User login
Early heparin treatment linked to lower COVID-19 mortality
Early treatment with low-molecular-weight heparin (LMWH) reduces the risk for death in patients with COVID-19, a retrospective cohort study shows.
Heparin could reduce the risk for blood clots, Andrea De Vito, MD, of the unit of infectious diseases at the University of Sassari, Italy, said during his online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
“Several studies try to describe the role played by coagulopathies in COVID-19 death,” but the mechanism causing them is still unclear, Dr. De Vito explained.
Some guidelines have suggested heparin as a treatment for hospitalized COVID-19 patients, but few have looked at nonhospitalized patients. In fact, the National Institutes of Health discourages the use of heparin in nonhospitalized COVID-19 patients, and guidance for the home care of COVID-19 patients from the World Health Organization doesn’t mention heparin treatment at all, he said.
To examine the benefits of early heparin – whether administered at home or in the hospital – Dr. De Vito and colleagues looked at a cohort of older adults with COVID-19 who were evaluated or treated at an Italian university hospital.
“Some patients were hospitalized immediately after symptoms onset; other people preferred to call their general practitioner and started the treatment at home,” Dr. De Vito said in an interview. “Other people were hospitalized for worsening of symptoms later in the course of the disease.”
Of the 734 patients, 296 received heparin within 5 days of the onset of symptoms or a positive COVID-19 test. Of the remaining 438 patients, 196 received LMWH treatment later during the disease course, and the rest never received LMWH.
All patients who received early heparin were treated with LMWH 4,000 IU, or 6,000 IU if their body mass index was above 30 kg/m2. This was reduced to 2,000 IU if estimated glomerular filtration rate (eGFR) dropped below 30 mL/min. None of the patients had previously received heparin.
Median age was slightly younger for patients who received early heparin than for those who did not (76.8 vs. 78.5 years).
Other demographic characteristics, such as sex and BMI, were similar in the two groups, as were rates of comorbidities, such as hypertension, cardiovascular disease, diabetes, chronic obstructive pulmonary disease, kidney disease, and neurologic conditions. Also similar were the frequency of symptoms (such as fever, cough, and shortness of breath) and rates of treatment with remdesivir or steroids.
Rates of hospital admission were not significantly different between patients who received early heparin and those who did not (65% vs. 61%). There was also no significant difference in use of a venturi mask (35% vs. 28%), noninvasive ventilation (13% vs. 14%), or intubation (5% vs. 8%).
However, rates of death were significantly lower in patients who received early heparin than in those who did not (13% vs. 25%; P < .0001).
There was a trend toward shorter hospital stays for patients treated with early heparin, but the difference was not significant (median, 10 vs. 13 days; P = .08).
Researchers also conducted a separate analysis of 219 COVID-19 patients who received LMWH at home, regardless of when during their disease course they received it. These patients were significantly less likely to be hospitalized than were patients who did not receive LMWH at home (odds ratio, 0.2; P < .0001).
Comparatively, early heparin treatment had a greater effect on the risk for death and the risk for hospitalization than did other factors.
“Thromboemboli are a major complication of COVID. There is good consensus that hospitalized patients with COVID should receive anticoagulants prophylactically, although the best dose is being studied,” said Judy Stone, MD, an infectious disease physician and journalist who was not involved in the study.
“This study extends those findings of benefit from anticoagulants to nonhospitalized patients, with fewer deaths in those treated with low-molecular-weight heparin,” Dr. Stone told this news organization. “The major limitation is that the study is retrospective and observational. The next step would be to confirm these findings prospectively, randomizing a similar group to LMWH or no anticoagulation.”
Another limitation of the study is that some of the patients lived in nursing homes and might have received care from nurses that eliminated the need for hospitalization, Dr. De Vito added.
The study did not note any external funding. The authors have disclosed no relevant financial relationships. Dr. Stone is a member of the advisory committee for the C-Path CURE Drug Repurposing Collaboratory (CDRC) program and has written for Medscape.
A version of this article first appeared on Medscape.com.
Early treatment with low-molecular-weight heparin (LMWH) reduces the risk for death in patients with COVID-19, a retrospective cohort study shows.
Heparin could reduce the risk for blood clots, Andrea De Vito, MD, of the unit of infectious diseases at the University of Sassari, Italy, said during his online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
“Several studies try to describe the role played by coagulopathies in COVID-19 death,” but the mechanism causing them is still unclear, Dr. De Vito explained.
Some guidelines have suggested heparin as a treatment for hospitalized COVID-19 patients, but few have looked at nonhospitalized patients. In fact, the National Institutes of Health discourages the use of heparin in nonhospitalized COVID-19 patients, and guidance for the home care of COVID-19 patients from the World Health Organization doesn’t mention heparin treatment at all, he said.
To examine the benefits of early heparin – whether administered at home or in the hospital – Dr. De Vito and colleagues looked at a cohort of older adults with COVID-19 who were evaluated or treated at an Italian university hospital.
“Some patients were hospitalized immediately after symptoms onset; other people preferred to call their general practitioner and started the treatment at home,” Dr. De Vito said in an interview. “Other people were hospitalized for worsening of symptoms later in the course of the disease.”
Of the 734 patients, 296 received heparin within 5 days of the onset of symptoms or a positive COVID-19 test. Of the remaining 438 patients, 196 received LMWH treatment later during the disease course, and the rest never received LMWH.
All patients who received early heparin were treated with LMWH 4,000 IU, or 6,000 IU if their body mass index was above 30 kg/m2. This was reduced to 2,000 IU if estimated glomerular filtration rate (eGFR) dropped below 30 mL/min. None of the patients had previously received heparin.
Median age was slightly younger for patients who received early heparin than for those who did not (76.8 vs. 78.5 years).
Other demographic characteristics, such as sex and BMI, were similar in the two groups, as were rates of comorbidities, such as hypertension, cardiovascular disease, diabetes, chronic obstructive pulmonary disease, kidney disease, and neurologic conditions. Also similar were the frequency of symptoms (such as fever, cough, and shortness of breath) and rates of treatment with remdesivir or steroids.
Rates of hospital admission were not significantly different between patients who received early heparin and those who did not (65% vs. 61%). There was also no significant difference in use of a venturi mask (35% vs. 28%), noninvasive ventilation (13% vs. 14%), or intubation (5% vs. 8%).
However, rates of death were significantly lower in patients who received early heparin than in those who did not (13% vs. 25%; P < .0001).
There was a trend toward shorter hospital stays for patients treated with early heparin, but the difference was not significant (median, 10 vs. 13 days; P = .08).
Researchers also conducted a separate analysis of 219 COVID-19 patients who received LMWH at home, regardless of when during their disease course they received it. These patients were significantly less likely to be hospitalized than were patients who did not receive LMWH at home (odds ratio, 0.2; P < .0001).
Comparatively, early heparin treatment had a greater effect on the risk for death and the risk for hospitalization than did other factors.
“Thromboemboli are a major complication of COVID. There is good consensus that hospitalized patients with COVID should receive anticoagulants prophylactically, although the best dose is being studied,” said Judy Stone, MD, an infectious disease physician and journalist who was not involved in the study.
“This study extends those findings of benefit from anticoagulants to nonhospitalized patients, with fewer deaths in those treated with low-molecular-weight heparin,” Dr. Stone told this news organization. “The major limitation is that the study is retrospective and observational. The next step would be to confirm these findings prospectively, randomizing a similar group to LMWH or no anticoagulation.”
Another limitation of the study is that some of the patients lived in nursing homes and might have received care from nurses that eliminated the need for hospitalization, Dr. De Vito added.
The study did not note any external funding. The authors have disclosed no relevant financial relationships. Dr. Stone is a member of the advisory committee for the C-Path CURE Drug Repurposing Collaboratory (CDRC) program and has written for Medscape.
A version of this article first appeared on Medscape.com.
Early treatment with low-molecular-weight heparin (LMWH) reduces the risk for death in patients with COVID-19, a retrospective cohort study shows.
Heparin could reduce the risk for blood clots, Andrea De Vito, MD, of the unit of infectious diseases at the University of Sassari, Italy, said during his online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
“Several studies try to describe the role played by coagulopathies in COVID-19 death,” but the mechanism causing them is still unclear, Dr. De Vito explained.
Some guidelines have suggested heparin as a treatment for hospitalized COVID-19 patients, but few have looked at nonhospitalized patients. In fact, the National Institutes of Health discourages the use of heparin in nonhospitalized COVID-19 patients, and guidance for the home care of COVID-19 patients from the World Health Organization doesn’t mention heparin treatment at all, he said.
To examine the benefits of early heparin – whether administered at home or in the hospital – Dr. De Vito and colleagues looked at a cohort of older adults with COVID-19 who were evaluated or treated at an Italian university hospital.
“Some patients were hospitalized immediately after symptoms onset; other people preferred to call their general practitioner and started the treatment at home,” Dr. De Vito said in an interview. “Other people were hospitalized for worsening of symptoms later in the course of the disease.”
Of the 734 patients, 296 received heparin within 5 days of the onset of symptoms or a positive COVID-19 test. Of the remaining 438 patients, 196 received LMWH treatment later during the disease course, and the rest never received LMWH.
All patients who received early heparin were treated with LMWH 4,000 IU, or 6,000 IU if their body mass index was above 30 kg/m2. This was reduced to 2,000 IU if estimated glomerular filtration rate (eGFR) dropped below 30 mL/min. None of the patients had previously received heparin.
Median age was slightly younger for patients who received early heparin than for those who did not (76.8 vs. 78.5 years).
Other demographic characteristics, such as sex and BMI, were similar in the two groups, as were rates of comorbidities, such as hypertension, cardiovascular disease, diabetes, chronic obstructive pulmonary disease, kidney disease, and neurologic conditions. Also similar were the frequency of symptoms (such as fever, cough, and shortness of breath) and rates of treatment with remdesivir or steroids.
Rates of hospital admission were not significantly different between patients who received early heparin and those who did not (65% vs. 61%). There was also no significant difference in use of a venturi mask (35% vs. 28%), noninvasive ventilation (13% vs. 14%), or intubation (5% vs. 8%).
However, rates of death were significantly lower in patients who received early heparin than in those who did not (13% vs. 25%; P < .0001).
There was a trend toward shorter hospital stays for patients treated with early heparin, but the difference was not significant (median, 10 vs. 13 days; P = .08).
Researchers also conducted a separate analysis of 219 COVID-19 patients who received LMWH at home, regardless of when during their disease course they received it. These patients were significantly less likely to be hospitalized than were patients who did not receive LMWH at home (odds ratio, 0.2; P < .0001).
Comparatively, early heparin treatment had a greater effect on the risk for death and the risk for hospitalization than did other factors.
“Thromboemboli are a major complication of COVID. There is good consensus that hospitalized patients with COVID should receive anticoagulants prophylactically, although the best dose is being studied,” said Judy Stone, MD, an infectious disease physician and journalist who was not involved in the study.
“This study extends those findings of benefit from anticoagulants to nonhospitalized patients, with fewer deaths in those treated with low-molecular-weight heparin,” Dr. Stone told this news organization. “The major limitation is that the study is retrospective and observational. The next step would be to confirm these findings prospectively, randomizing a similar group to LMWH or no anticoagulation.”
Another limitation of the study is that some of the patients lived in nursing homes and might have received care from nurses that eliminated the need for hospitalization, Dr. De Vito added.
The study did not note any external funding. The authors have disclosed no relevant financial relationships. Dr. Stone is a member of the advisory committee for the C-Path CURE Drug Repurposing Collaboratory (CDRC) program and has written for Medscape.
A version of this article first appeared on Medscape.com.
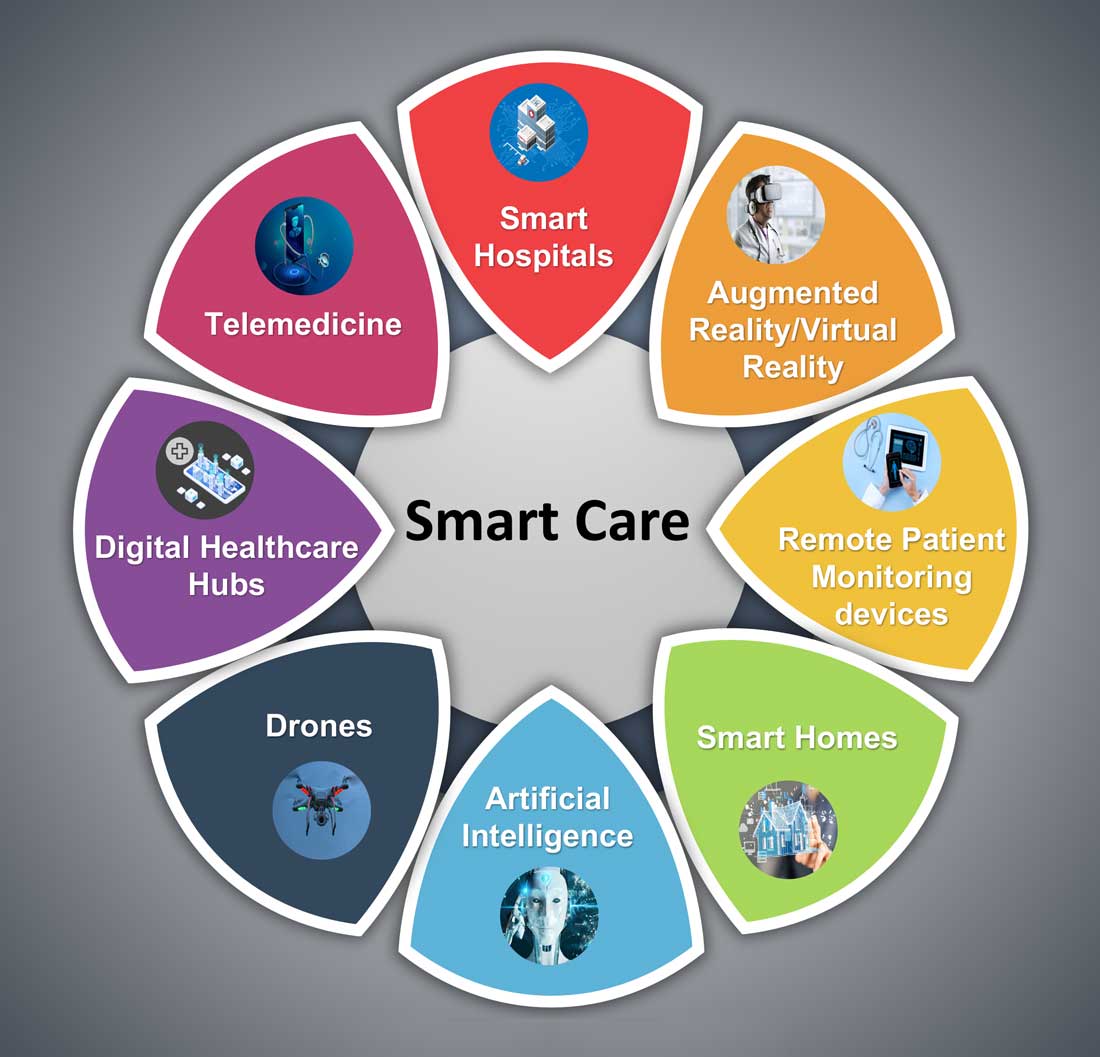
Hospital medicine and the future of smart care
People often overestimate what will happen in the next two years and underestimate what will happen in ten. – Bill Gates
The COVID-19 pandemic set in motion a series of innovations catalyzing the digital transformation of the health care landscape.
Telemedicine use exploded over the last 12 months to the point that it has almost become ubiquitous. With that, we saw a rapid proliferation of wearables and remote patient monitoring devices. Thanks to virtual care, care delivery is no longer strictly dependent on having onsite specialists, and care itself is not confined to the boundaries of hospitals or doctors’ offices anymore.
We saw the formation of the digital front door and the emergence of new virtual care sites like virtual urgent care, virtual home health, virtual office visits, virtual hospital at home that allowed clinical care to be delivered safely outside the boundaries of hospitals. Nonclinical public places like gyms, schools, and community centers were being transformed into virtual health care portals that brought care closer to the people.
Inside the hospital, we saw a fusion of traditional inpatient care and virtual care. Onsite hospital teams embraced telemedicine during the pandemic for various reasons; to conserve personal protective equipment (PPE), limit exposure, boost care capacity, improve access to specialists at distant sites, and bring family memberse to “webside” who cannot be at a patient’s bedside.
In clinical trials as well, virtual care is a welcome change. According to one survey1, most trial participants favored the use of telehealth services for clinical trials, as these helped them stay engaged, compliant, monitored, and on track while remaining at home. Furthermore, we are seeing the integration of artificial intelligence (AI) into telehealth, whether it is to aid physicians in clinical decision-making or to generate reminders to help patients with chronic disease management. However, this integration is only beginning to scratch the surface of the combination of two technologies’ real potential.
What’s next?
Based on these trends, it should be no surprise that digital health will become a vital sign for health care organizations.
