines and Related Biological Products Advisory Committee, which is expected to meet on Dec. 10, will review the trial data before the agency grants an EUA to Pfizer or Moderna. Then the FDA will post the data publicly.
The next step is for the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention to look at the data and decide who in the United States should receive the vaccine first, she pointed out. And both Pfizer and Moderna have shown their data to advisory panels of outside experts.
“There’s a lot of redundancy, and a lot of people are looking at the data,” Dr. Alexander said. “So I don’t think we’re cutting corners to get it out there more quickly.”
A version of this article originally appeared on Medscape.com.
CDC panel delves into priorities for COVID vaccine distribution
On Monday, members of an influential federal panel delved into the challenges ahead in deciding who will get the first doses of COVID-19 vaccines, including questions about which healthcare workers need those initial vaccinations the most.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) did not take any votes or seek to establish formal positions. Instead, the meeting served as a forum for experts to discuss the thorny issues ahead. The US Food and Drug Administration (FDA) could make a decision next month regarding clearance for the first COVID-19 vaccine.
An FDA advisory committee will meet December 10 to review the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, in partnership with BioNTech. Moderna Inc said on November 16 that it expects to soon ask the FDA for an EUA of its rival COVID vaccine.
ACIP will face a two-part task after the FDA clears COVID-19 vaccines, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. ACIP will need to first decide whether to recommend use of the vaccine and then address the “complicated and difficult” question of which groups should get the initial limited quantities.
“There aren’t any perfect decisions,” she told the ACIP members. “I know this is something that most of you didn’t anticipate doing, making these kinds of huge decisions in the midst of a pandemic.”
There has been considerable public discussion of prioritization of COVID-19 vaccines, including a set of recommendations offered by a special committee created by the National Academies of Sciences, Engineering and Medicine. In addition, CDC staff and members of ACIP outlined what they termed the “four ethical principles” meant to guide these decisions in a November 23 report in the agency’s Morbidity and Mortality Weekly Report. These four principles are to maximize benefits and minimize harms; promote justice; mitigate health inequities; and promote transparency.
But as the issuing of the first EUA nears, it falls to ACIP to move beyond endorsing broad goals. The panel will need to make decisions as to which groups will have to wait for COVID-19 vaccines.
ACIP members on Monday delved into these kinds of more detailed questions, using a proposed three-stage model as a discussion point.
In phase 1a of this model, healthcare workers and residents of long-term care facilities would be the first people to be vaccinated. Phase 1b would include those deemed essential workers, including police officers, firefighters, and those in education, transportation, food, and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years and older.
ACIP member Grace M. Lee, MD, MPH, of Stanford University, Stanford, California, questioned whether healthcare workers who are not seeing patients in person should wait to get the vaccines. There has been a marked rise in the use of telehealth during the pandemic, which has spared some clinicians from in-person COVID-19 patient visits in their practices.
“Close partnership with our public health colleagues will be critically important to make sure that we are not trying to vaccinate 100% of our healthcare workforce, if some proportion of our workforce can work from home,” Lee said.
ACIP member Pablo Sánchez, MD, of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, concurred. Some clinicians, he noted, may have better access to personal protective equipment than others, he said.
“Unfortunately, not all healthcare workers are equal in terms of risk,” Sánchez said. “Within institutions, we’re going to have to prioritize which ones will get” the vaccine.
Clinicians may also make judgments about their own risk and need for early access to COVID-19 vaccinations, Sánchez said.
“I’m 66, and I’d rather give it to somebody much older and sicker than me,” he said.
Broader access
Fairly large populations will essentially be competing for limited doses of the first vaccines to reach the market.
The overlap is significant in the four priority groups put forward by CDC. The CDC staff estimated that about 21 million people would fall into the healthcare personnel category, which includes hospital staff, pharmacists, and those working in long-term care facilities. There are about 87 million people in the essential workers groups. More than 100 million adults in the United States, such as those with diabetes and cancers, fall into the high-risk medical conditions group. Another 53 million people are aged 65 and older.
Department of Health and Human Services Secretary Alex Azar on November 18 said the federal government expects to have about 40 million doses of these two vaccines by the end of December, which is enough to provide the two-dose regimen for about 20 million. If all goes as expected, Pfizer and Moderna will ramp up production.
Moderna has said that it expects by the end of this year to have approximately 20 million doses of its vaccine ready to ship in the United States and that it is on track to manufacture 500 million to 1 billion doses globally in 2021. Pfizer and BioNTech have said they expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion doses by the end of 2021.
At the Monday meeting, several ACIP panelists stressed the need to ensure that essential workers get early doses of vaccines.
In many cases, these workers serve in jobs with significant public interaction and live in poor communities. They put themselves and their families at risk. Many of them lack the resources to take precautions available to those better able to isolate, said ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, Washington.
“These essential workers are out there putting themselves at risk to allow the rest of us to socially distance,” she said. “Recognizing that not all of them may want to be vaccinated at this stage, we need to provide them with the opportunity early on in the process.”
In Bell’s view, the initial rollout of COVID-19 vaccines will send an important message about sharing this resource.
“If we’re serious about valuing equity, we need to have that baked in early on in the vaccination program,” she said.
Bell also said she was in favor of including people living in nursing homes in the initial wave of vaccinations. Concerns were raised about the frailty of this population.
“Given the mortality impact on the healthcare system from the number of nursing home residents that have been dying, I think on balance it makes sense to include them in phase 1a,” Bell said.
Other ACIP panelists said missteps with early vaccination of people in nursing homes could undermine faith in the treatments. Because of the ages and medical conditions of people in nursing homes, many of them may die after receiving the COVID-19 vaccine. Such deaths would not be associated with vaccine, but the medical community would not yet have evidence to disprove a connection.
There could be a backlash, with people falsely linking the death of a grandparent to the vaccine.
Fellow ACIP member Robert L. Atmar, MD, Baylor College of Medicine, Houston, Texas, was among those who had raised concerns about including people living in long-term care facilities in phase 1a. He said there are not yet enough data to judge the balance of benefits and harms of vaccination for this population.
The Pfizer and Moderna vaccines are “reactagenic,” meaning people may not feel well in the days after receiving the shots. The symptoms could lead to additional health evaluations of older people in nursing homes as clinicians try to figure out whether the patient’s reactions to the vaccine are caused by some condition or infection, Atmar said.
“Those of us who see these patients in the hospital recognize that there are often medical interventions that are done in the pursuit of a diagnosis, of a change in clinical status, that in and of themselves can lead to harm,” Atmar said.
Clinicians likely will have to encourage their patients of all ages to receive second doses of COVID-19 vaccines, despite the malaise they may provoke.
“We really need to make patients aware that this is not going to be a walk in the park. I mean, they’re going to know they had a vaccine, they’re probably not going to feel wonderful, but they’ve got to come back for that second dose,” said Sandra Adamson Fryhofer, MD, who represented the American Medical Association.
ACIP is expected to meet again to offer specific recommendations on the Pfizer and Moderna vaccines. ACIP’s recommendations trigger reimbursement processes, Azar said at a Tuesday press conference. ACIP’s work will inform decisions made by the federal government and governors about deploying shipments of COVID-19 vaccines, he said.
“At the end of the day, that is a decision, though, of the US government to make, which is where to recommend the prioritization,” Azar said. “It will be our nation’s governors in implementing the distribution plans to tell us” where to ship the vaccine.
This article first appeared on Medscape.com.
On Monday, members of an influential federal panel delved into the challenges ahead in deciding who will get the first doses of COVID-19 vaccines, including questions about which healthcare workers need those initial vaccinations the most.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) did not take any votes or seek to establish formal positions. Instead, the meeting served as a forum for experts to discuss the thorny issues ahead. The US Food and Drug Administration (FDA) could make a decision next month regarding clearance for the first COVID-19 vaccine.
An FDA advisory committee will meet December 10 to review the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, in partnership with BioNTech. Moderna Inc said on November 16 that it expects to soon ask the FDA for an EUA of its rival COVID vaccine.
ACIP will face a two-part task after the FDA clears COVID-19 vaccines, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. ACIP will need to first decide whether to recommend use of the vaccine and then address the “complicated and difficult” question of which groups should get the initial limited quantities.
“There aren’t any perfect decisions,” she told the ACIP members. “I know this is something that most of you didn’t anticipate doing, making these kinds of huge decisions in the midst of a pandemic.”
