User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Pandemic effect: All other health care visits can wait
according to survey conducted at the end of April.
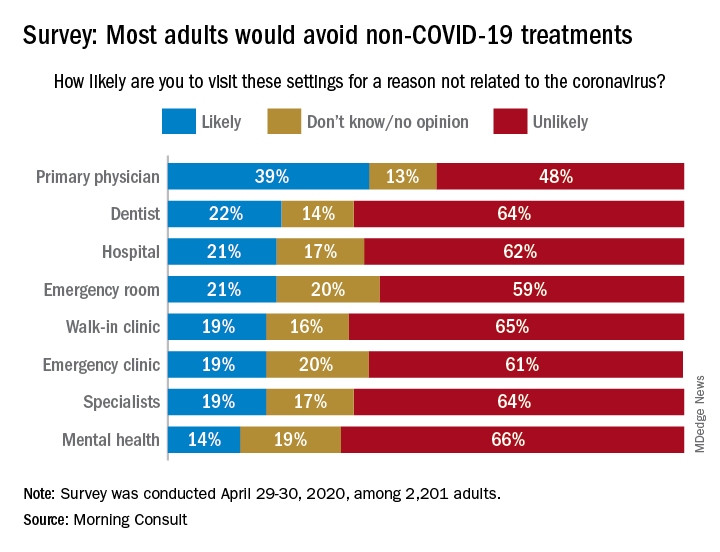
When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
according to survey conducted at the end of April.

When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
according to survey conducted at the end of April.

When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
FDA grants EUA to muscle stimulator to reduce mechanical ventilator usage
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
Fountains of Wayne, and a hospitalist’s first day, remembered
Like many in the health care field, I have found it hard to watch the news over these past couple of months when it seems that almost every story is about COVID-19 or its repercussions. Luckily, I have two young daughters who “encourage” me to listen to the Frozen 2 soundtrack instead of putting on the evening news when I get home from work. Still, news manages to seep through my defenses. As I scrolled through some headlines recently, I learned of the death of musician Adam Schlesinger from COVID-19. He wasn’t a household name, but his death still hit me in unexpected ways.
I started internship in late June 2005, in a city (Portland, Ore.) about as different from my previous home (Dallas) as any two places can possibly be. I think the day before internship started still ranks as the most nervous of my life. I’m not sure how I slept at all that night, but somehow I did and arrived at the Portland Veterans Affairs Hospital the following morning to start my new career.
And then … nothing happened. Early on that first day, the electronic medical records crashed, and no patients were admitted during our time on “short call.” My upper level resident took care of the one or two established patients on the team (both discharged), so I ended the day with records that would not be broken during the remainder of my residency: 0 notes written, 0 patients seen. Perhaps the most successful first day that any intern, anywhere has ever had, although it prepared me quite poorly for all the subsequent days.
Since I had some time on my hands, I made the 20-minute walk to one of my new hometown’s record stores where Fountains of Wayne (FOW) was playing an acoustic in-store set. Their album from a few years prior, “Welcome Interstate Managers,” was in heavy rotation when I made the drive from Dallas to Portland. It was (and is) a great album for long drives – melodic, catchy, and (mostly) up-tempo. Adam and the band’s singer, Chris Collingwood, played several songs that night on the store’s stage. Then they headed out to the next city, and I headed back home and on to many far-busier days of residency.
We would cross paths again a decade later. I moved back to Texas and became a hospitalist. It turns out that, if you have enough hospitalists of a certain age and if enough of those hospitalists have unearned confidence in their musical ability, then a covers band will undoubtedly be formed. And so, it happened here in San Antonio. We were not selective in our song choices – we played songs from every decade of the last 50 years, bands as popular as the Beatles and as indie as the Rentals. And we played some FOW.
Our band (which will go nameless here so that our YouTube recordings are more difficult to find) played a grand total of one gig during our years of intermittent practicing. That one gig was my wedding rehearsal dinner and the penultimate song we played was “Stacy’s Mom,” which is notable for being both FOW’s biggest hit and a completely inappropriate song to play at a wedding rehearsal dinner. The crowd was probably around the same size as the one that had seen Adam and Chris play in Portland 10 years prior. I don’t think the applause we received was quite as genuine or deserved, though.
After Adam and Chris played their gig, there was an autograph session and I took home a signed poster. Last year, I decided to take it out of storage and hang it in my office. The date of the show and the first day of my physician career, a date now nearly 15 years ago, is written in psychedelic typography at the bottom. The store that I went to that day is no longer there, a victim of progress like so many other record stores across the country. Another location of the same store is still open in Portland. I hope that it and all the other small book and music stores across the country can survive this current crisis, but I know that many will not.
So, here’s to you Adam, and to all the others who have lost their lives to this terrible illness. As a small token of remembrance, I’ll be playing some Fountains of Wayne on the drive home tonight. It’s not quite the same as playing it on a cross-country drive, but hopefully, we will all be able to do that again soon.
Dr. Sehgal is a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. He is a member of the editorial advisory board for The Hospitalist.
Like many in the health care field, I have found it hard to watch the news over these past couple of months when it seems that almost every story is about COVID-19 or its repercussions. Luckily, I have two young daughters who “encourage” me to listen to the Frozen 2 soundtrack instead of putting on the evening news when I get home from work. Still, news manages to seep through my defenses. As I scrolled through some headlines recently, I learned of the death of musician Adam Schlesinger from COVID-19. He wasn’t a household name, but his death still hit me in unexpected ways.
I started internship in late June 2005, in a city (Portland, Ore.) about as different from my previous home (Dallas) as any two places can possibly be. I think the day before internship started still ranks as the most nervous of my life. I’m not sure how I slept at all that night, but somehow I did and arrived at the Portland Veterans Affairs Hospital the following morning to start my new career.
And then … nothing happened. Early on that first day, the electronic medical records crashed, and no patients were admitted during our time on “short call.” My upper level resident took care of the one or two established patients on the team (both discharged), so I ended the day with records that would not be broken during the remainder of my residency: 0 notes written, 0 patients seen. Perhaps the most successful first day that any intern, anywhere has ever had, although it prepared me quite poorly for all the subsequent days.
Since I had some time on my hands, I made the 20-minute walk to one of my new hometown’s record stores where Fountains of Wayne (FOW) was playing an acoustic in-store set. Their album from a few years prior, “Welcome Interstate Managers,” was in heavy rotation when I made the drive from Dallas to Portland. It was (and is) a great album for long drives – melodic, catchy, and (mostly) up-tempo. Adam and the band’s singer, Chris Collingwood, played several songs that night on the store’s stage. Then they headed out to the next city, and I headed back home and on to many far-busier days of residency.
We would cross paths again a decade later. I moved back to Texas and became a hospitalist. It turns out that, if you have enough hospitalists of a certain age and if enough of those hospitalists have unearned confidence in their musical ability, then a covers band will undoubtedly be formed. And so, it happened here in San Antonio. We were not selective in our song choices – we played songs from every decade of the last 50 years, bands as popular as the Beatles and as indie as the Rentals. And we played some FOW.
Our band (which will go nameless here so that our YouTube recordings are more difficult to find) played a grand total of one gig during our years of intermittent practicing. That one gig was my wedding rehearsal dinner and the penultimate song we played was “Stacy’s Mom,” which is notable for being both FOW’s biggest hit and a completely inappropriate song to play at a wedding rehearsal dinner. The crowd was probably around the same size as the one that had seen Adam and Chris play in Portland 10 years prior. I don’t think the applause we received was quite as genuine or deserved, though.
After Adam and Chris played their gig, there was an autograph session and I took home a signed poster. Last year, I decided to take it out of storage and hang it in my office. The date of the show and the first day of my physician career, a date now nearly 15 years ago, is written in psychedelic typography at the bottom. The store that I went to that day is no longer there, a victim of progress like so many other record stores across the country. Another location of the same store is still open in Portland. I hope that it and all the other small book and music stores across the country can survive this current crisis, but I know that many will not.
So, here’s to you Adam, and to all the others who have lost their lives to this terrible illness. As a small token of remembrance, I’ll be playing some Fountains of Wayne on the drive home tonight. It’s not quite the same as playing it on a cross-country drive, but hopefully, we will all be able to do that again soon.
Dr. Sehgal is a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. He is a member of the editorial advisory board for The Hospitalist.
Like many in the health care field, I have found it hard to watch the news over these past couple of months when it seems that almost every story is about COVID-19 or its repercussions. Luckily, I have two young daughters who “encourage” me to listen to the Frozen 2 soundtrack instead of putting on the evening news when I get home from work. Still, news manages to seep through my defenses. As I scrolled through some headlines recently, I learned of the death of musician Adam Schlesinger from COVID-19. He wasn’t a household name, but his death still hit me in unexpected ways.
I started internship in late June 2005, in a city (Portland, Ore.) about as different from my previous home (Dallas) as any two places can possibly be. I think the day before internship started still ranks as the most nervous of my life. I’m not sure how I slept at all that night, but somehow I did and arrived at the Portland Veterans Affairs Hospital the following morning to start my new career.
