User login
Bleeding esophageal varices: Who should receive a shunt?
A transjugular intrahepatic portosystemic shunt (TIPS) has been shown in randomized controlled trials to be effective for:
- Secondary prevention of variceal bleeding
- Controlling refractory ascites in patients with liver cirrhosis.
In addition, findings from retrospective case series have suggested that it helps in cases of:
- Acute variceal bleeding refractory to endoscopic therapy
- Gastropathy due to portal hypertension
- Bleeding gastric varices
- Refractory hepatic hydrothorax
- Hepatorenal syndrome
- Budd-Chiari syndrome
- Veno-occlusive disease
- Hepatopulmonary syndrome.
Here, we discuss the indications for a TIPS in cirrhotic patients with esophageal variceal bleeding.
CIRRHOSIS CAN LEAD TO PORTAL HYPERTENSION, BLEEDING
Cirrhosis of the liver alters the hepatic architecture. Development of regenerating nodules and deposition of connective tissue between these nodules increase the resistance to portal blood flow, which can lead to portal hypertension.1
Esophageal variceal bleeding is a complication of portal hypertension and a major cause of death in patients with liver cirrhosis. Combined treatment with vasoactive drugs, prophylactic antibiotics, and endoscopic band ligation is the standard of care for patients with acute bleeding. However, this treatment fails in about 10% to 15% of these patients. A TIPS creates a connection between the portal and hepatic veins, resulting in portal decompression and homeostasis.2
PRE-TIPS EVALUATION
Patients being considered for a TIPS should be medically assessed before the procedure. The workup should include the following:
- Routine blood tests, including blood type and screen (indirect Coombs test), complete blood cell count, basic metabolic panel, liver function tests, prothrombin time, and partial thromboplastin time
- Doppler ultrasonography of the liver to ensure that the portal and hepatic veins are patent
- Echocardiography to assess pulmonary arterial pressure and right-side heart function
- The hepatic venous pressure gradient, which is measured at the time of TIPS placement, reflects the degree of portal hypertension. A hepatic vein is catheterized, and the right atrial pressure or the free hepatic venous pressure is subtracted from the wedged hepatic venous pressure. The gradient is normally 1 to 5 mm Hg. A gradient greater than 5 mm Hg indicates portal hypertension, and esophageal varices may start to bleed when the gradient is greater than 12 mm Hg. The goal of TIPS placement is to reduce the gradient to less than 12 mm Hg, or at least by 50%.
Heart failure is a contraindication
Pulmonary hypertension may follow TIPS placement because the shunt increases venous return to the heart. Additionally, systemic vascular resistance decreases in patients who have a shunt. This further worsens the hyperdynamic circulatory state already present in patients with cirrhosis. Cardiac output increases in response to these changes. When the heart’s ability to handle this “volume overload” is exceeded, pulmonary venous pressures rise, with increasing ventilation-perfusion mismatch, hypoxia, and pulmonary vasoconstriction; pulmonary edema may ensue.
Congestive heart failure, severe tricuspid regurgitation, and severe pulmonary hypertension (mean pulmonary pressures > 45 mm Hg) are therefore considered absolute contraindications to TIPS placement.3,4 This is why echocardiography is recommended to assess pulmonary pressure along with the size and function of the right side of the heart before proceeding with TIPS insertion.
Other considerations
TIPS insertion is not recommended in patients with active hepatic encephalopathy, which should be adequately controlled before insertion of a TIPS. This can be achieved with lactulose and rifaximin. Lactulose is a laxative; the recommended target is 3 to 4 bowel movements daily. Rifaximin is a poorly absorbed antibiotic that has a wide spectrum of coverage, affecting gram-negative and gram-positive aerobes and anaerobes. It wipes out the gut bacteria and so decreases the production of ammonia by the gut.
Paracentesis is recommended before TIPS placement if a large volume of ascites is present. Draining the fluid allows the liver to drop down and makes it easier to access the portal vein from the hepatic vein.
WHEN TO CONSIDER A TIPS IN ESOPHAGEAL VARICEAL BLEEDING
Acute bleeding refractory to endoscopic therapy
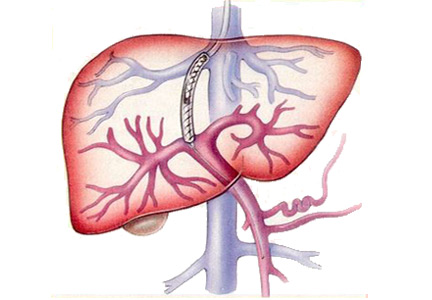
A TIPS remains the only choice to control acute variceal bleeding refractory to medical and endoscopic therapy (Figure 1), with a success rate of 90% to 100%.5 The urgency of TIPS placement is an independent predictor of early mortality.
Esophageal variceal rebleeding
Once varices bleed, the risk of rebleeding is higher than 50%, and rebleeding is associated with a high mortality rate. TIPS should be considered if nonselective beta-blockers and surveillance with upper endoscopy and banding fail to prevent rebleeding, with many studies showing a TIPS to be superior to pharmacologic and endoscopic therapies.6
A meta-analysis in 1999 by Papatheodoridis et al6 found that variceal rebleeding was significantly more frequent with endoscopic therapies, at 47% vs 19% with a TIPS, but the incidence of hepatic encephalopathy was higher with TIPS (34% vs 19%; P < .001), and there was no difference in mortality rates.
Hepatic encephalopathy occurs in 15% to 25% of patients after TIPS procedures. Risk factors include advanced age, poor renal function, and a history of hepatic encephalopathy. Hepatic encephalopathy can be managed with lactulose or rifaximin, or both (see above). Narcotics, antihistamines, and benzodiazepines should be avoided. In rare cases (5%) when hepatic encephalopathy is refractory to medical therapy, liver transplant should be considered.
A surgical distal splenorenal shunt is another option for patients with refractory or recurrent variceal bleeding. In a large randomized controlled trial,7 140 cirrhotic patients with recurrent variceal bleeding were randomized to receive either a distal splenorenal shunt or a TIPS. At a mean follow-up of 48 months, there was no difference in the rates of rebleeding between the two groups (5.5% with a surgical shunt vs 10.5% with a TIPS, P = .29) or in hepatic encephalopathy (50% in both groups). Survival rates were comparable between the two groups at 2 years (81% with a surgical shunt vs 88% with a TIPS) and 5 years (62% vs 61%).
Early use of TIPS after first variceal bleeding
In a 2010 randomized controlled trial,8 63 patients with cirrhosis (Child-Pugh class B or C) and acute variceal bleeding who had received standard medical and endoscopic therapy were randomized to receive either a TIPS within 72 hours of admission or long-term conservative treatment with nonselective beta-blockers and endoscopic band ligation. The 1-year actuarial probability of remaining free of rebleeding or failure to control bleeding was 50% in the conservative treatment group vs 97% in the early-TIPS group (P < .001). The 1-year actuarial survival rate was 61% in the conservative treatment group vs 86% in the early-TIPS group (P < .001).
The authors8 concluded that early use of TIPS in patients with cirrhosis and Child-Pugh scores of 7 to 13 who were hospitalized for acute variceal bleeding was associated with significant reductions in rates of treatment failure and mortality.
- Brenner D, Rippe RA. Pathogenesis of hepatic fibrosis. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 4th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
- Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol 2011; 9:936–946.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology 1994; 19:129–132.
- Rodríguez-Laiz JM, Bañares R, Echenagusia A, et al. Effects of transjugular intrahepatic portasystemic shunt (TIPS) on splanchnic and systemic hemodynamics, and hepatic function in patients with portal hypertension. Preliminary results. Dig Dis Sci 1995; 40:2121–2127.
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology 1999; 30:612–622.
- Henderson JM, Boyer TD, Kutner MH, et al; DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systemic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006; 130:1643–1651.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
A transjugular intrahepatic portosystemic shunt (TIPS) has been shown in randomized controlled trials to be effective for:
- Secondary prevention of variceal bleeding
- Controlling refractory ascites in patients with liver cirrhosis.
In addition, findings from retrospective case series have suggested that it helps in cases of:
- Acute variceal bleeding refractory to endoscopic therapy
- Gastropathy due to portal hypertension
- Bleeding gastric varices
- Refractory hepatic hydrothorax
- Hepatorenal syndrome
- Budd-Chiari syndrome
- Veno-occlusive disease
- Hepatopulmonary syndrome.
Here, we discuss the indications for a TIPS in cirrhotic patients with esophageal variceal bleeding.
CIRRHOSIS CAN LEAD TO PORTAL HYPERTENSION, BLEEDING
Cirrhosis of the liver alters the hepatic architecture. Development of regenerating nodules and deposition of connective tissue between these nodules increase the resistance to portal blood flow, which can lead to portal hypertension.1
Esophageal variceal bleeding is a complication of portal hypertension and a major cause of death in patients with liver cirrhosis. Combined treatment with vasoactive drugs, prophylactic antibiotics, and endoscopic band ligation is the standard of care for patients with acute bleeding. However, this treatment fails in about 10% to 15% of these patients. A TIPS creates a connection between the portal and hepatic veins, resulting in portal decompression and homeostasis.2
PRE-TIPS EVALUATION
Patients being considered for a TIPS should be medically assessed before the procedure. The workup should include the following:
- Routine blood tests, including blood type and screen (indirect Coombs test), complete blood cell count, basic metabolic panel, liver function tests, prothrombin time, and partial thromboplastin time
- Doppler ultrasonography of the liver to ensure that the portal and hepatic veins are patent
- Echocardiography to assess pulmonary arterial pressure and right-side heart function
- The hepatic venous pressure gradient, which is measured at the time of TIPS placement, reflects the degree of portal hypertension. A hepatic vein is catheterized, and the right atrial pressure or the free hepatic venous pressure is subtracted from the wedged hepatic venous pressure. The gradient is normally 1 to 5 mm Hg. A gradient greater than 5 mm Hg indicates portal hypertension, and esophageal varices may start to bleed when the gradient is greater than 12 mm Hg. The goal of TIPS placement is to reduce the gradient to less than 12 mm Hg, or at least by 50%.
Heart failure is a contraindication
Pulmonary hypertension may follow TIPS placement because the shunt increases venous return to the heart. Additionally, systemic vascular resistance decreases in patients who have a shunt. This further worsens the hyperdynamic circulatory state already present in patients with cirrhosis. Cardiac output increases in response to these changes. When the heart’s ability to handle this “volume overload” is exceeded, pulmonary venous pressures rise, with increasing ventilation-perfusion mismatch, hypoxia, and pulmonary vasoconstriction; pulmonary edema may ensue.
Congestive heart failure, severe tricuspid regurgitation, and severe pulmonary hypertension (mean pulmonary pressures > 45 mm Hg) are therefore considered absolute contraindications to TIPS placement.3,4 This is why echocardiography is recommended to assess pulmonary pressure along with the size and function of the right side of the heart before proceeding with TIPS insertion.
