User login
PARAGON-HF: Optimal systolic pressure in HFpEF is 120-129 mm Hg
A target systolic blood pressure (SBP) of 120-129 mm Hg in patients with heart failure with preserved ejection fraction proved to be the sweet spot with the lowest rates of major adverse cardiovascular and renal events in a new analysis from the landmark PARAGON-HF trial.
This finding from the largest-ever randomized, controlled study in heart failure with preserved ejection fraction (HFpEF) strengthens support for current U.S. joint hypertension guidelines, which call for a target SBP less than 130 mm Hg in patients with HFpEF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803), a recommendation based upon weak evidence until now. That’s because the SPRINT trial, the major impetus for adoption of intensive blood pressure control in the current guidelines, excluded patients with symptomatic HF, Scott D. Solomon, MD, and coinvestigators noted in their new analysis. The study was published in the Journal of the American College of Cardiology and had been planned for presentation during the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
The new analysis from PARAGON-HF (Prospective Comparison of ARNI with ARB Global Outcomes in HFpEF) also ruled out the SBP-lowering effect of sacubitril/valsartan (Entresto) as the explanation for the combination drug’s demonstrated beneficial impact on outcomes in the subgroup with an SBP of 120-129 mm Hg. That wasn’t actually a surprise. Indeed, the new study had two hypotheses: one, that the relationship between SBP and cardiovascular and renal outcomes in HFpEF would follow a J-shaped curve, and two, that sacubitril/valsartan’s blood pressure–lowering effect would not account for the drug’s outcome benefits in the subset of HFpEF patients with an SBP in the sweet spot of 120-129 mm Hg. Both hypotheses were borne out, noted Dr. Solomon, professor of medicine at Harvard Medical School and director of noninvasive cardiology at Brigham and Women’s Hospital, both in Boston.
“These data strongly support that additional mechanisms other than blood pressure–lowering account for the benefit. But this is not surprising. The same can be said for most of the therapies that work in heart failure,” he said in an interview.
Take, for example, spironolactone. In TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), another major trial in which Dr. Solomon played a leadership role, the beneficial effect of spironolactone on clinical outcomes also proved unrelated to the drug’s blood pressure–lowering effect.
Other known effects of sacubitril/valsartan, a novel angiotensin receptor–neprilysin inhibitor, or ARNI, might in theory account for the observed clinical benefits in ARNI-treated patients with an on-treatment SBP of 120-129 mm Hg in PARAGON-HF. These include improved left atrial remodeling, an increase in natriuretic peptides, and improved myocardial relaxation. However, the current lack of understanding of the basic mechanistic processes underlying the varied clinical expressions of HFpEF is a major factor contributing to the lack of any proven-effective therapy for this extremely common and costly disorder, according to Dr. Solomon and coinvestigators.
In contrast to HFpEF, for which to date there is no proven treatment, heart failure with reduced ejection fraction sacubitril/valsartan has a class I recommendation on the strength of its performance in significantly reducing cardiovascular deaths and heart failure hospitalizations in the PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371:993-1004).
PARAGON-HF included 4,822 patients with symptomatic HFpEF who were randomized to sacubitril/valsartan at 97/103 mg b.i.d. or valsartan at 160 mg b.i.d. As previously reported (N Engl J Med. 2019 Oct 24;381[17]:1609-20), at an average follow-up of 35 months, the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – occurred at a rate of 12.8 events per 100 patient-years in the sacubitril/valsartan group and 14.6 per 100 patient-years in the valsartan arm, for a 13% relative risk reduction that narrowly missed statistical significance (P = .059).
However, sacubitril/valsartan showed significant benefit on some prespecified secondary endpoints, including worsening renal function, change in New York Heart Association class, and quality of life. Women, who notably accounted for 52% of study participants, appeared to benefit from sacubitril/valsartan more than men as evidenced by their 27% relative risk reduction in the primary endpoint. Also, in the roughly half of PARAGON-HF participants with a baseline left ventricular ejection fraction of 45%-57%, treatment with sacubitril/valsartan resulted in a statistically significant 22% relative risk reduction in the primary endpoint, compared with valsartan alone.
SBP and cardiovascular outcomes in HFpEF
In the new analysis, Dr. Solomon and coworkers examined outcomes based on baseline and mean achieved SBP quartiles regardless of treatment arm. In an unadjusted analysis, the primary composite endpoint occurred at a rate of 15.2 events/100 patient-years in HFpEF patients with an achieved SBP below 120 mm Hg, 11.4/100 patient-years at 120-129 mm Hg, 12.2/100 patient-years at 130-139 mm Hg, and 15.6/100 patient-years at 140 mm Hg or more. Further, in a multivariate regression analysis extensively adjusted for atrial fibrillation, sex, race, and numerous other potential confounders, the group with an achieved SBP of 120-129 mm Hg continued to fare best. The adjusted risks for the primary endpoint were 11% and 21% higher in patients in the first and third quartiles of achieved SBP, compared with those at 120-129 mm Hg, although neither trend reached statistical significance. But patients in the top quartile, with an achieved SBP of 140 mm Hg or more, had a highly significant 56% increase in risk, compared with patients in the second-lowest SBP quartile.
Change in blood pressure from baseline to week 48 had no impact on quality of life or high-sensitivity troponin T. However, each 10–mm Hg lowering of SBP was associated with a modest 2.1% reduction in log-transformed N-terminal of the prohormone brain natriuretic peptide.
Sacubitril/valsartan reduced SBP by an average of 5.2 mm Hg more than valsartan alone at 4 weeks regardless of baseline SBP. And the combo drug had a significantly greater SBP-lowering effect in women than men, by a margin of 6.3 mm Hg versus 4.0 mm Hg. But a Cox regression analysis showed that in women, as in the study population as a whole, sacubitril/valsartan’s SBP-lowering effects didn’t account for the drug’s impact on outcomes.
In an editorial accompanying publication of the new PARAGON-HF blood pressure analysis (J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.024), Hector O. Ventura, MD, and colleagues at the Ochsner Clinic in New Orleans observed that the study results “lend some credence to the prognostic relationship of blood pressure in HFpEF, but whether they should serve as a therapeutic target or are merely a prognostic surrogate determined by other pathogenic factors, such as vascular ventricular uncoupling or aortic stiffness on one hand when blood pressure is greater than 140 mm Hg, or a reduced cardiac performance indicated by reduced blood pressure to less than 120 mm Hg, remains uncertain.”
“What is certain, however, is that the relationship and contributions of hypertension in manifest HFpEF are complex, multifactorial and likely go well beyond a simplistic framework of hemodynamic influences,” they added.
Dr. Solomon has received research grants from and serves as a consultant to Novartis, which funded PARAGON-HF, and has similar financial relationships with more than a dozen other pharmaceutical companies. Dr. Ventura reported having no relevant financial interests.
SOURCE: Solomon SD et al. J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.009.
A target systolic blood pressure (SBP) of 120-129 mm Hg in patients with heart failure with preserved ejection fraction proved to be the sweet spot with the lowest rates of major adverse cardiovascular and renal events in a new analysis from the landmark PARAGON-HF trial.
This finding from the largest-ever randomized, controlled study in heart failure with preserved ejection fraction (HFpEF) strengthens support for current U.S. joint hypertension guidelines, which call for a target SBP less than 130 mm Hg in patients with HFpEF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803), a recommendation based upon weak evidence until now. That’s because the SPRINT trial, the major impetus for adoption of intensive blood pressure control in the current guidelines, excluded patients with symptomatic HF, Scott D. Solomon, MD, and coinvestigators noted in their new analysis. The study was published in the Journal of the American College of Cardiology and had been planned for presentation during the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
The new analysis from PARAGON-HF (Prospective Comparison of ARNI with ARB Global Outcomes in HFpEF) also ruled out the SBP-lowering effect of sacubitril/valsartan (Entresto) as the explanation for the combination drug’s demonstrated beneficial impact on outcomes in the subgroup with an SBP of 120-129 mm Hg. That wasn’t actually a surprise. Indeed, the new study had two hypotheses: one, that the relationship between SBP and cardiovascular and renal outcomes in HFpEF would follow a J-shaped curve, and two, that sacubitril/valsartan’s blood pressure–lowering effect would not account for the drug’s outcome benefits in the subset of HFpEF patients with an SBP in the sweet spot of 120-129 mm Hg. Both hypotheses were borne out, noted Dr. Solomon, professor of medicine at Harvard Medical School and director of noninvasive cardiology at Brigham and Women’s Hospital, both in Boston.
“These data strongly support that additional mechanisms other than blood pressure–lowering account for the benefit. But this is not surprising. The same can be said for most of the therapies that work in heart failure,” he said in an interview.
Take, for example, spironolactone. In TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), another major trial in which Dr. Solomon played a leadership role, the beneficial effect of spironolactone on clinical outcomes also proved unrelated to the drug’s blood pressure–lowering effect.
Other known effects of sacubitril/valsartan, a novel angiotensin receptor–neprilysin inhibitor, or ARNI, might in theory account for the observed clinical benefits in ARNI-treated patients with an on-treatment SBP of 120-129 mm Hg in PARAGON-HF. These include improved left atrial remodeling, an increase in natriuretic peptides, and improved myocardial relaxation. However, the current lack of understanding of the basic mechanistic processes underlying the varied clinical expressions of HFpEF is a major factor contributing to the lack of any proven-effective therapy for this extremely common and costly disorder, according to Dr. Solomon and coinvestigators.
In contrast to HFpEF, for which to date there is no proven treatment, heart failure with reduced ejection fraction sacubitril/valsartan has a class I recommendation on the strength of its performance in significantly reducing cardiovascular deaths and heart failure hospitalizations in the PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371:993-1004).
PARAGON-HF included 4,822 patients with symptomatic HFpEF who were randomized to sacubitril/valsartan at 97/103 mg b.i.d. or valsartan at 160 mg b.i.d. As previously reported (N Engl J Med. 2019 Oct 24;381[17]:1609-20), at an average follow-up of 35 months, the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – occurred at a rate of 12.8 events per 100 patient-years in the sacubitril/valsartan group and 14.6 per 100 patient-years in the valsartan arm, for a 13% relative risk reduction that narrowly missed statistical significance (P = .059).
However, sacubitril/valsartan showed significant benefit on some prespecified secondary endpoints, including worsening renal function, change in New York Heart Association class, and quality of life. Women, who notably accounted for 52% of study participants, appeared to benefit from sacubitril/valsartan more than men as evidenced by their 27% relative risk reduction in the primary endpoint. Also, in the roughly half of PARAGON-HF participants with a baseline left ventricular ejection fraction of 45%-57%, treatment with sacubitril/valsartan resulted in a statistically significant 22% relative risk reduction in the primary endpoint, compared with valsartan alone.
SBP and cardiovascular outcomes in HFpEF
In the new analysis, Dr. Solomon and coworkers examined outcomes based on baseline and mean achieved SBP quartiles regardless of treatment arm. In an unadjusted analysis, the primary composite endpoint occurred at a rate of 15.2 events/100 patient-years in HFpEF patients with an achieved SBP below 120 mm Hg, 11.4/100 patient-years at 120-129 mm Hg, 12.2/100 patient-years at 130-139 mm Hg, and 15.6/100 patient-years at 140 mm Hg or more. Further, in a multivariate regression analysis extensively adjusted for atrial fibrillation, sex, race, and numerous other potential confounders, the group with an achieved SBP of 120-129 mm Hg continued to fare best. The adjusted risks for the primary endpoint were 11% and 21% higher in patients in the first and third quartiles of achieved SBP, compared with those at 120-129 mm Hg, although neither trend reached statistical significance. But patients in the top quartile, with an achieved SBP of 140 mm Hg or more, had a highly significant 56% increase in risk, compared with patients in the second-lowest SBP quartile.
Change in blood pressure from baseline to week 48 had no impact on quality of life or high-sensitivity troponin T. However, each 10–mm Hg lowering of SBP was associated with a modest 2.1% reduction in log-transformed N-terminal of the prohormone brain natriuretic peptide.
Sacubitril/valsartan reduced SBP by an average of 5.2 mm Hg more than valsartan alone at 4 weeks regardless of baseline SBP. And the combo drug had a significantly greater SBP-lowering effect in women than men, by a margin of 6.3 mm Hg versus 4.0 mm Hg. But a Cox regression analysis showed that in women, as in the study population as a whole, sacubitril/valsartan’s SBP-lowering effects didn’t account for the drug’s impact on outcomes.
