User login
Akathisia: “Ants in the Pants”
Potentially poor outcome if untreated
Case
The patient is a 65-year-old female with increasing anxiety and agitation. She completed cycle 2 of chemotherapy for breast cancer several hours ago. Her premedication was Reglan (metoclopramide); her only other medication is tamoxifen. Other than breast cancer, she suffers only from osteoarthritis.
She is found pacing about the ward – almost uncontrollably. She feels she must move, only to have to stop and, shortly afterwards, feels the urge to move again. This has never happened to her before. She must move despite being fatigued. She also complains of an odd overall feeling; something akin to “ant in the pants.” She is nervous and exhausted. What is her diagnosis and what clues to it are in her presentation?
Background
The word “akathisia” is derived from the Greek language and means “unable to sit.” It is thought to occur as a consequence of dopaminergic blockade in the midbrain region. The decrease in dopaminergic activity leads to a subsequent decrease in inhibitory motor control which, in turn, manifests as involuntary movements.
In this malady, the patient is seen as perpetually in motion. The patient feels the need to move until they must stop. But once static, they have the urge to move again. They pace, they rock and they ‘fidget’ – they just cannot sit still. This feeling has been likened to having “ants in the pants.” Patients become anxious, agitated, and suffer from insomnia. They cannot rest.
If left unresolved akathisia can torment patients to sheer exhaustion. For some it serves as a harbinger of suicide. This toxicity is more commonly seen in the psychiatric pharmacy with the most common offender being haloperidol. The causative agents of the least notoriety are the non-antipsychotics.
Diagnosis and treatment
Akathisia is an extrapyramidal symptom found largely but NOT exclusively with psychiatric medications. There are drugs in the non-psychiatric field that can also cause it, including antiemetics (e.g., metoclopramide), antihypertensives (e.g., diltiazem), and narcotics (e.g., cocaine). Metoclopramide is given under circumstances ranging from diabetic gastroparesis to premedicating chemotherapy. It is a peripheral and centrally acting dopamine antagonist. There are no lab tests or radiographic workups to diagnose akathisia. Its manifestations are erratic and disturbing, and the prognosis is doleful if unresolved.
The primary intervention for the treatment of akathisia is its recognition and the discontinuation of the offending drug. Beyond this, for symptomatic care, there is a compendium of case reports and small studies supporting many drugs, but only a few have received consistent recommendation. Beta-adrenergic antagonists, such as propranolol, are considered the gold standard, the first choice for the treatment of akathisia. Their toxicities include orthostatic hypotension and bradycardia. Additionally, they are contraindicated in the setting of asthma.
Anticholinergics, such as benztropine (cogentin) and trihexylphenidyl (artane) are considered in the literature as 2nd line treatments, behind beta-blockers. However, the data advocating their use is limited. They have multiple side-effects including sedation, memory impairment, visual impairment, and urinary retention. They are also contraindicated in patients with closed-angle glaucoma.
An equivalent alternative to beta-blockers could also be the 5HT2a receptor antagonists such as mirtazapine (remeron) and cyproheptadine (periactin). This class of medications is thought to act by an inhibitory control of dopaminergic neurons. Sedation and weight gain are the primary toxicities, and they are contraindicated in patients who are breastfeeding.
Benzodiazepines, such as clonazepam (klonopin), have shown some efficacy in improving symptoms but the data is very limited. The risk of tolerance and dependence, coupled with the problems of sedation impacting the elderly, prompts their placement in reserve. Vitamin B6 (pyridoxine), when given in a high dose format, causes significant improvement in akathisia. However, it can cause headache and nausea. Chronic administration of high doses has also been found to cause a severe and irreversible sensory neuropathy as well as lead to seizures. Many other agents have been studied, but the data are too small to warrant recommendation.
Conclusion
Akathisia remains an extreme reaction to drugs not always in the psychotropic class. The hospitalist will likely deal with the acute onset, a dramatic form, and a potentially poor outcome if untreated. The patient’s only true defense is the physician’s clinical acumen and their ability to recognize it.
Dr. Killeen is a physician in Tampa, Fla. He practices internal medicine, hematology, and oncology, and has worked in hospice and hospital medicine.
Recommended reading
Van Gool AR, Doorduijn JK, Sevnaeve C. Severe akathisia as a side effect of metoclopramide. Pharm World Sci. 2010; 32(6):704-706.
Loonen AJM, Stahl SM. The mechanism of drug-induced akathisia. CNS Spectr. 2010;15(11):491-494.
Forcen FE, Matsoukas K, Alici Y. Antipsychotic-induced akathisia in delirium: A systemic review. Palliat Support Care. 2016;14(1):77-84.
Sethuram K, Gedzior J. Akathisia: Case presentation and review of newer treatment agents. Psychiatric Annals. 2014;44(8):391-396.
Pringsheim T, et al. The assessment and treatment of antipsychotic-induced akathisia. Can J Psychiatry. 2018;63(11): 719-729.
Tachere RO, Mandana M. Beyond anxiety and agitation: A clinical approach to akathisia. Royal Australian Coll Gen Practitioners. 2017;46(5): 296-298.
Key points
- Although associated more with psychiatric medications, akathisia can occur with non-psychotropics as well.
- To recognize the illness, the clinician must notice the repetitive involuntary movements and pacing as well as the “ants in the pants” fidgeting involved.
- Primary treatment consists of medication discontinuation with pharmaceutical intervention as a backup.
- Recognition is the key to successful treatment.
Classic signs of akathisia
- Fidgeting – “ants in the pants”
- Swinging the legs while seated
- Rocking from foot to foot
- Walking while in a static position
- Inability to sit or stand still – pacing
- Onset appears with the initiation or dose adjustment of an offending drug
Quiz
1. Which of the following findings occur in Akathisia?
A. Fidgeting
B. Pacing
C. Swinging the legs while seated
D. All the above
Answer: D
Akathisia is manifest as involuntary hyperactivity of the extremities, particularly the lower extremities. People feel the urge to move, to continue endlessly in motion, stopping only when fatigue sets in. The fidgeting has been described by patients as feeling like “ants in the pants.”
2. Which of the following interventions are used to treat akathisia?
A. Drug discontinuation
B. Propranolol
C. Mirtazapine
D. All the above
Answer: D
All the interventions mentioned are used to treat akathisia. The foremost is to stop the offending drug. Failing this, propranolol is the “gold standard” while 5HT2a antagonists, such as mirtazapine, are favored when beta-blockers either fail or are contraindicated.
3. The use of pyridoxine (Vitamin B6) in the treatment of akathisia is associated with what toxicities?
A. Headache
B. Nausea
C. Seizures
D. All the above
Answer: D
The use of Vitamin B6 in the treatment of akathisia has several drawbacks. Its administration is associated with headache and nausea, and high dose usage increases the risk of seizure.
4. If unresolved, akathisia can lead to which of the following?
A. Insomnia
B. Suicide
C. Physical exhaustion
D. All the above
Answer: D
Akathisia, left unrecognized and untreated, can eventually lead to physical exhaustion, and is compounded by difficulties in trying to rest, hence insomnia. The physical and mental torment of this malady can lead to suicide.
Potentially poor outcome if untreated
Potentially poor outcome if untreated
Case
The patient is a 65-year-old female with increasing anxiety and agitation. She completed cycle 2 of chemotherapy for breast cancer several hours ago. Her premedication was Reglan (metoclopramide); her only other medication is tamoxifen. Other than breast cancer, she suffers only from osteoarthritis.
She is found pacing about the ward – almost uncontrollably. She feels she must move, only to have to stop and, shortly afterwards, feels the urge to move again. This has never happened to her before. She must move despite being fatigued. She also complains of an odd overall feeling; something akin to “ant in the pants.” She is nervous and exhausted. What is her diagnosis and what clues to it are in her presentation?
Background
The word “akathisia” is derived from the Greek language and means “unable to sit.” It is thought to occur as a consequence of dopaminergic blockade in the midbrain region. The decrease in dopaminergic activity leads to a subsequent decrease in inhibitory motor control which, in turn, manifests as involuntary movements.
In this malady, the patient is seen as perpetually in motion. The patient feels the need to move until they must stop. But once static, they have the urge to move again. They pace, they rock and they ‘fidget’ – they just cannot sit still. This feeling has been likened to having “ants in the pants.” Patients become anxious, agitated, and suffer from insomnia. They cannot rest.
If left unresolved akathisia can torment patients to sheer exhaustion. For some it serves as a harbinger of suicide. This toxicity is more commonly seen in the psychiatric pharmacy with the most common offender being haloperidol. The causative agents of the least notoriety are the non-antipsychotics.
Diagnosis and treatment
Akathisia is an extrapyramidal symptom found largely but NOT exclusively with psychiatric medications. There are drugs in the non-psychiatric field that can also cause it, including antiemetics (e.g., metoclopramide), antihypertensives (e.g., diltiazem), and narcotics (e.g., cocaine). Metoclopramide is given under circumstances ranging from diabetic gastroparesis to premedicating chemotherapy. It is a peripheral and centrally acting dopamine antagonist. There are no lab tests or radiographic workups to diagnose akathisia. Its manifestations are erratic and disturbing, and the prognosis is doleful if unresolved.
The primary intervention for the treatment of akathisia is its recognition and the discontinuation of the offending drug. Beyond this, for symptomatic care, there is a compendium of case reports and small studies supporting many drugs, but only a few have received consistent recommendation. Beta-adrenergic antagonists, such as propranolol, are considered the gold standard, the first choice for the treatment of akathisia. Their toxicities include orthostatic hypotension and bradycardia. Additionally, they are contraindicated in the setting of asthma.
Anticholinergics, such as benztropine (cogentin) and trihexylphenidyl (artane) are considered in the literature as 2nd line treatments, behind beta-blockers. However, the data advocating their use is limited. They have multiple side-effects including sedation, memory impairment, visual impairment, and urinary retention. They are also contraindicated in patients with closed-angle glaucoma.
An equivalent alternative to beta-blockers could also be the 5HT2a receptor antagonists such as mirtazapine (remeron) and cyproheptadine (periactin). This class of medications is thought to act by an inhibitory control of dopaminergic neurons. Sedation and weight gain are the primary toxicities, and they are contraindicated in patients who are breastfeeding.
Benzodiazepines, such as clonazepam (klonopin), have shown some efficacy in improving symptoms but the data is very limited. The risk of tolerance and dependence, coupled with the problems of sedation impacting the elderly, prompts their placement in reserve. Vitamin B6 (pyridoxine), when given in a high dose format, causes significant improvement in akathisia. However, it can cause headache and nausea. Chronic administration of high doses has also been found to cause a severe and irreversible sensory neuropathy as well as lead to seizures. Many other agents have been studied, but the data are too small to warrant recommendation.
Conclusion
Akathisia remains an extreme reaction to drugs not always in the psychotropic class. The hospitalist will likely deal with the acute onset, a dramatic form, and a potentially poor outcome if untreated. The patient’s only true defense is the physician’s clinical acumen and their ability to recognize it.
Dr. Killeen is a physician in Tampa, Fla. He practices internal medicine, hematology, and oncology, and has worked in hospice and hospital medicine.
Recommended reading
Van Gool AR, Doorduijn JK, Sevnaeve C. Severe akathisia as a side effect of metoclopramide. Pharm World Sci. 2010; 32(6):704-706.
Loonen AJM, Stahl SM. The mechanism of drug-induced akathisia. CNS Spectr. 2010;15(11):491-494.
Forcen FE, Matsoukas K, Alici Y. Antipsychotic-induced akathisia in delirium: A systemic review. Palliat Support Care. 2016;14(1):77-84.
Sethuram K, Gedzior J. Akathisia: Case presentation and review of newer treatment agents. Psychiatric Annals. 2014;44(8):391-396.
Pringsheim T, et al. The assessment and treatment of antipsychotic-induced akathisia. Can J Psychiatry. 2018;63(11): 719-729.
Tachere RO, Mandana M. Beyond anxiety and agitation: A clinical approach to akathisia. Royal Australian Coll Gen Practitioners. 2017;46(5): 296-298.
Key points
- Although associated more with psychiatric medications, akathisia can occur with non-psychotropics as well.
- To recognize the illness, the clinician must notice the repetitive involuntary movements and pacing as well as the “ants in the pants” fidgeting involved.
- Primary treatment consists of medication discontinuation with pharmaceutical intervention as a backup.
- Recognition is the key to successful treatment.
Classic signs of akathisia
- Fidgeting – “ants in the pants”
- Swinging the legs while seated
- Rocking from foot to foot
- Walking while in a static position
- Inability to sit or stand still – pacing
- Onset appears with the initiation or dose adjustment of an offending drug
Quiz
1. Which of the following findings occur in Akathisia?
A. Fidgeting
B. Pacing
C. Swinging the legs while seated
D. All the above
Answer: D
Akathisia is manifest as involuntary hyperactivity of the extremities, particularly the lower extremities. People feel the urge to move, to continue endlessly in motion, stopping only when fatigue sets in. The fidgeting has been described by patients as feeling like “ants in the pants.”