The next 12 to 24 months will set new standards for digital health and play a significant role in defining the next generation of virtual care. There are projections that global health care industry revenues will exceed $2.6 trillion by 2025, with AI and telehealth playing a prominent role in this growth.2 According to estimates, telehealth itself will be a $175 billion market by 2026 and approximately one in three patient encounters will go virtual.3,4 Moreover, virtual care will continue to make exciting transformations, helping to make quality care accessible to everyone in innovative ways. For example, the University of Cincinnati has recently developed a pilot project using a drone equipped with video technology, artificial intelligence, sensors, and first aid kits to go to hard-to-reach areas to deliver care via telemedicine.5
Smart hospitals
In coming years, we can expect the integration of AI, augmented reality (AR), and virtual reality (VR) into telemedicine at lightning speed – and at a much larger scale – that will enable surgeons from different parts of the globe to perform procedures remotely and more precisely.
AI is already gaining traction in different fields within health care – whether it’s predicting length of stay in the ICU, or assisting in triage decisions, or reading radiological images, to name just a few. The Mayo Clinic is using AI and computer-aided decision-making tools to predict the risk of surgery and potential post-op complications, which could allow even better collaboration between medical and surgical teams. We hear about the “X-ray” vision offered to proceduralists using HoloLens – mixed reality smartglasses – a technology that enables them to perform procedures more precisely. Others project that there will be more sensors and voice recognition tools in the OR that will be used to gather data to develop intelligent algorithms, and to build a safety net for interventionalists that can notify them of potential hazards or accidental sterile field breaches. The insights gained will be used to create best practices and even allow some procedures to be performed outside the traditional OR setting.
Additionally, we are seeing the development of “smart” patient rooms. For example, one health system in Florida is working on deploying Amazon Alexa in 2,500 patient rooms to allow patients to connect more easily to their care team members. In the not-so-distant future, smart hospitals with smart patient rooms and smart ORs equipped with telemedicine, AI, AR, mixed reality, and computer-aided decision-making tools will no longer be an exception.
Smart homes for smart care
Smart homes with technologies like gas detectors, movement sensors, and sleep sensors will continue to evolve. According to one estimate, the global smart home health care market was $8.7 billion in 2019, and is expected to be $96.2 billion by 2030.6
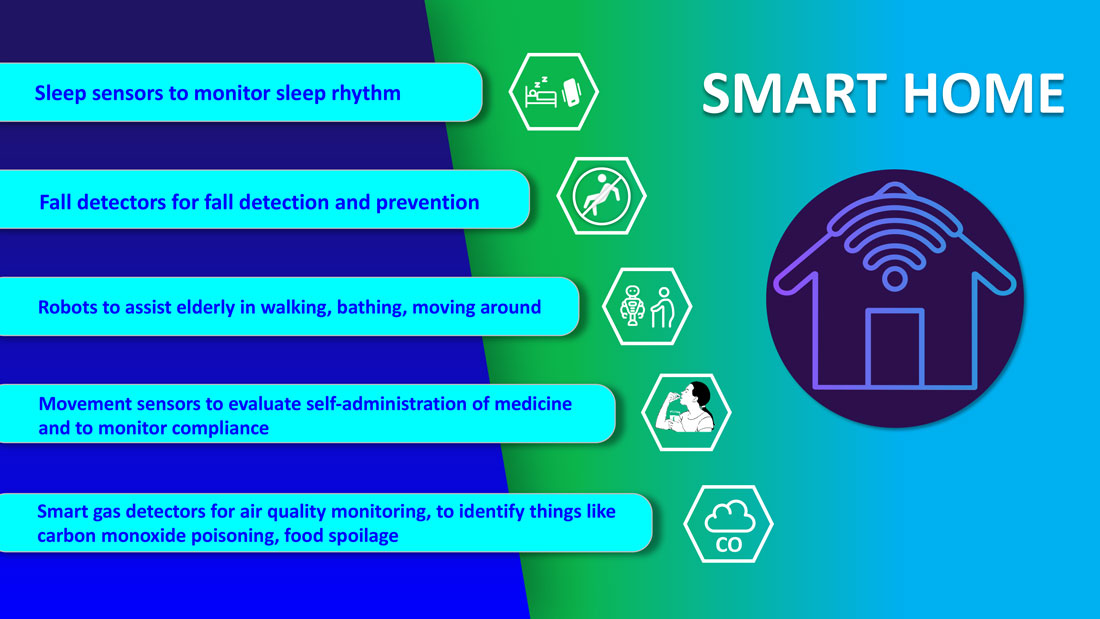
Smart technologies will have applications in fall detection and prevention, evaluation of self-administration of medicine, sleep rhythm monitoring, air quality monitoring for the detection of abnormal gas levels, and identification of things like carbon monoxide poisoning or food spoilage. In coming years, expect to see more virtual medical homes and digital health care complexes. Patients, from the convenience of their homes, might be able to connect to a suite of caregivers, all working collaboratively to provide more coordinated, effective care. The “hospital at home” model that started with six hospitals has already grown to over 100 hospitals across 29 states. The shift from onsite specialists to onscreen specialists will continue, providing greater access to specialized services.
With these emerging trends, it can be anticipated that much acute care will be provided to patients outside the hospital – either under the hospital at home model, via drone technology using telemedicine, through smart devices in smart homes, or via wearables and artificial intelligence. Hence, hospitals’ configuration in the future will be much different and more compact than currently, and many hospitals will be reserved for trauma patients, casualties of natural disasters, higher acuity diseases requiring complex procedures, and other emergencies.
The role of hospitalists has evolved over the years and is still evolving. It should be no surprise if, in the future, we work alongside a digital hospitalist twin to provide better and more personalized care to our patients. Change is uncomfortable but it is inevitable. When COVID hit, we were forced to find innovative ways to deliver care to our patients. One thing is for certain: post-pandemic (AD, or After Disease) we are not going back to a Before COVID (BC) state in terms of virtual care. With the new dawn of digital era, the crucial questions to address will be: What will the future role of a hospitalist look like? How can we leverage technology and embrace our flexibility to adapt to these trends? How can we apply the lessons learned during the pandemic to propel hospital medicine into the future? And is it time to rethink our role and even reclassify ourselves – from hospitalists to Acute Care Experts (ACE) or Primary Acute Care Physicians?
Dr. Zia is a hospitalist, physician advisor, and founder of Virtual Hospitalist - a telemedicine company with a 360-degree care model for hospital patients.
References
1. www.subjectwell.com/news/data-shows-a-majority-of-patients-remain-interested-in-clinical-trials-during-the-coronavirus-pandemic/
2. ww2.frost.com/news/press-releases/technology-innovations-and-virtual-consultations-drive-healthcare-2025/
3. www.gminsights.com/industry-analysis/telemedicine-market
4. www.healthcareitnews.com/blog/frost-sullivans-top-10-predictions-healthcare-2021
5. www.uc.edu/news/articles/2021/03/virtual-medicine--new-uc-telehealth-drone-makes-house-calls.html
6. www.psmarketresearch.com/market-analysis/smart-home-healthcare-market
People often overestimate what will happen in the next two years and underestimate what will happen in ten. – Bill Gates
The COVID-19 pandemic set in motion a series of innovations catalyzing the digital transformation of the health care landscape.
Telemedicine use exploded over the last 12 months to the point that it has almost become ubiquitous. With that, we saw a rapid proliferation of wearables and remote patient monitoring devices. Thanks to virtual care, care delivery is no longer strictly dependent on having onsite specialists, and care itself is not confined to the boundaries of hospitals or doctors’ offices anymore.
We saw the formation of the digital front door and the emergence of new virtual care sites like virtual urgent care, virtual home health, virtual office visits, virtual hospital at home that allowed clinical care to be delivered safely outside the boundaries of hospitals. Nonclinical public places like gyms, schools, and community centers were being transformed into virtual health care portals that brought care closer to the people.
Inside the hospital, we saw a fusion of traditional inpatient care and virtual care. Onsite hospital teams embraced telemedicine during the pandemic for various reasons; to conserve personal protective equipment (PPE), limit exposure, boost care capacity, improve access to specialists at distant sites, and bring family memberse to “webside” who cannot be at a patient’s bedside.
In clinical trials as well, virtual care is a welcome change. According to one survey1, most trial participants favored the use of telehealth services for clinical trials, as these helped them stay engaged, compliant, monitored, and on track while remaining at home. Furthermore, we are seeing the integration of artificial intelligence (AI) into telehealth, whether it is to aid physicians in clinical decision-making or to generate reminders to help patients with chronic disease management. However, this integration is only beginning to scratch the surface of the combination of two technologies’ real potential.
What’s next?
Based on these trends, it should be no surprise that digital health will become a vital sign for health care organizations.
The next 12 to 24 months will set new standards for digital health and play a significant role in defining the next generation of virtual care. There are projections that global health care industry revenues will exceed $2.6 trillion by 2025, with AI and telehealth playing a prominent role in this growth.2 According to estimates, telehealth itself will be a $175 billion market by 2026 and approximately one in three patient encounters will go virtual.3,4 Moreover, virtual care will continue to make exciting transformations, helping to make quality care accessible to everyone in innovative ways. For example, the University of Cincinnati has recently developed a pilot project using a drone equipped with video technology, artificial intelligence, sensors, and first aid kits to go to hard-to-reach areas to deliver care via telemedicine.5
Smart hospitals
In coming years, we can expect the integration of AI, augmented reality (AR), and virtual reality (VR) into telemedicine at lightning speed – and at a much larger scale – that will enable surgeons from different parts of the globe to perform procedures remotely and more precisely.

AI is already gaining traction in different fields within health care – whether it’s predicting length of stay in the ICU, or assisting in triage decisions, or reading radiological images, to name just a few. The Mayo Clinic is using AI and computer-aided decision-making tools to predict the risk of surgery and potential post-op complications, which could allow even better collaboration between medical and surgical teams. We hear about the “X-ray” vision offered to proceduralists using HoloLens – mixed reality smartglasses – a technology that enables them to perform procedures more precisely. Others project that there will be more sensors and voice recognition tools in the OR that will be used to gather data to develop intelligent algorithms, and to build a safety net for interventionalists that can notify them of potential hazards or accidental sterile field breaches. The insights gained will be used to create best practices and even allow some procedures to be performed outside the traditional OR setting.
Additionally, we are seeing the development of “smart” patient rooms. For example, one health system in Florida is working on deploying Amazon Alexa in 2,500 patient rooms to allow patients to connect more easily to their care team members. In the not-so-distant future, smart hospitals with smart patient rooms and smart ORs equipped with telemedicine, AI, AR, mixed reality, and computer-aided decision-making tools will no longer be an exception.
Smart homes for smart care
Smart homes with technologies like gas detectors, movement sensors, and sleep sensors will continue to evolve. According to one estimate, the global smart home health care market was $8.7 billion in 2019, and is expected to be $96.2 billion by 2030.6

Smart technologies will have applications in fall detection and prevention, evaluation of self-administration of medicine, sleep rhythm monitoring, air quality monitoring for the detection of abnormal gas levels, and identification of things like carbon monoxide poisoning or food spoilage. In coming years, expect to see more virtual medical homes and digital health care complexes. Patients, from the convenience of their homes, might be able to connect to a suite of caregivers, all working collaboratively to provide more coordinated, effective care. The “hospital at home” model that started with six hospitals has already grown to over 100 hospitals across 29 states. The shift from onsite specialists to onscreen specialists will continue, providing greater access to specialized services.
With these emerging trends, it can be anticipated that much acute care will be provided to patients outside the hospital – either under the hospital at home model, via drone technology using telemedicine, through smart devices in smart homes, or via wearables and artificial intelligence. Hence, hospitals’ configuration in the future will be much different and more compact than currently, and many hospitals will be reserved for trauma patients, casualties of natural disasters, higher acuity diseases requiring complex procedures, and other emergencies.
The role of hospitalists has evolved over the years and is still evolving. It should be no surprise if, in the future, we work alongside a digital hospitalist twin to provide better and more personalized care to our patients. Change is uncomfortable but it is inevitable. When COVID hit, we were forced to find innovative ways to deliver care to our patients. One thing is for certain: post-pandemic (AD, or After Disease) we are not going back to a Before COVID (BC) state in terms of virtual care. With the new dawn of digital era, the crucial questions to address will be: What will the future role of a hospitalist look like? How can we leverage technology and embrace our flexibility to adapt to these trends? How can we apply the lessons learned during the pandemic to propel hospital medicine into the future? And is it time to rethink our role and even reclassify ourselves – from hospitalists to Acute Care Experts (ACE) or Primary Acute Care Physicians?
Dr. Zia is a hospitalist, physician advisor, and founder of Virtual Hospitalist - a telemedicine company with a 360-degree care model for hospital patients.
References
1. www.subjectwell.com/news/data-shows-a-majority-of-patients-remain-interested-in-clinical-trials-during-the-coronavirus-pandemic/
2. ww2.frost.com/news/press-releases/technology-innovations-and-virtual-consultations-drive-healthcare-2025/
3. www.gminsights.com/industry-analysis/telemedicine-market
4. www.healthcareitnews.com/blog/frost-sullivans-top-10-predictions-healthcare-2021
5. www.uc.edu/news/articles/2021/03/virtual-medicine--new-uc-telehealth-drone-makes-house-calls.html
6. www.psmarketresearch.com/market-analysis/smart-home-healthcare-market
People often overestimate what will happen in the next two years and underestimate what will happen in ten. – Bill Gates
The COVID-19 pandemic set in motion a series of innovations catalyzing the digital transformation of the health care landscape.
Telemedicine use exploded over the last 12 months to the point that it has almost become ubiquitous. With that, we saw a rapid proliferation of wearables and remote patient monitoring devices. Thanks to virtual care, care delivery is no longer strictly dependent on having onsite specialists, and care itself is not confined to the boundaries of hospitals or doctors’ offices anymore.
We saw the formation of the digital front door and the emergence of new virtual care sites like virtual urgent care, virtual home health, virtual office visits, virtual hospital at home that allowed clinical care to be delivered safely outside the boundaries of hospitals. Nonclinical public places like gyms, schools, and community centers were being transformed into virtual health care portals that brought care closer to the people.
Inside the hospital, we saw a fusion of traditional inpatient care and virtual care. Onsite hospital teams embraced telemedicine during the pandemic for various reasons; to conserve personal protective equipment (PPE), limit exposure, boost care capacity, improve access to specialists at distant sites, and bring family memberse to “webside” who cannot be at a patient’s bedside.
In clinical trials as well, virtual care is a welcome change. According to one survey1, most trial participants favored the use of telehealth services for clinical trials, as these helped them stay engaged, compliant, monitored, and on track while remaining at home. Furthermore, we are seeing the integration of artificial intelligence (AI) into telehealth, whether it is to aid physicians in clinical decision-making or to generate reminders to help patients with chronic disease management. However, this integration is only beginning to scratch the surface of the combination of two technologies’ real potential.
What’s next?
Based on these trends, it should be no surprise that digital health will become a vital sign for health care organizations.
The next 12 to 24 months will set new standards for digital health and play a significant role in defining the next generation of virtual care. There are projections that global health care industry revenues will exceed $2.6 trillion by 2025, with AI and telehealth playing a prominent role in this growth.2 According to estimates, telehealth itself will be a $175 billion market by 2026 and approximately one in three patient encounters will go virtual.3,4 Moreover, virtual care will continue to make exciting transformations, helping to make quality care accessible to everyone in innovative ways. For example, the University of Cincinnati has recently developed a pilot project using a drone equipped with video technology, artificial intelligence, sensors, and first aid kits to go to hard-to-reach areas to deliver care via telemedicine.5
Smart hospitals
In coming years, we can expect the integration of AI, augmented reality (AR), and virtual reality (VR) into telemedicine at lightning speed – and at a much larger scale – that will enable surgeons from different parts of the globe to perform procedures remotely and more precisely.

AI is already gaining traction in different fields within health care – whether it’s predicting length of stay in the ICU, or assisting in triage decisions, or reading radiological images, to name just a few. The Mayo Clinic is using AI and computer-aided decision-making tools to predict the risk of surgery and potential post-op complications, which could allow even better collaboration between medical and surgical teams. We hear about the “X-ray” vision offered to proceduralists using HoloLens – mixed reality smartglasses – a technology that enables them to perform procedures more precisely. Others project that there will be more sensors and voice recognition tools in the OR that will be used to gather data to develop intelligent algorithms, and to build a safety net for interventionalists that can notify them of potential hazards or accidental sterile field breaches. The insights gained will be used to create best practices and even allow some procedures to be performed outside the traditional OR setting.
Additionally, we are seeing the development of “smart” patient rooms. For example, one health system in Florida is working on deploying Amazon Alexa in 2,500 patient rooms to allow patients to connect more easily to their care team members. In the not-so-distant future, smart hospitals with smart patient rooms and smart ORs equipped with telemedicine, AI, AR, mixed reality, and computer-aided decision-making tools will no longer be an exception.
