User login
New safety data regarding COVID vaccines
from the French National Agency for the Safety of Medicines and Health Products (ANSM).
The rare condition — more common in men than in women — is characterized by the sudden onset of severe pain in the shoulder, followed by arm paralysis. Its etiopathogenesis is not well understood, but vaccines, in particular the flu vaccine, have been implicated in some cases, the report states.
Six serious cases of the syndrome related to the Comirnaty (Pfizer) vaccine were reported by healthcare professionals and vaccinated individuals or their family and friends since the start of the monitoring program. Four of these cases occurred from September 3 to 16.
All six cases involved patients 19 to 69 years of age — two women and four men — who developed symptoms in the 50 days after vaccination. Half were reported after the first dose and half after the second dose. Four of the patients are currently recovering; the outcomes of the other two are unknown.
In the case of the Spikevax vaccine (Moderna), two cases of Parsonage-Turner syndrome were reported after vaccination (plus one that occurred after 50 days, which is currently being managed). The onset of symptoms in these two men — one in his early 30s and one in his early 60s — occurred less than 18 days after vaccination. One occurred after the first dose and one after the second dose. This timing indicates a possible link between the syndrome and the vaccine. Both men are currently in recovery.
This signal of mRNA vaccines is now “officially recognized,” according to the Pfizer and Moderna reports.
It is also considered a “potential signal” in the Vaxzevria (AstraZeneca) pharmacovigilance report, released October 8, which describes eight cases of Parsonage-Turner syndrome after vaccination.
Safety profile of mRNA COVID vaccines in youth
Between June 15, when children 12 years and older became eligible for vaccination, and August 26, there were 591 reports of potential adverse events — out of 6 million Pfizer doses administered — in 12- to 18-year-old children.
Of the 591 cases, 35.2% were deemed serious. The majority of these were cases of reactogenicity, malaise, or postvaccine discomfort (25%), followed by instances of myocarditis and pericarditis (15.9% and 7.2%, respectively). In eight of 10 cases, one of the first symptom reported was chest pain.
Myocarditis occurred in 39.4% of people after the first injection (mean time to onset, 13 days) and 54.5% after the second (mean time to onset, 4 days). Recorded progress was favorable in nearly nine of 10 cases.
Pericarditis occurred in 53.3% of people after the first injection (mean time to onset, 13 days), and 40.0% after the second (mean time to onset, 4 days).
Three cases of multisystem inflammatory syndrome in children (MISC) were reported after monitoring ended.
For this age group, “all reported events will continue to be monitored, especially serious events and multisystem inflammatory syndrome in children,” report authors conclude.
Data for adverse events after the Moderna vaccine remain limited, but the report stipulates that “the adverse events reported in 12- to 18-year-olds who received an injection do not display any particular pattern, compared with those reported in older subjects, with the exception of a roughly 100-fold lower incidence of reported adverse effects in the 12- to 17-year age group.”
No safety warnings for pregnant women
The pharmacovigilance report — which covered the period from December 27, 2020 to September 9, 2021 — “raises no safety warnings for pregnant or nursing women with any of the COVID-19 vaccines.” In addition, two recent studies — one published in JAMA and one in the New England Journal of Medicine — have shown no link between spontaneous miscarriage and mRNA vaccines.
“Moreover, it should be stressed that current data from the international literature consistently show that maternal SARS COV-2 infection increases the risk for fetal, maternal, and neonatal complications, and that this risk may increase with the arrival of the Alpha and Delta variants,” they write. “It is therefore important to reiterate the current recommendations to vaccinate all pregnant women, regardless of the stage of pregnancy.”
Some adverse effects, such as thromboembolic effects, in utero death, HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, and uterine contractions, will continue to be monitored.
Questions regarding menstrual disorders
As for gynecological disorders reported after vaccination, questions still remain. “In most of the reported cases, it is difficult to accurately determine whether the vaccine played a role in the occurrence of menstrual/genital bleeding,” the authors of the pharmacovigilance monitoring report state.
“Nonetheless, these cases warrant attention,” they add, and further discussions with the French National Association of Obstetricians and Gynecologists and the French Society of Endocrinology are needed in regard to these potential safety signals.
A version of this article first appeared on Medscape.com.
from the French National Agency for the Safety of Medicines and Health Products (ANSM).
The rare condition — more common in men than in women — is characterized by the sudden onset of severe pain in the shoulder, followed by arm paralysis. Its etiopathogenesis is not well understood, but vaccines, in particular the flu vaccine, have been implicated in some cases, the report states.
Six serious cases of the syndrome related to the Comirnaty (Pfizer) vaccine were reported by healthcare professionals and vaccinated individuals or their family and friends since the start of the monitoring program. Four of these cases occurred from September 3 to 16.
All six cases involved patients 19 to 69 years of age — two women and four men — who developed symptoms in the 50 days after vaccination. Half were reported after the first dose and half after the second dose. Four of the patients are currently recovering; the outcomes of the other two are unknown.
In the case of the Spikevax vaccine (Moderna), two cases of Parsonage-Turner syndrome were reported after vaccination (plus one that occurred after 50 days, which is currently being managed). The onset of symptoms in these two men — one in his early 30s and one in his early 60s — occurred less than 18 days after vaccination. One occurred after the first dose and one after the second dose. This timing indicates a possible link between the syndrome and the vaccine. Both men are currently in recovery.
This signal of mRNA vaccines is now “officially recognized,” according to the Pfizer and Moderna reports.
It is also considered a “potential signal” in the Vaxzevria (AstraZeneca) pharmacovigilance report, released October 8, which describes eight cases of Parsonage-Turner syndrome after vaccination.
Safety profile of mRNA COVID vaccines in youth
Between June 15, when children 12 years and older became eligible for vaccination, and August 26, there were 591 reports of potential adverse events — out of 6 million Pfizer doses administered — in 12- to 18-year-old children.
Of the 591 cases, 35.2% were deemed serious. The majority of these were cases of reactogenicity, malaise, or postvaccine discomfort (25%), followed by instances of myocarditis and pericarditis (15.9% and 7.2%, respectively). In eight of 10 cases, one of the first symptom reported was chest pain.
Myocarditis occurred in 39.4% of people after the first injection (mean time to onset, 13 days) and 54.5% after the second (mean time to onset, 4 days). Recorded progress was favorable in nearly nine of 10 cases.
Pericarditis occurred in 53.3% of people after the first injection (mean time to onset, 13 days), and 40.0% after the second (mean time to onset, 4 days).
Three cases of multisystem inflammatory syndrome in children (MISC) were reported after monitoring ended.
For this age group, “all reported events will continue to be monitored, especially serious events and multisystem inflammatory syndrome in children,” report authors conclude.
Data for adverse events after the Moderna vaccine remain limited, but the report stipulates that “the adverse events reported in 12- to 18-year-olds who received an injection do not display any particular pattern, compared with those reported in older subjects, with the exception of a roughly 100-fold lower incidence of reported adverse effects in the 12- to 17-year age group.”
No safety warnings for pregnant women
The pharmacovigilance report — which covered the period from December 27, 2020 to September 9, 2021 — “raises no safety warnings for pregnant or nursing women with any of the COVID-19 vaccines.” In addition, two recent studies — one published in JAMA and one in the New England Journal of Medicine — have shown no link between spontaneous miscarriage and mRNA vaccines.
“Moreover, it should be stressed that current data from the international literature consistently show that maternal SARS COV-2 infection increases the risk for fetal, maternal, and neonatal complications, and that this risk may increase with the arrival of the Alpha and Delta variants,” they write. “It is therefore important to reiterate the current recommendations to vaccinate all pregnant women, regardless of the stage of pregnancy.”
Some adverse effects, such as thromboembolic effects, in utero death, HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, and uterine contractions, will continue to be monitored.
Questions regarding menstrual disorders
As for gynecological disorders reported after vaccination, questions still remain. “In most of the reported cases, it is difficult to accurately determine whether the vaccine played a role in the occurrence of menstrual/genital bleeding,” the authors of the pharmacovigilance monitoring report state.
