User login
Sport-related concussion: How best to help young athletes
› Require athletes who sustain a concussion to wait a minimum of 7 to 10 days before returning to full unrestricted activity. C
› Ensure that any player diagnosed with concussion follows a guided return-to-play progression, supervised by an athletic trainer or physical therapist experienced in post-concussion care. C
› Advise patients who are old enough to drive not to do so for at least 24 hours after a concussion. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year in the United States, more than 44 million young people participate in sports activities.1 Yet the number of concussions incurred annually by children and adolescents engaged in sports and recreational play has been underestimated for years, and largely unknown.1,2
Some estimates were based solely on the number of young athletes treated in emergency departments or sports concussion clinics. Others focused only on team players of middle school or high school age, excluding younger children who were hit in the head on playgrounds or during other recreational activities. What’s more, large numbers of concussions—as many as 4 in 10 incurred by high school athletes—were never reported to a coach or medical professional.3
In a new study published in the journal Pediatrics in June, researchers used national databases and current literature to provide what they believe to be “the most accurate and precise estimate of youth concussion” thus far: Between 1.1 and 1.9 million sports- and recreation-related concussions occur among US youth ages 18 or younger annually.1
Among young people playing team sports, concussions are between 2 and 7 times more likely to occur during competitive games than in practice sessions.4-7 Boys on football and ice hockey teams have the highest rates of concussion in young athletes.For overall number of concussions, however, girls on soccer teams are second only to football players.4 Female soccer players are more likely than male soccer players to sustain concussions during equal number of hours of play.4,7
An increase in incidence. The incidence of concussion among young athletes appears to have increased in the past decade, a likely result of greater involvement in team sports, an increasing focus on safeguarding young people from the potential dangers associated with a blow to the brain, and better diagnostic techniques.4,8-10 And a recent study based on data from electronic medical records at a large regional pediatric health care network found that more than three-quarters of young people with sports-related concussions were first seen in a primary care setting.2
With this in mind, we present a comprehensive update of the evidence regarding the diagnosis and management of sport-related concussion. The recommendations we include are consistent with professional association guidelines.8-10 Although we focus on concussion in children and adolescents involved in athletic activities, the principles generally apply to patients of all ages and to concussions that may not be sports related.
Removal from play: A vital first step
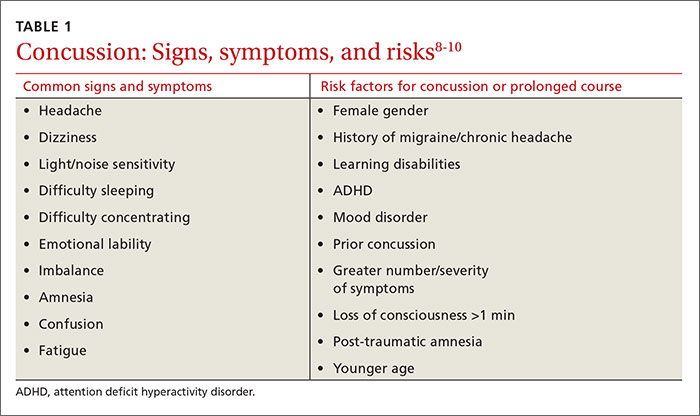
Whenever you conduct a physical exam for a young athlete, remind him or her—and the patient’s parents—that after a blow to the head, immediate removal from play is critical. Concussion is caused by a direct or indirect force to the brain that results in a transient disturbance in brain function,8-10 manifested by alterations in neurocognitive and motor function. While the signs and symptoms (TABLE 1)8-10 resolve within 10 days of injury in about 90% of cases, those who incur additional head impact within 24 hours have a higher symptom burden and prolonged recovery period.11 Even without repetitive impact, younger athletes may take longer to recover.8-10
The initial assessment
A child or adolescent who sustains a suspected concussion should be seen by a physician within 24 to 48 hours. Whether the initial assessment occurs in your office or on the sidelines of a game, it is important to confirm the time the incident occurred and the mechanism of injury.
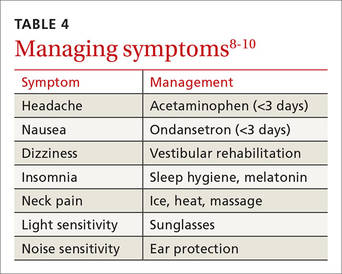
Concussion is diagnosed by a combination of history, physical exam, and objective testing when symptoms or exam findings associated with mild brain trauma—headache, dizziness, light and/or noise sensitivity, among others—closely follow a head injury.8-10 Certain maneuvers—assessing eye movements by asking the athlete to look in various directions, for instance, then to follow a pen or finger as you move it closer to his or her face—may provoke dizziness, headache, or other symptoms of concussion that were not apparent initially.
The differential diagnosis includes cervical musculoskeletal injury, craniofacial injury, epidural and subdural hematoma, heat-related illness, uncomplicated headache and migraine, upper respiratory infection, and vertigo.8-10
Tools aid in diagnosis
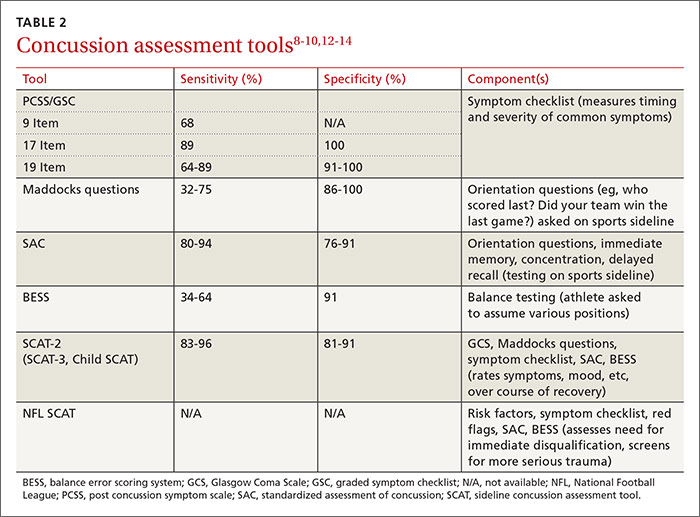
Many clinical assessment tools exist to aid in the diagnosis of concussion (TABLE 2).8-10,12-14 Any one of these tools, many of which use combinations of symptom checklists, balance exams, and cognitive assessments, may be included in your evaluation. No single tool has been found to be superior to any other.8-10 A combination of tools may improve diagnostic accuracy, but assessment tools should not be the sole basis used to diagnose or rule out concussion.
Any child or adolescent who had a blow to the head and at least one sign or symptom of concussion should be evaluated as soon as possible and assessed again later that day or the next day if any reason for concern remains.
Neuropsychological (NP) testing may involve computerized tests developed specifically for athletes. Patients may be required to react to objects that appear on a screen, for example, in a way that tests memory, performance, and reaction time. Because cognitive recovery often lags behind symptom resolution, NP testing may identify subtle brain deficits even in athletes who are asymptomatic at rest or with exercise. In general, NP testing has a sensitivity of 71% to 88% for athletes with concussion,10 but it is most beneficial when baseline test results are available. Interpretation of NP testing should be done only by qualified clinicians.
While NP testing may provide additional prognostic information, it should not alter the management of athletes who are symptomatic either at rest or with exercise.15 Nor is NP testing vital, as concussion can be accurately diagnosed and adequately managed without it.
Neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), is often used unnecessarily in the initial assessment of a patient who sustained a possible concussion.8-10 In fact, neuroimaging should be reserved for cases in which it is necessary to rule out more serious pathology: intracranial or subdural hematoma or a craniofacial injury, for example, in patients with clinical findings that are red flags. These red flags include focal neurologic deficits, continuing nausea/vomiting, or persistent disorientation (TABLE 3),8-10 or symptoms that worsen or persist beyond a few weeks. In such cases, further evaluation—with MRI of the brain, formal NP testing, and/or referral to a neurologist, physiatrist, or other physician who specializes in concussion care—is indicated.

Concussion management: Rest is key
While there is a dearth of high-quality studies on the management of sport-related concussion across all age groups, standardized protocols for both children and adults have been adopted in most clinical settings.8-10,16,17 The protocols provide a framework for an individualized treatment plan. Yet their use among primary care physicians is inconsistent.18-20
Traditionally, concussion management begins with relative physical and cognitive rest to allow the brain time to recover.8-10 Recent randomized controlled trials have challenged this premise by suggesting that mild to moderate physical activity for post-concussion patients who are mildly symptomatic does not adversely affect recovery.21,22 These studies have significant limitations, however, and further research is needed to provide specific guidance on this aspect of concussion management before it is adopted.
Physical restrictions include organized sports, recreational activity, recess, and physical education classes. Walking is permitted unless it exacerbates symptoms. These restrictions should continue until the patient is symptom-free.
Cognitive restrictions include modifications at school and at home. Once an athlete is able to concentrate and tolerate visual and auditory stimuli, he or she may return to school. But classroom modifications should be considered, possibly including shortened school days, extra time for testing and homework, help with note taking, and restrictions from classes likely to provoke symptoms, such as computer science or music. Limiting use of mobile devices, television viewing, noisy environments, and other possible provocations may help speed symptom resolution. These restrictions, too, should remain in place until the patient is symptom-free.
Driving is often not addressed by physicians managing the care of athletes with concussion, but evidence suggests it should be. A study of patients presenting to the emergency department found that within 24 hours of a concussion diagnosis, individuals had an impaired response to traffic hazards.23,24 And Canadian clinical practice guidelines recommend that athletes with mild traumatic brain injury (TBI) avoid driving within the first 24 hours.25
While American guidelines are silent on the question of driving for this patient population, we recommend that athletes with concussion be restricted from driving and engaging in other risky complex tasks, such as welding or shop class, for at least 24 hours. For many athletes diagnosed with concussion, driving restrictions of longer duration may be necessary based on their symptom profile and neurocognitive test results. Continued dizziness or visual deficits would pose a greater risk than fatigue or short-term memory loss, for example.
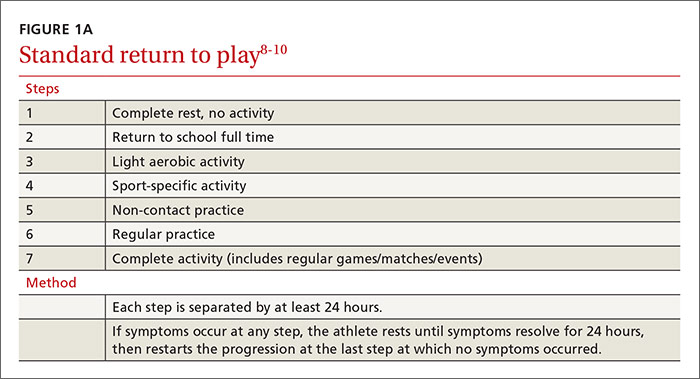
Overseeing the return to play
Return-to-activity progression follows a stepwise protocol, with 6 steps that the injured athlete must complete before resuming full activity (FIGURE 1A).8-10 This stepwise progression begins only when athletes are symptom free, even during provocative maneuvers; have had a normal neurologic exam, are back to school full time with no restriction; are off any medications prescribed for concussion symptoms (TABLE 4),8-10 and when neurocognitive testing, if performed, is back to baseline. If an athlete develops symptoms at any stage of the progression, rest is required until he or she remains asymptomatic for at least 24 hours. The progression is then restarted at the last stage at which the patient was symptom free.
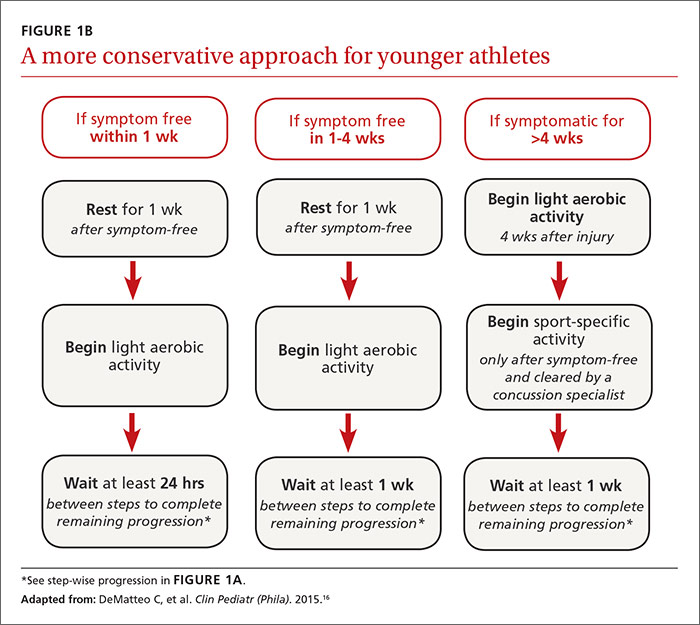
Some individualization, of course, is recommended here, too. Younger athletes and those with a prior history of concussion may require 10 days or more to complete all the steps, allowing an extra day at various steps. Neurologic maturation affects recovery time, and for younger individuals, a more conservative return-to-play protocol based on initial concussion symptom duration has been proposed (FIGURE 1B).16
Return to activity is often supervised by a certified athletic trainer at the athlete’s school. In the event that no athletic trainer is available, patients may be referred to physical therapists with experience in monitoring injured athletes.26 Anyone involved in the patient’s care, including the athlete himself, may use a symptom checklist to monitor recovery.
Although there is no evidence that the ongoing use of a symptom checklist affects the course of recovery, its use is often helpful in identifying specific symptoms that can be managed by means other than physical and cognitive rest—a sleep hygiene program for an individual with lingering difficulty sleeping, for example, or the continued application of ice, heat, and massage for persistent neck pain.
Checklist monitoring may be especially helpful for athletes whose symptoms extend beyond 10 days or who have multiple symptoms. Final clearance once all the steps have been completed requires follow-up with a health care provider.
Is a symptom-free waiting period necessary?
There is no evidence suggesting a need for a symptom-free waiting period before starting the return-to-play protocol.10,27 Because a repeat concussion is most likely within 7 to 10 days of the initial injury,8,9 however, most athletes should not return to contact play during that time frame, regardless of symptom resolution.
It is helpful to have asymptomatic athletes participate in non-contact activity before the 7 to 10 days are up, however. Doing so can help prevent deconditioning and injury upon return to contact sport, as there is evidence of increased risk of lower-extremity injury in the 90 days after concussion.28
What to tell athletes—and parents—about repetitive head trauma
There is growing concern about the long-term risks of concussion and repetitive head impact that may manifest as chronic traumatic encephalopathy (CTE) and chronic neurocognitive impairment (CNI) later in life. Indeed, some data strongly suggest—but do not definitively prove—a relationship between repetitive head injury and chronic neurodegenerative disease.8-10 You can tell worried patients or parents, however, that the majority of research on CTE and CNI has been based on professional football players.
Studies of long-term effects of soccer heading have shown conflicting results, with some finding cognitive impairment, altered postural control, and anatomic changes of the brain, while others found no effect on encephalopathy, concussion symptoms, or neurocognitive performance.29-36Here, too, most studies showing negative effects of soccer heading involved professional athletes.
Repetitive sub-concussive impact in high school football athletes has been found to induce biochemical changes to the brain,37 but the long-term effects are unknown. And, while concussion in high school athletes has been associated with short-term cognitive impairment, altered neurochemistry, and evidence of increased symptoms on baseline neurocognitive testing,8-10,38 no studies have linked concussion during middle school or high school with CNI. What’s more, a long-term (50-year) follow-up study of individuals who played football in high school found no difference in rates of neurodegenerative disease compared with age-matched controls.39
A new study of high school and college football players (mean age: 17.4 years) presented at the American Academy of Neurology 2016 Sports Concussion Conference in Chicago in July, however, found significant alterations in white matter 6 months post injury.40 The researchers compared 17 athletes with sport-related concussion with matched controls, using diffusion tensor imaging and diffusion kurtosis tensor imaging as biomarkers of brain recovery. The concussed athletes underwent MRI and symptom assessment at 24 hours, 8 days, and 6 months. The controls followed identical protocols.
At the 6-month assessment, there were no differences between the concussed group and the controls in terms of self-reported concussion symptoms, cognition, or balance. However, the concussed athletes had widespread decreased mean diffusivity compared with the controls. Despite the lack of clinical symptoms, the concussed athletes showed significant alterations in white matter “that were related to initial symptom severity ratings,” the authors concluded. These findings have implications both for determination of recovery from concussion and concussion management, they added.40
Although there is no way to eliminate all concussions, limited evidence suggests that improving athletic technique, limiting contact at practices, better enforcement of game rules, and rule changes regarding physical contact may decrease concussion risk.41-43 Many youth sports organizations have developed policies placing restrictions on head impact during practices and games. Studies are ongoing, too, to see if better headgear—or requiring helmets for soccer players—makes a difference.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, 203 East Fourth Avenue, Ranson, WV 25438; [email protected].
1. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics. 2016; June 20 [Epub ahead of print].
2. Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 2016; May 31 [Epub ahead of print].
3. Mihalik JK, Guskiewicz KM, Valovich McLeod TC, et al. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Ath Tr. 2013;48:645-653.
4. Marar M, McIlvain NM, Fields SK, et al. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747.
5. Kontos AP, Elbin RJ, Fazio-Sumrock VC. Incidence of sports-related concussion among youth football players aged 8-12 years. J Pediatr. 2013;163:717-720.
6. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatr. 2015;169:659-665.
7. Comstock RD, Currie DW, Pierpont LA, et al. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169:830-837.
8. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15-26.
9. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258.
10. Giza CC, Kutcher JS, Ashwal S, et al. Summary of the evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257.
11. Terwilliger VK, Pratson L, Vaughan CG, et al. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J Neurotrauma. 2016;33:761-765.
12. Putukian M, Echemendia R, Dettwiler-Danspeckgruber A. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. 2015;25:36-42.
13. Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050-1057.
14. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139-152.
15. Shrier I. Neuropsychological testing and concussions: a reasoned approach. Clin J Sport Med. 2012;22:211-213.
16. DeMatteo C, Stazyk K, Singh SK, et al. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila). 2015;54:152-163.
17. Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers Association position statement: management of sport concussion. J Athl Train. 2014;49:245-265.
18. Stoller J, Carson JD, Garel A, et al. Do family physicians, emergency department physicians, and pediatricians give consistent sport-related concussion management advice? Can Fam Physician. 2014;60:548, 550-552.
19. Lebrun CM, Mrazik M, Prasad AS, et al. Sport concussion knowledge base, clinical practices and needs for continuing medical education: a survey of family physicians and cross-border comparison. Br J Sports Med. 2013;47:54-59.
20. Zemek R, Eady K, Moreau K, et al. Knowledge of paediatric concussion among front-line primary care providers. Paediatr Child Health. 2014;19:475-480.
21. Maerlender A, Rieman W, Lichtenstein J, et al. Programmed physical exertion in recovery from sports-related concussion: a randomized pilot study. Dev Neuropsychol. 2015;40:273-278.
22. Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2015; July 24 [Epub ahead of print].
23. Preece MH, Horswill MS, Langlois JA, et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378.
24. Baker A, Unsworth CA, Lannin NA. Fitness-to-drive after mild traumatic brain injury: mapping the time trajectory of recovery in the acute stages post injury. Accid Anal Prev. 2015;79:50-55.
25. Marshall S, Bayley M, McCullagh S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257-267.
26. Yorke AM, Littleton S, Alsalaheen BA. Concussion attitudes and beliefs, knowledge, and clinical practice: a survey of physical therapists. Phys Ther. Available at: http://dx.doi.org/10.2522/ptj.20140598. Accessed January 21, 2016.
27. McCrea M, Guskiewicz K, Randolph C, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876-883.
28. Brooks MA, Peterson K, Biese K, et al. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742-747.
29. Witol AD, Webbe FM. Soccer heading frequency predicts neuropsychological deficits. Arch Clin Neuropsychol. 2003;18:397-417.
30. Haran FJ, Tierney R, Wright WG, et al. Acute changes in postural control after soccer heading. Int J Sports Med. 2013;34:350-354.
31. Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850-857.
32. Jordan SE, Green GA, Galanty HL, et al. Acute and chronic brain injury in United States national team soccer players. Am J Sports Med. 1996;24:205-210.
33. Kontos AP, Dolese A, Elbin RJ, et al. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011;25:1234-1241.
34. Straume-Naesheim TM, Andersen TE, Dvorak J, et al. Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br J Sports Med. 2005;39:70-77.
35. Stephens R, Rutherford A, Potter D, et al. Neuropsychological impairment as a consequence of football (soccer) play and football heading: a preliminary analysis and report on school students (13-16 years). Child Neuropsychol. 2005;11:513-526.
36. Stephens R, Rutherford A, Potter D, et al. Neuropsychological consequence of soccer play in adolescent UK school team soccer players. J Neuropsychiatry Clin Neurosci. 2010;22:295-303.
37. Poole VN, Breedlove EL, Shenk TE, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol. 2015;40:12-17.
38. Mannix R, Iverson GL, Maxwell B, et al. Multiple prior concussions are associated with symptoms in high school athletes. Ann Clin Trans Neurol. 2014;1:433-438.
39. Savica R, Parisi JE, Wold LE, et al. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87:335-340.
40. Lancaster M, Muftuler T, Olson D, et al. Chronic white matter changes following sport-related concussion measured by diffusion tensor and diffusion kurtosis imaging. Paper presented at: American Academy of Neurology 2016 Sports Concussion Conference; July 8-10, 2016; Chicago, Ill.
41. Kerr ZY, Yeargin SW, Valovich McLeod TC, et al. Comprehensive coach education reduces head impact exposures in American youth football. Orthop J Sports Med. 2015;3(ecollection):e232596711561545.
42. Black AM, Macpherson AK, Hagel BE, et al. Policy change eliminating body checking in non-elite ice hockey leads to a threefold reduction in injury and concussion risk in 11- and 12-year-old players. Br J Sports Med. 2016;50:55-61.
43. Council on Sports Medicine and Fitness. Tackling in youth football. Policy Statement of the American Academy of Pediatrics. Pediatrics. 2015;136:e1419-e1430.
› Require athletes who sustain a concussion to wait a minimum of 7 to 10 days before returning to full unrestricted activity. C
› Ensure that any player diagnosed with concussion follows a guided return-to-play progression, supervised by an athletic trainer or physical therapist experienced in post-concussion care. C
› Advise patients who are old enough to drive not to do so for at least 24 hours after a concussion. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year in the United States, more than 44 million young people participate in sports activities.1 Yet the number of concussions incurred annually by children and adolescents engaged in sports and recreational play has been underestimated for years, and largely unknown.1,2
Some estimates were based solely on the number of young athletes treated in emergency departments or sports concussion clinics. Others focused only on team players of middle school or high school age, excluding younger children who were hit in the head on playgrounds or during other recreational activities. What’s more, large numbers of concussions—as many as 4 in 10 incurred by high school athletes—were never reported to a coach or medical professional.3
In a new study published in the journal Pediatrics in June, researchers used national databases and current literature to provide what they believe to be “the most accurate and precise estimate of youth concussion” thus far: Between 1.1 and 1.9 million sports- and recreation-related concussions occur among US youth ages 18 or younger annually.1
Among young people playing team sports, concussions are between 2 and 7 times more likely to occur during competitive games than in practice sessions.4-7 Boys on football and ice hockey teams have the highest rates of concussion in young athletes.For overall number of concussions, however, girls on soccer teams are second only to football players.4 Female soccer players are more likely than male soccer players to sustain concussions during equal number of hours of play.4,7
An increase in incidence. The incidence of concussion among young athletes appears to have increased in the past decade, a likely result of greater involvement in team sports, an increasing focus on safeguarding young people from the potential dangers associated with a blow to the brain, and better diagnostic techniques.4,8-10 And a recent study based on data from electronic medical records at a large regional pediatric health care network found that more than three-quarters of young people with sports-related concussions were first seen in a primary care setting.2
With this in mind, we present a comprehensive update of the evidence regarding the diagnosis and management of sport-related concussion. The recommendations we include are consistent with professional association guidelines.8-10 Although we focus on concussion in children and adolescents involved in athletic activities, the principles generally apply to patients of all ages and to concussions that may not be sports related.
Removal from play: A vital first step
Whenever you conduct a physical exam for a young athlete, remind him or her—and the patient’s parents—that after a blow to the head, immediate removal from play is critical. Concussion is caused by a direct or indirect force to the brain that results in a transient disturbance in brain function,8-10 manifested by alterations in neurocognitive and motor function. While the signs and symptoms (TABLE 1)8-10 resolve within 10 days of injury in about 90% of cases, those who incur additional head impact within 24 hours have a higher symptom burden and prolonged recovery period.11 Even without repetitive impact, younger athletes may take longer to recover.8-10

The initial assessment
A child or adolescent who sustains a suspected concussion should be seen by a physician within 24 to 48 hours. Whether the initial assessment occurs in your office or on the sidelines of a game, it is important to confirm the time the incident occurred and the mechanism of injury.
Concussion is diagnosed by a combination of history, physical exam, and objective testing when symptoms or exam findings associated with mild brain trauma—headache, dizziness, light and/or noise sensitivity, among others—closely follow a head injury.8-10 Certain maneuvers—assessing eye movements by asking the athlete to look in various directions, for instance, then to follow a pen or finger as you move it closer to his or her face—may provoke dizziness, headache, or other symptoms of concussion that were not apparent initially.
The differential diagnosis includes cervical musculoskeletal injury, craniofacial injury, epidural and subdural hematoma, heat-related illness, uncomplicated headache and migraine, upper respiratory infection, and vertigo.8-10
Tools aid in diagnosis
Many clinical assessment tools exist to aid in the diagnosis of concussion (TABLE 2).8-10,12-14 Any one of these tools, many of which use combinations of symptom checklists, balance exams, and cognitive assessments, may be included in your evaluation. No single tool has been found to be superior to any other.8-10 A combination of tools may improve diagnostic accuracy, but assessment tools should not be the sole basis used to diagnose or rule out concussion.