Smart homes for smart care
Smart homes with technologies like gas detectors, movement sensors, and sleep sensors will continue to evolve. According to one estimate, the global smart home health care market was $8.7 billion in 2019, and is expected to be $96.2 billion by 2030.6

Smart technologies will have applications in fall detection and prevention, evaluation of self-administration of medicine, sleep rhythm monitoring, air quality monitoring for the detection of abnormal gas levels, and identification of things like carbon monoxide poisoning or food spoilage. In coming years, expect to see more virtual medical homes and digital health care complexes. Patients, from the convenience of their homes, might be able to connect to a suite of caregivers, all working collaboratively to provide more coordinated, effective care. The “hospital at home” model that started with six hospitals has already grown to over 100 hospitals across 29 states. The shift from onsite specialists to onscreen specialists will continue, providing greater access to specialized services.
With these emerging trends, it can be anticipated that much acute care will be provided to patients outside the hospital – either under the hospital at home model, via drone technology using telemedicine, through smart devices in smart homes, or via wearables and artificial intelligence. Hence, hospitals’ configuration in the future will be much different and more compact than currently, and many hospitals will be reserved for trauma patients, casualties of natural disasters, higher acuity diseases requiring complex procedures, and other emergencies.
The role of hospitalists has evolved over the years and is still evolving. It should be no surprise if, in the future, we work alongside a digital hospitalist twin to provide better and more personalized care to our patients. Change is uncomfortable but it is inevitable. When COVID hit, we were forced to find innovative ways to deliver care to our patients. One thing is for certain: post-pandemic (AD, or After Disease) we are not going back to a Before COVID (BC) state in terms of virtual care. With the new dawn of digital era, the crucial questions to address will be: What will the future role of a hospitalist look like? How can we leverage technology and embrace our flexibility to adapt to these trends? How can we apply the lessons learned during the pandemic to propel hospital medicine into the future? And is it time to rethink our role and even reclassify ourselves – from hospitalists to Acute Care Experts (ACE) or Primary Acute Care Physicians?
Dr. Zia is a hospitalist, physician advisor, and founder of Virtual Hospitalist - a telemedicine company with a 360-degree care model for hospital patients.
References
1. www.subjectwell.com/news/data-shows-a-majority-of-patients-remain-interested-in-clinical-trials-during-the-coronavirus-pandemic/
2. ww2.frost.com/news/press-releases/technology-innovations-and-virtual-consultations-drive-healthcare-2025/
3. www.gminsights.com/industry-analysis/telemedicine-market
4. www.healthcareitnews.com/blog/frost-sullivans-top-10-predictions-healthcare-2021
5. www.uc.edu/news/articles/2021/03/virtual-medicine--new-uc-telehealth-drone-makes-house-calls.html
6. www.psmarketresearch.com/market-analysis/smart-home-healthcare-market
Bullying in academic medicine rife, underreported
Bullying in academic medicine, especially among women, is rife, underreported, and remains largely unaddressed, new research suggests.
Investigators reviewed close to 70 studies, encompassing over 82,000 medical consultants or trainees in academic medical settings, and found that men were identified as the most common perpetrators – close to 70% of respondents – whereas women were the most common victims (56%).
Collectively, respondents in all of the studies identified the most common bullies to be consultants (54%), followed by residents (22%), and nurses (15%).
Disturbingly, less than one-third of victims overall reported that they were bullied, and close to 60% who formally reported the abuse said they did not have a positive outcome.
“We found that bullies are commonly men and senior consultants, while more than half of their victims are women,” senior author Harriette G.C. Van Spall, MD, MPH, associate professor of medicine and director of e-health and virtual care, Division of Cardiology, McMaster University, Hamilton, Ont., said in an interview.
“The greatest barriers to addressing academic bullying are the fear of reprisal, lack of impact of reporting, and non-enforcement of anti-bullying policies,” she added.
The study was published online July 12 in BMJ Open.
Personal experience
“Some behaviors were excruciating to deal with, protesting against them would bring more on, and every day was filled with dread. It took sheer will to show up at work to care for patients, to complete research I was leading, and to have hope, and my academic output, income, and personal well-being dropped during those years,” she added.
Dr. Van Spall thought the subject “merited research because our performance as clinicians, researchers, and educators relies on our work environment.”
To investigate, the researchers reviewed 68 studies (n = 82,349 respondents) conducted between 1999 and 2021 in academic medical settings, in which victims were either consultants or trainees. Many of the studies (31) were conducted in the U.S.
Other countries included the United Kingdom, Canada, Australia, Pakistan, Egypt, Iran, Turkey, New Zealand, Lithuania, Greece, India, Germany, Nigeria, Oman, and Finland.
Studies were required to describe the method and impact of bullying; characteristics of the perpetrators and victims; or interventions that were used to address the bullying.
“Bullying” was defined as “the abuse of authority by a perpetrator who targets the victim in an academic setting through punishing behaviors that include overwork, destabilization, and isolation in order to impede the education or career of the target.”
Systemic sexism
Bullying behaviors, reported in 28 studies (n = 35,779 respondents), were grouped into destabilization, threats to professional status, overwork, and isolation, with overwork found to be the most common form of bullying.
The most common impact of being bullied was psychological distress, reported by 39.1% of respondents in 14 studies, followed by considerations of quitting (35.9%; 7 studies), and worsening of clinical performance (34.6%, 8 studies).
“Among demographic groups, men were identified as the most common perpetrators (67.2% of 4,722 respondents in 5 studies) and women the most common victims (56.2% of 15,246 respondents in 27 studies),” the authors report.
“Academic medicine in many institutions is encumbered by systemic sexism that is evident in processes around remuneration, recognition, opportunities for advancement, and leadership positions,” said Dr. Van Spall.
“There are fewer women at decision-making tables in academic medicine, the climb is uphill at the best of times, and women are likely easier targets for bullies, as their voices are easier to drown out,” she added.
She noted that many men do “exhibit wonderful attributes of professionalism and decency,” but “some in positions of power are given impunity by virtue of other accomplishments.”
Multiple deterrents
Thirty-one studies (n = 15,868) described characteristics of the bullies and showed the most common to be consultants (53.6% [30 studies]), residents (22% [22 studies]), and nurses (14.9% [21 studies]).
Only a minority of victims (28.9% of 9,410 victims [10 studies]) formally reported the bullying. The researchers identified multiple deterrents to reporting.
When a formal complaint was submitted (n = 1,139 respondents), it most frequently had no perceived effect (35.6%); more than one-fifth (21.9%) experienced worsening of the bullying, and only 13.7% reported improvement.
The common institutional facilitators of bullying, described in 25 studies, included lack of enforcement of anti-bullying policies (13 studies), the hierarchical structure of medicine (7 studies), and normalization of bullying (10 studies).
Forty-nine studies looked at strategies to address academic bullying, including anti-bullying policies, mandatory workshops on mistreatment, establishing an anti-bullying oversight committee, and institutional support for victims. However, the studies testing the effectiveness of these interventions “had a high risk of bias.”
Support available
Commenting on the research for this news organization, Roberta Gebhard, DO, past president of the American Medical Women’s Association (AMWA) and a member of the advisory board for Physician Just Equity, called it a “good study, large, international, and well-written.”
Dr. Gebhard, a member of the Governing Council for the American Medical Association Women Physician Section, was not associated with this study but said she is currently researching women who left medical school and residency.
“A common reason for leaving is being bullied. Bullying is often not reported and if reported, often not addressed. Or, if addressed, the person who reports it is often retaliated against, which is a common experience, especially in women.”
She advised female physicians who are bullied to get support from other female physicians – for example, by joining the AMWA, which has an online women’s leadership group.
“Having other women physicians throughout the country you can call for advice and support can be helpful,” said Dr. Gebhard, a family practice physician based in Grand Island, New York.
Dr. Van Spall receives support from the Canadian Institutes of Health Research, the Heart and Stroke Foundation, the Women As One Escalator Award, and McMaster Department of Medicine. The study authors and Dr. Gebhard have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bullying in academic medicine, especially among women, is rife, underreported, and remains largely unaddressed, new research suggests.
Investigators reviewed close to 70 studies, encompassing over 82,000 medical consultants or trainees in academic medical settings, and found that men were identified as the most common perpetrators – close to 70% of respondents – whereas women were the most common victims (56%).
Collectively, respondents in all of the studies identified the most common bullies to be consultants (54%), followed by residents (22%), and nurses (15%).
Disturbingly, less than one-third of victims overall reported that they were bullied, and close to 60% who formally reported the abuse said they did not have a positive outcome.
“We found that bullies are commonly men and senior consultants, while more than half of their victims are women,” senior author Harriette G.C. Van Spall, MD, MPH, associate professor of medicine and director of e-health and virtual care, Division of Cardiology, McMaster University, Hamilton, Ont., said in an interview.
“The greatest barriers to addressing academic bullying are the fear of reprisal, lack of impact of reporting, and non-enforcement of anti-bullying policies,” she added.
The study was published online July 12 in BMJ Open.
Personal experience
“Some behaviors were excruciating to deal with, protesting against them would bring more on, and every day was filled with dread. It took sheer will to show up at work to care for patients, to complete research I was leading, and to have hope, and my academic output, income, and personal well-being dropped during those years,” she added.
Dr. Van Spall thought the subject “merited research because our performance as clinicians, researchers, and educators relies on our work environment.”
To investigate, the researchers reviewed 68 studies (n = 82,349 respondents) conducted between 1999 and 2021 in academic medical settings, in which victims were either consultants or trainees. Many of the studies (31) were conducted in the U.S.
Other countries included the United Kingdom, Canada, Australia, Pakistan, Egypt, Iran, Turkey, New Zealand, Lithuania, Greece, India, Germany, Nigeria, Oman, and Finland.
Studies were required to describe the method and impact of bullying; characteristics of the perpetrators and victims; or interventions that were used to address the bullying.
“Bullying” was defined as “the abuse of authority by a perpetrator who targets the victim in an academic setting through punishing behaviors that include overwork, destabilization, and isolation in order to impede the education or career of the target.”
Systemic sexism
Bullying behaviors, reported in 28 studies (n = 35,779 respondents), were grouped into destabilization, threats to professional status, overwork, and isolation, with overwork found to be the most common form of bullying.
The most common impact of being bullied was psychological distress, reported by 39.1% of respondents in 14 studies, followed by considerations of quitting (35.9%; 7 studies), and worsening of clinical performance (34.6%, 8 studies).
“Among demographic groups, men were identified as the most common perpetrators (67.2% of 4,722 respondents in 5 studies) and women the most common victims (56.2% of 15,246 respondents in 27 studies),” the authors report.
“Academic medicine in many institutions is encumbered by systemic sexism that is evident in processes around remuneration, recognition, opportunities for advancement, and leadership positions,” said Dr. Van Spall.
“There are fewer women at decision-making tables in academic medicine, the climb is uphill at the best of times, and women are likely easier targets for bullies, as their voices are easier to drown out,” she added.
She noted that many men do “exhibit wonderful attributes of professionalism and decency,” but “some in positions of power are given impunity by virtue of other accomplishments.”
Multiple deterrents
Thirty-one studies (n = 15,868) described characteristics of the bullies and showed the most common to be consultants (53.6% [30 studies]), residents (22% [22 studies]), and nurses (14.9% [21 studies]).
Only a minority of victims (28.9% of 9,410 victims [10 studies]) formally reported the bullying. The researchers identified multiple deterrents to reporting.
When a formal complaint was submitted (n = 1,139 respondents), it most frequently had no perceived effect (35.6%); more than one-fifth (21.9%) experienced worsening of the bullying, and only 13.7% reported improvement.
The common institutional facilitators of bullying, described in 25 studies, included lack of enforcement of anti-bullying policies (13 studies), the hierarchical structure of medicine (7 studies), and normalization of bullying (10 studies).
Forty-nine studies looked at strategies to address academic bullying, including anti-bullying policies, mandatory workshops on mistreatment, establishing an anti-bullying oversight committee, and institutional support for victims. However, the studies testing the effectiveness of these interventions “had a high risk of bias.”
Support available
Commenting on the research for this news organization, Roberta Gebhard, DO, past president of the American Medical Women’s Association (AMWA) and a member of the advisory board for Physician Just Equity, called it a “good study, large, international, and well-written.”
Dr. Gebhard, a member of the Governing Council for the American Medical Association Women Physician Section, was not associated with this study but said she is currently researching women who left medical school and residency.
“A common reason for leaving is being bullied. Bullying is often not reported and if reported, often not addressed. Or, if addressed, the person who reports it is often retaliated against, which is a common experience, especially in women.”
She advised female physicians who are bullied to get support from other female physicians – for example, by joining the AMWA, which has an online women’s leadership group.
“Having other women physicians throughout the country you can call for advice and support can be helpful,” said Dr. Gebhard, a family practice physician based in Grand Island, New York.
Dr. Van Spall receives support from the Canadian Institutes of Health Research, the Heart and Stroke Foundation, the Women As One Escalator Award, and McMaster Department of Medicine. The study authors and Dr. Gebhard have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bullying in academic medicine, especially among women, is rife, underreported, and remains largely unaddressed, new research suggests.
Investigators reviewed close to 70 studies, encompassing over 82,000 medical consultants or trainees in academic medical settings, and found that men were identified as the most common perpetrators – close to 70% of respondents – whereas women were the most common victims (56%).
Collectively, respondents in all of the studies identified the most common bullies to be consultants (54%), followed by residents (22%), and nurses (15%).
Disturbingly, less than one-third of victims overall reported that they were bullied, and close to 60% who formally reported the abuse said they did not have a positive outcome.
“We found that bullies are commonly men and senior consultants, while more than half of their victims are women,” senior author Harriette G.C. Van Spall, MD, MPH, associate professor of medicine and director of e-health and virtual care, Division of Cardiology, McMaster University, Hamilton, Ont., said in an interview.
“The greatest barriers to addressing academic bullying are the fear of reprisal, lack of impact of reporting, and non-enforcement of anti-bullying policies,” she added.
The study was published online July 12 in BMJ Open.
Personal experience
“Some behaviors were excruciating to deal with, protesting against them would bring more on, and every day was filled with dread. It took sheer will to show up at work to care for patients, to complete research I was leading, and to have hope, and my academic output, income, and personal well-being dropped during those years,” she added.
Dr. Van Spall thought the subject “merited research because our performance as clinicians, researchers, and educators relies on our work environment.”
To investigate, the researchers reviewed 68 studies (n = 82,349 respondents) conducted between 1999 and 2021 in academic medical settings, in which victims were either consultants or trainees. Many of the studies (31) were conducted in the U.S.
Other countries included the United Kingdom, Canada, Australia, Pakistan, Egypt, Iran, Turkey, New Zealand, Lithuania, Greece, India, Germany, Nigeria, Oman, and Finland.
Studies were required to describe the method and impact of bullying; characteristics of the perpetrators and victims; or interventions that were used to address the bullying.
“Bullying” was defined as “the abuse of authority by a perpetrator who targets the victim in an academic setting through punishing behaviors that include overwork, destabilization, and isolation in order to impede the education or career of the target.”
Systemic sexism
Bullying behaviors, reported in 28 studies (n = 35,779 respondents), were grouped into destabilization, threats to professional status, overwork, and isolation, with overwork found to be the most common form of bullying.
The most common impact of being bullied was psychological distress, reported by 39.1% of respondents in 14 studies, followed by considerations of quitting (35.9%; 7 studies), and worsening of clinical performance (34.6%, 8 studies).
“Among demographic groups, men were identified as the most common perpetrators (67.2% of 4,722 respondents in 5 studies) and women the most common victims (56.2% of 15,246 respondents in 27 studies),” the authors report.
“Academic medicine in many institutions is encumbered by systemic sexism that is evident in processes around remuneration, recognition, opportunities for advancement, and leadership positions,” said Dr. Van Spall.
“There are fewer women at decision-making tables in academic medicine, the climb is uphill at the best of times, and women are likely easier targets for bullies, as their voices are easier to drown out,” she added.
She noted that many men do “exhibit wonderful attributes of professionalism and decency,” but “some in positions of power are given impunity by virtue of other accomplishments.”
Multiple deterrents
Thirty-one studies (n = 15,868) described characteristics of the bullies and showed the most common to be consultants (53.6% [30 studies]), residents (22% [22 studies]), and nurses (14.9% [21 studies]).
Only a minority of victims (28.9% of 9,410 victims [10 studies]) formally reported the bullying. The researchers identified multiple deterrents to reporting.
When a formal complaint was submitted (n = 1,139 respondents), it most frequently had no perceived effect (35.6%); more than one-fifth (21.9%) experienced worsening of the bullying, and only 13.7% reported improvement.
The common institutional facilitators of bullying, described in 25 studies, included lack of enforcement of anti-bullying policies (13 studies), the hierarchical structure of medicine (7 studies), and normalization of bullying (10 studies).
Forty-nine studies looked at strategies to address academic bullying, including anti-bullying policies, mandatory workshops on mistreatment, establishing an anti-bullying oversight committee, and institutional support for victims. However, the studies testing the effectiveness of these interventions “had a high risk of bias.”
Support available
Commenting on the research for this news organization, Roberta Gebhard, DO, past president of the American Medical Women’s Association (AMWA) and a member of the advisory board for Physician Just Equity, called it a “good study, large, international, and well-written.”
