User login
Managing dyspepsia
› Review the medications taken by patients who suffer from dyspepsia, as many drugs—bisphosphonates, antibiotics, steroids, and nonsteroidal anti-inflammatory drugs, among others—are associated with this condition. B
› Order an esophagogastroduodenoscopy for patients ages 55 years or older with new-onset dyspepsia and those who have red flags for more serious conditions, eg, a history of upper gastrointestinal (GI) cancer, unintended weight loss, GI bleeding, dysphagia, or a palpable mass. C
› Prescribe acid suppression therapy as first-line treatment for patients who have dyspepsia but are at low risk or have tested negative for Helicobacter pylori infection. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, an estimated 25% to 30% of the US population suffers from dyspepsia.1 Most self-treat with home remedies and over-the-counter products, but others seek medical care. Dyspepsia accounts for an estimated 2% to 5% of primary care visits annually,2 mostly by patients who are found to have no organic, or structural, cause for their symptoms.1,3
Such patients are said to have functional dyspepsia (FD), a category that applies to about two-thirds of those with dyspepsia.1 A small number of cases are categorized as organic dyspepsia, indicating the presence of a clear structural or anatomic cause, such as an ulcer or mass. The remainder are said to have undifferentiated dyspepsia, which simply means that their signs and symptoms do not rise to the level for which further investigation is warranted and thus it is not known whether it is functional or organic.
There are many possible causes of FD, ranging from medications3,4 to abnormal gastroduodenal motility5,6 to Helicobacter pylori infection,7 and a comprehensive differential diagnosis. The first step in an investigation is to rule out red flags suggestive of gastrointestinal (GI) cancer or other serious disorders.
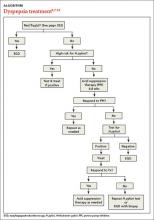
Patients with FD, like the vast majority of those you’ll treat in a primary care setting, suffer significant morbidity. Most have chronic symptoms, with intermittent flare-ups interspersed with periods of remission.8 In the text and dyspepsia treatment ALGORITHM5,7-12 that follow, you’ll find an evidence-based patient management approach.
Symptoms and causes: What to look for
The primary symptoms of dyspepsia include bothersome postprandial fullness, early satiety, and epigastric pain and burning. To meet the Rome criteria for dyspepsia, these symptoms must have been present for the last 3 months and have had an onset ≥6 months prior to diagnosis.2 Recurrent belching and nausea are also common, but are not included in the Rome diagnostic criteria.
Symptom severity is a poor predictor of the seriousness of the condition, however, and more intense symptoms are no more likely than milder cases to have an organic cause.13,14 Indeed, anxiety is a common comorbidity in patients with FD and a risk factor for the diagnosis. Compared with the general public, patients with FD have been found to have higher levels of anxiety, chronic tension, hostility, and hypochondriasis, and a tendency to be more pessimistic.15
Possible causes of FD
While the etiology of organic dyspepsia is clear, the cause of FD is often far more difficult to determine.
Medication use should always be considered, as many types of drugs—including bisphosphonates, antibiotics, narcotics, steroids, iron, metformin, and nonsteroidal anti-inflammatory drugs (NSAIDs)—are associated with dyspepsia.3,4
Gastroduodenal motility and accommodation, which has been found in numerous studies of patients with FD, is a proposed etiology.5,6
Visceral hypersensitivity also appears to play a role. In one study of patients with severe dyspepsia, 87% of those with FD had a reduced or altered GI pain threshold, compared with 20% of those with organic dyspepsia.16
H pylori, commonly linked to peptic ulcer disease (PUD), is also associated with both organic dyspepsia and FD.17,18 The gram-negative rod-shaped bacterium is present in approximately half of the population worldwide, but is more common in developing nations.7H pylori immunoglobulin G (IgG) is more prevalent in patients with dyspepsia, particularly in those younger than 30 years of age. The exact mechanism by which H pylori causes non-ulcerative dyspepsia is not clear, but inflammation, dysmotility, visceral hypersensitivity, and alteration of acid secretion have all been proposed.17
Dysfunctional intestinal epithelium is increasingly being considered in the pathophysiology of dyspepsia, among other conditions. Researchers theorize that certain foods, toxins, infections, and/or other stressors lead to changes in the structure and function of tight junctions, resulting in increased intestinal permeability.19 This in turn is thought to allow the outflow of antigens through the leaky epithelium and to stimulate an immune response—a process that may play a role in the increased GI inflammation or hypersensitivity associated with dyspepsia. The “leaky gut” theory may eventually lead to new ways to treat dyspepsia, but thus far, highquality evidence of the efficacy of treatments aimed at this mechanism is lacking.
A range of disorders included in the differential
The primary differential diagnosis for dyspepsia includes gastroesophageal reflux disease (GERD), esophagitis, chronic PUD (including both gastric and duodenal ulcers), and malignancy. The differential may also include biliary disorder, pancreatitis, hepatitis, or other liver disease; chronic abdominal wall pain, irritable bowel syndrome, motility disorders, or infiltrative diseases of the stomach (eosinophilic gastritis, Crohn’s disease, sarcoidosis); celiac disease and food sensitivities/allergies, including gluten, lactose, and other intolerances; cardiac disease, including acute coronary syndrome, myocardial infarction, and arrhythmias; intestinal angina; small intestine bacterial overgrowth; heavy metal toxicity; and hypercalcemia.8
Ulcers are found in approximately 10% of patients undergoing evaluation for dyspepsia.8 Previously, PUD was almost exclusively due to H pylori infection. In developed countries, however, chronic use of NSAIDs, including aspirin, has increased, and is now responsible for most ulcer diseases.20,21 The combination of H pylori infection and NSAID usage appears to be synergistic, with the risk of uncomplicated PUD estimated to be 17.5 times higher among those who test positive for H pylori and take NSAIDs vs a 3- to 4-fold increase in ulcer incidence among those with either of these risk factors alone.22
The work-up starts with a search for red flags
Symptom severity is a poor predictor of the seriousness of dyspepsia; more intense symptoms are no more likely than milder cases to have an organic cause.
Evaluation of a patient with dyspepsia begins with a thorough history. Start by determining whether the patient has any red flags, or alarm features, that may be associated with a more serious condition—particularly an underlying malignancy. One or more of the following is an indication for an esophagogastroduodenoscopy (EGD):5,8,12
• family and/or personal history of upper GI cancer
• unintended weight loss
• GI bleeding
• progressive dysphagia
• unexplained iron-deficiency anemia
• persistent vomiting
• palpable mass or lymphadenopathy
• jaundice.
While it is important to rule out these red flags, they are poor predictors of malignancy.23,24 With the exception of a single study, their positive predictive value was a mere 1%.8 Their usefulness lies in their ability to exclude malignancy, however; when none of these features is present, the negative predictive value for malignancy is >97%.8
Age is also a risk factor. In addition to red flags, EGD is recommended by the American Gastroenterological Association (AGA) for patients with new-onset dyspepsia who are 55 years or older—an age at which upper GI malignancy becomes more common. A repeat EGD is rarely indicated, unless Barrett’s esophagus or severe erosive esophagitis is found on the initial EGD.25
Physical exam, H pylori evaluation follow
A physical examination of all patients presenting with symptoms suggestive of dyspepsia is crucial. While the exam is usually normal, it may reveal epigastric tenderness on abdominal palpation. Rebound tenderness, guarding, or evidence of other abnormalities should raise the prospect of alternative diagnoses. GERD, for example, has many symptoms in common with dyspepsia, but is a more likely diagnosis in a patient who has retrosternal burning discomfort and regurgitation and reports that symptoms worsen at night and when lying down.
Lab work has limited value. Although laboratory work is not specifically addressed in the AGA guidelines (except for H pylori testing), a complete blood count is a reasonable part of an initial evaluation of dyspepsia to check for anemia. Other routine blood work is not needed, but further lab testing may be warranted based on the history, exam, and differential diagnosis.
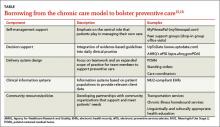
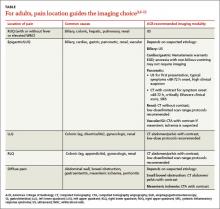
H pylori risk. Because of the association between dyspepsia and H pylori, evaluating the patient’s risk for infection with this bacterium, based primarily on his or her current and previous living conditions (TABLE 1),9 is the next step. Although a test for H pylori could be included in the initial work-up of all patients with dyspepsia, a better—and more cost-effective—strategy is to initially test only those at high risk. (More on testing and treating H pylori in a bit.)
Initiate acid suppression therapy for low-risk patients
First-line treatment for patients with dyspepsia who have no red flags for malignancy or other serious conditions and either are not at high risk for H pylori or are at high risk but have been tested for it and had negative results is a 4- to 8-week course of acid suppression therapy. Patients at low risk for H pylori should be tested for the bacterium only if therapy fails to alleviate their symptoms.9
H2RAs or PPIs? A look at the evidence
In a Cochrane review, both H2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) were significantly more effective than placebo for treating FD.26 However, H2RAs can lead to tachyphylaxis—an acute decrease in response to a drug—within 2 to 6 weeks, thus limiting their long-term efficacy.27
PPIs appear to be more effective than H2RAs, and are the AGA’s acid suppression drug of choice.11 The CADET study, a randomized controlled trial comparing PPIs (omeprazole 20 mg/d) with an H2RA (ranitidine 150 mg BID) and a prokinetic agent (cisapride 20 mg BID) as well as placebo for dyspepsia, found the PPI to be superior to the H2RA at 6 months.28 In a systematic review, the number needed to treat with PPI therapy for improvement of dyspepsia symptoms was 9.29
There is no specified time limit for the use of PPIs. AGA guidelines recommend that patients who respond to initial therapy stop treatment after 4 to 8 weeks.11 If symptoms recur, another course of the same treatment is justified; if necessary, therapy can continue long term. However, patients should be made aware of the risk for vitamin deficiency, osteoporosis, and fracture, as well as arrhythmias, Clostridium difficile infection, and rebound upon abrupt discontinuation of PPIs.
When to test for H pylori ...
Empiric treatment for H pylori is not recommended. Thus, testing is indicated for patients who have risk factors for the bacterium or who fail to respond to acid suppression therapy. There are various ways to test for the presence of H pylori. Which test you choose depends, in part, on patient-specific factors.
Serology. IgG serology testing is extremely useful in patients who have never been diagnosed with H pylori. It is best suited for those who are currently taking proton pump inhibitors (PPIs) or who recently completed a course of antibiotics, since neither medication affects the results of the serology test.
Serology testing should not be used, however, for any patient who was previously diagnosed with or treated for H pylori, because this type of test cannot distinguish between an active or past infection. The IgG serology test has a sensitivity of 87% and a specificity of 67%.30
Stool antigen. Stool tests using monoclonal antibodies to detect the presence of H pylori have a sensitivity of 87% to 92% and a specificity of 70%. Stool antigen is also an excellent post-treatment test to confirm that H pylori has been eradicated.31
Stool testing has some drawbacks, however. PPIs can decrease the sensitivity and should be discontinued at least 2 weeks prior to stool testing.32 In addition, a stool test for H pylori is not accurate if the patient has an acute GI bleed.
Urea breath testing. This is the most sensitive and specific test for active H pylori infection (90%-96% sensitivity and 88%-96% specificity).33 PPIs can lower the sensitivity of the test, however, and are typically discontinued at least 2 weeks prior to testing. Urea breath testing, like stool testing, is an excellent way to confirm that H pylori has been eradicated after treatment. However, it is more expensive than other tests for H pylori and often inconvenient to obtain.13
An EGD is indicated for a patient who has failed to respond to acid suppression therapy and has a negative serology, stool antigen, or urea breath test for H pylori.
Biopsy-based testing for H pylori is performed with EGD and is therefore reserved for patients who have red flags or other indications of a need for invasive testing. There are 3 types of biopsy-based tests: urease (sensitivity, 70%-90%; specificity, 95%); histology (87%-92% and 70%, respectively); and culture (85%-88% and 69%, respectively). Overall, the specificity is slightly better than that of noninvasive testing, but the sensitivity can be lowered by recent use of PPIs, bismuth, or antibiotics.12,34
... and how to treat it
H pylori infection is associated with an increased risk of noncardiac gastric adenocarcinoma, but a decreased risk of cardiac gastric adenocarcinoma and esophageal adenocarcinoma.35,36 Thus, the potential to reduce the risk of gastric cancer is not considered an indication for H pylori treatment. The possibility of improving dyspepsia symptoms is a reason to treat H pylori infection, although eradicating it does not always do so.
In a 2006 Cochrane Review, treating H pylori had a small but statistically significant benefit for patients with FD (NNT=14).37 A 2011 study on the effects of H pylori eradication on symptoms and quality of life in primary care patients with FD revealed a 12.5% improvement in quality of life and a 10.6% improvement in symptoms.38
The triple therapy regimen (a PPI + amoxicillin + clarithromycin) is the most common first-line H pylori treatment in the United States, and a good initial choice in regions in which clarithromycin resistance is low (TABLE 2).39-44 The standard duration is 7 days. A 2013 Cochrane Review showed that a longer duration (14 days) increased the rate of eradication (82% vs 73%), but this remains controversial.39 The addition of bismuth subsalicylate to the triple therapy regimen has been shown to increase the eradication rate of H pylori by approximately 10%.45 Adding probiotics (saccharomyces or lactobacillus) appears to increase eradication rates, as well.40
Sequential therapy consists of a 5-day course of treatment in which a PPI and amoxicillin are taken twice a day, followed by another 5-day course of a PPI, clarithromycin, and metronidazole. A recent meta-analysis of sequential therapy showed that it is superior to 7-day triple therapy but equivalent to 14-day triple therapy.40
LOAD (levofloxacin, omeprazole, nitazoxanide, and doxycycline) therapy for 7 to 10 days can be used in place of triple therapy in areas of high resistance or for persistent H pylori. In one study, the H pylori eradication rate for a 7-day course of LOAD therapy—levofloxacin and doxycycline taken once a day, omeprazole before breakfast, and nitazoxanide twice daily—was 90% vs 73.3% for a 7-day course of triple therapy.41
Quadruple therapy has 2 variations: bismuth-based and non-bismuth (concomitant) therapy. The latter uses the base triple therapy and adds either metronidazole or tinidazole for 7 to 14 days. In a multicenter randomized trial, this concomitant therapy was found to have similar efficacy to sequential therapy.42
Bismuth-based quad therapy includes a PPI, bismuth, metronidazole, and tetracycline. A meta-analysis found it to have a higher rate of eradication than triple therapy for patients with antibiotic resistance.43,44
For persistent H pylori, a PPI, levofloxacin, and amoxicillin for 10 days has been shown to be more effective and better tolerated than quadruple therapy.12
Confirmation is indicated when symptoms persist
If dyspepsia symptoms persist after H pylori treatment, it is reasonable to retest to confirm that the infection has in fact been eradicated. Confirmation is also indicated if the patient has an H pylori-associated ulcer or a prior history of gastric cancer.
Retesting should be performed at least 4 to 6 weeks after treatment is completed. If H pylori has not been eradicated, you can try another regimen. If retesting confirms eradication and symptoms persist, EGD with biopsy is indicated. Although EGD typically has a very low yield, even for patients with red flags, this invasive test often provides reassurance and increased satisfaction for patients with persistent symptoms.46
More options for challenging cases
Managing FD is challenging when both initial acid suppression therapy and H pylori eradication fail. Unproven but low-risk treatments include modification of eating habits (eg, eating slower, not gulping food), reducing stress, discontinuing medications that may be related to symptoms, avoiding foods that seem to exacerbate symptoms, and cutting down or eliminating tobacco, caffeine, alcohol, and carbonated beverages.8 Bismuth salts have been shown to be superior to placebo for the treatment of dyspepsia.25 Small studies have also demonstrated a favorable risk–benefit ratio for peppermint oil and caraway oil for the treatment of FD.47 Prokinetics have shown efficacy compared with placebo, although a Cochrane review questioned their efficacy based on publication bias.26
There is no good evidence of efficacy for over-the-counter antacids, such as TUMS, or for GI “cocktails” (antacid, antispasmotic, and lidocaine), sucralfate, psychological interventions (eg, cognitive behavioral therapy, relaxation therapy, or hypnosis), or antidepressants.48,49 Several recent randomized controlled trials have shown the efficacy of acupuncture for the treatment of dyspepsia.49,50 Ginger may also be helpful; it has been found to help with nausea in other GI conditions, but it’s uncertain whether it can help patients with dyspepsia.51
CORRESPONDENCE
Michael Malone, MD, 845 Fishburn Road, Hershey, PA 17053; [email protected]
1. Shaib Y, El-Serag HB. The prevalence and risk factors of functional dyspepsia in a multiethnic population in the United States. Am J Gastroenterol. 2004;99:2210-2216.
2. Talley NJ. Dyspepsia: management guidelines for the millennium. Gut. 2002;50(suppl 4):iv72–iv78.
3. Harmon RC, Peura DA. Evaluation and management of dyspepsia. Therap Adv Gastroenterol. 2010;3:87–98.
4. Bazaldua OV, Schneider FD. Evaluation and management of dyspepsia. Am Fam Physician. 1999;60:1773-1784.
5. Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479.
6. Haag S, Talley NJ, Holtmann G. Symptom patterns in functional dyspepsia and irritable bowel syndrome: relationship to disturbances in gastric emptying and response to a nutrient challenge in consulters and non-consulters. Gut. 2004;53:1445-1451.
7. Malfertheiner P, Megraud F, O’Morain CA, et al; European Helicobacter Study Group. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-664.
8. Talley NJ, Vakil NB, Moayyedi P. American Gastroenterological Association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756-1780.
9. Moayyedi P, Axon AT. The usefulness of the likelihood ratio in the diagnosis of dyspepsia and gastroesophageal reflux disease. Am J Gastroenterol. 1999;94:3122-3125.
10. McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597-1604.
11. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391.
12. Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori Infection. Am J Gastroenterol. 2007;102:1808-1825.
13. Moayyedi P, Talley NJ, Fennerty MB, et al. Can the clinical history distinguish between organic and functional dyspepsia? JAMA. 2006;295:1566-1576.
14. Eslick GD, Howell SC, Hammer J, et al. Empirically derived symptom sub-groups correspond poorly with diagnostic criteria for functional dyspepsia and irritable bowel syndrome. A factor and cluster analysis of a patient sample. Aliment Pharmacol Ther. 2004;19:133-140.
15. Aro P, Talley NJ, Ronkainen J, et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population-based study. Gastroenterology. 2009;137:94-100.
16. Mertz H, Fullerton S, Naliboff B, et al. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut. 1998;42:814-822.
17. O’Morain C. Role of Helicobacter pylori in functional dyspepsia. World J Gastroenterol. 2006;12:2677-2680.
18. Shmuely H, Obure S, Passaro DJ, et al. Dyspepsia symptoms and Helicobacter pylori infection, Nakuru, Kenya. Emerg Infect Dis. 2003;9:1103-1107.
19. Barbara G, Zecchi L, Barbaro R, et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46(suppl):S52-S55.
20. Liu NJ, Lee CS, Tang JH, et al. Outcomes of bleeding peptic ulcers: a prospective study. J Gastroenterol Hepatol. 2008;23:e340-e347.
21. Ramsoekh D, van Leerdam ME, Rauws EA, et al. Outcome of peptic ulcer bleeding, nonsteroidal anti-inflammatory drug use, and Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2005;3:859-864.
22. Papatheodoridis GV, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol. 2006;4:130-142.
23. Bai Y, Li ZS, Zou DW, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: an endoscopic database review of 102,665 patients from 1996 to 2006. Gut. 2010;59:722-728.
24. Vakil N. Dyspepsia, peptic ulcer, and H. pylori: a remembrance of things past. Am J Gastroenterol. 2010;105:572-574.
25. Shaheen NJ, Weinberg DS, Denberg TD, et al; Clinical Guidelines Committee of the American College of Physicians. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2012;157:808-816.
26. Moayyedi P, Soo S, Deeks J, et al. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;(4):CD001960.
27. Chiu CT, Hsu CM, Wang CC, et al. Randomised clinical trial: sodium alginate oral suspension is non-inferior to omeprazole in the treatment of patients with non-erosive gastroesophageal disease. Aliment Pharmacol Ther. 2013;38:1054-1064.
28. Veldhuyzen van Zanten SJ, Chiba N, Armstrong D, et al. A randomized trial comparing omeprazole, ranitidine, cisapride, or placebo in helicobacter pylori negative, primary care patients with dyspepsia: the CADET-HN Study. Am J Gastroenterol. 2005;100:1477-1488.
29. Moayyedi P, Delaney BC, Vakil N, et al. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329-1337.
30. Garza-González E, Bosques-Padilla FJ, Tijerina-Menchaca R, et al. Comparison of endoscopy-based and serum-based methods for the diagnosis of Helicobacter pylori. Can J Gastroenterol. 2003;17:101-106.
31. Kodama M, Murakami K, Okimoto T, et al. Influence of proton pump inhibitor treatment on Helicobacter pylori stool antigen test. World J Gastroenterol. 2012;18:44-48.
32. Shimoyama T. Stool antigen tests for the management of Helicobacter pylori infection. World J Gastroenterol. 2013;19:8188-8191.
33. Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2330-2338.
34. Gisbert J, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863.
35. Kamangar F, Dawsey SM, Blaser MJ, et al. Opposing risks of gastric cardiac and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445-1452.
36. Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prevent Res (Phila). 2008;1:329-338.
37. Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;(2):CD002096.
38. Mazzoleni LE, Sander GB, Francesconi CF, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med. 2011;171:1929-1936.
39. Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;(12):CD008337.
40. Zou J, Dong J, Yu X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:97-107.
41. Basu PP, Rayapudi K, Pacana T, et al. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011;106:1970-1975.
42. Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with 4 drugs are equally effective for eradication of H. pylori infection. Clin Gastroenterol Hepatol. 2010;8:36–41.
43. Osato R, Reddy R, Reddy SG, et al. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med. 2001;161:1217-1220.
44. Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple firstline therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343-357.
45. Hinostroza Morales D, Díaz Ferrer J. Addition of bismuth subsalicylate to triple eradication therapy for Helicobacter pylori infection: efficiency and adverse events. Rev Gastroenterol Peru. 2014;34:315-320.
46. Rabeneck L, Wristers K, Souchek J, et al. Impact of upper endoscopy on satisfaction in patients with previously uninvestigated dyspepsia. Gastrointest Endosc. 2003;57:295-299.
47. Hojo M, Miwa H, Yokoyama T, et al. Treatment of functional dyspepsia with antianxiety or antidepressive agents: systematic review. J Gastroenterol. 2005;40:1036-1042.
48. Soo S, Moayyedi P, Deeks J, et al. Psychological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2005;(2):CD002301.
49. Lima FA, Ferreira LE, Pace FH. Acupuncture effectiveness as a complementary therapy in functional dyspepsia patients. Arq Gastroenterol. 2013;50:202-207.
50. Ma TT, Yu SY, Li Y, et al. Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia. Aliment Pharmacol Ther. 2012;35:552-561.
51. Koretz RL, Rotblatt M. Complementary and alternative medicine in gastroenterology: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2004;2:957-967.
› Review the medications taken by patients who suffer from dyspepsia, as many drugs—bisphosphonates, antibiotics, steroids, and nonsteroidal anti-inflammatory drugs, among others—are associated with this condition. B
› Order an esophagogastroduodenoscopy for patients ages 55 years or older with new-onset dyspepsia and those who have red flags for more serious conditions, eg, a history of upper gastrointestinal (GI) cancer, unintended weight loss, GI bleeding, dysphagia, or a palpable mass. C
› Prescribe acid suppression therapy as first-line treatment for patients who have dyspepsia but are at low risk or have tested negative for Helicobacter pylori infection. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, an estimated 25% to 30% of the US population suffers from dyspepsia.1 Most self-treat with home remedies and over-the-counter products, but others seek medical care. Dyspepsia accounts for an estimated 2% to 5% of primary care visits annually,2 mostly by patients who are found to have no organic, or structural, cause for their symptoms.1,3
Such patients are said to have functional dyspepsia (FD), a category that applies to about two-thirds of those with dyspepsia.1 A small number of cases are categorized as organic dyspepsia, indicating the presence of a clear structural or anatomic cause, such as an ulcer or mass. The remainder are said to have undifferentiated dyspepsia, which simply means that their signs and symptoms do not rise to the level for which further investigation is warranted and thus it is not known whether it is functional or organic.
There are many possible causes of FD, ranging from medications3,4 to abnormal gastroduodenal motility5,6 to Helicobacter pylori infection,7 and a comprehensive differential diagnosis. The first step in an investigation is to rule out red flags suggestive of gastrointestinal (GI) cancer or other serious disorders.
Patients with FD, like the vast majority of those you’ll treat in a primary care setting, suffer significant morbidity. Most have chronic symptoms, with intermittent flare-ups interspersed with periods of remission.8 In the text and dyspepsia treatment ALGORITHM5,7-12 that follow, you’ll find an evidence-based patient management approach.
Symptoms and causes: What to look for
The primary symptoms of dyspepsia include bothersome postprandial fullness, early satiety, and epigastric pain and burning. To meet the Rome criteria for dyspepsia, these symptoms must have been present for the last 3 months and have had an onset ≥6 months prior to diagnosis.2 Recurrent belching and nausea are also common, but are not included in the Rome diagnostic criteria.
Symptom severity is a poor predictor of the seriousness of the condition, however, and more intense symptoms are no more likely than milder cases to have an organic cause.13,14 Indeed, anxiety is a common comorbidity in patients with FD and a risk factor for the diagnosis. Compared with the general public, patients with FD have been found to have higher levels of anxiety, chronic tension, hostility, and hypochondriasis, and a tendency to be more pessimistic.15
Possible causes of FD
While the etiology of organic dyspepsia is clear, the cause of FD is often far more difficult to determine.
Medication use should always be considered, as many types of drugs—including bisphosphonates, antibiotics, narcotics, steroids, iron, metformin, and nonsteroidal anti-inflammatory drugs (NSAIDs)—are associated with dyspepsia.3,4
Gastroduodenal motility and accommodation, which has been found in numerous studies of patients with FD, is a proposed etiology.5,6
Visceral hypersensitivity also appears to play a role. In one study of patients with severe dyspepsia, 87% of those with FD had a reduced or altered GI pain threshold, compared with 20% of those with organic dyspepsia.16
H pylori, commonly linked to peptic ulcer disease (PUD), is also associated with both organic dyspepsia and FD.17,18 The gram-negative rod-shaped bacterium is present in approximately half of the population worldwide, but is more common in developing nations.7H pylori immunoglobulin G (IgG) is more prevalent in patients with dyspepsia, particularly in those younger than 30 years of age. The exact mechanism by which H pylori causes non-ulcerative dyspepsia is not clear, but inflammation, dysmotility, visceral hypersensitivity, and alteration of acid secretion have all been proposed.17
Dysfunctional intestinal epithelium is increasingly being considered in the pathophysiology of dyspepsia, among other conditions. Researchers theorize that certain foods, toxins, infections, and/or other stressors lead to changes in the structure and function of tight junctions, resulting in increased intestinal permeability.19 This in turn is thought to allow the outflow of antigens through the leaky epithelium and to stimulate an immune response—a process that may play a role in the increased GI inflammation or hypersensitivity associated with dyspepsia. The “leaky gut” theory may eventually lead to new ways to treat dyspepsia, but thus far, highquality evidence of the efficacy of treatments aimed at this mechanism is lacking.
A range of disorders included in the differential
The primary differential diagnosis for dyspepsia includes gastroesophageal reflux disease (GERD), esophagitis, chronic PUD (including both gastric and duodenal ulcers), and malignancy. The differential may also include biliary disorder, pancreatitis, hepatitis, or other liver disease; chronic abdominal wall pain, irritable bowel syndrome, motility disorders, or infiltrative diseases of the stomach (eosinophilic gastritis, Crohn’s disease, sarcoidosis); celiac disease and food sensitivities/allergies, including gluten, lactose, and other intolerances; cardiac disease, including acute coronary syndrome, myocardial infarction, and arrhythmias; intestinal angina; small intestine bacterial overgrowth; heavy metal toxicity; and hypercalcemia.8
Ulcers are found in approximately 10% of patients undergoing evaluation for dyspepsia.8 Previously, PUD was almost exclusively due to H pylori infection. In developed countries, however, chronic use of NSAIDs, including aspirin, has increased, and is now responsible for most ulcer diseases.20,21 The combination of H pylori infection and NSAID usage appears to be synergistic, with the risk of uncomplicated PUD estimated to be 17.5 times higher among those who test positive for H pylori and take NSAIDs vs a 3- to 4-fold increase in ulcer incidence among those with either of these risk factors alone.22
The work-up starts with a search for red flags
Symptom severity is a poor predictor of the seriousness of dyspepsia; more intense symptoms are no more likely than milder cases to have an organic cause.
Evaluation of a patient with dyspepsia begins with a thorough history. Start by determining whether the patient has any red flags, or alarm features, that may be associated with a more serious condition—particularly an underlying malignancy. One or more of the following is an indication for an esophagogastroduodenoscopy (EGD):5,8,12
• family and/or personal history of upper GI cancer
• unintended weight loss
• GI bleeding
• progressive dysphagia
• unexplained iron-deficiency anemia
• persistent vomiting
• palpable mass or lymphadenopathy
• jaundice.
While it is important to rule out these red flags, they are poor predictors of malignancy.23,24 With the exception of a single study, their positive predictive value was a mere 1%.8 Their usefulness lies in their ability to exclude malignancy, however; when none of these features is present, the negative predictive value for malignancy is >97%.8
Age is also a risk factor. In addition to red flags, EGD is recommended by the American Gastroenterological Association (AGA) for patients with new-onset dyspepsia who are 55 years or older—an age at which upper GI malignancy becomes more common. A repeat EGD is rarely indicated, unless Barrett’s esophagus or severe erosive esophagitis is found on the initial EGD.25
Physical exam, H pylori evaluation follow
A physical examination of all patients presenting with symptoms suggestive of dyspepsia is crucial. While the exam is usually normal, it may reveal epigastric tenderness on abdominal palpation. Rebound tenderness, guarding, or evidence of other abnormalities should raise the prospect of alternative diagnoses. GERD, for example, has many symptoms in common with dyspepsia, but is a more likely diagnosis in a patient who has retrosternal burning discomfort and regurgitation and reports that symptoms worsen at night and when lying down.
Lab work has limited value. Although laboratory work is not specifically addressed in the AGA guidelines (except for H pylori testing), a complete blood count is a reasonable part of an initial evaluation of dyspepsia to check for anemia. Other routine blood work is not needed, but further lab testing may be warranted based on the history, exam, and differential diagnosis.
H pylori risk. Because of the association between dyspepsia and H pylori, evaluating the patient’s risk for infection with this bacterium, based primarily on his or her current and previous living conditions (TABLE 1),9 is the next step. Although a test for H pylori could be included in the initial work-up of all patients with dyspepsia, a better—and more cost-effective—strategy is to initially test only those at high risk. (More on testing and treating H pylori in a bit.)
Initiate acid suppression therapy for low-risk patients
First-line treatment for patients with dyspepsia who have no red flags for malignancy or other serious conditions and either are not at high risk for H pylori or are at high risk but have been tested for it and had negative results is a 4- to 8-week course of acid suppression therapy. Patients at low risk for H pylori should be tested for the bacterium only if therapy fails to alleviate their symptoms.9
H2RAs or PPIs? A look at the evidence
In a Cochrane review, both H2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) were significantly more effective than placebo for treating FD.26 However, H2RAs can lead to tachyphylaxis—an acute decrease in response to a drug—within 2 to 6 weeks, thus limiting their long-term efficacy.27
PPIs appear to be more effective than H2RAs, and are the AGA’s acid suppression drug of choice.11 The CADET study, a randomized controlled trial comparing PPIs (omeprazole 20 mg/d) with an H2RA (ranitidine 150 mg BID) and a prokinetic agent (cisapride 20 mg BID) as well as placebo for dyspepsia, found the PPI to be superior to the H2RA at 6 months.28 In a systematic review, the number needed to treat with PPI therapy for improvement of dyspepsia symptoms was 9.29
There is no specified time limit for the use of PPIs. AGA guidelines recommend that patients who respond to initial therapy stop treatment after 4 to 8 weeks.11 If symptoms recur, another course of the same treatment is justified; if necessary, therapy can continue long term. However, patients should be made aware of the risk for vitamin deficiency, osteoporosis, and fracture, as well as arrhythmias, Clostridium difficile infection, and rebound upon abrupt discontinuation of PPIs.
When to test for H pylori ...
Empiric treatment for H pylori is not recommended. Thus, testing is indicated for patients who have risk factors for the bacterium or who fail to respond to acid suppression therapy. There are various ways to test for the presence of H pylori. Which test you choose depends, in part, on patient-specific factors.
Serology. IgG serology testing is extremely useful in patients who have never been diagnosed with H pylori. It is best suited for those who are currently taking proton pump inhibitors (PPIs) or who recently completed a course of antibiotics, since neither medication affects the results of the serology test.
Serology testing should not be used, however, for any patient who was previously diagnosed with or treated for H pylori, because this type of test cannot distinguish between an active or past infection. The IgG serology test has a sensitivity of 87% and a specificity of 67%.30
Stool antigen. Stool tests using monoclonal antibodies to detect the presence of H pylori have a sensitivity of 87% to 92% and a specificity of 70%. Stool antigen is also an excellent post-treatment test to confirm that H pylori has been eradicated.31
Stool testing has some drawbacks, however. PPIs can decrease the sensitivity and should be discontinued at least 2 weeks prior to stool testing.32 In addition, a stool test for H pylori is not accurate if the patient has an acute GI bleed.
Urea breath testing. This is the most sensitive and specific test for active H pylori infection (90%-96% sensitivity and 88%-96% specificity).33 PPIs can lower the sensitivity of the test, however, and are typically discontinued at least 2 weeks prior to testing. Urea breath testing, like stool testing, is an excellent way to confirm that H pylori has been eradicated after treatment. However, it is more expensive than other tests for H pylori and often inconvenient to obtain.13
An EGD is indicated for a patient who has failed to respond to acid suppression therapy and has a negative serology, stool antigen, or urea breath test for H pylori.
Biopsy-based testing for H pylori is performed with EGD and is therefore reserved for patients who have red flags or other indications of a need for invasive testing. There are 3 types of biopsy-based tests: urease (sensitivity, 70%-90%; specificity, 95%); histology (87%-92% and 70%, respectively); and culture (85%-88% and 69%, respectively). Overall, the specificity is slightly better than that of noninvasive testing, but the sensitivity can be lowered by recent use of PPIs, bismuth, or antibiotics.12,34
... and how to treat it
H pylori infection is associated with an increased risk of noncardiac gastric adenocarcinoma, but a decreased risk of cardiac gastric adenocarcinoma and esophageal adenocarcinoma.35,36 Thus, the potential to reduce the risk of gastric cancer is not considered an indication for H pylori treatment. The possibility of improving dyspepsia symptoms is a reason to treat H pylori infection, although eradicating it does not always do so.
In a 2006 Cochrane Review, treating H pylori had a small but statistically significant benefit for patients with FD (NNT=14).37 A 2011 study on the effects of H pylori eradication on symptoms and quality of life in primary care patients with FD revealed a 12.5% improvement in quality of life and a 10.6% improvement in symptoms.38
The triple therapy regimen (a PPI + amoxicillin + clarithromycin) is the most common first-line H pylori treatment in the United States, and a good initial choice in regions in which clarithromycin resistance is low (TABLE 2).39-44 The standard duration is 7 days. A 2013 Cochrane Review showed that a longer duration (14 days) increased the rate of eradication (82% vs 73%), but this remains controversial.39 The addition of bismuth subsalicylate to the triple therapy regimen has been shown to increase the eradication rate of H pylori by approximately 10%.45 Adding probiotics (saccharomyces or lactobacillus) appears to increase eradication rates, as well.40
Sequential therapy consists of a 5-day course of treatment in which a PPI and amoxicillin are taken twice a day, followed by another 5-day course of a PPI, clarithromycin, and metronidazole. A recent meta-analysis of sequential therapy showed that it is superior to 7-day triple therapy but equivalent to 14-day triple therapy.40
LOAD (levofloxacin, omeprazole, nitazoxanide, and doxycycline) therapy for 7 to 10 days can be used in place of triple therapy in areas of high resistance or for persistent H pylori. In one study, the H pylori eradication rate for a 7-day course of LOAD therapy—levofloxacin and doxycycline taken once a day, omeprazole before breakfast, and nitazoxanide twice daily—was 90% vs 73.3% for a 7-day course of triple therapy.41
Quadruple therapy has 2 variations: bismuth-based and non-bismuth (concomitant) therapy. The latter uses the base triple therapy and adds either metronidazole or tinidazole for 7 to 14 days. In a multicenter randomized trial, this concomitant therapy was found to have similar efficacy to sequential therapy.42
Bismuth-based quad therapy includes a PPI, bismuth, metronidazole, and tetracycline. A meta-analysis found it to have a higher rate of eradication than triple therapy for patients with antibiotic resistance.43,44
For persistent H pylori, a PPI, levofloxacin, and amoxicillin for 10 days has been shown to be more effective and better tolerated than quadruple therapy.12
Confirmation is indicated when symptoms persist
If dyspepsia symptoms persist after H pylori treatment, it is reasonable to retest to confirm that the infection has in fact been eradicated. Confirmation is also indicated if the patient has an H pylori-associated ulcer or a prior history of gastric cancer.
Retesting should be performed at least 4 to 6 weeks after treatment is completed. If H pylori has not been eradicated, you can try another regimen. If retesting confirms eradication and symptoms persist, EGD with biopsy is indicated. Although EGD typically has a very low yield, even for patients with red flags, this invasive test often provides reassurance and increased satisfaction for patients with persistent symptoms.46
More options for challenging cases
Managing FD is challenging when both initial acid suppression therapy and H pylori eradication fail. Unproven but low-risk treatments include modification of eating habits (eg, eating slower, not gulping food), reducing stress, discontinuing medications that may be related to symptoms, avoiding foods that seem to exacerbate symptoms, and cutting down or eliminating tobacco, caffeine, alcohol, and carbonated beverages.8 Bismuth salts have been shown to be superior to placebo for the treatment of dyspepsia.25 Small studies have also demonstrated a favorable risk–benefit ratio for peppermint oil and caraway oil for the treatment of FD.47 Prokinetics have shown efficacy compared with placebo, although a Cochrane review questioned their efficacy based on publication bias.26
There is no good evidence of efficacy for over-the-counter antacids, such as TUMS, or for GI “cocktails” (antacid, antispasmotic, and lidocaine), sucralfate, psychological interventions (eg, cognitive behavioral therapy, relaxation therapy, or hypnosis), or antidepressants.48,49 Several recent randomized controlled trials have shown the efficacy of acupuncture for the treatment of dyspepsia.49,50 Ginger may also be helpful; it has been found to help with nausea in other GI conditions, but it’s uncertain whether it can help patients with dyspepsia.51
CORRESPONDENCE
Michael Malone, MD, 845 Fishburn Road, Hershey, PA 17053; [email protected]
› Review the medications taken by patients who suffer from dyspepsia, as many drugs—bisphosphonates, antibiotics, steroids, and nonsteroidal anti-inflammatory drugs, among others—are associated with this condition. B
› Order an esophagogastroduodenoscopy for patients ages 55 years or older with new-onset dyspepsia and those who have red flags for more serious conditions, eg, a history of upper gastrointestinal (GI) cancer, unintended weight loss, GI bleeding, dysphagia, or a palpable mass. C
› Prescribe acid suppression therapy as first-line treatment for patients who have dyspepsia but are at low risk or have tested negative for Helicobacter pylori infection. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, an estimated 25% to 30% of the US population suffers from dyspepsia.1 Most self-treat with home remedies and over-the-counter products, but others seek medical care. Dyspepsia accounts for an estimated 2% to 5% of primary care visits annually,2 mostly by patients who are found to have no organic, or structural, cause for their symptoms.1,3
Such patients are said to have functional dyspepsia (FD), a category that applies to about two-thirds of those with dyspepsia.1 A small number of cases are categorized as organic dyspepsia, indicating the presence of a clear structural or anatomic cause, such as an ulcer or mass. The remainder are said to have undifferentiated dyspepsia, which simply means that their signs and symptoms do not rise to the level for which further investigation is warranted and thus it is not known whether it is functional or organic.
There are many possible causes of FD, ranging from medications3,4 to abnormal gastroduodenal motility5,6 to Helicobacter pylori infection,7 and a comprehensive differential diagnosis. The first step in an investigation is to rule out red flags suggestive of gastrointestinal (GI) cancer or other serious disorders.
Patients with FD, like the vast majority of those you’ll treat in a primary care setting, suffer significant morbidity. Most have chronic symptoms, with intermittent flare-ups interspersed with periods of remission.8 In the text and dyspepsia treatment ALGORITHM5,7-12 that follow, you’ll find an evidence-based patient management approach.
Symptoms and causes: What to look for
The primary symptoms of dyspepsia include bothersome postprandial fullness, early satiety, and epigastric pain and burning. To meet the Rome criteria for dyspepsia, these symptoms must have been present for the last 3 months and have had an onset ≥6 months prior to diagnosis.2 Recurrent belching and nausea are also common, but are not included in the Rome diagnostic criteria.
Symptom severity is a poor predictor of the seriousness of the condition, however, and more intense symptoms are no more likely than milder cases to have an organic cause.13,14 Indeed, anxiety is a common comorbidity in patients with FD and a risk factor for the diagnosis. Compared with the general public, patients with FD have been found to have higher levels of anxiety, chronic tension, hostility, and hypochondriasis, and a tendency to be more pessimistic.15
Possible causes of FD
While the etiology of organic dyspepsia is clear, the cause of FD is often far more difficult to determine.
Medication use should always be considered, as many types of drugs—including bisphosphonates, antibiotics, narcotics, steroids, iron, metformin, and nonsteroidal anti-inflammatory drugs (NSAIDs)—are associated with dyspepsia.3,4
Gastroduodenal motility and accommodation, which has been found in numerous studies of patients with FD, is a proposed etiology.5,6
Visceral hypersensitivity also appears to play a role. In one study of patients with severe dyspepsia, 87% of those with FD had a reduced or altered GI pain threshold, compared with 20% of those with organic dyspepsia.16
H pylori, commonly linked to peptic ulcer disease (PUD), is also associated with both organic dyspepsia and FD.17,18 The gram-negative rod-shaped bacterium is present in approximately half of the population worldwide, but is more common in developing nations.7H pylori immunoglobulin G (IgG) is more prevalent in patients with dyspepsia, particularly in those younger than 30 years of age. The exact mechanism by which H pylori causes non-ulcerative dyspepsia is not clear, but inflammation, dysmotility, visceral hypersensitivity, and alteration of acid secretion have all been proposed.17
Dysfunctional intestinal epithelium is increasingly being considered in the pathophysiology of dyspepsia, among other conditions. Researchers theorize that certain foods, toxins, infections, and/or other stressors lead to changes in the structure and function of tight junctions, resulting in increased intestinal permeability.19 This in turn is thought to allow the outflow of antigens through the leaky epithelium and to stimulate an immune response—a process that may play a role in the increased GI inflammation or hypersensitivity associated with dyspepsia. The “leaky gut” theory may eventually lead to new ways to treat dyspepsia, but thus far, highquality evidence of the efficacy of treatments aimed at this mechanism is lacking.
A range of disorders included in the differential
The primary differential diagnosis for dyspepsia includes gastroesophageal reflux disease (GERD), esophagitis, chronic PUD (including both gastric and duodenal ulcers), and malignancy. The differential may also include biliary disorder, pancreatitis, hepatitis, or other liver disease; chronic abdominal wall pain, irritable bowel syndrome, motility disorders, or infiltrative diseases of the stomach (eosinophilic gastritis, Crohn’s disease, sarcoidosis); celiac disease and food sensitivities/allergies, including gluten, lactose, and other intolerances; cardiac disease, including acute coronary syndrome, myocardial infarction, and arrhythmias; intestinal angina; small intestine bacterial overgrowth; heavy metal toxicity; and hypercalcemia.8
Ulcers are found in approximately 10% of patients undergoing evaluation for dyspepsia.8 Previously, PUD was almost exclusively due to H pylori infection. In developed countries, however, chronic use of NSAIDs, including aspirin, has increased, and is now responsible for most ulcer diseases.20,21 The combination of H pylori infection and NSAID usage appears to be synergistic, with the risk of uncomplicated PUD estimated to be 17.5 times higher among those who test positive for H pylori and take NSAIDs vs a 3- to 4-fold increase in ulcer incidence among those with either of these risk factors alone.22
The work-up starts with a search for red flags
Symptom severity is a poor predictor of the seriousness of dyspepsia; more intense symptoms are no more likely than milder cases to have an organic cause.
Evaluation of a patient with dyspepsia begins with a thorough history. Start by determining whether the patient has any red flags, or alarm features, that may be associated with a more serious condition—particularly an underlying malignancy. One or more of the following is an indication for an esophagogastroduodenoscopy (EGD):5,8,12
• family and/or personal history of upper GI cancer
• unintended weight loss
• GI bleeding
• progressive dysphagia
• unexplained iron-deficiency anemia
• persistent vomiting
• palpable mass or lymphadenopathy
• jaundice.
While it is important to rule out these red flags, they are poor predictors of malignancy.23,24 With the exception of a single study, their positive predictive value was a mere 1%.8 Their usefulness lies in their ability to exclude malignancy, however; when none of these features is present, the negative predictive value for malignancy is >97%.8
Age is also a risk factor. In addition to red flags, EGD is recommended by the American Gastroenterological Association (AGA) for patients with new-onset dyspepsia who are 55 years or older—an age at which upper GI malignancy becomes more common. A repeat EGD is rarely indicated, unless Barrett’s esophagus or severe erosive esophagitis is found on the initial EGD.25
Physical exam, H pylori evaluation follow
A physical examination of all patients presenting with symptoms suggestive of dyspepsia is crucial. While the exam is usually normal, it may reveal epigastric tenderness on abdominal palpation. Rebound tenderness, guarding, or evidence of other abnormalities should raise the prospect of alternative diagnoses. GERD, for example, has many symptoms in common with dyspepsia, but is a more likely diagnosis in a patient who has retrosternal burning discomfort and regurgitation and reports that symptoms worsen at night and when lying down.
Lab work has limited value. Although laboratory work is not specifically addressed in the AGA guidelines (except for H pylori testing), a complete blood count is a reasonable part of an initial evaluation of dyspepsia to check for anemia. Other routine blood work is not needed, but further lab testing may be warranted based on the history, exam, and differential diagnosis.
H pylori risk. Because of the association between dyspepsia and H pylori, evaluating the patient’s risk for infection with this bacterium, based primarily on his or her current and previous living conditions (TABLE 1),9 is the next step. Although a test for H pylori could be included in the initial work-up of all patients with dyspepsia, a better—and more cost-effective—strategy is to initially test only those at high risk. (More on testing and treating H pylori in a bit.)
Initiate acid suppression therapy for low-risk patients
First-line treatment for patients with dyspepsia who have no red flags for malignancy or other serious conditions and either are not at high risk for H pylori or are at high risk but have been tested for it and had negative results is a 4- to 8-week course of acid suppression therapy. Patients at low risk for H pylori should be tested for the bacterium only if therapy fails to alleviate their symptoms.9
H2RAs or PPIs? A look at the evidence
In a Cochrane review, both H2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) were significantly more effective than placebo for treating FD.26 However, H2RAs can lead to tachyphylaxis—an acute decrease in response to a drug—within 2 to 6 weeks, thus limiting their long-term efficacy.27
PPIs appear to be more effective than H2RAs, and are the AGA’s acid suppression drug of choice.11 The CADET study, a randomized controlled trial comparing PPIs (omeprazole 20 mg/d) with an H2RA (ranitidine 150 mg BID) and a prokinetic agent (cisapride 20 mg BID) as well as placebo for dyspepsia, found the PPI to be superior to the H2RA at 6 months.28 In a systematic review, the number needed to treat with PPI therapy for improvement of dyspepsia symptoms was 9.29
There is no specified time limit for the use of PPIs. AGA guidelines recommend that patients who respond to initial therapy stop treatment after 4 to 8 weeks.11 If symptoms recur, another course of the same treatment is justified; if necessary, therapy can continue long term. However, patients should be made aware of the risk for vitamin deficiency, osteoporosis, and fracture, as well as arrhythmias, Clostridium difficile infection, and rebound upon abrupt discontinuation of PPIs.
When to test for H pylori ...
Empiric treatment for H pylori is not recommended. Thus, testing is indicated for patients who have risk factors for the bacterium or who fail to respond to acid suppression therapy. There are various ways to test for the presence of H pylori. Which test you choose depends, in part, on patient-specific factors.
Serology. IgG serology testing is extremely useful in patients who have never been diagnosed with H pylori. It is best suited for those who are currently taking proton pump inhibitors (PPIs) or who recently completed a course of antibiotics, since neither medication affects the results of the serology test.
Serology testing should not be used, however, for any patient who was previously diagnosed with or treated for H pylori, because this type of test cannot distinguish between an active or past infection. The IgG serology test has a sensitivity of 87% and a specificity of 67%.30
Stool antigen. Stool tests using monoclonal antibodies to detect the presence of H pylori have a sensitivity of 87% to 92% and a specificity of 70%. Stool antigen is also an excellent post-treatment test to confirm that H pylori has been eradicated.31
Stool testing has some drawbacks, however. PPIs can decrease the sensitivity and should be discontinued at least 2 weeks prior to stool testing.32 In addition, a stool test for H pylori is not accurate if the patient has an acute GI bleed.
Urea breath testing. This is the most sensitive and specific test for active H pylori infection (90%-96% sensitivity and 88%-96% specificity).33 PPIs can lower the sensitivity of the test, however, and are typically discontinued at least 2 weeks prior to testing. Urea breath testing, like stool testing, is an excellent way to confirm that H pylori has been eradicated after treatment. However, it is more expensive than other tests for H pylori and often inconvenient to obtain.13
An EGD is indicated for a patient who has failed to respond to acid suppression therapy and has a negative serology, stool antigen, or urea breath test for H pylori.
Biopsy-based testing for H pylori is performed with EGD and is therefore reserved for patients who have red flags or other indications of a need for invasive testing. There are 3 types of biopsy-based tests: urease (sensitivity, 70%-90%; specificity, 95%); histology (87%-92% and 70%, respectively); and culture (85%-88% and 69%, respectively). Overall, the specificity is slightly better than that of noninvasive testing, but the sensitivity can be lowered by recent use of PPIs, bismuth, or antibiotics.12,34
... and how to treat it
H pylori infection is associated with an increased risk of noncardiac gastric adenocarcinoma, but a decreased risk of cardiac gastric adenocarcinoma and esophageal adenocarcinoma.35,36 Thus, the potential to reduce the risk of gastric cancer is not considered an indication for H pylori treatment. The possibility of improving dyspepsia symptoms is a reason to treat H pylori infection, although eradicating it does not always do so.
In a 2006 Cochrane Review, treating H pylori had a small but statistically significant benefit for patients with FD (NNT=14).37 A 2011 study on the effects of H pylori eradication on symptoms and quality of life in primary care patients with FD revealed a 12.5% improvement in quality of life and a 10.6% improvement in symptoms.38
The triple therapy regimen (a PPI + amoxicillin + clarithromycin) is the most common first-line H pylori treatment in the United States, and a good initial choice in regions in which clarithromycin resistance is low (TABLE 2).39-44 The standard duration is 7 days. A 2013 Cochrane Review showed that a longer duration (14 days) increased the rate of eradication (82% vs 73%), but this remains controversial.39 The addition of bismuth subsalicylate to the triple therapy regimen has been shown to increase the eradication rate of H pylori by approximately 10%.45 Adding probiotics (saccharomyces or lactobacillus) appears to increase eradication rates, as well.40
Sequential therapy consists of a 5-day course of treatment in which a PPI and amoxicillin are taken twice a day, followed by another 5-day course of a PPI, clarithromycin, and metronidazole. A recent meta-analysis of sequential therapy showed that it is superior to 7-day triple therapy but equivalent to 14-day triple therapy.40
LOAD (levofloxacin, omeprazole, nitazoxanide, and doxycycline) therapy for 7 to 10 days can be used in place of triple therapy in areas of high resistance or for persistent H pylori. In one study, the H pylori eradication rate for a 7-day course of LOAD therapy—levofloxacin and doxycycline taken once a day, omeprazole before breakfast, and nitazoxanide twice daily—was 90% vs 73.3% for a 7-day course of triple therapy.41
Quadruple therapy has 2 variations: bismuth-based and non-bismuth (concomitant) therapy. The latter uses the base triple therapy and adds either metronidazole or tinidazole for 7 to 14 days. In a multicenter randomized trial, this concomitant therapy was found to have similar efficacy to sequential therapy.42
Bismuth-based quad therapy includes a PPI, bismuth, metronidazole, and tetracycline. A meta-analysis found it to have a higher rate of eradication than triple therapy for patients with antibiotic resistance.43,44
For persistent H pylori, a PPI, levofloxacin, and amoxicillin for 10 days has been shown to be more effective and better tolerated than quadruple therapy.12
Confirmation is indicated when symptoms persist
If dyspepsia symptoms persist after H pylori treatment, it is reasonable to retest to confirm that the infection has in fact been eradicated. Confirmation is also indicated if the patient has an H pylori-associated ulcer or a prior history of gastric cancer.
