User login
Is the "breast is best" mantra an oversimplification?
The benefits of breastfeeding for infants have long been touted as numerous and supported by overwhelming evidence. The World Health Organization (WHO), American College of Obstetricians and Gynecologists, American Academy of Pediatrics (AAP), and American Academy of Family Physicians all strongly recommend exclusive breastfeeding for the first 6 months of life, citing numerous health benefits for child and mother. These groups recommend that some breastfeeding be continued through the first 12 months of life, or longer, as desired (the WHO extends the recommendation to 2 years).1-4 In 2000, the Surgeon General of the United States released a strategic plan to increase rates of breastfeeding,5 setting goals (by 2010) of:
- 75% of mothers leaving the hospital breastfeeding
- 50% of babies breastfeeding at 6 months
- 25% of babies breastfeeding at 1 year.
Massive public health campaigns citing data for the many benefits of breastfeeding have been launched with the goal of increasing the breastfeeding rate. In 2014, statistics offered a testament to the success of these campaigns6:
- 82.5% of infants had been breastfed “ever”
- 55.3% were breastfed “some”
- 24.9% were breastfed exclusively through 6 months of age
- 33.7% were breastfed “some” at 12 months.
Breastfeeding advocacy has become clouded
In recent years, an increasing number of researchers, physicians, and authors have begun to question whether, in the United States, the benefits of breastfeeding children are exaggerated and the emphasis on breastfeeding might be leading to feelings of inadequacy, guilt, and anxiety among mothers.7-13 In 2016, the US Preventive Services Task Force (USPSTF) amended its recommendation to “promote and support breastfeeding” to simply “support breastfeeding”—a change that created substantial debate and prompted the Task Force to clarify its stance in changing the language: In its response to public comment, the USPSTF said that its position regarding promotion had not changed, but the language in the original statement had been revised to “ensure that the autonomy of women is respected.” 2,14-16
In contrast, others suggest counseling women on the risks of formula feeding rather than on the benefits of breastfeeding, citing substantial health outcome distinctions.17 Indeed, wide-ranging conclusions have been drawn from the same data on the topic, potentially creating uncertainty for physicians on how best to counsel women on their choice of how to feed their infant.
In this article, we address this uncertainty by utilizing the most recent and comprehensive data to examine infant health outcomes. When possible, the number needed to treat (NNT) for a given outcome has been calculated or approximated, allowing the reader to estimate the likelihood of benefit for an individual mother–infant dyad. Exercise caution when interpreting the NNT, however: The numbers suggest causality that cannot be definitively established using the observational data on which those numbers are based.
Continue to: Infectious disease
Infectious disease
Acute otitis media. Exclusive breastfeeding for 6 months is associated with a 43% reduction in the risk of acute otitis media (AOM) by 2 years of age (odds ratio [OR]=0.57; 95% confidence interval [CI], 0.44-0.75). Beyond 2 years of age, or when comparing “ever” and “never” breastfeeding, the effect disappears. All studies in this meta-analysis had serious limitations.18
Nearly half of children will have at least one case of AOM by one year of age; 80%, by 2 years.19,20 Since the introduction of the heptavalent pneumococcal conjugate vaccine, the rate of AOM at 2 years has fallen by as much as 20%.21 Assuming an incidence of 60% to 80% of AOM by 2 years, only 2 or 3 infants need to be exclusively breastfed for 6 months to prevent a single case of AOM.18 Prevention of AOM through breastfeeding may be related to head position during feeding, antibacterial effects of breast milk, protective oral microbiome in the breastfed infant pharynx, and/or prevention of primary viral upper respiratory infection (URI), which nearly always precedes AOM.18,19
Upper and lower respiratory tract infections. Infants who are exclusively breastfed for 4 months and partially breastfed after 4 months have a lower risk of URI (OR=0.65; 95% CI, 0.51-0.83) and of lower respiratory tract infection (LRTI; OR=0.50; 95% CI, 0.32-0.72).22
The effect is stronger for URI among infants exclusively breastfed for at least 6 months (OR=0.37; 95% CI, 0.18-0.74), but is no longer significant by that time for LRTI (OR=0.33; 95% CI, 0.08-1.40). Importantly, AOM was included in the URI group, and, as previously discussed, AOM has independently been shown to have an inverse relationship with breastfeeding duration.
At 7 to 12 months of age, no association was seen between breastfeeding and the incidence of URI. Curiously, an association with LRTI was again detected for infants breastfed exclusively for 4 months and partially thereafter, but was not detected with exclusive breastfeeding for at least 6 months (OR=0.46; 95% CI, 0.31-0.69). In this study, in the first 6 months of life, 40% of infants had a URI and 8% had an LRTI. The findings in this cohort suggest an NNT of 6 or 7 for prevention of URI and an NNT of 25 for prevention of LRTI in the first 6 months of life.22
Continue to: Children younger than 2 years are...
Children younger than 2 years are estimated to have approximately 6 bouts of the common cold a year, and essentially 100% have at least one bout—perhaps lowering the NNT for URI if applied widely. However, these data are not divided into 6-month intervals, making accurate extrapolation difficult.23
Gastrointestinal infection. The rate of diarrheal illness in the first year of life is lower in infants who are exclusively breastfed for at least 4 months and partially breastfed after.
Both the Promotion of Breastfeeding Intervention Trial (PROBIT; a clinical trial in which infants were randomized to a breastfeeding education intervention or standard care) and a 2010 prospective cohort study in the Netherlands of more than 3400 infants found a reduction in the risk of one or more gastrointestinal (GI) infections at a similar rate.22,24
- In PROBIT, 9.1% of infants in the intervention group, compared to 13.2% in the standard care group (OR=0.60; 95% CI, 0.40-0.91), had one or more GI infections at 12 months of age.24
- In the 2010 Netherlands cohort, 8% of infants had a GI infection by 6 months of age. Infants breastfed exclusively for at least 4 or 6 months had a decreased risk for GI infection (respectively: adjusted OR=0.41; 95% CI, 0.26-0.64 and adjusted OR=0.46; 95% CI, 0.14-1.59). No such association was found for any feeding group 7 to 12 months of age.22
These studies are notable for the low incidence of GI infection, which is frequently cited as 1.3 to 2.3 episodes per child per year in children younger than 3 years in the United States.25 However, that high incidence has likely declined significantly since the introduction of rotavirus vaccine in 2006. In the years following the introduction of the vaccine, infant visits for gastroenteritis decreased by >90% in all care settings in the South, Northeast, and Midwest regions of the United States and by 53% to 63% in the West region.26 Recent accurate epidemiologic information, in an era of significantly higher vaccination rates, is lacking.
Assuming the low incidence of GI infection reported in PROBIT and the Netherlands trials, about 25 to 30 infants need to be exclusively breastfed for 4 to 6 months to prevent a single GI infection during the first 6 to 12 months of life.22,24 Assuming a 60% incidence by age 12 months before introduction of the rotavirus vaccine, the NNT would be approximately 4.24 The true number is likely somewhere between those 2 NNTs.
Continue to: Hospitalization
Hospitalization
Risk of infection is decreased. A large cohort study in Scotland, involving more than 500,000 children, found an association between exclusive breastfeeding for 6 to 8 weeks and decreased risk of hospitalization within the first 6 months of life. Formula-fed and mixed-fed infants had an increased hazard ratio (HR) for hospitalization for common childhood illness (HR=1.40; 95% CI, 1.35-1.45 for formula-fed infants and HR=1.18; 95% CI, 1.11-1.25 for mixed-fed infants).27 The study also found increased rates of hospitalization for conditions for which other meta-analyses have failed to show a protective effect from breastfeeding—leading to suspicion of residual confounding in the study. Another United Kingdom cohort demonstrated lower rates of hospitalization for GI infection (NNT=171) and LRTI (NNT=115) among exclusively breastfed infants by 8 months of age.28
Risk of neonatal readmission is increased. Late preterm infants who are exclusively breastfed are nearly twice as likely to be hospitalized as breastfed term or non-breastfed preterm infants, primarily due to dehydration, failure to thrive, weight loss, and hyperbilirubinemia. In fact, exclusive breastfeeding at discharge from the hospital is likely the single greatest risk factor for hospital readmission in newborns.29,30 Term infants who are exclusively breastfed are more likely to be hospitalized compared to formula-fed or mixed-fed infants, due to hyperbilirubinemia, dehydration, hypernatremia, and weight loss (number needed to harm (NNH)=71).30-32 For weight loss >10% of birth weight with or without hospitalization, the NNH for breastfed infants is 13.32
Many of these hospitalizations and events could be avoided with appropriate monitoring and medically indicated supplementation; the likelihood of long-term harm is low. Formula supplementation is often avoided if possible in hospitals to promote exclusive breastfeeding; however, several small randomized clinical trials have demonstrated that limited formula supplementation in breastfed infants does not affect the breastfeeding continuation rate at 3 and 6 months, and, therefore, might be a way to decrease infant rehospitalization.33,34
Necrotizing enterocolitis
In preterm infants, breastfeeding has been associated with a lower rate of necrotizing enterocolitis. In the 2007 Agency for Healthcare Research and Quality report, the association was found to be only marginally statistically significant, and the authors warned that, first, evidence is old and heterogeneous and, second, present preterm formula is much different than the formula used in earlier studies of preterm infant nutrition and necrotizing enterocolitis.35 A 2012 Cochrane review included newer studies in its analysis but reached the same conclusion on the quality and heterogeneity of available evidence, with a NNT of 25.36
Continue to: Sudden infant death syndrome
Sudden infant death syndrome
There is a statistically significant association between sudden infant death syndrome (SIDS) and feeding method. Infants whose cause of death is SIDS are approximately one half as likely to have been breastfed as matched controls.35,37
In 2005, AAP did not recommend breastfeeding as a means to reduce the risk of SIDS because available evidence was mixed, and studies at the time were poorly controlled.38 Since that time, case-control meta-analyses have shed additional light on the association between SIDS and feeding method.35,37
The protective effect exists for any amount of breastfeeding and is stronger for exclusive breastfeeding, suggesting a protective role—not simply an association. Caution should be employed with this conclusion, however, because the studies included in the meta-analysis used univariate analysis primarily and did not control sufficiently for known confounders. In addition, the authors warn that publication bias might overestimate the association.38
Potential mechanisms of a protective role include decreased risk of infection and greater arousability from sleep in breastfed infants. Assuming a protective role, available data suggest that more than 3500 infants need to be breastfed to prevent one case of SIDS.39
Continue to: Allergic disease
Allergic disease
Asthma. There is evidence of a small protective effect of breastfeeding “ever” on asthma at 5 to 18 years of age in high-income countries (OR=0.90; 95% CI, 0.83-0.97). A family history of asthma or atopy did not affect this finding. The authors note there is some evidence of publication bias in this review, which is the largest and most comprehensive on the topic.40
With a lifetime prevalence of asthma in the United States of approximately 13.2%, this association would confer an NNT of roughly 76.41 Earlier, the literature demonstrated mixed and conflicting evidence, and some experts suggested an effect only when there is a family history of asthma or atopy.36
Eczema. For children younger than 2 years, there is low-grade- and very-low-grade-quality evidence that exclusive breastfeeding longer than 3 to 4 months is associated with a reduced risk of eczema (OR=0.74; 95% CI, 0.57-0.97).40
Previously, data suggested that this association existed only in children who had a family history of atopy.35 The protective association, however, exists regardless of family history and does not persist beyond 2 years of age. The authors noted evidence of publication bias, reverse causation, and misdiagnosis of early childhood rashes as eczema as limitations of their findings.40
Continue to: Reliable epidemiologic evidence...
Reliable epidemiologic evidence on the incidence of eczema in infants in the United States is limited, but the prevalence in the United States in children younger than 17 years is approximately 10.7% (with wide regional variation). Extrapolating these data generously, the NNT to prevent eczema in the first 2 years of life could be estimated at approximately 36.42
Allergic rhinitis. There is low-grade- and very-low-grade-quality evidence that more breastfeeding, compared to less breastfeeding, is associated with a lower risk of allergic rhinitis in children younger than 5 years (OR=0.79; 95% CI, 0.63-0.98). The association exists regardless of family history and disappears after 5 years of age. The differentiation of allergic rhinitis from rhinovirus infection (for which there is higher-quality evidence of a protective effect with breastfeeding) must be considered when interpreting these data.40
Reliable epidemiologic evidence on allergic rhinitis in children younger than 5 years is lacking, and incidence varies by region. A rough estimate, using data from 6- and 7-year-olds, indicates an NNT of 54 to 70.43
Food allergy. There is no evidence to suggest an association between breastfeeding and food allergy, either as protective or as a risk factor, and studies are limited.40 Interestingly, as data accumulate associating early exposure to foods with protection, some authors have proposed reexamining the recommendation from WHO and US health organizations for exclusive breastfeeding for the first 6 months of life.7,44
Continue to: Dental health
Dental health
Dental caries. There is consistent evidence that breastfeeding beyond 12 months of age is associated with the development of dental caries of deciduous teeth to 6 years of age (OR=2.90; 95% CI, 2.33-3.60). Many of the studies that showed this association did not control for the introduction of sugary foods and drinks, and there was a trend toward publication bias showing the association.45
Dental malocclusion. There is consistent evidence for approximately a two-thirds reduction in malocclusions in deciduous teeth in breastfed infants (OR=0.32; 95% CI, 0.25-0.40). Although the large majority of these data come from low-income and middle-income countries, the incidence of malocclusion is not thought to be associated with socioeconomic status, as so many other breastfeeding outcomes are.46
Childhood leukemia
In the largest meta-analysis available, a statistically significant inverse relationship between any breastfeeding for >6 months and childhood leukemia is evident in developed countries (OR=0.84; 95% CI, 0.78-0.91), although significant heterogeneity among studies and lack of control for confounding variables are significant limitations. In particular, an association has been demonstrated with acute lymphoblastic leukemia (ALL) but not with acute myelogenous leukemia.47 Given the rarity of childhood ALL, approximately 12,500 infants would need to be breastfed to prevent one case.48
Continue to: Long-term outcomes
Long-term outcomes
Cognitive development. Several studies conducted in developed countries have linked breastfeeding to positive cognitive outcomes in children, including higher intelligence quotient (IQ).35,49-52
These effects are conflicting, however, in studies that include sibling analysis and ones that control for maternal IQ.8,35,43,52-54 In the 2013 WHO meta-analysis, breastfeeding was associated with an increase of 2.2 points on normalized testing when only high-quality studies were included.51 A 2015 meta-analysis identified 4 high-quality studies with a large sample size and recall time <3 years, which demonstrated a mean difference of 1.76 points in IQ (95% CI, 0.25-3.26) in childhood and adolescence.52 Although statistically significant, this modest increase is of questionable clinical benefit and of unknown duration.
Obesity. The relationship between breastfeeding and obesity later in life is debatable. A large, systematic 2014 review of 15 cohort and 10 cross-sectional studies found a significantly reduced risk of childhood obesity among children who were breastfed (adjusted OR=0.78; 95% CI, 0.74-0.81).55 However, the review included studies that controlled for different confounders, and smaller effects were found in studies in which more confounders were taken into account.
The 2013 WHO meta-analysis found a small (approximately 10%) reduction in the prevalence of overweight or obese children, but cautioned that residual confounding and publication bias were likely.51 At 6.5 and 11.5 years of follow-up, PROBIT failed to demonstrate a protective effect for exclusively or “ever” breastfed infants.56 Sibling analysis similarly fails to demonstrate a statistically significant relationship.8
Continue to: A 2015 meta-analysis of 23 high-quality studies...
A 2015 meta-analysis of 23 high-quality studies with a sample size >1500 children and controlled for important confounders showed a pooled reduction in the prevalence of overweight or obesity of 13% (95% CI, 6-19).57 The protection in this meta-analysis showed a dilution of the effect as the participants aged and an inverse relationship of the effect with sample size.
Breastfeeding is, therefore, unlikely to play a significant, if any, role in combatting the obesity epidemic.
Hypertension. A meta-analysis of high-quality trials demonstrates a <1 mm Hg reduction in systolic blood pressure and no significant difference in diastolic pressure in breastfed infants.57 Similarly, no significant effect of breastfeeding on blood pressure has been demonstrated in trials of preterm infants.51
Type 2 diabetes. Available data are limited and heterogeneous for the association between breastfeeding and later development of type 2 diabetes. Only 2 high-quality trials were identified in the 2013 WHO meta-analysis, and their results conflict.51 A 2015 meta-analysis identified only 3 high-quality studies, without a statistically significant relationship.57
Dyslipidemia. Although earlier data suggested an association between breastfeeding and reduced cholesterol levels later in life, the 2013 WHO meta-analysis and a 2015 meta-analysis concluded that no association exists. The limited data available for preterm infants conflict.51,57
Growth. There is no evidence that feeding method has a short- or long-term effect on weight gain or length gain in preterm or term infants.35,36,58
Death. No clear association has been found between mortality and breastfeeding status in developed countries, except for the association with SIDS.35
Continue to: What issues frame and guide counseling on breastfeeding?
What issues frame and guide counseling on breastfeeding?
There is that “problem” with the evidence. The evidence for infant breastfeeding status and its association with health outcomes faces significant limitations; the great majority of those limitations tend to overestimate the benefits of breastfeeding. Nearly all evidence is based on observational studies, in which causality cannot be determined and self-selection bias, recall bias, and residual confounding limit the value or strength of the findings.
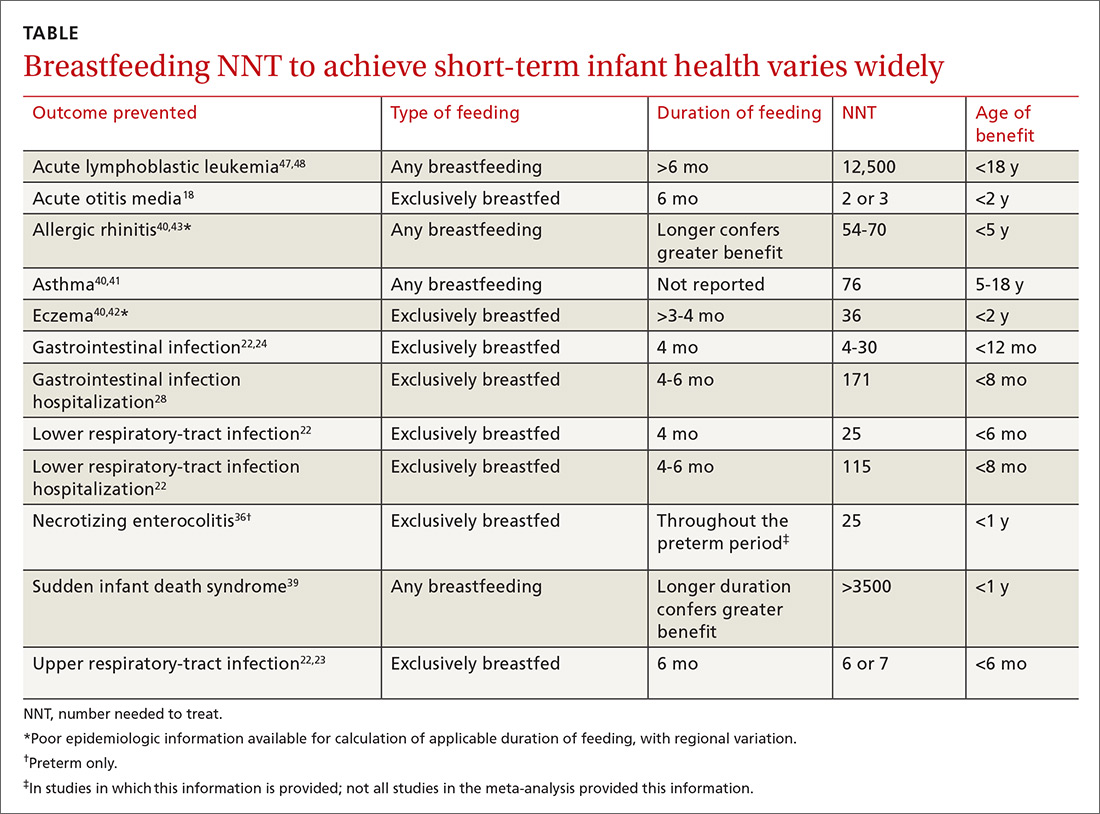
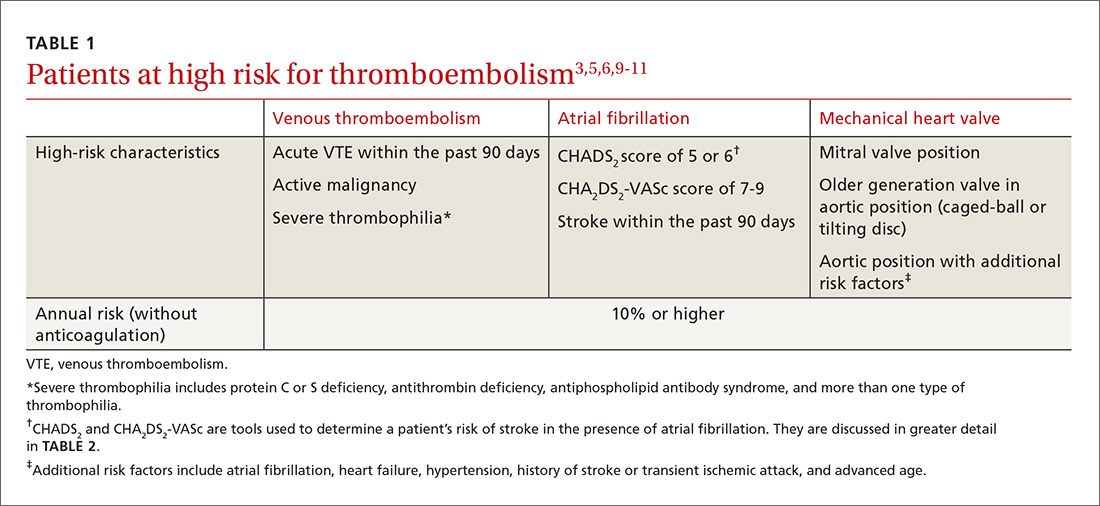
Breastfeeding rates are strongly socially patterned alongside socioeconomic status, race, and education level, all of which are simultaneously strongly tied to short- and long-term health outcomes.6 Other factors limiting the strength of the data set include varying definitions of infant feeding practices in different studies, varying definitions of outcomes and diseases, reverse causation, and evidence of publication bias in many meta-analyses. Given these shortcomings, the NNTs in this article probably represent a best-case scenario for breastfeeding outcomes for infants in the United States (TABLE 118,22-24,28,36,39-43,47,48).
Data need to be put into context. The NNTs for many breastfeeding outcomes (TABLE) compare favorably with other recommended interventions, particularly for other preventive care measures. Two examples: 81 mg/d aspirin for a 50-year-old man has an NNT of 35 to 45 for preventing nonfatal myocardial infarction, and the number needed to invite to screen with mammography to prevent one breast cancer death for a 50-year-old woman is 1339.59,60
In both of these examples, >95% of patients will not benefit from the intervention, yet these preventive measures are routinely recommended and have a significant impact at the public health level. Notably, these outcomes are more serious than most breastfeeding outcomes; have a longer-lasting effect, better-quality data, and better data for potential harms; are causally linked to the intervention; and require much less effort and commitment of time than breastfeeding.
The question must be reckoned with: Can advocacy be harmful?
In recent years, a growing number of concerns have been raised about:
- the potential harms of breastfeeding advocacy
- exaggeration of the benefits of breastfeeding
- promotion of breastfeeding at the expense of evidence-based medicine.
The “Ten Steps to Successful Breastfeeding” program of the Baby-friendly Hospital Initiative (BFHI; launched by UNICEF and WHO) has come under scrutiny because of an increasing number of reports of sudden unexpected postnatal collapse; fall injuries; modeling and encouragement of unsafe sleep practices; an overly rigid resistance to the use of formula supplementation; and the ban on pacifier use.61,62 The BFHI, promoted by the Centers for Disease Control and Prevention, is increasingly being adopted by hospitals with the expressed goal of increasing the breastfeeding rate from birth to discharge.
Continue to: Some of the "Ten Steps"...
Some of the “Ten Steps,” such as the call for skin-to-skin care and 24-hour rooming-in, have well-established benefit yet, when performed without supervision, can have the rare but serious unintended consequences of sudden unexpected postnatal collapse (the incidence of which may be higher than that of SIDS) and unsafe sleeping practices.62,63
Furthermore, despite evidence that early formula supplementation, when medically necessary, does not adversely impact the breastfeeding rate, the “Ten Steps” program advises that giving formula before breast milk comes in might “lead to failure to breastfeed.”33,34,61,63
Similarly, the ban on pacifiers is contrary to available evidence. The use of pacifiers before last sleep is more protective against SIDS than breastfeeding (NNT=2733), and there is evidence at one hospital that BFHI-inspired pacifier restriction is associated with a decrease in the rate of breastfeeding.64,65
Other harms of advocacy are even more poorly studied. Most of the evidence for harm comes from the psychology and social science literature—not the medical literature, perhaps because the prevailing opinion in the medical community is that breastfeeding has overwhelming evidence for benefit. In fact, in the USPSTF’s 2008 recommendation, the evidence review of breastfeeding promotion practices in primary care did not identify a single study that measured harm; in the 2016 update of that recommendation, only 2 such studies were identified.15,66
The literature that does investigate harm consistently finds that women who have difficulty breastfeeding or choose formula feeding report feelings of inadequacy, guilt, loss of agency, anxiety, and physical pain during breastfeeding that interferes with 1) their ability to bond or otherwise care for their infant and 2) competing work obligations.11-13,67-69 Given the lack of attention paid to these variables in the medical literature, it is the individual mother who is best positioned to weigh these factors against the benefits of breastfeeding.
Continue to: Shared decision-making is best—for mother and baby
Shared decision-making is best—for mother and baby
Breastfeeding might prevent certain infections in as many as 50% of infants, but a mother unable to breastfeed can take solace in the fact that >95% of breastfed infants will not realize any benefit from the preventive potential of breastfeeding in regard to hospitalization or allergic disease, and >99% will not realize benefit from either the prevention of SIDS or ALL, or from improvement in long-term health measures (except for, perhaps, a slightly higher IQ). The “breast is best” mantra is likely true at a public-health level; for the individual mother–infant dyad, however, where there is a need to balance personal, social, family, and financial factors, that mantra is an oversimplification.
Regrettably, there is a paucity of data on the risks of breastfeeding promotion—an area that deserves more study. Balancing the abundant, but often limited-quality, data on the benefits of breastfeeding and the sheer lack of data regarding the risks of advocacy represents a clinical and an ethical challenge for physicians. It is a challenge that can only be resolved through individualization of care and shared decision-making, in which the physician is expert on the benefits of breastfeeding, and the mother is expert on the personal circumstances to be weighed against those benefits.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: World Health Organization, and New York, NY: UNICEF; 2003. Available at: www.who.int/maternal_child_adolescent/documents/9241562218/en/. Accessed April 4, 2018.
2. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Breastfeeding Expert Work Group. Committee Opinion No. 658: Optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2016;127:e86-e92.
3. Gartner LM, Morton J, Lawrence RA, et al; American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496-506.
4. Breastfeeding (policy statement). Leawood, KS: American Academy of Family Physicians; 2007. Available at: https://www.aafp.org/about/policies/all/breastfeeding.html. Accessed April 3, 2018.
5. Office of the Surgeon General (US); Centers for Disease Control and Prevention (US); Office on Women’s Health (US). The Surgeon General’s call to action to support breastfeeding. Rockville, MD: US Department of Health and Human Services; 2011. Available at: www.surgeongeneral.gov/library/calls/breastfeeding/index.html. Updated August 12, 2014. Accessed April 4, 2018.
6. Breastfeeding: data & statistics. Atlanta, GA: Centers for Disease Control and Prevention; December 11, 2017. Available at: http://www.cdc.gov/breastfeeding/data/. Accessed May 17, 2018.
7. Fewtrell M, Wilson DC, Booth I, et al. A. Six months of exclusive breast feeding: how good is the evidence? BMJ. 2010;342:c5955.
8. Colen CG, Ramey DM. Is breast truly best? Estimating the effect of breastfeeding on long-term child wellbeing in the United States using sibling comparisons. Soc Sci Med. 2014;109:55-65.
9. Wolf J. Is Breast Best? Taking on the Breastfeeding Experts and the New High Stakes of Motherhood. New York, NY: NYU Press; 2010.
10. Tuteur A. Push Back: Guilt in the Age of Natural Parenting. New York, NY: HarperCollins Publishers; 2016.
11. Lee E. Health, morality, and infant feeding: British mothers’ experiences of formula milk use in the early weeks. Sociol Health Illn. 2007;29:1075-1090.
12. Williams K, Donaghue N, Kurz T. “Giving guilt the flick”?: an investigation of mothers’ talk about guilt in relation to infant feeding. Psychol Women Q. 2013;37:97-112.
13. Fahlquist JN, Roeser S. Ethical problems with information on infant feeding in developed countries. J Health Polit Policy Law. 2012;37:155-160.
14. U.S. Preventive Services Task Force. Final Recommendation Statement. Breastfeeding: Counseling. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breastfeeding-counseling. Accessed April 4, 2018.
15. US Preventive Services Task Force. Primary Care Interventions to Support Breastfeeding: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1688-1693.
16. Zahn CM, Hanley LE. Concerns over USPSTF draft recommendation on breastfeeding interventions [letter]. Washington, DC: The American College of Obstetricians and Gynecologists; May 18, 2016. Available at: https://www.acog.org/-/media/Departments/Breastfeeding/Breast-Feeding-ACOG-USPSTF.pdf?dmc=1&ts=20180518T1850056558. Accessed May 22, 2018.
17. Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2:222-231.
18. Bowatte G, Tham R, Allen KJ, et al. Breastfeeding and childhood acute otitis media: a systematic review and meta-analysis. Acta Paediatr. 2015;104:85-95.
19. Chonmaitree T, Trujillo R, Jennings K, et al. Acute otitis media and other complications of viral respiratory infection. Pediatrics. 2016;137:e20153555.
20. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
21. Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118:865-873.
22. Duijts L, Jaddoe VW, Hofman A, et al. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126:e18-e25.
23. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186:190-199.
24. Kramer MS, Chalmers B, Hodnett ED, et al; PROBIT Study Group (Promotion of Breastfeeding Intervention Trial). Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413-420.
25. Dennehy PH. Acute diarrheal disease in children: epidemiology, prevention, and treatment. Infect Dis Clin North Am. 2005;19:585-602.
26. Cortese MM, Tate JE, Simonsen L, et al. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatric Infect Dis J. 2010;29:489-494.
27. Ajetunmobi OM, Whyte B, Chalmers J, et al. Breastfeeding is associated with reduced childhood hospitalization: evidence from a Scottish birth cohort (1997-2009). J Pediatr. 2015;166:620-625.
28. Quigley MA, Kelly YJ, Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics. 2007;119:e837-e842.
29. Radtke JV. The paradox of breastfeeding-associated morbidity among late preterm infants. J Obstet Gynecol Neonatal Nurs. 2011;40:9-24.
30. Escobar GJ, Gonzales VM, Armstrong M, et al. Rehospitalization for neonatal dehydration: a nested case-control study. Arch Pediatr Adolesc Med. 2002;156:155-161.
31. Salas AA, Salazar J, Burgoa CV, et al. Significant weight loss in breastfed term infants readmitted for hyperbilirubinemia. BMC Pediatr. 2009;9:82.
32. Tarcan A, Tiker F, Vatandaş NS, et al. Weight loss and hypernatremia in breast-fed babies: frequency in neonates with non-hemolytic jaundice. J Paediatr Child Health. 2005;41:484-487.
33. Flaherman VJ, Aby J, Burgos AE, et al. Effect of early limited formula on duration and exclusivity of breastfeeding in at-risk infants: an RCT. Pediatrics. 2013;131:1059-1065.
34. Straňák Z, Feyereislova S, Černá M, et al. J. Limited amount of formula may facilitate breastfeeding: randomized, controlled trial to compare standard clinical practice versus limited supplemental feeding. Denning PW, ed. PLoS One. 2016;11:e0150053.
35. Ip S, Chung M, Raman G, et al. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Rockville, MD: Agency for Healthcare Research and Quality (US); 2007. Evidence Reports/Technology Assessments, No. 153. Available at: www.ncbi.nlm.nih.gov/books/NBK38337/. Accessed April 3, 2018.
36. Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014;(4):CD002971.
37. Hauck FR, Thompson JM, Tanabe KO, et al. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128:103-110.
38. American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116:1245-1255.
39. Moon RY, Fu L. Sudden infant death syndrome: an update. Pediatr Rev. 2012;33:314-320.
40. Lodge CJ, Tan DJ, Lau MX, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104:38-53.
41. Brim SN, Rudd RA, Funk RH, et al. Asthma prevalence among US children in underrepresented minority populations: American Indian/Alaska Native, Chinese, Filipino, and Asian Indian. Pediatrics. 2008;122:e217-e222.
42. Shaw TF, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
43. Mallol J, Crane J, von Mutius E, et al. The international study of asthma and allergies in childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr). 2013;41:73-85.
44. Flohr C, Nagel G, Weinmayr G, et al. Lack of evidence for a protective effect of prolonged breastfeeding on childhood eczema: lessons from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. Br J Dermatol. 2011;165:1280-1289.
45. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
46. Peres KG, Cascaes AM, Nascimento GG, et al. Effect of breastfeeding on malocclusions: a systematic review and meta-analysis. Acta Paediatr. 2015;104:54-61.
47. Amitya EL, Keinan-Boker L. Breastfeeding and childhood leukemia incidence: a meta-analysis and systematic review. JAMA Pediatr. 2015;169:e151025.
48. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943-1955.
49. Guxens M, Mendez MA, Moltó-Puigmartí C, et al. Breastfeeding, long-chain polyunsaturated fatty acids in colostrum, and infant mental development.
2012;129:1134-1140.
51. Horta BL, Victora CG. Long-term effects of breastfeeding: a systematic review. Geneva, Switzerland: World Health Organization; 2013. Available at: http://apps.who.int/iris/bitstream/10665/79198/1/9789241505307_eng.pdf. Accessed August 16, 2016.
52. Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104:14-19.
53. Der G, Batty GD, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. 2006;333:945.
54. Sajjad A, Tharner A, Kiefte-de Jong JC, et al. Breastfeeding duration and non-verbal IQ in children. J Epidemiol Community Health 2015;69:775-781.
55. Yan J, Liu L, Zhu Y, et al. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267.
56. Martin RM, Patel R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
57. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure, and type 2 diabetes: systematic review and meta-analysis. Acta Paediatr. 2015;104:30-37.
58. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Syst Rev. 2012;15:CD003517.
59. U.S. Preventive Services Task Force. Final recommendation statement: aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Published September 2017. Accessed April 3, 2018.
60. U.S. Preventive Services Task Force. Screening for breast cancer. Available at: www.uspreventiveservicestaskforce.org/Page/SupportingDoc/breast-cancer-screening/final-evidence-summary9. Published November 2009. Accessed April 2, 2018.
61. Bass JL, Gartley T, Kleinman R. Unintended consequences of current breastfeeding initiatives. JAMA Pediatr. 2016;170:923-924.
62. Feldman-Winter L, Goldsmith JP; Committee on Fetus and Newborn; Task Force on Sudden Infant Death Syndrome. Safe sleep and skin-to-skin care in the neonatal period for healthy term newborns. Pediatrics. 2016;138:e20161889.
63. The Mother and Child Health and Education Trust. Ten steps to successful breastfeeding. Available at: www.tensteps.org. Published November 8, 2017. Accessed April 3, 2018.
64. Hauck FR, Omojokun OO, Siadaty MS. Do pacifiers reduce the risk of sudden infant death syndrome? A meta-analysis. Pediatrics. 2005;116:e716-e723.
65. Kair LR, Kenron D, Etheredge K, et al. Pacifier restriction and exclusive breastfeeding. Pediatrics. 2013;131:e1101-e1107.
66. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
67. Wolf JB. Is breast really best? Risk and total motherhood in the National Breastfeeding Awareness Campaign. J Health Polit Policy Law. 2007;32:595-636.
68. Marshall JL, Godfrey M, Renfrew MJ. Being a ‘good mother’: managing breastfeeding and merging identities. Soc Sci Med. 2007;65:2147-2159.
69. Kelleher CM. The physical challenges of early breastfeeding. Soc Sci Med. 2006;63:2727-2738.
The benefits of breastfeeding for infants have long been touted as numerous and supported by overwhelming evidence. The World Health Organization (WHO), American College of Obstetricians and Gynecologists, American Academy of Pediatrics (AAP), and American Academy of Family Physicians all strongly recommend exclusive breastfeeding for the first 6 months of life, citing numerous health benefits for child and mother. These groups recommend that some breastfeeding be continued through the first 12 months of life, or longer, as desired (the WHO extends the recommendation to 2 years).1-4 In 2000, the Surgeon General of the United States released a strategic plan to increase rates of breastfeeding,5 setting goals (by 2010) of:
- 75% of mothers leaving the hospital breastfeeding
- 50% of babies breastfeeding at 6 months
- 25% of babies breastfeeding at 1 year.
Massive public health campaigns citing data for the many benefits of breastfeeding have been launched with the goal of increasing the breastfeeding rate. In 2014, statistics offered a testament to the success of these campaigns6:
- 82.5% of infants had been breastfed “ever”
- 55.3% were breastfed “some”
- 24.9% were breastfed exclusively through 6 months of age
- 33.7% were breastfed “some” at 12 months.
Breastfeeding advocacy has become clouded
In recent years, an increasing number of researchers, physicians, and authors have begun to question whether, in the United States, the benefits of breastfeeding children are exaggerated and the emphasis on breastfeeding might be leading to feelings of inadequacy, guilt, and anxiety among mothers.7-13 In 2016, the US Preventive Services Task Force (USPSTF) amended its recommendation to “promote and support breastfeeding” to simply “support breastfeeding”—a change that created substantial debate and prompted the Task Force to clarify its stance in changing the language: In its response to public comment, the USPSTF said that its position regarding promotion had not changed, but the language in the original statement had been revised to “ensure that the autonomy of women is respected.” 2,14-16
In contrast, others suggest counseling women on the risks of formula feeding rather than on the benefits of breastfeeding, citing substantial health outcome distinctions.17 Indeed, wide-ranging conclusions have been drawn from the same data on the topic, potentially creating uncertainty for physicians on how best to counsel women on their choice of how to feed their infant.
In this article, we address this uncertainty by utilizing the most recent and comprehensive data to examine infant health outcomes. When possible, the number needed to treat (NNT) for a given outcome has been calculated or approximated, allowing the reader to estimate the likelihood of benefit for an individual mother–infant dyad. Exercise caution when interpreting the NNT, however: The numbers suggest causality that cannot be definitively established using the observational data on which those numbers are based.
Continue to: Infectious disease
Infectious disease
Acute otitis media. Exclusive breastfeeding for 6 months is associated with a 43% reduction in the risk of acute otitis media (AOM) by 2 years of age (odds ratio [OR]=0.57; 95% confidence interval [CI], 0.44-0.75). Beyond 2 years of age, or when comparing “ever” and “never” breastfeeding, the effect disappears. All studies in this meta-analysis had serious limitations.18
Nearly half of children will have at least one case of AOM by one year of age; 80%, by 2 years.19,20 Since the introduction of the heptavalent pneumococcal conjugate vaccine, the rate of AOM at 2 years has fallen by as much as 20%.21 Assuming an incidence of 60% to 80% of AOM by 2 years, only 2 or 3 infants need to be exclusively breastfed for 6 months to prevent a single case of AOM.18 Prevention of AOM through breastfeeding may be related to head position during feeding, antibacterial effects of breast milk, protective oral microbiome in the breastfed infant pharynx, and/or prevention of primary viral upper respiratory infection (URI), which nearly always precedes AOM.18,19
Upper and lower respiratory tract infections. Infants who are exclusively breastfed for 4 months and partially breastfed after 4 months have a lower risk of URI (OR=0.65; 95% CI, 0.51-0.83) and of lower respiratory tract infection (LRTI; OR=0.50; 95% CI, 0.32-0.72).22
The effect is stronger for URI among infants exclusively breastfed for at least 6 months (OR=0.37; 95% CI, 0.18-0.74), but is no longer significant by that time for LRTI (OR=0.33; 95% CI, 0.08-1.40). Importantly, AOM was included in the URI group, and, as previously discussed, AOM has independently been shown to have an inverse relationship with breastfeeding duration.
At 7 to 12 months of age, no association was seen between breastfeeding and the incidence of URI. Curiously, an association with LRTI was again detected for infants breastfed exclusively for 4 months and partially thereafter, but was not detected with exclusive breastfeeding for at least 6 months (OR=0.46; 95% CI, 0.31-0.69). In this study, in the first 6 months of life, 40% of infants had a URI and 8% had an LRTI. The findings in this cohort suggest an NNT of 6 or 7 for prevention of URI and an NNT of 25 for prevention of LRTI in the first 6 months of life.22
Continue to: Children younger than 2 years are...
Children younger than 2 years are estimated to have approximately 6 bouts of the common cold a year, and essentially 100% have at least one bout—perhaps lowering the NNT for URI if applied widely. However, these data are not divided into 6-month intervals, making accurate extrapolation difficult.23
Gastrointestinal infection. The rate of diarrheal illness in the first year of life is lower in infants who are exclusively breastfed for at least 4 months and partially breastfed after.
Both the Promotion of Breastfeeding Intervention Trial (PROBIT; a clinical trial in which infants were randomized to a breastfeeding education intervention or standard care) and a 2010 prospective cohort study in the Netherlands of more than 3400 infants found a reduction in the risk of one or more gastrointestinal (GI) infections at a similar rate.22,24
- In PROBIT, 9.1% of infants in the intervention group, compared to 13.2% in the standard care group (OR=0.60; 95% CI, 0.40-0.91), had one or more GI infections at 12 months of age.24
- In the 2010 Netherlands cohort, 8% of infants had a GI infection by 6 months of age. Infants breastfed exclusively for at least 4 or 6 months had a decreased risk for GI infection (respectively: adjusted OR=0.41; 95% CI, 0.26-0.64 and adjusted OR=0.46; 95% CI, 0.14-1.59). No such association was found for any feeding group 7 to 12 months of age.22
These studies are notable for the low incidence of GI infection, which is frequently cited as 1.3 to 2.3 episodes per child per year in children younger than 3 years in the United States.25 However, that high incidence has likely declined significantly since the introduction of rotavirus vaccine in 2006. In the years following the introduction of the vaccine, infant visits for gastroenteritis decreased by >90% in all care settings in the South, Northeast, and Midwest regions of the United States and by 53% to 63% in the West region.26 Recent accurate epidemiologic information, in an era of significantly higher vaccination rates, is lacking.
Assuming the low incidence of GI infection reported in PROBIT and the Netherlands trials, about 25 to 30 infants need to be exclusively breastfed for 4 to 6 months to prevent a single GI infection during the first 6 to 12 months of life.22,24 Assuming a 60% incidence by age 12 months before introduction of the rotavirus vaccine, the NNT would be approximately 4.24 The true number is likely somewhere between those 2 NNTs.
Continue to: Hospitalization
Hospitalization
Risk of infection is decreased. A large cohort study in Scotland, involving more than 500,000 children, found an association between exclusive breastfeeding for 6 to 8 weeks and decreased risk of hospitalization within the first 6 months of life. Formula-fed and mixed-fed infants had an increased hazard ratio (HR) for hospitalization for common childhood illness (HR=1.40; 95% CI, 1.35-1.45 for formula-fed infants and HR=1.18; 95% CI, 1.11-1.25 for mixed-fed infants).27 The study also found increased rates of hospitalization for conditions for which other meta-analyses have failed to show a protective effect from breastfeeding—leading to suspicion of residual confounding in the study. Another United Kingdom cohort demonstrated lower rates of hospitalization for GI infection (NNT=171) and LRTI (NNT=115) among exclusively breastfed infants by 8 months of age.28
Risk of neonatal readmission is increased. Late preterm infants who are exclusively breastfed are nearly twice as likely to be hospitalized as breastfed term or non-breastfed preterm infants, primarily due to dehydration, failure to thrive, weight loss, and hyperbilirubinemia. In fact, exclusive breastfeeding at discharge from the hospital is likely the single greatest risk factor for hospital readmission in newborns.29,30 Term infants who are exclusively breastfed are more likely to be hospitalized compared to formula-fed or mixed-fed infants, due to hyperbilirubinemia, dehydration, hypernatremia, and weight loss (number needed to harm (NNH)=71).30-32 For weight loss >10% of birth weight with or without hospitalization, the NNH for breastfed infants is 13.32
Many of these hospitalizations and events could be avoided with appropriate monitoring and medically indicated supplementation; the likelihood of long-term harm is low. Formula supplementation is often avoided if possible in hospitals to promote exclusive breastfeeding; however, several small randomized clinical trials have demonstrated that limited formula supplementation in breastfed infants does not affect the breastfeeding continuation rate at 3 and 6 months, and, therefore, might be a way to decrease infant rehospitalization.33,34
Necrotizing enterocolitis
In preterm infants, breastfeeding has been associated with a lower rate of necrotizing enterocolitis. In the 2007 Agency for Healthcare Research and Quality report, the association was found to be only marginally statistically significant, and the authors warned that, first, evidence is old and heterogeneous and, second, present preterm formula is much different than the formula used in earlier studies of preterm infant nutrition and necrotizing enterocolitis.35 A 2012 Cochrane review included newer studies in its analysis but reached the same conclusion on the quality and heterogeneity of available evidence, with a NNT of 25.36
Continue to: Sudden infant death syndrome
Sudden infant death syndrome
There is a statistically significant association between sudden infant death syndrome (SIDS) and feeding method. Infants whose cause of death is SIDS are approximately one half as likely to have been breastfed as matched controls.35,37
In 2005, AAP did not recommend breastfeeding as a means to reduce the risk of SIDS because available evidence was mixed, and studies at the time were poorly controlled.38 Since that time, case-control meta-analyses have shed additional light on the association between SIDS and feeding method.35,37
The protective effect exists for any amount of breastfeeding and is stronger for exclusive breastfeeding, suggesting a protective role—not simply an association. Caution should be employed with this conclusion, however, because the studies included in the meta-analysis used univariate analysis primarily and did not control sufficiently for known confounders. In addition, the authors warn that publication bias might overestimate the association.38
Potential mechanisms of a protective role include decreased risk of infection and greater arousability from sleep in breastfed infants. Assuming a protective role, available data suggest that more than 3500 infants need to be breastfed to prevent one case of SIDS.39
Continue to: Allergic disease
Allergic disease
Asthma. There is evidence of a small protective effect of breastfeeding “ever” on asthma at 5 to 18 years of age in high-income countries (OR=0.90; 95% CI, 0.83-0.97). A family history of asthma or atopy did not affect this finding. The authors note there is some evidence of publication bias in this review, which is the largest and most comprehensive on the topic.40
With a lifetime prevalence of asthma in the United States of approximately 13.2%, this association would confer an NNT of roughly 76.41 Earlier, the literature demonstrated mixed and conflicting evidence, and some experts suggested an effect only when there is a family history of asthma or atopy.36
Eczema. For children younger than 2 years, there is low-grade- and very-low-grade-quality evidence that exclusive breastfeeding longer than 3 to 4 months is associated with a reduced risk of eczema (OR=0.74; 95% CI, 0.57-0.97).40
Previously, data suggested that this association existed only in children who had a family history of atopy.35 The protective association, however, exists regardless of family history and does not persist beyond 2 years of age. The authors noted evidence of publication bias, reverse causation, and misdiagnosis of early childhood rashes as eczema as limitations of their findings.40
Continue to: Reliable epidemiologic evidence...
Reliable epidemiologic evidence on the incidence of eczema in infants in the United States is limited, but the prevalence in the United States in children younger than 17 years is approximately 10.7% (with wide regional variation). Extrapolating these data generously, the NNT to prevent eczema in the first 2 years of life could be estimated at approximately 36.42
Allergic rhinitis. There is low-grade- and very-low-grade-quality evidence that more breastfeeding, compared to less breastfeeding, is associated with a lower risk of allergic rhinitis in children younger than 5 years (OR=0.79; 95% CI, 0.63-0.98). The association exists regardless of family history and disappears after 5 years of age. The differentiation of allergic rhinitis from rhinovirus infection (for which there is higher-quality evidence of a protective effect with breastfeeding) must be considered when interpreting these data.40
Reliable epidemiologic evidence on allergic rhinitis in children younger than 5 years is lacking, and incidence varies by region. A rough estimate, using data from 6- and 7-year-olds, indicates an NNT of 54 to 70.43
Food allergy. There is no evidence to suggest an association between breastfeeding and food allergy, either as protective or as a risk factor, and studies are limited.40 Interestingly, as data accumulate associating early exposure to foods with protection, some authors have proposed reexamining the recommendation from WHO and US health organizations for exclusive breastfeeding for the first 6 months of life.7,44
Continue to: Dental health
Dental health
Dental caries. There is consistent evidence that breastfeeding beyond 12 months of age is associated with the development of dental caries of deciduous teeth to 6 years of age (OR=2.90; 95% CI, 2.33-3.60). Many of the studies that showed this association did not control for the introduction of sugary foods and drinks, and there was a trend toward publication bias showing the association.45
Dental malocclusion. There is consistent evidence for approximately a two-thirds reduction in malocclusions in deciduous teeth in breastfed infants (OR=0.32; 95% CI, 0.25-0.40). Although the large majority of these data come from low-income and middle-income countries, the incidence of malocclusion is not thought to be associated with socioeconomic status, as so many other breastfeeding outcomes are.46
Childhood leukemia
In the largest meta-analysis available, a statistically significant inverse relationship between any breastfeeding for >6 months and childhood leukemia is evident in developed countries (OR=0.84; 95% CI, 0.78-0.91), although significant heterogeneity among studies and lack of control for confounding variables are significant limitations. In particular, an association has been demonstrated with acute lymphoblastic leukemia (ALL) but not with acute myelogenous leukemia.47 Given the rarity of childhood ALL, approximately 12,500 infants would need to be breastfed to prevent one case.48
Continue to: Long-term outcomes
Long-term outcomes
Cognitive development. Several studies conducted in developed countries have linked breastfeeding to positive cognitive outcomes in children, including higher intelligence quotient (IQ).35,49-52
These effects are conflicting, however, in studies that include sibling analysis and ones that control for maternal IQ.8,35,43,52-54 In the 2013 WHO meta-analysis, breastfeeding was associated with an increase of 2.2 points on normalized testing when only high-quality studies were included.51 A 2015 meta-analysis identified 4 high-quality studies with a large sample size and recall time <3 years, which demonstrated a mean difference of 1.76 points in IQ (95% CI, 0.25-3.26) in childhood and adolescence.52 Although statistically significant, this modest increase is of questionable clinical benefit and of unknown duration.
Obesity. The relationship between breastfeeding and obesity later in life is debatable. A large, systematic 2014 review of 15 cohort and 10 cross-sectional studies found a significantly reduced risk of childhood obesity among children who were breastfed (adjusted OR=0.78; 95% CI, 0.74-0.81).55 However, the review included studies that controlled for different confounders, and smaller effects were found in studies in which more confounders were taken into account.
The 2013 WHO meta-analysis found a small (approximately 10%) reduction in the prevalence of overweight or obese children, but cautioned that residual confounding and publication bias were likely.51 At 6.5 and 11.5 years of follow-up, PROBIT failed to demonstrate a protective effect for exclusively or “ever” breastfed infants.56 Sibling analysis similarly fails to demonstrate a statistically significant relationship.8
Continue to: A 2015 meta-analysis of 23 high-quality studies...
A 2015 meta-analysis of 23 high-quality studies with a sample size >1500 children and controlled for important confounders showed a pooled reduction in the prevalence of overweight or obesity of 13% (95% CI, 6-19).57 The protection in this meta-analysis showed a dilution of the effect as the participants aged and an inverse relationship of the effect with sample size.
Breastfeeding is, therefore, unlikely to play a significant, if any, role in combatting the obesity epidemic.