2. Which of the following interventions are used to treat akathisia?
A. Drug discontinuation
B. Propranolol
C. Mirtazapine
D. All the above
Answer: D
All the interventions mentioned are used to treat akathisia. The foremost is to stop the offending drug. Failing this, propranolol is the “gold standard” while 5HT2a antagonists, such as mirtazapine, are favored when beta-blockers either fail or are contraindicated.
3. The use of pyridoxine (Vitamin B6) in the treatment of akathisia is associated with what toxicities?
A. Headache
B. Nausea
C. Seizures
D. All the above
Answer: D
The use of Vitamin B6 in the treatment of akathisia has several drawbacks. Its administration is associated with headache and nausea, and high dose usage increases the risk of seizure.
4. If unresolved, akathisia can lead to which of the following?
A. Insomnia
B. Suicide
C. Physical exhaustion
D. All the above
Answer: D
Akathisia, left unrecognized and untreated, can eventually lead to physical exhaustion, and is compounded by difficulties in trying to rest, hence insomnia. The physical and mental torment of this malady can lead to suicide.
Case
The patient is a 65-year-old female with increasing anxiety and agitation. She completed cycle 2 of chemotherapy for breast cancer several hours ago. Her premedication was Reglan (metoclopramide); her only other medication is tamoxifen. Other than breast cancer, she suffers only from osteoarthritis.
She is found pacing about the ward – almost uncontrollably. She feels she must move, only to have to stop and, shortly afterwards, feels the urge to move again. This has never happened to her before. She must move despite being fatigued. She also complains of an odd overall feeling; something akin to “ant in the pants.” She is nervous and exhausted. What is her diagnosis and what clues to it are in her presentation?
Background
The word “akathisia” is derived from the Greek language and means “unable to sit.” It is thought to occur as a consequence of dopaminergic blockade in the midbrain region. The decrease in dopaminergic activity leads to a subsequent decrease in inhibitory motor control which, in turn, manifests as involuntary movements.
In this malady, the patient is seen as perpetually in motion. The patient feels the need to move until they must stop. But once static, they have the urge to move again. They pace, they rock and they ‘fidget’ – they just cannot sit still. This feeling has been likened to having “ants in the pants.” Patients become anxious, agitated, and suffer from insomnia. They cannot rest.
If left unresolved akathisia can torment patients to sheer exhaustion. For some it serves as a harbinger of suicide. This toxicity is more commonly seen in the psychiatric pharmacy with the most common offender being haloperidol. The causative agents of the least notoriety are the non-antipsychotics.
Diagnosis and treatment
Akathisia is an extrapyramidal symptom found largely but NOT exclusively with psychiatric medications. There are drugs in the non-psychiatric field that can also cause it, including antiemetics (e.g., metoclopramide), antihypertensives (e.g., diltiazem), and narcotics (e.g., cocaine). Metoclopramide is given under circumstances ranging from diabetic gastroparesis to premedicating chemotherapy. It is a peripheral and centrally acting dopamine antagonist. There are no lab tests or radiographic workups to diagnose akathisia. Its manifestations are erratic and disturbing, and the prognosis is doleful if unresolved.
The primary intervention for the treatment of akathisia is its recognition and the discontinuation of the offending drug. Beyond this, for symptomatic care, there is a compendium of case reports and small studies supporting many drugs, but only a few have received consistent recommendation. Beta-adrenergic antagonists, such as propranolol, are considered the gold standard, the first choice for the treatment of akathisia. Their toxicities include orthostatic hypotension and bradycardia. Additionally, they are contraindicated in the setting of asthma.
Anticholinergics, such as benztropine (cogentin) and trihexylphenidyl (artane) are considered in the literature as 2nd line treatments, behind beta-blockers. However, the data advocating their use is limited. They have multiple side-effects including sedation, memory impairment, visual impairment, and urinary retention. They are also contraindicated in patients with closed-angle glaucoma.
An equivalent alternative to beta-blockers could also be the 5HT2a receptor antagonists such as mirtazapine (remeron) and cyproheptadine (periactin). This class of medications is thought to act by an inhibitory control of dopaminergic neurons. Sedation and weight gain are the primary toxicities, and they are contraindicated in patients who are breastfeeding.
Benzodiazepines, such as clonazepam (klonopin), have shown some efficacy in improving symptoms but the data is very limited. The risk of tolerance and dependence, coupled with the problems of sedation impacting the elderly, prompts their placement in reserve. Vitamin B6 (pyridoxine), when given in a high dose format, causes significant improvement in akathisia. However, it can cause headache and nausea. Chronic administration of high doses has also been found to cause a severe and irreversible sensory neuropathy as well as lead to seizures. Many other agents have been studied, but the data are too small to warrant recommendation.
Conclusion
Akathisia remains an extreme reaction to drugs not always in the psychotropic class. The hospitalist will likely deal with the acute onset, a dramatic form, and a potentially poor outcome if untreated. The patient’s only true defense is the physician’s clinical acumen and their ability to recognize it.
Dr. Killeen is a physician in Tampa, Fla. He practices internal medicine, hematology, and oncology, and has worked in hospice and hospital medicine.
Recommended reading
Van Gool AR, Doorduijn JK, Sevnaeve C. Severe akathisia as a side effect of metoclopramide. Pharm World Sci. 2010; 32(6):704-706.
Loonen AJM, Stahl SM. The mechanism of drug-induced akathisia. CNS Spectr. 2010;15(11):491-494.
Forcen FE, Matsoukas K, Alici Y. Antipsychotic-induced akathisia in delirium: A systemic review. Palliat Support Care. 2016;14(1):77-84.
Sethuram K, Gedzior J. Akathisia: Case presentation and review of newer treatment agents. Psychiatric Annals. 2014;44(8):391-396.
Pringsheim T, et al. The assessment and treatment of antipsychotic-induced akathisia. Can J Psychiatry. 2018;63(11): 719-729.
Tachere RO, Mandana M. Beyond anxiety and agitation: A clinical approach to akathisia. Royal Australian Coll Gen Practitioners. 2017;46(5): 296-298.
Key points
- Although associated more with psychiatric medications, akathisia can occur with non-psychotropics as well.
- To recognize the illness, the clinician must notice the repetitive involuntary movements and pacing as well as the “ants in the pants” fidgeting involved.
- Primary treatment consists of medication discontinuation with pharmaceutical intervention as a backup.
- Recognition is the key to successful treatment.
Classic signs of akathisia
- Fidgeting – “ants in the pants”
- Swinging the legs while seated
- Rocking from foot to foot
- Walking while in a static position
- Inability to sit or stand still – pacing
- Onset appears with the initiation or dose adjustment of an offending drug
Quiz
1. Which of the following findings occur in Akathisia?
A. Fidgeting
B. Pacing
C. Swinging the legs while seated
D. All the above
Answer: D
Akathisia is manifest as involuntary hyperactivity of the extremities, particularly the lower extremities. People feel the urge to move, to continue endlessly in motion, stopping only when fatigue sets in. The fidgeting has been described by patients as feeling like “ants in the pants.”
2. Which of the following interventions are used to treat akathisia?
A. Drug discontinuation
B. Propranolol
C. Mirtazapine
D. All the above
Answer: D
All the interventions mentioned are used to treat akathisia. The foremost is to stop the offending drug. Failing this, propranolol is the “gold standard” while 5HT2a antagonists, such as mirtazapine, are favored when beta-blockers either fail or are contraindicated.
3. The use of pyridoxine (Vitamin B6) in the treatment of akathisia is associated with what toxicities?
A. Headache
B. Nausea
C. Seizures
D. All the above
Answer: D
The use of Vitamin B6 in the treatment of akathisia has several drawbacks. Its administration is associated with headache and nausea, and high dose usage increases the risk of seizure.
4. If unresolved, akathisia can lead to which of the following?
A. Insomnia
B. Suicide
C. Physical exhaustion
D. All the above
Answer: D
Akathisia, left unrecognized and untreated, can eventually lead to physical exhaustion, and is compounded by difficulties in trying to rest, hence insomnia. The physical and mental torment of this malady can lead to suicide.
More from DAPA-HF: Dapagliflozin quickly reduces heart failure events
Dapagliflozin’s benefits in patients with heart failure with reduced ejection fraction appeared quickly after treatment began, and patients who had been hospitalized for heart failure within the prior year got the biggest boost from the drug, according to secondary analyses of the more than 4,700-patient DAPA-HF trial.
Dapagliflozin’s significant reduction of the incidence of cardiovascular death or worsening heart failure became apparent in DAPA-HF within 28 days after patients started treatment, by which time those on the study drug had a 49% cut in this combined endpoint, compared with patients on placebo, David D. Berg, MD, and associates said in a recent report published in JAMA Cardiology.
Their analyses also showed that the absolute reduction linked with dapagliflozin treatment for this primary endpoint of the study (which classified worsening heart failure as either hospitalization for heart failure or an urgent visit because of heart failure that required intravenous therapy) was greatest, 10% during 2 years of follow-up, among the roughly one-quarter of enrolled patients who had been hospitalized for heart failure within 12 months of entering the study. Patients previously hospitalized for heart failure more than 12 months before they entered DAPA-HF had a 4% absolute cut in their primary-outcome events during the trial, and those who had never been hospitalized for heart failure had a 2% absolute benefit, compared with placebo, during 2 years of follow-up.
These findings were consistent with the timing of benefits for patients with heart failure with reduced ejection fraction (HFrEF) in recent studies of two other drugs from the same class, the sodium-glucose cotransporter (SGLT) inhibitors, including empagliflozin (Jardiance, which inhibits SGLT-2) in the EMPEROR-Reduced trial, and sotagliflozin (Zynquista, which inhibits both SGLT1 and -2) in the SOLOIST-WHF trial, noted Gregg C. Fonarow, MD, and Clyde W. Yancy, MD, in an editor’s note that accompanied the new report.
The new findings show “the opportunity to expeditiously implement this remarkable class of therapy for HFrEF is now compelling and deserves disruptive efforts to ensure comprehensive treatment and the best patient outcomes,” wrote Dr. Fonarow, a professor of medicine at the University of California, Los Angeles, and Dr. Yancy, a professor of medicine at Northwestern University, Chicago.
But despite these new findings, their exact meaning remains unclear in terms of when to start dapagliflozin (or a different drug from the same class), compared with the other drug classes that have proven highly effective in patients with HFrEF, and exactly how long after hospitalization for heart failure dapagliflozin can safely and effectively begin.
Data needed on starting an SGLT inhibitor soon after hospitalization in patients without diabetes
“DAPA-HF showed that, in patients with or without diabetes, an SGLT2 inhibitor reduced the risk of cardiovascular death or worsening heart failure in patients with stable HFrEF. SOLOIST-WHF looked strictly at patients with diabetes, and showed that a combined SGLT1 and SGLT2 inhibitor could reduce the risk of cardiovascular death or worsening heart failure in patients with recently decompensated heart failure,” Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston, noted in an interview. “What we don’t have is a trial focused exclusively on enrolling patients while hospitalized with acute heart failure, irrespective of whether they have diabetes, and testing the immediate clinical efficacy and safety of starting an SGLT2 inhibitor. That is what we are testing with the ongoing DAPA ACT HF-TIMI 68 trial.”
In addition, updated recommendations from the American College of Cardiology on initiating drug therapy in patients newly diagnosed with HFrEF that appeared in early 2021 promoted a sequence that starts most patients on sacubitril/valsartan (Entresto) and a beta-blocker, followed by a diuretic (when needed), a mineralocorticoid receptor agonist, and then an SGLT inhibitor. The recommendations note that starting a patient on all these drug classes could take 3-6 months.
“There are intense debates about the optimal sequence for introducing these therapies, and I don’t think we have solid data to suggest that one sequence is clearly better than another,” noted Dr. Berg. “A one-size-fits-all approach probably doesn’t make sense. For example, each of these therapies has a different set of effects on heart rate and blood pressure, and each has a unique side effect profile, so clinicians will often need to tailor the treatment approach to the patient. And, of course, cost is an important consideration. Although the optimal time to start an SGLT2 inhibitor remains uncertain, the results of our analysis suggest that waiting may result in preventable adverse heart failure events.”
DAPA-HF randomized 4,744 patients with HFrEF and in New York Heart Association functional class II-IV at 410 sites in 20 countries. The incidence of the primary, combined endpoint fell by 26% with dapagliflozin treatment, compared with placebo, during a median 18-month follow-up. Among the study cohort 27% of patients had been hospitalized for heart failure within a year of their entry, 20% had been hospitalized for heart failure more than 1 year before entry, and 53% had no history of a hospitalization for heart failure.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Berg has received research support through his institution from AstraZeneca. Dr. Fonarow has received personal fees from AstraZeneca and from numerous other companies. Dr. Yancy’s spouse works for Abbott Laboratories.