Retesting should be performed at least 4 to 6 weeks after treatment is completed. If H pylori has not been eradicated, you can try another regimen. If retesting confirms eradication and symptoms persist, EGD with biopsy is indicated. Although EGD typically has a very low yield, even for patients with red flags, this invasive test often provides reassurance and increased satisfaction for patients with persistent symptoms.46
More options for challenging cases
Managing FD is challenging when both initial acid suppression therapy and H pylori eradication fail. Unproven but low-risk treatments include modification of eating habits (eg, eating slower, not gulping food), reducing stress, discontinuing medications that may be related to symptoms, avoiding foods that seem to exacerbate symptoms, and cutting down or eliminating tobacco, caffeine, alcohol, and carbonated beverages.8 Bismuth salts have been shown to be superior to placebo for the treatment of dyspepsia.25 Small studies have also demonstrated a favorable risk–benefit ratio for peppermint oil and caraway oil for the treatment of FD.47 Prokinetics have shown efficacy compared with placebo, although a Cochrane review questioned their efficacy based on publication bias.26
There is no good evidence of efficacy for over-the-counter antacids, such as TUMS, or for GI “cocktails” (antacid, antispasmotic, and lidocaine), sucralfate, psychological interventions (eg, cognitive behavioral therapy, relaxation therapy, or hypnosis), or antidepressants.48,49 Several recent randomized controlled trials have shown the efficacy of acupuncture for the treatment of dyspepsia.49,50 Ginger may also be helpful; it has been found to help with nausea in other GI conditions, but it’s uncertain whether it can help patients with dyspepsia.51
CORRESPONDENCE
Michael Malone, MD, 845 Fishburn Road, Hershey, PA 17053; [email protected]
1. Shaib Y, El-Serag HB. The prevalence and risk factors of functional dyspepsia in a multiethnic population in the United States. Am J Gastroenterol. 2004;99:2210-2216.
2. Talley NJ. Dyspepsia: management guidelines for the millennium. Gut. 2002;50(suppl 4):iv72–iv78.
3. Harmon RC, Peura DA. Evaluation and management of dyspepsia. Therap Adv Gastroenterol. 2010;3:87–98.
4. Bazaldua OV, Schneider FD. Evaluation and management of dyspepsia. Am Fam Physician. 1999;60:1773-1784.
5. Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479.
6. Haag S, Talley NJ, Holtmann G. Symptom patterns in functional dyspepsia and irritable bowel syndrome: relationship to disturbances in gastric emptying and response to a nutrient challenge in consulters and non-consulters. Gut. 2004;53:1445-1451.
7. Malfertheiner P, Megraud F, O’Morain CA, et al; European Helicobacter Study Group. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-664.
8. Talley NJ, Vakil NB, Moayyedi P. American Gastroenterological Association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756-1780.
9. Moayyedi P, Axon AT. The usefulness of the likelihood ratio in the diagnosis of dyspepsia and gastroesophageal reflux disease. Am J Gastroenterol. 1999;94:3122-3125.
10. McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597-1604.
11. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391.
12. Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori Infection. Am J Gastroenterol. 2007;102:1808-1825.
13. Moayyedi P, Talley NJ, Fennerty MB, et al. Can the clinical history distinguish between organic and functional dyspepsia? JAMA. 2006;295:1566-1576.
14. Eslick GD, Howell SC, Hammer J, et al. Empirically derived symptom sub-groups correspond poorly with diagnostic criteria for functional dyspepsia and irritable bowel syndrome. A factor and cluster analysis of a patient sample. Aliment Pharmacol Ther. 2004;19:133-140.
15. Aro P, Talley NJ, Ronkainen J, et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population-based study. Gastroenterology. 2009;137:94-100.
16. Mertz H, Fullerton S, Naliboff B, et al. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut. 1998;42:814-822.
17. O’Morain C. Role of Helicobacter pylori in functional dyspepsia. World J Gastroenterol. 2006;12:2677-2680.
18. Shmuely H, Obure S, Passaro DJ, et al. Dyspepsia symptoms and Helicobacter pylori infection, Nakuru, Kenya. Emerg Infect Dis. 2003;9:1103-1107.
19. Barbara G, Zecchi L, Barbaro R, et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46(suppl):S52-S55.
20. Liu NJ, Lee CS, Tang JH, et al. Outcomes of bleeding peptic ulcers: a prospective study. J Gastroenterol Hepatol. 2008;23:e340-e347.
21. Ramsoekh D, van Leerdam ME, Rauws EA, et al. Outcome of peptic ulcer bleeding, nonsteroidal anti-inflammatory drug use, and Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2005;3:859-864.
22. Papatheodoridis GV, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol. 2006;4:130-142.
23. Bai Y, Li ZS, Zou DW, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: an endoscopic database review of 102,665 patients from 1996 to 2006. Gut. 2010;59:722-728.
24. Vakil N. Dyspepsia, peptic ulcer, and H. pylori: a remembrance of things past. Am J Gastroenterol. 2010;105:572-574.
25. Shaheen NJ, Weinberg DS, Denberg TD, et al; Clinical Guidelines Committee of the American College of Physicians. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2012;157:808-816.
26. Moayyedi P, Soo S, Deeks J, et al. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;(4):CD001960.
27. Chiu CT, Hsu CM, Wang CC, et al. Randomised clinical trial: sodium alginate oral suspension is non-inferior to omeprazole in the treatment of patients with non-erosive gastroesophageal disease. Aliment Pharmacol Ther. 2013;38:1054-1064.
28. Veldhuyzen van Zanten SJ, Chiba N, Armstrong D, et al. A randomized trial comparing omeprazole, ranitidine, cisapride, or placebo in helicobacter pylori negative, primary care patients with dyspepsia: the CADET-HN Study. Am J Gastroenterol. 2005;100:1477-1488.
29. Moayyedi P, Delaney BC, Vakil N, et al. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329-1337.
30. Garza-González E, Bosques-Padilla FJ, Tijerina-Menchaca R, et al. Comparison of endoscopy-based and serum-based methods for the diagnosis of Helicobacter pylori. Can J Gastroenterol. 2003;17:101-106.
31. Kodama M, Murakami K, Okimoto T, et al. Influence of proton pump inhibitor treatment on Helicobacter pylori stool antigen test. World J Gastroenterol. 2012;18:44-48.
32. Shimoyama T. Stool antigen tests for the management of Helicobacter pylori infection. World J Gastroenterol. 2013;19:8188-8191.
33. Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2330-2338.
34. Gisbert J, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863.
35. Kamangar F, Dawsey SM, Blaser MJ, et al. Opposing risks of gastric cardiac and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445-1452.
36. Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prevent Res (Phila). 2008;1:329-338.
37. Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;(2):CD002096.
38. Mazzoleni LE, Sander GB, Francesconi CF, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med. 2011;171:1929-1936.
39. Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;(12):CD008337.
40. Zou J, Dong J, Yu X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:97-107.
41. Basu PP, Rayapudi K, Pacana T, et al. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011;106:1970-1975.
42. Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with 4 drugs are equally effective for eradication of H. pylori infection. Clin Gastroenterol Hepatol. 2010;8:36–41.
43. Osato R, Reddy R, Reddy SG, et al. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med. 2001;161:1217-1220.
44. Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple firstline therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343-357.
45. Hinostroza Morales D, Díaz Ferrer J. Addition of bismuth subsalicylate to triple eradication therapy for Helicobacter pylori infection: efficiency and adverse events. Rev Gastroenterol Peru. 2014;34:315-320.
46. Rabeneck L, Wristers K, Souchek J, et al. Impact of upper endoscopy on satisfaction in patients with previously uninvestigated dyspepsia. Gastrointest Endosc. 2003;57:295-299.
47. Hojo M, Miwa H, Yokoyama T, et al. Treatment of functional dyspepsia with antianxiety or antidepressive agents: systematic review. J Gastroenterol. 2005;40:1036-1042.
48. Soo S, Moayyedi P, Deeks J, et al. Psychological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2005;(2):CD002301.
49. Lima FA, Ferreira LE, Pace FH. Acupuncture effectiveness as a complementary therapy in functional dyspepsia patients. Arq Gastroenterol. 2013;50:202-207.
50. Ma TT, Yu SY, Li Y, et al. Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia. Aliment Pharmacol Ther. 2012;35:552-561.
51. Koretz RL, Rotblatt M. Complementary and alternative medicine in gastroenterology: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2004;2:957-967.
1. Shaib Y, El-Serag HB. The prevalence and risk factors of functional dyspepsia in a multiethnic population in the United States. Am J Gastroenterol. 2004;99:2210-2216.
2. Talley NJ. Dyspepsia: management guidelines for the millennium. Gut. 2002;50(suppl 4):iv72–iv78.
3. Harmon RC, Peura DA. Evaluation and management of dyspepsia. Therap Adv Gastroenterol. 2010;3:87–98.
4. Bazaldua OV, Schneider FD. Evaluation and management of dyspepsia. Am Fam Physician. 1999;60:1773-1784.
5. Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479.
6. Haag S, Talley NJ, Holtmann G. Symptom patterns in functional dyspepsia and irritable bowel syndrome: relationship to disturbances in gastric emptying and response to a nutrient challenge in consulters and non-consulters. Gut. 2004;53:1445-1451.
7. Malfertheiner P, Megraud F, O’Morain CA, et al; European Helicobacter Study Group. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-664.
8. Talley NJ, Vakil NB, Moayyedi P. American Gastroenterological Association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756-1780.
9. Moayyedi P, Axon AT. The usefulness of the likelihood ratio in the diagnosis of dyspepsia and gastroesophageal reflux disease. Am J Gastroenterol. 1999;94:3122-3125.
10. McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597-1604.
11. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391.
12. Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori Infection. Am J Gastroenterol. 2007;102:1808-1825.
13. Moayyedi P, Talley NJ, Fennerty MB, et al. Can the clinical history distinguish between organic and functional dyspepsia? JAMA. 2006;295:1566-1576.
14. Eslick GD, Howell SC, Hammer J, et al. Empirically derived symptom sub-groups correspond poorly with diagnostic criteria for functional dyspepsia and irritable bowel syndrome. A factor and cluster analysis of a patient sample. Aliment Pharmacol Ther. 2004;19:133-140.
15. Aro P, Talley NJ, Ronkainen J, et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population-based study. Gastroenterology. 2009;137:94-100.
16. Mertz H, Fullerton S, Naliboff B, et al. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut. 1998;42:814-822.
17. O’Morain C. Role of Helicobacter pylori in functional dyspepsia. World J Gastroenterol. 2006;12:2677-2680.
18. Shmuely H, Obure S, Passaro DJ, et al. Dyspepsia symptoms and Helicobacter pylori infection, Nakuru, Kenya. Emerg Infect Dis. 2003;9:1103-1107.
19. Barbara G, Zecchi L, Barbaro R, et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46(suppl):S52-S55.
20. Liu NJ, Lee CS, Tang JH, et al. Outcomes of bleeding peptic ulcers: a prospective study. J Gastroenterol Hepatol. 2008;23:e340-e347.
21. Ramsoekh D, van Leerdam ME, Rauws EA, et al. Outcome of peptic ulcer bleeding, nonsteroidal anti-inflammatory drug use, and Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2005;3:859-864.
22. Papatheodoridis GV, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol. 2006;4:130-142.
23. Bai Y, Li ZS, Zou DW, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: an endoscopic database review of 102,665 patients from 1996 to 2006. Gut. 2010;59:722-728.
24. Vakil N. Dyspepsia, peptic ulcer, and H. pylori: a remembrance of things past. Am J Gastroenterol. 2010;105:572-574.
25. Shaheen NJ, Weinberg DS, Denberg TD, et al; Clinical Guidelines Committee of the American College of Physicians. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2012;157:808-816.
26. Moayyedi P, Soo S, Deeks J, et al. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;(4):CD001960.
27. Chiu CT, Hsu CM, Wang CC, et al. Randomised clinical trial: sodium alginate oral suspension is non-inferior to omeprazole in the treatment of patients with non-erosive gastroesophageal disease. Aliment Pharmacol Ther. 2013;38:1054-1064.
28. Veldhuyzen van Zanten SJ, Chiba N, Armstrong D, et al. A randomized trial comparing omeprazole, ranitidine, cisapride, or placebo in helicobacter pylori negative, primary care patients with dyspepsia: the CADET-HN Study. Am J Gastroenterol. 2005;100:1477-1488.
29. Moayyedi P, Delaney BC, Vakil N, et al. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329-1337.
30. Garza-González E, Bosques-Padilla FJ, Tijerina-Menchaca R, et al. Comparison of endoscopy-based and serum-based methods for the diagnosis of Helicobacter pylori. Can J Gastroenterol. 2003;17:101-106.
31. Kodama M, Murakami K, Okimoto T, et al. Influence of proton pump inhibitor treatment on Helicobacter pylori stool antigen test. World J Gastroenterol. 2012;18:44-48.
32. Shimoyama T. Stool antigen tests for the management of Helicobacter pylori infection. World J Gastroenterol. 2013;19:8188-8191.
33. Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2330-2338.
34. Gisbert J, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863.
35. Kamangar F, Dawsey SM, Blaser MJ, et al. Opposing risks of gastric cardiac and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445-1452.
36. Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prevent Res (Phila). 2008;1:329-338.
37. Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;(2):CD002096.
38. Mazzoleni LE, Sander GB, Francesconi CF, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med. 2011;171:1929-1936.
39. Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;(12):CD008337.
40. Zou J, Dong J, Yu X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:97-107.
41. Basu PP, Rayapudi K, Pacana T, et al. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011;106:1970-1975.
42. Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with 4 drugs are equally effective for eradication of H. pylori infection. Clin Gastroenterol Hepatol. 2010;8:36–41.
43. Osato R, Reddy R, Reddy SG, et al. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med. 2001;161:1217-1220.
44. Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple firstline therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343-357.
45. Hinostroza Morales D, Díaz Ferrer J. Addition of bismuth subsalicylate to triple eradication therapy for Helicobacter pylori infection: efficiency and adverse events. Rev Gastroenterol Peru. 2014;34:315-320.
46. Rabeneck L, Wristers K, Souchek J, et al. Impact of upper endoscopy on satisfaction in patients with previously uninvestigated dyspepsia. Gastrointest Endosc. 2003;57:295-299.
47. Hojo M, Miwa H, Yokoyama T, et al. Treatment of functional dyspepsia with antianxiety or antidepressive agents: systematic review. J Gastroenterol. 2005;40:1036-1042.
48. Soo S, Moayyedi P, Deeks J, et al. Psychological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2005;(2):CD002301.
49. Lima FA, Ferreira LE, Pace FH. Acupuncture effectiveness as a complementary therapy in functional dyspepsia patients. Arq Gastroenterol. 2013;50:202-207.
50. Ma TT, Yu SY, Li Y, et al. Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia. Aliment Pharmacol Ther. 2012;35:552-561.
51. Koretz RL, Rotblatt M. Complementary and alternative medicine in gastroenterology: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2004;2:957-967.
Improving our approach to preventive care
› Avoid scheduling annual visits exclusively for preventive care. A
› Institute simple practice changes to improve the preventive services you provide, such as implementing standing orders for influenza vaccines. A
› Adopt components of the chronic care model for preventive services wherever possible—using ancillary providers to remind patients to undergo colorectal cancer screening and recommending apps that support self-management, for example. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For well over a century, the periodic health exam has been associated with the delivery of preventive services1—a model widely accepted by physicians and patients alike. Approximately 8% of ambulatory care visits are for check-ups, and more than 20% of US residents schedule a health exam annually.2
Periodic health exams, however, do not result in optimal preventive care. Evidence suggests that important preventive services, such as dietary counseling, occur at only 8% of such visits; that just 10 seconds, on average, is devoted to smoking cessation; and that 80% of the preventive services patients receive are delivered outside of scheduled health exams.2 Because physicians and patients alike understandably prioritize acute problems, any discussion of health maintenance issues during a periodic check-up is likely to occur despite the visit’s agenda, not as part of it.3-7
Primary care physicians and practices are increasingly being held accountable for their performance on preventive measures. With that in mind, being familiar with evidence-based guidelines relating to the delivery of preventive services in ambulatory care settings, such as those developed by the US Preventive Services Task Force (USPSTF),8 is crucial. Identifying elements of the chronic care model that can be effectively applied to preventive care—and recognizing that the reactive acute care model is ineffective for both chronic illness and preventive services—is essential, as well.
Preventive care that's evidence-based
Good practice guidelines should have the following features, according to the Institute of Medicine:9
• Validity. Application would lead to the desired health and cost outcomes.
• Reliability/reproducibility. Others using the same data/interpretation would reach the same conclusion.
• Clinical applicability. Patient populations are appropriately defined.
• Clinical flexibility. Known or generally expected exceptions are identified.
• Clarity. Guideline uses unambiguous language with precise definitions.
• Multidisciplinary process. Developed with the participation of all key stakeholders.
• Scheduled review. Periodically reviewed and revised to incorporate new evidence/changing consensus.
• Documentation. Procedures used in development are well documented.
The USPSTF guidelines are consistent with these attributes.8
Some brief interventions fail
Guidelines are useful as practice standards for establishing preventive care goals, but their existence alone does not ensure the delivery of high-quality preventive services in an office setting. One key factor is time. It is estimated that a primary care physician with an average-sized patient panel would require an additional 7.4 hours per day to achieve 100% compliance with all of the USPSTF recommendations.10
Counseling, in particular, is an intervention that may be more effective in theory than in practice. Evidence suggests that even when primary care providers are trained in preventive counseling (and many are not), many brief interventions are not effective at creating sustained behavior change or health improvement.11
Others are effective
That’s not to say that there’s little that can be done. Use of standing orders for influenza vaccination is one example of an effective and easily implemented preventive measure that requires little or no additional physician time. Yet some doctors are resistant, fearing loss of control or lawsuits. In fact, the National Vaccine Injury Compensation Program specifically provides protection against vaccinerelated malpractice claims.12 A standing order for office staff to arrange a screening mammogram is another effective intervention; it has been shown to improve screening rates by as much as 30%.13
Lessons from chronic illness management
Chronic illness care and preventive care have much in common.14 Both acknowledge the need for proactive screening and counseling to bring about behavior change. In addition, both require ongoing care and follow-up, as well as depend on links to community resources. And finally, both are resource-intensive—too resource-intensive, some say, to be delivered in a cost-effective manner.
The Chronic Care Model has been proposed as a framework for improving preventive services (TABLE).15,16 Evidence suggests adopting some key components of chronic care can lead to significant gains in the delivery of preventive care, with the largest improvement seen when multiple components are used simultaneously.17-20
Self-management support. A growing body of evidence shows that self-management activities are associated with improved outcomes.21 Instituting a peer support group for pregnant women at a federally qualified health center, for example, led to a 7% absolute risk reduction in preterm births.22
Not all efforts to encourage self-management are effective, however, and evaluation is crucial to determine what works. In one large-scale trial, the use of patient reminder cards to facilitate a reduction in health risk behaviors such as tobacco use, risky drinking, unhealthy dietary patterns, and physical inactivity led to fewer health risk assessments being performed, fewer individual counseling encounters, and no change in these behaviors.23
Decision support. Clinical decision support systems, which generate patient-specific evidence-based assessments and/or recommendations that are actionable as part of the workflow at the point of care, have been found to improve care.24,25 An example of this is a prompt that reminds the physician to discuss chemoprevention with a patient at high risk for breast cancer.
Delivery system design. The patient-centered medical home (PCMH) is a well-known example of a redesign of health care delivery.26 Conversion to this model is associated with a small positive effect on preventive interventions.27 However, the persistence of a fee-for-service payment system—which does not include physician reimbursement for some of the added services incorporated into the PCMH—limits the implementation of the PCMH model.28
Many practices are improving health care delivery by using nonphysicians for various tasks related to preventive care. Care managers, for example, typically have smaller caseloads and focus on reducing unnecessary treatment for patients with high-risk conditions, such as congestive heart failure, while patient navigators generally have less clinical expertise but more knowledge of community services.
In one study, practice-initiated phone conversations with nonphysicians increased colorectal cancer screening by up to 40%.29 And in one pilot program, the use of ancillary providers led to an increase in colorectal cancer screening by as much as 123%.30 The human touch seems to be key to the success of these interventions. Passive reminders, such as videos being shown on waiting room televisions, have not proven to be effective.31
Clinical information systems. Early on, the power of electronic health records (EHRs) to improve practices’ delivery of preventive services was recognized. As early as 1995, the use of a reminder system to highlight such services during acute care visits was linked to improvements in counseling about smoking cessation and higher rates of cervical cancer screening, among other preventive measures.32,33 Overall, the use of EHRs alone has been shown to improve rates of preventive services by as much as 66%, with most practices reporting improvements of at least 20%.34
Today, EHRs that are Meaningful Use Stage 2-compliant have the tools needed to improve care. Requirements include the ability to generate patient registries of all those with a given disease and to identify patients on the registry who have not received needed care.35 To improve preventive care, registries should focus on the mitigation of risk factors, such as identifying—and contacting—patients with diabetes who are in need of, or overdue for, an annual eye exam.
Trials using the registry function, in combination with automated messaging to deliver targeted information to various patient groups (identified by the demographic information available from the EHR), are ongoing.
Community resources. Many clinicians have informal referral relationships with community organizations, such as the YMCA. Physician practices that establish links to community resources have the potential to have a large effect on unhealthy behaviors. (See “Putting theory into practice: 2 cases”.) However, a systematic review found that, while evidence to support such connections is mainly positive, research is limited and further evaluation is needed.36
The Practical Playbook (practicalplaybook.org)37 developed by the deBeaumont Foundation, Duke Department of Community and Family Medicine, and the Centers for Disease Control and Prevention, offers concrete examples of how physician practices are linking with community resources to improve the health of the population. For example, Duke University’s “Just for Us” program provides in-home chronic illness care to 350 high-risk elderly individuals. The LATCH program connects thousands of Latino immigrants to health care services and culturally and linguistically appropriate health education classes.37
CASE 1
Dominic B, a 53-year-old patient, has scheduled a visit for a cough that has persisted for 4 weeks. The patient is a nonsmoker, is married, and has no first-degree relatives with cancer. But when you review his chart before the visit, you note that he missed his 6-month check-up for hypertension and hyperlipidemia. In addition, your electronic health record (EHR ) flags the fact that he has not undergone colorectal screening and that his immunizations are not current. Because you have standing orders in place, your medical assistant gives him a flu shot and a pamphlet providing information on colorectal cancer screening before you enter the room.
During the visit, Mr. B mentions that his father, age 82, recently had a heart attack. This event—reinforced by the postcards and phone messages he received from your office after he missed his 6-month follow-up—prompted him to reluctantly admit it was “time for a check-up.” You take the patient’s blood pressure (BP) and review his lipid panel (blood work was ordered prior to his visit) with him. He is relieved to know that he will not need to be on a statin and agrees to be screened for colorectal cancer using a sensitive stool study.
Before he leaves, the patient requests medication for erectile dysfunction—a problem he never reported before. You ask him to keep a diary and return in 2 months, and promise to discuss his erectile dysfunction at that time.
CASE 2
A review of your practice’s patient registry reveals that Gladys P, age 55, is behind on breast and cervical cancer screening. She has had only a few sporadic office visits, the last of which was for bronchitis 18 months ago. At that time, the patient’s systolic BP was 162 mm Hg. You told her you would recheck it in 6 weeks, but she failed to return for follow-up.
Ms. P smokes, but has no other chronic diseases and takes no medications. There is no record of a mammogram or Pap smear, and you don’t know whether she sees a gynecologist routinely. Your office contacts her and discovers that you are the only doctor she sees. The patient tells the medical assistant who placed the call that her car broke down but she has not had money to repair or replace it, so she has had no way to get to your office.
Your staff arranges for her to get a ride from a community volunteer group, first to the nearby hospital for a mammogram and then to your office, where you perform a Pap smear and address her elevated BP and smoking. You are rewarded for the counseling and preventive care with a letter and a bonus check from Ms. P’s insurance carrier, congratulating you on your quality improvement efforts.
Growing emphasis on quality
Systemic changes in the US health care system are occurring rapidly, with an emphasis on quality and improved outcomes. Many physicians are now required to submit data to external agencies for payment, and much of the data is grounded in preventive standards. Medicare’s Physicians Quality Reporting System requires that all Medicare providers provide data on preventive and chronic illness care. Rates of vaccination, obesity screening, and tobacco use screening are examples of preventive services that will be reported publicly on the Centers for Medicare & Medicaid Services’ Physician Compare Web site.38
Physicians who work in accountable care organizations are required to meet quality standards on the delivery of certain preventive services, including breast cancer screening, colorectal cancer screening, influenza and pneumonia immunization, body mass index screening and follow-up, tobacco use screening and cessation intervention, screening for high blood pressure and followup, and screening for clinical depression and follow-up.39 As patients discover that the Affordable Care Act mandates that preventive services be covered with no cost sharing, they are likely to become more receptive to physician attempts to provide them.40,41
CORRESPONDENCE
Gerald Liu, MD, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604-3207; [email protected]
1. Han P. Historical changes in the objectives of the periodic health exam. Ann Intern Med. 1997;127:910-917.
2. Mehrota A, Zaslavsky A, Anyanian J. Preventive health examinations and preventive gynecologic examinations in the United States. Arch Intern Med. 2007;167:1876-1883.
3. Jean CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166-171.
4. McGinnis JM, Foege WH. The immediate versus the important. JAMA. 2004;291:1263-1264.
5. Crabtree BF, Miller WL, Tallia AF, et al. Delivery of clinical preventive services in family medicine offices. Ann Fam Med. 2005;3:430-435.
6. US Department of Health and Human Services. August 28, 2013. Healthy People 2020. Available at: www.healthypeople.gov. Accessed September 13, 2013.
7. Pollak KI, Krause KM, Yarnall KS, et al. Estimated time spent on preventive services by primary care physicians. BMC Health Serv Res. 2008;8:245.
8. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC: Office of Disease Prevention and Health Promotion; 1996.
9. Field MJ, Lohr KN, Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
10. Yarnell K, Pollak K, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635-641.
11. Butler CC, Simpson SA, Hood K, et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: a cluster randomized trial. BMJ. 2013;346:f1191.
12. Yonas M, Nowalk M, Zimmerman R, et al. Examining structural and clinical factors associated with implementation of standing orders for adult immunization. J Healthcare Qual. 2012;34:34-42.
13. Donahue K, Plescia M, Stafford K. Do standing orders help with chronic disease care and health maintenance in ambulatory practice? J Fam Pract. 2010;59:226-227.
14. Glasgow R, Orleans T, Wagner E. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2002;79.
15. Wagner EH. Chronic disease management: What will it take to improve care for chronic illness? Effective Clin Pract. 1998;1:2-4.
16. Barr V, Robinson S, Marin-Link B, et al. Chronic care model: an integration of concepts and strategies from population health promotion and the chronic care model. Hosp Q. 2003;7:73-83.
17. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775-1779.
18. Moore LG. Escaping the tyranny of the urgent by delivering planned care. Fam Pract Manage. 2006;13:37-40.
19. Tsai AC, Morton SC, Mangione CM, et al. A meta-analysis of interventions to improve care for chronic illnesses. Am J Managed Care. 2005;11:478-488.
20. Coleman K, Austin BT, Brach C, et al. Evidence on the chronic care model in the new millenium. Health Affairs. 2009;28:75-85.
21. Pearson ML, Mattke S, Shaw R, et al. Patient Self-Management Support Programs: An Evaluation. Final Contract Report. Publication No. 08-0011. Rockville, MD: Agency for Healthcare Research and Quality. November 2007.
22. Feder J. Restructuring care in a federally qualified health center to better meet patients’ needs. Health Affairs. 2011. Available at: http://content.healthaffairs.org/content/30/3/419.full.html. Accessed February 13, 2014.
23. Hung D, Rundall T, Tallia A, et al. Rethinking prevention in primary care: applying the chronic care model to address health risk behaviors. Milbank Q. 2007;85:69-91.
24. Lobach D, Sanders GD, Bright TJ, et al. Enabling Health Care Decisionmaking Through Clinical Decision Support and Knowledge Management. Evidence Report No. 203. Publication No. 12-E001-EF. Rockville, MD: Agency for Healthcare Research and Quality. April 2012.
25. Kawamoto K, Houlihan C, Balas E, et al. Improving clinical practice using clinical decision support systems: a systemic review of trials to identify features critical to success. BMJ. 2005;330:765.
26. American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American Osteopathic Association. Joint Principles of the Patient-Centered Medical Home. March 2007. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/dam/AAFP/documents/practice_management/pcmh/initiatives/PCMHJoint.pdf. Accessed May 7, 2015.
27. Agency for Healthcare Research and Quality. The Patient-Centered Medical Home. Closing the Quality Gap: Revisiting the State of the Science: Evidence Report/Technology Assessment Executive Summary No. 208. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/391/1178/EvidReport208_CQGPatientCenteredMedicalHome_FinalReport_20120703.pdf. Accessed May 7, 2015.
28. Peikes D, Zutshi A, Genevro J, et al. Early Evidence on the Patient-Centered Medical Home. Final Report. Publication No. 12-0020-EF. Rockville, MD: Agency for Healthcare Research and Quality. February 2012.
29. Liu G, Perkins A. Using a lay cancer screening navigator to increase colorectal cancer screening rates. J Am Board Fam Med. 2015;28:280-282.
30. Baker N, Parsons M, Donnelly S, et al. Improving colon cancer screening rates in primary care: a pilot study emphasising the role of the medical assistant. Qual Saf Health Care. 2009;18:355-359.
31. Holden D, Jonas D, Portersfield D. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668-676.
32. Dexheimer JW, Talbot TR, Sanders DL, et al. Prompting clinicians about preventive care measures: a systematic review of randomized controlled trials. J Am Med Inf Assoc. 2008;15:311-320.
33. Shea S, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inf Assoc. 1996;3:399-409.
34. Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144:742-752.
35. Future of Family Medicine Leadership Committee. The future of family medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2(Suppl 1):S3-S32.
36. Porterfield D, Hinnant L, Kane H, et al. Linkages Between Clinical Practices and Community Organizations for Prevention: Final Report. Rockville, MD: Agency for Healthcare Research and Quality. October 2010.
37. deBeaumont Foundation, Duke Community and Family Medicine, Centers for Disease Control and Prevention. A Practical Playbook. Available at: https://practicalplaybook.org. Accessed March 29, 2015.
38. Physician Quality Reporting System. Centers for Medicare and Medicaid Services. Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS. Accessed March 29, 2015.
39. RTI International. Accountable Care Organization 2014 Program Analysis Quality Performance Standards Narrative Measure Specifications. 2014. Centers for Medicare and Medicaid Services Web site. Available at: http://www.cms.gov/Medicare/Medicare-Feefor-Service-Payment/sharedsavingsprogram/Downloads/ACONarrativeMeasures-Specs.pdf. Accessed May 7, 2015.
40. Koh K, Sebelius K. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363:1296-1299.
41. Rosenbaum S. The patient protection and affordable care act: Implications for public health policy and practice. Public Health Rep. 2011;126:130-135.
› Avoid scheduling annual visits exclusively for preventive care. A
› Institute simple practice changes to improve the preventive services you provide, such as implementing standing orders for influenza vaccines. A
› Adopt components of the chronic care model for preventive services wherever possible—using ancillary providers to remind patients to undergo colorectal cancer screening and recommending apps that support self-management, for example. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For well over a century, the periodic health exam has been associated with the delivery of preventive services1—a model widely accepted by physicians and patients alike. Approximately 8% of ambulatory care visits are for check-ups, and more than 20% of US residents schedule a health exam annually.2
Periodic health exams, however, do not result in optimal preventive care. Evidence suggests that important preventive services, such as dietary counseling, occur at only 8% of such visits; that just 10 seconds, on average, is devoted to smoking cessation; and that 80% of the preventive services patients receive are delivered outside of scheduled health exams.2 Because physicians and patients alike understandably prioritize acute problems, any discussion of health maintenance issues during a periodic check-up is likely to occur despite the visit’s agenda, not as part of it.3-7
Primary care physicians and practices are increasingly being held accountable for their performance on preventive measures. With that in mind, being familiar with evidence-based guidelines relating to the delivery of preventive services in ambulatory care settings, such as those developed by the US Preventive Services Task Force (USPSTF),8 is crucial. Identifying elements of the chronic care model that can be effectively applied to preventive care—and recognizing that the reactive acute care model is ineffective for both chronic illness and preventive services—is essential, as well.
Preventive care that's evidence-based
Good practice guidelines should have the following features, according to the Institute of Medicine:9
• Validity. Application would lead to the desired health and cost outcomes.
• Reliability/reproducibility. Others using the same data/interpretation would reach the same conclusion.
• Clinical applicability. Patient populations are appropriately defined.
• Clinical flexibility. Known or generally expected exceptions are identified.
• Clarity. Guideline uses unambiguous language with precise definitions.
• Multidisciplinary process. Developed with the participation of all key stakeholders.
• Scheduled review. Periodically reviewed and revised to incorporate new evidence/changing consensus.
• Documentation. Procedures used in development are well documented.
The USPSTF guidelines are consistent with these attributes.8
Some brief interventions fail
Guidelines are useful as practice standards for establishing preventive care goals, but their existence alone does not ensure the delivery of high-quality preventive services in an office setting. One key factor is time. It is estimated that a primary care physician with an average-sized patient panel would require an additional 7.4 hours per day to achieve 100% compliance with all of the USPSTF recommendations.10
Counseling, in particular, is an intervention that may be more effective in theory than in practice. Evidence suggests that even when primary care providers are trained in preventive counseling (and many are not), many brief interventions are not effective at creating sustained behavior change or health improvement.11
Others are effective
That’s not to say that there’s little that can be done. Use of standing orders for influenza vaccination is one example of an effective and easily implemented preventive measure that requires little or no additional physician time. Yet some doctors are resistant, fearing loss of control or lawsuits. In fact, the National Vaccine Injury Compensation Program specifically provides protection against vaccinerelated malpractice claims.12 A standing order for office staff to arrange a screening mammogram is another effective intervention; it has been shown to improve screening rates by as much as 30%.13
Lessons from chronic illness management
Chronic illness care and preventive care have much in common.14 Both acknowledge the need for proactive screening and counseling to bring about behavior change. In addition, both require ongoing care and follow-up, as well as depend on links to community resources. And finally, both are resource-intensive—too resource-intensive, some say, to be delivered in a cost-effective manner.
The Chronic Care Model has been proposed as a framework for improving preventive services (TABLE).15,16 Evidence suggests adopting some key components of chronic care can lead to significant gains in the delivery of preventive care, with the largest improvement seen when multiple components are used simultaneously.17-20
Self-management support. A growing body of evidence shows that self-management activities are associated with improved outcomes.21 Instituting a peer support group for pregnant women at a federally qualified health center, for example, led to a 7% absolute risk reduction in preterm births.22
Not all efforts to encourage self-management are effective, however, and evaluation is crucial to determine what works. In one large-scale trial, the use of patient reminder cards to facilitate a reduction in health risk behaviors such as tobacco use, risky drinking, unhealthy dietary patterns, and physical inactivity led to fewer health risk assessments being performed, fewer individual counseling encounters, and no change in these behaviors.23
Decision support. Clinical decision support systems, which generate patient-specific evidence-based assessments and/or recommendations that are actionable as part of the workflow at the point of care, have been found to improve care.24,25 An example of this is a prompt that reminds the physician to discuss chemoprevention with a patient at high risk for breast cancer.
Delivery system design. The patient-centered medical home (PCMH) is a well-known example of a redesign of health care delivery.26 Conversion to this model is associated with a small positive effect on preventive interventions.27 However, the persistence of a fee-for-service payment system—which does not include physician reimbursement for some of the added services incorporated into the PCMH—limits the implementation of the PCMH model.28
Many practices are improving health care delivery by using nonphysicians for various tasks related to preventive care. Care managers, for example, typically have smaller caseloads and focus on reducing unnecessary treatment for patients with high-risk conditions, such as congestive heart failure, while patient navigators generally have less clinical expertise but more knowledge of community services.
In one study, practice-initiated phone conversations with nonphysicians increased colorectal cancer screening by up to 40%.29 And in one pilot program, the use of ancillary providers led to an increase in colorectal cancer screening by as much as 123%.30 The human touch seems to be key to the success of these interventions. Passive reminders, such as videos being shown on waiting room televisions, have not proven to be effective.31
Clinical information systems. Early on, the power of electronic health records (EHRs) to improve practices’ delivery of preventive services was recognized. As early as 1995, the use of a reminder system to highlight such services during acute care visits was linked to improvements in counseling about smoking cessation and higher rates of cervical cancer screening, among other preventive measures.32,33 Overall, the use of EHRs alone has been shown to improve rates of preventive services by as much as 66%, with most practices reporting improvements of at least 20%.34
Today, EHRs that are Meaningful Use Stage 2-compliant have the tools needed to improve care. Requirements include the ability to generate patient registries of all those with a given disease and to identify patients on the registry who have not received needed care.35 To improve preventive care, registries should focus on the mitigation of risk factors, such as identifying—and contacting—patients with diabetes who are in need of, or overdue for, an annual eye exam.
Trials using the registry function, in combination with automated messaging to deliver targeted information to various patient groups (identified by the demographic information available from the EHR), are ongoing.
Community resources. Many clinicians have informal referral relationships with community organizations, such as the YMCA. Physician practices that establish links to community resources have the potential to have a large effect on unhealthy behaviors. (See “Putting theory into practice: 2 cases”.) However, a systematic review found that, while evidence to support such connections is mainly positive, research is limited and further evaluation is needed.36
The Practical Playbook (practicalplaybook.org)37 developed by the deBeaumont Foundation, Duke Department of Community and Family Medicine, and the Centers for Disease Control and Prevention, offers concrete examples of how physician practices are linking with community resources to improve the health of the population. For example, Duke University’s “Just for Us” program provides in-home chronic illness care to 350 high-risk elderly individuals. The LATCH program connects thousands of Latino immigrants to health care services and culturally and linguistically appropriate health education classes.37
CASE 1
Dominic B, a 53-year-old patient, has scheduled a visit for a cough that has persisted for 4 weeks. The patient is a nonsmoker, is married, and has no first-degree relatives with cancer. But when you review his chart before the visit, you note that he missed his 6-month check-up for hypertension and hyperlipidemia. In addition, your electronic health record (EHR ) flags the fact that he has not undergone colorectal screening and that his immunizations are not current. Because you have standing orders in place, your medical assistant gives him a flu shot and a pamphlet providing information on colorectal cancer screening before you enter the room.
During the visit, Mr. B mentions that his father, age 82, recently had a heart attack. This event—reinforced by the postcards and phone messages he received from your office after he missed his 6-month follow-up—prompted him to reluctantly admit it was “time for a check-up.” You take the patient’s blood pressure (BP) and review his lipid panel (blood work was ordered prior to his visit) with him. He is relieved to know that he will not need to be on a statin and agrees to be screened for colorectal cancer using a sensitive stool study.
Before he leaves, the patient requests medication for erectile dysfunction—a problem he never reported before. You ask him to keep a diary and return in 2 months, and promise to discuss his erectile dysfunction at that time.
CASE 2
A review of your practice’s patient registry reveals that Gladys P, age 55, is behind on breast and cervical cancer screening. She has had only a few sporadic office visits, the last of which was for bronchitis 18 months ago. At that time, the patient’s systolic BP was 162 mm Hg. You told her you would recheck it in 6 weeks, but she failed to return for follow-up.
Ms. P smokes, but has no other chronic diseases and takes no medications. There is no record of a mammogram or Pap smear, and you don’t know whether she sees a gynecologist routinely. Your office contacts her and discovers that you are the only doctor she sees. The patient tells the medical assistant who placed the call that her car broke down but she has not had money to repair or replace it, so she has had no way to get to your office.
Your staff arranges for her to get a ride from a community volunteer group, first to the nearby hospital for a mammogram and then to your office, where you perform a Pap smear and address her elevated BP and smoking. You are rewarded for the counseling and preventive care with a letter and a bonus check from Ms. P’s insurance carrier, congratulating you on your quality improvement efforts.
Growing emphasis on quality
Systemic changes in the US health care system are occurring rapidly, with an emphasis on quality and improved outcomes. Many physicians are now required to submit data to external agencies for payment, and much of the data is grounded in preventive standards. Medicare’s Physicians Quality Reporting System requires that all Medicare providers provide data on preventive and chronic illness care. Rates of vaccination, obesity screening, and tobacco use screening are examples of preventive services that will be reported publicly on the Centers for Medicare & Medicaid Services’ Physician Compare Web site.38
Physicians who work in accountable care organizations are required to meet quality standards on the delivery of certain preventive services, including breast cancer screening, colorectal cancer screening, influenza and pneumonia immunization, body mass index screening and follow-up, tobacco use screening and cessation intervention, screening for high blood pressure and followup, and screening for clinical depression and follow-up.39 As patients discover that the Affordable Care Act mandates that preventive services be covered with no cost sharing, they are likely to become more receptive to physician attempts to provide them.40,41
CORRESPONDENCE
Gerald Liu, MD, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604-3207; [email protected]
› Avoid scheduling annual visits exclusively for preventive care. A
› Institute simple practice changes to improve the preventive services you provide, such as implementing standing orders for influenza vaccines. A
› Adopt components of the chronic care model for preventive services wherever possible—using ancillary providers to remind patients to undergo colorectal cancer screening and recommending apps that support self-management, for example. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For well over a century, the periodic health exam has been associated with the delivery of preventive services1—a model widely accepted by physicians and patients alike. Approximately 8% of ambulatory care visits are for check-ups, and more than 20% of US residents schedule a health exam annually.2
Periodic health exams, however, do not result in optimal preventive care. Evidence suggests that important preventive services, such as dietary counseling, occur at only 8% of such visits; that just 10 seconds, on average, is devoted to smoking cessation; and that 80% of the preventive services patients receive are delivered outside of scheduled health exams.2 Because physicians and patients alike understandably prioritize acute problems, any discussion of health maintenance issues during a periodic check-up is likely to occur despite the visit’s agenda, not as part of it.3-7
Primary care physicians and practices are increasingly being held accountable for their performance on preventive measures. With that in mind, being familiar with evidence-based guidelines relating to the delivery of preventive services in ambulatory care settings, such as those developed by the US Preventive Services Task Force (USPSTF),8 is crucial. Identifying elements of the chronic care model that can be effectively applied to preventive care—and recognizing that the reactive acute care model is ineffective for both chronic illness and preventive services—is essential, as well.
Preventive care that's evidence-based
Good practice guidelines should have the following features, according to the Institute of Medicine:9
• Validity. Application would lead to the desired health and cost outcomes.
• Reliability/reproducibility. Others using the same data/interpretation would reach the same conclusion.
• Clinical applicability. Patient populations are appropriately defined.
• Clinical flexibility. Known or generally expected exceptions are identified.
• Clarity. Guideline uses unambiguous language with precise definitions.
• Multidisciplinary process. Developed with the participation of all key stakeholders.
• Scheduled review. Periodically reviewed and revised to incorporate new evidence/changing consensus.
• Documentation. Procedures used in development are well documented.
The USPSTF guidelines are consistent with these attributes.8
Some brief interventions fail
Guidelines are useful as practice standards for establishing preventive care goals, but their existence alone does not ensure the delivery of high-quality preventive services in an office setting. One key factor is time. It is estimated that a primary care physician with an average-sized patient panel would require an additional 7.4 hours per day to achieve 100% compliance with all of the USPSTF recommendations.10
Counseling, in particular, is an intervention that may be more effective in theory than in practice. Evidence suggests that even when primary care providers are trained in preventive counseling (and many are not), many brief interventions are not effective at creating sustained behavior change or health improvement.11
Others are effective
That’s not to say that there’s little that can be done. Use of standing orders for influenza vaccination is one example of an effective and easily implemented preventive measure that requires little or no additional physician time. Yet some doctors are resistant, fearing loss of control or lawsuits. In fact, the National Vaccine Injury Compensation Program specifically provides protection against vaccinerelated malpractice claims.12 A standing order for office staff to arrange a screening mammogram is another effective intervention; it has been shown to improve screening rates by as much as 30%.13
Lessons from chronic illness management
Chronic illness care and preventive care have much in common.14 Both acknowledge the need for proactive screening and counseling to bring about behavior change. In addition, both require ongoing care and follow-up, as well as depend on links to community resources. And finally, both are resource-intensive—too resource-intensive, some say, to be delivered in a cost-effective manner.
The Chronic Care Model has been proposed as a framework for improving preventive services (TABLE).15,16 Evidence suggests adopting some key components of chronic care can lead to significant gains in the delivery of preventive care, with the largest improvement seen when multiple components are used simultaneously.17-20
Self-management support. A growing body of evidence shows that self-management activities are associated with improved outcomes.21 Instituting a peer support group for pregnant women at a federally qualified health center, for example, led to a 7% absolute risk reduction in preterm births.22
Not all efforts to encourage self-management are effective, however, and evaluation is crucial to determine what works. In one large-scale trial, the use of patient reminder cards to facilitate a reduction in health risk behaviors such as tobacco use, risky drinking, unhealthy dietary patterns, and physical inactivity led to fewer health risk assessments being performed, fewer individual counseling encounters, and no change in these behaviors.23
Decision support. Clinical decision support systems, which generate patient-specific evidence-based assessments and/or recommendations that are actionable as part of the workflow at the point of care, have been found to improve care.24,25 An example of this is a prompt that reminds the physician to discuss chemoprevention with a patient at high risk for breast cancer.
Delivery system design. The patient-centered medical home (PCMH) is a well-known example of a redesign of health care delivery.26 Conversion to this model is associated with a small positive effect on preventive interventions.27 However, the persistence of a fee-for-service payment system—which does not include physician reimbursement for some of the added services incorporated into the PCMH—limits the implementation of the PCMH model.28
Many practices are improving health care delivery by using nonphysicians for various tasks related to preventive care. Care managers, for example, typically have smaller caseloads and focus on reducing unnecessary treatment for patients with high-risk conditions, such as congestive heart failure, while patient navigators generally have less clinical expertise but more knowledge of community services.
In one study, practice-initiated phone conversations with nonphysicians increased colorectal cancer screening by up to 40%.29 And in one pilot program, the use of ancillary providers led to an increase in colorectal cancer screening by as much as 123%.30 The human touch seems to be key to the success of these interventions. Passive reminders, such as videos being shown on waiting room televisions, have not proven to be effective.31
Clinical information systems. Early on, the power of electronic health records (EHRs) to improve practices’ delivery of preventive services was recognized. As early as 1995, the use of a reminder system to highlight such services during acute care visits was linked to improvements in counseling about smoking cessation and higher rates of cervical cancer screening, among other preventive measures.32,33 Overall, the use of EHRs alone has been shown to improve rates of preventive services by as much as 66%, with most practices reporting improvements of at least 20%.34
Today, EHRs that are Meaningful Use Stage 2-compliant have the tools needed to improve care. Requirements include the ability to generate patient registries of all those with a given disease and to identify patients on the registry who have not received needed care.35 To improve preventive care, registries should focus on the mitigation of risk factors, such as identifying—and contacting—patients with diabetes who are in need of, or overdue for, an annual eye exam.
Trials using the registry function, in combination with automated messaging to deliver targeted information to various patient groups (identified by the demographic information available from the EHR), are ongoing.
Community resources. Many clinicians have informal referral relationships with community organizations, such as the YMCA. Physician practices that establish links to community resources have the potential to have a large effect on unhealthy behaviors. (See “Putting theory into practice: 2 cases”.) However, a systematic review found that, while evidence to support such connections is mainly positive, research is limited and further evaluation is needed.36
The Practical Playbook (practicalplaybook.org)37 developed by the deBeaumont Foundation, Duke Department of Community and Family Medicine, and the Centers for Disease Control and Prevention, offers concrete examples of how physician practices are linking with community resources to improve the health of the population. For example, Duke University’s “Just for Us” program provides in-home chronic illness care to 350 high-risk elderly individuals. The LATCH program connects thousands of Latino immigrants to health care services and culturally and linguistically appropriate health education classes.37
CASE 1
Dominic B, a 53-year-old patient, has scheduled a visit for a cough that has persisted for 4 weeks. The patient is a nonsmoker, is married, and has no first-degree relatives with cancer. But when you review his chart before the visit, you note that he missed his 6-month check-up for hypertension and hyperlipidemia. In addition, your electronic health record (EHR ) flags the fact that he has not undergone colorectal screening and that his immunizations are not current. Because you have standing orders in place, your medical assistant gives him a flu shot and a pamphlet providing information on colorectal cancer screening before you enter the room.
During the visit, Mr. B mentions that his father, age 82, recently had a heart attack. This event—reinforced by the postcards and phone messages he received from your office after he missed his 6-month follow-up—prompted him to reluctantly admit it was “time for a check-up.” You take the patient’s blood pressure (BP) and review his lipid panel (blood work was ordered prior to his visit) with him. He is relieved to know that he will not need to be on a statin and agrees to be screened for colorectal cancer using a sensitive stool study.
Before he leaves, the patient requests medication for erectile dysfunction—a problem he never reported before. You ask him to keep a diary and return in 2 months, and promise to discuss his erectile dysfunction at that time.
CASE 2
A review of your practice’s patient registry reveals that Gladys P, age 55, is behind on breast and cervical cancer screening. She has had only a few sporadic office visits, the last of which was for bronchitis 18 months ago. At that time, the patient’s systolic BP was 162 mm Hg. You told her you would recheck it in 6 weeks, but she failed to return for follow-up.
Ms. P smokes, but has no other chronic diseases and takes no medications. There is no record of a mammogram or Pap smear, and you don’t know whether she sees a gynecologist routinely. Your office contacts her and discovers that you are the only doctor she sees. The patient tells the medical assistant who placed the call that her car broke down but she has not had money to repair or replace it, so she has had no way to get to your office.
Your staff arranges for her to get a ride from a community volunteer group, first to the nearby hospital for a mammogram and then to your office, where you perform a Pap smear and address her elevated BP and smoking. You are rewarded for the counseling and preventive care with a letter and a bonus check from Ms. P’s insurance carrier, congratulating you on your quality improvement efforts.
Growing emphasis on quality
Systemic changes in the US health care system are occurring rapidly, with an emphasis on quality and improved outcomes. Many physicians are now required to submit data to external agencies for payment, and much of the data is grounded in preventive standards. Medicare’s Physicians Quality Reporting System requires that all Medicare providers provide data on preventive and chronic illness care. Rates of vaccination, obesity screening, and tobacco use screening are examples of preventive services that will be reported publicly on the Centers for Medicare & Medicaid Services’ Physician Compare Web site.38
Physicians who work in accountable care organizations are required to meet quality standards on the delivery of certain preventive services, including breast cancer screening, colorectal cancer screening, influenza and pneumonia immunization, body mass index screening and follow-up, tobacco use screening and cessation intervention, screening for high blood pressure and followup, and screening for clinical depression and follow-up.39 As patients discover that the Affordable Care Act mandates that preventive services be covered with no cost sharing, they are likely to become more receptive to physician attempts to provide them.40,41
CORRESPONDENCE
Gerald Liu, MD, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604-3207; [email protected]
1. Han P. Historical changes in the objectives of the periodic health exam. Ann Intern Med. 1997;127:910-917.
2. Mehrota A, Zaslavsky A, Anyanian J. Preventive health examinations and preventive gynecologic examinations in the United States. Arch Intern Med. 2007;167:1876-1883.
3. Jean CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166-171.
4. McGinnis JM, Foege WH. The immediate versus the important. JAMA. 2004;291:1263-1264.
5. Crabtree BF, Miller WL, Tallia AF, et al. Delivery of clinical preventive services in family medicine offices. Ann Fam Med. 2005;3:430-435.
6. US Department of Health and Human Services. August 28, 2013. Healthy People 2020. Available at: www.healthypeople.gov. Accessed September 13, 2013.
7. Pollak KI, Krause KM, Yarnall KS, et al. Estimated time spent on preventive services by primary care physicians. BMC Health Serv Res. 2008;8:245.
8. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC: Office of Disease Prevention and Health Promotion; 1996.
9. Field MJ, Lohr KN, Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
10. Yarnell K, Pollak K, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635-641.
11. Butler CC, Simpson SA, Hood K, et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: a cluster randomized trial. BMJ. 2013;346:f1191.
12. Yonas M, Nowalk M, Zimmerman R, et al. Examining structural and clinical factors associated with implementation of standing orders for adult immunization. J Healthcare Qual. 2012;34:34-42.
13. Donahue K, Plescia M, Stafford K. Do standing orders help with chronic disease care and health maintenance in ambulatory practice? J Fam Pract. 2010;59:226-227.
14. Glasgow R, Orleans T, Wagner E. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2002;79.
15. Wagner EH. Chronic disease management: What will it take to improve care for chronic illness? Effective Clin Pract. 1998;1:2-4.