Hypertension. A meta-analysis of high-quality trials demonstrates a <1 mm Hg reduction in systolic blood pressure and no significant difference in diastolic pressure in breastfed infants.57 Similarly, no significant effect of breastfeeding on blood pressure has been demonstrated in trials of preterm infants.51
Type 2 diabetes. Available data are limited and heterogeneous for the association between breastfeeding and later development of type 2 diabetes. Only 2 high-quality trials were identified in the 2013 WHO meta-analysis, and their results conflict.51 A 2015 meta-analysis identified only 3 high-quality studies, without a statistically significant relationship.57
Dyslipidemia. Although earlier data suggested an association between breastfeeding and reduced cholesterol levels later in life, the 2013 WHO meta-analysis and a 2015 meta-analysis concluded that no association exists. The limited data available for preterm infants conflict.51,57
Growth. There is no evidence that feeding method has a short- or long-term effect on weight gain or length gain in preterm or term infants.35,36,58
Death. No clear association has been found between mortality and breastfeeding status in developed countries, except for the association with SIDS.35
Continue to: What issues frame and guide counseling on breastfeeding?
What issues frame and guide counseling on breastfeeding?
There is that “problem” with the evidence. The evidence for infant breastfeeding status and its association with health outcomes faces significant limitations; the great majority of those limitations tend to overestimate the benefits of breastfeeding. Nearly all evidence is based on observational studies, in which causality cannot be determined and self-selection bias, recall bias, and residual confounding limit the value or strength of the findings.
Breastfeeding rates are strongly socially patterned alongside socioeconomic status, race, and education level, all of which are simultaneously strongly tied to short- and long-term health outcomes.6 Other factors limiting the strength of the data set include varying definitions of infant feeding practices in different studies, varying definitions of outcomes and diseases, reverse causation, and evidence of publication bias in many meta-analyses. Given these shortcomings, the NNTs in this article probably represent a best-case scenario for breastfeeding outcomes for infants in the United States (TABLE 118,22-24,28,36,39-43,47,48).
Data need to be put into context. The NNTs for many breastfeeding outcomes (TABLE) compare favorably with other recommended interventions, particularly for other preventive care measures. Two examples: 81 mg/d aspirin for a 50-year-old man has an NNT of 35 to 45 for preventing nonfatal myocardial infarction, and the number needed to invite to screen with mammography to prevent one breast cancer death for a 50-year-old woman is 1339.59,60
In both of these examples, >95% of patients will not benefit from the intervention, yet these preventive measures are routinely recommended and have a significant impact at the public health level. Notably, these outcomes are more serious than most breastfeeding outcomes; have a longer-lasting effect, better-quality data, and better data for potential harms; are causally linked to the intervention; and require much less effort and commitment of time than breastfeeding.
The question must be reckoned with: Can advocacy be harmful?
In recent years, a growing number of concerns have been raised about:
- the potential harms of breastfeeding advocacy
- exaggeration of the benefits of breastfeeding
- promotion of breastfeeding at the expense of evidence-based medicine.
The “Ten Steps to Successful Breastfeeding” program of the Baby-friendly Hospital Initiative (BFHI; launched by UNICEF and WHO) has come under scrutiny because of an increasing number of reports of sudden unexpected postnatal collapse; fall injuries; modeling and encouragement of unsafe sleep practices; an overly rigid resistance to the use of formula supplementation; and the ban on pacifier use.61,62 The BFHI, promoted by the Centers for Disease Control and Prevention, is increasingly being adopted by hospitals with the expressed goal of increasing the breastfeeding rate from birth to discharge.
Continue to: Some of the "Ten Steps"...
Some of the “Ten Steps,” such as the call for skin-to-skin care and 24-hour rooming-in, have well-established benefit yet, when performed without supervision, can have the rare but serious unintended consequences of sudden unexpected postnatal collapse (the incidence of which may be higher than that of SIDS) and unsafe sleeping practices.62,63
Furthermore, despite evidence that early formula supplementation, when medically necessary, does not adversely impact the breastfeeding rate, the “Ten Steps” program advises that giving formula before breast milk comes in might “lead to failure to breastfeed.”33,34,61,63
Similarly, the ban on pacifiers is contrary to available evidence. The use of pacifiers before last sleep is more protective against SIDS than breastfeeding (NNT=2733), and there is evidence at one hospital that BFHI-inspired pacifier restriction is associated with a decrease in the rate of breastfeeding.64,65
Other harms of advocacy are even more poorly studied. Most of the evidence for harm comes from the psychology and social science literature—not the medical literature, perhaps because the prevailing opinion in the medical community is that breastfeeding has overwhelming evidence for benefit. In fact, in the USPSTF’s 2008 recommendation, the evidence review of breastfeeding promotion practices in primary care did not identify a single study that measured harm; in the 2016 update of that recommendation, only 2 such studies were identified.15,66
The literature that does investigate harm consistently finds that women who have difficulty breastfeeding or choose formula feeding report feelings of inadequacy, guilt, loss of agency, anxiety, and physical pain during breastfeeding that interferes with 1) their ability to bond or otherwise care for their infant and 2) competing work obligations.11-13,67-69 Given the lack of attention paid to these variables in the medical literature, it is the individual mother who is best positioned to weigh these factors against the benefits of breastfeeding.
Continue to: Shared decision-making is best—for mother and baby
Shared decision-making is best—for mother and baby
Breastfeeding might prevent certain infections in as many as 50% of infants, but a mother unable to breastfeed can take solace in the fact that >95% of breastfed infants will not realize any benefit from the preventive potential of breastfeeding in regard to hospitalization or allergic disease, and >99% will not realize benefit from either the prevention of SIDS or ALL, or from improvement in long-term health measures (except for, perhaps, a slightly higher IQ). The “breast is best” mantra is likely true at a public-health level; for the individual mother–infant dyad, however, where there is a need to balance personal, social, family, and financial factors, that mantra is an oversimplification.
Regrettably, there is a paucity of data on the risks of breastfeeding promotion—an area that deserves more study. Balancing the abundant, but often limited-quality, data on the benefits of breastfeeding and the sheer lack of data regarding the risks of advocacy represents a clinical and an ethical challenge for physicians. It is a challenge that can only be resolved through individualization of care and shared decision-making, in which the physician is expert on the benefits of breastfeeding, and the mother is expert on the personal circumstances to be weighed against those benefits.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine, 101 Heart Drive, Greenville, NC 27834; [email protected].
The benefits of breastfeeding for infants have long been touted as numerous and supported by overwhelming evidence. The World Health Organization (WHO), American College of Obstetricians and Gynecologists, American Academy of Pediatrics (AAP), and American Academy of Family Physicians all strongly recommend exclusive breastfeeding for the first 6 months of life, citing numerous health benefits for child and mother. These groups recommend that some breastfeeding be continued through the first 12 months of life, or longer, as desired (the WHO extends the recommendation to 2 years).1-4 In 2000, the Surgeon General of the United States released a strategic plan to increase rates of breastfeeding,5 setting goals (by 2010) of:
- 75% of mothers leaving the hospital breastfeeding
- 50% of babies breastfeeding at 6 months
- 25% of babies breastfeeding at 1 year.
Massive public health campaigns citing data for the many benefits of breastfeeding have been launched with the goal of increasing the breastfeeding rate. In 2014, statistics offered a testament to the success of these campaigns6:
- 82.5% of infants had been breastfed “ever”
- 55.3% were breastfed “some”
- 24.9% were breastfed exclusively through 6 months of age
- 33.7% were breastfed “some” at 12 months.
Breastfeeding advocacy has become clouded
In recent years, an increasing number of researchers, physicians, and authors have begun to question whether, in the United States, the benefits of breastfeeding children are exaggerated and the emphasis on breastfeeding might be leading to feelings of inadequacy, guilt, and anxiety among mothers.7-13 In 2016, the US Preventive Services Task Force (USPSTF) amended its recommendation to “promote and support breastfeeding” to simply “support breastfeeding”—a change that created substantial debate and prompted the Task Force to clarify its stance in changing the language: In its response to public comment, the USPSTF said that its position regarding promotion had not changed, but the language in the original statement had been revised to “ensure that the autonomy of women is respected.” 2,14-16
In contrast, others suggest counseling women on the risks of formula feeding rather than on the benefits of breastfeeding, citing substantial health outcome distinctions.17 Indeed, wide-ranging conclusions have been drawn from the same data on the topic, potentially creating uncertainty for physicians on how best to counsel women on their choice of how to feed their infant.
In this article, we address this uncertainty by utilizing the most recent and comprehensive data to examine infant health outcomes. When possible, the number needed to treat (NNT) for a given outcome has been calculated or approximated, allowing the reader to estimate the likelihood of benefit for an individual mother–infant dyad. Exercise caution when interpreting the NNT, however: The numbers suggest causality that cannot be definitively established using the observational data on which those numbers are based.
Continue to: Infectious disease
Infectious disease
Acute otitis media. Exclusive breastfeeding for 6 months is associated with a 43% reduction in the risk of acute otitis media (AOM) by 2 years of age (odds ratio [OR]=0.57; 95% confidence interval [CI], 0.44-0.75). Beyond 2 years of age, or when comparing “ever” and “never” breastfeeding, the effect disappears. All studies in this meta-analysis had serious limitations.18
Nearly half of children will have at least one case of AOM by one year of age; 80%, by 2 years.19,20 Since the introduction of the heptavalent pneumococcal conjugate vaccine, the rate of AOM at 2 years has fallen by as much as 20%.21 Assuming an incidence of 60% to 80% of AOM by 2 years, only 2 or 3 infants need to be exclusively breastfed for 6 months to prevent a single case of AOM.18 Prevention of AOM through breastfeeding may be related to head position during feeding, antibacterial effects of breast milk, protective oral microbiome in the breastfed infant pharynx, and/or prevention of primary viral upper respiratory infection (URI), which nearly always precedes AOM.18,19
Upper and lower respiratory tract infections. Infants who are exclusively breastfed for 4 months and partially breastfed after 4 months have a lower risk of URI (OR=0.65; 95% CI, 0.51-0.83) and of lower respiratory tract infection (LRTI; OR=0.50; 95% CI, 0.32-0.72).22
The effect is stronger for URI among infants exclusively breastfed for at least 6 months (OR=0.37; 95% CI, 0.18-0.74), but is no longer significant by that time for LRTI (OR=0.33; 95% CI, 0.08-1.40). Importantly, AOM was included in the URI group, and, as previously discussed, AOM has independently been shown to have an inverse relationship with breastfeeding duration.
At 7 to 12 months of age, no association was seen between breastfeeding and the incidence of URI. Curiously, an association with LRTI was again detected for infants breastfed exclusively for 4 months and partially thereafter, but was not detected with exclusive breastfeeding for at least 6 months (OR=0.46; 95% CI, 0.31-0.69). In this study, in the first 6 months of life, 40% of infants had a URI and 8% had an LRTI. The findings in this cohort suggest an NNT of 6 or 7 for prevention of URI and an NNT of 25 for prevention of LRTI in the first 6 months of life.22
Continue to: Children younger than 2 years are...
Children younger than 2 years are estimated to have approximately 6 bouts of the common cold a year, and essentially 100% have at least one bout—perhaps lowering the NNT for URI if applied widely. However, these data are not divided into 6-month intervals, making accurate extrapolation difficult.23
Gastrointestinal infection. The rate of diarrheal illness in the first year of life is lower in infants who are exclusively breastfed for at least 4 months and partially breastfed after.
Both the Promotion of Breastfeeding Intervention Trial (PROBIT; a clinical trial in which infants were randomized to a breastfeeding education intervention or standard care) and a 2010 prospective cohort study in the Netherlands of more than 3400 infants found a reduction in the risk of one or more gastrointestinal (GI) infections at a similar rate.22,24
- In PROBIT, 9.1% of infants in the intervention group, compared to 13.2% in the standard care group (OR=0.60; 95% CI, 0.40-0.91), had one or more GI infections at 12 months of age.24
- In the 2010 Netherlands cohort, 8% of infants had a GI infection by 6 months of age. Infants breastfed exclusively for at least 4 or 6 months had a decreased risk for GI infection (respectively: adjusted OR=0.41; 95% CI, 0.26-0.64 and adjusted OR=0.46; 95% CI, 0.14-1.59). No such association was found for any feeding group 7 to 12 months of age.22
These studies are notable for the low incidence of GI infection, which is frequently cited as 1.3 to 2.3 episodes per child per year in children younger than 3 years in the United States.25 However, that high incidence has likely declined significantly since the introduction of rotavirus vaccine in 2006. In the years following the introduction of the vaccine, infant visits for gastroenteritis decreased by >90% in all care settings in the South, Northeast, and Midwest regions of the United States and by 53% to 63% in the West region.26 Recent accurate epidemiologic information, in an era of significantly higher vaccination rates, is lacking.
Assuming the low incidence of GI infection reported in PROBIT and the Netherlands trials, about 25 to 30 infants need to be exclusively breastfed for 4 to 6 months to prevent a single GI infection during the first 6 to 12 months of life.22,24 Assuming a 60% incidence by age 12 months before introduction of the rotavirus vaccine, the NNT would be approximately 4.24 The true number is likely somewhere between those 2 NNTs.
Continue to: Hospitalization
Hospitalization
Risk of infection is decreased. A large cohort study in Scotland, involving more than 500,000 children, found an association between exclusive breastfeeding for 6 to 8 weeks and decreased risk of hospitalization within the first 6 months of life. Formula-fed and mixed-fed infants had an increased hazard ratio (HR) for hospitalization for common childhood illness (HR=1.40; 95% CI, 1.35-1.45 for formula-fed infants and HR=1.18; 95% CI, 1.11-1.25 for mixed-fed infants).27 The study also found increased rates of hospitalization for conditions for which other meta-analyses have failed to show a protective effect from breastfeeding—leading to suspicion of residual confounding in the study. Another United Kingdom cohort demonstrated lower rates of hospitalization for GI infection (NNT=171) and LRTI (NNT=115) among exclusively breastfed infants by 8 months of age.28
Risk of neonatal readmission is increased. Late preterm infants who are exclusively breastfed are nearly twice as likely to be hospitalized as breastfed term or non-breastfed preterm infants, primarily due to dehydration, failure to thrive, weight loss, and hyperbilirubinemia. In fact, exclusive breastfeeding at discharge from the hospital is likely the single greatest risk factor for hospital readmission in newborns.29,30 Term infants who are exclusively breastfed are more likely to be hospitalized compared to formula-fed or mixed-fed infants, due to hyperbilirubinemia, dehydration, hypernatremia, and weight loss (number needed to harm (NNH)=71).30-32 For weight loss >10% of birth weight with or without hospitalization, the NNH for breastfed infants is 13.32
Many of these hospitalizations and events could be avoided with appropriate monitoring and medically indicated supplementation; the likelihood of long-term harm is low. Formula supplementation is often avoided if possible in hospitals to promote exclusive breastfeeding; however, several small randomized clinical trials have demonstrated that limited formula supplementation in breastfed infants does not affect the breastfeeding continuation rate at 3 and 6 months, and, therefore, might be a way to decrease infant rehospitalization.33,34
Necrotizing enterocolitis
In preterm infants, breastfeeding has been associated with a lower rate of necrotizing enterocolitis. In the 2007 Agency for Healthcare Research and Quality report, the association was found to be only marginally statistically significant, and the authors warned that, first, evidence is old and heterogeneous and, second, present preterm formula is much different than the formula used in earlier studies of preterm infant nutrition and necrotizing enterocolitis.35 A 2012 Cochrane review included newer studies in its analysis but reached the same conclusion on the quality and heterogeneity of available evidence, with a NNT of 25.36
Continue to: Sudden infant death syndrome
Sudden infant death syndrome
There is a statistically significant association between sudden infant death syndrome (SIDS) and feeding method. Infants whose cause of death is SIDS are approximately one half as likely to have been breastfed as matched controls.35,37
In 2005, AAP did not recommend breastfeeding as a means to reduce the risk of SIDS because available evidence was mixed, and studies at the time were poorly controlled.38 Since that time, case-control meta-analyses have shed additional light on the association between SIDS and feeding method.35,37
The protective effect exists for any amount of breastfeeding and is stronger for exclusive breastfeeding, suggesting a protective role—not simply an association. Caution should be employed with this conclusion, however, because the studies included in the meta-analysis used univariate analysis primarily and did not control sufficiently for known confounders. In addition, the authors warn that publication bias might overestimate the association.38
Potential mechanisms of a protective role include decreased risk of infection and greater arousability from sleep in breastfed infants. Assuming a protective role, available data suggest that more than 3500 infants need to be breastfed to prevent one case of SIDS.39
Continue to: Allergic disease
Allergic disease
Asthma. There is evidence of a small protective effect of breastfeeding “ever” on asthma at 5 to 18 years of age in high-income countries (OR=0.90; 95% CI, 0.83-0.97). A family history of asthma or atopy did not affect this finding. The authors note there is some evidence of publication bias in this review, which is the largest and most comprehensive on the topic.40
With a lifetime prevalence of asthma in the United States of approximately 13.2%, this association would confer an NNT of roughly 76.41 Earlier, the literature demonstrated mixed and conflicting evidence, and some experts suggested an effect only when there is a family history of asthma or atopy.36
Eczema. For children younger than 2 years, there is low-grade- and very-low-grade-quality evidence that exclusive breastfeeding longer than 3 to 4 months is associated with a reduced risk of eczema (OR=0.74; 95% CI, 0.57-0.97).40
Previously, data suggested that this association existed only in children who had a family history of atopy.35 The protective association, however, exists regardless of family history and does not persist beyond 2 years of age. The authors noted evidence of publication bias, reverse causation, and misdiagnosis of early childhood rashes as eczema as limitations of their findings.40
Continue to: Reliable epidemiologic evidence...
Reliable epidemiologic evidence on the incidence of eczema in infants in the United States is limited, but the prevalence in the United States in children younger than 17 years is approximately 10.7% (with wide regional variation). Extrapolating these data generously, the NNT to prevent eczema in the first 2 years of life could be estimated at approximately 36.42
Allergic rhinitis. There is low-grade- and very-low-grade-quality evidence that more breastfeeding, compared to less breastfeeding, is associated with a lower risk of allergic rhinitis in children younger than 5 years (OR=0.79; 95% CI, 0.63-0.98). The association exists regardless of family history and disappears after 5 years of age. The differentiation of allergic rhinitis from rhinovirus infection (for which there is higher-quality evidence of a protective effect with breastfeeding) must be considered when interpreting these data.40
Reliable epidemiologic evidence on allergic rhinitis in children younger than 5 years is lacking, and incidence varies by region. A rough estimate, using data from 6- and 7-year-olds, indicates an NNT of 54 to 70.43
Food allergy. There is no evidence to suggest an association between breastfeeding and food allergy, either as protective or as a risk factor, and studies are limited.40 Interestingly, as data accumulate associating early exposure to foods with protection, some authors have proposed reexamining the recommendation from WHO and US health organizations for exclusive breastfeeding for the first 6 months of life.7,44
Continue to: Dental health
Dental health
Dental caries. There is consistent evidence that breastfeeding beyond 12 months of age is associated with the development of dental caries of deciduous teeth to 6 years of age (OR=2.90; 95% CI, 2.33-3.60). Many of the studies that showed this association did not control for the introduction of sugary foods and drinks, and there was a trend toward publication bias showing the association.45
Dental malocclusion. There is consistent evidence for approximately a two-thirds reduction in malocclusions in deciduous teeth in breastfed infants (OR=0.32; 95% CI, 0.25-0.40). Although the large majority of these data come from low-income and middle-income countries, the incidence of malocclusion is not thought to be associated with socioeconomic status, as so many other breastfeeding outcomes are.46
Childhood leukemia
In the largest meta-analysis available, a statistically significant inverse relationship between any breastfeeding for >6 months and childhood leukemia is evident in developed countries (OR=0.84; 95% CI, 0.78-0.91), although significant heterogeneity among studies and lack of control for confounding variables are significant limitations. In particular, an association has been demonstrated with acute lymphoblastic leukemia (ALL) but not with acute myelogenous leukemia.47 Given the rarity of childhood ALL, approximately 12,500 infants would need to be breastfed to prevent one case.48
Continue to: Long-term outcomes
Long-term outcomes
Cognitive development. Several studies conducted in developed countries have linked breastfeeding to positive cognitive outcomes in children, including higher intelligence quotient (IQ).35,49-52
These effects are conflicting, however, in studies that include sibling analysis and ones that control for maternal IQ.8,35,43,52-54 In the 2013 WHO meta-analysis, breastfeeding was associated with an increase of 2.2 points on normalized testing when only high-quality studies were included.51 A 2015 meta-analysis identified 4 high-quality studies with a large sample size and recall time <3 years, which demonstrated a mean difference of 1.76 points in IQ (95% CI, 0.25-3.26) in childhood and adolescence.52 Although statistically significant, this modest increase is of questionable clinical benefit and of unknown duration.
Obesity. The relationship between breastfeeding and obesity later in life is debatable. A large, systematic 2014 review of 15 cohort and 10 cross-sectional studies found a significantly reduced risk of childhood obesity among children who were breastfed (adjusted OR=0.78; 95% CI, 0.74-0.81).55 However, the review included studies that controlled for different confounders, and smaller effects were found in studies in which more confounders were taken into account.
The 2013 WHO meta-analysis found a small (approximately 10%) reduction in the prevalence of overweight or obese children, but cautioned that residual confounding and publication bias were likely.51 At 6.5 and 11.5 years of follow-up, PROBIT failed to demonstrate a protective effect for exclusively or “ever” breastfed infants.56 Sibling analysis similarly fails to demonstrate a statistically significant relationship.8
Continue to: A 2015 meta-analysis of 23 high-quality studies...
A 2015 meta-analysis of 23 high-quality studies with a sample size >1500 children and controlled for important confounders showed a pooled reduction in the prevalence of overweight or obesity of 13% (95% CI, 6-19).57 The protection in this meta-analysis showed a dilution of the effect as the participants aged and an inverse relationship of the effect with sample size.
Breastfeeding is, therefore, unlikely to play a significant, if any, role in combatting the obesity epidemic.
Hypertension. A meta-analysis of high-quality trials demonstrates a <1 mm Hg reduction in systolic blood pressure and no significant difference in diastolic pressure in breastfed infants.57 Similarly, no significant effect of breastfeeding on blood pressure has been demonstrated in trials of preterm infants.51
Type 2 diabetes. Available data are limited and heterogeneous for the association between breastfeeding and later development of type 2 diabetes. Only 2 high-quality trials were identified in the 2013 WHO meta-analysis, and their results conflict.51 A 2015 meta-analysis identified only 3 high-quality studies, without a statistically significant relationship.57
Dyslipidemia. Although earlier data suggested an association between breastfeeding and reduced cholesterol levels later in life, the 2013 WHO meta-analysis and a 2015 meta-analysis concluded that no association exists. The limited data available for preterm infants conflict.51,57
Growth. There is no evidence that feeding method has a short- or long-term effect on weight gain or length gain in preterm or term infants.35,36,58
Death. No clear association has been found between mortality and breastfeeding status in developed countries, except for the association with SIDS.35
Continue to: What issues frame and guide counseling on breastfeeding?
What issues frame and guide counseling on breastfeeding?
There is that “problem” with the evidence. The evidence for infant breastfeeding status and its association with health outcomes faces significant limitations; the great majority of those limitations tend to overestimate the benefits of breastfeeding. Nearly all evidence is based on observational studies, in which causality cannot be determined and self-selection bias, recall bias, and residual confounding limit the value or strength of the findings.
Breastfeeding rates are strongly socially patterned alongside socioeconomic status, race, and education level, all of which are simultaneously strongly tied to short- and long-term health outcomes.6 Other factors limiting the strength of the data set include varying definitions of infant feeding practices in different studies, varying definitions of outcomes and diseases, reverse causation, and evidence of publication bias in many meta-analyses. Given these shortcomings, the NNTs in this article probably represent a best-case scenario for breastfeeding outcomes for infants in the United States (TABLE 118,22-24,28,36,39-43,47,48).
Data need to be put into context. The NNTs for many breastfeeding outcomes (TABLE) compare favorably with other recommended interventions, particularly for other preventive care measures. Two examples: 81 mg/d aspirin for a 50-year-old man has an NNT of 35 to 45 for preventing nonfatal myocardial infarction, and the number needed to invite to screen with mammography to prevent one breast cancer death for a 50-year-old woman is 1339.59,60
In both of these examples, >95% of patients will not benefit from the intervention, yet these preventive measures are routinely recommended and have a significant impact at the public health level. Notably, these outcomes are more serious than most breastfeeding outcomes; have a longer-lasting effect, better-quality data, and better data for potential harms; are causally linked to the intervention; and require much less effort and commitment of time than breastfeeding.
The question must be reckoned with: Can advocacy be harmful?
In recent years, a growing number of concerns have been raised about:
- the potential harms of breastfeeding advocacy
- exaggeration of the benefits of breastfeeding
- promotion of breastfeeding at the expense of evidence-based medicine.
The “Ten Steps to Successful Breastfeeding” program of the Baby-friendly Hospital Initiative (BFHI; launched by UNICEF and WHO) has come under scrutiny because of an increasing number of reports of sudden unexpected postnatal collapse; fall injuries; modeling and encouragement of unsafe sleep practices; an overly rigid resistance to the use of formula supplementation; and the ban on pacifier use.61,62 The BFHI, promoted by the Centers for Disease Control and Prevention, is increasingly being adopted by hospitals with the expressed goal of increasing the breastfeeding rate from birth to discharge.
Continue to: Some of the "Ten Steps"...
Some of the “Ten Steps,” such as the call for skin-to-skin care and 24-hour rooming-in, have well-established benefit yet, when performed without supervision, can have the rare but serious unintended consequences of sudden unexpected postnatal collapse (the incidence of which may be higher than that of SIDS) and unsafe sleeping practices.62,63
Furthermore, despite evidence that early formula supplementation, when medically necessary, does not adversely impact the breastfeeding rate, the “Ten Steps” program advises that giving formula before breast milk comes in might “lead to failure to breastfeed.”33,34,61,63
Similarly, the ban on pacifiers is contrary to available evidence. The use of pacifiers before last sleep is more protective against SIDS than breastfeeding (NNT=2733), and there is evidence at one hospital that BFHI-inspired pacifier restriction is associated with a decrease in the rate of breastfeeding.64,65
Other harms of advocacy are even more poorly studied. Most of the evidence for harm comes from the psychology and social science literature—not the medical literature, perhaps because the prevailing opinion in the medical community is that breastfeeding has overwhelming evidence for benefit. In fact, in the USPSTF’s 2008 recommendation, the evidence review of breastfeeding promotion practices in primary care did not identify a single study that measured harm; in the 2016 update of that recommendation, only 2 such studies were identified.15,66
The literature that does investigate harm consistently finds that women who have difficulty breastfeeding or choose formula feeding report feelings of inadequacy, guilt, loss of agency, anxiety, and physical pain during breastfeeding that interferes with 1) their ability to bond or otherwise care for their infant and 2) competing work obligations.11-13,67-69 Given the lack of attention paid to these variables in the medical literature, it is the individual mother who is best positioned to weigh these factors against the benefits of breastfeeding.
Continue to: Shared decision-making is best—for mother and baby
Shared decision-making is best—for mother and baby
Breastfeeding might prevent certain infections in as many as 50% of infants, but a mother unable to breastfeed can take solace in the fact that >95% of breastfed infants will not realize any benefit from the preventive potential of breastfeeding in regard to hospitalization or allergic disease, and >99% will not realize benefit from either the prevention of SIDS or ALL, or from improvement in long-term health measures (except for, perhaps, a slightly higher IQ). The “breast is best” mantra is likely true at a public-health level; for the individual mother–infant dyad, however, where there is a need to balance personal, social, family, and financial factors, that mantra is an oversimplification.
Regrettably, there is a paucity of data on the risks of breastfeeding promotion—an area that deserves more study. Balancing the abundant, but often limited-quality, data on the benefits of breastfeeding and the sheer lack of data regarding the risks of advocacy represents a clinical and an ethical challenge for physicians. It is a challenge that can only be resolved through individualization of care and shared decision-making, in which the physician is expert on the benefits of breastfeeding, and the mother is expert on the personal circumstances to be weighed against those benefits.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: World Health Organization, and New York, NY: UNICEF; 2003. Available at: www.who.int/maternal_child_adolescent/documents/9241562218/en/. Accessed April 4, 2018.
2. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Breastfeeding Expert Work Group. Committee Opinion No. 658: Optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2016;127:e86-e92.
3. Gartner LM, Morton J, Lawrence RA, et al; American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496-506.
4. Breastfeeding (policy statement). Leawood, KS: American Academy of Family Physicians; 2007. Available at: https://www.aafp.org/about/policies/all/breastfeeding.html. Accessed April 3, 2018.
5. Office of the Surgeon General (US); Centers for Disease Control and Prevention (US); Office on Women’s Health (US). The Surgeon General’s call to action to support breastfeeding. Rockville, MD: US Department of Health and Human Services; 2011. Available at: www.surgeongeneral.gov/library/calls/breastfeeding/index.html. Updated August 12, 2014. Accessed April 4, 2018.
6. Breastfeeding: data & statistics. Atlanta, GA: Centers for Disease Control and Prevention; December 11, 2017. Available at: http://www.cdc.gov/breastfeeding/data/. Accessed May 17, 2018.
7. Fewtrell M, Wilson DC, Booth I, et al. A. Six months of exclusive breast feeding: how good is the evidence? BMJ. 2010;342:c5955.
8. Colen CG, Ramey DM. Is breast truly best? Estimating the effect of breastfeeding on long-term child wellbeing in the United States using sibling comparisons. Soc Sci Med. 2014;109:55-65.
9. Wolf J. Is Breast Best? Taking on the Breastfeeding Experts and the New High Stakes of Motherhood. New York, NY: NYU Press; 2010.
10. Tuteur A. Push Back: Guilt in the Age of Natural Parenting. New York, NY: HarperCollins Publishers; 2016.
11. Lee E. Health, morality, and infant feeding: British mothers’ experiences of formula milk use in the early weeks. Sociol Health Illn. 2007;29:1075-1090.
12. Williams K, Donaghue N, Kurz T. “Giving guilt the flick”?: an investigation of mothers’ talk about guilt in relation to infant feeding. Psychol Women Q. 2013;37:97-112.
13. Fahlquist JN, Roeser S. Ethical problems with information on infant feeding in developed countries. J Health Polit Policy Law. 2012;37:155-160.
14. U.S. Preventive Services Task Force. Final Recommendation Statement. Breastfeeding: Counseling. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breastfeeding-counseling. Accessed April 4, 2018.
15. US Preventive Services Task Force. Primary Care Interventions to Support Breastfeeding: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1688-1693.
16. Zahn CM, Hanley LE. Concerns over USPSTF draft recommendation on breastfeeding interventions [letter]. Washington, DC: The American College of Obstetricians and Gynecologists; May 18, 2016. Available at: https://www.acog.org/-/media/Departments/Breastfeeding/Breast-Feeding-ACOG-USPSTF.pdf?dmc=1&ts=20180518T1850056558. Accessed May 22, 2018.
17. Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2:222-231.
18. Bowatte G, Tham R, Allen KJ, et al. Breastfeeding and childhood acute otitis media: a systematic review and meta-analysis. Acta Paediatr. 2015;104:85-95.
19. Chonmaitree T, Trujillo R, Jennings K, et al. Acute otitis media and other complications of viral respiratory infection. Pediatrics. 2016;137:e20153555.
20. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
21. Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118:865-873.
22. Duijts L, Jaddoe VW, Hofman A, et al. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126:e18-e25.
23. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186:190-199.
24. Kramer MS, Chalmers B, Hodnett ED, et al; PROBIT Study Group (Promotion of Breastfeeding Intervention Trial). Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413-420.
25. Dennehy PH. Acute diarrheal disease in children: epidemiology, prevention, and treatment. Infect Dis Clin North Am. 2005;19:585-602.
26. Cortese MM, Tate JE, Simonsen L, et al. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatric Infect Dis J. 2010;29:489-494.
27. Ajetunmobi OM, Whyte B, Chalmers J, et al. Breastfeeding is associated with reduced childhood hospitalization: evidence from a Scottish birth cohort (1997-2009). J Pediatr. 2015;166:620-625.
28. Quigley MA, Kelly YJ, Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics. 2007;119:e837-e842.
29. Radtke JV. The paradox of breastfeeding-associated morbidity among late preterm infants. J Obstet Gynecol Neonatal Nurs. 2011;40:9-24.
30. Escobar GJ, Gonzales VM, Armstrong M, et al. Rehospitalization for neonatal dehydration: a nested case-control study. Arch Pediatr Adolesc Med. 2002;156:155-161.
31. Salas AA, Salazar J, Burgoa CV, et al. Significant weight loss in breastfed term infants readmitted for hyperbilirubinemia. BMC Pediatr. 2009;9:82.
32. Tarcan A, Tiker F, Vatandaş NS, et al. Weight loss and hypernatremia in breast-fed babies: frequency in neonates with non-hemolytic jaundice. J Paediatr Child Health. 2005;41:484-487.
33. Flaherman VJ, Aby J, Burgos AE, et al. Effect of early limited formula on duration and exclusivity of breastfeeding in at-risk infants: an RCT. Pediatrics. 2013;131:1059-1065.
34. Straňák Z, Feyereislova S, Černá M, et al. J. Limited amount of formula may facilitate breastfeeding: randomized, controlled trial to compare standard clinical practice versus limited supplemental feeding. Denning PW, ed. PLoS One. 2016;11:e0150053.
35. Ip S, Chung M, Raman G, et al. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Rockville, MD: Agency for Healthcare Research and Quality (US); 2007. Evidence Reports/Technology Assessments, No. 153. Available at: www.ncbi.nlm.nih.gov/books/NBK38337/. Accessed April 3, 2018.
36. Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014;(4):CD002971.
37. Hauck FR, Thompson JM, Tanabe KO, et al. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128:103-110.
38. American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116:1245-1255.
39. Moon RY, Fu L. Sudden infant death syndrome: an update. Pediatr Rev. 2012;33:314-320.
40. Lodge CJ, Tan DJ, Lau MX, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104:38-53.
41. Brim SN, Rudd RA, Funk RH, et al. Asthma prevalence among US children in underrepresented minority populations: American Indian/Alaska Native, Chinese, Filipino, and Asian Indian. Pediatrics. 2008;122:e217-e222.
42. Shaw TF, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
43. Mallol J, Crane J, von Mutius E, et al. The international study of asthma and allergies in childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr). 2013;41:73-85.
44. Flohr C, Nagel G, Weinmayr G, et al. Lack of evidence for a protective effect of prolonged breastfeeding on childhood eczema: lessons from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. Br J Dermatol. 2011;165:1280-1289.
45. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
46. Peres KG, Cascaes AM, Nascimento GG, et al. Effect of breastfeeding on malocclusions: a systematic review and meta-analysis. Acta Paediatr. 2015;104:54-61.
47. Amitya EL, Keinan-Boker L. Breastfeeding and childhood leukemia incidence: a meta-analysis and systematic review. JAMA Pediatr. 2015;169:e151025.
48. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943-1955.
49. Guxens M, Mendez MA, Moltó-Puigmartí C, et al. Breastfeeding, long-chain polyunsaturated fatty acids in colostrum, and infant mental development.
2012;129:1134-1140.
51. Horta BL, Victora CG. Long-term effects of breastfeeding: a systematic review. Geneva, Switzerland: World Health Organization; 2013. Available at: http://apps.who.int/iris/bitstream/10665/79198/1/9789241505307_eng.pdf. Accessed August 16, 2016.
52. Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104:14-19.
53. Der G, Batty GD, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. 2006;333:945.
54. Sajjad A, Tharner A, Kiefte-de Jong JC, et al. Breastfeeding duration and non-verbal IQ in children. J Epidemiol Community Health 2015;69:775-781.
55. Yan J, Liu L, Zhu Y, et al. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267.
56. Martin RM, Patel R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
57. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure, and type 2 diabetes: systematic review and meta-analysis. Acta Paediatr. 2015;104:30-37.
58. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Syst Rev. 2012;15:CD003517.
59. U.S. Preventive Services Task Force. Final recommendation statement: aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Published September 2017. Accessed April 3, 2018.
60. U.S. Preventive Services Task Force. Screening for breast cancer. Available at: www.uspreventiveservicestaskforce.org/Page/SupportingDoc/breast-cancer-screening/final-evidence-summary9. Published November 2009. Accessed April 2, 2018.
61. Bass JL, Gartley T, Kleinman R. Unintended consequences of current breastfeeding initiatives. JAMA Pediatr. 2016;170:923-924.
62. Feldman-Winter L, Goldsmith JP; Committee on Fetus and Newborn; Task Force on Sudden Infant Death Syndrome. Safe sleep and skin-to-skin care in the neonatal period for healthy term newborns. Pediatrics. 2016;138:e20161889.
63. The Mother and Child Health and Education Trust. Ten steps to successful breastfeeding. Available at: www.tensteps.org. Published November 8, 2017. Accessed April 3, 2018.
64. Hauck FR, Omojokun OO, Siadaty MS. Do pacifiers reduce the risk of sudden infant death syndrome? A meta-analysis. Pediatrics. 2005;116:e716-e723.
65. Kair LR, Kenron D, Etheredge K, et al. Pacifier restriction and exclusive breastfeeding. Pediatrics. 2013;131:e1101-e1107.
66. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
67. Wolf JB. Is breast really best? Risk and total motherhood in the National Breastfeeding Awareness Campaign. J Health Polit Policy Law. 2007;32:595-636.
68. Marshall JL, Godfrey M, Renfrew MJ. Being a ‘good mother’: managing breastfeeding and merging identities. Soc Sci Med. 2007;65:2147-2159.
69. Kelleher CM. The physical challenges of early breastfeeding. Soc Sci Med. 2006;63:2727-2738.
1. Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: World Health Organization, and New York, NY: UNICEF; 2003. Available at: www.who.int/maternal_child_adolescent/documents/9241562218/en/. Accessed April 4, 2018.
2. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Breastfeeding Expert Work Group. Committee Opinion No. 658: Optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2016;127:e86-e92.
3. Gartner LM, Morton J, Lawrence RA, et al; American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496-506.
4. Breastfeeding (policy statement). Leawood, KS: American Academy of Family Physicians; 2007. Available at: https://www.aafp.org/about/policies/all/breastfeeding.html. Accessed April 3, 2018.
5. Office of the Surgeon General (US); Centers for Disease Control and Prevention (US); Office on Women’s Health (US). The Surgeon General’s call to action to support breastfeeding. Rockville, MD: US Department of Health and Human Services; 2011. Available at: www.surgeongeneral.gov/library/calls/breastfeeding/index.html. Updated August 12, 2014. Accessed April 4, 2018.
6. Breastfeeding: data & statistics. Atlanta, GA: Centers for Disease Control and Prevention; December 11, 2017. Available at: http://www.cdc.gov/breastfeeding/data/. Accessed May 17, 2018.
7. Fewtrell M, Wilson DC, Booth I, et al. A. Six months of exclusive breast feeding: how good is the evidence? BMJ. 2010;342:c5955.
8. Colen CG, Ramey DM. Is breast truly best? Estimating the effect of breastfeeding on long-term child wellbeing in the United States using sibling comparisons. Soc Sci Med. 2014;109:55-65.
9. Wolf J. Is Breast Best? Taking on the Breastfeeding Experts and the New High Stakes of Motherhood. New York, NY: NYU Press; 2010.
10. Tuteur A. Push Back: Guilt in the Age of Natural Parenting. New York, NY: HarperCollins Publishers; 2016.
11. Lee E. Health, morality, and infant feeding: British mothers’ experiences of formula milk use in the early weeks. Sociol Health Illn. 2007;29:1075-1090.
12. Williams K, Donaghue N, Kurz T. “Giving guilt the flick”?: an investigation of mothers’ talk about guilt in relation to infant feeding. Psychol Women Q. 2013;37:97-112.
13. Fahlquist JN, Roeser S. Ethical problems with information on infant feeding in developed countries. J Health Polit Policy Law. 2012;37:155-160.
14. U.S. Preventive Services Task Force. Final Recommendation Statement. Breastfeeding: Counseling. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breastfeeding-counseling. Accessed April 4, 2018.
15. US Preventive Services Task Force. Primary Care Interventions to Support Breastfeeding: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1688-1693.
16. Zahn CM, Hanley LE. Concerns over USPSTF draft recommendation on breastfeeding interventions [letter]. Washington, DC: The American College of Obstetricians and Gynecologists; May 18, 2016. Available at: https://www.acog.org/-/media/Departments/Breastfeeding/Breast-Feeding-ACOG-USPSTF.pdf?dmc=1&ts=20180518T1850056558. Accessed May 22, 2018.
17. Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2:222-231.
18. Bowatte G, Tham R, Allen KJ, et al. Breastfeeding and childhood acute otitis media: a systematic review and meta-analysis. Acta Paediatr. 2015;104:85-95.
19. Chonmaitree T, Trujillo R, Jennings K, et al. Acute otitis media and other complications of viral respiratory infection. Pediatrics. 2016;137:e20153555.
20. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
21. Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118:865-873.
22. Duijts L, Jaddoe VW, Hofman A, et al. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126:e18-e25.
23. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186:190-199.
24. Kramer MS, Chalmers B, Hodnett ED, et al; PROBIT Study Group (Promotion of Breastfeeding Intervention Trial). Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413-420.
25. Dennehy PH. Acute diarrheal disease in children: epidemiology, prevention, and treatment. Infect Dis Clin North Am. 2005;19:585-602.
26. Cortese MM, Tate JE, Simonsen L, et al. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatric Infect Dis J. 2010;29:489-494.
27. Ajetunmobi OM, Whyte B, Chalmers J, et al. Breastfeeding is associated with reduced childhood hospitalization: evidence from a Scottish birth cohort (1997-2009). J Pediatr. 2015;166:620-625.
28. Quigley MA, Kelly YJ, Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics. 2007;119:e837-e842.
29. Radtke JV. The paradox of breastfeeding-associated morbidity among late preterm infants. J Obstet Gynecol Neonatal Nurs. 2011;40:9-24.
30. Escobar GJ, Gonzales VM, Armstrong M, et al. Rehospitalization for neonatal dehydration: a nested case-control study. Arch Pediatr Adolesc Med. 2002;156:155-161.
31. Salas AA, Salazar J, Burgoa CV, et al. Significant weight loss in breastfed term infants readmitted for hyperbilirubinemia. BMC Pediatr. 2009;9:82.
32. Tarcan A, Tiker F, Vatandaş NS, et al. Weight loss and hypernatremia in breast-fed babies: frequency in neonates with non-hemolytic jaundice. J Paediatr Child Health. 2005;41:484-487.
33. Flaherman VJ, Aby J, Burgos AE, et al. Effect of early limited formula on duration and exclusivity of breastfeeding in at-risk infants: an RCT. Pediatrics. 2013;131:1059-1065.
34. Straňák Z, Feyereislova S, Černá M, et al. J. Limited amount of formula may facilitate breastfeeding: randomized, controlled trial to compare standard clinical practice versus limited supplemental feeding. Denning PW, ed. PLoS One. 2016;11:e0150053.
35. Ip S, Chung M, Raman G, et al. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Rockville, MD: Agency for Healthcare Research and Quality (US); 2007. Evidence Reports/Technology Assessments, No. 153. Available at: www.ncbi.nlm.nih.gov/books/NBK38337/. Accessed April 3, 2018.
36. Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014;(4):CD002971.
37. Hauck FR, Thompson JM, Tanabe KO, et al. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128:103-110.
38. American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116:1245-1255.
39. Moon RY, Fu L. Sudden infant death syndrome: an update. Pediatr Rev. 2012;33:314-320.
40. Lodge CJ, Tan DJ, Lau MX, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104:38-53.
41. Brim SN, Rudd RA, Funk RH, et al. Asthma prevalence among US children in underrepresented minority populations: American Indian/Alaska Native, Chinese, Filipino, and Asian Indian. Pediatrics. 2008;122:e217-e222.
42. Shaw TF, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
43. Mallol J, Crane J, von Mutius E, et al. The international study of asthma and allergies in childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr). 2013;41:73-85.
44. Flohr C, Nagel G, Weinmayr G, et al. Lack of evidence for a protective effect of prolonged breastfeeding on childhood eczema: lessons from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. Br J Dermatol. 2011;165:1280-1289.
45. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
46. Peres KG, Cascaes AM, Nascimento GG, et al. Effect of breastfeeding on malocclusions: a systematic review and meta-analysis. Acta Paediatr. 2015;104:54-61.
47. Amitya EL, Keinan-Boker L. Breastfeeding and childhood leukemia incidence: a meta-analysis and systematic review. JAMA Pediatr. 2015;169:e151025.
48. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943-1955.
49. Guxens M, Mendez MA, Moltó-Puigmartí C, et al. Breastfeeding, long-chain polyunsaturated fatty acids in colostrum, and infant mental development.
2012;129:1134-1140.
51. Horta BL, Victora CG. Long-term effects of breastfeeding: a systematic review. Geneva, Switzerland: World Health Organization; 2013. Available at: http://apps.who.int/iris/bitstream/10665/79198/1/9789241505307_eng.pdf. Accessed August 16, 2016.
52. Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104:14-19.
53. Der G, Batty GD, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. 2006;333:945.
54. Sajjad A, Tharner A, Kiefte-de Jong JC, et al. Breastfeeding duration and non-verbal IQ in children. J Epidemiol Community Health 2015;69:775-781.
55. Yan J, Liu L, Zhu Y, et al. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267.
56. Martin RM, Patel R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
57. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure, and type 2 diabetes: systematic review and meta-analysis. Acta Paediatr. 2015;104:30-37.
58. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Syst Rev. 2012;15:CD003517.
59. U.S. Preventive Services Task Force. Final recommendation statement: aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Published September 2017. Accessed April 3, 2018.
60. U.S. Preventive Services Task Force. Screening for breast cancer. Available at: www.uspreventiveservicestaskforce.org/Page/SupportingDoc/breast-cancer-screening/final-evidence-summary9. Published November 2009. Accessed April 2, 2018.
61. Bass JL, Gartley T, Kleinman R. Unintended consequences of current breastfeeding initiatives. JAMA Pediatr. 2016;170:923-924.
62. Feldman-Winter L, Goldsmith JP; Committee on Fetus and Newborn; Task Force on Sudden Infant Death Syndrome. Safe sleep and skin-to-skin care in the neonatal period for healthy term newborns. Pediatrics. 2016;138:e20161889.
63. The Mother and Child Health and Education Trust. Ten steps to successful breastfeeding. Available at: www.tensteps.org. Published November 8, 2017. Accessed April 3, 2018.
64. Hauck FR, Omojokun OO, Siadaty MS. Do pacifiers reduce the risk of sudden infant death syndrome? A meta-analysis. Pediatrics. 2005;116:e716-e723.
65. Kair LR, Kenron D, Etheredge K, et al. Pacifier restriction and exclusive breastfeeding. Pediatrics. 2013;131:e1101-e1107.
66. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
67. Wolf JB. Is breast really best? Risk and total motherhood in the National Breastfeeding Awareness Campaign. J Health Polit Policy Law. 2007;32:595-636.
68. Marshall JL, Godfrey M, Renfrew MJ. Being a ‘good mother’: managing breastfeeding and merging identities. Soc Sci Med. 2007;65:2147-2159.
69. Kelleher CM. The physical challenges of early breastfeeding. Soc Sci Med. 2006;63:2727-2738.
From The Journal of Family Practice | 2018;67(6):E1-E9.
PRACTICE RECOMMENDATIONS
› Encourage breastfeeding for its potential to reduce the risk of acute otitis media, upper- and lower-respiratory infections, gastrointestinal infection, and dental malocclusion. A
› Promote breastfeeding for its potential to make a small difference in intelligence quotient and the incidence of overweight and obesity—but not for any other significant impact on long-term health. B
› Consider the needs and preferences of the individual when advocating breastfeeding so as to avoid potentially engendering maternal feelings of guilt and inadequacy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Cognitive bias: Its influence on clinical diagnosis
CASE A patient with a history of drug-seeking behavior asks to be seen by you for lower back pain. Your impression upon entering the examination room is that the patient appears to be in minimal pain. A review of the patient’s chart leads you to suspect that the patient’s past behavior pattern is the reason for the visit. You find yourself downplaying his reports of weight loss, changed bowel habits, and lower extremity weakness—despite the fact that these complaints might have led you to consider more concerning causes of back pain in a different patient.
This situation is not uncommon. At one time or another, it’s likely that we have all placed an undue emphasis on a patient’s social background to reinforce a pre-existing opinion of the likely diagnosis. Doing so is an example of both anchoring and confirmation biases—just 2 of the many biases known to influence critical thinking in clinical practice (and which we’ll describe in a bit).
Reconsidering the diagnostic process. Previous attempts to address the issue of incorrect diagnosis and medical error have focused on systems-based approaches such as adopting electronic medical records to avert prescribing errors or eliminating confusing abbreviations in documentation.
Graber et al reviewed 100 errors involving internists and found that 46% of the errors resulted from a combination of systems-based and cognitive reasoning factors.2 More surprisingly, 28% of errors were attributable to reasoning failures alone.2 Singh et al showed that in one primary care network, most errors occurred during the patient-doctor encounter, with 56% involving errors in history taking and 47% involving oversights in the physical examination.3 Furthermore, most of the errors occurred in the context of common conditions such as pneumonia and congestive heart failure—rather than esoteric diseases—implying that the failures were due to errors in the diagnostic process rather than from a lack of knowledge.3
An understanding of the diagnostic process and the etiology of diagnostic error is of utmost importance in primary care. Family physicians who, on a daily basis, see a high volume of patients with predominantly low-acuity conditions, must be vigilant for the rare life-threatening condition that may mimic a more benign disease. It is in this setting that cognitive errors may abound, leading to both patient harm and emotional stress in physicians.3
This article reviews the current understanding of the cognitive pathways involved in diagnostic decision making, explains the factors that contribute to diagnostic errors, and summarizes the current research aimed at preventing these errors.
Continue to: The diagnostic process, as currently understood
The diagnostic process, as currently understood
Much of what is understood about the cognitive processes involved in diagnostic reasoning is built on research done in the field of behavioral science—specifically, the foundational work by psychologists Amos Tversky and Daniel Kahneman in the 1970s.4 Only relatively recently has the medical field begun to apply the findings of this research in its attempt to understand how clinicians diagnose.1 This work led to the description of 2 main cognitive pathways described by Croskerry and others.5
Type 1 processing, also referred to as the “intuitive” approach, uses a rapid, largely subconscious pattern-recognition method. Much in the same way one recognizes a familiar face, the clinician using a type 1 process rapidly comes to a conclusion by seeing a recognizable pattern among the patient’s signs and symptoms. For example, crushing chest pain radiating to the left arm instantly brings to mind a myocardial infarction without the clinician methodically formulating a differential diagnosis.4,5
Type 2 processing is an “analytic” approach in which the provider considers the salient characteristics of the case, generates a list of hypotheses, and proceeds to systematically test them and come to a more definitive conclusion.5 For example, an intern encountering a patient with a painfully swollen knee will consider the possibilities of septic arthritis, Lyme disease, and gout, and then carefully determine the likelihood of each disease based on the evidence available at the time.
How the processes work in practice. While these 2 pathways are well studied within behavioral circles and are even supported by neurobiologic evidence, most clinical encounters incorporate both methodologies in a parallel system known as the “dual-process” theory (FIGURE).4-6
For example, during an initial visit for back pain, a patient may begin by relaying that the discomfort began after lifting a heavy object. Immediately the clinician, using a type 1 process, will suspect a simple lumbar strain. However, upon further questioning, the patient reveals that the pain occurs at rest and wakes him from sleep; these characteristics are atypical for a simple strain. At this point, the clinician may switch to a type 2 analytic approach and generate a broad differential that includes infection and malignancy.
Continue to: Heuristics: Indispensable, yet susceptible to bias
Heuristics: Indispensable, yet susceptible to bias
Heuristics are cognitive shortcuts often operating subconsciously to solve problems more quickly and efficiently than if the problem were analyzed and solved deductively.7 The act of driving a car, for instance, is a complex everyday task wherein the use of heuristics is not just efficient but essential. Deliberately analyzing and consciously considering every action required in daily living prior to execution would be impractical and even dangerous.
Heuristics also have a role in the practice of medicine. When presented with a large volume of low-acuity patients, the primary care provider would find it impractical to formulate an extensive differential and test each diagnosis before devising a plan of action. Using heuristics during clinical decision-making, however, does make the clinician more vulnerable to biases, which are described in the text that follows.