Other considerations
TIPS insertion is not recommended in patients with active hepatic encephalopathy, which should be adequately controlled before insertion of a TIPS. This can be achieved with lactulose and rifaximin. Lactulose is a laxative; the recommended target is 3 to 4 bowel movements daily. Rifaximin is a poorly absorbed antibiotic that has a wide spectrum of coverage, affecting gram-negative and gram-positive aerobes and anaerobes. It wipes out the gut bacteria and so decreases the production of ammonia by the gut.
Paracentesis is recommended before TIPS placement if a large volume of ascites is present. Draining the fluid allows the liver to drop down and makes it easier to access the portal vein from the hepatic vein.
WHEN TO CONSIDER A TIPS IN ESOPHAGEAL VARICEAL BLEEDING
Acute bleeding refractory to endoscopic therapy
A TIPS remains the only choice to control acute variceal bleeding refractory to medical and endoscopic therapy (Figure 1), with a success rate of 90% to 100%.5 The urgency of TIPS placement is an independent predictor of early mortality.
Esophageal variceal rebleeding
Once varices bleed, the risk of rebleeding is higher than 50%, and rebleeding is associated with a high mortality rate. TIPS should be considered if nonselective beta-blockers and surveillance with upper endoscopy and banding fail to prevent rebleeding, with many studies showing a TIPS to be superior to pharmacologic and endoscopic therapies.6
A meta-analysis in 1999 by Papatheodoridis et al6 found that variceal rebleeding was significantly more frequent with endoscopic therapies, at 47% vs 19% with a TIPS, but the incidence of hepatic encephalopathy was higher with TIPS (34% vs 19%; P < .001), and there was no difference in mortality rates.
Hepatic encephalopathy occurs in 15% to 25% of patients after TIPS procedures. Risk factors include advanced age, poor renal function, and a history of hepatic encephalopathy. Hepatic encephalopathy can be managed with lactulose or rifaximin, or both (see above). Narcotics, antihistamines, and benzodiazepines should be avoided. In rare cases (5%) when hepatic encephalopathy is refractory to medical therapy, liver transplant should be considered.
A surgical distal splenorenal shunt is another option for patients with refractory or recurrent variceal bleeding. In a large randomized controlled trial,7 140 cirrhotic patients with recurrent variceal bleeding were randomized to receive either a distal splenorenal shunt or a TIPS. At a mean follow-up of 48 months, there was no difference in the rates of rebleeding between the two groups (5.5% with a surgical shunt vs 10.5% with a TIPS, P = .29) or in hepatic encephalopathy (50% in both groups). Survival rates were comparable between the two groups at 2 years (81% with a surgical shunt vs 88% with a TIPS) and 5 years (62% vs 61%).
Early use of TIPS after first variceal bleeding
In a 2010 randomized controlled trial,8 63 patients with cirrhosis (Child-Pugh class B or C) and acute variceal bleeding who had received standard medical and endoscopic therapy were randomized to receive either a TIPS within 72 hours of admission or long-term conservative treatment with nonselective beta-blockers and endoscopic band ligation. The 1-year actuarial probability of remaining free of rebleeding or failure to control bleeding was 50% in the conservative treatment group vs 97% in the early-TIPS group (P < .001). The 1-year actuarial survival rate was 61% in the conservative treatment group vs 86% in the early-TIPS group (P < .001).
The authors8 concluded that early use of TIPS in patients with cirrhosis and Child-Pugh scores of 7 to 13 who were hospitalized for acute variceal bleeding was associated with significant reductions in rates of treatment failure and mortality.
A transjugular intrahepatic portosystemic shunt (TIPS) has been shown in randomized controlled trials to be effective for:
- Secondary prevention of variceal bleeding
- Controlling refractory ascites in patients with liver cirrhosis.
In addition, findings from retrospective case series have suggested that it helps in cases of:
- Acute variceal bleeding refractory to endoscopic therapy
- Gastropathy due to portal hypertension
- Bleeding gastric varices
- Refractory hepatic hydrothorax
- Hepatorenal syndrome
- Budd-Chiari syndrome
- Veno-occlusive disease
- Hepatopulmonary syndrome.
Here, we discuss the indications for a TIPS in cirrhotic patients with esophageal variceal bleeding.
CIRRHOSIS CAN LEAD TO PORTAL HYPERTENSION, BLEEDING
Cirrhosis of the liver alters the hepatic architecture. Development of regenerating nodules and deposition of connective tissue between these nodules increase the resistance to portal blood flow, which can lead to portal hypertension.1
Esophageal variceal bleeding is a complication of portal hypertension and a major cause of death in patients with liver cirrhosis. Combined treatment with vasoactive drugs, prophylactic antibiotics, and endoscopic band ligation is the standard of care for patients with acute bleeding. However, this treatment fails in about 10% to 15% of these patients. A TIPS creates a connection between the portal and hepatic veins, resulting in portal decompression and homeostasis.2
PRE-TIPS EVALUATION
Patients being considered for a TIPS should be medically assessed before the procedure. The workup should include the following:
- Routine blood tests, including blood type and screen (indirect Coombs test), complete blood cell count, basic metabolic panel, liver function tests, prothrombin time, and partial thromboplastin time
- Doppler ultrasonography of the liver to ensure that the portal and hepatic veins are patent
- Echocardiography to assess pulmonary arterial pressure and right-side heart function
- The hepatic venous pressure gradient, which is measured at the time of TIPS placement, reflects the degree of portal hypertension. A hepatic vein is catheterized, and the right atrial pressure or the free hepatic venous pressure is subtracted from the wedged hepatic venous pressure. The gradient is normally 1 to 5 mm Hg. A gradient greater than 5 mm Hg indicates portal hypertension, and esophageal varices may start to bleed when the gradient is greater than 12 mm Hg. The goal of TIPS placement is to reduce the gradient to less than 12 mm Hg, or at least by 50%.
Heart failure is a contraindication
Pulmonary hypertension may follow TIPS placement because the shunt increases venous return to the heart. Additionally, systemic vascular resistance decreases in patients who have a shunt. This further worsens the hyperdynamic circulatory state already present in patients with cirrhosis. Cardiac output increases in response to these changes. When the heart’s ability to handle this “volume overload” is exceeded, pulmonary venous pressures rise, with increasing ventilation-perfusion mismatch, hypoxia, and pulmonary vasoconstriction; pulmonary edema may ensue.
Congestive heart failure, severe tricuspid regurgitation, and severe pulmonary hypertension (mean pulmonary pressures > 45 mm Hg) are therefore considered absolute contraindications to TIPS placement.3,4 This is why echocardiography is recommended to assess pulmonary pressure along with the size and function of the right side of the heart before proceeding with TIPS insertion.
Other considerations
TIPS insertion is not recommended in patients with active hepatic encephalopathy, which should be adequately controlled before insertion of a TIPS. This can be achieved with lactulose and rifaximin. Lactulose is a laxative; the recommended target is 3 to 4 bowel movements daily. Rifaximin is a poorly absorbed antibiotic that has a wide spectrum of coverage, affecting gram-negative and gram-positive aerobes and anaerobes. It wipes out the gut bacteria and so decreases the production of ammonia by the gut.
Paracentesis is recommended before TIPS placement if a large volume of ascites is present. Draining the fluid allows the liver to drop down and makes it easier to access the portal vein from the hepatic vein.
WHEN TO CONSIDER A TIPS IN ESOPHAGEAL VARICEAL BLEEDING
Acute bleeding refractory to endoscopic therapy
A TIPS remains the only choice to control acute variceal bleeding refractory to medical and endoscopic therapy (Figure 1), with a success rate of 90% to 100%.5 The urgency of TIPS placement is an independent predictor of early mortality.
Esophageal variceal rebleeding
Once varices bleed, the risk of rebleeding is higher than 50%, and rebleeding is associated with a high mortality rate. TIPS should be considered if nonselective beta-blockers and surveillance with upper endoscopy and banding fail to prevent rebleeding, with many studies showing a TIPS to be superior to pharmacologic and endoscopic therapies.6
A meta-analysis in 1999 by Papatheodoridis et al6 found that variceal rebleeding was significantly more frequent with endoscopic therapies, at 47% vs 19% with a TIPS, but the incidence of hepatic encephalopathy was higher with TIPS (34% vs 19%; P < .001), and there was no difference in mortality rates.
Hepatic encephalopathy occurs in 15% to 25% of patients after TIPS procedures. Risk factors include advanced age, poor renal function, and a history of hepatic encephalopathy. Hepatic encephalopathy can be managed with lactulose or rifaximin, or both (see above). Narcotics, antihistamines, and benzodiazepines should be avoided. In rare cases (5%) when hepatic encephalopathy is refractory to medical therapy, liver transplant should be considered.
A surgical distal splenorenal shunt is another option for patients with refractory or recurrent variceal bleeding. In a large randomized controlled trial,7 140 cirrhotic patients with recurrent variceal bleeding were randomized to receive either a distal splenorenal shunt or a TIPS. At a mean follow-up of 48 months, there was no difference in the rates of rebleeding between the two groups (5.5% with a surgical shunt vs 10.5% with a TIPS, P = .29) or in hepatic encephalopathy (50% in both groups). Survival rates were comparable between the two groups at 2 years (81% with a surgical shunt vs 88% with a TIPS) and 5 years (62% vs 61%).
Early use of TIPS after first variceal bleeding
In a 2010 randomized controlled trial,8 63 patients with cirrhosis (Child-Pugh class B or C) and acute variceal bleeding who had received standard medical and endoscopic therapy were randomized to receive either a TIPS within 72 hours of admission or long-term conservative treatment with nonselective beta-blockers and endoscopic band ligation. The 1-year actuarial probability of remaining free of rebleeding or failure to control bleeding was 50% in the conservative treatment group vs 97% in the early-TIPS group (P < .001). The 1-year actuarial survival rate was 61% in the conservative treatment group vs 86% in the early-TIPS group (P < .001).
The authors8 concluded that early use of TIPS in patients with cirrhosis and Child-Pugh scores of 7 to 13 who were hospitalized for acute variceal bleeding was associated with significant reductions in rates of treatment failure and mortality.
- Brenner D, Rippe RA. Pathogenesis of hepatic fibrosis. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 4th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
- Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol 2011; 9:936–946.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology 1994; 19:129–132.
- Rodríguez-Laiz JM, Bañares R, Echenagusia A, et al. Effects of transjugular intrahepatic portasystemic shunt (TIPS) on splanchnic and systemic hemodynamics, and hepatic function in patients with portal hypertension. Preliminary results. Dig Dis Sci 1995; 40:2121–2127.
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology 1999; 30:612–622.
- Henderson JM, Boyer TD, Kutner MH, et al; DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systemic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006; 130:1643–1651.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
- Brenner D, Rippe RA. Pathogenesis of hepatic fibrosis. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 4th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
- Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol 2011; 9:936–946.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology 1994; 19:129–132.