There has been considerable public discussion of prioritization of COVID-19 vaccines, including a set of recommendations offered by a special committee created by the National Academies of Sciences, Engineering and Medicine. In addition, CDC staff and members of ACIP outlined what they termed the “four ethical principles” meant to guide these decisions in a November 23 report in the agency’s Morbidity and Mortality Weekly Report. These four principles are to maximize benefits and minimize harms; promote justice; mitigate health inequities; and promote transparency.
But as the issuing of the first EUA nears, it falls to ACIP to move beyond endorsing broad goals. The panel will need to make decisions as to which groups will have to wait for COVID-19 vaccines.
ACIP members on Monday delved into these kinds of more detailed questions, using a proposed three-stage model as a discussion point.
In phase 1a of this model, healthcare workers and residents of long-term care facilities would be the first people to be vaccinated. Phase 1b would include those deemed essential workers, including police officers, firefighters, and those in education, transportation, food, and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years and older.
ACIP member Grace M. Lee, MD, MPH, of Stanford University, Stanford, California, questioned whether healthcare workers who are not seeing patients in person should wait to get the vaccines. There has been a marked rise in the use of telehealth during the pandemic, which has spared some clinicians from in-person COVID-19 patient visits in their practices.
“Close partnership with our public health colleagues will be critically important to make sure that we are not trying to vaccinate 100% of our healthcare workforce, if some proportion of our workforce can work from home,” Lee said.
ACIP member Pablo Sánchez, MD, of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, concurred. Some clinicians, he noted, may have better access to personal protective equipment than others, he said.
“Unfortunately, not all healthcare workers are equal in terms of risk,” Sánchez said. “Within institutions, we’re going to have to prioritize which ones will get” the vaccine.
Clinicians may also make judgments about their own risk and need for early access to COVID-19 vaccinations, Sánchez said.
“I’m 66, and I’d rather give it to somebody much older and sicker than me,” he said.
Broader access
Fairly large populations will essentially be competing for limited doses of the first vaccines to reach the market.
The overlap is significant in the four priority groups put forward by CDC. The CDC staff estimated that about 21 million people would fall into the healthcare personnel category, which includes hospital staff, pharmacists, and those working in long-term care facilities. There are about 87 million people in the essential workers groups. More than 100 million adults in the United States, such as those with diabetes and cancers, fall into the high-risk medical conditions group. Another 53 million people are aged 65 and older.
Department of Health and Human Services Secretary Alex Azar on November 18 said the federal government expects to have about 40 million doses of these two vaccines by the end of December, which is enough to provide the two-dose regimen for about 20 million. If all goes as expected, Pfizer and Moderna will ramp up production.
Moderna has said that it expects by the end of this year to have approximately 20 million doses of its vaccine ready to ship in the United States and that it is on track to manufacture 500 million to 1 billion doses globally in 2021. Pfizer and BioNTech have said they expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion doses by the end of 2021.
At the Monday meeting, several ACIP panelists stressed the need to ensure that essential workers get early doses of vaccines.
In many cases, these workers serve in jobs with significant public interaction and live in poor communities. They put themselves and their families at risk. Many of them lack the resources to take precautions available to those better able to isolate, said ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, Washington.
“These essential workers are out there putting themselves at risk to allow the rest of us to socially distance,” she said. “Recognizing that not all of them may want to be vaccinated at this stage, we need to provide them with the opportunity early on in the process.”
In Bell’s view, the initial rollout of COVID-19 vaccines will send an important message about sharing this resource.
“If we’re serious about valuing equity, we need to have that baked in early on in the vaccination program,” she said.
Bell also said she was in favor of including people living in nursing homes in the initial wave of vaccinations. Concerns were raised about the frailty of this population.
“Given the mortality impact on the healthcare system from the number of nursing home residents that have been dying, I think on balance it makes sense to include them in phase 1a,” Bell said.
Other ACIP panelists said missteps with early vaccination of people in nursing homes could undermine faith in the treatments. Because of the ages and medical conditions of people in nursing homes, many of them may die after receiving the COVID-19 vaccine. Such deaths would not be associated with vaccine, but the medical community would not yet have evidence to disprove a connection.
There could be a backlash, with people falsely linking the death of a grandparent to the vaccine.
Fellow ACIP member Robert L. Atmar, MD, Baylor College of Medicine, Houston, Texas, was among those who had raised concerns about including people living in long-term care facilities in phase 1a. He said there are not yet enough data to judge the balance of benefits and harms of vaccination for this population.
The Pfizer and Moderna vaccines are “reactagenic,” meaning people may not feel well in the days after receiving the shots. The symptoms could lead to additional health evaluations of older people in nursing homes as clinicians try to figure out whether the patient’s reactions to the vaccine are caused by some condition or infection, Atmar said.
“Those of us who see these patients in the hospital recognize that there are often medical interventions that are done in the pursuit of a diagnosis, of a change in clinical status, that in and of themselves can lead to harm,” Atmar said.
Clinicians likely will have to encourage their patients of all ages to receive second doses of COVID-19 vaccines, despite the malaise they may provoke.
“We really need to make patients aware that this is not going to be a walk in the park. I mean, they’re going to know they had a vaccine, they’re probably not going to feel wonderful, but they’ve got to come back for that second dose,” said Sandra Adamson Fryhofer, MD, who represented the American Medical Association.
ACIP is expected to meet again to offer specific recommendations on the Pfizer and Moderna vaccines. ACIP’s recommendations trigger reimbursement processes, Azar said at a Tuesday press conference. ACIP’s work will inform decisions made by the federal government and governors about deploying shipments of COVID-19 vaccines, he said.
“At the end of the day, that is a decision, though, of the US government to make, which is where to recommend the prioritization,” Azar said. “It will be our nation’s governors in implementing the distribution plans to tell us” where to ship the vaccine.
This article first appeared on Medscape.com.
On Monday, members of an influential federal panel delved into the challenges ahead in deciding who will get the first doses of COVID-19 vaccines, including questions about which healthcare workers need those initial vaccinations the most.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) did not take any votes or seek to establish formal positions. Instead, the meeting served as a forum for experts to discuss the thorny issues ahead. The US Food and Drug Administration (FDA) could make a decision next month regarding clearance for the first COVID-19 vaccine.
An FDA advisory committee will meet December 10 to review the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, in partnership with BioNTech. Moderna Inc said on November 16 that it expects to soon ask the FDA for an EUA of its rival COVID vaccine.
ACIP will face a two-part task after the FDA clears COVID-19 vaccines, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. ACIP will need to first decide whether to recommend use of the vaccine and then address the “complicated and difficult” question of which groups should get the initial limited quantities.
“There aren’t any perfect decisions,” she told the ACIP members. “I know this is something that most of you didn’t anticipate doing, making these kinds of huge decisions in the midst of a pandemic.”
There has been considerable public discussion of prioritization of COVID-19 vaccines, including a set of recommendations offered by a special committee created by the National Academies of Sciences, Engineering and Medicine. In addition, CDC staff and members of ACIP outlined what they termed the “four ethical principles” meant to guide these decisions in a November 23 report in the agency’s Morbidity and Mortality Weekly Report. These four principles are to maximize benefits and minimize harms; promote justice; mitigate health inequities; and promote transparency.
But as the issuing of the first EUA nears, it falls to ACIP to move beyond endorsing broad goals. The panel will need to make decisions as to which groups will have to wait for COVID-19 vaccines.
ACIP members on Monday delved into these kinds of more detailed questions, using a proposed three-stage model as a discussion point.
In phase 1a of this model, healthcare workers and residents of long-term care facilities would be the first people to be vaccinated. Phase 1b would include those deemed essential workers, including police officers, firefighters, and those in education, transportation, food, and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years and older.
ACIP member Grace M. Lee, MD, MPH, of Stanford University, Stanford, California, questioned whether healthcare workers who are not seeing patients in person should wait to get the vaccines. There has been a marked rise in the use of telehealth during the pandemic, which has spared some clinicians from in-person COVID-19 patient visits in their practices.
“Close partnership with our public health colleagues will be critically important to make sure that we are not trying to vaccinate 100% of our healthcare workforce, if some proportion of our workforce can work from home,” Lee said.
ACIP member Pablo Sánchez, MD, of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, concurred. Some clinicians, he noted, may have better access to personal protective equipment than others, he said.
“Unfortunately, not all healthcare workers are equal in terms of risk,” Sánchez said. “Within institutions, we’re going to have to prioritize which ones will get” the vaccine.