Biases
Bias is the psychological tendency to make a decision based on incomplete information and subjective factors rather than empirical evidence.4
Anchoring. One of the best-known biases, described in both behavioral science and medical literature, is anchoring. With this bias, the clinician fixates on a particular aspect of the patient’s initial presentation, excluding other more relevant clinical facts.8
A busy clinician, for example, may be notified by a medical assistant that the patient in Room One is complaining about fatigue and seems very depressed. The clinician then unduly anchors his thought process to this initial label of a depressed patient and, without much deliberation, prescribes an antidepressant medication. Had the physician inquired about skin and hair changes (unusual in depression), the more probable diagnosis of hypothyroidism would have come to mind.
Continue to: Premature closure...
Premature closure is another well-known bias associated with diagnostic errors.2,6 This is the tendency to cease inquiry once a possible solution for a problem is found. As the name implies, premature closure leads to an incomplete investigation of the problem and perhaps to incorrect conclusions.
If police arrested a potential suspect in a crime and halted the investigation, it’s possible the true culprit might not be found. In medicine, a classic example would be a junior clinician presented with a case of rectal bleeding in a 75-year-old man who has experienced weight loss and a change in bowel movements. The clinician observes a small nonfriable external hemorrhoid, incorrectly attributes the patient’s symptoms to that finding, and does not pursue the more appropriate investigation for gastrointestinal malignancy.
Interconnected biases. Often diagnostic errors are the result of multiple interconnected biases. For example, a busy emergency department physician is told that an unconscious patient smells of alcohol, so he is “probably drunk and just needs to sleep it off” (anchoring bias). The physician then examines the patient, who is barely arousable and indeed has a heavy odor of alcohol. The physician, therefore, decides not to order a basic laboratory work-up (premature closure). Because of this, the physician misses the correct and life-threatening diagnosis of a mental status change due to alcoholic ketoacidosis.6
Numerous other biases have been identified and studied.4,8 While an in-depth examination of all biases is beyond the scope of this article, some of those most relevant to medical practice are listed and briefly defined in the TABLE.4,8
Multiple studies point to the central role biases play in diagnostic error. A systematic review by Saposnik et al found that physician cognitive biases were associated with diagnostic errors in 36.5% to 77% of case studies, and that 71% of the studies reviewed found an association between cognitive errors and therapeutic errors.6 In experimental studies, cognitive biases have also been shown to decrease accuracy in the interpretation of radiologic studies and electrocardiograms.9 In one case review, cognitive errors were identified in 74% of cases where an actual medical error had been committed.2
Continue to: The human component: When the patient is "difficult"
The human component: When the patient is “difficult”
Failures in reasoning are not solely responsible for diagnostic errors. One increasingly scrutinized cause of impaired clinical judgment is the physician-patient relationship, especially one involving a “difficult” patient. Additionally, the medical literature is beginning to highlight the strong correlation between clinician fatigue or burnout and diagnostic errors.10
Patient-specific factors clearly impact the likelihood of diagnostic error. One randomized controlled trial showed that patients with disruptive behaviors negatively influence the accuracy of clinicians’ diagnoses.11 In this study, family medicine residents made 42% more diagnostic errors when evaluating complex clinical presentations involving patients with negative interpersonal characteristics (demeaning, aggressive, or demanding communication styles). Even with simple clinical problems, difficult patient behaviors were associated with a 6% higher rate of error than when such behaviors were absent, although this finding did not reach statistical significance.11
Researchers have proposed the “resource depletion” theory as an explanation for this finding.11 A patient with difficult behaviors will require additional cognitive resources from the physician to manage those behaviors.11 This leaves less cognitive capacity for solving the diagnostic problem.11 Furthermore, Riskin et al demonstrated that pediatric intensive care teams committed increased rates of medical errors and experienced poorer team performance when exposed to simulated families displaying rude behavior.12 Clearly, the power of the patient-physician relationship cannot be overstated when discussing diagnostic error.
Continue to: Strategies for reducing errors in the diagnostic process
Strategies for reducing errors in the diagnostic process
Although the mental pathways involved in diagnostic reasoning have become better elucidated, there is still considerable controversy and uncertainty surrounding effective ways to counter errors. In their review of the literature, Norman et al concluded that diagnostic errors are multifactorial and that strategies that solely educate novice clinicians about biases are unlikely to lead to significant gains because of “limited transfer.”9 That is, in simply teaching the theory of cognitive errors before trainees have had time to accumulate real-world experience, they do not learn how to apply corrective solutions.
Graber et al argue that mental shortcuts are often a beneficial behavior, and it would be unrealistic and perhaps even detrimental to eliminate them completely from clinical judgment.13 Despite the controversy, several corrective methods have been proposed and have shown promise. Two such methods are medical education on cognitive error and the use of differential diagnosis generators.2
Medical education on cognitive error. If heuristics and biases are acquired subconscious patterns of thinking, then it would be logical to assume that the most effective way to prevent their intrusion into the clinical decision-making process would be to intervene when the art of diagnosis is taught. Graber et al reference several small studies that demonstrated a small improvement in diagnostic accuracy when learners were educated about cognitive biases and clinical judgment.13
Additionally, with medical students, Mamede et al describe how structured reflection during case-based learning enhanced diagnostic accuracy.14 However, none of these studies have proven that increased awareness of cognitive biases results in fewer delayed or missed diagnoses in clinical practice. Clearly, further research is needed to determine whether the skills gained in the classroom would be transferable to clinical practice and result in lower rates of delayed or missed diagnoses. Future studies could also investigate if these findings are replicable when applied to more experienced clinicians rather than medical students and residents.
Continue to: Differential diagnosis generators
Differential diagnosis generators.
However, few randomized controlled studies have investigated whether the use of a DDx generator reduces diagnostic error, and evidence is lacking to prove their usefulness in clinical practice. Furthermore, while an exhaustive list of possible diagnoses may be helpful, some proposed diagnoses may be irrelevant and may distract from timely attention being paid to more likely possibilities. Additionally, forming an extensive DDx list during every patient encounter would significantly add to the physician’s workload and could contribute to physician burnout.
Selective use? We believe that DDx generators would be best used selectively as a safeguard for the clinician who becomes aware of an increased risk of diagnostic error in a particular patient. As previously discussed, errors involving cognitive processes are more often errors of improper reasoning rather than of insufficient knowledge.3 The DDx generator then serves as a way of double-checking to ensure that additional diagnoses are being considered. This can be especially helpful when facing patients who display difficult behaviors or when the clinician’s cognitive reserve is depleted by other factors.
DDx generators may also help the physician expand his or her differential diagnosis when a patient is failing to improve despite appropriately treating the working diagnosis.
Another option worth studying? Future studies could also investigate whether discussing a case with another clinician is an effective way to reduce cognitive biases and diagnostic errors.
Continue to: Looking foward
Looking forward
More research will hopefully lead to corrective solutions. But it is also likely that solutions will require additional time and resources on the part of already overburdened providers. Thus, new challenges will arise in applying remedies to the current model of health care management and reimbursement.
Despite clinically useful advances in technology and science, family physicians are left with the unsettling conclusion that the most common source of error may also be the most difficult to change: physicians themselves. Fortunately, history has shown that the field of medicine can overcome even the most ingrained and harmful tendencies of the human mind, including prejudice and superstition.16,17 This next challenge will be no exception.
CORRESPONDENCE
Thomas Yuen, MD, Crozer Keystone Family Medicine Residency, 1260 East Woodland Avenue, Suite 200, Philadelphia, PA 19064; [email protected].
1. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
2. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165:1493-1499.
3. Singh H, Giardina TD, Meyer AN, et al. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med. 2013;173:418-425.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131.
5. Croskerry P. A universal model of diagnostic reasoning. Acad Med. 2009;84:1022-1028.
6. Saposnik G, Redelmeier D, Ruff CC, et al. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138.
7. Gigerenzer G, Gaissmaier W. Heuristic decision making. Annu Rev Psychol. 2011;62:451-482.
8. Wellbery C. Flaws in clinical reasoning: a common cause of diagnostic error. Am Fam Physician. 2011;84:1042-1048.
9. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30.
10. Lockley SW, Cronin JW, Evans EE, et al. Effect of reducing interns’ weekly work hours on sleep and attentional failures. NEJM. 2004;351:1829-1837.
11. Schmidt HG, Van Gog T, Schuit SC, et al. Do patients’ disruptive behaviours influence the accuracy of a doctor’s diagnosis? A randomised experiment. BMJ Qual Saf. 2017;26:19-23.
12. Riskin A, Erez A, Foulk TA, et al. Rudeness and medical team performance. Pediatrics. 2017;139:e20162305.
13. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77:981-992.
14. Mamede S, Van Gog T, Sampaio AM, et al. How can students’ diagnostic competence benefit most from practice with clinical cases? The effects of structured reflection on future diagnosis of the same and novel diseases. Acad Med. 2014;89:121-127.
15. Bond WF, Schwartz LM, Weaver KR, et al. Differential diagnosis generators: an evaluation of currently available computer programs. J Gen Intern Med. 2012;27:213-219.
16. Porter R. The Greatest Benefit to Mankind: A Medical History of Humanity. New York, NY: W.W. Norton and Company, Inc.;1999.
17. Lazarus BA. The practice of medicine and prejudice in a New England town: the founding of Mount Sinai Hospital, Hartford, Connecticut. J Am Ethn Hist. 1991;10:21-41.
CASE A patient with a history of drug-seeking behavior asks to be seen by you for lower back pain. Your impression upon entering the examination room is that the patient appears to be in minimal pain. A review of the patient’s chart leads you to suspect that the patient’s past behavior pattern is the reason for the visit. You find yourself downplaying his reports of weight loss, changed bowel habits, and lower extremity weakness—despite the fact that these complaints might have led you to consider more concerning causes of back pain in a different patient.
This situation is not uncommon. At one time or another, it’s likely that we have all placed an undue emphasis on a patient’s social background to reinforce a pre-existing opinion of the likely diagnosis. Doing so is an example of both anchoring and confirmation biases—just 2 of the many biases known to influence critical thinking in clinical practice (and which we’ll describe in a bit).
Reconsidering the diagnostic process. Previous attempts to address the issue of incorrect diagnosis and medical error have focused on systems-based approaches such as adopting electronic medical records to avert prescribing errors or eliminating confusing abbreviations in documentation.
Graber et al reviewed 100 errors involving internists and found that 46% of the errors resulted from a combination of systems-based and cognitive reasoning factors.2 More surprisingly, 28% of errors were attributable to reasoning failures alone.2 Singh et al showed that in one primary care network, most errors occurred during the patient-doctor encounter, with 56% involving errors in history taking and 47% involving oversights in the physical examination.3 Furthermore, most of the errors occurred in the context of common conditions such as pneumonia and congestive heart failure—rather than esoteric diseases—implying that the failures were due to errors in the diagnostic process rather than from a lack of knowledge.3
An understanding of the diagnostic process and the etiology of diagnostic error is of utmost importance in primary care. Family physicians who, on a daily basis, see a high volume of patients with predominantly low-acuity conditions, must be vigilant for the rare life-threatening condition that may mimic a more benign disease. It is in this setting that cognitive errors may abound, leading to both patient harm and emotional stress in physicians.3
This article reviews the current understanding of the cognitive pathways involved in diagnostic decision making, explains the factors that contribute to diagnostic errors, and summarizes the current research aimed at preventing these errors.
Continue to: The diagnostic process, as currently understood
The diagnostic process, as currently understood
Much of what is understood about the cognitive processes involved in diagnostic reasoning is built on research done in the field of behavioral science—specifically, the foundational work by psychologists Amos Tversky and Daniel Kahneman in the 1970s.4 Only relatively recently has the medical field begun to apply the findings of this research in its attempt to understand how clinicians diagnose.1 This work led to the description of 2 main cognitive pathways described by Croskerry and others.5
Type 1 processing, also referred to as the “intuitive” approach, uses a rapid, largely subconscious pattern-recognition method. Much in the same way one recognizes a familiar face, the clinician using a type 1 process rapidly comes to a conclusion by seeing a recognizable pattern among the patient’s signs and symptoms. For example, crushing chest pain radiating to the left arm instantly brings to mind a myocardial infarction without the clinician methodically formulating a differential diagnosis.4,5
Type 2 processing is an “analytic” approach in which the provider considers the salient characteristics of the case, generates a list of hypotheses, and proceeds to systematically test them and come to a more definitive conclusion.5 For example, an intern encountering a patient with a painfully swollen knee will consider the possibilities of septic arthritis, Lyme disease, and gout, and then carefully determine the likelihood of each disease based on the evidence available at the time.
How the processes work in practice. While these 2 pathways are well studied within behavioral circles and are even supported by neurobiologic evidence, most clinical encounters incorporate both methodologies in a parallel system known as the “dual-process” theory (FIGURE).4-6
For example, during an initial visit for back pain, a patient may begin by relaying that the discomfort began after lifting a heavy object. Immediately the clinician, using a type 1 process, will suspect a simple lumbar strain. However, upon further questioning, the patient reveals that the pain occurs at rest and wakes him from sleep; these characteristics are atypical for a simple strain. At this point, the clinician may switch to a type 2 analytic approach and generate a broad differential that includes infection and malignancy.
Continue to: Heuristics: Indispensable, yet susceptible to bias
Heuristics: Indispensable, yet susceptible to bias
Heuristics are cognitive shortcuts often operating subconsciously to solve problems more quickly and efficiently than if the problem were analyzed and solved deductively.7 The act of driving a car, for instance, is a complex everyday task wherein the use of heuristics is not just efficient but essential. Deliberately analyzing and consciously considering every action required in daily living prior to execution would be impractical and even dangerous.
Heuristics also have a role in the practice of medicine. When presented with a large volume of low-acuity patients, the primary care provider would find it impractical to formulate an extensive differential and test each diagnosis before devising a plan of action. Using heuristics during clinical decision-making, however, does make the clinician more vulnerable to biases, which are described in the text that follows.
Biases
Bias is the psychological tendency to make a decision based on incomplete information and subjective factors rather than empirical evidence.4
Anchoring. One of the best-known biases, described in both behavioral science and medical literature, is anchoring. With this bias, the clinician fixates on a particular aspect of the patient’s initial presentation, excluding other more relevant clinical facts.8
A busy clinician, for example, may be notified by a medical assistant that the patient in Room One is complaining about fatigue and seems very depressed. The clinician then unduly anchors his thought process to this initial label of a depressed patient and, without much deliberation, prescribes an antidepressant medication. Had the physician inquired about skin and hair changes (unusual in depression), the more probable diagnosis of hypothyroidism would have come to mind.
Continue to: Premature closure...
Premature closure is another well-known bias associated with diagnostic errors.2,6 This is the tendency to cease inquiry once a possible solution for a problem is found. As the name implies, premature closure leads to an incomplete investigation of the problem and perhaps to incorrect conclusions.
If police arrested a potential suspect in a crime and halted the investigation, it’s possible the true culprit might not be found. In medicine, a classic example would be a junior clinician presented with a case of rectal bleeding in a 75-year-old man who has experienced weight loss and a change in bowel movements. The clinician observes a small nonfriable external hemorrhoid, incorrectly attributes the patient’s symptoms to that finding, and does not pursue the more appropriate investigation for gastrointestinal malignancy.
Interconnected biases. Often diagnostic errors are the result of multiple interconnected biases. For example, a busy emergency department physician is told that an unconscious patient smells of alcohol, so he is “probably drunk and just needs to sleep it off” (anchoring bias). The physician then examines the patient, who is barely arousable and indeed has a heavy odor of alcohol. The physician, therefore, decides not to order a basic laboratory work-up (premature closure). Because of this, the physician misses the correct and life-threatening diagnosis of a mental status change due to alcoholic ketoacidosis.6
Numerous other biases have been identified and studied.4,8 While an in-depth examination of all biases is beyond the scope of this article, some of those most relevant to medical practice are listed and briefly defined in the TABLE.4,8
Multiple studies point to the central role biases play in diagnostic error. A systematic review by Saposnik et al found that physician cognitive biases were associated with diagnostic errors in 36.5% to 77% of case studies, and that 71% of the studies reviewed found an association between cognitive errors and therapeutic errors.6 In experimental studies, cognitive biases have also been shown to decrease accuracy in the interpretation of radiologic studies and electrocardiograms.9 In one case review, cognitive errors were identified in 74% of cases where an actual medical error had been committed.2
Continue to: The human component: When the patient is "difficult"
The human component: When the patient is “difficult”
Failures in reasoning are not solely responsible for diagnostic errors. One increasingly scrutinized cause of impaired clinical judgment is the physician-patient relationship, especially one involving a “difficult” patient. Additionally, the medical literature is beginning to highlight the strong correlation between clinician fatigue or burnout and diagnostic errors.10
Patient-specific factors clearly impact the likelihood of diagnostic error. One randomized controlled trial showed that patients with disruptive behaviors negatively influence the accuracy of clinicians’ diagnoses.11 In this study, family medicine residents made 42% more diagnostic errors when evaluating complex clinical presentations involving patients with negative interpersonal characteristics (demeaning, aggressive, or demanding communication styles). Even with simple clinical problems, difficult patient behaviors were associated with a 6% higher rate of error than when such behaviors were absent, although this finding did not reach statistical significance.11
Researchers have proposed the “resource depletion” theory as an explanation for this finding.11 A patient with difficult behaviors will require additional cognitive resources from the physician to manage those behaviors.11 This leaves less cognitive capacity for solving the diagnostic problem.11 Furthermore, Riskin et al demonstrated that pediatric intensive care teams committed increased rates of medical errors and experienced poorer team performance when exposed to simulated families displaying rude behavior.12 Clearly, the power of the patient-physician relationship cannot be overstated when discussing diagnostic error.
Continue to: Strategies for reducing errors in the diagnostic process
Strategies for reducing errors in the diagnostic process
Although the mental pathways involved in diagnostic reasoning have become better elucidated, there is still considerable controversy and uncertainty surrounding effective ways to counter errors. In their review of the literature, Norman et al concluded that diagnostic errors are multifactorial and that strategies that solely educate novice clinicians about biases are unlikely to lead to significant gains because of “limited transfer.”9 That is, in simply teaching the theory of cognitive errors before trainees have had time to accumulate real-world experience, they do not learn how to apply corrective solutions.
Graber et al argue that mental shortcuts are often a beneficial behavior, and it would be unrealistic and perhaps even detrimental to eliminate them completely from clinical judgment.13 Despite the controversy, several corrective methods have been proposed and have shown promise. Two such methods are medical education on cognitive error and the use of differential diagnosis generators.2
Medical education on cognitive error. If heuristics and biases are acquired subconscious patterns of thinking, then it would be logical to assume that the most effective way to prevent their intrusion into the clinical decision-making process would be to intervene when the art of diagnosis is taught. Graber et al reference several small studies that demonstrated a small improvement in diagnostic accuracy when learners were educated about cognitive biases and clinical judgment.13
Additionally, with medical students, Mamede et al describe how structured reflection during case-based learning enhanced diagnostic accuracy.14 However, none of these studies have proven that increased awareness of cognitive biases results in fewer delayed or missed diagnoses in clinical practice. Clearly, further research is needed to determine whether the skills gained in the classroom would be transferable to clinical practice and result in lower rates of delayed or missed diagnoses. Future studies could also investigate if these findings are replicable when applied to more experienced clinicians rather than medical students and residents.
Continue to: Differential diagnosis generators
Differential diagnosis generators.
However, few randomized controlled studies have investigated whether the use of a DDx generator reduces diagnostic error, and evidence is lacking to prove their usefulness in clinical practice. Furthermore, while an exhaustive list of possible diagnoses may be helpful, some proposed diagnoses may be irrelevant and may distract from timely attention being paid to more likely possibilities. Additionally, forming an extensive DDx list during every patient encounter would significantly add to the physician’s workload and could contribute to physician burnout.
Selective use? We believe that DDx generators would be best used selectively as a safeguard for the clinician who becomes aware of an increased risk of diagnostic error in a particular patient. As previously discussed, errors involving cognitive processes are more often errors of improper reasoning rather than of insufficient knowledge.3 The DDx generator then serves as a way of double-checking to ensure that additional diagnoses are being considered. This can be especially helpful when facing patients who display difficult behaviors or when the clinician’s cognitive reserve is depleted by other factors.
DDx generators may also help the physician expand his or her differential diagnosis when a patient is failing to improve despite appropriately treating the working diagnosis.
Another option worth studying? Future studies could also investigate whether discussing a case with another clinician is an effective way to reduce cognitive biases and diagnostic errors.
Continue to: Looking foward
Looking forward
More research will hopefully lead to corrective solutions. But it is also likely that solutions will require additional time and resources on the part of already overburdened providers. Thus, new challenges will arise in applying remedies to the current model of health care management and reimbursement.
Despite clinically useful advances in technology and science, family physicians are left with the unsettling conclusion that the most common source of error may also be the most difficult to change: physicians themselves. Fortunately, history has shown that the field of medicine can overcome even the most ingrained and harmful tendencies of the human mind, including prejudice and superstition.16,17 This next challenge will be no exception.
CORRESPONDENCE
Thomas Yuen, MD, Crozer Keystone Family Medicine Residency, 1260 East Woodland Avenue, Suite 200, Philadelphia, PA 19064; [email protected].
CASE A patient with a history of drug-seeking behavior asks to be seen by you for lower back pain. Your impression upon entering the examination room is that the patient appears to be in minimal pain. A review of the patient’s chart leads you to suspect that the patient’s past behavior pattern is the reason for the visit. You find yourself downplaying his reports of weight loss, changed bowel habits, and lower extremity weakness—despite the fact that these complaints might have led you to consider more concerning causes of back pain in a different patient.
This situation is not uncommon. At one time or another, it’s likely that we have all placed an undue emphasis on a patient’s social background to reinforce a pre-existing opinion of the likely diagnosis. Doing so is an example of both anchoring and confirmation biases—just 2 of the many biases known to influence critical thinking in clinical practice (and which we’ll describe in a bit).
Reconsidering the diagnostic process. Previous attempts to address the issue of incorrect diagnosis and medical error have focused on systems-based approaches such as adopting electronic medical records to avert prescribing errors or eliminating confusing abbreviations in documentation.
Graber et al reviewed 100 errors involving internists and found that 46% of the errors resulted from a combination of systems-based and cognitive reasoning factors.2 More surprisingly, 28% of errors were attributable to reasoning failures alone.2 Singh et al showed that in one primary care network, most errors occurred during the patient-doctor encounter, with 56% involving errors in history taking and 47% involving oversights in the physical examination.3 Furthermore, most of the errors occurred in the context of common conditions such as pneumonia and congestive heart failure—rather than esoteric diseases—implying that the failures were due to errors in the diagnostic process rather than from a lack of knowledge.3
An understanding of the diagnostic process and the etiology of diagnostic error is of utmost importance in primary care. Family physicians who, on a daily basis, see a high volume of patients with predominantly low-acuity conditions, must be vigilant for the rare life-threatening condition that may mimic a more benign disease. It is in this setting that cognitive errors may abound, leading to both patient harm and emotional stress in physicians.3
This article reviews the current understanding of the cognitive pathways involved in diagnostic decision making, explains the factors that contribute to diagnostic errors, and summarizes the current research aimed at preventing these errors.
Continue to: The diagnostic process, as currently understood
The diagnostic process, as currently understood
Much of what is understood about the cognitive processes involved in diagnostic reasoning is built on research done in the field of behavioral science—specifically, the foundational work by psychologists Amos Tversky and Daniel Kahneman in the 1970s.4 Only relatively recently has the medical field begun to apply the findings of this research in its attempt to understand how clinicians diagnose.1 This work led to the description of 2 main cognitive pathways described by Croskerry and others.5
Type 1 processing, also referred to as the “intuitive” approach, uses a rapid, largely subconscious pattern-recognition method. Much in the same way one recognizes a familiar face, the clinician using a type 1 process rapidly comes to a conclusion by seeing a recognizable pattern among the patient’s signs and symptoms. For example, crushing chest pain radiating to the left arm instantly brings to mind a myocardial infarction without the clinician methodically formulating a differential diagnosis.4,5
Type 2 processing is an “analytic” approach in which the provider considers the salient characteristics of the case, generates a list of hypotheses, and proceeds to systematically test them and come to a more definitive conclusion.5 For example, an intern encountering a patient with a painfully swollen knee will consider the possibilities of septic arthritis, Lyme disease, and gout, and then carefully determine the likelihood of each disease based on the evidence available at the time.
How the processes work in practice. While these 2 pathways are well studied within behavioral circles and are even supported by neurobiologic evidence, most clinical encounters incorporate both methodologies in a parallel system known as the “dual-process” theory (FIGURE).4-6
For example, during an initial visit for back pain, a patient may begin by relaying that the discomfort began after lifting a heavy object. Immediately the clinician, using a type 1 process, will suspect a simple lumbar strain. However, upon further questioning, the patient reveals that the pain occurs at rest and wakes him from sleep; these characteristics are atypical for a simple strain. At this point, the clinician may switch to a type 2 analytic approach and generate a broad differential that includes infection and malignancy.
Continue to: Heuristics: Indispensable, yet susceptible to bias
Heuristics: Indispensable, yet susceptible to bias
Heuristics are cognitive shortcuts often operating subconsciously to solve problems more quickly and efficiently than if the problem were analyzed and solved deductively.7 The act of driving a car, for instance, is a complex everyday task wherein the use of heuristics is not just efficient but essential. Deliberately analyzing and consciously considering every action required in daily living prior to execution would be impractical and even dangerous.
Heuristics also have a role in the practice of medicine. When presented with a large volume of low-acuity patients, the primary care provider would find it impractical to formulate an extensive differential and test each diagnosis before devising a plan of action. Using heuristics during clinical decision-making, however, does make the clinician more vulnerable to biases, which are described in the text that follows.
Biases
Bias is the psychological tendency to make a decision based on incomplete information and subjective factors rather than empirical evidence.4
Anchoring. One of the best-known biases, described in both behavioral science and medical literature, is anchoring. With this bias, the clinician fixates on a particular aspect of the patient’s initial presentation, excluding other more relevant clinical facts.8
A busy clinician, for example, may be notified by a medical assistant that the patient in Room One is complaining about fatigue and seems very depressed. The clinician then unduly anchors his thought process to this initial label of a depressed patient and, without much deliberation, prescribes an antidepressant medication. Had the physician inquired about skin and hair changes (unusual in depression), the more probable diagnosis of hypothyroidism would have come to mind.
Continue to: Premature closure...
Premature closure is another well-known bias associated with diagnostic errors.2,6 This is the tendency to cease inquiry once a possible solution for a problem is found. As the name implies, premature closure leads to an incomplete investigation of the problem and perhaps to incorrect conclusions.
If police arrested a potential suspect in a crime and halted the investigation, it’s possible the true culprit might not be found. In medicine, a classic example would be a junior clinician presented with a case of rectal bleeding in a 75-year-old man who has experienced weight loss and a change in bowel movements. The clinician observes a small nonfriable external hemorrhoid, incorrectly attributes the patient’s symptoms to that finding, and does not pursue the more appropriate investigation for gastrointestinal malignancy.
Interconnected biases. Often diagnostic errors are the result of multiple interconnected biases. For example, a busy emergency department physician is told that an unconscious patient smells of alcohol, so he is “probably drunk and just needs to sleep it off” (anchoring bias). The physician then examines the patient, who is barely arousable and indeed has a heavy odor of alcohol. The physician, therefore, decides not to order a basic laboratory work-up (premature closure). Because of this, the physician misses the correct and life-threatening diagnosis of a mental status change due to alcoholic ketoacidosis.6
Numerous other biases have been identified and studied.4,8 While an in-depth examination of all biases is beyond the scope of this article, some of those most relevant to medical practice are listed and briefly defined in the TABLE.4,8
Multiple studies point to the central role biases play in diagnostic error. A systematic review by Saposnik et al found that physician cognitive biases were associated with diagnostic errors in 36.5% to 77% of case studies, and that 71% of the studies reviewed found an association between cognitive errors and therapeutic errors.6 In experimental studies, cognitive biases have also been shown to decrease accuracy in the interpretation of radiologic studies and electrocardiograms.9 In one case review, cognitive errors were identified in 74% of cases where an actual medical error had been committed.2
Continue to: The human component: When the patient is "difficult"
The human component: When the patient is “difficult”
Failures in reasoning are not solely responsible for diagnostic errors. One increasingly scrutinized cause of impaired clinical judgment is the physician-patient relationship, especially one involving a “difficult” patient. Additionally, the medical literature is beginning to highlight the strong correlation between clinician fatigue or burnout and diagnostic errors.10
Patient-specific factors clearly impact the likelihood of diagnostic error. One randomized controlled trial showed that patients with disruptive behaviors negatively influence the accuracy of clinicians’ diagnoses.11 In this study, family medicine residents made 42% more diagnostic errors when evaluating complex clinical presentations involving patients with negative interpersonal characteristics (demeaning, aggressive, or demanding communication styles). Even with simple clinical problems, difficult patient behaviors were associated with a 6% higher rate of error than when such behaviors were absent, although this finding did not reach statistical significance.11
Researchers have proposed the “resource depletion” theory as an explanation for this finding.11 A patient with difficult behaviors will require additional cognitive resources from the physician to manage those behaviors.11 This leaves less cognitive capacity for solving the diagnostic problem.11 Furthermore, Riskin et al demonstrated that pediatric intensive care teams committed increased rates of medical errors and experienced poorer team performance when exposed to simulated families displaying rude behavior.12 Clearly, the power of the patient-physician relationship cannot be overstated when discussing diagnostic error.
Continue to: Strategies for reducing errors in the diagnostic process
Strategies for reducing errors in the diagnostic process
Although the mental pathways involved in diagnostic reasoning have become better elucidated, there is still considerable controversy and uncertainty surrounding effective ways to counter errors. In their review of the literature, Norman et al concluded that diagnostic errors are multifactorial and that strategies that solely educate novice clinicians about biases are unlikely to lead to significant gains because of “limited transfer.”9 That is, in simply teaching the theory of cognitive errors before trainees have had time to accumulate real-world experience, they do not learn how to apply corrective solutions.
Graber et al argue that mental shortcuts are often a beneficial behavior, and it would be unrealistic and perhaps even detrimental to eliminate them completely from clinical judgment.13 Despite the controversy, several corrective methods have been proposed and have shown promise. Two such methods are medical education on cognitive error and the use of differential diagnosis generators.2
Medical education on cognitive error. If heuristics and biases are acquired subconscious patterns of thinking, then it would be logical to assume that the most effective way to prevent their intrusion into the clinical decision-making process would be to intervene when the art of diagnosis is taught. Graber et al reference several small studies that demonstrated a small improvement in diagnostic accuracy when learners were educated about cognitive biases and clinical judgment.13
Additionally, with medical students, Mamede et al describe how structured reflection during case-based learning enhanced diagnostic accuracy.14 However, none of these studies have proven that increased awareness of cognitive biases results in fewer delayed or missed diagnoses in clinical practice. Clearly, further research is needed to determine whether the skills gained in the classroom would be transferable to clinical practice and result in lower rates of delayed or missed diagnoses. Future studies could also investigate if these findings are replicable when applied to more experienced clinicians rather than medical students and residents.
Continue to: Differential diagnosis generators
Differential diagnosis generators.
However, few randomized controlled studies have investigated whether the use of a DDx generator reduces diagnostic error, and evidence is lacking to prove their usefulness in clinical practice. Furthermore, while an exhaustive list of possible diagnoses may be helpful, some proposed diagnoses may be irrelevant and may distract from timely attention being paid to more likely possibilities. Additionally, forming an extensive DDx list during every patient encounter would significantly add to the physician’s workload and could contribute to physician burnout.
Selective use? We believe that DDx generators would be best used selectively as a safeguard for the clinician who becomes aware of an increased risk of diagnostic error in a particular patient. As previously discussed, errors involving cognitive processes are more often errors of improper reasoning rather than of insufficient knowledge.3 The DDx generator then serves as a way of double-checking to ensure that additional diagnoses are being considered. This can be especially helpful when facing patients who display difficult behaviors or when the clinician’s cognitive reserve is depleted by other factors.
DDx generators may also help the physician expand his or her differential diagnosis when a patient is failing to improve despite appropriately treating the working diagnosis.
Another option worth studying? Future studies could also investigate whether discussing a case with another clinician is an effective way to reduce cognitive biases and diagnostic errors.
Continue to: Looking foward
Looking forward
More research will hopefully lead to corrective solutions. But it is also likely that solutions will require additional time and resources on the part of already overburdened providers. Thus, new challenges will arise in applying remedies to the current model of health care management and reimbursement.
Despite clinically useful advances in technology and science, family physicians are left with the unsettling conclusion that the most common source of error may also be the most difficult to change: physicians themselves. Fortunately, history has shown that the field of medicine can overcome even the most ingrained and harmful tendencies of the human mind, including prejudice and superstition.16,17 This next challenge will be no exception.
CORRESPONDENCE
Thomas Yuen, MD, Crozer Keystone Family Medicine Residency, 1260 East Woodland Avenue, Suite 200, Philadelphia, PA 19064; [email protected].
1. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
2. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165:1493-1499.
3. Singh H, Giardina TD, Meyer AN, et al. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med. 2013;173:418-425.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131.
5. Croskerry P. A universal model of diagnostic reasoning. Acad Med. 2009;84:1022-1028.
6. Saposnik G, Redelmeier D, Ruff CC, et al. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138.
7. Gigerenzer G, Gaissmaier W. Heuristic decision making. Annu Rev Psychol. 2011;62:451-482.
8. Wellbery C. Flaws in clinical reasoning: a common cause of diagnostic error. Am Fam Physician. 2011;84:1042-1048.
9. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30.
10. Lockley SW, Cronin JW, Evans EE, et al. Effect of reducing interns’ weekly work hours on sleep and attentional failures. NEJM. 2004;351:1829-1837.
11. Schmidt HG, Van Gog T, Schuit SC, et al. Do patients’ disruptive behaviours influence the accuracy of a doctor’s diagnosis? A randomised experiment. BMJ Qual Saf. 2017;26:19-23.
12. Riskin A, Erez A, Foulk TA, et al. Rudeness and medical team performance. Pediatrics. 2017;139:e20162305.
13. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77:981-992.
14. Mamede S, Van Gog T, Sampaio AM, et al. How can students’ diagnostic competence benefit most from practice with clinical cases? The effects of structured reflection on future diagnosis of the same and novel diseases. Acad Med. 2014;89:121-127.
15. Bond WF, Schwartz LM, Weaver KR, et al. Differential diagnosis generators: an evaluation of currently available computer programs. J Gen Intern Med. 2012;27:213-219.
16. Porter R. The Greatest Benefit to Mankind: A Medical History of Humanity. New York, NY: W.W. Norton and Company, Inc.;1999.
17. Lazarus BA. The practice of medicine and prejudice in a New England town: the founding of Mount Sinai Hospital, Hartford, Connecticut. J Am Ethn Hist. 1991;10:21-41.
1. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
2. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165:1493-1499.
3. Singh H, Giardina TD, Meyer AN, et al. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med. 2013;173:418-425.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131.
5. Croskerry P. A universal model of diagnostic reasoning. Acad Med. 2009;84:1022-1028.
6. Saposnik G, Redelmeier D, Ruff CC, et al. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138.
7. Gigerenzer G, Gaissmaier W. Heuristic decision making. Annu Rev Psychol. 2011;62:451-482.
8. Wellbery C. Flaws in clinical reasoning: a common cause of diagnostic error. Am Fam Physician. 2011;84:1042-1048.
9. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30.
10. Lockley SW, Cronin JW, Evans EE, et al. Effect of reducing interns’ weekly work hours on sleep and attentional failures. NEJM. 2004;351:1829-1837.
11. Schmidt HG, Van Gog T, Schuit SC, et al. Do patients’ disruptive behaviours influence the accuracy of a doctor’s diagnosis? A randomised experiment. BMJ Qual Saf. 2017;26:19-23.
12. Riskin A, Erez A, Foulk TA, et al. Rudeness and medical team performance. Pediatrics. 2017;139:e20162305.
13. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77:981-992.
14. Mamede S, Van Gog T, Sampaio AM, et al. How can students’ diagnostic competence benefit most from practice with clinical cases? The effects of structured reflection on future diagnosis of the same and novel diseases. Acad Med. 2014;89:121-127.
15. Bond WF, Schwartz LM, Weaver KR, et al. Differential diagnosis generators: an evaluation of currently available computer programs. J Gen Intern Med. 2012;27:213-219.
16. Porter R. The Greatest Benefit to Mankind: A Medical History of Humanity. New York, NY: W.W. Norton and Company, Inc.;1999.
17. Lazarus BA. The practice of medicine and prejudice in a New England town: the founding of Mount Sinai Hospital, Hartford, Connecticut. J Am Ethn Hist. 1991;10:21-41.
PRACTICE RECOMMENDATIONS
› Acquire a basic understanding of key cognitive biases to better appreciate how they could interfere with your diagnostic reasoning. C
› Consider using a differential diagnosis generator as a safeguard if you suspect an increased risk of diagnostic error in a particular patient. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Newer cholesterol-lowering agents: What you must know
Over the past 3 decades, age-adjusted mortality for cardiovascular disease (CVD) in the United States has dropped by more than 50%.1 While multiple factors have contributed to this remarkable decline, the introduction and widespread use of statin therapy is unquestionably one key factor. Despite nearly overwhelming evidence that statins effectively lower low-density lipoprotein cholesterol (LDL-C) and predictably reduce cardiovascular events, less than half of patients with clinical coronary heart disease receive high-intensity statin therapy, leaving this population at increased risk for future events.2
Statins: Latest evidence on risks and benefits
Statins aren’t perfect. Not every patient is able to achieve the desired LDL-C lowering with statin therapy, and some patients develop adverse effects such as myopathy, new-onset diabetes, and occasionally hemorrhagic stroke. A recent report puts the risks of statin therapy in perspective, estimating that the treatment of 10,000 patients for 5 years would cause one case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 hemorrhagic strokes.3 The same treatment would avert approximately 1000 CVD events among those with preexisting disease, and approximately 500 CVD events among those with elevated risk, but no preexisting disease.3
In blinded randomized controlled trials, statin therapy is associated with relatively few adverse events (AEs). In open-label observational studies, however, substantially more AEs are reported. During the blinded phase of one recent study, muscle-related AEs and erectile dysfunction were reported at a similar rate by participants randomly assigned to receive atorvastatin or placebo. During the nonblinded nonrandomized phase, complaints of muscle-related AEs were 41% more likely in participants taking statins compared with those who were not.4
Statin therapy offers predictable CVD risk reduction. The evidence report accompanying the 2016 US Preventive Services Task Force (USPSTF) guidelines on statins for the prevention of CVD states that the use of low- or moderate-dose statin therapy was associated with an approximately 30% relative risk reduction (RRR) in CVD events and in CVD deaths, and a 10% to 15% RRR in all-cause mortality.5 Those with greater baseline CVD risk will have greater absolute risk reduction (ARR) than those with low baseline risk.5
How effective are non-statin therapies?
Multiple studies have demonstrated that some drugs can favorably modify lipid levels but not improve patient outcomes—eg, niacin, fibrates, and omega-3 fatty acids. The therapies that do improve outcomes are those that act via upregulation of LDL-receptor expression: statins, ezetimibe, bile acid sequestrants, dietary interventions, and ileal bypass surgery.
A recent meta-analysis found that with a 38.7-mg/dL (1-mmol/L) reduction in LDL-C level, the relative risk for major vascular events was 0.77 (95% CI, 0.71-0.84) for statins and 0.75 (95% CI, 0.66-0.86) for monotherapy with non-statin interventions that upregulate LDL-receptor expression.6
Ezetimibe. Less impressive is the incremental benefit of adding some non-statin therapies to effective statin therapy. The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) reported that adding ezetimibe to effective statin therapy in stable patients with previous acute coronary syndrome reduced the LDL-C level from 69.5 mg/dL to 53.7 mg/dL (TABLE7-13).8 After 7 years of treatment, relative risk of atherosclerotic cardiovascular disease (ASCVD) outcomes decreased by 5.8%; absolute decrease in risk was 2%: from 34.7% to 32.7% (number needed to treat [NNT]=50).8 Consider adding ezetimibe to maximally-tolerated statin therapy for patients not meeting LDL-C goals with a statin alone.
Continue to: A new class to lower LDL-C: PCSK9 inhibitors
A new class to lower LDL-C: PCSK9 inhibitors
It is clear that additional approaches to LDL-C reduction are needed. A new drug class that effectively lowers LDL-C levels is monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9). PCSK9 activity is directly proportional to the circulating LDL-C level: gene mutations that increase PCSK9 function are one cause of elevated LDL-C and CVD risk in familial hypercholesterolemia (FH),14 whereas mutations that decrease PCSK9 activity are associated with a decrease in LDL-C levels and risk of ASCVD.15
Circulating PCSK9 initiates LDL-receptor clearance by binding to the LDL receptor; the complex is then taken into the hepatocyte, where it undergoes degradation, and the receptor is not recycled to the cell’s surface. The resultant decreased level of cholesterol within the hepatocyte upregulates HMG-CoA reductase (the enzyme that controls the rate-limiting step in cholesterol production and is targeted by statin therapy) and LDL-receptor activity to increase the available cholesterol in the hepatocyte. Unfortunately, statins promote the upregulation of both the LDL receptor and PCSK9, thereby limiting their LDL-C-lowering potency. Combined inhibition of HMG-CoA reductase with statins and PCSK9 with monoclonal antibodies exerts additive reductions in LDL-C.16
Evolocumab and alirocumab—monoclonal antibodies that prevent circulating PCSK9 from binding to the LDL receptor—have been approved by the US Food and Drug Administration (FDA) for use as adjuncts to diet and maximally-tolerated statin therapy in adults who have heterozygous familial hypercholesterolemia (HeFH) or clinical ASCVD and who must further lower LDL-C levels. The addition of a PCSK9 inhibitor to statin therapy consistently results in an incremental decrease in LDL-C of around 60%.10,11 Much of the data supporting the use of PCSK9 inhibitors are disease-oriented. Among patients with angiographic coronary disease treated with statins, the addition of evolocumab resulted in regression of atherosclerotic plaque measured by intravascular ultrasound after 18 months of treatment.10
Continue to: PCSK9 inhibitors reduce adverse CVD events when added to a statin
PCSK9 inhibitors reduce adverse CVD events when added to a statin. In a study designed to evaluate AEs and LDL-C lowering with evolocumab, a prespecified exploratory outcome was the incidence of adjudicated CVD events. After one year of therapy, the rate of events was reduced from 2.18% in the standard-therapy group to 0.95% in the evolocumab group—a relative decrease of 53%, but an absolute decrease of 1.23% (NNT=81).17
A similar reduction in the rate of major adverse CVD events was found in adding alirocumab to ongoing statin therapy. In a post hoc analysis of patients who received either adjunctive alirocumab or placebo, CVD events (death from coronary heart disease, nonfatal myocardial infarction [MI], fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) were 1.7% vs 3.3% (hazard ratio=0.52; 95% confidence interval, 0.31-0.90).11
FOURIER, the first major trial designed to evaluate cardiovascular outcomes with PCSK9 therapy, showed that adding evolocumab to effective statin therapy reduced the average LDL-C level from 92 mg/dL to 30 mg/dL.12 Evolocumab decreased the composite CVD outcome (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) over 2.2 years from 11.3% to 9.8%—a 15% RRR and a 1.5% ARR (NNT=67). Most of the participants were receiving high-intensity statin therapy at study entry. AEs were similar between the study groups.12
A prespecified analysis of FOURIER data found that evolocumab did not increase the risk of new-onset diabetes in patients without diabetes or prediabetes at baseline. Fasting plasma glucose and hemoglobin A1c levels in the evolocumab and placebo groups remained similar throughout the trial in patients with diabetes, prediabetes, or normoglycemia.18 Additionally, a randomized trial involving patients who received either evolocumab or placebo in addition to statin therapy found no significant difference in cognitive function between the groups over a median of 19 months.19
Continue to: Effective, but expensive
Effective, but expensive. At its current list price of approximately $14,000 per year,9 evolocumab, added to standard therapy in patients with ASCVD, exceeds the generally accepted cost-effectiveness threshold of $150,000 per quality-adjusted life year (QALY) achieved.20 Similar analysis in patients with HeFH estimated a cost of $503,000 per QALY achieved with evolocumab.21 The outcomes of cost-effectiveness analyses hinge on the event rate in the study population and the threshold for initiating therapy. For the FOURIER trial participants, with an annual event rate of 4.2 per 100 patient-years, a net annual price of approximately $6700 would be necessary to meet a $150,000 per QALY threshold.22
At 2015 prices, the addition of PCSK9 inhibitor therapy for all eligible patients would reduce cardiovascular care costs by an estimated $29 billion over 5 years but would also increase drug costs by an estimated $592 billion, representing a 38% increase over 2015 prescription drug expenditures.21 Treatment of less than 20 million US adults with evolocumab at the cost of this single drug would match the entire cost for all other prescription pharmaceuticals for all diseases in the United States combined.23
In 2012, 27.9% of US adults ages 40 years and older were taking prescribed lipid-lowering treatment; 23.2% were taking only statins.24 If the
Until the cost of PCSK9 inhibitors decreases to a justifiable level and outcomes of longer term studies are available, consider prescribing other adjunctive treatments for patients who have not achieved LDL-C goals with statin therapy alone. Generally, reserve use of PCSK9 inhibitors for the highest-risk adults: those with HeFH or clinical ASCVD who must further lower LDL-C levels. Some insurers, including Medicare, are covering PCSK9 inhibitors, but many patients have difficulty obtaining coverage.27
Continue to: CETP inhibitors: Not FDA approved
CETP inhibitors: Not FDA approved
In a recent trial of the cholesteryl ester transfer protein (CETP) inhibitor evacetrapib, the drug had favorable effects on lipid biomarkers but did not improve cardiovascular outcomes.28 More recently, the CETP inhibitor anacetrapib was shown to decrease the composite outcome of coronary death, MI, or coronary revascularization in adults with established ASCVD who were receiving high-intensity atorvastatin therapy.13 At the trial midpoint, mean high-density lipoprotein (HDL) cholesterol levels increased by 43 mg/dL in the anacetrapib group compared with that of the placebo group (a relative difference of 104%); mean non-HDL cholesterol decreased by 17 mg/dL, a relative difference of −18%. Over a median follow-up period of 4.1 years, the addition of anacetrapib was associated with a 9% RRR and a 1% absolute reduction in the composite outcome over a statin alone (NNT=100).13 At this point, the manufacturers of both agents have halted efforts to gain FDA approval.
Future directions
Newer strategies to inhibit PCSK9 function are under development. Small peptides that inhibit PCSK9 interaction with the LDL receptor offer the potential advantage of oral administration, as opposed to the currently available injectable anti-PCSK9 antibodies.29 A recent trial found that inhibition of PCSK9 messenger RNA (mRNA) synthesis with the small interfering RNA (siRNA) molecule inclisiran lowered LDL-C in patients with high cardiovascular risk and elevated LDL-C levels despite aggressive statin therapy.30 The effect of these strategies on cardiovascular outcomes remains unproven.
CORRESPONDENCE
Jonathon Firnhaber, MD, Department of Family Medicine, Brody School of Medicine, 101 Heart Drive, Mail Stop 654, Greenville, NC 27834; [email protected].
1. Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths — trends and projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157.
2. Rodriguez F, Harrington RA. Cholesterol, cardiovascular risk, statins, PCSK9 inhibitors, and the future of LDL-C lowering. JAMA. 2016;316:1967-1968.
3. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561.
4. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473-2481.
5. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024.
6. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289-1297.
7. GoodRx. Ezetimibe. Available at: https://www.goodrx.com/ezetimibe. Accessed May 2, 2018.
8. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
9. American Journal of Managed Care. Outcomes-based pricing for PCSK9 inhibitors. Available at: http://www.ajmc.com/contributor/inmaculada-hernandez-pharmd/2017/09/outcomes-based-pricing-for-pcsk9-inhibitors. Accessed May 2, 2018.
10. Nicholls S, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384.
11. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
12. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
13. HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217-1227.
14. Hunt SC, Hopkins PN, Bulka K, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089-1093.
15. Cohen J, Pertsemlidis A, Kotowski I, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161-165.
16. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
17. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
18. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941-950.
19. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633-643.
20. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304-2322.
21. Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743-753.
22. Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069-1078.
23. Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420.
24. Gu Q, Paulose-Ram R, Burt VL, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS data Brief. 2014;177:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db177.pdf. Accessed May 2, 2018.
25. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
26. Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force Recommendations vs the ACC/AHA Guidelines. JAMA. 2017;317:1563-1567.
27
28. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933-1942.
29. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
30. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430-1440.
Over the past 3 decades, age-adjusted mortality for cardiovascular disease (CVD) in the United States has dropped by more than 50%.1 While multiple factors have contributed to this remarkable decline, the introduction and widespread use of statin therapy is unquestionably one key factor. Despite nearly overwhelming evidence that statins effectively lower low-density lipoprotein cholesterol (LDL-C) and predictably reduce cardiovascular events, less than half of patients with clinical coronary heart disease receive high-intensity statin therapy, leaving this population at increased risk for future events.2
Statins: Latest evidence on risks and benefits
Statins aren’t perfect. Not every patient is able to achieve the desired LDL-C lowering with statin therapy, and some patients develop adverse effects such as myopathy, new-onset diabetes, and occasionally hemorrhagic stroke. A recent report puts the risks of statin therapy in perspective, estimating that the treatment of 10,000 patients for 5 years would cause one case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 hemorrhagic strokes.3 The same treatment would avert approximately 1000 CVD events among those with preexisting disease, and approximately 500 CVD events among those with elevated risk, but no preexisting disease.3
In blinded randomized controlled trials, statin therapy is associated with relatively few adverse events (AEs). In open-label observational studies, however, substantially more AEs are reported. During the blinded phase of one recent study, muscle-related AEs and erectile dysfunction were reported at a similar rate by participants randomly assigned to receive atorvastatin or placebo. During the nonblinded nonrandomized phase, complaints of muscle-related AEs were 41% more likely in participants taking statins compared with those who were not.4
Statin therapy offers predictable CVD risk reduction. The evidence report accompanying the 2016 US Preventive Services Task Force (USPSTF) guidelines on statins for the prevention of CVD states that the use of low- or moderate-dose statin therapy was associated with an approximately 30% relative risk reduction (RRR) in CVD events and in CVD deaths, and a 10% to 15% RRR in all-cause mortality.5 Those with greater baseline CVD risk will have greater absolute risk reduction (ARR) than those with low baseline risk.5
How effective are non-statin therapies?
Multiple studies have demonstrated that some drugs can favorably modify lipid levels but not improve patient outcomes—eg, niacin, fibrates, and omega-3 fatty acids. The therapies that do improve outcomes are those that act via upregulation of LDL-receptor expression: statins, ezetimibe, bile acid sequestrants, dietary interventions, and ileal bypass surgery.
A recent meta-analysis found that with a 38.7-mg/dL (1-mmol/L) reduction in LDL-C level, the relative risk for major vascular events was 0.77 (95% CI, 0.71-0.84) for statins and 0.75 (95% CI, 0.66-0.86) for monotherapy with non-statin interventions that upregulate LDL-receptor expression.6
Ezetimibe. Less impressive is the incremental benefit of adding some non-statin therapies to effective statin therapy. The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) reported that adding ezetimibe to effective statin therapy in stable patients with previous acute coronary syndrome reduced the LDL-C level from 69.5 mg/dL to 53.7 mg/dL (TABLE7-13).8 After 7 years of treatment, relative risk of atherosclerotic cardiovascular disease (ASCVD) outcomes decreased by 5.8%; absolute decrease in risk was 2%: from 34.7% to 32.7% (number needed to treat [NNT]=50).8 Consider adding ezetimibe to maximally-tolerated statin therapy for patients not meeting LDL-C goals with a statin alone.