In an editorial accompanying publication of the new PARAGON-HF blood pressure analysis (J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.024), Hector O. Ventura, MD, and colleagues at the Ochsner Clinic in New Orleans observed that the study results “lend some credence to the prognostic relationship of blood pressure in HFpEF, but whether they should serve as a therapeutic target or are merely a prognostic surrogate determined by other pathogenic factors, such as vascular ventricular uncoupling or aortic stiffness on one hand when blood pressure is greater than 140 mm Hg, or a reduced cardiac performance indicated by reduced blood pressure to less than 120 mm Hg, remains uncertain.”
“What is certain, however, is that the relationship and contributions of hypertension in manifest HFpEF are complex, multifactorial and likely go well beyond a simplistic framework of hemodynamic influences,” they added.
Dr. Solomon has received research grants from and serves as a consultant to Novartis, which funded PARAGON-HF, and has similar financial relationships with more than a dozen other pharmaceutical companies. Dr. Ventura reported having no relevant financial interests.
SOURCE: Solomon SD et al. J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.009.
A target systolic blood pressure (SBP) of 120-129 mm Hg in patients with heart failure with preserved ejection fraction proved to be the sweet spot with the lowest rates of major adverse cardiovascular and renal events in a new analysis from the landmark PARAGON-HF trial.
This finding from the largest-ever randomized, controlled study in heart failure with preserved ejection fraction (HFpEF) strengthens support for current U.S. joint hypertension guidelines, which call for a target SBP less than 130 mm Hg in patients with HFpEF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803), a recommendation based upon weak evidence until now. That’s because the SPRINT trial, the major impetus for adoption of intensive blood pressure control in the current guidelines, excluded patients with symptomatic HF, Scott D. Solomon, MD, and coinvestigators noted in their new analysis. The study was published in the Journal of the American College of Cardiology and had been planned for presentation during the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
The new analysis from PARAGON-HF (Prospective Comparison of ARNI with ARB Global Outcomes in HFpEF) also ruled out the SBP-lowering effect of sacubitril/valsartan (Entresto) as the explanation for the combination drug’s demonstrated beneficial impact on outcomes in the subgroup with an SBP of 120-129 mm Hg. That wasn’t actually a surprise. Indeed, the new study had two hypotheses: one, that the relationship between SBP and cardiovascular and renal outcomes in HFpEF would follow a J-shaped curve, and two, that sacubitril/valsartan’s blood pressure–lowering effect would not account for the drug’s outcome benefits in the subset of HFpEF patients with an SBP in the sweet spot of 120-129 mm Hg. Both hypotheses were borne out, noted Dr. Solomon, professor of medicine at Harvard Medical School and director of noninvasive cardiology at Brigham and Women’s Hospital, both in Boston.
“These data strongly support that additional mechanisms other than blood pressure–lowering account for the benefit. But this is not surprising. The same can be said for most of the therapies that work in heart failure,” he said in an interview.
Take, for example, spironolactone. In TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), another major trial in which Dr. Solomon played a leadership role, the beneficial effect of spironolactone on clinical outcomes also proved unrelated to the drug’s blood pressure–lowering effect.
Other known effects of sacubitril/valsartan, a novel angiotensin receptor–neprilysin inhibitor, or ARNI, might in theory account for the observed clinical benefits in ARNI-treated patients with an on-treatment SBP of 120-129 mm Hg in PARAGON-HF. These include improved left atrial remodeling, an increase in natriuretic peptides, and improved myocardial relaxation. However, the current lack of understanding of the basic mechanistic processes underlying the varied clinical expressions of HFpEF is a major factor contributing to the lack of any proven-effective therapy for this extremely common and costly disorder, according to Dr. Solomon and coinvestigators.
In contrast to HFpEF, for which to date there is no proven treatment, heart failure with reduced ejection fraction sacubitril/valsartan has a class I recommendation on the strength of its performance in significantly reducing cardiovascular deaths and heart failure hospitalizations in the PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371:993-1004).
PARAGON-HF included 4,822 patients with symptomatic HFpEF who were randomized to sacubitril/valsartan at 97/103 mg b.i.d. or valsartan at 160 mg b.i.d. As previously reported (N Engl J Med. 2019 Oct 24;381[17]:1609-20), at an average follow-up of 35 months, the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – occurred at a rate of 12.8 events per 100 patient-years in the sacubitril/valsartan group and 14.6 per 100 patient-years in the valsartan arm, for a 13% relative risk reduction that narrowly missed statistical significance (P = .059).
However, sacubitril/valsartan showed significant benefit on some prespecified secondary endpoints, including worsening renal function, change in New York Heart Association class, and quality of life. Women, who notably accounted for 52% of study participants, appeared to benefit from sacubitril/valsartan more than men as evidenced by their 27% relative risk reduction in the primary endpoint. Also, in the roughly half of PARAGON-HF participants with a baseline left ventricular ejection fraction of 45%-57%, treatment with sacubitril/valsartan resulted in a statistically significant 22% relative risk reduction in the primary endpoint, compared with valsartan alone.
SBP and cardiovascular outcomes in HFpEF
In the new analysis, Dr. Solomon and coworkers examined outcomes based on baseline and mean achieved SBP quartiles regardless of treatment arm. In an unadjusted analysis, the primary composite endpoint occurred at a rate of 15.2 events/100 patient-years in HFpEF patients with an achieved SBP below 120 mm Hg, 11.4/100 patient-years at 120-129 mm Hg, 12.2/100 patient-years at 130-139 mm Hg, and 15.6/100 patient-years at 140 mm Hg or more. Further, in a multivariate regression analysis extensively adjusted for atrial fibrillation, sex, race, and numerous other potential confounders, the group with an achieved SBP of 120-129 mm Hg continued to fare best. The adjusted risks for the primary endpoint were 11% and 21% higher in patients in the first and third quartiles of achieved SBP, compared with those at 120-129 mm Hg, although neither trend reached statistical significance. But patients in the top quartile, with an achieved SBP of 140 mm Hg or more, had a highly significant 56% increase in risk, compared with patients in the second-lowest SBP quartile.
Change in blood pressure from baseline to week 48 had no impact on quality of life or high-sensitivity troponin T. However, each 10–mm Hg lowering of SBP was associated with a modest 2.1% reduction in log-transformed N-terminal of the prohormone brain natriuretic peptide.
Sacubitril/valsartan reduced SBP by an average of 5.2 mm Hg more than valsartan alone at 4 weeks regardless of baseline SBP. And the combo drug had a significantly greater SBP-lowering effect in women than men, by a margin of 6.3 mm Hg versus 4.0 mm Hg. But a Cox regression analysis showed that in women, as in the study population as a whole, sacubitril/valsartan’s SBP-lowering effects didn’t account for the drug’s impact on outcomes.
In an editorial accompanying publication of the new PARAGON-HF blood pressure analysis (J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.024), Hector O. Ventura, MD, and colleagues at the Ochsner Clinic in New Orleans observed that the study results “lend some credence to the prognostic relationship of blood pressure in HFpEF, but whether they should serve as a therapeutic target or are merely a prognostic surrogate determined by other pathogenic factors, such as vascular ventricular uncoupling or aortic stiffness on one hand when blood pressure is greater than 140 mm Hg, or a reduced cardiac performance indicated by reduced blood pressure to less than 120 mm Hg, remains uncertain.”
“What is certain, however, is that the relationship and contributions of hypertension in manifest HFpEF are complex, multifactorial and likely go well beyond a simplistic framework of hemodynamic influences,” they added.
Dr. Solomon has received research grants from and serves as a consultant to Novartis, which funded PARAGON-HF, and has similar financial relationships with more than a dozen other pharmaceutical companies. Dr. Ventura reported having no relevant financial interests.
SOURCE: Solomon SD et al. J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.009.
FROM ACC 2020
COVID-19 in pediatric patients: What the hospitalist needs to know
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
Coronavirus stays in aerosols for hours, on surfaces for days
according to a new study.
The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
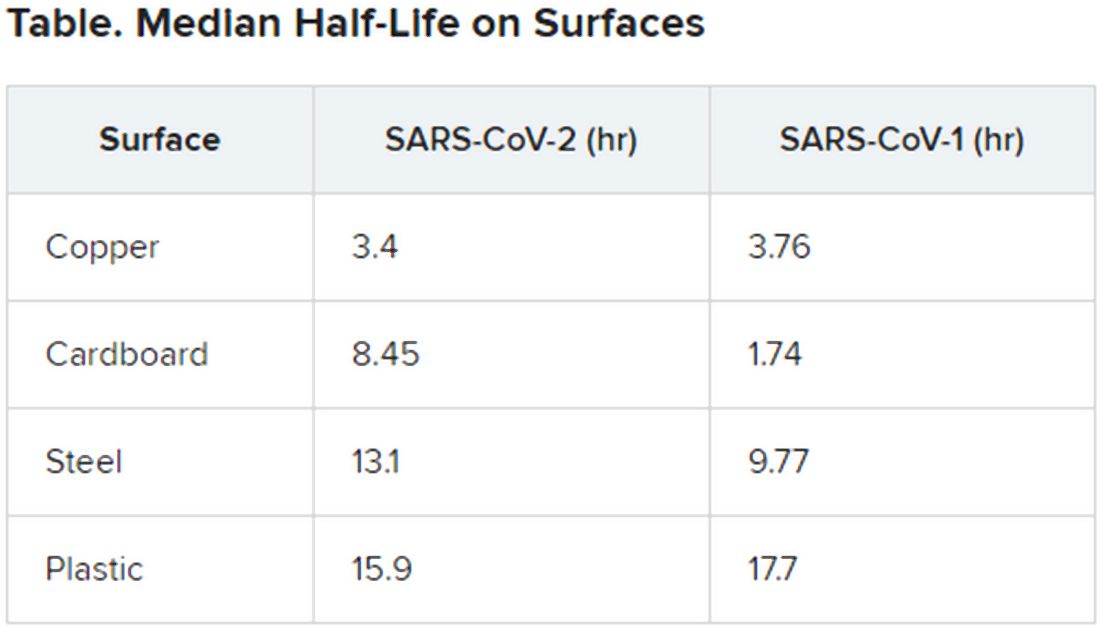
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
according to a new study.

The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
according to a new study.

The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Potential GI manifestation, transmission of coronavirus
The novel coronavirus (2019-nCoV) shows evidence of causing gastrointestinal symptoms and has the potential to be transmitted by the fecal-oral route, according to a new report from physicians at Shanghai Jiao Tong University, published online (Gastroenterology. 2020 March 3. doi: 10.1053/j.gastro.2020.02.054).
The virus’s respiratory symptoms are well documented and suggest primary transmission by droplet or contact, while other symptoms such as diarrhea, nausea, vomiting, and abdominal discomfort are less common and appear to vary between populations. The SARS coronavirus showed up in stool, even sometimes in patients discharged from the hospital. In a study of hospitalized patients in Wuhan, China, 10.1% of coronavirus patients had diarrhea and nausea in the 1-2 days before onset of fever and dyspnea. The first U.S. patient to be diagnosed had a 2-day history of nausea and vomiting, and had a loose bowel movement on the second day in the hospital. Clinicians later confirmed the presence of viral RNA in both the patient’s stool and airway.
The authors say that researchers in China have isolated viral RNA from the stool of two patients (unpublished), and it has been found in saliva, suggesting the possibility of the salivary gland as an infection or transmission route.
The authors maintain that previous studies likely overlooked or neglected patients who had mild intestinal symptoms. “Many efforts should be made to be alert on the initial digestive symptoms of COVID-19 for early detection, early diagnosis, early isolation and early intervention,” the authors wrote.
Like other coronaviruses, it appears that 2019-nCoV infects cells through an interaction between viral transmembrane spike glycoprotein (S-protein) receptor-binding domain, and the cell receptors angiotensin-converting enzyme 2 (ACE-2) and host cellular transmembrane serine protease (TMPRSS). Transcriptome analysis has shown that human lung AT2 cells express ACE-2 and TMPRSS, but esophagus upper and stratified epithelial cells also express both factors, as do stratified epithelial cells and absorptive enterocytes in the ileum and colon.
The researchers call for investigation into ACE-2 fusion proteins and TMPRSS inhibitors for diagnosis, prophylaxis, or treatment of COVID-19.
