User login
Standardizing your approach to dizziness and vertigo
Dizziness. Vertigo. These 2 terms are often used interchangeably by patients, with the sensations described as imbalance, lightheadedness, disorientation, presyncope, confusion—among others. While dizziness is a broad term that is often used to describe all the aforementioned sensations, including vertigo, true vertigo (a specific type of dizziness) is defined as the perception of movement within one’s visual field while stationary.1 Because patients are not usually aware of the distinction, their reports of signs and symptoms can cause much confusion for health care providers, thereby delaying a diagnosis.
International studies have reported the prevalence of both dizziness and vertigo to be between 15% and 36%.2,3 Over half of all patients with dizziness and vertigo are cared for by the family physician (FP), and the sensations combined account for approximately 5% of all family medicine visits.4,5 Additionally, between 2.5% and 4% of all emergency department (ED) visits stem from complaints of dizziness and vertigo, with an incidence of up to 25% in those >65 years of age.6,7
Causes of dizziness and vertigo are broad, ranging from the benign to the life-threatening. It has been reported that upwards of 50% of patients presenting to the FP’s office for dizziness leave without a diagnosis.8 Given the confusion surrounding the terms and their broad differential, this review aims to provide FPs with the tools to accurately discern benign from ominous causes.
Nonvestibular benign causes vastly outnumber life-threatening ones
Causes of dizziness are classified as either vestibular (these cause true vertigo) or nonvestibular in origin, with nonvestibular causes being more common.7
Nonvestibular etiologies: Numerous and varied
Nonvestibular causes are broad, spanning many different body systems. Cardiovascular causes of dizziness may include orthostatic hypotension, cardiac arrhythmia, myocardial infarction, and carotid artery stenosis.4,9 Metabolic causes include complications of diabetes such as hypoglycemia and peripheral neuropathy.4,9 Psychiatric conditions such as anxiety, depression, and bipolar disorder can manifest as dizziness, disorientation, or psychogenic vertigo.4,10 Medications including nonsteroidal anti-inflammatory drugs, anticonvulsants, antipsychotics, and sedatives can all contribute to dizziness.11 Other causes of dizziness include Parkinson’s disease, musculoskeletal disorders, and gait disorders.4,9 Especially in the elderly, sensory deficit (peripheral neuropathy), poor vision, and polypharmacy (≥5 medications) are common causes of dizziness.12
Vestibular etiologies of dizziness = true vertigo
Vestibular causes of a patient’s feelings of dizziness manifest as true vertigo and can be categorized as either central (a dysfunction of one or more parts of the central nervous system that help process balance and spatial information or along the pathway where these sensations are interpreted) or peripheral (a dysfunction of the balance organs of the inner ear) in origin.
Central vestibular causes include vertebrobasilar ischemic stroke, vertebrobasilar insufficiency (transient ischemic attack), vestibular migraines, and meningioma of the cerebellopontine angle and posterior fossa.13
Continue to: Peripheral vestibular causes
Peripheral vestibular causes. Benign paroxysmal positional vertigo (BPPV) represents the most common peripheral diagnosis. It is caused by dislodged otoliths in the posterior semicircular canal. While the majority of BPPV cases are idiopathic in nature, up to 15% may result from previous head injury.14 Other peripheral vestibular causes include vestibular neuronitis, viral labyrinthitis, Meniere’s disease, vestibular schwannoma, perilymphatic fistula, superior semicircular canal dehiscence (SSCD), and head trauma (basilar skull fracture).13
Start with a history: Is it dizziness or true vertigo?
The clinical history typically guides the differential diagnosis (FIGURE). Identifying true vertigo from among other sensations helps to limit the differential because true vertigo is caused by vestibular etiologies only. True vertigo is often reported by patients as “seeing the room spin;” this stems from the perception of motion.1 A notable exception is that patients with orthostatic hypotension will often describe spinning sensations lasting seconds to minutes when they rise from a seated or supine position.
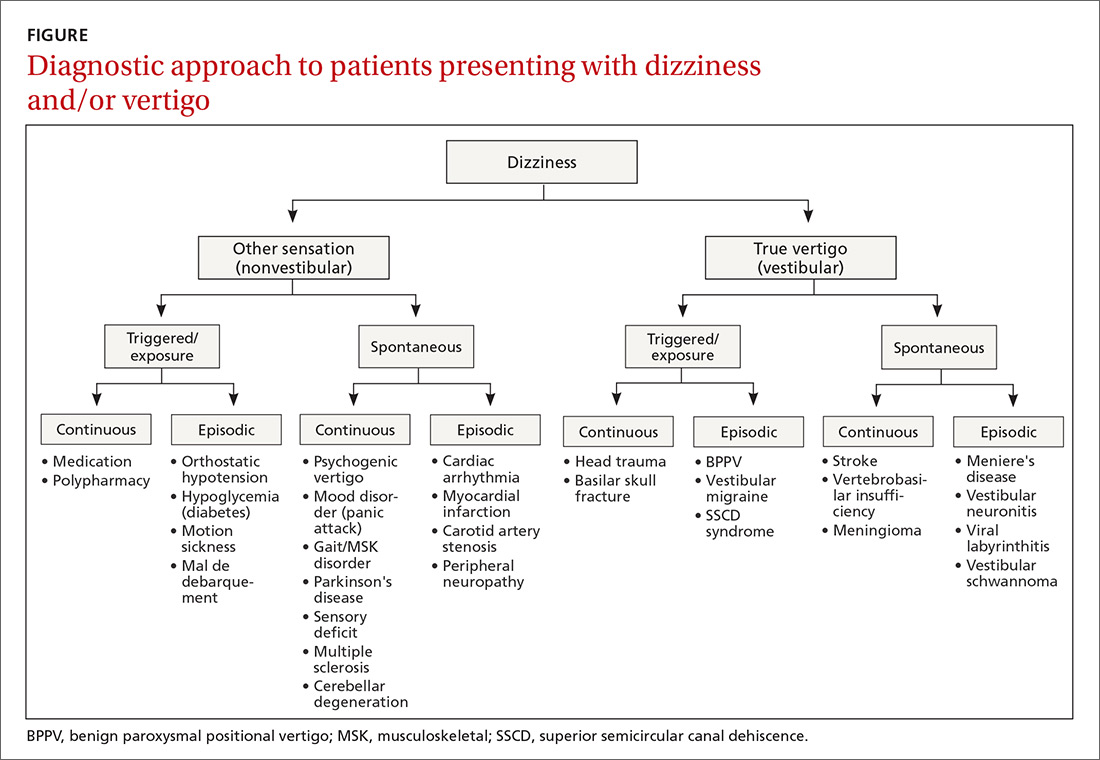
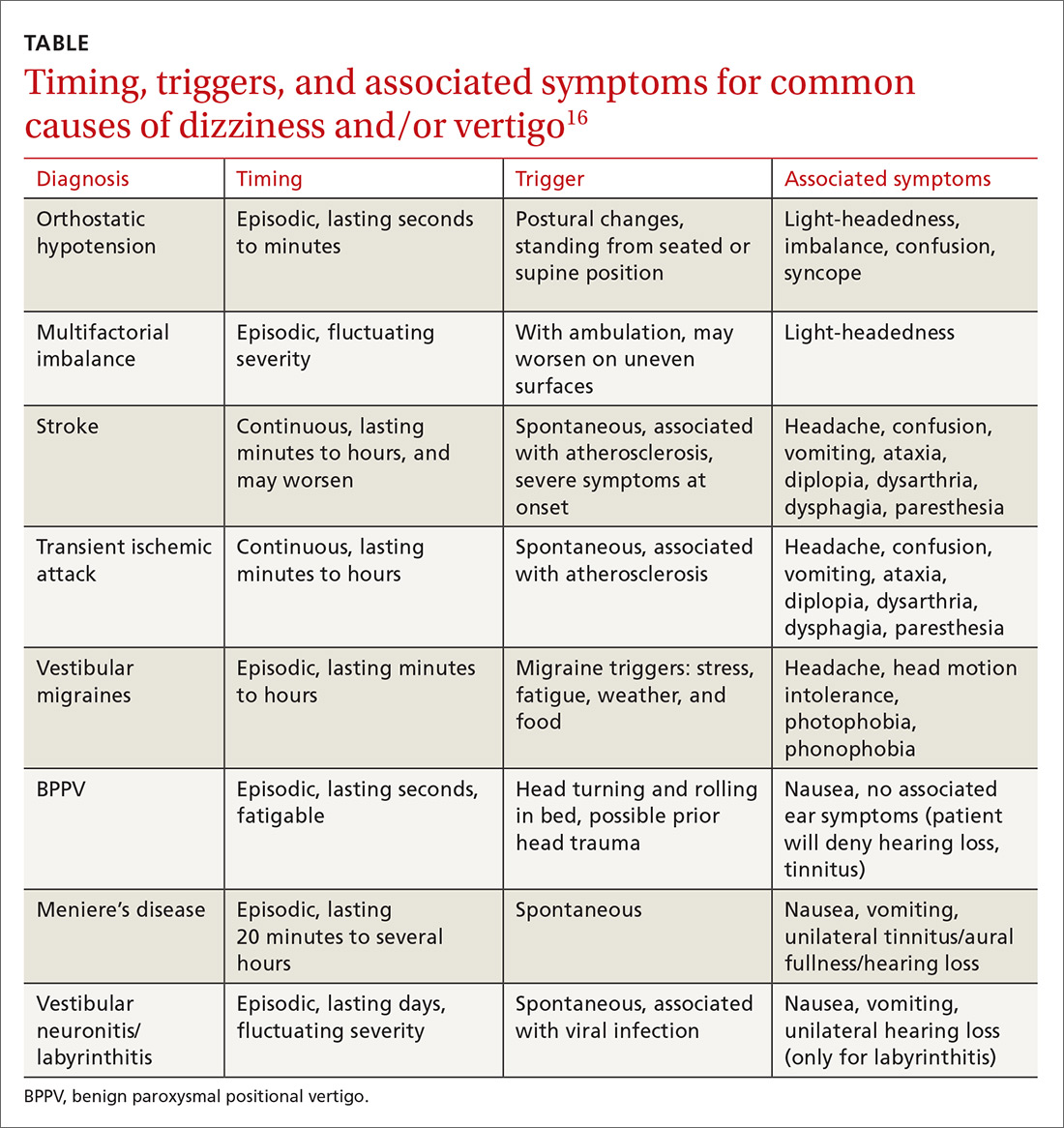
Never depend solely, however, on patient-reported sensations, as not all patients with true vertigo report spinning, and some patients with nonvestibular causes interpret their dizziness as a spinning sensation.15 Therefore, it is important to tease out specifics about the timing, triggers, and associated symptoms in order to further delineate possible causes (TABLE).16
Make a list of current medications. Gather a comprehensive list of current medications, especially from elderly patients, because polypharmacy is a major contributor to dizziness in this population.12 Keep in mind that elderly patients presenting with dizziness/vertigo may have multifactorial balance difficulties, which can be revealed by a detailed history.
Physical exam: May be broad or focused
Given the broad range of causes for dizziness, cardiovascular, head/neck, and neurologic examinations may be performed as part of the work-up, as the clinical history warrants. More typically, time is spent ruling out the following common causes.
Continue to: Orthostatic hypotension
Orthostatic hypotension. Orthostatic vitals are recommended initially in all patients with dizziness, although these may be normal in patients with orthostatic hypotension.17 A diagnosis of orthostatic hypotension can be made with systolic blood pressure decreases of 20 mm Hg or diastolic pressure decreases of ≥10 mm Hg within 3 minutes of standing.18 An increase in heart rate >30 beats per minute after rising from a supine position may indicate autonomic disturbances such as postural orthostatic tachycardia syndrome.19 However, physical examination findings alone are insufficient to make the diagnosis of orthostatic hypotension, and determining the underlying cause of the orthostatic hypotension (dehydration, cardiac dysfunction, pure-autonomic failure, medication adverse effect) is vital.18
BPPV. Perform the Dix-Hallpike maneuver (see https://collections.lib.utah.edu/details?id=177177 for a demonstration of the maneuver) on patients presenting with dizziness with features suggestive of BPPV (eg, attacks of dizziness triggered by head movements).20,21
As BPPV is the most common cause of vestibular dizziness, a negative Dix-Hallpike can be helpful in refining the differential diagnosis.20,21 The maneuver begins with the patient seated, looking directly ahead. To test the left side, ask the patient to turn his/her head 45 degrees to the left. Then direct the patient to lie back, so that the patient’s head is off the edge of the examination table and hyperextended, while maintaining the same head orientation. To test the right side, repeat the procedure with the patient turning his/her head to the right.
Torsional nystagmus is necessary for a positive Dix-Hallpike, which is diagnostic for BPPV. The laterality of BPPV can be determined by paying attention to the fast phase of the torsional nystagmus; the superior pole of the eye beats toward the affected side.14 The patient may report severe dizziness or vertigo during the Dix-Hallpike, but without torsional nystagmus, the test is negative, and the patient does not have BPPV.14
Neurologic causes. Perform a complete neurologic examination in patients who clearly do not have a history of orthostatic hypotension and who have a Dix-Hallpike test that is negative or not indicated.4 Also perform cerebellar testing including rapid-alternating movements, a finger-to-nose test, and a heel-to-shin test. Round out the neurologic exam with an assessment of gait and a Romberg’s test (see https://www.youtube.com/watch?v=U5a4lbmwmOw for a demonstration of Romberg’s test). Romberg’s test is performed by having the patient place his/her feet together with hands at sides and eyes closed. The patient is observed for up to a minute, with a positive test denoted by a loss of balance.
Continue to: Abnormal gait may indicate...
Abnormal gait may indicate peripheral neuropathy, while a positive Romberg’s test suggests involvement of the proprioceptive receptors and/or their pathway.
Central/peripheral vestibular causes. The head impulse, nystagmus, test of skew (HINTS) examination can differentiate between central and peripheral vestibular causes of dizziness and rule out stroke (a central vestibular cause).22 (See https://collections.lib.utah.edu/details?id=177180 for a video demonstrating the steps involved in performing the HINTS examination.) The head impulse (HI) portion of the exam is performed by moving the patient’s head side to side, while having the patient focus on the examiner’s nose. Rapid movements of both eyes (“abnormal” HI) suggest a peripheral etiology, while no eye movement with gaze fixated on the examiner’s nose (“normal” HI) is concerning for stroke or another central cause of vertigo.22
Nystagmus is assessed by having the patient follow the examiner’s finger as it moves in a horizontal direction. Spontaneous horizontal unidirectional nystagmus suggests a peripheral cause, while vertical or torsional bidirectional (direction-changing) nystagmus points to a central cause.22
The test of skew is executed by covering and uncovering each of the patient’s eyes, while asking the patient to look ahead. Vertical deviation of the eye after uncovering suggests a central etiology, more specifically one involving the brainstem.22
Diagnostic testing/imaging has a limited, but pivotal role
There is a limited role for routine laboratory testing in patients with dizziness. However, for those patients with underlying medical conditions (eg, diabetes), which may contribute to the symptoms, routine blood work can be ordered (ie, finger-stick blood glucose test).22
Continue to: More worrisome suspicions
More worrisome suspicions. Patients suspected of cardiac causes should have a full cardiac work-up performed.22 For suspected stroke, brain tumor, or head trauma, specific computed tomography or magnetic resonance imaging can be arranged.22 Carotid doppler can be used if dizziness is suspected to be caused by orthostatic hypotension or a vascular cause.23
Audiologic and vestibular testing. Audiologic testing is not routinely recommended and is only warranted in instances when patients report hearing loss or changes. Referral to an otolaryngologist for vestibular testing is warranted once life-threatening and alternate etiologies have been ruled out, and a vestibular disorder remains at the top of the differential.24
Treatment hinges on cause and may be multifaceted
Treatment hinges on the specific cause of the patient’s dizziness and may involve useful maneuvers, medication, physiotherapy, or perhaps even surgery.
Employ a particle repositioning maneuver for BPPV
A positive Dix-Hallpike test should prompt the use of a particle repositioning maneuver (PRM) to treat BPPV.21 The goal of PRMs, such as the Epley maneuver (see https://www.youtube.com/watch?v=9SLm76jQg3g for a demonstration of this maneuver), is to move the head in such a way as to return displaced otoliths in the semicircular canal back to the utricle. The Epley maneuver is specific for treating posterior semicircular canal BPPV, which is the most common variant.
Performing the Epley maneuver. To perform the Epley PRM for correction of an otolith in the left posterior semicircular canal, ask the patient to sit and look straight ahead. Lay the patient back, while asking the patient to turn his/her head 45 degrees to the left side. Then ask the patient to turn his/her head 45 degrees to the right side. Instruct the patient to maintain the same 45-degree head orientation, while rolling over to his/her right shoulder, ending in the right decubitus position. Conclude the maneuver by having the patient sit up.
Continue to: Performing the barbecue roll maneuver
Performing the barbecue roll maneuver. Different PRMs exist to treat less common variants of BPPV, including the “barbecue roll” maneuver for horizontal BPPV (see https://www.youtube.com/watch?v=mwTmM6uF5yA for a demonstration of this maneuver).25 The barbecue roll maneuver is initiated with the patient looking ahead and lying back. For a left-sided horizontal canal otolith, the patient first turns to the left decubitus position, then moves clockwise to the right decubitus position, stopping at each position for approximately 20 seconds, all while maintaining a straight head position. The patient then turns clockwise into a prone position, pausing, and finally turning into the left decubitus position again. The maneuver is completed with the patient sitting up.
Medications are used to treat symptoms and/or underlying causes
Adjustments in antihypertensives can be made in cases of orthostatic hypotension.17 Antiemetics (ondansetron, promethazine, metoclopramide), antihistamines (meclizine, dimenhydrinate, diphenhydramine), and benzodiazepines (lorazepam, diazepam) may be used during acute and brief vertiginous episodes to decrease symptom severity after central causes have been ruled out.26,27 However, patients with BPPV should avoid these medications as they may blunt central compensation and increase the risk of falls.27 Research has shown betahistine to improve vertigo control only in patients with Meniere’s disease and only when taken regularly and prophylactically.28 Therefore, do not prescribe betahistine for all other causes of dizziness/vertigo.28
Consider physiotherapy
All patients with dizziness/vertigo, and particularly those presenting with primary balance concerns, may benefit from vestibular rehabilitation therapy (VRT). This is an exercise-based program focusing on habituation of dizziness and improvement of postural stability.29 VRT can improve dizziness associated with central and peripheral vestibular lesions, vertigo of uncertain etiology, and psychogenic vertigo.30 Typically, the vestibular physiotherapist will provide home exercises for the patient, reducing the cost and inconvenience of attending multiple sessions.
Surgery and referrals
Referrals for surgery are rare and are typically reserved for refractory causes of vestibular disease, such as Meniere’s disease, BPPV, SSCD syndrome.31
Referral to the ED is warranted for symptom control if an acute vertiginous episode is refractory to initial management. Emergent or urgent neurology consultation is indicated for suspected or confirmed central disorders. Urgent cardiology referral is recommended for patients with symptoms of presyncope/syncope, arrhythmia, or persistent orthostatic hypotension after conservative management. Outpatient referral to an otolaryngologist is warranted if the patient has failed a course of balance physiotherapy, has a persistently positive Dix-Hallpike test after a PRM and vestibular/balance physiotherapy, or has asymmetric hearing loss.
Continue to: Management starts with primary and secondary prevention
Management starts with primary and secondary prevention
Patient education is essential for avoiding potential triggers of dizziness. Patients with orthostatic hypotension should be educated about the need to correct the underlying mechanism, including the need for adequate hydration and recognition of offending medications and contributory conditions/situations (caffeine, heat, standing quickly).17 Encouraging balance maintenance through exercise and physiotherapy can help with gait and musculoskeletal disorders, and reducing harmful habits (smoking, poor diet, no exercise) can lead to overall improved cardiovascular health.32 Advise those with Meniere’s disease to avoid potential triggers such as caffeine, high sodium foods, and alcohol.33
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Otology/Neurotology, Assistant Professor, Department of Otolaryngology, Queen's University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected].
1. Bisdorff A, Von Brevern M, Lempert T, et al. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19:1-13.
2. Mendel B, Bergenius J, Langius-Eklöf A. Dizziness: a common, troublesome symptom but often treatable. J Vestib Res. 2010;20:391-398.
3. Gopinath B, McMahon CM, Rochtchina E, et al. Dizziness and vertigo in an older population: the Blue Mountains prospective cross‐sectional study. Clin Otolaryngol. 2009;34:552-556.
4. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-368.
5. Sloan PD. Dizziness in primary care. Results from the National Ambulatory Care Survey. Fam Pract. 1989;29:33-38.
6. Kerber KA, Meurer WJ, West BT, et al. Dizziness presentations in US emergency departments, 1995–2004. Acad Emerg Med. 2008;15:744-750.
7. Newman-Toker DE, Hsieh YH, Camargo CA Jr, et al. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. 2008;83:765-775.
8. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: vertigo and dizziness. Can Fam Physician. 2007;53:1959.
9. Chan Y. Differential diagnosis of dizziness. Curr Opin in Otolaryngol Head Neck Surg. 2009;17:200-203.
10. Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of chronic dizziness. Arch Otolaryngol Head Neck Surg. 2007;133:170-176.
11. Kutz JW Jr. The dizzy patient. Med Clin North Am. 2010;94:989-1002.
12. Jahn K, Kressig RW, Bridenbaugh SA, et al. Dizziness and unstable gait in old age: etiology, diagnosis and treatment. Dtsch Ärztebl Int. 2015;112:387-393.
13. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
14. Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). CMAJ. 2003;169:681-693.
15. Newman-Toker DE, Dy FJ, Stanton VA, et al. How often is dizziness from primary cardiovascular disease true vertigo? A systematic review. J Gen Intern Med. 2008;23:2087-2094.
16. Newman-Toker DE, Edlow JA. TiTrATE: a novel, evidence-based approach to diagnosing acute dizziness and vertigo. Neurol Clin. 2015;33:577-599.
17. Shibao C, Lipsitz LA, Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. J Clin Hypertens (Greenwich). 2013;15:147-153.
18. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125-126.
19. Agarwal AK, Garg R, Ritch A, et al. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478-480.
20. Halker RB, Barrs DM, Wellik KE, et al. Establishing a diagnosis of benign paroxysmal positional vertigo through the dix-hallpike and side-lying maneuvers: a critically appraised topic. Neurologist. 2008;14:201-204.
21. Hilton MP, Pinder DK. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2014;(12):CD003162.
22. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome. Stroke. 2009;40:3504-3510.
23. Hamaguchi T, Iwasa K, Okino S, et al. Carotid duplex ultrasonography during head-up tilt in patients with orthostatic hypotension. Eur Neurol. 2007;57:219-222.
24. Canadian Society of Otolaryngology - Head & Neck Surgery. Five Things Physicians and Patients Should Question [Internet]. Choosing Wisely Canada. 2016 [cited 2017 August 17]. Available at: https://choosingwiselycanada.org/wp-content/uploads/2017/02/Hospital-medicine.pdf. Accessed August 30, 2017.
25. Lee SH, Kim JS. Benign paroxysmal positional vertigo. J Clin Neurol. 2010;6:51-63.
26. Zatonski T, Temporale H, Holanowska J, et al. Current views of treatment of vertigo and dizziness. J Med Diagn Meth. 2014;2:150.
27. Wipperman J. Dizziness and vertigo. Prim Care Clin Office Pract. 2014;41:115-131
28. Murdin L, Hussain K, Schilder AG. Betahistine for symptoms of vertigo. Cochrane Database Syst Rev. 2016;(6):CD010696.
29. Han BI, Song HS, Kim JS. Vestibular rehabilitation therapy: review of indications, mechanisms, and key exercises. J Clin Neurol. 2011;7:184-196.
30. Jung JY, Kim JS, Chung PS, et al. Effect of vestibular rehabilitation on dizziness in the elderly. Am J Otolaryngol. 2009;30:295-299.
31. Semaan MT, Megerian CA. Meniere’s disease: a challenging and relentless disorder. Otolaryngol Clin North Am. 2011;44:383-403.
32. Pirker W, Katzenschlager R. Gait disorders in adults and the elderly. Wien Klin Wochenschr. 2017;129:81-95.
33. Kirby SE, Yardley L. Physical and psychological triggers for attacks in Ménière’s disease: the patient perspective. Psychother Psychosom. 2012;81:396-398.
Dizziness. Vertigo. These 2 terms are often used interchangeably by patients, with the sensations described as imbalance, lightheadedness, disorientation, presyncope, confusion—among others. While dizziness is a broad term that is often used to describe all the aforementioned sensations, including vertigo, true vertigo (a specific type of dizziness) is defined as the perception of movement within one’s visual field while stationary.1 Because patients are not usually aware of the distinction, their reports of signs and symptoms can cause much confusion for health care providers, thereby delaying a diagnosis.
International studies have reported the prevalence of both dizziness and vertigo to be between 15% and 36%.2,3 Over half of all patients with dizziness and vertigo are cared for by the family physician (FP), and the sensations combined account for approximately 5% of all family medicine visits.4,5 Additionally, between 2.5% and 4% of all emergency department (ED) visits stem from complaints of dizziness and vertigo, with an incidence of up to 25% in those >65 years of age.6,7
Causes of dizziness and vertigo are broad, ranging from the benign to the life-threatening. It has been reported that upwards of 50% of patients presenting to the FP’s office for dizziness leave without a diagnosis.8 Given the confusion surrounding the terms and their broad differential, this review aims to provide FPs with the tools to accurately discern benign from ominous causes.
Nonvestibular benign causes vastly outnumber life-threatening ones
Causes of dizziness are classified as either vestibular (these cause true vertigo) or nonvestibular in origin, with nonvestibular causes being more common.7
Nonvestibular etiologies: Numerous and varied
Nonvestibular causes are broad, spanning many different body systems. Cardiovascular causes of dizziness may include orthostatic hypotension, cardiac arrhythmia, myocardial infarction, and carotid artery stenosis.4,9 Metabolic causes include complications of diabetes such as hypoglycemia and peripheral neuropathy.4,9 Psychiatric conditions such as anxiety, depression, and bipolar disorder can manifest as dizziness, disorientation, or psychogenic vertigo.4,10 Medications including nonsteroidal anti-inflammatory drugs, anticonvulsants, antipsychotics, and sedatives can all contribute to dizziness.11 Other causes of dizziness include Parkinson’s disease, musculoskeletal disorders, and gait disorders.4,9 Especially in the elderly, sensory deficit (peripheral neuropathy), poor vision, and polypharmacy (≥5 medications) are common causes of dizziness.12
Vestibular etiologies of dizziness = true vertigo
Vestibular causes of a patient’s feelings of dizziness manifest as true vertigo and can be categorized as either central (a dysfunction of one or more parts of the central nervous system that help process balance and spatial information or along the pathway where these sensations are interpreted) or peripheral (a dysfunction of the balance organs of the inner ear) in origin.
Central vestibular causes include vertebrobasilar ischemic stroke, vertebrobasilar insufficiency (transient ischemic attack), vestibular migraines, and meningioma of the cerebellopontine angle and posterior fossa.13
Continue to: Peripheral vestibular causes
Peripheral vestibular causes. Benign paroxysmal positional vertigo (BPPV) represents the most common peripheral diagnosis. It is caused by dislodged otoliths in the posterior semicircular canal. While the majority of BPPV cases are idiopathic in nature, up to 15% may result from previous head injury.14 Other peripheral vestibular causes include vestibular neuronitis, viral labyrinthitis, Meniere’s disease, vestibular schwannoma, perilymphatic fistula, superior semicircular canal dehiscence (SSCD), and head trauma (basilar skull fracture).13
Start with a history: Is it dizziness or true vertigo?
The clinical history typically guides the differential diagnosis (FIGURE). Identifying true vertigo from among other sensations helps to limit the differential because true vertigo is caused by vestibular etiologies only. True vertigo is often reported by patients as “seeing the room spin;” this stems from the perception of motion.1 A notable exception is that patients with orthostatic hypotension will often describe spinning sensations lasting seconds to minutes when they rise from a seated or supine position.

Never depend solely, however, on patient-reported sensations, as not all patients with true vertigo report spinning, and some patients with nonvestibular causes interpret their dizziness as a spinning sensation.15 Therefore, it is important to tease out specifics about the timing, triggers, and associated symptoms in order to further delineate possible causes (TABLE).16

Make a list of current medications. Gather a comprehensive list of current medications, especially from elderly patients, because polypharmacy is a major contributor to dizziness in this population.12 Keep in mind that elderly patients presenting with dizziness/vertigo may have multifactorial balance difficulties, which can be revealed by a detailed history.
Physical exam: May be broad or focused
Given the broad range of causes for dizziness, cardiovascular, head/neck, and neurologic examinations may be performed as part of the work-up, as the clinical history warrants. More typically, time is spent ruling out the following common causes.
Continue to: Orthostatic hypotension
Orthostatic hypotension. Orthostatic vitals are recommended initially in all patients with dizziness, although these may be normal in patients with orthostatic hypotension.17 A diagnosis of orthostatic hypotension can be made with systolic blood pressure decreases of 20 mm Hg or diastolic pressure decreases of ≥10 mm Hg within 3 minutes of standing.18 An increase in heart rate >30 beats per minute after rising from a supine position may indicate autonomic disturbances such as postural orthostatic tachycardia syndrome.19 However, physical examination findings alone are insufficient to make the diagnosis of orthostatic hypotension, and determining the underlying cause of the orthostatic hypotension (dehydration, cardiac dysfunction, pure-autonomic failure, medication adverse effect) is vital.18
BPPV. Perform the Dix-Hallpike maneuver (see https://collections.lib.utah.edu/details?id=177177 for a demonstration of the maneuver) on patients presenting with dizziness with features suggestive of BPPV (eg, attacks of dizziness triggered by head movements).20,21
As BPPV is the most common cause of vestibular dizziness, a negative Dix-Hallpike can be helpful in refining the differential diagnosis.20,21 The maneuver begins with the patient seated, looking directly ahead. To test the left side, ask the patient to turn his/her head 45 degrees to the left. Then direct the patient to lie back, so that the patient’s head is off the edge of the examination table and hyperextended, while maintaining the same head orientation. To test the right side, repeat the procedure with the patient turning his/her head to the right.
Torsional nystagmus is necessary for a positive Dix-Hallpike, which is diagnostic for BPPV. The laterality of BPPV can be determined by paying attention to the fast phase of the torsional nystagmus; the superior pole of the eye beats toward the affected side.14 The patient may report severe dizziness or vertigo during the Dix-Hallpike, but without torsional nystagmus, the test is negative, and the patient does not have BPPV.14
Neurologic causes. Perform a complete neurologic examination in patients who clearly do not have a history of orthostatic hypotension and who have a Dix-Hallpike test that is negative or not indicated.4 Also perform cerebellar testing including rapid-alternating movements, a finger-to-nose test, and a heel-to-shin test. Round out the neurologic exam with an assessment of gait and a Romberg’s test (see https://www.youtube.com/watch?v=U5a4lbmwmOw for a demonstration of Romberg’s test). Romberg’s test is performed by having the patient place his/her feet together with hands at sides and eyes closed. The patient is observed for up to a minute, with a positive test denoted by a loss of balance.
Continue to: Abnormal gait may indicate...
Abnormal gait may indicate peripheral neuropathy, while a positive Romberg’s test suggests involvement of the proprioceptive receptors and/or their pathway.
Central/peripheral vestibular causes. The head impulse, nystagmus, test of skew (HINTS) examination can differentiate between central and peripheral vestibular causes of dizziness and rule out stroke (a central vestibular cause).22 (See https://collections.lib.utah.edu/details?id=177180 for a video demonstrating the steps involved in performing the HINTS examination.) The head impulse (HI) portion of the exam is performed by moving the patient’s head side to side, while having the patient focus on the examiner’s nose. Rapid movements of both eyes (“abnormal” HI) suggest a peripheral etiology, while no eye movement with gaze fixated on the examiner’s nose (“normal” HI) is concerning for stroke or another central cause of vertigo.22
Nystagmus is assessed by having the patient follow the examiner’s finger as it moves in a horizontal direction. Spontaneous horizontal unidirectional nystagmus suggests a peripheral cause, while vertical or torsional bidirectional (direction-changing) nystagmus points to a central cause.22
The test of skew is executed by covering and uncovering each of the patient’s eyes, while asking the patient to look ahead. Vertical deviation of the eye after uncovering suggests a central etiology, more specifically one involving the brainstem.22
Diagnostic testing/imaging has a limited, but pivotal role
There is a limited role for routine laboratory testing in patients with dizziness. However, for those patients with underlying medical conditions (eg, diabetes), which may contribute to the symptoms, routine blood work can be ordered (ie, finger-stick blood glucose test).22
Continue to: More worrisome suspicions
More worrisome suspicions. Patients suspected of cardiac causes should have a full cardiac work-up performed.22 For suspected stroke, brain tumor, or head trauma, specific computed tomography or magnetic resonance imaging can be arranged.22 Carotid doppler can be used if dizziness is suspected to be caused by orthostatic hypotension or a vascular cause.23
Audiologic and vestibular testing. Audiologic testing is not routinely recommended and is only warranted in instances when patients report hearing loss or changes. Referral to an otolaryngologist for vestibular testing is warranted once life-threatening and alternate etiologies have been ruled out, and a vestibular disorder remains at the top of the differential.24
Treatment hinges on cause and may be multifaceted
Treatment hinges on the specific cause of the patient’s dizziness and may involve useful maneuvers, medication, physiotherapy, or perhaps even surgery.
Employ a particle repositioning maneuver for BPPV
A positive Dix-Hallpike test should prompt the use of a particle repositioning maneuver (PRM) to treat BPPV.21 The goal of PRMs, such as the Epley maneuver (see https://www.youtube.com/watch?v=9SLm76jQg3g for a demonstration of this maneuver), is to move the head in such a way as to return displaced otoliths in the semicircular canal back to the utricle. The Epley maneuver is specific for treating posterior semicircular canal BPPV, which is the most common variant.
Performing the Epley maneuver. To perform the Epley PRM for correction of an otolith in the left posterior semicircular canal, ask the patient to sit and look straight ahead. Lay the patient back, while asking the patient to turn his/her head 45 degrees to the left side. Then ask the patient to turn his/her head 45 degrees to the right side. Instruct the patient to maintain the same 45-degree head orientation, while rolling over to his/her right shoulder, ending in the right decubitus position. Conclude the maneuver by having the patient sit up.
Continue to: Performing the barbecue roll maneuver
Performing the barbecue roll maneuver. Different PRMs exist to treat less common variants of BPPV, including the “barbecue roll” maneuver for horizontal BPPV (see https://www.youtube.com/watch?v=mwTmM6uF5yA for a demonstration of this maneuver).25 The barbecue roll maneuver is initiated with the patient looking ahead and lying back. For a left-sided horizontal canal otolith, the patient first turns to the left decubitus position, then moves clockwise to the right decubitus position, stopping at each position for approximately 20 seconds, all while maintaining a straight head position. The patient then turns clockwise into a prone position, pausing, and finally turning into the left decubitus position again. The maneuver is completed with the patient sitting up.
Medications are used to treat symptoms and/or underlying causes
Adjustments in antihypertensives can be made in cases of orthostatic hypotension.17 Antiemetics (ondansetron, promethazine, metoclopramide), antihistamines (meclizine, dimenhydrinate, diphenhydramine), and benzodiazepines (lorazepam, diazepam) may be used during acute and brief vertiginous episodes to decrease symptom severity after central causes have been ruled out.26,27 However, patients with BPPV should avoid these medications as they may blunt central compensation and increase the risk of falls.27 Research has shown betahistine to improve vertigo control only in patients with Meniere’s disease and only when taken regularly and prophylactically.28 Therefore, do not prescribe betahistine for all other causes of dizziness/vertigo.28
Consider physiotherapy
All patients with dizziness/vertigo, and particularly those presenting with primary balance concerns, may benefit from vestibular rehabilitation therapy (VRT). This is an exercise-based program focusing on habituation of dizziness and improvement of postural stability.29 VRT can improve dizziness associated with central and peripheral vestibular lesions, vertigo of uncertain etiology, and psychogenic vertigo.30 Typically, the vestibular physiotherapist will provide home exercises for the patient, reducing the cost and inconvenience of attending multiple sessions.
Surgery and referrals
Referrals for surgery are rare and are typically reserved for refractory causes of vestibular disease, such as Meniere’s disease, BPPV, SSCD syndrome.31
Referral to the ED is warranted for symptom control if an acute vertiginous episode is refractory to initial management. Emergent or urgent neurology consultation is indicated for suspected or confirmed central disorders. Urgent cardiology referral is recommended for patients with symptoms of presyncope/syncope, arrhythmia, or persistent orthostatic hypotension after conservative management. Outpatient referral to an otolaryngologist is warranted if the patient has failed a course of balance physiotherapy, has a persistently positive Dix-Hallpike test after a PRM and vestibular/balance physiotherapy, or has asymmetric hearing loss.
Continue to: Management starts with primary and secondary prevention
Management starts with primary and secondary prevention
Patient education is essential for avoiding potential triggers of dizziness. Patients with orthostatic hypotension should be educated about the need to correct the underlying mechanism, including the need for adequate hydration and recognition of offending medications and contributory conditions/situations (caffeine, heat, standing quickly).17 Encouraging balance maintenance through exercise and physiotherapy can help with gait and musculoskeletal disorders, and reducing harmful habits (smoking, poor diet, no exercise) can lead to overall improved cardiovascular health.32 Advise those with Meniere’s disease to avoid potential triggers such as caffeine, high sodium foods, and alcohol.33
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Otology/Neurotology, Assistant Professor, Department of Otolaryngology, Queen's University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected].
Dizziness. Vertigo. These 2 terms are often used interchangeably by patients, with the sensations described as imbalance, lightheadedness, disorientation, presyncope, confusion—among others. While dizziness is a broad term that is often used to describe all the aforementioned sensations, including vertigo, true vertigo (a specific type of dizziness) is defined as the perception of movement within one’s visual field while stationary.1 Because patients are not usually aware of the distinction, their reports of signs and symptoms can cause much confusion for health care providers, thereby delaying a diagnosis.
International studies have reported the prevalence of both dizziness and vertigo to be between 15% and 36%.2,3 Over half of all patients with dizziness and vertigo are cared for by the family physician (FP), and the sensations combined account for approximately 5% of all family medicine visits.4,5 Additionally, between 2.5% and 4% of all emergency department (ED) visits stem from complaints of dizziness and vertigo, with an incidence of up to 25% in those >65 years of age.6,7
Causes of dizziness and vertigo are broad, ranging from the benign to the life-threatening. It has been reported that upwards of 50% of patients presenting to the FP’s office for dizziness leave without a diagnosis.8 Given the confusion surrounding the terms and their broad differential, this review aims to provide FPs with the tools to accurately discern benign from ominous causes.
Nonvestibular benign causes vastly outnumber life-threatening ones
Causes of dizziness are classified as either vestibular (these cause true vertigo) or nonvestibular in origin, with nonvestibular causes being more common.7
Nonvestibular etiologies: Numerous and varied
Nonvestibular causes are broad, spanning many different body systems. Cardiovascular causes of dizziness may include orthostatic hypotension, cardiac arrhythmia, myocardial infarction, and carotid artery stenosis.4,9 Metabolic causes include complications of diabetes such as hypoglycemia and peripheral neuropathy.4,9 Psychiatric conditions such as anxiety, depression, and bipolar disorder can manifest as dizziness, disorientation, or psychogenic vertigo.4,10 Medications including nonsteroidal anti-inflammatory drugs, anticonvulsants, antipsychotics, and sedatives can all contribute to dizziness.11 Other causes of dizziness include Parkinson’s disease, musculoskeletal disorders, and gait disorders.4,9 Especially in the elderly, sensory deficit (peripheral neuropathy), poor vision, and polypharmacy (≥5 medications) are common causes of dizziness.12
Vestibular etiologies of dizziness = true vertigo
Vestibular causes of a patient’s feelings of dizziness manifest as true vertigo and can be categorized as either central (a dysfunction of one or more parts of the central nervous system that help process balance and spatial information or along the pathway where these sensations are interpreted) or peripheral (a dysfunction of the balance organs of the inner ear) in origin.
Central vestibular causes include vertebrobasilar ischemic stroke, vertebrobasilar insufficiency (transient ischemic attack), vestibular migraines, and meningioma of the cerebellopontine angle and posterior fossa.13
Continue to: Peripheral vestibular causes
Peripheral vestibular causes. Benign paroxysmal positional vertigo (BPPV) represents the most common peripheral diagnosis. It is caused by dislodged otoliths in the posterior semicircular canal. While the majority of BPPV cases are idiopathic in nature, up to 15% may result from previous head injury.14 Other peripheral vestibular causes include vestibular neuronitis, viral labyrinthitis, Meniere’s disease, vestibular schwannoma, perilymphatic fistula, superior semicircular canal dehiscence (SSCD), and head trauma (basilar skull fracture).13
Start with a history: Is it dizziness or true vertigo?
The clinical history typically guides the differential diagnosis (FIGURE). Identifying true vertigo from among other sensations helps to limit the differential because true vertigo is caused by vestibular etiologies only. True vertigo is often reported by patients as “seeing the room spin;” this stems from the perception of motion.1 A notable exception is that patients with orthostatic hypotension will often describe spinning sensations lasting seconds to minutes when they rise from a seated or supine position.