Continue to: A new class to lower LDL-C: PCSK9 inhibitors
A new class to lower LDL-C: PCSK9 inhibitors
It is clear that additional approaches to LDL-C reduction are needed. A new drug class that effectively lowers LDL-C levels is monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9). PCSK9 activity is directly proportional to the circulating LDL-C level: gene mutations that increase PCSK9 function are one cause of elevated LDL-C and CVD risk in familial hypercholesterolemia (FH),14 whereas mutations that decrease PCSK9 activity are associated with a decrease in LDL-C levels and risk of ASCVD.15
Circulating PCSK9 initiates LDL-receptor clearance by binding to the LDL receptor; the complex is then taken into the hepatocyte, where it undergoes degradation, and the receptor is not recycled to the cell’s surface. The resultant decreased level of cholesterol within the hepatocyte upregulates HMG-CoA reductase (the enzyme that controls the rate-limiting step in cholesterol production and is targeted by statin therapy) and LDL-receptor activity to increase the available cholesterol in the hepatocyte. Unfortunately, statins promote the upregulation of both the LDL receptor and PCSK9, thereby limiting their LDL-C-lowering potency. Combined inhibition of HMG-CoA reductase with statins and PCSK9 with monoclonal antibodies exerts additive reductions in LDL-C.16
Evolocumab and alirocumab—monoclonal antibodies that prevent circulating PCSK9 from binding to the LDL receptor—have been approved by the US Food and Drug Administration (FDA) for use as adjuncts to diet and maximally-tolerated statin therapy in adults who have heterozygous familial hypercholesterolemia (HeFH) or clinical ASCVD and who must further lower LDL-C levels. The addition of a PCSK9 inhibitor to statin therapy consistently results in an incremental decrease in LDL-C of around 60%.10,11 Much of the data supporting the use of PCSK9 inhibitors are disease-oriented. Among patients with angiographic coronary disease treated with statins, the addition of evolocumab resulted in regression of atherosclerotic plaque measured by intravascular ultrasound after 18 months of treatment.10
Continue to: PCSK9 inhibitors reduce adverse CVD events when added to a statin
PCSK9 inhibitors reduce adverse CVD events when added to a statin. In a study designed to evaluate AEs and LDL-C lowering with evolocumab, a prespecified exploratory outcome was the incidence of adjudicated CVD events. After one year of therapy, the rate of events was reduced from 2.18% in the standard-therapy group to 0.95% in the evolocumab group—a relative decrease of 53%, but an absolute decrease of 1.23% (NNT=81).17
A similar reduction in the rate of major adverse CVD events was found in adding alirocumab to ongoing statin therapy. In a post hoc analysis of patients who received either adjunctive alirocumab or placebo, CVD events (death from coronary heart disease, nonfatal myocardial infarction [MI], fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) were 1.7% vs 3.3% (hazard ratio=0.52; 95% confidence interval, 0.31-0.90).11
FOURIER, the first major trial designed to evaluate cardiovascular outcomes with PCSK9 therapy, showed that adding evolocumab to effective statin therapy reduced the average LDL-C level from 92 mg/dL to 30 mg/dL.12 Evolocumab decreased the composite CVD outcome (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) over 2.2 years from 11.3% to 9.8%—a 15% RRR and a 1.5% ARR (NNT=67). Most of the participants were receiving high-intensity statin therapy at study entry. AEs were similar between the study groups.12
A prespecified analysis of FOURIER data found that evolocumab did not increase the risk of new-onset diabetes in patients without diabetes or prediabetes at baseline. Fasting plasma glucose and hemoglobin A1c levels in the evolocumab and placebo groups remained similar throughout the trial in patients with diabetes, prediabetes, or normoglycemia.18 Additionally, a randomized trial involving patients who received either evolocumab or placebo in addition to statin therapy found no significant difference in cognitive function between the groups over a median of 19 months.19
Continue to: Effective, but expensive
Effective, but expensive. At its current list price of approximately $14,000 per year,9 evolocumab, added to standard therapy in patients with ASCVD, exceeds the generally accepted cost-effectiveness threshold of $150,000 per quality-adjusted life year (QALY) achieved.20 Similar analysis in patients with HeFH estimated a cost of $503,000 per QALY achieved with evolocumab.21 The outcomes of cost-effectiveness analyses hinge on the event rate in the study population and the threshold for initiating therapy. For the FOURIER trial participants, with an annual event rate of 4.2 per 100 patient-years, a net annual price of approximately $6700 would be necessary to meet a $150,000 per QALY threshold.22
At 2015 prices, the addition of PCSK9 inhibitor therapy for all eligible patients would reduce cardiovascular care costs by an estimated $29 billion over 5 years but would also increase drug costs by an estimated $592 billion, representing a 38% increase over 2015 prescription drug expenditures.21 Treatment of less than 20 million US adults with evolocumab at the cost of this single drug would match the entire cost for all other prescription pharmaceuticals for all diseases in the United States combined.23
In 2012, 27.9% of US adults ages 40 years and older were taking prescribed lipid-lowering treatment; 23.2% were taking only statins.24 If the
Until the cost of PCSK9 inhibitors decreases to a justifiable level and outcomes of longer term studies are available, consider prescribing other adjunctive treatments for patients who have not achieved LDL-C goals with statin therapy alone. Generally, reserve use of PCSK9 inhibitors for the highest-risk adults: those with HeFH or clinical ASCVD who must further lower LDL-C levels. Some insurers, including Medicare, are covering PCSK9 inhibitors, but many patients have difficulty obtaining coverage.27
Continue to: CETP inhibitors: Not FDA approved
CETP inhibitors: Not FDA approved
In a recent trial of the cholesteryl ester transfer protein (CETP) inhibitor evacetrapib, the drug had favorable effects on lipid biomarkers but did not improve cardiovascular outcomes.28 More recently, the CETP inhibitor anacetrapib was shown to decrease the composite outcome of coronary death, MI, or coronary revascularization in adults with established ASCVD who were receiving high-intensity atorvastatin therapy.13 At the trial midpoint, mean high-density lipoprotein (HDL) cholesterol levels increased by 43 mg/dL in the anacetrapib group compared with that of the placebo group (a relative difference of 104%); mean non-HDL cholesterol decreased by 17 mg/dL, a relative difference of −18%. Over a median follow-up period of 4.1 years, the addition of anacetrapib was associated with a 9% RRR and a 1% absolute reduction in the composite outcome over a statin alone (NNT=100).13 At this point, the manufacturers of both agents have halted efforts to gain FDA approval.
Future directions
Newer strategies to inhibit PCSK9 function are under development. Small peptides that inhibit PCSK9 interaction with the LDL receptor offer the potential advantage of oral administration, as opposed to the currently available injectable anti-PCSK9 antibodies.29 A recent trial found that inhibition of PCSK9 messenger RNA (mRNA) synthesis with the small interfering RNA (siRNA) molecule inclisiran lowered LDL-C in patients with high cardiovascular risk and elevated LDL-C levels despite aggressive statin therapy.30 The effect of these strategies on cardiovascular outcomes remains unproven.
CORRESPONDENCE
Jonathon Firnhaber, MD, Department of Family Medicine, Brody School of Medicine, 101 Heart Drive, Mail Stop 654, Greenville, NC 27834; [email protected].
Over the past 3 decades, age-adjusted mortality for cardiovascular disease (CVD) in the United States has dropped by more than 50%.1 While multiple factors have contributed to this remarkable decline, the introduction and widespread use of statin therapy is unquestionably one key factor. Despite nearly overwhelming evidence that statins effectively lower low-density lipoprotein cholesterol (LDL-C) and predictably reduce cardiovascular events, less than half of patients with clinical coronary heart disease receive high-intensity statin therapy, leaving this population at increased risk for future events.2
Statins: Latest evidence on risks and benefits
Statins aren’t perfect. Not every patient is able to achieve the desired LDL-C lowering with statin therapy, and some patients develop adverse effects such as myopathy, new-onset diabetes, and occasionally hemorrhagic stroke. A recent report puts the risks of statin therapy in perspective, estimating that the treatment of 10,000 patients for 5 years would cause one case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 hemorrhagic strokes.3 The same treatment would avert approximately 1000 CVD events among those with preexisting disease, and approximately 500 CVD events among those with elevated risk, but no preexisting disease.3
In blinded randomized controlled trials, statin therapy is associated with relatively few adverse events (AEs). In open-label observational studies, however, substantially more AEs are reported. During the blinded phase of one recent study, muscle-related AEs and erectile dysfunction were reported at a similar rate by participants randomly assigned to receive atorvastatin or placebo. During the nonblinded nonrandomized phase, complaints of muscle-related AEs were 41% more likely in participants taking statins compared with those who were not.4
Statin therapy offers predictable CVD risk reduction. The evidence report accompanying the 2016 US Preventive Services Task Force (USPSTF) guidelines on statins for the prevention of CVD states that the use of low- or moderate-dose statin therapy was associated with an approximately 30% relative risk reduction (RRR) in CVD events and in CVD deaths, and a 10% to 15% RRR in all-cause mortality.5 Those with greater baseline CVD risk will have greater absolute risk reduction (ARR) than those with low baseline risk.5
How effective are non-statin therapies?
Multiple studies have demonstrated that some drugs can favorably modify lipid levels but not improve patient outcomes—eg, niacin, fibrates, and omega-3 fatty acids. The therapies that do improve outcomes are those that act via upregulation of LDL-receptor expression: statins, ezetimibe, bile acid sequestrants, dietary interventions, and ileal bypass surgery.
A recent meta-analysis found that with a 38.7-mg/dL (1-mmol/L) reduction in LDL-C level, the relative risk for major vascular events was 0.77 (95% CI, 0.71-0.84) for statins and 0.75 (95% CI, 0.66-0.86) for monotherapy with non-statin interventions that upregulate LDL-receptor expression.6
Ezetimibe. Less impressive is the incremental benefit of adding some non-statin therapies to effective statin therapy. The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) reported that adding ezetimibe to effective statin therapy in stable patients with previous acute coronary syndrome reduced the LDL-C level from 69.5 mg/dL to 53.7 mg/dL (TABLE7-13).8 After 7 years of treatment, relative risk of atherosclerotic cardiovascular disease (ASCVD) outcomes decreased by 5.8%; absolute decrease in risk was 2%: from 34.7% to 32.7% (number needed to treat [NNT]=50).8 Consider adding ezetimibe to maximally-tolerated statin therapy for patients not meeting LDL-C goals with a statin alone.
Continue to: A new class to lower LDL-C: PCSK9 inhibitors
A new class to lower LDL-C: PCSK9 inhibitors
It is clear that additional approaches to LDL-C reduction are needed. A new drug class that effectively lowers LDL-C levels is monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9). PCSK9 activity is directly proportional to the circulating LDL-C level: gene mutations that increase PCSK9 function are one cause of elevated LDL-C and CVD risk in familial hypercholesterolemia (FH),14 whereas mutations that decrease PCSK9 activity are associated with a decrease in LDL-C levels and risk of ASCVD.15
Circulating PCSK9 initiates LDL-receptor clearance by binding to the LDL receptor; the complex is then taken into the hepatocyte, where it undergoes degradation, and the receptor is not recycled to the cell’s surface. The resultant decreased level of cholesterol within the hepatocyte upregulates HMG-CoA reductase (the enzyme that controls the rate-limiting step in cholesterol production and is targeted by statin therapy) and LDL-receptor activity to increase the available cholesterol in the hepatocyte. Unfortunately, statins promote the upregulation of both the LDL receptor and PCSK9, thereby limiting their LDL-C-lowering potency. Combined inhibition of HMG-CoA reductase with statins and PCSK9 with monoclonal antibodies exerts additive reductions in LDL-C.16
Evolocumab and alirocumab—monoclonal antibodies that prevent circulating PCSK9 from binding to the LDL receptor—have been approved by the US Food and Drug Administration (FDA) for use as adjuncts to diet and maximally-tolerated statin therapy in adults who have heterozygous familial hypercholesterolemia (HeFH) or clinical ASCVD and who must further lower LDL-C levels. The addition of a PCSK9 inhibitor to statin therapy consistently results in an incremental decrease in LDL-C of around 60%.10,11 Much of the data supporting the use of PCSK9 inhibitors are disease-oriented. Among patients with angiographic coronary disease treated with statins, the addition of evolocumab resulted in regression of atherosclerotic plaque measured by intravascular ultrasound after 18 months of treatment.10
Continue to: PCSK9 inhibitors reduce adverse CVD events when added to a statin
PCSK9 inhibitors reduce adverse CVD events when added to a statin. In a study designed to evaluate AEs and LDL-C lowering with evolocumab, a prespecified exploratory outcome was the incidence of adjudicated CVD events. After one year of therapy, the rate of events was reduced from 2.18% in the standard-therapy group to 0.95% in the evolocumab group—a relative decrease of 53%, but an absolute decrease of 1.23% (NNT=81).17
A similar reduction in the rate of major adverse CVD events was found in adding alirocumab to ongoing statin therapy. In a post hoc analysis of patients who received either adjunctive alirocumab or placebo, CVD events (death from coronary heart disease, nonfatal myocardial infarction [MI], fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) were 1.7% vs 3.3% (hazard ratio=0.52; 95% confidence interval, 0.31-0.90).11
FOURIER, the first major trial designed to evaluate cardiovascular outcomes with PCSK9 therapy, showed that adding evolocumab to effective statin therapy reduced the average LDL-C level from 92 mg/dL to 30 mg/dL.12 Evolocumab decreased the composite CVD outcome (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) over 2.2 years from 11.3% to 9.8%—a 15% RRR and a 1.5% ARR (NNT=67). Most of the participants were receiving high-intensity statin therapy at study entry. AEs were similar between the study groups.12
A prespecified analysis of FOURIER data found that evolocumab did not increase the risk of new-onset diabetes in patients without diabetes or prediabetes at baseline. Fasting plasma glucose and hemoglobin A1c levels in the evolocumab and placebo groups remained similar throughout the trial in patients with diabetes, prediabetes, or normoglycemia.18 Additionally, a randomized trial involving patients who received either evolocumab or placebo in addition to statin therapy found no significant difference in cognitive function between the groups over a median of 19 months.19
Continue to: Effective, but expensive
Effective, but expensive. At its current list price of approximately $14,000 per year,9 evolocumab, added to standard therapy in patients with ASCVD, exceeds the generally accepted cost-effectiveness threshold of $150,000 per quality-adjusted life year (QALY) achieved.20 Similar analysis in patients with HeFH estimated a cost of $503,000 per QALY achieved with evolocumab.21 The outcomes of cost-effectiveness analyses hinge on the event rate in the study population and the threshold for initiating therapy. For the FOURIER trial participants, with an annual event rate of 4.2 per 100 patient-years, a net annual price of approximately $6700 would be necessary to meet a $150,000 per QALY threshold.22
At 2015 prices, the addition of PCSK9 inhibitor therapy for all eligible patients would reduce cardiovascular care costs by an estimated $29 billion over 5 years but would also increase drug costs by an estimated $592 billion, representing a 38% increase over 2015 prescription drug expenditures.21 Treatment of less than 20 million US adults with evolocumab at the cost of this single drug would match the entire cost for all other prescription pharmaceuticals for all diseases in the United States combined.23
In 2012, 27.9% of US adults ages 40 years and older were taking prescribed lipid-lowering treatment; 23.2% were taking only statins.24 If the
Until the cost of PCSK9 inhibitors decreases to a justifiable level and outcomes of longer term studies are available, consider prescribing other adjunctive treatments for patients who have not achieved LDL-C goals with statin therapy alone. Generally, reserve use of PCSK9 inhibitors for the highest-risk adults: those with HeFH or clinical ASCVD who must further lower LDL-C levels. Some insurers, including Medicare, are covering PCSK9 inhibitors, but many patients have difficulty obtaining coverage.27
Continue to: CETP inhibitors: Not FDA approved
CETP inhibitors: Not FDA approved
In a recent trial of the cholesteryl ester transfer protein (CETP) inhibitor evacetrapib, the drug had favorable effects on lipid biomarkers but did not improve cardiovascular outcomes.28 More recently, the CETP inhibitor anacetrapib was shown to decrease the composite outcome of coronary death, MI, or coronary revascularization in adults with established ASCVD who were receiving high-intensity atorvastatin therapy.13 At the trial midpoint, mean high-density lipoprotein (HDL) cholesterol levels increased by 43 mg/dL in the anacetrapib group compared with that of the placebo group (a relative difference of 104%); mean non-HDL cholesterol decreased by 17 mg/dL, a relative difference of −18%. Over a median follow-up period of 4.1 years, the addition of anacetrapib was associated with a 9% RRR and a 1% absolute reduction in the composite outcome over a statin alone (NNT=100).13 At this point, the manufacturers of both agents have halted efforts to gain FDA approval.
Future directions
Newer strategies to inhibit PCSK9 function are under development. Small peptides that inhibit PCSK9 interaction with the LDL receptor offer the potential advantage of oral administration, as opposed to the currently available injectable anti-PCSK9 antibodies.29 A recent trial found that inhibition of PCSK9 messenger RNA (mRNA) synthesis with the small interfering RNA (siRNA) molecule inclisiran lowered LDL-C in patients with high cardiovascular risk and elevated LDL-C levels despite aggressive statin therapy.30 The effect of these strategies on cardiovascular outcomes remains unproven.
CORRESPONDENCE
Jonathon Firnhaber, MD, Department of Family Medicine, Brody School of Medicine, 101 Heart Drive, Mail Stop 654, Greenville, NC 27834; [email protected].
1. Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths — trends and projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157.
2. Rodriguez F, Harrington RA. Cholesterol, cardiovascular risk, statins, PCSK9 inhibitors, and the future of LDL-C lowering. JAMA. 2016;316:1967-1968.
3. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561.
4. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473-2481.
5. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024.
6. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289-1297.
7. GoodRx. Ezetimibe. Available at: https://www.goodrx.com/ezetimibe. Accessed May 2, 2018.
8. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
9. American Journal of Managed Care. Outcomes-based pricing for PCSK9 inhibitors. Available at: http://www.ajmc.com/contributor/inmaculada-hernandez-pharmd/2017/09/outcomes-based-pricing-for-pcsk9-inhibitors. Accessed May 2, 2018.
10. Nicholls S, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384.
11. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
12. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
13. HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217-1227.
14. Hunt SC, Hopkins PN, Bulka K, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089-1093.
15. Cohen J, Pertsemlidis A, Kotowski I, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161-165.
16. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
17. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
18. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941-950.
19. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633-643.
20. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304-2322.
21. Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743-753.
22. Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069-1078.
23. Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420.
24. Gu Q, Paulose-Ram R, Burt VL, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS data Brief. 2014;177:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db177.pdf. Accessed May 2, 2018.
25. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
26. Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force Recommendations vs the ACC/AHA Guidelines. JAMA. 2017;317:1563-1567.
27
28. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933-1942.
29. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
30. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430-1440.
1. Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths — trends and projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157.
2. Rodriguez F, Harrington RA. Cholesterol, cardiovascular risk, statins, PCSK9 inhibitors, and the future of LDL-C lowering. JAMA. 2016;316:1967-1968.
3. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561.
4. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473-2481.
5. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024.
6. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289-1297.
7. GoodRx. Ezetimibe. Available at: https://www.goodrx.com/ezetimibe. Accessed May 2, 2018.
8. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
9. American Journal of Managed Care. Outcomes-based pricing for PCSK9 inhibitors. Available at: http://www.ajmc.com/contributor/inmaculada-hernandez-pharmd/2017/09/outcomes-based-pricing-for-pcsk9-inhibitors. Accessed May 2, 2018.
10. Nicholls S, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384.
11. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
12. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
13. HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217-1227.
14. Hunt SC, Hopkins PN, Bulka K, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089-1093.
15. Cohen J, Pertsemlidis A, Kotowski I, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161-165.
16. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
17. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
18. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941-950.
19. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633-643.
20. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304-2322.
21. Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743-753.
22. Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069-1078.
23. Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420.
24. Gu Q, Paulose-Ram R, Burt VL, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS data Brief. 2014;177:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db177.pdf. Accessed May 2, 2018.
25. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
26. Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force Recommendations vs the ACC/AHA Guidelines. JAMA. 2017;317:1563-1567.
27
28. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933-1942.
29. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
30. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430-1440.
From The Journal of Family Practice | 2018;67(6):339-341,344-345.
PRACTICE RECOMMENDATIONS
› Consider adding ezetimibe to maximally tolerated statin therapy for patients not meeting low-density lipoprotein cholesterol (LDL-C) goals with a statin alone. B
› Limit consideration of PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors to adults at highest risk: those with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease who must further lower LDL-C levels. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Parkinson’s disease: A treatment guide
Parkinson’s disease (PD) can be a tough diagnosis to navigate. Patients with this neurologic movement disorder can present with a highly variable constellation of symptoms,1 ranging from the well-known tremor and bradykinesia to difficulties with activities of daily living (particularly dressing and getting out of a car2) to nonspecific symptoms, such as pain, fatigue, hyposmia, and erectile dysfunction.3
Furthermore, medications more recently approved by the US Food and Drug Administration (FDA) have left many health care providers confused about what constitutes appropriate first-, second-, and third-line therapies, as well as add-on therapy for symptoms secondary to dopaminergic agents. What follows is a stepwise approach to managing PD that incorporates these newer therapies so that you can confidently and effectively manage patients with PD with little or no consultation.
First, though, we review who’s at greatest risk—and what you’ll see.
Family history tops list of risk factors for PD
While PD occurs in less than 1% of the population ≥40 years of age, its prevalence increases with age, becoming significantly higher by age 60 years, with a slight predominance toward males.4
A variety of factors increase the risk of developing PD. A well-conducted meta-analysis showed that the strongest risk factor is having a family member, particularly a first-degree relative, with a history of PD or tremor.5 Repeated head injury, with or without loss of consciousness, is also a factor;5 risk increases with each occurrence.6 Other risk factors include exposure to pesticides, rural living, and exposure to well water.5
Researchers have conducted several studies regarding the effects of elevated cholesterol and hypertension on the risk of PD, but results are still without consensus.5 A study published in 2017 reported a significantly increased risk of PD associated with having hepatitis B or C, but the mechanism for the association—including whether it is a consequence of treatment—is unknown.7
Smoking and coffee drinking. Researchers have found that cigarette smoking, beer consumption, and high coffee intake are protective against PD,5 but the benefits are outweighed by the risks associated with these strategies.8 The most practical protective factors are a high dietary intake of vitamin E and increased nut consumption.9 Dietary vitamin E can be found in almonds, spinach, sweet potatoes, sunflower seeds, and avocados. Studies have not found the same benefit with vitamin E supplements.9
Dx seldom requires testing, but may take time to come into focus
Motor symptoms. The key diagnostic criterium for PD is bradykinesia with at least one of the following: muscular rigidity, resting tremor (particularly a pill-rolling tremor) that improves with purposeful function, or postural instability.2 Other physical findings may include masking of facies and speech changes, such as becoming quiet, stuttering, or speaking monotonously without inflection.1 Cogwheeling, stooped posture, and a shuffling gait or difficulty initiating gait (freezing) are all neurologic signs that point toward a PD diagnosis.2
A systematic review found that the clinical features most strongly associated with a diagnosis of PD were trouble turning in bed, a shuffling gait, tremor, difficulty opening jars, micrographia, and loss of balance.10 Typically these symptoms are asymmetric.1
Symptoms that point to other causes. Falling within the first year of symptoms is strongly associated with movement disorders other than PD—notably progressive supranuclear palsy.11 Other symptoms that point toward an alternate diagnosis include a poor response to levodopa, symmetry at the onset of symptoms, rapid progression of disease, and the absence of a tremor.11 It is important to ensure that the patient is not experiencing drug-induced symptoms as can occur with some antipsychotics and antiemetics.
Nonmotor symptoms. Neuropsychiatric symptoms are common in patients with PD. Up to 58% of patients experience depression, and 49% complain of anxiety.12 Hallucinations are present in many patients and are more commonly visual than auditory in nature.13 Patients experience fatigue, daytime sleepiness, and inner restlessness at higher rates than do age-matched controls.3 Research also shows that symptoms such as constipation, mood disorders, erectile dysfunction, and hyposmia may predate the onset of motor symptoms.5
Insomnia is a common symptom that is likely multifactorial in etiology. Causes to consider include motor disturbance, nocturia, reversal of sleep patterns, and reemergence of PD symptoms after a period of quiescence.14 Additionally, hypersalivation and PD dementia can develop as complications of PD.
A clinical diagnosis. Although PD can be difficult to diagnose in the early stages, the diagnosis seldom requires testing.2 A recent systematic review concluded that a clinical diagnosis of PD, when compared with pathology, was correct 74% of the time when the diagnosis was made by nonexperts and correct 84% of the time when the diagnosis was made by movement disorder experts.15
Imaging. Computed tomography and magnetic resonance imaging can be useful in ruling out other diagnoses in the differential, including vascular disease and normal pressure hydrocephalus,2 but will not reveal findings suggestive of PD.
Other diagnostic tests. A levodopa challenge can confirm PD if the diagnosis is unclear.11 In addition, an olfactory test (presenting various odors to the patient for identification) can differentiate PD from progressive supranuclear palsy and corticobasal degeneration; however, it will not distinguish PD from multiple system atrophy.11 If the diagnosis remains unclear, consider a consultation with a neurologist.
Treatment centers on alleviating motor symptoms
The general guiding principle of therapy (TABLE16,17) is to alleviate the motor symptoms (bradykinesia, rigidity, and postural instability) associated with the disease. Experts recommend that treatment commence when symptoms begin to have disabling effects or become a source of discomfort for the patient.1
Carbidopa/levodopa is still often the first choice
Multiple systematic reviews support the use of carbidopa/levodopa as first-line treatment, with the dose kept as low as possible to maintain function, while minimizing motor fluctuations (also referred to as “off” time symptoms) and dyskinesia.11,16 Initial dosing is carbidopa 25 mg/levodopa 100 mg tid. Each can be titrated up to address symptoms to a maximum daily dosing of carbidopa 200 mg/levodopa 2000 mg.17
“Off” time—the return of Parkinson symptoms when the medication’s effect wanes—can become more unpredictable and more difficult to manage as the disease advances.11 Of note: The American Academy of Neurology (AAN) says there is no improvement in the amount of off time a patient experiences by changing to a sustained-release form of carbidopa/levodopa compared with an immediate-release version.11 In addition to the on-off phenomenon, common adverse effects associated with carbidopa/levodopa include nausea, somnolence, dizziness, and headaches. Less common adverse effects include orthostatic hypotension, confusion, and hallucinations.17
Other medications for the treatment of motor symptoms
Second-line agents include dopamine agonists (pramipexole, ropinirole, and bromocriptine) and monoamine oxidase type B (MAO-B) inhibitors (selegiline, rasagiline) (TABLE16,17). The dopamine agonists work by directly stimulating dopamine receptors, while the MAO-B inhibitors block dopamine metabolism, thus enhancing dopaminergic activity in the substantia nigra.
The pros/cons of these 2 classes. Research shows that both dopamine agonists and MAO-B inhibitors are less effective than carbidopa/levodopa at quelling the motor symptoms associated with PD. They can, however, delay the onset of motor complications when compared with carbidopa/levodopa.16
One randomized trial found no long-term benefits to beginning treatment with a levodopa-sparing therapy; however, few patients with earlier disease onset (<60 years of age) were included in the study.18 Given the typically longer duration of their illness, there is potential for this group of patients to develop a higher rate of motor symptoms secondary to carbidopa/levodopa. Thus, considering dopamine agonists and MAO-B inhibitors as initial therapy in patients ages <60 years may be helpful, since they typically will be taking medication longer.
Dopamine agonists. Pramipexole and ropinirole can be used as monotherapy or as an adjunct to levodopa to treat bradykinesia, postural instability, and rigidity. Bromocriptine, an ergot-derived dopamine agonist, is considered an agent of last resort because additional monitoring is required. Potential adverse effects mandate baseline testing and annual repeat testing, including measures of erythrocyte sedimentation rate and renal function and a chest x-ray.16 Consider this agent only if all second- and third-line therapies have provided inadequate control.16
Adverse effects. Dopamine agonists cause such adverse effects as orthostatic hypotension, drowsiness, dizziness, insomnia, abnormal dreams, nausea, constipation, and hallucinations. A Cochrane review notes that these adverse effects have led to higher drop-out rates than seen for carbidopa/levodopa in studies that compared the 2.19
Patients should be counseled about an additional adverse effect associated with dopamine agonists—the possible development of an impulse-control disorder, such as gambling, binge eating, or hypersexuality.1 If a patient develops any of these behaviors, promptly lower the dose of the dopamine agonist or stop the medication.16
The MAO-B inhibitors selegiline and rasagiline may also be considered for initial therapy but are more commonly used as adjunct therapy. Use of selegiline as monotherapy for PD is an off-label indication. Adverse effects for this class of agents include headache, dizziness, insomnia, nausea, and hypotension.
Add-on therapy to treat the adverse effects of primary therapy
Dopaminergic therapies come at the price of the development of off-time motor symptoms and dyskinesia.1,20 In general, these complications are managed by the addition of a dopamine agonist, MAO-B inhibitor, or a catechol-O-methyltransferase (COMT) inhibitor (entacapone).1
Rasagiline and entacapone are a good place to start and should be offered to patients to reduce off-time symptoms, according to the AAN (a Level A recommendation based on multiple high-level studies; see here for an explanation of Strength of Recommendation).
The newest medication, safinamide, has been shown to increase “on” time by one hour per day when compared with placebo; however, it has not yet been tested against existing therapies.21 Other medications that can be considered to reduce drug-induced motor complications include pergolide, pramipexole, ropinirole, and tolcapone.20 Carbidopa/levodopa and bromocriptine are not recommended for the treatment of dopaminergic motor complications.20 Both sustained-release carbidopa/levodopa and bromocriptine are no longer recommended to decrease off time due to ineffectiveness.20
The only medication that has evidence for reducing dyskinesias in patients with PD is amantadine;20 however, it has no effect on other motor symptoms and should not be considered first line.16 Additionally, as an antiviral agent active against some strains of influenza, it should not be taken 2 weeks before or after receiving the influenza vaccine.
When tremor dominates …
For many patients with PD, tremor is more difficult to treat than is bradykinesia, rigidity, and gait disturbance.16 For patients with tremor-predominant PD (characterized by prominent tremor of one or more limbs and a relative lack of significant rigidity and bradykinesia), first-line treatment choices are dopamine agonists (ropinirole, pramipexole), carbidopa/levodopa, and anticholinergic medications, including benztropine and trihexyphenidyl.22 Second-line choices include clozapine, amantadine, clonazepam, and propranolol.22
Treating nonmotor symptoms
Treatment of hypersalivation should start with an evaluation by a speech pathologist. If it doesn’t improve, then adjuvant treatment with glycopyrrolate may be considered.16 Carbidopa/levodopa has the best evidence for treating periodic limb movements of sleep,14 although dopamine agonists may also be considered.16 More research is needed to find an effective therapy to improve insomnia in patients with PD, but for now consider a nighttime dose of carbidopa/levodopa or melatonin.14
Treating cognitive disorders associated with PD
Depression. Treatment of depression in patients with PD is difficult. Multiple systematic reviews have been unable to find a difference in those treated with antidepressants and those not.23 In practice, the use of tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), and a combination of an SSRI and a norepinephrine reuptake inhibitor are commonly used. Additionally, some evidence suggests that pramipexole improves depressive symptoms, but additional research is needed.1
Dementia. Dementia occurs in up to 83% of those who have had PD for more than 20 years.1 Treatment includes the use of rivastigmine (a cholinesterase inhibitor).1 Further research is needed to determine whether donepezil improves dementia symptoms in patients with PD.1
Psychotic symptoms. Query patients and their families periodically about hallucinations and delusions.16 If such symptoms are present and not well tolerated by the patient and/or family, treatment options include quetiapine and clozapine.1 While clozapine is more effective, it requires frequent hematologic monitoring due to the risk of agranulocytosis.1 And quetiapine carries a black box warning about early death. Exercise caution when prescribing these medications, particularly if a patient is cognitively impaired, and always start with low doses.1
A newer medication, pimavanserin (a second-generation antipsychotic), was recently approved by the FDA to treat hallucinations and delusions of PD psychosis, although any improvement this agent provides may not be clinically significant.24 Unlike clozapine, no additional monitoring is needed and there are no significant safety concerns with the use of pimavanserin, which makes it a reasonable first choice for hallucinations and delusions. Other neuroleptic medications should not be used as they tend to worsen Parkinson symptoms.1
Consider tai chi, physical therapy to reduce falls
One study showed that tai chi, performed for an hour twice weekly, was significantly more effective at reducing falls when compared to the same amount of resistance training and strength training, and that the benefits remained 3 months after the completion of the 24-week study.25 To date, tai chi is the only intervention that has been shown to affect fall risk.
Guidelines recommend that physical therapy be available to all patients.16 A Cochrane review performed in 2013 determined that physical therapy improves walking endurance and balance but does not affect quality of life in terms of fear of falling.26
When meds no longer help, consider deep brain stimulation as a last resort
Deep brain stimulation consists of surgical implantation of a device to deliver electrical current to a targeted area of the brain. It can be considered for patients with PD who are no longer responsive to carbidopa/levodopa, not experiencing neuropsychiatric symptoms, and are experiencing significant motor complications despite optimal medical management.14 Referral to a specialist is recommended for these patients to assess their candidacy for this procedure.
Prognosis: Largely unchanged
While medications can improve quality of life and function, PD remains a chronic and progressive disorder that is associated with significant morbidity. A study performed in 2013 showed that older age at onset, cognitive dysfunction, and motor symptoms nonresponsive to levodopa were associated with faster progression toward disability.27
Keep an eye on patients’ bone mineral density (BMD), as patients with PD tend to have lower BMD,28 a 2-fold increase in the risk of fracture for both men and women,29 and a higher prevalence of vitamin D deficiency.30
Also, watch for signs of infection because the most commonly cited cause of death in those with PD is pneumonia rather than a complication of the disease itself.11
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 777 South Clinton Avenue, Rochester, NY 14620; [email protected].
1. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896-912.
2. Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055-2066.
3. Todorova A, Jenner P, Chaudhuri K. Non-motor Parkinson’s: integral to motor Parkinson’s, yet often neglected. Pract Neurol. 2014;14:310-322.
4. Pringsheim T, Jette N, Frolkis A, et al. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1583-1590.
5. Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol. 2012;72:893-901.
6. Dick FD, De Palma G, Ahmadi A, et al. Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occup Environ Med. 2007;64:666-672.
7. Pakpoor J, Noyce A, Goldacre R, et al. Viral hepatitis and Parkinson disease: a national record-linkage study. Neurology. 2017;88:1630-1633.
8. Hern T, Newton W. Does coffee protect against the development of Parkinson disease (PD)? J Fam Pract. 2000;49:685-686.
9. Zhang SM, Hernán MA, Chen H, et al. Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology. 2002;59:1161-1169.
10. Rao G, Fisch L, Srinivasan S, et al. Does this patient have Parkinson disease? JAMA. 2003;289:347-353.
11. Suchowersky O, Reich S, Perlmutter J, et al. Practice Parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:968-975.
12. Aarsland D, Brønnick K, Ehrt U, et al. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry. 2007;78:36-42.
13. Inzelberg R, Kipervasser S, Korczyn AD. Auditory hallucinations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;64:533-535.
14. Zesiewicz TA, Sullivan KL, Arnulf I, et al. Practice Parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74:924-931.
15. Rizzo G, Copetti M, Arcuti S, et al. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;86:566-576.
16. National Institute for Heath and Care Excellence. Parkinson’s disease in adults. NICE guideline NG 71. 2017. Available at: https://www.nice.org.uk/guidance/ng71. Accessed March 27, 2018.
17. Lexicomp version 4.0.1. Wolters Kluwer; Copyright 2017. Available at: https://online.lexi.com/lco/action/home. Accessed March 27, 2018.
18. Lang AE, Marras C. Initiating dopaminergic treatment in Parkinson’s disease. Lancet. 2014;384:1164-1166.
19. Stowe RL, Ives NJ, Clarke C, et al. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;CD006564.
20. Pahwa R, Factor SA, Lyons KE, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:983-995.
21. Schapira AH, Fox SH, Hauser RA, et al. Assessment of safety and efficacy of safinamide as a levodopa adjunct in patients with Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol. 2017;74:216-224.
22. Marjama-Lyons J, Koller W. Tremor-predominant Parkinson’s disease. Approaches to treatment. Drugs Aging. 2000;16:273-278.
23. Price A, Rayner L, Okon-Rocha E, et al. Antidepressants for the treatment of depression in neurological disorders: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2011;82:914-923.
24. Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomized placebo-controlled phase 3 trial. Lancet. 2014;383:533-540.
25. Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366:511-519.
26. Tomlinson CL, Patel S, Meek C, et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev. 2012;CD002817.
27. Velseboer DC, Broeders M, Post B, et al. Prognostic factors of motor impairment, disability, and quality of life in newly diagnosed PD. Neurology. 2013;80:627-633.
28. Cronin H, Casey MC, Inderhaugh J, et al. Osteoporosis in patients with Parkinson’s disease. J Am Geriatr Soc. 2006;54:1797-1798.
29. Tan L, Wang Y, Zhou L, et al. Parkinson’s disease and risk of fracture: a meta-analysis of prospective cohort studies. PLoS One. 2014;9:e94379.
30. Evatt ML, Delong MR, Khazai N, et al. Prevalence of vitamin D insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol. 2008;65:1348-1352.
Parkinson’s disease (PD) can be a tough diagnosis to navigate. Patients with this neurologic movement disorder can present with a highly variable constellation of symptoms,1 ranging from the well-known tremor and bradykinesia to difficulties with activities of daily living (particularly dressing and getting out of a car2) to nonspecific symptoms, such as pain, fatigue, hyposmia, and erectile dysfunction.3
Furthermore, medications more recently approved by the US Food and Drug Administration (FDA) have left many health care providers confused about what constitutes appropriate first-, second-, and third-line therapies, as well as add-on therapy for symptoms secondary to dopaminergic agents. What follows is a stepwise approach to managing PD that incorporates these newer therapies so that you can confidently and effectively manage patients with PD with little or no consultation.
First, though, we review who’s at greatest risk—and what you’ll see.
Family history tops list of risk factors for PD
While PD occurs in less than 1% of the population ≥40 years of age, its prevalence increases with age, becoming significantly higher by age 60 years, with a slight predominance toward males.4
A variety of factors increase the risk of developing PD. A well-conducted meta-analysis showed that the strongest risk factor is having a family member, particularly a first-degree relative, with a history of PD or tremor.5 Repeated head injury, with or without loss of consciousness, is also a factor;5 risk increases with each occurrence.6 Other risk factors include exposure to pesticides, rural living, and exposure to well water.5
Researchers have conducted several studies regarding the effects of elevated cholesterol and hypertension on the risk of PD, but results are still without consensus.5 A study published in 2017 reported a significantly increased risk of PD associated with having hepatitis B or C, but the mechanism for the association—including whether it is a consequence of treatment—is unknown.7
Smoking and coffee drinking. Researchers have found that cigarette smoking, beer consumption, and high coffee intake are protective against PD,5 but the benefits are outweighed by the risks associated with these strategies.8 The most practical protective factors are a high dietary intake of vitamin E and increased nut consumption.9 Dietary vitamin E can be found in almonds, spinach, sweet potatoes, sunflower seeds, and avocados. Studies have not found the same benefit with vitamin E supplements.9
Dx seldom requires testing, but may take time to come into focus
Motor symptoms. The key diagnostic criterium for PD is bradykinesia with at least one of the following: muscular rigidity, resting tremor (particularly a pill-rolling tremor) that improves with purposeful function, or postural instability.2 Other physical findings may include masking of facies and speech changes, such as becoming quiet, stuttering, or speaking monotonously without inflection.1 Cogwheeling, stooped posture, and a shuffling gait or difficulty initiating gait (freezing) are all neurologic signs that point toward a PD diagnosis.2
A systematic review found that the clinical features most strongly associated with a diagnosis of PD were trouble turning in bed, a shuffling gait, tremor, difficulty opening jars, micrographia, and loss of balance.10 Typically these symptoms are asymmetric.1
Symptoms that point to other causes. Falling within the first year of symptoms is strongly associated with movement disorders other than PD—notably progressive supranuclear palsy.11 Other symptoms that point toward an alternate diagnosis include a poor response to levodopa, symmetry at the onset of symptoms, rapid progression of disease, and the absence of a tremor.11 It is important to ensure that the patient is not experiencing drug-induced symptoms as can occur with some antipsychotics and antiemetics.
Nonmotor symptoms. Neuropsychiatric symptoms are common in patients with PD. Up to 58% of patients experience depression, and 49% complain of anxiety.12 Hallucinations are present in many patients and are more commonly visual than auditory in nature.13 Patients experience fatigue, daytime sleepiness, and inner restlessness at higher rates than do age-matched controls.3 Research also shows that symptoms such as constipation, mood disorders, erectile dysfunction, and hyposmia may predate the onset of motor symptoms.5
Insomnia is a common symptom that is likely multifactorial in etiology. Causes to consider include motor disturbance, nocturia, reversal of sleep patterns, and reemergence of PD symptoms after a period of quiescence.14 Additionally, hypersalivation and PD dementia can develop as complications of PD.
A clinical diagnosis. Although PD can be difficult to diagnose in the early stages, the diagnosis seldom requires testing.2 A recent systematic review concluded that a clinical diagnosis of PD, when compared with pathology, was correct 74% of the time when the diagnosis was made by nonexperts and correct 84% of the time when the diagnosis was made by movement disorder experts.15
Imaging. Computed tomography and magnetic resonance imaging can be useful in ruling out other diagnoses in the differential, including vascular disease and normal pressure hydrocephalus,2 but will not reveal findings suggestive of PD.
Other diagnostic tests. A levodopa challenge can confirm PD if the diagnosis is unclear.11 In addition, an olfactory test (presenting various odors to the patient for identification) can differentiate PD from progressive supranuclear palsy and corticobasal degeneration; however, it will not distinguish PD from multiple system atrophy.11 If the diagnosis remains unclear, consider a consultation with a neurologist.
Treatment centers on alleviating motor symptoms
The general guiding principle of therapy (TABLE16,17) is to alleviate the motor symptoms (bradykinesia, rigidity, and postural instability) associated with the disease. Experts recommend that treatment commence when symptoms begin to have disabling effects or become a source of discomfort for the patient.1
Carbidopa/levodopa is still often the first choice
Multiple systematic reviews support the use of carbidopa/levodopa as first-line treatment, with the dose kept as low as possible to maintain function, while minimizing motor fluctuations (also referred to as “off” time symptoms) and dyskinesia.11,16 Initial dosing is carbidopa 25 mg/levodopa 100 mg tid. Each can be titrated up to address symptoms to a maximum daily dosing of carbidopa 200 mg/levodopa 2000 mg.17
“Off” time—the return of Parkinson symptoms when the medication’s effect wanes—can become more unpredictable and more difficult to manage as the disease advances.11 Of note: The American Academy of Neurology (AAN) says there is no improvement in the amount of off time a patient experiences by changing to a sustained-release form of carbidopa/levodopa compared with an immediate-release version.11 In addition to the on-off phenomenon, common adverse effects associated with carbidopa/levodopa include nausea, somnolence, dizziness, and headaches. Less common adverse effects include orthostatic hypotension, confusion, and hallucinations.17
Other medications for the treatment of motor symptoms
Second-line agents include dopamine agonists (pramipexole, ropinirole, and bromocriptine) and monoamine oxidase type B (MAO-B) inhibitors (selegiline, rasagiline) (TABLE16,17). The dopamine agonists work by directly stimulating dopamine receptors, while the MAO-B inhibitors block dopamine metabolism, thus enhancing dopaminergic activity in the substantia nigra.
The pros/cons of these 2 classes. Research shows that both dopamine agonists and MAO-B inhibitors are less effective than carbidopa/levodopa at quelling the motor symptoms associated with PD. They can, however, delay the onset of motor complications when compared with carbidopa/levodopa.16
One randomized trial found no long-term benefits to beginning treatment with a levodopa-sparing therapy; however, few patients with earlier disease onset (<60 years of age) were included in the study.18 Given the typically longer duration of their illness, there is potential for this group of patients to develop a higher rate of motor symptoms secondary to carbidopa/levodopa. Thus, considering dopamine agonists and MAO-B inhibitors as initial therapy in patients ages <60 years may be helpful, since they typically will be taking medication longer.
Dopamine agonists. Pramipexole and ropinirole can be used as monotherapy or as an adjunct to levodopa to treat bradykinesia, postural instability, and rigidity. Bromocriptine, an ergot-derived dopamine agonist, is considered an agent of last resort because additional monitoring is required. Potential adverse effects mandate baseline testing and annual repeat testing, including measures of erythrocyte sedimentation rate and renal function and a chest x-ray.16 Consider this agent only if all second- and third-line therapies have provided inadequate control.16
Adverse effects. Dopamine agonists cause such adverse effects as orthostatic hypotension, drowsiness, dizziness, insomnia, abnormal dreams, nausea, constipation, and hallucinations. A Cochrane review notes that these adverse effects have led to higher drop-out rates than seen for carbidopa/levodopa in studies that compared the 2.19
Patients should be counseled about an additional adverse effect associated with dopamine agonists—the possible development of an impulse-control disorder, such as gambling, binge eating, or hypersexuality.1 If a patient develops any of these behaviors, promptly lower the dose of the dopamine agonist or stop the medication.16
The MAO-B inhibitors selegiline and rasagiline may also be considered for initial therapy but are more commonly used as adjunct therapy. Use of selegiline as monotherapy for PD is an off-label indication. Adverse effects for this class of agents include headache, dizziness, insomnia, nausea, and hypotension.
Add-on therapy to treat the adverse effects of primary therapy
Dopaminergic therapies come at the price of the development of off-time motor symptoms and dyskinesia.1,20 In general, these complications are managed by the addition of a dopamine agonist, MAO-B inhibitor, or a catechol-O-methyltransferase (COMT) inhibitor (entacapone).1
Rasagiline and entacapone are a good place to start and should be offered to patients to reduce off-time symptoms, according to the AAN (a Level A recommendation based on multiple high-level studies; see here for an explanation of Strength of Recommendation).
The newest medication, safinamide, has been shown to increase “on” time by one hour per day when compared with placebo; however, it has not yet been tested against existing therapies.21 Other medications that can be considered to reduce drug-induced motor complications include pergolide, pramipexole, ropinirole, and tolcapone.20 Carbidopa/levodopa and bromocriptine are not recommended for the treatment of dopaminergic motor complications.20 Both sustained-release carbidopa/levodopa and bromocriptine are no longer recommended to decrease off time due to ineffectiveness.20
The only medication that has evidence for reducing dyskinesias in patients with PD is amantadine;20 however, it has no effect on other motor symptoms and should not be considered first line.16 Additionally, as an antiviral agent active against some strains of influenza, it should not be taken 2 weeks before or after receiving the influenza vaccine.
When tremor dominates …
For many patients with PD, tremor is more difficult to treat than is bradykinesia, rigidity, and gait disturbance.16 For patients with tremor-predominant PD (characterized by prominent tremor of one or more limbs and a relative lack of significant rigidity and bradykinesia), first-line treatment choices are dopamine agonists (ropinirole, pramipexole), carbidopa/levodopa, and anticholinergic medications, including benztropine and trihexyphenidyl.22 Second-line choices include clozapine, amantadine, clonazepam, and propranolol.22
Treating nonmotor symptoms
Treatment of hypersalivation should start with an evaluation by a speech pathologist. If it doesn’t improve, then adjuvant treatment with glycopyrrolate may be considered.16 Carbidopa/levodopa has the best evidence for treating periodic limb movements of sleep,14 although dopamine agonists may also be considered.16 More research is needed to find an effective therapy to improve insomnia in patients with PD, but for now consider a nighttime dose of carbidopa/levodopa or melatonin.14
Treating cognitive disorders associated with PD
Depression. Treatment of depression in patients with PD is difficult. Multiple systematic reviews have been unable to find a difference in those treated with antidepressants and those not.23 In practice, the use of tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), and a combination of an SSRI and a norepinephrine reuptake inhibitor are commonly used. Additionally, some evidence suggests that pramipexole improves depressive symptoms, but additional research is needed.1
Dementia. Dementia occurs in up to 83% of those who have had PD for more than 20 years.1 Treatment includes the use of rivastigmine (a cholinesterase inhibitor).1 Further research is needed to determine whether donepezil improves dementia symptoms in patients with PD.1
Psychotic symptoms. Query patients and their families periodically about hallucinations and delusions.16 If such symptoms are present and not well tolerated by the patient and/or family, treatment options include quetiapine and clozapine.1 While clozapine is more effective, it requires frequent hematologic monitoring due to the risk of agranulocytosis.1 And quetiapine carries a black box warning about early death. Exercise caution when prescribing these medications, particularly if a patient is cognitively impaired, and always start with low doses.1
A newer medication, pimavanserin (a second-generation antipsychotic), was recently approved by the FDA to treat hallucinations and delusions of PD psychosis, although any improvement this agent provides may not be clinically significant.24 Unlike clozapine, no additional monitoring is needed and there are no significant safety concerns with the use of pimavanserin, which makes it a reasonable first choice for hallucinations and delusions. Other neuroleptic medications should not be used as they tend to worsen Parkinson symptoms.1
Consider tai chi, physical therapy to reduce falls
One study showed that tai chi, performed for an hour twice weekly, was significantly more effective at reducing falls when compared to the same amount of resistance training and strength training, and that the benefits remained 3 months after the completion of the 24-week study.25 To date, tai chi is the only intervention that has been shown to affect fall risk.
Guidelines recommend that physical therapy be available to all patients.16 A Cochrane review performed in 2013 determined that physical therapy improves walking endurance and balance but does not affect quality of life in terms of fear of falling.26
When meds no longer help, consider deep brain stimulation as a last resort
Deep brain stimulation consists of surgical implantation of a device to deliver electrical current to a targeted area of the brain. It can be considered for patients with PD who are no longer responsive to carbidopa/levodopa, not experiencing neuropsychiatric symptoms, and are experiencing significant motor complications despite optimal medical management.14 Referral to a specialist is recommended for these patients to assess their candidacy for this procedure.
Prognosis: Largely unchanged
While medications can improve quality of life and function, PD remains a chronic and progressive disorder that is associated with significant morbidity. A study performed in 2013 showed that older age at onset, cognitive dysfunction, and motor symptoms nonresponsive to levodopa were associated with faster progression toward disability.27
Keep an eye on patients’ bone mineral density (BMD), as patients with PD tend to have lower BMD,28 a 2-fold increase in the risk of fracture for both men and women,29 and a higher prevalence of vitamin D deficiency.30
Also, watch for signs of infection because the most commonly cited cause of death in those with PD is pneumonia rather than a complication of the disease itself.11
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 777 South Clinton Avenue, Rochester, NY 14620; [email protected].
Parkinson’s disease (PD) can be a tough diagnosis to navigate. Patients with this neurologic movement disorder can present with a highly variable constellation of symptoms,1 ranging from the well-known tremor and bradykinesia to difficulties with activities of daily living (particularly dressing and getting out of a car2) to nonspecific symptoms, such as pain, fatigue, hyposmia, and erectile dysfunction.3
Furthermore, medications more recently approved by the US Food and Drug Administration (FDA) have left many health care providers confused about what constitutes appropriate first-, second-, and third-line therapies, as well as add-on therapy for symptoms secondary to dopaminergic agents. What follows is a stepwise approach to managing PD that incorporates these newer therapies so that you can confidently and effectively manage patients with PD with little or no consultation.
First, though, we review who’s at greatest risk—and what you’ll see.