And then … nothing happened. Early on that first day, the electronic medical records crashed, and no patients were admitted during our time on “short call.” My upper level resident took care of the one or two established patients on the team (both discharged), so I ended the day with records that would not be broken during the remainder of my residency: 0 notes written, 0 patients seen. Perhaps the most successful first day that any intern, anywhere has ever had, although it prepared me quite poorly for all the subsequent days.
Since I had some time on my hands, I made the 20-minute walk to one of my new hometown’s record stores where Fountains of Wayne (FOW) was playing an acoustic in-store set. Their album from a few years prior, “Welcome Interstate Managers,” was in heavy rotation when I made the drive from Dallas to Portland. It was (and is) a great album for long drives – melodic, catchy, and (mostly) up-tempo. Adam and the band’s singer, Chris Collingwood, played several songs that night on the store’s stage. Then they headed out to the next city, and I headed back home and on to many far-busier days of residency.
We would cross paths again a decade later. I moved back to Texas and became a hospitalist. It turns out that, if you have enough hospitalists of a certain age and if enough of those hospitalists have unearned confidence in their musical ability, then a covers band will undoubtedly be formed. And so, it happened here in San Antonio. We were not selective in our song choices – we played songs from every decade of the last 50 years, bands as popular as the Beatles and as indie as the Rentals. And we played some FOW.
Our band (which will go nameless here so that our YouTube recordings are more difficult to find) played a grand total of one gig during our years of intermittent practicing. That one gig was my wedding rehearsal dinner and the penultimate song we played was “Stacy’s Mom,” which is notable for being both FOW’s biggest hit and a completely inappropriate song to play at a wedding rehearsal dinner. The crowd was probably around the same size as the one that had seen Adam and Chris play in Portland 10 years prior. I don’t think the applause we received was quite as genuine or deserved, though.
After Adam and Chris played their gig, there was an autograph session and I took home a signed poster. Last year, I decided to take it out of storage and hang it in my office. The date of the show and the first day of my physician career, a date now nearly 15 years ago, is written in psychedelic typography at the bottom. The store that I went to that day is no longer there, a victim of progress like so many other record stores across the country. Another location of the same store is still open in Portland. I hope that it and all the other small book and music stores across the country can survive this current crisis, but I know that many will not.
So, here’s to you Adam, and to all the others who have lost their lives to this terrible illness. As a small token of remembrance, I’ll be playing some Fountains of Wayne on the drive home tonight. It’s not quite the same as playing it on a cross-country drive, but hopefully, we will all be able to do that again soon.
Dr. Sehgal is a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. He is a member of the editorial advisory board for The Hospitalist.
Doctor with a mask: Enhancing communication and empathy
Delivering a goodbye monologue to an elderly patient, I said: “Tomorrow, my colleague Dr. XYZ, who is an excellent physician, will be here in my place, and I will leave a detailed sign out for them.” I was on the last day of a 7-day-long block on hospital medicine service. Typically, when I say goodbye, some patients respond “thank you, enjoy your time,” some don’t care, and some show disappointment at the transition. This patient became uneasy, choking back tears, and said: “But, I don’t want a new doctor. You know me well. ... They don’t even allow my family in the hospital.”
That expression of anxiety, of having to build rapport with a new provider, concerns about continuity of care, and missing support of family members were not alien to me. As I instinctively took a step toward him to offer a comforting hug, an unsolicited voice in my head said, “social distancing.” I steered back, handing him a box of tissues. I continued: “You have come a long way, and things are looking good from here,” providing more details before I left the room. There was a change in my practice that week. I didn’t shake hands with my patients; I didn’t sit on any unassigned chair; I had no family members in the room asking me questions or supporting my patients. I was trying to show empathy or a smile behind a mask and protective eyewear. The business card with photograph had become more critical than ever for patients to “see” their doctor.
Moving from room to room and examining patients, it felt like the coronavirus was changing the practice of medicine beyond concerns of virus transmission, losing a patient, or putting in extra hours. I realized I was missing so-called “nonverbal communication” amid social distancing: facial expressions, social touch, and the support of family or friends to motivate or destress patients. With no visitors and curbed health care staff entries into patient’s rooms, social distancing was amounting to social isolation. My protective gear and social distancing seemed to be reducing my perceived empathy with patients, and the ability to build a good patient-physician relationship.
Amid alarms, beeps, and buzzes, patients were not only missing their families but also the familiar faces of their physicians. I needed to raise my game while embracing the “new normal” of health care. Cut to the next 13 patients: I paid more attention to voice, tone, and posture. I called patient families from the bedside instead of the office. I translated my emotions with words, loud and clear, replacing “your renal function looks better” (said without a smile) with “I am happy to see your renal function better.”
Through years of practice, I felt prepared to deal with feelings of denial, grief, anxiety, and much more, but the emotions arising as a result of this pandemic were unique. “I knew my mother was old, and this day would come,” said one of the inconsolable family members of a critically ill patient. “However, I wished to be at her side that day, not like this.” I spend my days listening to patient and family concerns about unemployment with quarantine, fears of spreading the disease to loved ones, and the possibility of medications not working.
After a long day, I went back to that first elderly patient to see if he was comfortable with the transition of care. I did a video conference with his daughter, and repeated my goodbyes. The patient smiled and said: “Doc, you deserve a break.” That day I learned about the challenges of good clinical rounding in coronavirus times, and how to overcome them. For “millennial” physicians, it is our first pandemic, and we are learning from it every day.
Driving home through empty streets, I concluded that my answers to the clinical questions asked by patients and families lean heavily on ever-changing data, and the treatments offered have yet to prove their mettle. As a result, I will continue to focus as much on the time-tested fundamentals of clinical practice: communication and empathy. I cannot allow the social distancing and the mask to hide my compassion, or take away from patient satisfaction. Shifting gears, I turned on my car radio, using music to reset my mind before attending to my now-homeschooling kids.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Wong CK et al. Effect of facemasks on empathy and relational continuity: A randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200.
2. Little P et al. Randomised controlled trial of a brief intervention targeting predominantly nonverbal communication in general practice consultations. Br J Gen Pract. 2015;65(635):e351-6.
3. Varghese A. A doctor’s touch. TEDGlobal 2011. 2011 Jul. https://www.ted.com/talks/abraham_verghese_a_doctor_s_touch?language=en
Delivering a goodbye monologue to an elderly patient, I said: “Tomorrow, my colleague Dr. XYZ, who is an excellent physician, will be here in my place, and I will leave a detailed sign out for them.” I was on the last day of a 7-day-long block on hospital medicine service. Typically, when I say goodbye, some patients respond “thank you, enjoy your time,” some don’t care, and some show disappointment at the transition. This patient became uneasy, choking back tears, and said: “But, I don’t want a new doctor. You know me well. ... They don’t even allow my family in the hospital.”
That expression of anxiety, of having to build rapport with a new provider, concerns about continuity of care, and missing support of family members were not alien to me. As I instinctively took a step toward him to offer a comforting hug, an unsolicited voice in my head said, “social distancing.” I steered back, handing him a box of tissues. I continued: “You have come a long way, and things are looking good from here,” providing more details before I left the room. There was a change in my practice that week. I didn’t shake hands with my patients; I didn’t sit on any unassigned chair; I had no family members in the room asking me questions or supporting my patients. I was trying to show empathy or a smile behind a mask and protective eyewear. The business card with photograph had become more critical than ever for patients to “see” their doctor.
Moving from room to room and examining patients, it felt like the coronavirus was changing the practice of medicine beyond concerns of virus transmission, losing a patient, or putting in extra hours. I realized I was missing so-called “nonverbal communication” amid social distancing: facial expressions, social touch, and the support of family or friends to motivate or destress patients. With no visitors and curbed health care staff entries into patient’s rooms, social distancing was amounting to social isolation. My protective gear and social distancing seemed to be reducing my perceived empathy with patients, and the ability to build a good patient-physician relationship.
Amid alarms, beeps, and buzzes, patients were not only missing their families but also the familiar faces of their physicians. I needed to raise my game while embracing the “new normal” of health care. Cut to the next 13 patients: I paid more attention to voice, tone, and posture. I called patient families from the bedside instead of the office. I translated my emotions with words, loud and clear, replacing “your renal function looks better” (said without a smile) with “I am happy to see your renal function better.”
Through years of practice, I felt prepared to deal with feelings of denial, grief, anxiety, and much more, but the emotions arising as a result of this pandemic were unique. “I knew my mother was old, and this day would come,” said one of the inconsolable family members of a critically ill patient. “However, I wished to be at her side that day, not like this.” I spend my days listening to patient and family concerns about unemployment with quarantine, fears of spreading the disease to loved ones, and the possibility of medications not working.
After a long day, I went back to that first elderly patient to see if he was comfortable with the transition of care. I did a video conference with his daughter, and repeated my goodbyes. The patient smiled and said: “Doc, you deserve a break.” That day I learned about the challenges of good clinical rounding in coronavirus times, and how to overcome them. For “millennial” physicians, it is our first pandemic, and we are learning from it every day.