Any child or adolescent who had a blow to the head and at least one sign or symptom of concussion should be evaluated as soon as possible and assessed again later that day or the next day if any reason for concern remains.
Neuropsychological (NP) testing may involve computerized tests developed specifically for athletes. Patients may be required to react to objects that appear on a screen, for example, in a way that tests memory, performance, and reaction time. Because cognitive recovery often lags behind symptom resolution, NP testing may identify subtle brain deficits even in athletes who are asymptomatic at rest or with exercise. In general, NP testing has a sensitivity of 71% to 88% for athletes with concussion,10 but it is most beneficial when baseline test results are available. Interpretation of NP testing should be done only by qualified clinicians.
While NP testing may provide additional prognostic information, it should not alter the management of athletes who are symptomatic either at rest or with exercise.15 Nor is NP testing vital, as concussion can be accurately diagnosed and adequately managed without it.
Neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), is often used unnecessarily in the initial assessment of a patient who sustained a possible concussion.8-10 In fact, neuroimaging should be reserved for cases in which it is necessary to rule out more serious pathology: intracranial or subdural hematoma or a craniofacial injury, for example, in patients with clinical findings that are red flags. These red flags include focal neurologic deficits, continuing nausea/vomiting, or persistent disorientation (TABLE 3),8-10 or symptoms that worsen or persist beyond a few weeks. In such cases, further evaluation—with MRI of the brain, formal NP testing, and/or referral to a neurologist, physiatrist, or other physician who specializes in concussion care—is indicated.

Concussion management: Rest is key
While there is a dearth of high-quality studies on the management of sport-related concussion across all age groups, standardized protocols for both children and adults have been adopted in most clinical settings.8-10,16,17 The protocols provide a framework for an individualized treatment plan. Yet their use among primary care physicians is inconsistent.18-20
Traditionally, concussion management begins with relative physical and cognitive rest to allow the brain time to recover.8-10 Recent randomized controlled trials have challenged this premise by suggesting that mild to moderate physical activity for post-concussion patients who are mildly symptomatic does not adversely affect recovery.21,22 These studies have significant limitations, however, and further research is needed to provide specific guidance on this aspect of concussion management before it is adopted.
Physical restrictions include organized sports, recreational activity, recess, and physical education classes. Walking is permitted unless it exacerbates symptoms. These restrictions should continue until the patient is symptom-free.
Cognitive restrictions include modifications at school and at home. Once an athlete is able to concentrate and tolerate visual and auditory stimuli, he or she may return to school. But classroom modifications should be considered, possibly including shortened school days, extra time for testing and homework, help with note taking, and restrictions from classes likely to provoke symptoms, such as computer science or music. Limiting use of mobile devices, television viewing, noisy environments, and other possible provocations may help speed symptom resolution. These restrictions, too, should remain in place until the patient is symptom-free.
Driving is often not addressed by physicians managing the care of athletes with concussion, but evidence suggests it should be. A study of patients presenting to the emergency department found that within 24 hours of a concussion diagnosis, individuals had an impaired response to traffic hazards.23,24 And Canadian clinical practice guidelines recommend that athletes with mild traumatic brain injury (TBI) avoid driving within the first 24 hours.25
While American guidelines are silent on the question of driving for this patient population, we recommend that athletes with concussion be restricted from driving and engaging in other risky complex tasks, such as welding or shop class, for at least 24 hours. For many athletes diagnosed with concussion, driving restrictions of longer duration may be necessary based on their symptom profile and neurocognitive test results. Continued dizziness or visual deficits would pose a greater risk than fatigue or short-term memory loss, for example.

Overseeing the return to play
Return-to-activity progression follows a stepwise protocol, with 6 steps that the injured athlete must complete before resuming full activity (FIGURE 1A).8-10 This stepwise progression begins only when athletes are symptom free, even during provocative maneuvers; have had a normal neurologic exam, are back to school full time with no restriction; are off any medications prescribed for concussion symptoms (TABLE 4),8-10 and when neurocognitive testing, if performed, is back to baseline. If an athlete develops symptoms at any stage of the progression, rest is required until he or she remains asymptomatic for at least 24 hours. The progression is then restarted at the last stage at which the patient was symptom free.

Some individualization, of course, is recommended here, too. Younger athletes and those with a prior history of concussion may require 10 days or more to complete all the steps, allowing an extra day at various steps. Neurologic maturation affects recovery time, and for younger individuals, a more conservative return-to-play protocol based on initial concussion symptom duration has been proposed (FIGURE 1B).16

Return to activity is often supervised by a certified athletic trainer at the athlete’s school. In the event that no athletic trainer is available, patients may be referred to physical therapists with experience in monitoring injured athletes.26 Anyone involved in the patient’s care, including the athlete himself, may use a symptom checklist to monitor recovery.
Although there is no evidence that the ongoing use of a symptom checklist affects the course of recovery, its use is often helpful in identifying specific symptoms that can be managed by means other than physical and cognitive rest—a sleep hygiene program for an individual with lingering difficulty sleeping, for example, or the continued application of ice, heat, and massage for persistent neck pain.
Checklist monitoring may be especially helpful for athletes whose symptoms extend beyond 10 days or who have multiple symptoms. Final clearance once all the steps have been completed requires follow-up with a health care provider.
Is a symptom-free waiting period necessary?
There is no evidence suggesting a need for a symptom-free waiting period before starting the return-to-play protocol.10,27 Because a repeat concussion is most likely within 7 to 10 days of the initial injury,8,9 however, most athletes should not return to contact play during that time frame, regardless of symptom resolution.
It is helpful to have asymptomatic athletes participate in non-contact activity before the 7 to 10 days are up, however. Doing so can help prevent deconditioning and injury upon return to contact sport, as there is evidence of increased risk of lower-extremity injury in the 90 days after concussion.28
What to tell athletes—and parents—about repetitive head trauma
There is growing concern about the long-term risks of concussion and repetitive head impact that may manifest as chronic traumatic encephalopathy (CTE) and chronic neurocognitive impairment (CNI) later in life. Indeed, some data strongly suggest—but do not definitively prove—a relationship between repetitive head injury and chronic neurodegenerative disease.8-10 You can tell worried patients or parents, however, that the majority of research on CTE and CNI has been based on professional football players.
Studies of long-term effects of soccer heading have shown conflicting results, with some finding cognitive impairment, altered postural control, and anatomic changes of the brain, while others found no effect on encephalopathy, concussion symptoms, or neurocognitive performance.29-36Here, too, most studies showing negative effects of soccer heading involved professional athletes.
Repetitive sub-concussive impact in high school football athletes has been found to induce biochemical changes to the brain,37 but the long-term effects are unknown. And, while concussion in high school athletes has been associated with short-term cognitive impairment, altered neurochemistry, and evidence of increased symptoms on baseline neurocognitive testing,8-10,38 no studies have linked concussion during middle school or high school with CNI. What’s more, a long-term (50-year) follow-up study of individuals who played football in high school found no difference in rates of neurodegenerative disease compared with age-matched controls.39
A new study of high school and college football players (mean age: 17.4 years) presented at the American Academy of Neurology 2016 Sports Concussion Conference in Chicago in July, however, found significant alterations in white matter 6 months post injury.40 The researchers compared 17 athletes with sport-related concussion with matched controls, using diffusion tensor imaging and diffusion kurtosis tensor imaging as biomarkers of brain recovery. The concussed athletes underwent MRI and symptom assessment at 24 hours, 8 days, and 6 months. The controls followed identical protocols.
At the 6-month assessment, there were no differences between the concussed group and the controls in terms of self-reported concussion symptoms, cognition, or balance. However, the concussed athletes had widespread decreased mean diffusivity compared with the controls. Despite the lack of clinical symptoms, the concussed athletes showed significant alterations in white matter “that were related to initial symptom severity ratings,” the authors concluded. These findings have implications both for determination of recovery from concussion and concussion management, they added.40
Although there is no way to eliminate all concussions, limited evidence suggests that improving athletic technique, limiting contact at practices, better enforcement of game rules, and rule changes regarding physical contact may decrease concussion risk.41-43 Many youth sports organizations have developed policies placing restrictions on head impact during practices and games. Studies are ongoing, too, to see if better headgear—or requiring helmets for soccer players—makes a difference.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, 203 East Fourth Avenue, Ranson, WV 25438; [email protected].
› Require athletes who sustain a concussion to wait a minimum of 7 to 10 days before returning to full unrestricted activity. C
› Ensure that any player diagnosed with concussion follows a guided return-to-play progression, supervised by an athletic trainer or physical therapist experienced in post-concussion care. C
› Advise patients who are old enough to drive not to do so for at least 24 hours after a concussion. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year in the United States, more than 44 million young people participate in sports activities.1 Yet the number of concussions incurred annually by children and adolescents engaged in sports and recreational play has been underestimated for years, and largely unknown.1,2
Some estimates were based solely on the number of young athletes treated in emergency departments or sports concussion clinics. Others focused only on team players of middle school or high school age, excluding younger children who were hit in the head on playgrounds or during other recreational activities. What’s more, large numbers of concussions—as many as 4 in 10 incurred by high school athletes—were never reported to a coach or medical professional.3
In a new study published in the journal Pediatrics in June, researchers used national databases and current literature to provide what they believe to be “the most accurate and precise estimate of youth concussion” thus far: Between 1.1 and 1.9 million sports- and recreation-related concussions occur among US youth ages 18 or younger annually.1
Among young people playing team sports, concussions are between 2 and 7 times more likely to occur during competitive games than in practice sessions.4-7 Boys on football and ice hockey teams have the highest rates of concussion in young athletes.For overall number of concussions, however, girls on soccer teams are second only to football players.4 Female soccer players are more likely than male soccer players to sustain concussions during equal number of hours of play.4,7
An increase in incidence. The incidence of concussion among young athletes appears to have increased in the past decade, a likely result of greater involvement in team sports, an increasing focus on safeguarding young people from the potential dangers associated with a blow to the brain, and better diagnostic techniques.4,8-10 And a recent study based on data from electronic medical records at a large regional pediatric health care network found that more than three-quarters of young people with sports-related concussions were first seen in a primary care setting.2
With this in mind, we present a comprehensive update of the evidence regarding the diagnosis and management of sport-related concussion. The recommendations we include are consistent with professional association guidelines.8-10 Although we focus on concussion in children and adolescents involved in athletic activities, the principles generally apply to patients of all ages and to concussions that may not be sports related.
Removal from play: A vital first step
Whenever you conduct a physical exam for a young athlete, remind him or her—and the patient’s parents—that after a blow to the head, immediate removal from play is critical. Concussion is caused by a direct or indirect force to the brain that results in a transient disturbance in brain function,8-10 manifested by alterations in neurocognitive and motor function. While the signs and symptoms (TABLE 1)8-10 resolve within 10 days of injury in about 90% of cases, those who incur additional head impact within 24 hours have a higher symptom burden and prolonged recovery period.11 Even without repetitive impact, younger athletes may take longer to recover.8-10

The initial assessment
A child or adolescent who sustains a suspected concussion should be seen by a physician within 24 to 48 hours. Whether the initial assessment occurs in your office or on the sidelines of a game, it is important to confirm the time the incident occurred and the mechanism of injury.
Concussion is diagnosed by a combination of history, physical exam, and objective testing when symptoms or exam findings associated with mild brain trauma—headache, dizziness, light and/or noise sensitivity, among others—closely follow a head injury.8-10 Certain maneuvers—assessing eye movements by asking the athlete to look in various directions, for instance, then to follow a pen or finger as you move it closer to his or her face—may provoke dizziness, headache, or other symptoms of concussion that were not apparent initially.
The differential diagnosis includes cervical musculoskeletal injury, craniofacial injury, epidural and subdural hematoma, heat-related illness, uncomplicated headache and migraine, upper respiratory infection, and vertigo.8-10
Tools aid in diagnosis
Many clinical assessment tools exist to aid in the diagnosis of concussion (TABLE 2).8-10,12-14 Any one of these tools, many of which use combinations of symptom checklists, balance exams, and cognitive assessments, may be included in your evaluation. No single tool has been found to be superior to any other.8-10 A combination of tools may improve diagnostic accuracy, but assessment tools should not be the sole basis used to diagnose or rule out concussion.

Any child or adolescent who had a blow to the head and at least one sign or symptom of concussion should be evaluated as soon as possible and assessed again later that day or the next day if any reason for concern remains.
Neuropsychological (NP) testing may involve computerized tests developed specifically for athletes. Patients may be required to react to objects that appear on a screen, for example, in a way that tests memory, performance, and reaction time. Because cognitive recovery often lags behind symptom resolution, NP testing may identify subtle brain deficits even in athletes who are asymptomatic at rest or with exercise. In general, NP testing has a sensitivity of 71% to 88% for athletes with concussion,10 but it is most beneficial when baseline test results are available. Interpretation of NP testing should be done only by qualified clinicians.
While NP testing may provide additional prognostic information, it should not alter the management of athletes who are symptomatic either at rest or with exercise.15 Nor is NP testing vital, as concussion can be accurately diagnosed and adequately managed without it.
Neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), is often used unnecessarily in the initial assessment of a patient who sustained a possible concussion.8-10 In fact, neuroimaging should be reserved for cases in which it is necessary to rule out more serious pathology: intracranial or subdural hematoma or a craniofacial injury, for example, in patients with clinical findings that are red flags. These red flags include focal neurologic deficits, continuing nausea/vomiting, or persistent disorientation (TABLE 3),8-10 or symptoms that worsen or persist beyond a few weeks. In such cases, further evaluation—with MRI of the brain, formal NP testing, and/or referral to a neurologist, physiatrist, or other physician who specializes in concussion care—is indicated.

Concussion management: Rest is key
While there is a dearth of high-quality studies on the management of sport-related concussion across all age groups, standardized protocols for both children and adults have been adopted in most clinical settings.8-10,16,17 The protocols provide a framework for an individualized treatment plan. Yet their use among primary care physicians is inconsistent.18-20
Traditionally, concussion management begins with relative physical and cognitive rest to allow the brain time to recover.8-10 Recent randomized controlled trials have challenged this premise by suggesting that mild to moderate physical activity for post-concussion patients who are mildly symptomatic does not adversely affect recovery.21,22 These studies have significant limitations, however, and further research is needed to provide specific guidance on this aspect of concussion management before it is adopted.
Physical restrictions include organized sports, recreational activity, recess, and physical education classes. Walking is permitted unless it exacerbates symptoms. These restrictions should continue until the patient is symptom-free.
Cognitive restrictions include modifications at school and at home. Once an athlete is able to concentrate and tolerate visual and auditory stimuli, he or she may return to school. But classroom modifications should be considered, possibly including shortened school days, extra time for testing and homework, help with note taking, and restrictions from classes likely to provoke symptoms, such as computer science or music. Limiting use of mobile devices, television viewing, noisy environments, and other possible provocations may help speed symptom resolution. These restrictions, too, should remain in place until the patient is symptom-free.
Driving is often not addressed by physicians managing the care of athletes with concussion, but evidence suggests it should be. A study of patients presenting to the emergency department found that within 24 hours of a concussion diagnosis, individuals had an impaired response to traffic hazards.23,24 And Canadian clinical practice guidelines recommend that athletes with mild traumatic brain injury (TBI) avoid driving within the first 24 hours.25
While American guidelines are silent on the question of driving for this patient population, we recommend that athletes with concussion be restricted from driving and engaging in other risky complex tasks, such as welding or shop class, for at least 24 hours. For many athletes diagnosed with concussion, driving restrictions of longer duration may be necessary based on their symptom profile and neurocognitive test results. Continued dizziness or visual deficits would pose a greater risk than fatigue or short-term memory loss, for example.

Overseeing the return to play
Return-to-activity progression follows a stepwise protocol, with 6 steps that the injured athlete must complete before resuming full activity (FIGURE 1A).8-10 This stepwise progression begins only when athletes are symptom free, even during provocative maneuvers; have had a normal neurologic exam, are back to school full time with no restriction; are off any medications prescribed for concussion symptoms (TABLE 4),8-10 and when neurocognitive testing, if performed, is back to baseline. If an athlete develops symptoms at any stage of the progression, rest is required until he or she remains asymptomatic for at least 24 hours. The progression is then restarted at the last stage at which the patient was symptom free.

Some individualization, of course, is recommended here, too. Younger athletes and those with a prior history of concussion may require 10 days or more to complete all the steps, allowing an extra day at various steps. Neurologic maturation affects recovery time, and for younger individuals, a more conservative return-to-play protocol based on initial concussion symptom duration has been proposed (FIGURE 1B).16