Family history tops list of risk factors for PD
While PD occurs in less than 1% of the population ≥40 years of age, its prevalence increases with age, becoming significantly higher by age 60 years, with a slight predominance toward males.4
A variety of factors increase the risk of developing PD. A well-conducted meta-analysis showed that the strongest risk factor is having a family member, particularly a first-degree relative, with a history of PD or tremor.5 Repeated head injury, with or without loss of consciousness, is also a factor;5 risk increases with each occurrence.6 Other risk factors include exposure to pesticides, rural living, and exposure to well water.5
Researchers have conducted several studies regarding the effects of elevated cholesterol and hypertension on the risk of PD, but results are still without consensus.5 A study published in 2017 reported a significantly increased risk of PD associated with having hepatitis B or C, but the mechanism for the association—including whether it is a consequence of treatment—is unknown.7
Smoking and coffee drinking. Researchers have found that cigarette smoking, beer consumption, and high coffee intake are protective against PD,5 but the benefits are outweighed by the risks associated with these strategies.8 The most practical protective factors are a high dietary intake of vitamin E and increased nut consumption.9 Dietary vitamin E can be found in almonds, spinach, sweet potatoes, sunflower seeds, and avocados. Studies have not found the same benefit with vitamin E supplements.9
Dx seldom requires testing, but may take time to come into focus
Motor symptoms. The key diagnostic criterium for PD is bradykinesia with at least one of the following: muscular rigidity, resting tremor (particularly a pill-rolling tremor) that improves with purposeful function, or postural instability.2 Other physical findings may include masking of facies and speech changes, such as becoming quiet, stuttering, or speaking monotonously without inflection.1 Cogwheeling, stooped posture, and a shuffling gait or difficulty initiating gait (freezing) are all neurologic signs that point toward a PD diagnosis.2
A systematic review found that the clinical features most strongly associated with a diagnosis of PD were trouble turning in bed, a shuffling gait, tremor, difficulty opening jars, micrographia, and loss of balance.10 Typically these symptoms are asymmetric.1
Symptoms that point to other causes. Falling within the first year of symptoms is strongly associated with movement disorders other than PD—notably progressive supranuclear palsy.11 Other symptoms that point toward an alternate diagnosis include a poor response to levodopa, symmetry at the onset of symptoms, rapid progression of disease, and the absence of a tremor.11 It is important to ensure that the patient is not experiencing drug-induced symptoms as can occur with some antipsychotics and antiemetics.
Nonmotor symptoms. Neuropsychiatric symptoms are common in patients with PD. Up to 58% of patients experience depression, and 49% complain of anxiety.12 Hallucinations are present in many patients and are more commonly visual than auditory in nature.13 Patients experience fatigue, daytime sleepiness, and inner restlessness at higher rates than do age-matched controls.3 Research also shows that symptoms such as constipation, mood disorders, erectile dysfunction, and hyposmia may predate the onset of motor symptoms.5
Insomnia is a common symptom that is likely multifactorial in etiology. Causes to consider include motor disturbance, nocturia, reversal of sleep patterns, and reemergence of PD symptoms after a period of quiescence.14 Additionally, hypersalivation and PD dementia can develop as complications of PD.
A clinical diagnosis. Although PD can be difficult to diagnose in the early stages, the diagnosis seldom requires testing.2 A recent systematic review concluded that a clinical diagnosis of PD, when compared with pathology, was correct 74% of the time when the diagnosis was made by nonexperts and correct 84% of the time when the diagnosis was made by movement disorder experts.15
Imaging. Computed tomography and magnetic resonance imaging can be useful in ruling out other diagnoses in the differential, including vascular disease and normal pressure hydrocephalus,2 but will not reveal findings suggestive of PD.
Other diagnostic tests. A levodopa challenge can confirm PD if the diagnosis is unclear.11 In addition, an olfactory test (presenting various odors to the patient for identification) can differentiate PD from progressive supranuclear palsy and corticobasal degeneration; however, it will not distinguish PD from multiple system atrophy.11 If the diagnosis remains unclear, consider a consultation with a neurologist.
Treatment centers on alleviating motor symptoms
The general guiding principle of therapy (TABLE16,17) is to alleviate the motor symptoms (bradykinesia, rigidity, and postural instability) associated with the disease. Experts recommend that treatment commence when symptoms begin to have disabling effects or become a source of discomfort for the patient.1
Carbidopa/levodopa is still often the first choice
Multiple systematic reviews support the use of carbidopa/levodopa as first-line treatment, with the dose kept as low as possible to maintain function, while minimizing motor fluctuations (also referred to as “off” time symptoms) and dyskinesia.11,16 Initial dosing is carbidopa 25 mg/levodopa 100 mg tid. Each can be titrated up to address symptoms to a maximum daily dosing of carbidopa 200 mg/levodopa 2000 mg.17
“Off” time—the return of Parkinson symptoms when the medication’s effect wanes—can become more unpredictable and more difficult to manage as the disease advances.11 Of note: The American Academy of Neurology (AAN) says there is no improvement in the amount of off time a patient experiences by changing to a sustained-release form of carbidopa/levodopa compared with an immediate-release version.11 In addition to the on-off phenomenon, common adverse effects associated with carbidopa/levodopa include nausea, somnolence, dizziness, and headaches. Less common adverse effects include orthostatic hypotension, confusion, and hallucinations.17
Other medications for the treatment of motor symptoms
Second-line agents include dopamine agonists (pramipexole, ropinirole, and bromocriptine) and monoamine oxidase type B (MAO-B) inhibitors (selegiline, rasagiline) (TABLE16,17). The dopamine agonists work by directly stimulating dopamine receptors, while the MAO-B inhibitors block dopamine metabolism, thus enhancing dopaminergic activity in the substantia nigra.
The pros/cons of these 2 classes. Research shows that both dopamine agonists and MAO-B inhibitors are less effective than carbidopa/levodopa at quelling the motor symptoms associated with PD. They can, however, delay the onset of motor complications when compared with carbidopa/levodopa.16
One randomized trial found no long-term benefits to beginning treatment with a levodopa-sparing therapy; however, few patients with earlier disease onset (<60 years of age) were included in the study.18 Given the typically longer duration of their illness, there is potential for this group of patients to develop a higher rate of motor symptoms secondary to carbidopa/levodopa. Thus, considering dopamine agonists and MAO-B inhibitors as initial therapy in patients ages <60 years may be helpful, since they typically will be taking medication longer.
Dopamine agonists. Pramipexole and ropinirole can be used as monotherapy or as an adjunct to levodopa to treat bradykinesia, postural instability, and rigidity. Bromocriptine, an ergot-derived dopamine agonist, is considered an agent of last resort because additional monitoring is required. Potential adverse effects mandate baseline testing and annual repeat testing, including measures of erythrocyte sedimentation rate and renal function and a chest x-ray.16 Consider this agent only if all second- and third-line therapies have provided inadequate control.16
Adverse effects. Dopamine agonists cause such adverse effects as orthostatic hypotension, drowsiness, dizziness, insomnia, abnormal dreams, nausea, constipation, and hallucinations. A Cochrane review notes that these adverse effects have led to higher drop-out rates than seen for carbidopa/levodopa in studies that compared the 2.19
Patients should be counseled about an additional adverse effect associated with dopamine agonists—the possible development of an impulse-control disorder, such as gambling, binge eating, or hypersexuality.1 If a patient develops any of these behaviors, promptly lower the dose of the dopamine agonist or stop the medication.16
The MAO-B inhibitors selegiline and rasagiline may also be considered for initial therapy but are more commonly used as adjunct therapy. Use of selegiline as monotherapy for PD is an off-label indication. Adverse effects for this class of agents include headache, dizziness, insomnia, nausea, and hypotension.
Add-on therapy to treat the adverse effects of primary therapy
Dopaminergic therapies come at the price of the development of off-time motor symptoms and dyskinesia.1,20 In general, these complications are managed by the addition of a dopamine agonist, MAO-B inhibitor, or a catechol-O-methyltransferase (COMT) inhibitor (entacapone).1
Rasagiline and entacapone are a good place to start and should be offered to patients to reduce off-time symptoms, according to the AAN (a Level A recommendation based on multiple high-level studies; see here for an explanation of Strength of Recommendation).
The newest medication, safinamide, has been shown to increase “on” time by one hour per day when compared with placebo; however, it has not yet been tested against existing therapies.21 Other medications that can be considered to reduce drug-induced motor complications include pergolide, pramipexole, ropinirole, and tolcapone.20 Carbidopa/levodopa and bromocriptine are not recommended for the treatment of dopaminergic motor complications.20 Both sustained-release carbidopa/levodopa and bromocriptine are no longer recommended to decrease off time due to ineffectiveness.20
The only medication that has evidence for reducing dyskinesias in patients with PD is amantadine;20 however, it has no effect on other motor symptoms and should not be considered first line.16 Additionally, as an antiviral agent active against some strains of influenza, it should not be taken 2 weeks before or after receiving the influenza vaccine.
When tremor dominates …
For many patients with PD, tremor is more difficult to treat than is bradykinesia, rigidity, and gait disturbance.16 For patients with tremor-predominant PD (characterized by prominent tremor of one or more limbs and a relative lack of significant rigidity and bradykinesia), first-line treatment choices are dopamine agonists (ropinirole, pramipexole), carbidopa/levodopa, and anticholinergic medications, including benztropine and trihexyphenidyl.22 Second-line choices include clozapine, amantadine, clonazepam, and propranolol.22
Treating nonmotor symptoms
Treatment of hypersalivation should start with an evaluation by a speech pathologist. If it doesn’t improve, then adjuvant treatment with glycopyrrolate may be considered.16 Carbidopa/levodopa has the best evidence for treating periodic limb movements of sleep,14 although dopamine agonists may also be considered.16 More research is needed to find an effective therapy to improve insomnia in patients with PD, but for now consider a nighttime dose of carbidopa/levodopa or melatonin.14
Treating cognitive disorders associated with PD
Depression. Treatment of depression in patients with PD is difficult. Multiple systematic reviews have been unable to find a difference in those treated with antidepressants and those not.23 In practice, the use of tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), and a combination of an SSRI and a norepinephrine reuptake inhibitor are commonly used. Additionally, some evidence suggests that pramipexole improves depressive symptoms, but additional research is needed.1
Dementia. Dementia occurs in up to 83% of those who have had PD for more than 20 years.1 Treatment includes the use of rivastigmine (a cholinesterase inhibitor).1 Further research is needed to determine whether donepezil improves dementia symptoms in patients with PD.1
Psychotic symptoms. Query patients and their families periodically about hallucinations and delusions.16 If such symptoms are present and not well tolerated by the patient and/or family, treatment options include quetiapine and clozapine.1 While clozapine is more effective, it requires frequent hematologic monitoring due to the risk of agranulocytosis.1 And quetiapine carries a black box warning about early death. Exercise caution when prescribing these medications, particularly if a patient is cognitively impaired, and always start with low doses.1
A newer medication, pimavanserin (a second-generation antipsychotic), was recently approved by the FDA to treat hallucinations and delusions of PD psychosis, although any improvement this agent provides may not be clinically significant.24 Unlike clozapine, no additional monitoring is needed and there are no significant safety concerns with the use of pimavanserin, which makes it a reasonable first choice for hallucinations and delusions. Other neuroleptic medications should not be used as they tend to worsen Parkinson symptoms.1
Consider tai chi, physical therapy to reduce falls
One study showed that tai chi, performed for an hour twice weekly, was significantly more effective at reducing falls when compared to the same amount of resistance training and strength training, and that the benefits remained 3 months after the completion of the 24-week study.25 To date, tai chi is the only intervention that has been shown to affect fall risk.
Guidelines recommend that physical therapy be available to all patients.16 A Cochrane review performed in 2013 determined that physical therapy improves walking endurance and balance but does not affect quality of life in terms of fear of falling.26
When meds no longer help, consider deep brain stimulation as a last resort
Deep brain stimulation consists of surgical implantation of a device to deliver electrical current to a targeted area of the brain. It can be considered for patients with PD who are no longer responsive to carbidopa/levodopa, not experiencing neuropsychiatric symptoms, and are experiencing significant motor complications despite optimal medical management.14 Referral to a specialist is recommended for these patients to assess their candidacy for this procedure.
Prognosis: Largely unchanged
While medications can improve quality of life and function, PD remains a chronic and progressive disorder that is associated with significant morbidity. A study performed in 2013 showed that older age at onset, cognitive dysfunction, and motor symptoms nonresponsive to levodopa were associated with faster progression toward disability.27
Keep an eye on patients’ bone mineral density (BMD), as patients with PD tend to have lower BMD,28 a 2-fold increase in the risk of fracture for both men and women,29 and a higher prevalence of vitamin D deficiency.30
Also, watch for signs of infection because the most commonly cited cause of death in those with PD is pneumonia rather than a complication of the disease itself.11
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 777 South Clinton Avenue, Rochester, NY 14620; [email protected].
1. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896-912.
2. Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055-2066.
3. Todorova A, Jenner P, Chaudhuri K. Non-motor Parkinson’s: integral to motor Parkinson’s, yet often neglected. Pract Neurol. 2014;14:310-322.
4. Pringsheim T, Jette N, Frolkis A, et al. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1583-1590.
5. Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol. 2012;72:893-901.
6. Dick FD, De Palma G, Ahmadi A, et al. Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occup Environ Med. 2007;64:666-672.
7. Pakpoor J, Noyce A, Goldacre R, et al. Viral hepatitis and Parkinson disease: a national record-linkage study. Neurology. 2017;88:1630-1633.
8. Hern T, Newton W. Does coffee protect against the development of Parkinson disease (PD)? J Fam Pract. 2000;49:685-686.
9. Zhang SM, Hernán MA, Chen H, et al. Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology. 2002;59:1161-1169.
10. Rao G, Fisch L, Srinivasan S, et al. Does this patient have Parkinson disease? JAMA. 2003;289:347-353.
11. Suchowersky O, Reich S, Perlmutter J, et al. Practice Parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:968-975.
12. Aarsland D, Brønnick K, Ehrt U, et al. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry. 2007;78:36-42.
13. Inzelberg R, Kipervasser S, Korczyn AD. Auditory hallucinations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;64:533-535.
14. Zesiewicz TA, Sullivan KL, Arnulf I, et al. Practice Parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74:924-931.
15. Rizzo G, Copetti M, Arcuti S, et al. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;86:566-576.
16. National Institute for Heath and Care Excellence. Parkinson’s disease in adults. NICE guideline NG 71. 2017. Available at: https://www.nice.org.uk/guidance/ng71. Accessed March 27, 2018.
17. Lexicomp version 4.0.1. Wolters Kluwer; Copyright 2017. Available at: https://online.lexi.com/lco/action/home. Accessed March 27, 2018.
18. Lang AE, Marras C. Initiating dopaminergic treatment in Parkinson’s disease. Lancet. 2014;384:1164-1166.
19. Stowe RL, Ives NJ, Clarke C, et al. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;CD006564.
20. Pahwa R, Factor SA, Lyons KE, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:983-995.
21. Schapira AH, Fox SH, Hauser RA, et al. Assessment of safety and efficacy of safinamide as a levodopa adjunct in patients with Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol. 2017;74:216-224.
22. Marjama-Lyons J, Koller W. Tremor-predominant Parkinson’s disease. Approaches to treatment. Drugs Aging. 2000;16:273-278.
23. Price A, Rayner L, Okon-Rocha E, et al. Antidepressants for the treatment of depression in neurological disorders: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2011;82:914-923.
24. Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomized placebo-controlled phase 3 trial. Lancet. 2014;383:533-540.
25. Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366:511-519.
26. Tomlinson CL, Patel S, Meek C, et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev. 2012;CD002817.
27. Velseboer DC, Broeders M, Post B, et al. Prognostic factors of motor impairment, disability, and quality of life in newly diagnosed PD. Neurology. 2013;80:627-633.
28. Cronin H, Casey MC, Inderhaugh J, et al. Osteoporosis in patients with Parkinson’s disease. J Am Geriatr Soc. 2006;54:1797-1798.
29. Tan L, Wang Y, Zhou L, et al. Parkinson’s disease and risk of fracture: a meta-analysis of prospective cohort studies. PLoS One. 2014;9:e94379.
30. Evatt ML, Delong MR, Khazai N, et al. Prevalence of vitamin D insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol. 2008;65:1348-1352.
1. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896-912.
2. Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055-2066.
3. Todorova A, Jenner P, Chaudhuri K. Non-motor Parkinson’s: integral to motor Parkinson’s, yet often neglected. Pract Neurol. 2014;14:310-322.
4. Pringsheim T, Jette N, Frolkis A, et al. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1583-1590.
5. Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol. 2012;72:893-901.
6. Dick FD, De Palma G, Ahmadi A, et al. Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occup Environ Med. 2007;64:666-672.
7. Pakpoor J, Noyce A, Goldacre R, et al. Viral hepatitis and Parkinson disease: a national record-linkage study. Neurology. 2017;88:1630-1633.
8. Hern T, Newton W. Does coffee protect against the development of Parkinson disease (PD)? J Fam Pract. 2000;49:685-686.
9. Zhang SM, Hernán MA, Chen H, et al. Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology. 2002;59:1161-1169.
10. Rao G, Fisch L, Srinivasan S, et al. Does this patient have Parkinson disease? JAMA. 2003;289:347-353.
11. Suchowersky O, Reich S, Perlmutter J, et al. Practice Parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:968-975.
12. Aarsland D, Brønnick K, Ehrt U, et al. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry. 2007;78:36-42.
13. Inzelberg R, Kipervasser S, Korczyn AD. Auditory hallucinations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;64:533-535.
14. Zesiewicz TA, Sullivan KL, Arnulf I, et al. Practice Parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74:924-931.
15. Rizzo G, Copetti M, Arcuti S, et al. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;86:566-576.
16. National Institute for Heath and Care Excellence. Parkinson’s disease in adults. NICE guideline NG 71. 2017. Available at: https://www.nice.org.uk/guidance/ng71. Accessed March 27, 2018.
17. Lexicomp version 4.0.1. Wolters Kluwer; Copyright 2017. Available at: https://online.lexi.com/lco/action/home. Accessed March 27, 2018.
18. Lang AE, Marras C. Initiating dopaminergic treatment in Parkinson’s disease. Lancet. 2014;384:1164-1166.
19. Stowe RL, Ives NJ, Clarke C, et al. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;CD006564.
20. Pahwa R, Factor SA, Lyons KE, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:983-995.
21. Schapira AH, Fox SH, Hauser RA, et al. Assessment of safety and efficacy of safinamide as a levodopa adjunct in patients with Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol. 2017;74:216-224.
22. Marjama-Lyons J, Koller W. Tremor-predominant Parkinson’s disease. Approaches to treatment. Drugs Aging. 2000;16:273-278.
23. Price A, Rayner L, Okon-Rocha E, et al. Antidepressants for the treatment of depression in neurological disorders: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2011;82:914-923.
24. Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomized placebo-controlled phase 3 trial. Lancet. 2014;383:533-540.
25. Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366:511-519.
26. Tomlinson CL, Patel S, Meek C, et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev. 2012;CD002817.
27. Velseboer DC, Broeders M, Post B, et al. Prognostic factors of motor impairment, disability, and quality of life in newly diagnosed PD. Neurology. 2013;80:627-633.
28. Cronin H, Casey MC, Inderhaugh J, et al. Osteoporosis in patients with Parkinson’s disease. J Am Geriatr Soc. 2006;54:1797-1798.
29. Tan L, Wang Y, Zhou L, et al. Parkinson’s disease and risk of fracture: a meta-analysis of prospective cohort studies. PLoS One. 2014;9:e94379.
30. Evatt ML, Delong MR, Khazai N, et al. Prevalence of vitamin D insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol. 2008;65:1348-1352.
From The Journal of Family Practice | 2018;67(5):276-279,284-286.
PRACTICE RECOMMENDATIONS
› Use carbidopa/levodopa as first-line treatment for most patients with Parkinson's disease. A
› Prescribe rasagiline or entacapone for the treatment of motor fluctuations secondary to dopaminergic therapies. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hypothermia in adults: A strategy for detection and Tx
CASE
Patrick S, an 85-year-old man with multiple medical problems, was brought to his primary care provider after being found at home with altered mental status. His caretaker reported that Mr. S had been using extra blankets in bed and sleeping more, but he hadn’t had significant outdoor exposure. Measurement of his vital signs revealed tachycardia, tachypnea, hypotension, and a rectal temperature of 32°C (89.6°F).
How would you proceed with the care of this patient?
What is accidental hypothermia?
Accidental hypothermia is an unintentional drop in core body temperature to <35°C (<95°F). Mild hypothermia is defined as a core body temperature of 32°C to 35°C (90°F - 95°F); moderate hypothermia, 28°C to 32°C (82°F - 90°F); and severe hypothermia, <28°C (<82°F).1
The International Commission for Mountain Emergency Medicine divides hypothermia into 5 categories, emphasizing the clinical features of each stage as a guide to treatment (TABLE 1).2 These categories were adopted to help prehospital rescuers estimate the severity of hypothermia using physical symptoms. For example, most patients stop shivering at approximately 30°C (86°F)—the “moderate (HT II)” category of hypothermia—although this response varies widely from patient to patient. Notably, there are reports in the literature of survival in hypothermia with a temperature as low as 13.7°C (56.7°F) and with cardiac arrest for as long as 8 hours and 40 minutes, although these events are rare.3
Each year, approximately 700 deaths in the United States are the result of hypothermia.4 Between 1995 to 2004 in the United States, it is estimated that 15,574 visits were made to a health care provider or facility for hypothermia and other cold-related concerns.5 Based on reports in the international literature, the incidence of nonlethal hypothermia is much greater than the incidence of lethal hypothermia.5 Almost half of deaths from hypothermia are in people older than age 65 years; the male to female ratio is 2.5:1.1
Variables that predispose the body to temperature dysregulation include extremes of age, comorbid conditions, intoxication, chronic cold exposure, immersion accident, mental illness, impaired shivering, and lack of acclimatization.1 The most common causes of death associated with hypothermia are falls, drownings, and cardiovascular disease.4 In a 2008 study, hypothermia and other cold-related morbidity emergency department (ED) visits required more transfers of patients to a critical care unit than any other reason for visiting an ED (risk ratio, 6.73; 95% confidence interval, 1.8-25).5 Mortality among inpatients whose hypothermia is classified as moderate or severe reaches as high as 40%.3
More than just cold-weather exposure
Accidental hypothermia occurs when heat loss is superseded by the body’s ability to generate heat. It commonly happens in cold environments but can also occur at higher temperatures if the body’s thermoregulatory system malfunctions.
Environmental or iatrogenic factors (ie, primary hypothermia), such as wind, water immersion, wetness, aggressive fluid resuscitation, and heat stroke treatment can make people more susceptible to hypothermia. Medical conditions (ie, secondary hypothermia), such as burns, exfoliative dermatitis, severe psoriasis, hypoadrenalism, hypopituitarism, hypothyroidism, acute spinal cord transection, head trauma, stroke, tumor, pneumonia, Wernicke’s disease (encephalopathy), and sepsis can also predispose to hypothermia.1 Drugs, such as ethanol, phenothiazines, and sedative–hypnotics may decrease the hypothermia threshold.1 (For information on preventing hypothermia, see TABLE 2.6)
Pathophysiology: The role of the hypothalamus
Humans maintain body temperature by balancing heat production and heat loss to the environment. Heat is lost through the skin and lungs by 5 different mechanisms: radiation, conduction, convection, evaporation, and respiration. Convective heat loss to cold air and conductive heat loss to water are the most common mechanisms of accidental hypothermia.7
To maintain temperature homeostasis at 37°C (98.6°F) (±0.5°C [±0.9°F]), the hypothalamus receives input from central and peripheral thermal receptors and stimulates heat production through shivering, increasing the basal metabolic rate 2-fold to 5-fold.1 The hypothalamus also increases thyroid, catecholamine, and adrenal activity to increase the body’s production of heat and raise core temperature.
Heat conservation occurs by activation of sympathetically mediated vasoconstriction, reducing conduction to the skin, where cooling is greatest. After time, temperature regulation in the body becomes overwhelmed and catecholamine levels return to a pre-hypothermic state.
At 35°C (95°F), neurologic function begins to decline; at 32°C (89.6°F), metabolism, ventilation, and cardiac output decrease until shivering ceases. Changes in peripheral blood flow can create a false warming sensation, causing a person to remove clothing, a phenomenon referred to as paradoxical undressing. As hypothermia progresses, the neurologic, respiratory, and cardiac systems continue to slow until there is eventual cardiorespiratory failure.
Assessment and diagnosis
History and physical examination. A high index of suspicion for the diagnosis of hypothermia is essential, especially when caring for the elderly or patients presenting with unexplained illness. Often, symptoms of a primary condition may overshadow those reflecting hypothermia. In a multicenter survey that reviewed 428 cases of accidental hypothermia in the United States, 44% of patients had an underlying illness; 18%, coexisting infection; 19%, trauma; and 6%, overdose.3
There are no strict diagnostic criteria for hypothermia other than a core body temperature <35°C (<95°F). Standard thermometers often do not read below 34.4°C (93.2°F), so it is recommended that a rectal thermometer capable of reading low body temperatures be used for accurate measurement.
Hypothermic patients can exhibit a variety of symptoms, depending on the degree of decrease in core body temperature1:
- A mildly hypothermic patient might present with any combination of tachypnea, tachycardia, ataxia, impaired judgment, shivering, and vasoconstriction.
- Moderate hypothermia typically manifests as a decreased heart rate, decreased blood pressure, decreased level of consciousness, decreased respiratory effort, dilated pupils, extinction of shivering, and hyporeflexia. Cardiac abnormalities, such as atrial fibrillation and junctional bradycardia, may be seen in moderate hypothermia.
- Severe hypothermia presents with apnea, coma, nonreactive pupils, oliguria, areflexia, hypotension, bradycardia, and continued cardiac abnormalities, such as ventricular arrhythmias and asystole.
Laboratory evaluation. No specific laboratory tests are needed to diagnose hypothermia. General lab tests, however, may help determine whether hypothermia is the result, or the cause, of the clinical scenario. Recommended laboratory tests for making that determination include a complete blood count (CBC), chemistry panel, arterial blood gases, fingerstick glucose, and coagulation panel.
Results of lab tests may be abnormal because of the body’s decreased core body temperature. White blood cells and platelets in the CBC, for example, may be decreased due to splenic sequestration; these findings reverse with rewarming. With every 1°C (1.8°F) drop in core body temperature, hematocrit increases 2%.3 Sodium, chloride, and magnesium concentrations do not display consistent abnormalities with any core body temperature >25°C (77°F),3,8 but potassium levels may fluctuate because of acid-base changes that occur during rewarming.1 Creatinine and creatine kinase levels may be increased secondary to rhabdomyolysis or acute tubular necrosis.1
Arterial blood gases typically show metabolic acidosis or respiratory alkalosis, or both.8 Prothrombin time and partial thromboplastin time are typically elevated in vivo, secondary to temperature-dependent enzymes in the coagulation cascade, but are reported normal in a blood specimen that is heated to 37°C (98.6°F) prior to analysis.1,8
Both hyperglycemia and hypoglycemia can be associated with hypothermia. The lactate level can be elevated, due to hypoperfusion. Hepatic impairment may be seen secondary to decreased cardiac output. An increase in the lipase level may also occur.3
When a hypothermic patient fails to respond to rewarming, or there is no clear source of cold exposure, consider testing for other causes of the problem, including hypothyroidism and adrenal insufficiency (see “Differential diagnosis”). Hypothermia may also decrease thyroid function in people with preexisting disease.
Other laboratory studies that can be considered include fibrinogen, blood-alcohol level, urine toxicology screen, and blood and fluid cultures.3
Imaging. Imaging studies are not performed routinely in the setting of hypothermia; however:
- Chest radiography can be considered to assess for aspiration pneumonia, vascular congestion, and pulmonary edema.
- Computed tomography (CT) of the head is helpful in the setting of trauma or if mental status does not clear with rewarming.3
- Bedside ultrasonography can assess for cardiac activity, volume status, pulmonary edema, free fluid, and trauma. (See "Point-of-care ultrasound: Coming soon to primary care?" J Fam Pract. 2018;67:70-80.)
Electrocardiography. An electrocardiogram is essential to evaluate for arrhythmias. Findings associated with hypothermia are prolongation of PR, QRS, and QT intervals; ST-segment elevation, T-wave inversion; and Osborn waves (J waves), which represent a positive deflection at the termination of the QRS complex with associated J-point elevation.8 Osborn waves generally present when the core body temperature is <32°C (89.6°F) and become larger as the core body temperature drops further.3
Differential diagnosis. Hypothermia is most commonly caused by environmental exposure, but the differential diagnosis is broad: many medical conditions, as well as drug and alcohol intoxication, can contribute to hypothermia (TABLE 31).
Treatment: Usually unnecessary, sometimes crucial
Most patients with mild hypothermia recover completely with little intervention. These patients should be evaluated for cognitive irregularities and observed in the ED before discharge.9 Moderate and severe hypothermia patients should be assessed using pre-hospital protocols and given cardiopulmonary resuscitation (CPR) for cardiac arrest. Pre-hospital providers should rely more on symptoms in guiding their treatment response because core body temperature measurements can be difficult to obtain, and the response to a drop in core body temperature varies from patient to patient.10
Early considerations: Airway, breathing, circulation (ABC)
A first responder might have difficulty palpating the pulse of a hypothermic patient if that patient’s cardiopulmonary effort is diminished.9 This inability to palpate a pulse should not delay treatment unless the patient presents with lethal injury; the scene is unsafe; the chest is too stiff for CPR; do-not-resuscitate status is present; or the patient was buried in an avalanche for ≥35 minutes and the airway is filled with snow (FIGURE3,11,12). Pulse should be checked carefully for 60 seconds. If pulses are not present, CPR should be initiated.
Prevention of further heat loss should begin promptly for hypothermic patients who retain a perfusing rhythm.11 Lifesaving interventions, such as airway management, vascular access for volume replenishment, and defibrillation for ventricular tachycardia or ventricular fibrillation should be carried out according to Advanced Cardiac Life Support protocols.11 Patients in respiratory distress or incapable of protecting their airway because of altered mental status should undergo endotracheal intubation. Fluid resuscitation with isotonic crystalloid fluids, warmed to 40°C (104°F) to 42°C (~107°F) and delivered through 2 large-bore, peripheral intravenous (IV) needles, can be considered.
Special care should be taken when moving a hypothermic patient. Excessive movement can lead to stimulation of the irritable hypothermic heart and cause an arrhythmia.
Medical therapy. Caution is advised because the reduced metabolism of a hypothermic patient can lead to potentially toxic accumulation of drugs peripherally. In fact, outcomes have not been positively influenced by routine use of medications, other than treatment of ventricular fibrillation with amiodarone.11 Any intravenous (IV) drug should be held until the patient’s core temperature is >30°C (>86°F).11
Vasopressors can be beneficial during rewarming for a patient in cardiac arrest and are a reasonable consideration.2 Nitroglycerin, in conjunction with active external rewarming, can increase the overall hourly temperature gain in a moderately hypothermic patient.13
Rewarming. The extent of rewarming required can be predicted by the severity of hypothermia (FIGURE3,11,12). Mildly hypothermic patients can generally be rewarmed using passive external measures. Patients with moderate hypothermia benefit from active rewarming in addition to passive measures. Intervention for severe hypothermia requires external rewarming and internal warming, with admission to the intensive care unit.
Treatment plans for severely hypothermic patients differ, depending on whether the person has a perfusing or nonperfusing cardiac rhythm. Patients who maintain a perfusing rhythm can be rewarmed using external methods (although core rewarming is used more often). Patients who do not have a perfusing rhythm require more invasive procedures.11 When using any rewarming method, afterdrop phenomenon can occur: ie, vasodilation, brought on by rewarming, causes a drop in core body temperature, as cooler peripheral blood returns to the central circulation. This effect may be reduced by focused rewarming of the trunk prior to rewarming the extremities.3
Rewarming for mild hypothermia patients begins with passive external techniques. First, the patient is moved away from the environment for protection from further exposure. Next, wet or damaged clothing is removed, blankets or foil insulators are applied, and room temperature is maintained at ≥28°C (82°F).3,11,13,14
If the patient’s temperature does not normalize, or if the patient presented with moderate or severe hypothermia, rewarming is continued with active external and internal measures. Active external rewarming can supplement passive measures using radiant heat from warmed blankets, air rewarming devices, and heating pads.3,13,14 Active internal rewarming techniques rely on invasive measures to raise the core temperature. Heated crystalloid IV fluids do not treat hypothermia, but do help reduce further heat loss and can be helpful in patients in need of volume resuscitation.3,13
Severely hypothermic patients might require more invasive active internal rewarming techniques, such as body-cavity lavage and extracorporeal methods. Body-cavity lavage can be facilitated with large volumes (10-120 L) of warm fluid at 40°C to 42°C, circulated through the thoracic or abdominal cavities to raise core body temperature 3°C to 6°C per hour.3,13
Extracorporeal rewarming can be achieved through hemodialysis, continuous arteriovenous rewarming (CAVR), continuous veno-venous rewarming (CVVR), or cardiopulmonary bypass.3,13 Research has shown cardiopulmonary bypass to be the most effective technique, with as high as a 7°C rise in core body temperature per hour; CVVR and CAVR are less invasive, however, and more readily available in hospitals.3,11,13
Rewarming interventions should continue until return of spontaneous circulation and core body temperature reaches 32°C (89.6°F) to 34°C (93.2°F).11 Overall, resuscitation efforts may take longer than normal due to the need for rewarming and should continue until the patient has achieved a normal temperature of 37°C (97.8°F).
Prognosis varies with severity, the health of the patient
In healthy, mildly hypothermic patients, full recovery is common if heat loss is minimized and the cause is treated. Moderately hypothermic patients who receive proper care can also have a favorable result. Outcomes for severe hypothermia vary with duration, comorbidities, and severity of core body temperature loss.15
Immediate initiation of rewarming by pre-hospital providers improves outcomes, and higher mortality has been demonstrated with hospital admission temperatures <35°C (95°F).15 Almost 100% of primary hypothermia patients with cardiac stability who were treated using active external and minimally invasive rewarming techniques survived with an intact neurologic system.12 Fifty percent of patients who endured cardiac arrest or who were treated with extracorporeal rewarming had an intact neurologic system. In cardiac arrest cases without significant underlying disease or trauma, and in which hypoxia did not precede hypothermia, full recovery is possible (and has been observed).12
CASE
Mr. S was given a diagnosis of mild to moderate hypothermia and transferred to the nearest ED for further treatment. His age had put him at increased risk of hypothermia. The work-up included laboratory testing (CBC, chemistry panel, thyroid-stimulating hormone, urinalysis, and blood cultures), electrocardiography, chest radiography, and CT of the head.
The chest radiograph showed pneumonia. Based on the results of blood culture, bacterial infection (pneumonia) was determined to be the underlying cause of hypothermia. Mr. S was started on antibiotics.
CORRESPONDENCE
Natasha J. Pyzocha, DO, Bldg 1058, 1856 Irwin Dr, Fort Carson, CO 80913; [email protected].
1. McCullough L, Arora S. Diagnosis and treatment of hypothermia. Am Fam Physician. 2004;70:2325-2332.
2. Durrer B, Brugger H, Syme D; International Commission for Mountain Emergency Medicine. The medical on-site treatment of hypothermia: ICAR-MEDCOM recommendation. High Alt Med Biol. 2003;4.
3. Rischall ML, Rowland-Fisher A. Evidence-based management of accidental hypothermia in the emergency department. Emerg Med Pract. 2016;18:1-18.
4. Study: Hypothermia-related deaths—United States, 2003-2004. Atlanta, GA: Centers for Disease Control and Prevention; 2005. Available at: www.cdc.gov/media/pressrel/fs050224.htm. Accessed March 1, 2018.
5. Baumgartner EA, Belson M, Rubin C, et al. Hypothermia and other cold-related morbidity emergency department visits: United States, 1995-2004. Wilderness Environ Med. 2008;19:233-237.
6. Centers for Disease Control and Prevention. Preventing injuries associated with extreme cold. Int J Trauma Nurs. 2001;7:26-30.
7. Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992;10:311-327.
8. Mechem CC. Hypothermia and hyperthermia. In: Lanken PN, Manaker S, Hanson CW III, eds. The Intensive Care Unit Manual. Philadelphia: WB Saunders; 2000.
9. Weinberg AD. Hypothermia. Ann Emerg Med. 1993;22:370-377.
10. Zafren K, Giesbrecht GG, Danzl DF, et al. Wilderness Medical Society practice guidelines for the out-of-hospital evaluation and treatment of accidental hypothermia. Wilderness Environ Med. 2014;25:425-445.
11. Web-based integrated 2010 & 2015 guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 10: Special Circumstances of Resuscitation. Dallas, TX: American Heart Association; 2017. Available at: https://eccguidelines.heart.org/index.php/circulation/cpr-ecc-guidelines-2/part-10-special-circumstances-of-resuscitation. Accessed March 1, 2018.
12. Brown DJ, Brugger H, Boyd J, et al. Accidental hypothermia. N Engl J Med. 2012;367:1930-1938.
13. Petrone P, Asensio JA, Marini CP. Management of accidental hypothermia and cold injury. Curr Probl Surg. 2014;51:417-431.
14. Fudge J. Preventing and managing hypothermia and frostbite injury. Sports Health. 2016;8:133-139.
15. Martin RS, Kilgo PD, Miller PR, et al. Injury-associated hypothermia: an analysis of the 2004 National Trauma Data Bank. Shock. 2005;24:114-118.
CASE
Patrick S, an 85-year-old man with multiple medical problems, was brought to his primary care provider after being found at home with altered mental status. His caretaker reported that Mr. S had been using extra blankets in bed and sleeping more, but he hadn’t had significant outdoor exposure. Measurement of his vital signs revealed tachycardia, tachypnea, hypotension, and a rectal temperature of 32°C (89.6°F).
How would you proceed with the care of this patient?
What is accidental hypothermia?
Accidental hypothermia is an unintentional drop in core body temperature to <35°C (<95°F). Mild hypothermia is defined as a core body temperature of 32°C to 35°C (90°F - 95°F); moderate hypothermia, 28°C to 32°C (82°F - 90°F); and severe hypothermia, <28°C (<82°F).1
The International Commission for Mountain Emergency Medicine divides hypothermia into 5 categories, emphasizing the clinical features of each stage as a guide to treatment (TABLE 1).2 These categories were adopted to help prehospital rescuers estimate the severity of hypothermia using physical symptoms. For example, most patients stop shivering at approximately 30°C (86°F)—the “moderate (HT II)” category of hypothermia—although this response varies widely from patient to patient. Notably, there are reports in the literature of survival in hypothermia with a temperature as low as 13.7°C (56.7°F) and with cardiac arrest for as long as 8 hours and 40 minutes, although these events are rare.3
Each year, approximately 700 deaths in the United States are the result of hypothermia.4 Between 1995 to 2004 in the United States, it is estimated that 15,574 visits were made to a health care provider or facility for hypothermia and other cold-related concerns.5 Based on reports in the international literature, the incidence of nonlethal hypothermia is much greater than the incidence of lethal hypothermia.5 Almost half of deaths from hypothermia are in people older than age 65 years; the male to female ratio is 2.5:1.1
Variables that predispose the body to temperature dysregulation include extremes of age, comorbid conditions, intoxication, chronic cold exposure, immersion accident, mental illness, impaired shivering, and lack of acclimatization.1 The most common causes of death associated with hypothermia are falls, drownings, and cardiovascular disease.4 In a 2008 study, hypothermia and other cold-related morbidity emergency department (ED) visits required more transfers of patients to a critical care unit than any other reason for visiting an ED (risk ratio, 6.73; 95% confidence interval, 1.8-25).5 Mortality among inpatients whose hypothermia is classified as moderate or severe reaches as high as 40%.3
More than just cold-weather exposure
Accidental hypothermia occurs when heat loss is superseded by the body’s ability to generate heat. It commonly happens in cold environments but can also occur at higher temperatures if the body’s thermoregulatory system malfunctions.
Environmental or iatrogenic factors (ie, primary hypothermia), such as wind, water immersion, wetness, aggressive fluid resuscitation, and heat stroke treatment can make people more susceptible to hypothermia. Medical conditions (ie, secondary hypothermia), such as burns, exfoliative dermatitis, severe psoriasis, hypoadrenalism, hypopituitarism, hypothyroidism, acute spinal cord transection, head trauma, stroke, tumor, pneumonia, Wernicke’s disease (encephalopathy), and sepsis can also predispose to hypothermia.1 Drugs, such as ethanol, phenothiazines, and sedative–hypnotics may decrease the hypothermia threshold.1 (For information on preventing hypothermia, see TABLE 2.6)
Pathophysiology: The role of the hypothalamus
Humans maintain body temperature by balancing heat production and heat loss to the environment. Heat is lost through the skin and lungs by 5 different mechanisms: radiation, conduction, convection, evaporation, and respiration. Convective heat loss to cold air and conductive heat loss to water are the most common mechanisms of accidental hypothermia.7
To maintain temperature homeostasis at 37°C (98.6°F) (±0.5°C [±0.9°F]), the hypothalamus receives input from central and peripheral thermal receptors and stimulates heat production through shivering, increasing the basal metabolic rate 2-fold to 5-fold.1 The hypothalamus also increases thyroid, catecholamine, and adrenal activity to increase the body’s production of heat and raise core temperature.
Heat conservation occurs by activation of sympathetically mediated vasoconstriction, reducing conduction to the skin, where cooling is greatest. After time, temperature regulation in the body becomes overwhelmed and catecholamine levels return to a pre-hypothermic state.
At 35°C (95°F), neurologic function begins to decline; at 32°C (89.6°F), metabolism, ventilation, and cardiac output decrease until shivering ceases. Changes in peripheral blood flow can create a false warming sensation, causing a person to remove clothing, a phenomenon referred to as paradoxical undressing. As hypothermia progresses, the neurologic, respiratory, and cardiac systems continue to slow until there is eventual cardiorespiratory failure.
Assessment and diagnosis
History and physical examination. A high index of suspicion for the diagnosis of hypothermia is essential, especially when caring for the elderly or patients presenting with unexplained illness. Often, symptoms of a primary condition may overshadow those reflecting hypothermia. In a multicenter survey that reviewed 428 cases of accidental hypothermia in the United States, 44% of patients had an underlying illness; 18%, coexisting infection; 19%, trauma; and 6%, overdose.3
There are no strict diagnostic criteria for hypothermia other than a core body temperature <35°C (<95°F). Standard thermometers often do not read below 34.4°C (93.2°F), so it is recommended that a rectal thermometer capable of reading low body temperatures be used for accurate measurement.
Hypothermic patients can exhibit a variety of symptoms, depending on the degree of decrease in core body temperature1:
- A mildly hypothermic patient might present with any combination of tachypnea, tachycardia, ataxia, impaired judgment, shivering, and vasoconstriction.
- Moderate hypothermia typically manifests as a decreased heart rate, decreased blood pressure, decreased level of consciousness, decreased respiratory effort, dilated pupils, extinction of shivering, and hyporeflexia. Cardiac abnormalities, such as atrial fibrillation and junctional bradycardia, may be seen in moderate hypothermia.
- Severe hypothermia presents with apnea, coma, nonreactive pupils, oliguria, areflexia, hypotension, bradycardia, and continued cardiac abnormalities, such as ventricular arrhythmias and asystole.
Laboratory evaluation. No specific laboratory tests are needed to diagnose hypothermia. General lab tests, however, may help determine whether hypothermia is the result, or the cause, of the clinical scenario. Recommended laboratory tests for making that determination include a complete blood count (CBC), chemistry panel, arterial blood gases, fingerstick glucose, and coagulation panel.
Results of lab tests may be abnormal because of the body’s decreased core body temperature. White blood cells and platelets in the CBC, for example, may be decreased due to splenic sequestration; these findings reverse with rewarming. With every 1°C (1.8°F) drop in core body temperature, hematocrit increases 2%.3 Sodium, chloride, and magnesium concentrations do not display consistent abnormalities with any core body temperature >25°C (77°F),3,8 but potassium levels may fluctuate because of acid-base changes that occur during rewarming.1 Creatinine and creatine kinase levels may be increased secondary to rhabdomyolysis or acute tubular necrosis.1
Arterial blood gases typically show metabolic acidosis or respiratory alkalosis, or both.8 Prothrombin time and partial thromboplastin time are typically elevated in vivo, secondary to temperature-dependent enzymes in the coagulation cascade, but are reported normal in a blood specimen that is heated to 37°C (98.6°F) prior to analysis.1,8
Both hyperglycemia and hypoglycemia can be associated with hypothermia. The lactate level can be elevated, due to hypoperfusion. Hepatic impairment may be seen secondary to decreased cardiac output. An increase in the lipase level may also occur.3
When a hypothermic patient fails to respond to rewarming, or there is no clear source of cold exposure, consider testing for other causes of the problem, including hypothyroidism and adrenal insufficiency (see “Differential diagnosis”). Hypothermia may also decrease thyroid function in people with preexisting disease.
Other laboratory studies that can be considered include fibrinogen, blood-alcohol level, urine toxicology screen, and blood and fluid cultures.3
Imaging. Imaging studies are not performed routinely in the setting of hypothermia; however:
- Chest radiography can be considered to assess for aspiration pneumonia, vascular congestion, and pulmonary edema.
- Computed tomography (CT) of the head is helpful in the setting of trauma or if mental status does not clear with rewarming.3
- Bedside ultrasonography can assess for cardiac activity, volume status, pulmonary edema, free fluid, and trauma. (See "Point-of-care ultrasound: Coming soon to primary care?" J Fam Pract. 2018;67:70-80.)
Electrocardiography. An electrocardiogram is essential to evaluate for arrhythmias. Findings associated with hypothermia are prolongation of PR, QRS, and QT intervals; ST-segment elevation, T-wave inversion; and Osborn waves (J waves), which represent a positive deflection at the termination of the QRS complex with associated J-point elevation.8 Osborn waves generally present when the core body temperature is <32°C (89.6°F) and become larger as the core body temperature drops further.3
Differential diagnosis. Hypothermia is most commonly caused by environmental exposure, but the differential diagnosis is broad: many medical conditions, as well as drug and alcohol intoxication, can contribute to hypothermia (TABLE 31).
Treatment: Usually unnecessary, sometimes crucial
Most patients with mild hypothermia recover completely with little intervention. These patients should be evaluated for cognitive irregularities and observed in the ED before discharge.9 Moderate and severe hypothermia patients should be assessed using pre-hospital protocols and given cardiopulmonary resuscitation (CPR) for cardiac arrest. Pre-hospital providers should rely more on symptoms in guiding their treatment response because core body temperature measurements can be difficult to obtain, and the response to a drop in core body temperature varies from patient to patient.10
Early considerations: Airway, breathing, circulation (ABC)
A first responder might have difficulty palpating the pulse of a hypothermic patient if that patient’s cardiopulmonary effort is diminished.9 This inability to palpate a pulse should not delay treatment unless the patient presents with lethal injury; the scene is unsafe; the chest is too stiff for CPR; do-not-resuscitate status is present; or the patient was buried in an avalanche for ≥35 minutes and the airway is filled with snow (FIGURE3,11,12). Pulse should be checked carefully for 60 seconds. If pulses are not present, CPR should be initiated.
Prevention of further heat loss should begin promptly for hypothermic patients who retain a perfusing rhythm.11 Lifesaving interventions, such as airway management, vascular access for volume replenishment, and defibrillation for ventricular tachycardia or ventricular fibrillation should be carried out according to Advanced Cardiac Life Support protocols.11 Patients in respiratory distress or incapable of protecting their airway because of altered mental status should undergo endotracheal intubation. Fluid resuscitation with isotonic crystalloid fluids, warmed to 40°C (104°F) to 42°C (~107°F) and delivered through 2 large-bore, peripheral intravenous (IV) needles, can be considered.
Special care should be taken when moving a hypothermic patient. Excessive movement can lead to stimulation of the irritable hypothermic heart and cause an arrhythmia.
Medical therapy. Caution is advised because the reduced metabolism of a hypothermic patient can lead to potentially toxic accumulation of drugs peripherally. In fact, outcomes have not been positively influenced by routine use of medications, other than treatment of ventricular fibrillation with amiodarone.11 Any intravenous (IV) drug should be held until the patient’s core temperature is >30°C (>86°F).11
Vasopressors can be beneficial during rewarming for a patient in cardiac arrest and are a reasonable consideration.2 Nitroglycerin, in conjunction with active external rewarming, can increase the overall hourly temperature gain in a moderately hypothermic patient.13
Rewarming. The extent of rewarming required can be predicted by the severity of hypothermia (FIGURE3,11,12). Mildly hypothermic patients can generally be rewarmed using passive external measures. Patients with moderate hypothermia benefit from active rewarming in addition to passive measures. Intervention for severe hypothermia requires external rewarming and internal warming, with admission to the intensive care unit.
Treatment plans for severely hypothermic patients differ, depending on whether the person has a perfusing or nonperfusing cardiac rhythm. Patients who maintain a perfusing rhythm can be rewarmed using external methods (although core rewarming is used more often). Patients who do not have a perfusing rhythm require more invasive procedures.11 When using any rewarming method, afterdrop phenomenon can occur: ie, vasodilation, brought on by rewarming, causes a drop in core body temperature, as cooler peripheral blood returns to the central circulation. This effect may be reduced by focused rewarming of the trunk prior to rewarming the extremities.3
Rewarming for mild hypothermia patients begins with passive external techniques. First, the patient is moved away from the environment for protection from further exposure. Next, wet or damaged clothing is removed, blankets or foil insulators are applied, and room temperature is maintained at ≥28°C (82°F).3,11,13,14
If the patient’s temperature does not normalize, or if the patient presented with moderate or severe hypothermia, rewarming is continued with active external and internal measures. Active external rewarming can supplement passive measures using radiant heat from warmed blankets, air rewarming devices, and heating pads.3,13,14 Active internal rewarming techniques rely on invasive measures to raise the core temperature. Heated crystalloid IV fluids do not treat hypothermia, but do help reduce further heat loss and can be helpful in patients in need of volume resuscitation.3,13
Severely hypothermic patients might require more invasive active internal rewarming techniques, such as body-cavity lavage and extracorporeal methods. Body-cavity lavage can be facilitated with large volumes (10-120 L) of warm fluid at 40°C to 42°C, circulated through the thoracic or abdominal cavities to raise core body temperature 3°C to 6°C per hour.3,13
Extracorporeal rewarming can be achieved through hemodialysis, continuous arteriovenous rewarming (CAVR), continuous veno-venous rewarming (CVVR), or cardiopulmonary bypass.3,13 Research has shown cardiopulmonary bypass to be the most effective technique, with as high as a 7°C rise in core body temperature per hour; CVVR and CAVR are less invasive, however, and more readily available in hospitals.3,11,13
Rewarming interventions should continue until return of spontaneous circulation and core body temperature reaches 32°C (89.6°F) to 34°C (93.2°F).11 Overall, resuscitation efforts may take longer than normal due to the need for rewarming and should continue until the patient has achieved a normal temperature of 37°C (97.8°F).
Prognosis varies with severity, the health of the patient
In healthy, mildly hypothermic patients, full recovery is common if heat loss is minimized and the cause is treated. Moderately hypothermic patients who receive proper care can also have a favorable result. Outcomes for severe hypothermia vary with duration, comorbidities, and severity of core body temperature loss.15
Immediate initiation of rewarming by pre-hospital providers improves outcomes, and higher mortality has been demonstrated with hospital admission temperatures <35°C (95°F).15 Almost 100% of primary hypothermia patients with cardiac stability who were treated using active external and minimally invasive rewarming techniques survived with an intact neurologic system.12 Fifty percent of patients who endured cardiac arrest or who were treated with extracorporeal rewarming had an intact neurologic system. In cardiac arrest cases without significant underlying disease or trauma, and in which hypoxia did not precede hypothermia, full recovery is possible (and has been observed).12
CASE
Mr. S was given a diagnosis of mild to moderate hypothermia and transferred to the nearest ED for further treatment. His age had put him at increased risk of hypothermia. The work-up included laboratory testing (CBC, chemistry panel, thyroid-stimulating hormone, urinalysis, and blood cultures), electrocardiography, chest radiography, and CT of the head.
The chest radiograph showed pneumonia. Based on the results of blood culture, bacterial infection (pneumonia) was determined to be the underlying cause of hypothermia. Mr. S was started on antibiotics.
CORRESPONDENCE
Natasha J. Pyzocha, DO, Bldg 1058, 1856 Irwin Dr, Fort Carson, CO 80913; [email protected].
CASE
Patrick S, an 85-year-old man with multiple medical problems, was brought to his primary care provider after being found at home with altered mental status. His caretaker reported that Mr. S had been using extra blankets in bed and sleeping more, but he hadn’t had significant outdoor exposure. Measurement of his vital signs revealed tachycardia, tachypnea, hypotension, and a rectal temperature of 32°C (89.6°F).
How would you proceed with the care of this patient?
What is accidental hypothermia?