Clinicians may also make judgments about their own risk and need for early access to COVID-19 vaccinations, Sánchez said.
“I’m 66, and I’d rather give it to somebody much older and sicker than me,” he said.
Broader access
Fairly large populations will essentially be competing for limited doses of the first vaccines to reach the market.
The overlap is significant in the four priority groups put forward by CDC. The CDC staff estimated that about 21 million people would fall into the healthcare personnel category, which includes hospital staff, pharmacists, and those working in long-term care facilities. There are about 87 million people in the essential workers groups. More than 100 million adults in the United States, such as those with diabetes and cancers, fall into the high-risk medical conditions group. Another 53 million people are aged 65 and older.
Department of Health and Human Services Secretary Alex Azar on November 18 said the federal government expects to have about 40 million doses of these two vaccines by the end of December, which is enough to provide the two-dose regimen for about 20 million. If all goes as expected, Pfizer and Moderna will ramp up production.
Moderna has said that it expects by the end of this year to have approximately 20 million doses of its vaccine ready to ship in the United States and that it is on track to manufacture 500 million to 1 billion doses globally in 2021. Pfizer and BioNTech have said they expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion doses by the end of 2021.
At the Monday meeting, several ACIP panelists stressed the need to ensure that essential workers get early doses of vaccines.
In many cases, these workers serve in jobs with significant public interaction and live in poor communities. They put themselves and their families at risk. Many of them lack the resources to take precautions available to those better able to isolate, said ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, Washington.
“These essential workers are out there putting themselves at risk to allow the rest of us to socially distance,” she said. “Recognizing that not all of them may want to be vaccinated at this stage, we need to provide them with the opportunity early on in the process.”
In Bell’s view, the initial rollout of COVID-19 vaccines will send an important message about sharing this resource.
“If we’re serious about valuing equity, we need to have that baked in early on in the vaccination program,” she said.
Bell also said she was in favor of including people living in nursing homes in the initial wave of vaccinations. Concerns were raised about the frailty of this population.
“Given the mortality impact on the healthcare system from the number of nursing home residents that have been dying, I think on balance it makes sense to include them in phase 1a,” Bell said.
Other ACIP panelists said missteps with early vaccination of people in nursing homes could undermine faith in the treatments. Because of the ages and medical conditions of people in nursing homes, many of them may die after receiving the COVID-19 vaccine. Such deaths would not be associated with vaccine, but the medical community would not yet have evidence to disprove a connection.
There could be a backlash, with people falsely linking the death of a grandparent to the vaccine.
Fellow ACIP member Robert L. Atmar, MD, Baylor College of Medicine, Houston, Texas, was among those who had raised concerns about including people living in long-term care facilities in phase 1a. He said there are not yet enough data to judge the balance of benefits and harms of vaccination for this population.
The Pfizer and Moderna vaccines are “reactagenic,” meaning people may not feel well in the days after receiving the shots. The symptoms could lead to additional health evaluations of older people in nursing homes as clinicians try to figure out whether the patient’s reactions to the vaccine are caused by some condition or infection, Atmar said.
“Those of us who see these patients in the hospital recognize that there are often medical interventions that are done in the pursuit of a diagnosis, of a change in clinical status, that in and of themselves can lead to harm,” Atmar said.
Clinicians likely will have to encourage their patients of all ages to receive second doses of COVID-19 vaccines, despite the malaise they may provoke.
“We really need to make patients aware that this is not going to be a walk in the park. I mean, they’re going to know they had a vaccine, they’re probably not going to feel wonderful, but they’ve got to come back for that second dose,” said Sandra Adamson Fryhofer, MD, who represented the American Medical Association.
ACIP is expected to meet again to offer specific recommendations on the Pfizer and Moderna vaccines. ACIP’s recommendations trigger reimbursement processes, Azar said at a Tuesday press conference. ACIP’s work will inform decisions made by the federal government and governors about deploying shipments of COVID-19 vaccines, he said.
“At the end of the day, that is a decision, though, of the US government to make, which is where to recommend the prioritization,” Azar said. “It will be our nation’s governors in implementing the distribution plans to tell us” where to ship the vaccine.
This article first appeared on Medscape.com.
50.6 million tobacco users are not a homogeneous group
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.

with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.

with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.

with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
FROM MMWR
Immunodeficiency strongly linked to mental illness, suicidal behavior
Patients with a primary humoral immunodeficiency (PID) are 91% more likely to have a psychiatric disorder and 84% more likely to exhibit suicidal behavior, compared against those without the condition, new research shows.
Results showed that this association, which was stronger in women, could not be fully explained by comorbid autoimmune diseases or by familial confounding.
These findings have important clinical implications, study investigator Josef Isung, MD, PhD, Centre for Psychiatry Research, Karolinska Institute, Stockholm, Sweden, told Medscape Medical News.
Clinicians managing patients with PID “should be aware of this increased association with psychiatric disorders and perhaps screen for them,” said Isung.
The study was published in the November issue of JAMA Psychiatry.
Registry study
Mounting evidence suggests immune disruption plays a role in psychiatric disorders through a range of mechanisms, including altered neurodevelopment. However, little is known about the neuropsychiatric consequences resulting from the underproduction of homeostatic antibodies.
They’re associated with an increased risk for recurrent infections and of developing autoimmune diseases.
The immunodeficiency can be severe, even life threatening, but can also be relatively mild. One of the less severe PID types is selective IgA deficiency, which is linked to increased infections within the mucosa-associated lymphoid tissue (MALT), an important immune barrier.
Experts have long suspected that infections within the MALT are associated with certain forms of psychopathology in children, particularly obsessive-compulsive disorder and chronic tic disorders.
While patients with this selective IgA subtype may be at some increased risk for infection and autoimmune disease, their overall health otherwise is good, said Isung.
The prevalence of PIDs ranges from about 1:250 to 1:20,000, depending on the type of humoral immunodeficiency, although most would fall into the relatively rare category, he added.
Using several linked national Swedish registries, researchers identified individuals with any PID diagnosis affecting immunoglobulin levels, their full siblings, and those with a lifetime diagnosis of selective IgA deficiency. In addition, they collected data on autoimmune diseases.
The study outcome was a lifetime record of a psychiatric disorder, a suicide attempt, or death by suicide.
Strong link to autism
Researchers identified 8378 patients (59% women) with PID affecting immunoglobulin levels (median age at first diagnosis, 47.8 years). They compared this group with almost 14.3 million subjects without PID.
In those with PID, 27.6% had an autoimmune disease vs 6.8% of those without PID, a statistically significant difference (P < .001).
About 20.5% of those with PID and 10.7% of unexposed subjects had at least one diagnosis of a psychiatric disorder.
In a model adjusted for year of birth, sex, and history of autoimmune disease, subjects with PID had a 91% higher likelihood of any psychiatric disorder (adjusted odds ratio [AOR] 1.91; 95% CI, 1.81 - 2.01; P < .001) vs their counterparts without PID.
The AORs for individual psychiatric disorders ranged from 1.34 (95% CI, 1.17 - 1.54; P < .001) for schizophrenia and other psychotic disorders to 2.99 (95% CI, 2.42 - 3.70; P < .001) for autism spectrum disorders (ASDs)
It’s unclear why the association with PID was strongest for autism, “but being a neurodevelopmental disorder, maybe autism is logically more associated with this type of disruption,” said Isung.
Research suggests that immunologic disruption may play a role in ASD, either through altered maternal immune function in utero or through immune disruption after birth, the researchers note.
Compared to those without PID, individuals with it had a significantly increased likelihood of any suicidal behavior (AOR, 1.84; 95% CI, 1.66 - 2.04, P < .001) as well as individual outcomes of death by suicide and suicide attempts.
The association with psychiatric disorders and suicidal behavior was markedly stronger for exposure to both PID and autoimmune disease than for exposure to either of these alone, which suggest an additive effect for these immune-related conditions.
Sex differences
“It was unclear to us why women seemed particularly vulnerable,” said Isung. He noted that PIDs are generally about as common in women as in men, but women tend to have higher rates of psychiatric disorders.
The analysis of the sibling cohort also revealed an elevated risk for psychiatric disorders, including ASD and suicidal behavior, but to a lesser degree.
“From this we could infer that at least part of the associations would be genetic, but part would be related to the disruption in itself,” said Isung.