Return to activity is often supervised by a certified athletic trainer at the athlete’s school. In the event that no athletic trainer is available, patients may be referred to physical therapists with experience in monitoring injured athletes.26 Anyone involved in the patient’s care, including the athlete himself, may use a symptom checklist to monitor recovery.
Although there is no evidence that the ongoing use of a symptom checklist affects the course of recovery, its use is often helpful in identifying specific symptoms that can be managed by means other than physical and cognitive rest—a sleep hygiene program for an individual with lingering difficulty sleeping, for example, or the continued application of ice, heat, and massage for persistent neck pain.
Checklist monitoring may be especially helpful for athletes whose symptoms extend beyond 10 days or who have multiple symptoms. Final clearance once all the steps have been completed requires follow-up with a health care provider.
Is a symptom-free waiting period necessary?
There is no evidence suggesting a need for a symptom-free waiting period before starting the return-to-play protocol.10,27 Because a repeat concussion is most likely within 7 to 10 days of the initial injury,8,9 however, most athletes should not return to contact play during that time frame, regardless of symptom resolution.
It is helpful to have asymptomatic athletes participate in non-contact activity before the 7 to 10 days are up, however. Doing so can help prevent deconditioning and injury upon return to contact sport, as there is evidence of increased risk of lower-extremity injury in the 90 days after concussion.28
What to tell athletes—and parents—about repetitive head trauma
There is growing concern about the long-term risks of concussion and repetitive head impact that may manifest as chronic traumatic encephalopathy (CTE) and chronic neurocognitive impairment (CNI) later in life. Indeed, some data strongly suggest—but do not definitively prove—a relationship between repetitive head injury and chronic neurodegenerative disease.8-10 You can tell worried patients or parents, however, that the majority of research on CTE and CNI has been based on professional football players.
Studies of long-term effects of soccer heading have shown conflicting results, with some finding cognitive impairment, altered postural control, and anatomic changes of the brain, while others found no effect on encephalopathy, concussion symptoms, or neurocognitive performance.29-36Here, too, most studies showing negative effects of soccer heading involved professional athletes.
Repetitive sub-concussive impact in high school football athletes has been found to induce biochemical changes to the brain,37 but the long-term effects are unknown. And, while concussion in high school athletes has been associated with short-term cognitive impairment, altered neurochemistry, and evidence of increased symptoms on baseline neurocognitive testing,8-10,38 no studies have linked concussion during middle school or high school with CNI. What’s more, a long-term (50-year) follow-up study of individuals who played football in high school found no difference in rates of neurodegenerative disease compared with age-matched controls.39
A new study of high school and college football players (mean age: 17.4 years) presented at the American Academy of Neurology 2016 Sports Concussion Conference in Chicago in July, however, found significant alterations in white matter 6 months post injury.40 The researchers compared 17 athletes with sport-related concussion with matched controls, using diffusion tensor imaging and diffusion kurtosis tensor imaging as biomarkers of brain recovery. The concussed athletes underwent MRI and symptom assessment at 24 hours, 8 days, and 6 months. The controls followed identical protocols.
At the 6-month assessment, there were no differences between the concussed group and the controls in terms of self-reported concussion symptoms, cognition, or balance. However, the concussed athletes had widespread decreased mean diffusivity compared with the controls. Despite the lack of clinical symptoms, the concussed athletes showed significant alterations in white matter “that were related to initial symptom severity ratings,” the authors concluded. These findings have implications both for determination of recovery from concussion and concussion management, they added.40
Although there is no way to eliminate all concussions, limited evidence suggests that improving athletic technique, limiting contact at practices, better enforcement of game rules, and rule changes regarding physical contact may decrease concussion risk.41-43 Many youth sports organizations have developed policies placing restrictions on head impact during practices and games. Studies are ongoing, too, to see if better headgear—or requiring helmets for soccer players—makes a difference.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, 203 East Fourth Avenue, Ranson, WV 25438; [email protected].
1. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics. 2016; June 20 [Epub ahead of print].
2. Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 2016; May 31 [Epub ahead of print].
3. Mihalik JK, Guskiewicz KM, Valovich McLeod TC, et al. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Ath Tr. 2013;48:645-653.
4. Marar M, McIlvain NM, Fields SK, et al. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747.
5. Kontos AP, Elbin RJ, Fazio-Sumrock VC. Incidence of sports-related concussion among youth football players aged 8-12 years. J Pediatr. 2013;163:717-720.
6. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatr. 2015;169:659-665.
7. Comstock RD, Currie DW, Pierpont LA, et al. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169:830-837.
8. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15-26.
9. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258.
10. Giza CC, Kutcher JS, Ashwal S, et al. Summary of the evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257.
11. Terwilliger VK, Pratson L, Vaughan CG, et al. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J Neurotrauma. 2016;33:761-765.
12. Putukian M, Echemendia R, Dettwiler-Danspeckgruber A. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. 2015;25:36-42.
13. Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050-1057.
14. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139-152.
15. Shrier I. Neuropsychological testing and concussions: a reasoned approach. Clin J Sport Med. 2012;22:211-213.
16. DeMatteo C, Stazyk K, Singh SK, et al. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila). 2015;54:152-163.
17. Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers Association position statement: management of sport concussion. J Athl Train. 2014;49:245-265.
18. Stoller J, Carson JD, Garel A, et al. Do family physicians, emergency department physicians, and pediatricians give consistent sport-related concussion management advice? Can Fam Physician. 2014;60:548, 550-552.
19. Lebrun CM, Mrazik M, Prasad AS, et al. Sport concussion knowledge base, clinical practices and needs for continuing medical education: a survey of family physicians and cross-border comparison. Br J Sports Med. 2013;47:54-59.
20. Zemek R, Eady K, Moreau K, et al. Knowledge of paediatric concussion among front-line primary care providers. Paediatr Child Health. 2014;19:475-480.
21. Maerlender A, Rieman W, Lichtenstein J, et al. Programmed physical exertion in recovery from sports-related concussion: a randomized pilot study. Dev Neuropsychol. 2015;40:273-278.
22. Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2015; July 24 [Epub ahead of print].
23. Preece MH, Horswill MS, Langlois JA, et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378.
24. Baker A, Unsworth CA, Lannin NA. Fitness-to-drive after mild traumatic brain injury: mapping the time trajectory of recovery in the acute stages post injury. Accid Anal Prev. 2015;79:50-55.
25. Marshall S, Bayley M, McCullagh S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257-267.
26. Yorke AM, Littleton S, Alsalaheen BA. Concussion attitudes and beliefs, knowledge, and clinical practice: a survey of physical therapists. Phys Ther. Available at: http://dx.doi.org/10.2522/ptj.20140598. Accessed January 21, 2016.
27. McCrea M, Guskiewicz K, Randolph C, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876-883.
28. Brooks MA, Peterson K, Biese K, et al. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742-747.
29. Witol AD, Webbe FM. Soccer heading frequency predicts neuropsychological deficits. Arch Clin Neuropsychol. 2003;18:397-417.
30. Haran FJ, Tierney R, Wright WG, et al. Acute changes in postural control after soccer heading. Int J Sports Med. 2013;34:350-354.
31. Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850-857.
32. Jordan SE, Green GA, Galanty HL, et al. Acute and chronic brain injury in United States national team soccer players. Am J Sports Med. 1996;24:205-210.
33. Kontos AP, Dolese A, Elbin RJ, et al. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011;25:1234-1241.
34. Straume-Naesheim TM, Andersen TE, Dvorak J, et al. Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br J Sports Med. 2005;39:70-77.
35. Stephens R, Rutherford A, Potter D, et al. Neuropsychological impairment as a consequence of football (soccer) play and football heading: a preliminary analysis and report on school students (13-16 years). Child Neuropsychol. 2005;11:513-526.
36. Stephens R, Rutherford A, Potter D, et al. Neuropsychological consequence of soccer play in adolescent UK school team soccer players. J Neuropsychiatry Clin Neurosci. 2010;22:295-303.
37. Poole VN, Breedlove EL, Shenk TE, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol. 2015;40:12-17.
38. Mannix R, Iverson GL, Maxwell B, et al. Multiple prior concussions are associated with symptoms in high school athletes. Ann Clin Trans Neurol. 2014;1:433-438.
39. Savica R, Parisi JE, Wold LE, et al. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87:335-340.
40. Lancaster M, Muftuler T, Olson D, et al. Chronic white matter changes following sport-related concussion measured by diffusion tensor and diffusion kurtosis imaging. Paper presented at: American Academy of Neurology 2016 Sports Concussion Conference; July 8-10, 2016; Chicago, Ill.
41. Kerr ZY, Yeargin SW, Valovich McLeod TC, et al. Comprehensive coach education reduces head impact exposures in American youth football. Orthop J Sports Med. 2015;3(ecollection):e232596711561545.
42. Black AM, Macpherson AK, Hagel BE, et al. Policy change eliminating body checking in non-elite ice hockey leads to a threefold reduction in injury and concussion risk in 11- and 12-year-old players. Br J Sports Med. 2016;50:55-61.
43. Council on Sports Medicine and Fitness. Tackling in youth football. Policy Statement of the American Academy of Pediatrics. Pediatrics. 2015;136:e1419-e1430.
1. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics. 2016; June 20 [Epub ahead of print].
2. Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 2016; May 31 [Epub ahead of print].
3. Mihalik JK, Guskiewicz KM, Valovich McLeod TC, et al. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Ath Tr. 2013;48:645-653.
4. Marar M, McIlvain NM, Fields SK, et al. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747.
5. Kontos AP, Elbin RJ, Fazio-Sumrock VC. Incidence of sports-related concussion among youth football players aged 8-12 years. J Pediatr. 2013;163:717-720.
6. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatr. 2015;169:659-665.
7. Comstock RD, Currie DW, Pierpont LA, et al. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169:830-837.
8. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15-26.
9. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258.
10. Giza CC, Kutcher JS, Ashwal S, et al. Summary of the evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257.
11. Terwilliger VK, Pratson L, Vaughan CG, et al. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J Neurotrauma. 2016;33:761-765.
12. Putukian M, Echemendia R, Dettwiler-Danspeckgruber A. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. 2015;25:36-42.
13. Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050-1057.
14. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139-152.
15. Shrier I. Neuropsychological testing and concussions: a reasoned approach. Clin J Sport Med. 2012;22:211-213.
16. DeMatteo C, Stazyk K, Singh SK, et al. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila). 2015;54:152-163.
17. Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers Association position statement: management of sport concussion. J Athl Train. 2014;49:245-265.
18. Stoller J, Carson JD, Garel A, et al. Do family physicians, emergency department physicians, and pediatricians give consistent sport-related concussion management advice? Can Fam Physician. 2014;60:548, 550-552.
19. Lebrun CM, Mrazik M, Prasad AS, et al. Sport concussion knowledge base, clinical practices and needs for continuing medical education: a survey of family physicians and cross-border comparison. Br J Sports Med. 2013;47:54-59.
20. Zemek R, Eady K, Moreau K, et al. Knowledge of paediatric concussion among front-line primary care providers. Paediatr Child Health. 2014;19:475-480.
21. Maerlender A, Rieman W, Lichtenstein J, et al. Programmed physical exertion in recovery from sports-related concussion: a randomized pilot study. Dev Neuropsychol. 2015;40:273-278.
22. Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2015; July 24 [Epub ahead of print].
23. Preece MH, Horswill MS, Langlois JA, et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378.
24. Baker A, Unsworth CA, Lannin NA. Fitness-to-drive after mild traumatic brain injury: mapping the time trajectory of recovery in the acute stages post injury. Accid Anal Prev. 2015;79:50-55.
25. Marshall S, Bayley M, McCullagh S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257-267.
26. Yorke AM, Littleton S, Alsalaheen BA. Concussion attitudes and beliefs, knowledge, and clinical practice: a survey of physical therapists. Phys Ther. Available at: http://dx.doi.org/10.2522/ptj.20140598. Accessed January 21, 2016.
27. McCrea M, Guskiewicz K, Randolph C, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876-883.
28. Brooks MA, Peterson K, Biese K, et al. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742-747.
29. Witol AD, Webbe FM. Soccer heading frequency predicts neuropsychological deficits. Arch Clin Neuropsychol. 2003;18:397-417.
30. Haran FJ, Tierney R, Wright WG, et al. Acute changes in postural control after soccer heading. Int J Sports Med. 2013;34:350-354.
31. Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850-857.
32. Jordan SE, Green GA, Galanty HL, et al. Acute and chronic brain injury in United States national team soccer players. Am J Sports Med. 1996;24:205-210.
33. Kontos AP, Dolese A, Elbin RJ, et al. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011;25:1234-1241.
34. Straume-Naesheim TM, Andersen TE, Dvorak J, et al. Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br J Sports Med. 2005;39:70-77.
35. Stephens R, Rutherford A, Potter D, et al. Neuropsychological impairment as a consequence of football (soccer) play and football heading: a preliminary analysis and report on school students (13-16 years). Child Neuropsychol. 2005;11:513-526.
36. Stephens R, Rutherford A, Potter D, et al. Neuropsychological consequence of soccer play in adolescent UK school team soccer players. J Neuropsychiatry Clin Neurosci. 2010;22:295-303.
37. Poole VN, Breedlove EL, Shenk TE, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol. 2015;40:12-17.
38. Mannix R, Iverson GL, Maxwell B, et al. Multiple prior concussions are associated with symptoms in high school athletes. Ann Clin Trans Neurol. 2014;1:433-438.
39. Savica R, Parisi JE, Wold LE, et al. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87:335-340.
40. Lancaster M, Muftuler T, Olson D, et al. Chronic white matter changes following sport-related concussion measured by diffusion tensor and diffusion kurtosis imaging. Paper presented at: American Academy of Neurology 2016 Sports Concussion Conference; July 8-10, 2016; Chicago, Ill.
41. Kerr ZY, Yeargin SW, Valovich McLeod TC, et al. Comprehensive coach education reduces head impact exposures in American youth football. Orthop J Sports Med. 2015;3(ecollection):e232596711561545.
42. Black AM, Macpherson AK, Hagel BE, et al. Policy change eliminating body checking in non-elite ice hockey leads to a threefold reduction in injury and concussion risk in 11- and 12-year-old players. Br J Sports Med. 2016;50:55-61.
43. Council on Sports Medicine and Fitness. Tackling in youth football. Policy Statement of the American Academy of Pediatrics. Pediatrics. 2015;136:e1419-e1430.
From The Journal of Family Practice | 2016;65(8):538-544,546.
The benefits of doing ultrasound exams in your office
Point-of-care ultrasound is increasingly being integrated into clinical practice, as an adjunct to the physical examination and patient history,1 and into medical school curricula across North America.2,3 Research confirms that this technology improves patient survival in emergency medicine settings;4 however, the benefits of point-of-care ultrasound administered by family physicians (FPs) in the office setting are less well documented.
Here we provide a comprehensive review of the indications for ultrasound in the office setting, which range from diagnosing musculoskeletal injuries and guiding injections to screening for abdominal aortic aneurysm (AAA). We also address the accuracy and cost-effectiveness of ultrasound use and the training needed to make family medicine ultrasound (FAMUS) successful.
Ultrasound: A useful screening tool for abdominal aortic aneurysm
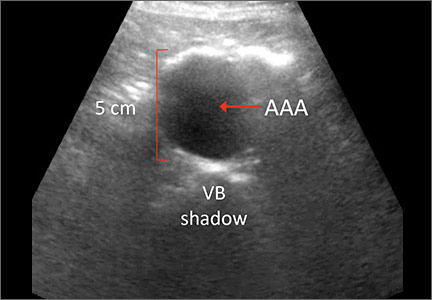
The US Preventive Services Task Force (USPSTF) recommends one-time screening for abdominal aortic aneurysm (AAA) in men ages 65 to 75 years who have ever smoked (See: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/abdominal-aortic-aneurysm-screening.) Ultrasound is a reliable tool for identifying AAA5 (FIGURE 1); its sensitivity and specificity range from 94% to 98.9% and 98% to 100%, respectively.6-9 It is also superior to physical examination for AAAs,10 which has a sensitivity of 29% for small AAAs (30-39 mm) and 76% for larger AAAs (>50 mm).11
Most importantly, research has demonstrated that long-term mortality benefits are associated with ultrasound screening of asymptomatic patients for AAA. For example, one study found that screening asymptomatic men ages 65 to 74 (a population-based sample, with no particular risk factors) for AAA resulted in a reduction in all-cause mortality and that the benefit of AAA-related mortality continued to accumulate throughout follow-up.12
In fact, nationwide programs to screen for AAA using ultrasound have been established in England, Northern Ireland, Scotland, Sweden, the United States, and Wales to help prevent deaths associated with AAA rupture.13 Despite the documented benefits of ultrasound screening for AAA, a large retrospective cohort study conducted in an American integrated health care system found that only about 9% of patients eligible for screening according to USPSTF guidelines were screened for AAA with ultrasound in primary care practices in 2012.14
While most AAA screening occurs in the hospital, screening for the condition can be just as easily and effectively performed in an FP’s office or outpatient clinic. A Canadian prospective observational study demonstrated that aortic diameter measurements were comparable whether they were obtained by ultrasound performed by an office-based physician (who had completed an emergency ultrasonography course and performed at least 50 ultrasonographer-supervised ultrasound scans of the aorta), or by a hospital-based technologist whose scans were then reviewed by a radiologist.15 (See the TABLE for an overview of the research involving family medicine ultrasound.)
The office-based scans had a high degree of correlation (0.81) with the hospital-based ones, a sensitivity and specificity of 100%, and lasted a mean of 3.5 minutes. The researchers concluded that ultrasound screening for AAA can be safely performed in the office setting by FPs who are trained to use point-of-care ultrasound technology, and that the screening can be completed within the time constraints of a typical family practice office visit.15
In a separate study, cardiologists compared hand-held ultrasound screening for AAA to standard 2-dimensional echocardiography. This study found that screening for AAA in an outpatient clinic with a hand-held ultrasound device is feasible and accurate with a sensitivity of 88% and a specificity of 98%.16
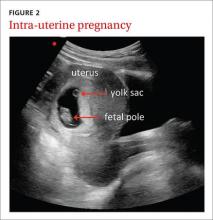
Ultrasound in the obstetrician’s office—and the FP’s office, too
The use of ultrasound in obstetrics (FIGURE 2) is particularly well documented, with evidence supporting the use of FAMUS for various obstetrical indications dating back 30 years.17 The American Academy of Family Physicians has a position paper endorsing diagnostic ultrasound for women’s health care and has offered obstetric ultrasound courses organized by, and for, FPs since 1989.18
In a prospective observational study conducted in the United Kingdom, an FP and a nurse midwife used ultrasound to assess 240 pregnant women presenting with vaginal bleeding in early pregnancy.19 Fetal heartbeat detection by an office ultrasound scan predicted fetal progression to 20 weeks with a sensitivity of 97% and a specificity of 98%. The clinicians also detected anomalies such as molar pregnancy, blighted ovum, and ectopic pregnancy.
FAMUS and its ability to accurately estimate delivery date was examined in another prospective study involving 186 patients at a community health center.20 Accuracy for the estimated date of delivery was 96% using stratified confidence intervals for first-, second-, and third-trimester examinations. The office-based ultrasound scans also detected one case of placenta previa, one fetal death, and 2 unsuspected twin pregnancies. Another study showed no difference in estimations of gestational age provided by ultrasound performed by supervised FP residents with 3 years’ ultrasound training (including 3 lectures per year and an annual 4-hour workshop), and radiologists.21
Further evidence that FAMUS can confirm fetal death and multiple gestations was provided by a retrospective review of almost 498 obstetric ultrasound examinations.22 FPs accurately predicted the presence or absence of fetal death, multiple gestations, and the estimated date of confinement. Another study demonstrated that 86% of 248 FP obstetrical scans were judged acceptable by a radiologist, 10% were repeated due to technical errors and subsequently found to be acceptable, and 3% were unacceptable and referred for formal ultrasound.23 These scans were performed by FPs who completed 5 days of theory and hands-on training and 3 half-days of apprenticeship in an ultrasound laboratory.
In a study conducted in Tanzania, bedside ultrasound scans performed by nurse midwives had 100% agreement with scans performed by a sonographer when evaluating for twins, the presence of fetal heartbeat, or fetal positioning. Overall, bedside ultrasound aided in the diagnosis (39%) and management plan (22%) of 542 patients.24 It is important to note, as highlighted in a multisite study, that consultation with specialists when appropriate is paramount to the successful use of ultrasound by the FP for prenatal care.25
Guiding joint injections, assessing LV function
Sports/exercise medicine. FPs with expertise in sports and exercise medicine commonly use office ultrasound to diagnose musculoskeletal (MSK) injuries, including rotator cuff tears, muscle ruptures, tendinitis, and bursitis.26 It is superior to magnetic resonance imaging (MRI) in terms of cost-to-benefit ratio, precision, and sensitivity (due, in part, to the fact that clinicians can obtain patient feedback during the examination).26 In addition, a review of office-based procedures for MSK indications demonstrated the usefulness of ultrasound for the guidance of joint aspirations and joint and tendon injections.27 Ultrasound guidance is commonly used to ensure procedural accuracy during aspirations and injections of the shoulder (glenohumeral joint; subacromial bursa), elbow, wrist (carpal tunnel tendons), hip, knee, and ankle.27-29
Cardiology (FIGURE 3). General practitioners in Norway found that 8 hours of training on a hand-held ultrasound device was sufficient to assess left ventricular function with a sensitivity and specificity of 78% and 83%, respectively.30 Their measurements of septal mitral annular excursion (a surrogate measurement of left ventricular function) were similar to those of a cardiologist using the same device and added no more than 5 minutes to the examination.
Other uses. In a separate study, military FPs with 16 hours of training found that FAMUS was easy to learn and effective in the outpatient and inpatient setting for the detection of AAA, trauma, musculoskeletal injuries, and certain obstetric, echocardiographic, and biliary indications.31 They reported that the average time spent per ultrasound examination was one to 5 minutes for the majority of the indications.
The authors of a retrospective study involving a suburban family practice reported that FAMUS was successfully used to identify the causes of epigastric and right upper quadrant pain, and to check post-void residual urinary bladder volume.32
The ultrasound-assisted physical examination can detect pathologies not apparent on history and physical examination alone (FIGURES 4 and 5). In one study, an FP used ultrasound in the office to identify pathologies in 31% of patients that were not detected on physical examination alone. The pathologies included AAAs, a thyroid cyst, mitral stenosis, gallstones, renal cysts, urinary retention, hydronephrosis, ectopic kidney, and an endometrial tumor.33
| 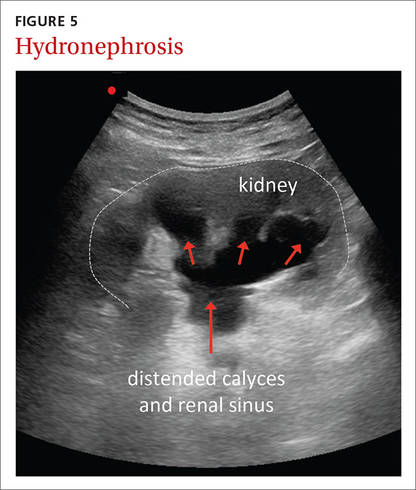
|
In another study, an FP performed ultrasound examinations on 189 patients during their annual exams.34 The technology identified pathologies that were not suspected after clinical assessment in 22% of these patients. With the emphasis in the current clinical landscape on choosing diagnostic tests wisely, it will be important to determine if findings like these positively impact patient care.35,36
Portable ultrasound machines are affordable
The relative affordability of portable ultrasound contributes to the cost-effectiveness of FAMUS. For FPs seeking to initiate an office-based ultrasound program, expenses to consider include the price of the machine itself, which ranges from $7500 to $50,000, depending on the technology included. Other expenses include the cost of disposables (eg, ultrasound gel and disinfectant wipes or spray), which may total about $400 per year.
In-office exams facilitate savings elsewhere. Other factors that contribute to the cost-effectiveness of FAMUS include reduced radiologist expenses and hospital visits. The cost savings of in-office ultrasound was highlighted almost 30 years ago when the cost of a FAMUS obstetrical scan was reported to be half that of a radiologist scan.23 This same study reported that increased costs for additional investigations caused by incidental findings using FAMUS could be offset by the decreased costs associated with an earlier diagnosis of serious conditions.23
A 2002 study demonstrated that office-based FAMUS scans (N=131) reduced the number of hospital scans, emergency admissions, and outpatient and inpatient hospital visits.37 Although the unit cost of a FAMUS scan was higher than an inpatient one, the total cost of the FAMUS scan was lower due to decreased hospital visits. In addition, research has shown that patients are more satisfied with office-based ultrasound examinations and prefer ultrasound performed by their FP to hospital-based ultrasound scans.31,37
Training: Cost and availability
Training in office-based ultrasound is available at the undergraduate, postgraduate, and continuing medical education levels. Undergraduate bedside ultrasound education is evident in medical schools around the globe including in Australia, Austria, Canada, China, Germany, France, the United States, and the United Kingdom.3 In an American survey of family medicine residency programs published in 2015, only 2.2% reported an established ultrasound curriculum; however, 29% had started a program within the past year.38 In Canada, one- and 2-day bedside ultrasound courses are offered to family medicine residents at a number of universities. And continuing medical education (CME) courses in bedside ultrasound are available to physicians on a regular basis internationally.39 In North America, CME courses exist specifically for urban and rural family medicine clinicians,40-43 and offer training for a wide range of applications.
Courses are often available for $1000 to $2000. Many of these courses run over a one- to 3-day period. Some provide a general overview of ultrasound for the primary care physician while others specialize in topics such as musculoskeletal uses, obstetric uses, or emergency department echocardiography.40-44
Challenges remain
More research is necessary to demonstrate that office-based ultrasound produces patient outcomes that are comparable to those resulting from hospital-based ultrasound. Also, bedside ultrasound is only as good as the operator who performs the examination,45 which highlights the importance of developing bedside ultrasound training programs tailored for FPs. National policies are essential for standardizing indications, training, and credentialing so that this effective tool can be used in a safe and effective manner.
CORRESPONDENCE
Peter Steinmetz, MD, CCFP, St. Mary’s Hospital, 3830 Ave Lacombe, Montreal, Quebec, Canada H3T1M5; [email protected].
ACKNOWLEDGEMENTS
We thank Assistant Professor Marion Dove, MD, CCFP, Department of Family Medicine, McGill University, for her suggestions and critical review of an earlier version of the manuscript. The term FAMUS is pending registration and is advertised with the Canadian Intellectual Property Office (Steinmetz, Volume 63, Issue 3217).
1. Solomon SD, Saldana F. Point-of-care ultrasound in medical education - stop listening and look. N Engl J Med. 2014;370:1083-1085.
2. Bahner DP, Goldman E, Way D, et al. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89:1681-1686.
3. Steinmetz P, Dobrescu O, Oleskevich S, et al. Bedside ultrasound education in Canadian medical schools: a national survey. Can Med Educ J. 2016;7:e78-e86.
4. Deshpande R, Akhtar S, Haddadin AS. Utility of ultrasound in the ICU. Curr Opin Anaesthesiol. 2014;27:123-132.
5. Wilmink ABM, Hubbard CSFF, Quick CRG. Quality of the measurement of the infrarenal aortic diameter by ultrasound. J Med Screen. 1997;4:49-53.
6. Costantino TG, Bruno EC, Handly N, et al. Accuracy of emergency medicine ultrasound in the evaluation of abdominal aortic aneurysm. J Emerg Med. 2005;29:455-460.
7. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
8. Nusbaum JW, Freimanis AK, Thomford NR. Echography in the diagnosis of abdominal aortic aneurysm. Arch Surg. 1971;102:385-388.
9. Wilmink ABM, Forshaw M, Quick CRG, et al. Accuracy of serial screening for abdominal aortic aneurysms by ultrasound. J Med Screen. 2002;9:125-127.
10. Lynch RM. Accuracy of abdominal examination in the diagnosis of non-ruptured abdominal aortic aneurysm. Accid Emerg Nurs. 2004;12:99-107.
11. Lederle FA, Simel DL. The rational clinical examination. Does this patient have abdominal aortic aneurysm? JAMA. 1999;281:77-82.
12. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656.
13. Stather PW, Dattani N, Bown MJ, et al. International variations in AAA screening. Eur J Vasc Endovasc Surg. 2013;45:231-234.
14. Ruff AL, Teng K, Hu B, et al. Screening for abdominal aortic aneurysms in outpatient primary care clinics. Am J Med. 2015;128:283-288.
15. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-178.
16. Vourvouri EC, Poldermans D, Schinkel AF, et al. Abdominal aortic aneurysm screening using a hand-held ultrasound device. “A pilot study”. Eur J Vasc Endovasc Surg. 2001;22:352-354.
17. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
18. American Academy of Family Physicians. Position Paper: Diagnostic ultrasonography in women’s health care. 2013. Available at http://www.aafp.org/about/policies/all/ultrasonography-diagnostic.html. Accessed 2013.
19. Everett CB, Preece E. Women with bleeding in the first 20 weeks of pregnancy: value of general practice ultrasound in detecting fetal heart movement. Br J Gen Pract. 1996;46:7-9.
20. Rodney WM, Prislin MD, Orientale E, et al. Family practice obstetric ultrasound in an urban community health center. Birth outcomes and examination accuracy of the initial 227 cases. J Fam Pract. 1990;30:163-168.
21. Keith R, Frisch L. Fetal biometry: a comparison of family physicians and radiologists. Fam Med. 2001;33:111-114.
22. Ornstein SM, Smith MA, Peggs J, et al. Obstetric ultrasound by family physicians. Adequacy as assessed by pregnancy outcome. J Fam Pract. 1990;30:403-408.
23. Hahn RG, Ho S, Roi LD, et al. Cost-effectiveness of office obstetrical ultrasound in family practice: preliminary considerations. J Am Board Fam Pract. 1988;1:33-38.
24. Stein W, Katunda I, Butoto C. A two-level ultrasonographic service in a maternity care unit of a rural district hospital in Tanzania. Trop Doct. 2008;38:125-126.
25. Morgan WC, Rodney WM, Hahn R, et al. Ultrasound for the primary care physician. Applications in family-centered obstetrics. Postgrad Med. 1988;83:103-107.
26. Coris EE, Pescasio M, Zwygart K, et al. Office-based ultrasound in sports medicine practice. Clin J Sport Med. 2011;21:57-61.
27. Royall NA, Farrin E, Bahner DP, et al. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2:57-66.
28. Bianchi S, Martinoli C. Ultrasound of the musculoskeletal system. Berlin; New York: Springer; 2007.
29. Narouze SN. Atlas of ultrasound-guided procedures in interventional pain management. New York: Springer; 2011.
30. Mjolstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
31. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-477.
32. Chan VSP, Piterman L, McCall L. Use of clinical ultrasonography in an Australian suburban family practice: its indications and findings. Hong Kong Practitioner. 1999;21:405-415.
33. Siepel T, Clifford DS, James PA, et al. The ultrasound-assisted physical examination in the periodic health evaluation of the elderly. J Fam Pract. 2000;49:628-632.
34. Rosenthal TC, Siepel T, Zubler J, et al. The use of ultrasonography to scan the abdomen of patients presenting for routine physical examinations. J Fam Pract. 1994;38:380-385.
35. Choosing Wisely Canada. Available at: http://www.choosingwiselycanada.org. Accessed 2016.
36. Hale I. Add to cart? Can Fam Physician. 2015;61:937-939.
37. Wordsworth S, Scott A. Ultrasound scanning by general practitioners: is it worthwhile? J Public Health Med. 2002;24:88-94.
38. Hall JW, Holman H, Bornemann P, et al. Point of Care Ultrasound in Family Medicine Residency Programs: A CERA Study. Fam Med. 2015;47:706-711.
39. WINFOCUS-World Interactive Network Focused on Critical UltraSound. Available at: http://www.winfocus.it/#winfocus. Accessed 2016.
40. McGill University. McGill Ultrasound Evaluation Program (MUSE). Bedside ultrasound course for primary care clinicians (MUSE 1.0). Available at: www.mcgill.ca/medsimcentre/muse. Accessed 2016.
41. The University of British Columbia. Faculty of Medicine UBC CPD (Continuing Professional Development). CPD/CME courses. Available at: http://ubccpd.ca/courses?combine=ultrasound&field_target_audience_tid=3&field_learning_type_tid=All&field_location_tid=All&field_cost_tid=All&
field_credit_type_tid=All&field_number_of_credits_tid=All&field_event_date_value_1%5Bvalue%5D%5Bdate%5D=. Accessed 2016.
42. Emergency Department Echo (EDE). Available at: www.edecourse.com. Accessed 2016.
43. McGill University. MUSE 2.0 Advanced Bedside Ultrasound Course. Available at: http://www.mcgill.ca/medsimcentre/channels/event/muse-20-advanced-bedside-ultrasound-course-256963. Accessed 2016.
44. Gulfcoast Ultrasound Institute. Available at: https://www.gcus.com. Accessed 2016.
45. Allen GM, Wilson DJ. Ultrasound in sports medicine—a critical evaluation. Eur J Radiol. 2007;62:79-85.
Point-of-care ultrasound is increasingly being integrated into clinical practice, as an adjunct to the physical examination and patient history,1 and into medical school curricula across North America.2,3 Research confirms that this technology improves patient survival in emergency medicine settings;4 however, the benefits of point-of-care ultrasound administered by family physicians (FPs) in the office setting are less well documented.
Here we provide a comprehensive review of the indications for ultrasound in the office setting, which range from diagnosing musculoskeletal injuries and guiding injections to screening for abdominal aortic aneurysm (AAA). We also address the accuracy and cost-effectiveness of ultrasound use and the training needed to make family medicine ultrasound (FAMUS) successful.
Ultrasound: A useful screening tool for abdominal aortic aneurysm
The US Preventive Services Task Force (USPSTF) recommends one-time screening for abdominal aortic aneurysm (AAA) in men ages 65 to 75 years who have ever smoked (See: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/abdominal-aortic-aneurysm-screening.) Ultrasound is a reliable tool for identifying AAA5 (FIGURE 1); its sensitivity and specificity range from 94% to 98.9% and 98% to 100%, respectively.6-9 It is also superior to physical examination for AAAs,10 which has a sensitivity of 29% for small AAAs (30-39 mm) and 76% for larger AAAs (>50 mm).11
Most importantly, research has demonstrated that long-term mortality benefits are associated with ultrasound screening of asymptomatic patients for AAA. For example, one study found that screening asymptomatic men ages 65 to 74 (a population-based sample, with no particular risk factors) for AAA resulted in a reduction in all-cause mortality and that the benefit of AAA-related mortality continued to accumulate throughout follow-up.12
In fact, nationwide programs to screen for AAA using ultrasound have been established in England, Northern Ireland, Scotland, Sweden, the United States, and Wales to help prevent deaths associated with AAA rupture.13 Despite the documented benefits of ultrasound screening for AAA, a large retrospective cohort study conducted in an American integrated health care system found that only about 9% of patients eligible for screening according to USPSTF guidelines were screened for AAA with ultrasound in primary care practices in 2012.14
While most AAA screening occurs in the hospital, screening for the condition can be just as easily and effectively performed in an FP’s office or outpatient clinic. A Canadian prospective observational study demonstrated that aortic diameter measurements were comparable whether they were obtained by ultrasound performed by an office-based physician (who had completed an emergency ultrasonography course and performed at least 50 ultrasonographer-supervised ultrasound scans of the aorta), or by a hospital-based technologist whose scans were then reviewed by a radiologist.15 (See the TABLE for an overview of the research involving family medicine ultrasound.)
The office-based scans had a high degree of correlation (0.81) with the hospital-based ones, a sensitivity and specificity of 100%, and lasted a mean of 3.5 minutes. The researchers concluded that ultrasound screening for AAA can be safely performed in the office setting by FPs who are trained to use point-of-care ultrasound technology, and that the screening can be completed within the time constraints of a typical family practice office visit.15
In a separate study, cardiologists compared hand-held ultrasound screening for AAA to standard 2-dimensional echocardiography. This study found that screening for AAA in an outpatient clinic with a hand-held ultrasound device is feasible and accurate with a sensitivity of 88% and a specificity of 98%.16
Ultrasound in the obstetrician’s office—and the FP’s office, too
The use of ultrasound in obstetrics (FIGURE 2) is particularly well documented, with evidence supporting the use of FAMUS for various obstetrical indications dating back 30 years.17 The American Academy of Family Physicians has a position paper endorsing diagnostic ultrasound for women’s health care and has offered obstetric ultrasound courses organized by, and for, FPs since 1989.18
In a prospective observational study conducted in the United Kingdom, an FP and a nurse midwife used ultrasound to assess 240 pregnant women presenting with vaginal bleeding in early pregnancy.19 Fetal heartbeat detection by an office ultrasound scan predicted fetal progression to 20 weeks with a sensitivity of 97% and a specificity of 98%. The clinicians also detected anomalies such as molar pregnancy, blighted ovum, and ectopic pregnancy.
FAMUS and its ability to accurately estimate delivery date was examined in another prospective study involving 186 patients at a community health center.20 Accuracy for the estimated date of delivery was 96% using stratified confidence intervals for first-, second-, and third-trimester examinations. The office-based ultrasound scans also detected one case of placenta previa, one fetal death, and 2 unsuspected twin pregnancies. Another study showed no difference in estimations of gestational age provided by ultrasound performed by supervised FP residents with 3 years’ ultrasound training (including 3 lectures per year and an annual 4-hour workshop), and radiologists.21
Further evidence that FAMUS can confirm fetal death and multiple gestations was provided by a retrospective review of almost 498 obstetric ultrasound examinations.22 FPs accurately predicted the presence or absence of fetal death, multiple gestations, and the estimated date of confinement. Another study demonstrated that 86% of 248 FP obstetrical scans were judged acceptable by a radiologist, 10% were repeated due to technical errors and subsequently found to be acceptable, and 3% were unacceptable and referred for formal ultrasound.23 These scans were performed by FPs who completed 5 days of theory and hands-on training and 3 half-days of apprenticeship in an ultrasound laboratory.
In a study conducted in Tanzania, bedside ultrasound scans performed by nurse midwives had 100% agreement with scans performed by a sonographer when evaluating for twins, the presence of fetal heartbeat, or fetal positioning. Overall, bedside ultrasound aided in the diagnosis (39%) and management plan (22%) of 542 patients.24 It is important to note, as highlighted in a multisite study, that consultation with specialists when appropriate is paramount to the successful use of ultrasound by the FP for prenatal care.25
Guiding joint injections, assessing LV function
Sports/exercise medicine. FPs with expertise in sports and exercise medicine commonly use office ultrasound to diagnose musculoskeletal (MSK) injuries, including rotator cuff tears, muscle ruptures, tendinitis, and bursitis.26 It is superior to magnetic resonance imaging (MRI) in terms of cost-to-benefit ratio, precision, and sensitivity (due, in part, to the fact that clinicians can obtain patient feedback during the examination).26 In addition, a review of office-based procedures for MSK indications demonstrated the usefulness of ultrasound for the guidance of joint aspirations and joint and tendon injections.27 Ultrasound guidance is commonly used to ensure procedural accuracy during aspirations and injections of the shoulder (glenohumeral joint; subacromial bursa), elbow, wrist (carpal tunnel tendons), hip, knee, and ankle.27-29
Cardiology (FIGURE 3). General practitioners in Norway found that 8 hours of training on a hand-held ultrasound device was sufficient to assess left ventricular function with a sensitivity and specificity of 78% and 83%, respectively.30 Their measurements of septal mitral annular excursion (a surrogate measurement of left ventricular function) were similar to those of a cardiologist using the same device and added no more than 5 minutes to the examination.
Other uses. In a separate study, military FPs with 16 hours of training found that FAMUS was easy to learn and effective in the outpatient and inpatient setting for the detection of AAA, trauma, musculoskeletal injuries, and certain obstetric, echocardiographic, and biliary indications.31 They reported that the average time spent per ultrasound examination was one to 5 minutes for the majority of the indications.
The authors of a retrospective study involving a suburban family practice reported that FAMUS was successfully used to identify the causes of epigastric and right upper quadrant pain, and to check post-void residual urinary bladder volume.32
The ultrasound-assisted physical examination can detect pathologies not apparent on history and physical examination alone (FIGURES 4 and 5). In one study, an FP used ultrasound in the office to identify pathologies in 31% of patients that were not detected on physical examination alone. The pathologies included AAAs, a thyroid cyst, mitral stenosis, gallstones, renal cysts, urinary retention, hydronephrosis, ectopic kidney, and an endometrial tumor.33