- Rodríguez-Laiz JM, Bañares R, Echenagusia A, et al. Effects of transjugular intrahepatic portasystemic shunt (TIPS) on splanchnic and systemic hemodynamics, and hepatic function in patients with portal hypertension. Preliminary results. Dig Dis Sci 1995; 40:2121–2127.
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology 1999; 30:612–622.
- Henderson JM, Boyer TD, Kutner MH, et al; DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systemic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006; 130:1643–1651.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
When should brain imaging precede lumbar puncture in cases of suspected bacterial meningitis?
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
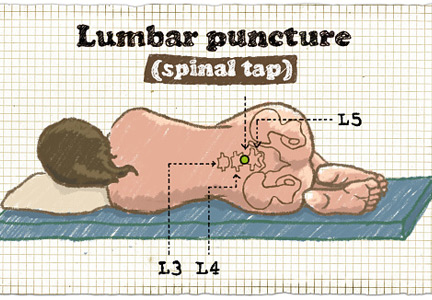
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
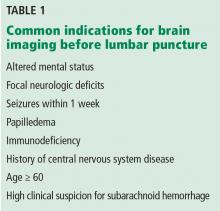
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
What is the role of roflumilast in chronic obstructive pulmonary disease?
Roflumilast has been shown to reduce rates of acute exacerbation in patients with severe chronic obstructive pulmonary disease (COPD), ie, forced expiratory volume in 1 second (FEV1) less than 50% with symptoms of chronic bronchitis and a history of exacerbations.
Roflumilast is a selective phosphodiesterase 4 (PDE4) inhibitor that acts on airway smooth muscle cells and various inflammatory cells. By blocking PDE4, roflumilast raises cyclic adenosine monophosphate levels within these cells, curtailing the inflammatory response.1,2
Roflumilast is not a bronchodilator, although modest improvements in FEV1 have been documented in clinical trials when it was used as maintenance therapy.
TRIALS OF ROFLUMILAST
Several trials have investigated the efficacy of roflumilast in COPD (Table 1).
The RECORD trial
The RECORD trial1 in 2005 was the first large randomized controlled trial of roflumilast in moderate to severe COPD. At a dose of 500 µg orally daily, there was a modest but statistically significant improvement in the postbronchodilator FEV1. There was also improvement in the St. George Respiratory Questionnaire score in the treatment arm, but this was not statistically significant. The study also found a reduction in acute exacerbations of COPD with roflumilast, which was a secondary end point.1
The results of this study spurred interest in roflumilast as well as criticism of the design of the study. First, COPD patients on inhaled maintenance therapy such as an inhaled corticosteroid and long-acting beta-agonist combination or a long-acting muscarinic antagonist had their medications held during the study. Second, the average FEV1 was 54% of predicted, indicative of a study population with less severe disease.1
The RATIO trial
Taking into account the results of the RECORD trial, the RATIO trial3 in 2007 recruited patients with more severe COPD—ie, Global Initiative for Chronic Obstructive Lung Disease (GOLD) class III and IV—and included the rate of acute exacerbations as a primary end point. Maintenance therapy with inhaled corticosteroids was continued in patients already taking them. However, long-acting beta-agonists and long-acting muscarinic antagonist therapies were held.3
Again, roflumilast improved postbronchodilator FEV1 compared with placebo. A reduction in acute exacerbations was seen but was not statistically significant except in subgroup analysis, where a statistically significant reduction in acute exacerbations was noted for patients with very severe (GOLD class IV) COPD.3
Post hoc analysis from the RATIO trial suggested that patients with chronic bronchitis and patients with a history of frequent exacerbations were more likely to respond to roflumilast.2
The EOS and HELIOS trials
In 2009, the results of the EOS and HELIOS trials of roflumilast in patients with severe COPD were published.4 These trials allowed continuation of long-acting beta-agonists and muscarinic antagonists. The prebronchodilator FEV1 improved modestly when roflumilast was added to a long-acting bronchodilator. These studies ran for only 24 weeks, and the rate of acute exacerbations was not a primary end point, although the results did show a trend toward reduction of exacerbations.4
The AURA and HERMES trials
Also in 2009 was the publication of the results of two 52-week placebo-controlled trials (AURA and HERMES) of roflumilast in patients with severe COPD with chronic bronchitis and a history of frequent exacerbations.5 Maintenance therapy with long-acting beta-agonists was continued, whereas inhaled corticosteroids and long-acting muscarinic antagonists were held. Statistically significant improvements in prebronchodilator FEV1 and reduction in the rate of exacerbations were observed in the roflumilast group (17% reduction, 95% confidence interval 8–25, P < .0003).5
The REACT trial
The REACT trial6 randomized 1,945 patients with severe COPD already on maximal recommended combination inhaled corticosteroid and long-acting beta-agonist therapy to receive either roflumilast or placebo. The patients’ ratio of FEV1 to forced vital capacity was less than 70%, their postbronchodilator FEV1 was less than 50%, and they had chronic bronchitis and a history of at least two acute exacerbations during the past year. They had also been on combination therapy for the previous year. Patients who were on long-acting muscarinic-antagonist therapy (70% of the cohort) were included, and continued with their medication.
Patients were followed for 52 weeks. There was a significant reduction in the rate of exacerbations in the roflumilast group vs placebo (0.823 vs 0.959; risk ratio 0.858; 95% confidence interval 0.740–0.995; P = .0424).6 As in previous trials, the roflumilast group showed an improvement in postbronchodilator FEV1. The study also showed a reduction in hospital admissions in the treatment group.6
ADVERSE EFFECTS OF ROFLUMILAST
Roflumilast is known to have adverse effects significant enough to reduce compliance, the most common being diarrhea, weight loss, and nausea.2,6,7 In the REACT trial,6 11% of patients in the roflumilast group vs 5% in the placebo group dropped out of the study because of adverse drug effects. Diarrhea was reported in 10% and weight loss in 9% of patients taking roflumilast. Weight loss has been shown to be reversible upon stopping roflumilast.2 There has been no evidence of increased risk of death or serious adverse events in studies of roflumilast in patients with COPD.2 However, the benefit-to-harm ratio suggests that roflumilast provides a net benefit only in patients at high risk of severe exacerbations.7
- Rabe KF, Bateman ED, O’Donnell DE, Witte S, Bredenbroker D, Bethke TD. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomized controlled trial. Lancet 2005; 366:63–71.
- Field SK. Roflumilast, a novel phosphodiesterase 4 inhibitor, for COPD patients with a history of exacerbations. Clin Med Insights Circ Respir Pulm Med 2011; 5:57–70.
- Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176:154–161.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomized clinical trials. Lancet 2009; 374:695–703.
- Calverley PM, Rabe KF, Goehring U-M, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomized clinical trials. Lancet 2009; 374:684–95.
- Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015; 385:857–866.
- Yu T, Fain K, Boyd CM, et al. Benefits and harms of roflumilast in moderate to severe COPD. Thorax 2014; 69:616–622.
Roflumilast has been shown to reduce rates of acute exacerbation in patients with severe chronic obstructive pulmonary disease (COPD), ie, forced expiratory volume in 1 second (FEV1) less than 50% with symptoms of chronic bronchitis and a history of exacerbations.
Roflumilast is a selective phosphodiesterase 4 (PDE4) inhibitor that acts on airway smooth muscle cells and various inflammatory cells. By blocking PDE4, roflumilast raises cyclic adenosine monophosphate levels within these cells, curtailing the inflammatory response.1,2
Roflumilast is not a bronchodilator, although modest improvements in FEV1 have been documented in clinical trials when it was used as maintenance therapy.
TRIALS OF ROFLUMILAST
Several trials have investigated the efficacy of roflumilast in COPD (Table 1).
The RECORD trial
The RECORD trial1 in 2005 was the first large randomized controlled trial of roflumilast in moderate to severe COPD. At a dose of 500 µg orally daily, there was a modest but statistically significant improvement in the postbronchodilator FEV1. There was also improvement in the St. George Respiratory Questionnaire score in the treatment arm, but this was not statistically significant. The study also found a reduction in acute exacerbations of COPD with roflumilast, which was a secondary end point.1
The results of this study spurred interest in roflumilast as well as criticism of the design of the study. First, COPD patients on inhaled maintenance therapy such as an inhaled corticosteroid and long-acting beta-agonist combination or a long-acting muscarinic antagonist had their medications held during the study. Second, the average FEV1 was 54% of predicted, indicative of a study population with less severe disease.1
The RATIO trial
Taking into account the results of the RECORD trial, the RATIO trial3 in 2007 recruited patients with more severe COPD—ie, Global Initiative for Chronic Obstructive Lung Disease (GOLD) class III and IV—and included the rate of acute exacerbations as a primary end point. Maintenance therapy with inhaled corticosteroids was continued in patients already taking them. However, long-acting beta-agonists and long-acting muscarinic antagonist therapies were held.3
Again, roflumilast improved postbronchodilator FEV1 compared with placebo. A reduction in acute exacerbations was seen but was not statistically significant except in subgroup analysis, where a statistically significant reduction in acute exacerbations was noted for patients with very severe (GOLD class IV) COPD.3
Post hoc analysis from the RATIO trial suggested that patients with chronic bronchitis and patients with a history of frequent exacerbations were more likely to respond to roflumilast.2
The EOS and HELIOS trials
In 2009, the results of the EOS and HELIOS trials of roflumilast in patients with severe COPD were published.4 These trials allowed continuation of long-acting beta-agonists and muscarinic antagonists. The prebronchodilator FEV1 improved modestly when roflumilast was added to a long-acting bronchodilator. These studies ran for only 24 weeks, and the rate of acute exacerbations was not a primary end point, although the results did show a trend toward reduction of exacerbations.4
The AURA and HERMES trials
Also in 2009 was the publication of the results of two 52-week placebo-controlled trials (AURA and HERMES) of roflumilast in patients with severe COPD with chronic bronchitis and a history of frequent exacerbations.5 Maintenance therapy with long-acting beta-agonists was continued, whereas inhaled corticosteroids and long-acting muscarinic antagonists were held. Statistically significant improvements in prebronchodilator FEV1 and reduction in the rate of exacerbations were observed in the roflumilast group (17% reduction, 95% confidence interval 8–25, P < .0003).5
The REACT trial
The REACT trial6 randomized 1,945 patients with severe COPD already on maximal recommended combination inhaled corticosteroid and long-acting beta-agonist therapy to receive either roflumilast or placebo. The patients’ ratio of FEV1 to forced vital capacity was less than 70%, their postbronchodilator FEV1 was less than 50%, and they had chronic bronchitis and a history of at least two acute exacerbations during the past year. They had also been on combination therapy for the previous year. Patients who were on long-acting muscarinic-antagonist therapy (70% of the cohort) were included, and continued with their medication.