The authors also noted that COVID-19 has been linked to mild to moderate liver injury as revealed by elevated aminotransferases, hypoproteinemia and prothrombin time prolongation. This also has precedent in that the SARS coronavirus can infect the liver, and biopsies revealed mitoses and apoptosis, along with other abnormalities. SARS-associated hepatitis may be the result of viral hepatitis, immune overreaction, or a secondary effect of antiviral medications or other drugs. Little is known to date about the ability of 2019-nCoV to infect the liver, but single-cell RNA sequencing data from two distinct cohorts showed more ACE-2 expression in cholangiocytes (59.7%) than hepatocytes (2.6%), which indicates that the virus might directly affect intrahepatic bile ducts.
The authors had no sources of funding or financial conflicts.
SOURCE: GU J et al. Gastroenterology. 2020 March 3. doi: 10.1053/j.gastro.2020.02.054.
*This story was updated on 4/10.2020.
The novel coronavirus (2019-nCoV) shows evidence of causing gastrointestinal symptoms and has the potential to be transmitted by the fecal-oral route, according to a new report from physicians at Shanghai Jiao Tong University, published online (Gastroenterology. 2020 March 3. doi: 10.1053/j.gastro.2020.02.054).
The virus’s respiratory symptoms are well documented and suggest primary transmission by droplet or contact, while other symptoms such as diarrhea, nausea, vomiting, and abdominal discomfort are less common and appear to vary between populations. The SARS coronavirus showed up in stool, even sometimes in patients discharged from the hospital. In a study of hospitalized patients in Wuhan, China, 10.1% of coronavirus patients had diarrhea and nausea in the 1-2 days before onset of fever and dyspnea. The first U.S. patient to be diagnosed had a 2-day history of nausea and vomiting, and had a loose bowel movement on the second day in the hospital. Clinicians later confirmed the presence of viral RNA in both the patient’s stool and airway.
The authors say that researchers in China have isolated viral RNA from the stool of two patients (unpublished), and it has been found in saliva, suggesting the possibility of the salivary gland as an infection or transmission route.
The authors maintain that previous studies likely overlooked or neglected patients who had mild intestinal symptoms. “Many efforts should be made to be alert on the initial digestive symptoms of COVID-19 for early detection, early diagnosis, early isolation and early intervention,” the authors wrote.
Like other coronaviruses, it appears that 2019-nCoV infects cells through an interaction between viral transmembrane spike glycoprotein (S-protein) receptor-binding domain, and the cell receptors angiotensin-converting enzyme 2 (ACE-2) and host cellular transmembrane serine protease (TMPRSS). Transcriptome analysis has shown that human lung AT2 cells express ACE-2 and TMPRSS, but esophagus upper and stratified epithelial cells also express both factors, as do stratified epithelial cells and absorptive enterocytes in the ileum and colon.
The researchers call for investigation into ACE-2 fusion proteins and TMPRSS inhibitors for diagnosis, prophylaxis, or treatment of COVID-19.
The authors also noted that COVID-19 has been linked to mild to moderate liver injury as revealed by elevated aminotransferases, hypoproteinemia and prothrombin time prolongation. This also has precedent in that the SARS coronavirus can infect the liver, and biopsies revealed mitoses and apoptosis, along with other abnormalities. SARS-associated hepatitis may be the result of viral hepatitis, immune overreaction, or a secondary effect of antiviral medications or other drugs. Little is known to date about the ability of 2019-nCoV to infect the liver, but single-cell RNA sequencing data from two distinct cohorts showed more ACE-2 expression in cholangiocytes (59.7%) than hepatocytes (2.6%), which indicates that the virus might directly affect intrahepatic bile ducts.
The authors had no sources of funding or financial conflicts.
SOURCE: GU J et al. Gastroenterology. 2020 March 3. doi: 10.1053/j.gastro.2020.02.054.
*This story was updated on 4/10.2020.
The novel coronavirus (2019-nCoV) shows evidence of causing gastrointestinal symptoms and has the potential to be transmitted by the fecal-oral route, according to a new report from physicians at Shanghai Jiao Tong University, published online (Gastroenterology. 2020 March 3. doi: 10.1053/j.gastro.2020.02.054).
The virus’s respiratory symptoms are well documented and suggest primary transmission by droplet or contact, while other symptoms such as diarrhea, nausea, vomiting, and abdominal discomfort are less common and appear to vary between populations. The SARS coronavirus showed up in stool, even sometimes in patients discharged from the hospital. In a study of hospitalized patients in Wuhan, China, 10.1% of coronavirus patients had diarrhea and nausea in the 1-2 days before onset of fever and dyspnea. The first U.S. patient to be diagnosed had a 2-day history of nausea and vomiting, and had a loose bowel movement on the second day in the hospital. Clinicians later confirmed the presence of viral RNA in both the patient’s stool and airway.
The authors say that researchers in China have isolated viral RNA from the stool of two patients (unpublished), and it has been found in saliva, suggesting the possibility of the salivary gland as an infection or transmission route.
The authors maintain that previous studies likely overlooked or neglected patients who had mild intestinal symptoms. “Many efforts should be made to be alert on the initial digestive symptoms of COVID-19 for early detection, early diagnosis, early isolation and early intervention,” the authors wrote.
Like other coronaviruses, it appears that 2019-nCoV infects cells through an interaction between viral transmembrane spike glycoprotein (S-protein) receptor-binding domain, and the cell receptors angiotensin-converting enzyme 2 (ACE-2) and host cellular transmembrane serine protease (TMPRSS). Transcriptome analysis has shown that human lung AT2 cells express ACE-2 and TMPRSS, but esophagus upper and stratified epithelial cells also express both factors, as do stratified epithelial cells and absorptive enterocytes in the ileum and colon.
The researchers call for investigation into ACE-2 fusion proteins and TMPRSS inhibitors for diagnosis, prophylaxis, or treatment of COVID-19.
The authors also noted that COVID-19 has been linked to mild to moderate liver injury as revealed by elevated aminotransferases, hypoproteinemia and prothrombin time prolongation. This also has precedent in that the SARS coronavirus can infect the liver, and biopsies revealed mitoses and apoptosis, along with other abnormalities. SARS-associated hepatitis may be the result of viral hepatitis, immune overreaction, or a secondary effect of antiviral medications or other drugs. Little is known to date about the ability of 2019-nCoV to infect the liver, but single-cell RNA sequencing data from two distinct cohorts showed more ACE-2 expression in cholangiocytes (59.7%) than hepatocytes (2.6%), which indicates that the virus might directly affect intrahepatic bile ducts.
The authors had no sources of funding or financial conflicts.
SOURCE: GU J et al. Gastroenterology. 2020 March 3. doi: 10.1053/j.gastro.2020.02.054.
*This story was updated on 4/10.2020.
FROM GASTROENTEROLOGY
ICH survival lags in the community setting
LOS ANGELES – Although recent findings from circumscribed patient populations enrolled in intervention studies have shown improved survival rates in patients with a recent intracerebral hemorrhagic stroke, data from a large, observational study in the Netherlands suggested a much darker real-world picture, with a 6-month mortality of 64% identified in a total cohort of nearly 15,000 people followed prospectively starting in 1990.
In striking contrast to the survival pattern over time of patients in the same Dutch study who had a first acute ischemic stroke, which showed a statistically significant and meaningful cut in mortality for ischemic stroke patients during the 25-year period examined, survival rates for patients during the first months following a first intracerebral hemorrhage (ICH) stayed flat during 1991-2015, Reem Waziry, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“The promising treatment advances [applied to patients] in the recent ICH trials may not be reflected in community-based treatment,” suggested Dr. Waziry, a research and teaching fellow in clinical epidemiology at the Harvard School of Public Health in Boston.
The data she reported came from the Rotterdam Study, which followed unselected, older people in the Rotterdam community with no stroke history, and during 25 years of monitoring identified 162 incident ICH strokes and 988 acute ischemic strokes. Concurrently with Dr. Waziry’s talk at the conference, the data she reported were published in Stroke. The data she reported also showed that, during the 25 years studied, mortality at 3 years following a first ICH stroke rose to 73% on average.
During her talk, Dr. Waziry also presented an unpublished comparison of the 64% 6-month mortality in the Rotterdam Study with the 3- to 6-month mortality reported in the control arms of four recent, randomized intervention trials, including the MISTIE III trial. Among the four randomized trials Dr. Waziry selected to make this post-hoc comparison, the study with the highest mortality among control patients was MISTIE III, which showed about 25% mortality after 6 months. In contrast, the 19% 6-month mortality among ischemic stroke patients in the Rotterdam Study was roughly similar to the mortality seem in the control arms of some recent studies of interventions for patients with acute ischemic stroke.
The Rotterdam Study receives no commercial funding. Dr. Waziry had no disclosures.
SOURCE: Waziry R et al. ISC 2020, Abstract LB14.
LOS ANGELES – Although recent findings from circumscribed patient populations enrolled in intervention studies have shown improved survival rates in patients with a recent intracerebral hemorrhagic stroke, data from a large, observational study in the Netherlands suggested a much darker real-world picture, with a 6-month mortality of 64% identified in a total cohort of nearly 15,000 people followed prospectively starting in 1990.
In striking contrast to the survival pattern over time of patients in the same Dutch study who had a first acute ischemic stroke, which showed a statistically significant and meaningful cut in mortality for ischemic stroke patients during the 25-year period examined, survival rates for patients during the first months following a first intracerebral hemorrhage (ICH) stayed flat during 1991-2015, Reem Waziry, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“The promising treatment advances [applied to patients] in the recent ICH trials may not be reflected in community-based treatment,” suggested Dr. Waziry, a research and teaching fellow in clinical epidemiology at the Harvard School of Public Health in Boston.
The data she reported came from the Rotterdam Study, which followed unselected, older people in the Rotterdam community with no stroke history, and during 25 years of monitoring identified 162 incident ICH strokes and 988 acute ischemic strokes. Concurrently with Dr. Waziry’s talk at the conference, the data she reported were published in Stroke. The data she reported also showed that, during the 25 years studied, mortality at 3 years following a first ICH stroke rose to 73% on average.
During her talk, Dr. Waziry also presented an unpublished comparison of the 64% 6-month mortality in the Rotterdam Study with the 3- to 6-month mortality reported in the control arms of four recent, randomized intervention trials, including the MISTIE III trial. Among the four randomized trials Dr. Waziry selected to make this post-hoc comparison, the study with the highest mortality among control patients was MISTIE III, which showed about 25% mortality after 6 months. In contrast, the 19% 6-month mortality among ischemic stroke patients in the Rotterdam Study was roughly similar to the mortality seem in the control arms of some recent studies of interventions for patients with acute ischemic stroke.
The Rotterdam Study receives no commercial funding. Dr. Waziry had no disclosures.
SOURCE: Waziry R et al. ISC 2020, Abstract LB14.
LOS ANGELES – Although recent findings from circumscribed patient populations enrolled in intervention studies have shown improved survival rates in patients with a recent intracerebral hemorrhagic stroke, data from a large, observational study in the Netherlands suggested a much darker real-world picture, with a 6-month mortality of 64% identified in a total cohort of nearly 15,000 people followed prospectively starting in 1990.
In striking contrast to the survival pattern over time of patients in the same Dutch study who had a first acute ischemic stroke, which showed a statistically significant and meaningful cut in mortality for ischemic stroke patients during the 25-year period examined, survival rates for patients during the first months following a first intracerebral hemorrhage (ICH) stayed flat during 1991-2015, Reem Waziry, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“The promising treatment advances [applied to patients] in the recent ICH trials may not be reflected in community-based treatment,” suggested Dr. Waziry, a research and teaching fellow in clinical epidemiology at the Harvard School of Public Health in Boston.
The data she reported came from the Rotterdam Study, which followed unselected, older people in the Rotterdam community with no stroke history, and during 25 years of monitoring identified 162 incident ICH strokes and 988 acute ischemic strokes. Concurrently with Dr. Waziry’s talk at the conference, the data she reported were published in Stroke. The data she reported also showed that, during the 25 years studied, mortality at 3 years following a first ICH stroke rose to 73% on average.
During her talk, Dr. Waziry also presented an unpublished comparison of the 64% 6-month mortality in the Rotterdam Study with the 3- to 6-month mortality reported in the control arms of four recent, randomized intervention trials, including the MISTIE III trial. Among the four randomized trials Dr. Waziry selected to make this post-hoc comparison, the study with the highest mortality among control patients was MISTIE III, which showed about 25% mortality after 6 months. In contrast, the 19% 6-month mortality among ischemic stroke patients in the Rotterdam Study was roughly similar to the mortality seem in the control arms of some recent studies of interventions for patients with acute ischemic stroke.
The Rotterdam Study receives no commercial funding. Dr. Waziry had no disclosures.
SOURCE: Waziry R et al. ISC 2020, Abstract LB14.