“Nonetheless, these cases warrant attention,” they add, and further discussions with the French National Association of Obstetricians and Gynecologists and the French Society of Endocrinology are needed in regard to these potential safety signals.
A version of this article first appeared on Medscape.com.
from the French National Agency for the Safety of Medicines and Health Products (ANSM).
The rare condition — more common in men than in women — is characterized by the sudden onset of severe pain in the shoulder, followed by arm paralysis. Its etiopathogenesis is not well understood, but vaccines, in particular the flu vaccine, have been implicated in some cases, the report states.
Six serious cases of the syndrome related to the Comirnaty (Pfizer) vaccine were reported by healthcare professionals and vaccinated individuals or their family and friends since the start of the monitoring program. Four of these cases occurred from September 3 to 16.
All six cases involved patients 19 to 69 years of age — two women and four men — who developed symptoms in the 50 days after vaccination. Half were reported after the first dose and half after the second dose. Four of the patients are currently recovering; the outcomes of the other two are unknown.
In the case of the Spikevax vaccine (Moderna), two cases of Parsonage-Turner syndrome were reported after vaccination (plus one that occurred after 50 days, which is currently being managed). The onset of symptoms in these two men — one in his early 30s and one in his early 60s — occurred less than 18 days after vaccination. One occurred after the first dose and one after the second dose. This timing indicates a possible link between the syndrome and the vaccine. Both men are currently in recovery.
This signal of mRNA vaccines is now “officially recognized,” according to the Pfizer and Moderna reports.
It is also considered a “potential signal” in the Vaxzevria (AstraZeneca) pharmacovigilance report, released October 8, which describes eight cases of Parsonage-Turner syndrome after vaccination.
Safety profile of mRNA COVID vaccines in youth
Between June 15, when children 12 years and older became eligible for vaccination, and August 26, there were 591 reports of potential adverse events — out of 6 million Pfizer doses administered — in 12- to 18-year-old children.
Of the 591 cases, 35.2% were deemed serious. The majority of these were cases of reactogenicity, malaise, or postvaccine discomfort (25%), followed by instances of myocarditis and pericarditis (15.9% and 7.2%, respectively). In eight of 10 cases, one of the first symptom reported was chest pain.
Myocarditis occurred in 39.4% of people after the first injection (mean time to onset, 13 days) and 54.5% after the second (mean time to onset, 4 days). Recorded progress was favorable in nearly nine of 10 cases.
Pericarditis occurred in 53.3% of people after the first injection (mean time to onset, 13 days), and 40.0% after the second (mean time to onset, 4 days).
Three cases of multisystem inflammatory syndrome in children (MISC) were reported after monitoring ended.
For this age group, “all reported events will continue to be monitored, especially serious events and multisystem inflammatory syndrome in children,” report authors conclude.
Data for adverse events after the Moderna vaccine remain limited, but the report stipulates that “the adverse events reported in 12- to 18-year-olds who received an injection do not display any particular pattern, compared with those reported in older subjects, with the exception of a roughly 100-fold lower incidence of reported adverse effects in the 12- to 17-year age group.”
No safety warnings for pregnant women
The pharmacovigilance report — which covered the period from December 27, 2020 to September 9, 2021 — “raises no safety warnings for pregnant or nursing women with any of the COVID-19 vaccines.” In addition, two recent studies — one published in JAMA and one in the New England Journal of Medicine — have shown no link between spontaneous miscarriage and mRNA vaccines.
“Moreover, it should be stressed that current data from the international literature consistently show that maternal SARS COV-2 infection increases the risk for fetal, maternal, and neonatal complications, and that this risk may increase with the arrival of the Alpha and Delta variants,” they write. “It is therefore important to reiterate the current recommendations to vaccinate all pregnant women, regardless of the stage of pregnancy.”
Some adverse effects, such as thromboembolic effects, in utero death, HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, and uterine contractions, will continue to be monitored.
Questions regarding menstrual disorders
As for gynecological disorders reported after vaccination, questions still remain. “In most of the reported cases, it is difficult to accurately determine whether the vaccine played a role in the occurrence of menstrual/genital bleeding,” the authors of the pharmacovigilance monitoring report state.
“Nonetheless, these cases warrant attention,” they add, and further discussions with the French National Association of Obstetricians and Gynecologists and the French Society of Endocrinology are needed in regard to these potential safety signals.
A version of this article first appeared on Medscape.com.
Even one vaccinated member can cut family’s COVID risk
The chances are reduced even further with each additional vaccinated or otherwise immune family member, according to new data.
Lead author Peter Nordström, MD, PhD, with the unit of geriatric medicine, Umeå (Sweden) University, said in an interview the message is important for public health: “When you vaccinate, you do not just protect yourself but also your relatives.”
The findings were published online on Oct. 11, 2021, in JAMA Internal Medicine.
Researchers analyzed data from 1,789,728 individuals from 814,806 families from nationwide registries in Sweden. All individuals had acquired immunity either from previously being infected with SARS-CoV-2 or by being fully vaccinated (that is, having received two doses of the Moderna, Pfizer, or Oxford/AstraZeneca vaccines). Persons were considered for inclusion until May 26, 2021.
Each person with immunity was matched in a 1:1 ratio to a person without immunity from a cohort of individuals with families that had from two to five members. Families with more than five members were excluded because of small sample sizes.
Primarily nonimmune families in which there was one immune family member had a 45%-61% lower risk of contracting COVID-19 (hazard ratio, 0.39-0.55; 95% confidence interval, 0.37-0.61; P < .001).
The risk reduction increased to 75%-86% when two family members were immune (HR, 0.14-0.25; 95% CI, 0.11-0.27; P < .001).
It increased to 91%-94% when three family members were immune (HR, 0.06-0.09; 95% CI, 0.04-0.10; P < .001) and to 97% with four immune family members (HR, 0.03; 95% CI, 0.02-0.05; P < .001).
“The results were similar for the outcome of COVID-19 infection that was severe enough to warrant a hospital stay,” the authors wrote. They listed as an example that, in three-member families in which two members were immune, the remaining nonimmune family member had an 80% lower risk for hospitalization (HR, 0.20; 95% CI, 0.10-0.43; P < .001).
Global implications
Dr. Nordström said the team used the family setting because it was more easily identifiable as a cohort with the national registries and because COVID-19 is spread among people in close contact with each other. The findings have implications for other groups that spend large amounts of time together and for herd immunity, he added.
The findings may be particularly welcome in regions of the world where vaccination rates are very low. The authors noted that most of the global population has not yet been vaccinated and that “it is anticipated that most of the population in low-income countries will be unable to receive a vaccine in 2021, with current vaccination rates suggesting that completely inoculating 70%-85% of the global population may take up to 5 years.”
Jill Foster, MD, a pediatric infectious disease specialist at the University of Minnesota, Minneapolis, said in an interview she agrees that the news could encourage countries that have very low vaccination rates.
This study may help motivate areas with few resources to start small, she said: “Even one is better than zero.”
She added that this news could also help ease the minds of families that have immunocompromised members or in which there are children who are too young to be vaccinated.
With these data, she said, people can see there’s something they can do to help protect a family member.
Dr. Foster said that although it’s intuitive to think that the more vaccinated people there are in a family, the safer people are, “it’s really nice to see the data coming out of such a large dataset.”
The authors acknowledged that a limitation of the study is that, at the time the study was conducted, the Delta variant was uncommon in Sweden. It is therefore unclear whether the findings regarding immunity are still relevant in Sweden and elsewhere now that the Delta strain is dominant.
The authors reported no relevant financial relationships. Dr. Foster has received grant support from Moderna.
A version of this article first appeared on Medscape.com.
The chances are reduced even further with each additional vaccinated or otherwise immune family member, according to new data.
Lead author Peter Nordström, MD, PhD, with the unit of geriatric medicine, Umeå (Sweden) University, said in an interview the message is important for public health: “When you vaccinate, you do not just protect yourself but also your relatives.”