Driving home through empty streets, I concluded that my answers to the clinical questions asked by patients and families lean heavily on ever-changing data, and the treatments offered have yet to prove their mettle. As a result, I will continue to focus as much on the time-tested fundamentals of clinical practice: communication and empathy. I cannot allow the social distancing and the mask to hide my compassion, or take away from patient satisfaction. Shifting gears, I turned on my car radio, using music to reset my mind before attending to my now-homeschooling kids.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Wong CK et al. Effect of facemasks on empathy and relational continuity: A randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200.
2. Little P et al. Randomised controlled trial of a brief intervention targeting predominantly nonverbal communication in general practice consultations. Br J Gen Pract. 2015;65(635):e351-6.
3. Varghese A. A doctor’s touch. TEDGlobal 2011. 2011 Jul. https://www.ted.com/talks/abraham_verghese_a_doctor_s_touch?language=en
Delivering a goodbye monologue to an elderly patient, I said: “Tomorrow, my colleague Dr. XYZ, who is an excellent physician, will be here in my place, and I will leave a detailed sign out for them.” I was on the last day of a 7-day-long block on hospital medicine service. Typically, when I say goodbye, some patients respond “thank you, enjoy your time,” some don’t care, and some show disappointment at the transition. This patient became uneasy, choking back tears, and said: “But, I don’t want a new doctor. You know me well. ... They don’t even allow my family in the hospital.”
That expression of anxiety, of having to build rapport with a new provider, concerns about continuity of care, and missing support of family members were not alien to me. As I instinctively took a step toward him to offer a comforting hug, an unsolicited voice in my head said, “social distancing.” I steered back, handing him a box of tissues. I continued: “You have come a long way, and things are looking good from here,” providing more details before I left the room. There was a change in my practice that week. I didn’t shake hands with my patients; I didn’t sit on any unassigned chair; I had no family members in the room asking me questions or supporting my patients. I was trying to show empathy or a smile behind a mask and protective eyewear. The business card with photograph had become more critical than ever for patients to “see” their doctor.
Moving from room to room and examining patients, it felt like the coronavirus was changing the practice of medicine beyond concerns of virus transmission, losing a patient, or putting in extra hours. I realized I was missing so-called “nonverbal communication” amid social distancing: facial expressions, social touch, and the support of family or friends to motivate or destress patients. With no visitors and curbed health care staff entries into patient’s rooms, social distancing was amounting to social isolation. My protective gear and social distancing seemed to be reducing my perceived empathy with patients, and the ability to build a good patient-physician relationship.
Amid alarms, beeps, and buzzes, patients were not only missing their families but also the familiar faces of their physicians. I needed to raise my game while embracing the “new normal” of health care. Cut to the next 13 patients: I paid more attention to voice, tone, and posture. I called patient families from the bedside instead of the office. I translated my emotions with words, loud and clear, replacing “your renal function looks better” (said without a smile) with “I am happy to see your renal function better.”
Through years of practice, I felt prepared to deal with feelings of denial, grief, anxiety, and much more, but the emotions arising as a result of this pandemic were unique. “I knew my mother was old, and this day would come,” said one of the inconsolable family members of a critically ill patient. “However, I wished to be at her side that day, not like this.” I spend my days listening to patient and family concerns about unemployment with quarantine, fears of spreading the disease to loved ones, and the possibility of medications not working.
After a long day, I went back to that first elderly patient to see if he was comfortable with the transition of care. I did a video conference with his daughter, and repeated my goodbyes. The patient smiled and said: “Doc, you deserve a break.” That day I learned about the challenges of good clinical rounding in coronavirus times, and how to overcome them. For “millennial” physicians, it is our first pandemic, and we are learning from it every day.
Driving home through empty streets, I concluded that my answers to the clinical questions asked by patients and families lean heavily on ever-changing data, and the treatments offered have yet to prove their mettle. As a result, I will continue to focus as much on the time-tested fundamentals of clinical practice: communication and empathy. I cannot allow the social distancing and the mask to hide my compassion, or take away from patient satisfaction. Shifting gears, I turned on my car radio, using music to reset my mind before attending to my now-homeschooling kids.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Wong CK et al. Effect of facemasks on empathy and relational continuity: A randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200.
2. Little P et al. Randomised controlled trial of a brief intervention targeting predominantly nonverbal communication in general practice consultations. Br J Gen Pract. 2015;65(635):e351-6.
3. Varghese A. A doctor’s touch. TEDGlobal 2011. 2011 Jul. https://www.ted.com/talks/abraham_verghese_a_doctor_s_touch?language=en
Case reports illustrate heterogeneity of skin manifestations in COVID patients
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
FDA tightens requirements for COVID-19 antibody tests
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
Hydroxychloroquine-triggered QTc-interval prolongations mount in COVID-19 patients
The potential for serious arrhythmias from hydroxychloroquine treatment of COVID-19 patients received further documentation from a pair of studies released on May 1, casting further doubt on whether the uncertain benefit from this or related drugs to infected patients is worth the clear risks the agents pose.
A report from 90 confirmed COVID-19 patients treated with hydroxychloroquine at one Boston hospital during March-April 2020 identified a significantly prolonged, corrected QT (QTc) interval of at least 500 msec in 18 patients (20%), which included 10 patients whose QTc rose by at least 60 msec above baseline, and a total of 21 patients (23%) having a notable prolongation (JAMA Cardiol. 2020 May 4. doi: 10.1001/jamacardio.2020.1834). This series included one patient who developed torsades de pointes following treatment with hydroxychloroquine and azithromycin, “which to our knowledge has yet to be reported elsewhere in the literature,” the report said.
The second report, from a single center in Lyon, France, included 40 confirmed COVID-19 patients treated with hydroxychloroquine during 2 weeks in late March, and found that 37 (93%) had some increase in the QTc interval, including 14 patients (36%) with an increase of at least 60 msec, and 7 patients (18%) whose QTc rose to at least 500 msec (JAMA Cardiol. 2020 May. doi: 10.1001/jamacardio.2020.1787). However, none of the 40 patients in this series developed an identified ventricular arrhythmia. All patients in both studies received hydroxychloroquine for at least 1 day, and roughly half the patients in each series also received concurrent azithromycin, another drug that can prolong the QTc interval and that has been frequently used in combination with hydroxychloroquine as an unproven COVID-19 treatment cocktail.
These two reports, as well as prior report from Brazil on COVID-19 patients treated with chloroquine diphosphate (JAMA Netw Open. 2020;3[4]:e208857), “underscore the potential risk associated with widespread use of hydroxychloroquine and the combination of hydroxychloroquine and azithromycin in ambulatory patients with known or suspected COVID-19. Understanding whether this risk is worth taking in the absence of evidence of therapeutic efficacy creates a knowledge gap that needs to be addressed,” wrote Robert O. Bonow, MD, a professor of medicine at Northwestern University in Chicago, and coauthors in an editorial that accompanied the two reports (JAMA Cardiol. 2020 May 4;doi: 10.1001/jamacardio.2020.1782). The editorial cited two recently-begun prospective trials, ORCHID and RECOVERY, that are more systematically assessing the safety and efficacy of hydroxychloroquine treatment in COVID-19 patients.
The findings lend further support to a Safety Communication from the U.S. Food and Drug Administration on April 24 that reminded clinicians that the Emergency Use Authorization for hydroxychloroquine and chloroquine in COVID-19 patients that the FDA issued on March 28 applied to only certain hospitalized patients or those enrolled in clinical trials. The Safety Communication also said that agency was aware of reports of adverse arrhythmia events when COVID-19 patients received these drugs outside a hospital setting as well as uninfected people who had received one of these drugs for preventing infection.
In addition, leaders of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society on April 10 issued a summary of considerations when using hydroxychloroquine and azithromycin to treat COVID-19 patients, and noted that a way to minimized the risk from these drugs is to withhold them from patients with a QTc interval of 500 msec or greater at baseline (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). The summary also highlighted the need for regular ECG monitoring of COVID-19 patients who receive drugs that can prolong the QTc interval, and recommended withdrawing treatment from patients when their QTc exceeds the 500 msec threshold.
None of the authors of the two reports and editorial had relevant commercial disclosures.
The potential for serious arrhythmias from hydroxychloroquine treatment of COVID-19 patients received further documentation from a pair of studies released on May 1, casting further doubt on whether the uncertain benefit from this or related drugs to infected patients is worth the clear risks the agents pose.
A report from 90 confirmed COVID-19 patients treated with hydroxychloroquine at one Boston hospital during March-April 2020 identified a significantly prolonged, corrected QT (QTc) interval of at least 500 msec in 18 patients (20%), which included 10 patients whose QTc rose by at least 60 msec above baseline, and a total of 21 patients (23%) having a notable prolongation (JAMA Cardiol. 2020 May 4. doi: 10.1001/jamacardio.2020.1834). This series included one patient who developed torsades de pointes following treatment with hydroxychloroquine and azithromycin, “which to our knowledge has yet to be reported elsewhere in the literature,” the report said.