Accidental hypothermia is an unintentional drop in core body temperature to <35°C (<95°F). Mild hypothermia is defined as a core body temperature of 32°C to 35°C (90°F - 95°F); moderate hypothermia, 28°C to 32°C (82°F - 90°F); and severe hypothermia, <28°C (<82°F).1
The International Commission for Mountain Emergency Medicine divides hypothermia into 5 categories, emphasizing the clinical features of each stage as a guide to treatment (TABLE 1).2 These categories were adopted to help prehospital rescuers estimate the severity of hypothermia using physical symptoms. For example, most patients stop shivering at approximately 30°C (86°F)—the “moderate (HT II)” category of hypothermia—although this response varies widely from patient to patient. Notably, there are reports in the literature of survival in hypothermia with a temperature as low as 13.7°C (56.7°F) and with cardiac arrest for as long as 8 hours and 40 minutes, although these events are rare.3
Each year, approximately 700 deaths in the United States are the result of hypothermia.4 Between 1995 to 2004 in the United States, it is estimated that 15,574 visits were made to a health care provider or facility for hypothermia and other cold-related concerns.5 Based on reports in the international literature, the incidence of nonlethal hypothermia is much greater than the incidence of lethal hypothermia.5 Almost half of deaths from hypothermia are in people older than age 65 years; the male to female ratio is 2.5:1.1
Variables that predispose the body to temperature dysregulation include extremes of age, comorbid conditions, intoxication, chronic cold exposure, immersion accident, mental illness, impaired shivering, and lack of acclimatization.1 The most common causes of death associated with hypothermia are falls, drownings, and cardiovascular disease.4 In a 2008 study, hypothermia and other cold-related morbidity emergency department (ED) visits required more transfers of patients to a critical care unit than any other reason for visiting an ED (risk ratio, 6.73; 95% confidence interval, 1.8-25).5 Mortality among inpatients whose hypothermia is classified as moderate or severe reaches as high as 40%.3
More than just cold-weather exposure
Accidental hypothermia occurs when heat loss is superseded by the body’s ability to generate heat. It commonly happens in cold environments but can also occur at higher temperatures if the body’s thermoregulatory system malfunctions.
Environmental or iatrogenic factors (ie, primary hypothermia), such as wind, water immersion, wetness, aggressive fluid resuscitation, and heat stroke treatment can make people more susceptible to hypothermia. Medical conditions (ie, secondary hypothermia), such as burns, exfoliative dermatitis, severe psoriasis, hypoadrenalism, hypopituitarism, hypothyroidism, acute spinal cord transection, head trauma, stroke, tumor, pneumonia, Wernicke’s disease (encephalopathy), and sepsis can also predispose to hypothermia.1 Drugs, such as ethanol, phenothiazines, and sedative–hypnotics may decrease the hypothermia threshold.1 (For information on preventing hypothermia, see TABLE 2.6)
Pathophysiology: The role of the hypothalamus
Humans maintain body temperature by balancing heat production and heat loss to the environment. Heat is lost through the skin and lungs by 5 different mechanisms: radiation, conduction, convection, evaporation, and respiration. Convective heat loss to cold air and conductive heat loss to water are the most common mechanisms of accidental hypothermia.7
To maintain temperature homeostasis at 37°C (98.6°F) (±0.5°C [±0.9°F]), the hypothalamus receives input from central and peripheral thermal receptors and stimulates heat production through shivering, increasing the basal metabolic rate 2-fold to 5-fold.1 The hypothalamus also increases thyroid, catecholamine, and adrenal activity to increase the body’s production of heat and raise core temperature.
Heat conservation occurs by activation of sympathetically mediated vasoconstriction, reducing conduction to the skin, where cooling is greatest. After time, temperature regulation in the body becomes overwhelmed and catecholamine levels return to a pre-hypothermic state.
At 35°C (95°F), neurologic function begins to decline; at 32°C (89.6°F), metabolism, ventilation, and cardiac output decrease until shivering ceases. Changes in peripheral blood flow can create a false warming sensation, causing a person to remove clothing, a phenomenon referred to as paradoxical undressing. As hypothermia progresses, the neurologic, respiratory, and cardiac systems continue to slow until there is eventual cardiorespiratory failure.
Assessment and diagnosis
History and physical examination. A high index of suspicion for the diagnosis of hypothermia is essential, especially when caring for the elderly or patients presenting with unexplained illness. Often, symptoms of a primary condition may overshadow those reflecting hypothermia. In a multicenter survey that reviewed 428 cases of accidental hypothermia in the United States, 44% of patients had an underlying illness; 18%, coexisting infection; 19%, trauma; and 6%, overdose.3
There are no strict diagnostic criteria for hypothermia other than a core body temperature <35°C (<95°F). Standard thermometers often do not read below 34.4°C (93.2°F), so it is recommended that a rectal thermometer capable of reading low body temperatures be used for accurate measurement.
Hypothermic patients can exhibit a variety of symptoms, depending on the degree of decrease in core body temperature1:
- A mildly hypothermic patient might present with any combination of tachypnea, tachycardia, ataxia, impaired judgment, shivering, and vasoconstriction.
- Moderate hypothermia typically manifests as a decreased heart rate, decreased blood pressure, decreased level of consciousness, decreased respiratory effort, dilated pupils, extinction of shivering, and hyporeflexia. Cardiac abnormalities, such as atrial fibrillation and junctional bradycardia, may be seen in moderate hypothermia.
- Severe hypothermia presents with apnea, coma, nonreactive pupils, oliguria, areflexia, hypotension, bradycardia, and continued cardiac abnormalities, such as ventricular arrhythmias and asystole.
Laboratory evaluation. No specific laboratory tests are needed to diagnose hypothermia. General lab tests, however, may help determine whether hypothermia is the result, or the cause, of the clinical scenario. Recommended laboratory tests for making that determination include a complete blood count (CBC), chemistry panel, arterial blood gases, fingerstick glucose, and coagulation panel.
Results of lab tests may be abnormal because of the body’s decreased core body temperature. White blood cells and platelets in the CBC, for example, may be decreased due to splenic sequestration; these findings reverse with rewarming. With every 1°C (1.8°F) drop in core body temperature, hematocrit increases 2%.3 Sodium, chloride, and magnesium concentrations do not display consistent abnormalities with any core body temperature >25°C (77°F),3,8 but potassium levels may fluctuate because of acid-base changes that occur during rewarming.1 Creatinine and creatine kinase levels may be increased secondary to rhabdomyolysis or acute tubular necrosis.1
Arterial blood gases typically show metabolic acidosis or respiratory alkalosis, or both.8 Prothrombin time and partial thromboplastin time are typically elevated in vivo, secondary to temperature-dependent enzymes in the coagulation cascade, but are reported normal in a blood specimen that is heated to 37°C (98.6°F) prior to analysis.1,8
Both hyperglycemia and hypoglycemia can be associated with hypothermia. The lactate level can be elevated, due to hypoperfusion. Hepatic impairment may be seen secondary to decreased cardiac output. An increase in the lipase level may also occur.3
When a hypothermic patient fails to respond to rewarming, or there is no clear source of cold exposure, consider testing for other causes of the problem, including hypothyroidism and adrenal insufficiency (see “Differential diagnosis”). Hypothermia may also decrease thyroid function in people with preexisting disease.
Other laboratory studies that can be considered include fibrinogen, blood-alcohol level, urine toxicology screen, and blood and fluid cultures.3
Imaging. Imaging studies are not performed routinely in the setting of hypothermia; however:
- Chest radiography can be considered to assess for aspiration pneumonia, vascular congestion, and pulmonary edema.
- Computed tomography (CT) of the head is helpful in the setting of trauma or if mental status does not clear with rewarming.3
- Bedside ultrasonography can assess for cardiac activity, volume status, pulmonary edema, free fluid, and trauma. (See "Point-of-care ultrasound: Coming soon to primary care?" J Fam Pract. 2018;67:70-80.)
Electrocardiography. An electrocardiogram is essential to evaluate for arrhythmias. Findings associated with hypothermia are prolongation of PR, QRS, and QT intervals; ST-segment elevation, T-wave inversion; and Osborn waves (J waves), which represent a positive deflection at the termination of the QRS complex with associated J-point elevation.8 Osborn waves generally present when the core body temperature is <32°C (89.6°F) and become larger as the core body temperature drops further.3
Differential diagnosis. Hypothermia is most commonly caused by environmental exposure, but the differential diagnosis is broad: many medical conditions, as well as drug and alcohol intoxication, can contribute to hypothermia (TABLE 31).
Treatment: Usually unnecessary, sometimes crucial
Most patients with mild hypothermia recover completely with little intervention. These patients should be evaluated for cognitive irregularities and observed in the ED before discharge.9 Moderate and severe hypothermia patients should be assessed using pre-hospital protocols and given cardiopulmonary resuscitation (CPR) for cardiac arrest. Pre-hospital providers should rely more on symptoms in guiding their treatment response because core body temperature measurements can be difficult to obtain, and the response to a drop in core body temperature varies from patient to patient.10
Early considerations: Airway, breathing, circulation (ABC)
A first responder might have difficulty palpating the pulse of a hypothermic patient if that patient’s cardiopulmonary effort is diminished.9 This inability to palpate a pulse should not delay treatment unless the patient presents with lethal injury; the scene is unsafe; the chest is too stiff for CPR; do-not-resuscitate status is present; or the patient was buried in an avalanche for ≥35 minutes and the airway is filled with snow (FIGURE3,11,12). Pulse should be checked carefully for 60 seconds. If pulses are not present, CPR should be initiated.
Prevention of further heat loss should begin promptly for hypothermic patients who retain a perfusing rhythm.11 Lifesaving interventions, such as airway management, vascular access for volume replenishment, and defibrillation for ventricular tachycardia or ventricular fibrillation should be carried out according to Advanced Cardiac Life Support protocols.11 Patients in respiratory distress or incapable of protecting their airway because of altered mental status should undergo endotracheal intubation. Fluid resuscitation with isotonic crystalloid fluids, warmed to 40°C (104°F) to 42°C (~107°F) and delivered through 2 large-bore, peripheral intravenous (IV) needles, can be considered.
Special care should be taken when moving a hypothermic patient. Excessive movement can lead to stimulation of the irritable hypothermic heart and cause an arrhythmia.
Medical therapy. Caution is advised because the reduced metabolism of a hypothermic patient can lead to potentially toxic accumulation of drugs peripherally. In fact, outcomes have not been positively influenced by routine use of medications, other than treatment of ventricular fibrillation with amiodarone.11 Any intravenous (IV) drug should be held until the patient’s core temperature is >30°C (>86°F).11
Vasopressors can be beneficial during rewarming for a patient in cardiac arrest and are a reasonable consideration.2 Nitroglycerin, in conjunction with active external rewarming, can increase the overall hourly temperature gain in a moderately hypothermic patient.13
Rewarming. The extent of rewarming required can be predicted by the severity of hypothermia (FIGURE3,11,12). Mildly hypothermic patients can generally be rewarmed using passive external measures. Patients with moderate hypothermia benefit from active rewarming in addition to passive measures. Intervention for severe hypothermia requires external rewarming and internal warming, with admission to the intensive care unit.
Treatment plans for severely hypothermic patients differ, depending on whether the person has a perfusing or nonperfusing cardiac rhythm. Patients who maintain a perfusing rhythm can be rewarmed using external methods (although core rewarming is used more often). Patients who do not have a perfusing rhythm require more invasive procedures.11 When using any rewarming method, afterdrop phenomenon can occur: ie, vasodilation, brought on by rewarming, causes a drop in core body temperature, as cooler peripheral blood returns to the central circulation. This effect may be reduced by focused rewarming of the trunk prior to rewarming the extremities.3
Rewarming for mild hypothermia patients begins with passive external techniques. First, the patient is moved away from the environment for protection from further exposure. Next, wet or damaged clothing is removed, blankets or foil insulators are applied, and room temperature is maintained at ≥28°C (82°F).3,11,13,14
If the patient’s temperature does not normalize, or if the patient presented with moderate or severe hypothermia, rewarming is continued with active external and internal measures. Active external rewarming can supplement passive measures using radiant heat from warmed blankets, air rewarming devices, and heating pads.3,13,14 Active internal rewarming techniques rely on invasive measures to raise the core temperature. Heated crystalloid IV fluids do not treat hypothermia, but do help reduce further heat loss and can be helpful in patients in need of volume resuscitation.3,13
Severely hypothermic patients might require more invasive active internal rewarming techniques, such as body-cavity lavage and extracorporeal methods. Body-cavity lavage can be facilitated with large volumes (10-120 L) of warm fluid at 40°C to 42°C, circulated through the thoracic or abdominal cavities to raise core body temperature 3°C to 6°C per hour.3,13
Extracorporeal rewarming can be achieved through hemodialysis, continuous arteriovenous rewarming (CAVR), continuous veno-venous rewarming (CVVR), or cardiopulmonary bypass.3,13 Research has shown cardiopulmonary bypass to be the most effective technique, with as high as a 7°C rise in core body temperature per hour; CVVR and CAVR are less invasive, however, and more readily available in hospitals.3,11,13
Rewarming interventions should continue until return of spontaneous circulation and core body temperature reaches 32°C (89.6°F) to 34°C (93.2°F).11 Overall, resuscitation efforts may take longer than normal due to the need for rewarming and should continue until the patient has achieved a normal temperature of 37°C (97.8°F).
Prognosis varies with severity, the health of the patient
In healthy, mildly hypothermic patients, full recovery is common if heat loss is minimized and the cause is treated. Moderately hypothermic patients who receive proper care can also have a favorable result. Outcomes for severe hypothermia vary with duration, comorbidities, and severity of core body temperature loss.15
Immediate initiation of rewarming by pre-hospital providers improves outcomes, and higher mortality has been demonstrated with hospital admission temperatures <35°C (95°F).15 Almost 100% of primary hypothermia patients with cardiac stability who were treated using active external and minimally invasive rewarming techniques survived with an intact neurologic system.12 Fifty percent of patients who endured cardiac arrest or who were treated with extracorporeal rewarming had an intact neurologic system. In cardiac arrest cases without significant underlying disease or trauma, and in which hypoxia did not precede hypothermia, full recovery is possible (and has been observed).12
CASE
Mr. S was given a diagnosis of mild to moderate hypothermia and transferred to the nearest ED for further treatment. His age had put him at increased risk of hypothermia. The work-up included laboratory testing (CBC, chemistry panel, thyroid-stimulating hormone, urinalysis, and blood cultures), electrocardiography, chest radiography, and CT of the head.
The chest radiograph showed pneumonia. Based on the results of blood culture, bacterial infection (pneumonia) was determined to be the underlying cause of hypothermia. Mr. S was started on antibiotics.
CORRESPONDENCE
Natasha J. Pyzocha, DO, Bldg 1058, 1856 Irwin Dr, Fort Carson, CO 80913; [email protected].
1. McCullough L, Arora S. Diagnosis and treatment of hypothermia. Am Fam Physician. 2004;70:2325-2332.
2. Durrer B, Brugger H, Syme D; International Commission for Mountain Emergency Medicine. The medical on-site treatment of hypothermia: ICAR-MEDCOM recommendation. High Alt Med Biol. 2003;4.
3. Rischall ML, Rowland-Fisher A. Evidence-based management of accidental hypothermia in the emergency department. Emerg Med Pract. 2016;18:1-18.
4. Study: Hypothermia-related deaths—United States, 2003-2004. Atlanta, GA: Centers for Disease Control and Prevention; 2005. Available at: www.cdc.gov/media/pressrel/fs050224.htm. Accessed March 1, 2018.
5. Baumgartner EA, Belson M, Rubin C, et al. Hypothermia and other cold-related morbidity emergency department visits: United States, 1995-2004. Wilderness Environ Med. 2008;19:233-237.
6. Centers for Disease Control and Prevention. Preventing injuries associated with extreme cold. Int J Trauma Nurs. 2001;7:26-30.
7. Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992;10:311-327.
8. Mechem CC. Hypothermia and hyperthermia. In: Lanken PN, Manaker S, Hanson CW III, eds. The Intensive Care Unit Manual. Philadelphia: WB Saunders; 2000.
9. Weinberg AD. Hypothermia. Ann Emerg Med. 1993;22:370-377.
10. Zafren K, Giesbrecht GG, Danzl DF, et al. Wilderness Medical Society practice guidelines for the out-of-hospital evaluation and treatment of accidental hypothermia. Wilderness Environ Med. 2014;25:425-445.
11. Web-based integrated 2010 & 2015 guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 10: Special Circumstances of Resuscitation. Dallas, TX: American Heart Association; 2017. Available at: https://eccguidelines.heart.org/index.php/circulation/cpr-ecc-guidelines-2/part-10-special-circumstances-of-resuscitation. Accessed March 1, 2018.
12. Brown DJ, Brugger H, Boyd J, et al. Accidental hypothermia. N Engl J Med. 2012;367:1930-1938.
13. Petrone P, Asensio JA, Marini CP. Management of accidental hypothermia and cold injury. Curr Probl Surg. 2014;51:417-431.
14. Fudge J. Preventing and managing hypothermia and frostbite injury. Sports Health. 2016;8:133-139.
15. Martin RS, Kilgo PD, Miller PR, et al. Injury-associated hypothermia: an analysis of the 2004 National Trauma Data Bank. Shock. 2005;24:114-118.
1. McCullough L, Arora S. Diagnosis and treatment of hypothermia. Am Fam Physician. 2004;70:2325-2332.
2. Durrer B, Brugger H, Syme D; International Commission for Mountain Emergency Medicine. The medical on-site treatment of hypothermia: ICAR-MEDCOM recommendation. High Alt Med Biol. 2003;4.
3. Rischall ML, Rowland-Fisher A. Evidence-based management of accidental hypothermia in the emergency department. Emerg Med Pract. 2016;18:1-18.
4. Study: Hypothermia-related deaths—United States, 2003-2004. Atlanta, GA: Centers for Disease Control and Prevention; 2005. Available at: www.cdc.gov/media/pressrel/fs050224.htm. Accessed March 1, 2018.
5. Baumgartner EA, Belson M, Rubin C, et al. Hypothermia and other cold-related morbidity emergency department visits: United States, 1995-2004. Wilderness Environ Med. 2008;19:233-237.
6. Centers for Disease Control and Prevention. Preventing injuries associated with extreme cold. Int J Trauma Nurs. 2001;7:26-30.
7. Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992;10:311-327.
8. Mechem CC. Hypothermia and hyperthermia. In: Lanken PN, Manaker S, Hanson CW III, eds. The Intensive Care Unit Manual. Philadelphia: WB Saunders; 2000.
9. Weinberg AD. Hypothermia. Ann Emerg Med. 1993;22:370-377.
10. Zafren K, Giesbrecht GG, Danzl DF, et al. Wilderness Medical Society practice guidelines for the out-of-hospital evaluation and treatment of accidental hypothermia. Wilderness Environ Med. 2014;25:425-445.
11. Web-based integrated 2010 & 2015 guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 10: Special Circumstances of Resuscitation. Dallas, TX: American Heart Association; 2017. Available at: https://eccguidelines.heart.org/index.php/circulation/cpr-ecc-guidelines-2/part-10-special-circumstances-of-resuscitation. Accessed March 1, 2018.
12. Brown DJ, Brugger H, Boyd J, et al. Accidental hypothermia. N Engl J Med. 2012;367:1930-1938.
13. Petrone P, Asensio JA, Marini CP. Management of accidental hypothermia and cold injury. Curr Probl Surg. 2014;51:417-431.
14. Fudge J. Preventing and managing hypothermia and frostbite injury. Sports Health. 2016;8:133-139.
15. Martin RS, Kilgo PD, Miller PR, et al. Injury-associated hypothermia: an analysis of the 2004 National Trauma Data Bank. Shock. 2005;24:114-118.
PRACTICE RECOMMENDATIONS
› Measure the patient's temperature with a rectal thermometer capable of reading a temperature <35°C (<95°F) when hypothermia is suspected. C
› Begin prevention of further heat loss promptly for hypothermic patients who retain a perfusing rhythm. C
› Do not consider a patient dead until body temperature has normalized. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Biopsies for skin cancer detection: Dispelling the myths
Once it’s determined that a growth requires a biopsy, there is often uncertainty about which type of biopsy to perform. Insufficient knowledge of, and/or experience with, the various biopsy modalities may deter FPs from performing skin biopsies when they are indicated. To help fill the knowledge gaps and better position FPs to tackle skin cancer in its earliest stages, this article identifies and dispels 5 of the most common myths surrounding skin biopsies for the detection of basal and squamous cell carcinoma and melanoma.
MYTH #1
A punch biopsy is always preferred for suspected melanoma because it gets full depth.
A deep shave biopsy (saucerization)—not a punch biopsy—is usually the procedure of choice when biopsying a lesion suspected to be melanoma.2 The National Comprehensive Cancer Network (NCCN) "Melanoma Clinical Practice Guidelines in Oncology" state that an excisional biopsy (elliptical, punch, or saucerization) with a 1- to 3-mm margin is the preferred method of biopsy for suspected melanoma.3 However, a punch biopsy should be performed only if a 1- to 3-mm margin all around a suspected melanoma can be obtained. Otherwise, a saucerization or elliptical excision is preferred.3
The saucerization technique generally permits optimal sampling in terms of both the breadth and depth of the growth, providing the pathologist with sufficient tissue from both the epidermis and dermis (FIGURE 1).
Why are breadth/depth important? Breadth is important because showing the pathologist the epidermis (especially the edge) of a suspected melanocytic tumor allows for detection of pagetoid spread (upward movement through the epidermis) of melanocytes and of single melanocytes at the edge of a tumor. Single melanocytes at the edge of a tumor and pagetoid spread are histologic features of melanoma that help to distinguish these lesions from nevi, which tend to have nested melanocytes.2
Depth is important because it predicts prognosis and impacts management. For tumors 0.8 mm to 1 mm deep, a sentinel lymph node biopsy (SLNB) should be considered.3,4 Although the tumor depth threshold for a SLNB is still debated, most skin cancer experts in the United States agree that a melanoma thicker than 1 mm qualifies for this procedure. Some melanomas with high-risk features (such as ulceration) qualify for an SNLB even if they are <1 mm in depth.5 An SLNB provides prognostic information, and a positive SLNB directly affects staging.
[polldaddy:9990508]
Avoid partial biopsies. For tumors that have been partially biopsied with a punch or shallow shave biopsy, evaluation of the remaining neoplasm after subsequent excision leads to tumor upstaging in 21% of patients, with 10% qualifying for an SLNB.6 Thus, the goal should always be to obtain the entire depth of the tumor with the initial biopsy.
In addition, surgical margins are determined by primary tumor depth. To ensure a depth greater than 1 mm, aim to obtain a tissue specimen that is at least as thick as a dime (1.3 mm).
Because the goal is to avoid partial sampling, a challenge exists when the suspicious growth is large. Many melanomas are broader than a centimeter. And while punch biopsies ensure a depth of 1 mm or more, they risk missing the thickest portion of the tumor.7
Partial sampling of large melanocytic tumors with punch biopsies can lead to sampling error.8 Ng et al9 found there was a significant increase in histopathologic misdiagnosis with a punch biopsy of part of a melanoma (odds ratio [OR]=16.6; 95% confidence interval [CI], 10-27; P<.001) and with shallow shave biopsy (OR=2.6; 95% CI, 1.2-5.7; P=.02) compared with excisional biopsy (including saucerization).9 Punch biopsy of part of a melanoma was also associated with increased odds of misdiagnosis with an adverse outcome (OR=20; 95% CI, 10-41; P<.001).
Punch biopsies do, however, offer a reasonable alternative when the melanoma is too broad for a complete saucerization. In these cases, consider multiple 4- to 6-mm punch biopsies to reduce the risk of sampling error.
Avoid performing punch biopsies <4 mm, as the breadth of tissue is inadequate. For example, even with dermoscopy, facial lentigo maligna melanoma is often difficult to differentiate from pigmented actinic keratosis and solar lentigines. (See JFP’s Watch and Learn Video on dermoscopy.) A broad shave biopsy is the preferred method of biopsy for lentigo maligna melanoma in situ according to the NCCN.3 And there have been several reports showing that the results of shave biopsies of melanocytic lesions are cosmetically acceptable to patients.10,11
If the biopsy confirms malignancy, a larger surgery with suturing will be needed. The most important issue to keep in mind is that if partial sampling leads to a benign diagnosis of a suspicious lesion, then the remainder of the lesion must be excised and sent for pathology.
Saucerization is also the preferred biopsy type for basal cell and squamous cell carcinomas (SCCs). Studies have shown tumor depth is the most important factor in predicting metastasis of SCC, as well as tumor relapse rate, making accurate identification of the depth of the tumor important for both management and prognosis.12,13 Determining the thickness of the SCC is important for guiding management. SCC in situ is more amenable than invasive SCC to topical therapy or electrodesiccation and curettage.
What you’ll need. FPs can perform saucerization quickly and easily in the office during a standard 15-minute visit. Of course, it is essential to have all the necessary materials available. The key materials needed are lidocaine and epinephrine, a sharp razor blade such as a DermaBlade, and something for hemostasis (aluminum chloride and/or an electrosurgical instrument). Cotton-tipped applicators to apply the aluminum chloride and needles and syringes to administer the local anesthetic are also needed. (See JFP’s Watch and Learn Video on shave biopsy.) A quick saucerization eliminates the need for the patient to return for an elliptical excision and prevents a delayed diagnosis that can occur as a result of a long wait to see a dermatologist.
As a final note, the pathology order form should be completed with information on biopsy type, clinical presentation, differential diagnosis, and whether or not the full lesion was excised.
MYTH #2
A wide excisional biopsy is required for a suspected melanoma.
While complete excision of the entire tumor does allow the pathologist to evaluate the entire growth, wide (>3 mm) margins on the initial biopsy are not necessary. In fact, there are potential disadvantages to full excisional biopsy.
For example, seborrheic keratoses and other benign growths can mimic melanoma. Neither the physician nor the patient wants to learn that a large elliptical wound was created for a growth that turned out to be a benign seborrheic keratosis. Saucerization provides the pathologist with the entire lesion, and the resulting shallow wound heals as a round scar that is most often acceptable to patients.10,11,14 In addition, excisional biopsies carry a higher risk of infection than does saucerization.
Even when the index of suspicion is high for melanoma, a wide margin is not indicated. NCCN guidelines suggest that the margins around a suspected melanoma on initial biopsy not exceed 3 mm to avoid disrupting the accuracy of an SLNB (FIGURE 2).3
In addition, time constraints (elliptical excisional biopsies can take up to one hour, especially when a layered closure is performed) and a lack of surgical training may prohibit FPs from performing excisions.
One study found that while dermatologists prefer shave biopsies (80.5%), surgeons prefer excisional biopsies (46.3%) and primary care physicians prefer punch biopsies (44%) for biopsy of a growth suspicious for melanoma.7 In fact, of the biopsies FPs perform, only 29% are of the shave variety.7
However, deep shave biopsies can be performed quickly, with the whole process taking less than 5 minutes. We advocate performing them at the time of presentation, as the evidence shows that deep shave biopsies of suspected melanoma are reliable and accurate in 97% of cases.15
MYTH #3
A partial biopsy can make the cancer spread.
There is no evidence to support that a partial biopsy has any effect on the local recurrence or metastatic potential of malignant melanoma.16 In fact, a biopsy elicits an inflammatory response that activates the patient’s immune system and often causes tumor lysis. Some tumors may even resolve after biopsy. In our clinical practice, we have had several cases of basal cell carcinoma resolve after a biopsy without additional treatment.
MYTH #4
If after performing a deep shave biopsy, tumor or pigment remains, you must leave it because a second biopsy specimen can’t be added to the first.
If pigment is visible after an initial shave or punch biopsy, it is reasonable to obtain additional tissue from the base of the biopsy site. While the deeper tissue cannot be added to the initial specimen for the purposes of Breslow’s depth, it is still helpful for the pathologist to have the sample so that he or she can analyze the tumor cells in the dermis. (Melanoma tumor depth is measured as the maximum distance between malignant cells and the top of the granular layer.17) In these situations, be sure to let the pathologist know that there are 2 specimens in the container.
In general, it is valuable to get as much of the tissue as possible at the time of the initial biopsy. One way to avoid leaving tumor at the base of the biopsy is to look at
MYTH #5
Epinephrine cannot be used for biopsies on the fingers, toes, nose, or penis.
Lidocaine with epinephrine is safe to use in areas with end-arteries, such as the fingers, toes, nose (FIGURE 4), and penis. There is no evidence to support the notion that local anesthesia with vasoconstriction can cause necrosis in these areas, and no case of necrosis has been reported since the introduction of commercial lidocaine with epinephrine in 1948.18
In addition to an absence of complications, epinephrine supplementation results in a relatively bloodless operating field and longer effectiveness of local anesthesia, as a study of more than 10,000 ear and nose surgeries using epinephrine-supplemented local anesthetics showed.19 The relative absence of blood in the operating field significantly reduces the duration of surgery and increases the healing rate because less electrocautery is needed.19
Similarly, the addition of epinephrine in digital blocks minimizes the need for tourniquets and large volumes of anesthetic and provides better and longer pain control during procedures.20 This topic was addressed by Prabhakar et al in a Cochrane Review in 2015.21 While digital surgeries are common, there were only 4 randomized controlled studies addressing the use of epinephrine in digital blocks. In these studies, there were no reports of adverse events, such as ischemia distal to the injection site. Evidence suggests that epinephrine in digital blocks can even be used safely in patients with vascular disease.22
And while the use of epinephrine with lidocaine in sites with end-arteries is beneficial for hemostasis and does not seem to pose a risk of ischemia, it is prudent to use the smallest volume of epinephrine (with lidocaine) needed to achieve anesthesia for the site.
CORRESPONDENCE
Elizabeth V. Seiverling, MD, 300 Southborough Drive, Suite 201, South Portland, ME 04106; [email protected].
1. Kerr OA, Tidman MJ, Walker JJ, et al. The profile of dermatological problems in primary care. Clin Exp Dermatol. 2010;35:380-383.
2. Hosler GA, Patterson JW. Lentigines, nevi, and melanomas. In: Patterson JW, ed. Weedon’s Skin Pathology. Elsevier; 2015:32,837-901.
3. Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. J Natl Compr Canc Netw. 2009;7:250-275.
4. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge S, Greene F, et al, eds. AJCC Cancer Staging Manual. Springer International Publishing; 2017;8:563-585.
5. American Joint Committee on Cancer. Implementation of AJCC 8th Edition Cancer Staging System. Available at: https://cancerstaging.org/About/news/Pages/Implementation-of-AJCC-8th-Edition-Cancer-Staging-System.aspx. Accessed April 2, 2018.
6. Karimipour DJ, Schwartz JL, Wang TS, et al. Microstaging accuracy after subtotal incisional biopsy of cutaneous melanoma. J Am Acad Dermatol. 2005;52:798-802.
7. Kaiser S, Vassell R, Pinckney RG, et al. Clinical impact of biopsy method on the quality of surgical management in melanoma. J Surg Oncol. 2014;109:775-779.
8. Montgomery BD, Sadler GM. Punch biopsy of pigmented lesions is potentially hazardous. Can Fam Physician. 2009;55:24.
9. Ng JC, Swain S, Dowling JP, et al. The impact of partial biopsy on histopathologic diagnosis of cutaneous melanoma: experience of an Australian tertiary referral service. Arch Dermatol. 2010;146:234-239.
10. Gambichler T, Senger E, Rapp S, et al. Deep shave excision of macular melanocytic nevi with the razor blade biopsy technique. Dermatol Surg. 2000;26:662-666.
11. Ferrandiz L, Moreno-Ramirez D, Camacho FM. Shave excision of common acquired melanocytic nevi: cosmetic outcome, recurrences, and complications. Dermatol Surg. 2005;31(9 Pt 1):1112-1115.
12. D'souza G, Carey TE, William WN Jr, et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J Acquir Immune Defic Syndr. 2014;65:603-610.
13. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer; 2010.
14. Elston DM, Stratman EJ, Miller SJ, et al. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
15. Zager JS, Hochwald SN, Marzban SS, et al. Shave biopsy is a safe and accurate method for the initial evaluation of melanoma. J Am Coll Surg. 2011;212:454-460.
16. Chanda JJ, Callen JP. Adverse effect of melanoma incision. J Am Acad Dermatol. 1985;13:519-522.
17. Noroozi N, Zakerolhosseini A. Computerized measurement of melanocytic tumor depth in skin histopathological images. Micron. 2015;77:44-56.
18. Nielsen LJ, Lumholt P, Hölmich LR. [Local anaesthesia with vasoconstrictor is safe to use in areas with end-arteries in fingers, toes, noses and ears]. Ugeskr Laeger. 2014;176(44).
19. Häfner HM, Röcken M, Breuninger H. Epinephrine-supplemented local anesthetics for ear and nose surgery: clinical use without complications in more than 10,000 surgical procedures. J Dtsch Dermatol Ges. 2005;3:195-199.
20. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
21. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
22. Ilicki J. Safety of epinephrine in digital nerve blocks: a literature review. J Emerg Med. 2015;49:799-809.
Once it’s determined that a growth requires a biopsy, there is often uncertainty about which type of biopsy to perform. Insufficient knowledge of, and/or experience with, the various biopsy modalities may deter FPs from performing skin biopsies when they are indicated. To help fill the knowledge gaps and better position FPs to tackle skin cancer in its earliest stages, this article identifies and dispels 5 of the most common myths surrounding skin biopsies for the detection of basal and squamous cell carcinoma and melanoma.
MYTH #1
A punch biopsy is always preferred for suspected melanoma because it gets full depth.
A deep shave biopsy (saucerization)—not a punch biopsy—is usually the procedure of choice when biopsying a lesion suspected to be melanoma.2 The National Comprehensive Cancer Network (NCCN) "Melanoma Clinical Practice Guidelines in Oncology" state that an excisional biopsy (elliptical, punch, or saucerization) with a 1- to 3-mm margin is the preferred method of biopsy for suspected melanoma.3 However, a punch biopsy should be performed only if a 1- to 3-mm margin all around a suspected melanoma can be obtained. Otherwise, a saucerization or elliptical excision is preferred.3
The saucerization technique generally permits optimal sampling in terms of both the breadth and depth of the growth, providing the pathologist with sufficient tissue from both the epidermis and dermis (FIGURE 1).
Why are breadth/depth important? Breadth is important because showing the pathologist the epidermis (especially the edge) of a suspected melanocytic tumor allows for detection of pagetoid spread (upward movement through the epidermis) of melanocytes and of single melanocytes at the edge of a tumor. Single melanocytes at the edge of a tumor and pagetoid spread are histologic features of melanoma that help to distinguish these lesions from nevi, which tend to have nested melanocytes.2
Depth is important because it predicts prognosis and impacts management. For tumors 0.8 mm to 1 mm deep, a sentinel lymph node biopsy (SLNB) should be considered.3,4 Although the tumor depth threshold for a SLNB is still debated, most skin cancer experts in the United States agree that a melanoma thicker than 1 mm qualifies for this procedure. Some melanomas with high-risk features (such as ulceration) qualify for an SNLB even if they are <1 mm in depth.5 An SLNB provides prognostic information, and a positive SLNB directly affects staging.
[polldaddy:9990508]
Avoid partial biopsies. For tumors that have been partially biopsied with a punch or shallow shave biopsy, evaluation of the remaining neoplasm after subsequent excision leads to tumor upstaging in 21% of patients, with 10% qualifying for an SLNB.6 Thus, the goal should always be to obtain the entire depth of the tumor with the initial biopsy.
In addition, surgical margins are determined by primary tumor depth. To ensure a depth greater than 1 mm, aim to obtain a tissue specimen that is at least as thick as a dime (1.3 mm).
Because the goal is to avoid partial sampling, a challenge exists when the suspicious growth is large. Many melanomas are broader than a centimeter. And while punch biopsies ensure a depth of 1 mm or more, they risk missing the thickest portion of the tumor.7
Partial sampling of large melanocytic tumors with punch biopsies can lead to sampling error.8 Ng et al9 found there was a significant increase in histopathologic misdiagnosis with a punch biopsy of part of a melanoma (odds ratio [OR]=16.6; 95% confidence interval [CI], 10-27; P<.001) and with shallow shave biopsy (OR=2.6; 95% CI, 1.2-5.7; P=.02) compared with excisional biopsy (including saucerization).9 Punch biopsy of part of a melanoma was also associated with increased odds of misdiagnosis with an adverse outcome (OR=20; 95% CI, 10-41; P<.001).
Punch biopsies do, however, offer a reasonable alternative when the melanoma is too broad for a complete saucerization. In these cases, consider multiple 4- to 6-mm punch biopsies to reduce the risk of sampling error.
Avoid performing punch biopsies <4 mm, as the breadth of tissue is inadequate. For example, even with dermoscopy, facial lentigo maligna melanoma is often difficult to differentiate from pigmented actinic keratosis and solar lentigines. (See JFP’s Watch and Learn Video on dermoscopy.) A broad shave biopsy is the preferred method of biopsy for lentigo maligna melanoma in situ according to the NCCN.3 And there have been several reports showing that the results of shave biopsies of melanocytic lesions are cosmetically acceptable to patients.10,11
If the biopsy confirms malignancy, a larger surgery with suturing will be needed. The most important issue to keep in mind is that if partial sampling leads to a benign diagnosis of a suspicious lesion, then the remainder of the lesion must be excised and sent for pathology.
Saucerization is also the preferred biopsy type for basal cell and squamous cell carcinomas (SCCs). Studies have shown tumor depth is the most important factor in predicting metastasis of SCC, as well as tumor relapse rate, making accurate identification of the depth of the tumor important for both management and prognosis.12,13 Determining the thickness of the SCC is important for guiding management. SCC in situ is more amenable than invasive SCC to topical therapy or electrodesiccation and curettage.
What you’ll need. FPs can perform saucerization quickly and easily in the office during a standard 15-minute visit. Of course, it is essential to have all the necessary materials available. The key materials needed are lidocaine and epinephrine, a sharp razor blade such as a DermaBlade, and something for hemostasis (aluminum chloride and/or an electrosurgical instrument). Cotton-tipped applicators to apply the aluminum chloride and needles and syringes to administer the local anesthetic are also needed. (See JFP’s Watch and Learn Video on shave biopsy.) A quick saucerization eliminates the need for the patient to return for an elliptical excision and prevents a delayed diagnosis that can occur as a result of a long wait to see a dermatologist.
As a final note, the pathology order form should be completed with information on biopsy type, clinical presentation, differential diagnosis, and whether or not the full lesion was excised.
MYTH #2
A wide excisional biopsy is required for a suspected melanoma.
While complete excision of the entire tumor does allow the pathologist to evaluate the entire growth, wide (>3 mm) margins on the initial biopsy are not necessary. In fact, there are potential disadvantages to full excisional biopsy.
For example, seborrheic keratoses and other benign growths can mimic melanoma. Neither the physician nor the patient wants to learn that a large elliptical wound was created for a growth that turned out to be a benign seborrheic keratosis. Saucerization provides the pathologist with the entire lesion, and the resulting shallow wound heals as a round scar that is most often acceptable to patients.10,11,14 In addition, excisional biopsies carry a higher risk of infection than does saucerization.
Even when the index of suspicion is high for melanoma, a wide margin is not indicated. NCCN guidelines suggest that the margins around a suspected melanoma on initial biopsy not exceed 3 mm to avoid disrupting the accuracy of an SLNB (FIGURE 2).3
In addition, time constraints (elliptical excisional biopsies can take up to one hour, especially when a layered closure is performed) and a lack of surgical training may prohibit FPs from performing excisions.
One study found that while dermatologists prefer shave biopsies (80.5%), surgeons prefer excisional biopsies (46.3%) and primary care physicians prefer punch biopsies (44%) for biopsy of a growth suspicious for melanoma.7 In fact, of the biopsies FPs perform, only 29% are of the shave variety.7
However, deep shave biopsies can be performed quickly, with the whole process taking less than 5 minutes. We advocate performing them at the time of presentation, as the evidence shows that deep shave biopsies of suspected melanoma are reliable and accurate in 97% of cases.15
MYTH #3
A partial biopsy can make the cancer spread.
There is no evidence to support that a partial biopsy has any effect on the local recurrence or metastatic potential of malignant melanoma.16 In fact, a biopsy elicits an inflammatory response that activates the patient’s immune system and often causes tumor lysis. Some tumors may even resolve after biopsy. In our clinical practice, we have had several cases of basal cell carcinoma resolve after a biopsy without additional treatment.
MYTH #4
If after performing a deep shave biopsy, tumor or pigment remains, you must leave it because a second biopsy specimen can’t be added to the first.
If pigment is visible after an initial shave or punch biopsy, it is reasonable to obtain additional tissue from the base of the biopsy site. While the deeper tissue cannot be added to the initial specimen for the purposes of Breslow’s depth, it is still helpful for the pathologist to have the sample so that he or she can analyze the tumor cells in the dermis. (Melanoma tumor depth is measured as the maximum distance between malignant cells and the top of the granular layer.17) In these situations, be sure to let the pathologist know that there are 2 specimens in the container.
In general, it is valuable to get as much of the tissue as possible at the time of the initial biopsy. One way to avoid leaving tumor at the base of the biopsy is to look at
MYTH #5
Epinephrine cannot be used for biopsies on the fingers, toes, nose, or penis.
Lidocaine with epinephrine is safe to use in areas with end-arteries, such as the fingers, toes, nose (FIGURE 4), and penis. There is no evidence to support the notion that local anesthesia with vasoconstriction can cause necrosis in these areas, and no case of necrosis has been reported since the introduction of commercial lidocaine with epinephrine in 1948.18
In addition to an absence of complications, epinephrine supplementation results in a relatively bloodless operating field and longer effectiveness of local anesthesia, as a study of more than 10,000 ear and nose surgeries using epinephrine-supplemented local anesthetics showed.19 The relative absence of blood in the operating field significantly reduces the duration of surgery and increases the healing rate because less electrocautery is needed.19
Similarly, the addition of epinephrine in digital blocks minimizes the need for tourniquets and large volumes of anesthetic and provides better and longer pain control during procedures.20 This topic was addressed by Prabhakar et al in a Cochrane Review in 2015.21 While digital surgeries are common, there were only 4 randomized controlled studies addressing the use of epinephrine in digital blocks. In these studies, there were no reports of adverse events, such as ischemia distal to the injection site. Evidence suggests that epinephrine in digital blocks can even be used safely in patients with vascular disease.22
And while the use of epinephrine with lidocaine in sites with end-arteries is beneficial for hemostasis and does not seem to pose a risk of ischemia, it is prudent to use the smallest volume of epinephrine (with lidocaine) needed to achieve anesthesia for the site.
CORRESPONDENCE
Elizabeth V. Seiverling, MD, 300 Southborough Drive, Suite 201, South Portland, ME 04106; [email protected].
Once it’s determined that a growth requires a biopsy, there is often uncertainty about which type of biopsy to perform. Insufficient knowledge of, and/or experience with, the various biopsy modalities may deter FPs from performing skin biopsies when they are indicated. To help fill the knowledge gaps and better position FPs to tackle skin cancer in its earliest stages, this article identifies and dispels 5 of the most common myths surrounding skin biopsies for the detection of basal and squamous cell carcinoma and melanoma.
MYTH #1
A punch biopsy is always preferred for suspected melanoma because it gets full depth.
A deep shave biopsy (saucerization)—not a punch biopsy—is usually the procedure of choice when biopsying a lesion suspected to be melanoma.2 The National Comprehensive Cancer Network (NCCN) "Melanoma Clinical Practice Guidelines in Oncology" state that an excisional biopsy (elliptical, punch, or saucerization) with a 1- to 3-mm margin is the preferred method of biopsy for suspected melanoma.3 However, a punch biopsy should be performed only if a 1- to 3-mm margin all around a suspected melanoma can be obtained. Otherwise, a saucerization or elliptical excision is preferred.3
The saucerization technique generally permits optimal sampling in terms of both the breadth and depth of the growth, providing the pathologist with sufficient tissue from both the epidermis and dermis (FIGURE 1).
Why are breadth/depth important? Breadth is important because showing the pathologist the epidermis (especially the edge) of a suspected melanocytic tumor allows for detection of pagetoid spread (upward movement through the epidermis) of melanocytes and of single melanocytes at the edge of a tumor. Single melanocytes at the edge of a tumor and pagetoid spread are histologic features of melanoma that help to distinguish these lesions from nevi, which tend to have nested melanocytes.2
Depth is important because it predicts prognosis and impacts management. For tumors 0.8 mm to 1 mm deep, a sentinel lymph node biopsy (SLNB) should be considered.3,4 Although the tumor depth threshold for a SLNB is still debated, most skin cancer experts in the United States agree that a melanoma thicker than 1 mm qualifies for this procedure. Some melanomas with high-risk features (such as ulceration) qualify for an SNLB even if they are <1 mm in depth.5 An SLNB provides prognostic information, and a positive SLNB directly affects staging.
[polldaddy:9990508]
Avoid partial biopsies. For tumors that have been partially biopsied with a punch or shallow shave biopsy, evaluation of the remaining neoplasm after subsequent excision leads to tumor upstaging in 21% of patients, with 10% qualifying for an SLNB.6 Thus, the goal should always be to obtain the entire depth of the tumor with the initial biopsy.
In addition, surgical margins are determined by primary tumor depth. To ensure a depth greater than 1 mm, aim to obtain a tissue specimen that is at least as thick as a dime (1.3 mm).
Because the goal is to avoid partial sampling, a challenge exists when the suspicious growth is large. Many melanomas are broader than a centimeter. And while punch biopsies ensure a depth of 1 mm or more, they risk missing the thickest portion of the tumor.7
Partial sampling of large melanocytic tumors with punch biopsies can lead to sampling error.8 Ng et al9 found there was a significant increase in histopathologic misdiagnosis with a punch biopsy of part of a melanoma (odds ratio [OR]=16.6; 95% confidence interval [CI], 10-27; P<.001) and with shallow shave biopsy (OR=2.6; 95% CI, 1.2-5.7; P=.02) compared with excisional biopsy (including saucerization).9 Punch biopsy of part of a melanoma was also associated with increased odds of misdiagnosis with an adverse outcome (OR=20; 95% CI, 10-41; P<.001).
Punch biopsies do, however, offer a reasonable alternative when the melanoma is too broad for a complete saucerization. In these cases, consider multiple 4- to 6-mm punch biopsies to reduce the risk of sampling error.
Avoid performing punch biopsies <4 mm, as the breadth of tissue is inadequate. For example, even with dermoscopy, facial lentigo maligna melanoma is often difficult to differentiate from pigmented actinic keratosis and solar lentigines. (See JFP’s Watch and Learn Video on dermoscopy.) A broad shave biopsy is the preferred method of biopsy for lentigo maligna melanoma in situ according to the NCCN.3 And there have been several reports showing that the results of shave biopsies of melanocytic lesions are cosmetically acceptable to patients.10,11
If the biopsy confirms malignancy, a larger surgery with suturing will be needed. The most important issue to keep in mind is that if partial sampling leads to a benign diagnosis of a suspicious lesion, then the remainder of the lesion must be excised and sent for pathology.
Saucerization is also the preferred biopsy type for basal cell and squamous cell carcinomas (SCCs). Studies have shown tumor depth is the most important factor in predicting metastasis of SCC, as well as tumor relapse rate, making accurate identification of the depth of the tumor important for both management and prognosis.12,13 Determining the thickness of the SCC is important for guiding management. SCC in situ is more amenable than invasive SCC to topical therapy or electrodesiccation and curettage.
What you’ll need. FPs can perform saucerization quickly and easily in the office during a standard 15-minute visit. Of course, it is essential to have all the necessary materials available. The key materials needed are lidocaine and epinephrine, a sharp razor blade such as a DermaBlade, and something for hemostasis (aluminum chloride and/or an electrosurgical instrument). Cotton-tipped applicators to apply the aluminum chloride and needles and syringes to administer the local anesthetic are also needed. (See JFP’s Watch and Learn Video on shave biopsy.) A quick saucerization eliminates the need for the patient to return for an elliptical excision and prevents a delayed diagnosis that can occur as a result of a long wait to see a dermatologist.
As a final note, the pathology order form should be completed with information on biopsy type, clinical presentation, differential diagnosis, and whether or not the full lesion was excised.
MYTH #2
A wide excisional biopsy is required for a suspected melanoma.
While complete excision of the entire tumor does allow the pathologist to evaluate the entire growth, wide (>3 mm) margins on the initial biopsy are not necessary. In fact, there are potential disadvantages to full excisional biopsy.
For example, seborrheic keratoses and other benign growths can mimic melanoma. Neither the physician nor the patient wants to learn that a large elliptical wound was created for a growth that turned out to be a benign seborrheic keratosis. Saucerization provides the pathologist with the entire lesion, and the resulting shallow wound heals as a round scar that is most often acceptable to patients.10,11,14 In addition, excisional biopsies carry a higher risk of infection than does saucerization.
Even when the index of suspicion is high for melanoma, a wide margin is not indicated. NCCN guidelines suggest that the margins around a suspected melanoma on initial biopsy not exceed 3 mm to avoid disrupting the accuracy of an SLNB (FIGURE 2).3
In addition, time constraints (elliptical excisional biopsies can take up to one hour, especially when a layered closure is performed) and a lack of surgical training may prohibit FPs from performing excisions.
One study found that while dermatologists prefer shave biopsies (80.5%), surgeons prefer excisional biopsies (46.3%) and primary care physicians prefer punch biopsies (44%) for biopsy of a growth suspicious for melanoma.7 In fact, of the biopsies FPs perform, only 29% are of the shave variety.7
However, deep shave biopsies can be performed quickly, with the whole process taking less than 5 minutes. We advocate performing them at the time of presentation, as the evidence shows that deep shave biopsies of suspected melanoma are reliable and accurate in 97% of cases.15
MYTH #3
A partial biopsy can make the cancer spread.
There is no evidence to support that a partial biopsy has any effect on the local recurrence or metastatic potential of malignant melanoma.16 In fact, a biopsy elicits an inflammatory response that activates the patient’s immune system and often causes tumor lysis. Some tumors may even resolve after biopsy. In our clinical practice, we have had several cases of basal cell carcinoma resolve after a biopsy without additional treatment.
MYTH #4
If after performing a deep shave biopsy, tumor or pigment remains, you must leave it because a second biopsy specimen can’t be added to the first.
If pigment is visible after an initial shave or punch biopsy, it is reasonable to obtain additional tissue from the base of the biopsy site. While the deeper tissue cannot be added to the initial specimen for the purposes of Breslow’s depth, it is still helpful for the pathologist to have the sample so that he or she can analyze the tumor cells in the dermis. (Melanoma tumor depth is measured as the maximum distance between malignant cells and the top of the granular layer.17) In these situations, be sure to let the pathologist know that there are 2 specimens in the container.
In general, it is valuable to get as much of the tissue as possible at the time of the initial biopsy. One way to avoid leaving tumor at the base of the biopsy is to look at
MYTH #5
Epinephrine cannot be used for biopsies on the fingers, toes, nose, or penis.
Lidocaine with epinephrine is safe to use in areas with end-arteries, such as the fingers, toes, nose (FIGURE 4), and penis. There is no evidence to support the notion that local anesthesia with vasoconstriction can cause necrosis in these areas, and no case of necrosis has been reported since the introduction of commercial lidocaine with epinephrine in 1948.18
In addition to an absence of complications, epinephrine supplementation results in a relatively bloodless operating field and longer effectiveness of local anesthesia, as a study of more than 10,000 ear and nose surgeries using epinephrine-supplemented local anesthetics showed.19 The relative absence of blood in the operating field significantly reduces the duration of surgery and increases the healing rate because less electrocautery is needed.19
Similarly, the addition of epinephrine in digital blocks minimizes the need for tourniquets and large volumes of anesthetic and provides better and longer pain control during procedures.20 This topic was addressed by Prabhakar et al in a Cochrane Review in 2015.21 While digital surgeries are common, there were only 4 randomized controlled studies addressing the use of epinephrine in digital blocks. In these studies, there were no reports of adverse events, such as ischemia distal to the injection site. Evidence suggests that epinephrine in digital blocks can even be used safely in patients with vascular disease.22
And while the use of epinephrine with lidocaine in sites with end-arteries is beneficial for hemostasis and does not seem to pose a risk of ischemia, it is prudent to use the smallest volume of epinephrine (with lidocaine) needed to achieve anesthesia for the site.