16. Barr V, Robinson S, Marin-Link B, et al. Chronic care model: an integration of concepts and strategies from population health promotion and the chronic care model. Hosp Q. 2003;7:73-83.
17. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775-1779.
18. Moore LG. Escaping the tyranny of the urgent by delivering planned care. Fam Pract Manage. 2006;13:37-40.
19. Tsai AC, Morton SC, Mangione CM, et al. A meta-analysis of interventions to improve care for chronic illnesses. Am J Managed Care. 2005;11:478-488.
20. Coleman K, Austin BT, Brach C, et al. Evidence on the chronic care model in the new millenium. Health Affairs. 2009;28:75-85.
21. Pearson ML, Mattke S, Shaw R, et al. Patient Self-Management Support Programs: An Evaluation. Final Contract Report. Publication No. 08-0011. Rockville, MD: Agency for Healthcare Research and Quality. November 2007.
22. Feder J. Restructuring care in a federally qualified health center to better meet patients’ needs. Health Affairs. 2011. Available at: http://content.healthaffairs.org/content/30/3/419.full.html. Accessed February 13, 2014.
23. Hung D, Rundall T, Tallia A, et al. Rethinking prevention in primary care: applying the chronic care model to address health risk behaviors. Milbank Q. 2007;85:69-91.
24. Lobach D, Sanders GD, Bright TJ, et al. Enabling Health Care Decisionmaking Through Clinical Decision Support and Knowledge Management. Evidence Report No. 203. Publication No. 12-E001-EF. Rockville, MD: Agency for Healthcare Research and Quality. April 2012.
25. Kawamoto K, Houlihan C, Balas E, et al. Improving clinical practice using clinical decision support systems: a systemic review of trials to identify features critical to success. BMJ. 2005;330:765.
26. American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American Osteopathic Association. Joint Principles of the Patient-Centered Medical Home. March 2007. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/dam/AAFP/documents/practice_management/pcmh/initiatives/PCMHJoint.pdf. Accessed May 7, 2015.
27. Agency for Healthcare Research and Quality. The Patient-Centered Medical Home. Closing the Quality Gap: Revisiting the State of the Science: Evidence Report/Technology Assessment Executive Summary No. 208. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/391/1178/EvidReport208_CQGPatientCenteredMedicalHome_FinalReport_20120703.pdf. Accessed May 7, 2015.
28. Peikes D, Zutshi A, Genevro J, et al. Early Evidence on the Patient-Centered Medical Home. Final Report. Publication No. 12-0020-EF. Rockville, MD: Agency for Healthcare Research and Quality. February 2012.
29. Liu G, Perkins A. Using a lay cancer screening navigator to increase colorectal cancer screening rates. J Am Board Fam Med. 2015;28:280-282.
30. Baker N, Parsons M, Donnelly S, et al. Improving colon cancer screening rates in primary care: a pilot study emphasising the role of the medical assistant. Qual Saf Health Care. 2009;18:355-359.
31. Holden D, Jonas D, Portersfield D. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668-676.
32. Dexheimer JW, Talbot TR, Sanders DL, et al. Prompting clinicians about preventive care measures: a systematic review of randomized controlled trials. J Am Med Inf Assoc. 2008;15:311-320.
33. Shea S, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inf Assoc. 1996;3:399-409.
34. Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144:742-752.
35. Future of Family Medicine Leadership Committee. The future of family medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2(Suppl 1):S3-S32.
36. Porterfield D, Hinnant L, Kane H, et al. Linkages Between Clinical Practices and Community Organizations for Prevention: Final Report. Rockville, MD: Agency for Healthcare Research and Quality. October 2010.
37. deBeaumont Foundation, Duke Community and Family Medicine, Centers for Disease Control and Prevention. A Practical Playbook. Available at: https://practicalplaybook.org. Accessed March 29, 2015.
38. Physician Quality Reporting System. Centers for Medicare and Medicaid Services. Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS. Accessed March 29, 2015.
39. RTI International. Accountable Care Organization 2014 Program Analysis Quality Performance Standards Narrative Measure Specifications. 2014. Centers for Medicare and Medicaid Services Web site. Available at: http://www.cms.gov/Medicare/Medicare-Feefor-Service-Payment/sharedsavingsprogram/Downloads/ACONarrativeMeasures-Specs.pdf. Accessed May 7, 2015.
40. Koh K, Sebelius K. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363:1296-1299.
41. Rosenbaum S. The patient protection and affordable care act: Implications for public health policy and practice. Public Health Rep. 2011;126:130-135.
1. Han P. Historical changes in the objectives of the periodic health exam. Ann Intern Med. 1997;127:910-917.
2. Mehrota A, Zaslavsky A, Anyanian J. Preventive health examinations and preventive gynecologic examinations in the United States. Arch Intern Med. 2007;167:1876-1883.
3. Jean CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166-171.
4. McGinnis JM, Foege WH. The immediate versus the important. JAMA. 2004;291:1263-1264.
5. Crabtree BF, Miller WL, Tallia AF, et al. Delivery of clinical preventive services in family medicine offices. Ann Fam Med. 2005;3:430-435.
6. US Department of Health and Human Services. August 28, 2013. Healthy People 2020. Available at: www.healthypeople.gov. Accessed September 13, 2013.
7. Pollak KI, Krause KM, Yarnall KS, et al. Estimated time spent on preventive services by primary care physicians. BMC Health Serv Res. 2008;8:245.
8. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC: Office of Disease Prevention and Health Promotion; 1996.
9. Field MJ, Lohr KN, Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
10. Yarnell K, Pollak K, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635-641.
11. Butler CC, Simpson SA, Hood K, et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: a cluster randomized trial. BMJ. 2013;346:f1191.
12. Yonas M, Nowalk M, Zimmerman R, et al. Examining structural and clinical factors associated with implementation of standing orders for adult immunization. J Healthcare Qual. 2012;34:34-42.
13. Donahue K, Plescia M, Stafford K. Do standing orders help with chronic disease care and health maintenance in ambulatory practice? J Fam Pract. 2010;59:226-227.
14. Glasgow R, Orleans T, Wagner E. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2002;79.
15. Wagner EH. Chronic disease management: What will it take to improve care for chronic illness? Effective Clin Pract. 1998;1:2-4.
16. Barr V, Robinson S, Marin-Link B, et al. Chronic care model: an integration of concepts and strategies from population health promotion and the chronic care model. Hosp Q. 2003;7:73-83.
17. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775-1779.
18. Moore LG. Escaping the tyranny of the urgent by delivering planned care. Fam Pract Manage. 2006;13:37-40.
19. Tsai AC, Morton SC, Mangione CM, et al. A meta-analysis of interventions to improve care for chronic illnesses. Am J Managed Care. 2005;11:478-488.
20. Coleman K, Austin BT, Brach C, et al. Evidence on the chronic care model in the new millenium. Health Affairs. 2009;28:75-85.
21. Pearson ML, Mattke S, Shaw R, et al. Patient Self-Management Support Programs: An Evaluation. Final Contract Report. Publication No. 08-0011. Rockville, MD: Agency for Healthcare Research and Quality. November 2007.
22. Feder J. Restructuring care in a federally qualified health center to better meet patients’ needs. Health Affairs. 2011. Available at: http://content.healthaffairs.org/content/30/3/419.full.html. Accessed February 13, 2014.
23. Hung D, Rundall T, Tallia A, et al. Rethinking prevention in primary care: applying the chronic care model to address health risk behaviors. Milbank Q. 2007;85:69-91.
24. Lobach D, Sanders GD, Bright TJ, et al. Enabling Health Care Decisionmaking Through Clinical Decision Support and Knowledge Management. Evidence Report No. 203. Publication No. 12-E001-EF. Rockville, MD: Agency for Healthcare Research and Quality. April 2012.
25. Kawamoto K, Houlihan C, Balas E, et al. Improving clinical practice using clinical decision support systems: a systemic review of trials to identify features critical to success. BMJ. 2005;330:765.
26. American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American Osteopathic Association. Joint Principles of the Patient-Centered Medical Home. March 2007. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/dam/AAFP/documents/practice_management/pcmh/initiatives/PCMHJoint.pdf. Accessed May 7, 2015.
27. Agency for Healthcare Research and Quality. The Patient-Centered Medical Home. Closing the Quality Gap: Revisiting the State of the Science: Evidence Report/Technology Assessment Executive Summary No. 208. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/391/1178/EvidReport208_CQGPatientCenteredMedicalHome_FinalReport_20120703.pdf. Accessed May 7, 2015.
28. Peikes D, Zutshi A, Genevro J, et al. Early Evidence on the Patient-Centered Medical Home. Final Report. Publication No. 12-0020-EF. Rockville, MD: Agency for Healthcare Research and Quality. February 2012.
29. Liu G, Perkins A. Using a lay cancer screening navigator to increase colorectal cancer screening rates. J Am Board Fam Med. 2015;28:280-282.
30. Baker N, Parsons M, Donnelly S, et al. Improving colon cancer screening rates in primary care: a pilot study emphasising the role of the medical assistant. Qual Saf Health Care. 2009;18:355-359.
31. Holden D, Jonas D, Portersfield D. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668-676.
32. Dexheimer JW, Talbot TR, Sanders DL, et al. Prompting clinicians about preventive care measures: a systematic review of randomized controlled trials. J Am Med Inf Assoc. 2008;15:311-320.
33. Shea S, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inf Assoc. 1996;3:399-409.
34. Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144:742-752.
35. Future of Family Medicine Leadership Committee. The future of family medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2(Suppl 1):S3-S32.
36. Porterfield D, Hinnant L, Kane H, et al. Linkages Between Clinical Practices and Community Organizations for Prevention: Final Report. Rockville, MD: Agency for Healthcare Research and Quality. October 2010.
37. deBeaumont Foundation, Duke Community and Family Medicine, Centers for Disease Control and Prevention. A Practical Playbook. Available at: https://practicalplaybook.org. Accessed March 29, 2015.
38. Physician Quality Reporting System. Centers for Medicare and Medicaid Services. Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS. Accessed March 29, 2015.
39. RTI International. Accountable Care Organization 2014 Program Analysis Quality Performance Standards Narrative Measure Specifications. 2014. Centers for Medicare and Medicaid Services Web site. Available at: http://www.cms.gov/Medicare/Medicare-Feefor-Service-Payment/sharedsavingsprogram/Downloads/ACONarrativeMeasures-Specs.pdf. Accessed May 7, 2015.
40. Koh K, Sebelius K. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363:1296-1299.
41. Rosenbaum S. The patient protection and affordable care act: Implications for public health policy and practice. Public Health Rep. 2011;126:130-135.
Cirrhosis complications: Keeping them under control
› Prescribe low-dose diuretics and recommend sodium restriction for patients with cirrhosis who have grade 2 (moderate) ascites. C
› Initiate treatment with beta-blockers to prevent variceal bleeding in all patients with medium or large varices, as well as in those with small varices who also have red wale signs and/or Child-Pugh Class B or C cirrhosis. A
› Consider evaluation for liver transplantation for a patient with cirrhosis who has experienced a major complication (eg, ascites, hepatic encephalopathy, or variceal hemorrhage) or one who has a model for end-stage liver disease (MELD) score ≥15. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joe M, age 59, seeks care at the local emergency department (ED) for shortness of breath. He also complains that his abdomen has been getting “bigger and bigger.” The ED physician recognizes that he is suffering from cirrhosis with secondary ascites and admits him. A paracentesis is performed and 7 L of fluid are removed. The patient is started on furosemide 40 mg/d and the health care team educates him about the relationship between his alcohol consumption and his enlarging abdomen. At discharge, he is told to follow up with his primary care physician.
Two weeks later, the patient arrives at your clinic for followup. What is the next step in managing this patient?
Cirrhosis—the end stage of chronic liver disease characterized by inflammation and fibrosis—is a relatively common and often fatal diagnosis. In the United States, an estimated 633,000 adults have cirrhosis,1 and each year approximately 32,000 people die from the condition.2 The most common causes of cirrhosis are heavy alcohol use, chronic hepatitis B or C infection, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis.3 Cirrhosis typically involves degeneration and necrosis of hepatocytes, which are replaced by fibrotic tissues and regenerative nodules, leading to loss of liver function.4
Patients with cirrhosis can be treated as outpatients—that is, until they decompensate. Obviously, treatment specific to the underlying causes of cirrhosis, such as interferon for a patient with hepatitis and abstinence for a patient with alcohol-related liver disease, should be the first concern. However, this article focuses on the family physician’s role in identifying and treating several of the most common complications of cirrhosis, including ascites, variceal bleeding, hepatic encephalopathy, and hepatorenal syndrome. We will also cover which patients should be referred for evaluation for liver transplantation. (For a guide to providing patient education for individuals with cirrhosis, see “Dx cirrhosis: What to teach your patient”.3,5-10)
Sodium restriction, diuretics are first steps for ascites
The goals of ascites treatment are to prevent or relieve dyspnea and abdominal pain and to prevent life-threatening complications, such as spontaneous bacterial peritonitis (SBP) and hepatorenal syndrome.11 Patient education is key regarding weight gain; that’s why it’s important to instruct patients to contact you if they gain more than 2 lbs/d for 3 consecutive days or more than 10 lbs.12
Approximately 10% of patients with ascites respond well to sodium restriction alone (1500-2000 mg/d).11 In addition to sodium restriction, patients with grade 2 ascites (moderate ascites with proportionate abdominal distension) should receive a low-dose diuretic, such as spironolactone (initial dose, 50-100 mg/d; increase up to 200-300 mg/d)13 or amiloride (5-10 mg/d).5
Painful gynecomastia and hyperkalemia are the most common adverse effects of spironolactone.13 Amiloride has fewer adverse effects than spironolactone, but is less effective.11 Low-dose furosemide (20-40 mg/d) may be added, although weight loss should be monitored to watch for excessive diuresis, which can lead to renal failure, hyponatremia, or encephalopathy.5,13 Also monitor electrolytes to watch for hypokalemia or hyponatremia.13
Recommended weight loss to prevent renal failure is 300 to 500 g/d (.66-1.1 lbs/d) for patients without peripheral edema, and 800 to 1000 g/d (1.7-2.2 lbs/d) for patients with peripheral edema.5,13
Patients with grade 3 (tense) or refractory ascites should have large-volume paracentesis (LVP) plus an albumin infusion.5 LVP (removal of >5 L of fluid) is more effective, faster, and has less risk of adverse effects than increasing the dosage of the patient’s diuretic.5,13 LVP can be done in an outpatient setting and is considered safe—even for patients with a prolonged prothrombin time.13,14 Rare complications of LVP include significant bleeding at the puncture site, infection, and intestinal perforation.5
Diuretics should be prescribed after LVP to prevent ascites recurrence.5 Plasma expanders can prevent hepatorenal syndrome, ascites recurrence, and dilutional hyponatremia.5,11 Albumin is the most efficacious of these agents;5,14 it is administered intravenously at a dose of 8 to 10 g/L of fluid removed.13,15
Take steps to prevent variceal bleeding
Soon after a patient is diagnosed with cirrhosis, he or she should undergo esophagogastroduodenoscopy to screen for the presence and size of varices.16 Although they can’t prevent esophagogastric varices, nonselective beta-blockers (NSBBs) are the gold standard for preventing first variceal hemorrhage in patients with small varices with red wale signs on the varices and/or Child-Pugh Class B or C cirrhosis (TABLE17), and in all patients with medium or large varices.18 Propranolol is usually started at 20 mg BID, or nadolol is started at 20 to 40 mg/d.16 The NSBB dose is adjusted to the maximum tolerated dose, which occurs when the patient's heart rate is reduced to 55 to 60 beats/min.
NSBBs are associated with poor survival in patients with refractory ascites and thus are contraindicated in these patients.19 NSBBs also should not be taken by patients with SBP because use of these medications is associated with worse outcomes compared to those not receiving NSBBs.20
Endoscopic variceal ligation is an alternative to NSBBs for the primary prophylaxis of variceal hemorrhage in patients with medium to large varices.18 In particular, ligation should be considered for patients with high-risk varices in whom beta-blockers are contraindicated or must be discontinued because of adverse effects.21
Avoid nitrates in patients with varices because these agents do not prevent first variceal hemorrhage and have been associated with higher mortality rates in patients older than 50.16 There is no significant additional benefit or mortality reduction associated with adding a nitrate to an NSBB.22 Transjugular intrahepatic portosystemic shunt (TIPS) or surgically created shunts are reserved for patients for whom medical therapy fails.18
Mental status changes suggest hepatic encephalopathy
Hepatic encephalopathy is a reversible impairment of neuropsychiatric function that is associated with impaired hepatic function. Because a patient with encephalopathy presents with an altered mental status, he or she may need to be admitted to the hospital for evaluation, diagnosis, and treatment.
The goals of hepatic encephalopathy treatment are to identify and correct precipitating causes and lower serum ammonia concentrations to improve mental status.15 Nutritional support should be provided without protein restriction unless the patient is severely proteinintolerant.23 The recommended initial therapy is lactulose 30 to 45 mL 2 to 4 times per day, to decrease absorption of ammonia in the gut. The dose should be titrated until patients have 2 to 3 soft stools daily.24
For patients who can’t tolerate lactulose or whose mental status doesn’t improve within 48 hours, rifaximin 400 mg orally 3 times daily or 550 mg 2 times daily is recommended.25 Neomycin 500 mg orally 3 times a day or 1 g twice daily is a second-line agent reserved for patients who are unable to take rifaximin; however, its efficacy is not well established, and neomycin has been associated with ototoxicity and nephrotoxicity.24
Watch for signs of kidney failure
Hepatorenal syndrome is renal failure induced by severe hepatic injury and characterized by azotemia and decreased renal blood flow and glomerular filtration rate.15 It is a diagnosis of exclusion. Hepatorenal syndrome is typically caused by arterial vasodilation in the splanchnic circulation in patients with portal hypertension.15,26,27 Type 1 hepatorenal syndrome is characterized by at least a 2-fold increase in serum creatinine to a level of >2.5 mg/dL over more than 2 weeks. Patients typically have urine output <400 to 500 mL/d. Type 2 hepatorenal syndrome is characterized by less severe renal impairment; it is associated with ascites that does not improve with diuretics.28
Patients with hepatorenal syndrome should not use any nephrotoxic agents, such as nonsteroidal anti-inflammatory drugs. Inpatient treatment is usually required and may include norepinephrine with albumin, terlipressin with midodrine, or octreotide and albumin. Patients who fail to respond to medical therapy may benefit from TIPS as a bridge until they can undergo liver transplantation.29
When to consider liver transplantation
The appropriateness and timing of liver transplantation should be determined on a case-by-case basis. For some patients with cirrhosis, transplantation may be the definitive treatment. For example, in some patients with hepatocellular carcinoma (HCC), liver transplantation is an option because transplantation can cure the tumor and underlying cirrhosis. However, while transplantation is a suitable option for early HCC in patients with cirrhosis, it has been shown to have limited efficacy in patients with advanced disease who are not selected using specific criteria.30
Referral for evaluation for transplantation should be considered once a patient with cirrhosis experiences a major complication (eg, ascites, variceal hemorrhage, or hepatic encephalopathy).31 Another criterion for timing and allocation of liver transplantation is based on the statistical model for end-stage liver disease (MELD), which is used to predict 3-month survival in patients with cirrhosis based on the relationships between serum bilirubin, serum creatinine, and international normalized ratio values.15 Liver transplantation should be considered for patients with a MELD score ≥15.15,31 Such patients should be promptly referred to a liver transplantation specialist to allow sufficient time for the appropriate psychosocial assessments and medical evaluations, and for patients and their families to receive appropriate education on things like the risks and benefits of transplantation.15
Patients with cirrhosis should be educated about complications of their condition, including ascites, esophageal varices, hepatic encephalopathy, hepatorenal syndrome, spontaneous bacterial peritonitis, and hepatocellular carcinoma (HCC).5 It’s important to explain that they will need to be evaluated every 6 months with serology and ultrasound to assess disease changes.6 Annual screening for HCC should be done with ultrasound or computed tomography scanning with or without alpha-fetoprotein.6
Ensure that your patient knows that he needs to receive the recommended immunizations. The Centers for Disease Control and Prevention recommends that patients with cirrhosis should receive annual influenza, pneumococcal 23, and hepatitis A and B series vaccinations.7
Advise patients with cirrhosis to be cautious when taking any medications. Patients with cirrhosis should avoid nonsteroidal anti-inflammatory drugs because these medications encourage sodium retention, which can exacerbate ascites.6 Acetaminophen use is discouraged, but should not be harmful unless the patient takes >2 g/d.8
Emphasize the importance of eating a healthy diet. Malnutrition is common in patients with cirrhosis3 and correlates with more severe disease and poorer outcomes, including mortality.9 Nutritional recommendations for patients with alcohol-related liver disease include thiamine 50 mg orally or intramuscularly, and riboflavin and pyridoxine in the recommended daily doses.10 Advise patients to take other vitamins, as needed, to treat any deficiencies.9
CASE › After evaluating Mr. M, you prescribe spironolactone 100 mg/d and furosemide 40 mg twice a day to address ascites, and propranolol—which you titrate to 80 mg twice a day—to prevent variceal hemorrhage. Mr. M is maintained on these medications and returns with his daughter, as he has been doing every 2 to 3 months. He is excited that he breathes easily as long as he avoids salt and takes his medications. He continues to see his hepatologist regularly, and his last paracentesis was 4 months ago. He has not used any alcohol since he was taught about the relationship between alcohol and his breathing.
CORRESPONDENCE
Suzanne Minor, MD, FAAF P, Assistant Professor of Family Medicine, Department of Humanities, Health, & Society, Florida International University, Herbert Wertheim College of Medicine, 11200 SW 8th Street, AHC II, 554A, Miami, FL 33199; [email protected]
1. Scaglion S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: A population-based study. J Clin Gastroenterol. 2014. October 8. [Epub ahead of print.]
2. Murphy SL, Xu JQ, Kochanek KD. Deaths: Final data for 2010. National Vital Statistics Reports. National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_04.pdf. Accessed April 30, 2015.
3. National Institute of Diabetes and Digestive and Kidney Diseases. Cirrhosis. National Institute of Diabetes and Digestive and Kidney Diseases Web site. Available at: http://www.niddk.nih.gov/health-information/health-topics/liver-disease/cirrhosis/Pages/facts.aspx. Accessed April 30, 2015.
4. Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20:7312-7324.
5. Ginès P, Cárdenas A, Arroyo V, et al. Management of cirrhosis and ascites. N Eng J Med. 2004;350:1646-1654.
6. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
7. Centers for Disease Control and Prevention. 2015 recommended immunizations for adults: By age. Centers for Disease Control and Prevention Web site. Available from: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule-easy-read.pdf. Accessed April 28, 2015.
8. Bacon BR. Cirrhosis and its complications. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012. Available from: http://accessmedicine.mhmedical.com/content.aspx?bookid=1130§ionid=79748841. Accessed April 28, 2015.
9. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105:14-32.
10. National Institute on Alcohol Abuse and Alcoholism. Alcohol Alert. Alcoholic liver disease. U.S. Department of Health & Human Services. 2005. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/aa64/aa64.htm. Accessed April 18, 2015.
11. Kashani A, Landaverde C, Medici V, et al. Fluid retention in cirrhosis: pathophysiology and management. QJM. 2008;101:71-85.
12. Chalasani NP, Vuppalanchi RK. Ascites: A common problem in people with cirrhosis. July 2013. American College of Gastroenterology Web site. Available at: http://patients.gi.org/topics/ascites/. Accessed April 28, 2015.
13. Kuiper JJ, van Buuren HR, de Man RA. Ascites in cirrhosis: a review of management and complications. Neth J Med. 2007;65:283-288.
14. Biecker E. Diagnosis and therapy of ascites in liver cirrhosis. World J Gastroentol. 2011;17:1237-1248.
15. Heidelbaugh JJ, Sherbondy M. Cirrhosis and chronic liver failure: Part II. Complications and treatment. Am Fam Physician. 2006;74:767-776.
16. Garcia-Tsao G, Lim JK; Members of Veterans Affairs Hepatitis C Resource Center Program. Management and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program. Am J Gastroenterol. 2009;104:1802-1829.
17. Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting shortterm survival among cirrhotics. Hepatology. 1987;7:660-664.
18. Garcia-Tsao G, Sanyal AJ, Grace ND, et al; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938.
19. Serste T, Melot C, Francoz C, et al. Deleterious effects of betablockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017-1022.
20. Mandorfer M, Bota S, Schwabl P, et al. Nonselective b blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:1680–1690.e1.
21. Sarin SK, Lamba GS, Kumar M, et al. Comparison of endoscopic ligation and propranolol for the primary prevention of variceal bleeding. N Engl J Med. 1999;340:988-993.
22. Garcia-Pagan JC, Feu F, Bosch J, et al. Propranolol compared with propranolol plus isosorbide-5-mononitrate for portal hypertension in cirrhosis. A randomized controlled study. Ann Intern Med. 1991;114:869-873.
23. Amodio P, Bemeur C, Butterworth R, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58:325-336.
24. Sharma P, Sharma BC. Disaccharides in the treatment of hepatic encephalopathy. Metab Brain Dis. 2013;28:313-320.
25. Jiang Q, Jiang XH, Zheng MH, et al. Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta-analysis. Eur J Gastroenterol Hepatol. 2008;20:1064-1070.
26. Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290.
27. Iwakiri Y. The molecules: mechanisms of arterial vasodilatation observed in the splanchnic and systemic circulation in portal hypertension. J Clin Gastroenterol. 2007;41(Suppl 3):S288-S294.
28. Epstein M, Berk DP, Hollenberg NK, et al. Renal failure in the patient with cirrhosis. The role of active vasoconstriction. Am J Med. 1970;49:175-185.
29. Singh V, Ghosh S, Singh B, et al. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol. 2012;56:1293-1298.
30. Chua TC, Saxena A, Chu F, et al. Hepatic resection for transplantable hepatocellular carcinoma for patients within Milan and UCSF criteria. Am J Clin Oncol. 2012;35:141-145.
31. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144-1165.
› Prescribe low-dose diuretics and recommend sodium restriction for patients with cirrhosis who have grade 2 (moderate) ascites. C
› Initiate treatment with beta-blockers to prevent variceal bleeding in all patients with medium or large varices, as well as in those with small varices who also have red wale signs and/or Child-Pugh Class B or C cirrhosis. A
› Consider evaluation for liver transplantation for a patient with cirrhosis who has experienced a major complication (eg, ascites, hepatic encephalopathy, or variceal hemorrhage) or one who has a model for end-stage liver disease (MELD) score ≥15. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joe M, age 59, seeks care at the local emergency department (ED) for shortness of breath. He also complains that his abdomen has been getting “bigger and bigger.” The ED physician recognizes that he is suffering from cirrhosis with secondary ascites and admits him. A paracentesis is performed and 7 L of fluid are removed. The patient is started on furosemide 40 mg/d and the health care team educates him about the relationship between his alcohol consumption and his enlarging abdomen. At discharge, he is told to follow up with his primary care physician.
Two weeks later, the patient arrives at your clinic for followup. What is the next step in managing this patient?
Cirrhosis—the end stage of chronic liver disease characterized by inflammation and fibrosis—is a relatively common and often fatal diagnosis. In the United States, an estimated 633,000 adults have cirrhosis,1 and each year approximately 32,000 people die from the condition.2 The most common causes of cirrhosis are heavy alcohol use, chronic hepatitis B or C infection, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis.3 Cirrhosis typically involves degeneration and necrosis of hepatocytes, which are replaced by fibrotic tissues and regenerative nodules, leading to loss of liver function.4
Patients with cirrhosis can be treated as outpatients—that is, until they decompensate. Obviously, treatment specific to the underlying causes of cirrhosis, such as interferon for a patient with hepatitis and abstinence for a patient with alcohol-related liver disease, should be the first concern. However, this article focuses on the family physician’s role in identifying and treating several of the most common complications of cirrhosis, including ascites, variceal bleeding, hepatic encephalopathy, and hepatorenal syndrome. We will also cover which patients should be referred for evaluation for liver transplantation. (For a guide to providing patient education for individuals with cirrhosis, see “Dx cirrhosis: What to teach your patient”.3,5-10)
Sodium restriction, diuretics are first steps for ascites
The goals of ascites treatment are to prevent or relieve dyspnea and abdominal pain and to prevent life-threatening complications, such as spontaneous bacterial peritonitis (SBP) and hepatorenal syndrome.11 Patient education is key regarding weight gain; that’s why it’s important to instruct patients to contact you if they gain more than 2 lbs/d for 3 consecutive days or more than 10 lbs.12
Approximately 10% of patients with ascites respond well to sodium restriction alone (1500-2000 mg/d).11 In addition to sodium restriction, patients with grade 2 ascites (moderate ascites with proportionate abdominal distension) should receive a low-dose diuretic, such as spironolactone (initial dose, 50-100 mg/d; increase up to 200-300 mg/d)13 or amiloride (5-10 mg/d).5
Painful gynecomastia and hyperkalemia are the most common adverse effects of spironolactone.13 Amiloride has fewer adverse effects than spironolactone, but is less effective.11 Low-dose furosemide (20-40 mg/d) may be added, although weight loss should be monitored to watch for excessive diuresis, which can lead to renal failure, hyponatremia, or encephalopathy.5,13 Also monitor electrolytes to watch for hypokalemia or hyponatremia.13
Recommended weight loss to prevent renal failure is 300 to 500 g/d (.66-1.1 lbs/d) for patients without peripheral edema, and 800 to 1000 g/d (1.7-2.2 lbs/d) for patients with peripheral edema.5,13
Patients with grade 3 (tense) or refractory ascites should have large-volume paracentesis (LVP) plus an albumin infusion.5 LVP (removal of >5 L of fluid) is more effective, faster, and has less risk of adverse effects than increasing the dosage of the patient’s diuretic.5,13 LVP can be done in an outpatient setting and is considered safe—even for patients with a prolonged prothrombin time.13,14 Rare complications of LVP include significant bleeding at the puncture site, infection, and intestinal perforation.5
Diuretics should be prescribed after LVP to prevent ascites recurrence.5 Plasma expanders can prevent hepatorenal syndrome, ascites recurrence, and dilutional hyponatremia.5,11 Albumin is the most efficacious of these agents;5,14 it is administered intravenously at a dose of 8 to 10 g/L of fluid removed.13,15
Take steps to prevent variceal bleeding
Soon after a patient is diagnosed with cirrhosis, he or she should undergo esophagogastroduodenoscopy to screen for the presence and size of varices.16 Although they can’t prevent esophagogastric varices, nonselective beta-blockers (NSBBs) are the gold standard for preventing first variceal hemorrhage in patients with small varices with red wale signs on the varices and/or Child-Pugh Class B or C cirrhosis (TABLE17), and in all patients with medium or large varices.18 Propranolol is usually started at 20 mg BID, or nadolol is started at 20 to 40 mg/d.16 The NSBB dose is adjusted to the maximum tolerated dose, which occurs when the patient's heart rate is reduced to 55 to 60 beats/min.
NSBBs are associated with poor survival in patients with refractory ascites and thus are contraindicated in these patients.19 NSBBs also should not be taken by patients with SBP because use of these medications is associated with worse outcomes compared to those not receiving NSBBs.20
Endoscopic variceal ligation is an alternative to NSBBs for the primary prophylaxis of variceal hemorrhage in patients with medium to large varices.18 In particular, ligation should be considered for patients with high-risk varices in whom beta-blockers are contraindicated or must be discontinued because of adverse effects.21
Avoid nitrates in patients with varices because these agents do not prevent first variceal hemorrhage and have been associated with higher mortality rates in patients older than 50.16 There is no significant additional benefit or mortality reduction associated with adding a nitrate to an NSBB.22 Transjugular intrahepatic portosystemic shunt (TIPS) or surgically created shunts are reserved for patients for whom medical therapy fails.18
Mental status changes suggest hepatic encephalopathy
Hepatic encephalopathy is a reversible impairment of neuropsychiatric function that is associated with impaired hepatic function. Because a patient with encephalopathy presents with an altered mental status, he or she may need to be admitted to the hospital for evaluation, diagnosis, and treatment.
The goals of hepatic encephalopathy treatment are to identify and correct precipitating causes and lower serum ammonia concentrations to improve mental status.15 Nutritional support should be provided without protein restriction unless the patient is severely proteinintolerant.23 The recommended initial therapy is lactulose 30 to 45 mL 2 to 4 times per day, to decrease absorption of ammonia in the gut. The dose should be titrated until patients have 2 to 3 soft stools daily.24
For patients who can’t tolerate lactulose or whose mental status doesn’t improve within 48 hours, rifaximin 400 mg orally 3 times daily or 550 mg 2 times daily is recommended.25 Neomycin 500 mg orally 3 times a day or 1 g twice daily is a second-line agent reserved for patients who are unable to take rifaximin; however, its efficacy is not well established, and neomycin has been associated with ototoxicity and nephrotoxicity.24
Watch for signs of kidney failure
Hepatorenal syndrome is renal failure induced by severe hepatic injury and characterized by azotemia and decreased renal blood flow and glomerular filtration rate.15 It is a diagnosis of exclusion. Hepatorenal syndrome is typically caused by arterial vasodilation in the splanchnic circulation in patients with portal hypertension.15,26,27 Type 1 hepatorenal syndrome is characterized by at least a 2-fold increase in serum creatinine to a level of >2.5 mg/dL over more than 2 weeks. Patients typically have urine output <400 to 500 mL/d. Type 2 hepatorenal syndrome is characterized by less severe renal impairment; it is associated with ascites that does not improve with diuretics.28
Patients with hepatorenal syndrome should not use any nephrotoxic agents, such as nonsteroidal anti-inflammatory drugs. Inpatient treatment is usually required and may include norepinephrine with albumin, terlipressin with midodrine, or octreotide and albumin. Patients who fail to respond to medical therapy may benefit from TIPS as a bridge until they can undergo liver transplantation.29
When to consider liver transplantation
The appropriateness and timing of liver transplantation should be determined on a case-by-case basis. For some patients with cirrhosis, transplantation may be the definitive treatment. For example, in some patients with hepatocellular carcinoma (HCC), liver transplantation is an option because transplantation can cure the tumor and underlying cirrhosis. However, while transplantation is a suitable option for early HCC in patients with cirrhosis, it has been shown to have limited efficacy in patients with advanced disease who are not selected using specific criteria.30
Referral for evaluation for transplantation should be considered once a patient with cirrhosis experiences a major complication (eg, ascites, variceal hemorrhage, or hepatic encephalopathy).31 Another criterion for timing and allocation of liver transplantation is based on the statistical model for end-stage liver disease (MELD), which is used to predict 3-month survival in patients with cirrhosis based on the relationships between serum bilirubin, serum creatinine, and international normalized ratio values.15 Liver transplantation should be considered for patients with a MELD score ≥15.15,31 Such patients should be promptly referred to a liver transplantation specialist to allow sufficient time for the appropriate psychosocial assessments and medical evaluations, and for patients and their families to receive appropriate education on things like the risks and benefits of transplantation.15
Patients with cirrhosis should be educated about complications of their condition, including ascites, esophageal varices, hepatic encephalopathy, hepatorenal syndrome, spontaneous bacterial peritonitis, and hepatocellular carcinoma (HCC).5 It’s important to explain that they will need to be evaluated every 6 months with serology and ultrasound to assess disease changes.6 Annual screening for HCC should be done with ultrasound or computed tomography scanning with or without alpha-fetoprotein.6
Ensure that your patient knows that he needs to receive the recommended immunizations. The Centers for Disease Control and Prevention recommends that patients with cirrhosis should receive annual influenza, pneumococcal 23, and hepatitis A and B series vaccinations.7
Advise patients with cirrhosis to be cautious when taking any medications. Patients with cirrhosis should avoid nonsteroidal anti-inflammatory drugs because these medications encourage sodium retention, which can exacerbate ascites.6 Acetaminophen use is discouraged, but should not be harmful unless the patient takes >2 g/d.8
Emphasize the importance of eating a healthy diet. Malnutrition is common in patients with cirrhosis3 and correlates with more severe disease and poorer outcomes, including mortality.9 Nutritional recommendations for patients with alcohol-related liver disease include thiamine 50 mg orally or intramuscularly, and riboflavin and pyridoxine in the recommended daily doses.10 Advise patients to take other vitamins, as needed, to treat any deficiencies.9
CASE › After evaluating Mr. M, you prescribe spironolactone 100 mg/d and furosemide 40 mg twice a day to address ascites, and propranolol—which you titrate to 80 mg twice a day—to prevent variceal hemorrhage. Mr. M is maintained on these medications and returns with his daughter, as he has been doing every 2 to 3 months. He is excited that he breathes easily as long as he avoids salt and takes his medications. He continues to see his hepatologist regularly, and his last paracentesis was 4 months ago. He has not used any alcohol since he was taught about the relationship between alcohol and his breathing.
CORRESPONDENCE
Suzanne Minor, MD, FAAF P, Assistant Professor of Family Medicine, Department of Humanities, Health, & Society, Florida International University, Herbert Wertheim College of Medicine, 11200 SW 8th Street, AHC II, 554A, Miami, FL 33199; [email protected]
› Prescribe low-dose diuretics and recommend sodium restriction for patients with cirrhosis who have grade 2 (moderate) ascites. C
› Initiate treatment with beta-blockers to prevent variceal bleeding in all patients with medium or large varices, as well as in those with small varices who also have red wale signs and/or Child-Pugh Class B or C cirrhosis. A
› Consider evaluation for liver transplantation for a patient with cirrhosis who has experienced a major complication (eg, ascites, hepatic encephalopathy, or variceal hemorrhage) or one who has a model for end-stage liver disease (MELD) score ≥15. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joe M, age 59, seeks care at the local emergency department (ED) for shortness of breath. He also complains that his abdomen has been getting “bigger and bigger.” The ED physician recognizes that he is suffering from cirrhosis with secondary ascites and admits him. A paracentesis is performed and 7 L of fluid are removed. The patient is started on furosemide 40 mg/d and the health care team educates him about the relationship between his alcohol consumption and his enlarging abdomen. At discharge, he is told to follow up with his primary care physician.
Two weeks later, the patient arrives at your clinic for followup. What is the next step in managing this patient?
Cirrhosis—the end stage of chronic liver disease characterized by inflammation and fibrosis—is a relatively common and often fatal diagnosis. In the United States, an estimated 633,000 adults have cirrhosis,1 and each year approximately 32,000 people die from the condition.2 The most common causes of cirrhosis are heavy alcohol use, chronic hepatitis B or C infection, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis.3 Cirrhosis typically involves degeneration and necrosis of hepatocytes, which are replaced by fibrotic tissues and regenerative nodules, leading to loss of liver function.4
Patients with cirrhosis can be treated as outpatients—that is, until they decompensate. Obviously, treatment specific to the underlying causes of cirrhosis, such as interferon for a patient with hepatitis and abstinence for a patient with alcohol-related liver disease, should be the first concern. However, this article focuses on the family physician’s role in identifying and treating several of the most common complications of cirrhosis, including ascites, variceal bleeding, hepatic encephalopathy, and hepatorenal syndrome. We will also cover which patients should be referred for evaluation for liver transplantation. (For a guide to providing patient education for individuals with cirrhosis, see “Dx cirrhosis: What to teach your patient”.3,5-10)
Sodium restriction, diuretics are first steps for ascites
The goals of ascites treatment are to prevent or relieve dyspnea and abdominal pain and to prevent life-threatening complications, such as spontaneous bacterial peritonitis (SBP) and hepatorenal syndrome.11 Patient education is key regarding weight gain; that’s why it’s important to instruct patients to contact you if they gain more than 2 lbs/d for 3 consecutive days or more than 10 lbs.12
Approximately 10% of patients with ascites respond well to sodium restriction alone (1500-2000 mg/d).11 In addition to sodium restriction, patients with grade 2 ascites (moderate ascites with proportionate abdominal distension) should receive a low-dose diuretic, such as spironolactone (initial dose, 50-100 mg/d; increase up to 200-300 mg/d)13 or amiloride (5-10 mg/d).5
Painful gynecomastia and hyperkalemia are the most common adverse effects of spironolactone.13 Amiloride has fewer adverse effects than spironolactone, but is less effective.11 Low-dose furosemide (20-40 mg/d) may be added, although weight loss should be monitored to watch for excessive diuresis, which can lead to renal failure, hyponatremia, or encephalopathy.5,13 Also monitor electrolytes to watch for hypokalemia or hyponatremia.13
Recommended weight loss to prevent renal failure is 300 to 500 g/d (.66-1.1 lbs/d) for patients without peripheral edema, and 800 to 1000 g/d (1.7-2.2 lbs/d) for patients with peripheral edema.5,13
Patients with grade 3 (tense) or refractory ascites should have large-volume paracentesis (LVP) plus an albumin infusion.5 LVP (removal of >5 L of fluid) is more effective, faster, and has less risk of adverse effects than increasing the dosage of the patient’s diuretic.5,13 LVP can be done in an outpatient setting and is considered safe—even for patients with a prolonged prothrombin time.13,14 Rare complications of LVP include significant bleeding at the puncture site, infection, and intestinal perforation.5
Diuretics should be prescribed after LVP to prevent ascites recurrence.5 Plasma expanders can prevent hepatorenal syndrome, ascites recurrence, and dilutional hyponatremia.5,11 Albumin is the most efficacious of these agents;5,14 it is administered intravenously at a dose of 8 to 10 g/L of fluid removed.13,15
Take steps to prevent variceal bleeding
Soon after a patient is diagnosed with cirrhosis, he or she should undergo esophagogastroduodenoscopy to screen for the presence and size of varices.16 Although they can’t prevent esophagogastric varices, nonselective beta-blockers (NSBBs) are the gold standard for preventing first variceal hemorrhage in patients with small varices with red wale signs on the varices and/or Child-Pugh Class B or C cirrhosis (TABLE17), and in all patients with medium or large varices.18 Propranolol is usually started at 20 mg BID, or nadolol is started at 20 to 40 mg/d.16 The NSBB dose is adjusted to the maximum tolerated dose, which occurs when the patient's heart rate is reduced to 55 to 60 beats/min.
NSBBs are associated with poor survival in patients with refractory ascites and thus are contraindicated in these patients.19 NSBBs also should not be taken by patients with SBP because use of these medications is associated with worse outcomes compared to those not receiving NSBBs.20
Endoscopic variceal ligation is an alternative to NSBBs for the primary prophylaxis of variceal hemorrhage in patients with medium to large varices.18 In particular, ligation should be considered for patients with high-risk varices in whom beta-blockers are contraindicated or must be discontinued because of adverse effects.21
Avoid nitrates in patients with varices because these agents do not prevent first variceal hemorrhage and have been associated with higher mortality rates in patients older than 50.16 There is no significant additional benefit or mortality reduction associated with adding a nitrate to an NSBB.22 Transjugular intrahepatic portosystemic shunt (TIPS) or surgically created shunts are reserved for patients for whom medical therapy fails.18
Mental status changes suggest hepatic encephalopathy
Hepatic encephalopathy is a reversible impairment of neuropsychiatric function that is associated with impaired hepatic function. Because a patient with encephalopathy presents with an altered mental status, he or she may need to be admitted to the hospital for evaluation, diagnosis, and treatment.
The goals of hepatic encephalopathy treatment are to identify and correct precipitating causes and lower serum ammonia concentrations to improve mental status.15 Nutritional support should be provided without protein restriction unless the patient is severely proteinintolerant.23 The recommended initial therapy is lactulose 30 to 45 mL 2 to 4 times per day, to decrease absorption of ammonia in the gut. The dose should be titrated until patients have 2 to 3 soft stools daily.24
For patients who can’t tolerate lactulose or whose mental status doesn’t improve within 48 hours, rifaximin 400 mg orally 3 times daily or 550 mg 2 times daily is recommended.25 Neomycin 500 mg orally 3 times a day or 1 g twice daily is a second-line agent reserved for patients who are unable to take rifaximin; however, its efficacy is not well established, and neomycin has been associated with ototoxicity and nephrotoxicity.24
Watch for signs of kidney failure
Hepatorenal syndrome is renal failure induced by severe hepatic injury and characterized by azotemia and decreased renal blood flow and glomerular filtration rate.15 It is a diagnosis of exclusion. Hepatorenal syndrome is typically caused by arterial vasodilation in the splanchnic circulation in patients with portal hypertension.15,26,27 Type 1 hepatorenal syndrome is characterized by at least a 2-fold increase in serum creatinine to a level of >2.5 mg/dL over more than 2 weeks. Patients typically have urine output <400 to 500 mL/d. Type 2 hepatorenal syndrome is characterized by less severe renal impairment; it is associated with ascites that does not improve with diuretics.28
Patients with hepatorenal syndrome should not use any nephrotoxic agents, such as nonsteroidal anti-inflammatory drugs. Inpatient treatment is usually required and may include norepinephrine with albumin, terlipressin with midodrine, or octreotide and albumin. Patients who fail to respond to medical therapy may benefit from TIPS as a bridge until they can undergo liver transplantation.29
When to consider liver transplantation
The appropriateness and timing of liver transplantation should be determined on a case-by-case basis. For some patients with cirrhosis, transplantation may be the definitive treatment. For example, in some patients with hepatocellular carcinoma (HCC), liver transplantation is an option because transplantation can cure the tumor and underlying cirrhosis. However, while transplantation is a suitable option for early HCC in patients with cirrhosis, it has been shown to have limited efficacy in patients with advanced disease who are not selected using specific criteria.30
Referral for evaluation for transplantation should be considered once a patient with cirrhosis experiences a major complication (eg, ascites, variceal hemorrhage, or hepatic encephalopathy).31 Another criterion for timing and allocation of liver transplantation is based on the statistical model for end-stage liver disease (MELD), which is used to predict 3-month survival in patients with cirrhosis based on the relationships between serum bilirubin, serum creatinine, and international normalized ratio values.15 Liver transplantation should be considered for patients with a MELD score ≥15.15,31 Such patients should be promptly referred to a liver transplantation specialist to allow sufficient time for the appropriate psychosocial assessments and medical evaluations, and for patients and their families to receive appropriate education on things like the risks and benefits of transplantation.15
Patients with cirrhosis should be educated about complications of their condition, including ascites, esophageal varices, hepatic encephalopathy, hepatorenal syndrome, spontaneous bacterial peritonitis, and hepatocellular carcinoma (HCC).5 It’s important to explain that they will need to be evaluated every 6 months with serology and ultrasound to assess disease changes.6 Annual screening for HCC should be done with ultrasound or computed tomography scanning with or without alpha-fetoprotein.6
Ensure that your patient knows that he needs to receive the recommended immunizations. The Centers for Disease Control and Prevention recommends that patients with cirrhosis should receive annual influenza, pneumococcal 23, and hepatitis A and B series vaccinations.7
Advise patients with cirrhosis to be cautious when taking any medications. Patients with cirrhosis should avoid nonsteroidal anti-inflammatory drugs because these medications encourage sodium retention, which can exacerbate ascites.6 Acetaminophen use is discouraged, but should not be harmful unless the patient takes >2 g/d.8
Emphasize the importance of eating a healthy diet. Malnutrition is common in patients with cirrhosis3 and correlates with more severe disease and poorer outcomes, including mortality.9 Nutritional recommendations for patients with alcohol-related liver disease include thiamine 50 mg orally or intramuscularly, and riboflavin and pyridoxine in the recommended daily doses.10 Advise patients to take other vitamins, as needed, to treat any deficiencies.9
CASE › After evaluating Mr. M, you prescribe spironolactone 100 mg/d and furosemide 40 mg twice a day to address ascites, and propranolol—which you titrate to 80 mg twice a day—to prevent variceal hemorrhage. Mr. M is maintained on these medications and returns with his daughter, as he has been doing every 2 to 3 months. He is excited that he breathes easily as long as he avoids salt and takes his medications. He continues to see his hepatologist regularly, and his last paracentesis was 4 months ago. He has not used any alcohol since he was taught about the relationship between alcohol and his breathing.
CORRESPONDENCE
Suzanne Minor, MD, FAAF P, Assistant Professor of Family Medicine, Department of Humanities, Health, & Society, Florida International University, Herbert Wertheim College of Medicine, 11200 SW 8th Street, AHC II, 554A, Miami, FL 33199; [email protected]
1. Scaglion S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: A population-based study. J Clin Gastroenterol. 2014. October 8. [Epub ahead of print.]
2. Murphy SL, Xu JQ, Kochanek KD. Deaths: Final data for 2010. National Vital Statistics Reports. National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_04.pdf. Accessed April 30, 2015.
3. National Institute of Diabetes and Digestive and Kidney Diseases. Cirrhosis. National Institute of Diabetes and Digestive and Kidney Diseases Web site. Available at: http://www.niddk.nih.gov/health-information/health-topics/liver-disease/cirrhosis/Pages/facts.aspx. Accessed April 30, 2015.
4. Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20:7312-7324.
5. Ginès P, Cárdenas A, Arroyo V, et al. Management of cirrhosis and ascites. N Eng J Med. 2004;350:1646-1654.
6. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
7. Centers for Disease Control and Prevention. 2015 recommended immunizations for adults: By age. Centers for Disease Control and Prevention Web site. Available from: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule-easy-read.pdf. Accessed April 28, 2015.
8. Bacon BR. Cirrhosis and its complications. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012. Available from: http://accessmedicine.mhmedical.com/content.aspx?bookid=1130§ionid=79748841. Accessed April 28, 2015.
9. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105:14-32.
10. National Institute on Alcohol Abuse and Alcoholism. Alcohol Alert. Alcoholic liver disease. U.S. Department of Health & Human Services. 2005. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/aa64/aa64.htm. Accessed April 18, 2015.
11. Kashani A, Landaverde C, Medici V, et al. Fluid retention in cirrhosis: pathophysiology and management. QJM. 2008;101:71-85.
12. Chalasani NP, Vuppalanchi RK. Ascites: A common problem in people with cirrhosis. July 2013. American College of Gastroenterology Web site. Available at: http://patients.gi.org/topics/ascites/. Accessed April 28, 2015.
13. Kuiper JJ, van Buuren HR, de Man RA. Ascites in cirrhosis: a review of management and complications. Neth J Med. 2007;65:283-288.
14. Biecker E. Diagnosis and therapy of ascites in liver cirrhosis. World J Gastroentol. 2011;17:1237-1248.
15. Heidelbaugh JJ, Sherbondy M. Cirrhosis and chronic liver failure: Part II. Complications and treatment. Am Fam Physician. 2006;74:767-776.
16. Garcia-Tsao G, Lim JK; Members of Veterans Affairs Hepatitis C Resource Center Program. Management and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program. Am J Gastroenterol. 2009;104:1802-1829.
17. Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting shortterm survival among cirrhotics. Hepatology. 1987;7:660-664.
18. Garcia-Tsao G, Sanyal AJ, Grace ND, et al; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938.
19. Serste T, Melot C, Francoz C, et al. Deleterious effects of betablockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017-1022.
20. Mandorfer M, Bota S, Schwabl P, et al. Nonselective b blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:1680–1690.e1.
21. Sarin SK, Lamba GS, Kumar M, et al. Comparison of endoscopic ligation and propranolol for the primary prevention of variceal bleeding. N Engl J Med. 1999;340:988-993.
22. Garcia-Pagan JC, Feu F, Bosch J, et al. Propranolol compared with propranolol plus isosorbide-5-mononitrate for portal hypertension in cirrhosis. A randomized controlled study. Ann Intern Med. 1991;114:869-873.
23. Amodio P, Bemeur C, Butterworth R, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58:325-336.
24. Sharma P, Sharma BC. Disaccharides in the treatment of hepatic encephalopathy. Metab Brain Dis. 2013;28:313-320.
25. Jiang Q, Jiang XH, Zheng MH, et al. Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta-analysis. Eur J Gastroenterol Hepatol. 2008;20:1064-1070.
26. Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290.
27. Iwakiri Y. The molecules: mechanisms of arterial vasodilatation observed in the splanchnic and systemic circulation in portal hypertension. J Clin Gastroenterol. 2007;41(Suppl 3):S288-S294.
28. Epstein M, Berk DP, Hollenberg NK, et al. Renal failure in the patient with cirrhosis. The role of active vasoconstriction. Am J Med. 1970;49:175-185.
29. Singh V, Ghosh S, Singh B, et al. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol. 2012;56:1293-1298.
30. Chua TC, Saxena A, Chu F, et al. Hepatic resection for transplantable hepatocellular carcinoma for patients within Milan and UCSF criteria. Am J Clin Oncol. 2012;35:141-145.
31. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144-1165.
1. Scaglion S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: A population-based study. J Clin Gastroenterol. 2014. October 8. [Epub ahead of print.]
2. Murphy SL, Xu JQ, Kochanek KD. Deaths: Final data for 2010. National Vital Statistics Reports. National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_04.pdf. Accessed April 30, 2015.
3. National Institute of Diabetes and Digestive and Kidney Diseases. Cirrhosis. National Institute of Diabetes and Digestive and Kidney Diseases Web site. Available at: http://www.niddk.nih.gov/health-information/health-topics/liver-disease/cirrhosis/Pages/facts.aspx. Accessed April 30, 2015.
4. Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20:7312-7324.