REPORTING FROM ISC 2020
Get With the Guidelines – Stroke targets ICH
The Get With the Guidelines – Stroke program is finally turning its attention to hemorrhagic strokes after having spurred improved patient management performance from participating U.S. stroke centers since its start in 2003 with a focus on acute ischemic stroke.
The advisers who craft policy for Get With the Guidelines – Stroke (GWTG–S) are planning to launch a pilot program later in 2020 that will initiate data monitoring and quality improvement aimed at optimizing care for patients following an intracerebral hemorrhage (ICH) starting at 15 U.S. stroke centers, with announcement of these 15 participating centers expected later in 2020. The program will start by targeting nine specific, evidence-based, key aspects of the acute management of ICH patients, said Kevin N. Sheth, MD, professor of neurology and neurosurgery, and chief of neurocritical care and emergency neurology at Yale University in New Haven, Conn, and a volunteer expert who is part of the team developing the ICH initiative.
According to Dr. Sheth, the nine imperatives of acute ICH care that the program plans to monitor at participating centers are:
- Obtain a baseline severity score.
- Identify etiology as spontaneous or treatment related.
- Perform coagulopathy reversal or anticoagulant reversal.
- Administer venous thromboembolism prophylaxis.
- Apply dysphagia screening within 24 hours, and delay oral intake until patient passes dysphagia screen.
- Provide patient management in a multidisciplinary stroke or ICU unit.
- Prescribe appropriate blood pressure treatment at discharge.
- Perform assessment for rehabilitation.
- Avoid prescribing corticosteroids and other contraindicated drugs.
GWTG–S is adopting these metrics for assessing the acute care of ICH patients based largely on the recommendations of an expert 2018 panel organized by the American Heart Association and American Stroke. Association that proposed a set of performance measures for the care of ICH patients. This set of performance measures served as the primary basis for designing the new GWTG–S program, along with considerations of feasibility for collecting data on these measures, Dr. Sheth said in an interview. “We hope to make it easy” for centers to collect the data needed to participate.
The existing GWTG–S program is now 17-years old, and has spread to nearly 2,400 U.S. stroke centers as of early 2020, but the time has come to broaden its reach to patients with ICH and the programs that treat these patients, Dr. Sheth said. After years of nihilism about the prospects for patients following an ICH stroke, survival rates have increased, presenting “an opportunity to optimize care, for quality improvement,” he explained. “It’s a huge shift.” ICH patients “do better than we used to think.”
The Get With the Guidelines – Stroke program is finally turning its attention to hemorrhagic strokes after having spurred improved patient management performance from participating U.S. stroke centers since its start in 2003 with a focus on acute ischemic stroke.
The advisers who craft policy for Get With the Guidelines – Stroke (GWTG–S) are planning to launch a pilot program later in 2020 that will initiate data monitoring and quality improvement aimed at optimizing care for patients following an intracerebral hemorrhage (ICH) starting at 15 U.S. stroke centers, with announcement of these 15 participating centers expected later in 2020. The program will start by targeting nine specific, evidence-based, key aspects of the acute management of ICH patients, said Kevin N. Sheth, MD, professor of neurology and neurosurgery, and chief of neurocritical care and emergency neurology at Yale University in New Haven, Conn, and a volunteer expert who is part of the team developing the ICH initiative.
According to Dr. Sheth, the nine imperatives of acute ICH care that the program plans to monitor at participating centers are:
- Obtain a baseline severity score.
- Identify etiology as spontaneous or treatment related.
- Perform coagulopathy reversal or anticoagulant reversal.
- Administer venous thromboembolism prophylaxis.
- Apply dysphagia screening within 24 hours, and delay oral intake until patient passes dysphagia screen.
- Provide patient management in a multidisciplinary stroke or ICU unit.
- Prescribe appropriate blood pressure treatment at discharge.
- Perform assessment for rehabilitation.
- Avoid prescribing corticosteroids and other contraindicated drugs.
GWTG–S is adopting these metrics for assessing the acute care of ICH patients based largely on the recommendations of an expert 2018 panel organized by the American Heart Association and American Stroke. Association that proposed a set of performance measures for the care of ICH patients. This set of performance measures served as the primary basis for designing the new GWTG–S program, along with considerations of feasibility for collecting data on these measures, Dr. Sheth said in an interview. “We hope to make it easy” for centers to collect the data needed to participate.
The existing GWTG–S program is now 17-years old, and has spread to nearly 2,400 U.S. stroke centers as of early 2020, but the time has come to broaden its reach to patients with ICH and the programs that treat these patients, Dr. Sheth said. After years of nihilism about the prospects for patients following an ICH stroke, survival rates have increased, presenting “an opportunity to optimize care, for quality improvement,” he explained. “It’s a huge shift.” ICH patients “do better than we used to think.”
The Get With the Guidelines – Stroke program is finally turning its attention to hemorrhagic strokes after having spurred improved patient management performance from participating U.S. stroke centers since its start in 2003 with a focus on acute ischemic stroke.
The advisers who craft policy for Get With the Guidelines – Stroke (GWTG–S) are planning to launch a pilot program later in 2020 that will initiate data monitoring and quality improvement aimed at optimizing care for patients following an intracerebral hemorrhage (ICH) starting at 15 U.S. stroke centers, with announcement of these 15 participating centers expected later in 2020. The program will start by targeting nine specific, evidence-based, key aspects of the acute management of ICH patients, said Kevin N. Sheth, MD, professor of neurology and neurosurgery, and chief of neurocritical care and emergency neurology at Yale University in New Haven, Conn, and a volunteer expert who is part of the team developing the ICH initiative.
According to Dr. Sheth, the nine imperatives of acute ICH care that the program plans to monitor at participating centers are:
- Obtain a baseline severity score.
- Identify etiology as spontaneous or treatment related.
- Perform coagulopathy reversal or anticoagulant reversal.
- Administer venous thromboembolism prophylaxis.
- Apply dysphagia screening within 24 hours, and delay oral intake until patient passes dysphagia screen.
- Provide patient management in a multidisciplinary stroke or ICU unit.
- Prescribe appropriate blood pressure treatment at discharge.
- Perform assessment for rehabilitation.
- Avoid prescribing corticosteroids and other contraindicated drugs.
GWTG–S is adopting these metrics for assessing the acute care of ICH patients based largely on the recommendations of an expert 2018 panel organized by the American Heart Association and American Stroke. Association that proposed a set of performance measures for the care of ICH patients. This set of performance measures served as the primary basis for designing the new GWTG–S program, along with considerations of feasibility for collecting data on these measures, Dr. Sheth said in an interview. “We hope to make it easy” for centers to collect the data needed to participate.
The existing GWTG–S program is now 17-years old, and has spread to nearly 2,400 U.S. stroke centers as of early 2020, but the time has come to broaden its reach to patients with ICH and the programs that treat these patients, Dr. Sheth said. After years of nihilism about the prospects for patients following an ICH stroke, survival rates have increased, presenting “an opportunity to optimize care, for quality improvement,” he explained. “It’s a huge shift.” ICH patients “do better than we used to think.”
Treating COVID-19 in patients with diabetes
Patients with diabetes may be at extra risk for coronavirus disease (COVID-19) mortality, and doctors treating them need to keep up with the latest guidelines and expert advice.
Most health advisories about COVID-19 mention diabetes as one of the high-risk categories for the disease, likely because early data coming out of China, where the disease was first reported, indicated an elevated case-fatality rate for COVID-19 patients who also had diabetes.
In an article published in JAMA, Zunyou Wu, MD, and Jennifer M. McGoogan, PhD, summarized the findings from a February report on 44,672 confirmed cases of the disease from the Chinese Center for Disease Control and Prevention. The overall case-fatality rate (CFR) at that stage was 2.3% (1,023 deaths of the 44,672 confirmed cases). The data indicated that the CFR was elevated among COVID-19 patients with preexisting comorbid conditions, specifically, cardiovascular disease (CFR, 10.5%), diabetes (7.3%), chronic respiratory disease (6.3%), hypertension (6%), and cancer (5.6%).
The data also showed an aged-related trend in the CFR, with patients aged 80 years or older having a CFR of 14.8% and those aged 70-79 years, a rate of 8.0%, while there were no fatal cases reported in patients aged 9 years or younger (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
Those findings have been echoed by the U.S. Centers of Disease Control and Prevention. The American Diabetes Association and the American Association of Clinical Endocrinologists have in turn referenced the CDC in their COVID-19 guidance recommendations for patients with diabetes.
Guidelines were already in place for treatment of infections in patients with diabetes, and
In general, patients with diabetes – especially those whose disease is not controlled, or not well controlled – can be more susceptible to more common infections, such as influenza and pneumonia, possibly because hyperglycemia can subdue immunity by disrupting function of the white blood cells.
Glucose control is key
An important factor in any form of infection control in patients with diabetes seems to be whether or not a patient’s glucose levels are well controlled, according to comments from members of the editorial advisory board for Clinical Endocrinology News. Good glucose control, therefore, could be instrumental in reducing both the risk for and severity of infection.
Paul Jellinger, MD, of the Center for Diabetes & Endocrine Care, Hollywood, Fla., said that, over the years, he had not observed higher infection rates in general in patients with hemoglobin A1c levels below 7, or even higher. However, “a bigger question for me, given the broad category of ‘diabetes’ listed as a risk for serious coronavirus complications by the CDC, has been: Just which individuals with diabetes are really at risk? Are patients with well-controlled diabetes at increased risk as much as those with significant hyperglycemia and uncontrolled diabetes? In my view, not likely.”
Alan Jay Cohen, MD, agreed with Dr. Jellinger. “Many patients have called the office in the last 10 days to ask if there are special precautions they should take because they are reading that they are in the high-risk group because they have diabetes. Many of them are in superb, or at least pretty good, control. I have not seen where they have had a higher incidence of infection than the general population, and I have not seen data with COVID-19 that specifically demonstrates that a person with diabetes in good control has an increased risk,” he said.
“My recommendations to these patients have been the same as those given to the general population,” added Dr. Cohen, medical director at Baptist Medical Group: The Endocrine Clinic, Memphis.
Herbert I. Rettinger, MD, also conceded that poorly controlled blood sugars and confounding illnesses, such as renal and cardiac conditions, are common in patients with long-standing diabetes, but “there is a huge population of patients with type 1 diabetes, and very few seem to be more susceptible to infection. Perhaps I am missing those with poor diet and glucose control.”
Philip Levy, MD, picked up on that latter point, emphasizing that “endocrinologists take care of fewer patients with diabetes than do primary care physicians. Most patients with type 2 diabetes are not seen by us unless the PCP has problems [treating them],” so it could be that PCPs may see a higher number of patients who are at a greater risk for infections.
Ultimately, “good glucose control is very helpful in avoiding infections,” said Dr. Levy, of the Banner University Medical Group Endocrinology & Diabetes, Phoenix.
For sick patients
Guidelines for patients at the Joslin Diabetes Center in Boston advise patients who are feeling sick to continue taking their diabetes medications, unless instructed otherwise by their providers, and to monitor their glucose more frequently because it can spike suddenly.
Patients with type 1 diabetes should check for ketones if their glucose passes 250 mg/dL, according to the guidelines, and patients should remain hydrated at all times and get plenty of rest.
“Sick-day guidelines definitely apply, but patients should be advised to get tested if they have any symptoms they are concerned about,” said Dr. Rettinger, of the Endocrinology Medical Group of Orange County, Orange, Calif.
If patients with diabetes develop COVID-19, then home management may still be possible, according to Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues (Diabetes Metab Syndr. 2020 Mar 10;14[3]:211-2. doi: 10.1016/j.dsx.2020.03.002).
Dr. Rettinger agreed, noting that home management would be feasible as long as “everything is going well, that is, the patient is not experiencing respiratory problems or difficulties in controlling glucose levels. Consider patients with type 1 diabetes who have COVID-19 as you would a nursing home patient – ever vigilant.”
Dr. Gupta and coauthors also recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve. However, the ADA warns in its guidelines that patients should “be aware that some constant glucose monitoring sensors (Dexcom G5, Medtronic Enlite, and Guardian) are impacted by acetaminophen (Tylenol), and that patients should check with finger sticks to ensure accuracy [if they are taking acetaminophen].”
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often, the authors wrote, cautioning that “frequent changes in dosage and correctional bolus may be required to maintain normoglycemia.” Dr Rettinger emphasized that “hyperglycemia, as always, is best treated with fluids and insulin and frequent checks of sugars to be sure the treatment regimen is successful.”