| 
|
In another study, an FP performed ultrasound examinations on 189 patients during their annual exams.34 The technology identified pathologies that were not suspected after clinical assessment in 22% of these patients. With the emphasis in the current clinical landscape on choosing diagnostic tests wisely, it will be important to determine if findings like these positively impact patient care.35,36
Portable ultrasound machines are affordable
The relative affordability of portable ultrasound contributes to the cost-effectiveness of FAMUS. For FPs seeking to initiate an office-based ultrasound program, expenses to consider include the price of the machine itself, which ranges from $7500 to $50,000, depending on the technology included. Other expenses include the cost of disposables (eg, ultrasound gel and disinfectant wipes or spray), which may total about $400 per year.
In-office exams facilitate savings elsewhere. Other factors that contribute to the cost-effectiveness of FAMUS include reduced radiologist expenses and hospital visits. The cost savings of in-office ultrasound was highlighted almost 30 years ago when the cost of a FAMUS obstetrical scan was reported to be half that of a radiologist scan.23 This same study reported that increased costs for additional investigations caused by incidental findings using FAMUS could be offset by the decreased costs associated with an earlier diagnosis of serious conditions.23
A 2002 study demonstrated that office-based FAMUS scans (N=131) reduced the number of hospital scans, emergency admissions, and outpatient and inpatient hospital visits.37 Although the unit cost of a FAMUS scan was higher than an inpatient one, the total cost of the FAMUS scan was lower due to decreased hospital visits. In addition, research has shown that patients are more satisfied with office-based ultrasound examinations and prefer ultrasound performed by their FP to hospital-based ultrasound scans.31,37
Training: Cost and availability
Training in office-based ultrasound is available at the undergraduate, postgraduate, and continuing medical education levels. Undergraduate bedside ultrasound education is evident in medical schools around the globe including in Australia, Austria, Canada, China, Germany, France, the United States, and the United Kingdom.3 In an American survey of family medicine residency programs published in 2015, only 2.2% reported an established ultrasound curriculum; however, 29% had started a program within the past year.38 In Canada, one- and 2-day bedside ultrasound courses are offered to family medicine residents at a number of universities. And continuing medical education (CME) courses in bedside ultrasound are available to physicians on a regular basis internationally.39 In North America, CME courses exist specifically for urban and rural family medicine clinicians,40-43 and offer training for a wide range of applications.
Courses are often available for $1000 to $2000. Many of these courses run over a one- to 3-day period. Some provide a general overview of ultrasound for the primary care physician while others specialize in topics such as musculoskeletal uses, obstetric uses, or emergency department echocardiography.40-44
Challenges remain
More research is necessary to demonstrate that office-based ultrasound produces patient outcomes that are comparable to those resulting from hospital-based ultrasound. Also, bedside ultrasound is only as good as the operator who performs the examination,45 which highlights the importance of developing bedside ultrasound training programs tailored for FPs. National policies are essential for standardizing indications, training, and credentialing so that this effective tool can be used in a safe and effective manner.
CORRESPONDENCE
Peter Steinmetz, MD, CCFP, St. Mary’s Hospital, 3830 Ave Lacombe, Montreal, Quebec, Canada H3T1M5; [email protected].
ACKNOWLEDGEMENTS
We thank Assistant Professor Marion Dove, MD, CCFP, Department of Family Medicine, McGill University, for her suggestions and critical review of an earlier version of the manuscript. The term FAMUS is pending registration and is advertised with the Canadian Intellectual Property Office (Steinmetz, Volume 63, Issue 3217).
Point-of-care ultrasound is increasingly being integrated into clinical practice, as an adjunct to the physical examination and patient history,1 and into medical school curricula across North America.2,3 Research confirms that this technology improves patient survival in emergency medicine settings;4 however, the benefits of point-of-care ultrasound administered by family physicians (FPs) in the office setting are less well documented.
Here we provide a comprehensive review of the indications for ultrasound in the office setting, which range from diagnosing musculoskeletal injuries and guiding injections to screening for abdominal aortic aneurysm (AAA). We also address the accuracy and cost-effectiveness of ultrasound use and the training needed to make family medicine ultrasound (FAMUS) successful.
Ultrasound: A useful screening tool for abdominal aortic aneurysm
The US Preventive Services Task Force (USPSTF) recommends one-time screening for abdominal aortic aneurysm (AAA) in men ages 65 to 75 years who have ever smoked (See: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/abdominal-aortic-aneurysm-screening.) Ultrasound is a reliable tool for identifying AAA5 (FIGURE 1); its sensitivity and specificity range from 94% to 98.9% and 98% to 100%, respectively.6-9 It is also superior to physical examination for AAAs,10 which has a sensitivity of 29% for small AAAs (30-39 mm) and 76% for larger AAAs (>50 mm).11
Most importantly, research has demonstrated that long-term mortality benefits are associated with ultrasound screening of asymptomatic patients for AAA. For example, one study found that screening asymptomatic men ages 65 to 74 (a population-based sample, with no particular risk factors) for AAA resulted in a reduction in all-cause mortality and that the benefit of AAA-related mortality continued to accumulate throughout follow-up.12
In fact, nationwide programs to screen for AAA using ultrasound have been established in England, Northern Ireland, Scotland, Sweden, the United States, and Wales to help prevent deaths associated with AAA rupture.13 Despite the documented benefits of ultrasound screening for AAA, a large retrospective cohort study conducted in an American integrated health care system found that only about 9% of patients eligible for screening according to USPSTF guidelines were screened for AAA with ultrasound in primary care practices in 2012.14
While most AAA screening occurs in the hospital, screening for the condition can be just as easily and effectively performed in an FP’s office or outpatient clinic. A Canadian prospective observational study demonstrated that aortic diameter measurements were comparable whether they were obtained by ultrasound performed by an office-based physician (who had completed an emergency ultrasonography course and performed at least 50 ultrasonographer-supervised ultrasound scans of the aorta), or by a hospital-based technologist whose scans were then reviewed by a radiologist.15 (See the TABLE for an overview of the research involving family medicine ultrasound.)
The office-based scans had a high degree of correlation (0.81) with the hospital-based ones, a sensitivity and specificity of 100%, and lasted a mean of 3.5 minutes. The researchers concluded that ultrasound screening for AAA can be safely performed in the office setting by FPs who are trained to use point-of-care ultrasound technology, and that the screening can be completed within the time constraints of a typical family practice office visit.15
In a separate study, cardiologists compared hand-held ultrasound screening for AAA to standard 2-dimensional echocardiography. This study found that screening for AAA in an outpatient clinic with a hand-held ultrasound device is feasible and accurate with a sensitivity of 88% and a specificity of 98%.16
Ultrasound in the obstetrician’s office—and the FP’s office, too
The use of ultrasound in obstetrics (FIGURE 2) is particularly well documented, with evidence supporting the use of FAMUS for various obstetrical indications dating back 30 years.17 The American Academy of Family Physicians has a position paper endorsing diagnostic ultrasound for women’s health care and has offered obstetric ultrasound courses organized by, and for, FPs since 1989.18
In a prospective observational study conducted in the United Kingdom, an FP and a nurse midwife used ultrasound to assess 240 pregnant women presenting with vaginal bleeding in early pregnancy.19 Fetal heartbeat detection by an office ultrasound scan predicted fetal progression to 20 weeks with a sensitivity of 97% and a specificity of 98%. The clinicians also detected anomalies such as molar pregnancy, blighted ovum, and ectopic pregnancy.
FAMUS and its ability to accurately estimate delivery date was examined in another prospective study involving 186 patients at a community health center.20 Accuracy for the estimated date of delivery was 96% using stratified confidence intervals for first-, second-, and third-trimester examinations. The office-based ultrasound scans also detected one case of placenta previa, one fetal death, and 2 unsuspected twin pregnancies. Another study showed no difference in estimations of gestational age provided by ultrasound performed by supervised FP residents with 3 years’ ultrasound training (including 3 lectures per year and an annual 4-hour workshop), and radiologists.21
Further evidence that FAMUS can confirm fetal death and multiple gestations was provided by a retrospective review of almost 498 obstetric ultrasound examinations.22 FPs accurately predicted the presence or absence of fetal death, multiple gestations, and the estimated date of confinement. Another study demonstrated that 86% of 248 FP obstetrical scans were judged acceptable by a radiologist, 10% were repeated due to technical errors and subsequently found to be acceptable, and 3% were unacceptable and referred for formal ultrasound.23 These scans were performed by FPs who completed 5 days of theory and hands-on training and 3 half-days of apprenticeship in an ultrasound laboratory.
In a study conducted in Tanzania, bedside ultrasound scans performed by nurse midwives had 100% agreement with scans performed by a sonographer when evaluating for twins, the presence of fetal heartbeat, or fetal positioning. Overall, bedside ultrasound aided in the diagnosis (39%) and management plan (22%) of 542 patients.24 It is important to note, as highlighted in a multisite study, that consultation with specialists when appropriate is paramount to the successful use of ultrasound by the FP for prenatal care.25
Guiding joint injections, assessing LV function
Sports/exercise medicine. FPs with expertise in sports and exercise medicine commonly use office ultrasound to diagnose musculoskeletal (MSK) injuries, including rotator cuff tears, muscle ruptures, tendinitis, and bursitis.26 It is superior to magnetic resonance imaging (MRI) in terms of cost-to-benefit ratio, precision, and sensitivity (due, in part, to the fact that clinicians can obtain patient feedback during the examination).26 In addition, a review of office-based procedures for MSK indications demonstrated the usefulness of ultrasound for the guidance of joint aspirations and joint and tendon injections.27 Ultrasound guidance is commonly used to ensure procedural accuracy during aspirations and injections of the shoulder (glenohumeral joint; subacromial bursa), elbow, wrist (carpal tunnel tendons), hip, knee, and ankle.27-29
Cardiology (FIGURE 3). General practitioners in Norway found that 8 hours of training on a hand-held ultrasound device was sufficient to assess left ventricular function with a sensitivity and specificity of 78% and 83%, respectively.30 Their measurements of septal mitral annular excursion (a surrogate measurement of left ventricular function) were similar to those of a cardiologist using the same device and added no more than 5 minutes to the examination.
Other uses. In a separate study, military FPs with 16 hours of training found that FAMUS was easy to learn and effective in the outpatient and inpatient setting for the detection of AAA, trauma, musculoskeletal injuries, and certain obstetric, echocardiographic, and biliary indications.31 They reported that the average time spent per ultrasound examination was one to 5 minutes for the majority of the indications.
The authors of a retrospective study involving a suburban family practice reported that FAMUS was successfully used to identify the causes of epigastric and right upper quadrant pain, and to check post-void residual urinary bladder volume.32
The ultrasound-assisted physical examination can detect pathologies not apparent on history and physical examination alone (FIGURES 4 and 5). In one study, an FP used ultrasound in the office to identify pathologies in 31% of patients that were not detected on physical examination alone. The pathologies included AAAs, a thyroid cyst, mitral stenosis, gallstones, renal cysts, urinary retention, hydronephrosis, ectopic kidney, and an endometrial tumor.33