Dapagliflozin’s benefits in patients with heart failure with reduced ejection fraction appeared quickly after treatment began, and patients who had been hospitalized for heart failure within the prior year got the biggest boost from the drug, according to secondary analyses of the more than 4,700-patient DAPA-HF trial.
Dapagliflozin’s significant reduction of the incidence of cardiovascular death or worsening heart failure became apparent in DAPA-HF within 28 days after patients started treatment, by which time those on the study drug had a 49% cut in this combined endpoint, compared with patients on placebo, David D. Berg, MD, and associates said in a recent report published in JAMA Cardiology.
Their analyses also showed that the absolute reduction linked with dapagliflozin treatment for this primary endpoint of the study (which classified worsening heart failure as either hospitalization for heart failure or an urgent visit because of heart failure that required intravenous therapy) was greatest, 10% during 2 years of follow-up, among the roughly one-quarter of enrolled patients who had been hospitalized for heart failure within 12 months of entering the study. Patients previously hospitalized for heart failure more than 12 months before they entered DAPA-HF had a 4% absolute cut in their primary-outcome events during the trial, and those who had never been hospitalized for heart failure had a 2% absolute benefit, compared with placebo, during 2 years of follow-up.
These findings were consistent with the timing of benefits for patients with heart failure with reduced ejection fraction (HFrEF) in recent studies of two other drugs from the same class, the sodium-glucose cotransporter (SGLT) inhibitors, including empagliflozin (Jardiance, which inhibits SGLT-2) in the EMPEROR-Reduced trial, and sotagliflozin (Zynquista, which inhibits both SGLT1 and -2) in the SOLOIST-WHF trial, noted Gregg C. Fonarow, MD, and Clyde W. Yancy, MD, in an editor’s note that accompanied the new report.
The new findings show “the opportunity to expeditiously implement this remarkable class of therapy for HFrEF is now compelling and deserves disruptive efforts to ensure comprehensive treatment and the best patient outcomes,” wrote Dr. Fonarow, a professor of medicine at the University of California, Los Angeles, and Dr. Yancy, a professor of medicine at Northwestern University, Chicago.
But despite these new findings, their exact meaning remains unclear in terms of when to start dapagliflozin (or a different drug from the same class), compared with the other drug classes that have proven highly effective in patients with HFrEF, and exactly how long after hospitalization for heart failure dapagliflozin can safely and effectively begin.
Data needed on starting an SGLT inhibitor soon after hospitalization in patients without diabetes
“DAPA-HF showed that, in patients with or without diabetes, an SGLT2 inhibitor reduced the risk of cardiovascular death or worsening heart failure in patients with stable HFrEF. SOLOIST-WHF looked strictly at patients with diabetes, and showed that a combined SGLT1 and SGLT2 inhibitor could reduce the risk of cardiovascular death or worsening heart failure in patients with recently decompensated heart failure,” Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston, noted in an interview. “What we don’t have is a trial focused exclusively on enrolling patients while hospitalized with acute heart failure, irrespective of whether they have diabetes, and testing the immediate clinical efficacy and safety of starting an SGLT2 inhibitor. That is what we are testing with the ongoing DAPA ACT HF-TIMI 68 trial.”
In addition, updated recommendations from the American College of Cardiology on initiating drug therapy in patients newly diagnosed with HFrEF that appeared in early 2021 promoted a sequence that starts most patients on sacubitril/valsartan (Entresto) and a beta-blocker, followed by a diuretic (when needed), a mineralocorticoid receptor agonist, and then an SGLT inhibitor. The recommendations note that starting a patient on all these drug classes could take 3-6 months.
“There are intense debates about the optimal sequence for introducing these therapies, and I don’t think we have solid data to suggest that one sequence is clearly better than another,” noted Dr. Berg. “A one-size-fits-all approach probably doesn’t make sense. For example, each of these therapies has a different set of effects on heart rate and blood pressure, and each has a unique side effect profile, so clinicians will often need to tailor the treatment approach to the patient. And, of course, cost is an important consideration. Although the optimal time to start an SGLT2 inhibitor remains uncertain, the results of our analysis suggest that waiting may result in preventable adverse heart failure events.”
DAPA-HF randomized 4,744 patients with HFrEF and in New York Heart Association functional class II-IV at 410 sites in 20 countries. The incidence of the primary, combined endpoint fell by 26% with dapagliflozin treatment, compared with placebo, during a median 18-month follow-up. Among the study cohort 27% of patients had been hospitalized for heart failure within a year of their entry, 20% had been hospitalized for heart failure more than 1 year before entry, and 53% had no history of a hospitalization for heart failure.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Berg has received research support through his institution from AstraZeneca. Dr. Fonarow has received personal fees from AstraZeneca and from numerous other companies. Dr. Yancy’s spouse works for Abbott Laboratories.
Dapagliflozin’s benefits in patients with heart failure with reduced ejection fraction appeared quickly after treatment began, and patients who had been hospitalized for heart failure within the prior year got the biggest boost from the drug, according to secondary analyses of the more than 4,700-patient DAPA-HF trial.
Dapagliflozin’s significant reduction of the incidence of cardiovascular death or worsening heart failure became apparent in DAPA-HF within 28 days after patients started treatment, by which time those on the study drug had a 49% cut in this combined endpoint, compared with patients on placebo, David D. Berg, MD, and associates said in a recent report published in JAMA Cardiology.
Their analyses also showed that the absolute reduction linked with dapagliflozin treatment for this primary endpoint of the study (which classified worsening heart failure as either hospitalization for heart failure or an urgent visit because of heart failure that required intravenous therapy) was greatest, 10% during 2 years of follow-up, among the roughly one-quarter of enrolled patients who had been hospitalized for heart failure within 12 months of entering the study. Patients previously hospitalized for heart failure more than 12 months before they entered DAPA-HF had a 4% absolute cut in their primary-outcome events during the trial, and those who had never been hospitalized for heart failure had a 2% absolute benefit, compared with placebo, during 2 years of follow-up.
These findings were consistent with the timing of benefits for patients with heart failure with reduced ejection fraction (HFrEF) in recent studies of two other drugs from the same class, the sodium-glucose cotransporter (SGLT) inhibitors, including empagliflozin (Jardiance, which inhibits SGLT-2) in the EMPEROR-Reduced trial, and sotagliflozin (Zynquista, which inhibits both SGLT1 and -2) in the SOLOIST-WHF trial, noted Gregg C. Fonarow, MD, and Clyde W. Yancy, MD, in an editor’s note that accompanied the new report.
The new findings show “the opportunity to expeditiously implement this remarkable class of therapy for HFrEF is now compelling and deserves disruptive efforts to ensure comprehensive treatment and the best patient outcomes,” wrote Dr. Fonarow, a professor of medicine at the University of California, Los Angeles, and Dr. Yancy, a professor of medicine at Northwestern University, Chicago.
But despite these new findings, their exact meaning remains unclear in terms of when to start dapagliflozin (or a different drug from the same class), compared with the other drug classes that have proven highly effective in patients with HFrEF, and exactly how long after hospitalization for heart failure dapagliflozin can safely and effectively begin.
Data needed on starting an SGLT inhibitor soon after hospitalization in patients without diabetes
“DAPA-HF showed that, in patients with or without diabetes, an SGLT2 inhibitor reduced the risk of cardiovascular death or worsening heart failure in patients with stable HFrEF. SOLOIST-WHF looked strictly at patients with diabetes, and showed that a combined SGLT1 and SGLT2 inhibitor could reduce the risk of cardiovascular death or worsening heart failure in patients with recently decompensated heart failure,” Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston, noted in an interview. “What we don’t have is a trial focused exclusively on enrolling patients while hospitalized with acute heart failure, irrespective of whether they have diabetes, and testing the immediate clinical efficacy and safety of starting an SGLT2 inhibitor. That is what we are testing with the ongoing DAPA ACT HF-TIMI 68 trial.”
In addition, updated recommendations from the American College of Cardiology on initiating drug therapy in patients newly diagnosed with HFrEF that appeared in early 2021 promoted a sequence that starts most patients on sacubitril/valsartan (Entresto) and a beta-blocker, followed by a diuretic (when needed), a mineralocorticoid receptor agonist, and then an SGLT inhibitor. The recommendations note that starting a patient on all these drug classes could take 3-6 months.
“There are intense debates about the optimal sequence for introducing these therapies, and I don’t think we have solid data to suggest that one sequence is clearly better than another,” noted Dr. Berg. “A one-size-fits-all approach probably doesn’t make sense. For example, each of these therapies has a different set of effects on heart rate and blood pressure, and each has a unique side effect profile, so clinicians will often need to tailor the treatment approach to the patient. And, of course, cost is an important consideration. Although the optimal time to start an SGLT2 inhibitor remains uncertain, the results of our analysis suggest that waiting may result in preventable adverse heart failure events.”
DAPA-HF randomized 4,744 patients with HFrEF and in New York Heart Association functional class II-IV at 410 sites in 20 countries. The incidence of the primary, combined endpoint fell by 26% with dapagliflozin treatment, compared with placebo, during a median 18-month follow-up. Among the study cohort 27% of patients had been hospitalized for heart failure within a year of their entry, 20% had been hospitalized for heart failure more than 1 year before entry, and 53% had no history of a hospitalization for heart failure.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Berg has received research support through his institution from AstraZeneca. Dr. Fonarow has received personal fees from AstraZeneca and from numerous other companies. Dr. Yancy’s spouse works for Abbott Laboratories.
FROM JAMA CARDIOLOGY
Ivabradine knocks down heart rate, symptoms in POTS
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
Quick byte: Curing diabetes
Harvard biologist Doug Melton, PhD, is exploring the use of stem cells to create replacement beta cells that produce insulin, according to Time magazine.
In 2014, he co-founded Semma Therapeutics to develop the technology, which was acquired by Vertex Pharmaceuticals.
“The company has created a small, implantable device that holds millions of replacement beta cells, letting glucose and insulin through but keeping immune cells out. ‘If it works in people as well as it does in animals, it’s possible that people will not be diabetic,’ said Dr. Melton, co-director of the Harvard Stem Cell Institute and an investigator of the Howard Hughes Medical Institute. ‘They will eat and drink and play like those of us who are not.’”
Reference
Steinberg D. 12 innovations that will change health care and medicine in the 2020s. Time. 2019 Oct 25. https://time.com/5710295/top-health-innovations/ Accessed Dec 5, 2019.
Harvard biologist Doug Melton, PhD, is exploring the use of stem cells to create replacement beta cells that produce insulin, according to Time magazine.
In 2014, he co-founded Semma Therapeutics to develop the technology, which was acquired by Vertex Pharmaceuticals.
“The company has created a small, implantable device that holds millions of replacement beta cells, letting glucose and insulin through but keeping immune cells out. ‘If it works in people as well as it does in animals, it’s possible that people will not be diabetic,’ said Dr. Melton, co-director of the Harvard Stem Cell Institute and an investigator of the Howard Hughes Medical Institute. ‘They will eat and drink and play like those of us who are not.’”
Reference
Steinberg D. 12 innovations that will change health care and medicine in the 2020s. Time. 2019 Oct 25. https://time.com/5710295/top-health-innovations/ Accessed Dec 5, 2019.
Harvard biologist Doug Melton, PhD, is exploring the use of stem cells to create replacement beta cells that produce insulin, according to Time magazine.
In 2014, he co-founded Semma Therapeutics to develop the technology, which was acquired by Vertex Pharmaceuticals.
“The company has created a small, implantable device that holds millions of replacement beta cells, letting glucose and insulin through but keeping immune cells out. ‘If it works in people as well as it does in animals, it’s possible that people will not be diabetic,’ said Dr. Melton, co-director of the Harvard Stem Cell Institute and an investigator of the Howard Hughes Medical Institute. ‘They will eat and drink and play like those of us who are not.’”
Reference
Steinberg D. 12 innovations that will change health care and medicine in the 2020s. Time. 2019 Oct 25. https://time.com/5710295/top-health-innovations/ Accessed Dec 5, 2019.
Roots of physician burnout: It’s the work load
Work load, not personal vulnerability, may be at the root of the current physician burnout crisis, a recent study has concluded.
The cutting-edge research utilized cognitive theory and work load analysis to get at the source of burnout among practitioners. The findings indicate that, although some institutions continue to emphasize personal responsibility of physicians to address the issue, it may be the amount and structure of the work itself that triggers burnout in doctors.
“We evaluated the cognitive load of a clinical workday in a national sample of U.S. physicians and its relationship with burnout and professional satisfaction,” wrote Elizabeth Harry, MD, SFHM, a hospitalist at the University of Colorado at Denver, Aurora and coauthors. The results were reported in the Joint Commission Journal on Quality and Patient Safety.
The researchers investigated whether task load correlated with burnout scores in a large national study of U.S. physicians from October 2017 to March 2018.