An analysis examining selective IgA subtype also revealed a link with psychiatric disorders and suicidal behavior, suggesting this link is not exclusive to severe PID cases.
“Our conclusion here was that it seems like PID itself, or the immune disruption in itself, could explain the association rather than the burden of illness,” said Isung.
However, he acknowledged that the long-term stress and mental health fallout of having a chronic illness like PID may also explain some of the increased risk for psychiatric disorders.
This study, he said, provides more evidence that immune disruptions affect neurodevelopment and the brain. However, he added, the underlying mechanism still isn’t fully understood.
The results highlight the need to raise awareness of the association between immunodeficiency and mental illness, including suicidality among clinicians, patients, and advocates.
These findings may also have implications in patients with other immune deficiencies, said Isung, noting, “it would be interesting to further explore associations with other immunocompromised populations.”
No surprises
Commenting on the findings for Medscape Medical News, Igor Galynker, MD, professor of psychiatry at Icahn School of Medicine at Mount Sinai, New York City, said the study was “very well-done” and used “reliable and well-controlled” databases.
However, he added, the results “are neither particularly dramatic nor conclusive” as it makes sense that medical illnesses like PID would “increase risk of psychopathology,” said Galynker.
PID patients are much more likely to have contact with clinicians and to receive a psychiatric diagnosis, he said.
“People with a chronic illness are more stressed and generally have high incidences of depression, anxiety, and suicidal behavior. In addition to that, they may be more likely to be diagnosed with those conditions because they see a clinician more frequently.”
However, that reasoning doesn’t apply to autism, which manifests in early childhood and so is unlikely to be the result of stress, said Galynker, which is why he believes the finding that ASD is the psychiatric outcome most strongly associated with PID is “the most convincing.”
Galynker wasn’t surprised that the association between PID and psychiatric illnesses, and suicidal behaviors, was stronger among women.
“Women attempt suicide four times more often than men to begin with, so you would expect this to be more pronounced” in those with PID.
The study was supported by grants from the Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institute; Stockholm Care Services; the Soderstrom Konig Foundation; and the Fredrik & Ingrid Thurings Foundation. Isung and Galynker have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with a primary humoral immunodeficiency (PID) are 91% more likely to have a psychiatric disorder and 84% more likely to exhibit suicidal behavior, compared against those without the condition, new research shows.
Results showed that this association, which was stronger in women, could not be fully explained by comorbid autoimmune diseases or by familial confounding.
These findings have important clinical implications, study investigator Josef Isung, MD, PhD, Centre for Psychiatry Research, Karolinska Institute, Stockholm, Sweden, told Medscape Medical News.
Clinicians managing patients with PID “should be aware of this increased association with psychiatric disorders and perhaps screen for them,” said Isung.
The study was published in the November issue of JAMA Psychiatry.
Registry study
Mounting evidence suggests immune disruption plays a role in psychiatric disorders through a range of mechanisms, including altered neurodevelopment. However, little is known about the neuropsychiatric consequences resulting from the underproduction of homeostatic antibodies.
They’re associated with an increased risk for recurrent infections and of developing autoimmune diseases.
The immunodeficiency can be severe, even life threatening, but can also be relatively mild. One of the less severe PID types is selective IgA deficiency, which is linked to increased infections within the mucosa-associated lymphoid tissue (MALT), an important immune barrier.
Experts have long suspected that infections within the MALT are associated with certain forms of psychopathology in children, particularly obsessive-compulsive disorder and chronic tic disorders.
While patients with this selective IgA subtype may be at some increased risk for infection and autoimmune disease, their overall health otherwise is good, said Isung.
The prevalence of PIDs ranges from about 1:250 to 1:20,000, depending on the type of humoral immunodeficiency, although most would fall into the relatively rare category, he added.
Using several linked national Swedish registries, researchers identified individuals with any PID diagnosis affecting immunoglobulin levels, their full siblings, and those with a lifetime diagnosis of selective IgA deficiency. In addition, they collected data on autoimmune diseases.
The study outcome was a lifetime record of a psychiatric disorder, a suicide attempt, or death by suicide.
Strong link to autism
Researchers identified 8378 patients (59% women) with PID affecting immunoglobulin levels (median age at first diagnosis, 47.8 years). They compared this group with almost 14.3 million subjects without PID.
In those with PID, 27.6% had an autoimmune disease vs 6.8% of those without PID, a statistically significant difference (P < .001).
About 20.5% of those with PID and 10.7% of unexposed subjects had at least one diagnosis of a psychiatric disorder.
In a model adjusted for year of birth, sex, and history of autoimmune disease, subjects with PID had a 91% higher likelihood of any psychiatric disorder (adjusted odds ratio [AOR] 1.91; 95% CI, 1.81 - 2.01; P < .001) vs their counterparts without PID.
The AORs for individual psychiatric disorders ranged from 1.34 (95% CI, 1.17 - 1.54; P < .001) for schizophrenia and other psychotic disorders to 2.99 (95% CI, 2.42 - 3.70; P < .001) for autism spectrum disorders (ASDs)
It’s unclear why the association with PID was strongest for autism, “but being a neurodevelopmental disorder, maybe autism is logically more associated with this type of disruption,” said Isung.
Research suggests that immunologic disruption may play a role in ASD, either through altered maternal immune function in utero or through immune disruption after birth, the researchers note.
Compared to those without PID, individuals with it had a significantly increased likelihood of any suicidal behavior (AOR, 1.84; 95% CI, 1.66 - 2.04, P < .001) as well as individual outcomes of death by suicide and suicide attempts.
The association with psychiatric disorders and suicidal behavior was markedly stronger for exposure to both PID and autoimmune disease than for exposure to either of these alone, which suggest an additive effect for these immune-related conditions.
Sex differences
“It was unclear to us why women seemed particularly vulnerable,” said Isung. He noted that PIDs are generally about as common in women as in men, but women tend to have higher rates of psychiatric disorders.
The analysis of the sibling cohort also revealed an elevated risk for psychiatric disorders, including ASD and suicidal behavior, but to a lesser degree.
“From this we could infer that at least part of the associations would be genetic, but part would be related to the disruption in itself,” said Isung.
An analysis examining selective IgA subtype also revealed a link with psychiatric disorders and suicidal behavior, suggesting this link is not exclusive to severe PID cases.
“Our conclusion here was that it seems like PID itself, or the immune disruption in itself, could explain the association rather than the burden of illness,” said Isung.
However, he acknowledged that the long-term stress and mental health fallout of having a chronic illness like PID may also explain some of the increased risk for psychiatric disorders.
This study, he said, provides more evidence that immune disruptions affect neurodevelopment and the brain. However, he added, the underlying mechanism still isn’t fully understood.
The results highlight the need to raise awareness of the association between immunodeficiency and mental illness, including suicidality among clinicians, patients, and advocates.
These findings may also have implications in patients with other immune deficiencies, said Isung, noting, “it would be interesting to further explore associations with other immunocompromised populations.”
No surprises
Commenting on the findings for Medscape Medical News, Igor Galynker, MD, professor of psychiatry at Icahn School of Medicine at Mount Sinai, New York City, said the study was “very well-done” and used “reliable and well-controlled” databases.
However, he added, the results “are neither particularly dramatic nor conclusive” as it makes sense that medical illnesses like PID would “increase risk of psychopathology,” said Galynker.
PID patients are much more likely to have contact with clinicians and to receive a psychiatric diagnosis, he said.
“People with a chronic illness are more stressed and generally have high incidences of depression, anxiety, and suicidal behavior. In addition to that, they may be more likely to be diagnosed with those conditions because they see a clinician more frequently.”
However, that reasoning doesn’t apply to autism, which manifests in early childhood and so is unlikely to be the result of stress, said Galynker, which is why he believes the finding that ASD is the psychiatric outcome most strongly associated with PID is “the most convincing.”
Galynker wasn’t surprised that the association between PID and psychiatric illnesses, and suicidal behaviors, was stronger among women.
“Women attempt suicide four times more often than men to begin with, so you would expect this to be more pronounced” in those with PID.
The study was supported by grants from the Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institute; Stockholm Care Services; the Soderstrom Konig Foundation; and the Fredrik & Ingrid Thurings Foundation. Isung and Galynker have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with a primary humoral immunodeficiency (PID) are 91% more likely to have a psychiatric disorder and 84% more likely to exhibit suicidal behavior, compared against those without the condition, new research shows.