The findings were published online on Oct. 11, 2021, in JAMA Internal Medicine.
Researchers analyzed data from 1,789,728 individuals from 814,806 families from nationwide registries in Sweden. All individuals had acquired immunity either from previously being infected with SARS-CoV-2 or by being fully vaccinated (that is, having received two doses of the Moderna, Pfizer, or Oxford/AstraZeneca vaccines). Persons were considered for inclusion until May 26, 2021.
Each person with immunity was matched in a 1:1 ratio to a person without immunity from a cohort of individuals with families that had from two to five members. Families with more than five members were excluded because of small sample sizes.
Primarily nonimmune families in which there was one immune family member had a 45%-61% lower risk of contracting COVID-19 (hazard ratio, 0.39-0.55; 95% confidence interval, 0.37-0.61; P < .001).
The risk reduction increased to 75%-86% when two family members were immune (HR, 0.14-0.25; 95% CI, 0.11-0.27; P < .001).
It increased to 91%-94% when three family members were immune (HR, 0.06-0.09; 95% CI, 0.04-0.10; P < .001) and to 97% with four immune family members (HR, 0.03; 95% CI, 0.02-0.05; P < .001).
“The results were similar for the outcome of COVID-19 infection that was severe enough to warrant a hospital stay,” the authors wrote. They listed as an example that, in three-member families in which two members were immune, the remaining nonimmune family member had an 80% lower risk for hospitalization (HR, 0.20; 95% CI, 0.10-0.43; P < .001).
Global implications
Dr. Nordström said the team used the family setting because it was more easily identifiable as a cohort with the national registries and because COVID-19 is spread among people in close contact with each other. The findings have implications for other groups that spend large amounts of time together and for herd immunity, he added.
The findings may be particularly welcome in regions of the world where vaccination rates are very low. The authors noted that most of the global population has not yet been vaccinated and that “it is anticipated that most of the population in low-income countries will be unable to receive a vaccine in 2021, with current vaccination rates suggesting that completely inoculating 70%-85% of the global population may take up to 5 years.”
Jill Foster, MD, a pediatric infectious disease specialist at the University of Minnesota, Minneapolis, said in an interview she agrees that the news could encourage countries that have very low vaccination rates.
This study may help motivate areas with few resources to start small, she said: “Even one is better than zero.”
She added that this news could also help ease the minds of families that have immunocompromised members or in which there are children who are too young to be vaccinated.
With these data, she said, people can see there’s something they can do to help protect a family member.
Dr. Foster said that although it’s intuitive to think that the more vaccinated people there are in a family, the safer people are, “it’s really nice to see the data coming out of such a large dataset.”
The authors acknowledged that a limitation of the study is that, at the time the study was conducted, the Delta variant was uncommon in Sweden. It is therefore unclear whether the findings regarding immunity are still relevant in Sweden and elsewhere now that the Delta strain is dominant.
The authors reported no relevant financial relationships. Dr. Foster has received grant support from Moderna.
A version of this article first appeared on Medscape.com.
The chances are reduced even further with each additional vaccinated or otherwise immune family member, according to new data.
Lead author Peter Nordström, MD, PhD, with the unit of geriatric medicine, Umeå (Sweden) University, said in an interview the message is important for public health: “When you vaccinate, you do not just protect yourself but also your relatives.”
The findings were published online on Oct. 11, 2021, in JAMA Internal Medicine.
Researchers analyzed data from 1,789,728 individuals from 814,806 families from nationwide registries in Sweden. All individuals had acquired immunity either from previously being infected with SARS-CoV-2 or by being fully vaccinated (that is, having received two doses of the Moderna, Pfizer, or Oxford/AstraZeneca vaccines). Persons were considered for inclusion until May 26, 2021.
Each person with immunity was matched in a 1:1 ratio to a person without immunity from a cohort of individuals with families that had from two to five members. Families with more than five members were excluded because of small sample sizes.
Primarily nonimmune families in which there was one immune family member had a 45%-61% lower risk of contracting COVID-19 (hazard ratio, 0.39-0.55; 95% confidence interval, 0.37-0.61; P < .001).
The risk reduction increased to 75%-86% when two family members were immune (HR, 0.14-0.25; 95% CI, 0.11-0.27; P < .001).
It increased to 91%-94% when three family members were immune (HR, 0.06-0.09; 95% CI, 0.04-0.10; P < .001) and to 97% with four immune family members (HR, 0.03; 95% CI, 0.02-0.05; P < .001).
“The results were similar for the outcome of COVID-19 infection that was severe enough to warrant a hospital stay,” the authors wrote. They listed as an example that, in three-member families in which two members were immune, the remaining nonimmune family member had an 80% lower risk for hospitalization (HR, 0.20; 95% CI, 0.10-0.43; P < .001).
Global implications
Dr. Nordström said the team used the family setting because it was more easily identifiable as a cohort with the national registries and because COVID-19 is spread among people in close contact with each other. The findings have implications for other groups that spend large amounts of time together and for herd immunity, he added.
The findings may be particularly welcome in regions of the world where vaccination rates are very low. The authors noted that most of the global population has not yet been vaccinated and that “it is anticipated that most of the population in low-income countries will be unable to receive a vaccine in 2021, with current vaccination rates suggesting that completely inoculating 70%-85% of the global population may take up to 5 years.”
Jill Foster, MD, a pediatric infectious disease specialist at the University of Minnesota, Minneapolis, said in an interview she agrees that the news could encourage countries that have very low vaccination rates.
This study may help motivate areas with few resources to start small, she said: “Even one is better than zero.”
She added that this news could also help ease the minds of families that have immunocompromised members or in which there are children who are too young to be vaccinated.
With these data, she said, people can see there’s something they can do to help protect a family member.
Dr. Foster said that although it’s intuitive to think that the more vaccinated people there are in a family, the safer people are, “it’s really nice to see the data coming out of such a large dataset.”
The authors acknowledged that a limitation of the study is that, at the time the study was conducted, the Delta variant was uncommon in Sweden. It is therefore unclear whether the findings regarding immunity are still relevant in Sweden and elsewhere now that the Delta strain is dominant.
The authors reported no relevant financial relationships. Dr. Foster has received grant support from Moderna.
A version of this article first appeared on Medscape.com.
CDC: Children just as vulnerable to COVID as adults
The study, which focused on 1,000 schools in Arizona’s Maricopa and Pima counties, found that there were 113 COVID-19 outbreaks in schools without mask requirements in the first month of in-person learning. There were 16 outbreaks in schools with mask requirements.
“Masks in schools work to protect our children, to keep them and their school communities safe, and to keep them in school for in-person learning,” CDC Director Rochelle Walensky, MD, said at an Oct. 13 White House briefing.
But, she said, more than 95% of schools across the country had remained open through the end of September, despite 1,800 school closures affecting nearly 1 million students.
Protection for children in school is just one piece of the puzzle, Dr. Walensky said – there must also be COVID-safe practices at home to limit transmission. A CDC study published in October found that children had similar infection rates, compared with adults, confirming there is risk to people of all ages.
“For those children not yet eligible for vaccination, the best protection we can provide them is to make sure everyone around them in the household is vaccinated and to make sure they’re wearing a mask in school and during indoor extracurricular activities,” Dr. Walensky said.
Meanwhile, Pfizer’s vaccine for children ages 5-11 may be approved by early November. The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee will meet Oct. 26 to discuss available data, and the CDC’s Advisory Committee on Immunization Practices will meet Nov. 2. A decision is expected soon after.
A version of this article first appeared on WebMD.com.
The study, which focused on 1,000 schools in Arizona’s Maricopa and Pima counties, found that there were 113 COVID-19 outbreaks in schools without mask requirements in the first month of in-person learning. There were 16 outbreaks in schools with mask requirements.
“Masks in schools work to protect our children, to keep them and their school communities safe, and to keep them in school for in-person learning,” CDC Director Rochelle Walensky, MD, said at an Oct. 13 White House briefing.