Dr. Gebhard, a member of the Governing Council for the American Medical Association Women Physician Section, was not associated with this study but said she is currently researching women who left medical school and residency.
“A common reason for leaving is being bullied. Bullying is often not reported and if reported, often not addressed. Or, if addressed, the person who reports it is often retaliated against, which is a common experience, especially in women.”
She advised female physicians who are bullied to get support from other female physicians – for example, by joining the AMWA, which has an online women’s leadership group.
“Having other women physicians throughout the country you can call for advice and support can be helpful,” said Dr. Gebhard, a family practice physician based in Grand Island, New York.
Dr. Van Spall receives support from the Canadian Institutes of Health Research, the Heart and Stroke Foundation, the Women As One Escalator Award, and McMaster Department of Medicine. The study authors and Dr. Gebhard have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Record number of U.S. drug overdoses in 2020
More Americans died from drug overdoses in 2020 than in any other year, the CDC said July 14.
, according to the provisional data the National Center for Health Statistics reported.
The spikes are largely attributed to the rise in use of fentanyl and other synthetic opioids.
The Washington Post reported that more than 69,000 overdose deaths involved opioids, up from 50,963 in 2019.
Amid the crush of overdoses, the White House announced that President Joe Biden has nominated Rahul Gupta, MD, to lead the White House Office of National Drug Control Policy.
Dr. Gupta is a former health commissioner of West Virginia, and is chief medical and health officer for the March of Dimes.
“Dr. Gupta led efforts in West Virginia to address the opioid crisis, gaining national prominence as a leader in tackling this issue,” March of Dimes President and CEO Stacey Stewart said in a statement. “At March of Dimes, he has advocated for policies and programs to prevent and treat substance use, with a focus on the safety and care of pregnant women and infants.”
Healthday contributed to this report. A version of this article first appeared on WebMD.com.
More Americans died from drug overdoses in 2020 than in any other year, the CDC said July 14.
, according to the provisional data the National Center for Health Statistics reported.
The spikes are largely attributed to the rise in use of fentanyl and other synthetic opioids.
The Washington Post reported that more than 69,000 overdose deaths involved opioids, up from 50,963 in 2019.
Amid the crush of overdoses, the White House announced that President Joe Biden has nominated Rahul Gupta, MD, to lead the White House Office of National Drug Control Policy.
Dr. Gupta is a former health commissioner of West Virginia, and is chief medical and health officer for the March of Dimes.
“Dr. Gupta led efforts in West Virginia to address the opioid crisis, gaining national prominence as a leader in tackling this issue,” March of Dimes President and CEO Stacey Stewart said in a statement. “At March of Dimes, he has advocated for policies and programs to prevent and treat substance use, with a focus on the safety and care of pregnant women and infants.”
Healthday contributed to this report. A version of this article first appeared on WebMD.com.
More Americans died from drug overdoses in 2020 than in any other year, the CDC said July 14.
, according to the provisional data the National Center for Health Statistics reported.
The spikes are largely attributed to the rise in use of fentanyl and other synthetic opioids.
The Washington Post reported that more than 69,000 overdose deaths involved opioids, up from 50,963 in 2019.
Amid the crush of overdoses, the White House announced that President Joe Biden has nominated Rahul Gupta, MD, to lead the White House Office of National Drug Control Policy.
Dr. Gupta is a former health commissioner of West Virginia, and is chief medical and health officer for the March of Dimes.
“Dr. Gupta led efforts in West Virginia to address the opioid crisis, gaining national prominence as a leader in tackling this issue,” March of Dimes President and CEO Stacey Stewart said in a statement. “At March of Dimes, he has advocated for policies and programs to prevent and treat substance use, with a focus on the safety and care of pregnant women and infants.”
Healthday contributed to this report. A version of this article first appeared on WebMD.com.
State-of-the-art psych unit designed with recovery in mind
Calming wall colors, nature-themed murals, and soft nighttime lighting are all part of a unique new state-of-the-art inpatient psychiatric unit that focuses especially on children and adolescents who have experienced significant trauma.
The 16-bed unit, which has been in the works for 3½ years and opened June 30 at the University of Maryland Medical Center (UMMC), in Baltimore, Maryland, treats youth aged 5 to 17 years. It has separate wings for younger children and for adolescents.
“We offer a really warm and welcoming environment that we think is going to promote health and healing,” the unit’s head, Sarah Edwards, DO, director of child and adolescent psychiatry at UMMC and assistant professor of psychiatry, University of Maryland School of Medicine (UMSOM), Baltimore, said in an interview.
Previous research shows that 1 in 4 children experience some kind of maltreatment, whether physical, sexual, or emotional, and that 1 in 5 develop a diagnosable mental health disorder.
, Dr. Edwards noted. Recent data show that the rate of suicidal ideation among youth has increased significantly during the COVID-19 crisis.
“Urban children have unfortunately suffered a lot of what we call traumatic stress, so they might be victims of physical or sexual abuse but also face layers of stressful situations – for example, living in unsafe neighborhoods and attending schools that might not be so welcoming and safe,” said Dr. Edwards.
Safety first
Typical conditions treated at the new unit will include depression, anxiety, attention-deficit/hyperactivity disorder, psychotic spectrum, as well as trauma disorders.
Some of these young patients have been through the foster care system and show signs of trauma and poor attachment, Dr. Edwards noted. As a result, they may have difficulty regulating their thoughts and emotions and at times exhibit dangerous behavior.
The new unit is designed both architecturally and clinically to deliver “trauma-informed” care. This type of approach “recognizes the pervasive nature of trauma” and promotes settings that facilitate recovery, Dr. Edwards added.
The idea is to treat individuals “in a way that doesn’t re-traumatize them or make their condition worse,” she added.
Safety is of the utmost importance in the unit, Jill RachBeisel, MD, chief of psychiatry at UMMC and professor and chair in the department of psychiatry at UMSOM, said in an interview.
“Health care workers must recognize and respond to the effects of trauma – and one very important way is to provide care in settings that emphasize physical and emotional safety, which helps instill a sense of control and empowerment,” Dr. RachBeisel said.
Providing youth with options is an important way to provide that sense of control, Dr. Edwards added. For example, residents can choose their own music in their bedroom, such as sounds of nature, running water, or birds chirping. They can also draw or write personal notes on a large whiteboard in their unit.
Circadian-rhythm lighting
Other unique elements of the new unit include walls painted soothing shades and murals of natural scenery, created by a local artist.
These murals perfectly capture “the kind of overall spirit of what we were trying to induce,” said Dr. Edwards.
A part of the unit dubbed the “front porch” has a large mural depicting “a landscape of beautiful trees and water and animals,” she noted. Kids can gather here to relax or just hang out.
The lighting at the unit mirrors circadian rhythms. It’s brighter during the day to promote wakefulness and participation in activities and gradually dims toward the evening hours to help induce restful nighttime sleep.
Safe and empowering and adopt productive behaviors and coping skills, Dr. Edwards noted.
The staff for the interprofessional unit includes psychiatrists, psychologists, psychiatric nurses, occupational therapists, and others trained in pediatric care.
Advice for other centers
“Our new unit is designed to provide the highest standard in mental health care and incorporates a high-tech approach to create a calming, soothing, and engaging setting,” said Dr. RachBeisel.
School-transition specialists help connect discharged patients and their families to vital services and peer support. These services represent “an essential component of the continuum of care” for youth experiencing mental distress, she added.
Other organizations considering establishing a similar type of psychiatric unit should consult all stakeholders.
“We had staff, no matter what their role, be part of every step of this process, including helping with the design, picking out furniture they thought would make the most sense, and helping choose the artwork,” she said.
It is also important to incorporate feedback from youth themselves, Dr. Edwards added.
A version of this article first appeared on Medscape.com.
Calming wall colors, nature-themed murals, and soft nighttime lighting are all part of a unique new state-of-the-art inpatient psychiatric unit that focuses especially on children and adolescents who have experienced significant trauma.
The 16-bed unit, which has been in the works for 3½ years and opened June 30 at the University of Maryland Medical Center (UMMC), in Baltimore, Maryland, treats youth aged 5 to 17 years. It has separate wings for younger children and for adolescents.
“We offer a really warm and welcoming environment that we think is going to promote health and healing,” the unit’s head, Sarah Edwards, DO, director of child and adolescent psychiatry at UMMC and assistant professor of psychiatry, University of Maryland School of Medicine (UMSOM), Baltimore, said in an interview.
Previous research shows that 1 in 4 children experience some kind of maltreatment, whether physical, sexual, or emotional, and that 1 in 5 develop a diagnosable mental health disorder.
, Dr. Edwards noted. Recent data show that the rate of suicidal ideation among youth has increased significantly during the COVID-19 crisis.
“Urban children have unfortunately suffered a lot of what we call traumatic stress, so they might be victims of physical or sexual abuse but also face layers of stressful situations – for example, living in unsafe neighborhoods and attending schools that might not be so welcoming and safe,” said Dr. Edwards.
Safety first
Typical conditions treated at the new unit will include depression, anxiety, attention-deficit/hyperactivity disorder, psychotic spectrum, as well as trauma disorders.
Some of these young patients have been through the foster care system and show signs of trauma and poor attachment, Dr. Edwards noted. As a result, they may have difficulty regulating their thoughts and emotions and at times exhibit dangerous behavior.
The new unit is designed both architecturally and clinically to deliver “trauma-informed” care. This type of approach “recognizes the pervasive nature of trauma” and promotes settings that facilitate recovery, Dr. Edwards added.
The idea is to treat individuals “in a way that doesn’t re-traumatize them or make their condition worse,” she added.
Safety is of the utmost importance in the unit, Jill RachBeisel, MD, chief of psychiatry at UMMC and professor and chair in the department of psychiatry at UMSOM, said in an interview.
“Health care workers must recognize and respond to the effects of trauma – and one very important way is to provide care in settings that emphasize physical and emotional safety, which helps instill a sense of control and empowerment,” Dr. RachBeisel said.
Providing youth with options is an important way to provide that sense of control, Dr. Edwards added. For example, residents can choose their own music in their bedroom, such as sounds of nature, running water, or birds chirping. They can also draw or write personal notes on a large whiteboard in their unit.
Circadian-rhythm lighting
Other unique elements of the new unit include walls painted soothing shades and murals of natural scenery, created by a local artist.
These murals perfectly capture “the kind of overall spirit of what we were trying to induce,” said Dr. Edwards.
A part of the unit dubbed the “front porch” has a large mural depicting “a landscape of beautiful trees and water and animals,” she noted. Kids can gather here to relax or just hang out.
The lighting at the unit mirrors circadian rhythms. It’s brighter during the day to promote wakefulness and participation in activities and gradually dims toward the evening hours to help induce restful nighttime sleep.
Safe and empowering and adopt productive behaviors and coping skills, Dr. Edwards noted.
The staff for the interprofessional unit includes psychiatrists, psychologists, psychiatric nurses, occupational therapists, and others trained in pediatric care.
Advice for other centers
“Our new unit is designed to provide the highest standard in mental health care and incorporates a high-tech approach to create a calming, soothing, and engaging setting,” said Dr. RachBeisel.
School-transition specialists help connect discharged patients and their families to vital services and peer support. These services represent “an essential component of the continuum of care” for youth experiencing mental distress, she added.
Other organizations considering establishing a similar type of psychiatric unit should consult all stakeholders.
“We had staff, no matter what their role, be part of every step of this process, including helping with the design, picking out furniture they thought would make the most sense, and helping choose the artwork,” she said.
It is also important to incorporate feedback from youth themselves, Dr. Edwards added.
A version of this article first appeared on Medscape.com.
Calming wall colors, nature-themed murals, and soft nighttime lighting are all part of a unique new state-of-the-art inpatient psychiatric unit that focuses especially on children and adolescents who have experienced significant trauma.
The 16-bed unit, which has been in the works for 3½ years and opened June 30 at the University of Maryland Medical Center (UMMC), in Baltimore, Maryland, treats youth aged 5 to 17 years. It has separate wings for younger children and for adolescents.
“We offer a really warm and welcoming environment that we think is going to promote health and healing,” the unit’s head, Sarah Edwards, DO, director of child and adolescent psychiatry at UMMC and assistant professor of psychiatry, University of Maryland School of Medicine (UMSOM), Baltimore, said in an interview.
Previous research shows that 1 in 4 children experience some kind of maltreatment, whether physical, sexual, or emotional, and that 1 in 5 develop a diagnosable mental health disorder.
, Dr. Edwards noted. Recent data show that the rate of suicidal ideation among youth has increased significantly during the COVID-19 crisis.
“Urban children have unfortunately suffered a lot of what we call traumatic stress, so they might be victims of physical or sexual abuse but also face layers of stressful situations – for example, living in unsafe neighborhoods and attending schools that might not be so welcoming and safe,” said Dr. Edwards.
Safety first
Typical conditions treated at the new unit will include depression, anxiety, attention-deficit/hyperactivity disorder, psychotic spectrum, as well as trauma disorders.
Some of these young patients have been through the foster care system and show signs of trauma and poor attachment, Dr. Edwards noted. As a result, they may have difficulty regulating their thoughts and emotions and at times exhibit dangerous behavior.
The new unit is designed both architecturally and clinically to deliver “trauma-informed” care. This type of approach “recognizes the pervasive nature of trauma” and promotes settings that facilitate recovery, Dr. Edwards added.
The idea is to treat individuals “in a way that doesn’t re-traumatize them or make their condition worse,” she added.
Safety is of the utmost importance in the unit, Jill RachBeisel, MD, chief of psychiatry at UMMC and professor and chair in the department of psychiatry at UMSOM, said in an interview.
“Health care workers must recognize and respond to the effects of trauma – and one very important way is to provide care in settings that emphasize physical and emotional safety, which helps instill a sense of control and empowerment,” Dr. RachBeisel said.
Providing youth with options is an important way to provide that sense of control, Dr. Edwards added. For example, residents can choose their own music in their bedroom, such as sounds of nature, running water, or birds chirping. They can also draw or write personal notes on a large whiteboard in their unit.
Circadian-rhythm lighting
Other unique elements of the new unit include walls painted soothing shades and murals of natural scenery, created by a local artist.
These murals perfectly capture “the kind of overall spirit of what we were trying to induce,” said Dr. Edwards.
A part of the unit dubbed the “front porch” has a large mural depicting “a landscape of beautiful trees and water and animals,” she noted. Kids can gather here to relax or just hang out.
The lighting at the unit mirrors circadian rhythms. It’s brighter during the day to promote wakefulness and participation in activities and gradually dims toward the evening hours to help induce restful nighttime sleep.
Safe and empowering and adopt productive behaviors and coping skills, Dr. Edwards noted.
The staff for the interprofessional unit includes psychiatrists, psychologists, psychiatric nurses, occupational therapists, and others trained in pediatric care.
Advice for other centers
“Our new unit is designed to provide the highest standard in mental health care and incorporates a high-tech approach to create a calming, soothing, and engaging setting,” said Dr. RachBeisel.
School-transition specialists help connect discharged patients and their families to vital services and peer support. These services represent “an essential component of the continuum of care” for youth experiencing mental distress, she added.
Other organizations considering establishing a similar type of psychiatric unit should consult all stakeholders.
“We had staff, no matter what their role, be part of every step of this process, including helping with the design, picking out furniture they thought would make the most sense, and helping choose the artwork,” she said.
It is also important to incorporate feedback from youth themselves, Dr. Edwards added.
A version of this article first appeared on Medscape.com.
“Enough English” to be at risk
A hectic Friday morning at the hospital seemed less stressful amid morning greetings and humor from colleagues. In a team room full of hospitalists, life and death are often discussed in detail, ranging from medical discussions to joys and frustrations of the day to philosophy, politics, and more. It is almost impossible to miss something interesting.
People breaking into their native languages over the phone call from home always make me smile. The mention of a “complicated Indian patient unable to use interpreter” caught my attention.
My friend and colleague asked if I would be willing to take over the patient since I could speak Hindi. I was doubtful if I would add anything to make a meaningful difference, given the patient wasn’t even participating in a conversation. However, my colleague’s concern for the patient and faith in me was enough to say, “Sure, let me add her to my list.”
At the bedside, it felt like a classic “acute on chronic” hot mess situation. The patient presented with a generalized rash, anasarca, renal failure, multifocal pneumonia, and delirium. All I could gather from the patient were some incomprehensible words that sounded like Hindi. I called the family to obtain some history and to provide updates. Her son was excited to hear from me, and it didn’t take him long to guess that I was from India. But that could still mean that I might speak any of the twenty-two or more Indian languages.
Answering my questions one by one in perfectly understandable English, he was short and sweet. Suspicious of missing out on details, I offered hesitantly, “You could speak in Hindi with me.” Then came a flood of information with the details, concerns, questions, and what was lost in the translation.
We all attend to patients and families with limited English proficiency (LEP), immigrants, and nonimmigrants. LEP is a term used to describe individuals who do not speak English as their primary language and have a limited ability to read, speak, write, or understand English.1 Recent data from the American Community Survey (2005-2009) reports that 8.6% of the population (24 million Americans) have LEP.2 It’s a large and growing population that needs help overcoming language barriers and the appropriate use of professional medical interpreter services – a backbone to safe, quality, and cost-effective patient care.