Results showed that this association, which was stronger in women, could not be fully explained by comorbid autoimmune diseases or by familial confounding.
These findings have important clinical implications, study investigator Josef Isung, MD, PhD, Centre for Psychiatry Research, Karolinska Institute, Stockholm, Sweden, told Medscape Medical News.
Clinicians managing patients with PID “should be aware of this increased association with psychiatric disorders and perhaps screen for them,” said Isung.
The study was published in the November issue of JAMA Psychiatry.
Registry study
Mounting evidence suggests immune disruption plays a role in psychiatric disorders through a range of mechanisms, including altered neurodevelopment. However, little is known about the neuropsychiatric consequences resulting from the underproduction of homeostatic antibodies.
They’re associated with an increased risk for recurrent infections and of developing autoimmune diseases.
The immunodeficiency can be severe, even life threatening, but can also be relatively mild. One of the less severe PID types is selective IgA deficiency, which is linked to increased infections within the mucosa-associated lymphoid tissue (MALT), an important immune barrier.
Experts have long suspected that infections within the MALT are associated with certain forms of psychopathology in children, particularly obsessive-compulsive disorder and chronic tic disorders.
While patients with this selective IgA subtype may be at some increased risk for infection and autoimmune disease, their overall health otherwise is good, said Isung.
The prevalence of PIDs ranges from about 1:250 to 1:20,000, depending on the type of humoral immunodeficiency, although most would fall into the relatively rare category, he added.
Using several linked national Swedish registries, researchers identified individuals with any PID diagnosis affecting immunoglobulin levels, their full siblings, and those with a lifetime diagnosis of selective IgA deficiency. In addition, they collected data on autoimmune diseases.
The study outcome was a lifetime record of a psychiatric disorder, a suicide attempt, or death by suicide.
Strong link to autism
Researchers identified 8378 patients (59% women) with PID affecting immunoglobulin levels (median age at first diagnosis, 47.8 years). They compared this group with almost 14.3 million subjects without PID.
In those with PID, 27.6% had an autoimmune disease vs 6.8% of those without PID, a statistically significant difference (P < .001).
About 20.5% of those with PID and 10.7% of unexposed subjects had at least one diagnosis of a psychiatric disorder.
In a model adjusted for year of birth, sex, and history of autoimmune disease, subjects with PID had a 91% higher likelihood of any psychiatric disorder (adjusted odds ratio [AOR] 1.91; 95% CI, 1.81 - 2.01; P < .001) vs their counterparts without PID.
The AORs for individual psychiatric disorders ranged from 1.34 (95% CI, 1.17 - 1.54; P < .001) for schizophrenia and other psychotic disorders to 2.99 (95% CI, 2.42 - 3.70; P < .001) for autism spectrum disorders (ASDs)
It’s unclear why the association with PID was strongest for autism, “but being a neurodevelopmental disorder, maybe autism is logically more associated with this type of disruption,” said Isung.
Research suggests that immunologic disruption may play a role in ASD, either through altered maternal immune function in utero or through immune disruption after birth, the researchers note.
Compared to those without PID, individuals with it had a significantly increased likelihood of any suicidal behavior (AOR, 1.84; 95% CI, 1.66 - 2.04, P < .001) as well as individual outcomes of death by suicide and suicide attempts.
The association with psychiatric disorders and suicidal behavior was markedly stronger for exposure to both PID and autoimmune disease than for exposure to either of these alone, which suggest an additive effect for these immune-related conditions.
Sex differences
“It was unclear to us why women seemed particularly vulnerable,” said Isung. He noted that PIDs are generally about as common in women as in men, but women tend to have higher rates of psychiatric disorders.
The analysis of the sibling cohort also revealed an elevated risk for psychiatric disorders, including ASD and suicidal behavior, but to a lesser degree.
“From this we could infer that at least part of the associations would be genetic, but part would be related to the disruption in itself,” said Isung.
An analysis examining selective IgA subtype also revealed a link with psychiatric disorders and suicidal behavior, suggesting this link is not exclusive to severe PID cases.
“Our conclusion here was that it seems like PID itself, or the immune disruption in itself, could explain the association rather than the burden of illness,” said Isung.
However, he acknowledged that the long-term stress and mental health fallout of having a chronic illness like PID may also explain some of the increased risk for psychiatric disorders.
This study, he said, provides more evidence that immune disruptions affect neurodevelopment and the brain. However, he added, the underlying mechanism still isn’t fully understood.
The results highlight the need to raise awareness of the association between immunodeficiency and mental illness, including suicidality among clinicians, patients, and advocates.
These findings may also have implications in patients with other immune deficiencies, said Isung, noting, “it would be interesting to further explore associations with other immunocompromised populations.”
No surprises
Commenting on the findings for Medscape Medical News, Igor Galynker, MD, professor of psychiatry at Icahn School of Medicine at Mount Sinai, New York City, said the study was “very well-done” and used “reliable and well-controlled” databases.
However, he added, the results “are neither particularly dramatic nor conclusive” as it makes sense that medical illnesses like PID would “increase risk of psychopathology,” said Galynker.
PID patients are much more likely to have contact with clinicians and to receive a psychiatric diagnosis, he said.
“People with a chronic illness are more stressed and generally have high incidences of depression, anxiety, and suicidal behavior. In addition to that, they may be more likely to be diagnosed with those conditions because they see a clinician more frequently.”
However, that reasoning doesn’t apply to autism, which manifests in early childhood and so is unlikely to be the result of stress, said Galynker, which is why he believes the finding that ASD is the psychiatric outcome most strongly associated with PID is “the most convincing.”
Galynker wasn’t surprised that the association between PID and psychiatric illnesses, and suicidal behaviors, was stronger among women.
“Women attempt suicide four times more often than men to begin with, so you would expect this to be more pronounced” in those with PID.
The study was supported by grants from the Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institute; Stockholm Care Services; the Soderstrom Konig Foundation; and the Fredrik & Ingrid Thurings Foundation. Isung and Galynker have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Can mental health teams de-escalate crises in NYC?
“Defund the police”: It’s a slogan, or perhaps a battle cry, that has emerged from the Black Lives Matter movement as a response to race-related police brutality and concerns that people of color are profiled, targeted, arrested, charged, manhandled, and killed by law enforcement in a disproportionate and unjust manner. It crosses into our realm as psychiatrists as mental health emergency calls are handled by the police and not by mental health professionals. The result is sometimes tragic: As many as half of police shootings involve people with psychiatric disorders, and the hope is that many of the police shootings could be avoided if crises were handed by mental health clinicians instead of, or in cooperation with, the police.
At best, police officers receive a week of specialized, crisis intervention training about how to approach those with psychiatric disorders; most officers receive no training. This leaves psychiatry as the only field where medical crises are routinely handled by the police – it is demeaning and embarrassing for some of our patients and dangerous for others. The reality remains, however, that there are times when psychiatric disorders result in violent behavior, and patients being taken for involuntary treatment often resist transport, so either way there is risk, both to the patient and to anyone who responds to a call for assistance.
Early this month, the office of New York City Mayor Bill de Blasio announced that a major change would be made in how mental health calls to 911 are handled in two “high-need” areas. The mayor’s website states:
“Beginning in February 2021, new Mental Health Teams will use their physical and mental health expertise, and experience in crisis response to de-escalate emergency situations, will help reduce the number of times police will need to respond to 911 mental health calls in these precincts. These teams will have the expertise to respond to a range of behavioral health problems, such as suicide attempts, substance misuse, and serious mental illness, as well as physical health problems, which can be exacerbated by or mask mental health problems. NYC Health + Hospitals will train and provide ongoing technical assistance and support. In selecting team members for this program, FDNY will prioritize professionals with significant experience with mental health crises.”
The press release goes on to say that, in situations where there is a weapon or reason to believe there is a risk of violence, the police will be dispatched along with the new mental health team.
“This is the first time in our history that health professionals will be the default responders to mental health emergencies,” New York City First Lady Chirlane McCray said as she announced the new program. “Treating mental health crises as mental health challenges and not public safety ones is the modern and more appropriate approach.”