But, she said, more than 95% of schools across the country had remained open through the end of September, despite 1,800 school closures affecting nearly 1 million students.
Protection for children in school is just one piece of the puzzle, Dr. Walensky said – there must also be COVID-safe practices at home to limit transmission. A CDC study published in October found that children had similar infection rates, compared with adults, confirming there is risk to people of all ages.
“For those children not yet eligible for vaccination, the best protection we can provide them is to make sure everyone around them in the household is vaccinated and to make sure they’re wearing a mask in school and during indoor extracurricular activities,” Dr. Walensky said.
Meanwhile, Pfizer’s vaccine for children ages 5-11 may be approved by early November. The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee will meet Oct. 26 to discuss available data, and the CDC’s Advisory Committee on Immunization Practices will meet Nov. 2. A decision is expected soon after.
A version of this article first appeared on WebMD.com.
The study, which focused on 1,000 schools in Arizona’s Maricopa and Pima counties, found that there were 113 COVID-19 outbreaks in schools without mask requirements in the first month of in-person learning. There were 16 outbreaks in schools with mask requirements.
“Masks in schools work to protect our children, to keep them and their school communities safe, and to keep them in school for in-person learning,” CDC Director Rochelle Walensky, MD, said at an Oct. 13 White House briefing.
But, she said, more than 95% of schools across the country had remained open through the end of September, despite 1,800 school closures affecting nearly 1 million students.
Protection for children in school is just one piece of the puzzle, Dr. Walensky said – there must also be COVID-safe practices at home to limit transmission. A CDC study published in October found that children had similar infection rates, compared with adults, confirming there is risk to people of all ages.
“For those children not yet eligible for vaccination, the best protection we can provide them is to make sure everyone around them in the household is vaccinated and to make sure they’re wearing a mask in school and during indoor extracurricular activities,” Dr. Walensky said.
Meanwhile, Pfizer’s vaccine for children ages 5-11 may be approved by early November. The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee will meet Oct. 26 to discuss available data, and the CDC’s Advisory Committee on Immunization Practices will meet Nov. 2. A decision is expected soon after.
A version of this article first appeared on WebMD.com.
No short-term death risk in elderly after COVID-19 vaccines
and launched an investigation into the safety of the BNT162b2 vaccine (Comirnaty; Pfizer-BioNTech).
Now, the results of that investigation and of a subsequent larger study of nursing home residents in Norway have shown no increased risk for short-term mortality following COVID-19 vaccination in the overall population of elderly patients. The new research also showed clear evidence of a survival benefit compared with the unvaccinated population, Anette Hylen Ranhoff, MD, PhD, said at the annual meeting of the European Geriatric Medicine Society, held in a hybrid format in Athens, Greece, and online.
“We found no evidence of increased short-term mortality among vaccinated older individuals, and particularly not among the nursing home patients,” said Dr. Ranhoff, a senior researcher at the Norwegian Institute of Public Health and professor at University of Bergen, Norway. “But we think that this [lower] mortality risk was most likely a sort of ‘healthy-vaccinee’ effect, which means that people who were a bit more healthy were vaccinated, and not those who were the very, very most frail.”
“We have more or less the same data in France about events, with very high rates of vaccination,” said session moderator Athanase Benetos MD, PhD, professor and chairman of geriatric medicine at the University Hospital of Nancy in France, who was not involved in the study.
“In my department, a month after the end of the vaccination and at the same time while the pandemic in the city was going up, we had a 90% decrease in mortality from COVID in the nursing homes,” he told Dr. Ranhoff.
Potential risks
Frail elderly patients were not included in clinical trials of COVID-19 vaccines, and although previous studies have shown a low incidence of local or systemic reactions to vaccination among older people, “we think that quite mild adverse events following vaccination could trigger and destabilize a frail person,” Dr. Ranhoff said.
As reported Jan. 15, 2021, in BMJ, investigation by the Norwegian Medicines Agency (NOMA) into 13 of the 23 reported cases concluded that common adverse reactions associated with mRNA vaccines could have contributed to the deaths of some of the frail elderly patients
Steinar Madsen, MD, NOMA medical director, told BMJ “we are not alarmed or worried about this, because these are very rare occurrences and they occurred in very frail patients with very serious disease.”
Health authorities investigate
In response to the report and at the request of the Norwegian Public Health Institute and NOMA, Dr. Ranhoff and colleagues investigated the first 100 deaths among nursing-home residents who received the vaccine. The team consisted of three geriatricians and an infectious disease specialist who sees patients in nursing homes.
They looked at each patient’s clinical course before and after vaccination, their health trajectory and life expectancy at the time of vaccination, new symptoms following vaccination, and the time from vaccination to new symptoms and to death.
In addition, the investigators evaluated Clinical Frailty Scale (CFS) scores for each patient. CFS scores range from 1 (very fit) to 9 (terminally ill, with a life expectancy of less than 6 months who are otherwise evidently frail).
The initial investigation found that among 95 evaluable patients, the association between vaccination and death was “probable” in 10, “possible” in 26, and “unlikely” in 59.
The mean time from vaccination to symptoms was 1.4 days in the probable cases, 2.5 days in the possible cases, and 4.7 days in the unlikely cases.
The mean time from vaccination to death was 3.1, 8.3, and 8.2 days, respectively.
In all three categories, the patients had mean CFS scores ranging from 7.6 to 7.9, putting them in the “severely frail” category, defined as people who are completely dependent for personal care but seem stable and not at high risk for dying.
“We have quite many nursing home residents in Norway, 35,000; more than 80% have dementia, and the mean age is 85 years. We know that approximately 45 people die every day in these nursing homes, and their mean age of death is 87.5 years,” Dr. Ranhoff said.
Population-wide study
Dr. Ranhoff and colleagues also looked more broadly into the question of potential vaccine-related mortality in the total population of older people in Norway from the day of vaccination to follow-up at 3 weeks.
They conducted a matched cohort study to investigate the relationship between the mRNA SARS-CoV-2 vaccine and overall death among persons aged 65 and older in the general population, and across four groups: patients receiving home-based care, long-term nursing home patients, short-term nursing home patients, and those not receiving health services.
The researchers identified a total of 967,786 residents of Norway aged 65 and over at the start of the country’s vaccination campaign at the end of December, 2020, and they matched vaccinated individuals with unvaccinated persons based on demographic, geographic, and clinical risk group factors.
Dr. Ranhoff showed Kaplan-Meier survival curves for the total population and for each of the health-service states. In all cases there was a clear survival benefit for vaccinated vs. unvaccinated patients. She did not, however, provide specific numbers or hazard ratios for the differences between vaccinated and unvaccinated individuals in each of the comparisons.
The study was supported by the Norwegian Institute of Public Health. Dr. Ranhoff and Dr. Benetos reported no conflicts of interest.
and launched an investigation into the safety of the BNT162b2 vaccine (Comirnaty; Pfizer-BioNTech).
Now, the results of that investigation and of a subsequent larger study of nursing home residents in Norway have shown no increased risk for short-term mortality following COVID-19 vaccination in the overall population of elderly patients. The new research also showed clear evidence of a survival benefit compared with the unvaccinated population, Anette Hylen Ranhoff, MD, PhD, said at the annual meeting of the European Geriatric Medicine Society, held in a hybrid format in Athens, Greece, and online.
“We found no evidence of increased short-term mortality among vaccinated older individuals, and particularly not among the nursing home patients,” said Dr. Ranhoff, a senior researcher at the Norwegian Institute of Public Health and professor at University of Bergen, Norway. “But we think that this [lower] mortality risk was most likely a sort of ‘healthy-vaccinee’ effect, which means that people who were a bit more healthy were vaccinated, and not those who were the very, very most frail.”
“We have more or less the same data in France about events, with very high rates of vaccination,” said session moderator Athanase Benetos MD, PhD, professor and chairman of geriatric medicine at the University Hospital of Nancy in France, who was not involved in the study.