The following day at bedside rounds, the nurse reported that the patient was looking and responding better. She could cooperate with interpreter services and could speak “some English.” Over the years, one thing that sounds more alarming than “no English” is “some English” or “enough English.” Around noon I received a page that the patient was refusing intravenous Lasix. At the bedside, however, the patient seemed unaware of the perceived refusal. Further discussions with the nurse lead to a familiar culprit, a relatively common gesture in South Asian cultures, a head bobble or shake.
The nurse reported that the patient shook her head side to side, seemed upset, and said “NO” when trying to administer the medication. On the other hand, the patient reported that she was upset to be at the hospital but had “NO” problem with the medicine.
My patient’s “some English” was indeed “enough English” to put her at risk due to medical error, which is highly likely when patients or providers can speak or understand a language to “get by” or to “make do.” Like my patient, the LEP patient population is more likely to experience medical errors, longer hospital stays, hospital-acquired complications, surgical delays, and readmissions. They are also less likely to receive preventive care, have access to regular care, or be satisfied with their care. They are much more likely to have adverse effects from drug complications, poor understanding of diagnoses, a greater risk of being misunderstood by their physicians or ancillary staff, and less likely to follow physician instructions.3-5 One study analyzed over 1,000 adverse-incident reports from six Joint Commission-accredited hospitals for LEP and English-speaking patients and found that 49% of LEP patients experienced physical harm versus 29.5% of English-speaking patients.6
I updated the patient’s LEP status that was missing in the chart, likely due to altered mental status at the time of admission. Reliable language and English proficiency data are usually entered at the patient’s point of entry with documentation of the language services required during the patient-provider encounter. The U.S. Census Bureau’s operational definition for LEP is a patient’s self-assessed ability to speak English less than “very well,” but how well it correlates with a patient’s actual English ability needs more study. Also, one’s self-assessed perception of ability might vary day to day, and language ability, by itself, is not static; it can differ from moment to moment and situation to situation. It may be easier to understand words in English when the situation is simple and less stressful than when things are complicated and stressful.
With a definition of LEP rather vague and the term somewhat derogatory, its meaning is open to interpretation. One study found that though speaking English less than “very well” was the most sensitive measure for identifying all of the patients who reported that they were unable to communicate effectively with their physicians, it was also the least specific.7 This lower specificity could lead to misclassification of some patients as LEP who are, in fact, able to effectively communicate in English with their physicians. This type of misclassification might lead to costly language assistance and carry the potential to cause conflicts between patient and provider. Telling a patient or family that they may have a “limited English proficiency” when they have believed otherwise and feel confident about their skills may come as a challenge. Some patients may also pretend to understand English to avoid being embarrassed about their linguistic abilities or perceive that they might be judged on their abilities in general.
Exiting the room, I gently reminded the RN to use the interpreter services. “Who has never been guilty of using an ad hoc interpreter or rushing through a long interpreter phone call due to time constraints?” I thought. A study from 2011 found that 43% of hospitalized patients with LEP had communicated without an interpreter present during admission, and 40% had communicated without an interpreter present after admission.8 In other words, a system in place does not mean service in use. But, the use of a trained interpreter is not only an obligation for care providers but a right for patients as per legal requirements of Title VI of the Civil Rights Act and the Standards for Culturally and Linguistically Appropriate Services (CLAS) by the Department of Health and Human Services’ (HSS) Office of Minority Health.9 In January 2010, The Joint Commission released a set of new and revised standards for patient-centered communication as part of an initiative to advance effective communication, cultural competence, and patient- and family-centered care.
Despite the requirements and availability of qualified medical interpreter services, there are multiple perceived and experienced barriers to the use of interpreter services. The most common one is that what comes as a free service for patients is a time commitment for providers. A long list of patients, acuity of the situation, and ease of use/availability of translation aids can change the calculus. One may be able to bill a prolonged service code (99354-99357) in addition to the appropriate E/M code, although a patient cannot be billed for the actual service provided by the interpreter. Longstanding CMS policy also permits reimbursement for translation/interpretation activities, so long as they are not included and paid for as part of the rate for direct service.10
The patient, however, insisted that she would rather have her son as the interpreter on the 3-way over the phone (OPI) conference call for interpretation. “He speaks good English and knows my medical history well,” she said. I counseled the patient on the benefits of using interpreter services and explained how to use the call button light and the visual aids.
Placing emphasis on educating patients about the benefits of using, and risks of not using, interpreter services is as essential as emphasizing that care providers use the services. Some patients may voluntarily choose to provide their own interpreter. Use of family members, friends, or unqualified staff as interpreters is one of the most commonly reported causes of errors by frontline staff. Using in-language collateral may help these patients understand how medical interpretation may create a better patient experience and outcome. A short factsheet, in different languages, on qualified interpreters’ expected benefits: meaning-for-meaning communication, impartiality, medical privacy, and improved patient safety and satisfaction, can also come in handy.
However, if the patient still refuses, providers should document the refusal of the offer of free language services, the name of the interpreter designated by the patient, the interpreter’s relationship to the LEP person, and the time or portions of the patient encounter that the interpreter’s services were used. Yet, language interpretation alone can be inadequate without document translation. According to one study, despite the availability of on-site professional interpreter services, hospitalized patients who do not speak English are less likely to have signed consent forms in their medical records.11 Health care professionals, therefore, need well-translated documents to treat LEP patients. Translated documents of consent forms for medical procedures, post-discharge instructions, prescription and medical device labels, and drug usage information may enhance informed decision making, safety and reduce stress and medical errors.
An unpopular and underused service needs it all: availability, convenience, monitoring, reporting, and team effort. Due to the sheer unpopularity and underuse of interpreter services, institutions should enhance ease of availability, monitor the use and quality of interpreter services, and optimize reporting of language-related errors. Ease of availability goes hand in hand with tapping local resources. Over the years, and even more so during the pandemic, in-person interpretation has transitioned to telephonic or video interpretation due to availability, safety, and cost issues. There are challenges in translating a language, and the absence of a visual channel adds another layer of complexity.
The current body of evidence does not indicate a superior interpreting method. Still, in one study providers and interpreters exposed to all three methods were more critical of remote methods and preferred videoconferencing to the telephone as a remote method. The significantly shorter phone interviews raised questions about the prospects of miscommunication in telephonic interpretation, given the absence of a visual channel.12
One way to bypass language barriers is to recognize the value added by hiring and training bilingual health care providers and fostering cultural competence. International medical graduates in many parts of the country aid in closing language barriers. Language-concordant care enhances trust between patients and physicians, optimizes health outcomes, and advances health equity for diverse populations.13-15 The presence of bilingual providers means more effective and timelier communication and improved patient satisfaction. But, according to a Doximity study, there is a significant “language gap” between those languages spoken by physicians and their patients.16 Hospitals, therefore, should assess, qualify, and incentivize staff who can serve as on-site medical interpreters for patients as a means to facilitate language concordant care for LEP patients.
The Agency of Healthcare Research and Quality (AHRQ) also has a guide on how hospitals can better identify, report, monitor, and prevent medical errors in patients with LEP. Included is the TeamSTEPPS LEP module to help develop and deploy a customized plan to train staff in teamwork skills and lead a medical teamwork improvement initiative.17
“Without my family, I was scared that nobody would understand me”
Back to the case. My patient was recovering well, and I was tying up loose ends on the switch day for the hospitalist teams.
“You will likely be discharged in a couple of days,” I said. She and the family were grateful and satisfied with the care. She had used the interpreter services and also received ethnocultural and language concordant and culturally competent care. Reducing language barriers is one of the crucial ways to reduce racial and ethnic disparities in quality of care and health outcomes, and it starts – in many cases – with identifying LEP patients. Proper use and monitoring of interpreter services, reporting language-related errors, hiring and testing bilingual staff’s language proficiency, and educating staff on cultural awareness are essential strategies for caring for LEP patients.
At my weeks’ end, in my handoff note to the incoming providers, I highlighted: “Patient will benefit from a Hindi speaking provider, Limited English Proficiency.”
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Questions and Answers. Limited English Proficiency: A federal interagency website. www.lep.gov/commonly-asked-questions.
2. United States Census Bureau. Percent of people 5 years and over who speak English less than ‘very well’. www.census.gov/library/visualizations/interactive/people-that-speak-english-less-than-very-well.html.
3. Jacobs EA, et al. Overcoming language barriers in health care: Costs and benefits of interpreter services. Am J Public Health. 2004;94(5):866–869. doi: 10.2105/ajph.94.5.866.
4. Gandhi TK, et al. Drug complications in outpatients. J Gen Intern Med. 2000;15(3):149–154. doi: 10.1046/j.1525-1497.2000.04199.x.
5. Karliner LS, et al. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2007;42(2):727–754. doi: 10.1111/j.1475-6773.2006.00629.x.
6. Divi C, et al. Language proficiency and adverse events in US hospitals: a pilot study. Int J Qual Health Care. 2007 Apr;19(2):60-7. doi: 10.1093/intqhc/mzl069.
7. Karliner LS, et al. Identification of limited English proficient patients in clinical care. J Gen Intern Med. 2008;23(10):1555-1560. doi:10.1007/s11606-008-0693-y.
8. Schenker Y, et al. Patterns of interpreter use for hospitalized patients with limited English proficiency. J Gen Intern Med. 2011 Jul;26(7):712-7. doi: 10.1007/s11606-010-1619-z.
9. Office of Minority Health, US Department of Health and Human Services. National Standards for Culturally and Linguistically Appropriate Services in Health Care: Final Report. Washington, DC: US Department of Health and Human Services; 2001. https://minorityhealth.hhs.gov/assets/pdf/checked/finalreport.pdf.
10. www.medicaid.gov/medicaid/financial-management/medicaid-administrative-claiming/translation-and-interpretation-services/index.html
11. Schenker Y, et al. The Impact of Language Barriers on Documentation of Informed Consent at a Hospital with On-Site Interpreter Services. J Gen Intern Med. 2007 Nov;22 Suppl 2(Suppl 2):294-9. doi: 10.1007/s11606-007-0359-1.
12. Locatis C, et al. Comparing in-person, video, and telephonic medical interpretation. J Gen Intern Med. 2010;25(4):345-350. doi:10.1007/s11606-009-1236-x.
13. Dunlap JL, et al. The effects of language concordant care on patient satisfaction and clinical understanding for Hispanic pediatric surgery patients. J Pediatr Surg. 2015 Sep;50(9):1586-9. doi: 10.1016/j.jpedsurg.2014.12.020.
14. Diamond L, et al. A Systematic Review of the Impact of Patient–Physician Non-English Language Concordance on Quality of Care and Outcomes. J Gen Intern Med. 2019 Aug;34(8):1591-1606. doi: 10.1007/s11606-019-04847-5.
15. Ngo-Metzger Q, et al. Providing high-quality care for limited English proficient patients: the importance of language concordance and interpreter use. J Gen Intern Med. 2007 Nov;22 Suppl 2(Suppl 2):324-30. doi: 10.1007/s11606-007-0340-z.
16. https://press.doximity.com/articles/first-ever-national-study-to-examine-different-languages-spoken-by-us-doctors.
17. Agency for Healthcare Research and Quality. Patients with Limited English Proficiency. www.ahrq.gov/teamstepps/lep/index.html.
A hectic Friday morning at the hospital seemed less stressful amid morning greetings and humor from colleagues. In a team room full of hospitalists, life and death are often discussed in detail, ranging from medical discussions to joys and frustrations of the day to philosophy, politics, and more. It is almost impossible to miss something interesting.
People breaking into their native languages over the phone call from home always make me smile. The mention of a “complicated Indian patient unable to use interpreter” caught my attention.
My friend and colleague asked if I would be willing to take over the patient since I could speak Hindi. I was doubtful if I would add anything to make a meaningful difference, given the patient wasn’t even participating in a conversation. However, my colleague’s concern for the patient and faith in me was enough to say, “Sure, let me add her to my list.”
At the bedside, it felt like a classic “acute on chronic” hot mess situation. The patient presented with a generalized rash, anasarca, renal failure, multifocal pneumonia, and delirium. All I could gather from the patient were some incomprehensible words that sounded like Hindi. I called the family to obtain some history and to provide updates. Her son was excited to hear from me, and it didn’t take him long to guess that I was from India. But that could still mean that I might speak any of the twenty-two or more Indian languages.
Answering my questions one by one in perfectly understandable English, he was short and sweet. Suspicious of missing out on details, I offered hesitantly, “You could speak in Hindi with me.” Then came a flood of information with the details, concerns, questions, and what was lost in the translation.
We all attend to patients and families with limited English proficiency (LEP), immigrants, and nonimmigrants. LEP is a term used to describe individuals who do not speak English as their primary language and have a limited ability to read, speak, write, or understand English.1 Recent data from the American Community Survey (2005-2009) reports that 8.6% of the population (24 million Americans) have LEP.2 It’s a large and growing population that needs help overcoming language barriers and the appropriate use of professional medical interpreter services – a backbone to safe, quality, and cost-effective patient care.
The following day at bedside rounds, the nurse reported that the patient was looking and responding better. She could cooperate with interpreter services and could speak “some English.” Over the years, one thing that sounds more alarming than “no English” is “some English” or “enough English.” Around noon I received a page that the patient was refusing intravenous Lasix. At the bedside, however, the patient seemed unaware of the perceived refusal. Further discussions with the nurse lead to a familiar culprit, a relatively common gesture in South Asian cultures, a head bobble or shake.
The nurse reported that the patient shook her head side to side, seemed upset, and said “NO” when trying to administer the medication. On the other hand, the patient reported that she was upset to be at the hospital but had “NO” problem with the medicine.
My patient’s “some English” was indeed “enough English” to put her at risk due to medical error, which is highly likely when patients or providers can speak or understand a language to “get by” or to “make do.” Like my patient, the LEP patient population is more likely to experience medical errors, longer hospital stays, hospital-acquired complications, surgical delays, and readmissions. They are also less likely to receive preventive care, have access to regular care, or be satisfied with their care. They are much more likely to have adverse effects from drug complications, poor understanding of diagnoses, a greater risk of being misunderstood by their physicians or ancillary staff, and less likely to follow physician instructions.3-5 One study analyzed over 1,000 adverse-incident reports from six Joint Commission-accredited hospitals for LEP and English-speaking patients and found that 49% of LEP patients experienced physical harm versus 29.5% of English-speaking patients.6
I updated the patient’s LEP status that was missing in the chart, likely due to altered mental status at the time of admission. Reliable language and English proficiency data are usually entered at the patient’s point of entry with documentation of the language services required during the patient-provider encounter. The U.S. Census Bureau’s operational definition for LEP is a patient’s self-assessed ability to speak English less than “very well,” but how well it correlates with a patient’s actual English ability needs more study. Also, one’s self-assessed perception of ability might vary day to day, and language ability, by itself, is not static; it can differ from moment to moment and situation to situation. It may be easier to understand words in English when the situation is simple and less stressful than when things are complicated and stressful.
With a definition of LEP rather vague and the term somewhat derogatory, its meaning is open to interpretation. One study found that though speaking English less than “very well” was the most sensitive measure for identifying all of the patients who reported that they were unable to communicate effectively with their physicians, it was also the least specific.7 This lower specificity could lead to misclassification of some patients as LEP who are, in fact, able to effectively communicate in English with their physicians. This type of misclassification might lead to costly language assistance and carry the potential to cause conflicts between patient and provider. Telling a patient or family that they may have a “limited English proficiency” when they have believed otherwise and feel confident about their skills may come as a challenge. Some patients may also pretend to understand English to avoid being embarrassed about their linguistic abilities or perceive that they might be judged on their abilities in general.
Exiting the room, I gently reminded the RN to use the interpreter services. “Who has never been guilty of using an ad hoc interpreter or rushing through a long interpreter phone call due to time constraints?” I thought. A study from 2011 found that 43% of hospitalized patients with LEP had communicated without an interpreter present during admission, and 40% had communicated without an interpreter present after admission.8 In other words, a system in place does not mean service in use. But, the use of a trained interpreter is not only an obligation for care providers but a right for patients as per legal requirements of Title VI of the Civil Rights Act and the Standards for Culturally and Linguistically Appropriate Services (CLAS) by the Department of Health and Human Services’ (HSS) Office of Minority Health.9 In January 2010, The Joint Commission released a set of new and revised standards for patient-centered communication as part of an initiative to advance effective communication, cultural competence, and patient- and family-centered care.
Despite the requirements and availability of qualified medical interpreter services, there are multiple perceived and experienced barriers to the use of interpreter services. The most common one is that what comes as a free service for patients is a time commitment for providers. A long list of patients, acuity of the situation, and ease of use/availability of translation aids can change the calculus. One may be able to bill a prolonged service code (99354-99357) in addition to the appropriate E/M code, although a patient cannot be billed for the actual service provided by the interpreter. Longstanding CMS policy also permits reimbursement for translation/interpretation activities, so long as they are not included and paid for as part of the rate for direct service.10
The patient, however, insisted that she would rather have her son as the interpreter on the 3-way over the phone (OPI) conference call for interpretation. “He speaks good English and knows my medical history well,” she said. I counseled the patient on the benefits of using interpreter services and explained how to use the call button light and the visual aids.