The second report, from a single center in Lyon, France, included 40 confirmed COVID-19 patients treated with hydroxychloroquine during 2 weeks in late March, and found that 37 (93%) had some increase in the QTc interval, including 14 patients (36%) with an increase of at least 60 msec, and 7 patients (18%) whose QTc rose to at least 500 msec (JAMA Cardiol. 2020 May. doi: 10.1001/jamacardio.2020.1787). However, none of the 40 patients in this series developed an identified ventricular arrhythmia. All patients in both studies received hydroxychloroquine for at least 1 day, and roughly half the patients in each series also received concurrent azithromycin, another drug that can prolong the QTc interval and that has been frequently used in combination with hydroxychloroquine as an unproven COVID-19 treatment cocktail.
These two reports, as well as prior report from Brazil on COVID-19 patients treated with chloroquine diphosphate (JAMA Netw Open. 2020;3[4]:e208857), “underscore the potential risk associated with widespread use of hydroxychloroquine and the combination of hydroxychloroquine and azithromycin in ambulatory patients with known or suspected COVID-19. Understanding whether this risk is worth taking in the absence of evidence of therapeutic efficacy creates a knowledge gap that needs to be addressed,” wrote Robert O. Bonow, MD, a professor of medicine at Northwestern University in Chicago, and coauthors in an editorial that accompanied the two reports (JAMA Cardiol. 2020 May 4;doi: 10.1001/jamacardio.2020.1782). The editorial cited two recently-begun prospective trials, ORCHID and RECOVERY, that are more systematically assessing the safety and efficacy of hydroxychloroquine treatment in COVID-19 patients.
The findings lend further support to a Safety Communication from the U.S. Food and Drug Administration on April 24 that reminded clinicians that the Emergency Use Authorization for hydroxychloroquine and chloroquine in COVID-19 patients that the FDA issued on March 28 applied to only certain hospitalized patients or those enrolled in clinical trials. The Safety Communication also said that agency was aware of reports of adverse arrhythmia events when COVID-19 patients received these drugs outside a hospital setting as well as uninfected people who had received one of these drugs for preventing infection.
In addition, leaders of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society on April 10 issued a summary of considerations when using hydroxychloroquine and azithromycin to treat COVID-19 patients, and noted that a way to minimized the risk from these drugs is to withhold them from patients with a QTc interval of 500 msec or greater at baseline (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). The summary also highlighted the need for regular ECG monitoring of COVID-19 patients who receive drugs that can prolong the QTc interval, and recommended withdrawing treatment from patients when their QTc exceeds the 500 msec threshold.
None of the authors of the two reports and editorial had relevant commercial disclosures.
The potential for serious arrhythmias from hydroxychloroquine treatment of COVID-19 patients received further documentation from a pair of studies released on May 1, casting further doubt on whether the uncertain benefit from this or related drugs to infected patients is worth the clear risks the agents pose.
A report from 90 confirmed COVID-19 patients treated with hydroxychloroquine at one Boston hospital during March-April 2020 identified a significantly prolonged, corrected QT (QTc) interval of at least 500 msec in 18 patients (20%), which included 10 patients whose QTc rose by at least 60 msec above baseline, and a total of 21 patients (23%) having a notable prolongation (JAMA Cardiol. 2020 May 4. doi: 10.1001/jamacardio.2020.1834). This series included one patient who developed torsades de pointes following treatment with hydroxychloroquine and azithromycin, “which to our knowledge has yet to be reported elsewhere in the literature,” the report said.
The second report, from a single center in Lyon, France, included 40 confirmed COVID-19 patients treated with hydroxychloroquine during 2 weeks in late March, and found that 37 (93%) had some increase in the QTc interval, including 14 patients (36%) with an increase of at least 60 msec, and 7 patients (18%) whose QTc rose to at least 500 msec (JAMA Cardiol. 2020 May. doi: 10.1001/jamacardio.2020.1787). However, none of the 40 patients in this series developed an identified ventricular arrhythmia. All patients in both studies received hydroxychloroquine for at least 1 day, and roughly half the patients in each series also received concurrent azithromycin, another drug that can prolong the QTc interval and that has been frequently used in combination with hydroxychloroquine as an unproven COVID-19 treatment cocktail.
These two reports, as well as prior report from Brazil on COVID-19 patients treated with chloroquine diphosphate (JAMA Netw Open. 2020;3[4]:e208857), “underscore the potential risk associated with widespread use of hydroxychloroquine and the combination of hydroxychloroquine and azithromycin in ambulatory patients with known or suspected COVID-19. Understanding whether this risk is worth taking in the absence of evidence of therapeutic efficacy creates a knowledge gap that needs to be addressed,” wrote Robert O. Bonow, MD, a professor of medicine at Northwestern University in Chicago, and coauthors in an editorial that accompanied the two reports (JAMA Cardiol. 2020 May 4;doi: 10.1001/jamacardio.2020.1782). The editorial cited two recently-begun prospective trials, ORCHID and RECOVERY, that are more systematically assessing the safety and efficacy of hydroxychloroquine treatment in COVID-19 patients.
The findings lend further support to a Safety Communication from the U.S. Food and Drug Administration on April 24 that reminded clinicians that the Emergency Use Authorization for hydroxychloroquine and chloroquine in COVID-19 patients that the FDA issued on March 28 applied to only certain hospitalized patients or those enrolled in clinical trials. The Safety Communication also said that agency was aware of reports of adverse arrhythmia events when COVID-19 patients received these drugs outside a hospital setting as well as uninfected people who had received one of these drugs for preventing infection.
In addition, leaders of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society on April 10 issued a summary of considerations when using hydroxychloroquine and azithromycin to treat COVID-19 patients, and noted that a way to minimized the risk from these drugs is to withhold them from patients with a QTc interval of 500 msec or greater at baseline (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). The summary also highlighted the need for regular ECG monitoring of COVID-19 patients who receive drugs that can prolong the QTc interval, and recommended withdrawing treatment from patients when their QTc exceeds the 500 msec threshold.
None of the authors of the two reports and editorial had relevant commercial disclosures.
FROM JAMA CARDIOLOGY
COVID-19: To have and to hold ... in quarantine
Tips for marriage survival during a pandemic
Most married couples vowed to stay with their partners during sickness and health, but none of us vowed to remain trapped with our loved ones behind the same four walls, all day, every day, for an unknown period of time. We didn’t sign up for this! Some romantics may be titillated by the prospect, while more independent partners may panic at the mere thought of spending all day and night with their loved ones.
Because of the swift implementation of the lifestyle-altering restrictions, couples did not have ample time to mentally and physically prepare. A lack of preparation and loss of control heightens our emotions. It can make couples more susceptible to engage in unhealthy styles of communication and destructive behaviors that are harmful to their relationships.
There are psychological reasons that “absence makes the heart grow fonder.” Distance from your partner is not just a clever way to make your partner appreciate and desire you more. It is human nature to habituate to what is part of your daily life. For instance, when your partner is away from you while on a work trip, you may find the first night or two alone relaxing; but by day 3, you begin to miss your partner’s hugs and kisses, smell, and touch. And after many days apart, you may even miss the incessant nagging that secretly motivates you. Physical distance from our partners essentially gives us the ability to long for and appreciate each other. Our brains are wired to pay more attention to things that are novel and exciting and less interested in what is in our everyday lives.
Separation gives us the ability to miss our partners, while quarantine does the complete opposite.
To avoid contemplating how to murder one’s spouse before quarantine ends, partners can strengthen their relationships by using the strategies I’ve outlined below, which are loosely based on dialectical behavior therapy (DBT). These strategies can be useful for anyone – providers and patients alike – going through these struggles.
Dialectical behavior therapy was developed by psychologist Marsha Linehan PhD, to help regulate emotions for people diagnosed with borderline personality disorder. These skills help to identify thoughts and feelings, to accept one’s inner emotional world and outward behaviors. The idea is that, once you can recognize and accept, then change is possible. The “dialectic” in dialectical behavior therapy implies that one is attempting to find a balance between acceptance and change. All of us can benefit from these skills, especially emotionally volatile couples who are trapped together in quarantine.
Radically accept what is uncertain in your lives
Radical acceptance is a practice used in DBT in situations that are out of our control, such as the COVID-19 pandemic. Radically accept that you and your partner are trapped in quarantine without attempting to place blame on our government, your spouse, your boss, and even yourself. Radical acceptance is exactly what the name implies. Accept your current situation for what it is and not what you hoped it to be.
Accept the unknown and unanswered questions such as when will this quarantine end? Will there be a summer camp? Will I get back to my office this summer? Will my children even return to school in the fall? The acceptance of what is out of your control will ultimately decrease your mental time spent worrying and obsessing about the uncertainties of your post-quarantine life and instead provide you more time to be present with your spouse.
Remain mindful during all communication with your spouse. To stay in the moment, you need to be aware of your bodily reactions to distress and notice when your heart rate increases, breathing becomes more shallow, stomach muscles tighten, and when your thoughts become more negative. Mindfulness skills enable us to use physiological changes in our body to become aware of our emotions. You can use your partner’s nonverbal body language and tone of voice to gauge that person’s emotional reactivity.