5. Ginès P, Cárdenas A, Arroyo V, et al. Management of cirrhosis and ascites. N Eng J Med. 2004;350:1646-1654.
6. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
7. Centers for Disease Control and Prevention. 2015 recommended immunizations for adults: By age. Centers for Disease Control and Prevention Web site. Available from: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule-easy-read.pdf. Accessed April 28, 2015.
8. Bacon BR. Cirrhosis and its complications. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012. Available from: http://accessmedicine.mhmedical.com/content.aspx?bookid=1130§ionid=79748841. Accessed April 28, 2015.
9. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105:14-32.
10. National Institute on Alcohol Abuse and Alcoholism. Alcohol Alert. Alcoholic liver disease. U.S. Department of Health & Human Services. 2005. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/aa64/aa64.htm. Accessed April 18, 2015.
11. Kashani A, Landaverde C, Medici V, et al. Fluid retention in cirrhosis: pathophysiology and management. QJM. 2008;101:71-85.
12. Chalasani NP, Vuppalanchi RK. Ascites: A common problem in people with cirrhosis. July 2013. American College of Gastroenterology Web site. Available at: http://patients.gi.org/topics/ascites/. Accessed April 28, 2015.
13. Kuiper JJ, van Buuren HR, de Man RA. Ascites in cirrhosis: a review of management and complications. Neth J Med. 2007;65:283-288.
14. Biecker E. Diagnosis and therapy of ascites in liver cirrhosis. World J Gastroentol. 2011;17:1237-1248.
15. Heidelbaugh JJ, Sherbondy M. Cirrhosis and chronic liver failure: Part II. Complications and treatment. Am Fam Physician. 2006;74:767-776.
16. Garcia-Tsao G, Lim JK; Members of Veterans Affairs Hepatitis C Resource Center Program. Management and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program. Am J Gastroenterol. 2009;104:1802-1829.
17. Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting shortterm survival among cirrhotics. Hepatology. 1987;7:660-664.
18. Garcia-Tsao G, Sanyal AJ, Grace ND, et al; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938.
19. Serste T, Melot C, Francoz C, et al. Deleterious effects of betablockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017-1022.
20. Mandorfer M, Bota S, Schwabl P, et al. Nonselective b blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:1680–1690.e1.
21. Sarin SK, Lamba GS, Kumar M, et al. Comparison of endoscopic ligation and propranolol for the primary prevention of variceal bleeding. N Engl J Med. 1999;340:988-993.
22. Garcia-Pagan JC, Feu F, Bosch J, et al. Propranolol compared with propranolol plus isosorbide-5-mononitrate for portal hypertension in cirrhosis. A randomized controlled study. Ann Intern Med. 1991;114:869-873.
23. Amodio P, Bemeur C, Butterworth R, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58:325-336.
24. Sharma P, Sharma BC. Disaccharides in the treatment of hepatic encephalopathy. Metab Brain Dis. 2013;28:313-320.
25. Jiang Q, Jiang XH, Zheng MH, et al. Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta-analysis. Eur J Gastroenterol Hepatol. 2008;20:1064-1070.
26. Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290.
27. Iwakiri Y. The molecules: mechanisms of arterial vasodilatation observed in the splanchnic and systemic circulation in portal hypertension. J Clin Gastroenterol. 2007;41(Suppl 3):S288-S294.
28. Epstein M, Berk DP, Hollenberg NK, et al. Renal failure in the patient with cirrhosis. The role of active vasoconstriction. Am J Med. 1970;49:175-185.
29. Singh V, Ghosh S, Singh B, et al. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol. 2012;56:1293-1298.
30. Chua TC, Saxena A, Chu F, et al. Hepatic resection for transplantable hepatocellular carcinoma for patients within Milan and UCSF criteria. Am J Clin Oncol. 2012;35:141-145.
31. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144-1165.
Your postop patient is confused and agitated—next steps?
› Conduct a baseline cognitive assessment during your patient’s routine visits and preoperative assessments to gauge his or her risk for delirium. A
› Work with the hospital team to implement nonpharmacologic interventions, such as reorienting the patient to day and time and avoiding sensory deprivation, as an initial treatment for delirium. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Mark Q, age 80, is admitted to the hospital to undergo hemicolectomy for colon cancer. His medical history includes hypertension, benign prostatic hyperplasia, and colon cancer. He did well immediately postop, but when you make morning rounds the day after his surgery, you notice that he is confused and agitated. Mr. Q’s chart reveals that earlier that morning, he pulled out his Foley catheter and intravenous (IV) line when his nurse declined his request to walk him to the bathroom.
How would you proceed?
Up to 50% of older adults who undergo surgical procedures develop delirium—a disturbance in attention and awareness accompanied by changes in cognition.1 Older adults are at heightened risk for this postoperative complication for several reasons. For one thing, older patients have a reduced capacity for homeostatic regulation when they undergo anesthesia and surgery.2 For another, age-related changes in brain neurochemistry and drug metabolism increase the likelihood of adverse drug effects, including those that could precipitate delirirum.3
Although postop delirium is a common complication in older patients, it sometimes goes unrecognized. Missed or delayed diagnosis of delirium can result in patients exhibiting behaviors that can compromise their safety, delay recuperation, and result in longer hospital stays, a greater financial burden, and increased morbidity and mortality.4 The American Geriatric Society recently published clinical guidelines and a best practices statement for preventing and treating postop delirium in patients ages >65 years.1,5 This article describes steps family physicians can take to assess their patients’ risk of delirium before they undergo surgery, and to recognize and treat delirium in the postop period.
Defining delirium
According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), the criteria for delirium are:6
A. A disturbance in attention (ie, reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment).
B. The disturbance develops over a short time (usually hours to a few days), represents a change from baseline attention and awareness, and tends to fluctuate in severity during the course of a day.
C. An additional disturbance in cognition (eg, memory deficits, disorientation, language, visuospatial ability, perception).
D. The disturbances in attention, awareness, and cognition aren’t better explained by another preexisting or evolving neurocognitive disorder and don’t occur in the context of a severely reduced level of arousal.
E. History, physical, or laboratory findings show that the disturbance is caused by the direct physiologic consequences of a general medical condition, substance intoxication/withdrawal, exposure to a toxin, or multiple etiologies.
The 3 subtypes of delirium are based on patients’ psychomotor activity.7 In hyperactive delirium, patients exhibit heightened arousal, restlessness, agitation, hallucinations, and inappropriate behavior. Hypoactive delirium is characterized by lethargy, reduced motor activity, incoherent speech, and lack of interest. Mixed delirium consists of a combination of hyperactive and hypoactive signs and symptoms.
Gauge risk before patients undergo surgery
Family physicians can assess their patients’ risk for developing delirium by conducting baseline screening during routine office visits as well as during preoperative evaluations. Factors that increase postop delirium risk include:1
• age >65 years
• dementia
• poor vision
• decreased hearing
• severe illness
• infection.
Routine cognitive screening can be done easily and efficiently using readily available tools such as the Alzheimer Association’s Cognitive Assessment Toolkit.8 This toolkit includes 3 brief, validated screening tools to identify patients with probable cognitive impairment: the General Practitioner Assessment of Cognition, the Memory Impairment Screen, and the Mini-Cog.
If preop screening indicates that the patient is at increased risk for delirium, the family physician should work with hospital’s interdisciplinary teams to institute prevention measures, such as the Hospital Elder Life Program (HELP).9 This program offers a structured curriculum for instructing volunteers to deliver daily orientation, early mobilization, feeding assistance, therapeutic activities, and other measures to help prevent delirium.
Prompt screening after surgery is essential, too
In addition to preop delirium risk assessment, all patients who undergo surgery should receive daily delirium screening during the first postoperative week. The Confusion Assessment Method (CAM) is a quick screening tool for assessing a patient’s level of arousal and consciousness.10 Based on the results of 7 high-quality studies (N=1071), CAM has a sensitivity of 94% (95% confidence interval [CI], 91%-97%) and specificity of 89% (95% CI, 85%-94%).11,12
Feature 1 of CAM, “Acute onset and fluctuating course,” requires that you compare the patient’s current mental status to his or her pre-hospital baseline mental status; the baseline status should be obtained from a family member, caretaker, or clinician who has observed the patient over time.10 This is intended to determine if the patient has experienced an acute change in mental status (eg, attention, orientation, cognition), usually over the course of hours to days.10 Feature 2, “Inattention,” is used to determine if the patient has a reduced ability to maintain attention to external stimuli and to appropriately shift attention to new external stimuli, and if the patient is unaware or out of touch with the environment.10 Feature 3, “Disorganized thinking,” is used to assess the patient’s organization of thought as expressed by speech or writing. Disorganized thinking typically manifests as rambling and irrelevant or incoherent speech.10 Feature 4, “Altered level of consciousness,” is used to rate the patient’s alertness level.10
A positive screen for delirium requires the presence of Feature 1 (acute onset and/or fluctuation) and Feature 2, plus either Feature 3 or Feature 4.
Is delirium—or something else—at work?
If an older adult is exhibiting cognitive and/or behavioral disturbances after undergoing surgery, it’s important to discern if these manifestations are the result of delirium, a preexisting psychiatric disorder, or some other cause if the patient has a clear sensorium (ALGORITHM).6,13,14
Delirium. If a patient’s CAM screen suggests delirium, conduct a thorough assessment for the signs and symptoms of delirium to determine if the patient meets DSM-5 criteria for the diagnosis.1 In order to avoid missing hypoactive, subtle, or atypical cases of delirium, conduct a thorough medical record and medications review, and gather assessments from the nursing staff and other team members regarding the patient’s behavior.
Preexisting psychiatric disorder. It’s important to differentiate psychiatric symptoms from those of a superimposed delirium.13 Because patients with preoperative depressive symptoms may be at increased risk for postop delirium, pre-surgical psychiatric evaluations are important for identifying even subtle psychopathological symptoms.15 (The psychiatric interview is the gold standard for diagnosis.16) For patients who have an established psychiatric diagnosis, consider consulting with the psychiatrist who is managing the patient’s psychiatric care.13
Other causes. If a patient who is exhibiting postop cognitive and/or behavioral disturbances has a reasonably accurate memory and a correct orientation for time, place, and person, interviews with the patient and caregivers (along with the psychiatric interview) will likely reveal potential causes for the behavioral problems.13
Is the patient suffering from dehydration? Drug withdrawal?
Assessment for an underlying organic cause must be performed because specific treatment for the underlying diagnosis may improve delirium.17 Common causes include hypoxia, infection, dehydration, acute metabolic disturbance, endocrinopathies, cardiac or vascular disorders, and drug withdrawal.13 An appropriate diagnostic work-up might consist of serum urea, glucose, electrolytes, liver function tests, arterial blood gas analyses, urinalysis, nutritional evaluation, electrocardiogram, and a complete blood count.
Ask patients about their use of alcohol and benzodiazepines, and consider alcohol or drug withdrawal as potential etiologies.18 Patients with delirium should also be assessed for iatrogenic hospital-related factors that could be causing or contributing to the condition, such as immobilization or malnutrition.13
Medications are a common culprit: Approximately 40% of cases of delirium are related to medication use.18 Commonly used postop medications such as analgesics, sedatives, proton pump inhibitors, and others can cause delirium.19 Carefully review the patient’s medication list.13 Medication-induced delirium is influenced by the number of medications taken (generally >3),20 the use of psychoactive medications,21 and the specific agent's anticholinergic potential.22 The 2012 updated Beers Criteria (American Geriatrics Society) is a useful resource for determining if “inappropriate polypharmacy” is the cause of postop delirium.23
Inadequate pain control. In a multisite trial,24 patients who received <10 mg/d of parenteral morphine sulfate equivalents were more likely to develop delirium than patients who received more analgesia. In cognitively intact patients, severe pain significantly increased the risk of delirium. With the exception of meperidine, opioids do not precipitate delirium in patients with acute pain.24 Not treating pain or administering very low—or excessively high—doses of opioids is associated with an increased risk of delirium for both cognitively intact and impaired patients.24
Constipation can contribute to the development of delirium.25 After surgery, patients tend to be less mobile and may be receiving medications that can cause constipation, such as opioids, iron, calcium, and channel blockers. Preventing and treating constipation in postop patients can reduce delirium risk.25
Begin treatment with nonpharmacologic measures
Regardless of whether a patient suffers from hyperactive, hypoactive, or mixed delirium, nonpharmacologic interventions are firstline treatment.19 Such interventions can help patients develop a sense of control over their environment, which can help relieve agitation.13 Because environmental shifts contribute to the development of delirium, avoiding transfers and securing a single room can be helpful.19 Patients with delirium have altered perceptions, and may view normal objects and routine clinician actions as harmful and threatening. Therefore, it is helpful to avoid sensory deprivation by making sure patients have access to their eyeglasses and hearing aids, and to provide nonthreatening cognitive/environmental stimulation.1,13,19 Patients should be encouraged to resume walking as soon as possible.1,19 Other nonpharmacologic interventions are listed in the TABLE.1,13,19
Safety issues must also be addressed.17 Patients with mixed or hyperactive delirium may become agitated, which can lead them to pull tubes, drains, or lines, as occurred with Mr. Q. Patients with hypoactive delirium may be prone to wandering, or receive less attention due to their hypoactive state.17 All patients with delirium are at risk of falls.
Patients should be evaluated for these risks to determine whether assigning a "sitter" or transfer to a stepdown unit or intensive care unit is warranted.17 Restraints are not recommended because they can exacerbate delirium and lead to injuries.26
Pharmacologic treatment should be reserved for patients whose behavior compromises their safety, and implemented only when the cause of the delirium is known. The primary objectives of drug therapy are to achieve and maintain safe and rapid behavioral control so the patient can receive necessary medical care, and to enhance functional recovery.14 The choice of a specific medication is individualized and depends on each patient’s clinical condition.14
For a patient with hyperactive delirium, an antipsychotic typically is the treatment of choice because these medications are dopamine receptor antagonists, and excessive dopamine transmission has been implicated in this type of delirium.27 Haloperidol often is the preferred treatment; a low-dose oral form is recommended for older patients who exhibit severe agitation because there is less risk of QT prolongation compared to IV administration.28
Second-generation antipsychotics (eg, risperidone, olanzapine, and quetiapine) are increasingly used due to their lower risk for adverse extrapyramidal symptoms, which are common in older patients.29-31 Despite this, increasing data show that morbidity with these agents may be underestimated, and the risks of adverse effects may vary among the medications in this class.32
For hypoactive or mixed delirium, nonpharmacologic interventions should be the mainstay of treatment. When medications are used, they should be used to target the underlying etiology of delirium (eg, treating a urinary tract infection with an antibiotic).33
A few final words about medication use for delirium ... Most medications that modify symptoms of delirium can actually prolong the delirium.33 Therefore, it's important to carefully consider the balance between effectively managing symptoms and causing adverse effects. Because older adults have increased sensitivity to medications, always start with small dosages and titrate to effect.34 Benzodiazepines and other hypnotics should be avoided in older patients, except when treating alcohol or benzodiazepine withdrawal.35
CASE › Mr. Q’s postop delirium screen is positive, and assessment for underlying causes reveals that he is suffering from postoperative pain and is constipated. Due to roommate noise and insomnia, he is transferred to a private room, where quiet times are observed. He receives oxycodone 5 mg every 4 hours for his pain and senna 30 mg at bedtime and a bisacodyl rectal suppository 10 mg/d for constipation. After 3 days Mr. Q’s postop pain and delirium resolves, and he is discharged home.
CORRESPONDENCE
Jackson Ng, MD, Teresa Lang Research Center, New York Hospital Queens, 56-45 Main St., Flushing, NY 11355; [email protected]
1. American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220:136-148.
2. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
3. O’Keeffe ST, Ní Chonchubhair A. Postoperative delirium in the elderly. Br J Anaesth. 1994;73:673-687.
4. Mangnall LT, Gallagher R, Stein-Parbury J. Postoperative delirium after colorectal surgery in older patients. Am J Crit Care. 2011;20:45-55.
5. American Geriatrics Society. American Geriatrics Society Clinical Practice Guideline for Postoperative Delirium in Older Adults: November 2014. American Geriatrics Society Web site. Available at: http://geriatricscareonline.org/ProductAbstract/americangeriatrics-society-clinical-practice-guideline-for-postoperativedelirium-in-older-adults/CL018. Accessed April 7, 2015.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing: 2013.
7. Potter J, George J; Guideline Development Group. The prevention, diagnosis and management of delirium in older people: concise guidelines. Clin Med. 2006;6:303-308.
8. Alzheimer’s Association. Cognitive Assessment Toolkit: A guide to detect cognitive impairment quickly and efficiently during the Medicare Annual Wellness Visit. 1999. Alzheimer’s Association Web site. Available at: http://www.alz.org/documents_custom/The%20Cognitive%20Assessment%20Toolkit%20Copy_v1.pdf. Accessed April 6, 2015.
9. The Hospital Elder Life Program. Hospital Elder Life Program (HELP) for Prevention of Delirium. The Hospital Elder Life Program Web site. Available at: http://www.hospitalelderlifeprogram.org. Accessed April 9, 2015.
10. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
11. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823-830.
12. Pisani MA, Araujo KL, Van Ness PH, et al. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121.
13. Simon L, Jewell N, Brokel J. Management of acute delirium in hospitalized elderly: a process improvement project. Geriatr Nurs. 1997;18:150-154.
14. Fish DN. Treatment of delirium in the critically ill patient. Clin Pharm. 1991;10:456-466.
15. Böhner H, Hummel TC, Habel U, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238:149-156.
16. Nordgaard J, Sass LA, Parnas J. The psychiatric interview: validity, structure, and subjectivity. Eur Arch Psychiatry Clin Neurosci. 2013;263:353-364.
17. Robinson TN, Eiseman B. Postoperative delirium in the elderly: diagnosis and management. Clin Interven Aging. 2008;3:351-355.
18. Demeure MJ, Fain MJ. The elderly surgical patient and postoperative delirium. J Am Coll Surg. 2006;203:752-757.
19. Ghandour A, Saab R, Mehr DR. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60:726-734.
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852-857.
21. Gaudreau JD, Gagnon P, Roy MA, et al. Association between psychoactive medications and delirium in hospitalized patients: a critical review. Psychosomatics. 2005;46:302-316.
22. Tune L, Carr S, Cooper T, et al. Association of anticholinergic activity of prescribed medications with postoperative delirium. J Neuropsychiatry Clin Neurosci. 1993;5:208-210.
23. Hitzeman N, Belsky K. Appropriate use of polypharmacy for older patients. Am Fam Physician. 2013;87:483-484.
24. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
25. Ross DD, Alexander CS. Management of common symptoms in terminally ill patients: Part II. Constipation, delirium, and dyspnea. Am Fam Physician. 2001;64:1019-1027.
26. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1-20.
27. Mantz J, Hemmings HC, Boddaert J. Case scenario: postoperative delirium in elderly surgical patients. Anesthesiology. 2010;112:189-195.
28. Gleason OC. Delirium. Am Fam Physician. 2003;67:1027-1034.
29. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol. 2004;19:125-127.
30. Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics. 2002;43:171-174.
31. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444-449.
32. Kohen I, Lester PE, Lam S. Antipsychotic treatments for the elderly: efficacy and safety of aripiprazole. Neuropsychiatr Dis Treat. 2010;6:47-58.
33. Farrell TW, Dosa D. The assessment and management of hypoactive delirium. Geriatrics for the Practicing Physician. 2007;90:393-395.
34. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
35. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80:388-393.
› Conduct a baseline cognitive assessment during your patient’s routine visits and preoperative assessments to gauge his or her risk for delirium. A
› Work with the hospital team to implement nonpharmacologic interventions, such as reorienting the patient to day and time and avoiding sensory deprivation, as an initial treatment for delirium. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Mark Q, age 80, is admitted to the hospital to undergo hemicolectomy for colon cancer. His medical history includes hypertension, benign prostatic hyperplasia, and colon cancer. He did well immediately postop, but when you make morning rounds the day after his surgery, you notice that he is confused and agitated. Mr. Q’s chart reveals that earlier that morning, he pulled out his Foley catheter and intravenous (IV) line when his nurse declined his request to walk him to the bathroom.
How would you proceed?
Up to 50% of older adults who undergo surgical procedures develop delirium—a disturbance in attention and awareness accompanied by changes in cognition.1 Older adults are at heightened risk for this postoperative complication for several reasons. For one thing, older patients have a reduced capacity for homeostatic regulation when they undergo anesthesia and surgery.2 For another, age-related changes in brain neurochemistry and drug metabolism increase the likelihood of adverse drug effects, including those that could precipitate delirirum.3
Although postop delirium is a common complication in older patients, it sometimes goes unrecognized. Missed or delayed diagnosis of delirium can result in patients exhibiting behaviors that can compromise their safety, delay recuperation, and result in longer hospital stays, a greater financial burden, and increased morbidity and mortality.4 The American Geriatric Society recently published clinical guidelines and a best practices statement for preventing and treating postop delirium in patients ages >65 years.1,5 This article describes steps family physicians can take to assess their patients’ risk of delirium before they undergo surgery, and to recognize and treat delirium in the postop period.
Defining delirium
According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), the criteria for delirium are:6
A. A disturbance in attention (ie, reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment).
B. The disturbance develops over a short time (usually hours to a few days), represents a change from baseline attention and awareness, and tends to fluctuate in severity during the course of a day.
C. An additional disturbance in cognition (eg, memory deficits, disorientation, language, visuospatial ability, perception).
D. The disturbances in attention, awareness, and cognition aren’t better explained by another preexisting or evolving neurocognitive disorder and don’t occur in the context of a severely reduced level of arousal.
E. History, physical, or laboratory findings show that the disturbance is caused by the direct physiologic consequences of a general medical condition, substance intoxication/withdrawal, exposure to a toxin, or multiple etiologies.
The 3 subtypes of delirium are based on patients’ psychomotor activity.7 In hyperactive delirium, patients exhibit heightened arousal, restlessness, agitation, hallucinations, and inappropriate behavior. Hypoactive delirium is characterized by lethargy, reduced motor activity, incoherent speech, and lack of interest. Mixed delirium consists of a combination of hyperactive and hypoactive signs and symptoms.
Gauge risk before patients undergo surgery
Family physicians can assess their patients’ risk for developing delirium by conducting baseline screening during routine office visits as well as during preoperative evaluations. Factors that increase postop delirium risk include:1
• age >65 years
• dementia
• poor vision
• decreased hearing
• severe illness
• infection.
Routine cognitive screening can be done easily and efficiently using readily available tools such as the Alzheimer Association’s Cognitive Assessment Toolkit.8 This toolkit includes 3 brief, validated screening tools to identify patients with probable cognitive impairment: the General Practitioner Assessment of Cognition, the Memory Impairment Screen, and the Mini-Cog.
If preop screening indicates that the patient is at increased risk for delirium, the family physician should work with hospital’s interdisciplinary teams to institute prevention measures, such as the Hospital Elder Life Program (HELP).9 This program offers a structured curriculum for instructing volunteers to deliver daily orientation, early mobilization, feeding assistance, therapeutic activities, and other measures to help prevent delirium.
Prompt screening after surgery is essential, too
In addition to preop delirium risk assessment, all patients who undergo surgery should receive daily delirium screening during the first postoperative week. The Confusion Assessment Method (CAM) is a quick screening tool for assessing a patient’s level of arousal and consciousness.10 Based on the results of 7 high-quality studies (N=1071), CAM has a sensitivity of 94% (95% confidence interval [CI], 91%-97%) and specificity of 89% (95% CI, 85%-94%).11,12
Feature 1 of CAM, “Acute onset and fluctuating course,” requires that you compare the patient’s current mental status to his or her pre-hospital baseline mental status; the baseline status should be obtained from a family member, caretaker, or clinician who has observed the patient over time.10 This is intended to determine if the patient has experienced an acute change in mental status (eg, attention, orientation, cognition), usually over the course of hours to days.10 Feature 2, “Inattention,” is used to determine if the patient has a reduced ability to maintain attention to external stimuli and to appropriately shift attention to new external stimuli, and if the patient is unaware or out of touch with the environment.10 Feature 3, “Disorganized thinking,” is used to assess the patient’s organization of thought as expressed by speech or writing. Disorganized thinking typically manifests as rambling and irrelevant or incoherent speech.10 Feature 4, “Altered level of consciousness,” is used to rate the patient’s alertness level.10
A positive screen for delirium requires the presence of Feature 1 (acute onset and/or fluctuation) and Feature 2, plus either Feature 3 or Feature 4.
Is delirium—or something else—at work?
If an older adult is exhibiting cognitive and/or behavioral disturbances after undergoing surgery, it’s important to discern if these manifestations are the result of delirium, a preexisting psychiatric disorder, or some other cause if the patient has a clear sensorium (ALGORITHM).6,13,14
Delirium. If a patient’s CAM screen suggests delirium, conduct a thorough assessment for the signs and symptoms of delirium to determine if the patient meets DSM-5 criteria for the diagnosis.1 In order to avoid missing hypoactive, subtle, or atypical cases of delirium, conduct a thorough medical record and medications review, and gather assessments from the nursing staff and other team members regarding the patient’s behavior.
Preexisting psychiatric disorder. It’s important to differentiate psychiatric symptoms from those of a superimposed delirium.13 Because patients with preoperative depressive symptoms may be at increased risk for postop delirium, pre-surgical psychiatric evaluations are important for identifying even subtle psychopathological symptoms.15 (The psychiatric interview is the gold standard for diagnosis.16) For patients who have an established psychiatric diagnosis, consider consulting with the psychiatrist who is managing the patient’s psychiatric care.13
Other causes. If a patient who is exhibiting postop cognitive and/or behavioral disturbances has a reasonably accurate memory and a correct orientation for time, place, and person, interviews with the patient and caregivers (along with the psychiatric interview) will likely reveal potential causes for the behavioral problems.13
Is the patient suffering from dehydration? Drug withdrawal?
Assessment for an underlying organic cause must be performed because specific treatment for the underlying diagnosis may improve delirium.17 Common causes include hypoxia, infection, dehydration, acute metabolic disturbance, endocrinopathies, cardiac or vascular disorders, and drug withdrawal.13 An appropriate diagnostic work-up might consist of serum urea, glucose, electrolytes, liver function tests, arterial blood gas analyses, urinalysis, nutritional evaluation, electrocardiogram, and a complete blood count.
Ask patients about their use of alcohol and benzodiazepines, and consider alcohol or drug withdrawal as potential etiologies.18 Patients with delirium should also be assessed for iatrogenic hospital-related factors that could be causing or contributing to the condition, such as immobilization or malnutrition.13
Medications are a common culprit: Approximately 40% of cases of delirium are related to medication use.18 Commonly used postop medications such as analgesics, sedatives, proton pump inhibitors, and others can cause delirium.19 Carefully review the patient’s medication list.13 Medication-induced delirium is influenced by the number of medications taken (generally >3),20 the use of psychoactive medications,21 and the specific agent's anticholinergic potential.22 The 2012 updated Beers Criteria (American Geriatrics Society) is a useful resource for determining if “inappropriate polypharmacy” is the cause of postop delirium.23
Inadequate pain control. In a multisite trial,24 patients who received <10 mg/d of parenteral morphine sulfate equivalents were more likely to develop delirium than patients who received more analgesia. In cognitively intact patients, severe pain significantly increased the risk of delirium. With the exception of meperidine, opioids do not precipitate delirium in patients with acute pain.24 Not treating pain or administering very low—or excessively high—doses of opioids is associated with an increased risk of delirium for both cognitively intact and impaired patients.24
Constipation can contribute to the development of delirium.25 After surgery, patients tend to be less mobile and may be receiving medications that can cause constipation, such as opioids, iron, calcium, and channel blockers. Preventing and treating constipation in postop patients can reduce delirium risk.25
Begin treatment with nonpharmacologic measures
Regardless of whether a patient suffers from hyperactive, hypoactive, or mixed delirium, nonpharmacologic interventions are firstline treatment.19 Such interventions can help patients develop a sense of control over their environment, which can help relieve agitation.13 Because environmental shifts contribute to the development of delirium, avoiding transfers and securing a single room can be helpful.19 Patients with delirium have altered perceptions, and may view normal objects and routine clinician actions as harmful and threatening. Therefore, it is helpful to avoid sensory deprivation by making sure patients have access to their eyeglasses and hearing aids, and to provide nonthreatening cognitive/environmental stimulation.1,13,19 Patients should be encouraged to resume walking as soon as possible.1,19 Other nonpharmacologic interventions are listed in the TABLE.1,13,19
Safety issues must also be addressed.17 Patients with mixed or hyperactive delirium may become agitated, which can lead them to pull tubes, drains, or lines, as occurred with Mr. Q. Patients with hypoactive delirium may be prone to wandering, or receive less attention due to their hypoactive state.17 All patients with delirium are at risk of falls.
Patients should be evaluated for these risks to determine whether assigning a "sitter" or transfer to a stepdown unit or intensive care unit is warranted.17 Restraints are not recommended because they can exacerbate delirium and lead to injuries.26
Pharmacologic treatment should be reserved for patients whose behavior compromises their safety, and implemented only when the cause of the delirium is known. The primary objectives of drug therapy are to achieve and maintain safe and rapid behavioral control so the patient can receive necessary medical care, and to enhance functional recovery.14 The choice of a specific medication is individualized and depends on each patient’s clinical condition.14
For a patient with hyperactive delirium, an antipsychotic typically is the treatment of choice because these medications are dopamine receptor antagonists, and excessive dopamine transmission has been implicated in this type of delirium.27 Haloperidol often is the preferred treatment; a low-dose oral form is recommended for older patients who exhibit severe agitation because there is less risk of QT prolongation compared to IV administration.28
Second-generation antipsychotics (eg, risperidone, olanzapine, and quetiapine) are increasingly used due to their lower risk for adverse extrapyramidal symptoms, which are common in older patients.29-31 Despite this, increasing data show that morbidity with these agents may be underestimated, and the risks of adverse effects may vary among the medications in this class.32
For hypoactive or mixed delirium, nonpharmacologic interventions should be the mainstay of treatment. When medications are used, they should be used to target the underlying etiology of delirium (eg, treating a urinary tract infection with an antibiotic).33
A few final words about medication use for delirium ... Most medications that modify symptoms of delirium can actually prolong the delirium.33 Therefore, it's important to carefully consider the balance between effectively managing symptoms and causing adverse effects. Because older adults have increased sensitivity to medications, always start with small dosages and titrate to effect.34 Benzodiazepines and other hypnotics should be avoided in older patients, except when treating alcohol or benzodiazepine withdrawal.35
CASE › Mr. Q’s postop delirium screen is positive, and assessment for underlying causes reveals that he is suffering from postoperative pain and is constipated. Due to roommate noise and insomnia, he is transferred to a private room, where quiet times are observed. He receives oxycodone 5 mg every 4 hours for his pain and senna 30 mg at bedtime and a bisacodyl rectal suppository 10 mg/d for constipation. After 3 days Mr. Q’s postop pain and delirium resolves, and he is discharged home.
CORRESPONDENCE
Jackson Ng, MD, Teresa Lang Research Center, New York Hospital Queens, 56-45 Main St., Flushing, NY 11355; [email protected]
› Conduct a baseline cognitive assessment during your patient’s routine visits and preoperative assessments to gauge his or her risk for delirium. A
› Work with the hospital team to implement nonpharmacologic interventions, such as reorienting the patient to day and time and avoiding sensory deprivation, as an initial treatment for delirium. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Mark Q, age 80, is admitted to the hospital to undergo hemicolectomy for colon cancer. His medical history includes hypertension, benign prostatic hyperplasia, and colon cancer. He did well immediately postop, but when you make morning rounds the day after his surgery, you notice that he is confused and agitated. Mr. Q’s chart reveals that earlier that morning, he pulled out his Foley catheter and intravenous (IV) line when his nurse declined his request to walk him to the bathroom.
How would you proceed?
Up to 50% of older adults who undergo surgical procedures develop delirium—a disturbance in attention and awareness accompanied by changes in cognition.1 Older adults are at heightened risk for this postoperative complication for several reasons. For one thing, older patients have a reduced capacity for homeostatic regulation when they undergo anesthesia and surgery.2 For another, age-related changes in brain neurochemistry and drug metabolism increase the likelihood of adverse drug effects, including those that could precipitate delirirum.3
Although postop delirium is a common complication in older patients, it sometimes goes unrecognized. Missed or delayed diagnosis of delirium can result in patients exhibiting behaviors that can compromise their safety, delay recuperation, and result in longer hospital stays, a greater financial burden, and increased morbidity and mortality.4 The American Geriatric Society recently published clinical guidelines and a best practices statement for preventing and treating postop delirium in patients ages >65 years.1,5 This article describes steps family physicians can take to assess their patients’ risk of delirium before they undergo surgery, and to recognize and treat delirium in the postop period.
Defining delirium
According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), the criteria for delirium are:6
A. A disturbance in attention (ie, reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment).
B. The disturbance develops over a short time (usually hours to a few days), represents a change from baseline attention and awareness, and tends to fluctuate in severity during the course of a day.
C. An additional disturbance in cognition (eg, memory deficits, disorientation, language, visuospatial ability, perception).
D. The disturbances in attention, awareness, and cognition aren’t better explained by another preexisting or evolving neurocognitive disorder and don’t occur in the context of a severely reduced level of arousal.
E. History, physical, or laboratory findings show that the disturbance is caused by the direct physiologic consequences of a general medical condition, substance intoxication/withdrawal, exposure to a toxin, or multiple etiologies.
The 3 subtypes of delirium are based on patients’ psychomotor activity.7 In hyperactive delirium, patients exhibit heightened arousal, restlessness, agitation, hallucinations, and inappropriate behavior. Hypoactive delirium is characterized by lethargy, reduced motor activity, incoherent speech, and lack of interest. Mixed delirium consists of a combination of hyperactive and hypoactive signs and symptoms.
Gauge risk before patients undergo surgery
Family physicians can assess their patients’ risk for developing delirium by conducting baseline screening during routine office visits as well as during preoperative evaluations. Factors that increase postop delirium risk include:1
• age >65 years
• dementia
• poor vision
• decreased hearing
• severe illness
• infection.
Routine cognitive screening can be done easily and efficiently using readily available tools such as the Alzheimer Association’s Cognitive Assessment Toolkit.8 This toolkit includes 3 brief, validated screening tools to identify patients with probable cognitive impairment: the General Practitioner Assessment of Cognition, the Memory Impairment Screen, and the Mini-Cog.
If preop screening indicates that the patient is at increased risk for delirium, the family physician should work with hospital’s interdisciplinary teams to institute prevention measures, such as the Hospital Elder Life Program (HELP).9 This program offers a structured curriculum for instructing volunteers to deliver daily orientation, early mobilization, feeding assistance, therapeutic activities, and other measures to help prevent delirium.
Prompt screening after surgery is essential, too
In addition to preop delirium risk assessment, all patients who undergo surgery should receive daily delirium screening during the first postoperative week. The Confusion Assessment Method (CAM) is a quick screening tool for assessing a patient’s level of arousal and consciousness.10 Based on the results of 7 high-quality studies (N=1071), CAM has a sensitivity of 94% (95% confidence interval [CI], 91%-97%) and specificity of 89% (95% CI, 85%-94%).11,12
Feature 1 of CAM, “Acute onset and fluctuating course,” requires that you compare the patient’s current mental status to his or her pre-hospital baseline mental status; the baseline status should be obtained from a family member, caretaker, or clinician who has observed the patient over time.10 This is intended to determine if the patient has experienced an acute change in mental status (eg, attention, orientation, cognition), usually over the course of hours to days.10 Feature 2, “Inattention,” is used to determine if the patient has a reduced ability to maintain attention to external stimuli and to appropriately shift attention to new external stimuli, and if the patient is unaware or out of touch with the environment.10 Feature 3, “Disorganized thinking,” is used to assess the patient’s organization of thought as expressed by speech or writing. Disorganized thinking typically manifests as rambling and irrelevant or incoherent speech.10 Feature 4, “Altered level of consciousness,” is used to rate the patient’s alertness level.10
A positive screen for delirium requires the presence of Feature 1 (acute onset and/or fluctuation) and Feature 2, plus either Feature 3 or Feature 4.
Is delirium—or something else—at work?
If an older adult is exhibiting cognitive and/or behavioral disturbances after undergoing surgery, it’s important to discern if these manifestations are the result of delirium, a preexisting psychiatric disorder, or some other cause if the patient has a clear sensorium (ALGORITHM).6,13,14
Delirium. If a patient’s CAM screen suggests delirium, conduct a thorough assessment for the signs and symptoms of delirium to determine if the patient meets DSM-5 criteria for the diagnosis.1 In order to avoid missing hypoactive, subtle, or atypical cases of delirium, conduct a thorough medical record and medications review, and gather assessments from the nursing staff and other team members regarding the patient’s behavior.
Preexisting psychiatric disorder. It’s important to differentiate psychiatric symptoms from those of a superimposed delirium.13 Because patients with preoperative depressive symptoms may be at increased risk for postop delirium, pre-surgical psychiatric evaluations are important for identifying even subtle psychopathological symptoms.15 (The psychiatric interview is the gold standard for diagnosis.16) For patients who have an established psychiatric diagnosis, consider consulting with the psychiatrist who is managing the patient’s psychiatric care.13
Other causes. If a patient who is exhibiting postop cognitive and/or behavioral disturbances has a reasonably accurate memory and a correct orientation for time, place, and person, interviews with the patient and caregivers (along with the psychiatric interview) will likely reveal potential causes for the behavioral problems.13
Is the patient suffering from dehydration? Drug withdrawal?
Assessment for an underlying organic cause must be performed because specific treatment for the underlying diagnosis may improve delirium.17 Common causes include hypoxia, infection, dehydration, acute metabolic disturbance, endocrinopathies, cardiac or vascular disorders, and drug withdrawal.13 An appropriate diagnostic work-up might consist of serum urea, glucose, electrolytes, liver function tests, arterial blood gas analyses, urinalysis, nutritional evaluation, electrocardiogram, and a complete blood count.
Ask patients about their use of alcohol and benzodiazepines, and consider alcohol or drug withdrawal as potential etiologies.18 Patients with delirium should also be assessed for iatrogenic hospital-related factors that could be causing or contributing to the condition, such as immobilization or malnutrition.13
Medications are a common culprit: Approximately 40% of cases of delirium are related to medication use.18 Commonly used postop medications such as analgesics, sedatives, proton pump inhibitors, and others can cause delirium.19 Carefully review the patient’s medication list.13 Medication-induced delirium is influenced by the number of medications taken (generally >3),20 the use of psychoactive medications,21 and the specific agent's anticholinergic potential.22 The 2012 updated Beers Criteria (American Geriatrics Society) is a useful resource for determining if “inappropriate polypharmacy” is the cause of postop delirium.23
Inadequate pain control. In a multisite trial,24 patients who received <10 mg/d of parenteral morphine sulfate equivalents were more likely to develop delirium than patients who received more analgesia. In cognitively intact patients, severe pain significantly increased the risk of delirium. With the exception of meperidine, opioids do not precipitate delirium in patients with acute pain.24 Not treating pain or administering very low—or excessively high—doses of opioids is associated with an increased risk of delirium for both cognitively intact and impaired patients.24
Constipation can contribute to the development of delirium.25 After surgery, patients tend to be less mobile and may be receiving medications that can cause constipation, such as opioids, iron, calcium, and channel blockers. Preventing and treating constipation in postop patients can reduce delirium risk.25
Begin treatment with nonpharmacologic measures
Regardless of whether a patient suffers from hyperactive, hypoactive, or mixed delirium, nonpharmacologic interventions are firstline treatment.19 Such interventions can help patients develop a sense of control over their environment, which can help relieve agitation.13 Because environmental shifts contribute to the development of delirium, avoiding transfers and securing a single room can be helpful.19 Patients with delirium have altered perceptions, and may view normal objects and routine clinician actions as harmful and threatening. Therefore, it is helpful to avoid sensory deprivation by making sure patients have access to their eyeglasses and hearing aids, and to provide nonthreatening cognitive/environmental stimulation.1,13,19 Patients should be encouraged to resume walking as soon as possible.1,19 Other nonpharmacologic interventions are listed in the TABLE.1,13,19
Safety issues must also be addressed.17 Patients with mixed or hyperactive delirium may become agitated, which can lead them to pull tubes, drains, or lines, as occurred with Mr. Q. Patients with hypoactive delirium may be prone to wandering, or receive less attention due to their hypoactive state.17 All patients with delirium are at risk of falls.
Patients should be evaluated for these risks to determine whether assigning a "sitter" or transfer to a stepdown unit or intensive care unit is warranted.17 Restraints are not recommended because they can exacerbate delirium and lead to injuries.26
Pharmacologic treatment should be reserved for patients whose behavior compromises their safety, and implemented only when the cause of the delirium is known. The primary objectives of drug therapy are to achieve and maintain safe and rapid behavioral control so the patient can receive necessary medical care, and to enhance functional recovery.14 The choice of a specific medication is individualized and depends on each patient’s clinical condition.14
For a patient with hyperactive delirium, an antipsychotic typically is the treatment of choice because these medications are dopamine receptor antagonists, and excessive dopamine transmission has been implicated in this type of delirium.27 Haloperidol often is the preferred treatment; a low-dose oral form is recommended for older patients who exhibit severe agitation because there is less risk of QT prolongation compared to IV administration.28
Second-generation antipsychotics (eg, risperidone, olanzapine, and quetiapine) are increasingly used due to their lower risk for adverse extrapyramidal symptoms, which are common in older patients.29-31 Despite this, increasing data show that morbidity with these agents may be underestimated, and the risks of adverse effects may vary among the medications in this class.32
For hypoactive or mixed delirium, nonpharmacologic interventions should be the mainstay of treatment. When medications are used, they should be used to target the underlying etiology of delirium (eg, treating a urinary tract infection with an antibiotic).33
A few final words about medication use for delirium ... Most medications that modify symptoms of delirium can actually prolong the delirium.33 Therefore, it's important to carefully consider the balance between effectively managing symptoms and causing adverse effects. Because older adults have increased sensitivity to medications, always start with small dosages and titrate to effect.34 Benzodiazepines and other hypnotics should be avoided in older patients, except when treating alcohol or benzodiazepine withdrawal.35
CASE › Mr. Q’s postop delirium screen is positive, and assessment for underlying causes reveals that he is suffering from postoperative pain and is constipated. Due to roommate noise and insomnia, he is transferred to a private room, where quiet times are observed. He receives oxycodone 5 mg every 4 hours for his pain and senna 30 mg at bedtime and a bisacodyl rectal suppository 10 mg/d for constipation. After 3 days Mr. Q’s postop pain and delirium resolves, and he is discharged home.
CORRESPONDENCE
Jackson Ng, MD, Teresa Lang Research Center, New York Hospital Queens, 56-45 Main St., Flushing, NY 11355; [email protected]
1. American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220:136-148.
2. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
3. O’Keeffe ST, Ní Chonchubhair A. Postoperative delirium in the elderly. Br J Anaesth. 1994;73:673-687.
4. Mangnall LT, Gallagher R, Stein-Parbury J. Postoperative delirium after colorectal surgery in older patients. Am J Crit Care. 2011;20:45-55.
5. American Geriatrics Society. American Geriatrics Society Clinical Practice Guideline for Postoperative Delirium in Older Adults: November 2014. American Geriatrics Society Web site. Available at: http://geriatricscareonline.org/ProductAbstract/americangeriatrics-society-clinical-practice-guideline-for-postoperativedelirium-in-older-adults/CL018. Accessed April 7, 2015.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing: 2013.
7. Potter J, George J; Guideline Development Group. The prevention, diagnosis and management of delirium in older people: concise guidelines. Clin Med. 2006;6:303-308.
8. Alzheimer’s Association. Cognitive Assessment Toolkit: A guide to detect cognitive impairment quickly and efficiently during the Medicare Annual Wellness Visit. 1999. Alzheimer’s Association Web site. Available at: http://www.alz.org/documents_custom/The%20Cognitive%20Assessment%20Toolkit%20Copy_v1.pdf. Accessed April 6, 2015.
9. The Hospital Elder Life Program. Hospital Elder Life Program (HELP) for Prevention of Delirium. The Hospital Elder Life Program Web site. Available at: http://www.hospitalelderlifeprogram.org. Accessed April 9, 2015.
10. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
11. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823-830.
12. Pisani MA, Araujo KL, Van Ness PH, et al. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121.
13. Simon L, Jewell N, Brokel J. Management of acute delirium in hospitalized elderly: a process improvement project. Geriatr Nurs. 1997;18:150-154.
14. Fish DN. Treatment of delirium in the critically ill patient. Clin Pharm. 1991;10:456-466.
15. Böhner H, Hummel TC, Habel U, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238:149-156.
16. Nordgaard J, Sass LA, Parnas J. The psychiatric interview: validity, structure, and subjectivity. Eur Arch Psychiatry Clin Neurosci. 2013;263:353-364.
17. Robinson TN, Eiseman B. Postoperative delirium in the elderly: diagnosis and management. Clin Interven Aging. 2008;3:351-355.
18. Demeure MJ, Fain MJ. The elderly surgical patient and postoperative delirium. J Am Coll Surg. 2006;203:752-757.
19. Ghandour A, Saab R, Mehr DR. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60:726-734.
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852-857.
21. Gaudreau JD, Gagnon P, Roy MA, et al. Association between psychoactive medications and delirium in hospitalized patients: a critical review. Psychosomatics. 2005;46:302-316.
22. Tune L, Carr S, Cooper T, et al. Association of anticholinergic activity of prescribed medications with postoperative delirium. J Neuropsychiatry Clin Neurosci. 1993;5:208-210.
23. Hitzeman N, Belsky K. Appropriate use of polypharmacy for older patients. Am Fam Physician. 2013;87:483-484.
24. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
25. Ross DD, Alexander CS. Management of common symptoms in terminally ill patients: Part II. Constipation, delirium, and dyspnea. Am Fam Physician. 2001;64:1019-1027.
26. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1-20.
27. Mantz J, Hemmings HC, Boddaert J. Case scenario: postoperative delirium in elderly surgical patients. Anesthesiology. 2010;112:189-195.
28. Gleason OC. Delirium. Am Fam Physician. 2003;67:1027-1034.
29. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol. 2004;19:125-127.
30. Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics. 2002;43:171-174.
31. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444-449.
32. Kohen I, Lester PE, Lam S. Antipsychotic treatments for the elderly: efficacy and safety of aripiprazole. Neuropsychiatr Dis Treat. 2010;6:47-58.
33. Farrell TW, Dosa D. The assessment and management of hypoactive delirium. Geriatrics for the Practicing Physician. 2007;90:393-395.
34. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
35. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80:388-393.
1. American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220:136-148.
2. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
3. O’Keeffe ST, Ní Chonchubhair A. Postoperative delirium in the elderly. Br J Anaesth. 1994;73:673-687.
4. Mangnall LT, Gallagher R, Stein-Parbury J. Postoperative delirium after colorectal surgery in older patients. Am J Crit Care. 2011;20:45-55.
5. American Geriatrics Society. American Geriatrics Society Clinical Practice Guideline for Postoperative Delirium in Older Adults: November 2014. American Geriatrics Society Web site. Available at: http://geriatricscareonline.org/ProductAbstract/americangeriatrics-society-clinical-practice-guideline-for-postoperativedelirium-in-older-adults/CL018. Accessed April 7, 2015.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing: 2013.
7. Potter J, George J; Guideline Development Group. The prevention, diagnosis and management of delirium in older people: concise guidelines. Clin Med. 2006;6:303-308.
8. Alzheimer’s Association. Cognitive Assessment Toolkit: A guide to detect cognitive impairment quickly and efficiently during the Medicare Annual Wellness Visit. 1999. Alzheimer’s Association Web site. Available at: http://www.alz.org/documents_custom/The%20Cognitive%20Assessment%20Toolkit%20Copy_v1.pdf. Accessed April 6, 2015.
9. The Hospital Elder Life Program. Hospital Elder Life Program (HELP) for Prevention of Delirium. The Hospital Elder Life Program Web site. Available at: http://www.hospitalelderlifeprogram.org. Accessed April 9, 2015.
10. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
11. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823-830.
12. Pisani MA, Araujo KL, Van Ness PH, et al. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121.
13. Simon L, Jewell N, Brokel J. Management of acute delirium in hospitalized elderly: a process improvement project. Geriatr Nurs. 1997;18:150-154.
14. Fish DN. Treatment of delirium in the critically ill patient. Clin Pharm. 1991;10:456-466.
15. Böhner H, Hummel TC, Habel U, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238:149-156.
16. Nordgaard J, Sass LA, Parnas J. The psychiatric interview: validity, structure, and subjectivity. Eur Arch Psychiatry Clin Neurosci. 2013;263:353-364.
17. Robinson TN, Eiseman B. Postoperative delirium in the elderly: diagnosis and management. Clin Interven Aging. 2008;3:351-355.
18. Demeure MJ, Fain MJ. The elderly surgical patient and postoperative delirium. J Am Coll Surg. 2006;203:752-757.
19. Ghandour A, Saab R, Mehr DR. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60:726-734.
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852-857.
21. Gaudreau JD, Gagnon P, Roy MA, et al. Association between psychoactive medications and delirium in hospitalized patients: a critical review. Psychosomatics. 2005;46:302-316.
22. Tune L, Carr S, Cooper T, et al. Association of anticholinergic activity of prescribed medications with postoperative delirium. J Neuropsychiatry Clin Neurosci. 1993;5:208-210.
23. Hitzeman N, Belsky K. Appropriate use of polypharmacy for older patients. Am Fam Physician. 2013;87:483-484.
24. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
25. Ross DD, Alexander CS. Management of common symptoms in terminally ill patients: Part II. Constipation, delirium, and dyspnea. Am Fam Physician. 2001;64:1019-1027.
26. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1-20.
27. Mantz J, Hemmings HC, Boddaert J. Case scenario: postoperative delirium in elderly surgical patients. Anesthesiology. 2010;112:189-195.
28. Gleason OC. Delirium. Am Fam Physician. 2003;67:1027-1034.
29. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol. 2004;19:125-127.
30. Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics. 2002;43:171-174.
31. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444-449.
32. Kohen I, Lester PE, Lam S. Antipsychotic treatments for the elderly: efficacy and safety of aripiprazole. Neuropsychiatr Dis Treat. 2010;6:47-58.
33. Farrell TW, Dosa D. The assessment and management of hypoactive delirium. Geriatrics for the Practicing Physician. 2007;90:393-395.
34. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
35. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80:388-393.
Accidental scratch—or a sign of self-cutting?
› Examine the forearms and legs of all patients ages 11 to 19 years as part of the routine health assessment, looking specifically for injuries that may be self-inflicted. C
› Make an immediate referral for outpatient psychotherapy for any patient with self-cutting behavior who admits to recent or current suicidal ideation or a plan. C
› Facilitate a direct transfer to the local emergency department for psychiatric evaluation for any patient with self-cutting behavior who admits to current suicidal intent. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice R, a 14-year-old student, comes to your office for a preparticipation exam in advance of volleyball season. During the exam, you note several scratches on her left forearm. When you ask about them, Alice tells you she scratched herself when she accidentally brushed up against some bushes while walking home from school. Her explanation strikes you as odd, given that it’s been rather cold out, and it seems likely that she would have been wearing a jacket.
If Alice were your patient, how would you proceed?
Few health disorders are as clouded in mystery as self-cutting behavior in adolescents. Self-cutting is often overlooked or undetected by the medical community.1 When examining an adolescent, a family physician (FP) may pay minimal attention to the patient’s forearms and legs, but such attention can provide clues to critical health information. Relatively minor injuries in a physically active adolescent might be easy to dismiss as “normal,” but knowing the types of injuries to look for—and what to ask your young patients—can help you identify injuries that are self-inflicted and intervene accordingly.
Being aware of self-cutting, understanding its potential sequelae, and having the skills necessary to develop an individualized treatment plan are essential tools for appropriately managing this behavior.2 Failure to recognize and address self-cutting in an adolescent has immediate consequences, such as the exacerbation of other psychiatric disorders or an increased risk of suicide. Potential longer-term consequences include an increased risk of premature death for adults who engaged in self-cutting as adolescents.3,4
For many young people, self-injury occurs only a few times, but the behavior may increase in frequency and severity when combined with other psychosocial factors.5 FPs can play a crucial role by identifying cutting behavior, providing medical treatment, educating patients and families about self-cutting, making an appropriate mental health referral, coordinating multidisciplinary collaboration, and, ultimately, supporting the patient and his or her family.
Cutting is a common form of self-harm
Some adolescents use sharp or rough objects to inflict injuries on their arms, legs, or other parts of their body. Individuals may cut, scratch, burn, abrade, or prick the skin repeatedly, often leaving scars. They may then attempt to hide the resulting injuries with clothing.6 Most self-cutting injuries are superficial, but can result in scarring. Severe injuries, such as lacerated tendons, penetrated major blood vessels, or disfiguring scars, are uncommon.