Placing emphasis on educating patients about the benefits of using, and risks of not using, interpreter services is as essential as emphasizing that care providers use the services. Some patients may voluntarily choose to provide their own interpreter. Use of family members, friends, or unqualified staff as interpreters is one of the most commonly reported causes of errors by frontline staff. Using in-language collateral may help these patients understand how medical interpretation may create a better patient experience and outcome. A short factsheet, in different languages, on qualified interpreters’ expected benefits: meaning-for-meaning communication, impartiality, medical privacy, and improved patient safety and satisfaction, can also come in handy.
However, if the patient still refuses, providers should document the refusal of the offer of free language services, the name of the interpreter designated by the patient, the interpreter’s relationship to the LEP person, and the time or portions of the patient encounter that the interpreter’s services were used. Yet, language interpretation alone can be inadequate without document translation. According to one study, despite the availability of on-site professional interpreter services, hospitalized patients who do not speak English are less likely to have signed consent forms in their medical records.11 Health care professionals, therefore, need well-translated documents to treat LEP patients. Translated documents of consent forms for medical procedures, post-discharge instructions, prescription and medical device labels, and drug usage information may enhance informed decision making, safety and reduce stress and medical errors.
An unpopular and underused service needs it all: availability, convenience, monitoring, reporting, and team effort. Due to the sheer unpopularity and underuse of interpreter services, institutions should enhance ease of availability, monitor the use and quality of interpreter services, and optimize reporting of language-related errors. Ease of availability goes hand in hand with tapping local resources. Over the years, and even more so during the pandemic, in-person interpretation has transitioned to telephonic or video interpretation due to availability, safety, and cost issues. There are challenges in translating a language, and the absence of a visual channel adds another layer of complexity.
The current body of evidence does not indicate a superior interpreting method. Still, in one study providers and interpreters exposed to all three methods were more critical of remote methods and preferred videoconferencing to the telephone as a remote method. The significantly shorter phone interviews raised questions about the prospects of miscommunication in telephonic interpretation, given the absence of a visual channel.12
One way to bypass language barriers is to recognize the value added by hiring and training bilingual health care providers and fostering cultural competence. International medical graduates in many parts of the country aid in closing language barriers. Language-concordant care enhances trust between patients and physicians, optimizes health outcomes, and advances health equity for diverse populations.13-15 The presence of bilingual providers means more effective and timelier communication and improved patient satisfaction. But, according to a Doximity study, there is a significant “language gap” between those languages spoken by physicians and their patients.16 Hospitals, therefore, should assess, qualify, and incentivize staff who can serve as on-site medical interpreters for patients as a means to facilitate language concordant care for LEP patients.
The Agency of Healthcare Research and Quality (AHRQ) also has a guide on how hospitals can better identify, report, monitor, and prevent medical errors in patients with LEP. Included is the TeamSTEPPS LEP module to help develop and deploy a customized plan to train staff in teamwork skills and lead a medical teamwork improvement initiative.17
“Without my family, I was scared that nobody would understand me”
Back to the case. My patient was recovering well, and I was tying up loose ends on the switch day for the hospitalist teams.
“You will likely be discharged in a couple of days,” I said. She and the family were grateful and satisfied with the care. She had used the interpreter services and also received ethnocultural and language concordant and culturally competent care. Reducing language barriers is one of the crucial ways to reduce racial and ethnic disparities in quality of care and health outcomes, and it starts – in many cases – with identifying LEP patients. Proper use and monitoring of interpreter services, reporting language-related errors, hiring and testing bilingual staff’s language proficiency, and educating staff on cultural awareness are essential strategies for caring for LEP patients.
At my weeks’ end, in my handoff note to the incoming providers, I highlighted: “Patient will benefit from a Hindi speaking provider, Limited English Proficiency.”
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Questions and Answers. Limited English Proficiency: A federal interagency website. www.lep.gov/commonly-asked-questions.
2. United States Census Bureau. Percent of people 5 years and over who speak English less than ‘very well’. www.census.gov/library/visualizations/interactive/people-that-speak-english-less-than-very-well.html.
3. Jacobs EA, et al. Overcoming language barriers in health care: Costs and benefits of interpreter services. Am J Public Health. 2004;94(5):866–869. doi: 10.2105/ajph.94.5.866.
4. Gandhi TK, et al. Drug complications in outpatients. J Gen Intern Med. 2000;15(3):149–154. doi: 10.1046/j.1525-1497.2000.04199.x.
5. Karliner LS, et al. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2007;42(2):727–754. doi: 10.1111/j.1475-6773.2006.00629.x.
6. Divi C, et al. Language proficiency and adverse events in US hospitals: a pilot study. Int J Qual Health Care. 2007 Apr;19(2):60-7. doi: 10.1093/intqhc/mzl069.
7. Karliner LS, et al. Identification of limited English proficient patients in clinical care. J Gen Intern Med. 2008;23(10):1555-1560. doi:10.1007/s11606-008-0693-y.
8. Schenker Y, et al. Patterns of interpreter use for hospitalized patients with limited English proficiency. J Gen Intern Med. 2011 Jul;26(7):712-7. doi: 10.1007/s11606-010-1619-z.
9. Office of Minority Health, US Department of Health and Human Services. National Standards for Culturally and Linguistically Appropriate Services in Health Care: Final Report. Washington, DC: US Department of Health and Human Services; 2001. https://minorityhealth.hhs.gov/assets/pdf/checked/finalreport.pdf.
10. www.medicaid.gov/medicaid/financial-management/medicaid-administrative-claiming/translation-and-interpretation-services/index.html
11. Schenker Y, et al. The Impact of Language Barriers on Documentation of Informed Consent at a Hospital with On-Site Interpreter Services. J Gen Intern Med. 2007 Nov;22 Suppl 2(Suppl 2):294-9. doi: 10.1007/s11606-007-0359-1.
12. Locatis C, et al. Comparing in-person, video, and telephonic medical interpretation. J Gen Intern Med. 2010;25(4):345-350. doi:10.1007/s11606-009-1236-x.
13. Dunlap JL, et al. The effects of language concordant care on patient satisfaction and clinical understanding for Hispanic pediatric surgery patients. J Pediatr Surg. 2015 Sep;50(9):1586-9. doi: 10.1016/j.jpedsurg.2014.12.020.
14. Diamond L, et al. A Systematic Review of the Impact of Patient–Physician Non-English Language Concordance on Quality of Care and Outcomes. J Gen Intern Med. 2019 Aug;34(8):1591-1606. doi: 10.1007/s11606-019-04847-5.
15. Ngo-Metzger Q, et al. Providing high-quality care for limited English proficient patients: the importance of language concordance and interpreter use. J Gen Intern Med. 2007 Nov;22 Suppl 2(Suppl 2):324-30. doi: 10.1007/s11606-007-0340-z.
16. https://press.doximity.com/articles/first-ever-national-study-to-examine-different-languages-spoken-by-us-doctors.
17. Agency for Healthcare Research and Quality. Patients with Limited English Proficiency. www.ahrq.gov/teamstepps/lep/index.html.
A hectic Friday morning at the hospital seemed less stressful amid morning greetings and humor from colleagues. In a team room full of hospitalists, life and death are often discussed in detail, ranging from medical discussions to joys and frustrations of the day to philosophy, politics, and more. It is almost impossible to miss something interesting.
People breaking into their native languages over the phone call from home always make me smile. The mention of a “complicated Indian patient unable to use interpreter” caught my attention.
My friend and colleague asked if I would be willing to take over the patient since I could speak Hindi. I was doubtful if I would add anything to make a meaningful difference, given the patient wasn’t even participating in a conversation. However, my colleague’s concern for the patient and faith in me was enough to say, “Sure, let me add her to my list.”
At the bedside, it felt like a classic “acute on chronic” hot mess situation. The patient presented with a generalized rash, anasarca, renal failure, multifocal pneumonia, and delirium. All I could gather from the patient were some incomprehensible words that sounded like Hindi. I called the family to obtain some history and to provide updates. Her son was excited to hear from me, and it didn’t take him long to guess that I was from India. But that could still mean that I might speak any of the twenty-two or more Indian languages.
Answering my questions one by one in perfectly understandable English, he was short and sweet. Suspicious of missing out on details, I offered hesitantly, “You could speak in Hindi with me.” Then came a flood of information with the details, concerns, questions, and what was lost in the translation.
We all attend to patients and families with limited English proficiency (LEP), immigrants, and nonimmigrants. LEP is a term used to describe individuals who do not speak English as their primary language and have a limited ability to read, speak, write, or understand English.1 Recent data from the American Community Survey (2005-2009) reports that 8.6% of the population (24 million Americans) have LEP.2 It’s a large and growing population that needs help overcoming language barriers and the appropriate use of professional medical interpreter services – a backbone to safe, quality, and cost-effective patient care.
The following day at bedside rounds, the nurse reported that the patient was looking and responding better. She could cooperate with interpreter services and could speak “some English.” Over the years, one thing that sounds more alarming than “no English” is “some English” or “enough English.” Around noon I received a page that the patient was refusing intravenous Lasix. At the bedside, however, the patient seemed unaware of the perceived refusal. Further discussions with the nurse lead to a familiar culprit, a relatively common gesture in South Asian cultures, a head bobble or shake.
The nurse reported that the patient shook her head side to side, seemed upset, and said “NO” when trying to administer the medication. On the other hand, the patient reported that she was upset to be at the hospital but had “NO” problem with the medicine.
My patient’s “some English” was indeed “enough English” to put her at risk due to medical error, which is highly likely when patients or providers can speak or understand a language to “get by” or to “make do.” Like my patient, the LEP patient population is more likely to experience medical errors, longer hospital stays, hospital-acquired complications, surgical delays, and readmissions. They are also less likely to receive preventive care, have access to regular care, or be satisfied with their care. They are much more likely to have adverse effects from drug complications, poor understanding of diagnoses, a greater risk of being misunderstood by their physicians or ancillary staff, and less likely to follow physician instructions.3-5 One study analyzed over 1,000 adverse-incident reports from six Joint Commission-accredited hospitals for LEP and English-speaking patients and found that 49% of LEP patients experienced physical harm versus 29.5% of English-speaking patients.6
I updated the patient’s LEP status that was missing in the chart, likely due to altered mental status at the time of admission. Reliable language and English proficiency data are usually entered at the patient’s point of entry with documentation of the language services required during the patient-provider encounter. The U.S. Census Bureau’s operational definition for LEP is a patient’s self-assessed ability to speak English less than “very well,” but how well it correlates with a patient’s actual English ability needs more study. Also, one’s self-assessed perception of ability might vary day to day, and language ability, by itself, is not static; it can differ from moment to moment and situation to situation. It may be easier to understand words in English when the situation is simple and less stressful than when things are complicated and stressful.
With a definition of LEP rather vague and the term somewhat derogatory, its meaning is open to interpretation. One study found that though speaking English less than “very well” was the most sensitive measure for identifying all of the patients who reported that they were unable to communicate effectively with their physicians, it was also the least specific.7 This lower specificity could lead to misclassification of some patients as LEP who are, in fact, able to effectively communicate in English with their physicians. This type of misclassification might lead to costly language assistance and carry the potential to cause conflicts between patient and provider. Telling a patient or family that they may have a “limited English proficiency” when they have believed otherwise and feel confident about their skills may come as a challenge. Some patients may also pretend to understand English to avoid being embarrassed about their linguistic abilities or perceive that they might be judged on their abilities in general.
Exiting the room, I gently reminded the RN to use the interpreter services. “Who has never been guilty of using an ad hoc interpreter or rushing through a long interpreter phone call due to time constraints?” I thought. A study from 2011 found that 43% of hospitalized patients with LEP had communicated without an interpreter present during admission, and 40% had communicated without an interpreter present after admission.8 In other words, a system in place does not mean service in use. But, the use of a trained interpreter is not only an obligation for care providers but a right for patients as per legal requirements of Title VI of the Civil Rights Act and the Standards for Culturally and Linguistically Appropriate Services (CLAS) by the Department of Health and Human Services’ (HSS) Office of Minority Health.9 In January 2010, The Joint Commission released a set of new and revised standards for patient-centered communication as part of an initiative to advance effective communication, cultural competence, and patient- and family-centered care.
Despite the requirements and availability of qualified medical interpreter services, there are multiple perceived and experienced barriers to the use of interpreter services. The most common one is that what comes as a free service for patients is a time commitment for providers. A long list of patients, acuity of the situation, and ease of use/availability of translation aids can change the calculus. One may be able to bill a prolonged service code (99354-99357) in addition to the appropriate E/M code, although a patient cannot be billed for the actual service provided by the interpreter. Longstanding CMS policy also permits reimbursement for translation/interpretation activities, so long as they are not included and paid for as part of the rate for direct service.10
The patient, however, insisted that she would rather have her son as the interpreter on the 3-way over the phone (OPI) conference call for interpretation. “He speaks good English and knows my medical history well,” she said. I counseled the patient on the benefits of using interpreter services and explained how to use the call button light and the visual aids.
Placing emphasis on educating patients about the benefits of using, and risks of not using, interpreter services is as essential as emphasizing that care providers use the services. Some patients may voluntarily choose to provide their own interpreter. Use of family members, friends, or unqualified staff as interpreters is one of the most commonly reported causes of errors by frontline staff. Using in-language collateral may help these patients understand how medical interpretation may create a better patient experience and outcome. A short factsheet, in different languages, on qualified interpreters’ expected benefits: meaning-for-meaning communication, impartiality, medical privacy, and improved patient safety and satisfaction, can also come in handy.
However, if the patient still refuses, providers should document the refusal of the offer of free language services, the name of the interpreter designated by the patient, the interpreter’s relationship to the LEP person, and the time or portions of the patient encounter that the interpreter’s services were used. Yet, language interpretation alone can be inadequate without document translation. According to one study, despite the availability of on-site professional interpreter services, hospitalized patients who do not speak English are less likely to have signed consent forms in their medical records.11 Health care professionals, therefore, need well-translated documents to treat LEP patients. Translated documents of consent forms for medical procedures, post-discharge instructions, prescription and medical device labels, and drug usage information may enhance informed decision making, safety and reduce stress and medical errors.
An unpopular and underused service needs it all: availability, convenience, monitoring, reporting, and team effort. Due to the sheer unpopularity and underuse of interpreter services, institutions should enhance ease of availability, monitor the use and quality of interpreter services, and optimize reporting of language-related errors. Ease of availability goes hand in hand with tapping local resources. Over the years, and even more so during the pandemic, in-person interpretation has transitioned to telephonic or video interpretation due to availability, safety, and cost issues. There are challenges in translating a language, and the absence of a visual channel adds another layer of complexity.
The current body of evidence does not indicate a superior interpreting method. Still, in one study providers and interpreters exposed to all three methods were more critical of remote methods and preferred videoconferencing to the telephone as a remote method. The significantly shorter phone interviews raised questions about the prospects of miscommunication in telephonic interpretation, given the absence of a visual channel.12
One way to bypass language barriers is to recognize the value added by hiring and training bilingual health care providers and fostering cultural competence. International medical graduates in many parts of the country aid in closing language barriers. Language-concordant care enhances trust between patients and physicians, optimizes health outcomes, and advances health equity for diverse populations.13-15 The presence of bilingual providers means more effective and timelier communication and improved patient satisfaction. But, according to a Doximity study, there is a significant “language gap” between those languages spoken by physicians and their patients.16 Hospitals, therefore, should assess, qualify, and incentivize staff who can serve as on-site medical interpreters for patients as a means to facilitate language concordant care for LEP patients.
The Agency of Healthcare Research and Quality (AHRQ) also has a guide on how hospitals can better identify, report, monitor, and prevent medical errors in patients with LEP. Included is the TeamSTEPPS LEP module to help develop and deploy a customized plan to train staff in teamwork skills and lead a medical teamwork improvement initiative.17
“Without my family, I was scared that nobody would understand me”
Back to the case. My patient was recovering well, and I was tying up loose ends on the switch day for the hospitalist teams.
“You will likely be discharged in a couple of days,” I said. She and the family were grateful and satisfied with the care. She had used the interpreter services and also received ethnocultural and language concordant and culturally competent care. Reducing language barriers is one of the crucial ways to reduce racial and ethnic disparities in quality of care and health outcomes, and it starts – in many cases – with identifying LEP patients. Proper use and monitoring of interpreter services, reporting language-related errors, hiring and testing bilingual staff’s language proficiency, and educating staff on cultural awareness are essential strategies for caring for LEP patients.
At my weeks’ end, in my handoff note to the incoming providers, I highlighted: “Patient will benefit from a Hindi speaking provider, Limited English Proficiency.”
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Questions and Answers. Limited English Proficiency: A federal interagency website. www.lep.gov/commonly-asked-questions.
2. United States Census Bureau. Percent of people 5 years and over who speak English less than ‘very well’. www.census.gov/library/visualizations/interactive/people-that-speak-english-less-than-very-well.html.