The practice of mindfulness leads to an increased emotional intelligence. The goal is to have enough self-awareness and emotional understanding of your partner and enough empathy to know when a conversation is becoming too emotionally charged and to let it go and back off. Mindfulness is not nagging your partner to remember to change the heating unit filters with a reminder of what happened years ago when this wasn’t done promptly – without first checking in to make sure your partner is emotionally ready for this type of conversation.
When we have strong emotions, we are using the more primitive parts of our brain that induce a fight or flight reaction. These emotional reactions overshadow the more advanced prefrontal region of our brain that stores our rational thoughts and reasoning skills, a concept identified by psychologist Daniel Goleman as “emotional hijacking.”
Use distress tolerance skills to deal with negative emotions
Distress tolerance is an individual’s ability to manage feelings in response to stress. Distress tolerance skills are aimed at helping one manage intense emotions without worsening a situation by engaging in behaviors that are destructive and may exacerbate the problem. The goal is to tolerate the stress while with your partner and not respond negatively or in a way that is harmful to the integrity of your relationship.
To prioritize your relationship, this may mean that you choose not to react negatively when your partner makes a passive-aggressive comment on how you spent your day during quarantine since you still have a pile of laundry on your bedroom floor and overflowing dishes in the kitchen sink. A high level of distress tolerance will enable you to not overreact or withdraw from your spouse when flooded with emotions of anger or sadness.
Distraction techniques are a type of distress tolerance skill. You can engage in activities that keep you distracted and require your full attention. When things get heated between you and your spouse during quarantine, try to obtain some distance from each other to cool down and engage in an activity that involves your full concentration.
Many of us have been surprised by our hidden talents that were discovered during the quarantine. Use the time away from your partner to distract yourself with your new passion for writing, baking, organizing, and even your newfound love of balloon artistry. Do an activity that engages your mind and provides you the necessary physical and mental time away from your partner to deescalate. You can always revisit the initial cause of the conflict when both you and your partner are not emotionally charged. You can also distract yourself with self-soothing tactics such as taking a warm bath or a reading good book. Perhaps distract yourself by giving back to others and spending time planning a drive-by surprise party for your sister’s birthday next month. It can be helpful to distract yourself by comparing yourself to others less fortunate than you or a time in your life when you and your partner were struggling much worse than now, to provide perspective. The goal is not to add to your distress but instead, provide yourself a sense of perspective.
Use interpersonal effectiveness skills to establish a healthy relationship
Be gentle in all your communications with your partner, think about your spouse’s perspective, show empathy and interest in what your partner has to say by your verbal communication or body language, such as maintaining eye contact, and offer recognitional cues, such as “uh-huh” and “oh, really.” Avoid communication that is at all invalidating. Never start a sentence with “YOU” while having heated conversations with your spouse; instead, use “I feel” statements. This type of communication avoids the blame game that gets many couples into trouble.
Instead, communicate how you feel while not necessarily blaming your spouse but rather expressing your emotions. This will ultimately lead to less defensive communication from your partner. Remember that not all communication is for the sole purpose of communicating. Much of the time, communication is used as an attempt for one partner to connect with the other partner. Couples may say that they have difficulty with communication when it is not the communication that is the issue but instead the underlying disconnect of the couple.
This disconnect usually manifests while couples are communicating, and therefore, can be misconstrued as solely a communication issue by the couple. When your partner asks you to stop staring at your phone during dinner, it is not necessarily that your spouse is attempting to control you or wants to engage in some deep conversation, but more likely a bid to try to connect with you. Your partner is attempting to tell you that he or she feels disconnected, misses you, and wants to reconnect.
Provide validation and acceptance to your partner
Focus on your partner’s strengths and accept the weaknesses. Accept that your partner is scattered, disorganized, and takes at least 20 minutes to find the phone and keys every morning. Remember that during your courtship days, you found your partner’s flighty attributes to be endearing. Do the same for your strengths and weaknesses.
Accept that the pandemic is unpredictable and that you may need to strengthen your ability to be flexible and more adaptable. This will ultimately lead to feeling less disappointment by your partner and more accepting of shortcomings. Acceptance of your imperfections will improve your sense of worth and confidence and lessen negative emotions, such as guilt, regret, and shame.
Accept the fact that, as similar as we all are, we use different methods to recharge ourselves. In contrast, your spouse needs alone time without distractions to reboot mentally and prepare for the following day. In the pre-pandemic world, if there were a mismatch in what a couple needed to feel rejuvenated, they could independently compensate and search for fulfillment outside of the home. Before stay-at-home orders were rolled out throughout the country, spouses had ample opportunities to spend time away from their partners at work, dinner with friends, or while squeezing in a 7 p.m. yoga sculpt class – barely getting home in time to kiss our children goodnight – with a few minutes to spare to engage in mundane conversation with our partners before our nighttime routine of TV commenced. Unfortunately, COVID-19 has made it very hard for couples to carve out that time for compensatory activities outside of the home.
Remember that you are a team
Remind yourself of the reason why you initially fell in love with your partner. Teammates do not keep score or compete with one another. They support each other when one player is not feeling well, and they make sacrifices for the betterment of the team.
Your marriage vows included “through sickness and health” and now should include “through quarantine.”
Dr. Abraham is a psychiatrist in private practice in Philadelphia. She has no disclosures.
Tips for marriage survival during a pandemic
Tips for marriage survival during a pandemic
Most married couples vowed to stay with their partners during sickness and health, but none of us vowed to remain trapped with our loved ones behind the same four walls, all day, every day, for an unknown period of time. We didn’t sign up for this! Some romantics may be titillated by the prospect, while more independent partners may panic at the mere thought of spending all day and night with their loved ones.
Because of the swift implementation of the lifestyle-altering restrictions, couples did not have ample time to mentally and physically prepare. A lack of preparation and loss of control heightens our emotions. It can make couples more susceptible to engage in unhealthy styles of communication and destructive behaviors that are harmful to their relationships.
There are psychological reasons that “absence makes the heart grow fonder.” Distance from your partner is not just a clever way to make your partner appreciate and desire you more. It is human nature to habituate to what is part of your daily life. For instance, when your partner is away from you while on a work trip, you may find the first night or two alone relaxing; but by day 3, you begin to miss your partner’s hugs and kisses, smell, and touch. And after many days apart, you may even miss the incessant nagging that secretly motivates you. Physical distance from our partners essentially gives us the ability to long for and appreciate each other. Our brains are wired to pay more attention to things that are novel and exciting and less interested in what is in our everyday lives.
Separation gives us the ability to miss our partners, while quarantine does the complete opposite.
To avoid contemplating how to murder one’s spouse before quarantine ends, partners can strengthen their relationships by using the strategies I’ve outlined below, which are loosely based on dialectical behavior therapy (DBT). These strategies can be useful for anyone – providers and patients alike – going through these struggles.
Dialectical behavior therapy was developed by psychologist Marsha Linehan PhD, to help regulate emotions for people diagnosed with borderline personality disorder. These skills help to identify thoughts and feelings, to accept one’s inner emotional world and outward behaviors. The idea is that, once you can recognize and accept, then change is possible. The “dialectic” in dialectical behavior therapy implies that one is attempting to find a balance between acceptance and change. All of us can benefit from these skills, especially emotionally volatile couples who are trapped together in quarantine.
Radically accept what is uncertain in your lives
Radical acceptance is a practice used in DBT in situations that are out of our control, such as the COVID-19 pandemic. Radically accept that you and your partner are trapped in quarantine without attempting to place blame on our government, your spouse, your boss, and even yourself. Radical acceptance is exactly what the name implies. Accept your current situation for what it is and not what you hoped it to be.
Accept the unknown and unanswered questions such as when will this quarantine end? Will there be a summer camp? Will I get back to my office this summer? Will my children even return to school in the fall? The acceptance of what is out of your control will ultimately decrease your mental time spent worrying and obsessing about the uncertainties of your post-quarantine life and instead provide you more time to be present with your spouse.
Remain mindful during all communication with your spouse. To stay in the moment, you need to be aware of your bodily reactions to distress and notice when your heart rate increases, breathing becomes more shallow, stomach muscles tighten, and when your thoughts become more negative. Mindfulness skills enable us to use physiological changes in our body to become aware of our emotions. You can use your partner’s nonverbal body language and tone of voice to gauge that person’s emotional reactivity.
The practice of mindfulness leads to an increased emotional intelligence. The goal is to have enough self-awareness and emotional understanding of your partner and enough empathy to know when a conversation is becoming too emotionally charged and to let it go and back off. Mindfulness is not nagging your partner to remember to change the heating unit filters with a reminder of what happened years ago when this wasn’t done promptly – without first checking in to make sure your partner is emotionally ready for this type of conversation.
When we have strong emotions, we are using the more primitive parts of our brain that induce a fight or flight reaction. These emotional reactions overshadow the more advanced prefrontal region of our brain that stores our rational thoughts and reasoning skills, a concept identified by psychologist Daniel Goleman as “emotional hijacking.”
Use distress tolerance skills to deal with negative emotions
Distress tolerance is an individual’s ability to manage feelings in response to stress. Distress tolerance skills are aimed at helping one manage intense emotions without worsening a situation by engaging in behaviors that are destructive and may exacerbate the problem. The goal is to tolerate the stress while with your partner and not respond negatively or in a way that is harmful to the integrity of your relationship.