Never depend solely, however, on patient-reported sensations, as not all patients with true vertigo report spinning, and some patients with nonvestibular causes interpret their dizziness as a spinning sensation.15 Therefore, it is important to tease out specifics about the timing, triggers, and associated symptoms in order to further delineate possible causes (TABLE).16

Make a list of current medications. Gather a comprehensive list of current medications, especially from elderly patients, because polypharmacy is a major contributor to dizziness in this population.12 Keep in mind that elderly patients presenting with dizziness/vertigo may have multifactorial balance difficulties, which can be revealed by a detailed history.
Physical exam: May be broad or focused
Given the broad range of causes for dizziness, cardiovascular, head/neck, and neurologic examinations may be performed as part of the work-up, as the clinical history warrants. More typically, time is spent ruling out the following common causes.
Continue to: Orthostatic hypotension
Orthostatic hypotension. Orthostatic vitals are recommended initially in all patients with dizziness, although these may be normal in patients with orthostatic hypotension.17 A diagnosis of orthostatic hypotension can be made with systolic blood pressure decreases of 20 mm Hg or diastolic pressure decreases of ≥10 mm Hg within 3 minutes of standing.18 An increase in heart rate >30 beats per minute after rising from a supine position may indicate autonomic disturbances such as postural orthostatic tachycardia syndrome.19 However, physical examination findings alone are insufficient to make the diagnosis of orthostatic hypotension, and determining the underlying cause of the orthostatic hypotension (dehydration, cardiac dysfunction, pure-autonomic failure, medication adverse effect) is vital.18
BPPV. Perform the Dix-Hallpike maneuver (see https://collections.lib.utah.edu/details?id=177177 for a demonstration of the maneuver) on patients presenting with dizziness with features suggestive of BPPV (eg, attacks of dizziness triggered by head movements).20,21
As BPPV is the most common cause of vestibular dizziness, a negative Dix-Hallpike can be helpful in refining the differential diagnosis.20,21 The maneuver begins with the patient seated, looking directly ahead. To test the left side, ask the patient to turn his/her head 45 degrees to the left. Then direct the patient to lie back, so that the patient’s head is off the edge of the examination table and hyperextended, while maintaining the same head orientation. To test the right side, repeat the procedure with the patient turning his/her head to the right.
Torsional nystagmus is necessary for a positive Dix-Hallpike, which is diagnostic for BPPV. The laterality of BPPV can be determined by paying attention to the fast phase of the torsional nystagmus; the superior pole of the eye beats toward the affected side.14 The patient may report severe dizziness or vertigo during the Dix-Hallpike, but without torsional nystagmus, the test is negative, and the patient does not have BPPV.14
Neurologic causes. Perform a complete neurologic examination in patients who clearly do not have a history of orthostatic hypotension and who have a Dix-Hallpike test that is negative or not indicated.4 Also perform cerebellar testing including rapid-alternating movements, a finger-to-nose test, and a heel-to-shin test. Round out the neurologic exam with an assessment of gait and a Romberg’s test (see https://www.youtube.com/watch?v=U5a4lbmwmOw for a demonstration of Romberg’s test). Romberg’s test is performed by having the patient place his/her feet together with hands at sides and eyes closed. The patient is observed for up to a minute, with a positive test denoted by a loss of balance.
Continue to: Abnormal gait may indicate...
Abnormal gait may indicate peripheral neuropathy, while a positive Romberg’s test suggests involvement of the proprioceptive receptors and/or their pathway.
Central/peripheral vestibular causes. The head impulse, nystagmus, test of skew (HINTS) examination can differentiate between central and peripheral vestibular causes of dizziness and rule out stroke (a central vestibular cause).22 (See https://collections.lib.utah.edu/details?id=177180 for a video demonstrating the steps involved in performing the HINTS examination.) The head impulse (HI) portion of the exam is performed by moving the patient’s head side to side, while having the patient focus on the examiner’s nose. Rapid movements of both eyes (“abnormal” HI) suggest a peripheral etiology, while no eye movement with gaze fixated on the examiner’s nose (“normal” HI) is concerning for stroke or another central cause of vertigo.22
Nystagmus is assessed by having the patient follow the examiner’s finger as it moves in a horizontal direction. Spontaneous horizontal unidirectional nystagmus suggests a peripheral cause, while vertical or torsional bidirectional (direction-changing) nystagmus points to a central cause.22
The test of skew is executed by covering and uncovering each of the patient’s eyes, while asking the patient to look ahead. Vertical deviation of the eye after uncovering suggests a central etiology, more specifically one involving the brainstem.22
Diagnostic testing/imaging has a limited, but pivotal role
There is a limited role for routine laboratory testing in patients with dizziness. However, for those patients with underlying medical conditions (eg, diabetes), which may contribute to the symptoms, routine blood work can be ordered (ie, finger-stick blood glucose test).22
Continue to: More worrisome suspicions
More worrisome suspicions. Patients suspected of cardiac causes should have a full cardiac work-up performed.22 For suspected stroke, brain tumor, or head trauma, specific computed tomography or magnetic resonance imaging can be arranged.22 Carotid doppler can be used if dizziness is suspected to be caused by orthostatic hypotension or a vascular cause.23
Audiologic and vestibular testing. Audiologic testing is not routinely recommended and is only warranted in instances when patients report hearing loss or changes. Referral to an otolaryngologist for vestibular testing is warranted once life-threatening and alternate etiologies have been ruled out, and a vestibular disorder remains at the top of the differential.24
Treatment hinges on cause and may be multifaceted
Treatment hinges on the specific cause of the patient’s dizziness and may involve useful maneuvers, medication, physiotherapy, or perhaps even surgery.
Employ a particle repositioning maneuver for BPPV
A positive Dix-Hallpike test should prompt the use of a particle repositioning maneuver (PRM) to treat BPPV.21 The goal of PRMs, such as the Epley maneuver (see https://www.youtube.com/watch?v=9SLm76jQg3g for a demonstration of this maneuver), is to move the head in such a way as to return displaced otoliths in the semicircular canal back to the utricle. The Epley maneuver is specific for treating posterior semicircular canal BPPV, which is the most common variant.
Performing the Epley maneuver. To perform the Epley PRM for correction of an otolith in the left posterior semicircular canal, ask the patient to sit and look straight ahead. Lay the patient back, while asking the patient to turn his/her head 45 degrees to the left side. Then ask the patient to turn his/her head 45 degrees to the right side. Instruct the patient to maintain the same 45-degree head orientation, while rolling over to his/her right shoulder, ending in the right decubitus position. Conclude the maneuver by having the patient sit up.
Continue to: Performing the barbecue roll maneuver
Performing the barbecue roll maneuver. Different PRMs exist to treat less common variants of BPPV, including the “barbecue roll” maneuver for horizontal BPPV (see https://www.youtube.com/watch?v=mwTmM6uF5yA for a demonstration of this maneuver).25 The barbecue roll maneuver is initiated with the patient looking ahead and lying back. For a left-sided horizontal canal otolith, the patient first turns to the left decubitus position, then moves clockwise to the right decubitus position, stopping at each position for approximately 20 seconds, all while maintaining a straight head position. The patient then turns clockwise into a prone position, pausing, and finally turning into the left decubitus position again. The maneuver is completed with the patient sitting up.
Medications are used to treat symptoms and/or underlying causes
Adjustments in antihypertensives can be made in cases of orthostatic hypotension.17 Antiemetics (ondansetron, promethazine, metoclopramide), antihistamines (meclizine, dimenhydrinate, diphenhydramine), and benzodiazepines (lorazepam, diazepam) may be used during acute and brief vertiginous episodes to decrease symptom severity after central causes have been ruled out.26,27 However, patients with BPPV should avoid these medications as they may blunt central compensation and increase the risk of falls.27 Research has shown betahistine to improve vertigo control only in patients with Meniere’s disease and only when taken regularly and prophylactically.28 Therefore, do not prescribe betahistine for all other causes of dizziness/vertigo.28
Consider physiotherapy
All patients with dizziness/vertigo, and particularly those presenting with primary balance concerns, may benefit from vestibular rehabilitation therapy (VRT). This is an exercise-based program focusing on habituation of dizziness and improvement of postural stability.29 VRT can improve dizziness associated with central and peripheral vestibular lesions, vertigo of uncertain etiology, and psychogenic vertigo.30 Typically, the vestibular physiotherapist will provide home exercises for the patient, reducing the cost and inconvenience of attending multiple sessions.
Surgery and referrals
Referrals for surgery are rare and are typically reserved for refractory causes of vestibular disease, such as Meniere’s disease, BPPV, SSCD syndrome.31
Referral to the ED is warranted for symptom control if an acute vertiginous episode is refractory to initial management. Emergent or urgent neurology consultation is indicated for suspected or confirmed central disorders. Urgent cardiology referral is recommended for patients with symptoms of presyncope/syncope, arrhythmia, or persistent orthostatic hypotension after conservative management. Outpatient referral to an otolaryngologist is warranted if the patient has failed a course of balance physiotherapy, has a persistently positive Dix-Hallpike test after a PRM and vestibular/balance physiotherapy, or has asymmetric hearing loss.
Continue to: Management starts with primary and secondary prevention
Management starts with primary and secondary prevention
Patient education is essential for avoiding potential triggers of dizziness. Patients with orthostatic hypotension should be educated about the need to correct the underlying mechanism, including the need for adequate hydration and recognition of offending medications and contributory conditions/situations (caffeine, heat, standing quickly).17 Encouraging balance maintenance through exercise and physiotherapy can help with gait and musculoskeletal disorders, and reducing harmful habits (smoking, poor diet, no exercise) can lead to overall improved cardiovascular health.32 Advise those with Meniere’s disease to avoid potential triggers such as caffeine, high sodium foods, and alcohol.33
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Otology/Neurotology, Assistant Professor, Department of Otolaryngology, Queen's University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected].
1. Bisdorff A, Von Brevern M, Lempert T, et al. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19:1-13.
2. Mendel B, Bergenius J, Langius-Eklöf A. Dizziness: a common, troublesome symptom but often treatable. J Vestib Res. 2010;20:391-398.
3. Gopinath B, McMahon CM, Rochtchina E, et al. Dizziness and vertigo in an older population: the Blue Mountains prospective cross‐sectional study. Clin Otolaryngol. 2009;34:552-556.
4. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-368.
5. Sloan PD. Dizziness in primary care. Results from the National Ambulatory Care Survey. Fam Pract. 1989;29:33-38.
6. Kerber KA, Meurer WJ, West BT, et al. Dizziness presentations in US emergency departments, 1995–2004. Acad Emerg Med. 2008;15:744-750.
7. Newman-Toker DE, Hsieh YH, Camargo CA Jr, et al. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. 2008;83:765-775.
8. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: vertigo and dizziness. Can Fam Physician. 2007;53:1959.
9. Chan Y. Differential diagnosis of dizziness. Curr Opin in Otolaryngol Head Neck Surg. 2009;17:200-203.
10. Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of chronic dizziness. Arch Otolaryngol Head Neck Surg. 2007;133:170-176.
11. Kutz JW Jr. The dizzy patient. Med Clin North Am. 2010;94:989-1002.
12. Jahn K, Kressig RW, Bridenbaugh SA, et al. Dizziness and unstable gait in old age: etiology, diagnosis and treatment. Dtsch Ärztebl Int. 2015;112:387-393.
13. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
14. Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). CMAJ. 2003;169:681-693.
15. Newman-Toker DE, Dy FJ, Stanton VA, et al. How often is dizziness from primary cardiovascular disease true vertigo? A systematic review. J Gen Intern Med. 2008;23:2087-2094.
16. Newman-Toker DE, Edlow JA. TiTrATE: a novel, evidence-based approach to diagnosing acute dizziness and vertigo. Neurol Clin. 2015;33:577-599.
17. Shibao C, Lipsitz LA, Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. J Clin Hypertens (Greenwich). 2013;15:147-153.
18. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125-126.
19. Agarwal AK, Garg R, Ritch A, et al. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478-480.
20. Halker RB, Barrs DM, Wellik KE, et al. Establishing a diagnosis of benign paroxysmal positional vertigo through the dix-hallpike and side-lying maneuvers: a critically appraised topic. Neurologist. 2008;14:201-204.
21. Hilton MP, Pinder DK. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2014;(12):CD003162.
22. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome. Stroke. 2009;40:3504-3510.
23. Hamaguchi T, Iwasa K, Okino S, et al. Carotid duplex ultrasonography during head-up tilt in patients with orthostatic hypotension. Eur Neurol. 2007;57:219-222.
24. Canadian Society of Otolaryngology - Head & Neck Surgery. Five Things Physicians and Patients Should Question [Internet]. Choosing Wisely Canada. 2016 [cited 2017 August 17]. Available at: https://choosingwiselycanada.org/wp-content/uploads/2017/02/Hospital-medicine.pdf. Accessed August 30, 2017.
25. Lee SH, Kim JS. Benign paroxysmal positional vertigo. J Clin Neurol. 2010;6:51-63.
26. Zatonski T, Temporale H, Holanowska J, et al. Current views of treatment of vertigo and dizziness. J Med Diagn Meth. 2014;2:150.
27. Wipperman J. Dizziness and vertigo. Prim Care Clin Office Pract. 2014;41:115-131
28. Murdin L, Hussain K, Schilder AG. Betahistine for symptoms of vertigo. Cochrane Database Syst Rev. 2016;(6):CD010696.
29. Han BI, Song HS, Kim JS. Vestibular rehabilitation therapy: review of indications, mechanisms, and key exercises. J Clin Neurol. 2011;7:184-196.
30. Jung JY, Kim JS, Chung PS, et al. Effect of vestibular rehabilitation on dizziness in the elderly. Am J Otolaryngol. 2009;30:295-299.
31. Semaan MT, Megerian CA. Meniere’s disease: a challenging and relentless disorder. Otolaryngol Clin North Am. 2011;44:383-403.
32. Pirker W, Katzenschlager R. Gait disorders in adults and the elderly. Wien Klin Wochenschr. 2017;129:81-95.
33. Kirby SE, Yardley L. Physical and psychological triggers for attacks in Ménière’s disease: the patient perspective. Psychother Psychosom. 2012;81:396-398.
1. Bisdorff A, Von Brevern M, Lempert T, et al. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19:1-13.
2. Mendel B, Bergenius J, Langius-Eklöf A. Dizziness: a common, troublesome symptom but often treatable. J Vestib Res. 2010;20:391-398.
3. Gopinath B, McMahon CM, Rochtchina E, et al. Dizziness and vertigo in an older population: the Blue Mountains prospective cross‐sectional study. Clin Otolaryngol. 2009;34:552-556.
4. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-368.
5. Sloan PD. Dizziness in primary care. Results from the National Ambulatory Care Survey. Fam Pract. 1989;29:33-38.
6. Kerber KA, Meurer WJ, West BT, et al. Dizziness presentations in US emergency departments, 1995–2004. Acad Emerg Med. 2008;15:744-750.
7. Newman-Toker DE, Hsieh YH, Camargo CA Jr, et al. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. 2008;83:765-775.
8. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: vertigo and dizziness. Can Fam Physician. 2007;53:1959.
9. Chan Y. Differential diagnosis of dizziness. Curr Opin in Otolaryngol Head Neck Surg. 2009;17:200-203.
10. Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of chronic dizziness. Arch Otolaryngol Head Neck Surg. 2007;133:170-176.
11. Kutz JW Jr. The dizzy patient. Med Clin North Am. 2010;94:989-1002.
12. Jahn K, Kressig RW, Bridenbaugh SA, et al. Dizziness and unstable gait in old age: etiology, diagnosis and treatment. Dtsch Ärztebl Int. 2015;112:387-393.
13. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
14. Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). CMAJ. 2003;169:681-693.
15. Newman-Toker DE, Dy FJ, Stanton VA, et al. How often is dizziness from primary cardiovascular disease true vertigo? A systematic review. J Gen Intern Med. 2008;23:2087-2094.
16. Newman-Toker DE, Edlow JA. TiTrATE: a novel, evidence-based approach to diagnosing acute dizziness and vertigo. Neurol Clin. 2015;33:577-599.
17. Shibao C, Lipsitz LA, Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. J Clin Hypertens (Greenwich). 2013;15:147-153.
18. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125-126.
19. Agarwal AK, Garg R, Ritch A, et al. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478-480.
20. Halker RB, Barrs DM, Wellik KE, et al. Establishing a diagnosis of benign paroxysmal positional vertigo through the dix-hallpike and side-lying maneuvers: a critically appraised topic. Neurologist. 2008;14:201-204.
21. Hilton MP, Pinder DK. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2014;(12):CD003162.
22. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome. Stroke. 2009;40:3504-3510.
23. Hamaguchi T, Iwasa K, Okino S, et al. Carotid duplex ultrasonography during head-up tilt in patients with orthostatic hypotension. Eur Neurol. 2007;57:219-222.
24. Canadian Society of Otolaryngology - Head & Neck Surgery. Five Things Physicians and Patients Should Question [Internet]. Choosing Wisely Canada. 2016 [cited 2017 August 17]. Available at: https://choosingwiselycanada.org/wp-content/uploads/2017/02/Hospital-medicine.pdf. Accessed August 30, 2017.
25. Lee SH, Kim JS. Benign paroxysmal positional vertigo. J Clin Neurol. 2010;6:51-63.
26. Zatonski T, Temporale H, Holanowska J, et al. Current views of treatment of vertigo and dizziness. J Med Diagn Meth. 2014;2:150.
27. Wipperman J. Dizziness and vertigo. Prim Care Clin Office Pract. 2014;41:115-131
28. Murdin L, Hussain K, Schilder AG. Betahistine for symptoms of vertigo. Cochrane Database Syst Rev. 2016;(6):CD010696.
29. Han BI, Song HS, Kim JS. Vestibular rehabilitation therapy: review of indications, mechanisms, and key exercises. J Clin Neurol. 2011;7:184-196.
30. Jung JY, Kim JS, Chung PS, et al. Effect of vestibular rehabilitation on dizziness in the elderly. Am J Otolaryngol. 2009;30:295-299.
31. Semaan MT, Megerian CA. Meniere’s disease: a challenging and relentless disorder. Otolaryngol Clin North Am. 2011;44:383-403.
32. Pirker W, Katzenschlager R. Gait disorders in adults and the elderly. Wien Klin Wochenschr. 2017;129:81-95.
33. Kirby SE, Yardley L. Physical and psychological triggers for attacks in Ménière’s disease: the patient perspective. Psychother Psychosom. 2012;81:396-398.
From The Journal of Family Practice | 2018;67(8):490-492,495-498.
PRACTICE RECOMMENDATIONS
› Employ the Dix-Hallpike maneuver to diagnose patients presenting with dizziness with features suggestive of benign paroxysmal positional vertigo (BPPV). A
› Use the head impulse, nystagmus, test of skew (HINTS) examination to differentiate between central and peripheral vestibular causes of dizziness and rule out stroke. B
› Prescribe betahistine only for patients with Meniere’s disease and not for patients with other causes of dizziness and/or vertigo. B
› Rely on antiemetics, antihistamines, and benzodiazepines to manage acute and brief episodes of vertigo, but not to treat BPPV because they blunt central compensation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Beat the heat: Identification and Tx of heat-related illness
Heat-related illnesses can affect people of any age who are subjected to extreme heat and humidity regardless of physical fitness level or baseline health status. The most serious of the heat-related illnesses is heat stroke. Prompt identification, early initiation of cooling measures (including cold-water immersion [CWI]), and transport to a higher level of care, when appropriate, are imperative. This article reviews heat-related illness identification, as well as management strategies.
Heat-related illnesses: From the benign to the severe
Some of the less severe forms of heat-related illness include heat cramps (which are due to dehydration and salt loss), heat rash, and heat edema. Heat rash and heat edema are benign. Heat rash typically resolves with cooler clothing and a cooler environment. Heat edema tends to improve after sleeping in a cooler environment with legs elevated. Heat syncope is the result of decreased cerebral perfusion due to fluid loss and vasodilation that results in a distributive hypovolemia. It commonly occurs after vigorous exercise when the athlete is standing still.
Heat exhaustion requires a more careful clinical assessment. It is the inability to continue activity in the heat, often with weakness and collapse. Also due to salt and water losses, it results in cardiovascular output that is insufficient to meet the circulatory and metabolic demands of the body. The body temperature is often elevated but <40° C (104° F), vomiting can occur, and mild central nervous system (CNS) dysfunction may be present.
Heat stroke is the most severe form of heat-related illness and can be life-threatening.1
It is important to understand that these heat-related illnesses do not progress along a continuum. Patients develop heat stroke without having had milder forms of heat illness, and patients with a milder type of heat illness usually do not progress to heat stroke.
Heat stroke: Definition, types, risk factors
Heat stroke is defined as a core body temperature ≥40° C (104° F) with CNS dysfunction in the setting of environmental heat stress. The mortality rate can reach over 50%.2-6
There are 2 main types of heat stroke: exertional heat stroke and nonexertional (classic) heat stroke. Exertional heat stroke more commonly affects healthy, young people, such as athletes or military personnel. Classic (nonexertional) heat stroke patients are typically elderly and/or have a chronic illness, although occasionally it involves children who are unable to escape from a hot environment.5,7 While exertional heat stroke typically develops over a period of a few hours in participants of prolonged activities, such as marathons, classic heat stroke in the elderly typically develops over a period of days in the setting of high environmental temperatures. In both conditions, there is an inability to maintain a normal body temperature leading to CNS dysregulation with altered mental status and often multisystem organ dysfunction.7
Continue to: Risk factors
Risk factors. Heat-related illness can affect patients of all ages and levels of physical fitness; however, certain factors place patients at increased risk. These include physical deconditioning, dehydration, high levels of exercise intensity, obesity, elevated environmental temperatures, sleep deprivation, certain medications, alcohol and drug abuse, concurrent illness, and wearing excessive clothing or equipment. It is imperative that severe cases of heat illness be identified early and treatment be initiated rapidly, as delays in cooling can significantly increase the fatality rate.5
Management: First suspect the diagnosis
Health care providers must first suspect heat-related illness and then accurately diagnose it. It is important to differentiate heat-related illness from syncope, cardiac abnormalities, gastroenteritis, hypoglycemia, and other entities that require alternate management. For cases of collapse, syncope or near-syncope, or altered mental status during exertion, heat stroke should be the default diagnosis until proven otherwise.
Obtain a core body temperature. While attending to airway, breathing, and circulation, obtain a core body temperature. Rectal (or esophageal) core temperatures provide a reliable reading that can assist in determining the severity of the heat illness. Axillary, tympanic, temporal, oral, and skin temperatures are affected by environmental factors and are not accurate determinants of core body temperature.8
Once heat stroke is diagnosed, the physician must immediately initiate cooling by removing clothing, placing the patient in the shade or an air-conditioned area, and beginning aggressive cooling measures (more on this in a bit). While field management requires an accurate diagnosis of the severity of a patient’s heat-related illness, one should not delay treatment in order to obtain a rectal temperature.
When treating the milder forms of heat illness, administer oral or intravenous (IV) isotonic fluids. For heat cramps, stretching the affected muscle can help. For heat syncope, lying the patient down and elevating the legs restores perfusion. Patients with heat exhaustion will require some cooling measures such as relocation to a shaded area, removal of excess clothing, and the use of cold towels, along with hydration and elevation of the feet.
Continue to: Cooling techniques for heat stroke
Cooling techniques for heat stroke
In order to adequately cool a patient suffering from heat stroke, health care providers must create a gradient for heat to escape the body through the skin into the environment by conduction, convection, or evaporation.3 Cooling heat stroke patients to less than 40° C (104° F) within 30 minutes after collapse decreases the fatality rate to almost zero.8
CWI comes out on top. CWI, also called an ice-bath, is typically performed in the field. The patient is submerged up to the neck in a tub containing ice and water. Circulating the water and ice mixture helps accelerate cooling.
There have been differences in opinion regarding which cooling method is superior3 (TABLE 13,8,9). Traditionally, there were some concerns that CWI might actually increase body temperature via peripheral vasoconstriction and shivering. But current research suggests that for exertional heat stroke, CWI to promote conductive cooling is the most effective strategy.3,8,10,11 A review of cooling rates in healthy hyperthermic athletes and heat stroke victims showed that ice-water immersion or CWI at 1° to 14° C (35.6°-57.2° F) is superior to all other types of cooling, including ice packs, fans, and partial-body ice-water immersion.10
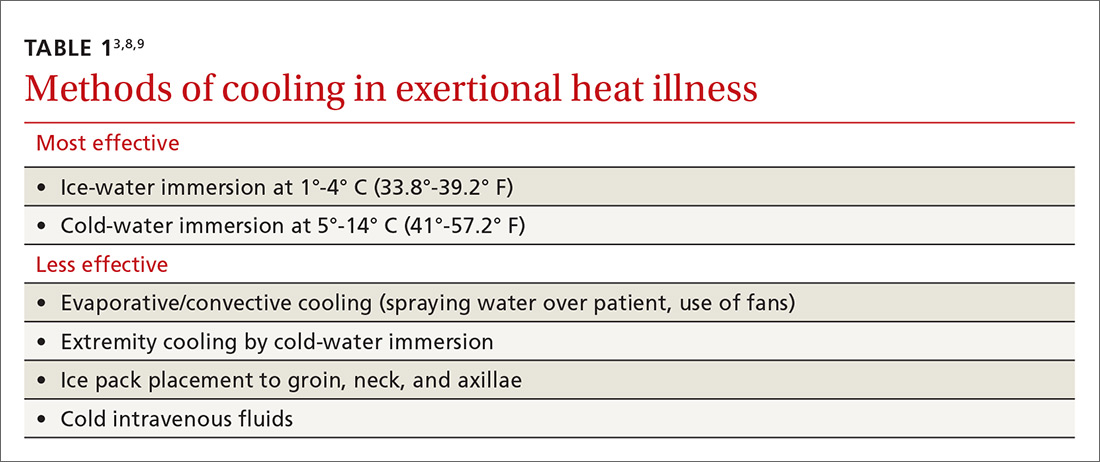
Furthermore, a 2015 meta-analysis looking at optimal procedures for cooling found that CWI cooled patients twice as fast as passive cooling (without any treatment).11 When cooling with CWI, core temperature drops about 0.2° C/min (0.36° F/min).10 Therefore, the temperature can be expected to drop about 1° C (1.8° F) for every 5 minutes of immersion. When unable to monitor a rectal temperature continuously, 10 to 15 minutes of immersion should get most patients below 40° C (104° F).
Extremity cooling. While CWI is the standard for cases of exertional heat illness, whole-body immersion is not always possible. In such cases, extremity cooling can be an effective body cooling method for exertional heat-related illness.12 Research has shown evaporative and convective cooling methods to have benefits for nonexertional heat-related illnesses.3,8,9 These methods usually involve directing air currents over exposed skin and spraying water on the affected individual.3
Contine to: Guidlines for transport
Guidelines for transport: Cool first, transport second
Most patients suspected of suffering from heat stroke should be transported to a hospital for further evaluation because of the high morbidity and mortality rates associated with it. However, cooling techniques should be implemented while awaiting transport. The current standard is “cool first, transport second.”7 Cooling interventions should continue in the ambulance if the core body temperature is still elevated. Techniques that can be used include the use of air conditioning, convective methods, and administration of IV fluids. As previously discussed, core body temperature should be continuously monitored. Cooling measures should be discontinued only when the patient’s rectal temperature reaches 38.9° C (102° F). Overly aggressive prehospital cooling beyond this point can result in prolonged hypothermia as well as cardiac arrhythmias.6
Monitoring and further evaluation
Monitoring patients with heat-related illness can be difficult, especially when utilizing CWI, as this may limit the ability to use devices such as a cardiac monitor or to continuously monitor rectal temperature. Beyond lowering core body temperature to below 39° C (102.2° F), early evaluation and treatment of other organ systems is vital, keeping in mind that these patients may develop multisystem organ failure. The initial work-up is listed in TABLE 2.
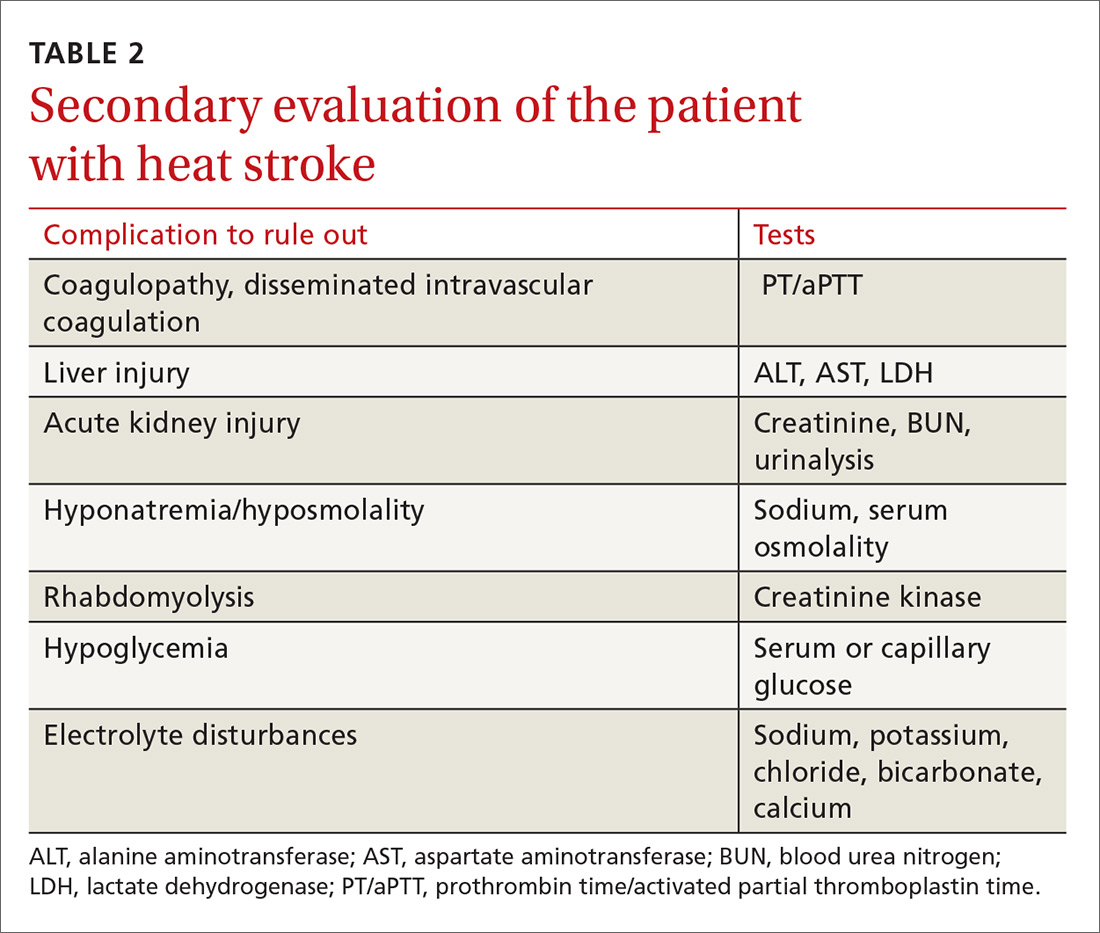
Depending on the severity of the injury and whether you suspect another diagnosis at work, additional studies may include urine output monitoring with a Foley catheter, electrocardiogram, chest radiograph, toxicology screen, a serum lactate level, and cardiac biomarkers.
Imaging. When evaluating for heat stroke, it usually isn’t necessary to obtain head imaging initially, as there are rarely abnormal findings in the early stages. Imaging may be obtained, however, if there is concern about a head injury or if neurologic abnormalities persist into later stages of treatment.5
Pharmacologic agents have not been shown to be of benefit in the treatment of heat-related illness. While dantrolene is commonly used in the treatment of neuroleptic malignant syndrome and malignant hyperthermia, the literature has not described any benefit associated with this agent in relation to heat-related illness. The same goes for antipyretics. Researchers have hypothesized that the reason these agents are ineffective is because body temperature is raised via a different mechanism in these conditions vs heat stroke.3
Continue to: Prevention
Prevention: Modifications and acclimatization are key
People who know they will be exposed to extreme heat should attempt to modify activities. There are many predisposing risk factors ranging from fever and illness to fatigue and dehydration. Risks can be minimized with physiologic adaptation through acclimatization, as well as making various behavioral changes such as adjusting activities, ensuring adequate hydration, and wearing appropriate clothing.13
Certain types of equipment, such as football helmets, can increase the risk of heat-related illness because they prevent heat exchange; however, the benefits sometimes outweigh the risks. With this in mind, consider modification of clothing and equipment if possible.1
In order to prevent heat-related illness, individuals should prehydrate prior to an event and replace fluids orally in order to prevent a >2% loss in body weight. Greater than a 2% loss directly correlates with increased core temperatures during exercise.1
Care should also be taken to perform regular physical activity prior to extreme heat exposure.1 Heat acclimatization takes place when a person’s body adapts to a hotter climate than they are accustomed to. This process can take up to 2 weeks, but once heat acclimation is accomplished, the person will have undergone physical changes, such as reduced metabolic heat production, which will decrease the risk of heat-related illness.13
Return to activity: Customize the approach
Each heat-related injury case is different; thus, return to activity should be individualized. In patients whose heat injury was believed to be secondary to a modifiable risk factor, efforts should be made to correct the predisposing factors that placed the patient at increased risk in the first place.
Additionally, the patient should allow sufficient time to recover. Guidelines recommend at least 1 to 2 weeks recovery before return to activity after heat stroke.8 Moreover, a graded return to activity, starting in a cool environment, is recommended. Gradual introduction of activity in the heat with close monitoring can help with acclimatization and help identify participants who continue to have cooling dysregulation. In the military and among athletes, tools such as heat-tolerance testing can be used to gauge the person’s readiness to return to play or duty.8 Heat tolerance testing is performed in a lab using continuous core temperature monitoring while having the subject exercise in a heated room.
CORRESPONDENCE
Scott Kinkade, MD, EdD, MA303 Medical Sciences Building, DC032.00, Columbia, MO 65212; [email protected].
1. Lipman GS, Eifling KP, Ellis MA, et al. Wilderness Medical Society practice guidelines for the prevention and treatment of heat-related illness: 2014 update. Wilderness Environ Med. 2014;25(4 Suppl):S55-S65.
2. Update: Heat injuries, active component, U.S. Armed Forces, 2014. MSMR. 2015;22:17-20.
3. Gaudio FG, Grissom CK. Cooling methods in heat stroke. J Emerg Med. 2016;50:607-616.
4. Hess JJ, Saha S, Luber G. Summertime acute heat illness in U.S. emergency departments from 2006 through 2010: analysis of a nationally representative sample. Environ Health Perspect. 2014;122:1209-1215.
5. People’s Liberation Army Professional Committee of Critical Care Medicine. Expert consensus on standardized diagnosis and treatment for heat stroke. Mil Med Res. 2016;3:1.
6. Stewart TE, Whitford AC. Dangers of prehospital cooling: a case report of afterdrop in a patient with exertional heat stroke. J Emerg Med. 2015;49:630-633.
7. Chan YK, Mamat M. Management of heat stroke. Trends Anaesthesia Crit Care. 2015;5:65-69.
8. Casa DJ, Armstrong LE, Kenny GP, et al. Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012;11:115-123.
9. Demartini JK, Casa DJ, Stearns R, et al. Effectiveness of cold water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc. 2015;47:240-245.
10. Casa DJ, McDermott BP, Lee EC, et al. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35:141-149.
11. Zhang Y, Davis JK, Casa DJ, et al. Optimizing cold water immersion for exercise-induced hyperthermia: a meta-analysis. Med Sci Sports Exerc. 2015;47:2464-2472.
12. DeGroot DW, Kenefick RW, Sawka MN. Impact of arm immersion cooling during ranger training on exertional heat illness and treatment costs. Mil Med. 2015;180:1178-1183.
13. Epstein Y, Druyan A, Heled Y. Heat injury prevention—a military perspective. J Strength Cond Res. 2012;26 (suppl 2):S82-S86.
Heat-related illnesses can affect people of any age who are subjected to extreme heat and humidity regardless of physical fitness level or baseline health status. The most serious of the heat-related illnesses is heat stroke. Prompt identification, early initiation of cooling measures (including cold-water immersion [CWI]), and transport to a higher level of care, when appropriate, are imperative. This article reviews heat-related illness identification, as well as management strategies.
Heat-related illnesses: From the benign to the severe
Some of the less severe forms of heat-related illness include heat cramps (which are due to dehydration and salt loss), heat rash, and heat edema. Heat rash and heat edema are benign. Heat rash typically resolves with cooler clothing and a cooler environment. Heat edema tends to improve after sleeping in a cooler environment with legs elevated. Heat syncope is the result of decreased cerebral perfusion due to fluid loss and vasodilation that results in a distributive hypovolemia. It commonly occurs after vigorous exercise when the athlete is standing still.
Heat exhaustion requires a more careful clinical assessment. It is the inability to continue activity in the heat, often with weakness and collapse. Also due to salt and water losses, it results in cardiovascular output that is insufficient to meet the circulatory and metabolic demands of the body. The body temperature is often elevated but <40° C (104° F), vomiting can occur, and mild central nervous system (CNS) dysfunction may be present.
Heat stroke is the most severe form of heat-related illness and can be life-threatening.1
It is important to understand that these heat-related illnesses do not progress along a continuum. Patients develop heat stroke without having had milder forms of heat illness, and patients with a milder type of heat illness usually do not progress to heat stroke.
Heat stroke: Definition, types, risk factors
Heat stroke is defined as a core body temperature ≥40° C (104° F) with CNS dysfunction in the setting of environmental heat stress. The mortality rate can reach over 50%.2-6
There are 2 main types of heat stroke: exertional heat stroke and nonexertional (classic) heat stroke. Exertional heat stroke more commonly affects healthy, young people, such as athletes or military personnel. Classic (nonexertional) heat stroke patients are typically elderly and/or have a chronic illness, although occasionally it involves children who are unable to escape from a hot environment.5,7 While exertional heat stroke typically develops over a period of a few hours in participants of prolonged activities, such as marathons, classic heat stroke in the elderly typically develops over a period of days in the setting of high environmental temperatures. In both conditions, there is an inability to maintain a normal body temperature leading to CNS dysregulation with altered mental status and often multisystem organ dysfunction.7
Continue to: Risk factors
Risk factors. Heat-related illness can affect patients of all ages and levels of physical fitness; however, certain factors place patients at increased risk. These include physical deconditioning, dehydration, high levels of exercise intensity, obesity, elevated environmental temperatures, sleep deprivation, certain medications, alcohol and drug abuse, concurrent illness, and wearing excessive clothing or equipment. It is imperative that severe cases of heat illness be identified early and treatment be initiated rapidly, as delays in cooling can significantly increase the fatality rate.5
Management: First suspect the diagnosis
Health care providers must first suspect heat-related illness and then accurately diagnose it. It is important to differentiate heat-related illness from syncope, cardiac abnormalities, gastroenteritis, hypoglycemia, and other entities that require alternate management. For cases of collapse, syncope or near-syncope, or altered mental status during exertion, heat stroke should be the default diagnosis until proven otherwise.
Obtain a core body temperature. While attending to airway, breathing, and circulation, obtain a core body temperature. Rectal (or esophageal) core temperatures provide a reliable reading that can assist in determining the severity of the heat illness. Axillary, tympanic, temporal, oral, and skin temperatures are affected by environmental factors and are not accurate determinants of core body temperature.8
Once heat stroke is diagnosed, the physician must immediately initiate cooling by removing clothing, placing the patient in the shade or an air-conditioned area, and beginning aggressive cooling measures (more on this in a bit). While field management requires an accurate diagnosis of the severity of a patient’s heat-related illness, one should not delay treatment in order to obtain a rectal temperature.
When treating the milder forms of heat illness, administer oral or intravenous (IV) isotonic fluids. For heat cramps, stretching the affected muscle can help. For heat syncope, lying the patient down and elevating the legs restores perfusion. Patients with heat exhaustion will require some cooling measures such as relocation to a shaded area, removal of excess clothing, and the use of cold towels, along with hydration and elevation of the feet.
Continue to: Cooling techniques for heat stroke
Cooling techniques for heat stroke
In order to adequately cool a patient suffering from heat stroke, health care providers must create a gradient for heat to escape the body through the skin into the environment by conduction, convection, or evaporation.3 Cooling heat stroke patients to less than 40° C (104° F) within 30 minutes after collapse decreases the fatality rate to almost zero.8
CWI comes out on top. CWI, also called an ice-bath, is typically performed in the field. The patient is submerged up to the neck in a tub containing ice and water. Circulating the water and ice mixture helps accelerate cooling.
There have been differences in opinion regarding which cooling method is superior3 (TABLE 13,8,9). Traditionally, there were some concerns that CWI might actually increase body temperature via peripheral vasoconstriction and shivering. But current research suggests that for exertional heat stroke, CWI to promote conductive cooling is the most effective strategy.3,8,10,11 A review of cooling rates in healthy hyperthermic athletes and heat stroke victims showed that ice-water immersion or CWI at 1° to 14° C (35.6°-57.2° F) is superior to all other types of cooling, including ice packs, fans, and partial-body ice-water immersion.10

Furthermore, a 2015 meta-analysis looking at optimal procedures for cooling found that CWI cooled patients twice as fast as passive cooling (without any treatment).11 When cooling with CWI, core temperature drops about 0.2° C/min (0.36° F/min).10 Therefore, the temperature can be expected to drop about 1° C (1.8° F) for every 5 minutes of immersion. When unable to monitor a rectal temperature continuously, 10 to 15 minutes of immersion should get most patients below 40° C (104° F).
Extremity cooling. While CWI is the standard for cases of exertional heat illness, whole-body immersion is not always possible. In such cases, extremity cooling can be an effective body cooling method for exertional heat-related illness.12 Research has shown evaporative and convective cooling methods to have benefits for nonexertional heat-related illnesses.3,8,9 These methods usually involve directing air currents over exposed skin and spraying water on the affected individual.3
Contine to: Guidlines for transport
Guidelines for transport: Cool first, transport second
Most patients suspected of suffering from heat stroke should be transported to a hospital for further evaluation because of the high morbidity and mortality rates associated with it. However, cooling techniques should be implemented while awaiting transport. The current standard is “cool first, transport second.”7 Cooling interventions should continue in the ambulance if the core body temperature is still elevated. Techniques that can be used include the use of air conditioning, convective methods, and administration of IV fluids. As previously discussed, core body temperature should be continuously monitored. Cooling measures should be discontinued only when the patient’s rectal temperature reaches 38.9° C (102° F). Overly aggressive prehospital cooling beyond this point can result in prolonged hypothermia as well as cardiac arrhythmias.6
Monitoring and further evaluation
Monitoring patients with heat-related illness can be difficult, especially when utilizing CWI, as this may limit the ability to use devices such as a cardiac monitor or to continuously monitor rectal temperature. Beyond lowering core body temperature to below 39° C (102.2° F), early evaluation and treatment of other organ systems is vital, keeping in mind that these patients may develop multisystem organ failure. The initial work-up is listed in TABLE 2.

Depending on the severity of the injury and whether you suspect another diagnosis at work, additional studies may include urine output monitoring with a Foley catheter, electrocardiogram, chest radiograph, toxicology screen, a serum lactate level, and cardiac biomarkers.
Imaging. When evaluating for heat stroke, it usually isn’t necessary to obtain head imaging initially, as there are rarely abnormal findings in the early stages. Imaging may be obtained, however, if there is concern about a head injury or if neurologic abnormalities persist into later stages of treatment.5
Pharmacologic agents have not been shown to be of benefit in the treatment of heat-related illness. While dantrolene is commonly used in the treatment of neuroleptic malignant syndrome and malignant hyperthermia, the literature has not described any benefit associated with this agent in relation to heat-related illness. The same goes for antipyretics. Researchers have hypothesized that the reason these agents are ineffective is because body temperature is raised via a different mechanism in these conditions vs heat stroke.3
Continue to: Prevention
Prevention: Modifications and acclimatization are key
People who know they will be exposed to extreme heat should attempt to modify activities. There are many predisposing risk factors ranging from fever and illness to fatigue and dehydration. Risks can be minimized with physiologic adaptation through acclimatization, as well as making various behavioral changes such as adjusting activities, ensuring adequate hydration, and wearing appropriate clothing.13
Certain types of equipment, such as football helmets, can increase the risk of heat-related illness because they prevent heat exchange; however, the benefits sometimes outweigh the risks. With this in mind, consider modification of clothing and equipment if possible.1
In order to prevent heat-related illness, individuals should prehydrate prior to an event and replace fluids orally in order to prevent a >2% loss in body weight. Greater than a 2% loss directly correlates with increased core temperatures during exercise.1
Care should also be taken to perform regular physical activity prior to extreme heat exposure.1 Heat acclimatization takes place when a person’s body adapts to a hotter climate than they are accustomed to. This process can take up to 2 weeks, but once heat acclimation is accomplished, the person will have undergone physical changes, such as reduced metabolic heat production, which will decrease the risk of heat-related illness.13
Return to activity: Customize the approach
Each heat-related injury case is different; thus, return to activity should be individualized. In patients whose heat injury was believed to be secondary to a modifiable risk factor, efforts should be made to correct the predisposing factors that placed the patient at increased risk in the first place.
Additionally, the patient should allow sufficient time to recover. Guidelines recommend at least 1 to 2 weeks recovery before return to activity after heat stroke.8 Moreover, a graded return to activity, starting in a cool environment, is recommended. Gradual introduction of activity in the heat with close monitoring can help with acclimatization and help identify participants who continue to have cooling dysregulation. In the military and among athletes, tools such as heat-tolerance testing can be used to gauge the person’s readiness to return to play or duty.8 Heat tolerance testing is performed in a lab using continuous core temperature monitoring while having the subject exercise in a heated room.
CORRESPONDENCE
Scott Kinkade, MD, EdD, MA303 Medical Sciences Building, DC032.00, Columbia, MO 65212; [email protected].
Heat-related illnesses can affect people of any age who are subjected to extreme heat and humidity regardless of physical fitness level or baseline health status. The most serious of the heat-related illnesses is heat stroke. Prompt identification, early initiation of cooling measures (including cold-water immersion [CWI]), and transport to a higher level of care, when appropriate, are imperative. This article reviews heat-related illness identification, as well as management strategies.
Heat-related illnesses: From the benign to the severe
Some of the less severe forms of heat-related illness include heat cramps (which are due to dehydration and salt loss), heat rash, and heat edema. Heat rash and heat edema are benign. Heat rash typically resolves with cooler clothing and a cooler environment. Heat edema tends to improve after sleeping in a cooler environment with legs elevated. Heat syncope is the result of decreased cerebral perfusion due to fluid loss and vasodilation that results in a distributive hypovolemia. It commonly occurs after vigorous exercise when the athlete is standing still.
Heat exhaustion requires a more careful clinical assessment. It is the inability to continue activity in the heat, often with weakness and collapse. Also due to salt and water losses, it results in cardiovascular output that is insufficient to meet the circulatory and metabolic demands of the body. The body temperature is often elevated but <40° C (104° F), vomiting can occur, and mild central nervous system (CNS) dysfunction may be present.
Heat stroke is the most severe form of heat-related illness and can be life-threatening.1
It is important to understand that these heat-related illnesses do not progress along a continuum. Patients develop heat stroke without having had milder forms of heat illness, and patients with a milder type of heat illness usually do not progress to heat stroke.
Heat stroke: Definition, types, risk factors
Heat stroke is defined as a core body temperature ≥40° C (104° F) with CNS dysfunction in the setting of environmental heat stress. The mortality rate can reach over 50%.2-6
There are 2 main types of heat stroke: exertional heat stroke and nonexertional (classic) heat stroke. Exertional heat stroke more commonly affects healthy, young people, such as athletes or military personnel. Classic (nonexertional) heat stroke patients are typically elderly and/or have a chronic illness, although occasionally it involves children who are unable to escape from a hot environment.5,7 While exertional heat stroke typically develops over a period of a few hours in participants of prolonged activities, such as marathons, classic heat stroke in the elderly typically develops over a period of days in the setting of high environmental temperatures. In both conditions, there is an inability to maintain a normal body temperature leading to CNS dysregulation with altered mental status and often multisystem organ dysfunction.7
Continue to: Risk factors
Risk factors. Heat-related illness can affect patients of all ages and levels of physical fitness; however, certain factors place patients at increased risk. These include physical deconditioning, dehydration, high levels of exercise intensity, obesity, elevated environmental temperatures, sleep deprivation, certain medications, alcohol and drug abuse, concurrent illness, and wearing excessive clothing or equipment. It is imperative that severe cases of heat illness be identified early and treatment be initiated rapidly, as delays in cooling can significantly increase the fatality rate.5
Management: First suspect the diagnosis
Health care providers must first suspect heat-related illness and then accurately diagnose it. It is important to differentiate heat-related illness from syncope, cardiac abnormalities, gastroenteritis, hypoglycemia, and other entities that require alternate management. For cases of collapse, syncope or near-syncope, or altered mental status during exertion, heat stroke should be the default diagnosis until proven otherwise.
Obtain a core body temperature. While attending to airway, breathing, and circulation, obtain a core body temperature. Rectal (or esophageal) core temperatures provide a reliable reading that can assist in determining the severity of the heat illness. Axillary, tympanic, temporal, oral, and skin temperatures are affected by environmental factors and are not accurate determinants of core body temperature.8
Once heat stroke is diagnosed, the physician must immediately initiate cooling by removing clothing, placing the patient in the shade or an air-conditioned area, and beginning aggressive cooling measures (more on this in a bit). While field management requires an accurate diagnosis of the severity of a patient’s heat-related illness, one should not delay treatment in order to obtain a rectal temperature.
When treating the milder forms of heat illness, administer oral or intravenous (IV) isotonic fluids. For heat cramps, stretching the affected muscle can help. For heat syncope, lying the patient down and elevating the legs restores perfusion. Patients with heat exhaustion will require some cooling measures such as relocation to a shaded area, removal of excess clothing, and the use of cold towels, along with hydration and elevation of the feet.
Continue to: Cooling techniques for heat stroke
Cooling techniques for heat stroke
In order to adequately cool a patient suffering from heat stroke, health care providers must create a gradient for heat to escape the body through the skin into the environment by conduction, convection, or evaporation.3 Cooling heat stroke patients to less than 40° C (104° F) within 30 minutes after collapse decreases the fatality rate to almost zero.8
CWI comes out on top. CWI, also called an ice-bath, is typically performed in the field. The patient is submerged up to the neck in a tub containing ice and water. Circulating the water and ice mixture helps accelerate cooling.
There have been differences in opinion regarding which cooling method is superior3 (TABLE 13,8,9). Traditionally, there were some concerns that CWI might actually increase body temperature via peripheral vasoconstriction and shivering. But current research suggests that for exertional heat stroke, CWI to promote conductive cooling is the most effective strategy.3,8,10,11 A review of cooling rates in healthy hyperthermic athletes and heat stroke victims showed that ice-water immersion or CWI at 1° to 14° C (35.6°-57.2° F) is superior to all other types of cooling, including ice packs, fans, and partial-body ice-water immersion.10

Furthermore, a 2015 meta-analysis looking at optimal procedures for cooling found that CWI cooled patients twice as fast as passive cooling (without any treatment).11 When cooling with CWI, core temperature drops about 0.2° C/min (0.36° F/min).10 Therefore, the temperature can be expected to drop about 1° C (1.8° F) for every 5 minutes of immersion. When unable to monitor a rectal temperature continuously, 10 to 15 minutes of immersion should get most patients below 40° C (104° F).
Extremity cooling. While CWI is the standard for cases of exertional heat illness, whole-body immersion is not always possible. In such cases, extremity cooling can be an effective body cooling method for exertional heat-related illness.12 Research has shown evaporative and convective cooling methods to have benefits for nonexertional heat-related illnesses.3,8,9 These methods usually involve directing air currents over exposed skin and spraying water on the affected individual.3
Contine to: Guidlines for transport
Guidelines for transport: Cool first, transport second
Most patients suspected of suffering from heat stroke should be transported to a hospital for further evaluation because of the high morbidity and mortality rates associated with it. However, cooling techniques should be implemented while awaiting transport. The current standard is “cool first, transport second.”7 Cooling interventions should continue in the ambulance if the core body temperature is still elevated. Techniques that can be used include the use of air conditioning, convective methods, and administration of IV fluids. As previously discussed, core body temperature should be continuously monitored. Cooling measures should be discontinued only when the patient’s rectal temperature reaches 38.9° C (102° F). Overly aggressive prehospital cooling beyond this point can result in prolonged hypothermia as well as cardiac arrhythmias.6
Monitoring and further evaluation
Monitoring patients with heat-related illness can be difficult, especially when utilizing CWI, as this may limit the ability to use devices such as a cardiac monitor or to continuously monitor rectal temperature. Beyond lowering core body temperature to below 39° C (102.2° F), early evaluation and treatment of other organ systems is vital, keeping in mind that these patients may develop multisystem organ failure. The initial work-up is listed in TABLE 2.