Because self-cutting behavior often remains private or intentionally hidden, its true incidence is unclear.6 However, self-cutting is not rare. In published literature, statistics for self-cutting often are grouped with those for other forms of non-suicidal self-injury (NSSI), including burning, hair-pulling, self-hitting, and self-poisoning. These self-harm behaviors have been reported in more than 10% of ninth graders7 and college students,8 and in up to 4% of adults.9 The lifetime prevalence of self-cutting is estimated to be 11.5%,10 and research suggests that the frequency of adolescent self-cutting may be increasing.5,11,12
Adolescent girls are 2 to 4 times more likely than adolescent boys to engage in NSSI.7,12 Girls primarily cut, scratch, or otherwise injure their skin, whereas boys more commonly hit or burn themselves, and inflict more injuries to the face, chest, and genitals.7,13 Most adolescents who self-cut do so only on their arms (67%).10
Not every patient who self-cuts has a psychiatric illness
Adolescents who cut themselves do not fit neatly within a typical profile.7,14,15 Self-harm in adolescents appears to be associated with a range of psychological factors, including internalizing disorders (eg, depression, anxiety, eating disorders), mood regulation difficulties (eg, impulsivity and related impulse control disorders, borderline personality traits/disorder), negative affect (eg, sadness, anxiousness, anger, stress, low self-esteem), and poor coping strategies (eg, avoidance, internalizing, substance use).14
The strong empirical relationship between psychological factors and self-harm has led many researchers and clinicians to view self-harm as a symptom of a psychiatric disorder.16 A study of psychiatric disorders among 44 adolescents (41 girls) with self-cutting behavior found a strong association with certain internalizing disorders: 63% had major depressive disorder, 37% suffered from anxiety, and 15% had an eating disorder.15 However, viewing self-cutting primarily as a manifestation of a psychiatric disorder doesn’t fully explain the behavior.
Self-harm behaviors occur across many disorders and are not unique to any single diagnosis.16 Moreover, evidence suggests that many (if not most) adolescents who self-harm do not fit the profile of psychiatric and social distress that has been well described in the literature.14,15 That is, many adolescents who self-harm may be categorized as psychologically “normal,” meaning they don’t meet the criteria for a diagnosis of depression, anxiety, or impulsivity.14,15
Being female is a strong predisposing factor for self-cutting.11 This may be because females have higher rates of depression and tend to internalize, whereas males tend to externalize and may underreport self-harm. Research suggests that modeling of self-cutting behavior by, for example, posting videos online may encourage other females to self-cut, and that contagion (ie, group cutting) is a factor.17 Self-injury is especially common in adolescent girls who have a history of physical abuse.18 In a study of patients with a history of cutting and suicidality, exposure to physical or sexual abuse, physical or emotional neglect, and chaotic family life during childhood and adolescence was associated with more frequent and more severe cutting.5,19
Cutting may help patients cope with emotional distress
Although adolescents may cut for many reasons, in general, the behavior is a coping strategy for affect regulation; cutting appears to displace emotional pain or relieve emotional blunting.2,16 Cutting provides an immediate—albeit unhealthy and temporary—method of coping. However, this is often followed by shame and low self-esteem, and the underlying emotional distress returns. Others may use self-cutting as a means of obtaining cathartic release, responding to peer pressure, or inflicting self-punishment.9 Adolescents with limited interpersonal skills may use cutting to affect relationships by, for example, communicating their distress to others and, in turn, eliciting sympathy, status, or camaraderie. If the adolescent who self-cuts interprets the resulting responses as positive, the cutting behavior is reinforced.20
The importance of the pain associated with self-injury is unclear. In an Internet survey of 128 adolescents who injured themselves, 43% reported the injuries often or always caused pain, whereas 25% said such injuries never caused pain.21 Some research suggests that self-inflicted trauma (including pain) may provide emotional relief by increasing serotonin levels, or may deliver desired euphoria by releasing endorphins.22
Self-cutting, suicide, and mortality risk. Suicide does not appear to be the intent or motivation of most adolescents who self-cut.11,12 Adolescents tend to cut themselves to “make life feel better,” not to end their life. However, the intent of self-cutting may change over time, and may lead adolescents to adopt more lethal forms of injury. In a study of adolescents receiving treatment for major depressive disorder, NSSI was found to be a strong predictor of suicidal behavior.23
Although self-harm and self-cutting occur more frequently in females, suicide is observed significantly more often in males and individuals with multiple self-harm episodes.3,24 Further, males who use analgesics to relieve the pain of cutting are at especially high risk for suicide.25
In general, individuals who self-harm have an increased risk of premature death.3,4 In a cohort study, more than 30,000 individuals with self-harm who presented to emergency departments in England had a mean loss of 31.4 years of life compared to the general population.4 Also, adolescent self-cutting is associated with adverse childhood experiences (eg, maltreatment), and these experiences are associated with early death in adults.26
A structured approach to assessment and care
If during the course of a physical examination you notice injuries such as cuts, scratches, burns, or rub marks, be especially suspicious of self-injury if they are located in areas of the body that the patient could easily reach. Also consider the possibility that the injuries may be a direct result of child abuse trauma. If you suspect physical or sexual abuse or neglect, federal law mandates you report such concerns to the appropriate state child protective services agency.
As you might expect, it’s important to use a nonjudgmental, empathic, and supportive approach when speaking to the patient about his or her motivation for cutting.5 A review of 74 studies found that attitudes of hospital staff, especially physicians, largely were negative toward patients who engaged in self-harm.27 One approach to talking to patients about self-cutting involves asking questions based on motivational interviewing techniques. (See “Talking to patients about self-harm: 5 questions to ask” below.5)
Be sure to document the location(s) and extent of the injury, and estimate the timeframe of the cutting based on the age of any scars. You’ll also need to treat the wounds and administer tetanus immunization, as appropriate.
Assess for additional risks, especially suicide. Such risks may include other behavioral issues (eg, alcohol or substance use, promiscuity, antisocial behavior), academic problems, or eating disorders. In addition, evaluate for prior and/or current mental health concerns, family dysfunction and conflict, and acute or chronic patient or family psychosocial stressors.
Assessment of suicidality should include direct queries about past, recent, and current suicidal ideation, intent, and plan. Further details on how to evaluate suicidality, including red flags to watch for, are available from the American Academy of Pediatrics at http://pediatrics.aappublications.org/content/105/4/871.full.pdf.
When evaluating a patient whose injuries might be self-inflicted, family physicians can use a subset of motivational interviewing (MI) techniques to promote a positive and supportive atmosphere for the patient, with the goal of making it easier for the patient to discuss self-injury.
Kerr et al5 suggests that family physicians can use a set of questions based on MI techniques to facilitate discussion of self-injury and prompt a patient to consider seeking help for his or her self-injury. Such questions might include:
1. What effect is self-cutting having on your life?
2. While it seems like self-cutting serves a function for you, what disadvantages are there if you continue to cut yourself?
3. What factors may motivate you to stop self-injuring right now?
4. How would your life be different right now if you were not self-cutting?
5. What do you think you would need in the way of help in order to stop self-cutting?
A patient who self-cuts and expresses the intent to commit suicide should be directly transferred to the emergency department for a psychiatric evaluation. A patient who admits he has been thinking about suicide or a suicide plan but does not state an intent to commit suicide should receive an immediate mental health referral to a psychotherapist or psychiatrist. Patients who have engaged in longstanding self-cutting should be referred to a therapist with experience in treating childhood trauma, especially if the patient has a history of behavioral or mental health disorders.12,28
The role of the family
Family members often will not have known about the cutting behavior. Family and caregivers should be educated about self-cutting (eg, its use as a coping strategy, the complexity of contributing factors), ways to provide a safe environment (eg, increased adult supervision, safeguarding of sharp objects), and the importance of mental health treatment. Positive family support is critical in addressing the patient’s self-cutting and underlying factors.
Determine if family intervention is needed. If family stressors, conflict, or dysfunction is identified as a contributing factor, recommend family counseling.
Several treatment options but few specifically for cutting
Many adolescents who self-cut want to stop cutting. In a survey of self-injured adolescents, 37% wanted to stop the behavior.14 However, even with treatment, cutting behavior often continues because cutting as a coping strategy may feel highly effective in the moment and can become addictive.29 Also, videos with explicit imagery of self-cutting are readily available on various Web sites and could normalize and reinforce the behavior.30
There are few evidence-based treatments for self-harm in general, let alone specifically for adolescent self-cutting.12,31 For adolescents with self-harm behaviors, individual cognitive behavioral therapy, dialectical behavioral therapy, group developmental therapy, multisystemic therapy, family intervention, psychotropic medication, and inpatient psychiatric treatment may help reduce risks and improve psychosocial functioning.12,31 Psychotropic medication has been shown to relieve psychiatric symptoms in patients who self-harm, but its effectiveness in reducing self-cutting behavior is unclear.12
CASE › After you speak to Alice with her parents out of the room, she admits that she had scratched her arms several times in the past few weeks because she felt stressed about her grades in certain classes. She says she’d done this scratching before as a coping mechanism, but never thought about suicide. With Alice’s permission, you discuss these incidents with her parents. You refer her to a psychotherapist to begin counseling, and ask that she return in 3 months so that you can monitor her progress.
CORRESPONDENCE
Jerry G. Jones, MD, University of Arkansas for Medical Sciences, College of Medicine, Department of Pediatrics, 1 Children’s Way, Slot 512-24A, Little Rock, AR 72202; [email protected]
1. Whitlock J, Muehlenkamp J, Purington A, et al. Nonsuicidal self-injury in a college population: general trends and sex differences. J Am Coll Health. 2011;59:691-698.
2. Shain BN; American Academy of Pediatrics Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2007;120:669-676.
3. Miller M, Hempstead K, Nguyen T, et al. Method choice in nonfatal self-harm as a predictor of subsequent episodes of self-harm and suicide: implications for clinical practice. Am J Public Health. 2013;103:e61-e68.
4. Bergen H, Hawton K, Walters K. Premature death after self-harm: a multicentre cohort study. Lancet. 2012;380:1568-1574.
5. Kerr PL, Muehlenkamp JJ, Turner JM. Nonsuicidal self-injury: a review of current research for family medicine and primary care physicians. J Am Board Fam Med. 2010;23:240-259.
6. Fordam K, Bailham D. Self-harm in young people. In: Beinart H, Kennedy P, Llewelyn S, eds. Clinical Psychology in Practice. West Sussex, England: BPS Blackwell; 2009:73-84.
7. Barrocas AL, Hankin BL, Young JF, et al. Rates of nonsuicidal self-injury in youth: age, sex, and behavioral methods in a community sample. Pediatrics. 2012;130:39-45.
8. Kuentzel JG, Arble E, Boutros N, et al. Nonsuicidal self-injury in an ethnically diverse college sample. Am J Orthopsychiatry. 2012;82:291-297.
9. Taylor JD. Cutting, piercing, and self-mutilation. In: Bryant CD, ed. Handbook of Deviant Behavior. New York, NY: Routledge; 2011:305-312.
10. Laukkanen E, Rissanen ML, Tolmunen T, et al. Adolescent self-cutting elsewhere than on the arms reveals more serious psychiatric symptoms. Eur Child Adolesc Psychiatry. 2013;22:501-510.
11. Hall B, Place M. Cutting to cope – a modern adolescent phenomenon. Child Care Health Dev. 2010;36:623-629.
12. Wood A. Self-harm in adolescents. Advances in Psychiatric Treatment. 2009;15:434-441.
13. Sornberger MJ, Heath NL, Toste JR, et al. Non suicidal self-injury and gender: patterns of prevalence, methods, and locations among adolescents. Suicide Life Threatening Behav. 2012;42:266-278.
14. Stanford S, Jones MP. Psychological subtyping finds pathological, impulsive, and “normal” groups among adolescents who self-harm. J Child Psychol Psychiatry. 2009;50:807-815.
15. Hintikka J, Tolmunen T, Rissanen ML, et al. Mental disorders in self-cutting adolescents. J Adolesc Health. 2009;44:464-467.
16. Nock MK. Why do people hurt themselves? New insights into the nature and functions of self-injury. Curr Dir Psychol Sci. 2009;18:78-83.
17. Hawton K, Harriss L, Rodham K. How adolescents who cut themselves differ from those who take overdoses. Eur Child Adolesc Psychiatry. 2010;19:513-523.
18. Swannell S, Martin G, Page A, et al. Child maltreatment, subsequent non-suicidal self-injury and the mediating role of dissociation, alexithymia and self-blame. Child Abuse Negl. 2012;36:572-584.
19. van der Kolk BA, Perry JC, Herman JL. Childhood origins of self-destructive behavior. Am J Psychiatry. 1991;148:1665-1671.
20. Brickell CM, Jellinek MS. Self-injury: why teens do it, how to help. March 2014. Contemporary Pediatrics Web site. Available at: http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/content/tags/borderline-personalitydisorder/self-injury-why-teens-do-it-how. Accessed March 4, 2015.
21. Murray CD, Warren A, Foxe J. An Internet survey of adolescent self-injuries. Australian e-Journal for the Advancement of Mental Health. 2005;4:7-9.
22. Fikke LT, Melinder A, Landro NI. The effects of acute tryptophan depletion on impulsivity and mood in adolescents engaging in non-suicidal self-injury. Hum Psychopharmacol. 2013;28:61-71.
23. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168:495-501.
24. Hawton K, Bergen H, Kapur N, et al. Repetition of self-harm and suicide following self-harm in children and adolescents: findings from the Multicentre Study of Self-harm in England. J Child Psychol Psychiatry. 2012;53:1212-1219.
25. Matsumoto T, Imamura F, Chiba Y, et al. Analgesia during self-cutting: clinical implications and the association with suicidal ideation. Psychiatry Clin Neurosci. 2008;62:355-358.
26. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) study. Am J Prev Med. 1998;14:245-258.
27. Saunders KE, Hawton K, Fortune S, et al. Attitudes and knowledge of clinical staff regarding people who self-harm: a systematic review. J Affect Disord. 2012;139:205-216.
28. Harrington R, Pickles A, Aglan A, et al. Early adult outcomes of adolescents who deliberately poisoned themselves. J Am Acad Child Adolesc Psychiatry. 2006;45:337-345.
29. Puskar KR, Bernardo L, Hatam M, et al. Self-cutting behaviors in adolescents. J Emerg Nurs. 2006;32:444-446.
30. Lewis SP, Heath NL, St. Denis JM, et al. The scope of nonsuicidal self-injury on YouTube. Pediatrics. 2011;127:e552-e557.
31. Gonzales AH, Bergstrom L. Adolescent non-suicidal self-injury (NSSI) interventions. J Child Adolesc Psychiatr Nurs. 2013;26:124-130.
› Examine the forearms and legs of all patients ages 11 to 19 years as part of the routine health assessment, looking specifically for injuries that may be self-inflicted. C
› Make an immediate referral for outpatient psychotherapy for any patient with self-cutting behavior who admits to recent or current suicidal ideation or a plan. C
› Facilitate a direct transfer to the local emergency department for psychiatric evaluation for any patient with self-cutting behavior who admits to current suicidal intent. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice R, a 14-year-old student, comes to your office for a preparticipation exam in advance of volleyball season. During the exam, you note several scratches on her left forearm. When you ask about them, Alice tells you she scratched herself when she accidentally brushed up against some bushes while walking home from school. Her explanation strikes you as odd, given that it’s been rather cold out, and it seems likely that she would have been wearing a jacket.
If Alice were your patient, how would you proceed?
Few health disorders are as clouded in mystery as self-cutting behavior in adolescents. Self-cutting is often overlooked or undetected by the medical community.1 When examining an adolescent, a family physician (FP) may pay minimal attention to the patient’s forearms and legs, but such attention can provide clues to critical health information. Relatively minor injuries in a physically active adolescent might be easy to dismiss as “normal,” but knowing the types of injuries to look for—and what to ask your young patients—can help you identify injuries that are self-inflicted and intervene accordingly.
Being aware of self-cutting, understanding its potential sequelae, and having the skills necessary to develop an individualized treatment plan are essential tools for appropriately managing this behavior.2 Failure to recognize and address self-cutting in an adolescent has immediate consequences, such as the exacerbation of other psychiatric disorders or an increased risk of suicide. Potential longer-term consequences include an increased risk of premature death for adults who engaged in self-cutting as adolescents.3,4
For many young people, self-injury occurs only a few times, but the behavior may increase in frequency and severity when combined with other psychosocial factors.5 FPs can play a crucial role by identifying cutting behavior, providing medical treatment, educating patients and families about self-cutting, making an appropriate mental health referral, coordinating multidisciplinary collaboration, and, ultimately, supporting the patient and his or her family.
Cutting is a common form of self-harm
Some adolescents use sharp or rough objects to inflict injuries on their arms, legs, or other parts of their body. Individuals may cut, scratch, burn, abrade, or prick the skin repeatedly, often leaving scars. They may then attempt to hide the resulting injuries with clothing.6 Most self-cutting injuries are superficial, but can result in scarring. Severe injuries, such as lacerated tendons, penetrated major blood vessels, or disfiguring scars, are uncommon.
Because self-cutting behavior often remains private or intentionally hidden, its true incidence is unclear.6 However, self-cutting is not rare. In published literature, statistics for self-cutting often are grouped with those for other forms of non-suicidal self-injury (NSSI), including burning, hair-pulling, self-hitting, and self-poisoning. These self-harm behaviors have been reported in more than 10% of ninth graders7 and college students,8 and in up to 4% of adults.9 The lifetime prevalence of self-cutting is estimated to be 11.5%,10 and research suggests that the frequency of adolescent self-cutting may be increasing.5,11,12
Adolescent girls are 2 to 4 times more likely than adolescent boys to engage in NSSI.7,12 Girls primarily cut, scratch, or otherwise injure their skin, whereas boys more commonly hit or burn themselves, and inflict more injuries to the face, chest, and genitals.7,13 Most adolescents who self-cut do so only on their arms (67%).10
Not every patient who self-cuts has a psychiatric illness
Adolescents who cut themselves do not fit neatly within a typical profile.7,14,15 Self-harm in adolescents appears to be associated with a range of psychological factors, including internalizing disorders (eg, depression, anxiety, eating disorders), mood regulation difficulties (eg, impulsivity and related impulse control disorders, borderline personality traits/disorder), negative affect (eg, sadness, anxiousness, anger, stress, low self-esteem), and poor coping strategies (eg, avoidance, internalizing, substance use).14
The strong empirical relationship between psychological factors and self-harm has led many researchers and clinicians to view self-harm as a symptom of a psychiatric disorder.16 A study of psychiatric disorders among 44 adolescents (41 girls) with self-cutting behavior found a strong association with certain internalizing disorders: 63% had major depressive disorder, 37% suffered from anxiety, and 15% had an eating disorder.15 However, viewing self-cutting primarily as a manifestation of a psychiatric disorder doesn’t fully explain the behavior.
Self-harm behaviors occur across many disorders and are not unique to any single diagnosis.16 Moreover, evidence suggests that many (if not most) adolescents who self-harm do not fit the profile of psychiatric and social distress that has been well described in the literature.14,15 That is, many adolescents who self-harm may be categorized as psychologically “normal,” meaning they don’t meet the criteria for a diagnosis of depression, anxiety, or impulsivity.14,15
Being female is a strong predisposing factor for self-cutting.11 This may be because females have higher rates of depression and tend to internalize, whereas males tend to externalize and may underreport self-harm. Research suggests that modeling of self-cutting behavior by, for example, posting videos online may encourage other females to self-cut, and that contagion (ie, group cutting) is a factor.17 Self-injury is especially common in adolescent girls who have a history of physical abuse.18 In a study of patients with a history of cutting and suicidality, exposure to physical or sexual abuse, physical or emotional neglect, and chaotic family life during childhood and adolescence was associated with more frequent and more severe cutting.5,19
Cutting may help patients cope with emotional distress
Although adolescents may cut for many reasons, in general, the behavior is a coping strategy for affect regulation; cutting appears to displace emotional pain or relieve emotional blunting.2,16 Cutting provides an immediate—albeit unhealthy and temporary—method of coping. However, this is often followed by shame and low self-esteem, and the underlying emotional distress returns. Others may use self-cutting as a means of obtaining cathartic release, responding to peer pressure, or inflicting self-punishment.9 Adolescents with limited interpersonal skills may use cutting to affect relationships by, for example, communicating their distress to others and, in turn, eliciting sympathy, status, or camaraderie. If the adolescent who self-cuts interprets the resulting responses as positive, the cutting behavior is reinforced.20
The importance of the pain associated with self-injury is unclear. In an Internet survey of 128 adolescents who injured themselves, 43% reported the injuries often or always caused pain, whereas 25% said such injuries never caused pain.21 Some research suggests that self-inflicted trauma (including pain) may provide emotional relief by increasing serotonin levels, or may deliver desired euphoria by releasing endorphins.22
Self-cutting, suicide, and mortality risk. Suicide does not appear to be the intent or motivation of most adolescents who self-cut.11,12 Adolescents tend to cut themselves to “make life feel better,” not to end their life. However, the intent of self-cutting may change over time, and may lead adolescents to adopt more lethal forms of injury. In a study of adolescents receiving treatment for major depressive disorder, NSSI was found to be a strong predictor of suicidal behavior.23
Although self-harm and self-cutting occur more frequently in females, suicide is observed significantly more often in males and individuals with multiple self-harm episodes.3,24 Further, males who use analgesics to relieve the pain of cutting are at especially high risk for suicide.25
In general, individuals who self-harm have an increased risk of premature death.3,4 In a cohort study, more than 30,000 individuals with self-harm who presented to emergency departments in England had a mean loss of 31.4 years of life compared to the general population.4 Also, adolescent self-cutting is associated with adverse childhood experiences (eg, maltreatment), and these experiences are associated with early death in adults.26
A structured approach to assessment and care
If during the course of a physical examination you notice injuries such as cuts, scratches, burns, or rub marks, be especially suspicious of self-injury if they are located in areas of the body that the patient could easily reach. Also consider the possibility that the injuries may be a direct result of child abuse trauma. If you suspect physical or sexual abuse or neglect, federal law mandates you report such concerns to the appropriate state child protective services agency.
As you might expect, it’s important to use a nonjudgmental, empathic, and supportive approach when speaking to the patient about his or her motivation for cutting.5 A review of 74 studies found that attitudes of hospital staff, especially physicians, largely were negative toward patients who engaged in self-harm.27 One approach to talking to patients about self-cutting involves asking questions based on motivational interviewing techniques. (See “Talking to patients about self-harm: 5 questions to ask” below.5)
Be sure to document the location(s) and extent of the injury, and estimate the timeframe of the cutting based on the age of any scars. You’ll also need to treat the wounds and administer tetanus immunization, as appropriate.
Assess for additional risks, especially suicide. Such risks may include other behavioral issues (eg, alcohol or substance use, promiscuity, antisocial behavior), academic problems, or eating disorders. In addition, evaluate for prior and/or current mental health concerns, family dysfunction and conflict, and acute or chronic patient or family psychosocial stressors.
Assessment of suicidality should include direct queries about past, recent, and current suicidal ideation, intent, and plan. Further details on how to evaluate suicidality, including red flags to watch for, are available from the American Academy of Pediatrics at http://pediatrics.aappublications.org/content/105/4/871.full.pdf.
When evaluating a patient whose injuries might be self-inflicted, family physicians can use a subset of motivational interviewing (MI) techniques to promote a positive and supportive atmosphere for the patient, with the goal of making it easier for the patient to discuss self-injury.
Kerr et al5 suggests that family physicians can use a set of questions based on MI techniques to facilitate discussion of self-injury and prompt a patient to consider seeking help for his or her self-injury. Such questions might include:
1. What effect is self-cutting having on your life?
2. While it seems like self-cutting serves a function for you, what disadvantages are there if you continue to cut yourself?
3. What factors may motivate you to stop self-injuring right now?
4. How would your life be different right now if you were not self-cutting?
5. What do you think you would need in the way of help in order to stop self-cutting?
A patient who self-cuts and expresses the intent to commit suicide should be directly transferred to the emergency department for a psychiatric evaluation. A patient who admits he has been thinking about suicide or a suicide plan but does not state an intent to commit suicide should receive an immediate mental health referral to a psychotherapist or psychiatrist. Patients who have engaged in longstanding self-cutting should be referred to a therapist with experience in treating childhood trauma, especially if the patient has a history of behavioral or mental health disorders.12,28
The role of the family
Family members often will not have known about the cutting behavior. Family and caregivers should be educated about self-cutting (eg, its use as a coping strategy, the complexity of contributing factors), ways to provide a safe environment (eg, increased adult supervision, safeguarding of sharp objects), and the importance of mental health treatment. Positive family support is critical in addressing the patient’s self-cutting and underlying factors.
Determine if family intervention is needed. If family stressors, conflict, or dysfunction is identified as a contributing factor, recommend family counseling.
Several treatment options but few specifically for cutting
Many adolescents who self-cut want to stop cutting. In a survey of self-injured adolescents, 37% wanted to stop the behavior.14 However, even with treatment, cutting behavior often continues because cutting as a coping strategy may feel highly effective in the moment and can become addictive.29 Also, videos with explicit imagery of self-cutting are readily available on various Web sites and could normalize and reinforce the behavior.30
There are few evidence-based treatments for self-harm in general, let alone specifically for adolescent self-cutting.12,31 For adolescents with self-harm behaviors, individual cognitive behavioral therapy, dialectical behavioral therapy, group developmental therapy, multisystemic therapy, family intervention, psychotropic medication, and inpatient psychiatric treatment may help reduce risks and improve psychosocial functioning.12,31 Psychotropic medication has been shown to relieve psychiatric symptoms in patients who self-harm, but its effectiveness in reducing self-cutting behavior is unclear.12
CASE › After you speak to Alice with her parents out of the room, she admits that she had scratched her arms several times in the past few weeks because she felt stressed about her grades in certain classes. She says she’d done this scratching before as a coping mechanism, but never thought about suicide. With Alice’s permission, you discuss these incidents with her parents. You refer her to a psychotherapist to begin counseling, and ask that she return in 3 months so that you can monitor her progress.
CORRESPONDENCE
Jerry G. Jones, MD, University of Arkansas for Medical Sciences, College of Medicine, Department of Pediatrics, 1 Children’s Way, Slot 512-24A, Little Rock, AR 72202; [email protected]
› Examine the forearms and legs of all patients ages 11 to 19 years as part of the routine health assessment, looking specifically for injuries that may be self-inflicted. C
› Make an immediate referral for outpatient psychotherapy for any patient with self-cutting behavior who admits to recent or current suicidal ideation or a plan. C
› Facilitate a direct transfer to the local emergency department for psychiatric evaluation for any patient with self-cutting behavior who admits to current suicidal intent. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice R, a 14-year-old student, comes to your office for a preparticipation exam in advance of volleyball season. During the exam, you note several scratches on her left forearm. When you ask about them, Alice tells you she scratched herself when she accidentally brushed up against some bushes while walking home from school. Her explanation strikes you as odd, given that it’s been rather cold out, and it seems likely that she would have been wearing a jacket.
If Alice were your patient, how would you proceed?
Few health disorders are as clouded in mystery as self-cutting behavior in adolescents. Self-cutting is often overlooked or undetected by the medical community.1 When examining an adolescent, a family physician (FP) may pay minimal attention to the patient’s forearms and legs, but such attention can provide clues to critical health information. Relatively minor injuries in a physically active adolescent might be easy to dismiss as “normal,” but knowing the types of injuries to look for—and what to ask your young patients—can help you identify injuries that are self-inflicted and intervene accordingly.
Being aware of self-cutting, understanding its potential sequelae, and having the skills necessary to develop an individualized treatment plan are essential tools for appropriately managing this behavior.2 Failure to recognize and address self-cutting in an adolescent has immediate consequences, such as the exacerbation of other psychiatric disorders or an increased risk of suicide. Potential longer-term consequences include an increased risk of premature death for adults who engaged in self-cutting as adolescents.3,4
For many young people, self-injury occurs only a few times, but the behavior may increase in frequency and severity when combined with other psychosocial factors.5 FPs can play a crucial role by identifying cutting behavior, providing medical treatment, educating patients and families about self-cutting, making an appropriate mental health referral, coordinating multidisciplinary collaboration, and, ultimately, supporting the patient and his or her family.
Cutting is a common form of self-harm
Some adolescents use sharp or rough objects to inflict injuries on their arms, legs, or other parts of their body. Individuals may cut, scratch, burn, abrade, or prick the skin repeatedly, often leaving scars. They may then attempt to hide the resulting injuries with clothing.6 Most self-cutting injuries are superficial, but can result in scarring. Severe injuries, such as lacerated tendons, penetrated major blood vessels, or disfiguring scars, are uncommon.
Because self-cutting behavior often remains private or intentionally hidden, its true incidence is unclear.6 However, self-cutting is not rare. In published literature, statistics for self-cutting often are grouped with those for other forms of non-suicidal self-injury (NSSI), including burning, hair-pulling, self-hitting, and self-poisoning. These self-harm behaviors have been reported in more than 10% of ninth graders7 and college students,8 and in up to 4% of adults.9 The lifetime prevalence of self-cutting is estimated to be 11.5%,10 and research suggests that the frequency of adolescent self-cutting may be increasing.5,11,12
Adolescent girls are 2 to 4 times more likely than adolescent boys to engage in NSSI.7,12 Girls primarily cut, scratch, or otherwise injure their skin, whereas boys more commonly hit or burn themselves, and inflict more injuries to the face, chest, and genitals.7,13 Most adolescents who self-cut do so only on their arms (67%).10
Not every patient who self-cuts has a psychiatric illness
Adolescents who cut themselves do not fit neatly within a typical profile.7,14,15 Self-harm in adolescents appears to be associated with a range of psychological factors, including internalizing disorders (eg, depression, anxiety, eating disorders), mood regulation difficulties (eg, impulsivity and related impulse control disorders, borderline personality traits/disorder), negative affect (eg, sadness, anxiousness, anger, stress, low self-esteem), and poor coping strategies (eg, avoidance, internalizing, substance use).14
The strong empirical relationship between psychological factors and self-harm has led many researchers and clinicians to view self-harm as a symptom of a psychiatric disorder.16 A study of psychiatric disorders among 44 adolescents (41 girls) with self-cutting behavior found a strong association with certain internalizing disorders: 63% had major depressive disorder, 37% suffered from anxiety, and 15% had an eating disorder.15 However, viewing self-cutting primarily as a manifestation of a psychiatric disorder doesn’t fully explain the behavior.
Self-harm behaviors occur across many disorders and are not unique to any single diagnosis.16 Moreover, evidence suggests that many (if not most) adolescents who self-harm do not fit the profile of psychiatric and social distress that has been well described in the literature.14,15 That is, many adolescents who self-harm may be categorized as psychologically “normal,” meaning they don’t meet the criteria for a diagnosis of depression, anxiety, or impulsivity.14,15
Being female is a strong predisposing factor for self-cutting.11 This may be because females have higher rates of depression and tend to internalize, whereas males tend to externalize and may underreport self-harm. Research suggests that modeling of self-cutting behavior by, for example, posting videos online may encourage other females to self-cut, and that contagion (ie, group cutting) is a factor.17 Self-injury is especially common in adolescent girls who have a history of physical abuse.18 In a study of patients with a history of cutting and suicidality, exposure to physical or sexual abuse, physical or emotional neglect, and chaotic family life during childhood and adolescence was associated with more frequent and more severe cutting.5,19
Cutting may help patients cope with emotional distress
Although adolescents may cut for many reasons, in general, the behavior is a coping strategy for affect regulation; cutting appears to displace emotional pain or relieve emotional blunting.2,16 Cutting provides an immediate—albeit unhealthy and temporary—method of coping. However, this is often followed by shame and low self-esteem, and the underlying emotional distress returns. Others may use self-cutting as a means of obtaining cathartic release, responding to peer pressure, or inflicting self-punishment.9 Adolescents with limited interpersonal skills may use cutting to affect relationships by, for example, communicating their distress to others and, in turn, eliciting sympathy, status, or camaraderie. If the adolescent who self-cuts interprets the resulting responses as positive, the cutting behavior is reinforced.20
The importance of the pain associated with self-injury is unclear. In an Internet survey of 128 adolescents who injured themselves, 43% reported the injuries often or always caused pain, whereas 25% said such injuries never caused pain.21 Some research suggests that self-inflicted trauma (including pain) may provide emotional relief by increasing serotonin levels, or may deliver desired euphoria by releasing endorphins.22
Self-cutting, suicide, and mortality risk. Suicide does not appear to be the intent or motivation of most adolescents who self-cut.11,12 Adolescents tend to cut themselves to “make life feel better,” not to end their life. However, the intent of self-cutting may change over time, and may lead adolescents to adopt more lethal forms of injury. In a study of adolescents receiving treatment for major depressive disorder, NSSI was found to be a strong predictor of suicidal behavior.23
Although self-harm and self-cutting occur more frequently in females, suicide is observed significantly more often in males and individuals with multiple self-harm episodes.3,24 Further, males who use analgesics to relieve the pain of cutting are at especially high risk for suicide.25
In general, individuals who self-harm have an increased risk of premature death.3,4 In a cohort study, more than 30,000 individuals with self-harm who presented to emergency departments in England had a mean loss of 31.4 years of life compared to the general population.4 Also, adolescent self-cutting is associated with adverse childhood experiences (eg, maltreatment), and these experiences are associated with early death in adults.26
A structured approach to assessment and care
If during the course of a physical examination you notice injuries such as cuts, scratches, burns, or rub marks, be especially suspicious of self-injury if they are located in areas of the body that the patient could easily reach. Also consider the possibility that the injuries may be a direct result of child abuse trauma. If you suspect physical or sexual abuse or neglect, federal law mandates you report such concerns to the appropriate state child protective services agency.
As you might expect, it’s important to use a nonjudgmental, empathic, and supportive approach when speaking to the patient about his or her motivation for cutting.5 A review of 74 studies found that attitudes of hospital staff, especially physicians, largely were negative toward patients who engaged in self-harm.27 One approach to talking to patients about self-cutting involves asking questions based on motivational interviewing techniques. (See “Talking to patients about self-harm: 5 questions to ask” below.5)
Be sure to document the location(s) and extent of the injury, and estimate the timeframe of the cutting based on the age of any scars. You’ll also need to treat the wounds and administer tetanus immunization, as appropriate.
Assess for additional risks, especially suicide. Such risks may include other behavioral issues (eg, alcohol or substance use, promiscuity, antisocial behavior), academic problems, or eating disorders. In addition, evaluate for prior and/or current mental health concerns, family dysfunction and conflict, and acute or chronic patient or family psychosocial stressors.
Assessment of suicidality should include direct queries about past, recent, and current suicidal ideation, intent, and plan. Further details on how to evaluate suicidality, including red flags to watch for, are available from the American Academy of Pediatrics at http://pediatrics.aappublications.org/content/105/4/871.full.pdf.
When evaluating a patient whose injuries might be self-inflicted, family physicians can use a subset of motivational interviewing (MI) techniques to promote a positive and supportive atmosphere for the patient, with the goal of making it easier for the patient to discuss self-injury.
Kerr et al5 suggests that family physicians can use a set of questions based on MI techniques to facilitate discussion of self-injury and prompt a patient to consider seeking help for his or her self-injury. Such questions might include:
1. What effect is self-cutting having on your life?
2. While it seems like self-cutting serves a function for you, what disadvantages are there if you continue to cut yourself?
3. What factors may motivate you to stop self-injuring right now?
4. How would your life be different right now if you were not self-cutting?
5. What do you think you would need in the way of help in order to stop self-cutting?
A patient who self-cuts and expresses the intent to commit suicide should be directly transferred to the emergency department for a psychiatric evaluation. A patient who admits he has been thinking about suicide or a suicide plan but does not state an intent to commit suicide should receive an immediate mental health referral to a psychotherapist or psychiatrist. Patients who have engaged in longstanding self-cutting should be referred to a therapist with experience in treating childhood trauma, especially if the patient has a history of behavioral or mental health disorders.12,28
The role of the family
Family members often will not have known about the cutting behavior. Family and caregivers should be educated about self-cutting (eg, its use as a coping strategy, the complexity of contributing factors), ways to provide a safe environment (eg, increased adult supervision, safeguarding of sharp objects), and the importance of mental health treatment. Positive family support is critical in addressing the patient’s self-cutting and underlying factors.
Determine if family intervention is needed. If family stressors, conflict, or dysfunction is identified as a contributing factor, recommend family counseling.
Several treatment options but few specifically for cutting
Many adolescents who self-cut want to stop cutting. In a survey of self-injured adolescents, 37% wanted to stop the behavior.14 However, even with treatment, cutting behavior often continues because cutting as a coping strategy may feel highly effective in the moment and can become addictive.29 Also, videos with explicit imagery of self-cutting are readily available on various Web sites and could normalize and reinforce the behavior.30
There are few evidence-based treatments for self-harm in general, let alone specifically for adolescent self-cutting.12,31 For adolescents with self-harm behaviors, individual cognitive behavioral therapy, dialectical behavioral therapy, group developmental therapy, multisystemic therapy, family intervention, psychotropic medication, and inpatient psychiatric treatment may help reduce risks and improve psychosocial functioning.12,31 Psychotropic medication has been shown to relieve psychiatric symptoms in patients who self-harm, but its effectiveness in reducing self-cutting behavior is unclear.12
CASE › After you speak to Alice with her parents out of the room, she admits that she had scratched her arms several times in the past few weeks because she felt stressed about her grades in certain classes. She says she’d done this scratching before as a coping mechanism, but never thought about suicide. With Alice’s permission, you discuss these incidents with her parents. You refer her to a psychotherapist to begin counseling, and ask that she return in 3 months so that you can monitor her progress.
CORRESPONDENCE
Jerry G. Jones, MD, University of Arkansas for Medical Sciences, College of Medicine, Department of Pediatrics, 1 Children’s Way, Slot 512-24A, Little Rock, AR 72202; [email protected]
1. Whitlock J, Muehlenkamp J, Purington A, et al. Nonsuicidal self-injury in a college population: general trends and sex differences. J Am Coll Health. 2011;59:691-698.
2. Shain BN; American Academy of Pediatrics Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2007;120:669-676.
3. Miller M, Hempstead K, Nguyen T, et al. Method choice in nonfatal self-harm as a predictor of subsequent episodes of self-harm and suicide: implications for clinical practice. Am J Public Health. 2013;103:e61-e68.
4. Bergen H, Hawton K, Walters K. Premature death after self-harm: a multicentre cohort study. Lancet. 2012;380:1568-1574.
5. Kerr PL, Muehlenkamp JJ, Turner JM. Nonsuicidal self-injury: a review of current research for family medicine and primary care physicians. J Am Board Fam Med. 2010;23:240-259.
6. Fordam K, Bailham D. Self-harm in young people. In: Beinart H, Kennedy P, Llewelyn S, eds. Clinical Psychology in Practice. West Sussex, England: BPS Blackwell; 2009:73-84.
7. Barrocas AL, Hankin BL, Young JF, et al. Rates of nonsuicidal self-injury in youth: age, sex, and behavioral methods in a community sample. Pediatrics. 2012;130:39-45.
8. Kuentzel JG, Arble E, Boutros N, et al. Nonsuicidal self-injury in an ethnically diverse college sample. Am J Orthopsychiatry. 2012;82:291-297.
9. Taylor JD. Cutting, piercing, and self-mutilation. In: Bryant CD, ed. Handbook of Deviant Behavior. New York, NY: Routledge; 2011:305-312.
10. Laukkanen E, Rissanen ML, Tolmunen T, et al. Adolescent self-cutting elsewhere than on the arms reveals more serious psychiatric symptoms. Eur Child Adolesc Psychiatry. 2013;22:501-510.
11. Hall B, Place M. Cutting to cope – a modern adolescent phenomenon. Child Care Health Dev. 2010;36:623-629.
12. Wood A. Self-harm in adolescents. Advances in Psychiatric Treatment. 2009;15:434-441.
13. Sornberger MJ, Heath NL, Toste JR, et al. Non suicidal self-injury and gender: patterns of prevalence, methods, and locations among adolescents. Suicide Life Threatening Behav. 2012;42:266-278.
14. Stanford S, Jones MP. Psychological subtyping finds pathological, impulsive, and “normal” groups among adolescents who self-harm. J Child Psychol Psychiatry. 2009;50:807-815.
15. Hintikka J, Tolmunen T, Rissanen ML, et al. Mental disorders in self-cutting adolescents. J Adolesc Health. 2009;44:464-467.
16. Nock MK. Why do people hurt themselves? New insights into the nature and functions of self-injury. Curr Dir Psychol Sci. 2009;18:78-83.
17. Hawton K, Harriss L, Rodham K. How adolescents who cut themselves differ from those who take overdoses. Eur Child Adolesc Psychiatry. 2010;19:513-523.
18. Swannell S, Martin G, Page A, et al. Child maltreatment, subsequent non-suicidal self-injury and the mediating role of dissociation, alexithymia and self-blame. Child Abuse Negl. 2012;36:572-584.
19. van der Kolk BA, Perry JC, Herman JL. Childhood origins of self-destructive behavior. Am J Psychiatry. 1991;148:1665-1671.
20. Brickell CM, Jellinek MS. Self-injury: why teens do it, how to help. March 2014. Contemporary Pediatrics Web site. Available at: http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/content/tags/borderline-personalitydisorder/self-injury-why-teens-do-it-how. Accessed March 4, 2015.
21. Murray CD, Warren A, Foxe J. An Internet survey of adolescent self-injuries. Australian e-Journal for the Advancement of Mental Health. 2005;4:7-9.
22. Fikke LT, Melinder A, Landro NI. The effects of acute tryptophan depletion on impulsivity and mood in adolescents engaging in non-suicidal self-injury. Hum Psychopharmacol. 2013;28:61-71.
23. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168:495-501.
24. Hawton K, Bergen H, Kapur N, et al. Repetition of self-harm and suicide following self-harm in children and adolescents: findings from the Multicentre Study of Self-harm in England. J Child Psychol Psychiatry. 2012;53:1212-1219.
25. Matsumoto T, Imamura F, Chiba Y, et al. Analgesia during self-cutting: clinical implications and the association with suicidal ideation. Psychiatry Clin Neurosci. 2008;62:355-358.
26. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) study. Am J Prev Med. 1998;14:245-258.
27. Saunders KE, Hawton K, Fortune S, et al. Attitudes and knowledge of clinical staff regarding people who self-harm: a systematic review. J Affect Disord. 2012;139:205-216.
28. Harrington R, Pickles A, Aglan A, et al. Early adult outcomes of adolescents who deliberately poisoned themselves. J Am Acad Child Adolesc Psychiatry. 2006;45:337-345.
29. Puskar KR, Bernardo L, Hatam M, et al. Self-cutting behaviors in adolescents. J Emerg Nurs. 2006;32:444-446.
30. Lewis SP, Heath NL, St. Denis JM, et al. The scope of nonsuicidal self-injury on YouTube. Pediatrics. 2011;127:e552-e557.
31. Gonzales AH, Bergstrom L. Adolescent non-suicidal self-injury (NSSI) interventions. J Child Adolesc Psychiatr Nurs. 2013;26:124-130.
1. Whitlock J, Muehlenkamp J, Purington A, et al. Nonsuicidal self-injury in a college population: general trends and sex differences. J Am Coll Health. 2011;59:691-698.
2. Shain BN; American Academy of Pediatrics Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2007;120:669-676.
3. Miller M, Hempstead K, Nguyen T, et al. Method choice in nonfatal self-harm as a predictor of subsequent episodes of self-harm and suicide: implications for clinical practice. Am J Public Health. 2013;103:e61-e68.
4. Bergen H, Hawton K, Walters K. Premature death after self-harm: a multicentre cohort study. Lancet. 2012;380:1568-1574.
5. Kerr PL, Muehlenkamp JJ, Turner JM. Nonsuicidal self-injury: a review of current research for family medicine and primary care physicians. J Am Board Fam Med. 2010;23:240-259.
6. Fordam K, Bailham D. Self-harm in young people. In: Beinart H, Kennedy P, Llewelyn S, eds. Clinical Psychology in Practice. West Sussex, England: BPS Blackwell; 2009:73-84.
7. Barrocas AL, Hankin BL, Young JF, et al. Rates of nonsuicidal self-injury in youth: age, sex, and behavioral methods in a community sample. Pediatrics. 2012;130:39-45.
8. Kuentzel JG, Arble E, Boutros N, et al. Nonsuicidal self-injury in an ethnically diverse college sample. Am J Orthopsychiatry. 2012;82:291-297.
9. Taylor JD. Cutting, piercing, and self-mutilation. In: Bryant CD, ed. Handbook of Deviant Behavior. New York, NY: Routledge; 2011:305-312.
10. Laukkanen E, Rissanen ML, Tolmunen T, et al. Adolescent self-cutting elsewhere than on the arms reveals more serious psychiatric symptoms. Eur Child Adolesc Psychiatry. 2013;22:501-510.
11. Hall B, Place M. Cutting to cope – a modern adolescent phenomenon. Child Care Health Dev. 2010;36:623-629.
12. Wood A. Self-harm in adolescents. Advances in Psychiatric Treatment. 2009;15:434-441.
13. Sornberger MJ, Heath NL, Toste JR, et al. Non suicidal self-injury and gender: patterns of prevalence, methods, and locations among adolescents. Suicide Life Threatening Behav. 2012;42:266-278.
14. Stanford S, Jones MP. Psychological subtyping finds pathological, impulsive, and “normal” groups among adolescents who self-harm. J Child Psychol Psychiatry. 2009;50:807-815.
15. Hintikka J, Tolmunen T, Rissanen ML, et al. Mental disorders in self-cutting adolescents. J Adolesc Health. 2009;44:464-467.
16. Nock MK. Why do people hurt themselves? New insights into the nature and functions of self-injury. Curr Dir Psychol Sci. 2009;18:78-83.
17. Hawton K, Harriss L, Rodham K. How adolescents who cut themselves differ from those who take overdoses. Eur Child Adolesc Psychiatry. 2010;19:513-523.
18. Swannell S, Martin G, Page A, et al. Child maltreatment, subsequent non-suicidal self-injury and the mediating role of dissociation, alexithymia and self-blame. Child Abuse Negl. 2012;36:572-584.
19. van der Kolk BA, Perry JC, Herman JL. Childhood origins of self-destructive behavior. Am J Psychiatry. 1991;148:1665-1671.
20. Brickell CM, Jellinek MS. Self-injury: why teens do it, how to help. March 2014. Contemporary Pediatrics Web site. Available at: http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/content/tags/borderline-personalitydisorder/self-injury-why-teens-do-it-how. Accessed March 4, 2015.
21. Murray CD, Warren A, Foxe J. An Internet survey of adolescent self-injuries. Australian e-Journal for the Advancement of Mental Health. 2005;4:7-9.
22. Fikke LT, Melinder A, Landro NI. The effects of acute tryptophan depletion on impulsivity and mood in adolescents engaging in non-suicidal self-injury. Hum Psychopharmacol. 2013;28:61-71.
23. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168:495-501.
24. Hawton K, Bergen H, Kapur N, et al. Repetition of self-harm and suicide following self-harm in children and adolescents: findings from the Multicentre Study of Self-harm in England. J Child Psychol Psychiatry. 2012;53:1212-1219.
25. Matsumoto T, Imamura F, Chiba Y, et al. Analgesia during self-cutting: clinical implications and the association with suicidal ideation. Psychiatry Clin Neurosci. 2008;62:355-358.
26. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) study. Am J Prev Med. 1998;14:245-258.
27. Saunders KE, Hawton K, Fortune S, et al. Attitudes and knowledge of clinical staff regarding people who self-harm: a systematic review. J Affect Disord. 2012;139:205-216.
28. Harrington R, Pickles A, Aglan A, et al. Early adult outcomes of adolescents who deliberately poisoned themselves. J Am Acad Child Adolesc Psychiatry. 2006;45:337-345.
29. Puskar KR, Bernardo L, Hatam M, et al. Self-cutting behaviors in adolescents. J Emerg Nurs. 2006;32:444-446.
30. Lewis SP, Heath NL, St. Denis JM, et al. The scope of nonsuicidal self-injury on YouTube. Pediatrics. 2011;127:e552-e557.
31. Gonzales AH, Bergstrom L. Adolescent non-suicidal self-injury (NSSI) interventions. J Child Adolesc Psychiatr Nurs. 2013;26:124-130.
RADIOLOGY REPORT: An imaging guide to abdominal pain
› Choose ultrasonography as the initial imaging test for patients with pain in the right upper quadrant. C
› Order computed tomography with contrast of the abdomen and/or pelvis for adults with acute pain of new onset in the right or left lower quadrant, or both. C
› Recommend ultrasound with graded compression as the initial imaging modality for children younger than 14 years who have acute right lower quadrant pain. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Harry L, age 54, presents with acute onset abdominal pain that began 2 days ago. He reports mild nausea but no vomiting, non-bloody diarrhea, and feeling feverish. The patient denies increased pain with movement.
If Mr. L were your patient, how would you proceed?
Although acute nontraumatic abdominal pain accounts for only about 1.5% of physician office visits, it is the cause of approximately 8% of visits—more than 11 million, a year—to US emergency departments.1,2 In most cases, the location of the pain, coupled with the patient history, physical exam, and clinical judgment, lead to the differential diagnosis and determines what type of imaging, if any, is needed.
Benign self-limiting causes of abdominal pain, such as constipation or gastroenteritis, may not require any imaging. However, if the patient has abdominal pain and fever, is older than age 65, or presents with peritoneal signs and symptoms, imaging will be a crucial diagnostic aid.3,4
With that in mind, the American College of Radiology (ACR) has developed a 9-point scoring criteria based on both the site and common causes of abdominal pain to determine the best imaging modality to use to rule in—or out—serious disease and conditions for which surgery is required. A rating of 1, 2, or 3 indicates that imaging is usually not appropriate; a rating of 4, 5, or 6 means the imaging test may be appropriate, and a rating of 7, 8, or 9 indicates that the recommended test is usually appropriate. The panel of experts who developed the scores considered the relative radiation level of each imaging modality, as well.5
In the text and TABLE3,6-12 that follow, you’ll find the most likely clinical diagnoses and the optimal tests for abdominal pain, based largely on where it hurts.
CASE 1 › Appendicitis: An 8-year-old girl presented with acute right lower quadrant pain and underwent an ultrasound (left), which showed a dilated ~2 cm (normal <6 mm) non-compressible appendix consistent with acute appendicitis. There was no significant free fluid within the right lower quadrant. Follow-up computed tomography (CT) scan (middle) with IV and oral contrast confirmed the dilated appendix with no evidence of perforation or abscess formation.
CASE 2 › Small bowel obstruction: A 54-year-old man sought care for abdominal pain and distention. A CT with IV and oral contrast (right) revealed multiple, fluid-filled dilated loops of small bowel (arrows) with bowel wall thickening, edema, and inflammatory stranding. The short segment of decreased enhancement (arrowhead) was consistent with bowel ischemia.
Right upper quadrant pain: Beware of acute biliary disease
Abdominal pain of the right upper quadrant (RUQ) is typically related to biliary, colonic, hepatic, or renal causes.12 Because of infection and the potential need for surgical intervention, untreated acute biliary disease can become life-threatening, particularly in the elderly.4
The ACR recommends ultrasound (US) as the initial imaging study for RUQ pain, regardless of whether the patient is febrile or has an elevated white blood cell count or a positive Murphy’s sign (demonstrated at youtube.com/watch?v=9L7N89sOSuc) (score=9). A 2012 meta-analysis found that US has a sensitivity of 81% and specificity of 83% for diagnosing acute cholecystitis.6
Although cholescintigraphy has a higher sensitivity and specificity (96% and 90%, respectively), US remains the initial study of choice because of its availability, study time, and the lack of ionizing radiation. For equivocal findings, computed tomography (CT), magnetic resonance imaging (MRI), and cholescintigraphy have similar levels of evidence.6
Epigastric/left upper quadrant pain: Consider pancreatits
Epigastric and left upper quadrant (LUQ) pain may have a gastric, biliary, pancreatic, vascular, renal, or cardiac etiology.12 The ACR criteria for testing depends on the type of pain suspected.
Pancreatitis. The Revised Atlanta Classification of Acute Pancreatitis requires 2 of the 3 classic criteria for a pancreatitis diagnosis: 1) abdominal pain suggestive of pancreatitis, 2) serum amylase and lipase levels ≥3 times the normal level, and 3) characteristic findings on imaging.13 The ACR recommends US as the initial imaging modality for suspected acute pancreatitis when it is the initial presentation, the patient has typical abdominal pain and increased serum amylase and lipase, and symptom onset was <48 to 72 hours before the patient sought care (score=9).7
For patients who are critically ill, meet the criteria for systemic inflammatory response syndrome (SIRS), have severe clinical scores on either the Acute Physiology and Chronic Health Evaluation (APACHE) II (available at clincalc.com/icumortality/apacheii.aspx) or Bedside Index for Severity in Acute Appendicitis (BISAP; mdcalc.com/bisap-score-for-pancreatitis-mortality/), or who present >48 to 72 hours after onset of symptoms, abdominal CT with contrast is recommended (score=8).
Urolithiasis. Patients with abdominal pain from a presumed renal source should undergo a non-contrast CT of the abdomen and pelvis for initial imaging, according to the ACR (score=8). The sensitivity is 95% to 96% and specificity is 98%.8 To limit radiation exposure, low-dose protocols and limiting scan range are preferred.