| 
|
In another study, an FP performed ultrasound examinations on 189 patients during their annual exams.34 The technology identified pathologies that were not suspected after clinical assessment in 22% of these patients. With the emphasis in the current clinical landscape on choosing diagnostic tests wisely, it will be important to determine if findings like these positively impact patient care.35,36
Portable ultrasound machines are affordable
The relative affordability of portable ultrasound contributes to the cost-effectiveness of FAMUS. For FPs seeking to initiate an office-based ultrasound program, expenses to consider include the price of the machine itself, which ranges from $7500 to $50,000, depending on the technology included. Other expenses include the cost of disposables (eg, ultrasound gel and disinfectant wipes or spray), which may total about $400 per year.
In-office exams facilitate savings elsewhere. Other factors that contribute to the cost-effectiveness of FAMUS include reduced radiologist expenses and hospital visits. The cost savings of in-office ultrasound was highlighted almost 30 years ago when the cost of a FAMUS obstetrical scan was reported to be half that of a radiologist scan.23 This same study reported that increased costs for additional investigations caused by incidental findings using FAMUS could be offset by the decreased costs associated with an earlier diagnosis of serious conditions.23
A 2002 study demonstrated that office-based FAMUS scans (N=131) reduced the number of hospital scans, emergency admissions, and outpatient and inpatient hospital visits.37 Although the unit cost of a FAMUS scan was higher than an inpatient one, the total cost of the FAMUS scan was lower due to decreased hospital visits. In addition, research has shown that patients are more satisfied with office-based ultrasound examinations and prefer ultrasound performed by their FP to hospital-based ultrasound scans.31,37
Training: Cost and availability
Training in office-based ultrasound is available at the undergraduate, postgraduate, and continuing medical education levels. Undergraduate bedside ultrasound education is evident in medical schools around the globe including in Australia, Austria, Canada, China, Germany, France, the United States, and the United Kingdom.3 In an American survey of family medicine residency programs published in 2015, only 2.2% reported an established ultrasound curriculum; however, 29% had started a program within the past year.38 In Canada, one- and 2-day bedside ultrasound courses are offered to family medicine residents at a number of universities. And continuing medical education (CME) courses in bedside ultrasound are available to physicians on a regular basis internationally.39 In North America, CME courses exist specifically for urban and rural family medicine clinicians,40-43 and offer training for a wide range of applications.
Courses are often available for $1000 to $2000. Many of these courses run over a one- to 3-day period. Some provide a general overview of ultrasound for the primary care physician while others specialize in topics such as musculoskeletal uses, obstetric uses, or emergency department echocardiography.40-44
Challenges remain
More research is necessary to demonstrate that office-based ultrasound produces patient outcomes that are comparable to those resulting from hospital-based ultrasound. Also, bedside ultrasound is only as good as the operator who performs the examination,45 which highlights the importance of developing bedside ultrasound training programs tailored for FPs. National policies are essential for standardizing indications, training, and credentialing so that this effective tool can be used in a safe and effective manner.
CORRESPONDENCE
Peter Steinmetz, MD, CCFP, St. Mary’s Hospital, 3830 Ave Lacombe, Montreal, Quebec, Canada H3T1M5; [email protected].
ACKNOWLEDGEMENTS
We thank Assistant Professor Marion Dove, MD, CCFP, Department of Family Medicine, McGill University, for her suggestions and critical review of an earlier version of the manuscript. The term FAMUS is pending registration and is advertised with the Canadian Intellectual Property Office (Steinmetz, Volume 63, Issue 3217).
1. Solomon SD, Saldana F. Point-of-care ultrasound in medical education - stop listening and look. N Engl J Med. 2014;370:1083-1085.
2. Bahner DP, Goldman E, Way D, et al. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89:1681-1686.
3. Steinmetz P, Dobrescu O, Oleskevich S, et al. Bedside ultrasound education in Canadian medical schools: a national survey. Can Med Educ J. 2016;7:e78-e86.
4. Deshpande R, Akhtar S, Haddadin AS. Utility of ultrasound in the ICU. Curr Opin Anaesthesiol. 2014;27:123-132.
5. Wilmink ABM, Hubbard CSFF, Quick CRG. Quality of the measurement of the infrarenal aortic diameter by ultrasound. J Med Screen. 1997;4:49-53.
6. Costantino TG, Bruno EC, Handly N, et al. Accuracy of emergency medicine ultrasound in the evaluation of abdominal aortic aneurysm. J Emerg Med. 2005;29:455-460.
7. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
8. Nusbaum JW, Freimanis AK, Thomford NR. Echography in the diagnosis of abdominal aortic aneurysm. Arch Surg. 1971;102:385-388.
9. Wilmink ABM, Forshaw M, Quick CRG, et al. Accuracy of serial screening for abdominal aortic aneurysms by ultrasound. J Med Screen. 2002;9:125-127.
10. Lynch RM. Accuracy of abdominal examination in the diagnosis of non-ruptured abdominal aortic aneurysm. Accid Emerg Nurs. 2004;12:99-107.
11. Lederle FA, Simel DL. The rational clinical examination. Does this patient have abdominal aortic aneurysm? JAMA. 1999;281:77-82.
12. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656.
13. Stather PW, Dattani N, Bown MJ, et al. International variations in AAA screening. Eur J Vasc Endovasc Surg. 2013;45:231-234.
14. Ruff AL, Teng K, Hu B, et al. Screening for abdominal aortic aneurysms in outpatient primary care clinics. Am J Med. 2015;128:283-288.
15. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-178.
16. Vourvouri EC, Poldermans D, Schinkel AF, et al. Abdominal aortic aneurysm screening using a hand-held ultrasound device. “A pilot study”. Eur J Vasc Endovasc Surg. 2001;22:352-354.
17. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
18. American Academy of Family Physicians. Position Paper: Diagnostic ultrasonography in women’s health care. 2013. Available at http://www.aafp.org/about/policies/all/ultrasonography-diagnostic.html. Accessed 2013.
19. Everett CB, Preece E. Women with bleeding in the first 20 weeks of pregnancy: value of general practice ultrasound in detecting fetal heart movement. Br J Gen Pract. 1996;46:7-9.
20. Rodney WM, Prislin MD, Orientale E, et al. Family practice obstetric ultrasound in an urban community health center. Birth outcomes and examination accuracy of the initial 227 cases. J Fam Pract. 1990;30:163-168.
21. Keith R, Frisch L. Fetal biometry: a comparison of family physicians and radiologists. Fam Med. 2001;33:111-114.
22. Ornstein SM, Smith MA, Peggs J, et al. Obstetric ultrasound by family physicians. Adequacy as assessed by pregnancy outcome. J Fam Pract. 1990;30:403-408.
23. Hahn RG, Ho S, Roi LD, et al. Cost-effectiveness of office obstetrical ultrasound in family practice: preliminary considerations. J Am Board Fam Pract. 1988;1:33-38.
24. Stein W, Katunda I, Butoto C. A two-level ultrasonographic service in a maternity care unit of a rural district hospital in Tanzania. Trop Doct. 2008;38:125-126.
25. Morgan WC, Rodney WM, Hahn R, et al. Ultrasound for the primary care physician. Applications in family-centered obstetrics. Postgrad Med. 1988;83:103-107.
26. Coris EE, Pescasio M, Zwygart K, et al. Office-based ultrasound in sports medicine practice. Clin J Sport Med. 2011;21:57-61.
27. Royall NA, Farrin E, Bahner DP, et al. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2:57-66.
28. Bianchi S, Martinoli C. Ultrasound of the musculoskeletal system. Berlin; New York: Springer; 2007.
29. Narouze SN. Atlas of ultrasound-guided procedures in interventional pain management. New York: Springer; 2011.
30. Mjolstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
31. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-477.
32. Chan VSP, Piterman L, McCall L. Use of clinical ultrasonography in an Australian suburban family practice: its indications and findings. Hong Kong Practitioner. 1999;21:405-415.
33. Siepel T, Clifford DS, James PA, et al. The ultrasound-assisted physical examination in the periodic health evaluation of the elderly. J Fam Pract. 2000;49:628-632.
34. Rosenthal TC, Siepel T, Zubler J, et al. The use of ultrasonography to scan the abdomen of patients presenting for routine physical examinations. J Fam Pract. 1994;38:380-385.
35. Choosing Wisely Canada. Available at: http://www.choosingwiselycanada.org. Accessed 2016.
36. Hale I. Add to cart? Can Fam Physician. 2015;61:937-939.
37. Wordsworth S, Scott A. Ultrasound scanning by general practitioners: is it worthwhile? J Public Health Med. 2002;24:88-94.
38. Hall JW, Holman H, Bornemann P, et al. Point of Care Ultrasound in Family Medicine Residency Programs: A CERA Study. Fam Med. 2015;47:706-711.
39. WINFOCUS-World Interactive Network Focused on Critical UltraSound. Available at: http://www.winfocus.it/#winfocus. Accessed 2016.
40. McGill University. McGill Ultrasound Evaluation Program (MUSE). Bedside ultrasound course for primary care clinicians (MUSE 1.0). Available at: www.mcgill.ca/medsimcentre/muse. Accessed 2016.
41. The University of British Columbia. Faculty of Medicine UBC CPD (Continuing Professional Development). CPD/CME courses. Available at: http://ubccpd.ca/courses?combine=ultrasound&field_target_audience_tid=3&field_learning_type_tid=All&field_location_tid=All&field_cost_tid=All&
field_credit_type_tid=All&field_number_of_credits_tid=All&field_event_date_value_1%5Bvalue%5D%5Bdate%5D=. Accessed 2016.
42. Emergency Department Echo (EDE). Available at: www.edecourse.com. Accessed 2016.
43. McGill University. MUSE 2.0 Advanced Bedside Ultrasound Course. Available at: http://www.mcgill.ca/medsimcentre/channels/event/muse-20-advanced-bedside-ultrasound-course-256963. Accessed 2016.
44. Gulfcoast Ultrasound Institute. Available at: https://www.gcus.com. Accessed 2016.
45. Allen GM, Wilson DJ. Ultrasound in sports medicine—a critical evaluation. Eur J Radiol. 2007;62:79-85.
1. Solomon SD, Saldana F. Point-of-care ultrasound in medical education - stop listening and look. N Engl J Med. 2014;370:1083-1085.
2. Bahner DP, Goldman E, Way D, et al. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89:1681-1686.
3. Steinmetz P, Dobrescu O, Oleskevich S, et al. Bedside ultrasound education in Canadian medical schools: a national survey. Can Med Educ J. 2016;7:e78-e86.
4. Deshpande R, Akhtar S, Haddadin AS. Utility of ultrasound in the ICU. Curr Opin Anaesthesiol. 2014;27:123-132.
5. Wilmink ABM, Hubbard CSFF, Quick CRG. Quality of the measurement of the infrarenal aortic diameter by ultrasound. J Med Screen. 1997;4:49-53.
6. Costantino TG, Bruno EC, Handly N, et al. Accuracy of emergency medicine ultrasound in the evaluation of abdominal aortic aneurysm. J Emerg Med. 2005;29:455-460.
7. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
8. Nusbaum JW, Freimanis AK, Thomford NR. Echography in the diagnosis of abdominal aortic aneurysm. Arch Surg. 1971;102:385-388.
9. Wilmink ABM, Forshaw M, Quick CRG, et al. Accuracy of serial screening for abdominal aortic aneurysms by ultrasound. J Med Screen. 2002;9:125-127.
10. Lynch RM. Accuracy of abdominal examination in the diagnosis of non-ruptured abdominal aortic aneurysm. Accid Emerg Nurs. 2004;12:99-107.
11. Lederle FA, Simel DL. The rational clinical examination. Does this patient have abdominal aortic aneurysm? JAMA. 1999;281:77-82.
12. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656.
13. Stather PW, Dattani N, Bown MJ, et al. International variations in AAA screening. Eur J Vasc Endovasc Surg. 2013;45:231-234.
14. Ruff AL, Teng K, Hu B, et al. Screening for abdominal aortic aneurysms in outpatient primary care clinics. Am J Med. 2015;128:283-288.
15. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-178.
16. Vourvouri EC, Poldermans D, Schinkel AF, et al. Abdominal aortic aneurysm screening using a hand-held ultrasound device. “A pilot study”. Eur J Vasc Endovasc Surg. 2001;22:352-354.
17. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
18. American Academy of Family Physicians. Position Paper: Diagnostic ultrasonography in women’s health care. 2013. Available at http://www.aafp.org/about/policies/all/ultrasonography-diagnostic.html. Accessed 2013.
19. Everett CB, Preece E. Women with bleeding in the first 20 weeks of pregnancy: value of general practice ultrasound in detecting fetal heart movement. Br J Gen Pract. 1996;46:7-9.
20. Rodney WM, Prislin MD, Orientale E, et al. Family practice obstetric ultrasound in an urban community health center. Birth outcomes and examination accuracy of the initial 227 cases. J Fam Pract. 1990;30:163-168.
21. Keith R, Frisch L. Fetal biometry: a comparison of family physicians and radiologists. Fam Med. 2001;33:111-114.
22. Ornstein SM, Smith MA, Peggs J, et al. Obstetric ultrasound by family physicians. Adequacy as assessed by pregnancy outcome. J Fam Pract. 1990;30:403-408.
23. Hahn RG, Ho S, Roi LD, et al. Cost-effectiveness of office obstetrical ultrasound in family practice: preliminary considerations. J Am Board Fam Pract. 1988;1:33-38.
24. Stein W, Katunda I, Butoto C. A two-level ultrasonographic service in a maternity care unit of a rural district hospital in Tanzania. Trop Doct. 2008;38:125-126.
25. Morgan WC, Rodney WM, Hahn R, et al. Ultrasound for the primary care physician. Applications in family-centered obstetrics. Postgrad Med. 1988;83:103-107.
26. Coris EE, Pescasio M, Zwygart K, et al. Office-based ultrasound in sports medicine practice. Clin J Sport Med. 2011;21:57-61.
27. Royall NA, Farrin E, Bahner DP, et al. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2:57-66.
28. Bianchi S, Martinoli C. Ultrasound of the musculoskeletal system. Berlin; New York: Springer; 2007.
29. Narouze SN. Atlas of ultrasound-guided procedures in interventional pain management. New York: Springer; 2011.
30. Mjolstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
31. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-477.
32. Chan VSP, Piterman L, McCall L. Use of clinical ultrasonography in an Australian suburban family practice: its indications and findings. Hong Kong Practitioner. 1999;21:405-415.
33. Siepel T, Clifford DS, James PA, et al. The ultrasound-assisted physical examination in the periodic health evaluation of the elderly. J Fam Pract. 2000;49:628-632.
34. Rosenthal TC, Siepel T, Zubler J, et al. The use of ultrasonography to scan the abdomen of patients presenting for routine physical examinations. J Fam Pract. 1994;38:380-385.
35. Choosing Wisely Canada. Available at: http://www.choosingwiselycanada.org. Accessed 2016.
36. Hale I. Add to cart? Can Fam Physician. 2015;61:937-939.
37. Wordsworth S, Scott A. Ultrasound scanning by general practitioners: is it worthwhile? J Public Health Med. 2002;24:88-94.
38. Hall JW, Holman H, Bornemann P, et al. Point of Care Ultrasound in Family Medicine Residency Programs: A CERA Study. Fam Med. 2015;47:706-711.
39. WINFOCUS-World Interactive Network Focused on Critical UltraSound. Available at: http://www.winfocus.it/#winfocus. Accessed 2016.
40. McGill University. McGill Ultrasound Evaluation Program (MUSE). Bedside ultrasound course for primary care clinicians (MUSE 1.0). Available at: www.mcgill.ca/medsimcentre/muse. Accessed 2016.
41. The University of British Columbia. Faculty of Medicine UBC CPD (Continuing Professional Development). CPD/CME courses. Available at: http://ubccpd.ca/courses?combine=ultrasound&field_target_audience_tid=3&field_learning_type_tid=All&field_location_tid=All&field_cost_tid=All&
field_credit_type_tid=All&field_number_of_credits_tid=All&field_event_date_value_1%5Bvalue%5D%5Bdate%5D=. Accessed 2016.
42. Emergency Department Echo (EDE). Available at: www.edecourse.com. Accessed 2016.
43. McGill University. MUSE 2.0 Advanced Bedside Ultrasound Course. Available at: http://www.mcgill.ca/medsimcentre/channels/event/muse-20-advanced-bedside-ultrasound-course-256963. Accessed 2016.
44. Gulfcoast Ultrasound Institute. Available at: https://www.gcus.com. Accessed 2016.
45. Allen GM, Wilson DJ. Ultrasound in sports medicine—a critical evaluation. Eur J Radiol. 2007;62:79-85.
Shortness of breath: Looking beyond the usual suspects
› Consider diagnoses other than asthma, COPD, heart failure, and pneumonia in patients with persistent or progressive dyspnea. C
› Avoid steroids in patients with acute pericarditis because research shows that they increase the risk of recurrence. B
› Consider anticoagulation with warfarin in patients with pulmonary arterial hypertension and cor pulmonale. Evidence shows that it improves survival and quality of life. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joan C is a 68-year-old woman who presents to the office complaining of an enlarging left chest wall mass that appeared within the past month. She was treated for small-cell lung cancer 11 years ago. She has a 45 pack-year smoking history (she quit when she received the diagnosis) and has heart failure, which is controlled. Your examination reveals a large (5 cm) firm mass on her left chest wall. There is no erythema or tenderness. She has no other complaints. You recommend surgical biopsy and refer her to surgery.
Ms. C returns to your office several days later complaining of new and worsening shortness of breath with exertion that began the previous day. The presentation is similar to prior asthma exacerbation episodes. She denies any cough, fever, chest pain, symptoms at rest, or hemoptysis. On exam she appears comfortable and not in any acute distress. You refill her albuterol.
The next day you learn that she is being admitted to the hospital with respiratory distress. An x-ray of her chest shows a concerning mass in her right upper lung.
Dyspnea is an uncomfortable awareness of breathing that occurs when complex neurochemical pathways used to maintain oxygenation and ventilation are disrupted. (See "The variable, and subjective, process of dyspnea"1-5). Sometimes described as air hunger, increased work of breathing, chest tightness, or chest constriction, the symptom is usually disproportionate to the patient’s level of exertion.
The variable, and subjective, process of dyspnea
The mechanism of action of shortness of breath is a complex and incompletely understood one that involves the central and peripheral nervous systems and neurochemical modulators. In the central nervous system, the medullary respiratory center likely relays increased oxygen demand to the anterior insula. The anterior insula, which is where dyspnea is perceived as unpleasant, then simultaneously disseminates this information to the cerebral cortex and the respiratory muscles to increase respiration and oxygen.1-3
The peripheral nervous system measures current oxygen flux and lung mechanics through pulmonary stretch mechanoreceptors, pulmonary irritant receptors, and alveolar C fibers. Input from all of these receptors ascends the respiratory pathway and affects how dyspnea is perceived. For example, a patient may complain of shortness of breath because the medullary respiratory center interprets input from activated pulmonary muscular stretch receptors in the setting of discordant oxygen (measured via peripheral chemoreceptors) and carbon dioxide levels (measured by medullary chemoreceptors) as an increased work of breathing.2,4,5
Neurochemical dissociation, which is the difference between the brain’s desired oxygen level and the amount it gets, is one potential hypothesis to explain why dyspnea is subjective and variable.2,5 One patient may complain of moderate or severe shortness of breath because he or she has a large dissociation between desired and actual oxygenation despite having only mild to moderate disease severity. However, another patient may report mild dyspnea despite having severe disease because his or her dissociation is small.
Take, for example, a patient who has had an acute myocardial infarction. Such patients often complain of significant difficulty breathing, likely because of the acute and sudden neurochemical dissociation that occurs with the infarction. On the other hand, a patient with gradually worsening moderate heart failure may complain of only mild dyspnea because the change in the patient’s perception of the ability to breathe is slow and small.
Most of the time dyspnea is due to either a primary lung or cardiovascular problem such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism (PE), pneumonia, congestive heart failure (CHF), or myocardial infarction. However, many other illnesses can also produce this symptom (TABLE 1). This article will review the uncommon etiologies of dyspnea that should be considered when the usual suspects have been eliminated.
Cardiovascular culprits
Dyspnea is a common symptom with cardiovascular diseases because cardiac output relates directly to tissue oxygenation. Any pathology that decreases the ability of the heart and blood vessels to transport oxygen will likely trigger discord between the central, peripheral, and neurochemical respiratory centers. Two uncommon cardiovascular etiologies of dyspnea are pericarditis and myocarditis.
Pericarditis
Pericarditis is generally a self-limited condition that responds promptly to initial treatment, although it can cause significant morbidity and mortality. One study showed that acute pericarditis accounted for 5% of patients presenting to the emergency department with non-ischemic chest pain.6 Another study found that the in-hospital mortality rate for acute pericarditis was 1.1%.7
Pericarditis causes dyspnea by restricting the heart’s ability to relax, thus decreasing preload and cardiac output. This occurs with large effusions (>20 mm in width on echocardiography) and can lead to cardiac tamponade—a medical emergency that should be suspected in patients with muffled heart sounds, hypotension, and increased jugular venous distention (Beck’s triad).
Pericarditis etiologies include:
- infectious causes (viral and bacterial entities, myocarditis),
- rheumatologic causes (gout, systemic lupus erythematosus, tumor necrosis factor receptor-associated periodic syndrome [TRAPS], familial Mediterranean fever),
- post-cardiac injury syndromes (either of the acute [2-4 days post injury] or late [Dressler syndrome] variety),
- metabolic disorders (hypothyroid disease, dialysis-related conditions), and
- malignancy.
More than 80% of pericarditis cases in developed countries are idiopathic and are assumed to have a viral source.8
Diagnosis. Acute pericarditis is diagnosed when 2 or more of the following symptoms are present:
- pleuritic chest pain radiating to the trapezius that is relieved by leaning forward
- pericardial friction rub
- electrocardiographic changes showing ST segment elevation in all leads but aVR and V1 and diffuse PR interval depression
- pericardial effusion on echocardiography.
Treatment. Treat non-severe and non-life threatening pericarditis with nonsteroidal anti-inflammatory drugs (NSAIDs). Avoid steroids because research has shown that they increase the risk for developing recurrent pericarditis.8 Hospitalize patients with large pericardial effusions and consider them for pericardiocentesis. Treat cardiac tamponade with urgent pericardiocentesis and hospitalization.
Myocarditis
Myocarditis can have a variety of etiologies (TABLE 29,10). Myocarditis causes dyspnea either by causing pericardial effusion or heart failure.
Diagnosis. Myocarditis can be difficult to diagnose. Suspect it in any patient with cardiogenic shock, acute or subacute left ventricular dysfunction, or myocardial damage from a non-coronary artery disease source. Echocardiography and cardiac serum biomarkers can help diagnose myocarditis, but the diagnostic gold standard remains myocardial biopsy.
Treatment. Treatment is focused on 2 goals: treating the specific etiology suspected and stabilizing any hemodynamic instability. Patients with mild cases can be treated and monitored in the outpatient setting.
Immunosuppressive therapy with immunoglobulin or steroids is not routinely recommended, but a trial may be considered in children, patients with severe hemodynamic compromise, or patients with giant cell arteritis, another autoimmune condition, sarcoidosis, or eosinophilic or non-viral myocarditis.
Because of the risk of sudden death from ventricular arrhythmias, any patient with cardiac symptoms such as chest pain, dyspnea, or palpitations should be admitted for cardiopulmonary monitoring. Patients with heart failure secondary to myocarditis should be treated according to the American Heart Association treatment guidelines for heart failure (available at: http://circ.ahajournals.org/content/128/16/e240.extract). Some patients may benefit from surgical interventions such as percutaneous cardiopulmonary support, extracorporeal membrane oxygenation, mechanical circulatory support, and left ventricular assistive devices. Ventricular arrhythmias may require implantable defibrillators or pacemakers.10

Pulmonary causes
Shortness of breath is common with most pulmonary diseases, although it may not be an initial symptom and may have an insidious onset. It occurs once oxygenation of blood becomes inadequate, resulting in peripheral nervous system activation and neurochemical dissociation. Most patients with a pulmonary infection, asthma exacerbation, or COPD will have dyspnea. Once infection, asthma, and COPD have been ruled out, other pathologic processes that interrupt oxygenation should be considered. Unlike COPD and infections, patients with lung cancer may not have dyspnea until the end stages of their disease.11 The following entities should be considered in patients with dyspnea when more common causes have been eliminated.
Restrictive lung diseases
Restrictive lung disease occurs when functional lung volume is decreased, either by an intrinsic or extrinsic source. As a result, these lung diseases cover a wide variety of pathologies and disease processes including interstitial lung diseases (which we’ll discuss here), environmental exposures, neuromuscular diseases, and other forms of chest wall dysfunction.
Interstitial lung disease occurs in the presence of lung parenchymal scarring or thickening, which can have many causes including pulmonary fibrosis, connective tissue diseases (eg, sarcoidosis or rheumatoid arthritis), and inflammatory processes (eg, hypersensitivity pneumonitis and coal worker's pneumoconiosis). Dyspnea results because parenchymal thickening decreases oxygen diffusion between the alveolar and capillary endothelium. Additionally, the lung’s ability to exchange air is restricted by parenchymal stiffness and decreased total lung and functional lung capacity. Treatment is disease specific.
Idiopathic pulmonary fibrosis is the most common interstitial pneumonia with a prevalence of 13 to 20 per 100,000 people.12 It commonly affects men between the ages of 50 and 75 years. Risk factors include cigarette smoking, dust exposure (to metals, woods, vegetables), and exposure to livestock or other animals.12 Suspect it when you have a middle-aged farmer or mill worker who complains of shortness of breath.
Treatment recommendations have changed recently and now consist of using only nintedanib (a tyrosine-kinase inhibitor), antacid medication, and pirfenidone. Anticoagulation (with warfarin), steroids, other immunologic agents including azathioprine, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors are not recommended.13
Pulmonary arterial hypertension and cor pulmonale
Pulmonary arterial hypertension (PAH) is defined as a mean resting precapillary pulmonary artery pressure >25 mm Hg or >30 mm Hg with activity. It can be idiopathic or caused by a variety of agents, diseases, and conditions (TABLE 314). PAH is rare (15 in one million adults) and underdiagnosed, and more often occurs in 20- to 30-year-old black women.14
Suspect PAH in younger, otherwise healthy patients who complain of exertional dyspnea, fatigue, chest pain, or palpitations who do not have any other heart or lung disease signs or symptoms. A diagnosis of PAH is often delayed because patients are worked up for other etiologies such as CHF, coronary artery disease, PE, and COPD.
Diagnosis. When PAH is suspected, the initial work-up should include:
- an echocardiogram with a possible bubble study,
- arterial blood gas measurements,
- complete blood count,
- complete metabolic panel,
- human immunodeficiency virus (HIV) testing,
- thyroid-stimulating hormone levels,
- chest x-ray (which is abnormal in 90% of patients and shows right ventricular enlargement, a prominent central pulmonary artery, or peripheral hypovascularity),14
- electrocardiogram (to rule out other acute cardiac etiologies, but not to diagnosis PAH because of poor sensitivity and specificity),
- liver ultrasound, and
- pulmonary function tests.
If clinically suggested, tests for anticentromere antibody, antinuclear antibodies, anti-Scl-70 antibodies, and ribonucleoprotein antibodies should be ordered, as well as sickle cell screening, cardiac magnetic resonance imaging, and chest computed tomography. A right heart catheterization is required to confirm PAH and determine disease severity.
Vasoreactivity testing helps guide treatment because it identifies which patients will benefit from calcium channel blockers. The 6-minute walk test is the best way to estimate prognosis and disease severity. It is a simple test you can perform in the office by measuring how far your patient can walk in 6 minutes. Miyamoto et al showed the test to be predictive of survival in idiopathic PAH.15 A lung biopsy is never indicated or needed for diagnosis, disease severity classification, or prognosis.
Treatment. Collaboration between primary and subspecialty physicians is usually recommended because PAH treatment requires advanced testing such as right heart catheterization or vasoreactivity testing. Research has shown anticoagulation with warfarin prolongs survival and improves quality of life.16 Oxygen may improve symptomatic control and should be started for anyone with saturation less than 90%.
Newer medications that target various pathways resulting in vasodilation include prostacyclin analogues (epoprostenol, iloprost, treprostinil), endothelin receptor antagonists (ambrisentan, bosentan), and phosphodiesterase type 5 inhibitors (sildenafil, tadalafil).14