Depending on the severity of the injury and whether you suspect another diagnosis at work, additional studies may include urine output monitoring with a Foley catheter, electrocardiogram, chest radiograph, toxicology screen, a serum lactate level, and cardiac biomarkers.
Imaging. When evaluating for heat stroke, it usually isn’t necessary to obtain head imaging initially, as there are rarely abnormal findings in the early stages. Imaging may be obtained, however, if there is concern about a head injury or if neurologic abnormalities persist into later stages of treatment.5
Pharmacologic agents have not been shown to be of benefit in the treatment of heat-related illness. While dantrolene is commonly used in the treatment of neuroleptic malignant syndrome and malignant hyperthermia, the literature has not described any benefit associated with this agent in relation to heat-related illness. The same goes for antipyretics. Researchers have hypothesized that the reason these agents are ineffective is because body temperature is raised via a different mechanism in these conditions vs heat stroke.3
Continue to: Prevention
Prevention: Modifications and acclimatization are key
People who know they will be exposed to extreme heat should attempt to modify activities. There are many predisposing risk factors ranging from fever and illness to fatigue and dehydration. Risks can be minimized with physiologic adaptation through acclimatization, as well as making various behavioral changes such as adjusting activities, ensuring adequate hydration, and wearing appropriate clothing.13
Certain types of equipment, such as football helmets, can increase the risk of heat-related illness because they prevent heat exchange; however, the benefits sometimes outweigh the risks. With this in mind, consider modification of clothing and equipment if possible.1
In order to prevent heat-related illness, individuals should prehydrate prior to an event and replace fluids orally in order to prevent a >2% loss in body weight. Greater than a 2% loss directly correlates with increased core temperatures during exercise.1
Care should also be taken to perform regular physical activity prior to extreme heat exposure.1 Heat acclimatization takes place when a person’s body adapts to a hotter climate than they are accustomed to. This process can take up to 2 weeks, but once heat acclimation is accomplished, the person will have undergone physical changes, such as reduced metabolic heat production, which will decrease the risk of heat-related illness.13
Return to activity: Customize the approach
Each heat-related injury case is different; thus, return to activity should be individualized. In patients whose heat injury was believed to be secondary to a modifiable risk factor, efforts should be made to correct the predisposing factors that placed the patient at increased risk in the first place.
Additionally, the patient should allow sufficient time to recover. Guidelines recommend at least 1 to 2 weeks recovery before return to activity after heat stroke.8 Moreover, a graded return to activity, starting in a cool environment, is recommended. Gradual introduction of activity in the heat with close monitoring can help with acclimatization and help identify participants who continue to have cooling dysregulation. In the military and among athletes, tools such as heat-tolerance testing can be used to gauge the person’s readiness to return to play or duty.8 Heat tolerance testing is performed in a lab using continuous core temperature monitoring while having the subject exercise in a heated room.
CORRESPONDENCE
Scott Kinkade, MD, EdD, MA303 Medical Sciences Building, DC032.00, Columbia, MO 65212; [email protected].
1. Lipman GS, Eifling KP, Ellis MA, et al. Wilderness Medical Society practice guidelines for the prevention and treatment of heat-related illness: 2014 update. Wilderness Environ Med. 2014;25(4 Suppl):S55-S65.
2. Update: Heat injuries, active component, U.S. Armed Forces, 2014. MSMR. 2015;22:17-20.
3. Gaudio FG, Grissom CK. Cooling methods in heat stroke. J Emerg Med. 2016;50:607-616.
4. Hess JJ, Saha S, Luber G. Summertime acute heat illness in U.S. emergency departments from 2006 through 2010: analysis of a nationally representative sample. Environ Health Perspect. 2014;122:1209-1215.
5. People’s Liberation Army Professional Committee of Critical Care Medicine. Expert consensus on standardized diagnosis and treatment for heat stroke. Mil Med Res. 2016;3:1.
6. Stewart TE, Whitford AC. Dangers of prehospital cooling: a case report of afterdrop in a patient with exertional heat stroke. J Emerg Med. 2015;49:630-633.
7. Chan YK, Mamat M. Management of heat stroke. Trends Anaesthesia Crit Care. 2015;5:65-69.
8. Casa DJ, Armstrong LE, Kenny GP, et al. Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012;11:115-123.
9. Demartini JK, Casa DJ, Stearns R, et al. Effectiveness of cold water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc. 2015;47:240-245.
10. Casa DJ, McDermott BP, Lee EC, et al. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35:141-149.
11. Zhang Y, Davis JK, Casa DJ, et al. Optimizing cold water immersion for exercise-induced hyperthermia: a meta-analysis. Med Sci Sports Exerc. 2015;47:2464-2472.
12. DeGroot DW, Kenefick RW, Sawka MN. Impact of arm immersion cooling during ranger training on exertional heat illness and treatment costs. Mil Med. 2015;180:1178-1183.
13. Epstein Y, Druyan A, Heled Y. Heat injury prevention—a military perspective. J Strength Cond Res. 2012;26 (suppl 2):S82-S86.
1. Lipman GS, Eifling KP, Ellis MA, et al. Wilderness Medical Society practice guidelines for the prevention and treatment of heat-related illness: 2014 update. Wilderness Environ Med. 2014;25(4 Suppl):S55-S65.
2. Update: Heat injuries, active component, U.S. Armed Forces, 2014. MSMR. 2015;22:17-20.
3. Gaudio FG, Grissom CK. Cooling methods in heat stroke. J Emerg Med. 2016;50:607-616.
4. Hess JJ, Saha S, Luber G. Summertime acute heat illness in U.S. emergency departments from 2006 through 2010: analysis of a nationally representative sample. Environ Health Perspect. 2014;122:1209-1215.
5. People’s Liberation Army Professional Committee of Critical Care Medicine. Expert consensus on standardized diagnosis and treatment for heat stroke. Mil Med Res. 2016;3:1.
6. Stewart TE, Whitford AC. Dangers of prehospital cooling: a case report of afterdrop in a patient with exertional heat stroke. J Emerg Med. 2015;49:630-633.
7. Chan YK, Mamat M. Management of heat stroke. Trends Anaesthesia Crit Care. 2015;5:65-69.
8. Casa DJ, Armstrong LE, Kenny GP, et al. Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012;11:115-123.
9. Demartini JK, Casa DJ, Stearns R, et al. Effectiveness of cold water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc. 2015;47:240-245.
10. Casa DJ, McDermott BP, Lee EC, et al. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35:141-149.
11. Zhang Y, Davis JK, Casa DJ, et al. Optimizing cold water immersion for exercise-induced hyperthermia: a meta-analysis. Med Sci Sports Exerc. 2015;47:2464-2472.
12. DeGroot DW, Kenefick RW, Sawka MN. Impact of arm immersion cooling during ranger training on exertional heat illness and treatment costs. Mil Med. 2015;180:1178-1183.
13. Epstein Y, Druyan A, Heled Y. Heat injury prevention—a military perspective. J Strength Cond Res. 2012;26 (suppl 2):S82-S86.
Nonpharmacologic treatment of chronic pain: What works?
In 2017, the American College of Physicians (ACP) published a clinical practice guideline on the management of low back pain (LBP) that states: “For patients with chronic low back pain, clinicians and patients should initially select nonpharmacologic treatment…”1
This represents a significant shift in clinical practice, as treatment of pain syndromes often starts with analgesics and other medication therapy. This recommendation highlights the need for physicians to place nonpharmacologic therapies front and center in the management of chronic pain syndromes. But recommending nonpharmacologic therapies often represents a daunting task for physicians, as this category encompasses a broad range of treatments, some of which are considered “alternative” and others that are less familiar to physicians.

This article discusses 3 categories of nonpharmacologic therapies in detail: exercise-based therapies, mind-body therapies, and complementary modalities, and answers the question: Which nonpharmacologic treatments should you recommend for specific pain conditions?
In answering the question, we will provide a brief synopsis of several treatments within these 3 broad categories to allow a framework to discuss them with your patients, and we will summarize the evidence for these therapies when used for 3 common pain conditions: chronic LBP, osteoarthritis (OA), and fibromyalgia. Finally, we will offer suggestions on how to utilize these therapies within the context of a patient’s treatment plan.
This review is not without limitations. The quality of evidence is sometimes difficult to evaluate when considering nonpharmacologic therapies and can vary significantly among modalities. We sought to include the highest quality systematic reviews available to best reflect the current state of the evidence. We included Cochrane-based reviews when possible and provided evidence ratings using the Strength of Recommendation Taxonomy (SORT) system2 in the hope of helping you best counsel your patients on the appropriate use of available options.
Exercise-based therapies: Options to get patients moving
Therapeutic exercise is broadly defined as physical activity that contributes to enhanced aerobic capacity, strength, and/or flexibility, although health benefits are derived from lower-intensity physical activity even when these parameters do not change. Therapeutic exercise has well-documented salubrious effects including decreased all-cause mortality, improved physical fitness, and improvement in a variety of chronic pain conditions. In a 2017 Cochrane review of aerobic exercise for fibromyalgia, pain scores improved by 18%, compared with controls, although the quality of evidence was low (6 trials; n=351).3
Yoga is a system of physical postures and breathing and meditation practices based in Hindu philosophy. Most yoga classes and research protocols involve some combination of these elements.
Continue to: There is a growing body of research demonstrating...
There is a growing body of research demonstrating the benefits and safety of yoga for the treatment of chronic pain. Multiple reviews have evaluated the effectiveness of yoga in the treatment of chronic LBP with fairly consistent results. A 2017 Cochrane review (12 trials; n=1080) found moderate evidence of improvement in functional outcomes, although the magnitude of benefit was small.4 Chou et al found low-quality evidence of improvement in pain and function with yoga compared with usual care, education, and other exercise therapy (14 trials; n=1431).5
Tai chi is a centuries-old system of slow, deliberate, flowing movements based in the Chinese martial arts. The gentle movements make this a particularly appealing treatment for those who may have difficulty with other forms of exercise, such as the elderly and patients with OA. Tai chi is effective for treating a variety of conditions such as back pain, knee pain, and fibromyalgia. Multiple reviews have shown effectiveness in the treatment of OA.6,7
A 2016 randomized controlled trial (RCT) compared a 12-week course of tai chi to standard physical therapy (PT) for knee OA (n=204).8 The authors found that both strategies yielded similar improvement in pain and function, but that the tai chi group had better outcomes in secondary measures of depression and quality of life.8 Chou et al also found tai chi effective for chronic LBP (2 trials; n=480)5 (TABLE 13-5,7,9-13).

Counsel patients seeking to learn tai chi that it takes time to learn all the postures. Beginner classes typically offer the most detailed instruction and are best suited to patients new to the activity.
Mind-body/behavioral therapies: Taking on a greater role
Mind-body therapies are becoming increasingly important in the management of chronic pain syndromes because of an improved understanding of chronic pain pathophysiology. Studies have shown chronic pain can induce changes in the cortex, which can affect pain processing and perpetuate the experience of pain. Mind-body therapies have the potential to directly address brain centers affected by chronic pain.14 In addition, mind-body therapies can improve coexisting psychological symptoms and coping skills.
Continue to: Psychological therapies
Psychological therapies for the treatment of chronic pain are generally based on a cognitive-behavioral theoretical platform. Cognitive processes surrounding the experience (or avoidance) of pain are thought to exacerbate pain symptoms. Patients are encouraged to shift their mental framework away from a pain-oriented focus and toward a personal goal-oriented focus.15
Overall, research has found cognitive behavioral therapies (CBT) to be effective in the management of chronic pain. A 2012 Cochrane review of psychological therapies used in the treatment of nonspecific chronic pain found CBT particularly effective at pain reduction and improvement in disability and pain-related coping skills (35 trials; n=4788).15
Psychological therapy is generally delivered in a face-to-face encounter, either individually or in a group setting; however, a 2014 Cochrane review suggests that Web-based interventions are efficacious as well.16 Low-quality evidence in a 2013 Cochrane review of CBT for fibromyalgia demonstrated a medium-sized effect of CBT on pain at long-term follow-up (23 trials; n=2031)17 (TABLE 25,17-25).
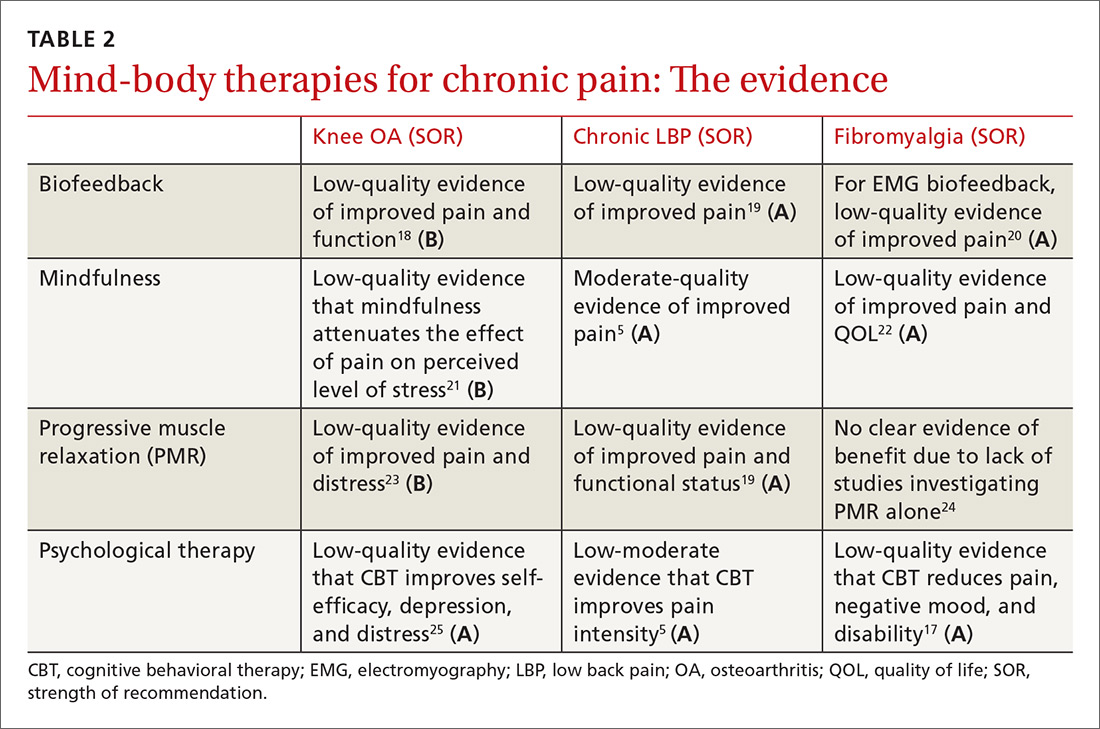
Biofeedback therapy gives patients real-time information about body processes to help bring those processes under voluntary control. Biofeedback devices measure parameters such as heart rate, blood pressure, and muscle tension and give patients visual or auditory cues to help bring those parameters into desired ranges. There is evidence of benefit in a variety of pain conditions including fibromyalgia, arthritis, LBP, and headache.18,19,26
Many psychologists are trained in biofeedback. A trained therapist usually guides biofeedback interventions initially, but patients can then utilize the skills independently. Devices can be purchased for home use. Phone-based applications are available and can be used, as well.
Continue to: Mindfulness
Mindfulness. Based on Eastern meditative traditions, mindfulness interventions focus on breathing and other body sensations as a means of bringing attention to the felt experience of the present moment. Mindfulness encourages a practice of detached observation with openness and curiosity, which allows for a reframing of experience. The growing body of mindfulness literature points to its effectiveness in a variety of pain conditions. A 2017 meta-analysis of mindfulness for pain conditions found a medium-sized effect on pain based on low-quality evidence (30 trials; n=2292).27
Participants can be taught in a series of group sessions (instruct interested patients to look for classes in their geographic area) or individually through a number of resources such as online audios, books, and smartphone applications.
Progressive muscle relaxation is a relaxation technique consisting of serially tightening and releasing different muscle groups to induce relaxation. Careful attention is paid to the somatic experience of tensing and releasing. Researchers have studied this technique for a variety of pain conditions, with the strongest effects observed in those with arthritis and those with LBP.19,28A variety of health care professionals can administer this therapy in office-based settings, and Internet-based audio recordings are available for home practice.
Complementary modalities for chronic pain
Complementary modalities are frequent additions to pain treatment plans. Spinal manipulative therapy (SMT) and massage therapy are regarded as biomechanical interventions, while acupuncture is categorized as a bio-energetic intervention. As a group, these treatments can address structural issues that may be contributing to pain conditions.
SMT is practiced by chiropractors, osteopathic physicians, and physical therapists. SMT improves function through the use of thrust techniques—quick, high-velocity, low-amplitude force applied to a joint, as well as other manual non-thrust techniques sometimes referred to as “mobilization” techniques. Experts have proposed multiple mechanisms of action for spinal manipulation and mobilization techniques, but ultimately SMT attempts to improve joint range of motion.
Continue to: SMT is most often studied for...
SMT is most often studied for the management of spinal pain. The authors of a 2017 systematic review and meta-analysis of 15 RCTs (n=1711) found moderate-quality evidence that SMT improves pain and function in chronic LBP at up to 6 weeks of follow-up.29 A 2017 systematic review performed for an ACP clinical practice guideline on the management of LBP found low-quality evidence of improvement in pain with SMT compared with an inactive treatment, although the magnitude of benefit was small.5 The authors also noted moderate-quality evidence that the benefits of SMT are comparable to other active treatments.5
Massage therapy is commonly used for a variety of pain conditions, but is most studied for LBP. A 2017 systematic review found low-quality evidence of short-term pain relief with massage therapy compared with other active interventions, although the effects were small.5 A 2015 Cochrane review of 25 RCTs (n=3096) found low-quality evidence of benefit for massage in chronic LBP when compared with both active and inactive controls.30
There was a small functional difference when compared with inactive controls. This review highlights the likely short-lived benefit of massage therapy. Although some studies have hinted at longer-term relief with massage therapy, the majority of the literature suggests the benefit is limited to immediate and short-term relief. Massage therapy is safe, although patients with central sensitization should be cautioned that more aggressive massage treatments may cause a flare of myofascial pain.
Acupuncture is one element of traditional Chinese medicine (TCM). And while the holistic system of TCM also includes herbal medicine, nutrition, meditative practices, and movement, acupuncture is often practiced as an independent therapy. In the United States, licensed acupuncturists and physicians provide the therapy. Training and licensing laws vary by state, as does insurance coverage.
Pain is the most common reason that people in the United States seek acupuncture therapy. It is not surprising then that the majority of research surrounding acupuncture involves its use for pain conditions. Chou et al reviewed acupuncture for chronic LBP in 2017 (32 trials; n=5931).5 Acupuncture improved both pain and function compared to inactive controls. In addition, 3 trials compared acupuncture to standard medications and found acupuncture to be superior at providing pain relief.
Continue to: In the management of headache pain...
In the management of headache pain, the literature has consistently found acupuncture to be beneficial in the prevention of migraine headaches. A 2016 Cochrane review found acupuncture beneficial compared to no treatment (4 trials; n=2199) or sham acupuncture (10 trials; n=1534), with benefit similar to prophylactic medications but with fewer adverse effects (3 trials; n=744).31
Evidence for benefit in OA pain has been mixed, but a 2016 meta-analysis evaluating 10 trials (n=2007) found acupuncture improved both short-term pain and functional outcome measures when compared with either no treatment or a sham control.32 There have also been reviews showing short-term benefit in fibromyalgia pain (TABLE 35,33-38).33

Building an effective treatment plan
When creating a treatment plan for chronic pain, it’s helpful to keep the following points in mind:
- Emphasize active treatments. Most traditional medical treatments and many complementary therapies are passive, meaning a patient receives a treatment with little agency in its implementation. Active therapies, such as exercise or relaxation practices, engage patients and improve pain-related coping skills. Active treatments promote self-efficacy, which is associated with improved outcomes in chronic pain.39
- Use treatments from different categories. Just as it is uncommon to choose multiple medications from the same pharmaceutical class, avoid recommending more than one nonpharmacologic treatment from each category. For example, adding chiropractic therapy to a treatment plan of PT, osteopathic manipulation, and massage isn’t likely to add significant benefit because all of these are structural therapies. Addition of a mind-body therapy would likely be a better choice. Consider the template provided when putting together a pain management plan (FIGURE).
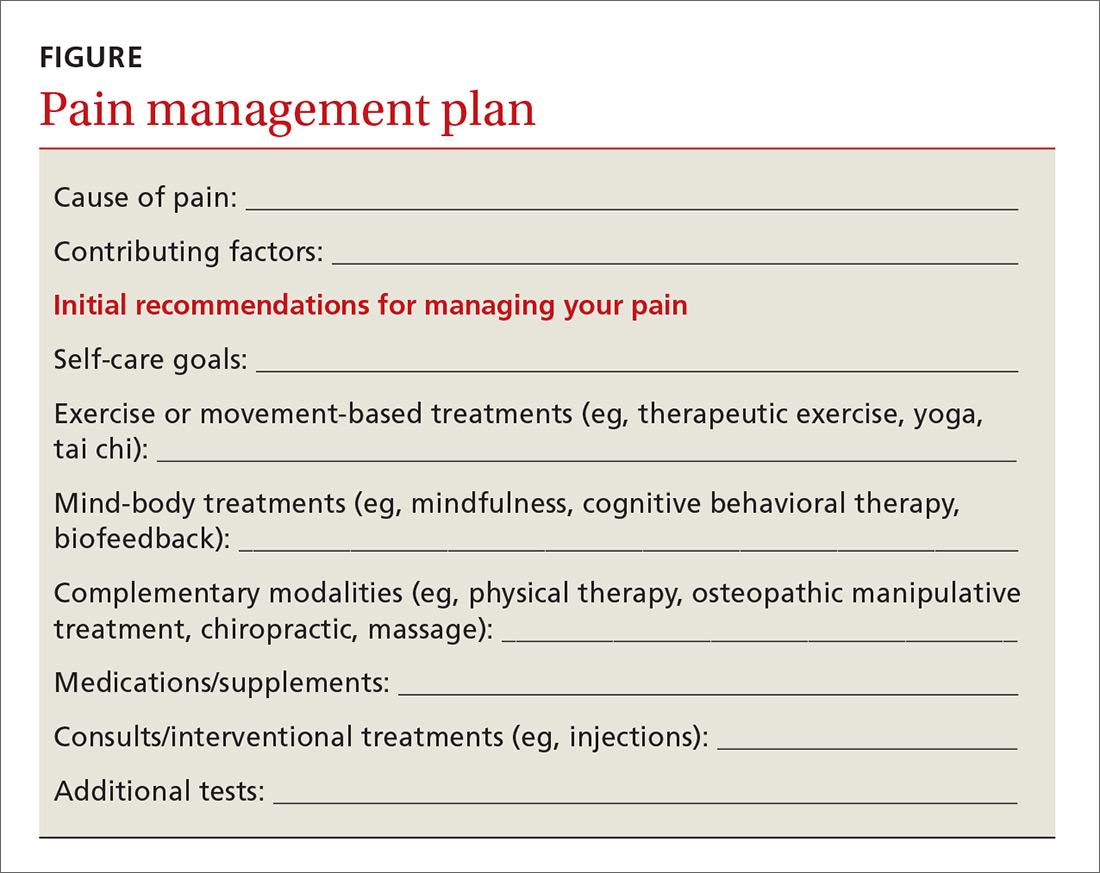
Continue to: Good plan, but how did the office visit go?
Good plan, but how did the office visit go?
A 2006 study by Laerum et al provided unique insights into the best ways to manage chronic pain.40 The authors asked patients a simple question: “What makes a good back consult?” The answers were deceptively simple, but serve as an excellent resource when working with patients to address their pain.
Patients indicated that taking their pain seriously was key to a good back consult. Other factors that were important to patients included: receiving an explanation of what is causing the pain, addressing psychosocial factors, and discussing what could be done.40 The following tips can help you address these patient priorities:
- Explain the underlying cause of the pain. Explaining the complex interplay of factors affecting pain helps patients understand why nonpharmacologic therapies are important. As an example, patients may accept mindfulness meditation as a treatment option if they understand that their chronic LBP is modulated in the brain.
- Address lifestyle and psychosocial issues. Pain syndromes cause far-reaching problems ranging from sleep dysfunction and weight gain to disrupted relationships and loss of employment. Explicitly addressing these issues helps patients cope better with these realities and gives clinicians more therapeutic targets.
The Veterans Affairs Health System offers a self-administered personal health inventory that can facilitate a patient-driven discussion about self-care. (See the Personal Health Inventory form available at: https://www.va.gov/PATIENTCENTEREDCARE/docs/PHI_Short_508.pdf.) In addition to identifying areas for growth, the inventory can highlight what is going well for a patient, adding an element of optimism that is often lacking in office visits for pain problems.
- Discuss what can be done in a way that empowers patients. Moving past medications when discussing pain treatment plans can be challenging. The goal of such discussions is to be as comprehensive as possible by including self-management aspects and nonpharmacologic approaches, in addition to appropriate medications. But this doesn’t all have to be done at once. Help patients set realistic goals for lifestyle-related change, and start with 1 or 2 nonpharmacologic therapies first. This approach both empowers patients and provides them with new treatment options that offer the hope of improved function.
CORRESPONDENCE
Russell Lemmon, DO, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Qaseem A, Wilt TJ, McLean RM, et al, for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Int Med. 2017;166:514-530.
2. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69:548-556.
3. Bidonde J, Busch AJ, Schachter CL, et al. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. 2017;(6):CD012700.
4. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev: 2017;(1):CD010671.
5. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166:493-505.
6. Hall A, Copsey B, Richmond H, et al. Effectiveness of tai chi for chronic musculoskeletal pain conditions: updated systematic review and meta-analysis. Phys Ther. 2017;97:227-238.
7. Ye J, Cai S, Zhong W, et al. Effects of tai chi for patients with knee osteoarthritis: a systematic review. J Phys Ther Sci. 2014;26:1133-1137.
8. Wang C, Schmid CH, Iversen MD, et al. Comparative effectiveness of tai chi versus physical therapy for knee osteoarthritis. Ann Int Med. 2016;165:77-86.
9. Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil. 2017;31:596-611.
10. Busch AJ, Webber SC, Richards RS, et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev. 2013;(12):CD010884.
11. Bidonde J, Busch AJ, Webber SC, et al. Aquatic exercise training for fibromyalgia. Cochrane Database Syst Rev. 2014;(10):CD011336.
12. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
13. Langhorst J, Klose P, Dobos GJ, et al. Efficacy and safety of meditative movement therapies in fibromyalgia syndrome: a systematic review and meta-analysis of randomized controlled trials. Rheumatol Int. 2013;33:193-207.
14. Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;(41 Suppl):66-72.
15. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;(11):CD007407.
16. Eccleston C, Fisher E, Craig L, et al. Psychological therapies (internet-delivered) for the management of chronic pain in adults. Cochrane Database Syst Rev. 2014;(2):CD010152.
17. Bernardy K, Klose P, Busch AJ, et al. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev. 2013;(9):CD009796.
18. Shull PB, Silder A, Shultz R, et al. Six-week gait retraining program reduces knee adduction moment, reduces pain, and improves function for individuals with medial compartment knee osteoarthritis. J Orthop Res. 2013;31:1020-1025.
19. Henschke N, Ostelo RW, van Tulder MW, et al. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev. 2010;(7):CD002014.
20. Glombiewski JA, Sawyer AT, Gutermann J, et al. Psychological treatments for fibromyalgia: a meta-analysis. Pain. 2010;151:280-295.
21. Lee AC, Harvey WF, Price LL, et al. Mindfulness is associated with psychological health and moderates pain in knee osteoarthritis. Osteoarthritis Cartilage. 2017;25:824-831.
22. Lauche R, Cramer H, Dobos G, et al. A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. J Psychosom Res. 2013;75:500-510.
23. Gay MC, Philippot P, Luminet O. Differential effectiveness of psychological interventions for reducing osteoarthritis pain: a comparison of Erickson hypnosis and Jacobson relaxation. Eur J Pain. 2002;6:1-16.
24. Meeus M, Nijs J, Vanderheiden T, et al. The effect of relaxation therapy on autonomic functioning, symptoms and daily functioning, in patients with chronic fatigue syndrome or fibromyalgia: a systematic review. Clin Rehabil. 2015;29:221-233.
25. Briani RV, Ferreira AS, Pazzinatto MF, et al. What interventions can improve quality of life or psychosocial factors of individuals with knee osteoarthritis? A systematic review with meta-analysis of primary outcomes from randomised controlled trials. Br J Sports Med. 2018. doi: 10.1136/bjsports-2017-098099.
26. Glombiewski JA, Bernardy K, Häuser W. Efficacy of EMG- and EEG-biofeedback in fibromyalgia syndrome: a meta-analysis and a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:962741.
27. Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51:199-213.
28. Kwekkeboom KL, Gretarsdottir E. Systematic review of relaxation interventions for pain. J Nurs Scholarsh. 2006;38:269-277.
29. Paige NM, Miake-Lye IM, Booth MS, et al. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain. Systematic review and meta-analysis. JAMA. 2017;317:1451-1460.
30. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015;(9):CD001929.
31. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;(6):CD001218.
32. Lin X, Huang K, Zhu G, et al. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J Bone Joint Surg Am. 2016;98:1578-1585.
33. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
34. Salamh P, Cook C, Reiman MP, et al. Treatment effectiveness and fidelity of manual therapy to the knee: a systematic review and meta-analysis. Musculoskeletal Care. 2017;15:238-248.
35. Posadzki P. Is spinal manipulation effective for pain? An overview of systematic reviews. Pain Med. 2012;13:754-761.
36. Perlman AI, Ali A, Njike VY, et al. Massage therapy for osteoarthritis of the knee: a randomized dose-finding trial. PLoS One. 2012;7:e30248.
37. Kalichman L. Massage therapy for fibromyalgia symptoms. Rheumatol Int. 2010;30:1151-1157.
38. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
39. Somers TJ, Wren AA, Shelby RA. The context of pain in arthritis: self-efficacy for managing pain and other symptoms. Curr Pain Headache Rep. 2012;16:502-508.
40. Laerum E, Indahl A, Skouen JS. What is “the good back-consultation”? A combined qualitative and quantitative study of chronic low back pain patients’ interaction with and perceptions of consultations with specialists. J Rehabil Med. 2006;38:255-262.
In 2017, the American College of Physicians (ACP) published a clinical practice guideline on the management of low back pain (LBP) that states: “For patients with chronic low back pain, clinicians and patients should initially select nonpharmacologic treatment…”1
This represents a significant shift in clinical practice, as treatment of pain syndromes often starts with analgesics and other medication therapy. This recommendation highlights the need for physicians to place nonpharmacologic therapies front and center in the management of chronic pain syndromes. But recommending nonpharmacologic therapies often represents a daunting task for physicians, as this category encompasses a broad range of treatments, some of which are considered “alternative” and others that are less familiar to physicians.

This article discusses 3 categories of nonpharmacologic therapies in detail: exercise-based therapies, mind-body therapies, and complementary modalities, and answers the question: Which nonpharmacologic treatments should you recommend for specific pain conditions?
In answering the question, we will provide a brief synopsis of several treatments within these 3 broad categories to allow a framework to discuss them with your patients, and we will summarize the evidence for these therapies when used for 3 common pain conditions: chronic LBP, osteoarthritis (OA), and fibromyalgia. Finally, we will offer suggestions on how to utilize these therapies within the context of a patient’s treatment plan.
This review is not without limitations. The quality of evidence is sometimes difficult to evaluate when considering nonpharmacologic therapies and can vary significantly among modalities. We sought to include the highest quality systematic reviews available to best reflect the current state of the evidence. We included Cochrane-based reviews when possible and provided evidence ratings using the Strength of Recommendation Taxonomy (SORT) system2 in the hope of helping you best counsel your patients on the appropriate use of available options.
Exercise-based therapies: Options to get patients moving
Therapeutic exercise is broadly defined as physical activity that contributes to enhanced aerobic capacity, strength, and/or flexibility, although health benefits are derived from lower-intensity physical activity even when these parameters do not change. Therapeutic exercise has well-documented salubrious effects including decreased all-cause mortality, improved physical fitness, and improvement in a variety of chronic pain conditions. In a 2017 Cochrane review of aerobic exercise for fibromyalgia, pain scores improved by 18%, compared with controls, although the quality of evidence was low (6 trials; n=351).3
Yoga is a system of physical postures and breathing and meditation practices based in Hindu philosophy. Most yoga classes and research protocols involve some combination of these elements.
Continue to: There is a growing body of research demonstrating...
There is a growing body of research demonstrating the benefits and safety of yoga for the treatment of chronic pain. Multiple reviews have evaluated the effectiveness of yoga in the treatment of chronic LBP with fairly consistent results. A 2017 Cochrane review (12 trials; n=1080) found moderate evidence of improvement in functional outcomes, although the magnitude of benefit was small.4 Chou et al found low-quality evidence of improvement in pain and function with yoga compared with usual care, education, and other exercise therapy (14 trials; n=1431).5
Tai chi is a centuries-old system of slow, deliberate, flowing movements based in the Chinese martial arts. The gentle movements make this a particularly appealing treatment for those who may have difficulty with other forms of exercise, such as the elderly and patients with OA. Tai chi is effective for treating a variety of conditions such as back pain, knee pain, and fibromyalgia. Multiple reviews have shown effectiveness in the treatment of OA.6,7
A 2016 randomized controlled trial (RCT) compared a 12-week course of tai chi to standard physical therapy (PT) for knee OA (n=204).8 The authors found that both strategies yielded similar improvement in pain and function, but that the tai chi group had better outcomes in secondary measures of depression and quality of life.8 Chou et al also found tai chi effective for chronic LBP (2 trials; n=480)5 (TABLE 13-5,7,9-13).

Counsel patients seeking to learn tai chi that it takes time to learn all the postures. Beginner classes typically offer the most detailed instruction and are best suited to patients new to the activity.
Mind-body/behavioral therapies: Taking on a greater role
Mind-body therapies are becoming increasingly important in the management of chronic pain syndromes because of an improved understanding of chronic pain pathophysiology. Studies have shown chronic pain can induce changes in the cortex, which can affect pain processing and perpetuate the experience of pain. Mind-body therapies have the potential to directly address brain centers affected by chronic pain.14 In addition, mind-body therapies can improve coexisting psychological symptoms and coping skills.
Continue to: Psychological therapies
Psychological therapies for the treatment of chronic pain are generally based on a cognitive-behavioral theoretical platform. Cognitive processes surrounding the experience (or avoidance) of pain are thought to exacerbate pain symptoms. Patients are encouraged to shift their mental framework away from a pain-oriented focus and toward a personal goal-oriented focus.15
Overall, research has found cognitive behavioral therapies (CBT) to be effective in the management of chronic pain. A 2012 Cochrane review of psychological therapies used in the treatment of nonspecific chronic pain found CBT particularly effective at pain reduction and improvement in disability and pain-related coping skills (35 trials; n=4788).15
Psychological therapy is generally delivered in a face-to-face encounter, either individually or in a group setting; however, a 2014 Cochrane review suggests that Web-based interventions are efficacious as well.16 Low-quality evidence in a 2013 Cochrane review of CBT for fibromyalgia demonstrated a medium-sized effect of CBT on pain at long-term follow-up (23 trials; n=2031)17 (TABLE 25,17-25).

Biofeedback therapy gives patients real-time information about body processes to help bring those processes under voluntary control. Biofeedback devices measure parameters such as heart rate, blood pressure, and muscle tension and give patients visual or auditory cues to help bring those parameters into desired ranges. There is evidence of benefit in a variety of pain conditions including fibromyalgia, arthritis, LBP, and headache.18,19,26
Many psychologists are trained in biofeedback. A trained therapist usually guides biofeedback interventions initially, but patients can then utilize the skills independently. Devices can be purchased for home use. Phone-based applications are available and can be used, as well.
Continue to: Mindfulness
Mindfulness. Based on Eastern meditative traditions, mindfulness interventions focus on breathing and other body sensations as a means of bringing attention to the felt experience of the present moment. Mindfulness encourages a practice of detached observation with openness and curiosity, which allows for a reframing of experience. The growing body of mindfulness literature points to its effectiveness in a variety of pain conditions. A 2017 meta-analysis of mindfulness for pain conditions found a medium-sized effect on pain based on low-quality evidence (30 trials; n=2292).27
Participants can be taught in a series of group sessions (instruct interested patients to look for classes in their geographic area) or individually through a number of resources such as online audios, books, and smartphone applications.
Progressive muscle relaxation is a relaxation technique consisting of serially tightening and releasing different muscle groups to induce relaxation. Careful attention is paid to the somatic experience of tensing and releasing. Researchers have studied this technique for a variety of pain conditions, with the strongest effects observed in those with arthritis and those with LBP.19,28A variety of health care professionals can administer this therapy in office-based settings, and Internet-based audio recordings are available for home practice.
Complementary modalities for chronic pain
Complementary modalities are frequent additions to pain treatment plans. Spinal manipulative therapy (SMT) and massage therapy are regarded as biomechanical interventions, while acupuncture is categorized as a bio-energetic intervention. As a group, these treatments can address structural issues that may be contributing to pain conditions.
SMT is practiced by chiropractors, osteopathic physicians, and physical therapists. SMT improves function through the use of thrust techniques—quick, high-velocity, low-amplitude force applied to a joint, as well as other manual non-thrust techniques sometimes referred to as “mobilization” techniques. Experts have proposed multiple mechanisms of action for spinal manipulation and mobilization techniques, but ultimately SMT attempts to improve joint range of motion.
Continue to: SMT is most often studied for...
SMT is most often studied for the management of spinal pain. The authors of a 2017 systematic review and meta-analysis of 15 RCTs (n=1711) found moderate-quality evidence that SMT improves pain and function in chronic LBP at up to 6 weeks of follow-up.29 A 2017 systematic review performed for an ACP clinical practice guideline on the management of LBP found low-quality evidence of improvement in pain with SMT compared with an inactive treatment, although the magnitude of benefit was small.5 The authors also noted moderate-quality evidence that the benefits of SMT are comparable to other active treatments.5
Massage therapy is commonly used for a variety of pain conditions, but is most studied for LBP. A 2017 systematic review found low-quality evidence of short-term pain relief with massage therapy compared with other active interventions, although the effects were small.5 A 2015 Cochrane review of 25 RCTs (n=3096) found low-quality evidence of benefit for massage in chronic LBP when compared with both active and inactive controls.30
There was a small functional difference when compared with inactive controls. This review highlights the likely short-lived benefit of massage therapy. Although some studies have hinted at longer-term relief with massage therapy, the majority of the literature suggests the benefit is limited to immediate and short-term relief. Massage therapy is safe, although patients with central sensitization should be cautioned that more aggressive massage treatments may cause a flare of myofascial pain.
Acupuncture is one element of traditional Chinese medicine (TCM). And while the holistic system of TCM also includes herbal medicine, nutrition, meditative practices, and movement, acupuncture is often practiced as an independent therapy. In the United States, licensed acupuncturists and physicians provide the therapy. Training and licensing laws vary by state, as does insurance coverage.
Pain is the most common reason that people in the United States seek acupuncture therapy. It is not surprising then that the majority of research surrounding acupuncture involves its use for pain conditions. Chou et al reviewed acupuncture for chronic LBP in 2017 (32 trials; n=5931).5 Acupuncture improved both pain and function compared to inactive controls. In addition, 3 trials compared acupuncture to standard medications and found acupuncture to be superior at providing pain relief.
Continue to: In the management of headache pain...
In the management of headache pain, the literature has consistently found acupuncture to be beneficial in the prevention of migraine headaches. A 2016 Cochrane review found acupuncture beneficial compared to no treatment (4 trials; n=2199) or sham acupuncture (10 trials; n=1534), with benefit similar to prophylactic medications but with fewer adverse effects (3 trials; n=744).31
Evidence for benefit in OA pain has been mixed, but a 2016 meta-analysis evaluating 10 trials (n=2007) found acupuncture improved both short-term pain and functional outcome measures when compared with either no treatment or a sham control.32 There have also been reviews showing short-term benefit in fibromyalgia pain (TABLE 35,33-38).33

Building an effective treatment plan
When creating a treatment plan for chronic pain, it’s helpful to keep the following points in mind:
- Emphasize active treatments. Most traditional medical treatments and many complementary therapies are passive, meaning a patient receives a treatment with little agency in its implementation. Active therapies, such as exercise or relaxation practices, engage patients and improve pain-related coping skills. Active treatments promote self-efficacy, which is associated with improved outcomes in chronic pain.39
- Use treatments from different categories. Just as it is uncommon to choose multiple medications from the same pharmaceutical class, avoid recommending more than one nonpharmacologic treatment from each category. For example, adding chiropractic therapy to a treatment plan of PT, osteopathic manipulation, and massage isn’t likely to add significant benefit because all of these are structural therapies. Addition of a mind-body therapy would likely be a better choice. Consider the template provided when putting together a pain management plan (FIGURE).

Continue to: Good plan, but how did the office visit go?
Good plan, but how did the office visit go?
A 2006 study by Laerum et al provided unique insights into the best ways to manage chronic pain.40 The authors asked patients a simple question: “What makes a good back consult?” The answers were deceptively simple, but serve as an excellent resource when working with patients to address their pain.
Patients indicated that taking their pain seriously was key to a good back consult. Other factors that were important to patients included: receiving an explanation of what is causing the pain, addressing psychosocial factors, and discussing what could be done.40 The following tips can help you address these patient priorities:
- Explain the underlying cause of the pain. Explaining the complex interplay of factors affecting pain helps patients understand why nonpharmacologic therapies are important. As an example, patients may accept mindfulness meditation as a treatment option if they understand that their chronic LBP is modulated in the brain.
- Address lifestyle and psychosocial issues. Pain syndromes cause far-reaching problems ranging from sleep dysfunction and weight gain to disrupted relationships and loss of employment. Explicitly addressing these issues helps patients cope better with these realities and gives clinicians more therapeutic targets.
The Veterans Affairs Health System offers a self-administered personal health inventory that can facilitate a patient-driven discussion about self-care. (See the Personal Health Inventory form available at: https://www.va.gov/PATIENTCENTEREDCARE/docs/PHI_Short_508.pdf.) In addition to identifying areas for growth, the inventory can highlight what is going well for a patient, adding an element of optimism that is often lacking in office visits for pain problems.
- Discuss what can be done in a way that empowers patients. Moving past medications when discussing pain treatment plans can be challenging. The goal of such discussions is to be as comprehensive as possible by including self-management aspects and nonpharmacologic approaches, in addition to appropriate medications. But this doesn’t all have to be done at once. Help patients set realistic goals for lifestyle-related change, and start with 1 or 2 nonpharmacologic therapies first. This approach both empowers patients and provides them with new treatment options that offer the hope of improved function.
CORRESPONDENCE
Russell Lemmon, DO, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
In 2017, the American College of Physicians (ACP) published a clinical practice guideline on the management of low back pain (LBP) that states: “For patients with chronic low back pain, clinicians and patients should initially select nonpharmacologic treatment…”1
This represents a significant shift in clinical practice, as treatment of pain syndromes often starts with analgesics and other medication therapy. This recommendation highlights the need for physicians to place nonpharmacologic therapies front and center in the management of chronic pain syndromes. But recommending nonpharmacologic therapies often represents a daunting task for physicians, as this category encompasses a broad range of treatments, some of which are considered “alternative” and others that are less familiar to physicians.

This article discusses 3 categories of nonpharmacologic therapies in detail: exercise-based therapies, mind-body therapies, and complementary modalities, and answers the question: Which nonpharmacologic treatments should you recommend for specific pain conditions?
In answering the question, we will provide a brief synopsis of several treatments within these 3 broad categories to allow a framework to discuss them with your patients, and we will summarize the evidence for these therapies when used for 3 common pain conditions: chronic LBP, osteoarthritis (OA), and fibromyalgia. Finally, we will offer suggestions on how to utilize these therapies within the context of a patient’s treatment plan.
This review is not without limitations. The quality of evidence is sometimes difficult to evaluate when considering nonpharmacologic therapies and can vary significantly among modalities. We sought to include the highest quality systematic reviews available to best reflect the current state of the evidence. We included Cochrane-based reviews when possible and provided evidence ratings using the Strength of Recommendation Taxonomy (SORT) system2 in the hope of helping you best counsel your patients on the appropriate use of available options.
Exercise-based therapies: Options to get patients moving
Therapeutic exercise is broadly defined as physical activity that contributes to enhanced aerobic capacity, strength, and/or flexibility, although health benefits are derived from lower-intensity physical activity even when these parameters do not change. Therapeutic exercise has well-documented salubrious effects including decreased all-cause mortality, improved physical fitness, and improvement in a variety of chronic pain conditions. In a 2017 Cochrane review of aerobic exercise for fibromyalgia, pain scores improved by 18%, compared with controls, although the quality of evidence was low (6 trials; n=351).3
Yoga is a system of physical postures and breathing and meditation practices based in Hindu philosophy. Most yoga classes and research protocols involve some combination of these elements.
Continue to: There is a growing body of research demonstrating...
There is a growing body of research demonstrating the benefits and safety of yoga for the treatment of chronic pain. Multiple reviews have evaluated the effectiveness of yoga in the treatment of chronic LBP with fairly consistent results. A 2017 Cochrane review (12 trials; n=1080) found moderate evidence of improvement in functional outcomes, although the magnitude of benefit was small.4 Chou et al found low-quality evidence of improvement in pain and function with yoga compared with usual care, education, and other exercise therapy (14 trials; n=1431).5
Tai chi is a centuries-old system of slow, deliberate, flowing movements based in the Chinese martial arts. The gentle movements make this a particularly appealing treatment for those who may have difficulty with other forms of exercise, such as the elderly and patients with OA. Tai chi is effective for treating a variety of conditions such as back pain, knee pain, and fibromyalgia. Multiple reviews have shown effectiveness in the treatment of OA.6,7
A 2016 randomized controlled trial (RCT) compared a 12-week course of tai chi to standard physical therapy (PT) for knee OA (n=204).8 The authors found that both strategies yielded similar improvement in pain and function, but that the tai chi group had better outcomes in secondary measures of depression and quality of life.8 Chou et al also found tai chi effective for chronic LBP (2 trials; n=480)5 (TABLE 13-5,7,9-13).

Counsel patients seeking to learn tai chi that it takes time to learn all the postures. Beginner classes typically offer the most detailed instruction and are best suited to patients new to the activity.
Mind-body/behavioral therapies: Taking on a greater role
Mind-body therapies are becoming increasingly important in the management of chronic pain syndromes because of an improved understanding of chronic pain pathophysiology. Studies have shown chronic pain can induce changes in the cortex, which can affect pain processing and perpetuate the experience of pain. Mind-body therapies have the potential to directly address brain centers affected by chronic pain.14 In addition, mind-body therapies can improve coexisting psychological symptoms and coping skills.
Continue to: Psychological therapies
Psychological therapies for the treatment of chronic pain are generally based on a cognitive-behavioral theoretical platform. Cognitive processes surrounding the experience (or avoidance) of pain are thought to exacerbate pain symptoms. Patients are encouraged to shift their mental framework away from a pain-oriented focus and toward a personal goal-oriented focus.15
Overall, research has found cognitive behavioral therapies (CBT) to be effective in the management of chronic pain. A 2012 Cochrane review of psychological therapies used in the treatment of nonspecific chronic pain found CBT particularly effective at pain reduction and improvement in disability and pain-related coping skills (35 trials; n=4788).15
Psychological therapy is generally delivered in a face-to-face encounter, either individually or in a group setting; however, a 2014 Cochrane review suggests that Web-based interventions are efficacious as well.16 Low-quality evidence in a 2013 Cochrane review of CBT for fibromyalgia demonstrated a medium-sized effect of CBT on pain at long-term follow-up (23 trials; n=2031)17 (TABLE 25,17-25).