To prioritize your relationship, this may mean that you choose not to react negatively when your partner makes a passive-aggressive comment on how you spent your day during quarantine since you still have a pile of laundry on your bedroom floor and overflowing dishes in the kitchen sink. A high level of distress tolerance will enable you to not overreact or withdraw from your spouse when flooded with emotions of anger or sadness.
Distraction techniques are a type of distress tolerance skill. You can engage in activities that keep you distracted and require your full attention. When things get heated between you and your spouse during quarantine, try to obtain some distance from each other to cool down and engage in an activity that involves your full concentration.
Many of us have been surprised by our hidden talents that were discovered during the quarantine. Use the time away from your partner to distract yourself with your new passion for writing, baking, organizing, and even your newfound love of balloon artistry. Do an activity that engages your mind and provides you the necessary physical and mental time away from your partner to deescalate. You can always revisit the initial cause of the conflict when both you and your partner are not emotionally charged. You can also distract yourself with self-soothing tactics such as taking a warm bath or a reading good book. Perhaps distract yourself by giving back to others and spending time planning a drive-by surprise party for your sister’s birthday next month. It can be helpful to distract yourself by comparing yourself to others less fortunate than you or a time in your life when you and your partner were struggling much worse than now, to provide perspective. The goal is not to add to your distress but instead, provide yourself a sense of perspective.
Use interpersonal effectiveness skills to establish a healthy relationship
Be gentle in all your communications with your partner, think about your spouse’s perspective, show empathy and interest in what your partner has to say by your verbal communication or body language, such as maintaining eye contact, and offer recognitional cues, such as “uh-huh” and “oh, really.” Avoid communication that is at all invalidating. Never start a sentence with “YOU” while having heated conversations with your spouse; instead, use “I feel” statements. This type of communication avoids the blame game that gets many couples into trouble.
Instead, communicate how you feel while not necessarily blaming your spouse but rather expressing your emotions. This will ultimately lead to less defensive communication from your partner. Remember that not all communication is for the sole purpose of communicating. Much of the time, communication is used as an attempt for one partner to connect with the other partner. Couples may say that they have difficulty with communication when it is not the communication that is the issue but instead the underlying disconnect of the couple.
This disconnect usually manifests while couples are communicating, and therefore, can be misconstrued as solely a communication issue by the couple. When your partner asks you to stop staring at your phone during dinner, it is not necessarily that your spouse is attempting to control you or wants to engage in some deep conversation, but more likely a bid to try to connect with you. Your partner is attempting to tell you that he or she feels disconnected, misses you, and wants to reconnect.
Provide validation and acceptance to your partner
Focus on your partner’s strengths and accept the weaknesses. Accept that your partner is scattered, disorganized, and takes at least 20 minutes to find the phone and keys every morning. Remember that during your courtship days, you found your partner’s flighty attributes to be endearing. Do the same for your strengths and weaknesses.
Accept that the pandemic is unpredictable and that you may need to strengthen your ability to be flexible and more adaptable. This will ultimately lead to feeling less disappointment by your partner and more accepting of shortcomings. Acceptance of your imperfections will improve your sense of worth and confidence and lessen negative emotions, such as guilt, regret, and shame.
Accept the fact that, as similar as we all are, we use different methods to recharge ourselves. In contrast, your spouse needs alone time without distractions to reboot mentally and prepare for the following day. In the pre-pandemic world, if there were a mismatch in what a couple needed to feel rejuvenated, they could independently compensate and search for fulfillment outside of the home. Before stay-at-home orders were rolled out throughout the country, spouses had ample opportunities to spend time away from their partners at work, dinner with friends, or while squeezing in a 7 p.m. yoga sculpt class – barely getting home in time to kiss our children goodnight – with a few minutes to spare to engage in mundane conversation with our partners before our nighttime routine of TV commenced. Unfortunately, COVID-19 has made it very hard for couples to carve out that time for compensatory activities outside of the home.
Remember that you are a team
Remind yourself of the reason why you initially fell in love with your partner. Teammates do not keep score or compete with one another. They support each other when one player is not feeling well, and they make sacrifices for the betterment of the team.
Your marriage vows included “through sickness and health” and now should include “through quarantine.”
Dr. Abraham is a psychiatrist in private practice in Philadelphia. She has no disclosures.
Most married couples vowed to stay with their partners during sickness and health, but none of us vowed to remain trapped with our loved ones behind the same four walls, all day, every day, for an unknown period of time. We didn’t sign up for this! Some romantics may be titillated by the prospect, while more independent partners may panic at the mere thought of spending all day and night with their loved ones.
Because of the swift implementation of the lifestyle-altering restrictions, couples did not have ample time to mentally and physically prepare. A lack of preparation and loss of control heightens our emotions. It can make couples more susceptible to engage in unhealthy styles of communication and destructive behaviors that are harmful to their relationships.
There are psychological reasons that “absence makes the heart grow fonder.” Distance from your partner is not just a clever way to make your partner appreciate and desire you more. It is human nature to habituate to what is part of your daily life. For instance, when your partner is away from you while on a work trip, you may find the first night or two alone relaxing; but by day 3, you begin to miss your partner’s hugs and kisses, smell, and touch. And after many days apart, you may even miss the incessant nagging that secretly motivates you. Physical distance from our partners essentially gives us the ability to long for and appreciate each other. Our brains are wired to pay more attention to things that are novel and exciting and less interested in what is in our everyday lives.
Separation gives us the ability to miss our partners, while quarantine does the complete opposite.
To avoid contemplating how to murder one’s spouse before quarantine ends, partners can strengthen their relationships by using the strategies I’ve outlined below, which are loosely based on dialectical behavior therapy (DBT). These strategies can be useful for anyone – providers and patients alike – going through these struggles.
Dialectical behavior therapy was developed by psychologist Marsha Linehan PhD, to help regulate emotions for people diagnosed with borderline personality disorder. These skills help to identify thoughts and feelings, to accept one’s inner emotional world and outward behaviors. The idea is that, once you can recognize and accept, then change is possible. The “dialectic” in dialectical behavior therapy implies that one is attempting to find a balance between acceptance and change. All of us can benefit from these skills, especially emotionally volatile couples who are trapped together in quarantine.
Radically accept what is uncertain in your lives
Radical acceptance is a practice used in DBT in situations that are out of our control, such as the COVID-19 pandemic. Radically accept that you and your partner are trapped in quarantine without attempting to place blame on our government, your spouse, your boss, and even yourself. Radical acceptance is exactly what the name implies. Accept your current situation for what it is and not what you hoped it to be.
Accept the unknown and unanswered questions such as when will this quarantine end? Will there be a summer camp? Will I get back to my office this summer? Will my children even return to school in the fall? The acceptance of what is out of your control will ultimately decrease your mental time spent worrying and obsessing about the uncertainties of your post-quarantine life and instead provide you more time to be present with your spouse.
Remain mindful during all communication with your spouse. To stay in the moment, you need to be aware of your bodily reactions to distress and notice when your heart rate increases, breathing becomes more shallow, stomach muscles tighten, and when your thoughts become more negative. Mindfulness skills enable us to use physiological changes in our body to become aware of our emotions. You can use your partner’s nonverbal body language and tone of voice to gauge that person’s emotional reactivity.
The practice of mindfulness leads to an increased emotional intelligence. The goal is to have enough self-awareness and emotional understanding of your partner and enough empathy to know when a conversation is becoming too emotionally charged and to let it go and back off. Mindfulness is not nagging your partner to remember to change the heating unit filters with a reminder of what happened years ago when this wasn’t done promptly – without first checking in to make sure your partner is emotionally ready for this type of conversation.
When we have strong emotions, we are using the more primitive parts of our brain that induce a fight or flight reaction. These emotional reactions overshadow the more advanced prefrontal region of our brain that stores our rational thoughts and reasoning skills, a concept identified by psychologist Daniel Goleman as “emotional hijacking.”
Use distress tolerance skills to deal with negative emotions
Distress tolerance is an individual’s ability to manage feelings in response to stress. Distress tolerance skills are aimed at helping one manage intense emotions without worsening a situation by engaging in behaviors that are destructive and may exacerbate the problem. The goal is to tolerate the stress while with your partner and not respond negatively or in a way that is harmful to the integrity of your relationship.
To prioritize your relationship, this may mean that you choose not to react negatively when your partner makes a passive-aggressive comment on how you spent your day during quarantine since you still have a pile of laundry on your bedroom floor and overflowing dishes in the kitchen sink. A high level of distress tolerance will enable you to not overreact or withdraw from your spouse when flooded with emotions of anger or sadness.
Distraction techniques are a type of distress tolerance skill. You can engage in activities that keep you distracted and require your full attention. When things get heated between you and your spouse during quarantine, try to obtain some distance from each other to cool down and engage in an activity that involves your full concentration.