New York City is not the first city to employ this model. In the United States, the CAHOOTS (Crisis Assistance Helping Out on the Streets) program in Eugene, Ore., has been run by the White Bird Clinic since 1989 as part of a community policing initiative. Last year, the team responded to 24,000 calls and police backup was required on only 150 of those responses. The CAHOOTS website states:
“The CAHOOTS model has been in the spotlight recently as our nation struggles to reimagine public safety. The program mobilizes two-person teams consisting of a medic (a nurse, paramedic, or EMT) and a crisis worker who has substantial training and experience in the mental health field. The CAHOOTS teams deal with a wide range of mental health-related crises, including conflict resolution, welfare checks, substance abuse, suicide threats, and more, relying on trauma-informed de-escalation and harm reduction techniques. CAHOOTS staff are not law enforcement officers and do not carry weapons; their training and experience are the tools they use to ensure a non-violent resolution of crisis situations. They also handle non-emergent medical issues, avoiding costly ambulance transport and emergency room treatment.”
Other cities in the United States are also looking at implementing programs where mental health teams, and not the police, respond to emergency calls. Last year, Oakland, Calif.’s city council invested $40,000 in research to assess how they could best implement a program like the one in Eugene. They hope to begin the Mobile Assistance Community Responders of Oakland (MACROS) next year. Sigal Samuel writes in a Vox article, “The goal is to launch the pilot next year with funding from the city budget, and although supporters are not yet sure what its size and duration will be, they’re hopeful it’ll make a big difference to Oakland’s overpoliced community of people without homes. They were among those who first called for a non-policing approach.”
The model is not unique to the United States. In 2005, Stockholm started a program with a psychiatric ambulance – equipped with comfortable seating rather than a stretcher – to respond to mental health emergencies. The ambulance responds to 130 calls a month. It is staffed with a driver and two psychiatric nurses, and for half of the calls, the police also come. While the Swedish program was not about removing resources from the police, it has relieved the police of the responsibility for many psychiatric emergencies.
The New York City program will be modeled after the CAHOOTS initiative in Eugene. It differs from the mobile crisis response services in many other cities because CAHOOTS is hooked directly into the 911 emergency services system. Its website notes that the program has saved money:
“The cost savings are considerable. The CAHOOTS program budget is about $2.1 million annually, while the combined annual budgets for the Eugene and Springfield police departments are $90 million. In 2017, the CAHOOTS teams answered 17% of the Eugene Police Department’s overall call volume. The program saves the city of Eugene an estimated $8.5 million in public safety spending annually.”
Some worry there is an unpredictable aspect to calls for psychiatric emergencies, and the potential for mental health professions to be injured or killed. Annette Hanson, MD, a forensic psychiatrist at University of Maryland, Baltimore, voiced her concerns, “While multidisciplinary teams are useful, there have been rare cases of violence against responding mental health providers. People with serious mental illness are rarely violent but their dangerousness is unpredictable and cannot be predicted by case screening.”
Daniel Felts is a mental health crisis counselor who has worked at CAHOOTS for the past 4* years. He has responded to about 8,000 calls, and called for police backup only three times to request an immediate "Code 3 cover" when someone's safety has been in danger. Mr. Felts calls the police about once a month for concerns that do not require an immediate response for safety.* “Over the last 4 years, I am only aware of three instances when a team member’s safety was compromised because of a client’s violent behavior. No employee has been seriously physically harmed. In 30 years, with hundreds of thousands (millions?) of calls responded to, no CAHOOTS worker has ever been killed, shot, or stabbed in the line of duty,” Mr. Felts noted.
Emergency calls are screened. “It is not uncommon for CAHOOTS to be dispatched to ‘stage’ for calls involving active disputes or acutely suicidal individuals where means are present. “Staging” entails us parking roughly a mile away while police make first contact and advise whether it is safe for CAHOOTS to engage.”
Mr. Felts went on to discuss the program’s relationship with the community. “ and how we operate. Having operated in Eugene for 30 years, our service is well understood to be one that does not kill, harm, or violate personal boundaries or liberties.”
Would a program like the ones in Stockholm or in Eugene work in other places? Eugene is a city with a population of 172,000 with a low crime rate. Whether a program implemented in one city can be mimicked in another very different city is not clear.
Paul Appelbaum, MD, a forensic psychiatrist at Columbia University, New York, is optimistic about New York City’s forthcoming program.
“The proposed pilot project in NYC is a real step forward. Work that we’ve done looking at fatal encounters involving the police found that roughly 25% of all deaths at the hands of the police are of people with mental illness. In many of those cases, police were initially called to bring people who were clearly troubled for psychiatric evaluation, but as the situation escalated, the police turned to their weapons to control it, which led to a fatal outcome. Taking police out of the picture whenever possible in favor of trained mental health personnel is clearly a better approach. It will be important for the city to collect good outcome data to enable independent evaluation of the pilot project – not something that political entities are inclined toward, but a critical element in assessing the effectiveness of this approach.”
There are questions that remain about the new program. Mayor de Blasio’s office has not released information about which areas of the city are being chosen for the new program, how much the program will cost, or what the funding source will be. If it can be implemented safely and effectively, it has the potential to provide more sensitive care to patients in crisis, and to save lives.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2018). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore.
*Correction, 11/27/2020: An earlier version of this article misstated the number of years Daniel Felts has worked at CAHOOTS.
“Defund the police”: It’s a slogan, or perhaps a battle cry, that has emerged from the Black Lives Matter movement as a response to race-related police brutality and concerns that people of color are profiled, targeted, arrested, charged, manhandled, and killed by law enforcement in a disproportionate and unjust manner. It crosses into our realm as psychiatrists as mental health emergency calls are handled by the police and not by mental health professionals. The result is sometimes tragic: As many as half of police shootings involve people with psychiatric disorders, and the hope is that many of the police shootings could be avoided if crises were handed by mental health clinicians instead of, or in cooperation with, the police.
At best, police officers receive a week of specialized, crisis intervention training about how to approach those with psychiatric disorders; most officers receive no training. This leaves psychiatry as the only field where medical crises are routinely handled by the police – it is demeaning and embarrassing for some of our patients and dangerous for others. The reality remains, however, that there are times when psychiatric disorders result in violent behavior, and patients being taken for involuntary treatment often resist transport, so either way there is risk, both to the patient and to anyone who responds to a call for assistance.
Early this month, the office of New York City Mayor Bill de Blasio announced that a major change would be made in how mental health calls to 911 are handled in two “high-need” areas. The mayor’s website states:
“Beginning in February 2021, new Mental Health Teams will use their physical and mental health expertise, and experience in crisis response to de-escalate emergency situations, will help reduce the number of times police will need to respond to 911 mental health calls in these precincts. These teams will have the expertise to respond to a range of behavioral health problems, such as suicide attempts, substance misuse, and serious mental illness, as well as physical health problems, which can be exacerbated by or mask mental health problems. NYC Health + Hospitals will train and provide ongoing technical assistance and support. In selecting team members for this program, FDNY will prioritize professionals with significant experience with mental health crises.”
The press release goes on to say that, in situations where there is a weapon or reason to believe there is a risk of violence, the police will be dispatched along with the new mental health team.
“This is the first time in our history that health professionals will be the default responders to mental health emergencies,” New York City First Lady Chirlane McCray said as she announced the new program. “Treating mental health crises as mental health challenges and not public safety ones is the modern and more appropriate approach.”
New York City is not the first city to employ this model. In the United States, the CAHOOTS (Crisis Assistance Helping Out on the Streets) program in Eugene, Ore., has been run by the White Bird Clinic since 1989 as part of a community policing initiative. Last year, the team responded to 24,000 calls and police backup was required on only 150 of those responses. The CAHOOTS website states:
“The CAHOOTS model has been in the spotlight recently as our nation struggles to reimagine public safety. The program mobilizes two-person teams consisting of a medic (a nurse, paramedic, or EMT) and a crisis worker who has substantial training and experience in the mental health field. The CAHOOTS teams deal with a wide range of mental health-related crises, including conflict resolution, welfare checks, substance abuse, suicide threats, and more, relying on trauma-informed de-escalation and harm reduction techniques. CAHOOTS staff are not law enforcement officers and do not carry weapons; their training and experience are the tools they use to ensure a non-violent resolution of crisis situations. They also handle non-emergent medical issues, avoiding costly ambulance transport and emergency room treatment.”
Other cities in the United States are also looking at implementing programs where mental health teams, and not the police, respond to emergency calls. Last year, Oakland, Calif.’s city council invested $40,000 in research to assess how they could best implement a program like the one in Eugene. They hope to begin the Mobile Assistance Community Responders of Oakland (MACROS) next year. Sigal Samuel writes in a Vox article, “The goal is to launch the pilot next year with funding from the city budget, and although supporters are not yet sure what its size and duration will be, they’re hopeful it’ll make a big difference to Oakland’s overpoliced community of people without homes. They were among those who first called for a non-policing approach.”