Patients were followed for 52 weeks. There was a significant reduction in the rate of exacerbations in the roflumilast group vs placebo (0.823 vs 0.959; risk ratio 0.858; 95% confidence interval 0.740–0.995; P = .0424).6 As in previous trials, the roflumilast group showed an improvement in postbronchodilator FEV1. The study also showed a reduction in hospital admissions in the treatment group.6
ADVERSE EFFECTS OF ROFLUMILAST
Roflumilast is known to have adverse effects significant enough to reduce compliance, the most common being diarrhea, weight loss, and nausea.2,6,7 In the REACT trial,6 11% of patients in the roflumilast group vs 5% in the placebo group dropped out of the study because of adverse drug effects. Diarrhea was reported in 10% and weight loss in 9% of patients taking roflumilast. Weight loss has been shown to be reversible upon stopping roflumilast.2 There has been no evidence of increased risk of death or serious adverse events in studies of roflumilast in patients with COPD.2 However, the benefit-to-harm ratio suggests that roflumilast provides a net benefit only in patients at high risk of severe exacerbations.7
Roflumilast has been shown to reduce rates of acute exacerbation in patients with severe chronic obstructive pulmonary disease (COPD), ie, forced expiratory volume in 1 second (FEV1) less than 50% with symptoms of chronic bronchitis and a history of exacerbations.
Roflumilast is a selective phosphodiesterase 4 (PDE4) inhibitor that acts on airway smooth muscle cells and various inflammatory cells. By blocking PDE4, roflumilast raises cyclic adenosine monophosphate levels within these cells, curtailing the inflammatory response.1,2
Roflumilast is not a bronchodilator, although modest improvements in FEV1 have been documented in clinical trials when it was used as maintenance therapy.
TRIALS OF ROFLUMILAST
Several trials have investigated the efficacy of roflumilast in COPD (Table 1).
The RECORD trial
The RECORD trial1 in 2005 was the first large randomized controlled trial of roflumilast in moderate to severe COPD. At a dose of 500 µg orally daily, there was a modest but statistically significant improvement in the postbronchodilator FEV1. There was also improvement in the St. George Respiratory Questionnaire score in the treatment arm, but this was not statistically significant. The study also found a reduction in acute exacerbations of COPD with roflumilast, which was a secondary end point.1
The results of this study spurred interest in roflumilast as well as criticism of the design of the study. First, COPD patients on inhaled maintenance therapy such as an inhaled corticosteroid and long-acting beta-agonist combination or a long-acting muscarinic antagonist had their medications held during the study. Second, the average FEV1 was 54% of predicted, indicative of a study population with less severe disease.1
The RATIO trial
Taking into account the results of the RECORD trial, the RATIO trial3 in 2007 recruited patients with more severe COPD—ie, Global Initiative for Chronic Obstructive Lung Disease (GOLD) class III and IV—and included the rate of acute exacerbations as a primary end point. Maintenance therapy with inhaled corticosteroids was continued in patients already taking them. However, long-acting beta-agonists and long-acting muscarinic antagonist therapies were held.3
Again, roflumilast improved postbronchodilator FEV1 compared with placebo. A reduction in acute exacerbations was seen but was not statistically significant except in subgroup analysis, where a statistically significant reduction in acute exacerbations was noted for patients with very severe (GOLD class IV) COPD.3
Post hoc analysis from the RATIO trial suggested that patients with chronic bronchitis and patients with a history of frequent exacerbations were more likely to respond to roflumilast.2
The EOS and HELIOS trials
In 2009, the results of the EOS and HELIOS trials of roflumilast in patients with severe COPD were published.4 These trials allowed continuation of long-acting beta-agonists and muscarinic antagonists. The prebronchodilator FEV1 improved modestly when roflumilast was added to a long-acting bronchodilator. These studies ran for only 24 weeks, and the rate of acute exacerbations was not a primary end point, although the results did show a trend toward reduction of exacerbations.4
The AURA and HERMES trials
Also in 2009 was the publication of the results of two 52-week placebo-controlled trials (AURA and HERMES) of roflumilast in patients with severe COPD with chronic bronchitis and a history of frequent exacerbations.5 Maintenance therapy with long-acting beta-agonists was continued, whereas inhaled corticosteroids and long-acting muscarinic antagonists were held. Statistically significant improvements in prebronchodilator FEV1 and reduction in the rate of exacerbations were observed in the roflumilast group (17% reduction, 95% confidence interval 8–25, P < .0003).5
The REACT trial
The REACT trial6 randomized 1,945 patients with severe COPD already on maximal recommended combination inhaled corticosteroid and long-acting beta-agonist therapy to receive either roflumilast or placebo. The patients’ ratio of FEV1 to forced vital capacity was less than 70%, their postbronchodilator FEV1 was less than 50%, and they had chronic bronchitis and a history of at least two acute exacerbations during the past year. They had also been on combination therapy for the previous year. Patients who were on long-acting muscarinic-antagonist therapy (70% of the cohort) were included, and continued with their medication.
Patients were followed for 52 weeks. There was a significant reduction in the rate of exacerbations in the roflumilast group vs placebo (0.823 vs 0.959; risk ratio 0.858; 95% confidence interval 0.740–0.995; P = .0424).6 As in previous trials, the roflumilast group showed an improvement in postbronchodilator FEV1. The study also showed a reduction in hospital admissions in the treatment group.6
ADVERSE EFFECTS OF ROFLUMILAST
Roflumilast is known to have adverse effects significant enough to reduce compliance, the most common being diarrhea, weight loss, and nausea.2,6,7 In the REACT trial,6 11% of patients in the roflumilast group vs 5% in the placebo group dropped out of the study because of adverse drug effects. Diarrhea was reported in 10% and weight loss in 9% of patients taking roflumilast. Weight loss has been shown to be reversible upon stopping roflumilast.2 There has been no evidence of increased risk of death or serious adverse events in studies of roflumilast in patients with COPD.2 However, the benefit-to-harm ratio suggests that roflumilast provides a net benefit only in patients at high risk of severe exacerbations.7
- Rabe KF, Bateman ED, O’Donnell DE, Witte S, Bredenbroker D, Bethke TD. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomized controlled trial. Lancet 2005; 366:63–71.
- Field SK. Roflumilast, a novel phosphodiesterase 4 inhibitor, for COPD patients with a history of exacerbations. Clin Med Insights Circ Respir Pulm Med 2011; 5:57–70.
- Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176:154–161.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomized clinical trials. Lancet 2009; 374:695–703.
- Calverley PM, Rabe KF, Goehring U-M, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomized clinical trials. Lancet 2009; 374:684–95.
- Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015; 385:857–866.
- Yu T, Fain K, Boyd CM, et al. Benefits and harms of roflumilast in moderate to severe COPD. Thorax 2014; 69:616–622.
- Rabe KF, Bateman ED, O’Donnell DE, Witte S, Bredenbroker D, Bethke TD. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomized controlled trial. Lancet 2005; 366:63–71.
- Field SK. Roflumilast, a novel phosphodiesterase 4 inhibitor, for COPD patients with a history of exacerbations. Clin Med Insights Circ Respir Pulm Med 2011; 5:57–70.
- Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176:154–161.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomized clinical trials. Lancet 2009; 374:695–703.
- Calverley PM, Rabe KF, Goehring U-M, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomized clinical trials. Lancet 2009; 374:684–95.
- Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015; 385:857–866.
- Yu T, Fain K, Boyd CM, et al. Benefits and harms of roflumilast in moderate to severe COPD. Thorax 2014; 69:616–622.
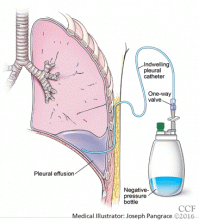
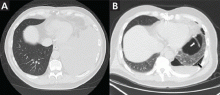
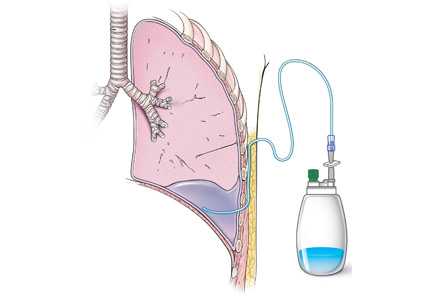
When should an indwelling pleural catheter be considered for malignant pleural effusion?
An indwelling pleural catheter should be considered when a malignant pleural effusion causes symptoms and recurs after thoracentesis, especially in patients with short to intermediate life expectancy or trapped lung, or who underwent unsuccessful pleurodesis.1
MALIGNANT PLEURAL EFFUSION
Malignant pleural effusion affects about 150,000 people in the United States each year. It occurs in 15% of patients with advanced malignancies, most often lung cancer, breast cancer, lymphoma, and ovarian cancer, which account for more than 50% of cases.2
In most patients with malignant pleural effusion, disabling dyspnea causes poor quality of life. The prognosis is unfavorable, with life expectancy of 3 to 12 months. Patients with poor performance status and lower glucose concentrations in the pleural fluid face a worse prognosis and a shorter life expectancy.2
In general, management focuses on relieving symptoms rather than on cure. Symptoms can be controlled by thoracentesis, but if the effusion recurs, the patient needs repeated visits to the emergency room or clinic or a hospital admission to drain the fluid. Frequent hospital visits can be grueling for a patient with a poor functional status, and so can the adverse effects of repeated thoracentesis. For that reason, an early palliative approach to malignant pleural effusion in patients with cancer and a poor prognosis leads to better symptom control and a better quality of life.3 Multiple treatments can be offered to control the symptoms in patients with recurrent malignant pleural effusion (Table 1).
PLEURODESIS HAS BEEN THE TREATMENT OF CHOICE
Pleurodesis has been the treatment of choice for malignant pleural effusion for decades. In this procedure, adhesion of the visceral and parietal pleura is achxieved by inducing inflammation either mechanically or chemically between the pleural surfaces. Injection of a sclerosant into the pleural space generates the inflammation. The sclerosant can be introduced through a chest tube or thoracoscope such as in video-assisted thoracic surgery or medical pleuroscopy. The use of talc is associated with a higher success rate than other sclerosing agents such as bleomycin and doxycycline.4
The downside of this procedure is that pleural effusion recurs in 10% to 40% of cases, and patients require 2 to 4 days in the hospital. Also, the use of talc can lead to acute lung injury–acute respiratory distress syndrome, a rare but potentially life-threatening complication. The incidence of this complication may be related to particle size, with small particles posing a higher risk than large ones.5,6
PLACEMENT OF AN INDWELLING PLEURAL CATHETER
Indwelling pleural catheters are currently used as palliative therapy for patients with recurrent malignant pleural effusion who suffer from respiratory distress due to rapid reaccumulation of pleural fluids that require multiple thoracentesis procedures.
An indwelling pleural catheter is contraindicated in patients with uncontrolled coagulopathy, multiloculated pleural effusions, or extensive malignancy in the skin.3 Other factors that need to be considered are the patient’s social circumstances: ie, the patient must be in a clean and safe environment and must have insurance coverage for the supplies.