Biofeedback therapy gives patients real-time information about body processes to help bring those processes under voluntary control. Biofeedback devices measure parameters such as heart rate, blood pressure, and muscle tension and give patients visual or auditory cues to help bring those parameters into desired ranges. There is evidence of benefit in a variety of pain conditions including fibromyalgia, arthritis, LBP, and headache.18,19,26
Many psychologists are trained in biofeedback. A trained therapist usually guides biofeedback interventions initially, but patients can then utilize the skills independently. Devices can be purchased for home use. Phone-based applications are available and can be used, as well.
Continue to: Mindfulness
Mindfulness. Based on Eastern meditative traditions, mindfulness interventions focus on breathing and other body sensations as a means of bringing attention to the felt experience of the present moment. Mindfulness encourages a practice of detached observation with openness and curiosity, which allows for a reframing of experience. The growing body of mindfulness literature points to its effectiveness in a variety of pain conditions. A 2017 meta-analysis of mindfulness for pain conditions found a medium-sized effect on pain based on low-quality evidence (30 trials; n=2292).27
Participants can be taught in a series of group sessions (instruct interested patients to look for classes in their geographic area) or individually through a number of resources such as online audios, books, and smartphone applications.
Progressive muscle relaxation is a relaxation technique consisting of serially tightening and releasing different muscle groups to induce relaxation. Careful attention is paid to the somatic experience of tensing and releasing. Researchers have studied this technique for a variety of pain conditions, with the strongest effects observed in those with arthritis and those with LBP.19,28A variety of health care professionals can administer this therapy in office-based settings, and Internet-based audio recordings are available for home practice.
Complementary modalities for chronic pain
Complementary modalities are frequent additions to pain treatment plans. Spinal manipulative therapy (SMT) and massage therapy are regarded as biomechanical interventions, while acupuncture is categorized as a bio-energetic intervention. As a group, these treatments can address structural issues that may be contributing to pain conditions.
SMT is practiced by chiropractors, osteopathic physicians, and physical therapists. SMT improves function through the use of thrust techniques—quick, high-velocity, low-amplitude force applied to a joint, as well as other manual non-thrust techniques sometimes referred to as “mobilization” techniques. Experts have proposed multiple mechanisms of action for spinal manipulation and mobilization techniques, but ultimately SMT attempts to improve joint range of motion.
Continue to: SMT is most often studied for...
SMT is most often studied for the management of spinal pain. The authors of a 2017 systematic review and meta-analysis of 15 RCTs (n=1711) found moderate-quality evidence that SMT improves pain and function in chronic LBP at up to 6 weeks of follow-up.29 A 2017 systematic review performed for an ACP clinical practice guideline on the management of LBP found low-quality evidence of improvement in pain with SMT compared with an inactive treatment, although the magnitude of benefit was small.5 The authors also noted moderate-quality evidence that the benefits of SMT are comparable to other active treatments.5
Massage therapy is commonly used for a variety of pain conditions, but is most studied for LBP. A 2017 systematic review found low-quality evidence of short-term pain relief with massage therapy compared with other active interventions, although the effects were small.5 A 2015 Cochrane review of 25 RCTs (n=3096) found low-quality evidence of benefit for massage in chronic LBP when compared with both active and inactive controls.30
There was a small functional difference when compared with inactive controls. This review highlights the likely short-lived benefit of massage therapy. Although some studies have hinted at longer-term relief with massage therapy, the majority of the literature suggests the benefit is limited to immediate and short-term relief. Massage therapy is safe, although patients with central sensitization should be cautioned that more aggressive massage treatments may cause a flare of myofascial pain.
Acupuncture is one element of traditional Chinese medicine (TCM). And while the holistic system of TCM also includes herbal medicine, nutrition, meditative practices, and movement, acupuncture is often practiced as an independent therapy. In the United States, licensed acupuncturists and physicians provide the therapy. Training and licensing laws vary by state, as does insurance coverage.
Pain is the most common reason that people in the United States seek acupuncture therapy. It is not surprising then that the majority of research surrounding acupuncture involves its use for pain conditions. Chou et al reviewed acupuncture for chronic LBP in 2017 (32 trials; n=5931).5 Acupuncture improved both pain and function compared to inactive controls. In addition, 3 trials compared acupuncture to standard medications and found acupuncture to be superior at providing pain relief.
Continue to: In the management of headache pain...
In the management of headache pain, the literature has consistently found acupuncture to be beneficial in the prevention of migraine headaches. A 2016 Cochrane review found acupuncture beneficial compared to no treatment (4 trials; n=2199) or sham acupuncture (10 trials; n=1534), with benefit similar to prophylactic medications but with fewer adverse effects (3 trials; n=744).31
Evidence for benefit in OA pain has been mixed, but a 2016 meta-analysis evaluating 10 trials (n=2007) found acupuncture improved both short-term pain and functional outcome measures when compared with either no treatment or a sham control.32 There have also been reviews showing short-term benefit in fibromyalgia pain (TABLE 35,33-38).33

Building an effective treatment plan
When creating a treatment plan for chronic pain, it’s helpful to keep the following points in mind:
- Emphasize active treatments. Most traditional medical treatments and many complementary therapies are passive, meaning a patient receives a treatment with little agency in its implementation. Active therapies, such as exercise or relaxation practices, engage patients and improve pain-related coping skills. Active treatments promote self-efficacy, which is associated with improved outcomes in chronic pain.39
- Use treatments from different categories. Just as it is uncommon to choose multiple medications from the same pharmaceutical class, avoid recommending more than one nonpharmacologic treatment from each category. For example, adding chiropractic therapy to a treatment plan of PT, osteopathic manipulation, and massage isn’t likely to add significant benefit because all of these are structural therapies. Addition of a mind-body therapy would likely be a better choice. Consider the template provided when putting together a pain management plan (FIGURE).

Continue to: Good plan, but how did the office visit go?
Good plan, but how did the office visit go?
A 2006 study by Laerum et al provided unique insights into the best ways to manage chronic pain.40 The authors asked patients a simple question: “What makes a good back consult?” The answers were deceptively simple, but serve as an excellent resource when working with patients to address their pain.
Patients indicated that taking their pain seriously was key to a good back consult. Other factors that were important to patients included: receiving an explanation of what is causing the pain, addressing psychosocial factors, and discussing what could be done.40 The following tips can help you address these patient priorities:
- Explain the underlying cause of the pain. Explaining the complex interplay of factors affecting pain helps patients understand why nonpharmacologic therapies are important. As an example, patients may accept mindfulness meditation as a treatment option if they understand that their chronic LBP is modulated in the brain.
- Address lifestyle and psychosocial issues. Pain syndromes cause far-reaching problems ranging from sleep dysfunction and weight gain to disrupted relationships and loss of employment. Explicitly addressing these issues helps patients cope better with these realities and gives clinicians more therapeutic targets.
The Veterans Affairs Health System offers a self-administered personal health inventory that can facilitate a patient-driven discussion about self-care. (See the Personal Health Inventory form available at: https://www.va.gov/PATIENTCENTEREDCARE/docs/PHI_Short_508.pdf.) In addition to identifying areas for growth, the inventory can highlight what is going well for a patient, adding an element of optimism that is often lacking in office visits for pain problems.
- Discuss what can be done in a way that empowers patients. Moving past medications when discussing pain treatment plans can be challenging. The goal of such discussions is to be as comprehensive as possible by including self-management aspects and nonpharmacologic approaches, in addition to appropriate medications. But this doesn’t all have to be done at once. Help patients set realistic goals for lifestyle-related change, and start with 1 or 2 nonpharmacologic therapies first. This approach both empowers patients and provides them with new treatment options that offer the hope of improved function.
CORRESPONDENCE
Russell Lemmon, DO, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Qaseem A, Wilt TJ, McLean RM, et al, for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Int Med. 2017;166:514-530.
2. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69:548-556.
3. Bidonde J, Busch AJ, Schachter CL, et al. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. 2017;(6):CD012700.
4. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev: 2017;(1):CD010671.
5. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166:493-505.
6. Hall A, Copsey B, Richmond H, et al. Effectiveness of tai chi for chronic musculoskeletal pain conditions: updated systematic review and meta-analysis. Phys Ther. 2017;97:227-238.
7. Ye J, Cai S, Zhong W, et al. Effects of tai chi for patients with knee osteoarthritis: a systematic review. J Phys Ther Sci. 2014;26:1133-1137.
8. Wang C, Schmid CH, Iversen MD, et al. Comparative effectiveness of tai chi versus physical therapy for knee osteoarthritis. Ann Int Med. 2016;165:77-86.
9. Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil. 2017;31:596-611.
10. Busch AJ, Webber SC, Richards RS, et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev. 2013;(12):CD010884.
11. Bidonde J, Busch AJ, Webber SC, et al. Aquatic exercise training for fibromyalgia. Cochrane Database Syst Rev. 2014;(10):CD011336.
12. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
13. Langhorst J, Klose P, Dobos GJ, et al. Efficacy and safety of meditative movement therapies in fibromyalgia syndrome: a systematic review and meta-analysis of randomized controlled trials. Rheumatol Int. 2013;33:193-207.
14. Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;(41 Suppl):66-72.
15. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;(11):CD007407.
16. Eccleston C, Fisher E, Craig L, et al. Psychological therapies (internet-delivered) for the management of chronic pain in adults. Cochrane Database Syst Rev. 2014;(2):CD010152.
17. Bernardy K, Klose P, Busch AJ, et al. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev. 2013;(9):CD009796.
18. Shull PB, Silder A, Shultz R, et al. Six-week gait retraining program reduces knee adduction moment, reduces pain, and improves function for individuals with medial compartment knee osteoarthritis. J Orthop Res. 2013;31:1020-1025.
19. Henschke N, Ostelo RW, van Tulder MW, et al. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev. 2010;(7):CD002014.
20. Glombiewski JA, Sawyer AT, Gutermann J, et al. Psychological treatments for fibromyalgia: a meta-analysis. Pain. 2010;151:280-295.
21. Lee AC, Harvey WF, Price LL, et al. Mindfulness is associated with psychological health and moderates pain in knee osteoarthritis. Osteoarthritis Cartilage. 2017;25:824-831.
22. Lauche R, Cramer H, Dobos G, et al. A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. J Psychosom Res. 2013;75:500-510.
23. Gay MC, Philippot P, Luminet O. Differential effectiveness of psychological interventions for reducing osteoarthritis pain: a comparison of Erickson hypnosis and Jacobson relaxation. Eur J Pain. 2002;6:1-16.
24. Meeus M, Nijs J, Vanderheiden T, et al. The effect of relaxation therapy on autonomic functioning, symptoms and daily functioning, in patients with chronic fatigue syndrome or fibromyalgia: a systematic review. Clin Rehabil. 2015;29:221-233.
25. Briani RV, Ferreira AS, Pazzinatto MF, et al. What interventions can improve quality of life or psychosocial factors of individuals with knee osteoarthritis? A systematic review with meta-analysis of primary outcomes from randomised controlled trials. Br J Sports Med. 2018. doi: 10.1136/bjsports-2017-098099.
26. Glombiewski JA, Bernardy K, Häuser W. Efficacy of EMG- and EEG-biofeedback in fibromyalgia syndrome: a meta-analysis and a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:962741.
27. Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51:199-213.
28. Kwekkeboom KL, Gretarsdottir E. Systematic review of relaxation interventions for pain. J Nurs Scholarsh. 2006;38:269-277.
29. Paige NM, Miake-Lye IM, Booth MS, et al. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain. Systematic review and meta-analysis. JAMA. 2017;317:1451-1460.
30. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015;(9):CD001929.
31. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;(6):CD001218.
32. Lin X, Huang K, Zhu G, et al. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J Bone Joint Surg Am. 2016;98:1578-1585.
33. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
34. Salamh P, Cook C, Reiman MP, et al. Treatment effectiveness and fidelity of manual therapy to the knee: a systematic review and meta-analysis. Musculoskeletal Care. 2017;15:238-248.
35. Posadzki P. Is spinal manipulation effective for pain? An overview of systematic reviews. Pain Med. 2012;13:754-761.
36. Perlman AI, Ali A, Njike VY, et al. Massage therapy for osteoarthritis of the knee: a randomized dose-finding trial. PLoS One. 2012;7:e30248.
37. Kalichman L. Massage therapy for fibromyalgia symptoms. Rheumatol Int. 2010;30:1151-1157.
38. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
39. Somers TJ, Wren AA, Shelby RA. The context of pain in arthritis: self-efficacy for managing pain and other symptoms. Curr Pain Headache Rep. 2012;16:502-508.
40. Laerum E, Indahl A, Skouen JS. What is “the good back-consultation”? A combined qualitative and quantitative study of chronic low back pain patients’ interaction with and perceptions of consultations with specialists. J Rehabil Med. 2006;38:255-262.
1. Qaseem A, Wilt TJ, McLean RM, et al, for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Int Med. 2017;166:514-530.
2. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69:548-556.
3. Bidonde J, Busch AJ, Schachter CL, et al. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. 2017;(6):CD012700.
4. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev: 2017;(1):CD010671.
5. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166:493-505.
6. Hall A, Copsey B, Richmond H, et al. Effectiveness of tai chi for chronic musculoskeletal pain conditions: updated systematic review and meta-analysis. Phys Ther. 2017;97:227-238.
7. Ye J, Cai S, Zhong W, et al. Effects of tai chi for patients with knee osteoarthritis: a systematic review. J Phys Ther Sci. 2014;26:1133-1137.
8. Wang C, Schmid CH, Iversen MD, et al. Comparative effectiveness of tai chi versus physical therapy for knee osteoarthritis. Ann Int Med. 2016;165:77-86.
9. Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil. 2017;31:596-611.
10. Busch AJ, Webber SC, Richards RS, et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev. 2013;(12):CD010884.
11. Bidonde J, Busch AJ, Webber SC, et al. Aquatic exercise training for fibromyalgia. Cochrane Database Syst Rev. 2014;(10):CD011336.
12. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
13. Langhorst J, Klose P, Dobos GJ, et al. Efficacy and safety of meditative movement therapies in fibromyalgia syndrome: a systematic review and meta-analysis of randomized controlled trials. Rheumatol Int. 2013;33:193-207.
14. Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;(41 Suppl):66-72.
15. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;(11):CD007407.
16. Eccleston C, Fisher E, Craig L, et al. Psychological therapies (internet-delivered) for the management of chronic pain in adults. Cochrane Database Syst Rev. 2014;(2):CD010152.
17. Bernardy K, Klose P, Busch AJ, et al. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev. 2013;(9):CD009796.
18. Shull PB, Silder A, Shultz R, et al. Six-week gait retraining program reduces knee adduction moment, reduces pain, and improves function for individuals with medial compartment knee osteoarthritis. J Orthop Res. 2013;31:1020-1025.
19. Henschke N, Ostelo RW, van Tulder MW, et al. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev. 2010;(7):CD002014.
20. Glombiewski JA, Sawyer AT, Gutermann J, et al. Psychological treatments for fibromyalgia: a meta-analysis. Pain. 2010;151:280-295.
21. Lee AC, Harvey WF, Price LL, et al. Mindfulness is associated with psychological health and moderates pain in knee osteoarthritis. Osteoarthritis Cartilage. 2017;25:824-831.
22. Lauche R, Cramer H, Dobos G, et al. A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. J Psychosom Res. 2013;75:500-510.
23. Gay MC, Philippot P, Luminet O. Differential effectiveness of psychological interventions for reducing osteoarthritis pain: a comparison of Erickson hypnosis and Jacobson relaxation. Eur J Pain. 2002;6:1-16.
24. Meeus M, Nijs J, Vanderheiden T, et al. The effect of relaxation therapy on autonomic functioning, symptoms and daily functioning, in patients with chronic fatigue syndrome or fibromyalgia: a systematic review. Clin Rehabil. 2015;29:221-233.
25. Briani RV, Ferreira AS, Pazzinatto MF, et al. What interventions can improve quality of life or psychosocial factors of individuals with knee osteoarthritis? A systematic review with meta-analysis of primary outcomes from randomised controlled trials. Br J Sports Med. 2018. doi: 10.1136/bjsports-2017-098099.
26. Glombiewski JA, Bernardy K, Häuser W. Efficacy of EMG- and EEG-biofeedback in fibromyalgia syndrome: a meta-analysis and a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:962741.
27. Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51:199-213.
28. Kwekkeboom KL, Gretarsdottir E. Systematic review of relaxation interventions for pain. J Nurs Scholarsh. 2006;38:269-277.
29. Paige NM, Miake-Lye IM, Booth MS, et al. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain. Systematic review and meta-analysis. JAMA. 2017;317:1451-1460.
30. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015;(9):CD001929.
31. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;(6):CD001218.
32. Lin X, Huang K, Zhu G, et al. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J Bone Joint Surg Am. 2016;98:1578-1585.
33. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
34. Salamh P, Cook C, Reiman MP, et al. Treatment effectiveness and fidelity of manual therapy to the knee: a systematic review and meta-analysis. Musculoskeletal Care. 2017;15:238-248.
35. Posadzki P. Is spinal manipulation effective for pain? An overview of systematic reviews. Pain Med. 2012;13:754-761.
36. Perlman AI, Ali A, Njike VY, et al. Massage therapy for osteoarthritis of the knee: a randomized dose-finding trial. PLoS One. 2012;7:e30248.
37. Kalichman L. Massage therapy for fibromyalgia symptoms. Rheumatol Int. 2010;30:1151-1157.
38. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
39. Somers TJ, Wren AA, Shelby RA. The context of pain in arthritis: self-efficacy for managing pain and other symptoms. Curr Pain Headache Rep. 2012;16:502-508.
40. Laerum E, Indahl A, Skouen JS. What is “the good back-consultation”? A combined qualitative and quantitative study of chronic low back pain patients’ interaction with and perceptions of consultations with specialists. J Rehabil Med. 2006;38:255-262.
From The Journal of Family Practice | 2018;67(8):474-477,480-483.
PRACTICE RECOMMENDATIONS
› Recommend tai chi as an exercise modality for patients with osteoarthritis. A
› Recommend mindfulness training for patients with chronic low back pain (LBP). A
› Recommend a trial of either acupuncture or spinal manipulation for patients with chronic LBP. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Thrombocytopenia and neutropenia: A structured approach to evaluation
Thrombocytopenia and neutropenia are commonly encountered laboratory abnormalities. The presence of either requires that you promptly evaluate for life-threatening causes and identify the appropriate etiology. This article identifies key questions to ask. It also includes algorithms and tables that will facilitate your evaluation of patients with isolated thrombocytopenia or isolated neutropenia and speed the way toward appropriate treatment.
Thrombocytopenia: A look at the numbers
Thrombocytopenia is defined as a platelet count <150,000/mcL.1 The blood abnormality is either suspected based on the patient’s signs or symptoms, such as ecchymoses, petechiae, purpura, epistaxis, gingival bleeding, or melena, or it is incidentally discovered during review of a complete blood count (CBC).
The development of clinical symptoms is closely related to the severity of the thrombocytopenia, with platelet counts <30,000/mcL more likely to result in clinical symptoms with minor trauma and counts <5,000/mcL potentially resulting in spontaneous bleeding. While most patients will have asymptomatic, incidentally-found thrombocytopenia, and likely a benign etiology, those with the signs/symptoms just described, evidence of infection, or thrombosis are more likely to have a serious etiology and require an expedited work-up. Although pregnancy may be associated with thrombocytopenia, this review confines itself to the causes of thrombocytopenia in non-pregnant adults.
Rule out pseudothrombocytopenia
When isolated thrombocytopenia is discovered incidentally in an asymptomatic person, the first step is to perform a repeat CBC with a peripheral smear to confirm the presence of thrombocytopenia, rule out laboratory error, and assess for platelet clumping. If thrombocytopenia is confirmed and platelet clumping is present, it may be due to the calcium chelator in the ethylenediaminetetraacetic anticoagulant contained within the laboratory transport tube; this cause of pseudothrombocytopenia occurs in up to 0.29% of the population.1 Obtaining a platelet count from a citrated or heparinized tube avoids this phenomenon.
Is the patient’s thrombocytopenia drug induced?
Once true thrombocytopenia is confirmed, the next step is to review the patient’s prescribed medications, as well as any illicit drugs used, for potential causes of drug-induced thrombocytopenia. DITP can be either immune-mediated or nonimmune-mediated.
Immune-mediated DITP typically occurs within 1 to 2 weeks of medication exposure and begins to improve within 1 to 2 days of stopping the offending drug.2 (See TABLE 13 for a list of medications that can induce thrombocytopenia.) It should be noted that most patients who take the medications listed in TABLE 1 do not experience thrombocytopenia; nonetheless, it is a potential risk associated with their use.
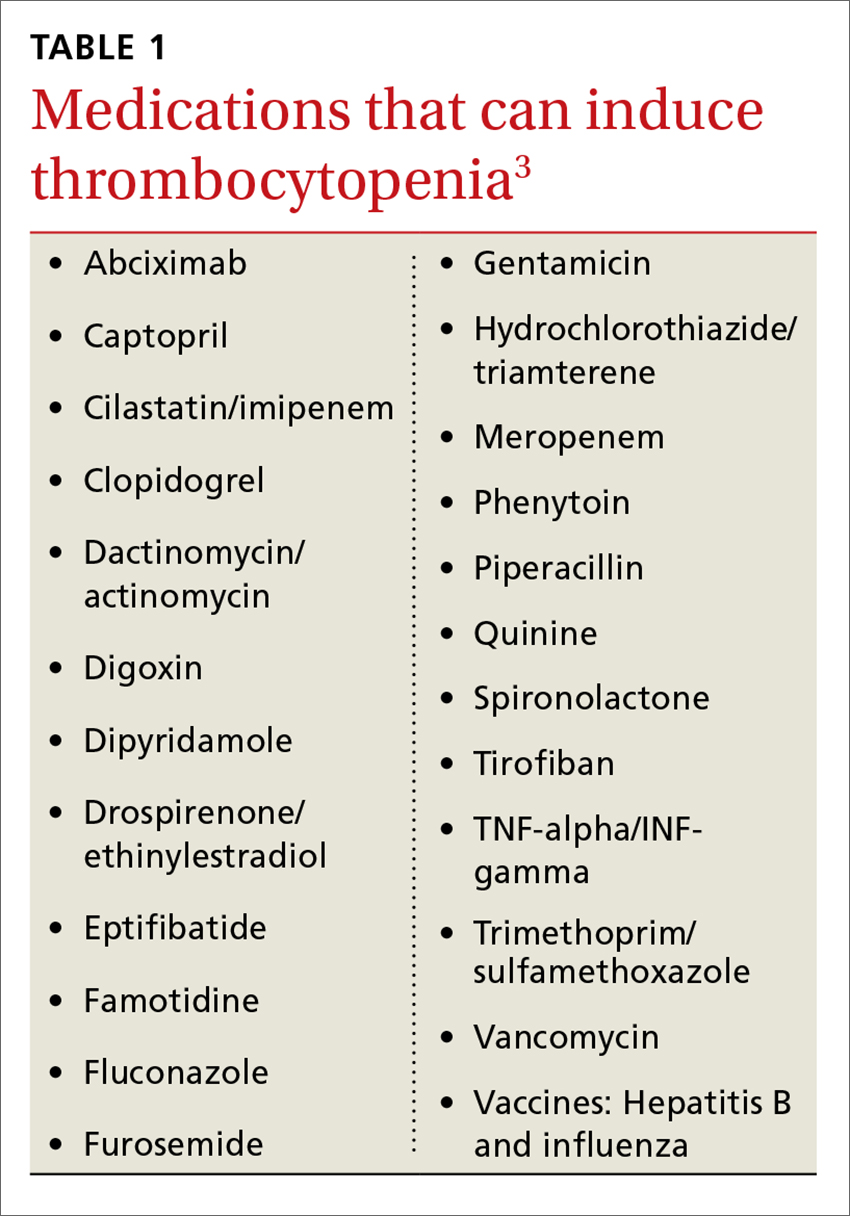
Heparin-induced thrombocytopenia (HIT) is a unique form of immune-mediated DITP in that it is caused by antibody complexes, resulting in platelet activation, clumping, and thrombotic events.4 HIT occurs <1% of patients in intensive care units, but can occur in any patient on long-term heparin therapy. It manifests as a >50% drop in platelet count within 5 to 14 days of the introduction of heparin; however, in those previously exposed to heparin, it can occur within 24 hours.4,5
Continue to: Non-immune-mediated DITP
Non-immune-mediated DITP, resulting from myelosuppression, chemotherapeutic agents, or valproic acid, is less common.1,2
Acute and chronic alcohol use. Although alcohol is not a drug per se, it can also result in thrombocytopenia. The mechanism is the direct suppression of bone marrow, although alcohol also causes B12 and folate deficiency, further contributing to the development of the blood abnormality.1
Is there thrombosis?
In addition to exploring a connection between thrombocytopenia and the drugs a patient is taking, it’s also important to look for evidence of thrombosis. The causes of thrombocytopenia that paradoxically result in thrombosis are: disseminated intravascular coagulation, hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, catastrophic antiphospholipid antibody syndrome, and the previously mentioned HIT. TABLE 24,6-9 outlines the clinical settings, laboratory findings, and treatments of thrombocytopenia associated with thrombosis.
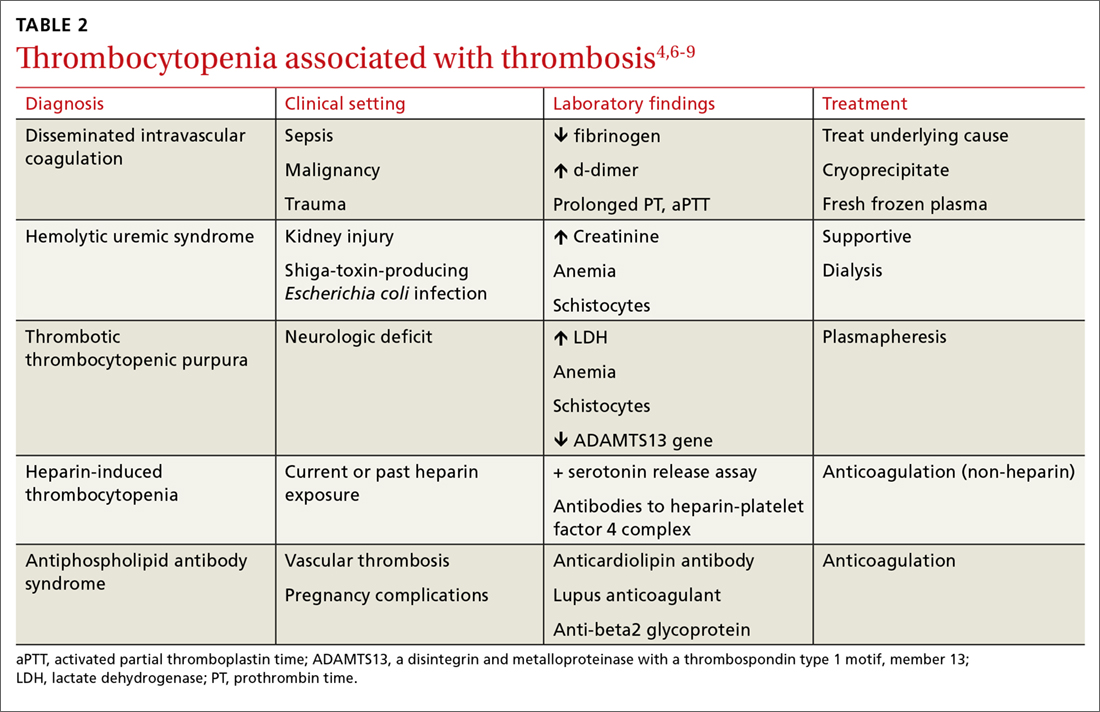
Is an infectious cause to blame?
If the patient is ill, consider infectious causes of thrombocytopenia. Thrombocytopenia associated with infection may result from an immune-mediated response to an illness itself, to treatment of an illness, to splenic sequestration, or to bone marrow suppression. TABLE 31,9-11 lists common infections that may cause thrombocytopenia.
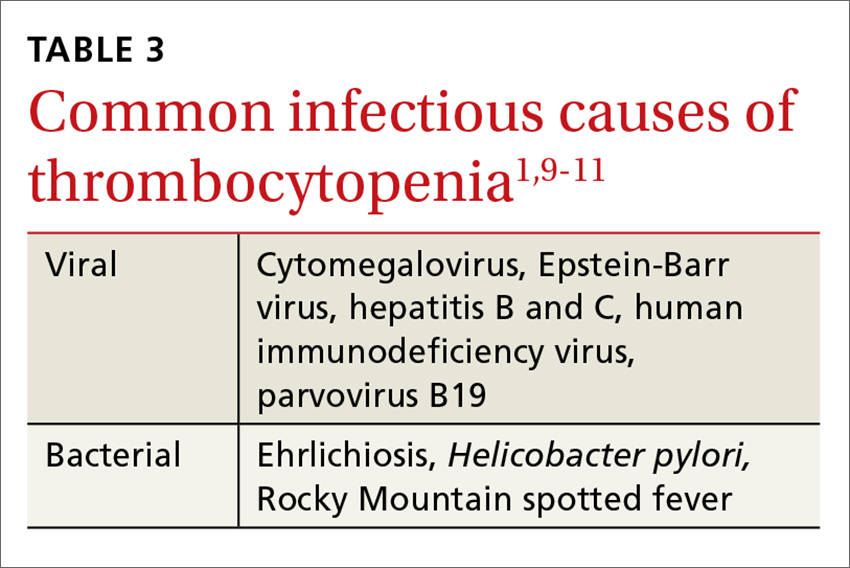
Of note, infection with Helicobacter pylori can cause asymptomatic thrombocytopenia via an immune-mediated mechanism.12 Eradication of H pylori results in a variable elevation in platelets, on average 30,000/mcL in 50% of patients with the infection.13
Is there pancytopenia?
A review of the peripheral smear, with attention to abnormalities in other cell lines, may assist in arriving at a diagnosis. If the peripheral smear reveals pancytopenia, then, in addition to many of the etiologies described earlier, one should also consider vitamin B12 or folate deficiency, copper deficiency, drug- and viral-induced aplastic anemia, paroxysmal nocturnal hemoglobinuria, leukemias, myelodysplastic disorders, and systemic lupus erythematosis.14 Pancytopenia is also seen with hypersplenism, which is often associated with cirrhosis.15 If the etiology isn’t readily apparent, a bone marrow biopsy may be required.
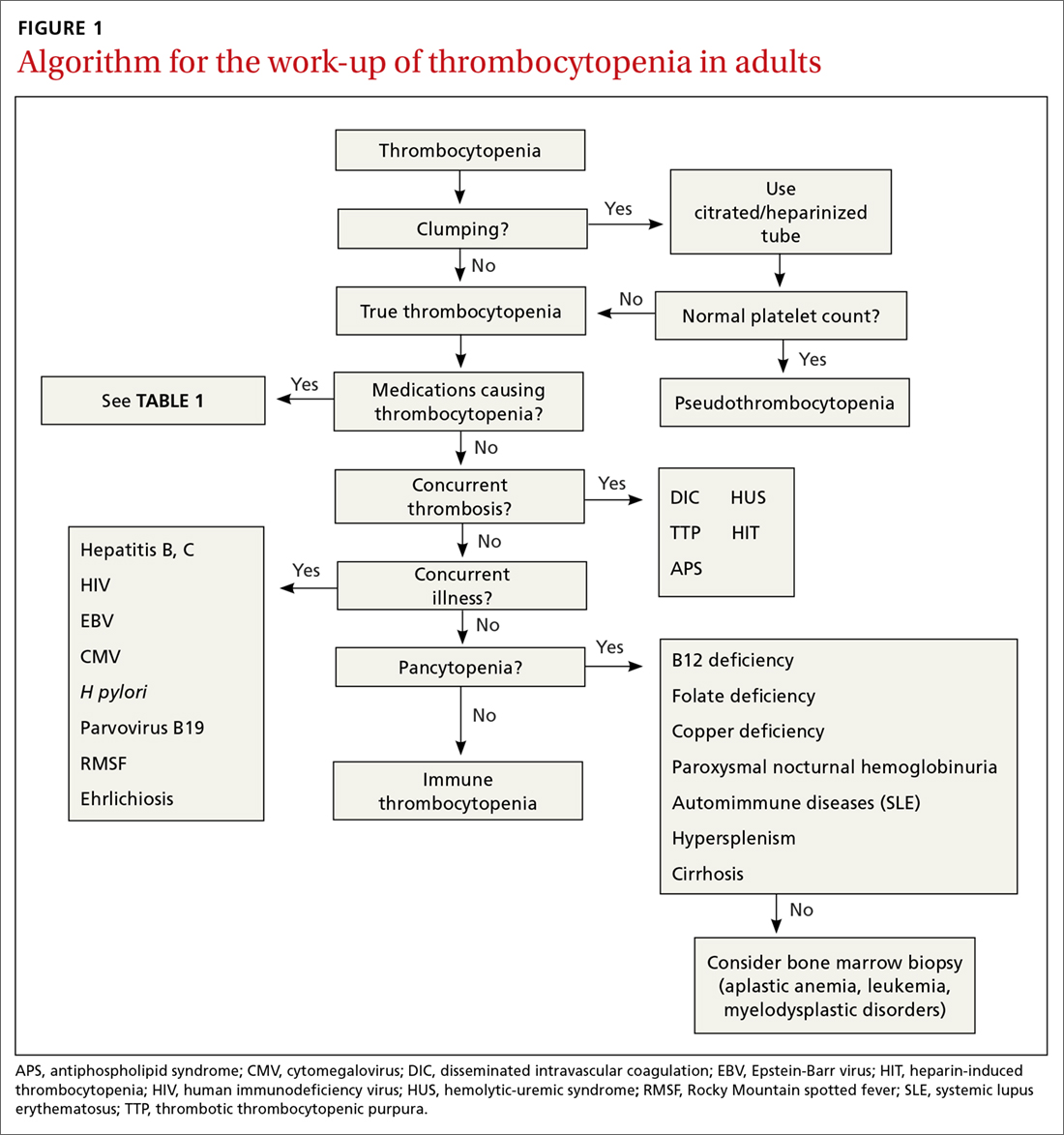
Continue to: Is immune thrombocytopenia to blame?
Is immune thrombocytopenia to blame?
Immune thrombocytopenia (ITP) is an autoimmune disorder resulting in the destruction of normal platelets and may be primary or secondary to processes described previously (HIT, H pylori infection, etc). Consider ITP if, after a thorough work-up, a cause of isolated thrombocytopenia is not identified.16 Treatment for ITP is outlined in TABLE 4.16 FIGURE 1 is an algorithm for the complete evaluation of thrombocytopenia in adults.
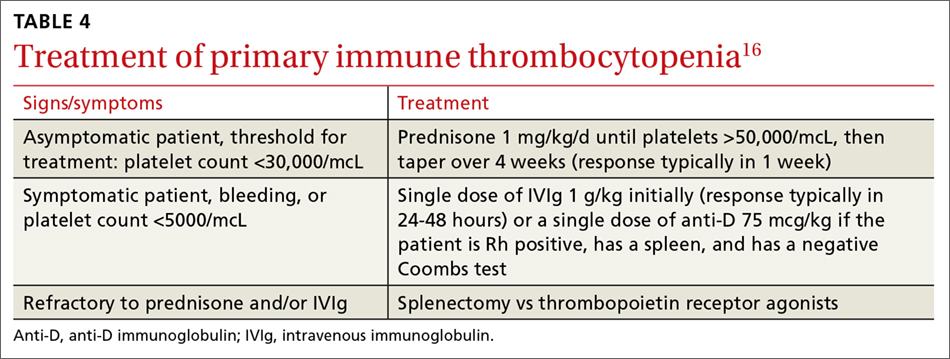
Treatment: Platelet transfusions
In general, patients who are not actively bleeding are considered stable and do not require platelet transfusions to minimize their risk of bleeding or prevent bleeding during a planned procedure unless their platelet count falls below the levels specified in TABLE 5.17 For patients who are actively bleeding, a more aggressive approach may be required. Locally-derived transfusion protocols typically guide transfusions for the actively hemorrhaging patient. The American Association of Blood Banks has put forth evidence-based guidelines for platelet transfusions when a patient is given a diagnosis of thrombocytopenia (see TABLE 5).17 Single-donor platelets have a shelf life of 3 to 5 days, and one unit will raise platelets 30,000 to 50,000/mcL.
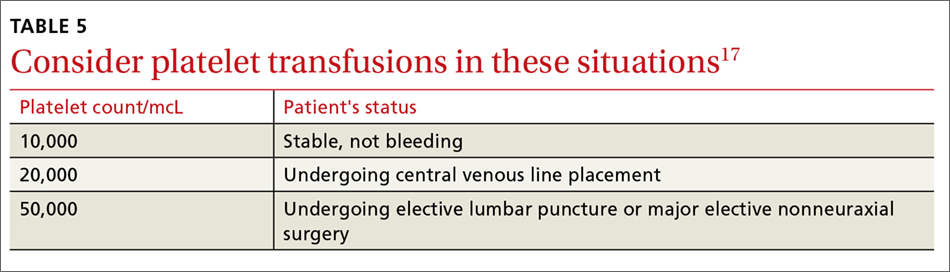
Neutropenia: Prevalence varies by ethnicity
An absolute neutrophil count (ANC) of <1500 cells/mcL traditionally defines neutropenia, with an ANC of 1000 to 1500 cells/mcL constituting mild neutropenia; 500 to 999 cells/mcL, moderate; and <500 cells/mcL, severe.18 Similar to the evaluation of thrombocytopenia, it is important to repeat the CBC prior to initiating a work-up in order to confirm that the neutropenia is not a laboratory error. Additionally, patients with signs or symptoms of infection should be worked up expeditiously.
The prevalence of neutropenia varies by ethnicity. According to the National Health and Nutrition Examination Survey 1999 to 2004, the prevalence was 4.5%, 0.79%, and 0.38% in black, white, and Mexican-American participants, respectively.19 FIGURE 2 outlines the outpatient work-up of adult patients with neutropenia not related to chemotherapy.
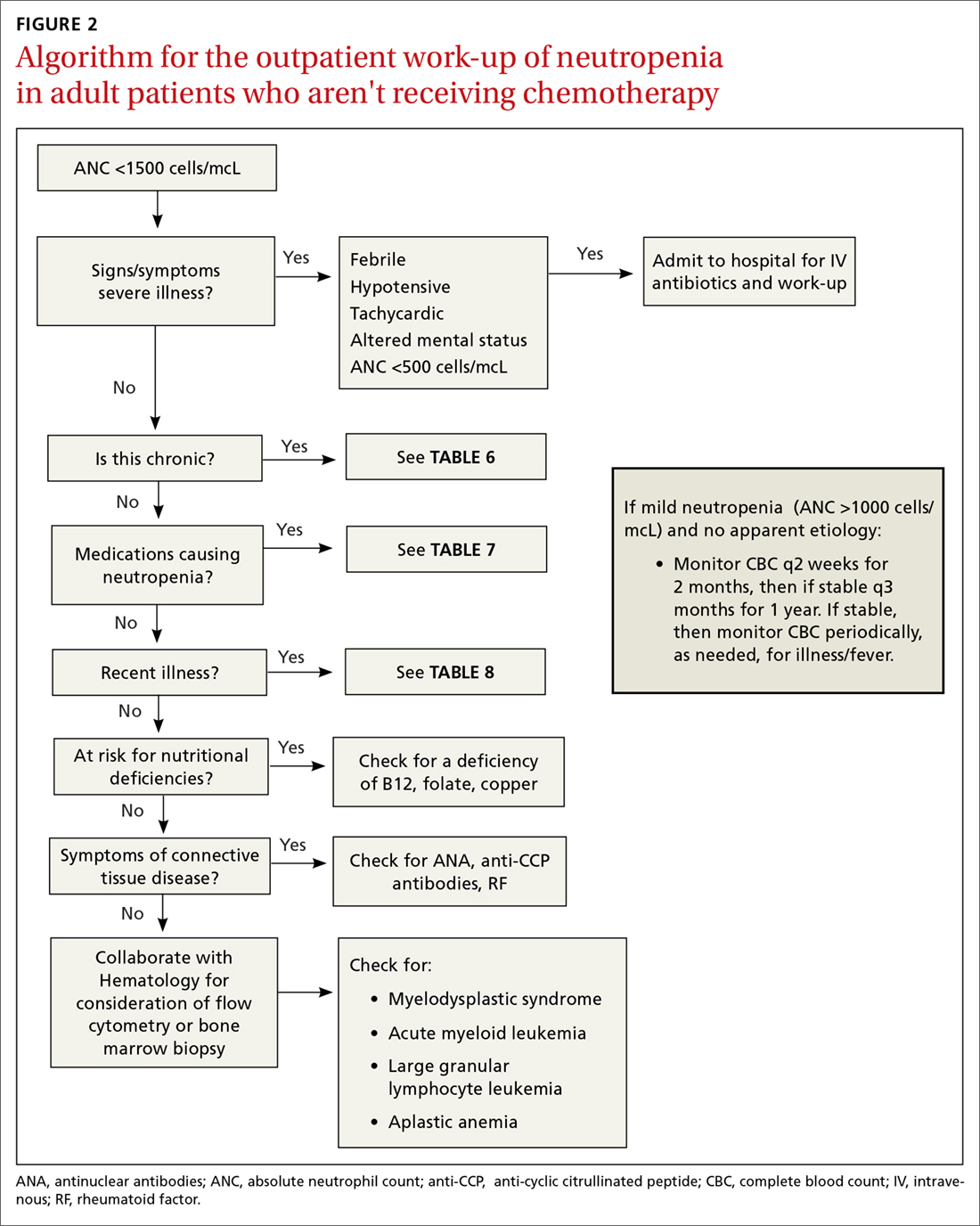
Continue to: Is the patient severely ill?
Is the patient severely ill?
The prognosis of the patient is related both to the etiology of the neutropenia, as well as to the nadir of the neutrophil count. Patients who have an ANC <500 cells/mcL or who have inadequate bone marrow reserves are at highest risk for an overwhelming infection.20,21 The absence of oral ulcers and gingivitis and/or the presence of purulent material at the site of an infection are signs of adequate bone marrow reserves.
Additionally, neutropenia may be the source—or the result—of a serious life-threatening illness. This distinction may not be readily apparent at the time of the patient’s presentation. If signs or symptoms of a severe illness are apparent (fever, hypotension, tachycardia, ANC <500 cells/mcL), admit the patient to the hospital for evaluation and initiation of antibiotics.
Is the neutropenia chronic?
A review of previous CBCs will identify whether this condition is new or chronic. A persistent, mild neutropenia (ANC 1000-1500 cells/mcL) in a healthy individual is consistent with benign familial or ethnic neutropenia (see TABLE 6).20 If prior CBCs are unavailable, then a diagnosis of chronic neutropenia may be established by verifying the persistence of mild neutropenia over time.

Cyclic neutropenia is a periodic neutropenia (occurring every 2-5 weeks) associated with mild illnesses that are related to the nadir of the neutrophil count. The diagnosis is established by obtaining serial CBCs twice weekly for 4 to 6 weeks, which reflect cycling of the neutrophil count.20,22
Are any medications contributing to the neutropenia?
Medications that suppress bone marrow or that interfere with other immune-mediated processes are the most common cause of acquired neutropenia.23 Drug-induced agranulocytosis is defined as an ANC <500 cells/mcL due to exposure to a drug that results in immunologic or cytotoxic destruction of neutrophils.24
A systematic review of case reports of drug-induced agranulocytosis (a decrease in peripheral neutrophil count to <500 cells/mcL) revealed that although at least 125 drugs were probably related to agranulocytosis, only 11 drugs were responsible for 50% of cases (carbimazole, clozapine, dapsone, dipyrone, methimazole, penicillin G, procainamide, propylthiouracil, rituximab, sulfasalazine, and ticlopidine), and fatality rates were higher (10% vs 3%) among those patients with a nadir <100 cells/mcL.25 TABLE 725 lists medications that can be associated with agranulocytosis. Depending on prior exposure to a drug, neutropenia/agranulocytosis can occur within hours to months of exposure to the causal drug and can take a few days to 3 weeks to resolve after cessation.25,26
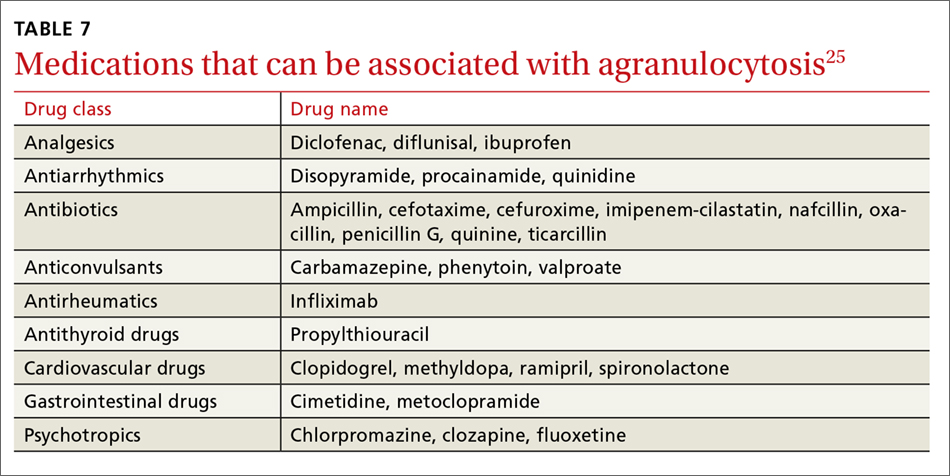
Continue to: Has the patient had any recent illnesses?
Has the patient had any recent illnesses?
The usual response to an infection is an increase in neutrophil count. However, certain bacterial, rickettsial, parasitic, and viral infections can result in neutropenia (see TABLE 823,27-29). Viral infections may cause transient neutropenia because of either bone marrow suppression or increased peripheral destruction, while neutropenia related to an overwhelming bacterial infection results from the depletion of bone marrow reserves.23,27

Do you suspect a nutritional deficiency?
Patients with a nutritional deficiency of B12, folate, or copper are likely to exhibit a deficiency in more than just neutrophils.23,27 In developed countries, people with neutropenia may have a history of malnutrition due to a disease (eg, anorexia nervosa) or surgery (eg, gastric bypass) that causes severe calorie restriction.20
Does your patient have symptoms of a connective tissue disease?
Neutropenia, in association with arthralgias, joint swelling, splenomegaly, or rash may be a manifestation of an underlying collagen vascular disorder, such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE).20 If the clinical scenario supports one of these diagnoses, undertake or refer the patient for a rheumatologic evaluation. This may include studies of anti-cyclic citrullinated peptide antibodies, rheumatoid factor to evaluate for RA, and/or antinuclear antibodies to evaluate for SLE.30,31 While most neutropenias associated with autoimmune disease are mild, neutropenia associated with Felty syndrome (RA, splenomegaly, and neutropenia) may be severe (ANC <100 cells/mcL).20,23
Is the etiology unclear?
Patients with moderate to severe neutropenia without an apparent etiology, in the setting of aplastic anemia, or in the presence of splenomegaly and/or lymphadenopathy, should undergo a hematologic evaluation and/or bone marrow biopsy, given that hematologic malignancy is a potential cause.20,27
The treatment of neutropenia hinges on correctly identifying the etiology of the diminished neutrophil count. If the cause is a medication, infection, underlying rheumatologic condition, or nutritional deficiency, then either treating the entity or withdrawing the offending medication should result in resolution of the neutropenia. If the cause is determined to be familial or ethnic, then patient reassurance is all that is required.
CORRESPONDENCE
Richard W. Temple, MD, FAAFP, CDR MC USN, Camp Lejeune Family Medicine Residency, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547-2538; [email protected].
1. Wong EY, Rose MG. Why does my patient have thrombocytopenia? Hematol Oncol Clin North Am. 2012;26:231-252.
2. Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357:580-587.
3. University of Oklahoma Health Sciences Center. Database for Drug–induced thrombocytopenia from group patient reports: an update. Available at: http://www.ouhsc.edu/platelets/InternetPostingGroupFrames2014.htm. Accessed May 7, 2018.
4. Sniecinski RM, Hursting MJ, Paidas MJ, et al. Etiology and assessment of hypercoagulability with lessons from heparin-induced thrombocytopenia. Anesth Analg. 2011;112:46-58.
5. Warkentin TE. Heparin-induced thrombocytopenia in critically ill patients. Crit Care Clin. 2011;27:805-823.
6. Connell NT, Sweeney JD. Does my patient have life- or limb-threatening thrombocytopenia? Hematol Oncol Clin North Am. 2012;26:369-382.
7. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654-666.
8. Hanly JG. Antiphospholipid syndrome: an overview. CMAJ. 2003;168:1675-1682.
9. Sekhon SS, Roy V. Thrombocytopenia in adults: a practical approach to evaluation and management. South Med J. 2006;99:491-498.
10. Gauer RL, Braun MM. Thrombocytopenia. Am Fam Physician. 2012;85:612-622.
11. Bratton RL, Corey R. Tick-borne disease. Am Fam Physician. 2005;71:2323-2330.
12. Yeh JJ, Tsai S, Wu DC, et al. P-selectin-dependent platelet aggregation and apoptosis may explain the decrease in platelet count during Helicobacter pylori infection. Blood. 2010;115:4247-4253.
13. Stasi R, Sarpatwari A, Segal JB, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systemic review. Blood. 2009;113:1231-1240.
14. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
15. Peck-Radosavljevic M. Hypersplenism. Eur J Gastroenterol Hepatol. 2001;13:317-323.
16. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190-4207.
17. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Int Med. 2015;162:205-213.
18. Palmblad J, Dufour C, Papadaki HA. How we diagnose neutropenia in the adult and elderly patient. Haematologica. 2014;99:1130-1133.
19. Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146:486-492.
20. Gibson C, Berliner N. How we evaluate and treat neutropenia in adults. Blood. 2014;124:1251-1258.
21. Urabe A. Clinical features of the neutropenic host: definitions and initial evaluation. CID. 2004;39(suppl 1):S53-S55.
22. Dale DC, Hammond WP 4th. Cyclic neutropenia: a clinical review. Blood Rev. 1988;2:178-185.
23. Munshi HG, Montgomery RB. Severe neutropenia: a diagnostic approach. West J Med. 2000;172:248-252.
24. Pisciotta AV. Drug-induced agranulocytosis peripheral destruction of polymorphonuclear leukocytes and their marrow precursors. Blood Rev. 1990;4:226-237.
25. Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146:657-665.
26. Bhatt V, Saleem A. Review: drug-induced neutropenia – pathophysiology, clinical features, and management. Ann Clin Lab Sci. 2004;34:131-137.
27. Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin Hematol. 2013;50:198-206.
28. Bakken JS, Krueth J, Wilson-Nordskog C, et al. Clinical and laboratory characteristics of human granulcytic ehrlichiosis. JAMA. 1996;275:199-205.
29. Hall GW, Schwartz RP. White blood cell count and differential in Rocky Mountain spotted fever. NC Med J. 1979;40:212-214.
30. Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797-808.
31. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012; 64:2677-2686.
Thrombocytopenia and neutropenia are commonly encountered laboratory abnormalities. The presence of either requires that you promptly evaluate for life-threatening causes and identify the appropriate etiology. This article identifies key questions to ask. It also includes algorithms and tables that will facilitate your evaluation of patients with isolated thrombocytopenia or isolated neutropenia and speed the way toward appropriate treatment.
Thrombocytopenia: A look at the numbers
Thrombocytopenia is defined as a platelet count <150,000/mcL.1 The blood abnormality is either suspected based on the patient’s signs or symptoms, such as ecchymoses, petechiae, purpura, epistaxis, gingival bleeding, or melena, or it is incidentally discovered during review of a complete blood count (CBC).
The development of clinical symptoms is closely related to the severity of the thrombocytopenia, with platelet counts <30,000/mcL more likely to result in clinical symptoms with minor trauma and counts <5,000/mcL potentially resulting in spontaneous bleeding. While most patients will have asymptomatic, incidentally-found thrombocytopenia, and likely a benign etiology, those with the signs/symptoms just described, evidence of infection, or thrombosis are more likely to have a serious etiology and require an expedited work-up. Although pregnancy may be associated with thrombocytopenia, this review confines itself to the causes of thrombocytopenia in non-pregnant adults.
Rule out pseudothrombocytopenia
When isolated thrombocytopenia is discovered incidentally in an asymptomatic person, the first step is to perform a repeat CBC with a peripheral smear to confirm the presence of thrombocytopenia, rule out laboratory error, and assess for platelet clumping. If thrombocytopenia is confirmed and platelet clumping is present, it may be due to the calcium chelator in the ethylenediaminetetraacetic anticoagulant contained within the laboratory transport tube; this cause of pseudothrombocytopenia occurs in up to 0.29% of the population.1 Obtaining a platelet count from a citrated or heparinized tube avoids this phenomenon.
Is the patient’s thrombocytopenia drug induced?
Once true thrombocytopenia is confirmed, the next step is to review the patient’s prescribed medications, as well as any illicit drugs used, for potential causes of drug-induced thrombocytopenia. DITP can be either immune-mediated or nonimmune-mediated.
Immune-mediated DITP typically occurs within 1 to 2 weeks of medication exposure and begins to improve within 1 to 2 days of stopping the offending drug.2 (See TABLE 13 for a list of medications that can induce thrombocytopenia.) It should be noted that most patients who take the medications listed in TABLE 1 do not experience thrombocytopenia; nonetheless, it is a potential risk associated with their use.