CORRESPONDENCE
Elizabeth V. Seiverling, MD, 300 Southborough Drive, Suite 201, South Portland, ME 04106; [email protected].
1. Kerr OA, Tidman MJ, Walker JJ, et al. The profile of dermatological problems in primary care. Clin Exp Dermatol. 2010;35:380-383.
2. Hosler GA, Patterson JW. Lentigines, nevi, and melanomas. In: Patterson JW, ed. Weedon’s Skin Pathology. Elsevier; 2015:32,837-901.
3. Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. J Natl Compr Canc Netw. 2009;7:250-275.
4. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge S, Greene F, et al, eds. AJCC Cancer Staging Manual. Springer International Publishing; 2017;8:563-585.
5. American Joint Committee on Cancer. Implementation of AJCC 8th Edition Cancer Staging System. Available at: https://cancerstaging.org/About/news/Pages/Implementation-of-AJCC-8th-Edition-Cancer-Staging-System.aspx. Accessed April 2, 2018.
6. Karimipour DJ, Schwartz JL, Wang TS, et al. Microstaging accuracy after subtotal incisional biopsy of cutaneous melanoma. J Am Acad Dermatol. 2005;52:798-802.
7. Kaiser S, Vassell R, Pinckney RG, et al. Clinical impact of biopsy method on the quality of surgical management in melanoma. J Surg Oncol. 2014;109:775-779.
8. Montgomery BD, Sadler GM. Punch biopsy of pigmented lesions is potentially hazardous. Can Fam Physician. 2009;55:24.
9. Ng JC, Swain S, Dowling JP, et al. The impact of partial biopsy on histopathologic diagnosis of cutaneous melanoma: experience of an Australian tertiary referral service. Arch Dermatol. 2010;146:234-239.
10. Gambichler T, Senger E, Rapp S, et al. Deep shave excision of macular melanocytic nevi with the razor blade biopsy technique. Dermatol Surg. 2000;26:662-666.
11. Ferrandiz L, Moreno-Ramirez D, Camacho FM. Shave excision of common acquired melanocytic nevi: cosmetic outcome, recurrences, and complications. Dermatol Surg. 2005;31(9 Pt 1):1112-1115.
12. D'souza G, Carey TE, William WN Jr, et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J Acquir Immune Defic Syndr. 2014;65:603-610.
13. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer; 2010.
14. Elston DM, Stratman EJ, Miller SJ, et al. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
15. Zager JS, Hochwald SN, Marzban SS, et al. Shave biopsy is a safe and accurate method for the initial evaluation of melanoma. J Am Coll Surg. 2011;212:454-460.
16. Chanda JJ, Callen JP. Adverse effect of melanoma incision. J Am Acad Dermatol. 1985;13:519-522.
17. Noroozi N, Zakerolhosseini A. Computerized measurement of melanocytic tumor depth in skin histopathological images. Micron. 2015;77:44-56.
18. Nielsen LJ, Lumholt P, Hölmich LR. [Local anaesthesia with vasoconstrictor is safe to use in areas with end-arteries in fingers, toes, noses and ears]. Ugeskr Laeger. 2014;176(44).
19. Häfner HM, Röcken M, Breuninger H. Epinephrine-supplemented local anesthetics for ear and nose surgery: clinical use without complications in more than 10,000 surgical procedures. J Dtsch Dermatol Ges. 2005;3:195-199.
20. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
21. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
22. Ilicki J. Safety of epinephrine in digital nerve blocks: a literature review. J Emerg Med. 2015;49:799-809.
1. Kerr OA, Tidman MJ, Walker JJ, et al. The profile of dermatological problems in primary care. Clin Exp Dermatol. 2010;35:380-383.
2. Hosler GA, Patterson JW. Lentigines, nevi, and melanomas. In: Patterson JW, ed. Weedon’s Skin Pathology. Elsevier; 2015:32,837-901.
3. Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. J Natl Compr Canc Netw. 2009;7:250-275.
4. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge S, Greene F, et al, eds. AJCC Cancer Staging Manual. Springer International Publishing; 2017;8:563-585.
5. American Joint Committee on Cancer. Implementation of AJCC 8th Edition Cancer Staging System. Available at: https://cancerstaging.org/About/news/Pages/Implementation-of-AJCC-8th-Edition-Cancer-Staging-System.aspx. Accessed April 2, 2018.
6. Karimipour DJ, Schwartz JL, Wang TS, et al. Microstaging accuracy after subtotal incisional biopsy of cutaneous melanoma. J Am Acad Dermatol. 2005;52:798-802.
7. Kaiser S, Vassell R, Pinckney RG, et al. Clinical impact of biopsy method on the quality of surgical management in melanoma. J Surg Oncol. 2014;109:775-779.
8. Montgomery BD, Sadler GM. Punch biopsy of pigmented lesions is potentially hazardous. Can Fam Physician. 2009;55:24.
9. Ng JC, Swain S, Dowling JP, et al. The impact of partial biopsy on histopathologic diagnosis of cutaneous melanoma: experience of an Australian tertiary referral service. Arch Dermatol. 2010;146:234-239.
10. Gambichler T, Senger E, Rapp S, et al. Deep shave excision of macular melanocytic nevi with the razor blade biopsy technique. Dermatol Surg. 2000;26:662-666.
11. Ferrandiz L, Moreno-Ramirez D, Camacho FM. Shave excision of common acquired melanocytic nevi: cosmetic outcome, recurrences, and complications. Dermatol Surg. 2005;31(9 Pt 1):1112-1115.
12. D'souza G, Carey TE, William WN Jr, et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J Acquir Immune Defic Syndr. 2014;65:603-610.
13. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer; 2010.
14. Elston DM, Stratman EJ, Miller SJ, et al. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
15. Zager JS, Hochwald SN, Marzban SS, et al. Shave biopsy is a safe and accurate method for the initial evaluation of melanoma. J Am Coll Surg. 2011;212:454-460.
16. Chanda JJ, Callen JP. Adverse effect of melanoma incision. J Am Acad Dermatol. 1985;13:519-522.
17. Noroozi N, Zakerolhosseini A. Computerized measurement of melanocytic tumor depth in skin histopathological images. Micron. 2015;77:44-56.
18. Nielsen LJ, Lumholt P, Hölmich LR. [Local anaesthesia with vasoconstrictor is safe to use in areas with end-arteries in fingers, toes, noses and ears]. Ugeskr Laeger. 2014;176(44).
19. Häfner HM, Röcken M, Breuninger H. Epinephrine-supplemented local anesthetics for ear and nose surgery: clinical use without complications in more than 10,000 surgical procedures. J Dtsch Dermatol Ges. 2005;3:195-199.
20. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
21. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
22. Ilicki J. Safety of epinephrine in digital nerve blocks: a literature review. J Emerg Med. 2015;49:799-809.
From The Journal of Family Practice | 2018;67(5):270-274.
The naloxone option
More than 64,000 people in the United States died of drug overdoses in 2016.1 Of those overdose deaths, more than 34,000 were related to the use of natural (eg, codeine, morphine); synthetic (eg, fentanyl); and semisynthetic (eg, oxycodone, hydrocodone) opioids.1 The number of drug-overdose fatalities (driven largely by opioids) has increased so dramatically in recent years that drug overdose is now the leading cause of intentional and unintentional injury-related death in the United States.2 Furthermore, opioid use is increasing among college students, with many injecting these agents.3 Those injecting (as opposed to other routes of delivery) have the highest death rate.4
The Department of Health and Human Services has identified 3 important issues to address with regard to the opioid epidemic: prescriber education, community naloxone access, and better interventions (such as naloxone overdose-reversal take-home kits) for people with opioid use disorders and/or a history of overdoses.5 (For more on overdose reversal kits, see “What FPs need to know about naloxone kits,” a 3-in-3 video.) With these goals in mind, we provide the following review of naloxone dosing and postoverdose treatment.
Steps FPs can take to reverse the overdose
Opioids act on delta, kappa, and mu receptors in the brain to produce analgesic effects,6 but, in large quantities, their mu receptor activity can cause fatal respiratory depression.7 Some of the most commonly abused opioids are heroin and the prescription opioids fentanyl, oxycodone, and hydrocodone.8
People who have overdosed on opioids generally present with evidence of obtundation, miosis, and difficulty breathing. Respiratory failure is the most common cause of death.9 Hypothermia, compartment syndrome, rhabdomyolysis, renal failure, and acute pulmonary edema are less common complications. Overdoses and these medical issues can potentially be reversed and/or mitigated by naloxone administration.10,11
Naloxone and its routes of administration. Naloxone is the agent of choice in overdose situations.12 It works as an antagonist of the delta, kappa, and mu receptors,6,13 has a rapid onset of action, and is associated with minimal adverse effects.14
Naloxone can be administered via the intravenous (IV), intranasal, intramuscular, subcutaneous, intraosseous, or endotracheal routes.6 Although IV administration has been the most common and is still generally preferred in the hospital setting, the intranasal route has gained favor, partly because it can be difficult to establish an IV in IV drug users and partly because it is easier for nonmedical people to administer.6
In addition, the nasal mucosa has an abundant blood supply resulting in rapid absorption. The drug reaches the systemic circulation quickly and avoids first-pass hepatic metabolism.6 Intranasal route absorption is enhanced by deep inhalation and patient cooperation, but it can still be effective in an unconscious patient. Response time is nearly the same as that with IV administration (both act within 1-2 mins).6
Naloxone has a short duration of action (shorter than that of some opiates), and its duration of action is influenced by the pharmacology and toxicity of the overdose drug.15 The serum half-life in adults ranges from 30 to 81 mins, and clinical impact varies from minutes to an hour.15 Thus, even if a patient initially improves after administration, close observation is mandatory due to the frequent need for repeat naloxone dosing.
Adverse effects. Naloxone is considered safe, with relatively few adverse effects and doesn’t have any effects on someone who isn’t experiencing an opioid overdose or currently on opioids.15 The only downside is that naloxone administration to an opioid-dependent person often precipitates an acute withdrawal event, characterized by global pain, agitation, generalized distress, and gastrointestinal complaints, including vomiting and diarrhea. Although withdrawal is not life-threatening, it can cause great discomfort.
Getting a handle on naloxone dosing
The starting dose of naloxone used to be 0.04 mg, but this was later increased to 0.4 mg. The advent and high overdose lethality of more potent drugs like fentanyl and carfentanil has made low-dose naloxone less effective.12
Currently, 1 mg is often the initial recommendation, but doses of 2 to 4 mg are not uncommon, and multiple administrations or continuous IV administration are frequently needed to reverse severe toxicities, such as those involving fentanyl or longer-action opioids like methadone. Anyone exhibiting difficulty breathing mandates a starting naloxone dose of at least 1 to 2 mg.12,16 In addition to breathing, additional doses are indicated clinically by medical parameters such as vital signs, ocular pupil diameter, and/or alertness.6
Intranasal administration often utilizes up to 4 mg of naloxone in one nostril, followed by a titrated additional administration in the other nostril. In life-threatening circumstances, especially those in which a patient is exhibiting respiratory depression, a much larger quantity of naloxone—up to 10 mg—may be administered by trained medical personnel.12,16 In the end, all dosing varies and must be individualized to the patient’s signs and symptoms. Those who have overdosed require prolonged monitoring to treat potential complications.
Emergency assistance and transport. Because of the dangers that can result from opioid toxicities, any hint or evidence of physiologic compromise merits a 911 call for emergency medical assistance and transport to a hospital emergency department (ED). Hospitalization is at the physician’s discretion.
Expanding the availability of naloxone in the community
The availability of naloxone overdose-reversal kits is growing among hospitals, other types of health care facilities, first responders, medical offices, and the general public. Distributing the kits to opioid users and their families has wide support but remains controversial (more on this in a bit).
Support even includes that from the current US Surgeon General, Jerome Adams, MD, MPH, who noted in a statement on April 5, 2018, the lifesaving success of opioid-overdose reversal naloxone kits by medical personnel, first responders, and other people. As a result, he formally recommended that more Americans keep such kits available in order to be able to quickly diminish opioid toxicities.17,18 His advice was especially directed toward people at risk for an opioid overdose or anyone associated with opioid drug users.
Prehospital management of overdoses is ideally managed by emergency medical service (EMS) personnel,10 but even nonmedical people can safely administer naloxone. About 10,000 overdose cases were documented to have been reversed by nonmedical providers between 1996 and 2010.10 Many states have laws limiting the civil and criminal liability for naloxone administrators. New Mexico was the first state to legally allow naloxone administration by individuals without a prescription.7 Pharmacists often participate in efforts to counter opioid drug overdose deaths by offering naloxone administration kits, along with training about techniques of use, to people filling opioid prescriptions and to household members and/or other individuals in the social support network of an opioid user.6 Some physicians co-prescribe naloxone to patients along with opioid therapies during long-term pain management. Such dual prescribing is encouraged by many clinics.19 This method has decreased opioid overdose deaths in North Carolina,20 in its army base at Fort Bragg,19 and in California.21
The issue of “risk compensation”
To those who say that having naloxone available to users of opioids or those in their social network promotes even riskier behavior resulting in increased overdoses, research points to just the opposite. A nonrandomized study that examined co-prescribing naloxone to patients on chronic opioid therapy for non-cancer-related pain, documented fewer opioid-related ED visits following use by prescribers and patients at community health centers.22 Other research has demonstrated a reduced number of community-level opioid overdose deaths once opioid overdose education and community naloxone distribution were implemented.23,24
After the overdose: Getting patients into treatment
After reversing initial toxicities, a protracted period of assessment is required to assure patient safety. Beyond prolonged observation after an overdose, it is critical to recommend and provide long-term substance abuse therapies. Simply reversing the overdose is not medically sufficient, even if postoverdose patients refuse such treatment referrals. The fact that many of these people subsequently die is evidence of the importance of adhering to a formal, long-term chemical dependence intervention program.
Persistent diligence is usually needed to convince a patient who has recovered from an acute drug overdose event to accept a treatment referral. Some EDs institute special teams to facilitate such referrals, using a multidisciplinary approach, including substance abuse counselors and social workers. Referral agencies are also sometimes included to aid patient acceptance and retention in drug abuse treatment interventions. (See "Resources" below for more information.)
SIDEBAR
Resources
- The Centers for Disease Control and Prevention’s Guideline for Prescribing Opioids for Chronic Pain. Available at: https://www.cdc.gov/drugoverdose/prescribing/guideline.html.
- National Institute on Drug Abuse. Available at: https://www.drugabuse.gov.
- Substance Abuse and Mental Health Services Administration. Available at: https://www.samhsa.gov/find-help/national-helpline.
- Your state’s prescription drug monitoring program. Available at: https://www.cdc.gov/drugoverdose/pdmp/states.html.
CORRESPONDENCE
Steven Lippmann, MD, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. National Institute on Drug Abuse. Overdose death rates. Revised September 2017. Available at: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed April 11, 2018.
2. Xu J, Murphy SL, Kochanek KD, et al. Deaths: final data for 2013. Nat Vital Stat Syst. 2016;64:1-119.
3. McCabe SE, West BT, Teter CJ, et al. Trends in medical use, diversion, and nonmedical use of prescription medications among college students from 2003 to 2013: connecting the dots. Addict Behav. 2014;39:1176-1182.
4. Green TC, Heimer R, Grau LE. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and naloxone distribution programs in the United States. Addiction. 2008;103:979-989.
5. US Department of Health and Human Services. HHS takes strong steps to address opioid-drug related overdose, death and dependence. March 26, 2015. Available at: http://wayback.archive-it.org/3926/20170127185704/https://www.hhs.gov/about/news/2015/03/26/hhs-takes-strong-steps-to-address-opioid-drug-related-overdose-death-and-dependence.html. Accessed April 16, 2018.
6. Robinson A, Wermeling DP. Intranasal naloxone administration for treatment of opioid overdose. Am J Health Syst Pharm. 2014;71:2129-2135.
7. Doyon S, Aks SE, Schaeffer S. Expanding access to naloxone in the United States. J Med Toxicol. 2014;10:431-434.
8. National Institute on Drug Abuse. Which classes of prescription drugs are commonly misused? Available at: https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/which-classes-prescription-drugs-are-commonly-misused. Accessed April 16, 2018.
9. Boom M, Niesters M, Sarton E, et al. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharm Des. 2012;18:5994-6004.
10. Weaver L, Palombi L, Bastianelli KMS. Naloxone administration for opioid overdose reversal in the prehospital setting: implications for pharmacists. J Pharm Pract. 2018;31:91-98.
11. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367:146-155.
12. Jordan MR, Morrisonponce D. Naloxone. StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK441910/. Accessed September 1, 2017.
13. Wilkerson RG, Kim HK, Windsor TA, et al. The opioid epidemic in the United States. Emerg Med Clin North Am. 2016;34:e1-e23.
14. Jeffery RM, Dickinson L, Ng ND, et al. Naloxone administration for suspected opioid overdose: an expanded scope of practice by a basic life support collegiate-based emergency medical services agency. J Am Coll Health. 2017;65:212-216.
15. Drugs.com. Naloxone. Available at: https://www.drugs.com/pro/naloxone.html. Accessed April 16, 2018.
16. Prabhu A, Abaid B, Naik S, et al. Naloxone for opioid overdoses. Internet and Psychiatry 2017. Available at: https://www.internetandpsychiatry.com/wp/editorials/naloxone-for-opioid-overdoses/. Accessed September 19, 2017.
17. HHS.gov. Surgeon General releases advisory on naloxone, an opioid overdose-reversing drug. Available at: https://www.hhs.gov/about/news/2018/04/05/surgeon-general-releases-advisory-on-naloxone-an-opioid-overdose-reversing-drug.html. Accessed April 16, 2018.
18. US Department of Health and Human Services. Surgeongeneral.gov. Surgeon General’s advisory on naloxone and opioid overdose. Available at: https://www.surgeongeneral.gov/priorities/opioid-overdose-prevention/naloxone-advisory.html. Accessed April 16, 2018.
19. Behar E, Rowe C, Santos GM, et al. Acceptability of naloxone co-prescription among primary care providers treating patients on long-term opioid therapy for pain. J Gen Intern Med. 2017;32:291-295.
20. Albert S, Brason FW 2nd, Sanford CK, et al. Project Lazarus: community‐based overdose prevention in rural North Carolina. Pain Med. 2011;12:S77-S85.
21. Rowe C, Santos GM, Vittinghoff E, et al. Predictors of participant engagement and naloxone utilization in a community‐based naloxone distribution program. Addiction. 2015;110:1301-1310.
22. Coffin PO, Behar E, Rowe C, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. 2016;165:245-252.
23. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174.
24. Bird SM, McAuley A, Perry S, et al. Effectiveness of Scotland’s National Naloxone Programme for reducing opioid-related deaths: a before (2006-10) versus after (2011-13) comparison. Addiction. 2016;111:883-891.
More than 64,000 people in the United States died of drug overdoses in 2016.1 Of those overdose deaths, more than 34,000 were related to the use of natural (eg, codeine, morphine); synthetic (eg, fentanyl); and semisynthetic (eg, oxycodone, hydrocodone) opioids.1 The number of drug-overdose fatalities (driven largely by opioids) has increased so dramatically in recent years that drug overdose is now the leading cause of intentional and unintentional injury-related death in the United States.2 Furthermore, opioid use is increasing among college students, with many injecting these agents.3 Those injecting (as opposed to other routes of delivery) have the highest death rate.4
The Department of Health and Human Services has identified 3 important issues to address with regard to the opioid epidemic: prescriber education, community naloxone access, and better interventions (such as naloxone overdose-reversal take-home kits) for people with opioid use disorders and/or a history of overdoses.5 (For more on overdose reversal kits, see “What FPs need to know about naloxone kits,” a 3-in-3 video.) With these goals in mind, we provide the following review of naloxone dosing and postoverdose treatment.
Steps FPs can take to reverse the overdose
Opioids act on delta, kappa, and mu receptors in the brain to produce analgesic effects,6 but, in large quantities, their mu receptor activity can cause fatal respiratory depression.7 Some of the most commonly abused opioids are heroin and the prescription opioids fentanyl, oxycodone, and hydrocodone.8
People who have overdosed on opioids generally present with evidence of obtundation, miosis, and difficulty breathing. Respiratory failure is the most common cause of death.9 Hypothermia, compartment syndrome, rhabdomyolysis, renal failure, and acute pulmonary edema are less common complications. Overdoses and these medical issues can potentially be reversed and/or mitigated by naloxone administration.10,11
Naloxone and its routes of administration. Naloxone is the agent of choice in overdose situations.12 It works as an antagonist of the delta, kappa, and mu receptors,6,13 has a rapid onset of action, and is associated with minimal adverse effects.14
Naloxone can be administered via the intravenous (IV), intranasal, intramuscular, subcutaneous, intraosseous, or endotracheal routes.6 Although IV administration has been the most common and is still generally preferred in the hospital setting, the intranasal route has gained favor, partly because it can be difficult to establish an IV in IV drug users and partly because it is easier for nonmedical people to administer.6
In addition, the nasal mucosa has an abundant blood supply resulting in rapid absorption. The drug reaches the systemic circulation quickly and avoids first-pass hepatic metabolism.6 Intranasal route absorption is enhanced by deep inhalation and patient cooperation, but it can still be effective in an unconscious patient. Response time is nearly the same as that with IV administration (both act within 1-2 mins).6
Naloxone has a short duration of action (shorter than that of some opiates), and its duration of action is influenced by the pharmacology and toxicity of the overdose drug.15 The serum half-life in adults ranges from 30 to 81 mins, and clinical impact varies from minutes to an hour.15 Thus, even if a patient initially improves after administration, close observation is mandatory due to the frequent need for repeat naloxone dosing.
Adverse effects. Naloxone is considered safe, with relatively few adverse effects and doesn’t have any effects on someone who isn’t experiencing an opioid overdose or currently on opioids.15 The only downside is that naloxone administration to an opioid-dependent person often precipitates an acute withdrawal event, characterized by global pain, agitation, generalized distress, and gastrointestinal complaints, including vomiting and diarrhea. Although withdrawal is not life-threatening, it can cause great discomfort.
Getting a handle on naloxone dosing
The starting dose of naloxone used to be 0.04 mg, but this was later increased to 0.4 mg. The advent and high overdose lethality of more potent drugs like fentanyl and carfentanil has made low-dose naloxone less effective.12
Currently, 1 mg is often the initial recommendation, but doses of 2 to 4 mg are not uncommon, and multiple administrations or continuous IV administration are frequently needed to reverse severe toxicities, such as those involving fentanyl or longer-action opioids like methadone. Anyone exhibiting difficulty breathing mandates a starting naloxone dose of at least 1 to 2 mg.12,16 In addition to breathing, additional doses are indicated clinically by medical parameters such as vital signs, ocular pupil diameter, and/or alertness.6
Intranasal administration often utilizes up to 4 mg of naloxone in one nostril, followed by a titrated additional administration in the other nostril. In life-threatening circumstances, especially those in which a patient is exhibiting respiratory depression, a much larger quantity of naloxone—up to 10 mg—may be administered by trained medical personnel.12,16 In the end, all dosing varies and must be individualized to the patient’s signs and symptoms. Those who have overdosed require prolonged monitoring to treat potential complications.
Emergency assistance and transport. Because of the dangers that can result from opioid toxicities, any hint or evidence of physiologic compromise merits a 911 call for emergency medical assistance and transport to a hospital emergency department (ED). Hospitalization is at the physician’s discretion.
Expanding the availability of naloxone in the community
The availability of naloxone overdose-reversal kits is growing among hospitals, other types of health care facilities, first responders, medical offices, and the general public. Distributing the kits to opioid users and their families has wide support but remains controversial (more on this in a bit).
Support even includes that from the current US Surgeon General, Jerome Adams, MD, MPH, who noted in a statement on April 5, 2018, the lifesaving success of opioid-overdose reversal naloxone kits by medical personnel, first responders, and other people. As a result, he formally recommended that more Americans keep such kits available in order to be able to quickly diminish opioid toxicities.17,18 His advice was especially directed toward people at risk for an opioid overdose or anyone associated with opioid drug users.
Prehospital management of overdoses is ideally managed by emergency medical service (EMS) personnel,10 but even nonmedical people can safely administer naloxone. About 10,000 overdose cases were documented to have been reversed by nonmedical providers between 1996 and 2010.10 Many states have laws limiting the civil and criminal liability for naloxone administrators. New Mexico was the first state to legally allow naloxone administration by individuals without a prescription.7 Pharmacists often participate in efforts to counter opioid drug overdose deaths by offering naloxone administration kits, along with training about techniques of use, to people filling opioid prescriptions and to household members and/or other individuals in the social support network of an opioid user.6 Some physicians co-prescribe naloxone to patients along with opioid therapies during long-term pain management. Such dual prescribing is encouraged by many clinics.19 This method has decreased opioid overdose deaths in North Carolina,20 in its army base at Fort Bragg,19 and in California.21
The issue of “risk compensation”
To those who say that having naloxone available to users of opioids or those in their social network promotes even riskier behavior resulting in increased overdoses, research points to just the opposite. A nonrandomized study that examined co-prescribing naloxone to patients on chronic opioid therapy for non-cancer-related pain, documented fewer opioid-related ED visits following use by prescribers and patients at community health centers.22 Other research has demonstrated a reduced number of community-level opioid overdose deaths once opioid overdose education and community naloxone distribution were implemented.23,24
After the overdose: Getting patients into treatment
After reversing initial toxicities, a protracted period of assessment is required to assure patient safety. Beyond prolonged observation after an overdose, it is critical to recommend and provide long-term substance abuse therapies. Simply reversing the overdose is not medically sufficient, even if postoverdose patients refuse such treatment referrals. The fact that many of these people subsequently die is evidence of the importance of adhering to a formal, long-term chemical dependence intervention program.
Persistent diligence is usually needed to convince a patient who has recovered from an acute drug overdose event to accept a treatment referral. Some EDs institute special teams to facilitate such referrals, using a multidisciplinary approach, including substance abuse counselors and social workers. Referral agencies are also sometimes included to aid patient acceptance and retention in drug abuse treatment interventions. (See "Resources" below for more information.)
SIDEBAR
Resources
- The Centers for Disease Control and Prevention’s Guideline for Prescribing Opioids for Chronic Pain. Available at: https://www.cdc.gov/drugoverdose/prescribing/guideline.html.
- National Institute on Drug Abuse. Available at: https://www.drugabuse.gov.
- Substance Abuse and Mental Health Services Administration. Available at: https://www.samhsa.gov/find-help/national-helpline.
- Your state’s prescription drug monitoring program. Available at: https://www.cdc.gov/drugoverdose/pdmp/states.html.
CORRESPONDENCE
Steven Lippmann, MD, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
More than 64,000 people in the United States died of drug overdoses in 2016.1 Of those overdose deaths, more than 34,000 were related to the use of natural (eg, codeine, morphine); synthetic (eg, fentanyl); and semisynthetic (eg, oxycodone, hydrocodone) opioids.1 The number of drug-overdose fatalities (driven largely by opioids) has increased so dramatically in recent years that drug overdose is now the leading cause of intentional and unintentional injury-related death in the United States.2 Furthermore, opioid use is increasing among college students, with many injecting these agents.3 Those injecting (as opposed to other routes of delivery) have the highest death rate.4
The Department of Health and Human Services has identified 3 important issues to address with regard to the opioid epidemic: prescriber education, community naloxone access, and better interventions (such as naloxone overdose-reversal take-home kits) for people with opioid use disorders and/or a history of overdoses.5 (For more on overdose reversal kits, see “What FPs need to know about naloxone kits,” a 3-in-3 video.) With these goals in mind, we provide the following review of naloxone dosing and postoverdose treatment.
Steps FPs can take to reverse the overdose
Opioids act on delta, kappa, and mu receptors in the brain to produce analgesic effects,6 but, in large quantities, their mu receptor activity can cause fatal respiratory depression.7 Some of the most commonly abused opioids are heroin and the prescription opioids fentanyl, oxycodone, and hydrocodone.8
People who have overdosed on opioids generally present with evidence of obtundation, miosis, and difficulty breathing. Respiratory failure is the most common cause of death.9 Hypothermia, compartment syndrome, rhabdomyolysis, renal failure, and acute pulmonary edema are less common complications. Overdoses and these medical issues can potentially be reversed and/or mitigated by naloxone administration.10,11
Naloxone and its routes of administration. Naloxone is the agent of choice in overdose situations.12 It works as an antagonist of the delta, kappa, and mu receptors,6,13 has a rapid onset of action, and is associated with minimal adverse effects.14
Naloxone can be administered via the intravenous (IV), intranasal, intramuscular, subcutaneous, intraosseous, or endotracheal routes.6 Although IV administration has been the most common and is still generally preferred in the hospital setting, the intranasal route has gained favor, partly because it can be difficult to establish an IV in IV drug users and partly because it is easier for nonmedical people to administer.6
In addition, the nasal mucosa has an abundant blood supply resulting in rapid absorption. The drug reaches the systemic circulation quickly and avoids first-pass hepatic metabolism.6 Intranasal route absorption is enhanced by deep inhalation and patient cooperation, but it can still be effective in an unconscious patient. Response time is nearly the same as that with IV administration (both act within 1-2 mins).6
Naloxone has a short duration of action (shorter than that of some opiates), and its duration of action is influenced by the pharmacology and toxicity of the overdose drug.15 The serum half-life in adults ranges from 30 to 81 mins, and clinical impact varies from minutes to an hour.15 Thus, even if a patient initially improves after administration, close observation is mandatory due to the frequent need for repeat naloxone dosing.
Adverse effects. Naloxone is considered safe, with relatively few adverse effects and doesn’t have any effects on someone who isn’t experiencing an opioid overdose or currently on opioids.15 The only downside is that naloxone administration to an opioid-dependent person often precipitates an acute withdrawal event, characterized by global pain, agitation, generalized distress, and gastrointestinal complaints, including vomiting and diarrhea. Although withdrawal is not life-threatening, it can cause great discomfort.
Getting a handle on naloxone dosing
The starting dose of naloxone used to be 0.04 mg, but this was later increased to 0.4 mg. The advent and high overdose lethality of more potent drugs like fentanyl and carfentanil has made low-dose naloxone less effective.12
Currently, 1 mg is often the initial recommendation, but doses of 2 to 4 mg are not uncommon, and multiple administrations or continuous IV administration are frequently needed to reverse severe toxicities, such as those involving fentanyl or longer-action opioids like methadone. Anyone exhibiting difficulty breathing mandates a starting naloxone dose of at least 1 to 2 mg.12,16 In addition to breathing, additional doses are indicated clinically by medical parameters such as vital signs, ocular pupil diameter, and/or alertness.6
Intranasal administration often utilizes up to 4 mg of naloxone in one nostril, followed by a titrated additional administration in the other nostril. In life-threatening circumstances, especially those in which a patient is exhibiting respiratory depression, a much larger quantity of naloxone—up to 10 mg—may be administered by trained medical personnel.12,16 In the end, all dosing varies and must be individualized to the patient’s signs and symptoms. Those who have overdosed require prolonged monitoring to treat potential complications.
Emergency assistance and transport. Because of the dangers that can result from opioid toxicities, any hint or evidence of physiologic compromise merits a 911 call for emergency medical assistance and transport to a hospital emergency department (ED). Hospitalization is at the physician’s discretion.
Expanding the availability of naloxone in the community
The availability of naloxone overdose-reversal kits is growing among hospitals, other types of health care facilities, first responders, medical offices, and the general public. Distributing the kits to opioid users and their families has wide support but remains controversial (more on this in a bit).
Support even includes that from the current US Surgeon General, Jerome Adams, MD, MPH, who noted in a statement on April 5, 2018, the lifesaving success of opioid-overdose reversal naloxone kits by medical personnel, first responders, and other people. As a result, he formally recommended that more Americans keep such kits available in order to be able to quickly diminish opioid toxicities.17,18 His advice was especially directed toward people at risk for an opioid overdose or anyone associated with opioid drug users.
Prehospital management of overdoses is ideally managed by emergency medical service (EMS) personnel,10 but even nonmedical people can safely administer naloxone. About 10,000 overdose cases were documented to have been reversed by nonmedical providers between 1996 and 2010.10 Many states have laws limiting the civil and criminal liability for naloxone administrators. New Mexico was the first state to legally allow naloxone administration by individuals without a prescription.7 Pharmacists often participate in efforts to counter opioid drug overdose deaths by offering naloxone administration kits, along with training about techniques of use, to people filling opioid prescriptions and to household members and/or other individuals in the social support network of an opioid user.6 Some physicians co-prescribe naloxone to patients along with opioid therapies during long-term pain management. Such dual prescribing is encouraged by many clinics.19 This method has decreased opioid overdose deaths in North Carolina,20 in its army base at Fort Bragg,19 and in California.21
The issue of “risk compensation”
To those who say that having naloxone available to users of opioids or those in their social network promotes even riskier behavior resulting in increased overdoses, research points to just the opposite. A nonrandomized study that examined co-prescribing naloxone to patients on chronic opioid therapy for non-cancer-related pain, documented fewer opioid-related ED visits following use by prescribers and patients at community health centers.22 Other research has demonstrated a reduced number of community-level opioid overdose deaths once opioid overdose education and community naloxone distribution were implemented.23,24
After the overdose: Getting patients into treatment
After reversing initial toxicities, a protracted period of assessment is required to assure patient safety. Beyond prolonged observation after an overdose, it is critical to recommend and provide long-term substance abuse therapies. Simply reversing the overdose is not medically sufficient, even if postoverdose patients refuse such treatment referrals. The fact that many of these people subsequently die is evidence of the importance of adhering to a formal, long-term chemical dependence intervention program.
Persistent diligence is usually needed to convince a patient who has recovered from an acute drug overdose event to accept a treatment referral. Some EDs institute special teams to facilitate such referrals, using a multidisciplinary approach, including substance abuse counselors and social workers. Referral agencies are also sometimes included to aid patient acceptance and retention in drug abuse treatment interventions. (See "Resources" below for more information.)
SIDEBAR
Resources
- The Centers for Disease Control and Prevention’s Guideline for Prescribing Opioids for Chronic Pain. Available at: https://www.cdc.gov/drugoverdose/prescribing/guideline.html.
- National Institute on Drug Abuse. Available at: https://www.drugabuse.gov.
- Substance Abuse and Mental Health Services Administration. Available at: https://www.samhsa.gov/find-help/national-helpline.
- Your state’s prescription drug monitoring program. Available at: https://www.cdc.gov/drugoverdose/pdmp/states.html.
CORRESPONDENCE
Steven Lippmann, MD, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. National Institute on Drug Abuse. Overdose death rates. Revised September 2017. Available at: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed April 11, 2018.
2. Xu J, Murphy SL, Kochanek KD, et al. Deaths: final data for 2013. Nat Vital Stat Syst. 2016;64:1-119.
3. McCabe SE, West BT, Teter CJ, et al. Trends in medical use, diversion, and nonmedical use of prescription medications among college students from 2003 to 2013: connecting the dots. Addict Behav. 2014;39:1176-1182.
4. Green TC, Heimer R, Grau LE. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and naloxone distribution programs in the United States. Addiction. 2008;103:979-989.
5. US Department of Health and Human Services. HHS takes strong steps to address opioid-drug related overdose, death and dependence. March 26, 2015. Available at: http://wayback.archive-it.org/3926/20170127185704/https://www.hhs.gov/about/news/2015/03/26/hhs-takes-strong-steps-to-address-opioid-drug-related-overdose-death-and-dependence.html. Accessed April 16, 2018.
6. Robinson A, Wermeling DP. Intranasal naloxone administration for treatment of opioid overdose. Am J Health Syst Pharm. 2014;71:2129-2135.
7. Doyon S, Aks SE, Schaeffer S. Expanding access to naloxone in the United States. J Med Toxicol. 2014;10:431-434.
8. National Institute on Drug Abuse. Which classes of prescription drugs are commonly misused? Available at: https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/which-classes-prescription-drugs-are-commonly-misused. Accessed April 16, 2018.
9. Boom M, Niesters M, Sarton E, et al. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharm Des. 2012;18:5994-6004.
10. Weaver L, Palombi L, Bastianelli KMS. Naloxone administration for opioid overdose reversal in the prehospital setting: implications for pharmacists. J Pharm Pract. 2018;31:91-98.
11. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367:146-155.
12. Jordan MR, Morrisonponce D. Naloxone. StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK441910/. Accessed September 1, 2017.
13. Wilkerson RG, Kim HK, Windsor TA, et al. The opioid epidemic in the United States. Emerg Med Clin North Am. 2016;34:e1-e23.
14. Jeffery RM, Dickinson L, Ng ND, et al. Naloxone administration for suspected opioid overdose: an expanded scope of practice by a basic life support collegiate-based emergency medical services agency. J Am Coll Health. 2017;65:212-216.
15. Drugs.com. Naloxone. Available at: https://www.drugs.com/pro/naloxone.html. Accessed April 16, 2018.
16. Prabhu A, Abaid B, Naik S, et al. Naloxone for opioid overdoses. Internet and Psychiatry 2017. Available at: https://www.internetandpsychiatry.com/wp/editorials/naloxone-for-opioid-overdoses/. Accessed September 19, 2017.
17. HHS.gov. Surgeon General releases advisory on naloxone, an opioid overdose-reversing drug. Available at: https://www.hhs.gov/about/news/2018/04/05/surgeon-general-releases-advisory-on-naloxone-an-opioid-overdose-reversing-drug.html. Accessed April 16, 2018.
18. US Department of Health and Human Services. Surgeongeneral.gov. Surgeon General’s advisory on naloxone and opioid overdose. Available at: https://www.surgeongeneral.gov/priorities/opioid-overdose-prevention/naloxone-advisory.html. Accessed April 16, 2018.
19. Behar E, Rowe C, Santos GM, et al. Acceptability of naloxone co-prescription among primary care providers treating patients on long-term opioid therapy for pain. J Gen Intern Med. 2017;32:291-295.
20. Albert S, Brason FW 2nd, Sanford CK, et al. Project Lazarus: community‐based overdose prevention in rural North Carolina. Pain Med. 2011;12:S77-S85.
21. Rowe C, Santos GM, Vittinghoff E, et al. Predictors of participant engagement and naloxone utilization in a community‐based naloxone distribution program. Addiction. 2015;110:1301-1310.
22. Coffin PO, Behar E, Rowe C, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. 2016;165:245-252.
23. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174.
24. Bird SM, McAuley A, Perry S, et al. Effectiveness of Scotland’s National Naloxone Programme for reducing opioid-related deaths: a before (2006-10) versus after (2011-13) comparison. Addiction. 2016;111:883-891.
1. National Institute on Drug Abuse. Overdose death rates. Revised September 2017. Available at: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed April 11, 2018.
2. Xu J, Murphy SL, Kochanek KD, et al. Deaths: final data for 2013. Nat Vital Stat Syst. 2016;64:1-119.
3. McCabe SE, West BT, Teter CJ, et al. Trends in medical use, diversion, and nonmedical use of prescription medications among college students from 2003 to 2013: connecting the dots. Addict Behav. 2014;39:1176-1182.
4. Green TC, Heimer R, Grau LE. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and naloxone distribution programs in the United States. Addiction. 2008;103:979-989.
5. US Department of Health and Human Services. HHS takes strong steps to address opioid-drug related overdose, death and dependence. March 26, 2015. Available at: http://wayback.archive-it.org/3926/20170127185704/https://www.hhs.gov/about/news/2015/03/26/hhs-takes-strong-steps-to-address-opioid-drug-related-overdose-death-and-dependence.html. Accessed April 16, 2018.
6. Robinson A, Wermeling DP. Intranasal naloxone administration for treatment of opioid overdose. Am J Health Syst Pharm. 2014;71:2129-2135.
7. Doyon S, Aks SE, Schaeffer S. Expanding access to naloxone in the United States. J Med Toxicol. 2014;10:431-434.
8. National Institute on Drug Abuse. Which classes of prescription drugs are commonly misused? Available at: https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/which-classes-prescription-drugs-are-commonly-misused. Accessed April 16, 2018.
9. Boom M, Niesters M, Sarton E, et al. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharm Des. 2012;18:5994-6004.
10. Weaver L, Palombi L, Bastianelli KMS. Naloxone administration for opioid overdose reversal in the prehospital setting: implications for pharmacists. J Pharm Pract. 2018;31:91-98.
11. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367:146-155.
12. Jordan MR, Morrisonponce D. Naloxone. StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK441910/. Accessed September 1, 2017.
13. Wilkerson RG, Kim HK, Windsor TA, et al. The opioid epidemic in the United States. Emerg Med Clin North Am. 2016;34:e1-e23.
14. Jeffery RM, Dickinson L, Ng ND, et al. Naloxone administration for suspected opioid overdose: an expanded scope of practice by a basic life support collegiate-based emergency medical services agency. J Am Coll Health. 2017;65:212-216.
15. Drugs.com. Naloxone. Available at: https://www.drugs.com/pro/naloxone.html. Accessed April 16, 2018.
16. Prabhu A, Abaid B, Naik S, et al. Naloxone for opioid overdoses. Internet and Psychiatry 2017. Available at: https://www.internetandpsychiatry.com/wp/editorials/naloxone-for-opioid-overdoses/. Accessed September 19, 2017.
17. HHS.gov. Surgeon General releases advisory on naloxone, an opioid overdose-reversing drug. Available at: https://www.hhs.gov/about/news/2018/04/05/surgeon-general-releases-advisory-on-naloxone-an-opioid-overdose-reversing-drug.html. Accessed April 16, 2018.
18. US Department of Health and Human Services. Surgeongeneral.gov. Surgeon General’s advisory on naloxone and opioid overdose. Available at: https://www.surgeongeneral.gov/priorities/opioid-overdose-prevention/naloxone-advisory.html. Accessed April 16, 2018.
19. Behar E, Rowe C, Santos GM, et al. Acceptability of naloxone co-prescription among primary care providers treating patients on long-term opioid therapy for pain. J Gen Intern Med. 2017;32:291-295.
20. Albert S, Brason FW 2nd, Sanford CK, et al. Project Lazarus: community‐based overdose prevention in rural North Carolina. Pain Med. 2011;12:S77-S85.
21. Rowe C, Santos GM, Vittinghoff E, et al. Predictors of participant engagement and naloxone utilization in a community‐based naloxone distribution program. Addiction. 2015;110:1301-1310.
22. Coffin PO, Behar E, Rowe C, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. 2016;165:245-252.
23. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174.
24. Bird SM, McAuley A, Perry S, et al. Effectiveness of Scotland’s National Naloxone Programme for reducing opioid-related deaths: a before (2006-10) versus after (2011-13) comparison. Addiction. 2016;111:883-891.
From The Journal of Family Practice | 2018;67(5):288-290,292.
Periprocedural management of oral anticoagulation: When and how to hit “pause”
Debra P is a 62-year-old African American woman who calls your office to report that she has an upcoming routine colonoscopy planned in 2 weeks. She has been taking warfarin for the past 2 years for ischemic stroke prevention secondary to atrial fibrillation (AF), and her gastroenterologist recommended that she contact her family physician (FP) to discuss periprocedural anticoagulation plans. Ms. P is currently taking warfarin 5 mg on Mondays, Wednesdays, and Fridays, and 2.5 mg all other days of the week. Her international normalized ratio (INR) was 2.3 when it was last checked 2 weeks ago, and it has been stable and within goal range for the past 6 months. Her medical history includes AF, well-controlled hypertension, and type 2 diabetes mellitus, as well as gout and stage 3 chronic kidney disease. Ms. P denies any history of stroke or transient ischemic attack (TIA). She is requesting instructions on how to manage her warfarin before and after her upcoming colonoscopy.
Jerry Q is a 68-year-old Caucasian man with longstanding osteoarthritis who is scheduled to undergo a total left knee arthroplasty in one week. His orthopedic surgeon recommended that he contact his FP for instructions regarding managing apixaban perioperatively. Jerry has been taking apixaban 5 mg bid for the past 9 months due to a history of recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE) (both unprovoked). Mr. Q had been taking warfarin following his first DVT 4 years ago, but, after reporting that INR monitoring was a burden, he was started on apixaban. The patient has normal renal function and is relatively healthy otherwise. How should apixaban be managed before and after his upcoming surgery?
Each year, approximately 15% to 20% of patients taking an oral anticoagulant undergo a procedure that carries a heightened risk for bleeding.1,2 Stopping oral anticoagulation is often necessary before—and sometimes briefly after—many of these procedures in order to minimize the risk of bleeding.3 This means that countless decisions must be made by health care providers each year regarding if, when, and how to pause and resume oral anticoagulation. These decisions are not always straightforward, especially when you consider the risks for thrombosis and bleeding that are unique to the procedure and to the individual patient.
With these variables in mind, the health care provider must make decisions regarding anticoagulation during the periprocedural period based on the following 5 questions:
- Will this patient need to have his/her oral anticoagulant stopped prior to the procedure?
- If the patient’s oral anticoagulation needs to be held, when should it be stopped and for how long?
- Will periprocedural bridging with a parenteral anticoagulant be necessary prior to the procedure?
- When should the patient resume his or her oral anticoagulant after the procedure, and at what dosage?
- Will bridging with a parenteral anticoagulant be necessary after the procedure?
Before addressing these 5 questions, though, physicians must assess patients’ thrombotic and bleeding risks.4-6
Anticoagulant regimens and the risks of discontinuing them
The 2 most common indications for long-term oral anticoagulation are venous thromboembolism (VTE), which occurs in approximately one million Americans every year,7,8 and stroke prevention in the setting of AF (AF occurs in 3-6 million US adults per year).6
Warfarin is also often used in patients with mechanical heart valves for long-term stroke prevention; however, direct oral anticoagulants (DOACs) are not recommended for patients with mechanical heart valves because trials have not yet demonstrated their safety or efficacy in this population.4,5,9
Who’s at highest risk for an acute thromboembolic event?
When planning for interruptions in oral anticoagulation, it is important to identify patients at highest risk for an acute thromboembolic event. Patients with 10% or higher annual risk for VTE or ischemic stroke are generally placed into this high-risk category (TABLE 13,5,6,9-11).3 Keep in mind that the absolute risk for thromboembolism during a brief period of oral coagulation interruption is relatively low, even in those patients considered to be at high risk. Using a mathematical approach (although simplistic), a patient with a 10% annual risk for a thromboembolic event would have <0.3% chance for developing such an event in the acute phase, even if their anticoagulation was withheld for up to 10 days ([10%/365 days] × 10 days).
Patients with mechanical heart valves. Nearly all patients with a mechanical heart valve are at moderate to high risk for ischemic stroke.3
For patients with AF, the CHADS2 and CHA2DS2-VASc scoring tools can be used to estimate annual thrombosis risk based on the presence of risk factors (TABLE 210,11).6,9-11 It should be noted, however, that these scoring tools have not been validated specifically for periprocedural risk estimations. Nonetheless, the latest 2017 American College of Cardiology (ACC) guidelines recommend the use of the CHA2DS2-VASc scoring tool for making decisions regarding perioperative bridging in patients with AF.11
Patients with previous VTE. Multiple aspects of a patient’s past medical history need to be taken into account when estimating annual and acute risk for VTE. Patients at the highest risk for VTE recurrence (annual VTE risk ≥10%) include those with recent VTE (past 90 days), active malignancy, and/or severe thrombophilias (TABLE 13,5,6,9-11).3,5,6 Patients without any of these features can still be at moderate risk for recurrent VTE, as a single VTE without a clear provoking factor can confer a 5% to 10% annualized risk for recurrence.12,13 Previous proximal DVT and PE are associated with a higher risk for recurrence than a distal DVT, and males have a higher recurrence risk than females.5,12 There are scoring tools, such as DASH (D-dimer, Age, Sex, Hormones) and the “Men Continue and HERDOO2,” that can help estimate annualized risk for VTE recurrence; however, they are not validated (nor particularly useful) when making decisions in the perioperative period.14,15
Additional risk factors. Consider additional risk factors for thromboembolism, including estrogen/hormone replacement therapy, pregnancy, leg or hip fractures, immobility, trauma, spinal cord injury, central venous lines, congestive heart failure, thrombophilia, increased age, obesity, and varicose veins.5,16
In addition, some surgeries have a higher inherent risk for thrombosis. Major orthopedic surgery (knee and hip arthroplasty, hip fracture surgery) and surgeries for major trauma or spinal cord injuries are associated with an exceedingly high rate of VTE.17 Similarly, coronary artery bypass surgery, heart valve replacement, and carotid endarterectomies carry the highest risk for acute ischemic stroke.3
Who’s at highest risk for bleeding?
Establishing the bleeding risk associated with a procedure is imperative prior to urgent and elective surgeries to help determine when anticoagulation therapy should be discontinued and reinitiated, as well as whether bridging therapy is appropriate. The 2012 CHEST guidelines state that bleeding risk should be assessed based on timing of anticoagulation relative to surgery and whether the anticoagulation is being used as prophylaxis for, or treatment of, thromboembolism.3 Categorizing procedures as having a minimal, low, or high risk for bleeding can be helpful in making anticoagulation decisions (TABLE 3).3,18-21
In addition to the bleeding risk associated with procedures, patient-specific factors need to be considered. A bleeding event within the past 3 months, platelet abnormalities, a supratherapeutic INR at the time of surgery, a history of bleeding from previous bridging, a bleed history with a similar procedure, and a high HAS-BLED (Hypertension, Abnormal renal or liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol usage) score are all factors that elevate the risk for perioperative bleeding.10,11 Although validated only in patients taking warfarin, the HAS-BLED scoring system can be utilized in patients with AF to estimate annual risk for major bleeding (TABLE 210,11).10
With this risk information in mind, it’s time to move on to the 5 questions you’ll need to ask.
1. Should the patient’s oral anticoagulation be stopped prior to the upcoming procedure?
The answer, of course, hinges on the patient’s risk of bleeding.
Usually, it is not necessary to withhold any doses of oral anticoagulation if your patient is scheduled for a procedure with minimal risk for bleeding (TABLE 33,18-21).3 However, it may be reasonable to stop anticoagulation if your patient has additional features that predispose to high bleeding risk (eg, hemophilia, Von Willebrand disease, etc). The CHEST guidelines recommend adding an oral prohemostatic agent (eg, tranexamic acid) if anticoagulation will be continued during a dental procedure.3
If your patient is undergoing any other procedure that has a low to high risk for bleeding, oral anticoagulation should be withheld prior to the procedure in most instances,3,11 although there are exceptions. For example, cardiac procedures, such as AF catheter ablation and cardiac pacemaker placement, are often performed with uninterrupted oral anticoagulation despite their bleeding risk category.3
When in doubt, discuss the perceived bleeding and clotting risks directly with the specialist performing the procedure. In patients who have had a VTE or ischemic stroke within the past 3 months, consider postponing the invasive procedure until the patient is beyond this period of highest thrombotic risk.11
2. How far in advance of the procedure should the oral anticoagulant be withheld?
Warfarin may need to be stopped anywhere from 2 to 5 days prior to the procedure, depending on a number of variables.
Warfarin has a half-life of approximately 36 hours, so it can take 3 to 5 days for warfarin concentrations to drop to safe levels for procedures with low to moderate bleeding risk and 5 to 7 days for procedures with high bleeding risk.21 The 2012 CHEST guidelines recommend that warfarin therapy be discontinued 5 days prior to surgery to minimize the risk for bleeding.3 The Anticoagulation Forum, a leading expert panel that produced a set of useful anticoagulation guidelines in 2016, recommends stopping warfarin 4 to 5 days prior to a procedure.21 If the provider chooses to withhold warfarin before a procedure with minimal bleeding risk, it should be stopped 2 to 3 days prior.3
Consider checking INR values the week before. A 2017 consensus statement from the ACC recommends that the timing of warfarin discontinuation be based on an INR value taken 5 to 7 days prior to the surgical procedure.11 This allows for a more tailored approach to preparing the patient for surgery. If the INR is below goal range, warfarin may need to be withheld for only 3 to 4 days prior to a procedure. Conversely, INRs above goal range may require warfarin to be held 6 or more days, depending on the degree of INR elevation.
While not always feasible in clinical practice, the CHEST guidelines recommend obtaining an INR value the day prior to the procedure to determine if the INR value is low enough to proceed with surgery, or if a low dose of oral vitamin K needs to be administered to ensure that the INR is in a safe range the following day.3
DOACs
DOACs can be withheld for much shorter durations preoperatively than warfarin.