Catheters are 66 cm long and 15.5F and are made of silicone rubber with fenestrations along the distal 24 cm. They have a one-way valve at the proximal end that allows fluids and air to go out but not in (Figure 1).1 Several systems are commercially available in the United States.
The catheter is inserted and tunneled percutaneously with the patient under local anesthesia and conscious sedation (Figure 2). Insertion is a same-day outpatient procedure, and intermittent pleural fluid drainage can be done at home by a home heathcare provider or a trained family member.7
In a meta-analysis, insertion difficulties were reported in only 4% of cases, particularly in patients who underwent prior pleural interventions. Spontaneous pleurodesis occurred in 45% of patients at a mean of 52 days after insertion.8
After catheter insertion, the pleural space should be drained three times a week. No more than 1,000 mL of fluid should be removed at a time—or less if drainage causes chest pain or cough secondary to trapped lung (see below). When the drainage declines to 150 mL per session, the sessions can be reduced to twice a week. If the volume drops to less than 50 mL per session, imaging (computed tomography or bedside thoracic ultrasonography) is recommended to ensure the achievement of pleurodesis and to rule out catheter blockage.
A large multicenter randomized controlled trial9 compared indwelling pleural catheter therapy and chest tube insertion with talc pleurodesis. Both procedures relieved symptoms for the first 42 days, and there was no significant difference in quality of life. However, the median length of hospital stay was 4 days for the talc pleurodesis group compared with 0 days for the indwelling pleural catheter group. Twenty-two percent of the talc group required a further pleural procedure such as a video-assisted thoracic surgery or thoracoscopy, compared with 6% of the indwelling catheter group. On the other hand, 36% of those in the indwelling catheter group experienced nonserious adverse events such as pleural infections that mandated outpatient oral antibiotic therapy, cellulitis, and catheter blockage, compared with 7% of the talc group.9
Symptomatic, inoperable trapped lung is another condition for which an indwelling pleural catheter is a reasonable strategy compared with pleurodesis. Trapped lung is a condition in which the lung fails to fully expand despite proper pleural fluid removal, creating a vacuum space between the parietal and visceral pleura (Figure 3).
Patients with trapped lung complain of severe dull or sharp pain during drainage of pleural fluids due to stretching of the visceral pleura against the intrathoracic vacuum space. Trapped lung can be detected objectively by using intrathoracic manometry while draining fluids, looking for more than a 20-cm H2O drop in the intrathoracic pressure. Radiographically, this may be identified as a pneumothorax ex vacuo10 (ie, caused by inability of the lung to expand to fill the thoracic cavity after pleural fluid has been drained) and is not a procedure complication.
Placement of an indwelling pleural catheter is the treatment of choice for trapped lung, since chemical pleurodesis is not feasible without the potential of parietal and visceral pleural apposition. In a retrospective study of indwelling catheter placement for palliative symptom control, a catheter relieved symptoms, improved quality of life, and afforded a substantial increase in mobility.1,11
In another multicenter pilot study,12 rapid pleurodesis was achieved in 30 patients with recurrent malignant pleural effusion by combining chemical pleurodesis and indwelling catheter placement. Both were done under direct vision with medical thoracoscopy. Pleurodesis succeeded in 92% of patients by day 8 after the procedure. The hospital stay was reduced to a mean of 2 days after the procedure. In the catheter group, fluids were drained three times in the first day after the procedure and twice a day on the second and third days. Of the 30 patients in this study, 2 had fever, 1 needed to have the catheter replaced, and 1 contracted empyema.
AN EFFECTIVE INITIAL TREATMENT
Placement of an indwelling pleural catheter is an effective initial treatment for recurrent malignant pleural effusion. Compared with chemical pleurodesis, it has a comparable success rate and complication rate. It offers the advantages of being a same-day surgical procedure entailing a shorter hospital stay and less need for further pleural intervention. This treatment should be considered for patients with symptomatic malignant pleural effusion, especially those in whom symptomatic malignant pleural effusion recurred after thoracentesis.8
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40.
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013; 34:459–471.
- Thomas R, Francis R, Davies HE, Lee YC. Interventional therapies for malignant pleural effusions: the present and the future. Respirology 2014; 19:809–822.
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012; 83:91–98.
- Gonzalez AV, Bezwada V, Beamis JF Jr, Villanueva AG. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010; 137:1375–1381.
- Rossi VF, Vargas FS, Marchi E, et al. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur Respir J 2010; 35:396–401.
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142:394–400.
- Kheir F, Shawwa K, Alokla K, Omballi M, Alraiyes AH. Tunneled pleural catheter for the treatment of malignant pleural effusion: a systematic review and meta-analysis. Am J Ther 2015 Feb 2. [Epub ahead of print]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307:2383–2389.
- Ponrartana S, Laberge JM, Kerlan RK, Wilson MW, Gordon RL. Management of patients with “ex vacuo” pneumothorax after thoracentesis. Acad Radiol 2005; 12:980–986.
- Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009; 9:961–964.
- Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011; 139:1419–1423.
An indwelling pleural catheter should be considered when a malignant pleural effusion causes symptoms and recurs after thoracentesis, especially in patients with short to intermediate life expectancy or trapped lung, or who underwent unsuccessful pleurodesis.1
MALIGNANT PLEURAL EFFUSION
Malignant pleural effusion affects about 150,000 people in the United States each year. It occurs in 15% of patients with advanced malignancies, most often lung cancer, breast cancer, lymphoma, and ovarian cancer, which account for more than 50% of cases.2
In most patients with malignant pleural effusion, disabling dyspnea causes poor quality of life. The prognosis is unfavorable, with life expectancy of 3 to 12 months. Patients with poor performance status and lower glucose concentrations in the pleural fluid face a worse prognosis and a shorter life expectancy.2
In general, management focuses on relieving symptoms rather than on cure. Symptoms can be controlled by thoracentesis, but if the effusion recurs, the patient needs repeated visits to the emergency room or clinic or a hospital admission to drain the fluid. Frequent hospital visits can be grueling for a patient with a poor functional status, and so can the adverse effects of repeated thoracentesis. For that reason, an early palliative approach to malignant pleural effusion in patients with cancer and a poor prognosis leads to better symptom control and a better quality of life.3 Multiple treatments can be offered to control the symptoms in patients with recurrent malignant pleural effusion (Table 1).
PLEURODESIS HAS BEEN THE TREATMENT OF CHOICE
Pleurodesis has been the treatment of choice for malignant pleural effusion for decades. In this procedure, adhesion of the visceral and parietal pleura is achxieved by inducing inflammation either mechanically or chemically between the pleural surfaces. Injection of a sclerosant into the pleural space generates the inflammation. The sclerosant can be introduced through a chest tube or thoracoscope such as in video-assisted thoracic surgery or medical pleuroscopy. The use of talc is associated with a higher success rate than other sclerosing agents such as bleomycin and doxycycline.4
The downside of this procedure is that pleural effusion recurs in 10% to 40% of cases, and patients require 2 to 4 days in the hospital. Also, the use of talc can lead to acute lung injury–acute respiratory distress syndrome, a rare but potentially life-threatening complication. The incidence of this complication may be related to particle size, with small particles posing a higher risk than large ones.5,6
PLACEMENT OF AN INDWELLING PLEURAL CATHETER
Indwelling pleural catheters are currently used as palliative therapy for patients with recurrent malignant pleural effusion who suffer from respiratory distress due to rapid reaccumulation of pleural fluids that require multiple thoracentesis procedures.
An indwelling pleural catheter is contraindicated in patients with uncontrolled coagulopathy, multiloculated pleural effusions, or extensive malignancy in the skin.3 Other factors that need to be considered are the patient’s social circumstances: ie, the patient must be in a clean and safe environment and must have insurance coverage for the supplies.
Catheters are 66 cm long and 15.5F and are made of silicone rubber with fenestrations along the distal 24 cm. They have a one-way valve at the proximal end that allows fluids and air to go out but not in (Figure 1).1 Several systems are commercially available in the United States.
The catheter is inserted and tunneled percutaneously with the patient under local anesthesia and conscious sedation (Figure 2). Insertion is a same-day outpatient procedure, and intermittent pleural fluid drainage can be done at home by a home heathcare provider or a trained family member.7
In a meta-analysis, insertion difficulties were reported in only 4% of cases, particularly in patients who underwent prior pleural interventions. Spontaneous pleurodesis occurred in 45% of patients at a mean of 52 days after insertion.8
After catheter insertion, the pleural space should be drained three times a week. No more than 1,000 mL of fluid should be removed at a time—or less if drainage causes chest pain or cough secondary to trapped lung (see below). When the drainage declines to 150 mL per session, the sessions can be reduced to twice a week. If the volume drops to less than 50 mL per session, imaging (computed tomography or bedside thoracic ultrasonography) is recommended to ensure the achievement of pleurodesis and to rule out catheter blockage.
A large multicenter randomized controlled trial9 compared indwelling pleural catheter therapy and chest tube insertion with talc pleurodesis. Both procedures relieved symptoms for the first 42 days, and there was no significant difference in quality of life. However, the median length of hospital stay was 4 days for the talc pleurodesis group compared with 0 days for the indwelling pleural catheter group. Twenty-two percent of the talc group required a further pleural procedure such as a video-assisted thoracic surgery or thoracoscopy, compared with 6% of the indwelling catheter group. On the other hand, 36% of those in the indwelling catheter group experienced nonserious adverse events such as pleural infections that mandated outpatient oral antibiotic therapy, cellulitis, and catheter blockage, compared with 7% of the talc group.9
Symptomatic, inoperable trapped lung is another condition for which an indwelling pleural catheter is a reasonable strategy compared with pleurodesis. Trapped lung is a condition in which the lung fails to fully expand despite proper pleural fluid removal, creating a vacuum space between the parietal and visceral pleura (Figure 3).
Patients with trapped lung complain of severe dull or sharp pain during drainage of pleural fluids due to stretching of the visceral pleura against the intrathoracic vacuum space. Trapped lung can be detected objectively by using intrathoracic manometry while draining fluids, looking for more than a 20-cm H2O drop in the intrathoracic pressure. Radiographically, this may be identified as a pneumothorax ex vacuo10 (ie, caused by inability of the lung to expand to fill the thoracic cavity after pleural fluid has been drained) and is not a procedure complication.
Placement of an indwelling pleural catheter is the treatment of choice for trapped lung, since chemical pleurodesis is not feasible without the potential of parietal and visceral pleural apposition. In a retrospective study of indwelling catheter placement for palliative symptom control, a catheter relieved symptoms, improved quality of life, and afforded a substantial increase in mobility.1,11
In another multicenter pilot study,12 rapid pleurodesis was achieved in 30 patients with recurrent malignant pleural effusion by combining chemical pleurodesis and indwelling catheter placement. Both were done under direct vision with medical thoracoscopy. Pleurodesis succeeded in 92% of patients by day 8 after the procedure. The hospital stay was reduced to a mean of 2 days after the procedure. In the catheter group, fluids were drained three times in the first day after the procedure and twice a day on the second and third days. Of the 30 patients in this study, 2 had fever, 1 needed to have the catheter replaced, and 1 contracted empyema.