Heparin-induced thrombocytopenia (HIT) is a unique form of immune-mediated DITP in that it is caused by antibody complexes, resulting in platelet activation, clumping, and thrombotic events.4 HIT occurs <1% of patients in intensive care units, but can occur in any patient on long-term heparin therapy. It manifests as a >50% drop in platelet count within 5 to 14 days of the introduction of heparin; however, in those previously exposed to heparin, it can occur within 24 hours.4,5
Continue to: Non-immune-mediated DITP
Non-immune-mediated DITP, resulting from myelosuppression, chemotherapeutic agents, or valproic acid, is less common.1,2
Acute and chronic alcohol use. Although alcohol is not a drug per se, it can also result in thrombocytopenia. The mechanism is the direct suppression of bone marrow, although alcohol also causes B12 and folate deficiency, further contributing to the development of the blood abnormality.1
Is there thrombosis?
In addition to exploring a connection between thrombocytopenia and the drugs a patient is taking, it’s also important to look for evidence of thrombosis. The causes of thrombocytopenia that paradoxically result in thrombosis are: disseminated intravascular coagulation, hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, catastrophic antiphospholipid antibody syndrome, and the previously mentioned HIT. TABLE 24,6-9 outlines the clinical settings, laboratory findings, and treatments of thrombocytopenia associated with thrombosis.

Is an infectious cause to blame?
If the patient is ill, consider infectious causes of thrombocytopenia. Thrombocytopenia associated with infection may result from an immune-mediated response to an illness itself, to treatment of an illness, to splenic sequestration, or to bone marrow suppression. TABLE 31,9-11 lists common infections that may cause thrombocytopenia.

Of note, infection with Helicobacter pylori can cause asymptomatic thrombocytopenia via an immune-mediated mechanism.12 Eradication of H pylori results in a variable elevation in platelets, on average 30,000/mcL in 50% of patients with the infection.13
Is there pancytopenia?
A review of the peripheral smear, with attention to abnormalities in other cell lines, may assist in arriving at a diagnosis. If the peripheral smear reveals pancytopenia, then, in addition to many of the etiologies described earlier, one should also consider vitamin B12 or folate deficiency, copper deficiency, drug- and viral-induced aplastic anemia, paroxysmal nocturnal hemoglobinuria, leukemias, myelodysplastic disorders, and systemic lupus erythematosis.14 Pancytopenia is also seen with hypersplenism, which is often associated with cirrhosis.15 If the etiology isn’t readily apparent, a bone marrow biopsy may be required.

Continue to: Is immune thrombocytopenia to blame?
Is immune thrombocytopenia to blame?
Immune thrombocytopenia (ITP) is an autoimmune disorder resulting in the destruction of normal platelets and may be primary or secondary to processes described previously (HIT, H pylori infection, etc). Consider ITP if, after a thorough work-up, a cause of isolated thrombocytopenia is not identified.16 Treatment for ITP is outlined in TABLE 4.16 FIGURE 1 is an algorithm for the complete evaluation of thrombocytopenia in adults.

Treatment: Platelet transfusions
In general, patients who are not actively bleeding are considered stable and do not require platelet transfusions to minimize their risk of bleeding or prevent bleeding during a planned procedure unless their platelet count falls below the levels specified in TABLE 5.17 For patients who are actively bleeding, a more aggressive approach may be required. Locally-derived transfusion protocols typically guide transfusions for the actively hemorrhaging patient. The American Association of Blood Banks has put forth evidence-based guidelines for platelet transfusions when a patient is given a diagnosis of thrombocytopenia (see TABLE 5).17 Single-donor platelets have a shelf life of 3 to 5 days, and one unit will raise platelets 30,000 to 50,000/mcL.

Neutropenia: Prevalence varies by ethnicity
An absolute neutrophil count (ANC) of <1500 cells/mcL traditionally defines neutropenia, with an ANC of 1000 to 1500 cells/mcL constituting mild neutropenia; 500 to 999 cells/mcL, moderate; and <500 cells/mcL, severe.18 Similar to the evaluation of thrombocytopenia, it is important to repeat the CBC prior to initiating a work-up in order to confirm that the neutropenia is not a laboratory error. Additionally, patients with signs or symptoms of infection should be worked up expeditiously.
The prevalence of neutropenia varies by ethnicity. According to the National Health and Nutrition Examination Survey 1999 to 2004, the prevalence was 4.5%, 0.79%, and 0.38% in black, white, and Mexican-American participants, respectively.19 FIGURE 2 outlines the outpatient work-up of adult patients with neutropenia not related to chemotherapy.

Continue to: Is the patient severely ill?
Is the patient severely ill?
The prognosis of the patient is related both to the etiology of the neutropenia, as well as to the nadir of the neutrophil count. Patients who have an ANC <500 cells/mcL or who have inadequate bone marrow reserves are at highest risk for an overwhelming infection.20,21 The absence of oral ulcers and gingivitis and/or the presence of purulent material at the site of an infection are signs of adequate bone marrow reserves.
Additionally, neutropenia may be the source—or the result—of a serious life-threatening illness. This distinction may not be readily apparent at the time of the patient’s presentation. If signs or symptoms of a severe illness are apparent (fever, hypotension, tachycardia, ANC <500 cells/mcL), admit the patient to the hospital for evaluation and initiation of antibiotics.
Is the neutropenia chronic?
A review of previous CBCs will identify whether this condition is new or chronic. A persistent, mild neutropenia (ANC 1000-1500 cells/mcL) in a healthy individual is consistent with benign familial or ethnic neutropenia (see TABLE 6).20 If prior CBCs are unavailable, then a diagnosis of chronic neutropenia may be established by verifying the persistence of mild neutropenia over time.

Cyclic neutropenia is a periodic neutropenia (occurring every 2-5 weeks) associated with mild illnesses that are related to the nadir of the neutrophil count. The diagnosis is established by obtaining serial CBCs twice weekly for 4 to 6 weeks, which reflect cycling of the neutrophil count.20,22
Are any medications contributing to the neutropenia?
Medications that suppress bone marrow or that interfere with other immune-mediated processes are the most common cause of acquired neutropenia.23 Drug-induced agranulocytosis is defined as an ANC <500 cells/mcL due to exposure to a drug that results in immunologic or cytotoxic destruction of neutrophils.24
A systematic review of case reports of drug-induced agranulocytosis (a decrease in peripheral neutrophil count to <500 cells/mcL) revealed that although at least 125 drugs were probably related to agranulocytosis, only 11 drugs were responsible for 50% of cases (carbimazole, clozapine, dapsone, dipyrone, methimazole, penicillin G, procainamide, propylthiouracil, rituximab, sulfasalazine, and ticlopidine), and fatality rates were higher (10% vs 3%) among those patients with a nadir <100 cells/mcL.25 TABLE 725 lists medications that can be associated with agranulocytosis. Depending on prior exposure to a drug, neutropenia/agranulocytosis can occur within hours to months of exposure to the causal drug and can take a few days to 3 weeks to resolve after cessation.25,26

Continue to: Has the patient had any recent illnesses?
Has the patient had any recent illnesses?
The usual response to an infection is an increase in neutrophil count. However, certain bacterial, rickettsial, parasitic, and viral infections can result in neutropenia (see TABLE 823,27-29). Viral infections may cause transient neutropenia because of either bone marrow suppression or increased peripheral destruction, while neutropenia related to an overwhelming bacterial infection results from the depletion of bone marrow reserves.23,27

Do you suspect a nutritional deficiency?
Patients with a nutritional deficiency of B12, folate, or copper are likely to exhibit a deficiency in more than just neutrophils.23,27 In developed countries, people with neutropenia may have a history of malnutrition due to a disease (eg, anorexia nervosa) or surgery (eg, gastric bypass) that causes severe calorie restriction.20
Does your patient have symptoms of a connective tissue disease?
Neutropenia, in association with arthralgias, joint swelling, splenomegaly, or rash may be a manifestation of an underlying collagen vascular disorder, such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE).20 If the clinical scenario supports one of these diagnoses, undertake or refer the patient for a rheumatologic evaluation. This may include studies of anti-cyclic citrullinated peptide antibodies, rheumatoid factor to evaluate for RA, and/or antinuclear antibodies to evaluate for SLE.30,31 While most neutropenias associated with autoimmune disease are mild, neutropenia associated with Felty syndrome (RA, splenomegaly, and neutropenia) may be severe (ANC <100 cells/mcL).20,23
Is the etiology unclear?
Patients with moderate to severe neutropenia without an apparent etiology, in the setting of aplastic anemia, or in the presence of splenomegaly and/or lymphadenopathy, should undergo a hematologic evaluation and/or bone marrow biopsy, given that hematologic malignancy is a potential cause.20,27
The treatment of neutropenia hinges on correctly identifying the etiology of the diminished neutrophil count. If the cause is a medication, infection, underlying rheumatologic condition, or nutritional deficiency, then either treating the entity or withdrawing the offending medication should result in resolution of the neutropenia. If the cause is determined to be familial or ethnic, then patient reassurance is all that is required.
CORRESPONDENCE
Richard W. Temple, MD, FAAFP, CDR MC USN, Camp Lejeune Family Medicine Residency, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547-2538; [email protected].
Thrombocytopenia and neutropenia are commonly encountered laboratory abnormalities. The presence of either requires that you promptly evaluate for life-threatening causes and identify the appropriate etiology. This article identifies key questions to ask. It also includes algorithms and tables that will facilitate your evaluation of patients with isolated thrombocytopenia or isolated neutropenia and speed the way toward appropriate treatment.
Thrombocytopenia: A look at the numbers
Thrombocytopenia is defined as a platelet count <150,000/mcL.1 The blood abnormality is either suspected based on the patient’s signs or symptoms, such as ecchymoses, petechiae, purpura, epistaxis, gingival bleeding, or melena, or it is incidentally discovered during review of a complete blood count (CBC).
The development of clinical symptoms is closely related to the severity of the thrombocytopenia, with platelet counts <30,000/mcL more likely to result in clinical symptoms with minor trauma and counts <5,000/mcL potentially resulting in spontaneous bleeding. While most patients will have asymptomatic, incidentally-found thrombocytopenia, and likely a benign etiology, those with the signs/symptoms just described, evidence of infection, or thrombosis are more likely to have a serious etiology and require an expedited work-up. Although pregnancy may be associated with thrombocytopenia, this review confines itself to the causes of thrombocytopenia in non-pregnant adults.
Rule out pseudothrombocytopenia
When isolated thrombocytopenia is discovered incidentally in an asymptomatic person, the first step is to perform a repeat CBC with a peripheral smear to confirm the presence of thrombocytopenia, rule out laboratory error, and assess for platelet clumping. If thrombocytopenia is confirmed and platelet clumping is present, it may be due to the calcium chelator in the ethylenediaminetetraacetic anticoagulant contained within the laboratory transport tube; this cause of pseudothrombocytopenia occurs in up to 0.29% of the population.1 Obtaining a platelet count from a citrated or heparinized tube avoids this phenomenon.
Is the patient’s thrombocytopenia drug induced?
Once true thrombocytopenia is confirmed, the next step is to review the patient’s prescribed medications, as well as any illicit drugs used, for potential causes of drug-induced thrombocytopenia. DITP can be either immune-mediated or nonimmune-mediated.
Immune-mediated DITP typically occurs within 1 to 2 weeks of medication exposure and begins to improve within 1 to 2 days of stopping the offending drug.2 (See TABLE 13 for a list of medications that can induce thrombocytopenia.) It should be noted that most patients who take the medications listed in TABLE 1 do not experience thrombocytopenia; nonetheless, it is a potential risk associated with their use.

Heparin-induced thrombocytopenia (HIT) is a unique form of immune-mediated DITP in that it is caused by antibody complexes, resulting in platelet activation, clumping, and thrombotic events.4 HIT occurs <1% of patients in intensive care units, but can occur in any patient on long-term heparin therapy. It manifests as a >50% drop in platelet count within 5 to 14 days of the introduction of heparin; however, in those previously exposed to heparin, it can occur within 24 hours.4,5
Continue to: Non-immune-mediated DITP
Non-immune-mediated DITP, resulting from myelosuppression, chemotherapeutic agents, or valproic acid, is less common.1,2
Acute and chronic alcohol use. Although alcohol is not a drug per se, it can also result in thrombocytopenia. The mechanism is the direct suppression of bone marrow, although alcohol also causes B12 and folate deficiency, further contributing to the development of the blood abnormality.1
Is there thrombosis?
In addition to exploring a connection between thrombocytopenia and the drugs a patient is taking, it’s also important to look for evidence of thrombosis. The causes of thrombocytopenia that paradoxically result in thrombosis are: disseminated intravascular coagulation, hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, catastrophic antiphospholipid antibody syndrome, and the previously mentioned HIT. TABLE 24,6-9 outlines the clinical settings, laboratory findings, and treatments of thrombocytopenia associated with thrombosis.

Is an infectious cause to blame?
If the patient is ill, consider infectious causes of thrombocytopenia. Thrombocytopenia associated with infection may result from an immune-mediated response to an illness itself, to treatment of an illness, to splenic sequestration, or to bone marrow suppression. TABLE 31,9-11 lists common infections that may cause thrombocytopenia.

Of note, infection with Helicobacter pylori can cause asymptomatic thrombocytopenia via an immune-mediated mechanism.12 Eradication of H pylori results in a variable elevation in platelets, on average 30,000/mcL in 50% of patients with the infection.13
Is there pancytopenia?
A review of the peripheral smear, with attention to abnormalities in other cell lines, may assist in arriving at a diagnosis. If the peripheral smear reveals pancytopenia, then, in addition to many of the etiologies described earlier, one should also consider vitamin B12 or folate deficiency, copper deficiency, drug- and viral-induced aplastic anemia, paroxysmal nocturnal hemoglobinuria, leukemias, myelodysplastic disorders, and systemic lupus erythematosis.14 Pancytopenia is also seen with hypersplenism, which is often associated with cirrhosis.15 If the etiology isn’t readily apparent, a bone marrow biopsy may be required.

Continue to: Is immune thrombocytopenia to blame?
Is immune thrombocytopenia to blame?
Immune thrombocytopenia (ITP) is an autoimmune disorder resulting in the destruction of normal platelets and may be primary or secondary to processes described previously (HIT, H pylori infection, etc). Consider ITP if, after a thorough work-up, a cause of isolated thrombocytopenia is not identified.16 Treatment for ITP is outlined in TABLE 4.16 FIGURE 1 is an algorithm for the complete evaluation of thrombocytopenia in adults.

Treatment: Platelet transfusions
In general, patients who are not actively bleeding are considered stable and do not require platelet transfusions to minimize their risk of bleeding or prevent bleeding during a planned procedure unless their platelet count falls below the levels specified in TABLE 5.17 For patients who are actively bleeding, a more aggressive approach may be required. Locally-derived transfusion protocols typically guide transfusions for the actively hemorrhaging patient. The American Association of Blood Banks has put forth evidence-based guidelines for platelet transfusions when a patient is given a diagnosis of thrombocytopenia (see TABLE 5).17 Single-donor platelets have a shelf life of 3 to 5 days, and one unit will raise platelets 30,000 to 50,000/mcL.

Neutropenia: Prevalence varies by ethnicity
An absolute neutrophil count (ANC) of <1500 cells/mcL traditionally defines neutropenia, with an ANC of 1000 to 1500 cells/mcL constituting mild neutropenia; 500 to 999 cells/mcL, moderate; and <500 cells/mcL, severe.18 Similar to the evaluation of thrombocytopenia, it is important to repeat the CBC prior to initiating a work-up in order to confirm that the neutropenia is not a laboratory error. Additionally, patients with signs or symptoms of infection should be worked up expeditiously.
The prevalence of neutropenia varies by ethnicity. According to the National Health and Nutrition Examination Survey 1999 to 2004, the prevalence was 4.5%, 0.79%, and 0.38% in black, white, and Mexican-American participants, respectively.19 FIGURE 2 outlines the outpatient work-up of adult patients with neutropenia not related to chemotherapy.

Continue to: Is the patient severely ill?
Is the patient severely ill?
The prognosis of the patient is related both to the etiology of the neutropenia, as well as to the nadir of the neutrophil count. Patients who have an ANC <500 cells/mcL or who have inadequate bone marrow reserves are at highest risk for an overwhelming infection.20,21 The absence of oral ulcers and gingivitis and/or the presence of purulent material at the site of an infection are signs of adequate bone marrow reserves.
Additionally, neutropenia may be the source—or the result—of a serious life-threatening illness. This distinction may not be readily apparent at the time of the patient’s presentation. If signs or symptoms of a severe illness are apparent (fever, hypotension, tachycardia, ANC <500 cells/mcL), admit the patient to the hospital for evaluation and initiation of antibiotics.
Is the neutropenia chronic?
A review of previous CBCs will identify whether this condition is new or chronic. A persistent, mild neutropenia (ANC 1000-1500 cells/mcL) in a healthy individual is consistent with benign familial or ethnic neutropenia (see TABLE 6).20 If prior CBCs are unavailable, then a diagnosis of chronic neutropenia may be established by verifying the persistence of mild neutropenia over time.

Cyclic neutropenia is a periodic neutropenia (occurring every 2-5 weeks) associated with mild illnesses that are related to the nadir of the neutrophil count. The diagnosis is established by obtaining serial CBCs twice weekly for 4 to 6 weeks, which reflect cycling of the neutrophil count.20,22
Are any medications contributing to the neutropenia?
Medications that suppress bone marrow or that interfere with other immune-mediated processes are the most common cause of acquired neutropenia.23 Drug-induced agranulocytosis is defined as an ANC <500 cells/mcL due to exposure to a drug that results in immunologic or cytotoxic destruction of neutrophils.24
A systematic review of case reports of drug-induced agranulocytosis (a decrease in peripheral neutrophil count to <500 cells/mcL) revealed that although at least 125 drugs were probably related to agranulocytosis, only 11 drugs were responsible for 50% of cases (carbimazole, clozapine, dapsone, dipyrone, methimazole, penicillin G, procainamide, propylthiouracil, rituximab, sulfasalazine, and ticlopidine), and fatality rates were higher (10% vs 3%) among those patients with a nadir <100 cells/mcL.25 TABLE 725 lists medications that can be associated with agranulocytosis. Depending on prior exposure to a drug, neutropenia/agranulocytosis can occur within hours to months of exposure to the causal drug and can take a few days to 3 weeks to resolve after cessation.25,26

Continue to: Has the patient had any recent illnesses?
Has the patient had any recent illnesses?
The usual response to an infection is an increase in neutrophil count. However, certain bacterial, rickettsial, parasitic, and viral infections can result in neutropenia (see TABLE 823,27-29). Viral infections may cause transient neutropenia because of either bone marrow suppression or increased peripheral destruction, while neutropenia related to an overwhelming bacterial infection results from the depletion of bone marrow reserves.23,27

Do you suspect a nutritional deficiency?
Patients with a nutritional deficiency of B12, folate, or copper are likely to exhibit a deficiency in more than just neutrophils.23,27 In developed countries, people with neutropenia may have a history of malnutrition due to a disease (eg, anorexia nervosa) or surgery (eg, gastric bypass) that causes severe calorie restriction.20
Does your patient have symptoms of a connective tissue disease?
Neutropenia, in association with arthralgias, joint swelling, splenomegaly, or rash may be a manifestation of an underlying collagen vascular disorder, such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE).20 If the clinical scenario supports one of these diagnoses, undertake or refer the patient for a rheumatologic evaluation. This may include studies of anti-cyclic citrullinated peptide antibodies, rheumatoid factor to evaluate for RA, and/or antinuclear antibodies to evaluate for SLE.30,31 While most neutropenias associated with autoimmune disease are mild, neutropenia associated with Felty syndrome (RA, splenomegaly, and neutropenia) may be severe (ANC <100 cells/mcL).20,23
Is the etiology unclear?
Patients with moderate to severe neutropenia without an apparent etiology, in the setting of aplastic anemia, or in the presence of splenomegaly and/or lymphadenopathy, should undergo a hematologic evaluation and/or bone marrow biopsy, given that hematologic malignancy is a potential cause.20,27
The treatment of neutropenia hinges on correctly identifying the etiology of the diminished neutrophil count. If the cause is a medication, infection, underlying rheumatologic condition, or nutritional deficiency, then either treating the entity or withdrawing the offending medication should result in resolution of the neutropenia. If the cause is determined to be familial or ethnic, then patient reassurance is all that is required.
CORRESPONDENCE
Richard W. Temple, MD, FAAFP, CDR MC USN, Camp Lejeune Family Medicine Residency, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547-2538; [email protected].
1. Wong EY, Rose MG. Why does my patient have thrombocytopenia? Hematol Oncol Clin North Am. 2012;26:231-252.
2. Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357:580-587.
3. University of Oklahoma Health Sciences Center. Database for Drug–induced thrombocytopenia from group patient reports: an update. Available at: http://www.ouhsc.edu/platelets/InternetPostingGroupFrames2014.htm. Accessed May 7, 2018.
4. Sniecinski RM, Hursting MJ, Paidas MJ, et al. Etiology and assessment of hypercoagulability with lessons from heparin-induced thrombocytopenia. Anesth Analg. 2011;112:46-58.
5. Warkentin TE. Heparin-induced thrombocytopenia in critically ill patients. Crit Care Clin. 2011;27:805-823.
6. Connell NT, Sweeney JD. Does my patient have life- or limb-threatening thrombocytopenia? Hematol Oncol Clin North Am. 2012;26:369-382.
7. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654-666.
8. Hanly JG. Antiphospholipid syndrome: an overview. CMAJ. 2003;168:1675-1682.
9. Sekhon SS, Roy V. Thrombocytopenia in adults: a practical approach to evaluation and management. South Med J. 2006;99:491-498.
10. Gauer RL, Braun MM. Thrombocytopenia. Am Fam Physician. 2012;85:612-622.
11. Bratton RL, Corey R. Tick-borne disease. Am Fam Physician. 2005;71:2323-2330.
12. Yeh JJ, Tsai S, Wu DC, et al. P-selectin-dependent platelet aggregation and apoptosis may explain the decrease in platelet count during Helicobacter pylori infection. Blood. 2010;115:4247-4253.
13. Stasi R, Sarpatwari A, Segal JB, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systemic review. Blood. 2009;113:1231-1240.
14. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
15. Peck-Radosavljevic M. Hypersplenism. Eur J Gastroenterol Hepatol. 2001;13:317-323.
16. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190-4207.
17. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Int Med. 2015;162:205-213.
18. Palmblad J, Dufour C, Papadaki HA. How we diagnose neutropenia in the adult and elderly patient. Haematologica. 2014;99:1130-1133.
19. Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146:486-492.
20. Gibson C, Berliner N. How we evaluate and treat neutropenia in adults. Blood. 2014;124:1251-1258.
21. Urabe A. Clinical features of the neutropenic host: definitions and initial evaluation. CID. 2004;39(suppl 1):S53-S55.
22. Dale DC, Hammond WP 4th. Cyclic neutropenia: a clinical review. Blood Rev. 1988;2:178-185.
23. Munshi HG, Montgomery RB. Severe neutropenia: a diagnostic approach. West J Med. 2000;172:248-252.
24. Pisciotta AV. Drug-induced agranulocytosis peripheral destruction of polymorphonuclear leukocytes and their marrow precursors. Blood Rev. 1990;4:226-237.
25. Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146:657-665.
26. Bhatt V, Saleem A. Review: drug-induced neutropenia – pathophysiology, clinical features, and management. Ann Clin Lab Sci. 2004;34:131-137.
27. Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin Hematol. 2013;50:198-206.
28. Bakken JS, Krueth J, Wilson-Nordskog C, et al. Clinical and laboratory characteristics of human granulcytic ehrlichiosis. JAMA. 1996;275:199-205.
29. Hall GW, Schwartz RP. White blood cell count and differential in Rocky Mountain spotted fever. NC Med J. 1979;40:212-214.
30. Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797-808.
31. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012; 64:2677-2686.
1. Wong EY, Rose MG. Why does my patient have thrombocytopenia? Hematol Oncol Clin North Am. 2012;26:231-252.
2. Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357:580-587.
3. University of Oklahoma Health Sciences Center. Database for Drug–induced thrombocytopenia from group patient reports: an update. Available at: http://www.ouhsc.edu/platelets/InternetPostingGroupFrames2014.htm. Accessed May 7, 2018.
4. Sniecinski RM, Hursting MJ, Paidas MJ, et al. Etiology and assessment of hypercoagulability with lessons from heparin-induced thrombocytopenia. Anesth Analg. 2011;112:46-58.
5. Warkentin TE. Heparin-induced thrombocytopenia in critically ill patients. Crit Care Clin. 2011;27:805-823.
6. Connell NT, Sweeney JD. Does my patient have life- or limb-threatening thrombocytopenia? Hematol Oncol Clin North Am. 2012;26:369-382.
7. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654-666.
8. Hanly JG. Antiphospholipid syndrome: an overview. CMAJ. 2003;168:1675-1682.
9. Sekhon SS, Roy V. Thrombocytopenia in adults: a practical approach to evaluation and management. South Med J. 2006;99:491-498.
10. Gauer RL, Braun MM. Thrombocytopenia. Am Fam Physician. 2012;85:612-622.
11. Bratton RL, Corey R. Tick-borne disease. Am Fam Physician. 2005;71:2323-2330.
12. Yeh JJ, Tsai S, Wu DC, et al. P-selectin-dependent platelet aggregation and apoptosis may explain the decrease in platelet count during Helicobacter pylori infection. Blood. 2010;115:4247-4253.
13. Stasi R, Sarpatwari A, Segal JB, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systemic review. Blood. 2009;113:1231-1240.
14. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
15. Peck-Radosavljevic M. Hypersplenism. Eur J Gastroenterol Hepatol. 2001;13:317-323.
16. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190-4207.
17. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Int Med. 2015;162:205-213.
18. Palmblad J, Dufour C, Papadaki HA. How we diagnose neutropenia in the adult and elderly patient. Haematologica. 2014;99:1130-1133.
19. Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146:486-492.
20. Gibson C, Berliner N. How we evaluate and treat neutropenia in adults. Blood. 2014;124:1251-1258.
21. Urabe A. Clinical features of the neutropenic host: definitions and initial evaluation. CID. 2004;39(suppl 1):S53-S55.
22. Dale DC, Hammond WP 4th. Cyclic neutropenia: a clinical review. Blood Rev. 1988;2:178-185.
23. Munshi HG, Montgomery RB. Severe neutropenia: a diagnostic approach. West J Med. 2000;172:248-252.
24. Pisciotta AV. Drug-induced agranulocytosis peripheral destruction of polymorphonuclear leukocytes and their marrow precursors. Blood Rev. 1990;4:226-237.
25. Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146:657-665.
26. Bhatt V, Saleem A. Review: drug-induced neutropenia – pathophysiology, clinical features, and management. Ann Clin Lab Sci. 2004;34:131-137.
27. Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin Hematol. 2013;50:198-206.
28. Bakken JS, Krueth J, Wilson-Nordskog C, et al. Clinical and laboratory characteristics of human granulcytic ehrlichiosis. JAMA. 1996;275:199-205.
29. Hall GW, Schwartz RP. White blood cell count and differential in Rocky Mountain spotted fever. NC Med J. 1979;40:212-214.
30. Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797-808.
31. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012; 64:2677-2686.
From The Journal of Family Practice | 2018;67(7):E1-E8.
PRACTICE RECOMMENDATIONS
› Employ a systematic approach to the diagnosis and treatment of thrombocytopenia and neutropenia. C
› Do not transfuse platelets in patients with platelet counts >10,000/mcL who are stable and are not undergoing an invasive procedure. C
› Monitor patients on heparin therapy for >4 days for heparin-induced thrombocytopenia. C
› Monitor (for life) patients with a history of gastric bypass for the development of nutritional neutropenias. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How best to manage chronic cholestasis
CASE
A 44-year-old nurse describes persistent fatigue and itching over the last 2 months. She is taking ramipril 5 mg/d for hypertension and has a family history of rheumatic disease. Lab tests reveal a recurrent moderate elevation of gamma glutamyl-transpeptidase (gGT; 75 U/L) associated with, on some occasions, mild elevation of alanine aminotransferase (ALT) levels (100 U/L) of unknown origin. She has no history of hepatitis virus infection, hepatotoxic medications, or alcohol intake. She is overweight with a body mass index of 28.5 kg/m2 and a waist circumference of 99 cm (39 inches). Liver ultrasonography detects an enlarged liver with diffuse echostructure dishomogeneity, but no signs of cirrhosis or portal hypertension. The patient’s biliary tree is not dilated.
How would you proceed with the care of this patient?
Cholestasis is characterized by the alteration of bile flow through any part of the biliary system, from the hepatocyte basocellular membrane to the duodenum. The condition is classified as intrahepatic when the cause is a defect of hepatocellular function or obstruction of the biliary tree within the liver. The extrahepatic form includes all conditions obstructing bile flow in the main biliary tract (choledochus, common bile duct).
The key to successfully managing cholestasis lies in the early identification of subtle signs and symptoms before serious complications can arise. In the review that follows, we provide guidance for evaluating laboratory and imaging results that are vital to the accurate diagnosis of intrahepatic and extrahepatic cholestasis. We also detail treatment recommendations.
Clues—subtle and otherwise—of cholestasis
Clinical features of cholestasis include fatigue and itching all over the skin. The latter likely is caused by induction of the enzyme autotaxin, which produces the neuronal activator lysophosphatidic acid. Retention of pruritogenic substances that normally are excreted into bile might contribute to pruritus as well.1 Jaundice, dark urine, and pale and fatty stools occur with advanced disease. However, a cholestatic condition can be detected in asymptomatic patients with elevated biochemical markers.
Continue to: Mildly elevated gGT and/or alkaline phosphatase (ALP)
Mildly elevated gGT and/or alkaline phosphatase (ALP) (0.5-2.5 times the upper normal limit [UNL] or 19-95 U/L and 60-300 U/L, respectively2) in the presence of normal transaminase levels (<20 U/L) in an asymptomatic patient can indicate chronic liver disease. Signs suggestive of significant liver disease have been reported in many patients with gGT or ALP elevation with good sensitivity (65%) and specificity (83%) for a diagnosis of intrahepatic cholestasis.3 However, because abnormal gGT values are common and often resolve spontaneously, family physicians (FPs) may pay little attention to this finding, thus missing an opportunity for early identification and treatment.
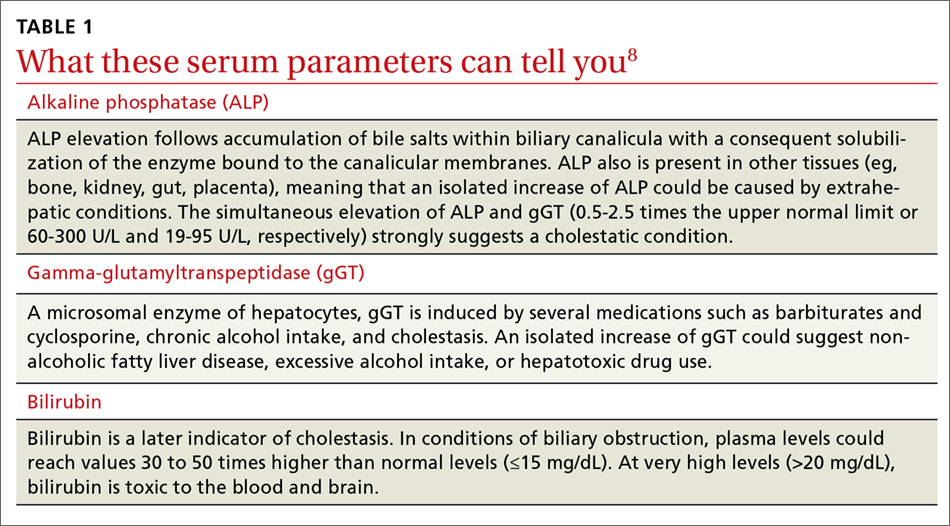
That’s why it’s important to schedule follow-up testing within 6 months for asymptomatic patients with abnormal laboratory findings. Persistent elevation of gGT alone or accompanied by ALP and ALT elevation (ALT >0.5 times the UNL or >18 U/L) is the most common feature of a chronic (>6 months) cholestatic condition.4 (In particular, elevated ALP levels appear to be associated with more aggressive disease and predict risk of liver transplantation or death in patients with primary biliary cholangitis (PBC).5,6 Lowering ALP levels is associated with improved disease outcomes, including transplant-free survival rates.5,7)
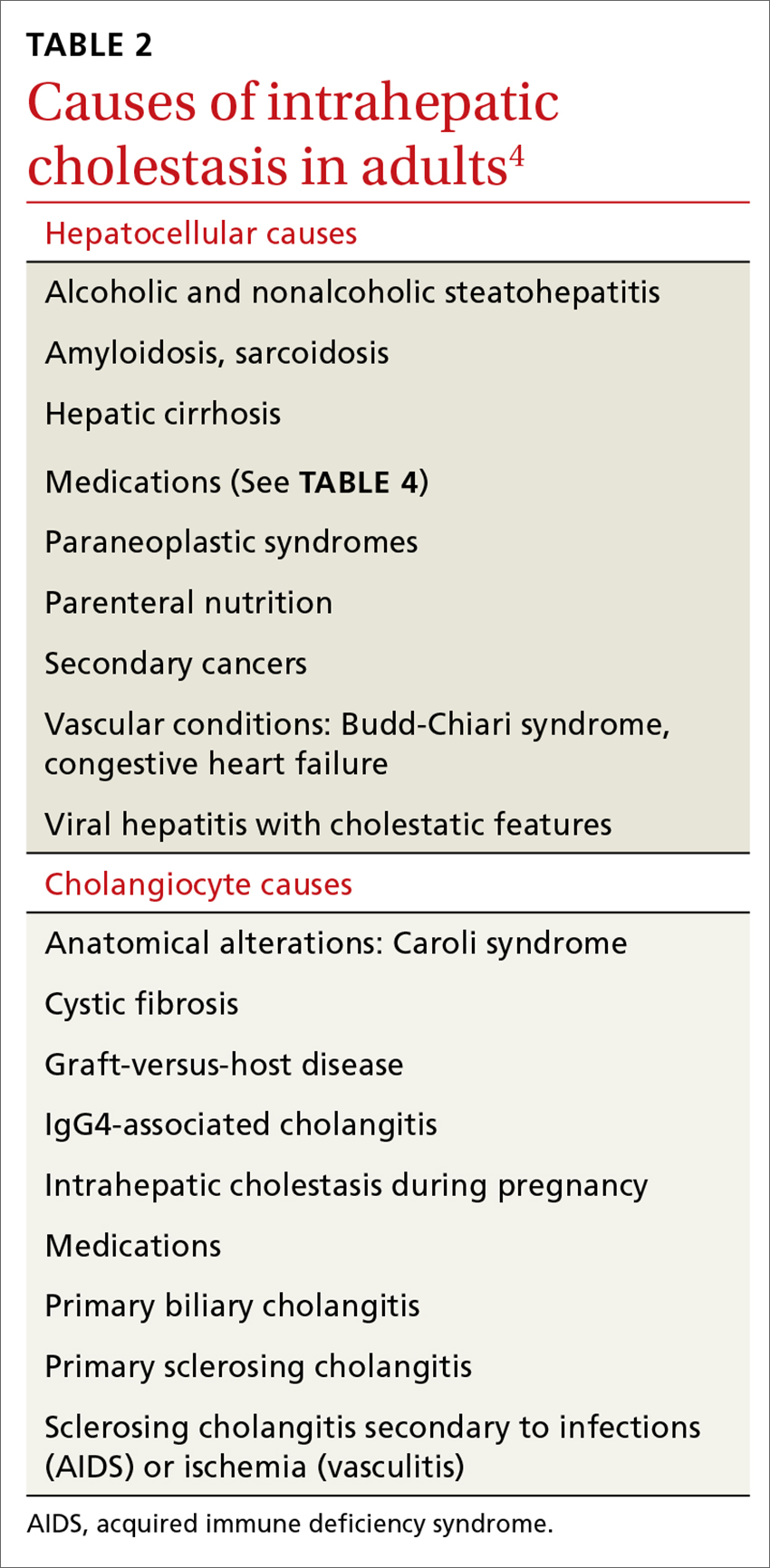
Elevated serum aminotransferase levels (aspartate aminotransferase [AST] >0.5 times the UNL or 17.5 U/L; ALT >0.5 times the UNL or >18 U/L) and bilirubin (>1.1 mg/dL), with predominance of the conjugated form (TABLE 18), suggest possible cholestasis. In light of such findings, a clinician’s next step should be to distinguish intrahepatic from extrahepatic conditions. (For a detailed list of the causes of intra- and extrahepatic cholestasis, see TABLES 24 and 3.9)

Patient’s history can provide important clues
A thorough patient history is especially important when cholestasis is suspected. Details about the patient’s occupation, environment, and lifestyle are key, as are the specifics of prescribed or over-the-counter medications and supplements that could be hepatotoxic (TABLE 410). A number of exogenous substances can cause liver injury, and the use of some herbal products (senna, black cohosh, greater celandine, kava) have been linked to hepatitis and cholestasis.11 Ask patients about alcohol use and history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders.