In regard to diabetic drug regimens, patients with type 1 or 2 disease should continue on their current medications, advised Yehuda Handelsman, MD. “Some, especially those on insulin, may require more of it. And the patient should increase fluid intake to prevent fluid depletion. We do not reduce antihyperglycemic medication to preserve fluids.
“As for hypoglycemia, we always aim for less to no hypoglycemia,” he continued. “Monitoring glucose and appropriate dosage is the way to go. In other words, do not reduce medications in sick patients who typically need more medication.”
Dr. Handelsman, medical director and principal investigator at Metabolic Institute of America, Tarzana, Calif., added that very sick patients who are hospitalized should be managed with insulin and that oral agents – particularly metformin and sodium-glucose transporter 2 inhibitors – should be stopped.
“Once the patient has recovered and stabilized, you can return to the prior regimen, and, even if the patient is still in hospital, noninsulin therapy can be reintroduced,” he said.
“This is standard procedure in very sick patients, especially those in critical care. Metformin may raise lactic acid levels, and the SGLT2 inhibitors cause volume contraction, fat metabolism, and acidosis,” he explained. “We also stop the glucagon-like peptide receptor–1 analogues, which can cause nausea and vomiting, and pioglitazone because it causes fluid overload.
“Only insulin can be used for acutely sick patients – those with sepsis, for example. The same would apply if they have severe breathing disorders, and definitely, if they are on a ventilator. This is also the time we stop aromatase inhibitor orals and we use insulin.”
Preventive measures
In the interest of maintaining good glucose control, patients also should monitor their glucose levels more frequently so that fluctuations can be detected early and quickly addressed with the appropriate medication adjustments, according to guidelines from the ADA and AACE. They should continue to follow a healthy diet that includes adequate protein and they should exercise regularly.
Patients should ensure that they have enough medication and testing supplies – for at least 14 days, and longer, if costs permit – in case they have to go into quarantine.
General preventive measures, such as frequent hand washing with soap and water, practicing good respiratory hygiene by sneezing or coughing into a facial tissue or bent elbow, also apply for reducing the risk of infection. Touching of the face should be avoided, as should nonessential travel and contact with infected individuals.
Patients with diabetes should always be current with their influenza and pneumonia shots.
Dr. Rettinger said that he always recommends the following preventative measures to his patients and he is using the current health crisis to reinforce them:
- Eat lots of multicolored fruits and vegetables.
- Eat yogurt and take probiotics to keep the intestinal biome strong and functional.
- Be extra vigilant regarding sugars and sugar control to avoid peaks and valleys wherever possible.
- Keep the immune system strong with at least 7-8 hours sleep and reduce stress levels whenever possible.
- Avoid crowds and handshaking.
- Wash hands regularly.
Possible therapies
There are currently no drugs that have been approved specifically for the treatment of COVID-19, although a vaccine against the disease is currently under development.
Dr. Gupta and his colleagues noted in their article that there have been reports of the anecdotal use of antiviral drugs such as lopinavir, ritonavir, interferon-beta, the RNA polymerase inhibitor remdesivir, and chloroquine.
However, Dr. Handelsman said that, as far as he knows, none of these drugs has been shown to be beneficial for COVID-19. “Some [providers] have tried Tamiflu, but with no clear outcomes, and for severely sick patients, they tried medications for anti-HIV, hepatitis C, and malaria, but so far, there has been no breakthrough.”
Dr. Cohen, Dr. Handelsman, Dr. Jellinger, Dr. Levy, and Dr. Rettinger are members of the editorial advisory board of Clinical Endocrinology News. Dr. Gupta and Dr. Wu, and their colleagues, reported no conflicts of interest.
Patients with diabetes may be at extra risk for coronavirus disease (COVID-19) mortality, and doctors treating them need to keep up with the latest guidelines and expert advice.
Most health advisories about COVID-19 mention diabetes as one of the high-risk categories for the disease, likely because early data coming out of China, where the disease was first reported, indicated an elevated case-fatality rate for COVID-19 patients who also had diabetes.
In an article published in JAMA, Zunyou Wu, MD, and Jennifer M. McGoogan, PhD, summarized the findings from a February report on 44,672 confirmed cases of the disease from the Chinese Center for Disease Control and Prevention. The overall case-fatality rate (CFR) at that stage was 2.3% (1,023 deaths of the 44,672 confirmed cases). The data indicated that the CFR was elevated among COVID-19 patients with preexisting comorbid conditions, specifically, cardiovascular disease (CFR, 10.5%), diabetes (7.3%), chronic respiratory disease (6.3%), hypertension (6%), and cancer (5.6%).
The data also showed an aged-related trend in the CFR, with patients aged 80 years or older having a CFR of 14.8% and those aged 70-79 years, a rate of 8.0%, while there were no fatal cases reported in patients aged 9 years or younger (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
Those findings have been echoed by the U.S. Centers of Disease Control and Prevention. The American Diabetes Association and the American Association of Clinical Endocrinologists have in turn referenced the CDC in their COVID-19 guidance recommendations for patients with diabetes.
Guidelines were already in place for treatment of infections in patients with diabetes, and
In general, patients with diabetes – especially those whose disease is not controlled, or not well controlled – can be more susceptible to more common infections, such as influenza and pneumonia, possibly because hyperglycemia can subdue immunity by disrupting function of the white blood cells.
Glucose control is key
An important factor in any form of infection control in patients with diabetes seems to be whether or not a patient’s glucose levels are well controlled, according to comments from members of the editorial advisory board for Clinical Endocrinology News. Good glucose control, therefore, could be instrumental in reducing both the risk for and severity of infection.
Paul Jellinger, MD, of the Center for Diabetes & Endocrine Care, Hollywood, Fla., said that, over the years, he had not observed higher infection rates in general in patients with hemoglobin A1c levels below 7, or even higher. However, “a bigger question for me, given the broad category of ‘diabetes’ listed as a risk for serious coronavirus complications by the CDC, has been: Just which individuals with diabetes are really at risk? Are patients with well-controlled diabetes at increased risk as much as those with significant hyperglycemia and uncontrolled diabetes? In my view, not likely.”
Alan Jay Cohen, MD, agreed with Dr. Jellinger. “Many patients have called the office in the last 10 days to ask if there are special precautions they should take because they are reading that they are in the high-risk group because they have diabetes. Many of them are in superb, or at least pretty good, control. I have not seen where they have had a higher incidence of infection than the general population, and I have not seen data with COVID-19 that specifically demonstrates that a person with diabetes in good control has an increased risk,” he said.
“My recommendations to these patients have been the same as those given to the general population,” added Dr. Cohen, medical director at Baptist Medical Group: The Endocrine Clinic, Memphis.
Herbert I. Rettinger, MD, also conceded that poorly controlled blood sugars and confounding illnesses, such as renal and cardiac conditions, are common in patients with long-standing diabetes, but “there is a huge population of patients with type 1 diabetes, and very few seem to be more susceptible to infection. Perhaps I am missing those with poor diet and glucose control.”
Philip Levy, MD, picked up on that latter point, emphasizing that “endocrinologists take care of fewer patients with diabetes than do primary care physicians. Most patients with type 2 diabetes are not seen by us unless the PCP has problems [treating them],” so it could be that PCPs may see a higher number of patients who are at a greater risk for infections.
Ultimately, “good glucose control is very helpful in avoiding infections,” said Dr. Levy, of the Banner University Medical Group Endocrinology & Diabetes, Phoenix.
For sick patients
Guidelines for patients at the Joslin Diabetes Center in Boston advise patients who are feeling sick to continue taking their diabetes medications, unless instructed otherwise by their providers, and to monitor their glucose more frequently because it can spike suddenly.
Patients with type 1 diabetes should check for ketones if their glucose passes 250 mg/dL, according to the guidelines, and patients should remain hydrated at all times and get plenty of rest.
“Sick-day guidelines definitely apply, but patients should be advised to get tested if they have any symptoms they are concerned about,” said Dr. Rettinger, of the Endocrinology Medical Group of Orange County, Orange, Calif.
If patients with diabetes develop COVID-19, then home management may still be possible, according to Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues (Diabetes Metab Syndr. 2020 Mar 10;14[3]:211-2. doi: 10.1016/j.dsx.2020.03.002).
Dr. Rettinger agreed, noting that home management would be feasible as long as “everything is going well, that is, the patient is not experiencing respiratory problems or difficulties in controlling glucose levels. Consider patients with type 1 diabetes who have COVID-19 as you would a nursing home patient – ever vigilant.”
Dr. Gupta and coauthors also recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve. However, the ADA warns in its guidelines that patients should “be aware that some constant glucose monitoring sensors (Dexcom G5, Medtronic Enlite, and Guardian) are impacted by acetaminophen (Tylenol), and that patients should check with finger sticks to ensure accuracy [if they are taking acetaminophen].”
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often, the authors wrote, cautioning that “frequent changes in dosage and correctional bolus may be required to maintain normoglycemia.” Dr Rettinger emphasized that “hyperglycemia, as always, is best treated with fluids and insulin and frequent checks of sugars to be sure the treatment regimen is successful.”
In regard to diabetic drug regimens, patients with type 1 or 2 disease should continue on their current medications, advised Yehuda Handelsman, MD. “Some, especially those on insulin, may require more of it. And the patient should increase fluid intake to prevent fluid depletion. We do not reduce antihyperglycemic medication to preserve fluids.
“As for hypoglycemia, we always aim for less to no hypoglycemia,” he continued. “Monitoring glucose and appropriate dosage is the way to go. In other words, do not reduce medications in sick patients who typically need more medication.”
Dr. Handelsman, medical director and principal investigator at Metabolic Institute of America, Tarzana, Calif., added that very sick patients who are hospitalized should be managed with insulin and that oral agents – particularly metformin and sodium-glucose transporter 2 inhibitors – should be stopped.
“Once the patient has recovered and stabilized, you can return to the prior regimen, and, even if the patient is still in hospital, noninsulin therapy can be reintroduced,” he said.
“This is standard procedure in very sick patients, especially those in critical care. Metformin may raise lactic acid levels, and the SGLT2 inhibitors cause volume contraction, fat metabolism, and acidosis,” he explained. “We also stop the glucagon-like peptide receptor–1 analogues, which can cause nausea and vomiting, and pioglitazone because it causes fluid overload.
“Only insulin can be used for acutely sick patients – those with sepsis, for example. The same would apply if they have severe breathing disorders, and definitely, if they are on a ventilator. This is also the time we stop aromatase inhibitor orals and we use insulin.”
Preventive measures
In the interest of maintaining good glucose control, patients also should monitor their glucose levels more frequently so that fluctuations can be detected early and quickly addressed with the appropriate medication adjustments, according to guidelines from the ADA and AACE. They should continue to follow a healthy diet that includes adequate protein and they should exercise regularly.
Patients should ensure that they have enough medication and testing supplies – for at least 14 days, and longer, if costs permit – in case they have to go into quarantine.
General preventive measures, such as frequent hand washing with soap and water, practicing good respiratory hygiene by sneezing or coughing into a facial tissue or bent elbow, also apply for reducing the risk of infection. Touching of the face should be avoided, as should nonessential travel and contact with infected individuals.
Patients with diabetes should always be current with their influenza and pneumonia shots.
Dr. Rettinger said that he always recommends the following preventative measures to his patients and he is using the current health crisis to reinforce them:
- Eat lots of multicolored fruits and vegetables.
- Eat yogurt and take probiotics to keep the intestinal biome strong and functional.
- Be extra vigilant regarding sugars and sugar control to avoid peaks and valleys wherever possible.
- Keep the immune system strong with at least 7-8 hours sleep and reduce stress levels whenever possible.
- Avoid crowds and handshaking.
- Wash hands regularly.
Possible therapies
There are currently no drugs that have been approved specifically for the treatment of COVID-19, although a vaccine against the disease is currently under development.
Dr. Gupta and his colleagues noted in their article that there have been reports of the anecdotal use of antiviral drugs such as lopinavir, ritonavir, interferon-beta, the RNA polymerase inhibitor remdesivir, and chloroquine.
However, Dr. Handelsman said that, as far as he knows, none of these drugs has been shown to be beneficial for COVID-19. “Some [providers] have tried Tamiflu, but with no clear outcomes, and for severely sick patients, they tried medications for anti-HIV, hepatitis C, and malaria, but so far, there has been no breakthrough.”
Dr. Cohen, Dr. Handelsman, Dr. Jellinger, Dr. Levy, and Dr. Rettinger are members of the editorial advisory board of Clinical Endocrinology News. Dr. Gupta and Dr. Wu, and their colleagues, reported no conflicts of interest.