AN EFFECTIVE INITIAL TREATMENT
Placement of an indwelling pleural catheter is an effective initial treatment for recurrent malignant pleural effusion. Compared with chemical pleurodesis, it has a comparable success rate and complication rate. It offers the advantages of being a same-day surgical procedure entailing a shorter hospital stay and less need for further pleural intervention. This treatment should be considered for patients with symptomatic malignant pleural effusion, especially those in whom symptomatic malignant pleural effusion recurred after thoracentesis.8
An indwelling pleural catheter should be considered when a malignant pleural effusion causes symptoms and recurs after thoracentesis, especially in patients with short to intermediate life expectancy or trapped lung, or who underwent unsuccessful pleurodesis.1
MALIGNANT PLEURAL EFFUSION
Malignant pleural effusion affects about 150,000 people in the United States each year. It occurs in 15% of patients with advanced malignancies, most often lung cancer, breast cancer, lymphoma, and ovarian cancer, which account for more than 50% of cases.2
In most patients with malignant pleural effusion, disabling dyspnea causes poor quality of life. The prognosis is unfavorable, with life expectancy of 3 to 12 months. Patients with poor performance status and lower glucose concentrations in the pleural fluid face a worse prognosis and a shorter life expectancy.2
In general, management focuses on relieving symptoms rather than on cure. Symptoms can be controlled by thoracentesis, but if the effusion recurs, the patient needs repeated visits to the emergency room or clinic or a hospital admission to drain the fluid. Frequent hospital visits can be grueling for a patient with a poor functional status, and so can the adverse effects of repeated thoracentesis. For that reason, an early palliative approach to malignant pleural effusion in patients with cancer and a poor prognosis leads to better symptom control and a better quality of life.3 Multiple treatments can be offered to control the symptoms in patients with recurrent malignant pleural effusion (Table 1).
PLEURODESIS HAS BEEN THE TREATMENT OF CHOICE
Pleurodesis has been the treatment of choice for malignant pleural effusion for decades. In this procedure, adhesion of the visceral and parietal pleura is achxieved by inducing inflammation either mechanically or chemically between the pleural surfaces. Injection of a sclerosant into the pleural space generates the inflammation. The sclerosant can be introduced through a chest tube or thoracoscope such as in video-assisted thoracic surgery or medical pleuroscopy. The use of talc is associated with a higher success rate than other sclerosing agents such as bleomycin and doxycycline.4
The downside of this procedure is that pleural effusion recurs in 10% to 40% of cases, and patients require 2 to 4 days in the hospital. Also, the use of talc can lead to acute lung injury–acute respiratory distress syndrome, a rare but potentially life-threatening complication. The incidence of this complication may be related to particle size, with small particles posing a higher risk than large ones.5,6
PLACEMENT OF AN INDWELLING PLEURAL CATHETER
Indwelling pleural catheters are currently used as palliative therapy for patients with recurrent malignant pleural effusion who suffer from respiratory distress due to rapid reaccumulation of pleural fluids that require multiple thoracentesis procedures.
An indwelling pleural catheter is contraindicated in patients with uncontrolled coagulopathy, multiloculated pleural effusions, or extensive malignancy in the skin.3 Other factors that need to be considered are the patient’s social circumstances: ie, the patient must be in a clean and safe environment and must have insurance coverage for the supplies.
Catheters are 66 cm long and 15.5F and are made of silicone rubber with fenestrations along the distal 24 cm. They have a one-way valve at the proximal end that allows fluids and air to go out but not in (Figure 1).1 Several systems are commercially available in the United States.
The catheter is inserted and tunneled percutaneously with the patient under local anesthesia and conscious sedation (Figure 2). Insertion is a same-day outpatient procedure, and intermittent pleural fluid drainage can be done at home by a home heathcare provider or a trained family member.7
In a meta-analysis, insertion difficulties were reported in only 4% of cases, particularly in patients who underwent prior pleural interventions. Spontaneous pleurodesis occurred in 45% of patients at a mean of 52 days after insertion.8
After catheter insertion, the pleural space should be drained three times a week. No more than 1,000 mL of fluid should be removed at a time—or less if drainage causes chest pain or cough secondary to trapped lung (see below). When the drainage declines to 150 mL per session, the sessions can be reduced to twice a week. If the volume drops to less than 50 mL per session, imaging (computed tomography or bedside thoracic ultrasonography) is recommended to ensure the achievement of pleurodesis and to rule out catheter blockage.
A large multicenter randomized controlled trial9 compared indwelling pleural catheter therapy and chest tube insertion with talc pleurodesis. Both procedures relieved symptoms for the first 42 days, and there was no significant difference in quality of life. However, the median length of hospital stay was 4 days for the talc pleurodesis group compared with 0 days for the indwelling pleural catheter group. Twenty-two percent of the talc group required a further pleural procedure such as a video-assisted thoracic surgery or thoracoscopy, compared with 6% of the indwelling catheter group. On the other hand, 36% of those in the indwelling catheter group experienced nonserious adverse events such as pleural infections that mandated outpatient oral antibiotic therapy, cellulitis, and catheter blockage, compared with 7% of the talc group.9
Symptomatic, inoperable trapped lung is another condition for which an indwelling pleural catheter is a reasonable strategy compared with pleurodesis. Trapped lung is a condition in which the lung fails to fully expand despite proper pleural fluid removal, creating a vacuum space between the parietal and visceral pleura (Figure 3).
Patients with trapped lung complain of severe dull or sharp pain during drainage of pleural fluids due to stretching of the visceral pleura against the intrathoracic vacuum space. Trapped lung can be detected objectively by using intrathoracic manometry while draining fluids, looking for more than a 20-cm H2O drop in the intrathoracic pressure. Radiographically, this may be identified as a pneumothorax ex vacuo10 (ie, caused by inability of the lung to expand to fill the thoracic cavity after pleural fluid has been drained) and is not a procedure complication.
Placement of an indwelling pleural catheter is the treatment of choice for trapped lung, since chemical pleurodesis is not feasible without the potential of parietal and visceral pleural apposition. In a retrospective study of indwelling catheter placement for palliative symptom control, a catheter relieved symptoms, improved quality of life, and afforded a substantial increase in mobility.1,11
In another multicenter pilot study,12 rapid pleurodesis was achieved in 30 patients with recurrent malignant pleural effusion by combining chemical pleurodesis and indwelling catheter placement. Both were done under direct vision with medical thoracoscopy. Pleurodesis succeeded in 92% of patients by day 8 after the procedure. The hospital stay was reduced to a mean of 2 days after the procedure. In the catheter group, fluids were drained three times in the first day after the procedure and twice a day on the second and third days. Of the 30 patients in this study, 2 had fever, 1 needed to have the catheter replaced, and 1 contracted empyema.
AN EFFECTIVE INITIAL TREATMENT
Placement of an indwelling pleural catheter is an effective initial treatment for recurrent malignant pleural effusion. Compared with chemical pleurodesis, it has a comparable success rate and complication rate. It offers the advantages of being a same-day surgical procedure entailing a shorter hospital stay and less need for further pleural intervention. This treatment should be considered for patients with symptomatic malignant pleural effusion, especially those in whom symptomatic malignant pleural effusion recurred after thoracentesis.8
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40.
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013; 34:459–471.
- Thomas R, Francis R, Davies HE, Lee YC. Interventional therapies for malignant pleural effusions: the present and the future. Respirology 2014; 19:809–822.
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012; 83:91–98.
- Gonzalez AV, Bezwada V, Beamis JF Jr, Villanueva AG. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010; 137:1375–1381.
- Rossi VF, Vargas FS, Marchi E, et al. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur Respir J 2010; 35:396–401.
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142:394–400.
- Kheir F, Shawwa K, Alokla K, Omballi M, Alraiyes AH. Tunneled pleural catheter for the treatment of malignant pleural effusion: a systematic review and meta-analysis. Am J Ther 2015 Feb 2. [Epub ahead of print]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307:2383–2389.
- Ponrartana S, Laberge JM, Kerlan RK, Wilson MW, Gordon RL. Management of patients with “ex vacuo” pneumothorax after thoracentesis. Acad Radiol 2005; 12:980–986.
- Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009; 9:961–964.
- Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011; 139:1419–1423.
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40.
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013; 34:459–471.
- Thomas R, Francis R, Davies HE, Lee YC. Interventional therapies for malignant pleural effusions: the present and the future. Respirology 2014; 19:809–822.
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012; 83:91–98.
- Gonzalez AV, Bezwada V, Beamis JF Jr, Villanueva AG. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010; 137:1375–1381.
- Rossi VF, Vargas FS, Marchi E, et al. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur Respir J 2010; 35:396–401.
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142:394–400.
- Kheir F, Shawwa K, Alokla K, Omballi M, Alraiyes AH. Tunneled pleural catheter for the treatment of malignant pleural effusion: a systematic review and meta-analysis. Am J Ther 2015 Feb 2. [Epub ahead of print]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307:2383–2389.
- Ponrartana S, Laberge JM, Kerlan RK, Wilson MW, Gordon RL. Management of patients with “ex vacuo” pneumothorax after thoracentesis. Acad Radiol 2005; 12:980–986.
- Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009; 9:961–964.
- Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011; 139:1419–1423.
Are there alternatives to surgery for Zenker diverticulum?
Conventional treatment for a large symptomatic Zenker diverticulum is to surgically either remove it (diverticulectomy) or obliterate it by repositioning and securing it after cutting into the cricopharyngeus muscle (diverticulopexy with cricopharyngeal myotomy). But high rates of complications with these procedures in elderly patients have led to the development of endoscopic procedures that are safer and have similar or higher success rates.
ESOPHAGEAL POUCH IN SPACE BETWEEN MUSCLES
Zenker diverticulum is an outpouching of the esophageal mucosa into the potential space of the Killian triangle, the area between the inferior pharyngeal constrictor and the cricopharyngeus muscle. Increased cricopharyngeal tone coupled with insufficient relaxation results in a pressure gradient that eventually causes esophageal outpouchings. The problem is induced by age-related fibrosis and atrophy, esophageal spasms related to gastroesophageal reflux disease, and idiopathic cricopharyngeal spasms.1
USUALLY PRESENTS WITH DYSPHAGIA
Zenker diverticulum has an estimated incidence of about 2 per 100,000 per year, but this is likely low because many cases are asymptomatic. It occurs mostly in men and people of Northern European descent in their 60s and 70s and rarely before age 40.2
About 80% to 90% of patients present with dysphagia. Other signs and symptoms include regurgitation of undigested foods and medications, halitosis, hoarseness, chronic cough, and aspiration.