As the delivery of health care becomes more complex, physicians are charged with ever-increasing amount of administrative and cognitive tasks. Recent evidence indicates that this growing complexity of work is tied to a greater risk of burnout in physicians, compared with workers in other fields. Cognitive load theory, pioneered by psychologist Jonathan Sweller, identified limitations in working memory that humans depend on to carry out cognitive tasks. Cognitive load refers to the amount of working memory used, which can be reduced in the presence of external emotional or physiological stressors. While a potential link between cognitive load and burnout may seem self-evident, the correlation between the cognitive load of physicians and burnout has not been evaluated in a large-scale study until recently.
Physician task load (PTL) was measured using the National Aeronautics and Space Administration Task Load Index (NASA-TLX), a validated questionnaire frequently used to evaluate the cognitive load of work environments, including health care environments. Four domains (perception of effort and mental, physical, and temporal demands) were used to calculate the total PTL score.
Burnout was evaluated using the Emotional Exhaustion and Depersonalization scales of the Maslach Burnout Inventory, a validated tool considered the gold standard for measurement.
The survey sample consisted of physicians of all specialties and was assembled using the American Medical Association Physician Masterfile, an almost complete record of all U.S. physicians independent of AMA membership. All responses were anonymous and participation was voluntary.
Results
Among 30,456 physicians who received the survey, 5,197 (17.1%) responded. In total, 5,276 physicians were included in the analysis.
The median age of respondents was 53 years, and 61.8% self-identified as male. Twenty-four specialties were identified: 23.8% were from a primary care discipline and internal medicine represented the largest respondent group (12.1%).
Almost half of respondents (49.7%) worked in private practice, and 44.8% had been in practice for 21 years or longer.
Overall, 44.0% had at least one symptom of burnout, 38.8% of participants scored in the high range for emotional exhaustion, and 27.4% scored in the high range for depersonalization. The mean score in task load dimension varied by specialty.
The mean PTL score was 260.9 (standard deviation, 71.4). The specialties with the highest PTL score were emergency medicine (369.8), urology (353.7), general surgery subspecialties (343.9), internal medicine subspecialties (342.2), and radiology (341.6).
Aside from specialty, PTL scores also varied by practice setting, gender, age, number of hours worked per week, number of nights on call per week, and years in practice.
The researchers observed a dose response relationship between PTL and risk of burnout. For every 40-point (10%) reduction in PTL, there was 33% lower odds of experiencing burnout (odds ratio, 0.67; 95% confidence interval, 0.65-0.70; P < .0001). Multivariable analyses also indicated that PTL was a significant predictor of burnout, independent of practice setting, specialty, age, gender, and hours worked.
Organizational strategies to reduce physician burnout
Coauthors of the study, Tait D. Shanafelt, MD, professor of medicine at Stanford (Calif.) University and Colin P. West, MD, PhD, of the Mayo Clinic in Rochester, Minn., are both experts on physician well-being and are passionate about finding new ways to reduce physician distress and improving health care delivery.
“Authentic efforts to address this problem must move beyond personal resilience,” Dr. Shanafelt said in an interview. “Organizations that fail to get serious about this issue are going to be left behind and struggle in the war for talent.
“Much like our efforts to improve quality, advancing clinician well-being requires organizations to make it a priority and establish the structure, process, and leadership to promote the desired outcomes,” said Dr. Shanafelt.
One potential strategy for improvement is appointing a chief wellness officer, a dedicated individual within the health care system that leads the organizational effort, explained Dr. Shanafelt. “Over 30 vanguard institutions across the United States have already taken this step.”
Dr. West, a coauthor of the study, explained that conducting an analysis of PTL is fairly straightforward for hospitals and individual institutions. “The NASA-TLX tool is widely available, free to use, and not overly complex, and it could be used to provide insight into physician effort and mental, physical, and temporal demand levels,” he said in an interview.
“Deeper evaluations could follow to identify specific potential solutions, particularly system-level approaches to alleviate PTL,” Dr. West explained. “In the short term, such analyses and solutions would have costs, but helping physicians work more optimally and with less chronic strain from excessive task load would save far more than these costs overall.”
Dr. West also noted that physician burnout is very expensive to a health care system, and strategies to promote physician well-being would be a prudent financial decision long term for health care organizations.
Dr. Harry, lead author of the study, agreed with Dr. West, noting that “quality improvement literature has demonstrated that improvements in inefficiencies that lead to increased demand in the workplace often has the benefit of reduced cost.
“Many studies have demonstrated the risk of turnover due to burnout and the significant cost of physician turn over,” she said in an interview. “This cost avoidance is well worth the investment in improved operations to minimize unnecessary task load.”
Dr. Harry also recommended the NASA-TLX tool as a free resource for health systems and organizations. She noted that future studies will further validate the reliability of the tool.
“At the core, we need to focus on system redesign at both the micro and the macro level,” Dr. Harry said. “Each health system will need to assess inefficiencies in their work flow, while regulatory bodies need to consider the downstream task load of mandates and reporting requirements, all of which contribute to more cognitive load.”
The study was supported by funding from the Stanford Medicine WellMD Center, the American Medical Association, and the Mayo Clinic department of medicine program on physician well-being. Coauthors Lotte N. Dyrbye, MD, and Dr. Shanafelt are coinventors of the Physician Well-being Index, Medical Student Well-Being Index, Nurse Well-Being, and Well-Being Index. Mayo Clinic holds the copyright to these instruments and has licensed them for external use. Dr. Dyrbye and Dr. Shanafelt receive a portion of any royalties paid to Mayo Clinic. All other authors reported no conflicts of interest.
Work load, not personal vulnerability, may be at the root of the current physician burnout crisis, a recent study has concluded.
The cutting-edge research utilized cognitive theory and work load analysis to get at the source of burnout among practitioners. The findings indicate that, although some institutions continue to emphasize personal responsibility of physicians to address the issue, it may be the amount and structure of the work itself that triggers burnout in doctors.
“We evaluated the cognitive load of a clinical workday in a national sample of U.S. physicians and its relationship with burnout and professional satisfaction,” wrote Elizabeth Harry, MD, SFHM, a hospitalist at the University of Colorado at Denver, Aurora and coauthors. The results were reported in the Joint Commission Journal on Quality and Patient Safety.
The researchers investigated whether task load correlated with burnout scores in a large national study of U.S. physicians from October 2017 to March 2018.
As the delivery of health care becomes more complex, physicians are charged with ever-increasing amount of administrative and cognitive tasks. Recent evidence indicates that this growing complexity of work is tied to a greater risk of burnout in physicians, compared with workers in other fields. Cognitive load theory, pioneered by psychologist Jonathan Sweller, identified limitations in working memory that humans depend on to carry out cognitive tasks. Cognitive load refers to the amount of working memory used, which can be reduced in the presence of external emotional or physiological stressors. While a potential link between cognitive load and burnout may seem self-evident, the correlation between the cognitive load of physicians and burnout has not been evaluated in a large-scale study until recently.
Physician task load (PTL) was measured using the National Aeronautics and Space Administration Task Load Index (NASA-TLX), a validated questionnaire frequently used to evaluate the cognitive load of work environments, including health care environments. Four domains (perception of effort and mental, physical, and temporal demands) were used to calculate the total PTL score.
Burnout was evaluated using the Emotional Exhaustion and Depersonalization scales of the Maslach Burnout Inventory, a validated tool considered the gold standard for measurement.
The survey sample consisted of physicians of all specialties and was assembled using the American Medical Association Physician Masterfile, an almost complete record of all U.S. physicians independent of AMA membership. All responses were anonymous and participation was voluntary.
Results
Among 30,456 physicians who received the survey, 5,197 (17.1%) responded. In total, 5,276 physicians were included in the analysis.
The median age of respondents was 53 years, and 61.8% self-identified as male. Twenty-four specialties were identified: 23.8% were from a primary care discipline and internal medicine represented the largest respondent group (12.1%).
Almost half of respondents (49.7%) worked in private practice, and 44.8% had been in practice for 21 years or longer.
Overall, 44.0% had at least one symptom of burnout, 38.8% of participants scored in the high range for emotional exhaustion, and 27.4% scored in the high range for depersonalization. The mean score in task load dimension varied by specialty.
The mean PTL score was 260.9 (standard deviation, 71.4). The specialties with the highest PTL score were emergency medicine (369.8), urology (353.7), general surgery subspecialties (343.9), internal medicine subspecialties (342.2), and radiology (341.6).
Aside from specialty, PTL scores also varied by practice setting, gender, age, number of hours worked per week, number of nights on call per week, and years in practice.
The researchers observed a dose response relationship between PTL and risk of burnout. For every 40-point (10%) reduction in PTL, there was 33% lower odds of experiencing burnout (odds ratio, 0.67; 95% confidence interval, 0.65-0.70; P < .0001). Multivariable analyses also indicated that PTL was a significant predictor of burnout, independent of practice setting, specialty, age, gender, and hours worked.
Organizational strategies to reduce physician burnout
Coauthors of the study, Tait D. Shanafelt, MD, professor of medicine at Stanford (Calif.) University and Colin P. West, MD, PhD, of the Mayo Clinic in Rochester, Minn., are both experts on physician well-being and are passionate about finding new ways to reduce physician distress and improving health care delivery.
“Authentic efforts to address this problem must move beyond personal resilience,” Dr. Shanafelt said in an interview. “Organizations that fail to get serious about this issue are going to be left behind and struggle in the war for talent.
“Much like our efforts to improve quality, advancing clinician well-being requires organizations to make it a priority and establish the structure, process, and leadership to promote the desired outcomes,” said Dr. Shanafelt.
One potential strategy for improvement is appointing a chief wellness officer, a dedicated individual within the health care system that leads the organizational effort, explained Dr. Shanafelt. “Over 30 vanguard institutions across the United States have already taken this step.”
Dr. West, a coauthor of the study, explained that conducting an analysis of PTL is fairly straightforward for hospitals and individual institutions. “The NASA-TLX tool is widely available, free to use, and not overly complex, and it could be used to provide insight into physician effort and mental, physical, and temporal demand levels,” he said in an interview.
“Deeper evaluations could follow to identify specific potential solutions, particularly system-level approaches to alleviate PTL,” Dr. West explained. “In the short term, such analyses and solutions would have costs, but helping physicians work more optimally and with less chronic strain from excessive task load would save far more than these costs overall.”
Dr. West also noted that physician burnout is very expensive to a health care system, and strategies to promote physician well-being would be a prudent financial decision long term for health care organizations.
Dr. Harry, lead author of the study, agreed with Dr. West, noting that “quality improvement literature has demonstrated that improvements in inefficiencies that lead to increased demand in the workplace often has the benefit of reduced cost.
“Many studies have demonstrated the risk of turnover due to burnout and the significant cost of physician turn over,” she said in an interview. “This cost avoidance is well worth the investment in improved operations to minimize unnecessary task load.”
Dr. Harry also recommended the NASA-TLX tool as a free resource for health systems and organizations. She noted that future studies will further validate the reliability of the tool.
“At the core, we need to focus on system redesign at both the micro and the macro level,” Dr. Harry said. “Each health system will need to assess inefficiencies in their work flow, while regulatory bodies need to consider the downstream task load of mandates and reporting requirements, all of which contribute to more cognitive load.”
The study was supported by funding from the Stanford Medicine WellMD Center, the American Medical Association, and the Mayo Clinic department of medicine program on physician well-being. Coauthors Lotte N. Dyrbye, MD, and Dr. Shanafelt are coinventors of the Physician Well-being Index, Medical Student Well-Being Index, Nurse Well-Being, and Well-Being Index. Mayo Clinic holds the copyright to these instruments and has licensed them for external use. Dr. Dyrbye and Dr. Shanafelt receive a portion of any royalties paid to Mayo Clinic. All other authors reported no conflicts of interest.
Work load, not personal vulnerability, may be at the root of the current physician burnout crisis, a recent study has concluded.
The cutting-edge research utilized cognitive theory and work load analysis to get at the source of burnout among practitioners. The findings indicate that, although some institutions continue to emphasize personal responsibility of physicians to address the issue, it may be the amount and structure of the work itself that triggers burnout in doctors.
“We evaluated the cognitive load of a clinical workday in a national sample of U.S. physicians and its relationship with burnout and professional satisfaction,” wrote Elizabeth Harry, MD, SFHM, a hospitalist at the University of Colorado at Denver, Aurora and coauthors. The results were reported in the Joint Commission Journal on Quality and Patient Safety.
The researchers investigated whether task load correlated with burnout scores in a large national study of U.S. physicians from October 2017 to March 2018.
As the delivery of health care becomes more complex, physicians are charged with ever-increasing amount of administrative and cognitive tasks. Recent evidence indicates that this growing complexity of work is tied to a greater risk of burnout in physicians, compared with workers in other fields. Cognitive load theory, pioneered by psychologist Jonathan Sweller, identified limitations in working memory that humans depend on to carry out cognitive tasks. Cognitive load refers to the amount of working memory used, which can be reduced in the presence of external emotional or physiological stressors. While a potential link between cognitive load and burnout may seem self-evident, the correlation between the cognitive load of physicians and burnout has not been evaluated in a large-scale study until recently.