Hematologic diseases
Hematologic diseases, including sickle cell disease, gammopathies, and malignancies, can cause dyspnea primarily by decreasing the body’s ability to transport oxygen. This usually is due to anemia, but it also can be caused by increased viscosity or sickling. Suspect a hematologic cause of dyspnea when a patient repeatedly returns to your office complaining of progressive dyspnea on exertion and possible Raynaud’s-like symptoms.
Sickle cell disease
Sickle cell disease is a heterogeneous genetic disease with varied physical manifestations. The sickling phenomenon occurs in patients who inherit the homozygous hemoglobin S trait or heterozygous hemoglobin S and C (hemoglobin SC) disease. Sickle cell patients develop dyspnea due to comorbid anemia, infectious processes, or cardiopulmonary disease.
Cardiac disease is common and an often unrecognized comorbidity. It is the leading cause of mortality in adults with sickle cell disease, resulting in 26% of deaths (usually from pulseless electrical activity, pulmonary emboli, multiorgan failure, or stroke).17 Nonfatal cardiac complications may also develop, including chronic heart disease from prolonged increased cardiac output (leading to ventricular hypertrophy), heart failure, or arrhythmias; non-atherosclerotic MI;18 and hemosiderosis-induced cardiomyopathy from repeat blood transfusions.
Pulmonary-related complications may be chronic or acute and may include restrictive lung disease, chronic hypoxemia, pulmonary hypertension, and interstitial fibrosis. Acute chest syndrome and cor pulmonale cause sudden pulmonary disease. Acute chest syndrome is often caused by pneumonia, in situ thrombosis infarction of the lung, or embolic infarction from fat or bone marrow. It is a medical emergency that should be considered in any patient with pulmonary symptoms, fever, chest pain, or cough and an infiltrate on chest x-ray.
Treatment for acute chest syndrome consists of oxygen, aggressive analgesia, antibiotics (if infection is suspected), and transfusions. Research has shown that steroids provide improvement, but result in more hospital readmissions.19
Multiple myeloma and other hematologic malignancies
Multiple myeloma and Waldenstrom macroglobulinemia (discussed here), as well as leukemia, and other hematologic malignancies, can cause dyspnea or dyspnea on exertion through anemia, increasing blood viscosity, or direct lung involvement.
Multiple myeloma, a plasma cell neoplasm, is associated with anemia in 73% of patients at time of diagnosis.20 This is because of bone marrow destruction. Anemia prevalence increases in patients treated with chemotherapy because of the agent's adverse effects. The decision to treat with irradiated, leukoreduced red cell transfusion is based on anemia severity, the presence of symptoms, and whether the patient is currently undergoing chemotherapy.
Waldenstrom macroglobulinemia is an IgM-specific monoclonal gammopathy associated with a lymphoplasmacytic lymphoma in the bone marrow. Dyspnea results from hyperviscosity syndrome, hemolytic or other anemias, and/or direct lung involvement including pleural effusion, pulmonary infiltrates, or a mass.
Hyperviscosity syndrome usually results in neurologic symptoms such as vision changes, headaches, vertigo, dizziness, dementia, or other changes in consciousness. Heart failure, which is often associated with comorbid anemia, can develop in severe cases.
Patients are generally asymptomatic if serum viscosity is <3 centipoises (cP). Symptoms increase in frequency and severity with increasing serum viscosity so that about two-thirds (67%) of patients have symptoms when viscosity is >4 cP and 75% have symptoms when viscosity is >5 cP.21
Neuromuscular diseases
Dyspnea occurs when respiratory muscles are weakened by neuromuscular diseases such as myasthenia gravis (discussed here), multiple sclerosis, or muscular dystrophy. Such diseases can cause respiratory insufficiency, increased rates of infection, or complete respiratory failure. Respiratory involvement is usually a manifestation of advanced disease. Suspect neuromuscular causes of dyspnea when you are seeing a patient admitted to the nursing home for long-term care because of profound weakness affecting their ability to do activities of daily living.
Myasthenia gravis
Myasthenia gravis, an autoimmune-mediated destruction of the postsynaptic acetylcholine receptors of the neuromuscular junction, is the most common disorder of neuromuscular transmission. It often affects the ocular (>50%; ptosis, diplopia), bulbar (15%; dysarthria, dysphagia, fatigable chewing), limb (<5%; usually proximal weakness), and respiratory muscles. Weakness typically fluctuates and worsens with muscle fatigue. Myasthenic crisis, an acute respiratory failure that occurs in 15% to 20% of patients, is often precipitated by an event such as surgery, an infection, or a medication change.22
Diagnosis. Myasthenia gravis is diagnosed by a clinical history and exam suggestive of the disease. Suspect it if signs and symptoms include weakness worse with fatigue especially of the ocular muscles (ptosis or diplopia), dysphagia, dysphonia, chewing difficulty, or limb weakness. Consider laboratory testing with an anti-acetylcholine receptor (AChR) antibody assay, an assay for muscle-specific kinase (MuSK) antibody, or an anti-striated muscle (anti-SM) antibody assay if the history and exam are suggestive of the disorder.
The most reliable test is the anti-AChR antibody assay, which is positive in 50% to 90% of patients with the disease.22 Less reliable is the anti-MuSK antibody assay, which can be positive in 40% to 60% of patients who are AChR-seronegative.23 An anti-striated muscle antibody assay is only helpful in patients with thymoma or onset of disease after age 40 years.24
Consider electrophysiologic tests, including repetitive nerve stimulation studies and single-fiber electromyography, if the above laboratory tests are inconclusive.25
Treatment depends on symptom severity and frequency. It can range from observation for mild occasional symptoms to chronic steroids and immunosuppressant medications in severe cases.
CASE › You see Ms. C in the intensive care unit the next day. She is intubated and has been responding poorly to the diuresis and breathing treatments used overnight. Her biopsy pathology results return and show recurrence of her small-cell lung cancer. She begins chemotherapy immediately and is extubated a few days later. She is discharged from the hospital a week later. Her shortness of breath is mild at this time, although she does require 2 liters of continuous oxygen.
CORRESPONDENCE
Christopher Taggart, MD, St. Mary’s Medical Center, Department of Family Medicine, 2698 Patterson Rd, Grand Junction, CO 81506; [email protected].
1. von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Resp Crit Care Med. 2008;177:1026-1032.
2. Thoma J, Gunten CV. Dyspnea. In: Bruera E, Higginson IJ, Ripamonti C, et al, eds. Textbook of Palliative Medicine. London: Hodder Arnold; 2009.
3. Manning H, Schwartzstein R. Pathophysiology of dyspnea. N Engl J Med. 1995;333;1547-1553.
4. O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:530-535.
5. O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148:1351-1357.
6. Seferovic PM, Ristic AD, Maksimovic R, et al. Pericardial syndromes: an update after the ESC guidelines 2004. Heart Fail Rev. 2013;18:255-266.
7. Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601-1606.
8. LeWinter MM. Acute pericarditis. N Engl J Med. 2014;371:2410-2416.
9. Pursnani A, Yee H, Slater W, et al. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Intern Med. 2009;150:225-226.
10. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738-747.
11. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000-1006.
12. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949-1961.
13. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3-e19.
14. Stringham R, Shah NR. Pulmonary arterial hypertension: an update on diagnosis and treatment. Am Fam Physician. 2010;82:370-377.
15. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlate and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487-492.
16. Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714-721.
17. Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patient with sickle cell disease. Am J Hematol. 2010;85:36-40.
18. Martin CR, Johnson CS, Cobb C, et al. D. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428-432.
19. Paul RN, Castro OL, Aggarwal A, et al. Acute chest syndrome: sickle cell disease. Eur J Haematol. 2011;87:191-207.
20. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060.
21. Crawford J, Cox EB, Cohen HJ. Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 1985;79:13-22.
22. Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32;215-226.
23. Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23:530-535.
24. Skeie GO, Mygland A, Aarli JA, et al. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99-104.
25. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006;16:459-467.
› Consider diagnoses other than asthma, COPD, heart failure, and pneumonia in patients with persistent or progressive dyspnea. C
› Avoid steroids in patients with acute pericarditis because research shows that they increase the risk of recurrence. B
› Consider anticoagulation with warfarin in patients with pulmonary arterial hypertension and cor pulmonale. Evidence shows that it improves survival and quality of life. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joan C is a 68-year-old woman who presents to the office complaining of an enlarging left chest wall mass that appeared within the past month. She was treated for small-cell lung cancer 11 years ago. She has a 45 pack-year smoking history (she quit when she received the diagnosis) and has heart failure, which is controlled. Your examination reveals a large (5 cm) firm mass on her left chest wall. There is no erythema or tenderness. She has no other complaints. You recommend surgical biopsy and refer her to surgery.
Ms. C returns to your office several days later complaining of new and worsening shortness of breath with exertion that began the previous day. The presentation is similar to prior asthma exacerbation episodes. She denies any cough, fever, chest pain, symptoms at rest, or hemoptysis. On exam she appears comfortable and not in any acute distress. You refill her albuterol.
The next day you learn that she is being admitted to the hospital with respiratory distress. An x-ray of her chest shows a concerning mass in her right upper lung.
Dyspnea is an uncomfortable awareness of breathing that occurs when complex neurochemical pathways used to maintain oxygenation and ventilation are disrupted. (See "The variable, and subjective, process of dyspnea"1-5). Sometimes described as air hunger, increased work of breathing, chest tightness, or chest constriction, the symptom is usually disproportionate to the patient’s level of exertion.
The variable, and subjective, process of dyspnea
The mechanism of action of shortness of breath is a complex and incompletely understood one that involves the central and peripheral nervous systems and neurochemical modulators. In the central nervous system, the medullary respiratory center likely relays increased oxygen demand to the anterior insula. The anterior insula, which is where dyspnea is perceived as unpleasant, then simultaneously disseminates this information to the cerebral cortex and the respiratory muscles to increase respiration and oxygen.1-3
The peripheral nervous system measures current oxygen flux and lung mechanics through pulmonary stretch mechanoreceptors, pulmonary irritant receptors, and alveolar C fibers. Input from all of these receptors ascends the respiratory pathway and affects how dyspnea is perceived. For example, a patient may complain of shortness of breath because the medullary respiratory center interprets input from activated pulmonary muscular stretch receptors in the setting of discordant oxygen (measured via peripheral chemoreceptors) and carbon dioxide levels (measured by medullary chemoreceptors) as an increased work of breathing.2,4,5
Neurochemical dissociation, which is the difference between the brain’s desired oxygen level and the amount it gets, is one potential hypothesis to explain why dyspnea is subjective and variable.2,5 One patient may complain of moderate or severe shortness of breath because he or she has a large dissociation between desired and actual oxygenation despite having only mild to moderate disease severity. However, another patient may report mild dyspnea despite having severe disease because his or her dissociation is small.
Take, for example, a patient who has had an acute myocardial infarction. Such patients often complain of significant difficulty breathing, likely because of the acute and sudden neurochemical dissociation that occurs with the infarction. On the other hand, a patient with gradually worsening moderate heart failure may complain of only mild dyspnea because the change in the patient’s perception of the ability to breathe is slow and small.
Most of the time dyspnea is due to either a primary lung or cardiovascular problem such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism (PE), pneumonia, congestive heart failure (CHF), or myocardial infarction. However, many other illnesses can also produce this symptom (TABLE 1). This article will review the uncommon etiologies of dyspnea that should be considered when the usual suspects have been eliminated.

Cardiovascular culprits
Dyspnea is a common symptom with cardiovascular diseases because cardiac output relates directly to tissue oxygenation. Any pathology that decreases the ability of the heart and blood vessels to transport oxygen will likely trigger discord between the central, peripheral, and neurochemical respiratory centers. Two uncommon cardiovascular etiologies of dyspnea are pericarditis and myocarditis.
Pericarditis
Pericarditis is generally a self-limited condition that responds promptly to initial treatment, although it can cause significant morbidity and mortality. One study showed that acute pericarditis accounted for 5% of patients presenting to the emergency department with non-ischemic chest pain.6 Another study found that the in-hospital mortality rate for acute pericarditis was 1.1%.7
Pericarditis causes dyspnea by restricting the heart’s ability to relax, thus decreasing preload and cardiac output. This occurs with large effusions (>20 mm in width on echocardiography) and can lead to cardiac tamponade—a medical emergency that should be suspected in patients with muffled heart sounds, hypotension, and increased jugular venous distention (Beck’s triad).
Pericarditis etiologies include:
- infectious causes (viral and bacterial entities, myocarditis),
- rheumatologic causes (gout, systemic lupus erythematosus, tumor necrosis factor receptor-associated periodic syndrome [TRAPS], familial Mediterranean fever),
- post-cardiac injury syndromes (either of the acute [2-4 days post injury] or late [Dressler syndrome] variety),
- metabolic disorders (hypothyroid disease, dialysis-related conditions), and
- malignancy.
More than 80% of pericarditis cases in developed countries are idiopathic and are assumed to have a viral source.8
Diagnosis. Acute pericarditis is diagnosed when 2 or more of the following symptoms are present:
- pleuritic chest pain radiating to the trapezius that is relieved by leaning forward
- pericardial friction rub
- electrocardiographic changes showing ST segment elevation in all leads but aVR and V1 and diffuse PR interval depression
- pericardial effusion on echocardiography.
Treatment. Treat non-severe and non-life threatening pericarditis with nonsteroidal anti-inflammatory drugs (NSAIDs). Avoid steroids because research has shown that they increase the risk for developing recurrent pericarditis.8 Hospitalize patients with large pericardial effusions and consider them for pericardiocentesis. Treat cardiac tamponade with urgent pericardiocentesis and hospitalization.
Myocarditis
Myocarditis can have a variety of etiologies (TABLE 29,10). Myocarditis causes dyspnea either by causing pericardial effusion or heart failure.
Diagnosis. Myocarditis can be difficult to diagnose. Suspect it in any patient with cardiogenic shock, acute or subacute left ventricular dysfunction, or myocardial damage from a non-coronary artery disease source. Echocardiography and cardiac serum biomarkers can help diagnose myocarditis, but the diagnostic gold standard remains myocardial biopsy.
Treatment. Treatment is focused on 2 goals: treating the specific etiology suspected and stabilizing any hemodynamic instability. Patients with mild cases can be treated and monitored in the outpatient setting.
Immunosuppressive therapy with immunoglobulin or steroids is not routinely recommended, but a trial may be considered in children, patients with severe hemodynamic compromise, or patients with giant cell arteritis, another autoimmune condition, sarcoidosis, or eosinophilic or non-viral myocarditis.
Because of the risk of sudden death from ventricular arrhythmias, any patient with cardiac symptoms such as chest pain, dyspnea, or palpitations should be admitted for cardiopulmonary monitoring. Patients with heart failure secondary to myocarditis should be treated according to the American Heart Association treatment guidelines for heart failure (available at: http://circ.ahajournals.org/content/128/16/e240.extract). Some patients may benefit from surgical interventions such as percutaneous cardiopulmonary support, extracorporeal membrane oxygenation, mechanical circulatory support, and left ventricular assistive devices. Ventricular arrhythmias may require implantable defibrillators or pacemakers.10

Pulmonary causes
Shortness of breath is common with most pulmonary diseases, although it may not be an initial symptom and may have an insidious onset. It occurs once oxygenation of blood becomes inadequate, resulting in peripheral nervous system activation and neurochemical dissociation. Most patients with a pulmonary infection, asthma exacerbation, or COPD will have dyspnea. Once infection, asthma, and COPD have been ruled out, other pathologic processes that interrupt oxygenation should be considered. Unlike COPD and infections, patients with lung cancer may not have dyspnea until the end stages of their disease.11 The following entities should be considered in patients with dyspnea when more common causes have been eliminated.
Restrictive lung diseases
Restrictive lung disease occurs when functional lung volume is decreased, either by an intrinsic or extrinsic source. As a result, these lung diseases cover a wide variety of pathologies and disease processes including interstitial lung diseases (which we’ll discuss here), environmental exposures, neuromuscular diseases, and other forms of chest wall dysfunction.
Interstitial lung disease occurs in the presence of lung parenchymal scarring or thickening, which can have many causes including pulmonary fibrosis, connective tissue diseases (eg, sarcoidosis or rheumatoid arthritis), and inflammatory processes (eg, hypersensitivity pneumonitis and coal worker's pneumoconiosis). Dyspnea results because parenchymal thickening decreases oxygen diffusion between the alveolar and capillary endothelium. Additionally, the lung’s ability to exchange air is restricted by parenchymal stiffness and decreased total lung and functional lung capacity. Treatment is disease specific.
Idiopathic pulmonary fibrosis is the most common interstitial pneumonia with a prevalence of 13 to 20 per 100,000 people.12 It commonly affects men between the ages of 50 and 75 years. Risk factors include cigarette smoking, dust exposure (to metals, woods, vegetables), and exposure to livestock or other animals.12 Suspect it when you have a middle-aged farmer or mill worker who complains of shortness of breath.
Treatment recommendations have changed recently and now consist of using only nintedanib (a tyrosine-kinase inhibitor), antacid medication, and pirfenidone. Anticoagulation (with warfarin), steroids, other immunologic agents including azathioprine, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors are not recommended.13
Pulmonary arterial hypertension and cor pulmonale
Pulmonary arterial hypertension (PAH) is defined as a mean resting precapillary pulmonary artery pressure >25 mm Hg or >30 mm Hg with activity. It can be idiopathic or caused by a variety of agents, diseases, and conditions (TABLE 314). PAH is rare (15 in one million adults) and underdiagnosed, and more often occurs in 20- to 30-year-old black women.14
Suspect PAH in younger, otherwise healthy patients who complain of exertional dyspnea, fatigue, chest pain, or palpitations who do not have any other heart or lung disease signs or symptoms. A diagnosis of PAH is often delayed because patients are worked up for other etiologies such as CHF, coronary artery disease, PE, and COPD.
Diagnosis. When PAH is suspected, the initial work-up should include:
- an echocardiogram with a possible bubble study,
- arterial blood gas measurements,
- complete blood count,
- complete metabolic panel,
- human immunodeficiency virus (HIV) testing,
- thyroid-stimulating hormone levels,
- chest x-ray (which is abnormal in 90% of patients and shows right ventricular enlargement, a prominent central pulmonary artery, or peripheral hypovascularity),14
- electrocardiogram (to rule out other acute cardiac etiologies, but not to diagnosis PAH because of poor sensitivity and specificity),
- liver ultrasound, and
- pulmonary function tests.
If clinically suggested, tests for anticentromere antibody, antinuclear antibodies, anti-Scl-70 antibodies, and ribonucleoprotein antibodies should be ordered, as well as sickle cell screening, cardiac magnetic resonance imaging, and chest computed tomography. A right heart catheterization is required to confirm PAH and determine disease severity.
Vasoreactivity testing helps guide treatment because it identifies which patients will benefit from calcium channel blockers. The 6-minute walk test is the best way to estimate prognosis and disease severity. It is a simple test you can perform in the office by measuring how far your patient can walk in 6 minutes. Miyamoto et al showed the test to be predictive of survival in idiopathic PAH.15 A lung biopsy is never indicated or needed for diagnosis, disease severity classification, or prognosis.
Treatment. Collaboration between primary and subspecialty physicians is usually recommended because PAH treatment requires advanced testing such as right heart catheterization or vasoreactivity testing. Research has shown anticoagulation with warfarin prolongs survival and improves quality of life.16 Oxygen may improve symptomatic control and should be started for anyone with saturation less than 90%.
Newer medications that target various pathways resulting in vasodilation include prostacyclin analogues (epoprostenol, iloprost, treprostinil), endothelin receptor antagonists (ambrisentan, bosentan), and phosphodiesterase type 5 inhibitors (sildenafil, tadalafil).14

Hematologic diseases
Hematologic diseases, including sickle cell disease, gammopathies, and malignancies, can cause dyspnea primarily by decreasing the body’s ability to transport oxygen. This usually is due to anemia, but it also can be caused by increased viscosity or sickling. Suspect a hematologic cause of dyspnea when a patient repeatedly returns to your office complaining of progressive dyspnea on exertion and possible Raynaud’s-like symptoms.
Sickle cell disease
Sickle cell disease is a heterogeneous genetic disease with varied physical manifestations. The sickling phenomenon occurs in patients who inherit the homozygous hemoglobin S trait or heterozygous hemoglobin S and C (hemoglobin SC) disease. Sickle cell patients develop dyspnea due to comorbid anemia, infectious processes, or cardiopulmonary disease.
Cardiac disease is common and an often unrecognized comorbidity. It is the leading cause of mortality in adults with sickle cell disease, resulting in 26% of deaths (usually from pulseless electrical activity, pulmonary emboli, multiorgan failure, or stroke).17 Nonfatal cardiac complications may also develop, including chronic heart disease from prolonged increased cardiac output (leading to ventricular hypertrophy), heart failure, or arrhythmias; non-atherosclerotic MI;18 and hemosiderosis-induced cardiomyopathy from repeat blood transfusions.
Pulmonary-related complications may be chronic or acute and may include restrictive lung disease, chronic hypoxemia, pulmonary hypertension, and interstitial fibrosis. Acute chest syndrome and cor pulmonale cause sudden pulmonary disease. Acute chest syndrome is often caused by pneumonia, in situ thrombosis infarction of the lung, or embolic infarction from fat or bone marrow. It is a medical emergency that should be considered in any patient with pulmonary symptoms, fever, chest pain, or cough and an infiltrate on chest x-ray.
Treatment for acute chest syndrome consists of oxygen, aggressive analgesia, antibiotics (if infection is suspected), and transfusions. Research has shown that steroids provide improvement, but result in more hospital readmissions.19
Multiple myeloma and other hematologic malignancies
Multiple myeloma and Waldenstrom macroglobulinemia (discussed here), as well as leukemia, and other hematologic malignancies, can cause dyspnea or dyspnea on exertion through anemia, increasing blood viscosity, or direct lung involvement.
Multiple myeloma, a plasma cell neoplasm, is associated with anemia in 73% of patients at time of diagnosis.20 This is because of bone marrow destruction. Anemia prevalence increases in patients treated with chemotherapy because of the agent's adverse effects. The decision to treat with irradiated, leukoreduced red cell transfusion is based on anemia severity, the presence of symptoms, and whether the patient is currently undergoing chemotherapy.
Waldenstrom macroglobulinemia is an IgM-specific monoclonal gammopathy associated with a lymphoplasmacytic lymphoma in the bone marrow. Dyspnea results from hyperviscosity syndrome, hemolytic or other anemias, and/or direct lung involvement including pleural effusion, pulmonary infiltrates, or a mass.
Hyperviscosity syndrome usually results in neurologic symptoms such as vision changes, headaches, vertigo, dizziness, dementia, or other changes in consciousness. Heart failure, which is often associated with comorbid anemia, can develop in severe cases.
Patients are generally asymptomatic if serum viscosity is <3 centipoises (cP). Symptoms increase in frequency and severity with increasing serum viscosity so that about two-thirds (67%) of patients have symptoms when viscosity is >4 cP and 75% have symptoms when viscosity is >5 cP.21
Neuromuscular diseases
Dyspnea occurs when respiratory muscles are weakened by neuromuscular diseases such as myasthenia gravis (discussed here), multiple sclerosis, or muscular dystrophy. Such diseases can cause respiratory insufficiency, increased rates of infection, or complete respiratory failure. Respiratory involvement is usually a manifestation of advanced disease. Suspect neuromuscular causes of dyspnea when you are seeing a patient admitted to the nursing home for long-term care because of profound weakness affecting their ability to do activities of daily living.
Myasthenia gravis
Myasthenia gravis, an autoimmune-mediated destruction of the postsynaptic acetylcholine receptors of the neuromuscular junction, is the most common disorder of neuromuscular transmission. It often affects the ocular (>50%; ptosis, diplopia), bulbar (15%; dysarthria, dysphagia, fatigable chewing), limb (<5%; usually proximal weakness), and respiratory muscles. Weakness typically fluctuates and worsens with muscle fatigue. Myasthenic crisis, an acute respiratory failure that occurs in 15% to 20% of patients, is often precipitated by an event such as surgery, an infection, or a medication change.22
Diagnosis. Myasthenia gravis is diagnosed by a clinical history and exam suggestive of the disease. Suspect it if signs and symptoms include weakness worse with fatigue especially of the ocular muscles (ptosis or diplopia), dysphagia, dysphonia, chewing difficulty, or limb weakness. Consider laboratory testing with an anti-acetylcholine receptor (AChR) antibody assay, an assay for muscle-specific kinase (MuSK) antibody, or an anti-striated muscle (anti-SM) antibody assay if the history and exam are suggestive of the disorder.
The most reliable test is the anti-AChR antibody assay, which is positive in 50% to 90% of patients with the disease.22 Less reliable is the anti-MuSK antibody assay, which can be positive in 40% to 60% of patients who are AChR-seronegative.23 An anti-striated muscle antibody assay is only helpful in patients with thymoma or onset of disease after age 40 years.24
Consider electrophysiologic tests, including repetitive nerve stimulation studies and single-fiber electromyography, if the above laboratory tests are inconclusive.25
Treatment depends on symptom severity and frequency. It can range from observation for mild occasional symptoms to chronic steroids and immunosuppressant medications in severe cases.
CASE › You see Ms. C in the intensive care unit the next day. She is intubated and has been responding poorly to the diuresis and breathing treatments used overnight. Her biopsy pathology results return and show recurrence of her small-cell lung cancer. She begins chemotherapy immediately and is extubated a few days later. She is discharged from the hospital a week later. Her shortness of breath is mild at this time, although she does require 2 liters of continuous oxygen.
CORRESPONDENCE
Christopher Taggart, MD, St. Mary’s Medical Center, Department of Family Medicine, 2698 Patterson Rd, Grand Junction, CO 81506; [email protected].
› Consider diagnoses other than asthma, COPD, heart failure, and pneumonia in patients with persistent or progressive dyspnea. C
› Avoid steroids in patients with acute pericarditis because research shows that they increase the risk of recurrence. B
› Consider anticoagulation with warfarin in patients with pulmonary arterial hypertension and cor pulmonale. Evidence shows that it improves survival and quality of life. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joan C is a 68-year-old woman who presents to the office complaining of an enlarging left chest wall mass that appeared within the past month. She was treated for small-cell lung cancer 11 years ago. She has a 45 pack-year smoking history (she quit when she received the diagnosis) and has heart failure, which is controlled. Your examination reveals a large (5 cm) firm mass on her left chest wall. There is no erythema or tenderness. She has no other complaints. You recommend surgical biopsy and refer her to surgery.
Ms. C returns to your office several days later complaining of new and worsening shortness of breath with exertion that began the previous day. The presentation is similar to prior asthma exacerbation episodes. She denies any cough, fever, chest pain, symptoms at rest, or hemoptysis. On exam she appears comfortable and not in any acute distress. You refill her albuterol.
The next day you learn that she is being admitted to the hospital with respiratory distress. An x-ray of her chest shows a concerning mass in her right upper lung.
Dyspnea is an uncomfortable awareness of breathing that occurs when complex neurochemical pathways used to maintain oxygenation and ventilation are disrupted. (See "The variable, and subjective, process of dyspnea"1-5). Sometimes described as air hunger, increased work of breathing, chest tightness, or chest constriction, the symptom is usually disproportionate to the patient’s level of exertion.
The variable, and subjective, process of dyspnea
The mechanism of action of shortness of breath is a complex and incompletely understood one that involves the central and peripheral nervous systems and neurochemical modulators. In the central nervous system, the medullary respiratory center likely relays increased oxygen demand to the anterior insula. The anterior insula, which is where dyspnea is perceived as unpleasant, then simultaneously disseminates this information to the cerebral cortex and the respiratory muscles to increase respiration and oxygen.1-3
The peripheral nervous system measures current oxygen flux and lung mechanics through pulmonary stretch mechanoreceptors, pulmonary irritant receptors, and alveolar C fibers. Input from all of these receptors ascends the respiratory pathway and affects how dyspnea is perceived. For example, a patient may complain of shortness of breath because the medullary respiratory center interprets input from activated pulmonary muscular stretch receptors in the setting of discordant oxygen (measured via peripheral chemoreceptors) and carbon dioxide levels (measured by medullary chemoreceptors) as an increased work of breathing.2,4,5
Neurochemical dissociation, which is the difference between the brain’s desired oxygen level and the amount it gets, is one potential hypothesis to explain why dyspnea is subjective and variable.2,5 One patient may complain of moderate or severe shortness of breath because he or she has a large dissociation between desired and actual oxygenation despite having only mild to moderate disease severity. However, another patient may report mild dyspnea despite having severe disease because his or her dissociation is small.
Take, for example, a patient who has had an acute myocardial infarction. Such patients often complain of significant difficulty breathing, likely because of the acute and sudden neurochemical dissociation that occurs with the infarction. On the other hand, a patient with gradually worsening moderate heart failure may complain of only mild dyspnea because the change in the patient’s perception of the ability to breathe is slow and small.
Most of the time dyspnea is due to either a primary lung or cardiovascular problem such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism (PE), pneumonia, congestive heart failure (CHF), or myocardial infarction. However, many other illnesses can also produce this symptom (TABLE 1). This article will review the uncommon etiologies of dyspnea that should be considered when the usual suspects have been eliminated.