Rare complications of Zenker diverticulum include recurrent pulmonary infection, tracheal fistula, ulceration, hemorrhage, squamous cell carcinoma (incidence 0.4% to 1.5%), vocal cord paralysis, and fistula to the prevertebral ligament with cervical osteomyelitis.1
The diagnosis is suspected based on symptoms but should be confirmed with a barium esophagram that shows an outpouching of contrast from the main contrast column.
A watch-and-wait approach should be used for patients with mild symptoms (ie, mild or intermittent dysphagia) and minor functional limitations. Patients should be counseled to eat small amounts of food at a time, to chew thoroughly, and to sip liquids between bites.
TREATMENT IS SURGERY OR ENDOSCOPY
Patients with more than mild symptoms who are candidates for intervention should be offered treatment. The goal is to open the septum between the diverticulum and the main esophageal lumen so that food can be propelled from the hypopharynx to the main esophageal lumen without obstruction. Because increased cricopharyngeal tone plays a large role in the development and propagation of a diverticulum, most experts recommend cricopharyngomyotomy in addition to any treatment strategy.
Open surgery
In centers that do not offer flexible endoscopy, and for patients with a large diverticulum, open surgery is the sole option. It is done under general anesthesia with a left cervical approach. The length of the cricopharyngeal myotomy can vary from 2 to 6 cm.3,4 This is followed by one of three options:
- Diverticulectomy
- Diverticulopexy, in which the diverticulum is repositioned and sutured against the prevertebral fascia
- Diverticular inversion, in which the diverticulum is inverted into the esophageal lumen and then oversewn.4
Success rates for open surgery vary from study to study but are usually around 90%.4 Complications are reported in 10% to 30% of cases and include mediastinitis, severe recurrent laryngeal nerve injury, and a 1% to 2% chance of death. These rates seem high, but the older age of most of these patients puts them at high risk.5
Endoscopic procedures
All endoscopic procedures involve incision of the wall separating the diverticulum from the esophageal lumen to relieve the obstruction.
Rigid endoscopy is used in centers that do not offer flexible endoscopy for patients who are not candidates for surgery. It is done under general anesthesia. With the patient’s neck completely hyperextended, a rigid endoscope is passed into the oral cavity, and diverticulotomy and cricopharyngeal myotomy are performed.
This technique has been extensively evaluated and, although effective, carries up to an 8% risk of complications, including perforation. It is not recommended for patients with a small diverticulum, a high body mass index, or difficulty with neck extension.6
Flexible endoscopy is the first-line therapy for most patients in many centers. With the flexible endoscope, the diverticulotomy and the cricopharyngeal myotomy can be performed in an outpatient endoscopy suite, and general anesthesia is not required.3
Initial studies of outcomes using these approaches have been promising,3,7–9 with substantial reduction in dysphagia, regurgitation, and chronic cough. Unfortunately, this technique is associated with a recurrence rate as high as 25%. However, repeat endoscopic therapy in patients with recurrence results in success rates similar to those for first-time treatment.
Complication rates are low. In a series of 150 patients, four adverse events occurred—three cases of fever and one of pneumonia. In addition, one patient had subcutaneous emphysema that resolved spontaneously.7
Improved device on horizon
A potentially major advance in the endoscopic treatment of Zenker diverticulum is the development of a diverticuloscope, a soft tube with a V-shaped end. This tube is inserted through the mouth with one leg of the “V” protecting the anterior esophageal wall and the other leg protecting the posterior diverticular lumen. The endoscope is passed through this tube to perform the diverticulotomy. In one report,10 diverticuloscope-guided diverticulotomy led to fewer complications, shorter procedural time, and higher success rates compared with the standard technique.10 This device is not yet available in the United States.
No head-to-head comparison of outcomes with various flexible endoscopic techniques for treating Zenker diverticulum has been published, nor are there data yet on the success of surgery if endoscopic therapy fails.
We recommend flexible endoscopy for the initial treatment of Zenker diverticulum in most patients, but its availability is limited in the United States, as many practitioners do not have adequate experience with the technique.
TAKE-HOME POINTS
- Zenker diverticulum is an outpouching of the esophageal mucosa. It is often asymptomatic. Patients with minimal symptoms and with no functional impairment can be followed conservatively.
- A head-to-head comparison of surgical and endoscopic therapy has not yet been done, and optimal patient selection criteria are lacking. Therefore, treatment recommendations are based primarily on expert opinion.
- We recommend flexible endoscopic therapy as initial treatment in patients with a small diverticulum, but this may be limited by the unavailability of a surgeon experienced in this technique.
- For a large diverticulum, we recommend an open cervical procedure involving excision of the diverticulum or a diverticulopexy for patients who are good surgical candidates.
- Ferreira LE, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus 2008; 21:1–8.
- Manno M, Manta R, Caruso A, et al. Alternative endoscopic treatment of Zenker’s diverticulum: a case series (with video). Gastrointest Endosc 2014; 79:168–170.
- Yuan Y, Zhao YF, Hu Y, Chen LQ. Surgical treatment of Zenker’s diverticulum. Dig Surg 2013; 30:207–218.
- Bizzotto A, Iacopini F, Landi R, Costamagna G. Zenker’s diverticulum: exploring treatment options. Acta Otorhinolaryngol Ital 2013; 33:219–229.
- Repici A, Pagano N, Fumagalli U, et. al. Transoral treatment of Zenker diverticulum: flexible endoscopy versus endoscopic stapling. A retrospective comparison of outcomes. Dis Esophagus 2011; 24:235–239.
- Law R, Katzka DA, Baron TH. Zenker’s diverticulum. Clin Gastroenterol Hepatol 2014; 12:1773–1782.
- Huberty V, El Bacha S, Blero D, Le Moine O, Hassid S, Devière J. Endoscopic treatment for Zenker’s diverticulum: long-term results (with video). Gastrointest Endosc 2013; 77:701–707.
- Case DJ, Baron TH. Flexible endoscopic management of Zenker diverticulum: the Mayo Clinic experience. Mayo Clin Proc 2010; 85:719–722.
- Al-Kadi AS, Maghrabi AA, Thomson D, Gillman LM, Dhalla S. Endoscopic treatment of Zenker diverticulum: results of a 7-year experience. J Am Coll Surg 2010; 211:239–243.
- Costamagna G, Iacopini F, Tringali A, et al. Flexible endoscopic Zenker’s diverticulotomy: cap-assisted technique vs diverticuloscope-assisted technique. Endoscopy 2007; 39:146–152.
Conventional treatment for a large symptomatic Zenker diverticulum is to surgically either remove it (diverticulectomy) or obliterate it by repositioning and securing it after cutting into the cricopharyngeus muscle (diverticulopexy with cricopharyngeal myotomy). But high rates of complications with these procedures in elderly patients have led to the development of endoscopic procedures that are safer and have similar or higher success rates.
ESOPHAGEAL POUCH IN SPACE BETWEEN MUSCLES
Zenker diverticulum is an outpouching of the esophageal mucosa into the potential space of the Killian triangle, the area between the inferior pharyngeal constrictor and the cricopharyngeus muscle. Increased cricopharyngeal tone coupled with insufficient relaxation results in a pressure gradient that eventually causes esophageal outpouchings. The problem is induced by age-related fibrosis and atrophy, esophageal spasms related to gastroesophageal reflux disease, and idiopathic cricopharyngeal spasms.1
USUALLY PRESENTS WITH DYSPHAGIA
Zenker diverticulum has an estimated incidence of about 2 per 100,000 per year, but this is likely low because many cases are asymptomatic. It occurs mostly in men and people of Northern European descent in their 60s and 70s and rarely before age 40.2
About 80% to 90% of patients present with dysphagia. Other signs and symptoms include regurgitation of undigested foods and medications, halitosis, hoarseness, chronic cough, and aspiration.
Rare complications of Zenker diverticulum include recurrent pulmonary infection, tracheal fistula, ulceration, hemorrhage, squamous cell carcinoma (incidence 0.4% to 1.5%), vocal cord paralysis, and fistula to the prevertebral ligament with cervical osteomyelitis.1
The diagnosis is suspected based on symptoms but should be confirmed with a barium esophagram that shows an outpouching of contrast from the main contrast column.
A watch-and-wait approach should be used for patients with mild symptoms (ie, mild or intermittent dysphagia) and minor functional limitations. Patients should be counseled to eat small amounts of food at a time, to chew thoroughly, and to sip liquids between bites.
TREATMENT IS SURGERY OR ENDOSCOPY
Patients with more than mild symptoms who are candidates for intervention should be offered treatment. The goal is to open the septum between the diverticulum and the main esophageal lumen so that food can be propelled from the hypopharynx to the main esophageal lumen without obstruction. Because increased cricopharyngeal tone plays a large role in the development and propagation of a diverticulum, most experts recommend cricopharyngomyotomy in addition to any treatment strategy.
Open surgery
In centers that do not offer flexible endoscopy, and for patients with a large diverticulum, open surgery is the sole option. It is done under general anesthesia with a left cervical approach. The length of the cricopharyngeal myotomy can vary from 2 to 6 cm.3,4 This is followed by one of three options:
- Diverticulectomy
- Diverticulopexy, in which the diverticulum is repositioned and sutured against the prevertebral fascia
- Diverticular inversion, in which the diverticulum is inverted into the esophageal lumen and then oversewn.4
Success rates for open surgery vary from study to study but are usually around 90%.4 Complications are reported in 10% to 30% of cases and include mediastinitis, severe recurrent laryngeal nerve injury, and a 1% to 2% chance of death. These rates seem high, but the older age of most of these patients puts them at high risk.5
Endoscopic procedures
All endoscopic procedures involve incision of the wall separating the diverticulum from the esophageal lumen to relieve the obstruction.
Rigid endoscopy is used in centers that do not offer flexible endoscopy for patients who are not candidates for surgery. It is done under general anesthesia. With the patient’s neck completely hyperextended, a rigid endoscope is passed into the oral cavity, and diverticulotomy and cricopharyngeal myotomy are performed.
This technique has been extensively evaluated and, although effective, carries up to an 8% risk of complications, including perforation. It is not recommended for patients with a small diverticulum, a high body mass index, or difficulty with neck extension.6
Flexible endoscopy is the first-line therapy for most patients in many centers. With the flexible endoscope, the diverticulotomy and the cricopharyngeal myotomy can be performed in an outpatient endoscopy suite, and general anesthesia is not required.3
Initial studies of outcomes using these approaches have been promising,3,7–9 with substantial reduction in dysphagia, regurgitation, and chronic cough. Unfortunately, this technique is associated with a recurrence rate as high as 25%. However, repeat endoscopic therapy in patients with recurrence results in success rates similar to those for first-time treatment.