Patients with diabetes may be at extra risk for coronavirus disease (COVID-19) mortality, and doctors treating them need to keep up with the latest guidelines and expert advice.
Most health advisories about COVID-19 mention diabetes as one of the high-risk categories for the disease, likely because early data coming out of China, where the disease was first reported, indicated an elevated case-fatality rate for COVID-19 patients who also had diabetes.
In an article published in JAMA, Zunyou Wu, MD, and Jennifer M. McGoogan, PhD, summarized the findings from a February report on 44,672 confirmed cases of the disease from the Chinese Center for Disease Control and Prevention. The overall case-fatality rate (CFR) at that stage was 2.3% (1,023 deaths of the 44,672 confirmed cases). The data indicated that the CFR was elevated among COVID-19 patients with preexisting comorbid conditions, specifically, cardiovascular disease (CFR, 10.5%), diabetes (7.3%), chronic respiratory disease (6.3%), hypertension (6%), and cancer (5.6%).
The data also showed an aged-related trend in the CFR, with patients aged 80 years or older having a CFR of 14.8% and those aged 70-79 years, a rate of 8.0%, while there were no fatal cases reported in patients aged 9 years or younger (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
Those findings have been echoed by the U.S. Centers of Disease Control and Prevention. The American Diabetes Association and the American Association of Clinical Endocrinologists have in turn referenced the CDC in their COVID-19 guidance recommendations for patients with diabetes.
Guidelines were already in place for treatment of infections in patients with diabetes, and
In general, patients with diabetes – especially those whose disease is not controlled, or not well controlled – can be more susceptible to more common infections, such as influenza and pneumonia, possibly because hyperglycemia can subdue immunity by disrupting function of the white blood cells.
Glucose control is key
An important factor in any form of infection control in patients with diabetes seems to be whether or not a patient’s glucose levels are well controlled, according to comments from members of the editorial advisory board for Clinical Endocrinology News. Good glucose control, therefore, could be instrumental in reducing both the risk for and severity of infection.
Paul Jellinger, MD, of the Center for Diabetes & Endocrine Care, Hollywood, Fla., said that, over the years, he had not observed higher infection rates in general in patients with hemoglobin A1c levels below 7, or even higher. However, “a bigger question for me, given the broad category of ‘diabetes’ listed as a risk for serious coronavirus complications by the CDC, has been: Just which individuals with diabetes are really at risk? Are patients with well-controlled diabetes at increased risk as much as those with significant hyperglycemia and uncontrolled diabetes? In my view, not likely.”
Alan Jay Cohen, MD, agreed with Dr. Jellinger. “Many patients have called the office in the last 10 days to ask if there are special precautions they should take because they are reading that they are in the high-risk group because they have diabetes. Many of them are in superb, or at least pretty good, control. I have not seen where they have had a higher incidence of infection than the general population, and I have not seen data with COVID-19 that specifically demonstrates that a person with diabetes in good control has an increased risk,” he said.
“My recommendations to these patients have been the same as those given to the general population,” added Dr. Cohen, medical director at Baptist Medical Group: The Endocrine Clinic, Memphis.
Herbert I. Rettinger, MD, also conceded that poorly controlled blood sugars and confounding illnesses, such as renal and cardiac conditions, are common in patients with long-standing diabetes, but “there is a huge population of patients with type 1 diabetes, and very few seem to be more susceptible to infection. Perhaps I am missing those with poor diet and glucose control.”
Philip Levy, MD, picked up on that latter point, emphasizing that “endocrinologists take care of fewer patients with diabetes than do primary care physicians. Most patients with type 2 diabetes are not seen by us unless the PCP has problems [treating them],” so it could be that PCPs may see a higher number of patients who are at a greater risk for infections.
Ultimately, “good glucose control is very helpful in avoiding infections,” said Dr. Levy, of the Banner University Medical Group Endocrinology & Diabetes, Phoenix.
For sick patients
Guidelines for patients at the Joslin Diabetes Center in Boston advise patients who are feeling sick to continue taking their diabetes medications, unless instructed otherwise by their providers, and to monitor their glucose more frequently because it can spike suddenly.
Patients with type 1 diabetes should check for ketones if their glucose passes 250 mg/dL, according to the guidelines, and patients should remain hydrated at all times and get plenty of rest.
“Sick-day guidelines definitely apply, but patients should be advised to get tested if they have any symptoms they are concerned about,” said Dr. Rettinger, of the Endocrinology Medical Group of Orange County, Orange, Calif.
If patients with diabetes develop COVID-19, then home management may still be possible, according to Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues (Diabetes Metab Syndr. 2020 Mar 10;14[3]:211-2. doi: 10.1016/j.dsx.2020.03.002).
Dr. Rettinger agreed, noting that home management would be feasible as long as “everything is going well, that is, the patient is not experiencing respiratory problems or difficulties in controlling glucose levels. Consider patients with type 1 diabetes who have COVID-19 as you would a nursing home patient – ever vigilant.”
Dr. Gupta and coauthors also recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve. However, the ADA warns in its guidelines that patients should “be aware that some constant glucose monitoring sensors (Dexcom G5, Medtronic Enlite, and Guardian) are impacted by acetaminophen (Tylenol), and that patients should check with finger sticks to ensure accuracy [if they are taking acetaminophen].”
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often, the authors wrote, cautioning that “frequent changes in dosage and correctional bolus may be required to maintain normoglycemia.” Dr Rettinger emphasized that “hyperglycemia, as always, is best treated with fluids and insulin and frequent checks of sugars to be sure the treatment regimen is successful.”
In regard to diabetic drug regimens, patients with type 1 or 2 disease should continue on their current medications, advised Yehuda Handelsman, MD. “Some, especially those on insulin, may require more of it. And the patient should increase fluid intake to prevent fluid depletion. We do not reduce antihyperglycemic medication to preserve fluids.
“As for hypoglycemia, we always aim for less to no hypoglycemia,” he continued. “Monitoring glucose and appropriate dosage is the way to go. In other words, do not reduce medications in sick patients who typically need more medication.”
Dr. Handelsman, medical director and principal investigator at Metabolic Institute of America, Tarzana, Calif., added that very sick patients who are hospitalized should be managed with insulin and that oral agents – particularly metformin and sodium-glucose transporter 2 inhibitors – should be stopped.
“Once the patient has recovered and stabilized, you can return to the prior regimen, and, even if the patient is still in hospital, noninsulin therapy can be reintroduced,” he said.
“This is standard procedure in very sick patients, especially those in critical care. Metformin may raise lactic acid levels, and the SGLT2 inhibitors cause volume contraction, fat metabolism, and acidosis,” he explained. “We also stop the glucagon-like peptide receptor–1 analogues, which can cause nausea and vomiting, and pioglitazone because it causes fluid overload.
“Only insulin can be used for acutely sick patients – those with sepsis, for example. The same would apply if they have severe breathing disorders, and definitely, if they are on a ventilator. This is also the time we stop aromatase inhibitor orals and we use insulin.”
Preventive measures
In the interest of maintaining good glucose control, patients also should monitor their glucose levels more frequently so that fluctuations can be detected early and quickly addressed with the appropriate medication adjustments, according to guidelines from the ADA and AACE. They should continue to follow a healthy diet that includes adequate protein and they should exercise regularly.
Patients should ensure that they have enough medication and testing supplies – for at least 14 days, and longer, if costs permit – in case they have to go into quarantine.
General preventive measures, such as frequent hand washing with soap and water, practicing good respiratory hygiene by sneezing or coughing into a facial tissue or bent elbow, also apply for reducing the risk of infection. Touching of the face should be avoided, as should nonessential travel and contact with infected individuals.
Patients with diabetes should always be current with their influenza and pneumonia shots.
Dr. Rettinger said that he always recommends the following preventative measures to his patients and he is using the current health crisis to reinforce them:
- Eat lots of multicolored fruits and vegetables.
- Eat yogurt and take probiotics to keep the intestinal biome strong and functional.
- Be extra vigilant regarding sugars and sugar control to avoid peaks and valleys wherever possible.
- Keep the immune system strong with at least 7-8 hours sleep and reduce stress levels whenever possible.
- Avoid crowds and handshaking.
- Wash hands regularly.
Possible therapies
There are currently no drugs that have been approved specifically for the treatment of COVID-19, although a vaccine against the disease is currently under development.
Dr. Gupta and his colleagues noted in their article that there have been reports of the anecdotal use of antiviral drugs such as lopinavir, ritonavir, interferon-beta, the RNA polymerase inhibitor remdesivir, and chloroquine.
However, Dr. Handelsman said that, as far as he knows, none of these drugs has been shown to be beneficial for COVID-19. “Some [providers] have tried Tamiflu, but with no clear outcomes, and for severely sick patients, they tried medications for anti-HIV, hepatitis C, and malaria, but so far, there has been no breakthrough.”
Dr. Cohen, Dr. Handelsman, Dr. Jellinger, Dr. Levy, and Dr. Rettinger are members of the editorial advisory board of Clinical Endocrinology News. Dr. Gupta and Dr. Wu, and their colleagues, reported no conflicts of interest.
COVID-19: Extra caution needed for patients with diabetes
Patients with diabetes may have an increased risk of developing coronavirus infection (COVID-19), along with increased risks of morbidity and mortality, according to researchers writing in Diabetes & Metabolic Syndrome.
Although relevant clinical data remain scarce, patients with diabetes should take extra precautions to avoid infection and, if infected, may require special care, reported Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues.
“The disease severity [with COVID-19] has varied from mild, self-limiting, flu-like illness to fulminant pneumonia, respiratory failure, and death,” the authors wrote.
As of March 16, 2020, the World Health Organization reported 167,515 confirmed cases of COVID-19 and 6,606 deaths from around the world, with a mortality rate of 3.9%. But the actual mortality rate may be lower, the authors suggested, because a study involving more than 1,000 confirmed cases reported a mortality rate of 1.4%.
“Considering that the number of unreported and unconfirmed cases is likely to be much higher than the reported cases, the actual mortality may be less than 1%, which is similar to that of severe seasonal influenza,” the authors said, in reference to an editorial by Anthony S. Fauci, MD, and colleagues in the New England Journal of Medicine. In addition, they noted, mortality rates may vary by region.
The largest study relevant to patients with diabetes, which involved 72,314 cases of COVID-19, showed that patients with diabetes had a threefold higher mortality rate than did those without diabetes (7.3% vs. 2.3%, respectively). These figures were reported by the Chinese Centre for Disease Control and Prevention.
However, data from smaller cohorts with diabetes and COVID-19 have yielded mixed results. For instance, one study, involving 140 patients from Wuhan, suggested that diabetes was not a risk factor for severe disease, and in an analysis of 11 studies reporting on laboratory abnormalities in patients with a diagnosis of COVID-19, raised blood sugar levels or diabetes were not mentioned among the predictors of severe disease.
“Our knowledge about the prevalence of COVID-19 and disease course in people with diabetes will evolve as more detailed analyses are carried out,” the authors wrote. “For now, it is reasonable to assume that people with diabetes are at increased risk of developing infection. Coexisting heart disease, kidney disease, advanced age, and frailty are likely to further increase the severity of disease.”
Prevention first
“It is important that people with diabetes maintain good glycemic control, because it might help in reducing the risk of infection and the severity,” the authors wrote.
In addition to more frequent monitoring of blood glucose levels, they recommended other preventive measures, such as getting adequate nutrition, exercising, and being current with vaccinations for influenza and pneumonia. The latter, they said, may also reduce the risk of secondary bacterial pneumonia after a respiratory viral infection.
In regard to nutrition, adequate protein intake is important and “any deficiencies of minerals and vitamins need to be taken care of,” they advised. Likewise, exercise is known to improve immunity and should continue, but they suggest avoiding gyms and swimming pools.
For patients with coexisting heart and/or kidney disease, they also recommended efforts to stabilize cardiac/renal status.
In addition, the general preventive measures, such as regular and thorough hand washing with soap and water, practicing good respiratory hygiene by sneezing and coughing into a bent elbow or a facial tissue, and avoiding contact with anyone who is infected, should be observed.
As with other patients with chronic diseases that are managed long-term medications, patients with diabetes should always ensure that they have a sufficient supply of their medications and refills, if possible.
After a diagnosis
If patients with diabetes develop COVID-19, then home management may still be possible, wrote the authors, who recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve.
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often. “Frequent changes in dosage and correctional bolus may be required to maintain normoglycemia,” they cautioned.