Radiography can be useful in patients with known kidney stone disease and previous films; however, the sensitivity in other patients is poor (58%-62%).8 Because pelviectasis and ureterectasis can take hours to develop, US will miss more than 30% of acute obstructions in patients who are not fully hydrated and is therefore not recommended as a first-line imaging modality.8 The sensitivity of US increases to 71% when it is combined with kidney, ureter, and bladder radiography, but is still lower than that of CT or IV urography.8
Left lower quadrant pain: Suspect sigmoid diverticulitis
The differential for left lower quadrant (LLQ) pain includes colonic, gynecologic, and renal etiologies.12 The most common cause in adults is acute sigmoid diverticulitis. Patients often present with the clinical triad of fever, LLQ pain, and leukocytosis.14 A decision to obtain imaging should be based on both the clinical presentation and examination. It may not be required for patients who have mild symptoms or have had previous episodes of diverticulitis.
Clinical scoring systems have been studied for LLQ pain. However, none has been validated in all settings and therefore no such system is routinely used.15 CT of the abdomen and pelvis with contrast media is the ACR’s recommendation for the initial imaging study (score=9). CT has a reported overall accuracy of 99%.9
CT can also assess the severity of disease and help determine medical vs surgical treatment.14 US using graded compression has a sensitivity of 77% to 98% and a specificity of 80% to 99%, but is limited by body habitus, technical expertise, and patient comfort. Therefore, US has not gained widespread use (score=4) for patients with LLQ pain.9,14 MRI is emerging as a potential option; however, longer scan times, cost, and availability continue to limit its use.14
Right lower quadrant pain: Is it appendicitis?
The differential for right lower quadrant (RLQ) pain, like that of LLQ pain, includes colonic, gynecologic, and renal etiologies.12 The most common cause of acute RLQ pain requiring surgery is appendicitis. History and physical exam achieve a diagnostic accuracy of 80%.16
If the diagnosis is clear, no imaging is warranted. In patients with equivocal clinical presentations, however, imaging is cost-effective and may reduce the rate of perforation, morbidity, mortality, and postoperative hospital stays.16 In addition, the accuracy of clinical diagnosis for elderly patients and women of childbearing age with RLQ pain tends to be lower than that of adult men. Therefore, some experts call for a lower imaging threshold for these populations.
CT of the abdomen and pelvis with contrast is the recommended initial imaging study in nonpregnant adults (score=8). CT has a sensitivity and specificity of 91% and 90%, respectively.10
CT without contrast is indicated for patients with RLQ pain who have a contraindication to contrast media, although the relative radiation level remains the same.
If limiting radiation exposure is especially important, consider US, followed by CT with contrast if US is inconclusive.10
Low-dose CT has been investigated as an alternative, but is not routinely used. A limited abdominal CT scan from the bottom of the body of the T10 vertebra to the top of the symphysis pubis allows for adequate evaluation and alternate diagnoses of concern when compared with full CT scans of the abdomen and pelvis.17 This limited CT scan has been found to result in a total body effective radiation dose reduction of 23% and, in women, a breast equivalent dose reduction of 85%, without missing a single case of acute appendicitis or pertinent alternative diagnoses.17
Diffuse abdominal pain: Suspect a blockage
Finally, some patients may present with diffuse or non-localizable pain with fever. The etiologies that often present with diffuse or nonspecific pain include small bowel obstruction and mesenteric ischemia.
Small bowel obstruction. When small bowel obstruction is suspected, CT of the abdomen and pelvis with contrast (score=9) is recommended. Oral contrast is not indicated if you suspect a high-grade obstruction, but may add functional information when only a partial or low-grade obstruction is suspected. The relative radiation level remains the same for both.11 For patients with pain and fever, postoperative or not, CT of the abdomen and pelvis with contrast is recommended (score=8).3
Mesenteric ischemia is associated with high morbidity and mortality rates (30%-90%).18,19 Acute mesenteric ischemia is most commonly secondary to embolism, followed by arterial thrombosis, non-occlusive ischemia, and less commonly, venous thrombosis.18 The typical presentation is pain out of proportion to the physical exam.19
Differentiating mesenteric ischemia from other causes of acute abdominal pain can be difficult. Patients with chronic mesenteric ischemia present with postprandial abdominal pain, weight loss, and food avoidance. Although radiography is often the initial test ordered, a negative test does not rule out mesenteric ischemia. Therefore, the ACR recommends CT angiography (CTA) of the abdomen with contrast for the evaluation of both acute and chronic mesenteric ischemia (score=9). US can be useful for excluding other causes of abdominal pain, as well as ischemia related to venous occlusion, but it has a low sensitivity (70%-89%)18 overall and therefore is not recommended as the initial test for acute or chronic mesenteric ischemia.
Magnetic resonance angiography (MRA) has a high sensitivity and specificity for severe stenosis or origin occlusions of the superior mesenteric artery and celiac axis; however, its ability to determine distal embolism and non-occlusive ischemia, and the length and availability of this test limit its usefulness.18
When the patient is a child
Imaging for bilious vomiting in infants up to 3 months varies based on age. In the first week of life, radiography of the abdomen is the ACR’s recommended first-line test (score=9).20 An upper GI series or contrast enema are also options, but less preferred due to their increased radiation exposure.20
For infants between one week and 3 months of age, an upper GI series is the study of choice (score=9) and radiography of the abdomen is second line (score=5). An upper GI series is recommended to evaluate non-bilious, intermittent non-projectile vomiting in those from birth to 3 months.20 Projectile non-bilious vomiting should be evaluated with US of the abdomen.20
In children with RLQ pain suggestive of acute appendicitis, US is the first line imaging method due to its relatively high sensitivity and specificity and lack of ionizing radiation (score=9).10 If US is inconclusive, then CT of the abdomen and pelvis with IV contrast, but not oral or rectal contrast, is recommended (score=7). Although MRI is a non-radiating modality, it should be reserved for use only in specialized pediatric facilities due to lack of experience, increased cost, and the usual need for sedation.10
CASE › Mr. L’s vital signs demonstrated mild tachycardia and his body temperature was 100.5° F. His physical exam revealed significant tenderness to palpation in the LL Q, but no rebound or guarding. A CT scan with contrast of the abdomen revealed diverticulitis without abscess or perforation. The patient was managed with a clear liquid diet and told to return to the clinic 2 days later.
CORRESPONDENCE
Heidi L. Gaddey, MD, Family Medicine Residency Program, University of Nebraska Medical Center and the 55th Medical Group, 2501 Capehart Road, Offutt Air Force Base, NE 68113; [email protected]
1. Centers for Disease Control and Prevention. National Ambulatory Medical Care Survey: 2010 Summary Tables. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/data/ahcd/namcs_summary/2010_namcs_web_tables.pdf. Accessed April 8, 2015.
2. Centers for Disease Control and Prevention. National Hospital Ambulatory Medical Care Survey: 2011 Emergency Department Summary Tables. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf. Accessed February 2, 2015.
3. American College of Radiology. ACR Appropriateness Criteria: Acute (Nonlocalized) abdominal pain and fever or suspected abdominal abscess. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69467/Narrative. Accessed December 15, 2014.
4. Lyon C, Clark DC. Diagnosis of acute abdominal pain in older patients. Am Fam Physician. 2006;74:1537-1544.
5. Crownover BK, Bepko JL. Appropriate and safe use of diagnostic imaging. Am Fam Physician. 2013;87:494-501.
6. American College of Radiology. ACR Appropriateness Criteria: right upper quadrant pain. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69474/Narrative/. Accessed December 15, 2014.
7. American College of Radiology. ACR Appropriateness Criteria: acute pancreatitis. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69468/Narrative. Accessed April 8, 2015.
8. American College of Radiology. ACR Appropriateness Criteria: acute onset flank pain—suspicion of stone disease. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69362/Narrative/. Accessed December 1, 2014.
9. American College of Radiology. ACR Appropriateness Criteria: left lower quadrant pain—suspected diverticulitis. American College of Radiology Web site. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/LeftLowerQuadrantPainSuspectedDiverticulitis.pdf. Accessed December 1, 2014.
10. American College of Radiology. ACR Appropriateness Criteria: right lower quadrant pain—suspected appendicitis. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69357/Narrative/. Accessed December 15, 2014.
11. American College of Radiology. ACR Appropriateness Criteria: suspected small-bowel obstruction. American College of Radiology Web site. Available at: http://www.acr.org/~/media/832F100277004BC69A8C818C7C9BFF33.pdf. Accessed December 10, 2014.
12. Cartwright SL, Knudson MP. Evaluation of acute abdominal pain in adults. Am Fam Physician. 2008;77:971-978.
13. Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262:751-764.
14. American College of Radiology. ACR Appropriateness Criteria: left lower quadrant pain—suspected diverticulitis. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69356/Narrative/. Accessed December 1, 2014.
15. Andeweg CS, Knobben L, Hendriks JC, et al. How to diagnose acute left-sided colonic diverticulitis: proposal for a clinical scoring system. Ann Surg. 2011;253:940-946.
16. Old JL, Dusing RW, Yap W, et al. Imaging for suspected appendicitis. Am Fam Physician. 2005;71:71-78.
17. Corwin MT, Chang M, Fananapazir G, et al. Accuracy and radiation dose reduction of a limited abdominopelvic CT in the diagnosis of acute appendicitis. Abdom Imaging. 2014; October 21 [Epub ahead of print].
18. American College of Radiology. ACR Appropriateness Criteria: imaging of mesenteric ischemia. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/70909/Narrative/. Accessed December 4, 2014.
19. Herbert GS, Steele SR. Acute and chronic mesenteric ischemia. Surg Clin North Am. 2007;87:1115-1134.
20. American College of Radiology. ACR Appropriateness Criteria: vomiting in infants up to 3 months of age. American College of Radiology Web site. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/VomitingInInfantsUpTo3MonthsOfAge.pdf. Accessed January 5, 2015.
› Choose ultrasonography as the initial imaging test for patients with pain in the right upper quadrant. C
› Order computed tomography with contrast of the abdomen and/or pelvis for adults with acute pain of new onset in the right or left lower quadrant, or both. C
› Recommend ultrasound with graded compression as the initial imaging modality for children younger than 14 years who have acute right lower quadrant pain. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Harry L, age 54, presents with acute onset abdominal pain that began 2 days ago. He reports mild nausea but no vomiting, non-bloody diarrhea, and feeling feverish. The patient denies increased pain with movement.
If Mr. L were your patient, how would you proceed?
Although acute nontraumatic abdominal pain accounts for only about 1.5% of physician office visits, it is the cause of approximately 8% of visits—more than 11 million, a year—to US emergency departments.1,2 In most cases, the location of the pain, coupled with the patient history, physical exam, and clinical judgment, lead to the differential diagnosis and determines what type of imaging, if any, is needed.
Benign self-limiting causes of abdominal pain, such as constipation or gastroenteritis, may not require any imaging. However, if the patient has abdominal pain and fever, is older than age 65, or presents with peritoneal signs and symptoms, imaging will be a crucial diagnostic aid.3,4
With that in mind, the American College of Radiology (ACR) has developed a 9-point scoring criteria based on both the site and common causes of abdominal pain to determine the best imaging modality to use to rule in—or out—serious disease and conditions for which surgery is required. A rating of 1, 2, or 3 indicates that imaging is usually not appropriate; a rating of 4, 5, or 6 means the imaging test may be appropriate, and a rating of 7, 8, or 9 indicates that the recommended test is usually appropriate. The panel of experts who developed the scores considered the relative radiation level of each imaging modality, as well.5
In the text and TABLE3,6-12 that follow, you’ll find the most likely clinical diagnoses and the optimal tests for abdominal pain, based largely on where it hurts.
CASE 1 › Appendicitis: An 8-year-old girl presented with acute right lower quadrant pain and underwent an ultrasound (left), which showed a dilated ~2 cm (normal <6 mm) non-compressible appendix consistent with acute appendicitis. There was no significant free fluid within the right lower quadrant. Follow-up computed tomography (CT) scan (middle) with IV and oral contrast confirmed the dilated appendix with no evidence of perforation or abscess formation.
CASE 2 › Small bowel obstruction: A 54-year-old man sought care for abdominal pain and distention. A CT with IV and oral contrast (right) revealed multiple, fluid-filled dilated loops of small bowel (arrows) with bowel wall thickening, edema, and inflammatory stranding. The short segment of decreased enhancement (arrowhead) was consistent with bowel ischemia.
Right upper quadrant pain: Beware of acute biliary disease
Abdominal pain of the right upper quadrant (RUQ) is typically related to biliary, colonic, hepatic, or renal causes.12 Because of infection and the potential need for surgical intervention, untreated acute biliary disease can become life-threatening, particularly in the elderly.4
The ACR recommends ultrasound (US) as the initial imaging study for RUQ pain, regardless of whether the patient is febrile or has an elevated white blood cell count or a positive Murphy’s sign (demonstrated at youtube.com/watch?v=9L7N89sOSuc) (score=9). A 2012 meta-analysis found that US has a sensitivity of 81% and specificity of 83% for diagnosing acute cholecystitis.6
Although cholescintigraphy has a higher sensitivity and specificity (96% and 90%, respectively), US remains the initial study of choice because of its availability, study time, and the lack of ionizing radiation. For equivocal findings, computed tomography (CT), magnetic resonance imaging (MRI), and cholescintigraphy have similar levels of evidence.6
Epigastric/left upper quadrant pain: Consider pancreatits
Epigastric and left upper quadrant (LUQ) pain may have a gastric, biliary, pancreatic, vascular, renal, or cardiac etiology.12 The ACR criteria for testing depends on the type of pain suspected.
Pancreatitis. The Revised Atlanta Classification of Acute Pancreatitis requires 2 of the 3 classic criteria for a pancreatitis diagnosis: 1) abdominal pain suggestive of pancreatitis, 2) serum amylase and lipase levels ≥3 times the normal level, and 3) characteristic findings on imaging.13 The ACR recommends US as the initial imaging modality for suspected acute pancreatitis when it is the initial presentation, the patient has typical abdominal pain and increased serum amylase and lipase, and symptom onset was <48 to 72 hours before the patient sought care (score=9).7
For patients who are critically ill, meet the criteria for systemic inflammatory response syndrome (SIRS), have severe clinical scores on either the Acute Physiology and Chronic Health Evaluation (APACHE) II (available at clincalc.com/icumortality/apacheii.aspx) or Bedside Index for Severity in Acute Appendicitis (BISAP; mdcalc.com/bisap-score-for-pancreatitis-mortality/), or who present >48 to 72 hours after onset of symptoms, abdominal CT with contrast is recommended (score=8).
Urolithiasis. Patients with abdominal pain from a presumed renal source should undergo a non-contrast CT of the abdomen and pelvis for initial imaging, according to the ACR (score=8). The sensitivity is 95% to 96% and specificity is 98%.8 To limit radiation exposure, low-dose protocols and limiting scan range are preferred.
Radiography can be useful in patients with known kidney stone disease and previous films; however, the sensitivity in other patients is poor (58%-62%).8 Because pelviectasis and ureterectasis can take hours to develop, US will miss more than 30% of acute obstructions in patients who are not fully hydrated and is therefore not recommended as a first-line imaging modality.8 The sensitivity of US increases to 71% when it is combined with kidney, ureter, and bladder radiography, but is still lower than that of CT or IV urography.8
Left lower quadrant pain: Suspect sigmoid diverticulitis
The differential for left lower quadrant (LLQ) pain includes colonic, gynecologic, and renal etiologies.12 The most common cause in adults is acute sigmoid diverticulitis. Patients often present with the clinical triad of fever, LLQ pain, and leukocytosis.14 A decision to obtain imaging should be based on both the clinical presentation and examination. It may not be required for patients who have mild symptoms or have had previous episodes of diverticulitis.
Clinical scoring systems have been studied for LLQ pain. However, none has been validated in all settings and therefore no such system is routinely used.15 CT of the abdomen and pelvis with contrast media is the ACR’s recommendation for the initial imaging study (score=9). CT has a reported overall accuracy of 99%.9
CT can also assess the severity of disease and help determine medical vs surgical treatment.14 US using graded compression has a sensitivity of 77% to 98% and a specificity of 80% to 99%, but is limited by body habitus, technical expertise, and patient comfort. Therefore, US has not gained widespread use (score=4) for patients with LLQ pain.9,14 MRI is emerging as a potential option; however, longer scan times, cost, and availability continue to limit its use.14
Right lower quadrant pain: Is it appendicitis?
The differential for right lower quadrant (RLQ) pain, like that of LLQ pain, includes colonic, gynecologic, and renal etiologies.12 The most common cause of acute RLQ pain requiring surgery is appendicitis. History and physical exam achieve a diagnostic accuracy of 80%.16
If the diagnosis is clear, no imaging is warranted. In patients with equivocal clinical presentations, however, imaging is cost-effective and may reduce the rate of perforation, morbidity, mortality, and postoperative hospital stays.16 In addition, the accuracy of clinical diagnosis for elderly patients and women of childbearing age with RLQ pain tends to be lower than that of adult men. Therefore, some experts call for a lower imaging threshold for these populations.
CT of the abdomen and pelvis with contrast is the recommended initial imaging study in nonpregnant adults (score=8). CT has a sensitivity and specificity of 91% and 90%, respectively.10
CT without contrast is indicated for patients with RLQ pain who have a contraindication to contrast media, although the relative radiation level remains the same.
If limiting radiation exposure is especially important, consider US, followed by CT with contrast if US is inconclusive.10
Low-dose CT has been investigated as an alternative, but is not routinely used. A limited abdominal CT scan from the bottom of the body of the T10 vertebra to the top of the symphysis pubis allows for adequate evaluation and alternate diagnoses of concern when compared with full CT scans of the abdomen and pelvis.17 This limited CT scan has been found to result in a total body effective radiation dose reduction of 23% and, in women, a breast equivalent dose reduction of 85%, without missing a single case of acute appendicitis or pertinent alternative diagnoses.17
Diffuse abdominal pain: Suspect a blockage
Finally, some patients may present with diffuse or non-localizable pain with fever. The etiologies that often present with diffuse or nonspecific pain include small bowel obstruction and mesenteric ischemia.
Small bowel obstruction. When small bowel obstruction is suspected, CT of the abdomen and pelvis with contrast (score=9) is recommended. Oral contrast is not indicated if you suspect a high-grade obstruction, but may add functional information when only a partial or low-grade obstruction is suspected. The relative radiation level remains the same for both.11 For patients with pain and fever, postoperative or not, CT of the abdomen and pelvis with contrast is recommended (score=8).3
Mesenteric ischemia is associated with high morbidity and mortality rates (30%-90%).18,19 Acute mesenteric ischemia is most commonly secondary to embolism, followed by arterial thrombosis, non-occlusive ischemia, and less commonly, venous thrombosis.18 The typical presentation is pain out of proportion to the physical exam.19
Differentiating mesenteric ischemia from other causes of acute abdominal pain can be difficult. Patients with chronic mesenteric ischemia present with postprandial abdominal pain, weight loss, and food avoidance. Although radiography is often the initial test ordered, a negative test does not rule out mesenteric ischemia. Therefore, the ACR recommends CT angiography (CTA) of the abdomen with contrast for the evaluation of both acute and chronic mesenteric ischemia (score=9). US can be useful for excluding other causes of abdominal pain, as well as ischemia related to venous occlusion, but it has a low sensitivity (70%-89%)18 overall and therefore is not recommended as the initial test for acute or chronic mesenteric ischemia.
Magnetic resonance angiography (MRA) has a high sensitivity and specificity for severe stenosis or origin occlusions of the superior mesenteric artery and celiac axis; however, its ability to determine distal embolism and non-occlusive ischemia, and the length and availability of this test limit its usefulness.18
When the patient is a child
Imaging for bilious vomiting in infants up to 3 months varies based on age. In the first week of life, radiography of the abdomen is the ACR’s recommended first-line test (score=9).20 An upper GI series or contrast enema are also options, but less preferred due to their increased radiation exposure.20
For infants between one week and 3 months of age, an upper GI series is the study of choice (score=9) and radiography of the abdomen is second line (score=5). An upper GI series is recommended to evaluate non-bilious, intermittent non-projectile vomiting in those from birth to 3 months.20 Projectile non-bilious vomiting should be evaluated with US of the abdomen.20
In children with RLQ pain suggestive of acute appendicitis, US is the first line imaging method due to its relatively high sensitivity and specificity and lack of ionizing radiation (score=9).10 If US is inconclusive, then CT of the abdomen and pelvis with IV contrast, but not oral or rectal contrast, is recommended (score=7). Although MRI is a non-radiating modality, it should be reserved for use only in specialized pediatric facilities due to lack of experience, increased cost, and the usual need for sedation.10
CASE › Mr. L’s vital signs demonstrated mild tachycardia and his body temperature was 100.5° F. His physical exam revealed significant tenderness to palpation in the LL Q, but no rebound or guarding. A CT scan with contrast of the abdomen revealed diverticulitis without abscess or perforation. The patient was managed with a clear liquid diet and told to return to the clinic 2 days later.
CORRESPONDENCE
Heidi L. Gaddey, MD, Family Medicine Residency Program, University of Nebraska Medical Center and the 55th Medical Group, 2501 Capehart Road, Offutt Air Force Base, NE 68113; [email protected]
› Choose ultrasonography as the initial imaging test for patients with pain in the right upper quadrant. C
› Order computed tomography with contrast of the abdomen and/or pelvis for adults with acute pain of new onset in the right or left lower quadrant, or both. C
› Recommend ultrasound with graded compression as the initial imaging modality for children younger than 14 years who have acute right lower quadrant pain. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Harry L, age 54, presents with acute onset abdominal pain that began 2 days ago. He reports mild nausea but no vomiting, non-bloody diarrhea, and feeling feverish. The patient denies increased pain with movement.
If Mr. L were your patient, how would you proceed?
Although acute nontraumatic abdominal pain accounts for only about 1.5% of physician office visits, it is the cause of approximately 8% of visits—more than 11 million, a year—to US emergency departments.1,2 In most cases, the location of the pain, coupled with the patient history, physical exam, and clinical judgment, lead to the differential diagnosis and determines what type of imaging, if any, is needed.
Benign self-limiting causes of abdominal pain, such as constipation or gastroenteritis, may not require any imaging. However, if the patient has abdominal pain and fever, is older than age 65, or presents with peritoneal signs and symptoms, imaging will be a crucial diagnostic aid.3,4
With that in mind, the American College of Radiology (ACR) has developed a 9-point scoring criteria based on both the site and common causes of abdominal pain to determine the best imaging modality to use to rule in—or out—serious disease and conditions for which surgery is required. A rating of 1, 2, or 3 indicates that imaging is usually not appropriate; a rating of 4, 5, or 6 means the imaging test may be appropriate, and a rating of 7, 8, or 9 indicates that the recommended test is usually appropriate. The panel of experts who developed the scores considered the relative radiation level of each imaging modality, as well.5
In the text and TABLE3,6-12 that follow, you’ll find the most likely clinical diagnoses and the optimal tests for abdominal pain, based largely on where it hurts.
CASE 1 › Appendicitis: An 8-year-old girl presented with acute right lower quadrant pain and underwent an ultrasound (left), which showed a dilated ~2 cm (normal <6 mm) non-compressible appendix consistent with acute appendicitis. There was no significant free fluid within the right lower quadrant. Follow-up computed tomography (CT) scan (middle) with IV and oral contrast confirmed the dilated appendix with no evidence of perforation or abscess formation.
CASE 2 › Small bowel obstruction: A 54-year-old man sought care for abdominal pain and distention. A CT with IV and oral contrast (right) revealed multiple, fluid-filled dilated loops of small bowel (arrows) with bowel wall thickening, edema, and inflammatory stranding. The short segment of decreased enhancement (arrowhead) was consistent with bowel ischemia.
Right upper quadrant pain: Beware of acute biliary disease
Abdominal pain of the right upper quadrant (RUQ) is typically related to biliary, colonic, hepatic, or renal causes.12 Because of infection and the potential need for surgical intervention, untreated acute biliary disease can become life-threatening, particularly in the elderly.4
The ACR recommends ultrasound (US) as the initial imaging study for RUQ pain, regardless of whether the patient is febrile or has an elevated white blood cell count or a positive Murphy’s sign (demonstrated at youtube.com/watch?v=9L7N89sOSuc) (score=9). A 2012 meta-analysis found that US has a sensitivity of 81% and specificity of 83% for diagnosing acute cholecystitis.6
Although cholescintigraphy has a higher sensitivity and specificity (96% and 90%, respectively), US remains the initial study of choice because of its availability, study time, and the lack of ionizing radiation. For equivocal findings, computed tomography (CT), magnetic resonance imaging (MRI), and cholescintigraphy have similar levels of evidence.6
Epigastric/left upper quadrant pain: Consider pancreatits
Epigastric and left upper quadrant (LUQ) pain may have a gastric, biliary, pancreatic, vascular, renal, or cardiac etiology.12 The ACR criteria for testing depends on the type of pain suspected.
Pancreatitis. The Revised Atlanta Classification of Acute Pancreatitis requires 2 of the 3 classic criteria for a pancreatitis diagnosis: 1) abdominal pain suggestive of pancreatitis, 2) serum amylase and lipase levels ≥3 times the normal level, and 3) characteristic findings on imaging.13 The ACR recommends US as the initial imaging modality for suspected acute pancreatitis when it is the initial presentation, the patient has typical abdominal pain and increased serum amylase and lipase, and symptom onset was <48 to 72 hours before the patient sought care (score=9).7
For patients who are critically ill, meet the criteria for systemic inflammatory response syndrome (SIRS), have severe clinical scores on either the Acute Physiology and Chronic Health Evaluation (APACHE) II (available at clincalc.com/icumortality/apacheii.aspx) or Bedside Index for Severity in Acute Appendicitis (BISAP; mdcalc.com/bisap-score-for-pancreatitis-mortality/), or who present >48 to 72 hours after onset of symptoms, abdominal CT with contrast is recommended (score=8).
Urolithiasis. Patients with abdominal pain from a presumed renal source should undergo a non-contrast CT of the abdomen and pelvis for initial imaging, according to the ACR (score=8). The sensitivity is 95% to 96% and specificity is 98%.8 To limit radiation exposure, low-dose protocols and limiting scan range are preferred.
Radiography can be useful in patients with known kidney stone disease and previous films; however, the sensitivity in other patients is poor (58%-62%).8 Because pelviectasis and ureterectasis can take hours to develop, US will miss more than 30% of acute obstructions in patients who are not fully hydrated and is therefore not recommended as a first-line imaging modality.8 The sensitivity of US increases to 71% when it is combined with kidney, ureter, and bladder radiography, but is still lower than that of CT or IV urography.8
Left lower quadrant pain: Suspect sigmoid diverticulitis
The differential for left lower quadrant (LLQ) pain includes colonic, gynecologic, and renal etiologies.12 The most common cause in adults is acute sigmoid diverticulitis. Patients often present with the clinical triad of fever, LLQ pain, and leukocytosis.14 A decision to obtain imaging should be based on both the clinical presentation and examination. It may not be required for patients who have mild symptoms or have had previous episodes of diverticulitis.
Clinical scoring systems have been studied for LLQ pain. However, none has been validated in all settings and therefore no such system is routinely used.15 CT of the abdomen and pelvis with contrast media is the ACR’s recommendation for the initial imaging study (score=9). CT has a reported overall accuracy of 99%.9
CT can also assess the severity of disease and help determine medical vs surgical treatment.14 US using graded compression has a sensitivity of 77% to 98% and a specificity of 80% to 99%, but is limited by body habitus, technical expertise, and patient comfort. Therefore, US has not gained widespread use (score=4) for patients with LLQ pain.9,14 MRI is emerging as a potential option; however, longer scan times, cost, and availability continue to limit its use.14
Right lower quadrant pain: Is it appendicitis?
The differential for right lower quadrant (RLQ) pain, like that of LLQ pain, includes colonic, gynecologic, and renal etiologies.12 The most common cause of acute RLQ pain requiring surgery is appendicitis. History and physical exam achieve a diagnostic accuracy of 80%.16
If the diagnosis is clear, no imaging is warranted. In patients with equivocal clinical presentations, however, imaging is cost-effective and may reduce the rate of perforation, morbidity, mortality, and postoperative hospital stays.16 In addition, the accuracy of clinical diagnosis for elderly patients and women of childbearing age with RLQ pain tends to be lower than that of adult men. Therefore, some experts call for a lower imaging threshold for these populations.
CT of the abdomen and pelvis with contrast is the recommended initial imaging study in nonpregnant adults (score=8). CT has a sensitivity and specificity of 91% and 90%, respectively.10
CT without contrast is indicated for patients with RLQ pain who have a contraindication to contrast media, although the relative radiation level remains the same.
If limiting radiation exposure is especially important, consider US, followed by CT with contrast if US is inconclusive.10
Low-dose CT has been investigated as an alternative, but is not routinely used. A limited abdominal CT scan from the bottom of the body of the T10 vertebra to the top of the symphysis pubis allows for adequate evaluation and alternate diagnoses of concern when compared with full CT scans of the abdomen and pelvis.17 This limited CT scan has been found to result in a total body effective radiation dose reduction of 23% and, in women, a breast equivalent dose reduction of 85%, without missing a single case of acute appendicitis or pertinent alternative diagnoses.17
Diffuse abdominal pain: Suspect a blockage
Finally, some patients may present with diffuse or non-localizable pain with fever. The etiologies that often present with diffuse or nonspecific pain include small bowel obstruction and mesenteric ischemia.
Small bowel obstruction. When small bowel obstruction is suspected, CT of the abdomen and pelvis with contrast (score=9) is recommended. Oral contrast is not indicated if you suspect a high-grade obstruction, but may add functional information when only a partial or low-grade obstruction is suspected. The relative radiation level remains the same for both.11 For patients with pain and fever, postoperative or not, CT of the abdomen and pelvis with contrast is recommended (score=8).3
Mesenteric ischemia is associated with high morbidity and mortality rates (30%-90%).18,19 Acute mesenteric ischemia is most commonly secondary to embolism, followed by arterial thrombosis, non-occlusive ischemia, and less commonly, venous thrombosis.18 The typical presentation is pain out of proportion to the physical exam.19
Differentiating mesenteric ischemia from other causes of acute abdominal pain can be difficult. Patients with chronic mesenteric ischemia present with postprandial abdominal pain, weight loss, and food avoidance. Although radiography is often the initial test ordered, a negative test does not rule out mesenteric ischemia. Therefore, the ACR recommends CT angiography (CTA) of the abdomen with contrast for the evaluation of both acute and chronic mesenteric ischemia (score=9). US can be useful for excluding other causes of abdominal pain, as well as ischemia related to venous occlusion, but it has a low sensitivity (70%-89%)18 overall and therefore is not recommended as the initial test for acute or chronic mesenteric ischemia.
Magnetic resonance angiography (MRA) has a high sensitivity and specificity for severe stenosis or origin occlusions of the superior mesenteric artery and celiac axis; however, its ability to determine distal embolism and non-occlusive ischemia, and the length and availability of this test limit its usefulness.18
When the patient is a child
Imaging for bilious vomiting in infants up to 3 months varies based on age. In the first week of life, radiography of the abdomen is the ACR’s recommended first-line test (score=9).20 An upper GI series or contrast enema are also options, but less preferred due to their increased radiation exposure.20
For infants between one week and 3 months of age, an upper GI series is the study of choice (score=9) and radiography of the abdomen is second line (score=5). An upper GI series is recommended to evaluate non-bilious, intermittent non-projectile vomiting in those from birth to 3 months.20 Projectile non-bilious vomiting should be evaluated with US of the abdomen.20
In children with RLQ pain suggestive of acute appendicitis, US is the first line imaging method due to its relatively high sensitivity and specificity and lack of ionizing radiation (score=9).10 If US is inconclusive, then CT of the abdomen and pelvis with IV contrast, but not oral or rectal contrast, is recommended (score=7). Although MRI is a non-radiating modality, it should be reserved for use only in specialized pediatric facilities due to lack of experience, increased cost, and the usual need for sedation.10
CASE › Mr. L’s vital signs demonstrated mild tachycardia and his body temperature was 100.5° F. His physical exam revealed significant tenderness to palpation in the LL Q, but no rebound or guarding. A CT scan with contrast of the abdomen revealed diverticulitis without abscess or perforation. The patient was managed with a clear liquid diet and told to return to the clinic 2 days later.
CORRESPONDENCE
Heidi L. Gaddey, MD, Family Medicine Residency Program, University of Nebraska Medical Center and the 55th Medical Group, 2501 Capehart Road, Offutt Air Force Base, NE 68113; [email protected]
1. Centers for Disease Control and Prevention. National Ambulatory Medical Care Survey: 2010 Summary Tables. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/data/ahcd/namcs_summary/2010_namcs_web_tables.pdf. Accessed April 8, 2015.
2. Centers for Disease Control and Prevention. National Hospital Ambulatory Medical Care Survey: 2011 Emergency Department Summary Tables. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf. Accessed February 2, 2015.
3. American College of Radiology. ACR Appropriateness Criteria: Acute (Nonlocalized) abdominal pain and fever or suspected abdominal abscess. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69467/Narrative. Accessed December 15, 2014.
4. Lyon C, Clark DC. Diagnosis of acute abdominal pain in older patients. Am Fam Physician. 2006;74:1537-1544.
5. Crownover BK, Bepko JL. Appropriate and safe use of diagnostic imaging. Am Fam Physician. 2013;87:494-501.
6. American College of Radiology. ACR Appropriateness Criteria: right upper quadrant pain. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69474/Narrative/. Accessed December 15, 2014.
7. American College of Radiology. ACR Appropriateness Criteria: acute pancreatitis. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69468/Narrative. Accessed April 8, 2015.
8. American College of Radiology. ACR Appropriateness Criteria: acute onset flank pain—suspicion of stone disease. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69362/Narrative/. Accessed December 1, 2014.
9. American College of Radiology. ACR Appropriateness Criteria: left lower quadrant pain—suspected diverticulitis. American College of Radiology Web site. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/LeftLowerQuadrantPainSuspectedDiverticulitis.pdf. Accessed December 1, 2014.
10. American College of Radiology. ACR Appropriateness Criteria: right lower quadrant pain—suspected appendicitis. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69357/Narrative/. Accessed December 15, 2014.
11. American College of Radiology. ACR Appropriateness Criteria: suspected small-bowel obstruction. American College of Radiology Web site. Available at: http://www.acr.org/~/media/832F100277004BC69A8C818C7C9BFF33.pdf. Accessed December 10, 2014.
12. Cartwright SL, Knudson MP. Evaluation of acute abdominal pain in adults. Am Fam Physician. 2008;77:971-978.
13. Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262:751-764.
14. American College of Radiology. ACR Appropriateness Criteria: left lower quadrant pain—suspected diverticulitis. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69356/Narrative/. Accessed December 1, 2014.
15. Andeweg CS, Knobben L, Hendriks JC, et al. How to diagnose acute left-sided colonic diverticulitis: proposal for a clinical scoring system. Ann Surg. 2011;253:940-946.
16. Old JL, Dusing RW, Yap W, et al. Imaging for suspected appendicitis. Am Fam Physician. 2005;71:71-78.
17. Corwin MT, Chang M, Fananapazir G, et al. Accuracy and radiation dose reduction of a limited abdominopelvic CT in the diagnosis of acute appendicitis. Abdom Imaging. 2014; October 21 [Epub ahead of print].
18. American College of Radiology. ACR Appropriateness Criteria: imaging of mesenteric ischemia. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/70909/Narrative/. Accessed December 4, 2014.
19. Herbert GS, Steele SR. Acute and chronic mesenteric ischemia. Surg Clin North Am. 2007;87:1115-1134.
20. American College of Radiology. ACR Appropriateness Criteria: vomiting in infants up to 3 months of age. American College of Radiology Web site. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/VomitingInInfantsUpTo3MonthsOfAge.pdf. Accessed January 5, 2015.
1. Centers for Disease Control and Prevention. National Ambulatory Medical Care Survey: 2010 Summary Tables. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/data/ahcd/namcs_summary/2010_namcs_web_tables.pdf. Accessed April 8, 2015.
2. Centers for Disease Control and Prevention. National Hospital Ambulatory Medical Care Survey: 2011 Emergency Department Summary Tables. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf. Accessed February 2, 2015.
3. American College of Radiology. ACR Appropriateness Criteria: Acute (Nonlocalized) abdominal pain and fever or suspected abdominal abscess. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69467/Narrative. Accessed December 15, 2014.
4. Lyon C, Clark DC. Diagnosis of acute abdominal pain in older patients. Am Fam Physician. 2006;74:1537-1544.
5. Crownover BK, Bepko JL. Appropriate and safe use of diagnostic imaging. Am Fam Physician. 2013;87:494-501.
6. American College of Radiology. ACR Appropriateness Criteria: right upper quadrant pain. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69474/Narrative/. Accessed December 15, 2014.
7. American College of Radiology. ACR Appropriateness Criteria: acute pancreatitis. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69468/Narrative. Accessed April 8, 2015.
8. American College of Radiology. ACR Appropriateness Criteria: acute onset flank pain—suspicion of stone disease. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69362/Narrative/. Accessed December 1, 2014.
9. American College of Radiology. ACR Appropriateness Criteria: left lower quadrant pain—suspected diverticulitis. American College of Radiology Web site. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/LeftLowerQuadrantPainSuspectedDiverticulitis.pdf. Accessed December 1, 2014.
10. American College of Radiology. ACR Appropriateness Criteria: right lower quadrant pain—suspected appendicitis. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69357/Narrative/. Accessed December 15, 2014.
11. American College of Radiology. ACR Appropriateness Criteria: suspected small-bowel obstruction. American College of Radiology Web site. Available at: http://www.acr.org/~/media/832F100277004BC69A8C818C7C9BFF33.pdf. Accessed December 10, 2014.
12. Cartwright SL, Knudson MP. Evaluation of acute abdominal pain in adults. Am Fam Physician. 2008;77:971-978.
13. Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262:751-764.
14. American College of Radiology. ACR Appropriateness Criteria: left lower quadrant pain—suspected diverticulitis. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/69356/Narrative/. Accessed December 1, 2014.
15. Andeweg CS, Knobben L, Hendriks JC, et al. How to diagnose acute left-sided colonic diverticulitis: proposal for a clinical scoring system. Ann Surg. 2011;253:940-946.
16. Old JL, Dusing RW, Yap W, et al. Imaging for suspected appendicitis. Am Fam Physician. 2005;71:71-78.
17. Corwin MT, Chang M, Fananapazir G, et al. Accuracy and radiation dose reduction of a limited abdominopelvic CT in the diagnosis of acute appendicitis. Abdom Imaging. 2014; October 21 [Epub ahead of print].
18. American College of Radiology. ACR Appropriateness Criteria: imaging of mesenteric ischemia. American College of Radiology Web site. Available at: https://acsearch.acr.org/docs/70909/Narrative/. Accessed December 4, 2014.
19. Herbert GS, Steele SR. Acute and chronic mesenteric ischemia. Surg Clin North Am. 2007;87:1115-1134.
20. American College of Radiology. ACR Appropriateness Criteria: vomiting in infants up to 3 months of age. American College of Radiology Web site. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/VomitingInInfantsUpTo3MonthsOfAge.pdf. Accessed January 5, 2015.
RADIOLOGY REPORT: Two radiologists offer 6 tips of the trade
1. Ask yourself the following before you order that test:
• Will imaging change management?
• Are there previous imaging results that provide diagnostic management information?
• Can the same information be obtained without exposure to ionizing radiation?
2. Call the radiologist to clarify the best initial imaging exam to answer a particular clinical question. A brief discussion of a patient’s presentation, initial work-up, and differential considerations will help the radiologist to ensure that he or she is performing the right exam to help guide management. Such initial discussions can reduce unnecessary, incorrect, or repeated exams and in some cases, may help to determine if a referral to a tertiary care center would be more appropriate.
3. Take into account available technology and patient allergies to determine the most expedient, efficacious exam to answer the clinical question.
Atopic individuals (particularly those with multiple severe allergies) and those with asthma are at heightened risk for allergic-like contrast reactions.1 A history of a prior allergic-like reaction to contrast is the most substantial risk factor for a recurrent adverse allergic reaction.1 In cases of prior contrast reaction, and when the benefits outweigh the risks, consider a pre-medication routine.
4. Provide the radiologist with a thorough history, which should include surgeries and pertinent laboratory values. Knowing the type of surgery a patient had and how recent it was will help guide the radiologist in evaluating for specific complications. For example, if a patient has had a recent laparoscopic cholecystectomy, complications can include bile leak, infection/abscess, hemorrhage, and intestinal injury.
Knowing this information will help avoid misinterpreting a small amount of expected free air or inflammatory change from acute bowel perforation with early phlegmon/abscess formation.
Similarly, it is vital for the radiologist to know if the patient being evaluated for right flank pain is febrile or has an elevated white blood cell count as an obstructed kidney that is infected may require emergent percutaneous drainage. On the other hand, an obstructed kidney without infection can, in most cases, be managed expectantly.
5. Include good provider contact information in the imaging request so any emergent findings can be readily communicated. Too few requests include provider contact information and when provided, it often leads to a time-consuming phone tree (or is outdated).
Routine exams should include the direct line of the clinic, receptionist, or provider’s desk. All emergent exams should include a current pager or cell phone number where the ordering physician or team can be reached directly.
6. Increase your knowledge of incidental findings and how to manage them. A good resource, “Managing incidental findings on abdominal CT: white paper of the ACR Incidental Findings Committee,” can be found at http://www.jacr.org/article/S1546-1440(10)00330-3/fulltext.
Reference
1. American College of Radiology. Committee on Drugs and Contrast Media. Manual on Contrast Media: Version 9. American College of Radiology Web site. Available at: http://www.acr.org/quality-safety/resources/contrast-manual. Accessed April 13, 2015.
1. Ask yourself the following before you order that test:
• Will imaging change management?
• Are there previous imaging results that provide diagnostic management information?
• Can the same information be obtained without exposure to ionizing radiation?
2. Call the radiologist to clarify the best initial imaging exam to answer a particular clinical question. A brief discussion of a patient’s presentation, initial work-up, and differential considerations will help the radiologist to ensure that he or she is performing the right exam to help guide management. Such initial discussions can reduce unnecessary, incorrect, or repeated exams and in some cases, may help to determine if a referral to a tertiary care center would be more appropriate.
3. Take into account available technology and patient allergies to determine the most expedient, efficacious exam to answer the clinical question.
Atopic individuals (particularly those with multiple severe allergies) and those with asthma are at heightened risk for allergic-like contrast reactions.1 A history of a prior allergic-like reaction to contrast is the most substantial risk factor for a recurrent adverse allergic reaction.1 In cases of prior contrast reaction, and when the benefits outweigh the risks, consider a pre-medication routine.
4. Provide the radiologist with a thorough history, which should include surgeries and pertinent laboratory values. Knowing the type of surgery a patient had and how recent it was will help guide the radiologist in evaluating for specific complications. For example, if a patient has had a recent laparoscopic cholecystectomy, complications can include bile leak, infection/abscess, hemorrhage, and intestinal injury.
Knowing this information will help avoid misinterpreting a small amount of expected free air or inflammatory change from acute bowel perforation with early phlegmon/abscess formation.
Similarly, it is vital for the radiologist to know if the patient being evaluated for right flank pain is febrile or has an elevated white blood cell count as an obstructed kidney that is infected may require emergent percutaneous drainage. On the other hand, an obstructed kidney without infection can, in most cases, be managed expectantly.
5. Include good provider contact information in the imaging request so any emergent findings can be readily communicated. Too few requests include provider contact information and when provided, it often leads to a time-consuming phone tree (or is outdated).
Routine exams should include the direct line of the clinic, receptionist, or provider’s desk. All emergent exams should include a current pager or cell phone number where the ordering physician or team can be reached directly.
6. Increase your knowledge of incidental findings and how to manage them. A good resource, “Managing incidental findings on abdominal CT: white paper of the ACR Incidental Findings Committee,” can be found at http://www.jacr.org/article/S1546-1440(10)00330-3/fulltext.
1. Ask yourself the following before you order that test:
• Will imaging change management?
• Are there previous imaging results that provide diagnostic management information?
• Can the same information be obtained without exposure to ionizing radiation?
2. Call the radiologist to clarify the best initial imaging exam to answer a particular clinical question. A brief discussion of a patient’s presentation, initial work-up, and differential considerations will help the radiologist to ensure that he or she is performing the right exam to help guide management. Such initial discussions can reduce unnecessary, incorrect, or repeated exams and in some cases, may help to determine if a referral to a tertiary care center would be more appropriate.
3. Take into account available technology and patient allergies to determine the most expedient, efficacious exam to answer the clinical question.
Atopic individuals (particularly those with multiple severe allergies) and those with asthma are at heightened risk for allergic-like contrast reactions.1 A history of a prior allergic-like reaction to contrast is the most substantial risk factor for a recurrent adverse allergic reaction.1 In cases of prior contrast reaction, and when the benefits outweigh the risks, consider a pre-medication routine.
4. Provide the radiologist with a thorough history, which should include surgeries and pertinent laboratory values. Knowing the type of surgery a patient had and how recent it was will help guide the radiologist in evaluating for specific complications. For example, if a patient has had a recent laparoscopic cholecystectomy, complications can include bile leak, infection/abscess, hemorrhage, and intestinal injury.
Knowing this information will help avoid misinterpreting a small amount of expected free air or inflammatory change from acute bowel perforation with early phlegmon/abscess formation.
Similarly, it is vital for the radiologist to know if the patient being evaluated for right flank pain is febrile or has an elevated white blood cell count as an obstructed kidney that is infected may require emergent percutaneous drainage. On the other hand, an obstructed kidney without infection can, in most cases, be managed expectantly.
5. Include good provider contact information in the imaging request so any emergent findings can be readily communicated. Too few requests include provider contact information and when provided, it often leads to a time-consuming phone tree (or is outdated).
Routine exams should include the direct line of the clinic, receptionist, or provider’s desk. All emergent exams should include a current pager or cell phone number where the ordering physician or team can be reached directly.
6. Increase your knowledge of incidental findings and how to manage them. A good resource, “Managing incidental findings on abdominal CT: white paper of the ACR Incidental Findings Committee,” can be found at http://www.jacr.org/article/S1546-1440(10)00330-3/fulltext.
Reference
1. American College of Radiology. Committee on Drugs and Contrast Media. Manual on Contrast Media: Version 9. American College of Radiology Web site. Available at: http://www.acr.org/quality-safety/resources/contrast-manual. Accessed April 13, 2015.
Reference
1. American College of Radiology. Committee on Drugs and Contrast Media. Manual on Contrast Media: Version 9. American College of Radiology Web site. Available at: http://www.acr.org/quality-safety/resources/contrast-manual. Accessed April 13, 2015.
RADIOLOGY REPORT: 2 cases to test your skills
CASE 1 ›
THE PATIENT: 28 year-old G0P0 woman
SIGNS & SYMPTOMS: Chronic pelvic fullness, acute lower abdominal/pelvic pain
A 28-year-old G0P0 woman with a history of chronic pelvic fullness presented with acute lower abdominal/pelvic pain. A pelvic exam showed mild cervical motion tenderness without vaginal discharge or palpable adnexal mass. Initial blood work was drawn and the patient was sent for a pelvic ultrasound with differential considerations including pelvic inflammatory disease, ovarian torsion, and intrauterine or ectopic pregnancy.
Transabdominal and transvaginal ultrasound (US) demonstrated unremarkable sonographic appearance of the uterus and bilateral ovaries. An evaluation of the adnexa was slightly limited secondary to multiple tortuous pelvic veins (FIGURE 1). The adnexa were otherwise grossly unremarkable and there was a small amount of free fluid within the pelvis.
Lab values came back and were remarkable for mild leukocytosis. The patient had a negative human chorionic gonadotropin test.
Given the patient’s progressive symptoms and mild leukocytosis, the physician ordered a computed tomography (CT) scan of the abdomen and pelvis with contrast.
The CT of the abdomen and pelvis with contrast revealed multiple dilated (>4 mm) tortuous periuterine vessels, left greater than right (FIGURE 2, short arrows). There was concurrent dilation of the left gonadal vein, which measured up to 7 to 8 mm (FIGURE 2, long arrow). No other acute intra-abdominal or pelvic process was identified. A trace amount of simple appearing free fluid was noted.
WHAT'S YOUR DIAGNOSIS?
The patient was given a diagnosis of pelvic congestion syndrome. She was managed conservatively and referred to an OB/GYN, who recommended a venogram and left gonadal vein coil embolization. After the procedure, the patient improved.
DISCUSSION
Patients with pelvic congestion syndrome typically present with chronic (>6 months), non-cyclical pelvic discomfort or fullness. The pathophysiology is similar to varicoceles in men and can be caused by incompetent valves, compression of the left renal vein by the superior mesenteric artery, or venous obstruction from an anatomic variant such as a retroaortic left renal vein. Treatment options include gonadal vein embolization and laparoscopic ligation.
Key imaging characteristics: Ultrasound, magnetic resonance imaging, and CT may show tortuous, dilated pelvic veins that measure >4 mm or a gonadal vein that measures >7 mm, consistent with pelvic or ovarian varices.
If there is clinical suspicion or imaging features suggestive of pelvic congestion, patients may require a pelvic venogram. Because established imaging criteria vary and initial imaging assessments can appear normal, pelvic congestion syndrome ultimately remains a clinical diagnosis.
CASE 2 ›
THE PATIENT: 64-year-old man with a history of diverticulosis
SIGNS & SYMPTOMS: Left lower quadrant pain
A 64-year-old man with a history of diverticulosis presented with 3 days of worsening left lower quadrant pain. Given the patient’s progressive symptoms and pain out of proportion to exam, acute diverticulitis was suspected. A computed tomography (CT) scan of the abdomen and pelvis with contrast was ordered to exclude complicating factors.
Axial CT images through the left lower quadrant demonstrated a 2.5 cm fat-containing ovoid lesion (FIGURE, star) with hyperemic rim (FIGURE, short arrows) abutting the lateral wall of the sigmoid colon with mild surrounding inflammatory change (FIGURE, long arrow).
There was no evidence of superimposed bowel obstruction, free air, or abscess formation. Scattered colonic diverticula were noted, without adjacent inflammatory change to suggest acute diverticulitis.
WHAT'S YOUR DIAGNOSIS?
The CT findings were consistent with a diagnosis of epiploic appendagitis. Subsequent lab work demonstrated no evidence of leukocytosis and a normal lactate value.
Given the benign and self-limiting nature of epiploic appendagitis, the patient was managed conservatively with oral anti-inflammatory medications (600 mg ibuprofen PO every 8 hours for 4-6 days) and released from the clinic with return precautions. Follow-up in 7 days demonstrated near-complete resolution of the patient’s symptoms.
DISCUSSION
Epiploic appendagitis is typically a nonsurgical, self-limiting process resulting from torsion of one or more epiploic appendages along the sigmoid, descending, or right hemicolon with resultant vascular occlusion that leads to ischemia.
Epiploic appendage ligation and resection is rare and usually reserved for when conservative management fails or there is new or worsening symptoms such as fever, progressive pain, or refractory nausea and vomiting.
Key imaging characteristics: 2-3 cm ovoid, fat-containing paracolic mass with thickened peritoneal lining and periappendiceal fat stranding. Classic features include a hyperattenuated central dot within the appendage corresponding to the thrombosed draining vein.
CASE 1 ›
THE PATIENT: 28 year-old G0P0 woman
SIGNS & SYMPTOMS: Chronic pelvic fullness, acute lower abdominal/pelvic pain
A 28-year-old G0P0 woman with a history of chronic pelvic fullness presented with acute lower abdominal/pelvic pain. A pelvic exam showed mild cervical motion tenderness without vaginal discharge or palpable adnexal mass. Initial blood work was drawn and the patient was sent for a pelvic ultrasound with differential considerations including pelvic inflammatory disease, ovarian torsion, and intrauterine or ectopic pregnancy.
Transabdominal and transvaginal ultrasound (US) demonstrated unremarkable sonographic appearance of the uterus and bilateral ovaries. An evaluation of the adnexa was slightly limited secondary to multiple tortuous pelvic veins (FIGURE 1). The adnexa were otherwise grossly unremarkable and there was a small amount of free fluid within the pelvis.
Lab values came back and were remarkable for mild leukocytosis. The patient had a negative human chorionic gonadotropin test.
Given the patient’s progressive symptoms and mild leukocytosis, the physician ordered a computed tomography (CT) scan of the abdomen and pelvis with contrast.
The CT of the abdomen and pelvis with contrast revealed multiple dilated (>4 mm) tortuous periuterine vessels, left greater than right (FIGURE 2, short arrows). There was concurrent dilation of the left gonadal vein, which measured up to 7 to 8 mm (FIGURE 2, long arrow). No other acute intra-abdominal or pelvic process was identified. A trace amount of simple appearing free fluid was noted.
WHAT'S YOUR DIAGNOSIS?
The patient was given a diagnosis of pelvic congestion syndrome. She was managed conservatively and referred to an OB/GYN, who recommended a venogram and left gonadal vein coil embolization. After the procedure, the patient improved.
DISCUSSION
Patients with pelvic congestion syndrome typically present with chronic (>6 months), non-cyclical pelvic discomfort or fullness. The pathophysiology is similar to varicoceles in men and can be caused by incompetent valves, compression of the left renal vein by the superior mesenteric artery, or venous obstruction from an anatomic variant such as a retroaortic left renal vein. Treatment options include gonadal vein embolization and laparoscopic ligation.