Complication rates are low. In a series of 150 patients, four adverse events occurred—three cases of fever and one of pneumonia. In addition, one patient had subcutaneous emphysema that resolved spontaneously.7
Improved device on horizon
A potentially major advance in the endoscopic treatment of Zenker diverticulum is the development of a diverticuloscope, a soft tube with a V-shaped end. This tube is inserted through the mouth with one leg of the “V” protecting the anterior esophageal wall and the other leg protecting the posterior diverticular lumen. The endoscope is passed through this tube to perform the diverticulotomy. In one report,10 diverticuloscope-guided diverticulotomy led to fewer complications, shorter procedural time, and higher success rates compared with the standard technique.10 This device is not yet available in the United States.
No head-to-head comparison of outcomes with various flexible endoscopic techniques for treating Zenker diverticulum has been published, nor are there data yet on the success of surgery if endoscopic therapy fails.
We recommend flexible endoscopy for the initial treatment of Zenker diverticulum in most patients, but its availability is limited in the United States, as many practitioners do not have adequate experience with the technique.
TAKE-HOME POINTS
- Zenker diverticulum is an outpouching of the esophageal mucosa. It is often asymptomatic. Patients with minimal symptoms and with no functional impairment can be followed conservatively.
- A head-to-head comparison of surgical and endoscopic therapy has not yet been done, and optimal patient selection criteria are lacking. Therefore, treatment recommendations are based primarily on expert opinion.
- We recommend flexible endoscopic therapy as initial treatment in patients with a small diverticulum, but this may be limited by the unavailability of a surgeon experienced in this technique.
- For a large diverticulum, we recommend an open cervical procedure involving excision of the diverticulum or a diverticulopexy for patients who are good surgical candidates.
Conventional treatment for a large symptomatic Zenker diverticulum is to surgically either remove it (diverticulectomy) or obliterate it by repositioning and securing it after cutting into the cricopharyngeus muscle (diverticulopexy with cricopharyngeal myotomy). But high rates of complications with these procedures in elderly patients have led to the development of endoscopic procedures that are safer and have similar or higher success rates.
ESOPHAGEAL POUCH IN SPACE BETWEEN MUSCLES
Zenker diverticulum is an outpouching of the esophageal mucosa into the potential space of the Killian triangle, the area between the inferior pharyngeal constrictor and the cricopharyngeus muscle. Increased cricopharyngeal tone coupled with insufficient relaxation results in a pressure gradient that eventually causes esophageal outpouchings. The problem is induced by age-related fibrosis and atrophy, esophageal spasms related to gastroesophageal reflux disease, and idiopathic cricopharyngeal spasms.1
USUALLY PRESENTS WITH DYSPHAGIA
Zenker diverticulum has an estimated incidence of about 2 per 100,000 per year, but this is likely low because many cases are asymptomatic. It occurs mostly in men and people of Northern European descent in their 60s and 70s and rarely before age 40.2
About 80% to 90% of patients present with dysphagia. Other signs and symptoms include regurgitation of undigested foods and medications, halitosis, hoarseness, chronic cough, and aspiration.
Rare complications of Zenker diverticulum include recurrent pulmonary infection, tracheal fistula, ulceration, hemorrhage, squamous cell carcinoma (incidence 0.4% to 1.5%), vocal cord paralysis, and fistula to the prevertebral ligament with cervical osteomyelitis.1
The diagnosis is suspected based on symptoms but should be confirmed with a barium esophagram that shows an outpouching of contrast from the main contrast column.
A watch-and-wait approach should be used for patients with mild symptoms (ie, mild or intermittent dysphagia) and minor functional limitations. Patients should be counseled to eat small amounts of food at a time, to chew thoroughly, and to sip liquids between bites.
TREATMENT IS SURGERY OR ENDOSCOPY
Patients with more than mild symptoms who are candidates for intervention should be offered treatment. The goal is to open the septum between the diverticulum and the main esophageal lumen so that food can be propelled from the hypopharynx to the main esophageal lumen without obstruction. Because increased cricopharyngeal tone plays a large role in the development and propagation of a diverticulum, most experts recommend cricopharyngomyotomy in addition to any treatment strategy.
Open surgery
In centers that do not offer flexible endoscopy, and for patients with a large diverticulum, open surgery is the sole option. It is done under general anesthesia with a left cervical approach. The length of the cricopharyngeal myotomy can vary from 2 to 6 cm.3,4 This is followed by one of three options:
- Diverticulectomy
- Diverticulopexy, in which the diverticulum is repositioned and sutured against the prevertebral fascia
- Diverticular inversion, in which the diverticulum is inverted into the esophageal lumen and then oversewn.4
Success rates for open surgery vary from study to study but are usually around 90%.4 Complications are reported in 10% to 30% of cases and include mediastinitis, severe recurrent laryngeal nerve injury, and a 1% to 2% chance of death. These rates seem high, but the older age of most of these patients puts them at high risk.5
Endoscopic procedures
All endoscopic procedures involve incision of the wall separating the diverticulum from the esophageal lumen to relieve the obstruction.
Rigid endoscopy is used in centers that do not offer flexible endoscopy for patients who are not candidates for surgery. It is done under general anesthesia. With the patient’s neck completely hyperextended, a rigid endoscope is passed into the oral cavity, and diverticulotomy and cricopharyngeal myotomy are performed.
This technique has been extensively evaluated and, although effective, carries up to an 8% risk of complications, including perforation. It is not recommended for patients with a small diverticulum, a high body mass index, or difficulty with neck extension.6
Flexible endoscopy is the first-line therapy for most patients in many centers. With the flexible endoscope, the diverticulotomy and the cricopharyngeal myotomy can be performed in an outpatient endoscopy suite, and general anesthesia is not required.3
Initial studies of outcomes using these approaches have been promising,3,7–9 with substantial reduction in dysphagia, regurgitation, and chronic cough. Unfortunately, this technique is associated with a recurrence rate as high as 25%. However, repeat endoscopic therapy in patients with recurrence results in success rates similar to those for first-time treatment.
Complication rates are low. In a series of 150 patients, four adverse events occurred—three cases of fever and one of pneumonia. In addition, one patient had subcutaneous emphysema that resolved spontaneously.7
Improved device on horizon
A potentially major advance in the endoscopic treatment of Zenker diverticulum is the development of a diverticuloscope, a soft tube with a V-shaped end. This tube is inserted through the mouth with one leg of the “V” protecting the anterior esophageal wall and the other leg protecting the posterior diverticular lumen. The endoscope is passed through this tube to perform the diverticulotomy. In one report,10 diverticuloscope-guided diverticulotomy led to fewer complications, shorter procedural time, and higher success rates compared with the standard technique.10 This device is not yet available in the United States.
No head-to-head comparison of outcomes with various flexible endoscopic techniques for treating Zenker diverticulum has been published, nor are there data yet on the success of surgery if endoscopic therapy fails.
We recommend flexible endoscopy for the initial treatment of Zenker diverticulum in most patients, but its availability is limited in the United States, as many practitioners do not have adequate experience with the technique.
TAKE-HOME POINTS
- Zenker diverticulum is an outpouching of the esophageal mucosa. It is often asymptomatic. Patients with minimal symptoms and with no functional impairment can be followed conservatively.
- A head-to-head comparison of surgical and endoscopic therapy has not yet been done, and optimal patient selection criteria are lacking. Therefore, treatment recommendations are based primarily on expert opinion.
- We recommend flexible endoscopic therapy as initial treatment in patients with a small diverticulum, but this may be limited by the unavailability of a surgeon experienced in this technique.
- For a large diverticulum, we recommend an open cervical procedure involving excision of the diverticulum or a diverticulopexy for patients who are good surgical candidates.
- Ferreira LE, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus 2008; 21:1–8.
- Manno M, Manta R, Caruso A, et al. Alternative endoscopic treatment of Zenker’s diverticulum: a case series (with video). Gastrointest Endosc 2014; 79:168–170.
- Yuan Y, Zhao YF, Hu Y, Chen LQ. Surgical treatment of Zenker’s diverticulum. Dig Surg 2013; 30:207–218.
- Bizzotto A, Iacopini F, Landi R, Costamagna G. Zenker’s diverticulum: exploring treatment options. Acta Otorhinolaryngol Ital 2013; 33:219–229.
- Repici A, Pagano N, Fumagalli U, et. al. Transoral treatment of Zenker diverticulum: flexible endoscopy versus endoscopic stapling. A retrospective comparison of outcomes. Dis Esophagus 2011; 24:235–239.
- Law R, Katzka DA, Baron TH. Zenker’s diverticulum. Clin Gastroenterol Hepatol 2014; 12:1773–1782.
- Huberty V, El Bacha S, Blero D, Le Moine O, Hassid S, Devière J. Endoscopic treatment for Zenker’s diverticulum: long-term results (with video). Gastrointest Endosc 2013; 77:701–707.
- Case DJ, Baron TH. Flexible endoscopic management of Zenker diverticulum: the Mayo Clinic experience. Mayo Clin Proc 2010; 85:719–722.
- Al-Kadi AS, Maghrabi AA, Thomson D, Gillman LM, Dhalla S. Endoscopic treatment of Zenker diverticulum: results of a 7-year experience. J Am Coll Surg 2010; 211:239–243.
- Costamagna G, Iacopini F, Tringali A, et al. Flexible endoscopic Zenker’s diverticulotomy: cap-assisted technique vs diverticuloscope-assisted technique. Endoscopy 2007; 39:146–152.
- Ferreira LE, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus 2008; 21:1–8.
- Manno M, Manta R, Caruso A, et al. Alternative endoscopic treatment of Zenker’s diverticulum: a case series (with video). Gastrointest Endosc 2014; 79:168–170.
- Yuan Y, Zhao YF, Hu Y, Chen LQ. Surgical treatment of Zenker’s diverticulum. Dig Surg 2013; 30:207–218.
- Bizzotto A, Iacopini F, Landi R, Costamagna G. Zenker’s diverticulum: exploring treatment options. Acta Otorhinolaryngol Ital 2013; 33:219–229.
- Repici A, Pagano N, Fumagalli U, et. al. Transoral treatment of Zenker diverticulum: flexible endoscopy versus endoscopic stapling. A retrospective comparison of outcomes. Dis Esophagus 2011; 24:235–239.
- Law R, Katzka DA, Baron TH. Zenker’s diverticulum. Clin Gastroenterol Hepatol 2014; 12:1773–1782.
- Huberty V, El Bacha S, Blero D, Le Moine O, Hassid S, Devière J. Endoscopic treatment for Zenker’s diverticulum: long-term results (with video). Gastrointest Endosc 2013; 77:701–707.
- Case DJ, Baron TH. Flexible endoscopic management of Zenker diverticulum: the Mayo Clinic experience. Mayo Clin Proc 2010; 85:719–722.
- Al-Kadi AS, Maghrabi AA, Thomson D, Gillman LM, Dhalla S. Endoscopic treatment of Zenker diverticulum: results of a 7-year experience. J Am Coll Surg 2010; 211:239–243.
- Costamagna G, Iacopini F, Tringali A, et al. Flexible endoscopic Zenker’s diverticulotomy: cap-assisted technique vs diverticuloscope-assisted technique. Endoscopy 2007; 39:146–152.