Physician task load (PTL) was measured using the National Aeronautics and Space Administration Task Load Index (NASA-TLX), a validated questionnaire frequently used to evaluate the cognitive load of work environments, including health care environments. Four domains (perception of effort and mental, physical, and temporal demands) were used to calculate the total PTL score.
Burnout was evaluated using the Emotional Exhaustion and Depersonalization scales of the Maslach Burnout Inventory, a validated tool considered the gold standard for measurement.
The survey sample consisted of physicians of all specialties and was assembled using the American Medical Association Physician Masterfile, an almost complete record of all U.S. physicians independent of AMA membership. All responses were anonymous and participation was voluntary.
Results
Among 30,456 physicians who received the survey, 5,197 (17.1%) responded. In total, 5,276 physicians were included in the analysis.
The median age of respondents was 53 years, and 61.8% self-identified as male. Twenty-four specialties were identified: 23.8% were from a primary care discipline and internal medicine represented the largest respondent group (12.1%).
Almost half of respondents (49.7%) worked in private practice, and 44.8% had been in practice for 21 years or longer.
Overall, 44.0% had at least one symptom of burnout, 38.8% of participants scored in the high range for emotional exhaustion, and 27.4% scored in the high range for depersonalization. The mean score in task load dimension varied by specialty.
The mean PTL score was 260.9 (standard deviation, 71.4). The specialties with the highest PTL score were emergency medicine (369.8), urology (353.7), general surgery subspecialties (343.9), internal medicine subspecialties (342.2), and radiology (341.6).
Aside from specialty, PTL scores also varied by practice setting, gender, age, number of hours worked per week, number of nights on call per week, and years in practice.
The researchers observed a dose response relationship between PTL and risk of burnout. For every 40-point (10%) reduction in PTL, there was 33% lower odds of experiencing burnout (odds ratio, 0.67; 95% confidence interval, 0.65-0.70; P < .0001). Multivariable analyses also indicated that PTL was a significant predictor of burnout, independent of practice setting, specialty, age, gender, and hours worked.
Organizational strategies to reduce physician burnout
Coauthors of the study, Tait D. Shanafelt, MD, professor of medicine at Stanford (Calif.) University and Colin P. West, MD, PhD, of the Mayo Clinic in Rochester, Minn., are both experts on physician well-being and are passionate about finding new ways to reduce physician distress and improving health care delivery.
“Authentic efforts to address this problem must move beyond personal resilience,” Dr. Shanafelt said in an interview. “Organizations that fail to get serious about this issue are going to be left behind and struggle in the war for talent.
“Much like our efforts to improve quality, advancing clinician well-being requires organizations to make it a priority and establish the structure, process, and leadership to promote the desired outcomes,” said Dr. Shanafelt.
One potential strategy for improvement is appointing a chief wellness officer, a dedicated individual within the health care system that leads the organizational effort, explained Dr. Shanafelt. “Over 30 vanguard institutions across the United States have already taken this step.”
Dr. West, a coauthor of the study, explained that conducting an analysis of PTL is fairly straightforward for hospitals and individual institutions. “The NASA-TLX tool is widely available, free to use, and not overly complex, and it could be used to provide insight into physician effort and mental, physical, and temporal demand levels,” he said in an interview.
“Deeper evaluations could follow to identify specific potential solutions, particularly system-level approaches to alleviate PTL,” Dr. West explained. “In the short term, such analyses and solutions would have costs, but helping physicians work more optimally and with less chronic strain from excessive task load would save far more than these costs overall.”
Dr. West also noted that physician burnout is very expensive to a health care system, and strategies to promote physician well-being would be a prudent financial decision long term for health care organizations.
Dr. Harry, lead author of the study, agreed with Dr. West, noting that “quality improvement literature has demonstrated that improvements in inefficiencies that lead to increased demand in the workplace often has the benefit of reduced cost.
“Many studies have demonstrated the risk of turnover due to burnout and the significant cost of physician turn over,” she said in an interview. “This cost avoidance is well worth the investment in improved operations to minimize unnecessary task load.”
Dr. Harry also recommended the NASA-TLX tool as a free resource for health systems and organizations. She noted that future studies will further validate the reliability of the tool.
“At the core, we need to focus on system redesign at both the micro and the macro level,” Dr. Harry said. “Each health system will need to assess inefficiencies in their work flow, while regulatory bodies need to consider the downstream task load of mandates and reporting requirements, all of which contribute to more cognitive load.”
The study was supported by funding from the Stanford Medicine WellMD Center, the American Medical Association, and the Mayo Clinic department of medicine program on physician well-being. Coauthors Lotte N. Dyrbye, MD, and Dr. Shanafelt are coinventors of the Physician Well-being Index, Medical Student Well-Being Index, Nurse Well-Being, and Well-Being Index. Mayo Clinic holds the copyright to these instruments and has licensed them for external use. Dr. Dyrbye and Dr. Shanafelt receive a portion of any royalties paid to Mayo Clinic. All other authors reported no conflicts of interest.
FROM THE JOINT COMMISSION JOURNAL ON QUALITY AND PATIENT SAFETY
CDC chief lays out attack plan for COVID variants
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
Alien cells may explain COVID-19 brain fog
, a new report suggests.
The authors report five separate post-mortem cases from patients who died with COVID-19 in which large cells resembling megakaryocytes were identified in cortical capillaries. Immunohistochemistry subsequently confirmed their megakaryocyte identity.
They point out that the finding is of interest as – to their knowledge – megakaryocytes have not been found in the brain before.
The observations are described in a research letter published online Feb. 12 in JAMA Neurology.
Bone marrow cells in the brain
Lead author David Nauen, MD, PhD, a neuropathologist from Johns Hopkins University, Baltimore, reported that he identified these cells in the first analysis of post-mortem brain tissue from a patient who had COVID-19.
“Some other viruses cause changes in the brain such as encephalopathy, and as neurologic symptoms are often reported in COVID-19, I was curious to see if similar effects were seen in brain post-mortem samples from patients who had died with the infection,” Dr. Nauen said.
On his first analysis of the brain tissue of a patient who had COVID-19, Dr. Nauen saw no evidence of viral encephalitis, but he observed some “unusually large” cells in the brain capillaries.
“I was taken aback; I couldn’t figure out what they were. Then I realized these cells were megakaryocytes from the bone marrow. I have never seen these cells in the brain before. I asked several colleagues and none of them had either. After extensive literature searches, I could find no evidence of megakaryocytes being in the brain,” Dr. Nauen noted.
Megakaryocytes, he explained, are “very large cells, and the brain capillaries are very small – just large enough to let red blood cells and lymphocytes pass through. To see these very large cells in such vessels is extremely unusual. It looks like they are causing occlusions.”
By occluding flow through individual capillaries, these large cells could cause ischemic alteration in a distinct pattern, potentially resulting in an atypical form of neurologic impairment, the authors suggest.
“This might alter the hemodynamics and put pressure on other vessels, possibly contributing to the increased risk of stroke that has been reported in COVID-19,” Dr. Nauen said. None of the samples he examined came from patients with COVID-19 who had had a stroke, he reported.
Other than the presence of megakaryocytes in the capillaries, the brain looked normal, he said. He has now examined samples from 15 brains of patients who had COVID-19 and megakaryocytes have been found in the brain capillaries in five cases.
New neurologic complication
Classic encephalitis found with other viruses has not been reported in brain post-mortem examinations from patients who had COVID-19, Dr. Nauen noted. “The cognitive issues such as grogginess associated with COVID-19 would indicate problems with the cortex but that hasn’t been documented. This occlusion of a multitude of tiny vessels by megalokaryocytes may offer some explanation of the cognitive issues. This is a new kind of vascular insult seen on pathology, and suggests a new kind of neurologic complication,” he added.
The big question is what these megakaryocytes are doing in the brain.
“Megakaryocytes are bone marrow cells. They are not immune cells. Their job is to produce platelets to help the blood clot. They are not normally found outside the bone marrow, but they have been reported in other organs in COVID-19 patients.
“But the big puzzle associated with finding them in the brain is how they get through the very fine network of blood vessels in the lungs. The geometry just doesn’t work. We don’t know which part of the COVID inflammatory response makes this happen,” said Dr. Nauen.
The authors suggest one possibility is that altered endothelial or other signaling is recruiting megakaryocytes into the circulation and somehow permitting them to pass through the lungs.
“We need to try and understand if there is anything distinctive about these megakaryocytes – which proteins are they expressing that may explain why they are behaving in such an unusual way,” said Dr. Nauen.
Noting that many patients with severe COVID-19 have problems with clotting, and megakaryocytes are part of the clotting system, he speculated that some sort of aberrant message is being sent to these cells.
“It is notable that we found megakaryocytes in cortical capillaries in 33% of cases examined. Because the standard brain autopsy sections taken sampled at random [are] only a minute portion of the cortical volume, finding these cells suggests the total burden could be considerable,” the authors wrote.
Dr. Nauen added that to his knowledge, this is the first report of such observations, and the next step is to look for similar findings in larger sample sizes.
A version of this article first appeared on Medscape.com.
, a new report suggests.
The authors report five separate post-mortem cases from patients who died with COVID-19 in which large cells resembling megakaryocytes were identified in cortical capillaries. Immunohistochemistry subsequently confirmed their megakaryocyte identity.
They point out that the finding is of interest as – to their knowledge – megakaryocytes have not been found in the brain before.
The observations are described in a research letter published online Feb. 12 in JAMA Neurology.
Bone marrow cells in the brain
Lead author David Nauen, MD, PhD, a neuropathologist from Johns Hopkins University, Baltimore, reported that he identified these cells in the first analysis of post-mortem brain tissue from a patient who had COVID-19.
“Some other viruses cause changes in the brain such as encephalopathy, and as neurologic symptoms are often reported in COVID-19, I was curious to see if similar effects were seen in brain post-mortem samples from patients who had died with the infection,” Dr. Nauen said.
On his first analysis of the brain tissue of a patient who had COVID-19, Dr. Nauen saw no evidence of viral encephalitis, but he observed some “unusually large” cells in the brain capillaries.
“I was taken aback; I couldn’t figure out what they were. Then I realized these cells were megakaryocytes from the bone marrow. I have never seen these cells in the brain before. I asked several colleagues and none of them had either. After extensive literature searches, I could find no evidence of megakaryocytes being in the brain,” Dr. Nauen noted.
Megakaryocytes, he explained, are “very large cells, and the brain capillaries are very small – just large enough to let red blood cells and lymphocytes pass through. To see these very large cells in such vessels is extremely unusual. It looks like they are causing occlusions.”
By occluding flow through individual capillaries, these large cells could cause ischemic alteration in a distinct pattern, potentially resulting in an atypical form of neurologic impairment, the authors suggest.
“This might alter the hemodynamics and put pressure on other vessels, possibly contributing to the increased risk of stroke that has been reported in COVID-19,” Dr. Nauen said. None of the samples he examined came from patients with COVID-19 who had had a stroke, he reported.
Other than the presence of megakaryocytes in the capillaries, the brain looked normal, he said. He has now examined samples from 15 brains of patients who had COVID-19 and megakaryocytes have been found in the brain capillaries in five cases.
New neurologic complication
Classic encephalitis found with other viruses has not been reported in brain post-mortem examinations from patients who had COVID-19, Dr. Nauen noted. “The cognitive issues such as grogginess associated with COVID-19 would indicate problems with the cortex but that hasn’t been documented. This occlusion of a multitude of tiny vessels by megalokaryocytes may offer some explanation of the cognitive issues. This is a new kind of vascular insult seen on pathology, and suggests a new kind of neurologic complication,” he added.
The big question is what these megakaryocytes are doing in the brain.
“Megakaryocytes are bone marrow cells. They are not immune cells. Their job is to produce platelets to help the blood clot. They are not normally found outside the bone marrow, but they have been reported in other organs in COVID-19 patients.
“But the big puzzle associated with finding them in the brain is how they get through the very fine network of blood vessels in the lungs. The geometry just doesn’t work. We don’t know which part of the COVID inflammatory response makes this happen,” said Dr. Nauen.
The authors suggest one possibility is that altered endothelial or other signaling is recruiting megakaryocytes into the circulation and somehow permitting them to pass through the lungs.
“We need to try and understand if there is anything distinctive about these megakaryocytes – which proteins are they expressing that may explain why they are behaving in such an unusual way,” said Dr. Nauen.
Noting that many patients with severe COVID-19 have problems with clotting, and megakaryocytes are part of the clotting system, he speculated that some sort of aberrant message is being sent to these cells.
“It is notable that we found megakaryocytes in cortical capillaries in 33% of cases examined. Because the standard brain autopsy sections taken sampled at random [are] only a minute portion of the cortical volume, finding these cells suggests the total burden could be considerable,” the authors wrote.