3. Jacobs EA, et al. Overcoming language barriers in health care: Costs and benefits of interpreter services. Am J Public Health. 2004;94(5):866–869. doi: 10.2105/ajph.94.5.866.
4. Gandhi TK, et al. Drug complications in outpatients. J Gen Intern Med. 2000;15(3):149–154. doi: 10.1046/j.1525-1497.2000.04199.x.
5. Karliner LS, et al. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2007;42(2):727–754. doi: 10.1111/j.1475-6773.2006.00629.x.
6. Divi C, et al. Language proficiency and adverse events in US hospitals: a pilot study. Int J Qual Health Care. 2007 Apr;19(2):60-7. doi: 10.1093/intqhc/mzl069.
7. Karliner LS, et al. Identification of limited English proficient patients in clinical care. J Gen Intern Med. 2008;23(10):1555-1560. doi:10.1007/s11606-008-0693-y.
8. Schenker Y, et al. Patterns of interpreter use for hospitalized patients with limited English proficiency. J Gen Intern Med. 2011 Jul;26(7):712-7. doi: 10.1007/s11606-010-1619-z.
9. Office of Minority Health, US Department of Health and Human Services. National Standards for Culturally and Linguistically Appropriate Services in Health Care: Final Report. Washington, DC: US Department of Health and Human Services; 2001. https://minorityhealth.hhs.gov/assets/pdf/checked/finalreport.pdf.
10. www.medicaid.gov/medicaid/financial-management/medicaid-administrative-claiming/translation-and-interpretation-services/index.html
11. Schenker Y, et al. The Impact of Language Barriers on Documentation of Informed Consent at a Hospital with On-Site Interpreter Services. J Gen Intern Med. 2007 Nov;22 Suppl 2(Suppl 2):294-9. doi: 10.1007/s11606-007-0359-1.
12. Locatis C, et al. Comparing in-person, video, and telephonic medical interpretation. J Gen Intern Med. 2010;25(4):345-350. doi:10.1007/s11606-009-1236-x.
13. Dunlap JL, et al. The effects of language concordant care on patient satisfaction and clinical understanding for Hispanic pediatric surgery patients. J Pediatr Surg. 2015 Sep;50(9):1586-9. doi: 10.1016/j.jpedsurg.2014.12.020.
14. Diamond L, et al. A Systematic Review of the Impact of Patient–Physician Non-English Language Concordance on Quality of Care and Outcomes. J Gen Intern Med. 2019 Aug;34(8):1591-1606. doi: 10.1007/s11606-019-04847-5.
15. Ngo-Metzger Q, et al. Providing high-quality care for limited English proficient patients: the importance of language concordance and interpreter use. J Gen Intern Med. 2007 Nov;22 Suppl 2(Suppl 2):324-30. doi: 10.1007/s11606-007-0340-z.
16. https://press.doximity.com/articles/first-ever-national-study-to-examine-different-languages-spoken-by-us-doctors.
17. Agency for Healthcare Research and Quality. Patients with Limited English Proficiency. www.ahrq.gov/teamstepps/lep/index.html.
Children and COVID: New vaccinations drop as the case count rises
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.
Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.

Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.

Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
FDA to warn J&J that vaccine can increase Guillain-Barré risk: Media
as early as July 13, according to multiple media reports.
Although the FDA is projected to add the new warning to the labeling for the vaccine, the agency still calculates the benefit of vaccination with the J&J product continues to outweigh the risk. Benefits include protection against the Delta variant and serious COVID-19 outcomes.
More than 100 cases of Guillain-Barré reported to the Vaccine Adverse Event Reporting System, a federal program for reporting vaccine issues, spurred the FDA to act.
Men and people older than 50 appear to be at highest risk, according to reports of a July 12 Centers for Disease Control and Prevention statement. The CDC also revealed that most cases occur about 2 weeks following immunization.
Guillain-Barré syndrome often causes muscle weakness and sometimes temporary paralysis. Most people who develop the rare syndrome recover.
Such was not the case for a 57-year-old man, the New York Times reported July 12. He had a history of both a heart attack and stroke in the previous 4 years and died in April after vaccination with the J&J vaccine and developing Guillain-Barré.
The new warning comes in the wake of a number of setbacks for the company’s COVID-19 vaccine. On April 13, the FDA and CDC both recommended a 10-day pause on administration of the J&J vaccine after reports of rare blood clot events emerged. In mid-June, the FDA requested that Johnson and Johnson discard millions of vaccine doses produced at a manufacturing facility in Baltimore.
The mRNA vaccines from Pfizer/BioNTech and Moderna are not affected by the new FDA warning.
The Biden administration is expected to make a formal announcement of the new warning for the Johnson and Johnson vaccine as early as July 13, the Times reports.
A version of this article first appeared on Medscape.com.
as early as July 13, according to multiple media reports.
Although the FDA is projected to add the new warning to the labeling for the vaccine, the agency still calculates the benefit of vaccination with the J&J product continues to outweigh the risk. Benefits include protection against the Delta variant and serious COVID-19 outcomes.
More than 100 cases of Guillain-Barré reported to the Vaccine Adverse Event Reporting System, a federal program for reporting vaccine issues, spurred the FDA to act.
Men and people older than 50 appear to be at highest risk, according to reports of a July 12 Centers for Disease Control and Prevention statement. The CDC also revealed that most cases occur about 2 weeks following immunization.
Guillain-Barré syndrome often causes muscle weakness and sometimes temporary paralysis. Most people who develop the rare syndrome recover.
Such was not the case for a 57-year-old man, the New York Times reported July 12. He had a history of both a heart attack and stroke in the previous 4 years and died in April after vaccination with the J&J vaccine and developing Guillain-Barré.
The new warning comes in the wake of a number of setbacks for the company’s COVID-19 vaccine. On April 13, the FDA and CDC both recommended a 10-day pause on administration of the J&J vaccine after reports of rare blood clot events emerged. In mid-June, the FDA requested that Johnson and Johnson discard millions of vaccine doses produced at a manufacturing facility in Baltimore.
The mRNA vaccines from Pfizer/BioNTech and Moderna are not affected by the new FDA warning.
The Biden administration is expected to make a formal announcement of the new warning for the Johnson and Johnson vaccine as early as July 13, the Times reports.
A version of this article first appeared on Medscape.com.
as early as July 13, according to multiple media reports.
Although the FDA is projected to add the new warning to the labeling for the vaccine, the agency still calculates the benefit of vaccination with the J&J product continues to outweigh the risk. Benefits include protection against the Delta variant and serious COVID-19 outcomes.
More than 100 cases of Guillain-Barré reported to the Vaccine Adverse Event Reporting System, a federal program for reporting vaccine issues, spurred the FDA to act.
Men and people older than 50 appear to be at highest risk, according to reports of a July 12 Centers for Disease Control and Prevention statement. The CDC also revealed that most cases occur about 2 weeks following immunization.
Guillain-Barré syndrome often causes muscle weakness and sometimes temporary paralysis. Most people who develop the rare syndrome recover.
Such was not the case for a 57-year-old man, the New York Times reported July 12. He had a history of both a heart attack and stroke in the previous 4 years and died in April after vaccination with the J&J vaccine and developing Guillain-Barré.
The new warning comes in the wake of a number of setbacks for the company’s COVID-19 vaccine. On April 13, the FDA and CDC both recommended a 10-day pause on administration of the J&J vaccine after reports of rare blood clot events emerged. In mid-June, the FDA requested that Johnson and Johnson discard millions of vaccine doses produced at a manufacturing facility in Baltimore.
The mRNA vaccines from Pfizer/BioNTech and Moderna are not affected by the new FDA warning.
The Biden administration is expected to make a formal announcement of the new warning for the Johnson and Johnson vaccine as early as July 13, the Times reports.
A version of this article first appeared on Medscape.com.
Standard medical mask can protect wearer from aerosols
A standard medical face mask is more effective at preventing the wearer from inhaling aerosols without causing substantial breathing resistance than various cloth, medical, or respirator masks, new research shows.
“Medical face masks with good filtration efficacies can provide even better protective effects than KN95 respirators,” Christian Sterr, MD, from Philipps University of Marburg (Germany), and colleagues wrote. “FFP2 respirators, on the other hand, could be useful in high-risk situations but require greater breathing effort and therefore physical stress for users.”
Extensive evidence has shown that face masks are an excellent form of source control, preventing infectious people from spreading the SARS-CoV-2 virus into the environment. But evidence has been less clear about how well masks protect the wearer from inhaling particles containing the virus.
The researchers conducted three experiments to test 32 different face masks. The findings were presented at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published online in PLOS One .
First they tested pressure drop, which “relates to how easily air can pass through the material,” said Chris Cappa, PhD, professor of civil and environmental engineering at the University of California, Davis, who was not involved with the study.
“Higher pressure drops mean that there is greater resistance to the air passing through. A higher pressure drop will typically mean breathing through the material will be slightly more challenging, compared to a low pressure drop. There is no relationship between pressure drop and the mask effectiveness,” he said in an interview.
Pressure drop was lowest with type II medical face masks, the typical three-ply surgical masks designed to stop large particles expelled by the wearer from entering the environment, was highest with respirators, including KN95 and FFP2 masks, and varied with the different cloth masks tested.
Next the researchers compared filtration efficacy, which “refers to how well the material removes particles from the air that passes through the mask material,” Dr. Cappa explained. They did this by placing each mask over the opening to an air collector that measured how many particles got through. “A mask that has 100% filtration efficacy will remove all particles from the air that passes through it and 0% means that no particles are removed.”
Cloth masks had the lowest filtration efficacy, at 28%. Certified face masks that met European Standards had a relatively high efficacy, at 70%; for uncertified face masks, filtration efficacy was 63%. As expected, KN95 and FFP2 masks had the highest filtration efficacy, at 94% and 98%, respectively.Finally, the researchers tested as-worn filtration efficacies. They placed each mask on a dummy head with an artificial airway that collected airborne particles. They then pumped a mixture of aerosol particles – ranging in size from 0.3 to 2.0 mcm – and particle-free pressurized air into the air-proof acrylic chamber in which the head was placed.
In this experiment, cloth masks and noncertified face masks were least effective, filtering less than 20% of aerosols. Interestingly, the cloth face mask with the highest filtration on its own (84%) had the lowest filtration efficacy (9%), apparently because of its very high pressure drop (breathing resistance). When more effort is required to breathe through a mask, more air can bypass the filtration system.
Type II medical face masks, however, filtered 47% of aerosols, KN95 masks filtered 41%, and FFP2 masks filtered 65%. Face shields did not prevent the inhalation of any aerosols.
“We know that face shields will only be effective in stopping very large droplets, essentially visible spittle,” Dr. Cappa explained. “Most of the particles that we exhale will travel right around a face shield.”
The “optimal mask effect is a combination of high filter performance and low filter resistance,” which applies to most of the FFP2 and medical type II face masks tested, Dr. Sterr and colleagues wrote. “The type II medical masks in our random sample showed very good as-worn filtration performances with a low additional work of breathing at the same time.”
Although this study showed how well different masks filtered out particles, it could not assess how well different masks prevent actual infection.
“Like any virus, SARS-CoV-2 can only infect people as long as it is viable,” the researchers wrote. “Moreover, a certain number of viable virus particles need to be inhaled to trigger an infection. Thus, the assessed filtration efficacy may differ from the provided protection rate against SARS-CoV-2.”
In addition, particles containing the virus could dry out while going through the mask and become less infectious. “Even a small reduction in inhaled particles might prevent infection or at least lead to a less severe infection,” they noted.
In fact, filtration efficacy does not necessarily indicate how well the mask filters out particles while being worn. “This might be due to the combined effects of mask fit and pressure drop of the mask material and therefore tendency for mask leakage,” the team wrote. “High pressure drop results in higher breathing resistance and therefore supports leakage, especially if combined to a loosely fitting mask.”
These findings are “in line with what we already knew,” Dr. Cappa explained. “Even if the mask material filters out nearly all particles that pass through it, as is the case for high-efficiency masks such as N95 and FFP2, if the mask does not fit well, then it will only provide moderate protection for the wearer.”
Although the findings reaffirm the different levels of filtration provided by various cloth masks, they do not “provide any guidance on which types of cloth masks are better or worse,” he said. But they do show that “medical face masks will generally provide more protection to the wearer.”
It’s not surprising that face shields offer little protection from aerosols, Dr. Cappa said, but they can provide added protection when worn with a mask.
“A face shield could prevent large droplets that might shoot out when a person coughs or sneezes from depositing on a person’s eye,” he pointed out. And it can help “redirect the plume of particles that an infected person exhales, which could be useful in close quarters. However, even then those particles will keep moving around and could be inhaled. A mask can really help to decrease the amount inhaled.”
The study did not use external funding. The authors and Dr. Cappa disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A standard medical face mask is more effective at preventing the wearer from inhaling aerosols without causing substantial breathing resistance than various cloth, medical, or respirator masks, new research shows.
“Medical face masks with good filtration efficacies can provide even better protective effects than KN95 respirators,” Christian Sterr, MD, from Philipps University of Marburg (Germany), and colleagues wrote. “FFP2 respirators, on the other hand, could be useful in high-risk situations but require greater breathing effort and therefore physical stress for users.”
Extensive evidence has shown that face masks are an excellent form of source control, preventing infectious people from spreading the SARS-CoV-2 virus into the environment. But evidence has been less clear about how well masks protect the wearer from inhaling particles containing the virus.
The researchers conducted three experiments to test 32 different face masks. The findings were presented at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published online in PLOS One .
First they tested pressure drop, which “relates to how easily air can pass through the material,” said Chris Cappa, PhD, professor of civil and environmental engineering at the University of California, Davis, who was not involved with the study.
“Higher pressure drops mean that there is greater resistance to the air passing through. A higher pressure drop will typically mean breathing through the material will be slightly more challenging, compared to a low pressure drop. There is no relationship between pressure drop and the mask effectiveness,” he said in an interview.
Pressure drop was lowest with type II medical face masks, the typical three-ply surgical masks designed to stop large particles expelled by the wearer from entering the environment, was highest with respirators, including KN95 and FFP2 masks, and varied with the different cloth masks tested.
Next the researchers compared filtration efficacy, which “refers to how well the material removes particles from the air that passes through the mask material,” Dr. Cappa explained. They did this by placing each mask over the opening to an air collector that measured how many particles got through. “A mask that has 100% filtration efficacy will remove all particles from the air that passes through it and 0% means that no particles are removed.”
Cloth masks had the lowest filtration efficacy, at 28%. Certified face masks that met European Standards had a relatively high efficacy, at 70%; for uncertified face masks, filtration efficacy was 63%. As expected, KN95 and FFP2 masks had the highest filtration efficacy, at 94% and 98%, respectively.Finally, the researchers tested as-worn filtration efficacies. They placed each mask on a dummy head with an artificial airway that collected airborne particles. They then pumped a mixture of aerosol particles – ranging in size from 0.3 to 2.0 mcm – and particle-free pressurized air into the air-proof acrylic chamber in which the head was placed.
In this experiment, cloth masks and noncertified face masks were least effective, filtering less than 20% of aerosols. Interestingly, the cloth face mask with the highest filtration on its own (84%) had the lowest filtration efficacy (9%), apparently because of its very high pressure drop (breathing resistance). When more effort is required to breathe through a mask, more air can bypass the filtration system.
Type II medical face masks, however, filtered 47% of aerosols, KN95 masks filtered 41%, and FFP2 masks filtered 65%. Face shields did not prevent the inhalation of any aerosols.
“We know that face shields will only be effective in stopping very large droplets, essentially visible spittle,” Dr. Cappa explained. “Most of the particles that we exhale will travel right around a face shield.”
The “optimal mask effect is a combination of high filter performance and low filter resistance,” which applies to most of the FFP2 and medical type II face masks tested, Dr. Sterr and colleagues wrote. “The type II medical masks in our random sample showed very good as-worn filtration performances with a low additional work of breathing at the same time.”
Although this study showed how well different masks filtered out particles, it could not assess how well different masks prevent actual infection.
“Like any virus, SARS-CoV-2 can only infect people as long as it is viable,” the researchers wrote. “Moreover, a certain number of viable virus particles need to be inhaled to trigger an infection. Thus, the assessed filtration efficacy may differ from the provided protection rate against SARS-CoV-2.”
In addition, particles containing the virus could dry out while going through the mask and become less infectious. “Even a small reduction in inhaled particles might prevent infection or at least lead to a less severe infection,” they noted.
In fact, filtration efficacy does not necessarily indicate how well the mask filters out particles while being worn. “This might be due to the combined effects of mask fit and pressure drop of the mask material and therefore tendency for mask leakage,” the team wrote. “High pressure drop results in higher breathing resistance and therefore supports leakage, especially if combined to a loosely fitting mask.”
These findings are “in line with what we already knew,” Dr. Cappa explained. “Even if the mask material filters out nearly all particles that pass through it, as is the case for high-efficiency masks such as N95 and FFP2, if the mask does not fit well, then it will only provide moderate protection for the wearer.”
Although the findings reaffirm the different levels of filtration provided by various cloth masks, they do not “provide any guidance on which types of cloth masks are better or worse,” he said. But they do show that “medical face masks will generally provide more protection to the wearer.”