The model is not unique to the United States. In 2005, Stockholm started a program with a psychiatric ambulance – equipped with comfortable seating rather than a stretcher – to respond to mental health emergencies. The ambulance responds to 130 calls a month. It is staffed with a driver and two psychiatric nurses, and for half of the calls, the police also come. While the Swedish program was not about removing resources from the police, it has relieved the police of the responsibility for many psychiatric emergencies.
The New York City program will be modeled after the CAHOOTS initiative in Eugene. It differs from the mobile crisis response services in many other cities because CAHOOTS is hooked directly into the 911 emergency services system. Its website notes that the program has saved money:
“The cost savings are considerable. The CAHOOTS program budget is about $2.1 million annually, while the combined annual budgets for the Eugene and Springfield police departments are $90 million. In 2017, the CAHOOTS teams answered 17% of the Eugene Police Department’s overall call volume. The program saves the city of Eugene an estimated $8.5 million in public safety spending annually.”
Some worry there is an unpredictable aspect to calls for psychiatric emergencies, and the potential for mental health professions to be injured or killed. Annette Hanson, MD, a forensic psychiatrist at University of Maryland, Baltimore, voiced her concerns, “While multidisciplinary teams are useful, there have been rare cases of violence against responding mental health providers. People with serious mental illness are rarely violent but their dangerousness is unpredictable and cannot be predicted by case screening.”
Daniel Felts is a mental health crisis counselor who has worked at CAHOOTS for the past 4* years. He has responded to about 8,000 calls, and called for police backup only three times to request an immediate "Code 3 cover" when someone's safety has been in danger. Mr. Felts calls the police about once a month for concerns that do not require an immediate response for safety.* “Over the last 4 years, I am only aware of three instances when a team member’s safety was compromised because of a client’s violent behavior. No employee has been seriously physically harmed. In 30 years, with hundreds of thousands (millions?) of calls responded to, no CAHOOTS worker has ever been killed, shot, or stabbed in the line of duty,” Mr. Felts noted.
Emergency calls are screened. “It is not uncommon for CAHOOTS to be dispatched to ‘stage’ for calls involving active disputes or acutely suicidal individuals where means are present. “Staging” entails us parking roughly a mile away while police make first contact and advise whether it is safe for CAHOOTS to engage.”
Mr. Felts went on to discuss the program’s relationship with the community. “ and how we operate. Having operated in Eugene for 30 years, our service is well understood to be one that does not kill, harm, or violate personal boundaries or liberties.”
Would a program like the ones in Stockholm or in Eugene work in other places? Eugene is a city with a population of 172,000 with a low crime rate. Whether a program implemented in one city can be mimicked in another very different city is not clear.
Paul Appelbaum, MD, a forensic psychiatrist at Columbia University, New York, is optimistic about New York City’s forthcoming program.
“The proposed pilot project in NYC is a real step forward. Work that we’ve done looking at fatal encounters involving the police found that roughly 25% of all deaths at the hands of the police are of people with mental illness. In many of those cases, police were initially called to bring people who were clearly troubled for psychiatric evaluation, but as the situation escalated, the police turned to their weapons to control it, which led to a fatal outcome. Taking police out of the picture whenever possible in favor of trained mental health personnel is clearly a better approach. It will be important for the city to collect good outcome data to enable independent evaluation of the pilot project – not something that political entities are inclined toward, but a critical element in assessing the effectiveness of this approach.”
There are questions that remain about the new program. Mayor de Blasio’s office has not released information about which areas of the city are being chosen for the new program, how much the program will cost, or what the funding source will be. If it can be implemented safely and effectively, it has the potential to provide more sensitive care to patients in crisis, and to save lives.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2018). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore.
*Correction, 11/27/2020: An earlier version of this article misstated the number of years Daniel Felts has worked at CAHOOTS.
“Defund the police”: It’s a slogan, or perhaps a battle cry, that has emerged from the Black Lives Matter movement as a response to race-related police brutality and concerns that people of color are profiled, targeted, arrested, charged, manhandled, and killed by law enforcement in a disproportionate and unjust manner. It crosses into our realm as psychiatrists as mental health emergency calls are handled by the police and not by mental health professionals. The result is sometimes tragic: As many as half of police shootings involve people with psychiatric disorders, and the hope is that many of the police shootings could be avoided if crises were handed by mental health clinicians instead of, or in cooperation with, the police.
At best, police officers receive a week of specialized, crisis intervention training about how to approach those with psychiatric disorders; most officers receive no training. This leaves psychiatry as the only field where medical crises are routinely handled by the police – it is demeaning and embarrassing for some of our patients and dangerous for others. The reality remains, however, that there are times when psychiatric disorders result in violent behavior, and patients being taken for involuntary treatment often resist transport, so either way there is risk, both to the patient and to anyone who responds to a call for assistance.
Early this month, the office of New York City Mayor Bill de Blasio announced that a major change would be made in how mental health calls to 911 are handled in two “high-need” areas. The mayor’s website states:
“Beginning in February 2021, new Mental Health Teams will use their physical and mental health expertise, and experience in crisis response to de-escalate emergency situations, will help reduce the number of times police will need to respond to 911 mental health calls in these precincts. These teams will have the expertise to respond to a range of behavioral health problems, such as suicide attempts, substance misuse, and serious mental illness, as well as physical health problems, which can be exacerbated by or mask mental health problems. NYC Health + Hospitals will train and provide ongoing technical assistance and support. In selecting team members for this program, FDNY will prioritize professionals with significant experience with mental health crises.”
The press release goes on to say that, in situations where there is a weapon or reason to believe there is a risk of violence, the police will be dispatched along with the new mental health team.
“This is the first time in our history that health professionals will be the default responders to mental health emergencies,” New York City First Lady Chirlane McCray said as she announced the new program. “Treating mental health crises as mental health challenges and not public safety ones is the modern and more appropriate approach.”
New York City is not the first city to employ this model. In the United States, the CAHOOTS (Crisis Assistance Helping Out on the Streets) program in Eugene, Ore., has been run by the White Bird Clinic since 1989 as part of a community policing initiative. Last year, the team responded to 24,000 calls and police backup was required on only 150 of those responses. The CAHOOTS website states:
“The CAHOOTS model has been in the spotlight recently as our nation struggles to reimagine public safety. The program mobilizes two-person teams consisting of a medic (a nurse, paramedic, or EMT) and a crisis worker who has substantial training and experience in the mental health field. The CAHOOTS teams deal with a wide range of mental health-related crises, including conflict resolution, welfare checks, substance abuse, suicide threats, and more, relying on trauma-informed de-escalation and harm reduction techniques. CAHOOTS staff are not law enforcement officers and do not carry weapons; their training and experience are the tools they use to ensure a non-violent resolution of crisis situations. They also handle non-emergent medical issues, avoiding costly ambulance transport and emergency room treatment.”
Other cities in the United States are also looking at implementing programs where mental health teams, and not the police, respond to emergency calls. Last year, Oakland, Calif.’s city council invested $40,000 in research to assess how they could best implement a program like the one in Eugene. They hope to begin the Mobile Assistance Community Responders of Oakland (MACROS) next year. Sigal Samuel writes in a Vox article, “The goal is to launch the pilot next year with funding from the city budget, and although supporters are not yet sure what its size and duration will be, they’re hopeful it’ll make a big difference to Oakland’s overpoliced community of people without homes. They were among those who first called for a non-policing approach.”
The model is not unique to the United States. In 2005, Stockholm started a program with a psychiatric ambulance – equipped with comfortable seating rather than a stretcher – to respond to mental health emergencies. The ambulance responds to 130 calls a month. It is staffed with a driver and two psychiatric nurses, and for half of the calls, the police also come. While the Swedish program was not about removing resources from the police, it has relieved the police of the responsibility for many psychiatric emergencies.
The New York City program will be modeled after the CAHOOTS initiative in Eugene. It differs from the mobile crisis response services in many other cities because CAHOOTS is hooked directly into the 911 emergency services system. Its website notes that the program has saved money:
“The cost savings are considerable. The CAHOOTS program budget is about $2.1 million annually, while the combined annual budgets for the Eugene and Springfield police departments are $90 million. In 2017, the CAHOOTS teams answered 17% of the Eugene Police Department’s overall call volume. The program saves the city of Eugene an estimated $8.5 million in public safety spending annually.”