Dr. Nauen added that to his knowledge, this is the first report of such observations, and the next step is to look for similar findings in larger sample sizes.
A version of this article first appeared on Medscape.com.
, a new report suggests.
The authors report five separate post-mortem cases from patients who died with COVID-19 in which large cells resembling megakaryocytes were identified in cortical capillaries. Immunohistochemistry subsequently confirmed their megakaryocyte identity.
They point out that the finding is of interest as – to their knowledge – megakaryocytes have not been found in the brain before.
The observations are described in a research letter published online Feb. 12 in JAMA Neurology.
Bone marrow cells in the brain
Lead author David Nauen, MD, PhD, a neuropathologist from Johns Hopkins University, Baltimore, reported that he identified these cells in the first analysis of post-mortem brain tissue from a patient who had COVID-19.
“Some other viruses cause changes in the brain such as encephalopathy, and as neurologic symptoms are often reported in COVID-19, I was curious to see if similar effects were seen in brain post-mortem samples from patients who had died with the infection,” Dr. Nauen said.
On his first analysis of the brain tissue of a patient who had COVID-19, Dr. Nauen saw no evidence of viral encephalitis, but he observed some “unusually large” cells in the brain capillaries.
“I was taken aback; I couldn’t figure out what they were. Then I realized these cells were megakaryocytes from the bone marrow. I have never seen these cells in the brain before. I asked several colleagues and none of them had either. After extensive literature searches, I could find no evidence of megakaryocytes being in the brain,” Dr. Nauen noted.
Megakaryocytes, he explained, are “very large cells, and the brain capillaries are very small – just large enough to let red blood cells and lymphocytes pass through. To see these very large cells in such vessels is extremely unusual. It looks like they are causing occlusions.”
By occluding flow through individual capillaries, these large cells could cause ischemic alteration in a distinct pattern, potentially resulting in an atypical form of neurologic impairment, the authors suggest.
“This might alter the hemodynamics and put pressure on other vessels, possibly contributing to the increased risk of stroke that has been reported in COVID-19,” Dr. Nauen said. None of the samples he examined came from patients with COVID-19 who had had a stroke, he reported.
Other than the presence of megakaryocytes in the capillaries, the brain looked normal, he said. He has now examined samples from 15 brains of patients who had COVID-19 and megakaryocytes have been found in the brain capillaries in five cases.
New neurologic complication
Classic encephalitis found with other viruses has not been reported in brain post-mortem examinations from patients who had COVID-19, Dr. Nauen noted. “The cognitive issues such as grogginess associated with COVID-19 would indicate problems with the cortex but that hasn’t been documented. This occlusion of a multitude of tiny vessels by megalokaryocytes may offer some explanation of the cognitive issues. This is a new kind of vascular insult seen on pathology, and suggests a new kind of neurologic complication,” he added.
The big question is what these megakaryocytes are doing in the brain.
“Megakaryocytes are bone marrow cells. They are not immune cells. Their job is to produce platelets to help the blood clot. They are not normally found outside the bone marrow, but they have been reported in other organs in COVID-19 patients.
“But the big puzzle associated with finding them in the brain is how they get through the very fine network of blood vessels in the lungs. The geometry just doesn’t work. We don’t know which part of the COVID inflammatory response makes this happen,” said Dr. Nauen.
The authors suggest one possibility is that altered endothelial or other signaling is recruiting megakaryocytes into the circulation and somehow permitting them to pass through the lungs.
“We need to try and understand if there is anything distinctive about these megakaryocytes – which proteins are they expressing that may explain why they are behaving in such an unusual way,” said Dr. Nauen.
Noting that many patients with severe COVID-19 have problems with clotting, and megakaryocytes are part of the clotting system, he speculated that some sort of aberrant message is being sent to these cells.
“It is notable that we found megakaryocytes in cortical capillaries in 33% of cases examined. Because the standard brain autopsy sections taken sampled at random [are] only a minute portion of the cortical volume, finding these cells suggests the total burden could be considerable,” the authors wrote.
Dr. Nauen added that to his knowledge, this is the first report of such observations, and the next step is to look for similar findings in larger sample sizes.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Victorious endurance: To pass the breaking point and not break
I’ve been thinking a lot about endurance recently.
COVID-19 is surging in the United States. Health care workers exhausted from the first and second waves are quickly reaching the verge of collapse. I’m seeing more and more heartbreaking articles about the bone-deep fatigue, fear, and frustration health care workers are facing, and I weep. As horrible as it is to be fighting this terrifying, little-understood, invisible virus, health care workers are also fighting an equally distressing war against misinformation, recklessness, apathy, and outright denial.
As if that wasn’t enough, we are also dealing with racial and social unrest not seen in decades. The most significant cultural divisions and political animosity perhaps since the Civil War. A contested election. The fraying of our democratic institutions and our standing in the global community. The weakest economy since the Great Depression. Record unemployment. Many individuals and families facing or already experiencing eviction and food insecurity. Record-setting fires, hurricanes, and other natural disasters that are only projected to intensify due to climate change.
That’s a lot to endure. And we don’t have much choice other than to live through it. Some of us will break under the strain; others will disengage by giving up clinical work or even leaving health care altogether. Some of us will pack it in and retire, walk away from relationships with family members or longtime friends, or even emigrate to another country (New Zealand, anyone?). Some of us will passively hunker down, letting the challenges of this time overwhelm us and just hoping we can hang on long enough to emerge, albeit beaten and scarred, on the other side.
But some of us will experience victorious endurance – the kind that doesn’t just accept suffering but finds a way to triumph over it. I came across the concept of victorious endurance in the Bible, but its origin is earlier, from classical Greece. It comes from the ancient Greek word hupomone, which literally means “abiding under” – as in disciplining oneself to bear up under a trial when one would more naturally rebel, or just give up. The ancient Greeks were big on virtues like self-control, long-suffering, and perseverance in the face of seemingly insurmountable difficulties; Odysseus was a poster child for hupomone. I believe the concept of victorious endurance can be applicable for people across many belief systems, philosophies, and ways of life.
The late William Barclay, former professor of divinity and biblical criticism at the University of Glasgow, Scotland, said of hupomone:
It is untranslatable. It does not describe the frame of mind which can sit down with folded hands and bowed head and let a torrent of troubles sweep over it in passive resignation. It describes the ability to bear things in such a triumphant way that it transfigures them. Chrysostom has a great panegyric on this hupomone. He calls it “the root of all goods, the mother of piety, the fruit that never withers, a fortress that is never taken, a harbour that knows no storms” and “the queen of virtues, the foundation of right actions, peace in war, calm in tempest, security in plots.” It is the courageous and triumphant ability to pass the breaking-point and not to break and always to greet the unseen with a cheer. It is the alchemy which transmutes tribulation into strength and glory.
Barclay further noted that “Cicero defines patientia, its Latin equivalent, as: ‘The voluntary and daily suffering of hard and difficult things, for the sake of honour and usefulness.”
In the midst of the most challenging public health emergency of our lifetimes, I am seeing hospitalists – and nurses, respiratory therapists, and countless other health care workers – doing exactly this, every day. I’m so incredibly proud of you all, and thankful beyond words.
I doubt that victorious endurance comes naturally to any of us; it’s something we work at, pursue and nurture. What’s the secret to cultivating victorious endurance in the midst of unimaginable stress? I’m pretty sure there’s no specific formula. I don’t mean to sound like a Pollyanna or to make light of the tumult and turmoil of these times, but here are a few things that, based on my own experiences, may help cultivate this valuable virtue.
Be part of a support network. In the midst of great stress, and especially during this time of social distancing, it’s especially tempting to just hunker down, close in on ourselves, and shut others out – sometimes even our closest friends and loved ones. Maintaining relationships is just too exhausting. But you need people who can come alongside you and offer words of encouragement when you are at your lowest. And there’s nothing that will bring out the best in you like being there to encourage and support someone else. We all need to both receive and to give emotional support at a time like this.
Take the long view. When we’re in the middle of a serious crisis, it seems like the problems we’re facing will last forever. There’s no light at the end of the tunnel, no port in the storm. But even this pandemic won’t last forever. If we can keep in mind the fact that things will eventually get better and that the current situation isn’t permanent, it can help us maintain our perspective and have more patience with the current dysfunction.
Focus on who you want to be in this moment. This is the hardest time most of us have ever lived through, both professionally and personally. But let me throw you a challenge. When you look back on this time from the perspective of five years from now, or maybe ten, how will you want to remember yourself? Who will you want to have been during this time? Looking back, what will make you proud of how you handled this challenge? Be that person.
Look for things to be thankful for. In the midst of the chaos that is our lives and our work right now, I believe we can still occasionally see moments of grace if we keep our eyes open for them. If we aren’t looking for them, we may miss them entirely. And those small moments of love, touches of compassion, displays of selflessness, and even flashes of victorious endurance in yourself or others are gifts to be treasured and held on to – to give thanks for.
Embrace a cause greater than yourself. May I suggest that one thing that might help our efforts to cultivate the virtue of victorious endurance during difficult times might be to embrace a cause that is bigger than yourself; that is, one that lures you to focus beyond your immediate circumstances? What are you passionate about, outside of your life’s normal routine?
If you don’t have a passion, consider what you might become passionate about, with a little effort. For some of us, like me, this will be our faith in God. For others it may be advocating for an end to racism or for broader social justice issues. Maybe it’s working to overcome our cultural and political divisions or to strengthen the institutions of our democracy. Perhaps it’s getting involved with efforts to mitigate climate change. Maybe it’s reaching out to the homeless or hungry in your own community or mentoring a child who is being left behind by the demands of remote learning.
Or perhaps what you embrace is even closer to home: maybe it’s working to eliminate health disparities in your institution or health system, or figuring out how to use technology and resources differently to improve how care is being delivered during or after this pandemic. Maybe it’s as simple as re-committing yourself to personally care for every patient you see today with the very best you have to offer, and with patience, compassion, and grace.
Find something that sets your heart on fire. Something that makes you want to take this difficult time and “transmute tribulation into strength and glory.” Something that, when you look back on these days, will make you thankful that you didn’t just hunker down and subsist through them. Instead, you accomplished great things; you learned; you contributed; and you grew stronger and better.
That’s victorious endurance.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey. This essay was published initially on The Hospital Leader, the official blog of SHM.
I’ve been thinking a lot about endurance recently.
COVID-19 is surging in the United States. Health care workers exhausted from the first and second waves are quickly reaching the verge of collapse. I’m seeing more and more heartbreaking articles about the bone-deep fatigue, fear, and frustration health care workers are facing, and I weep. As horrible as it is to be fighting this terrifying, little-understood, invisible virus, health care workers are also fighting an equally distressing war against misinformation, recklessness, apathy, and outright denial.
As if that wasn’t enough, we are also dealing with racial and social unrest not seen in decades. The most significant cultural divisions and political animosity perhaps since the Civil War. A contested election. The fraying of our democratic institutions and our standing in the global community. The weakest economy since the Great Depression. Record unemployment. Many individuals and families facing or already experiencing eviction and food insecurity. Record-setting fires, hurricanes, and other natural disasters that are only projected to intensify due to climate change.
That’s a lot to endure. And we don’t have much choice other than to live through it. Some of us will break under the strain; others will disengage by giving up clinical work or even leaving health care altogether. Some of us will pack it in and retire, walk away from relationships with family members or longtime friends, or even emigrate to another country (New Zealand, anyone?). Some of us will passively hunker down, letting the challenges of this time overwhelm us and just hoping we can hang on long enough to emerge, albeit beaten and scarred, on the other side.
But some of us will experience victorious endurance – the kind that doesn’t just accept suffering but finds a way to triumph over it. I came across the concept of victorious endurance in the Bible, but its origin is earlier, from classical Greece. It comes from the ancient Greek word hupomone, which literally means “abiding under” – as in disciplining oneself to bear up under a trial when one would more naturally rebel, or just give up. The ancient Greeks were big on virtues like self-control, long-suffering, and perseverance in the face of seemingly insurmountable difficulties; Odysseus was a poster child for hupomone. I believe the concept of victorious endurance can be applicable for people across many belief systems, philosophies, and ways of life.
The late William Barclay, former professor of divinity and biblical criticism at the University of Glasgow, Scotland, said of hupomone:
It is untranslatable. It does not describe the frame of mind which can sit down with folded hands and bowed head and let a torrent of troubles sweep over it in passive resignation. It describes the ability to bear things in such a triumphant way that it transfigures them. Chrysostom has a great panegyric on this hupomone. He calls it “the root of all goods, the mother of piety, the fruit that never withers, a fortress that is never taken, a harbour that knows no storms” and “the queen of virtues, the foundation of right actions, peace in war, calm in tempest, security in plots.” It is the courageous and triumphant ability to pass the breaking-point and not to break and always to greet the unseen with a cheer. It is the alchemy which transmutes tribulation into strength and glory.
Barclay further noted that “Cicero defines patientia, its Latin equivalent, as: ‘The voluntary and daily suffering of hard and difficult things, for the sake of honour and usefulness.”