Cardiovascular culprits
Dyspnea is a common symptom with cardiovascular diseases because cardiac output relates directly to tissue oxygenation. Any pathology that decreases the ability of the heart and blood vessels to transport oxygen will likely trigger discord between the central, peripheral, and neurochemical respiratory centers. Two uncommon cardiovascular etiologies of dyspnea are pericarditis and myocarditis.
Pericarditis
Pericarditis is generally a self-limited condition that responds promptly to initial treatment, although it can cause significant morbidity and mortality. One study showed that acute pericarditis accounted for 5% of patients presenting to the emergency department with non-ischemic chest pain.6 Another study found that the in-hospital mortality rate for acute pericarditis was 1.1%.7
Pericarditis causes dyspnea by restricting the heart’s ability to relax, thus decreasing preload and cardiac output. This occurs with large effusions (>20 mm in width on echocardiography) and can lead to cardiac tamponade—a medical emergency that should be suspected in patients with muffled heart sounds, hypotension, and increased jugular venous distention (Beck’s triad).
Pericarditis etiologies include:
- infectious causes (viral and bacterial entities, myocarditis),
- rheumatologic causes (gout, systemic lupus erythematosus, tumor necrosis factor receptor-associated periodic syndrome [TRAPS], familial Mediterranean fever),
- post-cardiac injury syndromes (either of the acute [2-4 days post injury] or late [Dressler syndrome] variety),
- metabolic disorders (hypothyroid disease, dialysis-related conditions), and
- malignancy.
More than 80% of pericarditis cases in developed countries are idiopathic and are assumed to have a viral source.8
Diagnosis. Acute pericarditis is diagnosed when 2 or more of the following symptoms are present:
- pleuritic chest pain radiating to the trapezius that is relieved by leaning forward
- pericardial friction rub
- electrocardiographic changes showing ST segment elevation in all leads but aVR and V1 and diffuse PR interval depression
- pericardial effusion on echocardiography.
Treatment. Treat non-severe and non-life threatening pericarditis with nonsteroidal anti-inflammatory drugs (NSAIDs). Avoid steroids because research has shown that they increase the risk for developing recurrent pericarditis.8 Hospitalize patients with large pericardial effusions and consider them for pericardiocentesis. Treat cardiac tamponade with urgent pericardiocentesis and hospitalization.
Myocarditis
Myocarditis can have a variety of etiologies (TABLE 29,10). Myocarditis causes dyspnea either by causing pericardial effusion or heart failure.
Diagnosis. Myocarditis can be difficult to diagnose. Suspect it in any patient with cardiogenic shock, acute or subacute left ventricular dysfunction, or myocardial damage from a non-coronary artery disease source. Echocardiography and cardiac serum biomarkers can help diagnose myocarditis, but the diagnostic gold standard remains myocardial biopsy.
Treatment. Treatment is focused on 2 goals: treating the specific etiology suspected and stabilizing any hemodynamic instability. Patients with mild cases can be treated and monitored in the outpatient setting.
Immunosuppressive therapy with immunoglobulin or steroids is not routinely recommended, but a trial may be considered in children, patients with severe hemodynamic compromise, or patients with giant cell arteritis, another autoimmune condition, sarcoidosis, or eosinophilic or non-viral myocarditis.
Because of the risk of sudden death from ventricular arrhythmias, any patient with cardiac symptoms such as chest pain, dyspnea, or palpitations should be admitted for cardiopulmonary monitoring. Patients with heart failure secondary to myocarditis should be treated according to the American Heart Association treatment guidelines for heart failure (available at: http://circ.ahajournals.org/content/128/16/e240.extract). Some patients may benefit from surgical interventions such as percutaneous cardiopulmonary support, extracorporeal membrane oxygenation, mechanical circulatory support, and left ventricular assistive devices. Ventricular arrhythmias may require implantable defibrillators or pacemakers.10

Pulmonary causes
Shortness of breath is common with most pulmonary diseases, although it may not be an initial symptom and may have an insidious onset. It occurs once oxygenation of blood becomes inadequate, resulting in peripheral nervous system activation and neurochemical dissociation. Most patients with a pulmonary infection, asthma exacerbation, or COPD will have dyspnea. Once infection, asthma, and COPD have been ruled out, other pathologic processes that interrupt oxygenation should be considered. Unlike COPD and infections, patients with lung cancer may not have dyspnea until the end stages of their disease.11 The following entities should be considered in patients with dyspnea when more common causes have been eliminated.
Restrictive lung diseases
Restrictive lung disease occurs when functional lung volume is decreased, either by an intrinsic or extrinsic source. As a result, these lung diseases cover a wide variety of pathologies and disease processes including interstitial lung diseases (which we’ll discuss here), environmental exposures, neuromuscular diseases, and other forms of chest wall dysfunction.
Interstitial lung disease occurs in the presence of lung parenchymal scarring or thickening, which can have many causes including pulmonary fibrosis, connective tissue diseases (eg, sarcoidosis or rheumatoid arthritis), and inflammatory processes (eg, hypersensitivity pneumonitis and coal worker's pneumoconiosis). Dyspnea results because parenchymal thickening decreases oxygen diffusion between the alveolar and capillary endothelium. Additionally, the lung’s ability to exchange air is restricted by parenchymal stiffness and decreased total lung and functional lung capacity. Treatment is disease specific.
Idiopathic pulmonary fibrosis is the most common interstitial pneumonia with a prevalence of 13 to 20 per 100,000 people.12 It commonly affects men between the ages of 50 and 75 years. Risk factors include cigarette smoking, dust exposure (to metals, woods, vegetables), and exposure to livestock or other animals.12 Suspect it when you have a middle-aged farmer or mill worker who complains of shortness of breath.
Treatment recommendations have changed recently and now consist of using only nintedanib (a tyrosine-kinase inhibitor), antacid medication, and pirfenidone. Anticoagulation (with warfarin), steroids, other immunologic agents including azathioprine, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors are not recommended.13
Pulmonary arterial hypertension and cor pulmonale
Pulmonary arterial hypertension (PAH) is defined as a mean resting precapillary pulmonary artery pressure >25 mm Hg or >30 mm Hg with activity. It can be idiopathic or caused by a variety of agents, diseases, and conditions (TABLE 314). PAH is rare (15 in one million adults) and underdiagnosed, and more often occurs in 20- to 30-year-old black women.14
Suspect PAH in younger, otherwise healthy patients who complain of exertional dyspnea, fatigue, chest pain, or palpitations who do not have any other heart or lung disease signs or symptoms. A diagnosis of PAH is often delayed because patients are worked up for other etiologies such as CHF, coronary artery disease, PE, and COPD.
Diagnosis. When PAH is suspected, the initial work-up should include:
- an echocardiogram with a possible bubble study,
- arterial blood gas measurements,
- complete blood count,
- complete metabolic panel,
- human immunodeficiency virus (HIV) testing,
- thyroid-stimulating hormone levels,
- chest x-ray (which is abnormal in 90% of patients and shows right ventricular enlargement, a prominent central pulmonary artery, or peripheral hypovascularity),14
- electrocardiogram (to rule out other acute cardiac etiologies, but not to diagnosis PAH because of poor sensitivity and specificity),
- liver ultrasound, and
- pulmonary function tests.
If clinically suggested, tests for anticentromere antibody, antinuclear antibodies, anti-Scl-70 antibodies, and ribonucleoprotein antibodies should be ordered, as well as sickle cell screening, cardiac magnetic resonance imaging, and chest computed tomography. A right heart catheterization is required to confirm PAH and determine disease severity.
Vasoreactivity testing helps guide treatment because it identifies which patients will benefit from calcium channel blockers. The 6-minute walk test is the best way to estimate prognosis and disease severity. It is a simple test you can perform in the office by measuring how far your patient can walk in 6 minutes. Miyamoto et al showed the test to be predictive of survival in idiopathic PAH.15 A lung biopsy is never indicated or needed for diagnosis, disease severity classification, or prognosis.
Treatment. Collaboration between primary and subspecialty physicians is usually recommended because PAH treatment requires advanced testing such as right heart catheterization or vasoreactivity testing. Research has shown anticoagulation with warfarin prolongs survival and improves quality of life.16 Oxygen may improve symptomatic control and should be started for anyone with saturation less than 90%.
Newer medications that target various pathways resulting in vasodilation include prostacyclin analogues (epoprostenol, iloprost, treprostinil), endothelin receptor antagonists (ambrisentan, bosentan), and phosphodiesterase type 5 inhibitors (sildenafil, tadalafil).14