Many of us have been surprised by our hidden talents that were discovered during the quarantine. Use the time away from your partner to distract yourself with your new passion for writing, baking, organizing, and even your newfound love of balloon artistry. Do an activity that engages your mind and provides you the necessary physical and mental time away from your partner to deescalate. You can always revisit the initial cause of the conflict when both you and your partner are not emotionally charged. You can also distract yourself with self-soothing tactics such as taking a warm bath or a reading good book. Perhaps distract yourself by giving back to others and spending time planning a drive-by surprise party for your sister’s birthday next month. It can be helpful to distract yourself by comparing yourself to others less fortunate than you or a time in your life when you and your partner were struggling much worse than now, to provide perspective. The goal is not to add to your distress but instead, provide yourself a sense of perspective.
Use interpersonal effectiveness skills to establish a healthy relationship
Be gentle in all your communications with your partner, think about your spouse’s perspective, show empathy and interest in what your partner has to say by your verbal communication or body language, such as maintaining eye contact, and offer recognitional cues, such as “uh-huh” and “oh, really.” Avoid communication that is at all invalidating. Never start a sentence with “YOU” while having heated conversations with your spouse; instead, use “I feel” statements. This type of communication avoids the blame game that gets many couples into trouble.
Instead, communicate how you feel while not necessarily blaming your spouse but rather expressing your emotions. This will ultimately lead to less defensive communication from your partner. Remember that not all communication is for the sole purpose of communicating. Much of the time, communication is used as an attempt for one partner to connect with the other partner. Couples may say that they have difficulty with communication when it is not the communication that is the issue but instead the underlying disconnect of the couple.
This disconnect usually manifests while couples are communicating, and therefore, can be misconstrued as solely a communication issue by the couple. When your partner asks you to stop staring at your phone during dinner, it is not necessarily that your spouse is attempting to control you or wants to engage in some deep conversation, but more likely a bid to try to connect with you. Your partner is attempting to tell you that he or she feels disconnected, misses you, and wants to reconnect.
Provide validation and acceptance to your partner
Focus on your partner’s strengths and accept the weaknesses. Accept that your partner is scattered, disorganized, and takes at least 20 minutes to find the phone and keys every morning. Remember that during your courtship days, you found your partner’s flighty attributes to be endearing. Do the same for your strengths and weaknesses.
Accept that the pandemic is unpredictable and that you may need to strengthen your ability to be flexible and more adaptable. This will ultimately lead to feeling less disappointment by your partner and more accepting of shortcomings. Acceptance of your imperfections will improve your sense of worth and confidence and lessen negative emotions, such as guilt, regret, and shame.
Accept the fact that, as similar as we all are, we use different methods to recharge ourselves. In contrast, your spouse needs alone time without distractions to reboot mentally and prepare for the following day. In the pre-pandemic world, if there were a mismatch in what a couple needed to feel rejuvenated, they could independently compensate and search for fulfillment outside of the home. Before stay-at-home orders were rolled out throughout the country, spouses had ample opportunities to spend time away from their partners at work, dinner with friends, or while squeezing in a 7 p.m. yoga sculpt class – barely getting home in time to kiss our children goodnight – with a few minutes to spare to engage in mundane conversation with our partners before our nighttime routine of TV commenced. Unfortunately, COVID-19 has made it very hard for couples to carve out that time for compensatory activities outside of the home.
Remember that you are a team
Remind yourself of the reason why you initially fell in love with your partner. Teammates do not keep score or compete with one another. They support each other when one player is not feeling well, and they make sacrifices for the betterment of the team.
Your marriage vows included “through sickness and health” and now should include “through quarantine.”
Dr. Abraham is a psychiatrist in private practice in Philadelphia. She has no disclosures.
FDA authorizes emergency use of remdesivir for COVID-19
The investigational antiviral drug, manufactured by Gilead Sciences Inc., was shown in a preliminary analysis of a National Institutes of Health (NIH) clinical trial to shorten recovery time in some patients, according to information presented during a White House press conference earlier this week. However, the results of the trial have not been published and little is known about how safe and effective it is in treating people in the hospital with COVID-19.
The emergency use authorization (EUA) designation means remdesivir can be distributed in the United States and administered intravenously by healthcare providers, as appropriate to treat severe disease. Those with severe disease, the FDA said in a press release, are patients with low blood oxygen levels or those who need oxygen therapy or more intensive support such as a mechanical ventilator.
“There’s tremendous interest among all parties to identify and arm ourselves with medicines to combat COVID-19, and through our Coronavirus Treatment Acceleration Program, the FDA is working around-the-clock and using every tool at our disposal to speed these efforts,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
The FDA writes, “Based on evaluation of the emergency use authorization criteria and the scientific evidence available, it was determined that it is reasonable to believe that remdesivir may be effective in treating COVID-19, and that, given there are no adequate, approved, or available alternative treatments, the known and potential benefits to treat this serious or life-threatening virus currently outweigh the known and potential risks of the drug’s use.”
The drug must be administered intravenously and the optimal dosing and duration are not yet known, the company said in a press release issued May 1.
In addition, Gilead advises that infusion-related reactions and liver transaminase elevations have been seen in patients treated with the drug.
“If signs and symptoms of a clinically significant infusion reaction occur, immediately discontinue administration of remdesivir and initiate appropriate treatment. Patients should have appropriate clinical and laboratory monitoring to aid in early detection of any potential adverse events. Monitor renal and hepatic function prior to initiating and daily during therapy with remdesivir; additionally monitor serum chemistries and hematology daily during therapy,” the company said.
Before granting the emergency use authorization, the FDA had allowed for study of the drug in clinical trials, as well as expanded access use for individual patients and through a multipatient expanded access program coordinated by Gilead.
“The EUA will be effective until the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biologics for prevention and treatment of COVID-19 is terminated and may be revised or revoked if it is determined the EUA no longer meets the statutory criteria for issuance,” the FDA said.
This article first appeared on Medscape.com.
The investigational antiviral drug, manufactured by Gilead Sciences Inc., was shown in a preliminary analysis of a National Institutes of Health (NIH) clinical trial to shorten recovery time in some patients, according to information presented during a White House press conference earlier this week. However, the results of the trial have not been published and little is known about how safe and effective it is in treating people in the hospital with COVID-19.
The emergency use authorization (EUA) designation means remdesivir can be distributed in the United States and administered intravenously by healthcare providers, as appropriate to treat severe disease. Those with severe disease, the FDA said in a press release, are patients with low blood oxygen levels or those who need oxygen therapy or more intensive support such as a mechanical ventilator.
“There’s tremendous interest among all parties to identify and arm ourselves with medicines to combat COVID-19, and through our Coronavirus Treatment Acceleration Program, the FDA is working around-the-clock and using every tool at our disposal to speed these efforts,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
The FDA writes, “Based on evaluation of the emergency use authorization criteria and the scientific evidence available, it was determined that it is reasonable to believe that remdesivir may be effective in treating COVID-19, and that, given there are no adequate, approved, or available alternative treatments, the known and potential benefits to treat this serious or life-threatening virus currently outweigh the known and potential risks of the drug’s use.”
The drug must be administered intravenously and the optimal dosing and duration are not yet known, the company said in a press release issued May 1.
In addition, Gilead advises that infusion-related reactions and liver transaminase elevations have been seen in patients treated with the drug.
“If signs and symptoms of a clinically significant infusion reaction occur, immediately discontinue administration of remdesivir and initiate appropriate treatment. Patients should have appropriate clinical and laboratory monitoring to aid in early detection of any potential adverse events. Monitor renal and hepatic function prior to initiating and daily during therapy with remdesivir; additionally monitor serum chemistries and hematology daily during therapy,” the company said.
Before granting the emergency use authorization, the FDA had allowed for study of the drug in clinical trials, as well as expanded access use for individual patients and through a multipatient expanded access program coordinated by Gilead.
“The EUA will be effective until the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biologics for prevention and treatment of COVID-19 is terminated and may be revised or revoked if it is determined the EUA no longer meets the statutory criteria for issuance,” the FDA said.
This article first appeared on Medscape.com.
The investigational antiviral drug, manufactured by Gilead Sciences Inc., was shown in a preliminary analysis of a National Institutes of Health (NIH) clinical trial to shorten recovery time in some patients, according to information presented during a White House press conference earlier this week. However, the results of the trial have not been published and little is known about how safe and effective it is in treating people in the hospital with COVID-19.
The emergency use authorization (EUA) designation means remdesivir can be distributed in the United States and administered intravenously by healthcare providers, as appropriate to treat severe disease. Those with severe disease, the FDA said in a press release, are patients with low blood oxygen levels or those who need oxygen therapy or more intensive support such as a mechanical ventilator.
“There’s tremendous interest among all parties to identify and arm ourselves with medicines to combat COVID-19, and through our Coronavirus Treatment Acceleration Program, the FDA is working around-the-clock and using every tool at our disposal to speed these efforts,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
The FDA writes, “Based on evaluation of the emergency use authorization criteria and the scientific evidence available, it was determined that it is reasonable to believe that remdesivir may be effective in treating COVID-19, and that, given there are no adequate, approved, or available alternative treatments, the known and potential benefits to treat this serious or life-threatening virus currently outweigh the known and potential risks of the drug’s use.”
The drug must be administered intravenously and the optimal dosing and duration are not yet known, the company said in a press release issued May 1.