In the midst of the most challenging public health emergency of our lifetimes, I am seeing hospitalists – and nurses, respiratory therapists, and countless other health care workers – doing exactly this, every day. I’m so incredibly proud of you all, and thankful beyond words.
I doubt that victorious endurance comes naturally to any of us; it’s something we work at, pursue and nurture. What’s the secret to cultivating victorious endurance in the midst of unimaginable stress? I’m pretty sure there’s no specific formula. I don’t mean to sound like a Pollyanna or to make light of the tumult and turmoil of these times, but here are a few things that, based on my own experiences, may help cultivate this valuable virtue.
Be part of a support network. In the midst of great stress, and especially during this time of social distancing, it’s especially tempting to just hunker down, close in on ourselves, and shut others out – sometimes even our closest friends and loved ones. Maintaining relationships is just too exhausting. But you need people who can come alongside you and offer words of encouragement when you are at your lowest. And there’s nothing that will bring out the best in you like being there to encourage and support someone else. We all need to both receive and to give emotional support at a time like this.
Take the long view. When we’re in the middle of a serious crisis, it seems like the problems we’re facing will last forever. There’s no light at the end of the tunnel, no port in the storm. But even this pandemic won’t last forever. If we can keep in mind the fact that things will eventually get better and that the current situation isn’t permanent, it can help us maintain our perspective and have more patience with the current dysfunction.
Focus on who you want to be in this moment. This is the hardest time most of us have ever lived through, both professionally and personally. But let me throw you a challenge. When you look back on this time from the perspective of five years from now, or maybe ten, how will you want to remember yourself? Who will you want to have been during this time? Looking back, what will make you proud of how you handled this challenge? Be that person.
Look for things to be thankful for. In the midst of the chaos that is our lives and our work right now, I believe we can still occasionally see moments of grace if we keep our eyes open for them. If we aren’t looking for them, we may miss them entirely. And those small moments of love, touches of compassion, displays of selflessness, and even flashes of victorious endurance in yourself or others are gifts to be treasured and held on to – to give thanks for.
Embrace a cause greater than yourself. May I suggest that one thing that might help our efforts to cultivate the virtue of victorious endurance during difficult times might be to embrace a cause that is bigger than yourself; that is, one that lures you to focus beyond your immediate circumstances? What are you passionate about, outside of your life’s normal routine?
If you don’t have a passion, consider what you might become passionate about, with a little effort. For some of us, like me, this will be our faith in God. For others it may be advocating for an end to racism or for broader social justice issues. Maybe it’s working to overcome our cultural and political divisions or to strengthen the institutions of our democracy. Perhaps it’s getting involved with efforts to mitigate climate change. Maybe it’s reaching out to the homeless or hungry in your own community or mentoring a child who is being left behind by the demands of remote learning.
Or perhaps what you embrace is even closer to home: maybe it’s working to eliminate health disparities in your institution or health system, or figuring out how to use technology and resources differently to improve how care is being delivered during or after this pandemic. Maybe it’s as simple as re-committing yourself to personally care for every patient you see today with the very best you have to offer, and with patience, compassion, and grace.
Find something that sets your heart on fire. Something that makes you want to take this difficult time and “transmute tribulation into strength and glory.” Something that, when you look back on these days, will make you thankful that you didn’t just hunker down and subsist through them. Instead, you accomplished great things; you learned; you contributed; and you grew stronger and better.
That’s victorious endurance.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey. This essay was published initially on The Hospital Leader, the official blog of SHM.
I’ve been thinking a lot about endurance recently.
COVID-19 is surging in the United States. Health care workers exhausted from the first and second waves are quickly reaching the verge of collapse. I’m seeing more and more heartbreaking articles about the bone-deep fatigue, fear, and frustration health care workers are facing, and I weep. As horrible as it is to be fighting this terrifying, little-understood, invisible virus, health care workers are also fighting an equally distressing war against misinformation, recklessness, apathy, and outright denial.
As if that wasn’t enough, we are also dealing with racial and social unrest not seen in decades. The most significant cultural divisions and political animosity perhaps since the Civil War. A contested election. The fraying of our democratic institutions and our standing in the global community. The weakest economy since the Great Depression. Record unemployment. Many individuals and families facing or already experiencing eviction and food insecurity. Record-setting fires, hurricanes, and other natural disasters that are only projected to intensify due to climate change.
That’s a lot to endure. And we don’t have much choice other than to live through it. Some of us will break under the strain; others will disengage by giving up clinical work or even leaving health care altogether. Some of us will pack it in and retire, walk away from relationships with family members or longtime friends, or even emigrate to another country (New Zealand, anyone?). Some of us will passively hunker down, letting the challenges of this time overwhelm us and just hoping we can hang on long enough to emerge, albeit beaten and scarred, on the other side.
But some of us will experience victorious endurance – the kind that doesn’t just accept suffering but finds a way to triumph over it. I came across the concept of victorious endurance in the Bible, but its origin is earlier, from classical Greece. It comes from the ancient Greek word hupomone, which literally means “abiding under” – as in disciplining oneself to bear up under a trial when one would more naturally rebel, or just give up. The ancient Greeks were big on virtues like self-control, long-suffering, and perseverance in the face of seemingly insurmountable difficulties; Odysseus was a poster child for hupomone. I believe the concept of victorious endurance can be applicable for people across many belief systems, philosophies, and ways of life.
The late William Barclay, former professor of divinity and biblical criticism at the University of Glasgow, Scotland, said of hupomone:
It is untranslatable. It does not describe the frame of mind which can sit down with folded hands and bowed head and let a torrent of troubles sweep over it in passive resignation. It describes the ability to bear things in such a triumphant way that it transfigures them. Chrysostom has a great panegyric on this hupomone. He calls it “the root of all goods, the mother of piety, the fruit that never withers, a fortress that is never taken, a harbour that knows no storms” and “the queen of virtues, the foundation of right actions, peace in war, calm in tempest, security in plots.” It is the courageous and triumphant ability to pass the breaking-point and not to break and always to greet the unseen with a cheer. It is the alchemy which transmutes tribulation into strength and glory.
Barclay further noted that “Cicero defines patientia, its Latin equivalent, as: ‘The voluntary and daily suffering of hard and difficult things, for the sake of honour and usefulness.”
In the midst of the most challenging public health emergency of our lifetimes, I am seeing hospitalists – and nurses, respiratory therapists, and countless other health care workers – doing exactly this, every day. I’m so incredibly proud of you all, and thankful beyond words.
I doubt that victorious endurance comes naturally to any of us; it’s something we work at, pursue and nurture. What’s the secret to cultivating victorious endurance in the midst of unimaginable stress? I’m pretty sure there’s no specific formula. I don’t mean to sound like a Pollyanna or to make light of the tumult and turmoil of these times, but here are a few things that, based on my own experiences, may help cultivate this valuable virtue.
Be part of a support network. In the midst of great stress, and especially during this time of social distancing, it’s especially tempting to just hunker down, close in on ourselves, and shut others out – sometimes even our closest friends and loved ones. Maintaining relationships is just too exhausting. But you need people who can come alongside you and offer words of encouragement when you are at your lowest. And there’s nothing that will bring out the best in you like being there to encourage and support someone else. We all need to both receive and to give emotional support at a time like this.
Take the long view. When we’re in the middle of a serious crisis, it seems like the problems we’re facing will last forever. There’s no light at the end of the tunnel, no port in the storm. But even this pandemic won’t last forever. If we can keep in mind the fact that things will eventually get better and that the current situation isn’t permanent, it can help us maintain our perspective and have more patience with the current dysfunction.
Focus on who you want to be in this moment. This is the hardest time most of us have ever lived through, both professionally and personally. But let me throw you a challenge. When you look back on this time from the perspective of five years from now, or maybe ten, how will you want to remember yourself? Who will you want to have been during this time? Looking back, what will make you proud of how you handled this challenge? Be that person.
Look for things to be thankful for. In the midst of the chaos that is our lives and our work right now, I believe we can still occasionally see moments of grace if we keep our eyes open for them. If we aren’t looking for them, we may miss them entirely. And those small moments of love, touches of compassion, displays of selflessness, and even flashes of victorious endurance in yourself or others are gifts to be treasured and held on to – to give thanks for.
Embrace a cause greater than yourself. May I suggest that one thing that might help our efforts to cultivate the virtue of victorious endurance during difficult times might be to embrace a cause that is bigger than yourself; that is, one that lures you to focus beyond your immediate circumstances? What are you passionate about, outside of your life’s normal routine?
If you don’t have a passion, consider what you might become passionate about, with a little effort. For some of us, like me, this will be our faith in God. For others it may be advocating for an end to racism or for broader social justice issues. Maybe it’s working to overcome our cultural and political divisions or to strengthen the institutions of our democracy. Perhaps it’s getting involved with efforts to mitigate climate change. Maybe it’s reaching out to the homeless or hungry in your own community or mentoring a child who is being left behind by the demands of remote learning.
Or perhaps what you embrace is even closer to home: maybe it’s working to eliminate health disparities in your institution or health system, or figuring out how to use technology and resources differently to improve how care is being delivered during or after this pandemic. Maybe it’s as simple as re-committing yourself to personally care for every patient you see today with the very best you have to offer, and with patience, compassion, and grace.
Find something that sets your heart on fire. Something that makes you want to take this difficult time and “transmute tribulation into strength and glory.” Something that, when you look back on these days, will make you thankful that you didn’t just hunker down and subsist through them. Instead, you accomplished great things; you learned; you contributed; and you grew stronger and better.
That’s victorious endurance.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey. This essay was published initially on The Hospital Leader, the official blog of SHM.
When the X-Waiver gets X’ed: Implications for hospitalists
There are two pandemics permeating the United States: COVID-19 and addiction. To date, more than 468,000 people have died from COVID-19 in the U.S. In the 12-month period ending in May 2020, over 80,000 died from a drug related cause – the highest number ever recorded in a year. Many of these deaths involved opioids.
COVID-19 has worsened outcomes for people with addiction. There is less access to treatment, increased isolation, and worsening psychosocial and economic stressors. These factors may drive new, increased, or more risky substance use and return to use for people in recovery. As hospitalists, we have been responders in both COVID-19 and our country’s worsening overdose and addiction crisis.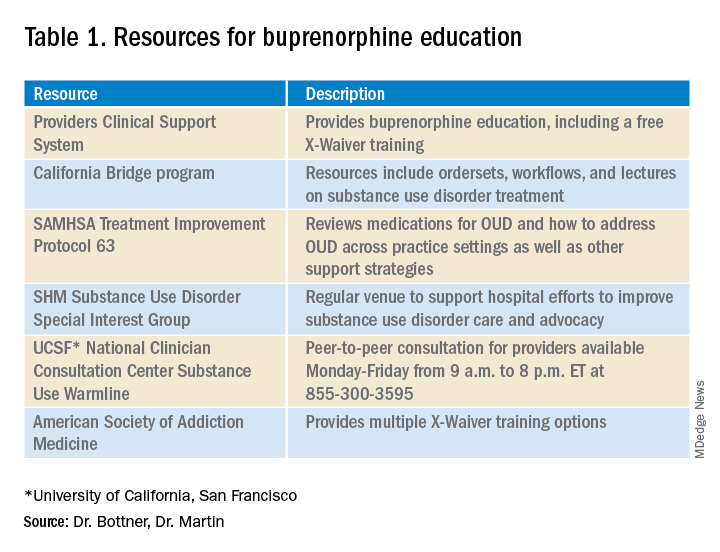
In December 2020’s Journal of Hospital Medicine article “Converging Crises: Caring for hospitalized adults with substance use disorder in the time of COVID-19”, Dr. Honora Englander and her coauthors called on hospitalists to actively engage patients with substance use disorders during hospitalization. The article highlights the colliding crises of addiction and COVID-19 and provides eight practical approaches for hospitalists to address substance use disorders during the pandemic, including initiating buprenorphine for opioid withdrawal and prescribing it for opioid use disorder (OUD) treatment.
Buprenorphine effectively treats opioid withdrawal, reduces OUD-related mortality, and decreases hospital readmissions related to OUD. To prescribe buprenorphine for OUD in the outpatient setting or on hospital discharge, providers need an X-Waiver. The X-Waiver is a result of the Drug Addiction Treatment Act 2000 (DATA 2000), which was enacted in 2000. It permits physicians to prescribe buprenorphine for OUD treatment after an 8-hour training. In 2016, the Comprehensive Addiction and Recovery Act extended buprenorphine prescribing to physician assistants (PAs) and advanced-practice nurses (APNs). However, PAs and APNs are required to complete a 24-hour training to receive the waiver.
On Jan. 14, 2021, the U.S. Department of Health and Human Services under the Trump administration announced it was removing the X-Waiver training previously required for physicians to prescribe this life-saving medication. However, on Jan. 20, 2021, the Biden administration froze the training requirement removal pending a 60-day review. The excitement about the waiver’s eradication further dampened on Jan. 25, when the plan was halted due to procedural factors coupled with the concern that HHS may not have the authority to void requirements mandated by Congress.