“In my department, a month after the end of the vaccination and at the same time while the pandemic in the city was going up, we had a 90% decrease in mortality from COVID in the nursing homes,” he told Dr. Ranhoff.
Potential risks
Frail elderly patients were not included in clinical trials of COVID-19 vaccines, and although previous studies have shown a low incidence of local or systemic reactions to vaccination among older people, “we think that quite mild adverse events following vaccination could trigger and destabilize a frail person,” Dr. Ranhoff said.
As reported Jan. 15, 2021, in BMJ, investigation by the Norwegian Medicines Agency (NOMA) into 13 of the 23 reported cases concluded that common adverse reactions associated with mRNA vaccines could have contributed to the deaths of some of the frail elderly patients
Steinar Madsen, MD, NOMA medical director, told BMJ “we are not alarmed or worried about this, because these are very rare occurrences and they occurred in very frail patients with very serious disease.”
Health authorities investigate
In response to the report and at the request of the Norwegian Public Health Institute and NOMA, Dr. Ranhoff and colleagues investigated the first 100 deaths among nursing-home residents who received the vaccine. The team consisted of three geriatricians and an infectious disease specialist who sees patients in nursing homes.
They looked at each patient’s clinical course before and after vaccination, their health trajectory and life expectancy at the time of vaccination, new symptoms following vaccination, and the time from vaccination to new symptoms and to death.
In addition, the investigators evaluated Clinical Frailty Scale (CFS) scores for each patient. CFS scores range from 1 (very fit) to 9 (terminally ill, with a life expectancy of less than 6 months who are otherwise evidently frail).
The initial investigation found that among 95 evaluable patients, the association between vaccination and death was “probable” in 10, “possible” in 26, and “unlikely” in 59.
The mean time from vaccination to symptoms was 1.4 days in the probable cases, 2.5 days in the possible cases, and 4.7 days in the unlikely cases.
The mean time from vaccination to death was 3.1, 8.3, and 8.2 days, respectively.
In all three categories, the patients had mean CFS scores ranging from 7.6 to 7.9, putting them in the “severely frail” category, defined as people who are completely dependent for personal care but seem stable and not at high risk for dying.
“We have quite many nursing home residents in Norway, 35,000; more than 80% have dementia, and the mean age is 85 years. We know that approximately 45 people die every day in these nursing homes, and their mean age of death is 87.5 years,” Dr. Ranhoff said.
Population-wide study
Dr. Ranhoff and colleagues also looked more broadly into the question of potential vaccine-related mortality in the total population of older people in Norway from the day of vaccination to follow-up at 3 weeks.
They conducted a matched cohort study to investigate the relationship between the mRNA SARS-CoV-2 vaccine and overall death among persons aged 65 and older in the general population, and across four groups: patients receiving home-based care, long-term nursing home patients, short-term nursing home patients, and those not receiving health services.
The researchers identified a total of 967,786 residents of Norway aged 65 and over at the start of the country’s vaccination campaign at the end of December, 2020, and they matched vaccinated individuals with unvaccinated persons based on demographic, geographic, and clinical risk group factors.
Dr. Ranhoff showed Kaplan-Meier survival curves for the total population and for each of the health-service states. In all cases there was a clear survival benefit for vaccinated vs. unvaccinated patients. She did not, however, provide specific numbers or hazard ratios for the differences between vaccinated and unvaccinated individuals in each of the comparisons.
The study was supported by the Norwegian Institute of Public Health. Dr. Ranhoff and Dr. Benetos reported no conflicts of interest.
and launched an investigation into the safety of the BNT162b2 vaccine (Comirnaty; Pfizer-BioNTech).
Now, the results of that investigation and of a subsequent larger study of nursing home residents in Norway have shown no increased risk for short-term mortality following COVID-19 vaccination in the overall population of elderly patients. The new research also showed clear evidence of a survival benefit compared with the unvaccinated population, Anette Hylen Ranhoff, MD, PhD, said at the annual meeting of the European Geriatric Medicine Society, held in a hybrid format in Athens, Greece, and online.
“We found no evidence of increased short-term mortality among vaccinated older individuals, and particularly not among the nursing home patients,” said Dr. Ranhoff, a senior researcher at the Norwegian Institute of Public Health and professor at University of Bergen, Norway. “But we think that this [lower] mortality risk was most likely a sort of ‘healthy-vaccinee’ effect, which means that people who were a bit more healthy were vaccinated, and not those who were the very, very most frail.”
“We have more or less the same data in France about events, with very high rates of vaccination,” said session moderator Athanase Benetos MD, PhD, professor and chairman of geriatric medicine at the University Hospital of Nancy in France, who was not involved in the study.
“In my department, a month after the end of the vaccination and at the same time while the pandemic in the city was going up, we had a 90% decrease in mortality from COVID in the nursing homes,” he told Dr. Ranhoff.
Potential risks
Frail elderly patients were not included in clinical trials of COVID-19 vaccines, and although previous studies have shown a low incidence of local or systemic reactions to vaccination among older people, “we think that quite mild adverse events following vaccination could trigger and destabilize a frail person,” Dr. Ranhoff said.
As reported Jan. 15, 2021, in BMJ, investigation by the Norwegian Medicines Agency (NOMA) into 13 of the 23 reported cases concluded that common adverse reactions associated with mRNA vaccines could have contributed to the deaths of some of the frail elderly patients
Steinar Madsen, MD, NOMA medical director, told BMJ “we are not alarmed or worried about this, because these are very rare occurrences and they occurred in very frail patients with very serious disease.”
Health authorities investigate
In response to the report and at the request of the Norwegian Public Health Institute and NOMA, Dr. Ranhoff and colleagues investigated the first 100 deaths among nursing-home residents who received the vaccine. The team consisted of three geriatricians and an infectious disease specialist who sees patients in nursing homes.
They looked at each patient’s clinical course before and after vaccination, their health trajectory and life expectancy at the time of vaccination, new symptoms following vaccination, and the time from vaccination to new symptoms and to death.
In addition, the investigators evaluated Clinical Frailty Scale (CFS) scores for each patient. CFS scores range from 1 (very fit) to 9 (terminally ill, with a life expectancy of less than 6 months who are otherwise evidently frail).
The initial investigation found that among 95 evaluable patients, the association between vaccination and death was “probable” in 10, “possible” in 26, and “unlikely” in 59.
The mean time from vaccination to symptoms was 1.4 days in the probable cases, 2.5 days in the possible cases, and 4.7 days in the unlikely cases.
The mean time from vaccination to death was 3.1, 8.3, and 8.2 days, respectively.
In all three categories, the patients had mean CFS scores ranging from 7.6 to 7.9, putting them in the “severely frail” category, defined as people who are completely dependent for personal care but seem stable and not at high risk for dying.
“We have quite many nursing home residents in Norway, 35,000; more than 80% have dementia, and the mean age is 85 years. We know that approximately 45 people die every day in these nursing homes, and their mean age of death is 87.5 years,” Dr. Ranhoff said.
Population-wide study
Dr. Ranhoff and colleagues also looked more broadly into the question of potential vaccine-related mortality in the total population of older people in Norway from the day of vaccination to follow-up at 3 weeks.
They conducted a matched cohort study to investigate the relationship between the mRNA SARS-CoV-2 vaccine and overall death among persons aged 65 and older in the general population, and across four groups: patients receiving home-based care, long-term nursing home patients, short-term nursing home patients, and those not receiving health services.
The researchers identified a total of 967,786 residents of Norway aged 65 and over at the start of the country’s vaccination campaign at the end of December, 2020, and they matched vaccinated individuals with unvaccinated persons based on demographic, geographic, and clinical risk group factors.
Dr. Ranhoff showed Kaplan-Meier survival curves for the total population and for each of the health-service states. In all cases there was a clear survival benefit for vaccinated vs. unvaccinated patients. She did not, however, provide specific numbers or hazard ratios for the differences between vaccinated and unvaccinated individuals in each of the comparisons.
The study was supported by the Norwegian Institute of Public Health. Dr. Ranhoff and Dr. Benetos reported no conflicts of interest.