When withholding anticoagulants, the goal is to have a low amount of anticoagulant effect (12%-25%) present during low-risk procedures and a nominal amount of anticoagulant effect (3%-6%) present for high-risk procedures.20 DOACs have much shorter half-lives than warfarin (7-19 hours vs 36-48 hours, respectively), so they can be withheld for much shorter durations preoperatively.20 For patients undergoing procedures that are considered to have a minimal risk for bleeding (such as minor dental and dermatologic procedures), DOACs do not generally need to be withheld; however, it may be ideal to time the procedure when the DOAC is at a trough concentration (before the next dose is due).3
DOACs generally need to be withheld for only 1 to 3 days prior to major surgical procedures in patients with normal renal function (creatinine clearance [CrCl] >30 mL/min using the Cockcroft-Gault formula).20 The available oral direct factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban) should generally be stopped 24 hours prior to a procedure that has a low bleeding risk, and 48 hours prior to procedures with high bleeding risk (TABLE 411,20).20 These medications may need to be withheld for an additional 1 to 2 days in patients with acute kidney injury or stage IV kidney disease.20
Dabigatran. About 80% of dabigatran is excreted renally, so its elimination is much more dependent on renal function than is that of the oral direct factor Xa inhibitors.20 Therefore, it generally needs to be withheld for at least 1 to 2 days longer than the oral factor Xa inhibitors unless CrCl >80 mL/min (TABLE 411,20).20
3. Is preoperative bridging with parenteral anticoagulation necessary?
In certain instances, patients who have a high thromboembolic risk and are discontinuing warfarin therapy may require bridging therapy with a low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH). If a patient’s CrCl is <30 mL/min, then UFH is the preferred agent for perioperative bridging.21
But before any decision is made, it’s best to have a good understanding of what the guidelines—and the literature—have to say.
Key studies and guidelines
The 2012 CHEST guidelines recommend providing bridge therapy for any patient at high risk for thromboembolism (>10% annual risk) and consideration of bridge therapy in the setting of moderate clotting risk (5%-10% annual risk), depending on specific patient and procedural risk factors (TABLE 13,5,6,9-11).3
In 2015, a landmark clinical trial was published that significantly shaped how patients taking warfarin are managed periprocedurally.22 The Bridge (Bridging anticoagulation in patients who require temporary interruption of warfarin therapy for an elective invasive procedure or surgery) trial was the first prospective, randomized controlled trial to assess the efficacy and safety of parenteral bridging in patients with AF taking warfarin and undergoing an elective surgery.
Patients in the trial received either dalteparin at a therapeutic dose of 100 IU/kg or a matching placebo administered subcutaneously bid from 3 days before the procedure until 24 hours before the procedure, and then for 5 to 10 days after the procedure. The incidence of thromboembolic events was not significantly lower in the dalteparin group than in the placebo group (0.3% vs 0.4%, respectively; P=.73), while major bleeding rates were nearly 3-fold higher in the dalteparin group (3.2% vs 1.3%; P=.005). The trial concluded that placebo “was noninferior to perioperative bridging with LMWH for the prevention of arterial thromboembolism and decreased the risk of major bleeding.”22
Patients excluded from the trial included those with a mechanical heart valve, or a recent (within 3 months) embolism, stroke, or TIA, and only 3% of enrolled patients would have been classified as having a high bleeding risk according to CHEST guidelines.3,22
A prospective observational registry study produced similar findings and found that those patients who received bridging had more bleeding events and a higher incidence of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days than those who did not receive bridging.23 Other retrospective cohort studies comparing bridging to no bridging strategies in patients taking warfarin for VTE, mechanical heart valves, or AF have also failed to show a reduction in the incidence of thrombotic events with LMWH bridging.24,25
In 2016, the European Society of Cardiology suggested that “bridging does not seem to be beneficial, except in patients with mechanical heart valves.”26 Similarly, the 2016 Anticoagulation Forum guidelines state that “most patients with VTE can safely interrupt warfarin for invasive procedures without bridge therapy,” and that bridge therapy should be “reserved for those at highest recurrent VTE risk (eg, VTE within the previous month; prior history of recurrent VTE during anticoagulation therapy interruption; undergoing a procedure with high inherent risk for VTE, such as joint replacement surgery or major abdominal cancer resection).”21 They go on to state that even in these high-risk groups, the clinical decision to use bridging therapy needs to carefully weigh the benefits against the potential risks of bleeding.21
Controversy also surrounds the intensity of LMWH bridging. The Anticoagulation Forum guidelines state that the use of prophylactic rather than therapeutic dose LMWH may be considered, while the CHEST guidelines do not make a firm recommendation regarding LMWH dose while bridging.3,21 Ultimately, in patients who receive perioperative bridging with LMWH, the CHEST guidelines recommend that it should be stopped 24 hours prior to the procedure and resumed in accordance with the bleeding risk of the procedure (ie, prophylactic doses may be appropriate within 24 hours postprocedure, while full treatment doses may need to be delayed for 48 to 72 hours if surgical bleeding risk is high).3 UFH bridge therapy may be stopped 4 to 6 hours prior to surgery.3
DOACs. Given the rapid onset and relatively short half-lives of DOACs, use of a parenteral bridging agent is generally not necessary or recommended before or after an invasive procedure in patients taking a DOAC.20
4. When should oral anticoagulation be resumed postoperatively, and at what intensity?
Warfarin can generally be resumed the same day as the procedure (in the evening), assuming there are no active bleeding complications.3,11 Once fully reversed, it generally takes around 5 days for warfarin to become fully therapeutic, so it can be started soon after surgery without increasing the risk for early postoperative bleeding.20
DOACs. Consider the patient’s individual and procedural risks for bleeding when determining when to resume a DOAC postoperatively. That’s because unlike warfarin, which takes several days to take full effect, DOACs provide a nearly immediate anticoagulation effect.20,21 For procedures that have a low bleeding risk, it is recommended to resume therapeutic anticoagulation 24 hours after the procedure has ended.3,11,20 For procedures that have a high risk for bleeding, resumption of therapeutic anticoagulation should be delayed until 48 to 72 hours after the procedure has ended.3,11,20
5. Is postoperative bridging with parenteral anticoagulation necessary?
Warfarin. If a patient was deemed to be at sufficient VTE risk to be bridged preoperatively, then that patient likely also should be bridged postoperatively, particularly if the surgery itself is associated with a heightened thrombotic risk. While warfarin can generally be resumed postoperatively the same day as the procedure, full therapeutic doses of a LMWH should not be initiated sooner than 24 hours postoperatively, and initiation should be delayed for 48 to 72 hours for procedures with the highest bleeding risk (such as neurosurgery).3,11,21 Prophylactic doses of LMWH can generally be resumed as early as 12 hours postoperatively for procedures with high VTE risk (such as major orthopedic surgery).17
DOACs. In patients undergoing a procedure that carries both a high thromboembolic and high bleeding risk (such as major orthopedic surgery), initiation of a full-dose DOAC may need to be delayed for 2 to 3 days; however, more immediate VTE prophylaxis is usually necessary.3,17 Prophylaxis after such procedures can begin 12 hours after the procedure with a low-intensity LMWH, which should be continued until it is deemed safe to resume full-intensity DOAC therapy.3,17,18 If the patient is undergoing major orthopedic surgery, an FDA-approved prophylactic dose of a DOAC could be a temporary alternative to LMWH.27
Ms. P’s upcoming colonoscopy may require a biopsy and would be classified as a procedure with low bleeding risk (per TABLE 3), so warfarin should be withheld prior to her procedure. You could check her INR 5 to 7 days before her colonoscopy to guide how many doses need to be withheld; however, given the patient’s tight INR control over the previous 6 months, you can assume her INR will be in goal range at that check. As a result, you recommend that she avoid an extra INR check and stop taking her warfarin 5 days prior to the colonoscopy.
Ms. P has a CHA2DS2VASc score of 3, which puts her at a relatively low risk for acute ischemic stroke over the next 1 to 2 weeks. Given the results of the BRIDGE trial, you recommend no parenteral bridging agent before or after her procedure. You also recommend that the patient resume her usual dose of warfarin the same day as her procedure (in the evening) unless her gastroenterologist recommends otherwise. You schedule her for a follow-up INR 5 to 7 days after her colonoscopy.
Mr. Q’s total knee arthroplasty (TKA)—a procedure associated with a high risk of bleeding—requires an interruption in his apixaban therapy. Additionally, he is at high risk for recurrent thromboembolism, given his history of recurrent, unprovoked DVTs; however, he is past the highest risk period (VTE within the past 3 months; his last one was 9 months ago). He is otherwise healthy and has normal renal function, so his apixaban should be withheld for a total of 4 doses (48 hours) prior to his procedure. He should resume his full dose of apixaban 48 to 72 hours after his procedure to minimize the risk for bleeding.
However, given that a TKA is a procedure associated with a high rate of postoperative VTE, initiate prophylactic anticoagulation (such as enoxaparin 40 mg subcutaneously daily or apixaban 2.5 mg PO bid) about 12 hours after the procedure and continue it until full-dose apixaban is resumed.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, 1000 E. University Ave., Dept. 3375, Laramie, WY 82071; [email protected].
1. Connelly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation. 2013;128:721-728.
3. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S.
4. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and newer oral anticoagulants for the long-term prevention and treatment of arterial and venous thromboembolism. Department of Veteran Affairs Evidence-Based Synthesis Project #09-010; 2012. Available at: https://www.ncbi.nlm.nih.gov/books/NBK97541/. Accessed October 15, 2017.
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and Expert Panel Report. Chest. 2016;149:315-352.
6. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:2246-2280.
7. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations — United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2012 June 8;61:401-404. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed October 15, 2017.
8. Anderson FA, Wheeler HB, Goldberg HJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933-938.
9. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451-2496.
10. Garwood CL, Korkis B, Grande D, et al. Anticoagulation bridge therapy in patients with atrial fibrillation: recent updates provide a rebalance of risk and benefit. Pharmacotherapy. 2017;37:712-714.
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2017;69:871-898.
12. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med. 2010;153:523-531.
13. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959-1967.
14. Tosetto A, Testa S, Martinelli I, et al. External validation of the DASH prediction rule: a retrospective cohort study. J Thromb Haemost. 2017;15:1963-1970.
15. Rodger MA, Le Gal G, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017;356:j1065.
16. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism.
17. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. Chest. 2008;133:381S-453S.
18. Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954-2962.
19. Eisen GM, Baron TH, Dominitz JA, et al. Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastrointest Endosc. 2002;55:775-779.
20. Burnett AE, Mahan CE, Vazquez SR. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
21. Witt DM, Clark NP, Kaatz S, et al. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:187-205.
22. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
23. Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131: 488-494.
24. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175;1163-1168.
25. Sjögren V, Grzymala-Lubanski B, Renlund H, et al. Safety and efficacy of bridging with low-molecular-weight heparin during temporary interruptions of warfarin: a register-based cohort study. Clin Appl Thromb Hemost. 2017;23:961-966.
26. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962.
27. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
Debra P is a 62-year-old African American woman who calls your office to report that she has an upcoming routine colonoscopy planned in 2 weeks. She has been taking warfarin for the past 2 years for ischemic stroke prevention secondary to atrial fibrillation (AF), and her gastroenterologist recommended that she contact her family physician (FP) to discuss periprocedural anticoagulation plans. Ms. P is currently taking warfarin 5 mg on Mondays, Wednesdays, and Fridays, and 2.5 mg all other days of the week. Her international normalized ratio (INR) was 2.3 when it was last checked 2 weeks ago, and it has been stable and within goal range for the past 6 months. Her medical history includes AF, well-controlled hypertension, and type 2 diabetes mellitus, as well as gout and stage 3 chronic kidney disease. Ms. P denies any history of stroke or transient ischemic attack (TIA). She is requesting instructions on how to manage her warfarin before and after her upcoming colonoscopy.
Jerry Q is a 68-year-old Caucasian man with longstanding osteoarthritis who is scheduled to undergo a total left knee arthroplasty in one week. His orthopedic surgeon recommended that he contact his FP for instructions regarding managing apixaban perioperatively. Jerry has been taking apixaban 5 mg bid for the past 9 months due to a history of recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE) (both unprovoked). Mr. Q had been taking warfarin following his first DVT 4 years ago, but, after reporting that INR monitoring was a burden, he was started on apixaban. The patient has normal renal function and is relatively healthy otherwise. How should apixaban be managed before and after his upcoming surgery?
Each year, approximately 15% to 20% of patients taking an oral anticoagulant undergo a procedure that carries a heightened risk for bleeding.1,2 Stopping oral anticoagulation is often necessary before—and sometimes briefly after—many of these procedures in order to minimize the risk of bleeding.3 This means that countless decisions must be made by health care providers each year regarding if, when, and how to pause and resume oral anticoagulation. These decisions are not always straightforward, especially when you consider the risks for thrombosis and bleeding that are unique to the procedure and to the individual patient.
With these variables in mind, the health care provider must make decisions regarding anticoagulation during the periprocedural period based on the following 5 questions:
- Will this patient need to have his/her oral anticoagulant stopped prior to the procedure?
- If the patient’s oral anticoagulation needs to be held, when should it be stopped and for how long?
- Will periprocedural bridging with a parenteral anticoagulant be necessary prior to the procedure?
- When should the patient resume his or her oral anticoagulant after the procedure, and at what dosage?
- Will bridging with a parenteral anticoagulant be necessary after the procedure?
Before addressing these 5 questions, though, physicians must assess patients’ thrombotic and bleeding risks.4-6
Anticoagulant regimens and the risks of discontinuing them
The 2 most common indications for long-term oral anticoagulation are venous thromboembolism (VTE), which occurs in approximately one million Americans every year,7,8 and stroke prevention in the setting of AF (AF occurs in 3-6 million US adults per year).6
Warfarin is also often used in patients with mechanical heart valves for long-term stroke prevention; however, direct oral anticoagulants (DOACs) are not recommended for patients with mechanical heart valves because trials have not yet demonstrated their safety or efficacy in this population.4,5,9
Who’s at highest risk for an acute thromboembolic event?
When planning for interruptions in oral anticoagulation, it is important to identify patients at highest risk for an acute thromboembolic event. Patients with 10% or higher annual risk for VTE or ischemic stroke are generally placed into this high-risk category (TABLE 13,5,6,9-11).3 Keep in mind that the absolute risk for thromboembolism during a brief period of oral coagulation interruption is relatively low, even in those patients considered to be at high risk. Using a mathematical approach (although simplistic), a patient with a 10% annual risk for a thromboembolic event would have <0.3% chance for developing such an event in the acute phase, even if their anticoagulation was withheld for up to 10 days ([10%/365 days] × 10 days).
Patients with mechanical heart valves. Nearly all patients with a mechanical heart valve are at moderate to high risk for ischemic stroke.3
For patients with AF, the CHADS2 and CHA2DS2-VASc scoring tools can be used to estimate annual thrombosis risk based on the presence of risk factors (TABLE 210,11).6,9-11 It should be noted, however, that these scoring tools have not been validated specifically for periprocedural risk estimations. Nonetheless, the latest 2017 American College of Cardiology (ACC) guidelines recommend the use of the CHA2DS2-VASc scoring tool for making decisions regarding perioperative bridging in patients with AF.11
Patients with previous VTE. Multiple aspects of a patient’s past medical history need to be taken into account when estimating annual and acute risk for VTE. Patients at the highest risk for VTE recurrence (annual VTE risk ≥10%) include those with recent VTE (past 90 days), active malignancy, and/or severe thrombophilias (TABLE 13,5,6,9-11).3,5,6 Patients without any of these features can still be at moderate risk for recurrent VTE, as a single VTE without a clear provoking factor can confer a 5% to 10% annualized risk for recurrence.12,13 Previous proximal DVT and PE are associated with a higher risk for recurrence than a distal DVT, and males have a higher recurrence risk than females.5,12 There are scoring tools, such as DASH (D-dimer, Age, Sex, Hormones) and the “Men Continue and HERDOO2,” that can help estimate annualized risk for VTE recurrence; however, they are not validated (nor particularly useful) when making decisions in the perioperative period.14,15
Additional risk factors. Consider additional risk factors for thromboembolism, including estrogen/hormone replacement therapy, pregnancy, leg or hip fractures, immobility, trauma, spinal cord injury, central venous lines, congestive heart failure, thrombophilia, increased age, obesity, and varicose veins.5,16
In addition, some surgeries have a higher inherent risk for thrombosis. Major orthopedic surgery (knee and hip arthroplasty, hip fracture surgery) and surgeries for major trauma or spinal cord injuries are associated with an exceedingly high rate of VTE.17 Similarly, coronary artery bypass surgery, heart valve replacement, and carotid endarterectomies carry the highest risk for acute ischemic stroke.3
Who’s at highest risk for bleeding?
Establishing the bleeding risk associated with a procedure is imperative prior to urgent and elective surgeries to help determine when anticoagulation therapy should be discontinued and reinitiated, as well as whether bridging therapy is appropriate. The 2012 CHEST guidelines state that bleeding risk should be assessed based on timing of anticoagulation relative to surgery and whether the anticoagulation is being used as prophylaxis for, or treatment of, thromboembolism.3 Categorizing procedures as having a minimal, low, or high risk for bleeding can be helpful in making anticoagulation decisions (TABLE 3).3,18-21
In addition to the bleeding risk associated with procedures, patient-specific factors need to be considered. A bleeding event within the past 3 months, platelet abnormalities, a supratherapeutic INR at the time of surgery, a history of bleeding from previous bridging, a bleed history with a similar procedure, and a high HAS-BLED (Hypertension, Abnormal renal or liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol usage) score are all factors that elevate the risk for perioperative bleeding.10,11 Although validated only in patients taking warfarin, the HAS-BLED scoring system can be utilized in patients with AF to estimate annual risk for major bleeding (TABLE 210,11).10
With this risk information in mind, it’s time to move on to the 5 questions you’ll need to ask.
1. Should the patient’s oral anticoagulation be stopped prior to the upcoming procedure?
The answer, of course, hinges on the patient’s risk of bleeding.
Usually, it is not necessary to withhold any doses of oral anticoagulation if your patient is scheduled for a procedure with minimal risk for bleeding (TABLE 33,18-21).3 However, it may be reasonable to stop anticoagulation if your patient has additional features that predispose to high bleeding risk (eg, hemophilia, Von Willebrand disease, etc). The CHEST guidelines recommend adding an oral prohemostatic agent (eg, tranexamic acid) if anticoagulation will be continued during a dental procedure.3
If your patient is undergoing any other procedure that has a low to high risk for bleeding, oral anticoagulation should be withheld prior to the procedure in most instances,3,11 although there are exceptions. For example, cardiac procedures, such as AF catheter ablation and cardiac pacemaker placement, are often performed with uninterrupted oral anticoagulation despite their bleeding risk category.3
When in doubt, discuss the perceived bleeding and clotting risks directly with the specialist performing the procedure. In patients who have had a VTE or ischemic stroke within the past 3 months, consider postponing the invasive procedure until the patient is beyond this period of highest thrombotic risk.11
2. How far in advance of the procedure should the oral anticoagulant be withheld?
Warfarin may need to be stopped anywhere from 2 to 5 days prior to the procedure, depending on a number of variables.
Warfarin has a half-life of approximately 36 hours, so it can take 3 to 5 days for warfarin concentrations to drop to safe levels for procedures with low to moderate bleeding risk and 5 to 7 days for procedures with high bleeding risk.21 The 2012 CHEST guidelines recommend that warfarin therapy be discontinued 5 days prior to surgery to minimize the risk for bleeding.3 The Anticoagulation Forum, a leading expert panel that produced a set of useful anticoagulation guidelines in 2016, recommends stopping warfarin 4 to 5 days prior to a procedure.21 If the provider chooses to withhold warfarin before a procedure with minimal bleeding risk, it should be stopped 2 to 3 days prior.3
Consider checking INR values the week before. A 2017 consensus statement from the ACC recommends that the timing of warfarin discontinuation be based on an INR value taken 5 to 7 days prior to the surgical procedure.11 This allows for a more tailored approach to preparing the patient for surgery. If the INR is below goal range, warfarin may need to be withheld for only 3 to 4 days prior to a procedure. Conversely, INRs above goal range may require warfarin to be held 6 or more days, depending on the degree of INR elevation.
While not always feasible in clinical practice, the CHEST guidelines recommend obtaining an INR value the day prior to the procedure to determine if the INR value is low enough to proceed with surgery, or if a low dose of oral vitamin K needs to be administered to ensure that the INR is in a safe range the following day.3
DOACs
DOACs can be withheld for much shorter durations preoperatively than warfarin.
When withholding anticoagulants, the goal is to have a low amount of anticoagulant effect (12%-25%) present during low-risk procedures and a nominal amount of anticoagulant effect (3%-6%) present for high-risk procedures.20 DOACs have much shorter half-lives than warfarin (7-19 hours vs 36-48 hours, respectively), so they can be withheld for much shorter durations preoperatively.20 For patients undergoing procedures that are considered to have a minimal risk for bleeding (such as minor dental and dermatologic procedures), DOACs do not generally need to be withheld; however, it may be ideal to time the procedure when the DOAC is at a trough concentration (before the next dose is due).3
DOACs generally need to be withheld for only 1 to 3 days prior to major surgical procedures in patients with normal renal function (creatinine clearance [CrCl] >30 mL/min using the Cockcroft-Gault formula).20 The available oral direct factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban) should generally be stopped 24 hours prior to a procedure that has a low bleeding risk, and 48 hours prior to procedures with high bleeding risk (TABLE 411,20).20 These medications may need to be withheld for an additional 1 to 2 days in patients with acute kidney injury or stage IV kidney disease.20
Dabigatran. About 80% of dabigatran is excreted renally, so its elimination is much more dependent on renal function than is that of the oral direct factor Xa inhibitors.20 Therefore, it generally needs to be withheld for at least 1 to 2 days longer than the oral factor Xa inhibitors unless CrCl >80 mL/min (TABLE 411,20).20
3. Is preoperative bridging with parenteral anticoagulation necessary?
In certain instances, patients who have a high thromboembolic risk and are discontinuing warfarin therapy may require bridging therapy with a low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH). If a patient’s CrCl is <30 mL/min, then UFH is the preferred agent for perioperative bridging.21
But before any decision is made, it’s best to have a good understanding of what the guidelines—and the literature—have to say.
Key studies and guidelines
The 2012 CHEST guidelines recommend providing bridge therapy for any patient at high risk for thromboembolism (>10% annual risk) and consideration of bridge therapy in the setting of moderate clotting risk (5%-10% annual risk), depending on specific patient and procedural risk factors (TABLE 13,5,6,9-11).3
In 2015, a landmark clinical trial was published that significantly shaped how patients taking warfarin are managed periprocedurally.22 The Bridge (Bridging anticoagulation in patients who require temporary interruption of warfarin therapy for an elective invasive procedure or surgery) trial was the first prospective, randomized controlled trial to assess the efficacy and safety of parenteral bridging in patients with AF taking warfarin and undergoing an elective surgery.
Patients in the trial received either dalteparin at a therapeutic dose of 100 IU/kg or a matching placebo administered subcutaneously bid from 3 days before the procedure until 24 hours before the procedure, and then for 5 to 10 days after the procedure. The incidence of thromboembolic events was not significantly lower in the dalteparin group than in the placebo group (0.3% vs 0.4%, respectively; P=.73), while major bleeding rates were nearly 3-fold higher in the dalteparin group (3.2% vs 1.3%; P=.005). The trial concluded that placebo “was noninferior to perioperative bridging with LMWH for the prevention of arterial thromboembolism and decreased the risk of major bleeding.”22
Patients excluded from the trial included those with a mechanical heart valve, or a recent (within 3 months) embolism, stroke, or TIA, and only 3% of enrolled patients would have been classified as having a high bleeding risk according to CHEST guidelines.3,22
A prospective observational registry study produced similar findings and found that those patients who received bridging had more bleeding events and a higher incidence of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days than those who did not receive bridging.23 Other retrospective cohort studies comparing bridging to no bridging strategies in patients taking warfarin for VTE, mechanical heart valves, or AF have also failed to show a reduction in the incidence of thrombotic events with LMWH bridging.24,25
In 2016, the European Society of Cardiology suggested that “bridging does not seem to be beneficial, except in patients with mechanical heart valves.”26 Similarly, the 2016 Anticoagulation Forum guidelines state that “most patients with VTE can safely interrupt warfarin for invasive procedures without bridge therapy,” and that bridge therapy should be “reserved for those at highest recurrent VTE risk (eg, VTE within the previous month; prior history of recurrent VTE during anticoagulation therapy interruption; undergoing a procedure with high inherent risk for VTE, such as joint replacement surgery or major abdominal cancer resection).”21 They go on to state that even in these high-risk groups, the clinical decision to use bridging therapy needs to carefully weigh the benefits against the potential risks of bleeding.21
Controversy also surrounds the intensity of LMWH bridging. The Anticoagulation Forum guidelines state that the use of prophylactic rather than therapeutic dose LMWH may be considered, while the CHEST guidelines do not make a firm recommendation regarding LMWH dose while bridging.3,21 Ultimately, in patients who receive perioperative bridging with LMWH, the CHEST guidelines recommend that it should be stopped 24 hours prior to the procedure and resumed in accordance with the bleeding risk of the procedure (ie, prophylactic doses may be appropriate within 24 hours postprocedure, while full treatment doses may need to be delayed for 48 to 72 hours if surgical bleeding risk is high).3 UFH bridge therapy may be stopped 4 to 6 hours prior to surgery.3
DOACs. Given the rapid onset and relatively short half-lives of DOACs, use of a parenteral bridging agent is generally not necessary or recommended before or after an invasive procedure in patients taking a DOAC.20
4. When should oral anticoagulation be resumed postoperatively, and at what intensity?
Warfarin can generally be resumed the same day as the procedure (in the evening), assuming there are no active bleeding complications.3,11 Once fully reversed, it generally takes around 5 days for warfarin to become fully therapeutic, so it can be started soon after surgery without increasing the risk for early postoperative bleeding.20
DOACs. Consider the patient’s individual and procedural risks for bleeding when determining when to resume a DOAC postoperatively. That’s because unlike warfarin, which takes several days to take full effect, DOACs provide a nearly immediate anticoagulation effect.20,21 For procedures that have a low bleeding risk, it is recommended to resume therapeutic anticoagulation 24 hours after the procedure has ended.3,11,20 For procedures that have a high risk for bleeding, resumption of therapeutic anticoagulation should be delayed until 48 to 72 hours after the procedure has ended.3,11,20
5. Is postoperative bridging with parenteral anticoagulation necessary?
Warfarin. If a patient was deemed to be at sufficient VTE risk to be bridged preoperatively, then that patient likely also should be bridged postoperatively, particularly if the surgery itself is associated with a heightened thrombotic risk. While warfarin can generally be resumed postoperatively the same day as the procedure, full therapeutic doses of a LMWH should not be initiated sooner than 24 hours postoperatively, and initiation should be delayed for 48 to 72 hours for procedures with the highest bleeding risk (such as neurosurgery).3,11,21 Prophylactic doses of LMWH can generally be resumed as early as 12 hours postoperatively for procedures with high VTE risk (such as major orthopedic surgery).17
DOACs. In patients undergoing a procedure that carries both a high thromboembolic and high bleeding risk (such as major orthopedic surgery), initiation of a full-dose DOAC may need to be delayed for 2 to 3 days; however, more immediate VTE prophylaxis is usually necessary.3,17 Prophylaxis after such procedures can begin 12 hours after the procedure with a low-intensity LMWH, which should be continued until it is deemed safe to resume full-intensity DOAC therapy.3,17,18 If the patient is undergoing major orthopedic surgery, an FDA-approved prophylactic dose of a DOAC could be a temporary alternative to LMWH.27
Ms. P’s upcoming colonoscopy may require a biopsy and would be classified as a procedure with low bleeding risk (per TABLE 3), so warfarin should be withheld prior to her procedure. You could check her INR 5 to 7 days before her colonoscopy to guide how many doses need to be withheld; however, given the patient’s tight INR control over the previous 6 months, you can assume her INR will be in goal range at that check. As a result, you recommend that she avoid an extra INR check and stop taking her warfarin 5 days prior to the colonoscopy.
Ms. P has a CHA2DS2VASc score of 3, which puts her at a relatively low risk for acute ischemic stroke over the next 1 to 2 weeks. Given the results of the BRIDGE trial, you recommend no parenteral bridging agent before or after her procedure. You also recommend that the patient resume her usual dose of warfarin the same day as her procedure (in the evening) unless her gastroenterologist recommends otherwise. You schedule her for a follow-up INR 5 to 7 days after her colonoscopy.
Mr. Q’s total knee arthroplasty (TKA)—a procedure associated with a high risk of bleeding—requires an interruption in his apixaban therapy. Additionally, he is at high risk for recurrent thromboembolism, given his history of recurrent, unprovoked DVTs; however, he is past the highest risk period (VTE within the past 3 months; his last one was 9 months ago). He is otherwise healthy and has normal renal function, so his apixaban should be withheld for a total of 4 doses (48 hours) prior to his procedure. He should resume his full dose of apixaban 48 to 72 hours after his procedure to minimize the risk for bleeding.
However, given that a TKA is a procedure associated with a high rate of postoperative VTE, initiate prophylactic anticoagulation (such as enoxaparin 40 mg subcutaneously daily or apixaban 2.5 mg PO bid) about 12 hours after the procedure and continue it until full-dose apixaban is resumed.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, 1000 E. University Ave., Dept. 3375, Laramie, WY 82071; [email protected].
Debra P is a 62-year-old African American woman who calls your office to report that she has an upcoming routine colonoscopy planned in 2 weeks. She has been taking warfarin for the past 2 years for ischemic stroke prevention secondary to atrial fibrillation (AF), and her gastroenterologist recommended that she contact her family physician (FP) to discuss periprocedural anticoagulation plans. Ms. P is currently taking warfarin 5 mg on Mondays, Wednesdays, and Fridays, and 2.5 mg all other days of the week. Her international normalized ratio (INR) was 2.3 when it was last checked 2 weeks ago, and it has been stable and within goal range for the past 6 months. Her medical history includes AF, well-controlled hypertension, and type 2 diabetes mellitus, as well as gout and stage 3 chronic kidney disease. Ms. P denies any history of stroke or transient ischemic attack (TIA). She is requesting instructions on how to manage her warfarin before and after her upcoming colonoscopy.
Jerry Q is a 68-year-old Caucasian man with longstanding osteoarthritis who is scheduled to undergo a total left knee arthroplasty in one week. His orthopedic surgeon recommended that he contact his FP for instructions regarding managing apixaban perioperatively. Jerry has been taking apixaban 5 mg bid for the past 9 months due to a history of recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE) (both unprovoked). Mr. Q had been taking warfarin following his first DVT 4 years ago, but, after reporting that INR monitoring was a burden, he was started on apixaban. The patient has normal renal function and is relatively healthy otherwise. How should apixaban be managed before and after his upcoming surgery?
Each year, approximately 15% to 20% of patients taking an oral anticoagulant undergo a procedure that carries a heightened risk for bleeding.1,2 Stopping oral anticoagulation is often necessary before—and sometimes briefly after—many of these procedures in order to minimize the risk of bleeding.3 This means that countless decisions must be made by health care providers each year regarding if, when, and how to pause and resume oral anticoagulation. These decisions are not always straightforward, especially when you consider the risks for thrombosis and bleeding that are unique to the procedure and to the individual patient.
With these variables in mind, the health care provider must make decisions regarding anticoagulation during the periprocedural period based on the following 5 questions:
- Will this patient need to have his/her oral anticoagulant stopped prior to the procedure?
- If the patient’s oral anticoagulation needs to be held, when should it be stopped and for how long?
- Will periprocedural bridging with a parenteral anticoagulant be necessary prior to the procedure?
- When should the patient resume his or her oral anticoagulant after the procedure, and at what dosage?
- Will bridging with a parenteral anticoagulant be necessary after the procedure?
Before addressing these 5 questions, though, physicians must assess patients’ thrombotic and bleeding risks.4-6
Anticoagulant regimens and the risks of discontinuing them
The 2 most common indications for long-term oral anticoagulation are venous thromboembolism (VTE), which occurs in approximately one million Americans every year,7,8 and stroke prevention in the setting of AF (AF occurs in 3-6 million US adults per year).6
Warfarin is also often used in patients with mechanical heart valves for long-term stroke prevention; however, direct oral anticoagulants (DOACs) are not recommended for patients with mechanical heart valves because trials have not yet demonstrated their safety or efficacy in this population.4,5,9
Who’s at highest risk for an acute thromboembolic event?
When planning for interruptions in oral anticoagulation, it is important to identify patients at highest risk for an acute thromboembolic event. Patients with 10% or higher annual risk for VTE or ischemic stroke are generally placed into this high-risk category (TABLE 13,5,6,9-11).3 Keep in mind that the absolute risk for thromboembolism during a brief period of oral coagulation interruption is relatively low, even in those patients considered to be at high risk. Using a mathematical approach (although simplistic), a patient with a 10% annual risk for a thromboembolic event would have <0.3% chance for developing such an event in the acute phase, even if their anticoagulation was withheld for up to 10 days ([10%/365 days] × 10 days).
Patients with mechanical heart valves. Nearly all patients with a mechanical heart valve are at moderate to high risk for ischemic stroke.3
For patients with AF, the CHADS2 and CHA2DS2-VASc scoring tools can be used to estimate annual thrombosis risk based on the presence of risk factors (TABLE 210,11).6,9-11 It should be noted, however, that these scoring tools have not been validated specifically for periprocedural risk estimations. Nonetheless, the latest 2017 American College of Cardiology (ACC) guidelines recommend the use of the CHA2DS2-VASc scoring tool for making decisions regarding perioperative bridging in patients with AF.11
Patients with previous VTE. Multiple aspects of a patient’s past medical history need to be taken into account when estimating annual and acute risk for VTE. Patients at the highest risk for VTE recurrence (annual VTE risk ≥10%) include those with recent VTE (past 90 days), active malignancy, and/or severe thrombophilias (TABLE 13,5,6,9-11).3,5,6 Patients without any of these features can still be at moderate risk for recurrent VTE, as a single VTE without a clear provoking factor can confer a 5% to 10% annualized risk for recurrence.12,13 Previous proximal DVT and PE are associated with a higher risk for recurrence than a distal DVT, and males have a higher recurrence risk than females.5,12 There are scoring tools, such as DASH (D-dimer, Age, Sex, Hormones) and the “Men Continue and HERDOO2,” that can help estimate annualized risk for VTE recurrence; however, they are not validated (nor particularly useful) when making decisions in the perioperative period.14,15
Additional risk factors. Consider additional risk factors for thromboembolism, including estrogen/hormone replacement therapy, pregnancy, leg or hip fractures, immobility, trauma, spinal cord injury, central venous lines, congestive heart failure, thrombophilia, increased age, obesity, and varicose veins.5,16
In addition, some surgeries have a higher inherent risk for thrombosis. Major orthopedic surgery (knee and hip arthroplasty, hip fracture surgery) and surgeries for major trauma or spinal cord injuries are associated with an exceedingly high rate of VTE.17 Similarly, coronary artery bypass surgery, heart valve replacement, and carotid endarterectomies carry the highest risk for acute ischemic stroke.3
Who’s at highest risk for bleeding?
Establishing the bleeding risk associated with a procedure is imperative prior to urgent and elective surgeries to help determine when anticoagulation therapy should be discontinued and reinitiated, as well as whether bridging therapy is appropriate. The 2012 CHEST guidelines state that bleeding risk should be assessed based on timing of anticoagulation relative to surgery and whether the anticoagulation is being used as prophylaxis for, or treatment of, thromboembolism.3 Categorizing procedures as having a minimal, low, or high risk for bleeding can be helpful in making anticoagulation decisions (TABLE 3).3,18-21
In addition to the bleeding risk associated with procedures, patient-specific factors need to be considered. A bleeding event within the past 3 months, platelet abnormalities, a supratherapeutic INR at the time of surgery, a history of bleeding from previous bridging, a bleed history with a similar procedure, and a high HAS-BLED (Hypertension, Abnormal renal or liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol usage) score are all factors that elevate the risk for perioperative bleeding.10,11 Although validated only in patients taking warfarin, the HAS-BLED scoring system can be utilized in patients with AF to estimate annual risk for major bleeding (TABLE 210,11).10
With this risk information in mind, it’s time to move on to the 5 questions you’ll need to ask.
1. Should the patient’s oral anticoagulation be stopped prior to the upcoming procedure?
The answer, of course, hinges on the patient’s risk of bleeding.
Usually, it is not necessary to withhold any doses of oral anticoagulation if your patient is scheduled for a procedure with minimal risk for bleeding (TABLE 33,18-21).3 However, it may be reasonable to stop anticoagulation if your patient has additional features that predispose to high bleeding risk (eg, hemophilia, Von Willebrand disease, etc). The CHEST guidelines recommend adding an oral prohemostatic agent (eg, tranexamic acid) if anticoagulation will be continued during a dental procedure.3
If your patient is undergoing any other procedure that has a low to high risk for bleeding, oral anticoagulation should be withheld prior to the procedure in most instances,3,11 although there are exceptions. For example, cardiac procedures, such as AF catheter ablation and cardiac pacemaker placement, are often performed with uninterrupted oral anticoagulation despite their bleeding risk category.3
When in doubt, discuss the perceived bleeding and clotting risks directly with the specialist performing the procedure. In patients who have had a VTE or ischemic stroke within the past 3 months, consider postponing the invasive procedure until the patient is beyond this period of highest thrombotic risk.11
2. How far in advance of the procedure should the oral anticoagulant be withheld?
Warfarin may need to be stopped anywhere from 2 to 5 days prior to the procedure, depending on a number of variables.
Warfarin has a half-life of approximately 36 hours, so it can take 3 to 5 days for warfarin concentrations to drop to safe levels for procedures with low to moderate bleeding risk and 5 to 7 days for procedures with high bleeding risk.21 The 2012 CHEST guidelines recommend that warfarin therapy be discontinued 5 days prior to surgery to minimize the risk for bleeding.3 The Anticoagulation Forum, a leading expert panel that produced a set of useful anticoagulation guidelines in 2016, recommends stopping warfarin 4 to 5 days prior to a procedure.21 If the provider chooses to withhold warfarin before a procedure with minimal bleeding risk, it should be stopped 2 to 3 days prior.3
Consider checking INR values the week before. A 2017 consensus statement from the ACC recommends that the timing of warfarin discontinuation be based on an INR value taken 5 to 7 days prior to the surgical procedure.11 This allows for a more tailored approach to preparing the patient for surgery. If the INR is below goal range, warfarin may need to be withheld for only 3 to 4 days prior to a procedure. Conversely, INRs above goal range may require warfarin to be held 6 or more days, depending on the degree of INR elevation.
While not always feasible in clinical practice, the CHEST guidelines recommend obtaining an INR value the day prior to the procedure to determine if the INR value is low enough to proceed with surgery, or if a low dose of oral vitamin K needs to be administered to ensure that the INR is in a safe range the following day.3
DOACs
DOACs can be withheld for much shorter durations preoperatively than warfarin.
When withholding anticoagulants, the goal is to have a low amount of anticoagulant effect (12%-25%) present during low-risk procedures and a nominal amount of anticoagulant effect (3%-6%) present for high-risk procedures.20 DOACs have much shorter half-lives than warfarin (7-19 hours vs 36-48 hours, respectively), so they can be withheld for much shorter durations preoperatively.20 For patients undergoing procedures that are considered to have a minimal risk for bleeding (such as minor dental and dermatologic procedures), DOACs do not generally need to be withheld; however, it may be ideal to time the procedure when the DOAC is at a trough concentration (before the next dose is due).3
DOACs generally need to be withheld for only 1 to 3 days prior to major surgical procedures in patients with normal renal function (creatinine clearance [CrCl] >30 mL/min using the Cockcroft-Gault formula).20 The available oral direct factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban) should generally be stopped 24 hours prior to a procedure that has a low bleeding risk, and 48 hours prior to procedures with high bleeding risk (TABLE 411,20).20 These medications may need to be withheld for an additional 1 to 2 days in patients with acute kidney injury or stage IV kidney disease.20
Dabigatran. About 80% of dabigatran is excreted renally, so its elimination is much more dependent on renal function than is that of the oral direct factor Xa inhibitors.20 Therefore, it generally needs to be withheld for at least 1 to 2 days longer than the oral factor Xa inhibitors unless CrCl >80 mL/min (TABLE 411,20).20
3. Is preoperative bridging with parenteral anticoagulation necessary?
In certain instances, patients who have a high thromboembolic risk and are discontinuing warfarin therapy may require bridging therapy with a low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH). If a patient’s CrCl is <30 mL/min, then UFH is the preferred agent for perioperative bridging.21
But before any decision is made, it’s best to have a good understanding of what the guidelines—and the literature—have to say.
Key studies and guidelines
The 2012 CHEST guidelines recommend providing bridge therapy for any patient at high risk for thromboembolism (>10% annual risk) and consideration of bridge therapy in the setting of moderate clotting risk (5%-10% annual risk), depending on specific patient and procedural risk factors (TABLE 13,5,6,9-11).3
In 2015, a landmark clinical trial was published that significantly shaped how patients taking warfarin are managed periprocedurally.22 The Bridge (Bridging anticoagulation in patients who require temporary interruption of warfarin therapy for an elective invasive procedure or surgery) trial was the first prospective, randomized controlled trial to assess the efficacy and safety of parenteral bridging in patients with AF taking warfarin and undergoing an elective surgery.
Patients in the trial received either dalteparin at a therapeutic dose of 100 IU/kg or a matching placebo administered subcutaneously bid from 3 days before the procedure until 24 hours before the procedure, and then for 5 to 10 days after the procedure. The incidence of thromboembolic events was not significantly lower in the dalteparin group than in the placebo group (0.3% vs 0.4%, respectively; P=.73), while major bleeding rates were nearly 3-fold higher in the dalteparin group (3.2% vs 1.3%; P=.005). The trial concluded that placebo “was noninferior to perioperative bridging with LMWH for the prevention of arterial thromboembolism and decreased the risk of major bleeding.”22
Patients excluded from the trial included those with a mechanical heart valve, or a recent (within 3 months) embolism, stroke, or TIA, and only 3% of enrolled patients would have been classified as having a high bleeding risk according to CHEST guidelines.3,22
A prospective observational registry study produced similar findings and found that those patients who received bridging had more bleeding events and a higher incidence of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days than those who did not receive bridging.23 Other retrospective cohort studies comparing bridging to no bridging strategies in patients taking warfarin for VTE, mechanical heart valves, or AF have also failed to show a reduction in the incidence of thrombotic events with LMWH bridging.24,25
In 2016, the European Society of Cardiology suggested that “bridging does not seem to be beneficial, except in patients with mechanical heart valves.”26 Similarly, the 2016 Anticoagulation Forum guidelines state that “most patients with VTE can safely interrupt warfarin for invasive procedures without bridge therapy,” and that bridge therapy should be “reserved for those at highest recurrent VTE risk (eg, VTE within the previous month; prior history of recurrent VTE during anticoagulation therapy interruption; undergoing a procedure with high inherent risk for VTE, such as joint replacement surgery or major abdominal cancer resection).”21 They go on to state that even in these high-risk groups, the clinical decision to use bridging therapy needs to carefully weigh the benefits against the potential risks of bleeding.21
Controversy also surrounds the intensity of LMWH bridging. The Anticoagulation Forum guidelines state that the use of prophylactic rather than therapeutic dose LMWH may be considered, while the CHEST guidelines do not make a firm recommendation regarding LMWH dose while bridging.3,21 Ultimately, in patients who receive perioperative bridging with LMWH, the CHEST guidelines recommend that it should be stopped 24 hours prior to the procedure and resumed in accordance with the bleeding risk of the procedure (ie, prophylactic doses may be appropriate within 24 hours postprocedure, while full treatment doses may need to be delayed for 48 to 72 hours if surgical bleeding risk is high).3 UFH bridge therapy may be stopped 4 to 6 hours prior to surgery.3
DOACs. Given the rapid onset and relatively short half-lives of DOACs, use of a parenteral bridging agent is generally not necessary or recommended before or after an invasive procedure in patients taking a DOAC.20
4. When should oral anticoagulation be resumed postoperatively, and at what intensity?
Warfarin can generally be resumed the same day as the procedure (in the evening), assuming there are no active bleeding complications.3,11 Once fully reversed, it generally takes around 5 days for warfarin to become fully therapeutic, so it can be started soon after surgery without increasing the risk for early postoperative bleeding.20
DOACs. Consider the patient’s individual and procedural risks for bleeding when determining when to resume a DOAC postoperatively. That’s because unlike warfarin, which takes several days to take full effect, DOACs provide a nearly immediate anticoagulation effect.20,21 For procedures that have a low bleeding risk, it is recommended to resume therapeutic anticoagulation 24 hours after the procedure has ended.3,11,20 For procedures that have a high risk for bleeding, resumption of therapeutic anticoagulation should be delayed until 48 to 72 hours after the procedure has ended.3,11,20
5. Is postoperative bridging with parenteral anticoagulation necessary?
Warfarin. If a patient was deemed to be at sufficient VTE risk to be bridged preoperatively, then that patient likely also should be bridged postoperatively, particularly if the surgery itself is associated with a heightened thrombotic risk. While warfarin can generally be resumed postoperatively the same day as the procedure, full therapeutic doses of a LMWH should not be initiated sooner than 24 hours postoperatively, and initiation should be delayed for 48 to 72 hours for procedures with the highest bleeding risk (such as neurosurgery).3,11,21 Prophylactic doses of LMWH can generally be resumed as early as 12 hours postoperatively for procedures with high VTE risk (such as major orthopedic surgery).17
DOACs. In patients undergoing a procedure that carries both a high thromboembolic and high bleeding risk (such as major orthopedic surgery), initiation of a full-dose DOAC may need to be delayed for 2 to 3 days; however, more immediate VTE prophylaxis is usually necessary.3,17 Prophylaxis after such procedures can begin 12 hours after the procedure with a low-intensity LMWH, which should be continued until it is deemed safe to resume full-intensity DOAC therapy.3,17,18 If the patient is undergoing major orthopedic surgery, an FDA-approved prophylactic dose of a DOAC could be a temporary alternative to LMWH.27
Ms. P’s upcoming colonoscopy may require a biopsy and would be classified as a procedure with low bleeding risk (per TABLE 3), so warfarin should be withheld prior to her procedure. You could check her INR 5 to 7 days before her colonoscopy to guide how many doses need to be withheld; however, given the patient’s tight INR control over the previous 6 months, you can assume her INR will be in goal range at that check. As a result, you recommend that she avoid an extra INR check and stop taking her warfarin 5 days prior to the colonoscopy.
Ms. P has a CHA2DS2VASc score of 3, which puts her at a relatively low risk for acute ischemic stroke over the next 1 to 2 weeks. Given the results of the BRIDGE trial, you recommend no parenteral bridging agent before or after her procedure. You also recommend that the patient resume her usual dose of warfarin the same day as her procedure (in the evening) unless her gastroenterologist recommends otherwise. You schedule her for a follow-up INR 5 to 7 days after her colonoscopy.
Mr. Q’s total knee arthroplasty (TKA)—a procedure associated with a high risk of bleeding—requires an interruption in his apixaban therapy. Additionally, he is at high risk for recurrent thromboembolism, given his history of recurrent, unprovoked DVTs; however, he is past the highest risk period (VTE within the past 3 months; his last one was 9 months ago). He is otherwise healthy and has normal renal function, so his apixaban should be withheld for a total of 4 doses (48 hours) prior to his procedure. He should resume his full dose of apixaban 48 to 72 hours after his procedure to minimize the risk for bleeding.
However, given that a TKA is a procedure associated with a high rate of postoperative VTE, initiate prophylactic anticoagulation (such as enoxaparin 40 mg subcutaneously daily or apixaban 2.5 mg PO bid) about 12 hours after the procedure and continue it until full-dose apixaban is resumed.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, 1000 E. University Ave., Dept. 3375, Laramie, WY 82071; [email protected].
1. Connelly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation. 2013;128:721-728.
3. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S.
4. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and newer oral anticoagulants for the long-term prevention and treatment of arterial and venous thromboembolism. Department of Veteran Affairs Evidence-Based Synthesis Project #09-010; 2012. Available at: https://www.ncbi.nlm.nih.gov/books/NBK97541/. Accessed October 15, 2017.
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and Expert Panel Report. Chest. 2016;149:315-352.
6. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:2246-2280.
7. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations — United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2012 June 8;61:401-404. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed October 15, 2017.
8. Anderson FA, Wheeler HB, Goldberg HJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933-938.
9. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451-2496.
10. Garwood CL, Korkis B, Grande D, et al. Anticoagulation bridge therapy in patients with atrial fibrillation: recent updates provide a rebalance of risk and benefit. Pharmacotherapy. 2017;37:712-714.
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2017;69:871-898.
12. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med. 2010;153:523-531.
13. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959-1967.
14. Tosetto A, Testa S, Martinelli I, et al. External validation of the DASH prediction rule: a retrospective cohort study. J Thromb Haemost. 2017;15:1963-1970.
15. Rodger MA, Le Gal G, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017;356:j1065.
16. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism.
17. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. Chest. 2008;133:381S-453S.
18. Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954-2962.
19. Eisen GM, Baron TH, Dominitz JA, et al. Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastrointest Endosc. 2002;55:775-779.
20. Burnett AE, Mahan CE, Vazquez SR. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
21. Witt DM, Clark NP, Kaatz S, et al. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:187-205.
22. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
23. Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131: 488-494.
24. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175;1163-1168.
25. Sjögren V, Grzymala-Lubanski B, Renlund H, et al. Safety and efficacy of bridging with low-molecular-weight heparin during temporary interruptions of warfarin: a register-based cohort study. Clin Appl Thromb Hemost. 2017;23:961-966.
26. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962.
27. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
1. Connelly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation. 2013;128:721-728.
3. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S.
4. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and newer oral anticoagulants for the long-term prevention and treatment of arterial and venous thromboembolism. Department of Veteran Affairs Evidence-Based Synthesis Project #09-010; 2012. Available at: https://www.ncbi.nlm.nih.gov/books/NBK97541/. Accessed October 15, 2017.
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and Expert Panel Report. Chest. 2016;149:315-352.
6. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:2246-2280.
7. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations — United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2012 June 8;61:401-404. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed October 15, 2017.
8. Anderson FA, Wheeler HB, Goldberg HJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933-938.
9. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451-2496.
10. Garwood CL, Korkis B, Grande D, et al. Anticoagulation bridge therapy in patients with atrial fibrillation: recent updates provide a rebalance of risk and benefit. Pharmacotherapy. 2017;37:712-714.
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2017;69:871-898.
12. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med. 2010;153:523-531.
13. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959-1967.
14. Tosetto A, Testa S, Martinelli I, et al. External validation of the DASH prediction rule: a retrospective cohort study. J Thromb Haemost. 2017;15:1963-1970.
15. Rodger MA, Le Gal G, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017;356:j1065.
16. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism.
17. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. Chest. 2008;133:381S-453S.
18. Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954-2962.
19. Eisen GM, Baron TH, Dominitz JA, et al. Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastrointest Endosc. 2002;55:775-779.
20. Burnett AE, Mahan CE, Vazquez SR. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
21. Witt DM, Clark NP, Kaatz S, et al. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:187-205.
22. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
23. Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131: 488-494.
24. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175;1163-1168.
25. Sjögren V, Grzymala-Lubanski B, Renlund H, et al. Safety and efficacy of bridging with low-molecular-weight heparin during temporary interruptions of warfarin: a register-based cohort study. Clin Appl Thromb Hemost. 2017;23:961-966.
26. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962.
27. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
PRACTICE RECOMMENDATIONS
› Don’t stop oral anticoagulation for procedures with minimal bleeding risk, such as minor dermatologic, dental, or ophthalmic procedures. C
› Reserve periprocedural bridging with a parenteral anticoagulant for those patients on warfarin who are at highest risk for thromboembolism (those with severe thrombophilia, active thrombosis, or mechanical heart valves). B
› Stop direct oral anticoagulants 24 to 48 hours prior to most invasive procedures, and do not employ periprocedural bridging. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series