Many of us continue to be hopeful that the X-Waiver will soon be gone. The Substance Abuse and Mental Health Services Administration has committed to working with federal agencies to increase access to buprenorphine. The Biden administration also committed to addressing our country’s addiction crisis, including a plan to “make effective prevention, treatment, and recovery services available to all, including through a $125 billion federal investment.”
Despite the pause on HHS’s recent attempt to “X the X-Waiver,” we now have renewed attention and interest in this critical issue and an opportunity for greater and longer-lasting legislative impact. SHM supports that Congress repeal the legislative requirement for buprenorphine training dictated by DATA 2000 so that it cannot be rolled back by future administrations. To further increase access to buprenorphine treatment, the training requirement should be removed for all providers who care for individuals with OUD.
The X-Waiver has been a barrier to hospitalist adoption of this critical, life-saving medication. HHS’s stance to nix the waiver, though fleeting, should be interpreted as an urgent call to the medical community, including us as hospitalists, to learn about buprenorphine with the many resources available (see table 1). As hospital medicine providers, we can order buprenorphine for patients with OUD during hospitalization. It is discharge prescriptions that have been limited to providers with an X-Waiver.
What can we do now to prepare for the eventual X-Waiver training removal? We can start by educating ourselves with the resources listed in table 1. Those of us who are already buprenorphine champions could lead trainings in our home institutions. In a future without the waiver there will be more flexibility to develop hospitalist-focused buprenorphine trainings, as the previous ones were geared for outpatient providers. Hospitalist organizations could support hospitalist-specific buprenorphine trainings and extend the models to include additional medications for addiction.
There is a large body of evidence regarding buprenorphine’s safety and efficacy in OUD treatment. With a worsening overdose crisis, there have been increasing opioid-related hospitalizations. When new medications for diabetes, hypertension, or DVT treatment become available, as hospitalists we incorporate them into our toolbox. As buprenorphine becomes more accessible, we can be leaders in further adopting it (and other substance use disorder medications while we are at it) as our standard of care for people with OUD.
Dr. Bottner is a physician assistant in the Division of Hospital Medicine at Dell Medical School at The University of Texas at Austin and director of the hospital’s Buprenorphine Team. Dr. Martin is a board-certified addiction medicine physician and hospitalist at University of California, San Francisco, and director of the Addiction Care Team at San Francisco General Hospital. Dr. Bottner and Dr. Martin colead the SHM Substance Use Disorder Special Interest Group.
There are two pandemics permeating the United States: COVID-19 and addiction. To date, more than 468,000 people have died from COVID-19 in the U.S. In the 12-month period ending in May 2020, over 80,000 died from a drug related cause – the highest number ever recorded in a year. Many of these deaths involved opioids.
COVID-19 has worsened outcomes for people with addiction. There is less access to treatment, increased isolation, and worsening psychosocial and economic stressors. These factors may drive new, increased, or more risky substance use and return to use for people in recovery. As hospitalists, we have been responders in both COVID-19 and our country’s worsening overdose and addiction crisis.
In December 2020’s Journal of Hospital Medicine article “Converging Crises: Caring for hospitalized adults with substance use disorder in the time of COVID-19”, Dr. Honora Englander and her coauthors called on hospitalists to actively engage patients with substance use disorders during hospitalization. The article highlights the colliding crises of addiction and COVID-19 and provides eight practical approaches for hospitalists to address substance use disorders during the pandemic, including initiating buprenorphine for opioid withdrawal and prescribing it for opioid use disorder (OUD) treatment.
Buprenorphine effectively treats opioid withdrawal, reduces OUD-related mortality, and decreases hospital readmissions related to OUD. To prescribe buprenorphine for OUD in the outpatient setting or on hospital discharge, providers need an X-Waiver. The X-Waiver is a result of the Drug Addiction Treatment Act 2000 (DATA 2000), which was enacted in 2000. It permits physicians to prescribe buprenorphine for OUD treatment after an 8-hour training. In 2016, the Comprehensive Addiction and Recovery Act extended buprenorphine prescribing to physician assistants (PAs) and advanced-practice nurses (APNs). However, PAs and APNs are required to complete a 24-hour training to receive the waiver.
On Jan. 14, 2021, the U.S. Department of Health and Human Services under the Trump administration announced it was removing the X-Waiver training previously required for physicians to prescribe this life-saving medication. However, on Jan. 20, 2021, the Biden administration froze the training requirement removal pending a 60-day review. The excitement about the waiver’s eradication further dampened on Jan. 25, when the plan was halted due to procedural factors coupled with the concern that HHS may not have the authority to void requirements mandated by Congress.
Many of us continue to be hopeful that the X-Waiver will soon be gone. The Substance Abuse and Mental Health Services Administration has committed to working with federal agencies to increase access to buprenorphine. The Biden administration also committed to addressing our country’s addiction crisis, including a plan to “make effective prevention, treatment, and recovery services available to all, including through a $125 billion federal investment.”
Despite the pause on HHS’s recent attempt to “X the X-Waiver,” we now have renewed attention and interest in this critical issue and an opportunity for greater and longer-lasting legislative impact. SHM supports that Congress repeal the legislative requirement for buprenorphine training dictated by DATA 2000 so that it cannot be rolled back by future administrations. To further increase access to buprenorphine treatment, the training requirement should be removed for all providers who care for individuals with OUD.
The X-Waiver has been a barrier to hospitalist adoption of this critical, life-saving medication. HHS’s stance to nix the waiver, though fleeting, should be interpreted as an urgent call to the medical community, including us as hospitalists, to learn about buprenorphine with the many resources available (see table 1). As hospital medicine providers, we can order buprenorphine for patients with OUD during hospitalization. It is discharge prescriptions that have been limited to providers with an X-Waiver.
What can we do now to prepare for the eventual X-Waiver training removal? We can start by educating ourselves with the resources listed in table 1. Those of us who are already buprenorphine champions could lead trainings in our home institutions. In a future without the waiver there will be more flexibility to develop hospitalist-focused buprenorphine trainings, as the previous ones were geared for outpatient providers. Hospitalist organizations could support hospitalist-specific buprenorphine trainings and extend the models to include additional medications for addiction.
There is a large body of evidence regarding buprenorphine’s safety and efficacy in OUD treatment. With a worsening overdose crisis, there have been increasing opioid-related hospitalizations. When new medications for diabetes, hypertension, or DVT treatment become available, as hospitalists we incorporate them into our toolbox. As buprenorphine becomes more accessible, we can be leaders in further adopting it (and other substance use disorder medications while we are at it) as our standard of care for people with OUD.
Dr. Bottner is a physician assistant in the Division of Hospital Medicine at Dell Medical School at The University of Texas at Austin and director of the hospital’s Buprenorphine Team. Dr. Martin is a board-certified addiction medicine physician and hospitalist at University of California, San Francisco, and director of the Addiction Care Team at San Francisco General Hospital. Dr. Bottner and Dr. Martin colead the SHM Substance Use Disorder Special Interest Group.
There are two pandemics permeating the United States: COVID-19 and addiction. To date, more than 468,000 people have died from COVID-19 in the U.S. In the 12-month period ending in May 2020, over 80,000 died from a drug related cause – the highest number ever recorded in a year. Many of these deaths involved opioids.
COVID-19 has worsened outcomes for people with addiction. There is less access to treatment, increased isolation, and worsening psychosocial and economic stressors. These factors may drive new, increased, or more risky substance use and return to use for people in recovery. As hospitalists, we have been responders in both COVID-19 and our country’s worsening overdose and addiction crisis.
In December 2020’s Journal of Hospital Medicine article “Converging Crises: Caring for hospitalized adults with substance use disorder in the time of COVID-19”, Dr. Honora Englander and her coauthors called on hospitalists to actively engage patients with substance use disorders during hospitalization. The article highlights the colliding crises of addiction and COVID-19 and provides eight practical approaches for hospitalists to address substance use disorders during the pandemic, including initiating buprenorphine for opioid withdrawal and prescribing it for opioid use disorder (OUD) treatment.
Buprenorphine effectively treats opioid withdrawal, reduces OUD-related mortality, and decreases hospital readmissions related to OUD. To prescribe buprenorphine for OUD in the outpatient setting or on hospital discharge, providers need an X-Waiver. The X-Waiver is a result of the Drug Addiction Treatment Act 2000 (DATA 2000), which was enacted in 2000. It permits physicians to prescribe buprenorphine for OUD treatment after an 8-hour training. In 2016, the Comprehensive Addiction and Recovery Act extended buprenorphine prescribing to physician assistants (PAs) and advanced-practice nurses (APNs). However, PAs and APNs are required to complete a 24-hour training to receive the waiver.
On Jan. 14, 2021, the U.S. Department of Health and Human Services under the Trump administration announced it was removing the X-Waiver training previously required for physicians to prescribe this life-saving medication. However, on Jan. 20, 2021, the Biden administration froze the training requirement removal pending a 60-day review. The excitement about the waiver’s eradication further dampened on Jan. 25, when the plan was halted due to procedural factors coupled with the concern that HHS may not have the authority to void requirements mandated by Congress.
Many of us continue to be hopeful that the X-Waiver will soon be gone. The Substance Abuse and Mental Health Services Administration has committed to working with federal agencies to increase access to buprenorphine. The Biden administration also committed to addressing our country’s addiction crisis, including a plan to “make effective prevention, treatment, and recovery services available to all, including through a $125 billion federal investment.”
Despite the pause on HHS’s recent attempt to “X the X-Waiver,” we now have renewed attention and interest in this critical issue and an opportunity for greater and longer-lasting legislative impact. SHM supports that Congress repeal the legislative requirement for buprenorphine training dictated by DATA 2000 so that it cannot be rolled back by future administrations. To further increase access to buprenorphine treatment, the training requirement should be removed for all providers who care for individuals with OUD.
The X-Waiver has been a barrier to hospitalist adoption of this critical, life-saving medication. HHS’s stance to nix the waiver, though fleeting, should be interpreted as an urgent call to the medical community, including us as hospitalists, to learn about buprenorphine with the many resources available (see table 1). As hospital medicine providers, we can order buprenorphine for patients with OUD during hospitalization. It is discharge prescriptions that have been limited to providers with an X-Waiver.
What can we do now to prepare for the eventual X-Waiver training removal? We can start by educating ourselves with the resources listed in table 1. Those of us who are already buprenorphine champions could lead trainings in our home institutions. In a future without the waiver there will be more flexibility to develop hospitalist-focused buprenorphine trainings, as the previous ones were geared for outpatient providers. Hospitalist organizations could support hospitalist-specific buprenorphine trainings and extend the models to include additional medications for addiction.
There is a large body of evidence regarding buprenorphine’s safety and efficacy in OUD treatment. With a worsening overdose crisis, there have been increasing opioid-related hospitalizations. When new medications for diabetes, hypertension, or DVT treatment become available, as hospitalists we incorporate them into our toolbox. As buprenorphine becomes more accessible, we can be leaders in further adopting it (and other substance use disorder medications while we are at it) as our standard of care for people with OUD.
Dr. Bottner is a physician assistant in the Division of Hospital Medicine at Dell Medical School at The University of Texas at Austin and director of the hospital’s Buprenorphine Team. Dr. Martin is a board-certified addiction medicine physician and hospitalist at University of California, San Francisco, and director of the Addiction Care Team at San Francisco General Hospital. Dr. Bottner and Dr. Martin colead the SHM Substance Use Disorder Special Interest Group.
New child COVID-19 cases decline as total passes 3 million
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
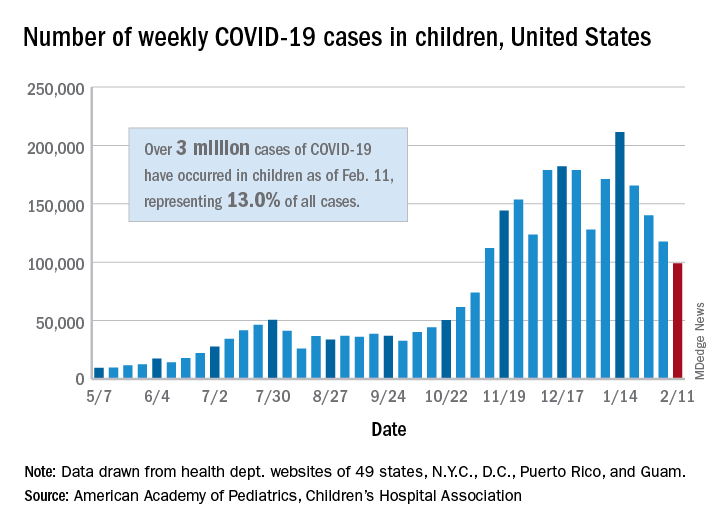
It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.


