It’s not surprising that face shields offer little protection from aerosols, Dr. Cappa said, but they can provide added protection when worn with a mask.
“A face shield could prevent large droplets that might shoot out when a person coughs or sneezes from depositing on a person’s eye,” he pointed out. And it can help “redirect the plume of particles that an infected person exhales, which could be useful in close quarters. However, even then those particles will keep moving around and could be inhaled. A mask can really help to decrease the amount inhaled.”
The study did not use external funding. The authors and Dr. Cappa disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A standard medical face mask is more effective at preventing the wearer from inhaling aerosols without causing substantial breathing resistance than various cloth, medical, or respirator masks, new research shows.
“Medical face masks with good filtration efficacies can provide even better protective effects than KN95 respirators,” Christian Sterr, MD, from Philipps University of Marburg (Germany), and colleagues wrote. “FFP2 respirators, on the other hand, could be useful in high-risk situations but require greater breathing effort and therefore physical stress for users.”
Extensive evidence has shown that face masks are an excellent form of source control, preventing infectious people from spreading the SARS-CoV-2 virus into the environment. But evidence has been less clear about how well masks protect the wearer from inhaling particles containing the virus.
The researchers conducted three experiments to test 32 different face masks. The findings were presented at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published online in PLOS One .
First they tested pressure drop, which “relates to how easily air can pass through the material,” said Chris Cappa, PhD, professor of civil and environmental engineering at the University of California, Davis, who was not involved with the study.
“Higher pressure drops mean that there is greater resistance to the air passing through. A higher pressure drop will typically mean breathing through the material will be slightly more challenging, compared to a low pressure drop. There is no relationship between pressure drop and the mask effectiveness,” he said in an interview.
Pressure drop was lowest with type II medical face masks, the typical three-ply surgical masks designed to stop large particles expelled by the wearer from entering the environment, was highest with respirators, including KN95 and FFP2 masks, and varied with the different cloth masks tested.
Next the researchers compared filtration efficacy, which “refers to how well the material removes particles from the air that passes through the mask material,” Dr. Cappa explained. They did this by placing each mask over the opening to an air collector that measured how many particles got through. “A mask that has 100% filtration efficacy will remove all particles from the air that passes through it and 0% means that no particles are removed.”
Cloth masks had the lowest filtration efficacy, at 28%. Certified face masks that met European Standards had a relatively high efficacy, at 70%; for uncertified face masks, filtration efficacy was 63%. As expected, KN95 and FFP2 masks had the highest filtration efficacy, at 94% and 98%, respectively.Finally, the researchers tested as-worn filtration efficacies. They placed each mask on a dummy head with an artificial airway that collected airborne particles. They then pumped a mixture of aerosol particles – ranging in size from 0.3 to 2.0 mcm – and particle-free pressurized air into the air-proof acrylic chamber in which the head was placed.
In this experiment, cloth masks and noncertified face masks were least effective, filtering less than 20% of aerosols. Interestingly, the cloth face mask with the highest filtration on its own (84%) had the lowest filtration efficacy (9%), apparently because of its very high pressure drop (breathing resistance). When more effort is required to breathe through a mask, more air can bypass the filtration system.
Type II medical face masks, however, filtered 47% of aerosols, KN95 masks filtered 41%, and FFP2 masks filtered 65%. Face shields did not prevent the inhalation of any aerosols.
“We know that face shields will only be effective in stopping very large droplets, essentially visible spittle,” Dr. Cappa explained. “Most of the particles that we exhale will travel right around a face shield.”
The “optimal mask effect is a combination of high filter performance and low filter resistance,” which applies to most of the FFP2 and medical type II face masks tested, Dr. Sterr and colleagues wrote. “The type II medical masks in our random sample showed very good as-worn filtration performances with a low additional work of breathing at the same time.”
Although this study showed how well different masks filtered out particles, it could not assess how well different masks prevent actual infection.
“Like any virus, SARS-CoV-2 can only infect people as long as it is viable,” the researchers wrote. “Moreover, a certain number of viable virus particles need to be inhaled to trigger an infection. Thus, the assessed filtration efficacy may differ from the provided protection rate against SARS-CoV-2.”
In addition, particles containing the virus could dry out while going through the mask and become less infectious. “Even a small reduction in inhaled particles might prevent infection or at least lead to a less severe infection,” they noted.
In fact, filtration efficacy does not necessarily indicate how well the mask filters out particles while being worn. “This might be due to the combined effects of mask fit and pressure drop of the mask material and therefore tendency for mask leakage,” the team wrote. “High pressure drop results in higher breathing resistance and therefore supports leakage, especially if combined to a loosely fitting mask.”
These findings are “in line with what we already knew,” Dr. Cappa explained. “Even if the mask material filters out nearly all particles that pass through it, as is the case for high-efficiency masks such as N95 and FFP2, if the mask does not fit well, then it will only provide moderate protection for the wearer.”
Although the findings reaffirm the different levels of filtration provided by various cloth masks, they do not “provide any guidance on which types of cloth masks are better or worse,” he said. But they do show that “medical face masks will generally provide more protection to the wearer.”
It’s not surprising that face shields offer little protection from aerosols, Dr. Cappa said, but they can provide added protection when worn with a mask.
“A face shield could prevent large droplets that might shoot out when a person coughs or sneezes from depositing on a person’s eye,” he pointed out. And it can help “redirect the plume of particles that an infected person exhales, which could be useful in close quarters. However, even then those particles will keep moving around and could be inhaled. A mask can really help to decrease the amount inhaled.”
The study did not use external funding. The authors and Dr. Cappa disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Malpractice claims from the COVID-19 pandemic: More questions than answers
Editor’s note: This article has been provided by The Doctors Company, the exclusively endorsed medical malpractice carrier for the Society of Hospital Medicine.
The pandemic has raised pressing questions around preventive measures, vaccines, and safe treatment, but it has also obscured one key lingering uncertainty for medical professionals: Where are all the medical malpractice claims?
A variety of factors create a cloud of uncertainty around when, if ever, we will see the claims we expected from care provided just before the pandemic, much less claims deriving from care during the pandemic of both COVID-19 and non–COVID-19 patients.
Malpractice claims take time to surface
We won’t know until 2022 or later whether there will be an increase in claims related to the pandemic. When a medical error occurs, it’s not like an automobile accident. Everybody nearby knows when there’s been an automobile accident because they hear screeching tires, a loud crash, and then sirens. But when a medical error occurs, generally speaking, neither the doctor nor the patient immediately knows that something is amiss. It can take months or years for people to realize that something untoward has occurred.
Claims from medical errors that occurred before the pandemic bring additional uncertainties. In 2020, we saw fewer than expected overall claims filed from events occurring 18-24 months before the pandemic. In total, 20% fewer claims were filed than in 2019. This may have had to do with courts shutting down, people being reluctant to meet with attorneys to discuss a claim, and/or lawyers working from home. We may see these claims filed later than expected, or maybe we won’t see them at all.
But without a doubt, pandemic-related claims will be filed. The pandemic’s impact on physicians increases the risk of claims. Burnout is a major cause of medical errors, and a recent study found that out of 60 countries, U.S. health care providers showed the highest rates of burnout. We’re concerned about the stress affecting physicians’ performance – not just the physical stress of the demands put on them while treating COVID-19 patients, but all of the worry. For instance, a lot of doctors at the start of this pandemic stayed at hotels because they didn’t want to bring the virus home to their families – if they got exposed. Those sorts of stressors from life disruptions, on top of the stress of treating COVID-19 patients and the stress of treating non–COVID-19 patients within overtaxed health care systems, contribute to the possibilities for error.
Immunity protections are not fail-safe
And while health care providers have medical liability protections during the pandemic, these protections may not prevent claims. Health care provider pandemic-related liability laws vary from state to state, and they will be tested in the courts as to whether they’re constitutional. For example, there is pending legislation in New York state that would repeal the provider protections created there at the start of the pandemic. Further, some expert witnesses will couch their statements in terms of what it takes to get around one of these statutes. Therefore, physicians do have reason for concern, even in states with strong liability protections.
The following case example, which is one of about 40 COVID-19–related claims made against our members so far, is a poster child for why these protections are necessary: A quadriplegic patient with COVID-19 had reached the point of organ failure before he reached the ED. There was really nothing medical science could do for him at that point, in terms of a chance at recovery. Therefore, the patient’s physician and conservator placed him in assisted living for palliative care. This was a sad but reasonable decision during a pandemic, with hospital beds needed for patients with a shot at surviving. Following that patient’s death, the physician is being sued.
Defending claims regarding treatment vs. regarding infection control
We are very confident in our ability to protect our members against claims where they are being sued over the treatment of the disease. Claims arising out of treatment are not concerning to us because there is no cure for COVID-19 – one can only treat the symptoms as the virus runs its course.
On the other hand, suits harder to defend would be those that revolve around transmitting the disease because providers didn’t follow guidelines from the Centers for Disease Control and Prevention or there wasn’t enough personal protective equipment. That’s why we stress the importance of following CDC guidelines, and why we’ve taken proactive steps to communicate with the entire medical community throughout the pandemic as part of our commitment to serve those who provide care.
Mr. White is chief operating officer at The Doctors Company. The guidelines suggested here are not rules, do not constitute legal advice, and do not ensure a successful outcome. The ultimate decision regarding the appropriateness of any treatment must be made by each health care provider considering the circumstances of the individual situation and in accordance with the laws of the jurisdiction in which the care is rendered.
Editor’s note: This article has been provided by The Doctors Company, the exclusively endorsed medical malpractice carrier for the Society of Hospital Medicine.
The pandemic has raised pressing questions around preventive measures, vaccines, and safe treatment, but it has also obscured one key lingering uncertainty for medical professionals: Where are all the medical malpractice claims?
A variety of factors create a cloud of uncertainty around when, if ever, we will see the claims we expected from care provided just before the pandemic, much less claims deriving from care during the pandemic of both COVID-19 and non–COVID-19 patients.
Malpractice claims take time to surface
We won’t know until 2022 or later whether there will be an increase in claims related to the pandemic. When a medical error occurs, it’s not like an automobile accident. Everybody nearby knows when there’s been an automobile accident because they hear screeching tires, a loud crash, and then sirens. But when a medical error occurs, generally speaking, neither the doctor nor the patient immediately knows that something is amiss. It can take months or years for people to realize that something untoward has occurred.
Claims from medical errors that occurred before the pandemic bring additional uncertainties. In 2020, we saw fewer than expected overall claims filed from events occurring 18-24 months before the pandemic. In total, 20% fewer claims were filed than in 2019. This may have had to do with courts shutting down, people being reluctant to meet with attorneys to discuss a claim, and/or lawyers working from home. We may see these claims filed later than expected, or maybe we won’t see them at all.
But without a doubt, pandemic-related claims will be filed. The pandemic’s impact on physicians increases the risk of claims. Burnout is a major cause of medical errors, and a recent study found that out of 60 countries, U.S. health care providers showed the highest rates of burnout. We’re concerned about the stress affecting physicians’ performance – not just the physical stress of the demands put on them while treating COVID-19 patients, but all of the worry. For instance, a lot of doctors at the start of this pandemic stayed at hotels because they didn’t want to bring the virus home to their families – if they got exposed. Those sorts of stressors from life disruptions, on top of the stress of treating COVID-19 patients and the stress of treating non–COVID-19 patients within overtaxed health care systems, contribute to the possibilities for error.
Immunity protections are not fail-safe
And while health care providers have medical liability protections during the pandemic, these protections may not prevent claims. Health care provider pandemic-related liability laws vary from state to state, and they will be tested in the courts as to whether they’re constitutional. For example, there is pending legislation in New York state that would repeal the provider protections created there at the start of the pandemic. Further, some expert witnesses will couch their statements in terms of what it takes to get around one of these statutes. Therefore, physicians do have reason for concern, even in states with strong liability protections.
The following case example, which is one of about 40 COVID-19–related claims made against our members so far, is a poster child for why these protections are necessary: A quadriplegic patient with COVID-19 had reached the point of organ failure before he reached the ED. There was really nothing medical science could do for him at that point, in terms of a chance at recovery. Therefore, the patient’s physician and conservator placed him in assisted living for palliative care. This was a sad but reasonable decision during a pandemic, with hospital beds needed for patients with a shot at surviving. Following that patient’s death, the physician is being sued.
Defending claims regarding treatment vs. regarding infection control
We are very confident in our ability to protect our members against claims where they are being sued over the treatment of the disease. Claims arising out of treatment are not concerning to us because there is no cure for COVID-19 – one can only treat the symptoms as the virus runs its course.
On the other hand, suits harder to defend would be those that revolve around transmitting the disease because providers didn’t follow guidelines from the Centers for Disease Control and Prevention or there wasn’t enough personal protective equipment. That’s why we stress the importance of following CDC guidelines, and why we’ve taken proactive steps to communicate with the entire medical community throughout the pandemic as part of our commitment to serve those who provide care.
Mr. White is chief operating officer at The Doctors Company. The guidelines suggested here are not rules, do not constitute legal advice, and do not ensure a successful outcome. The ultimate decision regarding the appropriateness of any treatment must be made by each health care provider considering the circumstances of the individual situation and in accordance with the laws of the jurisdiction in which the care is rendered.
Editor’s note: This article has been provided by The Doctors Company, the exclusively endorsed medical malpractice carrier for the Society of Hospital Medicine.
The pandemic has raised pressing questions around preventive measures, vaccines, and safe treatment, but it has also obscured one key lingering uncertainty for medical professionals: Where are all the medical malpractice claims?
A variety of factors create a cloud of uncertainty around when, if ever, we will see the claims we expected from care provided just before the pandemic, much less claims deriving from care during the pandemic of both COVID-19 and non–COVID-19 patients.
Malpractice claims take time to surface
We won’t know until 2022 or later whether there will be an increase in claims related to the pandemic. When a medical error occurs, it’s not like an automobile accident. Everybody nearby knows when there’s been an automobile accident because they hear screeching tires, a loud crash, and then sirens. But when a medical error occurs, generally speaking, neither the doctor nor the patient immediately knows that something is amiss. It can take months or years for people to realize that something untoward has occurred.
Claims from medical errors that occurred before the pandemic bring additional uncertainties. In 2020, we saw fewer than expected overall claims filed from events occurring 18-24 months before the pandemic. In total, 20% fewer claims were filed than in 2019. This may have had to do with courts shutting down, people being reluctant to meet with attorneys to discuss a claim, and/or lawyers working from home. We may see these claims filed later than expected, or maybe we won’t see them at all.
But without a doubt, pandemic-related claims will be filed. The pandemic’s impact on physicians increases the risk of claims. Burnout is a major cause of medical errors, and a recent study found that out of 60 countries, U.S. health care providers showed the highest rates of burnout. We’re concerned about the stress affecting physicians’ performance – not just the physical stress of the demands put on them while treating COVID-19 patients, but all of the worry. For instance, a lot of doctors at the start of this pandemic stayed at hotels because they didn’t want to bring the virus home to their families – if they got exposed. Those sorts of stressors from life disruptions, on top of the stress of treating COVID-19 patients and the stress of treating non–COVID-19 patients within overtaxed health care systems, contribute to the possibilities for error.
Immunity protections are not fail-safe
And while health care providers have medical liability protections during the pandemic, these protections may not prevent claims. Health care provider pandemic-related liability laws vary from state to state, and they will be tested in the courts as to whether they’re constitutional. For example, there is pending legislation in New York state that would repeal the provider protections created there at the start of the pandemic. Further, some expert witnesses will couch their statements in terms of what it takes to get around one of these statutes. Therefore, physicians do have reason for concern, even in states with strong liability protections.
The following case example, which is one of about 40 COVID-19–related claims made against our members so far, is a poster child for why these protections are necessary: A quadriplegic patient with COVID-19 had reached the point of organ failure before he reached the ED. There was really nothing medical science could do for him at that point, in terms of a chance at recovery. Therefore, the patient’s physician and conservator placed him in assisted living for palliative care. This was a sad but reasonable decision during a pandemic, with hospital beds needed for patients with a shot at surviving. Following that patient’s death, the physician is being sued.
Defending claims regarding treatment vs. regarding infection control
We are very confident in our ability to protect our members against claims where they are being sued over the treatment of the disease. Claims arising out of treatment are not concerning to us because there is no cure for COVID-19 – one can only treat the symptoms as the virus runs its course.
On the other hand, suits harder to defend would be those that revolve around transmitting the disease because providers didn’t follow guidelines from the Centers for Disease Control and Prevention or there wasn’t enough personal protective equipment. That’s why we stress the importance of following CDC guidelines, and why we’ve taken proactive steps to communicate with the entire medical community throughout the pandemic as part of our commitment to serve those who provide care.
Mr. White is chief operating officer at The Doctors Company. The guidelines suggested here are not rules, do not constitute legal advice, and do not ensure a successful outcome. The ultimate decision regarding the appropriateness of any treatment must be made by each health care provider considering the circumstances of the individual situation and in accordance with the laws of the jurisdiction in which the care is rendered.