In addition, Gilead advises that infusion-related reactions and liver transaminase elevations have been seen in patients treated with the drug.
“If signs and symptoms of a clinically significant infusion reaction occur, immediately discontinue administration of remdesivir and initiate appropriate treatment. Patients should have appropriate clinical and laboratory monitoring to aid in early detection of any potential adverse events. Monitor renal and hepatic function prior to initiating and daily during therapy with remdesivir; additionally monitor serum chemistries and hematology daily during therapy,” the company said.
Before granting the emergency use authorization, the FDA had allowed for study of the drug in clinical trials, as well as expanded access use for individual patients and through a multipatient expanded access program coordinated by Gilead.
“The EUA will be effective until the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biologics for prevention and treatment of COVID-19 is terminated and may be revised or revoked if it is determined the EUA no longer meets the statutory criteria for issuance,” the FDA said.
This article first appeared on Medscape.com.
CMS hikes telephone visit payments during pandemic
Physicians who are conducting telephone visits during the COVID-19 pandemic will be paid at a higher rate, more closely aligning the rates with payments for face-to-face visits.
On April 30, officials at the Centers for Medicare & Medicaid Services announced the temporary telephone visit rate change and expanded the scope of services that are eligible telephone visits to include many behavioral health and patient education services.
Rates for telephone visits will jump from $14-$41 per visit to about $46-$110. The pay increase is retroactive to March 1, 2020.
The move was welcomed by the American College of Physicians, but the organization said more needs to be done in order help maintain the financial stability of physician practices.
“ACP has repeatedly requested this change from CMS as the country has been dealing with the COVID-19 national emergency, and we are heartened that they have heard our concerns,” ACP President Jacqueline Fincher, MD, said in a statement. “More still needs to be done to ensure that physician practices are able to remain operational and care for their patients, but this change in payment policy addresses one of the biggest issues facing physicians as they struggle to make up for lost revenue and provide appropriate care to patients.”
CMS also is expanding payment availability for audio-only telemedicine services by waiving the video requirement for certain evaluation and management services. The move is aimed at reaching Medicare beneficiaries who may not have access to video technology or choose not to use it.
“This is a major victory for medicine that will enable physicians to care for their patients, especially their elderly patients with chronic conditions who may not have access to audio-visual technology or high-speed Internet,” Patrice Harris, MD, president of the American Medical Association, said in a statement. “This change will help patients address their health challenges that existed before COVID-19.”
Shawn Martin, senior vice president at the American Academy of Family Physicians, said his group is pleased to see CMS roll out this change and noted that it is especially important for patients with underlying health conditions. “This is the only connectivity they may have with a health care system for their ongoing health care needs.”
Samuel Jones, MD, chair of the Health Affairs Committee at the American College of Cardiology, highlighted the expansion and coverage of audio-only telemedicine appointments as a huge plus for patient access.*
“There was a huge hunger to say, ‘Can we just have improvement in the reimbursement for telephone, which is providing a good service, our patients our asking for it,’ and we were able to get that,” Dr. Jones said in an interview. “It really was, I think, a good thing for patient care.”
Dr. Jones also suggested that the temporary policy be extended after the COVID-19 crisis is over.
“Telemedicine is here to stay,” he said. “But if all of these relaxations suddenly go away with a snap of the finger, or if the reimbursement [is lowered], if all that changes as soon as this emergency declaration is over, we are going to have a hard time.”
The pay increase for telephone services was part of a broader package of increased regulatory flexibility CMS rolled out, including expanding the types of providers who can order a COVID-19 test.
*Correction, 5/5/2020: An earlier version of this story misstated Dr. Samuel Jones' affiliation. He is the chair of the Health Affairs Committee at the American College of Cardiology.
Physicians who are conducting telephone visits during the COVID-19 pandemic will be paid at a higher rate, more closely aligning the rates with payments for face-to-face visits.
On April 30, officials at the Centers for Medicare & Medicaid Services announced the temporary telephone visit rate change and expanded the scope of services that are eligible telephone visits to include many behavioral health and patient education services.
Rates for telephone visits will jump from $14-$41 per visit to about $46-$110. The pay increase is retroactive to March 1, 2020.
The move was welcomed by the American College of Physicians, but the organization said more needs to be done in order help maintain the financial stability of physician practices.
“ACP has repeatedly requested this change from CMS as the country has been dealing with the COVID-19 national emergency, and we are heartened that they have heard our concerns,” ACP President Jacqueline Fincher, MD, said in a statement. “More still needs to be done to ensure that physician practices are able to remain operational and care for their patients, but this change in payment policy addresses one of the biggest issues facing physicians as they struggle to make up for lost revenue and provide appropriate care to patients.”
CMS also is expanding payment availability for audio-only telemedicine services by waiving the video requirement for certain evaluation and management services. The move is aimed at reaching Medicare beneficiaries who may not have access to video technology or choose not to use it.
“This is a major victory for medicine that will enable physicians to care for their patients, especially their elderly patients with chronic conditions who may not have access to audio-visual technology or high-speed Internet,” Patrice Harris, MD, president of the American Medical Association, said in a statement. “This change will help patients address their health challenges that existed before COVID-19.”
Shawn Martin, senior vice president at the American Academy of Family Physicians, said his group is pleased to see CMS roll out this change and noted that it is especially important for patients with underlying health conditions. “This is the only connectivity they may have with a health care system for their ongoing health care needs.”
Samuel Jones, MD, chair of the Health Affairs Committee at the American College of Cardiology, highlighted the expansion and coverage of audio-only telemedicine appointments as a huge plus for patient access.*
“There was a huge hunger to say, ‘Can we just have improvement in the reimbursement for telephone, which is providing a good service, our patients our asking for it,’ and we were able to get that,” Dr. Jones said in an interview. “It really was, I think, a good thing for patient care.”
Dr. Jones also suggested that the temporary policy be extended after the COVID-19 crisis is over.
“Telemedicine is here to stay,” he said. “But if all of these relaxations suddenly go away with a snap of the finger, or if the reimbursement [is lowered], if all that changes as soon as this emergency declaration is over, we are going to have a hard time.”
The pay increase for telephone services was part of a broader package of increased regulatory flexibility CMS rolled out, including expanding the types of providers who can order a COVID-19 test.
*Correction, 5/5/2020: An earlier version of this story misstated Dr. Samuel Jones' affiliation. He is the chair of the Health Affairs Committee at the American College of Cardiology.
Physicians who are conducting telephone visits during the COVID-19 pandemic will be paid at a higher rate, more closely aligning the rates with payments for face-to-face visits.
On April 30, officials at the Centers for Medicare & Medicaid Services announced the temporary telephone visit rate change and expanded the scope of services that are eligible telephone visits to include many behavioral health and patient education services.
Rates for telephone visits will jump from $14-$41 per visit to about $46-$110. The pay increase is retroactive to March 1, 2020.
The move was welcomed by the American College of Physicians, but the organization said more needs to be done in order help maintain the financial stability of physician practices.
“ACP has repeatedly requested this change from CMS as the country has been dealing with the COVID-19 national emergency, and we are heartened that they have heard our concerns,” ACP President Jacqueline Fincher, MD, said in a statement. “More still needs to be done to ensure that physician practices are able to remain operational and care for their patients, but this change in payment policy addresses one of the biggest issues facing physicians as they struggle to make up for lost revenue and provide appropriate care to patients.”
CMS also is expanding payment availability for audio-only telemedicine services by waiving the video requirement for certain evaluation and management services. The move is aimed at reaching Medicare beneficiaries who may not have access to video technology or choose not to use it.
“This is a major victory for medicine that will enable physicians to care for their patients, especially their elderly patients with chronic conditions who may not have access to audio-visual technology or high-speed Internet,” Patrice Harris, MD, president of the American Medical Association, said in a statement. “This change will help patients address their health challenges that existed before COVID-19.”
Shawn Martin, senior vice president at the American Academy of Family Physicians, said his group is pleased to see CMS roll out this change and noted that it is especially important for patients with underlying health conditions. “This is the only connectivity they may have with a health care system for their ongoing health care needs.”
Samuel Jones, MD, chair of the Health Affairs Committee at the American College of Cardiology, highlighted the expansion and coverage of audio-only telemedicine appointments as a huge plus for patient access.*
“There was a huge hunger to say, ‘Can we just have improvement in the reimbursement for telephone, which is providing a good service, our patients our asking for it,’ and we were able to get that,” Dr. Jones said in an interview. “It really was, I think, a good thing for patient care.”
Dr. Jones also suggested that the temporary policy be extended after the COVID-19 crisis is over.
“Telemedicine is here to stay,” he said. “But if all of these relaxations suddenly go away with a snap of the finger, or if the reimbursement [is lowered], if all that changes as soon as this emergency declaration is over, we are going to have a hard time.”
The pay increase for telephone services was part of a broader package of increased regulatory flexibility CMS rolled out, including expanding the types of providers who can order a COVID-19 test.
*Correction, 5/5/2020: An earlier version of this story misstated Dr. Samuel Jones' affiliation. He is the chair of the Health Affairs Committee at the American College of Cardiology.