Hematologic diseases
Hematologic diseases, including sickle cell disease, gammopathies, and malignancies, can cause dyspnea primarily by decreasing the body’s ability to transport oxygen. This usually is due to anemia, but it also can be caused by increased viscosity or sickling. Suspect a hematologic cause of dyspnea when a patient repeatedly returns to your office complaining of progressive dyspnea on exertion and possible Raynaud’s-like symptoms.
Sickle cell disease
Sickle cell disease is a heterogeneous genetic disease with varied physical manifestations. The sickling phenomenon occurs in patients who inherit the homozygous hemoglobin S trait or heterozygous hemoglobin S and C (hemoglobin SC) disease. Sickle cell patients develop dyspnea due to comorbid anemia, infectious processes, or cardiopulmonary disease.
Cardiac disease is common and an often unrecognized comorbidity. It is the leading cause of mortality in adults with sickle cell disease, resulting in 26% of deaths (usually from pulseless electrical activity, pulmonary emboli, multiorgan failure, or stroke).17 Nonfatal cardiac complications may also develop, including chronic heart disease from prolonged increased cardiac output (leading to ventricular hypertrophy), heart failure, or arrhythmias; non-atherosclerotic MI;18 and hemosiderosis-induced cardiomyopathy from repeat blood transfusions.
Pulmonary-related complications may be chronic or acute and may include restrictive lung disease, chronic hypoxemia, pulmonary hypertension, and interstitial fibrosis. Acute chest syndrome and cor pulmonale cause sudden pulmonary disease. Acute chest syndrome is often caused by pneumonia, in situ thrombosis infarction of the lung, or embolic infarction from fat or bone marrow. It is a medical emergency that should be considered in any patient with pulmonary symptoms, fever, chest pain, or cough and an infiltrate on chest x-ray.
Treatment for acute chest syndrome consists of oxygen, aggressive analgesia, antibiotics (if infection is suspected), and transfusions. Research has shown that steroids provide improvement, but result in more hospital readmissions.19
Multiple myeloma and other hematologic malignancies
Multiple myeloma and Waldenstrom macroglobulinemia (discussed here), as well as leukemia, and other hematologic malignancies, can cause dyspnea or dyspnea on exertion through anemia, increasing blood viscosity, or direct lung involvement.
Multiple myeloma, a plasma cell neoplasm, is associated with anemia in 73% of patients at time of diagnosis.20 This is because of bone marrow destruction. Anemia prevalence increases in patients treated with chemotherapy because of the agent's adverse effects. The decision to treat with irradiated, leukoreduced red cell transfusion is based on anemia severity, the presence of symptoms, and whether the patient is currently undergoing chemotherapy.
Waldenstrom macroglobulinemia is an IgM-specific monoclonal gammopathy associated with a lymphoplasmacytic lymphoma in the bone marrow. Dyspnea results from hyperviscosity syndrome, hemolytic or other anemias, and/or direct lung involvement including pleural effusion, pulmonary infiltrates, or a mass.
Hyperviscosity syndrome usually results in neurologic symptoms such as vision changes, headaches, vertigo, dizziness, dementia, or other changes in consciousness. Heart failure, which is often associated with comorbid anemia, can develop in severe cases.
Patients are generally asymptomatic if serum viscosity is <3 centipoises (cP). Symptoms increase in frequency and severity with increasing serum viscosity so that about two-thirds (67%) of patients have symptoms when viscosity is >4 cP and 75% have symptoms when viscosity is >5 cP.21
Neuromuscular diseases
Dyspnea occurs when respiratory muscles are weakened by neuromuscular diseases such as myasthenia gravis (discussed here), multiple sclerosis, or muscular dystrophy. Such diseases can cause respiratory insufficiency, increased rates of infection, or complete respiratory failure. Respiratory involvement is usually a manifestation of advanced disease. Suspect neuromuscular causes of dyspnea when you are seeing a patient admitted to the nursing home for long-term care because of profound weakness affecting their ability to do activities of daily living.
Myasthenia gravis
Myasthenia gravis, an autoimmune-mediated destruction of the postsynaptic acetylcholine receptors of the neuromuscular junction, is the most common disorder of neuromuscular transmission. It often affects the ocular (>50%; ptosis, diplopia), bulbar (15%; dysarthria, dysphagia, fatigable chewing), limb (<5%; usually proximal weakness), and respiratory muscles. Weakness typically fluctuates and worsens with muscle fatigue. Myasthenic crisis, an acute respiratory failure that occurs in 15% to 20% of patients, is often precipitated by an event such as surgery, an infection, or a medication change.22
Diagnosis. Myasthenia gravis is diagnosed by a clinical history and exam suggestive of the disease. Suspect it if signs and symptoms include weakness worse with fatigue especially of the ocular muscles (ptosis or diplopia), dysphagia, dysphonia, chewing difficulty, or limb weakness. Consider laboratory testing with an anti-acetylcholine receptor (AChR) antibody assay, an assay for muscle-specific kinase (MuSK) antibody, or an anti-striated muscle (anti-SM) antibody assay if the history and exam are suggestive of the disorder.
The most reliable test is the anti-AChR antibody assay, which is positive in 50% to 90% of patients with the disease.22 Less reliable is the anti-MuSK antibody assay, which can be positive in 40% to 60% of patients who are AChR-seronegative.23 An anti-striated muscle antibody assay is only helpful in patients with thymoma or onset of disease after age 40 years.24
Consider electrophysiologic tests, including repetitive nerve stimulation studies and single-fiber electromyography, if the above laboratory tests are inconclusive.25
Treatment depends on symptom severity and frequency. It can range from observation for mild occasional symptoms to chronic steroids and immunosuppressant medications in severe cases.
CASE › You see Ms. C in the intensive care unit the next day. She is intubated and has been responding poorly to the diuresis and breathing treatments used overnight. Her biopsy pathology results return and show recurrence of her small-cell lung cancer. She begins chemotherapy immediately and is extubated a few days later. She is discharged from the hospital a week later. Her shortness of breath is mild at this time, although she does require 2 liters of continuous oxygen.
CORRESPONDENCE
Christopher Taggart, MD, St. Mary’s Medical Center, Department of Family Medicine, 2698 Patterson Rd, Grand Junction, CO 81506; [email protected].
1. von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Resp Crit Care Med. 2008;177:1026-1032.
2. Thoma J, Gunten CV. Dyspnea. In: Bruera E, Higginson IJ, Ripamonti C, et al, eds. Textbook of Palliative Medicine. London: Hodder Arnold; 2009.
3. Manning H, Schwartzstein R. Pathophysiology of dyspnea. N Engl J Med. 1995;333;1547-1553.
4. O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:530-535.
5. O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148:1351-1357.
6. Seferovic PM, Ristic AD, Maksimovic R, et al. Pericardial syndromes: an update after the ESC guidelines 2004. Heart Fail Rev. 2013;18:255-266.
7. Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601-1606.
8. LeWinter MM. Acute pericarditis. N Engl J Med. 2014;371:2410-2416.
9. Pursnani A, Yee H, Slater W, et al. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Intern Med. 2009;150:225-226.
10. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738-747.
11. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000-1006.
12. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949-1961.
13. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3-e19.
14. Stringham R, Shah NR. Pulmonary arterial hypertension: an update on diagnosis and treatment. Am Fam Physician. 2010;82:370-377.
15. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlate and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487-492.
16. Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714-721.
17. Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patient with sickle cell disease. Am J Hematol. 2010;85:36-40.
18. Martin CR, Johnson CS, Cobb C, et al. D. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428-432.
19. Paul RN, Castro OL, Aggarwal A, et al. Acute chest syndrome: sickle cell disease. Eur J Haematol. 2011;87:191-207.
20. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060.
21. Crawford J, Cox EB, Cohen HJ. Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 1985;79:13-22.
22. Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32;215-226.
23. Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23:530-535.
24. Skeie GO, Mygland A, Aarli JA, et al. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99-104.
25. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006;16:459-467.
1. von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Resp Crit Care Med. 2008;177:1026-1032.
2. Thoma J, Gunten CV. Dyspnea. In: Bruera E, Higginson IJ, Ripamonti C, et al, eds. Textbook of Palliative Medicine. London: Hodder Arnold; 2009.
3. Manning H, Schwartzstein R. Pathophysiology of dyspnea. N Engl J Med. 1995;333;1547-1553.
4. O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:530-535.
5. O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148:1351-1357.
6. Seferovic PM, Ristic AD, Maksimovic R, et al. Pericardial syndromes: an update after the ESC guidelines 2004. Heart Fail Rev. 2013;18:255-266.
7. Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601-1606.
8. LeWinter MM. Acute pericarditis. N Engl J Med. 2014;371:2410-2416.
9. Pursnani A, Yee H, Slater W, et al. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Intern Med. 2009;150:225-226.
10. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738-747.
11. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000-1006.
12. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949-1961.
13. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3-e19.
14. Stringham R, Shah NR. Pulmonary arterial hypertension: an update on diagnosis and treatment. Am Fam Physician. 2010;82:370-377.
15. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlate and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487-492.
16. Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714-721.
17. Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patient with sickle cell disease. Am J Hematol. 2010;85:36-40.
18. Martin CR, Johnson CS, Cobb C, et al. D. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428-432.
19. Paul RN, Castro OL, Aggarwal A, et al. Acute chest syndrome: sickle cell disease. Eur J Haematol. 2011;87:191-207.
20. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060.
21. Crawford J, Cox EB, Cohen HJ. Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 1985;79:13-22.
22. Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32;215-226.
23. Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23:530-535.
24. Skeie GO, Mygland A, Aarli JA, et al. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99-104.
25. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006;16:459-467.
Cervical cancer screening: How our approach may change
› Screen for cervical cancer in women ages 21 to 29 using cytology alone every 3 years. For women ages 30 to 65, screening with a combination of cytology and human papillomavirus (HPV) testing every 5 years is the preferred option. A
› Be aware of the alternative guideline proposed by several specialty organizations: All women ages 25 to 64 should receive primary HPV screening every 3 years with the FDA-approved HPV test. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
If the cervical cytology report you receive for a woman in her mid-20s is negative, how soon would you plan to repeat testing? Recommendations from the United States Preventive Services Task Force (USPSTF) and other leading organizations advise a combination of cytology and human papillomavirus (HPV) testing at specified intervals depending on a patient’s age. However, a study published in 2015 analyzed data from a statewide registry on provider behavior and found a wide array of screening intervals in practice and infrequent use of HPV testing.1 Clearly, adherence to published guidelines has been inconsistent.
Now, recommendations by several specialty groups are evolving based on newer evidence regarding HPV testing. These alternative guidelines recommend primary high-risk HPV testing for all women. This change is the topic of much national debate and is being researched for the USPSTF’s 2018 update on cervical cancer screening.
In this article, I review the USPSTF’s present recommendations and look ahead to how “best practices” for cervical cancer screening may be changing.
Current cervical cancer screening guidelines
Many subspecialty organizations and government agencies publish cervical cancer screening guidelines. The USPSTF guidelines, reviewed here, are evidence based, frequently updated, and widely used by primary care providers (TABLE).2,3 These guidelines recommend initiating cytology testing at age 21 and, if results are normal, repeating every 3 years. Reflex HPV testing is recommended if cytology results reveal atypical squamous cells of undetermined significance (ASCUS). For women ages 30 to 65, the preferred option is to undergo a combination of cytology and HPV testing every 5 years.2 Women older than 65 may discontinue screening.2 HPV immunization status does not affect USPSTF recommendations. Nationwide rates of HPV vaccination among females ages 13 to 17 vary among states, from ≤49% to ≥70%.4
What the guidelines do, and do not, cover. The USPSTF screening intervals apply as long as testing results are normal.2 These guidelines apply to all women regardless of the age at which they began sexual activity. These guidelines do not apply to women who have had abnormal cytology or HPV results and have not undergone adequate follow-up to ensure their lesion has cleared.5 These guidelines also do not apply to women who are immunosuppressed, who were exposed to diethylstilbestrol (DES) in utero, who have had a hysterectomy for non-oncologic reasons, or who have had cervical cancer.5 A woman may stop routine screening after age 65 if she has had adequate follow-up including either 3 negative cytology samples or 2 negative co-tests (cytology and HPV test) in the last 10 years.6 A woman may also discontinue screening if she has had a total hysterectomy and has no history of cervical dysplasia.7
Evidence behind the guidelines. The USPSTF guidelines were updated to their current state in 2012 reflecting a growing body of evidence that, for women 30 years and older, detection of cervical intraepithelial neoplasia (CIN) 3+ lesions improves with HPV co-testing. The supporting studies also found that the risk of a high-grade lesion appearing 5 years following co-testing was equivalent to the risk seen with cytology samples alone taken at 3-year intervals.8 The sensitivity of a single cytology test is only about 50%.9 A patient’s risk of cervical cancer 18 months after 3 negative cytology tests is about 1.5/100,000.10 The risk at 36 months following 3 negative cytology results is about 4.5/100,000. Annual screening would require almost 100,000 women to be screened to detect 3 additional cases of cervical cancer.10
Additional benefits of the updated USPSTF guidelines. The updated strategy decreases the number of visits for patients and the number of colposcopies, minimizing harm and patient anxiety. The current management algorithms also recommend more conservative management of women in their early 20s who have reported abnormal cytology, as the likelihood of their lesion clearing within 12 to 24 months is high.5 The recommendation does not call for high-risk HPV testing of women ages 21 to 29 because the infection is highly prevalent in this age group and is also likely to clear before any significant pathology arises. This avoids unnecessary and potentially harmful treatment of younger women.11
What about the alternative screening guideline?
In early 2015, the American Society for Colposcopy and Cervical Pathology (ASCCP) and the Society of Gynecologic Oncology (SGO) co-sponsored an expert panel representing several specialty societies. The panel released interim guidelines for cervical cancer screening reflecting a growing body of evidence that favors HPV testing as the primary modality.12 Additionally, in January 2016, these guidelines received an evidence-level B rating from the American Congress of Obstetricians and Gynecologists.13 Primary HPV screening is also the topic of research and discussion for USPSTF’s pending 2018 update of cervical cancer screening strategies.14
The alternative algorithm from the ASCCP and SGO recommends cervical cancer screening with HPV testing alone starting at the age of 25 and, if results are negative, repeating at 3-year intervals.12 If a patient tests positive for any of the 14 identified high-risk HPV types, reflex cytology is indicated with a referral for colposcopy if an abnormality is identified.12 If the cytology result is normal, follow-up with another HPV test in 12 months is recommended.12
Over the last 12 years, multiple international studies have demonstrated the efficacy of high-risk HPV testing in primary screening for cervical cancer.15 The most recent study conducted in the United States from 2008 to 2011—Addressing THE Need for Advanced HPV Diagnostics (ATHENA)—enrolled 42,000 women older than 25 years to compare the screening modalities of cytology alone, cytology and HPV testing combined, and HPV testing alone.16 The purpose of the study was to determine the safety of the cobas HPV test as a co-test and as a primary screening modality in women older than 25 years. (Many HPV tests are commercially available, but only the cobas HPV test is FDA-approved for primary screening.12)
ATHENA researchers concluded that HPV testing was more sensitive than cytology, but less specific.16 The researchers also concluded that adding cytology to the HPV test increased the sensitivity by less than 5% and that HPV was better at detecting CIN 2+ lesions than cytology alone.12,16 Another recently published study conducted at a tertiary care hospital with a smaller sample (1000 patients) corroborated the ATHENA results.17
For patients in their late 20s, this alternative strategy may increase the number of subsequent colposcopies. However, during the clinical trials just described, the absolute number of colposcopies needed to detect high-grade disease was the same as seen with the current guidelines. This finding indicates that, with the current algorithm, clinically significant pathology due to high-risk HPV may be missed in the 25-to-29 age group.8
Looking ahead. The alternative screening strategy is already being adopted in Australia, the Netherlands, and the United Kingdom.15 For providers in the United States considering this alternative strategy, the recommendation is to initiate cervical cancer screening with cytology alone at age 21, manage results appropriately, and then transition the patient to primary HPV testing with the FDA-approved test at age 25.12 This recommendation may be modified in the future. However the guidelines might change, patients will benefit only if the guidelines are implemented consistently in practice.
CORRESPONDENCE
Sabrina Hofmeister, DO, 1121 E. North Ave, Milwaukee, WI 53212; [email protected].
1. Kim JJ, Campos NG, Sy S, et al. Inefficiencies and high-value improvements in U.S. cervical cancer screening practice: a cost-effective analysis. Ann Intern Med. 2015;163:589-597.
2. Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880-891.
3. U.S. Preventive Services Task Force. Understanding How the USPSTF Works: USPSTF 101. Available at: http://www.uspreventiveservicestaskforce.org/Page/Name/understanding-how-the-uspstf-works. Accessed June 8, 2016.
4. Reagan-Steiner S, Yankey D, Jeyarajah J, et al; Centers for Disease Control and Prevention. National, regional, state, and selected local vaccination coverage among adolescents aged 13-17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784-792.
5. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16:175-204.
6. Vesco KK, Whitlock EP, Eder M, et al. Risk factors and other epidemiologic considerations for cervical cancer screening: a narrative review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:687-697.
7. Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papilloma virus test results. J Low Genit Tract Dis. 2016;20:119-125.
8. Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
9. Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589-1597.
10. Sawaya GF, Sung HY, Kinney W, et al. Cervical cancer after multiple negative tests in long-term members of a prepaid health plan. Acta Cytol. 2005;49:391-397.
11. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813-819.
12. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk HPV testing for cervical cancer screening: interim clinical guidance. J Low Genit Tract Dis. 2015;19:91-96.
13. American Congress of Obstetricians and Gynecologists. Practice Bulletin No. 157: Cervical cancer screening and prevention. Obstet Gynecol. 2016;127:e1-e20.
14. U.S. Preventive Services Task Force. Final Research Plan: Cervical Cancer: Screening. October 2015. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/final-research-plan/cervical-cancer-screening2. Accessed June 22, 2016.
15. Ronco G, Dillner J, Elfström KM, et al; International HPV Working Group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
16. Stoler MH, Wright TC Jr, Sharma A, et al. High-risk human papilloma virus testing in women with ASC-US cytology: results from the ATHENA HPV study. Am J Clin Path. 2011;135:468-475.
17. Choi JW, Kim Y, Lee JH, et al. The clinical performance of primary HPV screening, primary HPV screening plus cytology cotesting, and cytology alone at a tertiary care hospital. Cancer Cytopathol. 2016;124:144-152.
› Screen for cervical cancer in women ages 21 to 29 using cytology alone every 3 years. For women ages 30 to 65, screening with a combination of cytology and human papillomavirus (HPV) testing every 5 years is the preferred option. A
› Be aware of the alternative guideline proposed by several specialty organizations: All women ages 25 to 64 should receive primary HPV screening every 3 years with the FDA-approved HPV test. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
If the cervical cytology report you receive for a woman in her mid-20s is negative, how soon would you plan to repeat testing? Recommendations from the United States Preventive Services Task Force (USPSTF) and other leading organizations advise a combination of cytology and human papillomavirus (HPV) testing at specified intervals depending on a patient’s age. However, a study published in 2015 analyzed data from a statewide registry on provider behavior and found a wide array of screening intervals in practice and infrequent use of HPV testing.1 Clearly, adherence to published guidelines has been inconsistent.
Now, recommendations by several specialty groups are evolving based on newer evidence regarding HPV testing. These alternative guidelines recommend primary high-risk HPV testing for all women. This change is the topic of much national debate and is being researched for the USPSTF’s 2018 update on cervical cancer screening.
In this article, I review the USPSTF’s present recommendations and look ahead to how “best practices” for cervical cancer screening may be changing.
Current cervical cancer screening guidelines
Many subspecialty organizations and government agencies publish cervical cancer screening guidelines. The USPSTF guidelines, reviewed here, are evidence based, frequently updated, and widely used by primary care providers (TABLE).2,3 These guidelines recommend initiating cytology testing at age 21 and, if results are normal, repeating every 3 years. Reflex HPV testing is recommended if cytology results reveal atypical squamous cells of undetermined significance (ASCUS). For women ages 30 to 65, the preferred option is to undergo a combination of cytology and HPV testing every 5 years.2 Women older than 65 may discontinue screening.2 HPV immunization status does not affect USPSTF recommendations. Nationwide rates of HPV vaccination among females ages 13 to 17 vary among states, from ≤49% to ≥70%.4
What the guidelines do, and do not, cover. The USPSTF screening intervals apply as long as testing results are normal.2 These guidelines apply to all women regardless of the age at which they began sexual activity. These guidelines do not apply to women who have had abnormal cytology or HPV results and have not undergone adequate follow-up to ensure their lesion has cleared.5 These guidelines also do not apply to women who are immunosuppressed, who were exposed to diethylstilbestrol (DES) in utero, who have had a hysterectomy for non-oncologic reasons, or who have had cervical cancer.5 A woman may stop routine screening after age 65 if she has had adequate follow-up including either 3 negative cytology samples or 2 negative co-tests (cytology and HPV test) in the last 10 years.6 A woman may also discontinue screening if she has had a total hysterectomy and has no history of cervical dysplasia.7
Evidence behind the guidelines. The USPSTF guidelines were updated to their current state in 2012 reflecting a growing body of evidence that, for women 30 years and older, detection of cervical intraepithelial neoplasia (CIN) 3+ lesions improves with HPV co-testing. The supporting studies also found that the risk of a high-grade lesion appearing 5 years following co-testing was equivalent to the risk seen with cytology samples alone taken at 3-year intervals.8 The sensitivity of a single cytology test is only about 50%.9 A patient’s risk of cervical cancer 18 months after 3 negative cytology tests is about 1.5/100,000.10 The risk at 36 months following 3 negative cytology results is about 4.5/100,000. Annual screening would require almost 100,000 women to be screened to detect 3 additional cases of cervical cancer.10
Additional benefits of the updated USPSTF guidelines. The updated strategy decreases the number of visits for patients and the number of colposcopies, minimizing harm and patient anxiety. The current management algorithms also recommend more conservative management of women in their early 20s who have reported abnormal cytology, as the likelihood of their lesion clearing within 12 to 24 months is high.5 The recommendation does not call for high-risk HPV testing of women ages 21 to 29 because the infection is highly prevalent in this age group and is also likely to clear before any significant pathology arises. This avoids unnecessary and potentially harmful treatment of younger women.11
What about the alternative screening guideline?
In early 2015, the American Society for Colposcopy and Cervical Pathology (ASCCP) and the Society of Gynecologic Oncology (SGO) co-sponsored an expert panel representing several specialty societies. The panel released interim guidelines for cervical cancer screening reflecting a growing body of evidence that favors HPV testing as the primary modality.12 Additionally, in January 2016, these guidelines received an evidence-level B rating from the American Congress of Obstetricians and Gynecologists.13 Primary HPV screening is also the topic of research and discussion for USPSTF’s pending 2018 update of cervical cancer screening strategies.14
The alternative algorithm from the ASCCP and SGO recommends cervical cancer screening with HPV testing alone starting at the age of 25 and, if results are negative, repeating at 3-year intervals.12 If a patient tests positive for any of the 14 identified high-risk HPV types, reflex cytology is indicated with a referral for colposcopy if an abnormality is identified.12 If the cytology result is normal, follow-up with another HPV test in 12 months is recommended.12
Over the last 12 years, multiple international studies have demonstrated the efficacy of high-risk HPV testing in primary screening for cervical cancer.15 The most recent study conducted in the United States from 2008 to 2011—Addressing THE Need for Advanced HPV Diagnostics (ATHENA)—enrolled 42,000 women older than 25 years to compare the screening modalities of cytology alone, cytology and HPV testing combined, and HPV testing alone.16 The purpose of the study was to determine the safety of the cobas HPV test as a co-test and as a primary screening modality in women older than 25 years. (Many HPV tests are commercially available, but only the cobas HPV test is FDA-approved for primary screening.12)
ATHENA researchers concluded that HPV testing was more sensitive than cytology, but less specific.16 The researchers also concluded that adding cytology to the HPV test increased the sensitivity by less than 5% and that HPV was better at detecting CIN 2+ lesions than cytology alone.12,16 Another recently published study conducted at a tertiary care hospital with a smaller sample (1000 patients) corroborated the ATHENA results.17
For patients in their late 20s, this alternative strategy may increase the number of subsequent colposcopies. However, during the clinical trials just described, the absolute number of colposcopies needed to detect high-grade disease was the same as seen with the current guidelines. This finding indicates that, with the current algorithm, clinically significant pathology due to high-risk HPV may be missed in the 25-to-29 age group.8
Looking ahead. The alternative screening strategy is already being adopted in Australia, the Netherlands, and the United Kingdom.15 For providers in the United States considering this alternative strategy, the recommendation is to initiate cervical cancer screening with cytology alone at age 21, manage results appropriately, and then transition the patient to primary HPV testing with the FDA-approved test at age 25.12 This recommendation may be modified in the future. However the guidelines might change, patients will benefit only if the guidelines are implemented consistently in practice.
CORRESPONDENCE
Sabrina Hofmeister, DO, 1121 E. North Ave, Milwaukee, WI 53212; [email protected].
› Screen for cervical cancer in women ages 21 to 29 using cytology alone every 3 years. For women ages 30 to 65, screening with a combination of cytology and human papillomavirus (HPV) testing every 5 years is the preferred option. A
› Be aware of the alternative guideline proposed by several specialty organizations: All women ages 25 to 64 should receive primary HPV screening every 3 years with the FDA-approved HPV test. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
If the cervical cytology report you receive for a woman in her mid-20s is negative, how soon would you plan to repeat testing? Recommendations from the United States Preventive Services Task Force (USPSTF) and other leading organizations advise a combination of cytology and human papillomavirus (HPV) testing at specified intervals depending on a patient’s age. However, a study published in 2015 analyzed data from a statewide registry on provider behavior and found a wide array of screening intervals in practice and infrequent use of HPV testing.1 Clearly, adherence to published guidelines has been inconsistent.
Now, recommendations by several specialty groups are evolving based on newer evidence regarding HPV testing. These alternative guidelines recommend primary high-risk HPV testing for all women. This change is the topic of much national debate and is being researched for the USPSTF’s 2018 update on cervical cancer screening.
In this article, I review the USPSTF’s present recommendations and look ahead to how “best practices” for cervical cancer screening may be changing.
Current cervical cancer screening guidelines
Many subspecialty organizations and government agencies publish cervical cancer screening guidelines. The USPSTF guidelines, reviewed here, are evidence based, frequently updated, and widely used by primary care providers (TABLE).2,3 These guidelines recommend initiating cytology testing at age 21 and, if results are normal, repeating every 3 years. Reflex HPV testing is recommended if cytology results reveal atypical squamous cells of undetermined significance (ASCUS). For women ages 30 to 65, the preferred option is to undergo a combination of cytology and HPV testing every 5 years.2 Women older than 65 may discontinue screening.2 HPV immunization status does not affect USPSTF recommendations. Nationwide rates of HPV vaccination among females ages 13 to 17 vary among states, from ≤49% to ≥70%.4
What the guidelines do, and do not, cover. The USPSTF screening intervals apply as long as testing results are normal.2 These guidelines apply to all women regardless of the age at which they began sexual activity. These guidelines do not apply to women who have had abnormal cytology or HPV results and have not undergone adequate follow-up to ensure their lesion has cleared.5 These guidelines also do not apply to women who are immunosuppressed, who were exposed to diethylstilbestrol (DES) in utero, who have had a hysterectomy for non-oncologic reasons, or who have had cervical cancer.5 A woman may stop routine screening after age 65 if she has had adequate follow-up including either 3 negative cytology samples or 2 negative co-tests (cytology and HPV test) in the last 10 years.6 A woman may also discontinue screening if she has had a total hysterectomy and has no history of cervical dysplasia.7
Evidence behind the guidelines. The USPSTF guidelines were updated to their current state in 2012 reflecting a growing body of evidence that, for women 30 years and older, detection of cervical intraepithelial neoplasia (CIN) 3+ lesions improves with HPV co-testing. The supporting studies also found that the risk of a high-grade lesion appearing 5 years following co-testing was equivalent to the risk seen with cytology samples alone taken at 3-year intervals.8 The sensitivity of a single cytology test is only about 50%.9 A patient’s risk of cervical cancer 18 months after 3 negative cytology tests is about 1.5/100,000.10 The risk at 36 months following 3 negative cytology results is about 4.5/100,000. Annual screening would require almost 100,000 women to be screened to detect 3 additional cases of cervical cancer.10
Additional benefits of the updated USPSTF guidelines. The updated strategy decreases the number of visits for patients and the number of colposcopies, minimizing harm and patient anxiety. The current management algorithms also recommend more conservative management of women in their early 20s who have reported abnormal cytology, as the likelihood of their lesion clearing within 12 to 24 months is high.5 The recommendation does not call for high-risk HPV testing of women ages 21 to 29 because the infection is highly prevalent in this age group and is also likely to clear before any significant pathology arises. This avoids unnecessary and potentially harmful treatment of younger women.11
What about the alternative screening guideline?
In early 2015, the American Society for Colposcopy and Cervical Pathology (ASCCP) and the Society of Gynecologic Oncology (SGO) co-sponsored an expert panel representing several specialty societies. The panel released interim guidelines for cervical cancer screening reflecting a growing body of evidence that favors HPV testing as the primary modality.12 Additionally, in January 2016, these guidelines received an evidence-level B rating from the American Congress of Obstetricians and Gynecologists.13 Primary HPV screening is also the topic of research and discussion for USPSTF’s pending 2018 update of cervical cancer screening strategies.14
The alternative algorithm from the ASCCP and SGO recommends cervical cancer screening with HPV testing alone starting at the age of 25 and, if results are negative, repeating at 3-year intervals.12 If a patient tests positive for any of the 14 identified high-risk HPV types, reflex cytology is indicated with a referral for colposcopy if an abnormality is identified.12 If the cytology result is normal, follow-up with another HPV test in 12 months is recommended.12
Over the last 12 years, multiple international studies have demonstrated the efficacy of high-risk HPV testing in primary screening for cervical cancer.15 The most recent study conducted in the United States from 2008 to 2011—Addressing THE Need for Advanced HPV Diagnostics (ATHENA)—enrolled 42,000 women older than 25 years to compare the screening modalities of cytology alone, cytology and HPV testing combined, and HPV testing alone.16 The purpose of the study was to determine the safety of the cobas HPV test as a co-test and as a primary screening modality in women older than 25 years. (Many HPV tests are commercially available, but only the cobas HPV test is FDA-approved for primary screening.12)
ATHENA researchers concluded that HPV testing was more sensitive than cytology, but less specific.16 The researchers also concluded that adding cytology to the HPV test increased the sensitivity by less than 5% and that HPV was better at detecting CIN 2+ lesions than cytology alone.12,16 Another recently published study conducted at a tertiary care hospital with a smaller sample (1000 patients) corroborated the ATHENA results.17
For patients in their late 20s, this alternative strategy may increase the number of subsequent colposcopies. However, during the clinical trials just described, the absolute number of colposcopies needed to detect high-grade disease was the same as seen with the current guidelines. This finding indicates that, with the current algorithm, clinically significant pathology due to high-risk HPV may be missed in the 25-to-29 age group.8
Looking ahead. The alternative screening strategy is already being adopted in Australia, the Netherlands, and the United Kingdom.15 For providers in the United States considering this alternative strategy, the recommendation is to initiate cervical cancer screening with cytology alone at age 21, manage results appropriately, and then transition the patient to primary HPV testing with the FDA-approved test at age 25.12 This recommendation may be modified in the future. However the guidelines might change, patients will benefit only if the guidelines are implemented consistently in practice.
CORRESPONDENCE
Sabrina Hofmeister, DO, 1121 E. North Ave, Milwaukee, WI 53212; [email protected].
1. Kim JJ, Campos NG, Sy S, et al. Inefficiencies and high-value improvements in U.S. cervical cancer screening practice: a cost-effective analysis. Ann Intern Med. 2015;163:589-597.
2. Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880-891.
3. U.S. Preventive Services Task Force. Understanding How the USPSTF Works: USPSTF 101. Available at: http://www.uspreventiveservicestaskforce.org/Page/Name/understanding-how-the-uspstf-works. Accessed June 8, 2016.
4. Reagan-Steiner S, Yankey D, Jeyarajah J, et al; Centers for Disease Control and Prevention. National, regional, state, and selected local vaccination coverage among adolescents aged 13-17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784-792.
5. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16:175-204.
6. Vesco KK, Whitlock EP, Eder M, et al. Risk factors and other epidemiologic considerations for cervical cancer screening: a narrative review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:687-697.
7. Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papilloma virus test results. J Low Genit Tract Dis. 2016;20:119-125.
8. Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
9. Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589-1597.
10. Sawaya GF, Sung HY, Kinney W, et al. Cervical cancer after multiple negative tests in long-term members of a prepaid health plan. Acta Cytol. 2005;49:391-397.
11. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813-819.
12. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk HPV testing for cervical cancer screening: interim clinical guidance. J Low Genit Tract Dis. 2015;19:91-96.
13. American Congress of Obstetricians and Gynecologists. Practice Bulletin No. 157: Cervical cancer screening and prevention. Obstet Gynecol. 2016;127:e1-e20.
14. U.S. Preventive Services Task Force. Final Research Plan: Cervical Cancer: Screening. October 2015. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/final-research-plan/cervical-cancer-screening2. Accessed June 22, 2016.
15. Ronco G, Dillner J, Elfström KM, et al; International HPV Working Group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
16. Stoler MH, Wright TC Jr, Sharma A, et al. High-risk human papilloma virus testing in women with ASC-US cytology: results from the ATHENA HPV study. Am J Clin Path. 2011;135:468-475.
17. Choi JW, Kim Y, Lee JH, et al. The clinical performance of primary HPV screening, primary HPV screening plus cytology cotesting, and cytology alone at a tertiary care hospital. Cancer Cytopathol. 2016;124:144-152.
1. Kim JJ, Campos NG, Sy S, et al. Inefficiencies and high-value improvements in U.S. cervical cancer screening practice: a cost-effective analysis. Ann Intern Med. 2015;163:589-597.
2. Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880-891.
3. U.S. Preventive Services Task Force. Understanding How the USPSTF Works: USPSTF 101. Available at: http://www.uspreventiveservicestaskforce.org/Page/Name/understanding-how-the-uspstf-works. Accessed June 8, 2016.
4. Reagan-Steiner S, Yankey D, Jeyarajah J, et al; Centers for Disease Control and Prevention. National, regional, state, and selected local vaccination coverage among adolescents aged 13-17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784-792.
5. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16:175-204.
6. Vesco KK, Whitlock EP, Eder M, et al. Risk factors and other epidemiologic considerations for cervical cancer screening: a narrative review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:687-697.
7. Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papilloma virus test results. J Low Genit Tract Dis. 2016;20:119-125.
8. Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
9. Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589-1597.
10. Sawaya GF, Sung HY, Kinney W, et al. Cervical cancer after multiple negative tests in long-term members of a prepaid health plan. Acta Cytol. 2005;49:391-397.
11. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813-819.
12. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk HPV testing for cervical cancer screening: interim clinical guidance. J Low Genit Tract Dis. 2015;19:91-96.
13. American Congress of Obstetricians and Gynecologists. Practice Bulletin No. 157: Cervical cancer screening and prevention. Obstet Gynecol. 2016;127:e1-e20.
14. U.S. Preventive Services Task Force. Final Research Plan: Cervical Cancer: Screening. October 2015. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/final-research-plan/cervical-cancer-screening2. Accessed June 22, 2016.
15. Ronco G, Dillner J, Elfström KM, et al; International HPV Working Group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
16. Stoler MH, Wright TC Jr, Sharma A, et al. High-risk human papilloma virus testing in women with ASC-US cytology: results from the ATHENA HPV study. Am J Clin Path. 2011;135:468-475.
17. Choi JW, Kim Y, Lee JH, et al. The clinical performance of primary HPV screening, primary HPV screening plus cytology cotesting, and cytology alone at a tertiary care hospital. Cancer Cytopathol. 2016;124:144-152.