Some worry there is an unpredictable aspect to calls for psychiatric emergencies, and the potential for mental health professions to be injured or killed. Annette Hanson, MD, a forensic psychiatrist at University of Maryland, Baltimore, voiced her concerns, “While multidisciplinary teams are useful, there have been rare cases of violence against responding mental health providers. People with serious mental illness are rarely violent but their dangerousness is unpredictable and cannot be predicted by case screening.”
Daniel Felts is a mental health crisis counselor who has worked at CAHOOTS for the past 4* years. He has responded to about 8,000 calls, and called for police backup only three times to request an immediate "Code 3 cover" when someone's safety has been in danger. Mr. Felts calls the police about once a month for concerns that do not require an immediate response for safety.* “Over the last 4 years, I am only aware of three instances when a team member’s safety was compromised because of a client’s violent behavior. No employee has been seriously physically harmed. In 30 years, with hundreds of thousands (millions?) of calls responded to, no CAHOOTS worker has ever been killed, shot, or stabbed in the line of duty,” Mr. Felts noted.
Emergency calls are screened. “It is not uncommon for CAHOOTS to be dispatched to ‘stage’ for calls involving active disputes or acutely suicidal individuals where means are present. “Staging” entails us parking roughly a mile away while police make first contact and advise whether it is safe for CAHOOTS to engage.”
Mr. Felts went on to discuss the program’s relationship with the community. “ and how we operate. Having operated in Eugene for 30 years, our service is well understood to be one that does not kill, harm, or violate personal boundaries or liberties.”
Would a program like the ones in Stockholm or in Eugene work in other places? Eugene is a city with a population of 172,000 with a low crime rate. Whether a program implemented in one city can be mimicked in another very different city is not clear.
Paul Appelbaum, MD, a forensic psychiatrist at Columbia University, New York, is optimistic about New York City’s forthcoming program.
“The proposed pilot project in NYC is a real step forward. Work that we’ve done looking at fatal encounters involving the police found that roughly 25% of all deaths at the hands of the police are of people with mental illness. In many of those cases, police were initially called to bring people who were clearly troubled for psychiatric evaluation, but as the situation escalated, the police turned to their weapons to control it, which led to a fatal outcome. Taking police out of the picture whenever possible in favor of trained mental health personnel is clearly a better approach. It will be important for the city to collect good outcome data to enable independent evaluation of the pilot project – not something that political entities are inclined toward, but a critical element in assessing the effectiveness of this approach.”
There are questions that remain about the new program. Mayor de Blasio’s office has not released information about which areas of the city are being chosen for the new program, how much the program will cost, or what the funding source will be. If it can be implemented safely and effectively, it has the potential to provide more sensitive care to patients in crisis, and to save lives.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2018). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore.
*Correction, 11/27/2020: An earlier version of this article misstated the number of years Daniel Felts has worked at CAHOOTS.
COVID-19 cases in children continue to set records
As far as the pandemic is concerned, it seems like a pretty small thing. A difference of just 0.3%. Children now represent 11.8% of all COVID-19 cases that have occurred since the beginning of the pandemic, compared with 11.5% 1 week ago, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Hiding behind that 0.3%, however, is a much larger number: 144,145. That is the number of new child cases that occurred during the week that ended Nov. 19, and it’s the highest weekly figure yet, eclipsing the previous high of 111,946 from the week of Nov. 12, the AAP and the CHA said in their latest COVID-19 report. For the week ending Nov. 19, children represented 14.1% of all new cases, up from 14.0% the week before.
In the United States, more than 1.18 million children have been infected by the coronavirus since the beginning of the pandemic, with the total among all ages topping 10 million in 49 states (New York is not providing age distribution), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP/CHA data show. That works out to 11.8% of all cases.
The overall rate of child COVID-19 cases is now up to 1,573 per 100,000 children nationally, with considerable variation seen among the states. The lowest rates can be found in Vermont (344 per 100,000), Maine (452), and Hawaii (675), and the highest in North Dakota (5,589), South Dakota (3,993), and Wisconsin (3,727), the AAP and CHA said in the report.
Comparisons between states are somewhat problematic, though, because “each state makes different decisions about how to report the age distribution of COVID-19 cases, and as a result the age range for reported cases varies by state. … It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states at this time,” the two organizations noted.
Five more COVID-19–related deaths in children were reported during the week of Nov. 19, bringing the count to 138 and holding at just 0.06% of the total for all ages, based on data from 43 states and New York City. Children’s share of hospitalizations increased slightly in the last week, rising from 1.7% to 1.8% in the 24 states (and NYC) that are reporting such data. The total number of child hospitalizations in those jurisdictions is just over 6,700, the AAP and CHA said.
As far as the pandemic is concerned, it seems like a pretty small thing. A difference of just 0.3%. Children now represent 11.8% of all COVID-19 cases that have occurred since the beginning of the pandemic, compared with 11.5% 1 week ago, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Hiding behind that 0.3%, however, is a much larger number: 144,145. That is the number of new child cases that occurred during the week that ended Nov. 19, and it’s the highest weekly figure yet, eclipsing the previous high of 111,946 from the week of Nov. 12, the AAP and the CHA said in their latest COVID-19 report. For the week ending Nov. 19, children represented 14.1% of all new cases, up from 14.0% the week before.
In the United States, more than 1.18 million children have been infected by the coronavirus since the beginning of the pandemic, with the total among all ages topping 10 million in 49 states (New York is not providing age distribution), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP/CHA data show. That works out to 11.8% of all cases.
The overall rate of child COVID-19 cases is now up to 1,573 per 100,000 children nationally, with considerable variation seen among the states. The lowest rates can be found in Vermont (344 per 100,000), Maine (452), and Hawaii (675), and the highest in North Dakota (5,589), South Dakota (3,993), and Wisconsin (3,727), the AAP and CHA said in the report.
Comparisons between states are somewhat problematic, though, because “each state makes different decisions about how to report the age distribution of COVID-19 cases, and as a result the age range for reported cases varies by state. … It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states at this time,” the two organizations noted.
Five more COVID-19–related deaths in children were reported during the week of Nov. 19, bringing the count to 138 and holding at just 0.06% of the total for all ages, based on data from 43 states and New York City. Children’s share of hospitalizations increased slightly in the last week, rising from 1.7% to 1.8% in the 24 states (and NYC) that are reporting such data. The total number of child hospitalizations in those jurisdictions is just over 6,700, the AAP and CHA said.
As far as the pandemic is concerned, it seems like a pretty small thing. A difference of just 0.3%. Children now represent 11.8% of all COVID-19 cases that have occurred since the beginning of the pandemic, compared with 11.5% 1 week ago, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Hiding behind that 0.3%, however, is a much larger number: 144,145. That is the number of new child cases that occurred during the week that ended Nov. 19, and it’s the highest weekly figure yet, eclipsing the previous high of 111,946 from the week of Nov. 12, the AAP and the CHA said in their latest COVID-19 report. For the week ending Nov. 19, children represented 14.1% of all new cases, up from 14.0% the week before.
In the United States, more than 1.18 million children have been infected by the coronavirus since the beginning of the pandemic, with the total among all ages topping 10 million in 49 states (New York is not providing age distribution), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP/CHA data show. That works out to 11.8% of all cases.
The overall rate of child COVID-19 cases is now up to 1,573 per 100,000 children nationally, with considerable variation seen among the states. The lowest rates can be found in Vermont (344 per 100,000), Maine (452), and Hawaii (675), and the highest in North Dakota (5,589), South Dakota (3,993), and Wisconsin (3,727), the AAP and CHA said in the report.
Comparisons between states are somewhat problematic, though, because “each state makes different decisions about how to report the age distribution of COVID-19 cases, and as a result the age range for reported cases varies by state. … It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states at this time,” the two organizations noted.
Five more COVID-19–related deaths in children were reported during the week of Nov. 19, bringing the count to 138 and holding at just 0.06% of the total for all ages, based on data from 43 states and New York City. Children’s share of hospitalizations increased slightly in the last week, rising from 1.7% to 1.8% in the 24 states (and NYC) that are reporting such data. The total number of child hospitalizations in those jurisdictions is just over 6,700, the AAP and CHA said.
Concussion linked to risk for dementia, Parkinson’s disease, and ADHD
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From Family Medicine and Community Health