FROM EUGMS 2021
Omega-3s tame inflammation in elderly COVID-19 patients
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUGMS
Resident physician work-hour regulations associated with improved physician safety and health
Background: In 2011, the Accreditation Council for Graduate Medical Education (ACGME) enacted a consecutive work-hour restriction of 16 hours for first-year residents. Reports of these changes have focused on patient safety, resident education, and resident well-being. The impact on resident safety had not been addressed.
Study design: Prospective cohort study.
Setting: U.S. Academic institutions training resident physicians.
Synopsis: This study compared first-year resident physicians from 2002 to 2007 (pre-implementation) and 2014 to 2017 (post-implementation). In all, 5,680 pre-implementation residents and 9,596 post-implementation residents consented to the study. With the 2011 ACGME restriction, the risk of motor vehicle crash decreased 24% (relative risk [RR] .76; .67-.85), and percutaneous injury risk decreased more than 40% (RR .54; .48-.61). Although weekly work hours were significantly higher pre-implementation, self-reported hours involved in patient care were similar for both groups.
While this large, well-powered study suggests extended work-hour restrictions for resident physicians improve their safety, the study is limited by self-reporting of resident physicians. As the ACGME has re-introduced extended duration shifts for first-year resident physicians, hospitalists should advocate for objective physician safety studies in relation to extended-hour shifts.
Bottom line: The 2011 ACGME work-hour reform for first-year physicians improved their safety and health.
Citation: Weaver MD et al. The association between resident physician work-hour regulations and physician safety and health. Am J Med. 2020 July;133(7):e343-54.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: In 2011, the Accreditation Council for Graduate Medical Education (ACGME) enacted a consecutive work-hour restriction of 16 hours for first-year residents. Reports of these changes have focused on patient safety, resident education, and resident well-being. The impact on resident safety had not been addressed.
Study design: Prospective cohort study.
Setting: U.S. Academic institutions training resident physicians.
Synopsis: This study compared first-year resident physicians from 2002 to 2007 (pre-implementation) and 2014 to 2017 (post-implementation). In all, 5,680 pre-implementation residents and 9,596 post-implementation residents consented to the study. With the 2011 ACGME restriction, the risk of motor vehicle crash decreased 24% (relative risk [RR] .76; .67-.85), and percutaneous injury risk decreased more than 40% (RR .54; .48-.61). Although weekly work hours were significantly higher pre-implementation, self-reported hours involved in patient care were similar for both groups.
While this large, well-powered study suggests extended work-hour restrictions for resident physicians improve their safety, the study is limited by self-reporting of resident physicians. As the ACGME has re-introduced extended duration shifts for first-year resident physicians, hospitalists should advocate for objective physician safety studies in relation to extended-hour shifts.
Bottom line: The 2011 ACGME work-hour reform for first-year physicians improved their safety and health.
Citation: Weaver MD et al. The association between resident physician work-hour regulations and physician safety and health. Am J Med. 2020 July;133(7):e343-54.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: In 2011, the Accreditation Council for Graduate Medical Education (ACGME) enacted a consecutive work-hour restriction of 16 hours for first-year residents. Reports of these changes have focused on patient safety, resident education, and resident well-being. The impact on resident safety had not been addressed.
Study design: Prospective cohort study.
Setting: U.S. Academic institutions training resident physicians.
Synopsis: This study compared first-year resident physicians from 2002 to 2007 (pre-implementation) and 2014 to 2017 (post-implementation). In all, 5,680 pre-implementation residents and 9,596 post-implementation residents consented to the study. With the 2011 ACGME restriction, the risk of motor vehicle crash decreased 24% (relative risk [RR] .76; .67-.85), and percutaneous injury risk decreased more than 40% (RR .54; .48-.61). Although weekly work hours were significantly higher pre-implementation, self-reported hours involved in patient care were similar for both groups.
While this large, well-powered study suggests extended work-hour restrictions for resident physicians improve their safety, the study is limited by self-reporting of resident physicians. As the ACGME has re-introduced extended duration shifts for first-year resident physicians, hospitalists should advocate for objective physician safety studies in relation to extended-hour shifts.
Bottom line: The 2011 ACGME work-hour reform for first-year physicians improved their safety and health.
Citation: Weaver MD et al. The association between resident physician work-hour regulations and physician safety and health. Am J Med. 2020 July;133(7):e343-54.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Children and COVID-19: U.S. adds latest million cases in record time
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
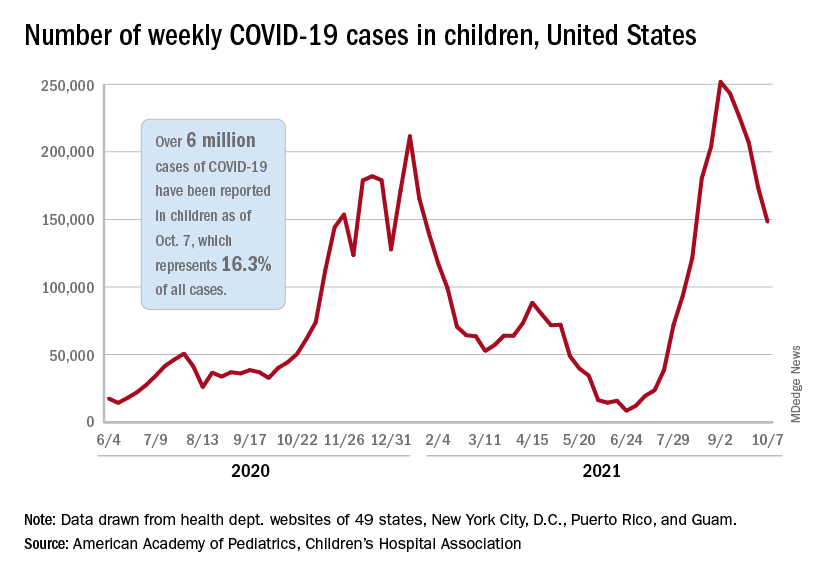
The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
Autopsy findings reveal venous thromboembolism in patients with COVID-19
Background: Despite the increased mortality rate of the novel coronavirus compared with influenza, little is understood about its pathogenicity. Prior studies have identified D-dimer levels, high Sequential Organ Failure Assessment score, and older age as markers for more severe disease and mortality. The specific cause of death of COVID-19 remains largely unknown.
Study design: Prospective cohort study.
Setting: Single academic center in Germany.
Synopsis: A complete autopsy was performed on 12 consecutive COVID-19 patient deaths at a single center. Seven had evidence of venous thromboembolism (VTE): three with bilateral lower extremity deep venous thrombosis (DVT) and four with massive pulmonary embolism/associated lower-extremity DVT. Prior to death, VTE was suspected clinically in only a single patient.
This small case series piques interest in the potential underrecognized thromboembolic pathology of COVID-19. While not practice changing, this study highlights the importance of hospitalists staying attuned to further studies regarding VTE prophylaxis in COVID-19.
Bottom line: Autopsies of COVID-19 patients revealed a high incidence of thromboembolic events; COVID-19–induced coagulopathy may play an underrecognized role in pathogenesis.
Citation: Wichmann D et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268-77.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Despite the increased mortality rate of the novel coronavirus compared with influenza, little is understood about its pathogenicity. Prior studies have identified D-dimer levels, high Sequential Organ Failure Assessment score, and older age as markers for more severe disease and mortality. The specific cause of death of COVID-19 remains largely unknown.
Study design: Prospective cohort study.
Setting: Single academic center in Germany.
Synopsis: A complete autopsy was performed on 12 consecutive COVID-19 patient deaths at a single center. Seven had evidence of venous thromboembolism (VTE): three with bilateral lower extremity deep venous thrombosis (DVT) and four with massive pulmonary embolism/associated lower-extremity DVT. Prior to death, VTE was suspected clinically in only a single patient.
This small case series piques interest in the potential underrecognized thromboembolic pathology of COVID-19. While not practice changing, this study highlights the importance of hospitalists staying attuned to further studies regarding VTE prophylaxis in COVID-19.