Continue to: Indicators pointing to cholestasis? It's time for ultrasonography
Indicators pointing to cholestasis? It’s time for ultrasonography
While biopsy is considered the gold standard for diagnosing and staging chronic cholestatic liver disease and can exclude an extrahepatic obstruction, it should be employed only if blood tests have been confirmed, second-level tests have been performed, and ultrasound is inconclusive.12 (More on biopsy in a bit.)
Ultrasonography is a low-cost, widely available, noninvasive test that allows easy identification of extrahepatic dilatation of the biliary tree and sometimes the underlying cause, as well. Ultrasonography identifies extrahepatic cholestasis by allowing visualization of an enlarged choledochus (>7 mm) or common hepatic duct (>5 mm) and an intrahepatic bile duct diameter that is more than 40% larger than adjacent branches of the portal vein.13 However, ultrasonography has a low diagnostic sensitivity for many conditions (eg, 15% to 89% for detecting common bile duct stones),14 requiring other diagnostic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), before reaching a diagnosis.
For asymptomatic patients with cirrhosis or those at an early stage of liver disease, ultrasound at 6-month intervals combined with serum liver function tests can be useful to track disease progression and screen for hepatocellular carcinoma or cholangiocarcinoma.15,16
New noninvasive methods. Noninvasive tools for evaluating the presence and severity of liver fibrosis and for differentiating cirrhosis from noncirrhotic conditions have positive predictive values >85% to 90% for some chronic liver diseases.17 Transient elastography, which assesses liver stiffness, is one such method. Although it is often used successfully, morbid obesity, small intercostal spaces, and ascites limit its diagnostic capability.18 Recently, some questions about the validity of elastography to assess the extent of fibrosis in patients with chronic cholestatic conditions have been reported.19,20
Suspect intrahepatic cholestasis? Your next steps
If imaging techniques do not show bile duct obstruction and you suspect the intrahepatic form, second-level tests could have strategic importance. This is where antimitochondrial antibodies (AMAs) come in. AMAs are immunoglobulins (IgG and IgM) directed against mitochondrial antigens. They are important markers for PBC, which is a T-lymphocyte-mediated attack on small intralobular bile ducts resulting in their gradual destruction and eventual disappearance. The sustained loss of intralobular bile ducts leads to signs and symptoms of cholestasis and eventually results in cirrhosis and liver failure.
AMA serum levels show high sensitivity and specificity (90% and 95%, respectively) for PBC.21 Some PBC patients (<5%) show histologic confirmation of the disease, but have negative AMA tests (AMA negative PBC or autoimmune cholangitis).22 Therefore, according to the American Association for the Study of Liver Diseases, diagnosis of PBC is guided by the combination of serologic, biochemical, and histologic criteria.23 Many PBC patients with or without a positive AMA (≥1:40) also have positive circulating antinuclear antibodies (ANA; ≥1:80). The recent availability of lab tests for antibodies (anti-M2, anti-gp120, anti-sp100) has allowed identification of subgroups of patients who have a more aggressive form of PBC. Patients with PBC often have elevated levels of circulating IgM (>280 mg/dL).
Continue to: Other circulating antibodies
Other circulating antibodies can help discriminate among cholestatic disorders. In particular, positive tests for perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are found in 25% to 95% of patients with primary sclerosing cholangitis (PSC), a chronic progressive disorder of unknown etiology that is characterized by inflammation, fibrosis, and stricturing of medium and large ducts of the intrahepatic and extrahepatic biliary tree.24 Anti-smooth muscle antibodies (SMA) can be observed in both PSC and autoimmune hepatitis.
Finally, there are syndromes with serologic and histologic overlap that are characterized by the simultaneous presence of PBC with autoimmune hepatitis or PSC or overlap of PSC with autoimmune hepatitis.
Liver biopsy fills in the rest of the diagnostic picture
Unfortunately, blood tests reveal little about organ integrity and are not useful for disease staging. The decision to perform a liver biopsy should be based on several factors, including the patient’s age, serum parameters, the need to stage the disease, therapy choices, and prognosis.12 One should also consider that biopsy is a costly procedure with potentially serious adverse effects; it should not be repeated frequently. However, when a biopsy is done, it provides critical information, including damage to medium-sized intrahepatic bile ducts with neoductular formation or bile duct scars and strictures.
Treating intrahepatic cholestasis
Although FPs often can provide most—or even all—of the care for patients with stable conditions, a specialist consultation might recommend further testing to identify the underlying disease, which is essential to establish the most appropriate treatment.
Treatment of patients with PBC is based on administering hydrophilic secondary bile salt ursodeoxycholic acid (UDCA) 15 mg/kg/d, which is used to equilibrate the ratio between hydrophilic and hydrophobic bile salts in the liver and bile,25 and is the only treatment approved by the US Food and Drug Administration (FDA) for PBC.4 Tauroursodeoxycholate is better absorbed than UDCA, and, although partially deconjugated and reconjugated with glycine, it undergoes reduced biotransformation to more hydrophobic metabolites and has benefits, including antioxidant, immunomodulation, and neuroprotective effects over UDCA—especially for long-term therapy in PBC.26 However, it is not used often in clinical practice.
Continue to: Bile acid administration counters the cytotoxic effect...
Bile acid administration counters the cytotoxic effect of hydrophobic bile salts. Although it seems that UDCA might improve biochemical and histologic features of the disease at earlier stages (I-II), it fails in patients with more advanced disease.27 In addition, monitoring and defining response to UDCA is inconsistent, partly because of variations in guideline criteria.28,29
Recently a new molecule, obeticholic acid (OCA), has been approved by the FDA. A farnesoid X receptor agonist, OCA is indicated for treating patients who do not tolerate UDCA or as an adjunct to UDCA in those with a partial response to UDCA, defined as lowering ALP levels by <1.5 times the baseline value after 12 months of treatment.
Treating PSC is more complex. Combination therapy with prednisone and azathioprine is recommended only when there is an overlap syndrome between PSC and autoimmune hepatitis.4 UDCA at a high dosage (15-20 mg/kg/d) is used to facilitate long-lasting biochemical remission. These patients also need to be monitored for inflammatory bowel diseases, which affect up to 75% of patients,30 and for cholangiocarcinoma, which is a life-limiting complication because of a lack of therapy options. Finally, these patients might need endoscopic-guided dilatation of the biliary tree when they have evidence of dominant fibrotic strictures of the greater bile ducts.14,31
Addressing the systemic effects of intrahepatic cholestasis
Pruritus. A number of potential pruritogens, including bile salts, endogenous opioids, histamine, serotonin, and lisophosphatidic acid (LPA), can be targeted to relieve pruritus.
- Bile acid resin binders such as cholestyramine are the first step for treating pruritus. UDCA also can be useful, mainly for intrahepatic cholestasis during pregnancy. Rifampicin, 300 mg/d, improves cholestatic pruritus, but is associated with hepatotoxicity and a number of severe reactions, such as nausea, loss of appetite, hemolytic anemia, and thrombocytopenia.31
- Most evidence favors a role for opioids in relieving itch, and micro-opioid receptor antagonists (naltrexone, naloxone, nalmefene) that exert an antipruritic effect can be effective.
- Sertraline (a selective serotonin reuptake inhibitor), 50 to 75 mg/d, usually is well tolerated in patients with chronic cholestasis and exerts a beneficial effect on pruritus in approximately 40% of patients.32
- Extracorporeal albumin dialysis removes albumin-bound pruritogens and has been found to be effective in patients with liver failure. Steroids and UV light also can be used in select patients.
- The potent neuronal activator LPA and its converting enzyme autotaxin have been identified in the serum of patients with cholestatic pruritus; experimental modalities using LPA antagonists are ongoing for treating pruritus in patients who do not respond to other medications.33
Continue to: Malnutrition
Malnutrition. Many patients with cholestasis are at risk for malnutrition, which can be exacerbated in those with cirrhosis. Causes of malnutrition include poor oral intake, malabsorption, or dental problems that prevent the patient from chewing. Assess the nutritional status of every patient with chronic cholestasis, and stress the importance of multivitamin supplementation to reverse systemic alterations caused by malnutrition.34
When the patient has advanced disease
Despite progress in diagnostic techniques, life expectancy and quality of life for patients with advanced cholestatic conditions remain poor. Patients routinely experience fatigue, pruritus, and complications of cirrhosis including ascites, encephalopathy, and bleeding. Cholestasis also carries the risk of life-threatening complications, partly because of comorbidities such as osteoporosis and malabsorption.
Liver transplantation can improve the life expectancy of patients with advanced disease, but because of long waiting lists, candidates for transplant often die before an organ becomes available. For many patients who are not in end-stage condition, targeted therapy is crucial to slow disease progression and is recommended along with hepatitis A and B vaccinations and nutritional counseling.35
Extrahepatic cholestasis is suspected? How to proceed
Computer tomography (CT) is recommended for better identification of neoplastic causes of biliary obstruction and for staging purposes. MRCP is an excellent noninvasive imaging technique for evaluating biliary ducts.36
MRCP has 92% to 93% sensitivity and 97% to 98% specificity for diagnosing biliary duct stones.37 MRCP also is the first-choice modality for evaluating bile ducts in patients with suspected PSC. If performed in expert centers, the diagnostic accuracy reaches that of ERCP. A meta-analysis of studies from 2000 to 2006 has shown a sensitivity of 86% and specificity of 94% for diagnosing PSC.38
Endoscopic ultrasonography, which uses an ultrasonographic probe, allows clinicians to evaluate the integrity of the biliary and pancreatic ducts and is effective for diagnosing and staging cancer of the ampulla of Vater (sensitivity 93% vs 7% for abdominal ultrasonography and 29% for CT), and identifying biliary stones and biliary tree strictures.
Continue to: ERCP
ERCP is widely employed for diagnosing and treating pancreatobiliary diseases; however, its use has dropped over the last 10 years because of the risk of complications. ERCP is nearly exclusively used as a therapeutic procedure for pancreatic sphincterotomy, biliary dilatations, and removing biliary stones. It also has a diagnostic role in dominant stenosis or suspected biliary malignancy using brushing cytology and sampling biopsies of the bile ducts.
Treating extrahepatic cholestasis
Treatment of the different underlying conditions that cause extrahepatic cholestasis is surgical. Thus, the potential surgical techniques that can resolve or improve an extrahepatic cholestatic condition are guided by the surgeon and beyond the scope of this article.
Treating osteopenia: A concern for intra- and extrahepatic cholestasis
Vitamin D deficiency as a consequence of reduced intestinal absorption (poor availability of bile salts) or decreased hepatic activation to 25,OH-cholecalcipherol in both intrahepatic and extrahepatic cholestasis can lead to reduced bone formation.39 However, osteopenia can occur even in early stages of the disease. Prescribing bisphosphonates, in combination with calcium and vitamin D3, to improve bone mineral density is a good practice.40
CASE
Blood tests and ultrasound imaging suggest the presence of a chronic liver disease. Other lab tests indicate that the patient has an ALP level 3 times normal. This finding, together with the other tests, points to a likely diagnosis of intrahepatic cholestatic liver disease. Serology confirms positivity for ANA (1:160) and AMA (1:640). The clinician suspects PBC, so the patient is referred to a liver specialist for further evaluation and to determine whether a liver biopsy is needed.
The liver specialist confirms the diagnosis of PBC, performs a transient elastographym, which indicates a low-grade liver fibrosis (F1 out of 4), and starts therapy with UDCA.
CORRESPONDENCE
Ignazio Grattagliano, MD, Italian College of General Practitioners and Primary Care, Via del Sansovino 179, 50142, Florence, Italy; [email protected].
1. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
2. Deska Pagana K, Pagana TJ. Mosby’s Diagnostic and Laboratory Test Reference. 13th ed. St. Louis, MO: Elsevier; 2017.
3. Sapey T, Mendler MH, Guyader D, et al. Respective value of alkaline phosphatase, gamma-glutamyl transpeptidase and 5’ nucleotidase serum activity in the diagnosis of cholestasis: a prospective study of 80 patients. J Clin Gastroenterol. 2000;30:259-263.
4. European Association for the Study of the Liver. EASL Clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267.
5. Lammers WJ, van Buuren HR, Hirschfield GM, et al; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.
6. Trivedi PJ, Corpechot C, Pares A, et al. Risk stratification in autoimmune cholestatic liver diseases: opportunities for clinicians and trialists. Hepatology. 2016;63:644-659.
7. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804-1812.
8. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223-2230.
9. Assy N, Jacob G, Spira G, et al. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262.
10. Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatology. 2011;53:1377-1387.
11. US Food and Drug Administration. Food. Consumer advisory: kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://wayback.archive-it.org/7993/20171114232640/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm085482.htm. Accessed June 19, 2018.
12. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
13. Rogoveanu I, Gheonea DI, Saftoiu A, et al. The role of imaging methods in identifying the causes of extrahepatic cholestasis. J Gastrointestin Liver Dis. 2006;15:265-271.
14. Gotthardt DN, Rudolph G, Klöters-Plachky P, et al. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527-534.
15. Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2015;11:38-46.
16. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
17. Pinzani M, Vizzutti F, Arena U, et al. Technology insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
18. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
19. Van Gossum A, Pironi L, Messing B, et al. Transient elastography (FibroScan) is not correlated with liver fibrosis but with cholestasis in patients with long-term home parenteral nutrition. JPEN. 2015;39:719-724.
20. Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723.
21. European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172.
22. Ozaslan E, Efe C, Gokbulut Ozaslan N. The diagnosis of antimitochondrial antibody-negative primary biliary cholangitis. Clin Res Hepatol Gastroenterol. 2016;40:553-561.
23. Lindor KD, Gershwin ME, Poupon R, et al; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308.
24. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791.
25. Dilger K, Hohenester S, Winkler-Budenhofer U, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133-140.
26. Invernizzi P, Setchell KD, Crosignani A, et al. Differences in the metabolism and disposition of ursodeoxycholic acid and of its taurine-conjugated species in patients with primary biliary cirrhosis. Hepatology. 1999;29:320-327.
27. Jorgensen R, Angulo P, Dickson ER, et al. Results of long-term ursodiol treatment for patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2647-2650.
28. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715-720.
29. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877.
30. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY). 2011;7:235-241.
31. Rodriguez HJ, Bass NM. Primary sclerosing cholangitis. Semin Gastrointest Dis. 2003;14:189-198.
32. Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23:3418-3426.
33. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
34. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis. Gastroenterol Clin Biol. 2008;32:265-273.
35. Fagiuoli S, Colli A, Bruno R, et al; 2011 AISF Single Topic Group. Management of infections pre- and post-liver transplantation: report of an AISF consensus conference. J Hepatol. 2014;60:1075-1089.
36. Kanaan Z, Antaki F. Magnetic resonance cholangiopancreatography still plays a role in the preoperative evaluation of choledocholithiasis and biliary pathology. J Am Coll Surg. 2016;222:325-326.
37. McMahon CJ. The relative roles of magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound in diagnosis of common bile duct calculi: a critically appraised topic. Abdom Imaging. 2008;33:6-9.
38. Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139-1151.
39. Wimalawansa SJ, Razzaque DMS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. 2017. doi: 10.1016/j.jsbmb.2017.12.009.
40. Yadav A, Carey EJ. Osteoporosis in chronic liver disease. Nutr Clin Pract. 2013;28:52-64.
CASE
A 44-year-old nurse describes persistent fatigue and itching over the last 2 months. She is taking ramipril 5 mg/d for hypertension and has a family history of rheumatic disease. Lab tests reveal a recurrent moderate elevation of gamma glutamyl-transpeptidase (gGT; 75 U/L) associated with, on some occasions, mild elevation of alanine aminotransferase (ALT) levels (100 U/L) of unknown origin. She has no history of hepatitis virus infection, hepatotoxic medications, or alcohol intake. She is overweight with a body mass index of 28.5 kg/m2 and a waist circumference of 99 cm (39 inches). Liver ultrasonography detects an enlarged liver with diffuse echostructure dishomogeneity, but no signs of cirrhosis or portal hypertension. The patient’s biliary tree is not dilated.
How would you proceed with the care of this patient?
Cholestasis is characterized by the alteration of bile flow through any part of the biliary system, from the hepatocyte basocellular membrane to the duodenum. The condition is classified as intrahepatic when the cause is a defect of hepatocellular function or obstruction of the biliary tree within the liver. The extrahepatic form includes all conditions obstructing bile flow in the main biliary tract (choledochus, common bile duct).
The key to successfully managing cholestasis lies in the early identification of subtle signs and symptoms before serious complications can arise. In the review that follows, we provide guidance for evaluating laboratory and imaging results that are vital to the accurate diagnosis of intrahepatic and extrahepatic cholestasis. We also detail treatment recommendations.
Clues—subtle and otherwise—of cholestasis
Clinical features of cholestasis include fatigue and itching all over the skin. The latter likely is caused by induction of the enzyme autotaxin, which produces the neuronal activator lysophosphatidic acid. Retention of pruritogenic substances that normally are excreted into bile might contribute to pruritus as well.1 Jaundice, dark urine, and pale and fatty stools occur with advanced disease. However, a cholestatic condition can be detected in asymptomatic patients with elevated biochemical markers.
Continue to: Mildly elevated gGT and/or alkaline phosphatase (ALP)
Mildly elevated gGT and/or alkaline phosphatase (ALP) (0.5-2.5 times the upper normal limit [UNL] or 19-95 U/L and 60-300 U/L, respectively2) in the presence of normal transaminase levels (<20 U/L) in an asymptomatic patient can indicate chronic liver disease. Signs suggestive of significant liver disease have been reported in many patients with gGT or ALP elevation with good sensitivity (65%) and specificity (83%) for a diagnosis of intrahepatic cholestasis.3 However, because abnormal gGT values are common and often resolve spontaneously, family physicians (FPs) may pay little attention to this finding, thus missing an opportunity for early identification and treatment.

That’s why it’s important to schedule follow-up testing within 6 months for asymptomatic patients with abnormal laboratory findings. Persistent elevation of gGT alone or accompanied by ALP and ALT elevation (ALT >0.5 times the UNL or >18 U/L) is the most common feature of a chronic (>6 months) cholestatic condition.4 (In particular, elevated ALP levels appear to be associated with more aggressive disease and predict risk of liver transplantation or death in patients with primary biliary cholangitis (PBC).5,6 Lowering ALP levels is associated with improved disease outcomes, including transplant-free survival rates.5,7)

Elevated serum aminotransferase levels (aspartate aminotransferase [AST] >0.5 times the UNL or 17.5 U/L; ALT >0.5 times the UNL or >18 U/L) and bilirubin (>1.1 mg/dL), with predominance of the conjugated form (TABLE 18), suggest possible cholestasis. In light of such findings, a clinician’s next step should be to distinguish intrahepatic from extrahepatic conditions. (For a detailed list of the causes of intra- and extrahepatic cholestasis, see TABLES 24 and 3.9)

Patient’s history can provide important clues
A thorough patient history is especially important when cholestasis is suspected. Details about the patient’s occupation, environment, and lifestyle are key, as are the specifics of prescribed or over-the-counter medications and supplements that could be hepatotoxic (TABLE 410). A number of exogenous substances can cause liver injury, and the use of some herbal products (senna, black cohosh, greater celandine, kava) have been linked to hepatitis and cholestasis.11 Ask patients about alcohol use and history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders.

Continue to: Indicators pointing to cholestasis? It's time for ultrasonography
Indicators pointing to cholestasis? It’s time for ultrasonography
While biopsy is considered the gold standard for diagnosing and staging chronic cholestatic liver disease and can exclude an extrahepatic obstruction, it should be employed only if blood tests have been confirmed, second-level tests have been performed, and ultrasound is inconclusive.12 (More on biopsy in a bit.)
Ultrasonography is a low-cost, widely available, noninvasive test that allows easy identification of extrahepatic dilatation of the biliary tree and sometimes the underlying cause, as well. Ultrasonography identifies extrahepatic cholestasis by allowing visualization of an enlarged choledochus (>7 mm) or common hepatic duct (>5 mm) and an intrahepatic bile duct diameter that is more than 40% larger than adjacent branches of the portal vein.13 However, ultrasonography has a low diagnostic sensitivity for many conditions (eg, 15% to 89% for detecting common bile duct stones),14 requiring other diagnostic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), before reaching a diagnosis.
For asymptomatic patients with cirrhosis or those at an early stage of liver disease, ultrasound at 6-month intervals combined with serum liver function tests can be useful to track disease progression and screen for hepatocellular carcinoma or cholangiocarcinoma.15,16
New noninvasive methods. Noninvasive tools for evaluating the presence and severity of liver fibrosis and for differentiating cirrhosis from noncirrhotic conditions have positive predictive values >85% to 90% for some chronic liver diseases.17 Transient elastography, which assesses liver stiffness, is one such method. Although it is often used successfully, morbid obesity, small intercostal spaces, and ascites limit its diagnostic capability.18 Recently, some questions about the validity of elastography to assess the extent of fibrosis in patients with chronic cholestatic conditions have been reported.19,20
Suspect intrahepatic cholestasis? Your next steps
If imaging techniques do not show bile duct obstruction and you suspect the intrahepatic form, second-level tests could have strategic importance. This is where antimitochondrial antibodies (AMAs) come in. AMAs are immunoglobulins (IgG and IgM) directed against mitochondrial antigens. They are important markers for PBC, which is a T-lymphocyte-mediated attack on small intralobular bile ducts resulting in their gradual destruction and eventual disappearance. The sustained loss of intralobular bile ducts leads to signs and symptoms of cholestasis and eventually results in cirrhosis and liver failure.
AMA serum levels show high sensitivity and specificity (90% and 95%, respectively) for PBC.21 Some PBC patients (<5%) show histologic confirmation of the disease, but have negative AMA tests (AMA negative PBC or autoimmune cholangitis).22 Therefore, according to the American Association for the Study of Liver Diseases, diagnosis of PBC is guided by the combination of serologic, biochemical, and histologic criteria.23 Many PBC patients with or without a positive AMA (≥1:40) also have positive circulating antinuclear antibodies (ANA; ≥1:80). The recent availability of lab tests for antibodies (anti-M2, anti-gp120, anti-sp100) has allowed identification of subgroups of patients who have a more aggressive form of PBC. Patients with PBC often have elevated levels of circulating IgM (>280 mg/dL).
Continue to: Other circulating antibodies
Other circulating antibodies can help discriminate among cholestatic disorders. In particular, positive tests for perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are found in 25% to 95% of patients with primary sclerosing cholangitis (PSC), a chronic progressive disorder of unknown etiology that is characterized by inflammation, fibrosis, and stricturing of medium and large ducts of the intrahepatic and extrahepatic biliary tree.24 Anti-smooth muscle antibodies (SMA) can be observed in both PSC and autoimmune hepatitis.
Finally, there are syndromes with serologic and histologic overlap that are characterized by the simultaneous presence of PBC with autoimmune hepatitis or PSC or overlap of PSC with autoimmune hepatitis.
Liver biopsy fills in the rest of the diagnostic picture
Unfortunately, blood tests reveal little about organ integrity and are not useful for disease staging. The decision to perform a liver biopsy should be based on several factors, including the patient’s age, serum parameters, the need to stage the disease, therapy choices, and prognosis.12 One should also consider that biopsy is a costly procedure with potentially serious adverse effects; it should not be repeated frequently. However, when a biopsy is done, it provides critical information, including damage to medium-sized intrahepatic bile ducts with neoductular formation or bile duct scars and strictures.
Treating intrahepatic cholestasis
Although FPs often can provide most—or even all—of the care for patients with stable conditions, a specialist consultation might recommend further testing to identify the underlying disease, which is essential to establish the most appropriate treatment.
Treatment of patients with PBC is based on administering hydrophilic secondary bile salt ursodeoxycholic acid (UDCA) 15 mg/kg/d, which is used to equilibrate the ratio between hydrophilic and hydrophobic bile salts in the liver and bile,25 and is the only treatment approved by the US Food and Drug Administration (FDA) for PBC.4 Tauroursodeoxycholate is better absorbed than UDCA, and, although partially deconjugated and reconjugated with glycine, it undergoes reduced biotransformation to more hydrophobic metabolites and has benefits, including antioxidant, immunomodulation, and neuroprotective effects over UDCA—especially for long-term therapy in PBC.26 However, it is not used often in clinical practice.
Continue to: Bile acid administration counters the cytotoxic effect...
Bile acid administration counters the cytotoxic effect of hydrophobic bile salts. Although it seems that UDCA might improve biochemical and histologic features of the disease at earlier stages (I-II), it fails in patients with more advanced disease.27 In addition, monitoring and defining response to UDCA is inconsistent, partly because of variations in guideline criteria.28,29
Recently a new molecule, obeticholic acid (OCA), has been approved by the FDA. A farnesoid X receptor agonist, OCA is indicated for treating patients who do not tolerate UDCA or as an adjunct to UDCA in those with a partial response to UDCA, defined as lowering ALP levels by <1.5 times the baseline value after 12 months of treatment.
Treating PSC is more complex. Combination therapy with prednisone and azathioprine is recommended only when there is an overlap syndrome between PSC and autoimmune hepatitis.4 UDCA at a high dosage (15-20 mg/kg/d) is used to facilitate long-lasting biochemical remission. These patients also need to be monitored for inflammatory bowel diseases, which affect up to 75% of patients,30 and for cholangiocarcinoma, which is a life-limiting complication because of a lack of therapy options. Finally, these patients might need endoscopic-guided dilatation of the biliary tree when they have evidence of dominant fibrotic strictures of the greater bile ducts.14,31
Addressing the systemic effects of intrahepatic cholestasis
Pruritus. A number of potential pruritogens, including bile salts, endogenous opioids, histamine, serotonin, and lisophosphatidic acid (LPA), can be targeted to relieve pruritus.
- Bile acid resin binders such as cholestyramine are the first step for treating pruritus. UDCA also can be useful, mainly for intrahepatic cholestasis during pregnancy. Rifampicin, 300 mg/d, improves cholestatic pruritus, but is associated with hepatotoxicity and a number of severe reactions, such as nausea, loss of appetite, hemolytic anemia, and thrombocytopenia.31
- Most evidence favors a role for opioids in relieving itch, and micro-opioid receptor antagonists (naltrexone, naloxone, nalmefene) that exert an antipruritic effect can be effective.
- Sertraline (a selective serotonin reuptake inhibitor), 50 to 75 mg/d, usually is well tolerated in patients with chronic cholestasis and exerts a beneficial effect on pruritus in approximately 40% of patients.32
- Extracorporeal albumin dialysis removes albumin-bound pruritogens and has been found to be effective in patients with liver failure. Steroids and UV light also can be used in select patients.
- The potent neuronal activator LPA and its converting enzyme autotaxin have been identified in the serum of patients with cholestatic pruritus; experimental modalities using LPA antagonists are ongoing for treating pruritus in patients who do not respond to other medications.33
Continue to: Malnutrition
Malnutrition. Many patients with cholestasis are at risk for malnutrition, which can be exacerbated in those with cirrhosis. Causes of malnutrition include poor oral intake, malabsorption, or dental problems that prevent the patient from chewing. Assess the nutritional status of every patient with chronic cholestasis, and stress the importance of multivitamin supplementation to reverse systemic alterations caused by malnutrition.34
When the patient has advanced disease
Despite progress in diagnostic techniques, life expectancy and quality of life for patients with advanced cholestatic conditions remain poor. Patients routinely experience fatigue, pruritus, and complications of cirrhosis including ascites, encephalopathy, and bleeding. Cholestasis also carries the risk of life-threatening complications, partly because of comorbidities such as osteoporosis and malabsorption.
Liver transplantation can improve the life expectancy of patients with advanced disease, but because of long waiting lists, candidates for transplant often die before an organ becomes available. For many patients who are not in end-stage condition, targeted therapy is crucial to slow disease progression and is recommended along with hepatitis A and B vaccinations and nutritional counseling.35
Extrahepatic cholestasis is suspected? How to proceed
Computer tomography (CT) is recommended for better identification of neoplastic causes of biliary obstruction and for staging purposes. MRCP is an excellent noninvasive imaging technique for evaluating biliary ducts.36
MRCP has 92% to 93% sensitivity and 97% to 98% specificity for diagnosing biliary duct stones.37 MRCP also is the first-choice modality for evaluating bile ducts in patients with suspected PSC. If performed in expert centers, the diagnostic accuracy reaches that of ERCP. A meta-analysis of studies from 2000 to 2006 has shown a sensitivity of 86% and specificity of 94% for diagnosing PSC.38
Endoscopic ultrasonography, which uses an ultrasonographic probe, allows clinicians to evaluate the integrity of the biliary and pancreatic ducts and is effective for diagnosing and staging cancer of the ampulla of Vater (sensitivity 93% vs 7% for abdominal ultrasonography and 29% for CT), and identifying biliary stones and biliary tree strictures.
Continue to: ERCP
ERCP is widely employed for diagnosing and treating pancreatobiliary diseases; however, its use has dropped over the last 10 years because of the risk of complications. ERCP is nearly exclusively used as a therapeutic procedure for pancreatic sphincterotomy, biliary dilatations, and removing biliary stones. It also has a diagnostic role in dominant stenosis or suspected biliary malignancy using brushing cytology and sampling biopsies of the bile ducts.
Treating extrahepatic cholestasis
Treatment of the different underlying conditions that cause extrahepatic cholestasis is surgical. Thus, the potential surgical techniques that can resolve or improve an extrahepatic cholestatic condition are guided by the surgeon and beyond the scope of this article.
Treating osteopenia: A concern for intra- and extrahepatic cholestasis
Vitamin D deficiency as a consequence of reduced intestinal absorption (poor availability of bile salts) or decreased hepatic activation to 25,OH-cholecalcipherol in both intrahepatic and extrahepatic cholestasis can lead to reduced bone formation.39 However, osteopenia can occur even in early stages of the disease. Prescribing bisphosphonates, in combination with calcium and vitamin D3, to improve bone mineral density is a good practice.40
CASE
Blood tests and ultrasound imaging suggest the presence of a chronic liver disease. Other lab tests indicate that the patient has an ALP level 3 times normal. This finding, together with the other tests, points to a likely diagnosis of intrahepatic cholestatic liver disease. Serology confirms positivity for ANA (1:160) and AMA (1:640). The clinician suspects PBC, so the patient is referred to a liver specialist for further evaluation and to determine whether a liver biopsy is needed.
The liver specialist confirms the diagnosis of PBC, performs a transient elastographym, which indicates a low-grade liver fibrosis (F1 out of 4), and starts therapy with UDCA.
CORRESPONDENCE
Ignazio Grattagliano, MD, Italian College of General Practitioners and Primary Care, Via del Sansovino 179, 50142, Florence, Italy; [email protected].
CASE
A 44-year-old nurse describes persistent fatigue and itching over the last 2 months. She is taking ramipril 5 mg/d for hypertension and has a family history of rheumatic disease. Lab tests reveal a recurrent moderate elevation of gamma glutamyl-transpeptidase (gGT; 75 U/L) associated with, on some occasions, mild elevation of alanine aminotransferase (ALT) levels (100 U/L) of unknown origin. She has no history of hepatitis virus infection, hepatotoxic medications, or alcohol intake. She is overweight with a body mass index of 28.5 kg/m2 and a waist circumference of 99 cm (39 inches). Liver ultrasonography detects an enlarged liver with diffuse echostructure dishomogeneity, but no signs of cirrhosis or portal hypertension. The patient’s biliary tree is not dilated.
How would you proceed with the care of this patient?
Cholestasis is characterized by the alteration of bile flow through any part of the biliary system, from the hepatocyte basocellular membrane to the duodenum. The condition is classified as intrahepatic when the cause is a defect of hepatocellular function or obstruction of the biliary tree within the liver. The extrahepatic form includes all conditions obstructing bile flow in the main biliary tract (choledochus, common bile duct).
The key to successfully managing cholestasis lies in the early identification of subtle signs and symptoms before serious complications can arise. In the review that follows, we provide guidance for evaluating laboratory and imaging results that are vital to the accurate diagnosis of intrahepatic and extrahepatic cholestasis. We also detail treatment recommendations.
Clues—subtle and otherwise—of cholestasis
Clinical features of cholestasis include fatigue and itching all over the skin. The latter likely is caused by induction of the enzyme autotaxin, which produces the neuronal activator lysophosphatidic acid. Retention of pruritogenic substances that normally are excreted into bile might contribute to pruritus as well.1 Jaundice, dark urine, and pale and fatty stools occur with advanced disease. However, a cholestatic condition can be detected in asymptomatic patients with elevated biochemical markers.
Continue to: Mildly elevated gGT and/or alkaline phosphatase (ALP)
Mildly elevated gGT and/or alkaline phosphatase (ALP) (0.5-2.5 times the upper normal limit [UNL] or 19-95 U/L and 60-300 U/L, respectively2) in the presence of normal transaminase levels (<20 U/L) in an asymptomatic patient can indicate chronic liver disease. Signs suggestive of significant liver disease have been reported in many patients with gGT or ALP elevation with good sensitivity (65%) and specificity (83%) for a diagnosis of intrahepatic cholestasis.3 However, because abnormal gGT values are common and often resolve spontaneously, family physicians (FPs) may pay little attention to this finding, thus missing an opportunity for early identification and treatment.

That’s why it’s important to schedule follow-up testing within 6 months for asymptomatic patients with abnormal laboratory findings. Persistent elevation of gGT alone or accompanied by ALP and ALT elevation (ALT >0.5 times the UNL or >18 U/L) is the most common feature of a chronic (>6 months) cholestatic condition.4 (In particular, elevated ALP levels appear to be associated with more aggressive disease and predict risk of liver transplantation or death in patients with primary biliary cholangitis (PBC).5,6 Lowering ALP levels is associated with improved disease outcomes, including transplant-free survival rates.5,7)

Elevated serum aminotransferase levels (aspartate aminotransferase [AST] >0.5 times the UNL or 17.5 U/L; ALT >0.5 times the UNL or >18 U/L) and bilirubin (>1.1 mg/dL), with predominance of the conjugated form (TABLE 18), suggest possible cholestasis. In light of such findings, a clinician’s next step should be to distinguish intrahepatic from extrahepatic conditions. (For a detailed list of the causes of intra- and extrahepatic cholestasis, see TABLES 24 and 3.9)

Patient’s history can provide important clues
A thorough patient history is especially important when cholestasis is suspected. Details about the patient’s occupation, environment, and lifestyle are key, as are the specifics of prescribed or over-the-counter medications and supplements that could be hepatotoxic (TABLE 410). A number of exogenous substances can cause liver injury, and the use of some herbal products (senna, black cohosh, greater celandine, kava) have been linked to hepatitis and cholestasis.11 Ask patients about alcohol use and history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders.

Continue to: Indicators pointing to cholestasis? It's time for ultrasonography
Indicators pointing to cholestasis? It’s time for ultrasonography
While biopsy is considered the gold standard for diagnosing and staging chronic cholestatic liver disease and can exclude an extrahepatic obstruction, it should be employed only if blood tests have been confirmed, second-level tests have been performed, and ultrasound is inconclusive.12 (More on biopsy in a bit.)
Ultrasonography is a low-cost, widely available, noninvasive test that allows easy identification of extrahepatic dilatation of the biliary tree and sometimes the underlying cause, as well. Ultrasonography identifies extrahepatic cholestasis by allowing visualization of an enlarged choledochus (>7 mm) or common hepatic duct (>5 mm) and an intrahepatic bile duct diameter that is more than 40% larger than adjacent branches of the portal vein.13 However, ultrasonography has a low diagnostic sensitivity for many conditions (eg, 15% to 89% for detecting common bile duct stones),14 requiring other diagnostic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), before reaching a diagnosis.
For asymptomatic patients with cirrhosis or those at an early stage of liver disease, ultrasound at 6-month intervals combined with serum liver function tests can be useful to track disease progression and screen for hepatocellular carcinoma or cholangiocarcinoma.15,16
New noninvasive methods. Noninvasive tools for evaluating the presence and severity of liver fibrosis and for differentiating cirrhosis from noncirrhotic conditions have positive predictive values >85% to 90% for some chronic liver diseases.17 Transient elastography, which assesses liver stiffness, is one such method. Although it is often used successfully, morbid obesity, small intercostal spaces, and ascites limit its diagnostic capability.18 Recently, some questions about the validity of elastography to assess the extent of fibrosis in patients with chronic cholestatic conditions have been reported.19,20
Suspect intrahepatic cholestasis? Your next steps
If imaging techniques do not show bile duct obstruction and you suspect the intrahepatic form, second-level tests could have strategic importance. This is where antimitochondrial antibodies (AMAs) come in. AMAs are immunoglobulins (IgG and IgM) directed against mitochondrial antigens. They are important markers for PBC, which is a T-lymphocyte-mediated attack on small intralobular bile ducts resulting in their gradual destruction and eventual disappearance. The sustained loss of intralobular bile ducts leads to signs and symptoms of cholestasis and eventually results in cirrhosis and liver failure.
AMA serum levels show high sensitivity and specificity (90% and 95%, respectively) for PBC.21 Some PBC patients (<5%) show histologic confirmation of the disease, but have negative AMA tests (AMA negative PBC or autoimmune cholangitis).22 Therefore, according to the American Association for the Study of Liver Diseases, diagnosis of PBC is guided by the combination of serologic, biochemical, and histologic criteria.23 Many PBC patients with or without a positive AMA (≥1:40) also have positive circulating antinuclear antibodies (ANA; ≥1:80). The recent availability of lab tests for antibodies (anti-M2, anti-gp120, anti-sp100) has allowed identification of subgroups of patients who have a more aggressive form of PBC. Patients with PBC often have elevated levels of circulating IgM (>280 mg/dL).
Continue to: Other circulating antibodies
Other circulating antibodies can help discriminate among cholestatic disorders. In particular, positive tests for perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are found in 25% to 95% of patients with primary sclerosing cholangitis (PSC), a chronic progressive disorder of unknown etiology that is characterized by inflammation, fibrosis, and stricturing of medium and large ducts of the intrahepatic and extrahepatic biliary tree.24 Anti-smooth muscle antibodies (SMA) can be observed in both PSC and autoimmune hepatitis.
Finally, there are syndromes with serologic and histologic overlap that are characterized by the simultaneous presence of PBC with autoimmune hepatitis or PSC or overlap of PSC with autoimmune hepatitis.
Liver biopsy fills in the rest of the diagnostic picture
Unfortunately, blood tests reveal little about organ integrity and are not useful for disease staging. The decision to perform a liver biopsy should be based on several factors, including the patient’s age, serum parameters, the need to stage the disease, therapy choices, and prognosis.12 One should also consider that biopsy is a costly procedure with potentially serious adverse effects; it should not be repeated frequently. However, when a biopsy is done, it provides critical information, including damage to medium-sized intrahepatic bile ducts with neoductular formation or bile duct scars and strictures.
Treating intrahepatic cholestasis
Although FPs often can provide most—or even all—of the care for patients with stable conditions, a specialist consultation might recommend further testing to identify the underlying disease, which is essential to establish the most appropriate treatment.
Treatment of patients with PBC is based on administering hydrophilic secondary bile salt ursodeoxycholic acid (UDCA) 15 mg/kg/d, which is used to equilibrate the ratio between hydrophilic and hydrophobic bile salts in the liver and bile,25 and is the only treatment approved by the US Food and Drug Administration (FDA) for PBC.4 Tauroursodeoxycholate is better absorbed than UDCA, and, although partially deconjugated and reconjugated with glycine, it undergoes reduced biotransformation to more hydrophobic metabolites and has benefits, including antioxidant, immunomodulation, and neuroprotective effects over UDCA—especially for long-term therapy in PBC.26 However, it is not used often in clinical practice.
Continue to: Bile acid administration counters the cytotoxic effect...
Bile acid administration counters the cytotoxic effect of hydrophobic bile salts. Although it seems that UDCA might improve biochemical and histologic features of the disease at earlier stages (I-II), it fails in patients with more advanced disease.27 In addition, monitoring and defining response to UDCA is inconsistent, partly because of variations in guideline criteria.28,29
Recently a new molecule, obeticholic acid (OCA), has been approved by the FDA. A farnesoid X receptor agonist, OCA is indicated for treating patients who do not tolerate UDCA or as an adjunct to UDCA in those with a partial response to UDCA, defined as lowering ALP levels by <1.5 times the baseline value after 12 months of treatment.
Treating PSC is more complex. Combination therapy with prednisone and azathioprine is recommended only when there is an overlap syndrome between PSC and autoimmune hepatitis.4 UDCA at a high dosage (15-20 mg/kg/d) is used to facilitate long-lasting biochemical remission. These patients also need to be monitored for inflammatory bowel diseases, which affect up to 75% of patients,30 and for cholangiocarcinoma, which is a life-limiting complication because of a lack of therapy options. Finally, these patients might need endoscopic-guided dilatation of the biliary tree when they have evidence of dominant fibrotic strictures of the greater bile ducts.14,31
Addressing the systemic effects of intrahepatic cholestasis
Pruritus. A number of potential pruritogens, including bile salts, endogenous opioids, histamine, serotonin, and lisophosphatidic acid (LPA), can be targeted to relieve pruritus.
- Bile acid resin binders such as cholestyramine are the first step for treating pruritus. UDCA also can be useful, mainly for intrahepatic cholestasis during pregnancy. Rifampicin, 300 mg/d, improves cholestatic pruritus, but is associated with hepatotoxicity and a number of severe reactions, such as nausea, loss of appetite, hemolytic anemia, and thrombocytopenia.31
- Most evidence favors a role for opioids in relieving itch, and micro-opioid receptor antagonists (naltrexone, naloxone, nalmefene) that exert an antipruritic effect can be effective.
- Sertraline (a selective serotonin reuptake inhibitor), 50 to 75 mg/d, usually is well tolerated in patients with chronic cholestasis and exerts a beneficial effect on pruritus in approximately 40% of patients.32
- Extracorporeal albumin dialysis removes albumin-bound pruritogens and has been found to be effective in patients with liver failure. Steroids and UV light also can be used in select patients.
- The potent neuronal activator LPA and its converting enzyme autotaxin have been identified in the serum of patients with cholestatic pruritus; experimental modalities using LPA antagonists are ongoing for treating pruritus in patients who do not respond to other medications.33
Continue to: Malnutrition
Malnutrition. Many patients with cholestasis are at risk for malnutrition, which can be exacerbated in those with cirrhosis. Causes of malnutrition include poor oral intake, malabsorption, or dental problems that prevent the patient from chewing. Assess the nutritional status of every patient with chronic cholestasis, and stress the importance of multivitamin supplementation to reverse systemic alterations caused by malnutrition.34
When the patient has advanced disease
Despite progress in diagnostic techniques, life expectancy and quality of life for patients with advanced cholestatic conditions remain poor. Patients routinely experience fatigue, pruritus, and complications of cirrhosis including ascites, encephalopathy, and bleeding. Cholestasis also carries the risk of life-threatening complications, partly because of comorbidities such as osteoporosis and malabsorption.
Liver transplantation can improve the life expectancy of patients with advanced disease, but because of long waiting lists, candidates for transplant often die before an organ becomes available. For many patients who are not in end-stage condition, targeted therapy is crucial to slow disease progression and is recommended along with hepatitis A and B vaccinations and nutritional counseling.35
Extrahepatic cholestasis is suspected? How to proceed
Computer tomography (CT) is recommended for better identification of neoplastic causes of biliary obstruction and for staging purposes. MRCP is an excellent noninvasive imaging technique for evaluating biliary ducts.36
MRCP has 92% to 93% sensitivity and 97% to 98% specificity for diagnosing biliary duct stones.37 MRCP also is the first-choice modality for evaluating bile ducts in patients with suspected PSC. If performed in expert centers, the diagnostic accuracy reaches that of ERCP. A meta-analysis of studies from 2000 to 2006 has shown a sensitivity of 86% and specificity of 94% for diagnosing PSC.38
Endoscopic ultrasonography, which uses an ultrasonographic probe, allows clinicians to evaluate the integrity of the biliary and pancreatic ducts and is effective for diagnosing and staging cancer of the ampulla of Vater (sensitivity 93% vs 7% for abdominal ultrasonography and 29% for CT), and identifying biliary stones and biliary tree strictures.
Continue to: ERCP
ERCP is widely employed for diagnosing and treating pancreatobiliary diseases; however, its use has dropped over the last 10 years because of the risk of complications. ERCP is nearly exclusively used as a therapeutic procedure for pancreatic sphincterotomy, biliary dilatations, and removing biliary stones. It also has a diagnostic role in dominant stenosis or suspected biliary malignancy using brushing cytology and sampling biopsies of the bile ducts.
Treating extrahepatic cholestasis
Treatment of the different underlying conditions that cause extrahepatic cholestasis is surgical. Thus, the potential surgical techniques that can resolve or improve an extrahepatic cholestatic condition are guided by the surgeon and beyond the scope of this article.
Treating osteopenia: A concern for intra- and extrahepatic cholestasis
Vitamin D deficiency as a consequence of reduced intestinal absorption (poor availability of bile salts) or decreased hepatic activation to 25,OH-cholecalcipherol in both intrahepatic and extrahepatic cholestasis can lead to reduced bone formation.39 However, osteopenia can occur even in early stages of the disease. Prescribing bisphosphonates, in combination with calcium and vitamin D3, to improve bone mineral density is a good practice.40
CASE
Blood tests and ultrasound imaging suggest the presence of a chronic liver disease. Other lab tests indicate that the patient has an ALP level 3 times normal. This finding, together with the other tests, points to a likely diagnosis of intrahepatic cholestatic liver disease. Serology confirms positivity for ANA (1:160) and AMA (1:640). The clinician suspects PBC, so the patient is referred to a liver specialist for further evaluation and to determine whether a liver biopsy is needed.
The liver specialist confirms the diagnosis of PBC, performs a transient elastographym, which indicates a low-grade liver fibrosis (F1 out of 4), and starts therapy with UDCA.
CORRESPONDENCE
Ignazio Grattagliano, MD, Italian College of General Practitioners and Primary Care, Via del Sansovino 179, 50142, Florence, Italy; [email protected].
1. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
2. Deska Pagana K, Pagana TJ. Mosby’s Diagnostic and Laboratory Test Reference. 13th ed. St. Louis, MO: Elsevier; 2017.
3. Sapey T, Mendler MH, Guyader D, et al. Respective value of alkaline phosphatase, gamma-glutamyl transpeptidase and 5’ nucleotidase serum activity in the diagnosis of cholestasis: a prospective study of 80 patients. J Clin Gastroenterol. 2000;30:259-263.
4. European Association for the Study of the Liver. EASL Clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267.
5. Lammers WJ, van Buuren HR, Hirschfield GM, et al; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.
6. Trivedi PJ, Corpechot C, Pares A, et al. Risk stratification in autoimmune cholestatic liver diseases: opportunities for clinicians and trialists. Hepatology. 2016;63:644-659.
7. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804-1812.
8. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223-2230.
9. Assy N, Jacob G, Spira G, et al. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262.
10. Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatology. 2011;53:1377-1387.
11. US Food and Drug Administration. Food. Consumer advisory: kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://wayback.archive-it.org/7993/20171114232640/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm085482.htm. Accessed June 19, 2018.
12. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
13. Rogoveanu I, Gheonea DI, Saftoiu A, et al. The role of imaging methods in identifying the causes of extrahepatic cholestasis. J Gastrointestin Liver Dis. 2006;15:265-271.
14. Gotthardt DN, Rudolph G, Klöters-Plachky P, et al. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527-534.
15. Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2015;11:38-46.
16. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
17. Pinzani M, Vizzutti F, Arena U, et al. Technology insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
18. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
19. Van Gossum A, Pironi L, Messing B, et al. Transient elastography (FibroScan) is not correlated with liver fibrosis but with cholestasis in patients with long-term home parenteral nutrition. JPEN. 2015;39:719-724.
20. Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723.
21. European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172.
22. Ozaslan E, Efe C, Gokbulut Ozaslan N. The diagnosis of antimitochondrial antibody-negative primary biliary cholangitis. Clin Res Hepatol Gastroenterol. 2016;40:553-561.
23. Lindor KD, Gershwin ME, Poupon R, et al; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308.
24. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791.
25. Dilger K, Hohenester S, Winkler-Budenhofer U, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133-140.
26. Invernizzi P, Setchell KD, Crosignani A, et al. Differences in the metabolism and disposition of ursodeoxycholic acid and of its taurine-conjugated species in patients with primary biliary cirrhosis. Hepatology. 1999;29:320-327.
27. Jorgensen R, Angulo P, Dickson ER, et al. Results of long-term ursodiol treatment for patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2647-2650.
28. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715-720.
29. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877.
30. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY). 2011;7:235-241.
31. Rodriguez HJ, Bass NM. Primary sclerosing cholangitis. Semin Gastrointest Dis. 2003;14:189-198.
32. Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23:3418-3426.
33. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
34. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis. Gastroenterol Clin Biol. 2008;32:265-273.
35. Fagiuoli S, Colli A, Bruno R, et al; 2011 AISF Single Topic Group. Management of infections pre- and post-liver transplantation: report of an AISF consensus conference. J Hepatol. 2014;60:1075-1089.
36. Kanaan Z, Antaki F. Magnetic resonance cholangiopancreatography still plays a role in the preoperative evaluation of choledocholithiasis and biliary pathology. J Am Coll Surg. 2016;222:325-326.
37. McMahon CJ. The relative roles of magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound in diagnosis of common bile duct calculi: a critically appraised topic. Abdom Imaging. 2008;33:6-9.
38. Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139-1151.
39. Wimalawansa SJ, Razzaque DMS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. 2017. doi: 10.1016/j.jsbmb.2017.12.009.
40. Yadav A, Carey EJ. Osteoporosis in chronic liver disease. Nutr Clin Pract. 2013;28:52-64.
1. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
2. Deska Pagana K, Pagana TJ. Mosby’s Diagnostic and Laboratory Test Reference. 13th ed. St. Louis, MO: Elsevier; 2017.
3. Sapey T, Mendler MH, Guyader D, et al. Respective value of alkaline phosphatase, gamma-glutamyl transpeptidase and 5’ nucleotidase serum activity in the diagnosis of cholestasis: a prospective study of 80 patients. J Clin Gastroenterol. 2000;30:259-263.
4. European Association for the Study of the Liver. EASL Clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267.
5. Lammers WJ, van Buuren HR, Hirschfield GM, et al; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.
6. Trivedi PJ, Corpechot C, Pares A, et al. Risk stratification in autoimmune cholestatic liver diseases: opportunities for clinicians and trialists. Hepatology. 2016;63:644-659.
7. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804-1812.
8. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223-2230.
9. Assy N, Jacob G, Spira G, et al. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262.
10. Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatology. 2011;53:1377-1387.
11. US Food and Drug Administration. Food. Consumer advisory: kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://wayback.archive-it.org/7993/20171114232640/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm085482.htm. Accessed June 19, 2018.
12. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
13. Rogoveanu I, Gheonea DI, Saftoiu A, et al. The role of imaging methods in identifying the causes of extrahepatic cholestasis. J Gastrointestin Liver Dis. 2006;15:265-271.
14. Gotthardt DN, Rudolph G, Klöters-Plachky P, et al. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527-534.
15. Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2015;11:38-46.
16. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
17. Pinzani M, Vizzutti F, Arena U, et al. Technology insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
18. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
19. Van Gossum A, Pironi L, Messing B, et al. Transient elastography (FibroScan) is not correlated with liver fibrosis but with cholestasis in patients with long-term home parenteral nutrition. JPEN. 2015;39:719-724.
20. Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723.
21. European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172.
22. Ozaslan E, Efe C, Gokbulut Ozaslan N. The diagnosis of antimitochondrial antibody-negative primary biliary cholangitis. Clin Res Hepatol Gastroenterol. 2016;40:553-561.
23. Lindor KD, Gershwin ME, Poupon R, et al; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308.
24. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791.
25. Dilger K, Hohenester S, Winkler-Budenhofer U, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133-140.
26. Invernizzi P, Setchell KD, Crosignani A, et al. Differences in the metabolism and disposition of ursodeoxycholic acid and of its taurine-conjugated species in patients with primary biliary cirrhosis. Hepatology. 1999;29:320-327.
27. Jorgensen R, Angulo P, Dickson ER, et al. Results of long-term ursodiol treatment for patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2647-2650.
28. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715-720.
29. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877.
30. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY). 2011;7:235-241.
31. Rodriguez HJ, Bass NM. Primary sclerosing cholangitis. Semin Gastrointest Dis. 2003;14:189-198.
32. Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23:3418-3426.
33. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
34. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis. Gastroenterol Clin Biol. 2008;32:265-273.
35. Fagiuoli S, Colli A, Bruno R, et al; 2011 AISF Single Topic Group. Management of infections pre- and post-liver transplantation: report of an AISF consensus conference. J Hepatol. 2014;60:1075-1089.
36. Kanaan Z, Antaki F. Magnetic resonance cholangiopancreatography still plays a role in the preoperative evaluation of choledocholithiasis and biliary pathology. J Am Coll Surg. 2016;222:325-326.
37. McMahon CJ. The relative roles of magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound in diagnosis of common bile duct calculi: a critically appraised topic. Abdom Imaging. 2008;33:6-9.
38. Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139-1151.
39. Wimalawansa SJ, Razzaque DMS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. 2017. doi: 10.1016/j.jsbmb.2017.12.009.
40. Yadav A, Carey EJ. Osteoporosis in chronic liver disease. Nutr Clin Pract. 2013;28:52-64.
From The Journal of Family Practice | 2018;67(7):E9-E15.
PRACTICE RECOMMENDATIONS
› Suspect intrahepatic cholestasis in a patient with pruritus, normal transaminases, and mildly elevated gamma glutamyl-transpeptidase and alkaline phosphatase levels. A
› Use ultrasonography as a first-line diagnostic tool for cholestasis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Diabetes in the elderly: Matching meds to needs
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2
Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; [email protected].
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2

Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; [email protected].
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2

Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; [email protected].
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
From The Journal of Family Practice | 2018;67(7):408-410,412-415.
PRACTICE RECOMMENDATIONS
› Allow higher A1C goals for elderly patients who have such comorbid conditions as cognitive dysfunction, dementia, or cardiovascular or renal disease. B
› Look to metformin first in most instances if there are no contraindications. Monitor renal function frequently and vitamin B12 levels periodically. B
› Consider glucagon-like peptide-1 receptor agonists for patients who also have established cardiovascular disease, or consider starting basal insulin instead of using multiple oral agents. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Blood pressure targets: How low should you go (and for whom)?
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.
Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.
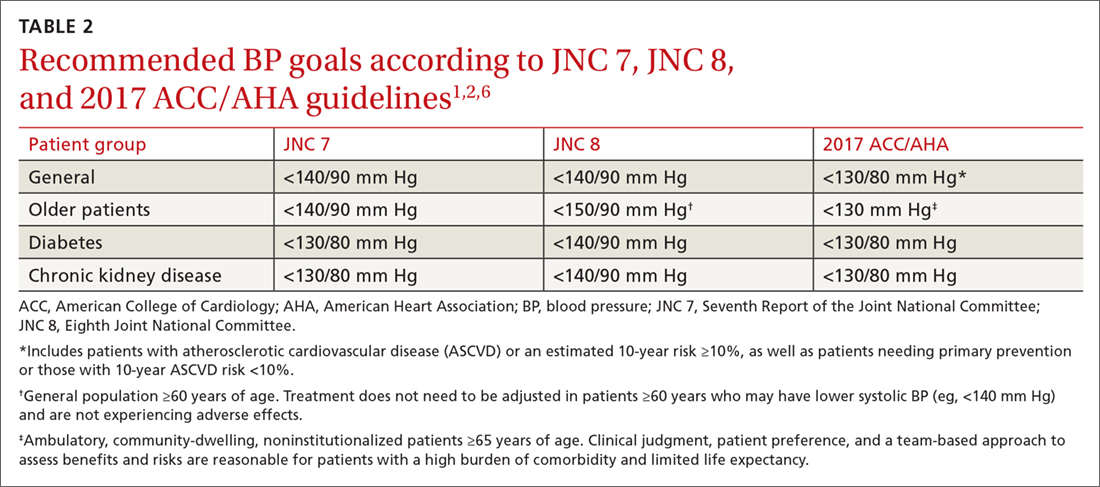
SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.
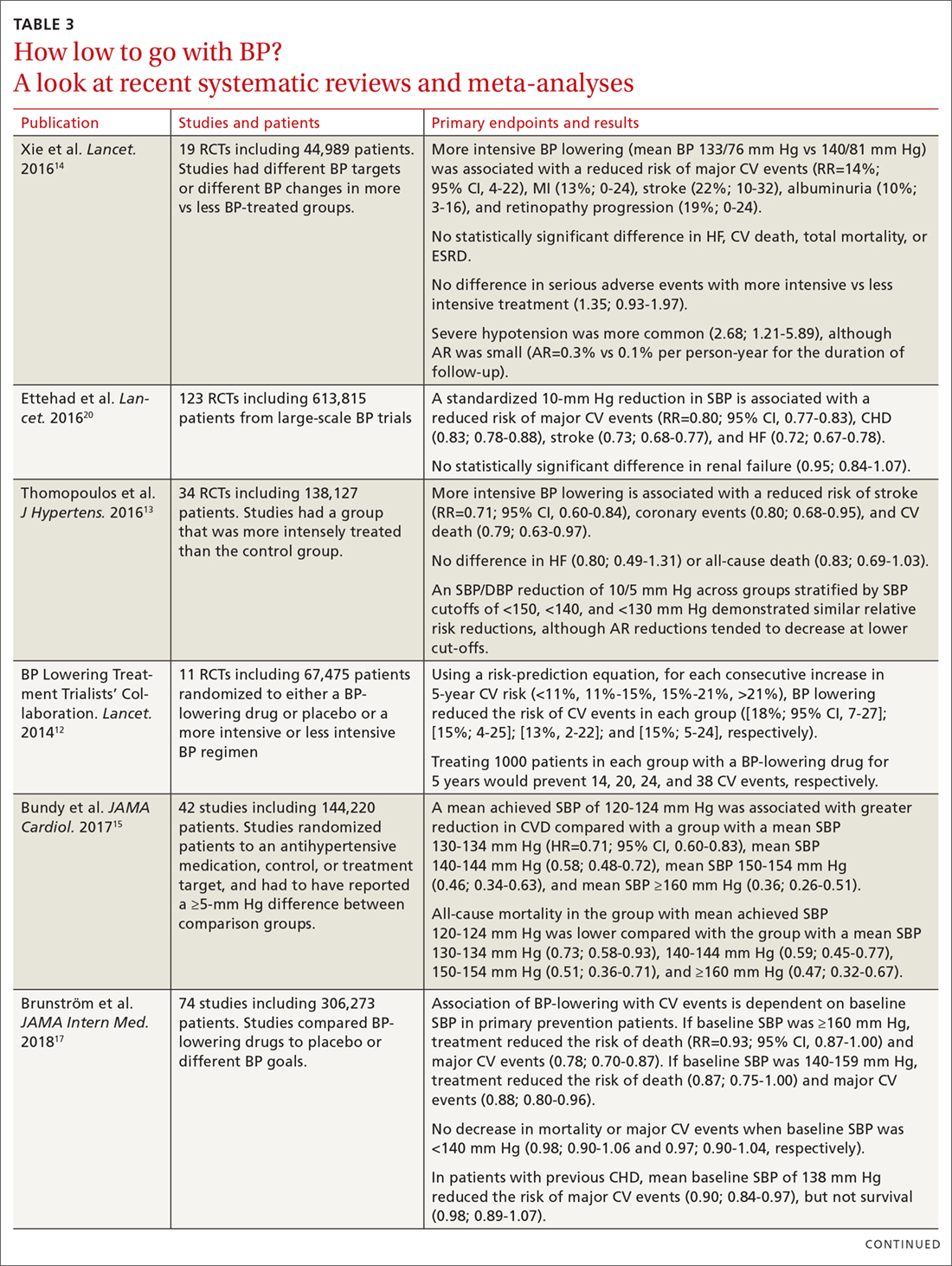
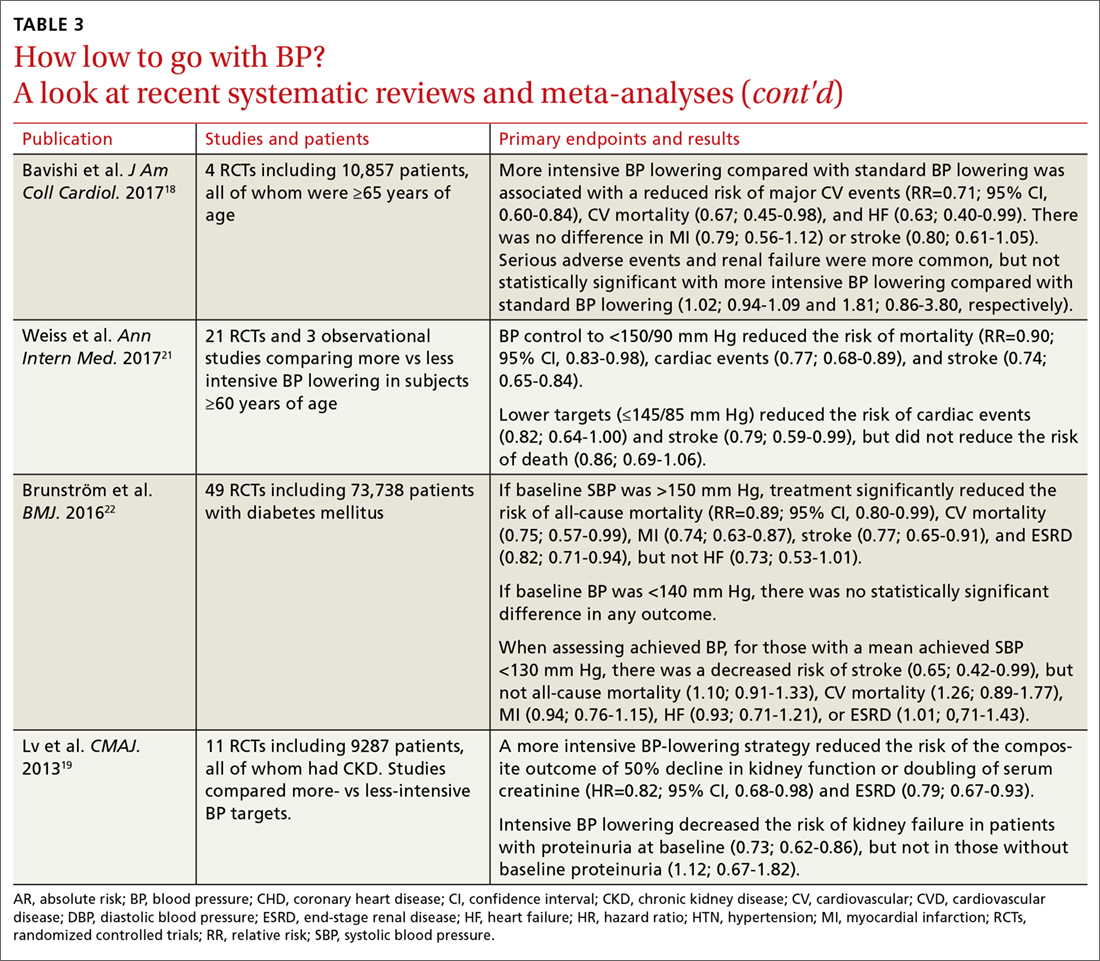
Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.
Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; [email protected].
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.

Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.

SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.


Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.

Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; [email protected].
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.

Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.

SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.


Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.

Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; [email protected].
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
PRACTICE RECOMMENDATIONS
› Treat adults with hypertension and cardiovascular disease or those at high risk (≥10%) of an atherosclerotic cardiovascular disease (ASCVD) event to a blood pressure (BP) goal <130/80 mm Hg. A for systolic BP goal; C for diastolic BP goal.
› Treat adults with hypertension and a low risk of a cardiovascular event (ie, primary prevention and ASCVD <10%) to a BP goal <130/80 mm Hg. B for systolic BP goal; C for diastolic BP goal.
› Treat ambulatory, community-dwelling, noninstitutionalized older patients to a systolic BP goal <130 mm Hg. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
When the answer to vaccines is “No”
We all know how challenging and time-consuming it can be to convince vaccine-hesitant patients that vaccinations are what is best for them and their children. Patients are bombarded with misinformation through the news and social media that seeds or “confirms” their doubts about vaccines. And for our part, we have only a few minutes during an office visit to refute all of the false claims that are a mere click or scroll away.
To better prepare for this challenge, this article details a practical approach to discussing vaccines with your patients. Using the patient-friendly language and evidence described here, you will be well positioned to refute 13 common vaccine misconceptions and overcome the barriers that stand in the way of these lifesaving interventions.
A few important baseline concepts
In discussing vaccination with our patients, it is important to keep the following in mind:
Patients don’t refuse vaccinations just to make our lives difficult. They truly are trying to make the best decisions they can for themselves and their families. Recognizing this can significantly reduce frustration levels.
Time well spent. While educating patients about the value of vaccines takes time, the return is worth it. The more consistently we offer vaccines, along with the reasons they are important, the more likely patients are to give vaccines a second thought. In fact, studies show that provider recommendation is the most important factor in patients’ decisions to vaccinate.1
Approach matters. In all other aspects of medicine, we attempt to use a participatory approach, involving our patients in decisions regarding their health care. When discussing vaccines, however, a participatory approach (eg, “What do you want to do about vaccines today?”) can introduce doubt into patients’ minds. Studies show that a presumptive approach (eg, “Today we are going to provide the tetanus, human papillomavirus [HPV], and meningitis vaccines”) is a much more effective way to get patients to vaccinate.2
[polldaddy:10018427]
Continue to: Barriers to counseling
Barriers to counseling. Health care providers report a variety of barriers to effective vaccine counseling (limited time and resources, lack of confidence in addressing patients’ concerns, etc).3 In addition, providers sometimes worry that strong encouragement of vaccination will create an adversarial relationship with vaccine-hesitant patients. Developing a good rapport and trusting relationship, as well as using motivational interviewing approaches, can help communicate the importance of vaccines, while leaving patients with the sense that you have heard them and respect their intentions. (See “Facilitate vaccine discussions using these 2 approaches.” 4-7)
SIDEBAR
Facilitate vaccine discussions using these 2 approaches4-7
C.A.S.E.
Corroborate
Acknowledge concerns and find some point on which you can agree.
Example: "It sounds like we both want to keep your child healthy and safe."
About me
Describe what you have done to build your expertise on the subject.
Example: "I have been practicing medicine for 15 years and have spent a great deal of time researching the data on vaccinations."
Science
Review the data and science behind vaccines.
Example: "Vaccines are more rigorously studied and safer than almost any other intervention we have in medicine."
Explain/advise
Explain your recommendations, based on the science.
Example: "This is why I vaccinate my children, and this is why I recommend this vaccine for your child."
3As
Ask
Don't stop at a patient's first "No." Respectfully dig a bit deeper.
Example: "What questions do you have about the vaccines we are recommending today? Tell me what worries you about them."
Acknowledge
Acknowledge your patient's concerns.
Example: "You are obviously a very devoted parent, and I know that you are trying to make the best decision you can for your child. With everything we see on the news and social media, it's not always easy to know what to believe about vaccines."
Advise
Advise patients/parents of the facts about vaccines and provide a strong recommendation to vaccinate.
Example: "Depending on the year, influenza kills 12,000 to 56,000 people annually; the vast majority of those who die did not receive the flu vaccine.7 My family and I get the flu shot every year, and I strongly encourage you and your children to get this lifesaving vaccine."
Continue to: If at first you don't succeed...
If at first you don’t succeed, try again because patients often have an experience that changes their mind. Perhaps a friend died of throat cancer or a family member developed a complication of the flu that required hospitalization. You never know when something will influence patients’ choices.
Don’t wait for scheduled well visits. Use every patient encounter as a means to catch patients up on missing vaccinations.
Common misconceptions and concerns and how to counter them
1. I’ve heard that vaccines can actually make you sick.
When patients raise this concern, start with an explanation of how vaccines work. Explain that our bodies protect us from foreign invaders (such as viruses and bacteria) by mounting an immune response when we are exposed to these proteins. Vaccinations work by exploiting this immune response; they expose the body to killed or weakened viral or bacterial proteins in a safe and controlled manner. In this way, our immune system will have already developed antibodies to these invaders by the time we are exposed to an active infection.
To use an analogy to war, instead of being subjected to a surprise attack where we suffer large losses in the battle, vaccination prepares us with weapons (antibodies) to defend ourselves so that our bodies are now able to successfully fight off that attack.
Because the majority of vaccines are killed virus vaccines, they cannot cause the illness against which they are meant to protect. Triggering the immune system may make some recipients feel a little “under the weather” for a day or 2, but they do not make us “sick.”
Live attenuated vaccines are similarly safe for those with a healthy immune system. We don’t administer them, however, to people who have a weakened immune system (eg, pregnant women, newborns, people with acquired immunodeficiency virus, or patients receiving chemotherapy or other types of immunosuppression) because these patients could develop the illness that we are trying to protect against.
Continue to: 2. Don't vaccines cause autism? Aren't they toxic to the nervous system?
2. Don’t vaccines cause autism? Aren’t they toxic to the nervous system?
The largest setback to vaccination efforts in recent history was a 1998 study by Andrew Wakefield that suggested that vaccination (specifically the mercury [in the form of thimerosal] present in the measles, mumps, rubella [MMR] vaccine) was linked to the development of autism.8 This research was subsequently debunked,9 and the author of the 1998 study was stripped of his medical license for falsifying data. However, the damage to vaccination efforts had already been done.
Aluminum. Thimerosal is not the only agent that patients may find concerning. Some also worry about the aluminum content of vaccines. Aluminum works as an additive to boost the body’s immune response to a vaccine. It is used only in killed virus vaccines—not in live attenuated ones. The Agency for Toxic Substances and Disease Registry monitors minimum risk levels (MRLs) of aluminum and other compounds in potentially hazardous substances. The amount of aluminum in vaccines is far below the MRL for aluminum, which is 1 mg/kg/d.10 (See “The facts about thimerosal and aluminum in vaccines.”11-16)
SIDEBAR
The facts about thimerosal and aluminum in vaccines
Thimerosal
Ethyl-mercury was used (in the form of thimerosal) as a preservative to prevent bacterial and fungal contamination of vaccines. Since 2001, however, thimerosal has been removed from all US-licensed vaccines—except multidose vials of influenza vaccine—as a precautionary measure (and not for any reproducible evidence of harm). The multidose flu vial contains <0.01% thimerosal.11
Ethyl-mercury is cleared from the body much more rapidly than methyl-mercury (the kind found in certain types of fish) and is less toxic.12
Since the removal of thimerosal from vaccines, the Centers for Disease Control and Prevention notes that the rates of autism have actually increased.13
Even Autism Speaks, the leading organization dedicated to advocacy for patients with autism and their families, denies a link between vaccines and autism.14
Aluminum
We are exposed to aluminum in products we use extensively every day, such as pots and pans, aluminum foil, seasonings, cereal, baby formula, paints, fuels, and antiperspirants.15
Infants are exposed to about 4.4 mg of aluminum in the vaccines typically administered in the first 6 months of life.16 However, infants typically ingest more than that during the first 6 months of life. Breast milk contains about 7 mg over 6 months; milk-based formulas contain about 38 mg over 6 months; and soy-based formulas contain about 117 mg over 6 months.16
Contine to: 3. I'm healthy. I never get sick. Why do I need vaccinations?
3. I’m healthy. I never get sick. Why do I need vaccinations?
A good way to counter this comment is to respond: “Saying you don’t need vaccinations because you never get sick is like saying you don’t need to wear a seat belt because you’ve never been in a car accident.” Advise patients that we seek to vaccinate all members of a community—not just those who are sick or at high risk—to protect ourselves and to provide “herd immunity.” It’s important to explain that herd immunity is resistance to the spread of a contagious disease that results if a sufficiently high number of people (depending on the illness, typically 80%-95%) are immune to the disease, especially through vaccination.17,18 If vaccination levels fall, we see a rise in cases of vaccine-preventable illness (as was seen during the 2017 measles outbreak in a community in Minnesota).19
Even though many of us may not suffer severe consequences of an infection, we can still pass that infection to others. While the whooping cough that a healthy 35-year-old gets may cause only prolonged annoyance or time off from work, it can kill the baby that is sitting next to that adult on the plane or bus.
4. Isn’t it true that we see fewer serious illnesses because of improved hygiene and sanitation, rather than vaccines?
Our current US sanitation standards were established under the Safe Drinking Water Act of 1974.20 While improvements in hygiene, sanitation, nutrition, and other public health measures have undoubtedly decreased the spread of disease and improved survival rates, there is no denying the significant drop in disease that occurs after the introduction of a vaccine for a particular illness or the increase in cases of that disease when vaccination rates drop off.
By the early 1990s, our current sanitation standards were already well established. Yet we didn’t see a significant decrease in the incidence of infections with Haemophilus influenzae type b (Hib) until after the conjugate Hib vaccines were introduced (dropping from about 20,000 cases/year to 1419 cases/year by 1993).21
In Britain, a drop in the rate of pertussis (whooping cough) vaccination in 1974 resulted in an epidemic of more than 100,000 cases and 36 deaths by 1978. There was no decrease in hygiene or sanitation standards to explain this rise.21
Continue to: 5. Vaccines are just another way for "big pharma" to make "big money."
5. Vaccines are just another way for “big pharma” to make “big money.”
Patients may benefit from knowing that in the earlier days of vaccines, pharmaceutical companies actually moved away from production of vaccines because they were not very profitable. These days, with worldwide distribution, drug companies are back in the swing of making vaccines and, as we would expect from all companies, are in business to make a profit.
That said, health care providers receive no payments from drug companies for offering vaccines or for offering one vaccine over another. The reason we recommend vaccination is because we know it is best for our patients’ health and the health of the community.

6. We don’t see polio anymore. Why do I need the vaccine?
One of the factors contributing to the rise in antivaccine sentiment is that we rarely see vaccine-preventable illnesses (such as polio, measles, and mumps). But the absence of these illnesses is precisely due to prior years’ vaccination efforts.
Smallpox, a deadly and disfiguring disease that killed many millions of people and contributed to the downfall of the Roman, Aztec, and Incan empires, was eradicated from the planet in 1979, thanks to focused vaccination efforts by the World Health Organization. Vaccination works, but we have to keep at it.
While we no longer see as many of these vaccine-preventable illnesses in the United States, they are still present in other parts of the world. Our world is much smaller than it used to be. International travel is common, and illnesses can be reintroduced into a community with relative ease. We must remain vigilant.
Continue to: 7. I heard that vaccines are made from aborted fetal tissue.
7. I heard that vaccines are made from aborted fetal tissue.
There are 5 vaccines (varicella, rubella, hepatitis A, shingles, and rabies vaccines) that were originally made using aborted fetal tissue. In 1960, tissue from 2 fetuses aborted by maternal choice (and not for the purpose of vaccine production) was used to propagate cell lines that are still used in vaccine development today.
Human cells provide advantages for vaccine production that other cells do not. Some viruses do not grow well in animal cells. Animal cells can introduce contamination by bacteria and viruses that are not carried in human cell lines. Vaccine production can be hindered or halted, resulting in a vaccine shortage, if animal products used in development are threatened (eg, if an illness strikes egg-producing chickens; eggs are used to make the influenza vaccine).22
Some patients, particularly those who are Catholic, may have concerns about these vaccines. The National Catholic Bioethics Center has prepared a statement regarding the use of these vaccines that may help settle any moral dilemmas.23 It reads:
“The cell lines under consideration were begun using cells taken from one or more fetuses aborted almost 40 years ago. Since that time, the cell lines have grown independently. It is important to note that descendent cells are not the cells of the aborted child.”
“One is morally free to use the vaccine regardless of its historical association with abortion. The reason is that the risk to public health, if one chooses not to vaccinate, outweighs the legitimate concern about the origins of the vaccine. This is especially important for parents, who have a moral obligation to protect the life and health of their children and those around them.”
Continue to: 8. Vaccines aren't studied—or monitored—thoroughly enough.
8. Vaccines aren’t studied—or monitored— thoroughly enough.
Patients would benefit from knowing that vaccines are some of the most thoroughly studied products brought to market. They undergo rigorous testing and oversight, from both public and private organizations, for 10 to 15 years before being released for distribution. Post-licensure monitoring is ongoing, and the manufacturer may voluntarily participate in Phase IV trials to continue to test the safety and efficacy of a vaccine after release to market.
Monitoring adverse effects. In addition, in 1990, the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration established the Vaccine Adverse Events Reporting System (VAERS) to “detect possible signals of adverse events associated with vaccines.”24 Most events reported are coincidental, but some common mild adverse events (like redness and swelling at the injection site) are often underreported.
Serious events are always thoroughly investigated and are often found unrelated. However, rare associations have been found. For example, an intestinal problem called intussusception, related to the original rotavirus vaccine, was discovered, and the vaccine causing it was removed from the market.25 A new, safer rotavirus vaccine option is now available. Patients need to know that we do have an effective system of checks and balances in which we can place our trust.
9. People can become paralyzed or stop breathing after receiving a vaccination. Why run those risks?
One of the most feared reactions to vaccination is Guillain-Barré syndrome (GBS), which can cause paralysis. The CDC estimates the risk for GBS associated with the flu vaccine, for example, to be 1 to 2 cases per 1 million people vaccinated.26 Another potential concern is the rate of anaphylaxis following vaccination. However, in a 2016 study in the Journal of Allergy and Clinical Immunology, the rate of anaphylaxis for all vaccines combined was only 1.31 per 1 million vaccines.27
The risk of developing severe complications from an illness is much greater than that of developing complications from the vaccine meant to protect a person against that illness. In the United States, the population-based risk for influenza-related hospitalization in children, for example, is as high as 150 in 100,000 with as many as 125 deaths annually.26
Continue to: 10. Isn't vaccination a personal choice? How does my health/illness impact the community?
10. Isn’t vaccination a personal choice? How does my health/illness impact the community?
Patients may not realize that most viruses are contagious from 1 to 2 days before symptoms appear, which means we can spread an illness before we even know we have it. Protecting oneself also protects those around us.
Economic concerns. There’s also the economic impact of these illnesses to consider. This includes the personal cost of being out of school or work for an extended period and the cost of a patient’s care, which can become astronomical if hospitalization is required and which can become the country’s problem if a person lacks sufficient health insurance coverage.
A study looking at the cost of 4 major adult vaccine-preventable illnesses (influenza, pneumococcal disease, shingles, and whooping cough) in the United States in 2013 estimated the annual cost for these illnesses in adults ≥50 years to be $26.5 billion.28 And that doesn’t include the cost of childhood vaccine-preventable diseases.
Countering 3 concerns about childhood vaccinations
1. I can’t afford vaccines for my child.
The Vaccines for Children program is a federally-funded program that covers the cost of all vaccines for children younger than 19 years of age who are Medicaid-eligible, American Indian, Alaskan Native, uninsured, or underinsured.29 Although there may be a small administration fee charged by the provider’s office, the vaccine is free.
2. Don’t all of the vaccines recommended for children overwhelm their immune systems?
Children are exposed to so many more proteins on a daily basis (by crawling around on the floor, putting their hands in their mouths, attending school or day care, etc) than they are ever exposed to in a series of vaccines.30 Exposure to these proteins in their environment and to those in vaccines only serves to boost their immunity and keep them healthier in the long run.
And thanks to advances in vaccine production, the immunologic load in vaccines is far less than it used to be. The 14 vaccines given today contain <200 bacterial and viral proteins or polysaccharides, compared with the >3000 of these immunologic components in the 7 vaccines administered in 1980.31
Continue to: Influenza vaccine: Patient-friendly talking points
SIDEBAR
Influenza vaccine: Patient-friendly talking points
- Some people think that getting the flu is no big deal. While it is true that the flu takes a greater toll on the very young and very old, the chronically ill, and the immune compromised, even healthy people can become seriously ill or die. The Centers for Disease Control and Prevention estimates that the flu is responsible for 140,000 to 720,000 hospitalizations and 12,000 to 56,000 deaths in the United States every year.7 Of those who die from the flu, approximately 80% did not receive a flu shot.36 Of children who died from the flu between 2004 and 2012, more than 40% had no risk factors for complications.37
- The flu shot is a killed virus vaccine, so it can't give you the flu. People sometimes feel under the weather (achy, low-grade fever) after a vaccine, but this is considered normal and evidence that your body's immune system is "revving up."
- It takes 2 weeks before the vaccine becomes effective so a person can still get the flu during that time. This is why it is so important to get the vaccine earlier in the fall, before the flu season takes hold.
- The "stomach flu" is not the flu. The flu vaccine does not protect against the "stomach flu" or other flu-like illnesses.
- The flu vaccine is not perfect. It is an educated guess as to which strains will be circulating that year. (At its best, the flu vaccine is about 60% effective.38) However, it makes the chance of getting the flu less likely and significantly decreases the odds of severe complications/death.
- Egg allergies are no longer a reason to avoid the flu vaccine. There is an egg-free vaccine called Flublok (for those ≥18 years of age). In 2016-17, the Advisory Committee on Immunization Practices changed the recommendations for flu vaccine in egg-allergic people. The recommendations say that if reactions are mild, or you can eat cooked eggs without a problem, you can receive a flu vaccine. If you have severe reactions, such as trouble breathing or recurrent vomiting, you can still receive the flu vaccine, but must be monitored by a health care provider who can recognize and respond to a severe allergic reaction.39
Continue to: 3. Why don't we adhere to Dr. Sears' vaccine schedule?
3. Why don’t we adhere to Dr. Sears’ vaccine schedule?
There are multiple ways in which Dr. Robert Sears’ book, The Vaccine Book: Making the Right Decision for your Child, published in 2007, misrepresents vaccine science and leads patients astray in making decisions regarding vaccinations.32 Most important to note is that Dr. Sears’ Alternative Vaccine Schedule, which seeks to make it so that children do not receive more than 2 vaccinations per office visit, would require visits to a health care provider at 2, 3, 4, 5, 6, 7, 9, 12, 15, 18, and 21 months, and at 2, 2.5, 3, 3.5, 4, 5, and 6 years of age. This significantly increases the number of office visits and needle sticks, and raises the age at which vaccines are given, increasing the risk of illness outbreaks and decreasing the likelihood that parents would return to the office to complete the full series.
Acceptance of influenza and HPV vaccines remains a challenge
We are significantly less successful at getting parents and patients to agree to influenza and HPV vaccines than to the other vaccines we offer. The influenza vaccine success rate in 2016 was 59% in children and 43.3% in adults.33 Compared to the Tdap vaccine (88%) and the meningococcal vaccine (82%), which are offered at the same age as the HPV vaccine, success rates for HPV vaccine are significantly lower. In 2016, only 60.4% of boys and girls were current on their first HPV injection and only 43.3% were up to date with the full series.34
Newness of vaccines a factor?
Perhaps it is because the recommendations for these 2 vaccines are relatively new, and people don’t yet grasp the seriousness and scope of the diseases. Until 2010, the flu shot was recommended only for the very young, the elderly, and the medically high risk.
Similarly, the HPV vaccine was originally introduced for girls in 2006 and wasn’t recommended for boys until 2011.
Continue to: Human papillomavirus vaccine: Patient-friendly talking points
SIDEBAR
Human papillomavirus vaccine: Patient-friendly talking points
- Human papillomavirus (HPV) causes genital warts and cancer of the cervix, vagina, vulva, anus, rectum, penis, and oropharynx.
- The HPV vaccine is a cancer prevention vaccine. The 9-valent vaccine is active against 2 genital wart-causing strains and 7 cancer-causing strains of HPV.
- HPV is highly prevalent; 79 million Americans are currently infected, nearly 14 million people become newly infected each year, and nearly all of us will be exposed at some point in our sexual lives.40
- There are often no outward signs of infection, so it is a difficult infection to avoid.
- It takes no high-risk sexual activity to be exposed to the HPV virus.
- The HPV vaccine is recommended for both boys and girls usually around age 11 to 12 years (but as early as 9 years and as late as 26 years is acceptable). If the first vaccine is administered before 15 years of age, only 2 injections are needed 6 to 12 months apart. If the first vaccine is administered after 15 years of age, 3 injections are needed at 0, 2 months, and 6 months.41
- Completing the series before sexual activity begins is the best way to protect our children because the vaccine is a preventive measure, not a treatment.
- The HPV vaccine is highly effective with >90% efficacy against high-risk cancer-causing strains.42
- The HPV vaccine offers long-term protection. The vaccine has been on the market since 2006, and immunity has not yet diminished. Further monitoring is ongoing.43
- The HPV vaccine is covered under the Vaccines For Children program until age 19 years. Then it is up to individual insurance plans to cover it.
- The HPV vaccine does not cause infertility.44 HPV infection, on the other hand, can lead to fertility problems if, for example, treatment for cervical precancer or cancer requires partial removal of the cervix or a hysterectomy.
- The HPV vaccine does not cause autoimmune diseases.45,46 Studies show no difference between vaccinated and unvaccinated groups in rates of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, type 1 diabetes mellitus, multiple sclerosis, Hashimoto's thyroiditis, Graves' disease, and others.
- The HPV vaccine does not encourage earlier sexual activity. There was no earlier incidence of outcomes related to sexual activity (pregnancy, sexually transmitted infection testing or diagnosis, or contraceptive counseling) in vaccinated vs unvaccinated patients studied.47
Continue to: A sensitive subject
A sensitive subject. Discussion of a vaccine related to a child’s sexual health makes some parents uncomfortable. Studies show that focusing on the cancer prevention aspects of the vaccine, rather than on sexual transmission of HPV, results in greater vaccine acceptance.35
However, if discussion of sexual transmission is unavoidable, remind parents to consider their own adolescence and whether they chose to share everything with their parents. Point out that there were probably things they did that they later looked back on and thought, “What was I thinking?” Their children, no matter how wonderful and levelheaded they are, will be no different. And, as much as parents don’t want to think about it, some kids will suffer unwanted sexual contact. Shouldn’t parents protect their children as best as they can?
A teen’s right to choose? Some states have passed a Mature Minor Doctrine, which provides for mature, unemancipated teens to make their own medical decisions regarding such issues as sexuality, mental health, and drug and alcohol use without their parents’ consent. In these states, teens may elect to receive the HPV vaccine without parental permission. (Check your state’s laws for specifics, and see the 2 boxes with patient-friendly talking points for influenza vaccine7,36-39 and human papillomavirus vaccine.40-47)
CORRESPONDENCE
Gretchen LaSalle, MD, MultiCare Rockwood Clinic, 2214 East 29th Avenue, Spokane, WA 99203; [email protected].
1. Paterson P, Meurice F, Stanberry LR, et al. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700-6706.
2. Opel DJ, Heritage J, Taylor J, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132:1037-1046.
3. Palmer J, Carrico C, Costanzo C. Identifying and overcoming perceived barriers of providers towards vaccination: a literature review. J Vaccines. 2015;1-7.
4. Autism Science Foundation. Making the CASE for vaccines: a new model for talking to patients about vaccines. Available at: http://autismsciencefoundation.org/wp-content/uploads/2015/12/Making-the-CASE-for-Vaccines-Guide_final.pdf. Accessed April 8, 2018.
5. Jacobson RM, Van Etta L, Bahta L. The C.A.S.E approach: guidance for talking to vaccine-hesitant patients. Minn Med. 2013;96:49-50.
6. Henrickson NB, Opel DJ, Grothaus L, et al. Physician communication training and parental vaccine hesitancy: a randomized trial. Pediatrics. 2015;136:70-79.
7. Centers for Disease Control and Prevention. Key facts about seasonal flu vaccine. Available at: https://www.cdc.gov/flu/protect/keyfacts.htm. Accessed April 8, 2018.
8. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641.
9. Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32:3623-3629.
10. Agency for Toxic Substances & Disease Registry. Minimal risk levels for hazardous substances. Available at: https://www.atsdr.cdc.gov/mrls/mrllist.asp#34tag. Accessed April 8, 2018.
11. US Food and Drug Administration. Thimerosal and vaccines. Available at: https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed April 8, 2018.
12. Hviid A, Stellfeld M, Wohlfahrt J, et al. Association between thimerosal-containing vaccine and autism. JAMA. 2003;290:1763-1766.
13. Centers for Disease Control and Prevention. Thimerosal in vaccines. Available at: https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed May 8, 2018.
14. Autism Speaks. Frequently asked questions. Available at: https://www.autismspeaks.org/what-autism/faq. Accessed April 8, 2018.
15. Agency for Toxic Substances & Disease Registry. Toxic substances portal-aluminum. Public Health Statement for Aluminum, CAS #7429-90-5. Available at: https://www.atsdr.cdc.gov/PHS/PHS.asp?id=1076&tid=34. Accessed April 8, 2018.
16. Children’s Hospital of Philadelphia. Vaccine ingredients-aluminum. Available at: www.chop.edu/centers-programs/vaccine-education-center/vaccine-ingredients/aluminum. Accessed April 8, 2018.
17. Orenstein W, Seib K. Mounting a good offense against measles. N Engl J Med. 2014;371:1661-1663.
18. Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med. 2012;55:72-77.
19. Hall V, Banerjee E, Kenyon C, et al. Measles outbreak – Minnesota April-May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:713-717.
20. The National Academies of Sciences Engineering Medicine. History of U.S. water and wastewater systems. Privatization of Water Services in the United States: an Assessment of Issues and Experience. Washington, DC: The National Academies Press; 2002:29-40. Available at: https://www.nap.edu/read/10135/chapter/4#35. Accessed May 7, 2018.
21. World Health Organization. Global vaccine safety. Six common misconceptions about immunization. Available at: http://www.who.int/vaccine_safety/initiative/detection/immunization_misconceptions/en/index1.html. Accessed May 7, 2018.
22. The history of vaccines. Human cell strains in vaccine development. Available at: https://www.historyofvaccines.org/content/articles/human-cell-strains-vaccine-development. Accessed April 8, 2018.
23. The National Catholic Bioethics Center. Frequently asked questions. Available at: https://www.ncbcenter.org/resources/frequently-asked-questions/use-vaccines/. Accessed April 8, 2018.
24. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015;33:4398-4405.
25. Foster S. Rotavirus vaccine and intussusception. J Pediatr Pharmacol Ther. 2007;12:4-7.
26. Mistry RD, Fischer JB, Prasad PA, et al. Severe complications of influenza-like illnesses. Pediatrics. 2014;134:e684-e690.
27. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
28. McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36:259-273.
29. Centers for Disease Control and Prevention. Vaccines for Children (VFC) Program. Available at: https://www.cdc.gov/features/vfcprogram/index.html. Accessed April 8, 2018.
30. Plotkin S, Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis. 2009;48:456-461.
31. Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics. 2002;109:124-129.
32. Offit PA, Moser CA. The problem with Dr. Bob’s alternative vaccine schedule. Pediatrics. 2009;123:e164-e169.
33. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. April 8. 2018.
34. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state and selected local area vaccination coverage among adolescents aged 13-17 years – United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
35. Thomas TL. Cancer prevention: HPV vaccination. Semin Oncol Nurs. 2016:32:273-280.
36. Centers for Disease Control and Prevention. Estimating seasonal influenza-associated deaths in the United States. Available at: https://www.cdc.gov/flu/about/disease/US_flu-related_deaths.htm. Accessed May 8, 2018.
37. Wong KK, Jain S, Blanton L, et al. Influenza-associated pediatric deaths in the United States: 2004-2012. Pediatrics. 2013;132:796-804.
38. Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed April 8, 2018.
39. Centers for Disease Control and Prevention. Influenza (flu). Flu vaccine and people with egg allergies. Available at: https://www.cdc.gov/flu/protect/vaccine/egg-allergies.htm. Accessed April 8, 2018.
40. Centers for Disease Control and Prevention. For parents: vaccines for your children. HPV vaccine for preteens and teens. Available at: https://www.cdc.gov/vaccines/parents/diseases/teen/hpv.html. Accessed April 8, 2018.
41. Centers for Disease Control and Prevention. Vaccines and preventable diseases. HPV vaccine recommendations. Available at: https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed May 7, 2018.
42. Cutts FT, Franceschi S, Goldie S, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ. 2007;85:719-726.
43. De Vincenzo R, Conte C, Ricci C, et al. Long-term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999-1010.
44. McInerney KA, Hatch EE, Wesselink AK. The effect of vaccination against human papillomavirus on fecundability. Paedeatr Perinat Epidemiol. 2017;31:531-536.
45. Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193-203.
46. Vichnin M, Bonanni P, Klein NP, et al. An overview of quadrivalent human papillomavirus vaccine safety: 2006-2015. Ped Infect Dis J. 2015;34:983-991.
47. Bednarczyk RA, Davis R, Ault K, et al. Sexual activity-related outcomes after human papillomavirus vaccination of 11-to-12-year-olds. Pediatrics. 2012;130:798-805.
We all know how challenging and time-consuming it can be to convince vaccine-hesitant patients that vaccinations are what is best for them and their children. Patients are bombarded with misinformation through the news and social media that seeds or “confirms” their doubts about vaccines. And for our part, we have only a few minutes during an office visit to refute all of the false claims that are a mere click or scroll away.
To better prepare for this challenge, this article details a practical approach to discussing vaccines with your patients. Using the patient-friendly language and evidence described here, you will be well positioned to refute 13 common vaccine misconceptions and overcome the barriers that stand in the way of these lifesaving interventions.
A few important baseline concepts
In discussing vaccination with our patients, it is important to keep the following in mind:
Patients don’t refuse vaccinations just to make our lives difficult. They truly are trying to make the best decisions they can for themselves and their families. Recognizing this can significantly reduce frustration levels.
Time well spent. While educating patients about the value of vaccines takes time, the return is worth it. The more consistently we offer vaccines, along with the reasons they are important, the more likely patients are to give vaccines a second thought. In fact, studies show that provider recommendation is the most important factor in patients’ decisions to vaccinate.1
Approach matters. In all other aspects of medicine, we attempt to use a participatory approach, involving our patients in decisions regarding their health care. When discussing vaccines, however, a participatory approach (eg, “What do you want to do about vaccines today?”) can introduce doubt into patients’ minds. Studies show that a presumptive approach (eg, “Today we are going to provide the tetanus, human papillomavirus [HPV], and meningitis vaccines”) is a much more effective way to get patients to vaccinate.2
[polldaddy:10018427]
Continue to: Barriers to counseling
Barriers to counseling. Health care providers report a variety of barriers to effective vaccine counseling (limited time and resources, lack of confidence in addressing patients’ concerns, etc).3 In addition, providers sometimes worry that strong encouragement of vaccination will create an adversarial relationship with vaccine-hesitant patients. Developing a good rapport and trusting relationship, as well as using motivational interviewing approaches, can help communicate the importance of vaccines, while leaving patients with the sense that you have heard them and respect their intentions. (See “Facilitate vaccine discussions using these 2 approaches.” 4-7)
SIDEBAR
Facilitate vaccine discussions using these 2 approaches4-7
C.A.S.E.
Corroborate
Acknowledge concerns and find some point on which you can agree.
Example: "It sounds like we both want to keep your child healthy and safe."
About me
Describe what you have done to build your expertise on the subject.
Example: "I have been practicing medicine for 15 years and have spent a great deal of time researching the data on vaccinations."
Science
Review the data and science behind vaccines.
Example: "Vaccines are more rigorously studied and safer than almost any other intervention we have in medicine."
Explain/advise
Explain your recommendations, based on the science.
Example: "This is why I vaccinate my children, and this is why I recommend this vaccine for your child."
3As
Ask
Don't stop at a patient's first "No." Respectfully dig a bit deeper.
Example: "What questions do you have about the vaccines we are recommending today? Tell me what worries you about them."
Acknowledge
Acknowledge your patient's concerns.
Example: "You are obviously a very devoted parent, and I know that you are trying to make the best decision you can for your child. With everything we see on the news and social media, it's not always easy to know what to believe about vaccines."
Advise
Advise patients/parents of the facts about vaccines and provide a strong recommendation to vaccinate.
Example: "Depending on the year, influenza kills 12,000 to 56,000 people annually; the vast majority of those who die did not receive the flu vaccine.7 My family and I get the flu shot every year, and I strongly encourage you and your children to get this lifesaving vaccine."
Continue to: If at first you don't succeed...
If at first you don’t succeed, try again because patients often have an experience that changes their mind. Perhaps a friend died of throat cancer or a family member developed a complication of the flu that required hospitalization. You never know when something will influence patients’ choices.
Don’t wait for scheduled well visits. Use every patient encounter as a means to catch patients up on missing vaccinations.
Common misconceptions and concerns and how to counter them
1. I’ve heard that vaccines can actually make you sick.
When patients raise this concern, start with an explanation of how vaccines work. Explain that our bodies protect us from foreign invaders (such as viruses and bacteria) by mounting an immune response when we are exposed to these proteins. Vaccinations work by exploiting this immune response; they expose the body to killed or weakened viral or bacterial proteins in a safe and controlled manner. In this way, our immune system will have already developed antibodies to these invaders by the time we are exposed to an active infection.
To use an analogy to war, instead of being subjected to a surprise attack where we suffer large losses in the battle, vaccination prepares us with weapons (antibodies) to defend ourselves so that our bodies are now able to successfully fight off that attack.
Because the majority of vaccines are killed virus vaccines, they cannot cause the illness against which they are meant to protect. Triggering the immune system may make some recipients feel a little “under the weather” for a day or 2, but they do not make us “sick.”
Live attenuated vaccines are similarly safe for those with a healthy immune system. We don’t administer them, however, to people who have a weakened immune system (eg, pregnant women, newborns, people with acquired immunodeficiency virus, or patients receiving chemotherapy or other types of immunosuppression) because these patients could develop the illness that we are trying to protect against.
Continue to: 2. Don't vaccines cause autism? Aren't they toxic to the nervous system?
2. Don’t vaccines cause autism? Aren’t they toxic to the nervous system?
The largest setback to vaccination efforts in recent history was a 1998 study by Andrew Wakefield that suggested that vaccination (specifically the mercury [in the form of thimerosal] present in the measles, mumps, rubella [MMR] vaccine) was linked to the development of autism.8 This research was subsequently debunked,9 and the author of the 1998 study was stripped of his medical license for falsifying data. However, the damage to vaccination efforts had already been done.
Aluminum. Thimerosal is not the only agent that patients may find concerning. Some also worry about the aluminum content of vaccines. Aluminum works as an additive to boost the body’s immune response to a vaccine. It is used only in killed virus vaccines—not in live attenuated ones. The Agency for Toxic Substances and Disease Registry monitors minimum risk levels (MRLs) of aluminum and other compounds in potentially hazardous substances. The amount of aluminum in vaccines is far below the MRL for aluminum, which is 1 mg/kg/d.10 (See “The facts about thimerosal and aluminum in vaccines.”11-16)
SIDEBAR
The facts about thimerosal and aluminum in vaccines
Thimerosal
Ethyl-mercury was used (in the form of thimerosal) as a preservative to prevent bacterial and fungal contamination of vaccines. Since 2001, however, thimerosal has been removed from all US-licensed vaccines—except multidose vials of influenza vaccine—as a precautionary measure (and not for any reproducible evidence of harm). The multidose flu vial contains <0.01% thimerosal.11
Ethyl-mercury is cleared from the body much more rapidly than methyl-mercury (the kind found in certain types of fish) and is less toxic.12
Since the removal of thimerosal from vaccines, the Centers for Disease Control and Prevention notes that the rates of autism have actually increased.13
Even Autism Speaks, the leading organization dedicated to advocacy for patients with autism and their families, denies a link between vaccines and autism.14
Aluminum
We are exposed to aluminum in products we use extensively every day, such as pots and pans, aluminum foil, seasonings, cereal, baby formula, paints, fuels, and antiperspirants.15
Infants are exposed to about 4.4 mg of aluminum in the vaccines typically administered in the first 6 months of life.16 However, infants typically ingest more than that during the first 6 months of life. Breast milk contains about 7 mg over 6 months; milk-based formulas contain about 38 mg over 6 months; and soy-based formulas contain about 117 mg over 6 months.16
Contine to: 3. I'm healthy. I never get sick. Why do I need vaccinations?
3. I’m healthy. I never get sick. Why do I need vaccinations?
A good way to counter this comment is to respond: “Saying you don’t need vaccinations because you never get sick is like saying you don’t need to wear a seat belt because you’ve never been in a car accident.” Advise patients that we seek to vaccinate all members of a community—not just those who are sick or at high risk—to protect ourselves and to provide “herd immunity.” It’s important to explain that herd immunity is resistance to the spread of a contagious disease that results if a sufficiently high number of people (depending on the illness, typically 80%-95%) are immune to the disease, especially through vaccination.17,18 If vaccination levels fall, we see a rise in cases of vaccine-preventable illness (as was seen during the 2017 measles outbreak in a community in Minnesota).19
Even though many of us may not suffer severe consequences of an infection, we can still pass that infection to others. While the whooping cough that a healthy 35-year-old gets may cause only prolonged annoyance or time off from work, it can kill the baby that is sitting next to that adult on the plane or bus.
4. Isn’t it true that we see fewer serious illnesses because of improved hygiene and sanitation, rather than vaccines?
Our current US sanitation standards were established under the Safe Drinking Water Act of 1974.20 While improvements in hygiene, sanitation, nutrition, and other public health measures have undoubtedly decreased the spread of disease and improved survival rates, there is no denying the significant drop in disease that occurs after the introduction of a vaccine for a particular illness or the increase in cases of that disease when vaccination rates drop off.
By the early 1990s, our current sanitation standards were already well established. Yet we didn’t see a significant decrease in the incidence of infections with Haemophilus influenzae type b (Hib) until after the conjugate Hib vaccines were introduced (dropping from about 20,000 cases/year to 1419 cases/year by 1993).21
In Britain, a drop in the rate of pertussis (whooping cough) vaccination in 1974 resulted in an epidemic of more than 100,000 cases and 36 deaths by 1978. There was no decrease in hygiene or sanitation standards to explain this rise.21
Continue to: 5. Vaccines are just another way for "big pharma" to make "big money."
5. Vaccines are just another way for “big pharma” to make “big money.”
Patients may benefit from knowing that in the earlier days of vaccines, pharmaceutical companies actually moved away from production of vaccines because they were not very profitable. These days, with worldwide distribution, drug companies are back in the swing of making vaccines and, as we would expect from all companies, are in business to make a profit.
That said, health care providers receive no payments from drug companies for offering vaccines or for offering one vaccine over another. The reason we recommend vaccination is because we know it is best for our patients’ health and the health of the community.