Concerning diabetic drug regimens, they suggest patients avoid antihyperglycemic agents that can cause volume depletion or hypoglycemia and, if necessary, that they reduce oral antidiabetic drugs and follow sick-day guidelines.
For hospitalized patients, the investigators strengthened that statement, advising that oral agents need to be stopped, particularly sodium-glucose cotransporter 2 inhibitors and metformin. “Insulin is the preferred agent for control of hyperglycemia in hospitalized sick patients,” they wrote.
Untested therapies
The authors also discussed a range of untested therapies that may help fight COVID-19, such as antiviral drugs (such as lopinavir and ritonavir), zinc nanoparticles, and vitamin C. Supplementing those recommendations, Dr. Gupta and colleagues provided a concise review of COVID-19 epidemiology and extant data relevant to patients with diabetes.
The investigators reported no conflicts of interest.
SOURCE: Gupta et al. Diabetes Metab Syndr. 2020;14(3):211-12.
Patients with diabetes may have an increased risk of developing coronavirus infection (COVID-19), along with increased risks of morbidity and mortality, according to researchers writing in Diabetes & Metabolic Syndrome.
Although relevant clinical data remain scarce, patients with diabetes should take extra precautions to avoid infection and, if infected, may require special care, reported Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues.
“The disease severity [with COVID-19] has varied from mild, self-limiting, flu-like illness to fulminant pneumonia, respiratory failure, and death,” the authors wrote.
As of March 16, 2020, the World Health Organization reported 167,515 confirmed cases of COVID-19 and 6,606 deaths from around the world, with a mortality rate of 3.9%. But the actual mortality rate may be lower, the authors suggested, because a study involving more than 1,000 confirmed cases reported a mortality rate of 1.4%.
“Considering that the number of unreported and unconfirmed cases is likely to be much higher than the reported cases, the actual mortality may be less than 1%, which is similar to that of severe seasonal influenza,” the authors said, in reference to an editorial by Anthony S. Fauci, MD, and colleagues in the New England Journal of Medicine. In addition, they noted, mortality rates may vary by region.
The largest study relevant to patients with diabetes, which involved 72,314 cases of COVID-19, showed that patients with diabetes had a threefold higher mortality rate than did those without diabetes (7.3% vs. 2.3%, respectively). These figures were reported by the Chinese Centre for Disease Control and Prevention.
However, data from smaller cohorts with diabetes and COVID-19 have yielded mixed results. For instance, one study, involving 140 patients from Wuhan, suggested that diabetes was not a risk factor for severe disease, and in an analysis of 11 studies reporting on laboratory abnormalities in patients with a diagnosis of COVID-19, raised blood sugar levels or diabetes were not mentioned among the predictors of severe disease.
“Our knowledge about the prevalence of COVID-19 and disease course in people with diabetes will evolve as more detailed analyses are carried out,” the authors wrote. “For now, it is reasonable to assume that people with diabetes are at increased risk of developing infection. Coexisting heart disease, kidney disease, advanced age, and frailty are likely to further increase the severity of disease.”
Prevention first
“It is important that people with diabetes maintain good glycemic control, because it might help in reducing the risk of infection and the severity,” the authors wrote.
In addition to more frequent monitoring of blood glucose levels, they recommended other preventive measures, such as getting adequate nutrition, exercising, and being current with vaccinations for influenza and pneumonia. The latter, they said, may also reduce the risk of secondary bacterial pneumonia after a respiratory viral infection.
In regard to nutrition, adequate protein intake is important and “any deficiencies of minerals and vitamins need to be taken care of,” they advised. Likewise, exercise is known to improve immunity and should continue, but they suggest avoiding gyms and swimming pools.
For patients with coexisting heart and/or kidney disease, they also recommended efforts to stabilize cardiac/renal status.
In addition, the general preventive measures, such as regular and thorough hand washing with soap and water, practicing good respiratory hygiene by sneezing and coughing into a bent elbow or a facial tissue, and avoiding contact with anyone who is infected, should be observed.
As with other patients with chronic diseases that are managed long-term medications, patients with diabetes should always ensure that they have a sufficient supply of their medications and refills, if possible.
After a diagnosis
If patients with diabetes develop COVID-19, then home management may still be possible, wrote the authors, who recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve.
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often. “Frequent changes in dosage and correctional bolus may be required to maintain normoglycemia,” they cautioned.
Concerning diabetic drug regimens, they suggest patients avoid antihyperglycemic agents that can cause volume depletion or hypoglycemia and, if necessary, that they reduce oral antidiabetic drugs and follow sick-day guidelines.
For hospitalized patients, the investigators strengthened that statement, advising that oral agents need to be stopped, particularly sodium-glucose cotransporter 2 inhibitors and metformin. “Insulin is the preferred agent for control of hyperglycemia in hospitalized sick patients,” they wrote.
Untested therapies
The authors also discussed a range of untested therapies that may help fight COVID-19, such as antiviral drugs (such as lopinavir and ritonavir), zinc nanoparticles, and vitamin C. Supplementing those recommendations, Dr. Gupta and colleagues provided a concise review of COVID-19 epidemiology and extant data relevant to patients with diabetes.
The investigators reported no conflicts of interest.
SOURCE: Gupta et al. Diabetes Metab Syndr. 2020;14(3):211-12.
Patients with diabetes may have an increased risk of developing coronavirus infection (COVID-19), along with increased risks of morbidity and mortality, according to researchers writing in Diabetes & Metabolic Syndrome.
Although relevant clinical data remain scarce, patients with diabetes should take extra precautions to avoid infection and, if infected, may require special care, reported Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues.
“The disease severity [with COVID-19] has varied from mild, self-limiting, flu-like illness to fulminant pneumonia, respiratory failure, and death,” the authors wrote.
As of March 16, 2020, the World Health Organization reported 167,515 confirmed cases of COVID-19 and 6,606 deaths from around the world, with a mortality rate of 3.9%. But the actual mortality rate may be lower, the authors suggested, because a study involving more than 1,000 confirmed cases reported a mortality rate of 1.4%.
“Considering that the number of unreported and unconfirmed cases is likely to be much higher than the reported cases, the actual mortality may be less than 1%, which is similar to that of severe seasonal influenza,” the authors said, in reference to an editorial by Anthony S. Fauci, MD, and colleagues in the New England Journal of Medicine. In addition, they noted, mortality rates may vary by region.
The largest study relevant to patients with diabetes, which involved 72,314 cases of COVID-19, showed that patients with diabetes had a threefold higher mortality rate than did those without diabetes (7.3% vs. 2.3%, respectively). These figures were reported by the Chinese Centre for Disease Control and Prevention.
However, data from smaller cohorts with diabetes and COVID-19 have yielded mixed results. For instance, one study, involving 140 patients from Wuhan, suggested that diabetes was not a risk factor for severe disease, and in an analysis of 11 studies reporting on laboratory abnormalities in patients with a diagnosis of COVID-19, raised blood sugar levels or diabetes were not mentioned among the predictors of severe disease.
“Our knowledge about the prevalence of COVID-19 and disease course in people with diabetes will evolve as more detailed analyses are carried out,” the authors wrote. “For now, it is reasonable to assume that people with diabetes are at increased risk of developing infection. Coexisting heart disease, kidney disease, advanced age, and frailty are likely to further increase the severity of disease.”
Prevention first
“It is important that people with diabetes maintain good glycemic control, because it might help in reducing the risk of infection and the severity,” the authors wrote.
In addition to more frequent monitoring of blood glucose levels, they recommended other preventive measures, such as getting adequate nutrition, exercising, and being current with vaccinations for influenza and pneumonia. The latter, they said, may also reduce the risk of secondary bacterial pneumonia after a respiratory viral infection.
In regard to nutrition, adequate protein intake is important and “any deficiencies of minerals and vitamins need to be taken care of,” they advised. Likewise, exercise is known to improve immunity and should continue, but they suggest avoiding gyms and swimming pools.
For patients with coexisting heart and/or kidney disease, they also recommended efforts to stabilize cardiac/renal status.
In addition, the general preventive measures, such as regular and thorough hand washing with soap and water, practicing good respiratory hygiene by sneezing and coughing into a bent elbow or a facial tissue, and avoiding contact with anyone who is infected, should be observed.
As with other patients with chronic diseases that are managed long-term medications, patients with diabetes should always ensure that they have a sufficient supply of their medications and refills, if possible.
After a diagnosis
If patients with diabetes develop COVID-19, then home management may still be possible, wrote the authors, who recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve.
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often. “Frequent changes in dosage and correctional bolus may be required to maintain normoglycemia,” they cautioned.
Concerning diabetic drug regimens, they suggest patients avoid antihyperglycemic agents that can cause volume depletion or hypoglycemia and, if necessary, that they reduce oral antidiabetic drugs and follow sick-day guidelines.
For hospitalized patients, the investigators strengthened that statement, advising that oral agents need to be stopped, particularly sodium-glucose cotransporter 2 inhibitors and metformin. “Insulin is the preferred agent for control of hyperglycemia in hospitalized sick patients,” they wrote.
Untested therapies
The authors also discussed a range of untested therapies that may help fight COVID-19, such as antiviral drugs (such as lopinavir and ritonavir), zinc nanoparticles, and vitamin C. Supplementing those recommendations, Dr. Gupta and colleagues provided a concise review of COVID-19 epidemiology and extant data relevant to patients with diabetes.
The investigators reported no conflicts of interest.
SOURCE: Gupta et al. Diabetes Metab Syndr. 2020;14(3):211-12.
FROM DIABETES & METABOLIC SYNDROME
FDA provides flexibility to improve COVID-19 test availability
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
CDC expert answers top COVID-19 questions
With new developments daily and lingering uncertainty about COVID-19, questions about testing and treatment for the coronavirus are at the forefront.
To address these top questions, Jay C. Butler, MD, deputy director for infectious diseases at the Centers for Disease Control and Prevention, sat down with JAMA editor Howard Bauchner, MD, to discuss the latest data on COVID-19 and to outline updated guidance from the agency. The following question-and-answer session was part of a live stream interview hosted by JAMA on March 16, 2020. The questions have been edited for length and clarity.
What test is being used to identify COVID-19?
In the United States, the most common and widely available test is the RT-polymerase chain reaction (rRT-PCR), which over the past few weeks has become available at public health labs across the country, Dr. Butler said during the JAMA interview. Capacity for the test is now possible in all 50 states and in Washington, D.C.
“More recently, there’s been a number of commercial labs that have come online to be able to do the testing,” Dr. Butler said. “Additionally, a number of academic centers are now able to run [Food and Drug Administration]–approved testing using slightly different PCR platforms.”
How accurate is the test?
Dr. Butler called PCR the “gold standard,” for testing COVID-19, and said it’s safe to say the test’s likelihood of identifying infection or past infection is extremely high. However, data on test sensitivity is limited.
“This may be frustrating to those of us who really like to know specifics of how to interpret the test results, but it’s important to keep in mind, we’re talking about a virus that we didn’t know existed 3 months ago,” he said.
At what point does a person with coronavirus test positive?
When exactly a test becomes positive is an unknown, Dr. Butler said. The assumption is that a patient who tests positive is more likely to be infectious, and data suggest the level of infectiousness is greatest after the onset of symptoms.
“There is at least some anecdotal reports that suggest that transmission could occur before onset of symptoms, but the data is still very limited,” he said. “Of course that has big implications in terms of how well we can really slow the spread of the virus.”
Who should get tested?
Dr. Butler said the focus should be individuals who are symptomatic with evidence of respiratory tract infection. People who are concerned about the virus and want a test are not the target.
“It’s important when talking to patients to help them to understand, this is different than a test for HIV or hepatitis C, where much of the message is: ‘Please get tested.’ ” he said. “This a situation where we’re trying to diagnose an acute infection. We do have a resource that may become limited again as some of the equipment required for running the test or collecting the specimen may come into short supply, so we want to focus on those people who are symptomatic and particularly on people who may be at higher risk of more severe illness.”
If a previously infected patient tests negative, can they still shed virus?
The CDC is currently analyzing how a negative PCR test relates to viral load, according to Dr. Butler. He added there have been situations in which a patient has twice tested negative for the virus, but a third swab resulted in a weakly positive result.
“It’s not clear if those are people who are actually infectious,” he said. “The PCR is detecting viral RNA, it doesn’t necessarily indicate there is viable virus present in the respiratory tract. So in general, I think it is safe to go back to work, but a positive test in a situation like that can be very difficult to interpret because we think it probably doesn’t reflect infectivity, but we don’t know for sure.”