Bottom line: Autopsies of COVID-19 patients revealed a high incidence of thromboembolic events; COVID-19–induced coagulopathy may play an underrecognized role in pathogenesis.
Citation: Wichmann D et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268-77.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Despite the increased mortality rate of the novel coronavirus compared with influenza, little is understood about its pathogenicity. Prior studies have identified D-dimer levels, high Sequential Organ Failure Assessment score, and older age as markers for more severe disease and mortality. The specific cause of death of COVID-19 remains largely unknown.
Study design: Prospective cohort study.
Setting: Single academic center in Germany.
Synopsis: A complete autopsy was performed on 12 consecutive COVID-19 patient deaths at a single center. Seven had evidence of venous thromboembolism (VTE): three with bilateral lower extremity deep venous thrombosis (DVT) and four with massive pulmonary embolism/associated lower-extremity DVT. Prior to death, VTE was suspected clinically in only a single patient.
This small case series piques interest in the potential underrecognized thromboembolic pathology of COVID-19. While not practice changing, this study highlights the importance of hospitalists staying attuned to further studies regarding VTE prophylaxis in COVID-19.
Bottom line: Autopsies of COVID-19 patients revealed a high incidence of thromboembolic events; COVID-19–induced coagulopathy may play an underrecognized role in pathogenesis.
Citation: Wichmann D et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268-77.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Rivaroxaban’s single daily dose may lead to higher bleeding risk than other DOACs
The results, which were published in the Annals of Internal Medicine, could help guide DOAC selection for high-risk groups with a prior history of peptic ulcer disease or major GI bleeding, said lead study authors Arnar Bragi Ingason, MD and Einar S. Björnsson, MD, PhD, in an email.
DOACs treat conditions such as atrial fibrillation, venous thromboembolism, and ischemic stroke and are known to cause GI bleeding. Previous studies have suggested that rivaroxaban poses a higher GI-bleeding risk than other DOACs.
These studies, which used large administrative databases, “had an inherent risk of selection bias due to insurance status, age, and comorbidities due to their origin from insurance/administrative databases. In addition, they lacked phenotypic details on GI bleeding events,” said Dr. Björnsson and Dr. Ingason, who are both of Landspitali University Hospital, Reykjavik, Iceland,
Daily dosage may exacerbate risk
Rivaroxaban is administered as a single daily dose, compared with apixaban’s and dabigatran’s twice-daily regimens. “We hypothesized that this may lead to a greater variance in drug plasma concentration, making these patients more susceptible to GI bleeding,” the lead authors said.
Using data from the Icelandic Medicine Registry, a national database of outpatient prescription information, they compared rates of GI bleeding among new users of apixaban, dabigatran, and rivaroxaban from 2014 to 2019. Overall, 5,868 patients receiving one of the DOACs took part in the study. Among these participants, 3,217 received rivaroxaban, 2,157 received apixaban, and 494 received dabigatran. The researchers used inverse probability weighting, Kaplan–Meier survival estimates, and Cox regression to compare GI bleeding.
Compared with dabigatran, rivaroxaban was associated with a 63%-104% higher overall risk for GI bleeding and 39%-95% higher risk for major GI bleeding. Rivaroxaban also had a 40%-42% higher overall risk for GI bleeding and 49%-50% higher risk for major GI bleeding, compared with apixaban.
The investigators were surprised by the low rate of upper GI bleeding for dabigatran, compared with the other two drugs. “However, these results must be interpreted in the context that the dabigatran group was relatively small,” said Dr. Björnsson and Dr. Ingason via email.
Overall, the study cohort was small, compared with previous registry studies.
Investigators also did not account for account for socioeconomic status or lifestyle factors, such as alcohol consumption or smoking. “However, because the cost of all DOACs is similar in Iceland, selection bias due to socioeconomic status is unlikely,” the investigators reported in their paper. “We are currently working on comparing the rates of thromboembolisms and overall major bleeding events between the drugs,” the lead authors said.
Clinicians should consider location of bleeding
Though retrospective, the study by Ingason et. al. “is likely as close as is feasible to a randomized trial as is possible,” said Don C. Rockey, MD, a professor of medicine at the Medical University of South Carolina, Charleston, in an interview.
“From the clinician’s perspective, it is important to take away that there may be differences among the DOACs in terms of where in the GI tract the bleeding occurs,” said Dr. Rockey. In the study, the greatest differences appeared to be in the upper GI tract, with rivaroxaban outpacing apixaban and dabigatran. In patients who are at risk for upper GI bleeding, it may be reasonable to consider use of dabigatran or apixaban, he suggested.
“A limitation of the study is that it is likely underpowered overall,” said Dr. Rockey. It also wasn’t clear how many deaths occurred either directly from GI bleeding or as a complication of GI bleeding, he said.The study also didn’t differentiate major bleeding among DOACs specifically in the upper or lower GI tract, Dr. Rockey added.
Other studies yield similar results
Dr. Ingason and Dr. Björnsson said their work complements previous studies, and Neena S. Abraham, MD, MSc , who has conducted a similar investigation to the new study, agreed with that statement.
Data from the last 4 years overwhelmingly show that rivaroxaban is most likely to cause GI bleeding, said Dr. Abraham, professor of medicine and a consultant with Mayo Clinic’s division of gastroenterology and hepatology, in an interview.
A comparative safety study Dr. Abraham coauthored in 2017 of rivaroxaban, apixaban, and dabigatran in a much larger U.S. cohort of 372,380 patients revealed that rivaroxaban had the worst GI bleeding profile. Apixaban was 66% safer than rivaroxaban and 64% safer than dabigatran to prevent gastrointestinal bleeding.
“I believe our group was the first to conduct this study and show clinically significant differences in GI safety of the available direct oral anticoagulants,” she said. Other investigators have since published similar results, and the topic of the new study needs no further investigation, according to Dr. Abraham.
“It is time for physicians to choose a better choice when prescribing a direct oral anticoagulant to their atrial fibrillation patients, and that choice is not rivaroxaban,” she said.
The Icelandic Centre for Research and the Landspítali University Hospital Research Fund provided funds for this study. Dr. Ingason, Dr. Björnsson, Dr. Rockey, and Dr. Abraham reported no disclosures.
The results, which were published in the Annals of Internal Medicine, could help guide DOAC selection for high-risk groups with a prior history of peptic ulcer disease or major GI bleeding, said lead study authors Arnar Bragi Ingason, MD and Einar S. Björnsson, MD, PhD, in an email.
DOACs treat conditions such as atrial fibrillation, venous thromboembolism, and ischemic stroke and are known to cause GI bleeding. Previous studies have suggested that rivaroxaban poses a higher GI-bleeding risk than other DOACs.
These studies, which used large administrative databases, “had an inherent risk of selection bias due to insurance status, age, and comorbidities due to their origin from insurance/administrative databases. In addition, they lacked phenotypic details on GI bleeding events,” said Dr. Björnsson and Dr. Ingason, who are both of Landspitali University Hospital, Reykjavik, Iceland,
Daily dosage may exacerbate risk
Rivaroxaban is administered as a single daily dose, compared with apixaban’s and dabigatran’s twice-daily regimens. “We hypothesized that this may lead to a greater variance in drug plasma concentration, making these patients more susceptible to GI bleeding,” the lead authors said.
Using data from the Icelandic Medicine Registry, a national database of outpatient prescription information, they compared rates of GI bleeding among new users of apixaban, dabigatran, and rivaroxaban from 2014 to 2019. Overall, 5,868 patients receiving one of the DOACs took part in the study. Among these participants, 3,217 received rivaroxaban, 2,157 received apixaban, and 494 received dabigatran. The researchers used inverse probability weighting, Kaplan–Meier survival estimates, and Cox regression to compare GI bleeding.