6. We don’t see polio anymore. Why do I need the vaccine?
One of the factors contributing to the rise in antivaccine sentiment is that we rarely see vaccine-preventable illnesses (such as polio, measles, and mumps). But the absence of these illnesses is precisely due to prior years’ vaccination efforts.
Smallpox, a deadly and disfiguring disease that killed many millions of people and contributed to the downfall of the Roman, Aztec, and Incan empires, was eradicated from the planet in 1979, thanks to focused vaccination efforts by the World Health Organization. Vaccination works, but we have to keep at it.
While we no longer see as many of these vaccine-preventable illnesses in the United States, they are still present in other parts of the world. Our world is much smaller than it used to be. International travel is common, and illnesses can be reintroduced into a community with relative ease. We must remain vigilant.
Continue to: 7. I heard that vaccines are made from aborted fetal tissue.
7. I heard that vaccines are made from aborted fetal tissue.
There are 5 vaccines (varicella, rubella, hepatitis A, shingles, and rabies vaccines) that were originally made using aborted fetal tissue. In 1960, tissue from 2 fetuses aborted by maternal choice (and not for the purpose of vaccine production) was used to propagate cell lines that are still used in vaccine development today.
Human cells provide advantages for vaccine production that other cells do not. Some viruses do not grow well in animal cells. Animal cells can introduce contamination by bacteria and viruses that are not carried in human cell lines. Vaccine production can be hindered or halted, resulting in a vaccine shortage, if animal products used in development are threatened (eg, if an illness strikes egg-producing chickens; eggs are used to make the influenza vaccine).22
Some patients, particularly those who are Catholic, may have concerns about these vaccines. The National Catholic Bioethics Center has prepared a statement regarding the use of these vaccines that may help settle any moral dilemmas.23 It reads:
“The cell lines under consideration were begun using cells taken from one or more fetuses aborted almost 40 years ago. Since that time, the cell lines have grown independently. It is important to note that descendent cells are not the cells of the aborted child.”
“One is morally free to use the vaccine regardless of its historical association with abortion. The reason is that the risk to public health, if one chooses not to vaccinate, outweighs the legitimate concern about the origins of the vaccine. This is especially important for parents, who have a moral obligation to protect the life and health of their children and those around them.”
Continue to: 8. Vaccines aren't studied—or monitored—thoroughly enough.
8. Vaccines aren’t studied—or monitored— thoroughly enough.
Patients would benefit from knowing that vaccines are some of the most thoroughly studied products brought to market. They undergo rigorous testing and oversight, from both public and private organizations, for 10 to 15 years before being released for distribution. Post-licensure monitoring is ongoing, and the manufacturer may voluntarily participate in Phase IV trials to continue to test the safety and efficacy of a vaccine after release to market.
Monitoring adverse effects. In addition, in 1990, the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration established the Vaccine Adverse Events Reporting System (VAERS) to “detect possible signals of adverse events associated with vaccines.”24 Most events reported are coincidental, but some common mild adverse events (like redness and swelling at the injection site) are often underreported.
Serious events are always thoroughly investigated and are often found unrelated. However, rare associations have been found. For example, an intestinal problem called intussusception, related to the original rotavirus vaccine, was discovered, and the vaccine causing it was removed from the market.25 A new, safer rotavirus vaccine option is now available. Patients need to know that we do have an effective system of checks and balances in which we can place our trust.
9. People can become paralyzed or stop breathing after receiving a vaccination. Why run those risks?
One of the most feared reactions to vaccination is Guillain-Barré syndrome (GBS), which can cause paralysis. The CDC estimates the risk for GBS associated with the flu vaccine, for example, to be 1 to 2 cases per 1 million people vaccinated.26 Another potential concern is the rate of anaphylaxis following vaccination. However, in a 2016 study in the Journal of Allergy and Clinical Immunology, the rate of anaphylaxis for all vaccines combined was only 1.31 per 1 million vaccines.27
The risk of developing severe complications from an illness is much greater than that of developing complications from the vaccine meant to protect a person against that illness. In the United States, the population-based risk for influenza-related hospitalization in children, for example, is as high as 150 in 100,000 with as many as 125 deaths annually.26
Continue to: 10. Isn't vaccination a personal choice? How does my health/illness impact the community?
10. Isn’t vaccination a personal choice? How does my health/illness impact the community?
Patients may not realize that most viruses are contagious from 1 to 2 days before symptoms appear, which means we can spread an illness before we even know we have it. Protecting oneself also protects those around us.
Economic concerns. There’s also the economic impact of these illnesses to consider. This includes the personal cost of being out of school or work for an extended period and the cost of a patient’s care, which can become astronomical if hospitalization is required and which can become the country’s problem if a person lacks sufficient health insurance coverage.
A study looking at the cost of 4 major adult vaccine-preventable illnesses (influenza, pneumococcal disease, shingles, and whooping cough) in the United States in 2013 estimated the annual cost for these illnesses in adults ≥50 years to be $26.5 billion.28 And that doesn’t include the cost of childhood vaccine-preventable diseases.
Countering 3 concerns about childhood vaccinations
1. I can’t afford vaccines for my child.
The Vaccines for Children program is a federally-funded program that covers the cost of all vaccines for children younger than 19 years of age who are Medicaid-eligible, American Indian, Alaskan Native, uninsured, or underinsured.29 Although there may be a small administration fee charged by the provider’s office, the vaccine is free.
2. Don’t all of the vaccines recommended for children overwhelm their immune systems?
Children are exposed to so many more proteins on a daily basis (by crawling around on the floor, putting their hands in their mouths, attending school or day care, etc) than they are ever exposed to in a series of vaccines.30 Exposure to these proteins in their environment and to those in vaccines only serves to boost their immunity and keep them healthier in the long run.
And thanks to advances in vaccine production, the immunologic load in vaccines is far less than it used to be. The 14 vaccines given today contain <200 bacterial and viral proteins or polysaccharides, compared with the >3000 of these immunologic components in the 7 vaccines administered in 1980.31
Continue to: Influenza vaccine: Patient-friendly talking points
SIDEBAR
Influenza vaccine: Patient-friendly talking points
- Some people think that getting the flu is no big deal. While it is true that the flu takes a greater toll on the very young and very old, the chronically ill, and the immune compromised, even healthy people can become seriously ill or die. The Centers for Disease Control and Prevention estimates that the flu is responsible for 140,000 to 720,000 hospitalizations and 12,000 to 56,000 deaths in the United States every year.7 Of those who die from the flu, approximately 80% did not receive a flu shot.36 Of children who died from the flu between 2004 and 2012, more than 40% had no risk factors for complications.37
- The flu shot is a killed virus vaccine, so it can't give you the flu. People sometimes feel under the weather (achy, low-grade fever) after a vaccine, but this is considered normal and evidence that your body's immune system is "revving up."
- It takes 2 weeks before the vaccine becomes effective so a person can still get the flu during that time. This is why it is so important to get the vaccine earlier in the fall, before the flu season takes hold.
- The "stomach flu" is not the flu. The flu vaccine does not protect against the "stomach flu" or other flu-like illnesses.
- The flu vaccine is not perfect. It is an educated guess as to which strains will be circulating that year. (At its best, the flu vaccine is about 60% effective.38) However, it makes the chance of getting the flu less likely and significantly decreases the odds of severe complications/death.
- Egg allergies are no longer a reason to avoid the flu vaccine. There is an egg-free vaccine called Flublok (for those ≥18 years of age). In 2016-17, the Advisory Committee on Immunization Practices changed the recommendations for flu vaccine in egg-allergic people. The recommendations say that if reactions are mild, or you can eat cooked eggs without a problem, you can receive a flu vaccine. If you have severe reactions, such as trouble breathing or recurrent vomiting, you can still receive the flu vaccine, but must be monitored by a health care provider who can recognize and respond to a severe allergic reaction.39
Continue to: 3. Why don't we adhere to Dr. Sears' vaccine schedule?
3. Why don’t we adhere to Dr. Sears’ vaccine schedule?
There are multiple ways in which Dr. Robert Sears’ book, The Vaccine Book: Making the Right Decision for your Child, published in 2007, misrepresents vaccine science and leads patients astray in making decisions regarding vaccinations.32 Most important to note is that Dr. Sears’ Alternative Vaccine Schedule, which seeks to make it so that children do not receive more than 2 vaccinations per office visit, would require visits to a health care provider at 2, 3, 4, 5, 6, 7, 9, 12, 15, 18, and 21 months, and at 2, 2.5, 3, 3.5, 4, 5, and 6 years of age. This significantly increases the number of office visits and needle sticks, and raises the age at which vaccines are given, increasing the risk of illness outbreaks and decreasing the likelihood that parents would return to the office to complete the full series.
Acceptance of influenza and HPV vaccines remains a challenge
We are significantly less successful at getting parents and patients to agree to influenza and HPV vaccines than to the other vaccines we offer. The influenza vaccine success rate in 2016 was 59% in children and 43.3% in adults.33 Compared to the Tdap vaccine (88%) and the meningococcal vaccine (82%), which are offered at the same age as the HPV vaccine, success rates for HPV vaccine are significantly lower. In 2016, only 60.4% of boys and girls were current on their first HPV injection and only 43.3% were up to date with the full series.34
Newness of vaccines a factor?
Perhaps it is because the recommendations for these 2 vaccines are relatively new, and people don’t yet grasp the seriousness and scope of the diseases. Until 2010, the flu shot was recommended only for the very young, the elderly, and the medically high risk.
Similarly, the HPV vaccine was originally introduced for girls in 2006 and wasn’t recommended for boys until 2011.
Continue to: Human papillomavirus vaccine: Patient-friendly talking points
SIDEBAR
Human papillomavirus vaccine: Patient-friendly talking points
- Human papillomavirus (HPV) causes genital warts and cancer of the cervix, vagina, vulva, anus, rectum, penis, and oropharynx.
- The HPV vaccine is a cancer prevention vaccine. The 9-valent vaccine is active against 2 genital wart-causing strains and 7 cancer-causing strains of HPV.
- HPV is highly prevalent; 79 million Americans are currently infected, nearly 14 million people become newly infected each year, and nearly all of us will be exposed at some point in our sexual lives.40
- There are often no outward signs of infection, so it is a difficult infection to avoid.
- It takes no high-risk sexual activity to be exposed to the HPV virus.
- The HPV vaccine is recommended for both boys and girls usually around age 11 to 12 years (but as early as 9 years and as late as 26 years is acceptable). If the first vaccine is administered before 15 years of age, only 2 injections are needed 6 to 12 months apart. If the first vaccine is administered after 15 years of age, 3 injections are needed at 0, 2 months, and 6 months.41
- Completing the series before sexual activity begins is the best way to protect our children because the vaccine is a preventive measure, not a treatment.
- The HPV vaccine is highly effective with >90% efficacy against high-risk cancer-causing strains.42
- The HPV vaccine offers long-term protection. The vaccine has been on the market since 2006, and immunity has not yet diminished. Further monitoring is ongoing.43
- The HPV vaccine is covered under the Vaccines For Children program until age 19 years. Then it is up to individual insurance plans to cover it.
- The HPV vaccine does not cause infertility.44 HPV infection, on the other hand, can lead to fertility problems if, for example, treatment for cervical precancer or cancer requires partial removal of the cervix or a hysterectomy.
- The HPV vaccine does not cause autoimmune diseases.45,46 Studies show no difference between vaccinated and unvaccinated groups in rates of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, type 1 diabetes mellitus, multiple sclerosis, Hashimoto's thyroiditis, Graves' disease, and others.
- The HPV vaccine does not encourage earlier sexual activity. There was no earlier incidence of outcomes related to sexual activity (pregnancy, sexually transmitted infection testing or diagnosis, or contraceptive counseling) in vaccinated vs unvaccinated patients studied.47
Continue to: A sensitive subject
A sensitive subject. Discussion of a vaccine related to a child’s sexual health makes some parents uncomfortable. Studies show that focusing on the cancer prevention aspects of the vaccine, rather than on sexual transmission of HPV, results in greater vaccine acceptance.35
However, if discussion of sexual transmission is unavoidable, remind parents to consider their own adolescence and whether they chose to share everything with their parents. Point out that there were probably things they did that they later looked back on and thought, “What was I thinking?” Their children, no matter how wonderful and levelheaded they are, will be no different. And, as much as parents don’t want to think about it, some kids will suffer unwanted sexual contact. Shouldn’t parents protect their children as best as they can?
A teen’s right to choose? Some states have passed a Mature Minor Doctrine, which provides for mature, unemancipated teens to make their own medical decisions regarding such issues as sexuality, mental health, and drug and alcohol use without their parents’ consent. In these states, teens may elect to receive the HPV vaccine without parental permission. (Check your state’s laws for specifics, and see the 2 boxes with patient-friendly talking points for influenza vaccine7,36-39 and human papillomavirus vaccine.40-47)
CORRESPONDENCE
Gretchen LaSalle, MD, MultiCare Rockwood Clinic, 2214 East 29th Avenue, Spokane, WA 99203; [email protected].
We all know how challenging and time-consuming it can be to convince vaccine-hesitant patients that vaccinations are what is best for them and their children. Patients are bombarded with misinformation through the news and social media that seeds or “confirms” their doubts about vaccines. And for our part, we have only a few minutes during an office visit to refute all of the false claims that are a mere click or scroll away.
To better prepare for this challenge, this article details a practical approach to discussing vaccines with your patients. Using the patient-friendly language and evidence described here, you will be well positioned to refute 13 common vaccine misconceptions and overcome the barriers that stand in the way of these lifesaving interventions.
A few important baseline concepts
In discussing vaccination with our patients, it is important to keep the following in mind:
Patients don’t refuse vaccinations just to make our lives difficult. They truly are trying to make the best decisions they can for themselves and their families. Recognizing this can significantly reduce frustration levels.
Time well spent. While educating patients about the value of vaccines takes time, the return is worth it. The more consistently we offer vaccines, along with the reasons they are important, the more likely patients are to give vaccines a second thought. In fact, studies show that provider recommendation is the most important factor in patients’ decisions to vaccinate.1
Approach matters. In all other aspects of medicine, we attempt to use a participatory approach, involving our patients in decisions regarding their health care. When discussing vaccines, however, a participatory approach (eg, “What do you want to do about vaccines today?”) can introduce doubt into patients’ minds. Studies show that a presumptive approach (eg, “Today we are going to provide the tetanus, human papillomavirus [HPV], and meningitis vaccines”) is a much more effective way to get patients to vaccinate.2
[polldaddy:10018427]
Continue to: Barriers to counseling
Barriers to counseling. Health care providers report a variety of barriers to effective vaccine counseling (limited time and resources, lack of confidence in addressing patients’ concerns, etc).3 In addition, providers sometimes worry that strong encouragement of vaccination will create an adversarial relationship with vaccine-hesitant patients. Developing a good rapport and trusting relationship, as well as using motivational interviewing approaches, can help communicate the importance of vaccines, while leaving patients with the sense that you have heard them and respect their intentions. (See “Facilitate vaccine discussions using these 2 approaches.” 4-7)
SIDEBAR
Facilitate vaccine discussions using these 2 approaches4-7
C.A.S.E.
Corroborate
Acknowledge concerns and find some point on which you can agree.
Example: "It sounds like we both want to keep your child healthy and safe."
About me
Describe what you have done to build your expertise on the subject.
Example: "I have been practicing medicine for 15 years and have spent a great deal of time researching the data on vaccinations."
Science
Review the data and science behind vaccines.
Example: "Vaccines are more rigorously studied and safer than almost any other intervention we have in medicine."
Explain/advise
Explain your recommendations, based on the science.
Example: "This is why I vaccinate my children, and this is why I recommend this vaccine for your child."
3As
Ask
Don't stop at a patient's first "No." Respectfully dig a bit deeper.
Example: "What questions do you have about the vaccines we are recommending today? Tell me what worries you about them."
Acknowledge
Acknowledge your patient's concerns.
Example: "You are obviously a very devoted parent, and I know that you are trying to make the best decision you can for your child. With everything we see on the news and social media, it's not always easy to know what to believe about vaccines."
Advise
Advise patients/parents of the facts about vaccines and provide a strong recommendation to vaccinate.
Example: "Depending on the year, influenza kills 12,000 to 56,000 people annually; the vast majority of those who die did not receive the flu vaccine.7 My family and I get the flu shot every year, and I strongly encourage you and your children to get this lifesaving vaccine."
Continue to: If at first you don't succeed...
If at first you don’t succeed, try again because patients often have an experience that changes their mind. Perhaps a friend died of throat cancer or a family member developed a complication of the flu that required hospitalization. You never know when something will influence patients’ choices.
Don’t wait for scheduled well visits. Use every patient encounter as a means to catch patients up on missing vaccinations.
Common misconceptions and concerns and how to counter them
1. I’ve heard that vaccines can actually make you sick.
When patients raise this concern, start with an explanation of how vaccines work. Explain that our bodies protect us from foreign invaders (such as viruses and bacteria) by mounting an immune response when we are exposed to these proteins. Vaccinations work by exploiting this immune response; they expose the body to killed or weakened viral or bacterial proteins in a safe and controlled manner. In this way, our immune system will have already developed antibodies to these invaders by the time we are exposed to an active infection.
To use an analogy to war, instead of being subjected to a surprise attack where we suffer large losses in the battle, vaccination prepares us with weapons (antibodies) to defend ourselves so that our bodies are now able to successfully fight off that attack.
Because the majority of vaccines are killed virus vaccines, they cannot cause the illness against which they are meant to protect. Triggering the immune system may make some recipients feel a little “under the weather” for a day or 2, but they do not make us “sick.”
Live attenuated vaccines are similarly safe for those with a healthy immune system. We don’t administer them, however, to people who have a weakened immune system (eg, pregnant women, newborns, people with acquired immunodeficiency virus, or patients receiving chemotherapy or other types of immunosuppression) because these patients could develop the illness that we are trying to protect against.
Continue to: 2. Don't vaccines cause autism? Aren't they toxic to the nervous system?
2. Don’t vaccines cause autism? Aren’t they toxic to the nervous system?
The largest setback to vaccination efforts in recent history was a 1998 study by Andrew Wakefield that suggested that vaccination (specifically the mercury [in the form of thimerosal] present in the measles, mumps, rubella [MMR] vaccine) was linked to the development of autism.8 This research was subsequently debunked,9 and the author of the 1998 study was stripped of his medical license for falsifying data. However, the damage to vaccination efforts had already been done.
Aluminum. Thimerosal is not the only agent that patients may find concerning. Some also worry about the aluminum content of vaccines. Aluminum works as an additive to boost the body’s immune response to a vaccine. It is used only in killed virus vaccines—not in live attenuated ones. The Agency for Toxic Substances and Disease Registry monitors minimum risk levels (MRLs) of aluminum and other compounds in potentially hazardous substances. The amount of aluminum in vaccines is far below the MRL for aluminum, which is 1 mg/kg/d.10 (See “The facts about thimerosal and aluminum in vaccines.”11-16)
SIDEBAR
The facts about thimerosal and aluminum in vaccines
Thimerosal
Ethyl-mercury was used (in the form of thimerosal) as a preservative to prevent bacterial and fungal contamination of vaccines. Since 2001, however, thimerosal has been removed from all US-licensed vaccines—except multidose vials of influenza vaccine—as a precautionary measure (and not for any reproducible evidence of harm). The multidose flu vial contains <0.01% thimerosal.11
Ethyl-mercury is cleared from the body much more rapidly than methyl-mercury (the kind found in certain types of fish) and is less toxic.12
Since the removal of thimerosal from vaccines, the Centers for Disease Control and Prevention notes that the rates of autism have actually increased.13
Even Autism Speaks, the leading organization dedicated to advocacy for patients with autism and their families, denies a link between vaccines and autism.14
Aluminum
We are exposed to aluminum in products we use extensively every day, such as pots and pans, aluminum foil, seasonings, cereal, baby formula, paints, fuels, and antiperspirants.15
Infants are exposed to about 4.4 mg of aluminum in the vaccines typically administered in the first 6 months of life.16 However, infants typically ingest more than that during the first 6 months of life. Breast milk contains about 7 mg over 6 months; milk-based formulas contain about 38 mg over 6 months; and soy-based formulas contain about 117 mg over 6 months.16
Contine to: 3. I'm healthy. I never get sick. Why do I need vaccinations?
3. I’m healthy. I never get sick. Why do I need vaccinations?
A good way to counter this comment is to respond: “Saying you don’t need vaccinations because you never get sick is like saying you don’t need to wear a seat belt because you’ve never been in a car accident.” Advise patients that we seek to vaccinate all members of a community—not just those who are sick or at high risk—to protect ourselves and to provide “herd immunity.” It’s important to explain that herd immunity is resistance to the spread of a contagious disease that results if a sufficiently high number of people (depending on the illness, typically 80%-95%) are immune to the disease, especially through vaccination.17,18 If vaccination levels fall, we see a rise in cases of vaccine-preventable illness (as was seen during the 2017 measles outbreak in a community in Minnesota).19
Even though many of us may not suffer severe consequences of an infection, we can still pass that infection to others. While the whooping cough that a healthy 35-year-old gets may cause only prolonged annoyance or time off from work, it can kill the baby that is sitting next to that adult on the plane or bus.
4. Isn’t it true that we see fewer serious illnesses because of improved hygiene and sanitation, rather than vaccines?
Our current US sanitation standards were established under the Safe Drinking Water Act of 1974.20 While improvements in hygiene, sanitation, nutrition, and other public health measures have undoubtedly decreased the spread of disease and improved survival rates, there is no denying the significant drop in disease that occurs after the introduction of a vaccine for a particular illness or the increase in cases of that disease when vaccination rates drop off.
By the early 1990s, our current sanitation standards were already well established. Yet we didn’t see a significant decrease in the incidence of infections with Haemophilus influenzae type b (Hib) until after the conjugate Hib vaccines were introduced (dropping from about 20,000 cases/year to 1419 cases/year by 1993).21
In Britain, a drop in the rate of pertussis (whooping cough) vaccination in 1974 resulted in an epidemic of more than 100,000 cases and 36 deaths by 1978. There was no decrease in hygiene or sanitation standards to explain this rise.21
Continue to: 5. Vaccines are just another way for "big pharma" to make "big money."
5. Vaccines are just another way for “big pharma” to make “big money.”
Patients may benefit from knowing that in the earlier days of vaccines, pharmaceutical companies actually moved away from production of vaccines because they were not very profitable. These days, with worldwide distribution, drug companies are back in the swing of making vaccines and, as we would expect from all companies, are in business to make a profit.
That said, health care providers receive no payments from drug companies for offering vaccines or for offering one vaccine over another. The reason we recommend vaccination is because we know it is best for our patients’ health and the health of the community.

6. We don’t see polio anymore. Why do I need the vaccine?
One of the factors contributing to the rise in antivaccine sentiment is that we rarely see vaccine-preventable illnesses (such as polio, measles, and mumps). But the absence of these illnesses is precisely due to prior years’ vaccination efforts.
Smallpox, a deadly and disfiguring disease that killed many millions of people and contributed to the downfall of the Roman, Aztec, and Incan empires, was eradicated from the planet in 1979, thanks to focused vaccination efforts by the World Health Organization. Vaccination works, but we have to keep at it.
While we no longer see as many of these vaccine-preventable illnesses in the United States, they are still present in other parts of the world. Our world is much smaller than it used to be. International travel is common, and illnesses can be reintroduced into a community with relative ease. We must remain vigilant.
Continue to: 7. I heard that vaccines are made from aborted fetal tissue.
7. I heard that vaccines are made from aborted fetal tissue.
There are 5 vaccines (varicella, rubella, hepatitis A, shingles, and rabies vaccines) that were originally made using aborted fetal tissue. In 1960, tissue from 2 fetuses aborted by maternal choice (and not for the purpose of vaccine production) was used to propagate cell lines that are still used in vaccine development today.
Human cells provide advantages for vaccine production that other cells do not. Some viruses do not grow well in animal cells. Animal cells can introduce contamination by bacteria and viruses that are not carried in human cell lines. Vaccine production can be hindered or halted, resulting in a vaccine shortage, if animal products used in development are threatened (eg, if an illness strikes egg-producing chickens; eggs are used to make the influenza vaccine).22
Some patients, particularly those who are Catholic, may have concerns about these vaccines. The National Catholic Bioethics Center has prepared a statement regarding the use of these vaccines that may help settle any moral dilemmas.23 It reads:
“The cell lines under consideration were begun using cells taken from one or more fetuses aborted almost 40 years ago. Since that time, the cell lines have grown independently. It is important to note that descendent cells are not the cells of the aborted child.”
“One is morally free to use the vaccine regardless of its historical association with abortion. The reason is that the risk to public health, if one chooses not to vaccinate, outweighs the legitimate concern about the origins of the vaccine. This is especially important for parents, who have a moral obligation to protect the life and health of their children and those around them.”
Continue to: 8. Vaccines aren't studied—or monitored—thoroughly enough.
8. Vaccines aren’t studied—or monitored— thoroughly enough.
Patients would benefit from knowing that vaccines are some of the most thoroughly studied products brought to market. They undergo rigorous testing and oversight, from both public and private organizations, for 10 to 15 years before being released for distribution. Post-licensure monitoring is ongoing, and the manufacturer may voluntarily participate in Phase IV trials to continue to test the safety and efficacy of a vaccine after release to market.
Monitoring adverse effects. In addition, in 1990, the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration established the Vaccine Adverse Events Reporting System (VAERS) to “detect possible signals of adverse events associated with vaccines.”24 Most events reported are coincidental, but some common mild adverse events (like redness and swelling at the injection site) are often underreported.
Serious events are always thoroughly investigated and are often found unrelated. However, rare associations have been found. For example, an intestinal problem called intussusception, related to the original rotavirus vaccine, was discovered, and the vaccine causing it was removed from the market.25 A new, safer rotavirus vaccine option is now available. Patients need to know that we do have an effective system of checks and balances in which we can place our trust.
9. People can become paralyzed or stop breathing after receiving a vaccination. Why run those risks?
One of the most feared reactions to vaccination is Guillain-Barré syndrome (GBS), which can cause paralysis. The CDC estimates the risk for GBS associated with the flu vaccine, for example, to be 1 to 2 cases per 1 million people vaccinated.26 Another potential concern is the rate of anaphylaxis following vaccination. However, in a 2016 study in the Journal of Allergy and Clinical Immunology, the rate of anaphylaxis for all vaccines combined was only 1.31 per 1 million vaccines.27
The risk of developing severe complications from an illness is much greater than that of developing complications from the vaccine meant to protect a person against that illness. In the United States, the population-based risk for influenza-related hospitalization in children, for example, is as high as 150 in 100,000 with as many as 125 deaths annually.26
Continue to: 10. Isn't vaccination a personal choice? How does my health/illness impact the community?
10. Isn’t vaccination a personal choice? How does my health/illness impact the community?
Patients may not realize that most viruses are contagious from 1 to 2 days before symptoms appear, which means we can spread an illness before we even know we have it. Protecting oneself also protects those around us.
Economic concerns. There’s also the economic impact of these illnesses to consider. This includes the personal cost of being out of school or work for an extended period and the cost of a patient’s care, which can become astronomical if hospitalization is required and which can become the country’s problem if a person lacks sufficient health insurance coverage.
A study looking at the cost of 4 major adult vaccine-preventable illnesses (influenza, pneumococcal disease, shingles, and whooping cough) in the United States in 2013 estimated the annual cost for these illnesses in adults ≥50 years to be $26.5 billion.28 And that doesn’t include the cost of childhood vaccine-preventable diseases.
Countering 3 concerns about childhood vaccinations
1. I can’t afford vaccines for my child.
The Vaccines for Children program is a federally-funded program that covers the cost of all vaccines for children younger than 19 years of age who are Medicaid-eligible, American Indian, Alaskan Native, uninsured, or underinsured.29 Although there may be a small administration fee charged by the provider’s office, the vaccine is free.
2. Don’t all of the vaccines recommended for children overwhelm their immune systems?
Children are exposed to so many more proteins on a daily basis (by crawling around on the floor, putting their hands in their mouths, attending school or day care, etc) than they are ever exposed to in a series of vaccines.30 Exposure to these proteins in their environment and to those in vaccines only serves to boost their immunity and keep them healthier in the long run.
And thanks to advances in vaccine production, the immunologic load in vaccines is far less than it used to be. The 14 vaccines given today contain <200 bacterial and viral proteins or polysaccharides, compared with the >3000 of these immunologic components in the 7 vaccines administered in 1980.31
Continue to: Influenza vaccine: Patient-friendly talking points
SIDEBAR
Influenza vaccine: Patient-friendly talking points
- Some people think that getting the flu is no big deal. While it is true that the flu takes a greater toll on the very young and very old, the chronically ill, and the immune compromised, even healthy people can become seriously ill or die. The Centers for Disease Control and Prevention estimates that the flu is responsible for 140,000 to 720,000 hospitalizations and 12,000 to 56,000 deaths in the United States every year.7 Of those who die from the flu, approximately 80% did not receive a flu shot.36 Of children who died from the flu between 2004 and 2012, more than 40% had no risk factors for complications.37
- The flu shot is a killed virus vaccine, so it can't give you the flu. People sometimes feel under the weather (achy, low-grade fever) after a vaccine, but this is considered normal and evidence that your body's immune system is "revving up."
- It takes 2 weeks before the vaccine becomes effective so a person can still get the flu during that time. This is why it is so important to get the vaccine earlier in the fall, before the flu season takes hold.
- The "stomach flu" is not the flu. The flu vaccine does not protect against the "stomach flu" or other flu-like illnesses.
- The flu vaccine is not perfect. It is an educated guess as to which strains will be circulating that year. (At its best, the flu vaccine is about 60% effective.38) However, it makes the chance of getting the flu less likely and significantly decreases the odds of severe complications/death.
- Egg allergies are no longer a reason to avoid the flu vaccine. There is an egg-free vaccine called Flublok (for those ≥18 years of age). In 2016-17, the Advisory Committee on Immunization Practices changed the recommendations for flu vaccine in egg-allergic people. The recommendations say that if reactions are mild, or you can eat cooked eggs without a problem, you can receive a flu vaccine. If you have severe reactions, such as trouble breathing or recurrent vomiting, you can still receive the flu vaccine, but must be monitored by a health care provider who can recognize and respond to a severe allergic reaction.39
Continue to: 3. Why don't we adhere to Dr. Sears' vaccine schedule?
3. Why don’t we adhere to Dr. Sears’ vaccine schedule?
There are multiple ways in which Dr. Robert Sears’ book, The Vaccine Book: Making the Right Decision for your Child, published in 2007, misrepresents vaccine science and leads patients astray in making decisions regarding vaccinations.32 Most important to note is that Dr. Sears’ Alternative Vaccine Schedule, which seeks to make it so that children do not receive more than 2 vaccinations per office visit, would require visits to a health care provider at 2, 3, 4, 5, 6, 7, 9, 12, 15, 18, and 21 months, and at 2, 2.5, 3, 3.5, 4, 5, and 6 years of age. This significantly increases the number of office visits and needle sticks, and raises the age at which vaccines are given, increasing the risk of illness outbreaks and decreasing the likelihood that parents would return to the office to complete the full series.
Acceptance of influenza and HPV vaccines remains a challenge
We are significantly less successful at getting parents and patients to agree to influenza and HPV vaccines than to the other vaccines we offer. The influenza vaccine success rate in 2016 was 59% in children and 43.3% in adults.33 Compared to the Tdap vaccine (88%) and the meningococcal vaccine (82%), which are offered at the same age as the HPV vaccine, success rates for HPV vaccine are significantly lower. In 2016, only 60.4% of boys and girls were current on their first HPV injection and only 43.3% were up to date with the full series.34
Newness of vaccines a factor?
Perhaps it is because the recommendations for these 2 vaccines are relatively new, and people don’t yet grasp the seriousness and scope of the diseases. Until 2010, the flu shot was recommended only for the very young, the elderly, and the medically high risk.
Similarly, the HPV vaccine was originally introduced for girls in 2006 and wasn’t recommended for boys until 2011.
Continue to: Human papillomavirus vaccine: Patient-friendly talking points
SIDEBAR
Human papillomavirus vaccine: Patient-friendly talking points
- Human papillomavirus (HPV) causes genital warts and cancer of the cervix, vagina, vulva, anus, rectum, penis, and oropharynx.
- The HPV vaccine is a cancer prevention vaccine. The 9-valent vaccine is active against 2 genital wart-causing strains and 7 cancer-causing strains of HPV.
- HPV is highly prevalent; 79 million Americans are currently infected, nearly 14 million people become newly infected each year, and nearly all of us will be exposed at some point in our sexual lives.40
- There are often no outward signs of infection, so it is a difficult infection to avoid.
- It takes no high-risk sexual activity to be exposed to the HPV virus.
- The HPV vaccine is recommended for both boys and girls usually around age 11 to 12 years (but as early as 9 years and as late as 26 years is acceptable). If the first vaccine is administered before 15 years of age, only 2 injections are needed 6 to 12 months apart. If the first vaccine is administered after 15 years of age, 3 injections are needed at 0, 2 months, and 6 months.41
- Completing the series before sexual activity begins is the best way to protect our children because the vaccine is a preventive measure, not a treatment.
- The HPV vaccine is highly effective with >90% efficacy against high-risk cancer-causing strains.42
- The HPV vaccine offers long-term protection. The vaccine has been on the market since 2006, and immunity has not yet diminished. Further monitoring is ongoing.43
- The HPV vaccine is covered under the Vaccines For Children program until age 19 years. Then it is up to individual insurance plans to cover it.
- The HPV vaccine does not cause infertility.44 HPV infection, on the other hand, can lead to fertility problems if, for example, treatment for cervical precancer or cancer requires partial removal of the cervix or a hysterectomy.
- The HPV vaccine does not cause autoimmune diseases.45,46 Studies show no difference between vaccinated and unvaccinated groups in rates of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, type 1 diabetes mellitus, multiple sclerosis, Hashimoto's thyroiditis, Graves' disease, and others.
- The HPV vaccine does not encourage earlier sexual activity. There was no earlier incidence of outcomes related to sexual activity (pregnancy, sexually transmitted infection testing or diagnosis, or contraceptive counseling) in vaccinated vs unvaccinated patients studied.47
Continue to: A sensitive subject
A sensitive subject. Discussion of a vaccine related to a child’s sexual health makes some parents uncomfortable. Studies show that focusing on the cancer prevention aspects of the vaccine, rather than on sexual transmission of HPV, results in greater vaccine acceptance.35
However, if discussion of sexual transmission is unavoidable, remind parents to consider their own adolescence and whether they chose to share everything with their parents. Point out that there were probably things they did that they later looked back on and thought, “What was I thinking?” Their children, no matter how wonderful and levelheaded they are, will be no different. And, as much as parents don’t want to think about it, some kids will suffer unwanted sexual contact. Shouldn’t parents protect their children as best as they can?
A teen’s right to choose? Some states have passed a Mature Minor Doctrine, which provides for mature, unemancipated teens to make their own medical decisions regarding such issues as sexuality, mental health, and drug and alcohol use without their parents’ consent. In these states, teens may elect to receive the HPV vaccine without parental permission. (Check your state’s laws for specifics, and see the 2 boxes with patient-friendly talking points for influenza vaccine7,36-39 and human papillomavirus vaccine.40-47)
CORRESPONDENCE
Gretchen LaSalle, MD, MultiCare Rockwood Clinic, 2214 East 29th Avenue, Spokane, WA 99203; [email protected].
1. Paterson P, Meurice F, Stanberry LR, et al. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700-6706.
2. Opel DJ, Heritage J, Taylor J, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132:1037-1046.
3. Palmer J, Carrico C, Costanzo C. Identifying and overcoming perceived barriers of providers towards vaccination: a literature review. J Vaccines. 2015;1-7.
4. Autism Science Foundation. Making the CASE for vaccines: a new model for talking to patients about vaccines. Available at: http://autismsciencefoundation.org/wp-content/uploads/2015/12/Making-the-CASE-for-Vaccines-Guide_final.pdf. Accessed April 8, 2018.
5. Jacobson RM, Van Etta L, Bahta L. The C.A.S.E approach: guidance for talking to vaccine-hesitant patients. Minn Med. 2013;96:49-50.
6. Henrickson NB, Opel DJ, Grothaus L, et al. Physician communication training and parental vaccine hesitancy: a randomized trial. Pediatrics. 2015;136:70-79.
7. Centers for Disease Control and Prevention. Key facts about seasonal flu vaccine. Available at: https://www.cdc.gov/flu/protect/keyfacts.htm. Accessed April 8, 2018.
8. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641.
9. Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32:3623-3629.
10. Agency for Toxic Substances & Disease Registry. Minimal risk levels for hazardous substances. Available at: https://www.atsdr.cdc.gov/mrls/mrllist.asp#34tag. Accessed April 8, 2018.
11. US Food and Drug Administration. Thimerosal and vaccines. Available at: https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed April 8, 2018.
12. Hviid A, Stellfeld M, Wohlfahrt J, et al. Association between thimerosal-containing vaccine and autism. JAMA. 2003;290:1763-1766.
13. Centers for Disease Control and Prevention. Thimerosal in vaccines. Available at: https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed May 8, 2018.
14. Autism Speaks. Frequently asked questions. Available at: https://www.autismspeaks.org/what-autism/faq. Accessed April 8, 2018.
15. Agency for Toxic Substances & Disease Registry. Toxic substances portal-aluminum. Public Health Statement for Aluminum, CAS #7429-90-5. Available at: https://www.atsdr.cdc.gov/PHS/PHS.asp?id=1076&tid=34. Accessed April 8, 2018.
16. Children’s Hospital of Philadelphia. Vaccine ingredients-aluminum. Available at: www.chop.edu/centers-programs/vaccine-education-center/vaccine-ingredients/aluminum. Accessed April 8, 2018.
17. Orenstein W, Seib K. Mounting a good offense against measles. N Engl J Med. 2014;371:1661-1663.
18. Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med. 2012;55:72-77.
19. Hall V, Banerjee E, Kenyon C, et al. Measles outbreak – Minnesota April-May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:713-717.
20. The National Academies of Sciences Engineering Medicine. History of U.S. water and wastewater systems. Privatization of Water Services in the United States: an Assessment of Issues and Experience. Washington, DC: The National Academies Press; 2002:29-40. Available at: https://www.nap.edu/read/10135/chapter/4#35. Accessed May 7, 2018.
21. World Health Organization. Global vaccine safety. Six common misconceptions about immunization. Available at: http://www.who.int/vaccine_safety/initiative/detection/immunization_misconceptions/en/index1.html. Accessed May 7, 2018.
22. The history of vaccines. Human cell strains in vaccine development. Available at: https://www.historyofvaccines.org/content/articles/human-cell-strains-vaccine-development. Accessed April 8, 2018.
23. The National Catholic Bioethics Center. Frequently asked questions. Available at: https://www.ncbcenter.org/resources/frequently-asked-questions/use-vaccines/. Accessed April 8, 2018.
24. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015;33:4398-4405.
25. Foster S. Rotavirus vaccine and intussusception. J Pediatr Pharmacol Ther. 2007;12:4-7.
26. Mistry RD, Fischer JB, Prasad PA, et al. Severe complications of influenza-like illnesses. Pediatrics. 2014;134:e684-e690.
27. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
28. McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36:259-273.
29. Centers for Disease Control and Prevention. Vaccines for Children (VFC) Program. Available at: https://www.cdc.gov/features/vfcprogram/index.html. Accessed April 8, 2018.
30. Plotkin S, Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis. 2009;48:456-461.
31. Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics. 2002;109:124-129.
32. Offit PA, Moser CA. The problem with Dr. Bob’s alternative vaccine schedule. Pediatrics. 2009;123:e164-e169.
33. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. April 8. 2018.
34. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state and selected local area vaccination coverage among adolescents aged 13-17 years – United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
35. Thomas TL. Cancer prevention: HPV vaccination. Semin Oncol Nurs. 2016:32:273-280.
36. Centers for Disease Control and Prevention. Estimating seasonal influenza-associated deaths in the United States. Available at: https://www.cdc.gov/flu/about/disease/US_flu-related_deaths.htm. Accessed May 8, 2018.
37. Wong KK, Jain S, Blanton L, et al. Influenza-associated pediatric deaths in the United States: 2004-2012. Pediatrics. 2013;132:796-804.
38. Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed April 8, 2018.
39. Centers for Disease Control and Prevention. Influenza (flu). Flu vaccine and people with egg allergies. Available at: https://www.cdc.gov/flu/protect/vaccine/egg-allergies.htm. Accessed April 8, 2018.
40. Centers for Disease Control and Prevention. For parents: vaccines for your children. HPV vaccine for preteens and teens. Available at: https://www.cdc.gov/vaccines/parents/diseases/teen/hpv.html. Accessed April 8, 2018.
41. Centers for Disease Control and Prevention. Vaccines and preventable diseases. HPV vaccine recommendations. Available at: https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed May 7, 2018.
42. Cutts FT, Franceschi S, Goldie S, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ. 2007;85:719-726.
43. De Vincenzo R, Conte C, Ricci C, et al. Long-term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999-1010.
44. McInerney KA, Hatch EE, Wesselink AK. The effect of vaccination against human papillomavirus on fecundability. Paedeatr Perinat Epidemiol. 2017;31:531-536.
45. Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193-203.
46. Vichnin M, Bonanni P, Klein NP, et al. An overview of quadrivalent human papillomavirus vaccine safety: 2006-2015. Ped Infect Dis J. 2015;34:983-991.
47. Bednarczyk RA, Davis R, Ault K, et al. Sexual activity-related outcomes after human papillomavirus vaccination of 11-to-12-year-olds. Pediatrics. 2012;130:798-805.
1. Paterson P, Meurice F, Stanberry LR, et al. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700-6706.
2. Opel DJ, Heritage J, Taylor J, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132:1037-1046.
3. Palmer J, Carrico C, Costanzo C. Identifying and overcoming perceived barriers of providers towards vaccination: a literature review. J Vaccines. 2015;1-7.
4. Autism Science Foundation. Making the CASE for vaccines: a new model for talking to patients about vaccines. Available at: http://autismsciencefoundation.org/wp-content/uploads/2015/12/Making-the-CASE-for-Vaccines-Guide_final.pdf. Accessed April 8, 2018.
5. Jacobson RM, Van Etta L, Bahta L. The C.A.S.E approach: guidance for talking to vaccine-hesitant patients. Minn Med. 2013;96:49-50.
6. Henrickson NB, Opel DJ, Grothaus L, et al. Physician communication training and parental vaccine hesitancy: a randomized trial. Pediatrics. 2015;136:70-79.
7. Centers for Disease Control and Prevention. Key facts about seasonal flu vaccine. Available at: https://www.cdc.gov/flu/protect/keyfacts.htm. Accessed April 8, 2018.
8. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641.
9. Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32:3623-3629.
10. Agency for Toxic Substances & Disease Registry. Minimal risk levels for hazardous substances. Available at: https://www.atsdr.cdc.gov/mrls/mrllist.asp#34tag. Accessed April 8, 2018.
11. US Food and Drug Administration. Thimerosal and vaccines. Available at: https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed April 8, 2018.
12. Hviid A, Stellfeld M, Wohlfahrt J, et al. Association between thimerosal-containing vaccine and autism. JAMA. 2003;290:1763-1766.
13. Centers for Disease Control and Prevention. Thimerosal in vaccines. Available at: https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed May 8, 2018.
14. Autism Speaks. Frequently asked questions. Available at: https://www.autismspeaks.org/what-autism/faq. Accessed April 8, 2018.
15. Agency for Toxic Substances & Disease Registry. Toxic substances portal-aluminum. Public Health Statement for Aluminum, CAS #7429-90-5. Available at: https://www.atsdr.cdc.gov/PHS/PHS.asp?id=1076&tid=34. Accessed April 8, 2018.
16. Children’s Hospital of Philadelphia. Vaccine ingredients-aluminum. Available at: www.chop.edu/centers-programs/vaccine-education-center/vaccine-ingredients/aluminum. Accessed April 8, 2018.
17. Orenstein W, Seib K. Mounting a good offense against measles. N Engl J Med. 2014;371:1661-1663.
18. Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med. 2012;55:72-77.
19. Hall V, Banerjee E, Kenyon C, et al. Measles outbreak – Minnesota April-May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:713-717.
20. The National Academies of Sciences Engineering Medicine. History of U.S. water and wastewater systems. Privatization of Water Services in the United States: an Assessment of Issues and Experience. Washington, DC: The National Academies Press; 2002:29-40. Available at: https://www.nap.edu/read/10135/chapter/4#35. Accessed May 7, 2018.
21. World Health Organization. Global vaccine safety. Six common misconceptions about immunization. Available at: http://www.who.int/vaccine_safety/initiative/detection/immunization_misconceptions/en/index1.html. Accessed May 7, 2018.
22. The history of vaccines. Human cell strains in vaccine development. Available at: https://www.historyofvaccines.org/content/articles/human-cell-strains-vaccine-development. Accessed April 8, 2018.
23. The National Catholic Bioethics Center. Frequently asked questions. Available at: https://www.ncbcenter.org/resources/frequently-asked-questions/use-vaccines/. Accessed April 8, 2018.
24. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015;33:4398-4405.
25. Foster S. Rotavirus vaccine and intussusception. J Pediatr Pharmacol Ther. 2007;12:4-7.
26. Mistry RD, Fischer JB, Prasad PA, et al. Severe complications of influenza-like illnesses. Pediatrics. 2014;134:e684-e690.
27. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
28. McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36:259-273.
29. Centers for Disease Control and Prevention. Vaccines for Children (VFC) Program. Available at: https://www.cdc.gov/features/vfcprogram/index.html. Accessed April 8, 2018.
30. Plotkin S, Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis. 2009;48:456-461.
31. Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics. 2002;109:124-129.
32. Offit PA, Moser CA. The problem with Dr. Bob’s alternative vaccine schedule. Pediatrics. 2009;123:e164-e169.
33. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. April 8. 2018.
34. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state and selected local area vaccination coverage among adolescents aged 13-17 years – United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
35. Thomas TL. Cancer prevention: HPV vaccination. Semin Oncol Nurs. 2016:32:273-280.
36. Centers for Disease Control and Prevention. Estimating seasonal influenza-associated deaths in the United States. Available at: https://www.cdc.gov/flu/about/disease/US_flu-related_deaths.htm. Accessed May 8, 2018.
37. Wong KK, Jain S, Blanton L, et al. Influenza-associated pediatric deaths in the United States: 2004-2012. Pediatrics. 2013;132:796-804.
38. Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed April 8, 2018.
39. Centers for Disease Control and Prevention. Influenza (flu). Flu vaccine and people with egg allergies. Available at: https://www.cdc.gov/flu/protect/vaccine/egg-allergies.htm. Accessed April 8, 2018.
40. Centers for Disease Control and Prevention. For parents: vaccines for your children. HPV vaccine for preteens and teens. Available at: https://www.cdc.gov/vaccines/parents/diseases/teen/hpv.html. Accessed April 8, 2018.
41. Centers for Disease Control and Prevention. Vaccines and preventable diseases. HPV vaccine recommendations. Available at: https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed May 7, 2018.
42. Cutts FT, Franceschi S, Goldie S, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ. 2007;85:719-726.
43. De Vincenzo R, Conte C, Ricci C, et al. Long-term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999-1010.
44. McInerney KA, Hatch EE, Wesselink AK. The effect of vaccination against human papillomavirus on fecundability. Paedeatr Perinat Epidemiol. 2017;31:531-536.
45. Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193-203.
46. Vichnin M, Bonanni P, Klein NP, et al. An overview of quadrivalent human papillomavirus vaccine safety: 2006-2015. Ped Infect Dis J. 2015;34:983-991.
47. Bednarczyk RA, Davis R, Ault K, et al. Sexual activity-related outcomes after human papillomavirus vaccination of 11-to-12-year-olds. Pediatrics. 2012;130:798-805.
From The Journal of Family Practice | 2018;67(6):348-351,359-364.
PRACTICE RECOMMENDATIONS
› Use a presumptive approach when discussing vaccines with patients/parents. A
› Offer vaccines at every opportunity; provider recommendation is the most important factor in getting patients to vaccinate. A
› Focus on the cancer prevention aspect of the human papillomavirus vaccine to improve rates of vaccine acceptance. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series