Do we have an adequate supply of tests in the United States?
The CDC has addressed supply concerns by broadening the number of PCR platforms that can be used to run COVID-19 analyses, Dr. Butler said. Expansion of these platforms has been one way the government is furthering testing options and enabling consumer labs and academic centers to contribute to testing.
When can people who test positive go back to work?
The CDC is still researching that question and reviewing the data, Dr. Butler said. The current recommendation is that a patient who tests positive is considered clear to return to work after two negative tests at least 24 hours apart, following the resolution of symptoms. The CDC has not yet made an official recommendation on an exact time frame, but the CDC is considering a 14-day minimum of quarantine.
“The one caveat I’ll add is that someone who is a health care worker, even if they have resolved symptoms, it’s still a good idea to wear a surgical mask [when they return to work], just as an extra precaution.”
What do we know about immunity? Can patients get reinfected?
Long-term immunity after exposure and infection is virtually unknown, Dr. Butler said. Investigators know those with COVID-19 have an antibody response, but whether that is protective or not, is unclear. In regard to older coronaviruses, such as those that cause colds, patients generally develop an antibody response and may have a period of immunity, but that immunity eventually wanes and reinfection can occur.
What is the latest on therapies?
A number of trials are underway in China and in the United States to test possible therapies for COVID-19, Dr. Butler said. One of the candidate drugs is the broad spectrum antiviral drug remdesivir, which was developed for the treatment of the Ebola virus. Additionally, the National Institutes of Health is studying the potential for monoclonal antibodies to treat COVID-19.
“Of course these are drugs not yet FDA approved,” he said. “We all want to have them in our toolbox as soon as possible, but we want to make sure these drugs are going to benefit and not harm, and that they really do have the utility that we hope for.”
Is there specific guidance for healthcare workers about COVID-19?
Health care workers have a much higher likelihood of being exposed or exposing others who are at high risk of severe infection, Dr. Butler said. That’s why, if a health care worker becomes infected and recovers, it’s still important to take extra precautions when going back to work, such as wearing a mask.
“These are recommendations that are in-draft,” he said. “I want to be clear, I’m floating concepts out there that people can consider. ... I recognize as a former infection control medical director at a hospital that sometimes you have to adapt those guidelines based on your local conditions.”
With new developments daily and lingering uncertainty about COVID-19, questions about testing and treatment for the coronavirus are at the forefront.
To address these top questions, Jay C. Butler, MD, deputy director for infectious diseases at the Centers for Disease Control and Prevention, sat down with JAMA editor Howard Bauchner, MD, to discuss the latest data on COVID-19 and to outline updated guidance from the agency. The following question-and-answer session was part of a live stream interview hosted by JAMA on March 16, 2020. The questions have been edited for length and clarity.
What test is being used to identify COVID-19?
In the United States, the most common and widely available test is the RT-polymerase chain reaction (rRT-PCR), which over the past few weeks has become available at public health labs across the country, Dr. Butler said during the JAMA interview. Capacity for the test is now possible in all 50 states and in Washington, D.C.
“More recently, there’s been a number of commercial labs that have come online to be able to do the testing,” Dr. Butler said. “Additionally, a number of academic centers are now able to run [Food and Drug Administration]–approved testing using slightly different PCR platforms.”
How accurate is the test?
Dr. Butler called PCR the “gold standard,” for testing COVID-19, and said it’s safe to say the test’s likelihood of identifying infection or past infection is extremely high. However, data on test sensitivity is limited.
“This may be frustrating to those of us who really like to know specifics of how to interpret the test results, but it’s important to keep in mind, we’re talking about a virus that we didn’t know existed 3 months ago,” he said.
At what point does a person with coronavirus test positive?
When exactly a test becomes positive is an unknown, Dr. Butler said. The assumption is that a patient who tests positive is more likely to be infectious, and data suggest the level of infectiousness is greatest after the onset of symptoms.
“There is at least some anecdotal reports that suggest that transmission could occur before onset of symptoms, but the data is still very limited,” he said. “Of course that has big implications in terms of how well we can really slow the spread of the virus.”
Who should get tested?
Dr. Butler said the focus should be individuals who are symptomatic with evidence of respiratory tract infection. People who are concerned about the virus and want a test are not the target.
“It’s important when talking to patients to help them to understand, this is different than a test for HIV or hepatitis C, where much of the message is: ‘Please get tested.’ ” he said. “This a situation where we’re trying to diagnose an acute infection. We do have a resource that may become limited again as some of the equipment required for running the test or collecting the specimen may come into short supply, so we want to focus on those people who are symptomatic and particularly on people who may be at higher risk of more severe illness.”
If a previously infected patient tests negative, can they still shed virus?
The CDC is currently analyzing how a negative PCR test relates to viral load, according to Dr. Butler. He added there have been situations in which a patient has twice tested negative for the virus, but a third swab resulted in a weakly positive result.
“It’s not clear if those are people who are actually infectious,” he said. “The PCR is detecting viral RNA, it doesn’t necessarily indicate there is viable virus present in the respiratory tract. So in general, I think it is safe to go back to work, but a positive test in a situation like that can be very difficult to interpret because we think it probably doesn’t reflect infectivity, but we don’t know for sure.”
Do we have an adequate supply of tests in the United States?
The CDC has addressed supply concerns by broadening the number of PCR platforms that can be used to run COVID-19 analyses, Dr. Butler said. Expansion of these platforms has been one way the government is furthering testing options and enabling consumer labs and academic centers to contribute to testing.
When can people who test positive go back to work?
The CDC is still researching that question and reviewing the data, Dr. Butler said. The current recommendation is that a patient who tests positive is considered clear to return to work after two negative tests at least 24 hours apart, following the resolution of symptoms. The CDC has not yet made an official recommendation on an exact time frame, but the CDC is considering a 14-day minimum of quarantine.
“The one caveat I’ll add is that someone who is a health care worker, even if they have resolved symptoms, it’s still a good idea to wear a surgical mask [when they return to work], just as an extra precaution.”
What do we know about immunity? Can patients get reinfected?
Long-term immunity after exposure and infection is virtually unknown, Dr. Butler said. Investigators know those with COVID-19 have an antibody response, but whether that is protective or not, is unclear. In regard to older coronaviruses, such as those that cause colds, patients generally develop an antibody response and may have a period of immunity, but that immunity eventually wanes and reinfection can occur.
What is the latest on therapies?
A number of trials are underway in China and in the United States to test possible therapies for COVID-19, Dr. Butler said. One of the candidate drugs is the broad spectrum antiviral drug remdesivir, which was developed for the treatment of the Ebola virus. Additionally, the National Institutes of Health is studying the potential for monoclonal antibodies to treat COVID-19.
“Of course these are drugs not yet FDA approved,” he said. “We all want to have them in our toolbox as soon as possible, but we want to make sure these drugs are going to benefit and not harm, and that they really do have the utility that we hope for.”
Is there specific guidance for healthcare workers about COVID-19?
Health care workers have a much higher likelihood of being exposed or exposing others who are at high risk of severe infection, Dr. Butler said. That’s why, if a health care worker becomes infected and recovers, it’s still important to take extra precautions when going back to work, such as wearing a mask.
“These are recommendations that are in-draft,” he said. “I want to be clear, I’m floating concepts out there that people can consider. ... I recognize as a former infection control medical director at a hospital that sometimes you have to adapt those guidelines based on your local conditions.”
With new developments daily and lingering uncertainty about COVID-19, questions about testing and treatment for the coronavirus are at the forefront.
To address these top questions, Jay C. Butler, MD, deputy director for infectious diseases at the Centers for Disease Control and Prevention, sat down with JAMA editor Howard Bauchner, MD, to discuss the latest data on COVID-19 and to outline updated guidance from the agency. The following question-and-answer session was part of a live stream interview hosted by JAMA on March 16, 2020. The questions have been edited for length and clarity.
What test is being used to identify COVID-19?
In the United States, the most common and widely available test is the RT-polymerase chain reaction (rRT-PCR), which over the past few weeks has become available at public health labs across the country, Dr. Butler said during the JAMA interview. Capacity for the test is now possible in all 50 states and in Washington, D.C.
“More recently, there’s been a number of commercial labs that have come online to be able to do the testing,” Dr. Butler said. “Additionally, a number of academic centers are now able to run [Food and Drug Administration]–approved testing using slightly different PCR platforms.”
How accurate is the test?
Dr. Butler called PCR the “gold standard,” for testing COVID-19, and said it’s safe to say the test’s likelihood of identifying infection or past infection is extremely high. However, data on test sensitivity is limited.
“This may be frustrating to those of us who really like to know specifics of how to interpret the test results, but it’s important to keep in mind, we’re talking about a virus that we didn’t know existed 3 months ago,” he said.
At what point does a person with coronavirus test positive?
When exactly a test becomes positive is an unknown, Dr. Butler said. The assumption is that a patient who tests positive is more likely to be infectious, and data suggest the level of infectiousness is greatest after the onset of symptoms.
“There is at least some anecdotal reports that suggest that transmission could occur before onset of symptoms, but the data is still very limited,” he said. “Of course that has big implications in terms of how well we can really slow the spread of the virus.”
Who should get tested?
Dr. Butler said the focus should be individuals who are symptomatic with evidence of respiratory tract infection. People who are concerned about the virus and want a test are not the target.
“It’s important when talking to patients to help them to understand, this is different than a test for HIV or hepatitis C, where much of the message is: ‘Please get tested.’ ” he said. “This a situation where we’re trying to diagnose an acute infection. We do have a resource that may become limited again as some of the equipment required for running the test or collecting the specimen may come into short supply, so we want to focus on those people who are symptomatic and particularly on people who may be at higher risk of more severe illness.”
If a previously infected patient tests negative, can they still shed virus?
The CDC is currently analyzing how a negative PCR test relates to viral load, according to Dr. Butler. He added there have been situations in which a patient has twice tested negative for the virus, but a third swab resulted in a weakly positive result.
“It’s not clear if those are people who are actually infectious,” he said. “The PCR is detecting viral RNA, it doesn’t necessarily indicate there is viable virus present in the respiratory tract. So in general, I think it is safe to go back to work, but a positive test in a situation like that can be very difficult to interpret because we think it probably doesn’t reflect infectivity, but we don’t know for sure.”
Do we have an adequate supply of tests in the United States?
The CDC has addressed supply concerns by broadening the number of PCR platforms that can be used to run COVID-19 analyses, Dr. Butler said. Expansion of these platforms has been one way the government is furthering testing options and enabling consumer labs and academic centers to contribute to testing.
When can people who test positive go back to work?
The CDC is still researching that question and reviewing the data, Dr. Butler said. The current recommendation is that a patient who tests positive is considered clear to return to work after two negative tests at least 24 hours apart, following the resolution of symptoms. The CDC has not yet made an official recommendation on an exact time frame, but the CDC is considering a 14-day minimum of quarantine.
“The one caveat I’ll add is that someone who is a health care worker, even if they have resolved symptoms, it’s still a good idea to wear a surgical mask [when they return to work], just as an extra precaution.”
What do we know about immunity? Can patients get reinfected?
Long-term immunity after exposure and infection is virtually unknown, Dr. Butler said. Investigators know those with COVID-19 have an antibody response, but whether that is protective or not, is unclear. In regard to older coronaviruses, such as those that cause colds, patients generally develop an antibody response and may have a period of immunity, but that immunity eventually wanes and reinfection can occur.
What is the latest on therapies?
A number of trials are underway in China and in the United States to test possible therapies for COVID-19, Dr. Butler said. One of the candidate drugs is the broad spectrum antiviral drug remdesivir, which was developed for the treatment of the Ebola virus. Additionally, the National Institutes of Health is studying the potential for monoclonal antibodies to treat COVID-19.
“Of course these are drugs not yet FDA approved,” he said. “We all want to have them in our toolbox as soon as possible, but we want to make sure these drugs are going to benefit and not harm, and that they really do have the utility that we hope for.”
Is there specific guidance for healthcare workers about COVID-19?
Health care workers have a much higher likelihood of being exposed or exposing others who are at high risk of severe infection, Dr. Butler said. That’s why, if a health care worker becomes infected and recovers, it’s still important to take extra precautions when going back to work, such as wearing a mask.
“These are recommendations that are in-draft,” he said. “I want to be clear, I’m floating concepts out there that people can consider. ... I recognize as a former infection control medical director at a hospital that sometimes you have to adapt those guidelines based on your local conditions.”