Compared with dabigatran, rivaroxaban was associated with a 63%-104% higher overall risk for GI bleeding and 39%-95% higher risk for major GI bleeding. Rivaroxaban also had a 40%-42% higher overall risk for GI bleeding and 49%-50% higher risk for major GI bleeding, compared with apixaban.
The investigators were surprised by the low rate of upper GI bleeding for dabigatran, compared with the other two drugs. “However, these results must be interpreted in the context that the dabigatran group was relatively small,” said Dr. Björnsson and Dr. Ingason via email.
Overall, the study cohort was small, compared with previous registry studies.
Investigators also did not account for account for socioeconomic status or lifestyle factors, such as alcohol consumption or smoking. “However, because the cost of all DOACs is similar in Iceland, selection bias due to socioeconomic status is unlikely,” the investigators reported in their paper. “We are currently working on comparing the rates of thromboembolisms and overall major bleeding events between the drugs,” the lead authors said.
Clinicians should consider location of bleeding
Though retrospective, the study by Ingason et. al. “is likely as close as is feasible to a randomized trial as is possible,” said Don C. Rockey, MD, a professor of medicine at the Medical University of South Carolina, Charleston, in an interview.
“From the clinician’s perspective, it is important to take away that there may be differences among the DOACs in terms of where in the GI tract the bleeding occurs,” said Dr. Rockey. In the study, the greatest differences appeared to be in the upper GI tract, with rivaroxaban outpacing apixaban and dabigatran. In patients who are at risk for upper GI bleeding, it may be reasonable to consider use of dabigatran or apixaban, he suggested.
“A limitation of the study is that it is likely underpowered overall,” said Dr. Rockey. It also wasn’t clear how many deaths occurred either directly from GI bleeding or as a complication of GI bleeding, he said.The study also didn’t differentiate major bleeding among DOACs specifically in the upper or lower GI tract, Dr. Rockey added.
Other studies yield similar results
Dr. Ingason and Dr. Björnsson said their work complements previous studies, and Neena S. Abraham, MD, MSc , who has conducted a similar investigation to the new study, agreed with that statement.
Data from the last 4 years overwhelmingly show that rivaroxaban is most likely to cause GI bleeding, said Dr. Abraham, professor of medicine and a consultant with Mayo Clinic’s division of gastroenterology and hepatology, in an interview.
A comparative safety study Dr. Abraham coauthored in 2017 of rivaroxaban, apixaban, and dabigatran in a much larger U.S. cohort of 372,380 patients revealed that rivaroxaban had the worst GI bleeding profile. Apixaban was 66% safer than rivaroxaban and 64% safer than dabigatran to prevent gastrointestinal bleeding.
“I believe our group was the first to conduct this study and show clinically significant differences in GI safety of the available direct oral anticoagulants,” she said. Other investigators have since published similar results, and the topic of the new study needs no further investigation, according to Dr. Abraham.
“It is time for physicians to choose a better choice when prescribing a direct oral anticoagulant to their atrial fibrillation patients, and that choice is not rivaroxaban,” she said.
The Icelandic Centre for Research and the Landspítali University Hospital Research Fund provided funds for this study. Dr. Ingason, Dr. Björnsson, Dr. Rockey, and Dr. Abraham reported no disclosures.
The results, which were published in the Annals of Internal Medicine, could help guide DOAC selection for high-risk groups with a prior history of peptic ulcer disease or major GI bleeding, said lead study authors Arnar Bragi Ingason, MD and Einar S. Björnsson, MD, PhD, in an email.
DOACs treat conditions such as atrial fibrillation, venous thromboembolism, and ischemic stroke and are known to cause GI bleeding. Previous studies have suggested that rivaroxaban poses a higher GI-bleeding risk than other DOACs.
These studies, which used large administrative databases, “had an inherent risk of selection bias due to insurance status, age, and comorbidities due to their origin from insurance/administrative databases. In addition, they lacked phenotypic details on GI bleeding events,” said Dr. Björnsson and Dr. Ingason, who are both of Landspitali University Hospital, Reykjavik, Iceland,
Daily dosage may exacerbate risk
Rivaroxaban is administered as a single daily dose, compared with apixaban’s and dabigatran’s twice-daily regimens. “We hypothesized that this may lead to a greater variance in drug plasma concentration, making these patients more susceptible to GI bleeding,” the lead authors said.
Using data from the Icelandic Medicine Registry, a national database of outpatient prescription information, they compared rates of GI bleeding among new users of apixaban, dabigatran, and rivaroxaban from 2014 to 2019. Overall, 5,868 patients receiving one of the DOACs took part in the study. Among these participants, 3,217 received rivaroxaban, 2,157 received apixaban, and 494 received dabigatran. The researchers used inverse probability weighting, Kaplan–Meier survival estimates, and Cox regression to compare GI bleeding.
Compared with dabigatran, rivaroxaban was associated with a 63%-104% higher overall risk for GI bleeding and 39%-95% higher risk for major GI bleeding. Rivaroxaban also had a 40%-42% higher overall risk for GI bleeding and 49%-50% higher risk for major GI bleeding, compared with apixaban.
The investigators were surprised by the low rate of upper GI bleeding for dabigatran, compared with the other two drugs. “However, these results must be interpreted in the context that the dabigatran group was relatively small,” said Dr. Björnsson and Dr. Ingason via email.
Overall, the study cohort was small, compared with previous registry studies.
Investigators also did not account for account for socioeconomic status or lifestyle factors, such as alcohol consumption or smoking. “However, because the cost of all DOACs is similar in Iceland, selection bias due to socioeconomic status is unlikely,” the investigators reported in their paper. “We are currently working on comparing the rates of thromboembolisms and overall major bleeding events between the drugs,” the lead authors said.
Clinicians should consider location of bleeding
Though retrospective, the study by Ingason et. al. “is likely as close as is feasible to a randomized trial as is possible,” said Don C. Rockey, MD, a professor of medicine at the Medical University of South Carolina, Charleston, in an interview.
“From the clinician’s perspective, it is important to take away that there may be differences among the DOACs in terms of where in the GI tract the bleeding occurs,” said Dr. Rockey. In the study, the greatest differences appeared to be in the upper GI tract, with rivaroxaban outpacing apixaban and dabigatran. In patients who are at risk for upper GI bleeding, it may be reasonable to consider use of dabigatran or apixaban, he suggested.
“A limitation of the study is that it is likely underpowered overall,” said Dr. Rockey. It also wasn’t clear how many deaths occurred either directly from GI bleeding or as a complication of GI bleeding, he said.The study also didn’t differentiate major bleeding among DOACs specifically in the upper or lower GI tract, Dr. Rockey added.
Other studies yield similar results
Dr. Ingason and Dr. Björnsson said their work complements previous studies, and Neena S. Abraham, MD, MSc , who has conducted a similar investigation to the new study, agreed with that statement.
Data from the last 4 years overwhelmingly show that rivaroxaban is most likely to cause GI bleeding, said Dr. Abraham, professor of medicine and a consultant with Mayo Clinic’s division of gastroenterology and hepatology, in an interview.
A comparative safety study Dr. Abraham coauthored in 2017 of rivaroxaban, apixaban, and dabigatran in a much larger U.S. cohort of 372,380 patients revealed that rivaroxaban had the worst GI bleeding profile. Apixaban was 66% safer than rivaroxaban and 64% safer than dabigatran to prevent gastrointestinal bleeding.
“I believe our group was the first to conduct this study and show clinically significant differences in GI safety of the available direct oral anticoagulants,” she said. Other investigators have since published similar results, and the topic of the new study needs no further investigation, according to Dr. Abraham.
“It is time for physicians to choose a better choice when prescribing a direct oral anticoagulant to their atrial fibrillation patients, and that choice is not rivaroxaban,” she said.
The Icelandic Centre for Research and the Landspítali University Hospital Research Fund provided funds for this study. Dr. Ingason, Dr. Björnsson, Dr. Rockey, and Dr. Abraham reported no disclosures.
FROM ANNALS OF INTERNAL MEDICINE
New reports help nail down myocarditis risk with COVID-19 vaccine
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.