Key imaging characteristics: Ultrasound, magnetic resonance imaging, and CT may show tortuous, dilated pelvic veins that measure >4 mm or a gonadal vein that measures >7 mm, consistent with pelvic or ovarian varices.
If there is clinical suspicion or imaging features suggestive of pelvic congestion, patients may require a pelvic venogram. Because established imaging criteria vary and initial imaging assessments can appear normal, pelvic congestion syndrome ultimately remains a clinical diagnosis.
CASE 2 ›
THE PATIENT: 64-year-old man with a history of diverticulosis
SIGNS & SYMPTOMS: Left lower quadrant pain
A 64-year-old man with a history of diverticulosis presented with 3 days of worsening left lower quadrant pain. Given the patient’s progressive symptoms and pain out of proportion to exam, acute diverticulitis was suspected. A computed tomography (CT) scan of the abdomen and pelvis with contrast was ordered to exclude complicating factors.
Axial CT images through the left lower quadrant demonstrated a 2.5 cm fat-containing ovoid lesion (FIGURE, star) with hyperemic rim (FIGURE, short arrows) abutting the lateral wall of the sigmoid colon with mild surrounding inflammatory change (FIGURE, long arrow).
There was no evidence of superimposed bowel obstruction, free air, or abscess formation. Scattered colonic diverticula were noted, without adjacent inflammatory change to suggest acute diverticulitis.
WHAT'S YOUR DIAGNOSIS?
The CT findings were consistent with a diagnosis of epiploic appendagitis. Subsequent lab work demonstrated no evidence of leukocytosis and a normal lactate value.
Given the benign and self-limiting nature of epiploic appendagitis, the patient was managed conservatively with oral anti-inflammatory medications (600 mg ibuprofen PO every 8 hours for 4-6 days) and released from the clinic with return precautions. Follow-up in 7 days demonstrated near-complete resolution of the patient’s symptoms.
DISCUSSION
Epiploic appendagitis is typically a nonsurgical, self-limiting process resulting from torsion of one or more epiploic appendages along the sigmoid, descending, or right hemicolon with resultant vascular occlusion that leads to ischemia.
Epiploic appendage ligation and resection is rare and usually reserved for when conservative management fails or there is new or worsening symptoms such as fever, progressive pain, or refractory nausea and vomiting.
Key imaging characteristics: 2-3 cm ovoid, fat-containing paracolic mass with thickened peritoneal lining and periappendiceal fat stranding. Classic features include a hyperattenuated central dot within the appendage corresponding to the thrombosed draining vein.
CASE 1 ›
THE PATIENT: 28 year-old G0P0 woman
SIGNS & SYMPTOMS: Chronic pelvic fullness, acute lower abdominal/pelvic pain
A 28-year-old G0P0 woman with a history of chronic pelvic fullness presented with acute lower abdominal/pelvic pain. A pelvic exam showed mild cervical motion tenderness without vaginal discharge or palpable adnexal mass. Initial blood work was drawn and the patient was sent for a pelvic ultrasound with differential considerations including pelvic inflammatory disease, ovarian torsion, and intrauterine or ectopic pregnancy.
Transabdominal and transvaginal ultrasound (US) demonstrated unremarkable sonographic appearance of the uterus and bilateral ovaries. An evaluation of the adnexa was slightly limited secondary to multiple tortuous pelvic veins (FIGURE 1). The adnexa were otherwise grossly unremarkable and there was a small amount of free fluid within the pelvis.
Lab values came back and were remarkable for mild leukocytosis. The patient had a negative human chorionic gonadotropin test.
Given the patient’s progressive symptoms and mild leukocytosis, the physician ordered a computed tomography (CT) scan of the abdomen and pelvis with contrast.
The CT of the abdomen and pelvis with contrast revealed multiple dilated (>4 mm) tortuous periuterine vessels, left greater than right (FIGURE 2, short arrows). There was concurrent dilation of the left gonadal vein, which measured up to 7 to 8 mm (FIGURE 2, long arrow). No other acute intra-abdominal or pelvic process was identified. A trace amount of simple appearing free fluid was noted.
WHAT'S YOUR DIAGNOSIS?
The patient was given a diagnosis of pelvic congestion syndrome. She was managed conservatively and referred to an OB/GYN, who recommended a venogram and left gonadal vein coil embolization. After the procedure, the patient improved.
DISCUSSION
Patients with pelvic congestion syndrome typically present with chronic (>6 months), non-cyclical pelvic discomfort or fullness. The pathophysiology is similar to varicoceles in men and can be caused by incompetent valves, compression of the left renal vein by the superior mesenteric artery, or venous obstruction from an anatomic variant such as a retroaortic left renal vein. Treatment options include gonadal vein embolization and laparoscopic ligation.
Key imaging characteristics: Ultrasound, magnetic resonance imaging, and CT may show tortuous, dilated pelvic veins that measure >4 mm or a gonadal vein that measures >7 mm, consistent with pelvic or ovarian varices.
If there is clinical suspicion or imaging features suggestive of pelvic congestion, patients may require a pelvic venogram. Because established imaging criteria vary and initial imaging assessments can appear normal, pelvic congestion syndrome ultimately remains a clinical diagnosis.
CASE 2 ›
THE PATIENT: 64-year-old man with a history of diverticulosis
SIGNS & SYMPTOMS: Left lower quadrant pain
A 64-year-old man with a history of diverticulosis presented with 3 days of worsening left lower quadrant pain. Given the patient’s progressive symptoms and pain out of proportion to exam, acute diverticulitis was suspected. A computed tomography (CT) scan of the abdomen and pelvis with contrast was ordered to exclude complicating factors.
Axial CT images through the left lower quadrant demonstrated a 2.5 cm fat-containing ovoid lesion (FIGURE, star) with hyperemic rim (FIGURE, short arrows) abutting the lateral wall of the sigmoid colon with mild surrounding inflammatory change (FIGURE, long arrow).
There was no evidence of superimposed bowel obstruction, free air, or abscess formation. Scattered colonic diverticula were noted, without adjacent inflammatory change to suggest acute diverticulitis.
WHAT'S YOUR DIAGNOSIS?
The CT findings were consistent with a diagnosis of epiploic appendagitis. Subsequent lab work demonstrated no evidence of leukocytosis and a normal lactate value.
Given the benign and self-limiting nature of epiploic appendagitis, the patient was managed conservatively with oral anti-inflammatory medications (600 mg ibuprofen PO every 8 hours for 4-6 days) and released from the clinic with return precautions. Follow-up in 7 days demonstrated near-complete resolution of the patient’s symptoms.
DISCUSSION
Epiploic appendagitis is typically a nonsurgical, self-limiting process resulting from torsion of one or more epiploic appendages along the sigmoid, descending, or right hemicolon with resultant vascular occlusion that leads to ischemia.
Epiploic appendage ligation and resection is rare and usually reserved for when conservative management fails or there is new or worsening symptoms such as fever, progressive pain, or refractory nausea and vomiting.
Key imaging characteristics: 2-3 cm ovoid, fat-containing paracolic mass with thickened peritoneal lining and periappendiceal fat stranding. Classic features include a hyperattenuated central dot within the appendage corresponding to the thrombosed draining vein.
When to recommend cognitive behavioral therapy
› Tell patients who are potential candidates for cognitive behavioral therapy (CBT) that it has been demonstrated to be effective in treating anxiety and trauma-related disorders. A
› Motivate patients by pointing out that CBT is short-term therapy that is cost-effective and has the potential to be more beneficial than medication. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Darla S, a 42-year-old being treated for gastrointestinal (GI) distress, has undergone multiple tests over the course of the year, including a colonoscopy, an endoscopy, and a food allergy work-up. All had negative results. Medication trials—with proton pump inhibitors, H2 receptor antagonists, and prokinetics, among others—have not brought her any relief. The patient recently began taking sertraline 200 mg/d, which seemed to be helping. But on her latest visit, Ms. S requests a prescription for a sleeping pill. When asked what’s been keeping her up, the patient confides that she recently began having nightmares relating to a sexual assault that occurred several years ago.
If Ms. S were your patient, what would you recommend?
Family physicians (FPs) often encounter patients who are experiencing psychological distress, particularly anxiety.1 This may become evident when you’re treating one problem, such as low back pain or GI distress, but come to realize that anxiety is a key contributing factor or cause. Or you may discover that an anxiety or trauma-related disorder is complicating or interfering with treatment—preventing a patient with heart disease from quitting smoking, exercising regularly, or following a heart-healthy diet, for example.
Psychotropic medication is an option in such cases, of course. But the drugs often have adverse effects or interact with other medications the patient is taking, and their effects typically last only as long as the course of treatment. Being familiar with effective nonpharmacologic treatments—most notably, cognitive behavioral therapy (CBT)—will help you provide such patients with optimal care.
Advantages. CBT has several advantages that supportive counseling, traditional psychotherapy, and other nonpharmacologic treatments for psychological disorders do not: It is time-limited, typically lasting 9 to 12 weeks; skill-based; and goal-oriented. It also has a large amount of data to support it.2-4
Chances are you are familiar with the basic elements of CBT—challenging problematic beliefs, ensuring an increase in pleasant activities, and providing extended exposure to places or activities that trigger avoidance and/or arousal so that these responses are gradually diminished.2 However, there is not one single model of CBT. Rather, there are specific protocols for the conditions included in this review (TABLE 1).5
But before we get to the protocols, let’s first look at the evidence.
Meta-analyses demonstrate efficacy and effectiveness
Multiple studies and meta-analyses have consistently found CBT to reduce symptoms associated with anxiety and trauma-related disorders. Foremost among them are a metaanalysis by Hofmann and Smits6 of randomized placebo-controlled studies that assessed CBT’s efficacy and a meta-analysis by Stewart and Chambless7 that focused instead on effectiveness studies—ie, those assessing CBT in less-controlled, real-world practice. The findings are highlighted in TABLE 2.6,7
A 2012 review of meta-analyses of CBT8 for a broader range of psychological disorders found it to be more effective in treating generalized anxiety disorder (GAD), obsessive compulsive disorder (OCD), panic disorder (PD), posttraumatic stress disorder (PTSD), and social anxiety disorder (SAD) than control conditions, such as placebo, and often more effective than other treatments. Notably, CBT was shown to be more effective than relaxation therapy for PD, more effective in the long term than psychopharmacology for SAD, and more effective than supportive counseling for PTSD.
Cognitive processing and exposure therapy for PTSD
Two types of CBT have been found to be particularly effective in treating PTSD: cognitive processing therapy and exposure therapy. Each has specific protocols, although treatment often has some components of each.9
Cognitive processing therapy, a firstline treatment for PTSD, was initially developed for the treatment of rape victims,10 but has been found to be effective in treating combat-related PTSD, as well.11 It incorporates the core elements of cognitive therapy—identifying false or unhelpful trauma-related thoughts, then evaluating the evidence for and against them so the patient learns to consider whether these problematic thoughts are the result of cognitive bias or error and develop more realistic and/or useful thoughts. Cognitive processing therapy, however, focuses primarily on issues of safety, danger, and trust relating to patients’ views of themselves, others, and the world. Patients are asked to write, and then read, a narrative of the trauma they endured to help them challenge troubling thoughts about it.10
A woman undergoing treatment for PTSD relating to a sexual assault, for example, may initially think, “All men are bad.” Challenging this thought by examining evidence for and against it may help her replace it with the more realistic belief that some—but not all—men are bad.
Exposure therapy, which is also a firstline treatment for PTSD,12-14 involves presenting the frightening stimuli to patients in a safe environment so that they can learn a new way of responding.9 If a patient is afraid of a specific location because she was assaulted there, for instance, slowly exposing her to the site while ensuring her safety can help her anxiety diminish. Depending on the circumstances, exposure may be conducted in vivo (tangible stimuli), achieved through mental imagery (of a combat zone where an improvised explosive device detonated, for example), or both.
Prolonged exposure has been shown to be very effective in treating PTSD resulting from a variety of traumatic events. Other aspects of treatment include education about the disorder and breathing retraining to reduce arousal and increase the patient’s ability to relax.15
Generalized anxiety disorder: Worry exposure and relaxation
CBT for GAD has 5 components:
- education about the disorder
- cognitive restructuring
- progressive muscle relaxation
- worry exposure
- in vivo exposure.
Relaxation training is a crucial part of treatment for GAD, perhaps more so than for other anxiety disorders.16 Cognitive restructuring is vital, as well. This involves the use of the Socratic means of questioning, asking “Tell me what you mean by ‘horrible,’” for example, and “What about that is of most concern to you?”
Worry exposure occurs by instructing the patient to engage in prolonged worry about one particular topic, rather than jumping from one worrisome subject to another. The single focus reduces the distress that worry causes, thereby decreasing the time spent worrying.17 As treatment progresses, the patient is taught to set aside a specific time to worry. Worrying outside of the designated “worry time” is not allowed.18,19
Panic disorder: Recognizing what's behind physical symptoms
Treatment for PD combines education about the disorder, cognitive restructuring, and exposure.
Education helps the patient understand the reason the increased arousal response occurs at seemingly random times—recognizing that he or she is interpreting normal physiological sensations negatively, for example, and that the physical response is the body’s way of protecting itself.
Cognitive restructuring helps patients reformulate their view of the relationship between physical symptoms and panic attacks. An individual might learn to interpret a rapid heart rate as an indication that his heart is working harder and getting stronger, for instance, rather than as a symptom of cardiovascular distress.
Interoceptive exposure therapy teaches patients to identify their internal physical cues (eg, shortness of breath, shakiness, and tachycardia) and then deliberately induce them—by breathing through a straw, climbing a flight of stairs, or spinning in a chair, for example. With repeated exposure, patients learn that the physical sensations are not dangerous, and the anxiety associated with them decreases.
In vivo exposure involves the creation of a “fear hierarchy” of places and activities that the patient avoids due to fear of having a panic attack, then gradually exposing him or her to them.18,20
Obsessive-compulsive disorder: Exposure and response
Exposure and response prevention (ERP) is the primary treatment for OCD. However, this seemingly straightforward behavioral treatment can be very challenging to implement because the compulsion that reduces a patient’s anxiety may be a mental act—silently repeating a number or phrase until the distress is released, for example—and thus unobservable.
Treatment consists of first helping the patient recognize his or her recurrent thoughts, behaviors, or mental acts, then identifying triggers for these compulsions. Next, the patient is gradually exposed to these triggers without being allowed to engage in the compulsive response that typically follows.21,22 For example, a clinician may have a patient obsessed with germs pick objects out of the trash during a therapy session but not allow hand washing afterwards or repeatedly write or say a number or word that normally elicits compulsive behavior but prevent the patient from engaging in it.
Social anxiety disorder: Group therapy
Group therapy, in which the group setting itself becomes a type of exposure, is a very effective treatment for SAD.23 This can be challenging, however, as patients with this disorder may be less likely to seek treatment if they know they will be put into a group. Individual treatment is another option for patients with SAD, and can be equally effective.24
Cognitive restructuring of anxiety-provoking thoughts (eg, “Everyone will think I’m stupid”) and exposure to social situations and cues that patients with this disorder typically avoid are other key components of treatment.25,26 Exposure often occurs outside of the therapy setting. Patients may be instructed to go to a cafeteria and have lunch alone without looking at their phone or reading a book, for instance, or to go to a coffee shop and strike up a conversation with someone of the opposite sex while in line. Exposures within the therapeutic setting may involve associates of the therapist to help create an anxiety-provoking environment—eg, having a patient give an impromptu speech in front of an attractive associate of the opposite sex.
Where psychopharmacology fits in
While CBT is clearly a viable alternative to medication, psychopharmacology is sometimes indicated for anxiety or trauma-related disorders, depending on the diagnosis and on whether psychotherapy is ongoing.27 Evidence shows that specific types of drugs are effective for treating some anxiety-related disorders, while other medications may worsen symptoms (eg, selective serotonin reuptake inhibitors have demonstrated effectiveness in the treatment of PD, while benzodiazepines are contraindicated for patients with PTSD).27-29 Other research has found that a combined approach (psychotherapy plus medication) can be effective for the treatment of some anxiety disorders, including OCD.30 Although the combination may initially assist patients in their efforts to manage troublesome symptoms, in some cases it may limit the gains made from CBT.31
Talking to patients about CBT
In discussing treatment options with patients with anxiety or trauma-related disorders (TABLE 3),2-4,10,12-14,32-34 it is important to note that psychotherapy—and particularly CBT—may be more cost-effective and have longerlasting effects than medication.32-34 Explain that it is a short-term treatment (typically lasting 9 to 12 weeks) but has been found to have long-term results.2-4,6,7 Point out, too, that patients who engage in CBT are likely to learn new skills, some of which may last a lifetime—and do not have to worry about adverse effects or potential drug-drug interactions as they would if they opted for psychopharmacology instead.
Finally, tell patients that you have vetted the practitioners you refer patients to and that you will continue to see them while they undergo treatment to ensure that the CBT is progressing well and following the established protocol.
CASE › Ms. S’s primary care physician considers prescribing alprazolam, but is concerned because this anti-anxiety medication can be habit-forming. Noting that although the patient is already taking sertraline, her distress related to the trauma appears to be worsening, the doctor suggests Ms. S try CBT. He explains that CBT is time-limited but has been found to have substantial long-lasting benefits for women who, like her, have been victims of sexual assault. The physician also tells Ms. S that CBT follows a specific protocol that typically consists of 9 to 12 weekly sessions; includes homework assignments and often follows a manual; is goal-oriented and measurable; and focuses on changing present behavior, thoughts, and feelings.
When Ms. S agrees to a referral, her physician assures her that he has vetted the practitioner and asks her to come in after 12 weeks of CBT so he can monitor her progress.
CORRESPONDENCE
Scott Coffey, PhD, Department of Psychiatry and Human Behavior, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected]
1. Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146:317-325.
2. Beck JS. Cognitive Behavioral Therapy: Basics and Beyond. 2nd ed. New York, NY: Guilford Press; 2011.
3. Dobson KS, ed. Handbook of Cognitive-Behavioral Therapies. 2nd ed. New York, NY: Guilford Press; 2002.
4. Hollon SD, Beck AT. Cognitive and cognitive-behavioral therapies. In: Bergin AE, Garfield SL, eds. Handbook of Psychotherapy and Behavior Change. 4th ed. Hoboken, NJ: John Wiley & Sons; 1994:428-466.
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
6. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621-632.
7. Stewart RE, Chambless DL. Cognitive–behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77:595-606.
8. Hofmann SG, Asnaani A, Vonk IJ, et al. The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognitive Ther Res. 2012;36:427-440.
9. Cahill SP, Rothbaum BO, Resick PA, et al. Cognitive-behavioral therapy for adults. In: Foa EB, Keane TM, Friedman MJ, et al, eds. Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. 2nd ed. New York, NY: Guilford Press; 2009:139-222.
10. Resick PA, Schnicke M. Cognitive Processing Therapy for Rape Victims: A Treatment Manual. Newbury Park, CA: Sage Publications; 1993.
11. Monson CM, Schnurr PP, Resick PA, et al. Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. J Consult Clin Psychol. 2006;74:898-907.
12. Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2007;(3):CD003388.
13. Bisson JI, Ehlers A, Matthews R, et al. Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiatry. 2007;190:97-104.
14. Powers MB, Halpern JM, Ferenschak MP, et al. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin Psychol Rev. 2010;30:635-641.
15. Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide. New York, NY: Oxford University Press; 2007.
16. Borkovec T, Costello E. Efficacy of applied relaxation and cognitive- behavioral therapy in the treatment of generalized anxiety disorder. J Consult Clin Psychol. 1993;61:611-619.
17. Provencher MD, Dugas MJ, Ladouceur R. Efficacy of problemsolving training and cognitive exposure in the treatment of generalized anxiety disorder: a case replication series. Cognitive Behav Pract. 2004;11:404-414.
18. Craske MG, Barlow DH. Mastery of Your Anxiety and Worry: Workbook. 2nd ed. New York, NY: Oxford University Press; 2006.
19. Zinbarg RE, Craske MG, Barlow DH. Mastery of Your Anxiety and Worry: Therapist Guide. 2nd ed. New York, NY: Oxford University Press; 2006.
20. Craske MG, Barlow DH. Mastery of Your Anxiety and Panic: Therapist Guide. 4th ed. New York, NY: Oxford University Press; 2007.
21. Foa EB, Yadin E, Lichner TK. Exposure and Response (Ritual) Prevention for Obsessive-Compulsive Disorder: Therapist Guide. 2nd ed. New York, NY: Oxford University Press; 2012.
22. Yazdin E, Foa EB, Kuchner TK. Exposure and Response (Ritual) Prevention for Obsessive-Compulsive Disorder: Workbook. 2nd ed. New York, NY: Oxford University Press; 2012.
23. Heimberg RG, Juster HR, Hope DA, et al. Cognitive-behavioral group treatment: Description, case presentation, and empirical support. In: Stein MB, ed. Social Phobia: Clinical and Research Perspectives. Washington, DC: American Psychiatric Press; 1995:293-321.
24. Stangier U, Heidenreich T, Peitz M, et al. Cognitive therapy for social phobia: individual versus group treatment. Behav Res Ther. 2003;41:991-1007.
25. Hope DA, Heimberg RG, Turk CL. Managing Social Anxiety: A Cognitive-Behavioral Therapy Approach: Therapist Guide. 2nd ed. New York, NY: Oxford University Press; 2010.
26. Hope DA, Heimberg RG, Turk CL. Managing Social Anxiety: A Cognitive-Behavioral Therapy Approach: Workbook. 2nd ed. New York, NY: Oxford University Press; 2010.
27. Ravindran LN, Stein MB. The pharmacologic treatment of anxiety disorders: a review of progress. J Clin Psychiatry. 2010;7:839-854.
28. Bernardy NC. The role of benzodiazepines in the treatment of posttraumatic stress disorder (PTSD). PTSD Res Q. 2013;23:1-9.
29. Foa EB, Keane TM, Friedman MJ, et al, eds. Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. 2nd ed. New York, NY: Guilford Press; 2009.
30. Foa EB, Liebowitz MR, Kozak MJ, et al. Randomized, placebocontrolled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:151-161.
31. Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: Review and analysis. Clin Psychol: Sci Pract. 2005;12:72-86.
32. Antonuccio DO, Thomas M, Danton WG. A cost-effectiveness analysis of cognitive behavior therapy and fluoxetine (prozac) in the treatment of depression. Behav Ther. 1997;28:187-210.
33. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA. 2000;283:2529-2536.
34. Gould RA, Otto MW, Pollack MH, et al. Cognitive behavioral and pharmacological treatment of generalized anxiety disorder: A preliminary meta-analysis. Behav Ther. 1997;28:285-305.
› Tell patients who are potential candidates for cognitive behavioral therapy (CBT) that it has been demonstrated to be effective in treating anxiety and trauma-related disorders. A
› Motivate patients by pointing out that CBT is short-term therapy that is cost-effective and has the potential to be more beneficial than medication. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Darla S, a 42-year-old being treated for gastrointestinal (GI) distress, has undergone multiple tests over the course of the year, including a colonoscopy, an endoscopy, and a food allergy work-up. All had negative results. Medication trials—with proton pump inhibitors, H2 receptor antagonists, and prokinetics, among others—have not brought her any relief. The patient recently began taking sertraline 200 mg/d, which seemed to be helping. But on her latest visit, Ms. S requests a prescription for a sleeping pill. When asked what’s been keeping her up, the patient confides that she recently began having nightmares relating to a sexual assault that occurred several years ago.
If Ms. S were your patient, what would you recommend?
Family physicians (FPs) often encounter patients who are experiencing psychological distress, particularly anxiety.1 This may become evident when you’re treating one problem, such as low back pain or GI distress, but come to realize that anxiety is a key contributing factor or cause. Or you may discover that an anxiety or trauma-related disorder is complicating or interfering with treatment—preventing a patient with heart disease from quitting smoking, exercising regularly, or following a heart-healthy diet, for example.
Psychotropic medication is an option in such cases, of course. But the drugs often have adverse effects or interact with other medications the patient is taking, and their effects typically last only as long as the course of treatment. Being familiar with effective nonpharmacologic treatments—most notably, cognitive behavioral therapy (CBT)—will help you provide such patients with optimal care.
Advantages. CBT has several advantages that supportive counseling, traditional psychotherapy, and other nonpharmacologic treatments for psychological disorders do not: It is time-limited, typically lasting 9 to 12 weeks; skill-based; and goal-oriented. It also has a large amount of data to support it.2-4
Chances are you are familiar with the basic elements of CBT—challenging problematic beliefs, ensuring an increase in pleasant activities, and providing extended exposure to places or activities that trigger avoidance and/or arousal so that these responses are gradually diminished.2 However, there is not one single model of CBT. Rather, there are specific protocols for the conditions included in this review (TABLE 1).5
But before we get to the protocols, let’s first look at the evidence.
Meta-analyses demonstrate efficacy and effectiveness
Multiple studies and meta-analyses have consistently found CBT to reduce symptoms associated with anxiety and trauma-related disorders. Foremost among them are a metaanalysis by Hofmann and Smits6 of randomized placebo-controlled studies that assessed CBT’s efficacy and a meta-analysis by Stewart and Chambless7 that focused instead on effectiveness studies—ie, those assessing CBT in less-controlled, real-world practice. The findings are highlighted in TABLE 2.6,7
A 2012 review of meta-analyses of CBT8 for a broader range of psychological disorders found it to be more effective in treating generalized anxiety disorder (GAD), obsessive compulsive disorder (OCD), panic disorder (PD), posttraumatic stress disorder (PTSD), and social anxiety disorder (SAD) than control conditions, such as placebo, and often more effective than other treatments. Notably, CBT was shown to be more effective than relaxation therapy for PD, more effective in the long term than psychopharmacology for SAD, and more effective than supportive counseling for PTSD.
Cognitive processing and exposure therapy for PTSD
Two types of CBT have been found to be particularly effective in treating PTSD: cognitive processing therapy and exposure therapy. Each has specific protocols, although treatment often has some components of each.9
Cognitive processing therapy, a firstline treatment for PTSD, was initially developed for the treatment of rape victims,10 but has been found to be effective in treating combat-related PTSD, as well.11 It incorporates the core elements of cognitive therapy—identifying false or unhelpful trauma-related thoughts, then evaluating the evidence for and against them so the patient learns to consider whether these problematic thoughts are the result of cognitive bias or error and develop more realistic and/or useful thoughts. Cognitive processing therapy, however, focuses primarily on issues of safety, danger, and trust relating to patients’ views of themselves, others, and the world. Patients are asked to write, and then read, a narrative of the trauma they endured to help them challenge troubling thoughts about it.10
A woman undergoing treatment for PTSD relating to a sexual assault, for example, may initially think, “All men are bad.” Challenging this thought by examining evidence for and against it may help her replace it with the more realistic belief that some—but not all—men are bad.
Exposure therapy, which is also a firstline treatment for PTSD,12-14 involves presenting the frightening stimuli to patients in a safe environment so that they can learn a new way of responding.9 If a patient is afraid of a specific location because she was assaulted there, for instance, slowly exposing her to the site while ensuring her safety can help her anxiety diminish. Depending on the circumstances, exposure may be conducted in vivo (tangible stimuli), achieved through mental imagery (of a combat zone where an improvised explosive device detonated, for example), or both.
Prolonged exposure has been shown to be very effective in treating PTSD resulting from a variety of traumatic events. Other aspects of treatment include education about the disorder and breathing retraining to reduce arousal and increase the patient’s ability to relax.15
Generalized anxiety disorder: Worry exposure and relaxation
CBT for GAD has 5 components:
- education about the disorder
- cognitive restructuring
- progressive muscle relaxation
- worry exposure
- in vivo exposure.
Relaxation training is a crucial part of treatment for GAD, perhaps more so than for other anxiety disorders.16 Cognitive restructuring is vital, as well. This involves the use of the Socratic means of questioning, asking “Tell me what you mean by ‘horrible,’” for example, and “What about that is of most concern to you?”
Worry exposure occurs by instructing the patient to engage in prolonged worry about one particular topic, rather than jumping from one worrisome subject to another. The single focus reduces the distress that worry causes, thereby decreasing the time spent worrying.17 As treatment progresses, the patient is taught to set aside a specific time to worry. Worrying outside of the designated “worry time” is not allowed.18,19
Panic disorder: Recognizing what's behind physical symptoms
Treatment for PD combines education about the disorder, cognitive restructuring, and exposure.
Education helps the patient understand the reason the increased arousal response occurs at seemingly random times—recognizing that he or she is interpreting normal physiological sensations negatively, for example, and that the physical response is the body’s way of protecting itself.
Cognitive restructuring helps patients reformulate their view of the relationship between physical symptoms and panic attacks. An individual might learn to interpret a rapid heart rate as an indication that his heart is working harder and getting stronger, for instance, rather than as a symptom of cardiovascular distress.
Interoceptive exposure therapy teaches patients to identify their internal physical cues (eg, shortness of breath, shakiness, and tachycardia) and then deliberately induce them—by breathing through a straw, climbing a flight of stairs, or spinning in a chair, for example. With repeated exposure, patients learn that the physical sensations are not dangerous, and the anxiety associated with them decreases.
In vivo exposure involves the creation of a “fear hierarchy” of places and activities that the patient avoids due to fear of having a panic attack, then gradually exposing him or her to them.18,20
Obsessive-compulsive disorder: Exposure and response
Exposure and response prevention (ERP) is the primary treatment for OCD. However, this seemingly straightforward behavioral treatment can be very challenging to implement because the compulsion that reduces a patient’s anxiety may be a mental act—silently repeating a number or phrase until the distress is released, for example—and thus unobservable.
Treatment consists of first helping the patient recognize his or her recurrent thoughts, behaviors, or mental acts, then identifying triggers for these compulsions. Next, the patient is gradually exposed to these triggers without being allowed to engage in the compulsive response that typically follows.21,22 For example, a clinician may have a patient obsessed with germs pick objects out of the trash during a therapy session but not allow hand washing afterwards or repeatedly write or say a number or word that normally elicits compulsive behavior but prevent the patient from engaging in it.
Social anxiety disorder: Group therapy
Group therapy, in which the group setting itself becomes a type of exposure, is a very effective treatment for SAD.23 This can be challenging, however, as patients with this disorder may be less likely to seek treatment if they know they will be put into a group. Individual treatment is another option for patients with SAD, and can be equally effective.24
Cognitive restructuring of anxiety-provoking thoughts (eg, “Everyone will think I’m stupid”) and exposure to social situations and cues that patients with this disorder typically avoid are other key components of treatment.25,26 Exposure often occurs outside of the therapy setting. Patients may be instructed to go to a cafeteria and have lunch alone without looking at their phone or reading a book, for instance, or to go to a coffee shop and strike up a conversation with someone of the opposite sex while in line. Exposures within the therapeutic setting may involve associates of the therapist to help create an anxiety-provoking environment—eg, having a patient give an impromptu speech in front of an attractive associate of the opposite sex.
Where psychopharmacology fits in
While CBT is clearly a viable alternative to medication, psychopharmacology is sometimes indicated for anxiety or trauma-related disorders, depending on the diagnosis and on whether psychotherapy is ongoing.27 Evidence shows that specific types of drugs are effective for treating some anxiety-related disorders, while other medications may worsen symptoms (eg, selective serotonin reuptake inhibitors have demonstrated effectiveness in the treatment of PD, while benzodiazepines are contraindicated for patients with PTSD).27-29 Other research has found that a combined approach (psychotherapy plus medication) can be effective for the treatment of some anxiety disorders, including OCD.30 Although the combination may initially assist patients in their efforts to manage troublesome symptoms, in some cases it may limit the gains made from CBT.31
Talking to patients about CBT
In discussing treatment options with patients with anxiety or trauma-related disorders (TABLE 3),2-4,10,12-14,32-34 it is important to note that psychotherapy—and particularly CBT—may be more cost-effective and have longerlasting effects than medication.32-34 Explain that it is a short-term treatment (typically lasting 9 to 12 weeks) but has been found to have long-term results.2-4,6,7 Point out, too, that patients who engage in CBT are likely to learn new skills, some of which may last a lifetime—and do not have to worry about adverse effects or potential drug-drug interactions as they would if they opted for psychopharmacology instead.
Finally, tell patients that you have vetted the practitioners you refer patients to and that you will continue to see them while they undergo treatment to ensure that the CBT is progressing well and following the established protocol.
CASE › Ms. S’s primary care physician considers prescribing alprazolam, but is concerned because this anti-anxiety medication can be habit-forming. Noting that although the patient is already taking sertraline, her distress related to the trauma appears to be worsening, the doctor suggests Ms. S try CBT. He explains that CBT is time-limited but has been found to have substantial long-lasting benefits for women who, like her, have been victims of sexual assault. The physician also tells Ms. S that CBT follows a specific protocol that typically consists of 9 to 12 weekly sessions; includes homework assignments and often follows a manual; is goal-oriented and measurable; and focuses on changing present behavior, thoughts, and feelings.
When Ms. S agrees to a referral, her physician assures her that he has vetted the practitioner and asks her to come in after 12 weeks of CBT so he can monitor her progress.
CORRESPONDENCE
Scott Coffey, PhD, Department of Psychiatry and Human Behavior, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected]
› Tell patients who are potential candidates for cognitive behavioral therapy (CBT) that it has been demonstrated to be effective in treating anxiety and trauma-related disorders. A
› Motivate patients by pointing out that CBT is short-term therapy that is cost-effective and has the potential to be more beneficial than medication. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Darla S, a 42-year-old being treated for gastrointestinal (GI) distress, has undergone multiple tests over the course of the year, including a colonoscopy, an endoscopy, and a food allergy work-up. All had negative results. Medication trials—with proton pump inhibitors, H2 receptor antagonists, and prokinetics, among others—have not brought her any relief. The patient recently began taking sertraline 200 mg/d, which seemed to be helping. But on her latest visit, Ms. S requests a prescription for a sleeping pill. When asked what’s been keeping her up, the patient confides that she recently began having nightmares relating to a sexual assault that occurred several years ago.
If Ms. S were your patient, what would you recommend?
Family physicians (FPs) often encounter patients who are experiencing psychological distress, particularly anxiety.1 This may become evident when you’re treating one problem, such as low back pain or GI distress, but come to realize that anxiety is a key contributing factor or cause. Or you may discover that an anxiety or trauma-related disorder is complicating or interfering with treatment—preventing a patient with heart disease from quitting smoking, exercising regularly, or following a heart-healthy diet, for example.
Psychotropic medication is an option in such cases, of course. But the drugs often have adverse effects or interact with other medications the patient is taking, and their effects typically last only as long as the course of treatment. Being familiar with effective nonpharmacologic treatments—most notably, cognitive behavioral therapy (CBT)—will help you provide such patients with optimal care.
Advantages. CBT has several advantages that supportive counseling, traditional psychotherapy, and other nonpharmacologic treatments for psychological disorders do not: It is time-limited, typically lasting 9 to 12 weeks; skill-based; and goal-oriented. It also has a large amount of data to support it.2-4
Chances are you are familiar with the basic elements of CBT—challenging problematic beliefs, ensuring an increase in pleasant activities, and providing extended exposure to places or activities that trigger avoidance and/or arousal so that these responses are gradually diminished.2 However, there is not one single model of CBT. Rather, there are specific protocols for the conditions included in this review (TABLE 1).5
But before we get to the protocols, let’s first look at the evidence.
Meta-analyses demonstrate efficacy and effectiveness
Multiple studies and meta-analyses have consistently found CBT to reduce symptoms associated with anxiety and trauma-related disorders. Foremost among them are a metaanalysis by Hofmann and Smits6 of randomized placebo-controlled studies that assessed CBT’s efficacy and a meta-analysis by Stewart and Chambless7 that focused instead on effectiveness studies—ie, those assessing CBT in less-controlled, real-world practice. The findings are highlighted in TABLE 2.6,7
A 2012 review of meta-analyses of CBT8 for a broader range of psychological disorders found it to be more effective in treating generalized anxiety disorder (GAD), obsessive compulsive disorder (OCD), panic disorder (PD), posttraumatic stress disorder (PTSD), and social anxiety disorder (SAD) than control conditions, such as placebo, and often more effective than other treatments. Notably, CBT was shown to be more effective than relaxation therapy for PD, more effective in the long term than psychopharmacology for SAD, and more effective than supportive counseling for PTSD.
Cognitive processing and exposure therapy for PTSD
Two types of CBT have been found to be particularly effective in treating PTSD: cognitive processing therapy and exposure therapy. Each has specific protocols, although treatment often has some components of each.9
Cognitive processing therapy, a firstline treatment for PTSD, was initially developed for the treatment of rape victims,10 but has been found to be effective in treating combat-related PTSD, as well.11 It incorporates the core elements of cognitive therapy—identifying false or unhelpful trauma-related thoughts, then evaluating the evidence for and against them so the patient learns to consider whether these problematic thoughts are the result of cognitive bias or error and develop more realistic and/or useful thoughts. Cognitive processing therapy, however, focuses primarily on issues of safety, danger, and trust relating to patients’ views of themselves, others, and the world. Patients are asked to write, and then read, a narrative of the trauma they endured to help them challenge troubling thoughts about it.10
A woman undergoing treatment for PTSD relating to a sexual assault, for example, may initially think, “All men are bad.” Challenging this thought by examining evidence for and against it may help her replace it with the more realistic belief that some—but not all—men are bad.
Exposure therapy, which is also a firstline treatment for PTSD,12-14 involves presenting the frightening stimuli to patients in a safe environment so that they can learn a new way of responding.9 If a patient is afraid of a specific location because she was assaulted there, for instance, slowly exposing her to the site while ensuring her safety can help her anxiety diminish. Depending on the circumstances, exposure may be conducted in vivo (tangible stimuli), achieved through mental imagery (of a combat zone where an improvised explosive device detonated, for example), or both.
Prolonged exposure has been shown to be very effective in treating PTSD resulting from a variety of traumatic events. Other aspects of treatment include education about the disorder and breathing retraining to reduce arousal and increase the patient’s ability to relax.15
Generalized anxiety disorder: Worry exposure and relaxation
CBT for GAD has 5 components:
- education about the disorder
- cognitive restructuring
- progressive muscle relaxation
- worry exposure
- in vivo exposure.
Relaxation training is a crucial part of treatment for GAD, perhaps more so than for other anxiety disorders.16 Cognitive restructuring is vital, as well. This involves the use of the Socratic means of questioning, asking “Tell me what you mean by ‘horrible,’” for example, and “What about that is of most concern to you?”
Worry exposure occurs by instructing the patient to engage in prolonged worry about one particular topic, rather than jumping from one worrisome subject to another. The single focus reduces the distress that worry causes, thereby decreasing the time spent worrying.17 As treatment progresses, the patient is taught to set aside a specific time to worry. Worrying outside of the designated “worry time” is not allowed.18,19
Panic disorder: Recognizing what's behind physical symptoms
Treatment for PD combines education about the disorder, cognitive restructuring, and exposure.
Education helps the patient understand the reason the increased arousal response occurs at seemingly random times—recognizing that he or she is interpreting normal physiological sensations negatively, for example, and that the physical response is the body’s way of protecting itself.
Cognitive restructuring helps patients reformulate their view of the relationship between physical symptoms and panic attacks. An individual might learn to interpret a rapid heart rate as an indication that his heart is working harder and getting stronger, for instance, rather than as a symptom of cardiovascular distress.
Interoceptive exposure therapy teaches patients to identify their internal physical cues (eg, shortness of breath, shakiness, and tachycardia) and then deliberately induce them—by breathing through a straw, climbing a flight of stairs, or spinning in a chair, for example. With repeated exposure, patients learn that the physical sensations are not dangerous, and the anxiety associated with them decreases.
In vivo exposure involves the creation of a “fear hierarchy” of places and activities that the patient avoids due to fear of having a panic attack, then gradually exposing him or her to them.18,20
Obsessive-compulsive disorder: Exposure and response
Exposure and response prevention (ERP) is the primary treatment for OCD. However, this seemingly straightforward behavioral treatment can be very challenging to implement because the compulsion that reduces a patient’s anxiety may be a mental act—silently repeating a number or phrase until the distress is released, for example—and thus unobservable.
Treatment consists of first helping the patient recognize his or her recurrent thoughts, behaviors, or mental acts, then identifying triggers for these compulsions. Next, the patient is gradually exposed to these triggers without being allowed to engage in the compulsive response that typically follows.21,22 For example, a clinician may have a patient obsessed with germs pick objects out of the trash during a therapy session but not allow hand washing afterwards or repeatedly write or say a number or word that normally elicits compulsive behavior but prevent the patient from engaging in it.
Social anxiety disorder: Group therapy
Group therapy, in which the group setting itself becomes a type of exposure, is a very effective treatment for SAD.23 This can be challenging, however, as patients with this disorder may be less likely to seek treatment if they know they will be put into a group. Individual treatment is another option for patients with SAD, and can be equally effective.24
Cognitive restructuring of anxiety-provoking thoughts (eg, “Everyone will think I’m stupid”) and exposure to social situations and cues that patients with this disorder typically avoid are other key components of treatment.25,26 Exposure often occurs outside of the therapy setting. Patients may be instructed to go to a cafeteria and have lunch alone without looking at their phone or reading a book, for instance, or to go to a coffee shop and strike up a conversation with someone of the opposite sex while in line. Exposures within the therapeutic setting may involve associates of the therapist to help create an anxiety-provoking environment—eg, having a patient give an impromptu speech in front of an attractive associate of the opposite sex.
Where psychopharmacology fits in
While CBT is clearly a viable alternative to medication, psychopharmacology is sometimes indicated for anxiety or trauma-related disorders, depending on the diagnosis and on whether psychotherapy is ongoing.27 Evidence shows that specific types of drugs are effective for treating some anxiety-related disorders, while other medications may worsen symptoms (eg, selective serotonin reuptake inhibitors have demonstrated effectiveness in the treatment of PD, while benzodiazepines are contraindicated for patients with PTSD).27-29 Other research has found that a combined approach (psychotherapy plus medication) can be effective for the treatment of some anxiety disorders, including OCD.30 Although the combination may initially assist patients in their efforts to manage troublesome symptoms, in some cases it may limit the gains made from CBT.31
Talking to patients about CBT
In discussing treatment options with patients with anxiety or trauma-related disorders (TABLE 3),2-4,10,12-14,32-34 it is important to note that psychotherapy—and particularly CBT—may be more cost-effective and have longerlasting effects than medication.32-34 Explain that it is a short-term treatment (typically lasting 9 to 12 weeks) but has been found to have long-term results.2-4,6,7 Point out, too, that patients who engage in CBT are likely to learn new skills, some of which may last a lifetime—and do not have to worry about adverse effects or potential drug-drug interactions as they would if they opted for psychopharmacology instead.
Finally, tell patients that you have vetted the practitioners you refer patients to and that you will continue to see them while they undergo treatment to ensure that the CBT is progressing well and following the established protocol.
CASE › Ms. S’s primary care physician considers prescribing alprazolam, but is concerned because this anti-anxiety medication can be habit-forming. Noting that although the patient is already taking sertraline, her distress related to the trauma appears to be worsening, the doctor suggests Ms. S try CBT. He explains that CBT is time-limited but has been found to have substantial long-lasting benefits for women who, like her, have been victims of sexual assault. The physician also tells Ms. S that CBT follows a specific protocol that typically consists of 9 to 12 weekly sessions; includes homework assignments and often follows a manual; is goal-oriented and measurable; and focuses on changing present behavior, thoughts, and feelings.
When Ms. S agrees to a referral, her physician assures her that he has vetted the practitioner and asks her to come in after 12 weeks of CBT so he can monitor her progress.
CORRESPONDENCE
Scott Coffey, PhD, Department of Psychiatry and Human Behavior, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected]
1. Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146:317-325.
2. Beck JS. Cognitive Behavioral Therapy: Basics and Beyond. 2nd ed. New York, NY: Guilford Press; 2011.
3. Dobson KS, ed. Handbook of Cognitive-Behavioral Therapies. 2nd ed. New York, NY: Guilford Press; 2002.
4. Hollon SD, Beck AT. Cognitive and cognitive-behavioral therapies. In: Bergin AE, Garfield SL, eds. Handbook of Psychotherapy and Behavior Change. 4th ed. Hoboken, NJ: John Wiley & Sons; 1994:428-466.
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
6. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621-632.
7. Stewart RE, Chambless DL. Cognitive–behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77:595-606.
8. Hofmann SG, Asnaani A, Vonk IJ, et al. The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognitive Ther Res. 2012;36:427-440.
9. Cahill SP, Rothbaum BO, Resick PA, et al. Cognitive-behavioral therapy for adults. In: Foa EB, Keane TM, Friedman MJ, et al, eds. Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. 2nd ed. New York, NY: Guilford Press; 2009:139-222.
10. Resick PA, Schnicke M. Cognitive Processing Therapy for Rape Victims: A Treatment Manual. Newbury Park, CA: Sage Publications; 1993.
11. Monson CM, Schnurr PP, Resick PA, et al. Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. J Consult Clin Psychol. 2006;74:898-907.
12. Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2007;(3):CD003388.
13. Bisson JI, Ehlers A, Matthews R, et al. Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiatry. 2007;190:97-104.
14. Powers MB, Halpern JM, Ferenschak MP, et al. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin Psychol Rev. 2010;30:635-641.
15. Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide. New York, NY: Oxford University Press; 2007.
16. Borkovec T, Costello E. Efficacy of applied relaxation and cognitive- behavioral therapy in the treatment of generalized anxiety disorder. J Consult Clin Psychol. 1993;61:611-619.
17. Provencher MD, Dugas MJ, Ladouceur R. Efficacy of problemsolving training and cognitive exposure in the treatment of generalized anxiety disorder: a case replication series. Cognitive Behav Pract. 2004;11:404-414.
18. Craske MG, Barlow DH. Mastery of Your Anxiety and Worry: Workbook. 2nd ed. New York, NY: Oxford University Press; 2006.
19. Zinbarg RE, Craske MG, Barlow DH. Mastery of Your Anxiety and Worry: Therapist Guide. 2nd ed. New York, NY: Oxford University Press; 2006.
20. Craske MG, Barlow DH. Mastery of Your Anxiety and Panic: Therapist Guide. 4th ed. New York, NY: Oxford University Press; 2007.
21. Foa EB, Yadin E, Lichner TK. Exposure and Response (Ritual) Prevention for Obsessive-Compulsive Disorder: Therapist Guide. 2nd ed. New York, NY: Oxford University Press; 2012.
22. Yazdin E, Foa EB, Kuchner TK. Exposure and Response (Ritual) Prevention for Obsessive-Compulsive Disorder: Workbook. 2nd ed. New York, NY: Oxford University Press; 2012.
23. Heimberg RG, Juster HR, Hope DA, et al. Cognitive-behavioral group treatment: Description, case presentation, and empirical support. In: Stein MB, ed. Social Phobia: Clinical and Research Perspectives. Washington, DC: American Psychiatric Press; 1995:293-321.
24. Stangier U, Heidenreich T, Peitz M, et al. Cognitive therapy for social phobia: individual versus group treatment. Behav Res Ther. 2003;41:991-1007.
25. Hope DA, Heimberg RG, Turk CL. Managing Social Anxiety: A Cognitive-Behavioral Therapy Approach: Therapist Guide. 2nd ed. New York, NY: Oxford University Press; 2010.
26. Hope DA, Heimberg RG, Turk CL. Managing Social Anxiety: A Cognitive-Behavioral Therapy Approach: Workbook. 2nd ed. New York, NY: Oxford University Press; 2010.
27. Ravindran LN, Stein MB. The pharmacologic treatment of anxiety disorders: a review of progress. J Clin Psychiatry. 2010;7:839-854.
28. Bernardy NC. The role of benzodiazepines in the treatment of posttraumatic stress disorder (PTSD). PTSD Res Q. 2013;23:1-9.
29. Foa EB, Keane TM, Friedman MJ, et al, eds. Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. 2nd ed. New York, NY: Guilford Press; 2009.
30. Foa EB, Liebowitz MR, Kozak MJ, et al. Randomized, placebocontrolled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:151-161.
31. Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: Review and analysis. Clin Psychol: Sci Pract. 2005;12:72-86.
32. Antonuccio DO, Thomas M, Danton WG. A cost-effectiveness analysis of cognitive behavior therapy and fluoxetine (prozac) in the treatment of depression. Behav Ther. 1997;28:187-210.
33. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA. 2000;283:2529-2536.
34. Gould RA, Otto MW, Pollack MH, et al. Cognitive behavioral and pharmacological treatment of generalized anxiety disorder: A preliminary meta-analysis. Behav Ther. 1997;28:285-305.
1. Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146:317-325.
2. Beck JS. Cognitive Behavioral Therapy: Basics and Beyond. 2nd ed. New York, NY: Guilford Press; 2011.
3. Dobson KS, ed. Handbook of Cognitive-Behavioral Therapies. 2nd ed. New York, NY: Guilford Press; 2002.
4. Hollon SD, Beck AT. Cognitive and cognitive-behavioral therapies. In: Bergin AE, Garfield SL, eds. Handbook of Psychotherapy and Behavior Change. 4th ed. Hoboken, NJ: John Wiley & Sons; 1994:428-466.
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
6. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621-632.
7. Stewart RE, Chambless DL. Cognitive–behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77:595-606.
8. Hofmann SG, Asnaani A, Vonk IJ, et al. The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognitive Ther Res. 2012;36:427-440.
9. Cahill SP, Rothbaum BO, Resick PA, et al. Cognitive-behavioral therapy for adults. In: Foa EB, Keane TM, Friedman MJ, et al, eds. Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. 2nd ed. New York, NY: Guilford Press; 2009:139-222.
10. Resick PA, Schnicke M. Cognitive Processing Therapy for Rape Victims: A Treatment Manual. Newbury Park, CA: Sage Publications; 1993.
11. Monson CM, Schnurr PP, Resick PA, et al. Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. J Consult Clin Psychol. 2006;74:898-907.
12. Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2007;(3):CD003388.
13. Bisson JI, Ehlers A, Matthews R, et al. Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiatry. 2007;190:97-104.
14. Powers MB, Halpern JM, Ferenschak MP, et al. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin Psychol Rev. 2010;30:635-641.
15. Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide. New York, NY: Oxford University Press; 2007.
16. Borkovec T, Costello E. Efficacy of applied relaxation and cognitive- behavioral therapy in the treatment of generalized anxiety disorder. J Consult Clin Psychol. 1993;61:611-619.
17. Provencher MD, Dugas MJ, Ladouceur R. Efficacy of problemsolving training and cognitive exposure in the treatment of generalized anxiety disorder: a case replication series. Cognitive Behav Pract. 2004;11:404-414.
18. Craske MG, Barlow DH. Mastery of Your Anxiety and Worry: Workbook. 2nd ed. New York, NY: Oxford University Press; 2006.
19. Zinbarg RE, Craske MG, Barlow DH. Mastery of Your Anxiety and Worry: Therapist Guide. 2nd ed. New York, NY: Oxford University Press; 2006.
20. Craske MG, Barlow DH. Mastery of Your Anxiety and Panic: Therapist Guide. 4th ed. New York, NY: Oxford University Press; 2007.
21. Foa EB, Yadin E, Lichner TK. Exposure and Response (Ritual) Prevention for Obsessive-Compulsive Disorder: Therapist Guide. 2nd ed. New York, NY: Oxford University Press; 2012.
22. Yazdin E, Foa EB, Kuchner TK. Exposure and Response (Ritual) Prevention for Obsessive-Compulsive Disorder: Workbook. 2nd ed. New York, NY: Oxford University Press; 2012.
23. Heimberg RG, Juster HR, Hope DA, et al. Cognitive-behavioral group treatment: Description, case presentation, and empirical support. In: Stein MB, ed. Social Phobia: Clinical and Research Perspectives. Washington, DC: American Psychiatric Press; 1995:293-321.
24. Stangier U, Heidenreich T, Peitz M, et al. Cognitive therapy for social phobia: individual versus group treatment. Behav Res Ther. 2003;41:991-1007.
25. Hope DA, Heimberg RG, Turk CL. Managing Social Anxiety: A Cognitive-Behavioral Therapy Approach: Therapist Guide. 2nd ed. New York, NY: Oxford University Press; 2010.
26. Hope DA, Heimberg RG, Turk CL. Managing Social Anxiety: A Cognitive-Behavioral Therapy Approach: Workbook. 2nd ed. New York, NY: Oxford University Press; 2010.
27. Ravindran LN, Stein MB. The pharmacologic treatment of anxiety disorders: a review of progress. J Clin Psychiatry. 2010;7:839-854.
28. Bernardy NC. The role of benzodiazepines in the treatment of posttraumatic stress disorder (PTSD). PTSD Res Q. 2013;23:1-9.
29. Foa EB, Keane TM, Friedman MJ, et al, eds. Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. 2nd ed. New York, NY: Guilford Press; 2009.
30. Foa EB, Liebowitz MR, Kozak MJ, et al. Randomized, placebocontrolled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:151-161.
31. Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: Review and analysis. Clin Psychol: Sci Pract. 2005;12:72-86.
32. Antonuccio DO, Thomas M, Danton WG. A cost-effectiveness analysis of cognitive behavior therapy and fluoxetine (prozac) in the treatment of depression. Behav Ther. 1997;28:187-210.
33. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA. 2000;283:2529-2536.
34. Gould RA, Otto MW, Pollack MH, et al. Cognitive behavioral and pharmacological treatment of generalized anxiety disorder: A preliminary meta-analysis. Behav Ther. 1997;28:285-305.
Prurigo nodularis: Picking the right treatment
› Start with topical corticosteroids under occlusion and periodically substitute with steroid-sparing agents (calcipotriol ointment or pimecrolimus 1% cream) for localized prurigo nodularis. B
› Consider adding oral antihistamines or montelukast to the initial regimen if a pruritic cause is suspected; alternatively, consider adding these agents if topical therapies alone do not effectively treat the prurigo nodules. C
› Turn to oral naltrexone, gabapentin, or pregabalin for more widespread or treatment-resistant cases. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 43-year-old woman arrives at your office with persistent itching on her arms and legs. For some time, she has used moisturizing lotions and herbal preparations suggested by her mother, but they have provided no relief. You note multiple 0.5- to 2-cm firm, excoriated nodules symmetrically distributed on her elbows and knees bilaterally. She has seasonal allergies and a history of childhood asthma. How would you care for this patient?
Treating prurigo nodularis (PN) can be a daunting task for even the most experienced clinician. Prurigo nodules are cutaneous lesions often produced by repetitive scratching—hence the nickname “picker’s nodules”—which may occur as sequelae of chronic pruritus or neurotic excoriations. Thus, PN can be classified as a subtype of neurodermatitis. The nodules can be intensely pruritic, resulting in an itch-scratch cycle that can be difficult to break.1,2 In this review, we examine evidence-based therapies for PN.
Key findings with prurigo nodularis
Typically, prurigo nodules are firm, hyperkeratotic, pruritic papules or nodules that range in diameter from a few millimeters to several centimeters. The lesions usually have eroded or ulcerated components secondary to repeated excoriation, which can eventually lead to scarring and changes in pigmentation. Patients can have one nodule or hundreds of lesions, depending on disease severity. The lesions tend to be distributed symmetrically and have a predilection for the extensor surfaces of the upper and lower limbs. The abdomen, posterior neck, upper and lower back, and buttocks are also commonly affected, whereas the face, palms, and flexural areas are rarely involved2-5 (FIGURE 1).
The differential diagnosis for PN includes dermatitis herpetiformis, scabies, lichen simplex chronicus, hypertrophic lichen planus, perforating disorders, atopic dermatitis, allergic contact dermatitis, neurotic excoriations, and multiple keratoacanthomas.4,5
PN prevalence and etiology are unknown. Although PN can occur at any age, the typical age range is 20 to 60 years, with middle-aged women most commonly affected. Patients who develop PN at a younger age are more likely to have an atopic diathesis.3,4
There is ongoing debate regarding whether PN is a primary cutaneous disease or a response to repetitive scratching provoked by a separate cause. PN has been associated with a variety of diseases, such as psychiatric disorders, atopic dermatitis, chronic renal failure, hyperthyroidism, iron-deficiency anemia, obstructive biliary disease, gastric malignancy, lymphoma, leukemia, human immunodeficiency virus (HIV), hepatitis B, and hepatitis C.2,3
Use the diagnostic work-up to focus on management decisions
When taking the history, first determine why patients are picking or scratching. If the lesions are pruritic or painful, look for a potential underlying cause of pruritic symptoms.6 If you identify an underlying dermatologic or systemic condition, treat that disorder first.1 For example, adequately treating a patient’s atopic dermatitis or hyperthyroidism may quell the pruritic symptoms and potentially make the prurigo nodules more responsive to symptomatic treatment or even obviate the need for such measures.
If treating the underlying cause of PN does not provide adequate relief, or if no cause for pruritic nodules can be found, the nodules may yet respond to symptomatic treatments targeted at decreasing pruritus and inflammation. In contrast, with patients who habitually scratch lesions they describe as non-pruritic, neurotic excoriations could be the source of PN, making the nodules less likely to respond to antipruritic therapies.4,7
Patient insights. Assessing whether patients have insight into their condition is also important. Some patients may be unaware that they are repetitively picking and scratching the affected areas and causing the development and perpetuation of the nodules. In cases associated with an underlying psychiatric component, such as delusional parasitosis, patients often lack insight into their condition and thus may benefit from treatment of psychiatric comorbidities.4,7
On physical exam, try to find lesions that have not been traumatized by patients. They can be useful in uncovering a primary cause, such as scabies, atopic dermatitis, lichenoid drug eruption, or simple xerosis.
If a diagnosis cannot be made clinically, consider obtaining a biopsy of a nontraumatized lesion. Traumatized lesions are typically unrevealing on histopathology. If the clinical assessment of pruritic lesions is indeterminate, laboratory tests that may prove helpful include, but are not limited to, thyroid-stimulating hormone levels, liver function tests, kidney function, a hepatitis panel, and HIV screening.
With severe refractory pruritus in which a primary cutaneous or systemic cause cannot be determined, evaluate for malignancy—especially polycythemia, lymphoma, or multiple myeloma—by ordering liver function tests (including lactate dehydrogenase), a complete blood count with differential, a basic metabolic panel, a chest x-ray, and possibly a serum protein electrophoresis.7
Available treatments
If the patient’s pruritic symptoms do not resolve and an underlying cause cannot be determined, direct treatment at decreasing pruritus either locally or systemically. Topical therapies, typically associated with fewer adverse effects, are preferable in localized cases of PN. In more severe, widespread, or recalcitrant disease, systemic agents may be necessary. Typical first-line treatments for PN aimed at decreasing pruritic symptoms include:
- topical antipruritics, such as ointments containing menthol or camphor; topical corticosteroids, with increased efficacy under occlusion as seen with flurandrenolide tape (Cordran tape)
- oral antihistamines, such as promethazine hydrochloride; oral antidepressants, such as doxepin
- intralesional corticosteroids—eg, triamcinolone acetonide (the concentration
used depends on the thickness of the lesion and how well the lesion responded to prior injections) - a short course of systemic corticosteroids, unless the patient has a comorbid condition that could be exacerbated by rapid tapering of corticosteroids (eg, psoriasis).
For patients with concomitant depression or anxiety, treatment with a selective serotonin reuptake inhibitor or anxiolytic, respectively, may be indicated.2-4 With the exception of topical corticosteroids8,9 and oral antihistamines,10 the aforementioned first-line treatments for PN are mostly based on clinical experience and anecdotal success with no studies to support their use.3 Furthermore, these treatments may be ineffective for many patients.11,12 We present our review of several studies in the literature examining potential therapies for PN.
Topical therapies
Calcipotriol vs betamethasone. A prospective, randomized, double-blind study that ran right/left comparisons of calcipotriol ointment (a vitamin D3 analog) and betamethasone ointment as treatment for PN in 9 patients showed that calcipotriol and betamethasone were both effective. However, calcipotriol ointment 50 mcg/g was more effective in reducing the number and size of nodules compared with 0.1% betamethasone valerate ointment.8
Topical corticosteroids have long been viewed as a first-line therapy for PN.2 However, given their potential for adverse effects with long-term use, such as skin atrophy, steroidsparing agents are preferred. Calcipotriol ointment can be useful as both a steroid-sparing and a keratolytic agent, as it inhibits keratinocyte proliferation.4,13 Corticosteroids and calcipotriol possess anti-inflammatory and antipruritic properties, likely explaining their efficacy in treating PN.4
Pimecrolimus and tacrolimus. The topical calcineurin inhibitors pimecrolimus and tacrolimus have been used successfully as steroid-sparing agents in treating atopic dermatitis.14 Their antipruritic effect, likely related to their influence on cutaneous sensory nerve fibers and inhibition of inflammatory cytokines, could also explain their efficacy in treating PN.15,16
A randomized, hydrocortisone-controlled, double-blind phase II trial sponsored by Novartis was designed as a right/left comparison study between pimecrolimus 1% cream and hydrocortisone 1% cream in 30 patients with non-atopic PN. When applied twice daily, each agent decreased pruritic symptoms and resolved scratch lesions to degrees that were statistically significant. However, an intention-to-treat analysis revealed no significant differences between pimecrolimus and hydrocortisone.15 In a prospective case series of 11 patients with PN, 2 out of 4 patients (50%) receiving tacrolimus 0.1% ointment and 5 out of 7 patients (71%) using pimecrolimus 1% cream experienced a reduction in pruritic symptoms and improvement of lesions by 50% or greater with twice daily application of their assigned calcineurin inhibitor.16
Before prescribing topical calcineurin inhibitors, inform patients of the black-box warning issued by the US Food and Drug Administration (FDA) regarding the theoretical increased risk of developing cutaneous malignancy and lymphoma. This warning is controversial because in clinical databases, the incidences of malignancy and lymphoma associated with topical calcineurin inhibitors are less than those observed in the general population.14
Capsaicin. Based on a prospective study of 33 patients with PN, topical capsaicin may be an effective treatment if administered 4 to 6 times daily for at least 2 weeks and up to 10 months.17 Patients may require up to 0.3% concentration for total resolution of pruritus. Importantly, capsaicin use may be limited by the high application frequency.
Systemic therapies
Fexofenadine and montelukast. Oral antihistamines have long been used as a first-line treatment for PN. Although clinical experience and anecdotal success support the use of various antihistamines, evidence-based literature exists only for fexofenadine and the leukotriene receptor antagonist montelukast. These oral agents also avoid potential unwanted effects of topical antihistamines, which may sensitize skin and increase the risk of developing allergic contact dermatitis.1
Whereas antihistamines exert their antipruritic effect by blocking histamine H1-receptors, montelukast decreases pruritic symptoms by antagonizing leukotriene receptors.10 In a prospective study of 12 patients with PN receiving fexofenadine 240 mg twice daily and montelukast 10 mg daily for 4 weeks, 9 of the 12 patients (75%) reported some degree of improvement.10 However, 5 of these 9 patients (56%) achieved only slight improvement. Level of improvement was based on how well the agents reduced the pruritus and lesion number.
Naltrexone. As an opioid antagonist, naltrexone is able to block endogenous opiates from binding to central opioid receptors and causing the sensation of pruritus. Accordingly, oral naltrexone can be used to treat PN, as shown in an open-label clinical trial in which 9 out of 17 patients (53%) achieved high antipruritic effect, defined as a reduction of pruritic symptoms by at least half.18
When selecting naltrexone to treat PN, prescribe a daily dose of 50 mg for an average of 4.7 months; up to 20 months of treatment may be required. If tachyphylaxis occurs, consider increasing the dose to 50 mg twice a day. Most patients should notice some level of antipruritic efficacy and varying degrees of lesion flattening, softening, or healing. However, exacerbation after therapy discontinuation may occur in 41% of patients. Adverse medication effects include fatigue, nausea, and dizziness.18
Gabapentin and pregabalin. In response to a report of a case series in which 4 patients with PN responded well to gabapentin,19 Mazza et al20 conducted a prospective study of pregabalin treatment for 30 patients with PN. Both gabapentin and pregabalin inhibit calcium influx and subsequent excitatory neurotransmitter release, the mechanism by which they likely decrease pruritus in patients with PN.20 In the pregabalin study, 23 out of 30 patients (77%) experienced complete resolution of pruritic symptoms and a reduction of prurigo nodules in number or flattening. The recommended dosage of pregabalin is 25 mg 3 times daily for 3 months, after which time clinical progress is assessed. If a patient is not lesion-free, continue pregabalin at a maintenance dose of 50 mg/d for up to 2 years. Adverse effects typically include headache, sedation, and dizziness.20
When to refer a dermatologist
Refer patients to a dermatologist if initial clinical findings suggest a need for further work-up to rule out primary cutaneous diseases, or if the therapies discussed (TABLE8-10,15-20) yield unsatisfactory results. Dermatologists can provide more advanced treatments that require close monitoring, such as phototherapy,21-24 cyclosporine,25 or thalidomide.26-28 Based on multiple case series and case reports, as well as our own personal experience (FIGURE 2), thalidomide is efficacious in treating PN. However, thalidomide is typically reserved for cases that are severe and treatment-recalcitrant due to the drug’s high cost, teratogenicity (pregnancy category X), and potentially irreversible peripheral neuropathy.29
Putting Tx options into practice
In addition to ruling out potential causes of pruritus and determining the best treatment for each individual with PN, assess for and appropriately treat any psychiatric comorbidities, which are often a psychological component of PN.
Localized PN. Start with topical corticosteroids under occlusion for localized PN. To avoid complications of long-term topical corticosteroid use, including dermal atrophy, periodically switch to a steroid-sparing agent, such as calcipotriol ointment or topical pimecrolimus. Less evidence is available to support the efficacy of tacrolimus ointment in PN treatment. Topical capsaicin is not as practical as other topical treatments since it needs to be applied 4 to 6 times daily. Oral antihistamines and montelukast may be added to the therapeutic regimen if there is a chronic pruritic component related to the lesions themselves or an underlying atopic diathesis fueling the itch-scratch cycle.
Widespread or treatment-resistant PN. Prescribe naltrexone, gabapentin, or pregabalin for more widespread disease or lesions resistant to conservative therapies. If you suspect a primary cutaneous disease as the underlying cause of pruritus or if topical and oral therapies do not achieve the desired therapeutic effect, refer to a dermatologist for further work-up and treatment.
How we would manage the case presented in the introduction. We would start the 43-year-old on topical corticosteroids under occlusion and periodically substitute calcipotriol ointment. (Given the unease that some patients might feel with the black-box warning on topical calcineurin inhibitors, we would likely try calcipotriol ointment as a courtesy before suggesting topical calcineurin inhibitors.) We would also prescribe an oral antihistamine at the start, given that her history of seasonal allergies and childhood asthma increases her chances of having an atopic component causing or exacerbating her disease. However, assessing her response to topical therapies before initiating an oral antihistamine would also be an appropriate strategy.
Unfortunately, PN is typically a chronic and often treatment-resistant disease with disappointing recurrence rates. As we learn more about the pathophysiology of PN, more effective therapies will hopefully emerge to improve the quality of life for these patients.
CORRESPONDENCE
Michael Saco, MD, Department of Dermatology & Cutaneous Surgery, University of South Florida, 13330 Laurel Drive, Tampa, FL 33612; [email protected]
1. Moses S. Pruritus. Am Fam Phys. 2003;68:1135-1142.
2. Jorizzo JL, Gatti S, Smith EB. Prurigo: a clinical review. J Am Acad Dermatol. 1981;4:723-728.
3. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-218.
4. Wallengren J. Prurigo: diagnosis and management. Am J Clin Dermatol. 2004;5:85-95.
5. Accioly-Filho LW, Nogueira A, Ramos-e-Silva M. Prurigo nodularis of Hyde: an update. J Eur Acad Dermatol Venereol. 2000;14:75-82.
6. Reamy BV, Bunt CW, Fletcher S. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
7. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450.
8. Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of Prurigo nodularis. Arch Dermatol. 2000;136:807-808.
9. Saraceno R, Chiricozzi A, Nisticò SP, et al. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21:363-366.
10. Shintani T, Ohata C, Koga H, et al. Combination therapy of fexofenadine and montelukast is effective in prurigo nodularis and pemphigoid nodularis. Dermatol Ther. 2014;27:135-139.
11. Paghdal KV, Schwartz R. Thalidomide and its dermatologic uses. Acta Dermatovenerol Croat. 2007;15:39-44.
12. Alfadley A, Al-Hawsawi K, Thestrup-Pedersen K, et al. Treatment of prurigo nodularis with thalidomide: a case report and review of the literature. Int J Dermatol. 2003;42:372-375.
13. Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
14. Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
15. Siepmann D, Lotts T, Blome C, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, doubleblind phase II trial. Dermatology. 2013;227:353-360.
16. Ständer S, Schürmeyer-Horst F, Luger TA, et al. Treatment of pruritic diseases with topical calcineurin inhibitors. Ther Clin Risk Manag. 2006;2:213-218.
17. Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471-478.
18. Metze D, Reimann S, Beissert S, et al. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41:533-539.
19. Gencoglan G, Inanir I, Gunduz K. Therapeutic hotline: treatment of prurigo nodularis and lichen simplex chronicus with gabapentin. Dermatol Ther. 2010;23:194-198.
20. Mazza M, Guerriero G, Marano G, et al. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38:16-18.
21. Hammes S, Hermann J, Roos S, et al. UVB 308-nm excimer light and bath PUVA: combination therapy is very effective in the treatment of prurigo nodularis. J Eur Acad Dermatol Venereol. 2011;25:799-803.
22. Rombold S, Lobisch K, Katzer K, et al. Efficacy of UVA1 phototherapy in 230 patients with various skin diseases. Photodermatol Photoimmunol Photomed. 2008;24:19-23.
23. Tamagawa-Mineoka R, Katoh N, Ueda E, et al. Narrow-band ultraviolet B phototherapy in patients with recalcitrant nodular prurigo. J Dermatol. 2007;34:691-695.
24. Saraceno R, Nisticò SP, Capriotti E, et al. Monochromatic excimer light (308 nm) in the treatment of prurigo nodularis. Photodermatol Photoimmunol Photomed. 2008;24:43-45.
25. Siepmann D, Luger TA, Ständer S. Antipruritic effect of cyclosporine microemulsion in prurigo nodularis: results of a case series. J Dtsch Dermatol Ges. 2008;6:941-946.
26. Orlando A, Renna S, Cottone M. Prurigo nodularis of Hyde treated with low-dose thalidomide. Eur Rev Med Pharmacol Sci. 2009;13:141-145.
27. Lan CC, Lin CL, Wu CS, et al. Treatment of idiopathic prurigo nodularis in Taiwanese patients with low-dose thalidomide. J Dermatol. 2007;34:237-242.
28. Taefehnorooz H, Truchetet F, Barbaud A, et al. Efficacy of thalidomide in the treatment of prurigo nodularis. Acta Derm Venereol. 2011;91:344-345.
29. Wu JJ, Huang DB, Pang KR, et al. Thalidomide: dermatological indications, mechanisms of action and side-effects. Br J Dermatol. 2005;153:254-273.
› Start with topical corticosteroids under occlusion and periodically substitute with steroid-sparing agents (calcipotriol ointment or pimecrolimus 1% cream) for localized prurigo nodularis. B
› Consider adding oral antihistamines or montelukast to the initial regimen if a pruritic cause is suspected; alternatively, consider adding these agents if topical therapies alone do not effectively treat the prurigo nodules. C
› Turn to oral naltrexone, gabapentin, or pregabalin for more widespread or treatment-resistant cases. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 43-year-old woman arrives at your office with persistent itching on her arms and legs. For some time, she has used moisturizing lotions and herbal preparations suggested by her mother, but they have provided no relief. You note multiple 0.5- to 2-cm firm, excoriated nodules symmetrically distributed on her elbows and knees bilaterally. She has seasonal allergies and a history of childhood asthma. How would you care for this patient?
Treating prurigo nodularis (PN) can be a daunting task for even the most experienced clinician. Prurigo nodules are cutaneous lesions often produced by repetitive scratching—hence the nickname “picker’s nodules”—which may occur as sequelae of chronic pruritus or neurotic excoriations. Thus, PN can be classified as a subtype of neurodermatitis. The nodules can be intensely pruritic, resulting in an itch-scratch cycle that can be difficult to break.1,2 In this review, we examine evidence-based therapies for PN.
Key findings with prurigo nodularis
Typically, prurigo nodules are firm, hyperkeratotic, pruritic papules or nodules that range in diameter from a few millimeters to several centimeters. The lesions usually have eroded or ulcerated components secondary to repeated excoriation, which can eventually lead to scarring and changes in pigmentation. Patients can have one nodule or hundreds of lesions, depending on disease severity. The lesions tend to be distributed symmetrically and have a predilection for the extensor surfaces of the upper and lower limbs. The abdomen, posterior neck, upper and lower back, and buttocks are also commonly affected, whereas the face, palms, and flexural areas are rarely involved2-5 (FIGURE 1).
The differential diagnosis for PN includes dermatitis herpetiformis, scabies, lichen simplex chronicus, hypertrophic lichen planus, perforating disorders, atopic dermatitis, allergic contact dermatitis, neurotic excoriations, and multiple keratoacanthomas.4,5
PN prevalence and etiology are unknown. Although PN can occur at any age, the typical age range is 20 to 60 years, with middle-aged women most commonly affected. Patients who develop PN at a younger age are more likely to have an atopic diathesis.3,4
There is ongoing debate regarding whether PN is a primary cutaneous disease or a response to repetitive scratching provoked by a separate cause. PN has been associated with a variety of diseases, such as psychiatric disorders, atopic dermatitis, chronic renal failure, hyperthyroidism, iron-deficiency anemia, obstructive biliary disease, gastric malignancy, lymphoma, leukemia, human immunodeficiency virus (HIV), hepatitis B, and hepatitis C.2,3
Use the diagnostic work-up to focus on management decisions
When taking the history, first determine why patients are picking or scratching. If the lesions are pruritic or painful, look for a potential underlying cause of pruritic symptoms.6 If you identify an underlying dermatologic or systemic condition, treat that disorder first.1 For example, adequately treating a patient’s atopic dermatitis or hyperthyroidism may quell the pruritic symptoms and potentially make the prurigo nodules more responsive to symptomatic treatment or even obviate the need for such measures.
If treating the underlying cause of PN does not provide adequate relief, or if no cause for pruritic nodules can be found, the nodules may yet respond to symptomatic treatments targeted at decreasing pruritus and inflammation. In contrast, with patients who habitually scratch lesions they describe as non-pruritic, neurotic excoriations could be the source of PN, making the nodules less likely to respond to antipruritic therapies.4,7
Patient insights. Assessing whether patients have insight into their condition is also important. Some patients may be unaware that they are repetitively picking and scratching the affected areas and causing the development and perpetuation of the nodules. In cases associated with an underlying psychiatric component, such as delusional parasitosis, patients often lack insight into their condition and thus may benefit from treatment of psychiatric comorbidities.4,7
On physical exam, try to find lesions that have not been traumatized by patients. They can be useful in uncovering a primary cause, such as scabies, atopic dermatitis, lichenoid drug eruption, or simple xerosis.
If a diagnosis cannot be made clinically, consider obtaining a biopsy of a nontraumatized lesion. Traumatized lesions are typically unrevealing on histopathology. If the clinical assessment of pruritic lesions is indeterminate, laboratory tests that may prove helpful include, but are not limited to, thyroid-stimulating hormone levels, liver function tests, kidney function, a hepatitis panel, and HIV screening.
With severe refractory pruritus in which a primary cutaneous or systemic cause cannot be determined, evaluate for malignancy—especially polycythemia, lymphoma, or multiple myeloma—by ordering liver function tests (including lactate dehydrogenase), a complete blood count with differential, a basic metabolic panel, a chest x-ray, and possibly a serum protein electrophoresis.7
Available treatments
If the patient’s pruritic symptoms do not resolve and an underlying cause cannot be determined, direct treatment at decreasing pruritus either locally or systemically. Topical therapies, typically associated with fewer adverse effects, are preferable in localized cases of PN. In more severe, widespread, or recalcitrant disease, systemic agents may be necessary. Typical first-line treatments for PN aimed at decreasing pruritic symptoms include:
- topical antipruritics, such as ointments containing menthol or camphor; topical corticosteroids, with increased efficacy under occlusion as seen with flurandrenolide tape (Cordran tape)
- oral antihistamines, such as promethazine hydrochloride; oral antidepressants, such as doxepin
- intralesional corticosteroids—eg, triamcinolone acetonide (the concentration
used depends on the thickness of the lesion and how well the lesion responded to prior injections) - a short course of systemic corticosteroids, unless the patient has a comorbid condition that could be exacerbated by rapid tapering of corticosteroids (eg, psoriasis).
For patients with concomitant depression or anxiety, treatment with a selective serotonin reuptake inhibitor or anxiolytic, respectively, may be indicated.2-4 With the exception of topical corticosteroids8,9 and oral antihistamines,10 the aforementioned first-line treatments for PN are mostly based on clinical experience and anecdotal success with no studies to support their use.3 Furthermore, these treatments may be ineffective for many patients.11,12 We present our review of several studies in the literature examining potential therapies for PN.
Topical therapies
Calcipotriol vs betamethasone. A prospective, randomized, double-blind study that ran right/left comparisons of calcipotriol ointment (a vitamin D3 analog) and betamethasone ointment as treatment for PN in 9 patients showed that calcipotriol and betamethasone were both effective. However, calcipotriol ointment 50 mcg/g was more effective in reducing the number and size of nodules compared with 0.1% betamethasone valerate ointment.8
Topical corticosteroids have long been viewed as a first-line therapy for PN.2 However, given their potential for adverse effects with long-term use, such as skin atrophy, steroidsparing agents are preferred. Calcipotriol ointment can be useful as both a steroid-sparing and a keratolytic agent, as it inhibits keratinocyte proliferation.4,13 Corticosteroids and calcipotriol possess anti-inflammatory and antipruritic properties, likely explaining their efficacy in treating PN.4
Pimecrolimus and tacrolimus. The topical calcineurin inhibitors pimecrolimus and tacrolimus have been used successfully as steroid-sparing agents in treating atopic dermatitis.14 Their antipruritic effect, likely related to their influence on cutaneous sensory nerve fibers and inhibition of inflammatory cytokines, could also explain their efficacy in treating PN.15,16
A randomized, hydrocortisone-controlled, double-blind phase II trial sponsored by Novartis was designed as a right/left comparison study between pimecrolimus 1% cream and hydrocortisone 1% cream in 30 patients with non-atopic PN. When applied twice daily, each agent decreased pruritic symptoms and resolved scratch lesions to degrees that were statistically significant. However, an intention-to-treat analysis revealed no significant differences between pimecrolimus and hydrocortisone.15 In a prospective case series of 11 patients with PN, 2 out of 4 patients (50%) receiving tacrolimus 0.1% ointment and 5 out of 7 patients (71%) using pimecrolimus 1% cream experienced a reduction in pruritic symptoms and improvement of lesions by 50% or greater with twice daily application of their assigned calcineurin inhibitor.16
Before prescribing topical calcineurin inhibitors, inform patients of the black-box warning issued by the US Food and Drug Administration (FDA) regarding the theoretical increased risk of developing cutaneous malignancy and lymphoma. This warning is controversial because in clinical databases, the incidences of malignancy and lymphoma associated with topical calcineurin inhibitors are less than those observed in the general population.14
Capsaicin. Based on a prospective study of 33 patients with PN, topical capsaicin may be an effective treatment if administered 4 to 6 times daily for at least 2 weeks and up to 10 months.17 Patients may require up to 0.3% concentration for total resolution of pruritus. Importantly, capsaicin use may be limited by the high application frequency.
Systemic therapies
Fexofenadine and montelukast. Oral antihistamines have long been used as a first-line treatment for PN. Although clinical experience and anecdotal success support the use of various antihistamines, evidence-based literature exists only for fexofenadine and the leukotriene receptor antagonist montelukast. These oral agents also avoid potential unwanted effects of topical antihistamines, which may sensitize skin and increase the risk of developing allergic contact dermatitis.1
Whereas antihistamines exert their antipruritic effect by blocking histamine H1-receptors, montelukast decreases pruritic symptoms by antagonizing leukotriene receptors.10 In a prospective study of 12 patients with PN receiving fexofenadine 240 mg twice daily and montelukast 10 mg daily for 4 weeks, 9 of the 12 patients (75%) reported some degree of improvement.10 However, 5 of these 9 patients (56%) achieved only slight improvement. Level of improvement was based on how well the agents reduced the pruritus and lesion number.
Naltrexone. As an opioid antagonist, naltrexone is able to block endogenous opiates from binding to central opioid receptors and causing the sensation of pruritus. Accordingly, oral naltrexone can be used to treat PN, as shown in an open-label clinical trial in which 9 out of 17 patients (53%) achieved high antipruritic effect, defined as a reduction of pruritic symptoms by at least half.18
When selecting naltrexone to treat PN, prescribe a daily dose of 50 mg for an average of 4.7 months; up to 20 months of treatment may be required. If tachyphylaxis occurs, consider increasing the dose to 50 mg twice a day. Most patients should notice some level of antipruritic efficacy and varying degrees of lesion flattening, softening, or healing. However, exacerbation after therapy discontinuation may occur in 41% of patients. Adverse medication effects include fatigue, nausea, and dizziness.18
Gabapentin and pregabalin. In response to a report of a case series in which 4 patients with PN responded well to gabapentin,19 Mazza et al20 conducted a prospective study of pregabalin treatment for 30 patients with PN. Both gabapentin and pregabalin inhibit calcium influx and subsequent excitatory neurotransmitter release, the mechanism by which they likely decrease pruritus in patients with PN.20 In the pregabalin study, 23 out of 30 patients (77%) experienced complete resolution of pruritic symptoms and a reduction of prurigo nodules in number or flattening. The recommended dosage of pregabalin is 25 mg 3 times daily for 3 months, after which time clinical progress is assessed. If a patient is not lesion-free, continue pregabalin at a maintenance dose of 50 mg/d for up to 2 years. Adverse effects typically include headache, sedation, and dizziness.20
When to refer a dermatologist
Refer patients to a dermatologist if initial clinical findings suggest a need for further work-up to rule out primary cutaneous diseases, or if the therapies discussed (TABLE8-10,15-20) yield unsatisfactory results. Dermatologists can provide more advanced treatments that require close monitoring, such as phototherapy,21-24 cyclosporine,25 or thalidomide.26-28 Based on multiple case series and case reports, as well as our own personal experience (FIGURE 2), thalidomide is efficacious in treating PN. However, thalidomide is typically reserved for cases that are severe and treatment-recalcitrant due to the drug’s high cost, teratogenicity (pregnancy category X), and potentially irreversible peripheral neuropathy.29
Putting Tx options into practice
In addition to ruling out potential causes of pruritus and determining the best treatment for each individual with PN, assess for and appropriately treat any psychiatric comorbidities, which are often a psychological component of PN.
Localized PN. Start with topical corticosteroids under occlusion for localized PN. To avoid complications of long-term topical corticosteroid use, including dermal atrophy, periodically switch to a steroid-sparing agent, such as calcipotriol ointment or topical pimecrolimus. Less evidence is available to support the efficacy of tacrolimus ointment in PN treatment. Topical capsaicin is not as practical as other topical treatments since it needs to be applied 4 to 6 times daily. Oral antihistamines and montelukast may be added to the therapeutic regimen if there is a chronic pruritic component related to the lesions themselves or an underlying atopic diathesis fueling the itch-scratch cycle.
Widespread or treatment-resistant PN. Prescribe naltrexone, gabapentin, or pregabalin for more widespread disease or lesions resistant to conservative therapies. If you suspect a primary cutaneous disease as the underlying cause of pruritus or if topical and oral therapies do not achieve the desired therapeutic effect, refer to a dermatologist for further work-up and treatment.
How we would manage the case presented in the introduction. We would start the 43-year-old on topical corticosteroids under occlusion and periodically substitute calcipotriol ointment. (Given the unease that some patients might feel with the black-box warning on topical calcineurin inhibitors, we would likely try calcipotriol ointment as a courtesy before suggesting topical calcineurin inhibitors.) We would also prescribe an oral antihistamine at the start, given that her history of seasonal allergies and childhood asthma increases her chances of having an atopic component causing or exacerbating her disease. However, assessing her response to topical therapies before initiating an oral antihistamine would also be an appropriate strategy.
Unfortunately, PN is typically a chronic and often treatment-resistant disease with disappointing recurrence rates. As we learn more about the pathophysiology of PN, more effective therapies will hopefully emerge to improve the quality of life for these patients.
CORRESPONDENCE
Michael Saco, MD, Department of Dermatology & Cutaneous Surgery, University of South Florida, 13330 Laurel Drive, Tampa, FL 33612; [email protected]
› Start with topical corticosteroids under occlusion and periodically substitute with steroid-sparing agents (calcipotriol ointment or pimecrolimus 1% cream) for localized prurigo nodularis. B
› Consider adding oral antihistamines or montelukast to the initial regimen if a pruritic cause is suspected; alternatively, consider adding these agents if topical therapies alone do not effectively treat the prurigo nodules. C
› Turn to oral naltrexone, gabapentin, or pregabalin for more widespread or treatment-resistant cases. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 43-year-old woman arrives at your office with persistent itching on her arms and legs. For some time, she has used moisturizing lotions and herbal preparations suggested by her mother, but they have provided no relief. You note multiple 0.5- to 2-cm firm, excoriated nodules symmetrically distributed on her elbows and knees bilaterally. She has seasonal allergies and a history of childhood asthma. How would you care for this patient?
Treating prurigo nodularis (PN) can be a daunting task for even the most experienced clinician. Prurigo nodules are cutaneous lesions often produced by repetitive scratching—hence the nickname “picker’s nodules”—which may occur as sequelae of chronic pruritus or neurotic excoriations. Thus, PN can be classified as a subtype of neurodermatitis. The nodules can be intensely pruritic, resulting in an itch-scratch cycle that can be difficult to break.1,2 In this review, we examine evidence-based therapies for PN.
Key findings with prurigo nodularis
Typically, prurigo nodules are firm, hyperkeratotic, pruritic papules or nodules that range in diameter from a few millimeters to several centimeters. The lesions usually have eroded or ulcerated components secondary to repeated excoriation, which can eventually lead to scarring and changes in pigmentation. Patients can have one nodule or hundreds of lesions, depending on disease severity. The lesions tend to be distributed symmetrically and have a predilection for the extensor surfaces of the upper and lower limbs. The abdomen, posterior neck, upper and lower back, and buttocks are also commonly affected, whereas the face, palms, and flexural areas are rarely involved2-5 (FIGURE 1).
The differential diagnosis for PN includes dermatitis herpetiformis, scabies, lichen simplex chronicus, hypertrophic lichen planus, perforating disorders, atopic dermatitis, allergic contact dermatitis, neurotic excoriations, and multiple keratoacanthomas.4,5
PN prevalence and etiology are unknown. Although PN can occur at any age, the typical age range is 20 to 60 years, with middle-aged women most commonly affected. Patients who develop PN at a younger age are more likely to have an atopic diathesis.3,4
There is ongoing debate regarding whether PN is a primary cutaneous disease or a response to repetitive scratching provoked by a separate cause. PN has been associated with a variety of diseases, such as psychiatric disorders, atopic dermatitis, chronic renal failure, hyperthyroidism, iron-deficiency anemia, obstructive biliary disease, gastric malignancy, lymphoma, leukemia, human immunodeficiency virus (HIV), hepatitis B, and hepatitis C.2,3
Use the diagnostic work-up to focus on management decisions
When taking the history, first determine why patients are picking or scratching. If the lesions are pruritic or painful, look for a potential underlying cause of pruritic symptoms.6 If you identify an underlying dermatologic or systemic condition, treat that disorder first.1 For example, adequately treating a patient’s atopic dermatitis or hyperthyroidism may quell the pruritic symptoms and potentially make the prurigo nodules more responsive to symptomatic treatment or even obviate the need for such measures.
If treating the underlying cause of PN does not provide adequate relief, or if no cause for pruritic nodules can be found, the nodules may yet respond to symptomatic treatments targeted at decreasing pruritus and inflammation. In contrast, with patients who habitually scratch lesions they describe as non-pruritic, neurotic excoriations could be the source of PN, making the nodules less likely to respond to antipruritic therapies.4,7
Patient insights. Assessing whether patients have insight into their condition is also important. Some patients may be unaware that they are repetitively picking and scratching the affected areas and causing the development and perpetuation of the nodules. In cases associated with an underlying psychiatric component, such as delusional parasitosis, patients often lack insight into their condition and thus may benefit from treatment of psychiatric comorbidities.4,7
On physical exam, try to find lesions that have not been traumatized by patients. They can be useful in uncovering a primary cause, such as scabies, atopic dermatitis, lichenoid drug eruption, or simple xerosis.
If a diagnosis cannot be made clinically, consider obtaining a biopsy of a nontraumatized lesion. Traumatized lesions are typically unrevealing on histopathology. If the clinical assessment of pruritic lesions is indeterminate, laboratory tests that may prove helpful include, but are not limited to, thyroid-stimulating hormone levels, liver function tests, kidney function, a hepatitis panel, and HIV screening.
With severe refractory pruritus in which a primary cutaneous or systemic cause cannot be determined, evaluate for malignancy—especially polycythemia, lymphoma, or multiple myeloma—by ordering liver function tests (including lactate dehydrogenase), a complete blood count with differential, a basic metabolic panel, a chest x-ray, and possibly a serum protein electrophoresis.7
Available treatments
If the patient’s pruritic symptoms do not resolve and an underlying cause cannot be determined, direct treatment at decreasing pruritus either locally or systemically. Topical therapies, typically associated with fewer adverse effects, are preferable in localized cases of PN. In more severe, widespread, or recalcitrant disease, systemic agents may be necessary. Typical first-line treatments for PN aimed at decreasing pruritic symptoms include:
- topical antipruritics, such as ointments containing menthol or camphor; topical corticosteroids, with increased efficacy under occlusion as seen with flurandrenolide tape (Cordran tape)
- oral antihistamines, such as promethazine hydrochloride; oral antidepressants, such as doxepin
- intralesional corticosteroids—eg, triamcinolone acetonide (the concentration
used depends on the thickness of the lesion and how well the lesion responded to prior injections) - a short course of systemic corticosteroids, unless the patient has a comorbid condition that could be exacerbated by rapid tapering of corticosteroids (eg, psoriasis).
For patients with concomitant depression or anxiety, treatment with a selective serotonin reuptake inhibitor or anxiolytic, respectively, may be indicated.2-4 With the exception of topical corticosteroids8,9 and oral antihistamines,10 the aforementioned first-line treatments for PN are mostly based on clinical experience and anecdotal success with no studies to support their use.3 Furthermore, these treatments may be ineffective for many patients.11,12 We present our review of several studies in the literature examining potential therapies for PN.
Topical therapies
Calcipotriol vs betamethasone. A prospective, randomized, double-blind study that ran right/left comparisons of calcipotriol ointment (a vitamin D3 analog) and betamethasone ointment as treatment for PN in 9 patients showed that calcipotriol and betamethasone were both effective. However, calcipotriol ointment 50 mcg/g was more effective in reducing the number and size of nodules compared with 0.1% betamethasone valerate ointment.8
Topical corticosteroids have long been viewed as a first-line therapy for PN.2 However, given their potential for adverse effects with long-term use, such as skin atrophy, steroidsparing agents are preferred. Calcipotriol ointment can be useful as both a steroid-sparing and a keratolytic agent, as it inhibits keratinocyte proliferation.4,13 Corticosteroids and calcipotriol possess anti-inflammatory and antipruritic properties, likely explaining their efficacy in treating PN.4
Pimecrolimus and tacrolimus. The topical calcineurin inhibitors pimecrolimus and tacrolimus have been used successfully as steroid-sparing agents in treating atopic dermatitis.14 Their antipruritic effect, likely related to their influence on cutaneous sensory nerve fibers and inhibition of inflammatory cytokines, could also explain their efficacy in treating PN.15,16
A randomized, hydrocortisone-controlled, double-blind phase II trial sponsored by Novartis was designed as a right/left comparison study between pimecrolimus 1% cream and hydrocortisone 1% cream in 30 patients with non-atopic PN. When applied twice daily, each agent decreased pruritic symptoms and resolved scratch lesions to degrees that were statistically significant. However, an intention-to-treat analysis revealed no significant differences between pimecrolimus and hydrocortisone.15 In a prospective case series of 11 patients with PN, 2 out of 4 patients (50%) receiving tacrolimus 0.1% ointment and 5 out of 7 patients (71%) using pimecrolimus 1% cream experienced a reduction in pruritic symptoms and improvement of lesions by 50% or greater with twice daily application of their assigned calcineurin inhibitor.16
Before prescribing topical calcineurin inhibitors, inform patients of the black-box warning issued by the US Food and Drug Administration (FDA) regarding the theoretical increased risk of developing cutaneous malignancy and lymphoma. This warning is controversial because in clinical databases, the incidences of malignancy and lymphoma associated with topical calcineurin inhibitors are less than those observed in the general population.14
Capsaicin. Based on a prospective study of 33 patients with PN, topical capsaicin may be an effective treatment if administered 4 to 6 times daily for at least 2 weeks and up to 10 months.17 Patients may require up to 0.3% concentration for total resolution of pruritus. Importantly, capsaicin use may be limited by the high application frequency.
Systemic therapies
Fexofenadine and montelukast. Oral antihistamines have long been used as a first-line treatment for PN. Although clinical experience and anecdotal success support the use of various antihistamines, evidence-based literature exists only for fexofenadine and the leukotriene receptor antagonist montelukast. These oral agents also avoid potential unwanted effects of topical antihistamines, which may sensitize skin and increase the risk of developing allergic contact dermatitis.1
Whereas antihistamines exert their antipruritic effect by blocking histamine H1-receptors, montelukast decreases pruritic symptoms by antagonizing leukotriene receptors.10 In a prospective study of 12 patients with PN receiving fexofenadine 240 mg twice daily and montelukast 10 mg daily for 4 weeks, 9 of the 12 patients (75%) reported some degree of improvement.10 However, 5 of these 9 patients (56%) achieved only slight improvement. Level of improvement was based on how well the agents reduced the pruritus and lesion number.
Naltrexone. As an opioid antagonist, naltrexone is able to block endogenous opiates from binding to central opioid receptors and causing the sensation of pruritus. Accordingly, oral naltrexone can be used to treat PN, as shown in an open-label clinical trial in which 9 out of 17 patients (53%) achieved high antipruritic effect, defined as a reduction of pruritic symptoms by at least half.18
When selecting naltrexone to treat PN, prescribe a daily dose of 50 mg for an average of 4.7 months; up to 20 months of treatment may be required. If tachyphylaxis occurs, consider increasing the dose to 50 mg twice a day. Most patients should notice some level of antipruritic efficacy and varying degrees of lesion flattening, softening, or healing. However, exacerbation after therapy discontinuation may occur in 41% of patients. Adverse medication effects include fatigue, nausea, and dizziness.18
Gabapentin and pregabalin. In response to a report of a case series in which 4 patients with PN responded well to gabapentin,19 Mazza et al20 conducted a prospective study of pregabalin treatment for 30 patients with PN. Both gabapentin and pregabalin inhibit calcium influx and subsequent excitatory neurotransmitter release, the mechanism by which they likely decrease pruritus in patients with PN.20 In the pregabalin study, 23 out of 30 patients (77%) experienced complete resolution of pruritic symptoms and a reduction of prurigo nodules in number or flattening. The recommended dosage of pregabalin is 25 mg 3 times daily for 3 months, after which time clinical progress is assessed. If a patient is not lesion-free, continue pregabalin at a maintenance dose of 50 mg/d for up to 2 years. Adverse effects typically include headache, sedation, and dizziness.20
When to refer a dermatologist
Refer patients to a dermatologist if initial clinical findings suggest a need for further work-up to rule out primary cutaneous diseases, or if the therapies discussed (TABLE8-10,15-20) yield unsatisfactory results. Dermatologists can provide more advanced treatments that require close monitoring, such as phototherapy,21-24 cyclosporine,25 or thalidomide.26-28 Based on multiple case series and case reports, as well as our own personal experience (FIGURE 2), thalidomide is efficacious in treating PN. However, thalidomide is typically reserved for cases that are severe and treatment-recalcitrant due to the drug’s high cost, teratogenicity (pregnancy category X), and potentially irreversible peripheral neuropathy.29
Putting Tx options into practice
In addition to ruling out potential causes of pruritus and determining the best treatment for each individual with PN, assess for and appropriately treat any psychiatric comorbidities, which are often a psychological component of PN.
Localized PN. Start with topical corticosteroids under occlusion for localized PN. To avoid complications of long-term topical corticosteroid use, including dermal atrophy, periodically switch to a steroid-sparing agent, such as calcipotriol ointment or topical pimecrolimus. Less evidence is available to support the efficacy of tacrolimus ointment in PN treatment. Topical capsaicin is not as practical as other topical treatments since it needs to be applied 4 to 6 times daily. Oral antihistamines and montelukast may be added to the therapeutic regimen if there is a chronic pruritic component related to the lesions themselves or an underlying atopic diathesis fueling the itch-scratch cycle.
Widespread or treatment-resistant PN. Prescribe naltrexone, gabapentin, or pregabalin for more widespread disease or lesions resistant to conservative therapies. If you suspect a primary cutaneous disease as the underlying cause of pruritus or if topical and oral therapies do not achieve the desired therapeutic effect, refer to a dermatologist for further work-up and treatment.
How we would manage the case presented in the introduction. We would start the 43-year-old on topical corticosteroids under occlusion and periodically substitute calcipotriol ointment. (Given the unease that some patients might feel with the black-box warning on topical calcineurin inhibitors, we would likely try calcipotriol ointment as a courtesy before suggesting topical calcineurin inhibitors.) We would also prescribe an oral antihistamine at the start, given that her history of seasonal allergies and childhood asthma increases her chances of having an atopic component causing or exacerbating her disease. However, assessing her response to topical therapies before initiating an oral antihistamine would also be an appropriate strategy.
Unfortunately, PN is typically a chronic and often treatment-resistant disease with disappointing recurrence rates. As we learn more about the pathophysiology of PN, more effective therapies will hopefully emerge to improve the quality of life for these patients.
CORRESPONDENCE
Michael Saco, MD, Department of Dermatology & Cutaneous Surgery, University of South Florida, 13330 Laurel Drive, Tampa, FL 33612; [email protected]
1. Moses S. Pruritus. Am Fam Phys. 2003;68:1135-1142.
2. Jorizzo JL, Gatti S, Smith EB. Prurigo: a clinical review. J Am Acad Dermatol. 1981;4:723-728.
3. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-218.
4. Wallengren J. Prurigo: diagnosis and management. Am J Clin Dermatol. 2004;5:85-95.
5. Accioly-Filho LW, Nogueira A, Ramos-e-Silva M. Prurigo nodularis of Hyde: an update. J Eur Acad Dermatol Venereol. 2000;14:75-82.
6. Reamy BV, Bunt CW, Fletcher S. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
7. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450.
8. Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of Prurigo nodularis. Arch Dermatol. 2000;136:807-808.
9. Saraceno R, Chiricozzi A, Nisticò SP, et al. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21:363-366.
10. Shintani T, Ohata C, Koga H, et al. Combination therapy of fexofenadine and montelukast is effective in prurigo nodularis and pemphigoid nodularis. Dermatol Ther. 2014;27:135-139.
11. Paghdal KV, Schwartz R. Thalidomide and its dermatologic uses. Acta Dermatovenerol Croat. 2007;15:39-44.
12. Alfadley A, Al-Hawsawi K, Thestrup-Pedersen K, et al. Treatment of prurigo nodularis with thalidomide: a case report and review of the literature. Int J Dermatol. 2003;42:372-375.
13. Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
14. Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
15. Siepmann D, Lotts T, Blome C, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, doubleblind phase II trial. Dermatology. 2013;227:353-360.
16. Ständer S, Schürmeyer-Horst F, Luger TA, et al. Treatment of pruritic diseases with topical calcineurin inhibitors. Ther Clin Risk Manag. 2006;2:213-218.
17. Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471-478.
18. Metze D, Reimann S, Beissert S, et al. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41:533-539.
19. Gencoglan G, Inanir I, Gunduz K. Therapeutic hotline: treatment of prurigo nodularis and lichen simplex chronicus with gabapentin. Dermatol Ther. 2010;23:194-198.
20. Mazza M, Guerriero G, Marano G, et al. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38:16-18.
21. Hammes S, Hermann J, Roos S, et al. UVB 308-nm excimer light and bath PUVA: combination therapy is very effective in the treatment of prurigo nodularis. J Eur Acad Dermatol Venereol. 2011;25:799-803.
22. Rombold S, Lobisch K, Katzer K, et al. Efficacy of UVA1 phototherapy in 230 patients with various skin diseases. Photodermatol Photoimmunol Photomed. 2008;24:19-23.
23. Tamagawa-Mineoka R, Katoh N, Ueda E, et al. Narrow-band ultraviolet B phototherapy in patients with recalcitrant nodular prurigo. J Dermatol. 2007;34:691-695.
24. Saraceno R, Nisticò SP, Capriotti E, et al. Monochromatic excimer light (308 nm) in the treatment of prurigo nodularis. Photodermatol Photoimmunol Photomed. 2008;24:43-45.
25. Siepmann D, Luger TA, Ständer S. Antipruritic effect of cyclosporine microemulsion in prurigo nodularis: results of a case series. J Dtsch Dermatol Ges. 2008;6:941-946.
26. Orlando A, Renna S, Cottone M. Prurigo nodularis of Hyde treated with low-dose thalidomide. Eur Rev Med Pharmacol Sci. 2009;13:141-145.
27. Lan CC, Lin CL, Wu CS, et al. Treatment of idiopathic prurigo nodularis in Taiwanese patients with low-dose thalidomide. J Dermatol. 2007;34:237-242.
28. Taefehnorooz H, Truchetet F, Barbaud A, et al. Efficacy of thalidomide in the treatment of prurigo nodularis. Acta Derm Venereol. 2011;91:344-345.
29. Wu JJ, Huang DB, Pang KR, et al. Thalidomide: dermatological indications, mechanisms of action and side-effects. Br J Dermatol. 2005;153:254-273.
1. Moses S. Pruritus. Am Fam Phys. 2003;68:1135-1142.
2. Jorizzo JL, Gatti S, Smith EB. Prurigo: a clinical review. J Am Acad Dermatol. 1981;4:723-728.
3. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-218.
4. Wallengren J. Prurigo: diagnosis and management. Am J Clin Dermatol. 2004;5:85-95.
5. Accioly-Filho LW, Nogueira A, Ramos-e-Silva M. Prurigo nodularis of Hyde: an update. J Eur Acad Dermatol Venereol. 2000;14:75-82.
6. Reamy BV, Bunt CW, Fletcher S. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
7. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450.
8. Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of Prurigo nodularis. Arch Dermatol. 2000;136:807-808.
9. Saraceno R, Chiricozzi A, Nisticò SP, et al. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21:363-366.
10. Shintani T, Ohata C, Koga H, et al. Combination therapy of fexofenadine and montelukast is effective in prurigo nodularis and pemphigoid nodularis. Dermatol Ther. 2014;27:135-139.
11. Paghdal KV, Schwartz R. Thalidomide and its dermatologic uses. Acta Dermatovenerol Croat. 2007;15:39-44.
12. Alfadley A, Al-Hawsawi K, Thestrup-Pedersen K, et al. Treatment of prurigo nodularis with thalidomide: a case report and review of the literature. Int J Dermatol. 2003;42:372-375.
13. Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
14. Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
15. Siepmann D, Lotts T, Blome C, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, doubleblind phase II trial. Dermatology. 2013;227:353-360.
16. Ständer S, Schürmeyer-Horst F, Luger TA, et al. Treatment of pruritic diseases with topical calcineurin inhibitors. Ther Clin Risk Manag. 2006;2:213-218.
17. Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471-478.
18. Metze D, Reimann S, Beissert S, et al. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41:533-539.
19. Gencoglan G, Inanir I, Gunduz K. Therapeutic hotline: treatment of prurigo nodularis and lichen simplex chronicus with gabapentin. Dermatol Ther. 2010;23:194-198.
20. Mazza M, Guerriero G, Marano G, et al. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38:16-18.
21. Hammes S, Hermann J, Roos S, et al. UVB 308-nm excimer light and bath PUVA: combination therapy is very effective in the treatment of prurigo nodularis. J Eur Acad Dermatol Venereol. 2011;25:799-803.
22. Rombold S, Lobisch K, Katzer K, et al. Efficacy of UVA1 phototherapy in 230 patients with various skin diseases. Photodermatol Photoimmunol Photomed. 2008;24:19-23.
23. Tamagawa-Mineoka R, Katoh N, Ueda E, et al. Narrow-band ultraviolet B phototherapy in patients with recalcitrant nodular prurigo. J Dermatol. 2007;34:691-695.
24. Saraceno R, Nisticò SP, Capriotti E, et al. Monochromatic excimer light (308 nm) in the treatment of prurigo nodularis. Photodermatol Photoimmunol Photomed. 2008;24:43-45.
25. Siepmann D, Luger TA, Ständer S. Antipruritic effect of cyclosporine microemulsion in prurigo nodularis: results of a case series. J Dtsch Dermatol Ges. 2008;6:941-946.
26. Orlando A, Renna S, Cottone M. Prurigo nodularis of Hyde treated with low-dose thalidomide. Eur Rev Med Pharmacol Sci. 2009;13:141-145.
27. Lan CC, Lin CL, Wu CS, et al. Treatment of idiopathic prurigo nodularis in Taiwanese patients with low-dose thalidomide. J Dermatol. 2007;34:237-242.
28. Taefehnorooz H, Truchetet F, Barbaud A, et al. Efficacy of thalidomide in the treatment of prurigo nodularis. Acta Derm Venereol. 2011;91:344-345.
29. Wu JJ, Huang DB, Pang KR, et al. Thalidomide: dermatological indications, mechanisms of action and side-effects. Br J Dermatol. 2005;153:254-273.